Literature Reviews
- Introduction
- Tutorials and resources
- Step 1: Literature search
- Step 2: Analysis, synthesis, critique
- Step 3: Writing the review
If you need any assistance, please contact the library staff at the Georgia Tech Library Help website .

Analysis, synthesis, critique
Literature reviews build a story. You are telling the story about what you are researching. Therefore, a literature review is a handy way to show that you know what you are talking about. To do this, here are a few important skills you will need.
Skill #1: Analysis
Analysis means that you have carefully read a wide range of the literature on your topic and have understood the main themes, and identified how the literature relates to your own topic. Carefully read and analyze the articles you find in your search, and take notes. Notice the main point of the article, the methodologies used, what conclusions are reached, and what the main themes are. Most bibliographic management tools have capability to keep notes on each article you find, tag them with keywords, and organize into groups.
Skill #2: Synthesis
After you’ve read the literature, you will start to see some themes and categories emerge, some research trends to emerge, to see where scholars agree or disagree, and how works in your chosen field or discipline are related. One way to keep track of this is by using a Synthesis Matrix .
Skill #3: Critique
As you are writing your literature review, you will want to apply a critical eye to the literature you have evaluated and synthesized. Consider the strong arguments you will make contrasted with the potential gaps in previous research. The words that you choose to report your critiques of the literature will be non-neutral. For instance, using a word like “attempted” suggests that a researcher tried something but was not successful. For example:
There were some attempts by Smith (2012) and Jones (2013) to integrate a new methodology in this process.
On the other hand, using a word like “proved” or a phrase like “produced results” evokes a more positive argument. For example:
The new methodologies employed by Blake (2014) produced results that provided further evidence of X.
In your critique, you can point out where you believe there is room for more coverage in a topic, or further exploration in in a sub-topic.
Need more help?
If you are looking for more detailed guidance about writing your dissertation, please contact the folks in the Georgia Tech Communication Center .
- << Previous: Step 1: Literature search
- Next: Step 3: Writing the review >>
- Last Updated: Apr 2, 2024 11:21 AM
- URL: https://libguides.library.gatech.edu/litreview

- Research Process
Systematic Literature Review or Literature Review?
- 3 minute read
- 44.4K views
Table of Contents
As a researcher, you may be required to conduct a literature review. But what kind of review do you need to complete? Is it a systematic literature review or a standard literature review? In this article, we’ll outline the purpose of a systematic literature review, the difference between literature review and systematic review, and other important aspects of systematic literature reviews.
What is a Systematic Literature Review?
The purpose of systematic literature reviews is simple. Essentially, it is to provide a high-level of a particular research question. This question, in and of itself, is highly focused to match the review of the literature related to the topic at hand. For example, a focused question related to medical or clinical outcomes.
The components of a systematic literature review are quite different from the standard literature review research theses that most of us are used to (more on this below). And because of the specificity of the research question, typically a systematic literature review involves more than one primary author. There’s more work related to a systematic literature review, so it makes sense to divide the work among two or three (or even more) researchers.
Your systematic literature review will follow very clear and defined protocols that are decided on prior to any review. This involves extensive planning, and a deliberately designed search strategy that is in tune with the specific research question. Every aspect of a systematic literature review, including the research protocols, which databases are used, and dates of each search, must be transparent so that other researchers can be assured that the systematic literature review is comprehensive and focused.
Most systematic literature reviews originated in the world of medicine science. Now, they also include any evidence-based research questions. In addition to the focus and transparency of these types of reviews, additional aspects of a quality systematic literature review includes:
- Clear and concise review and summary
- Comprehensive coverage of the topic
- Accessibility and equality of the research reviewed
Systematic Review vs Literature Review
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper. That is, it includes an introduction, description of the methods used, a discussion and conclusion, as well as a reference list or bibliography.
A systematic review, however, includes entirely different components that reflect the specificity of its research question, and the requirement for transparency and inclusion. For instance, the systematic review will include:
- Eligibility criteria for included research
- A description of the systematic research search strategy
- An assessment of the validity of reviewed research
- Interpretations of the results of research included in the review
As you can see, contrary to the general overview or summary of a topic, the systematic literature review includes much more detail and work to compile than a standard literature review. Indeed, it can take years to conduct and write a systematic literature review. But the information that practitioners and other researchers can glean from a systematic literature review is, by its very nature, exceptionally valuable.
This is not to diminish the value of the standard literature review. The importance of literature reviews in research writing is discussed in this article . It’s just that the two types of research reviews answer different questions, and, therefore, have different purposes and roles in the world of research and evidence-based writing.
Systematic Literature Review vs Meta Analysis
It would be understandable to think that a systematic literature review is similar to a meta analysis. But, whereas a systematic review can include several research studies to answer a specific question, typically a meta analysis includes a comparison of different studies to suss out any inconsistencies or discrepancies. For more about this topic, check out Systematic Review VS Meta-Analysis article.
Language Editing Plus
With Elsevier’s Language Editing Plus services , you can relax with our complete language review of your systematic literature review or literature review, or any other type of manuscript or scientific presentation. Our editors are PhD or PhD candidates, who are native-English speakers. Language Editing Plus includes checking the logic and flow of your manuscript, reference checks, formatting in accordance to your chosen journal and even a custom cover letter. Our most comprehensive editing package, Language Editing Plus also includes any English-editing needs for up to 180 days.

- Publication Recognition
How to Make a PowerPoint Presentation of Your Research Paper

- Manuscript Preparation
What is and How to Write a Good Hypothesis in Research?
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
- University Libraries
- Research Guides
- Reviewing Research: Literature Reviews, Scoping Reviews, Systematic Reviews
- Differentiating the Three Review Types
Reviewing Research: Literature Reviews, Scoping Reviews, Systematic Reviews: Differentiating the Three Review Types
- Framework, Protocol, and Writing Steps
- Working with Keywords/Subject Headings
- Citing Research
The Differences in the Review Types
Grant, M.J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. H ealth Information & Libraries Journal , 26: 91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x The objective of this study is to provide descriptive insight into the most common types of reviews, with illustrative examples from health and health information domains.
- What Type of Review is Right for you (Cornell University)
Literature Reviews
Literature Review: it is a product and a process.
As a product , it is a carefully written examination, interpretation, evaluation, and synthesis of the published literature related to your topic. It focuses on what is known about your topic and what methodologies, models, theories, and concepts have been applied to it by others.
The process is what is involved in conducting a review of the literature.
- It is ongoing
- It is iterative (repetitive)
- It involves searching for and finding relevant literature.
- It includes keeping track of your references and preparing and formatting them for the bibliography of your thesis
- Literature Reviews (University of North Carolina at Chapel Hill) This handout will explain what literature reviews are and offer insights into the form and construction of literature reviews in the humanities, social sciences, and sciences.
Scoping Reviews
Scoping reviews are a " preliminary assessment of potential size and scope of available research literature . Aims to identify nature and extent of research evidence (usually including ongoing research)." Grant and Booth (2009).
Scoping reviews are not mapping reviews: Scoping reviews are more topic based and mapping reviews are more question based.
- examining emerging evidence when specific questions are unclear - clarify definitions and conceptual boundaries
- identify and map the available evidence
- a scoping review is done prior to a systematic review
- to summarize and disseminate research findings in the research literature
- identify gaps with the intention of resolution by future publications
- Scoping review timeframe and limitations (Touro College of Pharmacy
Systematic Reviews
Many evidence-based disciplines use ‘systematic reviews," this type of review is a specific methodology that aims to comprehensively identify all relevant studies on a specific topic, and to select appropriate studies based on explicit criteria . ( https://cebma.org/faq/what-is-a-systematic-review/ )
- clearly defined search criteria
- an explicit reproducible methodology
- a systematic search of the literature with the defined criteria met
- assesses validity of the findings - no risk of bias
- a comprehensive report on the findings, apparent transparency in the results
- Better evidence for a better world Browsable collection of systematic reviews
- Systematic Reviews in the Health Sciences by Molly Maloney Last Updated Apr 23, 2024 515 views this year
- Next: Framework, Protocol, and Writing Steps >>
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Dissertation
- What is a Literature Review? | Guide, Template, & Examples
What is a Literature Review? | Guide, Template, & Examples
Published on 22 February 2022 by Shona McCombes . Revised on 7 June 2022.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research.
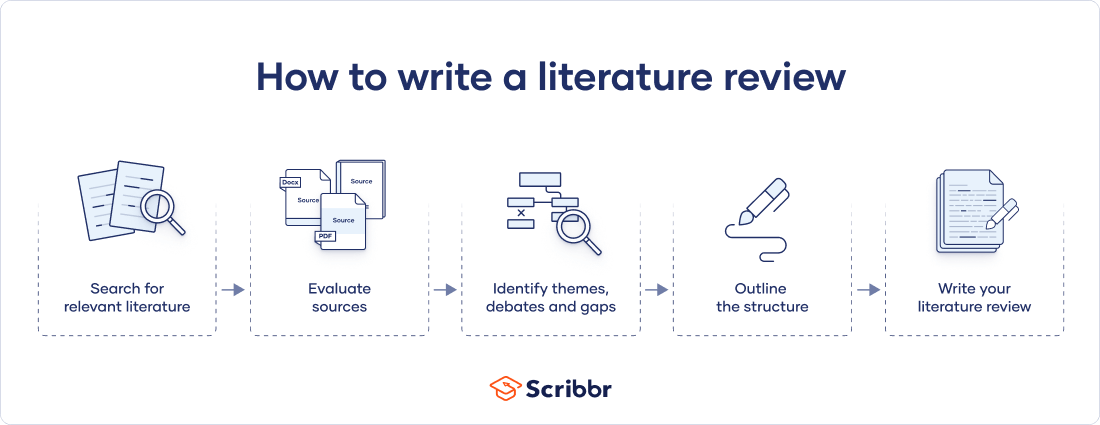
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarise sources – it analyses, synthesises, and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Be assured that you'll submit flawless writing. Upload your document to correct all your mistakes.

Table of contents
Why write a literature review, examples of literature reviews, step 1: search for relevant literature, step 2: evaluate and select sources, step 3: identify themes, debates and gaps, step 4: outline your literature review’s structure, step 5: write your literature review, frequently asked questions about literature reviews, introduction.
- Quick Run-through
- Step 1 & 2
When you write a dissertation or thesis, you will have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and scholarly context
- Develop a theoretical framework and methodology for your research
- Position yourself in relation to other researchers and theorists
- Show how your dissertation addresses a gap or contributes to a debate
You might also have to write a literature review as a stand-alone assignment. In this case, the purpose is to evaluate the current state of research and demonstrate your knowledge of scholarly debates around a topic.
The content will look slightly different in each case, but the process of conducting a literature review follows the same steps. We’ve written a step-by-step guide that you can follow below.
The only proofreading tool specialized in correcting academic writing
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Correct my document today
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research objectives and questions .
If you are writing a literature review as a stand-alone assignment, you will have to choose a focus and develop a central question to direct your search. Unlike a dissertation research question, this question has to be answerable without collecting original data. You should be able to answer it based only on a review of existing publications.
Make a list of keywords
Start by creating a list of keywords related to your research topic. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list if you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can use boolean operators to help narrow down your search:
Read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
To identify the most important publications on your topic, take note of recurring citations. If the same authors, books or articles keep appearing in your reading, make sure to seek them out.
You probably won’t be able to read absolutely everything that has been written on the topic – you’ll have to evaluate which sources are most relevant to your questions.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models and methods? Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- How does the publication contribute to your understanding of the topic? What are its key insights and arguments?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible, and make sure you read any landmark studies and major theories in your field of research.
You can find out how many times an article has been cited on Google Scholar – a high citation count means the article has been influential in the field, and should certainly be included in your literature review.
The scope of your review will depend on your topic and discipline: in the sciences you usually only review recent literature, but in the humanities you might take a long historical perspective (for example, to trace how a concept has changed in meaning over time).
Remember that you can use our template to summarise and evaluate sources you’re thinking about using!
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It’s important to keep track of your sources with references to avoid plagiarism . It can be helpful to make an annotated bibliography, where you compile full reference information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
You can use our free APA Reference Generator for quick, correct, consistent citations.
To begin organising your literature review’s argument and structure, you need to understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly-visual platforms like Instagram and Snapchat – this is a gap that you could address in your own research.
There are various approaches to organising the body of a literature review. You should have a rough idea of your strategy before you start writing.
Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarising sources in order.
Try to analyse patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organise your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text, your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
If you are writing the literature review as part of your dissertation or thesis, reiterate your central problem or research question and give a brief summary of the scholarly context. You can emphasise the timeliness of the topic (“many recent studies have focused on the problem of x”) or highlight a gap in the literature (“while there has been much research on x, few researchers have taken y into consideration”).
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, make sure to follow these tips:
- Summarise and synthesise: give an overview of the main points of each source and combine them into a coherent whole.
- Analyse and interpret: don’t just paraphrase other researchers – add your own interpretations, discussing the significance of findings in relation to the literature as a whole.
- Critically evaluate: mention the strengths and weaknesses of your sources.
- Write in well-structured paragraphs: use transitions and topic sentences to draw connections, comparisons and contrasts.
In the conclusion, you should summarise the key findings you have taken from the literature and emphasise their significance.
If the literature review is part of your dissertation or thesis, reiterate how your research addresses gaps and contributes new knowledge, or discuss how you have drawn on existing theories and methods to build a framework for your research. This can lead directly into your methodology section.
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a dissertation , thesis, research paper , or proposal .
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarise yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
McCombes, S. (2022, June 07). What is a Literature Review? | Guide, Template, & Examples. Scribbr. Retrieved 29 April 2024, from https://www.scribbr.co.uk/thesis-dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a dissertation proposal | a step-by-step guide, what is a theoretical framework | a step-by-step guide, what is a research methodology | steps & tips.

- University of Texas Libraries
Literature Reviews
Analyze results.
- What is a literature review?
- Steps in the Literature Review Process
- Define your research question
- Determine inclusion and exclusion criteria
- Choose databases and search
- Review Results
- Synthesize Results
- Librarian Support
Analysis should lead to insight. This is how you will contribute to the field.
- Analysis requires that you have an approach or a point of view to evaluate the material you found.
- Are there gaps in the literature?
- Where has significant research taken place, and who has done it?
- Is there consensus or debate on this topic?
- Which methodological approaches work best?
Analysis is the part of the literature review process where you justify why your research is needed, how others have not addressed it, and/or how your research advances the field.
Tips for Writing a Literature Review
Though this video is titled "Tips for Writing a Literature Review," the ideas expressed relate to being focused on the research topic and building a strong case, which is also part of the analysis phase.
- Last Updated: Oct 26, 2022 2:49 PM
- URL: https://guides.lib.utexas.edu/literaturereviews


About Systematic Reviews
Understanding the Differences Between a Systematic Review vs Literature Review

Automate every stage of your literature review to produce evidence-based research faster and more accurately.
Let’s look at these differences in further detail.
Goal of the Review
The objective of a literature review is to provide context or background information about a topic of interest. Hence the methodology is less comprehensive and not exhaustive. The aim is to provide an overview of a subject as an introduction to a paper or report. This overview is obtained firstly through evaluation of existing research, theories, and evidence, and secondly through individual critical evaluation and discussion of this content.
A systematic review attempts to answer specific clinical questions (for example, the effectiveness of a drug in treating an illness). Answering such questions comes with a responsibility to be comprehensive and accurate. Failure to do so could have life-threatening consequences. The need to be precise then calls for a systematic approach. The aim of a systematic review is to establish authoritative findings from an account of existing evidence using objective, thorough, reliable, and reproducible research approaches, and frameworks.
Level of Planning Required
The methodology involved in a literature review is less complicated and requires a lower degree of planning. For a systematic review, the planning is extensive and requires defining robust pre-specified protocols. It first starts with formulating the research question and scope of the research. The PICO’s approach (population, intervention, comparison, and outcomes) is used in designing the research question. Planning also involves establishing strict eligibility criteria for inclusion and exclusion of the primary resources to be included in the study. Every stage of the systematic review methodology is pre-specified to the last detail, even before starting the review process. It is recommended to register the protocol of your systematic review to avoid duplication. Journal publishers now look for registration in order to ensure the reviews meet predefined criteria for conducting a systematic review [1].
Search Strategy for Sourcing Primary Resources
Learn more about distillersr.
(Article continues below)
Quality Assessment of the Collected Resources
A rigorous appraisal of collected resources for the quality and relevance of the data they provide is a crucial part of the systematic review methodology. A systematic review usually employs a dual independent review process, which involves two reviewers evaluating the collected resources based on pre-defined inclusion and exclusion criteria. The idea is to limit bias in selecting the primary studies. Such a strict review system is generally not a part of a literature review.
Presentation of Results
Most literature reviews present their findings in narrative or discussion form. These are textual summaries of the results used to critique or analyze a body of literature about a topic serving as an introduction. Due to this reason, literature reviews are sometimes also called narrative reviews. To know more about the differences between narrative reviews and systematic reviews , click here.
A systematic review requires a higher level of rigor, transparency, and often peer-review. The results of a systematic review can be interpreted as numeric effect estimates using statistical methods or as a textual summary of all the evidence collected. Meta-analysis is employed to provide the necessary statistical support to evidence outcomes. They are usually conducted to examine the evidence present on a condition and treatment. The aims of a meta-analysis are to determine whether an effect exists, whether the effect is positive or negative, and establish a conclusive estimate of the effect [2].
Using statistical methods in generating the review results increases confidence in the review. Results of a systematic review are then used by clinicians to prescribe treatment or for pharmacovigilance purposes. The results of the review can also be presented as a qualitative assessment when the end goal is issuing recommendations or guidelines.
Risk of Bias
Literature reviews are mostly used by authors to provide background information with the intended purpose of introducing their own research later. Since the search for included primary resources is also less exhaustive, it is more prone to bias.
One of the main objectives for conducting a systematic review is to reduce bias in the evidence outcome. Extensive planning, strict eligibility criteria for inclusion and exclusion, and a statistical approach for computing the result reduce the risk of bias.
Intervention studies consider risk of bias as the “likelihood of inaccuracy in the estimate of causal effect in that study.” In systematic reviews, assessing the risk of bias is critical in providing accurate assessments of overall intervention effect [3].
With numerous review methods available for analyzing, synthesizing, and presenting existing scientific evidence, it is important for researchers to understand the differences between the review methods. Choosing the right method for a review is crucial in achieving the objectives of the research.
[1] “Systematic Review Protocols and Protocol Registries | NIH Library,” www.nihlibrary.nih.gov . https://www.nihlibrary.nih.gov/services/systematic-review-service/systematic-review-protocols-and-protocol-registries
[2] A. B. Haidich, “Meta-analysis in medical research,” Hippokratia , vol. 14, no. Suppl 1, pp. 29–37, Dec. 2010, [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3049418/#:~:text=Meta%2Danalyses%20are%20conducted%20to
3 Reasons to Connect

- En español – ExME
- Em português – EME
Systematic reviews vs meta-analysis: what’s the difference?
Posted on 24th July 2023 by Verónica Tanco Tellechea

You may hear the terms ‘systematic review’ and ‘meta-analysis being used interchangeably’. Although they are related, they are distinctly different. Learn more in this blog for beginners.
What is a systematic review?
According to Cochrane (1), a systematic review attempts to identify, appraise and synthesize all the empirical evidence to answer a specific research question. Thus, a systematic review is where you might find the most relevant, adequate, and current information regarding a specific topic. In the levels of evidence pyramid , systematic reviews are only surpassed by meta-analyses.
To conduct a systematic review, you will need, among other things:
- A specific research question, usually in the form of a PICO question.
- Pre-specified eligibility criteria, to decide which articles will be included or discarded from the review.
- To follow a systematic method that will minimize bias.
You can find protocols that will guide you from both Cochrane and the Equator Network , among other places, and if you are a beginner to the topic then have a read of an overview about systematic reviews.
What is a meta-analysis?
A meta-analysis is a quantitative, epidemiological study design used to systematically assess the results of previous research (2) . Usually, they are based on randomized controlled trials, though not always. This means that a meta-analysis is a mathematical tool that allows researchers to mathematically combine outcomes from multiple studies.
When can a meta-analysis be implemented?
There is always the possibility of conducting a meta-analysis, yet, for it to throw the best possible results it should be performed when the studies included in the systematic review are of good quality, similar designs, and have similar outcome measures.
Why are meta-analyses important?
Outcomes from a meta-analysis may provide more precise information regarding the estimate of the effect of what is being studied because it merges outcomes from multiple studies. In a meta-analysis, data from various trials are combined and generate an average result (1), which is portrayed in a forest plot diagram. Moreover, meta-analysis also include a funnel plot diagram to visually detect publication bias.
Conclusions
A systematic review is an article that synthesizes available evidence on a certain topic utilizing a specific research question, pre-specified eligibility criteria for including articles, and a systematic method for its production. Whereas a meta-analysis is a quantitative, epidemiological study design used to assess the results of articles included in a systematic-review.
Remember: All meta-analyses involve a systematic review, but not all systematic reviews involve a meta-analysis.
If you would like some further reading on this topic, we suggest the following:
The systematic review – a S4BE blog article
Meta-analysis: what, why, and how – a S4BE blog article
The difference between a systematic review and a meta-analysis – a blog article via Covidence
Systematic review vs meta-analysis: what’s the difference? A 5-minute video from Research Masterminds:
- About Cochrane reviews [Internet]. Cochranelibrary.com. [cited 2023 Apr 30]. Available from: https://www.cochranelibrary.com/about/about-cochrane-reviews
- Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37.
Verónica Tanco Tellechea
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

How to read a funnel plot
This blog introduces you to funnel plots, guiding you through how to read them and what may cause them to look asymmetrical.

Heterogeneity in meta-analysis
When you bring studies together in a meta-analysis, one of the things you need to consider is the variability in your studies – this is called heterogeneity. This blog presents the three types of heterogeneity, considers the different types of outcome data, and delves a little more into dealing with the variations.

Natural killer cells in glioblastoma therapy
As seen in a previous blog from Davide, modern neuroscience often interfaces with other medical specialities. In this blog, he provides a summary of new evidence about the potential of a therapeutic strategy born at the crossroad between neurology, immunology and oncology.
Literature Review vs Systematic Review
- Literature Review vs. Systematic Review
- Primary vs. Secondary Sources
- Databases and Articles
- Specific Journal or Article
Subject Guide

Definitions
It’s common to confuse systematic and literature reviews because both are used to provide a summary of the existent literature or research on a specific topic. Regardless of this commonality, both types of review vary significantly. The following table provides a detailed explanation as well as the differences between systematic and literature reviews.
Kysh, Lynn (2013): Difference between a systematic review and a literature review. [figshare]. Available at: http://dx.doi.org/10.6084/m9.figshare.766364
- << Previous: Home
- Next: Primary vs. Secondary Sources >>
- Last Updated: Dec 15, 2023 10:19 AM
- URL: https://libguides.sjsu.edu/LitRevVSSysRev

Guide to Thematic Analysis

- Abductive Thematic Analysis
- Collaborative Thematic Analysis
- Deductive Thematic Analysis
- How to Do Thematic Analysis
- Inductive Thematic Analysis
- Reflexive Thematic Analysis
- Advantages of Thematic Analysis
- Thematic Analysis for Case Studies
- Thematic Coding
- Disadvantages of Thematic Analysis
- Thematic Analysis in Educational Research
- Thematic Analysis Examples
- Thematic Analysis for Focus Groups
- Thematic Analysis vs. Grounded Theory
- What is Thematic Analysis?
- Increasing Rigor in Thematic Analysis
- Thematic Analysis for Interviews
- Introduction
What is a thematic literature review?
Advantages of a thematic literature review, structuring and writing a thematic literature review.
- Thematic Analysis in Mixed Methods Approach
- Thematic Analysis in Observations
- Peer Review in Thematic Analysis
- How to Present Thematic Analysis Results
- Thematic Analysis in Psychology
- Thematic Analysis of Secondary Data
- Thematic Analysis in Social Work
- Thematic Analysis Software
- Thematic Analysis in Surveys
- Thematic Analysis in UX Research
- Thematic vs. Content Analysis
- Thematic Analysis vs. Discourse Analysis
- Thematic Analysis vs. Framework Analysis
- Thematic Analysis vs. Narrative Analysis
- Thematic Analysis vs. Phenomenology
A thematic literature review serves as a critical tool for synthesizing research findings within a specific subject area. By categorizing existing literature into themes, this method offers a structured approach to identify and analyze patterns and trends across studies. The primary goal is to provide a clear and concise overview that aids scholars and practitioners in understanding the key discussions and developments within a field. Unlike traditional literature reviews , which may adopt a chronological approach or focus on individual studies, a thematic literature review emphasizes the aggregation of findings through key themes and thematic connections. This introduction sets the stage for a detailed examination of what constitutes a thematic literature review, its benefits, and guidance on effectively structuring and writing one.
A thematic literature review methodically organizes and examines a body of literature by identifying, analyzing, and reporting themes found within texts such as journal articles, conference proceedings, dissertations, and other forms of academic writing. While a particular journal article may offer some specific insight, a synthesis of knowledge through a literature review can provide a comprehensive overview of theories across relevant sources in a particular field.
Unlike other review types that might organize literature chronologically or by methodology , a thematic review focuses on recurring themes or patterns across a collection of works. This approach enables researchers to draw together previous research to synthesize findings from different research contexts and methodologies, highlighting the overarching trends and insights within a field.
At its core, a thematic approach to a literature review research project involves several key steps. Initially, it requires the comprehensive collection of relevant literature that aligns with the review's research question or objectives. Following this, the process entails a meticulous analysis of the texts to identify common themes that emerge across the studies. These themes are not pre-defined but are discovered through a careful reading and synthesis of the literature.
The thematic analysis process is iterative, often involving the refinement of themes as the review progresses. It allows for the integration of a broad range of literature, facilitating a multidimensional understanding of the research topic. By organizing literature thematically, the review illuminates how various studies contribute to each theme, providing insights into the depth and breadth of research in the area.
A thematic literature review thus serves as a foundational element in research, offering a nuanced and comprehensive perspective on a topic. It not only aids in identifying gaps in the existing literature but also guides future research directions by underscoring areas that warrant further investigation. Ultimately, a thematic literature review empowers researchers to construct a coherent narrative that weaves together disparate studies into a unified analysis.
Organize your literature search with ATLAS.ti
Collect and categorize documents to identify gaps and key findings with ATLAS.ti. Download a free trial.
Conducting a literature review thematically provides a comprehensive and nuanced synthesis of research findings, distinguishing it from other types of literature reviews. Its structured approach not only facilitates a deeper understanding of the subject area but also enhances the clarity and relevance of the review. Here are three significant advantages of employing a thematic analysis in literature reviews.
Enhanced understanding of the research field
Thematic literature reviews allow for a detailed exploration of the research landscape, presenting themes that capture the essence of the subject area. By identifying and analyzing these themes, reviewers can construct a narrative that reflects the complexity and multifaceted nature of the field.
This process aids in uncovering underlying patterns and relationships, offering a more profound and insightful examination of the literature. As a result, readers gain an enriched understanding of the key concepts, debates, and evolutionary trajectories within the research area.
Identification of research gaps and trends
One of the pivotal benefits of a thematic literature review is its ability to highlight gaps in the existing body of research. By systematically organizing the literature into themes, reviewers can pinpoint areas that are under-explored or warrant further investigation.
Additionally, this method can reveal emerging trends and shifts in research focus, guiding scholars toward promising areas for future study. The thematic structure thus serves as a roadmap, directing researchers toward uncharted territories and new research questions .
Facilitates comparative analysis and integration of findings
A thematic literature review excels in synthesizing findings from diverse studies, enabling a coherent and integrated overview. By concentrating on themes rather than individual studies, the review can draw comparisons and contrasts across different research contexts and methodologies . This comparative analysis enriches the review, offering a panoramic view of the field that acknowledges both consensus and divergence among researchers.
Moreover, the thematic framework supports the integration of findings, presenting a unified and comprehensive portrayal of the research area. Such integration is invaluable for scholars seeking to navigate the extensive body of literature and extract pertinent insights relevant to their own research questions or objectives.
The process of structuring and writing a thematic literature review is pivotal in presenting research in a clear, coherent, and impactful manner. This review type necessitates a methodical approach to not only unearth and categorize key themes but also to articulate them in a manner that is both accessible and informative to the reader. The following sections outline essential stages in the thematic analysis process for literature reviews , offering a structured pathway from initial planning to the final presentation of findings.
Identifying and categorizing themes
The initial phase in a thematic literature review is the identification of themes within the collected body of literature. This involves a detailed examination of texts to discern patterns, concepts, and ideas that recur across the research landscape. Effective identification hinges on a thorough and nuanced reading of the literature, where the reviewer actively engages with the content to extract and note significant thematic elements. Once identified, these themes must be meticulously categorized, often requiring the reviewer to discern between overarching themes and more nuanced sub-themes, ensuring a logical and hierarchical organization of the review content.
Analyzing and synthesizing themes
After categorizing the themes, the next step involves a deeper analysis and synthesis of the identified themes. This stage is critical for understanding the relationships between themes and for interpreting the broader implications of the thematic findings. Analysis may reveal how themes evolve over time, differ across methodologies or contexts, or converge to highlight predominant trends in the research area. Synthesis involves integrating insights from various studies to construct a comprehensive narrative that encapsulates the thematic essence of the literature, offering new interpretations or revealing gaps in existing research.
Presenting and discussing findings
The final stage of the thematic literature review is the discussion of the thematic findings in a research paper or presentation. This entails not only a descriptive account of identified themes but also a critical examination of their significance within the research field. Each theme should be discussed in detail, elucidating its relevance, the extent of research support, and its implications for future studies. The review should culminate in a coherent and compelling narrative that not only summarizes the key thematic findings but also situates them within the broader research context, offering valuable insights and directions for future inquiry.
Gain a comprehensive understanding of your data with ATLAS.ti
Analyze qualitative data with specific themes that offer insights. See how with a free trial.

Home » Education » Difference Between Literature Review and Systematic Review
Difference Between Literature Review and Systematic Review
Main difference – literature review vs systematic review.
Literature review and systematic review are two scholarly texts that help to introduce new knowledge to various fields. A literature review, which reviews the existing research and information on a selected study area, is a crucial element of a research study. A systematic review is also a type of a literature review. The main difference between literature review and systematic review is their focus on the research question ; a systematic review is focused on a specific research question whereas a literature review is not.
This article highlights,
1. What is a Literature Review? – Definition, Features, Characteristics
2. What is a Systematic Review? – Definition, Features, Characteristics

What is a Literature Review
A literature review is an indispensable element of a research study. This is where the researcher shows his knowledge on the subject area he or she is researching on. A literature review is a discussion on the already existing material in the subject area. Thus, this will require a collection of published (in print or online) work concerning the selected research area. In simple terms, a literature is a review of the literature in the related subject area.
A good literature review is a critical discussion, displaying the writer’s knowledge on relevant theories and approaches and awareness of contrasting arguments. A literature review should have the following features (Caulley, 1992)
- Compare and contrast different researchers’ views
- Identify areas in which researchers are in disagreement
- Group researchers who have similar conclusions
- Criticize the methodology
- Highlight exemplary studies
- Highlight gaps in research
- Indicate the connection between your study and previous studies
- Indicate how your study will contribute to the literature in general
- Conclude by summarizing what the literature indicates
The structure of a literature review is similar to that of an article or essay, unlike an annotated bibliography . The information that is collected is integrated into paragraphs based on their relevance. Literature reviews help researchers to evaluate the existing literature, to identify a gap in the research area, to place their study in the existing research and identify future research.

What is a Systematic Review
A systematic review is a type of systematic review that is focused on a particular research question . The main purpose of this type of research is to identify, review, and summarize the best available research on a specific research question. Systematic reviews are used mainly because the review of existing studies is often more convenient than conducting a new study. These are mostly used in the health and medical field, but they are not rare in fields such as social sciences and environmental science. Given below are the main stages of a systematic review:
- Defining the research question and identifying an objective method
- Searching for relevant data that from existing research studies that meet certain criteria (research studies must be reliable and valid).
- Extracting data from the selected studies (data such as the participants, methods, outcomes, etc.
- Assessing the quality of information
- Analyzing and combining all the data which would give an overall result.
Literature Review is a critical evaluation of the existing published work in a selected research area.
Systematic Review is a type of literature review that is focused on a particular research question.
Literature Review aims to review the existing literature, identify the research gap, place the research study in relation to other studies, to evaluate promising research methods, and to suggest further research.
Systematic Review aims to identify, review, and summarize the best available research on a specific research question.
Research Question
In Literature Review, a r esearch question is formed after writing the literature review and identifying the research gap.
In Systematic Review, a research question is formed at the beginning of the systematic review.
Research Study
Literature Review is an essential component of a research study and is done at the beginning of the study.
Systematic Review is not followed by a separate research study.
Caulley, D. N. “Writing a critical review of the literature.” La Trobe University: Bundoora (1992).
“Animated Storyboard: What Are Systematic Reviews?” . cccrg.cochrane.org . Cochrane Consumers and Communication . Retrieved 1 June 2016.
Image Courtesy: Pixabay
About the Author: Hasa
Hasanthi is a seasoned content writer and editor with over 8 years of experience. Armed with a BA degree in English and a knack for digital marketing, she explores her passions for literature, history, culture, and food through her engaging and informative writing.
You May Also Like These
Leave a reply cancel reply.
We use cookies on this site to enhance your experience
By clicking any link on this page you are giving your consent for us to set cookies.
A link to reset your password has been sent to your email.
Back to login
We need additional information from you. Please complete your profile first before placing your order.
Thank you. payment completed., you will receive an email from us to confirm your registration, please click the link in the email to activate your account., there was error during payment, orcid profile found in public registry, download history, difference between a literature review and a critical review.
- Charlesworth Author Services
- 08 October, 2021
As you read research papers, you may notice that there are two very different kinds of review of prior studies. Sometimes, this section of a paper is called a literature review, and at other times, it is referred to as a critical review or a critical context . These differences may be more commonly seen across different fields. Although both these sections are about reviewing prior and existing studies, this article aims to clarify the differences between the two.
Literature review
A literature review is a summary of prior or existing studies that are related to your own research paper . A literature review can be a part of a research paper or can form a paper in itself . For the former, the literature review is designed as a basis upon which your own current study is designed and built. The latter forms a synthesis of prior studies and is a way to highlight future research agendas or a framework.
Writing a literature review
In a literature review, you should attempt to discuss the arguments and findings in prior studies and then work to build on these studies as you develop your own research. You can also highlight the connection between existing and prior literature to demonstrate how the current study you are presenting can advance your knowledge in the field .
When performing a literature review, you should aim to summarise your discussions using a specific aspect of the literature, such as by topic, time, methodology/ design and findings . By doing so, you should be able to establish an effective way to present the relevant literature and demonstrate the connection between prior studies and your research.
Do note that a literature review does not include a presentation or discussion of any results or findings – this should come at a later point in the paper or study. You should also not impose your subjective viewpoints or opinions on the literature you discuss.
Critical review
A critical review is also a popular way of reviewing prior and existing studies. It can cover and discuss the main ideas or arguments in a book or an article, or it can review a specific concept, theme, theoretical perspective or key construct found in the existing literature .
However, the key feature that distinguishes a critical review from a literature review is that the former is more than just a summary of different topics or methodologies. It offers more of a reflection and critique of the concept in question, and is engaged by authors to more clearly contextualise their own research within the existing literature and to present their opinions, perspectives and approaches .
Given that a critical review is not just a summary of prior literature, it is generally not considered acceptable to follow the same strategy as for a literature review. Instead, aim to organise and structure your critical review in a way that would enable you to discuss the key concepts, assert your perspectives and locate your arguments and research within the existing body of work.
Structuring a critical review
A critical review would generally begin with an introduction to the concepts you would like to discuss. Depending on how broad the topics are, this can simply be a brief overview or it could set up a more complex framework. The discussion that follows through the rest of the review will then address and discuss your chosen themes or topics in more depth.
Writing a critical review
The discussion within a critical review will not only present and summarise themes but also critically engage with the varying arguments, writings and perspectives within those themes. One important thing to note is that, similar to a literature review , you should keep your personal opinions, likes and dislikes out of a review. Whether you personally agree with a study or argument – and whether you like it or not – is immaterial. Instead, you should focus upon the effectiveness and relevance of the arguments , considering such elements as the evidence provided, the interpretations and analysis of the data, whether or not a study may be biased in any way, what further questions or problems it raises or what outstanding gaps and issues need to be addressed.
In conclusion
Although a review of previous and existing literature can be performed and presented in different ways, in essence, any literature or critical review requires a solid understanding of the most prominent work in the field as it relates to your own study. Such an understanding is crucial and significant for you to build upon and synthesise the existing knowledge, and to create and contribute new knowledge to advance the field .
Read previous (fourth) in series: How to refer to other studies or literature in the different sections of a research paper
Maximise your publication success with Charlesworth Author Services .
Charlesworth Author Services, a trusted brand supporting the world’s leading academic publishers, institutions and authors since 1928.
To know more about our services, visit: Our Services
Share with your colleagues
Related articles.

Conducting a Literature Review
Charlesworth Author Services 10/03/2021 00:00:00


Important factors to consider as you Start to Plan your Literature Review
Charlesworth Author Services 06/10/2021 00:00:00

How to Structure and Write your Literature Review
Charlesworth Author Services 07/10/2021 00:00:00
Related webinars

Bitesize Webinar: How to write and structure your academic article for publication- Module 3: Understand the structure of an academic paper
Charlesworth Author Services 04/03/2021 00:00:00

Bitesize Webinar: How to write and structure your academic article for publication: Module 5: Conduct a Literature Review

Bitesize Webinar: How to write and structure your academic article for publication: Module 7: Write a strong theoretical framework section
Charlesworth Author Services 05/03/2021 00:00:00

Bitesize Webinar: How to write and structure your academic article for publication: Module 11: Know when your article is ready for submission
Literature search.

Why and How to do a literature search
Charlesworth Author Services 17/08/2020 00:00:00

Best tips to do a PubMed search
Charlesworth Author Services 26/08/2021 00:00:00

Best tips to do a Scopus search
Understanding the influence of different proxy perspectives in explaining the difference between self-rated and proxy-rated quality of life in people living with dementia: a systematic literature review and meta-analysis
- Open access
- Published: 24 April 2024
Cite this article
You have full access to this open access article

- Lidia Engel ORCID: orcid.org/0000-0002-7959-3149 1 ,
- Valeriia Sokolova 1 ,
- Ekaterina Bogatyreva 2 &
- Anna Leuenberger 2
217 Accesses
Explore all metrics
Proxy assessment can be elicited via the proxy-patient perspective (i.e., asking proxies to assess the patient’s quality of life (QoL) as they think the patient would respond) or proxy-proxy perspective (i.e., asking proxies to provide their own perspective on the patient’s QoL). This review aimed to identify the role of the proxy perspective in explaining the differences between self-rated and proxy-rated QoL in people living with dementia.
A systematic literate review was conducted by sourcing articles from a previously published review, supplemented by an update of the review in four bibliographic databases. Peer-reviewed studies that reported both self-reported and proxy-reported mean QoL estimates using the same standardized QoL instrument, published in English, and focused on the QoL of people with dementia were included. A meta-analysis was conducted to synthesize the mean differences between self- and proxy-report across different proxy perspectives.
The review included 96 articles from which 635 observations were extracted. Most observations extracted used the proxy-proxy perspective (79%) compared with the proxy-patient perspective (10%); with 11% of the studies not stating the perspective. The QOL-AD was the most commonly used measure, followed by the EQ-5D and DEMQOL. The standardized mean difference (SMD) between the self- and proxy-report was lower for the proxy-patient perspective (SMD: 0.250; 95% CI 0.116; 0.384) compared to the proxy-proxy perspective (SMD: 0.532; 95% CI 0.456; 0.609).
Different proxy perspectives affect the ratings of QoL, whereby adopting a proxy-proxy QoL perspective has a higher inter-rater gap in comparison with the proxy-patient perspective.
Similar content being viewed by others

Exploring self-report and proxy-report quality-of-life measures for people living with dementia in care homes
Convergent validity of EQ-5D with core outcomes in dementia: a systematic review

Proxy reporting of health-related quality of life for people with dementia: a psychometric solution
Avoid common mistakes on your manuscript.
Quality of life (QoL) has become an important outcome for research and practice but obtaining reliable and valid estimates remains a challenge in people living with dementia [ 1 ]. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria [ 2 ], dementia, termed as Major Neurocognitive Disorder (MND), involves a significant decline in at least one cognitive domain (executive function, complex attention, language, learning, memory, perceptual-motor, or social cognition), where the decline represents a change from a patient's prior level of cognitive ability, is persistent and progressive over time, is not associated exclusively with an episode of delirium, and reduces a person’s ability to perform everyday activities. Since dementia is one of the most pressing challenges for healthcare systems nowadays [ 3 ], it is critical to study its impact on QoL. The World Health Organization defines the concept of QoL as “individuals' perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns” [ 4 ]. It is a broad ranging concept incorporating in a complex way the persons' physical health, psychological state, level of independence, social relationships, personal beliefs, and their relationships to salient features of the environment.
Although there is evidence that people with mild to moderate dementia can reliably rate their own QoL [ 5 ], as the disease progresses, there is typically a decline in memory, attention, judgment, insight, and communication that may compromise self-reporting of QoL [ 6 ]. Additionally, behavioral symptoms, such as agitation, and affective symptoms, such as depression, may present another challenge in obtaining self-reported QoL ratings due to emotional shifts and unwillingness to complete the assessment [ 7 ]. Although QoL is subjective and should ideally be assessed from an individual’s own perspective [ 8 ], the decline in cognitive function emphasizes the need for proxy-reporting by family members, health professionals, or care staff who are asked to report on behalf of the person with dementia. However, proxy-reports are not substitutable for self-reports from people with dementia, as they offer supplementary insights, reflecting the perceptions and viewpoints of people surrounding the person with dementia [ 9 ].
Previous research has consistently highlighted a disagreement between self-rated and proxy-rated QoL in people living with dementia, with proxies generally providing lower ratings (indicating poorer QoL) compared with person’s own ratings [ 8 , 10 , 11 , 12 ]. Impairment in cognition associated with greater dementia severity has been found to be associated with larger difference between self-rating and proxy-rating obtained from family caregivers, as it becomes increasingly difficult for severely cognitively impaired individuals to respond to questions that require contemplation, introspection, and sustained attention [ 13 , 14 ]. Moreover, non-cognitive factors, such as awareness of disease and depressive symptoms play an important role when comparing QoL ratings between individuals with dementia and their proxies [ 15 ]. Qualitative evidence has also shown that people with dementia tend to compare themselves with their peers, whereas carers make comparisons with how the person used to be in the past [ 9 ]. The disagreement between self-reported QoL and carer proxy-rated QoL could be modulated by some personal, cognitive or relational factors, for example, the type of relationship or the frequency of contact maintained, person’s cognitive status, carer’s own feeling about dementia, carer’s mood, and perceived burden of caregiving [ 14 , 16 ]. Disagreement may also arise from the person with dementia’s problems to communicate symptoms, and proxies’ inability to recognize certain symptoms, like pain [ 17 ], or be impacted by the amount of time spent with the person with dementia [ 18 ]. This may also prevent proxies to rate accurately certain domains of QoL, with previous evidence showing higher level of agreement for observable domains, such as mobility, compared with less observable domains like emotional wellbeing [ 8 ]. Finally, agreement also depends on the type of proxy (i.e., informal/family carers or professional staff) and the nature of their relationship, for instance, proxy QoL scores provided by formal carers tend to be higher (reflecting better QoL) compared to the scores supplied by family members [ 19 , 20 ]. Staff members might associate residents’ QoL with the quality of care delivered or the stage of their cognitive impairment, whereas relatives often focus on comparison with the person’s QoL when they were younger, lived in their own home and did not have dementia [ 20 ].
What has been not been fully examined to date is the role of different proxy perspectives employed in QoL questionnaires in explaining disagreement between self-rated and proxy-rated scores in people with dementia. Pickard et al. (2005) have proposed a conceptual framework for proxy assessments that distinguish between the proxy-patient perspective (i.e., asking proxies to assess the patient’s QoL as they think the patient would respond) or proxy-proxy perspective (i.e., asking proxies to provide their own perspective on the patient’s QoL) [ 21 ]. In this context, the intra-proxy gap describes the differences between proxy-patient and proxy-proxy perspective, whereas the inter-rater gap is the difference between self-report and proxy-report [ 21 ].
Existing generic and dementia-specific QoL instruments specify the perspective explicitly in their instructions or imply the perspective indirectly in their wording. For example, the instructions of the Dementia Quality of Life Measure (DEMQOL) asks proxies to give the answer they think their relative would give (i.e., proxy-patient perspective) [ 22 ], whereas the family version of the Quality of Life in Alzheimer’s Disease (QOL-AD) instructs the proxies to rate their relative’s current situation as they (the proxy) see it (i.e., proxy-proxy perspective) [ 7 ]. Some instruments, like the EQ-5D measures, have two proxy versions for each respective perspective [ 23 , 24 ]. The Adult Social Care Outcome Toolkit (ASCOT) proxy version, on the other hand, asks proxies to complete the questions from both perspectives, from their own opinion and how they think the person would answer [ 25 ].
QoL scores generated using different perspectives are expected to differ, with qualitative evidence showing that carers rate the person with dementia’s QoL lower (worse) when instructed to comment from their own perspective than from the perspective of the person with dementia [ 26 ]. However, to our knowledge, no previous review has fully synthesized existing evidence in this area. Therefore, we aimed to undertake a systematic literature review to examine the role of different proxy-assessment perspectives in explaining differences between self-rated and proxy-rated QoL in people living with dementia. The review was conducted under the hypothesis that the difference in QoL estimates will be larger when adopting the proxy-proxy perspective compared with proxy-patient perspective.
The review was registered with the International Prospective Register of Systematic Reviews (CRD42022333542) and followed the Preferred Reporting Items System for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (see Appendix 1 ) [ 27 ].
Search strategy
This review used two approaches to obtain literature. First, primary articles from an existing review by Roydhouse et al. were retrieved [ 28 ]. The review included studies published from inception to February 2018 that compared self- and proxy-reports. Studies that focused explicitly on Alzheimer’s Disease or dementia were retrieved for the current review. Two reviewers conducted a full-text review to assess whether the eligibility criteria listed below for the respective study were met. An update of the Roydhouse et al. review was undertaken to capture more recent studies. The search strategy by Roydhouse et al. was amended and covered studies published after January 1, 2018, and was limited to studies within the context of dementia. The original search was undertaken over a three-week period (17/11/2021–9/12/2021) and then updated on July 3, 2023. Peer-reviewed literature was sourced from MEDLINE, CINAHL, and PsycINFO databases via EBSCOHost as well as EMBASE. Four main search term categories were used: (1) proxy terms (i.e., care*-report*), (2) QoL/ outcome terms (i.e., ‘quality of life’), (3) disease terms (i.e., ‘dementia’), and (4) pediatric terms (i.e., ‘pediatric*’) (for exclusion). Keywords were limited to appear in titles and abstracts only, and MeSH terms were included for all databases. A list of search strategy can be found in Appendix 2 . The first three search term categories were searched with AND, and the NOT function was used to exclude pediatric terms. A limiter was applied in all database searches to only include studies with human participants and articles published in English.
Selection criteria
Studies from all geographical locations were included in the review if they (1) were published in English in a peer-reviewed journal (conference abstracts, dissertations, a gray literature were excluded); (2) were primary studies (reviews were excluded); (3) clearly defined the disease of participants, which were limited to Alzheimer’s disease or dementia; (4) reported separate QoL scores for people with dementia (studies that included mixed populations had to report a separate QoL score for people with dementia to be considered); (5) were using a standardized and existing QoL instrument for assessment; and (6) provided a mean self-reported and proxy-reported QoL score for the same dyads sample (studies that reported means for non-matched samples were excluded) using the same QoL instrument.
Four reviewers (LE, VS, KB, AL) were grouped into two groups who independently screened the 179 full texts from the Roydhouse et. al (2022) study that included Alzheimer’s disease or dementia patients. If a discrepancy within the inclusion selection occurred, articles were discussed among all the reviewers until a consensus was reached. Studies identified from the database search were imported into EndNote [ 29 ]. Duplicates were removed through EndNote and then uploaded to Rayyan [ 30 ]. Each abstract was reviewed by two independent reviewers (any two from four reviewers). Disagreements regarding study inclusions were discussed between all reviewers until a consensus was reached. Full-text screening of each eligible article was completed by two independent reviewers (any two from four reviewers). Again, a discussion between all reviewers was used in case of disagreements.
Data extraction
A data extraction template was created in Microsoft Excel. The following information were extracted if available: country, study design, study sample, study setting, dementia type, disease severity, Mini-Mental Health State Exam (MMSE) score details, proxy type, perspective, living arrangements, QoL assessment measure/instrument, self-reported scores (mean, SD), proxy-reported scores (mean, SD), and agreement statistics. If a study reported the mean (SD) for the total score as well as for specific QoL domains of the measure, we extracted both. If studies reported multiple scores across different time points or subgroups, we extracted all scores. For interventional studies, scores from both the intervention group and the control group were recorded. In determining the proxy perspective, we relied on authors’ description in the article. If the perspective was not explicitly stated, we adopted the perspective of the instrument developers; where more perspectives were possible (e.g., in the case of the EQ-5D measures) and the perspective was not explicitly stated, it was categorized as ‘undefined.’ For agreement, we extracted the Intraclass Correlation Coefficient (ICC), a reliability index that reflects both degree of correlation and agreement between measurements of continuous variables. While there are different forms of ICC based on the model (1-way random effects, 2-wy random effects, or 2-way fixed effects), the type (single rater/measurement or the mean k raters/measurements), and definition of relationship [ 31 ], this level of information was not extracted due to insufficient information provided in the original studies. Values for ICC range between 0 and 1, with values interpreted as poor (less than 0.5), moderate (0.5–0.75), good (0.75–0.9), and excellent (greater than 0.9) reliability between raters [ 31 ].
Data synthesis and analysis
Characteristics of studies were summarized descriptively. Self-reported and proxy-reported means and SD were extracted from the full texts and the mean difference was calculated (or extracted if available) for each pair. Studies that reported median values instead of mean values were converted using the approach outlined by Wan et al. (2014) [ 32 ]. Missing SDs (5 studies, 20 observations) were obtained from standard errors or confidence intervals reported following the Cochrane guidelines [ 33 ]. Missing SDs (6 studies, 29 observations) in studies that only presented the mean value without any additional summary statistics were imputed using the prognostic method [ 34 ]. Thereby, we predicted the missing SDs by calculating the average SDs of observed studies with full information by the respective measure and source (self-report versus proxy-report).
A meta-analysis was performed in Stata (17.1 Stata Corp LLC, College Station, TX) to synthesize mean differences between self- and proxy-reported scores across different proxy perspectives. First, the pooled raw mean differences were calculated for each QoL measure separately, given differences in scales between measures. Secondly, we calculated the pooled standardized mean difference (SMD) for all studies stratified by proxy type (family carer, formal carers, mixed), dementia severity (mild, moderate, severe), and living arrangement (residential/institutional care, mixed). SMD accounts for the use of different measurement scales, where effect sizes were estimated using Cohen’s d. Random-effects models were used to allow for unexplained between-study variability based on the restricted maximum-likelihood (REML) estimator. The percentage of variability attributed to heterogeneity between the studies was assessed using the I 2 statistic; an I 2 of 0%-40% represents possibly unimportant heterogeneity, 30–60% moderate heterogeneity, 50–90% substantial heterogeneity, and 75%-100% considerable heterogeneity [ 35 ]. Chi-squared statistics (χ 2 ) provided evidence of heterogeneity, where a p -value of 0.1 was used as significance level. For studies that reported agreement statistics, based on ICC, we also ran a forest plot stratified by the study perspective. We also calculated Q statistic (Cochran’s test of homogeneity), which assesses whether observed differences in results are compatible with chance alone.
Risk of bias and quality assessment
The quality of studies was assessed using the using a checklist for assessing the quality of quantitative studies developed by Kmet et al. (2004) [ 36 ]. The checklist consists of 14 items and items are scored as ‘2’ (yes, item sufficiently addressed), ‘1’ (item partially addressed), ‘0’ (no, not addressed), or ‘not applicable.’ A summary score was calculated for each study by summing the total score obtained across relevant items and dividing by the total possible score. Scores were adjusted by excluding items that were not applicable from the total score. Quality assessment was undertaken by one reviewer, with 25% of the papers assessed independently by a second reviewer.
The PRISMA diagram in Fig. 1 shows that after the abstract and full-text screening, 38 studies from the database search and 58 studies from the Roydhouse et al. (2022) review were included in this review—a total of 96 studies. A list of all studies included and their characteristics can be found in Appendix 3.
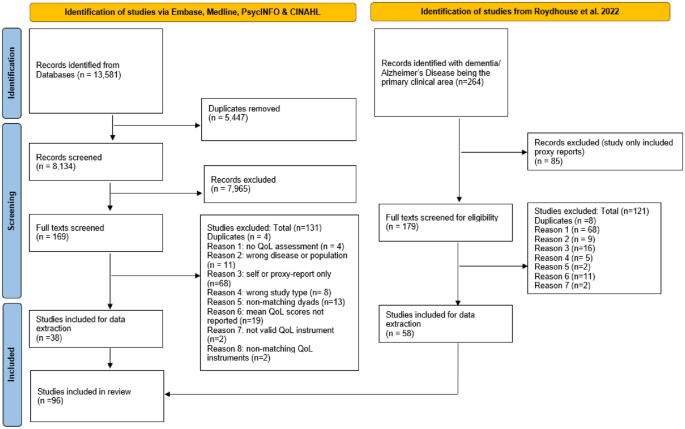
PRISMA 2020 flow diagram
General study characteristics
The 96 articles included in the review were published between 1999 and 2023 from across the globe; most studies (36%) were conducted in Europe. People with dementia in these studies were living in the community (67%), residential/institutional care (15%), as well as mixed dwelling settings (18%). Most proxy-reports were provided by family carers (85%) and only 8 studies (8%) included formal carers. The mean MMSE score for dementia and Alzheimer’s participants was 18.77 (SD = 4.34; N = 85 studies), which corresponds to moderate cognitive impairment [ 37 ]. Further characteristics of studies included are provided in Table 1 . The quality of studies included (see Appendix 4) was generally very good, scoring on average 91% (SD: 9.1) with scores ranging from 50 to 100%.
Quality of life measure and proxy perspective used
A total of 635 observations were recorded from the 96 studies. The majority of studies and observations extracted assumed the proxy-proxy perspective (77 studies, 501 observations), followed by the proxy-patient perspective (18 studies, 62 observations), with 18 studies (72 observations) not clearly defining the perspective. Table 2 provides a detailed overview of number of studies and observations across the respective QoL measures and proxy perspectives. Two studies (14 observations) adopted both perspectives within the same study design: one using the QOL-AD measure [ 5 ] and the second study exploring the EQ-5D-3L and EQ VAS [ 38 ]. Overall, the QOL-AD was the most often used QoL measure, followed by the EQ-5D and DEMQOL. Mean scores for specific QoL domains were accessible for the DEMQOL and QOL-AD. However, only the QOL-AD provided domain-specific mean scores from both proxy perspectives.
Mean scores and mean differences by proxy perspective and QoL measure
The raw mean scores for self-reported and proxy-reported QoL scores are provided in the Supplementary file 2. The pooled raw mean difference by proxy perspective and measure is shown in Table 3 . Regardless of the perspective adopted and the QoL instrument used, self-reported scores were higher (indicating better QoL) compared with proxy-reported scores, except for the DEMQOL, where proxies reported better QoL than people with dementia themselves. Most instruments were explored from one perspective, except for the EQ-5D-3L, EQ VAS, and QOL-AD, for which mean differences were available for both perspectives. For these three measures, mean differences were smaller when adopting the proxy-patient perspective compared with proxy-proxy perspective, although mean scores for the QOL-AD were slightly lower from the proxy-proxy perspective. I 2 statistics indicate considerable heterogeneity (I 2 > 75%) between studies. Mean differences by specific QoL domains are provided in Appendix 5, but only for the QOL-AD measure that was explored from both perspectives. Generally, mean differences appeared to be smaller for the proxy-proxy perspective than the proxy-patient perspective across all domains, except for ‘physical health’ and ‘doing chores around the house.’ However, results need to be interpreted carefully as proxy-patient perspective scores were derived from only one study.
Standardized mean differences by proxy perspective, stratified by proxy type, dementia severity, and living arrangement
Table 4 provides the SMD by proxy perspective, which adjusts for the different QoL measurement scales. Findings suggest that adopting the proxy-patient perspective results in lower SMDs (SMD: 0.250; 95% CI 0.116; 0.384) compared with the proxy-proxy perspective (SMD: 0.532; 95% CI 0.456; 0.609). The largest SMD was recorded for studies that did not define the study perspective (SMD: 0.594; 95% CI 0.469; 0.718). A comparison by different proxy types (formal carers, family carers, and mixed proxies) revealed some mixed results. When adopting the proxy-proxy perspective, the largest SMD was found for family carers (SMD: 0.556; 95% CI 0.465; 0.646) compared with formal carers (SMD: 0.446; 95% CI 0.305; 0.586) or mixed proxies (SMD: 0.335; 95% CI 0.211; 0.459). However, the opposite relationship was found when the proxy-patient perspective was used, where the smallest SMD was found for family carers compared with formal carers and mixed proxies. The SMD increased with greater level of dementia severity, suggesting a greater disagreement. However, compared with the proxy-proxy perspective, where self-reported scores were greater (i.e., better QoL) than proxy-reported scores across all dementia severity levels, the opposite was found when adopting the proxy-patient perspective, where proxies reported better QoL than people with dementia themselves, except for the severe subgroup. No clear trend was observed for different living settings, although the SMD appeared to be smaller for people with dementia living in residential care compared with those living in the community.
Direct proxy perspectives comparison studies
Two studies assessed both proxy perspectives within the same study design. Bosboom et al. (2012) found that compared with self-reported scores (mean: 34.7; SD: 5.3) using the QOL-AD, proxy scores using the proxy-patient perspective were closer to the self-reported scores (mean: 32.1; SD: 6.1) compared with the proxy-proxy perspective (mean: 29.5; SD: 5.4) [ 5 ]. Similar findings were reported by Leontjevas et al. (2016) using the EQ-5D-3L, including the EQ VAS, showing that the inter-proxy gap between self-report (EQ-5D-3L: 0.609; EQ VAS: 65.37) and proxy-report was smaller when adopting the proxy-patient perspective (EQ-5D-3L: 0.555; EQ VAS: 65.15) compared with the proxy-proxy perspective (EQ-5D-3L: 0.492; EQ VAS: 64.42) [ 38 ].
Inter-rater agreement (ICC) statistics
Six studies reported agreement statistics based on ICC, from which we extracted 17 observations that were included in the meta-analysis. Figure 2 shows the study-specific and overall estimates of ICC by the respective study perspective. The heterogeneity between studies was high ( I 2 = 88.20%), with a Q test score of 135.49 ( p < 0.001). While the overall ICC for the 17 observations was 0.3 (95% CI 0.22; 0.38), indicating low agreement, the level of agreement was slightly better when adopting a proxy-patient perspective (ICC: 0.36, 95% CI 0.23; 0.49) than a proxy-proxy perspective (ICC: 0.26, 95% CI 0.17; 0.35).
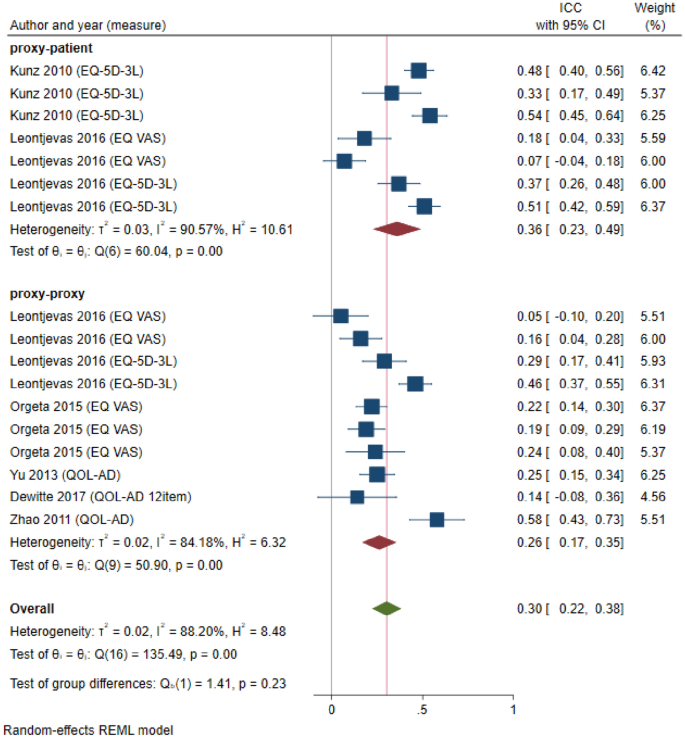
Forest plot depicting study-specific and overall ICC estimates by study perspective
While previous studies highlighted a disagreement between self-rated and proxy-rated QoL in people living with dementia, this review, for the first time, assessed the role of different proxy perspectives in explaining the inter-rater gap. Our findings align with the baseline hypothesis and indicate that QoL scores reported from the proxy-patient perspective are closer to self-reported QoL scores than the proxy-proxy perspective, suggesting that the proxy perspective does impact the inter-rater gap and should not be ignored. This finding was observed across different analyses conducted in this review (i.e., pooled raw mean difference, SMD, ICC analysis), which also confirms the results of two previous primary studies that adopted both proxy perspectives within the same study design [ 5 , 38 ]. Our findings emphasize the need for transparency in reporting the proxy perspective used in future studies, as it can impact results and interpretation. This was also noted by the recent ISPOR Proxy Task Force that developed a checklist of considerations when using proxy-reporting [ 39 ]. While consistency in proxy-reports is desirable, it is crucial to acknowledge that each proxy perspective holds significance in future research, depending on study objectives. It is evident that both proxy perspectives offer distinct insights—one encapsulating the perspectives of people with dementia, and the other reflecting the viewpoints of proxies. Therefore, in situations where self-report is unattainable due to advanced disease severity and the person’s perspective on their own QoL assessment is sought, it is recommended to use the proxy-patient perspective. Conversely, if the objective of future research is to encompass the viewpoints of proxies, opting for the proxy-proxy perspective is advisable. However, it is important to note that proxies may deviate from instructed perspectives, requiring future qualitative research to examine the adherence to proxy perspectives. Additionally, others have argued that proxy-reports should not substitute self-reports, and only serve as supplementary sources alongside patient self-reports whenever possible [ 9 ].
This review considered various QoL instruments, but most instruments adopted one specific proxy perspective, limiting detailed analyses. QoL instruments differ in their scope (generic versus disease-specific) as well as coverage of QoL domains. The QOL-AD, an Alzheimer's Disease-specific measure, was commonly used. Surprisingly, for this measure, the mean differences between self-reported and proxy-reported scores were smaller using the proxy-proxy perspective, contrary to the patterns observed with all other instruments. This may be due to the lack of studies reporting QOL-AD proxy scores from the proxy-patient perspective, as the study by Bosboom et al. (2012) found the opposite [ 5 ]. Previous research has also suggested that the inter-rater gap is dependent on the QoL domains and that the risk of bias is greater for more ‘subjective’ (less observable) domains such as emotions, feelings, and moods in comparison with observable, objective areas such as physical domains [ 8 , 40 ]. However, this review lacks sufficient observations for definitive results on QoL dimensions and their impact on self-proxy differences, emphasizing the need for future research in this area.
With regard to proxy type, there is an observable trend suggesting a wider inter-rater gap when family proxies are employed using the proxy-proxy perspective, in contrast to formal proxies. This variance might be attributed to the use of distinct anchoring points; family proxies tend to assess the individual's QoL in relation to their past self before having dementia, while formal caregivers may draw comparisons with other individuals with dementia under their care [ 41 ]. However, the opposite was found when the proxy-patient perspective was used, where family proxies scores seemed to align more closely with self-reported scores, resulting in lower SMD scores. This suggests that family proxies might possess a better ability to empathize with the perspective of the person with dementia compared to formal proxies. Nonetheless, it is important to interpret these findings cautiously, given the relatively small number of observations for formal caregiver reports. Additionally, other factors such as emotional connection, caregiver burden, and caregiver QoL may also impact proxy-reports by family proxies [ 14 , 16 ] that have not been explored in this review.
Our review found that the SMD between proxy and self-report increased with greater level of dementia severity, contrasting a previous study, which showed that cognitive impairment was not the primary factor that accounted for the differences in the QoL assessments between family proxies and the person with dementia [ 15 ]. However, it is noteworthy that different interpretations and classifications were used across studies to define mild, moderate, and severe dementia, which needs to be considered. Most studies used MMSE to define dementia severity levels. Given the MMSE’s role as a standard measure of cognitive function, the study findings are considered generalizable and clinically relevant for people with dementia across different dementia severity levels. When examining the role of the proxy perspective by level of severity, we found that compared with the proxy-proxy perspective, where self-reported scores were greater than proxy-reported scores across all dementia severity levels, the proxy-patient perspective yielded the opposite results, and proxies reported better QoL than people with dementia themselves, except for the severe subgroup. It is possible that in the early stages of dementia, the person with dementia has a greater awareness of increasing deficits, coupled with denial and lack of acceptance, leading to a more critical view of their own QoL than how proxies think they would rate their QoL. However, future studies are warranted, given the small number of observations adopting the proxy-patient perspective in our review.
The heterogeneity observed in the studies included was high, supporting the use of random-effects meta-analysis. This is not surprising given the diverse nature of studies included (i.e., RCTs, cross-sectional studies), differences in the population (i.e., people living in residential care versus community-dwelling people), mixed levels of dementia severity, and differences between instruments. While similar heterogeneity was observed in another review on a similar topic [ 42 ], our presentation of findings stratified by proxy type, dementia severity, and living arrangement attempted to account for such differences across studies.
Limitations and recommendations for future studies
Our review has some limitations. Firstly, proxy perspectives were categorized based on the authors' descriptions, but many papers did not explicitly state the perspective, which led to the use of assumptions based on instrument developers. Some studies may have modified the perspective's wording without reporting it. Due to lack of resources, we did not contact the authors of the original studies directly to seek clarification around the proxy perspective adopted. Regarding studies using the EQ-5D, which has two proxy perspectives, some studies did not specify which proxy version was used, suggesting the potential use of self-reported versions for proxies. In such cases, the proxy perspective was categorized as undefined. Despite accounting for factors like QoL measure, proxy type, setting, and dementia severity, we could not assess the impact of proxy characteristics (e.g., carer burden) or dementia type due to limited information provided in the studies. We also faced limitations in exploring the proxy perspective by QoL domains due to limited information. Further, not all studies outlined the data collection process in full detail. For example, it is possible that the proxy also assisted the person with dementia with their self-report, which could have resulted in biased estimates and the need for future studies applying blinding. Although we assessed the risk of bias of included studies, the checklist was not directly reflecting the purpose of our study that looked into inter-rater agreement. No checklist for this purpose currently exists. Finally, quality appraisal by a second reviewer was only conducted for the first 25% of the studies due to resource constraints and a low rate of disagreement between the two assessors. However, an agreement index between reviewers regarding the concordance in selecting full texts for inclusion and conducting risk of bias assessments was not calculated.
This review demonstrates that the choice of proxy perspective impacts the inter-rater gap. QoL scores from the proxy-patient perspective align more closely with self-reported scores than the proxy-proxy perspective. These findings contribute to the broader literature investigating factors influencing differences in QoL scores between proxies and individuals with dementia. While self-reported QoL is the gold standard, proxy-reports should be viewed as complements rather than substitutes. Both proxy perspectives offer unique insights, yet QoL assessments in people with dementia are complex. The difference in self- and proxy-reports can be influenced by various factors, necessitating further research before presenting definitive results that inform care provision and policy.
Data availability
All data associated with the systematic literature review are available in the supplementary file.
Moyle, W., & Murfield, J. E. (2013). Health-related quality of life in older people with severe dementia: Challenges for measurement and management. Expert Review of Pharmacoeconomics & Outcomes, 13 (1), 109–122. https://doi.org/10.1586/erp.12.84
Article Google Scholar
Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V., Paulsen, J. S., & Petersen, R. C. (2014). Classifying neurocognitive disorders: The DSM-5 approach. Nature reviews Neurology, 10 (11), 634–642. https://doi.org/10.1038/nrneurol.2014.181
Article PubMed Google Scholar
Health, The Lancet Regional., & – Europe. (2022). Challenges for addressing dementia. The Lancet Regional Health . https://doi.org/10.1016/j.lanepe.2022.100504
The WHOQOL Group. (1995). The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Social Science & Medicine, 41 (10), 1403–1409. https://doi.org/10.1016/0277-9536(95)00112-K
Bosboom, P. R., Alfonso, H., Eaton, J., & Almeida, O. P. (2012). Quality of life in Alzheimer’s disease: Different factors associated with complementary ratings by patients and family carers. International Psychogeriatrics, 24 (5), 708–721. https://doi.org/10.1017/S1041610211002493
Scholzel-Dorenbos, C. J., Rikkert, M. G., Adang, E. M., & Krabbe, P. F. (2009). The challenges of accurate measurement of health-related quality of life in frail elderly people and dementia. Journal of the American Geriatrics Society, 57 (12), 2356–2357. https://doi.org/10.1111/j.1532-5415.2009.02586.x
Logsdon, R. G., Gibbons, L. E., McCurry, S. M., & Teri, L. (2002). Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine, 64 (3), 510–519. https://doi.org/10.1097/00006842-200205000-00016
Hutchinson, C., Worley, A., Khadka, J., Milte, R., Cleland, J., & Ratcliffe, J. (2022). Do we agree or disagree? A systematic review of the application of preference-based instruments in self and proxy reporting of quality of life in older people. Social Science & Medicine, 305 , 115046. https://doi.org/10.1016/j.socscimed.2022.115046
Smith, S. C., Hendriks, A. A. J., Cano, S. J., & Black, N. (2020). Proxy reporting of health-related quality of life for people with dementia: A psychometric solution. Health and Quality of Life Outcomes, 18 (1), 148. https://doi.org/10.1186/s12955-020-01396-y
Article CAS PubMed PubMed Central Google Scholar
Andrieu, S., Coley, N., Rolland, Y., Cantet, C., Arnaud, C., Guyonnet, S., Nourhashemi, F., Grand, A., Vellas, B., & group, P. (2016). Assessing Alzheimer’s disease patients’ quality of life: Discrepancies between patient and caregiver perspectives. Alzheimer’s & Dementia, 12 (4), 427–437. https://doi.org/10.1016/j.jalz.2015.09.003
Jönsson, L., Andreasen, N., Kilander, L., Soininen, H., Waldemar, G., Nygaard, H., Winblad, B., Jonhagen, M. E., Hallikainen, M., & Wimo, A. (2006). Patient- and proxy-reported utility in Alzheimer disease using the EuroQoL. Alzheimer Disease & Associated Disorders, 20 (1), 49–55. https://doi.org/10.1097/01.wad.0000201851.52707.c9
Zucchella, C., Bartolo, M., Bernini, S., Picascia, M., & Sinforiani, E. (2015). Quality of life in Alzheimer disease: A comparison of patients’ and caregivers’ points of view. Alzheimer Disease & Associated Disorders, 29 (1), 50–54. https://doi.org/10.1097/WAD.0000000000000050
Article CAS Google Scholar
Buckley, T., Fauth, E. B., Morrison, A., Tschanz, J., Rabins, P. V., Piercy, K. W., Norton, M., & Lyketsos, C. G. (2012). Predictors of quality of life ratings for persons with dementia simultaneously reported by patients and their caregivers: The Cache County (Utah) study. International Psychogeriatrics, 24 (7), 1094–1102. https://doi.org/10.1017/S1041610212000063
Article PubMed PubMed Central Google Scholar
Schiffczyk, C., Romero, B., Jonas, C., Lahmeyer, C., Muller, F., & Riepe, M. W. (2010). Generic quality of life assessment in dementia patients: A prospective cohort study. BMC Neurology, 10 , 48. https://doi.org/10.1186/1471-2377-10-48
Sousa, M. F., Santos, R. L., Arcoverde, C., Simoes, P., Belfort, T., Adler, I., Leal, C., & Dourado, M. C. (2013). Quality of life in dementia: The role of non-cognitive factors in the ratings of people with dementia and family caregivers. International Psychogeriatrics, 25 (7), 1097–1105. https://doi.org/10.1017/S1041610213000410
Arons, A. M., Krabbe, P. F., Scholzel-Dorenbos, C. J., van der Wilt, G. J., & Rikkert, M. G. (2013). Quality of life in dementia: A study on proxy bias. BMC Medical Research Methodology, 13 , 110. https://doi.org/10.1186/1471-2288-13-110
Gomez-Gallego, M., Gomez-Garcia, J., & Ato-Lozano, E. (2015). Addressing the bias problem in the assessment of the quality of life of patients with dementia: Determinants of the accuracy and precision of the proxy ratings. The Journal of Nutrition, Health & Aging, 19 (3), 365–372. https://doi.org/10.1007/s12603-014-0564-7
Moon, H., Townsend, A. L., Dilworth-Anderson, P., & Whitlatch, C. J. (2016). Predictors of discrepancy between care recipients with mild-to-moderate dementia and their caregivers on perceptions of the care recipients’ quality of life. American Journal of Alzheimer’s Disease & Other Dementias, 31 (6), 508–515. https://doi.org/10.1177/1533317516653819
Crespo, M., Bernaldo de Quiros, M., Gomez, M. M., & Hornillos, C. (2012). Quality of life of nursing home residents with dementia: A comparison of perspectives of residents, family, and staff. The Gerontologist, 52 (1), 56–65. https://doi.org/10.1093/geront/gnr080
Griffiths, A. W., Smith, S. J., Martin, A., Meads, D., Kelley, R., & Surr, C. A. (2020). Exploring self-report and proxy-report quality-of-life measures for people living with dementia in care homes. Quality of Life Research, 29 (2), 463–472. https://doi.org/10.1007/s11136-019-02333-3
Pickard, A. S., & Knight, S. J. (2005). Proxy evaluation of health-related quality of life: A conceptual framework for understanding multiple proxy perspectives. Medical Care, 43 (5), 493–499. https://doi.org/10.1097/01.mlr.0000160419.27642.a8
Smith, S. C., Lamping, D. L., Banerjee, S., Harwood, R. H., Foley, B., Smith, P., Cook, J. C., Murray, J., Prince, M., Levin, E., Mann, A., & Knapp, M. (2007). Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychological Medicine, 37 (5), 737–746. https://doi.org/10.1017/S0033291706009469
Article CAS PubMed Google Scholar
Brooks, R. (1996). EuroQol: The current state of play. Health Policy, 37 (1), 53–72. https://doi.org/10.1016/0168-8510(96)00822-6
Herdman, M., Gudex, C., Lloyd, A., Janssen, M., Kind, P., Parkin, D., Bonsel, G., & Badia, X. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research, 20 (10), 1727–1736. https://doi.org/10.1007/s11136-011-9903-x
Rand, S., Caiels, J., Collins, G., & Forder, J. (2017). Developing a proxy version of the adult social care outcome toolkit (ASCOT). Health and Quality of Life Outcomes, 15 (1), 108. https://doi.org/10.1186/s12955-017-0682-0
Engel, L., Bucholc, J., Mihalopoulos, C., Mulhern, B., Ratcliffe, J., Yates, M., & Hanna, L. (2020). A qualitative exploration of the content and face validity of preference-based measures within the context of dementia. Health and Quality of Life Outcomes, 18 (1), 178. https://doi.org/10.1186/s12955-020-01425-w
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hrobjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal, 372 , n71. https://doi.org/10.1136/bmj.n71
Roydhouse, J. K., Cohen, M. L., Eshoj, H. R., Corsini, N., Yucel, E., Rutherford, C., Wac, K., Berrocal, A., Lanzi, A., Nowinski, C., Roberts, N., Kassianos, A. P., Sebille, V., King, M. T., Mercieca-Bebber, R., Force, I. P. T., & the, I. B. o. D. (2022). The use of proxies and proxy-reported measures: A report of the international society for quality of life research (ISOQOL) proxy task force. Quality of Life Research, 31 (2), 317–327. https://doi.org/10.1007/s11136-021-02937-8
The EndNote Team. (2013). EndNote (Version EndNote X9) [64 bit]. Philadelphia, PA: Clarivate.
Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan—A web and mobile app for systematic reviews. Systematic Reviews, 5 (1), 210. https://doi.org/10.1186/s13643-016-0384-4
Koo, T. K., & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15 (2), 155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Wan, X., Wang, W., Liu, J., & Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology, 14 , 135. https://doi.org/10.1186/1471-2288-14-135
Higgins JPT and Green S (editors). (2011). Cochrane handbook for systematic reviews of interventions.Version 5.1.0 [updated March 2011]. Retrieved 20 Jan 2023, from https://handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm
Ma, J., Liu, W., Hunter, A., & Zhang, W. (2008). Performing meta-analysis with incomplete statistical information in clinical trials. BMC Medical Research Methodology, 8 , 56. https://doi.org/10.1186/1471-2288-8-56
Deeks, J. J., Higgins, J. P. T., Altman, D. G., & on behalf of the Cochrane Statistical Methods Group. (2023). Chapter 10: Analysing data and undertaking meta-analyses. In J. Higgins & J. Thomas (Eds.), Cochrane Handbook for Systematic Reviews of Interventions. Version 6.4.
Kmet, L. M., Cook, L. S., & Lee, R. C. (2004). Standard quality assessment criteria for evaluating primary research papers from a variety of fields: Health and technology assessment unit. Alberta Heritage Foundation for Medical Research.
Lewis, T. J., & Trempe, C. L. (2017). Diagnosis of Alzheimer’s: Standard-of-care . USA: Elsevier Science & Technology.
Google Scholar
Leontjevas, R., Teerenstra, S., Smalbrugge, M., Koopmans, R. T., & Gerritsen, D. L. (2016). Quality of life assessments in nursing homes revealed a tendency of proxies to moderate patients’ self-reports. Journal of Clinical Epidemiology, 80 , 123–133. https://doi.org/10.1016/j.jclinepi.2016.07.009
Lapin, B., Cohen, M. L., Corsini, N., Lanzi, A., Smith, S. C., Bennett, A. V., Mayo, N., Mercieca-Bebber, R., Mitchell, S. A., Rutherford, C., & Roydhouse, J. (2023). Development of consensus-based considerations for use of adult proxy reporting: An ISOQOL task force initiative. Journal of Patient-Reported Outcomes, 7 (1), 52. https://doi.org/10.1186/s41687-023-00588-6
Li, M., Harris, I., & Lu, Z. K. (2015). Differences in proxy-reported and patient-reported outcomes: assessing health and functional status among medicare beneficiaries. BMC Medical Research Methodology . https://doi.org/10.1186/s12874-015-0053-7
Robertson, S., Cooper, C., Hoe, J., Lord, K., Rapaport, P., Marston, L., Cousins, S., Lyketsos, C. G., & Livingston, G. (2020). Comparing proxy rated quality of life of people living with dementia in care homes. Psychological Medicine, 50 (1), 86–95. https://doi.org/10.1017/S0033291718003987
Khanna, D., Khadka, J., Mpundu-Kaambwa, C., Lay, K., Russo, R., Ratcliffe, J., & Quality of Life in Kids: Key Evidence to Strengthen Decisions in Australia Project, T. (2022). Are We Agreed? Self- versus proxy-reporting of paediatric health-related quality of life (HRQoL) Using generic preference-based measures: A systematic review and meta-analysis. PharmacoEconomics, 40 (11), 1043–1067. https://doi.org/10.1007/s40273-022-01177-z
Download references
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was conducted without financial support.
Author information
Authors and affiliations.
Monash University Health Economics Group, School of Public Health and Preventive Medicine, Monash University, Level 4, 553 St. Kilda Road, Melbourne, VIC, 3004, Australia
Lidia Engel & Valeriia Sokolova
School of Health and Social Development, Deakin University, Burwood, VIC, Australia
Ekaterina Bogatyreva & Anna Leuenberger
You can also search for this author in PubMed Google Scholar
Contributions
LE contributed to the study conception and design. The original database search was performed by AL and later updated by VS. All authors were involved in the screening process, data extraction, and data analyses. Quality assessment was conducted by VS and LE. The first draft of the manuscript was written by LE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Lidia Engel .
Ethics declarations
Competing interests.
Lidia Engel is a member of the EuroQol Group.
Ethical approval
Not applicable.
Consent to participate
Consent to publish, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLSX 67 KB)
Supplementary file2 (docx 234 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Engel, L., Sokolova, V., Bogatyreva, E. et al. Understanding the influence of different proxy perspectives in explaining the difference between self-rated and proxy-rated quality of life in people living with dementia: a systematic literature review and meta-analysis. Qual Life Res (2024). https://doi.org/10.1007/s11136-024-03660-w
Download citation
Accepted : 27 March 2024
Published : 24 April 2024
DOI : https://doi.org/10.1007/s11136-024-03660-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Quality of Life
- Outcome measurement
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 04 May 2024
Effect of young age (below 40 years) on oncologic outcomes in Lebanese patients with breast cancer: a matched cohort study
- Eman Sbaity 1 ,
- Hani Tamim 2 ,
- Ghada El-Hajj Fuleihan 2 , 3 , 4 ,
- Jaber Abbas 1 ,
- Mariam Zahwe 5 ,
- Razan El Sayed 6 &
- Ali Shamseddine 7
BMC Cancer volume 24 , Article number: 560 ( 2024 ) Cite this article
Metrics details
Developing countries have a significantly higher incidence of breast cancer in patients younger than 40 years as compared to developed countries. This study aimed to examine if young age at diagnosis is an independent prognostic factor for worse survival outcomes in breast cancer as well as the effect of age on Disease-free survival (DFS) and local recurrence free survival (LRFS) after adjusting for various tumor characteristics, local and systemic treatments.
This is a secondary analysis of prospective cohort of patients from two existing databases. We identified patients with breast cancer aged 40 years or less and we matched them to those older than 40 years. We also matched based on stage and molecular subtypes. In cohort 1, we matched at a ratio of 1:1, while in cohort 2 we matched at a ratio of 1:3.
In cohort 1, Disease-free survival (DFS) at 5 years was significantly shorter for those younger than 40 years (75.6% and 92.7% respectively; p < 0.03). On multivariate analysis, only chemotherapy was found to be significant, while age was not found to be an independent predictor of prognosis. Local recurrence free survival at 5 years was similar between both age categories. Only hormonal therapy is a significant predictor for LRFS at 5 years. In the second cohort, DFS and LRFS at 3 years were similar between those younger and those older than 40 years. On multivariate analysis, no factor including age was found to be an independent predictor of prognosis.
Data in the literature is controversial on the effect of young age on breast cancer prognosis. Our findings could not demonstrate that age is an independent prognostic factor in our population. There is a need for outcomes from larger, prospective series that have longer follow-ups and more data from our region.
Peer Review reports
Breast cancer is the most common cancer in females globally, with 2.3 million women diagnosed with breast cancer in 2020 [ 1 ]. Median age of breast cancer varies between developed countries of 41.9 years [ 2 ] compared to 44–48 years in developing countries [ 3 , 4 ]. As for Lebanon, the median age is 49.8 years [ 5 ].
Although breast cancer does not commonly occur in patients younger than 40 years of age, it is a leading cause of death from cancer in this young population. The proportion of young patients diagnosed with breast cancer is much higher in developing countries, 19-33.3% in Arab countries [ 6 , 7 , 8 , 9 ] compared to developed countries, 5–7% in the United States of America. This may be explained by different population pyramid, environmental or genetic factors [ 10 ].
Reports of breast cancers in young patients show higher proportions of adverse clinic-pathologic features, Her2 neu expression, Estrogen receptors (ER)- and progesterone receptor (PR)-negative tumors, and high-grade tumors that tend to be larger and to involve regional lymph nodes [ 11 , 12 , 13 ]. In addition, authors documented a different distribution of molecular breast cancer subtypes between young breast cancer and older population [ 14 ].
The effect of young age on oncologic outcomes is controversial in the literature. Initially, physicians attributed the worse prognosis of breast cancer at a younger age to an advanced stage of presentation, adverse pathologic subtypes, and less aggressive treatments [ 15 , 16 , 17 , 18 ]. This is supported by results from a large series of patients showing young age to be an independent risk factor for worse disease-free survival (DFS), Distant Disease-Free Survival (DDFS), and overall survival (OS) [ 18 , 19 ]. Other authors consider young age as a surrogate marker of more advanced stage or more aggressive phenotypes resulting in worse prognosis.
Due to the perception of a more aggressive disease, young breast cancer patients frequently receive total mastectomies. Thus, it is of high clinical importance to understand whether breast cancer surgery (BCS) is associated with a higher risk of local recurrence to better counsel young patients. Many large retrospective series showed a higher rate of local recurrence in BCS as compared to total mastectomy performed at a young age, but no difference in survival [ 18 , 20 , 21 , 22 , 23 , 24 , 25 ]. Thus, when indicated, BCS is still a viable option for young patients..
The aim of this cohort study is to examine if young age at diagnosis is an independent prognostic factor for worse survival outcomes in breast cancer as well as the effect of age on DFS and local recurrence free survival (LRFS) after adjusting for various tumor characteristics, local and systemic treatments received.
Methodology
Study design.
We conducted a secondary analysis of two existing prospective cohorts of breast cancer patients. We matched patients younger than 40 years to those older than 40 years at a ratio of 1:3. We matched cases based on the stage of breast cancer at presentation and molecular subtypes.
Data sources
The study is a secondary analysis of two existing databases. The first is prospectively collected data of all Lebanese non-metastatic breast cancer patients who received any part of their treatment at the American University of Beirut Medical Center (AUBMC) between the years 2011–2014 to study the difference in outcomes between the two age groups (IRB study #IM.AS.17). It includes 123 Lebanese patients (with 47 patients below the age of 40 years). The second is an IRB-approved prospectively collected database of the clinical research unit at Basile Cancer Institute at AUBMC (BIO-2018-0302), including all consecutive breast cancer patients who have presented to AUBMC from October 2014 to December 2016. Informed consent to participate in the initial studies was obtained from all participants. We performed a comparison between both datasets regarding tumor characteristics and received treatments. This revealed statistically significant differences in both populations, so we decided to analyze each cohort separately.
Eligibility criteria
We included patients with:
Non-metastatic biopsy-proven breast cancer.
Lebanese women, older than 18 years.
Received any part of their treatment or followed up at AUBMC.
We excluded:
Male breast cancer patients.
Patients with unclassified tumor.
Patients with missing data on stage or ER, PR, or Her2 NEU or staging information.
Sampling Frame
At our institution, breast cancer patients are treated by dedicated breast surgical oncologists, breast medical oncologists, and breast radiation oncologists, ensuring similar and up-to-date treatment plans based on National Comprehensive Cancer Network (NCCN) guidelines, American Society of Cancer Oncology (ASCO), and ASBS (American Society of Breast Surgery). In addition, most of these patients are discussed in the weekly Breast Tumor Board before initiation of treatments.
Outcome measures
Primary outcome:
DFS at 3 years, defined by survival without clinical or radiological evidence of recurrence of disease, whether local, distant, or both.
Secondary outcomes:
Local recurrence Free Survival at 3 years (LRFS) defined by clinical or radiological evidence of disease in the affected breast or regional nodal basin.
Overall Survival (OS) at 3 years defined from time of diagnosis till date of death or last follow up.
Distant Metastasis Free Survival (DMFS) is defined from the date of diagnosis till the date of distant metastasis or till the date of last follow up in patients who did not experience metastasis.
Effect of various tumor grade, local and systemic treatments received on DFS, LRFS, and DMFS.
Statistical analysis
We conducted all analyses using SPSS Version 24, and statistical significance was assumed at a p < 0.05. Prognostic factors were compared between the two groups using chi-square test for categorical variables and independent t-test for continuous variables. We estimated DFS, LRFS, DMFS, and OS, using Kaplan–Meier method and Log-Rank test for the different survival curves between both age categories. We then performed a univariate analysis comparing the effect of each grade of tumor, chemotherapy, Hormonal therapy, Herceptin, type of surgery, radiation therapy, and BMI on DFS, LRFS, and DMFS at three years follow up. We used Cox regression analysis to assess how survival outcomes change between both age groups after controlling for grade of tumor, chemotherapy, hormonal therapy, Herceptin therapy, surgery type, and radiation therapy. COX Regression was done using the forward and backward methods.
Description
The first cohort included 122 breast cancer patients, 77 patients above the age of 40 years, and 45 patients below or equal to 40 years. We performed 1:1 matching, where 41 breast cancer patients aged 40 or younger were matched to 41 breast cancer patients older than 40. The patients’ molecular subtypes and stages are shown in Table 1 .
Comparison of treatments received
Total mastectomy was performed only in onethird of patients above 40 years as compared to 56% of patients below 40 years with p = 0.026. Radiotherapy and chemotherapy were administered in a similar proportion for both groups without any statistical difference. A higher proportion of patients in the younger subgroup received trastuzumab as compared to the older subgroup, 30% and 12.2% respectively ( p = 0.049). Similarly, patients in the younger subgroup are more likely to receive hormonal therapy, but without reaching a statistical significance. (Table 1 )
Only one death is documented in the older subgroup, and two deaths in the younger subgroup. Local recurrence is double in the younger age group, with 6 local recurrence events (14.6%) compared to 3 local recurrence events (7.3%) in the older age group. Distant metastasis occurred more in the younger age groupin 9 patients (22%) compared to 6 (14.6%) in the older age group.
Survival analysis
DFS at 5 years : in young patients, the DFS is 75.6% compared to 92.7% in older patients with p = 0.035. In patients who received chemotherapy, DFS is lower in the younger age group,74.1% compared to 100% in those older than 40 years, with a statistically significant p -value of 0.005. Similarly, in patients who received hormonal therapy, DFS is lower in the younger subgroup of patients, at76.5% compared to 96.8%, with a p -value of 0.016. On multivariate analysis, only chemotherapy was an independent prognostic factor for DFS at 5 years. Age was not found to be an independent prognostic factor for DFS. (Table 2 )
LRFS at 5 years : among those above 40 years, the is 92.7% (3 local recurrence events) and 87.8% (5 local recurrence events) among those below or equal to 40 years ( p = 0.361).
We did not find any clinically or statistically significant difference when stratifying LRFS at 5 years according to the different loco-regional and systemic therapies. Patients who underwent partial mastectomy have an LRFS of 96.4% in the older group and 94.4% in the younger group, with no statistical significance ( p = 0.655). For those who underwent total mastectomy, LRFS is 84.6% and 82.6% in the older and younger group, respectively ( p = 0.821). In multivariate cox regression analysis, only hormonal therapy was found to be a predictor for worse LRFS at 5 years, while age was not found to be an independent prognostic factor.
OS at 5 years : in the above 40 years group, the overall survival at 5 years is 97.6% and 95.1% in the below or equal to 40 years group, with no statistical significance ( p = 0.490) (Table 3 ).
There are a total of 399 patients in cohort 2, with 55 breast cancer patients aged 40 or younger matched to 165 breast cancer patients older than 40 years, at a 1:3 ratio. In the older age group, a total of 40 patients (28%) had grade 3 breast cancer, compared to 22 patients (44%) in the younger age category with a significant p -value of 0.03 (Table 4 ).
Mastectomy was performed more frequently in the older group, but the difference is not statistically significant ;76 (46.6%) patients above 40 years underwent total mastectomy as compared to 20 (37%) patients below 40 years ( p = 0.219). For radiation therapy, 67.7% of patients over 40 years received radiotherapy, and 64.8% of patients in the younger group ( P = 0.698). Both age subgroups received chemotherapy in a similar proportion, with 74.4% of patients in the above 40 years groupand 77.4% in the below or equal to 40 years group ( p -value of 0.663). A higher proportion of patients in the younger subgroup received trastuzumab and hormonal therapy as compared to the older subgroup but without reaching statistical significance. A total of 81.3% of patients younger than 40 years received hormonal therapy compared to 69.7% of patients older than 40 years ( p = 0.119).
No deaths are documented in any subgroup. Local recurrence did not occur in the younger group, and only 1 event (0.6%) occurred in the older group. Distant metastasis occurred in 1 patient (1.8%) in the below 40 years subgroup and in 6 patients (3.6%) in the older age group.
DFS at 3-years : is slightly shorter for the younger age patient category but without reaching statistical significance (97% versus 98.2%; p = 0.621). DFS is 100% for partial mastectomy in the younger group and 97.7% (2 events) in the older group ( p = 0.346). Among patients treated with total mastectomy, DFS is 97.4% in the older group and 95% in the younger group, with no statistical significance ( p = 0.645). On multivariate Cox regression analysis for DFS at 3 years for cohort 2, no factor was found to be a significant predictor of survival.
LRFS at 3 years : local recurrence occurred only in 1 patient (99.4%) in the younger group and none in the older group. ( p = 0.537). On multivariate Cox regression analysis for cohort 2, no factor was found to be a significant predictor of survival for LRFS at 3 years.
In our first cohort, the distribution of early and advanced breast cancer was similar. In the second cohort, older breast cancer patients were more likely to be present in the early stages. This result was similar to that reported by many authors who mentioned that young patients with breast cancer present with a more aggressive clinical picture and advanced stage as compared to older patients. Cancers in the younger age group are usually detected by the patients themselves and,consequently, are often bigger in size and more advanced than the screen-detected tumors in those above 40 years [ 26 , 27 ].
We had a similar proportion of molecular subtypes in both age categories, with a slightly higher proportion of Triple negative molecular subtype in our young patient population. This is different from the proportions known for breast cancer patients. The literature reports on a difference in the distribution of breast cancer molecular subtypes between younger and older patients [ 14 , 28 ]. Variability in molecular subtyping among different populations may be due to variability in pathology reviews and different definitions to categorize the 4 subtypes, where mainly the confusion happens with deciding on luminal A and B.
Age was not found to be an independent prognostic factor in our cohort of patients, which is matched for stage and molecular subtype and after adjusting for treatments received. DFS after 5 years was statistically lower in the younger group. However, LRFS at 3 years and 5 years were not statistically different between both age categories. Nixon et al. reported similar results where young breast cancer has a higher local recurrence rate and inferior DFS [ 12 , 13 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ]. Recurrence events in breast cancer usually happen at a median of 32 months, which falls within our follow-up period. Nevertheless, a longer follow-up is required to make a solid conclusion.
When we stratified the outcomes according to the type of surgery done, there was no significant difference in the DFS at 3 and 5 years and LRFS at 3 years in both age categories. This result was similar to the National Cancer Institute randomized study that showed similar local recurrence between both arms of treatment for BCS and total mastectomy [ 38 , 39 ]. Age was not found to be a predictor of local recurrence when total mastectomy was performed. However, in the Institut Gustave-Roussy Breast Cancer Group Distribution of local recurrence based on age grouping of less than and more than 40 years, there was a three times higher rate of local recurrence in the younger patients with BCS.
On univariate analysis of cohort 1, patients receiving radiotherapy and those below 40 years had lower 5-year DFS, but this difference was not statistically significant ( p = 0.093). We cannot compare our results to the literature because the majority of studies did not report on the use of radiation therapy after BCS, so we cannot identify those local recurrences secondary to lack of radiation. Similarly, it is not possible to discern the effect of post mastectomy radiation on preventing local recurrences.
In the strata of patients who received chemotherapy, DFS is lower in the younger age group, 74.1% compared to 100%, in those older than 40 years, with a statistically significant p -value. On multivariate analysis, only chemotherapy was an independent prognostic factor for DFS at 5 years. Age was not found to be an independent prognostic factor for DFS. Similarly, we did not find a statistically significant difference when we stratified LRFS at 5 years for the chemotherapy treatments. On the contrary, the Beadle et al. study [ 40 ] showed that local therapies did not affect the rate of local recurrence in young patients, but systemic therapy did affect it with statistical significance. This speaks about a different biology in young patients, where perhaps even in stage I, chemotherapy should be given to improve oncologic outcomes.
In the strata of patients in cohort 1, patients who received hormonal therapy had statistically significant lower DFS at 5 years in the younger subgroup of patients but not for LRFS at 5 years. On multivariate Cox regression analysis, only hormonal therapy was found to be a predictor for worse LRFS at 5 years, while age was not found to be an independent prognostic factor. ER-positive is reported in the literature to be associated with worse prognosis in the younger population. Some attribute this to the fact that young patients were not treated with hormonal therapy until recently. Consequently, the worse prognosis observed in the young population of ER-positive cancermay be due to the differential use of Tamoxifen. In addition, even after treating young breast cancer patients with Tamoxifen, there is high non-compliance. This was demonstrated by a systematic review by Murphy et al. [ 41 ].
Among those who received trastuzumab, LRFS is 100% in the older group and 83.3% in the younger group ( p = 0.315). For those who did not receive trastuzumab, LRFS is similar, with 91.7% in the older group and 89.3% in the younger group, with no statistical significance ( p = 0.629). Many studies that explored local recurrence in a large cohort of breast cancer patients included patients from the era before the introduction of trastuzumab in the treatment of Her 2 positive breast cancer. This is a major limitation of such studies because trastuzumab dramatically improved in oncologic outcomes when indicated.
Strengths and limitations
This study aimed to fill a gap in the literature where there is limited data from the modern era of effective surgery, chemotherapy, hormonal, and radiation therapy in young patients. The reported data on the effect of age on breast cancer outcomes is widely retrospective; our data is prospectively collected. Another strength of our study is matching the two age cohorts on the two most important baseline prognostic factors (stage and molecular phenotype). Data on local recurrence after BCT in young patients mainly camefrom series collected over long periods of time and did not receive the modern modalities of breast cancer treatments. Our cohort of patients is a modern cohort treated based on modern therapies.
Because there is no breast cancer national comprehensive database, we could not compare our cohort characteristics to national and thus decide on the generalizability of our results. We have a limited follow-up period for our patients, where the majority reach only 3 years of follow-up.
Data availability
The datasets analyzed in the current study are available on request from the corresponding author.
Abbreviations
Estrogen Receptors
Progesterone Receptor
Disease Free Survival
Distant Disease-Free Survival
Overall Survival
Breast Cancer Surgery
Local Recurrence Free Survival
American University of Beirut Medical Center
Human Epidermal Growth Factor Receptor 2
National Comprehensive Cancer Network
American Society of cancer Oncology
American Society of Breast Surgery
Distant Metastasis Free Survival
Breast-conserving therapy
WHO. Breast cancer 2021 [Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
Dafni U, Tsourti Z, Alatsathianos I. Breast Cancer statistics in the European Union: incidence and survival across European Countries. Breast Care. 2019;14(6):344–53.
Article PubMed PubMed Central Google Scholar
Obiorah CC, Abu EK. Breast cancer in Rivers State, Nigeria: ten-year review of the Port Harcourt cancer registry. South Afr J Oncol. 2019;3.
Mehdi I, Monem EA, Bahrani BJA, Kharusi SA, Nada AM, Lawati JA, et al. Age at diagnosis of female breast cancer in Oman: issues and implications. South Asian J Cancer. 2014;03(02):101–6.
Article Google Scholar
El Saghir NS. Increased age adjusted incidence rates in younger-aged groups at presentation. In Lebanon and arab countries. Implications for screening and for europeans, australians and americans of Arabic origins. Eur J Cancer Suppl. 2004;2(3):187–8.
Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (≤ 40 years) with breast cancer: young women with breast Cancer. J Surg Oncol. 2009;100(3):248–51.
Article PubMed Google Scholar
Elkum N, Dermime S, Ajarim D, Al-Zahrani A, Alsayed A, Tulbah A, et al. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007;7(1):222.
AlZaman AS, Mughal SA, AlZaman YS, AlZaman ES. Correlation between hormone receptor status and age, and its prognostic implications in breast cancer patients in Bahrain. SMJ. 2016;37(1):37–42.
Peng R, Wang S, Shi Y, Liu D, Teng X, Qin T, et al. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case–control study. The Breast. 2011;20(6):568–73.
Chouchane L, Boussen H, Sastry KSR. Breast cancer in arab populations: molecular characteristics and disease management implications. Lancet Oncol. 2013;14(10):e417–e24.
Article CAS PubMed Google Scholar
Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, et al. Very young women (< 35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13(2):273–9.
Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr. 1994(16):35–42.
Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. JCO. 1994;12(5):888–94.
Article CAS Google Scholar
Azim HA, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating Prognosis and Biology of breast Cancer arising in Young Women using gene expression profiling. Clin Cancer Res. 2012;18(5):1341–51.
Adami H-O, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast Cancer. N Engl J Med. 1986;315(9):559–63.
de la Rochefordière A, Campana F, Fenton J, Vilcoq JR, Fourquet A, Asselain B, et al. Age as prognostic factor in premenopausal breast carcinoma. The Lancet. 1993;341(8852):1039–43.
Dubsky PC, Gnant MFX, Taucher S, Roka S, Kandioler D, Pichler-Gebhard B, et al. Young Age as an independent adverse prognostic factor in Premenopausal patients with breast Cancer. Clin Breast Cancer. 2002;3(1):65–72.
Oh JL, Bonnen M, Outlaw ED, Schechter NR, Perkins GH, Strom EA, et al. The impact of young age on locoregional recurrence after doxorubicin-based breast conservation therapy in patients 40 years old or younger: how young is young? Int J Radiation Oncology*Biology*Physics. 2006;65(5):1345–52.
Liedtke C, Hess KR, Karn T, Rody A, Kiesel L, Hortobagyi GN, et al. The prognostic impact of age in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2013;138(2):591–9.
Recht A, Connolly JL, Schnitt SJ, Silver B, Rose MA, Love S, et al. The effect of young age on tumor recurrence in the treated breast after conservative surgery and radiotherapy. Int J Radiation Oncology*Biology*Physics. 1988;14(1):3–10.
Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, et al. Why are local recurrences after breast-conserving therapy more frequent in younger patients? JCO. 1990;8(4):591–8.
Kurtz JM, Spitalier J-M, Amalric R, Brandone H, Ayme Y, Bressac C, et al. Mammary recurrences in women younger than forty. Int J Radiation Oncology*Biology*Physics. 1988;15(2):271–6.
Kim SH, Simkovich-Heerdt A, Tran KN, Maclean B, Borgen PI. Women 35 years of age or younger have higher Locoregional Relapse Rates after undergoing breast conservation therapy. J Am Coll Surg. 1998;187(1):1–8.
van der Sangen MJC, Poortmans PMP, Scheepers SWM, Lemaire BMD, van Berlo CLH, Tjan-Heijnen VCG, et al. Prognosis following local recurrence after breast conserving treatment in young women with early breast cancer. Eur J Surg Oncol (EJSO). 2013;39(8):892–8.
Kroman N, Holtveg H, Wohlfahrt J, Jensen M-B, Mouridsen HT, Blichert-Toft M, et al. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer. 2004;100(4):688–93.
Ruddy KJ, Gelber S, Tamimi RM, Schapira L, Come SE, Meyer ME, et al. Breast cancer presentation and diagnostic delays in young women: breast ca Presentation in Young Women. Cancer. 2014;120(1):20–5.
Foxcroft LM, Evans EB, Porter AJ. The diagnosis of breast cancer in women younger than 40. The Breast. 2004;13(4):297–306.
Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131(3):1061–6.
Adami H-O, Malker B, Meirik O, Persson I, Bergkvist L, Stone B. Age as a prognostic factor in breast cancer. Cancer. 1985;56(4):898–902.
Fowble BL, Schultz DJ, Overmoyer B, Solin LJ, Fox K, Jardines L, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiation Oncology*Biology*Physics. 1994;30(1):23–33.
Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77(1):97–103.
Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko S-S, et al. Poor outcome of hormone receptor–positive breast Cancer at very young age is due to tamoxifen resistance: Nationwide Survival Data in Korea—A Report from the Korean breast Cancer Society. JCO. 2007;25(17):2360–8.
Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast Cancer in Young women: poor survival despite intensive treatment. PLoS ONE. 2009;4(11):e7695.
Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast Cancer mortality in women younger than Age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208(3):341–7.
Xiong Q, Valero V, Kau V, Kau S-W, Taylor S, Smith TL, et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the M. D. Anderson Cancer Center experience. Cancer. 2001;92(10):2523–8.
El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6(1):194.
Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage III breast cancer in young women1. J Am Coll Surg. 2000;190(5):523–9.
Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute randomized trial. Cancer. 2003;98(4):697–702.
Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast Cancer. N Engl J Med. 1995;332(14):907–11.
Beadle BM, Woodward WA, Tucker SL, Outlaw ED, Allen PK, Oh JL, et al. Ten-year recurrence rates in young women with breast cancer by locoregional treatment approach. Int J Radiat Oncol Biol Phys. 2009;73(3):734–44.
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–78.
Article CAS PubMed PubMed Central Google Scholar
Download references
Acknowledgements
The authors acknowledge the contribution of Ahmad Najia and Nadia Hoyek who were involved in this research under the Medical Research Volunteer Program (MRVP) at the American University of Beirut.
Author information
Authors and affiliations.
Department of Surgery, American University of Beirut Medical Center, P.O. Box: 11-0236, Riad El-Solh, Beirut, 1107 2020, Lebanon
Eman Sbaity & Jaber Abbas
Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Hani Tamim & Ghada El-Hajj Fuleihan
Calcium Metabolism and Osteoporosis Program, American University of Beirut, Beirut, Lebanon
Ghada El-Hajj Fuleihan
Scholars in HeAlth Research Program (SHARP), American University of Beirut Medical Center, Beirut, Lebanon
Faculty of Medicine, American University of Beirut, Beirut, Lebanon
Mariam Zahwe
Center for Translational Injury Research, Department of Surgery, McGovern Medical School, University of Texas Health Science Center (UT Health), Houston, USA
Razan El Sayed
Department of Hematology/Oncology, American University of Beirut Medical Center, Beirut, Lebanon
Ali Shamseddine
You can also search for this author in PubMed Google Scholar
Contributions
Methodology: E.S, H.T, G.E, A.S; Resources: A.S, J.A; Data Curation: E.S; Data Analysis: E.S, H.T; Writing - original draft: E.S; Writing – review & editing: H.T, G.E, A.S, J.A, M.Z, R.E. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Eman Sbaity .
Ethics declarations
Ethics approval and consent to participate.
This study is a secondary analysis of two IRB-approved databases at the American University of Beirut Medical Center. All methods of the initial studies were carried out in accordance with the Declaration of Helsinki guidelines and regulations and got approved by the Institutional Review Board of the American University of Beirut Medical Center. (IRB number: IM.AS.17) (BIO-2018-0302). Informed consent to participate in the study was obtained from all participants included in the initial studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sbaity, E., Tamim, H., El-Hajj Fuleihan, G. et al. Effect of young age (below 40 years) on oncologic outcomes in Lebanese patients with breast cancer: a matched cohort study. BMC Cancer 24 , 560 (2024). https://doi.org/10.1186/s12885-024-11910-w
Download citation
Received : 17 February 2023
Accepted : 22 January 2024
Published : 04 May 2024
DOI : https://doi.org/10.1186/s12885-024-11910-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 02 May 2024
Alteration of prothrombin time in Plasmodium falciparum and Plasmodium vivax infections with different levels of severity: a systematic review and meta-analysis
- Suriyan Sukati 1 , 2 ,
- Tirawat Wannatung 3 ,
- Thitinat Duangchan 1 , 2 ,
- Kwuntida Uthaisar Kotepui 8 ,
- Frederick Ramirez Masangkay 4 ,
- Ching-Ping Tseng 5 , 6 , 7 &
- Manas Kotepui 8
Scientific Reports volume 14 , Article number: 9816 ( 2024 ) Cite this article
137 Accesses
Metrics details
- Diagnostic markers
- Prognostic markers
Malaria infection leads to hematological abnormalities, including deranged prothrombin time (PT). Given the inconsistent findings regarding PT in malaria across different severities and between Plasmodium falciparum and P. vivax , this study aimed to synthesize available evidence on PT variations in clinical malaria. A systematic literature search was performed in PubMed, Embase, Scopus, Ovid, and Medline from 27 November 2021 to 2 March 2023 to obtain studies documenting PT in malaria. Study quality was evaluated using the Joanna Briggs Institute checklist, with data synthesized through both qualitative and quantitative methods, including meta-regression and subgroup analyses, to explore heterogeneity and publication bias. From 2767 articles, 21 studies were included. Most studies reported prolonged or increased PT in malaria patients compared to controls, a finding substantiated by the meta-analysis ( P < 0.01, Mean difference: 8.86 s, 95% CI 5.32–12.40 s, I 2 : 87.88%, 4 studies). Severe malaria cases also showed significantly higher PT than non-severe ones ( P = 0.03, Hedges’s g: 1.65, 95% CI 0.20–3.10, I 2 : 97.91%, 7 studies). No significant PT difference was observed between P. falciparum and P. vivax infections ( P = 0.88, Mean difference: 0.06, 95% CI − 0.691–0.8, I 2 : 65.09%, 2 studies). The relationship between PT and malaria-related mortality remains unclear, underscoring the need for further studies. PT is typically prolonged or increased in malaria, particularly in severe cases, with no notable difference between P. falciparum and P. vivax infections. The inconsistency in PT findings between fatal and non-fatal cases highlights a gap in current understanding, emphasizing the need for future studies to inform therapeutic strategies.
Similar content being viewed by others

Methemoglobin levels in malaria: a systematic review and meta-analysis of its association with Plasmodium falciparum and Plasmodium vivax infections and disease severity

Prevalence and outcomes of malaria as co-infection among patients with human African trypanosomiasis: a systematic review and meta-analysis

Association of reduced glutathione levels with Plasmodium falciparum and Plasmodium vivax malaria: a systematic review and meta-analysis
Introduction.
Malaria, caused by Plasmodium species infection, is a serious global health challenge. In 2021, over 200 million people were infected, leading to an estimated 619,000 deaths 1 . Infections by Plasmodium species are transmitted through the bites of infected female Anopheles mosquitoes 2 . The five most common Plasmodium species infecting humans are Plasmodium falciparum ( P. falciparum ), Plasmodium malariae ( P. malariae ), Plasmodium vivax ( P. vivax ), Plasmodium ovale ( P. ovale ), and Plasmodium knowlesi ( P. knowlesi ), with P. falciparum responsible for the most fatal infections. After transmission via mosquito bites, the malaria parasite travels to the liver, replicates in the host's liver cells, and then proceeds to infect red blood cells in the circulation 3 . Plasmodium infection can lead to a wide range of clinical symptoms, which may include fever, chills, headaches, nausea, vomiting, muscle aches, fatigue, and, in some cases, jaundice due to the destruction of red blood cells. Symptoms can vary from mild, uncomplicated malaria, which involves the aforementioned symptoms without signs of severe organ dysfunction, to severe malaria, characterized by more serious conditions such as impaired consciousness, respiratory distress, multiple convulsions, severe anemia, hemoglobinuria, acute kidney injury, hyperparasitemia, and complications leading to death 4 . Well-documented hematological changes occur during malaria infection, such as anemia, thrombocytopenia, and lymphocytopenia 5 , 6 . Moreover, severe malaria can result in significant liver damage, respiratory distress, renal failure, multi-organ dysfunction, abnormal bleeding, and cerebral malaria—a fatal neurological complication predominantly associated with P. falciparum infection 7 .
Coagulation, a critical process in hemostasis, involves a complex cascade of events that lead to the formation of a blood clot, preventing excessive bleeding when blood vessels are injured 8 , 9 . Initially, the process begins with the vascular injury, triggering the exposure of tissue factor, a key activator of the coagulation cascade. This exposure leads to the activation of the extrinsic pathway, marked by the conversion of prothrombin to thrombin. Thrombin then plays a central role in converting fibrinogen into fibrin, which forms the structural basis of a clot 10 . Simultaneously, the intrinsic pathway, initiated by contact activation factors within the blood (Factors XII, XI, VIII, IX), converges with the extrinsic pathway to amplify thrombin production. The common pathway, involving both extrinsic and intrinsic pathways, culminates in the stabilization of the fibrin clot alongside activated platelets, effectively sealing the site of vascular injury 8 , 9 , 10 .
Coagulation abnormalities are common laboratory findings in malaria infection, observable across the spectrum from uncomplicated to severe malaria 11 , 12 . Previous studies have reported that clinically apparent bleeding and disseminated intravascular coagulation (DIC), a severe coagulopathy, is associated with severe malaria infection and is more common in cases of cerebral malaria 13 , 14 . It has been reported that approximately 5–10% of severe malaria cases develop DIC, which is associated with high mortality 15 . The pathogenesis of Plasmodium species, specifically, P. falciparum is known to cause severe endothelial dysfunction in cases of both uncomplicated and fatal malaria due to its unique ability to sequester in the microvasculature and induce inflammatory responses 11 . Various procoagulants present during malaria infection, including tissue factor (TF) released from damaged vascular endothelial cells, the induction of TF expression in endothelial cells, exposed phosphatidylserine on infected red blood cells, lysis of activated platelets, macrophage migration inhibitory factor (MIF), and interleukin-6 cytokine (IL-6), are believed to activate the coagulation system 16 , 17 , 18 .
Routine laboratory coagulation tests, such as prothrombin time (PT) and activated partial thromboplastin time (APTT), are commonly used to investigate blood coagulation changes. PT reflects the activity of the extrinsic (factor VII) and common (factors V, X, II, and I) coagulation pathways, while APTT monitors the competency of the intrinsic (Factors XII, XI, VIII, IX) and common pathways 19 . Prior research indicates that the extent of coagulation derangement often corresponds with the severity and activity of the disease process 20 . Some studies indicate that patients with high parasitemia often experience alterations in coagulation tests, as high parasitemia is known to enhance fibrin formation and activate plasminogen, thus disturbing the coagulation system 21 , 22 , 23 . Notably, prolonged PT has been observed in P. falciparum infections characterized by high parasitemia 22 , suggesting that PT could potentially serve as a predictive marker for the clinical progression of Plasmodium infection. Nevertheless, the results of PT in malaria remain inconsistent across different studies. Therefore, this systematic review and meta-analysis aims to investigate differences in PT between malaria patients and uninfected controls, between varying degrees of disease severity, between different Plasmodium species, and between fatalities and survivors. Such a data collection would help in monitoring and predicting disease progression and could aid in preventing life-threatening complications of malaria infection through early detection and proper management of the hypercoagulable state.
Protocol and registration
The protocol of systematic review was registered at PROSPERO with a registration number CRD42022346003. The systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines 24 .
Data sources and searches
A comprehensive search was conducted in databases such as PubMed, Embase, Scopus, Ovid, and Medline from their inception up to March 2, 2023 (the final date of searches), to identify studies reporting on PT in malaria cases. To construct the search strategy, the search terms were combined with Boolean operators (AND, OR): (coagulation OR "blood clotting" OR "prothrombin time" OR prothrombin OR "russell viper venom time" OR "russells viper venom time" OR "thrombotest" OR "quick test"). Language restrictions were applied to include only English-language articles, without constraints on the year of publication. The details of the searches in all databases are listed in Table S1 .
Definitions
Severe P. falciparum malaria is characterized by the presence of P. falciparum asexual parasitaemia alongside one or more complications as specified by the WHO criteria for severe malaria such as impaired consciousness, prostration, severe malarial anemia, jaundice, acidosis, renal impairment, significant bleeding, shock, multiple convulsions, hypoglycemia, and hyperparasitemia 25 . Severe P. vivax malaria follows the same definition as severe P. falciparum malaria, except that it doesn't require any specified parasite density thresholds. Non-severe malaria, on the other hand, is identified by the detection of asexual Plasmodium parasitemia, yet with the absence of any complications stipulated by the WHO's criteria for severe malaria.
The outcomes of the systematic review were the difference in PT between the following groups of participants: (i) malaria cases and uninfected controls, (ii) severe and non-severe malaria cases, (iii) different Plasmodium species, and (iv) deaths and survivors.
Eligibility criteria
The PICo (P: population, I: Outcome of interest, Co: context) approach was used to include eligible studies. (i) P: patients with malaria in all clinical severity (asymptomatic, uncomplicated, severe, or fatal malaria). (ii) I: prothrombin time in qualitative (prolonged or normal) and quantitative using mean ± standard deviation (SD) for normally distributed data or median and interquartile range (IQR) for data not normally distributed. Co: worldwide or global. The inclusion criteria are: (i) original studies that investigated prothrombin time in patients with malaria; (ii) study designs that could be cross-sectional, cohort, or case–control studies; (iii) PT assessments conducted upon admission, before treatment. The exclusion criteria were: case reports/case series, clinical trials without baseline PT measurements, letters, communications, conference abstracts, book series, in vitro studies, articles not in English, and reviews/systematic reviews.
Study selection and data extraction
Study selection was performed by two review authors (MK and SS) independently using EndNote 20 for reference management. First, duplicate studies were excluded, and then titles and abstracts of the remaining studies were screened. Second, studies with irrelevant titles and abstracts were removed. Third, potentially relevant studies were examined for full-text, and ineligible studies were removed with specific reasons. Fourth, studies that met the eligibility criteria were included in the systematic review. After the studies were selected based on the eligibility criteria, the following data were extracted from each study using a pilot-tested, standard datasheet: name of the first author, publication year, study design, study location (year of conduction), characteristics of participants enrolled, Plasmodium spp., age range, PT in malaria and other groups, method for malaria detection, and method for PT. Data from each study were extracted by two authors (SS and MK). Any disagreements regarding study selection and data extraction between the two authors were resolved through discussion to reach a consensus.
Quality assessment
Two review authors (SS and TD) independently assessed the quality of included studies using the Joanna Briggs Institute (JBI) critical appraisal checklist for observational studies 26 . The checklist contains a set of questions that determine the internal and external validity of a study. These questions cover various aspects of study design, conduct, and reporting, including: sampling of study participants, sample size, study subjects and the setting, data analysis, methods used for the identification of the condition, a standard, reliable way for measurement of the condition, statistical analysis, confounding factors, identification of subpopulations, and measurement of outcome. Each item is scored as "yes," "no," "unclear," or "not applicable". The total score defines the overall quality of the study in which low, moderate, and the high quality if the total score were ≤ 50%, 51–74%, and ≥ 75%, respectively.
Data synthesis
Data synthesis was performed using qualitative and quantitative approaches. The qualitative involved the narrative description of the results of an individual study; meanwhile, the quantitative synthesis involved the pooling of results from several studies that reported the same outcome data. In the present study, the pooled mean difference (MD) or standardized mean difference (Hedge’s g) was used as the pooled effect estimates of PT between groups of participants including (i) malaria cases and uninfected controls, (ii) severe and non-severe malaria cases, (iii) and different Plasmodium species. The heterogeneity of the effect estimates were determined using the I 2 for inconsistency in which I 2 values between 25–50%, 51–75%, and > 75% indicated low, moderate, and significant heterogeneity, respectively 27 . In the meta-analysis comparing PT between groups of participants, the meta-regression and subgroup analyses were conducted to identify the source of the heterogeneity. These analyses were stratified based on several factors such as publication year, study design, country, continent, Plasmodium species, age groups, methods for malaria diagnosis, and methods for PT assessment. For any comparison involving more than ten studies, the potential publication bias and the influence of small-study effects were assessed using the funnel plots, contour-enhanced funnel plots, and Egger’s regression test 28 . All the computations were performed using Stata software version 17.0 (StataCorp LLC, College Station, TX).
Search results
From the identified 2767 articles across databases including PubMed, Embase, Scopus, Ovid, and Medline, 1041 duplicates were removed. This resulted in 1726 unique records, which were screened for relevance. Subsequently, 977 records not relevant to malaria, PT, or lacking abstracts were eliminated. Of the 749 reports marked for retrieval, 14 were inaccessible, leaving 735 for eligibility assessment. After rigorous evaluation, 715 were further excluded due to a variety of reasons including irrelevance to the study's focus, being animal/in vitro studies, reviews, non-English language studies, among others. Ultimately, 20 studies that met the selection criteria were identified. An additional study, identified from a reference list, was included, totaling 21 studies for review 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 (Fig. 1 ).
Study flow diagram showing the study selection processes.
Characteristics of the included studies
Table 1 summarize the characteristics of the 21 studies included in the review. Out of 21 studies analyzed, the majority (52.38%) were published between 2010 and 2019, with the common study design being retrospective (33.33%). Most research was conducted in Asia (61.90%), specifically India (38.1%). The Plasmodium species most studied was P. falciparum (42.86%). Study participants were mainly adults (33.33%) or unspecified (33.33%). Microscopy (52.38%) was the dominant method for malaria detection, while the majority of studies (66.67%) did not specify their PT measurement method (Table 1 ). Details of all included studies were demonstrated in Table S2 .
Quality of the included studies
For case–control studies, two studies 29 , 33 showed issues in group comparability regarding the presence or absence of disease (Table S3 ). Despite meeting the criteria for appropriate matching of cases and controls, the same identification criteria, and standard exposure measurement, both studies lacked the identification of and strategies for dealing with confounding factors. For cross-sectional studies, one study was included in the appraisal despite not clearly defining inclusion criteria, failing to identify confounding factors, and lacking appropriate statistical analysis 31 . One study, on the other hand, met all appraisal criteria 35 . Two studies 43 , 48 , while failing to fully address confounding factors, were also included due to their adherence to the remaining criteria.
For cohort studies, a significant number of studies all demonstrated a similar pattern 30 , 32 , 34 , 36 , 37 , 38 , 40 , 41 , 42 , 46 , 47 , 49 . They met most of the appraisal criteria but failed to identify or state strategies to handle confounding factors and did not employ strategies to address incomplete follow-up. Despite these shortcomings, they were all included in the appraisal. One study managed to identify and address confounding factors but did not manage to address incomplete follow-up 39 . One study did not adequately address confounding factors or incomplete follow-up and failed to use appropriate statistical analysis, yet was included 44 . Lastly, one study 45 faced a unique issue with unclear exposure measurement but was included despite this and the usual issues with confounding factors and incomplete follow-up.
Difference in PT between malaria patients and uninfected controls
Comparison of PT between malaria patients and uninfected controls were reported in eight studies 29 , 31 , 33 , 34 , 36 , 45 , 46 , 47 . Six studies demonstrated that PT was significantly higher in malaria patients than uninfected controls 29 , 33 , 34 , 36 , 46 , 47 while two studies reported that PT was prolonged in uncomplicated malaria (11/47 cases) as compared to normal uninfected controls 31 . PT was prolonged only in cerebral malaria but normal in non-cerebral severe malaria, uncomplicated malaria, non-malaria, and healthy controls 45 . The meta-analysis using the data from four studies that reported quantitative data of PT 29 , 33 , 36 , 47 showed a significantly higher PT in malaria patients as compared to uninfected controls ( P < 0.01, Mean difference: 8.859 s, 95% CI 5.315–12.403 s, I 2 : 87.88%, 4 studies Fig. 2 ).
The meta-analysis using the data from four studies showed significantly higher PT (unit in second) in malaria patients compared to uninfected controls. Abbreviations: N, number of participants; CI, confidence interval; SD, standard deviation.
Difference in PT between severe and non-severe malaria
Comparison of PT in severe and non-severe malaria were reported in 11 studies 31 , 35 , 37 , 40 , 41 , 42 , 43 , 44 , 45 , 48 , 49 . Out of 11 studies examining PT in severe and non-severe malaria, varied results were reported. Several studies 35 , 37 , 40 , 42 , 48 found PT to be higher in severe malaria than non-severe cases. One study found no significant difference in PT between severe and non-severe malaria 49 , while another reported normal PT levels in both conditions 41 . Interestingly, one study reported normal PT in severe malaria but prolonged PT in non-severe malaria 31 . Another study found prolonged PT in cerebral malaria but normal in non-cerebral severe malaria and non-severe malaria 45 , whereas one found prolonged PT in cerebral severe malaria but normal in non-severe malaria 43 . Finally, one study reported prolonged PT in both severe and non-severe malaria 44 . The meta-analysis using the data from seven studies that reported quantitative data of PT 35 , 37 , 40 , 42 , 44 , 48 , 49 showed a significantly higher PT in severe malaria than non-severe malaria ( P = 0.03, Hedges's g: 1.65, 95% CI 0.20–3.10, I 2 : 97.91%, 7 studies Fig. 3 ).
The meta-analysis using the data from seven studies showed significantly higher PT (unit in second) in severe malaria than non-severe malaria. Abbreviations: N, number of participants; CI, confidence interval; SD, standard deviation.
The meta-regression analysis was further performed to identify the source of heterogeneity of the effect estimate. The results showed that publication years, study design, country, continent, age group, Plasmodium species, diagnostic method for malaria, or method for PT measurement did not affect the pooled effect estimate (Table S4 ). Although these covariates did not affect the pooled effect estimate, the R 2 values indicated that the covariate, diagnostic method for malaria, explains 11.15% of the variation in PT differences between severe and non-severe malaria cases, indicating some level of explanatory power, though still relatively low. The subgroup analysis has been further performed to identify the source of heterogeneity of the effect estimate (Table 2 ). The subgroup analysis based on publication years showed no difference in PT for studies published from 2010 to 2019 ( P = 0.18), while those from 2000 to 2009 reported a significant increase in PT ( P < 0.01). The subgroup analysis based on study design showed that cross-sectional study subgroup yielded a significant increase in PT ( P = 0.04), while prospective studies subgroup showed no difference in PT ( P = 0.12). The subgroup analysis based on continent found a significant increase in PT for severe malaria cases in Asia ( P = 0.01). The subgroup analysis based on age showed a trend toward higher PT in adults with severe malaria, but no conclusive results for unspecified age or all age groups due to insufficient data. In the context of Plasmodium species, studies reporting on P. falciparum indicated a non-significant difference in PT ( P = 0.38). P. vivax studies revealed a non-significant difference in PT ( P = 0.48). Subgroup analysis based on diagnostic methods demonstrated a significant increase in PT for severe malaria when Microscopy/RDT was used ( P = 0.03), but not when Microscopy alone was used ( P = 0.65). Lastly, the method of PT measurement subgroup analysis showed a significant increase in PT in severe malaria for studies where the PT measurement method was not specified ( P = 0.01). The manual and optical clotting methods could not generate P -values due to insufficient data.
Difference in PT between different Plasmodium species
Comparison of PT in different Plasmodium species were reported in six studies 30 , 32 , 35 , 37 , 39 , 41 . There appears to be variance in the PT based on the Plasmodium species involved in the malaria infection. For instance, one study suggested PT prolongation in both P. vivax and P. falciparum malaria 32 . Contrarily, another study found PT to be prolonged in P. falciparum malaria, but normal in P. vivax malaria and mixed infections 39 . In mixed infection cases, PT was found to be significantly higher than in P. vivax mono-infection cases 30 . However, another investigation reported normal PT for both P. falciparum and P. vivax malaria 41 . Two additional studies did not provide qualitative results comparing PT between different Plasmodium species, but they provided quantitative data for the meta-analysis 35 , 37 . There was no significant difference in PT between patients with P. falciparum and those with P. vivax mono-infection ( P = 0.88, Mean difference: 0.06, 95% CI − 0.691–0.8, I 2 : 65.09%, 2 studies, Supplementary Fig. 1 ). There was no significant difference in PT between patients with P. falciparum / P. vivax mixed infections and those with P. vivax mono-infection ( P = 0.35, Mean difference: 4.37 s, 95% CI − 4.77–13.52 s, I 2 : 99.21%, 2 studies, Supplementary Fig. 2 ).
Difference in PT between fatal malaria and non-fatal malaria
The comparison of PT in fatal and non-fatal malaria cases has been addressed in two studies 31 . One study noted a significantly higher PT in fatal malaria cases compared to surviving cases 38 . Contrarily, another study reported PT as normal in instances of fatal malaria 31 .
Publication bias
Publication bias was not assessed using funnel plots, contour-enhanced funnel plots, or Egger’s regression test because the meta-analysis included fewer than ten studies.
Sensitivity analysis
The sensitivity analysis was conducted to assess the robustness of the meta-analysis findings. For assessing PT differences between malaria patients and uninfected controls, the meta-analysis demonstrated stability under both the fixed effect model and when calculating the standard mean difference (SMD) (Supplementary Figs. 3 and 4 , respectively). Furthermore, the leave-one-out sensitivity analysis did not identify any single study as an outlier that significantly influenced the overall pooled effect estimate (Fig. 4 ), affirming the consistency of these results. Similarly, the stability of the meta-analysis comparing PT between severe and non-severe malaria cases was evaluated. When the fixed effect model was applied, the analysis remained stable (Supplementary Fig. 5 ). However, the leave-one-out sensitivity analysis revealed the presence of study outliers that had a notable impact on the pooled effect estimate (Fig. 5 ). This highlights certain studies' influence on the overall meta-analysis outcome for PT differences between severe and non-severe malaria cases.
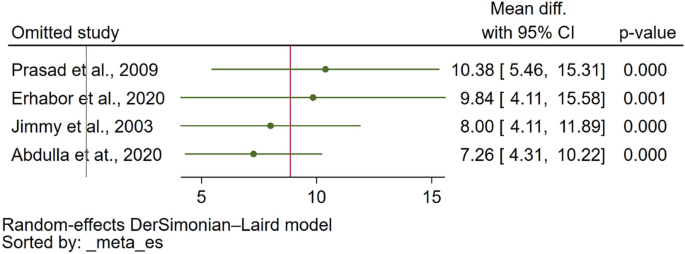
The leave-one-out meta-analysis of PT (unit in second) between malaria patients and uninfected controls showed no study outlier that affect the pooled effect estimate. Abbreviations: CI, confidence interval.
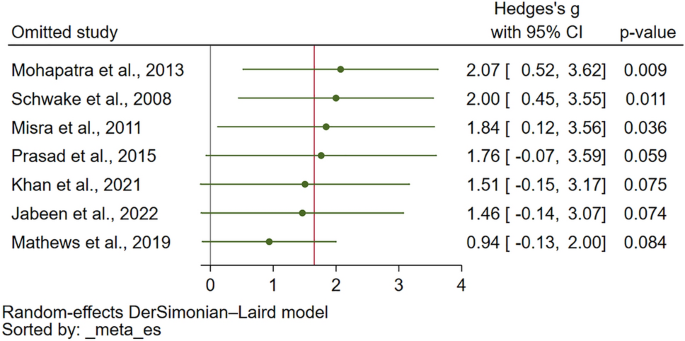
The leave-one-out meta-analysis of PT (unit in second) between severe and non-severe malaria showed study outliers that affect the pooled effect estimate. Abbreviations: CI, confidence interval.
Malaria's impact on blood coagulation, evidenced by alterations in prothrombin time (PT), underscores a complex interplay between the disease's pathophysiology and coagulation pathways 11 . The meta-analysis results also supported the finding from individual study in which PT was statistically increased in malaria patients as compared to uninfected controls. Furthermore, the meta-analysis result was stable when the statistical model or the pooled effect estimate were changed, indicating a certain degree of coagulation activation that causes the alteration of PT during Plasmodium infections. In uncomplicated malaria, prolonged PT could be observed between 3.77 and 16.7% in different studies 39 , 50 , 51 . The presence of a prolonged PT, along with clinical indications such as bleeding manifestations and standard coagulation laboratory tests such as APTT, D-dimers, and fibrinogen degradation products, can suggest the onset of DIC. Consequently, DIC can trigger both microvascular and macrovascular clotting, impairing blood circulation, which can ultimately result in the development of Multiple Organ Dysfunction Syndrome (MODS) 52 .
The systematic review revealed that patients suffering from severe malaria exhibited a prolonged PT in comparison to those with non-severe malaria. This finding was statistically significant, with PT levels being higher in severe malaria patients than in uninfected controls. Such observations align with the existing literature that links severe malaria, especially cases involving P. falciparum , to significant disruptions in coagulation pathways 47 . Specifically, P. falciparum -infected erythrocytes are known to exhibit elevated levels of tissue factor 16 , a key initiator of the coagulation cascade, which can lead to increased PT. The activation of the coagulation system, coupled with the impairment of anticoagulant mechanisms, contributes to a prothrombotic state 11 , thereby explaining the prolonged PT observed in severe malaria cases. This activation of the coagulation system, along with impaired anticoagulant mechanisms, likely contributes to a prothrombotic state, elucidating the extended PT observed in cases of severe malaria. Numerous studies have documented a trend towards more pronounced PT alterations in patients with severe malaria compared to those presenting with uncomplicated forms of the disease 20 , 32 , 43 , 47 , 53 , 54 , 55 .
The PT prolongation in severe malaria, observed in the range of 5.74–22.7% 56 , 57 , 58 , 59 , could be attributed to several mechanisms. One such mechanism might include the release of tissue factors from damaged vascular endothelial cells or the expression of activated tissue factor on endothelial cells, leading to consumptive coagulopathy due to the activation of the extrinsic coagulation pathway 16 , 60 . Nevertheless, certain studies showed that the proportion of prolonged PT was similar in both severe and uncomplicated malaria, or there was no significant difference in PT between the two clinical malaria groups 41 , 44 , 49 , 61 . Inconsistency was observed in the meta-analysis results, as alterations in the statistical model or the pooled effect estimate led to instability in the outcomes. However, based on the available evidence, it can be concluded that patients with severe malaria are more likely to exhibit prolonged or increased PT.
The systematic review highlighted the limited evidence differentiating PT between Plasmodium species. According to the meta-analysis, there were observed similarities in PT between patients with P. falciparum mono-infection and those with P. vivax mono-infection, as well as between patients with P. falciparum / P. vivax mixed infections and those with P. vivax mono-infection. These findings suggest that an increase or prolongation in PT can occur in P. falciparum , non- P. falciparum malaria, and mixed Plasmodium infections. Interestingly, prolonged PT was observed in patients suffering from both severe P. falciparum and severe P. vivax malaria. In a study that involved both P. vivax and P. falciparum 59 , there was a minimal difference in the proportion of prolonged PT, with severe P. falciparum malaria having a slightly higher proportion of prolonged PT than severe P. vivax malaria (10.6 vs. 5.74%). The slight variance in PT prolongation rates between these two Plasmodium species suggests that underlying mechanisms, such as differences in endothelial activation and microvascular dysfunction 62 , may contribute to this discrepancy. Nonetheless, the specific factors driving these subtle differences remain to be fully elucidated, highlighting an area for future research.
The systematic review of studies involving patients with both fatal and non-fatal malaria demonstrated varied results. The discrepancies in these findings may be attributable to the multifaceted nature of the factors contributing to the risk of death among malaria patients. For instance, those suffering from fatal malaria could potentially experience a higher consumption of coagulation factors than patients with non-fatal malaria 63 . This enhanced consumption of coagulation factors in fatal malaria cases may be linked to a heightened degree of coagulation activation. This heightened activation, in turn, could be driven by systemic endothelial activation, a common occurrence in severe or fatal malaria 64 . This scenario proposes a complex interplay between coagulation factors and the pathophysiology of malaria, suggesting that severe or fatal malaria cases exhibit a hyperactive coagulation state, leading to excessive consumption of coagulation factors, subsequently resulting in a more prolonged PT. However, it is important to underscore that while the correlation between fatal malaria and heightened coagulation activation seems plausible, the evidence is not wholly conclusive. The heterogeneous nature of these findings underscores the need for more in-depth investigations. Future studies should aim to elucidate the precise mechanisms by which malaria influences the coagulation pathway and the subsequent variations in PT, as well as the potential discrepancies in these effects between fatal and non-fatal cases.
The systematic review and meta-analysis of PT in malaria patients have several limitations. First, the significant heterogeneity observed across studies may compromise the validity of the findings. Despite the subgroup analysis and meta-regression performed to identify the source of heterogeneity, the variance remained largely unexplained, which implies that other factors unaccounted for in this study might be contributing to this heterogeneity. Second, the studies used for comparison were not homogenous in terms of patient characteristics, methods of PT measurement, diagnostic methods for malaria, and Plasmodium species, which could affect the comparison of PT among different studies. Moreover, most of the included studies used cross-sectional designs, limiting the ability to establish causality between malaria infection and increased PT. Finally, the limited number of studies and their geographic concentration also pose challenges in generalizing the findings globally.
For future research implications, it is recommended to perform more robust studies, such as randomized controlled trials or prospective cohort studies, which can help to provide a more accurate assessment of the PT in malaria patients and reduce bias. It would also be beneficial to include a more diverse sample, both in terms of geographical distribution and characteristics of patients, to allow for a broader understanding of the relationship between malaria and PT. There is also a need to investigate the exact biological mechanisms driving the observed differences in PT in patients with malaria, as this could provide a more in-depth understanding of the disease's pathophysiology and possibly lead to the development of novel therapeutic interventions.
The systematic review and meta-analysis revealed PT prolongation in malaria infections caused by P. falciparum and P. vivax , particularly in severe cases, yet found no significant difference in PT between the two species. The ambiguous results concerning PT in fatal versus non-fatal cases highlight existing research gaps. Future studies should focus on refining PT measurement methods, exploring the impact of treatment and comorbidities on PT, and unraveling the molecular mechanisms of coagulation alterations induced by malaria. Advancements in these areas promise to inform targeted therapies for malaria-associated coagulopathy, aiming to improve patient outcomes.
Data availability
All data relating to the present study are available in this manuscript and supplementary files.
WHO. World Malaria Report (World Health Organization, 2022).
Miller, L. H., Ackerman, H. C., Su, X. Z. & Wellems, T. E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 19 (2), 156–167 (2013).
Article CAS PubMed PubMed Central Google Scholar
Baer, K. et al. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 9 (2), 397–412 (2007).
Article CAS PubMed Google Scholar
Severe Falciparum Malaria. World Health Organization, communicable diseases cluster. Trans. R. Soc. Trop. Med. Hyg. 94 (Suppl 1), S1-90 (2000).
Google Scholar
Kotepui, M., Phunphuech, B., Phiwklam, N., Chupeerach, C. & Duangmano, S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar. J. 13 , 218 (2014).
Article PubMed PubMed Central Google Scholar
Maina, R. N. et al. Impact of Plasmodium falciparum infection on haematological parameters in children living in Western Kenya. Malar. J. 9 , S4 (2010).
Anghan, H., Sethi, P., Soneja, M., Mahajan, S. & Wig, N. Clinical and laboratory features associated with acute kidney injury in severe malaria. Indian J. Crit. Care Med. 22 (10), 718–722 (2018).
Palta, S., Saroa, R. & Palta, A. Overview of the coagulation system. Indian J. Anaesth. 58 (5), 515–523 (2014).
Chaudhry, R., Usama, S. M., Babiker, H. M.: Physiology, Coagulation Pathways. StatPearls Treasure Island (FL) (2023).
Norris, L. A. Blood coagulation. Best Pract. Res. Clin. Obstet. Gynaecol. 17 (3), 369–383 (2003).
Article PubMed Google Scholar
Angchaisuksiri, P. Coagulopathy in malaria. Thromb. Res. 133 (1), 5–9 (2014).
Grobusch, M. P. & Kremsner, P. G. Uncomplicated malaria. Curr. Top. Microbiol. Immunol. 295 , 83–104 (2005).
CAS PubMed Google Scholar
Milner, D. A. Jr. et al. The systemic pathology of cerebral malaria in African children. Front. Cell Infect. Microbiol. 4 , 104 (2014).
Sailo, L., Pradhan, D., Nongthombam, R. & Bhattacharyya, P. Disseminated intravascular coagulation in malaria: A case report. Niger. J. Med. 55 (2), 171–172 (2014).
Article Google Scholar
Francischetti, I. M., Seydel, K. B. & Monteiro, R. Q. Blood coagulation, inflammation, and malaria. Microcirculation 15 (2), 81–107 (2008).
Francischetti, I. M. et al. Plasmodium falciparum -infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J. Thromb. Haemost. 5 (1), 155–165 (2007).
Kern, P., Hemmer, C. J., Van Damme, J., Gruss, H. J. & Dietrich, M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 87 (2), 139–143 (1989).
Bridges, D. J. et al. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 115 (7), 1472–1474 (2010).
Chaudhry, R., Usama, S. M. & Babiker, H. M. Physiology, Coagulation Pathways. StatPearls Treasure Island (FL) (2022).
Pukrittayakamee, S. et al. Activation of the coagulation cascade in falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 83 (6), 762–766 (1989).
Horstmann, R. D. & Dietrich, M. Haemostatic alterations in malaria correlate to parasitaemia. Blut 51 (5), 329–335 (1985).
Rojanasthien, S., Surakamolleart, V., Boonpucknavig, S. & Isarangkura, P. Hematological and coagulation studies in malaria. J. Med. Assoc. Thai. 75 (Suppl 1), 190–194 (1992).
PubMed Google Scholar
Bashir, B. A. & Ahmed, M. S. Laboratory blood coagulation in Sudanese with falciparum malaria: a glance of change outcomes. Int. J. Blood Res. Disord. 7 , 1–5 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 18 (3), e1003583 (2021).
WHO. WHO Guidelines for malaria. Geneva: World Heath Organization; 2023. Available from: https://www.who.int/publications/i/item/guidelines-for-malaria . Accessed 9 Mar 2024.
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., Currie, M., Qureshi, R., Mattis, P., Lisy, K. & Mu, P.-F. Chapter 7: Systematic reviews of etiology and risk: JBI; 2020. Available from: https://synthesismanual.jbi.global . Accessed 22 Dec 2023.
Higgins, J. P. T., Chandler, J., Cumpston, M., Li, T., Page, M. J. & Welch, V. A. (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022. Available from: www.training.cochrane.org/handbook . Accessed 22 Dec 2023.
Cumpston, M. et al. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.ED000142 (2019).
Abdullah, S. J. et al. A severe case of falciparum malaria, 10 years after malaria eradication: A case report. Yale J. Biol. Med. 94 (2), 277–284 (2021).
PubMed PubMed Central Google Scholar
Akhlaq, A. et al. Neurological complications in patients with plasmodium vivax malaria from Karachi, Pakistan. J. R. Coll. Physicians Edinb. 48 (3), 198–201 (2018).
Amano, H., Sano, A., Araki, T. & Inoki, S. Fibrin-degradation products in falciparum malaria. Zentralbl Bakteriol Mikrobiol Hyg. A Med. Mikrobiol. Infekt. Parasitol. 250 (1–2), 242–247 (1981).
Dasgupta, A., Rai, S. & Das, G. A. Persistently elevated laboratory markers of thrombosis and fibrinolysis after clinical recovery in malaria points to residual and smouldering cellular damage. Indian J. Hematol. Blood Transfus. 28 (1), 29–36 (2012).
Erhabor, O., Abdulrahaman, A. & Erhabor, T. Effects of malaria parasitaemia on some haemostatic parameters among pregnant women of African descent in Specialist Hospital Sokoto, Nigeria. Hum. Antibodies 28 (1), 21–28 (2020).
Huson, M. A. M. et al. Impact of HIV infection on the haemostatic response during sepsis and malaria. Br. J. Haematol. 173 (6), 918–926 (2016).
Jabeen, S. et al. Alteration of coagulation profile in malaria patients and its correlation with the degree of parasitemia. Pak. Armed Forces Med. J. 72 (2), 513–517 (2022).
Jimmy, E. O., Saliu, I. & Ademowo, O. Fibrinopeptide-A and fibrinogen interactions in acute, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 97 (8), 879–881 (2003).
Khan, M., Nisar, H. & Mushahid, N. Clinical and lab profile of severe and uncomplicated malaria: A prospective study from Khuzdar Balochistan. Pak. J. Med. Sci. 37 (7), 1918–1923 (2021).
Khan, R., Quaiser, S. & Haque, S. F. Malarial acute kidney injury: Prognostic markers. Ann. Trop. Med. Public Health 6 (3), 280–284 (2013).
Kueh, Y. K. & Yeo, K. L. Haematological alterations in acute malaria. Scand. J. Haematol. 29 (2), 147–152 (1982).
Mathews, S. E., Bhagwati, M. M. & Agnihotri, V. Clinical spectrum of Plasmodium vivax infection, from benign to severe malaria: A tertiary care prospective study in adults from Delhi, India. Trop. Parasitol. 9 (2), 88–92 (2019).
Meltzer, E., Keller, S., Shmuel, S. & Schwartz, E. D-dimer levels in non-immune travelers with malaria. Travel Med. Infect. Dis. 27 , 104–106 (2019).
Misra, D. P., Das, S., Pattnaik, M., Singh, S. C. & Jena, R. K. Relationship of hepatic and renal dysfunction with haemorrheological parameters in Plasmodium falciparum malaria. J. Assoc. Physicians India 59 , 552–556 (2011).
Mohanty, D. et al. Fibrinolysis, inhibitors of blood coagulation, and monocyte derived coagulant activity in acute malaria. Am. J. Hematol. 54 (1), 23–29 (1997).
Mohapatra, S., Samantaray, J. C., Arulselvi, S. & Ghosh, A. Disseminated intravascular coagulation following malaria due to Plasmodium vivax : A thromboelastography-based study. Malar. J. 12 , 336 (2013).
Moxon, C. A. et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J. Thromb. Haemost. 13 (9), 1653–1664 (2015).
Moxon, C. A. et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122 (5), 842–851 (2013).
Prasad, R. et al. Coagulation status and platelet functions in children with severe falciparum malaria and their correlation of outcome. J. Trop. Pediatr. 55 (6), 374–378 (2009).
Prasad, R. & Mishra, O. P. Acute kidney injury in children with Plasmodium falciparum malaria: Determinants for mortality. Perit. Dial. Int. 36 (2), 213–217 (2016).
Schwake, L. et al. Early treatment of imported falciparum malaria in the intermediate and intensive care unit setting: an 8-year single-center retrospective study. Crit. Care 12 (1), R22 (2008).
Butler, T., Tong, M. J., Fletcher, J. R., Dostalek, R. J. & Robbins, T. O. Blood coagulation studies in Plasmodium falciparum malaria. Am. J. Med. Sci. 265 (1), 63–67 (1973).
Beale, P. J., Cormack, J. D. & Oldrey, T. B. Thrombocytopenia in malaria with immunoglobulin (IgM) changes. Br. Med. J. 1 (5796), 345–349 (1972).
Costello, R. A., Nehring, S. M. Disseminated intravascular coagulation. StatPearls, Treasure Island (FL) (2023).
Ali, H. et al. Parasite density and the spectrum of clinical illness in falciparum malaria. J. Coll. Phys. Surg. Pak. 18 (6), 362–368 (2008).
Moxon, C. A. et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J. Thromb. Haemost. 14 (2), 1653–1664 (2016).
Moxon, C. A. et al. Loss of protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria. J. Thromb. Haemost. 11 , 4 (2013).
Clemens, R. et al. Activation of the coagulation cascade in severe falciparum malaria through the intrinsic pathway. Br. J. Haematol. 87 (1), 100–105 (1994).
Holst, F. G. E. et al. Low levels of fibrin-stabilizing factor (factor XIII) in human Plasmodium falciparum malaria: Correlation with clinical severity. Am. J. Trop. Med. Hyg. 60 (1), 99–104 (1999).
Naqvi, R., Ahmad, E., Akhtar, F., Naqvi, A. & Rizbi, A. Outcome in severe acute renal failure associated with malaria. Nephrol. Dial. Transplant. 18 (9), 1820–1823 (2003).
Zubairi, A. B. S. et al. Severe Plasmodium vivax malaria in Pakistan. Emerg. Infect. Dis. 19 (11), 1851–1854 (2013).
Srichaikul, T. Hemostatic alterations in malaria. Southeast Asian J. Trop. Med. Public Health 24 (Suppl 1), 86–91 (1993).
Srichaikul, T., Puwasatien, P., Puwasatien, P., Karnjanajetanee, J. & Bokisch, V. Complement changes and disseminated intravascular coagulation in Plasmodium falciparum malaria. Lancet 305 (7910), 770–772 (1975).
Woodford, J. et al. Early endothelial activation precedes glycocalyx degradation and microvascular dysfunction in experimentally induced Plasmodium falciparum and Plasmodium vivax infection. Infect. Immun. 88 (5), e00895-e919 (2020).
Duangchan, T., Kotepui, M., Sukati, S., Rattanapan, Y. & Wangdi, K. A systematic review and meta-analysis of the proportion estimates of disseminated intravascular coagulation (DIC) in malaria. Trop. Med. Infect. Dis. 8 (6), 289 (2023).
Turner, G. D. et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145 (5), 1057–1069 (1994).
CAS PubMed PubMed Central Google Scholar
Download references
Author information
Authors and affiliations.
Medical Technology, School of Allied Health Sciences, Walailak University, Tha Sala, Nakhon Si Thammarat, Thailand
Suriyan Sukati & Thitinat Duangchan
Hematology and Transfusion Science Research Center, Walailak University, Tha Sala, Nakhon Si Thammarat, Thailand
Faculty of Medicine, Western University, Huai Krachao, Kanchanaburi, Thailand
Tirawat Wannatung
Department of Medical Technology, University of Santo Tomas, Manila, Philippines
Frederick Ramirez Masangkay
Department of Medical Biotechnology and Laboratory Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Ching-Ping Tseng
Graduate Institute of Biomedical Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Department of Laboratory Medicine, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan
Medical Technology Program, Faculty of Science, Nakhon Phanom University, Nakhon Phanom, Thailand
Kwuntida Uthaisar Kotepui & Manas Kotepui
You can also search for this author in PubMed Google Scholar
Contributions
M.K., S.S., T.W., and T.D. carried out the study design, study selection, data extraction, and statistical analysis. M.K., S.S., and T.D. drafted the manuscript. K.U.K., F.R.M., C.P.T. performed critical review and revision of the manuscript. All authors read and approved the final version of the manuscript. All authors consent to the publication of the final version of the manuscript.
Corresponding author
Correspondence to Manas Kotepui .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., supplementary information 3., supplementary information 4., supplementary information 5., supplementary information 6., supplementary information 7., supplementary information 8., supplementary information 9., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Sukati, S., Wannatung, T., Duangchan, T. et al. Alteration of prothrombin time in Plasmodium falciparum and Plasmodium vivax infections with different levels of severity: a systematic review and meta-analysis. Sci Rep 14 , 9816 (2024). https://doi.org/10.1038/s41598-024-60170-y
Download citation
Received : 14 August 2023
Accepted : 19 April 2024
Published : 02 May 2024
DOI : https://doi.org/10.1038/s41598-024-60170-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prothrombin time
- Coagulation
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Skill #1: Analysis. Analysis means that you have carefully read a wide range of the literature on your topic and have understood the main themes, and identified how the literature relates to your own topic. Carefully read and analyze the articles you find in your search, and take notes. Notice the main point of the article, the methodologies ...
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important ...
There are several fundamental differences between traditional SLRs and meta-analysis, distinguishing these popular methods for accumulating knowledge in a research domain. ... Review Manager (RevMan) is a web-based software that manages the entire literature review process and meta-analysis. The meta-analyst uploads all studies to RevMan ...
The difference between literature review and systematic review comes back to the initial research question. Whereas the systematic review is very specific and focused, the standard literature review is much more general. The components of a literature review, for example, are similar to any other research paper.
Literature Review: it is a product and a process. As a product, it is a carefully written examination, interpretation, evaluation, and synthesis of the published literature related to your topic.It focuses on what is known about your topic and what methodologies, models, theories, and concepts have been applied to it by others.. The process is what is involved in conducting a review of the ...
However, a literature review that only offers an arbitrary selection of evidence is often not fully representative of the state of existing knowledge, and the selection of some studies over others ultimately leads to what is known in statistical analysis as a sample selection bias - a type of bias caused by choosing a non-random sample of ...
A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research. There are five key steps to writing a literature review: Search for relevant literature. Evaluate sources. Identify themes, debates and gaps.
Analysis is the part of the literature review process where you justify why your research is needed, how others have not addressed it, and/or how your research advances the field. ... Though this video is titled "Tips for Writing a Literature Review," the ideas expressed relate to being focused on the research topic and building a strong case ...
relevance and the differences between the literature review and other forms of academic writing. The fundamental steps involved in undertaking a literature ... approach, the purpose of the review is to provide an analysis and synthesis of all the ACTIVITY 1.1 01-Coughlan-Ch-01.indd 2 05/02/2013 10:02:51 AM.
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
As mentioned previously, there are a number of existing guidelines for literature reviews. Depending on the methodology needed to achieve the purpose of the review, all types can be helpful and appropriate to reach a specific goal (for examples, please see Table 1).These approaches can be qualitative, quantitative, or have a mixed design depending on the phase of the review.
A literature review should connect to the study question, guide the study methodology, and be central in the discussion by indicating how the analyzed data advances what is known in the field. ... An introduction discussing the potential of CURES is followed by an analysis of the existing literature relevant to the design of CUREs that allows ...
The methodology involved in a literature review is less complicated and requires a lower degree of planning. For a systematic review, the planning is extensive and requires defining robust pre-specified protocols. It first starts with formulating the research question and scope of the research. The PICO's approach (population, intervention ...
A systematic review is an article that synthesizes available evidence on a certain topic utilizing a specific research question, pre-specified eligibility criteria for including articles, and a systematic method for its production. Whereas a meta-analysis is a quantitative, epidemiological study design used to assess the results of articles ...
A meta-analysis is a type of systematic review that summarises and compares data using statistical techniques. Aim/Definition. A scholarly literature review summarises evidence on a topic using a formal writing style and adopting qualitative data collection methods to select and interpret studies. Can involve some quantitative analysis.
Regardless of this commonality, both types of review vary significantly. The following table provides a detailed explanation as well as the differences between systematic and literature reviews. Kysh, Lynn (2013): Difference between a systematic review and a literature review.
A literature review is a systematic way of collecting and synthesizing previous research (Snyder, 2019).An integrative literature review provides an integration of the current state of knowledge as a way of generating new knowledge (Holton, 2002).HRDR is labeling Integrative Literature Review as one of the journal's four non-empirical research article types as in theory and conceptual ...
For scientific purposes, the term Literature Review is the one used most often. For more information on the Literature Review, click on that link under the Review By Type tab. The difference between a Systematic Review and a Narrative Review can be summarized as follows:
Advantages of a thematic literature review. Conducting a literature review thematically provides a comprehensive and nuanced synthesis of research findings, distinguishing it from other types of literature reviews. Its structured approach not only facilitates a deeper understanding of the subject area but also enhances the clarity and relevance of the review.
1. Introduction. Systematically reviewing a topic demands researchers to define, identify, select, and analyze relevant literature. Systematic literature reviews in medical sciences generally involve an exhaustive literature search process and rigid selection criteria, besides aiming the summation of evidence concerning a particular medical treatment, especially by means of meta-analyses ...
There is an important difference between the Literature review research and the Analysis research; the authors in analysis should have there own results for theoretical or conceptual model put by ...
A systematic review is also a type of a literature review. The main difference between literature review and systematic review is their focus on the research question; a systematic review is focused on a specific research question whereas a literature review is not. This article highlights, 1. What is a Literature Review?
It can cover and discuss the main ideas or arguments in a book or an article, or it can review a specific concept, theme, theoretical perspective or key construct found in the existing literature. However, the key feature that distinguishes a critical review from a literature review is that the former is more than just a summary of different ...
Purpose Proxy assessment can be elicited via the proxy-patient perspective (i.e., asking proxies to assess the patient's quality of life (QoL) as they think the patient would respond) or proxy-proxy perspective (i.e., asking proxies to provide their own perspective on the patient's QoL). This review aimed to identify the role of the proxy perspective in explaining the differences between ...
The literature reports on a difference in the distribution of breast cancer molecular subtypes between younger and older patients [14, 28]. Variability in molecular subtyping among different populations may be due to variability in pathology reviews and different definitions to categorize the 4 subtypes, where mainly the confusion happens with ...
The systematic review and meta-analysis revealed PT prolongation in malaria infections caused by P. falciparum and P. vivax, particularly in severe cases, yet found no significant difference in PT ...