
- < Previous
Home > ETD > OPEN_ACCESS_DISSERTATIONS > 1697

Open Access Dissertations
Quantifying human heat stress in working environments, and their relationship to atmospheric dynamics, due to global climate change.
Jonathan R. Buzan , Purdue University
Date of Award
Degree type.
Dissertation
Degree Name
Doctor of Philosophy (PhD)
Earth, Atmospheric, and Planetary Sciences
Committee Chair
Matthew Huber
Committee Member 1
H. Jay Melosh
Committee Member 2
Committee member 3.
Daniel Chavas
Committee Member 4
Thomas W. Hertel
Committee Member 5
Keith Oleson
Heat stress is a global issue that crosses socioeconomic status. Heat stress leads to reduced worker capacity on seasonal scales, and weekly to sub-daily timescales, incapacitation, morbidity, and mortality. This dissertation focuses on 2 distinct parts: quantification methods of heat stress, and heat stress applications. Quantification methods of heat stress Chapters 1–3 focus on historical analysis of heat stress. Chapter 1 is a detailed assessment of previous work in heat stress—methods, history, and future research outlook. Chapter 2 focuses on the implementation and quantification of a battery of heat stress metrics within the global circulation model framework. The ultimate outcome is a Fortran module, the HumanIndexMod [1], that may be run independently on individual datasets, or used with the Community Earth System Model 1, Community Land Model Version 5 (released February 2018 w/HumanIndexMod). Chapter 3 is an analysis of a battery of heat stress metrics with the focus on showing their differences in global circulation models, and thermodynamic predictability and scalability. Heat stress applications Chapters 4 and 5 focus on applications for physical impact modeling and economic outcomes. Chapter 4 quantifies labor impacts from heat stress due to the covariance or temperature, humidity, and radiation. My predictions of labor productivity losses from heat stress are amenable to Integrated Assessment Modeling. Chapter 5 is a preliminary economic impacts analysis–a 1st order sensitivity perturbation study for labor impacts–which will guide a flagship application for the Purdue University Big Idea Project, GLASS: Global to Local Analysis of Systems Sustainability. My labor productivity losses from heat stress will become a boundary condition for a series of sensitivity assessments intended to inform the policy making process to help achieve the United Nations Sustainability Development Goals.
Recommended Citation
Buzan, Jonathan R., "Quantifying Human Heat Stress in Working Environments, and Their Relationship to Atmospheric Dynamics, Due to Global Climate Change" (2018). Open Access Dissertations . 1697. https://docs.lib.purdue.edu/open_access_dissertations/1697
Since March 13, 2022
Advanced Search
- Notify me via email or RSS
- Purdue Libraries
- Purdue University Press Open Access Collections
Links for Authors
- Policies and Help Documentation
- Collections
- Disciplines
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
Physio-biochemical characterization of wheat genotypes under temperature stress
- Research Article
- Published: 26 December 2022
- Volume 29 , pages 131–143, ( 2023 )
Cite this article
- Ankita Pandey ORCID: orcid.org/0000-0003-2562-1228 1 , 2 ,
- Mamrutha Harohalli Masthigowda ORCID: orcid.org/0000-0003-0242-5654 1 ,
- Rakesh Kumar ORCID: orcid.org/0000-0003-0811-5657 1 , 3 ,
- Girish Chandra Pandey 2 ,
- Sushma M. Awaji 4 ,
- Gyanendra Singh ORCID: orcid.org/0000-0002-8507-9138 1 &
- Gyanendra Pratap Singh 1
630 Accesses
5 Citations
Explore all metrics
Thermal stress is a major abiotic stress in wheat and is highly complex in mechanism. A large area in northwestern plain zones (NWPZ), which is the wheat bowl of India is affected by heat stress. Climate change also causes an abrupt increase in temperature at different growth stages of wheat. Thus, wiser selection of stress tolerant varieties is an important strategy to combat the climate change effect. The present study aims for physiological and biochemical screening of timely sown NWPZ wheat varieties (WB2, HD3086, DBW88, DPW621-50, DBW17, HD2967 and PBW550) of India for their thermal stress tolerance along with heat tolerant (RAJ3765) and susceptible checks (RAJ4014) at seedling stage. The experiment was conducted in completely randomized design under controlled laboratory condition and heat stress was induced at 37 °C at seedling stage. Later different physio-biochemical traits were studied in both control and stress seedlings. All traits exhibited significant variations among genotypes under heat stress condition. Root and shoot weight, relative water content, chlorophyll content index and chlorophyll fluorescence reduced significantly, whereas membrane leakage, osmotic potential, catalase, ascorbate peroxidase, guaiacol peroxidase, malondialdehyde content and proline content were increased in stress plants. A tolerance matrix was prepared based on stress response of the genotypes for each trait and a final tolerance score was given to each genotype. Based on this tolerance matrix, DBW88 and PBW550 were identified as tolerant, DPW621-50, DBW17 and HD2967 as moderately susceptible and HD3086 and WB2 as susceptible to heat stress. Earlier studies parade that seedling level stress tolerance has high correlation with adult level stress tolerance under field condition in wheat. Hence, this study helps in wiser selection of varieties for sowing in NWPZ based on weather forecast of the location for creating varietal mosaic in context of climate change.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Drought Tolerance Strategies in Plants: A Mechanistic Approach
Muhammad Ilyas, Mohammad Nisar, … Abid Ullah

Drought Stress in Plants: An Overview

Heat stress effects and management in wheat. A review
Nurunnaher Akter & M. Rafiqul Islam
Abbreviations
- Heat stress
Chlorophyll content index
Chlorophyll fluorescence
Relative water content
Osmotic potential
Guaiacol peroxidase
Ascorbate peroxidase
Malondialdehyde content
Reactive oxygen species
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Article CAS Google Scholar
Akter N, Islam M (2017) Heat stress effects and management in wheat: a review. Agron Sustain Dev 37:37. https://doi.org/10.1007/s13593-017-0443-9
Asthir B, Bala S, Bains NS (2014) Effect of terminal heat stress on yield and yield attributes of wheat. Indian J Appl Res 4:1–2. https://doi.org/10.15373/2249555X/June2014/1
Article Google Scholar
Barrs H, Weatherley P (1962) A Re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428. https://doi.org/10.1071/BI9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Blum A, Zhang JX, Nguyen HT (1999) Consistent differences among wheat cultivars in osmotic adjustment and their relationship to plant production. Field Crop Res 64:287–291. https://doi.org/10.1016/S0378-4290(99)00064-7
Carillo P, Mastrolonardo G, Nacca F, Parisi D, Verlotta A, Fuggi A (2008) Nitrogen metabolism in durum wheat under salinity: accumulation of proline and glycine betaine. Funct Plant Biol 35:412–426. https://doi.org/10.1071/FP08108
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39:1–6. https://doi.org/10.1590/1678-4685-GMB-2015-0109
Chauhan BS, Singh B, Kaur P, Mahajan G, Randhawa RK, Singh H, Kang MS (2014) Global warming and its possible impact on agriculture in India. Adv Agron 123:65–121. https://doi.org/10.1016/B978-0-12-420225-2.00002-9
Daloza AS, Rydsaa JH, Hodnebrog O, Sillmann J, van Oorta B, Mohr CW, Agrawal M, Emberson L, Stordal F, Zhang T (2021) Direct and indirect impacts of climate change on wheat yield in the Indo-Gangetic plain in India. J Agri Food Res 4:100132. https://doi.org/10.1016/j.jafr.2021.100132
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Dhyani K, Ansari MW, Rao YR, Verma RS, Shukla A, Tuteja N (2013) Comparative physiological response of wheat genotypes under terminal heat stress. Plant Signal Behav 8:1–6. https://doi.org/10.4161/psb.24564
Dillard HR (2019) Global food and nutrition security: from challenges to solutions. Food Secur 11:249–252. https://doi.org/10.1007/S12571-019-00893-3
Ding S, Ali EF, Elmahdy AM, Ragab KE, Seleiman MF, Kheir AMS (2021) Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric Water Manag. https://doi.org/10.1016/j.agwat.2020.106626
Gupta NK, Agarwal S, Agarwal VP, Nathawat NS, Gupta S, Singh G (2013) Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol Plant 35:1837–1842. https://doi.org/10.1007/s11738-013-1221-1
Gupta R, Somanathan E, Dey S (2016) Global warming and local air pollution have reduced wheat yields in India. Clim Change 140:593–604. https://doi.org/10.1007/s10584-016-1878-8
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/IJMS14059643
Hossain A, Sarker MAZ, Saifuzzaman M, da Silva JAT, Lozovskaya MV, Akhter MM (2013) Evaluation of growth, yield, relative performance and heat susceptibility of eight wheat ( Triticum aestivum L.) genotypes grown under heat stress. Int J Plant Prod 7:615–636
Google Scholar
ICAR-IIWBR (2021) Director’s reports of AICRP on Wheat and Barley 2020–21. In: Singh GP (ed) AICRP on wheat and barley progress report 2020–21 crop improvement. ICAR—Indian Institute of Wheat and Barley Research, Karnal, Haryana, India, p 76
Iqbal M, Raja NI, Yasmeen F, Hussain M, Ejaz M, Shah MA (2017) Impacts of heat stress on wheat: a critical review. Adv Crop Sci Technol 5:1–9. https://doi.org/10.4172/2329-8863.1000251
Islam AU, Chhabra AK, Dhanda SS, Peerzada OH (2017) Genetic diversity, heritability and correlation studies for yield and its components in bread wheat under heat stress conditions. IOSR J Agric Vet Sci 5:71–77. https://doi.org/10.9790/2380-1005017177
Jagadish SK, Way DA, Sharkey TD (2021) Plant heat stress: concepts directing future research. Plant Cell Environ 44:1992–2005. https://doi.org/10.1111/pce.14050
Janda T, Khalil R, Tajti J, Pál M, Darkó E (2019) Responses of young wheat plants to moderate heat stress. Acta Physiol Plant 41:137. https://doi.org/10.1007/s11738-019-2930-x
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean ( Phaseolus vulgaris ) nodules under salt stress. J Plant Physiol 162:929–936. https://doi.org/10.1016/j.jplph.2004.10.005
Kaur R, Bedi S, Kaur P, Asthir B (2018) Biochemical evaluation in wheat ( Triticum aestivum L. ) under heat stress conditions. Ind J Agric Biochem 31:111–115. https://doi.org/10.5958/0974-4479.2018.00019.9
Khan M, Iqbal N, Masood A, Per T, Khan N (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8:11. https://doi.org/10.4161/psb.26374
Khan SU, Din JU, Qayyum A, Jan NE, Jenks MA (2015) Heat tolerance indicators in Pakistani wheat ( Triticum aestivum L .) genotypes. Acta Bot Croat 74:109–121. https://doi.org/10.1515/botcro-2015-0002
Khan NA, Khan S, Naz N, Shah M, Irfanullah AS, Sher H, Khan A (2017) Effect of heat stress on growth, physiological and biochemical activities of wheat ( Triticum aestivum L. ). IJB 11:173–183. https://doi.org/10.12692/ijb/11.4.173-183
Kumar R, Goswami S, Sharma S, Singh K, Gadpayle K, Kumar N (2012) Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H 2 O 2 and transcript of heat shock protein. Int J Plant Physiol Biochem 4:83–91. https://doi.org/10.5897/IJPPB12.008
Kumar S, Singh R, Nayyar H (2013) α-Tocopherol application modulates the response of wheat ( Triticum aestivum L. ) seedlings to elevated temperatures by mitigation of stress injury and enhancement of antioxidants. J Plant Growth Regul 32:307–314. https://doi.org/10.1007/s00344-012-9299-z
Kumar SN, Aggarwal PK, Rani DNS, Saxena R, Chauhan N, Jain S (2014) Vulnerability of wheat production to climate change in India. Clim Res 59:173–187. https://doi.org/10.3354/cr01212
Kumar R, Kaur A, Mamrutha HM, Grewal A (2017) Synergistic effect of cefotaxime and timentin to suppress the Agrobacterium overgrowth in wheat ( Triticum aestivum L.) transformation. Asian J Microbiol Biotechnol Environ Sci 19(4):961–967
Kumar A, Sharma S, Giri K, Goswami A, Chaudhary B, Sengar RS (2018) Morpho-physiological and biochemical characteristics of wheat ( Triticum aestivum L. ) Varieties under heat stress condition. Progress Agric 18:195–200. https://doi.org/10.5958/0976-4615.2018.00044.3
Kumar R, Masthigowda MH, Kaur A, Bhusal N, Pandey A, Kumar S, Mishra C, Singh G, Singh GP (2020) Identification and characterization of multiple abiotic stress tolerance genes in wheat. Mol Biol Rep 47:8629–8643. https://doi.org/10.1007/s11033-020-05906-5
Kumari M, Pudake RN, Singh VP, Joshi AK (2013) Association of stay green trait with canopy temperature depression and yield traits under terminal heat stress in wheat ( Triticum aestivum L. ). Euphytica 190:87–97. https://doi.org/10.1007/s10681-012-0780-3
Kumari A, Ranjan R, Roy C, Pal A, Kumar S (2020) Effect of heat stress on inter-relationship of physiological and biochemical traits with grain yield in wheat ( Triticum aestivum L. ). Curr Appl Sci Technol 39:19–29. https://doi.org/10.9734/cjast/2020/v39i1930786
Lamaoui M, Jemo M, Datla R, Bekkaoui F (2018) Heat and drought stresses in crops and approaches for their mitigation. Front Chem 6:26–30. https://doi.org/10.3389/fchem.2018.00026
Maia J, Voigt E, Macêdo C, Ferreira-Silva S, Silveira J (2010) Salt-induced changes in antioxidative enzyme activities in root tissues do not account for the differential salt tolerance of two cowpea cultivars. Braz J Plant Physiol 22:113–122. https://doi.org/10.1590/S1677-04202010000200005
Malhi GS, Kaur M, Kaushik P (2021) Impact of climate change on agriculture and its mitigation strategies: a review. Sustainability 13:1318. https://doi.org/10.3390/su13031318
Mamrutha HM, Khobra R, Sendhila R, Munjal R, Sai Prasad SV, Biradard S, Mavi GS, Dhar T, Bahadur R, Bhagwan JH, Prakash S, Singh H, Shukla RS, Srivastava M, Singh C, Gosavi AB, Salunke VD, Dhyani VC, Singh GP (2020a) Developing stress intensity index and prioritizing hotspot locations for screening wheat genotypes under climate change scenario. Ecol Ind 118:106714. https://doi.org/10.1016/j.ecolind.2020.106714
Mamrutha HM, Rinki K, Venkatesh K, Gopalareddy K, Khan H, Mishra CN, Kumar S, Kumar Y, Singh G, Singh GP (2020b) Impact of high night temperature stress on different growth stages of wheat. Plant Physiol Rep 25:707–715. https://doi.org/10.1007/s40502-020-00558-w
Mohan D, Singh SS, Gupta RK (2011) Vibrancy of the Indian wheat in upholding yield and quality under global environmental change. In: Wheat: productivity enhancement under changing climate. New Delhi, pp 87–94
Mohan D, Mamrutha HM, Tyagi BS (2017) Weather conditions favouring wheat ( Triticum aestivum L. ) productivity in hot climate of central India and congenial environment of north-western plains. Indian J Agric Sci 87:278–281
Mukherjee A, Simon Wang SY, Promchote P (2019) Examination of the climate factors that reduced wheat yield in northwest India during the 2000s. Water 11:43. https://doi.org/10.3390/w11020343
Pachauri RK, Meyer LA (2014) IPCC, 2014: climate change 2014: synthesis report. Contribution of working groups I, II and III... | Tribal Climate Change Guide. 10013/epic.45156.d001
Pandey GC, Mamrutha HM, Tiwari R, Sareen S, Bhatia S, Siwach P, Tiwari V, Sharma I (2015) Physiological traits associated with heat tolerance in bread wheat ( Triticum aestivum L. ). Physiol Mol Biol Plants 21:93–99. https://doi.org/10.1007/s12298-014-0267-x
Pandey GC, Mehta G, Sharma P, Sharma V (2019) Terminal heat tolerance in wheat: an overview. J Cereal Res 11:1–16. https://doi.org/10.25174/2249-4065/2019/79252
Poudel PB, Poudel MR (2020) Heat stress effects and tolerance in wheat: a review. J Biol Today’s World 9:217
CAS Google Scholar
Puthur JT (2016) Antioxidants and cellular antioxidation mechanism in plants. South Indian J Biol Sci 2:14–17
Qaseem MF, Qureshi R, Shaheen H (2020) Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat ( Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci Rep 9:6955. https://doi.org/10.1038/s41598-019-43477-z
Ramadas S, Kiran Kumar TM, Pratap Singh G (2020) Wheat production in India: trends and prospects. In: Shah F, Khan Z, Iqbal A, Turan M, Olgun M (eds) Recent advances in grain crops research. IntechOpen, London. https://doi.org/10.5772/INTECHOPEN.86341
Chapter Google Scholar
Rinki MHM, Kumar R, Tiwari V (2016) Comparison of seedling and adult stage heat stress tolerance in wheat. Wheat Barley News Lett 10:9
Sairam RK, Srivastava GC (2001) Water stress tolerance of wheat ( Triticum aestivum L. ): variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J Agron Crop Sci 186:63–70. https://doi.org/10.1046/J.1439-037X.2001.00461.X
Sattar A, Sher A, Ijaz M, Ul-Allah S, Rizwan MS, Hussain M et al (2020) Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 15:e0232974. https://doi.org/10.1371/journal.pone.0232974
Sharma DK, Andersen SB, Ottosen CO, Rosenqvist EW (2015) Heat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol Plant 153:284–298. https://doi.org/10.1111/ppl.12245
Sharma D, Tiwari R, Gupta VK, Rane J, Singh R (2018) Genotype and ambient temperature during growth can determine the quality of starch from wheat. J Cereal Sci 79:240–246. https://doi.org/10.1016/j.jcs.2017.11.006
Sharma D, Singh R, Tiwari R, Kumar R, Gupta V (2019) Wheat responses and tolerance to terminal heat stress: a review. In: Wheat production in changing environments, pp 149–173. https://doi.org/10.1007/978-981-13-6883-7_7
Sharma P, Singh R, Dahiya M, Kumar V, Kumar A, Sharma AK (2021) Screening of heat stress tolerant in early stage of wheat seedling using morphological parameters. Asian J Biol Sci 10:667. https://doi.org/10.5530/ajbls.2021.10.89
Sonkar G, Mall R, Banerjee T, Singh N, Kumar T, Chand R (2019) Vulnerability of Indian wheat against rising temperature and aerosols. Environ Pollut 254:112946. https://doi.org/10.1016/j.envpol.2019.07.114
Turkan I, Bor M, Ozdemir F, Koca H (2005) Differential response of lipid peroxidation and antioxidants in the leaves of drought tolerant P. Acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231. https://doi.org/10.1016/j.plantsci.2004.07.032
Wang X, Cai J, Liu F, Dai T, Cao W, Wollenweber B, Jiang D (2014) Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol Biochem 74:185–192. https://doi.org/10.1016/J.PLAPHY.2013.11.014
Xin M, Peng H, Ni Z, Yao Y, Hu Z, Sun Q (2019) Wheat responses and tolerance to high temperature. In: Wheat production in changing environments, pp 139–147. https://doi.org/10.1007/978-981-13-6883-7_6
Zampieri M, Ceglar A, Dentener F, Toreti A (2017) Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ Res Lett 12:064008. https://doi.org/10.1088/1748-9326/aa723b
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791. https://doi.org/10.1093/oxfordjournals.pcp.a078658
Download references
Acknowledgements
This work is financially supported by the Indian Council of Agricultural Research, New Delhi, India, under the Grant No. RSCIIWBRSIL201500400185.3 and ICAR Network Project on Functional Genomics and Genetic Modification in Crops (NPFGGM, Project No.1006474).
Author information
Authors and affiliations.
ICAR-Indian Institute of Wheat and Barley Research, Karnal, Haryana, 132001, India
Ankita Pandey, Mamrutha Harohalli Masthigowda, Rakesh Kumar, Gyanendra Singh & Gyanendra Pratap Singh
Biosciences and Biotechnology, Banasthali Vidyapith, Banasthali, Rajasthan, 304022, India
Ankita Pandey & Girish Chandra Pandey
University of California, Berkeley, CA, 94720, USA
Rakesh Kumar
ICAR-National Rice Research Institute, Cuttack, Odisha, 753006, India
Sushma M. Awaji
You can also search for this author in PubMed Google Scholar
Contributions
MHM conceived and designed the research with AP. AP conducted experiments. AP, RK and SMA collected the experimental data. AP, RK, GCP and MHM wrote the manuscript. MHM, GS and GPS edited the manuscript. All authors read and approved the manuscript for publication.
Corresponding author
Correspondence to Mamrutha Harohalli Masthigowda .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Ethics approval
The present research study does not involve any human participants, their data, or biological material.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Pandey, A., Harohalli Masthigowda, M., Kumar, R. et al. Physio-biochemical characterization of wheat genotypes under temperature stress. Physiol Mol Biol Plants 29 , 131–143 (2023). https://doi.org/10.1007/s12298-022-01267-4
Download citation
Received : 06 May 2022
Revised : 22 October 2022
Accepted : 05 December 2022
Published : 26 December 2022
Issue Date : January 2023
DOI : https://doi.org/10.1007/s12298-022-01267-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Physiological traits
- Biochemical traits
- Seedling stress
- Antioxidants
- Find a journal
- Publish with us
- Track your research
REVIEW article
The impact of heat stress in plant reproduction.

- Dipartimento di Bioscienze, Università degli Studi di Milano, Milano, Italy
The increment in global temperature reduces crop productivity, which in turn threatens food security. Currently, most of our food supply is produced by plants and the human population is estimated to reach 9 billion by 2050. Gaining insights into how plants navigate heat stress in their reproductive phase is essential for effectively overseeing the future of agricultural productivity. The reproductive success of numerous plant species can be jeopardized by just one exceptionally hot day. While the effects of heat stress on seedlings germination and root development have been extensively investigated, studies on reproduction are limited. The intricate processes of gamete development and fertilization unfold within a brief timeframe, largely concealed within the flower. Nonetheless, heat stress is known to have important effects on reproduction. Considering that heat stress typically affects both male and female reproductive structures concurrently, it remains crucial to identify cultivars with thermotolerance. In such cultivars, ovules and pollen can successfully undergo development despite the challenges posed by heat stress, enabling the completion of the fertilization process and resulting in a robust seed yield. Hereby, we review the current understanding of the molecular mechanisms underlying plant resistance to abiotic heat stress, focusing on the reproductive process in the model systems of Arabidopsis and Oryza sativa.
Introduction
Heat Stress (HS) causes substantial crop loss worldwide. The average global temperature is constantly increasing, and this change is expected to have deleterious effects on crop yield. A recent study showed that drought and, particularly, extreme heat episodes dramatically decreased cereal production by 9–10% between 1964 and 2007 ( Lesk et al., 2016 ). Average temperatures are estimated to rise by 2–3°C over the next 30 to 50 years. Given the fact that the human population is estimated to reach 9 billion by 2050, genetic improvement of tolerance traits to abiotic stresses on stable crops is an immediate priority. Europe recently experienced several heat waves, in 2003 the heat-related death toll ran into tens of thousands. Another heat wave in 2012 impacted crop productivity and yield of several important food species with a decrease of up to 40%, as for sunflowers for example ( Peng et al., 2004 ). Among all the documented losses, it was estimated that rice grain production decreased by 10% for each 1°C increase, and it has also been predicted that every 1°C increase reduces wheat production by 3 - 4% ( Xu et al., 2020 ; Wang et al., 2020 ). Similar deleterious effects have been shown for maize and barley, for which each day that the plants are exposed to a temperature over 30°C, yield is reduced by 1% ( Rezaei et al., 2015 ). The year 2016 ranks as the warmest on record and the year 2018 was the fourth warmest since 1880 (Source: NASA/GISS) confirming a continuous trend towards warmer climates. Since extreme climatic events, such as heat waves, are increasingly common, agriculture will face extraordinary challenges to sustain productivity ( Mulla et al., 2020 ).
Understanding how plants cope with HS during their reproductive phase is critical for managing the future of agricultural productivity, as most of our food supply is a product of plant reproduction. Even if the effect of temperature has been extensively studied using accessible plant tissues, such as leaves and roots, analysis on reproduction is often difficult because gametophyte development and fertilization are complex processes that occur during a narrow window of time and deep inside the flower. The effect of HS on plant reproduction is very wide affecting many reproductive tissues at the same time. HS leads to abnormalities in floral development, plants develop altered flower structures, with reduced flower size, or even the development of complete sterile flowers. These flower changes are translated into impaired pollination and fertilization processes that ultimately lead to reduced fruit and seed production. From the male side, HS impacts anther and pollen grain development leading to morphological abnormalities and displacement of the metabolic processes that impair pollen grain ability to germinate and to grow pollen tubes. From the female side, HS can disrupt gametogenesis, leading to abnormal development of the female gametophyte (embryo sac), this can affect the formation of essential components within the embryo sac, such as the gametes (egg cell). Impaired fertilization and embryo development are the ultimate consequences of HS which results in reduced and/or poor seed production ( Barnabás et al., 2008 ; Prasad et al., 2008 ; Bita and Gerats, 2013 ) ( Figure 1 ).
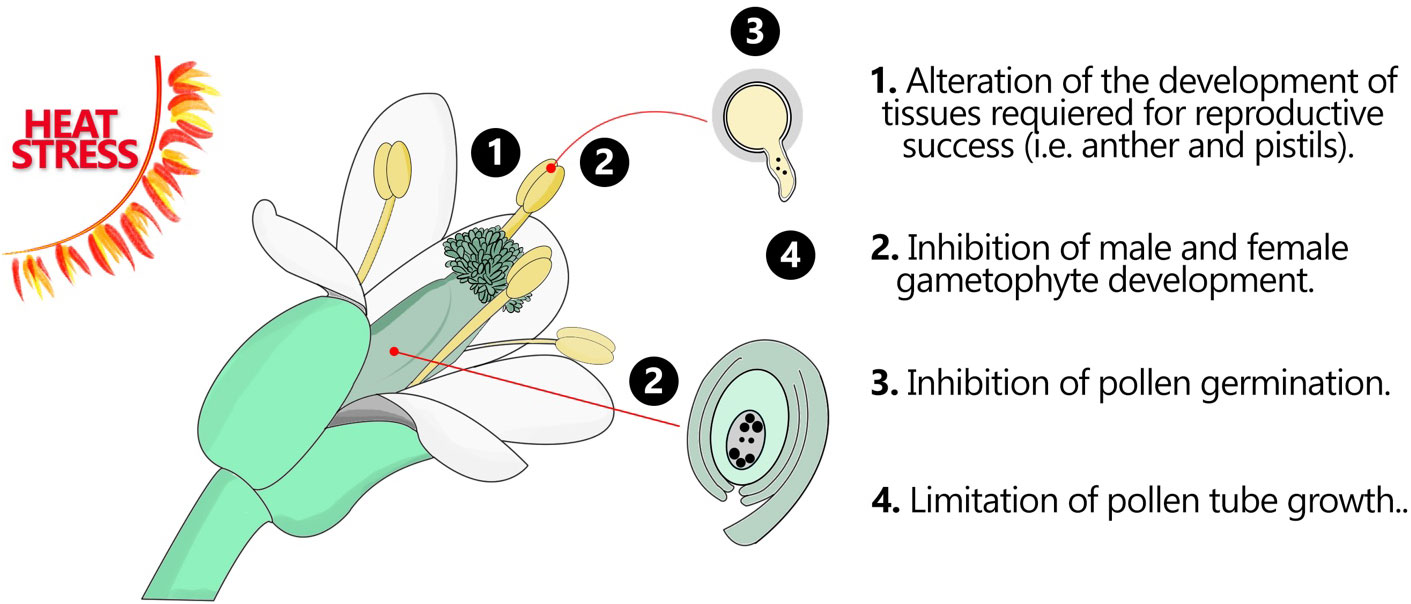
Figure 1 Flower organs and tissues in response HS. Heat stress can have significant impacts on various flower organs and tissues, leading to alterations in their development, structure, and function. These effects can disrupt normal floral development and ultimately impact reproductive success. This figure demonstrates how the fertile flower organs may respond to HS.
Many genes regulating responses and resistance to various biotic and abiotic stresses have been precisely identified. However, coordinated responses between roots and leaves at the whole plant level remain largely unknown. Roots and shoots communicate with each other to synchronize and optimize plant development and respond to environmental changes. Thus, the growth of these two structures is coordinated and this requires communication mediated by signal messengers that move between the aboveground and belowground structures ( Ko and Helariutta, 2017 ). The vascular system serves as an effective long‐distance communication system, with the phloem and xylem serving to input information relating to conditions ( Lucas et al., 2013 ). An interesting observation is that a stress applied to systemic tissues is sensed by the inflorescence in a short timeframe (minutes or a few hours) ( Choudhury et al., 2018 ), thus pointing to the existence of a fast communication pathway that most probably relies on (i) changes in electric potential, (ii) calcium ions (Ca 2+ ) and (iii) reactive oxygen species (ROS) ( Iba, 2002 ; Liao et al., 2017 ; Jespersen, 2020 ).
For this reason, understanding how environmental cues are sensed and transmitted to systemic organs of plants such as the inflorescence and how this impacts the flower development therefore seeds’ setting and plant reproduction in model species, such as Arabidopsis , could be relevant for the establishment of a baseline for crop improvement. Here we will provide an overview of the importance of molecular mechanisms underlying plant resistance to HS, focusing on the reproductive process in Arabidopsis thaliana and Oryza sativa , highlighting the role of Ca 2+ as a link between the perception of environmental signal and a physiological response.
Pollen and ovule development response to HS in Arabidopsis
Flowering plants, i.e. angiosperms, alternate between a highly reduced gametophytic (haploid) and sporophytic (diploid) generations ( Yadegari and Drews, 2004 ). The sporophyte is the multicellular diploid plant whereas the haploid structure called gametophyte is generated by meiotic cell division within the male and female reproductive organs ( Drews and Koltunow, 2011 ). In Arabidopsis, male gametophyte development occurs within stamens, composed of a filament and an anther. Within anthers, non-reproductive cells differentiate into specialized layers, including the tapetum, surrounding sporogenous cells ( Scott et al., 2004 ). Two distinct phases, microsporogenesis and microgametogenesis, produce mature pollen. Microsporogenesis involves meiosis in pollen mother cells, generating haploid microspores. After callose wall degeneration, individual microspores are released ( Borg et al., 2009 ). Subsequent mitotic divisions yield vegetative and generative cells. Asymmetric division in the first pollen mitosis determines unique gene expression profiles, defining structures and fates. A second mitosis produces twin sperm cells for double fertilization, leading to embryo and endosperm development ( Twell et al., 1998 ). Abiotic stress in pollen development was comprehensively studied in the last years and reviews were produced where pollen defects in different species were reviewed ( Chaturvedi et al., 2021 ).
In Arabidopsis was recently demonstrated that the male gametophyte (pollen) is particularly sensitive to heat fluctuations, causing defects in meiotic restitution ( De Storme and Geelen, 2020 ). More in detail meiosis and in particular meiotic recombination are highly sensitive to elevated temperatures, meiotic microtubule cytoskeleton resulted in an irregular spindle orientation, and aberrant cytokinesis that consequently led to the production of aneuploid male gametes ( De Storme and Geelen, 2020 ; Hedhly et al., 2020 ; De Jaeger-Braet et al., 2022 ). In Arabidopsis, the increase in crossover frequency at high temperatures was associated with elevated numbers of Type I interfering pathway crossover. Interestingly, the meiotic hyper-recombination observed in Arabidopsis resulted specific for HS, as plants subject to salt stress did not exhibit an increase in crossover frequency ( Modliszewski et al., 2018 ). Precisely because of the HS effect on chromosome segregation, high-temperature treatment has been proposed as a tool in plant breeding to induce genome elimination and haploid induction. Indeed, if applied to haploid inducer mutants, such as mutant for the CENTROMERE-SPECIFIC HISTONE H3, short-term HS increases the efficiency of haploid induction by ten times ( Ahmadli et al., 2023 ; Jin et al., 2023 ; Figure 2 ). Another aspect of male meiosis that is influenced by the HS is the duration of the different phases of meiosis. By performing live cell imaging on male meiocytes, De Jaeger-Braet et al. (2022) showed that the meiosis phase of meiocytes at a high temperature of 34°C is faster than at 21°C. By contrast, the pachytene/diakinesis phase gets prolonged at 34°C. The extension of this specific phase is recombination dependent since it was not detected in ataxia telangiectasia mutated ( atm ) mutant in which recombination is completely abolished.
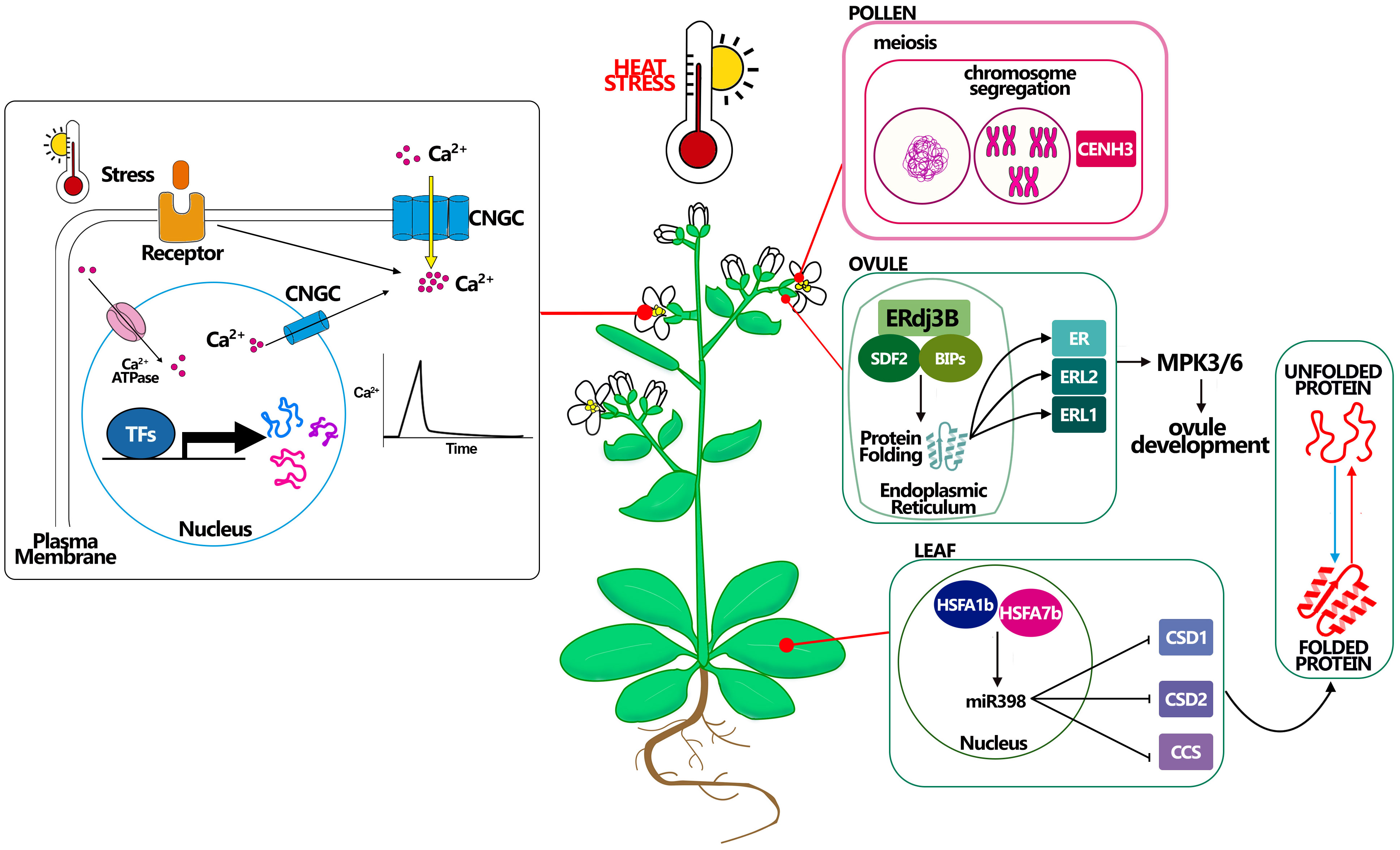
Figure 2 Molecular mechanisms connected with heat stress resistance in Arabidopsis. Heat affects plant Ca 2+ channels, inducing a transient increase in cytosolic Ca 2+ concentration. Stress sensors recognize environmental signals (i.e. heat) and activate plasma membrane-localized Ca 2+ channels, allowing Ca 2+ influx into the cytosol by the use of CNGCs channels. Cytosolic Ca 2+ ion acts as a second messenger that triggers specific cellular responses. Pollen CENH3 mediates genome elimination during HS, this mechanism could be better studied to understand how to maintain meiosis under HS. Ovule complex ERdj3B acting in complex with SDF2 and BIPs in the endoplasmic reticulum control the protein folding, being essential to the plant tolerate the HS, probably via the ERL proteins. A good example in leaf development for thermotolerance is giving by miR398 which expression is controlled by HSFA proteins and that promotes the resistance to stress controlling the correct folding of the proteins.
Regarding the female part of development studies using the model plant Arabidopsis, HS reduced the total number of ovules and increased ovule abortion ( Whittle et al., 2009 ). Furthermore, it has been described in Tomato that female pistils exposed to HS (32/26°C) and then crossed with pollen from plants grown in standard conditions (at 28/22°C) exhibited reduced fruit set and a reduced number of seeds per fruit compared with the control pistils of plants grown at 28/22°C ( Peet et al., 1998 ). The seed set was even more reduced in the reciprocal experiment using maize plants when transient HS was applied for three days on developing pollen that later was used to pollinate female flowers grown at optimal conditions ( Begcy et al., 2019 ). These were the only studies that described some of the female defects caused by HS, which remained largely unstudied.
In Arabidopsis, the female germline initiates during the initial phase of ovule development. This process begins with the differentiation of a distal subepidermal cell known as the megaspore mother cell, which undergoes meiosis to give rise to four haploid megaspores, typically arranged in a linear tetrad. Among these four megaspores, only one survives to become the functional megaspore, while the remaining three undergo programmed cell death. Following megasporogenesis, the functional megaspore proceeds to undergo a Polygonum-type pattern of megagametogenesis, leading to the formation of the embryo sac and female gamete. Integument development in Arabidopsis is a simple two-cell layered structure that develops around the embryo sac and after fertilization protects the developing embryo developing the seed coat ( Christensen et al., 2002 ; Yadegari and Drews, 2004 , Mendes et al., 2016 ).
Arabidopsis endoplasmic reticulum-localized DnaJ family 3B (ERdj3B) was recently described as an important factor for the correct development of ovule integuments by controlling the translocation of the ERECTA-family receptor kinases in the ecotype Landsberg ( Leng et al., 2022 ). ERdj3B is a component of the stromal cell-derived factor 2 (SDF2)–ERdj3B–binding immunoglobulin protein (BiP) chaperone complex, and has functions in protein folding, translocation, and quality control. ERdj3B was first described to be involved in thermotolerance during anther development ( Yamamoto et al., 2020 ) and was also described now to have complex functions not only in ovule integument development but also in HS response from the female side. Leng and collaborators ( Leng et al., 2022 ) further described that higher temperatures were shown to aggravate the defective phenotypes of erdj3b mutants, linking that the response to HS has more severe effects on ovule development when ERECTA-family receptor kinases are absent ( Figure 2 ). A recent study also described the TCP transcription factors inhibit the homeotic conversion of ovules into carpelloid structures under HS, ( Lan et al., 2023 ) reinforcing the fact that transcription factor complexes important for ovule identity can also be related to HS perception and could have significant implications for understanding the molecular mechanisms underlying the plant’s response to HS and how it affects reproductive development.
HS proteins and HS transcription factors in Arabidopsis
For quite some time in cellular biology, HS proteins have been recognized as those whose levels significantly rise when cells are cultivated at elevated temperatures, providing a form of resilience. It is now understood that these proteins play a role in assisting newly synthesized proteins in proper folding and safeguarding proteins that may otherwise misfold and lose their intended functional conformation during stressful events. Importantly, these proteins are not solely associated with HS but also have connections to other biotic and/or abiotic stress conditions. HS proteins (HSPs) besides stress-responsive genes ( Ul Haq et al., 2019 ), also are involved in plant growth and development under normal conditions, like the flowers, seeds, and fruits set development, in the tuberization ( Agrawal et al., 2013 ) and nutrient uptake ( Shekhar et al., 2016 ). Studies using Arabidopsis and crops (rice, maize, and wheat) showed that the basis of thermotolerance resides in the overexpression of HSP factors, which increase plant resistance to abiotic HS ( Ul Haq et al., 2019 ). These studies have focused on the analysis of transgenic plants under a broad spectrum of induced HS treatments, which makes the data extremely variable and not suitable for comparison purposes ( Yeh et al., 2012 ). The optimum scenario is to identify a stable accession or species that naturally overexpresses HSP factors that induce HS resistance. Furthermore, most of the thermotolerance studies are again based on seedling germination and root development, meanwhile, the reproductive phase is often not considered. Therefore, even though a plant can potentially tolerate HS at early phases of development, it might be HS susceptible at mature stages and hence sterile.
At the molecular level, the cellular response to HS is represented by the induction of HSP, a group of stress proteins that are classified as molecular chaperones and proteases. The molecular analysis of HSP promoters leads to the identification of the heat shock element (HSE), a stress-responsive promoter element essential for HS inducibility; this binding site is characterized by multiple adjacent 5`-nGAAn-3`. The position of HSEs in the genome is various and distances upstream of their transcription starting site. In vertebrates and plants, HSP transcription requires the transient binding of HS transcription factors (HSFs) to the HSEs present within their promoters ( Wu, 1995 ; Morimoto, 1998 ; Kovács et al., 2022 ). Plant HSFs are divided in three classes A, B and C. Class A HSFs typically contain one or two acidic AHA motifs and function as transcriptional activators, as indicated by Döring et al. (2000) . On the other hand, class B HSFs possess a B3 repressing domain, which has also been identified in 24 other transcription factors in Arabidopsis. Class C HSFs have not been thoroughly described ( Czarnecka-Verner et al., 2004 ; Ikeda and Ohme-Takagi, 2009 ; Guo et al., 2016 ). In contrast to the limited number of HSF members in vertebrates (4), Drosophila (1), Caenorhabditis elegans (1), and yeast (1), plant HSF families exhibit a considerable number of members derived from a complex, plant-specific superfamily, as highlighted by Wu (1995) and Morimoto (1998) .
The large size of the plant HSFs family inevitably complicates the unraveling of their function under stress conditions ( Scharf et al., 2012 and reviewed in Guo et al., 2016 ).
The identification of factors that allow plants to tolerate HS has been mainly performed in the model species Arabidopsis. Yet, research on economically relevant species has been performed ( Ul Haq et al., 2019 ). In Arabidopsis during the vegetative phase, the constitutive expression of the HS transcription factors HSFA1a, b, d, and e are responsible for triggering the HS response ( Yoshida et al., 2011 ). HSF1abde are responsible for basal thermotolerance and initiate the acquisition of thermotolerance. A second transcription factor from this family is HSFA2, the most highly heat-induced HSF. Remarkably, ectopic expression of HSFA2 was able to rescue the phenotype of the quadruple mutant hsaf1abde at reproductive stage ( Liu and Charng, 2013 ). This is partly explained by the fact that HSFA2 can induce its own expression. HSFA3, HSFA7a, and HSFA7b are also induced by HSFA2 and/or HSFA1 after HS ( Liu and Charng, 2013 ). Instead, the defective mutant hsfa2 is only impaired in maintaining the acquired thermotolerance after long recovery ( Charng et al., 2007 ). HSFs were extensively reviewed by Guo et al., 2016 .
Manipulation on genes that have the potential to improve HS tolerance has focused mainly on genes involved in the synthesis of HSP. Yet, no clear evidence regarding the improvement to HS resistance of such mutants is available. One of the first studies that reported how a HSP manipulated can improve the tolerance to HS was made using the HSF SQUAMOSA promoter binding protein-like7 (SPL7, Yamanouchi et al., 2002 ). Mutant plants lacking the function of spl7 develop more necrotic lesions on leaves under HS treatment (35°C for 24 hours followed by 42°C for 24 hours). spl7 mutant lines complemented with wild-type SPL7 were more resistant to HS showing no occurrence of necrotic lesions during the growth period ( Yamanouchi et al., 2002 ). Remarkably, a strong correlation between the inserted number of copies of the transgene and the reduction in the necrotic lesions was detected, suggesting that the “overexpression” of SPL7 might be helpful to improving HS tolerance ( Yamanouchi et al., 2002 ), any evidence in the reproductive part were studied. The sole HSF identified with a function in ovule development is HSFB2a. Plants with heterozygous mutations in HSFB2a display 50% sterile ovules and a significant decrease in both male and female transmission, suggesting that the gene’s absence adversely affects the development of both male and female germ lines. Even if is not an HS related phenotype is it very interesting to notice that the homozygous mutant was already sterile, with block during female gametophyte ( Wunderlich et al., 2014 ). A very interesting study demonstrated that heat-inducible miR398 that is directly activated by HSFA1b and HSFA7b is required for thermotolerance through the downregulation of its target genes CSD1, CSD2 and CCS which encode for copper chaperones. The corresponding mutations to csd1 , csd2 and ccs mutant plants are more heat-tolerant and the resistant transgenic plants expressing the miR398-resistant forms of CSD1, CSD2 or CCS were more sensitive to HS at 37°C ( Guan et al., 2013 , Figure 2 ). Studies involving miRNAs during reproductive tissues would be of outmost importance as in the last years were described to play several roles during reproduction ( Petrella et al., 2021 ).
Calcium as a signal for HS in Arabidopsis
In plants, calcium ion (Ca 2+ ) plays an important role both as a structural component of plant cell walls and membranes and as an intracellular second messenger. As second messenger, Ca 2+ is involved in an advanced network of signaling pathways taking part in various signaling processes generated in response to both biotic and abiotic stresses, as well as developmental stimuli ( Kudla et al., 2018 ; Resentini et al., 2021 ; Ghosh et al., 2022 ). In nature, plants must cope with both seasonal and diurnal temperature changes. Particularly, temperature fluctuations that occur in a single day can be dangerous for plants since they can face temperature stress more rapidly as compared to other stresses such as drought or salinity ( Larkindale and Knight, 2002 ; Ghosh et al., 2022 ). Therefore, like other organisms, plants have evolved defense mechanisms to efficiently cope with temperature stress and to prevent the disruption of multiple cellular processes, including protein folding, cytoskeletal organization, membrane stability, regulation of ROS and ion homeostasis ( Weigand et al., 2021 ; Ghosh et al., 2022 ).
It has been shown that plants exposed to a heat shock show a transient increase of the cytosolic Ca 2+ concentration ( Gong et al., 1998 ; Wu and Jinn, 2010 ). Such an increase was shown to depend on the activity of some members of the CYCLIC NUCLEOTIDE-GATED channels (CNGCs) family ( Finka et al., 2012 ; Cui et al., 2020 ). Interestingly, CNGCs have been implicated in diverse aspects of plant growth and development, such as pollen tube growth and fertility. Six CNGC members—CNGC7, 8, 9, 10, 16 and 18—have been reported as highly expressed in the pollen grain and pollen tube ( Frietsch et al., 2007 ; Tunc-Ozdemir et al., 2013 ). Among them, genetic evidence identifies CNGC16 as a critical component in maintaining pollen fertility under conditions of heat and drought stress ( Tunc-Ozdemir et al., 2013 ). The cngc16 mutant, in fact, showed more than a 10-fold stress-dependent loss in pollen fitness as well as seed set under HS and drought stress. At the same time, cngc16 mutant pollen exhibited attenuated expression of HS responsive genes ( Tunc-Ozdemir et al., 2013 ). Nonetheless, there are scant pieces of evidence directly supporting a role for Ca 2+ signals as an initial heat sensing response during plant reproduction ( Ghosh et al., 2022 ) ( Figure 2 ).
Weigand and colleagues in 2021 generated a reporter called CGf, a ratiometric, genetically encoded Ca 2+ indicator with a mCherry domain fused to the intensiometric Ca 2+ reporter GCaMP6f. By using this new tool, the authors showed that HS suppressed the tip-focused Ca 2+ oscillations in growing pollen tubes with the consequent growth arrest and even pollen tube tip rupture ( Weigand et al., 2021 ). This important result highlights the urgent need to better investigate the HS signaling in pollen tubes and better define the role of Ca 2+ signaling components in this response. It is obvious that the temperature stress, by affecting pollen tube development will lead to decreased fertility and reduced seed production. A good knowledge of the specific role of Ca 2+ signaling in a pollen tube, subjected to HS, will surely be instrumental to developing tailored strategies aimed at improving pollen resilience to HS. During fertilization process, synergids are an essential part of the female gametophyte. These cells are involved in guiding the pollen tube to the embryo sac and facilitating the entry of the male gamete, and commit programmed cell death upon pollen tube arrival ( Mendes et al., 2016 ). Ca 2+ spikes were detected in the reception and recognition of the pollen tube by the synergid cells and ultimately upon pollen tube burst and delivery of the male gametes and upon synergid cell death ( Ngo et al., 2014 ). Studies understanding how Ca 2+ spikes relationship with HS in the context of fertilization process is crucial for developing effective strategies to mitigate the negative impacts of HS on seed production and ensuring sustainable practices in the face of climate change.
HS effects in a crop of economic relevance, Oryza sativa
Rice, like several other cereal species, shows large adaptive phenotypic plasticity enabling yield stability across environments. However, high temperatures beyond the critical threshold of rice growth can cause severe reductions in grain yield and quality, particularly from the heading stage to the grain-filling stage. Generally, the process of male and female gametophyte formation in Oryza sativa is similar to what was described for Arabidopsis, is mainly divided into three stages: meiotic division of the spore mother cell, mitotic stage of functional spore cells, and mature stage of gametophyte ( Itoh et al., 2005 ). Research focusing on rice ovule and pollen development are limited for several reasons, the main one is the fact that the gametophytes are deeply embedded in the inflorescences also called panicles, because of their conic shape ( Li et al., 2023 ). Rice panicle comprises the main axis, a branch from the branch meristem, and a spikelet from the spikelet meristem. The spikelet meristem forms the sterile organs, glumes and lemmas that enclose the florets which on in its turn contains all the reproductive and fertile organs that give rise to seeds (grains) ( Itoh et al., 2005 ). The flowering phase in rice is highly sensitive to high temperatures. Two days of HS conditions resulted in an increase in the number of spikelets with non-viable pollen, meanwhile, four or more days of HS led to complete male sterility and several morphological defects on panicle development in heat-sensitive variety Nipponbare ( Figure 3 ) ( Endo et al., 2009 , Li et al., 2023 ). To gain insight into the molecular mechanism of heat-induced male sterility, Endo and collaborators analyzed transcriptional alteration in the anther under high-temperature conditions using DNA microarray. The identified high temperature-repressed genes, such as YY1 and YY2 were expressed predominantly in the tapetum at the uninucleate microspore stage. Among them two genes involved in lipid metabolism, a plant-specific cytochrome P450 and a GDSL type ligase were identified, suggesting that the composition of lipid derivatives in the pollen might be altered in anthers exposed to high temperatures ( Endo et al., 2009 ). Another study ( Zhang et al., 2012 ) evaluated transcriptomic changes accompanying HS in reproductive tissues, at early stages of development (pre- and during meiosis) from the heat-tolerant cultivar Indica-type 996, which exhibits better anther dehiscent, pollen fertility rate and final seed yield than heat-sensitive cultivar Indica-type 4628 ( Luo et al., 2005 ; Zhang et al., 2008 ). The predominant transcription factor gene families responsive to HS were HSF, NAC, AP2/ERF, WRKY, MYB, and C2H2, showing time-dependent gene expression pattern under short/middle-term HS (from 20 minutes to 8 hours). Furthermore, the promoter analysis of HS early up-regulated genes showed the important role of some specific motifs, such as HSE, GCC box, ABRE and CE3 in response to HS ( Zhang et al., 2012 , Figure 3 ). It is widely known that the HSE motif can be recognized and bound by HSF to respond to heat shock ( Yamamoto et al., 2005 ), while other motifs are linked to ethylene, ABA and Ca 2+ signaling, suggesting the existence of a complex crosstalk between several hormones and stimuli during heat shock ( Zhang et al., 2012 ). Recently another thermotolerant rice accession was described, T2- Jinxibai, that after 45°C for 24h, exhibited high resistance to HS and the seedlings exhibited a survival rate of 90.93% after heat treatment. Sixty transcription factors were differentially expressed in the thermotolerant accession including the members of the AP2/ERF, NAC, HSF, WRKY, and C2H2 families as seen for the thermotolerant Indica-type 996 cultivar ( He et al., 2023 , Figure 3 ).
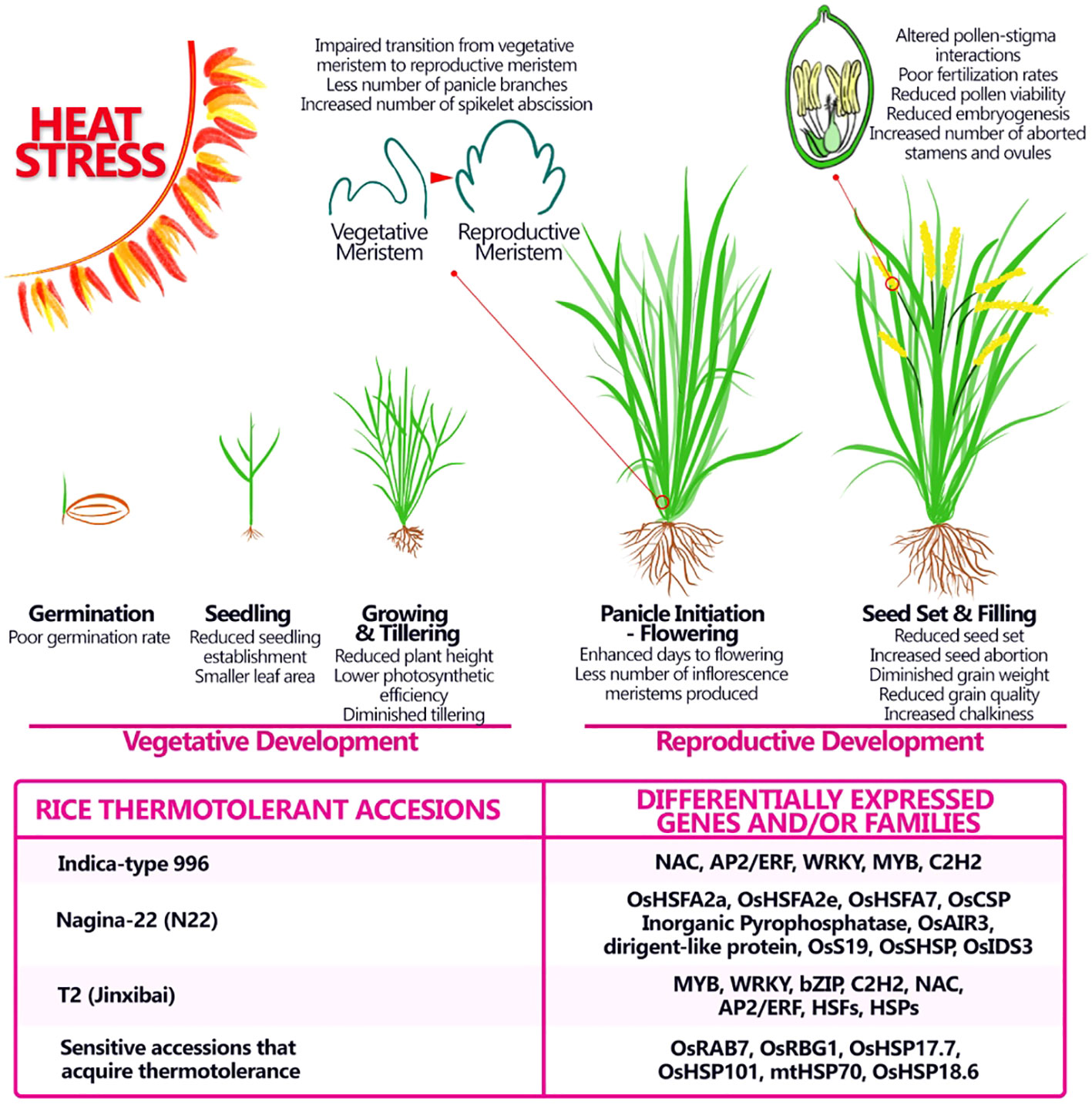
Figure 3 Adverse impact of heat stress on morphological and physiological aspects of rice at different stages of development and possible genes and/or families involved in thermotolerance. Heat stress affects rice productivity by affecting both vegetative and reproductive stages, still major effects of heat stress are seen during the reproductive stage and can provoke alterations in the number of panicles produced in the latest stages, modifying the starch content and therefore the quality of the grain. Some thermotolerant accessions were studied and specific gene or families involved in the thermotolerance were discovered.
Very interestingly, Jagadish et al. (2008) describes the heat tolerant cultivar Oryza sativa indica Nagina 22 (N22), which after a 6-hour high temperature treatment, still maintains a 71% of spikelet fertility. This is a positive significant value if compared with sensitive (Moroberekan - 18% fertility) or intermediate (IR64 - 48% fertility) cultivars ( Jagadish et al., 2010 ). Conducting a proteomic examination of the anthers in the heat-tolerant cultivar N22 unveiled the distinct expression patterns of 13 proteins. Among these, seven proteins demonstrated sequence similarities to potential cold shock protein (CSP), an inorganic pyrophosphatase, a serine protease (AIR3), a dirigent-like protein, a ribosomal protein (S19), a small heat shock protein (sHSP), and an iron deficiency protein (IDS3). These proteins are presumed contributors to the observed heightened heat tolerance in the cultivar. Particularly interesting are the stress-responsive cold and heat shock proteins identified ( Jagadish et al., 2010 , Figure 3 ), which will require further analysis to determine their role in HS tolerance. Consequently, it has been proposed that heat shock proteins contribute to higher tolerance to HS in rice. Supporting this hypothesis, Sailaja et al. (2015) reported that the expression of several heat shock expression factors (HSFS - OsHSFA2a, OsHSFA2e, and OsHSFA7) were highly upregulated in N22 plants when they were under heat treatment (42°C for 24 hours), with respect to the heat susceptible cultivar Vandana. Only two HSFS (OsHSFA2e and OsHSFA7) were upregulated under the same heat treatment in the susceptible cultivar Vandana, although the increase on the level of expression was minimal when compared with N22.
N22 cultivar also presented an improvement in the photosynthetic rate and chlorophyll fluorescence, and reduced transpiration rate under HS. These traits may help this cultivar to show better performance on HS conditions and hence superior yield ( Vivitha et al., 2018 ). The N22 cultivar was also used in another study to depict the global transcriptional response to HS in reproductive tissues, specifically during anthesis ( González -Schain et al., 2016 ). It has been well documented that anthesis in rice is the stage most sensitive to high temperatures ( Prasad et al., 2006 ; Jagadish et al., 2007 ), during which many physiological processes occur in less than one hour. Indeed, it was shown that reproductive tissue responds quickly, already after 30 minutes, to adjust their transcriptome to prevent damage produced by high temperature (38°C). Proper expression of protective chaperons in anthers at anthesis is needed to overcome stress damage and to ensure fertilization ( González -Schain et al., 2016 ).
Similar to Arabidopsis, in the case of rice, there are relatively few instances where the impact of temperature stress on female reproductive processes has been explored. However, a more extensive body of knowledge exists regarding the effects of HS on male reproductive functions.This is because pollen is easily accessible compared to ovules coupled with the notion that pollen exhibits greater sensitivity to HS than female reproductive organs in different crop plants ( Hedhly et al., 2009 ; Wang et al., 2021 ). However, recent studies revealed varying degrees of sensitivity of the pistil, ovaries, ovules, and gametophyte to the HS depending on rice varieties and developmental stages ( Wang et al., 2021 ; Shi et al., 2022 ). Shi and colleagues ( Shi et al., 2022 ) demonstrated the heat sensitivity of the pistil showing that HSed pistil pollinated with non-stressed pollen resulted in a significant reduction in spikelet fertility in the sensitive IR64 cultivar at 40°C. On the contrary, no sensitivity was observed in the N22 variety, indicating tolerance of N22 to HS also during pistil development. Interestingly, a significant proportion of ovules of IR64 variety subjected to HS were characterized by a non-corrected differentiation of megaspore mother cell or by the degeneration of all four megaspore cells instead of three after meiosis. All those effects resulted in a lesser proportion of viable embryo sacs, such as mature embryo sacs lacking the egg cell or the central cell ( Shi et al., 2022 ). In addition to the effects on gametophyte, a previous study reported the effects of heat on the tissues of the pistil. In particular, about half of the spikelets observed at the SEM microscope developed pistil hyperplasia, i.e., proliferated female organs or tissues, including multiple stigmata and/or ovaries, and differentiation of trichomes from ovary epidermis ( Takeoka et al., 1991 ). HS reduces the capacity of rice grain to assimilate supplies, such as starch and proteins, additionally also shortens grain-filling stage duration, leading to the reduction of grain weight and a chalky-appearing grains, greatly damaging their market value ( Kobata and Uemuki, 2004 ; Peng et al., 2004 ). Given that starch constitutes the primary component of grains, its deficiency is a key factor contributing to the reduction in grain weight under high temperatures. Consequently, transcriptomic studies have demonstrated that HS suppresses the expression of genes involved in starch biosynthesis while promoting the expression of enzymes responsible for starch consumption ( Yamakawa et al., 2007 ). For example, heightened temperatures led to increased expression levels of several α-amylase genes, namely Amy1A, Amy1C, Amy3A, Amy3D, and Amy3E , along with an elevation in enzyme activity. In contrast, the expression of starch biosynthetic genes such as granule-bound starch synthase I (GBSSI ) and a starch branching enzyme (BEIIb) was reduced ( Yamakawa et al., 2007 ). Subsequent research confirmed that the expression of Amy1A, Amy3C, and Amy3D in the endosperm during seed ripening significantly contributes to the production of chalky grains in high-temperature conditions ( Nakata et al., 2017 ). Furthermore, the downregulation of two key sucrose transporter genes, namely SUT1 and SUT2, under HS indicates a potential hindrance to the import of sucrose into the endosperm ( Yamakawa and Hakata, 2010 ).Those results were supported by a parallel metabolomic analysis showing that sucrose and amino acids accumulated, and the level of sugar phosphates and organic acids decreased in HS-ripened caryopses ( Yamakawa and Hakata, 2010 ). Thermotolerance in rice during both vegetative and reproductive growth without a yield penalty was recently identified by a natural quantitative trait locus (QTL), TT2 -THERMOTOLERANCE 2. TT2 encodes a Gγ subunit that codifies for a heterotrimeric GTP-binding proteins (G proteins), the thermotolerance was directly linked to the SCT1 (Sensing Ca 2+ Transcription factor 1) - dependent alteration of wax biosynthesis. The calmodulin–SCT1 interaction was attenuated by reduced heat-triggered Ca 2+ caused by disrupted TT2 ( Kan et al., 2022 ).
Recently, the allele of the TT1 gene coming from African rice ( O. glaberrima - CG14), which encodes for a 26S proteasome α2 subunit protein, boost thermotolerance by enhancing the recycling and elimination of denatured ubiquitinated proteins consequence of HS. Remarkably, plants harbouring the TT1 -CG14 allele greatly outperformed plants carrying the Asian rice ( TT1 - O. sativa spp. japonica ) allele in grain per plant production after heat treatment (12h at 38°C/12h at 35°C for 5 days). Yield superiority conferred by the TT1 -CG14 allele, was observed regardless if HS was applied during flowering or grain filling stages. These results validate the potential of the TT1 -CG14 allele for breeding heat tolerant crops ( Li et al., 2015 ).
Likewise, plants harbouring the TT3 QTL from CG14 presented higher survival rate at reproductive stage and improved grain yield after HS treatment (30 days at 38°C/34°C day/night) compared with plants carrying the Asian rice TT3-QTL (from O. sativa spp. japonica ). TT3 quantitative trait loci contains the TT3.1 and TT3.2 genes, TT3.1 is a RING-type E3 ligase and TT3.2 is a chloroplast precursor protein (ubiquitinated by TT3.1). After HS, TT3.2 is accumulated in chloroplasts causing damages to the photosystem II complex, compromising the thylakoid stability. Consequently, the improved E3 ubiquitin ligase activity of TT3.1-CG14 ubiquitinating TT3.2 for its rapid vacuolar degradation, protect the thylakoids from HS, hence increasing the thermotolerance of the plants ( Zhang et al., 2022 ).
El-Esawi and Alayafi (2019) demonstrated that the overexpression of OsRAB7 enhances not only HS tolerance but also increased grain yield. The RAB protein family is involved in multiple developmental processes and has been linked to tolerance to environmental stresses (reviewed in Tiwari et al., 2021 ). Transgenic plants overexpressing OsRAB7 presented an increment of nearly 40% in survival rate after a heat treatment (40°C day/32°C night, irrigated daily, for 10 days) with respect to the wild type individuals. Under HS conditions, both wild-type and overexpression lines presented diminishment in growth when compared to individuals growing in normal conditions. However, transgenic lines exhibited better growth performance when compared to the wild type under HS conditions. These findings suggest that the increased expression of OsRAB7 in transgenic rice plants positively influences their survival rate, growth, relative water content, and resilience against both drought and HSes. Given that OsRAB7 overexpression has been associated with improved salt tolerance in rice by enhancing stress signaling transduction through intracellular vesicle trafficking ( Peng et al., 2014 ), it is highly likely that heightened HS tolerance is also achievable through enhanced intracellular vesicle trafficking ( El-Esawi and Alayafi, 2019 ). The yield-related improved traits on the OsRAB7 overexpressing lines when compared with the wild type were: panicle length (+25%), number of spikelets per panicle (+11%), total number of spikelets per hill (+11%), number of filled grains per hill (+35%), filling rate (+21%), and total grain weight (+27%). It is important to notice that under normal conditions, the OsRAB7 overexpressing lines did not show any significant difference on yield traits compared to the wild type ( El-Esawi and Alayafi, 2019 ). These data are extremely important because represent one of the few examples of reported transgenic lines that display a better yield performance under HS conditions. Indeed, most of the improved stress tolerance transgenic lines are focused on the survival rate of the plants but no data regarding the effect on yield is shown. Recently, Lo et al (2020) identified a novel RICE BIG GRAIN 1 (RBG1) gene that is involved in auxin homeostasis and enhances cell division. The overexpression of RBG1 impacts several aspects of plant growth and development including a significant enhancement in the size of the panicle and seeds, when compared to wild-type plants. This positive yield effect, together with the fact that 31 members of the HSPs gene family resulted upregulated on RBG1 overexpression lines when compared with wild type plants, led the researchers to evaluate the performance of these lines under several stress conditions including HS (4 days at 42°C). Notably, the RBG1 overexpression lines showed a higher survival rate after recovery (≈80%) than the wild-type plants (≈20%). However, if the positive effect on yield properties that confers the overexpression of RBG1 is still manifested under HS conditions remains unclear. Undoubtedly, OsRAB7 and OsRBG1 represent excellent candidates to be used in future breeding programs focused on dealing with climate change and raising the global temperature. Nonetheless, the performance under HS conditions of some other important yield-related traits such as plant height, number of panicles per plant, number of primary and secondary branches on the main panicle and thousand seeds weight, remain largely unexplored for these interesting transgenic lines ( Lo et al., 2020 ).
Some natural allelic variations on SLENDER GUY 1 ( OsSLG1 ), a cytosolic tRNA 2-thiolation protein, confer higher thermotolerance at both seedling and reproductive stages ( Xu et al., 2020 ). The loss of function of SLG1 reduced more than 80% the survival rate of the seedlings after heat treatment. Interestingly, slg1 plants at reproductive stage after heat treatment (40°C for 5 days) showed reduced seed-setting rate generated by a large reduction on the number of pollen grains on the surface of the stigma and consequently of growing pollen-tubes. The authors proposed that the SLG1 tRNA-modification activity positively impacts the translation efficiency of the cell, thus modulating the concentration of HSPs and reducing the proportion of mis-folded proteins ( Xu et al., 2020 ). In consequence, augmented translational efficiency and fidelity resulted critically beneficial to tolerate high temperature stress.
HSPs along with HSFs are the most important players in heat response transcriptional regulatory networks. In 2003, Katiyar-Agarwal and colleagues demonstrated that overexpressing Arabidopsis HSP101 in rice under high-salinity conditions improved yield by enhancing pollen tube viability. Other studies involving HSPs demonstrated that the survival rate for rice plants overexpressing the sHSP17.7 after a 2-hour at 50°C treatment increased with respect to the control ( Murakami et al., 2004 ). In a clear example of “cross-tolerance”, rice lines overexpressing the sHSP17.7 protein were also capable of continuing to grow after a 6-day long drought period, while untransformed plants did not survive the treatment ( Sato and Yokoya, 2008 ). The mitochondrial HS protein mtHSP70 is apparently involved in conferring heat tolerance resistance to rice protoplasts when overexpressed. Heat treatment (15 minutes at 48°C) on untransformed rice protoplasts resulted in around 27% survival rate, meanwhile protoplast overexpressing mtHSP70 presented a 60% survival rate ( Qi et al., 2011 , Li et al., 2023 ). These authors suggest that overexpression of mtHSP70 promotes increased HS tolerance on rice protoplasts by inhibiting programmed cell death triggered by high temperature through the maintenance of the mitochondrial membrane potential and preventing reactive oxygen species signal amplification ( Qi et al., 2011 ). OsHSP18.6 is also capable of conferring enhanced HS tolerance when overexpressed. OsHSP18.6 overexpression lines displayed better root and shoot growth performance after a 3-week HS treatment (45°C/12h, 28°C/12h – Wang et al., 2015 ). Nonetheless, not all the HSPs increased HS tolerance when overexpressed. For example, overexpression of OsHsp17.0 and OsHsp23.7 did not improve HS tolerance with respect to wild type plants, but it enhanced salt stress and drought stress tolerances ( Zou et al., 2012 ).
Future perspectives
In response to HS, plants employ several mechanisms to maintain homeostasis and normal cellular functions. Understanding how these processes occur in the reproductive tissues of model species such as Arabidopsis is relevant for the establishment of a platform for advanced studies in crop species. Interestingly, HSPs are present not only in Arabidopsis but also in mammals, drosophila, yeast and so on. The studies of molecular mechanisms underlying tolerance of HS could also be important for understanding similar mechanisms in other species. The knowledge of the molecular mechanism due to HS derived from the characterization of putative thermotolerant related genes and pathways in model species might have a direct impact on other species, specifically in species with more economic relevance for humankind. Most of the consequences of climate change for agricultural production are expected to be negative, making the implementation of mitigation strategies much needed to adapt crops to these new conditions. Special attention is directed towards crops that are essential parts of the human caloric intake: rice, wheat, and maize ( FAO, 2023 ).
A plant exhibiting HS tolerance can sustain its regular growth and uphold, or even boost, total yield production in elevated temperature conditions by modifying metabolic and/or structural characteristics ( Wahid et al., 2007 ). The features associated with heat tolerance are influenced by multiple genes and are connected to the morphological and physiological adaptations in rice. However, there is a scarcity of information regarding stress avoidance and tolerance mechanisms specific to rice. Identification of the molecular basis of plant adaptation is fundamental to driving plant breeding into the development of novel varieties that can adapt to climate changes. In addition, genome editing tools could play a role in bolstering or hastening crop responses to climate change and/or biofortified crops to provide adequate nutritional quality to a growing population ( Leisner, 2020 ). Rice, like several other cereal species, shows large adaptive phenotypic plasticity enabling yield stability across environments.
Author contributions
FR: Conceptualization, Writing – original draft. GO-A: Conceptualization, Writing – original draft. MC: Writing – original draft. MM: Writing – original draft, Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. MM was supported by Linea 2 - PSR2021, Bioscience Department, University of Milan, and by MUR PRIN2022 (PRIN202223MMIRA_01). MC was supported by Linea 2 - PSR2021, Bioscience Department, University of Milan, and by CRISPit Project MSCA-2021-SE-01. FR was supported by MUR PRIN2017 (PRIN 2017ZBBYNC) and GO-A was supported by a PhD fellowship from the University of Milan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agrawal, L., Narula, K., Basu, S., Shekhar, S., Ghosh, S., Datta, A., et al. (2013). Comparative proteomics reveals a role for seed storage protein AmA1 in cellular growth, development, and nutrient accumulation. J. Proteome Res. 12 (11), 4904–4930. doi: 10.1021/pr4007987
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahmadli, U., Kalidass, M., Khaitova, L. C., Fuchs, J., Cuacos, M., Demidov, D., et al. (2023). High temperature increases centromere-mediated genome elimination frequency and enhances haploid induction in Arabidopsis. Plant Commun. 4 (3), 100507. doi: 10.1016/j.xplc.2022.100507
Barnabás, B., Jäger, K., Fehér, A. (2008). The effect of drought and HS on reproductive processes in cereals. Plant Cell Environ. 31 (1), 11–38. doi: 10.1111/j.1365-3040.2007.01727.x
Begcy, K., Nosenko, T., Zhou, L. Z., Fragner, L., Weckwerth, W., Dresselhaus, T. (2019). Male sterility in maize after transient HS during the tetrad stage of pollen development. Plant Physiol. 181 (2), 683–700. doi: 10.1104/pp.19.00707
Bita, C. E., Gerats, T. (2013). Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of HS-tolerant crops. Front. Plant Sci. 4, 273. doi: 10.3389/fpls.2013.00273
Borg, M., Brownfield, L., Twell, D. (2009). Male gametophyte development: a molecular perspective. J. Exp. Bot. 60 (5), 1465–1478. doi: 10.1093/jxb/ern355
Charng, Y., Liu, H., Liu, N., Chi, W., Wang, C., Chang, S., et al. (2007). A heat-inducible transcription factor, HsfA2, is required for extension for acquired thermotolerance in Arabidopsis. Plant Physiol. 143, 251–262. doi: 10.1104/pp.106.091322
Chaturvedi, P., Wiese, A. J., Ghatak, A., Záveská Drábková, L., Weckwerth, W., Honys, D. (2021). HS response mechanisms in pollen development. New Phytol. 231 (2), 571–585. doi: 10.1111/nph.17380
Choudhury, F. K., Devireddy, A. R., Azad, R. K., Shulaev, V., Mittler, R. (2018). Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 178 (4), 1461–1472. doi: 10.1104/pp.18.01031
Christensen, C. A., Gorsich, S. W., Brown, R. H., Jones, L. G., Brown, J., Shaw, J. M., et al. (2002). Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. The Plant Cell 14(9), 2215–2232. doi: 10.1105/tpc.002170
Cui, Y., Lu, S., Li, Z., Cheng, J., Hu, P., Zhu, T., et al. (2020). CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 183 (4), 1794–1808. doi: 10.1104/pp.20.00591
Czarnecka-Verner, E., Pan, S., Salem, T., Gurley, W. B. (2004). Plant class b HSFs inhibit transcription and exhibit affinity for TFIIB and TBP. Plant Mol. Biol. 56, 57–75. doi: 10.1007/s11103-004-2307-3
De Jaeger-Braet, J., Krause, L., Buchholz, A., Schnittger, A. (2022). HS reveals a specialized variant of the pachytene checkpoint in meiosis of Arabidopsis thaliana. Plant Cell. 34 (1), 433–454. doi: 10.1093/plcell/koab257
De Storme, N., Geelen, D. (2020). High temperatures alter cross-over distribution and induce male meiotic restitution in Arabidopsis thaliana. Commun. Biol. 3 (1), 187. doi: 10.1038/s42003-020-0897-1
Döring, P., Treuter, E., Kistner, C., Lyck, R., Chen, A., Nover, L. (2000). The role of AHA motifs in the activator function of tomato HS transcription factors HsfA1 and HsfA2. Plant Cell 12 (2), 265–278. doi: 10.1105/tpc.12.2.265
Drews, G. N., Koltunow, A. M. (2011). The female gametophyte. Arabidopsis Book 2011 (9). doi: 10.1199/tab.0155
CrossRef Full Text | Google Scholar
El-Esawi, M. A., Alayafi, A. A. (2019). Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 10 (1), 56. doi: 10.3390/genes10010056
Endo, M., Tsuchiya, T., Hamada, K., Kawamura, S., Yano, K., Ohshima, M., et al. (2009). High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 50 (11), 1911–1922. doi: 10.1093/pcp/pcp135
Finka, A., Cuendet, A. F. H., Maathuis, F. J., Saidi, Y., Goloubinoff, P. (2012). Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24 (8), 3333–3348. doi: 10.1105/tpc.112.095844
Frietsch, S., Wang, Y.-F., Sladek, C., Poulsen, L. R., Romanowsky, S. M., Schroeder., J. I., et al. (2007). A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. U.S.A. 104, 14531–14536. doi: 10.1073/pnas.0701781104
Ghosh, S., Bheri, M., Bisht, D., Pandey, G. K. (2022). Calcium signaling and transport machinery: Potential for development of stress tolerance in plants. Curr. Plant Biol. 29, 100235. doi: 10.1016/j.cpb.2022.100235
Gong, M., van der Luit, A. H., Knight, M. R., Trewavas, A. J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116 (1), 429–437. doi: 10.1104/pp.116.1.429
González-Schain, N., Dreni, L., Lawas, L. M., Galbiati, M., Colombo, L., Heuer, S., et al. (2016). Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of HS responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 57 (1), 57–68. doi: 10.1093/pcp/pcv174
Guan, Q., Lu, X., Zeng, H., Zhang, Y., Zhu, J. (2013). HS induction of mi r 398 triggers a regulatory loop that is critical for thermotolerance in a rabidopsis. Plant J. 74 (5), 840–851. doi: 10.1111/tpj.12169
Guo, M., Liu, J. H., Ma, X., Luo, D. X., Gong, Z. H., Lu, M. H. (2016). The plant HS transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00114
He, Y., Guan, H., Li, B., Zhang, S., Xu, Y., Yao, Y., et al. (2023). Transcriptome analysis reveals the dynamic and rapid transcriptional reprogramming involved in HS and identification of heat response genes in rice. Int. J. Mol. Sci. 24 (19), 14802. doi: 10.3390/ijms241914802
Hedhly, A., Hormaza, J. I., Herrero, M. (2009). Global warming and sexual plant reproduction. Trends Plant Sci. 14 (1), 30–36. doi: 10.1016/j.tplants.2008.11.001
Hedhly, A., Nestorova, A., Herrmann, A., Grossniklaus, U. (2020). Acute HS during stamen development affects both the germline and sporophytic lineages in Arabidopsis thaliana (L.) Heynh. Environ. Exp. Bot. 173, 103992. doi: 10.1016/j.envexpbot.2020.103992
Iba, K. (2002). Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 53 (1), 225–245. doi: 10.1146/annurev.arplant.53.100201.160729
Ikeda, M., Ohme-Takagi, M. (2009). A novel group of transcriptional repressors in arabidopsis. Plant Cell Physiol. 50 (5), 970–975. doi: 10.1093/pcp/pcp048
Itoh, J. I., Nonomura, K. I., Ikeda, K., Yamaki, S., Inukai, Y., Yamagishi, H., et al. (2005). Rice plant development: from zygote to spikelet. Plant Cell Physiol. 46 (1), 23–47. doi: 10.1093/pcp/pci501
Jagadish, S. V., Craufurd, P. Q., Wheeler, T. R. (2007). High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 58 (7), 1627–1635. doi: 10.1093/jxb/erm003
Jagadish, S. V. K., Craufurd, P. Q., Wheeler, T. R. (2008). Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci. 48 (3), 1140–1146. doi: 10.2135/cropsci2007.10.0559
Jagadish, S. V. K., Muthurajan, R., Oane, R., Wheeler, T. R., Heuer, S., Bennett, J., et al. (2010). Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 61 (1), 143–156. doi: 10.1093/jxb/erp289
Jespersen, D. (2020). Heat shock induced stress tolerance in plants: Physiological, biochemical, and molecular mechanisms of acquired tolerance. In Priming-mediated stress and cross-stress tolerance in crop plants. Acad. Press , 161–174. doi: 10.1016/B978-0-12-817892-8.00010-6
Jin, C., Sun, L., Trinh, H. K., Danny, G. (2023). HS promotes haploid formation during CENH3-mediated genome elimination in Arabidopsis. Plant Reprod. 36, 147–155. doi: 10.1007/s00497-023-00457-8
Kan, Y., Mu, X. R., Zhang, H., Gao, J., Shan, J. X., Ye, W. W., et al. (2022). TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 8, 53–67. doi: 10.1038/s41477-021-01039-0
Ko, D., Helariutta, Y. (2017). Shoot–root communication in flowering plants. Curr. Biol. 27 (17), R973–R978. doi: 10.1016/j.cub.2017.06.054
Kobata, T., Uemuki, N. (2004). High temperatures during the grain-filling period do not reduce the potential grain dry matter increase of rice. Agron. J. 96 (2), 406–414. doi: 10.2134/agronj2004.0406
Kovács, D., Kovács, M., Ahmed, S., Barna, J. (2022). Functional diversification of heat shock factors. Biol. futura 73, 427–439. doi: 10.1007/s42977-022-00138-z
Kudla, J., Becker, D., Grill, E., Hedrich, R., Hippler, M., Kummer, U., et al. (2018). Advances and current challenges in calcium signaling. New Phytol. 218 (2), 414–431. doi: 10.1111/nph.14966
Lan, J., Wang, N., Wang, Y., Jiang, Y., Yu, H., Cao, X., et al. (2023). Arabidopsis TCP4 transcription factor inhibits high temperature-induced homeotic conversion of ovules. Nat. Commun. 14 (1), 5673. doi: 10.1038/s41467-023-41416-1
Larkindale, J., Knight, M. R. (2002). Protection against HS-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 128 (2), 682–695. doi: 10.1104/pp.010320
Leisner, C. P. (2020). Climate change impacts on food security-focus on perennial cropping systems and nutritional value. Plant Sci. 293, 110412. doi: 10.1016/j.plantsci.2020.110412
Leng, Y. J., Yao, Y. S., Yang, K. Z., Wu, P. X., Xia, Y. X., Zuo, C. R., et al. (2022). Arabidopsis ERdj3B coordinates with ERECTA-family receptor kinases to regulate ovule development and the HS response. Plant Cell. 34 (10), 3665–3684. doi: 10.1093/plcell/koac226
Lesk, C., Rowhani, P., Ramankutty, N. (2016). Influence of extreme weather disasters on global crop production. Nature 529 (7584), 84–87. doi: 10.1038/nature16467
Li, X., Chao, D., Wu, Y., Huang, X., Chen, K., Cui, L., et al. (2015). Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 47 (7), 827–833. doi: 10.1038/ng.3305
Li, J. Y., Yang, C., Xu, J., Lu, H. P., Liu, J. X. (2023). The hot science in rice research: How rice plants cope with HS. Plant Cell Environ. 46 (4), 1087–1103. doi: 10.1111/pce.14509
Liao, C., Zheng, Y., Guo, Y. (2017). MYB30 transcription factor regulates oxidative and HS responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytol. 216 (1), 163–177. doi: 10.1111/nph.14679
Liu, H. C., Charng, Y. Y. (2013). Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 163 (1), 276–290. doi: 10.1104/pp.113.221168
Lo, S. F., Cheng, M. L., Hsing, Y. I. C., Chen, Y. S., Lee, K. W., Hong, Y. F., et al. (2020). Rice Big Grain 1 promotes cell division to enhance organ development, stress tolerance and grain yield. Plant Biotechnol. J. 18 (9), 1969–1983. doi: 10.1111/pbi.13357
Lucas, W. J., Groover, A., Lichtenberger, R., Furuta, K., Yadav, S. R., Helariutta, Y., et al. (2013). The plant vascular system: evolution, development and functions F. J. Integr. Plant Biol. 55 (4), 294–388. doi: 10.1111/jipb.12041
Luo, L., Liu, G., Xiao, Y., Tang, W., Chen, L. (2005). Influences of high-temperature stress on the fertility of pollen, spikelet and grain-weight in rice. J. Hunan Agri Univ 31, 593–596.
Google Scholar
Mendes, M. A., Guerra, R. F., Castelnovo, B., Silva-Velazquez, Y., Morandini, P., Manrique, S., et al. (2016). Live and let die: a REM complex promotes fertilization through synergid cell death in Arabidopsis. Development 143(15), 2780–2790. doi: 10.1242/dev.134916
Modliszewski, J. L., Wang, H., Albright, A. R., Lewis, S. M., Bennett, A. R., Huang, J., et al. (2018). Elevated temperature increases meiotic crossover frequency via the interfering (Type I) pathway in Arabidopsis thaliana. PloS Genet. 14 (5), e1007384. doi: 10.1371/journal.pgen.1007384
Morimoto, R. I. (1998). Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12 (24), 3788–3796. doi: 10.1101/gad.12.24.3788
Mulla, S., Singh, S. K., Singh, K. K., Praveen, B. (2020). Climate change and agriculture: a review of crop models. Global Climate Change Environ. policy: Agric. Perspect. , 423–435. doi: 10.1007/978-981-13-9570-3_15
Murakami, T., Matsuba, S., Funatsuki, Kawaguchi, K., Surayama, H., Tanida, M., et al. (2004). Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed. 13, 165–175. doi: 10.1023/B:MOLB.0000018764.30795.c1
Nakata, M., Fukamatsu, Y., Miyashita, T., Hakata, M., Kimura, R., Nakata, Y., et al. (2017). High temperature-induced expression of rice α-amylases in developing endosperm produces chalky grains. Front. Plant Sci. 8, 2089. doi: 10.3389/fpls.2017.02089
Ngo, Q. A., Vogler, H., Lituiev, D. S., Nestorova, A., Grossniklaus, U. (2014). A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell 29(4), 491–500. doi: 10.1016/j.devcel.2014.04.008
Peet, M. M., Sato, S., Gardner, R. G. (1998). Comparing HS effects on male-fertile and male-sterile tomatoes. Plant Cell Environment 21, 225–231. doi: 10.1046/j.1365-3040.1998.00281.x
Peng, X., Ding, X., Chang, T., Wang, Z., Liu, R., Zeng, X., et al. (2014). Overexpression of a vesicle trafficking gene, OsRab7, enhances salt tolerance in rice. Sci. World J. 2014, 483526. doi: 10.1155/2014/483526
Peng, S., Huang, J., Sheehy, J. E., Laza, R. C., Visperas, R. M., Zhong, X., et al. (2004). Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. 101 (27), 9971–9975. doi: 10.1073/pnas.0403720101
Petrella, R., Cucinotta, M., Mendes, M. A., Underwood, C. J., Colombo, L. (2021). The emerging role of small RNAs in ovule development, a kind of magic. Plant Reprod. 34 (4), 335–335. doi: 10.1007/s00497-021-00421-4
Prasad, P. V. V., Boote, K. J., Allen, L. H., Sheehy, J. E., Thomas, J. M. G. (2006). Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 95, (2–3), 398–411. doi: 10.1016/j.fcr.2005.04.008
Prasad, P. V. V., Staggenborg, S. A., Ristic, Z. (2008). Impacts of drought and/or HS on physiological, developmental, growth, and yield processes of crop plants. Response Crops to limited water: Understand model Water Stress effects Plant Growth processes 1, 301–355. doi: 10.2134/advagricsystmodel1.c11
Qi, Y., Wang, H., Zou, Y., Liu, C., Liu, Y., Wang, Y., et al. (2011). Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett. 585 (1), 231–239. doi: 10.1016/j.febslet.2010.11.051
Resentini, F., Ruberti, C., Grenzi, M., Bonza, M. C., Costa, A. (2021). The signatures of organellar calcium. Plant Physiol. 187 (4), 1985–2004. doi: 10.1093/plphys/kiab189
Rezaei, E. E., Webber, H., Gaiser, T., Naab, J., Ewert, F. (2015). HS in cereals: Mechanisms and modelling. Eur. J. Agron. 64, 98–113. doi: 10.1016/j.eja.2014.10.003
Sailaja, B., Subrahmanyam, D., Neelamraju, S., Vishnukiran, T., Rao, Y. V., Vijayalakshmi, P., et al. (2015). Integrated physiological, biochemical, and molecular analysis identifies important traits and mechanisms associated with differential response of rice genotypes to elevated temperature. Front. Plant Sci. 6, 1044. doi: 10.3389/fpls.2015.01044
Sato, Y., Yokoya, S. (2008). Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 27 (2), 329–334. doi: 10.1007/s00299-007-0470-0
Scharf, K. D., Berberich, T., Ebersberger, I., Nover, L. (2012). The plant HS transcription factor (Hsf) family: structure, function and evolution. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 1819 (2), 104–119. doi: 10.1016/j.bbagrm.2011.10.002
Scott, R. J., Spielman, M., Dickinson, H. (2004). Stamen structure and function. Plant Cell 16 (suppl_1), S46–S60. doi: 10.1105/tpc.017012
Shekhar, S., Mishra, D., Gayali, S., Buragohain, A. K., Chakraborty, S., Chakraborty, N. (2016). Comparison of proteomic and metabolomic profiles of two contrasting ecotypes of sweetpotato (Ipomoea batata L.). J. Proteomics 143, 306–317. doi: 10.1016/j.jprot.2016.03.028
Shi, W., Yang, J., Kumar, R., Zhang, X., Impa, S. M., Xiao, G., et al. (2022). HS during gametogenesis irreversibly damages female reproductive organ in rice. Rice 5 (1), 32. doi: 10.1186/s12284-022-00578-0
Takeoka, Y., Hiroi, K., Kitano, H., Wada, T. (1991). Pistil hyperplasia in rice spikelets as affected by HS. Sexual Plant Reprod. 4, 39–43. doi: 10.1007/BF00194570
The State of Food Security and Nutrition in the World (2023). Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum (Rome: FAO). doi: 10.4060/cc3017en
Tiwari, P., Srivastava, D., Chauhan, A. S., Indoliya, Y., Singh, P. K., Tiwari, S., et al. (2021). Root system architecture, physiological analysis and dynamic transcriptomics unravel the drought-responsive traits in rice genotypes. Ecotoxicol Environ. Saf. 207, 111252. doi: 10.1016/j.ecoenv.2020.111252
Tunc-Ozdemir, M., Tang, C., Ishka, M. R., Brown, E., Groves, N. R., Myers, C. T., et al. (2013). A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 161 (2), 1010–1020. doi: 10.1104/pp.112.206888
Twell, D., Park, S. K., Lalanne, E. (1998). Asymmetric division and cell-fate determination in developing pollen. Trends Plant Sci. 3 (8), 305–310. doi: 10.1105/tpc.002170
Ul Haq, S., Khan, A., Ali, M., Khattak, A. M., Gai, W. X., Zhang, H. X., et al. (2019). Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 20 (21), 5321. doi: 10.3390/ijms20215321
Vivitha, P., Raveendran, M., Vijayalakshmi, C., Vijayalakshmi, D. (2018). Genetic dissection of high temperature stress tolerance using photosynthesis parameters in QTL introgressed lines of rice cv. Improved White Ponni. Indian J. Plant Physiol. 23, 741–747. doi: 10.1007/s40502-018-0408-2
Wahid, A., Gelani, S., Ashraf, M., Foolad, M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61 (3), 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wang, W., Cai, C., He, J., Gu, J., Zhu, G., Zhang, W., et al. (2020). Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res. 248, 107605. doi: 10.1016/j.fcr.2019.107605
Wang, Y., Impa, S. M., Sunkar, R., Jagadish, S. V. K. (2021). The neglected other half - role of the pistil in plant HS responses. Plant Cell Environ. 44 (7), 2200–2210. doi: 10.1111/pce.14067
Wang, A., Yu, X., Mao, Y., Liu, Y., Liu, G., Liu, Y., et al. (2015). Overexpression of a small heat-shock-protein gene enhances tolerance to abiotic stresses in rice. Plant Breed. 134 (4), 384–393. doi: 10.1111/pbr.12289
Weigand, C., Kim, S. H., Brown, E., Medina, E., Mares, M., III, Miller, G., et al. (2021). A ratiometric calcium reporter CGf reveals calcium dynamics both in the single cell and whole plant levels under HS. Front. Plant Sci. 12, 777975. doi: 10.3389/fpls.2021.777975
Whittle, C. A., Otto, S. P., Johnston, M. O., Krochko, J. E. (2009). Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 87 (6), 650–657. doi: 10.1139/B09-030
Wu, C. (1995). Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11 (1), 441–469. doi: 10.1146/annurev.cb.11.110195.002301
Wu, H. C., Jinn, T. L. (2010). Heat shock-triggered Ca2+ mobilization accompanied by pectin methylesterase activity and cytosolic Ca2+ oscillation are crucial for plant thermotolerance. Plant Signaling Behav. 5 (10), 1252–1256. doi: 10.4161/psb.5.10.12607
Wunderlich, M., Gross-Hardt, R., Schöffl, F. (2014). Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol. Biol. 85 (6), 541–550. doi: 10.1007/s11103-014-0202-0
Xu, J., Henry, A., Sreenivasulu, N. (2020). Rice yield formation under high day and night temperatures—A prerequisite to ensure future food security. Plant Cell Environ. 43 (7), 1595–1608. doi: 10.1111/pce.13748
Xu, Y., Zhang, L., Ou, S., Wang, R., Wang, Y., Chu, C., et al. (2020). Natural variations of SLG1 confer high-temperature tolerance in indica rice. Nat. Commun. 11 (1), 5441. doi: 10.1038/s41467-020-19320-9
Yadegari, R., Drews, G. N. (2004). Female gametophyte development. Plant Cell 16 (suppl_1), S133–S141. doi: 10.1105/tpc.018192
Yamakawa, H., Hakata, M. (2010). Atlas of rice grain filling-related metabolism under high temperature: joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 51 (5), 795–809. doi: 10.1093/pcp/pcq034
Yamakawa, H., Hirose, T., Kuroda, M., Yamaguchi, T. (2007). Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol. 144 (1), 258–277. doi: 10.1104/pp.107.098665
Yamamoto, A., Mizukami, Y., Sakurai, H. (2005). Identification of a novel class of target genes and a novel type of binding sequence of heat shock transcription factor in Saccharomyces cerevisiae. J. Biol. Chem. 280 (12), 11911–11919. doi: 10.1074/jbc.M411256200
Yamamoto, M., Uji, S., Sugiyama, T., Sakamoto, T., Kimura, S., Endo, T., et al. (2020). ERdj3B-mediated quality control maintains anther development at high temperatures. Plant Physiol. 182, 1979–1990. doi: 10.1104/pp.19.01356
Yamanouchi, U., Yano, M., Lin, H., Ashikari, M., Yamada, K. (2002). A rice spotted leaf gene, Spl7, encodes a HS transcription factor protein. Proc. Natl. Acad. Sci. 99 (11), 7530–7535. doi: 10.1073/pnas.112209199
Yeh, C. H., Kaplinsky, N. J., Hu, C., Charng, Y. Y. (2012). Some like it hot, some like it warm: phenotyping to explore thermotolerance diversity. Plant Sci. 195, 10–23. doi: 10.1016/j.plantsci.2012.06.004
Yoshida, T., Ohama, N., Nakajima, J., Kidokoro, S., Mizoi, J., Nakashima, K., et al. (2011). Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet. Genomics 286, 321–332. doi: 10.1007/s00438-011-0647-7
Zhang, G., Chen, L., Zhang, S., Liu, G., Tang, W., Li, M. H., et al. (2008). Effects of high temperature stress on pollen characters and anther microstructure of rice. Acta Ecol. Sin. 28, 1089–1097. (In Chinese with English abstract)
Zhang, X., Li, J., Liu, A., Zou, J., Zhou, X., Xiang, J., et al. (2012). Expression profile in rice panicle: insights into heat response mechanism at reproductive stage. PloS One 7 (11), e49652. doi: 10.1371/journal.pone.0049652
Zhang, H., Zhou, J., Kan, Y., Shan, J., Ye, W., Dong, N., et al. (2022). A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 376 (6599), 1293–1300. doi: 10.1126/science.abo5721
Zou, J., Liu, C., Liu, A., Zou, D., Chen, X. (2012). Overexpression of OsHsp17. 0 and OsHsp23. 7 enhances drought and salt tolerance in rice. J. Plant Physiol. 169 (6), 628–635. doi: 10.1016/j.jplph.2011.12.014
Keywords: heat stress, plant reproduction, rice, Arabidopsis , calcium signaling, pollen development, ovule development
Citation: Resentini F, Orozco-Arroyo G, Cucinotta M and Mendes MA (2023) The impact of heat stress in plant reproduction. Front. Plant Sci. 14:1271644. doi: 10.3389/fpls.2023.1271644
Received: 02 August 2023; Accepted: 13 November 2023; Published: 07 December 2023.
Reviewed by:
Copyright © 2023 Resentini, Orozco-Arroyo, Cucinotta and Mendes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta A. Mendes, [email protected]
†These authors have contribute equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 June 2023
Impact of heat stress, water stress, and their combined effects on the metabolism and transcriptome of grape berries
- Seanna Hewitt 1 ,
- Esther Hernández-Montes 2 , 3 ,
- Amit Dhingra 1 , 4 &
- Markus Keller 1 , 2
Scientific Reports volume 13 , Article number: 9907 ( 2023 ) Cite this article
2938 Accesses
6 Citations
3 Altmetric
Metrics details
- Plant molecular biology
- Plant physiology
- Plant stress responses
Recurring heat and drought episodes present challenges to the sustainability of grape production worldwide. We investigated the impacts of heat and drought stress on transcriptomic and metabolic responses of berries from two wine grape varieties. Cabernet Sauvignon and Riesling grapevines were subjected to one of four treatments during early fruit ripening: (1) drought stress only, (2) heat stress only, (3) simultaneous drought and heat stress, (4) no drought or heat stress (control). Berry metabolites, especially organic acids, were analyzed, and time-course transcriptome analysis was performed on samples before, during, and after the stress episode. Both alone and in conjunction with water stress, heat stress had a much more significant impact on berry organic acid content, pH, and titratable acidity than water stress. This observation contrasts with previous reports for leaves, which responded more strongly to water stress, indicating that grape berries display a distinct, organ-specific response to environmental stresses. Consistent with the metabolic changes, the global transcriptomic analysis revealed that heat stress had a more significant impact on gene expression in grape berries than water stress in both varieties. The differentially expressed genes were those associated with the tricarboxylic acid cycle and glyoxylate cycle, mitochondrial electron transport and alternative respiration, glycolysis and gluconeogenesis, carbohydrate allocation, ascorbate metabolism, and abiotic stress signaling pathways. Knowledge regarding how environmental stresses, alone and in combination, impact the berry metabolism of different grape varieties will form the basis for developing recommendations for climate change mitigation strategies and genetic improvement.
Similar content being viewed by others
Climate change impacts and adaptations of wine production
Cornelis van Leeuwen, Giovanni Sgubin, … Gregory A. Gambetta

Spatial co-transcriptomics reveals discrete stages of the arbuscular mycorrhizal symbiosis
Karen Serrano, Margaret Bezrutczyk, … Benjamin Cole

Legume rhizodeposition promotes nitrogen fixation by soil microbiota under crop diversification
Mengjie Qiao, Ruibo Sun, … Yan Chen
Introduction
Grapevine ( Vitis spp.) is a valuable and versatile fruit crop worldwide, primarily grown in temperate regions characterized by warm and dry summers. Grape berries are used in producing juice, wine, distilled liquor, table grapes, and raisins 1 . In addition to its commercial diversity and high economic value, the grapevine has emerged as a model perennial fruit crop for the study of drought and heat tolerance, with many of a diverse array of cultivated varieties adapted to grow in temperate regions with Mediterranean climates 2 , 3 .
The rising temperature and declining water availability associated with global climate change pose challenges for high-quality wine grape production, especially in already warm and dry regions. Known effects of high temperature on grape quality include undesirable decreases in key chemical constituents like organic acids and anthocyanins and increases in sugar, leading to higher alcohol contents in wines 4 , 5 . Organic acids affect the organoleptic properties in wines, such as the perception of acidity and freshness, while also contributing to microbial stability during wine storage. Higher temperatures cause a general reduction of malic acid in grapes at maturity, with negative consequences for wine quality. However, the accumulated malic acid in grapes is consumed by respiration and gluconeogenesis processes throughout fruit maturation, and these processes are temperature-sensitive 6 . By comparison, temperature effects on the other major organic acid in grapes, tartaric acid, are largely unknown. This example underlies the importance of intensifying the study of temperature-dependent pathways involved in berry primary metabolism and the genes associated with these pathways during berry ripening.
Hand-in-hand with increasing temperature is an increased prevalence of drought conditions. Moderate water deficit can benefit grape production, promoting the accumulation of desirable metabolites such as anthocyanins and other phenolics 7 , 8 , 9 , 10 . Although the use of irrigation is becoming more common, a majority of grape-growing regions remain unirrigated. In those regions, the plants and berries may be exposed to periods of drought as part of their regular growing regime 2 . Severe and prolonged heat and drought episodes can be detrimental to wine grape production, hindering growth, negatively impacting yield and fruit metabolism, and on a global scale, impacting the distribution of winegrowing areas 11 , 12 . Even irrigated grape production regions are experiencing the impacts of climate change, with mountain snowpacks becoming less reliable as temperatures continue to rise 13 .
Adapting wine grape production to changing climatic conditions will require varietal diversification and integration of traits that improve resilience to heat and drought stress 3 , 5 . Further, identifying physiological, metabolic, and genetic factors contributing to abiotic stress tolerance and grape berry composition is urgently needed. While the effects of water stress and heat stress on grapevine leaf physiology have been well characterized 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , there is still much to be learned about the impacts of these stress factors—particularly in conjunction with one another—on the physiology, metabolism, and underlying gene expression in grape berries 22 . Several diverse hypotheses have been advanced to explain the roles of abiotic stresses on grape development. One such hypothesis is that water stress directly alters fruit metabolism via the influence of root-derived or locally produced abscisic acid (ABA) or via auxin signaling 23 , 24 . Another hypothesis states that variety-specific differences in hydraulic behavior translate into differences in the metabolic response of grape berries to water stress 25 . Alternatively, water stress may indirectly affect grape composition by reducing canopy size and density, which increases sun exposure of the fruit clusters and hence berry temperature 16 . A hybrid model of both the direct and indirect effects of water stress on berry metabolism and associated gene expression has also been proposed 26 , 27 . Thus, it remains unclear whether the presumed water stress effect on berry composition is direct or whether it is mediated by berry temperature (and light). The interaction between water deficit and temperature is expected to impact biological pathways associated with the production of key metabolites for wine production, such as sugars, organic acids, anthocyanins, and other phenolics, as well as diverse aroma volatiles that contribute to the flavor and quality of the wine 2 , 22 .
By testing the effects of drought and heat stress alone or in combination, this study sought to fill the knowledge gap in understanding the individual and interactive effects of water deficit and temperature on grape berry metabolism and underlying gene expression. We used pot-grown plants in climate-controlled growth chambers to avoid the effect of solar heating of grape berries under water stress that is inevitable under field conditions. We assayed key metabolites and conducted a global gene expression analysis in ripening berries of two contrasting wine grape varieties, Cabernet Sauvignon and Riesling. Overall, this study revealed genes and associated pathways involved in grape berry abiotic stress response and how these pathways vary under heat and drought stress by variety. Knowledge regarding how grapevines respond to the interaction between heat and drought stress and how grape ripening is impacted will form the basis for developing enhanced recommendations for irrigation management and grapevine genetic improvement in the context of climate change.
Materials and methods
Plant material and experimental conditions.
The experiment was carried out under controlled environmental conditions at the Irrigated Agriculture Research and Extension Center in Prosser, Washington, USA, in 2018 using two-year-old, own-rooted Riesling and Cabernet Sauvignon grapevines ( Vitis vinifera L.). The plants were sourced from Clean Plant Center Northwest, Washington State University, Prosser, WA. Plants were grown in 20-L pots filled with a mix of 50% (v/v) sandy loam soil, 25% peat moss, and 25% pumice; in addition, 3 g L −1 of dolomite was added to the mix. A complete slow-release fertilizer was applied at the six-leaf stage, at anthesis (beginning of bloom), and after fruit set. Vines were pruned to four shoots and thinned to one fruit cluster per shoot. Plants were grown outdoors until the first berries started to soften (9 August), then they were moved to four 11.3-m 2 growth chambers (TPRB-111, BioChambers, Winnipeg, MB, Canada) for acclimatization. During the 6-day acclimatization period, vines were irrigated daily to 100% of field capacity. Each growth chamber was equipped with temperature and light control. Chamber lighting consisted of 50% metal-halide lamps (186–204, Venture, Solon, OH, USA) and 50% high-pressure sodium-vapor lamps (HPS ET18, Sylvania, Danvers, MA, USA), providing a maximum photosynthetic photon flux of 1100 µmol m −2 s −1 at canopy height.
After the acclimatization period, four treatments were applied for 7 days: heat stress (HS), water stress (WS), heat and water stress (HWS), and no stress or control (C). Cabernet Sauvignon and Riesling vines (6 single-vine replicates per treatment) from the C and WS treatments were randomly assigned to two growth chambers. Vines from the HS and HWS treatments were randomly assigned to the other two chambers to account for possible chamber effects. Light conditions and temperature were programmed to change hourly to simulate the photoperiod and average hourly temperature registered in the field for the veraison (beginning of ripening) period of the two varieties from 2006 to 2016. The temperatures in the control treatment group changed diurnally from 15 °C (3:00–5:00) to 29 °C (13:00–16:00), and the relative humidity changed from 75 to 40%. In the heat stress chambers, hourly temperatures were increased by 10 °C relative to the control. Relative humidity in the HS chambers ranged from 50 to 25%. Irrigation treatments were established by measuring soil water content using a 20-cm TDR probe (HydroSense II, Campbell Scientific, Logan, UT, USA) and weighing the pots daily. Well-watered vines were irrigated daily to field capacity, and water-stressed vines were irrigated to approximately 50% of field capacity. After the stress period, a 7-day recovery period followed, using the control temperature schedule and well-watered conditions.
All methods were performed on grape plants that were cultivated for the purposes of the experiments, including the collection of plant material for all analysis, and all relevant institutional, national, and international guidelines and legislation were complied with.
Berry sample collection
Independent berry samples were collected for metabolite and transcriptomic analysis. Ten berries per plant were randomly collected for chemical analysis on day 7 of the stress period. Additionally, five berries per plant were randomly collected for transcriptomic analysis before the stress period started (day 0, ‘Before Stress’), on day 7 of the stress period (‘During Stress’), and on day 7 of the recovery period (‘After Stress’). All samples were collected into zip-lock plastic bags, flash-frozen in liquid nitrogen, and stored at − 80 °C.
Metabolite analysis
Berries were thawed at room temperature and weighed using a precision balance (AX205 DR, Mettler-Toledo, Greifensee, Switzerland), and juice was extracted manually by pressing the berries inside the plastic bags. The juice total soluble solids (TSS) concentration was analyzed using a benchtop refractometer (MT RE40D, Mettler-Toledo). The juice was stored in 15-mL centrifuge tubes at − 20 °C for organic acid analysis. Grape juice was thawed at room temperature, heated to 71 °C for 20 min 28 , and cooled to room temperature. Titratable acidity (TA) was analyzed to an end-point of pH 8.1 using an auto-titrator (Titrino plus 848, Metrohm, Herisau, Switzerland) connected to a compact sample changer (869 CSC, Metrohm). The pH was measured using an MP225 Quattro pH meter (Mettler-Toledo). The juice was centrifuged at 13,250 g for 15 min and filtered through a 0.45-µm membrane (Nanosep® Centrifugal Filters). The juice was diluted to analyze organic acids using an Agilent 7100 capillary electrophoresis system (Agilent, Santa Clara, CA, USA) following the protocol of the Agilent Organic Acids Solution kit (PN 5063–6510). Berry metabolite data were analyzed by three-way analysis of variance (ANOVA) using SAS University Edition (SAS Institute, Cary, NC, USA) to test for effects of temperature, water, variety, and their interactions.
RNA extraction and sequencing
Grape berries (seeds removed) were pulverized under liquid nitrogen using a mortar and pestle. Total RNA was extracted from three biological replicates, each replicate comprised of homogenate of 6 berries each, using a modified DEPC-CTAB protocol 29 , with three technical replicates performed for each biological replicate at the ‘Before Stress’, ‘During Stress’, and ‘After Stress’ time points. RNA was quality-checked on an agarose gel and was quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Following quality validation and quantification using a Qubit Fluorometer (Thermo Fisher Scientific) and Agilent Bioanalyzer (Santa Clara, CA, USA), cDNA libraries were prepared from the RNA and sequenced by BGI Genomics (Hong Kong, China) on an Illumina HiSeq 4000 platform as 2 × 150 paired-end reads.

Transcriptome assembly and treatment read mapping
The 2 × 150 paired-end fastq files generated using Illumina HiSeq 4000 were input into the CLC Genomics Workbench (ver. 8.5.1) for pre-processing and assembly according to published methods 30 , 31 . Briefly, the CLC Create Sequencing QC report tool was used to assess the quality and determine the amount of sequence to trim. The CLC Trim Sequence process was then used to trim quality scores with a limit of 0.001, corresponding to a Phred value of 30. The 13, 5′ terminal nucleotides were removed, as were any ambiguous nucleotides. Reads below length 51 were discarded. Overlapping pairs were merged using the ‘Merge Overlapping Pairs’ tool, following which a de novo assembly was performed with all trimmed, merged datasets. The de novo assembly resulted in the production of 195,894 contiguous sequences (contigs). Contigs with less than 2 × coverage and those less than 200 bp in length were eliminated. For each dataset (treatment/replicate), the original, non-trimmed reads were mapped back to the master assembly contig subset. Mapping resulted in the generation of individual treatment sample reads per contig. The master transcriptome was exported as a fasta file for functional annotation, and the read counts for each dataset were exported for normalization with the Reads Per Kilobase per Million reads (RPKM) method 32 . Contigs with RPKM values of less than 0.5 for all treatments and time points were filtered out. The final working dataset consisted of 87,867 contigs.
Differential expression analysis
Differentially expressed genes for each treatment, in comparison with the control, were identified using the time course, multi-series differential expression feature in the OmicsBox suite, which employs the maSigPro R package. The FDR-corrected cutoff was p ≤ 0.05. The statistical analysis ensured that genes that did not meet the assumption of equal variances were eliminated from the analysis. The differentially expressed genes (DEGs) and expression values were matched with their corresponding functional annotations (Supplementary File 1 ).
Functional annotation
The master transcriptome fasta file produced from the Illumina assembly was imported into OmicsBox 1.4.11 (BioBam Bioinformatics S.L., Valencia, Spain) for functional annotation of expressed contigs. Contig sequences were identified by a blastx alignment against the NCBI ‘Viridiplantae’ database with an e-value specification of 10.0E-3f. GO annotation was assigned using the ‘Mapping’ and ‘Annotation’ features using default parameters to generate a functionally annotated master assembly 33 .
Gene ontology enrichment analysis
GO enrichment analysis using Fisher’s exact test was conducted in OmicsBox to identify the cellular components, molecular functions, and biological processes that were over- or under-represented in the various stress treatments in comparison with the control berries for both grape varieties (FDR-corrected p -value < 0.001). Lists of the differentially expressed, functionally annotated genes were generated for the Cabernet Sauvignon and Riesling during HS, WS, and HWS. These lists served as the treatment datasets for enrichment analyses, and the master annotated transcriptome was used as the reference dataset. Prior to conducting enrichment analysis, the Go-Slim feature was used to reduce the number of GO terms present in the annotated reference transcriptome to overarching functions and processes displaying the greatest enrichment.
Results and discussion
Leaf physiology.
In conjunction with this study, we recently reported the impact of drought stress and heat stress in the same experiment on shoot growth, leaf physiological traits, abscisic acid (ABA) and proline levels, and expression of key genes involved in ABA and proline biosynthesis in leaves 21 . Water availability dominated the growth and leaf physiological responses, while heat stress only played a minor role, even with daily maximum leaf temperatures close to 40 °C. Compared with the control, WS significantly reduced shoot elongation, leaf water potential (Ψ l ), stomatal conductance (g s ), photosynthesis, and transpiration, while strongly increasing leaf ABA. By contrast, with the exception of Ψ l, heat stress rarely altered any of these physiological traits and did not impact shoot growth, though it tended to exacerbate the effect of water stress on leaf physiology. Riesling leaves seemed to be somewhat more sensitive to heat stress, especially in combination with water stress than were Cabernet Sauvignon leaves. For example, based on the decrease in g s , Riesling vines experienced severe water stress (g s < 0.05 mol H 2 O m −2 s −1 ) under both WS and HWS, but Cabernet Sauvignon only reached extreme stress under HWS 21 . In both varieties, however, midday Ψ l decreased from an average of − 0.8 MPa in the control to − 1.2 MPa under HS to -1.3 MPa under both WS and HWS. While sufficiently low to induce stomatal closure, these values are still high enough for grapevines to avoid xylem cavitation and leaf wilting 2 . All vines were exposed to the stress treatments for 7 days; thus, the mature leaves on which our measurements were conducted had developed under non-stress conditions. These results confirm recent data obtained using Malbec grapevines and show that leaves are relatively resilient with respect to heat stress, at least in the temperature range (≤ 40 °C) tested here 17 . Earlier work had found the optimum temperature for photosynthesis of grape leaves to be in the range of 25‒30 °C 34 .
Berry metabolites
In sharp contrast to most leaf physiological traits and shoot growth 21 , the variation in berry metabolite composition was dominated by the effect of temperature, while water availability played, at most, a minor role. The different measures of fruit composition in the berries collected after 7 days of stress (Fig. 1 ) indicated that these berries were in the middle of the ripening period, during which sugars accumulate rapidly and malic acid is degraded 35 . Despite the up to 70% reduction in leaf photosynthesis under WS 21 , neither berry weight nor TSS (which is a robust proxy for sugar concentration in ripening grapes) were significantly impacted by any of the stress treatments (Fig. 1 A and B). Similar results were found in a heat-stress experiment with Cabernet Sauvignon grapes 36 and Muscat Hamburg grapes 37 and in a water-stress experiment with different grape varieties 38 . In another study, however, in which Sémillon grapes were exposed to 40/25 °C day/night temperatures for 4 days at the beginning of ripening, heat stress inhibited both berry growth and sugar accumulation 39 . Therefore, the effect of heat stress might depend on the variety or the way treatments are applied. For example, while our study applied realistic, diurnal irradiance and temperature profiles, many other growth chamber studies used static day/night conditions. In both of our varieties, heat stress increased the berry pH (Fig. 1 C). In Riesling, the WS berries had an intermediate pH (Fig. 1 C). Heat stress (HS and HWS) decreased TA by 26–32%, malic acid (MalA) by 40–52%, and oxalic acid (OxA) by 20–42% in both varieties (Fig. 1 D), while water stress by itself had no effect (Fig. 1 E). Among organic acids, MalA is metabolized during grape ripening, while OxA continues to accumulate 40 . Two other organic acids, tartaric acid (TartA) and citric acid (CitA) displayed variety-specific responses with regard to the impact of stress on their accumulation. TartA, like OxA a derivative of ascorbic acid (vitamin C) metabolism 22 , was not significantly impacted in Cabernet Sauvignon under any stress condition but was elevated in Riesling under heat stress (Fig. 1 G). CitA was not significantly impacted by any stress condition in either variety (Fig. 1 H).
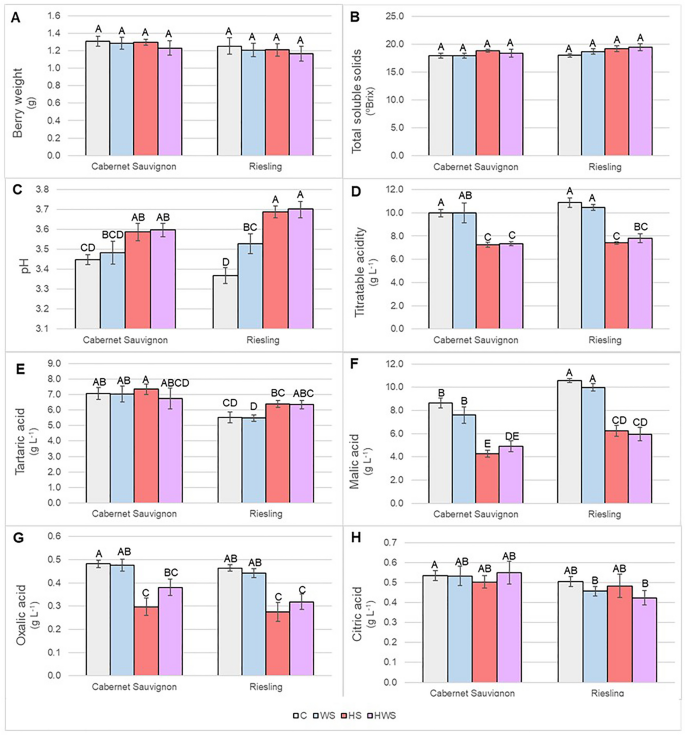
Effects of heat stress (HS), water stress (WS), and heat + water stress (HWS) at the beginning of fruit ripening on Cabernet Sauvignon and Riesling grape berry composition. Control (C) values are also shown for reference. Parameters measured included ( A ) berry weight, ( B ) total soluble solids, ( C ) pH, ( D ) titratable acidity, ( E ) tartaric acid, ( F ) malic acid, ( G ) oxalic acid, and ( H ) citric acid. Different letters indicate significant differences ( p < 0.05). Error bars represent standard error (n = 6).
In practice, the impact of elevated temperature during the growing season often requires acid addition to adjust wine acidity and reduce the pH to appropriate levels, an endeavor that can incur significant expenses. Unlike MalA, which is easily metabolized under heat stress 41 , 42 , TartA is much more stable at high temperatures 6 , 35 . In our study, heat stress during the early ripening phase slightly increased TartA in Riesling but not Cabernet Sauvignon berries. In contrast, Lecourieux et al. 36 , 43 reported an increase in Cabernet Sauvignon berries exposed to elevated temperature (~ 34 °C vs. 26 °C at veraison). It is conceivable that there may be varietal differences in the activation of antioxidative processes in an effort to maintain homeostasis under heat stress. Despite the limited response of TartA to temperature, the lower acidity of grape berries that accompanied exposure of grapevines to HS and HWS, but not WS, indicates that temperature has a particularly pronounced effect on fruit acidity (especially MalA and OxA), which is not brought about by water deficit alone. This is consistent with the hypothesis that the effects of water stress on grape composition, or at least on organic acid metabolism, may be indirect and mediated directly by temperature (and light), arising from a decrease in shoot growth under water stress and the associated increase in fruit exposure to sunlight, causing solar heating of the berries 16 . Though water stress decreased shoot growth in our study as well 21 , we excluded solar heating as a complicating variable by applying temperature treatments inside environmentally-controlled growth chambers. Unlike in many field experiments, moreover, leaf senescence (yellowing and abscission) was not observed with the treatment structure used in our study.
In summary, whereas water stress had a direct effect on leaf physiological processes (which may be mediated by hydraulic properties as well as hormones such as ABA), it did not affect berry organic acid metabolism in a similar way. The latter, instead, was much more strongly impacted by temperature. It is likely that the pronounced effect of temperature in grape berries compared with leaves is a consequence of the low transpiration rate and, thus, the poor evaporative cooling capacity of grape berries 44 . During ripening, moreover, water is supplied to the berries via the phloem rather than the xylem, so the berries are relatively well buffered against soil water deficit 38 , 45 .
Transcriptome assembly and annotation
Functional annotation of the 87,867 contigs from the final grape assembly dataset resulted in the assignation of blast results to 63,032 (71.73%) of contigs and functional annotation to a total of 55,182 contigs (62.80%) (Supplementary File 1 ).
Differentially expressed genes and enriched gene ontologies
A total of 5144; 4041; and 5283 annotated genes were significantly differentially expressed over time in HS, WS, and HWS treatments, respectively. Of these, 2001 HS, 656 WS, and 2056 HWS genes were differentially expressed in Cabernet Sauvignon, and 3,808 HS, 3498 WS, and 3912 HWS genes were differentially expressed in Riesling (Fig. 2 ). In addition, a set of 70 genes that displayed shared differential expression across all treatments and in both grape varieties was identified—these were classified as “core stress genes” (Fig. 2 , Supplementary File 2 ).

Numbers of genes that were significantly differentially expressed over time ( p < 0.05) compared to the control in berries of Cabernet Sauvignon and Riesling grapevines exposed to heat stress (HS), water stress (WS), or combined heat and water stress (HWS) at the beginning of fruit ripening. A further subset of 70 genes was identified among the shared HS, WS, and HWS DE genes—these were differentially expressed in all stress regimes in both grape varieties.
In Cabernet Sauvignon, gene expression response was consistent with the berry metabolite results, with the highest number of DEGs identified in the HWS and HS treatments, while WS elicited fewer expression changes in comparison with HS and HWS. Riesling displayed more DE genes in all treatments compared to Cabernet Sauvignon; this was particularly notable in the berries exposed to the WS treatment. Cabernet Sauvignon displayed fewer DEGs than Riesling, and this was particularly notable in the berries exposed to the WS treatment. The greater number of DEGs identified in Riesling (Fig. 2 ), and the elevated number of genes impacted by WS in this variety, suggests a heightened sensitivity of Riesling berries to both temperature and water stress compared to Cabernet Sauvignon, consistent with the physiological observations in leaves 21 .
Gene ontology enrichment analysis was conducted using lists of DE genes identified in HS, WS, and HWS treatments for each variety (6 lists total). Core stress ontologies enriched for both varieties in all stress treatments include GOs corresponding to transcription factor activity and gene expression regulation (GO:0003700, GO:0140110, GO:0003723, GO:0008135, GO:0090079); primary cellular metabolic processes, including the metabolism of carbohydrates, lipids, and organic substances (GO:0044238, GO:0005975, GO:0006629, GO:0006807, GO:0071704, GO:0006091); response to stresses and stimuli (GO:0006950, GO:0009607, GO:0042221); signal transduction (GO:0007165, GO:0023052, GO:0038023, GO:0060089, GO:0016301); enzymatic regulation of biological processes (GO:0065007, GO:0030234, GO:0003824, GO:0065009), transporter activity (GO:0006810, GO:0005215); and maintenance of cellular homeostasis (GO:0019725). Several shared ontologies enriched in both genotypes during the HS and HWS treatments, but not WS, included a response to abiotic stimulus (GO:0009628) and cell growth/differentiation (GO:0030154, GO:0040007). Additionally, several ontologies associated with the development of shoot and reproductive structures (GO:0048367, GO:0048608, GO:0061458) were enriched in both varieties during WS; however, during HS and HWS, these were only enriched in Riesling. Similarly, secondary metabolic processes (GO:0019748) were enriched for both varieties in the HWS treatment but only for Riesling in the HS and WS treatments. Compared to variety and stress-specific ontologies, the large number of shared enriched ontologies suggests that a common group of pathways is activated in response to heat and water stress in Cabernet Sauvignon and Riesling grape berries.
While the majority of the enriched ontologies identified were variety and stress agnostic, there were a few GOs identified that were enriched in a variety-specific manner. In Cabernet Sauvignon, chromatin binding (GO:0003682) and protein-containing complex binding (GO:004487) were enriched in the HS and HWS treatments only. In Riesling, unique GOs enriched in all stress treatments included circadian rhythm (GO:0007623) and response to radiation (GO:0009314); unique GOs enriched in HS and WS (but not HWS) included terms associated with epigenetic regulation of gene expression (GO:0006338, GO:0010468, GO:0040029); and unique GOs enriched in WS and HWS (but not HS) included carbohydrate binding (GO:0030246) (Table 1 ).
While the number and nature of the differentially expressed genes varied by treatment and stress type—with more variety- and stress-specific genes identified than shared DE genes (Fig. 2 )—interestingly, these diverse gene sets corresponded to highly similar enrichment results. This finding suggests that the berries of the two varieties seem to adopt different strategies at the gene expression level when exposed to individual or concomitant stress to activate different aspects of stress-responsive pathways and ultimately achieve similar biological outcomes. Overall, the enriched ontologies indicate a global stress response involving enhanced gene expression regulation, stress signal transduction, energy production, and primary metabolic activities.
Due to the correspondence with the TSS and organic acid assays performed, the pathways corresponding to gene ontologies ‘organic substance metabolic process’, ‘carbohydrate metabolic process,’ ‘primary metabolic process,’ ‘generation of precursor metabolites and energy’ were investigated in greater detail at the level of individual genes involved in the corresponding pathways. Additionally, to better understand the observation that variety and stress-specific gene expression patterns underly similar enriched gene ontologies, the pathways corresponding to transcription factor activity and stress signal transduction are also explored further, as the signaling-associated genes are expected to lend insight into variety and stress regime-specific initial response to heat and drought.
Organic acid metabolism
Organic acid content is a critical component of fruit and wine organoleptic quality. Most acids accumulate until grape berries undergo a metabolic shift at the onset of ripening. However, acid content, and therefore TA and pH, are highly influenced by genotype and environmental conditions 46 . High-temperature-driven reduction of organic acids, particularly malate, has been studied primarily in red grapevine varieties, including Shiraz 46 , Cabernet Sauvignon 43 , 47 , Muscat Hamburg 37 , and DCRF mutants (microvine) 42 . Recently, the effects of combined temperature and drought stress on two white varieties, Chardonnay and Xynisteri, were also studied 48 . Results of these studies and the present work suggest that WS and HS may impact grape berries of different varieties in different ways, with the metabolic and physiological responses much more pronounced under heat stress.
Several of the assayed organic acids are directly (MalA, CitA) or indirectly (OxA) associated with the TCA cycle and the glyoxylate cycle. The former pathway is responsible for the breakdown of pyruvate produced during glycolysis, the generation of donor molecules for mitochondrial electron transport and substrates for other metabolites, and the production of CO 2 , while the glyoxylate cycle is involved in the breakdown of fatty acids to produce substrates for gluconeogenesis. Significantly elevated expression of a DEG encoding the mitochondrial isoform of malate dehydrogenase (mMDH), which catalyzes the conversion of MalA to oxaloacetic acid (OAA), was observed in HS and HWS grapes, corresponding to reduced levels of MalA in the berries (Fig. 3 ). Glyoxysomal malate dehydrogenase (gMDH) also displayed high transcript abundance during stress. In contrast to MDH isoform expression, most other TCA and glyoxylate cycle enzyme-encoding genes displayed reduced expression during both HS and WS and especially under HWS (Fig. 3 ). The enzyme in the TCA cycle downstream of mMDH, citrate synthase (mCS), displayed reduced transcript abundance during WS and HWS in Cabernet Sauvignon, and during HS and HWS in Riesling, with significant changes in mCS expression only observed in Riesling. In the glyoxysome, expression of gCS displayed a similar pattern in Cabernet Sauvignon and Riesling during HWS; after stress, however, expression increased significantly in Riesling WS and HWS (Fig. 3 ). The expression changes in CS isoforms during stress corresponded to reduced CitA levels in Riesling, while no significant change in CitA accumulation was observed in Cabernet Sauvignon.
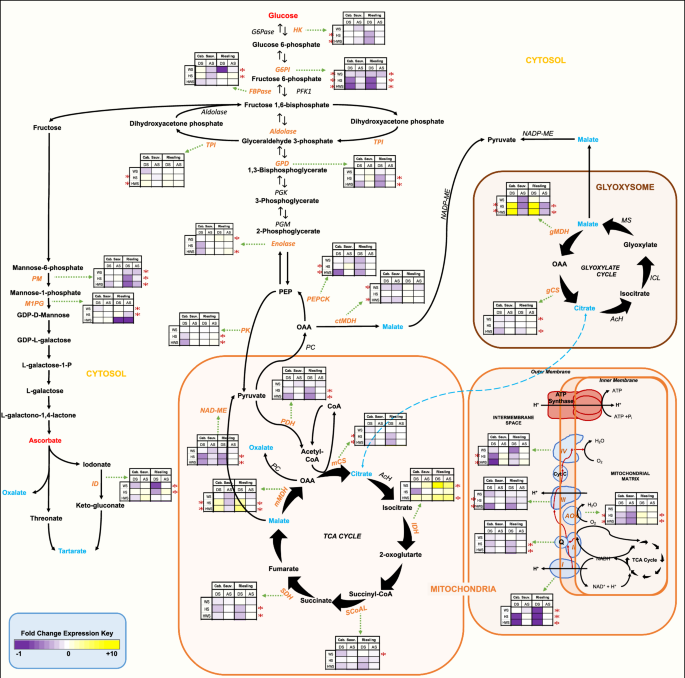
Organic acid metabolism (TCA cycle and glyoxylate cycle), mitochondrial electron transport, glycolysis/gluconeogenesis, and ascorbate metabolic pathways in berries of Cabernet Sauvignon and Riesling grapevines subjected to water stress (WS), heat stress (HS), or heat + water stress (HWS) at the beginning of fruit ripening. Bold blue text indicates organic acids assayed in this study: malic acid (MalA)/malate, citric acid (CitA)/citrate, oxalic acid (OxA)/oxalate, tartaric acid (TartA)/tartrate. Bold/italicized orange text indicates enzymes corresponding to significantly differentially expressed genes: organic acid metabolism/TCA cycle—pyruvate dehydrogenase (PDH), NAD malic enzyme (NAD-ME), mitochondrial malate dehydrogenase (mMDH), cytosolic malate dehydrogenase (ctMDH), mitochondrial citrate synthase (mCS), mitochondrial isocitrate dehydrogenase (mIDH), succinyl-CoA ligase (SCoAL), succinate dehydrogenase (SDH); mitochondrial electron transport—Complex I (NADH-uibquinone oxidoreductase), Complex II (succinate dehydrogenase), Complex III (Ubiquinol-cytochrome-c reductase), Complex IV (Cytochrome c oxidase), and alternative oxidase (AOX); glyoxylate cycle—glyoxysomal malate dehydrogenase (gMDH), glyoxysomal citrate synthase (gCS); glycolysis/gluconeogenesis—hexokinase (HK), glucose-6-phosphate isomerase (G6PI), fructose-1,6-bisphosphatase (FBPase), aldolase, triosephosphate isomerase (TPI), glyceraldehyde-3-phosphate dehydrogenase (GPD), enolase, pyruvate kinase (PK), phosphoenolpyruvate carboxykinase (PEPCK); ascorbate metabolism—phosphomannomutase (PM), mannose-1-phosphate guanylyltransferase (M1PG), and iodonate dehydrogenase (ID). Red arrows in mETC indicate the direction of electron flow. Dashed green arrows link DEGs to their corresponding expression heatmaps. Heatmap boxes show the expression of significant DEGs as fold change of each treatment during and after stress (DS and AS, respectively) normalized to the before stress control condition. Red asterisks indicate significant ( p < 0.05) differential expression over time (as assessed via MaSigPro) for Cabernet Sauvignon (left side of heatmaps) and Riesling (right side of heatmaps).
The third metabolite assayed, OxA, can be produced via a number of pathways, including decarboxylation of OAA and metabolism of ascorbate and/or glyoxylate (Fig. 3 ) 49 . Assuming a hypothetical, unidirectional production of TCA and glyoxylate cycle metabolites, the increased MDH and decreased CS transcript abundance would be expected to indicate a greater flux of OAA into the production of OxA. However, this is not what we observed in this study, as OxA levels decreased significantly in both Cabernet Sauvignon and Riesling under heat stress (Fig. 3 ), suggesting an alternative fate of OAA, such as a gluconeogenic precursor or biomineralization to calcium-oxalate 50 , 51 .
Venios et al. 20 described a comprehensive physiological response of grape berries to heat stress, characterized by reduced TA, reduced MalA, increased sugar-to-acid ratio, reduced flavanol, and anthocyanin production, and increased sugar content. The described compositional changes are consistent with those measured in the present study, particularly with regard to TA and MalA content. However, while previous studies have sought to elucidate the effects of heat on different stages of berry development and in different temperature regimes 46 , 52 , 53 , the present study explored the different effects that the interaction of heat and water stress have in both red and white grape varieties during the early ripening phase.
Carbohydrate metabolic processes—glycolysis and gluconeogenesis
It has been suggested that transcriptome remodeling in response to high temperature disrupts the synchrony of sugar and organic acid metabolism during grape berry development 42 . Consistent with this idea, our study found heat stress effects on organic acids but not sugar in ripening grape berries (Fig. 1 ). Thus, in conjunction with the assessment of organic acid metabolism at the transcriptome level, it was also of interest to observe expression patterns of genes involved in gluconeogenesis as well as glycolysis. Gluconeogenesis has a temperature optimum near 20 °C, decreasing to half the maximum rate at 30 °C 54 . Thus, the effects of elevated temperature are expected to elicit a particularly notable effect on gluconeogenic processes.
Hexokinase, the first committed enzyme in glycolysis, displayed a significant reduction in transcript abundance in Riesling during HS and HWS in comparison with the control; however, in Cabernet Sauvignon, no significant change in expression of this gene was observed under any of the stress conditions (Fig. 3 ). A similar trend was observed for the second step in the glycolytic pathway, phosphohexose isomerase, where significant decreases in gene expression were measured during WS, HS, and HWS in berries of both grape varieties, particularly at the peak of stress treatment.
Mitochondrial electron transport
In general, reduced expression of genes associated with mitochondrial ETC complexes I, II, III, and IV was observed during stress in both varieties. In most cases, HS and HWS had the greatest impact on the reduction of transcriptional activity. Transcription associated with alternative respiration (AOX homolog, ubiquinol oxidase 2) was elevated in Riesling but repressed in Cabernet Sauvignon—the AOX gene is activated in response to stress and plays a role in the reduction of ROS 55 . The heightened expression in Riesling may be indicative of an elevated ROS scavenging response in this variety. As Riesling berries, unlike those of Cabernet Sauvignon, do not accumulate anthocyanins, they might utilize an alternative strategy by which to mitigate oxidative stress, although both varieties displayed elevated expression of ROS scavenging genes in response to HS and HWS (Fig. 3 ).
Heat and water stress signal transduction pathways
In the general model for heat stress response in grape berries 20 , stress sensor proteins respond to elevated temperatures and transmit heat stress signals via ROS and secondary messengers that activate signal transducers, such as mitogen-activated protein kinases (MAPKs), that further relay the stress signal. This activates a transcriptional network comprised of stress-related proteins and chaperones (e.g., heat shock proteins [HSPs], heat stress transcription factors [HSFs]; ascorbate peroxidase [APX], and dehydroascorbate reductase [DHAR]) that ultimately confers tolerance to heat stress.
In both Cabernet Sauvignon and Riesling, several MAPK genes, numerous HSP genes, and several HSF , APX , and DHAR genes displayed significant responses to HS and HWS treatments (Fig. 4 , top). Unique to Riesling, many HSP s were significantly upregulated under WS as well, indicating these transcription factors may also play a role in the WS response, in addition to the HS response, of this variety. Several significant MAPK s were identified in both varieties, with more significant DEGs observed in Riesling, as well as a greater balance of upregulated to downregulated genes. Though Cabernet Sauvignon displayed fewer significant MAPK genes, nearly all were upregulated. HSP s, not surprisingly, represented a substantial portion of significant DEGs pertaining to the general heat stress pathway (Fig. 4 , top). Both Cabernet Sauvignon and Riesling had high numbers (i.e., 52 and 48) of upregulated DEGs in the HS and HWS treatment. As with MAPKs, Riesling displayed a high number of upregulated HSP s under WS, suggesting that HSPs play a role in the WS response in this variety (Fig. 4 , top). In addition to the MAPK and HSP primary signal transducers, differentially expressed HSF s were identified in both varieties, with stronger representation in Riesling. Multiple differentially expressed APX and DHAR genes were identified in both varieties, with a higher representation of upregulated DEGs in Cabernet Sauvignon HS and HWS treatments and a greater balance of upregulated and downregulated DEGs observed in Riesling. The greater balance of upregulated to downregulated genes in Riesling is suggestive of a more fine-tuned mechanism of stress response regulation, which may be necessary in the absence of pigmented antioxidants, like anthocyanins, that accumulate in red varieties. This is in contrast to Cabernet Sauvignon, which displayed fewer differentially expressed genes overall but for which a greater percentage of the DEGs were upregulated under HS and HWS treatments (Fig. 4 , top).
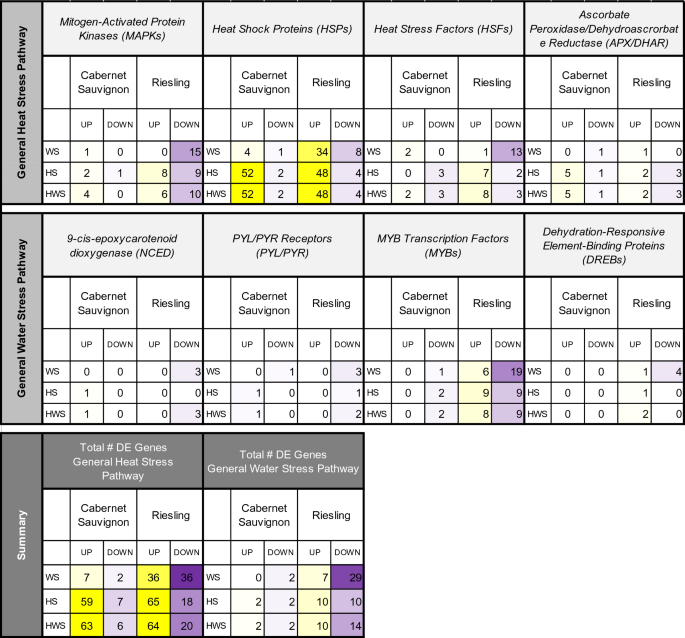
Number of general heat stress pathway and general water stress pathway genes that displayed a significant expression trend (as determined by MaSigPro) over time in comparison with the control in Cabernet Sauvignon and Riesling grape berries exposed to water stress (WS), heat stress (HS), and/or heat + water stress (HWS) treatments. Yellow indicates elevated expression, and purple indicates decreased expression, with the brightness of the color corresponding to the number of DE genes (darker = more DE genes). The total number of DE genes by treatment and grape variety is summarized at the bottom.
In the general model for response to water deficit 56 , drought response may be activated in both ABA-dependent and independent manners. In the case of the former, water deficit triggers the accumulation of ABA and ABA receptor activity (e.g., increased expression of NCED , PYR , and PYL ), which leads to activation of MYB/MYC transcription factors, which together coordinate the hormone-mediated drought response. In the case of the latter, drought conditions activate dehydration-responsive binding elements and cold-binding factors ( DREB s and CBF s), which coordinate the water stress response by MYC , MYB , and other transcription factors in a stress hormone-independent manner.
This study revealed that Cabernet Sauvignon berries displayed significant temporal upregulation of ABA receptor-encoding NCED and PYL/PYR genes in HS and HWS but not WS (Fig. 4 , middle), which contrasts with the response observed in leaves 21 . No significantly upregulated DEGs encoding ABA receptors were observed for Riesling, and conversely, all of the differentially expressed genes identified for this class were downregulated (primarily in the WS treatment). MYB/MYC genes were represented among the DEGs for both varieties; however, no significantly upregulated MYB/MYC DEGs were observed in Cabernet Sauvignon, and a larger number of these transcription factors, as well as a greater balance of upregulated and downregulated genes, was observed in Riesling (Fig. 4 , middle). Significant DREB genes were not represented in Cabernet Sauvignon at all, while Riesling displayed differential expression of DREB genes for all stress treatments (Fig. 4 , middle). Taken together, these findings suggest that, unlike their leaves, the berries of both grape varieties likely respond to water stress in an ABA-independent manner, although ABA-dependent mechanisms may be at play in the heat stress response of Cabernet Sauvignon. Moreover, the heightened number of significant DEGs for the WS treatment, as well as the greater balance of upregulated and downregulated genes, in Riesling indicates that the berries of this variety may be more sensitive to water stress than those of Cabernet Sauvignon, and therefore may require more fine-tuned regulation of its responses (Fig. 4 , bottom).
The results of this study highlight the effects of temperature and water availability and the combined effects of heat and water stress on grape berry metabolism and composition. Exposure to a 7-day episode of heat stress, and heat in combination with water stress, during early grape ripening, increased berry pH, decreased TA, and generally decreased organic acid content, but did not alter sugar content, in the berries of two distinct wine grape varieties. The finding that heat stress was more impactful than water stress at the metabolic level was paralleled at the transcriptome level in Cabernet Sauvignon but not Riesling. Moreover, we identified important differences in comparing the global and transcriptomic responses of Cabernet Sauvignon and Riesling berries to the different kinds of stresses, as well as the variation in responses of key metabolic and stress-responsive pathways including glycolysis and gluconeogenesis, the TCA and glyoxylate cycles, mETC and AOX, abscisic acid metabolism, and general heat and water stress response pathways. A more pronounced response to water stress was observed for Riesling berries in comparison with Cabernet Sauvignon berries at the transcriptome level, with a greater number of significantly differentially expressed genes and a greater balance of genes that were upregulated versus downregulated. Cabernet Sauvignon, on the other hand, displayed substantially fewer differentially expressed genes overall (~ 1/2 that of Riesling in HS, ~ 1/5 that of Riesling in WS, and ~ 1/2 that of Riesling in HWS [Fig. 2 ]), with a majority of the general heat and water stress pathway genes significantly upregulated in expression during stress. The similar berry metabolite-level responses of the two varieties, in conjunction with the notable differences in gene expression of key metabolic and stress-responsive pathways underlying similar enriched gene ontologies, suggests that these two varieties may have somewhat different genetic mechanisms for mitigating water stress and heat stress, alone or in combination, that ultimately result in a similar physiological outcome. The goal of this study was to investigate the short-term effects of heat stress, water stress, and the combined effects of both on berry metabolism and gene expression. Moving forward, the identification of physiological and genetic factors that contribute to both short and long-term abiotic stress tolerance of different grape varieties is of particular importance to devising viticultural and genetic strategies for the mitigation of abiotic stresses.
Data availability
The RNAseq datasets generated during the current study are available in the Short Read Archive on the NCBI database. The accession number is PRJNA928668, can be accessed here: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA928668 .
Food and Agriculture Organization of the United Nations. Table and dried grapes: Non-alcoholic products of the vitivinicultural sector intended for human consumption. Int. Organ. Vine Wine. FAO - OIV , ISBN: 978-92-5-109708-3 (2016).
Gambetta, G. A. et al. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 71 , 4658–4676 (2020).
Article CAS PubMed PubMed Central Google Scholar
Morales-Castilla, I. et al. Diversity buffers winegrowing regions from climate change losses. Proc. Natl. Acad. Sci. 117 , 2864–2869 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Gutiérrez-Gamboa, G., Zheng, W. & Martínez de Toda, F. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Food Res. Int. 139 , 109946 (2021).
Article PubMed Google Scholar
Suter, B., Destrac Irvine, A., Gowdy, M., Dai, Z. & van Leeuwen, C. Adapting wine grape ripening to global change requires a multi-trait approach. Front. Plant Sci. 12 , 36 (2021).
Article Google Scholar
Sweetman, C., Deluc, L. G., Cramer, G. R., Ford, C. M. & Soole, K. L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 70 , 1329–1344 (2009).
Article CAS PubMed Google Scholar
Santesteban, L. G., Miranda, C. & Royo, J. B. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. ‘Tempranillo’. Agric. Water Manag. 98 , 1171–1179 (2011).
Ju, Y. et al. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon ( Vitis Vinifera L.) grapes and wines. Plant Physiol. Biochem. 135 , 469–479 (2019).
Yang, B. et al. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 314 , 126170 (2020).
Casassa, L. F., Keller, M. & Harbertson, J. F. Regulated deficit irrigation alters anthocyanins, tannins and sensory properties of Cabernet Sauvignon grapes and wines. Molecules 20 , 7820–7844 (2015).
Cramer, G. R. Abiotic stress and plant responses from the whole vine to the genes. Aust. J. Grape Wine Res. 16 , 86–93 (2010).
Article CAS Google Scholar
Hannah, L. et al. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. 110 , 6907–6912 (2013).
Jones, G. V. et al. Climate change and its consequences for viticulture. In Woodhead Publishing Series in Food Science, Technology and Nutrition (ed. Reynolds, A. G.) (Woodhead Publishing, 2022).
Google Scholar
Chaves, M. M. et al. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 105 , 661–676 (2010).
Dal Santo, S. et al. Distinct transcriptome responses to water limitation in isohydric and anisohydric grapevine cultivars. BMC Genomics 17 , 1–19 (2016).
Keller, M. et al. Deficit irrigation alters grapevine growth, physiology, and fruit microclimate. Am. J. Enol. Vitic. 67 , 426–435 (2016).
Galat Giorgi, E., Sadras, V. O., Keller, M. & Perez Peña, J. Interactive effects of high temperature and water deficit on Malbec grapevines. Aust. J. grape wine Res. 25 , 345–356 (2019).
García, Y. G., Montoya, M. M. & Gutiérrez, P. A. Detection of RNA viruses in Cape gooseberry ( Physalis peruviana L.) by RNAseq using total RNA and dsRNA inputs. Arch. Phytopathol. Plant Prot. 53 , 395–413 (2020).
Liu, M., Ju, Y., Min, Z., Fang, Y. & Meng, J. Transcriptome analysis of grape leaves reveals insights into response to heat acclimation. Sci. Hortic. 272 , 109554 (2020).
Venios, X., Korkas, E., Nisiotou, A. & Banilas, G. Grapevine responses to heat stress and global warming. Plants 9 , 1754 (2020).
Article PubMed PubMed Central Google Scholar
Lehr, P. P., Hernández-Montes, E., Ludwig-Müller, J., Keller, M. & Zörb, C. Abscisic acid and proline are not equivalent markers for heat, drought and combined stress in grapevines. Aust. J. Grape Wine Res. 28 , 119–130 (2022).
Rienth, M. et al. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario–a review. Front. Plant Sci. 12 , 262 (2021).
Deluc, L. G. et al. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10 , 212 (2009).
Savoi, S. et al. Multi-omics and integrated network analyses reveal new insights into the systems relationships between metabolites, structural genes, and transcriptional regulators in developing grape berries ( Vitis vinifera L.) exposed to water deficit. Front. Plant Sci. 8 , 1124 (2017).
Hochberg, U., Degu, A., Cramer, G. R., Rachmilevitch, S. & Fait, A. Cultivar specific metabolic changes in grapevines berry skins in relation to deficit irrigation and hydraulic behavior. Plant Physiol. Biochem. 88 , 42–52 (2015).
Castellarin, S. D., Matthews, M. A., Di Gaspero, G. & Gambetta, G. A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227 , 101–112 (2007).
Palai, G., Caruso, G., Gucci, R. & D’Onofrio, C. Deficit irrigation differently affects aroma composition in berries of Vitis vinifera L. (cvs Sangiovese and Merlot) grafted on two rootstocks. Aust. J. Grape Wine Res. 28 (4), 590–606 (2022).
Threlfall, R., Main, G. & Morris, J. Effect of freezing grape berries and heating must samples on extraction of components and composition parameters of red wine grape varieties. Aust. J. Grape Wine Res. 12 , 161–169 (2006).
Gasic, K., Hernandez, A. & Korban, S. S. RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol. Biol. Report. 22 , 437–438 (2004).
Hewitt, S. L., Ghogare, R. & Dhingra, A. Glyoxylic acid overcomes 1-MCP-induced blockage of fruit ripening in Pyrus communis L. var. ‘D’Anjou’. Sci. Rep. 10 , 1–14 (2020).
Sharpe, R. M. et al. Concomitant phytonutrient and transcriptome analysis of mature fruit and leaf tissues of tomato ( Solanum lycopersicum L. Cv. Oregon Spring) grown using organic and conventional fertilizer. PLoS One 15 , e0227429 (2020).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5 , 621–628 (2008).
Götz, S. et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 36 , 3420–3435 (2008).
Greer, D. H. & Weedon, M. M. Modelling photosynthetic responses to temperature of grapevine ( Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant. Cell Environ. 35 , 1050–1064 (2012).
Hernández-Montes, E., Zhang, Y., Chang, B.-M., Shcherbatyuk, N. & Keller, M. Soft, sweet, and colorful: Stratified sampling reveals sequence of events at the onset of grape ripening. Am. J. Enol. Vitic. 72 , 137–151 (2021).
Lecourieux, F. et al. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing Cabernet Sauvignon grape berries. Front. Plant Sci. 8 , 53 (2017).
Carbonell-Bejerano, P. et al. Thermotolerance responses in ripening berries of Vitis vinifera L. cv Muscat Hamburg. Plant Cell Physiol. 54 , 1200–1216 (2013).
Keller, M., Zhang, Y. U. N., Shrestha, P. M., Biondi, M. & Bondada, B. R. Sugar demand of ripening grape berries leads to recycling of surplus phloem water via the xylem. Plant. Cell Environ. 38 , 1048–1059 (2015).
Greer, D. H. & Weston, C. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Funct. Plant Biol. 37 , 206–214 (2010).
Keller, M. & Shrestha, P. M. Solute accumulation differs in the vacuoles and apoplast of ripening grape berries. Planta 239 , 633–642 (2014).
Lakso, A. N. & Kliewer, W. M. The influence of temperature on malic acid metabolism in grape berries: I. Enzyme responses. Plant Physiol. 56 , 370–372 (1975).
Rienth, M. et al. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 16 , 164 (2016).
Lecourieux, D. et al. Proteomic and metabolomic profiling underlines the stage-and time-dependent effects of high temperature on grape berry metabolism. J. Integr. Plant Biol. 62 , 1132–1158 (2020).
Zhang, Y. & Keller, M. Grape berry transpiration is determined by vapor pressure deficit, cuticular conductance, and berry size. Am. J. Enol. Vitic. 66 , 454–462 (2015).
Zhang, Y. & Keller, M. Discharge of surplus phloem water may be required for normal grape ripening. J. Exp. Bot. 68 , 585–595 (2017).
CAS PubMed PubMed Central Google Scholar
Sweetman, C., Sadras, V. O., Hancock, R. D., Soole, K. L. & Ford, C. M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 65 , 5975–5988 (2014).
Ruffner, H. P., Hawker, J. S. & Hale, C. R. Temperature and enzymic control of malate metabolism in berries of Vitis vinifera. Phytochemistry 15 , 1877–1880 (1976).
Tzortzakis, N., Chrysargyris, A. & Aziz, A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 10 , 249 (2020).
Cai, X. et al. Expression analysis of oxalate metabolic pathway genes reveals oxalate regulation patterns in spinach. Molecules 23 , 1286 (2018).
Franceschi, V. R. & Nakata, P. A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 56 , 41–71 (2005).
DeBolt, S., Hardie, J. I. M., Tyerman, S. & Ford, C. M. Composition and synthesis of raphide crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: Ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Aust. J. Grape Wine Res. 10 , 134–142 (2004).
Pillet, J. et al. VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries. Plant Cell Physiol. 53 , 1776–1792 (2012).
Rienth, M. et al. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine ( Vitis vinifera ) fruit. BMC Plant Biol. 14 , 1–18 (2014).
Ruffner, H. P., Koblet, W. & Rast, D. Gluconeogenese in reifenden Beeren von Vitis vinifera. Vitis 13 , 19–328 (1975).
Hewitt, S. & Dhingra, A. Beyond ethylene: New insights regarding the role of AOX in the respiratory climacteric. Frontiers in Plant Science 11 , 543958 (2020).
Boudsocq, M. & Laurière, C. Osmotic signaling in plants. Multiple pathways mediated by emerging kinase families. Plant Physiol. 138 , 1185–1194 (2005).
Download references
Acknowledgements
We thank Noorani Barkat, Patrick Lehr, and Alan Kawakami for technical assistance and Dr. Tom Collins for the use of the capillary electrophoresis system. This work was funded by grants from the Specialty Crop Block Grant Program (grant number K1999) and the Washington State Grape and Wine Research Program to MK. The work was supported in part by the USDA National Institute of Food and Agriculture Hatch project 1000186 (MK lab) and Hatch project WNP00011 (AD lab), along with startup funds from AgriLife Research at Texas A&M University to AD.
Author information
Authors and affiliations.
Department of Horticulture, Washington State University, Pullman, WA, USA
Seanna Hewitt, Amit Dhingra & Markus Keller
Department of Viticulture and Enology, Irrigated Agriculture Research and Extension Center, Washington State University, Prosser, WA, USA
Esther Hernández-Montes & Markus Keller
Department of Agricultural Production, CEIGRAM, Universidad Politécnica de Madrid, Madrid, Spain
Esther Hernández-Montes
Department of Horticultural Sciences, Texas A&M University, College Station, TX, USA
Amit Dhingra
You can also search for this author in PubMed Google Scholar
Contributions
S.H conducted the transcriptome analysis and statistical analysis for all experiments. E.H.M conducted the stress treatments and metabolic analysis. S.H and E.H.M wrote the manuscript. M.K and A.D conceived the study, wrote the manuscript and contributed to data analysis and approach. All authors reviewed and approved the manuscript.
Corresponding authors
Correspondence to Amit Dhingra or Markus Keller .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hewitt, S., Hernández-Montes, E., Dhingra, A. et al. Impact of heat stress, water stress, and their combined effects on the metabolism and transcriptome of grape berries. Sci Rep 13 , 9907 (2023). https://doi.org/10.1038/s41598-023-36160-x
Download citation
Received : 20 January 2023
Accepted : 30 May 2023
Published : 19 June 2023
DOI : https://doi.org/10.1038/s41598-023-36160-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Insights into the cell-wall dynamics in grapevine berries during ripening and in response to biotic and abiotic stresses.
- Giulia Malacarne
- Jorge Lagreze
- Lorenza Dalla Costa
Plant Molecular Biology (2024)
The Germin-like protein gene OsGER4 is involved in heat stress response in rice root development
- Trang Thi Nguyen
- Dan The Pham
- Huong Thi Mai To
Functional & Integrative Genomics (2023)
Temperature variability during the growing season affects the quality attributes of table grapes in Pothwar—insight from a new emerging viticulture region in South Asia
- Rizwan Rafique
- Touqeer Ahmad
- Mukhtar Ahmed
International Journal of Biometeorology (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Genet
- PMC10308031
Insights into morphological and physio-biochemical adaptive responses in mungbean ( Vigna radiata L.) under heat stress
Ragini bhardwaj.
1 ICAR-National Bureau of Plant Genetic Resources, New Delhi, India
2 Department of Bioscience and Biotechnology, Banasthali Vidyapith University, Tonk Rajasthan, India
Jafar K. Lone
Renu pandey.
3 Division of Plant Physiology, ICAR-Indian Agricultural Research Institute, New Delhi, India
Nupur Mondal
4 Shivaji College, University of Delhi, New Delhi, India
R. Dhandapani
Surendra kumar meena.
5 Division of Crop Improvement, ICAR-Indian Grassland and Research Institute, Jhansi, India
Suphiya Khan
Anandan Annamalai , Indian Institute of Seed Science, India
Mungbean ( Vigna radiata L. Wilczek) is an important food legume crop which contributes significantly to nutritional and food security of South and Southeast Asia. The crop thrives in hot and humid weather conditions, with an optimal temperature range of 28°–35°C, and is mainly cultivated under rainfed environments. However, the rising global temperature has posed a serious threat to mungbean cultivation. Optimal temperature is a vital factor in cellular processes, and every crop species has evolved with its specific temperature tolerance ability. Moreover, variation within a crop species is inevitable, given the diverse environmental conditions under which it has evolved. For instance, various mungbean germplasm can grow and produce seeds in extreme ambient temperatures as low as 20°C or as high as 45°C. This range of variation in mungbean germplasm for heat tolerance plays a crucial role in developing heat tolerant and high yielding mungbean cultivars. However, heat tolerance is a complex mechanism which is extensively discussed in this manuscript; and at the same time individual genotypes have evolved with various ways of heat stress tolerance. Therefore, to enhance understanding towards such variability in mungbean germplasm, we studied morphological, anatomical, physiological, and biochemical traits which are responsive to heat stress in plants with more relevance to mungbean. Understanding heat stress tolerance attributing traits will help in identification of corresponding regulatory networks and associated genes, which will further help in devising suitable strategies to enhance heat tolerance in mungbean. The major pathways responsible for heat stress tolerance in plants are also discussed.
1 Introduction
Mungbean ( Vigna radiata L. Wilczek), also commonly called as greengram, is a leguminous crop. It is an annual grain legume crop, cultivated in different soil types of the South-East Asia and South East Africa, Australia, and South America ( Parihar et al., 2017 ). The crop requires warm-humid climatic conditions, with temperature ranging between 25°C and 35°C and a well distributed rainfall of 400–550 mm during growing season. Mungbean has a high range of storage protein (22%–27%) with sugar, minerals, and soluble dietary fibers ( Alom et al., 2014 ). Recently, the crop area and production has increased demand of plant-based protein with affordable market price, as a result, mungbean is now being commercially cultivated in large scale ( Keatinge et al., 2011 ). Global production of mungbean is around 6.0 million tones which comes from a cultivated area of about 7.3 million hectares ( Gayacharan et al., 2023 ). India alone produces mungbean up to 41% of the global production which makes it the largest producer of mungbean followed by Myanmar, Bangladesh and Pakistan ( Schreinemachers et al., 2019 ). Loam to sandy loam soils with good drainage are the best suited for mungbean cultivation. Because of its short life span, nitrogen-fixing ability, low water requirement, great biomass, and high yield mungbean is considered as one of the most important crops in agriculture ( Alom et al., 2014 ). However, the high variability in climatic conditions including rising temperature and unpredictable water deficit environments during its cropping season cause drastic reduction in mungbean productivity ( Singh et al., 2016 ). Several abiotic stresses such as heat, salinity, water-logging, and drought highly affects the growth and development in Mungbean ( Dreesen et al., 2012 ; Bita and Gerats, 2013 ; Kaur and Nayyar, 2015 ; Landi et al., 2017 ; Zandalinas et al., 2017 ).
Among various factors, global temperature rise is the major challenge in legume crop production. The erratic and low rainfall, soil desertification, evolving new races of pest and pathogens are some other problems associated with the global temperature rise, which are adversely impacting crop production across the globe. Amid climate change, cultivation of legume crops has become more challenging, as these are obligatory adapted to low input environments and are majorly cultivated in rainfed conditions. Legumes are highly impacted by insect, pest primarily attributed to their protein rich nature and narrow genetic base. The regional report of Middle East and North Africa (MENA) region, climate change adversely impacts pulses more than any other crop group ( Njuki et al., 2022 ). The regional report on MENA region indicated yield reduction due to climate change in pulses by 17.2%, followed by oil seeds (6.86%), cereals (4.18%) and fruits and vegetables (1.78%) ( Njuki et al., 2022 ). Similar impact of climate change is observed across the globe with varying severity. However, among all food sources, pulses are the only food for which increased consumption demand is predicted by 2050 ( Njuki et al., 2022 ).
Legumes including mungbean, which are grown in warm-humid climatic conditions are more affected by high temperature. In India, mungbean cultivation during summer season faces severe heat waves, and sometime temperature rises to 45°C, at which most of the cellular processes stop functioning. Various studies have demonstrated that under heat stress, significant yield losses occur in mungbean at reproductive stage of plant ( Hamada, 2001 ; Hall, 2010 ; Kumar et al., 2013 ; Kaur and Nayyar, 2015 ; Sharma et al., 2016 ; Priya et al., 2020 ). Studies also described that mungbean under heat stress at reproductive stage is more adversely impacted as compared to vegetative stage under heat stress ( Hall, 2010 ; Priya et al., 2020 ). Moreover, the male reproductive parts are more at risk to heat stress in comparison to the female reproductive parts in mungbean ( Dickson and Boettger, 1984 ; Monterroso and Wien, 1990 ). Further, it is also found that physiological processes in reproductive tissues of mungbean are more susceptible to heat stress ( Asseng et al., 2011 ; Kaur et al., 2015 ).
Heat stress triggers numerous physiological and biochemical processes in mungbean to counter the heat stress impact, but the crop yield is drastically reduced when severity of the heat stress is extreme ( HanumanthaRao et al., 2016 ; Sita et al., 2017 ). However, very less research has been done to understand the impact of heat stress on mungbean for yield attributing traits and the reproductive parts. Therefore, to increase the productivity of mungbean under heat stress environment, it is important to find out the physio-biochemical and molecular variations for high temperature stress tolerance in the mungbean germplasm, and probe the mechanisms leading to heat sensitivity in mungbean crop. In this review, we provide recent understanding of heat stress effects and tolerance mechanisms in mungbean, focusing on its morphological, and physio-biochemical responses under high temperature stress.
2 Impacts of heat stress in mungbean
Mungbean is a summer season crop that may be cultivated in all dry and semi-arid parts of the world. However, recent global average temperature rise has posed a threat for the mungbean crop production ( Abd El Lateff et al., 2018 ). The constant higher atmospheric temperature for longer duration is highly detrimental for the growth and physiological functions of various food crops ( Cao et al., 2011 ). Severity of the crop damage varies with the timing, duration and magnitude of the elevated temperature, as well as the genotype specific defense response. In mungbean, during the summer season where temperature rise above 40°C causes terminal heat stress during reproductive stage of the plants which is a major concern in mungbean productivity because it results in impaired anthesis, loss of pollen viability, reduced flower fertilization, increased flower drop and shortened period for grain filling ( HanumanthaRao et al., 2016 ; Basu et al., 2019 ) ( Figure 1 ; Figure 2 ). Even an increase in temperature by a few degrees changes crop cycle and accelerates flower drop and embryo abortion, and poor grain filling ( Kaur and Nayyar, 2015 ; Kaushal et al., 2016 ). Also, in kharif season mungbean temperature >40°C occur during early growth stages of the crop, causing similar problem particularly in northern parts of India ( HanumanthaRao et al., 2016 ).

General overview of impact of heat stress on physiological and metabolic pathways including morphological changes in plants under heat stress condition.

Impact of heat stress on morpho-anatomical features, reproductive biology and physio-biochemical properties of plants under heat stress. The corresponding adaptive responses of the plant under stress are also highlighted.
Reproductive organs are highly sensitive to high temperature stress. In mungbean, high temperature cause flower shedding as high as 79% ( Kumari and Varma, 1983 ). However, the genotypic variability in mungbean germplasm is observed attributing to specific or combination of heat stress tolerance mechanisms ( Kaur et al., 2015 ; Baroowa et al., 2016 ; Sharma et al., 2016 ). The effect of heat stress in mungbean is not thoroughly investigated yet, and it needs more in-depth research ( Kaur et al., 2015 ).
2.1 Morphological and anatomical changes in mungbean in response to heat stress
Heat stress can cause a range of modifications at morphological and anatomical levels in plants such as scorching of leaves and stems, loss of leaves, inhibition in shoots and roots growth, shrinking of seeds, and damage to fruits. As a result of these alterations, it consequently lead to reduced crop productivity ( Vollenweider and Günthardt-Goerg, 2005 ). In mungbean, heat stress causes many structural changes at morpho-anatomical level ( Figures 3A–H ). The different phases of reproductive stage such as pollen germination, loss of pollen viability, less pollen load on stigma, poor anther dehiscence, pollen sterility, and poor ovule viability lowers the crop yield ( Kaushal et al., 2013 ). In a study by Kaur et al. (2015) on two mungbean genotypes (SML 832 and SML 668), similar results were observed in response to heat stress treatment. They also observed decrease in plant biomass (16%–19%), total number of pod set, seed yield (35%–40%), number of filled pods (32%–38%), and seed number (43%–47%), due to high temperature exposure.

Typical morphological symptoms in mungbean plants in response to heat stress. Each genotype has a specific response to heat stress, e.g., flower drop, poor grain filling, pod discoloration, and leaf margin burning (A) , upward curvature of leaves to protect plant from heat from sunlight (B) , poor pod filling and reduced pod length (C) , early onset of maturity (D) , leaf abscission due to early onset of senescence to mobilize nutrients towards reproductive parts (E) , leaf margin burning (F) , almost entire flower drops due to high temperature during reproductive phase (G) , heat tolerant genotype with better pollen viability (H) than the heat susceptible one (I) , and seed shriveling due to heat stress during grain filling stage (J) .
Failure of ovule fertilization is often associated with plant productivity factors such as loss of pollen viability, loss of stigma receptivity and reduced pollen tube growth ( Hurkman et al., 2009 ; Patriyawaty et al., 2018 ). Pollen becomes often non-viable before fertilizing the flower ( Figures 3I,J ). It is also reported that the higher ambient temperature reduces the stigma receptivity to pollen causing embryo abortion and poor seed set ( Kaushal et al., 2013 ). Reductions in seed set per pod are reported to be correlated with the poor pollen tube growth and tropism defects ( Zinn et al., 2010 ). Moreover, heat stress can directly damage cell membranes, leading to changes in their permeability. The heat stress can alter the microtubules organization which not only affect the elongation, differentiation, and expansion of cells but also negatively impacts the cytoskeleton structure ( Rasheed, 2009 ; Bita and Gerats, 2013 ). In addition, heat stress also causes decrease in pod length, seed quality, seed size, and number of seeds per pod which ultimately reduces grain yield and quality in mungbean. Similarly, Sharma et al. (2016) reported that heat stress-induced oxidative stress caused chlorosis, leaf rolling, and leaf blistering in mungbean plants.
Several studies have confirmed the susceptibility of mungbean to rising temperatures ( Jha et al., 2017 ). High-temperature stress can have drastic impacts on plant growth and development, as well as on different physiological activities ( HanumanthaRao et al., 2016 ). For instance, mungbean may lose vigor due to extended exposure to high temperatures results in limiting seedling growth and development ( Kumar et al., 2011 ; Devasirvatham et al., 2012 ). Heat stress can also result in negative impacts on vegetative growth, including leaf senescence, chlorosis, necrosis, burning, and abscission, reduced internode elongation, and suppression of root and shoot development ( Kaushal et al., 2016 ; Sharma et al., 2016 ). Other effects of heat stress on mungbean include leaf curling, plant wilting, yellowing and blackening of leaves, reduction in plant height, reduced number of branches, and biomass ( Kaur et al., 2015 ). Figure 2 and Table 1 illustrate the impact of heat stress on mungbean’s morphological and anatomical traits.
Sources of heat stress tolerance identified in mungbean for various agro-morphological, physiological and biochemical traits.
2.2 Physiological changes in mungbean under heat stress
Heat stress can have several negative impacts on plant physiology, such as chlorophyll reduction, decreased photosynthesis, decreased transpiration, increased canopy temperature, and increased stomatal aperture ( Schoffl et al., 1998 ; Kaushal et al., 2013 ). These effects can ultimately lead to reduced plant productivity. Additionally, heat stress can cause membrane damage, protein degradation, and altered metabolism in plant cells ( Schoffl et al., 1998 ; Kaushal et al., 2013 ). Heat stress can also increase electrolyte leakage in plants due to altered membrane permeability by direct injuries, which affects the differentiation, elongation, and expansion of cells ( Rasheed, 2009 ; Bita and Gerats, 2013 ). Further, structural alterations in chloroplast protein complexes and reduction in enzyme activity occur due to the initial impacts of thermal stress ( Ahmad et al., 2010 ). Although a modest rise in ambient temperature generally promotes plant growth and development, it shortens the plant’s life span and results in a significant reduction in light uptake during the plant’s growth phase ( Kalaji et al., 2016 ). Additionally, plant water status is a critical component of plant survival under heat stress. Plants try to normalize their canopy temperature through increased transpiration rate, and therefore, heat stress in combination with soil moisture stress proves to be most detrimental to the plant ( Simões-Araújo et al., 2003 ). Unfortunately, most of the legume crops, including mungbean, are cultivated under rainfed conditions in tropical and sub-tropical regions. As a result, increasing global warming and adverse climatic conditions disrupt the monsoon seasons, resulting in uneven/less rainfall, which affects plant growth and development ( Simões-Araújo et al., 2003 ). Heat stress can rapidly reduce tissue water content despite ample availability of soil moisture, similar to drought stress conditions, leading to a disruption in nutrient uptake from roots ( Wahid et al., 2007 ). The drastic water loss due to high transpiration, particularly during the daytime, affects important physiological processes, ultimately resulting in reduced plant growth and development ( Fahad et al., 2017 ). Heat stress can also reduce seed viability and lower plant and yield quality. Moreover, plants exhibit programmed cell death in some specific cells and tissues under heat stress ( Anderson and Padhye, 2004 ).
In mungbean, the ideal temperature for growth and development is 28°C–30°C, and further every degree increase in temperature may reduce crop production by 35%–40% ( Tzudir et al., 2014 ; HanumanthaRao et al., 2016 ; Sharma et al., 2016 ). Mungbean plant can thrive well up to 40°C of temperature, after which flower shedding begins ( Zinn et al., 2010 ; Sita et al., 2017 ). Heat stress reduces leaf area and stomata openings which cause dramatic reduction in the carbon dioxide assimilation rate and photosynthesis during the vegetative stage of the mungbean ( HanumanthaRao et al., 2016 ). The increase in carbon dioxide (CO 2 ) content also causes stomatal closure, which hinders photosynthesis in the mungbean. High CO 2 concentration along with high ambient temperature, proves more detrimental for mungbean growth and development ( Reardon and Qaderi, 2017 ). In a study, CO 2 assimilation in mungbean was significantly reduced at 40°C, which had a direct impact on photosynthetic efficiency ( Karim et al., 2003 ). High temperatures also reported to reduce the chlorophyll and carotenoid levels, as well as the chlorophyll stability index ( Chand et al., 2018 ). A slightly higher temperature (36°C) temperature treatment on mungbean genotypes indicated that the exposure of plants to high temperature has greater adverse impact on leaf conductance at the pre-flowering stage than the blooming and grain filling stages, although high-temperature treatments had no effect on transpiration rate at any stage, but photosynthetic activity decreased at all three stages ( Islam, 2015 ). Similarly, another study on three prominent mungbean varieties viz., MH 421, MH 318, and Basanti indicated reduction in chlorophyll and carotenoid content, as well as a decrease in chlorophyll stability index in response to high temperature ( Chand et al., 2018 ). Under heat stress, sensitive genotypes (MH 318 and Basanti) showed higher losses in the physiological attributes stated above, whereas tolerant genotypes (MH 421) maintained high yield and physiological functioning ( Chand et al., 2018 ). Evidenced from numerous studies it is found that Photosynthesis is highly sensitive to high temperatures ( Sinsawat et al., 2004 ). Heat stress affects photosynthetic functions in mungbean by disrupting photosynthetic machinery, causing structural aberrations (particularly the thylakoid membrane) and alterations of chloroplast enzymes. Based on mungbean germplasm screening for heat responsive traits various important promising mungbean donors are identified ( Table 1 ). Also, impact of heat stress on physiological processes in various crops is listed in Table 1 and Figure 1 , Figure 2 .
2.3 Biochemical changes in mungbean under heat stress
High temperatures have a significant impact on the metabolism and biochemistry of mungbean plants. Under heat stress, the formation of reactive oxygen species (ROS), such as hydroxyl radical, singlet oxygen, superoxide radical and hydrogen peroxide, increases, causing protein degradation, membrane damage, and enzyme inactivation, and hence increases oxidative stress ( Liu and Huang, 2000 ). Long-term exposure to relatively high temperature stress can result in severe cellular injury or death, while at extremely high temperatures, this can occur in just minutes ( Wahid et al., 2007 ). These injuries, coupled with a lack of water content, can cause a reduction in ion flow and plant growth, as well as an increase in the generation of toxic compounds and reactive oxygen species ( Howarth, 2005 ). Some of the metabolic effects of heat stress on mungbean plants are briefly described in Figure 1 and Table 2 .
Candidate genes identified under heat stress tolerance in plants for various agro-morphological, physiological and biochemical traits
In mungbean, high temperature especially >40°/30°C (max/min) reduces leaf water potential and increases oxidative stress resulting in growth suppression and chlorosis which is linked to reduced crop yield ( Kumar and Wigge, 2010 ). High temperature stress can cause a considerable increase in hydrogen peroxide (H 2 O 2 ) concentration in mungbean as well as in other plants, which could be due to a reduction in catalase activity by blocking catalase production which lowers the enzyme’s steady-state level because of high turnover rate ( Scandalios et al., 1997 ). When plants are exposed to high levels of heat, they experience an oxidative burst (N, 1997) that can cause an increase in H 2 O 2 ( Levine et al., 1994 ; Baker and Orlandi, 1995 ). Antioxidant enzymes such as SOD and CAT become less active during heat shock, compromising the plant’s defenses and leading to increased levels of oxidant species ( Willekens et al., 1995 ; Foyer et al., 1997 ; Polle, 1997 ). This can have a negative impact on cellular metabolism, resulting in high levels of harmful compounds like malondialdehyde and H 2 O 2 that affect plant productivity ( Bita and Gerats, 2013 ). Lipid peroxidation under aerobic conditions, is a natural metabolic process that can be affected by ROS, causing damage to the cell membrane and impairing its function ( Blokhina et al., 2003 ). ( Heath and Packer, 1968 ). The presence of malondialdehyde (MDA) is an indication of oxidative damage, and it is created by the lipid peroxidation of the cell membrane ( Mandhania et al., 2006 ). It has been reported that high temperature stress on four mungbean genotypes (NCM 89, NM 20-21, NM 121-123, and NM 19-19) at seedling stage observed an increase in the levels of lipid peroxidation as there is a considerable increase in MDA concentration ( Mansoor and Naqvi, 2013 ). Studies in seedlings of four mungbean genotypes have shown that an increase in temperature can cause an increase in lipid peroxidation, leading to decreased net photosynthesis, water use efficiency, stomatal conductance, total chlorophyll, and nutrient partitioning in sensitive genotypes. Additionally, heat stress can negatively impact assimilate partitioning and apoplastic to symplastic phloem transport, resulting in a decrease in carbohydrate buildup and viability of pollen grains in mungbean ( Kaur et al., 2015 ; Taiz et al., 2015 ; Hanif and Wahid, 2018 ).
Seed shriveling in legumes is primarily linked to reduced synthesis of carbohydrates and storage proteins due to elevated temperature. The four enzymes viz., Adenosine Diphosphate Glucose Pyrophosphorylase, Starch Branching Enzyme, Starch Synthase, and Sucrose Synthase are crucial for the grain filling process ( Taiz et al., 2015 ). In general Sucrose synthase plays in important role in grain filling ( Basu et al., 2019 ), and temperature beyond a certain period affects the enzyme activities and lead to poor grain filling. Similarly, the functioning of Nitrate reductase is also diminished during heat stress, which is a most important enzyme of nitrogen (N) metabolism in plants. It catalyzes the reduction of inorganic N in the form of nitrate to organic form in translational process of proteins. Heat stress is known to adversely affect the nitrate reductase enzyme activity, which is more detrimental for leguminous crops affecting protein biosynthesis during grain filling and reproductive stages ( Klimenko et al., 2006 ; Farooq et al., 2017 ). Heat stress also impairs the nitrogen-fixing activity of mungbean by restricting the production of root hair and infection thread ( Bansal et al., 2014 ). Furthermore, heat stress reduces seed viability, plant and seed quality ( Anderson and Padhye, 2004 ). The nutritional value of seed is impaired mainly due to adverse impact of heat stress on synthesis and accumulation of protein and carbohydrates ( Taiz and Zeiger, 2010 ).
3 Adaptation strategies in mungbean to develop heat tolerant genotypes
Mungbean plant deploy a number of adaptation mechanisms such as changes in plant morphological and anatomical features, secondary metabolite production and their accumulation in target tissues/cells, production of anti-oxidants, stress hormones and proteins, and alteration in myriad of physiological activities ( Figure 2 ). The adaptation mechanisms that result in thermal tolerance in plants include secondary metabolites, heat-shock proteins (HSPs), ROS scavenging systems, and accumulation of suitable solutes ( Nakamoto and Hiyama, 1999 ; Wahid et al., 2007 ; Mittler et al., 2012 ; Hanif and Wahid, 2018 ). During heat stress, ROS produced by aerobic metabolism have a deleterious impact on cellular catabolism, causing lipid membrane peroxidation as well as damage to proteins and nucleic acids ( Bita and Gerats, 2013 ). To tolerate high temperatures, plants need to have sufficient levels of antioxidants ( Awasthi et al., 2015 ). When subjected to heat stress, plants generate and obtain new stress proteins, such as HSPs. These HSPs are molecular chaperones that play a crucial role in protein folding, translocation, proper aggregation, and degradation under both normal and stressful conditions ( Vierling, 1991 ). HSP100, HSP90, HSP70, HSP60, and the small heat-stress protein (sHSP) family ( Wang et al., 2004 ) are five heat-stress protein/chaperone families that play an important role in mitigating heat-stress, including protecting native proteins against denaturation. Isoprenoids, flavonoids, and carotenoids are secondary metabolites that inhibit peroxidase activity and promote high-temperature stress tolerance ( Havaux, 1998 ; Loreto et al., 1998 ; Rivero et al., 2004 ). When plants are exposed to heat stress, they undergo various interconnected morpho-physiological, biochemical, and molecular changes unique to their tolerance and adaptive nature to their surrounding environment, ultimately enabling them to restore redox homoeostasis ( Waseem et al., 2011 ) ( Figure 4 ). This indicates the necessity in developing heat stress tolerance in mungbean cultivars. Mungbean plants have adapted various types of defense mechanism to survive the heat stress, which are discussed in the following sections. Sources of tolerance identified by various workers are highlighted in Table 1 .

Different signaling and defense pathways in response to heat stress in plants. High temperature stress effects the plasma membrane to activate the calcium channels, which induces Ca 2+ influx, thus the MAPK cascade regulates and activates the various transcriptional factors which ultimately leads the accumulation of the stress responsive genes, the antioxidants and ROS.
3.1 Morphological and anatomical adaptations involved in mungbean response to heat stress
Increase in atmospheric temperature beyond a certain optimum point drastically reduces the plant growth and development as cellular processes require a specific temperature range. Plants have evolved with a great variability in their morphological adaptation as a part of survival mechanisms, such as early maturation, leaf rolling, and changing leaf orientation to cope up with the certain level of temperature deviations ( Srivastava et al., 2012 ). However, these adaptation mechanisms are often associated with yield losses under high temperature in many crop plants ( Adams et al., 2001 ; Rodriguez et al., 2008 ). Further rise in atmospheric temperature causes development of symptoms such as scorching of leaves and twigs, shoot and root growth inhibition, early onset of leaf senescence and abscission, and pod discoloration as an adaptation for plant survival. In response to heat stress, plant height is reduced in mungbean, which is specifically attributed to the disruption in cell elongation. This demonstrates that the mungbean plants exhibit the adaptation mechanism to respond heat stimuli. Certain important differences can be detected in the parameters like 100 seed weight, seeds/plant, pods/plant, seed yield, total biomass/plant, branches/plant, and plant height, etc. In mungbean as adaptation traits ( Sharma et al., 2016 ) ( Table 1 ). The reproductive phase of plant growth and development is highly sensitive to heat stress. To overcome certain level of higher temperature, plant deploy two very crucial mechanisms of plant defense viz. Dehydration of pollen grains and embryos, resulting in stress tolerance in the pollen grains, and prolonged dormancy in embryos under stressful conditions ( Zinn et al., 2010 ). As a result, pollen and embryos remain viable under higher temperature, however, the magnitude of tolerance varies among genotypes. Therefore, these traits are considered as important for development of heat-tolerant mungbean varieties ( Rainey and Griffiths, 2005 ).
In a study effects of heat stress on 41 mungbean genotypes for their vegetative and reproductive activities under controlled growing conditions was examined and a few tolerant lines for stress were identified ( Sharma et al., 2016 ). A field study in New Delhi in the Kharif seasons of 2014 and 2015 was conducted to assess the impact of high temperatures on seven mungbean genotypes in a rain-fed environment, and various heat stress responsive morphological traits were identified ( Chikukura et al., 2017 ). Night temperature rise is considered as more crucial for flower drop, as it is observed that under the higher temperature stress, the flower abscission is higher during the night as compared to the daytime ( Solai et al., 2015 ). Under high temperature stress, plants maintain their metabolites level required for flower and pollen and anther development. According to Banon et al. (2004) , the reduction of cell size and the closure of stomata can help to decrease excessive water loss and lead to increased stomatal density and enlarged xylem vessels in plants under heat stress. However, Shen et al. (2017) have noted that the anatomical changes in response to heat stress can vary among different species. Some of the important morphological, physiological, biochemical and anatomical responses of mungbean genotypes to heat stress tolerance are highlighted in Figure 1 , Figure 2 and Table 2 .
3.2 Physiological adaptation in response to heat stress
There are few studies on how high temperature stress affects stage-specific functional physiology in legumes from post-flowering to blooming. The reproductive stage is destined to be more impacted by and prone to temperature vagaries due to its delicate organelle constitution, even if heat stress sensitivity in plants changes with plant growth. Depending on the species and genotype, there are significant inter- and intra-specific variations that affect the response under heat stress ( Sakata and Higashitani, 2008 ; Bita and Gerats, 2013 ).
The persistence of photosynthesis in plants when they are exposed to stress conditions is supported by the stay-green (SGR) trait, which is also known as delayed leaf senescence. The maintenance of photosynthetic activity in plants under stressful conditions is facilitated by the stay-green (SGR) trait, also referred to as delayed leaf senescence ( de Souza Luche et al., 2015 ). Understanding the role of SGR in plants could lead to improved plant production and productivity ( Thomas and Ougham, 2014 ). During grain filling, the SGR characteristic allows plants to continue photosynthesizing by creating a senescence pattern that increases the amount of sugar produced by photosynthesis ( Pinto et al., 2016 ). According to recent research, SGR wheat genotypes demonstrated improved tolerance to high temperatures due to enhanced structural stability of the photosynthetic apparatus and lower accumulation of harmful reactive oxygen species ( Tian et al., 2012 ). The cultivar “Mairaj-2008” has also demonstrated superior ability to grow under heat stress compared to cultivars that lack the SGR trait and are more susceptible to heat damage ( Nawaz et al., 2013 ). Canopy temperature depression (CTD) could serve as a useful tool for selecting heat-tolerant genotypes based on observable differences in their traits ( Mason and Singh, 2014 ). Studies have shown that lower canopy temperatures promote better yield potential in wheat exposed to elevated temperatures, and CTD is effective in mitigating heat stress in wheat ( Amani et al., 1996 ; Fischer et al., 1998 ; Mason et al., 2013 ). Examination of stomatal behavior under stress conditions can be performed using a leaf porometer, which measures fluctuations in gaseous exchange rate that trigger stomatal opening ( Chandra et al., 2017 ). High-yielding cultivars with fully opened stomata have increased transpiration rates, improved CO2 and water vapor diffusion, and enhanced photosynthetic efficiency ( Condon et al., 2007 ).
Chlorophyll fluorescence (ChlF) is a non-invasive marker for photosystem II (PSII) quantum efficiency and can be used to assess early stress in plants, making it a useful tool for investigating plant heat stress tolerance ( Sharma et al., 2014 ; Kalaji et al., 2016 ). Studies have found that genotypes with high ChlF values, such as the heat-tolerant line RRR46 of common bean, outperform other lines when exposed to heat stress, indicating their potential for future breeding programs ( Stefanov et al., 2011 ). Membrane stability is another important physiological trait that can affect heat tolerance in plants, and higher membrane stability during grain filling has been shown to increase heat tolerance in wheat ( Gupta et al., 2013 ; Ramani et al., 2017 ).
Flag leaf photosynthetic efficiency has also been found to play a role in heat stress tolerance in crops. For example, the wheat cultivar “Jimai22”showed increased yield under heat stress and demonstrated PSII stability and significant carboxylation activity ( Feng et al., 2014 ). Heat-tolerant mungbean and lentil genotypes have also been found to exhibit better photosynthetic efficiencies under heat stress than heat-sensitive genotypes ( Sharma et al., 2016 ; IG3263, respectively). Conversely, decreased cellular thermostability in rice has been linked to potential reductions in crop output ( Maavimani and Saraswathi, 2014 ). Overall, physiological traits such as ChlF, membrane stability, and photosynthetic efficiency can provide important insights into plant heat stress tolerance and can be used to identify promising genotypes for breeding programs. A comprehensive list of mungbean genotypes based on physiological traits under heat stress tolerance is provided in Table 1 .
The traditional theme of research on legume reproduction has tremendously focused on vegetative stage of the plants rather than their physiological and molecular mechanisms underlying legume crop reproductive heat tolerance ( Lin et al., 1984 ; Valliyodan and Nguyen, 2006 ; Kumar, 2012 ; Sita et al., 2017 ). However, recent studies have shown a growing interest in understanding these mechanisms ( Devasirvatham et al., 2012 ; Kaushal et al., 2013 ; Sita et al., 2017 ; Patriyawaty et al., 2018 ). Despite this, there is still a lack of knowledge regarding the specific physiological and molecular mechanisms that allow legume crops to be resilient to heat stress during the reproductive stage ( Devasirvatham et al., 2012 ; Kaushal et al., 2013 ; Sita et al., 2017 ; Patriyawaty et al., 2018 ). One possible explanation for this lack of understanding is that many legume cultivars are difficult to transform genetically, which limits the availability of transgenic evidence ( Young and Udvardi, 2009 ; Song et al., 2013 ). However, on the basis of current research on other crop groups, particularly cereals, it is possible to suggest physiological and molecular explanations for legumes’ ability to withstand heat stress ( Devasirvatham et al., 2012 ; Kaushal et al., 2013 ; Sita et al., 2017 ; Patriyawaty et al., 2018 ).
3.3 Biochemical adaptation mechanisms involved in mungbean against heat stress
Plants have evolved with a number of biochemical adaptation mechanisms that allow them to tolerate high temperature stress, for example, secondary metabolites, ROS scavenging system and heat-shock proteins (HSPs) ( Nakamoto and Hiyama, 1999 ; Sakamoto and Murata, 2002 ; Wahid et al., 2007 ; Mittler et al., 2012 ). Plants use both non-enzymatic and enzymatic ROS scavenging defense mechanisms to combat ROS production. Non-enzymatic antioxidants such as glutathione (GSH) and ascorbic acid (ASC) collaborate with enzymatic antioxidants such as ascorbate peroxidase (APX), Catalase (CAT), superoxide dismutase (SOD), peroxidase (POX), and glutathione reductase (GR) to maintain high antioxidant levels required for plant heat tolerance. ( Suzuki et al., 2012 ; Awasthi et al., 2015 ). Isoprenoids, flavonoids, and carotenoids are secondary metabolites that inhibit peroxidase activity and promote high-temperature stress tolerance ( Havaux, 1998 ; Loreto et al., 1998 ; Rivero et al., 2004 ). Chickpea grown under high-temperature stress at 35/25 and 45/35°C (day/night, 12 h/12 h) conditions exhibited increased levels of antioxidants such as proline and glutathione ( Kumar et al., 2011 ). The non-enzymatic and enzymatic antioxidant pools are the most efficient and prominent defense mechanisms used by plants. Antioxidants with low molecular weight decrease oxidants without causing significant pro-oxidant activity.
The enormous generation of ROS occurs during heat stress which destroy proteins, lipids, nucleic acids, and carbohydrates ( Bita and Gerats, 2013 ). Mungbean plants can protect themselves from ROS by activating various types of non-enzymatic and enzymatic defensive mechanisms in different cells in plants ( Bita and Gerats, 2013 ). In an experiment under field conditions for heat stress, out of forty-one mungbean genotypes, only a few were found to be heat-tolerant viz., {"type":"entrez-nucleotide","attrs":{"text":"EC693357","term_id":"110007474","term_text":"EC693357"}} EC693357 , ML1299, {"type":"entrez-nucleotide","attrs":{"text":"EC693369","term_id":"110007486","term_text":"EC693369"}} EC693369 , Harsha, and {"type":"entrez-nucleotide","attrs":{"text":"EC693358","term_id":"110007475","term_text":"EC693358"}} EC693358 which suffered less oxidative damage than heat sensitive mungbean genotypes ( Sharma et al., 2016 ) and when compared to susceptible genotypes, there was increased APX activity in heat resistant genotypes. However, the activity of CAT was increased in both heat-resistant and heat-tolerant genotypes ( Sharma et al., 2016 ). As a result, mungbean genotypes that can withstand under heat stress during reproductive stages could be chosen for screening growth and productivity analysis. Some supplements, such as Ca, K, Mg, and N have been shown to reduce the harmfulness of ROS in plant cells by increasing anti-oxidants such as POD, SOD and CAT ( Waraich et al., 2012 ). Mungbean genotypes can be chosen based on the quantity of enzyme expression, with stress tolerant genotypes having more prominent activities than sensitive genotypes for breeding stress tolerant mungbean cultivars ( Kumar et al., 2013 ).
Aside from the anti-oxidative system, accumulation of various appropriate protective osmolytes such as sugar and their derivatives (polyols), ammonium-based products, proline, and some compounds of sulphonium-based derivatives have been shown in the protection and/or repair of molecules and structures damaged by ROS, as well as in ROS sequestration ( Sairam and Tyagi, 2004 ; Miller et al., 2007 ). As a result, osmotic adjustment is considered as one of the most promising mechanisms for drought and heat which can be accomplished by accumulating appropriate solutes (e.g., glycine betaine and proline) in protoplasm ( Chaves et al., 2003 ; Bartels and Sunkar, 2005 ). Proline is one of the prominent osmo-protectant, which also play a crucial role in cellular homeostasis. It also acts as a signaling molecule in triggering specific gene expression, in cell proliferation or cell death, and to alter mitochondrial functions. These are crucial processes for plants’ recovery after stress. Certain natural combinations of osmolytes are formed under abiotic stress conditions such as salt, drought, and heat ( Hare et al., 1998 ; Sakamoto and Murata, 2002 ). Plants may protect themselves by the accumulation of these solutes in order to increase the tolerance up to certain circumstances. These osmolytes help in increasing the stability of membrane bilayer and proteins. Defensive molecules such as flavonoids, anthocyanin, and plant steroids have been recognized as secondary metabolites that contribute to heat stress tolerance in plants. In response to heat stress, plants also increase their levels of several major phytohormones including ethylene (ET), salicylic acid (SA), and abscisic acid (ABA) to enhance their tolerance. Evaluation of these plant hormones in response to heat stress involves measuring various biochemical and physiological parameters, as well as assessing growth regulating parameters such as photosynthesis and biomass. Additionally, other hormones like auxin, gibberellins (GAs), cytokinins, abscisic acid, brassinosteroids (BRs), and jasmonic acid (JA) have been identified as contributing to heat stress tolerance in plants, as noted by various studies ( Peleg and Blumwald, 2011 ; 2011 ; Mittler et al., 2012 ; Zhou et al., 2014 ; Dobrá et al., 2015 ; Xia et al., 2015 ).
3.4 Production of heat shock and regulatory protein in response to heat stress
Plants possess an adaptive mechanism to manage heat stress via the production of stress proteins like heat shock proteins (HSPs). These HSPs act as molecular chaperones and assist in activities like protein folding, translocation, aggregation, and degradation in both regular and stressful environments ( Vierling, 1991 ). When subjected to sudden or constant temperature variations, plants increase their HSP production ( Schoffl et al., 1998 ; Nakamoto and Hiyama, 1999 ), highlighting the critical nature of HSPs when it comes to temperature stress across all species ( Vierling, 1991 ). Plants in dry and semi-arid regions can synthesize and store HSPs in large quantities. HSPs are only present during specific developmental stages like pollen formation, germination, fruit maturation, and embryogenesis. These proteins protect other proteins from denaturation that could be caused by high temperatures. In sensitive organs and tissues where HSPs accumulate quickly, they can safeguard the metabolic system of the cells, which makes them crucial in a plant’s stress response and overall survival. Numerous research studies have established a close relationship between the development of heat tolerance and the synthesis and buildup of HSPs. An instance of this is the elevated expression of HSP68, which is typically expressed constitutively in mitochondria, when potato, maize, tomato, soybean, and barley cells were placed under heat stress ( Neumann et al., 1993 ). HSP70, extensively studied, is believed to partake in several functions, including but not limited to the folding or assisting of proteins, translocation, protein translation, proteolysis, inhibition of aggregation, as well as the restoration of denatured proteins ( Zhang et al., 2005 ). Many plant species necessitate the induction of HSP70 for heat tolerance of cells and tissues post heat stress. Additionally, HSP101 has been deemed vital ( Schoffl et al., 1998 ). Furthermore, different tissues within the same plant manifest diverse abilities to produce specific proteins at 40°C and the magnitude and duration of the synthesis also differ. Five different types of heat-stress protein/chaperone families are reported; these are HSP100, HSP90, HSP70, HSP60 and the small heat-stress proteins (sHSPs) family ( Wang et al., 2004 ), that play an important role in heat-stress mitigation, including protecting native proteins against denaturation.
3.5 Mitigating heat stress through agricultural practices
Along with development of tolerant mungbean genotypes integration of heat stress mitigation strategies through appropriate agricultural practices will have greater impact on sustaining the mungbean production amid rising global temperature. Agricultural practices such as timely sowing of crop, selection of early short duration genotypes to escape peak heat stress period, seed priming to enhance the seed vigour and initiate heat tolerance defense mechanisms, irrigation management to enhance water use efficiency and maintain plant water potential, mulching and enhancing organic material to enhance soil water retention and reduce soil temperature. Mungbean sown during summer/spring season, particularly in northern part of India, face more threat of heat stress during later stage of crop growth ( HanumanthaRao et al., 2016 ). To overcome the terminal heat stress, early maturing mungbean cultivars with early seedling vigour are very crucial for sustaining mungbean production. Majority of mungbean cultivars matures in 60–70 days, however, the evaluation of mungbean collections reveals availability of mungbean germplasm which matures within 50 days of sowing ( Gayacharan et al., 2020 ). Seed priming is another promising tool for induction of artificial stress memory response in terms of accumulation of secondary metabolites, antioxidants, improved water plant water potential, etc. ( Ahmad et al., 2021 ; Chakraborty and Dwivedi, 2021 ; Tamindžić et al., 2023 ). Utilization of heat tolerant mungbean cultivars may further help in mitigating impact of heat stress in mungbean cultivation. Mungbean cultivars such as Pusa Vishal, SML 668, IPM 99-125, and few more listed in Table 1 are helping in sustaining mungbean cultivation during the summer/spring season in northern parts of India.
4 Signaling pathways/factors involved in heat stress tolerance in plants
Plants can detect even a slight deviation in temperature because of the sensing mechanism present on cell membrane through change in fluidity of membrane bilayer. This leads to the conformational changes and post-translation modifications such as phosphorylation/dephosphorylation processes ( Kaushal et al., 2016 ; Sehgal et al., 2016 ). In response to heat stress plants activate various signaling pathways that lead to the induction of heat stress tolerance genes. There are four major prominent signaling pathways viz. ABA, MAPK, ROS and Ca 2+ . Under high temperatures, ABA levels increase in plant tissues, leading to the activation of stress specific ABA signaling pathway. The ABA signaling pathway operates through ABA binding to receptors, which subsequently engage phosphatases and kinases to regulate downstream gene expression. In plants, this pathway triggers the activation of heat stress tolerance genes, such as HSP70, HSP90, and HSP101 ( Batcho et al., 2020 ; Priya et al., 2020 ). Similarly, the MAPK signaling cascade plays a critical role in activating MAPKs, which then phosphorylate transcription factors and trigger the induction of stress-responsive genes such as HSP17, HSP26, and HSP70 under heat stress in mung beans ( Wang et al., 2021 ). Additionally, the ROS signaling pathway has been linked to HSP70 and HSP90 induction under heat stress conditions, and it also interacts with other pathways like ABA and MAPK to regulate gene expression ( Banti et al., 2010 ).
Calcium (Ca 2+ ) is a ubiquitous secondary messenger that plays a critical role in plant stress responses, including heat stress. Under high temperatures, the heat shock reaction (HSR) is initially triggered by the detection of temperature increase by plasma membrane which then activates ion channels like Ca 2+ channels and induces an inward flux of Ca 2+ into cells ( Bokszczanin et al., 2013 ). According to several mechanisms reported, blockage of calcium channel or chelators causes inward flux of Ca 2+ ions which is a significant signal of heat stress. Ca 2+ interact with downstream targets, such as calmodulin and calcium-dependent protein kinases (CDPKs), leading to the activation of heat shock transcription factors (HSFs) and several other transcriptional factors such as WRKY39 and stress-responsive genes ( Li et al., 2011 ; 2010 ). In addition, this Ca 2+ influx induces the activation of another signaling cascade system including calcium-dependent protein kinases (CDPKs), mitogen-activated protein kinases (MAPKs), and NADPH oxidase, all of which cause the generation of ROS ( Sangwan et al., 2002 ; Suzuki et al., 2011 ) ( Figure 4 ). These different signaling and defensive pathways are evolutionary conserved processes in legumes and work similarly in almost all of the legume crops. In plants, the Ca 2+ signaling pathway has been shown to be involved in the induction of heat stress tolerance genes, such as HSP70 and HSP90 ( Zheng et al., 2020 ).
Heat stress activates various other signaling molecules such as PIPK (phosphatidylinositol-4-phosphate-5-kinase), PLD (phospholipase-D), phosphatidic acid, IP3 (D-myo-inositol-1,4,5-triphosphate), and PIP2 (phosphatidylinositol-4,5-bisphosphate). In plants, the activation of two UPR (Unfolded protein response) signaling pathways is triggered by heat stress, one in ER and the other in cytoplasm ( Sugio et al., 2009 ; Pincus et al., 2010 ; Deng et al., 2011 ). This leads to proteolytic cleavage in the membrane of the endoplasmic reticulum and activates different bZIP transcription factors (Tfr). ( Che et al., 2010 ; Deng et al., 2011 ). The accumulation of chaperones in ER, along with calcium signaling, activates brassinosteroid signaling, which in turn activates the transcription of heat-tolerant genes ( Che et al., 2010 ). In cytosol, the unfolded proteins activate the cytosolic UPR pathway for HSF and HSFA2 transcription factors to induce downstream heat stress responsive genes ( Sugio et al., 2009 ).
Plants also use phytohormones, such as ABA and brassinosteroids, as well as signaling molecules like nitric oxide to help them tolerate heat stress ( Hasanuzzaman et al., 2011 ; Asthir, 2015 ). Studies have shown that applying exogenous ABA to Phragmites communis led to a decrease in MDA and H 2 O 2 content and an increase in POX, APX, CAT, and SOD levels, which suggests less oxidative damage in treated plants compared to non-treated plants ( Ding et al., 2010 ). Similarly, spraying BRs on Phaseolus vulgaris increased yield and quality of pods, total phenolic acids in pods, and vegetative growth through the BRs signaling pathway ( El-Bassiony et al., 2012 ). Salicylic acid (SA) is also an important signaling molecule that influences plant growth and development under stress conditions and can act as a protectant under heat stress ( Yuan et al., 2008 ; Hasanuzzaman et al., 2013 ). Applying SA can increase enzyme activity, carotenoid and chlorophyll levels, photosynthetic rates, ion uptake, flower induction, plant growth, and thermogenesis, and affect the ethylene biosynthesis pathway ( Bajguz and Hayat, 2009 ).
Under heat stress tolerance, nitric oxide (NO) is considered as an another important signaling molecule that regulates various morpho-physiological and biochemical processes in a systematic concentration-dependent manner and acts as a redox-related signaling molecule in legumes ( Hasanuzzaman et al., 2013 ; 2012 ; 2011 ; Waraich et al., 2012 ; Fancy et al., 2017 ). Exogenous treatment of NO on heat-stressed plants increases the stability and shelf life of chlorophyll molecules, decreases H 2 O 2 content, and increases antioxidant enzyme activities by enhancing sodium nitropruside levels in plants ( Yang et al., 2006 ).
5 Molecular markers and candidate genes associated with heat stress tolerance in plants
The traditional breeding programs are crucial for the identification of stable genetic and genomic resources for their introduction into elite cultivars in addition to advances in biotechnological and molecular approaches ( Cabello et al., 2014 ). Recent efforts on developing new breeding methods have not been enough to cultivate heat tolerant varieties ( Grover et al., 2013 ). Therefore, development of heat tolerance variety is limited through breeding approach. In order to fill this gap, a unique approach has been adapted by breeders for cultivation of stress tolerance variety which is beneficial for developing stress tolerant genotypes with high productivity by interpreting the genomic regions on chromosomes responsible for tolerance ( Driedonks et al., 2016 ). Mungbean is one of the legumes which has rarely received the application of new breeding tools (NBTs) and technologies for understanding the basic tolerance mechanism. However, recent advancement in genome sequencing technologies and their implication in crop research programs has also helped in generating genomic resources in mungbean. Currently there are two genome assemblies (Vradiata_ver6, ASM158444v1) with full genome representation and one assembly (ASM18089v1) with partial genome representation ( Tangphatsornruang et al., 2009 ; Kang et al., 2014 ; Liu et al., 2016 ). The genome sequence information is helping in understanding the underlying molecular mechanisms involved in trait expressions.
The utilization of genome sequencing data and advanced genome annotation tools has facilitated the identification of key candidate genes that play essential roles in heat stress tolerance. The genes HSP60, HSP70, HSP90, HSP100, and smHSP are well-known molecular chaperones that are induced by heat stress and are critical in safeguarding plants against heat damage ( Kotak et al., 2007 ; Mishra et al., 2018 ). Additionally, CBF/DREB1 protein family members and LEA proteins have also been identified as stress-responsive proteins that protect plants from abiotic stresses, including heat stress ( Li et al., 2020 ). Another group of stress-responsive protein that is involved in protecting plants from heat stresses is LEA (Late embryogenesis abundant). In mungbean, some LEA genes viz. VrLEA-55, VrLEA-47, VrLEA-40, and VrLEA-2 were identified by Singh et al. (2022) , and reported their upregulation under heat stress conditions. Furthermore, genes encoding antioxidant enzymes such as APX, CAT, and SOD have been identified as significant candidates that aid in scavenging reactive oxygen species under stress conditions. A comprehensive list of identified candidate genes responsible for heat stress tolerance in various plant species is presented in Table 2 .
Plant breeding has become more efficient with the aid of Marker aided selection (MAS), however, simple sequence repeats (SSRs) and single nucleotide polymorphisms (SNPs) are commonly used for quantitative trait loci (QTL) analysis in breeding programs ( Das and Rao, 2015 ). Recent advances report that novel breeding approaches utilizing QTL mapping have been effective in developing heat stress tolerance in plants ( Jha et al., 2014 ; Shamsudin et al., 2016 ). Several QTLs for various traits associated with heat stress tolerance have been identified in different crops. For example, in cowpea, foundation genomic areas linked to heat stress tolerance were identified using SNP markers in a RIL population (CB27 9 IT82E-18), while QTLs associated with browning of seed coats were also discovered ( Lucas et al., 2013 ; Pottorff et al., 2014 ). In lentil, two QTLs ( qHt-ps and qHt-ss ) were characterized, which were linked to heat stress tolerance in pod set and seedling survival ( Singh et al., 2016 ). Similarly, in chickpeas, eight QTLs were identified, four of which were located on the CaLG05 genomic region, for pod set, pod filling, seed number, and grain yield with a combined phenotypic variation of up to 50% under heat stress tolerance, while as the remaining QTLs were located on the CaLG06 genomic regions. ( Paul et al., 2018 ). Jha et al. (2021) identified 37 major and 40 minor QTLs for heat stress tolerance using an inbred population in chickpea. They also identified 32 potential candidate genes in the QTL regions that encode HSPs and HSFs and are involved in the regulation of flowering time and pollen development.
Similarly, in non-legume crops such as wheat plants, QTLs associated with grain filling and leaf senescence were reported on chromosome number 5A and 1B ( Yang et al., 2002 ; Mason et al., 2010 ). Nine QTLs for tillering and grain filling, and three QTLs for green color were also identified in wheat ( Kumar and Wigge, 2010 ). In maize, six QTLs for pollen tube growth and five QTLs for pollen germination, as well as six QTLs for cellular membrane stability, were detected using RFLP mapping technique ( Ottaviano et al., 1991 ; Frova and Sari-Gorla, 1994 ). These QTLs and genes are evolutionarily conserved and exhibit mostly similar function in all legumes and other crops. A brief summary of different QTLs/genes associated with heat stress tolerance in legumes/crops is mentioned in Table 2 . Currently, the identification and characterization of marker genes associated with heat stress tolerance studies have gained more interest and require better attention and understanding in this field.
6 Conclusion and future perspective
Mungbean is a short-duration crop that thrives in a variety of soils and climates. However, its cultivation is increasingly being influenced by heat stress all over the world. To overcome drastic rise in global temperature amid climate change, a comprehensive and holistic approach is required to sustain crop production. The existing variability in ex situ collections conserved in seed genebanks or in situ on-farm should be the first target to find sources of heat tolerance. Further, utilization of such germplasm to incorporate various climate-smart features into mungbean through new breeding tools would enable mungbean cultivars to perform well in a variety of locations and adapt to diverse agro-climatic regions. In addition to recent advances in new breeding tools, the latest developments in genomics, transcriptomics and metabolomics fields could make a great impact on trait identification and may help to accelerate in developing desired mungbean cultivars. Regions of the genome linked to advantageous characteristics, heat tolerance, water-use efficiency, and the photosynthetic pathways, can also be targeted by combining the genome sequence and phenotyping data. Additionally, a thorough examination of the mungbean pan-genome variability should be performed to understand pan-genome variability at species level. Researchers are now well equipped to explore and exploit underlying molecular processes, which may pave the way for developing multi-stress tolerant mungbean that is best suited to adverse growing environments. Modern scientific tools such as mutational breeding and genome editing may prove very useful in creation of desired novel variability and development of heat tolerant genotypes. Integration of suitable agricultural practices to mitigate heat stress may further provide additional protection to crop cultivation amid rising global temperature.
Acknowledgments
Authors duly acknowledge ICAR-National Bureau of Plant Genetic Resources, New Delhi, ICAR-Indian Agricultural Research institute, New Delhi, Shivaji College, University of Delhi, New Delhi, ICAR-Indian Grassland and Research Institute, Jhansi, and Banasthali Vidyapith University, Tonk Rajasthan for providing necessary support for the study.
Author contributions
Author RB surveyed the literature and made initial draft of the manuscript. G outlined the manuscript’s content, improved its content and coordinated among the co-authors. SM and SK helped in improving initial draft. JL made significant changes and corrections in text and figures. RP, NM, and DR contributed in further improvement of the figures and scientific contents of physiological aspects. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Abd El Lateff E., Abd El-Salam M., Selim M., Tawfik M., Mohamad E.-K., Farrag A. (2018). Effect of climate change on mungbean growth and productivity under Egyptian conditions . Indian J. Agric. Sci . 2 , 16–23. [ Google Scholar ]
- Adams S. R., Cockshull K. E., Cave C. R. J. (2001). Effect of temperature on the growth and development of tomato fruits . Ann. Bot. 88 , 869–877. 10.1006/anbo.2001.1524 [ CrossRef ] [ Google Scholar ]
- Ahmad P., Jaleel C. A., Salem M. A., Nabi G., Sharma S. (2010). Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress . Crit. Rev. Biotechnol. 30 , 161–175. 10.3109/07388550903524243 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmad M., Waraich E. A., Hussain S., Ayyub C. M., Ahmad Z., Zulfiqar U. (2021). Improving heat stress tolerance in Camelina sativa and Brassica napus through thiourea seed priming . J. Plant Growth Regul. 41 , 2886–2902. 10.1007/s00344-021-10482-4 [ CrossRef ] [ Google Scholar ]
- Alom K. M., Rashid M. H., Biswas M. (2014). Genetic variability, correlation and path analysis in mungbean ( Vigna radiata L) . J. Environ. Sci. Nat. Resour . 7 , 131–138. 10.3329/jesnr.v7i1.22161 [ CrossRef ] [ Google Scholar ]
- Amani I., Fischer R. A., Reynolds M. P. (1996). Canopy temperature depression association with yield of irrigated spring wheat cultivars in a hot climate . J. Agron. Crop Sci. 176 , 119–129. 10.1111/j.1439-037x.1996.tb00454.x [ CrossRef ] [ Google Scholar ]
- Anderson J. A., Padhye S. R. (2004). Protein aggregation, radical scavenging capacity, and stability of hydrogen peroxide defense systems in heat-stressed vinca and sweet pea leaves . J. Am. Soc. Hortic . 129 , 54–59. 10.21273/jashs.129.1.0054 [ CrossRef ] [ Google Scholar ]
- Apel K., Hirt H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction . Annu. Rev. Plant Biol . 55 , 373–399. 10.1146/annurev.arplant.55.031903.141701 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ara N., Nakkanong K., Lv W., Yang J., Hu Z., Zhang M. (2013). Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“ Cucurbita maxima ” and “ Cucurbita moschata ”) and their interspecific inbred line “maxchata” . Int. J. Mol. Sci . 14 , 24008–24028. 10.3390/ijms141224008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ashraf M., Hafeez M. (2004). Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations . Biol. Plant . 48 , 81–86. 10.1023/B:BIOP.0000024279.44013.61 [ CrossRef ] [ Google Scholar ]
- Asseng S., Foster I. A. N., Turner N. C. (2011). The impact of temperature variability on wheat yields . Glob. Chang. Biol . 17 , 997–1012. 10.1111/j.1365-2486.2010.02262.x [ CrossRef ] [ Google Scholar ]
- Asthir B. (2015). Protective mechanisms of heat tolerance in crop plants . J. Plant Interact . 10 , 202–210. 10.1080/17429145.2015.1067726 [ CrossRef ] [ Google Scholar ]
- Awasthi R., Bhandari K., Nayyar H. (2015). Temperature stress and redox homeostasis in agricultural crops . Front. Environ . Sci. 3 , 11. 10.3389/fenvs.2015.00011 [ CrossRef ] [ Google Scholar ]
- Bajguz A., Hayat S. (2009). Effects of brassinosteroids on the plant responses to environmental stresses . Plant Physiol . biochem . 47 , 1–8. 10.1016/j.plaphy.2008.10.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baker C. J., Orlandi E. W. (1995). Active oxygen in plant pathogenesis . Annu. Rev. Phytopathol. 33 , 299–321. 10.1146/annurev.py.33.090195.001503 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bañon S., Fernandez J. A., Franco J. A., Torrecillas A., Alarcón J. J., Sánchez-Blanco M. J. (2004). Effects of water stress and night temperature preconditioning on water relations and morphological and anatomical changes of Lotus creticus plants . Sci. Hortic . 101 , 333–342. 10.1016/j.scienta.2003.11.007 [ CrossRef ] [ Google Scholar ]
- Bansal M., Kukreja K., Sunita S., Dudeja S. S. (2014). Symbiotic effectivity of high temperature tolerant mungbean ( Vigna radiata ) rhizobia under different temperature conditions . Int. J. Curr. Microbiol. Appl . Sci . 3 , 807–821. [ Google Scholar ]
- Banti V., Mafessoni F., Loreti E., Alpi A., Perata P. (2010). The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis . Plant Physiol. 152 , 1471–1483. 10.1104/pp.109.149815 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baroowa B., Gogoi N., Farooq M. (2016). Changes in physiological, biochemical and antioxidant enzyme activities of green gram ( Vigna radiata L) genotypes under drought . Acta Physiol . Plant 38 , 219. 10.1007/s11738-016-2230-7 [ CrossRef ] [ Google Scholar ]
- Bartels D., Sunkar R. (2005). Drought and salt tolerance in plants . Crit. Rev . Plant Sci. 24 , 23–58. 10.1080/07352680590910410 [ CrossRef ] [ Google Scholar ]
- Basu P. S., Pratap A., Gupta S., Sharma K., Tomar R., Singh N. P. (2019). Physiological traits for shortening crop duration and improving productivity of greengram ( Vigna radiata L. Wilczek ) under high temperature . Front. Plant Sci . 10 , 1508. 10.3389/fpls.2019.01508 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Batcho A. A., Sarwar M. B., Tariq L., Rashid B., Hassan S., Husnain T. (2020). Identification and characterisation of heat shock protein gene (HSP70) family and its expression in Agave sisalana under heat stress . J. Hortic. Sci. Biotechnol . 95 , 470–482. 10.1080/14620316.2019.1685412 [ CrossRef ] [ Google Scholar ]
- Bita C. E., Gerats T. (2013). Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops . Front. Plant Sci. 4 , 273. 10.3389/fpls.2013.00273 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blokhina O., Virolainen E., Fagerstedt K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: A review . Ann. Bot . 91 , 179–194. 10.1093/aob/mcf118 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bokszczanin K. L., Consortium S., Fragkostefanakis S. (2013). Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance . Front. Plant Sci . 4 , 315. 10.3389/fpls.2013.00315 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bolouri-Moghaddam M. R., Le Roy K., Xiang L., Rolland F., Van den Ende W. (2010). Sugar signalling and antioxidant network connections in plant cells . FEBS J. 277 , 2022–2037. 10.1111/j.1742-4658.2010.07633.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cabello J. V., Lodeyro A. F., Zurbriggen M. D. (2014). Novel perspectives for the engineering of abiotic stress tolerance in plants . Curr. Opin. Biotechnol . 26 , 62–70. 10.1016/j.copbio.2013.09.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cao D., Li H., Yi J., Zhang J., Che H., Cao J., et al. (2011). Antioxidant properties of the mung bean flavonoids on alleviating heat stress . PloS one 6 , e21071. 10.1371/journal.pone.0021071 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chakraborty P., Dwivedi P. (2021). Seed priming and its role in mitigating heat stress responses in crop plants . Curr. Opin. Biotechnol . 21 ( 2 ), 1718–1734. 10.1007/s42729-021-00474-4 [ CrossRef ] [ Google Scholar ]
- Chand G., Nandwal A. S., Kumar N., Devi S., Khajuria S. (2018). Yield and physiological responses of mungbean Vigna radita (L ) Wilczek genotypes to high temperature at reproductive stage . Legume Research-An Int. J. 41 , 557–562. 10.18805/lr-3795 [ CrossRef ] [ Google Scholar ]
- Chand G., Nandwal A. S., Dogra S., Sharma M. (2020). Biochemical response of mungbean [ Vigna radiata (L.) wilczek ] genotypes under terminal heat stress at reproductive stage . Int. J. Curr. Microbiol. App. Sci . 9 , 2975–2986. 10.20546/ijcmas.2020.907.351 [ CrossRef ] [ Google Scholar ]
- Chandra K., Prasad R., Thakur P., Madhukar K., Prasad L. C. (2017). Heat tolerance in wheat-A key strategy to combat climate change through molecular markers . Int. J. Curr. Microbiol. Appl . Sci. 6 , 662–675. 10.20546/ijcmas.2017.603.077 [ CrossRef ] [ Google Scholar ]
- Chaves M. M., Maroco J. P., Pereira J. S. (2003). Understanding plant responses to drought—From genes to the whole plant . Funct . Plant Biol . 30 , 239–264. 10.1071/FP02076 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Che P., Bussell J. D., Zhou W., Estavillo G. M., Pogson B. J., Smith S. M. (2010). Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in arabidopsis . Sci. Signal. 28 ( 3 ), ra69. 10.1126/scisignal.2001140 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen C., Chen Z. (2002). Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor . Plant Physiol. 129 , 706–716. 10.1104/pp.001057 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chikukura L., Bandyopadhyay S. K., Pathak H., Chakrabarti B. (2017). Effect of elevated temperature stress on growth, yield and yield attributes of mungbean ( Vigna radiata ) in semi-arid north-west India . Curr. Adv. Agric. Sci. Int. J. 9 , 18–22. 10.5958/2394-4471.2017.00003.X [ CrossRef ] [ Google Scholar ]
- Condon A. G., Reynolds M. P., Rebetzke G. J., Ginkel M. van, Richards R. A., Farquhar G. D. (2007). “ Using stomatal aperture-related traits to select for high yield potential in bread wheat ,” in Wheat Production in Stressed Environments: Proceedings of the 7th International Wheat Conference, Mar del Plata, Argentina, November–December 2005 (Springer; ), 617–624. [ Google Scholar ]
- Das G., Rao G. J. N. (2015). Molecular marker assisted gene stacking for biotic and abiotic stress resistance genes in an elite rice cultivar . Front. Plant Sci. 6 , 698. 10.3389/fpls.2015.00698 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Das S., Krishnan P., Nayak M., Ramakrishnan B. (2014). High temperature stress effects on pollens of rice ( Oryza sativa L) genotypes . Environ. Exp. Bot . 101 , 36–46. 10.1016/j.envexpbot.2014.01.004 [ CrossRef ] [ Google Scholar ]
- de Souza Luche H., da Silva J. A. G., Nornberg R., Zimmer C. M., Arenhardt E. G., da Rosa Caetano V., et al. (2015). Stay-green effects on adaptability and stability in wheat . Afr. J. Agric. Res . 10 , 1142–1149. [ Google Scholar ]
- Deng Y., Humbert S., Liu J.-X., Srivastava R., Rothstein S. J., Howell S. H. (2011). Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis . Proc. Natl. Acad. Sci . U.S.A. 108 , 7247–7252. 10.1073/pnas.1102117108 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Devasirvatham V., Gaur P. M., Mallikarjuna N., Tokachichu R. N., Trethowan R. M., Tan D. K. (2012). Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments . Funct. Plant Biol . 39 , 1009–1018. 10.1071/FP12033 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dickson M. H., Boettger M. A. (1984). Effect of high and low temperatures on pollen germination and seed set in snap beans . J. Am. Soc. Hortic. Sci. 109 , 372–374. 10.21273/JASHS.109.3.372 [ CrossRef ] [ Google Scholar ]
- Ding W., Song L., Wang X., Bi Y. (2010). Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis . Biol. Plant . 54 , 607–613. 10.1007/s10535-010-0110-3 [ CrossRef ] [ Google Scholar ]
- Djanaguiraman M., Prasad P. V. V., Al-Khatib K. (2011). Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress . Environ. Exp. Bot . 71 , 215–223. 10.1016/j.envexpbot.2010.12.006 [ CrossRef ] [ Google Scholar ]
- Djanaguiraman M., Prasad P. V. V., Boyle D. L., Schapaugh W. T. (2013). Soybean pollen anatomy, viability and pod set under high temperature stress . J. Agron. Crop Sci . 199 , 171–177. 10.1111/jac.12005 [ CrossRef ] [ Google Scholar ]
- Dobrá J., Černý M., Štorchová H., Dobrev P., Skalák J., Jedelský P. L., et al. (2015). The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis . Plant Sci. 231 , 52–61. 10.1016/j.plantsci.2014.11.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doke N. (1997). “ The oxidative burst: Roles in signal transduction and plant stress ,” in Oxidative stress and the molecular biology of antioxidant defenses . Editors Scandalios J. G. New York: Cold Spring Harbor Press; ), 785–813. [ Google Scholar ]
- Dreesen F. E., De Boeck H. J., Janssens I. A., Nijs I. (2012). Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community . Environ. Exp. Bot . 79 , 21–30. 10.1016/j.envexpbot.2012.01.005 [ CrossRef ] [ Google Scholar ]
- Driedonks N., Rieu I., Vriezen W. H. (2016). Breeding for plant heat tolerance at vegetative and reproductive stages . Plant Reprod. 29 , 67–79. 10.1007/s00497-016-0275-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Bassiony A. M., Ghoname A. A., El-Awadi M. E., Fawzy Z. F., Gruda N. (2012). Ameliorative effects of brassinosteroids on growth and productivity of snap beans grown under high temperature . Gesunde Pflanz. 64 , 175–182. 10.1007/s10343-012-0286-x [ CrossRef ] [ Google Scholar ]
- Escobar-Restrepo J.-M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W.-C., et al. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception . Sci 317 , 656–660. 10.1126/science.1143562 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fahad S., Hussain S., Saud S., Hassan S., Ihsan Z., Shah A. N., et al. (2016). Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature . Front. Plant Sci. 7 , 1250. 10.3389/fpls.2016.01250 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fahad S., Bajwa A. A., Nazir U., Anjum S. A., Farooq A., Zohaib A., et al. (2017). Crop production under drought and heat stress: Plant responses and management options . Front. Plant Sci. 8 , 1147. 10.3389/fpls.2017.01147 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fancy N. N., Bahlmann A.-K., Loake G. J. (2017). Nitric oxide function in plant abiotic stress . Plant Cell. Environ. 40 , 462–472. 10.1111/pce.12707 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Farooq M., Nadeem F., Gogoi N., Ullah A., Alghamdi S. S., Nayyar H., et al. (2017). Heat stress in grain legumes during reproductive and grain-filling phases . Crop. Pasture. Sci . 68 , 985–1005. 10.1071/cp17012 [ CrossRef ] [ Google Scholar ]
- Feng B., Liu P., Li G., Dong S. T., Wang F. H., Kong L. A., et al. (2014). Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties . J. Agron. Crop Sci . 200 , 143–155. 10.1111/jac.12045 [ CrossRef ] [ Google Scholar ]
- Fischer R. A., Rees D., Sayre K. D., Lu Z.-M., Condon A. G., Saavedra A. L. (1998). Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies . Crop Sci. 38 , 1467–1475. 10.2135/cropsci1998.0011183x003800060011x [ CrossRef ] [ Google Scholar ]
- Foyer C. H., Lopez-Delgado H., Dat J. F., Scott I. M. (1997). Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling . Physiol. Plant. 100 , 241–254. 10.1034/j.1399-3054.1997.1000205.x [ CrossRef ] [ Google Scholar ]
- Frova C., Sari-Gorla M. (1994). Quantitative trait loci (QTLs) for pollen thermotolerance detected in maize . Mol. Genet. Genom. MGG 245 , 424–430. 10.1007/BF00302254 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gayacharan, , Tripathi K., Meena S. K., Panwar B. S., Lal H., Rana J. C., et al. (2020). Understanding genetic variability in the mungbean ( Vigna radiata L) genepool . Ann. Appl. Biol. 177 ( 3 ), 346–357. 10.1111/aab.12624 [ CrossRef ] [ Google Scholar ]
- Gayacharan, , Parida S. K., Mondal N., Yadav R., Vishwakarma H., Rana J. C. (2023). Mining legume germplasm for genetic gains: An Indian perspective . Front. Genet. 14 , 996828. 10.3389/fgene.2023.996828 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Giorno F., Wolters-Arts M., Mariani C., Rieu I. (2013). Ensuring reproduction at high temperatures: The heat stress response during anther and pollen development . Plants 2 , 489–506. 10.3390/plants2030489 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grover A., Mittal D., Negi M., Lavania D. (2013). Generating high temperature tolerant transgenic plants: Achievements and challenges . Plant Sci. 205 , 38–47. 10.1016/j.plantsci.2013.01.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guna M., Ramanathan S., Geethalakshmi V., Chandrakumar K., Kokilavani S., Djanaguiraman M. (2022). Effect of high night temperature and CO 2 on yield and seed quality of summer green gram ( Vigna radiata ) under soil plant atmospheric research (SPAR) . J. Agric. Meteorol. 24 , 229–234. 10.54386/jam.v24i3.1685 [ CrossRef ] [ Google Scholar ]
- Gupta N. K., Agarwal S., Agarwal V. P., Nathawat N. S., Gupta S., Singh G. (2013). Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings . Acta Physiol. Plant. 35 , 1837–1842. 10.1007/s11738-013-1221-1 [ CrossRef ] [ Google Scholar ]
- Hall A. E. (2010). Breeding for heat tolerance . Plant Breed. Rev . 10 , 129–168. [ Google Scholar ]
- Hamada A. M. (2001). Alteration in growth and some relevant metabolic processes of broad bean plants during extreme temperatures exposure . Acta Physiol. Plant 23 , 193–200. 10.1007/s11738-001-0008-y [ CrossRef ] [ Google Scholar ]
- Hanif A., Wahid A. (2018). Seed yield loss in mungbean is associated to heat stress induced oxidative damage and loss of photosynthetic capacity in proximal trifoliate leaf . Pak. J. Agric. Sci. 55 . [ Google Scholar ]
- HanumanthaRao B., Nair R. M., Nayyar H. (2016). Salinity and high temperature tolerance in mungbean [ Vigna radiata (L) Wilczek] from a physiological perspective . Front. Plant Sci . 7 , 957. 10.3389/fpls.2016.00957 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hare P. D., Cress W. A., Van Staden J. (1998). Dissecting the roles of osmolyte accumulation during stress . Plant Cell. Environ. 21 , 535–553. 10.1046/j.1365-3040.1998.00309.x [ CrossRef ] [ Google Scholar ]
- Hasanuzzaman M., Hossain M. A., Fujita M. (2011). Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings . Plant Cell. Rep. 5 , 353–365. 10.1007/s11816-011-0189-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hasanuzzaman M., Hossain M. A., da Silva J. A. T., Fujita M. (2012). “ Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor ,” in Crop stress and its management: Perspectives and strategies . Editors Venkateswarlu B., Shanker A., Shanker C., Maheswari M. (Dordrecht: Springer; ). 10.1007/978-94-007-2220-0_8 [ CrossRef ] [ Google Scholar ]
- Hasanuzzaman M., Nahar K., Alam M. M., Roychowdhury R., Fujita M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants . Int. J. Mol. Sci. 14 , 9643–9684. 10.3390/ijms14059643 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Havaux M. (1998). Carotenoids as membrane stabilizers in chloroplasts . Trends Plant Sci. 3 , 147–151. 10.1016/s1360-1385(98)01200-x [ CrossRef ] [ Google Scholar ]
- Heath R. L., Packer L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation . Arch. Biochem. Biophys . 125 , 189–198. 10.1016/0003-9861(68)90654-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Howarth C. J. (2005). “ Genetic improvements of tolerance to high temperature ,” in Abiotic stresses: Plant resistance through breeding and molecular approaches . Editors Ashraf M., Harris P. J. C. (New York: Howarth Press Inc.). [ Google Scholar ]
- Hurkman W. J., Vensel W. H., Tanaka C. K., Whitehand L., Altenbach S. B. (2009). Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain . J. Cereal Sci . 49 , 12–23. 10.1016/j.jcs.2008.06.014 [ CrossRef ] [ Google Scholar ]
- Iqbal M., Ahmad M., Mustafa G. (2015). Impact of farm households’ adaptations to climate change on food security: Evidence from different agro-ecologies of Pakistan . Pak. Dev. Rev. 55 ( 4 ), 561–588. Part-II (Winter 2016). 10.30541/v55i4i-iipp.561-588 [ CrossRef ] [ Google Scholar ]
- Islam M. T. (2015). Effects of high temperature on photosynthesis and yield in mungbean . Bangladesh J. Bot . 44 , 451–454. 10.3329/bjb.v44i3.38553 [ CrossRef ] [ Google Scholar ]
- Jha U. C., Bohra A., Singh N. P. (2014). Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance . Plant Breed. 133 , 679–701. 10.1111/pbr.12217 [ CrossRef ] [ Google Scholar ]
- Jha U. C., Bohra A., Parida S. K., Jha R. (2017). Integrated “omics” approaches to sustain global productivity of major grain legumes under heat stress . Plant Breed. 136 , 437–459. 10.1111/pbr.12489 [ CrossRef ] [ Google Scholar ]
- Jha U. C., Nayyar H., Palakurthi R., Jha R., Valluri V., Bajaj P., et al. (2021). Major QTLs and potential candidate genes for heat stress tolerance identified in chickpea ( Cicer arietinum L ) . Front. Plant Sci . 12 , 655103. 10.3389/fpls.2021.655103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jincy M., Jeyakumar P., Boominathan P., Manivannan N., Varanavasiappan S., Rajendraprasad V. B. (2019). Effect of drought and high temperature stress on greengram ( Vigna radiata (L) Wilczek) at vegetative stage . Pharma Innov. 8 , 647–650. [ Google Scholar ]
- Jincy M., Prasad V. B. R., Senthil A., Jeyakumar P., Manivannan N. (2020). Physiological divergence in green gram [ Vigna radiata (L) wilczek] genotypes for drought and high temperature stress tolerance during flowering phase . Legume Res. 45 , 960. 10.18805/LR-4314 [ CrossRef ] [ Google Scholar ]
- Kalaji H. M., Jajoo A., Oukarroum A., Brestic M., Zivcak M., Samborska I. A., et al. (2016). Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions . Acta Physiol. Plant 38 , 102–111. 10.1007/s11738-016-2113-y [ CrossRef ] [ Google Scholar ]
- Kang Y. J., Kim S. K., Kim M. Y., Lestari P., Kim K. H., Ha B.-K., et al. (2014). Genome sequence of mungbean and insights into evolution within Vigna species . Nat. Commun . 5 , 5443. 10.1038/ncomms6443 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karim M. A., Fukamachi H., Komori S., Ogawa K., Hidaka T. (2003). Growth, yield and photosynthetic activity of Vigna radiata L. grown at different temperature and light levels . Plant Prod. Sci. 6 , 43–49. 10.1626/pps.6.43 [ CrossRef ] [ Google Scholar ]
- Kaur S., Nayyar H. (2015). Selenium fertilization to salt-stressed mungbean ( Vigna radiata L. Wilczek) plants reduces sodium uptake, improves reproductive function, pod set and seed yield . Sci. Hortic. 197 , 304–317. 10.1016/j.scienta.2015.09.048 [ CrossRef ] [ Google Scholar ]
- Kaur R., Bains T. S., Bindumadhava H., Nayyar H. (2015). Responses of mungbean ( Vigna radiata L) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits . Sci. Hortic. 197 , 527–541. 10.1016/j.scienta.2015.10.015 [ CrossRef ] [ Google Scholar ]
- Kaushal N., Awasthi R., Gupta K., Gaur P., Siddique K. H., Nayyar H. (2013). Heat-stress-induced reproductive failures in chickpea ( Cicer arietinum ) are associated with impaired sucrose metabolism in leaves and anthers . Funct. Plant Biol . 40 , 1334–1349. 10.1071/FP13082 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaushal N., Bhandari K., Siddique K. H., Nayyar H. (2016). Food crops face rising temperatures: An overview of responses, adaptive mechanisms, and approaches to improve heat tolerance . Cogent food Agric. 2 , 1134380. 10.1080/23311932.2015.1134380 [ CrossRef ] [ Google Scholar ]
- Keatinge J. D. H., Easdown W. J., Yang R. Y., Chadha M. L., Shanmugasundaram S. (2011). Overcoming chronic malnutrition in a future warming world: The key importance of mungbean and vegetable soybean . Euphytica 180 , 129–141. 10.1007/s10681-011-0401-6 [ CrossRef ] [ Google Scholar ]
- Klimenko S. B., Peshkova A. A., Dorofeev N. V. (2006). Nitrate reductase activity during heat shock in winter wheat . J. Stress Physiol . Biochem. 2 , 50–55. [ Google Scholar ]
- Königshofer H., Lechner S. (2002). Are polyamines involved in the synthesis of heat-shock proteins in cell suspension cultures of tobacco and alfalfa in response to high-temperature stress? Plant Physiol. biochem . 40 , 51–59. 10.1016/S0981-9428(01)01347-X [ CrossRef ] [ Google Scholar ]
- Kotak S., Larkindale J., Lee U., von Koskull-Döring P., Vierling E., Scharf K.-D. (2007). Complexity of the heat stress response in plants . Curr. Opin. Plant Biol . 10 , 310–316. 10.1016/j.pbi.2007.04.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar S. V., Wigge P. A. (2010). H2A.Z-Containing nucleosomes mediate the thermosensory response in arabidopsis . Cell. 140 , 136–147. 10.1016/j.cell.2009.11.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar S., Kaur R., Kaur N., Bhandhari K., Kaushal N., Gupta K., et al. (2011). Heat-stress induced inhibition in growth and chlorosis in mungbean ( Phaseolus aureus Roxb) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress . Acta Physiol. Plant. 33 , 2091–2101. 10.1007/s11738-011-0748-2 [ CrossRef ] [ Google Scholar ]
- Kumar P., Pal M., Joshi R., Sairam R. K. (2013). Yield, growth and physiological responses of mung bean [ Vigna radiata (L) Wilczek] genotypes to waterlogging at vegetative stage . Physiol. Mol. Biol. Plants. 19 , 209–220. 10.1007/s12298-012-0153-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar R. R. (2012). Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H2O2 and transcript of heat shock protein . Int. J. Plant Physiol. Biochem. 4 ( 4 ), 83–91. 10.5897/IJPPB12.008 [ CrossRef ] [ Google Scholar ]
- Kumari P., Varma S. K. (1983). Genotypic differences in flower production/shedding and yield in mungbean ( Vigna radiata ) . Acta Physiol. Plant 4 , 402–405. [ Google Scholar ]
- Landi S., Hausman J.-F., Guerriero G., Esposito S. (2017). Poaceae vs. Abiotic stress: Focus on drought and salt stress, recent insights and perspectives . Front. Plant Sci. 8 , 1214. 10.3389/fpls.2017.01214 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levine A., Tenhaken R., Dixon R., Lamb C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response . Cell. 79 , 583–593. 10.1016/0092-8674(94)90544-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li S., Zhou X., Chen L., Huang W., Yu D. (2010). Functional characterization of Arabidopsis thaliana WRKY39 in heat stress . Mol. Cells 29 , 475–483. 10.1007/s10059-010-0059-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li S., Fu Q., Chen L., Huang W., Yu D. (2011). Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance . Planta 233 , 1237–1252. 10.1007/s00425-011-1375-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li W., Chen Y., Ye M., Lu H., Wang D., Chen Q. (2020). Evolutionary history of the C-repeat binding factor/dehydration-responsive element-binding 1 (CBF/DREB1) protein family in 43 plant species and characterization of CBF/DREB1 proteins in Solanum tuberosum . BMC Evol. Biol . 20 , 142. 10.1186/s12862-020-01710-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lin C.-Y., Roberts J. K., Key J. L. (1984). Acquisition of thermotolerance in soybean seedlings: Synthesis and accumulation of heat shock proteins and their cellular localization . Plant Physiol. 74 , 152–160. 10.1104/pp.74.1.152 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu X., Huang B. (2000). Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass . Crop Sci. 40 , 503–510. 10.2135/cropsci2000.402503x [ CrossRef ] [ Google Scholar ]
- Liu Z.-W., Wu Z.-J., Li X.-H., Huang Y., Li H., Wang Y.-X., et al. (2016). Identification, classification, and expression profiles of heat shock transcription factors in tea plant ( Camellia sinensis ) under temperature stress . Gene 576 , 52–59. 10.1016/j.gene.2015.09.076 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Loreto F., Förster A., Dürr M., Csiky O., Seufert G. (1998). On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes . Plant Cell. Environ. 21 , 101–107. 10.1046/j.1365-3040.1998.00268.x [ CrossRef ] [ Google Scholar ]
- Lucas M. R., Ehlers J. D., Huynh B.-L., Diop N.-N., Roberts P. A., Close T. J. (2013). Markers for breeding heat-tolerant cowpea . Mol. Breed. 31 , 529–536. 10.1007/s11032-012-9810-z [ CrossRef ] [ Google Scholar ]
- Maavimani M., Saraswathi R. (2014). Anther characteristics and spikelet fertility in rice ( Oryza sativa L) under high temperature stress at anthesis . Indian J. Genet. Plant. Breed. 74 , 300–308. 10.5958/0975-6906.2014.00847.5 [ CrossRef ] [ Google Scholar ]
- Mandhania S., Madan S., Sawhney V. (2006). Antioxidant defense mechanism under salt stress in wheat seedlings . Biol. Plant. 50 , 227–231. 10.1007/s10535-006-0011-7 [ CrossRef ] [ Google Scholar ]
- Mansoor S., Naqvi F. N. (2013). Effect of heat stress on lipid peroxidation and antioxidant enzymes in mung bean ( Vigna radiata L) seedlings . Afr. J. Biotechnol. 12 . 10.4314/ajb.v12i21 [ CrossRef ] [ Google Scholar ]
- Mason R. E., Singh R. P. (2014). Considerations when deploying canopy temperature to select high yielding wheat breeding lines under drought and heat stress . Agronomy 4 , 191–201. 10.3390/agronomy4020191 [ CrossRef ] [ Google Scholar ]
- Mason R. E., Mondal S., Beecher F., Pacheco A., Jampala B., Ibrahim A., et al. (2010). QTL associated with heat susceptibility index in wheat ( Triticum aestivum L) under short-term reproductive stage heat stress . Euphytica 174 , 423–436. 10.1007/s10681-010-0151-x [ CrossRef ] [ Google Scholar ]
- Mason R. E., Hays D. B., Mondal S., Ibrahim A. M. H., Basnet B. R. (2013). QTL for yield, yield components and canopy temperature depression in wheat under late sown field conditions . Euphytica 194 , 243–259. 10.1007/s10681-013-0951-x [ CrossRef ] [ Google Scholar ]
- Mathur S., Agrawal D., Jajoo A. (2014). Photosynthesis: Response to high temperature stress . J. Photochem. Photobiol. B Biol. 137 , 116–126. 10.1016/j.jphotobiol.2014.01.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maulana F., Ayalew H., Anderson J. D., Kumssa T. T., Huang W., Ma X.-F. (2018). Genome-Wide association mapping of seedling heat tolerance in winter wheat . Front. Plant Sci. 9 , 1272. 10.3389/fpls.2018.01272 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller G., Suzuki N., Rizhsky L., Hegie A., Koussevitzky S., Mittler R. (2007). Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses . Plant Physiol. 144 , 1777–1785. 10.1104/pp.107.101436 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mishra D., Shekhar S., Singh D., Chakraborty S., Chakraborty N. (2018). “ Heat shock proteins and abiotic stress tolerance in plants ,” in Regulation of heat shock protein responses . Editors Asea A., Kaur P. (Cham: Springer; ) 13 . 10.1007/978-3-319-74715-6_3 [ CrossRef ] [ Google Scholar ]
- Mittler R., Finka A., Goloubinoff P. (2012). How do plants feel the heat? Trends biochem. Sci. 37 , 118–125. 10.1016/j.tibs.2011.11.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mohammed A. R., Tarpley L. (2010). Effects of high night temperature and spikelet position on yield-related parameters of rice ( Oryza sativa L) plants . Eur. J. Agron. 33 , 117–123. 10.1016/j.eja.2009.11.006 [ CrossRef ] [ Google Scholar ]
- Monterroso V. A., Wien H. C. (1990). Flower and pod abscission due to heat stress in beans . J. Am. Soc. Hortic. Sci. 115 , 631–634. 10.21273/jashs.115.4.631 [ CrossRef ] [ Google Scholar ]
- Nair R. M., Pandey A. K., War A. R., Hanumantharao B., Shwe T., Alam A., et al. (2019). Biotic and abiotic constraints in mungbean production-progress in genetic improvement . Front. Plant Sci. 10 , 1340. 10.3389/fpls.2019.01340 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakamoto H., Hiyama T. (1999). “ Heat-shock proteins and temperature stress ,” in Handbook of plant and crop stress (New York: Marcel Dekker; ), 399–416. [ Google Scholar ]
- Nawaz A., Farooq M., Cheema S. A., Wahid A. (2013). Differential response of wheat cultivars to terminal heat stress . Int. J. Agric. Biol. 15 . [ Google Scholar ]
- Neumann D., Emmermann M., Thierfelder J.-M., Zur Nieden U., Clericus M., Braun H.-P., et al. (1993). HSP68-a DnaK-like heat-stress protein of plant mitochondria . Planta 190 , 32–43. 10.1007/BF00195672 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Njuki J., Benin S., Marivoet W., Ulimwengu J. M., Mwongera C., Breisinger C., et al. (2022). “ Regional developments [in 2022 global food policy report] ,” in IFPRI book chapters , 114–145. [ Google Scholar ]
- Ottaviano E., Sari Gorla M., Pe E., Frova C. (1991). Molecular markers (RFLPs and HSPs) for the genetic dissection of thermotolerance in maize . Theor. Appl. Genet. 81 , 713–719. 10.1007/BF00224979 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Parihar A. K., Kumar A., Dixit G. P., Gupta S. (2017). Seasonal effects on outbreak of yellow mosaic disease in released cultivars of mungbean ( Vigna radiata ) and urdbean ( Vigna mungo ) . Indian J. Agric. Sci. 87 , 734–738. 10.56093/ijas.v87i6.70938 [ CrossRef ] [ Google Scholar ]
- Patriyawaty N. R., Rachaputi R. C., George D. (2018). Physiological mechanisms underpinning tolerance to high temperature stress during reproductive phase in mungbean ( Vigna radiata (L) Wilczek) . Environ. Exp. Bot. 150 , 188–197. 10.1016/j.envexpbot.2018.03.022 [ CrossRef ] [ Google Scholar ]
- Paul S., Das M. K., Baishya P., Ramteke A., Farooq M., Baroowa B., et al. (2017). Effect of high temperature on yield associated parameters and vascular bundle development in five potato cultivars . Sci. Hortic. 225 , 134–140. 10.1016/j.scienta.2017.06.061 [ CrossRef ] [ Google Scholar ]
- Paul P. J., Samineni S., Thudi M., Sajja S. B., Rathore A., Das R. R., et al. (2018). Molecular mapping of QTLs for heat tolerance in chickpea . Int. J. Mol. Sci. 19 , 2166. 10.3390/ijms19082166 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Peleg Z., Blumwald E. (2011). Hormone balance and abiotic stress tolerance in crop plants . Curr. Opin. Plant Biol. 14 , 290–295. 10.1016/j.pbi.2011.02.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pincus D., Chevalier M. W., Aragón T., van Anken E., Vidal S. E., El-Samad H., et al. (2010). BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response . PLoS Biol. 8 , e1000415. 10.1371/journal.pbio.1000415 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pinto R. S., Lopes M. S., Collins N. C., Reynolds M. P. (2016). Modelling and genetic dissection of staygreen under heat stress . Theor. Appl. Genet. 129 , 2055–2074. 10.1007/s00122-016-2757-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Polle A. (1997). Defense against photooxidative damage in plants . Cold Spring Harb. Monogr. Ser. 34 , 623–666. [ Google Scholar ]
- Pottorff M., Roberts P. A., Close T. J., Lonardi S., Wanamaker S., Ehlers J. D. (2014). Identification of candidate genes and molecular markers for heat-induced Brown discoloration of seed coats in cowpea [ Vigna unguiculata (L) Walp] . BMC genomics 15 , 328. 10.1186/1471-2164-15-328 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Prasad P. V. V., Djanaguiraman M., Prasad P. V. V., Djanaguiraman M. (2011). High night temperature decreases leaf photosynthesis and pollen function in grain sorghum . Funct. Plant Biol. 38 , 993–1003. 10.1071/FP11035 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Priya M., Pratap A., Sengupta D., Singh N. P., Jha U. C., Siddique K., et al. (2020). “ Mungbean and high temperature stress: Responses and strategies to improve heat tolerance ,” in Heat stress in food grain crops: Plant breeding and omics research . Editors Jha U. C., Nayyar H., Gupta S. (Singapore: Bentham Science Publishers; ), 144–170. [ Google Scholar ]
- Punia S., Punia M., Yadav R. (2019). Drought and heat tolerance in mungbean . Proceedings of national conference on harmony with nature in context of resource conservation and climate change (HARMONY - 2016) , 22–24. [ Google Scholar ]
- Rainey K. M., Griffiths P. D. (2005). Differential response of common bean genotypes to high temperature . J. Am. Soc. Hortic. Sci. 130 , 18–23. 10.21273/jashs.130.1.18 [ CrossRef ] [ Google Scholar ]
- Ramani H. R., Mandavia M. K., Dave R. A., Bambharolia R. P., Silungwe H., Garaniya N. H. (2017). Biochemical and physiological constituents and their correlation in wheat ( Triticum aestivum L) genotypes under high temperature at different development stages . Int. J. Plant Physiol. Biochem. 9 , 1–8. 10.5897/ijppb2015.0240 [ CrossRef ] [ Google Scholar ]
- Rasheed R. (2009). Salinity and extreme temperature effects on sprouting buds of sugarcane ( Saccharum officinarum L): Some histological and biochemical studies . PhD Thesis. Faisalabad Pakistan: University of Agriculture. [ Google Scholar ]
- Reardon M. E., Qaderi M. M. (2017). Individual and interactive effects of temperature, carbon dioxide and abscisic acid on mung bean ( Vigna radiata ) plants . J. Plant. Interact. 12 , 295–303. 10.1080/17429145.2017.1353654 [ CrossRef ] [ Google Scholar ]
- Rivero R. M., Ruiz J. M., Romero L. (2004). Oxidative metabolism in tomato plants subjected to heat stress . J. Hortic. Sci. Biotechnol. 79 , 560–564. 10.1080/14620316.2004.11511805 [ CrossRef ] [ Google Scholar ]
- Rodríguez M., Canales E., Borrás-Hidalgo O. (2005). Molecular aspects of abiotic stress in plants . Biotecnol. Apl. 22 , 1–10. [ Google Scholar ]
- Rodriguez R. J., Henson J., Van Volkenburgh E., Hoy M., Wright L., Beckwith F., et al. (2008). Stress tolerance in plants via habitat-adapted symbiosis . ISME J. 2 , 404–416. 10.1038/ismej.2007.106 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sairam R. K., Tyagi A. (2004). Physiology and molecular biology of salinity stress tolerance in plants . Curr. Sci. 86 , 407–421. [ Google Scholar ]
- Sakamoto A., Murata N. (2002). The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants . Plant, Cell. and Environ. 25 , 163–171. 10.1046/j.0016-8025.2001.00790.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sakata T., Higashitani A. (2008). Male sterility accompanied with abnormal anther development in plants-genes and environmental stresses with special reference to high temperature injury . Int. J. Plant Dev. Biol. 2 , 42–51. [ Google Scholar ]
- Samakovli D., Tichá T., Vavrdová T., Ovečka M., Luptovčiak I., Zapletalová V., et al. (2020). YODA-HSP90 module regulates phosphorylation-dependent inactivation of SPEECHLESS to control stomatal development under acute heat stress in Arabidopsis . Mol. Plant. 13 , 612–633. 10.1016/j.molp.2020.01.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sangwan V., Örvar B. L., Beyerly J., Hirt H., Dhindsa R. S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways . Plant J. 31 , 629–638. 10.1046/j.1365-313x.2002.01384.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sangwan S., Munjal R., Ram K., Kumar N. (2019). QTL mapping for morphological and physiological traits in RILs of spring wheat population of WH1021 × WH711 . J. Environ. Biol. 40 , 674–682. 10.22438/jeb/40/4/mrn-1002 [ CrossRef ] [ Google Scholar ]
- Sarieva G. E., Kenzhebaeva S. S., Lichtenthaler H. K. (2010). Adaptation potential of photosynthesis in wheat cultivars with a capability of leaf rolling under high temperature conditions . Russ. J. Plant Physiol. 57 , 28–36. 10.1134/S1021443710010048 [ CrossRef ] [ Google Scholar ]
- Scandalios J. G., Guan L., Polidoros A. N. (1997). Catalases in plants: Gene structure, properties, regulation, and expression . Cold Spring Harb. Protoc. 34 , 343–406. [ Google Scholar ]
- Schoffl F., Prandl R., Reindl A. (1998). Regulation of the heat-shock response . Plant Physiol. 117 , 1135–1141. 10.1104/pp.117.4.1135 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schreinemachers P., Sequeros T., Rani S., Rashid M. A., Gowdru N. V., Rahman M. S., et al. (2019). Counting the beans: Quantifying the adoption of improved mungbean varieties in South Asia and Myanmar . Food Secur. 11 , 623–634. 10.1007/s12571-019-00926-x [ CrossRef ] [ Google Scholar ]
- Sehgal A., Sita K., Nayyar H. (2016). Heat stress in plants: Sensing and defense mechanisms . J. Plant. Sci. Res. 32 , 195. [ Google Scholar ]
- Sekhar S., Kumar J., Mohanty S., Mohanty N., Panda R. S., Das S., et al. (2021). Identification of novel QTLs for grain fertility and associated traits to decipher poor grain filling of basal spikelets in dense panicle rice . Sci. Rep. 11 ( 1 ), 13617. 10.1038/s41598-021-93134-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shamsudin N. A. A., Swamy B. P. M., Ratnam W., StaCruz Ma. T., Raman A., Kumar A. (2016). Marker assisted pyramiding of drought yield QTLs into a popular Malaysian rice cultivar, MR219 . BMC Genet. 17 , 30. 10.1186/s12863-016-0334-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma D. K., Fernández J. O., Rosenqvist E., Ottosen C.-O., Andersen S. B. (2014). Genotypic response of detached leaves versus intact plants for chlorophyll fluorescence parameters under high temperature stress in wheat . J. Plant Physiol. 171 , 576–586. 10.1016/j.jplph.2013.09.025 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma L., Priya M., Bindumadhava H., Nair R. M., Nayyar H. (2016). Influence of high temperature stress on growth, phenology and yield performance of mungbean [ Vigna radiata (L) Wilczek] under managed growth conditions . Sci. Hortic. 213 , 379–391. 10.1016/j.scienta.2016.10.033 [ CrossRef ] [ Google Scholar ]
- Shen H. F., Zhao B., Xu J. J., Liang W., Huang W. M., Li H. H. (2017). Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron . S. Afr. J. Bot. 112 , 338–345. 10.1016/j.sajb.2017.06.018 [ CrossRef ] [ Google Scholar ]
- Sheoran S., Gupta M., Kumari S., Kumar S., Rakshit S. (2022). Meta-QTL analysis and candidate genes identification for various abiotic stresses in maize ( Zea mays L) and their implications in breeding programs . Mol. Breed. 42 , 26. 10.1007/s11032-022-01294-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siddiqui M. H., Al-Khaishany M. Y., Al-Qutami M. A., Al-Whaibi M. H., Grover A., Ali H. M., et al. (2015). Morphological and physiological characterization of different genotypes of faba bean under heat stress . Saudi J. Biol. Sci. 22 , 656–663. 10.1016/j.sjbs.2015.06.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simões-Araújo J. L., Rodrigues R. L., de A., Gerhardt L. B., Mondego J. M. C., Alves-Ferreira M., et al. (2002). Identification of differentially expressed genes by cDNA-AFLP technique during heat stress in cowpea nodules . FEBS Lett. 515 , 44–50. 10.1016/S0014-5793(02)02416-X [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simões-Araújo J. L., Rumjanek N. G., Margis-Pinheiro M. (2003). Small heat shock proteins genes are differentially expressed in distinct varieties of common bean . Braz. J. Plant Physiol. 15 , 33–41. 10.1590/S1677-04202003000100005 [ CrossRef ] [ Google Scholar ]
- Singh D. P., Singh B. B., Pratap A. (2016). Genetic improvement of mungbean and urdbean and their role in enhancing pulse production in India . Indian J. Genet. Plant. Breed. 76 , 550–567. 10.5958/0975-6906.2016.00072.9 [ CrossRef ] [ Google Scholar ]
- Singh H., Kaur P., Bal S. K., Choudhury B. U. (2021). Effect of elevated temperature on green gram [ Vigna radiata (L) Wilczek] performance under temperature gradient tunnel (TGT) environment in Punjab . J. Agric. Meteorol. 23 , 3–13. 10.54386/jam.v23i1.82 [ CrossRef ] [ Google Scholar ]
- Singh C. M., Kumar M., Pratap A., Tripathi A., Singh S., Mishra A., et al. (2022). Genome-Wide analysis of late embryogenesis abundant protein gene family in vigna species and expression of VrLEA encoding genes in Vigna glabrescens reveal its role in heat tolerance . Front. Plant Sci. 13 , 843107. 10.3389/fpls.2022.843107 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sinsawat V., Leipner J., Stamp P., Fracheboud Y. (2004). Effect of heat stress on the photosynthetic apparatus in maize ( Zea mays L) grown at control or high temperature . Environ. Exp. Bot. 52 , 123–129. 10.1016/j.envexpbot.2004.01.010 [ CrossRef ] [ Google Scholar ]
- Sita K., Sehgal A., HanumanthaRao B., Nair R. M., Vara Prasad P. V., Kumar S., et al. (2017). Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance . Front. Plant Sci. 8 , 1658. 10.3389/fpls.2017.01658 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Solai M., Vijayalakshmi C., Basu P. S. (2015). Effect of high temperature on flowering pattern, pollen germination and pod setting in green gram ( Vigna radiata (L) Wilczek) . Int. J. Plant Biol. 7 , 59–66. [ Google Scholar ]
- Song L., Ding W., Zhao M., Sun B., Zhang L. (2006). Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed . Plant Sci. 171 , 449–458. 10.1016/j.plantsci.2006.05.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Song J., Jiao L. F., Xiao K., Luan Z. S., Hu C. H., Shi B., et al. (2013). Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers . Anim. Feed Sci. Technol. 185 , 175–181. 10.1016/j.anifeedsci.2013.08.001 [ CrossRef ] [ Google Scholar ]
- Spiertz J. H. J., Hamer R. J., Xu H., Primo-Martin C., Don C., van der Putten P. E. L. (2006). Heat stress in wheat ( Triticum aestivum L): Effects on grain growth and quality traits , modelling quality traits and their genetic variability for wheat . Eur. J. Agron. 25 , 89–95. 10.1016/j.eja.2006.04.012 [ CrossRef ] [ Google Scholar ]
- Srivastava S., Pathak A. D., Gupta P. S., Shrivastava A. K., Srivastava A. K. (2012). Hydrogen peroxide-scavenging enzymes impart tolerance to high temperature induced oxidative stress in sugarcane . J. Environ. Biol. 33 ( 3 ), 657–661. [ PubMed ] [ Google Scholar ]
- Stefanov D., Petkova V., Denev I. D. (2011). Screening for heat tolerance in common bean ( Phaseolus vulgaris L) lines and cultivars using JIP-test . Sci. Hortic. 128 , 1–6. 10.1016/j.scienta.2010.12.003 [ CrossRef ] [ Google Scholar ]
- Sugio A., Dreos R., Aparicio F., Maule A. J. (2009). The cytosolic protein response as a subcomponent of the wider heat shock response in arabidopsis . Plant Cell. 21 , 642–654. 10.1105/tpc.108.062596 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Suzuki N., Miller G., Morales J., Shulaev V., Torres M. A., Mittler R. (2011). Respiratory burst oxidases: The engines of ROS signaling . Curr. Opin. Plant Biol. 14 , 691–699. 10.1016/j.pbi.2011.07.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Suzuki N., Koussevitzky S., Mittler R., Miller G. (2012). ROS and redox signalling in the response of plants to abiotic stress . Plant Cell. Environ. 35 , 259–270. 10.1111/j.1365-3040.2011.02336.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taiz L., Zeiger E. (2010). Plant physiol . Sunderland, MA: Sinauer Associates Inc. [ Google Scholar ]
- Taiz L., Zeiger E., Møller I. M., Murphy A. (2015). Plant physiol. and develop . Sunderland, USA: Sinauer Associates Incorporated. [ Google Scholar ]
- Tamindžić G., Ignjatov M., Miljaković D., Červenski J., Milošević D., Nikolić Z., et al. (2023). Seed priming treatments to improve heat stress tolerance of garden pea ( Pisum sativum L) . Agriculture 13 ( 2 ), 439. 10.3390/agriculture13020439 [ CrossRef ] [ Google Scholar ]
- Tangphatsornruang S., Somta P., Uthaipaisanwong P., Chanprasert J., Sangsrakru D., Seehalak W., et al. (2009). Characterization of microsatellites and gene contents from genome shotgun sequences of mungbean ( Vigna radiata (L) Wilczek ) . BMC Plant Biol. 9 , 137. 10.1186/1471-2229-9-137 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thomas H., Ougham H. (2014). The stay-green trait . J. Exp. Bot. 65 , 3889–3900. 10.1093/jxb/eru037 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tian Y., Chen J., Chen C., Deng A., Song Z., Zheng C., et al. (2012). Warming impacts on winter wheat phenophase and grain yield under field conditions in Yangtze Delta Plain, China . Field Crops Res. 134 , 193–199. 10.1016/j.fcr.2012.05.013 [ CrossRef ] [ Google Scholar ]
- Tiwari M., Kumar R., Min D., Jagadish S. V. K. (2022). Genetic and molecular mechanisms underlying root architecture and function under heat stress—a hidden story . Plant Cell. Environ. 45 , 771–788. 10.1111/pce.14266 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tzudir L., Bera P. S., Chakraborty P. K. (2014). Impact of temperature on the reproductive development in mungbean ( Vigna radiata ) varieties under different dates of sowing . Int. J. Bio-resour. Stress Manag. 5 , 194. 10.5958/0976-4038.2014.00555.7 [ CrossRef ] [ Google Scholar ]
- Valliyodan B., Nguyen H. T. (2006). Understanding regulatory networks and engineering for enhanced drought tolerance in plants . Curr. Opin. 9 , 189–195. 10.1016/j.pbi.2006.01.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vargas Y., Mayor-Duran V. M., Buendia H. F., Ruiz-Guzman H., Raatz B. (2021). Physiological and genetic characterization of heat stress effects in a common bean RIL population . PLOS ONE 16 , e0249859. 10.1371/journal.pone.0249859 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vierling E. (1991). The roles of heat shock proteins in plants . Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 , 579–620. 10.1146/annurev.pp.42.060191.003051 [ CrossRef ] [ Google Scholar ]
- Vollenweider P., Günthardt-Goerg M. S. (2005). Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage . Environ. Pollut. 137 , 455–465. 10.1016/j.envpol.2005.01.032 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wahid A., Gelani S., Ashraf M., Foolad M. R. (2007). Heat tolerance in plants: An overview . Environ. Exp. Bot. 61 , 199–223. 10.1016/j.envexpbot.2007.05.011 [ CrossRef ] [ Google Scholar ]
- Wang W., Vinocur B., Shoseyov O., Altman A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response . Trends Plant Sci. 9 , 244–252. 10.1016/j.tplants.2004.03.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang J., Wu B., Yin H., Fan Z., Li X., Ni S., et al. (2017). Overexpression of CaAPX induces orchestrated reactive oxygen scavenging and enhances cold and heat tolerances in tobacco . Bio. Med. Res. Int. 2017 , e4049534. 10.1155/2017/4049534 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Y., Reiter R. J., Chan Z. (2018). Phytomelatonin: A universal abiotic stress regulator . J. Exp. Bot. 69 , 963–974. 10.1093/jxb/erx473 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Y., Yu Y., Huang M., Gao P., Chen H., Liu M., et al. (2020). Transcriptomic and proteomic profiles of II YOU 838 ( Oryza sativa ) provide insights into heat stress tolerance in hybrid rice . PeerJ 8 , e8306. 10.7717/peerj.8306 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Q., Lu X., Chen X., Zhao L., Han M., Wang S., et al. (2021). Genome-wide identification and function analysis of HMAD gene family in cotton ( Gossypium spp ) . BMC Plant Biol. 21 , 386. 10.1186/s12870-021-03170-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waraich E. A., Ahmad R., Halim A., Aziz T. (2012). Alleviation of temperature stress by nutrient management in crop plants: A review . J. Soil Sci. Plant Nutr. 12 , 221–244. 10.4067/S0718-95162012000200003 [ CrossRef ] [ Google Scholar ]
- Waseem M., Ali A., Tahir M., Nadeem M. A., Ayub M., Tanveer A., et al. (2011). Mechanism of drought tolerance in plant and its management through different methods . C. J. Agric. Sci. 5 , 10–25. [ Google Scholar ]
- Willekens H., Inzé D., Van Montagu M., van Camp W. (1995). Catalases in plants . Mol. Breed. 1 , 207–228. 10.1007/BF02277422 [ CrossRef ] [ Google Scholar ]
- Xia X.-J., Zhou Y.-H., Shi K., Zhou J., Foyer C. H., Yu J.-Q. (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance . J. Exp. Bot. 66 , 2839–2856. 10.1093/jxb/erv089 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang J., Sears R. G., Gill B. S., Paulsen G. M. (2002). Quantitative and molecular characterization of heat tolerance in hexaploid wheat . Euphytica 126 , 275–282. 10.1023/A:1016350509689 [ CrossRef ] [ Google Scholar ]
- Yang J.-D., Yun J.-Y., Zhang T.-H., Zhao H.-L. (2006). Presoaking with nitric oxide donor SNP alleviates heat shock damages in mung bean leaf discs . Bot. Stud. 47 , 129–136. [ Google Scholar ]
- Young N. D., Udvardi M. (2009). Translating Medicago truncatula genomics to crop legumes . Curr. Opin. Plant Biol. 12 , 193–201. 10.1016/j.pbi.2008.11.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan Z.-C., Haudecoeur E., Faure D., Kerr K. F., Nester E. W. (2008). Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signal salicylic acid, indole-3-acetic acid and γ-amino butyric acid reveals signaling cross-talk and Agrobacterium-plant co-evolution . Cell. Microbiol. 10 , 2339–2354. 10.1111/j.1462-5822.2008.01215.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zandalinas S. I., Balfagón D., Arbona V., Gómez-Cadenas A. (2017). Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus . Front. Plant Sci. 8 , 953. 10.3389/fpls.2017.00953 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang J.-H., Huang W.-D., Liu Y.-P., Pan Q.-H. (2005). Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants ( Vitis vinifera L. Cv . Jingxiu) under cross-temperature stresses . J. Integr. Plant Biol. 47 , 959–970. 10.1111/j.1744-7909.2005.00109.x [ CrossRef ] [ Google Scholar ]
- Zhang X., Li J., Liu A., Zou J., Zhou X., Xiang J., et al. (2012). Expression profile in rice panicle: Insights into heat response mechanism at reproductive stage . PLOS ONE 7 , e49652. 10.1371/journal.pone.0049652 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zheng H., Wang W., Xu K., Xu Y., Ji D., Chen C., et al. (2020). Ca 2+ influences heat shock signal transduction in Pyropia haitanensis . Aquaculture 516 , 734618. 10.1016/j.aquaculture.2019.734618 [ CrossRef ] [ Google Scholar ]
- Zhou J., Wang J., Yu J.-Q., Chen Z. (2014). Role and regulation of autophagy in heat stress responses of tomato plants . Front. Plant Sci. 5 , 174. 10.3389/fpls.2014.00174 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zinn K. E., Tunc-Ozdemir M., Harper J. F. (2010). Temperature stress and plant sexual reproduction: Uncovering the weakest links . J. Exp. Bot. 61 , 1959–1968. 10.1093/jxb/erq053 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Heat stress is a global issue that crosses socioeconomic status. Heat stress leads to reduced worker capacity on seasonal scales, and weekly to sub-daily timescales, incapacitation, morbidity, and mortality. This dissertation focuses on 2 distinct parts: quantification methods of heat stress, and heat stress applications. Quantification methods of heat stress Chapters 1-3 focus on historical ...
Each QTL explains approximately 4.5-19.3% phenotypic variance, and a combination of the superior haplotype of three QTLs on chromosomes 1B, 5A, and 6D can improve the genetic effect of heat tolerance compared with a single locus (Mason et al. 2011). Pinto et al. (2010) also identified 16 QTLs associated with heat stress adaptive traits using ...
Thermal indices and occupational heat stress: a systematic review and meta-analysis. In: Effects of Heat on Behavioral and Physiological Mechanisms of the Human Thermoregulatory System during Rest, Exercise, and Work (PhD Thesis). Thessaly, Greece: University of Greece, chapt 6, 2020. Google Scholar; 58.
e heat is on Doctoral Dissertation Series 2018:61 9 789177 535379 ISBN 978-91-7753-537-9 ... ISRN LUTMDN/TMAT-1028-SE The heat is on Evaluation of workplace heat stress under a changing climate KARIN LUNDGREN KOWNACKI ERGONOMICS AND AEROSOL TECHNOLOGY | DESIGN SCIENCES LUND UNIVERSITY ... My PhD research has been a journey of a lifeti me ...
The introduction and use of heat stress indices and models to predict and quantify heat stress and heat strain has helped to reduce morbidity and mortality in industrial, military, sports, and leisure activities dramatically. Models used range from simple instruments that attempt to mimic the human-environment heat exchange to complex ...
Abstract and Figures. Wheat production requires at least ~ 2.4% increase per year rate by 2050 globally to meet food demands. However, heat stress results in serious yield loss of wheat worldwide ...
The overall effects of heat stress on morphological parameters of wheat are illustrated in Fig. 1. The temperature range for wheat seed germination is from 4 to 37 °C, where 12 °C to 25 °C is the optimal temperature range, however high temperature up to 40 °C adversely affects photosynthesis (Spilde 1989 ).
These data 1349 indicate that the reduction in V ̇ O2max under heat stress is associated with a rise in whole-body temperature, 1350 rather than the prevailing ambient conditions per se. Red ...
2. Plant Response to Heat Stress. Plant responses to HT vary with the degree of temperature, duration and plant type. At extreme HT, cellular damage or cell death may occur within minutes, which may lead to a catastrophic collapse of cellular organization [].Heat stress affects all aspects of plant processes like germination, growth, development, reproduction and yield [5,29-31].
Drought and heat stress are potential problems that can reduce wheat yield, particularly during the terminal growth stages in arid and semiarid regions of the. world. The current study intended to ...
neering courses in the areas of thermal stresses, directed MS and PhD thesis and dissertation research projects, including studies of thermal stresses in the thermal shroud of a space environmental simulation chamber, and conducted continuing education courses involving thermal stress applications for practicing engineers for more than 3 decades.
Heat interference 'often' was reported (in order of frequency) by 37.5% for daily travel, 34.5% for work, 29.9% for housework, 27.4% for sleeping and 25.9% for exercise. Health and well-being frequency outcomes are reported in table 3: 37.6% reported being very satisfied with their life, around 15% reported having very much energy in the ...
Graduate Theses, Dissertations, and Problem Reports 2020. Assessment of Heat Stress for Outdoor Work Conditions in Saudi Arabia. JAMAL ALANAZI. [email protected] Follow this and additional works at: https://researchrepository.wvu.edu/etd Part of the Ergonomics Commons, and the Industrial Engineering Commons. Recommended Citation.
Thermal stress is a major abiotic stress in wheat and is highly complex in mechanism. A large area in northwestern plain zones (NWPZ), which is the wheat bowl of India is affected by heat stress. Climate change also causes an abrupt increase in temperature at different growth stages of wheat. Thus, wiser selection of stress tolerant varieties is an important strategy to combat the climate ...
IntroductionHeat stress caused by high temperatures has important adverse effects on the safety and health status of humans and animals, and dietary interventions to alleviate heat stress in daily life are highly feasible.MethodsIn this study, the components of mung bean that have heat stress-regulating effects were characterized by in vitro antioxidant indicators and heat stress cell models ...
Genetic basis in response to heat stress in wheat. Heat stress tolerance is a quantitative trait contributed by many minor QTLs (Bohnert et al. 2006), and it is more difficult to measure phenotypic variation in response to heat stress compared with other agronomic traits.Therefore, there is very limited available information about the genetic basis of heat stress response in wheat, and none ...
Heat Stress (HS) causes substantial crop loss worldwide. The average global temperature is constantly increasing, and this change is expected to have deleterious effects on crop yield. ... FR was supported by MUR PRIN2017 (PRIN 2017ZBBYNC) and GO-A was supported by a PhD fellowship from the University of Milan. Conflict of interest.
3.2 The proportion of four protein fractions. The relative distribution of four protein fractions, that is, albumins, globulins, glutelins and prolamins in seeds of different genotypes, is given in Figures 2-5.The relative proportion of albumins and globulins was shown to decrease while glutelin and prolamins increase at high temperatures or under heat stress.
Molecular Studies on Bread Wheat (Triticum aestivum L.) for Drought Stress Tolerance. December 2019. Thesis for: PhD Thesis. Advisor: Prof. Hamdy Emara, Prof Ahmed Nower, Prof, Khaled Salem and ...
Revisiting summer infertility in the boar: impact of heat stress on the quality and DNA integrity of spermatozoa, and its mitigation by antioxidant therapy Thesis submitted by Santiago T. Peña Jr. DVM, MTVSc In January 2018 for the degree of Doctor of Philosophy College of Public Health, Medical and Veterinary Sciences James Cook University
Recurring heat and drought episodes present challenges to the sustainability of grape production worldwide. We investigated the impacts of heat and drought stress on transcriptomic and metabolic ...
2 Impacts of heat stress in mungbean. Mungbean is a summer season crop that may be cultivated in all dry and semi-arid parts of the world. However, recent global average temperature rise has posed a threat for the mungbean crop production (Abd El Lateff et al., 2018).The constant higher atmospheric temperature for longer duration is highly detrimental for the growth and physiological functions ...
The yield loss assessment over the control experiment had shown more significant loss under combined stress (55.96%), followed by drought (41.11%) and least affected by heat alone (4.77%). PCA ...