
- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search

IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
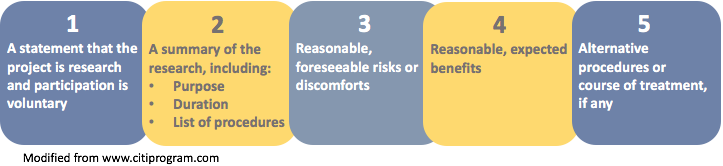
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
Informed consent guidance.
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/17/24
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Brief protocol for exempt research including data management and security questionnaire
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Child assent 12-14
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breathe (Sheff)
- v.14(2); 2018 Jun

How to obtain informed consent for research
1 University of Messina, “G. Martino” Hospital, Messina, Italy
Amelia Licari
2 University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [1]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [2]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [3].
Short abstract
The process of obtaining informed consent for clinical trials is tightly regulated; complications arise in circumstances when consent may be waived, or when needed from vulnerable populations http://ow.ly/rEMe30j5MVq
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [ 1 ]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [ 2 ]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [ 3 ].
The informed consent form must be written in language easily understood by the subjects, it must minimise the possibility of coercion or undue influence, and the subject must be given sufficient time to consider participation. However, informed consent is not merely a form that is signed, but is a process in which the subject has an understanding of the research and its risks, and it is tightly described in ethical codes and regulations for human subject research [ 2 ].
Educational aims
- To provide a comprehensive overview of issues in obtaining informed consent in clinical research.
- To describe the process of obtaining informed consent in clinical trials.
- To highlight the circumstances under which informed consent can be waived.
- To review the setting of obtaining informed consent from “vulnerable populations”.
The informed consent process
The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [ 4 ]. Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation. Conditions posing practical challenges in obtaining informed consent from the real subject may include situations of medical emergency or obtaining consent from “vulnerable” subjects and/or children [ 5 ].
Research-related information must be presented to enable people to voluntarily decide whether or not to participate as a research subject. For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject’s participation; a detailed description of study treatment or intervention and of any experimental procedures (including, in the case of randomised clinical trials (RCTs), also blinding and randomisation); a statement that participation in research is voluntary; probable risks and benefits associated with research participation; details of the nature of the illness and possible outcome if the condition is left untreated; availability, risks and benefits of alternative treatments; information about procedures adopted for ensuring data protection/confidentiality/privacy, including duration of storage of personal data; details about the handling of any incidental findings of the research; description of any planned genetic tests; details of insurance coverage in case of injury; reference contacts for any further answers to pertinent questions about the research and the subject’s rights and in case of any research-related injury to the subject; and any other information that seems necessary for an informed decision to be taken by the subject. Of particular importance, a statement offering the subject the opportunity to withdraw at any time from the research without consequences must be provided during the information disclosure [ 2 ]. Specific information should be provided in case of research projects involving children, incapacitated adults not able to give informed consent, illiterate populations, etc. (as will be described later in this article).
The information about the research should be given by a physician or by other individuals ( i.e. researchers) with appropriate scientific training and qualifications [ 6 ]. Furthermore, the location where the informed consent is being discussed, and the subject’s physical, emotional and psychological capability, must be taken into consideration when taking consent from a human subject.
Informed consent: when is it not necessary?
After institutional review board (IRB) or independent ethics committee approval is achieved, obtaining informed consent from each human subject prior to his/her participation in clinical trial is mandatory [ 5 ]. However, when specific circumstances occur, the informed consent can be waived, and “research without consent” is possible, which allows enrolment of patients without their consent, under strict regulation [ 7 ]. In order that research without consent is considered justifiable, the following three conditions have to be met: 1) it is impracticable to obtain consent, 2) the research does not infringe the principle of self-determination, and 3) the research provides significant clinical relevance [ 8 ].
The first condition, of “impracticability”, occurs when obtaining informed consent is burdened by high impact in terms of time and economic resources or could compromise the study’s validity [ 8 ]. The second condition means that, although physicians are requested to ensure that the patient has understood the aim of the research and the risks and/or benefits associated with study participation, the researchers are also advised to respect the patient’s decision-making capacity, not interfering with his/her decisions and acting always in the patient’s best interest [ 9 ]. The third condition leads to justification of waiving consent when the clinical relevance and public health importance are potentially high [ 8 ].
The formal literature identifies different types of RCTs and classifies them into three macro-areas: 1) RCTs based on infeasibility of informed consent; 2) RCTs that omit informed consent only for control groups; and 3) RCTs that omit informed consent entirely.
RCTs based on infeasibility of informed consent
Emergency clinical studies, involving critically ill subjects, represent an exception to the requirement of informed consent. The investigated life-saving therapy and the medical intervention may be required immediately, not permitting the researchers to wait and respect all procedures of obtaining informed consent. Within this context, the researchers will be able to proceed with patient recruitment, also without the subject’s consent to treatment, when, prior to the study, the IRB has ascertained the presence of mandatory conditions ( table 1 ) [ 10 ].
Table 1
Conditions to be met in emergency clinical study
Cluster randomised studies include cluster-cluster and individual-cluster research [ 11 ]. In cluster-cluster designs ( e.g. studies on infectious disease prevention), the intervention involves the entire target community, so that single subjects cannot refuse it [ 12 ]. Conversely, in individual-cluster designs ( e.g. studies on primary care), although the intervention involves all the selected community, the right to refuse treatment is allowed. Under this circumstance, the omission of informed consent is justified only when the treatment refusal undermines the validity of the research study and/or procedures [ 13 ].
RCTs that omit informed consent only for control groups
In Zelen’s single-consent model ( e.g. RCTs in infectious or oncological diseases), randomisation occurs prior to any consent, and informed consent is sought only from individuals assigned to experimental treatment [ 14 ]. In the control group, the physicians do not make substantial changes in routine patient care, so informed consent is not required for patient enrolment [ 8 ].
In order to improve study recruitment, Zelen developed the double-consent design. Specifically, informed consent is requested for subjects to be involved in the study but not for the randomisation, preventing psychological distress [ 14 ].
In follow-up studies, the nested consent model ( e.g. for single cohort studies) or cohort multiple RCTs model ( e.g. for multiple cohort studies) is applied. In these variants, patients give their consent for prospective follow-up; however, they remain blinded to any randomised experimental interventions [ 15 ].
In trials using the model of “consent to postponed information”, the informed consent process is carried out after the study is completed [ 16 ].
All these RCT types aim to avoid unnecessary stress in patients who will not receive the new promising experimental treatment. Moreover, these clinical study designs do not affect the standard therapeutic approach or infringe the rights of the patients in the control group; therefore, the clinical trial can proceed without obtaining informed consent [ 8 ].
RCTs that omit informed consent entirely
Based on the fact that patients are assigned to standard care interventions, no informed consent is sought either in low-risk pragmatic RCTs [ 17 ] or in prompted optional randomisation trials [ 18 , 19 ]. However, in a low-risk pragmatic RCT, patients do not have the possibility to choose one of the two standard treatments, whereas in a prompted optional randomisation trial, both the researchers and the enrolled patients can choose one type of treatment over another, despite the randomisation results [ 6 ].
Special needs: vulnerable patients
A “vulnerable population” is defined as a disadvantaged community subgroup unable to make informed choices, protect themselves from inherent or intended risks, or keep their own interests safeguarded [ 20 ]. In the health domain, “vulnerable populations” refers to physical vulnerability ( e.g. pregnant women, fetuses, children, orphans, students, employees, prisoners, the military, and those who are chronically or terminally ill), psychological vulnerability (cognitively and intellectually impaired individuals) and social vulnerability (those who are homeless, from ethnic minorities, are immigrants or refugees) [ 20 ].
Due to a compromised free will and inability to make conscious decisions, several ethical dilemmas (related to communications, privacy and treatment) often arise when research involves these populations. Guaranteeing protection of rights, safety, data privacy and confidentiality of vulnerable subjects are prerogatives of good clinical practice, and law dispositions are regulated and strictly monitored by the applicable authorities [ 21 ].
Physical vulnerability
For a long time, pregnant women were excluded from clinical research because of their “vulnerability”. Although pregnant women are able to make informed and conscious choices, they have been considered “vulnerable” due to the potential risks to the fetus, who is also considered as a “patient” [ 22 ]. More recently, with the consideration of pregnant women as “scientifically complex” rather than “vulnerable” subjects, it has been permitted to involve this category in research trials [ 23 ]. The “scientific complexity” reflects both ethical and physiological complexity. The ethical aspects are secondary to the need to find a balance between interests of the fetus and the mother. The physiological aspects are strictly related to the pregnancy status [ 24 ].
Research studies involving pregnant women and fetuses have to satisfy specific federal regulations ( table 2 ). The following appropriate precautions should be taken in research studies involving pregnant women: no pregnant woman may be involved as a subject in a human clinical research project unless the purpose of the research is to meet the health needs of the mother and the fetus will be placed at risk only to the minimum extent necessary to meet such needs, or the risk to the fetus is minimal [ 25 ].
Table 2
Conditions to be met in research studies involving pregnant women and fetuses
Researchers can enrol pregnant women only when the mother and/or the father are legally competent. In fact, the consent to participate in research may be either self-directed (only the mother’s consent is required) or made with the guidance of the woman’s partner. However, the father’s consent need not be obtained when: 1) the research activity is directed to the health needs of the mother; 2) the father’s identity is doubtful; 3) the father is absent; or 4) a pregnancy from rape has occurred [ 26 ]. The consent signature requirements from the mother and father are summarised in table 3 . Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient’s health (mother and/or fetus) will be in danger.
Table 3
Consent signature requirements for pregnant women and children
# : consent requirements are the same whether the risk is “no more than minimal” or “more than minimal”.
Medical students and employees, who take part in numerous aspects of patient care in primary, secondary and tertiary care settings, are often invited to participate in human studies as volunteers. Frequently, the requesting researcher is their supervisor or instructor, who may push them to participate in the study, which can negatively influence their decision and also violate the consent legitimacy. Therefore, in order to protect these subjects against “coercion” or “undue influence”, when an investigator wishes to recruit medical students or employees, they must first obtain IRB approval for inclusion in the study of these vulnerable subgroups [ 27 ].
Prisoners, defined as any individual involuntarily confined or detained in a penal institution, are considered as “vulnerable” because they may be coerced into study participation, and also, due to both cognitive and psychiatric disorders, they can show an impaired ability to provide voluntary informed consent [ 28 ]. To protect this population, the Office for Human Research Protections has stipulated federal regulations according to which the only studies that may involve prisoners are those with independent and valid reasons for involving them ( table 4 ) [ 25 ].
Table 4
Studies that may involve prisoners
Due to the context of war in which they work, as well as the critical care setting in which they are treated, military subjects often receive medical care and/or participate in biomedical research under an “implied consent” condition. Moreover, the superior–subordinate relationship contributes to favour coercion or undue influence, making this population vulnerable [ 29 ]. To curb this phenomenon and to ensure that participation is truly voluntary, the US Dept of Defense agencies have adopted requirements similar to those that govern medical research that applies to the civilian population. Accordingly, the medical research recruitment session happens in the absence of superiors, and the informed consent is obtained prior to participating in a medical research study. The presence of an ombudsman guarantees and verifies that the participation is voluntary and that the information provided during recruitment is complete, accurate and clear. A payment as an incentive is acceptable but it must not be used to legitimise a coercive interference. Additional protection is provided to students at service academies, especially those aged <18 years. However, when emergency research is conducted or the research study advances the development of a medical product needed by the armed forces, informed consent will not be required [ 29 ].
Psychological vulnerability
Mental disability may compromise the self-determination and decision-making capacities [ 30 ]. Researchers interested in enrolling individuals with cognitive disorders are invited to apply different strategies to promote a better understanding of information-gathering processes. Simplifying the questions and content, adopting supportive technologies, using a more simple language, and spending more time for the information process have been suggested as useful and valid measures. When all these strategies prove to be insufficient, the investigators are required to obtain consent from a legally authorised representative [ 30 ].
Social vulnerability
Similarly to other vulnerable populations, research involving the homeless, ethnic minorities, immigrants and refugees is regulated by laws and specific procedures. Cultural and language differences, “undocumented” migrant status, and the precarious legal positions of these subjects raise several ethical issues, such as whether the participation is truly voluntary, or there are unrealistic expectations, or any benefits for their “status”.
Obtaining informed consent in these groups is extremely complex. A friendly procedure has been identified as the best way to adequately involve these vulnerable groups. A health centre or community building could represent an accessible location. The reimbursement of travel expenses for applicants can be a valid solution to obtain a representative sample for the clinical research. Clear and simple language, emphasising confidentiality, with the help of professional interpreters, can tempt migrants to sign the consent form. Lastly, the possibility of receiving something back in return for their contribution may enable successful enrolment of migrants in research [ 31 ].
Special needs: children
Because of their young age as well as their limited emotional and intellectual abilities, children are considered to be legally incompetent to give valid informed consent; thus, to enrol a child in a research study, the permission by at least one parent or legal representative is mandatory ( table 3 ). For subjects aged <18 years, biological or adoptive parents or legal guardians (persons having both legal capacity and responsibility) can give consent on behalf of their child, exercising free power of choice without any form of coercion. While married mothers and fathers both have parental responsibility, unmarried parents can exert parental responsibility only if they are named individually on the child’s birth certificate. Also, divorced parents maintain parental responsibility, but it is necessary to know to whom the child’s custody has been assigned [ 32 ]. However, on this matter, the European laws and regulations are not harmonised and several discrepancies are present in each country [ 33 ].
Despite potential benefits for the research subjects, the failure of parents to give consent (or their refusal to give consent) is not a rare circumstance [ 34 ]. It can be the case that researchers are dealing with underage parents, so that, although underage parents are responsible for representing their children, as minors themselves they are not considered to be sufficiently mature; therefore, they will be not able to give valid consent. Literacy and socioeconomic levels have been identified as the most common reasons for parental non-response [ 34 ]. Clarity and adequate explanation of research information materials should be part of effective planning to overcome language and social barriers.
In clinical studies in which the adopted methodology constitutes “less than minimal risks” for children, passive parental consent represents a possible way to more easily obtain informed parental consent [ 34 ]. Furthermore, parents can be informed with regard to a possible study involving their children, and, at the time of data collection, only the child’s assent is required. In fact, although the child’s decision-making capacity and understanding of the research project in which he/she will be involved may be limited, the Medical Research Council have shown that, when study details are provided and communicated in a clear and adequate manner, the child can be able to reach a decision and participate consciously in the research [ 35 ]. “Assent” is the term coined to express the child’s willingness to participate in clinical trials despite their young age. The “assent” should include and respect the following key points: 1) helping the child to acquire disease awareness; 2) explaining the potential impact of the experimental treatment; 3) evaluating the child’s ability to understand and adapt to new situations or challenges; and 4) positively influencing the patient’s willingness to participate in clinical trials [ 36 ]. Although the “assent” is not mandatory for research offering a direct benefit for the child, it arises from the need to respect paediatric research subjects [ 37 ]. The evaluation of the capacity to provide the “assent” is based on developmental stage, intellectual abilities and life or disease experience. Usually, the cut-off age of 7 years is used for the beginning of logical thought processes and rational decision making [ 38 ]. However, “assent” for children aged <7 years can be also required once the ability to read and write has been verified [ 32 ]. Figures 1 and and2 2 summarise the parental and assent permission requirements, respectively.

Flow chart of parental permission requirements.

Flow chart of child assent requirements.
When conducting clinical research, the obtaining of informed consent is required. Informed consent is a procedure through which a competent subject, after having received and understood all the research-related information, can voluntarily provide his or her willingness to participate in a clinical trial. However, when it is impracticable to obtain consent, and the research does not infringe the principle of self-determination and also provides significant clinical relevance, the researcher is legally authorised to proceed without informed consent. Furthermore, in order to preserve the self-determination and decision-making rights, specific law dispositions are applied when vulnerable populations are enrolled in clinical trials.
Self-evaluation questions
- a) Diagnosis
- b) Risks and benefits of treatment
- c) Alternatives to treatment
- d) Family’s wishes
- a) When a minor is considered as emancipated
- b) When a patient is found to be incompetent
- c) When immediate treatment is necessary to prevent death or permanent impairment
- d) When the subject is aged >18 years
- a) Minor is married or divorced
- b) Minor on active duty in the US armed forces
- c) Minor is considered self-sufficient by a court
- d) Minor having a son
Suggested answers
- All research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent.
- Voluntary expression of consent and adequate information disclosure about the research are critical and essential elements of the informed consent process.
- When specific circumstances occur, informed consent can be waived: if it is impracticable to obtain consent, if the research does not infringe the principle of self-determination, and if the research provides significant clinical relevance.
- Participation of vulnerable patients in clinical trials is regulated by specific law dispositions.
Conflict of interest: None declared.

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )
- Study protocol
- Open access
- Published: 10 April 2024
Safety and efficacy of ketorolac in improving the prognosis of acute type A aortic dissection patients: a protocol of a randomized, double-blinded, and placebo-controlled study
- Zhikang Lv 1 ,
- Tuo Pan 2 ,
- Haitao Zhang 2 ,
- Yapeng Wang 2 ,
- Yusanjian Matniyaz 1 ,
- Yuxian Tang 3 ,
- Lichong Lu 1 &
- Dongjin Wang 1
Trials volume 25 , Article number: 250 ( 2024 ) Cite this article
234 Accesses
Metrics details
Acute type A aortic dissection (aTAAD) is a critical and life-threatening condition. Previous research has demonstrated that the use of ketorolac not only reduces the progression, incidence, and severity of aortic aneurysms in animal models, but also decreases postoperative mortality and complications in patients undergoing open abdominal aortic aneurysm replacement. However, there is a lack of studies investigating the efficacy of ketorolac in treating aTAAD in humans. Therefore, we conducted a study to evaluate the safety and efficacy of ketorolac in patients with aTAAD. Our hypothesis was that ketorolac treatment for aTAAD patients would meet safety indicators and effectively improve patient prognosis.
Methods/design
This study is a single-center, randomized, double-blinded, and placebo-controlled study. A total of 120 patients with aTAAD will be recruited and will be randomized into the ketorolac group and placebo group with a ratio of 1:1. Ketorolac tromethamine 60 mg per 2 ml will be intramuscularly injected within 2 h before surgery, followed by intramuscular injections of 30 mg per 1 ml BID. on the first and second postoperative days in the Ketorolac group, while 0.9% saline will be administered at the same dose, dosage form, and time in the placebo group. This study aims to evaluate the safety and efficacy of ketorolac in improving the prognosis of aTAAD. The primary endpoint is the composite endpoint event concerning drug-related adverse events. Secondary endpoints include drug-related adverse events, laboratory examination of blood, diagnostic imaging tests, clinical biomarkers, etc.
This study has been approved by the Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College (approval number: 2023–197-02). This study is designed to evaluate the safety and efficacy of ketorolac in patients with aTAAD. All participating patients will sign an informed consent form, and the trial results will be published in international peer-reviewed journals.
Trial registration
The Chinese Clinical Trial Registry ( http://www.chictr.org.cn ) ChiCTR2300074394. Registered on 4 October 2023.
Peer Review reports
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/ ).
Introduction
Background and rationale {6a}.
Acute type A aortic dissection (aTAAD) is considered the most perilous form of aortic disease [ 1 ]. Common causes include hypertension, medial degeneration (cystic medial necrosis), and Marfan syndrome [ 2 ]. Currently, rapid surgical reconstruction remains the primary treatment option for aTAAD [ 3 , 4 , 5 , 6 ]. Based on reported references, the in-hospital mortality rate after surgery stands at 25% [ 7 ]. Consequently, there is a pressing need to investigate alternative approaches that can effectively decrease surgical mortality.
Previous studies have shown that activated macrophages in mice secrete inflammatory factors, which can enhance migration and invasion capabilities, accelerate degradation of the aortic media, and contribute to the development of type A aortic dissection (TAAD) [ 8 , 9 , 10 ]. Additionally, Rho GTPase, an important regulator of cytoskeleton actin and intracellular signal transduction, has been found to be activated in TAAD [ 11 , 12 ]. In the literature, it has been observed that ketorolac, a nonsteroidal anti-inflammatory drug and inhibitor of the Rho GTPase signaling pathway, can reduce the progression, incidence, and severity of aortic disease in animal models [ 13 , 14 ].
Previous clinical research has suggested that ketorolac may reduce postoperative mortality, neurological complications, renal complications, and respiratory failure in patients undergoing open abdominal aortic aneurysm replacement surgery [ 15 ]. However, it is important to note that this study was retrospective and focused specifically on abdominal aortic aneurysm. Additionally, the 2022 American Heart Association (AHA) guidelines have reported controversy regarding the use of ketorolac in the analgesic treatment of aTAAD [ 16 ]. Therefore, our plan is to conduct a single-center, randomized, double-blinded, and placebo-controlled trial to investigate the safety and efficacy of ketorolac in improving the prognosis of aTAAD patients.
Objectives {7}
The objective of this study is to assess the safety and efficacy of ketorolac in improving the prognosis of aTAAD.

Trial design {8}
This study adopts a single-center, parallel-group design, employing randomization, double-blinding, and a placebo-controlled approach. It is a superiority test. Patients diagnosed with aTAAD will be actively recruited and subsequently randomized into either the ketorolac group or the placebo group, maintaining a 1:1 ratio. The study encompasses a follow-up period of 90 days.
Methods: participants, interventions, and outcomes
Study setting {9}.
This trial is conducted at a single center, specifically at the Department of Cardiac Surgery, Nanjing Drum Tower Hospital. Nanjing Drum Tower Hospital serves as a prominent heart center in Nanjing city, located in Jiangsu province, China. Inclusion criteria, as delineated below, are applied to assess the eligibility of patients for recruitment into the study.
Eligibility criteria {10}
The inclusion criteria are as follows:
Patients diagnosed with aTAAD require emergency operation;
Patients aged between 18 and 65; and
Signing an informed consent.
The exclusion criteria are as follows:
Long-term fasting or inability to self eat;
History of malignant tumors;
Weight < 50 kg;
Traumatic aortic dissection;
Patients with Marfan syndrome;
Unstable vital signs require mechanical assistance or rescue before surgery (IABP, ECMO, LVAD, etc.);
Patients required endotracheal intubation before surgery;
After admission, patients with consciousness disorder, central nervous system dysfunction, or evidence of cerebral hypoperfusion;
Preoperative hematemesis, black or bloody stools, or bowel dilation;
Limb ischemia before surgery;
Malperfusion syndrome before surgery;
Patients who need percutaneous interventions to relieve malperfusion;
History of digestive ulcer or chronic gastroenteritis;
Dialysis before admission or a history of renal insufficiency;
History of liver disease;
Allergies to aspirin and non-steroidal anti-inflammatory drugs like ketorolac tromethamine;
Chronic inflammatory diseases, autoimmune diseases, or other situations require long-term use of hormones or non-steroidal drugs;
No cerebral perfusion during deep hypothermic circulatory arrest;
History of Grade 4 surgery or acute myocardial infarction within 90 days;
History of cardiac surgery or operations on great vessels;
Pregnant and lactating women;
Refusing to participate in this clinical trial or sign an informed consent form;
Other situations where the subject deems it unsuitable to participate in this project.
Who will take informed consent? {26a}
Patients diagnosed with aTAAD and planning to undergo surgical treatment will undergo eligibility screening to determine their suitability for participation in this trial. Once the surgeon has assessed the patient as eligible, their family will be invited to meet with the research physician. During this meeting, their family will have the opportunity to ask any questions they may have and sign the informed consent form.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Consent will be sought for the retrospective review of participants’ medical records and the procurement of blood samples to evaluate inflammation, document adverse events, conduct comprehensive blood laboratory examinations, and analyze clinical biomarkers.
Interventions
Explanation for the choice of comparators {6b}.
Patients diagnosed with acute type A aortic dissection (aTAAD) will be randomly allocated to either the ketorolac group or the placebo group, maintaining a 1:1 ratio. The intervention entails the administration of ketorolac tromethamine or 0.9% saline to the respective subjects. Ketorolac tromethamine, serving as the interventional treatment in the trial, will be administered 2 h prior to surgery (60 mg per 2 ml) and will continue for the initial 2 days post-operation (30 mg per 1 ml BID). The use of 0.9% saline functions as an inert placebo in the trial.
Intervention description {11a}
The surgeries will be performed by Professor Dongjin Wang. The study will commence on the day the patients sign an informed consent form and receive their randomized numbers. The ketorolac group, consisting of patients with aTAAD, will be treated with ketorolac. They will receive a preoperative intramuscular injection of 60 mg per 2 ml ketorolac and a postoperative injection of 30 mg per 1 ml ketorolac BID for two consecutive days. The placebo group, also consisting of patients with aTAAD, will receive placebo treatment. They will receive a preoperative intramuscular injection of 2 ml 0.9% saline placebo and a postoperative injection of 1 ml 0.9% saline placebo BID for two consecutive days. During the administration of ketorolac, patients’ blood pressure and renal function will be routinely monitored, and targeted treatment methods such as sedation, hypotension, diuresis, and dialysis will be applied as necessary. Additionally, all included patients will receive the same medical and surgical treatments in accordance with the diagnosis and treatment guidelines for aTAAD. Aortic dissection tissue samples will be collected for laboratory study during the surgery. Both the ketorolac group and the placebo group patients will undergo follow-up examinations before surgery and on the 1st, 3rd, 5th, 7th, 30th, and 90th day after surgery. These examinations will include assessments of adverse drug events, cardiac ultrasound, chest X-ray, laboratory tests (blood routine examination, blood biochemistry, erythrocyte sedimentation rate, coagulation function, CK-MB, cardiac troponin I (cTnI), PCT, IL-6, CRP, myohemoglobin (MYO), type B natriuretic peptide (BNP), D-dimer, etc.) and clinical biomarkers (matrix metalloproteinase-9 (MMP-9), MMP-2, MMP-3, MMP-13, type III procollagen, etc.). CTA examination of the cervical, thoracic, and abdominal aorta artery will be performed before surgery and on the 30th and 90th days after surgery.
Criteria for discontinuing or modifying allocated interventions {11b}
Subjects can withdraw from the study at any time for any reason. Considering the safety of the subjects. When the subject experiences situations where it is not appropriate to continue the study, including worsening of the condition, serious adverse events, poor compliance, experiencing intolerable accidents such as bankruptcy or family misfortune, and unwillingness to sign an informed consent form. Patients can be terminated to continue this study.
Strategies to improve adherence to interventions {11c}
The administration of drugs and the collection of blood and tissue samples will be closely monitored by our specialized staff at our center. They will maintain close communication with the treating nurse and surgeons and monitor the progress of the trial.
Relevant concomitant care permitted or prohibited during the trial {11d}
All included patients will receive the same medical and surgical treatments in accordance with the diagnosis and treatment guidelines for aTAAD.
Provisions for post-trial care {30}
The sponsor has insurance that covers damage to research subjects resulting from injury or death caused by ketorolac. This insurance applies to any damage that becomes apparent during the trial and the subsequent 90-day follow-up period.
Outcomes {12}
Primary endpoint.
Efficacy: death, organ malperfusion syndrome, permanent dialysis, tracheotomy, neurological impairment, postoperative mechanical circulatory support, and unplanned cardiac reoperation.
Safety: gastrointestinal ulceration, gastrointestinal bleeding, gastrointestinal perforation, postoperative hemorrhage (postoperative hemorrhage exceeding 1000 ml within 24 h), renal failure, liver failure, drug allergies, and other severe adverse events following drug administration.
Secondary endpoints
Erythrocyte sedimentation rate (ESR), C-reactive protein, procalcitonin, SII [ 17 ], coagulation function (prothrombin time, activated partial prothrombin time, bleeding time), liver function (alanine transaminase, glutamic transaminase, lactate dehydrogenase, total bilirubin, direct bilirubin), renal function (creatinine, uric acid, eGFR), high-sensitivity troponin T, serum B-type natriuretic peptide, myocardial enzyme (creatine kinase, creatine kinase MB isoenzyme), and other laboratory examination of blood;
Chest X-ray (for pulmonary inflammation and effusion, etc.), CTA scan of the cervical, thoracic, and abdominal aorta artery (for aortic diameter and extent of dissection, etc.), cardiac ultrasound (for cardiac ejection fraction and left ventricular diastolic dysfunction, etc.), and other imaging examinations.
The patient’s cardiopulmonary bypass time during surgery, total surgical time, aortic cross-clamping time, and postoperative hospital stay;
Postoperative drainage color, drainage volume, postoperative patient blood transfusion, and transfusion volume;
The incidence of postoperative sternal infection, secondary debridement, secondary tracheal intubation, pneumonia, and delirium; and
Enzyme-linked immunosorbent assay (ELISA) will be performed to detect and measure IL-1β IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, TNF-α, INF-γ, granulocyte–macrophage colony-stimulating factor (GM-CSF) and calcitonin;
Safety assessment
Ketorolac has been used in clinical practice for many years and is generally well tolerated. According to the medication instructions, the primary adverse reactions associated with ketorolac are gastrointestinal reactions, gastric bleeding, and potential liver and kidney damage. Guidelines from the AHA suggest that ketorolac may also contribute to hypertension and kidney damage [ 16 ]. And some cardiovascular adverse events related to ketorolac should be included. Therefore, the safety assessment variables include death, organ malperfusion syndrome, hypertension (blood pressure > 140/90 mmHg), stage 2 AKI diagnosed by KIDGO criteria [ 18 ], tracheotomy, neurological impairment prior to discharge, postoperative mechanical circulation support, and postoperative cardiac arrest.
Participant timeline {13}
Table 1 shows the participant timeline.
Sample size {14}
The required sample size was calculated using the PASS (15.0.5) software. The primary endpoint of efficacy was used to calculate the sample size. In the literature, the total death rate of hospitalization after surgery was 25% [ 7 ], the incidence of organ malperfusion syndrome was 15–33% [ 19 , 20 ], permanent dialysis was 2.6% [ 21 ], tracheotomy was 8% [ 22 ], neurological impairment was 6.9% [ 22 ], postoperative mechanical circulatory support was 20% [ 23 ], unplanned cardiac reoperation was 4.2% [ 24 ], and postoperative cardiac arrest was 0.7–5.2% [ 25 ]. Therefore, we assumed that the incidence of composite endpoint events in the control group is 70%, and the incidence of composite endpoint events in the ketorolac group is reduced to 40%. Using PASS (15.0.5) software, when the sample size of the ketorolac group and the control group is 53 cases, this difference can be detected with over 90% confidence. Considering that the loss of follow-up rate is higher than 10%, the final total sample size of this experiment is 120 cases, with 60 cases in the experimental group and 60 cases in the control group.
Recruitment {15}
Commencing from August 2023, all patients with type A aortic dissection (aTAAD) undergoing treatment at our institution will be informed and invited to participate in this clinical trial. The study aims to enroll a total of 120 eligible patients from the cardiothoracic surgery department of Nanjing Drum Tower Hospital within the timeframe spanning August 2023 to December 2026. Our center, renowned for its expertise, conducts more than 250 surgical procedures for aTAAD annually. Anticipating a robust enrollment process, we project the successful recruitment of the targeted 120 patients within the designated timeframe.
Assignment of interventions: allocation
Sequence generation {16a}.
This study employs a block-randomized treatment allocation strategy to minimize selective bias in treatment assignment. The trial utilizes a block randomization approach, facilitated by SAS 9.4 statistical software, with participants allocated in a 1:1 ratio to the ketorolac and placebo groups. Sequentially numbered random codes are generated based on the “Central Code Random Number Table.” The random number table is provided by qualified professionals. As recruitment is finishing, participants can only obtain a random code, without knowledge of the corresponding medication.
Concealment mechanism {16b}
This trial employs a double-blind design to ensure the implementation of blinding throughout the experimental process. We conduct medication blinding based on randomized codes. Ketorolac and placebo drugs will be enclosed in identical envelopes. Participants, drug dispensing center staff, and trial personnel will be unable to discern the type of medication based on envelope appearance. Blinding will be accomplished by encoding the experimental and control drugs according to random information and placing them into indistinguishable envelopes. Each coded drug will have an accompanying emergency letter for unblinding in urgent situations. Monitors and researchers must remain blinded throughout, and records of the blinding process will be meticulously maintained.
After obtaining informed consent forms, clinical physicians, responsible for recruiting participants based on inclusion criteria, will sequentially assign random codes. A drug administrator, independent from the study intervention and evaluation, will dispense the drug based on the random code. The drug, enveloped in a standardized cover, will then be administered to the patient. The names and random codes of participants will be recorded and stored by clinical physicians, while the drug administrator will handle drug distribution and retrieval. Records of both dispensing and retrieval will be documented. Until the trial is unblinded, all involved personnel will remain unaware of the grouping of participants.
Implementation {16c}
Essentially, qualified professionals generate random codes, and a subgroup not involved in medication distribution independently packages and codes the drugs according to these random codes. This ensures a one-to-one correspondence between the random codes and the medications. Upon patient enrollment, the clinical doctor assesses eligibility, and patients are assigned sequential random codes. The medication administrator then dispenses the drugs based on these random codes.
Assignment of interventions: blinding
Who will be blinded {17a}.
This study adheres to a double-blinded design, ensuring that both the study staff (excluding those engaged in drug preparation) and the patients remain unaware of the treatment assignments.
Procedure for unblinding if needed {17b}
Emergency unblinding can be performed at any time if patients experience severe adverse events or unexpected worsening of their clinical status, as deemed necessary by the principal investigator or the sponsor. Participants are provided with “In case of emergency” cards, which should be carried at all times during the study and include an emergency phone number. The researcher must record the date, location, reason, person responsible for unblinding, main investigator, and relevant personnel in charge of the drug clinical trial institution. These details should be documented in the case report form (CRF). Subjects who undergo emergency unblinding will be considered as withdrawal cases.
Data collection and management
Plans for assessment and collection of outcomes {18a}.
Clinical data, including patient admission information, demographic data, laboratory blood examinations, and imaging examinations, will be collected from the electronic medical record (EMR). The CRFs will be initially filled out on paper and then entered into Microsoft Excel by the associated researchers. The follow-up period will last for 90 days, during which the researchers will record all medical data and laboratory results from any hospital visits. At the end of the follow-up period, the patients will be interviewed via telephone.
Plans to promote participant retention and complete follow-up {18b}
Telephone follow-up will be systematically conducted to prompt patients to attend follow-up appointments at 30 and 90 days post-surgery. Additionally, the trial will offer patients financial assistance, encompassing travel expenses and accommodations.
Data management {19}
Patient data will be initially sourced from the Electronic Medical Record (EMR) and subsequently transcribed onto the Case Report Form (CRF). The patient data will be securely stored in Excel format on a computer. The CRF will meticulously capture specific details of adverse events (AEs) experienced by the patients.
To ensure the quality of trial data, we employ data range checks. Prior to the commencement of the trial, expected ranges for each measurement variable are established, grounded in prior research, clinical guidelines, or professional consensus. These ranges are designed to be both reasonable and aligned with clinical practice. Pre-data entry, range checks are executed to eliminate potential human errors. Post data entry, the utilization of graphical tools and visualization methods such as histograms and box plots aids in identifying outliers or issues with data distribution. We also have dedicated data monitors who randomly inspect the CRFs completed by study personnel. Any identified outliers will be promptly documented and reported.
Confidentiality {27}
The original Case Report Forms (CRFs) and Informed Consent Forms (ICFs) will be securely stored in a locked filing cabinet within the Department of Cardio-Thoracic Surgery at Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School. Only data monitors possess the authorization to access and review the hard-copy documents within the filing cabinet. Patient identification details will not be disclosed in any publications. Following the publication of the article, trial data will be shared with other researchers through secure electronic means, such as email, to facilitate international prospective meta-analyses.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
All patients will provide aortic dissection tissue samples during surgery, which will be sent to the laboratory for hematoxylin–eosin staining, western blot, and immunofluorescence. Additionally, blood samples will be collected before surgery and at 1, 3, 5, and 7 days after surgery. These blood samples will also be sent to the laboratory for ELISA. The tissue specimens will be stored at room temperature after being embedded in paraffin or frozen in liquid nitrogen and stored in a refrigerator at − 80 ℃. Similarly, the blood samples will be centrifuged and stored in a refrigerator at − 80 ℃.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}.
Statistical analysis will be performed using SPSS software (version 26.0) and R (× 64 3.5.0). Continuous variables will be presented as mean ± standard deviation. The Kolmogorov–Smirnov test will assess normal distribution. If variables exhibit normal distribution, an independent sample t -test will be employed; otherwise, the Mann–Whitney U test will be utilized. Categorical variables’ frequency will be expressed as percentages and analyzed using the chi-square test or Fisher’s exact test. It should be noted that we are not conducting an Intention to Treat analysis due to the exclusion of patients with more than 10% missing data. Double-tailed p -values and 95% confidence intervals will be reported, with statistical significance defined as p < 0.05.
Interim analyses {21b}
Interim analysis will be performed. When we complete the enrollment of 60 patients, 30 of whom are in the ketorolac group and 30 in the placebo group, we will perform an interim analysis.
Methods for additional analyses (e.g., subgroup analyses) {20b}
There are no subgroup analyses planned.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
We allow patients to have 10% missing data, which will be deleted as blank. When the patient’s data is missing by more than 10%, the patient will be deleted.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
The datasets used and/or analyzed during the current study can be made available by the corresponding author upon reasonable request.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}.
This study is a single-center study conducted at Nanjing Drum Tower Hospital. The trial involves various roles:
Principle investigator: oversees the trial and has medical responsibility for the patients.
Data manager: organizes data capture and ensures data quality.
Study coordinator: handles trial registration, coordinates study visits, and prepares annual safety reports.
Study physician: obtains informed consent, and ensures follow-up according to the protocol.
A research assistant: randomizes the patients and administers the drug.
Statistician: creates random scales and provides consultation on statistical methods.
The study team holds weekly meetings. There is no trial steering committee or stakeholder and public involvement group.
Composition of the data monitoring committee, its role and reporting structure {21a}
The Data and Safety Monitoring Board (DSMB) is composed of a chair, who is a cardiologist, along with a cardiac surgeon, an independent statistician, a cardiac anesthesiologist with expertise in medical ethics and law, and a clinical pharmacologist. The DSMB will formulate tailored data security monitoring plans, taking into consideration the magnitude of associated risks. Given the high-risk nature of this study, an autonomous Data Security Monitoring Committee has been established to systematically oversee the accumulated data on security and effectiveness. The committee’s mandate is to provide well-informed recommendations regarding the continuation of the research.
Adverse event reporting and harms {22}
Any adverse events (AEs) that occur during the study period will be carefully documented, evaluated, and treated. AEs grading will be based on Common Terminology Criteria for Adverse Events V.5.0.In the case of serious AEs that pose a risk to the patient’s life, an immediate report will be submitted to the Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College. Additionally, the incidence of AEs will be calculated.
Frequency and plans for auditing trial conduct {23}
An independent study monitor will be appointed to conduct study-specific auditing. The monitor will visit one time for every 40 patients and verify the presence and completeness of the investigation file. Additionally, the monitor will review the following data for 25% of randomly selected patients: informed consents, inclusion and exclusion criteria, source data, and documentation of any missing or reported AEs.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
A “substantial amendment” is defined as an amendment that is likely to have a significant impact on various aspects of a trial. These aspects include the safety and well-being of the trial participants, the scientific value of the trial, the overall conduct and management of the trial, as well as the quality and safety of any intervention used in the trial. Whenever a substantial amendment occurs, it is mandatory to notify both the Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College, and the competent authority. On the other hand, non-substantial amendments are recorded and filed for documentation purposes. If any amendments directly affect or involve the participants, they are duly informed about the changes. In such cases, if necessary, additional consent will be requested and properly documented. Furthermore, it is essential to update the online trial registries to reflect the amendments made.
Dissemination plans {31a}
The findings of this research will be comprehensively disseminated through international peer-reviewed journals. The reporting will encompass both positive and negative outcomes. Additionally, a lay summary will be crafted, designed to be shared with all participating families, providing accessible information about the research outcomes.
This study presents the first randomized, double-blinded, and placebo-controlled trial conducted to evaluate the safety and efficacy of ketorolac in the treatment of aTAAD. The findings of this trial will serve as a valuable reference for the use of ketorolac in the treatment of aTAAD. If the hypothesis of this trial proves to be accurate, ketorolac treatment for aTAAD can be considered safe and potentially beneficial in reducing inflammatory biomarkers, such as IL-6, CRP, and PCT.
The guidelines state that ketorolac can increase hypertension and cause renal dysfunction, so patients with acute aortic syndrome should use it with caution [ 16 ]. However, the guidelines only mention aortic disease and do not specifically address the potential harm of ketorolac in aTAAD. There is also no clear literature support provided. Previous clinical studies have shown that ketorolac is safe for perioperative treatment of surgery [ 26 ], and it has also been found to be beneficial in the treatment of aTAAD [ 15 ]. Our preliminary basic experiments have confirmed the beneficial effects of ketorolac on animal aortic aneurysms, which align with the conclusions of published basic research papers [ 12 , 27 , 28 ]. From a clinical application perspective, patients in the ICU undergo strict monitoring of their blood pressure, urine output, etc. Active treatment methods can effectively control the hypertension and kidney damage that are concerning in the guidelines. Therefore, the use of ketorolac in the treatment of aTAAD remains a controversial issue.
We intend to reevaluate the safety and efficacy of ketorolac in the treatment of aTAAD. Our approach involves two main components: Firstly, we will conduct a randomized controlled trial. Secondly, we will collect aortic tissue samples from patients during surgery and perform a series of laboratory experiments to investigate the underlying mechanisms and pathways. By integrating both clinical trials and basic experiments, our study aims to offer scholars a solid reference framework and novel insights.
Trial status
The trial started patient recruitment in August 2023. The current protocol is version 2 of 20–3-2024. Currently (20th of March 2024), we have included fifty-five patients. The recruitment process will persist for a duration of 2 years, concluding in December 2026.
Availability of data and materials {29}
Upon reasonable request, the corresponding author will provide access to the datasets utilized and/or examined during the present investigation.
Abbreviations
Adverse events
American Heart Association
Acute type A aortic dissection
Type B natriuretic peptide
Case report form
Cardiac troponin I
Data and safety monitoring board
Enzyme-linked immunosorbent assay
Electronic medical record
Erythrocyte sedimentation rate
Granulocyte-macrophage colony-stimulating factor
Informed consent forms
Matrix metalloproteinase-9
Myohemoglobin
Protease-activated receptor-1
Quantitative real-time polymerase chain reaction
Type A aortic dissection
Evangelista A, Isselbacher EM, Bossone E, et al. Insights From the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research[J]. Circulation. 2018;137(17):1846–60.
Article PubMed Google Scholar
Sampson UK, Norman PE, Fowkes FG, et al. Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010[J]. Glob Heart. 2014;9(1):171–180.e10.
Sorber R, Hicks CW. Diagnosis and management of acute aortic syndromes: dissection, penetrating aortic ulcer, and intramural hematoma[J]. Curr Cardiol Rep. 2022;24(3):209–16.
Article PubMed PubMed Central Google Scholar
Malaisrie SC, Szeto WY, Halas M, et al. 2021 The American Association for Thoracic Surgery expert consensus document: surgical treatment of acute type A aortic dissection[J]. J Thorac Cardiovasc Surg. 2021;162(3):735–758.e2.
Macgillivray TE, Gleason TG, Patel HJ, et al. The Society of Thoracic Surgeons/American Association for Thoracic Surgery Clinical Practice Guidelines on the Management of Type B Aortic Dissection[J]. Ann Thorac Surg. 2022;113(4):1073–92.
Zhu Y, Lingala B, Baiocchi M, et al. Type A aortic dissection-experience over 5 decades: JACC Historical Breakthroughs in Perspective[J]. J Am Coll Cardiol. 2020;76(14):1703–13.
Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience[J]. J Thorac Cardiovasc Surg. 2005;129(1):112–22.
Li Y, Ren P, Dawson A, et al. Single-cell transcriptome analysis reveals dynamic cell populations and differential gene expression patterns in control and aneurysmal human aortic tissue[J]. Circulation. 2020;142(14):1374–88.
Article CAS PubMed PubMed Central Google Scholar
Cui H, Chen Y, Li K, et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection[J]. Eur Heart J. 2021;42(42):4373–85.
Article CAS PubMed Google Scholar
Luo W, Wang Y, Zhang L, et al. Critical role of cytosolic DNA and its sensing adaptor STING in aortic degeneration, dissection, and rupture[J]. Circulation. 2020;141(1):42–66.
Kann AP, Hung M, Wang W, et al. An injury-responsive Rac-to-Rho GTPase switch drives activation of muscle stem cells through rapid cytoskeletal remodeling[J]. Cell Stem Cell. 2022;29(6):933–947.e6.
Pan L, Bai P, Weng X, et al. Legumain is an endogenous modulator of integrin αvβ3 triggering vascular degeneration, dissection, and rupture[J]. Circulation. 2022;145(9):659–74.
Walton LJ, Franklin IJ, Bayston T, et al. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms[J]. Circulation. 1999;100(1):48–54.
King VL, Trivedi DB, Gitlin JM, et al. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice[J]. Arterioscler Thromb Vasc Biol. 2006;26(5):1137–43.
Nejim B, Weaver ML, Locham S, et al. Intravenous ketorolac is associated with reduced mortality and morbidity after open abdominal aortic aneurysm repair[J]. Vascular. 2021;29(1):15–26.
Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines[J]. Circulation. 2022;146(24):e334–482.
Li Z, Zhang H, Baraghtha S, et al. Short- and mid-term survival prediction in patients with acute type A aortic dissection undergoing surgical repair: based on the systemic immune-inflammation index[J]. J Inflamm Res. 2022;15:5785–99.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1)[J]. Crit Care. 2013;17(1):204.
Okita Y, Takamoto S, Ando M, et al. Surgical strategies in managing organ malperfusion as a complication of aortic dissection[J]. Eur J Cardiothorac Surg. 1995;9(5):242–6 discussion 247.
Fann JI, Sarris GE, Mitchell RS, et al. Treatment of patients with aortic dissection presenting with peripheral vascular complications[J]. Ann Surg. 1990;212(6):705–13.
Kouchoukos NT, Masetti P, Rokkas CK, et al. Hypothermic cardiopulmonary bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta[J]. Ann Thorac Surg. 2002;74(5):S1885-7 discussion S1892–8.
Inoue Y, Minatoya K, Oda T, et al. Surgical outcomes for acute type A aortic dissection with aggressive primary entry resection[J]. Eur J Cardiothorac Surg. 2016;50(3):567–73.
Nakai C, Izumi S, Haraguchi T, et al. Acute Type A AORTIC DISSECTION WITH CARDIOPULMONARY ARREST AT Presentation[J]. Ann Thorac Surg. 2021;112(4):1210–6.
Shah PR, Butterworth JFT. Adverse outcomes from reoperation after cardiac surgery: There’s more to it than blood[J]. J Thorac Cardiovasc Surg. 2017;154(3):936–7.
Michaelis P, Leone RJ. Cardiac arrest after cardiac surgery: an evidence-based resuscitation protocol[J]. Crit Care Nurse. 2019;39(1):15–25.
Corsini EM, Zhou N, Antonoff MB, et al. Postoperative bleeding and acute kidney injury in esophageal cancer patients receiving ketorolac[J]. Ann Thorac Surg. 2021;111(4):1111–7.
Claridge M, Hobbs S, Quick C, et al. Nonsteroidal antiinflammatory drugs are associated with increased aortic stiffness[J]. Vasc Health Risk Manag. 2005;1(2):149–53.
Mirarab Razi H, Mosleh N, Shomali T, et al. Deterioration of wound healing and intense suppression of MMP-9 mRNA expression after short-term administration of different topical glucocorticoids or NSAIDs in an avian model of corneal lesions[J]. Iran J Vet Res. 2021;22(3):188–94.
CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
We express our gratitude to the patients for their valuable time, dedication, and willingness to participate in this trial. We also extend our appreciation to Yi-wei Liu for their insightful consultations. Additionally, we would like to acknowledge the contributions of Yuan-xi Luo, Xin Li, Wen-xin Su, and You-ru Kong for their valuable suggestions on the protocol. Lastly, we would like to thank our colleagues at the Nanjing Drum Tower Hospital for their unwavering commitment and assistance in the manufacturing procedure.
This research is funded by the National Natural Science Foundation of China (Project approval number: 82241212) ( https://grants.nsfc.gov.cn ).
Author information
Authors and affiliations.
Department of Cardiac Surgery, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
Zhikang Lv, Yusanjian Matniyaz, Lichong Lu & Dongjin Wang
Department of Cardiac Surgery, Nanjing Drum Tower Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Nanjing, China
Tuo Pan, Haitao Zhang & Yapeng Wang
Department of Cardiac Surgery, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
Yuxian Tang
You can also search for this author in PubMed Google Scholar
Contributions
ZL: designing and overseeing data analysis plans, data quality checks, drafting the manuscript. WD: designing and overseeing data analysis plans, curating the final data set. LL: designing and overseeing data analysis plans, curating the final data set. PT: designing and overseeing data analysis plans, data quality checks, curating the final data set. ZH: data management. WS: data management. WY: data management. TY: study statistician. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Lichong Lu or Dongjin Wang .
Ethics declarations
Ethics approval and consent to participate {24}.
This study has been approved by the Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College (approval number: 2023–197-02). Written, informed consent to participate will be obtained from all participants.
Consent for publication {32}
Not applicable—no identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. The participant information materials and informed consent form are available from the corresponding author on request. On the completion of this trial, a journal article manuscript will be prepared to present the trial results.
Competing interests {28}
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lv, Z., Pan, T., Zhang, H. et al. Safety and efficacy of ketorolac in improving the prognosis of acute type A aortic dissection patients: a protocol of a randomized, double-blinded, and placebo-controlled study. Trials 25 , 250 (2024). https://doi.org/10.1186/s13063-024-08093-x
Download citation
Received : 11 December 2023
Accepted : 03 April 2024
Published : 10 April 2024
DOI : https://doi.org/10.1186/s13063-024-08093-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stanford type A aortic dissection
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Avoid Common Problems with Consent Forms. Read these tips! 1. ... Informed Consent . Required Element when applicable . Suggested Language 2. When writing your consent documents, always: ... when part of the research study (e.g., study on nursing students' learning of medical procedures). 3. Before submitting consent documents to the IRB:
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
This is a template to assist researchers in the design of their informed consent form. You must adapt this template to the requirements of your particular study, using the notes and suggestions provided. Before using this template, check whether your organisation provides a template consent form and if so, incorporate their requirements into ...
assist research proponents in the design of their informed consent forms (ICF). Researchers are encouraged to use this when creating their informed consent forms to best suit the design of their study. Use of alternative wording or format is allowed. 2. The informed consent form consists of two parts: the information sheet and the consent ...
If you are conducting a clinical study, please use the BC Clinical Research Informed Consent Form Guide and Template. Formatting Information: • Type size should be a minimum 12-point font. Consider using a 13 or 14-point font size if ... The title of the study on the consent form should be the same as the title in the application form
Page%1%of%4% Participant's initials: _____ IRB approval number: date: Approval
Step 1 - Download in PDF, Microsoft Word (.docx), or Open Document Text (.odt). Step 2 - The title of the research study being conducted must be included at the top of the consent form. Step 3 - Enter the following information related to the primary researcher in the fields provided: Step 4 - The purpose of the study, the procedures ...
mother tongue and English. Ideally, translated consent forms should be back-translated and compared with the original to ensure accuracy. For review purposes, both the local language and the English version of the ICF should be submitted. For multi-centre research studies, a common consent form will be taken as a minimum requirement to which ...
Your specific consent form should include information pertinent to your specific research project, and may need to be considerably different from this sample. The highlighted sections are to be filled in by you.) My name is (name of person doing project), and I am a (student/professor, etc.) at Union College. I am inviting you to participate in ...
Instructions: This document provides guidance and language necessary to populate the template informed consent form. The guidance incorporates the regulatory requirements for informed consent. The format may be modified or expanded as indicated to better meet the needs of the research design and/or participant population.
Include a statement in the consent form that informs participants they are participating in a research study conducted by UCR researchers. Describe the purpose(s) of the research study in lay terms. Include a statement that indicates why this is considered a research study. Provide definitions for specific research design features (e.g.,
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
The consent signature requirements from the mother and father are summarised in table 3. Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient's health (mother and/or fetus) will be in danger.
GUIDE FOR INFORMED CONSENT. Consent to participate in a research study should be understood as a process rather than an event. Researchers should plan for and articulate the steps by which consent is initially obtained and the steps by which it is reviewed throughout the study. In order for participants to give meaningful consent, they should ...
• For the consent form, modify the [bracketed, blue text] as appropriate for your study, or remove it if it is a note for yo u, the author. As you work, pl ease remove all prompts to the author. • Please use simple words and short sentences when adding study specific information to this consent form.
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
Guidance for Writing an Informed Consent Document version 2/26/2021 Page 1 of 5 Guidance for Writing an Informed Consent Document . Writing the consent form is an important part of the research process. The goal of the consent form is to present the details of the research study to the participant in a manner that provides enough
This template is a guide to help researchers design study information sheets and consent forms. It has been designed with reference to HRA Participant Information Sheet Preparation Guidance. Sample text is italicised. Alter or delete as required as you produce the draft. Standard required text is underlined.
Obtaining genuine informed consent from research participants is best thought of as a process of sharing information and addressing questions and concerns, rather than simply obtaining a signature on a prescribed form. It starts with the researcher developing an awareness of national or regional guidelines, and may involve discussions with, and ...
Informed Consent Template. Please Note: When using letterhead, use the letterhead appropriate for your research. Informed Consent Document. For the research study: The Effects of Age on the Internal and External Controls of Behavior and the Perception of those Controls Over Time. This study is being conducted by Sara Jones, a PhD Candidate at ...
Study Title: Psychobiology of acute stress and behavior Sponsor: UMass, Amherst, College of Social and Behavioral Sciences 1. WHAT IS THIS FORM? This form is called a Consent Form. It will give you information about the study so you can make an informed decision about participation in this research study. This consent form will
5.1.1. Obtain the current IRB-approved consent form. 5.1.2. Verify that you are using the most current IRB-approved version of the study specific consent form and that the consent form is in language understandable to the subject/representative. 5.1.3. Provide a copy of the consent form to the subject/representative. Whenever possible provide the
Obtaining consent from subjects prior to their participation in a study is the foundation of ethical research. To give informed consent, however, subjects must be given: ... this information should be clearly disclosed in informed consent forms. If the study collects reportable information (e.g., ongoing child abuse, imminent threat of harm to ...
WAIVER OF DOCUMENTATION OF INFORMED CONSENT 12 The IRB may approve a waiver of the requirement to document informed consent provided that one of the following apply: 45 CFR 46.117(c)(i) •The only record linking the subject and the research would be the consent document •The principal risk would be potential harm resulting from a breach of
All participating patients will sign an informed consent form, and the trial results will be published in international peer-reviewed journals. ... (aTAAD) is a critical and life-threatening condition. Previous research has demonstrated that the use of ketorolac not only reduces the progression, incidence, and severity of aortic aneurysms in ...