Preeclampsia: Recent Advances in Predicting, Preventing, and Managing the Maternal and Fetal Life-Threatening Condition
Kai-jung chang.
1 Department of Obstetrics and Gynecology, Taipei Tzu-Chi Hospital, The Buddhist Tzu-Chi Medical Foundation, Taipei 231, Taiwan

Kok-Min Seow
2 Department of Obstetrics and Gynecology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei 111, Taiwan
3 Department of Obstetrics and Gynecology, National Yang-Ming Chiao-Tung University, Taipei 112, Taiwan
Kuo-Hu Chen
4 School of Medicine, Tzu-Chi University, Hualien 970, Taiwan
Associated Data
Not applicable.
Preeclampsia accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies. Defined as gestational hypertension after 20 weeks of pregnancy and coexisting proteinuria or generalized edema, and certain forms of organ damage, it is life-threatening for both the mother and the fetus, in terms of increasing the rate of mortality and morbidity. Preeclamptic pregnancies are strongly associated with significantly higher medical costs. The maternal costs are related to the extra utility of the healthcare system, more resources used during hospitalization, and likely more surgical spending due to an elevated rate of cesarean deliveries. The infant costs also contribute to a large percentage of the expenses as the babies are prone to preterm deliveries and relevant or causative adverse events. Preeclampsia imposes a considerable financial burden on our societies. It is important for healthcare providers and policy-makers to recognize this phenomenon and allocate enough economic budgets and medical and social resources accordingly. The true cellular and molecular mechanisms underlying preeclampsia remain largely unexplained, which is assumed to be a two-stage process of impaired uteroplacental perfusion with or without prior defective trophoblast invasion (stage 1), followed by general endothelial dysfunction and vascular inflammation that lead to systemic organ damages (stage 2). Risk factors for preeclampsia including race, advanced maternal age, obesity, nulliparity, multi-fetal pregnancy, and co-existing medical disorders, can serve as warnings or markers that call for enhanced surveillance of maternal and fetal well-being. Doppler ultrasonography and biomarkers including the mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and serum pregnancy-associated plasma protein A (PAPP-A) can be used for the prediction of preeclampsia. For women perceived as high-risk individuals for developing preeclampsia, the administration of low-dose aspirin on a daily basis since early pregnancy has proven to be the most effective way to prevent preeclampsia. For preeclamptic females, relevant information, counseling, and suggestions should be provided to facilitate timely intervention or specialty referral. In pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance including the Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test can be arranged. If the results are unfavorable, early intervention and aggressive therapy should be considered. Affected females should have access to higher levels of obstetric units and neonatal institutes. Before, during, and after delivery, monitoring and preparation should be intensified for affected gravidas to avoid serious complications of preeclampsia. In severe cases, delivery of the fetus and the placenta is the ultimate solution to treat preeclampsia. The current review is a summary of recent advances regarding the knowledge of preeclampsia. However, the detailed etiology, pathophysiology, and effect of preeclampsia seem complicated, and further research to address the primary etiology and pathophysiology underlying the clinical manifestations and outcomes is warranted.
1. Introduction
Hypertensive disorder during pregnancy poses a substantial threat to both maternal and fetal health conditions [ 1 ]. Preeclampsia is one of the most well-known medical conditions that belong to this disease spectrum, which also accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies [ 2 , 3 ]. It is depicted as a gestational condition with a hypertensive disorder diagnosed after 20 weeks of gestation and coexisting proteinuria or generalized edema, and certain forms of hematologic disorders such as thrombocytopenia or signs of end organ damage including renal impairment, abnormal liver function, pulmonary edema, and cerebral and visual disturbance [ 4 , 5 ]. The definitions of gestational hypertension (pregnancy-induced hypertension) and preeclampsia are shown in Figure 1 . Serious or long-term complications may result when preeclampsia turns into a severe type or is left without being sufficiently treated. Multiorgan involvement may be seen in such cases, and the impairment of uteroplacental perfusion could potentially lead to gestational complications and poor fetal outcomes including intrauterine fetal growth restriction and preterm delivery. As the situation worsens, it may become life-threatening for both the mother and the fetus, in terms of increasing the rate of mortality and morbidity [ 5 ].

The definitions of gestational hypertension (pregnancy-induced hypertension) and preeclampsia.
One straightforward way to categorize preeclampsia is to subdivide it into early-onset and late-onset groups in accordance with the gestational age (GA). The cutoff point is usually set as GA 34 weeks or GA 37 weeks, and we can subcategorize preeclampsia into the early-onset (GA < 34 weeks), late-onset (GA ≥ 34 weeks), preterm (GA < 37 weeks), and term (GA ≥ 37weeks) subgroups ( Table 1 ). The diagnoses made at different timings during the pregnancy course may suggest different pathophysiologic and etiologic pathways [ 6 ].
Classification of preeclampsia according to gestational age.
| Gestational Age | Terminology |
|---|---|
| GA < 34 weeks | Early-onset preeclampsia |
| GA ≥ 34weeks | Late-onset preeclampsia |
| GA < 37w | Preterm preeclampsia |
| GA ≥ 37w | Term preeclampsia |
Preeclampsia should be viewed as a disease spectrum in which different subtypes may vary greatly in disease mechanisms and clinical presentations. The status of a previously normotensive pregnant woman developing new onset of hypertension after GA 20 weeks is termed “gestational hypertension” or “pregnancy induced hypertension”. If aside from gestational hypertension, a patient is also noted with proteinuria, thrombocytopenia, impairment in renal or liver function, cerebral symptoms, visual symptoms, or pulmonary edema, then she meets the diagnostic criteria of preeclampsia ( Figure 1 ). In terms of severity, preeclampsia could be classified as “nonsevere” or “severe” types ( Table 2 ), with the latter group exhibiting clinical features including blood pressure exceeding 160/100 mmHg, headache, visual disturbances, upper abdominal pain, oliguria, elevated serum creatinine, thrombocytopenia (<100,000/µL), elevated level of liver enzymes, fetal growth restriction, pulmonary edema, onset at an early gestational age, and the presence of convulsion (eclampsia) [ 7 ].
Classification of preeclampsia according to severity.
| Abnormality | Non-Severe Type | Severe Type |
|---|---|---|
| Systolic blood pressure | ≥140 mmHg | ≥160 mmHg |
| DBP | ≥90 mmHg | ≥110 mmHg |
| Thrombocytopenia (<10 /L) | Absent | Present |
| Abnormal liver function (liver enzymes two times the normal limits) | Absent | Present |
| Renal insufficiency (serum creatinine level exceeding 1.1 mg/dL or two times the normal limits) | Absent | Present |
| Proteinuria | Absent or Present | Absent or Present |
| New-onset headache | Absent | Present |
| Visual disturbance | Absent | Present |
| Upper abdominal pain | Absent | Present |
| Pulmonary edema | Absent | Present |
| Convulsion/Eclampsia | Absent | Present |
Although the definite cause of preeclampsia remains unknown to date, several hypotheses have been made to explain its pathophysiology. One of the most commonly accepted theories is the two-stage model, which proposes that inadequate trophoblast invasion would lead to shallow placentation and subsequent poor uteroplacental perfusion (stage I), thus causing widespread endothelial dysfunction and systemic clinical manifestations (stage II) [ 8 ]. The window between the first and second stages provides an optimal opportunity for prediction during the subclinical phase [ 5 ]. Known as a safe and effective drug in the prevention of pregnancy-related vascular disorders including but not limited to preeclampsia, aspirin has been applied for preeclampsia prevention with a low dosage starting as early as before GA 16 weeks and until approximately GA 36 weeks [ 9 ]. Nevertheless, non-pregnant women throughout the world enjoy the privilege of early prevention and intervention. Even if they do, sometimes preeclampsia may still develop. The only definite solution for preeclampsia is the delivery or termination of pregnancy. When a diagnosis is made, antihypertensive medication is, however, one of the most important treatments before delivery. Fluid control, prevention, and treatment for end organ damage should be applied as well [ 10 ].
Due to the notable prevalence and influence of preeclampsia in pregnancy, an understanding of preeclampsia, as thorough as possible, is crucial. The review aimed to summarize existing studies in the literature to explore the epidemiology, etiology (risk factors), socioeconomic burdens, pathophysiologic mechanisms, prediction, prevention, and treatment of preeclampsia. The cutting-edge studies will be analyzed and integrated into this review to provide state-of-the-art knowledge.
2. Materials and Methods
Searching terms and strategies in the literature.
The literature was searched to identify basic and clinical studies, which investigated the epidemiology, etiology (risk factors), socioeconomic burdens, and underlying pathophysiological mechanisms of preeclampsia, along with its prediction, prevention, and treatment. Figure 2 illustrates the flowchart of database searching, screening, and inclusion of the references that we selected from the literature. In this review, all of the articles were retrieved from the databases Medline and PubMed using the search terms “preeclampsia”, “gestational hypertension”, and “pregnancy induced hypertension” for the research topic. For screening and selection in the next stage, only full-text articles were considered for inclusion in further analysis. In the second stage, the articles published before 1983 were excluded to ensure the novelty of the current review. Duplicated articles were also excluded. From a total of 152 articles identified in the screening process, 126 potential articles (1983–2022) met the criteria for inclusion.

Flowchart of database searching, screening, and inclusion of the references selected from the literature.
Hereafter, two experts in the field independently inspected the contents of articles including demographics, research designs, and outcomes, and identified eligible basic and clinical studies for inclusion. The solicited articles with poor research designs, questionable sampling methods, or mismatched outcomes would be excluded at this stage. The discrepancies between the experts were discussed via mutual communication to reach a consensus. All eligible studies were included in the review using the search terms and strategies (identification from the database, screening of the studies, selection of potential articles, and final inclusion). Finally, a total of 103 articles were collected for review from 152 articles identified in the initial search.
3. Epidemiology, Etiology (Risk Factors), and Economic Burden of Preeclampsia
3.1. epidemiology and risk factors.
Preeclampsia is a gestational disorder affecting women worldwide from different nations, ethnicities, age groups, etc. Overall, a prevalence rate of approximately 2 to 15% of pregnant women is documented, with an average prevalence rate of approximately 4.6% [ 2 , 3 , 11 ]. The pathophysiology of preeclampsia is complex and remains incompletely unveiled, which makes it sensible that its prevalence and traits would vary under different circumstances. In other words, different populations with preeclampsia may display different prevalence, patterns, or distribution of risk factors and pregnancy outcomes. The study of its epidemiology and risk factors could thereby demonstrate its complexity and heterogeneity. Table 3 presents a list of risk factors for preeclampsia.
A brief summary of risk factors for preeclampsia.
| High-Risk Factors | Moderate/Other Risk Factors |
|---|---|
| Prior history of preeclampsia Chronic hypertension Pre-existing type I or type II diabetes mellitus Renal disease Autoimmune disease (esp. antiphospholipid syndrome, systemic lupus erythematous) Young maternal age (<25 years) | Nulliparity Advanced maternal age (>35 or >40 y/o) Family history of preeclampsia Long interpregnancy interval (>5 or >10 years) Maternal overweight/obesity (BMI > 30 or 35 kg/m ) Multifetal gestation Family history of early-onset cardiovascular disease History of SGA or adverse gestational outcomes Previous miscarriage with same partner Low maternal birthweight of preterm delivery Increased prepregnancy triglycerides Heritable thrombophilia Connective tissue disorder Vaginal bleeding in early pregnancy Gestational trophoblastic disease Drug abuse |
3.2. Race and Ethnicity
In a cross-sectional study conducted by Yang et al., a thorough comparison between the characteristics of preeclampsia among the Chinese and Swedish populations was made. The study included a total of 634,689 pregnancies, among which the Chinese and the Swedish exhibited similar prevalence rates of approximately 2 to 3%. However, there were marked variabilities in the other descriptive results. The maternal age, mean body mass index (BMI), and obesity rates were higher in Sweden, while more nulliparous women and cesarean deliveries were identified in their Chinese counterparts. The disease extent and pregnancy outcomes also differed. Mild preeclampsia was more common in the Swedish population, while there were more severe cases in China. The Chinese also had overall higher rates of stillbirth, preterm birth, and low birth weight. Ethnicity, lifestyle, metabolic perturbations, genetic factors, and seeking medical help may all contribute to these variabilities [ 11 ]. While race is a potential contributory factor, it may not be completely persuasive in this scenario since the Swedish comprised relatively richer ethnicities whereas the Chinese were primarily Hans.
The role of race in preeclampsia has been investigated in various studies. A review article written by Zhang et al. pointed out that African Americans had a higher rate and severity of preeclampsia, which was likely related to, if not directly resulting from, multifactorial causes including previous history of preeclampsia, system lupus erythematosus, sickle cell anemia, gestational diabetes mellitus, and a history of chronic hypertension [ 12 ]. Another study conducted by Ghosh et al. suggested that non-Hispanic women had higher odds of developing preeclampsia and had greater severity of disease, compared with Hispanic women and Asian/Pacific Islanders. An expert review written by Johnson also mentioned a higher risk of preeclampsia among Black, Native American, and Native Alaskan races. Nevertheless, it is worth noting that research focusing on the role of race or ethnicity in the disparities of preeclampsia shares some common limitations. Firstly, race or ethnicity is not a scientifically biological or genetic trait; rather, it is more often self-reported and thus may become subjective. Secondly, a person could belong to more than one racial or ethnic group instead of being assigned to one single category. Thirdly, a standardized method of classification used in medical research may fail to reflect the cultural, lingual, or historical origins and distributions in reality. Instead of serving as a direct or independent factor, the role that race or ethnicity plays in preeclampsia may correspond to the reflection or marker for the influence of cultural, socioeconomic, or healthcare resources, etc. [ 13 ].
Many real-world statistics suggest that advanced or extremely young maternal age is an important risk factor for preeclampsia. Furthermore, these mothers at risk may also face more adverse maternal and neonatal outcomes and hence should raise special concerns throughout their pregnancy courses.
A cohort study that included preeclamptic individuals from 1998–2014 in the U.S. suggested that women at extreme ages (<25 years or >45 years) tended to develop severe morbidities. Women younger than 25 years of age had a significantly higher rate of developing eclampsia. On the other hand, women more than 45 years old were more likely to suffer from acute heart failure and acute kidney injury (acute renal failure). The results suggested that both extremely young and elderly mothers were exposed to a greater threat during gestation but possibly from different perspectives [ 14 ].
Some studies focused mainly on the advanced maternal age (AMA) groups. A registry-based study in Finland suggested that women older than 35 years exhibited more preeclampsia, early and late preterm deliveries, cesarean deliveries, and poorer neonatal outcomes [ 15 ]. In a retrospective cross-sectional study in Indonesia conducted in 2016-2017, preeclamptic women over 35 years of age developed more severe complications in general, with postpartum hemorrhage in particular, while no significant increase in the developments of HELLP syndrome, visual disturbances, pulmonary edema, or eclampsia was identified. Regarding neonatal outcomes, there were more preterm deliveries (GA < 37 weeks), intrauterine growth restrictions, neonatal asphyxia, and neonatal infections in the group of advanced-age women [ 16 ].
3.4. Parity
Nulliparity has long been classified as a risk factor, as it may triple the risk for preeclampsia [ 17 ]. Some studies have concluded that nulliparous women were found with a higher percentage of preeclampsia compared to other cohorts [ 18 ]. Many hypotheses attribute this to immunological reasons. A feasible explanation is that a suboptimal maternal adaptation to fetal or paternal alloantigens may indirectly result in impaired uteroplacental perfusion, which accounts for the pathogenesis of preeclampsia [ 19 ]. Nulliparous women were also proposed to endure an “angiogenic imbalance”, manifested as a higher circulating sFlt1 level and sFlt1/PIGF ratio, which may also contribute to their tendency of developing preeclampsia [ 20 ]. From a fundamental viewpoint, meanwhile, there may be little difference between nulliparous and multiparous women regarding other risk factors including AMA, diabetes mellitus, multifetal gestations, etc. [ 21 ].
3.5. Obesity
Obesity is an alarming issue in the modern world and has proven to confer many hazards to human health. With time, it has raised concerns in both developed and developing countries. As one of the leading attributable risk factors, the mechanism of how obesity could potentially lead to preeclampsia has been studied. Obesity is known to be associated with systemic inflammatory reactions, insulin resistance, and oxidative stress. The pathways through which obesity could result in hypertensive disorder include increased oxidative stress, increased sympathetic tone, and increased expression of angiotensinogen [ 22 ]. Insulin resistance, on the other hand, is linked to reduced cytotrophoblast migration and consequent placental ischemia [ 23 ].
For pregnant women, maternal obesity, maternal overweight, and even a BMI increase within the normal range may indicate an increased risk of maternal and fetal morbidities, including preeclampsia. Accordingly, obese or overweight pregnant women should be advised to lose weight through diet control, a moderate amount of physical activity, and lifestyle modification.
3.6. Other Maternal Conditions
One of the most important risk factors for preeclampsia is a history of preeclampsia in a previous pregnancy. Women with preeclampsia in their first pregnancies have a notably higher risk of developing it again in their second pregnancies. Many cohort studies have demonstrated this phenomenon. [ref. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies]. Aside from her own medical history, a woman’s family history of preeclampsia should warrant special concerns as well, as a positive family history is also a powerful indicator of preeclampsia at all stages of the pregnancy course [ 24 , 25 ].
Multifetal gestation is associated with a three- to four-fold increased risk for preeclampsia. This may be more related to the gestation itself because multifetal gestation imposes a greater burden on the cardiovascular system. Individuals with multifetal gestations are frequently excluded from general studies, as they are often viewed as a special population. For these women who are diagnosed with preeclampsia, it may be confusing whether some of the adverse outcomes such as preterm deliveries are related to preeclampsia or multifetal gestation per se. Nevertheless, the fact that women with multifetal gestations are more prone to preeclampsia and serious complications should raise more attention, and allow an opportunity for screening, prevention, or early intervention [ 26 ].
Preeclamptic patients with pre-pregnancy chronic hypertension are classified to have “superimposed preeclampsia”. Chronic hypertension accounts for approximately 4% of pregnancies and is often associated with adverse gestational outcomes such as preeclampsia, preterm delivery, intrauterine fetal growth restriction, and placenta abruption. Approximately 20% of these patients eventually develop preeclampsia and tend to do so even earlier than the normotensive population. Poorer pregnancy outcomes are observed concurrently [ 27 ].
Both pre-existing type I and type II diabetes mellitus have been shown to possess a higher risk of preeclampsia. Statistically, 10–20% of diabetic women develop preeclampsia during pregnancy, which is a significantly higher percentage compared to their non-diabetic counterparts. On the other hand, gestational diabetes mellitus (GDM) is also considered to be an independent risk factor for preeclampsia by some researchers, while more investigations are required to determine whether GDM and preeclampsia share a common etiologic pathway [ 28 ].
While preeclamptic patients have an increased long-term risk of developing an end-stage renal disease, the renal disease itself may also serve as a risk factor for preeclampsia. To be more specific, microalbuminuria, diabetic nephropathy, and chronic kidney disease may predispose to preeclampsia [ 29 ]. This may be related to impaired glycocalyx integrity and alterations in the complement and renin-angiotensin-aldosterone systems [ 30 ].
Other than the aforementioned risk factors, mostly involving but not limited to pre-pregnancy health conditions, there are numerous other risk factors that could be mentioned. Aside from pre-existing hypertension, diabetes mellitus, and renal disease, particular medical conditions including autoimmune disease, periodontal disease, and antidepressant exposure have all been proven to play a part in this “disease of theories”. [ 31 , 32 , 33 ].
3.7. Socioeconomic Burden
Preeclampsia is one of the top causes of adverse maternal and fetal outcomes globally, and hence a short-term special medical care program is often required to take care of preeclamptic patients. This makes it not only a health-related issue but also a socioeconomic one, as greater manpower or resource consumption and extra spending in the healthcare system would be inevitable. Yet, few studies have aimed to make estimates of the potentially enormous socioeconomic burden related to preeclampsia. Relevant studies have made limited conclusions to date.
Jing Hao et al. conducted a retrospective study to investigate the economic burden of preeclampsia using data from the United States. Three cohorts were defined in this study: Women who had uncomplicated pregnancies until term, women with hypertension but not preeclampsia, and women diagnosed with preeclampsia. The maternal and infant costs were estimated from GA 20 weeks until 6 weeks postpartum for the former and 12 months post-delivery for the latter. The mean care cost of the preeclamptic group was $41,790 USD, which was significantly higher than the uncomplicated group ($13,187 USD) and the group with hypertension but without preeclampsia ($24,182 USD). The cost difference was largely dependent on the infant costs [ 34 ].
Another retrospective study focused on a similar issue in the United States was conducted by Warren et al. The maternal costs were estimated from 6 months before birth and 12 months afterward, while the infant costs were calculated until 12 months of age. The results suggested an estimated increased cost of $6583 USD per birth in the maternal model. On the other hand, the increased cost of the infant model was substantially influenced by the gestational age at birth. Costs devoted to the infant accounted for 26% of total healthcare costs at term delivery, and a tremendously increased percentage of 91% with deliveries at GA < 28 weeks. As a result of preeclampsia and preterm deliveries, these high costs were more closely related to adverse fetal or infant outcomes, including intraventricular hemorrhage, bronchopulmonary dysplasia, periventricular leukomalacia, and infant death [ 35 ].
An Irish study using data from the SCOPE (Screening for Pregnancy End Points) study disclosed a doubling average cost (5243 EUR) in preeclampsia-complicated pregnancies. The study included data from the initial antepartum visit until 12 months postpartum and drew the conclusion that these costs were primarily related to postpartum care, followed by antepartum and peripartum care, respectively. The increased medical costs were related to more and higher-level health services including antepartum examinations, more maternal hospitalization spending, longer infantile NICU stays, etc. [ 36 ].
Despite the lack of abundant research, present economic studies on preeclampsia in different parts of the world seem to have reached the consensus that preeclamptic pregnancies are strongly associated with significantly higher medical costs for both the mother and her baby. The maternal costs are related to the extra utility of the healthcare system, more resources used during hospitalization, and likely more surgical spending due to an elevated rate of cesarean deliveries. The infant costs also contribute to a large percentage of the expenses as the babies are prone to preterm deliveries and relevant or causative adverse events. Although studies cannot reflect the accurate amount of preeclampsia-related healthcare costs in reality, there is no doubt that preeclampsia imposes a considerable financial burden on our societies. It is important for healthcare providers and policy-makers to recognize this phenomenon and allocate enough economic budgets, medical, and social resources accordingly.
4. Pathophysiology of Preeclampsia
4.1. brief summary.
Preeclampsia has been termed a “disease of theories” by some as numerous studies have aimed to propose different concepts to explore its complex etiology and pathophysiology. Previous findings have suggested that the triggers of preeclampsia include placental factors and other predisposing maternal factors. The mechanisms of early- and late-onset preeclampsia may not be completely the same. Based on the current understanding of preeclampsia, the revised “two-stage model” has become one of the most widely accepted theories regarding its formation.
The classical two-stage model was first described in 1991, innovatively introducing the idea that preeclampsia should be viewed as a trophoblastic disease rather than merely a hypertensive disorder. In this model, the first stage of preeclampsia is described as the “placental stage”, in which deficient remodeling of spiral arteries results in impaired placental perfusion and placental ischemia. As the disease progresses with time and clinical maternal syndrome develops, it reaches the second stage [ 37 ]. The clinical manifestations of preeclampsia will be further discussed in the next section.
Ever since the initial proposal of the two-stage theory, ongoing research has expanded and refined our knowledge of the development of preeclampsia. Stage 1 is focused on the revised idea of impaired uteroplacental perfusion with or without poor placentation and subsequent spiral artery insufficiency. Stage 2 surrounds the concept that general endothelial dysfunction and vascular inflammation would lead to a systemic clinical response. Figure 3 displays the contributing factors and the two-stage models of preeclampsia.

Contributing factors and the two-stage models of preeclampsia.
4.2. Stage 1
In the current revised model, stage 1 is initiated when reduced placental perfusion develops. Poor placentation and the resultant deficient spiral artery remodeling in the intervillous space is one of the causes for this phase but may not be the sole mechanism. In addition to the impairment of placental perfusion, maternal factors are essential so as to result in the development of systemic maternal pathophysiological changes [ 37 ]. Stage 1 usually occurs in the first trimester, at the period of time when the deep invasion of the extravillous trophoblast (EVT) takes place. The migration of EVT cells into the decidua leads to the remodeling of maternal spiral arteries, which is a key element to uteroplacental perfusion and fetal blood supply. The process may initiate as early as before GA 8 weeks, while the establishment of uteroplacental circulation is completed at approximately GA 12 weeks. Hence, it is believed that stage 1 takes place before GA 12 to 20 weeks [ 38 ].
The differentiation and invasion of EVTs are regulated by various factors including cytokines, growth factors, chemokines, cell adhesion molecules, placental oxygen tension, extracellular matrix (ECM)-degrading enzymes, and membrane-bound cell surface peptidases. These factors are either directly or indirectly related to the differentiation and decidual invasion of EVT cells and may serve as markers for the first stage of preeclampsia formation [ 38 ]. When defective trophoblast invasion and insufficient transformation of the maternal uterine vasculature emerge, decreased maternal uterine blood flow follows, which may be detected and quantified by uterine artery Doppler studies. Persistent high vessel resistance in early pregnancy may suggest that the aforementioned phenomenon has occurred. Existing studies have demonstrated that the placental endothelial cells in women with high-resistance uterine arteries are more sensitive to TNFα and thus are more susceptible to cell injury and apoptosis.
In the normal process of trophoblast invasion, the resistance of uterine artery blood flow decreases, and the uterine artery blood flow increases in the term. The placenta is typically developed in the first trimester. If a relatively hypoxic environment is noted, the latter placental tissues may exhibit an altered balance of antioxidant enzyme activity. However, the histopathology findings of the placenta are nonspecific and not limited to preeclamptic pregnancies. These changes to the placenta can also be induced by other microscopic insults or toxins [ 39 ].
4.3. Stage 2
Stage 2 features the scenario where impaired uteroplacental perfusion interacts with other various maternal constitutional factors. Pathophysiological changes in the liver, kidney, and cardiovascular system are compatible with the concept of insufficient blood supply. Systemic endothelial dysfunction and injury are possible explanations for the maternal clinical manifestations and have been proven to be present in preeclamptic women.
One important issue that attracts interest is how the first stage links to or leads to the second stage. The clinical value of discovering the answer lies in the fact that it may shed light on a way to “prevent” the formation of preeclampsia, which will be further discussed in the article. One proposal suggests that microparticle particles produced during syncytiotrophoblast apoptosis may directly or indirectly result in endothelial dysfunction. An increased amount and concentration of inflammatory cells and substances have been found in women with preeclampsia, and they could potentially alter the systemic endothelial function. The renin-angiotensin system may also play a role in the process. In addition, some recent findings have suggested that vascular endothelial growth factor (VEGF) and placental growth factor (sFlt-1) could be involved in the linkage as well. Moreover, oxidative stress accumulated during the process may provide another possible explanation [ 40 ]. Table 4 lists the possible pathways and explanations of mechanisms underlying preeclampsia.
Possible pathways and explanations of mechanisms underlying preeclampsia.
| Possible Pathways | Explanations |
|---|---|
| Failure of maternal vascular adaptation | |
| Dysregulation of the renin-angiotensin system (RAS) | |
| Oxidative stress |
Various factors contribute to the regulation of artery compliance during pregnancy. A failure of maternal vascular adaptation can cause hypertensive disorders such as preeclampsia. Some circulating cytokines and growth factors at abnormal levels may inhibit normal calcium signaling events, thereby damaging cell-to-cell contacts of the endothelium and leading to endothelial dysfunction. Important markers include endothelin-1 (ET-1), interleukin-8 (IL-8), ELAM, and the endothelial leukocyte adhesion molecule-1 [ 41 ]. There is also sound evidence of decreased production or bioavailability of nitric oxide (NO)—a stimulant of smooth muscle relaxation—in preeclamptic pregnancies [ 42 ]. Other potential influential vasodilators include prostacyclin (PGI2) and the endothelium-derived hyperpolarizing factor (EDHF) [ 41 ].
As mentioned above, the dysregulation of the renin-angiotensin system (RAS) may participate in the pathogenesis of preeclampsia. In 2007, Florian Herse et al. published a study that included preeclamptic and non-preeclamptic women who had undergone cesarean deliveries. Genetic characteristics and histopathological results of the maternal and placental tissues of the participants were investigated. A 4-fold increase in the angiotensin II type 1 (AT1) receptor in the decidua was found in preeclamptic pregnancies. Increases in corresponding gene and protein expression were also confirmed by RT-PCR and immunohistochemistry studies. Circulating agonistic autoantibodies (AAs) targeting the AT1 receptor have been described previously, with the ability to cross the placenta and enter fetal circulation. AT1-AAs could induce calcium signaling and initiate events that would later lead to preeclampsia [ 43 ]. Roxanna A. Irani et al. published a study with similar findings in 2010. Animal experiments showed that pregnant mice with AT1-AA injections developed preeclamptic features and also had increased levels of antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1) and endoglin. Additionally, AT1-AA might be associated with increased TNF-α, indirectly causing damage to the endothelium and end organs [ 44 ].
Oxidative stress describes the imbalance between the formation of oxidative reactive species (ROS) and the antioxidant capacity of the body [ 45 ]. A causal role of oxidative stress in hypertension has been demonstrated in previous research, with multiple possible pathogenic pathways including the alteration of NO bioavailability or signaling. A reduction of oxidative stress has been observed in hypertensive cases who received antihypertensive treatment [ 46 ]. Oxidative stress in the healthy placenta may be important for its organogenesis, but excess levels in the impaired placenta would lead to increased circulating placenta debris, damaging the maternal endothelial cells in the term. As a major source of ROS production, the mitochondria have been found to be swollen in the trophoblasts of preeclamptic animal models, which plays a crucial role in cell apoptosis. Altogether, any errors in the maintenance of the oxygen pressure may bring about placental diseases and maternal complications, such as preeclampsia [ 46 ].
4.4. Limitations of the Placenta Model
Even though the two-stage theory is the mainstream explanation of the origin of preeclampsia, some argue that further evaluation is needed to determine the causative relation between trophoblast development and spiral artery transformation. For instance, previous case reports have pointed out similar findings of the uterine artery Doppler waveforms in extra-uterine pregnancies, suggesting that the resistance of uterine artery blood flow may not accurately reflect the consequences of trophoblast invasion [ 47 ].
Some have suggested that the result of Doppler studies may be a reflection of systemic vascular resistance changes but not on the uterine artery itself. The argument is based on the paradox that a “de-transformation” of spiral arteries does not occur when the vascular resistance of the uterine artery is noted in the third trimester [ 48 ]. In the meantime, while it is fairly certain that impaired uteroplacental perfusion is associated with subsequent endothelial dysfunction, almost all the supporting evidence of different hypotheses of its linkage raises some challenges. To date, it is believed that many potential mechanisms underlie preeclampsia, and the disease is caused by complicated interactions between maternal and environmental factors, and potentially more than that. The incomplete understanding of its pathogenesis continues to provoke further research.
5. Systemic Manifestations of Preeclampsia
Preeclampsia is a systemic disorder that may present with various symptoms and signs. The manifestation of preeclampsia is widely perceived to be centered around hypertension and proteinuria, but clinical presentations could be variable in essence. Different organs and systems could all be influenced by preeclampsia. Systemic manifestations of preeclampsia are shown in Figure 4 .

Systemic manifestations of preeclampsia.
According to the two-stage theory, preeclampsia proceeds into the clinical stage once the systemic vascular response and inflammation have taken place as a result of endothelial dysfunction. This aptly explains why preeclampsia is a global syndrome as the endothelium is distributed all over the body. The most famous affected organs and systems include the central nervous system, cardiovascular system, liver, and kidney.
5.1. Central Nervous System (CNS)
The brain is a vital organ that requires approximately 20% of available oxygen to maintain normal function. In most physiological conditions, cerebral blood flow (CBF) has sufficient capability to autoregulate and remains rather stable to cope with its high metabolic demand. However, once brain injury to a certain extent occurs, sequelae including acute severe hypertension, the loss of myogenic tone of the vascular smooth muscle, and uncontrolled vasoconstriction may lead to the failure of autoregulation. As they key to the delicate homeostasis of the brain environment, both hypoperfusion and hyperperfusion may break the balance and bring great harm. Insufficient CBF could lead to ischemic brain injury and ischemic stroke. Hyperperfusion, on the other hand, may disrupt the blood–brain barrier (BBB) and cause edema formation, which is one of the classic findings in preeclamptic and eclamptic patients [ 49 , 50 ].
CBF could be assessed in patients with preeclampsia via transcranial Doppler imaging. The middle cerebral artery is often chosen to be the target of examinations. Perhaps somewhat surprisingly, the cerebral flow index (CFI) appears to be normal in most women with preeclampsia. However, cerebral perfusion pressure (CPP) exhibits greater elevation in preeclamptic women and may serve as the key to brain injury among these women [ 51 ]. Brain damage has been demonstrated in autopsies and image studies [ 52 ]. It has been proven in some studies that elevated CPP corresponds to hypertension, and antihypertensive treatment that decreases CPP lowers the rate of cerebral complications in these patients [ 53 ].
CNS manifestations that are suggestive of severe disease status are headaches, visual disturbances, changes in consciousness, and seizures. The spectrum is coined “preeclamptic encephalopathy” [ 53 ]. Once a seizure takes place, the impression of eclampsia is almost certain after the exclusion of other previously known neurological conditions that may also lead to convulsion events. Eclampsia is one of the most serious forms of preeclampsia and is highly related to obstetric morbidities and mortalities. Management for eclampsia is similar to those for any form of severe preeclampsia, and most patients recover well without neurological sequelae [ 54 ].
Another frequent neurological finding is posterior reversible encephalopathy syndrome (PRES). PRES is a result of hypoxia and vasogenic edema of the brain that is often related to acute uncontrolled hypertension or systemic endothelial dysfunction. The syndrome progresses in a rather rapid manner but also resolves rapidly with a good prognosis once the trigger is withdrawn [ 55 ]. PRES is high among pregnant women with severe preeclampsia or eclampsia, and usually indicates a better prognosis than PRES in non-pregnant women or is associated with other causes [ 56 ].
Stroke—or a cerebrovascular accident—refers to a brain attack when impairment of part of the CBF or a burst in a brain blood vessel occurs. Strongly related to hypertensive disorders, cerebrovascular events are yet another complication that is significantly linked to preeclampsia. Although uncommon in pregnancy, it shares similar disease pathways and risk factors with strokes that take place in non-pregnant patients, and thus is indicative of an increased long-term risk for stroke events [ 57 ].
5.2. Cardiovascular Systems
Preeclampsia has been classified as one independent gender-specific risk factor for cardiovascular events by the American Heart Association (AHA). Studies have proven that women with gestational hypertensive disorders carry a 2- to 4-fold risk for cardiovascular diseases [ 58 ]. In fact, preeclampsia and cardiovascular diseases share many predisposing factors such as elevated blood pressure and increased BMI. The disease spectrum includes coronary heart disease, heart failure, and cardiovascular disease death, and the influences may be life-long [ 59 ].
The long-term cardiovascular sequelae not only affect the mother but have also proven to bring hazards to her children at the same time. Although the establishment of a dependency relationship is difficult, many studies have shown an increased rate of congenital heart disease and future cardiovascular morbidities for offspring. Some scholars believe, however, that the influences of cardiovascular risks on offspring are limited to term infants or cases with early preeclampsia [ 60 ].
The complex multifactorial nature of preeclampsia and cardiovascular diseases makes it hard to make a straightforward ascription of the latter to the former. There is also a lack of a standardized protocol for cardiovascular prevention. Nevertheless, medical staff and preeclamptic patients should keep in mind the importance of continuous screening and early intervention of cardiovascular diseases. Monitoring of the body weight, blood pressure, lipid level, and lifestyle should be performed every five years until the age of 50 when women would qualify for most other international regular cardiovascular risk assessment guidelines [ 61 ].
Preeclampsia-related liver disease is frequently seen in the third trimester. Liver involvement is rare but indicative of severe disease extent. The most notorious example is H(Hemolysis)EL(Elevated Liver Enzymes)LP(Low Platelet Count) syndrome, which is a variant of severe preeclampsia. According to the diagnostic criteria of Tennessee Classification and Mississippi Classification, an elevated liver enzyme is usually defined by an elevated AST or ALT ≥ 70 U/L, although blood tests often reveal a level ≥ 500 U/L. Thrombotic microangiopathy serves as one of the possible explanations, while periportal hemorrhage and necrosis have been observed in histopathology studies. As rare as it may be, the condition could lead to hepatic rupture [ 62 ]. Women treated with corticosteroids exhibit overall improved laboratory results including liver function tests. Administration after delivery helps to avoid a rebound and further complications. Nevertheless, the natural course of HELLP could not be altered by corticosteroids [ 63 ].
Other liver diseases associated with preeclampsia include acute fatty liver of pregnancy (AFLP), hepatic infarction, and rupture. In cases of AFLP, laboratory abnormalities include elevated liver enzymes, prolonged prothrombin time and partial thromboplastin time, and increased bilirubin levels. Other typical clinical symptoms comprise central nervous system involvements such as headache and consciousness disturbances, jaundice, and gastrointestinal symptoms including anorexia, abdominal discomfort, nausea, and vomiting. If the expression of long-chain 3-hydroxyacyl-CoA dehydrogenase is not evident, the prognosis is usually good [ 64 ].
Hepatic complications in pregnancy are rare but could be fatal. They are more likely to be found in preeclamptic or eclamptic cases and indicate severe disease status. Hence, prompt termination of pregnancy or delivery is often indicated. Liver transplantation may be considered in patients with a grave prognosis [ 63 ].
5.4. Kidney
The imbalance of the renin-angiotensin aldosterone system (RAAS) along with the imbalance between proangiogenic and anti-angiogenic factors may explain the relationship between preeclampsia and renal impairment. Similar to cardiovascular risks, preeclampsia shares common predisposing factors with renal risks and confers a higher risk of chronic kidney diseases later in life [ 65 ].
The activation of RAAS is normal during pregnancy, which results in a volume increase. However, excessive activation possibly related to sFlt-1 and AT1-AAs—as may be seen in preeclamptic subjects—could lead to preeclampsia or preeclampsia-like syndrome. Once the delicate balance is disrupted, hypertension and renal involvements may be seen [ 66 ]. Thrombotic microangiopathies in renal cells have been observed in histopathology studies, suggesting glomerular injury in preeclamptic patients [ 67 ].
When acute kidney injury occurs, an abrupt increase in serum creatinine and a decrease in urine output could be detected. However, both the glomerular filtration rate and serum creatinine level are not perfectly reliable markers during pregnancy, as physiological changes allow an increase in the former and a reduction in the latter. The diagnosis may rely on other clinical manifestations such as oliguria, proteinuria, and edema, and is thereby delayed in some cases [ 68 ].
The condition may be life threatening, but also tends to regress rapidly in the postpartum period. Nonetheless, it still warrants concern for screening for later renal diseases. There is a significant association between preeclampsia—the early-onset subgroup in particular—and future chronic kidney diseases, hypertensive diseases, and glomerular or proteinuric diseases. For preeclamptic women, a 10- to 12-fold increase in end-stage renal disease has been proposed in existing statistical analyses. Hence, further screening for kidney diseases years after pregnancy should be implemented [ 69 ].
5.5. Other Targets
Preeclampsia is a global disorder that may present with symptoms and pathologic findings all over the body. Aside from the vital organs, for example, the hematologic system is another commonly affected target. In a study conducted by Neelam Jhajharia et al., lower hemoglobin and platelets were found in these patients, while higher WBC and hematocrit were observed [ 70 ]. Similar findings could also be found in other studies [ 71 , 72 ]. Some parameters may vary from study to study, but a trend of decreased hemoglobin and platelet levels is almost always observed in data analyses. Marked thrombocytopenia signifies a severe disease form, as manifested in HELLP syndrome.
Gastrointestinal involvements are common in preeclamptic patients. Symptoms of nausea and vomiting are frequently experienced by them, and some women complain of indigestion. The more devastating complications include hepatic involvement as described earlier, and pancreatic involvement, namely referring to the increased risk of pancreatitis and necrosis of the pancreas [ 73 ].
Another classic clinical manifestation of preeclampsia is edema. It is worth noting that edema is not essential to the diagnosis of preeclampsia and is often observed in normal pregnancies as a result of the increase in body fluids. General swelling due to water and salt retention is especially prominent in preeclampsia due to elevated blood pressure and endothelial injury causing extravasation from the vessels. As rare as it may be, one of the most severe presentations of fluid overload is pulmonary edema, which has been reported in cases of severe preeclampsia [ 74 ].
6. Prediction and Prevention of Preeclampsia
The potential consequences of preeclampsia pose great threat and harm to mothers and their children, the medical system, and society worldwide. To prevent adverse outcomes, various strategies have been invented and studied, including diet control, exercise, and medication. Among them, the administration of low-dose aspirin has been proven to be one of the most effective ways to prevent the development and progression of preeclampsia.
In order to apply preventive methods in a cost-effective manner, a precise prediction model and timing would be required. According to the two-stage theory of its pathophysiology, the first stage of preeclampsia typically takes place in the first trimester, when inadequate trophoblast invasion leads to abnormal placentation and subsequent uteroplacental insufficiency. During this process, the patient is usually in her subclinical phase, which allows a window for screening and prevention.
6.1. Prediction Models
Apparently, a good prediction tool would provide many benefits to the early prevention and intervention of preeclampsia. Different professional organizations have thus far proposed their own prediction models based on the currently acknowledged risk factors.
An expert review written by Piya Chaemsaithong et al. made a detailed comparison between some of the most widely accepted prediction models ( Table 5 ). Risk factors such as a history of preeclampsia in previous pregnancies, chronic hypertension, autoimmune diseases, renal diseases, diabetes mellitus, and multifetal gestation are included in almost all the prediction models and are primarily considered to be “high” risk factors if the models made a segmentation between “high” risk and “moderate” risk factors. Other risk factors that are taken into consideration include nulliparity, advanced maternal age, maternal obesity, and family history, among others. Some are classified as “moderate” risk factors. A previous medical record and chronic hypertension are considered to be the two most important contributory risk factors [ 5 ].
A comparison between different prediction models of preeclampsia.
| Organization | NICE (National Institute for Health and Care Excellence) | ACOG (American College of Obstetricians and Gynecologists) | ISSHP (Iinternational Society for the Study of Hypertension) | FMF (Fetal Medicine Foundation) |
|---|---|---|---|---|
| Screening method | Based on numbers of risk factors High-risk factors: Previous pregnancy with preeclampsia Chronic hypertension Autoimmune disease Diabetes mellitus Moderate-risk factors: Nulliparity Age ≥ 40 y/o Interpregnancy interval ≥ 10 years Initial BMI ≥ 35 kg/m Family history of preeclampsia Multifetal pregnancy | Based on numbers of risk factors High-risk factors: Previous pregnancy with preeclampsia Chronic hypertension Autoimmune disease Diabetes mellitus Multifetal pregnancy Renal disease Moderate-risk factors: Nulliparity Age ≥ 35 y/o Interpregnancy interval ≥ 10 years Initial BMI ≥ 30 kg/m Family history of preeclampsia History of SGA or adverse outcomes Socioeconomic features | Based on numbers of risk factors High-risk factors: Previous pregnancy with preeclampsia Chronic hypertension Autoimmune disease Diabetes mellitus Renal disease Initial BMI ≥ 30 kg/m Moderate-risk factors: Nulliparity Age ≥ 35 y/o Family history of preeclampsia < 6m sexual relationship before pregnancy Connective tissue disorder | Bayes theorem: to combine the a priori risk from maternal characteristics and results of various biomarkers |
| Detection rate | Preterm: 41% Term: 34% | Preterm: 5% Term: 2% | Not documented | 8.2%, 64.0%, 71.8%, and 75.8% at 5%, 10%, 15%, and 20% fixed FPRs |
| False positive rate | Preterm: 10% Term: 10% | Preterm: 0.2% Term: 0.2% | Not documented |
Most prediction models have either low detection rates or high false-positive rates, however, and are insufficient for precise prediction. An alternative is to use the Bayes theorem and take the individual maternal history and characteristics into consideration. This competing model allows a more patient-specific and dynamic approach and is used by the Fetal Medicine Foundation (FMF) and is the only one that has undergone extensive internal and external validations. In addition to the checklist for risk factors, other maternal factors including the mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and serum pregnancy-associated plasma protein A (PAPP-A) are also taken into account [ 5 , 75 ]. The best timing of preeclampsia risk screening is around GA 11 to 13 weeks. As soon as the result is revealed, early prevention could be initiated if a high risk for preeclampsia is suspected [ 76 ].
A systematic review examined the performance of soluble fms-like tyrosine kinase-1 (sFlt-1), the placental growth factor (PlGF), and the sFlt-1/PlGF ratio in predicting adverse outcomes in women with preeclampsia. The literature search identified 33 eligible studies (n = 9426). Due to significant heterogeneity between studies, few studies (n = 4–8) were included in the final meta-analysis component. Nonetheless, both PlGF and the sFlt-1/PlGF ratio demonstrated areas ROC values between 0.68 and 0.87 for the prediction of composite adverse maternal and perinatal outcomes, preterm birth, and fetal growth restriction. Conclusively, PlGF and the sFlt-1/PlGF ratio show prognostic promise for adverse outcomes in preeclampsia, but study heterogeneity limits their clinical utility [ 77 ].
Currently, prediction models for gestational hypertension and preeclampsia have been developed with data and assumptions from developed countries. A review aimed to identify and assess the methodological quality of prediction models for gestational hypertension and pre-eclampsia with reference to their application in low-resource settings. The review retrieved 40 eligible articles and revealed 77% of all the prediction models’ combined biomarkers with maternal clinical characteristics. The biomarkers used as predictors in most models were PAPP-A and PlGF. Only five studies were conducted in low- and middle-income countries. Therefore, the review concluded that prediction models using maternal characteristics, with good discrimination and calibration, should be externally validated for use in low- and middle-income countries where biomarker assays are not routinely available [ 78 ].
6.2. Possible Preventative Measures
An article written by Sammya Bezerra Maia and Holanda Moura et al. offered a perspective on the potential preventative measures by classifying them as primary, secondary, or tertiary preventions. Primary prevention is unlikely to be successful since the concept is centered around the avoidance of pregnancy in high-risk populations and lifestyle modification in the whole population in order to decrease the incidence of preeclampsia. Secondary prevention focuses on the interruption of the pathogenic process before its development and is the main target of investigations. Tertiary prevention does not aim to prevent preeclampsia itself, but rather prevent its further complications. Aside from the aforementioned section on aspirin prevention, lifestyle management, nutritional supplementation, and antenatal surveillance may aid primary and secondary prevention. Accordingly, rest, exercise, diet modification such as a low-salt diet, and antioxidant use have all been suggested. Unfortunately, none of them have been proven to be effective [ 79 ]. In contrast, another systematic review solicited 28 RCTs studying the effects of various factors such as anticoagulants (heparin, enoxaparin, dalteparin, and nadroparin), aspirin, paravastatin, nitric oxide, yoga, micronutrients such as L-arginine, folic acid, vitamin E and C, phytonutrients, lycopene, and vitamin D alone or in combination with calcium. The results of this review showed that low-molecular-weight heparin, enoxaparin, yoga, L-arginine, folic acid, and vitamin D prevented preeclampsia alone or combined with calcium [ 80 ].
6.3. Low-Dose Aspirin
In fact, for women perceived as high-risk individuals for developing preeclampsia, the administration of low-dose aspirin on a daily basis since early pregnancy has proven to be the most effective way to prevent preeclampsia.
Aspirin is one of the oldest medications still in use to date. It is widely applied as an antithrombotic drug due to its effect on platelet inactivation. The mechanism of platelet inactivation relies on COX-1 inhibition, which blocks TXA2 synthesis. It is usually administered in a low dose (75–81 mg/day), which is sufficient for TXA2 but not PGI2 inhibition. The application of aspirin is primarily related to secondary prevention for cardiovascular diseases, while its use in primary prevention remains somewhat controversial. Another common use of aspirin is anticoagulant treatment in neurological diseases such as transient ischemic attack (TIA) or stroke. A recent history or increased risk for gastrointestinal bleeding, intracerebral bleeding, and other adverse events is worth noting, and aspirin should only be administered when the benefits outweigh the risks—primarily related to the increased bleeding tendency [ 76 ].
Ever since the first publication of a case report suggesting the role of aspirin in preeclampsia prevention in 1978, numerous studies have aimed to quantify the effects of aspirin on preventing preeclampsia but without a consensus owing to the heterogeneity of the study groups. Meanwhile, meta-analyses have suggested prophylactic aspirin use, which is best started before GA 16 weeks and ahead of the completion of placentation. Later, the ASPRE trial confirmed the effect of aspirin on early-onset preeclampsia. With good compliance, aspirin prophylaxis could reach 76–90% effect size [ 79 ].
A meta-analysis including a total of 18,907 participants in eight trials reported that the administration of aspirin was associated with a reduction in the risk of preterm preeclampsia (relative risk: 0.62; 95% confidence interval: 0.45–0.87), but there was no significant effect on term preeclampsia (relative risk: 0.92; 95% confidence interval: 0.70–1.21). The reduction in preterm preeclampsia was confined to the subgroup in which aspirin was initiated at ≤16 weeks of gestation and at a daily dose of ≥100 mg (relative risk: 0.33; 95% confidence interval: 0.19–0.57). Thus, aspirin can reduce the risk of preterm preeclampsia rather than term preeclampsia, and only when it is initiated at ≤16 weeks of gestation and at a daily dose of ≥100 mg [ 81 ].
Many organizations have proposed their own guidelines regarding the dosage and timing of aspirin use. Table 6 presents the current recommendations for the administration of low-dose aspirin in women at risk of future preeclampsia. While some differences exist, most agree on a daily dosage between 60 and 150 mg per day, and some have a precise dosage of 81 mg per day for low-dose aspirin tablets in the U.S. Administration is recommended late in the first trimester and could be initiated as early as GA 12 weeks and before GA 16 weeks. Low-dose aspirin is usually prescribed until the late preterm period. Bleeding disorders are uncommon, while some women might experience a certain degree of gastrointestinal discomfort [ 79 , 82 ].
Recommendations for administration of low-dose aspirin in women at risk of future preeclampsia.
| Organization | ACOG 2018 | USPSTF 2021 | NICE 2019 | ISSHP 2018 | FIGO 2019 |
|---|---|---|---|---|---|
| Recommend in high-risk patients Considered in patients with 1 or more moderate risk factors | Recommend in patients with ≥1 high-risk factors or 2 moderate-risk factors Considered in patients with ≥1 moderate risk factors | Recommend in high-risk patients Considered in patients with 1 or more moderate risk factors | Recommended in high-risk patients | Recommended in high-risk patients or when the risk is ≥1/100 | |
| Initiate between GA 12 and 28 weeks (best before GA 16 weeks) and use until delivery | Initiate ≥ GA 12 weeks | Initiate ≥ GA 12 weeks and use until delivery | Initiate ≤ GA 20 weeks (best before GA 16 weeks) | Initiate between GA 11 and 14 weeks and use until GA 36 weeks, delivery or preeclampsia | |
| 81 mg/day | 81 mg/day | 75–150 mg/day | 75–162 mg/day | 150 mg/day |
Low-dose aspirin use for preeclampsia prevention has been proven to be cost-effective and safe in pregnancy. Therefore, when the prediction model suggests a high risk of preeclampsia or when a mother carries some risk factors, low-dose aspirin should be initiated if no contraindications are identified.
7. Management of Preeclampsia
Table 7 is a summary of the current management practices of preeclampsia.
A summary of current management practices of preeclampsia.
| Management | Explanation |
|---|---|
| Blood pressure control | Choices for antihypertensive therapy during pregnancy are limited. The most frequently administered drugs include labetalol, hydralazine, and nifedipine. The former two come in intravenous and oral forms, of which intravenous injections are often used in severe or emergent conditions. Labetalol and nifedipine are more commonly recognized as the first antihypertensive medications for gestational hypertensive disorders. |
| Seizure prevention | Magnesium is the drug of choice for seizure prevention in preeclamptic and eclamptic cases. It is proven to be superior to other anticonvulsants and is associated with fewer side effects. The mechanism is primarily related to its calcium antagonistic effect and potential to function as an NMDA blocker. It is given with an initial loading dose followed by continuous infusion. Neurologic signs and respiratory patterns should be closely monitored to prevent toxicity. |
| Delivery and Termination of Pregnancy | The only way to stop or reverse the process of preeclampsia formation is delivery. Therefore, prompt delivery is indicated once the patient reaches term pregnancy. For preterm women with severe disease features, termination of delivery should be strongly considered, but risks higher neonatal morbidities and mortalities due to immaturity. In these cases, cortiocosteroids should be administered for fetal lung maturation before delivery if time allows. |
| Fluid management | Preeclamptic women often experience fluid overload, which could lead to serious complications such as pulmonary edema. Therefore, unnecessary fluids should be avoided. |
| Diet management | Most evidence regarding diet management against preeclampsia is not strongly convincing. However, maternal weight control, high fiber intake, probiotics use, calcium and vitamin D supplements, multivitamin and multimineral supplements, and avoidance of a high-salt diet and raw food are considered to be beneficial. A Mediterranean-style diet that is rich in vegetables, fruits, and healthy fats has also been proven to lower the risks of preeclampsia. |
| Exercise | Aerobic exercise is associated with a reduction of gestational hypertensive disorders as it promotes placentation and a healthier immune reaction in general. The frequency, intensity, type, and time of exercise should be an individualized plan discussed between the patient and physician based on the maternal condition. |
| Long-term follow-up | Even after delivery and the recovery of preeclampsia, women still bear an increased risk of developing cardiovascular, renal, and hepatic sequelae, along with other chronic diseases. Therefore, long-term follow-up for the patient’s health condition is indicated. |
7.1. Antihypertensive Treatment
Elevated blood pressure is essential to the diagnosis of preeclampsia and is associated with increased cardiac, vascular, and neurological risks. Therefore, antihypertensive medication should be administered for the control of blood pressure.
Some studies divide gestational hypertension into “severe” and “non-severe” groups, typically setting 160 mmHg as the cutoff point for systemic blood pressure (SBP). While the choice of antihypertensive regimen may be designed in an individualized manner, most clinicians agree with the initiation of antihypertensive therapy when the SBP exceeds 140 or 160 mmHg or when the diastolic blood pressure (DBP) reaches above 100 mmHg. The treatment target also differs between different guidelines; some set no specific treatment target, while some target an SBP below 110 mmHg or a DBP less than 80 to 90 mmHg [ 83 ].
The majority of common antihypertensive medications are contraindicated during pregnancy, so the choice of drugs is rather limited. Currently, almost all the approved medications belong to class C, including labetalol, hydralazine, nifedipine, methyldopa (class B), diazoxide, and the relatively contraindicated nitroprusside. The former three are used more frequently even though the FDA has not approved the usage of nifedipine in hypertension management [ 83 ].
7.1.1. Labetalol
Labetalol could be administered in the intravenous or oral form, while the intravenous form is more often used in hypertensive emergencies or grave conditions. It is a combined alpha- and beta-adrenoceptor-blocking agent with more potency on the beta receptor. It is widely used as an antihypertensive agent and has the advantage of exerting a minimal effect on heart rate and cardiac output. Side effects and adverse events are primarily related to the influence on the RAAS and respiratory system. Therefore, other alternatives should be contemplated for patients with asthma [ 84 ].
When given in preeclamptic pregnancies, labetalol also decreases proteinuria and perinatal deaths. No other antihypertensive medications have proven to produce similar effects [ 85 ]. In non-severe cases, the oral dosage of 200 to 1200 mg per day can be divided into two to three doses, depending on the individual condition [ 83 ]. On the other hand, an intravenous bolus of 20 mg labetalol is indicated in severe cases and could be followed by a double dose in ten minutes [ 86 ].
7.1.2. Hydralazine
Hydralazine is another popular medication of choice, which also comes in intravenous and oral forms similar to labetalol. It lowers blood pressure by acting as a direct arteriole vasodilator. Headache, flushing, chest discomfort, and gastrointestinal upset have been reported with hydralazine use. It is also known to be associated with drug-induced lupus syndrome [ 87 ]. However, drug toxicity is uncommon. The drug is fairly safe except for contraindication in women with coronary artery disease since the increased cardiac output and oxygen demand may be hazardous [ 87 ]. Its effects have been proven, but it may be less efficacious than other antihypertensive drugs such as labetalol and nifedipine and is perceived by some as a second-line choice instead of a first-line option [ 85 ]. In regular oral use for blood pressure control, the recommended regimen is to start with 10 mg four times per day with gradual adjustment. The maintenance dosage could be as much as 50 mg four times per day and still has room for titration as long as it does not exceed the daily maximum dosage of 300 mg [ 87 ].
7.1.3. Nifedipine
Nifedipine is a safe and effective oral drug for lowering blood pressure in preeclamptic patients. The advantages include its relatively low cost and wider accessibility [ 88 ]. This medication functions as a calcium channel blocker with a rapid vasodilating effect but is associated with few adverse events and a low risk of hypotension [ 89 ]. Headache, flushing, and palpitations are the most frequent complaints encountered [ 90 ]. Its simultaneous relaxing effect on the myometrium also makes it a tocolytic drug commonly used to avoid preterm delivery [ 90 ]. It is usually initiated with a 10 mg dose and could be repeated later [ 86 ].
7.2. Magnesium Sulfate
Magnesium sulfate is used extensively for the prevention of seizures in preeclampsia and recurrent seizures in eclampsia for a lengthy period of time. Compared to placebo and other anticonvulsants such as phenytoin and diazepam, magnesium sulfate has proven to be more effective with fewer side effects. Although the mechanism is not fully understood, several possible explanations have been proposed [ 91 ].
Since the twentieth century, the action of magnesium sulfate used in preeclampsia has been studied. It is less likely related to antihypertensive effects as eclampsia does not necessarily take place in a hypertensive condition. Instead, magnesium sulfate may function as a calcium antagonist and inhibit acetylcholine-calcium-dependent release. While calcium may induce vasospasm and activate smooth muscle constriction, magnesium works in the opposite fashion. Since cerebral vasospasm is a common finding in preeclamptic seizures, this may serve as a plausible explanation to justify the use of magnesium sulfate. Another hypothesis suggests magnesium functions as a blocker of NMDA receptors, and thus prevents calcium influx [ 92 , 93 ].
In women with severe preeclampsia or eclampsia, magnesium could be given with an initial loading dose via the intravenous or intramuscular route, followed by a maintenance infusion. To reach the therapeutic serum concentration of 3.5 to 7 mEq/L (4.2 to 8.4 mg/dL), the recommended loading dose is 6 g intramuscularly, 2 to 4 g intravenously with a rate of 1 g/min, or a combination of both. Different guidelines and studies may suggest a slight modification. Some minor side effects such as a warm sensation, flushing, nausea, and vomiting may be encountered within the therapeutic window. However, serious adverse events may occur if marked hypermagnesemia is noted. The loss of the normal patellar reflex may be seen when the serum concentration of magnesium reaches 8 to 10 mEq/L, and more devastating respiratory depression could result when the serum concentration of magnesium reaches or exceeds 13 mEq/L. Therefore, persistent monitoring of the neurological performance such as the presence of the patellar reflex, respiratory pattern, and urine output should be implemented to avoid magnesium toxicity and severe adverse events. If a dosing error or toxicity is noted, calcium gluconate could be administered as an antidote [ 94 ].
Overall, magnesium sulfate use in pregnant women is still considered to be safe as long as close surveillance is performed. Its effects on seizure prevention have been assuring, and associated morbidity and mortality rates are low.
7.3. Delivery and Termination of Pregnancy
Preeclampsia is a pregnancy-specific condition, which would require delivery or termination of pregnancy under certain circumstances. A dilemma between expectant management and delivery is sometimes faced, especially when the patient has not reached term pregnancy. Hence, the timing of delivery in preeclamptic patients has raised keen discussions.
Lucy C Chappell et al. conducted a randomized clinical trial on late-preterm preeclamptic patients to survey this issue. A total of 901 gravidas with preeclampsia from GA 34 weeks to less than GA 37 weeks were included and randomly allocated to expectant management or planned delivery evenly. Planned delivery was initiated within 48 h of randomization to allow corticosteroid use if needed, and labor induction was prioritized unless an indication for cesarean delivery existed. The maternal outcomes included severe hypertension, deficits, impairments of different organs, placenta abruption, and maternal death. The perinatal outcomes were a composite of NICU stays, neonatal deaths, and further neurological developments. The statistical findings suggested a significantly lower rate of maternal morbidities and mortalities but more adverse perinatal outcomes in the planned delivery group. The higher rate of adverse perinatal outcomes, however, was primarily related to NICU admissions due to preterm birth, and other neonatal outcomes were similar to those in the expectant group otherwise. The total maternal and neonatal medical costs were lower in the planned delivery group. Collectively, the results suggested planned delivery in women with late-preterm preeclampsia, but the risk of increased NICU admission, although not associated with further morbidities, should be informed and discussed with the patient [ 95 ].
A systematic review in 2017 solicited six articles regarding preterm preeclampsia and the timing of delivery. The subjects of discussion included both early-preterm and late-preterm women. The statistics suggested postponing delivery until GA 37 weeks for better fetal outcomes, and no severe maternal complications or fetal distress existed. However, delivery should be considered even with early preterm patients once severe maternal complications or impaired fetal well-being was noted. It is important to note that suggested delivery is not equivalent to an emergency delivery and should still allow a 24-h interval for preparation (e.g., corticosteroid use to promote fetal lung maturity) since an immediate delivery is often associated with greater risks. On the other hand, women who chose expectant management should receive close monitoring and medication if needed. For example, magnesium sulfate could be used to reduce the risk of eclampsia, and more recently, has been suggested as a neuroprotective medication for the fetus before GA 32 weeks in particular [ 96 ].
According to the most updated ACOG guidelines in 2018, the choice between expectant management and prompt delivery should be made based on gestational age, maternal condition, and fetal well-being. Those with reassuring antenatal testing, no signs of preterm labor, and no severe disease features make good candidates for expectant management until GA 37 weeks. There are no benefits to delaying delivery afterward. Conversely, those with potentially severe features and a worrisome fetal condition should be advised for immediate delivery. For patients with severe preeclampsia, early delivery indicates a trade-off between fetal benefits and maternal risks, and a thorough discussion between the medical team and the patient should be performed. Delivery should be considered at any time when the maternal or fetal condition deteriorates or becomes unstable regardless of the gestational age. A complete course of corticosteroid administration is not always necessary, especially when the woman has reached late preterm (GA ≥ 34 weeks) [ 97 ].
7.4. Fluid Management
Fluid management is important in women with preeclampsia because they are more prone to fluid overload, which could lead to pulmonary edema. However, research on an ideal fluid strategy has been limited to date. Present data fail to suggest an optimal regimen, in turn provoking future research [ 98 ]. Nevertheless, intravenous medication with fluids is almost inevitable for hospitalized patients and should therefore be administered with caution. For instance, some clinicians have recently suggested avoiding intravenous fluid preloads before epidural or spinal anesthesia [ 98 ].
7.5. Diet Management
Fl Diet and nutrient intake may impact the risks of preeclampsia, and some studies have aimed to seek the best policy for diet management. In 2022, BMJ published a review based on data from 2000 to 2021 regarding the effects of dietary factors, nutritional supplements, and maternal weight on preeclampsia. Some of the findings may be contradictory to public belief; for instance, a low-salt diet to prevent hypertension and antioxidant (e.g., Vit. C and E) supplements to relieve oxidative stress seem to be plausible ways to prevent preeclampsia, but the review fails to show enough evidence in reality. However, it is important to note that a low amount or lack of evidence does not imply that they are not helpful and should not be recommended. In fact, the dietary factors that have proven to reduce the risk of preeclampsia include maternal weight control, high fiber intake, probiotics use, calcium and vitamin D supplements, multivitamin and multimineral supplements, and the avoidance of a high-salt diet and raw food [ 99 ].
A cohort study conducted by Anum S. Minhas et al. suggested that a self-reported Mediterranean-style diet is associated with lower preeclampsia risks. A Mediterranean-style diet is rich in vegetables, fruits, and healthy fats, of which the coherence could be assessed with a food-frequency questionnaire, in which individuals answer the questions by recalling their eating habits regarding meat, seafood, vegetables, beans, fruits, oil, wine, sweetened beverages, and commercially baked foods. It has been previously proven to lower cardiovascular risks in the non-pregnant population, and this study further suggested that greater adherence is associated with >20% lower odds of developing preeclampsia in the pregnant population compared to women with less adherence [ 100 ].
7.6. Exercise
Many clinical and animal studies have identified significant or non-significant effects of exercise during pregnancy on the reduction of gestational hypertensive disorders including preeclampsia.
There are a few possible explanations in so for the results observed. To begin with, maternal exercise creates a transient hypoxic environment, which in turn promotes the compensatory proliferation of the trophoblastic, endothelial, and stromal cells of the placenta, and consequently leads to improved placentation. Secondly, exercise stimulates antioxidant pathways and increases the number of mitochondria, relieving much of the oxidative stress that is linked to preeclampsia. Thirdly, exercise has an anti-inflammatory effect, which allows the body to maintain a healthy immune reaction, thus reducing the abnormal immune response to the fetus that is witnessed in preeclamptic patients [ 101 ].
A review study in 2017 suggested that aerobic exercise is beneficial in pregnancy and should be encouraged. Whether or not aerobic exercise could reduce preeclampsia remained controversial in some studies, but overall, the review concluded that 30 to 60 min aerobic exercise two to seven times per week during pregnancy reduced the incidence of gestational hypertensive disorders and the rate of cesarean deliveries [ 102 ]. However, this may not be the perfect regimen for every pregnant woman. The pre-pregnancy physical activity levels and maternal condition should always be taken into consideration for physicians to offer the best advice on the frequency, intensity, type, and time of exercise [ 103 ].
7.7. Long-Term Follow Up
Last but not least, preeclampsia is a syndrome that develops before delivery, yet also demands extra healthcare in the long run. Long-term follow-up for potential complications is indicated since sequelae of the cardiovascular system, liver, and kidney could take place. Close surveillance for years is suggested, which requires alertness from a good medical team and good medical compliance from the patient herself.
8. Discussion
The true cellular and molecular mechanisms underlying preeclampsia remain largely unexplained, which are assumed to be a two-stage process of impaired uteroplacental perfusion with or without prior defective trophoblast invasion (stage 1), followed by general endothelial dysfunction and vascular inflammation that lead to systemic organ damages (stage 2). Although the causes of preeclampsia are multi-factorial and cannot be described in a simple way, the aforementioned theories may provide a reasonable explanation for the results observed in past studies. Nevertheless, the detailed etiology, pathophysiology, and effect of preeclampsia seem complicated and remain to be clarified.
As mentioned above, overweight including pre-pregnancy obesity and excessive weight gain during pregnancy predisposes women to the progression of preeclampsia. As a state of chronic inflammation, overweight will increase the risk of preeclampsia by means of activating macrophages, NK cells, and peripheral helper T cells within the placenta to produce inflammatory cytokines such as IL-6, IL-7, and TNF-α. Established on these findings and reasons, avoiding excessive weight gain before and during pregnancy, rather than merely using overweight as a predictor, may be the best strategy to prevent the occurrence of preeclampsia. Therefore, proper weight control for pregnant females can not only decrease the physical burden on the body but also reduce the risk of preeclampsia.
Well-recognized risk factors for preeclampsia include race, advanced maternal age, obesity, nulliparity, multi-fetal pregnancy, and co-existing medical disorders. These factors can serve as warnings or markers to label pregnant women who need enhanced surveillance of maternal and fetal well-being. For at-risk females, appropriate information, counseling, and suggestions should be provided to facilitate a timely intervention or specialty referral. For pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance including a Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test can be arranged. If the results are unfavorable, early intervention and aggressive therapy should be considered. Moreover, affected females should have access to higher levels of obstetric units and neonatal institutes. Before, during, and after delivery, monitoring and preparation should be intensified for affected gravidas to avoid serious complications of preeclampsia. In response to the potential physiological effect and psychological impact, consultation and discussion are usually beneficial for females diagnosed with preeclampsia. For severe cases, delivery of the fetus and placenta is the ultimate solution to treat preeclampsia. However, the determination of the appropriate timing for delivery depends on the severity of maternal preeclampsia and the maturity of the fetus.
It remains not fully understood with regard to the molecular level and pathologic mechanism of preeclampsia and its associated treatment. More studies are still required to investigate the role of anticoagulant therapy (such as aspirin, as described above) in preeclampsia. To minimize the heterogeneity of research in the future, the standardization of several critical factors in preeclampsia and the related treatment should be carefully considered. Two of the important factors are the timing and intensity of screening and intervention, which have a remarkable impact on the therapeutic effects. Furthermore, the severity and outcome in the individuals diagnosed with preeclampsia need standardization. Moreover, a larger sample size is also required to draw a reliable conclusion and to improve the reproducibility of the study result.
9. Conclusions
Preeclampsia accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies. It is life-threatening for both the mother and the fetus, in turn, increasing the rate of mortality and morbidity.
Preeclamptic pregnancies are strongly associated with significantly higher medical costs. The maternal costs are related to the extra utility of the healthcare system, more resources used during hospitalization, and likely more surgical spending due to an elevated rate of cesarean deliveries. The infant costs also contribute to a large percentage of the expenses as the babies are prone to preterm deliveries and relevant or causative adverse events. Preeclampsia imposes a considerable financial burden on our societies. It is important for healthcare providers and policy-makers to recognize this phenomenon and allocate enough economic budgets and medical and social resources accordingly.
The true cellular and molecular mechanisms underlying preeclampsia remain largely unexplained, which are assumed to be a two-stage process of impaired uteroplacental perfusion with or without prior defective trophoblast invasion (stage 1), followed by general endothelial dysfunction and vascular inflammation that lead to systemic organ damages (stage 2).
Risk factors for preeclampsia, including race, advanced maternal age, obesity, nulliparity, multi-fetal pregnancy, and co-existing medical disorders, can serve as warnings or markers that call for enhanced surveillance of maternal and fetal well-being. Doppler ultrasonography and biomarkers including the mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and serum pregnancy-associated plasma protein A (PAPP-A) can be used for the prediction of preeclampsia. For women perceived as high-risk individuals for developing preeclampsia, the administration of low-dose aspirin on a daily basis since early pregnancy has proven to be the most effective way to prevent preeclampsia. For preeclamptic females, relevant information, counseling, and suggestions should be provided to facilitate timely intervention or specialty referral. In pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance including a Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test can be arranged. If the results are unfavorable, early intervention and aggressive therapy should be considered. Affected females should have access to higher levels of obstetric units and neonatal institutes. Before, during, and after delivery, monitoring and preparation should be intensified for affected gravidas to avoid serious complications of preeclampsia. In severe cases, delivery of the fetus and placenta is the ultimate solution to treat preeclampsia.
Although the aforementioned theories may provide a reasonable explanation for the results observed in the past studies, the detailed etiology, pathophysiology, and effect of preeclampsia seem complicated, and further research to address the primary etiology and pathophysiology underlying the clinical manifestations and outcomes is warranted.
Funding Statement
This review and APC were funded by a grant from Taipei Tzu-Chi Hospital, Taiwan (TCRD-TPE-111-10) for K.-H.C. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in writing the manuscript, or in deciding to publish the results.
Author Contributions
K.-J.C., K.-M.S. and K.-H.C. conceived the review and designed the search methods for the literature; K.-J.C. and K.-H.C. collected the data in the literature; K.-J.C., K.-M.S. and K.-H.C. performed data analyses; K.-J.C. and K.-H.C. wrote the review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Case report
- Open access
- Published: 26 September 2018
Use of a qualitative case study to learn lessons from severe preeclampsia causing a maternal near-miss: a case report
- Moti Tolera ORCID: orcid.org/0000-0002-4739-1390 1 ,
- Alula M. Teklu 2 ,
- Abdurahman Ahmed 3 ,
- Abdiwahab Hashi 4 ,
- Lemessa Oljira 1 ,
- Zerihun Abebe 5 ,
- Wondimagegn Gezahegn 5 &
- Kahasse Gebre Kidan 5
Journal of Medical Case Reports volume 12 , Article number: 277 ( 2018 ) Cite this article
12k Accesses
3 Citations
Metrics details
Maternal mortality is a critical indicator in assessing the quality of services provided by a health care system. Approximately 99% of all maternal deaths occur in developing countries; where a majority of the causes of these deaths are preventable.
Case presentation
A 25-year-old, married, multigravida, black woman who has had six live births presented to a health center with the chief compliant of abnormal body swelling of 2 days’ duration and loss of consciousness. On arrival to the first contact health center her blood pressure was 170/105 mmHg and her temperature was 36.5 °C. She had generalized swelling, a history of blurred vision, and headache. She had no history of abortion, stillbirth, and cesarean section and no history of antenatal care follow-up. She gave birth to her previous children at home with no history of obstetric complications. The gestational age at the time of arrival was 37 weeks. She was referred to a general hospital for further management.
At the general hospital she was diagnosed as having severe preeclampsia and she was managed with magnesium sulfate and an antihypertensive medication for 2 days. She was counseled to have induction of labor by the attending physician but refused to give consent and went home. She returned to the referral hospital 2 days later after labor had started spontaneously at home and the delivery was a spontaneous vaginal delivery with outcome of a live male baby, his Apgar score was 6/10 immediately after birth and he weighed 1.9 kg.
Conclusions
If there were no previous obstetric problems, the women perceived that she will not face complications in her future pregnancies and stay home until she had developed life-threatening complications. If women visit health facilities and if the health care providers are responsive and there is robust referral in place, maternal and fetal complications will be prevented.
Peer Review reports
Maternal complications and mortality is a critical indicator to assess the quality of services provided by a health care systems [ 1 ]. A total of 10 million women worldwide are estimated to experience severe complications of pregnancy every year, with half a million of these dying as a result; out of all maternal deaths, 99% occur in developing countries including Ethiopia [ 2 ]. Even though some efforts were made by governments of Ethiopia to promote institutional deliveries, births continue to occur in the home settings [ 3 , 4 , 5 , 6 ]. Ethiopia is the fourth largest contributor country to global maternal deaths, next to India, Nigeria, and the Democratic Republic of Congo [ 7 ].
A maternal near-miss case is defined as “a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or within 42 days of termination of pregnancy” [ 8 ] and is used for monitoring the implementation of critical interventions in maternal health care. So, a systematic process should be in place to assess the quality of care with an assumption that all maternal deaths involve at least one life-threatening condition (organ dysfunction) [ 8 , 9 ]. Complications during pregnancy and childbirth can occur at any point of time; therefore, it is important to ensure readiness in terms of infrastructure, human resources, and equipment for timely management of complications at all basic and emergency obstetric care health facilities [ 1 ]. Direct obstetric complications account for 85% of maternal deaths as well as many acute and chronic illnesses [ 10 , 11 ]. Preeclampsia-eclampsia disorder is one of the direct pregnancy-related complications and is a pregnancy-specific hypertensive disorder affecting multiple systems and contribute a maternal mortality rate of 1.8% [ 12 , 13 ]. Eclampsia is defined as the occurrence of one or more convulsions superimposed on preeclampsia [ 4 ]. Preeclampsia is pregnancy-induced hypertension in association with proteinuria (> 0.3 g in 24 hours) ± edema and virtually any organ system may be affected. Severe preeclampsia is variously defined [ 1 , 4 ]. There is consensus that severe hypertension is confirmed with a diastolic blood pressure (BP) ≥ 110 mmHg on two occasions or systolic BP ≥ 170 mmHg on two occasions or significant proteinuria (at least 1 g/liter) [ 14 ]. Risk factors for preeclampsia include either a very young or advanced maternal age, multi-fetal pregnancy, hemolytic disease of the newborn, diabetes mellitus, chronic systemic hypertension, and renal disease [ 15 ]. In developing countries the incidence of eclampsia/preeclampsia poses a great problem in the field of obstetrics due to poor socioeconomic conditions and lack of antenatal care (ANC) follow-up [ 16 ]. Hence the aim of this case report was to explore a case of maternal near-miss from the woman’s home to her place of delivery and to share the lessons learnt from this case using a qualitative case study design, which is a rarely reported technique.
Materials and methods
The case study was conducted in one of general hospitals in the Somali regional state of Ethiopia. The hospital was established approximately 50 years ago. The study was conducted from February 1 to March 1, 2017; using an explorative qualitative case study design. The tools used to collect data were in-depth interviews (IDIs), key informant interviews (KIIs), and facility abstraction using World Health Organization (WHO) near-miss assessment tools [ 8 ]. Participants of this study were the mother herself, her sister, her male partner, maternal and child health (MCH) coordinators at the health facilities she has visited, the head of the health center/hospital, and the health service providers who assisted her both at first contact and at the referral hospital, such as the gynecologist, midwife, and general practitioner (GP). Purposive sampling was used to select the case as well the participants. A near-miss case was identified from among women with pregnancy-related complications whose diagnosis met the WHO near-miss criteria [ 8 ] and who were admitted to the obstetric unit of the hospital. Investigations were conducted for abnormalities, septicemia, anemia, and other organ dysfunction/failure. Data were collected for determining the nature of the obstetric complication, presence of organ system dysfunction/failure, and timing of near-miss events with respect to admission. Fetal outcome and Intensive Care Unit (ICU) admissions were also noted. Detailed information on maternal complication for the underlying cause and time period was obtained. The information was documented using the narrative qualitative method. For this, multiple interviews were carried out. The data collectors had many years of experience with qualitative case study data collection, verbatim transcription, and translation. The data collectors in the field transcribed audio-digital recordings into the local language and then translated the data into English. The data collectors also kept notes and kept records of field reports, field notes, completed questionnaires, and interview recordings. The inductive qualitative data analysis method was used to analyze the data collected. The data were entered, coded, categorized, and analyzed using NVIVO version 11 software. Consent for data collection:
◈ Ethical clearance for study was obtained from SPHMMC IRRB and local IRRB Ethio-Somali Regional Health Bureau (ESRHB).
◈ Official letters were taken to zonal health departments, Woreda Health Offices, health centers, and health post/kebele (neighborhood) administrators.
◈ The study participants were informed about the purpose of the study and written and informed verbal consent was taken.
◈ All participants’ right to self-determination was respected.
◈ The confidentiality and the privacy of the respondents were maintained.
◈ Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Patient information
The case was a 25-year-old married, multigravida, black woman. She was gravida 7 and para 6, all surviving. She lived in a rural pastoral area. She had no formal education and her livelihood was pastoralist. She was categorized into a low socioeconomic class. She had no history of abortion, stillbirth, cesarean section (C-section), or any obstetric complications.
History of current pregnancy
The current pregnancy was her seventh with a gestational age of approximately 37 weeks at presentation. The mother explained as her gestational age increased she started to develop generalized body swelling that started from her eyes, then face, and eventually migrated to her legs. She had history of blurred vision and headache. After two to three episodes of seizure, she was unconscious, and then she was taken to a nearby health facility (Health center) by her husband and mother.
“I was unconscious when my husband and my mother took me to the nearby health center.” The mother
At the first contact health center her axial temperature was 36.5 °C and her BP was 170/105 mmHg in supine position; she had three episodes of seizure and other clinical findings and no tests were found on the patient's chart.
After this episode, there was no altered state of consciousness and no seizure. She did not have, headache, nausea, visual disturbances, or fever; she had no history of head injury, central nervous infection, no history of alcohol intake, no history of seizure or epilepsy, and she was negative for stigmata of neurocutaneous syndrome.
Because of shortage of supply and instruments, except urine analysis (protein 3+), no other laboratory or other investigative tests were done at the health center. The health care providers at the primary health facility diagnosed her as having preeclampsia and referred her to the nearby general hospital with an ambulance.
Maternal condition on arrival at the general hospital
The hospital is named as a general hospital but is serving as a referral hospital in the region, providing a wide range of services including approximately 25 deliveries per day.
“It is serving beyond its capacity. If you look around the maternity area, many women were in labor there; since there is no place we can refer to, we don’t have a limit on admission, and we accept everyone. When you provide service beyond capacity, there will be quality problems. The delivery rate is extremely high in our situation. Sometimes there are up to 25 deliveries per day.” Hospital officer
The mother was visited by GPs, who were assigned to the emergency room at the hospital, as soon as she arrived. The family reported that they brought her to the health facility because she was sick due to an evil spirit and they had unsuccessfully tried spiritual treatment at their locality. Her vital signs on arrival at the general hospital were: BP 180/110 mmHg, temperature 37.6 °C, respiratory rate (RR) 22/minute, and her pulse rate (PR) was 82/minute. The health care providers counseled her family that they can use both modern and spiritual treatments in the hospital and then her family gave consent for admission. Then after the health care providers gave lifesaving emergency treatment using antihypertensive and anti-seizure medications, she was admitted to the obstetrics ward and she underwent full investigations including an ultrasound and laboratory investigations: urine analysis, complete blood count (CBC), and organ function tests like liver function test (LFT) and renal function test (RFT). The laboratory results showed 3+ protein for urine analysis dipstick test which is equivalent to 3 gm/l protein [ 14 ] and CBC was in normal range except for hemoglobin and platelets of 7 mg/dl and 100,000/mm 3 , respectively. Her aspartate aminotransferase (AST) result was raised (85 mg/dl) but the RFT result showed no increment in creatinine (1.5 mg/dl). She was finally diagnosed as having severe preeclampsia and full-term pregnancy (even though the diagnosis seemed eclampsia). The pregnancy type was singleton and in the cephalic position as reported by the radiologist. She was started on magnesium sulfate and an antihypertensive treatment (methyldopa). After her BP was controlled and she looked well, she was counseled for induction of labor due to the indication of (preeclamsia/) eclampsia. However, she refused the induction and went home; unfortunately, 2 days later she returned to the hospital soon after labor had started spontaneously at home. She was again admitted to the hospital for delivery. During this time she had no history of loss of consciousness, no history of seizure, no history of blurred vision, and no abnormal findings were detected. Because of abscence of cardiotocography (CTG) fetal heart monitoring was not done. The mother’s BP was 135/95 mmHg, PR was 80 beats/minute, and respiratory rate (RR) was 25/minute. She had pedal and periorbital edema. After 6 hours follow-up at labor and delivery ward, she gave birth to a live male baby with an Apgar score of 6/10 at 5 minutes after delivery and a weight of 1900 mg. The baby was admitted to the under-5 ward due to having a low birth weight (1.9Kg). She went home with orally administered methyldopa 250 mg to be taken twice a day.
“After referral to this facility, she was clerked at emergency ward by a general practitioner and then she was admitted and seen by the gynecologist. Then she had full investigations including an ultrasound and she was counseled for induction of delivery. However, she refused induction and preferred to go back home. Then she delivered spontaneously... The fetal outcome was a live but underweight male child... Now it is 24 hours since she delivered and the maternal status is good. ....We will transfer her to her baby in the ICU after we control her blood pressure.” MCH focal person of the hospital
The mother and her family described her condition during the pregnancy as very risky and she thinks she would have been died if she had not gone to the health facility and received treatment.
“…this was a very risky pregnancy, she was near to death, if she had no intervention here, and she was near to death...” The mother’s sister
“...after this pregnancy I decided to go to the health facility” The mother
She gave birth to all her six previous babies at home without any problems and was supported by a non-trained traditional birth attendant (n-TBA) because she believed visiting a health facility while healthy was not important during pregnancy. She had no history of ANC attendance for the current pregnancy as she felt well and had no previous complications.
“Truly speaking I did not attended any ANC for the benefit of my health and that of the fetus but if I get sick I will go to the health facility to get treatment…I had all my previous deliveries in my home without any complication, I think it is ok to deliver in my home when I have no health problems.” The mother
She believed that giving birth at a health institution is only necessary when there are problems during pregnancy that is why she presented to this health facility after her family perceived she had developed some complications.
“…it is very good to deliver in a health facility when you have a health concern. In my last delivery I went to the health center, all my body was swollen, to get treatment I went there.” The mother
The mother perceived that culture and religion are supportive of seeking health services when needed and religion does not prohibit her from seeking any health services except being attended to by male health care providers and “strange individuals,” meaning individuals who do not know her local language; she perceived the patriarchal system in her community affected her use of the health services she needed as men were the source of finance and were the decision makers.
“… but it is difficult to get delivery service from males and strange people.” The mother
“Husband is the source of income for the health care expenses and the family needs husbands who have a good attitude about modern health care utilization, so it is easy for the mother to use.” The husband
The MCH focal person stated that cases of eclampsia and preeclampsia were increasing in this region, and as the cause is unknown it needs further research.
“The cause of preeclampsia and eclampsia is unknown but cases are increasing in our region. It may be associated with their eating habits. One doctor is doing research into preeclampsia and eclampsia cases to investigate the cause of these problems .” MCH focal person of the hospital
The mother was asked about her personal feelings toward the health facility she was in and the health care providers caring for her; she was satisfied with the services the health care providers provided her but complained that the hospital had inadequate room for labor and childbirth.
“The health care providers were welcoming and friendly to the patients but in the hospital there are no adequate rooms for the women delivering.” The mother
The MCH focal person also discussed lack of an ICU for delivery. They also do not have enough space for delivery services and the delivery room is very congested. Women were delivering on the floor outside the room.
“It is known that we don’t have ICU services and this is a critical problem in our set up. I and the gynecologists tried a lot but still there is no solution. There are many problems with this but the main reason is that there is no space for establishing it. As you see the delivery ward is crowded, women are delivering on the floor and there is not even a little space. ” MCH focal person
The health care providers complained about the accessibility of ambulances; they reported that most of the ambulances which were provided by the Ministry of Health were assigned for other duties, rather than serving emergency health services or laboring mothers.
“…This may be the most common complaint related to transportation. It is thought that there should be an ambulance service for all mothers and the Ministry of Health distributed ambulances to all districts even though they are used for other purposes.” MCH focal person
The mother also described the quality of services starting from the kebele (neighborhood) she is living in, to the referral facility where she was treated and gave birth. The health facility near her has a shortage of human resources and supplies including medications, but at the referral health facility, she felt that it had enough human resources and they were serving the admitted patients day and night.
“….in our kebele the health center does not have enough health care providers and drugs. But it was okay in the hospital as health care providers were with us day and night.” The mother
The MCH focal person reported that the mother preferred the death of her child to a surgical intervention done to save the life of herself and her baby. The intention of the mother was to save her own life rather than saving her child, because it was expected that the mother could give birth to many children if she survived. A lot of time was spent counseling the mother to have a C-section and other interventions. Even the father’s focus was not on the baby but on the life of the mother.
“First, the community in our region doesn’t worry about their children. Even the mothers themselves don’t worry about their beloved child. A mother will prefer the death of her fetus rather than surgical delivery. They fear complications of operations and consider it as if we are going to slaughter them. One doctor is doing research on how long it takes to counsel and get consent for cesarean section. If you ask a woman about an operation, she asks ‘Could I survive without an operation despite the survival of the fetus?’ And if we say yes, she says, the child can die, I can have another child tomorrow and I don’t like to be operated on. So they don’t care about their child. All families, the father and the mother don’t focus on the child.” MCH focal person
The mother discussed that she had learnt many lessons from her current pregnancy complications, in addition to this, she learnt a lot about the advantages of using a health facility for the benefit of the mother and her baby, including about family planning.
“From this pregnancy I have learnt that going to the health facility is good for the health of the mother and baby….” The mother
In addition, there was poor interaction among the hospital staff. For example, the senior midwifery nurses do not consult the junior physicians on the assumption that these junior GPs lack experience. Moreover, rather than teaching the junior GPs the senior physicians accept the existing trends of the midwives and as a result there was poor communication between the GPs and other senior staff.
“The main thing is most GPs were new graduates and lack experience, second it is time consuming to make the decision. This system is well accepted by the seniors.” MCH focal person
The main delay before reaching the hospital was lack of transportation followed by lack of awareness about the disease. For example, if a woman is convulsing, the family believes that she was attacked by an evil spirit rather than disease.
“…Then they bring her to hospital after they failed many other trials. But lack of transportation is still a more severe problem and a mother will not die here if she is admitted early.” MCH focal person
Discussion and conclusions
This study found that previous pregnancy situation may repeat it self in the future pregnancies. Moreover, the health administrators use the ambulance for administrative purposes rather than for emergency health issues. On a positive note, attending a health facility is an opportunity for a mother to learn about existing health services in the facility such as family planning and the importance of ANC.
In the community, women who have had no obstetric problems during their previous pregnancies perceived that they will have no problems with their consecutive pregnancies and therefore feel that ANC and facility delivery was not useful. It was only necessary when severe complications occured that they attend health facilities. Moreover, women do not like to visit health facilities due to fear of pregnancy-related interventions like induction and instrumentation during delivery. Seizures due to eclampsia were perceived by the community as being caused by an evil spirit, and they prefer to take a preeclamptic/eclamptic mother to religious institutions rather than to health facilities. Women with preeclampsia/eclampsia may have underweight babies and visiting health facilities will be lifesaving for both the mother and her child.
Studies reported that not attending ANC, preeclampsia, multiple pregnancies, and bad obstetric history are significant maternal factors resulting in low birth weight babies and low birth weight babies are at a greater risk of having a disability and for diseases [ 17 , 18 , 19 ].
Preeclampsia/eclampsia is the result of ischemia in the tertiary villi of the placenta and affects all organs; the definitive treatment is termination of the pregnancy (removing the placenta). However, because of fear of instrumentation and the culture of being attended at home, the mother refused the procedure and went home to be attended at home; the labor spontaneously started at home after 2 days which may further affect the health of the mother and her baby.
Neonates delivered by multiparous women were at three-times greater risk of a low Apgar score compared with lower parity women, and both multiparity and low birth weight were independently associated with a low Apgar score (OR, 2.4) for low birth weight [ 20 ]. In this case, the mother is multiparous and her baby had a low birth weight and low Apgar score (6 at fifth minute). This shows that there is a need for further analytical research on the relationship between multiparity and low birth weight and low Apgar score.
An uneventful obstetric history often blinds multiparous women to possible dangers in childbirth, leads to low antenatal uptake, and late presentation to hospitals for obstetric complications. So robust patient education and use of accessible community health promotors like health extension workers (HEWs) and Ethiopia’s Women’s Development Army should be encouraged to improve antenatal coverage and early presentation to hospitals.
Abbreviations
Antenatal care
Aspartate aminotransferase
Blood pressure
Complete blood count
Cesarean section
Cardiotocography
General practitioner
Health extension workers
Intensive Care Unit
In-depth interviews
Institutional Research Review Board
Key informant interviews
Liver function test
Maternal and child health
Non-trained traditional birth attendant
Renal function test
Respiratory rate
Saint Paul’s Hospital Millennium Medical College
World Health Organization
India, Ministry of Health. Maternal near miss review: Operational Guidelines Ministry of Health & Family Welfare Government of India. Ned Delhi: Department of Maternal Health; 2014.
Google Scholar
Karolinski A, et al. The epidemiology of life threatening complications associated with reproductive process in public hospitals in Argentina. BJOG. 2013;120:1685–95.
Article CAS Google Scholar
National MDSR Task Force. Policy Brief: Recommendations on Quaility of Care in MNH Services. Addis Ababa: Ethiopian Ministry of Health; 2016.
Ethiopian Ministry of Health. Health Sector Transformation Plan 2015-2020. Addis Ababa: Ethiopian Ministry of Health; 2015.
Ethiopian Ministry of Health. Health Sector Development Program IV, 2010-2015. Addis Ababa: Ethiopian Ministry of Health; 2010.
Ethiopian Ministry of Health. National Guideline for Family Planning Services in Ethiopia. Addis Ababa: Family Planning Technical Working Group, Ethiopian Ministry of Health; 2011.
WHO. Trends in Maternal Mortality: 1990 to 2015. Geneva: WHO; 2015.
WHO. Report on the burden of endemic health care-associated infection worldwide. Geneva: WHO; 2011.
World Health Organization. Evaluating the quality of care for severe pregnancy complications: the WHO near-miss approach for maternal health. Swizerland: WHO; 2011.
FMoH (Federal Ministry of Health of Ethiopia). Health Service Devlopment Program IV (HSDP IV 2009/10-2014/15). Ethiopia: FMoH; 2010.
FMoH (Federal Ministry of Health of Ethiopia). National Baseline Assessment for Emergency Obstetric and Newborn Care in 2008. Addis Ababa: FMoH; 2009.
Abalos E, et al. Global and regional estimates of preeclampsia and eclampsia: a systemic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7.
Article Google Scholar
Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–400.
Tuffnell DJ, et al. The management of severe pre-eclampsia/eclampsia: Guideline No. 10(A). London: Royal College of Obstetrics and Gynecology; 2010. p. 1–11.
Ng EW, Waite K, Bennett M. Preeclampsia-Eclampsia Hypertensive Retinopathy. Honolulu: Retina Institute of Hawaii; 2011.
Khanum M, Ashraf F, Sahrin H. A Clinical Study of 100 Cases of Eclampsia In Rajshahi Medical College Hospital. TAJ. 2004;17(2):80–3.
Singh G, Chouhan R, Sidhu K. Maternal Factors for Low Birth Weight Babies. MJAFI. 2009;65:10–2.
CAS Google Scholar
Bugssa G, Dimtsu B, Alemayehu M. Socio demographic and maternal determinants of low birth weight at Mekelle Hhospital, nNorthern Ethiopia: a cross-sectional study. Am J Adv Drug Deliv. 2014;2(5):609–18.
WHO. WHA Global Nutrition Targets 2025: Low Birth Weight Policy Brief. Geneva: WHO; 2014.
Andrew HM, et al. Grand multiparity: is it still a risk in pregnancy? BMC Pregnancy Childbirth. 2013;13:241.
Download references
Acknowledgements
First of all I would like to acknowledge almighty God; my appreciation also goes to Dr Fante Belew who helped me in preparing and fine-tuning this manuscript.
Funding for this study was obtained from Department for International Development (DFID) through Federal Ministry of Health of Ethiopia.
Availability of data and materials
Data used for this manuscript are readily available.
Author information
Authors and affiliations.
Haramaya University School of Public health, Dire Dawa, Ethiopia
Moti Tolera & Lemessa Oljira
MERQ Consultancy, Addis Ababa, Ethiopia
Alula M. Teklu
Debre Berhan University, Debre Birhan, Ethiopia
Abdurahman Ahmed
Jigjiga University, Jigjiga, Ethiopia
Abdiwahab Hashi
St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Zerihun Abebe, Wondimagegn Gezahegn & Kahasse Gebre Kidan
You can also search for this author in PubMed Google Scholar
Contributions
Authors are wholly involved in the study right from the inception to write-up of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Moti Tolera .
Ethics declarations
Authors’ information.
The Author is an expert with different qualifications (MPH in Health services management, BSC in Public health, BSC in Computer science, Diploma in MLS) and is currently working as Head, Public Health and Health Policy, School of Public Health, Haramaya University, Ethiopia.
Ethics approval and consent to participate
Ethical clearance for the research was obtained from SPHMMC IRRBs. And participation in this study was completely voluntary. No personal identity was disclosed; information on the study participant is anonymous.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Tolera, M., Teklu, A.M., Ahmed, A. et al. Use of a qualitative case study to learn lessons from severe preeclampsia causing a maternal near-miss: a case report. J Med Case Reports 12 , 277 (2018). https://doi.org/10.1186/s13256-018-1821-x
Download citation
Received : 06 February 2018
Accepted : 28 August 2018
Published : 26 September 2018
DOI : https://doi.org/10.1186/s13256-018-1821-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Maternal near-miss
- Maternal complication

Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 26 June 2024
A dynamic prediction model for preeclampsia using the sFlt-1/PLGF ratio combined with multiple factors
- Guili Chen 1 na1 ,
- Yuanyuan Chen 3 na1 ,
- Yao Shi 1 ,
- Zhoufen Mao 1 ,
- Jiaqi Lou 2 &
- Jianting Ma 1
BMC Pregnancy and Childbirth volume 24 , Article number: 443 ( 2024 ) Cite this article
Metrics details
Preeclampsia (PE) is a pregnancy-related multi-organ disease and a significant cause of incidence rate and mortality of pregnant women and newborns worldwide. Delivery remains the only available treatment for PE. This study aims to establish a dynamic prediction model for PE.
A total of 737 patients who visited our hospital from January 2021 to June 2022 were identified according to the inclusion and exclusion criteria, forming the primary dataset. Additionally, 176 singleton pregnant women who visited our hospital from July 2022 to November 2022 comprised the verification set. We investigated different gestational weeks of sFlt-1/PLGF (soluble FMS-like tyrosine kinase-1, placental growth factor) ratio combined with maternal characteristics and routine prenatal laboratory results in order to predict PE in each trimester. Multivariate logistic regression was used to establish the prediction model for PE at different gestational weeks. The discrimination, calibration, and clinical validity were utilized to evaluate predictive models as well as models in external validation queues.
At 20–24 weeks, the obtained prediction model for PE yielded an area under the curve of 0.568 (95% confidence interval, 0.479–0.657). At 25–29 weeks, the obtained prediction model for PE yielded an area under the curve of 0.773 (95% confidence interval, 0.703–0.842)and 0.731 (95% confidence interval, 0.653–0.809) at 30–34 weeks. After adding maternal factors, uterine artery pulsation index(Ut-IP), and other laboratory indicators to the sFlt-1/PLGF ratio, the predicted performance of PE improved. It found that the AUC improved to 0.826(95% confidence interval, 0.748 ∼ 0.904) at 20–24 weeks, 0.879 (95% confidence interval, 0.823 ∼ 0.935) at 25–29 weeks, and 0.862(95% confidence interval, 0.799 ∼ 0.925) at 30–34 weeks.The calibration plot of the prediction model indicates good predictive accuracy between the predicted probability of PE and the observed probability. Furthermore, decision-curve analysis showed an excellent clinical application value of the models.
Using the sFlt-1/PLGF ratio combined with multiple factors at 25–29 weeks can effectively predict PE, but the significance of re-examination in late pregnancy is not significant.
Peer Review reports
Introduction
PE is a pregnancy-related multi-organ disease and a significant cause of maternal and neonatal morbidity and mortality worldwide, especially in developing countries [ 1 ]. PE can be defined as new-onset hypertension after 20 weeks of gestation (systolic blood pressure persistently ≥ 140 mm Hg or diastolic blood pressure persistently ≥ 90 mm Hg, or both) associated with proteinuria, or end-organ dysfunction, or both. Pregnant women with pre-existing chronic hypertension will be diagnosed with chronic hypertension complicated by PE if they develop new-onset proteinuria or end-organ dysfunction [ 2 ]. Early PE is diagnosed if the disease occurs at ≤ 34 weeks of gestation [ 3 ]. The pathophysiology of PE is not fully understood. It may be a combination of genetic and environmental factors and abnormal placental formation [ 4 ]. Impaired spiral arteriole remodeling, maternal vascular malperfusion, and abnormal cellular interactions in the early first trimester might increase the risk of developing PE. As pregnancy enters the second trimester, the diseased placenta progressively gradually secretes large amounts of anti-angiogenic factors, such as soluble fms-like tyrosine kinase-1 (sFlt1) that cause vascular inflammation, endothelial dysfunction, and maternal vascular injury [ 2 , 4 , 5 , 6 , 7 ]. Many studies have shown that circulating placental growth factor (PLGF) levels were decreased, and sFlt-1 levels were increased in PE. Predictions of PE were challenging because little is known about its etiology, various risk factors, and possible multiple pathogenic phenotypes. Previous studies have mostly focused on nested case-control studies [ 8 ], univariate analysis [ 9 ], or detection of the sFlt-1/PLGF ratio at a certain gestational week [ 10 ]. However, there have been relatively few predictions from continuous longitudinal and multivariate studies of sFlt-1/PLGF. A single detection indicator has limitations in predicting PE. Our study aims to investigate and compare the value of the sFlt-1/PLGF ratio at different gestational weeks combined with multiple factors in diagnosing PE, identify women at high risk of PE and provide early intervention to prevent and reduce adverse pregnancy outcomes.
Materials and methods
This observational, single-center, prospective study was conducted at the People’s Hospital of Yuyao (Zhejiang, China). The study included single pregnant women who came to our hospital for examination between 11 and 13 weeks. Pregnant women with fetal abnormalities, fetal loss, and transfer to other hospitals for examination and delivery due to other reasons were excluded from the study.
This study enrolled single pregnant women who visited our hospital from January 2021 to June 2022. All participants underwent routine prenatal examinations and were followed up until delivery. A total of 737 patients met the inclusion and exclusion criteria and comprised the training sets. From July 2022 to November 2022, an independent validation set of 176 singleton pregnant women was screened using the same criteria as the development dataset. All eligible women attending our hospital visit at 11 to 13 weeks gestation were provided with written information about the study, and those who agreed to participate provided written informed consent.
The maternal characteristics of pregnant women were obtained through consultation with professional medical practitioners during the initial visit to our hospital. This included maternal age, pre-pregnancy body mass index (BMI), mode of pregnancy, number of pregnancies, mean arterial pressure (MAP), previous delivery history, abortion history, previous pregnancy with gestational hypertension, history of chronic hypertension, chronic kidney disease, and immune system diseases. We also assessed whether aspirin was being taken during this pregnancy and checked for gestational diabetes and hypothyroidism due to pregnancy comorbidity. At 11 to 13 weeks gestation, laboratory indicators including pregnancy associated plasma protein-A (PAPP-A), PAPP-A MOM, hematocrit (HCT), fasting blood glucose (FBG), hemoglobin (Hb), and 25-hydroxyvitamins D were measured. Doppler ultrasound examinations were performed transabdominally to measure the pulsatility index and calculate the mean UtA-PI of the left and right arteries. Between 20 and 24 weeks of pregnancy, we checked for triglycerides(TG), total cholesterol(TC), uric acid(UC), and sFlt-1/PLGF. Then we re-measured sFlt-1/PLGF every five weeks at pregnancy 25–29 weeks and again at pregnancy 30–34 weeks.
All data were analyzed using SPSS 26.0 and R 4.2.2 software. The normality of the variables was assessed using either the Kolmogorov-Smirnov or Shapiro-Wilk test. Descriptive statistics were used to characterize the participants’ essential features. Group comparisons based on variable normality were conducted using Independent t-tests or Mann-Whitney U-tests. Categorical variables were summarized by counts and percentages, and compared using either Pearson’s chi-square or Fisher’s exact test. Potential risk factors for PE were identified as the best predictors through binary Logistic regression analysis, leading to the establishment of a logistic prediction model. The predictive performance was evaluated using receiver operating characteristic (ROC) curves and calibration plots, which assessed the consistency between predicted PE probabilities and actual outcomes. External validation of the model was performed with populations from different time periods within the same region, in addition to decision-curve analysis (DCA) being utilized to evaluate clinical validity. A probability value less than 0.05 was considered statistically significant.
A total of 737 pregnant women with singleton pregnancies were included in the study, of which 55 (7.5%) ultimately developed PE and were assigned to the study group. Among these, 3 pregnant women (0.41%) had early-onset PE, while the remaining 52 had late-onset PE. In the external test population of 176 cases, 13 patients (7.4%) had PE, with one pregnant woman (0.56%) having early-onset PE and the remaining 12 having late-onset PE. The basic sociodemographic characteristics, medical history, and laboratory data of all participants are presented in Table 1 . The age and nulliparous proportion of the PE patients were higher than those in the normal group ( p < 0.05). However, levels of PAPP-A and PAPP-A MOM were significantly lower in the PE patients ( p < 0.05). Pre-pregnancy BMI, MAP, hematocrit, fasting blood glucose, triglyceride, and uric acid levels were significantly higher in the PE patients compared to the normal group. The UtA-PI of the PE group was also significantly higher than that of the control group. Significant differences between the two groups were observed in aspirin use, gestational diabetes incidence, history of original hypertension, and mode of pregnancy( p < 0.05).
In Table 1 , it is evident that the sFlt-1/PLGF ratio varies across different gestational weeks. Specifically, the sFlt-1/PLGF ratio in the PE group was higher than that of the control group at 20–24 weeks, although this difference was not statistically significant ( p = 0.096). However, at 25–29 weeks and 30–34 weeks of pregnancy, the sFlt-1/PLGF ratio in the PE group was significantly higher than that of the control group ( p < 0.001).
The results of the single-factor logistic analysis using the collected data are presented in Table 2 . In addition to the sFlt-1/PLGF ratio, significant factors between the two groups included age, PAPP-A MOM, HCT, pre-pregnancy BMI, FBG, UC, UtA-PI, aspirin use, diabetes in pregnancy, history of delivery, primary hypertension and mode of pregnancy. Variables significantly associated with PE in univariate logistic regression were included in multivariate logistic regression. Six independent predictive factors were identified (Table 3 ), including sFlt-1/PLGF ratio at 25–29 weeks and 30–34 weeks, UtA-PI, PAPP-A MOM, uric acid levels, history of delivery and primary hypertension. The collinearity test results indicated that all variables had variance inflation factors less than 2, which suggests that collinearity can be ignored.
The area under the ROC curve (AUC) of the predictive model for PE obtained at 11 ∼ 13 weeks of pregnancy with maternal factors was 0.815 (95% confidence interval, 0.731 ∼ 0.898), with a sensitivity of 75.6% and a false positive rate of 18.9% (Fig. 1 A; Table 4 ). The AUC for sFlt-1/PLGF in predicting PE at 20 ∼ 24 weeks gestation was closer to 0.5 (Fig. 1 B; Table 4 ), indicating poor sensitivity and specificity. At 25–29 weeks of pregnancy, the AUC of the predictive model for PE with sFlt-1/PLGF ratio was found to be 0.776 (95% confidence interval, 0.704–0.848), with a sensitivity of 50.9% and a false positive rate of only 3.1%. The cut-off value for sFlt-1/PLGF is set at 34(Fig 1 C, Table 4 ). The AUC of the predictive model for PE obtained at 30–34 weeks of pregnancy with sFlt-1/PLGF ratio was 0.732(95% confidence interval, 0.65–0.814), with a sensitivity of 52.7% and a false positive rate of 4.4%. The cut-off value of sFlt-1/PLGF is 34(Fig. 1 D; Table 4 ). We combined other predictors to sFlt-1/PlGF using multiple logistic regression analysis to compare its performance. It found that the AUC improved to 0.826(95% confidence interval, 0.748 ∼ 0.904) at 20–24 weeks(Fig. 1 E; Table 4 ), 0.879 (95% confidence interval, 0.823 ∼ 0.935) at 25–29 weeks(Fig. 1 F; Table 4 ), and 0.862(95% confidence interval,0.799 ∼ 0.925) at 30–34 weeks(Fig. 1 G; Table 4 ). The calibration curve of the model (Fig. 2 , left) shows a calibration slope of 1 and an intercept of 0, indicating a good prediction accuracy between the predicted probability of PE and the observed probability. However, the calibration plot of the external validation model (Fig. 2 , right) shows a calibration slope of 0.940 and an intercept of -0.915, indicating the sensitivity and accuracy are relatively poor. The DCA plot indicated good positive net benefits of the predictive model (Fig. 3 , left) and External validation model (Fig. 3 , right) among majority threshold probabilities.
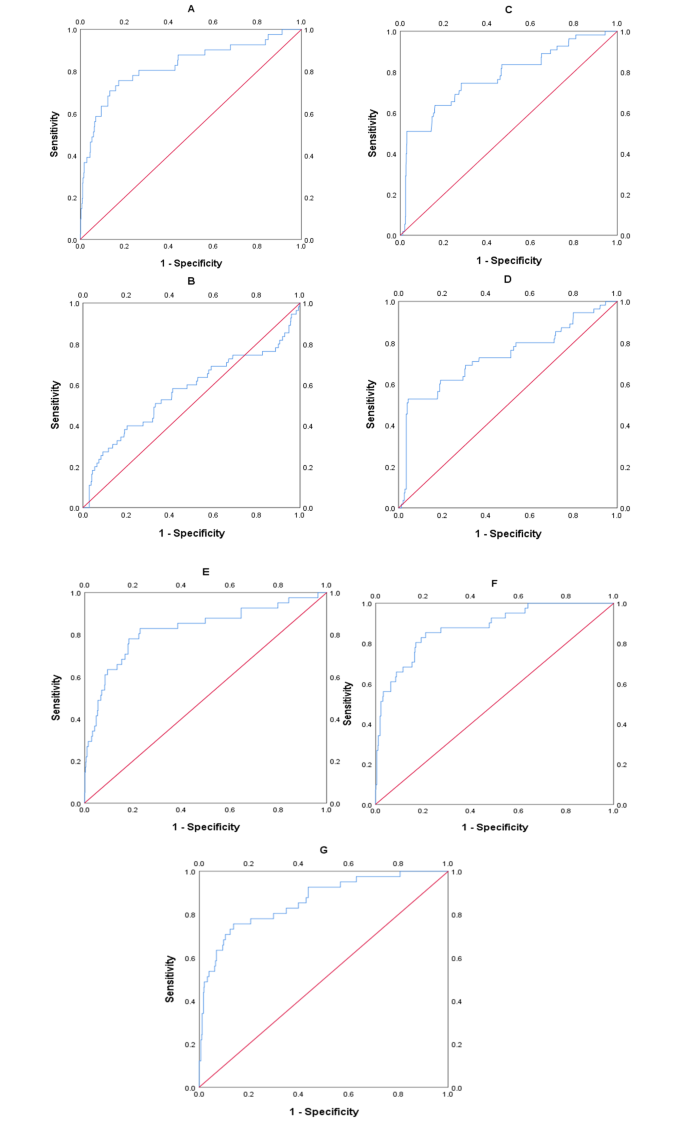
ROC curves of maternal factors, single-factor and multi-factor validation models. A shows the ROC curve of the maternal factors and UtA-PI. B shows the ROC curve of the predictive model for the sFlt-1/PLGF ratio from 20 to 24 weeks, C from 25 to 29 weeks, and D from 30 to 34 weeks. Figure E, F, G shows the ROC curve of the multi-factor prediction model corresponding to gestational age

Calibration curves. Validation models for all predictive factors. The left image shows the calibration map of the prediction model, and the right image shows the external validation calibration map

Decision curve analysis. On the left is the DCA of the prediction model, and on the right is the DCA of external validation
PE occurs in 2–8% of pregnancies worldwide, with estimates of at least 16% among low- and middle-income countries [ 5 , 10 ]. Our study’s findings indicate an incidence rate of PE at 7.5%, which is consistent with previous literature reports. The impact of PE on the health of mothers and newborns is severe. Women whoexperience PE during pregnancy appear tohavesignificantly increased future risk of vascular dementia [ 11 ]. Maternal hypertensive disorders of pregnancy(HDP), other than gestational hypertension, independently increase the risk of intellectual disability in offspring, in addition to the potential impacts of preterm birth and small-for-gestational-age (SGA) [ 12 ]. Currently, delivery remains the only available treatment for PE. Therefore, it is particularly important to predict the occurrence of PE early and provide preventive measures.
Many studies have found that angiogenic biomarker tests conducted in early pregnancy or before 20 weeks of gestation do not effectively predict the onset of PE [ 3 , 5 , 8 , 9 ]. Ohkuchi et al. [ 13 ] discovered that serum PlGF levels in women with singleton pregnancy at 9–13 weeks of gestation may be useful for predicting preterm PE. Additionally, Amylidi-Mohr’s study [ 14 ] demonstrated the effectiveness of first-trimester combined screening for PE using the FMF algorithm in their population. Guizani et al. [ 15 ] utilized the Fetal Medicine Foundation (FMF) at 11–13 weeks gestation to predict PE and found a detection rate of 80.6% for PE occurring before 37 weeks and 31.8% for PE occurring after 37 weeks when screening by maternal factors, MAP, UtA-PI, and sFlt. In our study, a combination of maternal factors and UtA-PI showed good predictive performance for precursors between 11 and 13 weeks (0.815;95% confidence interval:0.731–0.898). Several studies [ 16 , 17 ] have also shown that high-risk women who begin taking daily aspirin in early pregnancy significantly reduce their risk of developing PE, particularly premature PE. In our study, pregnant patients with high-risk factors for PE and those predicted to have PE at 11–13 weeks were administered a daily dose of 75 mg aspirin before 16 weeks, which was continued until 36 weeks of pregnancy. This prophylactic treatment resulted in a reduced incidence rate of early-onset PE (0.54%). According to Yemane’s study, the incidence of GH was 6% (4.9–8.5), and the likelihood of progression from GH to PE was 17.1% (13.4–23.8). Our data showed no statistically significant difference in previous GH history between the two groups, which may be attributed to the administration of aspirin prophylactic treatment to pregnant women with high-risk factors.
Some research studies [ 15 , 18 , 19 ] have indicated that the FMF algorithm is effective in predicting preterm PE. However, its performance in predicting late-onset PE ≥ 37 weeks is found to be poor. Nevertheless, both early-onset and late-onset PE lead to severe short-term and long-term complications for pregnant women and newborns. Therefore, we included TG, TC, and UC, as well as sFlt-1/PLGF levels detected during pregnancy at 20–24 weeks. Additionally, we repeated the measurement of sFlt-1/PLGF levels during pregnancy at 25–29 weeks and again at 30–34 weeks.
In our study, the levels of sFlt-1/PLGF in the PE group increased with gestational age and were significantly higher than those in the control group at 24–29 weeks and 30–34 weeks of pregnancy. Levine et al. [ 20 ] found that the sFlt1/PLGF ratio was significantly higher at 25 through 28 weeks among those who developed term PE compared to the controls. Moore Simas et al. [ 21 ] conducted a prospective study on 94 pregnant women between 22 and 36 weeks, and they observed that, compared to the control group, the increase in sFlt1/PLGF ratio in PE women became more significant with increasing gestational age. These findings are consistent with our research results. Fan Yu et al. [ 9 ] tested sFlt-1 and PLGF in pregnant women in southwestern China between 12 and 36 weeks of pregnancy (an average of 29 weeks) and used their ratio for predicting PE. They discovered that when the critical value of sFlt-1/PLGF ratio was set at 26.6, the area under the ROC curve was calculated as being high at 0.918, with both high sensitivity (85.42%) and specificity (96.27%). Karoline et al. [ 22 ] reported that the cut-off value for predicting PE in Austrians using sFlt-1/PLGF at 32 weeks of pregnancy was 10.3. In a cohort study of 97 pregnant women at gestational ages of 24–28 weeks in the south of Vietnam, Trung et al. [ 10 ] found that the sFlt-1/PLGF ratio had an area under the ROC curve of 80%, modest sensitivity (50.0%), and good specificity (86.6%) with a cut-off point of 4.3. Additionally, Viktorija et al. [ 23 ] conducted a case-control clinical study at the tertiary care center of the Hospital of Lithuanian University of Health Sciences Kauno Klinikos. On the day of PE diagnosis, the SFlt-1/PLGF ratio was tested and found to have a cut-off value of ≥ 35 (sensitivity 95.8%, specificity 96.2%) when the sensitivity and specificity for predicting PE were highest. Villa [ 24 ] conducted a nested case-control study on high-risk women and found that Serum sFlt-1/PlGF ratio over 40 at 26 + 0 to 28 + 0 weeks of gestation has high specificity and sensitivity in identifying women who developed early-onset PE disease. These studies demonstrate that cut-off values for diagnosis vary among different countries and regions, possibly due to factors such as race, genetics, living environments, and habits which are speculated to be related to the pathogenesis of PE. Our study aimed to investigate the dynamic changes in the sFlt-1/PLGF ratio during pregnancy from 20 to 34 weeks and its relationship with PE. The data revealed that in normal pregnant women, the sFlt-1/PLGF ratio peaked at 20 to 24 weeks of gestation, decreased until 25 to 29 weeks, and then gradually increased at 30 to 34 weeks. Conversely, in patients with PE, there was a significant increase in the sFlt-1/PLGF ratio as gestational age advanced from 20 to 34 weeks.
Dawson LM et al. [ 25 ] discovered that factors independently associated with an increased risk of PE included primiparous delivery, which aligns with our research findings. Furthermore, the SOGC clinical practice guidelines [ 26 ] also identify primiparous women as moderate risk factors. Our present results are consistent with several previous findings indicating that the Uric acid levels [ 27 , 28 ] in patients with PE were higher than those in normal pregnant women. However, maternal serum PAPP-A MOM [ 29 , 30 ] was significantly lower. Some studies [ 31 , 32 , 33 ] have demonstrated that the uterine artery pulsatility index measured by Doppler ultrasound is useful and effective for predicting PE and should be implemented in clinical practice. Chronic hypertension [ 34 , 35 ] was associated with an increased risk of PE, which is in line with our research findings.
After incorporating maternal factors, angiogenic markers, and Ut-PI into the predictive factors at 25–29 weeks of gestation, the AUC for predicting PE increased to 0.879, with a sensitivity of 85.4% and a false positive rate of 21%. The AUC also increased to 0.862 at 30–34 weeks, with a sensitivity of 75.6% and a false positive rate of 13.8%. The use of multiple factors for predicting PE at 25–29 weeks demonstrated good performance; however, its performance was relatively poor at 30–34 weeks. Additionally, early-onset PE occurring between 30 and 34 weeks can be diagnosed without prediction. Our research suggests that combining the sFlt-1/PLGF ratio with multiple factors at 25–29 weeks effectively predicts PE, but re-examination in late pregnancy does not hold significant significance according to our findings. Our prediction model performed better in calibration and discrimination than another model(Maric, I. 2020) [ 36 ]. We made a DCA to quantify the clinical usefulness of the models and found that our prediction model exhibited good performance. However, the sensitivity and accuracy of the calibration map for external validation models are relatively poor. This may be due to the limited sample size in external validation model research.
The strength of our study lies in its integration of maternal factors, biochemical markers, and dynamic angiogenesis factors at 20–34 weeks for the prediction of PE. At 20–24 weeks, maternal factors and biochemical markers can compensate for the relatively weak predictive performance of the sFlt-1/PLGF ratio. However, it is important to note that one limitation of this study is the insufficient number of samples. Additionally, as a single-center study, we did not monitor the sFlt-1/PLGF ratio throughout pregnancy or assess biochemical markers at different gestational weeks.
The sFlt-1/PLGF ratio combined with multiple factors at 25–29 weeks can effectively predict PE. However, the significance of re-examination in late pregnancy is not substantial.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
American College of Obstetricians and Gynecologists TFoHiP. Hypertens Pregnancy Obstet Gynecol. 2013;122(5):1122–31.
Google Scholar
Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–54.
Article CAS PubMed Google Scholar
Widmer M, Cuesta C, Khan KS, Conde-Agudelo A, Carroli G, Fusey S, et al. Accuracy of angiogenic biomarkers at ⩽ 20 weeks’ gestation in predicting the risk of pre-eclampsia: a WHO multicentre study. Pregnancy Hypertension: Int J Women’s Cardiovasc Health. 2015;5(4):330–8.
Article Google Scholar
Espinoza JVA, Pettker CM, Simhan H. Acog Obstet Gynecol. 2019;133(1):1.
Ma’ayeh M, Costantine MM. Prevention of preeclampsia. Semin Fetal Neonatal Med. 2020;25(5):101123.
Article PubMed PubMed Central Google Scholar
Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022;226(2):S1019–34.
Article CAS PubMed PubMed Central Google Scholar
Karumanchi SA, Libermann T. Discovery of antiangiogenic factors in the pathogenesis of preeclampsia. Am J Obstet Gynecol. 2022;226(2):S1035–S6.e5.
MG; AOOOACCRRCAGCCAKRGGKMGTT. First-trimester serum soluble fms-like tyrosine kinase-1, free vascular endothelial growth factor, placental growth factor and uterine artery Doppler in preeclampsia. J Perinatol. 2013;33(9):670–4.
Yu F, Bai Q, Zhang S, Jiang Y. Predictive value of soluble fms-like tyrosine kinase‐1 against placental growth factor for preeclampsia in a Chinese pregnant women population. J Clin Lab Anal. 2019;33(5).
Nguyen TH, Bui TC, Vo TM, Tran QM, Luu LT-T, Nguyen TD. Predictive value of the sFlt-1 and PlGF in women at risk for preeclampsia in the south of Vietnam. Pregnancy Hypertens. 2018;14:37–42.
Article PubMed Google Scholar
Carey C, Mulcahy E, McCarthy FP, Jennings E, Kublickiene K, Khashan A et al. Hypertensive disorders of pregnancy and the risk of maternal dementia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2024.
Wang LW, Lin HC, Tsai ML, Chang YT, Chang YC. Maternal hypertensive pregnancy disorders increase childhood intellectual disability hazards independently from preterm birth and small for gestational age. Early Hum Dev. 2023;185:105856.
Ohkuchi A, Takahashi K, Hirashima C, Suzuki H, Takahashi H, Nagayama S et al. Automated electrochemiluminescence immunoassay for serum PlGF levels in women with singleton pregnancy at 9–13 weeks of gestation predicts preterm preeclampsia: a retrospective cohort study. Hypertens Res. 2023.
Amylidi-Mohr S, Kubias J, Neumann S, Surbek D, Risch L, Raio L, et al. Reducing the risk of Preterm Preeclampsia: comparison of two first trimester screening and treatment strategies in a single centre in Switzerland. Geburtshilfe Frauenheilkd. 2021;81(12):1354–61.
Guizani M, Valsamis J, Dutemeyer V, Kang X, Ceccotti V, Khalife J, et al. First-Trimester Combined Multimarker prospective study for the detection of pregnancies at a high risk of developing Preeclampsia using the fetal Medicine Foundation-Algorithm. Fetal Diagn Ther. 2018;43(4):266–73.
Xiao Y, Ling Q, Yao M, Gu Y, Lan Y, Liu S et al. Aspirin 75 mg to prevent preeclampsia in high-risk pregnancies: a retrospective real-world study in China. Eur J Med Res. 2023;28(1).
Jacobsen E, Shanmugam V, Jagannathan J. Rosai–Dorfman Disease with activating KRAS Mutation — Response to Cobimetinib. N Engl J Med. 2017;377(24):2398–9.
Skråstad RB, Hov GG, Blaas HG, Romundstad PR, Salvesen K. Risk assessment for preeclampsia in nulliparous women at 11–13 weeks gestational age: prospective evaluation of two algorithms. BJOG: Int J Obstet Gynecol. 2014;122(13):1781–8.
Zwertbroek EF, Groen H, Fontanella F, Maggio L, Marchi L, Bilardo CM. Performance of the FMF First-Trimester Preeclampsia-Screening Algorithm in a High-Risk Population in the Netherlands. Fetal Diagn Ther. 2021;48(2):103–11.
Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in Preeclampsia. N Engl J Med. 2006;355(10):992–1005.
Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197(3):244.e1–.e8.
Mayer-Pickel K, Stern C, Eberhard K, Lang U, Obermayer-Pietsch B, Cervar-Zivkovic M. Comparison of mean platelet volume (MPV) and sFlt-1/PlGF ratio as predictive markers for preeclampsia. J Maternal-Fetal Neonatal Med. 2019;34(9):1407–14.
Taraseviciene V, Grybauskiene R, Maciuleviciene R. sFlt-1, PlGF, sFlt-1/PlGF ratio and uterine artery doppler for preeclampsia diagnostics. Med (Kaunas). 2016;52(6):349–53.
Villa PM, Hämäläinen E, Mäki A, Räikkönen K, Pesonen A-K, Taipale P et al. Vasoactive agents for the prediction of early- and late-onset preeclampsia in a high-risk cohort. BMC Pregnancy Childbirth. 2013;13(1).
Dawson LM, Parfrey PS, Hefferton D, Dicks EL, Cooper MJ, Young D, et al. Familial risk of Preeclampsia in Newfoundland. J Am Soc Nephrol. 2002;13(7):1901–6.
Magee LA, Smith GN, Bloch C, Cote AM, Jain V, Nerenberg K, et al. Guideline 426: Hypertensive disorders of pregnancy: diagnosis, prediction, Prevention, and management. J Obstet Gynaecol Can. 2022;44(5):547–71. e1.
Ness RB, Hubel CA. Risk for coronary artery Disease and Morbid Preeclampsia: a Commentary. Ann Epidemiol. 2005;15(9):726–33.
Barneo-Caragol C, Martínez-Morillo E, Rodríguez-González S, Lequerica-Fernández P, Vega-Naredo I, Álvarez FV. Increased serum strontium levels and altered oxidative stress status in early-onset preeclampsia. Free Radic Biol Med. 2019;138:1–9.
Honarjoo M, Kohan S, Zarean E, Tarrahi MJ. Assessment of β-human-derived chorionic gonadotrophic hormone (βhCG) and pregnancy-associated plasma protein A (PAPP-A) levels as predictive factors of preeclampsia in the first trimester among Iranian women: a cohort study. BMC Pregnancy Childbirth. 2019;19(1).
Ranta JK, Raatikainen K, Romppanen J, Pulkki K, Heinonen S. Decreased PAPP-A is associated with preeclampsia, premature delivery, and small for gestational age infants but not with placental abruption. Eur J Obstet Gynecol Reproductive Biology. 2011;157(1):48–52.
Article CAS Google Scholar
Das E, Singh V, Agrawal S, Pati SK. Prediction of Preeclampsia using first-trimester uterine artery doppler and pregnancy-Associated plasma Protein-A (PAPP-A): a prospective study in Chhattisgarh, India. Cureus. 2022;14(2):e22026.
PubMed PubMed Central Google Scholar
Guzmán YN, Uriel M, Ramírez AP, Romero XC. Uterine artery Pulsatility Index as a pre-eclampsia predictor in the 3 trimesters in women with Singleton pregnancies. Revista Brasileira De Ginecologia E Obstetrícia /. RBGO Gynecol Obstet. 2021;43(12):904–10.
Liu Y, Xie Z, Huang Y, Lu X, Yin F. Uterine arteries pulsatility index by Doppler ultrasound in the prediction of preeclampsia: an updated systematic review and meta-analysis. Arch Gynecol Obstet. 2023;309(2):427–37.
Lecarpentier E, Haddad B. Aspirin for the prevention of placenta-mediated complications in pregnant women with chronic hypertension. J Gynecol Obstet Hum Reprod. 2020;49(9):101845.
Advani R, Chandrasekaran S. Chronic hypertension diagnosed by the American Heart Association and American College of Cardiology criteria is associated with increased risk of developing hypertensive disorders of pregnancy. Am J Obstet Gynecol MFM. 2024;6(3):101269.
Maric I, Tsur A, Aghaeepour N, Montanari A, Stevenson DK, Shaw GM, et al. Early prediction of preeclampsia via machine learning. Am J Obstet Gynecol MFM. 2020;2(2):100100.
Download references
Acknowledgements
We would like to thank all the women who participated in this study.
This work was supported by the Project for Zhejiang Medical Technology and Hygiene Program(2021KY1073).
Author information
Guili Chen and Yuanyuan Chen contributed equally to this work.
Authors and Affiliations
The People’s Hospital of Yuyao, Zhejiang, 315400, China
Guili Chen, Yao Shi, Zhoufen Mao & Jianting Ma
Health Science Center, Ningbo University, Zhejiang, 315000, China
Ningbo University Medical Department, Zhejiang, 315000, China
Yuanyuan Chen
You can also search for this author in PubMed Google Scholar
Contributions
GL-C wrote the manuscript. JT-M made critical revisions to the manuscript for important intellectual content and contributed to the study concept, design, and implementation. GL-C, JQ-L, and ZF-M were responsible for data collection, input, and correction. GL-Cand YS contributed to data statistics and analysis. JT-M gave important advice on the manuscript. All the authors discussed the first draft of the paper and put forward suggestions for revision. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jianting Ma .
Ethics declarations
Ethics approval and consent to participate.
The study received approval from the Ethics Committee of Yuyao People’s Hospital (protocol code: 2020-09-016), and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Supplementary Information
The online version contains supplementary material available at https://www.scidb.cn/anonymous/ZmEyRWpl .
PE Manuscript Supplementary Material 1.
PE Manuscript Supplementary Material 2.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chen, G., Chen, Y., Shi, Y. et al. A dynamic prediction model for preeclampsia using the sFlt-1/PLGF ratio combined with multiple factors. BMC Pregnancy Childbirth 24 , 443 (2024). https://doi.org/10.1186/s12884-024-06627-4
Download citation
Received : 17 December 2023
Accepted : 09 June 2024
Published : 26 June 2024
DOI : https://doi.org/10.1186/s12884-024-06627-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Preeclampsia(PE)
- SFlt-1/PLGF ratio
- Dynamic prediction model
- Logistic models
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals
You are here
- Online First
- Achieved oxygen saturations and risk for bronchopulmonary dysplasia with pulmonary hypertension in preterm infants
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-0238-6472 Samuel J Gentle 1 ,
- Avinash Singh 2 ,
- http://orcid.org/0000-0002-3218-1024 Colm P Travers 1 ,
- Arie Nakhmani 2 ,
- Waldemar A Carlo 1 ,
- Namasivayam Ambalavanan 1
- 1 Department of Pediatrics , The University of Alabama at Birmingham , Birmingham , Alabama , USA
- 2 Department of Electrical and Computer Engineering , The University of Alabama at Birmingham , Birmingham , Alabama , USA
- Correspondence to Dr Samuel J Gentle, Department of Pediatrics, The University of Alabama at Birmingham, Birmingham, Alabama, USA; sjgentle{at}uabmc.edu
Objective Characterisation of oxygen saturation (SpO 2 )-related predictors that correspond with both bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) development and survival status in infants with BPD-PH may improve patient outcomes. This investigation assessed whether (1) infants with BPD-PH compared with infants with BPD alone, and (2) BPD-PH non-survivors compared with BPD-PH survivors would (a) achieve lower SpO 2 distributions, (b) have a higher fraction of inspired oxygen (FiO 2 ) exposure and (c) have a higher oxygen saturation index (OSI).
Design Case–control study between infants with BPD-PH (cases) and BPD alone (controls) and by survival status within cases.
Setting Single-centre study in the USA.
Patients Infants born at <29 weeks’ gestation and on respiratory support at 36 weeks’ postmenstrual age.
Exposures FiO 2 exposure, SpO 2 distributions and OSI were analysed over the week preceding BPD-PH diagnosis.
Main outcomes and measures BPD-PH, BPD alone and survival status in infants with BPD-PH.
Results 40 infants with BPD-PH were compared with 40 infants with BPD alone. Infants who developed BPD-PH achieved lower SpO 2 compared with infants with BPD (p<0.001), were exposed to a higher FiO 2 (0.50 vs 0.34; p=0.02) and had a higher OSI (4.3 vs 2.6; p=0.03). Compared with survivors, infants with BPD-PH who died achieved a lower SpO 2 (p<0.001) and were exposed to a higher FiO 2 (0.70 vs 0.42; p=0.049).
Conclusions SpO 2 -related predictors differed between infants with BPD-PH and BPD alone and among infants with BPD-PH by survival status. The OSI may provide a non-invasive predictor for BPD-PH in preterm infants.
- Neonatology
- Respiratory Medicine
- Intensive Care Units, Neonatal
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available upon reasonable request to the corresponding author (SG).
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/archdischild-2024-327014
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) increases children’s risk for adverse outcomes into adulthood; however, physicians are currently limited in their ability to predict which children will develop disease.
WHAT THIS STUDY ADDS
Achieved oxygen saturations (SpO 2 ) and the oxygen saturation index (OSI; a parameter which relates respiratory support to SpO 2 ) helped identify infants with BPD-PH. Infants with BPD-PH who died achieved a lower SpO 2 compared with survivors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
SpO 2 targeting may be a modifiable exposure that reduces infants’ risk for the development of BPD-PH. In addition, the OSI may be used as a non-invasive, continuous predictor for BPD-PH.
Introduction
Bronchopulmonary dysplasia (BPD) impacts ~50% of infants born extremely preterm, 1 and it is now known that up to 40% of these infants will have BPD-associated pulmonary hypertension (BPD-PH). 2 BPD-PH, the most severe endotype of BPD, has been reported to culminate in death in up to 48% of infants before the age of 2. 3 Therefore, identification of exposures and predictors for the development of BPD-PH are critical to improve outcomes in this vulnerable population.
Evidence from adults indicates that chronic exposure to high altitudes 4 5 and chronic hypoxia from lung disease 6 7 cause pulmonary vascular remodelling, right ventricular hypertrophy and PH. In lambs with PH, targeting pulse oximetry saturations (SpO 2 ) of 85–89% results in reduced pulmonary perfusion and higher pulmonary vascular resistance compared with higher SpO 2 targets. 8 9 It is unknown whether achieved SpO 2 is associated with preterm infants’ risk for PH. While an SpO 2 target of 91–95%, as compared with 85–89%, increased the need for supplemental oxygen at 36 weeks’ postmenstrual age (PMA) in a meta-analysis of the previous five large, randomised trials in preterm infants, none of these trials reported the outcome of PH. 10–12
As PH may limit gas exchange, previous investigators 13 14 have characterised PH severity using the relationship between oxygen administration and oxygen exchange using the oxygen saturation index (OSI) defined using the mean airway pressure (MAP), fraction of inspired oxygen (FiO 2 ) and SpO 2 (OSI=MAP×FiO 2 ×100/SpO 2 ). 15 We hypothesised that infants with BPD-PH (compared with infants with BPD alone) and non-survivors with BPD-PH (compared with survivors with BPD-PH) would spend a higher proportion of time (on average) with lower SpO 2 distributions and a higher ratio of oxygen delivery to diffusion as estimated by the OSI.
Patient population
This was a single-centre, observational investigation of data prospectively collected between 2018 and 2023 at the University of Alabama at Birmingham (UAB). High-fidelity, cardiorespiratory data were available for a discovery cohort of infants born between 2018 and 2020 as a secondary analysis of infants from UAB enrolled in the Prematurity-Related Ventilatory Control (PreVENT) study. 16 From this cohort, we included infants who were born at <29 weeks’ gestation and on any respiratory support at 36 weeks’ PMA with use of the grade BPD definition. 17 Infants were excluded if they had major congenital anomalies or genetic syndromes. At UAB, infants meeting these inclusion criteria are systematically screened for PH by echocardiography starting at a month of age, and then once a month until discharge. Infants were characterised as having BPD-PH if echocardiographic evidence of PH was demonstrated on any echocardiogram performed during hospitalisation using previously reported criteria. 18
Comparison groups for analyses were (1) infants with echocardiographic BPD-PH and infants with BPD alone and (2) within infants with BPD-PH by survival status up until discharge irrespective of postnatal age. Infants were matched by gestational age at birth (±1 week). The cardiorespiratory parameter analyses were constrained to the postnatal week preceding the first instance of echocardiographic evidence in infants with BPD-PH and, in infants with BPD alone, the week preceding a postnatal age-matched echocardiogram demonstrating absence of PH.
The predictive utility of the OSI was evaluated in this discovery cohort, which was further evaluated in a validation cohort born between 2021 and April 2023. As OSI values are reliant on MAP values for calculations, these analyses were limited to infants on continuous positive airway pressure, non-invasive ventilation or invasive ventilation.
Parameter comparisons from discovery cohort
In infants with any grade of BPD, 17 distributions of SpO 2 were recorded using Philips IntelliVue MP70 or MP50 monitors with Nellcor pulse oximetry sensors (Nellcor, Mansfield, Massachusetts) with an averaging time of 8 s. Data were collected and stored using the BedMaster system ( Excel Medical Electronics , Jupiter, Florida, USA) which collected data as .STP files, which were converted to HDF5 files and later analysed using MATLAB ( MathWorks , Natick, Massachusetts, USA) in-house code.
In infants with grade 2 BPD receiving positive airway pressure and grade 3 BPD, 17 predictive parameters considered included (1) FiO 2 , (2) the ratio of SpO 2 to FiO 2 and (3) OSI over the course of the postnatal week preceding echocardiographic PH. While this constrained the population analysed, infants with severe forms of BPD are at highest risk for long-term neurodevelopmental impairment. 17 Median data, derived from hourly data during this week, were collected from the electronic medical record with concurrent abstraction of SpO 2 , MAP and FiO 2 for OSI calculations. In infants on continuous positive airway pressure, the positive end expiratory pressure value was used as a surrogate for MAP. SpO 2 targets were also compared between groups to ensure differences in SpO 2 distributions were not impacted by targeting.
Parameter comparisons in the validation cohort
The ability of predictive parameters to accurately classify infants with BPD-PH was appraised using a receiver operating characteristic curve-informed cut-off value. These values were used to evaluate the sensitivity, specificity, negative predictive values and positive predictive values in the validation cohort.
Second, predictors with the greatest predictive utility, based on the predictor with the highest area under the curve (AUC), were further evaluated over the entirety of the hospitalisation, in conjunction with data from the discovery cohort, to determine whether predictive parameters could be functionally used for risk stratification within a specific postnatal time period either by (a) postnatal week or (b) PMA.
Power analysis
To our knowledge, SpO 2 distributions have not previously been compared between infants with BPD alone and those with BPD-PH. We have previously reported the duration of severe hypoxaemia between infants with BPD alone and those with BPD-PH in which a similar duration of time was spent <80% and <70% in both groups. 19 However, the distribution of SpO 2 has not been reported. Regarding the OSI, previous studies in infants with congenital diaphragmatic hernia (CDH) have reported an AUC of 0.71 for the detection of PH. 20 Using this AUC, an overall prevalence in our dataset of 50% (based on aforementioned matching approach), level of shrinkage of 0.90 and an expected Cox-Snell R-squared of 0.12 (based on the c-statistic and prevalence), we anticipated the need for 70 infants for model derivation and an additional 70 infants for model validation.
Statistical analysis
Distributions of achieved SpO 2 were compared using an Anderson-Darling test. Receiver operating characteristics (ROC) analysis identified optimal thresholds for BPD-PH accuracy. A Fisher’s exact test was used for analyses of categorical variables between comparison groups. Following tests of normality, the appropriate parametric or non-parametric test was used for continuous variables. Two-way analysis of variance was used for longitudinal OSI comparisons. Analyses were performed with SPSS V.28 and GraphPad Prism V.8.2.1 with a p<0.05 considered significant.
80 infants (40 with BPD-PH gestational age matched to 40 infants with BPD alone) were available from the UAB PreVENT cohort for parameter comparisons for the discovery cohort. The median gestational age and birth weight were 24.4 (IQR 23–26) vs 24.6 (IQR 23–26) weeks’ gestation (p=0.65) and 589 (IQR 529–660) vs 648 (IQR 581–851) g (p=0.004) in infants with BPD alone and BPD-PH, respectively. The respective proportion of infants with grade 1, 2 and 3 BPD among infants with BPD-PH was 40%, 33% and 23% (two infants died prior to 36 weeks’ PMA), respectively, as compared with 55%, 30% and 15% among infants with BPD alone (p=0.46). We previously reported demographic and clinical characteristics in this population, 17 with additional characteristics detailed in online supplemental table 1 .
Supplemental material
Bpd-ph versus bpd alone.
In the week preceding echocardiographic diagnosis, infants who developed BPD-PH achieved a lower SpO 2 compared with infants with BPD alone ( figure 1 ; p=0.0005) despite the similar SpO 2 alarm limits ( online supplemental table 2 ). Representative tracings from infants with BPD alone and BPD-PH are available in online supplemental figure 1 . Compared with infants with BPD alone, infants with BPD-PH were exposed to a higher FiO 2 (0.50 vs 0.34; p=0.02), had a higher OSI (4.26 vs 2.64; p=0.03) and had a similar SpO 2 :FiO 2 ratio (2.2 vs 2.9; p=0.09) in the week prior to echocardiographic identification of BPD-PH ( figure 2A–C ).
- Download figure
- Open in new tab
- Download powerpoint
Characterisation of achieved oxygen saturations (SpO 2 ) in the week preceding initial echocardiographic diagnosis of bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) as compared with saturations in infants with BPD without PH (BPD alone). The distribution of SpO 2 differed between infants with and without BPD-PH (p<0.001).
Differences in respiratory parameters between infants with bronchopulmonary dysplasia (BPD) alone and BPD-associated pulmonary hypertension (BPD-PH) (A–C) and among infants with BPD-PH by survival status (D–F). Parameters of comparison include fraction of inspired oxygen (FiO 2 ), oxygen saturation index (OSI) and ratio of oxygen saturation (SpO 2 ) and FiO 2 . *P<0.05.
In comparing these parameters, OSI had the highest AUC of the ROC curve (0.70), followed by FiO 2 (0.51) and SpO 2 :FiO 2 ratio (0.48) ( figure 3 ). The optimal cut-off value for OSI was determined to be 2.79, which was used to classify infants in the validation cohort. The validation cohort consisted of 87 infants (44 with BPD-PH and 45 with BPD alone). The median gestational age in infants with BPD-PH was 24.4 weeks (IQR 24–25), and 24.0 weeks (IQR 23–25) in infants with BPD alone. From this validation cohort, there were 26 infants with BPD-PH and 13 infants with BPD alone exposed to positive airway pressure for OSI performance appraisal. Test sensitivity was 92% (95% CI 75% to 99%) and specificity was 46% (95% CI 19% to 75%), with a positive predictive value of 77% and a negative predictive value of 75%.
Receiver operating characteristic curves for oxygen saturation index (OSI), fraction of inspired oxygen (FiO 2 ) and oxygen saturation (SpO 2 ):FiO 2 ratio. AUC, area under the curve; BPD, bronchopulmonary dysplasia; BPD-PH, BPD-associated pulmonary hypertension.
In longitudinal OSI comparisons, there was increasing discriminatory utility and separation in OSI values between infants with BPD alone and BPD-PH beyond 16 postnatal weeks and 36 weeks’ PMA ( figure 4A,B ).
Oxygen saturation index (OSI) over hospitalisation by postnatal week (A) and postmenstrual age (B). Data include infants from both the discovery and validation cohorts. Tables beneath panels indicate the number of unique patients per time period available for comparisons. *P<0.05. BPD, bronchopulmonary dysplasia; BPD-PH, BPD-associated pulmonary hypertension.
BPD-PH survivors versus BPD-PH non-survivors
When achieved SpO 2 was compared within infants with BPD-PH by survival status, infants with BPD-PH who died achieved lower SpO 2 compared with survivors ( figure 5 ; p=0.0005). Within infants with BPD-PH, non-survivors were exposed to a higher FiO 2 (p=0.049) and had a similar OSI and SpO 2 :FiO 2 ratio compared with survivors ( figure 2D–F ).
Characterisation of achieved oxygen saturations (SpO 2 ) in the week preceding initial echocardiographic diagnosis of bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) by survival status. The distribution of SpO 2 differed between non-survivors and survivors with BPD-PH (p<0.001).
In this prospective observational study, infants with BPD-PH achieved lower SpO 2 distributions compared with infants with BPD alone. In evaluating related predictive parameters to differentiate infants with BPD-PH from infants with BPD alone, infants with BPD-PH had a higher OSI in the week preceding echocardiographic diagnosis with progressive separation in OSI values after 36 weeks’ PMA. Whereas non-survivors with BPD-PH achieved a lower SpO 2 distribution compared with survivors, OSI was not predictive of survival.
Given that infants that developed BPD-PH achieved lower SpO 2 compared with infants with BPD alone, it is plausible that higher SpO 2 targets may reduce risk for BPD-PH development. The American Thoracic Society and the American Heart Association recommend targeting an SpO 2 of 92–95% in infants with established BPD-PH 21 ; however, there is currently limited evidence for what SpO 2 targeting strategy reduces risk for BPD-PH. In both the Surfactant, Positive Pressure, and Oxygenation Randomized Trial 10 and the Neonatal Oxygenation Prospective Meta-analysis, 22 progressive divergence in survival between randomisation groups occurred with increasing postnatal age starting about a month after randomisation, indicating that infants in the low SpO 2 group are at increased risk for late death. While PH was not routinely assessed in these trials, the time course of the progressive divergence is consistent with the natural history of PH in extremely preterm infants. 2 Evidence from the present study also identified differences in achieved SpO 2 distributions in infants with BPD-PH by survival status, which may further support SpO 2 targets higher than currently recommended. 21 Collectively, these data support the need for additional investigations evaluating the impact of SpO 2 targeting on both the development of BPD-PH and survival in infants with established BPD-PH.
Data from animal studies provide mechanistic associations between SpO 2 targets and pulmonary vascular resistance. In a model of term gestation lambs, pulmonary blood flow was assessed following asphyxia and randomisation to three SpO 2 targets: 85–89%, 90–94% and 95–99%. Lambs in the 95–99% SpO 2 target group had the highest pulmonary blood flow and lowest pulmonary vascular resistance. 9 However, as higher SpO 2 targets may increase retinopathy of prematurity (ROP) risk, 22 the benefits of higher SpO 2 targets must be considered in context to infants’ competing risk for ROP.
In addition to different SpO 2 distributions between infants with and without PH, infants with BPD-PH also had a higher OSI compared with infants with BPD. The OSI has been used in other clinical contexts to continuously measure the presence and severity of PH. In a cohort of infants (n=42) with CDH, an OSI ≥12.5 was associated with a high risk for PH, whereas an OSI ≥22 was associated both with extracorporeal membrane oxygenation and mortality. 23 Animal models have also substantiated the relationship between OSI and increasing pulmonary vascular resistance. 24 Receiver operating curve analysis in the present study identified an optimal OSI cut-off value of ~2.8 in discriminating between infants with BPD-PH from infants with BPD alone. Compared with infants with CDH, the predictive utility of this metric may be more limited given that infants at risk for BPD-PH may not be on a mode of respiratory support from which an OSI can be calculated. In our study, 44% of the discovery cohort and 55% of the validation cohort were on a mode of respiratory support without an estimated MAP precluding an OSI calculation. However, given that a higher proportion of infants with more severe endotypes of BPD develop BPD-PH, 2 the OSI may still have clinical value in infants at highest risk for BPD-PH. While FiO 2 differed between infants with BPD-PH and BPD alone (consistent with previous literature 18 25 ) and has the potential for more generalised applicability, the predictive utility, as estimated by the AUC, was only 0.51 compared with 0.70 using OSI. The magnitude of an individual patient’s FiO 2 may therefore be non-specific to the BPD-PH and may also correspond to other BPD phenotypes. 26
In longitudinal analyses not accounting for the timing of echocardiographic BPD-PH identification, the timing of OSI separation between infants with BPD alone and BPD-PH occurred at around 36 weeks’ PMA. This postnatal age of separation is consistent with the natural history of BPD-PH development reported in observational studies. 2 In considering plausible explanations for this timing of separation, we speculate that this may be driven by progressive intracardiac or extracardiac shunting in infants with BPD-PH, which, by potentially increasing the FiO 2 to SpO 2 ratio over time, would mathematically increase the OSI. Other changes in clinical management, such as subsequent increases in SpO 2 targets, would likely have a limited effect given the anticipated increase in both FiO 2 and SpO 2 . Use of the OSI during hospitalisation in infants with grade 2 and 3 BPD may therefore be used as a continuous biomarker to identify the development of BPD-PH. Moreover, the predictive utility of OSI, as assessed in the validation cohort, conferred a sensitivity of 92%, though the specificity of OSI was only 46% for which echocardiographic confirmation is warranted to further establish the presence or absence of BPD-PH.
There are many strengths with this analysis of SpO 2 achievement and related predictive parameters in infants with and without BPD-PH. The high-fidelity, prospective collection of cardiorespiratory parameters in a large cohort of infants with BPD-PH enabled a comparison of both SpO 2 achievement both between infants with and without BPD-PH and within infants with BPD-PH by survival status. Validation of predictive parameters identified within the discovery cohort and identification of the postnatal age of OSI separation further supports the application of OSI measurements in clinical practice. This observational study was within a single centre for which further evaluation is warranted. Observations within the discovery cohort related to achieved SpO 2 , FiO 2 and OSI were also limited to the week preceding echocardiographic diagnosis in infants with BPD-PH. Observational differences between infants with BPD alone and BPD-PH are limited to associations. Whether SpO 2 targeting has preventative and therapeutic utility for infants with BPD-PH needs further evaluation in randomised studies of differential SpO 2 targets.
In this observational cohort study comparing SpO 2 achievement between infants with BPD-PH and BPD alone, SpO 2 achievement differed between comparison groups and within infants with BPD-PH by survival status. The OSI, which relates oxygen administration to oxygen exchange, differentiated infants with BPD-PH from those with BPD alone with high sensitivity but low specificity with progressive discrimination following 36 weeks’ PMA. Further evidence to support the influence of SpO 2 targeting and the predictive utility of OSI in infants either at risk for or with established BPD-PH are warranted.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
The institutional review board provided approval prior to all analyses.
- Hansen NI , et al
- Arjaans S ,
- Zwart EAH ,
- Ploegstra M-J , et al
- Khemani E ,
- McElhinney DB ,
- Rhein L , et al
- Arias-stella J ,
- Penaloza D ,
- Marticorena E , et al
- Wright JL ,
- Lakshminrusimha S ,
- Swartz DD ,
- Gugino SF , et al
- Chandrasekharan P ,
- Network SSGotEKSNNR ,
- Schmidt B ,
- Asztalos EV , et al
- Thomas NJ ,
- Shaffer ML ,
- Willson DF , et al
- Adamson L ,
- Forster C , et al
- Ghuman AK ,
- Newth CJL ,
- Ambalavanan N ,
- Weese-Mayer DE ,
- Hibbs AM , et al
- Jensen EA ,
- Gantz MG , et al
- Foster C , et al
- Gentle SJ ,
- Travers CP ,
- Nakhmani A , et al
- Horn-Oudshoorn EJJ ,
- Vermeulen MJ ,
- Crossley KJ , et al
- Archer SL , et al
- Darlow BA ,
- Finer N , et al
- Knol R , et al
- Chandrasekharan PK ,
- Williams A , et al
- Clark M , et al
- White AM , et al
Contributors SG is guarantor and helped with manuscript conceptualisation, data curation, formal analysis, original draft preparation, review and editing, and provided final approval of the version to be published. AS and AN contributed to interpretation of data for the work, statistical analysis, manuscript review and editing, and provided final approval of the version to be published. CPT contributed to original draft preparation, review and editing, and provided final approval of the version to be published. WAC and NA contributed to conceptualisation, interpretation of data for the work, original draft preparation, review and editing, and provided final approval of the version to be published. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding NIH/NHLBI (U01 HL133536, U01 HL133536-05S1), National Heart, Lung, and Blood Institute (K23HL157618), American Heart Association (24CDA1275188), LDCC (U01 HL133708).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Online First
- Association between heavy alcohol consumption and cryptogenic ischaemic stroke in young adults: a case–control study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-8489-7051 Nicolas Martinez-Majander 1 ,
- Shakar Kutal 1 ,
- Pauli Ylikotila 2 ,
- Nilufer Yesilot 3 ,
- Lauri Tulkki 1 ,
- http://orcid.org/0000-0001-7530-818X Marialuisa Zedde 4 ,
- http://orcid.org/0000-0001-8815-2807 Tomi Sarkanen 5 , 6 ,
- Ulla Junttola 7 ,
- Annika Nordanstig 8 ,
- Annette Fromm 9 ,
- Kristina Ryliskiene 10 ,
- Radim Licenik 11 ,
- Phillip Ferdinand 12 ,
- Dalius Jatuzis 10 ,
- Liisa Kõrv 13 ,
- Janika Kõrv 13 ,
- http://orcid.org/0000-0001-8629-3315 Alessandro Pezzini 14 ,
- Suvi Tuohinen 15 ,
- Juha Sinisalo 15 ,
- Mika Lehto 16 ,
- Eva Gerdts 17 , 18 ,
- Essi Ryödi 19 ,
- Jaana Autere 20 ,
- Marja Hedman 21 ,
- Ana Catarina Fonseca 22 ,
- Ulrike Waje-Andreassen 9 ,
- Bettina von Sarnowski 23 ,
- Petra Redfors 8 ,
- Tiina Sairanen 1 ,
- Turgut Tatlisumak 8 ,
- Risto O Roine 2 ,
- Juha Huhtakangas 7 ,
- Heikki Numminen 5 ,
- Pekka Jäkälä 20 ,
- http://orcid.org/0000-0002-6630-6104 Jukka Putaala 1
- The SECRETO Study Group
- 1 Neurology , Helsinki University Hospital and University of Helsinki , Helsinki , Finland
- 2 Department of Neurology, Neurocenter , Turku University Hospital, University of Turku , Turku , Finland
- 3 Department of Neurology , Istanbul University Istanbul Faculty of Medicine , Istanbul , Turkey
- 4 Neurology , Azienda Ospedaliera Arcispedale Santa Maria Nuova - IRCCS , Reggio Emilia , Italy
- 5 Department of Neurology , Tampere University Hospital, Wellbeing Services County of Pirkanmaa , Tampere , Finland
- 6 Faculty of Medicine and Health Technology , Tampere University , Tampere , Finland
- 7 Clinical Neuroscience Research Unit and Department of Neurology , Oulu University Hospital , Oulu , Finland
- 8 Department of Neurology , Sahlgrenska University Hospital , Gothenburg , Sweden
- 9 Department of Neurology , Haukeland University Hospital , Bergen , Norway
- 10 Centre of Neurology , Vilnius University , Vilnius , Lithuania
- 11 Stroke , Peterborough City Hospital , Peterborough , UK
- 12 Neurosciences , University Hospitals of North Midlands NHS Trust , Stoke-on-Trent , UK
- 13 Department of Neurology and Neurosurgery , University of Tartu , Tartu , Estonia
- 14 Department of Medicine and Surgery, University of Parma and Stroke Care Program, Department of Emergency , Parma University Hospital , Parma , Italy
- 15 Department of Cardiology , Helsinki University Hospital and University of Helsinki , Helsinki , Finland
- 16 Department of Internal Medicine, Jorvi Hospital , HUS Helsinki University Hospital, Helsinki Finland, and University of Helsinki , Helsinki , Finland
- 17 Department of Heart Disease , Haukeland University Hospital , Bergen , Norway
- 18 Department of Clinical Science , University of Bergen , Bergen , Norway
- 19 Tampere Heart Hospital , Tampere University Hospital , Tampere , Finland
- 20 Neurocenter Neurology , Kuopio University Hospital and University of Eastern Finland , Kuopio , Finland
- 21 Heart Centre , Kuopio University Hospital , Kuopio , Finland
- 22 Department of Neurosciences and Mental Health (Neurology) , Hospital de Santa Maria-CHLN, Faculdade de Medicina, Universidade de Lisboa , Lisboa , Portugal
- 23 Department of Neurology , University Medicine Greifswald , Greifswald , Germany
- Correspondence to Nicolas Martinez-Majander, Neurology, HUS Helsinki University Hospital, Helsinki, Uusimaa, Finland; nicolas.martinez-majander{at}hus.fi
Background The underlying risk factors for young-onset cryptogenic ischaemic stroke (CIS) remain unclear. This multicentre study aimed to explore the association between heavy alcohol consumption and CIS with subgroup analyses stratified by sex and age.
Methods Altogether, 540 patients aged 18–49 years (median age 41; 47.2% women) with a recent CIS and 540 sex-matched and age-matched stroke-free controls were included. Heavy alcohol consumption was defined as >7 (women) and >14 (men) units per week or at least an average of two times per month ≥5 (women) and ≥7 (men) units per instance (binge drinking). A conditional logistic regression adjusting for age, sex, education, hypertension, cardiovascular diseases, diabetes, hypercholesterolaemia, current smoking, obesity, diet and physical inactivity was used to assess the independent association between alcohol consumption and CIS.
Results Patients were twice as more often heavy alcohol users compared with controls (13.7% vs 6.7%, p<0.001), were more likely to have hypertension and they were more often current smokers, overweight and physically inactive. In the entire study population, heavy alcohol consumption was independently associated with CIS (adjusted OR 2.11; 95% CI 1.22 to 3.63). In sex-specific analysis, heavy alcohol consumption was associated with CIS in men (2.72; 95% CI 1.25 to 5.92), but not in women (1.56; 95% CI 0.71 to 3.41). When exploring the association with binge drinking alone, a significant association was shown in the entire cohort (2.43; 95% CI 1.31 to 4.53) and in men (3.36; 95% CI 1.44 to 7.84), but not in women.
Conclusions Heavy alcohol consumption, particularly binge drinking, appears to be an independent risk factor in young men with CIS.
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/jnnp-2024-333759
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The incidence of young-onset ischaemic stroke (IS) is increasing, and this increase seems to be driven by the proportion of cryptogenic IS (CIS). Habitual risk factors, such as heavy alcohol consumption and binge drinking, seem more important in younger vs older individuals, but their association with young-onset CIS still remains understudied.
WHAT THIS STUDY ADDS
Our multicentre case–control Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers and Outcome study demonstrated a strong association between heavy alcohol consumption and binge drinking and young-onset CIS, particularly in men, independent from coexisting stroke risk factors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Future studies should more extensively explore the mechanisms and associated features increasing the risk of young-onset CIS in individuals with heavy drinking. Reducing heavy alcohol consumption remains one of the main targets of lifestyle interventions in young individuals to mitigate their risk of IS, including CIS.
Introduction
Large studies from the last decade have demonstrated a high prevalence of traditional risk factors in young patients with ischaemic stroke (IS). 1–3 There is evidence to suggest that also habitual risk factors may have a stronger role in the development of stroke in younger compared with older individuals. A German nationwide case–control study indicated that low physical activity and hypertension had the strongest association for young-onset IS, followed by smoking and heavy episodic alcohol consumption. 4 Interestingly also, a more recent prospective population-based incidence study suggested that the increase in the incidence of young-onset IS over the last decades was mainly driven by strokes in individuals without traditional risk factors and with undetermined causes. 5
Alcohol intoxication has long been known to be associated with young-onset IS. 6 Both recent and long-term heavy alcohol consumption, as well as binge drinking, can act as a chronic risk factor 7 8 and as a transient trigger 9 for young-onset IS. With long-term heavy drinking, up to 8–15 fold increases in the stroke risk in young patients have been shown. 7 8 In the Risk Factors for Ischemic and Intracerebral Hemorrhagic Stroke in 22 Countries (INTERSTROKE) study, a subgroup analysis of non-selected young patients with IS reported a 5-fold increased risk associated with binge drinking. 10
There is a special interest in studying risk factors for cryptogenic IS (CIS) as (1) they seem to drive the increasing incidence, (2) habitual risk factors seem more important in younger vs older individuals, (3) many other young-specific risk factors and lifestyle habits may have synergy with heavy alcohol consumption and binge drinking and (4) different patterns of alcohol consumption have not been assessed in a larger sample of young patients with CIS. In this study, we aimed to explore the association between heavy alcohol consumption and young-onset CIS in an international multicentre case–control study.
Study population
Between November 2013 and November 2022, 546 young patients with CIS and 546 sex-matched and age-matched stroke-free control subjects were enrolled in 19 European centres in the prospective multicentre SECRETO study (Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers and Outcome, NCT01934725 ). Patients aged 18–49 years and hospitalised due to first-ever imaging-positive IS of undetermined aetiology were included and examined according to a standardised protocol, as previously described. 11 12
All included patients underwent standardised and timely diagnostic workup to exclude definite causes of stroke. Investigations included brain MRI, imaging of intracranial and extracranial vessels with either CT angiography or MR angiography, laboratory testing per protocol, 12-lead ECG, continuous ECG for at least 24 hours, as well as both transthoracic echocardiogram and transoesophageal echocardiogram (TEE). Echocardiography studies were performed according to a standardised protocol. 13 Ancillary testing was carried out on the discretion of physician in charge. Stroke severity was reported with the National Institutes of Health Stroke Scale (NIHSS) score.
CIS was defined according to A-S-C-O classification as the absence of disease (grade 0), or any of grade II (causality uncertain) or grade III (unlikely a direct cause) pathology using diagnostic testing of highest grade of evidence. 14
One sex-matched and age-matched (±5 years) stroke-free control for each patient from the same region was searched locally at each study centre. Sources to identify control subjects included a random search through population registers where feasible, patients’ non-related proxies and hospital staff unrelated to the study.
Cardiovascular risk factors and comorbidities
Detailed clinical history was obtained from all participants using medical records and a structured interview during a study visit. Low level of education was classified as either primary or lower secondary education, or upper secondary education. Registered cardiovascular risk factors included hypertension (prior diagnosis of hypertension, prior antihypertensive medication or a mean of two office blood pressure measures 140/90 or over at study visit), diabetes mellitus (prior diagnosis of any diabetes and/or prior antidiabetic medication), hypercholesterolaemia (prior diagnosis of hypercholesterolaemia or antilipemic medication), cardiovascular disease (history of coronary heart disease, congestive heart failure, peripheral arterial disease or atrial fibrillation (AF)), current tobacco smoking (smoking at least one cigarette per day on average), waist-to-hip ratio (obesity defined as >0.85 in women and >0.90 in men), unhealthy diet, physical inactivity and heavy alcohol consumption. Physical inactivity was assessed using the short version of the International Physical Activity Questionnaire, 15 defined as not meeting any of the criteria for either moderate or high levels of physical activity. A modified version of the Mediterranean Diet Score was used to report participants’ diets, with a higher score indicating a healthier diet and a median of 24 points used as the cut-off for the dichotomous variable. 16
Adaptation of the WHO Alcohol, Smoking and Substance Involvement Screening Test was used to assess alcohol consumption with a structured interview both in patients and controls. 17 Heavy alcohol consumption was defined as >7 (women) and >14 (men) units per week or at least an average of two times per month ≥5 (women) and ≥7 (men) units per instance (binge drinking), according to Substance Abuse and Mental Health Services Administration (SAMHSA), US Department of Health and Human Services. 18
For this study, high-risk right-to-left shunt (RLS) was defined as PFO combined with atrial septal aneurysm in TEE, or a large shunt either in TEE or in transcranial Doppler with bubble study (TCD-BS). 13 19 A subcohort of stroke-free controls at selected study sites also underwent evaluation for RLS.
Statistical analysis
Univariate comparisons of baseline characteristics between patients and controls were assessed using statistical testing appropriate for matched case–control studies, for example, McNemar’s test for dichotomised variables, Paired t-test to compare normally distributed continuous variables, and Wilcoxon signed rank test for non-normally distributed continuous variables. Results are presented as absolute numbers (percentage), mean (SD or median (IQR). A p<0.05 was considered significant.
Data imputation was performed for variables with >10 missing values, namely waist circumference (percentage missing, 8.4%), hip circumference (8.7%) and diet score (14.9%). Multivariate imputation was performed with a Chained Equations R package. In the controls’ variables, there were no variables with >10 missing values and thus, no imputation was performed.
Any potential imbalances between patients and controls were addressed using conditional logistic regression analysis suitable for a matched case–control study, and adjusted OR and 95% CI were reported. Three models are presented: (1) unadjusted conditional logistic regression analysis, (2) adjusted for age, sex and level of education and (3) fully adjusted for age, sex, level of education and predefined vascular risk factors. Based on previous literature, potential confounding vascular risk factors included hypertension, hypercholesterolaemia any type of diabetes mellitus, current smoking, obesity, physical inactivity, unhealthy diet and any other cardiovascular disease. To explore any potential dose–response relationship between alcohol consumption and CIS, we performed additional exploratory conditional logistic regression analysis with quartiles of alcohol consumption, lowest quartile used as the reference group.
We studied potential interactions between heavy alcohol consumption, sex, age and the presence or absence of selected comorbidities, including current smoking, obesity, physical inactivity and high-risk RLS in a logistic regression model adjusted for age, sex, level of education and vascular risk factors by including the presence of the comorbidity as a covariate and further including an interaction term in the model.
Additional conditional logistic regression analyses with similar models were performed to explore the association stratified by sex for binge drinking alone with young-onset CIS. As an exploratory analysis, these were also performed for three predetermined age groups as well (18–34, 35–44 and 45–49 years).
Furthermore, to explore the robustness of the results, we assessed the association by comparing patients to controls who were identified strictly from population-based sources, that is, excluding those selected from for example, hospital staff and patients’ nonrelated proxies. This sensitivity analysis is also reported stratified by sex.
Statistical analyses were performed with IBM SPSS Statistics for Windows, V.29.0 (IBM).
To study associations, we included 540 patients with CIS (median age 41 years, IQR 34–46; 47.2% women) and 540 age-matched and sex-matched controls with detailed data on alcohol consumption available. In patients, the median delay from symptom onset to hospital admission was 0 (IQR 0–1) days and from admission to study inclusion/interview 6 (IQR 4–9) days. Median NIHSS score on admission was 2 (IQR 0–4, range 0–17). Of all patients, 25.7% had NIHSS score of 0, 50.6% had mild strokes (NIHSS 1–4), 14.0% had moderate strokes (NIHSS 5–9) and 9.9% had severe strokes (NIHSS≥10). Patients with heavy alcohol consumption had more severe strokes compared with patients without heavy alcohol consumption (mild strokes 44.6% vs 51.4%, moderate strokes 24.3% vs 12.3% and severe strokes 12.2% vs 9.5%, p=0.027).
Univariate comparison between patients and matched controls
In the entire study population, patients were more likely to be heavy alcohol users compared with controls (13.7% vs 6.7%, p<0.001) ( table 1 and figure 1 ). Case–control analyses stratified by age groups showed that this difference was significant in men aged 18–34 years (32.3% vs 11.3%, p=0.004) but not in other age groups ( online supplemental figure s1 ). Regarding other risk factors, patients were more likely to have a history of hypertension, and they were more often current smokers, overweight, less educated, physically inactive and had more unhealthy diet ( table 1 ). Overall, there were very few participants with pre-existing cardiovascular disease.
Supplemental material
- Download figure
- Open in new tab
- Download powerpoint
Comparison of heavy alcohol consumption for all study participants and stratified by sex and age group.
- View inline
Comparison of baseline characteristics of cardiovascular risk factors of young cryptogenic ischaemic stroke patients and stroke-free control subjects included in the study
Female patients had a lower level of education and more unhealthy diet compared with female controls. They were also more likely to be current smokers, obese and hypertensive. There was no difference in heavy alcohol consumption (11.8% vs 7.5%, p=0.099). Male patients were more frequently heavy alcohol users compared with male controls (15.4% vs 6.0%, p<0.001). Male patients also had a lower level of education, more unhealthy diet, they were more often obese, and current smokers ( table 1 ). In patients, the youngest males were more likely to be heavy alcohol users compared with other age groups (p<0.001) ( online supplemental figure s1 ). In female patients, there were no significant differences between age groups.
Association between heavy alcohol consumption and CIS
In the unadjusted model, a significant association between heavy alcohol consumption and young-onset CIS in the entire study population emerged (OR 2.56; 95% CI 1.61 to 4.06, p<0.001). This association remained significant after adjustment for demographics (OR 2.27; 95% CI 1.38 to 3.74, p=0.001) and for further vascular risk factors (OR 2.11; 95% CI 1.22 to 3.63, p=0.007) ( table 2 , figure 2 ). ORs and 95% CIs for each covariate appear in online supplemental table s1 .
Association between heavy alcohol consumption and cryptogenic ischaemic stroke, stratified by sex and age (for men only). Diabetes and cardiovascular disease included in the model only in older men (45–49 years) due to low prevalence of these risk factors in other groups.
ORs and 95% CI from conditional logistic regression on the association between heavy alcohol consumption, binge drinking and cryptogenic ischaemic stroke, also stratified by sex
Association between heavy alcohol consumption and CIS according to sex
In sex-specific analysis, the association between heavy alcohol consumption and CIS was significant in men, both in unadjusted analysis (OR 3.25; 95% CI 1.70 to 6.21, p<0.001) and when analysed further adjusting for demographics (OR 3.11; 95% CI 1.53 to 6.30, p=0.002) and for demographics and vascular risk factors (OR 2.72; 95% CI 1.25 to 5.92, p=0.012) ( table 2 , figure 2 , p for interaction 0.038). No association was found in women alone when adjusting for demographics (OR 1.58; 95% CI 0.77 to 3.25, p=0.215), nor in a fully adjusted model including demographics and vascular risk factors (OR 1.56; 95% CI 0.71 to 3.41, p=0.267) ( table 2 , figure 2 ). ORs and 95% CIs for each covariate are shown in online supplemental table s2 . In an exploratory analysis of men by age groups, in the youngest men, a significant association was shown between heavy alcohol consumption and CIS (OR 12.11; 95% CI 1.54 to 95.47, p=0.018) ( online supplemental table s3 ). However, there was no formal interaction between heavy alcohol consumption and age group in men (p for interaction 0.665). No association was seen in other age groups in men or any age group in women.
Association between binge drinking and CIS
Analysis of binge drinkers alone showed similar differences in univariate comparison in the entire study population (11.7% vs 4.8%, p<0.001) and in men (15.1% vs 4.9%, p<0.001) but not in women ( figure 3 ). Association with young-onset CIS was also significant in the entire population (fully adjusted OR 2.43; 95% CI 1.31 to 4.53, p=0.005) and in men (fully adjusted OR 3.36; 95% CI 1.44 to 7.84, p=0.005), but again, not in women (fully adjusted OR 1.50; 95% CI 0.58 to 3.92, p=0.404) ( table 2 , figure 2 ). Unadjusted and less-adjusted models and ORs for each covariate are shown in online supplemental tables s4 and s5 .
Comparison of binge drinking for all study participants and stratified by sex and age group.
Interaction assessment with other risk factors
In the entire cohort, there was no interaction between heavy alcohol consumption and current smoking (p=0.667), obesity (p=0.732) or physical inactivity (p=0.295) ( table 3 ). In men, we found no interaction between heavy alcohol consumption and current smoking (p=0.968), obesity (p=0.056) or physical inactivity (p=0.852). In women, no interaction between heavy alcohol consumption and current smoking (p=0.509), obesity (p=0.372) or physical inactivity (p=0.100) was found ( table 3 ). High-risk RLS was detected in 194 (37.9%) patients and 34 (8.8%) controls (p<0.001) of the investigated individuals. No difference in the prevalence of high-risk RLS among patients was observed between sexes (women 39.3% vs men 35.9%, p=0.419) or in controls (women 7.9% vs men 10.1%, p=0.466). Exploratory analysis showed no interaction between heavy alcohol consumption and high-risk RLS (p=0.108) ( online supplemental table s6 ).
Exploratory subgroup analyses on the association between heavy alcohol consumption and cryptogenic ischaemic stroke
Sensitivity analyses
When selecting case–control pairs with strictly population-based controls (n=316), patients with heavy alcohol consumption had a higher risk of CIS (unadjusted OR 2.25; 95% CI 1.50 to 4.05, p=0.007). The association remained significant when adjusted for demographics (OR 2.22; 95% CI 1.15 to 4.27, p=0.017), but not in the fully adjusted model (OR 1.97; 95% CI 0.94 to 4.10, p=0.071). In male patients with population-based male controls only, the association remained significant when adjusted for demographics (OR 3.13; 95% CI 1.29 to 7.58, p=0.012), but not in the fully adjusted model (OR 2.39; 95% CI 0.87 to 6.54, p=0.091). In women, no association was observed in any of the models. Unadjusted and less-adjusted models and ORs for each covariate are shown in online supplemental tables s7 and s8 . Again, in univariate analyses, particularly younger male patients were more likely to be binge drinkers than young male controls ( online supplemental table s3 and online supplemental figure s2 ). No dose-response was observed in further exploratory analysis in the entire study population nor in men alone ( online supplemental table s9 ).
In this multicentre case–control study, we detected a robust independent association between heavy alcohol consumption and binge drinking with young-onset CIS. This connection held true in young men, even after accounting for various well-known confounding factors, such as hypertension, physical inactivity, obesity and current smoking. Interestingly, this association was not observed in young women.
The present analysis adds to previous knowledge from meta-analysis of the associations between alcohol consumption and stroke by demonstrating that high alcohol consumption as well as binge drinking is an independent risk factor for IS, particularly in young men. 20–22 In smaller and older studies, both recent and long-term heavy alcohol consumption, as well as binge drinking, have been shown to act as a chronic risk factor 7 8 and as a transient trigger 9 for young-onset IS of any aetiology. With long-term heavy drinking, increases of up to 8–15 times in the stroke risk in young patients have been reported. 7 8 22 However, most of these studies included young IS patients with any type of aetiology, in contrast to a specific subgroup of CIS patients in our study.
In accordance with our findings, patients who were current drinkers in prior studies were more likely to be younger men, but also current smokers. This reflects reality, as for instance according to Centers for Disease Control and Prevention, heavy alcohol consumption and binge drinking are most common among younger adults aged 18–34 and among men. Furthermore, the INTERSTROKE study reported risk factors for a subgroup of young patients (<45 years) alone, such as a 5.4-fold risk for binge drinking stroke (71% were IS). 10 However, the INTERSTROKE study included IS of all aetiologies, in contrast to subgroups of CIS patients in our study. Also, the Stroke in Young Fabry Patients study reported that among patients with transient ischaemic attack or IS of any aetiology, heavy alcohol consumption was more common in men than in women, highlighted in individuals aged 18–24 years compared with other age groups. 2 The independent relation with heavy alcohol consumption only in men in our study might be explained by the higher prevalence of heavy alcohol consumption and binge drinking compared with female patients. Particularly binge drinking might further be associated with other less well-documented risk factors in men, such as illicit drug use and unhealthy diet. In our study, female patients also had other more frequent risk factors compared with female controls, namely hypertension and abdominal obesity. Other well-known risk factors in women include pregnancy, puerperium and the use of combined oral contraceptives. These sex-specific and gender-specific risk factors might diminish the effect of alcohol consumption alone compared with young men.
Several differing criteria for heavy alcohol consumption and binge drinking exist. Applying different criteria in different studies may evidently affect the comparison between studies. For instance, in the INTERSTROKE study, the limits for a high intake of alcohol were considerably higher than in the SECRETO study, >14 drinks for women and >21 drinks for men. 23 Binge drinking was defined as >5 drinks in 1 day at least once a month over the previous 12 months for both sexes. Their study referred to an older prospective population-based study and criteria from 1997, 24 compared with SAMHSA criteria used in SECRETO which were updated in 2015. This difference shows that the criteria have become considerably stricter over the years.
Mechanisms associated with heavy alcohol consumption and CIS include, for instance, adverse effects on hemostasis, fibrinolytics, blood clotting and subclinical cardiac arrhythmias, excluding documented AF. 25 Heavy drinkers may also be more likely to suffer from head and neck trauma predisposing cervical or intracranial artery dissection and subsequent IS, although such mechanisms are not likely in CIS. Moreover, excessive alcohol consumption can predispose other risk factors including hypertension and visceral obesity, but also more acute conditions such as cerebral vasospasm. 26 27 In our study, patients with heavy alcohol consumption also had more severe strokes based on unknown mechanisms. This might be caused by a potential covert embolism from heart or other unknown source or perhaps thrombus developing locally in larger arteries. Recent analyses of first SECRETO including 150 patients and controls also demonstrated that left atrial myopathy more than doubled the risk of young-onset CIS, but LA myopathy was not associated with heavy alcohol consumption itself. 28 Heavy drinkers may also harbour several other risk factors making them as potentially important target group for both primary and secondary preventive measures.
The most notable strengths of the SECRETO study include the robustness of the prespecified and published study protocol and an extensive and timely diagnostic workup for each participant. As described above, only patients with imaging verified IS were included to ensure the homogeneity of the study population and to exclude any stroke mimics. Furthermore, all participants were examined in a standardised manner and validated, structured questionnaires were used for data collection with high granularity. There were only a few missing data points, as patients and controls were personally interviewed. Sensitivity analyses were performed to demonstrate the robustness of the results, such as including only population-based controls. It was also possible to adjust for multiple relevant confounders in the multivariable analyses. As this study enrolled patients and controls in 19 centres across Europe, our results are considered generalisable to populations of European origin.
However, some limitations must be acknowledged. Although the aim was to enrol all consecutive patients, some selection bias might have occurred. For instance, as enrolled patients had relatively mild strokes on admission, it is possible that some patients with more debilitating symptoms may have been left out and thus, the results of this study are generalisable to minor to moderate strokes. However, prior studies have demonstrated that young IS patients tend to have overall lower NIHSS on admission than older patients. 29 Some selection bias might also be present when enrolling controls. For instance, controls with more frequent alcohol consumption might have been less willing to participate in this kind of study, possibly leading to overestimation of effect size. Our sensitivity analysis showed, however, that when restricting to population-based controls only, the effect size of heavy alcohol consumption remained similar and nearly significant even with a smaller sample size. As informed consent also from controls was required before collecting more data on baseline characteristics and risk factors, we were not able to describe in detail those stroke-free individuals who did not accept our study invitation. However, the prevalence of heavy alcohol consumption reported by SAMHSA was comparable with the prevalence in stroke-free controls in our study (7.1% in young adults aged 18 to 25 and 6.3% in adults aged 26 or older), 17 further supporting that there is no considerable selection bias with our control subjects. As the data for this study were collected mainly during hospitalisation or shortly after discharge, we were not able to report the frequency of, for instance, newly-onset AF later diagnosed with an implantable loop recorder (ILR) or home telemetry. However, the use of ILRs and external ECG recorders have become more prevalent only in recent years and were seldom used during our study’s recruitment. In addition, there are no strong recommendations to use of ILRs for screening AF in young stroke patients given their low expected yield.
Conclusions
Our multicentre case–control study demonstrated a strong association between heavy alcohol consumption and binge drinking and young-onset CIS, independent from coexisting stroke risk factors. Subgroup analyses confirmed these associations in men but not in women. Future studies should more extensively explore the mechanisms and associated features increasing the risk of young-onset CIS in individuals with heavy drinking, such as alcohol type, recent consumption prior to index stroke, cumulative lifetime risk of alcohol consumption and effects on the coagulation system. Reducing heavy alcohol consumption remains one of the main targets of lifestyle interventions in young individuals to mitigate their risk of IS, including CIS.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants and the study protocol was approved by the local ethics committees in each recruiting site. In Helsinki University Hospital, this was HUS/2684/2017. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We are indebted to Laura-Leena Kupari, RN, Jaana Koski, RN and Anu Eräkanto, Research Secretary, for their invaluable assist in conducting the study.
- Putaala J ,
- Yesilot N ,
- Waje-Andreassen U , et al
- von Sarnowski B ,
- Grittner U , et al
- Pezzini A ,
- Lodigiani C , et al
- Grittner U ,
- Rolfs A , et al
- Rothwell PM
- Hillbom M ,
- McNeil JJ ,
- O’Malley HM , et al
- Nightingale AL ,
- Haapaniemi H ,
- O’Donnell MJ , et al
- Martinez-Majander N ,
- Saeed S , et al
- Ylikotila P , et al
- Amarenco P ,
- Bogousslavsky J ,
- Caplan LR , et al
- Marshall AL ,
- Sjöström M , et al
- Panagiotakos DB ,
- Pitsavos C ,
- Arvaniti F , et al
- ↵ Substance abuse and mental health services administration . n.d. Available : https://www.samhsa.gov/
- Tsivgoulis G ,
- Stamboulis E ,
- Sharma VK , et al
- Larsson SC ,
- Wolk A , et al
- Reynolds K ,
- Nolen JDL , et al
- Irving H , et al
- O’Donnell M ,
- Rangarajan S , et al
- Kauhanen J ,
- Kaplan GA ,
- Goldberg DE , et al
- Saloheimo P ,
- Altura BM ,
- Altura BT ,
- Gebrewold A
- Sindre RB ,
- Putaala J , et al
- Fonarow GC ,
- Reeves MJ ,
- Zhao X , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
X @MlzZedde, @SarkanenTomi, @CatarinaACF
Contributors NM-M, SK and JP designed the study, acquired and analysed the data and prepared the first version of the manuscript. All authors acquired the data, critically reviewed and edited the manuscript and approved the final version of the manuscript. JP is the guarantor of this work.
Funding Helsinki and Uusimaa Hospital District research fund (TYH2014407, TYH2018318); Academy of Finland (286246, 318075, 322656); The Finnish Medical Foundation (5739); The Sigrid Jusélius Foundation (N/A), Sahlgrenska University Hospital (ALFGBG-726821).
Competing interests JP: shareholder of Olvi Oyj. TT: has served/serves on scientific advisory boards for Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Inventiva and Portola Pharm.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
Diagnose preeclampsia with severe features and order/initiate magnesium sulfate Counsel the patient regarding preeclampsia and provide delivery recommendations OR If the team does not correct the hypertension or fails to recognize preeclampsia with severe features and initiate magnesium sulfate within 10 minutes 4.0 Case Scenario:
April 17, 2020 Missouri AIM Collaborative Webinar Amanda Trudell DO MSCI FACOG Director Maternal Fetal Medicine Program Development Missouri Baptist Medical Center, St. Louis, MO. 33 yo G4P3003 @ 28w2d with chronic hypertension on labetalol 600 mg TID. The patient presents to the OB office with BP logs concerning for severe range HTN as an ...
2. Case presentation. This is a 35 year old patient, having had three in-utero fetal deaths at five months of pregnancy and one child by vaginal birth (11 years ago), with no particular pathological history, admitted for preeclampsia at 19 weeks of gestation and 4 days, with a systolic blood pressure at 170 mmHg and a diastolic blood pressure of 110 mmHg, headache and epigastric pain.
Pre-eclampsia in Pregnancy. Canterbury ANSC: Educational Case Study Series - June 2021Hypertensive disorders affect 8-20% of pregnant women and are associated with substantial complications for the woman and baby including increased risk of maternal morbidity and mortality, increased risk of still. irth, preterm delivery and intrauterine ...
Hypertensive disorders (preeclampsia and eclampsia) of pregnancy are a leading cause of maternal morbidity and mortality.1 Although the incidence of eclampsia has decreased, ... The case Ms. Susan Smith is a healthy 33-year-old G1P0 at 38 weeks gestation admitted for induction of labor due to gestational hypertension. Her baseline vital signs ...
A review article that summarizes the current knowledge and challenges of pre-eclampsia, a common disorder of pregnancy. It covers the definition, epidemiology, sub-types, causes, diagnosis, treatment, and long term consequences of pre-eclampsia.
Results Out of a total of 500 pregnant women, 31 developed preeclampsia; hence, the prevalence of preeclampsia was found to be 6.2%. Of the 31 preeclamptic women, majority belonged to the 20-29 ...
The complications of severe PE can be serious enough to cause fetal and maternal morbidity. Intravascular coagulopathy, renal failure, and HELLP syndrome [ 1, 2] are the commonest complications of severe PE. Severe PE increases the risk of adverse maternal outcome by 8.7-fold. [] Cerebral hemorrhage, pulmonary edema, renal failure, and/or ...
5.0 Case Flow/Algorithm with branch point and completion criteria: Simulation facilitator will introduce the scenario to the team outside the room and then bring primary bedside provider to the patient's room and then read them the patient scenario. The OB Nurse should then enter the room, assess the patient, and then call for assistance as
case study on pre eclampsia - Free download as Word Doc (.doc), PDF File (.pdf), Text File (.txt) or read online for free. This case study describes a 26-year-old pregnant woman admitted to the hospital for high blood pressure and labor pains. The patient has a history of hypertension during this pregnancy but no other major medical issues. A physical assessment was performed and found the ...
Abstract. Preeclampsia is clinically de ned by hypertensio n and proteinuria, with o r without pathologic edema that occurs a er 20 weeks '. gestation, but can also presen t up to 4-6 weeks post ...
UNFOLDING Clinical Reasoning Case Study: STUDENT Preeclampsia-Eclampsia History of Present Problem: Dana Myers is a 40-year-old woman, G-3 P-2 who is 34 weeks gestation. Her health care provider has been monitoring her weekly because her blood pressure has been increasing the past month and is currently 146/88. Last week she had 1+ non-pitting ...
Abstract. Preeclampsia is defined as BP > 140/90 mmHg on 2 occasions 4-6 hours apart, which occurs in pregnancy after 20th week of period of gestation, which involves multi organ system ...
1. Introduction. Hypertensive disorder during pregnancy poses a substantial threat to both maternal and fetal health conditions [].Preeclampsia is one of the most well-known medical conditions that belong to this disease spectrum, which also accounts for one of the most common documented gestational complications, with a prevalence of approximately 2 to 15% of all pregnancies [2,3].
The purpose of this study was to determine the risk factors which most influence the incidence of preeclampsia. This was an observational analytic study with case-control study design. The populations were all pregnant and postpartum mothers who were treated in RSUD dr. Soeselo Slawi from January to June 2018.
Background Maternal mortality is a critical indicator in assessing the quality of services provided by a health care system. Approximately 99% of all maternal deaths occur in developing countries; where a majority of the causes of these deaths are preventable. Case presentation A 25-year-old, married, multigravida, black woman who has had six live births presented to a health center with the ...
Case Presentation on Pre-eclampsia - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. This document contains a case study of a 27-year-old pregnant woman admitted to the hospital. She is a primigravida at 38 weeks and 6 days in latent labor with pre-eclampsia. Her chief complaints were leaking per vagina for 12 hours and lower abdominal pain ...
This case study discusses preeclampsia, a pregnancy complication characterized by high blood pressure and organ damage. It begins with an introduction defining preeclampsia and classifications based on blood pressure levels. It then covers causes, pathophysiology, risk factors, complications, signs and symptoms, diagnostic evaluation through history, physical exam and laboratory tests. The ...
s a pregnancy-specific hypertensive disorder with multisystem involvement. Severe preeclampsia can result in. both acute and long term complications for both the woman and her newborn. Maternal complications of severe preeclampsia include pulmonary edema, myocardial infarction, stroke, acute respirator. distress syndrome, coagulopathy, severe ...
Case Presentation. The patient was a 17-year-old male who was admitted to our hospital in May 2020 due to uncontrolled hypertension for 6 months and weakness of limbs for 20 days. Six months prior to admission, blood pressure of the patient was found to have increased to 200/120 mmHg during the physical examination.
Objective Preeclampsia (PE) is a pregnancy-related multi-organ disease and a significant cause of incidence rate and mortality of pregnant women and newborns worldwide. Delivery remains the only available treatment for PE. This study aims to establish a dynamic prediction model for PE. Methods A total of 737 patients who visited our hospital from January 2021 to June 2022 were identified ...
Case Study Preeclampsia - Free download as PDF File (.pdf), Text File (.txt) or read online for free. This 29-year-old pregnant woman with a history of preeclampsia presented to the emergency department at 29 weeks gestation with severe hypertension, headache, blurry vision and abdominal pain. Her blood pressure was over 200/120. She was transferred to the labor and delivery unit where she ...
Introduction. Bronchopulmonary dysplasia (BPD) impacts ~50% of infants born extremely preterm,1 and it is now known that up to 40% of these infants will have BPD-associated pulmonary hypertension (BPD-PH).2 BPD-PH, the most severe endotype of BPD, has been reported to culminate in death in up to 48% of infants before the age of 2.3 Therefore, identification of exposures and predictors for the ...
PDF | The trends of diseases has been changed from the last few years, now the burden of non-communicable diseases is increasing day by day. ... Case study, American Journal of Hypertension ...
Case Study (Preeclampsia) - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. This case study discusses preeclampsia, a condition characterized by high blood pressure and protein in the urine during pregnancy. Preeclampsia is a leading cause of maternal and infant illness and death. It occurs most often in first time mothers and those under 17 ...
Background The underlying risk factors for young-onset cryptogenic ischaemic stroke (CIS) remain unclear. This multicentre study aimed to explore the association between heavy alcohol consumption and CIS with subgroup analyses stratified by sex and age. Methods Altogether, 540 patients aged 18-49 years (median age 41; 47.2% women) with a recent CIS and 540 sex-matched and age-matched stroke ...