Literature Review vs Systematic Review
- Literature Review vs. Systematic Review
- Primary vs. Secondary Sources
- Databases and Articles
- Specific Journal or Article

Subject Guide

Definitions
It’s common to confuse systematic and literature reviews because both are used to provide a summary of the existent literature or research on a specific topic. Regardless of this commonality, both types of review vary significantly. The following table provides a detailed explanation as well as the differences between systematic and literature reviews.
Kysh, Lynn (2013): Difference between a systematic review and a literature review. [figshare]. Available at: http://dx.doi.org/10.6084/m9.figshare.766364
- << Previous: Home
- Next: Primary vs. Secondary Sources >>
- Last Updated: Dec 15, 2023 10:19 AM
- URL: https://libguides.sjsu.edu/LitRevVSSysRev
Penn State University Libraries
- Home-Articles and Databases
- Asking the clinical question
- PICO & Finding Evidence
- Evaluating the Evidence
- Systematic Review vs. Literature Review
- Ethical & Legal Issues for Nurses
- Nursing Library Instruction Course
- Data Management Toolkit This link opens in a new window
- Useful Nursing Resources
- Writing Resources
- LionSearch and Finding Articles
- The Catalog and Finding Books
Know the Difference! Systematic Review vs. Literature Review
It is common to confuse systematic and literature reviews as both are used to provide a summary of the existent literature or research on a specific topic. Even with this common ground, both types vary significantly. Please review the following chart (and its corresponding poster linked below) for the detailed explanation of each as well as the differences between each type of review.
- What's in a name? The difference between a Systematic Review and a Literature Review, and why it matters by Lynn Kysh, MLIS, University of Southern California - Norris Medical Library
- << Previous: Evaluating the Evidence
- Next: Ethical & Legal Issues for Nurses >>
- Last Updated: Mar 1, 2024 11:54 AM
- URL: https://guides.libraries.psu.edu/nursing

- Research Process
Systematic Literature Review or Literature Review?
- 3 minute read
- 43.7K views
Table of Contents
As a researcher, you may be required to conduct a literature review. But what kind of review do you need to complete? Is it a systematic literature review or a standard literature review? In this article, we’ll outline the purpose of a systematic literature review, the difference between literature review and systematic review, and other important aspects of systematic literature reviews.
What is a Systematic Literature Review?
The purpose of systematic literature reviews is simple. Essentially, it is to provide a high-level of a particular research question. This question, in and of itself, is highly focused to match the review of the literature related to the topic at hand. For example, a focused question related to medical or clinical outcomes.
The components of a systematic literature review are quite different from the standard literature review research theses that most of us are used to (more on this below). And because of the specificity of the research question, typically a systematic literature review involves more than one primary author. There’s more work related to a systematic literature review, so it makes sense to divide the work among two or three (or even more) researchers.
Your systematic literature review will follow very clear and defined protocols that are decided on prior to any review. This involves extensive planning, and a deliberately designed search strategy that is in tune with the specific research question. Every aspect of a systematic literature review, including the research protocols, which databases are used, and dates of each search, must be transparent so that other researchers can be assured that the systematic literature review is comprehensive and focused.
Most systematic literature reviews originated in the world of medicine science. Now, they also include any evidence-based research questions. In addition to the focus and transparency of these types of reviews, additional aspects of a quality systematic literature review includes:
- Clear and concise review and summary
- Comprehensive coverage of the topic
- Accessibility and equality of the research reviewed
Systematic Review vs Literature Review
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper. That is, it includes an introduction, description of the methods used, a discussion and conclusion, as well as a reference list or bibliography.
A systematic review, however, includes entirely different components that reflect the specificity of its research question, and the requirement for transparency and inclusion. For instance, the systematic review will include:
- Eligibility criteria for included research
- A description of the systematic research search strategy
- An assessment of the validity of reviewed research
- Interpretations of the results of research included in the review
As you can see, contrary to the general overview or summary of a topic, the systematic literature review includes much more detail and work to compile than a standard literature review. Indeed, it can take years to conduct and write a systematic literature review. But the information that practitioners and other researchers can glean from a systematic literature review is, by its very nature, exceptionally valuable.
This is not to diminish the value of the standard literature review. The importance of literature reviews in research writing is discussed in this article . It’s just that the two types of research reviews answer different questions, and, therefore, have different purposes and roles in the world of research and evidence-based writing.
Systematic Literature Review vs Meta Analysis
It would be understandable to think that a systematic literature review is similar to a meta analysis. But, whereas a systematic review can include several research studies to answer a specific question, typically a meta analysis includes a comparison of different studies to suss out any inconsistencies or discrepancies. For more about this topic, check out Systematic Review VS Meta-Analysis article.
Language Editing Plus
With Elsevier’s Language Editing Plus services , you can relax with our complete language review of your systematic literature review or literature review, or any other type of manuscript or scientific presentation. Our editors are PhD or PhD candidates, who are native-English speakers. Language Editing Plus includes checking the logic and flow of your manuscript, reference checks, formatting in accordance to your chosen journal and even a custom cover letter. Our most comprehensive editing package, Language Editing Plus also includes any English-editing needs for up to 180 days.

- Publication Recognition
How to Make a PowerPoint Presentation of Your Research Paper

- Manuscript Preparation
What is and How to Write a Good Hypothesis in Research?
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
Covidence website will be inaccessible as we upgrading our platform on Monday 23rd August at 10am AEST, / 2am CEST/1am BST (Sunday, 15th August 8pm EDT/5pm PDT)
The difference between a systematic review and a literature review
- Best Practice
Home | Blog | Best Practice | The difference between a systematic review and a literature review
Covidence takes a look at the difference between the two
Most of us are familiar with the terms systematic review and literature review. Both review types synthesise evidence and provide summary information. So what are the differences? What does systematic mean? And which approach is best 🤔 ?
‘ Systematic ‘ describes the review’s methods. It means that they are transparent, reproducible and defined before the search gets underway. That’s important because it helps to minimise the bias that would result from cherry-picking studies in a non-systematic way.
This brings us to literature reviews. Literature reviews don’t usually apply the same rigour in their methods. That’s because, unlike systematic reviews, they don’t aim to produce an answer to a clinical question. Literature reviews can provide context or background information for a new piece of research. They can also stand alone as a general guide to what is already known about a particular topic.
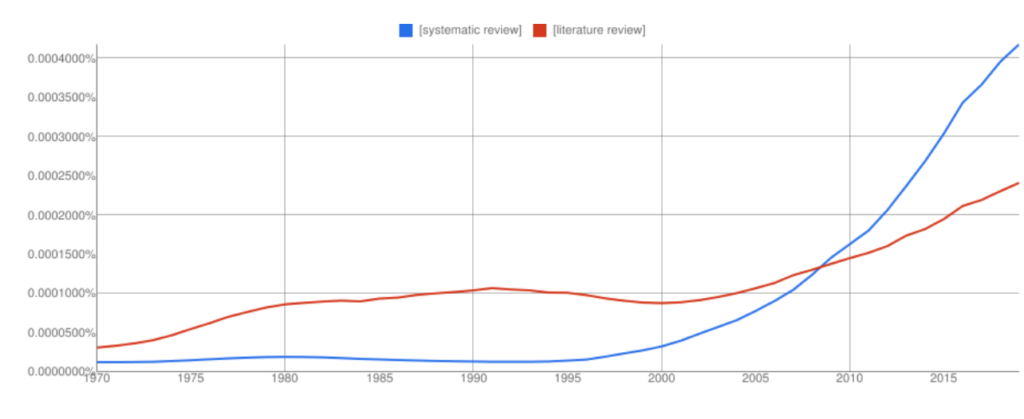
Interest in systematic reviews has grown in recent years and the frequency of ‘systematic reviews’ in Google books has overtaken ‘literature reviews’ (with all the usual Ngram Viewer warnings – it searches around 6% of all books, no journals).
Let’s take a look at the two review types in more detail to highlight some key similarities and differences 👀.
🙋🏾♂️ What is a systematic review?
Systematic reviews ask a specific question about the effectiveness of a treatment and answer it by summarising evidence that meets a set of pre-specified criteria.
The process starts with a research question and a protocol or research plan. A review team searches for studies to answer the question using a highly sensitive search strategy. The retrieved studies are then screened for eligibility using the inclusion and exclusion criteria (this is done by at least two people working independently). Next, the reviewers extract the relevant data and assess the quality of the included studies. Finally, the review team synthesises the extracted study data and presents the results. The process is shown in figure 2 .

The results of a systematic review can be presented in many ways and the choice will depend on factors such as the type of data. Some reviews use meta-analysis to produce a statistical summary of effect estimates. Other reviews use narrative synthesis to present a textual summary.
Covidence accelerates the screening, data extraction, and quality assessment stages of your systematic review. It provides simple workflows and easy collaboration with colleagues around the world.
When is it appropriate to do a systematic review?
If you have a clinical question about the effectiveness of a particular treatment or treatments, you could answer it by conducting a systematic review. Systematic reviews in clinical medicine often follow the PICO framework, which stands for:
👦 Population (or patients)
💊 Intervention
💊 Comparison
Here’s a typical example of a systematic review title that uses the PICO framework: Alarms [intervention] versus drug treatments [comparison] for the prevention of nocturnal enuresis [outcome] in children [population]
Key attributes
- Systematic reviews follow prespecified methods
- The methods are explicit and replicable
- The review team assesses the quality of the evidence and attempts to minimise bias
- Results and conclusions are based on the evidence
🙋🏻♀️ What is a literature review?
Literature reviews provide an overview of what is known about a particular topic. They evaluate the material, rather than simply restating it, but the methods used to do this are not usually prespecified and they are not described in detail in the review. The search might be comprehensive but it does not aim to be exhaustive. Literature reviews are also referred to as narrative reviews.
Literature reviews use a topical approach and often take the form of a discussion. Precision and replicability are not the focus, rather the author seeks to demonstrate their understanding and perhaps also present their work in the context of what has come before. Often, this sort of synthesis does not attempt to control for the author’s own bias. The results or conclusion of a literature review is likely to be presented using words rather than statistical methods.
When is it appropriate to do a literature review?
We’ve all written some form of literature review: they are a central part of academic research ✍🏾. Literature reviews often form the introduction to a piece of writing, to provide the context. They can also be used to identify gaps in the literature and the need to fill them with new research 📚.
- Literature reviews take a thematic approach
- They do not specify inclusion or exclusion criteria
- They do not answer a clinical question
- The conclusions might be influenced by the author’s own views
🙋🏽 Ok, but what is a systematic literature review?
A quick internet search retrieves a cool 200 million hits for ‘systematic literature review’. What strange hybrid is this 🤯🤯 ?
Systematic review methodology has its roots in evidence-based medicine but it quickly gained traction in other areas – the social sciences for example – where researchers recognise the value of being methodical and minimising bias. Systematic review methods are increasingly applied to the more traditional types of review, including literature reviews, hence the proliferation of terms like ‘systematic literature review’ and many more.
Beware of the labels 🚨. The terminology used to describe review types can vary by discipline and changes over time. To really understand how any review was done you will need to examine the methods critically and make your own assessment of the quality and reliability of each synthesis 🤓.
Review methods are evolving constantly as researchers find new ways to meet the challenge of synthesising the evidence. Systematic review methods have influenced many other review types, including the traditional literature review.
Covidence is a web-based tool that saves you time at the screening, selection, data extraction and quality assessment stages of your systematic review. It supports easy collaboration across teams and provides a clear overview of task status.
Get a glimpse inside Covidence and how it works

Laura Mellor. Portsmouth, UK
Perhaps you'd also like....

Data Extraction Tip 5: Communicate Regularly
The Covidence Global Scholarship recipients are putting evidence-based research into practice. We caught up with some of the winners to discover the impact of their work and find out more about their experiences.

Data Extraction Tip 4: Extract the Right Amount of Data

Data Extraction Tip 3: Pilot the Template
Better systematic review management, head office, working for an institution or organisation.
Find out why over 350 of the world’s leading institutions are seeing a surge in publications since using Covidence!
Request a consultation with one of our team members and start empowering your researchers:
By using our site you consent to our use of cookies to measure and improve our site’s performance. Please see our Privacy Policy for more information.
- Locations and Hours
- UCLA Library
- Research Guides
- Biomedical Library Guides
Systematic Reviews
- Types of Literature Reviews
What Makes a Systematic Review Different from Other Types of Reviews?
- Planning Your Systematic Review
- Database Searching
- Creating the Search
- Search Filters and Hedges
- Grey Literature
- Managing and Appraising Results
- Further Resources
Reproduced from Grant, M. J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26: 91–108. doi:10.1111/j.1471-1842.2009.00848.x
- << Previous: Home
- Next: Planning Your Systematic Review >>
- Last Updated: Apr 17, 2024 2:02 PM
- URL: https://guides.library.ucla.edu/systematicreviews
How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses
Affiliations.
- 1 Behavioural Science Centre, Stirling Management School, University of Stirling, Stirling FK9 4LA, United Kingdom; email: [email protected].
- 2 Department of Psychological and Behavioural Science, London School of Economics and Political Science, London WC2A 2AE, United Kingdom.
- 3 Department of Statistics, Northwestern University, Evanston, Illinois 60208, USA; email: [email protected].
- PMID: 30089228
- DOI: 10.1146/annurev-psych-010418-102803
Systematic reviews are characterized by a methodical and replicable methodology and presentation. They involve a comprehensive search to locate all relevant published and unpublished work on a subject; a systematic integration of search results; and a critique of the extent, nature, and quality of evidence in relation to a particular research question. The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information. We outline core standards and principles and describe commonly encountered problems. Although this guide targets psychological scientists, its high level of abstraction makes it potentially relevant to any subject area or discipline. We argue that systematic reviews are a key methodology for clarifying whether and how research findings replicate and for explaining possible inconsistencies, and we call for researchers to conduct systematic reviews to help elucidate whether there is a replication crisis.
Keywords: evidence; guide; meta-analysis; meta-synthesis; narrative; systematic review; theory.
- Guidelines as Topic
- Meta-Analysis as Topic*
- Publication Bias
- Review Literature as Topic
- Systematic Reviews as Topic*
University Libraries University of Nevada, Reno
- Skill Guides
- Subject Guides
Systematic, Scoping, and Other Literature Reviews: Overview
- Project Planning
What Is a Systematic Review?
Regular literature reviews are simply summaries of the literature on a particular topic. A systematic review, however, is a comprehensive literature review conducted to answer a specific research question. Authors of a systematic review aim to find, code, appraise, and synthesize all of the previous research on their question in an unbiased and well-documented manner. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) outline the minimum amount of information that needs to be reported at the conclusion of a systematic review project.
Other types of what are known as "evidence syntheses," such as scoping, rapid, and integrative reviews, have varying methodologies. While systematic reviews originated with and continue to be a popular publication type in medicine and other health sciences fields, more and more researchers in other disciplines are choosing to conduct evidence syntheses.
This guide will walk you through the major steps of a systematic review and point you to key resources including Covidence, a systematic review project management tool. For help with systematic reviews and other major literature review projects, please send us an email at [email protected] .
Getting Help with Reviews
Organization such as the Institute of Medicine recommend that you consult a librarian when conducting a systematic review. Librarians at the University of Nevada, Reno can help you:
- Understand best practices for conducting systematic reviews and other evidence syntheses in your discipline
- Choose and formulate a research question
- Decide which review type (e.g., systematic, scoping, rapid, etc.) is the best fit for your project
- Determine what to include and where to register a systematic review protocol
- Select search terms and develop a search strategy
- Identify databases and platforms to search
- Find the full text of articles and other sources
- Become familiar with free citation management (e.g., EndNote, Zotero)
- Get access to you and help using Covidence, a systematic review project management tool
Doing a Systematic Review
- Plan - This is the project planning stage. You and your team will need to develop a good research question, determine the type of review you will conduct (systematic, scoping, rapid, etc.), and establish the inclusion and exclusion criteria (e.g., you're only going to look at studies that use a certain methodology). All of this information needs to be included in your protocol. You'll also need to ensure that the project is viable - has someone already done a systematic review on this topic? Do some searches and check the various protocol registries to find out.
- Identify - Next, a comprehensive search of the literature is undertaken to ensure all studies that meet the predetermined criteria are identified. Each research question is different, so the number and types of databases you'll search - as well as other online publication venues - will vary. Some standards and guidelines specify that certain databases (e.g., MEDLINE, EMBASE) should be searched regardless. Your subject librarian can help you select appropriate databases to search and develop search strings for each of those databases.
- Evaluate - In this step, retrieved articles are screened and sorted using the predetermined inclusion and exclusion criteria. The risk of bias for each included study is also assessed around this time. It's best if you import search results into a citation management tool (see below) to clean up the citations and remove any duplicates. You can then use a tool like Rayyan (see below) to screen the results. You should begin by screening titles and abstracts only, and then you'll examine the full text of any remaining articles. Each study should be reviewed by a minimum of two people on the project team.
- Collect - Each included study is coded and the quantitative or qualitative data contained in these studies is then synthesized. You'll have to either find or develop a coding strategy or form that meets your needs.
- Explain - The synthesized results are articulated and contextualized. What do the results mean? How have they answered your research question?
- Summarize - The final report provides a complete description of the methods and results in a clear, transparent fashion.
Adapted from
Types of reviews, systematic review.
These types of studies employ a systematic method to analyze and synthesize the results of numerous studies. "Systematic" in this case means following a strict set of steps - as outlined by entities like PRISMA and the Institute of Medicine - so as to make the review more reproducible and less biased. Consistent, thorough documentation is also key. Reviews of this type are not meant to be conducted by an individual but rather a (small) team of researchers. Systematic reviews are widely used in the health sciences, often to find a generalized conclusion from multiple evidence-based studies.
Meta-Analysis
A systematic method that uses statistics to analyze the data from numerous studies. The researchers combine the data from studies with similar data types and analyze them as a single, expanded dataset. Meta-analyses are a type of systematic review.
Scoping Review
A scoping review employs the systematic review methodology to explore a broader topic or question rather than a specific and answerable one, as is generally the case with a systematic review. Authors of these types of reviews seek to collect and categorize the existing literature so as to identify any gaps.
Rapid Review
Rapid reviews are systematic reviews conducted under a time constraint. Researchers make use of workarounds to complete the review quickly (e.g., only looking at English-language publications), which can lead to a less thorough and more biased review.
Narrative Review
A traditional literature review that summarizes and synthesizes the findings of numerous original research articles. The purpose and scope of narrative literature reviews vary widely and do not follow a set protocol. Most literature reviews are narrative reviews.
Umbrella Review
Umbrella reviews are, essentially, systematic reviews of systematic reviews. These compile evidence from multiple review studies into one usable document.
Grant, Maria J., and Andrew Booth. “A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies.” Health Information & Libraries Journal , vol. 26, no. 2, 2009, pp. 91-108. doi: 10.1111/j.1471-1842.2009.00848.x .
- Next: Project Planning >>
Literature Review Research
Literature review vs. systematic review.
- Literature Review Process
- Finding Literature Reviews
- Helpful Tips and Resources
- Citing Sources This link opens in a new window
Resources for Systematic Reviews
- NIH Systematic Review Protocols and Protocol Registries Systematic review services and information from the National Institutes of Health.
- Purdue University Systematic Reviews LibGuide Purdue University has created this helpful online research guide on systematic reviews. Most content is available publicly but please note that some links are accessible only to Purdue students.
It is common to confuse literature and systematic reviews because both are used to provide a summary of the existing literature or research on a specific topic. Despite this commonality, these two reviews vary significantly. The table below highlights the differences.
Kysh, Lynn (2013). Difference between a systematic review and a literature review. figshare. Poster. https://doi.org/10.6084/m9.figshare.766364.v1
- << Previous: Home
- Next: Literature Review Process >>
- Last Updated: Apr 18, 2024 6:36 PM
- URL: https://tcsedsystem.libguides.com/literature_review
- En español – ExME
- Em português – EME
Traditional reviews vs. systematic reviews
Posted on 3rd February 2016 by Weyinmi Demeyin

Millions of articles are published yearly (1) , making it difficult for clinicians to keep abreast of the literature. Reviews of literature are necessary in order to provide clinicians with accurate, up to date information to ensure appropriate management of their patients. Reviews usually involve summaries and synthesis of primary research findings on a particular topic of interest and can be grouped into 2 main categories; the ‘traditional’ review and the ‘systematic’ review with major differences between them.
Traditional reviews provide a broad overview of a research topic with no clear methodological approach (2) . Information is collected and interpreted unsystematically with subjective summaries of findings. Authors aim to describe and discuss the literature from a contextual or theoretical point of view. Although the reviews may be conducted by topic experts, due to preconceived ideas or conclusions, they could be subject to bias.
Systematic reviews are overviews of the literature undertaken by identifying, critically appraising and synthesising results of primary research studies using an explicit, methodological approach(3). They aim to summarise the best available evidence on a particular research topic.
The main differences between traditional reviews and systematic reviews are summarised below in terms of the following characteristics: Authors, Study protocol, Research question, Search strategy, Sources of literature, Selection criteria, Critical appraisal, Synthesis, Conclusions, Reproducibility, and Update.
Traditional reviews
- Authors: One or more authors usually experts in the topic of interest
- Study protocol: No study protocol
- Research question: Broad to specific question, hypothesis not stated
- Search strategy: No detailed search strategy, search is probably conducted using keywords
- Sources of literature: Not usually stated and non-exhaustive, usually well-known articles. Prone to publication bias
- Selection criteria: No specific selection criteria, usually subjective. Prone to selection bias
- Critical appraisal: Variable evaluation of study quality or method
- Synthesis: Often qualitative synthesis of evidence
- Conclusions: Sometimes evidence based but can be influenced by author’s personal belief
- Reproducibility: Findings cannot be reproduced independently as conclusions may be subjective
- Update: Cannot be continuously updated
Systematic reviews
- Authors: Two or more authors are involved in good quality systematic reviews, may comprise experts in the different stages of the review
- Study protocol: Written study protocol which includes details of the methods to be used
- Research question: Specific question which may have all or some of PICO components (Population, Intervention, Comparator, and Outcome). Hypothesis is stated
- Search strategy: Detailed and comprehensive search strategy is developed
- Sources of literature: List of databases, websites and other sources of included studies are listed. Both published and unpublished literature are considered
- Selection criteria: Specific inclusion and exclusion criteria
- Critical appraisal: Rigorous appraisal of study quality
- Synthesis: Narrative, quantitative or qualitative synthesis
- Conclusions: Conclusions drawn are evidence based
- Reproducibility: Accurate documentation of method means results can be reproduced
- Update: Systematic reviews can be periodically updated to include new evidence
Decisions and health policies about patient care should be evidence based in order to provide the best treatment for patients. Systematic reviews provide a means of systematically identifying and synthesising the evidence, making it easier for policy makers and practitioners to assess such relevant information and hopefully improve patient outcomes.
- Fletcher RH, Fletcher SW. Evidence-Based Approach to the Medical Literature. Journal of General Internal Medicine. 1997; 12(Suppl 2):S5-S14. doi:10.1046/j.1525-1497.12.s2.1.x. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1497222/
- Rother ET. Systematic literature review X narrative review. Acta paul. enferm. [Internet]. 2007 June [cited 2015 Dec 25]; 20(2): v-vi. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-21002007000200001&lng=en. http://dx.doi.org/10.1590/S0103-21002007000200001
- Khan KS, Ter Riet G, Glanville J, Sowden AJ, Kleijnen J. Undertaking systematic reviews of research on effectiveness: CRD’s guidance for carrying out or commissioning reviews. NHS Centre for Reviews and Dissemination; 2001.
Weyinmi Demeyin
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Traditional reviews vs. systematic reviews
THE INFORMATION IS VERY MUCH VALUABLE, A LOT IS INDEED EXPECTED IN ORDER TO MASTER SYSTEMATIC REVIEW
Thank you very much for the information here. My question is : Is it possible for me to do a systematic review which is not directed toward patients but just a specific population? To be specific can I do a systematic review on the mental health needs of students?
Hi Rosemary, I wonder whether it would be useful for you to look at Module 1 of the Cochrane Interactive Learning modules. This is a free module, open to everyone (you will just need to register for a Cochrane account if you don’t already have one). This guides you through conducting a systematic review, with a section specifically around defining your research question, which I feel will help you in understanding your question further. Head to this link for more details: https://training.cochrane.org/interactivelearning
I wonder if you have had a search on the Cochrane Library as yet, to see what Cochrane systematic reviews already exist? There is one review, titled “Psychological interventions to foster resilience in healthcare students” which may be of interest: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013684/full You can run searches on the library by the population and intervention you are interested in.
I hope these help you start in your investigations. Best wishes. Emma.
La revisión sistemática vale si hay solo un autor?
HI Alex, so sorry for the delay in replying to you. Yes, that is a very good point. I have copied a paragraph from the Cochrane Handbook, here, which does say that for a Cochrane Review, you should have more than one author.
“Cochrane Reviews should be undertaken by more than one person. In putting together a team, authors should consider the need for clinical and methodological expertise for the review, as well as the perspectives of stakeholders. Cochrane author teams are encouraged to seek and incorporate the views of users, including consumers, clinicians and those from varying regions and settings to develop protocols and reviews. Author teams for reviews relevant to particular settings (e.g. neglected tropical diseases) should involve contributors experienced in those settings”.
Thank you for the discussion point, much appreciated.
Hello, I’d like to ask you a question: what’s the difference between systematic review and systematized review? In addition, if the screening process of the review was made by only one author, is still a systematic or is a systematized review? Thanks
Hi. This article from Grant & Booth is a really good one to look at explaining different types of reviews: https://onlinelibrary.wiley.com/doi/10.1111/j.1471-1842.2009.00848.x It includes Systematic Reviews and Systematized Reviews. In answer to your second question, have a look at this Chapter from the Cochrane handbook. It covers the question about ‘Who should do a systematic review’. https://training.cochrane.org/handbook/current/chapter-01
A really relevant part of this chapter is this: “Systematic reviews should be undertaken by a team. Indeed, Cochrane will not publish a review that is proposed to be undertaken by a single person. Working as a team not only spreads the effort, but ensures that tasks such as the selection of studies for eligibility, data extraction and rating the certainty of the evidence will be performed by at least two people independently, minimizing the likelihood of errors.”
I hope this helps with the question. Best wishes. Emma.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

What do trialists do about participants who are ‘lost to follow-up’?
Participants in clinical trials may exit the study prior to having their results collated; in this case, what do we do with their results?

Family Therapy approaches for Anorexia Nervosa
Is Family Therapy effective in the treatment of Anorexia Nervosa? Emily summarises a recent Cochrane Review in this blog and examines the evidence.

Antihypertensive drugs for primary prevention – at what blood pressure do we start treatment?
In this blog, Giorgio Karam examines the evidence on antihypertensive drugs for primary prevention – when do we start treatment?
- University Libraries
- Research Guides
- Reviewing Research: Literature Reviews, Scoping Reviews, Systematic Reviews
- Differentiating the Three Review Types
Reviewing Research: Literature Reviews, Scoping Reviews, Systematic Reviews: Differentiating the Three Review Types
- Framework, Protocol, and Writing Steps
- Working with Keywords/Subject Headings
- Citing Research
The Differences in the Review Types
Grant, M.J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. H ealth Information & Libraries Journal , 26: 91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x The objective of this study is to provide descriptive insight into the most common types of reviews, with illustrative examples from health and health information domains.
- What Type of Review is Right for you (Cornell University)
Literature Reviews
Literature Review: it is a product and a process.
As a product , it is a carefully written examination, interpretation, evaluation, and synthesis of the published literature related to your topic. It focuses on what is known about your topic and what methodologies, models, theories, and concepts have been applied to it by others.
The process is what is involved in conducting a review of the literature.
- It is ongoing
- It is iterative (repetitive)
- It involves searching for and finding relevant literature.
- It includes keeping track of your references and preparing and formatting them for the bibliography of your thesis
- Literature Reviews (University of North Carolina at Chapel Hill) This handout will explain what literature reviews are and offer insights into the form and construction of literature reviews in the humanities, social sciences, and sciences.
Scoping Reviews
Scoping reviews are a " preliminary assessment of potential size and scope of available research literature . Aims to identify nature and extent of research evidence (usually including ongoing research)." Grant and Booth (2009).
Scoping reviews are not mapping reviews: Scoping reviews are more topic based and mapping reviews are more question based.
- examining emerging evidence when specific questions are unclear - clarify definitions and conceptual boundaries
- identify and map the available evidence
- a scoping review is done prior to a systematic review
- to summarize and disseminate research findings in the research literature
- identify gaps with the intention of resolution by future publications
- Scoping review timeframe and limitations (Touro College of Pharmacy
Systematic Reviews
Many evidence-based disciplines use ‘systematic reviews," this type of review is a specific methodology that aims to comprehensively identify all relevant studies on a specific topic, and to select appropriate studies based on explicit criteria . ( https://cebma.org/faq/what-is-a-systematic-review/ )
- clearly defined search criteria
- an explicit reproducible methodology
- a systematic search of the literature with the defined criteria met
- assesses validity of the findings - no risk of bias
- a comprehensive report on the findings, apparent transparency in the results
- Better evidence for a better world Browsable collection of systematic reviews
- Systematic Reviews in the Health Sciences by Molly Maloney Last Updated Apr 23, 2024 465 views this year
- Next: Framework, Protocol, and Writing Steps >>
Systematic Reviews & Literature Reviews
Evidence synthesis: part 1.
This blog post is the first in a series exploring Evidence Synthesis . We’re going to start by looking at two types of evidence synthesis: literature reviews and systemic reviews . To help me with this topic I looked at a number of research guides from other institutions, e.g., Cornell University Libraries.
The Key Differences Between a Literature Review and a Systematic Review
Overall, while both literature reviews and systematic reviews involve reviewing existing research literature, systematic reviews adhere to more rigorous and transparent methods to minimize bias and provide robust evidence to inform decision-making in education and other fields. If you are interested in learning about other evidence synthesis this decision tree created by Cornell Libraries (Robinson, n.d.) is a nice visual introduction.
Along with exploring evidence synthesis I am also interested in generative A.I. I want to be transparent about how I used A.I. to create the table above. I fed this prompt into ChatGPT:
“ List the differences between a literature review and a systemic review for a graduate student of education “
I wanted to see what it would produce. I reformatted the list into a table so that it would be easier to compare and contrast these two reviews much like the one created by Cornell University Libraries (Kibbee, 2024). I think ChatGPT did a pretty good job. I did have to do quite a bit of editing, and make sure that what was created matched what I already knew. There are things ChatGPT left out, for example time frames, and how many people are needed for a systemic review, but we can revisit that in a later post.
Kibbee, M. (2024, April 10). Libguides: A guide to evidence synthesis: Cornell University Library Evidence Synthesis Service. Cornell University Library. https://guides.library.cornell.edu/evidence-synthesis/intro
- Blog Archive 2009-2018
- Library Hours
- Library Salons
- Library Spaces
- Library Workshops
- Reference Desk Questions
Subscribe to the Bank Street Library Blog
- About WordPress
- Get Involved
- WordPress.org
- Documentation
- Learn WordPress
SRJ Student Resource
Literature review vs research articles: how are they different.
Unlock the secrets of academic writing with our guide to the key differences between a literature review and a research paper! 📚 Dive into the world of scholarly exploration as we break down how a literature review illuminates existing knowledge, identifies gaps, and sets the stage for further research. 🌐 Then, gear up for the adventure of crafting a research paper, where you become the explorer, presenting your unique insights and discoveries through independent research. 🚀 Join us on this academic journey and discover the art of synthesizing existing wisdom and creating your own scholarly masterpiece! 🎓✨
We are always accepting submissions! Submit work within SRJ’s scope anytime while you’re a graduate student.
Leave a Reply Cancel reply
The act of commenting on this site is an opt-in action and San Jose State University may not be held liable for the information provided by participating in the activity.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Your go-to destination for graduate student research support
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 April 2024
Surgery is associated with better long-term outcomes than pharmacological treatment for obesity: a systematic review and meta-analysis
- Leonardo Zumerkorn Pipek 1 ,
- Walter Augusto Fabio Moraes 2 ,
- Rodrigo Massato Nobetani 2 ,
- Vitor Santos Cortez 2 ,
- Alberto Santos Condi 2 ,
- João Victor Taba 2 ,
- Rafaela Farias Vidigal Nascimento 3 ,
- Milena Oliveira Suzuki 2 ,
- Fernanda Sayuri do Nascimento 2 ,
- Vitoria Carneiro de Mattos 2 ,
- Leandro Ryuchi Iuamoto 4 ,
- Wu Tu Hsing 4 ,
- Luiz Augusto Carneiro-D’Albuquerque 5 ,
- Alberto Meyer 5 &
- Wellington Andraus 5
Scientific Reports volume 14 , Article number: 9521 ( 2024 ) Cite this article
150 Accesses
1 Altmetric
Metrics details
- Endocrine system and metabolic diseases
- Gastrointestinal diseases
Obesity is a highly prevalent disease with numerous complications. Both intensive medical treatment with the use of pharmacological drugs and bariatric surgery are current options. The objective of this meta-analysis was to compare, in the long-term, intensive medical treatment and surgery based on twelve parameters related to weight loss, cardiovascular and endocrine changes. A review of the literature was conducted in accordance with the PRISMA guidelines (PROSPERO: CRD42021265637). The literature screening was done from inception to October 2023 through PubMed, EMBASE and Web of Science databases. We included randomized clinical trials that had separate groups for medical treatment and bariatric surgery as an intervention for obesity. The risk of bias was assessed through RoB2. A meta-analysis was performed with measures of heterogeneity and publication bias. Subgroup analysis for each surgery type was performed. Data is presented as forest-plots. Reviewers independently identified 6719 articles and 6 papers with a total 427 patients were included. All studies were randomized controlled trials, three had a follow up of 5 years and two had a follow up of 10 years. Both groups demonstrated statistical significance for most parameters studied. Surgery was superior for weight loss (− 22.05 kg [− 28.86; − 15.23), total cholesterol (− 0.88 [− 1.59; − 0.17]), triglycerides (− 0.70 [− 0.82; − 0.59]), HDL (0.12 [0.02; 0.23]), systolic pressure (− 4.49 [− 7.65; − 1.33]), diastolic pressure (− 2.28 [− 4.25; − 0.31]), Hb glycated (− 0.97 [− 1.31; − 0.62]), HOMA IR (− 2.94; [− 3.52; − 2.35]) and cardiovascular risk (− 0.08; [− 0.10; − 0.05]). Patient in the surgical treatment group had better long term outcomes when compared to the non-surgical group for most clinical parameters.
Similar content being viewed by others

Short- and long-term safety and efficacy of bariatric surgery for severely obese adolescents: a narrative review
Weight-loss thresholds after bariatric surgery and cardiovascular outcomes: more is better

Associations between diet composition, dietary pattern, and weight outcomes after bariatric surgery: a systematic review
Introduction.
Obesity has been a known condition for over 2000 years 1 but that has become much more prevalent in recent decades. Despite great efforts to prevent this disease, the prevalence in adults in the United States has increased in recent decades and reached 42.4% in 2018. The GBD Obesity Study 2 Collaborators 2015 showed that this increasing trend occurred in more than 70 countries and is highly expressive in adolescents.
The classification of obesity is defined by a body mass index (BMI) greater than 30 kg/m 2 . The psychological damage that many of these patients suffer in a society governed by aesthetic standards is just one of the most visible and immediate consequences of obesity. Mortality from cardiovascular causes and its relationship with BMI has already been widely studied 3 , showing that the risk increases progressively with the increase of the index. Similarly, obesity was associated with a higher incidence of cancer 4 , respiratory 5 and metabolic 6 diseases.
In this context, the importance of effective treatment of this condition is clear, reducing mortality and improving the quality of life of these patients. While some benefits are evident with a loss of just 5% 6 of their weight, many patients require a more expressive loss to reduce the risks associated with obesity.
There are several treatments available for weight loss. Lifestyle changes, low calorie diet and increasing physical activity are the mainstay treatment for all patients 7 , 8 . Specific weight loss diets and exercise programs have also been developed for this purpose, yielding varying results. Finally, pharmacological, and surgical treatment has gained more attention in recent years for selected patients in whom other measures were insufficient.
Several studies have demonstrated the effectiveness of bariatric surgery in the short and medium term for the treatment of obesity. More recent studies have also shown that new drugs developed for weight loss may be a viable option for the treatment of this disease 8 , 9 . Comparison of these new drugs with surgical treatment is scarce in the literature and aimed only at evaluating changes related to weight loss in a short period of time.
This systematic review evaluated the hypothesis whether surgical treatment is superior than non-surgical treatment for patients with obesity. We evaluated the long-term effect of these treatments on anthropometric measures (weight, waist circumference, BMI) and on obesity related pathologies (triglycerides, LDL, HDL, total cholesterol, cardiovascular risk, systolic and diastolic blood pressure, HOMA and glycated hemoglobin).
Materials and methods
This systematic review was carried out in accordance with the items of Preferred Reports for Systematic Reviews and Protocol Meta-Analysis (PRISMA-P) 10 and assessing the methodological quality of systematic reviews (AMSTAR-2) guidelines 11 . This study was registered by the Prospective Register of Systematic Reviews (PROSPERO, 258667) before the research was carried out.
Drafting of the research question was based on the PICO strategy 12 , considering: P (Patients with obesity with indication for bariatric surgery based on BMI); I (Bariatric Surgery); C (Pharmacological treatment); O (Long term morbidity/mortality—at least 5 years of follow up).
Eligibility criteria
Inclusion criteria.
Types of studies: Randomized clinical trials.
Types of participants: Patients eligible for bariatric surgery, according to the American Society for Metabolic and Bariatric Surgery (ASMBS).
Types of intervention: Bariatric surgery or medical treatment.

Exclusion criteria
Studies were excluded if they: (1) did not have one group for each type of intervention (surgery or pharmacologic treatment); (2) had a heterogeneous population; (3) did not use a standard assessment method for the entire duration of the study, or did not have pre-assessment; (4) were not related to the question in the review; (5) were in a language other than English, Portuguese or Spanish; (6) were incomplete, unpublished or inaccessible to the authors.
Types of variables/parameters analyzed
Data was collected and arranged in tables, including the authors name, date and country of publication, number of participants included in the final analysis, sex, age, and body mass index.
Literature revision
The survey was from inception to October 10, 2023, without language restrictions, in the Medline database (via PubMed), EMBASE and Web of Science.
Using the search tool, we selected MeSH terms from the most relevant publications to conduct a new search to obtain articles that could be included in this systematic review. In addition, a manual search of theses, meetings, references, study records and contact with experts in the field was carried out.
Search strategy
The same keywords were used in all databases, according to each database input format.
The search strategy was:
(Bariatric Surgery) AND ((nonsurgical) OR (Orlistat) OR (phentermine) OR (topiramate) OR (lorcaserin) OR (naltrexone) OR (bupropion) OR (liraglutide) OR (conservative) OR (conventional) OR (Anti-Obesity Agents) OR (Intensive medical)) AND (obesity) → 3024.
(Bariatric Surgery) AND ((nonsurgical) OR (conservative) OR (Anti-Obesity Agents) OR (Intensive medical)) AND (obesity) → 4732.
Web of Science:
(Bariatric Surgery) AND ((nonsurgical) OR (conservative) OR (Anti-Obesity Agents) OR (Intensive medical)) AND (obesity) → 1772.
Data extraction
The data for each study was extracted independently by two authors. Disagreements were resolved by consensus. If no consensus was reached, a third author was consulted. Data extraction was carried out using the Rayyan tool— https://rayyan.qcri.org/ 13 .
All studies were analyzed by their titles and abstracts, according to inclusion and exclusion criteria. If the eligibility criteria was met, the full text would be extracted. All studies eligible for qualitative analysis are described in the “Results” section.
Missing data was clarified by contacting the authors directly.
Data validation
The risk of bias for intervention-type studies was analyzed using the guidelines of the Cochrane Back Review Group (CBRG) 14 .
Statistical analysis
As several studies of sufficient quality were available, a meta-analysis was carried out with measures of heterogeneity and publication bias. The data was presented through forest-plots, according to their statistical relevance.
Characteristics of study participants are presented as means, minimum and maximum values for quantitative variables, and as frequencies and percentages for qualitative variables. The prevalence values and 95% confidence intervals was calculated using the Wilson method To assess the global heterogeneity between the studies, Cochran's Q test was calculated, as well as the I2 (percentage of variation). The results of the studies' association measures and their respective 95% confidence intervals are presented in forest-plots.
Statistical analysis were performed using the Stata/MP 14.0 software for Windows.
Study selection
The electronic search found 9528 results for the keywords used. After removing 2809 duplicates and screening through abstract, we considered 55 potentially eligible studies for full-text analysis. Of these, 49 did not respect the exclusion criteria. Only 6 studies were considered eligible for qualitative analysis and 6 articles were eligible for meta-analysis [Fig. 1 ].
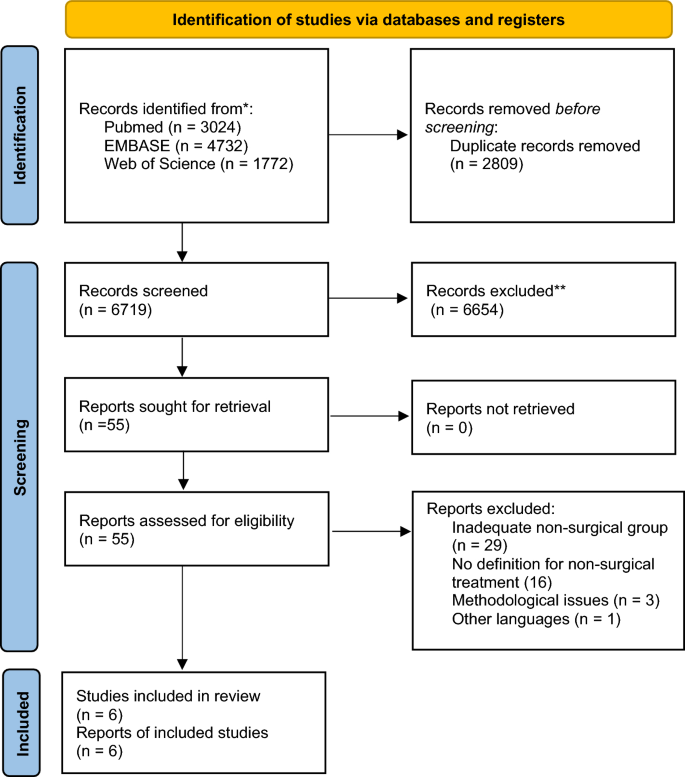
PRISMA 2020 flow diagram for new systematic reviews.
Many studies were excluded due to lack of description for the intervention in the non-surgical group.
Study characteristics
The following articles were included in the systematic review and meta-analysis 15 , 16 , 17 , 18 , 19 , 20 . In total, there were 427 participants. All studies were RCT. Four had a follow up of five years 15 , 16 , 19 and two had a follow up of 10 years 17 , 18 . Of the six eligible studies, two were undertaken in the United States of America 15 , 16 , two in Italy 17 , 19 , one in Australia 18 , and one in Singapore 20 . Study characteristics and detailed demographics can be found in Tables 1 and 2 . All studies included a group treated exclusively with intensive medical treatment (IMT). The definition of IMT differed between them but were considered if the patients had frequent follow up visits and were instructed on health habits including exercise and diet, with or without the use of pharmacological treatment.
There were four modalities of surgery used for weight loss: Roux-en-Y Gastric Bypass (RYGB) 15 , 17 , 18 , 19 , 20 ; Biliopancreatic diversion (BPD) 17 , 19 ; Laparoscopic Sleeve Gastrectomy (LSG) 15 , 16 ; Laparoscopic Adjustable Gastric Band (LAGB) 18 . The subgroup analysis for outcomes separated studies in RYGB, LSG and other types of surgery. The non-surgical treatment for obesity included one or the combination of the following medications: Orlistat, Phentermine, Naltrexone, Bupropion, Liraglutide, Lorcaserin, Sibutramine.
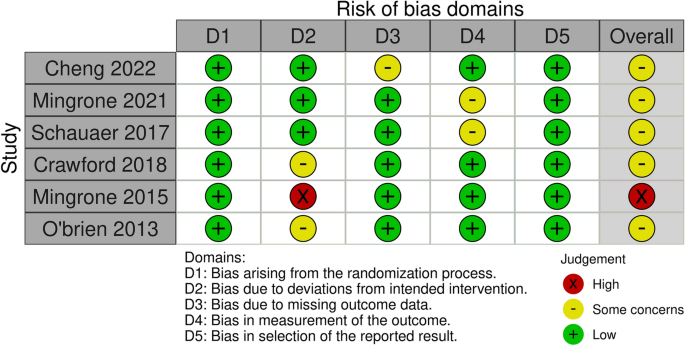
Risk of bias
After reading the articles included in the systematic review, the following elements were analyzed to determine the level of evidence: study design and selection, detection, loss, reporting and information bias. The summary of the risk of bias analysis for each of the included articles is presented in Fig. 2
Risk of bias analysis.
All studies had a low risk of bias for most criteria. In three of the studies, assessors were aware of the intervention received by study participants or the information was not available 16 , 17 , 20 . Three other studies 15 , 18 , 19 had bias regarding deviations from intended interventions due to the fact that an appropriate analysis to estimate the effects of assignment to intervention was not performed 15 ; patients assigned to the control group crossed over to the intervention group, and no measures were reportedly taken to balance that deviation 19 ; there was a significant loss of follow-up for all groups 20 .
All six studies had data on weight loss after treatment. Mean difference values and their respective 95% confidence intervals (95% CI) were calculated. In Fig. 3 A, the forest plot is shown. All publications found that surgical procedures were more efficient for long term weight loss. The global MD value was − 22.1 kg (95% CI [− 28.9; − 15.2). The measure of heterogeneity I2 (Higgins heterogeneity measure) was 77.8%, a value considered as high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).The subgroup analysis showed that there was not a significant difference between the types of surgery ( p = 0.30).
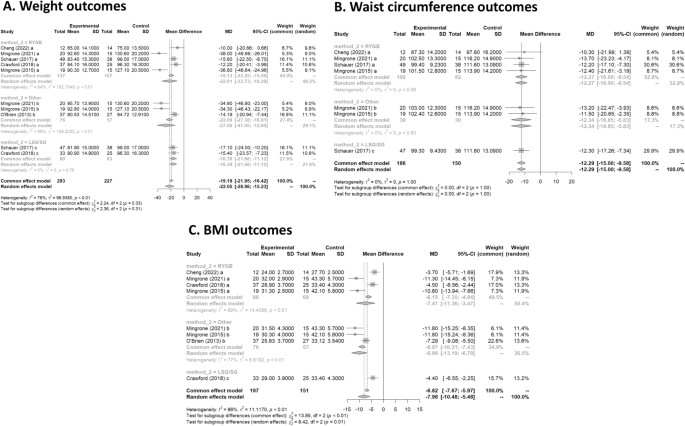
(A ) Weight outcomes; ( B ) Waist circumference outcomes; ( C ) BMI outcomes.
Waist circumference
Four studies had data on waist circumference 16 , 17 , 19 , 20 . In Fig. 3 B, the forest plot is shown. Patients treated with surgery had a mean difference of − 12.3 (95% CI [− 15.0; − 9.6]) compared to IMT. The measure of heterogeneity I2 (Higgins heterogeneity measure) was 0%, a value considered as low heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did not allow us to reject the null hypothesis of non-heterogeneity ( p = 0.99).
The subgroup analysis showed that there was not a significant difference between the types of surgery ( p = 0.99).
Five studies had data on BMI 16 , 17 , 18 , 19 , 20 . In Fig. 3 C, the forest plot is shown. Patients treated with surgery had a mean difference of − 8.0 (95% CI [− 10.5; − 5.5]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 84%, a value considered high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was a significant difference between the types of surgery ( p = 0.01). The group with LAGB and BPD surgery had the highest decrease in BMI, with a mean of − 10.0.
Triglycerides
Three studies had data on tryglycerides 17 , 19 , 20 . In Fig. 4 A, the forest plot is shown. Patients treated with surgery had a mean difference of − 0.7 (95% CI [− 0.8; − 0.6]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 50.4%, a value considered high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did not allow us to reject the null hypothesis of non-heterogeneity ( p = 0.08).
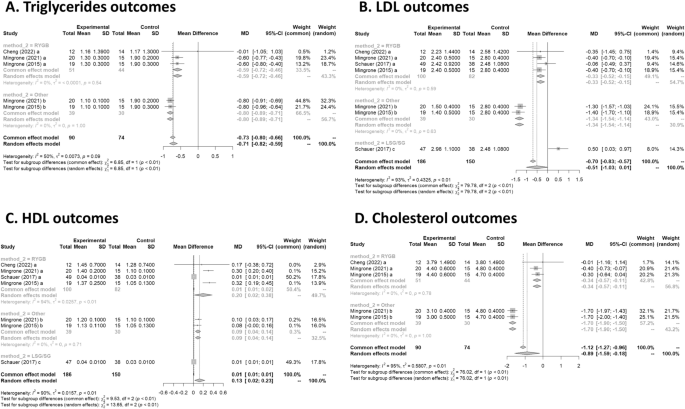
(A ) Triglycerides outcomes; ( B ) LDL outcomes; ( C ) HDL outcome; ( D ) Cholesterol outcomes.
The subgroup analysis showed that there was a significant difference between the types of surgery ( p = 0.01), with a worse outcome for RYGB.
Four studies had data on LDL 16 , 17 , 19 , 20 . In Fig. 4 B, the forest plot is shown. Patients treated with surgery had a mean difference of − 0.5 (95% CI [− 1.0; 0.0]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 92.7%, a value considered high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was a significant difference between the types of surgery ( p = 0.01). There was an increase of 0.5 in LDL for the LSG group. The group with LAGB and BPD surgery had the highest decrease in LDL, with a mean of − 1.3.
Four studies had data on HDL 16 , 17 , 19 , 20 . In Fig. 4 C, the forest plot is shown. Patients treated with surgery had a mean difference of 0.1 (95% CI [0.0; 0.2]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 90.5%, a value considered high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was a significant difference between the types of surgery ( p = 0.01). The group with RYGB surgery had the highest significant increase in HDL, with a mean of 0.2.
Cholesterol
Three studies had data on cholesterol 17 , 19 , 20 . In Fig. 4 D, the forest plot is shown. Patients treated with surgery had a mean difference of − 0.9 (95% CI [− 1.6; − 0.2]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 94.8%, a value considered as high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was a significant difference between the types of surgery ( p = 0.01). The group with LAGB and BPD surgery had the highest decrease in cholesterol, with a mean of − 1.7.
Cardiovascular risk
Two studies had data on cardiovascular risk 17 , 19 . In Fig. 5 A, the forest plot is shown. Patients treated with surgery had a mean difference of − 0.08 (95% CI [− 0.10; − 0.05]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 0%, a value considered as low heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did not allow us to reject the null hypothesis of non-heterogeneity ( p = 0.44).

(A ) Cardiovascular risk outcomes; ( B ) Systolic blood pressure outcomes; ( C ) Diastolic blood pressure outcomes; ( D ) HOMA outcomes; ( E ) Glycated Hemoglobin outcomes.
The subgroup analysis showed that there was no significant difference between the types of surgery ( p = 0.36).
Systolic blood pressure
Four studies had data on systolic blood pressure 16 , 17 , 19 , 20 . In Fig. 5 B, the forest plot is shown. Patients treated with surgery had a mean difference of − 4.49 (95% CI [− 7.65; − 1.33]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 71%, a value considered as high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was not a significant difference between the types of surgery ( p = 0.79).
Diastolic blood pressure
Four studies had data on diastolic blood pressure 16 , 17 , 19 , 20 . In Fig. 5 C, the forest plot is shown. Patients treated with surgery had a mean difference of − 2.28 (95% CI [− 4.25; − 0.31]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 60.5%, a value considered as high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was not a significant difference between the types of surgery ( p = 0.66).
Three studies had data on HOMA 15 , 17 , 19 . In Fig. 5 D, the forest plot is shown. Patients treated with surgery had a mean difference of − 2.94 (95% CI [− 3.52; − 2.35]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 14%, a value considered as low heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did not allow us to reject the null hypothesis of non-heterogeneity ( p = 0.32).
The subgroup analysis showed that there was no significant difference between the types of surgery ( p = 0.33).
Glycated Hemoglobin
Five studies had data on glycated haemoglobin 15 , 16 , 17 , 19 , 20 . In Fig. 5 E, the forest plot is shown. Patients treated with surgery had a mean difference of − 1.0(95% CI [− 1.3; − 0.6]) compared to IMT. The measure of heterogeneity I2 (Higgins’s heterogeneity measure) was 79.8%, a value considered as high heterogeneity. According to Cochran’s Q heterogeneity test, the sample evidence did allow us to reject the null hypothesis of non-heterogeneity ( p = 0.01).
The subgroup analysis showed that there was no significant difference between the types of surgery ( p = 0.98).
Obesity is defined as a BMI greater than or equal to 30 by the CDC and is currently among the most prevalent diseases in the world, in addition to being an important risk factor for many other diseases. It has high rates of morbidity and mortality 21 , 22 and, in this context, weight loss can bring countless positive impacts to the individual. Currently, there are several treatments for obesity, and we can divide them into non-surgical or surgical.
Non-surgical treatments include non-drug and drug treatments. Among the non-medicated, we can highlight the change in eating habits, regular physical exercise, and cognitive behavioral therapy 8 . Ideally, these measures should be implemented for all patients living with obesity, even for those who will undergo drug or surgical treatment. Recently, in addition to lifestyle change, neuromodulation with deep transcranial stimulation has also been studied and has shown effectiveness in weight loss reduction 23 .
A systematic review carried out in 2021, which analyzed 64 articles concluded that among the most effective non-surgical interventions are low-carbohydrate or low-fat diets and combined therapies. This study also showed that non-drug interventions, such as physical exercise, when used alone, are not very effective in reducing the weight of these patients Therefore, a combination of two or more therapies should be chosen 24 .
Pharmacological treatment must be chosen together with the patient. One or more drugs can be used, the main ones used being: Liraglutide, Semaglutide, Tirzepatide, Orlistat, Phentermine and Sibutramine 25 .
Liraglutide was recently approved for the treatment of obesity and is now one of the most widely used drugs. It acts as a GLP-1 receptor agonist 26 , 27 , 28 , enhancing its effects. This group of drugs is already known in the treatment of Type 2 Diabetes Mellitus, a condition that can often be associated with obesity 29 , 30 , since its pathophysiology involves increased insulin resistance. The main actions of this drug are: increased satiety due to a reduction in the speed of gastric emptying, increased insulin release and decreased glucagon release. Semaglutide is a drug with a similar mechanism of action who demonstrated not only a substantial weight loss 31 , but was also associated with a lower 10-year T2D risk in people with overweight or obesity after 2 years of follow up 32 . More recently, a new drug that combines GLP-1 and GIP receptor agonist, Tirzepatide, has shown even better results in the short term 33 .
Orlistat, in turn, reversibly inhibits the lipase enzyme 34 , which has the function of breaking down fat from food for its absorption, as well as inhibiting the absorption of ingested triglycerides. Thus, there is elimination of fat in the feces 35 . The main adverse effects are gastrointestinal symptoms, however this can be beneficial as it leads to a change in behavior, for example causing a lower consumption of foods rich in fat 36 .
Phentermine, an amphetamine analogue, can be used in conjunction with topiramate for the treatment of obesity. The mechanism of action of the drugs is not yet known, however, significant weight loss has already been observed, in addition to a reduction in the consumption of hypercaloric foods and a decrease in the speed of gastric emptying with the use of this combination of drugs 37 , 38 .
Sibutramine, widely used in the 1990s, acts to inhibit the reuptake of serotonin, norepinephrine, and dopamine 34 . Serotonin, in turn, activates POMC system neurons and inhibits NPY neurons, thereby promoting reduced appetite and increased satiety. Despite generating weight reduction 39 , some data show increased cardiovascular risk 40 , and therefore, it is no longer used as a first-line drug.
Among the possible surgeries, the most performed today are: Roux-en-Y Gastric Bypass (RYGB), Biliopancreatic diversion (BPD), Laparoscopic Sleeve Gastrectomy (LSG) and Laparoscopic Adjustable Gastric Band (LAGB). According to the NIH and the American Bariatric Society 41 , 42 , some indications for performing bariatric surgery are adults with BMI greater than or equal to 40 and adults with BMI greater than 35 accompanied by some comorbidity such as type 2 diabetes mellitus, obstructive sleep apnea or hypertension.
RYGB is one of the best-known procedures and its complications vary according to the surgical technique used. Some complications include gastric distention, ulcers, cholelithiasis, hernias, dumping syndrome, and hyperammonaemia encephalopathy.
BPD presents long-term nutritional complications, such as anemia, bone diseases and fat-soluble vitamin deficiency. This technique has high mortality rates, mainly due to the complexity of the technique.
Among the procedures described, LSG is the one with the fewest complications, being described in the literature bleeding or stenosis of the stoma. An alternative technique using endoscopy for sleeve gastroplasty has shown to be safe and efficient for weight loss after 104 weeks, with important improvements in metabolic comorbidities 43 .
The procedure with the lowest mortality rate is the LAGB 44 . Despite this, it can present complications such as obstruction, band erosion, band slippage and gastric prolapse, esophagitis, hernia, in addition to having a high rate of reoperation, reaching 50% of patients who underwent this surgery 45 .
In this article, we compare data on weight loss through intensive drug treatment, which includes changes in eating habits, physical exercise, and medications, and through surgical treatment. Both treatments showed that weight loss caused an improvement in the lipid panel, with a reduction in total cholesterol, triglycerides and LDL, an increase in HDL, improvement in systolic and diastolic blood pressure, decrease in glycated hemoglobin and insulin resistance (accessed through HOMA), in addition to reducing the risk for cardiovascular diseases.
Our systematic review confirmed the findings of individual studies that bariatric surgery has a greater potential for weight reduction, BMI and waist circumference, as already described in individual articles and widely in the literature. It should be noted that even in the long term, this difference remained. Similarly, a 2014 Cochrane systematic review 46 comparing RCT with more than 1 year of follow-up showed that all 7 articles included demonstrated an advantage of the surgical group. An article 47 on the use of pharmacological treatment for obesity showed that even recent drugs approved, including GLP 1 agonists, are not able to reduce weight to levels similar to those of bariatric surgery to date, despite the emergence of new drugs still in initial phase 48 . It is worth mentioning that in these studies the comparison time is relatively short (12 months) and that we do not have data on the long-term impact. Thus, in relation to long term weight loss, bariatric surgery is still the best option.
Most articles were not able to individually demonstrate that surgical treatment is superior to non-surgical in terms of pressure reduction. However, the result of the meta-analysis showed a superiority of the surgical group in relation to both systolic and diastolic pressure, more pronounced in the BPD group. Wang 49 performed a systematic review focused on the impact on pressure and demonstrated that there was a reduction in systolic and diastolic values, but the subgroup analysis showed that this occurs only in the RYGB groups for systolic pressure. Similarly, Schiavon also demonstrated a significant reduction in the need of blood pressure medication after 3 years in the RYGB group when compared intensive medical treatment for obesity 50 . This difference found in only one subtype of surgery seems to be just a reflection of the sample size, which can be interpreted that surgical treatment in general tends to reduce pressure to a greater extent than non-surgical treatment. The fact that different types of surgery are significant may reflect the studies selected in our meta-analysis, which have longer follow-ups.
In relation to both HOMA-IR and glycated Hb, there was a more significant improvement in the group that underwent surgery. The way in which the data on diabetes remission was reported in the articles did not allow a meta-analysis to be carried out with these data and, therefore, it was not included. However, individual data from the Mingrone 2015, Mingrone 2021 and Schauer articles showed that the surgery group had better results. A network meta-analysis from 2021 51 comparing the different types of metabolic surgery for the treatment of obesity and diabetes showed that RYGB was 20% more likely to result in remission of type 2 diabetes compared to SG. There was no significant difference between the other groups. Moreover, the effects of bariatric surgery on diabetes is not exclusive for patients with obesity, as shown by a study with patients with a BMI of 27–32 kg/m 2 that had a better glycemic control when treated with RYGB 20 . Regarding the lipid profile, Schauer's study was not able to demonstrate superiority in relation to LDL and HDL parameters. However, by combining the data from Mingrone's articles, it is possible to demonstrate that surgical treatment is superior. Regarding cholesterol reduction, Mingrone's studies showed that although RYGB and BDP were better in relation to non-surgical treatment, the BDP technique had a statistically greater reduction in relation to RYGB. This can be explained by the greater intestinal exclusion in BDP and, therefore, having a greater impact on lipid absorption. Despite Sayeed's study 52 et al. was not included in this meta-analysis due to the inadequate way of separating the groups for analysis, the results regarding the lipid profile showed that the group that received both interventions was superior to the exclusive non-surgical treatment. It is important to point out that despite a statistically significant difference between the groups, the effect size of this difference is probably not clinically significant.
The choice of treatment for obesity can also have an impact on several other patient comorbidities. Hossain et al. 53 performed a systematic review with 26 studies that showed that bariatric surgery appears to be more effective in the treatment of asthma. Similarly, a study by Crawford et al. 15 showed that there is a greater increase in bone turnover in groups undergoing bariatric surgery in relation to pharmacological treatment. Other than that, bariatric surgery is also demonstrated to be superior in the treatment of other obesity related pathologies, such as Non-Alcoholic Steatohepatitis (NASH), and in the treatment of obesity in adolescents 54 , 55 .
The effect of major cardiovascular adverse events (MACE) and mortality 56 have also been promising for bariatric surgery. A recent cohort comparing bariatric surgery in patients with obesity and use of GLP1-agonists inpatients with diabetes showed a lower risk of MACE in the surgical group 57 . The surgical treatment has also shown superiority when compared to medical treatment regarding the prevention of diabetic kidney disease in 5 years for patients with diabetes and obesity 58 . Boyers et al. evaluated the cost-effectiveness of surgical and pharmacological treatment in the treatment of obesity and found that RYGB should be the treatment of choice only if the optimization of health system costs is considered 59 .
Another important consideration is the fact that pharmacological and surgical treatment for obesity are not mutually exclusive. Most clinicians choose to combine both treatment modalities in practice to improve results. Weight gain after bariatric surgery is a known possibility, and for those patients, two-thirds of the weight regain can be safely lost with GLP1 agonist, providing clinicians with a therapeutic option for this clinical challenge.
Methodologies and limitations of the studies
Despite the large number of articles in the literature on the treatment of obesity, there are few RCTs comparing non-surgical and surgical treatment, and most of them only follow up in the short term. In addition, many articles do not adequately describe the strategy used in non-surgical treatment. This lack of data and standardization in this type of treatment can lead to bias and possibly the formation of extremely heterogeneous groups for analysis.
Most of the studies included in our systematic review have diabetes as an inclusion criteria. In this circumstance, our findings may not be generalized to patients with obesity without diabetes.
Another important limitation of our systematic review refers to pharmacological treatment in the non-surgical group. The use of GLP 1 agonists has great potential in the treatment of obesity, but they have only started to be used recently. As the purpose of our article is to assess the long-term impact, there are still few articles available that used this drug. The use of the most recent medications, such as Tirzepatide, could not be evaluated in our study, once there are no RCTs in the literature presenting its long-term effects. Those drugs proved to be very efficient and might have similar effect in the long term. Future systematic reviews may reveal a different results when including the new generation of weight loss medication.
Finally, choosing the most appropriate treatment often involves individual characteristics of each patient, and the impact on quality of life can be extremely subjective and difficult to assess.
Obesity is a disease that increases the morbidity and mortality of patients, contributing to several secondary diseases. This systematic review evaluated the impact on the main variables related to obesity in the long term. The findings indicated that both treatment modalities are efficacious in managing obesity; however, the surgical group demonstrated superior outcomes in comparison to the non-surgical group across most variables. Nonetheless, the advent of novel pharmacological treatments has shown promising potential. Further studies focusing on the long-term impacts of these new drug treatments should be undertaken to allow for a comprehensive comparison with non-surgical treatment methods.
Data availability
Data is provided within the manuscript or supplementary information files.
Bray, G. The Battle of the Bulge: A History of Obesity Research (Dorrance Pub., 2007).
Collaborators GBD 2015 O, Afshin, A., Forouzanfar, M. H., Reitsma, M. B., Sur, P., Estep, K. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 , 13–27 (2017).
Whitlock, G. et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 373 , 1083–1096 (2009).
Article PubMed Google Scholar
Steele, C. B. et al. Vital Signs: Trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb. Mortal. Wkly. Rep. 66 , 1052–1058 (2017).
Article PubMed PubMed Central Google Scholar
Goldhaber, S. Z. et al. Risk factors for pulmonary embolism. The Framingham Study. Am. J. Med. 74 , 1023–1028 (1983).
Article CAS PubMed Google Scholar
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346 , 393–403 (2002).
Bray, G. A., Frühbeck, G., Ryan, D. H. & Wilding, J. P. H. Management of obesity. The Lancet 387 , 1947–1956 (2016).
Article Google Scholar
Perdomo, C. M., Cohen, R. V., Sumithran, P., Clément, K. & Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. The Lancet 401 , 1116–1130 (2023).
Updike, W. H. et al. Is it time to expand glucagon-like peptide-1 receptor agonist use for weight loss in patients without diabetes?. Drugs 81 , 881–893 (2021).
Moher, D. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 , 1 (2015).
Shea, B. J. et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358 , j4008 (2017).
Brown, D. A review of the PubMed PICO Tool: using evidence-based practice in health education. Health Promot. Pract. 21 , 496–498 (2020).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 5 , 210 (2016).
Leeflang, M. M. G., Deeks, J. J., Takwoingi, Y. & Macaskill, P. Cochrane diagnostic test accuracy reviews. Syst. Rev. 2 , 82 (2013).
Crawford, M. R. et al. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr. Pract. 24 , 256–264 (2018).
Schauer, P. R. et al. Bariatric surgery versus intensive medical therapy for diabetes—5-Year outcomes. N. Engl. J. Med. 376 , 641–651 (2017).
Mingrone, G. et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-Year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet 397 , 293–304 (2021).
O’Brien, P. E., Brennan, L., Laurie, C. & Brown, W. Intensive medical weight loss or laparoscopic adjustable gastric banding in the treatment of mild to moderate obesity: Long-term follow-up of a prospective randomised trial. Obes. Surg. 23 , 1345–1353 (2013).
Mingrone, G. et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 Year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet 386 , 964–973 (2015).
Cheng, A. et al. Roux-en-Y gastric bypass versus best medical treatment for type 2 diabetes mellitus in adults with body mass index between 27 and 32 kg/m 2 : A 5-year randomized controlled trial. Diabetes Res. Clin. Pract. 188 , 109900 (2022).
Chooi, Y. C., Ding, C. & Magkos, F. The epidemiology of obesity. Metabolism 92 , 6–10 (2019).
Christensen, S. Recognizing obesity as a disease. J. Am. Assoc. Nurse Pract. 32 , 497–503 (2020).
Ferrulli, A. et al. Weight loss induced by deep transcranial magnetic stimulation in obesity: A randomized, double-blind, sham-controlled study. Diabetes Obes. Metab. 21 , 1849–1860 (2019).
Twells, L. K. et al. Nonsurgical weight loss interventions: A systematic review of systematic reviews and meta-analyses. Obes. Rev. 22 , e13320 (2021).
Rosa-Gonçalves, P. & Majerowicz, D. Pharmacotherapy of obesity: Limits and perspectives. Am. J. Cardiovasc. Drugs 19 , 349–364 (2019).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373 , 11–22 (2015).
Knudsen, L. B. & Lau, J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. (Lausanne) 10 , 155 (2019).
de Oca alejandra PZMTS, PelliTero S, PUig-DoMingo M. obesity and glP-1. Minerva Endocrinology 46 , 168–176 (2021).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444 , 840–846 (2006).
Article ADS CAS PubMed Google Scholar
Rubio-Almanza, M., Cámara-Gómez, R. & Merino-Torres, J. F. Obesity and type 2 diabetes: Also linked in therapeutic options. Endocrinol. Diabetes Nutr. 66 , 140–149 (2019).
Wharton, S. et al. Two-year effect of semaglutide 2.4 mg on control of eating in adults with overweight/obesity: STEP 5. Obesity 31 , 703–715 (2023).
Wilkinson, L. et al. Effect of semaglutide 2.4 mg once weekly on 10-year type 2 diabetes risk in adults with overweight or obesity. Obesity 31 , 2249–2259 (2023).
Frías, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385 , 503–515 (2021).
Son, J. W. & Kim, S. Comprehensive review of current and upcoming anti-obesity drugs. Diabetes Metab. J. 44 , 802–818 (2020).
Ballinger, A. & Peikin, S. R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 440 , 109–117 (2002).
Zhou, Y. H. et al. Effect of anti-obesity drug on cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled trials. PLoS One 7 , e39062 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar
Cosentino, G., Conrad, A. O. & Uwaifo, G. I. Phentermine and topiramate for the management of obesity: A review. Drug Des. Dev. Ther. 7 , 267–278 (2013).
CAS Google Scholar
Smith, S. M., Meyer, M. & Trinkley, K. E. Fentermina/topiramato (qsymia) para el tratamiento de obesidad. Ann. Pharmacother. 47 , 340–349 (2013).
Sharma, B. & Henderson, D. C. Sibutramine: Current status as an anti-obesity drug and its future perspectives. Expert Opin. Pharmacother. 9 , 2161–2173 (2008).
Tziomalos, K., Krassas, G. E. & Tzotzas, T. The use of sibutramine in the management of obesity and related disorders: An update. Vasc. Health Risk Manag. 5 , 441–452 (2009).
CAS PubMed PubMed Central Google Scholar
Burguera, B. et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J. Endocrinol. Invest. 30 , 844–852 (2007).
Grundy, S. M. et al. Gastrointestinal surgery for severe obesity. Ann. Intern. Med. 115 , 956–961 (1991).
Abu Dayyeh, B. K. et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. The Lancet 400 , 441–451 (2022).
Chapman, A. E. et al. Laparoscopic adjustable gastric banding in the treatment of obesity: A systematic literature review. Surgery 135 , 326–351 (2004).
Himpens, J. et al. Long-term outcomes of laparoscopic adjustable gastric banding. Arch. Surg. 146 , 802–807 (2011).
Colquitt, J. L., Pickett, K., Loveman, E. & Frampton, G. K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014 , CD003641 (2014).
Cotugno, M. et al. Clinical efficacy of bariatric surgery versus liraglutide in patients with type 2 diabetes and severe obesity: A 12-month retrospective evaluation. Acta Diabetol. 52 , 331–336 (2014).
Tan, Q. et al. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front. Endocrinol. (Lausanne) https://doi.org/10.3389/fendo.2022.838410 (2022).
Wang, L. et al. The impact of bariatric surgery versus non-surgical treatment on blood pressure: Systematic review and meta-analysis. Obes. Surg. 31 , 4970–4984 (2021).
Schiavon, C. A. et al. Three-year outcomes of bariatric surgery in patients with obesity and hypertension. Ann. Intern. Med. 173 , 685–693 (2020).
Currie, A. C., Askari, A., Fangueiro, A. & Mahawar, K. Network meta-analysis of metabolic surgery procedures for the treatment of obesity and diabetes. Obes. Surg. 31 , 4528–4541 (2021).
Ikramuddin, S. et al. Lifestyle intervention and medical management with vs without roux-en-y gastric bypass and control of hemoglobin a1c, ldl cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA J. Am. Med. Assoc. 319 , 266–278 (2018).
Hossain, N., Arhi, C. & Borg, C. M. Is bariatric surgery better than nonsurgical weight loss for improving asthma control? A systematic review. Obes. Surg. 31 , 1810–1832 (2021).
Järvholm, K. et al. Metabolic and bariatric surgery versus intensive non-surgical treatment for adolescents with severe obesity (AMOS2): A multicentre, randomised, controlled trial in Sweden. Lancet Child Adolesc. Health 7 , 249–260 (2023).
Verrastro, O. et al. Bariatric–metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): A multicentre, open-label, randomised trial. The Lancet 401 , 1786–1797 (2023).
Courcoulas, A. P. et al. Reduction in long-term mortality after sleeve gastrectomy and gastric bypass compared to nonsurgical patients with severe obesity. Ann. Surg. 277 , 442–448 (2023).
Stenberg, E. & Näslund, E. Major adverse cardiovascular events among patients with type-2 diabetes, a nationwide cohort study comparing primary metabolic and bariatric surgery to GLP-1 receptor agonist treatment. Int. J. Obes. 47 , 251–256 (2023).
Article CAS Google Scholar
Bjornstad, P. et al. Effect of surgical versus medical therapy on diabetic kidney disease over 5 years in severely obese adolescents with type 2 diabetes. Diabetes Care 43 , 187–195 (2020).
Boyers, D. et al. Cost-effectiveness of bariatric surgery and non-surgical weight management programmes for adults with severe obesity: A decision analysis model. Int. J. Obes. 45 , 2179–2190 (2021).
Download references
Acknowledgements
The authors are thankful to Justin Axel-Berg for the English corrections and Rossana V. Mendoza López for the statistical analysis.
Author information
Authors and affiliations.
Department of Neurology, Hospital das Clínicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, São Paulo, SP, Brazil
Leonardo Zumerkorn Pipek
Faculty of Medicine FMUSP, University of São Paulo, São Paulo, Brazil
Walter Augusto Fabio Moraes, Rodrigo Massato Nobetani, Vitor Santos Cortez, Alberto Santos Condi, João Victor Taba, Milena Oliveira Suzuki, Fernanda Sayuri do Nascimento & Vitoria Carneiro de Mattos
Centro Universitário FMABC, Santo André, São Paulo, Brazil
Rafaela Farias Vidigal Nascimento
Center of Acupuncture, Department of Orthopaedics and Traumatology, University of São Paulo, São Paulo, Brazil
Leandro Ryuchi Iuamoto & Wu Tu Hsing
Department of Gastroenterology, Hospital das Clínicas, HCFMUSP, Avenida Doutor Arnaldo, 455, São Paulo, Brazil
Luiz Augusto Carneiro-D’Albuquerque, Alberto Meyer & Wellington Andraus
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: L.Z.P., A.M. Methodology: L.Z.P., L.R.I., A.M. Formal analysis: L.Z.P., R.F.V.N., A.M. Data Curation: L.Z.P., W.A.F.B., R.M.N., V.S.C., A.S.C., J.V.T., R.F.V.N., M.O.S., F.S.N., V.C.M. Writing—Original Draft: L.Z.P., W.A.F.B., R.M.N., V.S.C., A.S.C., J.V.T., R.F.V.N., M.O.S., F.S.N., V.C.M. Writing—Review and Editing: L.Z.P., R.F.V.N., L.R.I., W.T.H., L.A.C., A.M., W.A. Visualization: L.Z.P., R.F.V.N., A.M. Supervision: L.R.I., W.T.H., L.A.C., A.M., W.A. Project administration: L.R.I., W.T.H., L.A.C., A.M., W.A.
Corresponding author
Correspondence to Alberto Meyer .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Pipek, L.Z., Moraes, W.A.F., Nobetani, R.M. et al. Surgery is associated with better long-term outcomes than pharmacological treatment for obesity: a systematic review and meta-analysis. Sci Rep 14 , 9521 (2024). https://doi.org/10.1038/s41598-024-57724-5
Download citation
Received : 19 November 2023
Accepted : 21 March 2024
Published : 25 April 2024
DOI : https://doi.org/10.1038/s41598-024-57724-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Long term outcome
- Pharmacological treatment
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Burden of Antimicrobial Resistance in Japan: A Systematic Literature Review and Meta-Analysis
- Original Research
- Open access
- Published: 25 April 2024
Cite this article
You have full access to this open access article

- Tetsuya Matsumoto 1 ,
- Akira Yuasa ORCID: orcid.org/0000-0002-3699-7231 2 ,
- Hiroyuki Matsuda 3 ,
- Dilinuer Ainiwaer 3 &
- Naohiro Yonemoto 2
121 Accesses
1 Altmetric
Explore all metrics
Introduction
Antimicrobial resistance (AMR) is one of the most serious public health challenges worldwide, including in Japan. However, there is limited evidence assessing the AMR burden in Japan. Thus, this systematic literature review (SLR) and meta-analysis (MA) were conducted to assess the clinical and economic burden of AMR in Japan.
Comprehensive literature searches were performed on EMBASE, MEDLINE, the Cochrane Library, and ICHUSHI between 2012 and 2022 following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. MA estimated a pooled effect between the two comparative arms (AMR vs. non-AMR). The results were reported in measures of odds ratios (ORs) for in-hospital mortality and in standardized mean differences (SMDs) for length of stay (LOS) and direct medical costs.
Literature searches identified 1256 de-duplicated records, of which 56 observational studies (English, n = 35; Japanese, n = 21) were included. Of note, twenty-two studies (39.3%) compared the AMR group with non-AMR group. In the SLR, in-hospital mortality, LOS, and direct medical costs were higher in the AMR group compared to the non-AMR group. Eight studies were selected for the MA. In the AMR group, the pooled estimate showed a statistically higher in-hospital mortality [random effect (RE)—OR 2.25, 95% CI 1.34–3.79; I 2 = 89%; τ 2 = 0.2257, p < 0.01], LOS (RE—SMD 0.37, 95% CI − 0.09–0.84; I 2 = 99%; τ 2 = 0.3600, p < 0.01), and direct medical cost (RE—SMD 0.53, 95% CI 0.43–0.62; I 2 = 0.0%; τ 2 = 0.0, p = 0.88) versus the non-AMR group.
Our study presents an overview of the clinical and economic burden of AMR in Japan. Patients with AMR infections experience significantly higher in-hospital mortality, LOS, and direct medical costs compared with patients without AMR infections.
Similar content being viewed by others
Estimating the burden of antimicrobial resistance: a systematic literature review.
Guideline Recommendations for Empirical Antimicrobial Therapy: An Appraisal of Research Evidence for Clinical Decision-Making in Ethiopia

Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review
Avoid common mistakes on your manuscript.
Antimicrobials are medications used to prevent and treat infections. These include antibiotics, antivirals, antifungals, and antiparasitic drugs. Antimicrobial resistance (AMR) occurs when pathogens develop mechanisms to thrive or survive in the presence of the drug. As a result, antimicrobials show reduced clinical efficacy, and infections become increasingly difficult or impossible to treat. These result in an increased risk of disease spread, severe illness, and death [ 1 ]. The irrational use of antibiotics is directly correlated with the increase in AMR and varies from country to country, depending on the respective antibiotic usage patterns [ 2 , 3 ].
The global review on AMR estimated 700,000 deaths in 2014 attributable to drug-resistant strains of common bacterial infections, human immunodeficiency virus (HIV), tuberculosis, and malaria [ 4 ]. However, bacterial AMR emerged as one of the leading public health threats of the twenty-first century, with 1.27 million deaths directly caused by bacterial AMR globally in 2019 [ 5 ]. The total annual number of deaths caused by AMR in Japan is unknown; however, a 2020 study by Tsuzuki et al. estimated that, in 2017, the number of deaths attributed to bloodstream infections (BSIs) caused by methicillin-resistant Staphylococcus aureus (MRSA) and fluoroquinolone-resistant Escherichia coli (FQREC) was approximately 8100 [ 6 ]. According to the Japan Nosocomial Infections Surveillance (JANIS) Annual Open Report 2022, the resistance rates of third-generation cephalosporin antibiotics, including cefotaxime and ceftazidime, were 26.8% and 12.8% for E. coli and 12.6% and 10.3% for Klebsiella pneumoniae , respectively, whereas for levofloxacin, the resistance rate to E. coli was 39.6% [ 7 ]. An analysis of 2289 hospitals across Japan, conducted as part of the JANIS program 2022, reported that the percentage of hospitals reporting at least one corresponding specific AMR bacteria in 2022 was the highest for MRSA (99.7%), followed by penicillin-resistant Streptococcus pneumoniae (PRSP; 53.9%) and carbapenem-resistant Enterobacteriaceae (CRE; 51.2%) [ 7 ].
Rising AMR has severe health and economic consequences [ 8 ]. A 2018 report by the Organization for Economic Co-operation and Development (OECD) that analyzed 33 OECD, European Union (EU), and European Economic Area countries estimated that AMR caused a total of $3.5 billion annually in health care costs. In Japan, Matsumoto et al. [ 9 ] estimated the clinical and economic outcomes of drug-resistant gram-negative pathogens in Japan and demonstrated that economic and clinical values could be considerably increased by reducing AMR levels in the country. It was reported that savings of ¥2.5 billion ($23 million) to ¥6.4 billion ($60 million) in hospitalization costs could be achieved in a year by reducing AMR by 50% in Japan [ 9 ].
In March 2021, the Japan Agency for Medical Research and Development (AMED) Public and Private Partnerships for Infectious Diseases R&D created the first edition of the Japanese version of the Priority Pathogen List, which was approved by seven academic societies and the Drug Discovery Promotion Review Committee [ 10 ].
In Japan, there is limited evidence assessing AMR burden. Since AMR strains and resistance rates that require attention differ from country to country, there is a need to appropriately assess the country-wise burden of AMR by referring to previous data and reports. This study aims to conduct a systematic literature review (SLR) and meta-analysis (MA) to determine the clinical and economic burden of AMR in Japan.
The searches were designed by considering the combination of sensitivity and specificity. The SLR was conducted in accordance with the general recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [ 11 ], the general principles of the Centre for Reviews and Dissemination (University of York) guidance [ 12 ] for undertaking reviews in health care, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 13 ].
Eligibility Criteria
Table 1 contains the eligibility criteria defined under the Population, Intervention, Comparison, Outcomes, and Study design (PICOS) framework [ 14 ]. The systematic literature search was conducted using a predefined search strategy to identify eligible studies; the search strategies are presented in Supplementary Table 1. The target population for this SLR was adult Japanese patients (≥ 18 years of age) with at least one AMR infectious disease for any treatment. The focus was on interventional and observational studies assessing the clinical burden [in-hospital mortality, and length of stay (LOS)—including intensive care unit (ICU) and isolation bed stays] and economic burden (direct medical costs, duration of antibiotics, and amount of antibiotic usage) of AMR in Japan.
The definition of AMR in this study refers to the first edition of the Japanese version of the Priority Pathogen List that was created by the AMED Public and Private Partnerships for Infectious Diseases R&D and which was approved by seven academic societies and the Drug Discovery Promotion Review Committee [ 10 ].
Data Sources
Evidence published between 2012 and 2022 was sourced from various databases, including EMBASE, MEDLINE, the Cochrane Library (via Ovid SP ® ), and ICHUSHI (a Japanese database). Gray literature and conference abstracts for the last 3 years were also hand-searched to identify records on the treatments used for AMR infection in Japan in the following databases: Infectious Diseases Society of America, European Congress of Clinical Microbiology & Infectious Diseases/European Society of Clinical Microbiology and Infectious Diseases, International Society for Pharmacoeconomics and Outcomes Research, The Japanese Association for Infectious Diseases, Japanese Society of Chemotherapy, and Japan Society of Environmental Infectious.
Search Strategy
The search strategy was developed by combining free-text words, indexing terms, and Boolean terms with terms pertinent to disease areas, interventions, and study designs. The search strings were modified to conform to the syntax of each database. According to the eligibility criteria, all retrieved studies were then evaluated. Two reviewers independently assessed the eligibility (inclusion/exclusion) of titles and abstracts identified during the search using predetermined criteria using the PICOS framework. Every instance of uncertainty or ambiguity was resolved by consulting a third independent reviewer. Publications selected as potentially relevant from the abstract screening were retained for the full-text review. Two independent reviewers evaluated full-text publications, and discrepancies were resolved by consulting a third independent reviewer.
Data Extraction
A standardized MS Excel ® data extraction template was used to conduct data extraction from the full-text studies identified by the searches. The key methodology, patient characteristics, and results were extracted and tabulated for each study. One researcher performed data extraction, which was checked by another independent researcher. The results of the data extraction were used for the feasibility assessment and the MA. Means, medians, standard deviations (SDs), 95% confidence intervals (95% CIs), and interquartile ranges (reporting 25th and 75th values) were used to report continuous outcomes, while counts and percentages were used to report categorical outcomes. For the comparison of direct medical costs in the MA, the selected cost data of the studies were adjusted to represent the values for 2022 in local currency [ 15 ]. The annual exchange rate for 2022 by the OECD was used to convert Japanese yen to United States (US) dollars ($1 = ¥131.498) [ 16 ]. Other cost data were presented as reported in the studies.
Quality Assessment
The quality of randomized controlled trial (RCT) studies included in the SLR was evaluated to ensure that this review's conclusions and findings are based on the best available evidence and to identify any potential sources of bias in the data. The quality of RCT studies retained for data extraction was assessed using a Cochrane risk of bias tool for randomized trials. This checklist (also called “RoB”) is the most recommended tool for RCTs. The RoB 2.0 tool is suitable for individually randomized, parallel-group, and cluster-randomized trials [ 17 ]. The Newcastle-Ottawa scales for cohort case-control studies were used to evaluate the risk of bias in each individual article included in this study [ 18 ] (Supplementary Table 2). Gray literature that did not encompass full-text articles was evaluated using the Authority, Accuracy, Coverage, Objectivity, Date, Significance checklist [ 19 ].
Data Analysis
Statistical analysis.
The MA estimated the clinical and economic burden between the two comparative arms: the AMR (resistant) and non-AMR (susceptible) arms. A high degree of heterogeneity was estimated because of differences in underlying diseases, causative organisms, patient backgrounds, and clinical characteristics among individual studies. The MA was performed only on studies when they used design and/or analysis for adjustment of confounders, such as propensity score matching performed on the two comparative arms. All analyses were performed using the R software environment for statistical computing and graphics (version 4.1 or above). The ‘meta,’ ‘metafor,’ package in R was used to conduct the analyses.
The MA was used to estimate the pooled effect between the two arms (i.e., summary of proportions, time-to-event outcomes, and mean of outcomes) across all eligible studies. The weighted average of each outcome measure of the studies was calculated using inverse variance weighting to estimate the pooled effect [ 20 ]. Both random effect (RE) and fixed effect (FE) models were applied; however, the RE model was selected as the primary model [ 20 ]. The results were reported with the measure of effect as odds ratios (ORs) and standardized mean differences (SMDs) with 95% CIs. In-hospital mortality was reported with dichotomous outcomes. Also, LOS and direct medical costs were reported as continuous outcomes. The Box-Cox method [ 21 ] was applied to studies not reporting mean and SD directly to estimate the mean and SD according to the reported sample size, median, minimum value, maximum value, first quartile, or third quartile.
Assessment of Statistical Heterogeneity and Publication Bias
Heterogeneity among the selected studies was evaluated using both the Q test and I 2 index [ 22 ]. In the Q test, a p value cutoff < 0.1 for the test for heterogeneity indicated presence of heterogeneity. The I 2 index (0–40% no heterogeneity, 30–60% moderate heterogeneity, 50–90% substantial heterogeneity, and 75–100% considerable heterogeneity) was used to describe the percentage of total variation across analyses due to heterogeneity [ 11 , 23 ]. In case of existing substantial heterogeneity in MA and sufficient studies, subgroup analysis was conducted according to the characteristic of the study or patient, including studies in MA. Moreover, as another analysis, the study with extreme value, which might lead to substantial heterogeneity, was excluded in MA.
Publication bias was assessed through funnel plot for base case analysis in this study, Egger’s test was not applicable because of the small number of studies for each outcome ( n < 10 studies) [ 24 , 25 ].
Ethical Approval
Ethics approval was not required for this study. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Study Overview
A search of the databases returned a total of 1262 results. Following the removal of duplicates and a thorough review, 57 records (consisting of 56 studies) that met the inclusion criteria were included in the SLR (Supplementary Table 3). Figure 1 presents the PRISMA flow diagram for the SLR, and Supplementary Table 4 presents the PRISMA check list.
PRISMA flow diagram for SLR. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SLR systematic literature review
Study Characteristics
For the SLR, a total of 56 studies (35 in English and 21 in Japanese) were included. The details of these studies are presented in Supplementary Table 5. All studies were conducted in Japan, with the majority being conducted in single-center settings (62.5%), followed by multicenter settings (33.9%) and unclear settings (3.6%). Fifty-three of the 56 studies (94.6%) were cohort studies, two (3.6%) were case-control studies, and one (1.8%) was a cross-sectional study. Twenty-two studies (39.3%) compared the AMR group with the non-AMR group. The overall sample size ranged from 33 to 7,772,050 participants, while the sample size for resistant populations ranged from 12 to 93,838 participants. Fourteen of the 56 studies (25.0%) included patients with BSI, nine (16.1%) included patients with pneumonia, four (7.1%) included patients with surgical site infection, and two studies each (3.6%) included patients with urinary tract infection and sepsis. One study each (1.8%) enrolled patients infected with vertebral osteomyelitis and invasive pneumococcal disease, and the remaining twenty-three studies (41.1%) included patients with multiple infections. Twenty-nine (51.8%) of the 56 studies were on MRSA, and S. aureus was the most common causative pathogen (53.6%). Table 2 presents details of the study characteristics.
Baseline Patient Characteristics
Supplementary Table 6 presents the baseline characteristics of the patients in the 56 included studies. The median age distribution of the population ranged from 60 to 89 years. The average body mass index (BMI) varied between 20.8 and 22.9 kg/m 2 . There were marginally more male than female participants. The majority of the population in this study included patients with MRSA infections. Average Sequential Organ Failure Assessment (SOFA) scores ranged between 4.7 and 8.5. A total of 51.8% of the patients had a SOFA score ≥ 2, indicating that the infection was associated with an increased risk of death or prolonged hospital stay. The median Charlson Comorbidity Index (CCI) score ranged from 0.99 to 3.9. The proportion of patients with CCI scores ≥ 3 and ≥ 2 was greater in the resistant group (69.0% and 45.4%, respectively) than in the susceptible group (35.0% and 35.9%, respectively). The most prevalent comorbidity was diabetes.
Clinical Outcomes
In-hospital mortality.
Forty studies reported in-hospital mortality (which includes 7-, 28-, 30-, and 90-day mortality) [ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 ]. See Supplementary Table 7 for more details and other results of the SLR.
Twenty-four studies evaluated MRSA [ 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 ], and of those, seventeen studies reported in-hospital mortality, which ranged from 0% [ 54 , 59 ] to 73.0% [ 44 ]. The MRSA group had significantly higher in-hospital mortality compared with the non-MRSA group (31.2% vs. 11.6%, p < 0.001) [ 48 ] and the methicillin-susceptible S. aureus (MSSA) group (36.7% vs. 15.0%, p = 0.012) [ 62 ]. In 2016, Uematsu et al. compared in-hospital mortality between an anti-MRSA group and a control group and found that the anti-MRSA group had significantly higher in-hospital mortality (22.6% vs. 6.2%, p < 0.001, respectively) than the control group [ 56 ].
Three studies [ 39 , 40 , 41 ] evaluated carbapenem-resistant patients, and the in-hospital mortality rate ranged from 8.1 to 36.4% [ 40 ]. In a 2022 study by Imai et al., the carbapenem-resistant group had higher in-hospital mortality than the susceptible group; however, the difference was not statistically significant (25.6% vs. 21.9%, p = 0.407, respectively) [ 41 ].
A significant association among lower BMI, lower Barthel Index, higher Hugh-Jones grade, higher A-DROP score, C-reactive protein ≥ 20 mg/dl or infiltration of at least two-thirds of one lung, mechanical ventilation at admission, interstitial lung disease, aspiration pneumonia, a high CCI score, a high SOFA score, and having a vancomycin minimum inhibitory concentration ≥ 1.5 µg/ml was observed with increased in-hospital mortality in the MRSA group. Meanwhile, in carbapenem-resistant patients, older age, male gender, immunosuppressive drug use, pneumonia, sepsis, and a CCI score ≥ 1 were significantly associated with increased in-hospital mortality.
Length of Stay
A total of thirty studies reported LOS [ 26 , 27 , 31 , 40 , 41 , 42 , 43 , 47 , 49 , 52 , 54 , 55 , 56 , 58 , 62 , 63 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 ], the details and results of which are presented in Supplementary Table 8. Sixteen studies investigated MRSA infections [ 42 , 43 , 47 , 49 , 52 , 54 , 55 , 56 , 58 , 62 , 63 , 65 , 66 , 68 , 69 , 76 ], for which the mean LOS ranged from 8.6 [ 54 ] to 123.6 days [ 68 ]. The MRSA population had a significantly longer LOS than the non-MRSA group (35.0 days vs. 14.0 days, p < 0.001, respectively) [ 63 ].
Carbapenem-resistant groups [ 40 , 41 , 67 , 72 , 74 , 77 ] were reported in six studies, and the median LOS ranged from 14.0 to 83.0 days [ 40 ]. Imai et al. demonstrated in 2022 that carbapenem-resistant infections had a significantly longer LOS than carbapenem-susceptible infections (64.0 days vs. 46.0 days, p < 0.001, respectively), and even after adjusting with the inverse probability of treatment weight method, a similar trend was observed (median: 63.0 days vs. 51.0 days, p = 0.004, respectively) [ 41 ].
Economic Outcomes
Direct medical costs.
In total, fifteen studies reported direct medical costs [ 41 , 56 , 58 , 59 , 62 , 63 , 65 , 67 , 68 , 69 , 70 , 71 , 73 , 79 , 80 ]. Supplementary Table 9 presents the details and results of the studies. Nine studies evaluated direct medical costs in the MRSA population [ 56 , 58 , 59 , 62 , 63 , 65 , 68 , 69 , 80 ] with a median hospitalization cost ranging from $496 [ 62 ] to $29,596 [ 65 ]. The median hospitalization cost in the MRSA group was significantly higher than that in the non-MRSA group ($12,156 vs. $4665, p < 0.001, respectively) [ 63 ], as well as in the MSSA group ($26,035 vs. $19,823, p = 0.036, and mean: $15,762 vs. $14,152, p < 0.001, both respectively) [ 58 ]. Tsuzuki et al., in 2021, also evaluated the 5-year total intervention cost (including drug, transplantation, radiation, surgery, and blood transfusion costs), which was $475,059 in the MRSA group and $344,418 in the MSSA group [ 62 ].
Two studies evaluated the costs in the carbapenem-resistant population [ 41 , 67 ]. The average hospitalization cost per patient in the carbapenem-resistant group was higher than that in the carbapenem-sensitive group ($1648 vs. $532, p < 0.001, respectively) [ 67 ]. In the carbapenem-resistant group, the median costs for consultation ($163 vs. $143, p = 0.238, respectively), laboratory tests ($2498 vs. $1845, p = 0.002, respectively), and hospital stay ($14,307 vs. $10,560, p < 0.001, respectively) were higher than those in the carbapenem-sensitive group [ 41 ]. In addition, the cost of the intervention, which includes medications and surgeries, was higher in the carbapenem-resistant group than in the carbapenem-sensitive group [ 41 , 67 ].
Amount of Antibiotic Usage
Only four studies [ 50 , 55 , 77 , 80 ] reported the amount of antibiotic usage; hence, very few data were available. Supplementary Table 10 presents the details and results of the studies. According to a study published in 2015 by Suzuki et al., between 2006 and 2008, the mean amount of vancomycin in MRSA patients was high (2,591.6 g and 2,563.5 g per year, respectively). In contrast, the amount decreased between 2007 and 2009 (2348.8 g and 2384.5 g, respectively). In a study by Ogasawara et al. in 2012, 20 of the 33 patients treated with meropenem were resistant to Pseudomonas aeruginosa . The daily dose of meropenem for the resistant group was 1, and for the non-resistant group, it was 1.5 (the unit of measurement was not specified) [ 77 ].
Duration of Antibiotics
In total, twelve studies reported the duration of antibiotics [ 37 , 38 , 49 , 55 , 59 , 63 , 73 , 74 , 75 , 77 , 79 , 81 ]. Supplementary Table 11 presents the details and results of the studies. The median duration of antibiotic use in MRSA patients ranged from 10.0 to 21.5 days [ 49 , 55 ], whereas for ESBL bacteremia, it ranged from 9.0 to 15.9 days [ 38 , 75 ]. In those studies, the duration of antibiotic use was reported for the MRSA (median: 24.0 days, p < 0.001) [ 63 ], carbapenem-resistant (mean: 14.9 days) [ 74 ], and meropenem-resistant (median: 9.0 days, p > 0.05) [ 77 ] populations, which required longer antibiotic treatment than the non-MRSA (median: 9.0 days), non-carbapenem-resistant (mean: 11.0 days), and non-meropenem-resistant (median: 7.0 days) populations.
Pooled Clinical Outcomes
A total of eight studies from the SLR were included for the MA, of which four [ 41 , 56 , 62 , 63 ], seven [ 41 , 56 , 62 , 63 , 72 , 75 , 77 ], and three [ 41 , 56 , 62 ] studies evaluated in-hospital mortality, LOS, and direct medical costs, respectively. The outcomes of the amount and the duration of antibiotics were not evaluated because of a very limited number of eligible studies.
Among the 20,387 patients from the four studies [ 41 , 56 , 62 , 63 ], the pooled estimate showed a statistically significantly higher mortality in the AMR group compared with the non-AMR group for both the FE (OR 3.19, 95% CI 2.92–3.48) and RE models (OR 2.25, 95% CI 1.34–3.79, I 2 = 89%, τ 2 = 0.2257, p < 0.01) (Fig. 2 ). The funnel plot of in-hospital mortality is shown in Supplementary Fig. 1. The plot of in-hospital mortality represents bias as the dots outside of the triangle.
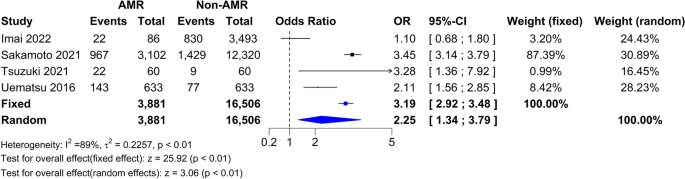
Forest plot of in-hospital mortality. AMR antimicrobial resistance, CI confidence interval, OR odds ratio
In-Hospital Mortality Sensitivity Analyses
A series of sensitivity analyses were conducted after excluding the carbapenem-resistant group. Analysis conducted after excluding the carbapenem-resistant population retained only the MRSA population [ 56 , 62 , 63 ], and the results suggested higher mortality in the AMR group versus the non-AMR group, with statistically significant ORs for the FE (OR 3.31, 95% CI 3.02–3.61) and RE (OR 2.84, 95% CI 1.95–4.14; I 2 = 79%, τ 2 = 0.0735, p < 0.01) models (Supplementary Fig. 2).
Additionally, a sensitivity analysis conducted after excluding the study that might include other antimicrobial resistance mechanisms [ 63 ], and it showed similar results (Supplementary Figs. 3 and 4). This study was defined as an outlier study based on the influence analysis.
Among the 20,662 patients from the seven studies [ 41 , 56 , 62 , 63 , 72 , 75 , 77 ], the pooled estimate showed a statistically significantly higher LOS in the AMR group versus the non-AMR group for FE (SMD 1.27, 95% CI 1.23–1.31) and a higher LOS in the AMR group versus the non-AMR group for RE (SMD 0.37, 95% CI − 0.09–0.84, I 2 = 99%, τ 2 = 0.3600, p < 0.01) models (Fig. 3 ). The funnel plot of length of stay was shown in Supplementary Fig. 5. The plot of length of stay represents bias as the dots outside of the triangle.
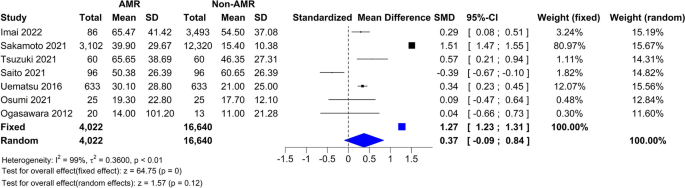
Forest plot of LOS. AMR antimicrobial resistance, CI confidence interval, LOS length of stay, SD standard deviation, SMD standardized mean difference
Length of Stay Sensitivity Analyses
A series of sensitivity analyses was conducted after excluding (1) MRSA, ESBL, and meropenem populations and (2) carbapenem-resistant, ESBL, and meropenem populations. The results of the sensitivity analysis conducted after excluding the carbapenem-resistant, ESBL, and meropenem populations retained only the MRSA population [ 56 , 62 , 63 ] (Supplementary Fig. 6). The results showed that the AMR group had a significantly longer LOS compared with the non-AMR group (for FE and RE models, respectively, SMD 1.35, 95% CI 1.31–1.39 and SMD 0.81, 95% CI 0.10–1.53, I 2 = 99%, τ 2 = 0.3905, p < 0.01). The results of the sensitivity analysis conducted after excluding the MRSA, ESBL, and meropenem populations retained only the carbapenem-resistant population [ 41 , 72 ] (Supplementary Fig. 7). The results showed that the AMR group had a longer LOS compared with the non-AMR group; however, the results were not statistically significant (for FE and RE models, respectively, SMD 0.05, 95% CI − 0.12 to 0.22 and SMD − 0.04, 95% CI − 0.71 to 0.63, I 2 = 93%, τ 2 = 0.2162, p < 0.01).
Additionally, a sensitivity analysis conducted after excluding the study [ 63 ] which might include other antimicrobial resistance mechanisms showed similar results (Supplemental Figs. 8–10). This study was defined as an outlier study based on the influence analysis.
Direct Medical Cost
Among the 4965 patients from the three studies [ 41 , 56 , 62 ], the pooled estimate showed a statistically significant increase in direct medical costs among the AMR groups compared with the non-AMR groups (for both FE and RE models, SMD 0.53, 95% CI 0.43–0.62, I 2 = 0.0%, τ 2 = 0.0, p = 0.88) (Fig. 4 ). The funnel plot of direct medical cost is shown in Supplementary Fig. 11. The plot did not show bias with the limitation of small sample size (i.e., study number) included in MA.
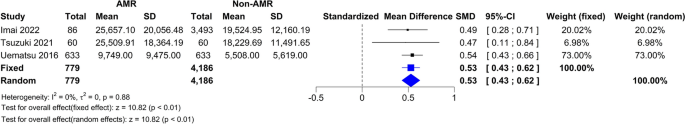
Forest plot of direct medical cost in US dollar. AMR antimicrobial resistance, CI confidence interval, SD standard deviation, SMD standardized mean difference, US United States
Direct Medical Cost Sensitivity Analyses
The sensitivity analysis was conducted after excluding the carbapenem-resistant population retained only the MRSA population [ 56 , 62 ] (Supplementary Fig. 12). The analysis showed significantly higher direct medical costs for the AMR group (for both FE and RE models, SMD 0.54, 95% CI 0.43–0.65, I 2 = 0.0%, τ 2 = 0.0, p = 0.71) compared to the non-AMR group.
This SLR and MA were conducted to determine the clinical and economic burden of AMR in Japan. In-hospital mortality and LOS were the clinical outcomes analyzed, while direct medical costs and the duration and amount of antibiotics were the economic outcomes.
Most of the studies (53.6%) included in the SLR evaluated patients with S. aureus infections. In a previous global SLR study, S. aureus was the most identified causative bacterium (23.4%) [ 82 ]. In Japan, nearly half of the cases were confirmed to be S. aureus [ 82 ]. The newly published Japanese National Action Plan on Antimicrobial Resistance (AMR) 2023–2027 reported that, as of 2020, the MRSA rate in Japan (48.1%) was higher compared to the EU (16.7%) and other developed countries [ 83 ]. In addition, this study found only two studies targeting vancomycin resistance. The reason for this is thought to be that the prevalence of vancomycin-resistant enterococci (VRE) is extremely low in Japan. In the 2022 report, the proportion of VRE in Enterococcus faecium was 2.6% [ 7 ]. On the other hand, the percentage of VRE in Europe and the US was reported to be 16.8% and 82.1%, respectively [ 84 , 85 ]. Additionally, AMR-related mortality rates have been reported to vary by region, with rates tending to be higher in developing countries [ 5 ]. This is thought to be influenced not only by the prevalence of AMR but also by access to medical care and the state of public health. In this way, to understand the burden of disease caused by AMR in a specific country or region and to compare existing research results, it is necessary to consider differences in the prevalence of AMR and other influences.
In Japan, a 2019 domestic survey reported that approximately 8000 people died in 2017 because of MRSA and FQREC, which are two of the most common drug-resistant bacteria [ 6 ]. Japan's AMR Countermeasure Action Plan 2016–2020 set a goal of “reducing the methicillin resistance rate of S. aureus to 20% or less in 2020,” but the actual annual rate of decrease was only 2% [ 86 ]. On the other hand, the UK has achieved an annual decrease rate of 5%. By thoroughly implementing infection prevention and control, and promoting the appropriate use of antimicrobials, it has been confirmed that Japan aims to reduce the drug resistance rate to < 20%, which is the same level as in other developed countries. Under these circumstances, countermeasures against MRSA are urgently needed in Japan, and this study was considered to be extremely useful for understanding the clinical and economic burden of AMR and MRSA in Japan.
According to our results, the MRSA group had significantly higher in-hospital mortality, LOS, and median or average hospitalization cost relative to the non-MRSA, MSSA, and control groups [ 51 , 56 , 58 , 62 , 63 , 65 ]. Even after adjusting for multiple variables such as age, sex, CCI, Barthel Index, nosocomial infection, BSI, ICU admission, surgery, and submission dates of positive S. aureus cultures, the correlation remained statistically significant [ 58 ]. Published results from developed countries were also in agreement with our results, in that the median LOS was longer for the MRSA group than for the MSSA group (9 days vs. 7 days, p = 0.045 from the US [ 87 ], respectively, and 22.5 days vs. 14 days from Canada [ 88 ], respectively).
The carbapenem-resistant population displayed a higher in-hospital mortality rate than the non-resistant population; however, the results were not statistically significant because the resistant group had a limited sample size [ 41 ], but LOS in the carbapenem-resistant population was significantly longer than that in the non-resistant population [ 58 ]. The multi-drug resistant (MDR) population also had a significantly longer LOS compared with the non-MDR population [ 78 ]. Hospitalization costs were also greater in the carbapenem-, penicillin-, oxacillin-, cephalosporin-, fluroquinolone-, and gentamicin-resistant groups than in the respective non-resistant groups [ 41 , 67 , 70 , 73 ]. Similar reviews conducted by Dadgostar et al. in 2019 [ 89 ] and Pulingam et al. in 2022 [ 3 ] also reported that AMR imposes a significant economic burden.
The results of our MA revealed that in-hospital mortality, hospital stays, and direct medical costs were significantly greater in the AMR group relative to the non-AMR group. Our results were consistent with another MA conducted by Poudel et al. in 2023, which reported that the resistant group had significantly higher mortality (OR 1.844 [95% CI 1.187–2.865]), LOS (mean: 7.4 days [95% CI 3.4–11.4]), and readmission (OR 1.492 [95% CI 1.231–1.807]). The same study also reported that costs attributable to the resistant group were higher in the resistant group compared with the non-resistant group [ 90 ]. Similarly, the MAs conducted by Cosgrove et al. in 2003 [ 91 ] (OR = 1.93, 95% CI 1.54–2.42, p < 0.001) and Rödenbeck et al. in 2023 [ 92 ] (OR 2.29, 95% CI 1.91–2.75) also suggested that the resistant group had significantly higher mortality than the susceptible group. Furthermore, the SLR conducted by Naylor et al. in 2018 [ 82 ] demonstrated that the resistant population had higher mortality and greater costs compared with the non-resistant population.
Several SLRs and MAs on the disease burden of AMR have been conducted globally [ 82 , 90 , 93 , 94 ]. These studies highlight that the global burden of AMR is substantial from both economic and clinical perspectives [ 90 ]. Resistant bacterial infections are associated with significant mortality [ 93 ]. There is considerable variability in burden estimates, which can lead to inaccurate intervention evaluations and poor policy or investment decisions [ 82 ]. AMR decision-making and policy should be driven by reliable, unbiased estimates of the effect size, which can be achieved through MAs [ 94 ].
Substantial heterogeneity between studies was observed in our MA because of the considerable diversity in antibiotic resistance. Pooled estimates were calculated to integrate studies regardless of the type of antibiotic resistance for the AMR and non-AMR comparisons, and the sensitivity analysis was conducted by sub-grouping antibiotic resistance types. While the MA results of in-hospital mortality and LOS in the pooled estimates showed high heterogeneity, a few results from the sensitivity analysis showed low heterogeneity. On the other hand, the heterogeneity in the pooled estimates and the sensitivity analysis of direct medical costs was consistent.
Although there is limited evidence in this literature review, most of the eligible studies used for the MA were published after 2016; therefore, there is a possibility of data overlapping among the multicenter studies involved in the MAs. For studies that adjusted patient background for both resistance and non-resistance groups, there is a possibility that the patients showed resistance to other antibiotics than those targeted in the study. In Japan, during the study period, the treatment environment has not significantly changed because of almost no new antibiotics being available; however, the prevalence of resistant bacteria may have been changing.
Conclusions
In Japan, AMR is associated with a significant clinical and economic burden that impacts both patients and society. There is a pressing need for payers, providers, and policymakers to make informed decisions regarding interventions to mitigate AMR-associated burdens. However, since AMR strains and resistance rates show country-wise diversity, appropriately assessing the burden of AMR by referring to existing data and reports is of utmost importance.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files. Except for confidential data associated with data analysis, all data are available from the corresponding author upon reasonable request.
World Health Organization. Antimicrobial resistance. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance .
Altarac D, Gutch M, Mueller J, Ronsheim M, Tommasi R, Perros M. Challenges and opportunities in the discovery, development, and commercialization of pathogen-targeted antibiotics. Drug Discov Today. 2021;26(9):2084–9.
Article CAS PubMed Google Scholar
Pulingam T, Parumasivam T, Gazzali AM, Sulaiman AM, Chee JY, Lakshmanan M, et al. Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022;170: 106103.
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations: The Review on Antimicrobial Resistance. 2016. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf .
Antimicrobial RC. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
Article Google Scholar
Tsuzuki S, Matsunaga N, Yahara K, Gu Y, Hayakawa K, Hirabayashi A, et al. National trend of blood-stream infection attributable deaths caused by Staphylococcus aureus and Escherichia coli in Japan. J Infect Chemother. 2020;26(4):367–71.
Japan Nosocomial Infections Surveillance (JANIS). Annual Open Report 2022. 2023. Available from: https://janis.mhlw.go.jp/english/report/open_report/2022/3/1/ken_Open_Report_Eng_202200_clsi2012.pdf .
Van Katwyk SR, Grimshaw JM, Hoffman SJ. Ten Years of inaction on antimicrobial resistance: an environmental scan of policies in Canada from 2008 to 2018. Healthc Policy. 2020;15(4):48–62.
PubMed PubMed Central Google Scholar
Matsumoto T, Darlington O, Miller R, Gordon J, McEwan P, Ohashi T, et al. Estimating the economic and clinical value of reducing antimicrobial resistance to three gram-negative pathogens in Japan. J Health Econ Outcomes Res. 2021;8(2):64–75.
Article PubMed PubMed Central Google Scholar
AMED Public and Private Partnerships for Infectious Diseases R&D. Priority Pathogens Lists for R&D of New Antibiotics (2021 version). 2021. Available from: https://id3catalyst.jp/apid/en/list.html .
The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. 2023. Available from: https://training.cochrane.org/handbook/current .
Centre for Reviews and Dissemination, University of York. Systematic Reviews: CRD's guidance for undertaking reviews in health care. 2009. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37(6):387.
Article PubMed Google Scholar
Medical Data Vision. Part #1: 2022 Reform of Medical Fee Structure; what you see, what you don’t. 2022. Available from: https://en.mdv.co.jp/ebm/column/article/13.html .
Organisation for Economic Co-operation and Development. OECD Exchange rates. 2022. Available from: https://data.oecd.org/conversion/exchange-rates.htm .
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7.
Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, et al., editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014.
Tyndall J. The AACODS checklist. 2010. Available from: https://www.library.sydney.edu.au/research/systematic-review/downloads/AACODS_Checklist.pdf .
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29.
McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J. 2020;62(1):69–98.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. 1st ed. Boca Raton: Chapman & Hall/CRC Press; 2021. p. 2021.
Book Google Scholar
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343: d4002.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Article CAS PubMed PubMed Central Google Scholar
Suehiro T, Takahashi Y, Okushita Y, Shiraishi A, Ogawa M, Imozuka K, et al. Detection of MRSA and ESBL-producing bacteria in emergency hospitalized patients [Japanese] [臨牀指針 緊急入院患者における MRSA および ESBL 産生菌の検出状況について]. Jpn J Clin Exp Med. 2012;89(6):813–5.
Google Scholar
Kohno J, Kawamura T, Kikuchi A, Akaishi T, Takayama S, Ishii T. A Japanese traditional medicine Hochuekkito promotes negative conversion of vancomycin-resistant Enterococci. Sci Rep. 2021;11(1):11300.
Hanada S, Iwata S, Kishi K, Morozumi M, Chiba N, Wajima T, et al. Host factors and biomarkers associated with poor outcomes in adults with invasive pneumococcal disease. PLoS One. 2016;11(1): e0147877.
Yamagata A, Ito A, Nakanishi Y, Ishida T. Prognostic factors in nursing and healthcare-associated pneumonia. J Infect Chemother. 2020;26(6):563–9.
Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985–95.
Hamada Y, Magarifuchi H, Oho M, Kusaba K, Nagasawa Z, Fukuoka M, et al. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: an eight-year retrospective comparison study. J Infect Chemother. 2015;21(7):527–30.
Hattori H, Maeda M, Nagatomo Y, Takuma T, Niki Y, Naito Y, et al. Epidemiology and risk factors for mortality in bloodstream infections: a single-center retrospective study in Japan. Am J Infect Control. 2018;46(12):e75–9.
Kainuma A, Momiyama K, Kimura T, Akiyama K, Inoue K, Naito Y, et al. An outbreak of fluoroquinolone-resistant Pseudomonas aeruginosa ST357 harboring the exoU gene. J Infect Chemother. 2018;24(8):615–22.
Yamada K, Imoto W, Yamairi K, Shibata W, Namikawa H, Yoshii N, et al. The intervention by an antimicrobial stewardship team can improve clinical and microbiological outcomes of resistant gram-negative bacteria. J Infect Chemother. 2019;25(12):1001–6.
Hanaoka N, Etoh S, Fujiyoshi N, Hirata T, Omura K. Antimicrobial therapy for multi-drug resistant Pseudomonas aeruginosa (MDRP) infection in patients with severe burns, admitted to Chiba emergency medical center [Japanese] [当医療センターでの重症熱傷患者における多剤耐性緑膿菌感染症に対しての抗菌薬療法]. Jpn J Intensive Care Med. 2013;37(4):311–8.
Nagao M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin Microbiol Infect. 2013;19(9):852–8.
Ueda S, Okada M, Ito T, Kobayashi K, Takahashi T, Marumo S, et al. Comparative study of the effects of different dosing frequencies of cefmetazole on urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli [Japanese] [基質特異性拡張型 β-ラクタマーゼ産生大腸菌に起因する尿路感染症に対するセフメタゾールの投与回数の違いによる有効性の比較検討]. J Jpn Soc Hosp Pharm. 2022;58(2):167–72.
Mitsuboshi S, Tsuruma N, Watanabe K, Takahashi S, Ito A, Nakashita M, et al. Advanced age is not a risk factor for mortality in patients with Bacteremia caused by extended-spectrum β-lactamase-producing organisms: a multicenter cohort Study. Jpn J Infect Dis. 2020;73(4):288–92.
Tetsuka N, Hirabayashi A, Matsumoto A, Oka K, Hara Y, Morioka H, et al. Molecular epidemiological analysis and risk factors for acquisition of carbapenemase-producing Enterobacter cloacae complex in a Japanese university hospital. Antimicrob Resist Infect Control. 2019;8:126.
Hayakawa K, Nakano R, Hase R, Shimatani M, Kato H, Hasumi J, et al. Comparison between IMP carbapenemase-producing Enterobacteriaceae and non-carbapenemase-producing Enterobacteriaceae: a multicentre prospective study of the clinical and molecular epidemiology of carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother. 2020;75(3):697–708.
Imai S, Inoue N, Nagai H. Economic and clinical burden from carbapenem-resistant bacterial infections and factors contributing: a retrospective study using electronic medical records in Japan. BMC Infect Dis. 2022;22(1):581.
Furukawa D, Asai S, Nakgohri T. Trend of methicillin - resistant Staphylococcus aureus isolation in gastroenterological surgery] [Japanese] [消化器外科におけるメチシリン耐性黄色ブドウ球菌検出の動向]. J Jpn Soc Surg Infect. 2012;9(2):143–50.
Sano T. MRSA infection of department of respiratory medicine [Japanese] [本学における MRSA 感染症の現状 呼吸器内科における MRSA 感染症の現状]. J Med Soc Toho Univ. 2012;59(6):320–2.
Iwabuchi H, Oguchi K. Actual status of sepsis and countermeasures [Japanese] [4. 敗血症の実態と対策]. J Jpn Soc Dial Ther. 2013;46(2):176–8.
The Japanese Society of Intensive Care Medicine CoSR. 2007 JSICM Sepsis 1st Registry: management of severe sepsis and septic shock in Japan. J Jpn Soc Intensive Care Med. 2013;20(2):329–34.
Nakatsuka Y, Morimoto C, Yasuda I, Tsuji T, Kaji Y, Yasuda T, et al. An analysis of the correlation between guidelines-concordant treatment and the treatment outcome on nursing and healthcare-associated pneumonia patients. Kansenshogaku Zasshi. 2013;87(6):739–45.
Sasaki S, Kuwana Y, Chimori A, Yoshida K. Epidemiological characterization of clinically isolated MRSA in Kinki Central Hospital, mainly for vancomycin MIC [Japanese] [近畿中央病院における MRSA 臨床分離株の疫学調査]. Med J Kinki Central Hosp. 2015;35:25–32.
Kawamura I, Sekiya N, Araoka H, Nei T, Harada S, Kurai H, et al. Surveillance of Methicillin-resistant Staphylococcus aureus in 7 Japanese Hospitals, 2015. Jpn J Infect Prevent Control. 2017;32(3):135–40.
Fukushima M, Maeda A, Soga H, Tomono Y, Machida S, Oi Z, et al. Effects of AST activity on MRSA bacteremia using the automatic simultaneous multi-item genetic testing system (Verigene system) [Japanese] [自動多項目同時遺伝子関連検査システム (Verigeneシステム) を利用した MRSA 菌血症に対する AST 活動の効果]. J Jpn Soc Hosp Pharm. 2022;58(5):545–50.
Mizokami F, Shibasaki M, Yoshizue Y, Noro T, Mizuno T, Furuta K. Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin Interv Aging. 2013;8:1015–21.
Shoji H, Urakawa T, Watanabe K, Hirano T, Katsumi K, Ohashi M, et al. Clinical features, outcomes, and survival factor in patients with vertebral osteomyelitis infected by methicillin-resistant staphylococci. J Orthop Sci. 2016;21(3):282–6.
Isobe M, Uejima E, Seki M, Yamagishi Y, Miyawaki K, Yabuno K, et al. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother. 2012;18(6):841–7.
Kaku N, Yanagihara K, Morinaga Y, Yamada K, Harada Y, Migiyama Y, et al. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J Infect Chemother. 2014;20(6):350–5.
Seki M, Takahashi H, Yamamoto N, Hamaguchi S, Ojima M, Hirose T, et al. Polymerase chain reaction-based active surveillance of MRSA in emergency department patients. Infect Drug Resist. 2015;8:113–8.
Shoji H, Maeda M, Shirakura T, Takuma T, Ugajin K, Fukuchi K, et al. More accurate measurement of vancomycin minimum inhibitory concentration indicates poor outcomes in meticillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2015;46(5):532–7.
Uematsu H, Yamashita K, Kunisawa S, Fushimi K, Imanaka Y. The economic burden of methicillin-resistant Staphylococcus aureus in community-onset pneumonia inpatients. Am J Infect Control. 2016;44(12):1628–33.
Shime N, Saito N, Bokui M, Sakane N, Kamimura M, Shinohara T, et al. Clinical outcomes after initial treatment of methicillin-resistant Staphylococcus aureus infections. Infect Drug Resist. 2018;11:1073–81.
Uematsu H, Yamashita K, Mizuno S, Kunisawa S, Shibayama K, Imanaka Y. Effect of methicillin-resistant Staphylococcus aureus in Japan. Am J Infect Control. 2018;46(10):1142–7.
Yuasa A, Murata T, Imai K, Yamamoto Y, Fujimoto Y. Treatment procedures and associated medical costs of methicillin-resistant Staphylococcus aureus infection in Japan: a retrospective analysis using a database of Japanese employment-based health insurance. SAGE Open Med. 2019;7:2050312119871181.
Miyazaki T, Yanagihara K, Kakeya H, Izumikawa K, Mukae H, Shindo Y, et al. Daily practice and prognostic factors for pneumonia caused by methicillin-resistant Staphylococcus aureus in Japan: a multicenter prospective observational cohort study. J Infect Chemother. 2020;26(2):242–51.
Umemura Y, Ogura H, Takuma K, Fujishima S, Abe T, Kushimoto S, et al. Current spectrum of causative pathogens in sepsis: a prospective nationwide cohort study in Japan. Int J Infect Dis. 2021;103:343–51.
Tsuzuki S, Yu J, Matsunaga N, Ohmagari N. Length of stay, hospitalisation costs and in-hospital mortality of methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia in Japan. Public Health. 2021;198:292–6.
Sakamoto Y, Yamauchi Y, Jo T, Michihata N, Hasegawa W, Takeshima H, et al. In-hospital mortality associated with community-acquired pneumonia due to methicillin-resistant Staphylococcus aureus : a matched-pair cohort study. BMC Pulm Med. 2021;21(1):345.
Shimizu M, Mihara T, Ohara J, Inoue K, Kinoshita M, Sawa T. Relationship between mortality and molecular epidemiology of methicillin-resistant Staphylococcus aureus bacteremia. PLoS One. 2022;17(7): e0271115.
Uematsu H, Yamashita K, Kunisawa S, Fushimi K, Imanaka Y. Estimating the disease burden of methicillin-resistant Staphylococcus aureus in Japan: retrospective database study of Japanese hospitals. PLoS One. 2017;12(6): e0179767.
Aoyagi T, Kaito C, Sekimizu K, Omae Y, Saito Y, Mao H, et al. Impact of psm-mec in the mobile genetic element on the clinical characteristics and outcome of SCCmec-II methicillin-resistant Staphylococcus aureus bacteraemia in Japan. Clin Microbiol Infect. 2014;20(9):912–9.
Asai N, Sakanashi D, Suematsu H, Kato H, Hagihara M, Nishiyama N, et al. The epidemiology and risk factor of carbapenem-resistant enterobacteriaceae colonization and infections: case control study in a single institute in Japan. J Infect Chemother. 2018;24(7):505–9.
Fukuda H, Sato D, Iwamoto T, Yamada K, Matsushita K. Healthcare resources attributable to methicillin-resistant Staphylococcus aureus orthopedic surgical site infections. Sci Rep. 2020;10(1):17059.
Kashimura N, Kusachi S, Konishi T, Shimizu J, Kusunoki M, Oka M, et al. Impact of surgical site infection after colorectal surgery on hospital stay and medical expenditure in Japan. Surg Today. 2012;42(7):639–45.
Naylor NR, Yamashita K, Iwami M, Kunisawa S, Mizuno S, Castro-Sanchez E, et al. Code-sharing in cost-of-illness calculations: an application to antibiotic-resistant bloodstream infections. Front Public Health. 2020;8: 562427.
Obara H, Saitou J, Fukuda H. Increased burden on medical resources of penicillin-resistant streptococcus pneumoniae infections: estimates using JANIS data. Jpn J Infect Prevent Control. 2015;30(3):165–73.
Saito S, Hayakawa K, Tsuzuki S, Ishikane M, Nagashima M, Mezaki K, et al. A matched case-case-control study of the impact of clinical outcomes and risk factors of patients with IMP-type carbapenemase-producing carbapenem-resistant enterobacteriaceae in Japan. Antimicrob Agents Chemother. 2021;65(3). https://doi.org/10.1128/aac.01483-20 .
Uryu K, Nishiura S, Yamamoto T, Umakosi T, Nisida M, Suzuki M, et al. Changes in medical treatment fees for pneumococcal pneumonia in our hospital before and after the introduction of DPC. J Jpn Soc Clin Pathway. 2012;14(2):113–21.
Maeda M, Oto Y, Murayama J, Minemura A, Baba T, Yoshida H, et al. A study of risk factors for antibiotic selective pressure in Carbapenem-treated patients [Japanese] [カルバペネム系薬投与患者における耐性菌選択リスク因子の検討]. J Showa Univ Soc. 2014;74(1):67–72.
Osumi T, Tanaka D, Eguchi T, Imai T, Shimizu H, Sakai M, et al. retrospective study of initiation of empirical therapies out of the antibacterial spectrum for urinary tract infections presumed to be caused by extended spectrum β-lactamases producing bacteria [Japanese] [ESBL 産生菌が原因と推定される尿路感染症にスペクトラムが外れた経験的治療を開始したことが及ぼす影響の後方視的検討]. J Jpn Soc Hosp Pharm. 2021;57(11):1215–20.
Kobayashi H, Moriyama Y, Kurosu H. Report methicillin-resistant Staphylococcus aureus hospital infection surveillance for the fiscal year 2011 [Japanese] [報告 2011年度の Methicillin-resistant Staphylococcus aureus 病院感染症サーベイランス]. Jpn J Infect Prevent Control. 2013;28(3):178–9.
Ogasahara Y, Nagasaki N, Ohno K, Harino T, Yoshida T, Maruko M. Factors causing P. aeruginosa resistance in patients treated with meropenem [Japanese] [Meropenem 投与患者における緑膿菌耐性化因子の検討]. Jpn J Infect Prevent Control. 2012;27(6):419–24.
Kosuge Y, Nagashima G, Enomoto K, Kato A, Noda M, Morishima H, et al. Clinical characteristics of multidrug-resistant bacteria infection with stroke [Japanese] [脳卒中患者における多剤耐性菌感染症の臨床検討]. Neurosurg Emerg. 2013;18(2):173–6.
Fukatsu M, Umemura T, Mizuno T, Ohguchi H, Iwatsu S, Matsumoto S, et al. Positive impacts of antimicrobial stewardship on intial eradication success and drug costs of treatment for clarithromycin-reisistant helicobacter pyloriInfections [Japanese] [薬剤師によるクラリスロマイシン耐性 Helicobacter pylori 感染に対する抗菌薬適正使用支援が初回除菌率と薬剤費に与える影響]. Jpn J Pharm Health Care Sci. 2021;47(11):609–15.
Article CAS Google Scholar
Suzuki T, Tsuchiya M, Niwa T, Watanabe T, Ohta H, Fukako A, et al. Cost effectiveness of controlling healthcare-associated spread of methicillin-resistant Staphylococcus aureus [Japanese] [当院における MRSA 感染制御活動の経済的評価に関する検討]. Jpn J Infect Prevent Control. 2015;30(2):91–6.
Goto Y, Hayashi M, Akasaki J, Ito M, Yamamoto K, Sawa A, et al. Study of risk factors for carriage of vancomycin-resistant enterococcus [Japanese] [バンコマイシン耐性腸球菌の保菌におけるリスク因子の検討]. J Jpn Soc Hospital Pharm. 2014;4:499–502.
Naylor NR, Atun R, Zhu N, Kulasabanathan K, Silva S, Chatterjee A, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7:58.
The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2023–2027. 2023. Available from: https://www.mhlw.go.jp/content/10900000/001096228.pdf .
Surveillance of antimicrobial resistance in Europe, 2020 data. 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Surveillance-antimicrobial-resistance-in-Europe-2020.pdf .
Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41(1):1–18.
The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2016–2020. 2016. Available from: https://www.mhlw.go.jp/content/10900000/0000138942.pdf .
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–74.
Thampi N, Showler A, Burry L, Bai AD, Steinberg M, Ricciuto DR, et al. Multicenter study of health care cost of patients admitted to hospital with Staphylococcus aureus bacteremia: impact of length of stay and intensity of care. Am J Infect Control. 2015;43(7):739–44.
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903–10.
Poudel AN, Zhu S, Cooper N, Little P, Tarrant C, Hickman M, et al. The economic burden of antibiotic resistance: a systematic review and meta-analysis. PLoS One. 2023;18(5): e0285170.
Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–9.
Rodenbeck M, Ayobami O, Eckmanns T, Pletz MW, Bleidorn J, Markwart R. Clinical epidemiology and case fatality due to antimicrobial resistance in Germany: a systematic review and meta-analysis, 1 January 2010 to 31 December 2021. Euro Surveill. 2023. https://doi.org/10.2807/1560-7917.ES.2023.28.20.2200672 .
MacKinnon MC, Sargeant JM, Pearl DL, Reid-Smith RJ, Carson CA, Parmley EJ, et al. Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):200.
Wozniak TM, Barnsbee L, Lee XJ, Pacella RE. Using the best available data to estimate the cost of antimicrobial resistance: a systematic review. Antimicrob Resist Infect Control. 2019;8:26.
Download references
Medical Writing, Editorial, and Other Assistance.
The authors’ heartfelt appreciation goes to Aditya K. Kumar and Anil Dasari from IQVIA India, and Karin Matsumoto and Yawen Dai from IQVIA Solutions Japan for their systematic literature review support; Annoda Kumar and Rosario Vivek from IQVIA India for providing medical writing support; and Todd D. Taylor from IQVIA Solutions Japan for providing editorial assistance; all of which was funded by Pfizer Japan Inc. in accordance with the Good Publication Practice (GPP3) guidelines. ( http://www.ismpp.org/gpp3 ).
Authorship.
All authors met all four requirements for authorship as outlined by the International Committee of Medical Journal Editors (ICMJE). All authors read and approved the final version of the manuscript.
This work, including the journal’s Rapid Service Fee, was supported by Pfizer Japan Inc.
Author information
Authors and affiliations.
Department of Infectious Diseases, School of Medicine, International University of Health and Welfare, Narita, Japan
Tetsuya Matsumoto
Japan Access & Value, Pfizer Japan Inc., Shinjuku Bunka Quint Building, 3-22-7, Yoyogi, Shibuya-ku, Tokyo, 151-8589, Japan
Akira Yuasa & Naohiro Yonemoto
Real World Evidence Solutions & HEOR, IQVIA Solutions Japan G.K., Tokyo, Japan
Hiroyuki Matsuda & Dilinuer Ainiwaer
You can also search for this author in PubMed Google Scholar
Contributions
Tetsuya Matsumoto, Akira Yuasa, and Naohiro Yonemoto conceptualized and designed the study. Hiroyuki Matsuda and Dilinuer Ainiwaer were responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Corresponding author
Correspondence to Akira Yuasa .
Ethics declarations
Conflict of interest.
The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: Tetsuya Matsumoto has been on the speakers’ bureau for Pfizer Japan Inc., KYORIN Pharmaceutical Co., Ltd., and MSD K.K. Akira Yuasa and Naohiro Yonemoto are full-time employees of Pfizer Japan Inc., and hold stocks and stock options from Pfizer Inc. Hiroyuki Matsuda and Dilinuer Ainiwaer are employees of IQVIA Solutions Japan G.K., which received funding from Pfizer Japan Inc. to undertake the research outlined in this study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: Akira Yuasa, Tetsuya Matsumoto. Systematic Literature Review on the Clinical and Economic Burdens of Antimicrobial Resistance in the Japanese Population. The 70th East Japan Chapter General Meeting of the Japanese Society of Chemotherapy, October 2023, Tokyo, Japan. Akira Yuasa, Naohiro Yonemoto, Hiroyuki Matsuda, et al. EPH230 Systematic Literature Review on the Clinical and Economic Burdens of Antimicrobial Resistance in the Japanese Population. ISPOR Europe 2023, November 2023, Copenhagen, Denmark.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 3071 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Matsumoto, T., Yuasa, A., Matsuda, H. et al. Burden of Antimicrobial Resistance in Japan: A Systematic Literature Review and Meta-Analysis. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-00960-z
Download citation
Received : 21 December 2023
Accepted : 11 March 2024
Published : 25 April 2024
DOI : https://doi.org/10.1007/s40121-024-00960-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antimicrobial resistance
- Meta-Analysis
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

ADHD Diagnosis and Treatment in Children and Adolescents
Comparative Effectiveness Review, No. 267
Investigators: Bradley S. Peterson , M.D., Joey Trampush , Ph.D., Margaret Maglione , M.P.P., Maria Bolshakova , B.S., Ph.D., Morah Brown , M.P.H., Mary Rozelle , P.A., Aneesa Motala , B.A., Sachi Yagyu , M.L.S., Jeremy Miles , Ph.D., Sheila Pakdaman , Ph.D., Mario Gastelum , M.P.H., Bich Thuy (Becky) Nguyen , M.P.H., Erin Tokutomi , M.P.H., Esther Lee , M.P.H. candidate, Jerusalem Z. Belay , M.P.H., Coleman Schaefer , M.P.H., Benjamin Coughlin , M.P.H., Karin Celosse , Psy.D, M.S.C.P., M.P.H., Sreya Molakalapalli , M.P.H., Brittany Shaw , M.P.H. candidate, Tanzina Sazmin , M.P.H., M.B.B.S., Anne N. Onyekwuluje , M.D., M.P.H., Danica Tolentino , M.S., and Susanne Hempel , Ph.D.
- Copyright and Permissions
The systematic review assessed evidence on the diagnosis, treatment, and monitoring of attention deficit hyperactivity disorder ( ADHD ) in children and adolescents to inform a planned update of the American Academy of Pediatrics ( AAP ) guidelines.
Data sources:
We searched PubMed ® , Embase ® , PsycINFO ® , ERIC, clinicaltrials.gov , and prior reviews for primary studies published since 1980. The report includes studies published to June 15, 2023.
Review methods:
The review followed a detailed protocol and was supported by a Technical Expert Panel. Citation screening was facilitated by machine learning; two independent reviewers screened full text citations for eligibility. We abstracted data using software designed for systematic reviews. Risk of bias assessments focused on key sources of bias for diagnostic and intervention studies. We conducted strength of evidence ( SoE ) and applicability assessments for key outcomes. The protocol for the review has been registered in PROSPERO (CRD42022312656).
Searches identified 23,139 citations, and 7,534 were obtained as full text. We included 550 studies reported in 1,097 publications (231 studies addressed diagnosis, 312 studies addressed treatment, and 10 studies addressed monitoring). Diagnostic studies reported on the diagnostic performance of numerous parental ratings, teacher rating scales, teen/child self-reports, clinician tools, neuropsychological tests, EEG approaches, imaging, and biomarkers. Multiple approaches showed promising diagnostic performance (e.g., using parental rating scales), although estimates of performance varied considerably across studies and the SoE was generally low. Few studies reported estimates for children under the age of 7. Treatment studies evaluated combined pharmacological and behavior approaches, medication approved by the Food and Drug Administration, other pharmacologic treatment, psychological/behavioral approaches, cognitive training, neurofeedback, neurostimulation, physical exercise, nutrition and supplements, integrative medicine, parent support, school interventions, and provider or model-of-care interventions. Medication treatment was associated with improved broadband scale scores and ADHD symptoms (high SoE) as well as function (moderate SoE), but also appetite suppression and adverse events (high SoE). Psychosocial interventions also showed improvement in ADHD symptoms based on moderate SoE. Few studies have evaluated combinations of pharmacological and youth-directed psychosocial interventions, and we did not find combinations that were systematically superior to monotherapy (low SoE). Published monitoring approaches for ADHD were limited and the SoE is insufficient.
Conclusion:
Many diagnostic tools are available to aid the diagnosis of ADHD , but few monitoring strategies have been studied. Medication therapies remain important treatment options, although with a risk of side effects, as the evidence base for psychosocial therapies strengthens and other nondrug treatment approaches emerge.
- Collapse All
- Acknowledgments
- Key Informants
- Technical Expert Panel
- Peer Reviewers
- Main Points
- Background and Purpose
- Strengths and Limitations
- Implications and Conclusions
- 1.1. Background
- 1.2. Purpose and Scope of the Systematic Review
- 2.1. Review Approach
- 2.2. Study Selection
- 2.3. Data Extraction
- 2.4. Risk of Bias Assessment
- 2.5. Data Synthesis and Analysis
- 2.6. Grading the Body of Evidence
- 2.7. Peer Review and Public Commentary
- 3. Results: Description of Included Evidence
- 4.1. KQ1, ADHD Diagnosis Key Points
- 4.2. KQ1, ADHD Diagnosis Summary of Findings
- 4.3. Summary ADHD Diagnosis by Tests for All Age Groups
- 4.4. KQ1a. What is the comparative diagnostic accuracy of approaches that can be used in the primary care practice setting or by specialists to diagnose ADHD among individuals younger than 7 years of age?
- 4.5. KQ1b. What is the comparative diagnostic accuracy of EEG, imaging, or approaches assessing executive function that can be used in the primary care practice setting or by specialists to diagnose ADHD among individuals aged 7 through 17?
- 4.6. KQ1c. For both populations, how does the comparative diagnostic accuracy of these approaches vary by clinical setting, including primary care or specialty clinic, or patient subgroup, including age, sex, or other risk factors associated with ADHD?
- 4.7. KQ1d. What are the adverse effects associated with being labeled correctly or incorrectly as having ADHD?
- 4.8. Summary of Findings. KQ1a–d
- 5.1. KQ2, ADHD Treatment Key Points
- 5.2. KQ2, ADHD Treatment Results
- 5.3. Effects by Intervention
- 5.4. KQ2a. How do these outcomes vary by presentation (inattentive, hyperactive/impulsive, and combined) or other co-occurring conditions?
- 5.6. KQ2b. What is the risk of diversion of pharmacologic treatment?
- 5.7. Summary of Findings KQ2a and KQ2b
- 6.1. Key Question (KQ) 3 ADHD Monitoring Key Points
- 6.2. KQ 3 ADHD Monitoring Summary of Findings
- Findings in Relation to the Decisional Dilemma(s)
- Findings in Relation to Existing Research Syntheses and Practice Guidelines
- Implications
- Applicability
- Abbreviations and Acronyms
- Appendix A. Methods
- Appendix B. List of Excluded and Background Studies
- Appendix C. Evidence Tables
- Appendix D. Critical Appraisal and Applicability Tables
- Appendix E. List of Included Studies
- Appendix F. Expert Guidance and Review
- Appendix G. PCORI Checklist
Suggested citation:
Peterson BS, Trampush J, Maglione M, Bolshakova M, Brown M, Rozelle M, Motala A, Yagyu S, Miles J, Pakdaman S, Gastelum M, Nguyen BT, Tokutomi E, Lee E, Belay JZ, Schaefer C, Coughlin B, Celosse K, Molakalapalli S, Shaw B, Sazmin T, Onyekwuluje AN, Tolentino D, Hempel S. ADHD Diagnosis and Treatment in Children and Adolescents. Comparative Effectiveness Review No. 267. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 75Q80120D00009.) AHRQ Publication No. 24-EHC003. PCORI Publication No. 2023-SR-03. Rockville, MD: Agency for Healthcare Research and Quality; March 2024. DOI: https://doi.org/ 10.23970/AHRQEPCCER267 . Posted final reports are located on the Effective Health Care Program search page .
This report is based on research conducted by the Southern California Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 75Q80120D00009). The Patient-Centered Outcomes Research Institute ® (PCORI ® ) funded the report (PCORI ® Publication No. 2023-SR-03). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ or PCORI ® , its Board of Governors or Methodology Committee. Therefore, no statement in this report should be construed as an official position of PCORI ® , AHRQ, or the U.S. Department of Health and Human Services.
None of the investigators have any affiliations or financial involvement that conflicts with the material presented in this report.
The information in this report is intended to help healthcare decision makers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of healthcare services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information, i.e., in the context of available resources and circumstances presented by individual patients.
This report is made available to the public under the terms of a licensing agreement between the author and the Agency for Healthcare Research and Quality. Most AHRQ documents are publicly available to use for noncommercial purposes (research, clinical or patient education, quality improvement projects) in the United States, and do not need specific permission to be reprinted and used unless they contain material that is copyrighted by others. Specific written permission is needed for commercial use (reprinting for sale, incorporation into software, incorporation into for-profit training courses) or for use outside of the U.S. If organizational policies require permission to adapt or use these materials, AHRQ will provide such permission in writing.
PCORI ® , AHRQ, or U.S. Department of Health and Human Services endorsement of any derivative products that may be developed from this report, such as clinical practice guidelines, other quality enhancement tools, or reimbursement or coverage policies, may not be stated or implied.
A representative from AHRQ served as a Contracting Officer’s Representative and reviewed the contract deliverables for adherence to contract requirements and quality. AHRQ did not directly participate in the literature search, determination of study eligibility criteria, data analysis, interpretation of data, or preparation or drafting of this report.
AHRQ and PCORI ® appreciate appropriate acknowledgment and citation of their work. Suggested language for acknowledgment: This work was based on an evidence report, ADHD Diagnosis and Treatment in Children and Adolescents, by the Evidence-based Practice Center Program at the Agency for Healthcare Research and Quality (AHRQ) and funded by PCORI ® .
- Cite this Page Peterson BS, Trampush J, Maglione M, et al. ADHD Diagnosis and Treatment in Children and Adolescents [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2024 Mar. (Comparative Effectiveness Review, No. 267.) doi: 10.23970/AHRQEPCCER267
- PDF version of this title (12M)
- Disable Glossary Links
Other titles in this collection
- AHRQ Comparative Effectiveness Reviews
Recent Activity
- ADHD Diagnosis and Treatment in Children and Adolescents ADHD Diagnosis and Treatment in Children and Adolescents
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
It's common to confuse systematic and literature reviews because both are used to provide a summary of the existent literature or research on a specific topic. Regardless of this commonality, both types of review vary significantly. The following table provides a detailed explanation as well as the differences between systematic and ...
Systematic Review vs. Literature Review. It is common to confuse systematic and literature reviews as both are used to provide a summary of the existent literature or research on a specific topic. Even with this common ground, both types vary significantly. Please review the following chart (and its corresponding poster linked below) for the ...
Learn the difference between systematic and literature reviews, their purposes, components, and examples. A systematic review is a focused and transparent search for evidence-based research, while a literature review is a general overview of a topic.
Introduction. A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
A systematic review follows explicit methodology to answer a well-defined research question by searching the literature comprehensively, evaluating the quantity and quality of research evidence rigorously, and analyzing the evidence to synthesize an answer to the research question. The evidence gathered in systematic reviews can be qualitative ...
Learn the difference between systematic and literature reviews, their methods, purposes and attributes. Covidence is a web-based tool that helps you conduct systematic reviews efficiently and collaboratively.
Learn what a systematic review is, how it differs from other types of reviews, and how to conduct one. See an example of a systematic review on probiotics and eczema, and compare it with a literature review and a meta-analysis.
Rapid review. Assessment of what is already known about a policy or practice issue, by using systematic review methods to search and critically appraise existing research. Completeness of searching determined by time constraints. Time-limited formal quality assessment. Typically narrative and tabular.
A systematic review collects secondary data, and is a synthesis of all available, relevant evidence which brings together all existing primary studies for review (Cochrane 2016).A systematic review differs from other types of literature review in several major ways.
Table 1: Literature reviews vs systematic reviews. Literature review Methodological stage Systematic review Introduces context and current thinking, often without a specific question, is general and covers several aspects of a topic. Focus of review Uses a precise question to produce evidence to underpin a piece of research.
The graphical output of meta-analysis is a forest plot which provides information on individual studies and the pooled effect. Systematic reviews of literature can be undertaken for all types of questions, and all types of study designs. This article highlights the key features of systematic reviews, and is designed to help readers understand ...
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
A systematic review, however, is a comprehensive literature review conducted to answer a specific research question. Authors of a systematic review aim to find, code, appraise, and synthesize all of the previous research on their question in an unbiased and well-documented manner.
Literature Review: Systematic Review: Definition. Qualitatively summarizes evidence on a topic using informal or subjective methods to collect and interpret studies: High-level overview of primary research on a focused question that identifies, selects, synthesizes, and appraises all high quality research evidence to that question ...
Systematic literature reviews (SRs) are a way of synthesising scientific evidence to answer a particular research question in a way that is transparent and reproducible, while seeking to include all published evidence on the topic and appraising the quality of th is evidence. SRs have become a major methodology
A literature review is a systematic way of collecting and synthesizing previous research (Snyder, 2019).An integrative literature review provides an integration of the current state of knowledge as a way of generating new knowledge (Holton, 2002).HRDR is labeling Integrative Literature Review as one of the journal's four non-empirical research article types as in theory and conceptual ...
They aim to summarise the best available evidence on a particular research topic. The main differences between traditional reviews and systematic reviews are summarised below in terms of the following characteristics: Authors, Study protocol, Research question, Search strategy, Sources of literature, Selection criteria, Critical appraisal ...
Literature Review: it is a product and a process. As a product, it is a carefully written examination, interpretation, evaluation, and synthesis of the published literature related to your topic.It focuses on what is known about your topic and what methodologies, models, theories, and concepts have been applied to it by others.. The process is what is involved in conducting a review of the ...
Acommon type of submission at any Journal is a review of the published information related to a topic.These are often returned to their authors without review, usually because they are literature reviews rather than systematic reviews. There is a big difference between the two (Table 1).Here, we summarise the differences, how they are used in academic work, and why a general literature review ...
Apart from systematic literature review, some other common types of literature review are1: The most commonly used form of review, however, is the systematic literature review. Compared to the other types of literature reviews described above, this one requires a more rigorous and well-defined approach. The systematic literature review can be ...
While quality assessment of individual studies may be mentioned in a literature review, it is typically less rigorous and systematic compared to a systematic review. Quality assessment of included studies is a critical component, involving the evaluation of study design, risk of bias, methodological rigor, and internal validity.
Unlock the secrets of academic writing with our guide to the key differences between a literature review and a research paper! 📚 Dive into the world of scholarly exploration as we break down how a literature review illuminates existing knowledge, identifies gaps, and sets the stage for further research. 🌐 Then, gear up for the adventure of crafting a research paper, where you become the ...
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
A systematic literature review design was used in this study following the guidelines of Paul and Criado (Citation 2020). There are various types of systematic literature reviews, including structured reviews, framework-based reviews, bibliometric reviews, and meta-analysis reviews. Among these review methods, we preferred the structured review ...
This systematic review was carried out in accordance with the items of Preferred Reports for Systematic Reviews and Protocol Meta-Analysis (PRISMA-P) 10 and assessing the methodological quality of ...
Systematic Literature Review on the Clinical and Economic Burdens of Antimicrobial Resistance in the Japanese Population. The 70th East Japan Chapter General Meeting of the Japanese Society of Chemotherapy, October 2023, Tokyo, Japan. Akira Yuasa, Naohiro Yonemoto, Hiroyuki Matsuda, et al. EPH230 Systematic Literature Review on the Clinical and ...
We abstracted data using software designed for systematic reviews. Risk of bias assessments focused on key sources of bias for diagnostic and intervention studies. We conducted strength of evidence and applicability assessments for key outcomes. The protocol for the review has been registered in PROSPERO (CRD42022312656).
Only clinical trials, meta-analysis, randomized controlled trials, reviews, and systematic reviews written in English were included. During the literature review, particular consideration was given to works published in the previous 5 years (January 2018 to December 2022).