An official website of the United States government
Here’s how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Dept. of Health & Human Services

Outlines the process for registering a clinical trial, updating the entry, and reporting results. Clinical trial sponsors are required to register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulations.
Guidance for grantees on registering and updating the record of a clinical trial
Issued by: National Institutes of Health (NIH)
Issue Date: August 01, 2019
COVID-19 is an emerging, rapidly evolving situation.
Get the latest public health information from CDC: https://www.coronavirus.gov Get the latest research information from NIH: https://www.nih.gov/coronavirus
ClinicalTrials.gov is a consumer-friendly database with information on clinical studies funded and/or sponsored by the NIH, other federal agencies, and private industry. The database was developed by NIH, through its National Library of Medicine (NLM), in collaboration with the Food and Drug Administration (FDA).
The Food and Drug Administration Amendments Act of 2007 (also called FDAAA) requires that clinical trial sponsors register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulation ( see Public Law 110-85, Title VIII ). Penalties for failure to register a trial with complete information may include civil monetary penalties and the withholding of federal grant funds.
Find more information on requirements and developing information concerning FDAAA . Clinical trial sponsors are required to register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulations.
Register Your Clinical Trial
Sponsors can register clinical studies on clinicaltrials.gov via a web-based data entry system called the Protocol Registration and Results System (PRS) . To register your clinical trial:
- Check to see whether your organization already has a PRS organization account .
- Apply for a PRS account. See how to apply for an account on ClinicalTrials.gov.
- Logon to PRS on the PRS Login Page .
- Enter the required and optional data elements.
- Preview, inspect, and submit the record.
Contact your organization's Clinicaltrials.gov account administrator to register your trial. If you do not know your account administrator, contact [email protected] for assistance.
Update Trial Information
You are responsible for maintaining the accuracy of the trial information in ClinicalTrials.gov and for reporting results. To update your trial information:
- Logon to Clinicaltrials.gov.
- Select MODIFY.
- Select EDIT next to your trial's listing.
- Select EDIT next to the block of information you wish to edit.
- Select OK at the bottom of the page when your editing is complete.
- Select RESET TO COMPLETED near the top of the page.
- Select APPROVE.
- Select RELEASE.
Report Results
You are required to submit results of data. It is important to note that results-reporting requires a fairly sophisticated knowledge of the study. To report your trial results:
- Logon to ClinicalTrials.gov.
- Scroll down to “For Completed Studies” and select ENTER RESULTS.
- Begin entering results.
Helpful Links and Resources
- Clinicaltrials.gov Assistance: [email protected] .
- Logon Clinicaltrials.gov .
- International Committee of Medical Journal Editors requirements: International Committee of Medical Journal Editors requirement .
- Public Law Information: FDAAA (Public Law 110-85) information .
- FAQs: Clinicaltrials.gov .
More on the FDAAA
Under the 1997 FDA Modernization Act, NIH was instructed to establish a database of therapeutic clinical trials that is understandable and accessible to the public. The purpose of this legislation was to make information about clinical trials available to members of the public who are suffering from diseases so they can apply to be research subjects.
ClinicalTrials.gov offers up-to-date information for locating federally and privately supported clinical trials for a wide range of diseases and conditions. A clinical trial (also clinical research) is a research study in human volunteers to answer specific health questions. Interventional trials determine whether experimental treatments or new ways of using known therapies are safe and effective under controlled environments. Observational trials address health issues in large groups of people or populations in natural settings.
The Food and Drug Administration Amendments Act of 2007 (also called FDAAA) was passed on September 27, 2007. The law now requires that the “Responsible Party” must register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulation ( see Public Law 110-85, Title VIII ). It also mandates that some previously optional data elements are now required. In general, the Responsible Party is defined as the sponsor of an applicable clinical trial. The law also allows the role of Responsible Party to be assigned to the Principal Investigator (PI) if the PI is responsible for conducting the trial, has access to and control over the data from the clinical trial, has the right to publish the results of the trial, and has the ability to meet all of FDAAA’s requirements for the submission of clinical trial information. For investigator-initiated clinical trials, NIH is generally not the sponsor and, as such, NIH would not be the Responsible Party. Under this law, the Responsible Party is accountable for compliance, including accuracy and completeness of the data. Penalties for failure to register a trial with complete information may include civil monetary penalties and the withholding of federal grant funds.
ClinicalTrials.gov also helps to register trials in accordance with the International Committee of Medical Journal Editors (ICMJE) initiative requiring prior entry of clinical trials in a public registry as a condition for publication.
HHS is committed to making its websites and documents accessible to the widest possible audience, including individuals with disabilities. We are in the process of retroactively making some documents accessible. If you need assistance accessing an accessible version of this document, please reach out to the [email protected] .
DISCLAIMER: The contents of this database lack the force and effect of law, except as authorized by law (including Medicare Advantage Rate Announcements and Advance Notices) or as specifically incorporated into a contract. The Department may not cite, use, or rely on any guidance that is not posted on the guidance repository, except to establish historical facts.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

ClinicalTrials.gov is a consumer-friendly database with information on clinical studies funded and/or sponsored by the NIH, other federal agencies, and private industry. The database was developed by NIH, through its National Library of Medicine (NLM), in collaboration with the Food and Drug Administration (FDA).
The Food and Drug Administration Amendments Act of 2007 (also called FDAAA) requires that clinical trial sponsors register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulation ( see Public Law 110-85, Title VIII ). Penalties for failure to register a trial with complete information may include civil monetary penalties and the withholding of federal grant funds.
Find more information on requirements and developing information concerning FDAAA . Clinical trial sponsors are required to register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulations.
Register Your Clinical Trial
Sponsors can register clinical studies on clinicaltrials.gov via a web-based data entry system called the Protocol Registration and Results System (PRS) . To register your clinical trial:
- Check to see whether your organization already has a PRS organization account .
- Apply for a PRS account. See how to apply for an account on ClinicalTrials.gov.
- Logon to PRS on the PRS Login Page .
- Enter the required and optional data elements.
- Preview, inspect, and submit the record.
Contact your organization's Clinicaltrials.gov account administrator to register your trial. If you do not know your account administrator, contact [email protected] for assistance.
Update Trial Information
You are responsible for maintaining the accuracy of the trial information in ClinicalTrials.gov and for reporting results. To update your trial information:
- Logon to Clinicaltrials.gov.
- Select MODIFY.
- Select EDIT next to your trial's listing.
- Select EDIT next to the block of information you wish to edit.
- Select OK at the bottom of the page when your editing is complete.
- Select RESET TO COMPLETED near the top of the page.
- Select APPROVE.
- Select RELEASE.
Report Results
You are required to submit results of data. It is important to note that results-reporting requires a fairly sophisticated knowledge of the study. To report your trial results:
- Logon to ClinicalTrials.gov.
- Scroll down to “For Completed Studies” and select ENTER RESULTS.
- Begin entering results.
Helpful Links and Resources
- Clinicaltrials.gov Assistance: [email protected].
- Logon Clinicaltrials.gov .
- International Committee of Medical Journal Editors requirements: International Committee of Medical Journal Editors requirement .
- Public Law Information: FDAAA (Public Law 110-85) information .
- FAQs: Clinicaltrials.gov .
More on the FDAAA
Under the 1997 FDA Modernization Act, NIH was instructed to establish a database of therapeutic clinical trials that is understandable and accessible to the public. The purpose of this legislation was to make information about clinical trials available to members of the public who are suffering from diseases so they can apply to be research subjects.
ClinicalTrials.gov offers up-to-date information for locating federally and privately supported clinical trials for a wide range of diseases and conditions. A clinical trial (also clinical research) is a research study in human volunteers to answer specific health questions. Interventional trials determine whether experimental treatments or new ways of using known therapies are safe and effective under controlled environments. Observational trials address health issues in large groups of people or populations in natural settings.
The Food and Drug Administration Amendments Act of 2007 (also called FDAAA) was passed on September 27, 2007. The law now requires that the “Responsible Party” must register and report results for certain clinical trials of drugs, biologics, and devices that are subject to FDA regulation ( see Public Law 110-85, Title VIII ). It also mandates that some previously optional data elements are now required. In general, the Responsible Party is defined as the sponsor of an applicable clinical trial. The law also allows the role of Responsible Party to be assigned to the Principal Investigator (PI) if the PI is responsible for conducting the trial, has access to and control over the data from the clinical trial, has the right to publish the results of the trial, and has the ability to meet all of FDAAA’s requirements for the submission of clinical trial information. For investigator-initiated clinical trials, NIH is generally not the sponsor and, as such, NIH would not be the Responsible Party. Under this law, the Responsible Party is accountable for compliance, including accuracy and completeness of the data. Penalties for failure to register a trial with complete information may include civil monetary penalties and the withholding of federal grant funds.
ClinicalTrials.gov also helps to register trials in accordance with the International Committee of Medical Journal Editors (ICMJE) initiative requiring prior entry of clinical trials in a public registry as a condition for publication.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Opportunities & Announcements
- Funding Strategy for Grants
- Grant Writing & Approval Process
- Managing Grants
- Clinical Research
- Small Business Research
What You Need to Know About ClinicalTrials.gov Registration and Results Reporting
In order to register a clinical trial in the National Library of Medicine’s (NLM) ClinicalTrials.gov registry, you or your designee will need to have a Protocol Registration System (PRS) individual or organization account. This account will allow you to create and update your clinical trials records as necessary. More details are available on the PRS website .
For more information about Clinicaltrials.gov registration and results submission, see Frequently Asked Questions about Clinicaltrials.gov Registration and Results Submission for NIMH-funded Clinical Trials .
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Clinical Research
What to submit.
We consider all types of clinical research. Original studies can be submitted in any of our content types .
Preparing your submission
Nature Medicine follows the recommendations from the International Committee of Medical Journal Editors ( ICMJE ) and the Enhancing the QUAlity and Transparency Of health Research ( EQUATOR ) Network for registration and reporting clinical studies.
Registration
All interventional trials must be registered before enrollment of the first participant. Trial registration records must be available in a primary register of the WHO International Clinical Trials Registry Platform ( ICTRP ), in ClinicalTrials.gov , or in any publicly accessible database that meets the minimum 24-item trial registration dataset .
The trial number must be clearly indicated in the abstract and methods section of the manuscript. Trials with retrospective registration or with registration in a database that is not publicly accessible cannot be considered.
Study protocol
To enable proper assessment of the work by our editorial team and referees, we request that a copy of the approved study protocol, including the statistical analysis plan, be included with the initial submission. Sensitive content can be redacted. If the original protocol is in a language other than English, a translation should be provided. Any deviations from the plan indicated in the trial registry or study protocol must be justified.
Interim analyses
Interim analyses should be pre-specified in the study protocol. Preliminary analyses of ongoing clinical trials that have not been pre-specified must be justified and are considered on a case-by-case basis. Unplanned post-hoc or exploratory analyses should be clearly indicated as such in the abstract and manuscript text.
Reporting guidelines
- Case reports or case series – if performed in the setting of a clinical trial evaluating effectiveness in a single patient, a CONSORT extension for N-of-1 trials must be provided; if treatment is provided outside a clinical trial (e.g., as compassionate use, or experimental observational study), authors must provide a statement that the research protocol was approved by IRB and ethics committees and participants gave written informed consent, according to CARE guidelines and in compliance with the Declaration of Helsinki principles.
- Randomized trials must conform to CONSORT 2010 guidelines , and the CONSORT checklist should be submitted with the manuscript and other materials, including the protocol. Non-randomized trials are encouraged to use the CONSORT principles and framework in the reporting of the results. Reports that do not conform to the CONSORT guidelines may need to be revised before formal review.
- Observational studies (cohort, case–control or cross-sectional designs) must be reported according to the STROBE statement .
- Systematic reviews and meta-analyses must follow the PRISMA guidelines.
- Studies reporting biomarkers in association with clinical outcomes should follow the STARD guidelines. For biomarker studies in oncology, we request that authors adhere to REMARK guidelines.
- Epidemiology studies are encouraged to follow the recommendation in the GATHER statement.
Data sharing
In accordance with Nature Research policy , a Data Availability Statement (DAS) must be included with all original research manuscripts. Nature Medicine is committed to transparency in data availability, and we request that authors provide a clear statement summarizing what data is available, when and to whom, and how to access it, and clearly stating any restrictions to data access.
Following ICMJE recommendations , clinical trials that began enrolling participants 1 January 2019 and later must include a data sharing plan in the trial's registration. If the data sharing plan changes after registration, this should be reflected in the statement submitted and published with the manuscript, and updated in the registry record.
Competing interests
In the interests of transparency and to help readers form their own judgments of potential bias, Nature Research journals require authors to declare any competing financial and/or non-financial interests in relation to the work described. The corresponding author is responsible for submitting a competing interests statement on behalf of all authors of the paper.
The role of the sponsor in any stage of the study, including manuscript preparation, must be acknowledged.
Additional Resources
Nature Research Editorial Policies
Nature Research competing interests policy
Editorial: Raising the bar for clinical research
Editorial: Confronting conflict of interest
Editorial: Clarifying access to data
Editorial: Redefining Medicine
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, list of registries, frequently asked questions.
What is a registry?
A registry is a collection of information about individuals, usually focused around a specific diagnosis or condition. Many registries collect information about people who have a specific disease or condition, while others seek participants of varying health status who may be willing to participate in research about a particular disease. Individuals provide information about themselves to these registries on a voluntary basis. Registries can be sponsored by a government agency, nonprofit organization, health care facility, or private company. It’s always good to check first to know who sponsors the registry – or – look for information on a registry’s site to know about their sponsor(s).
Why are registries needed?
Registries can provide health care professionals and researchers with first-hand information about people with certain conditions, both individually and as a group, and over time, to increase our understanding of that condition. Some registries collect information that can be used to track trends about the number of people with diseases, treatments, and more. Other registries invite people to sign up to be contacted about participating in clinical research. These ask very basic questions about health history that would help determine whether someone is possibly eligible to join a research study.
It sounds like these registries collect personal health information. Is there a risk that such information could be disclosed?
Government agencies have strict privacy requirements set by law such as the Federal Information Security Management Act (FISMA), and the Health Insurance Portability and Accountability Act (HIPAA). If registries have followed all of these rules, the likelihood of identifiable personal information being shared is very small.
What benefits will someone receive from participating in a registry?
Participation in a registry is likely to increase what we know about a specific condition, help health care professionals improve treatment, and allow researchers to design better studies on a particular condition, including development and testing of new treatments. Being part of a clinical trials registry can help people interested in participating in research connect with clinical investigators. However, individuals (and their families) who choose to participate in a registry should understand that participation will not guarantee a treatment or cure for their condition or that they will be eligible to join a study.
Who has access to the information in a registry?
Usually, a federally-funded registry has a very limited list of individuals (registry coordinator) who may have access to participants’ personal, identifying information. ;Those individuals must be specially trained and certified regarding information security requirements.
Who owns the data from a registry? Who makes decisions about how these data will be used?
The data collected in a disease registry is stripped of personal information. It belongs to the sponsor of the registry, and depending on how the registry is set up, may be shared with the participants and their families, and approved health care professionals and researchers. However, personal, identifying information is kept private. Usually, a registry has a governing committee that makes decisions about how the data can be used or shared.
Can a participant withdraw from the registry?
Yes. Registries are free and voluntary; there is no penalty for choosing to withdraw at any point.
Who should the participant contact with additional questions or concerns?
For any questions about participation or any issues that may arise, registries provide a contact, usually the registry coordinator.
How is a registry different from a clinical trial?
Registries focused on specific diseases or conditions collect information voluntarily from people with those conditions. Clinical trials registries collect basic health information from people who agree to be contacted about participating in future clinical trials or studies.
A clinical trial is the study of new ways to prevent, detect or treat diseases or conditions. Volunteering for a registry does not mean a person has signed up for a clinical trial. Participation in a disease registry can sometimes become a first step toward participation in a clinical trial, but registries and specific trials are not directly linked.
Disclaimer: The following listing is not intended to be comprehensive, and the inclusion of any particular organization on this list does not imply endorsement by the National Institutes of Health or the Department of Health and Human Services. Our intent is to provide information about registry efforts at the national level and therefore have not included many local groups that can offer valuable assistance to individuals and their families within a limited geographic area.
Alzheimer’s Prevention Registry
Autoimmune registry, autoimmune research network (arnet), breast cancer surveillance consortium, cancer genetics network, cascade fh registry, cchs now registry, cerebral palsy research network mycp, chromosome 8p registry, clinical trials public data share website, collaborative islet transplant registry, colon cancer family registry, congenital heart disease genetic network study (chd genes), congenital muscle disease international registry (cmdir), creatineinfo registry, cure rtd foundation, curedrpla global patient registry, cystic fibrosis foundation patient registry, development of a national incompatible kidney transplant registry, dominantly inherited alzheimer network (dian) — expanded registry, drug inducted liver injury network (dilin), ds-connect™: the down syndrome registry, dtrf desmoid tumor patient registry, the environmental polymorphisms registry (epr) — using dna to study disease, epithelioid hemangioendothelioma (ehe) global patient registry, eyegene ® : the national ophthalmic disease genotyping and phenotyping network, fanconi anemia patient registry, fd/mas patient registry, fecal microbiota transplant national registry, fibromuscular dysplasia (fmd) registry, foundation fighting blindness, foundation for sarcoidosis patient registry, frontotemporal degeneration (ftd) registry, genomeconnect, global genes rare-x patient communities, the global paroxysmal nocturnal hemoglobinuria (pnh) patient registry (iamrare.org), global prader-willi syndrome registry, global registry for inherited neuropathies (grin) registry, impact registry, diagnostic and interventional cardiac catheterization in congenital heart disease, inherited bone marrow failure syndrome, interagency registry for mechanically assisted circulatory support (intermacs), international registry of coronavirus exposure in pregnancy (ircep), international registry of werner syndrome, itp natural history study registry, kcnt1 epilepsy, krabbe community united research and engagement study (krabbecures), leigh syndrome global patient registry, lipedema foundation, lupus family registry and repository, monogenic diabetes at the university of chicago, mother to baby, multiple myeloma research foundation’s (mmrf) curecloud, myasthenia gravis patient registry, national addiction & hiv data archive program, national alopecia areata registry, national als registry, national and state cancer registries, national pediatric cardiology quality improvement collaborative, national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (gentac), nida center for genetics research, nidcd national temporal bone, hearing & balance pathology resource registry, nih human embryonic stem cell registry, nih national registry of u.s. myotonic dystrophy and u.s. facioscapulohumeral muscular dystrophy (fshd), oaa natural history patient registry, pediatric cardiac critical care consortium (pc4), pediatric imaging, neurocognition, and genetics (ping), pediatric pulmonary hypertension (pphnet) informatics registry, pku patient registry, pprom registry (preterm premature rupture of membranes), pregsource ® : crowdsourcing to understand pregnancy, the preeclampsia registry, priority (pregnancy coronavirus outcomes registry), pulmonary fibrosis, rare diseases registry program (radar), research registry for neonatal lupus, ray: research accelerated by you, sample collection registry, section on neonatal-perinatal medicine (sonpm), seer registries, severe chronic neutropenia international registry, shareforcures (breast cancer research registry by susan g. komen), simons searchlight (rare genetic neurodevelopmental disorders registry), society for thoracic surgeons society, congenital heart surgery database, syngap1 (mrd5) patient registry, tatton brown rahman syndrome (tbrs), usher syndrome registry, usidnet registry for patients with primary immunodeficiency diseases, virtual pediatric systems (vps).
This page last reviewed on April 17, 2024
Connect with Us
- More Social Media from NIH
Clinical Trial Registries, Results Databases, and Research Data Repositories
- First Online: 15 June 2023
Cite this chapter

- Karmela Krleža-Jerić ORCID: orcid.org/0000-0001-9377-0917 4 , 5 , 6 , 7 ,
- Mersiha Mahmić-Kaknjo ORCID: orcid.org/0000-0003-2738-8909 8 , 9 &
- Khaled El Emam ORCID: orcid.org/0000-0003-3325-4149 10 , 11
Part of the book series: Health Informatics ((HI))
589 Accesses
2 Altmetric
Trial registration, results disclosure, and sharing of analyzable individual participant data (IPD) are considered powerful tools for achieving higher levels of transparency and accountability for clinical trials. The emphasis on disseminating knowledge and growing demands for transparency in clinical research are contributing to a major paradigm shift in health research. In this new paradigm, knowledge will be generated from the culmination of all existing knowledge—not just from bits and parts of previous knowledge, as has been largely the case until now. Fully transparent clinical research diminishes publication bias, increases accountability, avoids unnecessary duplication of research (and thus avoid research waste), efficiently advances research, provides more reliable evidence for diagnostic and therapeutic interventions, regains public trust, and contributes to research integrity. Transparency of clinical trials, at a minimum, means sharing information about the trial design, conduct, and results, as well as the analyzable data. Not only must the information itself be explicitly documented, but an access location or medium for distribution also must be provided. Thus, transparency is realized by making research protocols, results, and cleaned and anonymized IPDs publicly available using well-defined, freely accessible electronic tools. Many electronic tools enabling sharing clinical trial information have emerged. These tools include registries hosting protocol data, results databases hosting aggregate data, and research data repositories hosting reusable and analyzable data sets and other research-related information. These tools are at different levels of development and are plagued with heterogeneity as international standards for trial registration do not yet address the sharing of individual patient data. Additionally, the need to measure and improve clinical trial transparency has led to development of specific electronic tools. This chapter is relevant for any professional involved in clinical trials and the use of the knowledge generated from them, including clinical and biomedical researchers, clinical trialists, systematic reviewers, information technology and informatics specialists, patients, journal editors, and public and private research funders and sponsors. Suggested competencies and learning activities for specific roles are presented at the end of the chapter.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
WHO. International Clinical Trials Registry Platform (ICTRP) [Internet]. [cited 2022 Mar 28]. https://www.who.int/clinical-trials-registry-platform .
WCRIF. Mission—World Conferences on Research Integrity [Internet]. [cited 2022 May 10]. https://wcrif.org/foundation/mission .
Vickers AJ. Sharing raw data from clinical trials: what progress since we first asked “Whose data set is it anyway?” Trials [Internet]. 2016;17:227. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4855346/ .
Krleža-Jerić K. Clinical trial registration: the differing views of industry, the WHO, and the Ottawa Group. PLoS Med. 2005;2:1093–7.
Article Google Scholar
Clinical Study Data Request [Internet]. [cited 2016 Aug 31]. https://www.clinicalstudydatarequest.com/ .
Project Data Sphere [Internet]. [cited 2016 Jul 31]. https://www.projectdatasphere.org/ .
YODA Project [Internet]. [cited 2016 Jul 18]. http://yoda.yale.edu/ .
Vivli—A global Clinical trial data sharing platform: proposal, definition and scope background and objectives. 2016;(June). https://vivli.org .
European Medicines Agency—Clinical data publication—Documents from advisory groups on clinical-trial data [Internet]. [cited 2018 Aug 20]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/document_listing/document_listing_000368.jsp&mid=WC0b01ac05809f3f12#section1 .
Krleža-Jerić K. Sharing of data from clinical trials and research integrity. In: Steneck N, Anderson M, Kleinert S, Mayer T, editor. Integrity in the Global Research Arena; Proceedings of the World Conference on Research Integrity; 3rd, Montreal 2013. World Scientific Publisher, Singapore. p 91.
Google Scholar
Krleža-Jerić K. Sharing of clinical trial data and research integrity. Period Biol. 2014;116(4):337–9.
Krleža-Jerić K. Clinical trials registries and results databases. In: Richesson RL, Andrews JE, editors. Clinical research informatics. London: Springer; 2012. p. 389–408.
Chapter Google Scholar
Chan AW, Hróbjartsson A. Promoting public access to clinical trial protocols: challenges and recommendations. Trials. 2018;19(1):116.
Article PubMed PubMed Central Google Scholar
Clinical Trial Transparency | Transparimed [Internet]. 2019 [cited 2022 Mar 30]. https://www.transparimed.org/ .
Krleza-Jeric K. Clinical trial registries, results databases, and research data repositories. In: Richesson RL, Andrews JE, editors. Clinical research informatics. 2nd ed. Springer International; 2019. p. 453–480.
Krleza-Jerić K, Chan AW, Dickersin K, Sim I, Grimshaw J, Gluud C. Principles for international registration of protocol information and results from human trials of health related interventions: Ottawa statement (part 1). BMJ [Internet]. 2005 [cited 2016 Jul 13];330(7497):956–8. http://www.ncbi.nlm.nih.gov/pubmed/15845980 .
Krleza-Jeric K. IMPACT Observatory [Internet]. MedILS. [cited 2022 Jun 4]. https://www.medils.org/research/impact-observatory .
RDA. About RDA | RDA [Internet]. 2016 [cited 2022 Jun 3]. https://www.rd-alliance.org/about-rda .
Good Pharma Scorecard | Bioethics International [Internet]. 2009 [cited 2022 Mar 30]. https://bioethicsinternational.org/good-pharma-scorecard/ .
Miller J, Ross JS, Wilenzick M, Mello MM. Sharing of clinical trial data and results reporting practices among large pharmaceutical companies: cross sectional descriptive study and pilot of a tool to improve company practices. BMJ. 2019;366:l4217.
Goldacre B, Devito NJ, Heneghan C, Irving F, Bacon S, Fleminger J, et al. Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource. BMJ [Internet]. 2018 [cited 2021 Nov 26];362:3218. https://doi.org/10.1136/bmj.k3218 .
WHO Global Observatory Clin trials. Number of clinical trials by year, country, region and income group [Internet]. 2022 [cited 2022 May 10]. https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/number-of-clinical-trials-by-year-country-who-region-and-income-group .
WHO News. Joint statement on public disclosure of results from clinical trials [Internet]. 2017 [cited 2022 May 27]. https://www.who.int/news/item/18-05-2017-joint-statement-on-registration .
Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol [Internet]. 1986 [cited 2016 Jul 13];4(10):1529–41. http://www.ncbi.nlm.nih.gov/pubmed/3760920 .
Bass A. Side effects; A prosecutor, whistelblower, and a bestselling antidepressant on trial. 1st ed. Algonquin Books of Chapel Hill; 2008. 260 p.
Gibson L. GlaxoSmithKline to publish clinical trials after US lawsuit. BMJ. 2004;328(7455):1513.
DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the international committee of medical journal editors. JAMA [Internet]. 2004 [cited 2016 Jul 12];292(11):1363–4. http://www.ncbi.nlm.nih.gov/pubmed/15355936 .
World Health Organisation. Ministerial Summit on Health Research Report by the Secretariat. 2005.
International Clinical Trials Registry Platform (ICRTP) [Internet]. [cited 2016 Jul 11]. https://www.who.int/clinical-trials-registry-platform .
Mahmić-Kaknjo M, Šimić J, Krleža-Jerić K. Setting the impact (Improve access to clinical trial data) observatory baseline. Biochem Med [Internet]. 2018;28(1):010201. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5806612/ .
ISRCTN. ISRCTN Registry [Internet]. [cited 2022 Jun 2]. https://www.isrctn.com/ .
NIH-NLM. ClinicalTrials.gov [Internet]. [cited 2022 May 27]. https://clinicaltrials.gov/ .
Cho SM, Serghiou S, Ioannidis JPA, Klassen TP, Contopoulos-Ioannidis DG. Large pediatric randomized clinical trials in ClinicalTrials.gov download large pediatric randomized clinical trials in ClinicalTrials.gov. Pediatrics. 2021;148(3):e2020049771.
Article PubMed Google Scholar
Chan AW, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet [Internet]. 2013 [cited 2022 Mar 26];381(9861):91–2. http://www.thelancet.com/article/S0140673612621606/fulltext .
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J a, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ [Internet]. 2013;346:e7586. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3541470&tool=pmcentrez&rendertype=abstract .
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Emam K el, Jonker E, Sampson M, Krleža-Jerić K, Neisa A. The use of electronic data capture tools in clinical trials: web-survey of 259 Canadian trials. J Med Internet Res [Internet]. 2009 [cited 2022 Mar 26];11(1). /pmc/articles/PMC2762772/.
Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols the SPIRIT-PRO extension. JAMA [Internet]. 2018;319(5):483–94. https://jamanetwork.com/ .
Dai L, Cheng CW, Tian R, Zhong LL, Li YP, Lyu AP, et al. Standard Protocol Items for Clinical Trials with Traditional Chinese Medicine 2018: recommendations, explanation and elaboration (SPIRIT-TCM Extension 2018). Chin J Integr Med [Internet]. 2019 [cited 2022 Mar 27];25(1):71–9. https://pubmed.ncbi.nlm.nih.gov/30484022/ .
Cruz Rivera S, Liu X, Chan AW, Denniston AK, Calvert MJ, Ashrafian H, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Lancet Digital Health. 2020;2(10):e549–60.
Porcino AJ, Shamseer L, Chan AW, Kravitz RL, Orkin A, Punja S, et al. SPIRIT extension and elaboration for n-of-1 trials: SPENT 2019 checklist. BMJ [Internet]. 2020 [cited 2022 Mar 27]; https://doi.org/10.1136/bmj.m122 . http://www.bmj.com/ .
EQUATOR. The EQUATOR Network | Enhancing the QUAlity and Transparency Of Health Research [Internet]. [cited 2022 May 26]. https://www.equator-network.org/ .
https://www.icmje.org/about-icmje/faqs/clinical-trials-registration/
WHO trial registration data set version 1.3.1. [Internet]. [cited 2022 Mar 24]. https://www.who.int/clinical-trials-registry-platform/network/who-data-set .
Bhatia P, Mohammed S. Observational study design: extending the standard. J Anaesthesiol Clin Pharmacol [Internet]. 2021 [cited 2022 Jan 7];37(3):317–8. https://pubmed.ncbi.nlm.nih.gov/34759537/ .
Bradley SH, Lloyd KE, Devito NJ. Automatic registration for UK trials. A welcome development, not a panacea. BMJ [Internet]. 2022 [cited 2022 Jan 29]; https://doi.org/10.1136/bmj.o41 .
UMIN-ICDR Individual Case data repository [Internet]. [cited 2018 Aug 11]. http://www.umin.ac.jp/icdr/index.html .
Banzi R, Canham S, Kuchinke W, Krleza-Jeric K, Demotes-Mainard J, Ohmann C. Evaluation of repositories for sharing individual-participant data from clinical studies. Trials [Internet]. 2019 [cited 2022 Feb 12];20(1). https://pubmed.ncbi.nlm.nih.gov/30876434/ .
Reveiz L, Chan AW, Krleza-Jerić K, Granados CE, Pinart M, Etxeandia I, et al. Reporting of methodologic information on trial registries for quality assessment: a study of trial records retrieved from the WHO search portal. PLoS One [Internet]. 2010;5(8):e12484. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0012484 .
Reveiz L, Krleza-Jerić K, Chan AW, de Aguiar S. Do trialists endorse clinical trial registration? Survey of a PubMed sample. Trials [Internet]. 2007;8:30. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2147029&tool=pmcentrez&rendertype=abstract .
Harriman SL, Patel J. When are clinical trials registered? An analysis of prospective versus retrospective registration. Trials [Internet]. 2016;17:187. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4832501/ .
Gray R, Brown E, Gray G. A review of prospective trial registration in the Journal of Advanced Nursing in 2018. J Adv Nurs [Internet]. 2019 Oct 1 [cited 2021 Nov 15];75(10):2051–3. https://onlinelibrary.wiley.com/doi/full/10.1111/jan.14090 .
Venugopal N, Saberwal G. A comparative analysis of important public clinical trial registries, and a proposal for an interim ideal one. Plos One [Internet]. 2021. https://doi.org/10.1371/journal.pone.0251191 .
Taylor NJ, Gorman DM. Registration and primary outcome reporting in behavioral health trials. BMC Med Res Methodol. 2022;22(1):41.
WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects—WMA—The World Medical Association [Internet]. 2013 [cited 2018 Aug 19]. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
Krleza-Jerić K, Lemmens T. 7th revision of the Declaration of Helsinki: good news for the transparency of clinical trials. Croat Med J [Internet]. 2009 [cited 2016 Jul 13];50(2):105–10. http://www.ncbi.nlm.nih.gov/pubmed/19399942 .
Goodyear MDE, Krleza-Jeric K, Lemmens T. The Declaration of Helsinki. BMJ [Internet]. 2007 [cited 2016 Jul 13];335(7621):624–5. http://www.ncbi.nlm.nih.gov/pubmed/17901471 .
Zarin DA, Tse T, Williams RJ, Rajakannan T. The status of trial registration eleven years after the ICMJE policy. N Engl J Med [Internet]. 2017;376(4):383–91. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5813248/ .
Rising K, Bacchetti P, Bero L. Reporting bias in drug trials submitted to the Food and Drug Administration: review of publication and presentation. Ioannidis J, editor. PLoS Med [Internet]. 2008;5(11):e217. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2586350/ .
CDISC [Internet]. [cited 2018 Aug 19]. https://www.cdisc.org/ .
Morley RL, Edmondson MJ, Rowlands C, Blazeby JM, Hinchliffe RJ. Registration and publication of emergency and elective randomised controlled trials in surgery: a cohort study from trial registries. BMJ Open [Internet]. 2018 Jul 1 [cited 2022 Mar 29];8(7). https://pubmed.ncbi.nlm.nih.gov/29982216/ .
Greiner B, Corcoran A, Wheeler D. Clinical trial registry searches are under-utilized in systematic reviews from critical care journals: a bibliometric analysis. J Crit Care. 2021;63:175–8.
Riedl D, Rothmund M, Darlington AS, Sodergren S, Crazzolara R, de Rojas T. Rare use of patient-reported outcomes—in childhood cancer clinical trials—a systematic review of clinical trial registries. Eur J Cancer [Internet]. 2021 [cited 2021 Nov 14];152:90–9. http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Yerokhin VV, Carr BK, Sneed G, Vassar M. Clinical trials registries are underused in the pregnancy and childbirth literature: a systematic review of the top 20 journals. BMC Res Notes. 2016;9(1):475.
Dear RF, Barratt AL, McGeechan K, Askie L, Simes J, Tattersall MHN. Landscape of cancer clinical trials in Australia: using trial registries to guide future research. Med J Aust [Internet]. 2011 [cited 2022 Mar 29];194(8):387–91. https://pubmed.ncbi.nlm.nih.gov/21495937/ .
Ecker BL, Brajcich BC, Ellis RJ, Ko CY, D’Angelica MI. Registry-based randomized clinical trials in surgery: working with ACS-NSQIP and the AHPBA to conduct pragmatic trials. J Surg Oncol [Internet]. 2022 [cited 2022 Jan 4];125(1):89–92. https://onlinelibrary.wiley.com/doi/10.1002/jso.26742 .
Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021. https://doi.org/10.1136/bmj.n71 .
Reddy AK, Anderson JM, Gray HM, Fishbeck K, Vassar M. Clinical trial registry use in orthopaedic surgery systematic reviews. J Bone Joint Surg Am [Internet]. 2021 [cited 2022 Mar 28];103(10):e41. https://pubmed.ncbi.nlm.nih.gov/33983151/ .
Food and Drug Administration Amendments Act (FDAAA) of 2007 [Internet]. Office of the Commissioner; [cited 2018 Aug 21]. https://www.fda.gov/regulatoryinformation/lawsenforcedbyfda/significantamendmentstothefdcact/foodanddrugadministrationamendmentsactof2007/default.htm .
Prospero-International prospective register of systematic reviews [Internet]. [cited 2018 Aug 20]. https://www.crd.york.ac.uk/prospero/ .
Krleža-Jeric K, Lemmens T, Reveiz L, Cuervo LG, Bero LA. Prospective registration and results disclosure of clinical trials in the Americas: a roadmap toward transparency. Rev Panam Salud Publica [Internet]. 2011 Jul [cited 2016 Jun 15];30(1):87–96. http://www.ncbi.nlm.nih.gov/pubmed/22159656 .
Australia Clinical Trials Toolkit | Australian Clinical Trials [Internet]. [cited 2018 Aug 21]. https://www.australianclinicaltrials.gov.au/clinical-trials-toolkit#overlay-context=home .
Pienaar E. Cochrane SA webinar: clinical trial registration and the Pan African Clinical Trials Registry—YouTube [Internet]. 2018 [cited 2022 May 7]. https://www.youtube.com/watch?v=BIMHoXXqyTA .
Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans [Internet]. TCPS 2. 2014. www.nserc-crsng.gc.ca .
Australia New Zealand Clinical Trials Registry. Data item definition/explanation [Internet]. [cited 2018 Aug 13]. http://www.anzctr.org.au/docs/ANZCTRDatafieldexplanationV5.pdf .
Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005. N Engl J Med [Internet]. 2005;353(26):2779–87. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1568386/ .
Askie LM, Hunter KE, Berber S, Langford A, Tan-Koay AG, Vu T, Sausa R, Seidler AL, Ko H SRJ. The Clinical Trials Landscape in Australia 2006–2015 [Internet]. Sydney: Australian New Zealand Clinical Trials Registry; 2017 [cited 2018 Aug 18]. 83 p. http://www.anzctr.org.au/docs/ClinicalTrialsInAustralia2006-2015.pdf#page=1&zoom=auto,557,766 .
Zarin DA, Califf RM. Trial reporting and the clinical trials enterprise. JAMA Intern Med [Internet]. 2021 [cited 2022 Mar 14];181(8):1131–2. https://pubmed.ncbi.nlm.nih.gov/34028521/ .
Speich B, Gloy VL, Klatte K, Gryaznov D, Taji Heravi A, Ghosh N, et al. Reliability of trial information across registries for trials with multiple registrations: a systematic review + supplemental content. JAMA Network Open [Internet]. 2021 [cited 2022 Jan 29];4(11):2128898. https://jamanetwork.com/ .
DeVito NJ, Goldacre B. Trends and variation in data quality and availability on the European Union Clinical Trials register: a cross-sectional study. Clin Trials [Internet]. 2022 [cited 2022 Mar 19]. https://pubmed.ncbi.nlm.nih.gov/35144496/ .
Paludan-Müller AS, Maclean-Nyegaard IR, Munkholm K. Substantial delays in clinical data published by the European Medicines Agency—a cross sectional study. J Clin Epidemiol [Internet]. 2022;146:68–76. https://doi.org/10.1016/j.jclinepi.2022.02.004 .
Ghersi D, Clarke M, Berlin J, Gülmezoglu AM, Kush R, Lumbiganon P, et al. Reporting the findings of clinical trials: a discussion paper. Bull World Health Organ [Internet]. 2008;86(6). http://www.icmje .
Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database—update and key issues. N Engl J Med [Internet]. 2011;364(9):852–860. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3066456/ .
Speich B, Gryaznov D, Busse JW, Gloy VL, Lohner S, Klatte K, et al. Nonregistration, discontinuation, and nonpublication of randomized trials: A repeated metaresearch analysis. Kesselheim AS, editor. PLoS Med [Internet]. 2022 [cited 2022 May 20];19(4):e1003980. https://dx.plos.org/10.1371/journal.pmed.1003980 .
Riedel N, Wieschowski S, Bruckner T, Holst MR, Kahrass H, Nury E, et al. Results dissemination from completed clinical trials conducted at German university medical centers remained delayed and incomplete. The 2014–2017 cohort. J Clin Epidemiol [Internet]. 2022 [cited 2022 May 5];144:1–7. https://pubmed.ncbi.nlm.nih.gov/34906673/ .
Moorthy VS, Karam G, Vannice KS, Kieny MP. Rationale for WHO’s new position calling for prompt reporting and public disclosure of interventional clinical trial results. PLoS Med. 2015;12(4):e1001819.
Krleza-Jeric K. International dialogue on the public reporting of clinical trial outcome and results—PROCTOR meeting. Croat Med J. 2008;49:267–8.
Krleža-Jerić K, Gabelica M, Banzi R, Krnić-Martinić M, Pulido B, Mahmić-Kaknjo M, et al. IMPACT Observatory: tracking the evolution of clinical trial data sharing and research integrity. Biochem Med (Zagreb) [Internet]. 2016 [cited 2022 Apr 3];26(3):308–17. https://pubmed.ncbi.nlm.nih.gov/27812300/ .
Mahmić-Kaknjo M, Šimić J, Krleža-Jerić K. Setting the IMPACT (Improve access to clinical trial data) observatory baseline. Biochem Med [Internet]. 2018 [cited 2022 Jun 4];28(1):010201. https://www.biochemia-medica.com/en/journal/28/10.11613/BM.2018.010201 .
Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gøtzsche PC, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet [Internet]. 2014 [cited 2016 Jul 14];383(9913):257–66. http://www.ncbi.nlm.nih.gov/pubmed/24411650 .
Ohmann C, Banzi R, Canham S, Battaglia S, Matei M, Ariyo C, et al. Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open [Internet]. 2017;7(12):e018647. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5736032/ .
World Health Organization. WHO statement on public disclosure of clinical trial results [Internet]. 2015 [cited 2016 Jul 11]. http://www.who.int/ictrp/results/reporting/en/ .
Woodcock J. FDA Takes action for failure to submit required clinical trial results information to ClinicalTrials.gov | FDA [Internet]. 2021 [cited 2022 Mar 30]. https://www.fda.gov/news-events/press-announcements/fda-takes-action-failure-submit-required-clinical-trial-results-information-clinicaltrialsgov .
European Medicines Agency. European Medicines Agency policy on publication of clinical data for medicinal products for human use [Internet]. 2019 [cited 2022 May 12]. https://www.ema.europa.eu/en/documents/other/european-medicines-agency-policy-publication-clinical-data-medicinal-products-human-use_en.pdf .
DeVito NJ, Goldacre B. New EU trial reporting regulations must be enforced. BMJ. 2022;376:o410.
Strzebonska K, Wasylewski MT, Zaborowska L, Riedel N, Wieschowski S, Strech D, et al. Results dissemination of registered clinical trials across Polish academic institutions: a cross-sectional analysis. BMJ Open. 2020;10:34666.
Mayo-Wilson E, Heyward J, Keyes A, Reynolds J, White S, Atri N, et al. Clinical trial registration and reporting: a survey of academic organizations in the United States. BMC Med. 2018;16(1):60.
European Medicine Agency. Clinical Trials Information System | European Medicines Agency [Internet]. 2022 [cited 2022 May 4]. https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trials-information-system .
EU Trials Tracker—Who’s not sharing clinical trial results? [Internet]. [cited 2022 Mar 30]. https://eu.trialstracker.net/ .
Dal-Ré R, Goldacre B, Mahillo-Fernández I, DeVito NJ. European non-commercial sponsors showed substantial variation in results reporting to the EU trial registry. J Clin Epidemiol. 2022;(142):161–70.
Gao L, Guo R, Han Z, Liu J, Chen X. Clinical trial reporting. Lancet [Internet]. 2020 [cited 2022 May 7];396(10261):1488–9. http://www.thelancet.com/article/S0140673620322650/fulltext .
Chan AW, Hróbjartsson A. Promoting public access to clinical trial protocols: challenges and recommendations. Trials [Internet]. 2018. https://doi.org/10.1186/s13063-018-2510-1 .
Bian ZX, Wu TX. Legislation for trial registration and data transparency. Trials [Internet]. 2010;11(1):64. https://doi.org/10.1186/1745-6215-11-64 .
Collins F. Has the revolution arrived? Nature [Internet]. 2010;464(7289):674–5. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5101928/ .
Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature [Internet]. 2003;422:835. https://doi.org/10.1038/nature01626
Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, et al. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Ethiop J Health Sci. 2017;27(4):315–8.
Gøtzsche PC. Why we need easy access to all data from all clinical trials and how to accomplish it. Trials [Internet]. 2011;12:249. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3264537/ .
Rockhold F, Bromley C, Wagner EK, Buyse M. Open science: the open clinical trials data journey. Clin Trials [Internet]. 2019 [cited 2022 Mar 19];16(5):539–46. https://journals.sagepub.com/doi/10.1177/1740774519865512 .
Rockhold F, Nisen P, Freeman A. Data sharing at a crossroads. NEJM. 2016;375:1115–7.
Re3Data; Registry of Research Data Repositories [Internet]. [cited 2018 Aug 11]. www.Re3data.org .
Edinburgh DataShare [Internet]. [cited 2018 Aug 18]. https://datashare.is.ed.ac.uk/ .
DRUM. Data Repository for U of M (DRUM) [Internet]. [cited 2022 Jun 12]. https://conservancy.umn.edu/drum .
Krleza-Jeric K, Hrynaszkiewicz I. Environmental scan of repositories of clinical research data: how far have we got with public disclosure of trial data? [Internet]. figshare; 2018. https://figshare.com/articles/Environmental_Scan_of_Repositories_of_Clinical_Research_Data_How_Far_Have_We_Got_With_Public_Disclosure_of_Trial_Data_/5755386 .
Gabelica M, Martinic MK, Luksic D, Krleza-Jeric K. Clinical trial transparency and data repositories; an environmental scan of the IMPACT (Improving access to clinical trial data) Observatory [Internet]. figshare; 2018. https://figshare.com/articles/Clinical_trial_transparency_and_data_repositories_an_environmental_scan_of_the_IMPACT_Improving_Access_to_Clinical_Trial_Data_Observatory/7390559 .
Krleža-Jerić K, Gabelica M, Banzi R, Martinić MK, Pulido B, Mahmić-Kaknjo M, et al. IMPACT observatory: tracking the evolution of clinical trial data sharing and research integrity. Biochem Med (Zagreb) [Internet]. 2016;26(3):308–17. http://www.ncbi.nlm.nih.gov/pubmed/27812300%0A , http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5082220 .
NIH. NIH Office of Data Science Strategy Announces New Initiative to Improve Access to NIH-funded Data | Data Science at NIH [Internet]. 2022 [cited 2022 May 28]. https://datascience.nih.gov/news/nih-office-of-data-science-strategy-announces-new-initiative-to-improve-data-access .
The Dataverse Project [Internet]. [cited 2018 Aug 18]. https://dataverse.org/ .
Harvard Dataverse [Internet]. [cited 2018 Aug 19]. https://dataverse.harvard.edu/ .
Research Data Alliance RDA [Internet]. [cited 2018 Aug 16]. https://www.rd-alliance.org/about-rda/who-rda.html .
CoreTrustSeal [Internet]. [cited 2018 Jun 27]. https://www.coretrustseal.org/about/ .
Pampel H, Vierkant P, Scholze F, Bertelmann R, Kindling M, Klump J, et al. Making research data repositories visible: the re3data.org registry. PLoS One. 2013;8(11):e78080.
Article CAS PubMed PubMed Central Google Scholar
Persistent Identifier [Internet]. [cited 2018 Aug 18]. https://en.wikipedia.org/wiki/Persistent_identifier .
DataCite [Internet]. [cited 2018 Aug 11]. https://www.datacite.org/index.html .
Scaiano M, Middleton G, Arbuckle L, Kolhatkar V, Peyton L, Dowling M, et al. A unified framework for evaluating the risk of re-identification of text de-identification tools. J Biomed Inform [Internet]. 2016 [cited 2022 May 10];63:174–83. https://doi.org/10.1016/j.jbi.2016.07.015 .
Willenborg L, de Waal T. Elements of statistical disclosure control. New York: Springer New York; 2001. (Lecture Notes in Statistics; vol. 155).
European Medicines Agency. European Medicines Agency policy on publication of clinical data for medicinal products for human use: policy 0070 [Internet]. 2014 [cited 2022 May 10]. www.ema.europa.eu/contact .
Health Canada. Guidance document on public release of clinical information: profile page—Canada.ca [Internet]. 2019 [cited 2022 May 10]. https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/profile-public-release-clinical-information-guidance.html .
El Emam K, Lucy Mosquera RH. Practical synthetic data generation balancing privacy and the broad availability of data. Sebastopol: O’Reilly Media, 2020 [Internet]. 2020 [cited 2022 May 10];166. https://www.oreilly.com/library/view/practical-synthetic-data/9781492072737/ .
El Emam K, Jonker E, Arbuckle L, Malin B. A systematic review of re-identification attacks on health data. PLoS One [Internet]. 2011. www.plosone.org .
MDR. ECRIN-MDR Wiki [Internet]. 2020 [cited 2022 Jun 14]. http://ecrin-mdr.online/index.php/Project_Overview .
Download references
Acknowledgments
The authors would like to thank Nevena Jeric, Apropo Media for graphic design.
The views expressed here are the authors’ and do not represent the views of any organization.
Author information
Authors and affiliations.
IMPACT Observatory, Mediterranean Institute for Life Sciences—MedILS, Split, Croatia
Karmela Krleža-Jerić
Croatian Association for Sciences and Arts-HDZU, Sarajevo, Bosnia and Herzegovina Croatian, Cochrane, London, UK
Vivli Cambridge Croatian, Cochrane, Split, Croatia
ISRCTN Registry Advisory Group, London, UK
Zenica Cantonal Hospital, Zenica, Bosnia and Herzegovina
Mersiha Mahmić-Kaknjo
Sarajevo Medical School, Sarajevo School of Science and Technology, Sarajevo, Bosnia and Herzegovina
School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON, Canada
Khaled El Emam
Children’s Hospital of Eastern Ontario Research Institute, Ottawa, ON, Canada
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Learning Health Sciences, University of Michigan School of Medicin, Ann Arbor, MI, USA
Rachel L. Richesson
School of Information, University of South Florida, Tampa, FL, USA
James E. Andrews
Medical Informatics and Clinical Epidemiology, Oregon Health and Science University, Portland, OR, USA
Kate Fultz Hollis
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Krleža-Jerić, K., Mahmić-Kaknjo, M., El Emam, K. (2023). Clinical Trial Registries, Results Databases, and Research Data Repositories. In: Richesson, R.L., Andrews, J.E., Fultz Hollis, K. (eds) Clinical Research Informatics. Health Informatics. Springer, Cham. https://doi.org/10.1007/978-3-031-27173-1_17
Download citation
DOI : https://doi.org/10.1007/978-3-031-27173-1_17
Published : 15 June 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-27172-4
Online ISBN : 978-3-031-27173-1
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Clinical Trials
Testing the Addition of High Dose, Targeted Radiation to the Usual Treatment for Locally-Advanced Inoperable Non-small Cell Lung Cancer
- Print details
Tab Title Description
- Observational study — observes people and measures outcomes without affecting results.
- Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices.
- Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
Study phase
During the early phases (phases 1 and 2), researchers assess safety, side effects, optimal dosages and risks/benefits. In the later phase (phase 3), researchers study whether the treatment works better than the current standard therapy. They also compare the safety of the new treatment with that of current treatments. Phase 3 trials include large numbers of people to make sure that the result is valid. There are also less common very early (phase 0) and later (phase 4) phases. Phase 0 trials are small trials that help researchers decide if a new agent should be tested in a phase 1 trial. Phase 4 trials look at long-term safety and effectiveness, after a new treatment has been approved and is on the market.
- Rochester, Minnesota: 24-001802
- Jacksonville, Florida: 24-001802
About this study
The purpose of this study is to compare the effect of adding stereotactic body radiation therapy (SBRT) to standard treatment (image guided radiation therapy [IGRT] and chemotherapy followed by immunotherapy with durvalumab) versus standard treatment alone in treating patients with non-small cell lung cancer that cannot be treated by surgery (inoperable).
Participation eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate. There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Inclusion Criteria: - Pathologically (histologically or cytologically) proven diagnosis of stage II or III (American Joint Committee on Cancer [AJCC] eighth edition) non-small cell lung cancer (NSCLC) with known PD-L1 status prior to registration - Patients must have an identified primary tumor and at least one nodal metastasis (peribronchial/hilar/intrapulmonary, mediastinal/subcarinal, supraclavicular/scalene) - Up to 4 cycles of systemic therapy received prior to registration for the current study cancer is allowable; any prior chemotherapy for a different cancer is also permissible - The patient must be deemed clinically appropriate for curative intent definitive combined modality therapy, based on the following staging assessments: - History/physical examination prior to registration; - Magnetic resonance imaging (MRI) scan of the brain (preferred) or CT scan of the brain (if available, contrast is preferred for all neuroimaging) prior to registration; - CT chest with IV contrast (if contrast is available and unless contraindicated, such as for abnormal kidney function) prior to registration. PET/CT may be used if the CT portion is of identical diagnostic quality as achieved in a stand-alone CT - No evidence of distant metastases based on FDG PET/CT scan obtain within 60 days of registration - Primary tumor = - Age >= 18 - Eastern Cooperative Oncology Group (ECOG) performance status 0-2 - Hematologic function (e.g. platelets, leukocytes, hemoglobin) amenable, at the discretion of the treating physician, to allow for treatment with chemotherapy and concurrent radiation therapy - Creatinine clearance >= 25 mL/min by the Cockcroft-Gault (C-G) equation - Subjects with non-malignant pleural effusion are eligible provided the effusion is not known or demonstrated to be an exudative effusion - If a pleural effusion is present, the following criteria must be met to exclude malignant involvement: - When pleural fluid is visible on both the CT scan and on a chest x-ray, a pleuracentesis is required to confirm that the pleural fluid is cytologically negative; - Effusions that are minimal (i.e., not visible on chest x-ray) that are too small to safely tap are eligible - Medical history consistent with the patient being amenable, at the discretion of the treating physician, to allow for treating with consolidation immunotherapy. Patients with known EGFR/ALK mutation at the time of registration are eligible, and these patients can be treated with consolidation durvalumab or chemotherapy at the discretion of the treating physician - Patients with a prior or concurrent malignancy whose natural history or treatment does not have the potential to interfere with the safety or efficacy assessment of the investigational regimen - Negative pregnancy test = childbearing potential - The patient or a legally authorized representative must provide study-specific informed consent prior to study entry and, for patients treated in the United States (U.S.), authorization permitting release of personal health information Exclusion Criteria: - Prior radiotherapy to the region of the study cancer that would result in overlap of radiation therapy fields that is determined by the treating physician to impede the treatment of the study malignancy - Patients without identifiable primary tumor and at least 1 pathologically enlarged lymph node are not eligible (T3-4N0 or T0N1-3 patients are not eligible). At least 1 radiographically-involved lymph node is required, but pathologic confirmation of involvement is not mandated - Centrally located primary tumor in significant overlap of the primary SBRT and nodal radiation fields. Centrally located is defined as within or touching the zone of the proximal bronchial tree, which is a volume 2 cm in all directions around the proximal bronchial tree (carina, right and left main bronchi, right and left upper lobe bronchi, intermedius bronchus, right middle lobe bronchus, lingular bronchus right and left lower lobe bronchi) - Participants who are pregnant or unwilling to discontinue nursing - Participants of childbearing potential (participants who may become pregnant or who may impregnate a partner) unwilling to use highly effective contraceptives during therapy and for the Food and Drug Administration (FDA)-labeled contraception timeframe required after the final dose of the selected chemotherapy regimen, because the treatment in this study may be significantly teratogenic
Note: Other protocol defined Inclusion/Exclusion criteria may apply.
Eligibility last updated 6/20/23. Questions regarding updates should be directed to the study team contact.
Participating Mayo Clinic locations
Study statuses change often. Please contact the study team for the most up-to-date information regarding possible participation.
More information
- Publications
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.13(4); 2023
- PMC10124266

Original research
Reporting of retrospective registration in clinical trial publications: a cross-sectional study of german trials, martin haslberger.
QUEST Center for Responsible Research, Berlin Institute of Health at Charité, Berlin, Germany
Stefanie Gestrich
Daniel strech, associated data.
bmjopen-2022-069553supp001.pdf
bmjopen-2022-069553supp002.pdf
Data are available in a public, open access repository. All code and the data for this study are available at https://github.com/mhaslberger/retrospective-registration . Data are also available in an OSF repository ( https://osf.io/8g5cf/ ).
Prospective registration has been widely implemented and accepted as a best practice in clinical research, but retrospective registration is still commonly found. We assessed to what extent retrospective registration is reported transparently in journal publications and investigated factors associated with transparent reporting.
We used a dataset of trials registered in ClinicalTrials.gov or Deutsches Register Klinischer Studien, with a German University Medical Center as the lead centre, completed in 2009–2017, and with a corresponding peer-reviewed results publication. We extracted all registration statements from results publications of retrospectively registered trials and assessed whether they mention or justify the retrospective registration. We analysed associations of retrospective registration and reporting thereof with registration number reporting, International Committee of Medical Journal Editors (ICMJE) membership/-following and industry sponsorship using χ 2 or Fisher exact test.
In the dataset of 1927 trials with a corresponding results publication, 956 (53.7%) were retrospectively registered. Of those, 2.2% (21) explicitly report the retrospective registration in the abstract and 3.5% (33) in the full text. In 2.1% (20) of publications, authors provide an explanation for the retrospective registration in the full text. Registration numbers were significantly underreported in abstracts of retrospectively registered trials compared with prospectively registered trials. Publications in ICMJE member journals did not have statistically significantly higher rates of both prospective registration and disclosure of retrospective registration, and publications in journals claiming to follow ICMJE recommendations showed statistically significantly lower rates compared with non-ICMJE-following journals. Industry sponsorship of trials was significantly associated with higher rates of prospective registration, but not with transparent registration reporting.
Conclusions
Contrary to ICMJE guidance, retrospective registration is disclosed and explained only in a small number of retrospectively registered studies. Disclosure of the retrospective nature of the registration would require a brief statement in the manuscript and could be easily implemented by journals.
Strengths and limitations of this study
- We use a large, high-quality dataset of all trials conducted at German university medical centres over a period of 9 years (2009–2017) and registered in two registries, with results publications determined by an extensive manual screening process.
- This study only includes trials led by German university medical centres, which might limit its generalisability to other regions. Follow-up for trial publications ends uniformly in 2020, meaning that older trials had longer follow-up for publication than newer trials in the dataset.
Introduction
Prospective registration of clinical trials (ie, registration before enrolment of the first participant) is an important practice to reduce biases in their conduct and reporting. 1 A number of ethical and legal documents call for prospective registration: The Declaration of Helsinki 2 and the WHO registry standards 3 state that prospective registration and results reporting of clinical trials are an ethical responsibility. European law, for example, explicitly, mandates prospective registration of pharmaceutical trials. 4 In addition, many journals, via the International Committee of Medical Journal Editors (ICMJE), encourage or require prospective registration with an appropriate registry before the first participant is enrolled for all trials they publish, as well as the reporting of trial registration numbers (TRNs) in publications for better findability. 5 6 Similarly, reporting guidelines such as Consolidated Standards of Reporting Trials 7 and Good Publication Practice 3 8 recommend the reporting of TRNs.
Prospective registration has been widely implemented and advocated for many reasons: to detect and mitigate publication bias (ie, the non-reporting of studies, or aspects of studies, that did not yield a positive result) and selective reporting (ie, the selective reporting of only statistically significant primary outcomes). Prospective registration allows for public scrutiny of trials, identification of research gaps and to support the coordination of efforts by preventing unnecessary duplication. 9 When trials are registered retrospectively, that is, their registry entry is created after study start, this undermines many of the reasons for registration. While prospective registration has increased over the past decade, retrospective registration is still widespread. 10–14 Some registries, such as Deutsches Register Klinischer Studien (DRKS) or the WHO’s International Clinical Trials Registry Platform, explicitly mark retrospectively registered entries as such, whereas others, such as ClinicalTrials.gov, do not. While some journal editors allow retrospectively registered trials to be published, others do not. Journals following ICMJE guidance should in principle mandate prospective registration, but this principle is not always enforced. 12 15 16 According to ICMJE guidance, journals should publish retrospectively registered studies only in exceptional cases, noting that ‘authors should indicate in the publication when registration was completed and why it was delayed. Editors should publish a statement indicating why an exception was allowed’. 5 This was investigated by previous studies which found that such reporting rarely happens. 17 18
Our study aims to investigate the conduct of retrospective registration and its transparent reporting in a larger sample. In a previous study in a cohort of 1509 trials conducted at German university medical centers (UMC), registered in DRKS or ClinicalTrials.gov, and reported as complete between 2009 and 2013, 75% were registered retrospectively. 19 This rate dropped to 46% for the 1658 trials completed between 2014 and 2017. 20 Using the data from these two studies on trials registered in two large registries, led by German UMCs, completed between 2009 and 2017 and with at least one available peer-reviewed results publication, 19 20 we investigate whether and how authors report retrospective registration in the results publication. We also explore how retrospective registration is associated with other practices such as TRN reporting.
Data sources and sample
We based our sample on two related projects that were conducted at our research group. 19 20 The projects have drawn a full sample (n=3113) of registry entries for interventional studies reported as complete between 2009 and 2017, led by a German UMC and registered in one of two registries: DRKS, which is the WHO primary trial registry for Germany, and ClinicalTrials.gov, which is also routinely used in Germany to register clinical research and accepted by the ICMJE. Our dataset also includes the earliest results publications found for 68.4% (2129/3113) of the trials, which were manually identified in different stages until 1 September 2020. We retrieved the combined data from the two projects from a GitHub repository ( https://github.com/maia-sh/intovalue-data , accessed on 22 February 2022). The final dataset is publicly available. 21
Eligibility criteria
We included any trial that (1) was registered as an interventional study in either the ClinicalTrials.gov or the DRKS database, (2) was completed between 2009 and 2017, (3) reports a German UMC listed as the responsible party or lead sponsor, or with a principal investigator from a German UMC and (4) has published results in a peer-reviewed journal. Detailed descriptions of how these variables were derived are provided in the original publications of the dataset. 19 20 Retrospective registration was determined based on the registration and study start dates in the registry entries: dates were set to the first of the respective month and studies with a registration date more than 1 month after start date counted as retrospectively registered. For trials that were registered in both registries, we kept the entry that was created earlier.
Data extraction
For all retrospectively registered trials, we manually searched the abstract and the full text of the publications, including editorial statements, whether they reported:
- The fact that the study was registered (binary).
- A TRN (binary).
- The exact wording used to report the registration, including any provided registration numbers (free text).
- The date of the retrospective registration (binary).
- The fact that the study was retrospectively registered (binary).
- We also assessed whether (binary) and how authors justified or explained the retrospective registration (free text).
One rater (MH) used the keywords ‘regist’, ‘nct’, ‘drks’, ‘eudra’, ‘retro’, ‘delay’ and ‘after’ to search for registration numbers and wording pointing to retrospective registration in all publications. We considered a retrospective registration statement transparent if the authors explicitly mentioned that the registration was retrospective, for example, ‘this study was retrospectively registered in (registry), (TRN)’. Reporting of the registration date alone was not considered as transparent reporting of retrospective registration, except if the date of registration was mentioned in combination with the study start date in the same paragraph.
ICMJE journals
We created additional variables for whether journals are ICMJE members or follow the ICMJE recommendations. 22
Cross-registrations
We classified all retrospectively registered studies in our sample that also report a registration in EudraCT in the publication as prospective, as registrations on the platform are required prior to the approval of regulatory agencies or research ethics committees. 4
Reliability assessment of ratings
To assess the reliability of the data extraction, another rater (SG) performed 3 validation steps: first, a sample of 100 publications was screened using the same extraction form, during the main screening to refine category definitions. Second, another sample of 100 publications for which no registration number reporting was noted by MH to check for false negative ratings. Third, all cases with either date or reporting of retrospective registration or justification were screened to check for false positives.
Associations between prospective registration and other variables
To test the strength of the associations between prospective registration and three variables, we used Pearson’s χ 2 independence test. These variables were: (1) publication in an ICMJE member journal or a journal following ICMJE recommendations, (2) reporting of a registration number and (3) industry funding.
Associations between reporting of retrospective registration and other variables
To test the strength of the associations between the reporting of retrospective registration and two binary variables, we used Fisher’s exact test, as case numbers were low. These variables are: (1) publication in an ICMJE member journal or a journal following ICMJE recommendations and (2) industry funding.
We used Microsoft Excel for data collection and R (V.4.0.3) for data analysis and visualisation.
We checked our manuscript against the Strengthening the Reporting of Observational Studies in Epidemiology checklist ( online supplemental table 1 ). 23
Supplementary data
Patient and public involvement.
No patient involved.
Sample of retrospectively registered trials
After applying the above-mentioned exclusion criteria, 1932 registered studies with an associated results publication remained. Of these, 1038 (54%) were retrospectively registered according to the information provided in ClinicalTrials.gov and DRKS. We screened these 1038 studies for our analysis. Five of the publications were excluded as they were mislabeled as results publications in the dataset. Another 77 (8%) of the publications provided a EudraCT number, in which case we reclassified the study as prospectively registered, leaving 956 studies. For statistical comparisons, we used the studies classified as prospectively registered (n=971) in the dataset as a control group. A flowchart of this study selection is provided in figure 1 . Basic characteristics of included trials are available in online supplemental table 2 .

Flowchart of inclusion/exclusion of studies. From the 1038 trials that were retrospectively registered in Clincialtrials.gov (CT.gov) or Deutsches Register Klinischer Studien (DRKS), we excluded 5 publications that clearly did not report clinical study results (eg, secondary analyses of CT data) and another 77 that reported EudraCT entries in the publications, resulting in 956 retrospectively registered studies from a total dataset of 1927 (971+956) studies.
Retrospective registration
Figure 2 shows the extent of retrospective registration over time, which has been falling steadily from 100% in 2004 to 25% in 2017.

Percentage of retrospectively registered (RR) trials over time (per study start year). Generalised additive model smoother laid over (blue) with 95% CI. Bubble sizes indicate the number of trials per year included in the dataset.
We describe associations between prospective registration and previously defined binary variables in table 1 . We found no statistically significant association between publication in ICMJE member journals and prospective registration (p=0.10). Similarly, we found no statistically significant association with prospective registration when also including publication in journals reporting to follow ICMJE recommendations (p=0.47). It is important to note here that the information on ICMJE-following is based on journals’ requests to be included on the ICMJE website as a journal following the ICMJE’s recommendations, 22 therefore our results suggest that journals requesting to be listed on the site often do not enforce the recommendations strongly. However, there are other journals, such as many PLOS journals, that are not featured on the ICMJE site, but implement the recommendations. Retrospectively registered trials, compared with prospectively registered trials, significantly underreported registration numbers in the abstract (p=0.0007). Industry sponsorship of trials was associated with prospective registration (p=0.002). In 31% (294/956) of trials, registration occurred between study completion and publication (median 370 days before publication). Another 3% (25/956) of trials were registered after publication (median 249 days after publication).
ICMJE, International Committee of Medical Journal Editors; TRN, trial registration number.
Reporting of registration
Table 2 summarises the prevalence of reporting of trial registration and the reporting of retrospective registration. In 82% (783/956) of the remaining results publications of retrospectively registered trials, the registration was explicitly reported in either the abstract or the full text. In all except four of these publications, the registration was mentioned by providing the registration number. In the other cases, the registration was mentioned but without reporting a registration number.
Number of retrospectively registered trials and prevalence of key retrospective registration reporting practices
*‘Other’ includes footnotes, sidebars, etc.
Reporting of retrospective registration
The rate of trials for which retrospective registration is reported transparently increased over the last years up to 15% in 2020 ( figure 3 ). Overall, among all 956 retrospectively registered clinical studies, 5% (47) mention explicitly that this registration was retrospective in the abstract or full text (see table 2 ). Among those cases, 20 give some explanation or justification for why registration was retrospective. In 7% (67) of cases, the authors reported the registration date alongside the registration statement, but in 35 of those, the date was provided without giving the necessary context that the registration was retrospective.

Percentage of retrospectively registered trials reporting retrospective registration transparently in the publication over time (per study publication year). Generalised additive model smoother laid over (blue) with 95% CI. Bubble sizes indicate the number of trials per year included in the dataset. Starting in 2013, some authors begin to report retrospective registration. 15% of publications of retrospectively registered trials from 2020 transparently report retrospective registration. Four trials were published before 2009—in all those cases, the study completion dates provided in the registry were after 2009. Study start dates were before 2005 and studies were registered in 2005 (3/4) or later (1/4).
Publications in ICMJE member journals did not have a statistically significantly higher rate of reporting of retrospective registration (13% vs 5%, p=0.18), whereas publications in ICMJE member or following journals had a significantly lower rate (2% vs 7%, p=0.004). We found no association with transparent reporting of retrospective registration for industry sponsored trials (2% vs 5%, p=0.16) ( table 3 ).
Associations between transparent reporting of retrospective registration and other variables
ICMJE, International Committee of Medical Journal Editors.
Justifications of retrospective registration
In 20 cases in which the retrospective nature of the registration was reported, the authors provided further information explaining or justifying the retrospective registration. Notably, 14 of the 20 studies (70%) that justified the retrospective registration were published in a single journal, PLOS ONE . Table 4 shows the main themes present in authors’ explanations, with text examples.
Main themes identified from authors’ explanations of retrospective reporting and example statements
In this study, we show that in a sample of 956 results publications from retrospectively registered clinical studies led by German UMCs and completed between 2009 and 2017, only a small number of publications (5%) make the retrospective nature of the registration transparent, and even fewer (2%) explain the reasons for retrospective registration. To our knowledge, two studies have previously quantified the transparent reporting of retrospective registration in journal publications: Al-Durra et al 17 found in a sample of 286 publications in ICMJE member journals and published in 2018 that only 3% (8/286) of papers of retrospectively registered trials in their sample include justifications or explanations for delayed registration. Similarly, Loder et al , 18 in their analysis of 70 papers submitted to the British Medical Journal from 2012 to 2015 and rejected for registration issues, found that 3% (2/70) disclosed the registration problem when published in another journal. Our study finds a slightly lower percentage of 2% for explanations of the reasons for retrospective registration, but a higher percentage of 5% for disclosure in a larger sample representing a broader selection of journals and extended time frame.
We found that publications were not significantly more often prospectively registered when they were published in ICMJE member journals or in journals following ICMJE recommendations, but showed a significantly higher rate of TRN reporting. A similar result was found by Al-Durra et al . 17 Further, we found that transparent reporting of retrospective registration does not happen significantly more often in publications in ICMJE member journals, and is even happening at a significantly lower rate in journals listed as following ICMJE recommendations.
There were different reasons for retrospective registration brought forth by authors, many of which have been described previously. 15 17 18 24 In some cases, authors raise points that lie outside their direct responsibility, such as delays caused by the registry or research not being legally required to be preregistered. Several other reasons provided were within authors’ control, such as logistic and administrative issues, miscommunication between researchers or unawareness of registration policies. In some cases, authors report registering a study to meet journal editorial policies even though registration would not be required for the kind of research otherwise. This is also possibly reflected in the fact that almost a third (31%) of retrospectively registered studies in our sample have been registered between study completion and publication. In one publication, the authors transparently describe that the registration occurred only when ‘results suggested a publication and further continuation of this research’, which has been previously described as ‘selective registration bias’ 17 and is explicitly called out in ICMJE guidance as it ‘meets none of the purposes of preregistration’. 5 Another identified theme revolves around the confidentiality of methods; however, in this case, many other details about the trial could have been preregistered.
Limitations
For feasibility and data quality reasons, our study was based on an existing validated dataset, containing only trials led by German UMCs, which might limit its generalisability to other regions. However, the sample also contained multicentre trials with other countries involved and is larger and from a wider variety of journals compared with previous studies. 17 18 Our analysis of retrospective registration is based on trial start dates and registration dates as provided by the two registries used for sampling: Clinicaltrials.gov and DRKS. It is possible that authors did not update their registry entries when delays to the start date occurred. For example, we did not specifically follow-up cases in which authors wrote that a trial was registered prospectively, but the registry dates did not reflect that statement. In order not to reduce the sample size, we also did not correct for varying follow-up in the identification of result publications, for example, by limiting our analysis to publications published within 2 years of trial completion. However, this means that the newer trials in the sample (ie, years 2016, 2017) might not reflect the complete research output of those years as some trials may not have been published by the end of follow-up in 2020 and were therefore excluded from the analysis. The numbers presented in figures 2 and 3 may overestimate the improvements in prospective registration as trials reporting results on time might likely generally show a higher quality of registration conduct and might therefore be registered prospectively at a higher rate.
In our analyses involving the classification into ICMJE-following and non-following journals, we relied on the data provided on the ICMJE website ( icmje.org ), which are self-reported by journals, that is, a journal must write to the ICMJE that they want to be included in the list. Thus, there are some journals missing in the ICMJE data and therefore in our dataset. For ICMJE member journals (n=12) on the other hand, there is a complete listing available.
The Declaration of Helsinki and other guidelines for responsible clinical research unanimously recommend prospective registration of all clinical research. 2 For clinical trials regulated by drug and device regulatory authorities, this was codified into law. 4 A major aim of prospective registration is to minimise the risk of undisclosed changes to the protocol after the study started and first results are analysed. When registration happens retrospectively, this major goal is not addressed. The reporting of study registration is generally considered a best practice to make a study more trustworthy. In the case of retrospective registration, in contrast, reporting registration without transparency on the retrospective nature should rather raise concerns as readers might wrongly interpret the mentioning of registration as a quality criterion. This could be considered ‘performative reproducibility’, that is, the ‘pretence of reproducibility without the reality’. 25 Journal editors and reviewers could enforce explicit reporting and explanation of retrospective registration, but we found that this rarely happens. To fulfil the ICMJE requirements on reporting retrospective registration, a simple note in the registration statement of the paper would suffice, such as: ‘This study was retrospectively registered as (TRN) at (Registry), (X) days after the trial started because (Reason)’.
Supplementary Material
Acknowledgments.
We thank Maia Salholz-Hillel for conceptual feedback and technical support. We thank Martin R. Holst and Dr Delwen Franzen for feedback on the manuscript.
Contributors: MH: Conceptualisation, methodology, investigation, analysis, writing—original draft, project management. SG: Methodology, investigation. DS: Conceptualisation, methodology, supervision, writing—review and editing, funding acquisition, guarantor.
Funding: This work was partly funded under a grant from the Federal Ministry of Education and Research of Germany (Bundesministerium fuer Bildung und Forschung—BMBF) (01PW18012). The funder was not involved in the study design, data collection, analysis, or interpretation, writing of the manuscript, or the decision to submit for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Ethics statements, patient consent for publication.
Not applicable.
Ethics approval
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
Submit Studies
Related Pages
- Login to ClinicalTrials.gov PRS
Do you or someone you know want to participate in a clinical study? See information for patients and families .
What Is the Purpose of Trial Registration and Results Submission?
Why do i need to register my trial and submit results to clinicaltrials.gov.
Registering clinical trials when they begin, providing timely updates, submitting summary results, and making this information publicly available fulfills a number of purposes and benefits a variety of people.
Trial Registry Purposes and Benefits for Various Groups
Results database purposes and benefits for various groups, required by law.
Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801) (PDF) requires Responsible Parties to register and submit summary results of clinical trials with ClinicalTrials.gov. The law applies to certain clinical trials of drugs (including biological products) and medical devices. For more information:
- See FDAAA 801 and the Final Rule to learn about Responsible Party, Applicable Clinical Trials, and deadlines for registration and results submission
- See the Protocol Registration Data Element Definitions , Expanded Access Data Element Definitions , and Results Data Element Definitions to learn about the specific data elements
- See History, Policies, and Laws to learn about other relevant laws, including the Food and Drug Administration Modernization Act
- Key FDAAA Issues (9:23) Deborah A. Zarin, MD, Director, ClinicalTrials.gov, National Library of Medicine Discusses key issues in the FDAAA related to registering trials and submitting results (March 2011)
The Final Rule for Clinical Trials Registration and Results Information Submission (42 CFR Part 11) clarifies and expands the regulatory requirements and procedures for submitting registration and results information for certain clinical trials to ClinicalTrials.gov, in accordance with Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801). The Final Rule has been in effect since January 18, 2017. For more information on the Final Rule:
- See the Final Rule Information page for details on data submission requirements for 42 CFR Part 11, including the formatting of certain types of clinical trial information.
- View the archived content for the Final Rule Webinar Series , designed to assist responsible parties further their understanding of the Final Rule.
- See the Checklist and Elaboration for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (PDF) (June 2018) for complete statutory definitions and more information on the meaning of applicable clinical trial.
- View Frequently Asked Questions (FAQs) about the Final Rule (42 CFR Part 11).
Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801) (PDF) requires responsible parties to register clinical trials and submit summary results to ClinicalTrials.gov. The law applies to certain clinical trials of drug, biological, and device products and has been in effect since September 27, 2007.
Required for Journal Publication
The International Committee of Medical Journal Editors (ICMJE) requires trial registration as a condition of the publication of research results generated by a clinical trial. To fulfill this obligation organizations and individuals can provide the World Health Organization (WHO) Trial Registration Data Set required by ICMJE to ClinicalTrials.gov or a WHO primary registry . The ICMJE expects authors to meet all results reporting requirements of their funding and regulatory agencies. If there are no such reporting requirements, the ICMJE encourages authors to submit results information to the same database on which their trials are registered.
See the ICMJE section of the Support Materials page or visit the ICMJE Web site .
Selected Trial Registration Laws and Policies
A summary of key laws and policies requiring clinical trial registration is provided in the table below:
- Submit Studies : This section of the site is a resource for clinical research professionals, sponsors, and investigators who are responsible for registration and results submission to ClinicalTrials.gov.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
- Systematic review update
- Open access
- Published: 23 April 2024
The usage of population and disease registries as pre-screening tools for clinical trials, a systematic review
- Juliette Foucher ORCID: orcid.org/0000-0001-9026-0268 1 , 2 ,
- Louisa Azizi 1 ,
- Linn Öijerstedt 1 , 2 ,
- Ulf Kläppe 1 , 2 &
- Caroline Ingre 1 , 2
Systematic Reviews volume 13 , Article number: 111 ( 2024 ) Cite this article
246 Accesses
6 Altmetric
Metrics details
This systematic review aims to outline the use of population and disease registries for clinical trial pre-screening.
Materials and methods
The search was conducted in the time period of January 2014 to December 2022 in three databases: MEDLINE, Embase, and Web of Science Core Collection. References were screened using the Rayyan software, firstly based on titles and abstracts only, and secondly through full text review. Quality of the included studies was assessed using the List of Included Studies and quality Assurance in Review tool, enabling inclusion of publications of only moderate to high quality.
The search originally identified 1430 citations, but only 24 studies were included, reporting the use of population and/or disease registries for trial pre-screening. Nine disease domains were represented, with 54% of studies using registries based in the USA, and 62.5% of the studies using national registries. Half of the studies reported usage for drug trials, and over 478,679 patients were identified through registries in this review. Main advantages of the pre-screening methodology were reduced financial burden and time reduction.
Discussion and conclusion
The use of registries for trial pre-screening increases reproducibility of the pre-screening process across trials and sites, allowing for implementation and improvement of a quality assurance process. Pre-screening strategies seem under-reported, and we encourage more trials to use and describe their pre-screening processes, as there is a need for standardized methodological guidelines.
Peer Review reports
Introduction
Clinical trials are essential in allowing the scientific transition from basic research to clinical practice, whether the trials are about drug development or other types of non-drug interventions [ 1 ]. Clinical trial protocols are the trial documents of reference, detailing every step for participants enrolled in the trial. One of the critical protocol sections, is the list of eligibility criteria [ 2 ]—if this list is clinical trial specific, it will often include recurring criteria within the same field or disease area, with trial specific cut-off differences [ 2 ]. For example, when conducting a trial for the neurodegenerative disease amyotrophic lateral sclerosis (ALS), it will in the majority of cases include vital capacity (VC) measurement as a trial eligibility criteria. Some ALS trials only include patients with a VC above 50% (NCT05633459), while others ask for patients with VC equal or superior to 65% (national clinical trial NCT, NCT04248465). Enrolling the right participants in a clinical trial is essential as it (1) could allow for a personalized medicine approach [ 3 , 4 , 5 , 6 ], (2) might be the only option to access drugs in development for patients suffering from diseases with no cures [ 7 , 8 , 9 , 10 ], (3) should ensure that motivated participants complete the entire study without dropping out, and thus ultimately maximize patient retention [ 11 , 12 , 13 , 14 ], and (4) in the end ensures good quality clinical trial data [ 15 ]. However, trial enrollment can also be challenging, especially to efficiently identify the above mentioned potentially eligible candidates during the pre-screening process [ 16 , 17 , 18 ]. This requires the identification of participants meeting the most stringent criteria and ultimately highly likely to be successful during the screening process. The pre-screening procedure is crucial as it decreases the screen failure rate, which drastically varies between trials across disease areas and countries [ 19 , 20 , 21 , 22 ]. Considering that screen failures are associated with participant burden while also negatively impacting the study budget, there is a need to develop clinical trial recruitment strategies targeting these aspects [ 23 , 24 , 25 , 26 ].
Typically, trial pre-screening is staff-bound with a designated staff member in charge of the pre-screening process. Research teams usually have team specific pre-screening processes, as there are no national consensus, guidelines nor universal standard operating procedures (SOPs) on how to conduct the pre-screening for clinical trials. A typical pre-screening process may include an internal check of the hospital medical journals in paper format, a review of electronic medical journals, a review of medical journals sent via traditional mail in case of a referral, direct emails from patients emailing a research team, and more [ 27 , 28 , 29 , 30 ]. This creates a pool of information derived from several sources, with no standardized system assuring quality and replicability, not allowing an audit trail for quality assurance and control, and overall creating inequitable trial access for patients [ 31 ]. Clinical trial eligibility criteria will often include both demographic data and disease specific information. This information is captured in most disease registries/population registries/patient registries, and there is an growing interest in using such registries for pre-screening due to the high quality and easy accessible data [ 32 , 33 , 34 , 35 ]. Such disease registries/population registries/patient registries are to be distinguished from other types of databases such as electronic health records (EHR). For the purpose of this review, we will use the term patient registry when referring to a specific database aiming to capture data on all patients from a patient population in a specific site, state, or country [ 32 , 36 , 37 ].
The usage of population and disease registries for trial pre-screening has previously been investigated through a literature review of the period 2004–2013, reporting limited registry use for clinical trial pre-screening, but advocating for a more systematic usage as this was deemed an efficient method [ 38 ]. The combined use of registries and medical record data has been described as optimizing trial recruitment [ 23 ], and we have since 2013 observed an explosion of clinical trials in many different fields as reported by the International Clinical Trial Registry Platform (ICTRP) of the World Health Organization (WHO) [ 39 ]. The ICTRP collects trial registration information from different databases such as Clinicaltrial.gov and reported 34,291 clinical trials in 2013 versus 59,964 clinical trials in 2021. The increasing number of clinical trials globally highlights the need for efficient and equitable pre-screening processes, but also for an updated review considering the last review on this topic was not conducted with a systematic methodology [ 38 ].
In this systematic review, we characterized the use of population and disease registries as a pre-screening tool for clinical trials not discriminating between drug and non-drug trials. We included publications published between January 2014 and December 2022, as a non-systematic review covered the 2004 to 2013 timeframe. We aimed to describe the type of registries used, disease areas, type of clinical trials linked to the registry-based pre-screening, and potential assets the method brought to the pre-screening process.
Inclusion and exclusion criteria
Citations and references obtained from the search were screened using the Rayyan software and our set of inclusion and exclusion criteria are listed in Table 1 . Eligible studies had to be in English, from peer-reviewed journals, reporting the use of population/patient/disease registries for trial pre-screening. Included studies also needed to be set in trials on patients and not on healthy individuals. Studies had to have been published in our targeted window between January 2014 and December 2022, and abstracts had to be available for review. Finally, we included studies of high to moderate quality, as evaluated through the List of Included Studies and quality Assurance in Review (LISA-R) tool. Since there was no standardized tool to judge the quality of the included studies, we developed a quality assurance tool, the LISA-R. This quality assurance checklist was developed using guidance provided on the Parsifal platform for systematic reviews, a platform providing support for researchers conducting reviews and wishing to establish new quality assurance tools. The tool consists of 11 items in which each item was judged on a two-level scale (yes/no) (LISA-R blank tool available in Supplementary material 2 ). For each “yes”, one point was attributed, giving a scale range from 0 to 11. An overall score > 8 was interpreted as high quality, 6–8 moderate quality, and < 6 low quality.
Search and selection strategy
The study protocol was registered in PROSPERO with the identification number CRD42023433968 and followed the PRISMA requirements [ 40 ]. A literature search was performed in the following databases: MEDLINE, Embase, and Web of Science Core Collection. The last search was conducted on June 22, 2023.
The search strategy was developed in MEDLINE (Ovid) in collaboration with librarians at the Karolinska Institutet University Library. For each search concept, medical subject headings (MeSH-terms) and free text terms were identified (Supplementary material 1 ). The search was then translated, with Polyglot Search Translator used for the translation of the controlled vocabulary [ 41 ], into the other databases.
Language restriction was made to English and the search was limited to years 2014–2022 as a previous non-systematic review covered the 2004–2013 period [ 38 ]. De-duplication was done using the method described by Bramer et al. [ 1 ]. One final step was added to compare digital object identifiers to finalize de-duplication. The full search strategies for all databases are available in supplementary material (Supplementary material 1 ). The review of papers was conducted by two of the authors (JF and LA) independently and then cross-checked. A third author (CI) was asked to solve selection conflicts if they arose, by setting-up a meeting where JF and LA could expose their process and CI could make the final decision. A first review process (phase 1) was done based on titles and abstracts only, while the second review was of full texts (phase 2). Only the publications of moderate and high quality as per the LISA-R tool were included in the final search (phase 3).
Data extraction
Data extraction was conducted by two of the authors (JF and LA) reading the full texts and summarizing information in table format through an excel form. This data extraction form was created for the sole purpose of this systematic review. The extraction form included the information we wished to extract from the included studies: trial type (drug trial versus non-drug trial), clinical trial name, NCT number, registry name and scope, patient population, and age. In order to specifically look into enrollment and pre-screening rates, we extracted the number of patients identified through the registries, number of patients eligible for the trial in question, and number of patients enrolled in the clinical trial. Different enrollment rates were calculated when possible and represented by percentages: (1) comparing the number of patient enrolled to the number identified in the pre-screening process and (2) comparing the number of patient enrolled to number of patients actually eligible after screening.
Review process
A total of 1430 citations were identified through the literature search. Out of them, 1369 were excluded based on titles and abstract review as they did not meet inclusion criteria (Table 1 ). One citation was excluded as a duplicate (Fig. 1 ). The 60 remaining publications were reviewed by reading the full text and 35 publications were subsequently excluded as they did not meet inclusion criteria. The remaining 25 publications were assessed using the LISA-R tool and the articles of low quality were excluded, ending up with 24 included papers (Supplementary material 3 a and b). The list of excluded papers is available upon request.
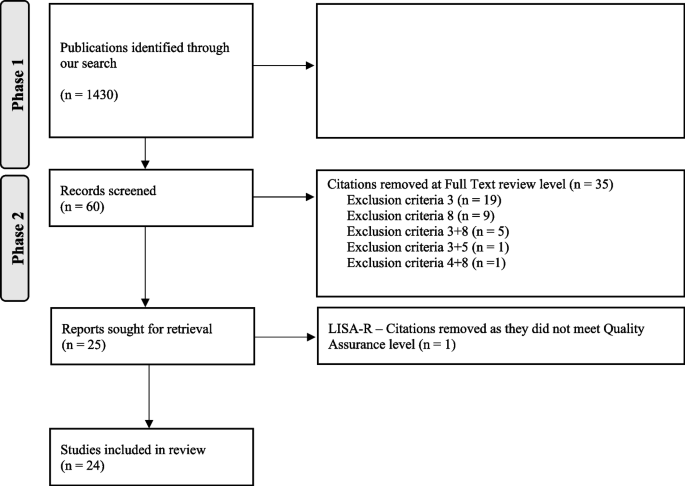
Flowchart of the selection process
Included articles—descriptive characteristics
Out of the 24 articles included and reporting the use of a population/disease registry for a clinical trial pre-screening [ 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 ], we identified nine disease domains/fields: nine articles were associated with oncology, three articles with the cardiovascular field, two papers with inflammatory diseases, one paper with autoimmune diseases, one paper with pulmonary diseases, one paper with hepatology, two papers with endocrinology, four papers with neurological and/or neuromuscular disorders, and one paper associated with metabolic disorders. Among the included articles, a majority were based on registries from the USA (13 out of 24) [ 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 ], with the rest describing either single-European-country registries or other international registries, and one registry from Israel [ 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 ]. Descriptive characteristics of the included papers are available in Supplementary material 4 .
Included articles—trial/registry and patient population’s characteristics
In terms of registry scope, 15 of the 24 included studies reported using national registries for trial pre-screening (Supplementary material 4 , papers 12/18/20/16/10/9/13/21/24/14/4/8/5/6/23) (respectively [ 42 , 43 , 45 , 46 , 48 , 49 , 50 , 51 , 54 , 57 , 58 , 59 , 62 , 63 , 65 ]), and 9 of 24 studies reported using local registries (Supplementary material 4 , papers 22/17/24/1/19/7/2/5/3) (respectively [ 44 , 53 , 54 , 55 , 56 , 60 , 61 , 62 , 64 ]). Local registries were either at a state level (Supplementary material 4 , papers 17 and 19) [ 53 , 56 ], or a specific site level (Supplementary material 4 , papers 22/1/7/2/3) (respectively [ 44 , 55 , 60 , 61 , 64 ]). Two included studies reported using a combination of local and national registries (Supplementary material 4 , papers 24 and 5) [ 54 , 62 ]. Only two international registries were reported being used for trial pre-screening (Supplementary material 4 , papers 15 and 11) [ 47 , 52 ]. We observed that 50% of the included studies reported pre-screening for drug trials (Supplementary material 4 , papers 22/16/15/10/21/1/14/8/7/2/6/23) (respectively [ 44 , 46 , 47 , 48 , 51 , 55 , 57 , 59 , 60 , 61 , 63 , 65 ]), and 50% of non-drug trials (Supplementary material 4 , papers 12/18/20/9//13/11/17/24/19/4/5/3) (respectively [ 42 , 43 , 45 , 49 , 50 , 52 , 53 , 54 , 56 , 58 , 62 , 64 ]). Only one registry and trial targeted a pediatric population (Supplementary material 4 , paper 14) [ 57 ]. In total, we estimate that over 478,679 patients were identified through registries in this review. However, three studies did not report any patient numbers [ 59 , 61 , 62 ]. The number of patients eligible in the individual studies varied between 59 and 16,091 [ 43 , 45 ].
Characteristics linked to the clinical trials, the registries, and the patient population included in this systematic review are available in Table 2 .
Included articles—patient trial enrollment out of registry pre-screening
Seven of the 24 papers included reported mock enrollment numbers as they were from retrospective studies, followed by a simulated enrollment performance out of registry usage for pre-screening [ 43 , 45 , 46 , 47 , 48 , 56 , 65 ]. Only 11 studies reported the full pre-screening process from patient identification to eligibility evaluation and finally trial enrollment, with highly heterogenous numbers and enrollment rates [ 42 , 43 , 44 , 45 , 47 , 51 , 52 , 53 , 54 , 56 , 58 ] (Table 2 ).
In the 11 papers that fully reported numbers from identified, eligible, and enrolled patients (Supplementary material 4 , papers 12/18/22/20/15/21/11/17/24/19/4) (respectively [ 42 , 43 , 44 , 45 , 47 , 51 , 52 , 53 , 54 , 56 , 58 ]), we observed different enrollment rates compared to patients first identified through their respective registries with a span of 0.87% [ 58 ] to 60% [ 42 ]. When calculating the enrollment rates compared to patients deemed eligible from their respective registries, results went to a span of 18.6% [ 58 ] to 99% [ 42 ].
Quality assessment and risk of bias
In order to assess quality of the included paper and their risk of bias (RoB), we developed a quality checklist available as Supplementary Table 2 . Over the 24 studies describing registry use for clinical trial pre-screening, the majority of the 11 quality questions selected in our checklist were met (Supplementary material 4 , papers 12/18/22/20/16/15/9/21/11/17/24/1/19/14/4/8/7/2/5/6/3/23) (respectively [ 42 , 43 , 44 , 45 , 46 , 47 , 49 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 ]), with only 2 studies checking for all items (Supplementary material 4 , papers 10 and 13) [ 48 , 50 ].
In 11 of 24 studies, NCT numbers were not mentioned (Supplementary material 4 , papers 22/16/9/24/1/14/4/8/7/2/6) (respectively [ 44 , 46 , 49 , 54 , 55 , 57 , 58 , 59 , 60 , 61 , 63 ]) even though trial names were documented in ten of them (Supplementary material 4 , papers 22/16/9/1/14/4/8/7/2/6) (respectively [ 44 , 46 , 49 , 55 , 57 , 58 , 59 , 60 , 61 , 63 ]). Conflict of interests were disclosed in the vast majority of the studies with only one paper not disclosing them (Supplementary material 4 , paper 19) [ 56 ].
In terms of pre-screening methodology, only six studies (Supplementary material 4 , papers 12/22/16/9/21/11) did not specify in what way the registry was used to perform trial pre-screening (respectively [ 42 , 44 , 46 , 49 , 51 , 52 ]).
Overall, with a majority of quality marks being met using the LISA-R tool, we estimate the quality of the studies included in this review from moderate to high, as the majority of them provide enough information to replicate their methods and findings in using a population registry for a clinical trial pre-screening.
We conducted a systematic review including 24 studies reporting the usage of population and disease registries for clinical trial pre-screening between January 2014 and December 2022. We aimed to describe the type of registries used, disease areas, type of clinical trials linked to the registry-based pre-screening, and potential assets the method brought to the pre-screening process. Our study shows that the use of registries for clinical trial pre-screening is very diverse in terms of registry type (international, national, local and statewide, local and site specific). We observed less diversity in terms of geography since a majority of the studies included in the review were using registries from the USA, with only one study using Nordic registry data. The US dominance is surprising knowing that for example the Nordic countries have been extensively described for their use of registries and registry-research [ 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 ]. This could possibly be explained by “recruitment registries” or “research ready cohorts” are being developed and were excluded from our review (Table 1 ) as they either include healthy participants who are at risk of developing diseases in the future or patients who are solely in registries due to their interest in participating in clinical trials [ 38 , 74 , 75 , 76 , 77 , 78 , 79 ]. Our search highlighted that such recruitment registries seem to be extensively used in Alzheimer and dementia research [ 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 ]. This could explain why patient registries may surprisingly not be the first in line of use for trial pre-screening, as “recruitment registries” are blooming to support different trials. However, recruitment registries should be carefully considered as they bring ethical concerns. Indeed, they can lead to consenting patients already enrolled in other trials, or having to deal with changes in patient’s disease status not being updated [ 88 ].
In terms of disease areas, 11 of the 24 included studies reported use in either cardiovascular health or oncology. This is aligned with the ICTRP website that reports oncology and cardiovascular trials at the 1 st and 3 rd position for the numbers of trials by health category (the 2 nd place being for neuropsychiatric conditions) [ 39 ]. We found half of drug trials (compared to other interventions) in our included studies with 12 publications of the 24 included reporting registry usage for drug trial pre-screening [ 44 , 46 , 47 , 48 , 51 , 55 , 57 , 59 , 60 , 61 , 63 , 65 ]. This reflects the importance of drug trials in the clinical trial landscape, but should also be considered in relation to the disease domains they are associated with, as certain diseases call more for drug trials than non-drug trials.
The main challenge of this systematic review was that it did not follow the traditional Patient/Exposure/Comparator/Outcome (PECO), or Patient/Intervention/Comparator/Outcome (PICO) as usually recommended [ 89 ]. The reason was that such traditional method did not fit our study purpose. If a PECO was to be outlined then our patient group could be people with diseases (as we are investigating patient registries/disease registries/population registries). The exposure could be to be included in a registry, with the consequence that the inclusion of all exposures indeed would not allow for intervention-specific conclusion. However, it does increase the generalizability of our results as we included registries from different disease areas. In this study, we could not define a control group or comparator group, since all included studies used different set-ups. For that reason, we did not specify the comparator group in our search strategy, and we included all control groups in our analyses. This could possibly have led to a dilution of the results, but we believe that it extended the external validity of our study. Regarding the outcome of the PECO, it could be the number of patients identified/eligible/enrolled for a clinical trial. However, here we aimed to describe the landscape of the usage of registries for clinical trials via a systematic review approach, meaning the final enrollment numbers were not an indicator of success or failure.
We observed a large variation in enrollments rates compared to patients deemed eligible from their respective registries. Higher and lower rates should not be interpreted as successes or failures, as these are directly linked to patient population, registry types, and moreover trial inclusion and exclusion criteria. One may identify a great number of patients in a registry but have very restrictive inclusion and exclusion criteria that will only make a fraction of your identified patients eligible, which should not be interpreted as a default in methodology. Similarly, only a fraction of the eligible patients will be enrolled due to various reasons: trial may be linked to a high patient burden leading to only a few patients consenting it, patients may live far away from the trial center and do not wish to travel, or patient may decline research participation for other reasons. However, these numbers should be considered when discussing pre-screening and recruitment methodology with respect of their specific constraints and challenges. This could allow study teams and field experts to better understand their recruitment workflow and the parameters influencing these rates.
Our second challenge was that available checklists of RoB tools did not fit our research question, leading us to develop our own quality assessment checklist of 11 items, the LISA-R tool (Supplementary material 2 ). This may be seen as a limitation as this did not allow us to produce a RoB score. However, the checklist allowed us to obtain a rigorous and traceable quality assessment tool. Through this checklist, we estimated that all included studies were either of high or moderate quality. If the checklist was developed using guidance provided on the Parsifal platform for systematic reviews, it was not tested prior to this review as it was specifically designed for this study, and is the pilot try of the LISA-R [ 90 ]. In the future, we aim like to validate this tool in a larger and dedicated study.
Thirdly, we only included studies published in English, and we do acknowledge that more studies fitting in our scope may have been published in other languages. However, as English is the main scientific language, we would not expect the additional studies to change our observations and conclusions.
Lastly, we need to acknowledge the risk of bias in registry inclusion. We know that certain registries may be highly effective at capturing patients, like the Swedish Motor Neuron Disease (MND) National Quality Registry including 99% of MND patients in the Stockholm Region [ 91 ]. However, this is not the case for all registries and varies geographically. For an optimal and efficient national registry based pre-screening, one would need to have 100% of a national patient population entered in the registry in order to be truly representative. We observed that five of the included studies reported using a local registry that was at center scale (Supplementary material 4 , papers 22/1/7/2/3) (respectively [ 44 , 55 , 60 , 61 , 64 ]), for which we would assume a 100% adherence between patient followed up at the site and the site registry. However, this would depend on site resources and site staff’s adherence to registering patients into the site’s registry. A recent Cochrane review on “ strategies to improve recruitment to randomized trials ” only mentioned two papers reporting pre-screening methods, both judged with high risk of bias and therefore not included in the final analyses, highlighting the blind spot surrounding trial pre-screening methodology [ 92 , 93 , 94 ].
Of the 24 included studies in our review, 14 reported benefits for using population registries in the trial pre-screening process [ 42 , 43 , 45 , 48 , 50 , 55 , 56 , 58 , 59 , 60 , 61 , 63 , 64 , 65 ]. Advantages of this methodology has been described as cost-efficient trial recruitment and benefits patients in countries with small populations or low population density in specific areas and also patients with rare diseases [ 55 , 61 , 65 , 95 , 96 ]. Ethically, using a population or disease registry for clinical trial pre-screening in a systematic manner would guarantee for all patients to be considered in the same equitable way, not discriminating between patients clinically followed up in large university hospital and patients living in remote areas. However, in order to be representative of the full disease population one would need to make sure 100% of patients are enrolled in their disease registries in order for it to be absolutely representative. Using registries only capturing a small portion of the patient population is introducing a potential risk of bias, and therefore efforts to capture all patients in disease registries should be maintained. Furthermore, the use of registries for pre-screening patients for clinical trials also gives an opportunity for a trial in a real-world setting and increases the evidence value of the trial [ 55 , 61 , 65 , 95 , 96 ].
Furthermore, using registries for trial pre-screening increases the reproducibility of the pre-screening process across trials, increases the chances of all registry patients to be considered, and allows for implementation and improvement of quality assurance processes. As most studies reported use of national registries, this highlights the consideration of a patient population on a national level, maximizing efficiency and representativity of the pre-screening process. However, to apply such methodology, it is essential that the consent forms include information about the data might be used to confirm clinical trial eligibility and that trials may be offered to the included patients [ 97 ]. One might argue that these benefits are not reflecting the current reality: since January 2014 there was a mean of 50,000 clinical trials running each year [ 39 ] and only 24 studies between 2014 and 2022 reported using population registries for pre-screening despite advantages with this method. However, the literature is known to under-report recruitment strategies in clinical trials, from protocols to publications [ 98 , 99 ]. This leads to restrictive data, as this systematic review only reflects research that reported registry use in a clinical trial pre-screening setting. It is important to consider more clinical trials may pre-screen and recruit patients from registries without reporting it neither in their protocol nor in their published methodology. This means that registry use for trial pre-screening may be much more important than reported in this review. Furthermore, studies reporting use of population and disease registries for trial pre-screening have failed to address questions around data privacy and protection. The majority of disease registries around the world are accessible by two types of users: patients, who may directly fill out information into the registries, and health care professionals. These registries have data agreement in place, regarding privacy, sharing, and use such as data extraction for research purposes. When pre-screening for clinical trials, clinical trial sponsors do request pre-screening logs. This is done for financial reasons, as clinical research teams do negotiate in their clinical trial budget to be compensated for the time spent pre-screening patients for a specific trial. Pre-screening logs are provided by sponsors and collect limited data respecting information privacy regulations applying locally, such as General Data Protection Regulation (GDPR) in the European Union. It is essential to continue using tools such as pre-screening logs to serve as buffers to minimize data sharing from registries to sponsors (most often pharmaceutical companies) and maintain compliance with information privacy regulations. The main difference linked to this aspect would be observed between the USA and Europe, as the US regulation allows for race data to be collected which is not approved in Europe. This is limiting the evaluation of racial representativeness in European clinical trials, which may be biased by enrolling a vast majority of Caucasian participants.
Finally, as artificial intelligence (AI) is being developed, studies are now reporting use of machine learning for patient pre-screening into trials: Su et al. recently cited a pilot trial from the Mayo Clinic in Rochester using an AI-based trial matching system [ 2 ]. The paper reported an enrollment increase of 80% due to the quick and accurate patient matching to the oncology trial run at the Mayo Clinic [ 2 ], a system that could be applied to patient registries. Oncology has also brought us algorithms for clinical trial pre-screening, specifically Evolutionary Strategy algorithms (ES algorithms) [ 100 , 101 ], that are commonly used in machine learning [ 102 ]. Ni et al. reported a 450% increase in efficacy of clinical trial pre-screening using electronic health record and not a patient registry, despite the fact that 10% of eligible patients were missed in the process [ 101 ]. More globally, data-driven technologies and strategies are more and more being reported in the literature, whether it is supporting prevention, diagnosis, or decision-making [ 103 , 104 , 105 , 106 ]. Such strategies’ impact on time optimization and associated cost reduction could be of great aid both to small trial centers working with limited staff and resources, and bigger trial centers dealing with a large volume of patients and trials.
Future studies are needed to address the limitations specific to certain disease fields to better describe the disease-specific needs around the use of registries for clinical trial pre-screening.
In conclusion, we aimed to describe the type of registries used, disease areas, type of clinical trials linked to the registry-based pre-screening, and potential assets the method brought to the pre-screening process. Only 24 studies between 2014 and 2022 reported using population and disease registries for clinical trial pre-screening despite time optimization and financial advantages using the method. A majority of the registries used were on a national level, and half of the trials for which pre-screening was performed were drug trials. Pre-screening strategies remain under-reported, and the use of population and disease registries for trial pre-screening may be much more important than what is described in this review, both for drug trials and non-drug trials. Our review is therefore stressing the need for standardized methodological guidelines for clinical trial pre-screening and encourages reporting of pre-screening processes in trial protocols and publications.
Availability of data and materials
Data is accessible upon request (full search and excel master document supporting screening, exported from Rayyan).

Abbreviations
Amyotrophic lateral sclerosis
Vital capacity
National clinical trial
Standard operating procedures
Electronic health record
International Clinical Trial Registry Platform
World Health Organization
Medical subject heading
List of Included Studies and quality Assurance in Review
Risk of bias
Patient/Exposure/Comparator/Outcome
Patient/Intervention/Comparator/Outcome
Motor neuron disease
Artificial intelligence
Evolutionary Strategy
Bramer WM, Giustini D, De Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. jmla. 2016;104. https://doi.org/10.5195/jmla.2016.24 .
Su Q, Cheng G, Huang J. A review of research on eligibility criteria for clinical trials. Clin Exp Med. 2023;23:1867–79.
Article PubMed Google Scholar
Miesbach W, Oldenburg J, Klamroth R, et al. Gentherapie der Hämophilie: Empfehlung der Gesellschaft für Thrombose- und Hämostaseforschung (GTH). Hamostaseologie. 2023;43:196–207.
Superchi C, Brion Bouvier F, Gerardi C, et al. Study designs for clinical trials applied to personalised medicine: a scoping review. BMJ Open. 2022;12:e052926.
Article PubMed PubMed Central Google Scholar
Chaytow H, Faller KME, Huang Y-T, et al. Spinal muscular atrophy: from approved therapies to future therapeutic targets for personalized medicine. Cell Rep Med. 2021;2:100346.
Article CAS PubMed PubMed Central Google Scholar
Sharma R. Innovative genoceuticals in human gene therapy solutions: challenges and safe clinical trials of orphan gene therapy products. CGT. 2024;24:46–72.
Article CAS Google Scholar
Tjeertes J, Bacino CA, Bichell TJ, et al. Enabling endpoint development for interventional clinical trials in individuals with Angelman syndrome: a prospective, longitudinal, observational clinical study (FREESIAS). J Neurodev Disord. 2023;15:22.
Sun Z, Zhang B, Peng Y. Development of novel treatments for amyotrophic lateral sclerosis. Metab Brain Dis. 2023. https://doi.org/10.1007/s11011-023-01334-z .
Gharat R, Dixit G, Khambete M, et al. Targets, trials and tribulations in Alzheimer therapeutics. Eur J Pharmacol. 2024;962:176230.
Article CAS PubMed Google Scholar
Revelles-Peñas L, Pastor-Navarro S, López-Piñero AA, Velasco-Tirado V. Use of a spinal cord stimulator to treat livedoid vasculopathy: Effective control of an untreatable disease. Estimulador medular en la vasculopatía livedoide: control eficaz de una patología intratable. Actas dermo-sifiliograficas. 2024:S0001-7310(23)00933-X. Advance online publication. https://doi.org/10.1016/j.ad.2023.02.037 .
Goddard-Eckrich D, Gatanaga OS, Thomas BV, et al. Characteristics of drug-involved black women under community supervision; implications for retention in HIV clinical trials and healthcare. Soc Work Health Care. 2024;63:35–52.
Chaudhari N, Ravi R, Gogtay N, et al. Recruitment and retention of the participants in clinical trials: challenges and solutions. Perspect Clin Res. 2020;11:64.
Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs. 2010;19:227–33.
Hadidi N, Lindquist R, Treat-Jacobson D, et al. Participant withdrawal: challenges and practical solutions for recruitment and retention in clinical trials. Creat Nurs. 2013;19:37–41.
Juni P. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–6.
Hamel LM, Penner LA, Albrecht TL, et al. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23:327–37.
Thoma A, Farrokhyar F, McKnight L, et al. Practical tips for surgical research: how to optimize patient recruitment. Can J Surg. 2010;53:205–10.
PubMed PubMed Central Google Scholar
Pinto BM, Dunsiger SI. The many faces of recruitment in a randomized controlled trial. Contemp Clin Trials. 2021;102:106285.
on behalf of the INVESTIGATE studies group, Hilton P, Buckley BS, et al. Understanding variations in patient screening and recruitment in a multicentre pilot randomised controlled trial: a vignette-based study. Trials. 2016;17:522.
Article PubMed Central Google Scholar
Parekh D, Patil VM, Nawale K, et al. Audit of screen failure in 15 randomised studies from a low and middle-income country. ecancer. 2022;16:1476. https://doi.org/10.3332/ecancer.2022.1476 .
Article Google Scholar
Mahajan P, Kulkarni A, Narayanswamy S, et al. Reasons why patients fail screening in Indian breast cancer trials. Perspect Clin Res. 2015;6:190.
Mckane A, Sima C, Ramanathan RK, et al. Determinants of patient screen failures in phase 1 clinical trials. Invest New Drugs. 2013;31:774–9.
Treweek S. Recruitment to trials - why is it hard and how might we make it less so? Trials. 2011;12:A110.
FDA. Enhancing the diversity of clinical trial populations — eligibility criteria, enrollment practices, and trial designs guidance for industry. Center for Biologics Evaluation and Research Center for Drug Evaluation and Research; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial .
Wong SE, North SA, Sweeney CJ, et al. Screen failure rates in contemporary randomized clinical phase II/III therapeutic trials in genitourinary malignancies. Clin Genitourin Cancer. 2018;16:e233–42.
DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–85.
Lombardo G, Couvert C, Kose M, et al. Electronic health records (EHRs) in clinical research and platform trials: application of the innovative EHR-based methods developed by EU-PEARL. J Biomed Inform. 2023;148:104553.
Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: a systematic review. A&D Transl Res & Clin Interv. 2019;5:751–70.
Task Force Participants, Vellas B, Hampel H, et al. Alzheimer’s disease therapeutic trials: EU/US task force report on recruitment, retention, and methodology. J Nutr Health Aging. 2012;16:339–45.
Kirn DR, Grill JD, Aisen P, et al. Centralizing prescreening data collection to inform data-driven approaches to clinical trial recruitment. Alzheimers Res Ther. 2023;15:88.
Acuña-Villaorduña A, Baranda JC, Boehmer J, et al. Equitable access to clinical trials: how do we achieve it? Am Soc Clin Oncol Educ Book. 2023;43:e389838.
Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for evaluating patient outcomes: a user’s guide. 3rd ed. Agency for Healthcare Research and Quality (US): Rockville; 2014. http://www.ncbi.nlm.nih.gov/books/NBK208616/ . Accessed 16 Jan 2024.
Google Scholar
Dreyer NA. Registries for robust evidence. JAMA. 2009;302:790.
Gabel RA. Patient registries, predictive models, and optimal care. JAMA. 2009;302:2662.
Bestehorn K. Medizinische Register: ein Beitrag zur Versorgungsforschung. Med Klin. 2005;100:722–8.
Williams WG. Uses and limitations of registry and academic databases. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:66–70.
Williams WG, McCrindle BW. Practical experience with databases for congenital heart disease: a registry versus an academic database. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:132–42.
Tan MH, Thomas M, MacEachern MP. Using registries to recruit subjects for clinical trials. Contemp Clin Trials. 2015;41:31–8.
Wold Health Organization W. International Clinical Trials Registry Platform (ICTRP). https://www.who.int/clinical-trials-registry-platform . Accessed 14 Dec 2023.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89.
Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. jmla. 2020;108. https://doi.org/10.5195/jmla.2020.834 .
Lasch F, Weber K, Koch A. Commentary: on the levels of patient selection in registry-based randomized controlled trials. Trials. 2019;20:100.
ASCEND Study Collaborative Group, Aung T, Haynes R, et al. Cost-effective recruitment methods for a large randomised trial in people with diabetes: A Study of Cardiovascular Events iN Diabetes (ASCEND). Trials. 2016;17:286.
Achiron A, Givon U, Magalashvili D, et al. Effect of Alfacalcidol on multiple sclerosis-related fatigue: a randomized, double-blind placebo-controlled study. Mult Scler. 2015;21:767–75.
Heywood J, Evangelou M, Goymer D, et al. Effective recruitment of participants to a phase I study using the internet and publicity releases through charities and patient organisations: analysis of the adaptive study of IL-2 dose on regulatory T cells in type 1 diabetes (DILT1D). Trials. 2015;16:86.
Oni C, Mitchell S, James K, Ng WF, Griffiths B, Hindmarsh V, Price E, Pease CT, Emery P, Lanyon P, Jones A, Bombardieri M, Sutcliffe N, Pitzalis C, Hunter J, Gupta M, McLaren J, Cooper A, Regan M, Giles I, …. UK Primary Sjögren’s Syndrome Registry. Eligibility for clinical trials in primary Sjögren's syndrome: lessons from the UK Primary Sjögren's Syndrome Registry. Rheumatology (Oxford, England). 2016;55(3):544–52. https://doi.org/10.1093/rheumatology/kev373 .
Darmon A, Bhatt DL, Elbez Y, et al. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J. 2018;39:750–757a.
Huebner H, Kurbacher CM, Kuesters G, et al. Heregulin (HRG) assessment for clinical trial eligibility testing in a molecular registry (PRAEGNANT) in Germany. BMC Cancer. 2020;20:1091.
van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncol. 2020;21:80–94.
theFilnemus Myotonic Dystrophy Study Group, De Antonio M, Dogan C, et al. The DM-scope registry: a rare disease innovative framework bridging the gap between research and medical care. Orphanet J Rare Dis. 2019;14:122.
Heidrich B, Cordes H-J, Klinker H, et al. Treatment extension of pegylated interferon alpha and ribavirin does not improve SVR in patients with genotypes 2/3 without rapid virological response (OPTEX Trial): a prospective, randomized, two-arm, multicentre phase IV clinical trial. PLoS One. 2015;10:e0128069.
Toth GG, Lansky A, Baumbach A, et al. Validation of the all-comers design: results of the TARGET-AC substudy. Am Heart J. 2020;221:148–54.
Brown JC, Troxel AB, Ky B, et al. A randomized phase II dose–response exercise trial among colon cancer survivors: purpose, study design, methods, and recruitment results. Contemp Clin Trials. 2016;47:366–75.
Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors: trial to reduce depressive symptoms. Psychooncology. 2014;23:507–15.
Xiang JJ, Roy A, Summers C, et al. Brief report: implementation of a universal prescreening protocol to increase recruitment to lung cancer studies at a Veterans Affairs cancer center. JTO Clin Res Rep. 2022;3:100357.
Russo R, Coultas D, Ashmore J, et al. Chronic obstructive pulmonary disease self-management activation research trial (COPD–SMART): results of recruitment and baseline patient characteristics. Contemp Clin Trials. 2015;41:192–201.
Tamborlane WV, Chang P, Kollman C, et al. Eligibility for clinical trials is limited for youth with type 2 diabetes: insights from the Pediatric Diabetes Consortium T2D Clinic Registry. Pediatr Diabetes. 2018;19:1379–84.
Danila MI, Chen L, Ruderman EM, et al. Evaluation of an intervention to support patient-rheumatologist conversations about escalating treatment in patients with rheumatoid arthritis: a proof-of-principle study. ACR Open Rheumatol. 2022;4:279–87.
Green MF, Bell JL, Hubbard CB, McCall SJ, McKinney MS, Riedel JE, Menendez CS, Abbruzzese JL, Strickler JH, Datto MB. Implementation of a Molecular Tumor Registry to Support the Adoption of Precision Oncology Within an Academic Medical Center: The Duke University Experience. JCO Precis Oncol. 2021;5:PO.21.00030. https://doi.org/10.1200/PO.21.00030 .
Guerra CE, Kelly S, Redlinger C, et al. Pancreatic cancer clinical treatment trials accrual: a closer look at participation rates. Am J Clin Oncol. 2021;44:227–31.
Wu J, Yakubov A, Abdul-Hay M, et al. Prescreening to increase therapeutic oncology trial enrollment at the largest public hospital in the United States. JCO Oncol Pract. 2022;18:e620–5.
Shadyab AH, LaCroix AZ, Feldman HH, et al. Recruitment of a multi-site randomized controlled trial of aerobic exercise for older adults with amnestic mild cognitive impairment: the EXERT trial. Alzheimers Dement. 2021;17:1808–17.
Mehta P, Raymond J, Han MK, et al. Recruitment of patients with amyotrophic lateral sclerosis for clinical trials and epidemiological studies: descriptive study of the National ALS Registry’s research notification mechanism. J Med Internet Res. 2021;23:e28021.
Valle CG, Camp LN, Diamond M, et al. Recruitment of young adult cancer survivors into a randomized controlled trial of an mHealth physical activity intervention. Trials. 2022;23:254.
Curtis JR, Wright NC, Xie F, et al. Use of health plan combined with registry data to predict clinical trial recruitment. Clin Trials. 2014;11:96–101.
Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic Health Registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533–54.
Børø S, Thoresen S, Boge Brant S, et al. Initial investigation of using Norwegian health data for the purpose of external comparator arms - an example for non-small cell lung cancer. Acta Oncol. 2023;62:1642–8.
Axelsson L, Alvariza A, Lindberg J, et al. Unmet palliative care needs among patients with end-stage kidney disease: a national registry study about the last week of life. J Pain Symptom Manage. 2018;55:236–44.
Nørgaard M, Mailhac A, Fagerlund K, et al. Treatment patterns, survival, and healthcare utilisation and costs in patients with locally advanced and metastatic bladder cancer in Denmark 2015–2020. Acta Oncol. 2023;62:1784–90.
Bakken IJ, Ariansen AMS, Knudsen GP, et al. The Norwegian Patient Registry and the Norwegian Registry for Primary Health Care: research potential of two nationwide health-care registries. Scand J Public Health. 2020;48:49–55.
Pol T, Karlström P, Lund LH. Heart failure registries – future directions. J Cardiol. 2024;83:84–90.
Pålsson S, Pivodic A, Grönlund MA, et al. Cataract surgery in patients with uveitis: data from the Swedish National Cataract Register. Acta Ophthalmol. 2023;101:376–83.
Annual report 2021 with results and improvements measures from the national quality register for lung cancer. Oslo: The Cancer Registry of Norway; 2022. https://www.kreftregisteret.no/Generelt/Rapporter/Arsrapport-fra-kvalitetsregistrene/Arsrapport-for-lungekreft/ .
Rimel BJ, Lester J, Sabacan L, et al. A novel clinical trial recruitment strategy for women’s cancer. Gynecol Oncol. 2015;138:445–8.
Kluding PM, Denton J, Jamison TR, et al. Frontiers: integration of a research participant registry with medical clinic registration and electronic health records. Clin Transl Sci. 2015;8:405–11.
Mudaranthakam DP, Thompson J, Hu J, et al. A Curated Cancer Clinical Outcomes Database (C3OD) for accelerating patient recruitment in cancer clinical trials. JAMIA Open. 2018;1:166–71.
Kannan V, Wilkinson KE, Varghese M, et al. Count me in: using a patient portal to minimize implicit bias in clinical research recruitment. J Am Med Inform Assoc. 2019;26:703–13.
McKinstry B, Sullivan FM, Vasishta S, et al. Cohort profile: the Scottish research register SHARE. A register of people interested in research participation linked to NHS data sets. BMJ Open. 2017;7:e013351.
Robotham D, Waterman S, Oduola S, et al. Facilitating mental health research for patients, clinicians and researchers: a mixed-method study. BMJ Open. 2016;6:e011127.
Vermunt L, Veal CD, Ter Meulen L, et al. European Prevention of Alzheimer’s Dementia Registry: recruitment and prescreening approach for a longitudinal cohort and prevention trials. Alzheimers Dement. 2018;14:837–42.
Zwan MD, Van Der Flier WM, Cleutjens S, et al. Dutch Brain Research Registry for study participant recruitment: design and first results. A&D Transl Res & Clin Interv. 2021;7:e12132.
Langbaum JB, Karlawish J, Roberts JS, et al. GeneMatch: a novel recruitment registry using at-home APOE genotyping to enhance referrals to Alzheimer’s prevention studies. Alzheimers Dement. 2019;15:515–24.
Grill JD, Hoang D, Gillen DL, et al. Constructing a local potential participant registry to improve Alzheimer’s disease clinical research recruitment. JAD. 2018;63:1055–63.
for the IMI-EPAD collaborators, Vermunt L, Muniz-Terrera G, et al. Prescreening for European Prevention of Alzheimer Dementia (EPAD) trial-ready cohort: impact of AD risk factors and recruitment settings. Alz Res Ther. 2020;12:8.
Harwood RH, Goldberg SE, Brand A, et al. Promoting Activity, Independence, and Stability in Early Dementia and mild cognitive impairment (PrAISED): randomised controlled trial. BMJ. 2023:e074787.
Jimenez-Maggiora GA, Bruschi S, Raman R, Langford O, Donohue M, Rafii MS, Sperling RA, Cummings JL, Aisen PS. TRC-PAD: Accelerating Recruitment of AD Clinical Trials through Innovative Information Technology. J Prev Alzheimers Dis. 2020;7(4):226–33. https://doi.org/10.14283/jpad.2020.48 .
Langbaum JB, High N, Nichols J, Kettenhoven C, Reiman EM, Tariot PN. The Alzheimer's Prevention Registry: A Large Internet-Based Participant Recruitment Registry to Accelerate Referrals to Alzheimer's-Focused Studies. J Prev Alzheimers Dis. 2020;7(4):242–50. https://doi.org/10.14283/jpad.2020.31 .
Milne R, Bunnik E, Tromp K, Bemelmans S, Badger S, Gove D, Maman M, Schermer M, Truyen L, Brayne C, Richard E. Ethical Issues in the Development of Readiness Cohorts in Alzheimer's Disease Research. J Prev Alzheimers Dis. 2017;4(2):125–31. https://doi.org/10.14283/jpad.2017.5 .
Morgan RL, Whaley P, Thayer KA, et al. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–31.
Kitchenham B. Guidelines for performing Systematic Literature Reviews in Software Engineering, Version 2.3, EBSE Technical Report EBSE-2007-01, Keele University and University of Durham. 2007.
Longinetti E, Regodón Wallin A, Samuelsson K, et al. The Swedish motor neuron disease quality registry. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:528–37.
Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2018. https://doi.org/10.1002/14651858.MR000013.pub6 .
Diguiseppi C, Goss C, Xu S, et al. Telephone screening for hazardous drinking among injured patients seen in acute care clinics: feasibility study. Alcohol Alcohol. 2006;41:438–45.
Graham A, Goss C, Xu S, et al. Effect of using different modes to administer the AUDIT-C on identification of hazardous drinking and acquiescence to trial participation among injured patients. Alcohol Alcohol. 2007;42:423–9.
Davis MK, Fine NM. An urgent need for data to drive decision making: rationale for the Canadian Registry for Amyloidosis Research. Can J Cardiol. 2020;36:447–9.
Ieva F, Gale CP, Sharples LD. Contemporary roles of registries in clinical cardiology: when do we need randomized trials? Expert Rev Cardiovasc Ther. 2014;12:1383–6.
Levesque E, Leclerc D, Puymirat J, et al. Developing registries of volunteers: key principles to manage issues regarding personal information protection. J Med Ethics. 2010;36:712–4.
Lasagna L. Problems in publication of clinical trial methodology. Clin Pharmacol Ther. 1979;25:751–3.
O’Sullivan Greene E, Shiely F. Recording and reporting of recruitment strategies in trial protocols, registries, and publications was nonexistent. J Clin Epidemiol. 2022;152:248–56.
Ni Y, Wright J, Perentesis J, et al. Increasing the efficiency of trial-patient matching: automated clinical trial eligibility pre-screening for pediatric oncology patients. BMC Med Inform Decis Mak. 2015;15:28.
Ni Y, Kennebeck S, Dexheimer JW, et al. Automated clinical trial eligibility prescreening: increasing the efficiency of patient identification for clinical trials in the emergency department. J Am Med Inform Assoc. 2015;22:166–78.
Kramer O. Evolution strategies. In: Machine learning for evolution strategies. Cham: Springer International Publishing; 2016. pp. 13–21. https://doi.org/10.1007/978-3-319-33383-0_2 .
Rahman MA, Moayedikia A, Wiil UK. Editorial: data-driven technologies for future healthcare systems. Front Med Technol. 2023;5:1183687. https://doi.org/10.3389/fmedt.2023.1183687 .
Subrahmanya SVG, Shetty DK, Patil V, Hameed BMZ, Paul R, Smriti K, Naik N, Somani BK. The role of data science in healthcare advancements: applications, benefits, and future prospects. Ir J Med Sci. 2022;191(4):1473–83. https://doi.org/10.1007/s11845-021-02730-z .
Sousa MJ, Pesqueira AM, Lemos C, Sousa M, Rocha Á. Decision-making based on big data analytics for people management in healthcare organizations. J Med Syst. 2019;43(9):290. https://doi.org/10.1007/s10916-019-1419-x .
Khan S, Khan HU, Nazir S. Systematic analysis of healthcare big data analytics for efficient care and disease diagnosing. Sci Rep. 2022;12(1):22377. https://doi.org/10.1038/s41598-022-26090-5 .
Download references
Acknowledgements
Authors would like to thank the librarians from the Karolinska Institutet Library and especially Emma-Lotta Säätelä, for their help supporting the literature search for this study. We also thank Dr. Wim Grooten who acted as a mentor during this project in systematic review methodology.
Open access funding provided by Karolinska Institute. This study was funded by Stoppa ALS.
Author information
Authors and affiliations.
Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Juliette Foucher, Louisa Azizi, Linn Öijerstedt, Ulf Kläppe & Caroline Ingre
Department of Neurology, Karolinska University Hospital, Stockholm, Sweden
Juliette Foucher, Linn Öijerstedt, Ulf Kläppe & Caroline Ingre
You can also search for this author in PubMed Google Scholar
Contributions
JF and CI designed the study. JF and LA conducted the search and screened all citations. CI resolved screened conflicts when they arose. All authors participated to the manuscript, with JF and LA being major contributors to the manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Juliette Foucher .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
C. Ingre has consulted for Cytokinetics, Pfizer, BioArctic, Novartis, Tikomed, Ferrer, Amylyx, and Mitsubishi and was a DMC member for Appelis Pharmaceutical. She is also a board member of Tobii Dynavox and of the Stiching TRICALS Foundation; all outside the submitted work. All other authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Foucher, J., Azizi, L., Öijerstedt, L. et al. The usage of population and disease registries as pre-screening tools for clinical trials, a systematic review. Syst Rev 13 , 111 (2024). https://doi.org/10.1186/s13643-024-02533-0
Download citation
Received : 29 January 2024
Accepted : 12 April 2024
Published : 23 April 2024
DOI : https://doi.org/10.1186/s13643-024-02533-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical trials
- Pre-screening
- Patient selection
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Participate in a Clinical Trial
Interested in a local clinical trial simply search by state, type of ms or keyword and see what's going on in your area., help further multiple sclerosis research, benefits and risks of participating in clinical trials.
- Playing an active role in your own healthcare
- Gaining access to new research treatments before they are widely available
- Helping others by contributing to medical research
- Unpleasant, serious or even life-threatening side effects to treatment
- Ineffective treatment
- The possibility of receiving a placebo or sugar pill instead of active medicine in controlled trials
- Increased demand on your time and attention than receiving standard treatment, such as extra trips to the doctor’s office or extra tests
NARCOMS Patient Registry
Want to post a trial.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Regulatory Information
- Search for FDA Guidance Documents
- Cancer Clinical Trial Eligibility Criteria: Performance Status
GUIDANCE DOCUMENT
Cancer Clinical Trial Eligibility Criteria: Performance Status Draft Guidance for Industry, IRBs, and Clinical Investigators April 2024
Not for implementation. Contains non-binding recommendations.
This guidance is being distributed for comment purposes only.
Submit Comments by 06/25/2024
Although you can comment on any guidance at any time (see 21 CFR 10.115(g)(5)), to ensure that the FDA considers your comment on a draft guidance before it begins work on the final version of the guidance, submit either online or written comments on the draft guidance before the close date.
If unable to submit comments online, please mail written comments to:
Dockets Management Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2024-D-1377
The purposes of eligibility criteria for cancer clinical trials are to select the intended patient population and reduce potential risks to trial participants. However, eligibility criteria are sometimes more restrictive than necessary, and expanding eligibility criteria to be more inclusive is one trial design consideration that may improve the diversity of clinical trial populations. This guidance is one in a series of guidances that provide recommendations regarding eligibility criteria for clinical trials of investigational drugs regulated by CDER and CBER for the treatment of cancer. Specifically, this guidance includes recommendations regarding expanding eligibility criteria to include patients with a wider range of performance status. This guidance is intended to assist interested parties, including sponsors and/or institutional review boards, who are responsible for the development and oversight of clinical trials.
Part 1. Overview Information
National Institutes of Health ( NIH )
P30 Center Core Grants
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
See Section III. 3. Additional Information on Eligibility .
This Notice of Funding Opportunity (NOFO) invites applications for Cystic Fibrosis (CF) Research and Translation Core Centers. CF Research and Translation Core Centers are designed to support both basic and clinical research on Cystic Fibrosis. CF Research and Translation Core Centers support three primary research-related activities: Research Core services; a Pilot and Feasibility program; and an Administrative Core with an enrichment program. Core Centers provide shared resources to support research to develop and test new therapies for CF and to foster collaborations among institutions with a strong existing research base in CF. The NIDDK currently supports seven CF Research and Translation Centers located at institutions with documented programs of research excellence in basic and clinical CF Research. Information about the currently funded CF Research and Translation Centers may be found at: https://www.niddk.nih.gov/research-funding/research-programs/cystic-fibrosis-research-translation-centers or https://www.cysticfibrosiscenters.org/ .
June 17, 2024
All applications are due by 5:00 PM local time of applicant organization.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
Not Applicable
It is critical that applicants follow the Multi-Project (M) Instructions in the How to Apply - Application Guide , except where instructed to do otherwise (in this NOFO or in a Notice from the NIH Guide for Grants and Contracts ). Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
Part 2. Full Text of Announcement
Section i. notice of funding opportunity description.
Program Objectives Cystic Fibrosis is one of the most common, life-limiting genetic diseases, and is estimated to affect 30,000 Americans. Individuals with CF have inherited two mutated copies of the gene, cystic fibrosis transmembrane conductance regulator (CFTR), in every tissue of the body. Although lung disease is the primary cause of death in CF, multiple other organ systems have altered functions including the liver, pancreas, bone, sweat glands, and gastrointestinal and reproductive systems.
More effective management of the pancreatic complications and the lung infections has extended median survival in the United States to over 40 years of age. All states in the U.S. now screen for CF at birth to enable presymptomatic support for nutrition and preventing infections that may further improve the quality of life for these patients. Children identified at birth avoid the malnutrition that was the common presenting sign for CF. In addition, these patients benefit from many new forms of therapy that are being developed, including highly effective modulator therapies (HEMT), to aggressively eradicate bacterial colonization of the lung, control inflammation and lung damage, improve management of diabetes and liver disease and provide nutritional support.
In addition to treating the signs, symptoms and complications of CF, researchers are investigating molecular and pharmacologic methods to treat the underlying cause of CF. Screening of small molecules has identified lead molecules that increase either expression, processing, trafficking or function of the CFTR protein. This approach has led to the development of the drug ivacaftor which is approved for patients with the G551D substitution in CFTR as well as other mutations. A combination drug lumacaftor/ivacaftor was then approved for patients with the F508del mutation, the most common of the more than 2000 known CF mutations. Another combination drug, ivacaftor/tezacaftor, was subsequently approved. Triple drug therapy (elexacaftor/ivacaftor/tezacaftor) was approved in 2019 and can be used by 90% of CF patients. The success of these drugs is spurring the development of additional drugs with more mutation targets and greater potency, and also targeting those patients who are not eligible for HEMT treatment. The ultimate goal is to have effective therapies for all patients with CF.
Despite current available therapies, CF related gastrointestinal and nutrition problems remain. Although pancreatic enzyme replacement provides therapeutic benefit, the effects are variable and malabsorption still remains an issue. Thus, better nutritional support and diet composition recommendations are needed. With HEMT therapy, reports of obesity and metabolic syndrome are now emerging, issues previously not observed in patients with CF.
Now that subjects with CF are living longer, there is an increased incidence of CF-related diabetes (CFRD). In fact, about 40% of patients with CF will develop diabetes by the age of 20. How mutated CFTR results in diabetes is still unclear. Recent guidelines developed by the Cystic Fibrosis Foundation and the American Diabetes Association suggest that all patients should be screened for the development of diabetes yearly by an oral glucose tolerance test starting at the age of 10. Insulin treatment of CF-related diabetes has been shown to improve survival and lung disease in patients with CF. How new CF drug treatments affect CFRD incidence and progression is not yet completely clear, but at least some patients appear to benefit.
Another increased CF morbidity, in part reflecting the longer CF lifespan, is CF associated liver disease (CFLD) which can affect up to 30% of patients. Clinically there is great variability in presentation, from having asymptomatic liver enzyme elevations to frank cirrhosis. The mechanism(s) responsible for CF disease found in the liver and bile ducts is incompletely understood. Whether or not new CF drug treatment benefits CFLD is not yet known. CF related renal disease is also becoming more prevalent with longer lifespans.
Gene therapy approaches to deliver a normal CFTR gene are also being pursued. This approach would allow treatment options for those patients ineligible or unable to tolerate HEMT therapy. New vectors with less toxicity have been developed. A question is at what age should gene therapy be considered? The availability of improved animal models such as the pig and ferret, which better mimic the human disease, aid in the testing of these new therapies. These encouraging findings suggest that collaborative efforts between basic and clinical researchers could foster the translation of novel basic research findings into preclinical testing in animal models and potential clinical applications.
CF research often requires the use of specialized technologies and resources to support a cohesive research effort. The goal of the Center is to make state-of-the-art technologies and resources readily accessible to a broad spectrum of investigators working on CF.
Center Structure and Activities
The NIDDK CF Research and Translation Centers are part of an integrated program of research support designed to enhance multidisciplinary research in pathogenesis and treatment of cystic fibrosis and its complications. The goal of these Cystic Fibrosis Research and Translation Core Centers is to support research to develop and test therapies for CF. These Centers will provide resources for communication and collaboration between basic and clinical researchers. CF Research and Translation Core Centers support three primary research-related activities:(1) Research Core Services that provide shared resources to enhance the efficiency of research and foster collaborations within and among institutions with strong existing bases of research on cystic fibrosis; (2) a Pilot and Feasibility Program designed to foster the development of new investigators and to provide seed-support for innovative high-risk projects; and (3) an Administrative Core with an Enrichment Program to promote interdisciplinary interaction and educational updates for investigators.
Institution and Research Base
A CF Research and Translation Center must be an identifiable unit within a single institution such as a university medical center, or within a consortium of cooperating institutions with complementary research bases. In either case, the Center applications must be associated with an existing program of excellence in basic and clinical CF biomedical research. Program excellence is measured through a consistent and outstanding record of productivity and peer-reviewed research funding in CF and related research areas. To justify Center support, there must be a significant research base of NIDDK-funded investigators pursuing research activities in Center topic areas as well as investigators with other sources of peer-reviewed support. NIDDK mission-related CF research should represent at least 40% of the research base. Major themes and NIDDK mission-related CF research should be relevant to NIDDK cystic fibrosis research interests which include mechanisms underlying CF disease (excluding those associated with the specific development of lung disease), development of therapeutic approaches targeting or applicable to multiple organs, and elucidating pathophysiology and/or therapy directed at diabetes, nutritional, gastrointestinal, renal, hepatic or gall bladder complications of CF. While additional research themes outside the NIDDK mission (e.g., pulmonary complications of CF) may also be described, and these research projects may utilize Center resources, applicant institutions should be pursuing major themes directly related to NIDDK research priorities. A high level of integration and close collaboration among Center personnel from diverse scientific disciplines is an important feature of a successful CF Research and Translation Center. Centers should be established around one or more central themes or focus areas that link Center investigators and their research programs
Administrative Core with an Enrichment Program
CF Research and Translational Center applications must include an administrative core that will be responsible for allocation and oversight of Center resources. The Administrative Core must have a process to a) assess the productivity, effectiveness, and appropriateness of Center activities; b) determine criteria and selection process for Center membership; and c) foster collaborations and scientific opportunities among its members through the planning of an enrichment program. CF Research and Translational Centers are encouraged to develop a Center website to communicate available resources to the participating Institution(s). The Center Director will provide oversight for the entire Center and should have appropriate administrative experience. One or more Associate Directors should be named who will be involved in the administrative, scientific, or educational enrichment efforts of the Center and who will serve as Acting Center Director in the absence of the Director. A process must be in place that would be used to recommend a successor to the Director, if needed.
The CF Research and Translation Core Center enrichment program must be designed to advance translational research in CF and promote scientific exchange among investigators with research interests in these topic areas, and to enhance interactions between CF researchers and investigators from other fields with relevant expertise. The enrichment program can support activities such as seminars, guest speakers, visiting scientists, consultants, and workshops.
Enrichment of the postdoctoral experience so as to better conduct research in cystic fibrosis is an associated activity of a CF Research and Translation Core Center. While stipends/salaries for fellows cannot be funded from Center funds, the establishment of a Center should provide an enhanced environment for research educational enrichment. Just as in the case of funding for individual research projects, funding for fellowships to increase the critical mass of early-stage investigators in CF research is strongly encouraged and should be sought from NIH NRSA, institutional training grants (e.g., T32, T35) and individual fellowships (e.g., F30, F32), and other sources such as private foundations, and commercial companies. No formal training support is provided by a CF Center grant.
- Biomedical Research Cores
CF Research and Translation Centers are designed around biomedical research cores that provide shared resources for essential services, techniques, or instrumentation to Center participants enabling them to conduct their funded individual research projects more efficiently and/or more effectively. Cores provide specialized technologies and expertise needed to accomplish the stated goals of the Center toward the development of therapies for CF. Each core should provide services to multiple funded research projects. Centers may propose either Institutional Cores or Expanded Cores. Whereas Institutional Cores support research at a single institution or set of cooperating Institutions, Expanded Cores serve specific scientific communities on a regional, national, or international level and should be based on unique expertise or technology available at the domestic Center. Expanded Cores are physically located within a funded United States Cystic Fibrosis Research and Translation Center and are located within the United States. A category of research base for Cores that are used as an Expanded Core should be considered the "extended research base." The extended research base for an Expanded Core could include all investigators who might expect to use the core in some way that are not located at the specific domestic Center. This might include investigators who would be expected to fully compensate the core service through a charge-back, and thus would not be obtaining direct financial assistance from the Center. The list could include investigators who use the core services but otherwise have no collaborative interactions with other Center investigators. The extended research base should be defined as an entity separate from the institutional research base. For review purposes, it will be evaluated as part of the Expanded Core, in order to distinguish it from the local institutional research base. Examples of types of biomedical core resources that would be considered responsive to this Funding Opportunity Announcement include:
- Collection, analysis, storage and distribution of data and samples;
- Provision of specialized tools and technologies or access to specialized expertise;
- Development, standardization and distribution of reagents and/or protocols;
- Recruitment of patients and coordination of patient studies;
- Development, beta-testing and dissemination of specialty assays, methods, and services on an Institutional or national level;
- Sharing of specialized tools, technologies and expertise among investigators.
In responding to this NOFO, applicants are encouraged to propose cores that address specific objectives based on the unique requirements of investigators at the applicant institution(s). Particular emphasis should be placed on services that support and foster interdisciplinary, integrated and translational approaches to research in the Center topic areas. Preference will be given to core support services that are not readily available or cost-effective when supplied from commercial sources, and techniques or technologies that may be technically challenging or require specialized expertise, equipment or infrastructure. Proposed Cores should not focus on pulmonary aspects of CF.
The need for core support from the CF Research and Translation Center must be well-justified, with a broad user base of NIDDK and other NIH-funded investigators pursuing research activities in Center topic areas, as well as CF investigators with other sources of peer-reviewed support. Participants in the CF Research and Translation Center program are encouraged to become fully integrated into, and synergistic with, other NIDDK- and NIH-funded Centers within their institutional setting. This includes the clinical research sites established by the Clinical and Translational Science Awards supported by the National Institutes of Health/NCATS ( https://ncats.nih.gov/research/research-activities/ctsa ) related NIH Roadmap activities, other NIH or IC clinical activities and any related NIDDK-funded Center programs such as the Nutrition Obesity Research Center (NORC) Program ( http://www.norccentral.org/ ), the NIDDK Diabetes Research Center (DRC) Program ( https://www.diabetescenters.org/ ), the Centers for Diabetes Translation Research (CDTR) ( https://www.diabetes-translation.org ) or the Digestive Diseases Research Centers. (https://www.niddk.nih.gov/research-funding/research-programs/digestive-disease-centers ).
Arrangements for sufficient space for core activities or for access to appropriate established facilities should be made. Centers are strongly encouraged to enter into cooperative arrangements with cores already established within their institution, or with other Centers in close proximity, when existing cores offer the services needed. These arrangements are important whenever greater efficiency or cost savings can be realized by such an agreement. However, it should be clear that the CF Research and Translation Center cores can function independently. It may be advantageous for a CF Research and Translation Center to provide support for appropriate personnel to work specifically with Center members in an existing facility/core (e.g., transgenic animal core) at the institution. In this case, the designated CF Research and Translation Center Core Director should work closely with the parent facility core Director to coordinate services, unless the same individual assumes both roles.
Pilot and Feasibility Program
The CF Research and Translation Core Center Pilot and Feasibility (P&F) program provides seed support for new and innovative research projects directed at basic biomedical, clinical and translational research questions relevant to cystic fibrosis complications in the mission of NIDDK. To be considered a viable P&F program, the Center must support a minimum of two pilot projects annually, up to a maximum of four pilot projects. It is anticipated that up to $75,000 in direct costs per year for up to two years will be provided for the majority of approved P&F projects. Efforts to increase the number of P&F awards and availability of funds for the program through the use of program income or alternative funding sources are particularly encouraged.
The P&F program is particularly directed at new and early stage investigators and established investigators new to CF research. Established CF investigators pursuing high impact/high risk projects that are a significant departure from their usual work are also eligible for support under the CF Research and Translation Core Center P&F program. P&F support is also encouraged for investigators underrepresented in research. This Program should be integrated into the overall research goals of the Center and make use of the resources provided by the Cores. P&F programs may also be structured to provide support for establishing interdisciplinary collaborations and to help forge new partnerships between basic scientists and clinical researchers. In general, NIDDK expects CF Research and Translation Centers to solicit investigators at affiliated hospitals or institutions to participate in the Center P&F program. While the distribution of P&F funds to be used in each award category is ultimately at the discretion of the Center P&F committee, it is expected that the Center P&F program will, where possible, place particular emphasis on funding innovative clinical and translational research projects. The focus of new P and F projects should be within the NIDDK mission. All new P and F projects will require prior approval by NIDDK.
CF Research and Translation Centers Directors Meetings
NIDDK is planning to convene a meeting every other year for Center Directors and Associate Directors. This meeting will allow exchange of information on available Core activities and advances between CF Research and Translation Centers.
See Section VIII. Other Information for award authorities and regulations.
Investigators proposing NIH-defined clinical trials may refer to the Research Methods Resources website for information about developing statistical methods and study designs.
Section II. Award Information
Grant: A financial assistance mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
The OER Glossary and the How to Apply - Application Guide provides details on these application types. Only those application types listed here are allowed for this NOFO.
Optional: Accepting applications that either propose or do not propose clinical trial(s).
NIDDK intends to commit $6.2 million in FY 2025 to fund 5 awards.
Application budgets are limited to $750,000 direct costs per year. Equipment costs (only allowed for first year) and indirect costs on subcontracts are not included in the $750,000 direct cost cap. Equipment requests are capped at $100,000 and may be limited by available funds. It is anticipated that the award budget will be directly correlated to the breadth, quality and relevance to Cystic Fibrosis of the research base being served by the Center.
The maximum project period may not exceed 5 years.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. Eligible Applicants
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Local Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
Federal Governments
- Eligible Agencies of the Federal Government
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Non-domestic (non-U.S.) Entities (Foreign Organization) are not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement , are not allowed.
Applicant organizations
Applicant organizations must complete and maintain the following registrations as described in the How to Apply- Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission, please reference NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications for additional information.
- NATO Commercial and Government Entity (NCAGE) Code – Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- Unique Entity Identifier (UEI) - A UEI is issued as part of the SAM.gov registration process. The same UEI must be used for all registrations, as well as on the grant application.
- eRA Commons - Once the unique organization identifier is established, organizations can register with eRA Commons in tandem with completing their Grants.gov registration; all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov – Applicants must have an active SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from diverse backgrounds, including underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for NIH support. See, Reminder: Notice of NIH's Encouragement of Applications Supporting Individuals from Underrepresented Ethnic and Racial Groups as well as Individuals with Disabilities, NOT-OD-22-019 .
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the How to Apply - Application Guide .
Because a Cystic Fibrosis Research Center has a large and complex administrative structure, the PD/PI must have strong leadership abilities and demonstrated proficiency in managing large, multi-component programs. In addition, the PD/PI should be a recognized leader and authority in the CF research community. The PD/PI must be willing to participate in meetings of the CF Center Directors which are scheduled in years 2 and 4 of the grant.
2. Cost Sharing
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement Section 1.2- Definitions of Terms.
3. Additional Information on Eligibility
Number of Applications
Only one application is allowed per Institution.
The NIH will not accept duplicate or highly overlapping applications under review at the same time per NIH Grants Policy Statement Section 2.3.7.4 Submission of Resubmission Application . This means that the NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review (see NIH Grants Policy Statement 2.3.9.4 Similar, Essentially Identical, or Identical Applications ).
Research Base: Successful CF Research and Translation Center applications require an existing program of excellence in biomedical research in Cystic Fibrosis. To justify Center support, the CF Research and Translation Center must serve a large research base of NIDDK and other NIH-funded investigators pursuing research activities in Center topic areas, as well as CF investigators with other sources of peer-reviewed support. NIDDK mission-related CF research should represent at least 40% of the research base. NIDDK mission-related CF research interests include mechanisms underlying CF disease (excluding those associated with the specific development of lung disease), development of therapeutic approaches targeting or applicable to multiple organs, and elucidating pathophysiology and/or therapy directed at diabetes, nutritional, gastrointestinal, renal, hepatic or gall bladder complications of CF. While additional research interests outside the NIDDK mission (e.g., pulmonary complications of CF) may also be described, and these research projects may utilize Center resources, applicant institutions should be pursuing major efforts directly related to NIDDK research priorities.
Section IV. Application and Submission Information
1. Requesting an Application Package
The application forms package specific to this opportunity must be accessed through ASSIST or an institutional system-to-system solution. A button to apply using ASSIST is available in Part 1 of this NOFO. See the administrative office for instructions if planning to use an institutional system-to-system solution.
2. Content and Form of Application Submission
It is critical that applicants follow the Multi-Project (M) Instructions in the How to Apply - Application Guide , except where instructed in this notice of funding opportunity to do otherwise and where instructions in the How to Apply - Application Guide are directly related to the Grants.gov downloadable forms currently used with most NIH opportunities. Conformance to the requirements in the How to Apply - Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
Letter of Intent
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows IC staff to estimate the potential review workload and plan the review.
By the date listed in Part 1. Overview Information , prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed activity
- Name(s), address(es), and telephone number(s) of the PD(s)/PI(s)
- Names of other key personnel
- Participating institution(s)
- Number and title of this funding opportunity
The letter of intent should be sent to:
John Connaughton, Ph.D. Chief, Scientific Review Branch National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Telephone: 301-594-7797 Email: [email protected]
Page Limitations
All page limitations described in the How to Apply- Application Guide and the Table of Page Limits must be followed.
Instructions for the Submission of Multi-Component Applications
The following section supplements the instructions found in How to Apply- Application Guide and should be used for preparing a multi-component application.
The application should consist of the following components:
- Overall: required
Administrative Core
- P and F Program
Overall Component
When preparing the application, use Component Type ‘Overall’.
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions, as noted.
SF424(R&R) Cover (Overall)
Complete entire form.
PHS 398 Cover Page Supplement (Overall)
Note: Human Embryonic Stem Cell lines from other components should be repeated in cell line table in Overall component.
Research & Related Other Project Information (Overall)
Follow standard instructions.
Project Summary/Abstract: Describe the scientific theme(s) of the CF Research and Translation Center and the need for a Center to support the investigators in the research base. Include the number of Center members and the overall direct costs present in the research base. Provide a brief overview of the research base as it relates to the theme(s) of the Center, as well as an overview of the biomedical research cores, and the pilot & feasibility and enrichment programs.
Project Narrative In 1-3 sentences describe the relevance of the research to be supported and facilitated by Center activities (Core services; Pilot and Feasibility, and Enrichment programs) on Cystic Fibrosis and public health.
Facilities and Other Resources: Describe the existing environment and facilities briefly in the context of how the Center will use or change existing access, space, and usage; include space maps as needed (see "Other Attachments") and letters of institutional commitment (see 'Letters of Support'). Scientific personnel and institutional resources capable of supporting the research base must be available.
Equipment: A general listing of major, shared pieces of equipment to be used by Center members should be provided. Note: Specific research core facilities, equipment and special resources should be listed in each proposed biomedical research core component.
Other Attachments: The following "Other Attachments" must be included with the Overall Component in order to aid in the review of applications. The filename provided for each attachment will be the name used for the bookmark in the application image. All attachments need to be in .pdf format.
Grant Support: Please title this attachment "Grant Support" and include all Federal and non-federal grant support for CF Research and Translation Center members. Complete and organize alphabetically by the last name of the Center Investigator who is listed as the Project Director/Principal Investigator (PD/PI) on the grant. Include Supporting Organization/Grant Numbers, Complete Grant Title, Project Period, Annual Direct Costs, and Identify Other NIDDK Centers (if the grant listed is also included in its research base). The attachment should include, in order: Current Grant Support (Table A.1), Other Grant Support (Table A.2) and Pending Grant Support (Table A.3). Examples of how Tables A.1, A.2 and A.3 might be formatted are provided for applicant assistance with this requirement ( https://www.niddk.nih.gov/research-funding/process/apply/funding-mechanisms/p30/cystic-fibrosis-research-translation-center-application-resources ). The 'Current Grant Support' table should include research project grants relevant to Cystic Fibrosis research, which are considered to be potential or likely users of the core services. 'Other Grant Support' should include infrastructure/services grants (other Centers or CTSA awards), training (such as T- or F-awards) grants, and other such grants that support CF-related research but would not be direct users of the core services.
Biographical Sketches of Center Research Base Investigators: Please title this attachment "Center Member Biographical Sketches." Provide biographical sketches for all Center members, as defined by the Center within the application, and organize them alphabetically by the last name of the Research Base Investigator, including any affiliated hospitals or proposed partners. These biographical sketches should use the NIH format. Description of Center Research Base Investigators: Please title this attachment Description of Center Research Base Investigators and organize alphabetically by Center Member (last name). Provide a narrative description of no more than one page per research base investigator. These narratives should include: the active grant number(s), title(s), and a few descriptive sentences of the investigator research projects, as well as a brief description regarding what aspect of the investigator research justifies the use of Center core facilities. In the description of the research base, include ONLY those grants awarded, or subcontracted, to investigators at the applicant institution or consortium, not to investigators at other locations. It is particularly important to provide a few sentences indicating the relatedness of a cited grant to research in cystic fibrosis when this is not readily apparent from the project title of the grant.
Center Collaborations: Please title this attachment Center Collaborations and organize alphabetically by Center Member (last name, first name). List all Center Members. Provide primary Department Affiliation, key words for research interests, names of other Center members who are collaborators (through publications, grants or research projects), and the number of collaborative publications (only those relevant to the CF Center).
Relation to Overall Center: Please title this attachment "Relation to Overall Center". Provide Charts and Tables related to the Institutional Commitment at the applicant institution, such as an organizational chart(s) to illustrate the structure, interactions, and leaders of the institution and CF Research and Translation Center.
Project/Performance Site Locations (Overall)
Enter primary site only.
A summary of Project/Performance Sites in the Overall section of the assembled application image in eRA Commons compiled from data collected in the other components will be generated upon submission.
Research and Related Senior/Key Person Profile (Overall)
Include only the Project Director/Principal Investigator (PD/PI) and any multi-PDs/PIs (if applicable to this NOFO) for the entire application.
A summary of Senior/Key Persons followed by their Biographical Sketches in the Overall section of the assembled application image in eRA Commons will be generated upon submission.
Budget (Overall)
The only budget information included in the Overall component is the Estimated Project Funding section of the SF424 (R&R) Cover.
A budget summary in the Overall section of the assembled application image in eRA Commons compiled from detailed budget data collected in the other components will be generated upon submission.
PHS 398 Research Plan (Overall)
Specific Aims: Describe the broad long-term objectives of the proposed CF Research and Translation Center. A strategic vision, theme and set of goals must be developed and described in the application. The plan should outline the existing skills and technologies available in the research base as well as other resources at the Institution(s). Provide a brief overview of the research base as it relates to the theme(s) of the Center, as well as a brief overview of the biomedical research cores, and the pilot & feasibility and enrichment programs. This plan should delineate how the Center will enhance ongoing projects, assist in the development of new projects, respond to future opportunities, and promote collaborations toward developing new treatments for CF.
Research Strategy: The Research Strategy should include the following sections instead of the ones listed in the application instructions.
Research Base :
The Center Core grant provides a mechanism for fostering interdisciplinary cooperation within a group of established investigators conducting high quality research on Cystic Fibrosis. Therefore, existence of a strong, substantial research base in CF is a fundamental requirement for, and the most important aspect of, the establishment of a Center. NIDDK mission-related CF research should represent at least 40% of the research base. NIDDK mission-related CF research includes mechanisms underlying CF disease (excluding those associated with the specific development of lung disease), development of therapeutic approaches targeting or applicable to multiple organs, and elucidating pathophysiology and/or therapy directed at diabetes, nutritional, gastrointestinal, renal, hepatic or gall bladder complications of CF. Applicants should describe the approach used in determining that their NIDDK mission-related CF research is at least 40% of the research base as well as the results of this analysis.
Applicants should include an overview of current research in CF and related areas being conducted at their institution in sufficient detail to allow reviewers to judge its extent and the interrelationship of ongoing research. The relevance to CF of all research included in the research base should be described. Applicants should indicate how the establishment of a Center will provide added dimensions, such as greater focus and increased cooperation, communication and collaboration that would not likely occur without Center resources.
A high level of integration and close collaboration among Center personnel from diverse scientific disciplines is an important feature of a successful CF Research and Translation Center. Accordingly, the applicant should clearly state considerations for Center membership with specific reference to the potential of members to form interactive, collaborative and synergistic relationships. Criteria for becoming a CF Research and Translation Center member should be clearly defined. Each Center, however, is expected to formulate these definitions based on its own situation. Center membership and affiliation are often open to those individuals who would like to promote the mission of the Center. Specific membership criteria, and any affiliation categories (if applicable), should be clearly defined by the Center Director in order to better organize and facilitate the focus of the Center's mission. Subsets of members based on their degree of participation or other quantitative measures are acceptable. Applicants should provide clear guidelines for a) how Center membership(s) is (are) defined; b) the application and selection processes for Center membership; and c) the obligations of Center membership. Suitable criteria include, but are not limited to, peer-reviewed independent funding, participation in CF-related research, and the need for the use of core facilities. All research base investigators should be Center members. Designation as a Center member without the need for the use of core facilities must be well justified.
Presentation of the research base in the application should be done in two ways: (1) by completing a Table (see "Other Attachments" (Grant Support, Biographical Sketches of Center Research Base Investigators, and Description of Center Research Base Investigators)) and (2) by a full narrative description of the CF and related research activities at the applicant institution and any collaborating institutions. This presentation should be organized into several areas of emphasis that demonstrate the research focus of the Center. The research of each Center participant should be discussed and interrelationships of research being conducted by Center participants should be highlighted. Since most, if not all, of the research base will have undergone separate peer review, the quality of the individual funded projects is already established. The more important aspects are: (a) interactions and interrelationships of the research efforts; (b) uses and benefits of core services; (c) plans to develop productive collaborations among Center investigators; and (d) willingness of Center investigators to contribute to the overall objectives of the CF Research and Translation Center.
Strategic Vision :
Theme- Provide the central theme(s) of the CF Research and Translation Center and the likely supported research, resources, and relevance to CF. The theme may be broad or focused, depending upon the goals of the Center. For clearer presentation, it is recommended that Center applicants subdivide the research base into areas of research emphasis or central research themes that link Center members and their research programs. Appropriate presentation of the research base is very important since its assessment is a primary emphasis in the evaluation of an application. Major themes should be relevant to NIDDK research interests related to cystic fibrosis which include mechanisms underlying the disease (excluding those associated with the specific development of lung disease), development of therapeutic approaches targeting or applicable to multiple organs, and elucidating pathophysiology and/or therapy directed at diabetes, and/or nutrition, gastrointestinal and liver complications of CF. While additional research themes outside the NIDDK mission (e.g., pulmonary complications of CF) may also be described and these research projects may utilize Center resources, applicant institutions should be pursuing major themes directly related to NIDDK research priorities.
Goals and Directions- Describe the current and future directions for the CF Research and Translation Center in the forthcoming project period. Indicate how the research supported by the Center impacts the understanding of CF and its complications. Describe the short, mid- and long-term goals and measures of success. Describe the likely advances expected in the field of CF. Describe the expected, widely-applicable research tools and scientific advances that will emerge from the Center emphasis. Describe any basic science work that has successfully been translated to the bedside or plans to enhance that translation in the next project period. Outline the impact of interdisciplinary studies and collaborations, especially among basic scientists and clinical researchers. Renewal applications must also describe the accomplishments of the Center in the preceding project period and how it intends to build upon its successes. These accomplishments should be presented, as appropriate in the areas of basic science and clinical research. The impact of Center-based science should be discussed in detail. Since one of the objectives of the Center is to extend research relevant to CF, new areas of research and acquisition of new funding should be highlighted in the overview. The progress and accomplishments in the research base, to development of multidisciplinary, collaborative, and cooperative interrelationships, and to alteration in the original Center design in order to meet the evolving needs of the research base should be discussed.
Biomedical Research Cores should be discussed and their role in the Strategic Vision described. Summarize the services and resources provided by the CF Research and Translation Center; describe how the biomedical research cores will address the scientific needs of the research base. Brief examples of ongoing or planned research should be discussed as appropriate with reference to the supporting Core. Proposed Cores should not focus on pulmonary aspects of CF.
Integration of investigators with multiple skills and talents- Outline steps the CF Research and Translation Center will take to promote interdisciplinary studies and collaborations, especially among basic scientists and clinical researchers. Describe the types of initiatives that will stimulate the teams and attract high-caliber professionals. Applicants should describe any academic and research partnerships that have been or will be pursued in order to advance the goals and mission of the Center.
Building research capacity- Provide details on the special talents and resources that will be drawn to and built upon at the CF Research and Translation Center. Indicate how these talents will be harnessed and used to promote new collaborations and produce multidimensional teams to address more complex questions. Describe academic and research partnerships that will be pursued by the CF Research and Translation Center to advance its goals and missions. Describe how the P and F program will help build research capacity. The focus of new P and F projects should be within the NIDDK mission. All new P and F projects will require prior approval by NIDDK.
Provide a plan to determine the need for services and instrumentation of the Center. Address the steps that will ensure that the CF Research and Translation Center proceeds at the cutting edge of technology and concepts. It is expected that biomedical research cores needs may change with time. Include information on the process of re-evaluation of needs and implementation of changes.
Within this section, describe the research capacity and clearly identifiable major scientific focus in CF research. The CF Research and Translation Center initiative fosters interdisciplinary cooperation among established investigators conducting high-quality research related to CF. Therefore, existence of a strong research capability in CF is fundamental to establishment of a new, or continuation of an existing, CF Research and Translation Center.
Innovation :
Address how the CF Research and Translation Center will not only evolve with the science conducted by the Center Investigators, but also challenge and seek to advance or change current research or clinical practice paradigms by using novel theoretical concepts, approaches or methodologies, instrumentation, or interventions. Explain how the synergy of the Center with the research base will lead to novel services and resources in the Cores and their application to important questions in CF research. Describe the potential for interdisciplinary collaborations among Center Investigators. For a Biomedical Research Core that by its nature is not innovative, describe how it is essential to advance the field.
Summarize the services and resources provided by the Center, and how they are managed and coordinated. Describe how the Center will address the scientific needs of the research base. Indicate if any of the proposed cores will utilize or expand cores already existing at the institution. Describe how the proposed Center will leverage existing resources and fill gaps in the services available. Also, describe how the Center will enhance the research base through enrichment activities.
Leveraging of existing resources is encouraged, particularly when this provides a range of services or efficiency that would not otherwise be available. Furthermore, applicants should demonstrate that support for the existing resource through the CF Research and Translation Center provides added value to the resource beyond that which would be provided by paying for use of the resource through a fee for service. Applicants from institutions that have a Clinical Translational Science Award (CTSA) funded by the NIH may wish to identify the CTSA as a resource for conducting the proposed research, if appropriate. In such cases, appropriate letters of support from the CTSA program director or principal investigator should be included with the application detailing plans for appropriate integration and synergy of the CF Research and Translation Center and CTSA activities (See Letters of Support).
In addition, applicants should address the potential for integration, harmonization, and enhancement of CF Research and Translation Center activities through cooperation with other NIH- supported core facilities at the applicant institution. Applicants are encouraged to suggest coordinated efforts, such as enrichment programs or pilot and feasibility programs. Other NIH-supported Centers and associated Cores at the institution should be identified, and assurances provided that overlap or redundancy in Center activities will be avoided unless expressly required to fulfill the mission of the CF Research and Translation Center.
For new applications: Emphasize the anticipated impact of the establishment of a CF Research and Translation Center on the research base. Include an indication of how the establishment of a CF Research and Translation Center will provide added dimensions and new opportunities for CF and related research, along with increased cooperation, communication, and collaboration among investigators.
For renewal applications: Briefly, describe the progress in the research base during the previous project period including development of multidisciplinary, collaborative, and cooperative interrelationships, and any alterations in the original Center design in order to meet the evolving needs of the research base. These accomplishments should be presented, as appropriate, in the areas of basic science, clinical research, public health, prevention, and translation. Since one of the objectives of the Center is to extend research relevant to CF, new areas of research and acquisition of new funding should be highlighted in the overview.
Progress Report Publications List (renewal applications only): List the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, and other printed materials that have resulted from the project since it was last reviewed competitively. This should be done by using the Consolidated Publications List (Table G) ( https://www.niddk.nih.gov/research-funding/process/apply/funding-mechanisms/p30/cystic-fibrosis-research-translation-center-application-resources ) which assists applicants with documenting the contribution of individual cores to peer-reviewed publications by the research base. Only list publications once. Within individual biomedical research core descriptions, refer to publications associated within the core by number as listed in the table of publications. Use an asterisk to indicate any publication that fails to cite Center grant support.
Letters of Support: Include any letters of support for the proposed Center by appropriate institutional officials. Letters should address the commitment of the parent organization, or any of its partners, to the CF Research and Translation Center and its goals. The parent institution is expected to recognize the CF Research and Translation Center as a formal organizational component and provide documented evidence of space dedicated to the needs of the Center, protected time to devote to Center activities, staff recruitment, dedicated equipment, or other financial support for the proposed Center. The parent institution should provide assurance of its commitment to continuing support of the CF Research and Translation Center in the event of a change in directorship and a well-defined plan for this eventuality should be in place. In addition, it is expected that the Institution will support the goal of providing to Center members priority access to Institution and Center facilities and services at minimal or reduced cost. Both the institution and pertinent departments must show a strong commitment to supporting the Center.
A letter from the PD/PI of any related NIH-funded T32 at the Center institution should be included that acknowledges and details how the PD/PI of the T32 intends to promote cohesive interactions between the two programs.
If collaborations are being developed between the CF Research and Translation Center and the local CTSA staff and/or research personnel, a letter of agreement from the CTSA PD(s)/PI(s) should be included. For any collaborations between the CTSA and a specific Biomedical Research Core, the letter of support should be included within the specific Biomedical Research Core component of the application.
Resource Sharing Plan : Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the How to Apply - Application Guide .
Other Plan(s):
All instructions in the How to Apply- Application Guide must be followed, with the following additional instructions:
- All applicants planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of direct costs requested for any one year, must address a Data Management and Sharing Plan.
Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in How to Apply- Application Guide ; any instructions provided here are in addition to the How to Apply - Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Overall)
When involving human subjects research, clinical research, and/or NIH-defined clinical trials follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, there must be at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record within the application. The study record(s) must be included in the component(s) where the work is being done, unless the same study spans multiple components. To avoid the creation of duplicate study records, a single study record with sufficient information for all involved components must be included in the Overall component when the same study spans multiple components.
Study Record: PHS Human Subjects and Clinical Trials Information
All instructions in the How to Apply - Application Guide must be followed.
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the How to Apply- Application Guide must be followed.
PHS Assignment Request Form (Overall)
All instructions in the How to Apply- Application Guide must be followed.
When preparing your application, use Component Type ‘Administrative Core’.
All instructions in the How to Apply- Application Guide must be followed, with the following additional instructions, as noted.
Note: Effective for due dates on or after January 25, 2023, the Data Management and Sharing Plan will be attached in the Other Plan(s) attachment in FORMS-H application forms packages. If required, the Data Management and Sharing (DMS) Plan must be provided in the Overall component.
SF424 (R&R) Cover (Administrative Core)
Complete only the following fields:
- Applicant Information
- Type of Applicant (optional)
- Descriptive Title of Applicant’s Project
- Proposed Project Start/Ending Dates
PHS 398 Cover Page Supplement (Administrative Core)
Enter Human Embryonic Stem Cells in each relevant component.
Research & Related Other Project Information (Administrative Core)
Human Subjects: Answer only the ‘Are Human Subjects Involved?’ and 'Is the Project Exempt from Federal regulations?’ questions.
Vertebrate Animals: Answer only the ‘Are Vertebrate Animals Used?’ question.
Project Narrative: Do not complete. Note: ASSIST screens will show an asterisk for this attachment indicating it is required. However, eRA systems only enforce this requirement in the Overall component and applications will not receive an error if omitted in other components.
Other Attachments: (renewal applications only; optional): Additional information related to the Center-supported Enrichment Program activities, such as agendas/announcements for Cystic Fibrosis Research and Translation Center retreats, symposia, workshops, meetings, specialized courses, seminar series, etc., illustrating the interactions among Center members and other investigators, as well as other educational opportunities may be included in the application. This should be loaded as a file in .pdf format titled "Enrichment Program".
Project /Performance Site Location(s) (Administrative Core)
List all performance sites that apply to the specific component.
Note: The Project Performance Site form allows up to 300 sites, prior to using additional attachment for additional entries.
Research & Related Senior/Key Person Profile (Administrative Core)
- In the Project Director/Principal Investigator section of the form, use Project Role of ‘Other’ with Category of ‘Project Lead’ and provide a valid eRA Commons ID in the Credential field.
- In the additional Senior/Key Profiles section, list Senior/Key persons that are working in the component.
- Include a single Biographical Sketch for each Senior/Key person listed in the application regardless of the number of components in which they participate. When a Senior/Key person is listed in multiple components, the Biographical Sketch can be included in any one component.
- If more than 100 Senior/Key persons are included in a component, the Additional Senior Key Person attachments should be used.
- The P30 Center Director PD/PI must be the Administrative Core Director. The Center Director PD/PI should be an experienced and respected scientist with a proven track record for obtaining NIH funding. She/he must be able to coordinate, integrate, and provide guidance in the establishment of new programs in CF research. If there are multiple PD/PIs, then these must be Co-Administrative Core Directors.
- In this component, also provide biographical sketches for any consultants. For renewal applications only, provide biographical sketches for the members of the External Advisory Committee. These biographical sketches should use the NIH format. In the additional Senior/Key Profiles section, list those Senior/Key persons in the Project Role of "Other" with Category of "Consultant" or "Advisory Committee." New applications should NOT contact potential External Advisory Committee (EAC) members nor provide names or biographical sketches for EAC members; overall qualifications and areas of scientific expertise for the EAC members may be included.
Budget (Administrative Core)
Budget forms appropriate for the specific component will be included in the application package.
Personnel: The Center Director must provide an at least 1.2 person month effort on the Administrative Core and a total of 2.4 person months effort distributed among the Administrative and other components of the Center. In a multiple-PD/PI application, the combined effort of the PD/PIs must equal 2.4 person months. Where possible and applicable, CF Research and Translation Centers are encouraged to leverage administrative resources in other NIH funded centers (e.g., Diabetes Research Centers, Centers for Diabetes Translation Research, Nutrition Obesity Research Centers, and Clinical and Translational Science Awards) to maximize efficiency of resources.
Travel: Include the costs of domestic and foreign travel only if the travel is directly related to the activities of the CF Research and Translational Center, including travel for external advisors and guest speakers. Include travel costs for the Center Director and Associate Director(s) to attend the CF Research and Translation Center Directors meetings in Bethesda, Maryland in years 2 and 4.
Equipment: If pieces of specialized equipment, or computers, costing more than $5,000 are requested, the application must identify similar equipment already available within the institution and provide a clear justification for purchase based on core service provided to CF Research and Translation Center investigators. Requests for general-purpose equipment should be included only after ascertaining the availability of such items within the institution. Justify the request based on this availability. Equipment may only be requested in the initial budget period, are limited to $100,000 and are subject to funding availability.
Supplies: Consumable supplies directly related to the operational aspects of the Administrative Core facilities are an allowable expense.
Consultants: Include costs associated with consultants (consultant fees, per diem, teleconferences and travel) when their services are required by the CF Research and Translation Center, such as the members of the External Advisory Board.
Other Expenses: Funds for supporting a CF Research and Translation Center website may be requested. Include funds to support Enrichment Program activities such as workshops, research fora, symposia, Center retreats and seminar series. Funds for Enrichment Program-associated activities such as the printing and distribution/mailing of brochures, programs, and meeting materials, as well as posters and other advertisement materials, may be requested.
Note: The R&R Budget form included in many of the component types allows for up to 100 Senior/Key Persons in section A and 100 Equipment Items in section C prior to using attachments for additional entries. All other SF424 (R&R) instructions apply.
PHS 398 Research Plan (Administrative Core)
Specific Aims: Clearly state how the Administrative Core will set the overall direction for the Center, prioritize Center resources, provide external review of Center functions and organize enrichment activities. In addition, there needs to be an indication of how the Administrative Core interacts with the other Cores and Programs to achieve the goals of the Center and ensure optimal utilization of Center Resources.
Research Strategy: The CF Research and Translation Center must be an identifiable organizational unit within a university medical center or a consortium of cooperating institutions including the university-affiliated Center. Such a Center will involve the interaction of broad and diverse elements; thus, lines of authority and approval by the appropriate institutional officials must be clearly specified. The Administrative Core plays a key role in the coordination and functioning of the Center.
The applicant should describe how the Administrative Core will take a leadership role in setting the overall direction of the Center. In addition, direct lines of communication between the Administrative Core and Biomedical Research Cores as well as with the P&F Program should be delineated, as all of these cores/programs serve critical roles for Center integration. Procedures for reviewing the utilization, quality and necessity of Core services must be described.
Centers should describe policies and procedures for changes in core function over the life of the Center. These will allow Centers to adapt to the development of new technologies, existing technologies may no longer be needed, or economic changes such as commercial services may replace core services. Provide a plan to determine the need for services and instrumentation of the Center.
Each applicant institution specifies one or more Center Director(s) (PD(s)/PI(s)) to be responsible for the scientific and administrative leadership of the Center. If multiple Center Directors are proposed, the application must document their complementary expertise. The Director(s) should be an experienced and respected scientist with a proven track record for obtaining NIH funding. She/he must be able to coordinate, integrate, and provide guidance in the establishment of new programs in CF and related research. One or more Associate Directors should be named who will be involved in the administrative, scientific, or educational enrichment efforts of the Center and will serve as Acting Center Director in the absence of the Director. A process must be described that would be used to recommend a successor to the Director, if needed.
Applicants should describe any educational enrichment opportunities provided by the CF Research and Translation Core Center for Center participants, and document ways the Center may facilitate, enhance or foster the institutional educational enrichment environment. Specifically, Center applicants should provide information on related educational/training programs at the Center institution(s) and describe how the CF Center will help to integrate, facilitate and enhance activities of trainees.
The CF Research and Translation Core Center enrichment program must be designed to advance translational research in CF and promote scientific exchange among investigators with research interests in these topic areas, and to enhance interactions between CF researchers and investigators from other fields with relevant expertise. The enrichment program can support activities such as seminars, guest speakers, visiting scientists, consultants, and workshops. The applicants should describe the enrichment program at the Center.
It is expected that organization of the Administrative Core will provide a supportive structure sufficient to ensure accomplishment of the following:
- Coordination and integration of the CF Research and Translation Center components and activities.
- Assessment of productivity, effectiveness, and appropriateness of the CF Research and Translation Center activities, assessment of scientific opportunities, and areas for collaboration among Center members.
- Develop criteria for Center membership and a process for reviewing and updating membership.
- Organization of CF Research and Translation Center enrichment activities, such as retreats, invitation of consultants, meetings, and seminars.
- Organization of the Internal and External Advisory Committees to coordinate and review Center activities.
- Recordkeeping of meeting minutes and measures of success including: use of Center facilities, publications, pilot and feasibility awards, and new grant applications resulting from preliminary data enabled by the CF Research and Translation Center.
- Interactions with other CF Research and Translation Centers, the NIDDK, and other appropriate individuals, groups, or organizations.
The administrative structure must include an Internal Advisory Committee (IAC) and an External Advisory Committee (EAC). New applications should not list members of the EAC but should describe what expertise is needed. Renewal applications must list the members of the Internal and External Advisory Committees and document their functions and effectiveness.
The final administrative structure of the Center will be left largely to the discretion of the applicant institution. However, past experience has demonstrated that the effective development of the Center programs requires close interaction between the Center Director, Core/Program Leaders, appropriate institutional administrative personnel, the staff of the awarding agency, and the members of the community in which the Center is located. Therefore, each Center applicant should establish an administrative structure that will permit the development of such interactions. Within this structure, each applicant institution must also establish a mechanism to oversee the use of funds for the proposed pilot and feasibility program. This mechanism must include the use of appropriate consultants for review from the scientific community outside the Center institution. Consultants who will serve on advisory committees should not be specifically identified in the application but the process by which they will be selected should be described. These same consultants may be utilized, if desired, for review of other activities of the Center.
Enrichment Program: The CF Research and Translation Center enrichment program should be designed to advance translational research in CF and promote scientific exchange among investigators with research interests in these topic areas, and to enhance interactions between CF researchers and investigators from other fields with relevant expertise. The enrichment program can support activities such as seminars, guest speakers, visiting scientists, consultants, and workshops. Applicants should describe any educational opportunities afforded by the CF Center for Center participants, and document ways the Center may facilitate, enhance or foster the institutional educational environment. Specifically, Center applicants should provide information on NIDDK or other NIH Institute T32 or K12 programs at the Center institution(s), and describe how the CF Center will help to integrate, facilitate and enhance activities of T32-supported trainees of K12/KL2-supported scholars.
Training postdoctoral fellows to conduct research in CF is an associated activity of a Center. While stipends for fellows cannot be funded from the Center, the establishment of a Center should provide an enhanced environment for educational and enrichment opportunities. Just as in the case of funding for individual research projects, funding for fellowships should be sought from NIH NRSA institutional training grants (e.g. T32, T35) and individual fellowships (e.g. F30, F32), and other sources such as private foundations, and commercial companies.
Letters of Support: A letter(s) from the PD/PI of any related NIH-funded T32 and/or T35 institutional training grant(s) at the Center institution should be included that acknowledges and details how the PD/PI of the T32 or T35 training grant intends to promote cohesive interactions between the two programs. Such letters should also briefly describe how any short-term (e.g. summer) medical student research programs, supported by NIH-funded T32 or T35 training grants, coordinate with Center activities related to the proposed Enrichment Program. Include any other letters of support for the proposed Core, as appropriate.
Resource Sharing Plan: Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the How to Apply- Application Guide.
Only limited items are allowed in the Appendix. Follow all instructions for the How to Apply- Application Guide ; any instructions provided here are in addition to those in the Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Administrative Core)
When involving human subjects research, clinical research, and/or NIH-defined clinical trials follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply- Application Guide, with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record.
All instructions in the SF424 (R&R) Application Guide must be followed.
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the How to Apply- Application Guide must be followed.
Biomedical Research Core
When preparing your application in ASSIST, use Component Type ‘Biomedical Research Core’. Separate 'Core' components should be created for each individual Biomedical Research Core proposed including optional Regional/National Shared Resources Cores. All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions, as noted.
A biomedical research core is a shared facility that provides a needed service to Center investigators enabling them to conduct their funded individual research projects more efficiently and/or more effectively. Cores should be designed to furnish a group of investigators with materials, techniques, determinations, instrumentations, and/or quality control to enhance research and contribute to cost-effectiveness. A recharge mechanism is acceptable to help defray costs to the Center. If such a cost recovery system is developed, a detailed charge justification must be presented. Participating Center members must also be informed to include such costs with their full budget justifications in their applications for individual grant support. Cores may be proposed to support any research activity of the Center, but usually fall into one of five categories: (1) provision of a technology that lends itself to automation or preparation in large batches; (2) complex instrumentation; (3) animal preparation, care and characterization; (4) clinical resources; and (5) service and user training. Limited developmental research is also an appropriate function of a core facility. Such activities, however, must be directly related to enhancing the function or utility of the Core. Proposed Cores should not focus on pulmonary aspects of CF.
Proposed Center research Cores may be an institutional shared research Core. In such cases, the research core support provided by the CF Research and Translation Center should be proportional to the use of the institutional research core by Center members.
SF424 (R&R) Cover (Biomedical Research Core)
Phs 398 cover page supplement (biomedical research core), research & related other project information (biomedical research core).
Facilities and Other Resources: The description of the physical arrangements, instrumentation and any other resources for the cores should be given special attention. Arrangements for sufficient space for core activities or for access to appropriate established facilities must be made. Centers are strongly encouraged to enter into cooperative arrangements with cores already established within their institution, or with other Centers in close proximity, when the existing cores offer the services needed. These arrangements are important whenever greater efficiency or cost savings can be realized by such an agreement. When proposing the use of a shared facility, details about access, fee-schedules, and prioritization of Center members' use of the shared facility must be described. It may be advantageous for a CF Research and Translation Center to provide support for appropriate personnel to work specifically for Center members in an existing facility/core (e.g., transgenic animal core) at the institution. In that case, the designated CF Research and Translation Center Core Director must work closely with the parent facility Core Director to coordinate services, unless the same individual assumes both roles.
Core Facility Use: Please title this attachment "Core Facility Use" and indicate each Core user, funded project that supports the Core use, period of Core use, services used and estimated use. Table E is provided for applicant assistance with this requirement ( https://www.niddk.nih.gov/research-funding/process/apply/funding-mechanisms/p30/cystic-fibrosis-research-translation-center-application-resources ).
Project /Performance Site Location(s) (Biomedical Research Core)
Research & related senior/key person profile (biomedical research core).
- Where appropriate, an established expert in the core activities may also be included as a consultant to the core.
Budget (Biomedical Research Core)
Personnel : A core Director must devote a minimum of 1.0 person month to the Core to ensure adequate oversight. A co-director or other professional or technical staff may be included. The salary amount charged to the CF Research and Translation Center grant must be commensurate with the time spent on Core activities and is subject to institutional and NIH salary policies. Stipends for graduate students and postdoctoral fellows are not appropriate for Biomedical Research Cores. The PD/PI must devote a total of 2.4 person months effort distributed among the Administrative and other components of the Center. In a multiple-PD/PI application, the combined effort of the PD/PIs must equal 2.4 person months.
Equipment : If specialized equipment costing more than $5,000 is requested, the application must provide a clear justification based on the Core services being provided to CF Research and Translation Center Investigators. Requests for general-purpose equipment should be included only after ascertaining the availability of such items within the institution. Justify the request based on this availability. A shared cost within the Institution may be required for equipment purchased through the Center. Requests for general-purpose equipment should be included only after ascertaining the availability of such items within the institution. Justify the request based on this availability. Equipment may only be requested in the initial year of the project period. Equipment requests are capped at $100,000 and may be limited by available funds.
Supplies : Consumable supplies directly related to the Core are an allowable expense. This includes office materials as well as laboratory supplies. The supply budgets of separately funded individual research projects must be appropriately reduced to reflect such support, thus eliminating duplication.
Research Patient Care Costs: Research patient care costs (both in-patient and out-patient) are an allowable expense. Attempts should be made to utilize existing clinical facilities, such as those supported by Clinical and Translational Science Awards (CTSAs) and individually supported beds. If the CTSA is to be used, include a letter of agreement from the PD/PI of the CTSA; such a letter (in .pdf format) may be attached as a 'Letter of support' for the appropriate Biomedical Research Core(s). Request costs relating to the clinical research efforts of CF Research and Translation Center investigators ONLY if there is no overlap with other funding. Costs already budgeted in individual projects should be appropriately reduced if such costs are to be transferred to the Center. The CF Research and Translation Center is not intended to be a facility for health care delivery. Thus, only those patient costs directly related to research activities may be charged to the Center.
Travel: Funds for Center investigators/faculty to attend national or international scientific meetings or workshops may not be requested. If well-justified and related directly to Core activities/functions, limited travel funds for Core professional staff may be requested to support travel to national scientific meetings/workshops.
Consultants : Include costs associated with consultants (e.g. consultant fees, per diem, teleconferences, and travel) when their services are required by the core.
Other Expenses: Funds for equipment maintenance/service contracts may be requested but should reflect an equivalent percentage of the service contract based on the overall use by the Center Investigators. The budget justification for any maintenance/service contracts should document usage of the equipment by Center members. Only in very rare cases should full support for a maintenance/service contract be requested, and strong justification must be provided in such cases.
PHS 398 Research Plan (Biomedical Research Core)
Specific Aims: Clearly state the aims of the Biomedical Core
Research Strategy: Cores may be based solely at the applicant institution or at multiple institutions through subcontracts. If subcontracts are to be utilized the applicant must clearly demonstrate how a cohesive and integrated operation will be ensured and describe the advantages of this approach to the performance of core functions. The Center may also provide resources for funded projects at collaborating institutions without a subcontractual arrangement with the parent institution. Proposed Center research cores may be an institutional shared research core. In such cases, the research core support provided by the CF Research and Translation Center should be proportional to the use of the institutional research core by Center members. As with other research cores, details about access and prioritization of center members to the shared research core(s) should be provided. Moreover, the applicant should document that the CF Research and Translation Center will be in a position to have some input to, and oversight of, the shared institutional core with respect to its management, planning for future changes and improvements, etc.
Expanded Cores : Centers are encouraged to propose Cores that provide unique expertise or technology to a community outside the Institution. These could be on a regional, national or an international level. An Expanded Core may define its own Center research base which is expanded to include investigators from outside the Center. It may include investigators that just use this resource or service but do not have a formal collaboration with other Center investigators. If an Expanded Core is proposed, please provide the basis for this justification.
Justification for proposing a core : The establishment and continued support of biomedical research cores within a Center are justified on the basis of use by independently funded Center investigators. The minimum requirement for establishing a core is significant usage by two or more investigators with independently-funded, peer-reviewed projects. While investigators holding awards from the Center pilot and feasibility program are appropriate users of the core facilities, their use does not contribute to justification for establishment or continued support of a core. Additionally, the minimum of two independently funded users does not in itself provide sufficient justification. Core usage should be documented in the "Core Facility Use" attachment in Other Attachments above. Please describe a justification for each proposed Core. Proposed Cores should not focus on pulmonary aspects of CF.
Recharge/program income system: A recharge system may be developed to allow investigators to utilize any core. Recharge fees are allowable budgetary items in the investigators' individual research project grants. A system of payment management/accounting must be established such that it is clear to the individual users, the institutional business office, and the NIDDK what the recharge system covers and how funds recovered are being used. Program income must be re-invested into direct support of Center-related activities and/or expenses and may not generate a profit for the Center. This will enable Center investigators to appropriately adjust the budgets on their own grants and ensure accountability. Please describe any recharge system that is used by the Center including the amount of funds received and how these funds were utilized.
Management of the Core and operational plan: The organization and proposed mode of operation of each Core should be presented. Included should be a plan for prioritizing investigator use of the Core as well as a definition of qualified users. If use by investigators outside the parent institution is proposed, the mechanism by which such investigators will apply and be evaluated and selected should be detailed. The definition of qualified users should not be too narrow. Some minor Core use could serve to entice established investigators in other scientific disciplines into CF research. Any proposed, ongoing or completed developmental efforts should be described. If the core is used to train investigators in special techniques, the mechanism for this training should be included.
Applicants should provide information on other programs supporting related resources at their institution and describe the nature of synergy and integration between the CF Research and Translation Center and these other activities. Applicants must also clearly describe how duplication or redundancies of effort, services and resources will be avoided.
Centers are strongly encouraged to enter into cooperative arrangements with cores already established within their institution, or with other Centers in close proximity, when the existing cores offer the services needed. These arrangements are important whenever greater efficiency or cost savings can be realized by such an agreement. However, it should be clear that the CF Research and Translation Center cores can function independently. It may be advantageous for a CF Center to provide support for appropriate personnel to work specifically for CF Center members in an existing facility/core (e.g., transgenic animal core) at the institution. In this case, the designated CF Center Core Director must work closely with the parent facility Core Director to coordinate services, unless the same individual assumes both roles. These arrangements are important whenever greater efficiency or cost savings can be realized by such an agreement. Therefore, financial justification such as comparative costs of other sources of proposed core services as well as plans for cost recovery from users should be detailed.
Because cooperation between CF Research and Translation Centers is also encouraged, but not required, any plans to work with one or more other established CF Research and Translation Centers may be provided along with a description of the benefit(s) to the Center being proposed. Benefits might include, but are not limited to, cost savings, ability to provide additional or improved services, or enhanced productivity by Center members.
Renewal applications: Information relative to cores in renewal applications should generally cover all of the same points as new applications. In addition, past performance and accomplishments should be described and highlighted. The effect of the service provided by a core on investigator productivity and cost effectiveness should also be addressed. In renewal applications, any changes should be carefully documented, especially modifications reflecting adaptation to new technologies and establishment of new Cores to reflect emerging areas of science or needs of the research base.
Progress Report Publication List: Core productivity and accomplishments as demonstrated by peer-reviewed research publications supported by the core should be documented; list the number(s) of the relevant publication(s) from Table G above ( https://www.niddk.nih.gov/research-funding/process/apply/funding-mechanisms/p30/cystic-fibrosis-research-translation-center-application-resources ).
Letters of Support: For Expanded Cores, include letters of support from outside investigators who would use these Cores. If a Clinical and Translational Science Awards (CTSA) is being used a letter of agreement from the PD/PI of the CTSA is required. This letter in .pdf format should be attached as a "Letter of support" for the appropriate Biomedical Research Core. Also, provide letters to address the career potential of, and institutional commitment to junior scientists who serve as core managers/technical directors. In addition, other letters of support for the proposed Core may be included, as appropriate.
PHS Human Subjects and Clinical Trials Information (Biomedical Research Core)
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the How to Apply- Application Guide must be followed.
When preparing your application in ASSIST, use Component Type ‘P and F Program’.
All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions, as noted.
SF424 (R&R) Cover (P and F Program)
Phs 398 cover page supplement (p and f program), research & related other project information (p and f program).
Other Attachments: The following "Other Attachments" must be included with the Pilot and Feasibility Program component in order to aid in the review of applications. The filename provided for each attachment will be the name used for the bookmark in the application image. All attachments should be in .pdf format.
Pilot Project Outcomes (renewal applications only): Please title this attachment "Pilot Project Outcomes" and list all Pilot Projects supported in full, or in part, by the CF Research and Translation Center. Provide information on the most recent 5 or, if applicable, 10-year period. Include the years funded, awardee, dates and amount of P&F funding, pilot project title, P&F award type (i.e. new investigator; established investigator), number of abstracts and publications derived from pilot support, subsequent grants funded or pending applications (including grant number/funding agency and project period), and whether the P&F awardee is still involved in CF research. Table F is provided for applicant assistance with this requirement ( https://www.niddk.nih.gov/research-funding/process/apply/funding-mechanisms/p30/cystic-fibrosis-research-translation-center-application-resources ).
Pilot Project Summary/Abstract (all applications): Please title this attachment "Pilot Project Information" and provide a Project Summary/Abstract for each proposed pilot and feasibility project, as well as the biographical sketch of the investigator for each of the proposed pilot and feasibility projects.
Project /Performance Site Location(s) (P and F Program)
Research & related senior/key person profile (p and f program).
- The P and F program must have a director who is an established investigator in cystic fibrosis research.
Budget (P and F Program)
Personnel: This category should include salary support for the P&F Program Director who must devote a minimum of 1.0 person month to ensure adequate oversight. The PD/PI must devote a total of 2.4 person months effort distributed among the Administrative and other components of the Center. In a multiple-PD/PI application, the combined effort of the PD/PIs must equal 2.4 person months. The salary amount charged to the CF Research and Translation Center grant must be commensurate with the time spent on P and F Program activities and is subject to institutional and NIH salary policies.
Consultants : Include costs associated with consultants (e.g. consultant fees/honoraria, per diem, and teleconferences) when their services are required by the Pilot and Feasibility Program, such as any external reviewers for P and F applications.
Other Expenses : Include funds to support individual Pilot & Feasibility projects. Each center must support a minimum of 2 P&F and a maximum of 4 projects which can be up to $75,000 per year each. The applicant should provide details on how F&A costs for P&F projects with partnering institutions will be handled.
PHS 398 Research Plan (P and F Program)
Specific Aims: Clearly state the aims of the Pilot and Feasibility Program.
Research Strategy: Describe the overall goals and structure of the Pilot and Feasibility Program. Describe its management plans, including both internal and external review mechanisms along with an outline of the plans for future years of the P and F Program. Also, included should be an assessment of the relevancy of the proposed individual pilot and feasibility studies and of the program as a whole to research on cystic fibrosis and to the specific goals, objectives and uniqueness of the individual Center program generally. A Pilot and Feasibility study provides modest research support for a limited time (one to two years) to enable eligible investigators to explore the feasibility of a concept related to the mission of the Center and generate sufficient data to pursue the project through other funding mechanisms. The pilot and feasibility studies are intended to: (1) provide initial support for new investigators; (2) allow exploration of possible innovative new leads or new directions for established investigators and (3) stimulate established investigators who are new to CF research to apply their expertise to research in this area. Pilot and feasibility study support is not intended for large projects by established investigators which would otherwise be submitted as separate research grant applications. Pilot and feasibility funds are also not intended to support or supplement ongoing funded research of an established investigator.
Requirements : Each Center must contain a pilot and feasibility program with a minimum of 2 projects. A maximum of 4 projects can be requested. The focus of new P and F projects should be within the NIDDK mission. All new P and F projects will require prior approval by NIDDK.
Eligibility and related guidelines: Investigators eligible for pilot and feasibility funding generally fall into three categories: (1) new investigators without current or past NIH research support (R01, P01) as a PD/PI (current or past support from other sources should have been modest); (2) established investigators with no previous work in CF who wish to apply their expertise to a problem in this area; and (3) established investigators who propose testing innovative ideas that represent a clear departure from ongoing research interests. It is expected that the majority of the investigators will fall into the first category. All eligible investigators, however, must have faculty appointments and be independent investigators. Postdoctoral fellows or their equivalents are not eligible. Each pilot and feasibility study proposal should state clearly the justification for eligibility of the investigator under one of the above three criteria.
A proposed pilot and feasibility study should present a testable hypothesis and clearly delineate the question being asked, detail the procedures to be followed, and discuss how the data will be analyzed. It must be on a topic related to the objectives of the Core Center. Projects should be focused, since funding for these studies is modest and is limited to two years or less. Any one investigator is eligible only once to receive this support, unless the additional proposed pilot and feasibility study constitutes a real departure from his/her ongoing research.
Pilot and Feasibility projects proposing clinical studies are encouraged. The National Center for Advancing Translational Sciences (NCATS) supports Clinical and Translational Science Awards (CTSA) nationwide, which provide services and resources to enhance clinical research ( https://ncats.nih.gov/research/research-activities/ctsa ). Research Centers supported by the NIDDK and other NIH Institutes and Centers are encouraged to collaborate with CTSAs to avoid duplication of effort and enhance utilization of services and resources.
Initial review and management of the pilot and feasibility program: By the very nature of this program, a significant responsibility for its management will be left to the Center administration during the project periods. The application should clearly describe and justify the pool from which potential pilot and feasibility applications will be solicited. This can be limited to investigators at the parent institution or expanded to include investigators at institutions with well-defined affiliation with the Center. Such an affiliation can occur either through a subcontractual relationship to support Core resources or through inclusion of funded projects at a collaborating institution in the research base utilizing the shared resources of the Center. The mechanisms by which information on the availability of pilot and feasibility awards will be disseminated and by which applicants will apply, undergo review, and be selected for these awards must be described and will be an important element in the review of the pilot and feasibility component of the Center.
Since pilot and feasibility studies can be awarded for any period of time up to two years, studies end at various times. In addition, the studies may also be terminated by the Center administration before their approved time limit for various reasons: e.g., (1) the investigator may receive outside funding for the project; (2) the project was found not to be feasible; (3) the investigator may leave the Center institution; etc. When this occurs, the Center may make new awards for pilot and feasibility studies with the remaining funds.
While a Center's administrative framework for management of the pilot and feasibility program is basically left up to each Center (the framework is subject to NIH peer review), certain minimal requirements must be met. There must also be a committee representing all the aspects of the Center which will assist the director in the management of the program. The major responsibilities of the director and the committee will be to:
- Maintain oversight and review of ongoing pilot and feasibility studies;
- Develop and maintain a mechanism for the oversight and review of ongoing P and F projects; this is especially important as a requirement for a second year of P and F support;
- Make recommendations regarding termination or other actions to the Center Executive Committee (or equivalent);
- Prepare and ensure appropriate distribution of announcements of the availability of pilot and feasibility funding;
- Arrange and preside over the scientific merit review of proposals. At least one reviewer from outside the parent institution must be used for each proposal. All reviewers should assign impact scores in accordance with the NIH system. Copies of all of the proposals with written documentation of their reviews, priority/impact scores, and final action must be retained by the Center;
- Maintain, insofar as is possible, a record of subsequent career events of each pilot and feasibility study recipient. This record must also be made available to reviewers at the time of the renewal application;
- Make recommendations to the Center Executive Committee (or equivalent) for final decisions. A record of actions by this committee must be documented.
- Ensure adequate institutional plans and procedures to assure compliance with applicable federal regulations and NIH policies for the protection of human research participants, including the evaluation of risks and protections in project proposals, appropriate ethical oversight of funded projects, and plans for monitoring data and safety in clinical research projects when human research subjects are involved in pilot and feasibility awards.
All applicants should describe how these requirements will be met and have been met in the case of renewal applications. Also, included should be an assessment of the relevancy of the proposed individual pilot and feasibility studies and of the program as a whole to research on cystic fibrosis and to the specific goals, objectives and uniqueness of the individual Center and of the Center program generally.
Pilot and Feasibility program in new application: Applicants should provide (see "Other Attachments") an abstract for each proposed pilot and feasibility project, and the biographical sketch of the investigator of the proposed pilot and feasibility project; these pilot and feasibility proposals are reviewed for scientific merit and eligibility by the applicant Institution's review group. These initial pilot and feasibility studies must have been reviewed by the Center in the manner proposed for review of future studies so that only those considered to be of the highest quality are included in the grant application. The amount of pilot and feasibility funds provided for the first year will be based on the review of the proposed studies. The budget for future years is recommended by the institutional review group based on the quality of the proposed pilot and feasibility studies, and the proposed method for management and review (as evidenced by this set of projects). Also considered will be the review group's evaluation of the future justification for continued pilot and feasibility support.
Pilot and feasibility program in renewal applications: Applicants should provide (see "Other Attachments") an abstract for each proposed pilot and feasibility project, and the biographical sketch of the investigator of the proposed pilot and feasibility project; these pilot and feasibility proposals are reviewed for scientific merit and eligibility by the applicant Institution's review group or process. These initial pilot and feasibility studies must have been reviewed by the Center in the manner proposed for review of future studies so that only those considered to be of the highest quality are included in the grant application.
After the initial review of pilot and feasibility proposals as described above, responsibility for review during the remainder of the project period will reside within the Center itself. After this internal review process, all new P and F projects will require prior approval by NIDDK. This approach provides each Center with the needed flexibility for effective and efficient management of the program. In renewal applications, the review of this program will be based on the past track record, the management of the program, and an assessment of overall potential needs and opportunities.
The historical overview will cover the pilot and feasibility program since the inception of the Center. This should include a list of all pilot and feasibility projects ever awarded. The pilot and feasibility program director may wish to highlight certain studies or certain aspects of the past studies. Collaborations which resulted in lasting relationships, acquisition of new skills by the study recipient, or other significant outcomes should be identified. The relationship of the scope of the various studies to that of the Center should be emphasized.
The description of Center management of the program will present in detail the current system used to manage the pilot and feasibility program, including its integration with and relationship to the rest of the administrative structure. The use of outside consultants for review should be included in the discussion. Important features of the solicitation process should be provided including the distribution and the number of respondents.
Funding levels for the pilot and feasibility program on renewal applications: The format for renewal of pilot and feasibility programs will depend on whether the applicant is requesting: (1) a number of pilot projects less than or equal to that for the previous project period, or (2) an increase in the number of pilot projects.
If the applicant wishes to maintain the same number of pilot projects in a renewal application, the recommendation of the NIH review group will be based on the overall performance of the center's pilot and feasibility program as documented in the application. This recommendation will be based on: (1) the extent to which awarded funds were fully utilized during the previous project period; (2) awards were made to investigators who fully met the eligibility criteria for pilot and feasibility support as outlined above; (3) Center-relatedness; and (4) success of previously supported pilot and feasibility studies (e.g., publications, subsequent independent R01 or other peer-reviewed support, and/or attraction of new investigator into Center related research).
Conversely, should the applicant institution feel that an increased level of funding for the pilot and feasibility program is justified, new pilot and feasibility studies, over and above the number currently awarded, must be submitted with the renewal applications. These proposals would be reviewed by the NIH review group in a fashion similar to the review of pilot and feasibility studies during the initial review. The NIH review group would assess the new proposals, along with the overall performance of the program during the previous grant period to arrive at a recommendation for a possible increased pilot and feasibility funding level.
Letters of Support: Include any letters of support for the Pilot and Feasibility Program by the appropriate institutional official at partnering organizations if applicable.
PHS Human Subjects and Clinical Trials Information (P and F Program)
For applications proposing P and F Programs that will accept projects that include Delayed Onset Human Subjects Research or Delayed Onset Clinical Trials, provide a plan to address the protection of human research participants, including the evaluation of risks and protections in project proposals, appropriate ethical oversight of funded projects, and plans for monitoring data and safety in clinical research projects.
3. Unique Entity Identifier and System for Award Management (SAM)
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
4. Submission Dates and Times
Part I. contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies) using ASSIST or other electronic submission systems. Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in How to Apply- Application Guide.
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review .
6. Funding Restrictions
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost.
7. Other Submission Requirements and Information
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
For information on how applications will be automatically assembled for review and funding consideration after submission, refer to: http://grants.nih.gov/grants/ElectronicReceipt/files/Electronic_Multi-project_Application_Image_Assembly.pdf .
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply - Application Guide . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) and component Project Leads must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile form . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review and responsiveness by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH. Applications that are incomplete, non-compliant and/or nonresponsive will not be reviewed.
Use of Common Data Elements in NIH-funded Research
Many NIH ICs encourage the use of common data elements (CDEs) in basic, clinical, and applied research, patient registries, and other human subject research to facilitate broader and more effective use of data and advance research across studies. CDEs are data elements that have been identified and defined for use in multiple data sets across different studies. Use of CDEs can facilitate data sharing and standardization to improve data quality and enable data integration from multiple studies and sources, including electronic health records. NIH ICs have identified CDEs for many clinical domains (e.g., neurological disease), types of studies (e.g. genome-wide association studies (GWAS)), types of outcomes (e.g., patient-reported outcomes), and patient registries (e.g., the Global Rare Diseases Patient Registry and Data Repository). NIH has established a Common Data Element (CDE) Resource Portal" ( https://cde.nlm.nih.gov/home / ) to assist investigators in identifying NIH-supported CDEs when developing protocols, case report forms, and other instruments for data collection. The Portal provides guidance about and access to NIH-supported CDE initiatives and other tools and resources for the appropriate use of CDEs and data standards in NIH-funded research. Investigators are encouraged to consult the Portal and describe in their applications any use they will make of NIH-supported CDEs in their projects.
Post Submission Materials
Applicants are required to follow the instructions for post-submission materials, as described in the policy .
Section V. Application Review Information
1. Criteria
Only the review criteria described below will be considered in the review process. Applications submitted to NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
For this P30 Notice of Funding Opportunity Announcement, note the following:
Reviewers will be asked to evaluate and score the following individual sections. The overall impact score is not the average for these components.
- Research Base, including the focus, quality of research, collaborations among members, relevance to the Center's stated research focus, and, for renewal applications, the growth, re-focusing, or evolution of the research base.
- Each Biomedical Research Core, including: the potential of the core to empower CF research by promoting new research directions and creating CF-specific research opportunities that are not available through commercial or institutional resources; the documented need for and use of the proposed services; number of users; qualifications of personnel; management, including prioritization and responsiveness to the needs of the users; quality control management; and any appropriate developmental work.
- Administrative Core, including committee structure, Center membership criteria, Enrichment Program and lines of communication.
- Pilot and Feasibility Program, including the organization of the overall process of solicitation, review, and monitoring of P and F projects, and for renewal applications the quality, type of recipient (new investigator, new investigator to field, established investigator with new direction) appropriateness and outcomes of supported P and F projects.
- CF Research and Translation Center Director (PD/PI), including leadership and commitment to the stated goals of the CF Research and Translation Center.
Center Pilot and Feasibility Programs have the option to support Clinical Trials.
A proposed Clinical Trial application may include study design, methods, and intervention that are not by themselves innovative but address important questions or unmet needs. Additionally, the results of the clinical trial may indicate that further clinical development of the intervention is unwarranted or lead to new avenues of scientific investigation.
Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the project proposed).
Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Significance
Does the project address an important problem or a critical barrier to progress in the field? Is the prior research that serves as the key support for the proposed project rigorous? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Specific to this NOFO: How much evidence is there that the research base to be supported by the Center demonstrates evidence of a strong and consistent record of publications and peer-reviewed funding related to the proposed focus of the Center? How well do the proposed Cores fill a need in the CF research Community and provide services that would otherwise be unavailable, or be more cost-effective if centralized? How well do the focus, relevance, interrelationships, quality, productivity, and, to some extent, quantity of the research base support the stated theme of the Center? Are the research base investigators receiving sufficient benefit to justify the services/programs supported by the Center? If collaborations with other CF Research and Translation Centers, or individuals outside of the research base exist, what level of enhancement results? How well do collaborations enhance the productivity or quality of the research base; enhance the services offered by the Center; strengthen the Enrichment Program; allow for a more efficient use of the Center's available resources; or otherwise benefit the research base?
In addition, for applications involving clinical trials
Are the scientific rationale and need for a clinical trial to test the proposed hypothesis or intervention well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms? For trials focusing on clinical or public health endpoints, is this clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy? For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Investigator(s)
Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
Specific to this NOFO: How appropriate are the proposed Center Director(s) and Associate Director(s)' administrative and leadership experience as well as available time to direct a multicomponent Center? How well-qualified and appropriate are the Core Directors? How willing are the Center investigators responsible for the individual components to interact with each other and contribute to the overall objectives of the CF Research and Translation Center?
With regard to the proposed leadership for the project, do the PD/PI(s) and key personnel have the expertise, experience, and ability to organize, manage and implement the proposed clinical trial and meet milestones and timelines? Do they have appropriate expertise in study coordination, data management and statistics? For a multicenter trial, is the organizational structure appropriate and does the application identify a core of potential center investigators and staffing for a coordinating center?
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Specific to this NOFO: What is the availability of the Cores' new methods, techniques, and/or resources through commercial or existing institutional resources? Is there evidence of Core adaptability to emerging areas of science or technology or changing Center needs, as appropriate relative to the purpose of the Core and the research supported by the Center? Does How well does the Center encourage high-risk, innovative ideas through their P and F program?
Does the design/research plan include innovative elements, as appropriate, that enhance its sensitivity, potential for information or potential to advance scientific knowledge or clinical practice?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have the investigators included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address:
1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of individuals of all ages (including children and older adults), justified in terms of the scientific goals and research strategy proposed?
Specific to this NOFO: How clear and appropriate are criteria for membership in the CF Research and Translation Center? How appropriate Is the administrative structure for monitoring Core usage, prioritization, and quality? How appropriate are the proposed Cores for the focus of the Center? Are the proposed Cores not focused on pulmonary aspects of CF? How much added value do the Cores provide relative to what is available elsewhere and how much stimulation is there for the development of new approaches? How appropriate is the structure to solicit, evaluate, award and monitor the pilot and feasibility studies?
How appropriate and relevant are the proposed cores and the modes of operation (such as potential utilization, prioritization of requests for services, cost-recovery, and quality control monitoring)? How well do the Cores provide opportunities not otherwise available to the investigators through other available federally-funded and/or institutional resources; represent appropriate cost savings/cost sharing advantage; and stimulate the development of new approaches? How justified, if proposed, is support through an Institutional Biomedical Research Core, and would it provide added value and access to the resource that is beyond that which would be provided for the use of the institutional core through a fee-for-service process? How well do the Research Cores support either new investigators or established investigators moving into CF research? How appropriate is administrative organization proposed for the following:(a) coordination of ongoing research between the separately funded projects and the Center, including mechanisms for internal monitoring;(b) establishment and maintenance of internal communication and cooperation among the Center investigators;(c) mechanism for selecting and replacing professional or technical personnel within the cores;(d) management capabilities, including fiscal administration, procurement, property and personnel management, planning, budgeting, and other appropriate capabilities? How efficient and effective are the use and/or planned use of the limited enrichment funds, including the contribution of these activities to the stated goals of the Center?
Does the application adequately address the following, if applicable
Study Design
Is the study design justified and appropriate to address primary and secondary outcome variable(s)/endpoints that will be clear, informative and relevant to the hypothesis being tested? Is the scientific rationale/premise of the study based on previously well-designed preclinical and/or clinical research? Given the methods used to assign participants and deliver interventions, is the study design adequately powered to answer the research question(s), test the proposed hypothesis/hypotheses, and provide interpretable results? Is the trial appropriately designed to conduct the research efficiently? Are the study populations (size, gender, age, demographic group), proposed intervention arms/dose, and duration of the trial, appropriate and well justified?
Are potential ethical issues adequately addressed? Is the process for obtaining informed consent or assent appropriate? Is the eligible population available? Are the plans for recruitment outreach, enrollment, retention, handling dropouts, missed visits, and losses to follow-up appropriate to ensure robust data collection? Are the planned recruitment timelines feasible and is the plan to monitor accrual adequate? Has the need for randomization (or not), masking (if appropriate), controls, and inclusion/exclusion criteria been addressed? Are differences addressed, if applicable, in the intervention effect due to sex/gender and race/ethnicity?
Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? Is there a plan to obtain required study agent(s)? Does the application propose to use existing available resources, as applicable?
Data Management and Statistical Analysis
Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions? Are the procedures for data management and quality control of data adequate at clinical site(s) or at center laboratories, as applicable? Have the methods for standardization of procedures for data management to assess the effect of the intervention and quality control been addressed? Is there a plan to complete data analysis within the proposed period of the award?
Environment
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
Specific to this NOFO: How committed is the Institution to the Center program, including lines of accountability, regarding management of the Center grant and the Institution's contribution to the management capabilities of the Center? How clear is the potential for interaction with scientists from other departments and institutions?
If proposed, are the administrative, data coordinating, enrollment and laboratory/testing centers, appropriate for the trial proposed?
Does the application adequately address the capability and ability to conduct the trial at the proposed site(s) or centers? Are the plans to add or drop enrollment centers, as needed, appropriate?
If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
If multi-sites/centers, is there evidence of the ability of the individual site or center to: (1) enroll the proposed numbers; (2) adhere to the protocol; (3) collect and transmit data in an accurate and timely fashion; and, (4) operate within the proposed organizational structure?
Additional Review Criteria - Overall
As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items.
Study Timeline
Specific to applications involving clinical trials
Is the study timeline described in detail, taking into account start-up activities, the anticipated rate of enrollment, and planned follow-up assessment? Is the projected timeline feasible and well justified? Does the project incorporate efficiencies and utilize existing resources (e.g., CTSAs, practice-based research networks, electronic medical records, administrative database, or patient registries) to increase the efficiency of participant enrollment and data collection, as appropriate?
Are potential challenges and corresponding solutions discussed (e.g., strategies that can be implemented in the event of enrollment shortfalls)?
Protections for Human Subjects
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
Inclusion of Women, Minorities, and Individuals Across the Lifespan
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
Vertebrate Animals
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
Not Applicable.
For Renewals, the committee will consider the progress made in the last funding period.
Research Base: What evidence is there that the Center demonstrates a stable or growing research base with strong and consistent record of scientific excellence and achievement reflected in an outstanding level of productivity and continuing success in securing peer-reviewed research funding? How well does the Center show evidence of fostering multi-disciplinary collaborations among its Center investigators? What new research directions have resulted from such collaborations?
Biomedical Research Cores: Are the number and impact of research publications that acknowledge the Center sufficient to justify each core? What significant fraction of papers a) acknowledge the Center and b) do not have core personnel as co-authors? Are the number and listing of Center investigators who have used the core and resultant key advances consistent with the level of core investment? Do the number and listing of investigators who have used the core multiple times indicate satisfaction and continuing need for core services? Are there sufficient numbers of users who are not Core personnel or their collaborators? Are the number and listing of users who are not Center personnel or members consistent with the best utilization of the Core by the community? Are the numbers of services/tests completed by each core indicative of sufficient utilization? Is the capacity of each core with current resources sufficient to serve the needs of the Center community? Does the Center provide evidence of ability to evolve cores to meet changing needs of the research community? Does the Center provide evidence of Program Income and/or sufficient institutional support? If applicable, has the national/regional shared resource core been effective as well as beneficial to both the Center members and partnering institution(s)?
Administrative Core (with Enrichment Program): How effective has the administrative structure proven to be? How effective has the oversight of Center activities, including the P and F and Enrichment programs been? How well does the Center website show evidence of continuing maintenance and a high level of quality and usability? Has the Enrichment program fostered multidisciplinary approaches to CF research and to attract new investigators or investigators with relevant expertise to CF research?
Pilot and Feasibility Program : How well justified and related to the goals of the Center are the numbers and types of P and F awards? How well utilized was the P and F program during the previous project period? Were awards made to investigators who fully met the eligibility criteria (as described in the NOFO) for pilot and feasibility support? Are data provided to document the outcome of all P and F projects completed in the last five years, including those that failed to lead to further funding? Has the P and F program led to publications of significant impact, subsequent independent R01 or other peer-reviewed support, and/or attracted new investigators into diabetes, nutrition/obesity, hepatic, renal or gastro-intestinal-related research? How well documented and consistent with the level of support provided are research papers generated under these awards, projects successfully funded with independent grants, and key advances linked to these awards? For those Centers who provided P and F support to investigators new to CF research and/or to experienced CF researchers with innovative ideas, how successful were the P and F awardees in their outcomes?
Additional Review Considerations - Overall
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Applications from Foreign Organizations
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Resource Sharing Plans
Reviewers will comment on whether the Resource Sharing Plan(s) (e.g., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
Authentication of Key Biological and/or Chemical Resources:
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) convened by NIDDK, in accordance with NIH peer review policy and procedures , using the stated review criteria. Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications will receive a written critique.
Applications may undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score.
Appeals of initial peer review will not be accepted for applications submitted in response to this NOFO.
Applications will be assigned on the basis of established PHS referral guidelines to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications submitted in response to this NOFO. Following initial peer review, recommended applications will receive a second level of review by the appropriate NIDDK Advisory Council. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access their Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. Award Notices
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient's business official.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions. Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this NOFO will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
Individual awards are based on the application submitted to, and as approved by, the NIH and are subject to the IC-specific terms and conditions identified in the NoA.
ClinicalTrials.gov: If an award provides for one or more clinical trials. By law (Title VIII, Section 801 of Public Law 110-85), the "responsible party" must register and submit results information for certain “applicable clinical trials” on the ClinicalTrials.gov Protocol Registration and Results System Information Website ( https://register.clinicaltrials.gov ). NIH expects registration and results reporting of all trials whether required under the law or not. For more information, see https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
Institutional Review Board or Independent Ethics Committee Approval: Grantee institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
Data and Safety Monitoring Requirements: The NIH policy for data and safety monitoring requires oversight and monitoring of all NIH-conducted or -supported human biomedical and behavioral intervention studies (clinical trials) to ensure the safety of participants and the validity and integrity of the data. Further information concerning these requirements is found at http://grants.nih.gov/grants/policy/hs/data_safety.htm and in the application instructions (SF424 (R&R) and PHS 398).
Investigational New Drug or Investigational Device Exemption Requirements: Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
Prior Approval of Pilot Projects
All recipient-selected Pilot and Feasibility projects require prior approval by NIDDK prior to initiation. The focus of new Pilot and Feasibility project should be within the NIDDK mission. A complete project application (including biographical sketches, personnel and budget justification, research plan, human and/or animal subject sections and any IACUC, IRB or other required documentation), and the critiques of the proposals by the Advisory Committee must be provided to NIDDK with the Just in Time information or prior to the start of the Pilot and Feasibility projects.
- The recipient institution will comply with the NIH Guidance on Changes That Involve Human Subjects in Active Awards and That Will Require Prior NIH Approval .
- The recipient institution will provide NIH with specific plans for data and safety monitoring, and will notify the IRB and NIH of serious adverse events and unanticipated problems, consistent with NIH DSMP policies .
- The recipient institution will provide NIH with written study protocols that address risks and protections for human subjects in accordance with NIH’s Instructions for Preparing the Human Subjects Section of the Research Plan
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities , including of note, but not limited to:
- Federal-wide Standard Terms and Conditions for Research Grants
- Prohibition on Certain Telecommunications and Video Surveillance Services or Equipment
- Acknowledgment of Federal Funding
If a recipient is successful and receives a Notice of Award, in accepting the award, the recipient agrees that any activities under the award are subject to all provisions currently in effect or implemented during the period of the award, other Department regulations and policies in effect at the time of the award, and applicable statutory provisions.
If a recipient receives and award, the recipient must follow all applicable nondiscrimination laws. The recipient agrees to this when registering in SAM.gov. The recipient must also submit an Assurance of Compliance ( HHS-690 ). To learn more, see the Laws and Regulations Enforced by the HHS Office for Civil Rights website .
HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to System for Award Management (SAM.gov) requirements. SAM.gov requires Federal agencies to review and consider information about an applicant in the designated integrity and performance system (currently SAM.gov) prior to making an award. An applicant can review and comment on any information in the responsibility/qualification records available in SAM.gov. NIH will consider any comments by the applicant, in addition to the information available in the responsibility/qualification records in SAM.gov, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 2 CFR Part 200.206 “Federal awarding agency review of risk posed by applicants.” This provision will apply to all NIH grants and cooperative agreements except fellowships.
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR Part 200.301.
The Federal Funding Accountability and Transparency Act of 2006 as amended (FFATA), includes a requirement for recipients of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All recipients of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over the threshold. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 2 CFR Part 200.113 and Appendix XII to 2 CFR Part 200, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (Responsibility/Qualification in SAM.gov, formerly FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 2 CFR Part 200 – Award Term and Condition for Recipient Integrity and Performance Matters.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten submission by the due date, and post-submission issues)
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information (Questions regarding application instructions, application processes, and NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-637-3015
Grants.gov Customer Support (Questions regarding Grants.gov registration and Workspace) Contact Center Telephone: 800-518-4726 Email: [email protected]
Thomas L Eggerman, MD, PhD. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Telephone: 301-594-8813 Email: [email protected]
Ryan Morris, PhD National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Telephone: 301-480-1296 Email: [email protected]
Christy Ezell National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Telephone: 301-443-9231 Email: [email protected]
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 200.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .
- Open access
- Published: 22 April 2024
NSABP FB-10: a phase Ib/II trial evaluating ado-trastuzumab emtansine (T-DM1) with neratinib in women with metastatic HER2-positive breast cancer
- Samuel A. Jacobs 1 ,
- Ying Wang 1 ,
- Jame Abraham 1 , 2 ,
- Huichen Feng 1 ,
- Alberto J. Montero 1 , 2 , 3 ,
- Corey Lipchik 1 ,
- Melanie Finnigan 1 ,
- Rachel C. Jankowitz 1 , 4 nAff5 ,
- Mohamad A. Salkeni 1 , 6 nAff7 ,
- Sai K. Maley 1 ,
- Shannon L. Puhalla 1 , 8 , 9 ,
- Fanny Piette 10 ,
- Katie Quinn 11 ,
- Kyle Chang 11 ,
- Rebecca J. Nagy 11 ,
- Carmen J. Allegra 1 , 12 ,
- Kelly Vehec 1 ,
- Norman Wolmark 1 , 8 ,
- Peter C. Lucas 1 , 8 , 9 , 13 ,
- Ashok Srinivasan 1 , 14 &
- Katherine L. Pogue-Geile 1
Breast Cancer Research volume 26 , Article number: 69 ( 2024 ) Cite this article
249 Accesses
1 Altmetric
Metrics details
We previously reported our phase Ib trial, testing the safety, tolerability, and efficacy of T-DM1 + neratinib in HER2-positive metastatic breast cancer patients. Patients with ERBB2 amplification in ctDNA had deeper and more durable responses. This study extends these observations with in-depth analysis of molecular markers and mechanisms of resistance in additional patients.
Forty-nine HER2-positive patients (determined locally) who progressed on-treatment with trastuzumab + pertuzumab were enrolled in this phase Ib/II study. Mutations and HER2 amplifications were assessed in ctDNA before (C1D1) and on-treatment (C2D1) with the Guardant360 assay. Archived tissue (TP0) and study entry biopsies (TP1) were assayed for whole transcriptome, HER2 copy number, and mutations, with Ampli-Seq, and centrally for HER2 with CLIA assays. Patient responses were assessed with RECIST v1.1, and Molecular Response with the Guardant360 Response algorithm.
The ORR in phase II was 7/22 (32%), which included all patients who had at least one dose of study therapy. In phase I, the ORR was 12/19 (63%), which included only patients who were considered evaluable, having received their first scan at 6 weeks. Central confirmation of HER2-positivity was found in 83% (30/36) of the TP0 samples. HER2-amplified ctDNA was found at C1D1 in 48% (20/42) of samples. Patients with ctHER2-amp versus non-amplified HER2 ctDNA determined in C1D1 ctDNA had a longer median progression-free survival (PFS): 480 days versus 60 days ( P = 0.015). Molecular Response scores were significantly associated with both PFS (HR 0.28, 0.09–0.90, P = 0.033) and best response ( P = 0.037). All five of the patients with ctHER2-amp at C1D1 who had undetectable ctDNA after study therapy had an objective response. Patients whose ctHER2-amp decreased on-treatment had better outcomes than patients whose ctHER2-amp remained unchanged. HER2 RNA levels show a correlation to HER2 CLIA IHC status and were significantly higher in patients with clinically documented responses compared to patients with progressive disease ( P = 0.03).
Conclusions
The following biomarkers were associated with better outcomes for patients treated with T-DM1 + neratinib: (1) ctHER2-amp (C1D1) or in TP1; (2) Molecular Response scores; (3) loss of detectable ctDNA; (4) RNA levels of HER2; and (5) on-treatment loss of detectable ctHER2-amp. HER2 transcriptional and IHC/FISH status identify HER2-low cases (IHC 1+ or IHC 2+ and FISH negative) in these heavily anti-HER2 treated patients. Due to the small number of patients and samples in this study, the associations we have shown are for hypothesis generation only and remain to be validated in future studies.
Clinical Trials registration NCT02236000
Introduction
In 2013, T-DM1 was the first HER2-targeted antibody–drug conjugate (ADC) granted FDA-approval for late-stage metastatic breast cancer after prior trastuzumab. In 2019, T-DM1 was approved as post-neoadjuvant therapy in patients with residual disease based on the KATHERINE trial, demonstrating that post-neoadjuvant T-DM1 was statistically more beneficial than trastuzumab, preventing recurrence of invasive disease or deaths in patients with residual disease in breast or lymph nodes after treatment with trastuzumab ± pertuzumab (hazard ratio for invasive disease or death, 0.05: 95% CI 0.039–0.64; P < 0.001) [ 1 ]. KATHERINE required archival HER2-positivity but did not mandate HER2 status at study entry. Because multiple studies have confirmed that HER2 status is plastic with conversion of HER2-positive disease to HER2-low (IHC = 0–1+ or IHC 2+ /FISH-negative) under pressure of therapy [ 2 , 3 , 4 ], this raised the question of ADC efficacy in HER2-low patients—either de novo (HR + /HER2-negative) or acquired from conversion of HER2-amplified to HER2-low. There are now several breast cancer-targeting ADCs in the pipeline in addition to the newly approved trastuzumab deruxtecan (T-DXd). Initial approval of T-DXd was for metastatic HER2-positive breast cancer after prior progression on multiple lines of anti-HER2 therapy (DESTINY-Breast01) [ 5 ]. In DESTINY-Breast03, T-DXd improved PFS and OS compared to T-DM1 [ 6 ] in patients with metastatic disease with progression on trastuzumab. In heavily pretreated HER2-low breast cancer patients, T-DXd was evaluated in a single arm phase II study. The objective response rate (ORR) to T-DXd by central review was 37%, with median duration of response of 10.4 months [ 7 ]. DESTINY-Breast04, a randomized, multicenter trial in patients with unresectable or metastatic HER2-low breast cancer, reported highly significant improvements in PFS and OS in patients receiving T-DXd compared to physician choice of treatment [ 8 ]—a particularly striking observation, because neither trastuzumab nor T-DM1 has shown consistent activity in HER2-low populations [ 9 ]. HER2 status (expression, mutation, amplification) is thus emerging as a predictor of clinical efficacy for anti-HER2-therapy. In our NSABP phase Ib trial of HER2-targeted therapies using T-DM1 + neratinib in HER2-positive patients, we showed a discordance in HER2 status between archival tissue and a liquid biopsy obtained at study entry. Loss of ctHER2-amp occurred in 63% (17 of 27) patients. Deeper and more durable responses were observed with T-DM1 + neratinib in patients with ctHER2-amplification [ 10 ]. We now report on an expanded cohort. Our aims were to: (1) confirm activity of T-DM1 + neratinib in patients progressing on a taxane with trastuzumab + pertuzumab (HP), (2) evaluate discordance in HER2 amplification between archived tissue, contemporaneous tissue, and blood, and (3) compare mutation and gene-expression profiles at different time points. Finally, in a subset of patients, we assessed response by RECIST 1.1 with the Guardant Molecular Response score [ 11 , 12 ].
Trial design
FB-10 was a single-arm, nonrandomized, unblinded clinical trial approved by participating institutions’ institutional review boards. Written informed consent was required. FB-10 was conducted according to Good Clinical Practices and the Declaration of Helsinki.
The phase Ib trial was a dose escalation study evaluating T-DM1 + neratinib in women with metastatic HER2-postive breast cancer based on local determination of HER2. Patients received 3.6 mg/kg T-DM1 intravenously on a 3-week cycle and oral neratinib was taken daily in one of four dose cohorts (120, 160, 200 and 240 mg). Twenty-seven patients enrolled, with 5 experiencing a dose-limited toxicity. Three withdrew early for other reasons (Fig. 1 ). Nineteen patients were evaluable for response, which required follow-up imaging after the second cycle of treatment (6 weeks). The recommended phase II dose of neratinib was determined to be 160 mg/d [ 10 ]; however, we did not detect a dose response, i.e., neratinib at 120 mg/d was as effective as higher doses and less toxic. [ 10 ]
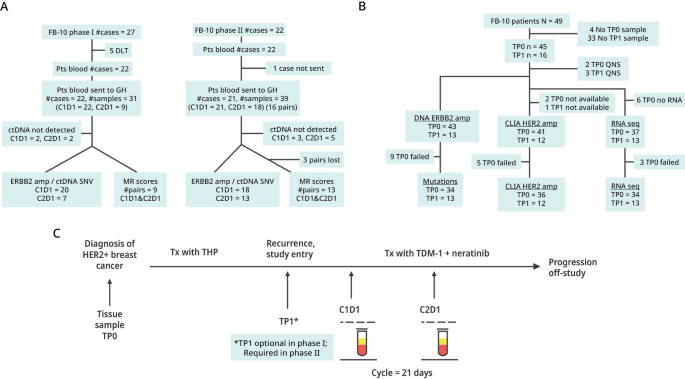
Remark Diagram of Blood and Tissue Samples: NSABP FB-10. A Blood samples collected from patients enrolled into FB-10 phase Ib and phase II, and successful assays for ctDNA analysis of HER2 amplification with Guardant360 assays. B Tissue samples collected from patients enrolled into FB-10 and their samples that were profiled for mutations and whole transcriptomic analysis and for ERBB2 amplification status with CLIA and AmpliSeq assays. C The timing and type of sample collections (tissue: TP0 or TP1, or blood: C1D1 or C2D1) are shown
The phase II expansion included all patients (N = 22) who received at least one dose of study therapy in the analysis of safety and efficacy. Eligibility criteria were identical in phase Ib and phase II [ 10 ]. All eligible patients had prior HP and a taxane as neoadjuvant therapy or for de novo metastatic disease, had measurable disease, were ECOG PS ≤ 2, with adequate hematologic, renal, and liver function. Patients with known stable brain metastases were eligible. Brain imaging at entry was not required. Treatment in phase II included T-DM1 at 3.6 mg/kg iv q 3 weeks and neratinib at 160 mg/day. Primary diarrhea prophylaxis was mandated as described in phase I [ 10 ].
Safety assessment
Safety assessment was similar in phase Ib and phase II including physical examination, interim history, and laboratory assessments. Patients remained on treatment until progressive disease or discontinuation because of withdrawal, physician discretion or toxicity. For phase Ib patients, adverse event (AE) assessment occurred on days 1, 8, and 15 of cycle 1; on day 1 of each cycle and for 30 days after therapy discontinuation or when alternate therapy began. Phase II AE assessments were made on day 1 of each cycle.
Response evaluation
In phase Ib, patients were assessed for best response beginning with their first follow-up scan after 2 cycles (6 weeks). Response by RECIST v1.1 was complete response (CR), partial response (PR), stable disease (SD), or progression (PD). In phase II, imaging studies were performed after every 3 cycles (9 weeks). The clinical benefit rate included all CR, PR, and SD patients with duration ≥ 180 days. Patients with stable disease of < 180 days were included with progressive disease patients. A confirmatory scan at least one month after the best response was not required in this study, perhaps accounting for partial responses of short duration in several patients.
Blood and tissue collection
Blood samples were required before treatment at cycle 1, day 1 (C1D1), and after treatment at cycle 2 day 1 (C2D1) for all patients in phase Ib and II (Fig. 1 A). Archived tissue (TP0) of diagnostic blocks or slides were required on all phase Ib and II patients which included 27 patients from phase Ib and 22 patients in phase II. (Fig. 1 B). Contemporaneous biopsy specimens or slides at study entry (TP1) were optional in phase Ib but in phase II, after enrollment of the first 6 patients, the study was amended to require a study entry biopsy. The timing and type of collections of samples are shown in Fig. 1 C.
ctDNA assessment
Samples were analyzed by the Guardant360 assay (Fig. 1 A), which detects single-nucleotide variants, indels, fusions, and copy number alterations in 74 genes. For HER2 amplification, a cutoff of ≥ 2.14 was used. Where amplification could not be determined because of failed assays or no blood, samples were categorized as indeterminant. Guardant Health (Guardant360 assay) is Clinical Laboratory Improvement Amendments (CLIA)-certified, College of American Pathologists-accredited, New York State Department of Health-approved laboratory.
ctDNA molecular response
Guardant360 Molecular Response is a next generation sequencing (NGS)‐based liquid biopsy that assesses changes in tumor‐derived cell‐free DNA (ctDNA) between baseline and an early on‐treatment timepoint in patients with solid tumor malignancies. It employs an algorithm to identify informative somatic single nucleotide variants (SNVs), insertions/deletions and gene fusions and calculates the percent ctDNA change between the two timepoints based on the mean variant allele frequency (VAF) between two [(mean VAF2/mean VAF1) −1 × 100%]. Using the Molecular Response panel and the Guardant bioinformatics pipeline, the change in ctDNA levels between baseline and the initial follow-up scan (6 weeks in phase I and 9 weeks in phase II) was calculated and the change in VAF determined. Molecular Response is calculated as the ratio of mean VAF on-treatment to baseline with a cutoff of 50%. Decreases in ctDNA of 50%‐100% during this timeframe are associated with clinical benefit in patients on anti‐cancer therapies [ 11 , 13 , 14 ]. Kaplan–Meier curves for PFS are generated for patients above and below a Molecular Response cut off. [ 11 ]
Isolation of nucleic acid
Tumor regions, defined by a certified pathologist, were macrodissected. DNA and RNA were isolated using the Qiagen AllPrep DNA/RNA kit, following the manufacturer’s recommendations but eliminating the xylene wash in the first step. Separate tissue sections were used for RNA and DNA isolation.
Whole transcriptomic profiling
10–30 ng of RNA from the phase II samples was reverse transcribed. cDNA libraries were constructed using whole transcriptomic Ampli-Seq kits, following the manufacturer’s instructions without a fragmentation step due to the small size of the RNAs. This same method did not work well for the phase Ib RNAs. To overcome this problem, phase Ib RNAs were made library-ready via the HTG EdgeSeq system and the HTP panel, which includes probes to interrogate 19,398 genes representing most of the human transcriptome (details in Additional file 1 ).
Breast cancer molecular subtypes were determined by applying the AIMs signature [ 15 ]. The 8-gene trastuzumab-benefit groups were determined using a validated signature. [ 16 , 17 ]
HER2 amplification status and analysis of variants in tissues
DNA sequencing was performed using a custom Ampli-Seq panel referred to as the NAR panel, amplifying 3,847 amplicons with 94.25% coverage of exons from 117 genes in HER2-activated pathways (Additional file 1 : Table S1). The panel was designed using the Thermo Fisher Ion AmpliSeq™ Designer tool ( https://www.ampliseq.com ). Libraries were constructed using 10 ng of DNA using the Ion AmpliSeq™ kit for Chef DL8. The Ion Chef instrument was used to template and load samples on Ion 550 chips. Up to 32 samples were barcoded, pooled, and sequenced on the S5 sequencer (ThermoFisher) following manufacturer’s instructions.
We have used two different criteria to identify variants in FB-10 tissue. For a conservative approach to variant selection, we selected variants with VAF ≥ 10% and for a less restrictive option we selected variants with VAF ≥ 5%. Additional details are included in Additional file 1 and the rationale for these approaches is discussed.
Ion Torrent data and the Ion Reporter software were used to determine HER2 copy number.
HER2 IHC FISH
HER2 status was also assessed in tissue samples with IHC and reflexively for FISH at the discretion of the Director at the CLIA laboratory (Magee Women’s Hospital, University of Pittsburgh Medical Center). Nine samples were equivocal IHC 0 or 1+ due to poor tissue quality prompting examination with FISH. Based on FISH, four were included as HER2-positive.
Statistical analysis
Phase Ib safety, tolerability, efficacy, and recommended phase 2 dose (RP2D) of neratinib in combination with T-DM1 was previously reported [ 10 ]. In the phase II expansion cohort, in which neratinib was administered at the RP2D of 160 mg/d, the intention was to confirm clinical efficacy and tolerability of the combination and to extend the correlative findings. Given the small sample size, the endpoint analyses remain descriptive.
The aim of the single-arm phase II expansion was to rule out the null hypothesis that the ORR was 25% with the alternative hypothesis of an ORR of 45%. With these assumptions, the sample size required was 22 and the decision rules are to declare success if > 8 responses; to declare failure if < 7 responses; and to consider the trial inconclusive if 7 or 8 responses (7/22 = 32%, 8/22 = 36%). At the outset of the study, we did not anticipate the large number of patients with loss of HER2-amplification in blood as determined by the Guardant assay. Thus, the subset analyses based upon HER-amplification detected in blood were performed post-hoc.
Patient characteristics
In the phase Ib portion of this study, 27 patients were enrolled between February 2015 and July 2017. Nineteen patients were evaluable having had at least one follow-up imaging study; three patients withdrew from the study in cycle 1 and 5 patients with a dose-limited toxicity in cycle 1 did not have an imaging assessment. All phase II patients who received at least one dose of study drugs were included in the analysis. Twenty-two patients were evaluable for toxicity and 20 were evaluated for efficacy with at least one scan performed after their third cycle. Two non-evaluable patients who withdrew from the study did not have their first scan but are included as PD. Median age was 55.5 years (range 32–70). Hormone status (ER and/or PR) was positive in 13 patients and negative in 9. All patients were HER2-positive at baseline by local determination (Additional file 1 : Table S3).
Similar to phase Ib patients, diarrhea was the most frequent toxicity in phase II: grade 2, 6 patients (27%); grade 3, 8 (36%). Other grade 3/4 toxicities included: thrombocytopenia, 2 patients (10%); transaminase elevation, 3 patients (15%); and pneumonitis, 1 patient (5%). There were no unanticipated toxicities in the phase II expansion.
Among 19 evaluable patients in phase Ib, there were 3 CRs and 9 PRs for an ORR of 63% (12/19) [ 10 ]. In phase II, including all patients who received at least one dose of therapy, there were 2 CRs, 5 PRs for an ORR of 32% (7/22), and 3 SDs of 180 days or longer making the clinical benefit rate (CBR) 45% (10/22). In phase Ib and II, nine patients had sustained objective responses lasting approximately 1 year or longer (range 343–1453 + days, Additional file 1 : Table S4; Additional file 2 : Table S5). Treatment was discontinued at or before the first scan in 15 patients for a variety of reasons, including 5 DLTs (all in phase I) and 10 with clinical progression in phase I and II.
ctDNA clearance and treatment response
Because clearance of ctDNA has been associated with treatment response, we compared the outcomes of patients who were positive or negative for ctDNA after study treatment. The response rate among the ctDNA-positive patients who were still ctDNA-positive after study therapy was 9/19 (47%), but the ctHER2 DNA-positive patients who became ctDNA-undetectable at C2D1, the response rate was 6/6 (100%), demonstrating that the loss of ctDNA was associated with a very good response.
HER2 amplification in tissues and blood
We assessed the HER2 amplification status of TP0 and TP1 with a CLIA HER2 IHC/FISH assay, and with an Ampli-Seq NGS assay. These tissue samples were also compared to the HER2 amplification status in blood samples collected at C1D1 and C2D1 (Fig. 2 B). There was good concordance between the CLIA HER2/FISH and Ampli-Seq assays (100% in TP1 tissues and 85% in all tissues), which demonstrated the technical accuracy of the Ampli-Seq. Concordance between TP0 tissue with IHC/FISH and C1D1 ctDNA was 71% (20/28). Concordance between C1D1 and C2D1 was 54% (14/26). Anti-HER2 treatment is potentially the causal reason for the discordance between C1D1 and C2D1, which showed a total loss of ctDNA in some samples and a loss of HER2 amplification in others.
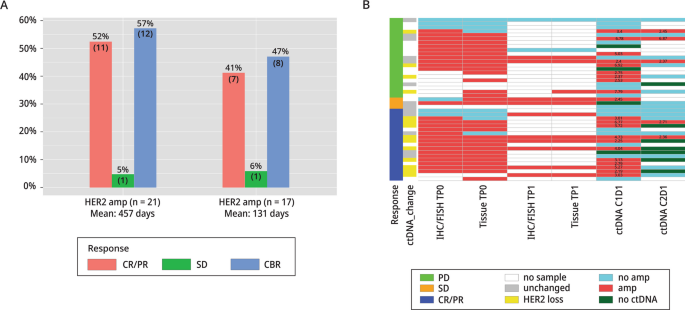
Amplification Status of Tissues and Blood: NSABP FB-10. A Response rates (CR/PR, CBR, and SD) for FB-10 HER2-amplified and non-amplified patients based on ctDNA results. B HER2-amplification status of FB-10 tumor tissues based on CLIA tests (IHC/FISH) and Ampli-Seq (Tissue) in baseline (TP0) and study entry (TP1) samples are shown. HER2-amplification status was determined in ctDNA at C1D1 and at C2D1 with the Guardant360 assays. Responses, amplification status, and changes in copy number in ctDNA between C1D1 and C2D1 are indicated as shown in the legend
Using the Guardant360 assay cut point of 2.14 for amplification among 43 C1D1 samples (22 in phase Ib, 21 in phase II), 6 patient samples were indeterminate (including 4 for which somatic mutations were not detected) (Fig. 2 B , dark green), 1 was not evaluable (NE), and 1 other failed quality control. Among the remaining 37 samples, there were 21/37 (57%) patients with amplification and 17/37 (46%) without. The objective response (CR, PR) rate was 55% (11/20) in amplified patients and 41% (7/17) in non-amplified patients. The CBR in patients with ctHER2-amplification was 12/21 (57%) and in non-amplified patients it was 8/17 (47%). There was one patient with SD who was ctHER2-amp indeterminate. Mean duration of response (CBR) was substantially longer in amplified patients, 457 days compared to 131 days in non-amplified patients ( P = 0.008) (Fig. 2 A; Additional file 1 : Table S4).
We compared progression-free survival (PFS) of patients whose ctDNA or tumor tissues had HER2 amplification to patients with no HER2 amplification. Patients with ctHER2-amp at C1D1 or in their TP1 tumor tissue had a significantly longer PFS than patients with no HER2 amplification ( Fig. 3 A–E ) .
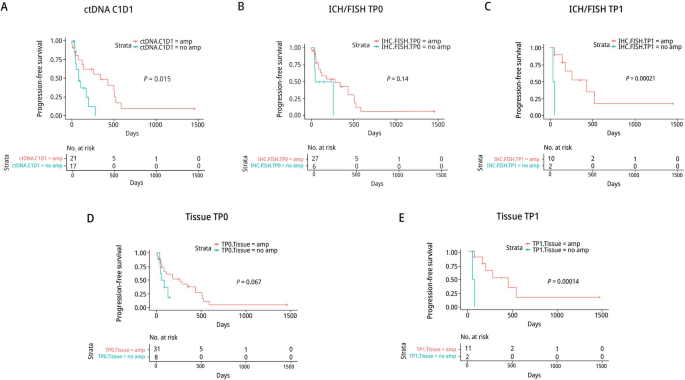
Association of HER2-amplification Status with Patient Outcomes: NSABP FB-10. A Kaplan-Meier plots of patients with or without HER2 amplification in ctDNA or in TP0 tissue ( B & D ), or in TP1 tissue ( C & E ) based on IHC/FISH ( B & C ) and on Ampli-Seq ( D & E )
In phase I and II there were 26 C1D1 and C2D1 pairs, 15 with and 11 without ctHER2-amp at C1D1. Among the 15 with ctHER2-amp at C1D1, 14 showed HER2 loss at C2D1 as defined by a loss ≥ 28% of HER2 copy number or no ctDNA detected. The ORR among these 14 patients was 71% (10/14). Of the 10 responders, 5 cleared ctDNA completely by C2D1, 3 had detectable ctDNA but no ctHER2-amp, and 2 were HER2-positive but the amplification level in C2D1 had decreased dramatically (Fig. 2 B; Additional file 2 : Table S5). The 2 remaining patients with detectable ctHER2-amp with no loss of HER2 amplification had PD, suggesting that a loss of ctDNA and/or a loss of HER2 ctDNA amplification was a marker for a good response to study therapy. However, in 11 patients with no HER2 ctDNA amplification at C1D1, the ORR was 45% (5/11), indicating that some non-amplified tumors were responsive to study treatment.
Molecular response by ctDNA
We assessed the association between molecular response and objective radiologic response (Fig. 4 A). A total of 21 patients (9 phase Ib and 12 phase II) had paired samples that met criteria for assessment of molecular response. Criteria included ≥ 1 alteration present in one of the paired samples plus a mutant molecule count of ≥ 15 in either sample. Molecular responders demonstrated a longer PFS compared to non-responders (median PFS 7.4 vs. 2.8, HR 0.28, 95%CI 0.09-0.90, P=0.033 using Wilcox test). [0.09–0.90, P = 0.033 using Wilcox test]. We also examined the association between molecular response and best RECIST response. Patients with CR/PR/SD had significantly lower Molecular Response values compared to patients with PD ( P = 0.037; Fig. 4 B ).
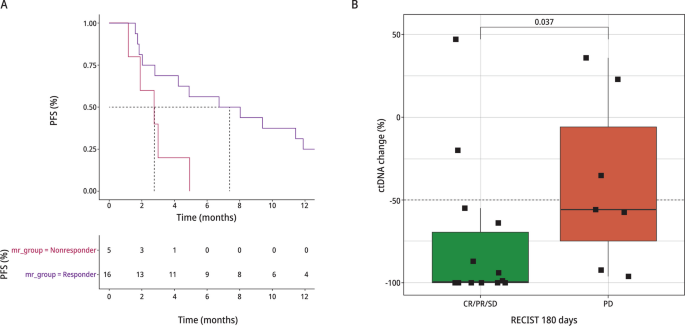
Molecular Response and Patient Outcomes: NSABP FB-10. A Kaplan–Meier curves showing association of MR with PFS using a molecular response cutoff of 50%. B Association between molecular response and best RECIST response
Mutations/variants in tissues and ctDNA
Because ERBB2 is the target of the study therapies, we have examined both tissue and ctDNA for mutations in the ERBB2 gene. No ERBB2 variants in tissue at a VAF of ≥ 10% were observed, however, in ctDNA 3 nonsynonymous, ERBB2 variants (I767M, V777L, and S310Y) were detected in the C1D1 samples from 3 patients. These variants have been associated with sensitivity to neratinib in breast cancer patients [ 18 ]. In this study, the patients whose tumors had a V777L or a S310Y mutation had a PR, but the one patient with a I767M mutation had PD with brain metastasis. The tumor with the I767M mutation also had a P1233L mutation [ 19 ]. Interestingly, in an exhaustive meta-analysis of 37,218 patients, including 11,906 primary tumor samples, 5,541 extracerebral metastasis samples, and with 1485 brain metastasis samples found that a nearby ERBB2 mutation (P1227S) was the only mutation restricted to brain metastasis. It is unknown whether any of these mutations played a role in the patient responses or the course of disease, but it is of interest to note them [ 20 ].
We examined DNA variants in all available TP0 and TP1 tissues using our NAR Ampli-Seq panel, which included ESR1, HER2, and 115 other genes in HER2-activated pathways. Based on our stringent criteria for variant detection, i.e., VAF ≥ 10%, plus other criteria as described in Additional file 1 , we identified 27 variants among 28 samples, representing 21 patients ( Fig. 5 A ) .
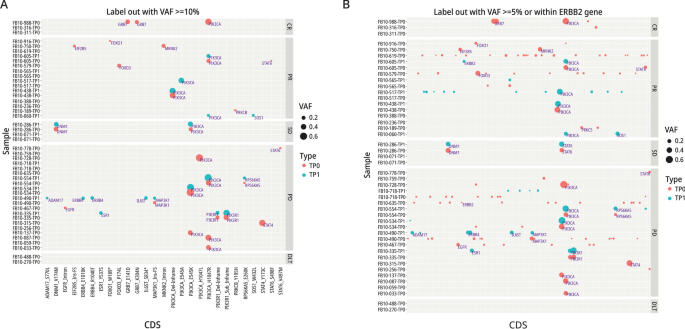
Variant Alleles in Patients and their Responses: NSABP FB-10. A Variants detected with a VAF of ≥ 10% in patients with PD, SD, PR or CR. *indicates a stop codon. B Variant alleles with a VAF of ≥ 5% in patients with PD, SD, PR or CR
The frequency of PIK3CA mutations among all sequenced patients was 34% (12/35), similar to that seen in other studies of unselected metastatic and early-stage breast cancer patients (cBioPortal). All of the mutations were in exons 9 and 20 at amino acid 545 and 1,047, respectively. These PIK3CA variants also have the highest VAFs (ranging from 10 to 72% across samples), however, PIK3CA mutations do not appear to influence patient outcomes, because response rates between PIK3CA mutant and WT tumors were similar: 42% (4/12) versus 45% (14/31), respectively. In one case, a PIK3CA mutation was detected only in TP1 but this patient had a PR, again indicating that PIK3CA is not a resistance marker for study therapy. Variants detected only in PD or CR patients represent potential resistance or sensitivity markers, respectively, to study therapy. Mutations detected only in TP1 samples among the 12 paired TP0/TP1 cases, included ADAM17_S770L, ERBB4_E1010K, ERBB4_R1040T, and IL6ST_S834* in one sample and an ESR1_EY537S mutation in another (Fig. 5 A). Both patients had PD, perhaps indicating that these mutations may have emerged in response to prior therapies. Details of variants are presented in Additional file 1 .
We examined the PAM50 subtypes and the 8-gene trastuzumab benefit signature in all available tissues [ 16 ]. Among the 29 patients with response and gene expression data for TP0 tissue, we found that 19/34 (56%) were HER2E, 8 (24%) were luminal B, 4 (12%) were basal, 2 (5.9%) were normal, and 1 (2.9%) was luminal A. Patients with luminal subtype tumors had a lower CR/PR response rate (1/8 [12.5%]) than patients with a non-luminal subtype (12/23 [52%]) (Additional file 2 : Table S5). Among the TP1 samples with gene expression data, the frequency of CR/PR was 1/4 in luminal patients and 5/9 in the non-luminal patients. Intrinsic subtypes differed between TP0 and TP1 tissues in some cases (Additional file 1 : Table S4). Although numbers are small, the frequency of CR/PR rates were consistently lower among the luminal patients than non-luminal patients.
The 8-gene trastuzumab signature is a validated signature for identifying patients with large-, moderate- or no-benefit from trastuzumab when added to chemotherapy in the adjuvant setting [ 16 , 17 ] and has been shown to associate with pCR rates in the neoadjuvant setting [ 21 , 22 ]. We questioned whether this signature may also show an association with response in the metastatic setting. Among the large-, moderate- and no- benefit groups the percent of CR/PR patients was 67% (4/6), 50% (9/18), and 29% (2/7), respectively (data in Additional file 2 : Table S5).
As expected, the level of HER2 RNA increased as the IHC status increased (i.e., 0, 1 + , 2 + , to 3 +) (Additional file 1 : Fig. S2). In TP1 samples they were concordant with patient responses suggesting that HER2 RNA expression in study entry is associated with response to T-DM1 + neratinib ( Fig. 6 ).
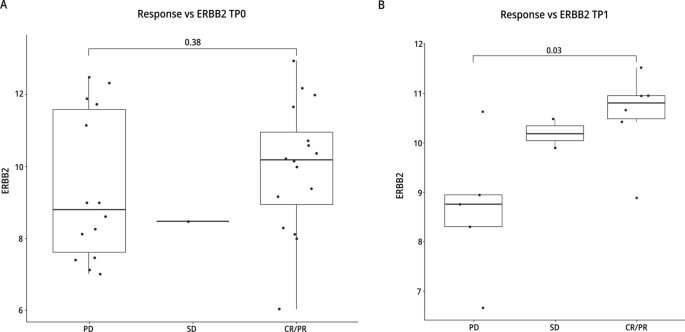
RNA Expression Levels and Response to Therapy. RNA expression levels in TP0 tissues ( A ) and in TP1 tissues ( B ) from patients with PD, SD or CR/PR. The units for RNA expression were log 2 expression values
Significant differences were detected in RNA levels between IHC 1 + and 3 + and between 2 + and 3 + but not between 0 and 1 + nor between 1 + and 2 + (Additional file 1 : Fig. S3). Although numbers are limited, these data show that the RNA levels are not different between 0 and 1 + . These patients may benefit from treatment with other ADCs. The DAISY and DESTINY-Breast04 trials signal that T-DXd may have significant activity in HER2-low patients [ 8 , 23 ].
Approximately 35% of HER2-positive patients may have a loss of HER2 amplification after undergoing chemotherapy + anti-HER2 therapy [ 2 , 3 ]. In a retrospective analysis of 525 patients who received neoadjuvant chemotherapy (NAC) + HP, 141 patients with residual disease had HER2 status determined pre-and post-NAC-HP. HER2 was concordant (positive/positive) in 84/141 (60%). HER2 protein expression was lost (IHC 0) in 13/57 (23%) and designated as HER2-low in 44/57 (77%) including IHC 1 + in 31 and IHC 2 + /FISH non-amplified in 13 [ 4 ]. HER2 intratumoral heterogeneity is likely one cause of discordant HER2 status between primary and post-treatment residual or metastatic disease [ 24 ]. Other possibilities include decreased HER2 expression, which could be a transient change or a result of the selection of HER2-low subclones [ 4 ].
We have assessed HER2 status before and after pre- and post-study therapy in not only solid tissue but also blood. We have determined the HER2 status in tissues with CLIA IHC/FISH assays, which is the gold standard for HER2 assessment, plus with Ampli-Seq, because it provided a quantitative analysis of HER2 copy number with a greater dynamic range. Ampli-Seq was able to detect a decrease in HER2 copy number in samples that had not lost HER2 amplification based on IHC/FISH. We have also monitored HER2 status in liquid biopsies, which has several advantages over genomic analysis of tissues. Blood has exposure to all potential metastatic sites allowing for the detection of different variants from different metastatic sites. Thus, blood may be more representative of the metastatic tumor than examination of a single biopsied lesion, and may reflect tumor evolution and intratumoral heterogeneity [ 25 ]. Blood samples are more easily collected, making multiple serial collections possible. Collecting multiple serial tissue samples is impractical, costly, and represents a much greater risk to patients than does serial collection of blood. The assessment of ctDNA is a powerful tool, showing very promising results to monitor tumor recurrence and response to therapy, but it does not yet replace the current gold standard, IHC/FISH, for the assessment of HER2 status in solid tumors. However, the monitoring of the HER2 status in ctDNA does provide an indicator of tumor response to therapy.
In our phase Ib/II study, HER2 tissue was amplified in the baseline samples (TP0) (pre- anti-HER2 therapy) in all patients by local determination, however, in liquid biopsies at C1D1 after chemotherapy + HP, HER2-amplification was detected in only 20/42 (48%) of patients. Patients with ctHER2-amp versus non-amplified HER2 ctDNA determined in C1D1 ctDNA had a longer median PFS, 480 days versus 60 days ( P = 0.015). It is expected that patients with HER2 amplification would respond to study therapy (chemotherapy + HP).
Loss of HER2 amplification observed after one cycle of study therapy may indicate that responders are either clearing ctDNA completely or that the amplification falls below the limit of detection. In the 5 cases who were ctHER2 DNA amplified, and completely cleared ctDNA, the response rate was 100%.
We applied a Molecular Response VAF ratio calculation to measure the change in ctDNA from baseline to C2D1, with the hypothesis that an early decrease in ctDNA levels would predict response to T-DM1 + neratinib therapy, as measured by PFS and RECIST response. Indeed, Molecular Response was associated with both PFS and best response to therapy. This should be confirmed in larger dataset, however, our findings are in line with other studies demonstrating the ability of ctDNA to predict short- and long-term efficacy. Early data from the PADA-1 trial suggests that changing therapy based on alterations detected in ctDNA, prior to evidence of progression via imaging, may provide clinical benefit. In that trial, patients with ER + /HER2-negative metastatic breast cancer being treated in the first line setting with an aromatase inhibitor (AI) + palbociclib were monitored for hotspot ESR1 alterations via ctDNA using digital droplet PCR (ddPCR). Patients with rising ESR1 VAF on therapy, but no synchronous evidence of disease progression via RECIST 1.1, were randomized to either continue receiving an AI + palbociclib or switched to fulvestrant + palbociclib. PADA-1 met its primary efficacy objective, with patients randomized to receive fulvestrant + palbociclib having a significantly longer PFS compared to those who stayed on an AI + palbociclib (median PFS 11.9 months [95% CI 9.1–13.6 months] versus 5.7 months [95% CI 3.9–7.5 months]; stratified HR 0.61 [95% CI 0.43–0.86], two-sided P = 0.004) [ 26 ]. More data on mutational evolution is needed to determine whether similar strategies employing ctDNA to inform change in therapy will be broadly applicable across breast cancer subtypes and therapy classes in order to further prolong OS.
Although a cross comparison of studies can be problematic, phase II and III studies suggest that as patients are more heavily treated with anti-HER2 regimens, the PFS and ORR decrease with each subsequent anti-HER2 therapy [ 27 , 28 , 29 , 30 ]. However, in a phase III randomized trial of trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in patients (N = 524) whose disease progressed on anti-HER2 therapy, the reported ORR for patients treated with T-DXd or T-DM1 were 79.7% versus 34.2%, respectively. The landmark analysis of PFS at 12 months was 75.8% with T-DXd as compared to 34.1% with T-DM1 (HR 0.28, 95% CI 0.22–0.37; P < 0.001) [ 6 ]. Although both T-DXd and T-DM1 have a trastuzumab backbone, there are substantial differences in the linker-payload chemistry, which favors an increased intracellular payload and a bystander effect with T-DXd [ 31 , 32 ].
We have shown in our study that the benefit from T-DM1 + neratinib is limited in HER2-non-amplified tumors. This finding is consistent with a study reporting a PFS with T-DM1 of 1.5 months [ 33 ] in patients discordant for HER2 in primary and metastatic tissue (HER2-positive/negative). Although loss of HER2-amplification appears to be one mechanism of resistance to T-DM1, half of the patients with HER2-amplified tumors did not respond to T-DM1 + neratinib, indicating that resistance to T-DM1 is not limited to loss of HER2-amplification. Many other mechanisms of resistance to T-DM1 have been proposed, such as altered cellular uptake, intracellular transport, and metabolism of the payload. [ 32 ]
Based on the low level of activity of T-DM1 monotherapy in patients failing HP, we speculate that the combination of T-DM1 and neratinib is more effective than T-DM1 monotherapy. We are unaware of any trials in HER2-positive breast cancer in which patients progressing on HP have been randomized to compare the response to T-DM1 as monotherapy with a combination of T-DM1 and an irreversible tyrosine kinase inhibitor (TKI). However, in preclinical lung models with ERBB2 mutation and/or amplification, the combination of T-DM1 + neratinib did show increased efficacy over monotherapy. Anecdotally, enhanced efficacy was demonstrated in a breast cancer patient progressing on monotherapy with T-DM1 who then responded with addition of neratinib. The mechanism of action of the antibody–drug conjugates (ADC) such as T-DM1 and T-DXd involves the recognition and binding of the trastuzumab backbone to the extracellular HER2 surface receptor. The ADC-protein complex is internalized with cleavage of the cytotoxic payload. Irreversible TKIs such as neratinib and afatinib (unlike reversible TKIs such as lapatinib) have been shown to enhance HER2 internalization and lysosomal sorting, which has the potential to increase uptake of bound ADC and release of their cytotoxic payload [ 34 ].
The KATHERINE [ 6 ] study established T-DM1 as a standard of care in early HER2-positive breast cancer patients with residual disease after neoadjuvant therapy [ 1 ]. DESTINY-Breast03 has clearly shown superiority of T-DXd over T-DM1 in HER2-positive metastatic disease. The currently recruiting DESTINY-Breast05 study will compare T-DXd with T-DM1 in high-risk HER2-positive patients with residual disease following NAC-HP (NCT04622319). Another study, DESTINY-Breast06 (NCT04494425), will address the question of HER2-low (IHC 1 + or IHC 2 + /FISH-negative or HER2 IHC > 0, < 1 +) in patients with metastatic hormone-positive disease with progression on at least two lines of endocrine therapy comparing T-DXd with investigator’s choice of chemotherapy [ 31 ]. The 30-40% of HER2-positive patients with residual or metastatic disease after neoadjuvant therapy who have lost HER2 amplification, while not directly being addressed with these ADCs studies, warrant further investigation with newer generation ADCs.
Our study has several limitations including the non-randomized design, the logistic difficulties in obtaining samples of blood and tissue on all patients and the small sample size, which limited its power and the ability to perform multivariant analysis. However, the strengths of our study include the multiple temporal sample collections, multiple assessments of HER2 status, and molecular assessment of DNA in both tissue and blood.
Despite the limitations of our study, the findings have generated several hypotheses that should be further investigated. First, retrospective confirmation in a large phase III study that loss of HER2-expression under the pressure of therapy can be detected with liquid biopsy; second, the overall response, depth, and duration of response to anti-HER2 therapy is greater in patients with HER2-amplified than in non-amplified patients; third, the activity of T-DM1 or other ADCs with a trastuzumab-backbone may be enhanced with addition of an irreversible TKI such as neratinib. This hypothesis, testing the interaction of an ADC with a TKI, reversible and irreversible, could be evaluated in patient-derived xenografts or other model systems and should be validated prior to a randomized trial. Finally, a small fraction of HER2-nonamplified patients did benefit from T-DM1 + neratinib. Possible explanations, which require further investigation, include a false negative assay, enhanced internalization of T-DM1 in presence of neratinib, or EGFR becoming the driver in patients with loss of HER2-amplification, and inhibition by neratinib [ 32 , 33 , 34 ]. We also realize that a low ctDNA fraction could have prevented the detection of ctHER2 amplification.
Gene expression analysis revealed several important observations. First, the level of HER2 RNA expression in TP1 tissues was closely correlated with the response rate to study therapy. Second, non-luminal subtypes had a better response rate than luminal tumors, although this difference was not statistically significant. Third, there was a non-significant association of the 8-gene trastuzumab benefit groups with the rate of responses to study therapy. Fourth, changes in intrinsic subtypes were seen between TP0 and TP1 tissue samples, indicating that these changes may be a result of tumors evolving to become resistant to HP. These results highlight the importance of collecting and monitoring molecular changes in tissue samples as patients move through their treatments.
PIK3CA mutations are a known oncogenic driver in breast cancer and drive therapeutic resistance in multiple HER2-targeted therapies [ 35 ]. In the EMILIA trial, patients with PIK3CA mutations, treated with capecitabine + lapatinib were associated with a shorter PFS than were patients with wild-type tumors; however, in patients treated with T-DM1 this was not the case [ 36 ]. We likewise see that in patients treated with T-DM1 + neratinib, PIK3CA mutations were not associated with outcomes. However, we cannot rule out the possibility that a subset of patients, refractory to T-DM1 + neratinib with PIK3CA mutations, may be responsive to PIK3CA inhibitors.
We demonstrate the usefulness of serial assessment of HER2 status in blood and tissue in patients with an initial diagnosis of HER2-positive disease. Loss of HER2 amplification in ctDNA, or the complete loss of ctDNA on treatment with T-DM1 + neratinib, was associated with clinical benefit. Further, we show that many of the patients with short-lived PR or PD were HER2-low in tissue. These patients may be better treated with the recently approved ADC, trastuzumab deruxtecan. We observed that the ADC, T-DM1, plus neratinib, was well tolerated. The combination with an irreversible tyrosine kinase inhibitor with other ADCs warrants investigation.
Availability of data and materials
Anonymized individual participant data that underlie the results reported in this article will be available in dbGAP or other publicly available site after publication.
von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Article Google Scholar
Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–9.
Article PubMed Google Scholar
Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15:7381–8.
Article CAS Google Scholar
Ferraro E, Safonov A, Wen HY, et al. Abstract P2-13-06: clinical implication of HER2 status change after neoadjuvant chemotherapy with Trastuzumab and Pertuzumab (HP) in patients with HER2-positive breast cancer. Cancer Res. 2022;82:2–13.
Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21.
Article CAS PubMed Google Scholar
Cortes J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54.
Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38:1887–96.
Article CAS PubMed PubMed Central Google Scholar
Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20.
Fehrenbacher L, Cecchini RS, Geyer CE Jr, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38:444–53.
Abraham J, Montero AJ, Jankowitz RC, et al. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. J Clin Oncol. 2019;37:2601–9.
Thompson JC, Carpenter EL, Silva BA, et al. Serial monitoring of circulating tumor DNA by next-generation gene sequencing as a biomarker of response and survival in patients with advanced NSCLC receiving pembrolizumab-based therapy. JCO Precis Oncol. 2021;5:510–24.
Zhang J-T, Liu S-Y, Gao W, et al. Longitudinal undetectable molecular residual disease defines potentially cured population in localized non-small cell lung cancer. Cancer Discov. 2022;12:1690–701.
Paik PK, Felip E, Veillon R, et al. Tepotinib in non–small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383:931–43.
Martínez-Sáez O, Pascual T, Brasó-Maristany F, et al. 5P Circulating tumour DNA (ctDNA) dynamics using a standardized multi-gene panel in advanced breast cancer patients (pts) treated with CDK4/6 inhibitors (CDK4/6i). Ann Oncol. 2020;31:S17.
Paquet ER, Hallett MT. Absolute assignment of breast cancer intrinsic molecular subtype. J Natl Cancer Inst. 2015;107:357.
Pogue-Geile KL, Kim C, Jeong JH, et al. Predicting degree of benefit from adjuvant trastuzumab in NSABP trial B-31. J Natl Cancer Inst. 2013;105:1782–8.
Pogue-Geile KL, Song N, Serie DJ, et al. Validation of the NSABP/NRG oncology 8-gene trastuzumab-benefit signature in alliance/NCCTG N9831. JNCI Cancer Spectr. 2020. https://doi.org/10.1093/jncics/pkaa058 .
Article PubMed PubMed Central Google Scholar
Gaibar M, Beltrán L, Romero-Lorca A, et al. Somatic mutations in HER2 and implications for current treatment paradigms in HER2-positive breast cancer. J Oncol. 2020;2020:6375956.
Ng CK, Martelotto LG, Gauthier A, et al. Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol. 2015;16:107.
Nguyen TT, Hamdan D, Angeli E, et al. Genomics of breast cancer brain metastases: a meta-analysis and therapeutic implications. Cancers (Basel). 2023;15:1728.
Jacobs SA, Robidoux A, Abraham J, et al. NSABP FB-7: a phase II randomized neoadjuvant trial with paclitaxel + trastuzumab and/or neratinib followed by chemotherapy and postoperative trastuzumab in HER2+ breast cancer. Breast Cancer Res. 2019;21:133.
Pogue-Geile K, Wang Y, Feng H, et al. Association of molecular signatures, mutations, and sTILs, with pCR in breast cancer patients in NRG Oncology/NSABP B-52. Cancer Res. 2019;79:4064.
Dieras V, Deluche E, Lusque A. Trastuzumab deruxtecan for advanced breast cancer patients, regardless of HER2 status: a phase II study with biomarkers analysis (DAISY). SABCS:PD8-02, 2021
Lee HJ, Kim JY, Park SY, et al. Clinicopathologic significance of the intratumoral heterogeneity of HER2 gene amplification in HER2-positive breast cancer patients treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144:570–8.
Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26:1859–64.
Bidard F-C, Hardy-Bessard A-C, Dalenc F, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising <em>ESR1</em> mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367–77.
Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99.
Conte B, Fabi A, Poggio F, et al. T-DM1 efficacy in patients with HER2-positive metastatic breast cancer progressing after a taxane plus pertuzumab and trastuzumab: an Italian multicenter observational study. Clin Breast Cancer. 2020;20:e181–7.
Yokoe T, Kurozumi S, Nozawa K, et al. Clinical benefit of treatment after trastuzumab emtansine for HER2-positive metastatic breast cancer: a real-world multi-centre cohort study in Japan (WJOG12519B). Breast Cancer. 2021;28:581–91.
Bardia A, Barrios C, Dent R et al: Abstract OT-03-09: Trastuzumab deruxtecan (T-DXd; DS-8201) vs investigator’s choice of chemotherapy in patients with hormone receptor-positive (HR+), HER2 low metastatic breast cancer whose disease has progressed on endocrine therapy in the metastatic setting: a randomized, global phase 3 trial (DESTINY-Breast06). Cancer Res. 2021; 81:OT-03-09-OT-03-09
Hunter FW, Barker HR, Lipert B, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. 2020;122:603–12.
Van Raemdonck E, Floris G, Berteloot P, et al. Efficacy of anti-HER2 therapy in metastatic breast cancer by discordance of HER2 expression between primary and metastatic breast cancer. Breast Cancer Res Treat. 2021;185:183–94.
Li BT, Michelini F, Misale S, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020;10:674–87.
Rasti AR, Guimaraes-Young A, Datko F, et al. PIK3CA mutations drive therapeutic resistance in human epidermal growth factor receptor 2–positive breast cancer. JCO Precis Oncol. 2022;6:e2100370.
Baselga J, Lewis Phillips GD, Verma S, et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res. 2016;22:3755–63.
Download references
Acknowledgments
We would also like to thank Wendy L. Rea, BA, for editing the manuscript.
We would like to thank our funders BCRF (CONS-20-009), Guardant Health Inc., Puma Biotechnology, Inc., and the NSABP Foundation. The NSABP Foundation received funding from Puma Biotechnology to conduct the clinical trial and for the collection of tissues and blood samples associated with this clinical trial. No authors received any direct funding for this research, but indirectly received salary support for efforts to conduct this research. The funder played no role in the design of the study, or collection, analysis, or interpretation of the data, or in the writing of the manuscript or submission thereof.
Author information
Rachel C. Jankowitz
Present address: University of Pennsylvania Perelman School of Medicine, State College, PA, USA
Mohamad A. Salkeni
Present address: Virginia Cancer Specialists, Fairfax, VA, USA
Authors and Affiliations
NSABP Foundation, Pittsburgh, PA, USA
Samuel A. Jacobs, Ying Wang, Jame Abraham, Huichen Feng, Alberto J. Montero, Corey Lipchik, Melanie Finnigan, Rachel C. Jankowitz, Mohamad A. Salkeni, Sai K. Maley, Shannon L. Puhalla, Carmen J. Allegra, Kelly Vehec, Norman Wolmark, Peter C. Lucas, Ashok Srinivasan & Katherine L. Pogue-Geile
Cleveland Clinic, Weston/Taussig Cancer Institute, Cleveland, OH, USA
Jame Abraham & Alberto J. Montero
University Hospitals/Seidman Cancer Center, Case Western Reserve University, Cleveland, OH, USA
Alberto J. Montero
University of Pittsburgh, Pittsburgh, PA, USA
National Institutes of Health, Washington, DC, USA
UPMC Hillman Cancer Center, Pittsburgh, PA, USA
Shannon L. Puhalla, Norman Wolmark & Peter C. Lucas
University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Shannon L. Puhalla & Peter C. Lucas
International Drug Development Institute, Louvain-la-Neuve, Belgium
Fanny Piette
Guardant Health, Redwood City, CA, USA
Katie Quinn, Kyle Chang & Rebecca J. Nagy
University of Florida Health, Gainesville, FL, USA
Carmen J. Allegra
Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
Peter C. Lucas
Autism Impact Fund, Pittsburgh, PA, USA
Ashok Srinivasan
You can also search for this author in PubMed Google Scholar
Contributions
Conception &/or Design: SAJ, YW, JA, HF, CL, KPG. Acquisition (of pts/materials) &/or Analysis: All authors: SAJ, YW, JA, HF, AJM, CL, MF, RCJ, AMS, SKM, SLP, FP, KQ, KC, RJN, CJA, KV, NW, PCL, AS, KP-G. Interpretation of the data: SAJ, YW, HF, FP, KQ, AS, KP-G. Has drafted the work or substantively revised it: SAJ, YW, AS, KP-G.
Corresponding author
Correspondence to Samuel A. Jacobs .
Ethics declarations
Ethics approval and consent to participate.
Central IRB approval provided by Adverra IRB, Columbia, MD.
Competing interests
AJ Montero: Honoraria: Celgene, AstraZeneca, OncoSec; Consulting/Advisory Role: New Century Health, Welwaze, Paragon healthcare; Research Funding: F. Hoffmann-La Roche Ltd, Basel, Switzerland; Uncompensated: Roche; Open Payments: https://openpaymentsdata.cms.gov/physician/618396 . K Quinn: Guardant Health Shareholder. K Chang: Guardant Health Shareholder. RJ Nagy: Guardant Health Shareholder. PC Lucas: Equity interest in AMGEN outside the submitted work. KL Pogue-Geile: Consulting for Bluestar BioAdvisors and Provisional Patents filed both outside the submitted work. All other authors declare no other potential conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Additional Patient information and Methodological Details.
Additional file 2
. All Molecular and Response Data Information for NSABP FB-10 Patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jacobs, S.A., Wang, Y., Abraham, J. et al. NSABP FB-10: a phase Ib/II trial evaluating ado-trastuzumab emtansine (T-DM1) with neratinib in women with metastatic HER2-positive breast cancer. Breast Cancer Res 26 , 69 (2024). https://doi.org/10.1186/s13058-024-01823-8
Download citation
Received : 05 October 2023
Accepted : 11 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s13058-024-01823-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Metastatic breast cancer
- ctDNA HER2 amplification
- Clinical trial
- Neratinib + t-DM1
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
8600 Rockville Pike, Bethesda, MD 20894. Go to the classic website. ClinicalTrials.gov. An official website of the U.S. Department of Health and Human Services, National Institutes of Health, National Library of Medicine, and National Center for Biotechnology Information.
ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Explore 492,685 research studies in all 50 states and in 223 countries.
Steps for Registering a Clinical Study. The steps on this page describe the overall process of registering studies. If you would like step-by-step instructions for entering registration information into the PRS, see the PRS Guided Tutorials. The tutorials include a quick overview guide called Entering a New Registration that briefly summarizes ...
The ClinicalTrials.gov Protocol Registration and Results System (PRS) is a Web-based data entry system used to register a clinical study or submit results information for a registered study. You must have an account in order to Login to ClinicalTrials.gov PRS. ClinicalTrials.gov allows the registration of clinical studies with human subjects ...
Sponsors can register clinical studies on clinicaltrials.gov via a web-based data entry system called the Protocol Registration and Results System (PRS). To register your clinical trial: ... A clinical trial (also clinical research) is a research study in human volunteers to answer specific health questions. Interventional trials determine ...
All NIH-funded clinical trials are expected to register and submit results information to Clinicaltrials.gov, as per the "NIH Policy on Dissemination of NIH-Funded Clinical Trial Information" for competing applications and contract proposals submitted on or after January 18, 2017 .This website provides resources for understanding and complying with this NIH policy and the federal regulations ...
Effective January 18, 2017, the NIH Policy on the Dissemination of NIH-funded Clinical Trials establishes the expectation for Clinicaltrials.gov registration of all NIH-funded clinical trials, funded in whole or in part by NIH, regardless of study phase, type of intervention, or whether the trial is subject to FDAAA. This policy applies to ...
Register Your Clinical Trial. Sponsors can register clinical studies on clinicaltrials.gov via a web-based data entry system called the Protocol Registration and Results System (PRS). To register your clinical trial: Check to see whether your organization already has a PRS organization account. (link is external) . Apply for a PRS account.
2. Requirements for Registering & Reporting NIH-funded Clinical Trials in ClinicalTrials.gov: Find resources for understanding and complying with the NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information and the federal regulations in FDAAA 801 as implemented by 42 CFR Part 11 (the Final Rule). (Source: NIH Grants & Funding)
NIH conducts clinical research trials for many diseases and conditions, including cancer, Alzheimer's disease, allergy and infectious diseases, and neurological disorders. To search for other diseases and conditions, you can visit ClinicalTrials.gov. This is a searchable registry and results database of federally and privately supported ...
The NIMH facilitates the registration of NIMH-sponsored trials by using the National Library of Medicines (NLM) Protocol Registration System (PRS). In order to create and update your trial record, you or your designee will need to have an NIMH user account (free of charge) with the NLMs PRS. To obtain a new user account, go to the PRS website.
Conclusion: Prospective registration of clinical trial is mandatory for more transparent research and sustaining the validity of evidence based practice and availability of reliable data. Clinical trials registration has the potential to contribute substantially to improve clinical trial transparency and reducing publication bias and selective ...
Clinical trial information means the data elements, including clinical trial registration information and clinical trial results information, that the responsible party is required to submit to ClinicalTrials.gov, as specified in section 402 (j) of the Public Health Service Act ( 42 U.S.C. 282 (j)) and this part.
The research community has embraced trial registration. Before the ICMJE policy, ClinicalTrials.gov, the largest trial registry at the time, contained 13,153 trials; this number climbed to 22,714 ...
Clinical trials registration seeks to regulate and streamline the clinical trials by mandating registration in various registries, through free for registration sites such as Clinical Trials Registry of India (CTRI). ... The ICMJE definition of a clinical trial is 'any research project that prospectively assigns people or a group of people to ...
Following ICMJE recommendations, clinical trials that began enrolling participants 1 January 2019 and later must include a data sharing plan in the trial's registration. If the data sharing plan ...
The mission of the WHO International Clinical Trials Registry Platform is to ensure that a complete view of research is accessible to all those involved in health care decision making. This will improve research transparency and will ultimately strengthen the validity and value of the scientific evidence base. Registration of all interventional trials is a scientific, ethical and moral ...
Clinical trials registries collect basic health information from people who agree to be contacted about participating in future clinical trials or studies. A clinical trial is the study of new ways to prevent, detect or treat diseases or conditions. Volunteering for a registry does not mean a person has signed up for a clinical trial.
Trial registration, results disclosure, and data sharing are considered powerful tools for achieving higher levels of transparency and accountability in clinical trials, as well as for improving the quality and integrity of research. Increasing emphasis on knowledge sharing and growing demands for transparency in clinical research are ...
Clinical Trial Registries. Clinical Research Support (CRS) provides expertise in federal regulations and policy that govern reporting requirements in ClinicalTrials.gov and the National Cancer Institute's Clinical Trials Reporting Program (CTRP). CRS staff work with Consortium investigators and their staff to ensure registration, accrual ...
Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best. ... with known EGFR/ALK mutation at the time of registration are eligible, and these ... Cancer Center Clinical Trials Referral Office (855) 776-0015. More ...
Introduction. Prospective registration of clinical trials (ie, registration before enrolment of the first participant) is an important practice to reduce biases in their conduct and reporting. 1 A number of ethical and legal documents call for prospective registration: The Declaration of Helsinki 2 and the WHO registry standards 3 state that prospective registration and results reporting of ...
Why Do I Need to Register My Trial and Submit Results to ClinicalTrials.gov? Required by Law. The Final Rule for Clinical Trials Registration and Results Information Submission (42 CFR Part 11) clarifies and expands the regulatory requirements and procedures for submitting registration and results information for certain clinical trials to ClinicalTrials.gov, in accordance with Section 801 of ...
Established in 1911, The University of Tennessee Health Science Center aims to improve human health through education, research, clinical care and public service. The UT Health Science Center campuses include colleges of Dentistry, Graduate Health Sciences, Health Professions, Medicine, Nursing and Pharmacy. Patient care, professional education and research are carried out at hospitals and ...
The ICTRP collects trial registration information from different databases such as Clinicaltrial.gov and reported 34,291 clinical trials in 2013 versus 59,964 clinical trials in 2021. ... Kose M, et al. Electronic health records (EHRs) in clinical research and platform trials: application of the innovative EHR-based methods developed by EU ...
Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. ... data and safety monitoring for clinical trials. For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1 ...
Home | National Multiple Sclerosis Society
Center for Biologics Evaluation and Research. The purposes of eligibility criteria for cancer clinical trials are to select the intended patient population and reduce potential risks to trial ...
Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. ... For applications proposing P and F Programs that will accept projects that include Delayed Onset Human Subjects Research or Delayed Onset Clinical Trials, provide a plan to address the protection of human research participants ...
Clinical Trials registration NCT02236000. We previously reported our phase Ib trial, testing the safety, tolerability, and efficacy of T-DM1 + neratinib in HER2-positive metastatic breast cancer patients. ... No authors received any direct funding for this research, but indirectly received salary support for efforts to conduct this research ...