
Home > USC Columbia > Engineering and Computing, College of > Biomedical Engineering > Biomedical Engineering Theses and Dissertations

Biomedical Engineering Theses and Dissertations
Theses/dissertations from 2023 2023.
Laboratory Management Models in Core Facilities , Karmen Michael Owen
Detecting Physiological Concentrations of Alzheimer’s Associated Amyloid-β Protein Utilizing a Cell-Based Response , Brittany Elizabeth Watson
Volume Change Measurements of Cancer Cells in a Microfluidics Platform , Yukuan Yu
Theses/Dissertations from 2022 2022
Investigating the Impact of Endothelial Dysfunction and Aging On Vascular Remodeling Using Mouse Models , Liya Du
Histomechanical Compatibility of Coronary Artery Bypass Grafts , Colton J. Kostelnik
The Effect of Pulsed Field DC Electrophoresis and Field Amplified Sample Stacking on the Microchip Electrophoretic Separation of Organic Dyes , Travis Geoffrey Stewart
Long Non-coding RNA PVT1 – An Exploratory Study in Ovarian and Endometrial Cancer , Kevin Tabury
Interrogating the Role of ING4 in Hematopoietic Stem Cells and Cancer , Zanshé Thompson
Theses/Dissertations from 2021 2021
An in Vitro Approach To Vascular Therapeutic Testing , Shahd Ali Hasanain
Age and Sex Dependency of Thoracic Aortic Aneurysm Progression in a Mouse Model of Marfan Syndrome , Nazli Gharraee
Impaired Metabolic Flexibility in a Mouse Model Of Leigh Syndrome , Richard Sterling McCain Jr
Vascular Endothelial Dysfunction and Effects on Arterial Wall Microstructure , Jeffrey Thomas Rodgers
The Immune Modulatory Role of Endocannabinoid Anandamide to Suppress Inflammation Through Regulation of Microrna and Microbiome , Muthanna Ali Sultan
Theses/Dissertations from 2020 2020
Advanced Geometric Analyses in Vascular Disease and Interventions , Dara Ahmadi Azar
Nitric Oxide Expression With Age and Diet in the Arterial Wall of Apoe Knockout Mice , Kara Cooper
cis -Resveratrol Upregulates Tyrosyl-tRNA Synthetase and Inhibits the Proliferation of Select Breast Cancer Cell Lines , Marion Cone Hope III
Using Human Granulosa Cells to Select the Most Competent Embryos for Uterine Transfer in in Vitro Fertilization Cycles , Richard John Kordus
Effect of Cannabinoid Treatment on Immune Cell Functions During Acute Lung Injury , Amira Kamil Mohammed
Theses/Dissertations from 2019 2019
Beneficial Effects of Resveratrol Against Colitis and Colorectal Cancer Mediated by the Host Microbiome, Epigenome, and Immune Response , Haider Rasheed Daham Alrafas
Three-Dimensional Plasma Cell Survival Microniche in Multiple Myeloma , Katrina A. Harmon
Adipose Tissue Engineering: A Therapeutic Strategy for the Treatment of Obesity and Glucose Intolerance , Michael A. Hendley
Role of P-Glycoprotein in Alzheimer’s Disease for Enhanced Brain Elimination of Amyloid-β , Hope Holt
Three-Dimensional Collagen Tubes for In Vitro Modeling , Rebecca Jones
Experimental Methods and Techniques for Improved Biomechanical Characterization of Diverse Murine Aortopathies , Brooks Alexander Lane
Experimental Study of Free-Solution Separation Under Pulsed Electrophoresis in Microchip , Xin Liu
Biophysical Analyses of Left Ventricular Remodeling Secondary to Myocardial Infarction and Left Ventricular Pressure Overload , William Manuel Torres
RAGE Expression and Inflammation in Alzheimer’s Disease: in Vitro Model Development and Investigation of a Potential Peptoid Inhibitor , Lauren Michell Wolf
Theses/Dissertations from 2018 2018
Identification of the Mechanisms Through Which Botanicals Attenuate Pathogenesis of Human Diseases , Esraah Alharris
A Comprehensive Reengineering Of The Hospital Emergency Triage System , Nicholas D. Boltin
Matrix Stiffness Modulates Mesenchymal Stem Cell Sensitivity to Geometric Asymmetry Signals , Maria Eugenia Piroli
Association Between Mechanics And Biology In Vascular Graft Remodeling , David Andrew Prim
Modulation Of Amyloid-β Aggregation Via Small Molecules And Glycine Zipper Alterations , Steven Zebulon Vance
Theses/Dissertations from 2017 2017
Atherosclerotic Plaque Adhesion Strength and its role in Plaque Rupture , Bilal Merei
Far-Field Optical Microscopy Based on Stimulated Emission Depletion , Yunxia Wang
Theses/Dissertations from 2016 2016
A Theoretical Study of Polymer based Drug Delivery Systems , Ebtisam Abdullatif Aldaais
Automated Image Analysis And Spatial Computational Modeling Of NF-kB In Cerebrovascular Endothelial Cells , Kasey Catalfomo
Therapeutic Potential Of Catechins And Derivatives For The Prevention Of Alzheimer’s Disease , Shelby Elaine Chastain
A Mechanical Approach to the Characterization of Material Failure of Atherosclerotic Lesions , Lindsey A. Davis
Enabling Studies to Optimize Biomaterials for the Treatment of Myocardial Infarction , Eva Adriana Romito
Theses/Dissertations from 2015 2015
Effects of Cell Adhesion Peptides, pH, and Matrix Shape on Maintenance of Breast Cancer Stem Cells in an Engineered Hydrogel Matrix , Leily Daneshian
Design and Development of a Ventilation Chamber for Testing Efficacy of Tracheal Stents , Caroline N. Horton
Material Considerations for Development of 3D Printed Bronchial and Tracheal Stents , Nidah M. Hussain
Theses/Dissertations from 2014 2014
Isolation of Natural Nrf2 Activators from American Ginseng , Akrm Abdalrahman
Genes Mediating Cardiac Remodeling During Pregnancy and the Early Post-Partum-Period in Mice , Esam Aljrbi
A Three-Dimensional in Vitromodel of Atherogenesis , Pin Hsuan Chang
Identifying Performance Criteria of Fully Bioresorbable Scaffolds for Endovascular Applications , Jahid Ferdous
Novel Conditioning Protocols Focusing on Oxygen Manipulation to Enhance Stem Cell Transplantation , Brandon William Hanna
Developing a Bioreactor for Biaxial Mechanical Testing and Conditioning of Vascular Tissue , Steve Marcous
Experimental and Theoretical Studies of Native and Engineered Vascular Tissue Mechanics , Boran Zhou
Theses/Dissertations from 2013 2013
Toward Directing Cell Fate: Carbon Nanotubes As Modulators of Extracellular and Transporters of Intracellular Cues , Qingsu Cheng
Biomechanics of Porcine Renal Artery and the Development of A Replacment Vessel , Mohamed Gabr
Osteon-Mimetic Nanocomposite Materials For Bone Regeneration , Ozan Karaman
Microencapsulation of a Connexin-43 Mimetic Peptide as a Novel Wound Healing Agent in an Ocular Injury Model , Keith Brian Moore
Gold Nanoparticles and Peptoids as Novel Inhibitors of Amyloid Beta Aggregation in Alzheimer's Disease , Kelly Ann Moore
Effect of Physiological Oxygen Levels On Osteogenic Differentiation of Adipose-Derived Stem Cells , Suchit Sahai
Investigation of START Domain Proteins in Human Luteinized Cells and COS-1 Cells , Bo Shi
Characterizing Hypoxia and Its Behavioral Effects In 3-Dimensional Cell Aggregates , Matthew Lorincz Skiles
The Effect of αCT-1 Peptide on Bone Marrow Stromal Cells Following Injury , Adam Clay Vandergriff
Theses/Dissertations from 2012 2012
Flow-Induced Forces Regulate the Development of Cardiac Valves , Stefanie Vawn Biechler
Stimulated Emission Depletion (STED) Microscopy and Pacific Orange Dye Optimization For H9C2 Cox-1 Imaging Via Indirect Immunocytochemistry , John Wesley Merriman
Vasculogenic Scaffolds: How Cell-Cell and Cell-Matrix Interactions Regulate Vascular Differentiation and Morphogenesis , Samantha Jo Stinson
A Novel Quantitative Mechanical Test of Atherosclerotic Plaque Stability , Ying Wang
Theses/Dissertations from 2011 2011
Synthesis and Characterization of Thermally Responsive Nanocapsules Surface Decorated With Folic Acid For Targeted Drug Delivery and Cancer Destruction , Kyle Bradley Gilstrap
Study of Polyphenols and Naphthalimide Analogs As Inhibitors of Amyloid- β Protein Aggregation In Alzheimer'S Disease , Chen Suo
Development and Characterization of Micro/Nano Scale Biomaterials For Biomedical Applications , WUJIE ZHANG
Theses/Dissertations from 2010 2010
Study of Structural and Physical Properties of Small Molecule and Nanoparticle Inhibitors of Amyloid-B Protein Fibril Formation In Alzheimer'S Disease , Deborah Soto-Ortega
Theses/Dissertations from 2009 2009
A Novel Technique for Fabricating Aligned Nanofibers by Using Solution Electrospinning , Ozan Karaman
Synthesis and Characterization of Injectable Star-shaped Poly(Lactide-co-Glycolide-co-Acrylate) Macromers , Jianping Wu
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Submissions
- University Libraries
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright

- Langson Library
- Science Library
- Grunigen Medical Library
- Law Library
- Connect From Off-Campus
- Accessibility
- Gateway Study Center

Email this link
Biomedical engineering.
- Find Articles
- Reference Sources
- Patent Information
- Professional & Career Information
- Bibliographic Management Software
- UC Irvine Departments & Sites
Research Grants & Funding Resources
- Dissertation/Thesis Preparation
- Media Resources Center
- Henry Stewart Talks: The Biomedical & Life Sciences Collection
- Engineering Ethics
- Things To Do & Things to Know
* Indicates UCI Access Only
- GrantForward GrantForward is a funding opportunity database and recommendation service built by academics for researchers
- SPIN - Sponsored Projects Information Network* SPIN is a searchable database made available by the UCI Office of Research that indexes approximately 8,000 opportunities. SPIN and IRIS include some overlap. Spin Userguide
- CORDIS - Community Research and Development Information Service This site provides information on current research projects in the European Union and gives access to documents concerning the EU Framework programmes as well as to other EU sites.
- CRISP - Computer Retrieval of Information on Scientific Projects Contains information on federally funded biomedical research projects. The database is maintained by the Office of Extramural Research at the National Institutes of Health.
- D&B Million Dollar Directory*
- Grants.gov Grants.gov is a central storehouse for information on over 1,000 U.S. Government grant programs offered by the 26 federal grant-making agencies and provides access to approximately $500 billion in annual awards.
- NIH Guide for Grants and Contracts NIH Funding Opportunities (NIH Guide for Grants and Contracts)
- NSF (National Science Foundation) A-Z Index of Funding Opportunities
- UROP Off-campus: Biomedical sciences For Undergraduates-These listings are identified by the program name, which is linked to a summary of the program and the program's official Web site.
- UROP Off-campus: Engineering, Computer Science, and Applied Sciences For Undergraduates-These listings are identified by the program name, which is linked to a summary of the program and the program's official Web site.
- UROP Off-campus: Physical Sciences For Undergraduates-These listings are identified by the program name, which is linked to a summary of the program and the program's official Web site.
- Annual register of grant support LB 2336 A5
- Grants Register
- Directory of research grants LB 2338 D5
- Fastweb Fastweb and the College Board offers scholarship searches for parents and students.
- FinAid: The Smart Student Guide to Financial Aid Resources on loans, scholarships, and financial aid of every kind; also on calculating needs, application forms, etc.
- Federal Students Aid Provides information on federal loans.
- Campus Grotto Offers a tool that lets students and parents compare loan options.
- PLUS Loans Explains how parents van borrow for their childrens's education.
- Research Grants & Resources
Proposal Preparation
- Developing And Writing Grant Proposals Useful for applying for federal grants. By the staff of the Catalog of Federal Domestic Assistance.
- EPA Grantwriting Tutorial Online tutorial from Purdue University in collaboration with the U.S. Environmental Protection Agency.
- Grants.gov: Applicant Resources Includes Developing a Grant Proposal, terminology proposal writing links, links to application packages, grantmaking agencies, types of grants, and more.
- All About Grants Tutorials For biomedical investigators applying for NIH research project grants. Maintained by its National Institute of Allergy and Infectious Diseases.
- Guide for Writing a Funding Proposal Written by S. Joseph Levine of Michigan State University. Offers excellent advice on all parts of the proposal. Includes a sample proposal and links to other proposal writing sites.
- Proposal Writer's Guide An excellent outline by Donald Thackrey for academic faculty and staff. Especially useful. A site maintained by the University of Michigan's Division of Research and Development Administration.
- Proposal Preparation and Submission A site maintained by the University of Michigan's Division of Research and Development Administration.
- Proposal Writing: Selected Web Sites Outstanding. Provides essential links for university scholars and researchers needing more than here on research funding in particular.
- Writing Winning Proposals: An Introduction Excellent advice from the ASME: American Society of Mechanical Engineers.
- Grant Proposal Writing Tips Some good, clearly-stated tips from the Corporation for Public Broadcasting, which reviews hundreds of proposals a year.
- GrantProposal.com Well organized site on proposal writing. Includes an overview, inquiry and cover letters, standard components of a proposal, a sample proposal, advice from funders, and more. An excellent section on researching funding opportunities is included also.
- How to Write a Mission Statement Outline by Janet M. Radtke on writing a mission statement for an organization. A site of the Los-Angeles based Grantsmanship Center.
- National Network of Grantmakers: NNG Common Grant Application This common grant application form is accepted by a number of foundations. It can also be useful in organizing the information needed in a project proposal. (pdf)
- Grants for Nonprofits Information for how to write specific grants for nonprofits, how to find appropriate funding sources for nonprofits and how to get grants from nonprofit organizations. Lots of easy hints.
- The Foundation Center's Proposal Writing Short Course Free, online course.
- The Foundation Center's Proposal Budgeting Basics Free, online course.
- The Foundation Center Webinars on Proposal Writing Basics Free; must register in advance.
- The Foundation Center's Guide to Proposal Writing, 5th ed. - An Audio Book! Free audio book from the Foundation Center. Listen online now or download for later listening.
- Sample Grant Proposals Proposals from the Idea Bank's Grant Writing Course. Most are successfully funded, and are for funding for fire departments.
- School Grants Offers a number of education-focused, successful, sample proposals. Most are directed to corporate or government funding sources and are downloadable in PDF format.
- Winning Grant Proposals Online Provides funded grant proposals for sale in a variety of categories. Particularly well-stocked with federal grant proposals. A site from the Grantsmanship Center, a Los-Angeles based proposal training center.
- Writing a Successful Grant Proposal An excellent and thorough guide for writing grant proposals, from the Minnesota Council on Foundations. FAQs include pros and cons of hiring a professional grantwriter and what to do if a proposal is funded.
Sources for Funding - Databases
There are several major databases to consult for seeking funding - the two largest are Pivot & COS. On the left sidebar, is a long list of resources that source funding opportunities for travel, research, grants & contracts.
- COS - Community of Science Funding Opportunities Funded at UCI by the UCI Libraries. Provides a user-friendly interface to a worldwide database of more than 25,000 records.
- COS Userguide
- COS Funding News
- COS Top Ten Funding Opportunities Of the more than 25,000 records, the records most viewed last week.
- << Previous: UC Irvine Departments & Sites
- Next: Dissertation/Thesis Preparation >>
- Last Updated: Apr 4, 2024 9:24 AM
- URL: https://guides.lib.uci.edu/engr_biomed
Off-campus? Please use the Software VPN and choose the group UCIFull to access licensed content. For more information, please Click here
Software VPN is not available for guests, so they may not have access to some content when connecting from off-campus.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Biomedical engineering articles from across Nature Portfolio
Biomedical engineering is a branch of engineering that applies principles and design concepts of engineering to healthcare. Biomedical engineers deal with medical devices such as imaging equipment, biocompatible materials such as prostheses or therapeutic biologicals, or processes such as regenerative tissue growth, for example.
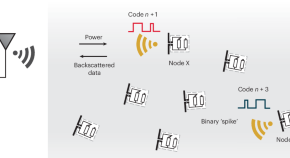
Wireless radiofrequency network of distributed microsensors
Distributed sensing of a dynamic environment is typically characterized by the sparsity of events, such as neuronal firing in the brain. Using the brain as inspiration, an event-driven communication strategy is developed that enables the efficient transmission, accurate retrieval and interpretation of sparse events across a network of thousands of wireless microsensors.
Latest Research and Reviews
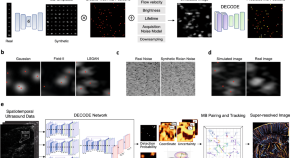
Context-aware deep learning enables high-efficacy localization of high concentration microbubbles for super-resolution ultrasound localization microscopy
Ultrasound localisation microscopy enables deep tissue microvascular imaging. Here, authors introduce LOCA-ULM, a deep learning pipeline enhancing localisation accuracy in high microbubble concentrations. LOCA-ULM reveals dense cerebrovascular networks and enhances the sensitivity of functional ULM.
- YiRang Shin
- Matthew R. Lowerison
- Pengfei Song
In vivo validation of highly customized cranial Ti-6AL-4V ELI prostheses fabricated through incremental forming and superplastic forming: an ovine model study
- Silvia Brogini
- Alberto Crovace
- Gianluca Giavaresi
Early gastric cancer detection and lesion segmentation based on deep learning and gastroscopic images
- Kezhi Zhang
- Haibao Wang
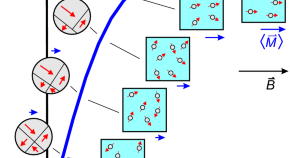
A comprehensive approach to characterize navigation instruments for magnetic guidance in biological systems
- Peter Blümler
- Fabian Raudzus
- Friederike Schmid

Exploring the electrical robustness of conductive textile fasteners for wearable devices in different human motion conditions
- Afonso Fortes Ferreira
- Helena Alves
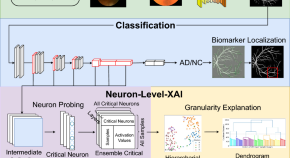
Neuron-level explainable AI for Alzheimer’s Disease assessment from fundus images
- Nooshin Yousefzadeh
- Charlie Tran
News and Comment

From equitable access to equitable innovation: rethinking bioengineering for global health
What does global health equity mean? In bioengineering, ‘equity’ is often interpreted as global ‘access’ to technologies, thereby neglecting wider structural inequalities. Here we suggest that concepts of equity need to be expanded to incorporate principles of equitable representation and recognition within the innovation ecosystem.
- Alice Street
- Maïwenn Kersaudy Kerhoas
- Zibusiso Ndlovu
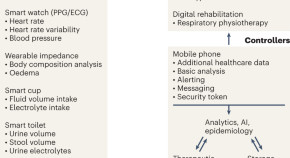
The potential of wearable sweat sensors in heart failure management
Wearable sweat sensors could be used to monitor patients with heart failure, providing a route to personalized and automated patient management in hospitals and at home.
- Noé Brasier
- Ole Frobert
- Roozbeh Ghaffari
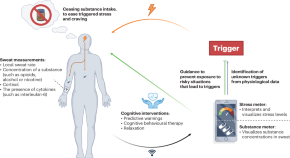
Towards on-skin analysis of sweat for managing disorders of substance abuse
A patient-centred system that leverages the analysis of sweat via wearable sensors may better support the management of patients with substance-use disorders.
- Noe Brasier
- Juliane R. Sempionatto
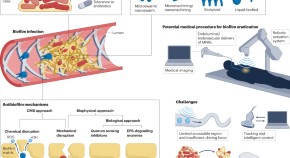
Micro- and nanorobots for biofilm eradication
Micro- and nanorobots present a promising approach for navigating within the body and eliminating biofilm infections. Their motion can be remotely controlled by external fields and tracked by clinical imaging. They can mechanically disrupt the biofilm matrix and kill the dormant bacterial cells synergistically, thereby improving the effectiveness of biofilm eradication.
- Staffan Kjelleberg
Innovating dialysis through computational modelling of hollow-fibre haemodialysers
Haemodialyser technology has not advanced much in decades, despite its unresolved shortcomings. Sophisticated new computational tools such as high-fidelity surrogate in silico dialyser models could reduce the time and expense of exploring alternative designs, dialysis dose and operating conditions compared with the current gold standard in vitro studies.
- Ruhit Sinha
- Michael V. Rocco
- Anne E. Staples
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- translation and innovation
- Case-Coulter Proposal
Case-Coulter Translational Research Partnership Proposal
Program synopsis.
Program year 2024 (PY24) is the third year of the fourth phase of the Case-Coulter Translational Research Partnership (CCTRP) to support collaborative translational research projects that address unmet or poorly met clinical needs. The CCTRP seeks to reduce the market risk of promising new clinical products arising from the research programs of BME and affiliated Case Western Reserve University faculty.
Successful projects will thus often focus on the development of prototype products, building relationships with companies, performing clinical feasibility studies, obtaining regulatory approval, and other activities that companies or business experts indicate are the essential last steps before a license can be negotiated or a startup launched.
An overarching goal of the program is to foster research and development work likely leading to commercially relevant translational technologies within a three- to five-year horizon. Because the goals of all Coulter projects are to reduce market risk, all proposals are expected to reflect genuine business input, and all projects must include a “business advisor” as an important member of the decision-making team.
The funding provided by the CCTRP should be considered as a “cooperative agreement” rather than a “grant." That is, the CCTRP staff, Technology Transfer Office (TTO) staff, and the Oversight Committee (OC) are partners in the project rather than a funding agency. Faculty should (and will be expected to) take advantage of the unique resources provided by the program and to include program staff in all major decisions. Project PI’s are expected to provide at least monthly updates on progress towards milestones, and timely quarterly reporting from the BME PI of each project will be a milestone of all projects and will factor into decisions about continued funding.
The CCTRP awards funding at two different levels, pilot and full projects.
PreProposal Due Date - March 15 C3i Program - April to June Proposals and Pitch Presentations Due - Approximately July Pitch Day - Approximately August Project Start Date - September 1
Proposal Information
The 2024 Case-Coulter Translational Research Partnership Program Request for Applications (RFA) is now open!
Helpful links:
- Pre-proposal cover page template - Due March 15
- Pre-proposal template - Due March 15
- Full proposal template and cover page template
- Pitch video template and pitch presentation template
All submissions should be directly submitted to Box or sent to [email protected] . You should receive a confirmation within one business day.
Point of Contact
Stephen Fening, PhD Associate Vice President for Research Professor, Biomedical Engineering Managing Director, Case-Coulter Translational Research Partnership Wickenden Building, Rm. 410 216.368.2639 office [email protected] Schedule a meeting with Steve .
Andrew Cornwell, PhD Associate Director, Case-Coulter Translational Research Partnership Wickenden Building, Rm. 411 216.502.2887 office [email protected] Schedule a meeting with Andy .
Schedule a Meeting With a Member of Our Team (Most Availability)
Pilot Projects
Pilot projects up to $25,000 can be applied for on a rolling basis but will be considered quarterly. Pilot projects will typically be for a six-month period with specific milestones defined with the intent of participation in the annual full Coulter award cycle. Apply using same instructions with a focus on the necessary steps that will position PI for a successful full CCTRP application the following year. The individual component sections can be scaled back, but the PI is responsible for making his/her case. There are no CCTRP OC presentations for pilot projects.
Full Projects
Full projects will typically be for an initial one-year period with specific milestones defined on a quarterly basis; in special circumstances, proposals can span more than one year but must be approved prior to submission. Continuation and renewal applications will be evaluated on a competitive basis with new applications, and must have a comparison of milestones achieved vs. those planned in the original award. An important part of the proposal is the commercialization opportunity and the idea or vision for the end product. Examples of preferred future outcomes include inventions, patents, improved diagnosis and treatment of disease, commercial products, licenses, commercial partnerships and start-up companies.
Project Stages
Stage 1 (pre-proposal).
A pre-proposal is required by March 15, 2024 . The pre-proposal should include the pre-proposal cover page and follow the two-page template (no more than 2 pages in length not including the cover page). The submission should be submitted via email to [email protected] no later than midnight of the posted due date. You will receive an email reply to each LOI submitted to confirm receipt no later than once business day after the submission deadline. If you do not receive a reply email by this time, then your LOI was not received and you should follow up with a call to one of the points of contact on the first page of this RFA.
Applicants are strongly encouraged to discuss their proposal with Steve Fening (Coulter Program Director, [email protected] ) or Andrew Cornwell (Associate Coulter Program Director, [email protected] ) prior to submission.
Stage 2 (Pre-proposal Review)
The pre-proposal stage will serve as a decision point to identify the proposals with the highest likelihood of success. Teams whose pre-proposals do not move to full proposals will have the opportunity to work with the CCTRP staff to strengthen their approach for a subsequent pilot proposal or for evaluation in the following year. The CCTRP OC will review pre-proposals and make recommendations on funding priority. This committee expertise includes that of professional investors, experienced entrepreneurs, medical and scientific experts, large industry, and technology transfer professionals.
Stage 3 (C3i Program)
The Case-Coulter TRP partners with the Clinical and Translational Science and Collaborative (CTSC) to provide an optional strategic development program for applicants who are invited to submit a full proposal and oral pitch. The goal of this program is to provide the necessary resources, including knowledge and market research funding, for applicants to assemble the best possible full application. While not mandatory, participation is strongly encouraged as the purpose of this program is to assist faculty investigators. The program will consist of didactic sessions and group mentoring and will be further described to those reaching this stage. Lecture slides for the C3i program will be posted on Box as they are available. Market research funding will be available although this is contingent upon participation in the program. This funding shall be used at the sole discretion of the CPD office to thoroughly uncover any hurdles between the technology and commercialization. While not exhaustive, this diligence process will be extremely important to the success of technology. Learn more about the C3i program .

Stage 4 (Full Proposal and Oral Pitch)
Applicants accepted past the pre-proposal stage will be asked to submit a full grant application (five-page limit) and to give a brief presentation to the OC. Based on the advice of the OC, the Coulter Principal Investigator (i.e., the BME chair) will make final funding decisions regarding each project.
Stage 5 (Project Selection)
Applicants will be notified in early August on the outcome of the evaluation of their proposal. The months of August will be spent meeting with awardees to inform of award requirements in more detail, getting award accounts set up and, where necessary, to refine the project plan, resolve OC questions, address issues on which the award might be contingent, etc. Awardees should expect to possibly have interactions/support from members of the OC over the course of the award period. Proposals selected for funding can expect to begin work September 1.
Budget and Project Period
Awards up to $200,000 will be considered if well-justified. Applicants should carefully determine the funds needed to achieve well-defined translational milestones. As the amount requested by a proposal increases, project milestones are expected to be increasingly related to mitigating market risk, increasingly achievable, and increasingly likely to lead to a license or startup in the short term. Funds may be used for salary support of faculty, graduate students and other research staff, but may not be used for general staff or administrative support or for tuition.
Operating supplies, minor equipment items, prototyping expenses, imaging time and travel directly associated with the research activity are examples of eligible budget items. Your budget should reflect an accurate assessment of what the PI thinks is needed to take this translational research project to the next step of commercialization. It is expected that the PI participate in the project in a meaningful way, thus a significant percentage of PI effort should be allocated towards the budget.
Total costs should not exceed $200,000 for the project period of 12 months, and all requests should not be for the maximum. Only well-justified proposals will be competitive for the maximum award. Indirect costs not to exceed 15% of direct cost are to be included in the total cost budget. Carry-over of funds beyond the initial interval must be approved in advance and will be based upon attainment of milestones; extensions may be approved for a maximum of six months based on written justification by the PI. Approval for requests for extensions of funding beyond the six-month end date are not likely to be granted.
Funding Disbursement and Reporting
Project funds are released on a quarterly basis, subject to the attainment of milestones and receipt of the quarterly report. The first Tranche begins in September, but subsequent are contingent upon milestone-based performance (typically on a quarterly basis). The CCTRP office wants to know if a project is not working as planned, as there may be other resources available, such as expertise from the CCTRP Oversight Committee (OC). Additionally, if a milestone cannot be achieved, the CCTRP office will work with awardees to determine appropriate changes that will enable the project to get back on track. The CCTRP office aims to be flexible within this framework to do what is best for technology commercialization. It is also expected the budget will reflect uniform activity throughout the year.
Program Eligibility
Each full proposal must have co-principal investigators:
- BME co-PIs must be permanent, full-time, tenure-track faculty within the professorial ranks (assistant/associate/full) of the Case Western Reserve University Department of Biomedical Engineering. Secondary faculty of the BME department may also be a co-PI. For secondary appointment consideration, respective applicants must apply to the BME department. More details will be provided to those prospective applicants who reach Stage 4 of the process.
- Clinical co-PIs must be full-time professional staff in one of the affiliated medical centers of Case Western Reserve University, including University Hospitals, Cleveland Clinic, MetroHealth Medical Center, and the Cleveland VA. Full-time clinical faculty in the School of Dental Medicine and the School of Nursing are also eligible as clinical co-PIs.
General Information
Criteria - Proposed research must relate directly to critical market-related milestones, as determined in conjunction with the project business advisor and industrial experts, which will ultimately lead to applications in health care. The objectives of the project should include an outcome that will benefit patients. Evaluation of each proposal will be on the basis of scientific merit, potential health care impact and significance, experience of the investigators, and the potential for commercialization.
IRB/IACUC - Approvals for animal and human subjects (if needed) must be completed by the time of final proposal review. The status of approvals must be clearly indicated in the proposal, and documentation of approval must be submitted prior to establishing the award accounts.
Templates - Templates are available for all three content submissions: Pre-proposal, proposal, and oral pitch. The template for the pre-proposal is available of the program's website in word formate. Other templates will be made available as needed.
Please submit all materials (pre-proposals, proposals, oral pitch) electronically to [email protected] .
Frequently Asked Questions
Who is involved with the Translational Research Partnership?
The Translational Research Partnership (TRP) leadership information is available on the CCRTP People page , and we are pleased to answer your questions or respond to comments at any time.
The TRP is overseen by an oversight committee (OC) that is responsible for project selection, guidance; mentoring is an important and critical role of the OC.
What are the requirements for submitting a proposal?
Many questions can be answered by reviewing the call for proposals listed above.
- Is the research translational?
- Is it based on sound scientific principles?
- Is the plan feasible?
- Are the milestones appropriate?
- Is the project scale appropriate for the amount of funding?
- Does the clinical member have patient contact?
- Does the clinician contribute effectively and substantially?
- Does the project address a clinical need that is an unmet need, under-met need, or improved method?
Other translational research considerations:
- Is a Product endpoint identified?
- What is its Intellectual Property (IP) potential?
- What is the market landscape?
- What is the regulatory landscape?
- What is the timeframe for realization?
- What is the potential for follow-on funding?
What are the important deadlines?
The funding cycle deadlines may be viewed in the timeline section above.
We use cookies to enhance our website for you. Proceed if you agree to this policy or learn more about it.
- Essay Database >
- Essays Samples >
- Essay Types >
- Research Proposal Example
Biomedical Engineering Research Proposals Samples For Students
2 samples of this type
If you're seeking an applicable way to streamline writing a Research Proposal about Biomedical Engineering, WowEssays.com paper writing service just might be able to help you out.
For starters, you should browse our huge collection of free samples that cover most various Biomedical Engineering Research Proposal topics and showcase the best academic writing practices. Once you feel that you've figured out the major principles of content presentation and taken away actionable insights from these expertly written Research Proposal samples, putting together your own academic work should go much smoother.
However, you might still find yourself in a situation when even using top-notch Biomedical Engineering Research Proposals doesn't allow you get the job done on time. In that case, you can get in touch with our experts and ask them to craft a unique Biomedical Engineering paper according to your individual specifications. Buy college research paper or essay now!
Example Of Approaches To Brain Stress Testing: Bold Magnetic Resonance With Computer-Controlled Research Proposal
Introduction.
Human brain is a complex organ and so are its diseases. Over the years numerous techniques, devices and processes have been developed that can help us to correctly access and diagnose the problem areas and provide solutions to them (14). Computer controlled delivery of carbon dioxide is a method that is conducted with the help of a gas blender that works to supply and alter carbon dioxide to the brain creating tensions in the brain (10) (16).
Carbon dioxide challenge
Free research proposal on research on pain and dementia residents.
Don't waste your time searching for a sample.
Get your research proposal done by professional writers!
Just from $10/page
Password recovery email has been sent to [email protected]
Use your new password to log in
You are not register!
By clicking Register, you agree to our Terms of Service and that you have read our Privacy Policy .
Now you can download documents directly to your device!
Check your email! An email with your password has already been sent to you! Now you can download documents directly to your device.
or Use the QR code to Save this Paper to Your Phone
The sample is NOT original!
Short on a deadline?
Don't waste time. Get help with 11% off using code - GETWOWED
No, thanks! I'm fine with missing my deadline
biomedical engineering Recently Published Documents
Total documents.
- Latest Documents
- Most Cited Documents
- Contributed Authors
- Related Sources
- Related Keywords
Ophthalmological instruments of Al-Halabi fill in a gap in the biomedical engineering history
Virus-like particles: revolutionary platforms for developing vaccines against emerging infectious diseases.
Virus-like particles (VLPs) are nanostructures that possess diverse applications in therapeutics, immunization, and diagnostics. With the recent advancements in biomedical engineering technologies, commercially available VLP-based vaccines are being extensively used to combat infectious diseases, whereas many more are in different stages of development in clinical studies. Because of their desired characteristics in terms of efficacy, safety, and diversity, VLP-based approaches might become more recurrent in the years to come. However, some production and fabrication challenges must be addressed before VLP-based approaches can be widely used in therapeutics. This review offers insight into the recent VLP-based vaccines development, with an emphasis on their characteristics, expression systems, and potential applicability as ideal candidates to combat emerging virulent pathogens. Finally, the potential of VLP-based vaccine as viable and efficient immunizing agents to induce immunity against virulent infectious agents, including, SARS-CoV-2 and protein nanoparticle-based vaccines has been elaborated. Thus, VLP vaccines may serve as an effective alternative to conventional vaccine strategies in combating emerging infectious diseases.
Biomedical Engineering Technologies
Biocybernetics and biomedical engineering – current trends and challenges, deep learning models principles applied to biomedical engineering, deep learning models evolution applied to biomedical engineering, ieee transactions on biomedical engineering (t-bme), biomedical engineering and occupational therapy approach in technologies for enhancement human labor and defense abilities, artificial intelligence models applied to biomedical engineering, introduction to cognitive science, cognitive computing, and human cognitive relation to help in the solution of artificial intelligence biomedical engineering problems, export citation format, share document.
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Active funding opportunity
Nsf 24-561: foundations for digital twins as catalyzers of biomedical technological innovation, program solicitation, document information, document history.
- Posted: March 22, 2024
Program Solicitation NSF 24-561
Full Proposal Deadline(s) (due by 5 p.m. submitting organization’s local time):
June 21, 2024
May 05, 2025
First Monday in May, Annually Thereafter
Important Information And Revision Notes
Any proposal submitted in response to this solicitation should be submitted in accordance with the NSF Proposal & Award Policies & Procedures Guide (PAPPG) that is in effect for the relevant due date to which the proposal is being submitted. The NSF PAPPG is regularly revised and it is the responsibility of the proposer to ensure that the proposal meets the requirements specified in this solicitation and the applicable version of the PAPPG. Submitting a proposal prior to a specified deadline does not negate this requirement.
Summary Of Program Requirements
General information.
Program Title:
Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech)
The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) program supports inherently interdisciplinary research projects that underpin the mathematical and engineering foundations behind the development and use of digital twins and synthetic data in biomedical and healthcare applications, with a particular focus on digital, in silico models used in the evaluation of medical devices and the relevance of the developed models in addressing current and emerging challenges affecting the development and assessment of biomedical technologies. The goal of the FDT-BioTech initiative is to catalyze biomedical technological innovation through new foundational development of methods and algorithms relevant to digital twins and synthetic humans.
Cognizant Program Officer(s):
Please note that the following information is current at the time of publishing. See program website for any updates to the points of contact.
Yulia R. Gel, MPS/DMS, telephone: (703) 292-7888, email: [email protected]
Zhilan J. Feng, MPS/DMS, telephone: (703) 292-7523, email: [email protected]
Stephanie George, ENG/CBET, telephone: (703) 292-7825, email: [email protected]
Varun Chandola, CISE/OAC, telephone: (703) 292-2656, email: [email protected]
Ashok Srinivasan, CISE/OAC, telephone: (703) 292-2122, email: [email protected]
Laura Biven, NIH, telephone: (301)480-4021, email: [email protected]
Fenglou Mao, NIH, telephone: (301)451-9389, email: [email protected]
Aldo Badano, FDA, telephone: (301) 796-2534, email: [email protected]
- 47.049 --- Mathematical and Physical Sciences
- 47.070 --- Computer and Information Science and Engineering
- 93.310 --- NIH Office of Data Science
Award Information
Anticipated Type of Award: Standard Grant or Continuing Grant
Estimated Number of Awards: 6 to 10
The number of awards will depend on the quality of the received proposals and the budget availability.
$4,000,000 to $5,000,000 in FY24, contingent on availability of funds.
The duration of the awards should be up to 3 years. The award size and duration should be consistent with the project scope.
Collaborative projects from multiple organizations are accepted, according to standard NSF procedures. The total budget (direct and indirect cost) for a collaborative project from multiple organizations must not exceed $1,000,000.
Eligibility Information
Who May Submit Proposals:
Proposals may only be submitted by the following: Institutions of Higher Education (IHEs) - Two- and four-year IHEs (including community colleges) accredited in, and having a campus located in the US, acting on behalf of their faculty members. Special Instructions for International Branch Campuses of US IHEs: If the proposal includes funding to be provided to an international branch campus of a US institution of higher education (including through use of subawards and consultant arrangements), the proposer must explain the benefit(s) to the project of performance at the international branch campus, and justify why the project activities cannot be performed at the US campus.
Who May Serve as PI:
There are no restrictions or limits.
Limit on Number of Proposals per Organization:
Limit on Number of Proposals per PI or co-PI: 1
An individual may serve as PI or co-PI on no more than ONE proposal. Participating in a proposal as other senior/key personnel does not count in this limit. Changes in investigator roles post-submission to meet the eligibility limits will not be allowed. It is the responsibility of the submitters to confirm that the entire team is within the eligibility guidelines.
Proposal Preparation and Submission Instructions
A. proposal preparation instructions.
- Letters of Intent: Not required
- Preliminary Proposal Submission: Not required
Full Proposals:
- Full Proposals submitted via Research.gov: NSF Proposal and Award Policies and Procedures Guide (PAPPG) guidelines apply. The complete text of the PAPPG is available electronically on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
- Full Proposals submitted via Grants.gov: NSF Grants.gov Application Guide: A Guide for the Preparation and Submission of NSF Applications via Grants.gov guidelines apply (Note: The NSF Grants.gov Application Guide is available on the Grants.gov website and on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=grantsgovguide ).
B. Budgetary Information
Cost Sharing Requirements:
Inclusion of voluntary committed cost sharing is prohibited.
Indirect Cost (F&A) Limitations:
Not Applicable
Other Budgetary Limitations:
C. Due Dates
Proposal review information criteria.
Merit Review Criteria:
National Science Board approved criteria. Additional merit review criteria apply. Please see the full text of this solicitation for further information.
Award Administration Information
Award Conditions:
Standard NSF award conditions apply.
Reporting Requirements:
Standard NSF reporting requirements apply.
I. Introduction
Digital twins offer tremendous potential to revolutionize healthcare delivery by enabling data-informed decision-making under uncertainty. The National Academies of Science, Engineering and Medicine (NASEM) published a report in 2023 entitled “Foundational Research Gaps and Future Directions for Digital Twins.” This report defines a digital twin as “a set of virtual information constructs that mimics the structure, context, and behavior of a natural, engineered, or social system (or system-of-systems), is dynamically updated with data from its physical twin, has a predictive capability, and informs decisions that realize value”. In addition, this report recognizes that in the healthcare sciences such virtual representations of human physiology and pathology have the potential to enable novel pathways for the development and evaluation of new biomedical technologies. Achieving this vision requires a convergent research approach that engages disciplines spanning mathematics, statistics, biomedical engineering, and computational sciences to address the broad range of emerging needs for developing foundational concepts behind digital twins. This anticipated paradigm shift hinges on fundamental scientific and engineering breakthroughs by interdisciplinary teams for developing, validating, and sharing human digital twin frameworks, capable of integrating data from individuals, populations, and devices to catalyze new discoveries and innovation in healthcare systems.
The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) program aims to accelerate innovations in biomedical technologies through development of principled mathematical, statistical, and engineering foundations for digital twins and synthetic human models in healthcare applications. The specific focus of FDT-BioTech is on digital, in silico models that could be used in the evaluation of medical devices and to advance regulatory sciences. The work is also expected to contribute more broadly to the development and implementation of human digital twins. This FDT-BioTech program provides an opportunity to form cohesive collaboration teams including mathematicians, statisticians, biomedical engineers, computer scientists, physicians, and experts from other domains. This collaboration will advance our understanding of foundational mechanisms behind computational representations of physiologic systems; verification, validation, and uncertainty quantification in a biomedical context; transferability, generalizability, and robustness; ethics, security, and privacy; and validation and sharing mechanisms, particularly in terms of regulatory relevance.
II. Program Description
This interagency solicitation is a collaboration between the U.S. National Science Foundation (NSF), National Institutes of Health (NIH) and Food and Drug Administration (FDA). The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) supports innovative and transformative research to advance the mathematical, statistical, and engineering approaches underpinning digital twins in biomedical and healthcare domains ultimately enabling unique tools for innovative evaluation of novel emerging technology that can potentially de-risk therapeutic, biologic, and medical device development and accelerate the introduction of safe and effective medical technologies for improved patient outcomes. Additionally, the emerging concepts of digital twins demonstrate a high potential to revolutionize preclinical and clinical research through reliable in silico investigations, as well as transform clinical practice by providing a framework for patient monitoring, management, and optimal decision making.
Furthermore, collectively, digital twins can be used to develop digital cohorts for accelerating innovation in biomedical technology. For instance, ensembles of digital twin humans could allow for on-demand enrollment of digital cohorts and pipelines for development, tuning, testing, and monitoring in the digital world. Digital study populations can display the variability observed in human populations, including under-represented subgroups and rare conditions, thereby addressing the fundamental problem of algorithmic and other biases which remains inaccessible with current paradigms. Ultimately, leveraging digital models of patients, disease processes, and medical devices is an agile modern approach to technological development and represents a paradigm shift in the development and evaluation of medical products and new technologies. However, achieving this vision hinges on fundamental advances in mathematics, statistics, computational sciences, and engineering.
Note: Projects may leverage virtual representations at multiple scales including a single physiologic or pathologic system, multiple systems, whole-humans, or populations; and be patient-specific or synthetically-derived. Virtual representations may include artificial intelligence (AI), first-principles, mechanistic, or empirical models. The virtual representations should be capable of interfacing with medical technologies and thus may include virtual representations of medical devices.
The rationale for FDT-BioTech is the current knowledge gaps that obstruct the development and use of digital twins in biomedical and other domains. Filling this gap requires novel crosscutting interdisciplinary approaches, where mathematical and statistical foundations play a pivotal role. Some examples of new developments in the foundation of digital twins with strong potential to spur new advances in biotechnology include but are not limited to the following:
- Computational representations of physiological systems at appropriate scales: The virtual representation of real-world physiology is at the core of a human digital twin. The human body is a dynamic and complex system whose behaviors are extremely difficult to model and predict. Tools to adequately build computational representations are lacking. New mathematical, statistical and machine learning methods are needed to enable novel computationally efficient pathways for the integration of prior information into the systematic combination of physical data and their digital counterparts. These strategies may include hybrid modeling approaches – combining mechanistic models, machine learning, and data-driven models – and surrogate models – statistical data-fit models, reduced order models, and simplified models. Furthermore, these models must be capable of assimilating dynamic multi-modal data at different spatial and temporal scales, dynamically updating and adapting, coupling multiphysics systems, and operating with limited data or accounting for extrapolation. These requirements may necessitate new model management workflows including assessing model evolution and drift. Understanding the tradeoffs associated with model and computational choices will increase confidence in predictive insights and digital twin-informed decision making. Moreover, digital twins not only integrate data streams from their physical twin but also data and outcomes from similar physical counterparts. New mathematical, statistical and machine learning methods are needed that could enable novel computationally efficient pathways for integration of prior information into the systematic combination of physical data and their digital counterparts.
- Verification, Validation, and Uncertainty Quantification (VVUQ): Appropriate verification, validation, and uncertainty quantification (VVUQ) are essential to build confidence and trust in digital twins. The complexity of the digital twin ecosystem may require new and advanced strategies and workflows that consider VVUQ as a continuous process. New data collection technologies (quality, source, structure) may affect algorithm or solution verification. Further, the state of the physical twin will evolve over time; and new strategies are needed to ensure the virtual representation accurately reflects these changes (i.e., adaptive model validation). The current lack of evidence of digital twin predictive capabilities adversely impacts the use of digital twins in the healthcare domain. There is a critical need for understanding the confidence interval of digital twin outputs while accounting for various types of uncertainties including modeling uncertainties, measurement and data uncertainties, and process uncertainties. One benefit of digital twins is the ability to test what-if scenarios, such as the performance of a therapeutic, biologic, or diagnostic device. However, to harness this potential, the outputs from the digital twin should be representative of the physical twin’s response (i.e. commutable) even when based on unseen data or extrapolation. New approaches, including but not limited to tools for causal inference, covariate adjustment, extreme value analysis, and neural solvers of partial differential equations, are needed for assessment of the digital twin utility in a broad range of settings.
- Transferability, Generalizability and Robustness : Most digital twins are designed with a particular purpose in mind. To leverage these digital twins for new purposes or scenarios (i.e. testing novel medical technologies), new techniques and tools are required to quantify and improve the transferability of digital twin predictions. There is also a need for techniques to advance the generalizability of evaluation findings on synthetic data from digital twin models to findings on patient data, including performance on various population subgroups. Another fundamental question is associated with the analysis of the robustness of the digital twin models, so that the medical devices designed and evaluated using digital twins are ensured to exhibit the expected standards of safety and effectiveness.
- Ethics, Security, and Privacy : Ethics, security, and privacy are critical to the success of digital twin ecosystems; and include fidelity and reliability of the models, security and access to data, recognition that data and models built on that data may be biased, and ethical use of the data and model outputs. The current limited understanding of the sources and types of biases has led to considerable concern in the community that synthetic human models, including digital twins, may inadvertently propagate or even further exacerbate current inequalities in healthcare delivery. For example, bias may be introduced in data measurement technologies, data labeling, data sources (i.e. is the data representative of the population, have rare conditions been included, small data sets); as well as models, algorithms, and decision-making processes based on this data. Understanding, measuring, and minimizing potential latent biases require novel or advanced methods of statistical inference as current approaches are underdeveloped. Further, strategies to ensure protection of privacy of individual’s data used to develop the DT (at various scale), and equitable impacts and distribution of resources within the context of digital twins are needed. Development of such foundational approaches have potential to accelerate the widespread adoption of digital twins not only in biomedical sciences but also numerous application domains.
- Validation and Sharing Mechanisms : There is a critical need to design computational infrastructure, platforms, and best practices for in silico databanks for medical technology evaluation with pre-defined data sequestration provisions. The lack of methods and platforms with broad involvement of the interdisciplinary scientific community substantially impacts the development, validation, and adoption of digital twins in biomedicine. Furthermore, innovative tools are needed for management, maintenance, service, test data reuse, and auditing of banks of digital twins under privacy- and integrity-preserving federated settings. This in turn will allow for a synergistic acceleration of innovation in a wide range of medical technology areas. Finally, the widespread adoption of digital twins and in silico models for human health will only be realized by more collaborative solutions to sharing and validation of models with established protocols between different digital twin sources as opposed to the status of a disconnected, site-specific collection of digital twin data and human in silico models.
The above list of themes provides examples for possible research initiatives that may be supported by the FDT-BioTech solicitation. Proposals with complementary aims, not listed here, will also be considered. Furthermore, these research themes are clearly not mutually exclusive, and a given project may address multiple themes.
Ethical, Legal, and Social Implications (ELSI): It is essential to recognize ethical, legal, and social implications (ELSI) during the development of human digital twins and synthetic humans. A digital twin ecosystem that does not include ESLI at the start will build inequity into core design and implementation principles perpetuating disparities in health, infrastructure, and resources. All proposals must identify potential ELS implications of the proposed work and outline ways to mitigate negative implications.
This program encourages teams to consider the generalizability of their approaches to other systems, populations, or non-biomedical applications.
In addition to the examples described in this solicitation, the program welcomes submissions of proposals that contain outcomes (methods and models) with a clear dissemination plan, made available as practical, open-source tools that industry can utilize in support of the development of new biomedical technologies. Such tools should be of production quality, shared with the research community, and facilitate interoperability with other tools and data infrastructure. Proposals targeting such tools should include project personnel with cyberinfrastructure development expertise. Furthermore, these tools can include innovative science-based approaches including methodologies and datasets and are meant to support innovators in early stages of development as they prepare toward securing premarket authorization by the FDA. Examples of regulatory science tools published by the Office of Science and Engineering Laboratories, Center for Devices and Radiological Health (OSEL/CDRH/FDA) are available in FDA’s catalog of regulatory science tools ( Catalog of Regulatory Science Tools to Help Assess New Medical Devices | FDA ). Furthermore, FDA will offer opportunities to the FDT-BioTech PIs to discuss and submit their software code implementing the developed methods and algorithms and receive feedback on its relevance to current and emerging regulatory science challenges within the precompetitive space.
This program welcomes the submission of proposals that include the participation of the full spectrum of diverse talent in STEM, e.g., as PI, co-PI, senior/key personnel, postdoctoral scholars, graduate or undergraduate students or trainees. This includes historically under-represented or underserved populations. It also includes diverse institutions including Minority-Serving Institutions (MSIs), Primarily Undergraduate Institutions (PUIs), and two-year colleges, as well as major research institutions. Proposals from EPSCoR (Established Program to Stimulate Competitive Research) jurisdictions are especially encouraged.
Successful projects are anticipated to be collaborative in nature and have at least two senior/key personnels, with participation from both the mathematical sciences and at least one of the domain knowledge disciplines such as the biomedical sciences or computer science with cyberinfrastructure development expertise. In particular, interdisciplinary teams with PI and co-PI from the mathematical sciences, biomedical sciences and computer science with cyberinfrastructure development expertise are encouraged. These requirements will help to ensure that the proposals are truly integrative.
III. Award Information
Anticipated Funding Amount: $4,000,000 to $5,000,000
Estimated program budget, number of awards and average award size/duration are subject to the availability of funds.
IV. Eligibility Information
Additional Eligibility Info:
A minimum of two collaborating Senior/Key Personnel, with participation from both the mathematical sciences and at least one of the domain knowledge disciplines such as the biomedical sciences or computer science with cyberinfrastructure development expertise is required. Interdisciplinary teams with PI and co-PI from the mathematical sciences, the biomedical sciences, and computer science with cyberinfrastructure development expertise are encouraged.
V. Proposal Preparation And Submission Instructions
Full Proposal Preparation Instructions : Proposers may opt to submit proposals in response to this Program Solicitation via Research.gov or Grants.gov.
- Full Proposals submitted via Research.gov: Proposals submitted in response to this program solicitation should be prepared and submitted in accordance with the general guidelines contained in the NSF Proposal and Award Policies and Procedures Guide (PAPPG). The complete text of the PAPPG is available electronically on the NSF website at: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg . Paper copies of the PAPPG may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-8134 or by e-mail from [email protected] . The Prepare New Proposal setup will prompt you for the program solicitation number.
- Full proposals submitted via Grants.gov: Proposals submitted in response to this program solicitation via Grants.gov should be prepared and submitted in accordance with the NSF Grants.gov Application Guide: A Guide for the Preparation and Submission of NSF Applications via Grants.gov . The complete text of the NSF Grants.gov Application Guide is available on the Grants.gov website and on the NSF website at: ( https://www.nsf.gov/publications/pub_summ.jsp?ods_key=grantsgovguide ). To obtain copies of the Application Guide and Application Forms Package, click on the Apply tab on the Grants.gov site, then click on the Apply Step 1: Download a Grant Application Package and Application Instructions link and enter the funding opportunity number, (the program solicitation number without the NSF prefix) and press the Download Package button. Paper copies of the Grants.gov Application Guide also may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-8134 or by e-mail from [email protected] .
In determining which method to utilize in the electronic preparation and submission of the proposal, please note the following:
Collaborative Proposals. All collaborative proposals submitted as separate submissions from multiple organizations must be submitted via Research.gov. PAPPG Chapter II.E.3 provides additional information on collaborative proposals.
See PAPPG Chapter II.D.2 for guidance on the required sections of a full research proposal submitted to NSF. Please note that the proposal preparation instructions provided in this program solicitation may deviate from the PAPPG instructions.
The following instructions supplement or deviate from the PAPPG:
Proposal Title : To facilitate timely processing, proposal titles must begin with FDT-BioTech, followed by a colon and the title of the project (i.e. FDT-BioTech: Title). The title of collaborative proposals submitted as separate submissions from multiple organizations should begin with the designation "Collaborative Research: FDT-BioTech:" All proposals in a collaborative project should have the same title. Please note that if submitting via Research.gov, the system will automatically insert the prepended title “Collaborative Research” when the collaborative set of proposals is created.
Project Description : In addition to the requirements specified in the PAPPG, the Project Description should clearly:
- Demonstrate the potential benefits of the proposed work for regulatory sciences.
- Include a separate section with a heading Ethics, Legal, and Social Implications (ELSI) that clearly identifies potential Ethics, Legal, and Social Implications (ELSI) in the proposed work and consider ways to mitigate negative implications.
- Explain how the proposed research effectively integrates diverse fields (e.g. mathematics, statistics, computational sciences, biomedical sciences, computer science, cyberinfrastructure development and engineering) to advance the foundation of digital twins.
- Address how the multidisciplinary group of researchers is appropriate to the project, how the team members provide distinct, complementary expertise to the project, and why all fields of expertise are needed to complete the proposed work represented on the team.
Cost Sharing:
D. Research.gov/Grants.gov Requirements
For Proposals Submitted Via Research.gov:
To prepare and submit a proposal via Research.gov, see detailed technical instructions available at: https://www.research.gov/research-portal/appmanager/base/desktop?_nfpb=true&_pageLabel=research_node_display&_nodePath=/researchGov/Service/Desktop/ProposalPreparationandSubmission.html . For Research.gov user support, call the Research.gov Help Desk at 1-800-381-1532 or e-mail [email protected] . The Research.gov Help Desk answers general technical questions related to the use of the Research.gov system. Specific questions related to this program solicitation should be referred to the NSF program staff contact(s) listed in Section VIII of this funding opportunity.
For Proposals Submitted Via Grants.gov:
Before using Grants.gov for the first time, each organization must register to create an institutional profile. Once registered, the applicant's organization can then apply for any federal grant on the Grants.gov website. Comprehensive information about using Grants.gov is available on the Grants.gov Applicant Resources webpage: https://www.grants.gov/web/grants/applicants.html . In addition, the NSF Grants.gov Application Guide (see link in Section V.A) provides instructions regarding the technical preparation of proposals via Grants.gov. For Grants.gov user support, contact the Grants.gov Contact Center at 1-800-518-4726 or by email: [email protected] . The Grants.gov Contact Center answers general technical questions related to the use of Grants.gov. Specific questions related to this program solicitation should be referred to the NSF program staff contact(s) listed in Section VIII of this solicitation.
Submitting the Proposal: Once all documents have been completed, the Authorized Organizational Representative (AOR) must submit the application to Grants.gov and verify the desired funding opportunity and agency to which the application is submitted. The AOR must then sign and submit the application to Grants.gov. The completed application will be transferred to Research.gov for further processing.
The NSF Grants.gov Proposal Processing in Research.gov informational page provides submission guidance to applicants and links to helpful resources including the NSF Grants.gov Application Guide , Grants.gov Proposal Processing in Research.gov how-to guide , and Grants.gov Submitted Proposals Frequently Asked Questions . Grants.gov proposals must pass all NSF pre-check and post-check validations in order to be accepted by Research.gov at NSF.
When submitting via Grants.gov, NSF strongly recommends applicants initiate proposal submission at least five business days in advance of a deadline to allow adequate time to address NSF compliance errors and resubmissions by 5:00 p.m. submitting organization's local time on the deadline. Please note that some errors cannot be corrected in Grants.gov. Once a proposal passes pre-checks but fails any post-check, an applicant can only correct and submit the in-progress proposal in Research.gov.
Proposers that submitted via Research.gov may use Research.gov to verify the status of their submission to NSF. For proposers that submitted via Grants.gov, until an application has been received and validated by NSF, the Authorized Organizational Representative may check the status of an application on Grants.gov. After proposers have received an e-mail notification from NSF, Research.gov should be used to check the status of an application.
VI. NSF Proposal Processing And Review Procedures
Proposals received by NSF are assigned to the appropriate NSF program for acknowledgement and, if they meet NSF requirements, for review. All proposals are carefully reviewed by a scientist, engineer, or educator serving as an NSF Program Officer, and usually by three to ten other persons outside NSF either as ad hoc reviewers, panelists, or both, who are experts in the particular fields represented by the proposal. These reviewers are selected by Program Officers charged with oversight of the review process. Proposers are invited to suggest names of persons they believe are especially well qualified to review the proposal and/or persons they would prefer not review the proposal. These suggestions may serve as one source in the reviewer selection process at the Program Officer's discretion. Submission of such names, however, is optional. Care is taken to ensure that reviewers have no conflicts of interest with the proposal. In addition, Program Officers may obtain comments from site visits before recommending final action on proposals. Senior NSF staff further review recommendations for awards. A flowchart that depicts the entire NSF proposal and award process (and associated timeline) is included in PAPPG Exhibit III-1.
A comprehensive description of the Foundation's merit review process is available on the NSF website at: https://www.nsf.gov/bfa/dias/policy/merit_review/ .
Proposers should also be aware of core strategies that are essential to the fulfillment of NSF's mission, as articulated in Leading the World in Discovery and Innovation, STEM Talent Development and the Delivery of Benefits from Research - NSF Strategic Plan for Fiscal Years (FY) 2022 - 2026 . These strategies are integrated in the program planning and implementation process, of which proposal review is one part. NSF's mission is particularly well-implemented through the integration of research and education and broadening participation in NSF programs, projects, and activities.
One of the strategic objectives in support of NSF's mission is to foster integration of research and education through the programs, projects, and activities it supports at academic and research institutions. These institutions must recruit, train, and prepare a diverse STEM workforce to advance the frontiers of science and participate in the U.S. technology-based economy. NSF's contribution to the national innovation ecosystem is to provide cutting-edge research under the guidance of the Nation's most creative scientists and engineers. NSF also supports development of a strong science, technology, engineering, and mathematics (STEM) workforce by investing in building the knowledge that informs improvements in STEM teaching and learning.
NSF's mission calls for the broadening of opportunities and expanding participation of groups, institutions, and geographic regions that are underrepresented in STEM disciplines, which is essential to the health and vitality of science and engineering. NSF is committed to this principle of diversity and deems it central to the programs, projects, and activities it considers and supports.
A. Merit Review Principles and Criteria
The National Science Foundation strives to invest in a robust and diverse portfolio of projects that creates new knowledge and enables breakthroughs in understanding across all areas of science and engineering research and education. To identify which projects to support, NSF relies on a merit review process that incorporates consideration of both the technical aspects of a proposed project and its potential to contribute more broadly to advancing NSF's mission "to promote the progress of science; to advance the national health, prosperity, and welfare; to secure the national defense; and for other purposes." NSF makes every effort to conduct a fair, competitive, transparent merit review process for the selection of projects.
1. Merit Review Principles
These principles are to be given due diligence by PIs and organizations when preparing proposals and managing projects, by reviewers when reading and evaluating proposals, and by NSF program staff when determining whether or not to recommend proposals for funding and while overseeing awards. Given that NSF is the primary federal agency charged with nurturing and supporting excellence in basic research and education, the following three principles apply:
- All NSF projects should be of the highest quality and have the potential to advance, if not transform, the frontiers of knowledge.
- NSF projects, in the aggregate, should contribute more broadly to achieving societal goals. These "Broader Impacts" may be accomplished through the research itself, through activities that are directly related to specific research projects, or through activities that are supported by, but are complementary to, the project. The project activities may be based on previously established and/or innovative methods and approaches, but in either case must be well justified.
- Meaningful assessment and evaluation of NSF funded projects should be based on appropriate metrics, keeping in mind the likely correlation between the effect of broader impacts and the resources provided to implement projects. If the size of the activity is limited, evaluation of that activity in isolation is not likely to be meaningful. Thus, assessing the effectiveness of these activities may best be done at a higher, more aggregated, level than the individual project.
With respect to the third principle, even if assessment of Broader Impacts outcomes for particular projects is done at an aggregated level, PIs are expected to be accountable for carrying out the activities described in the funded project. Thus, individual projects should include clearly stated goals, specific descriptions of the activities that the PI intends to do, and a plan in place to document the outputs of those activities.
These three merit review principles provide the basis for the merit review criteria, as well as a context within which the users of the criteria can better understand their intent.
2. Merit Review Criteria
All NSF proposals are evaluated through use of the two National Science Board approved merit review criteria. In some instances, however, NSF will employ additional criteria as required to highlight the specific objectives of certain programs and activities.
The two merit review criteria are listed below. Both criteria are to be given full consideration during the review and decision-making processes; each criterion is necessary but neither, by itself, is sufficient. Therefore, proposers must fully address both criteria. (PAPPG Chapter II.D.2.d(i). contains additional information for use by proposers in development of the Project Description section of the proposal). Reviewers are strongly encouraged to review the criteria, including PAPPG Chapter II.D.2.d(i), prior to the review of a proposal.
When evaluating NSF proposals, reviewers will be asked to consider what the proposers want to do, why they want to do it, how they plan to do it, how they will know if they succeed, and what benefits could accrue if the project is successful. These issues apply both to the technical aspects of the proposal and the way in which the project may make broader contributions. To that end, reviewers will be asked to evaluate all proposals against two criteria:
- Intellectual Merit: The Intellectual Merit criterion encompasses the potential to advance knowledge; and
- Broader Impacts: The Broader Impacts criterion encompasses the potential to benefit society and contribute to the achievement of specific, desired societal outcomes.
The following elements should be considered in the review for both criteria:
- Advance knowledge and understanding within its own field or across different fields (Intellectual Merit); and
- Benefit society or advance desired societal outcomes (Broader Impacts)?
- To what extent do the proposed activities suggest and explore creative, original, or potentially transformative concepts?
- Is the plan for carrying out the proposed activities well-reasoned, well-organized, and based on a sound rationale? Does the plan incorporate a mechanism to assess success?
- How well qualified is the individual, team, or organization to conduct the proposed activities?
- Are there adequate resources available to the PI (either at the home organization or through collaborations) to carry out the proposed activities?
Broader impacts may be accomplished through the research itself, through the activities that are directly related to specific research projects, or through activities that are supported by, but are complementary to, the project. NSF values the advancement of scientific knowledge and activities that contribute to achievement of societally relevant outcomes. Such outcomes include, but are not limited to: full participation of women, persons with disabilities, and other underrepresented groups in science, technology, engineering, and mathematics (STEM); improved STEM education and educator development at any level; increased public scientific literacy and public engagement with science and technology; improved well-being of individuals in society; development of a diverse, globally competitive STEM workforce; increased partnerships between academia, industry, and others; improved national security; increased economic competitiveness of the United States; and enhanced infrastructure for research and education.
Proposers are reminded that reviewers will also be asked to review the Data Management and Sharing Plan and the Mentoring Plan, as appropriate.
Additional Solicitation Specific Review Criteria
Regulatory science tools component for medical device evaluation :
The work to be funded by this solicitation must demonstrate the potential benefits for regulatory sciences. Proposals shall include a detailed section of how the proposed computational methods and tools developed under the awards will impact the early de-risking of new technology and contribute to facilitating regulatory evaluations including, for example, frequent interaction with OSEL/CDRH/FDA during the post-award period.
B. Review and Selection Process
Proposals submitted in response to this program solicitation will be reviewed by Ad hoc Review and/or Panel Review.
NSF will coordinate and manage the review of proposals jointly with NIH and FDA. The representatives from NIH and FDA may serve as panel observers. Relevant information about proposals and reviews of proposals will be shared with NIH and FDA as appropriate.
Reviewers will be asked to evaluate proposals using two National Science Board approved merit review criteria and, if applicable, additional program specific criteria. A summary rating and accompanying narrative will generally be completed and submitted by each reviewer and/or panel. The Program Officer assigned to manage the proposal's review will consider the advice of reviewers and will formulate a recommendation.
After scientific, technical and programmatic review and consideration of appropriate factors, the NSF Program Officer recommends to the cognizant Division Director whether the proposal should be declined or recommended for award. NSF strives to be able to tell proposers whether their proposals have been declined or recommended for funding within six months. Large or particularly complex proposals or proposals from new recipients may require additional review and processing time. The time interval begins on the deadline or target date, or receipt date, whichever is later. The interval ends when the Division Director acts upon the Program Officer's recommendation.
After programmatic approval has been obtained, the proposals recommended for funding will be forwarded to the Division of Grants and Agreements or the Division of Acquisition and Cooperative Support for review of business, financial, and policy implications. After an administrative review has occurred, Grants and Agreements Officers perform the processing and issuance of a grant or other agreement. Proposers are cautioned that only a Grants and Agreements Officer may make commitments, obligations or awards on behalf of NSF or authorize the expenditure of funds. No commitment on the part of NSF should be inferred from technical or budgetary discussions with a NSF Program Officer. A Principal Investigator or organization that makes financial or personnel commitments in the absence of a grant or cooperative agreement signed by the NSF Grants and Agreements Officer does so at their own risk.
Once an award or declination decision has been made, Principal Investigators are provided feedback about their proposals. In all cases, reviews are treated as confidential documents. Verbatim copies of reviews, excluding the names of the reviewers or any reviewer-identifying information, are sent to the Principal Investigator/Project Director by the Program Officer. In addition, the proposer will receive an explanation of the decision to award or decline funding.
VII. Award Administration Information
A. notification of the award.
Notification of the award is made to the submitting organization by an NSF Grants and Agreements Officer. Organizations whose proposals are declined will be advised as promptly as possible by the cognizant NSF Program administering the program. Verbatim copies of reviews, not including the identity of the reviewer, will be provided automatically to the Principal Investigator. (See Section VI.B. for additional information on the review process.)
B. Award Conditions
An NSF award consists of: (1) the award notice, which includes any special provisions applicable to the award and any numbered amendments thereto; (2) the budget, which indicates the amounts, by categories of expense, on which NSF has based its support (or otherwise communicates any specific approvals or disapprovals of proposed expenditures); (3) the proposal referenced in the award notice; (4) the applicable award conditions, such as Grant General Conditions (GC-1)*; or Research Terms and Conditions* and (5) any announcement or other NSF issuance that may be incorporated by reference in the award notice. Cooperative agreements also are administered in accordance with NSF Cooperative Agreement Financial and Administrative Terms and Conditions (CA-FATC) and the applicable Programmatic Terms and Conditions. NSF awards are electronically signed by an NSF Grants and Agreements Officer and transmitted electronically to the organization via e-mail.
*These documents may be accessed electronically on NSF's Website at https://www.nsf.gov/awards/managing/award_conditions.jsp?org=NSF . Paper copies may be obtained from the NSF Publications Clearinghouse, telephone (703) 292-8134 or by e-mail from [email protected] .
More comprehensive information on NSF Award Conditions and other important information on the administration of NSF awards is contained in the NSF Proposal & Award Policies & Procedures Guide (PAPPG) Chapter VII, available electronically on the NSF Website at https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
Administrative and National Policy Requirements
Build America, Buy America
As expressed in Executive Order 14005, Ensuring the Future is Made in All of America by All of America’s Workers (86 FR 7475), it is the policy of the executive branch to use terms and conditions of Federal financial assistance awards to maximize, consistent with law, the use of goods, products, and materials produced in, and services offered in, the United States.
Consistent with the requirements of the Build America, Buy America Act (Pub. L. 117-58, Division G, Title IX, Subtitle A, November 15, 2021), no funding made available through this funding opportunity may be obligated for an award unless all iron, steel, manufactured products, and construction materials used in the project are produced in the United States. For additional information, visit NSF’s Build America, Buy America webpage.
C. Reporting Requirements
For all multi-year grants (including both standard and continuing grants), the Principal Investigator must submit an annual project report to the cognizant Program Officer no later than 90 days prior to the end of the current budget period. (Some programs or awards require submission of more frequent project reports). No later than 120 days following expiration of a grant, the PI also is required to submit a final annual project report, and a project outcomes report for the general public.
Failure to provide the required annual or final annual project reports, or the project outcomes report, will delay NSF review and processing of any future funding increments as well as any pending proposals for all identified PIs and co-PIs on a given award. PIs should examine the formats of the required reports in advance to assure availability of required data.
PIs are required to use NSF's electronic project-reporting system, available through Research.gov, for preparation and submission of annual and final annual project reports. Such reports provide information on accomplishments, project participants (individual and organizational), publications, and other specific products and impacts of the project. Submission of the report via Research.gov constitutes certification by the PI that the contents of the report are accurate and complete. The project outcomes report also must be prepared and submitted using Research.gov. This report serves as a brief summary, prepared specifically for the public, of the nature and outcomes of the project. This report will be posted on the NSF website exactly as it is submitted by the PI.
More comprehensive information on NSF Reporting Requirements and other important information on the administration of NSF awards is contained in the NSF Proposal & Award Policies & Procedures Guide (PAPPG) Chapter VII, available electronically on the NSF Website at https://www.nsf.gov/publications/pub_summ.jsp?ods_key=pappg .
VIII. Agency Contacts
Please note that the program contact information is current at the time of publishing. See program website for any updates to the points of contact.
General inquiries regarding this program should be made to:
For questions related to the use of NSF systems contact:
- NSF Help Desk: 1-800-381-1532
- Research.gov Help Desk e-mail: [email protected]
For questions relating to Grants.gov contact:
Grants.gov Contact Center: If the Authorized Organizational Representatives (AOR) has not received a confirmation message from Grants.gov within 48 hours of submission of application, please contact via telephone: 1-800-518-4726; e-mail: [email protected] .
IX. Other Information
The NSF website provides the most comprehensive source of information on NSF Directorates (including contact information), programs and funding opportunities. Use of this website by potential proposers is strongly encouraged. In addition, "NSF Update" is an information-delivery system designed to keep potential proposers and other interested parties apprised of new NSF funding opportunities and publications, important changes in proposal and award policies and procedures, and upcoming NSF Grants Conferences . Subscribers are informed through e-mail or the user's Web browser each time new publications are issued that match their identified interests. "NSF Update" also is available on NSF's website .
Grants.gov provides an additional electronic capability to search for Federal government-wide grant opportunities. NSF funding opportunities may be accessed via this mechanism. Further information on Grants.gov may be obtained at https://www.grants.gov .
About The National Science Foundation
The National Science Foundation (NSF) is an independent Federal agency created by the National Science Foundation Act of 1950, as amended (42 USC 1861-75). The Act states the purpose of the NSF is "to promote the progress of science; [and] to advance the national health, prosperity, and welfare by supporting research and education in all fields of science and engineering."
NSF funds research and education in most fields of science and engineering. It does this through grants and cooperative agreements to more than 2,000 colleges, universities, K-12 school systems, businesses, informal science organizations and other research organizations throughout the US. The Foundation accounts for about one-fourth of Federal support to academic institutions for basic research.
NSF receives approximately 55,000 proposals each year for research, education and training projects, of which approximately 11,000 are funded. In addition, the Foundation receives several thousand applications for graduate and postdoctoral fellowships. The agency operates no laboratories itself but does support National Research Centers, user facilities, certain oceanographic vessels and Arctic and Antarctic research stations. The Foundation also supports cooperative research between universities and industry, US participation in international scientific and engineering efforts, and educational activities at every academic level.
Facilitation Awards for Scientists and Engineers with Disabilities (FASED) provide funding for special assistance or equipment to enable persons with disabilities to work on NSF-supported projects. See the NSF Proposal & Award Policies & Procedures Guide Chapter II.F.7 for instructions regarding preparation of these types of proposals.
The National Science Foundation has Telephonic Device for the Deaf (TDD) and Federal Information Relay Service (FIRS) capabilities that enable individuals with hearing impairments to communicate with the Foundation about NSF programs, employment or general information. TDD may be accessed at (703) 292-5090 and (800) 281-8749, FIRS at (800) 877-8339.
The National Science Foundation Information Center may be reached at (703) 292-5111.
Privacy Act And Public Burden Statements
The information requested on proposal forms and project reports is solicited under the authority of the National Science Foundation Act of 1950, as amended. The information on proposal forms will be used in connection with the selection of qualified proposals; and project reports submitted by proposers will be used for program evaluation and reporting within the Executive Branch and to Congress. The information requested may be disclosed to qualified reviewers and staff assistants as part of the proposal review process; to proposer institutions/grantees to provide or obtain data regarding the proposal review process, award decisions, or the administration of awards; to government contractors, experts, volunteers and researchers and educators as necessary to complete assigned work; to other government agencies or other entities needing information regarding proposers or nominees as part of a joint application review process, or in order to coordinate programs or policy; and to another Federal agency, court, or party in a court or Federal administrative proceeding if the government is a party. Information about Principal Investigators may be added to the Reviewer file and used to select potential candidates to serve as peer reviewers or advisory committee members. See System of Record Notices , NSF-50 , "Principal Investigator/Proposal File and Associated Records," and NSF-51 , "Reviewer/Proposal File and Associated Records.” Submission of the information is voluntary. Failure to provide full and complete information, however, may reduce the possibility of receiving an award.
An agency may not conduct or sponsor, and a person is not required to respond to, an information collection unless it displays a valid Office of Management and Budget (OMB) control number. The OMB control number for this collection is 3145-0058. Public reporting burden for this collection of information is estimated to average 120 hours per response, including the time for reviewing instructions. Send comments regarding the burden estimate and any other aspect of this collection of information, including suggestions for reducing this burden, to:
Suzanne H. Plimpton Reports Clearance Officer Policy Office, Division of Institution and Award Support Office of Budget, Finance, and Award Management National Science Foundation Alexandria, VA 22314

Pew Biomedical Scholars
The Pew Charitable Trusts has invited Duke to nominate a candidate for the 2025 Pew Biomedical Scholars Program. Please read the details below carefully. The Pew Scholars Program supports assistant professors of outstanding promise in science relevant to the advancement of human health.
There is an internal competition to select Duke's one permitted nominee, as detailed below.
- Duke Internal Deadline: April 14, 2024
- Sponsor Nomination Deadline: May 15, 2024
- Sponsor's Application Deadline : September 5, 2024
Candidates must meet all of the following eligibility requirements:
- Hold a doctorate in biomedical sciences, medicine, or a related field, including engineering or the physical sciences.
- As of Sept. 5, 2024, run an independent lab and hold a full-time appointment at the rank of assistant professor. (Appointments such as research assistant professor, adjunct assistant professor, assistant professor research track, visiting professor, or instructor are not eligible).
- Please note that the eligibility criteria above have been temporarily expanded to account for COVID-related lab shutdowns. Please direct any questions to the program office at [email protected] .
- May apply to the program a maximum of two times. All applicants must be nominated by their institution and must complete the 2025 online application.
- If applicants have appointments at more than one eligible nominating institution or affiliate, they may not reapply in a subsequent year from a different nominating entity.
- May not be nominated for the Pew Scholars Program and the Pew-Stewart Scholars Program for Cancer Research in the same year.
Based on their performance during their education and training, candidates should demonstrate outstanding promise as contributors in science relevant to human health. This program does not fund clinical trials research. Strong proposals will incorporate particularly creative and pioneering approaches to basic, translational, and applied biomedical research. Candidates whose work is based on biomedical principles but who bring in concepts and theories from more diverse fields are encouraged to apply.
Ideas with the potential to produce an unusually high impact are encouraged. Selection of the successful candidates will be based on a detailed description of the work that the applicant proposes to undertake, evaluations of the candidate’s performance, and notable past accomplishments, including honors, awards, and publications. In evaluating the candidates, the National Advisory Committee gives considerable weight to both the project proposal and the researcher, including evidence that the candidate is a successful independent investigator and has the skill set needed to carry out their high-impact proposal.
An award of $75,000 per year for four years will be provided to the sponsoring institution for use by the scholar, subject to annual review of the scholar’s progress. Grant agreements will be issued in August of the award year. The awarded funds may be used at the discretion of the Pew scholar, for personnel, equipment, supplies, or travel directly related to the scholar's research and as to best advance his or her research and career.
- The amount of the award that may be used for the principal investigator’s salary is limited to $12,500 per year (including benefits) or $50,000 over the duration of the grant. There are no limits on student or postdoctoral salaries.
- Not more than 8 percent ($24,000) of the total award value may be allocated for facilities and administration (F&A) charges or indirect costs (IDCs).
- Should the funds not be immediately required, they may be accumulated and carried over through the grant period and, with written approval of the program office, the grant may receive a no-cost extension for one additional year (without additional funds).
- Subawards are allowed.
2021: Zhao Zhang, Pharmacology and Cancer Biology
2015: Gianna Hammer, Immunology
2014: Lindsey Glickfield, Neurobiology
2014: Jeremy Kay, Duke Eye Center
2012 Donald Fox Department of Pharmacology & Cancer Biology Department of Cell Biology Research Field: Tissue Repair and Genome Stability David R. Sherwood, Ph.D. (2007) Kenneth Poss (2006) Raphael Valdivia (2003) Mark W. Grinstaff (1999) Thomas L. Ortel (1995) Jonathan S. Stamler (1993) Philip M. Rosoff (1987) Jack D. Keene (1985)
Owing to the sponsor's restriction on the number of applications that may be submitted from Duke, anyone wishing to pursue nomination should submit the following materials as one PDF: * Project summary - 2 pages * CV - 2 pages: PLEASE INCLUDE YOUR APPOINTMENT DATE on your CV, as well as, honors, awards, and publications
Please submit internal materials through My Research Proposal. (Code: ILN) https://www.grantinterface.com/sl/LFk4zS
Instructions for creating an account (if needed) and submitting your materials: https://ctsi.duke.edu/about-myresearchproposal

Search form
- Academic Advising & Support
- Academic Planning & Resources
- Environmental Changemakers Certificate
- Academic Opportunities & Research
- Financial Support
- Online Forms & Resources
- Calendar & Deadlines
- Career Resources
- Clubs and Organizations
- Frequently Asked Questions
- Advisors & Coordinators
- Graduate Studies Committee
- Curricular Practical Training
- New Graduate Students
- Ph.D. Milestones
- Policies & Procedures
- Teaching Assistant FAQs
- Career Development Resources
- Engineering Student Study/Meeting Space
- Free Software
- Message From Chair
- Facts & Figures
- Undergraduate
- Faculty & Staff
- Academic Employment
- Dept Events
- Discovery News
- Student Testimonials
- Give to BME
- Seminar Series
- E-Newsletter
- Message from Chair
- CEE Affiliates
- Give to CEE
- International Center Form
- MSE Business & Forms
- MSE 298 Seminars
- MSE Diversity & Inclusion
- Support MSE@UCI
- MAE Seminars
- Corporate Affiliates
- Interdisciplinary Graduate Programs
- All faculty & staff
- Dean's Office
- Development and External Relations
- Student Affairs
- Engineering Research Management
- UC Irvine Directory
- Proposal Text and Resources
- Early Career Opportunities
- Purchasing Requests
- Reimbursements
- Purchasing & Reimbursement Mission Statement
- Business Meetings/ Entertainment Guidelines
- Service Agreements
- Travel Guidelines
- Travel Tips
- UC Policies & Procedures
- Dean's Executive Office
- Chief Administrative Officers
- Personnel Unit
- Finance Unit
- Purchasing Unit
- Computing Unit
- Facilities Unit
- Curriculum, Analytical Studies, & Accreditation (CASA)
- Communications Office
- Development and External Relations Office
- Outreach Unit
- Office of Information Technology
- Faculty Websites
- Computer Labs & Laptops
- Engineering Facilities Request Form
- Safety Procedures
- Campus Evacuation Zones
- Environmental Health & Safety
- UCI Police Department
- Helpful Links
- At Your Service
- Zot! Portal
- FAQs for Engineering Instructors
- Spring Awards
- Process Improvement
- Alumni Spotlight
- Hall of Fame
- #ANTEATERENGINEER
- Ways to Give
- UCI Engineering Alumni Society
- UC Irvine Alumni Association
- Dean's Message
- Strategic Plan
- Facts and Figures
- Henry Samueli
- School Leadership
- Engineering Leadership Council
- Accreditation
- Orange County
- Got Questions?
- Enrollment and Degrees Awarded
- How to Apply
- Prospective Students
- Newly Admitted
- Majors and Minors Offered
- Programs and Concentrations
- Accelerated Status Program
- International Fellowships
- Meet Us on the Road
- Anteater Voices
- Ph.D. and Master's Inquiry Form
- Message from the Associate Dean
- UCI Engineering-LANL Graduate Fellowships
- Research Thrusts
- Research by Department
- Research Centers, Institutes and Facilities
- Undergraduate Research
- Interdisciplinary Science and Engineering Building (ISEB)
- Contracts & Grants / ERM
- Research and Proposal Development
- Annual Membership Levels
- Connect with Students
- Prototyping Services
- Sponsored Research
- External Relations Office
- Community College
- International
- IDEA - Inclusion, Diversity, Equity, Access
- Stacey Nicholas Office of Access and Inclusion
- Inclusion in Engineering Education
- Samueli Shoutouts
- Media Watch
- Dean's Report
- Social Media
- Style Guide
MRSEC/MSE Special Seminar: Low-Dimensional Quantum Materials Design Through Atomically Precise Film Synthesis

Abstract: Low-dimensional quantum materials are at the forefront of scientific exploration due to their extraordinary electronic and magnetic characteristics, distinct from those observed in bulk systems. Among the various synthesis techniques, molecular-beam epitaxy (MBE) emerges as a leading technique for developing these innovative materials. This thin-film deposition approach enables precise engineering of quantum materials, unlocking fascinating properties such as superconductivity, quantum magnetism and topological states. In this talk, I will discuss how to utilize MBE, along with characterization tools such as angle-resolved photoemission spectroscopy and resonant x-ray scattering, to unveil a completely new antiferromagnetic metal phase in transition metal oxide nickelate. Additionally, I will cover the exploration of the superconductivity in nickelates and iron-based chalcogenide. I will also share my vision and effort in pushing the boundaries of the MBE technique to further explore novel low-dimensional quantum materials.
Bio: Qi Song is currently a postdoctoral scholar in the Department of Material Science and Engineering at Cornell University. She earned her doctorate in physics from Fudan University in China, and then spent two years as a postdoctoral scholar at Harvard University before joining Cornell. Her research is centered around the exploration of low-dimensional quantum materials through molecular beam epitaxy, assisted by spectroscopy characterization.
Related Content
Upcoming events.
- 4 Apr MSE 298 Seminar: Materials Design For Next-Generation Li- and Na-ion Batteries
- 5 Apr EECS Seminar: Decentralized and Dispersed Computing for the Internet of Things
- 5 Apr CBE Distinguished Lecture: Synthetic Hydrogels as Extracellular Matrix Mimics - Engineering Materials for 4D Cell Culture
- 8 Apr MRSEC/MSE Special Seminar: Designing New Electronic Phases Intransition-Metal Oxide Thin Films
- 9 Apr MRSEC/MSE Special Seminar: Low-Dimensional Quantum Materials Design Through Atomically Precise Film Synthesis
News & Events

COMMENTS
All students must complete this written proposal and an oral presentation on their research within a maximum of one semester after passing the PhD Qualifying Examination or entering the PhD program, whichever is later. The format and length of the written portion of the DRP will follow the guidelines set forth by the National Institutes of ...
2.2 The Department of Biomedical Engineering. Within JHU, the BME PhD is hosted by the Department of Biomedical Engineering (BME), with Faculty both at the School of Medicine (SOM) and Whiting School of Engineering. The mission of BME is to advance human health by training biomedical engineers who will lead in Engineering the Future of Medicine ...
Research Proposal Template Biomedical Research: General Page 2 Methods . Human Subjects This section describes your proposed study population and, if the study is prospective, how you will identify and enroll participants and obtain their informed consent. The bulleted list gives examples of elements you may need to describe (the first three items
The PhD program in biomedical engineering is strongly interdisciplinary and designed to prepare students to apply engineering principles to problems in medicine and biology, to understand and model multiple attributes of living systems, and to use this knowledge to develop novel biomedical systems and devices.
Selection and investigation of a biomedical engineering research topic independently under the supervision of a faculty member. The student will carry out analytical and/or experimental work and prepare a project proposal including a literature review, and a written thesis. Each student must deliver a public lecture.
BMC Biomedical Engineering considers articles on all as-pects of biomedical engineering, including fundamental, translational and clinical research. It combines tools and methods from biology and medicine with mathematics, physical sciences and engineering towards the under-standing of human biology and disease and the improve-ment of human ...
Most good research proposals are usually between 2000 and 4000 words in length. A strong research proposal can and should make a positive first impression about your potential to become a good researcher. It should show those reading it that your ideas are focused, interesting and realistic. Although you should write the proposal yourself, it ...
DEPARTMENT OF MATERIALS SCIENCE AND ENGINEERING 201 DUPONT HALL. University of Delaware Newark, Delaware 19716 Ph: 302/831-2062 Fax: 302/831-4545. November 21, 2011. Dawn Elliott. Professor and Director Biomedical Engineering Program College of Engineering. University of Delaware.
biomedical engineering to produce high quality research 4. Disseminating individual work in written and oral formats to translate individual ... research proposal modelled on the NSERC CGS -M application format. Research Approach Presentations The second main activity in this course is a series of assignments that ends with a three-minute ...
Each PhD student enrolled in the Department of Biomedical Engineering (BME) PhD program is required to adhere to the following requirements and deadlines. Students requiring additional coursework will be notified of any required prerequisites as determined by the student's advisor and/or BME graduate program director.
Hyun Jung Kim, hk9746, Biomedical Engineering. Purpose. The purpose of the proposed research is to collect tissue, blood and fecal samples from patients undergoing standard of care for their gastrointestinal disease, including Inflammatory Bowel Disease (IBD), and Colorectal Cancer (CRC). Tissue and blood samples will be obtained during ...
Abstract. One of the major objectives of the TEMPUS IV CRH-BME Joint Project is to propose an updated vision of study programs and curricula in the field of biomedical engineering (BME) in Europe ...
Biomedical engineering is the application of engineering princip les and design concepts to medicine and biology for. healthcare purposes. This field s eeks to close the gap between engineering ...
Biomedical engineering: A brief survey into current challenges and a proposed research roadmap September 2012 International Journal of Healthcare Technology and Management 13(1/2/3):1 - 31
The research proposal gave me a better app ecia-14 58 21 7 tion about how new technology is created. The research proposal was one of the most cha!-21 43 29 7 lenging assignme)1tS I have had at OU. Writing a research proposal in this course helped with another course/courses taken afterwards 36 64 0 0 and/or a research project.
PDF. Three-Dimensional Plasma Cell Survival Microniche in Multiple Myeloma, Katrina A. Harmon. PDF. Adipose Tissue Engineering: A Therapeutic Strategy for the Treatment of Obesity and Glucose Intolerance, Michael A. Hendley. PDF. Role of P-Glycoprotein in Alzheimer's Disease for Enhanced Brain Elimination of Amyloid-β, Hope Holt. PDF
Biology and Medicine: Biomedical Engineering (sponsor) Genre: theses Subject: Biomedical engineering Collection: Biology and Medicine Biomedical Engineering Theses and Dissertations. ... All disciplines of biomedical research utilize in vitro models to answer a range of biological questions. Traditional in vitro models, two-dimensional (2D ...
Well organized site on proposal writing. Includes an overview, inquiry and cover letters, standard components of a proposal, a sample proposal, advice from funders, and more. An excellent section on researching funding opportunities is included also.
Biomedical engineering is a branch of engineering that applies principles and design concepts of engineering to healthcare. Biomedical engineers deal with medical devices such as imaging equipment ...
Each full proposal must have co-principal investigators: BME co-PIs must be permanent, full-time, tenure-track faculty within the professorial ranks (assistant/associate/full) of the Case Western Reserve University Department of Biomedical Engineering. Secondary faculty of the BME department may also be a co-PI.
Biomedical Engineering Research Proposals Samples For Students. 2 samples of this type. If you're seeking an applicable way to streamline writing a Research Proposal about Biomedical Engineering, WowEssays.com paper writing service just might be able to help you out. For starters, you should browse our huge collection of free samples that cover ...
An interactive workshop on 'The Critical Steps for Successful Research: The Research Proposal and Scientific Writing' was conducted in conjunction with the 64(th) Annual Conference of the Indian ...
With the recent advancements in biomedical engineering technologies, commercially available VLP-based vaccines are being extensively used to combat infectious diseases, whereas many more are in different stages of development in clinical studies. Because of their desired characteristics in terms of efficacy, safety, and diversity, VLP-based ...
The Foundations for Digital Twins as Catalyzers of Biomedical Technological Innovation (FDT-BioTech) program supports inherently interdisciplinary research projects that underpin the mathematical and engineering foundations behind the development and use of digital twins and synthetic data in biomedical and healthcare applications, with a ...
This program does not fund clinical trials research. Strong proposals will incorporate particularly creative and pioneering approaches to basic, translational, and applied biomedical research. Candidates whose work is based on biomedical principles but who bring in concepts and theories from more diverse fields are encouraged to apply.
Abstract: Low-dimensional quantum materials are at the forefront of scientific exploration due to their extraordinary electronic and magnetic characteristics, distinct from those observed in bulk systems.Among the various synthesis techniques, molecular-beam epitaxy (MBE) emerges as a leading technique for developing these innovative materials.