Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Tuberculosis articles from across Nature Portfolio
Tuberculosis (TB) is an infectious disease caused by strains of bacteria known as mycobacteria. The disease most commonly affects the lungs and can be fatal if not treated. However, most infected individuals show no disease symptoms. One third of the worlds population is thought to have been infected with TB.
Latest Research and Reviews
Prevalence of pulmonary tuberculosis and HIV infections and risk factors associated to tuberculosis in detained persons in Antananarivo, Madagascar
- Fanjasoa Rakotomanana
- Anou Dreyfus
- Rindra V. Randremanana
The recent rapid expansion of multidrug resistant Ural lineage Mycobacterium tuberculosis in Moldova
Chitwood et al. report on the rapid expansion of a Ural-lineage multidrug resistant strain of Mycobacterium tuberculosis in Moldova. This strain has an estimated reproduction number more than two times greater than otherwise similar drug susceptible strains.
- Melanie H. Chitwood
- Caroline Colijn
- Benjamin Sobkowiak
Genomic insights into anthropozoonotic tuberculosis in captive sun bears ( Helarctos malayanus ) and an Asiatic black bear ( Ursus thibetanus ) in Cambodia
- Kirsty Officer
- Timothy M. Walker
- Bethany Jackson
TOLLIP inhibits lipid accumulation and the integrated stress response in alveolar macrophages to control Mycobacterium tuberculosis infection
Toll-interacting protein (TOLLIP) prevents inflammation and lipid accumulation in alveolar macrophages to limit integrated stress response activation, macrophage necrosis and promote control of Mycobacterium tuberculosis .
- Sambasivan Venkatasubramanian
- Courtney R. Plumlee
- Javeed A. Shah
Multidrug-resistant tuberculosis
Multidrug-resistant tuberculosis (MDR-TB) is caused by Mycobacterium tuberculosis that is resistant to several first-line drugs. MDR-TB is an increasing public health challenge. In this Primer, Dheda et al. summarize the epidemiology and mechanisms, and discuss diagnosis, management and quality of life of patients with MDR-TB.
- Keertan Dheda
- Fuad Mirzayev
- Christoph Lange

Molecular docking, molecular dynamics simulations and binding free energy studies of interactions between Mycobacterium tuberculosis Pks13, PknG and bioactive constituents of extremophilic bacteria
- Kudakwashe Nyambo
- Kudzanai Ian Tapfuma
- Vuyo Mavumengwana
News and Comment
Restocking the tuberculosis drug arsenal
After many lean years, important progress has been made in updating the anti-tuberculosis drug armamentarium; a new drug that targets bacterial protein synthesis is one of several that could help transform the treatment of this neglected and deadly disease.
- Eric L. Nuermberger
- Richard E. Chaisson
Digital intervention improves tuberculosis treatment outcomes
An intervention that incorporates electronic pill boxes and remote adherence monitoring improved treatment success in patients with tuberculosis in Tibet — making this a promising strategy for low-resource settings.
- Karen O’Leary

A spotlight on the tuberculosis epidemic in South Africa
Tuberculosis is the leading cause of death from a single infectious agent, with over 25% of these occurring in the African region. Multi-drug resistant strains which do not respond to first-line antibiotics continue to emerge, putting at risk numerous public health strategies which aim to reduce incidence and mortality. Here, we speak with Professor Valerie Mizrahi, world-leading researcher and former director of the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town, regarding the tuberculosis burden in South Africa. We discuss the challenges faced by researchers, the lessons that need to be learnt and current innovations to better understand the overall response required to accelerate progress.
Presumed ocular tuberculosis – need for caution before considering anti-tubercular therapy
- Rohan Chawla
- Urvashi B. Singh
- Pradeep Venkatesh
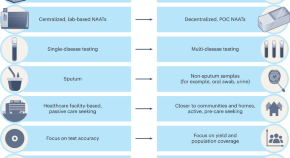
Transforming tuberculosis diagnosis
Diagnosis is the weakest aspect of tuberculosis (TB) care and control. We describe seven critical transitions that can close the massive TB diagnostic gap and enable TB programmes worldwide to recover from the pandemic setbacks.
- Madhukar Pai
- Puneet K. Dewan
- Soumya Swaminathan
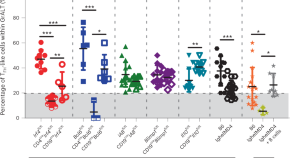
B cells and T follicular helper-like cells within lung granulomas are required for TB control
We show a crucial protective function for T follicular helper (T FH )-like cells localized within granuloma-associated lymphoid tissue for Mycobacterium tuberculosis control in mouse models of tuberculosis. Antigen-specific B cells contribute to this strategic localization and the maturation of cytokine-producing T FH -like cells.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Tuberculosis
Book editors.
- PMID: 30212088
- Bookshelf ID: NBK525174
- DOI: 10.1596/978-1-4648-0524-0_ch11
Despite 90 years of vaccination and 60 years of chemotherapy, tuberculosis (TB) remains the world’s leading cause of death from an infectious agent, exceeding human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) for the first time (WHO 2015b, 2016a). The World Health Organization (WHO) estimates that there are about 10.4 million new cases and 1.8 million deaths from TB each year. One-third of these new cases (about 3 million) remain unknown to the health system, and many are not receiving proper treatment.
Tuberculosis is an infectious bacterial disease caused by Mycobacterium tuberculosis (Mtb), which is transmitted between humans through the respiratory route and most commonly affects the lungs, but can damage any tissue. Only about 10 percent of individuals infected with Mtb progress to active TB disease within their lifetime; the remainder of persons infected successfully contain their infection. One of the challenges of TB is that the pathogen persists in many infected individuals in a latent state for many years and can be reactivated to cause disease. The risk of progression to TB disease after infection is highest soon after the initial infection and increases dramatically for persons co-infected with HIV/AIDS or other immune-compromising conditions.
Treatment of TB disease requires multiple drugs for many months. These long drug regimens are challenging for both patients and health care systems, especially in low- and middle-income countries (LMICs), where the disease burden often far outstrips local resources. In some areas, the incidence of drug-resistant TB, requiring even longer treatment regimens with drugs that are more expensive and difficult to tolerate, is increasing.
Diagnosis in LMICs is made primarily by microscopic examination of stained smears of sputum of suspected patients; however, smear microscopy is capable of detecting only 50–60 percent of all cases (smear-positive). More sensitive methods of diagnosing TB and detecting resistance to drugs have recently become available, although they are more expensive. The time between the onset of disease and when diagnosis is made and treatment is initiated is often protracted, and such delays allow the transmission of disease. Although bacille Calmette–Guérin (BCG) remains the world’s most widely used vaccine, its effectiveness is geographically highly variable and incomplete. Modeling suggests that more effective vaccines will likely be needed to drive tuberculosis toward elimination in high-incidence settings.
The basic strategy to combat TB has been, for 40 years, to provide diagnosis and treatment to individuals who are ill and who seek care at a health facility. The premise is that, if patients with active disease are cured, mortality will disappear, prevalence of disease will decline, transmission will decline, and therefore incidence should decline. The reality in many countries is more complex, and overall the decline in incidence (only about 1.5 percent per year) has been unacceptably slow.
Chemotherapy for TB is one of the most cost-effective of all health interventions (McKee and Atun 2006). This evidence has been central to the global promotion of the WHO and Stop TB Partnership policy of directly observed therapy, short course (DOTS) strategy, the package of measures combining best practices in the diagnosis and care of patients with TB (UN General Assembly 2000). The DOTS strategy to control tuberculosis promotes standardized treatment, with supervision and patient support that may include, but is far broader than, direct observation of therapy (DOT), where a health care worker personally observes the patient taking the medication (WHO 2013a).
Thanks in part to these efforts and national and international investments, much progress has been made in TB control over the past several decades. Between 1990 and 2010, absolute global mortality from TB declined 18.7 percent, from 1.47 million to 1.20 million (Lozano and others 2012) and by 22 percent between 2000 and 2015 (WHO 2016a). By 2015, an estimated 49 million lives had been saved (WHO 2016a). The internationally agreed targets for TB, embraced in the United Nations (UN) Millennium Development Goals (MDGs), sought “to halt and reverse the expanding incidence of tuberculosis by 2015,” and this target has been met to some extent in all six WHO regions and in most, but not all, of the world’s 22 high-burden countries (WHO 2014c).
Despite progress, major gaps persist. Although the Sustainable Development Goals (SDGs) seek to end the tuberculosis epidemic altogether (WHO 2015a, 2015c), the decline in incidence has been disappointing. One of every three TB patients remains “unknown to the health system,” many are undiagnosed and untreated, and case detection and treatment success rates remain too low in the high-burden countries. Ominously, rates of multidrug-resistant (MDR) TB—defined as resistance to the two major TB drugs, isoniazid and rifampicin—are rising globally (WHO 2011a) with the emergence of extensively drug-resistant (XDR) TB, resistant to many second-line drugs, as well as strains resistant to all current drugs (Dheda and others 2014; Udwadia and others 2012; Uplekar and others 2015). These are now primarily the result of transmission rather than inadequate treatment (Shah and others 2017).
Moreover, the TB problem has become more pressing because of co-infection with HIV/AIDS. While globally HIV/AIDS and TB co-infection represents only 11 percent of the total TB burden, in some areas of Sub-Saharan Africa with a high burden of TB, as many as three-quarters of TB patients are co-infected with HIV/AIDS. In those countries, efforts to control TB are overwhelmed by the rising number of TB cases occurring in parallel with the HIV/AIDS epidemic. And after decades of steady decline, the incidence of TB is also increasing in some high-income countries (HICs), mainly as the result of outbreaks in vulnerable groups (WHO 2015b).
If the ultimate goal of controlling an infectious disease is to interrupt transmission, turning the tide on TB will require early and accurate case detection, rapid commencement of and adherence to effective treatment that prevents transmission, and, where possible, preventive treatment of latent TB. It is universally understood that new strategies and more effective tools and interventions will be required to reach post-2015 targets (Bloom and Atun 2016; WHO 2015a). These interventions must be not only cost-effective, but also affordable and capable of having an impact on a very large scale.
TB control will need three new advances—development of new point-of-care diagnostics, more effective drug regimens to combat drug-susceptible and drug-resistant TB, and more effective vaccines. As argued in this chapter, these require new strategies and tools that include moving away from the traditional DOTS passive case finding and toward more active case finding in high-burden regions; service delivery that is targeted to the most vulnerable populations and integrated with other services, especially HIV/AIDS services; and care that is based at the primary health care and community levels. Specifically, in high-burden countries, many individuals with TB are asymptomatic, such that waiting for patients to become sick enough to seek care has not been sufficient to reduce transmission and incidence markedly (Bates and others 2012; Mao and others 2014; Willingham and others 2001; Wood and others 2007). A more active and aggressive approach is needed that tackles health system barriers to effective TB control.
The strategies for controlling TB recommended by the WHO have evolved significantly over time. In the early formulations, the central tenets of the global TB control strategy were clinical and programmatic in nature, focusing principally on the delivery of standardized drug regimens; the underlying assumption was that the problem could be solved largely by existing biomedical tools (Atun, McKee, and others 2005; Schouten and others 2011). Yet, in many LMICs, health system weaknesses in governance, financing, health workforce, procurement and supply chain management, and information systems have impeded TB control (Elzinga, Raviglione, and Maher 2004; Marais and others 2010; Travis and others 2004) and not been adequately addressed by TB control efforts. The current global TB strategy, formulated as the End TB Strategy, is the most comprehensive ever, with three major pillars:
Integrated, patient-centered care and prevention
Social and political action to address determinants of disease
Recognition of the urgent need for research to provide new tools (WHO 2015a).
Health systems are important and need to be strengthened. As with other health interventions, the success of tuberculosis treatment and control in a country is often determined by the strength of its health system (McKee and Atun 2006; WHO 2003). A health system can be defined in many ways, perhaps best as “all the activities whose primary purpose is to promote, restore, or maintain health” (WHO 2000, 5).
In a sense, the major risk factor for acquiring TB is breathing. Thus, people of all social and economic statuses are at risk. While TB disproportionately affects the poor, the narrative that TB is a disease only of the poor is misleading and counterproductive, if it leads either to further stigmatization of the disease or to the view that middle- and high-income countries need not worry about the disease. In the case of co-infection with HIV/AIDS, evidence suggests that HIV/AIDS is often more prevalent in better-off populations in Africa that suffer high rates of TB.
The analytical framework underlying this chapter defines key functions of the health system, ultimate goals, and contextual factors that affect the health system (figure 11.1). It builds on the WHO framework (WHO 2000) as well as health system frameworks developed by Frenk (1994), Hsiao and Heller (2007), and Roberts and others (2004), and national accounts (OECD, Eurostat, and WHO 2011). It also draws on earlier studies by Atun (2012); Atun and Coker (2008); Atun, Samyshkin, and others (2006); Samb and others (2009); and Swanson and others (2012).
The four key health system functions represented in the framework are as follows:
Governance and organization. The policy and regulatory environment; stewardship and regulatory functions of the ministry of health and its relation to other levels of the health system; and structural arrangements for insurers and purchasers, health care providers, and market regulators
Financing. The way funds are collected, funds and risks are pooled, finances are allocated, and health care providers are remunerated
Resource management. The way resources—physical, human, and intellectual—are generated and allocated, including their geographic and needs-based allocation
Service delivery. Both population- and individual-level public health interventions and health care services provided in community, primary health care, hospitals, and other health institutions.
Each of these functions is influenced by the economic, demographic, legal, cultural, and political context.
As the framework suggests, health system goals include better health, financial protection, and user satisfaction. Personal health services and public health interventions should be organized to achieve an appropriate balance of equity (including reducing out-of-pocket [OOP] expenditures and impoverishment of individuals and families), efficiency, effectiveness (that is, the extent to which interventions are evidence based and safe), responsiveness, equity, and client satisfaction (as perceived by the users of services).
This chapter is organized as follows. First, we provide a detailed discussion of the global burden of disease and clinical context, followed by a review of approaches to diagnosis, treatment, and prevention. The aim throughout is to approach TB through a health system lens and, in the latter part of the chapter, to provide recommendations for improving delivery strategies and strengthening health systems, including care, supply chain, and information systems. Because the current tools for combating TB are seriously inadequate, we conclude with sections on critical research and development and economic analyses of new interventions for diagnosis, treatment, and vaccines. Throughout, emphasis is placed on data or modeling of the economic costs and benefits, where available, of current or possible future interventions to combat this disease.
The chapter recommends moving toward active case finding in high-burden countries; greater investments in health systems; community-based rather than hospital-based service delivery; and greater support for research on new tools—that is, developing better diagnostics, treatment regimens, and vaccines. Most of these approaches were included in earlier WHO policies, but were not emphasized. They are now part of the WHO’s End TB Strategy, with which this report is fully consistent (WHO 2015a, 2015c).
© 2017 International Bank for Reconstruction and Development / The World Bank.
- Historical Trends, Current Burden, and Global Response
- Infection and Disease in Individuals and Populations
- TB Diagnosis and Screening
- TB Treatment
- TB Prevention
- Turning the Tide Against TB
- Research and Development
- Financing for TB Programs
- Economic Analyses and Cost-Effectiveness
- Extended Cost-Effectiveness Analysis of Universal Public Financing of TB Treatment
- Summary and Recommendations
Publication types
ORIGINAL RESEARCH article
Aberrant adaptive immune response underlies genetic susceptibility to tuberculosis.

- 1 BostonGene Laboratory, Waltham, United States
- 2 Abu Dhabi Stem Cells Center (ADSCC), Abu Dhabi, United Arab Emirates
- 3 Pirogov Russian National Research Medical University, Moscow, Moscow Oblast, Russia
- 4 Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Moscow , Russia, Moscow, Moscow Oblast, Russia
- 5 Institute of Clinical Molecular Biology, Faculty of Medicine, University of Kiel, Kiel, Schleswig-Holstein, Germany
- 6 Central Tuberculosis Research Institute (RAMS), Moscow, Moscow Oblast, Russia
- 7 Central European Institute of Technology (CEITEC), Brno, Olomouc, Czechia
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Mycobacterium tuberculosis (Mtb) remains a major threat worldwide, although only a fraction of infected individuals develops tuberculosis (TB). TB susceptibility is shaped by multiple genetic factors, and we performed comparative immunological analysis of two mouse strains to uncover relevant mechanisms underlying susceptibility and resistance. C57BL/6 mice are relatively TBresistant, whereas I/St mice are prone to develop severe TB, partly due to the MHC-II allelic variant that shapes suboptimal CD4 + T cell receptor repertoire. We investigated the repertoires of lunginfiltrating helper T cells and B cells at the peak of anti-Mtb immune responses in both strains. We found that lung CD4 + T cell repertoires of infected C57BL/6 but not I/St mice contained convergent TCR clusters with functionally confirmed Mtb specificity. Transcriptomic analysis revealed a more prominent Th1 signature in C57BL/6, and expression of pro-inflammatory IL-16 in I/St lunginfiltrating helper T cells. The two strains also showed distinct Th2 signatures. Furthermore, the humoral response of I/St mice was delayed, less focused, and dominated by IgG/IgM isotypes, whereas C57BL/6 mice generated more Mtb antigen-focused IgA response. We conclude that the inability of I/St mice to produce a timely and efficient anti-Mtb adaptive immune responses arises
Keywords: TCR repertoire, Tuberculosis, TB-susceptible mouse strain, CD4 + T cells, B cells, Immunoglobulins, Transcriptomic signatures
Received: 02 Feb 2024; Accepted: 11 Apr 2024.
Copyright: © 2024 Tsareva, Shelyakin, Shagina, Myshkin, Merzlyak, Kriukova, Apt, Linge, Chudakov and Britanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Irina Linge, Central Tuberculosis Research Institute (RAMS), Moscow, 107564, Moscow Oblast, Russia Olga Britanova, Institute of Clinical Molecular Biology, Faculty of Medicine, University of Kiel, Kiel, 24105, Schleswig-Holstein, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 14, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
Tuberculosis can have a lasting impact on the lung health of successfully treated individuals
by European Society of Clinical Microbiology and Infectious Diseases
New research being presented at this year's ESCMID Global Congress (formerly ECCMID) in Barcelona, Spain (27–30 April) has found compelling evidence that tuberculosis (TB) can have a lasting impact on the lungs of individuals who have been successfully treated for the disease.
TB survivors have smaller lungs with narrower airways and slower air flow, the analysis of data on tens of thousands of individuals from around the world found.
"This damage could have a profound effect on long-term health, reduce quality of life and affect ability to work and carry out day-to-day tasks," says lead researcher Dr. Sharenja Ratnakumar, of St George's, University of London, London, UK.
"And, with growing numbers of people being successfully treated for TB, the finding strongly indicates that post-TB lung disease is an under-recognized global challenge."
TB can be cured with antibiotics and, worldwide, an estimated 155 million people are alive today as a result of successful diagnosis and treatment of the bacterial infection.
However, although significant progress has been made in combating TB in recent decades, the number of new diagnoses has increased since the COVID-19 pandemic. Some 7.5 million were diagnosed globally in 2022—the highest number since monitoring began in 1995 and above the pre-COVID baseline of 7.1 million in 2019, according to WHO's 2023 Global Tuberculosis Report.
The burden is highest in sub-Saharan Africa and south east Asia but even low incidence countries such as the UK are seeing diagnoses increase. According to provisional data from the UK Health Security Agency, there were 4,850 new diagnoses in England in 2023. This is above pre-COVID levels and represents a rise of more than 10% on 2022, when there were 4,380 diagnoses.
Previous research has found that between 18% and >80% of survivors will be left with lung damage that reduces their quality of life and life expectancy but data on the size and type of respiratory impairment is scarce. To find out more, Dr. Ratnakumar and colleagues carried out a systematic review and meta-analysis of existing research on the topic.
The Medline, Embase and CINAHL databases were searched from 1/01/00 to 31/01/23 for studies that compared the lung function of individuals with a history of TB with that of healthy controls.
The meta-analysis included data on 75,631 individuals from 15 studies conducted in 17 countries with varying TB incidence and income levels.
The 7,377 TB survivors had an average age range of 11–65 years. Many of the studies were skewed towards a younger population (<50years) from mainly low- and middle-income countries.
Four measures of lung function were included in the analysis: forced expiratory volume in 1 second (FEV1, the volume of air can be forcefully exhaled in one second); forced vital capacity (FVC, the volume of air that can be forcefully exhaled in a single breath); FEV1/FVC ratio; FVC as a percentage of the predicted value (compares the volume to the average of a healthy person of the same age, sex and height).
The study found that, compared to the healthy controls, the participants with prior TB had significantly lower results on all four measures of lung function, with FEV1 more affected than FVC.
Dr. Ratnakumar says, "FEV1 was 230 milliliters lower compared to healthy controls and FVC was 140 milliliters lower. A decrease in FEV1 of 100 milliliters is considered clinically significant and is associated with an increased risk of cardiovascular and respiratory disease."
The results as a whole point to the TB survivors having smaller lungs (restrictive disease) and narrower airways with slower air flow (obstructive disease). This means that the breaths they take are smaller and take longer; breathing is less efficient and less able to respond to increased ventilatory demands such as during exercise.
Analysis of data from five of the studies showed the TB survivors to have 65% higher odds of airflow obstruction (AFO) than the healthy controls.
The results suggest TB can leave a lasting and widespread impact on the lungs, especially in terms of how the airways are structured. This valuable insight can help guide rehabilitation strategies and, in the longer term, aid in the development of new therapies, say the researchers.
Dr. Ratnakumar explains, "Our results strongly indicate that post-tuberculosis lung disease is an under-recognized global challenge—and one that has significant implications for clinical practice and policy.
"The focus, until now, has been on the treatment of acute TB, but even when treatment is successful, individuals can be left with significant lung damage.
"This can cause breathlessness that can affect their ability to work and go about their day-to-day lives and reduces their quality of life.
"This legacy of TB has been overlooked for too long and it is vital it is recognized.
"With an estimated 74 million lives saved through tuberculosis treatment between 2000 and 2020 and a rising life expectancy , there is an urgent need for evidence-based recommendations on the diagnosis, treatment and management of post-tuberculosis lung disease.
"Our study also provides compelling evidence that the long-term care of individuals with post-tuberculosis lung disease should be an explicit component of the WHO's End TB strategy."
Explore further
Feedback to editors

How trauma gets 'under the skin': Research investigates impaired muscle function caused by childhood trauma
36 minutes ago

New mechanism uncovered in early stages of Alzheimer's disease
52 minutes ago

Study reveals AI enhances physician-patient communication

Newly found rare cells could be a missing link in color perception

New vaccine strategy may mean the end of the line for endless boosters

Research explores why we remember what we remember
2 hours ago

Epilepsy drug prevents brain tumors in mice with neurofibromatosis type 1

New way found to treat early relapse in leukemia
3 hours ago

Researchers discover cause of a new rare genetic condition: Glutamine synthetase stabilization disorder

Microplastics make their way from the gut to other organs, researchers find
4 hours ago
Related Stories

COVID-19 pandemic could have led to 20,000 prostate cancer diagnosis being missed
Mar 18, 2024

New study reveals long-term mental health risks after COVID-19
Mar 21, 2024

Burden of visual impairment has increased globally, according to study
Dec 13, 2023

Fight against TB back on track after COVID disruptions: WHO
Nov 7, 2023
COVID-19 survivors may have higher risk of developing diabetes
Feb 13, 2023

One in five patients with rheumatoid arthritis went undiagnosed during the pandemic
Nov 3, 2022
Recommended for you

Specific nasal cells found to protect against COVID-19 in children
11 hours ago

Breakthrough aerosol human infection model gives hope for future TB vaccine development
5 hours ago

Large study finds antibiotics aren't effective for most lower tract respiratory infections
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

TB Epidemiologic Studies Consortium
The TB Epidemiologic Studies Consortium (TBESC) was established to strengthen, focus, and coordinate tuberculosis (TB) programmatic research. Since 2001, CDC has funded TBESC external partners to conduct epidemiologic and operational research to find better approaches to TB control and prevention.

TB Trials Consortium
The TB Trials Consortium (TBTC) is a collaboration of North American and international clinical investigators whose mission is to conduct programmatically relevant research concerning the diagnosis, clinical management, and prevention of TB infection and disease.
Behavioral and Social Science Research
Behavioral and social science research has the potential to make a tremendous impact on TB elimination efforts. This research is needed to 1) understand how behaviors of both patients and providers affect TB-related care seeking, diagnosis, treatment success, and prevention; and 2) understand how other social, cultural, and environmental influences affect health seeking and treatment outcomes related to TB.
Epidemiologic and Economic Modeling
Modeling epidemics and economics of disease provide useful information on how to prevent the greatest amount of disease with existing resources. In 2014, CDC funded a 5-year cooperative agreement, the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) Epidemiologic and Economic Modeling Agreement (NEEMA) , to support modeling activities to inform and, ultimately, improve the effectiveness of public health programs and activities supported by NCHHSTP.
- NEEMA Funded Projects by Topic Area – Tuberculosis
- NEEMA Published Papers – Tuberculosis
- NEEMA Abstracts- Tuberculosis
To receive email updates about this page, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Scoping Review
- Open access
- Published: 25 May 2023
Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis
- Nader Salari 1 , 2 ,
- Amir Hossein Kanjoori 3 ,
- Amin Hosseinian-Far 4 ,
- Razie Hasheminezhad 3 ,
- Kamran Mansouri 5 &
- Masoud Mohammadi ORCID: orcid.org/0000-0002-5722-8300 6
Infectious Diseases of Poverty volume 12 , Article number: 57 ( 2023 ) Cite this article
7393 Accesses
10 Citations
8 Altmetric
Metrics details
Tuberculosis is a bacterial infectious disease, which affects different parts of a human body, mainly lungs and can lead to the patient’s death. The aim of this study is to investigate the global prevalence of drug-resistant tuberculosis using a systematic review and meta-analysis.
In this study, the PubMed, Scopus, Web of Science, Embase, ScienceDirect and Google Scholar repositories were systematically searched to find studies reporting the global prevalence of drug-resistant tuberculosis. The search did not entail a lower time limit, and articles published up until August 2022 were considered. Random effects model was used to perform the analysis. The heterogeneity of the studies was examined with the I 2 test. Data analysis was conducted within the Comprehensive Meta-Analysis software.
In the review of 148 studies with a sample size of 318,430 people, the I 2 index showed high heterogeneity ( I 2 = 99.6), and accordingly random effects method was used to analyze the results. Publication bias was also examined using the Begg and Mazumdar correlation test which indicated the existence of publication bias in the studies ( P = 0.008). According to our meta-analysis, the global pooled prevalence of multi-drug resistant TB is 11.6% (95% CI : 9.1–14.5%).
Conclusions
The global prevalence of drug-resistant tuberculosis was found to be very high, thus health authorities should consider ways to control and manage the disease to prevent a wider spread of tuberculosis and potentially subsequent deaths.
Tuberculosis (TB) is one of the most common infectious diseases, which is the main cause of widespread mortality, especially among people living with HIV (PLHIV) [ 1 , 2 ]. The disease is caused by a type of bacteria called Mycobacterium TB [ 3 ]. Different types of TB are multi drug-resistant (MDR), pre-extensively drug-resistant (Pre-XDR), and extensively drug-resistant (XDR) [ 4 ]. TB usually affects the lungs, however it can also affect other parts of the body, such as the kidneys and the brain [ 4 ].
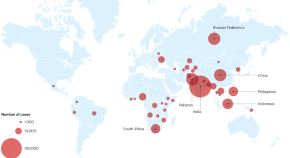
There were an estimated 450,000 incident cases of MDR in 2021, up 3.1% from 437,000 in 2020, three countries accounted for 42% of global cases in 202: India (26%), the Russian Federation (8.5%), and Pakistan (7.9%) [ 5 ]. A study by Baya et al., reported that the average age of patients was 39.31 ± 14.64 years, whilst 62.6% of patients were less than 40 years old. Patients were predominantly male 76.2%, and 77.1% were married [ 6 ]. Additionally, the prevalence of latent MDR TB has been reported in some countries of Eastern Europe and Central Asia, such as China (6 million people), India (4 million people), and Russia (1.8 million people) [ 4 , 5 , 6 ].
According to the existing literature, risk factors of tuberculosis include demographic characteristics such as gender, age, place of residence, education, marital status, bad habits such as alcohol abuse and smoking, and concomitant infections including diabetes mellitus, HIV, Acid-Fast Bacilli (AFB) smear, pulmonary space, history of tuberculosis, and history of anti-tuberculosis treatment are significant risk factors for MDR TB [ 7 ].
TB often impacts patients with other diseases such as diabetes, HIV, and chronic obstructive pulmonary disease (COPD) [ 7 ]. Cough or fever for > 2 weeks, weight loss, or hemoptysis are among the symptoms of TB which are also associated with lack of health insurance, tuberculin skin test, diagnosis through a process not entailing screening, and ethnicities other than Asian [ 8 ]. Complications of this disease include bronchial stenosis, severe airway obstruction, pneumonia, and hemoptysis, the most common of which is liver damage [ 9 , 10 ]. Vocal cord paralysis, associated with laryngeal TB, can also be found among the patients [ 10 ]. To treat TB, a combination of isoniazid, rifampin, ethambutol, and pyrazinamide, followed by a combination of isoniazid and rifampin are used [ 9 ].
Several studies have been conducted on the prevalence of drug-resistant tuberculosis worldwide. These studies have reported different rates, yet their reported results are heterogeneous and are not aligned. The aim of this systematic review and meta-analysis is to pool the reported results of the existing studies and offer a scientifically consistent prevalence for drug resistant TB. The findings of our study can provide useful insights to health policymakers to devised appropriate interventions, with a view to reducing the subsequent complications from the disease.
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The keywords of prevalence, drug-resistant tuberculosis, burden, outbreak and their combination using the (AND) and (OR) operators, were used to search the PubMed, Google Scholar, Science Direct, Embase, Scopus and Web of Science databases. The search was conducted with no lower time limit and until August 2022. The reference lists within the identified studies were also manually searched to ensure the comprehensive of the collected articles. The information of the identified studies was transferred into the EndNote reference management software, and studies that had reported the prevalence of drug-resistant tuberculosis by continent and were satisfying the inclusion criteria, were selected for final analysis.

Inclusion and exclusion criteria
The following criteria were used to keep an identified study in the systematic review and for meta-analysis: Studies that reported the prevalence of drug-resistant tuberculosis (including cross-sectional, case–control, and cohort studies), Studies with their full-text available, Studies that provided sufficient data (sample size, prevalence), Studies written and published in English. In contrary, the following criteria resulted in excluding identified articles: Case report studies, case series studies, duplicate studies and meta-analysis studies.
Study selection
Similarly, selection of studies was conducted in accordance with the PRISMA guidelines. Initially, articles that were duplicates in different databases were excluded, and only one copy was retained. Subsequently, the initial screening of articles was conducted through reviewing the titles and abstracts, and irrelevant articles were omitted based on the inclusion and exclusion criteria. Then their full text of articles was reviewed in line with the inclusion and exclusion criteria, and at this stage further irrelevant studies were removed. To avoid any potential bias, all the steps of reviews and data extraction were conducted by two reviewers independently. In cases where there was a difference of opinion between two reviewers, the review of the article was finalized by a third reviewer.
Quality evaluation
To evaluate the quality of articles, a checklist appropriate to observational studies was selected. The Strengthening the Reporting of Observational Studies in Epidemiology checklist (STROBE) consists of six scales including: title, abstract, introduction, methods, results, and discussion. In total, this instruction consists of 32 subscales. These 32 subscales denote different methodological aspects of the study, i.e., title, statement of the problem, study objectives, type of study, statistical population of the study, sampling method, determining the appropriate sample size, definition of variables and procedures, study data collection tools, statistical analysis methods and findings. Consider that the fulfilment of each of the subscales award a point, and based on this, articles with scores of 16 and above were considered to be of medium and high methodological quality articles respectively. Articles with a score below 16 were considered to be of poor quality and were therefore excluded from our study.
Data extraction
Data extraction was completed by two researchers using a different pre-prepared checklist. This checklist included: first author's name, year of publication, study location, sample size, age group of men and women, global prevalence of drug-resistant tuberculosis, and research instruments.
Statistical analysis
The extracted information were structured and were inputted into Comprehensive Meta-Analysis software (Version 2, Biostat, Inc., 14 North Dean Street, Englewood, NJ 07631 USA). The heterogeneity of the studies was then assessed using the I 2 test. In order to check the publication bias, the Begg’s test was used at a significance level of 0.1, and associated Funnel plots were drawn.
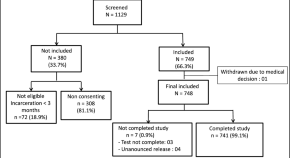
Following the initial search, 5109 articles were identified from the databases. An additional 60 related articles were also included following manual searches. Information of all identified articles were then transferred into the EndNote reference management software. Throughout the PRISMA’s identification stage, 2491 articles were excluded due to being repeated in various databases, and only one copy was retained. In the screening stage, the title and abstract of the studies were reviewed and 1964 further articles were excluded based on the inclusion and exclusion criteria. In the eligibility evaluation phase, 323 articles were omitted, after examination of the full text of the articles. As part of quality evaluation, and through the evaluation of the full text of the articles and based on the scores obtained from the STROBE checklist, studies with poor methodological quality were removed, and finally 148 studies were kept for analysis. All included studies were cross-sectional and most of the reviewed studies were conducted in Africa (continent). The information related to the 148 included studies is presented in Fig. 1 and Additional file 1 : Tables S1 to S6 [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 ].
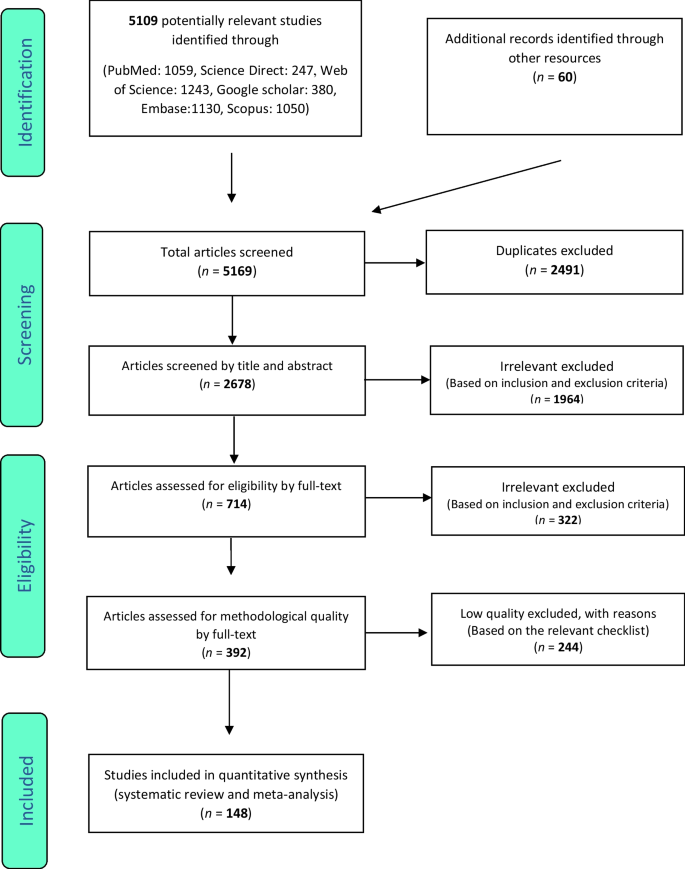
PRISMA flow diagram for study selection
Multi drug-resistant TB
In the review of 148 studies that had studied multi drug resistant TB (sample size of 318,430 people), the I 2 test showed a high heterogeneity ( I 2 = 99.6), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the global pooled prevalence of multi-drug resistant TB was found to be 11.6% (95% CI : 9.1–14.5%). Test of publication bias in the studies through the Begg and Mazumdar correlation test showed the existence of publication bias among the studies ( P = 0.008) (Table 1 ) (Figs. S1, S2 in Additional file 2 ).
Isoniazid resistant TB
In 98 studies with a focus on Isoniazid resistant TB (sample size of 102,260 people), the I 2 heterogeneity test showed a high heterogeneity ( I 2 = 99.03), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the pooled global prevalence of isoniazid resistant TB was found to be 15.7% (95% CI : 13.7–17.9%). The study of publication bias in the studies through the Begg and Mazumdar correlation test showed the existence of publication bias in the studies ( P = 0.02) (Table 1 ) (Figs. S3, S4 in Additional file 2 ).
Rifampin resistant TB
In the review of 109 studies that had researched rifampin resistant TB (sample size of 215,660 people), the I 2 heterogeneity test showed a high heterogeneity ( I 2 = 98.9), and similarly, random effects method was used to analyze the results. Based on the meta-analysis, the pooled global prevalence of rifampin- resistant TB was found as 9.4% (95% CI : 7.8–11.2%). The study of publication bias in the studies through the Begg and Mazumdar correlation test indicated the existence of publication bias in the studies ( P = 0.00045) (Table 1 ) (Figs. S5, S6 in Additional file 2 ).
Single drug resistant TB
In the review of 35 studies with a focus on single drug resistant TB (sample size of 45,147 people), the I 2 heterogeneity test showed a high heterogeneity ( I 2 = 98.5). Hence, random effects method was used to analyze the results. Considering the meta-analysis results, the pooled global prevalence of single drug resistant TB was found as 11.8% (95% CI : 9.2–15.2%). The study of publication bias in the studies through the Begg and Mazumdar correlation test showed the absence of publication bias in the studies ( P = 0.139) (Table 1 ) (Figs. S7, S8 in Additional file 2 ).
Extensive drug resistant TB
In the review of 56 studies on extensive drug resistant TB (sample size of 350,420 people), the I 2 heterogeneity test showed high heterogeneity ( I 2 = 98.8), and therefore, random effects method was used to analyze the results. Considering the meta-analysis results, the pooled global prevalence of extensive drug resistant TB was found to be 2.5% (95% CI : 2–3%). The study of publication bias using the Begg and Mazumdar correlation test indicated the absence of publication bias in the studies ( P = 0.938) (Table 1 ) (Figs. S9, S10 in Additional file 2 ).
Information in Table 2 outlines the subgroup analysis of the types of tuberculosis resistance among patients by gender, and by TB type. Accordingly, male patients have a higher prevalence in multi-drug resistant TB, Isoniazid resistant TB and Rifampin-resistant TB, compared to female patients, with prevalence of 20% (95% CI : 11.9–31.8%), 17.5% (95% CI : 9.6–29.8%), and 12.7% (95% CI : 5.7–25.9%) respectively. Given that the articles did not report gender-segregated data for single drug-resistant TB and extensively drug-resistant TB, the authors could not include these results in the subgroup analysis.
Tuberculosis is a very common infection with a bacterial agent called Mycobacterium [ 22 , 45 , 180 , 181 , 182 ]. MDR-TB is a strain of Tuberculosis (TB) that is resistant to at least two of the most important anti-tuberculosis drugs (INH and RIF) [ 180 , 181 , 182 , 183 , 184 , 185 ].
This systematic review and meta-analysis was conducted to identify and review existing research works that had examined prevalence of different types of TB. It was also aimed to obtain pooled prevalence of TB types globally. Accordingly, we did not find a specific study on the prevalence of drug-resistant tuberculosis at the global level, despite the fact that there are many articles that have reported the prevalence of this disease at country level, or at most in a continent.
Considering the reported results of an all included studies, the global pooled prevalence of different types of drug-resistant tuberculosis, namely MDR, Isoniazid (INH), Rifampcin (RIF), and XDR were calculated as 11.6%, 15.7%, 9.4%, and 2.5%, respectively.
Eastern European countries, the Russia and Central Asian countries, and parts of China have a high rate of MDR-TB infection [ 184 , 186 ]. In the study by Kindu Alem Mola et al., the authors reported that the level of MDR-TB in East Africa is higher than other regions globally [ 187 ]. In this work, based on the relevant reports from the World Health Organization (WHO) in 2015, the prevalence of global MDR TB in new and previous TB cases were 3.5% and 20.5%, respectively, while countries in southern regions of Africa have greater rates [ 187 , 188 ].
The main reasons for the emergence of MDR TB globally numerous [ 187 ], and they are mostly related to living conditions [ 189 ], lifestyle [ 190 ], previous medical history [ 111 , 191 ], history of diabetes [ 192 , 193 ] and Human Immunodeficiency Viruses (HIV) infection [ 194 ]. A study conducted in Ethiopia shows that HIV infection is one of the most important factors associated with MDR TB [ 187 , 195 ]. In addition, HIV patients, due to the length of hospitalization in hospitals with poorer hygiene and infection control, are more exposed to MDR TB and hence the rate of infection is higher among these patients [ 187 ]. In another study by Al-Derraji et al. [ 187 , 196 ], the incidence of MDR TB among HIV-positive patients was reported to be 20% higher compared to that of HIV-negatives [ 187 ].
In densely populated and poor families, the spread of TB disease is also more prevalent [ 187 ]. According to the literature, unhealthy or poor lifestyles which entail alcohol abuse, smoking, drug use, etc. are the main risk factors related to the spread of MDR TB [ 187 ]. It was also stated that smokers, especially men, are more likely to be infected with MDR TB compared to female smokers [ 187 , 197 , 198 , 199 ].
According to an article by Jilani Talha et al., tuberculosis complications are usually seen more among elderly patients, young children, people with severe respiratory disorders or patients who do not receive proper treatment. Accordingly, patients who do not receive proper treatment are more exposed to tuberculosis complications. Some of these complications are acute respiratory distress syndrome, extensive lung destruction, empyema, pneumothorax, disseminated tuberculosis infection (including tuberculosis meningitis), bronchiectasis, fibrothorax, aspergilloma, and hemoptysis [ 200 ].
According to a study conducted by Jilani et al., with a focus on treating active tuberculosis, a combination of drugs is required during the two intensive and the continuous phases; the first-line drugs that are the most common regimen for tuberculosis treatment include: (1) isoniazid, (2) rifampin, (3) ethambutol, and (4) pyrazinamide [ 200 ].
The intensive phase in the treatment of Tuberculosis includes the combination of the above 4 drugs that are prescribed for 2 months, yet the continuation phase includes the combination of isoniazid and rifampin for an additional 4 months. The second line drugs include: (1) Injectable aminoglycoside: streptomycin, amikacin, kanamycin; (2) Injectable polypeptides: viomycin and capreomycin; (3) Fluoroquinolones: levofloxacin, gatifloxacin, ofloxacin and moxifloxacin, and (4) Para-amino salicylic acid, ethionamide, cycloserine, prothionamide, trazodone, linezolid [ 200 ].
In a study conducted, the side effects of each anti-tuberculosis drug were described as follows: (1) Isoniazid: liver damage (fatigue, nausea, lethargy, abdominal pain, and vomiting), skin rash, numbness, headache and tingling of limbs; (2) Rifampin: jaundice, arthralgia (joint stiffness), and fever; (3) Ethambutol: visual impairment including blurred or reduced vision and blindness, liver damage, headache, and nausea, and (4) Pyrazinamide: nausea, painful or swollen joints, and liver damage [ 200 ]. According to the reported results of the same study, the highest prevalence of multi-drug resistant tuberculosis was reported in males.
Considering the ratio of infections among males vs females, one study reported that the split between males and females with multi-drug resistant tuberculosis was 70.4% and 29.6% respectively [ 201 ], In a study conducted in patients with resistant tuberculosis in Ghana, the ratio of males and females was 69.6% and 30.4% respectively [ 202 ], whilst in another study conducted in Egypt, the ratio of males and females was reported as 67.5% and 32.5%, respectively [ 203 ]. Moreover, in a similar research work conducted in Ethiopia 65.3% male and 34.7% female has multi drug resistant TB [ 204 ].
Our study shows that different strains of Tuberculosis, including drug-resistant TBs, have a high prevalence. On the other hand, these strains can be treated, and there are similar strategies and interventions to control existing and new infections. Considering the complications that this disease may cause, its control and management are vital, since it would be possible to reduce the Tuberculosis induced mortality rate through controlling its different strains.
The main limitation of the present meta-analysis is related to the significant publication bias among the identified studies, and therefore, the results should be considered with caution. Moreover, it is recommended that future meta-analysis studies in this field are conducted using more keywords and databases to potentially eliminate this bias.
According to the results of the present study, the global prevalence of multidrug-resistant, mono drug-resistant, isoniazid, and rifampicin tuberculosis are 11.6%, 11.8%, 15.7%, and 9.4%, respectively. The results of this study can offer some consistency to the heterogeneous results from studies conducted around the world and provide reliable insights to health policymakers. Such insights would be instrumental to devise appropriate preventive, therapeutic and diagnostic measures.
Availability of data and materials
Datasets are available through the corresponding author upon reasonable request.
Abbreviations
Drug susceptibility testing
Whole genome sequencing
Restriction fragment length polymorphism
Decontamination and Ziehl–Neelsen
Chest X-ray
Antimicrobial susceptibility testing
Confidence interval
Tuberculosis
Iacobino A, Fattorini L, Giannoni F. Drug-resistant tuberculosis 2020: where we stand. Appl Sci. 2020;10(6):2153.
Article CAS Google Scholar
Asgedom SW, Teweldemedhin M, Gebreyesus H. Prevalence of multidrug-resistant tuberculosis and associated factors in Ethiopia: a systematic review. J Pathogens. 2018;2018:7104921.
Article Google Scholar
Lange C, Chesov D, Heyckendorf J, Leung CC, Udwadia Z, Dheda K. Drug-resistant tuberculosis: an update on disease burden, diagnosis and treatment. Respirology. 2018;23(7):656–73.
Article PubMed Google Scholar
Mazurek GH. Division of tuberculosis elimination, national center for HIV, STD, and TB prevention, centers for disease control and prevention (CDC). Guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55.
PubMed Google Scholar
Global Tuberculosis Report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 .
Baya B, Achenbach CJ, Kone B, Toloba Y, Dabitao DK, Diarra B, et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. IJID. 2019;81:149–55.
Xi Y, Zhang W, Qiao R-J, Tang J. Risk factors for multidrug-resistant tuberculosis: a worldwide systematic review and meta-analysis. PLoS ONE. 2022;17(6): e0270003.
Article CAS PubMed PubMed Central Google Scholar
Miller LG, Asch SM, Yu EI, Knowles L, Gelberg L, Davidson P. A population-based survey of tuberculosis symptoms: how atypical are atypical presentations? Clin Infect Dis. 2000;30(2):293–9.
Article CAS PubMed Google Scholar
Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. DÄ International. 2019;116(43)
Hsu D, Irfan M, Jabeen K, Iqbal N, Hasan R, Migliori GB, et al. Post tuberculosis treatment infectious complications. IJID. 2020;92:S41–5.
Google Scholar
Wang W, Wang J, Zhao Q, Darling ND, Yu M, Zhou B, et al. Contribution of rural-to-urban migration in the prevalence of drug resistant tuberculosis in China. Eur J Clin Microbiol Infect Dis ESCMID. 2011;30(4):581–6.
Timire C, Metcalfe JZ, Chirenda J, Scholten JN, Manyame-Murwira B, Ngwenya M, et al. Prevalence of drug-resistant tuberculosis in Zimbabwe: a health facility-based cross-sectional survey. IJID. 2019;87:119–25.
CAS PubMed Google Scholar
Ahmad N, Javaid A, Sulaiman SA, Ming LC, Ahmad I, Khan AH. Resistance patterns, prevalence, and predictors of fluoroquinolones resistance in multidrug resistant tuberculosis patients. Braz J Infect Dis SBI. 2016;20(1):41–7.
Mohammed KAS, Khudhair GS, Bekheet A-R. Prevalence and drug resistance pattern of Mycobacterium tuberculosis isolated from tuberculosis patients in Basra, Iraq. Pol J Microbiol. 2022;71(2):205–15.
Article PubMed PubMed Central Google Scholar
Hu Y, Mathema B, Zhao Q, Chen L, Lu W, Wang W, et al. Acquisition of second-line drug resistance and extensive drug resistance during recent transmission of Mycobacterium tuberculosis in rural China. Clin Microbiol Infect ESCMID. 2015;21(12):1093.
Mazahir R, Beig FK, Ahmed Z, Alam S. Burden of tuberculosis among household children of adult multi drug resistant patients and their response to first line anti tubercular drugs. Egypt Pediatr Assoc Gazette. 2017;65(4):122–6.
Ayaz A, Hasan Z, Jafri S, Inayat R, Mangi R, Channa AA, et al. Characterizing Mycobacterium tuberculosis isolates from Karachi, Pakistan: drug resistance and genotypes. IJID. 2012;16(4):e303–9.
Al-Dabbagh M, Lapphra K, McGloin R, Inrig K, Schaaf HS, Marais BJ, et al. Drug-resistant tuberculosis: pediatric guidelines. PIDJ. 2011;30(6):501–5.
Yang Y, Zhou C, Shi L, Meng H, Yan H. Prevalence and characterization of drug-resistant tuberculosis in a local hospital of Northeast China. IJID. 2014;22:83–6.
Magula NP, Madala ND, Kriel Y, Bayi V, Duze NP, Manzini TC, et al. Prevalence of drug resistant tuberculosis in patients presenting with a large pericardial effusion at King Edward VIII Hospital. IJID. 2014;21:87.
Mahabeer P, Khan M, Mlisana K. Drug-resistant tuberculosis in children less than 5 years old with culture positive mycobacterium tuberculosis. IJID. 2016;45:212–3.
Berberian G, Gonzalez S, Reijtman V, Miño N, Casimir L, Sarkis C, et al. Seventeen years of drug-resistant tuberculosis in Argentinian children. IJID. 2016;45:387.
Singhal R, Arora J, Sah GC, Bhalla M, Sarin R, Prasad MV. Frequency of multi-drug resistance and mutations in Mycobacterium tuberculosis isolates from Punjab state of India. J Epidemiol Glob Health. 2017;7(3):175–80.
Prakash R, Kumar D, Gupta VK, Jain S, Chauhan DS, Tiwari PK, et al. Status of multidrug resistant tuberculosis (MDR-TB) among the Sahariya tribe of North Central India. J Infect Public Health. 2016;9(3):289–97.
Dujaili JA, Blebil AQ, Dujaili MA, Awaisu A, Hassali MA, Syed Sulaiman SA. Prevelance of pulmonary tuberculosis and multi drug resistant tuberculosis patients in baghdad, Iraq. Value Health. 2013;16(3):A82.
Santos LC, Bousquet Hde M, Pereira AM, Junqueira-Kipnis AP, Kipnis A. A high prevalence of resistance in new tuberculosis cases of midwestern Brazil. Infect Genet Evol. 2010;10(7):1052–7.
Kontsevaya I, Nikolayevskyy V, Kovalyov A, Ignatyeva O, Sadykhova A, Simak T, et al. Tuberculosis cases caused by heterogeneous infection in Eastern Europe and their influence on outcomes. Infect Genet Evol. 2017;48:76–82.
Bhembe NL, Green E. Molecular epidemiological study of multidrug-resistant tuberculosis isolated from sputum samples in Eastern Cape, South Africa. Infect Genet Evol. 2020;80: 104182.
Ghebremichael S, Petersson R, Koivula T, Pennhag A, Romanus V, Berggren I, et al. Molecular epidemiology of drug-resistant tuberculosis in Sweden. Microbes Infect. 2008;10(6):699–705.
Montoro E, Lemus D, Echemendía M, Armas L, González-Ochoa E, Llanes MJ, et al. Drug-resistant tuberculosis in Cuba results of the three global projects. Tuberculosis. 2006;86(3–4):319–23.
Pardini M, Niemann S, Varaine F, Iona E, Meacci F, Orrù G, et al. Characteristics of drug-resistant tuberculosis in Abkhazia (Georgia), a high-prevalence area in Eastern Europe. Tuberculosis. 2009;89(4):317–24.
Jiao W-W, Liu Z-G, Han R, Zhao X-Q, Dong F, Dong H-Y, et al. Prevalence of drug resistant Mycobacterium tuberculosis among children in China. Tuberculosis. 2015;95(3):315–20.
Brandao AP, Pinhata JMW, Simonsen V, Oliveira RS, Ghisi KT, Rabello MCS, et al. Transmission of Mycobacterium tuberculosis presenting unusually high discordance between genotypic and phenotypic resistance to rifampicin in an endemic tuberculosis setting. Tuberculosis. 2020;125: 102004.
Van Rie A, Warren R, Richardson M, Gie RP, Enarson DA, Beyers N, et al. Classification of drug-resistant tuberculosis in an epidemic area. Lancet. 2000;356(9223):22–5.
Chand K, Tewari S, Varghese S. Prevalence of drug resistant tuberculosis in armed forces-study from a tertiary referral chest diseases hospital at Pune. MJAFI. 2000;56(2):130–4.
Ismail NA, Mvusi L, Nanoo A, Dreyer A, Omar SV, Babatunde S, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18(7):779–87.
Daniel O, Osman E. Prevalence and risk factors associated with drug resistant TB in South West, Nigeria. Asian Pac J Trop Med. 2011;4(2):148–51.
Wang G, Peng YL, Zhang G, Zhang L, Xing J, Li D, et al. Sample survey of drug-resistant tuberculosis in Henan, China, 1996. Respirology. 2002;7(1):67–72.
Hannan MM, Peres H, Maltez F, Hayward AC, Machado J, Morgado A, et al. Investigation and control of a large outbreak of multi-drug resistant tuberculosis at a central Lisbon hospital. J Hosp Infect. 2001;47(2):91–7.
Wu B, Zhang L, Liu Z, He H, Pan A, Wang F, et al. Drug-resistant tuberculosis in Zhejiang Province, China: an updated analysis of time trends, 1999–2013. Glob Health Action. 2017;10(1):1293925.
Phyu S, Lwin T, Ti T, Maung W, Mar WW, Shein SS, et al. Drug-resistant tuberculosis in Yangon, Myanmar. Scand J Infect Dis. 2005;37(11–12):846–51.
Hu Y, Mathema B, Wang W, Hoffner S, Kreiswirth B, Xu B. Prevalence of multidrug-resistant pulmonary tuberculosis in counties with different duration of DOTS implementation in rural China. Microb Drug Resist. 2008;14(3):227–32.
Shah MA, Shah I. Increasing prevalence of pediatric drug-resistant tuberculosis in Mumbai, India, and its outcome. PIDJ. 2018;37(12):1261–3.
Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. Predominance of multi-drug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. J Med Microbiol. 2006;55(10):1413–8.
Kapata N, Chanda-Kapata P, Bates M, Mwaba P, Cobelens F, Grobusch MP, et al. Multidrug-resistant TB in Zambia: review of national data from 2000 to 2011. TM & IH. 2013;18(11):1386–91.
Kamolwat P, Nateniyom S, Chaiprasert A, Disratthakit A, Mahasirimongkol S, Yamada N, et al. Prevalence and associated risk factors of drug-resistant tuberculosis in Thailand: results from the fifth national anti-tuberculosis drug resistance survey. TM & IH. 2021;26(1):45–53.
Hu Y, Hoffner S, Wu L, Zhao Q, Jiang W, Xu B. Prevalence and genetic characterization of second-line drug-resistant and extensively drug-resistant Mycobacterium tuberculosis in Rural China. Antimicrob Agents Chemother. 2013;57(8):3857–63.
Huo F, Lu J, Zong Z, Jing W, Shi J, Ma Y, et al. Change in prevalence and molecular characteristics of isoniazid-resistant tuberculosis over a 10-year period in China. BMC Infect Dis. 2019;19(1):689.
Zhao LL, Chen Y, Chen ZN, Liu HC, Hu PL, Sun Q, et al. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Hunan, China. Antimicrob Agents Chemother. 2014;58(6):3475–80.
Daum LT, Konstantynovska OS, Solodiankin OS, Liashenko OO, Poteiko PI, Bolotin VI, et al. Next-generation sequencing for characterizing drug resistance-conferring Mycobacterium tuberculosis genes from clinical isolates in the Ukraine. J Clin Microbiol. 2018;56: e00009.
Dinic L, Akande P, Idigbe EO, Ani A, Onwujekwe D, Agbaji O, et al. Genetic determinants of drug-resistant tuberculosis among HIV-infected patients in Nigeria. J Clin Microbiol. 2012;50(9):2905–9.
Agarwal M, Gunal S, Durmaz R, Yang Z. Integration of Mycobacterium tuberculosi s drug susceptibility testing and genotyping with epidemiological data analysis to gain insight into the epidemiology of drug-resistant tuberculosis in Malatya, Turkey. J Clin Microbiol. 2010;48(9):3301–5.
Djuretic T, Herbert J, Drobniewski F, Yates M, Smith EG, Magee JG, et al. Antibiotic resistant tuberculosis in the United Kingdom: 1993–1999. Thorax. 2002;57(6):477–82.
Lv XT, Lu XW, Shi XY, Zhou L. Prevalence and risk factors of multi-drug resistant tuberculosis in Dalian, China. J Int Med Res. 2017;45(6):1779–86.
Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC Infect Dis. 2011;11:28.
Sharaf Eldin GS, Fadl-Elmula I, Ali MS, Ali AB, Salih AL, Mallard K, et al. Tuberculosis in Sudan: a study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect Dis. 2011;11:219.
Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15:461.
Ba Diallo A, Ossoga GW, Daneau G, Lo S, Ngandolo R, Djaibé CD, et al. Emergence and clonal transmission of multi-drug-resistant tuberculosis among patients in Chad. BMC Infect Dis. 2017;17(1):579.
Juma SP, Maro A, Pholwat S, Mpagama SG, Gratz J, Liyoyo A, et al. Underestimated pyrazinamide resistance may compromise outcomes of pyrazinamide containing regimens for treatment of drug susceptible and multi-drug-resistant tuberculosis in Tanzania. BMC Infect Dis. 2019;19(1):129.
Ogari CO, Nyamache AK, Nonoh J, Amukoye E. Prevalence and detection of drug resistant mutations in Mycobacterium tuberculosis among drug naïve patients in Nairobi, Kenya. BMC Infect Dis. 2019;19(1):279.
Saldanha N, Runwal K, Ghanekar C, Gaikwad S, Sane S, Pujari S. High prevalence of multi drug resistant tuberculosis in people living with HIV in Western India. BMC Infect Dis. 2019;19(1):391.
Gehre F, Otu J, Kendall L, Forson A, Kwara A, Kudzawu S, et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Med. 2016;14:160.
Diriba G, Kebede A, Tola HH, Alemu A, Tadesse M, Tesfaye E, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):54.
Cox HS, McDermid C, Azevedo V, Muller O, Coetzee D, Simpson J, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS ONE. 2010;5(11): e13901.
Hom JK, Wang B, Chetty S, Giddy J, Mazibuko M, Allen J, et al. Drug-resistant tuberculosis among HIV-infected patients starting antiretroviral therapy in Durban, South Africa. PLoS ONE. 2012;7(8): e43281.
Porwal C, Kaushik A, Makkar N, Banavaliker JN, Hanif M, Singla R, et al. Incidence and risk factors for extensively drug-resistant tuberculosis in Delhi region. PLoS ONE. 2013;8(2): e55299.
Isaakidis P, Das M, Kumar AMV, Peskett C, Khetarpal M, Bamne A, et al. Alarming levels of drug-resistant tuberculosis in HIV-infected patients in metropolitan Mumbai, India. PLoS ONE. 2014;9(10): e110461.
Ullah I, Shah AA, Basit A, Ali M, Khan A, Ullah U, et al. Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert MTB/RIF in Khyber Pakhtunkhwa, Pakistan: a retrospective study. BMC Infect Dis. 2016;16:143.
Mesfin EA, Beyene D, Tesfaye A, Admasu A, Addise D, Amare M, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS ONE. 2018;13(6): e0197737.
Kigozi E, Kasule GW, Musisi K, Lukoye D, Kyobe S, Katabazi FA, et al. Prevalence and patterns of rifampicin and isoniazid resistance conferring mutations in Mycobacterium tuberculosis isolates from Uganda. PLoS ONE. 2018;13(5): e0198091.
Shibabaw A, Gelaw B, Gebreyes W, Robinson R, Wang SH, Tessema B. The burden of pre-extensively and extensively drug-resistant tuberculosis among MDR-TB patients in the Amhara region, Ethiopia. PLoS ONE. 2020;15(2): e0229040.
Gilad J, Borer A, Riesenberg K, Peled N, Schlaeffer F. Epidemiology and ethnic distribution of multidrug-resistant tuberculosis in Southern Israel, 1992–1997. Chest. 2000;117(3):738–43.
Ramzan MM, Sabayev V, Anwar N, Patel A, Asnis D, Avaiya A, et al. Prevalence of drug resistant tuberculosis among asians: a flushing hospital experience. Chest. 2004;126(4, Supplement):753S.
Um S-J, Son C, Roh MS, Lee S-K, Kim KH, Huh J, et al. Prevalence Of Multi Drug Resistant Pulmonary Tuberculosis In Intermediate Endemism Country. A55 Multi-drug resistant and extensively drug-resistant tuberculosis: American Thoracic Society; 2011. p. A1824-A.
Diandé S, Badoum G, Combary A, Zombra I, Saouadogo T, Sawadogo LT, et al. Multidrug-resistant tuberculosis in Burkina Faso from 2006 to 2017: results of national surveys. Eur J Microbiol Immunol. 2019;9(1):23–8.
Becerril-Montes P, Said-Fernández S, Luna-Herrera J, Caballero-Olín G, Enciso-Moreno JA, Martínez-Rodríguez HG, et al. A population-based study of first and second-line drug-resistant tuberculosis in a high-burden area of the Mexico/United States border. Mem Inst Oswaldo Cruz. 2013;108(2):160–6.
Bastos GM, Cezar MC, Mello FC, Conde MB. Prevalence of primary drug resistance in pulmonary tuberculosis patients with no known risk factors for such. J Bras Pneumol. 2012;38(6):733.
Micheletti VCD, Moreira JS, Ribeiro MO, Kritski AL, Braga JU. Drug-resistant tuberculosis in subjects included in the second national survey on antituberculosis drug resistance in Porto Alegre, Brazil. J Bras Pneumol. 2014;40(2):155–63.
Zhao LL, Huang MX, Xiao TY, Liu HC, Li MC, Zhao XQ, et al. Prevalence, risk and genetic characteristics of drug-resistant tuberculosis in a tertiary care tuberculosis hospital in China. Infect Drug Resist. 2019;12:2457–65.
Migliori GB, Ortmann J, Girardi E, Besozzi G, Lange C, Cirillo DM, et al. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg Infect Dis. 2007;13(5):780.
Deng Y, Wang Y, Wang J, Jing H, Yu C, Wang H, et al. Laboratory-based surveillance of extensively drug-resistant tuberculosis, China. Emerg Infect Dis. 2011;17(3):495.
Wallengren K, Scano F, Nunn P, Margot B, Buthelezi SS, Williams B, et al. Drug-resistant tuberculosis, KwaZulu-Natal, South Africa, 2001–2007. Emerg Infect Dis. 2011;17(10):1913.
El Achkar S, Demanche C, Osman M, Rafei R, Ismail MB, Yaacoub H, et al. Drug-resistant tuberculosis, Lebanon, 2016–2017. Emerg Infect Dis. 2019;25(3):564.
Lee SW, Jeon K, Kim KH, Min KH. Multidrug-resistant pulmonary tuberculosis among young Korean soldiers in a communal setting. J Korean Med Sci. 2009;24(4):592–5.
Buyankhishig B, Naranbat N, Mitarai S, Rieder HL. Nationwide survey of anti-tuberculosis drug resistance in Mongolia. Int J Tuberculosis Lung Dis. 2011;15(9):1201–5.
Seddon JA, Hesseling AC, Marais BJ, Jordaan A, Victor T, Schaaf HS. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. IJTLD. 2012;16(7):928–33.
Bojorquez-Chapela I, Bäcker CE, Orejel I, López A, Díaz-Quiñonez A, Hernández-Serrato MI, et al. Drug resistance in Mexico: results from the national survey on drug-resistant tuberculosis. IJTLD. 2013;17(4):514–9.
Ei PW, Aung WW, Nyunt WW, Swe TL, Htwe MM, Win SM, et al. Extensively drug-resistant tuberculosis in Myanmar: burden and mutations causing second-line drug resistance. IJTLD. 2018;22(1):47–53.
Smith CM, Lessells R, Grant AD, Herbst K, Tanser F. Spatial clustering of drug-resistant tuberculosis in Hlabisa subdistrict, KwaZulu-Natal, 2011–2015. IJTLD. 2018;22(3):287–93.
Article CAS PubMed Central Google Scholar
Alikhanova N, Akhundova I, Seyfaddinova M, Mammadbayov E, Mirtskulava V, Rüsch-Gerdes S, et al. First national survey of anti-tuberculosis drug resistance in Azerbaijan and risk factors analysis. Public Health Action. 2014;4(Suppl 2):S17-23.
Tasbiti AH, Yari S, Ghanei M, Shokrgozar MA, Fateh A, Bahrmand A. Low levels of extensively drug-resistant tuberculosis among multidrug resistant tuberculosis isolates and their relationship to risk factors: surveillance in Tehran, Iran; 2006 to 2014. PHRP. 2017;8(2):116–23.
Cox HS, Orozco JD, Male R, Ruesch-Gerdes S, Falzon D, Small I, et al. Multidrug-resistant tuberculosis in central Asia. Emerg Infect Dis. 2004;10(5):865.
Otokunefor K, Otokunefor TV, Omakwele G. Multi-drug resistant Mycobacterium tuberculosis in Port Harcourt, Nigeria. Afr J Lab Med. 2018;7(2):805.
Mehdi RM, Reza MS, Mohammad R. Study prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Tuberculosis in East Azerbaijan province of Iran. HealthMED. 2012;6(9):3091–4.
Israel K, Jean-Baptiste G, Julceus E, Docteur W, Sohler N, editors. Prevalence of Multi-Drug Resistant Tuberculosis in Zanmi Lasante Network among Patients who had a Gene Xpert Testing from October 2014 to September 20152018: 9th Annual CUGH Conference.
Wang SF, Yang Z, Yu P, Hui Wen Z, Yan LZ. Prevalence and risk factors of primary drug-resistant tuberculosis in China. Biomed Environ Sci. 2016;29(2):91–8.
Wang D, Yang C, Kuang T, Lei H, Meng X, Tong A, et al. Prevalence of multidrug and extensively drug-resistant tuberculosis in Beijing, China: a hospital-based retrospective study. Jpn J Infect Dis. 2010;63(5):368–71.
Laghari GS, Hussain Z, Khemani L, Hussain SZM, Yaqoob U. Burden of drug-resistant pulmonary tuberculosis in Pakistani children: a cross-sectional study. F1000Research. 2019;8:344.
Afroz H, Ali MA, Fakruddin M, Kamrunnahar DS. Prevalence and treatment follow-up of drug-resistant extra-pulmonary tuberculosis in rural communities in Narshingdi, Bangladesh. Int J Adv Med. 2014;1:71–7.
Faridi M, Shukla I, Fatima N, Varshney S, Shameem M. Prevalence of primary pulmonary multi-drug resistant tuberculosis in and around Aligarh Region. J Med Microb Diagn. 2018;7(285):2161.
Aguiar F, Vieira M, Staviack A, Buarque C, Marsico A, Fonseca L, et al. Prevalence of anti-tuberculosis drug resistance in an HIV/AIDS reference hospital in Rio de Janeiro, Brazil. IJTLD. 2009;13(1):54–61.
CAS Google Scholar
Rashedi J, Mahdavi Poor B, Rafi A, Asgharzadeh M, Abdolalizadeh J, Moaddab SR. Multidrug-resistant tuberculosis in north-west of Iran and Republic of Azerbaijan: a major public health concern for Iranian people. J Res Health Sci. 2015;15(2):101–3.
Akhtar AM, Arif MA, Kanwal S, Majeed S. Prevalence and drug resistance pattern of MDR TB in retreatment cases of Punjab, Pakistan. JPMA. 2016;66(8):989–93.
Anunnatsiri S, Chetchotisakd P, Wanke C. Factors associated with treatment outcomes in pulmonary tuberculosis in northeastern Thailand. Southeast Asian J Trop Med Public Health. 2005;36(2):324–30.
Surucuoglu S, Ozkutuk N, Celik P, Gazi H, Dinc G, Kurutepe S, et al. Drug-resistant pulmonary tuberculosis in western Turkey: prevalence, clinical characteristics and treatment outcome. Ann Saudi Med. 2005;25(4):313–8.
Bhat J, Rao VG, Yadav R, Muniyandi M, Sharma R, Karfarma C, et al. Situation of drug resistant tuberculosis in Saharia tribe of central India. Indian J Med Res. 2015;141(5):636–9.
CAS PubMed PubMed Central Google Scholar
Kinjal R, Firoz G, Iva C, Gaurang K, Pradeep P. Study On Prevalence Of Drug Resistance And Genetic Mutation Pattern Among Suspected Drug Resistant Pulmonary Tuberculosis Cases In Jamnagar District. National Journal of Integrated Research in Medicine. 2019;10(4)
Jacobs MG, Pinto VL. Characterization of drug-resistant tuberculosis in Brazil, 2014. Epidemiologia e Serviços de Saúd. 2020;28: e2018294.
Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16(10):1193–201.
Abouyannis M, Dacombe R, Dambe I, Mpunga J, Faragher B, Gausi F, et al. Drug resistance of Mycobacterium tuberculosis in Malawi: a cross-sectional survey. Bull World Health Organ. 2014;92(11):798–806.
Abdella K, Abdissa K, Kebede W, Abebe G. Drug resistance patterns of Mycobacterium tuberculosis complex and associated factors among retreatment cases around Jimma, Southwest Ethiopia. BMC Public Health. 2015;15:599.
Weyer K, Brand J, Lancaster J, Levin J, Van der Walt M. Determinants of multidrug-resistant tuberculosis in South Africa: results from a national survey. SAMJ. 2007;97(11):1120–8.
Soliman NS. Prevalence of multidrug-resistant tuberculosis using phenotypic drug susceptibility testing and GeneXpert MTB/RIF with characterization of non-tuberculous mycobacteria using MALDI-TOF. EJMM. 2021;30(3):143–51.
Villa-Rosas C, Laniado-Laborín R, Oceguera-Palao L. Primary drug resistance in a region with high burden of tuberculosis. A critical problem. Salud Publica Mex. 2015;57(2):177–9.
Aia P, Kal M, Lavu E, John LN, Johnson K, Coulter C, et al. The burden of drug-resistant tuberculosis in Papua New Guinea: results of a large population-based survey. PLoS ONE. 2016;11(3): e0149806.
Hang NTL, Maeda S, Lien LT, Thuong PH, Hung NV, Thuy TB, et al. Primary drug-resistant tuberculosis in Hanoi, Viet Nam: present status and risk factors. PLoS ONE. 2013;8(8): e71867.
Minion J, Pai M. Assays for drug resistant tuberculosis in high burden countries reply. Lancet Infect Dis. 2011;11(3):162.
Gallo JF, Pinhata JMW, Simonsen V, Galesi VMN, Ferrazoli L, Oliveira RS. Prevalence, associated factors, outcomes and transmission of extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in São Paulo, Brazil: a cross-sectional study. ESCMID. 2018;24(8):889–95.
Bedaso MH, Kalil FS. Trends of Drug Resistance Tuberculosis from 2014 to 2018, Bale Zone, Oromia Region, Ethiopia. Infect Drug Resist. 2021;14:2073–8.
Anwaierjiang A, Wang Q, Liu H, Yin C, Xu M, Li M, et al. Prevalence and molecular characteristics based on whole genome sequencing of Mycobacterium tuberculosis resistant to four anti-tuberculosis drugs from Southern Xinjiang, China. Infect Drug Resist. 2021;14:3379–91.
Patil S, Nakate P, Patil S, Shelke Y. Prevalence of multi-drug resistant (MDR) pulmonary tuberculosis in a tertiary care rural hospital in Western Maharashtra, India. 2019.
Kulkarni GS, Palwe SD, Patil NP, Telkhade AJ, Kadukar J. Prevalence of multidrug-resistant tuberculosis at a regional drug-resistant tuberculosis center of Maharashtra. Indian J Respir Care. 2020;9(1):30.
Adwani S, Desai UD, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai. JKIMSU. 2016;5:13–9.
Dagne B, Desta K, Fekade R, Amare M, Tadesse M, Diriba G, et al. The Epidemiology of first and second-line drug-resistance Mycobacterium tuberculosis complex common species: evidence from selected TB treatment initiating centers in Ethiopia. PLoS ONE. 2021;16(1): e0245687.
Micheni LN, Kassaza K, Kinyi H, Ntulume I, Bazira J. Rifampicin and isoniazid drug resistance among patients diagnosed with pulmonary tuberculosis in southwestern Uganda. PLoS ONE. 2021;16(10): e0259221.
Muhmmad A, Muhammad N, Khan ZU, Jamal T, Ishaq M. Burden of multi-drug resistant and extensive drug resistant of Mycobacterium tuberculosis . KJMS. 2019;12(2):1.
Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, et al. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect. 2008;47(7):e57-63.
Lukoye D, Adatu F, Musisi K, Kasule GW, Were W, Odeke R, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS ONE. 2013;8(8): e70763.
Oyedeji GJ, Adeyemo C, Dissou A, Abiodun T, Alli OAT, Onaolapo OJ, et al. Prevalence of multi-drug resistant tuberculosis among tuberculosis patients attending chest clinics in Osun-State, Nigeria. Curr Pharm Biotechnol. 2020;21(10):939–47.
Masood R, Muhammad IN, Siddiqui T, Mushtaque M, Irshad A. High prevalence of DR-TB (drug-resistant tuberculosis): an Indicator of public health negligence. Pak J Pharm Sci. 2019;32(4):1529–36.
Safwat TM, Elmasry AA, Mohamed AKM. Prevalence of multi drug-resistant tuberculosis at Abbassia Chest Hospital from July 2006 to 2009. Egypt J Bronchol. 2011;5(2):124–30.
Abebe G, Abdissa K, Abdissa A, Apers L, Agonafir M, de Jong BC, et al. Relatively low primary drug resistant tuberculosis in southwestern Ethiopia. BMC Res Notes. 2012;5:225.
Yoshiyama T, Supawitkul S, Kunyanone N, Riengthong D, Yanai H, Abe C, et al. Prevalence of drug-resistant tuberculosis in an HIV endemic area in northern Thailand. Int J Tuberc Lung Dis. 2001;5(1):32–9.
Dewan P, Sosnovskaja A, Thomsen V, Cicenaite J, Laserson K, Johansen I, et al. High prevalence of drug-resistant tuberculosis, Republic of Lithuania, 2002. IJTLD. 2005;9(2):170–4.
Pleumpanupat W, Jittimanee S, Akarasewi P, Rienthong S, Jittimanee S, Chiewlian Y, et al. Resistance to anti-tuberculosis drugs among smear-positive cases in Thai prisons 2 years after the implementation of the DOTS strategy. IJTLD. 2003;7(5):472–7.
Sidze LK, Mouafo Tekwu E, Kuaban C, Assam Assam JP, Tedom JC, Eyangoh S, et al. Strong decrease in streptomycin-resistance and absence of XDR 12 years after the Reorganization of the National Tuberculosis Control Program in the Central Region of Cameroon. PLoS ONE. 2014;9(6): e98374.
Nigus DM, Lingerew W, Beyene B, Tamiru A, Lemma M, Melaku MY. Prevalence of multi drug resistant tuberculosis among presumptive multi drug resistant tuberculosis cases in Amhara National Regional State, Ethiopia. J Mycobac Dis. 2014;4(152):2161.
Alberte-Castiñeiras A, Brezmes-Valdivieso MF, Campos-Bueno A, Montes-Martinez I, López-Medrano R, Avellaneda C, et al. Drug-resistant tuberculosis in Castilla-León, Spain, 1996–2000. IJTLD. 2006;10(5):554–8.
Elmi OS, Hasan H, Abdullah S, Jeab MZM, Zilfalil B, Naing NN. Prevalence and associated factors with transmission of latent tuberculosis among household contacts of multi-drug resistant tuberculosis patients in Malaysia. World J Med Sci. 2014;10(3):285–94.
Amala SE, Silas G. The prevalence of tuberculosis (TB) and multiple drug resistant tuberculosis (MDR-TB) in Bayelsa state. NIGERIA AJHR. 2019;5:1–5.
Brito RC, Mello FCQ, Andrade MK, Oliveira H, Costa W, Matos HJ, et al. Drug-resistant tuberculosis in six hospitals in Rio de Janeiro, Brazil. IJTLD. 2010;14(1):24–33.
Wang G, Jiang G, Jing W, Zong Z, Yu X, Chen S, et al. Prevalence and molecular characterizations of seven additional drug resistance among multidrug-resistant tuberculosis in China: a subsequent study of a national survey. J Infect. 2021;82(3):371–7.
Xiang Y, Ying L, Liu J, Su Q, Shen J, Zhan J, et al. An epidemiological study of resistant tuberculosis in Chongqing, China. J Med Colleges of PLA. 2011;26(3):158–73.
Yang X, Yuan Y, Pang Y, Wang B, Bai Y, Wang Y, et al. The burden of MDR/XDR tuberculosis in coastal plains population of China. PLoS ONE. 2015;10(2): e0117361.
Zazueta-Beltran J, Leon-Sicairos N, Muro-Amador S, Flores-Gaxiola A, Velazquez-Roman J, Flores-Villasenor H, et al. Increasing drug resistance of Mycobacterium tuberculosis in Sinaloa, Mexico, 1997–2005. IJID. 2011;15(4):E272–6.
Pang Y, Zhu D, Zheng H, Shen J, Hu Y, Liu J, et al. Prevalence and molecular characterization of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis isolates from Southern China. BMC Infect Dis. 2017;17(1):711.
Lapphra K, Sutthipong C, Vanprapar N, Phongsamart W, Wittawatmongkol O, Udompornwattana S, et al. Drug-resistant tuberculosis in Thai children. IJID. 2012;16: e26.
Liu ZY, Shilkret KI, Finelli L. Epidemiology of drug-resistant tuberculosis in New Jersey from 1991 to 1995. Int J Epidemiol. 1998;27(1):121–6.
Huo F, Luo J, Shi J, Zong Z, Jing W, Dong W, et al. A 10-year comparative analysis shows that increasing prevalence of rifampin-resistant mycobacterium tuberculosis in china is associated with the transmission of strains harboring compensatory mutations. Antimicrob Agents Chemother. 2018;62(4): e02303.
Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Ghebremichael S, Hoffner S, et al. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates from Ethiopian pulmonary tuberculosis patients with and without human immunodeficiency virus infection. J Clin Microbiol. 2002;40(5):1636–43.
Amanullah F, Ashfaq M, Khowaja S, Parekh A, Salahuddin N, Lotia-Farrukh I, et al. High tuberculosis prevalence in children exposed at home to drug-resistant tuberculosis. IJTLD. 2014;18(5):520–7.
Ombura IP, Onyango N, Odera S, Mutua F, Nyagol J. Prevalence of drug resistance Mycobacterium tuberculosis among patients seen in coast Provincial General Hospital, Mombasa, Kenya. PLoS ONE. 2016;11(10): e0163994.
Gebeyehu M, Lemma E, Eyob G. Prevalence of drug resistant tuberculosis in Arsi Zone, Ethiopia. The Ethiopian Journal of Health Development. 2001;15(1).
Madukaji L, Okohu I, Usman S, Oyedum U, Enagi A, Usman A, et al. Early detection of Pre-XDR TB with line probe assay in a high TB burden country. Afr Health Sci. 2021;21(3):968–74.
Khunjeli R, Mohsin U, Shrestha S, Adhikari S, Srivastava B, Shrestha B. Prevalence of primary drug resistant tuberculosis in a tertiary care hospital. Nepal JCMC. 2014;4(4):36–8.
Pande JN, Singh UB, Sinha S, Agarwal RC, Singh SPN. Evaluation of risk factors and prevalence of drug resistant tuberculosis in north India. Chest. 2005;128(4):404S.
Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338(23):1641–9.
Committee TR. Drug-resistant Mycobacterium tuberculosis in Japan: a nationwide survey, 2002. IJTLD. 2007;11(10):1129–35.
Dorjee K, Sadutshang TD, Rana RS, Topgyal S, Phunkyi D, Choetso T, et al. High prevalence of rifampin-resistant tuberculosis in mountainous districts of India. IJTB. 2020;67(1):59–64.
Mohajeri P, Sadri H, Farahani A, Norozi B, Atashi S. Frequency of mutations associated with rifampicin resistance in Mycobacterium tuberculosis strains isolated from patients in west of Iran. Microb Drug Resist. 2015;21(3):315–9.
Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185–92.
Araya S, Negesso AE, Tamir Z. Rifampicin-resistant Mycobacterium tuberculosis among patients with presumptive tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist. 2020;13:3451–9.
Wang X, Fu Q, Li Z, Chen S, Liu Z, Nelson H, et al. Drug-resistant tuberculosis in Zhejiang province, China, 1999–2008. Emerg Infect Dis. 2012;18(3):496.
El Achkar S. Prevalence of drug-resistant tuberculosis assessed by next-generation sequencing: an 18-month nationwide study in Lebanon: Université de Lille; 2019.
Adejumo OA, Olusola-Faleye B, Adepoju V, Bowale A, Adesola S, Falana A, et al. Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. Afr Health Sci. 2018;18(3):472–8.
Gebrehiwet GB, Kahsay AG, Welekidan LN, Hagos AK, Abay GK, Hagos DG. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J Infect Dev Ctries. 2019;13(1):21–7.
Bitet DE, Kumurya SA, Joseph L, Bathelomow P. Rifampicin resistant tuberculosis among patients attending General Hospital, Kagarko, Kaduna State, Nigeria. Afr J Clin Exp Microbiol. 2020;21(3):250–4.
Awais M, Ahmad R, Jan F, Anwar B, Rehman R, Mujtaba G, et al. Prevalence and detection of drug-resistant tuberculosis in Hazara Division, Pakistan. Acad J Biotechnol. 2018;6(9):116–23.
Ikuabe PO, Ebuenyi ID. Prevalence of rifampicin resistance by automated Genexpert rifampicin assay in patients with pulmonary tuberculosis in Yenagoa, Nigeria. Pan Afr Med J. 2018;29:204.
Meaza A, Tesfaye E, Mohamed Z, Zerihun B, Seid G, Eshetu K, et al. Diagnostic accuracy of Truenat Tuberculosis and Rifampicin-Resistance assays in Addis Ababa, Ethiopia. PLoS ONE. 2021;16(12): e0261084.
Fadeyi A, Desalu OO, Ugwuoke C, Opanwa OA, Nwabuisi C, Salami AK. Prevalence of rifampicin-resistant tuberculosis among patients previously treated for pulmonary tuberculosis in North-Western, Nigeria. NMJ. 2017;58(6):161–6.
Elion Assiana DO, Abdul J, Linguissi LSG, Epola M, Vouvoungui JC, Mabiala A, et al. Epidemiological profile of multidrug-resistant and extensively drug-resistant Mycobacterium tubrculosis among Congolese patients. Ann Clin Microbiol Antimicrob. 2021;20(1):84.
Huo F, Zhang F, Xue Y, Shang Y, Liang Q, Ma Y, et al. Increased prevalence of levofloxacin-resistant Mycobacterium tuberculosis in China is associated with specific mutations within the gyrA gene. IJID. 2020;92:241–6.
Rivière E, Verboven L, Dippenaar A, Goossens S, De Vos E, Streicher E, et al. Variants in bedaquiline-candidate-resistance genes: prevalence in bedaquiline-naive patients, effect on MIC, and association with Mycobacterium tuberculosis Lineage. Antimicrob Agents Chemother. 2022;66(7): e0032222.
Latrilha FO, Simonsen V, Pinhata JM, Brandao AP, Galesi VMN, Waldman EA, et al. Transmission and prevalence of drug-resistant tuberculosis in a Brazilian setting under a directly observed therapy short-course strategy. Rev Soc Bras Med Trop. 2020;53: e20190404.
Ahmed I, Jabeen K, Inayat R, Hasan R. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob Agents Chemother. 2013;57(6):2522–5.
Kumar RS. Prevalence of pre-extensively drug-resistant tuberculosis and extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in South Tamil Nadu. Int J Sci Stud. 2020;8(9):96–9.
Chuchottaworn C. Extensively drug resistant tuberculosis (XDR-TB) in Chest Disease Institute, 197–205. J Med Assoc Thailand. 2010;93(1):34–7.
Pang Y, Lu J, Huo F, Ma Y, Zhao L, Li Y, et al. Prevalence and treatment outcome of extensively drug-resistant tuberculosis plus additional drug resistance from the National Clinical Center for Tuberculosis in China: a five-year review. J Infect. 2017;75(5):433–40.
Diriba G, Alemu A, Tola HH, Yenew B, Amare M, Eshetu K, et al. Pre-extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in Ethiopia: a laboratory-based surveillance study. IJID Regions. 2022;5:39–43.
Mellor Y, Herron D. Disease management: an introduction to tuberculosis. Aust Pharm. 2020;39(2):52–9.
Agyeman AA, Ofori-Asenso R. Tuberculosis—an overview. J Public Health Emerg. 2017;1(7):1–11.
Onyedum CC, Alobu I, Ukwaja KN. Prevalence of drug-resistant tuberculosis in Nigeria: a systematic review and meta-analysis. PLoS ONE. 2017;12(7): e0180996.
Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8(1):1–5.
Duan Q, Chen Z, Chen C, Zhang Z, Lu Z, Yang Y, et al. The prevalence of drug-resistant tuberculosis in mainland China: an updated systematic review and meta-analysis. PLoS ONE. 2016;11(2): e0148041.
Organization WH. Anti-tuberculosis drug resistance in the world. Report No. 4: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. Anti-tuberculosis drug resistance in the world Report No 4: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. 2008.
Molla KA, Reta MA, Ayene YY. Prevalence of multidrug-resistant tuberculosis in East Africa: a systematic review and meta-analysis. PLoS ONE. 2022;17(6): e0270272.
Migliori GB, Dheda K, Centis R, Mwaba P, Bates M, O’Grady J, et al. Review of multidrug-resistant and extensively drug-resistant TB: global perspectives with a focus on sub-Saharan Africa. Trop Med Int Health. 2010;15(9):1052–66.
Chen S, Huai P, Wang X, Zhong J, Wang X, Wang K, et al. Risk factors for multidrug resistance among previously treated patients with tuberculosis in eastern China: a case–control study. Int J Infect Dis. 2013;17(12):e1116–20.
Lema NA, Mbelele PM, Majigo M, Abade A, Matee MI. Risk factors associated with multidrug resistant tuberculosis among patients referred to Kibong’oto Infectious Disease Hospital in northern Tanzania. TJHR. 2016;18(4).
Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS ONE. 2015;10(3): e0119332.
Alkabab YM, Al-Abdely HM, Heysell SK. Diabetes-related tuberculosis in the Middle East: an urgent need for regional research. Int J Infect Dis. 2015;40:64–70.
Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):1–15.
Mesfin YM, Hailemariam D, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2014;9(1): e82235.
Demile B, Zenebu A, Shewaye H, Xia S, Guadie A. Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in a tertiary armed force referral and teaching hospital, Ethiopia. BMC Infect Dis. 2018;18(1):1–10.
Al-Darraji HAA, Tan C, Kamarulzaman A, Altice FL. Prevalence and correlates of latent tuberculosis infection among employees of a high security prison in Malaysia. Occup Environ Med. 2015;72(6):442–7.
Gobena D, Ameya G, Haile K, Abreha G, Worku Y, Debela T. Predictor of multidrug resistant tuberculosis in southwestern part of Ethiopia: a case control study. Ann Clin Microbiol Antimicrob. 2018;17(1):1–7.
Dujaili JA, Syed Sulaiman SA, Awaisu A, Muttalif AR, Blebil AQ. Outcomes of tuberculosis treatment: a retrospective cohort analysis of smoking versus non-smoking patients in Penang, Malaysia. J Public Health. 2011;19(2):183–9.
Rajendran M, Zaki RA, Aghamohammadi N. Contributing risk factors towards the prevalence of multidrug-resistant tuberculosis in Malaysia: a systematic review. Tuberculosis. 2020;122: 101925.
Jilani TN, Avula A, Gondal Z, Siddiqui AH. Active tuberculosis. 2018.
Dirie AMH, Çolakoğlu S, Abdulle OM, Abdi BM, Osman MA, Shire AM, Hussein AM. Prevalence of multidrug-resistant TB among smear-positive pulmonary TB patients in Banadir, Somalia: a multicenter study. Infect Drug Resist. 2022;15:7241–8.
Sylverken AA, Kwarteng A, Twumasi-Ankrah S, et al. The burden of drug resistance tuberculosis in Ghana; results of the First National Survey. PLoS ONE. 2021;16(6):1–14.
Emam SA, Kasem EM, Sedhom AE. Characteristics of multidrug resistant tuberculosis in Minia, Egypt. Medico Legal Updat. 2020;20(1):446–52.
Welekidan LN, Skjerve E, Dejene TA, et al. Characteristics of pulmonary multidrug-resistant tuberculosis patients in Tigray Region, Ethiopia: a cross-sectional study. PLoS ONE. 2020;15(8):1–20.
Download references
Acknowledgements
We would like to thank the support provided by the Student Research Committee of Kermanshah University of Medical Sciences.
By Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (50002460). This deputy has no role in the study process.
Author information
Authors and affiliations.
Department of Biostatistics, School of Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
Nader Salari
Sleep Disorders Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
Amir Hossein Kanjoori & Razie Hasheminezhad
Department of Business Systems & Operations, University of Northampton, Northampton, UK
Amin Hosseinian-Far
Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
Kamran Mansouri
Cellular and Molecular Research Center, Gerash University of Medical Sciences, Gerash, Iran
Masoud Mohammadi
You can also search for this author in PubMed Google Scholar
Contributions
NS and RH and AK contributed to the design, MM statistical analysis, participated in most of the study steps. MM and RH and AHF prepared the manuscript. AK and RH and KM assisted in designing the study, and helped in the interpretation of results. All authors have read and approved the content of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Masoud Mohammadi .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was received from the ethics committee of deputy of research and technology, Kermanshah University of Medical Sciences (50002460).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Supplementary Information
Additional file 1: table s1..
Summary of Characteristics of Included Studies of Prevalence of MDR-TB. TableS2. Summary of Characteristics of Included Studies of Prevalence of Isoniazid Resistant-TB . Table S3. Summary of Characteristics of Included Studies of Prevalence of Rifampcin Resistant-TB. Table S4. Summary of Characteristics of Included Studies of Prevalence of Single Drug Resistant-TB. Table S5. Summary of characteristics of included studies of prevalence of XDR-TB. TableS6. Summary of characteristics of included studies of prevalence of pre-XDR TB.
Additional file 2: Figure S1.
Forest plot of the global prevalence ofmulti-drug resistant TB based on the random effects method. Figure S2. Funnel plotof publication bias in reviewed studies. Figure S3. Forest plot of global prevalence of isoniazid resistant TB based on randomeffects method. Figure S4. Funnel plot of publication biasin reviewed studies. Figure S5. Forest plot ofglobal prevalence of rifampin-resistant TB based on random effects method. FigureS6. Funnel plot of publication bias in reviewed studies. FigureS7. Forest plot of globalprevalence of single drug resistant TB based on random effects method. FigureS8. Funnel plot of publication bias in reviewed studies. Figure S9. Forest plot of global prevalence of extensively drugresistant TB based on random effects method. Figure S10. Funnel Plot of Publication Bias in ReviewedStudies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Salari, N., Kanjoori, A.H., Hosseinian-Far, A. et al. Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis. Infect Dis Poverty 12 , 57 (2023). https://doi.org/10.1186/s40249-023-01107-x
Download citation
Received : 09 February 2023
Accepted : 16 May 2023
Published : 25 May 2023
DOI : https://doi.org/10.1186/s40249-023-01107-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drug-resistant tuberculosis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 15 April 2024
Increased tuberculosis case detection in Tanzanian children and adults using African giant pouched rats
- Tefera B. Agizew 1 , 2 ,
- Joseph Soka 1 ,
- Cynthia D. Fast 1 , 2 ,
- Stephen Mwimanzi 1 ,
- Gilbert Mwesiga 1 ,
- Nashon Edward 1 ,
- Marygiven Stephen 1 ,
- Reheme Kondo 1 ,
- Robert Burny 3 ,
- Christophe Cox 1 , 2 &
- Negussie Beyene 1 , 2 , 4
BMC Infectious Diseases volume 24 , Article number: 401 ( 2024 ) Cite this article
Metrics details
African giant pouched rats, trained by Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO), have demonstrated their ability to detect tuberculosis (TB) from sputum. We assessed rat-based case detection and compared the mycobacterium bacillary load (MTB-load) in children versus adults.
From January–December 2022, samples were collected prospectively from 69 Directly Observed Therapy (DOT) facilities’ presumed TB patients. Using an average of five rats, APOPO re-evaluated patients with bacteriologically negative (sputum-smear microscopy or Xpert MTB/RIF) results. Rat-positive samples were tested using concentrated smear light-emitting diode microscopy to confirm TB detection before treatment initiation. The rats’ identification of pulmonary TB is based on smelling TB-specific volatile organic compounds (VOCs) in sputum. Using STATA, Chi-square for odds ratio and confidence interval was calculated and evaluated: (1) the yield of rat-based TB detection compared to that of the health facilities; (2) rat-based TB detection in children versus adults; and (3) rats’ ability to detect TB across MTB-loads and between children and adults.
From 35,766 patients, 5.3% (1900/35,766) were smear-positive and 94.7% (33,866/35,766) were smear or Xpert-negatives at DOTS facility. Of those with negative results, 2029 TB cases were detected using rats, contributing to 52% (2029/3929 of total TB identified), which otherwise would have been missed. Compared to DOT facilities, rats were six-fold more likely to detect TB among Acid Fast Bacilli (AFB) 1+/scanty [90% (1829/2029) versus 60% (1139/1900), odds ratio, OR = 6.11, 95% confidence interval, CI: 5.14–7.26]; twice more likely to identify TB cases among children [71% (91/129) versus 51% (1795/3542), OR = 2.3, 95% CI: 1.59–3.42]; and twice more likely to identify TB cases among children with AFB 1+/scanty than adults with the same MTB-load [5% (86/1703) versus 3% (28/1067), OR = 2.0, 95% CI: 1.28–3.03].
Conclusions
Rats contributed over half of the TB cases identified in program settings, and children, especially those with a lower MTB-load, were more likely to be diagnosed with TB by rats. The chemical signatures, VOCs, were only available for adults, and further research describing the characteristics of VOCs in children versus adults may pave the way to enhance TB diagnosis in children.
Peer Review reports
Introduction
Despite the curable and preventable nature of tuberculosis (TB), about 10.6 million people fell ill with it and 1.6 million died in 2021 [ 1 ]. The African region accounts for nearly one-third of the estimated global burden of TB, and TB remains a public health problem in sub-Saharan African countries, Tanzania included. TB is declining by only 5% on average annually in Tanzania [ 1 ]. With this current rate of decline, considerable gaps persist in finding and treating TB cases timely, as a result of which achieving reduced TB transmission and TB cases per the END TB target may not be possible in the near future [ 2 ]. Therefore, scaling up of cheaper, faster, and more sustainable diagnostic tests to find more TB patients, especially those missed in routine program settings, is essential.
Over the years, African giant pouched rats ( Cricetomys ansorgei ), trained by Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO) to identify TB by smell, have demonstrated their ability to detect TB from sputum [ 3 , 4 , 5 ]. The trained rats have very high sample throughput, as they can screen 100 sputum samples in 20 minutes [ 6 ]. The rats’ identification of pulmonary TB is based on smelling TB-specific volatile organic compounds (VOCs) in sputum specimens [ 5 ]. By the end of 2022, APOPO’s TB Detection Programs had screened 870,777 sputum samples from 517,264 presumptive TB patients and found 26,084 newly diagnosed TB patients in three high-TB burden countries (Ethiopia, Mozambique, and Tanzania). At APOPO, we have generally done this by re-evaluating (second-line TB screening) sputum samples initially tested and declared bacteriologically negative by sputum-smear microscopy (smear) or Xpert MTB/RIF [ 4 ]. Before treatment initiation, all TB patients identified using rats are confirmed using the national standard diagnostics.
Due to the low bacillary load and lesser sensitivity of the available diagnostic methods, including culture, diagnosing TB in children is difficult, leaving them undiagnosed and therefore untreated [ 7 ]. Using APOPO rats, Mgode et al. reported on the diagnosis of TB in children (0–14 years) in 2018. They discovered 39% (208/539) contributions among pediatric TB cases reported, which translates to 62.8% additional cases (incremental yield) by rats to DOT facilities TB case detection [ 8 ]. There is limited data on rats’ abilities to detect TB in children versus adults. Other than the above report, all the previous studies were focused on adults [ 9 , 10 ]. In this study, we aim to evaluate: (1) the yield of rat-based TB case detection compared to that of the health facilities; (2) rat-based TB case detection in children versus adults; and (3) rats’ ability to detect TB across Mycobacterium TB bacillary loads (MTB-load) and between children and adults.
Study settings and designs
We conducted a prospective study using routinely collected data on presumptive TB patients enrolled in the national TB program’s routine care at 69 (58 Dar es Salaam, 11 Dodoma) DOT facilities from 1st of January to 31st of December 2022 in Tanzania.
Study population
The study population includes all individuals of all ages, including children, men, and women, who presented with symptoms of TB at study sites.
Tuberculosis screening
At the health facility visit, children (0–14 years old) and adults (15 years and above, hereafter referred to as adults) were screened for TB symptoms. Per the national TB guidelines, adults were screened for 4 TB symptoms (cough, fever, night sweats, and weight loss) of two or more weeks duration. Children were screened for weight loss or failure to thrive (no weight gain > 3 months), cough for ≥2 weeks, fever for ≥2 weeks, fatigue or reduced playfulness for ≥2 weeks, and profuse night sweats for ≥2 weeks [ 11 ]. Presumptive TB cases were defined when patients were screened positive for one or more of the TB symptoms using the above guide.
APOPO tuberculosis detection model using rats
APOPO sample collection, a rat-based assessment procedure, and a TB detection model using rats were reported in previous APOPO publications (Fig. 1 ) [ 3 , 12 ]. In summary, smear, which has very good specificity but very poor sensitivity, especially in sub-Saharan African settings [ 13 ], remains the most commonly used TB diagnostic method in low- and middle-income countries [ 4 ]. At APOPO, samples from smear- or Xpert MTB/RIF-negative presumptive TB patients are screened using detection rats, and rat-positive samples are confirmed using light-emitting diode fluorescence smear microscopy (LED-FM), yielding an annual average of a 40% increase (incremental yield) in smear-positive case detection [ 3 ]. Therefore, APOPO envisages targeting and reaching the missed TB cases to maximize TB case detection and treatment coverage. In this study, five rats on average sequentially evaluated the samples placed under 10 sniffing holes in the floor in a of rectangular chamber (205 cm long, 55 cm wide, and 55 cm high). The details of the evaluation setup have been reported elsewhere [ 14 ].
APOPO tuberculosis detection model using rats. Reproduced with permission [ 12 ]
Data collection
Data were collected using standardized case report forms (CRF) between January and December 2022. An APOPO-developed TB Laboratory Information System (TB-LIMS) was used to record the data. Logic checks were used to find inconsistencies, which were then verified against the original CRF after being discovered. Consistencies and missing data were, whenever possible, fixed by reviewing patient charts.
Statistical analysis
Data were analyzed using STATA statistical software 15 [ 15 ]. A Chi-square for odds ratio was calculated to analyze the demographic and laboratory parameters of the patients, and rats’ TB detection over the DOT facilities and compared the increase by MTB-load in children versus adults. We employed an odds ratio and a 95% confidence interval, and statistical significance was determined by P values less than 0.05.
From a total of 35,766 patients, 46,048 samples were screened between January and December 2022; Among these patients 5.3% (1900/35,766) were smear-positive and 94.7% (33,866/35,766) were smear or Xpert-negatives at DOTS facility (Fig. 2 a). Figure 2 b shows children and adults, respectively, were 8% (2508/33,243) and 92% (30,735/33,243). Among presumptive TB patients 2523, and 258 TB patients were not included for the odds ratio analysis due to missing age. Bacteriologically confirmed (smear at DOT facilities and rats’ detection verified by smear) cumulative TB cases were 11% (3929/35,766), whereas 5% (129/2508) and 12% (3542/30,735) were among children and adults, respectively. The median age was 36 years (interquartile range, 26–47 years), and 68% (2680/3903) were males (Fig. 2 b and Table 1 ).
A Rat indication and tuberculosis cases after LED smear confirmation among presumptive tuberculosis patients in Tanzania study sites from January to December 2022. Note: *Since the samples were not tested by culture, 17.8% does not truly represent sensitivity. Since the LED smear is not good enough in sensitivity, nor does 81.9% represent a true false positive. B Presumptive tuberculosis and bacteriologically confirmed tuberculosis cases in Tanzania study sites from January to December 2022. Note: *Children versus adults bacteriologically confirmed TB: 5% (129/2508) versus 12% (3542/30,735), odds ratio 0.42, 95% confidence interval: 0.35–0.50, p value, 0.001
TB detection using rats
Of the 3929 TB cases, APOPO rats’ detection contributed 52% (2029/3929) of the TB cases reported as smear-positive TB at DOT facilities that otherwise would have been missed by routine program. Compared to DOT facilities, the detection rats were six-fold more likely to detect TB among patients with Acid Fast Bacilli (AFB) smear 1+ or scanty [90% (1829/2029) versus 60% (1139/1900), odds ratio, OR = 6.11, 95% confidence interval, CI: 5.14–7.26]. The odds of identifying TB cases by rat among children were two-fold higher than adults [71% (91/129) versus 51% (1795/3542), OR = 2.3, 95% CI: 1.59–3.42] (Table 1 ). Furthermore, the odds of identifying TB cases by rats among children with AFB 1+ or scanty smear were two-fold higher than those among adults with the same MTB-load range [5% (86/1703) versus 3% (28/1067), OR = 2.0, 95% CI: 1.28–3.03] (Tables 2 and 3 and Figs. 3 and 4 ).
Tuberculosis cases among children and adults identified using rats by Mycobacterium bacillary load
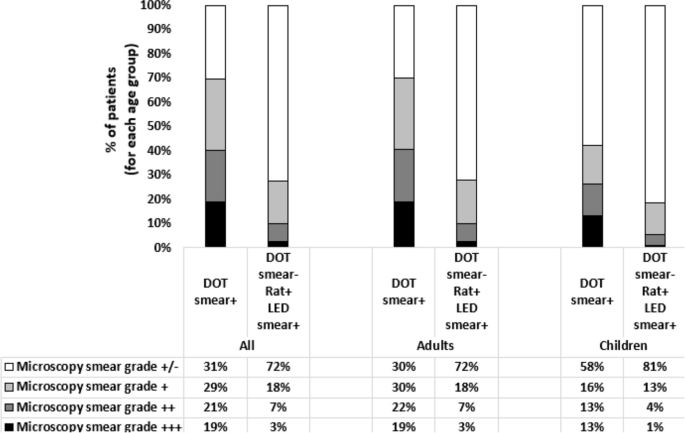
Tuberculosis cases detected by smear microscopy at DOTs and by rats at APOPO and confirmed by LED smear microscopy in Tanzania study sites from January to December 2022
Tuberculosis detection by smear at DOTs and by rats at APOPO confirmed by LED smear by Mycobacterium tuberculosis bacillary load
Higher proportions of AFB Scanty were identified among TB diagnosed by rats and confirmed by LED smear than those diagnosed at DOT, and this was similar among children and adults (Fig. 4 ).
On average, 2.36 (3459/1466), 3.40 (1233/363), 3.68 (545/148), and 3.75 (195/52) rats from a set of five rats indicated TB in those with AFB Scanty, 1+, 2+, and 3+, respectively More rats were able to identify TB as the MTB-load increased (Fig. 5 ).
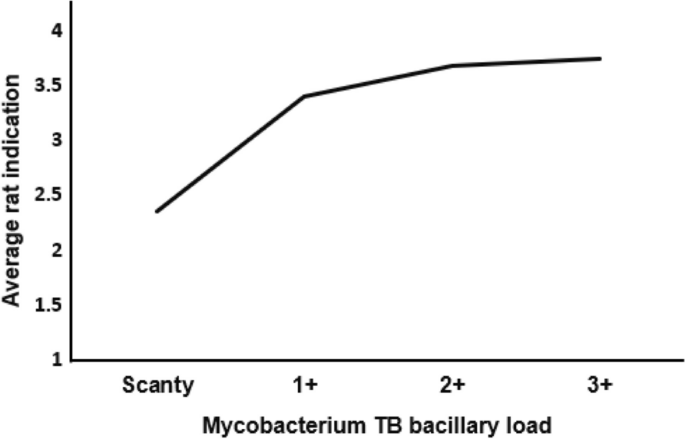
Average rat indication by Mycobacterium Tuberculosis bacillary load
Discussions
For more than a decade, APOPO-trained African giant pouched rats have been used to re-evaluate (second-line screening) sputum samples tested by smear under routine program conditions [ 3 , 4 ], and this has allowed us to compare the effect of rats on pulmonary TB case finding against DOT facilities. In the present study, rats demonstrated a 52% contribution in the detection of TB cases among patients who presented to routine DOT facilities with symptoms of TB and were diagnosed with TB. Rats were six times more likely than DOT facilities to uncover TB in patients with AFB smear 1+ or scanty, and this finding is similar to a previous study (five times more likely), though the latter was only applicable to children [ 4 ]. It is worth noting that if verification tests following the sputum samples’ rat indication as positive had been confirmed by molecular WHO-recommended rapid diagnostics, such as Xpert MTB/RIF Untra or Truenat, or by culture, which can detect TB with much lower concentrations of mycobacterium in a sample than concentrated LED microscopy, the contribution of rats would have been higher. Rats’ contribution has implications for Tanzania’s efforts to eliminate TB, especially as those who were identified using rats were deemed TB negative (missed TB cases) in the context of the standard TB diagnostic procedures and would have been left untreated otherwise.
We described elsewhere how rats can discriminate Mycobacterium TB by sniffing a variety of VOCs [ 3 , 5 ]. According to a recent meta-analysis (53,181 samples from 24,600 patients in seven studies), the sensitivity and specificity of TB screening using rats were 81.3 and 73.4%, respectively [ 16 ]. In our report, we further analyzed the rats’ responses to different MTB-loads with respect to their impact on children and adults with pulmonary TB.
TB detection using rats among children and adults
Comparing the effect of TB detection using rats in children to that in adults, rats were more than twice as likely to identify TB in children compared to adults, even though the likelihood of TB diagnosis among children with presumptive TB was lower by 58%. It should be noted that the increased TB detection among children was not only higher, but it was also significantly higher among children with a lesser bacillary load (AFB 1+ or scanty) compared to adults with a similar bacillary load. Our finding suggests that the rat-based technology could be used to improve pediatric TB case detection in high-burden countries such as Tanzania, where children are diagnosed with TB less frequently than adults by the standard of care.
In the previous study, rats contributed 39% (208/539) to pulmonary TB case detection reported in children (0–14 years), which was far less than our finding (71%, p < 0.001 ) [ 8 ].
APOPO’s rat technology has shown further improvement over time toward better rat and rat-handler training techniques, among other possibilities, which may explain the difference between the two reports. APOPO is working to advance its technologies further in this direction, and rats’ performance-rewarding systems are being computerized. The recent fully automated (i.e., controlling indication threshold time, notification of correct identification, reward delivery, and data recording are all computer controlled) rat cage is one step in this process and is currently being evaluated [ 14 ]. If validated, the automated system eliminates the effect of the human factor, i.e., rat handlers, who previously rewarded rats manually by providing food after a rat indicated the sample had TB. With the automated cage, APOPO anticipates improving and speeding up TB detection in Tanzania. In agreement with Mgode et al., our work has implications for developing rat-based TB diagnostic techniques or algorithms among children due to the challenges of detecting TB in this age group, where sputum quality and quantity remain a concern [ 7 , 8 ].
Fewer number of rats is needed to identify TB with a higher bacillary load
The direct relationship between smear positivity and MTB-load was expected [ 17 ]. Interestingly, we also observed that when the bacillary load increased, more and more rats identified TB cases, suggesting that fewer rats were needed to detect TB, and once again, the effect tends to be stronger in children than in adults. The properties of the chemical signature, VOCs, among various age groups have not yet been determined, and the previous report was for adults only [ 5 ]. Our research led us to two hypotheses: (1) children may have distinct or greater VOC characteristics, making it simpler to diagnose TB even when the bacillary load is lower than that of adults; and (2) probably, the less developed physiology may not let them to produce other odors that could be developed by adult patients, which in turn may somehow mask the odor from the characteristic VOCs. These merit additional investigation. On the other hand, sputum quality and quantity have an impact on the accuracy of TB diagnosis by smear or Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) [ 17 , 18 , 19 ], and in the same vein, higher-quality sputum makes it simpler for rats to detect TB.
Our study has some limitations. First, because the data were from a routine program setting, the age of certain TB cases was missing, which prevented them from being further analyzed. Rats were, however, four times more likely to detect TB than DOT among those with missing ages, and It’s likely that the conclusion would remain unchanged if the missing data were included. Second, health workers at various DOT facilities may not have received training before the data collection, and the TB screening and recording procedures may not have been consistent, which may result in inconsistent presumptive patient identification. Third, despite the fact that sputum induction is usual at DOT facilities, it was possible to collect insufficient sputum samples from children, which may have resulted in fewer pediatric TB diagnoses than in adult patients. Finally, for those samples evaluated by rats after the Xpert MTB/RIF negative test result, using the same sample for rats was not possible. Thus, a sister sample was collected and evaluated by rats. In such cases, we cannot rule out the possibility of inter-sample variations, i.e., the sister sample had a chance not to be negative if it was tested by Xpert MTB/RIF before rats’ evaluation.
In conclusion, in our high TB burden settings, APOPO rats led to more than half of the TB cases identified. Children had a higher rate of TB detection, particularly among those with lower bacillary loads. In the present study, rats demonstrated their essential role in TB control efforts, specifically in identifying TB cases that were overlooked in routine program diagnostic settings. Since rats were more easily able to detect TB in children than adults, and the data from prior reports about chemical signatures, VOCs, were only available for adults, further research describing the characteristics of VOCs in children versus adults may pave the way to enhance TB diagnosis in children. Like other TB diagnostic technologies, it’s likely that the quality of the sputum specimen influences the rat’s ability to detect TB by sniffing; therefore, advancements in this area make it simpler for the rats to recognize TB.
Availability of data and materials
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Although the patient-level data do not include patient names, this IRB decision is in the interest of ensuring patient confidentiality. An individual may email the lead author ( [email protected] ) or the APOPO TB Detection Research Department ( [email protected] ) to request the data.
Abbreviations
Acid fast bacilli
Anti-persoonsmijnen ontmijnende product ontwikkeling
Confidence interval
Case report form
Directly observed therapy
Light-emitting diode fluorescence smear microscopy
Mycobacterium Tuberculosis
South Texas Art Therapy Association
Tuberculosis
Volatile organic compound
Ziehl-Neelsen
World Health Organization (WHO). Global TB report 2022. Geneva, Switzerland: WHO; 2022. Accessed on 10 July 2023, and available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
Agizew TB, Dememew ZG, Leta T, Hiruy H, Tesema E, Abelti EA, et al. Prospects for tuberculosis elimination in Ethiopia: feasibility, challenges, and opportunities. Pan Afr Med J. 2022;43(146) https://doi.org/10.11604/pamj.2022.43.146.35557 .
Mulder C, Mgode GF, Ellis H, Valverde E, Beyene N, Cox C, et al. Accuracy of giant African pouched rats for diagnosing tuberculosis: comparison with culture and Xpert MTB/RIF. Int J Tuberc Lung Dis. 2017;21(11):1127–33. https://doi.org/10.5588/ijtld.17.0139 .
Article CAS PubMed Google Scholar
Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO). Annual report 2022. Morogoro, Tanzania; 2022. Accessed on 1 July 2023, and available from: https://apopo.org
Mgode GF, Weetjens BJ, Nawrath T, Lazar D, Cox C, Jubitana M, et al. Mycobacterium tuberculosis volatile s for diagnosis of tuberculosis by Cricetomys rats. Tuberculosis. 2012;92:535–42. https://doi.org/10.1016/j.tube.2012.07.006 .
Mulder C, Mgode G, Reid SE. Tuberculosis diagnostic technology: an African solution. . .think rats. Afr J Lab Med. 2017;6:a420. https://doi.org/10.4102/ajlm.v6i2.420 .
Article Google Scholar
Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Intl J Tuberc Lung Dis. 2001;5:594–603. https://pubmed.ncbi.nlm.nih.gov/11467365/
CAS Google Scholar
Mgode GF, Cox LC, Mwimanzi S, Mulder C. Pediatric tuberculosis detection using trained African giant pouched rats. Pediatr Res. 2018;84:99–103. https://doi.org/10.1038/pr.2018.40 .
Article PubMed Google Scholar
Poling A, Weetjens BJ, Cox C, Mgode G, Jubitana M, Kazwala R, et al. Using giant African pouched rats to detect tuberculosis in human sputum samples: 2009 findings. Am J Trop Med Hyg. 2010;83(6):1308–10. https://doi.org/10.4269/ajtmh.2010.10-0180 .
Article PubMed PubMed Central Google Scholar
Mahoney AM, Weetjens BJ, Cox C, et al. Using giant African pouched rats to detect tuberculosis in human sputum samples: 2010 findings. Pan Afr Med J. 2011;9:28. https://doi.org/10.4269/ajtmh.2010.10-0180 .
The United Republic of Tanzania, National Tuberculosis and leprosy program., National Tuberculosis Program Manual, Ministry of Health, Tanzania 2020. Accessed on 11 Sept 2023, and available from: https://ntlp.go.tz/site/assets/files/1081/ntlp_manual_2020_2021_1.pdf
Beyene N, Sitotaw LA, Telila EG, Gebre HA, Alemu NB, Burny R, et. al. The use of rats to detect drug-resistant TB. PHA Mach 2023; 13(1):64–69 . https://doi.org/10.5588/pha.22.0059 .
Alnour TMS. Smear microscopy as a diagnostic tool of tuberculosis: review of smear negative cases, frequency, risk factors, and prevention criteria. Indian J Tuberc. 2018;65(3):190–4. https://doi.org/10.1016/j.ijtb.2018.02.001 .
Beyene N, Mgode G, Burny R, Fast CD, Cox C, Fiebig L. A multidisciplinary approach towards finding and treating all tuberculosis patients. In: Rezaei N, editor. Tuberculosis: integrated studies for a complex disease. Switzerland: Springer-Verlag; 2023. p. 207–27.
Chapter Google Scholar
StataCorp. Stata statistical software: release 14. College Station, TX: StataCorp LP; 2015.
Google Scholar
Kanaan R, Farkas N, Heygi P, Soós A, Hegyi D, Németh K, et al. Rats sniff out pulmonary tuberculosis from sputum: a diagnostic accuracy meta-analysis. Sci Rep. 2021;11(1):1877. https://doi.org/10.1038/s41598-021-81086-x .
Article CAS PubMed PubMed Central Google Scholar
Lumb R, Van-Deun A, Ivan Bastian I, Mark F-GM. Laboratory diagnosis of tuberculosis by sputum microscopy: the handbook. Australia: global edition. SA Pathology: Adelaide, South Australia; 2013. https://catalogue.nla.gov.au/catalog/6448252
World Health Organization (WHO) same-day diagnosis of tuberculosis by microscopy 2011. WHO/HTM/TB/2011.7. Accessed on 17 Aug 2023, and available from: https://www.who.int/publications/i/item/WHO-HTM-TB-2011.7
Zimba O, Tamuhla T, Basotli J, Letsibogo G, Pals S, Mathebula U, et al. The effect of sputum quality and volume on the yield of bacteriologically-confirmed TB by Xpert MTB/RIF and smear microscopy among people living with HIV in Botswana. The Pan Afri Med Jour. 2019;33:110. https://doi.org/10.11604/pamj.2019.33.110.15319 .
Download references
Acknowledgements
The authors gratefully acknowledge the service of the APOPO TB research team, which carried out the day-to-day work of the evaluation of samples from DOT facilities. The authors would also like to thank the Tanzania Ministry of Health, the National TB and Leprosy Program, and health care workers at the collaborating DOT facilities in Dar es Salaam and Dodoma. Patient tracking by MKUTA is much appreciated.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the APOPO project. References in this manuscript to any specific commercial products, process, service, manufacturer, or company do not constitute its endorsement or recommendation by the donors or APOPO.
This research has been supported by Else Kröner-Fresenius-Stiftung (EKFS, Germany), the Light Foundation (Germany), the Polish embassy in Tanzania, and the Directorate General for Development Cooperation (DGD, Belgium), who supported the study financially. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO) Tuberculosis Department, Sokoine University of Agriculture, Morogoro, Tanzania
Tefera B. Agizew, Joseph Soka, Cynthia D. Fast, Stephen Mwimanzi, Gilbert Mwesiga, Nashon Edward, Marygiven Stephen, Reheme Kondo, Christophe Cox & Negussie Beyene
Department of Biology, University of Antwerp, Antwerp, Belgium
Tefera B. Agizew, Cynthia D. Fast, Christophe Cox & Negussie Beyene
APOPO, TB Detection Program, Eduardo Mondlane University, Maputo, Mozambique
Robert Burny
APOPO TB Research Project, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Negussie Beyene
You can also search for this author in PubMed Google Scholar
Contributions
T.A and J.S conceptualized and designed the study and conducted the statistical analysis. S.M, G.M, N.E, M.S., and R.K conducted the fieldwork and laboratory work. T.A, J.S, S.M, C.F, R.B, C.C, and N.B reviewed the collected data and participated in the writing of the results and discussions. All co-authors contributed to the writing and review of the manuscript.
Corresponding author
Correspondence to Tefera B. Agizew .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the Institutional Review Board ( IRB, Ref. nr. NMRI/HQ/R.8c/Vov.I/2066 ) at the Tanzanian National Institute for Medical Research (Dar es Salaam, Tanzania). Following IRB approval, patients were enrolled in the study, and data were collected as part of the national TB program’s routine TB diagnostic care activities. Therefore, the ethical committee waived the need for informed consent for this operational research that involved second-line TB screening following routine care.
Pertaining to the rats, all procedures were conducted with approval from the Institutional Committee for Research Involving Animals of the Sokoine University of Agriculture and in accordance with all relevant guidelines and regulations. The methods and results reported herein adhere to the National Centre for Replacement Refinement & Reduction of Animals in Research ARRIVE 2.0 guidelines. At the conclusion of all experiments, subjects remained housed according to the procedures described above with no animals sacrificed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Agizew, T.B., Soka, J., Fast, C.D. et al. Increased tuberculosis case detection in Tanzanian children and adults using African giant pouched rats. BMC Infect Dis 24 , 401 (2024). https://doi.org/10.1186/s12879-024-09313-0
Download citation
Received : 29 September 2023
Accepted : 10 April 2024
Published : 15 April 2024
DOI : https://doi.org/10.1186/s12879-024-09313-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Rat-based TB detection
- Mycobacterium bacillary load
- Pulmonary TB
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Health Popul Nutr
- v.28(2); 2010 Apr

Tuberculosis: A Global Health Problem
Tuberculosis (TB) is an ancient disease that has affected mankind for more than 4,000 years ( 1 ). It is a chronic disease caused by the bacillus Mycobacterium tuberculosis and spreads from person to person through air. TB usually affects the lungs but it can also affect other parts of the body, such as brain, intestines, kidneys, or the spine. Symptoms of TB depend on where in the body the TB bacteria are growing. In the cases of pulmonary TB, it may cause symptoms, such as chronic cough, pain in the chest, haemoptysis, weakness or fatigue, weight loss, fever, and night-sweats.
TB remains a leading cause of morbidity and mortality in developing countries, including Bangladesh. With the discovery of chemotherapy in the 1940s and adoption of the standardized short course in the 1980s, it was believed that TB would decline globally. Although a declining trend was observed in most developed countries, this was not evident in many developing countries ( 2 ). In developing countries, about 7% of all deaths are attributed to TB which is the most common cause of death from a single source of infection among adults ( 3 ). It is the first infectious disease declared by the World Health Organization (WHO) as a global health emergency ( 4 ). In 2007, it was estimated globally that there were 9.27 million incident cases of TB, 13.7 million prevalent cases, 1.32 million deaths from TB in HIV-negative and 0.45 million deaths in HIV-positive persons ( 5 ). Asia and Africa alone constitute 86% of all cases ( 5 ). Bangladesh ranked the 6th highest for the burden of TB among 22 high-burden countries in 2007, with 353,000 new cases, 70,000 deaths, and an incidence of 223/100,000 people per year ( 5 ).
Implementation of directly-observed therapy short course (DOTS) has been a ‘breakthrough’ in the control of tuberculosis. In many countries, it has become the cornerstone in the treatment of tuberculosis. The number of countries and the coverage of DOTS within the countries have increased over the years ( 5 ). Over the last 15 years, about 35 million people have been cured, and eight million deaths have been averted with the adoption of DOTS ( 6 ). Implementation of DOTS was started in 1993 in Bangladesh, and it gradually covered the whole country ( 7 ).
Men are more commonly affected than women. The case notifications in most countries are higher in males than in females. There were 1.4 million smear-positive TB cases in men and 775,000 in women in 2004 ( 8 ). The ratio of female to male TB cases notified globally is 0.47:0.67 ( 9 ). The reasons for these gender differences are not clear. These may be due to differences in the prevalence of infection, rate of progression from infection to disease, under-reporting of female cases, or the differences in access to services.
The association between poverty and TB is well-recognized, and the highest rates of TB were found in the poorest section of the community ( 10 ). TB occurs more frequently among low-income people living in overcrowded areas and persons with little schooling ( 11 ). Poverty may result in poor nutrition which may be associated with alterations in immune function. On the other hand, poverty resulting in overcrowded living conditions, poor ventilation, and poor hygiene-habits is likely to increase the risk of transmission of TB ( 12 ).
Various surveys have been conducted to understand the knowledge, attitudes, and practices regarding tuberculosis ( 13 – 14 ). One survey in India reported that most (93%) people had heard of TB but only 20.5% of the people demonstrated sufficient knowledge of TB ( 13 ). This issue of the Journal includes an article by Rundi who explored healthcare-seeking behaviour with regard to TB among the people of Sabah in East Malaysia and the impact of TB on patients and their families ( 15 ). The author used qualitative methods and interviewed patients with TB and their relatives. It was found that most (96%) respondents did not know the cause of TB. TB also affected life-styles of the people. The author emphasized the need to understand the reasons for misconceptions about TB and to address it through health education.
Better understanding of the prevalence of drug resistance against tuberculosis is one of the key elements in the control of TB. Drug resistance, in combination with other factors, results in increased morbidity and mortality due to tuberculosis. Drug-resistant strains of TB is rapidly emerging worldwide ( 16 ). The WHO reported alarming rise of not only multidrug-resistant (MDR) TB but also of XDR TB (extreme drug-resistant TB) globally. Both treatment and management of such cases are well beyond the capacity of any developing country. Globally, there were about 0.5 million cases of MDR TB. In Bangladesh, the MDR rate is 3.5% among new cases and 20% among previously-treated cases ( 5 ). The death rate in MDR cases is high (50–60%) and is often associated with a short span of disease (4–16 weeks) ( 17 ). Several factors have been identified for the development of MDR cases. These include non-adherence to therapy, lack of direct observed treatment, limited or interrupted drug supplies, poor quality of drugs, widespread availability of anti-TB drugs without prescription, poor medical management, and poorly-managed national control programmes ( 18 – 20 ). Continuation of the existing MDR surveillance is important to effectively plan for the treatment of MDR cases and implementation of the DOTS-Plus strategy. It requires rapid, concerted action and close collaboration among government, non-government and private organizations to control MDR tuberculosis ( 21 ).
The diagnosis of TB among children is difficult. Moreover, young children cannot produce sputum. Estimates indicate that children constitute about 10% of all new cases in high-burden areas ( 8 ). Clinical signs and symptoms and scoring system have been used for the diagnosis of TB among children ( 22 ). Various diagnostic techniques have been used for improving the diagnosis among children. These include culture, serodiagnosis, and nucleic acid amplification ( 23 ).
Many countries use BCG vaccine as part of their TB-control programme. The protective efficacy of BCG viccine against all forms of TB is about 50% but it was more in serious forms of infection (64% in cases of tuberculosis meningitis and 78% in disseminated infection) ( 24 ). Several new vaccines against TB are being developed. These vaccines are now being field-tested in different countries in different phases ( 25 ).
There are several challenges which need to be addressed for effective control of TB, particularly in developing countries. These include the development of an effective surveillance system, accelerated identification of cases, expansion of DOTS to hard-to-reach areas, strengthening of DOTS in urban settings, ensuring adequate staff and laboratory facilities, involvement of private practitioners, treatment facilities for MDR cases, identification of TB among children and extra-pulmonary cases, and effective coordination among healthcare providers ( 5 , 26 – 27 ). Moreover, the prevalence of TB is influenced by HIV, and effective control measures are needed for both the diseases.
Further research is warranted to improve diagnostics, develop new drugs and vaccines, simple and effective regimen for simultaneous treatment of TB and HIV, ways to improve programme effectiveness, and better understanding of the relationship between TB and chronic diseases, e.g. diabetes and smoking, and identify social and behavioural factors which limit the detection of cases ( 8 , 28 ).

IMAGES
VIDEO
COMMENTS
Tuberculosis (TB) is an infectious disease caused by strains of bacteria known as mycobacteria. The disease most commonly affects the lungs and can be fatal if not treated. However, most infected ...
Introduction. Tuberculosis (TB) represents a major global health threat that, despite being preventable and treatable, is the 13th leading cause of death worldwide and the second leading infectious killer after coronavirus disease 2019 (COVID-19) [1, 2].In the past decades, the TB burden has been slowly decreasing; however, with the emergence of COVID-19 and the current political conflicts ...
Cegielski JP, Chan PC, Lan Z, Udwadia ZF, Viiklepp P, Yim JJ, Menzies D. Aminoglycosides and Capreomycin in the Treatment of Multidrug-resistant Tuberculosis: Individual Patient Data Meta-analysis of 12 030 Patients From 25 Countries, 2009-2016. Clin Infect Dis. 2020 Oct 30:ciaa621. doi: 10.1093/cid/ciaa621.
Abstract. Tuberculosis (TB) is one of the most ancient diseases of mankind, with molecular evidence going back to over 17,000 years. In spite of newer modalities for diagnosis and treatment of TB, unfortunately, people are still suffering, and worldwide it is among the top 10 killer infectious diseases, second only to HIV.
29 Mar 2023. 19 Nov 2022. 08 Nov 2022. Tuberculosis Research and Treatment publishes original research articles and review articles related to all aspects of tuberculosis, from the immunological basis of disease to translational and clinical research.
Tuberculosis remains the leading cause of death from an infectious disease among adults worldwide, with more than 10 million people becoming newly sick from tuberculosis each year. Advances in diagnosis, including the use of rapid molecular testing and whole-genome sequencing in both sputum and non-sputum samples, could change this situation. Although little has changed in the treatment of ...
World TB Day on March 24, 2023, marks another year of both progress and setbacks for tuberculosis research and control efforts. The COVID-19 pandemic continues to impact tuberculosis case detection, treatment, and prevention, which has led to millions of missed cases. However, the promise of vaccines and continued developments in the diagnosis and treatment of tuberculosis are important steps ...
Tuberculosis remains a formidable global health challenge, causing substantial morbidity and mortality due to the limited protection in the lungs provided by the current BCG vaccine.1 The urgent need for an effective tuberculosis vaccine has prompted innovative approaches to accelerate vaccine development. One such approach is the use of controlled human infection models, which offer a unique ...
Tuberculosis is a speciality journal focusing on basic experimental research on tuberculosis. Tuberculosis aims to publish original research and reviews. It publishes articles on host response and immunology of tuberculosis and the molecular biology, genetics and physiology of the organism. View full aims & scope. $2490. Article publishing charge.
1. Introduction. Tuberculosis (TB) is a leading global public health problem, with high morbidity and mortality in humans. Until the COVID-19 pandemic, TB was still the leading cause of death from a single infectious agent, ranking above HIV/acquired immune deficiency syndrome [].The number of people newly diagnosed with TB fell from 7.1 million in 2019 to 5.8 million in 2020, and reduced ...
PMID: 34872367. Harrist AV, McDaniel CJ, Wortham JM, Althomsons SP. Developing National Genotype-Independent Indicators for Recent Mycobacterium Tuberculosis Transmission Using Pediatric Cases-United States, 2011-2017. Public Health Rep. 2021 Feb 19:33354920985215. doi: 10.1177/0033354920985215.
Tuberculosis is an infectious bacterial disease caused by Mycobacterium tuberculosis (Mtb), which is transmitted between humans through the respiratory route and most commonly affects the lungs, but can damage any tissue. Only about 10 percent of individuals infected with Mtb progress to active TB disease within their lifetime; the remainder of ...
Tuberculosis (TB) is an airborne disease caused by Mycobacterium tuberculosis (MTB) that usually affects the lungs leading to severe coughing, fever, and chest pains. Although current research in the past four years has provided valuable insight into TB transmission, diagnosis, and treatment, much remains to be discovered to effectively decrease the incidence of and eventually eradicate TB.
Abstract. Tuberculosis (TB) remains one of the deadliest infectious diseases responsible for millions of deaths annually across the world. In this paper we present a general overview of TB ...
Tuberculosis (TB) disease incidence has decreased steadily since 1993 (), a result of decades of work by local TB programs to detect, treat, and prevent TB disease and transmission.During 2020, a total of 7,163 TB cases were provisionally reported to CDC's National Tuberculosis Surveillance System (NTSS) by the 50 U.S. states and the District of Columbia (DC), a relative reduction of 20% ...
6 Central Tuberculosis Research Institute (RAMS), Moscow, Moscow Oblast, Russia 7 Central European Institute of Technology (CEITEC), Brno, Olomouc, Czechia The final, formatted version of the article will be published soon. Notify me Mycobacterium tuberculosis (Mtb) remains a major threat worldwide, although only a fraction of infected ...
New research being presented at this year's ESCMID Global Congress (formerly ECCMID) in Barcelona, Spain (27–30 April) has found compelling evidence that tuberculosis (TB) can have a lasting ...
TB Trials Consortium. The TB Trials Consortium (TBTC) is a collaboration of North American and international clinical investigators whose mission is to conduct programmatically relevant research concerning the diagnosis, clinical management, and prevention of TB infection and disease.. Behavioral and Social Science Research. Behavioral and social science research has the potential to make a ...
Tuberculosis is a bacterial infectious disease, which affects different parts of a human body, mainly lungs and can lead to the patient's death. The aim of this study is to investigate the global prevalence of drug-resistant tuberculosis using a systematic review and meta-analysis. In this study, the PubMed, Scopus, Web of Science, Embase, ScienceDirect and Google Scholar repositories were ...
Despite 90 years of vaccination and 60 years of chemotherapy, tuberculosis (TB) remains the world's leading cause of death from an infectious agent, exceeding human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) for the first time (WHO 2015b, 2016a). The World Health Organization (WHO) estimates that there are about 10.4 million new cases and 1.8 million deaths from TB ...
Background African giant pouched rats, trained by Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO), have demonstrated their ability to detect tuberculosis (TB) from sputum. We assessed rat-based case detection and compared the mycobacterium bacillary load (MTB-load) in children versus adults. Methods From January-December 2022, samples were collected prospectively from 69 ...
Tuberculosis (TB) is an ancient human disease caused by Mycobacterium tuberculosis which mainly affects the lungs, making pulmonary disease the most common presentation (K Zaman, 2010) [1]. However, TB is a multi-systemic disease with a protean presentation. The organ system most commonly affected includes the respiratory system, the gastrointestinal (GI) system, the lymphoreticular system ...
1 INTRODUCTION. Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (MTB) that poses a serious risk to human health. The 2022 World Health Organization Global Tuberculosis Report shows that the COVID-19 pandemic has reversed years of global progress in the fight against TB and continues to have a devastating impact on TB prevention and control. 1 ...
Tuberculosis (TB) is an ancient disease that has affected mankind for more than 4,000 years ( 1 ). It is a chronic disease caused by the bacillus Mycobacterium tuberculosis and spreads from person to person through air. TB usually affects the lungs but it can also affect other parts of the body, such as brain, intestines, kidneys, or the spine.