Advertisement

- Previous Issue
- Previous Article
- Next Article

The Peer Review Imperative
Threats to peer review, public trust in science and medicine, peer review matters: research quality and the public trust.
Michael M. Todd, M.D., served as Handling Editor for this article.
This article has a related Infographic on p. 17A.
Accepted for publication October 13, 2020.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
- Search Site
Evan D. Kharasch , Michael J. Avram , J. David Clark , Andrew J. Davidson , Timothy T. Houle , Jerrold H. Levy , Martin J. London , Daniel I. Sessler , Laszlo Vutskits; Peer Review Matters: Research Quality and the Public Trust. Anesthesiology 2021; 134:1–6 doi: https://doi.org/10.1097/ALN.0000000000003608
Download citation file:
- Ris (Zotero)
- Reference Manager
“Peer review grounds the public trust in the scientific and medical research enterprise…”

Image: Adobe Stock.
In an era of evidence-based medicine, peer review is an engine and protector of that evidence. Such evidence, vetted by and surviving the peer review process, serves to inform clinical decision-making, providing practitioners with the information to make diagnostic and therapeutic decisions. Unfortunately, there is recent and growing pressure to prioritize the speed of research dissemination, often at the expense of careful peer review. It is timely to remind readers and the public of the value brought by peer review, its benefits to patients, how much the public trust in science and medicine rests upon peer review, and how these have become vulnerable.
Peer review has been the foundation of scholarly publishing and scientific communication since the 1665 publication of the Philosophical Transactions of the Royal Society. The benefits and advantages of peer review in scientific research, and particularly medical research, are manifold and manifest. 1 Journals, editors, and peer reviewers hold serious responsibility as stewards of valid information, with accountability to the scientific community and an obligation to maintain the public trust. Anesthesiology states its aspiration and its responsibility on the cover of every issue: Trusted Evidence. Quality peer review (more specifically, closed or single-blind peer review, in which the identity of reviewers is confidential) is a foundational tenet of Anesthesiology.
Peer review grounds the public trust in the scientific and medical research enterprise, as well as the substantial public investment in scientific research. Peer review affords patients some degree of comfort in placing their trust in practitioners, knowing that they should be informed by the best possible, vetted evidence.
Quality peer review enriches and safeguards the scientific content, transparency, comprehensibility, and scientific integrity of published articles. It can enhance published research importance, originality, authenticity, scientific validity, adherence to experimental rigor, and correctness of results and interpretations and can identify errors in research execution. Peer review can help authors improve reporting quality, presentation clarity, and transparency, thereby enhancing comprehension and potential use by clinicians and scientists. Careful scrutiny can identify whether research has appropriate ethical principles, regulatory approvals, compliance, and equitable inclusion of both sexes. Peer review should consider the appropriateness of authorship and can detect duplicate publication, fabrication, falsification, plagiarism, and other misconduct.
Peer review should serve as a tempering factor on overenthusiastic authors and overstated conclusions, unwarranted extrapolations, conflation of association with causality, unsupported clinical recommendations, and spin. Spin is a well known, unfortunately common, and often insidious bias in the presentation and interpretation of results that seeks to convince readers that the beneficial effect of an experimental treatment exceeds what has actually been found or that minimizes untoward effects. 2–4
Manuscripts often change substantially between the initial submission and the revised and improved published version. Improvement during the peer review process is not apparent to readers, who only see the final, published article, but is well known to authors, reviewers, and editors. Peer review is a defining difference in an era of proliferating predatory journals and other forms of research dissemination. Anesthesiology reviewers and editors devote considerable effort in service to helping authors improve their scientific communications, whether published in this journal or if ultimately elsewhere.
In the domain of clinical research, peer review does not change the scientific premise of an investigation, the hypothesis, or the study design, although it frequently improves their communication. Peer review does not change clinical research data, although it often corrects, enhances, or strengthens the statistical analysis of those data and can markedly improve their presentation and clarity. More importantly, peer review can assess, correct, and improve the interpretation, meaning, importance, and communication of research results—and importantly, confirm that conclusions emanate strictly from those results. Peer review may occasionally fundamentally revise or even reverse clinical research interpretations and recommendations. Each of these many functions enhances reader understanding and should ultimately improve patient care.
Peer review is not a guarantee of truth, and it can be imperfect. Medical history provides many examples of peer-reviewed research that was later found to be incorrect, typically through error or occasionally from misconduct. However, peer review certainly was and remains an essential initial check and quality control that has weeded out, or corrected before publication, innumerable reports of research of insufficient quality or veracity that otherwise would have been published and thereby become publicly accessible. Additionally, science should be “self-correcting,” and peer review is one of the most important factors responsible for such correction. Peer review remains an element by which medical science achieves the “self-correction” that drives progress.
Quality peer review does take time. So also do the initial preparation of manuscripts and the modifications made by authors in response to peer review. Anesthesiology endeavors to provide both quality and timely peer review. Our time to first decision averages only 16 days.
The increasing emphasis on fast research dissemination, often absent quality peer review, comes mostly but not exclusively because of the immediacy of the internet and broader media and societal trends. In an era in which the companies whose major product is the immediacy of information are the economic leaders (Facebook, Twitter, Google, and Apple), it is unsurprising that the immediacy of information is challenging that of quality as the value proposition in the research marketplace. Nevertheless, fast is not synonymous with good. We believe that sacrificing quality on the altar of speed is unwise, benefits no one (except perhaps authors), and may ultimately diminish trust in medical research and possibly even worsen clinical care.
Another recent societal problem is the growing spillover of political and media communication trends into scientific communication. Almost half of Americans believe that science researchers overstate the implications of their research, and three in four think “the biggest problem with news about scientific research findings is the way news reporters cover it.” 5 Scientific conclusions may be perverted through internet-based campaigns of disinformation and misinformation and dissemination of misleading and biased information. 6 This threatens the public trust in the scientific enterprise and scientific knowledge. 7 Social media has made science and health vulnerable to strategic manipulation. 7 , 8 It is also “leaving peer-reviewed communication behind as some scientists begin to worry less about their citation index (which takes years to develop) and more about their Twitter response (measurable in hours).” 8 Peer-reviewed journals cannot reverse these trends, but they can at least ensure that scientific conclusions when presented are correct and clearly stated.
In addition to the premium on dissemination speed versus peer review quality, a new variant of rapid clinical research dissemination has emerged that abrogates peer review entirely: preprints. Preprints are research reports that are posted by authors in a publicly accessible online repository in place of or before publication in a peer-reviewed scholarly journal. The preprint concept is decades old, rooted in physics and mathematics, in which authors traditionally sent their hand- or typewritten manuscript draft to a few colleagues for feedback before submitting it to a journal for publication. With the advent of the internet, this process was replaced by preprint servers and public posting. With the creation of a preprint server for biology and the life sciences (bioRxiv.org), the posting of unreviewed manuscripts by basic biomedical scientists has exploded in popularity and practice. Next came the creation of medRxiv.org, a publicly accessible preprint server for disseminating unpublished and unreviewed clinical research results in their “preliminary form” 9 and more so a call for research funders to require mandatory posting of their grantees’ research reports first on preprint servers before peer-reviewed publication. 10 Lack of peer review is the hallmark of preprints.
The main arguments offered by proponents of preprints are the free and near-immediate access to research results, claimed acceleration of the progress of research by immediate dissemination without peer review, and the assumption that articles will be improved by feedback from a wider group of readers alongside formal review by a few experts. Specifically claimed advantages of preprints are that they bypass the peer review process that adversely delays the dissemination of research results and “lifesaving cures” and “the months-long turnaround time of the publishing process and share findings with the community more quickly.” 11 In addition it is claimed that preprints address “researchers recently becoming vocally frustrated about the lengthy process of distributing research through the conventional pipelines, numerous laments decrying increasingly impractical demands of journals and reviewers, complicated dynamics at play from both authors and publishers that can affect time to press” and enable “sharing papers online before (or instead of) publication in peer-reviewed journals.” 11
Preprints for clinical research have been justifiably criticized. 2 , 12–15 Most importantly, medical preprints lack safeguards afforded by peer review and increase the possibility of disseminating wrong or incorrectly interpreted results. Related concerns are that preprints are unnecessary for and potentially harmful to scientific progress and a significant threat with potential consequence to patient health and safety. Preprint server proponents “assume that most preprints would subsequently be peer reviewed,” 10 possibly before or after formal publication (if published), thus enabling correction or improvement (before or after publication). However, it is estimated that careful peer review of a manuscript takes 5 to 6 h. 1 , 16 It seems highly unlikely that busy scientists will surf the web in search of preprints on which to spend half a day providing concerted informative peer review.
Preprint enthusiasts claim that peer review after posting will provide scholarly input, facilitate preprint improvement, and enhance research quality. In fact, such peer review has been scant with biologic preprints, and it seems naïve to expect it with medical preprints. In reality, most preprints receive few comments, even fewer formal reviews, and many comments that are “counted” to support the notion that preprints do undergo peer review actually come through social media; a tweet is hardly a substantive review. The idea that comments on servers will replace quality peer review is not happening now and seems unlikely to transpire. Moreover, a survey found that the lack of peer review was an important reason why authors deliberately choose to post via preprint. 17 Additionally, postdissemination peer review takes longer than traditional prepublication peer review, and there remains concern by authors who do value peer review about the quality of the post-preprint peer review process and the quality of posted preprints. 17
Preprint server proponents state “the work in question would be available to interested readers while these processes (peer review) take place, which is more or less what happens in physics today.” 10 The lives of patients are different than the lives of subatomic particles. Preprints deliberately “decouples the dissemination of manuscripts from the much slower process of evaluation and certification.” 10 However, it is exactly that coupling that validates clinical research, benefits patients, improves health, and engenders public trust.
The potential for free and unfettered distribution of raw, unvetted, and potentially incorrect information to be consumed by clinicians and patients cannot be called a medical advance. Use of such information by news outlets and online web services to promote “new” and “latest” research further misinforms the public and patients and is a disservice.
Relegating peer review to the realm of option and afterthought is not in the interest of research quality and integrity or of patients and public health. There is no apparent value in abrogating peer review of clinical research and all its many attendant benefits in ensuring the quality of clinical research available to practitioners and patients. Practitioners and patients have historically not seen the unreviewed manuscript submissions that eventually become revised peer-reviewed publications. Doing so now, given the sizable fraction of clinical research manuscripts that are rejected for publication and the substantial changes in most that are published, by providing the public with unreviewed preprints seems to carry considerable risk.
An additional problem is that the same research report can be posted on several preprint servers or websites or multiple versions may exist on the same preprint site. Various versions may be the same or different, and the final peer-reviewed published article (if it ever exists) may bear little semblance to the various posted versions, which remain freely available. Which version is correct? Availability of various differing reports of the same research risks competing or incorrect information and can only generate confusion. Scientific publishing decades ago banned publication of the same research in multiple journals owing to concerns about data integrity and inappropriate reuse. Restarting this now, via preprints, seems unwise—especially in medicine.
The public cannot and should not be expected to differentiate between posting and peer-reviewed publication. Unfortunately, and worse, even some practitioners do not understand the difference. Posting is often referred to erroneously as publication. Indeed, even the world’s most prestigious scientific journals refer to posting as publication. 18 Such conflation blurs the validity of information. That peer-reviewed publications and preprints both receive digital object identifiers further blurs their distinction and may give the latter more apparent credibility in the eyes of the lay public. The preprint community (servers and scientists) continues to claim simultaneously that preprints are and are not publications, depending on how such claims meet their proclivities. Although the bioRxiv server contains the disclaimer “readers should be aware that articles on bioRxiv have not been finalized by authors, might contain errors, and report information that has not yet been accepted or endorsed in any way by the scientific or medical community” on a web page, 19 it is not on the preprint itself for readers to see (perhaps this disclaimer, and the one below, should appear on the cover page of every preprint and as a footnote on every page). Fortunately, the medRxiv home page ( http://www.medrxiv.org ) states the following disclaimer: “Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information.” Then why bother?
The popularity of preprints in the basic science world has exploded in the last 5 yr, with the number of documents posted to preprint servers increasing exponentially. 20 While acknowledging the noble reasons given by preprint servers and authors for the dissemination of research by posting, three other apparent reasons are less noble. The first is competition for research funding. Major research funders ( e.g. , the National Institutes of Health) do not allow citation of unpublished manuscripts in grant applications but do allow citation of preprints. 21 , 22 The second is the preoccupation of authors with the speed of availability. There is a growing (and disappointing) trend of authors perceiving a need to claim priority (“we are the first to report…”), grounded perhaps on fear of being “scooped.” The third is the pursuit of academic promotion, which is based largely on the number of peer-reviewed publications listed on a curriculum vitae . We now see faculty listing preprints in the peer-reviewed research publications section of their curriculum vitae. All these drivers (priority, science advancement, reputational reward, and financial return) 7 are investigator centric. They are neither quality-centric nor patient-centric.
Who benefits if clinical research quality is sacrificed at the altar of speed? Certainly, it is not patients, public health, or the public trust in science, medicine, and the research enterprise. Enthusiasm for preprints seems to be emanating mostly from investigators, presumably because of academic or other incentives, 23 including the desire for prominence and further funding. Is this why we do medical research? Should we be investigator- or patient-centric?
Little in the argumentation espoused by proponents of clinical preprints attends to their benefit to patients. Indeed, posted preprints without all the scrutiny and benefits of peer review may lack quality and validity and may report flawed data and conclusions, which may hurt patients. 17 , 23 As stated previously, “clinical studies of poor quality can harm patients who might start or stop therapy in response to faulty data, whereas little short-term harm would be expected from an unreviewed astronomy study.” 12
The importance of peer review in clinical research and the downside of its absence in posted preprints is illuminated by the COVID-19 pandemic. As of this date (October 1, 2020), there are 9,222 unreviewed COVID-19 SARS–CoV-2 preprints posted: 7,257 on medRxiv and 1,965 on bioRxiv. 24 To date, 33 COVID-19 articles have been retracted (0.37%), and 5 others have been temporarily retracted or have expressions of concern. 25 Of the 33 retractions, 11 (33%) were posted on an Rxiv server. The overall retraction rate in the general peer-reviewed literature is 0.04%. 26
Based upon one of the unreviewed COVID-19 medical preprints, 27 the Commissioner of the U.S. Food and Drug Administration (the government agency entrusted more than any other to protect public health) and the President of the United States announced that convalescent plasma from COVID-19 survivors was “safe and very effective” and had been “proven to reduce mortality by 35%.” 28 Although the Commissioner later, after scientific uproar over that misinformation, “corrected” his comment in a tweet (a back page retraction to a front page headline), 29 the preprint was used to justify a Food and Drug Administration decision to issue an emergency use authorization for convalescent plasma to treat severe COVID-19. Would these errors have been prevented by peer review? We will never know.
Even if priority in clinical (and basic) research is valued, compared to the unquestionable value of quality, clinical preprints have questionable necessity in establishing precedence in contemporary times. Clinical trials registration, which makes fully public the existence of all such research, establishes both who is doing what and when. Some investigators may even publish their entire clinical protocol, to further make their studies known and by whom and when.
For hundreds of years, patent medicines (exotic concoctions of substances, often addicting and sometimes toxic) were claimed to prevent or cure a panoply of illnesses, without any evidence of effectiveness or safety or warning of potential harm. These medical elixirs, the magic potions of snake oil salesmen and charlatans, were heavily advertised and promoted to ailing, sometimes desperate, and thoroughly unsuspecting citizens—all without any oversight, regulation, quality control, or peer review. It was not until the 20th century that medical peer review and the requirement for evidence of effectiveness and safety reigned in the “Wild West” and launched the modern era of medicine, yielding the scientific discovery, progress, and improvement in human health seen today. This era rests on the bedrock of peer review, the quality ideal, and the evidence that constitutes the foundation for evidence-based medicine.
Will clinical preprints become the patent medicines of the new millennium? Do they portend the unrestricted and unregulated spillage of anything claimed as research, by anyone, and absent the quality control afforded by peer review? Like the patent medicines of a bygone era, which were heavily promoted by the newly developed advertising industry, will “posted” clinical research become fodder for the medical advertising industry and media at large, pushing who knows what information and claims on practitioners and a public already deluged with endless promotions and claims with which they cannot keep up or verify? An unsuspecting public is incapable of differentiating between the “posting” of any research observation by anyone with access to a computer and proper scholarly “publication” of peer-reviewed results and conclusions. This is particularly true of vulnerable patients with severe and/or incurable diseases, who may grasp at anything. Moreover, continuous claims of “breakthroughs” and “proven treatments” based on preprints, followed by backpedaling after challenges and outcries, further reduces public confidence in the scientific endeavor as a whole. This can create the perception that clinical science is unreliable and might be a matter of turf wars and politics instead of reliable valid evidence.
Over the past century and throughout the world, legislation has been passed and government agencies have been created to protect the public and maintain their trust in the medicines they take. Few would advocate dismantling the protections against patent medicines. Why now consider dismantling the peer review process in clinical research?
In 2019, the editors of several journals expressed a well articulated principle that they will not accept clinical research manuscripts that had been previously posted to a preprint server. 30 Their rationale was that the benefit of preprint servers in clinical research did not outweigh the potential harm to patients and scientific integrity. Major specific concerns included: “1) Preprints may be perceived by some (and used by less scrupulous investigators) as evidence even though the studies have not gone through peer review and the public may not be able to discern an unreviewed preprint from a seminal article in a leading journal; 2) It seems unlikely that the kind of prepublication dialogue that has taken place in other academic disciplines (e.g. mathematics and physics) will take place in medicine or surgery because the incentives are very different; 3) Preprints may lead to multiple competing, and perhaps even conflicting, versions of the ‘same’ content being available online at the same time, which can cause (at least) confusion and (at most) grave harm; and 4) For the vast majority of medical diagnoses, a few months of review of a study’s findings do not make a difference; the pace of discovery and dissemination generally is adequate.” These editors’ concerns and approach merit consideration if not more widespread adoption.
The potential for practitioner and public confusion regarding the difference between unregulated preprints and peer-reviewed publication is substantial. Indeed, the posting of preprints is often incorrectly termed “publication.” Peer-reviewed publications versus posted “publications” will soon become a difference without a distinction. Moreover, authors cannot have it both ways. They cannot claim a preprint as a publication for purposes of a grant (and now in some universities potentially for purposes of a degree, appointment, and/or promotion), yet claim it is not a publication for the purposes of submission to a peer-reviewed journal that does not allow prior publication. More importantly, the peer review imperative in clinical research and the role it plays in research quality, the evidence base, and patient care, constitutes an obligation to patient safety that cannot and should not be abrogated.
Peer review, clinical research quality, and the public trust in clinical research all now face an unprecedented assault. Quality peer review is a foundational tenet of Anesthesiology and underlies the Trusted Evidence we publish. Quality, timely, and unpressured peer review will continue to be a hallmark of Anesthesiology , in service to readers, patients, and the public trust.
Acknowledgments
We thank Ryan Walther, Managing Editor, and Vicki Tedeschi, Director of Digital Communications, for their valuable insights.
Competing Interests
Dr. Clark has a consulting agreement with Teikoku Pharma USA (San Jose, California). Dr. Levy reports being on Advisory and Steering Committees for Instrumentation Laboratory (Bedford, Massachusetts), Merck & Co. (Kenilworth, New Jersey), and Octapharma (Lachen, Switzerland). Dr. London reports financial relationships with Wolters Kluwer UptoDate (Philadelphia, Pennsylvania) and Springer (journal honorarium; New York, New York). The remaining authors declare no competing interests.
Citing articles via
Most viewed, email alerts, related articles, social media, affiliations.
- ASA Practice Parameters
- Online First
- Author Resource Center
- About the Journal
- Editorial Board
- Rights & Permissions
- Online ISSN 1528-1175
- Print ISSN 0003-3022
- Anesthesiology
- ASA Monitor

- Terms & Conditions Privacy Policy
- Manage Cookie Preferences
- © Copyright 2024 American Society of Anesthesiologists
This Feature Is Available To Subscribers Only
Sign In or Create an Account

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Understanding Peer Review in Science

Peer review is an essential element of the scientific publishing process that helps ensure that research articles are evaluated, critiqued, and improved before release into the academic community. Take a look at the significance of peer review in scientific publications, the typical steps of the process, and and how to approach peer review if you are asked to assess a manuscript.
What Is Peer Review?
Peer review is the evaluation of work by peers, who are people with comparable experience and competency. Peers assess each others’ work in educational settings, in professional settings, and in the publishing world. The goal of peer review is improving quality, defining and maintaining standards, and helping people learn from one another.
In the context of scientific publication, peer review helps editors determine which submissions merit publication and improves the quality of manuscripts prior to their final release.
Types of Peer Review for Manuscripts
There are three main types of peer review:
- Single-blind review: The reviewers know the identities of the authors, but the authors do not know the identities of the reviewers.
- Double-blind review: Both the authors and reviewers remain anonymous to each other.
- Open peer review: The identities of both the authors and reviewers are disclosed, promoting transparency and collaboration.
There are advantages and disadvantages of each method. Anonymous reviews reduce bias but reduce collaboration, while open reviews are more transparent, but increase bias.
Key Elements of Peer Review
Proper selection of a peer group improves the outcome of the process:
- Expertise : Reviewers should possess adequate knowledge and experience in the relevant field to provide constructive feedback.
- Objectivity : Reviewers assess the manuscript impartially and without personal bias.
- Confidentiality : The peer review process maintains confidentiality to protect intellectual property and encourage honest feedback.
- Timeliness : Reviewers provide feedback within a reasonable timeframe to ensure timely publication.
Steps of the Peer Review Process
The typical peer review process for scientific publications involves the following steps:
- Submission : Authors submit their manuscript to a journal that aligns with their research topic.
- Editorial assessment : The journal editor examines the manuscript and determines whether or not it is suitable for publication. If it is not, the manuscript is rejected.
- Peer review : If it is suitable, the editor sends the article to peer reviewers who are experts in the relevant field.
- Reviewer feedback : Reviewers provide feedback, critique, and suggestions for improvement.
- Revision and resubmission : Authors address the feedback and make necessary revisions before resubmitting the manuscript.
- Final decision : The editor makes a final decision on whether to accept or reject the manuscript based on the revised version and reviewer comments.
- Publication : If accepted, the manuscript undergoes copyediting and formatting before being published in the journal.
Pros and Cons
While the goal of peer review is improving the quality of published research, the process isn’t without its drawbacks.
- Quality assurance : Peer review helps ensure the quality and reliability of published research.
- Error detection : The process identifies errors and flaws that the authors may have overlooked.
- Credibility : The scientific community generally considers peer-reviewed articles to be more credible.
- Professional development : Reviewers can learn from the work of others and enhance their own knowledge and understanding.
- Time-consuming : The peer review process can be lengthy, delaying the publication of potentially valuable research.
- Bias : Personal biases of reviews impact their evaluation of the manuscript.
- Inconsistency : Different reviewers may provide conflicting feedback, making it challenging for authors to address all concerns.
- Limited effectiveness : Peer review does not always detect significant errors or misconduct.
- Poaching : Some reviewers take an idea from a submission and gain publication before the authors of the original research.
Steps for Conducting Peer Review of an Article
Generally, an editor provides guidance when you are asked to provide peer review of a manuscript. Here are typical steps of the process.
- Accept the right assignment: Accept invitations to review articles that align with your area of expertise to ensure you can provide well-informed feedback.
- Manage your time: Allocate sufficient time to thoroughly read and evaluate the manuscript, while adhering to the journal’s deadline for providing feedback.
- Read the manuscript multiple times: First, read the manuscript for an overall understanding of the research. Then, read it more closely to assess the details, methodology, results, and conclusions.
- Evaluate the structure and organization: Check if the manuscript follows the journal’s guidelines and is structured logically, with clear headings, subheadings, and a coherent flow of information.
- Assess the quality of the research: Evaluate the research question, study design, methodology, data collection, analysis, and interpretation. Consider whether the methods are appropriate, the results are valid, and the conclusions are supported by the data.
- Examine the originality and relevance: Determine if the research offers new insights, builds on existing knowledge, and is relevant to the field.
- Check for clarity and consistency: Review the manuscript for clarity of writing, consistent terminology, and proper formatting of figures, tables, and references.
- Identify ethical issues: Look for potential ethical concerns, such as plagiarism, data fabrication, or conflicts of interest.
- Provide constructive feedback: Offer specific, actionable, and objective suggestions for improvement, highlighting both the strengths and weaknesses of the manuscript. Don’t be mean.
- Organize your review: Structure your review with an overview of your evaluation, followed by detailed comments and suggestions organized by section (e.g., introduction, methods, results, discussion, and conclusion).
- Be professional and respectful: Maintain a respectful tone in your feedback, avoiding personal criticism or derogatory language.
- Proofread your review: Before submitting your review, proofread it for typos, grammar, and clarity.
- Couzin-Frankel J (September 2013). “Biomedical publishing. Secretive and subjective, peer review proves resistant to study”. Science . 341 (6152): 1331. doi: 10.1126/science.341.6152.1331
- Lee, Carole J.; Sugimoto, Cassidy R.; Zhang, Guo; Cronin, Blaise (2013). “Bias in peer review”. Journal of the American Society for Information Science and Technology. 64 (1): 2–17. doi: 10.1002/asi.22784
- Slavov, Nikolai (2015). “Making the most of peer review”. eLife . 4: e12708. doi: 10.7554/eLife.12708
- Spier, Ray (2002). “The history of the peer-review process”. Trends in Biotechnology . 20 (8): 357–8. doi: 10.1016/S0167-7799(02)01985-6
- Squazzoni, Flaminio; Brezis, Elise; Marušić, Ana (2017). “Scientometrics of peer review”. Scientometrics . 113 (1): 501–502. doi: 10.1007/s11192-017-2518-4
Related Posts
Peer Review in Scientific Publications: Benefits, Critiques, & A Survival Guide
Affiliations.
- 1 Clinical Biochemistry, Department of Pediatric Laboratory Medicine, The Hospital for Sick Children, University of Toronto , Toronto, Ontario, Canada.
- 2 Clinical Biochemistry, Department of Pediatric Laboratory Medicine, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada; Chair, Communications and Publications Division (CPD), International Federation for Sick Clinical Chemistry (IFCC), Milan, Italy.
- PMID: 27683470
- PMCID: PMC4975196
Peer review has been defined as a process of subjecting an author's scholarly work, research or ideas to the scrutiny of others who are experts in the same field. It functions to encourage authors to meet the accepted high standards of their discipline and to control the dissemination of research data to ensure that unwarranted claims, unacceptable interpretations or personal views are not published without prior expert review. Despite its wide-spread use by most journals, the peer review process has also been widely criticised due to the slowness of the process to publish new findings and due to perceived bias by the editors and/or reviewers. Within the scientific community, peer review has become an essential component of the academic writing process. It helps ensure that papers published in scientific journals answer meaningful research questions and draw accurate conclusions based on professionally executed experimentation. Submission of low quality manuscripts has become increasingly prevalent, and peer review acts as a filter to prevent this work from reaching the scientific community. The major advantage of a peer review process is that peer-reviewed articles provide a trusted form of scientific communication. Since scientific knowledge is cumulative and builds on itself, this trust is particularly important. Despite the positive impacts of peer review, critics argue that the peer review process stifles innovation in experimentation, and acts as a poor screen against plagiarism. Despite its downfalls, there has not yet been a foolproof system developed to take the place of peer review, however, researchers have been looking into electronic means of improving the peer review process. Unfortunately, the recent explosion in online only/electronic journals has led to mass publication of a large number of scientific articles with little or no peer review. This poses significant risk to advances in scientific knowledge and its future potential. The current article summarizes the peer review process, highlights the pros and cons associated with different types of peer review, and describes new methods for improving peer review.
Keywords: journal; manuscript; open access; peer review; publication.
You are using an outdated browser . Please upgrade your browser today !
What Is Peer Review and Why Is It Important?
It’s one of the major cornerstones of the academic process and critical to maintaining rigorous quality standards for research papers. Whichever side of the peer review process you’re on, we want to help you understand the steps involved.
This post is part of a series that provides practical information and resources for authors and editors.
Peer review – the evaluation of academic research by other experts in the same field – has been used by the scientific community as a method of ensuring novelty and quality of research for more than 300 years. It is a testament to the power of peer review that a scientific hypothesis or statement presented to the world is largely ignored by the scholarly community unless it is first published in a peer-reviewed journal.
It is also safe to say that peer review is a critical element of the scholarly publication process and one of the major cornerstones of the academic process. It acts as a filter, ensuring that research is properly verified before being published. And it arguably improves the quality of the research, as the rigorous review by like-minded experts helps to refine or emphasise key points and correct inadvertent errors.
Ideally, this process encourages authors to meet the accepted standards of their discipline and in turn reduces the dissemination of irrelevant findings, unwarranted claims, unacceptable interpretations, and personal views.
If you are a researcher, you will come across peer review many times in your career. But not every part of the process might be clear to you yet. So, let’s have a look together!
Types of Peer Review
Peer review comes in many different forms. With single-blind peer review , the names of the reviewers are hidden from the authors, while double-blind peer review , both reviewers and authors remain anonymous. Then, there is open peer review , a term which offers more than one interpretation nowadays.
Open peer review can simply mean that reviewer and author identities are revealed to each other. It can also mean that a journal makes the reviewers’ reports and author replies of published papers publicly available (anonymized or not). The “open” in open peer review can even be a call for participation, where fellow researchers are invited to proactively comment on a freely accessible pre-print article. The latter two options are not yet widely used, but the Open Science movement, which strives for more transparency in scientific publishing, has been giving them a strong push over the last years.
If you are unsure about what kind of peer review a specific journal conducts, check out its instructions for authors and/or their editorial policy on the journal’s home page.
Why Should I Even Review?
To answer that question, many reviewers would probably reply that it simply is their “academic duty” – a natural part of academia, an important mechanism to monitor the quality of published research in their field. This is of course why the peer-review system was developed in the first place – by academia rather than the publishers – but there are also benefits.
Are you looking for the right place to publish your paper? Find out here whether a De Gruyter journal might be the right fit.
Besides a general interest in the field, reviewing also helps researchers keep up-to-date with the latest developments. They get to know about new research before everyone else does. It might help with their own research and/or stimulate new ideas. On top of that, reviewing builds relationships with prestigious journals and journal editors.
Clearly, reviewing is also crucial for the development of a scientific career, especially in the early stages. Relatively new services like Publons and ORCID Reviewer Recognition can support reviewers in getting credit for their efforts and making their contributions more visible to the wider community.
The Fundamentals of Reviewing
You have received an invitation to review? Before agreeing to do so, there are three pertinent questions you should ask yourself:
- Does the article you are being asked to review match your expertise?
- Do you have time to review the paper?
- Are there any potential conflicts of interest (e.g. of financial or personal nature)?
If you feel like you cannot handle the review for whatever reason, it is okay to decline. If you can think of a colleague who would be well suited for the topic, even better – suggest them to the journal’s editorial office.
But let’s assume that you have accepted the request. Here are some general things to keep in mind:
Please be aware that reviewer reports provide advice for editors to assist them in reaching a decision on a submitted paper. The final decision concerning a manuscript does not lie with you, but ultimately with the editor. It’s your expert guidance that is being sought.
Reviewing also needs to be conducted confidentially . The article you have been asked to review, including supplementary material, must never be disclosed to a third party. In the traditional single- or double-blind peer review process, your own anonymity will also be strictly preserved. Therefore, you should not communicate directly with the authors.
When writing a review, it is important to keep the journal’s guidelines in mind and to work along the building blocks of a manuscript (typically: abstract, introduction, methods, results, discussion, conclusion, references, tables, figures).
After initial receipt of the manuscript, you will be asked to supply your feedback within a specified period (usually 2-4 weeks). If at some point you notice that you are running out of time, get in touch with the editorial office as soon as you can and ask whether an extension is possible.
Some More Advice from a Journal Editor
- Be critical and constructive. An editor will find it easier to overturn very critical, unconstructive comments than to overturn favourable comments.
- Justify and specify all criticisms. Make specific references to the text of the paper (use line numbers!) or to published literature. Vague criticisms are unhelpful.
- Don’t repeat information from the paper , for example, the title and authors names, as this information already appears elsewhere in the review form.
- Check the aims and scope. This will help ensure that your comments are in accordance with journal policy and can be found on its home page.
- Give a clear recommendation . Do not put “I will leave the decision to the editor” in your reply, unless you are genuinely unsure of your recommendation.
- Number your comments. This makes it easy for authors to easily refer to them.
- Be careful not to identify yourself. Check, for example, the file name of your report if you submit it as a Word file.
Sticking to these rules will make the author’s life and that of the editors much easier!
Explore new perspectives on peer review in this collection of blog posts published during Peer Review Week 2021

[Title image by AndreyPopov/iStock/Getty Images Plus
David Sleeman
David Sleeman worked as a Senior Journals Manager in the field of Physical Sciences at De Gruyter.
You might also be interested in
Academia & Publishing
The Impact of Transformative Agreements on Scholarly Publishing
Our website is currently unavailable: cyberattacks on cultural heritage institutions, wie steht es um das wissenschaftliche erbe der ddr eine podiumsdiskussion, visit our shop.
De Gruyter publishes over 1,300 new book titles each year and more than 750 journals in the humanities, social sciences, medicine, mathematics, engineering, computer sciences, natural sciences, and law.
Pin It on Pinterest
- Technical Support
- Find My Rep
You are here
What is peer review.
Peer review is ‘a process where scientists (“peers”) evaluate the quality of other scientists’ work. By doing this, they aim to ensure the work is rigorous, coherent, uses past research and adds to what we already know.’ You can learn more in this explainer from the Social Science Space.

Peer review brings academic research to publication in the following ways:
- Evaluation – Peer review is an effective form of research evaluation to help select the highest quality articles for publication.
- Integrity – Peer review ensures the integrity of the publishing process and the scholarly record. Reviewers are independent of journal publications and the research being conducted.
- Quality – The filtering process and revision advice improve the quality of the final research article as well as offering the author new insights into their research methods and the results that they have compiled. Peer review gives authors access to the opinions of experts in the field who can provide support and insight.
Types of peer review
- Single-anonymized – the name of the reviewer is hidden from the author.
- Double-anonymized – names are hidden from both reviewers and the authors.
- Triple-anonymized – names are hidden from authors, reviewers, and the editor.
- Open peer review comes in many forms . At Sage we offer a form of open peer review on some journals via our Transparent Peer Review program , whereby the reviews are published alongside the article. The names of the reviewers may also be published, depending on the reviewers’ preference.
- Post publication peer review can offer useful interaction and a discussion forum for the research community. This form of peer review is not usual or appropriate in all fields.
To learn more about the different types of peer review, see page 14 of ‘ The Nuts and Bolts of Peer Review ’ from Sense about Science.
Please double check the manuscript submission guidelines of the journal you are reviewing in order to ensure that you understand the method of peer review being used.
- Journal Author Gateway
- Journal Editor Gateway
- Transparent Peer Review
- How to Review Articles
- Using Sage Track
- Peer Review Ethics
- Resources for Reviewers
- Reviewer Rewards
- Ethics & Responsibility
- Sage Editorial Policies
- Publication Ethics Policies
- Sage Chinese Author Gateway 中国作者资源
- Open Resources & Current Initiatives
- Discipline Hubs
What is peer review?
From a publisher’s perspective, peer review functions as a filter for content, directing better quality articles to better quality journals and so creating journal brands.
Running articles through the process of peer review adds value to them. For this reason publishers need to make sure that peer review is robust.
Editor Feedback
"Pointing out the specifics about flaws in the paper’s structure is paramount. Are methods valid, is data clearly presented, and are conclusions supported by data?” (Editor feedback)
“If an editor can read your comments and understand clearly the basis for your recommendation, then you have written a helpful review.” (Editor feedback)

Peer Review at Its Best
What peer review does best is improve the quality of published papers by motivating authors to submit good quality work – and helping to improve that work through the peer review process.
In fact, 90% of researchers feel that peer review improves the quality of their published paper (University of Tennessee and CIBER Research Ltd, 2013).
What the Critics Say
The peer review system is not without criticism. Studies show that even after peer review, some articles still contain inaccuracies and demonstrate that most rejected papers will go on to be published somewhere else.
However, these criticisms should be understood within the context of peer review as a human activity. The occasional errors of peer review are not reasons for abandoning the process altogether – the mistakes would be worse without it.
Improving Effectiveness
Some of the ways in which Wiley is seeking to improve the efficiency of the process, include:
- Reducing the amount of repeat reviewing by innovating around transferable peer review
- Providing training and best practice guidance to peer reviewers
- Improving recognition of the contribution made by reviewers
Visit our Peer Review Process and Types of Peer Review pages for additional detailed information on peer review.
Transparency in Peer Review
Wiley is committed to increasing transparency in peer review, increasing accountability for the peer review process and giving recognition to the work of peer reviewers and editors. We are also actively exploring other peer review models to give researchers the options that suit them and their communities.

- University of Texas Libraries
- UT Libraries
Social Work Research
- Peer Review & Evaluation
- Getting Started
- Encyclopedias & Handbooks
- Evidence Based Practice
- Reading Scholarly Articles
- Books & Dissertations
- Data and Statistics
- Film & Video
- Policy & Government Documents
- Trabajo Social
- Citing & Citation Management
- Research Funding
- Open Access
- Choosing & Assessing Journals
- Increasing Access to Your Work
- Tracking Your Impact
- Systematic Reviews
- Tests & Measures
- What is Peer Review?
- How to check peer review
Peer Review is a critical part of evaluating information. It is a process that journals use to ensure the articles they publish represent the best scholarship currently available, and articles from peer reviewed journal are often grounded in empirical research. When an article is submitted to a peer reviewed journal, the editors send it out to other scholars in the same field (the author's peers) to get their assessment of the quality of the scholarship, its relevance to the field, its appropriateness for the journal, etc. Sometimes, you'll see this referred to as "refereed."
Publications that don't use peer review (Time, Cosmo, Salon) rely on an editor to determine the value of an article. Their goal is mainly to educate or entertain the general public, not to support scholarly research.
Most library databases will have a search feature that allows you to limit your results to peer reviewed or scholarly sources.
If you can't tell whether or not a journal is peer-reviewed, check Ulrichsweb.
- Access the database here: Ulrichsweb
- Type in the title of the journal
- Peer-reviewed journals will have a referee jersey ("refereed" is another term for "peer-reviewed") - example below

Evaluation Criteria
Use the criteria below to help you evaluate a source. As you do, remember:
- Each criterion should be considered in the context of your research question . For example, currency changes if you are working on a current event vs. a historical topic.
- Weigh all four criteria when making your decision. For example, the information may appear accurate, but if the authority is suspect you may want to find a more authoritative source for your information.
Criteria to consider:
- Currency : When was the information published or last updated? Is it current enough for your topic?
- Relevance : Is this information that you are looking for? Is it related to your topic? Is it detailed enough to help you answer questions on your topic.
- Use Web of Science database and/or Google Scholar
- Accuracy : Is the information true? What information does the author cite or refer to? Can you find this information anywhere else? Can you find evidence to back it up from another resource? Are studies mentioned but not cited (this would be something to check on)? Can you locate those studies?
- Methodology: What type of study did they conduct? Is it an appropriate type of study to answer their research question? How many people were involved in the study? Is the sample size large and diverse enough to give trustworthy results?
- Purpose/perspective : What is the purpose of the information? Was it written to sell something or to convince you of something? Is this fact or opinion based? Is it unfairly biased?
Learn more about how to evaluate your source
- Evaluate Sources
Source Evaluation Chart
- Source Evaluation Chart If you find it helpful, use this chart to evaluate sources as you read them. This is just a sample of some of the questions you might ask.
- Last Updated: Jan 26, 2024 7:14 AM
- URL: https://guides.lib.utexas.edu/socialwork

- myState on Mississippi State University
- Directory on Mississippi State University
- Seminar Series
Peer Review and (Dis)Service to Research
Dr. Renee Clary
Peer review serves an essential role in research and its dissemination of results, though the quality of peer review varies greatly. This session will overview the essential variables for superior peer review, including the importance of reviewer confidentiality and security, to help you avoid being THAT Reviewer.
- Find Office of Research and Economic Development on Facebook
- Find Office of Research and Economic Development on X Twitter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE INDEX
- 01 May 2024
Plagiarism in peer-review reports could be the ‘tip of the iceberg’
- Jackson Ryan 0
Jackson Ryan is a freelance science journalist in Sydney, Australia.
You can also search for this author in PubMed Google Scholar
Time pressures and a lack of confidence could be prompting reviewers to plagiarize text in their reports. Credit: Thomas Reimer/Zoonar via Alamy
Mikołaj Piniewski is a researcher to whom PhD students and collaborators turn when they need to revise or refine a manuscript. The hydrologist, at the Warsaw University of Life Sciences, has a keen eye for problems in text — a skill that came in handy last year when he encountered some suspicious writing in peer-review reports of his own paper.
Last May, when Piniewski was reading the peer-review feedback that he and his co-authors had received for a manuscript they’d submitted to an environmental-science journal, alarm bells started ringing in his head. Comments by two of the three reviewers were vague and lacked substance, so Piniewski decided to run a Google search, looking at specific phrases and quotes the reviewers had used.
To his surprise, he found the comments were identical to those that were already available on the Internet, in multiple open-access review reports from publishers such as MDPI and PLOS. “I was speechless,” says Piniewski. The revelation caused him to go back to another manuscript that he had submitted a few months earlier, and dig out the peer-review reports he received for that. He found more plagiarized text. After e-mailing several collaborators, he assembled a team to dig deeper.

Meet this super-spotter of duplicated images in science papers
The team published the results of its investigation in Scientometrics in February 1 , examining dozens of cases of apparent plagiarism in peer-review reports, identifying the use of identical phrases across reports prepared for 19 journals. The team discovered exact quotes duplicated across 50 publications, saying that the findings are just “the tip of the iceberg” when it comes to misconduct in the peer-review system.
Dorothy Bishop, a former neuroscientist at the University of Oxford, UK, who has turned her attention to investigating research misconduct, was “favourably impressed” by the team’s analysis. “I felt the way they approached it was quite useful and might be a guide for other people trying to pin this stuff down,” she says.
Peer review under review
Piniewski and his colleagues conducted three analyses. First, they uploaded five peer-review reports from the two manuscripts that his laboratory had submitted to a rudimentary online plagiarism-detection tool . The reports had 44–100% similarity to previously published online content. Links were provided to the sources in which duplications were found.
The researchers drilled down further. They broke one of the suspicious peer-review reports down to fragments of one to three sentences each and searched for them on Google. In seconds, the search engine returned a number of hits: the exact phrases appeared in 22 open peer-review reports, published between 2021 and 2023.
The final analysis provided the most worrying results. They took a single quote — 43 words long and featuring multiple language errors, including incorrect capitalization — and pasted it into Google. The search revealed that the quote, or variants of it, had been used in 50 peer-review reports.
Predominantly, these reports were from journals published by MDPI, PLOS and Elsevier, and the team found that the amount of duplication increased year-on-year between 2021 and 2023. Whether this is because of an increase in the number of open-access peer-review reports during this time or an indication of a growing problem is unclear — but Piniewski thinks that it could be a little bit of both.
Why would a peer reviewer use plagiarized text in their report? The team says that some might be attempting to save time , whereas others could be motivated by a lack of confidence in their writing ability, for example, if they aren’t fluent in English.
The team notes that there are instances that might not represent misconduct. “A tolerable rephrasing of your own words from a different review? I think that’s fine,” says Piniewski. “But I imagine that most of these cases we found are actually something else.”
The source of the problem
Duplication and manipulation of peer-review reports is not a new phenomenon. “I think it’s now increasingly recognized that the manipulation of the peer-review process, which was recognized around 2010, was probably an indication of paper mills operating at that point,” says Jennifer Byrne, director of biobanking at New South Wales Health in Sydney, Australia, who also studies research integrity in scientific literature.
Paper mills — organizations that churn out fake research papers and sell authorships to turn a profit — have been known to tamper with reviews to push manuscripts through to publication, says Byrne.

The fight against fake-paper factories that churn out sham science
However, when Bishop looked at Piniewski’s case, she could not find any overt evidence of paper-mill activity. Rather, she suspects that journal editors might be involved in cases of peer-review-report duplication and suggests studying the track records of those who’ve allowed inadequate or plagiarized reports to proliferate.
Piniewski’s team is also concerned about the rise of duplications as generative artificial intelligence (AI) becomes easier to access . Although his team didn’t look for signs of AI use, its ability to quickly ingest and rephrase large swathes of text is seen as an emerging issue.
A preprint posted in March 2 showed evidence of researchers using AI chatbots to assist with peer review, identifying specific adjectives that could be hallmarks of AI-written text in peer-review reports .
Bishop isn’t as concerned as Piniewski about AI-generated reports, saying that it’s easy to distinguish between AI-generated text and legitimate reviewer commentary. “The beautiful thing about peer review,” she says, is that it is “one thing you couldn’t do a credible job with AI”.
Preventing plagiarism
Publishers seem to be taking action. Bethany Baker, a media-relations manager at PLOS, who is based in Cambridge, UK, told Nature Index that the PLOS Publication Ethics team “is investigating the concerns raised in the Scientometrics article about potential plagiarism in peer reviews”.

How big is science’s fake-paper problem?
An Elsevier representative told Nature Index that the publisher “can confirm that this matter has been brought to our attention and we are conducting an investigation”.
In a statement, the MDPI Research Integrity and Publication Ethics Team said that it has been made aware of potential misconduct by reviewers in its journals and is “actively addressing and investigating this issue”. It did not confirm whether this was related to the Scientometrics article.
One proposed solution to the problem is ensuring that all submitted reviews are checked using plagiarism-detection software. In 2022, exploratory work by Adam Day, a data scientist at Sage Publications, based in Thousand Oaks, California, identified duplicated text in peer-review reports that might be suggestive of paper-mill activity. Day offered a similar solution of using anti-plagiarism software , such as Turnitin.
Piniewski expects the problem to get worse in the coming years, but he hasn’t received any unusual peer-review reports since those that originally sparked his research. Still, he says that he’s now even more vigilant. “If something unusual occurs, I will spot it.”
doi: https://doi.org/10.1038/d41586-024-01312-0
Piniewski, M., Jarić, I., Koutsoyiannis, D. & Kundzewicz, Z. W. Scientometrics https://doi.org/10.1007/s11192-024-04960-1 (2024).
Article Google Scholar
Liang, W. et al. Preprint at arXiv https://doi.org/10.48550/arXiv.2403.07183 (2024).
Download references
Related Articles

- Peer review
- Research management

Algorithm ranks peer reviewers by reputation — but critics warn of bias
Nature Index 25 APR 24

Researchers want a ‘nutrition label’ for academic-paper facts
Nature Index 17 APR 24

Rwanda 30 years on: understanding the horror of genocide
Editorial 09 APR 24

Structure peer review to make it more robust
World View 16 APR 24

Is ChatGPT corrupting peer review? Telltale words hint at AI use
News 10 APR 24

How reliable is this research? Tool flags papers discussed on PubPeer
News 29 APR 24

Scientists urged to collect royalties from the ‘magic money tree’
Career Feature 25 APR 24

Silver Endowed Chair (Developmental Psychiatry)(Open Rank Faculty)
The Robert A. Silver Endowed Chair in Developmental Neurobiology leads an internationally recognized, competitively funded research program...
Tampa, Florida
University of South Florida - Department of Psychiatry & Behavioral Neurosciences
W2 Professorship with tenure track to W3 in Animal Husbandry (f/m/d)
The Faculty of Agricultural Sciences at the University of Göttingen invites applications for a temporary professorship with civil servant status (g...
Göttingen (Stadt), Niedersachsen (DE)
Georg-August-Universität Göttingen
Postdoctoral Associate- Cardiovascular Research
Houston, Texas (US)
Baylor College of Medicine (BCM)
Faculty Positions & Postdocs at Institute of Physics (IOP), Chinese Academy of Sciences
IOP is the leading research institute in China in condensed matter physics and related fields. Through the steadfast efforts of generations of scie...
Beijing, China
Institute of Physics (IOP), Chinese Academy of Sciences (CAS)
Director, NLM
Vacancy Announcement Department of Health and Human Services National Institutes of Health DIRECTOR, NATIONAL LIBRARY OF MEDICINE THE POSITION:...
Bethesda, Maryland
National Library of Medicine - Office of the Director
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 29 April 2024
Self-reported health related quality of life in children and adolescents with an eating disorder
- A. Wever ORCID: orcid.org/0000-0002-8877-5876 1 ,
- E. van Gerner 2 ,
- J.C.M Jansen 3 &
- B. Levelink 1
BMC Psychology volume 12 , Article number: 242 ( 2024 ) Cite this article
100 Accesses
Metrics details
Eating disorders in children and adolescents can have serious medical and psychological consequences. The objective of this retrospective quantitative study is to gain insight in self-reported Health Related Quality of Life (HRQoL) of children and adolescents with a DSM-5 diagnosis of an eating disorder.
Collect and analyse data of patients aged 8–18 years, receiving treatment for an eating disorder. At the start and end of treatment patients completed the KIDSCREEN-52, a questionnaire measuring HRQoL.
Data of 140 patients were analysed. Children diagnosed with Anorexia Nervosa, Bulimia Nervosa, and Other Specified Feeding or Eating Disorder all had lower HRQoL on multiple dimensions at the start of treatment, there is no statistically significant difference between these groups. In contrast, patients with Avoidant Restrictive Food Intake Disorder only had lower HRQoL for the dimension Physical Well-Being. HRQoL showed a significant improvement in many dimensions between start and end of treatment, but did not normalize compared to normative reference values of Dutch children.
The current study showed that self-reported HRQoL is low in children with eating disorders, both at the beginning but also at the end of treatment. This confirms the importance of continuing to invest in the various HRQoL domains.
Peer Review reports
Eating disorders in children and adolescents can have serious medical and psychological consequences and rank 12th on the list of physical and mental conditions amongst woman aged 15–19 years in high-income countries when looking at the global burden of disease [ 1 , 2 ]. The estimated lifetime prevalence of Anorexia Nervosa (AN) in woman is 1–4% and 1–2% for Bulimia Nervosa (BN), and the epidemiology is changing, with increasing rates of eating disorders in younger children, boys and minority groups [ 2 , 3 ].
The past two decades research on health-related quality of life (HRQoL) in patients with an eating disorder has increased [ 4 , 5 , 6 , 7 , 8 ]. HRQoL is a subjective evaluation of the overall health of an individual, as well as the health of underlying subdimensions of physical, psychological and social functioning [ 9 ]. Most studies have been conducted in adults and a recent review and meta-analysis both show that eating disorders are associated with significant impaired HRQoL compared with the healthy population [ 10 , 11 ]. To our knowledge only one other study evaluated the impact of eating disorders on HRQoL in children and adolescents. Jenkins et al. looked at the impact of eating disorders in a group of adolescents seeking treatment for AN, BN or eating disorder not otherwise specified (EDNOS) [ 6 ]. This study reported a poorer HRQoL measured with the SF-36 Health Survey in adolescents with an eating disorder compared with adolescent norms for the Swedish population [ 6 ]. Two studies included both children and adolescents. Weigel et al. examined the association between disorder specific factors, comorbidity and HRQoL in anorexia nervosa in adolescents and adults. HRQoL was measured using the visual analogue scale (EQ-VAS) a generic scale that does not look to different HRQoL domains [ 12 ]. Ackard et al. assessed quality of life in patients diagnosed with an eating disorder, mean age at initial assessment was 20.6 years (SD 5 8.3 years), with a range of 12–53 years. Children were not assessed separatly. Other studies in children and adolescents focused on disordered eating behaviours, but not diagnosed eating disorders [ 4 ]. A review of population-based studies showed that disordered eating attitudes and behaviours were associated with lower HRQoL in children and adolescents [ 9 ]. Herpertz-Dahlmann and colleagues found a poorer HRQoL in adolescents with self-reported disordered eating, and an association between eating disorder symptoms and psychopathology [ 13 ].
Because treating an eating disorder encompasses more than weight gain alone it is important to know the possible impact of an eating disorder on HRQoL [ 14 ]. As there are still few studies on self-reported HRQoL in children and adolescents with a diagnosed eating disorder, the primary aim of this study is to gain more insight in the different domains of self-reported HRQoL in a clinical sample of children and adolescents with a DSM-5 diagnosis of an eating disorder at the beginning of treatment. In addition, changes of HRQoL between start and end of treatment were evaluated to determine whether treatment influences HRQoL and if so which domains.
Participant
Data of patients who were diagnosed conform the Diagnostic and Statistical Manual of Mental Disorders (DSM) -IV-TR/DSM-5 criteria for an eating disorder, and receiving treatment between November 2006 and April 2019 at The Mutsaersstichting were used [ 15 , 16 ]. The Mutsaersstichting is a mental healthcare institute specialised in eating disorders in the Netherlands where children between 0 and 18 years receive both in- and outpatient treatment. At first presentation, every patient received an extensive consultation with a child and youth psychologist, a child and youth psychiatrist and a paediatrician. Based on this information DSM-IV-TR and DSM-5 classification were made. Patients diagnosed before 2014 were rediagnosed using the DSM-5 classification, especially using the new criteria for Avoidant Restrictive Food Intake Disorder (ARFID). Subsequently, a personalized treatment plan was presented to the family. Treatment always consisted of a combination of family-based treatment, individual treatment, group treatment and physical follow-up. Data from patients who met the DSM-5 diagnosis for AN, BN, ARFID, Binge Eating Disorder (BED), or OSFED were considered eligible for analyses. Because the study specifically focused on self-reported HRQoL, only data of children between the ages of 8 and 18 were included, since for younger children the parents completed the HRQoL questionnaire. Children and adolescents who only had HRQoL reports completed by the parents were excluded. Ethical approval was obtained from the medical ethics committee of the Maastricht University Medical Centre.
As part of the Routine Outcome Monitoring the KIDSCREEN-52 questionnaire was sent to every patient who sought treatment for an eating disorder at the Mutsaersstichting. Baseline characteristics and clinical data were collected at the start and end of treatment. At first consultation, patient characteristics including age, sex, underlying diseases, eating attitudes and behaviours, compensatory behaviour and sociodemographic data were obtained. Heart rate and blood pressure were measured with an oscillometric blood pressure machine and evaluated according to the Clinical Practice Guidline of the American Acadamy of Pediatrics [ 17 ]. In addition, a full physical examination was performed. Body Mass Index (BMI) was calculated from measured weight and height [ 18 ]. Growth charts designed by the Dutch organization for applied scientific research (TNO) were used to determine height for age (standard deviation, SD) and weight for height (SD) [ 19 , 20 ]. At the end-evaluation data was collected concerning most recent height, weight, BMI and eating attitudes and behaviours.
The KIDSCREEN-52 is a validated self-report questionnaire for measuring HRQoL in European children between 8 and 18 years old [ 21 , 22 , 23 , 24 , 25 ]. It consists of 52 questions, divided into 10 dimensions: Physical Well-being, Psychological Well-being, Moods and Emotions, Self-Perception, Autonomy, Parent Relations and Home Life, Social Support and Peers, School Environment, Social Acceptance (Bullying), and Financial Resources. The KIDSCREEN-52 uses 5-point Likert scale responses, within each different dimension the results are converted into a Rasch scale. Cronbach–alpha’s vary between 0.77 and 0.89 [ 25 ]. The results are transformed to a t-score, giving the children in the total reference population a mean t-score of 50 with a SD of 10. Specific reference populations are made by country, gender and age groups. The results of this study are compared with the validated normative reference values of Dutch children in the age between 8 and 18 years old [ 25 ]. Ulrike Ravens-Sieberer defined a mean t-score 0.5 SD below the mean t-score of the specific referential population of a country as a low HRQoL and a t-score 0.5 SD above the mean t-score of the referential population as high HRQoL [ 25 ].
Data analysis
All statistical analyses were performed using IBM SPSS Statistics version 25 [ 26 ]. The Mann-Withney U, χ 2 , and fisher exact test were used to determine whether there were statistical differences between all the children and adolescents included in this study and the children who completed the questionnaire at intake and end-evaluation. Paired t test was used to test for statistically significant differences in HRQoL between start- and end of treatment. To test the differences in t-score on the KIDSCREEN-52 stratified for DSM-classification, a one-way ANOVA and Welch test was done, with the Tukey’s Test as a post-hoc analysis. Statistically significance was considered when the result had a p value of < 0.05. Univariate regression analysis was done in the group diagnosed with AN to test whether there is an association between HRQoL and BMI, BMI SD, age, excessive exercise and binge eating. Since purging only occurred in four patients this could not be included in the analysis. Other DSM-5 diagnoses where not included due to small subgroup sample size.
Baseline characteristics
Data of 276 patients were analysed of which 140 were found eligible for this study (Fig. 1 ). Baseline characteristics are presented in Table 1 . The total population consisted primarily of female children and adolescents ( n = 119; 85%) with a mean age of 15.0 years ranging from 8 to 18 years. Almost half of the population was classified as AN ( n = 68; 48.6%). The mean weight for children with AN ( n = 67) was 44.1 kg (minimal weight 25 kg– maximal weight 59 kg) with a mean weight SD of -1.6 and mean BMI of 15.9 kg/m 2 (minimal BMI 11.9 kg/m 2 – maximal BMI 19.9 kg/m 2 ). Children with ARFID had a mean weight of 31.9 kg (minimal weight 18 kg– maximal weight 105 kg), mean weight SD– 0.6, mean BMI 15.9 kg/m 2 (minimal BMI 12.1 kg/m 2 – maximal BMI 36.3 kg/m 2 ). Only two patients were diagnosed with BED, this was too small a sample size to be included in results stratified for the DSM-5 criteria. No significant differences were found in the baseline characteristics between children who completed the KIDSCREEN-52 only at the beginning of treatment, and those who completed the questionnaire both at the start and end-evaluation ( n = 47), except for psychiatric co-morbidities ( X 2 (1) = 4.97; p = 0.026). Even though the effect size for this finding, Cramer’s V = 0.188, was weak, due to the known association between psychiatric co-morbidities and eating disorder symptoms, a comparison between HRQoL at the beginning and end of treatment was only made within the group of 47 patients that completed both questionnaires [ 13 , 27 , 28 ].
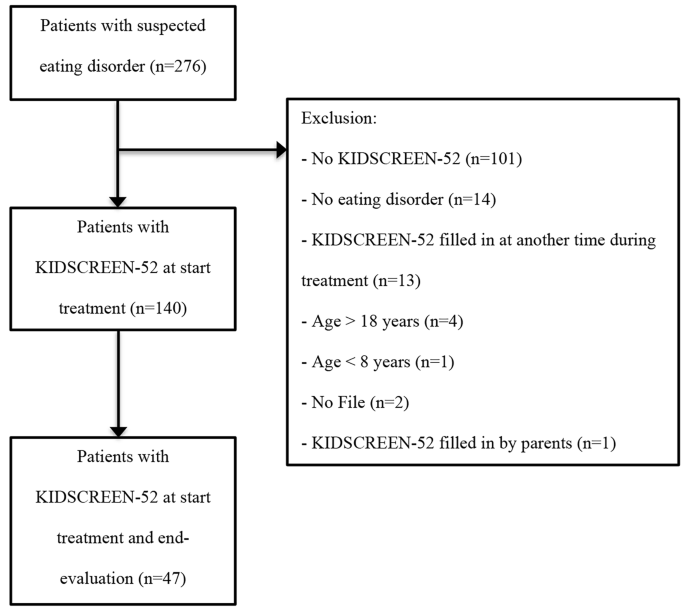
HRQoL at the start of treatment
Table 2 shows mean t-scores scored by children and adolescents on the KIDSCREEN-52 at the start of treatment, stratified for the DSM-5 criteria. Children with the diagnosis AN, BN and OSFED all had a lower HRQoL (≤ 0.5 SD of mean score) than the reference population for the dimensions Physical Well-being, Psychological Well-being, Moods and Emotions, Self-Perception, Autonomy, Financial Resources, Peers and Social Support, School Environment and Bullying. There were no statistically significant differences in t-scores between AN, BN and OSFED. This was confirmed with a Turkey’s post hoc test. Compared with the reference population the HRQoL in patients with ARFID was only lower for the dimension Physical Well-Being. For the dimensions Physical Well-being, Psychological Well-being, Moods and Emotions, Self-Perception, Autonomy, Parent Relations and Home Life and School Environment the t-scores of children with ARFID were significantly higher than those of the children who met criteria of all other eating disorders. Social Support and Peers was significantly higher in patients with ARFID compared to AN, but not with BN and OSFED. Univariate regression analysis in the group diagnosed with AN showed a significant association between a higher t-score on the domain Physical Well-being and higher BMI, BMI SD. Other variables were not associated with a higher or lower t-score.
HRQoL change between start and end of treatment
In Table 3 mean t-scores of the KIDSCREEN-52 at the start of treatment are compared with t-scores at the end evaluation. HRQoL showed a significant improvement in mean t-scores before and after treatment for Physical Well-being (t (46) = -4.4, p < 0.001), Psychological Well-being (t (45) = − 3.0, P = 0.004), Moods and emotions (t (45) = -3.3, p = 0.002) Self Perception (t (45) = -3.7, p = 0.001) and School environment (t (44) = -2.8, p = 0.008). However, after treatment the HRQoL for these dimensions did not normalize compared to normative reference values of Dutch children. The subgroup sample sizes were too small for findings relating to change in QoL before and after treatment to be stratified by diagnosis.
This study shows that the self-reported HRQoL in children and adolescents receiving outpatient treatment in the Netherlands for an eating disorder is significantly lower on multiple dimensions at the beginning and end of treatment compared with the reference population. Most studies that have been conducted in children and adolescents are population-based studies that focus on disordered eating behaviours, yet they also show a significantly decreased mental HRQoL [ 9 , 13 , 29 , 30 , 31 , 32 , 33 , 34 ]. The study of Jenskins, showed similar results in a group of sixty-seven adolescents seeking treatment for an eating disorder [ 6 ].
The domain physical well-being is significantly lower for all types of eating disorders. This finding replicates that of Winkler et al. in which compared to the controls, adult women with AN had significantly impaired HRQoL as measured by the Eating Disorders Quality of Life (EDQOL) scale including lower physical functioning [ 35 ]. Yet several other studies showed only a significantly lower mental component summary and normal levels in the psychical component summary scored with Short Form-36 Health Survey (SF-36) [ 4 , 6 , 14 , 36 ]. This difference could partially be explained by the use of different questionnaires, where some questionnaires could reflect the physical pathology of eating disorders rather than real physical health. The KIDSCREEN-52 for example specifically asked for fatigue, where other questionnaires ask for the ability to walk the stairs. When diagnosed with AN extensive exercise might be associated with the disease itself. Disease severity and duration of the eating disorder might also influence results. Children and adolescents in our study received one or more previous treatments in 55% of the patients and in 34% had a disease duration of more than one year. To gain more insight a univariate regression analysis was done in the group diagnosed with AN, which showed a significant association between a higher BMI and higher t-score on the domain Physical Well-being, suggesting that the results as shown within this study might be a reflection of real physical health rather than psychopathology.
When comparing AN, BN and OSFED this study does not find statistically significant differences similar as the meta-analysis on quality of life by Winkler et al. suggesting a similarity between these eating disorders with regard to HRQoL [ 35 ]. Notable exception to this are the children and adolescents with ARFID, who only score lower on the item Physical Well-being, unlike the children and adolescents classified with all other eating disorders who have lower scores on almost all HRQoL dimensions. This suggests that HRQoL affects children with ARFID differently. Hay et al. compared adults and adolescents from the age of 15 years with ARFID in the Australian population to other eating disorders and found, unlike the current study, a normal physical HRQoL and a significantly lower mental HRQoL [ 37 ]. A Dutch study by Krom et al., in which children were treated for ARFID in a Diagnostic Centre for Feeding Problems showed that the HRQoL, reported by their parents using TNO-AZL Preschool Children Quality of Life (TAPQOL) was significantly lower on the subscales appetite, lungs, stomach, motor functioning, and positive mood and liveliness, suggesting that both physical and mental HRQoL was affected [ 38 ]. The difference in mental HRQoL between the current study and the study by Krom et al. might be explained by an overestimation by parents of the child’s psychosocial functioning due to parent’s own concerns, and besides that it might be caused by age differences. Another explanation could be that ARFID differs from longer recognised disorders such as anorexia nervosa and bulimia nervosa in that they do not have a core psychopathology of body image disturbance or weight/shape overvaluation. Given that in adolescent and young adult women at least, it is clear that overvaluation of weight/shape is very strongly associated with impairment in quality of life including but not limited to the mental health domain, it is not too surprising that children and adolescents with a diagnosis of ARFID report relatively little impairment in mental HRQoL [ 39 , 40 ]. The lower physical HRQoL that is seen in the current study might be explained by nutritional deficits often seen in children with ARFID [ 41 ].
The HRQoL shows significant improvement after treatment in all dimensions except for Autonomy and Social Support and Peers. However, HRQoL does not normalize compared to the reference population, and stays significantly impaired. This finding is consistent with considerations of other studies, namely that symptom remission alone is not sufficient for improvement in quality of life [ 42 ]. Studies looking at the long-term effects of eating disorders show that the long-term HRQoL after treatment continues to improve but is still not normalized after 8- or 30-years [ 14 , 42 , 43 , 44 ]. Thus follow-up, with paying attention to HRQoL, should continue longer than the initial treatment. Similar to our results, greatest improvement in HRQoL was noted in the physical functioning domain [ 43 , 44 ]. With childhood and adolescence being a critical period of development, the current study underlines the importance of treatment in which the success of the treatment is not based on BMI or amount of food intake alone, but focuses on other quality of life factors, such as psychological well-being, autonomy and social support.
There are limitations to this study. Due to the small subgroup sample size findings in the change in HRQoL before and after treatment could not be stratified by diagnosis. This study enrolled participants during a 14-year period, this longer period could have confounded the results due to changes in the care and treatments. Also, the retrospective nature of this study and the use of a generic HRQoL scale needs to be taken into consideration. Using generic HRQoL scales could give an over or underestimation of the HRQoL, since it does not focus specific on eating disorders, and questions for example about physical wellbeing could be an expression of the eating disorder rather than healthy behaviour. Our patients received both in- and outpatient treatment, which implies a certain disease severity and might not be generalizable to patients in other settings. HRQoL at the start of treatment could be lower or higher depending on the setting. Even though the children who completed the KIDSCREEN-52 only at the beginning of treatment and those who completed the questionnaire both at the start and end-evaluation are comparable, a large number of patients did not fill in the KIDSCREEN-52 at end-evaluation which might influence the outcome of quality of life after treatment, especially if the patients that did recover are the ones that did not fill in the questionnaire.
However, despite the limitations this descriptive study gives insight in the self-reported HRQoL of children and adolescents in the Netherlands treated for an eating disorder. It shows a significant reduction in both mental and physical HRQoL compared to the reference population with the exception of ARFID in which only physical HRQoL is impaired. This study also shows that even after treatment, children do not achieve normal HRQoL, which poses a potential risk to their development. Long-term follow-up of these children seems important, and more research is needed focusing on the effect of using quality of life parameters as most important measurements for recovery.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Anorexia Nervosa
Avoidant Restrictive Food Intake Disorder
Binge Eating Disorder
Body Mass Index
Bulimia Nervosa
Diagnostic and Statistical Manual of Mental Disorders
Eating Disorders Quality of Life
Eating disorder not otherwise specified
Health-related quality of life
Standard deviation
36-Short Form-36 Health Survey
TNO-AZL Preschool Children Quality of Life
Erskine HE, Whiteford HA, Pike KM. The global burden of eating disorders. Curr Opin Psychiatry. 2016;29(6):346–53.
Article PubMed Google Scholar
Campbell K, Peebles R. Eating disorders in children and adolescents: state of the art review. Pediatrics. 2014;134(3):582–92.
Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr Opin Psychiatry. 2016;29(6):340–5.
Ackard DM, Richter S, Egan A, Engel S, Cronemeyer CL. The meaning of (quality of) life in patients with eating disorders: a comparison of generic and disease-specific measures across diagnosis and outcome. Int J Eat Disord. 2014;47(3):259–67.
Engel SG, Adair CE, Las Hayas C, Abraham S. Health-related quality of life and eating disorders: a review and update. Int J Eat Disord. 2009;42(2):179–87.
Jenkins PE, Hoste RR, Doyle AC, Eddy K, Crosby RD, Hill L, et al. Health-related quality of life among adolescents with eating disorders. J Psychosom Res. 2014;76(1):1–5.
Krom H, van der Sluijs Veer L, van Zundert S, Otten MA, Benninga M, Haverman L, Kindermann A. Health related quality of life of infants and children with avoidant restrictive food intake disorder. Int J Eat Disord. 2019;52(4):410–8.
Article PubMed PubMed Central Google Scholar
Chou B, Bohn JA, Mairs R. Acute abdominal pain caused by hematometra in an adolescent female: a case report. J Med Case Rep. 2016;10(1):369.
Wu XY, Yin WQ, Sun HW, Yang SX, Li XY, Liu HQ. The association between disordered eating and health-related quality of life among children and adolescents: a systematic review of population-based studies. PLoS ONE. 2019;14(10):e0222777.
Ágh T, Kovács G, Supina D, Pawaskar M, Herman BK, Vokó Z, Sheehan DV. A systematic review of the health-related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eat Weight Disord. 2016;21(3):353–64.
Winkler LA, Christiansen E, Lichtenstein MB, Hansen NB, Bilenberg N, Støving RK. Quality of life in eating disorders: a meta-analysis. Psychiatry Res. 2014;219(1):1–9.
Weigel A, Konig HH, Gumz A, Lowe B, Brettschneider C. Correlates of health related quality of life in anorexia nervosa. Int J Eat Disord. 2016;49(6):630–4.
Herpertz-Dahlmann B, Wille N, Holling H, Vloet TD, Ravens-Sieberer U, group Bs. Disordered eating behaviour and attitudes, associated psychopathology and health-related quality of life: results of the BELLA study. Eur Child Adolesc Psychiatry. 2008;17(Suppl 1):82–91.
de la Rie SM, Noordenbos G, van Furth EF. Quality of life and eating disorders. Qual Life Res. 2005;14(6):1511–22.
[APA] APA. Diagnostic and statistical manual of mental disorders: DSM-IV. (1994). Washington, DC:1994.
Association AP. Diagnostic and Statistical Manual of Mental Disorders. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed2013.
Abaied JL, Wagner C, Breslend NL, Flynn M. Respiratory sinus arrhythmia as a predictor of eating disorder symptoms in college students: moderation by responses to stress and parent psychological control. Eat Behav. 2016;21:109–15.
Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–94.
Schonbeck Y, Talma H, van Dommelen P, Bakker B, Buitendijk SE, HiraSing RA, van Buuren S. The world’s tallest nation has stopped growing taller: the height of Dutch children from 1955 to 2009. Pediatr Res. 2013;73(3):371–7.
Schonbeck Y, van Dommelen P, HiraSing RA, van Buuren S. Trend in height of Turkish and Moroccan children living in the Netherlands. PLoS ONE. 2015;10(5):e0124686.
Ravens-Sieberer U, Erhart M, Rajmil L, Herdman M, Auquier P, Bruil J, et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: a short measure for children and adolescents’ well-being and health-related quality of life. Qual Life Res. 2010;19(10):1487–500.
Ravens-Sieberer U, Gosch A, Rajmil L, Erhart M, Bruil J, Duer W, et al. KIDSCREEN-52 quality-of-life measure for children and adolescents. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):353–64.
Ravens-Sieberer U, Gosch A, Rajmil L, Erhart M, Bruil J, Power M, et al. The KIDSCREEN-52 quality of life measure for children and adolescents: psychometric results from a cross-cultural survey in 13 European countries. Value Health. 2008;11(4):645–58.
Ravens-Sieberer U, Herdman M, Devine J, Otto C, Bullinger M, Rose M, Klasen F. The European KIDSCREEN approach to measure quality of life and well-being in children: development, current application, and future advances. Qual Life Res. 2014;23(3):791–803.
Ravens-Sieberer U. The Kidscreen questionnaires: quality of life questionnaires for children and adolescents; handbook. Pabst Science Publ.; 2006.
IBM Corp. Released 2017. IBM SPSS statistics for Windows VA, NY: IBM Corp.
Padierna A, Quintana JM, Arostegui I, Gonzalez N, Horcajo MJ. The health-related quality of life in eating disorders. Qual Life Res. 2000;9(6):667–74.
Jenkins PE, Hoste RR, Meyer C, Blissett JM. Eating disorders and quality of life: a review of the literature. Clin Psychol Rev. 2011;31(1):113–21.
Ackard DM, Fulkerson JA, Neumark-Sztainer D. Psychological and behavioral risk profiles as they relate to eating disorder diagnoses and symptomatology among a school-based sample of youth. Int J Eat Disord. 2011;44(5):440–6.
Zeiler M, Waldherr K, Philipp J, Nitsch M, Dur W, Karwautz A, Wagner G. Prevalence of eating Disorder Risk and associations with Health-related quality of life: results from a large school-based Population Screening. Eur Eat Disord Rev. 2016;24(1):9–18.
Stice E, Marti CN, Shaw H, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders from a community sample of adolescents. J Abnorm Psychol. 2009;118(3):587–97.
Gelin Z, Fuso S, Hendrick S, Cook-Darzens S, Simon Y. The effects of a multiple family therapy on adolescents with eating disorders: an outcome study. Fam Process. 2015;54(1):160–72.
Kenny B, Bowe SJ, Taylor CB, Moodie M, Brown V, Hoban E, Williams J. Longitudinal relationships between sub-clinical depression, sub-clinical eating disorders and health-related quality of life in early adolescence. Int J Eat Disord. 2023;56(6):1114–24.
Mitchell TB, Steele RG. Bidirectional associations between Disordered Eating and Health-Related Quality of Life in Elementary School-Age Youth. J Pediatr Psychol. 2017;42(3):315–24.
PubMed Google Scholar
Winkler LA, Gudex C, Lichtenstein MB, Roder ME, Adair CE, Sjogren JM, Stoving RK. Explanatory factors for Disease-Specific Health-Related Quality of Life in Women with Anorexia Nervosa. J Clin Med. 2021;10(8).
Mitchison D, Hay P, Mond J, Slewa-Younan S. Self-reported history of anorexia nervosa and current quality of life: findings from a community-based study. Qual Life Res. 2013;22(2):273–81.
Hay P, Mitchison D, Collado AEL, Gonzalez-Chica DA, Stocks N, Touyz S. Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. J Eat Disord. 2017;5:21.
Krom H, van Oers HA, van der Sluijs Veer L, van Zundert SMC, Otten MGM, Haverman L, et al. Health-Related Quality of Life and Distress of parents of children with avoidant restrictive food intake disorder. J Pediatr Gastroenterol Nutr. 2021;73(1):115–24.
Mond JM, Hay PJ, Rodgers B, Owen C. Mental health impairment associated with eating-disorder features in a community sample of women. J Ment Health. 2011;20(5):456–66.
Wagner AF, Stefano EC, Cicero DC, Latner JD, Mond JM. Eating disorder features and quality of life: does gender matter? Qual Life Res. 2016;25(10):2603–10.
Bryant-Waugh R. Avoidant/Restrictive food intake disorder. Child Adolesc Psychiatr Clin N Am. 2019;28(4):557–65.
Pohjolainen V, Koponen S, Rasanen P, Roine RP, Sintonen H, Karlsson H. Long-term health-related quality of life in eating disorders. Qual Life Res. 2016;25(9):2341–6.
Dobrescu SR, Dinkler L, Gillberg C, Rastam M, Gillberg C, Wentz E. Anorexia nervosa: 30-year outcome. Br J Psychiatry. 2020;216(2):97–104.
Padierna A, Quintana JM, Arostegui I, Gonzalez N, Horcajo MJ. Changes in health related quality of life among patients treated for eating disorders. Qual Life Res. 2002;11(6):545–52.
Download references
Acknowledgements
Not applicable.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and affiliations.
Department of Paediatrics, Maastricht University Medical Centre (MUMC+), P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands
A. Wever & B. Levelink
Department of Primary care, Radboud University Medical Centre, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, The Netherlands
E. van Gerner
Department of Child and Youth Psychiatry, Postweg 88, 5915 HB, De Mutsaersstichting, Venlo, The Netherlands
J.C.M Jansen
You can also search for this author in PubMed Google Scholar
Contributions
CRediT author statement: A Wever: Writing - Original Draft, E. van Gerner: Formal analysis and Investigation, J.C.M. Jansen: Conceptualization and Writing - Review & Editing, B Levelink: Conceptualization and Writing - Review & Editing.
Corresponding author
Correspondence to A. Wever .
Ethics declarations
Ethics approval and consent to participate.
The medical ethics committee of the Maastricht University Medical Centre was consulted for ethical approval (METC 2019 − 1027). The medical ethics committee stated that the Medical Research Involving Human Subjects Act (WMO) does not apply to this study. The need for informed consent was waived by medical ethics committee of the Maastricht University Medical Centre, because Medical Research Involving Human Subjects Act (WMO) does not apply to this study. This research was performed following the Dutch legislation that applies to retrospective research, Agreement on Medical Treatment Act (Wet op de geneeskundige behandelingsovereenkomst, WGBO, in Dutch), from the Dutch Civil Code.
Consent for publication
Competing interests.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wever, A., van Gerner, E., Jansen, J. et al. Self-reported health related quality of life in children and adolescents with an eating disorder. BMC Psychol 12 , 242 (2024). https://doi.org/10.1186/s40359-024-01684-y
Download citation
Received : 27 November 2023
Accepted : 25 March 2024
Published : 29 April 2024
DOI : https://doi.org/10.1186/s40359-024-01684-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health Related Quality of Life
- Adolescents
- Eating disorder
- Anorexia nervosa
- Avoidant/Restrictive food intake disorder
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
- Open access
- Published: 02 May 2024
Determination of drug-related problems in the hematology service: a prospective interventional study
- Aslınur Albayrak ORCID: orcid.org/0000-0001-5862-4746 1 &
- Demircan Özbalcı 2
BMC Cancer volume 24 , Article number: 552 ( 2024 ) Cite this article
Metrics details
Patients with hematological malignancies often require multidrug therapy using a variety of antineoplastic agents and supportive care medications. This increases the risk of drug-related problems (DRPs). Determining DRPs in patients hospitalized in hematology services is important for patients to achieve their drug treatment goals and prevent adverse effects. This study aims to identify DRPs by the clinical pharmacist in the multidisciplinary team in patients hospitalized in the hematology service of a university hospital in Turkey.
This study was conducted prospectively between December 2022 and May 2023 in the hematology service of Suleyman Demirel University Research and Application Hospital in Isparta, Turkey. DRPs were determined using the Pharmaceutical Care Network Europe (PCNE) 9.1 Turkish version.
This study included 140 patients. Older age, longer hospital stay, presence of acute lymphoblastic leukemia, presence of comorbidities, higher number of medications used, and polypharmacy rate were statistically significantly higher in the DRP group than in the non-DRP group ( p < 0.05). According to multivariate logistic regression analysis, the probability of DRP in patients with polypharmacy was statistically significant 7.921 times (95% CI: 3.033–20.689) higher than in patients without polypharmacy ( p < 0.001).Every 5-day increase in the length of hospital stay increased the likelihood of DRP at a statistically significant level (OR = 1.476, 95% CI: 1.125–1.938 p = 0.005). In this study, at least one DRP was detected in 69 (49.3%) patients and the total number of DRPs was 152. Possible or actual adverse drug events (96.7%) were the most common DRPs. The most important cause of DRPs was drug choice (94.7%), and the highest frequency within its subcategories was the combination of inappropriate drugs (93.4%).
Conclusions
This study shows the importance of including a clinical pharmacist in a multidisciplinary team in identifying and preventing DRPs in the hematology service.
Peer Review reports
Hematological malignancies include a variety of diseases such as Hodgkin lymphoma, non-Hodgkin lymphoma, leukemias, and multiple myeloma [ 1 ]. New treatment strategies were developed for all these diseases and the survival time of patients was increased [ 2 , 3 , 4 ]. Hematological cancer patients require combination therapy using a variety of antineoplastic agents and supportive care medications [ 5 ]. Polypharmacy is the use of multiple medications and is common in this patient group [ 6 ]. Polypharmacy increases the risk of drug-related problems (DRPs) [ 7 ]. DRPs are defined as an event or situation involving medication that interferes with desired health outcomes. DRPs include inappropriate dosage and method of administration, drug-drug interactions, drug omissions and monitoring deficiencies, and adverse drug reactions [ 8 , 9 ]. This may fail to achieve drug therapy goals or harm the patient [ 10 ]. It also causes prolonged hospital stay, readmission, and increased mortality [ 11 , 12 , 13 ].
Within a multidisciplinary team, clinical pharmacists can detect and prevent DRPs early through comprehensive medication review [ 9 , 14 ]. Clinical pharmacy services are pretty new in Turkey. Although there have been postgraduate programs (master’s degree, doctorate) related to clinical pharmacy for years, there has been a clinical pharmacy specialty program since 2018 [ 15 ]. Only graduates of the clinical specialty program can work in public hospitals [ 16 ]. Therefore, the number of clinical pharmacists actively working in hospitals is relatively low.
The contributions of clinical pharmacists in identifying and preventing DRPs have been demonstrated in many clinical departments [ 14 , 17 , 18 , 19 , 20 ]. However, studies on determining DRPs in patients with hematological malignancy are limited [ 5 , 9 , 21 , 22 , 23 ]. In a study conducted in an onco-hematology and bone marrow transplant unit in Brazil [ 23 ], the frequency of DRPs was found to be 135 (9%). 135 interventions were performed by the pharmacist and 90% were accepted. In a study conducted in France [ 9 ], 552 (12.6%) DRPs were found. Medication problems were mostly related to anti-infective agents, and oncologists’ acceptance of interventions was found to be high (96%). In a study conducted in Korea [ 5 ], a total of 1187 DRPs were identified in 438 (23.9%) of 1836 hospitalized patients with hematological malignancy. Pharmacists’ intervention was accepted by 88.3%. In a study examining the clinical and economic impact of pharmacist interventions in an outpatient hematology-oncology department in France [ 24 ], a total of 1970 pharmacist interventions were performed, corresponding to an average of 3.5 pharmacist interventions/patient, and the total cost savings was €175,563. The clinical pharmacist’s cost-benefit ratio was found to be €3.7 for every €1 invested.
As far as it is known, no study shows that DRPs are determined by the clinician in the hematology service in Turkey. Therefore, this study aims to determine drug-related problems by a clinical pharmacist within the multidisciplinary team in patients with a diagnosis of hematological malignancy hospitalized in the hematology services of a university hospital in Turkey.
Study design
This study was conducted prospectively between December 2022 and May 2023 in the hematology service of Suleyman Demirel University Research and Application Hospital in Isparta, Turkey.
All patients over the age of 18 who were hospitalized in the hematology service for more than 24 h were included in the study. Only the first hospitalization of each patient was evaluated. Informed consent was obtained from all participants before they participated in the study. Ethics Committee approval was obtained from Suleyman Demirel University Faculty of Medicine Clinical Research Ethics Committee (Approval No:274, Date:28.09.2022).
The service where the research was conducted had 15 beds and two physicians and assistant physicians were working. There was no stem cell transplant unit in the hospital. Isparta was a small city with a population of 449,777 [ 25 ]. The hospital and patient population where the study was conducted were smaller than the hospitals in Turkey’s metropolitan cities.
Sample size
The sample size was calculated based on the approximate number of patients admitted to the hematology service during the previous 6 months. With the Raosoft sample size calculator, the sample size was found to be minimum 123 with a population size of 180, 5% margin of error, 95% confidence interval and 50% distribution rate [ 26 ].
Data collection
The clinical pharmacist in the study was an academic, did not routinely work in this hospital, and was present at the hospital for this study. The clinical pharmacist performed comprehensive medication reviews of patients and provided interventions. The patients’ socio-demographic characteristics, history, diagnosis, comorbidities, medications used, laboratory test results, and interventions were recorded in the data collection form by the clinical pharmacist. The patients’ data were obtained from the hospital database, patient files, and patients. In general, interventions were made through verbal communication. UpToDate® and Sanford Guide to Antimicrobial Therapy Mobile® software were used for the interventions [ 27 , 28 ]. The Lexicomp Drug Interactions® tool, accessed via UpToDate®, was used to identify drug-drug interactions [ 29 ]. According to Lexicomp Drug Interactions®, drug interactions consist of five categories. A -no known interaction, B- no action required, C -monitor therapy, D- consider changing therapy, X- avoid combination. The presence of at least one of the risk levels C, D, and X was defined as a potential drug-drug interactions because it was clinically significant [ 30 , 31 , 32 ]. Polypharmacy was defined as the use of 5 or more medications [ 33 , 34 ].
DRPs were determined using the Pharmaceutical Care Network Europe (PCNE) 9.1 Turkish version. PCNE 9.1 has 3 primary fields for problems, 9 primary fields for causes, 5 primary fields for planned interventions, 3 primary fields for acceptance level (of interventions), and 4 primary fields for status of the problem. Problems include treatment effectiveness and safety, while reasons include drug selection, drug form dose selection, and treatment duration [ 35 ].
Statistical analysis
Statistical analysis was performed using SPSS 20. Continuous variables were expressed as median-interquartile range, and categorical variables were expressed as percentage and frequency. The normality of the data was analysed with the Kolmogorov-Smirnov test. The Mann-Whitney U test was used to compare continuous independent variables, and the Chi-Square test was used for categorical variables. The Pearson Chi-Square (> 25), the Continuity Correction (5–25), and the Fisher’s Exact test (< 5) were used according to the number of cases. Multiple logistic regression analysis was performed to determine the best predictor(s) which effect on the presence of DRP. Any variable whose univariable test had a p value < 0.10 was accepted as a candidate for the multivariable model along with all variables of known clinical importance. Odds ratios, 95% confidence intervals and Wald statistics for each independent variable were also calculated. A p-value smaller than 0.05 was considered statistically significant.
This study included 140 patients. Almost half (55%) of the patients were male and the median age was 65 (55–74) years. The median length of hospital stay was 8 (5–14) days. The median number of medications used by the patients was 6 (4–7). Polypharmacy was present in 67% of the patients. Older age, longer hospital stay, presence of acute lymphoblastic leukemia, presence of comorbidities, higher number of medications used, and polypharmacy rate were statistically significantly higher in the DRP group than in the non-DRP group ( p < 0.05). Table 1 shows the socio-demographic and clinical characteristics of the patients.
At least one DRP was detected in 69 (49.3%) patients and the total number of DRPs was 152. Possible or actual adverse drug events (96.7%) were the most common DRPs. The most important cause of DRPs were drug choice (94.7%), and the highest frequency within its subcategories was the combination of inappropriate drugs (93.4%). Potential drug-drug interactions were detected in at least one C risk in 43 (30.7%) patients, at least one D risk in 11 (7.9%) patients, and at least one X risk in 6 patients (4.3%).
The clinical pharmacist performed 104 (68.4%) interventions on the prescriber, of which 100 (96.15%) were accepted and fully implemented. All 120 DRPs (78.9%) were resolved, and 28 DRPs (18.4%) were not possible or necessary to be resolved. Table 2 shows the classification of DRPs. Table 3 shows some examples of interventions performed by the clinical pharmacist. Anticancer drugs such as venetoclax, lenalidomide, and dasatinib were examples of potential drug-drug interactions. Table 4 shows the adverse effects that occurred. Drug-related nephrotoxicity was the most common adverse effect. Table 5 shows the results of the multivariate logistic regression analysis: factors most predictive of the presence of DRP. Polypharmacy and length of hospitalization were the most determinant factors in differentiating the groups with and without DRP, respectively. After adjustment for other factors, the likelihood of the presence of DRP was statistically significantly 7.921 folds (95% CI: 3.033–20.689) higher in patients with polypharmacy compared to patients without polypharmacy ( p < 0.001). On the other hand, each 5-day increase in the duration of hospitalization continued to increase the likelihood of the presence of DRP by a statistically significant (OR = 1.476, 95% CI: 1.125–1.938 p = 0.005).
In our study, 152 DRPs were identified and 120 DRPs were totally solved. This reveals the importance of involving the clinical pharmacist in a multidisciplinary team. The most common DRPs in our study were possible or actual adverse drug events. Since the patient population was generally elderly and cancer patients, they were exposed to polypharmacy and drug-drug interactions. Additionally, this was not surprising since the risk of exposure to possible or actual adverse drug events was high due to the anticancer medications they use [ 36 , 37 ]. Adverse drug events varied across studies. While this rate was 28.6% in the study conducted by Kim et al. [ 5 ] in the hematology service, it was 78.6% in the study conducted by Umar et al. [ 14 ] in the oncology service. Since Kim et al.‘s study [ 5 ] was retrospective, the rate of possible or actual adverse effects may have been found to be low. Additionally, although both studies used the PCNE classification system, it was not mentioned in Kim et al.‘s study which drug-drug interaction tool was used and which risk ratio for drug-drug interaction was considered clinically significant.
In our study, most of the causes of DRPs were related to drug selection and their subgroup, inappropriate combination of drugs. Drug-drug interaction rates in the studies were 14.3%, 7.4%, 13.6%, and 73.2%, respectively [ 5 , 9 , 14 , 23 ]. Differences in this rate may be due to polypharmacy rates, differences in healthcare services, and different drug-drug interaction software [ 38 , 39 ]. Most of the potential drug-drug interactions in our study were at risk C (monitor therapy). Therefore, in some drug-drug interactions that required monitoring, only the physician was informed, and in others, intervention was recommended to the prescriber. Drug-drug interactions were mostly related to supportive medications. In our study, anticancer drugs such as venetoclax, lenalidomide, bortezomib, and dasatinib had potential drug-drug interactions. Venetoclax had potential drug-drug interactions with verapamil-trandolapril at increased risk of D. Verapamil-trandolapril is a CYP3A4 inhibitor [ 40 ], and concomitant use with venetoclax increases the concentration of venetoclax. It is recommended that the dose of venetoclax be reduced by 50% [ 29 , 41 , 42 , 43 ]. Also, there was a potential drug-drug interaction at risk X (avoid combination) between dasatinib and pantoprazole. Concomitant use of these two agents decreases the concentration of dasatinib [ 44 ]. Bortezomib had potential drug-drug interactions at risk level C with antihypertensive drugs and drugs used in the treatment of benign prostatic hyperplasia, such as tamsulosin [ 29 ]. Bortezomib may have a blood pressure-lowering effect, so if used concomitantly with an antihypertensive drug or another drug that can lower blood pressure, the patient should be monitored for hypotension [ 45 , 46 ]. In our study, there was also a potential drug-drug interaction between bortezomib and diltiazem at risk level C. Diltiazem, as a CYP3A4 inhibitor, may increase bortezomib concentration [ 40 ]. The bortezomib prescribing information emphasizes that in this case, it should be monitored for toxicity and dose reduction should be made if necessary [ 29 , 47 ]. In our study, there was a potential drug-drug interaction between lenalidomide and dexamethasone. When lenalidomide and dexamethasone are used together, venous thromboembolism prophylaxis should be considered, as the thrombogenic activity of lenalidomide may increase [ 29 , 48 , 49 ]. Additionally, potential drug-drug interactions with antiemetics and opioid-derived analgesics were frequently observed in our study. Identifying, monitoring, and intervening when necessary, drug-drug interactions are very important in cancer patients, and clinical pharmacists have important roles in this regard [ 50 , 51 ].
Dose selection was the second important DRP in our study. Renal dosage adjustment of drugs is significant, especially in patients who develop acute kidney injury [ 52 ]. Even if the drugs are started at the correct dose, the dose of the drugs should be monitored and adjusted when necessary in case of liver and renal dysfunction [ 52 , 53 ]. In our study, antimicrobials were among the drugs that required dosage adjustment according to renal function. This was due to the fact that although infectious disease physicians started antimicrobials at the correct dose, these doses were sometimes not followed up later.
Drug-induced nephrotoxicity was a common adverse event in our study, similar to other studies [ 17 , 54 ]. Also, venetoclax-related hyperuricemia, hyperkalemia and neutropenia were observed in some patients. In a study investigating the incidence of venetoclax-related toxicity risk in British Columbia, hyperkalemia and hyperphosphatemia were observed in 9 patients (27%), and hyperuricemia was observed in 7 patients (21%) [ 55 ]. In their study by Koehler et al., venetoclax-related hyperkalemia (31%) and hyperuricemia (5%) were observed [ 56 ]. In our study, one acute lymphoblastic leukemia patient had vincristine-induced neuropathy. Vincristine-induced neuropathy is a common side effect and its incidence is between 30 and 40% [ 57 ].
The clinical pharmacist’s acceptance rate of the interventions was good. In general, interventions regarding renal and hepatic dosing were accepted. The clinical pharmacist did not intervene in some cases that required monitoring (for example, category C drug interactions) and only informed the physician. These were evaluated as not possible or necessary to resolve the problem.
One of the strengths of the study is that the acceptability of the interventions was higher than other studies [ 5 , 18 , 23 , 58 ]. Additionally, our study was the first study in Turkey to reveal DRPs in detail in this vulnerable patient population in the hematology service. One of the limitations of our study is that it was conducted in a single center and with a small number of patients. In addition, the clinical pharmacist in the study was an academician and did not work full-time in the hospital, but worked at certain times of the day. This may have caused some DRPs not to be determined.
According to our study, a high frequency of DRPs and possible or actual adverse drug events were detected in patients. Older age, longer hospital stay, presence of acute lymphoblastic leukemia, presence of comorbidities, higher number of medications used, and polypharmacy rate were statistically significantly higher in the DRP group than in the non-DRP group According to the results of multiple logistic regression analysis, polypharmacy and length of hospital stay were the most determining factors in distinguishing between groups with and without DRP. The most common DRP was related to possible or actual adverse drug events. The most common cause of DRPs was drug selection and its subgroup, inappropriate combination of drugs. Also, our study shows the importance of including a clinical pharmacist in a multidisciplinary team in identifying and preventing DRPs in the hematology service.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Allart-Vorelli P, Porro B, Baguet F, Michel A, Cousson-Gélie F. Haematological cancer and quality of life: a systematic literature review. Blood cancer J. 2015;5(4):e305–e.
Article CAS PubMed PubMed Central Google Scholar
Westerweel PE, Te Boekhorst PA, Levin M-D, Cornelissen JJ. New approaches and treatment combinations for the management of chronic myeloid leukemia. Front Oncol. 2019;9:665.
Article PubMed PubMed Central Google Scholar
Alanazi F, Kwa FA, Burchall G, Jackson DE. New generation drugs for treatment of multiple myeloma. Drug Discov Today. 2020;25(2):367–79.
Article CAS PubMed Google Scholar
Wang L, Li L-r, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. 2020;13:1–23.
Kim MG, Jeong CR, Kim HJ, Kim JH, Song Y-K, Kim KI, et al. Network analysis of drug-related problems in hospitalized patients with hematologic malignancies. Support Care Cancer. 2018;26:2737–42.
Article PubMed Google Scholar
Bushardt RL, Massey EB, Simpson TW, Ariail JC, Simpson KN. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3(2):383–9.
Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63(2):187–95.
Kaufmann CP, Stämpfli D, Hersberger KE, Lampert ML. Determination of risk factors for drug-related problems: a multidisciplinary triangulation process. BMJ Open. 2015;5(3):e006376.
Delpeuch A, Leveque D, Gourieux B, Herbrecht R. Impact of clinical pharmacy services in a hematology/oncology inpatient setting. Anticancer Res. 2015;35(1):457–60.
PubMed Google Scholar
Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
CAS PubMed Google Scholar
El Morabet N, Uitvlugt EB, van den Bemt BJ, van den Bemt PM, Janssen MJ, Karapinar-Çarkit F. Prevalence and preventability of drug‐related hospital readmissions: a systematic review. J Am Geriatr Soc. 2018;66(3):602–8.
Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41(2):192–9.
CAS Google Scholar
Reinau D, Furrer C, Stämpfli D, Bornand D, Meier CR. Evaluation of drug-related problems and subsequent clinical pharmacists’ interventions at a Swiss university hospital. J Clin Pharm Ther. 2019;44(6):924–31.
Umar RM, Apikoglu-Rabus S, Yumuk PF. Significance of a clinical pharmacist-led comprehensive medication management program for hospitalized oncology patients. Int J Clin Pharm. 2020;42:652–61.
Kara E, Okuyan B, Demirkan K, Sancar M. Question awaiting answer during the development and implementation stage of clinical pharmacy services in Turkey: who is clinical pharmacist? J Lit Pharm Sci. 2021;10(1):109–18.
Article Google Scholar
Law on Amending the Law on Pharmacists and Pharmacies and Certain Decree Laws. Official gazette of the Republic of Turkey, 29175, 14. Oct 2014. https://www.resmigazete.gov.tr/eskiler/2014/11/20141114-3.htm Accessed Sep 2023.
Albayrak A, Başgut B, Bıkmaz GA, Karahalil B. Clinical pharmacist assessment of drug-related problems among intensive care unit patients in a Turkish university hospital. BMC Health Serv Res. 2022;22(1):1–7.
Hailu BY, Berhe DF, Gudina EK, Gidey K, Getachew M. Drug related problems in admitted geriatric patients: the impact of clinical pharmacist interventions. BMC Geriatr. 2020;20(1):1–8.
Ali MAS, Khedr EMH, Ahmed FAH, Mohamed NNE. Clinical pharmacist interventions in managing drug-related problems in hospitalized patients with neurological diseases. Int J Clin Pharm. 2018;40:1257–64.
Malfará M, Pernassi M, Aragon D, Carlotti A. Impact of the clinical pharmacist interventions on prevention of pharmacotherapy related problems in the paediatric intensive care unit. Int J Clin Pharm. 2018;40:513–9.
Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26(4):866–72.
Farias TF, Aguiar KS, Rotta I, Belletti KMS, Carlotto J. Implementing a clinical pharmacy service in hematology. Einstein (São Paulo). 2016;14:384–90.
Visacri MB, Tavares MG, Barbosa CR, Duarte NC, Moriel P. Clinical pharmacy in onco-hematology and bone marrow transplant: a valuable contribution to improving patient safety. J Oncol Pharm Pract. 2021;27(5):1172–80.
De Grégori J, Pistre P, Boutet M, Porcher L, Devaux M, Pernot C, et al. Clinical and economic impact of pharmacist interventions in an ambulatory hematology–oncology department. J Oncol Pharm Pract. 2020;26(5):1172–9.
TUIK. The Results of Address Based Population Registration System. 2023. https://data.tuik.gov.tr/Bulten/Index?p=The-Results-of-Address-Based-Population-Registration-System-2023-49684&dil=2 . Accessed Feb 2024.
Raosoft Inc. (2004) RaoSoft® sample size calculator. http://www.raosoft.com/samplesize.html . Accessed Aug 2022.
UpToDate®. https://www.uptodate.com / Accessed Dec 2022.
Sandford J, Gilbert D, Mae Llering R, Sande M. Guide to antimicrobial therapy. Viena, Virginia: Antimicrobial therapy. Inc; 1997.
Google Scholar
Uptodate Lexicomp Drugs & Drug Interaction. https://www.uptodate.com/home/drugs-drug-interaction Accessed Dec 2022.
Kovačević M, Vezmar Kovačević S, Miljković B, Radovanović S, Stevanović P. The prevalence and preventability of potentially relevant drug-drug interactions in patients admitted for cardiovascular diseases: a cross‐sectional study. Int J Clin Pract. 2017;71(10):e13005.
Ren W, Liu Y, Zhang J, Fang Z, Fang H, Gong Y, et al. Prevalence of potential drug–drug interactions in outpatients of a general hospital in China: a retrospective investigation. Int J Clin Pharm. 2020;42:1190–6.
Luzze B, Atwiine B, Lugobe HM, Yadesa TM. Frequency, severity, and factors associated with clinically significant drug-drug interactions among patients with cancer attending Mbarara Regional Referral Hospital Cancer Unit, Uganda. BMC Cancer. 2022;22(1):1–11.
Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–95.
Turner JP, Jamsen KM, Shakib S, Singhal N, Prowse R, Bell JS. Polypharmacy cut-points in older people with cancer: how many medications are too many? Support Care Cancer. 2016;24:1831–40.
Pharmaceutical Care Network Europe. PCNE classification V 9.1. https://www.pcne.org/workinggroups/2/drug-related-problem-classification . 2020;0. Accessed Dec 2022.
Maggiore RJ, Gross CP, Hurria A. Polypharmacy in older adults with cancer. Oncologist. 2010;15(5):507–22.
Balducci L, Goetz-Parten D, Steinman MA. Polypharmacy and the management of the older cancer patient. Ann Oncol. 2013;24(Suppl 7):vii36–40.
Kheshti R, Aalipour M, Namazi S. A comparison of five common drug–drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract. 2016;5(4):257.
Hovstadius B, Petersson G. Factors leading to excessive polypharmacy. Clin Geriatr Med. 2012;28(2):159–72.
Renton KW. Inhibition of hepatic microsomal drug metabolism by the calcium channel blockers diltiazem and verapamil. Biochem Pharmacol. 1985;34(14):2549–53.
Lai C, Bhansali RS, Kuo EJ, Mannis G, Lin RJ. Older adults with newly diagnosed AML: hot topics for the practicing clinician. Am Soc Clin Oncol Educ Book. 2023;43:e390018.
US Food and Drug Administration. Venetoclax tablets. 2020 https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208573s020s021lbl.pdf . Accessed Feb 2024.
BCCA protocol. Therapy of Acute Myeloid Leukemia using Azacitidine and Venetoclax. 2022. http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Leukemia-BMT/ULKAMLAVEN_Protocol.pdf . Accessed Feb 2024.
Takahashi N, Miura M, Niioka T, Sawada K. Influence of H2-receptor antagonists and proton pump inhibitors on dasatinib pharmacokinetics in Japanese leukemia patients. Cancer Chemother Pharmacol. 2012;69:999–1004.
San Miguel J, Bladé J, Boccadoro M, Cavenagh J, Glasmacher A, Jagannath S, et al. A practical update on the use of bortezomib in the management of multiple myeloma. Oncologist. 2006;11(1):51–61.
Ho M, Moscvin M, Low SK, Evans B, Close S, Schlossman R, et al. Risk factors for the development of orthostatic hypotension during autologous stem cell transplant in patients with multiple myeloma. Leuk Lymphoma. 2022;63(10):2403–12.
US Food and Drug Administration. Bortezomi for Injection For subcutaneous or intravenous use. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021602s040lbl.pdf . Accessed Feb 2024.
Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496–520.
Falanga A, Ay C, Di Nisio M, Gerotziafas G, Jara-Palomares L, Langer F, et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34(5):452–67.
Ansari J. Drug interaction and pharmacist. J Young Pharm. 2010;2(3):326–31.
Blower P, De Wit R, Goodin S, Aapro M. Drug–drug interactions in oncology: why are they important and can they be minimized? Crit Rev Oncol Hematol. 2005;55(2):117–42.
Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65:757–73.
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–61.
Martins RR, Silva LT, Lopes FM. Impact of medication therapy management on pharmacotherapy safety in an intensive care unit. Int J Clin Pharm. 2019;41(1):179–88.
Lee C, Markarian A, Ladha F, Nakashima L, de Lemos M, Schaff K, et al. Real-world incidence of venetoclax toxicities in British Columbia. J Oncol Pharm Pract. 2022;28(5):1163–9.
Koehler AB, Leung N, Call TG, Rabe KG, Achenbach SJ, Ding W, et al. Incidence and risk of tumor lysis syndrome in patients with relapsed chronic lymphocytic leukemia (CLL) treated with venetoclax in routine clinical practice. Leuk Lymphoma. 2020;61(10):2383–8.
Li G-z, Hu Y-h, Li D-y, Zhang Y, Guo H-l, Li Y-m, et al. Vincristine-induced peripheral neuropathy: a mini-review. Neurotoxicology. 2020;81:161–71.
Ayhan YE, Karakurt S, Sancar M. The effect of the clinical pharmacist in minimizing drug-related problems and related costs in the intensive care unit in Turkey: a non‐randomized controlled study. J Clin Pharm Ther. 2022;47(11):1867–74.
Download references
Acknowledgements
Not applicable.
No funding provided.
Author information
Authors and affiliations.
Department of Clinical Pharmacy, Faculty of Pharmacy, Suleyman Demirel University, Isparta, Türkiye
Aslınur Albayrak
Department of Hematology, Faculty of Medicine, Suleyman Demirel University, Isparta, Türkiye
Demircan Özbalcı
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: AA, DÖ; Data Collection: AA; Analysis and interpretation of data: AA; Drafting of the manuscript: AA; Critical revision of the manuscript for important intellectual content: AA, DÖ. All the authors read and approved the final manuscript.
Corresponding author
Correspondence to Aslınur Albayrak .
Ethics declarations
Ethics approval and consent to participate.
Ethics Committee approval was obtained from Suleyman Demirel University Faculty of Medicine Clinical Research Ethics Committee (Approval No:274, Date:28.09.2022). We confirm that all methods were performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Albayrak, A., Özbalcı, D. Determination of drug-related problems in the hematology service: a prospective interventional study. BMC Cancer 24 , 552 (2024). https://doi.org/10.1186/s12885-024-12291-w
Download citation
Received : 08 November 2023
Accepted : 19 April 2024
Published : 02 May 2024
DOI : https://doi.org/10.1186/s12885-024-12291-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical pharmacist
- Drug related problem
- Hematologic malignancy
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Peer review is a mutual responsibility among fellow scientists, and scientists are expected, as part of the academic community, to take part in peer review. If one is to expect others to review their work, they should commit to reviewing the work of others as well, and put effort into it. 2) Be pleasant. If the paper is of low quality, suggest ...
The most common types are: Single-blind review. Double-blind review. Triple-blind review. Collaborative review. Open review. Relatedly, peer assessment is a process where your peers provide you with feedback on something you've written, based on a set of criteria or benchmarks from an instructor.
Peer review cannot improve poor research, but it can often "correct, enhance and strengthen the statistical analysis of data and can markedly improve presentation ... For any journal it is of great importance whether the paper is likely to be cited and you should help the Editor in Chief to make the decision as to whether the manuscript is a ...
More importantly, peer review can assess, correct, and improve the interpretation, meaning, importance, and communication of research results—and importantly, confirm that conclusions emanate strictly from those results. Peer review may occasionally fundamentally revise or even reverse clinical research interpretations and recommendations.
Peer review is important because it serves to uphold the quality of the literature as well as advance the scientific knowledgebase 10. In theory, peer reviewers serve to filter out poor research. ... the field and/or critical appraisal skills are respected enough to be entrusted with gauging the quality of scientific research. Working as a peer ...
Authors rely on the comments of reviewers to make sure that their work meets publication standards. Research has shown that authors place a great value on peer review. An important study of review quality reported a survey of authors (320 of 528 surveyed) and editors (3) on the quality of reviews. The editors represented three major nursing ...
The manuscript peer review process helps ensure scientific publications are credible and minimizes errors. Peer review is an essential element of the scientific publishing process that helps ensure that research articles are evaluated, critiqued, and improved before release into the academic community. Take a look at the significance of peer review in scientific publications, the typical steps ...
The peer-review process is the longstanding method by which research quality is assured. On the one hand, it aims to assess the quality of a manuscript, with the desired outcome being (in theory ...
The major advantage of a peer review process is that peer-reviewed articles provide a trusted form of scientific communication. Since scientific knowledge is cumulative and builds on itself, this trust is particularly important. Despite the positive impacts of peer review, critics argue that the peer review process stifles innovation in ...
It is also safe to say that peer review is a critical element of the scholarly publication process and one of the major cornerstones of the academic process. It acts as a filter, ensuring that research is properly verified before being published. And it arguably improves the quality of the research, as the rigorous review by like-minded experts ...
Background. Reviewers play a pivotal role in scholarly publishing. The peer review system exists to validate academic work, helps to improve the quality of published research, and increases networking possibilities within research communities. Despite criticisms, peer review is still the only widely accepted method for research validation and ...
Peer review brings academic research to publication in the following ways: Evaluation - Peer review is an effective form of research evaluation to help select the highest quality articles for publication.; Integrity - Peer review ensures the integrity of the publishing process and the scholarly record. Reviewers are independent of journal publications and the research being conducted.
Peer review is designed to assess the validity, quality and often the originality of articles for publication. Its ultimate purpose is to maintain the integrity of science by filtering out invalid or poor quality articles. From a publisher's perspective, peer review functions as a filter for content, directing better quality articles to ...
This editorial provides an overview of the importance of peer reviewing, generally and to the Review of Religious Research journal. Several practical recommendations are offered to reviewers. Following these practices will aid reviewers in communicating their feedback clearly to the editor and having it received well by authors.
Medical research goes through peer review before publication in a journal to ensure that the findings are reliable and suitable for the audience. Peer review is important for preventing false ...
This emphasizes a new level of awareness for editors and peer reviewers addressing objectivity and bias in reviews and, more broadly, how research is conducted. The goal of peer review is to provide the editor and author with comments that evaluate the soundness and validity of the research, the methodology, the results, and conclusions ...
Peer Review is a critical part of evaluating information. It is a process that journals use to ensure the articles they publish represent the best scholarship currently available, and articles from peer reviewed journal are often grounded in empirical research. When an article is submitted to a peer reviewed journal, the editors send it out to ...
Peer review is embedded in the core of our knowledge generation systems, perceived as a method for establishing quality or scholarly legitimacy for research, while also often distributing academic prestige and standing on individuals. Despite its critical importance, it curiously remains poorly understood in a number of dimensions. In order to address this, we have analysed peer review to ...
Peer review serves an essential role in research and its dissemination of results, though the quality of peer review varies greatly. This session will overview the essential variables for superior peer review, including the importance of reviewer confidentiality and security, to help you avoid being THAT Reviewer.
Peer review under review Piniewski and his colleagues conducted three analyses. First, they uploaded five peer-review reports from the two manuscripts that his laboratory had submitted to a ...
A review of how psychologists use tools of assessment to ensure reliability and validity in research
Peer Review reports. ... Long-term follow-up of these children seems important, and more research is needed focusing on the effect of using quality of life parameters as most important measurements for recovery. Data availability. No datasets were generated or analysed during the current study.
2.1. Definition of organizational culture. OC is a set of norms, values, beliefs, and attitudes that guide the actions of all organization members and have a significant impact on employee behavior (Schein, Citation 1992).Supporting Schein's definition, Denison et al. (Citation 2012) define OC as the underlying values, protocols, beliefs, and assumptions that organizational members hold, and ...
The findings highlighted the multidimensionality of HIV notification emphasizing the importance of tailored support and education to enhance the notification process for PLHIV and their networks. ... Peer Review reports. Introduction. HIV notification is a process through which people ... The study was carried out by a research team ...
The most important cause of DRPs was drug choice (94.7%), and the highest frequency within its subcategories was the combination of inappropriate drugs (93.4%). Conclusions. This study shows the importance of including a clinical pharmacist in a multidisciplinary team in identifying and preventing DRPs in the hematology service.