Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Stem-cell research articles from across Nature Portfolio
Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells are the source of all tissues, understanding their properties helps in our understanding of the healthy and diseased body's development and homeostasis.
Latest Research and Reviews
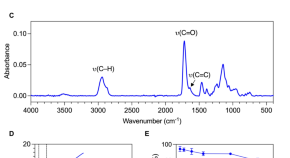
Considerations of growth factor and material use in bone tissue engineering using biodegradable scaffolds in vitro and in vivo
- Karen M. Marshall
- Jonathan P. Wojciechowski
- Richard O. C. Oreffo
Long-term lineage commitment in hematopoietic stem cell gene therapy
- Andrea Calabria
- Giulio Spinozzi
- Eugenio Montini
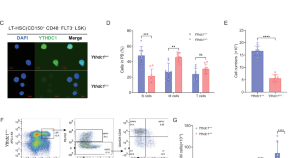
YTHDC1-mediated microRNA maturation is essential for hematopoietic stem cells maintenance
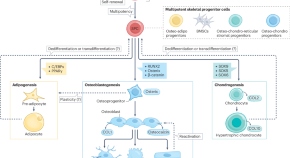
Skeletal stem and progenitor cells in bone physiology, ageing and disease
Various populations of skeletal stem and progenitor cells (SSPCs) exist within the native skeletal environments of humans and mice, with complex roles in the maintenance of bone tissue. This Review discusses the current state of knowledge of the identity and roles of SSPCs in skeletal health, disease and ageing.
- Seppe Melis
- Dana Trompet
- Christa Maes
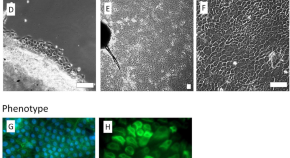
Bio-printing method as a novel approach to obtain a fibrin scaffold settled by limbal epithelial cells for corneal regeneration
- Krzysztof Pietryga
- Katarzyna Jesse
- Edward Wylęgała
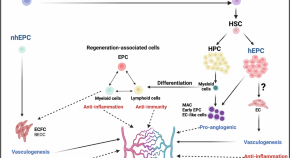
Anti-inflammatory Prowess of endothelial progenitor cells in the realm of biology and medicine
- Mehdi Hassanpour
- Amankeldi A. Salybkov
- Takayuki Asahara

News and Comment
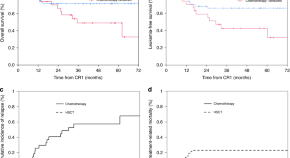
Comparison of allo-SCT versus consolidation chemotherapy as post-remission therapy in acute lymphoblastic leukaemia aged ≥55 years
- Yu-Qian Sun
(Donor) age is more than just a number…
- Amelia A. Langston
Impact of JACIE accreditation on safety of patient care: healthcare providers perspective from a tertiary care stem cell transplant center in Saudi Arabia
- Mohamed Bayoumy
- Ahlam Almasari
- Wasil Jastaniah

Wine without alcohol: medicine without doctors!
- Shaun R. McCann
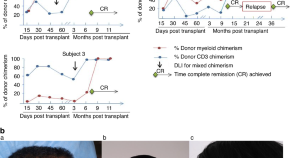
Durable remission of refractory and advanced stage mycosis fungoides/sezary syndrome utilizing an “outpatient” alemtuzumab, fludarabine-based reduced intensity allogeneic hematopoietic cell transplantation
- Ramaprasad Srinivasan
- Richard Childs
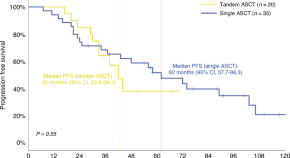
Real-world outcomes of tandem ASCT in newly diagnosed multiple myeloma patients with standard risk features: a single-center analysis
- Andrea Poveda-García
- Estela Ruiz
- Valentín Cabañas
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO