- Reference Manager
- Simple TEXT file

People also looked at
Systematic review article, new insights into pathophysiology of β-thalassemia.

- 1 Hematology Service, Virgen de la Arrixaca University Hospital, Murcia, Spain
- 2 Biomedical Research Institute of Murcia (IMIB), Murcia, Spain
- 3 Centro de Investigaci3n Biomédica en Red de Enfermedades Raras (CIBERER), Madrid, Spain
β-thalassemia is a disease caused by genetic mutations including a nucleotide change, small insertions or deletions in the β-globin gene, or in rare cases, gross deletions into the β-globin gene. These mutations affect globin-chain subunits within the hemoglobin tetramer what induces an imbalance in the α/β-globin chain ratio, with an excess of free α-globin chains that triggers the most important pathogenic events of the disease: ineffective erythropoiesis, chronic anemia/chronic hypoxia, compensatory hemopoietic expansion and iron overload. Based on advances in our knowledge of the pathophysiology of β-thalassemia, in recent years, emerging therapies and clinical trials are being conducted and are classified into three major categories based on the different approach features of the underlying pathophysiology: correction of the α/β-globin disregulation; improving iron overload and reverse ineffective erythropoiesis. However, pathways such as the dysregulation of transcriptional factors, activation of the inflammasome, or approach to mechanisms of bone mineral loss, remain unexplored for future therapeutic targets. In this review, we update the main pathophysiological pathways involved in β-thalassemia, focusing on the development of new therapies directed at new therapeutic targets.
Introduction
Thalassemias is an inherited hemoglobin disorder characterized by reduced or absent globin chain synthesis, resulting in variable clinical phenotypes from severe chronic anemia requiring lifelong transfusion and iron chelating therapy to asymptomatic individuals ( 1 ).
Traditionally, β-thalassemias have been more common in countries in the Mediterranean area, North and Central Africa, Southeast Asia, and the Middle East. However, as a result of migrations of populations, β-thalassemias are now encountered in other regions, such as Northern Europe and North America ( 2 ).
β -thalassemia has a broad clinical spectrum, and traditionally has been classified in the clinic in thalassemia major (TM), thalassemia intermedia (TI), and thalassemia minor ( Figure 1 ). The TM grouped patients with more severe anemia from an early age who require periodic blood transfusions associated with iron chelation for life, while thalassemia minor, the less severe manifestation, is characterized by people with mild asymptomatic anemia and a heterozygous condition (trait) for thalassemia. TI constituted a group with a variable clinical spectrum, from mild to moderate to moderately severe anemia, who do not require blood transfusions on a regular basis, sometimes only occasionally, but who do develop various complications of thalassemia such as extramedullary hematopoiesis, pulmonary hypertension, iron overload, leg ulcers, skeletal deformities, and growth retardation ( 3 , 4 ).
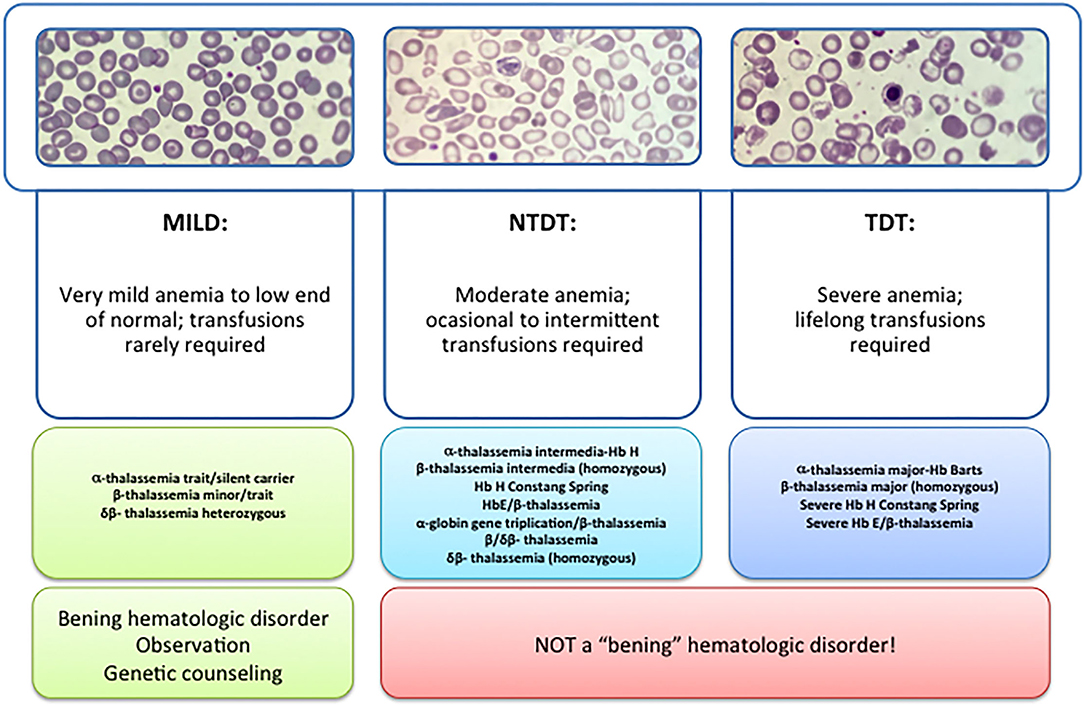
Figure 1 . Types of thalassemia. Genotype–Phenotype Association. α and β-thalassemias are genetically heterogeneous diseases. The clinical management with RBC transfusions is an essential factor in classifying them as either transfusion-dependent thalassemia (TDT) or non–transfusion-dependent thalassemia (NTDT). Patients with TDT need life-long regular transfusions for survival in early childhood while patients with NTDT do not need life-long regular transfusions for survival and normally have later in childhood or even in adulthood with mild/moderate anemia that requires only occasional or short-course regular transfusions under concrete clinical circumstances during times of erythroid stress (infection, pregnancy, surgery, or aplastic crisis); however, usually present the typical complications of TDT such as extramedullary hematopoiesis, iron overload, leg ulcers, and osteoporosis. Patients with TDT include those with β-thalassemia major or severe forms of β-thalassemia intermedia, HbE/β-thalassemia, or α-thalassemia/HbH disease. NTDT mainly encompasses three clinically distinct forms: β-thalassemia intermedia (β-TI), hemoglobin E/β- thalassemia (mild and moderate forms), and α-thalassemia intermedia (hemoglobin H disease).
Recently, this classification has changed due to better understanding of the pathophysiology of the disease and findings focused on the clinical management and complications of IT that show that these patients may present with the same serious complications as transfused patients later in life. In 2012, the International Thalassemia Federation adopted the new terminology for clinical classification of transfusion-dependent thalassemia (TDT) and non–transfusion-dependent thalassemia (NTDT) that groups in three different types in the clinic: α-thalassemia intermedia (hemoglobin H disease) and β-thalassemia intermedia (β-TI), hemoglobin E/β- thalassemia (mild and moderate forms). Differentiating a new patient with thalassemia as TDT or NTDT is essential and requires an accurate clinician's evaluation using various indicators such as hematological parameters, particularly baseline Hb levels, and follow-up for a minimum of 3 to 6 months to determine clinical severity is recommended before making a diagnosis of TDT or NTDT ( 5 , 6 ).
The three important pathophysiologic factors in β-thalassemias are: chronic anemia/hypoxia, ineffective erythropoiesis, and iron overload. The harshness of the disease depends mainly on molecular deficiencies. Chain imbalance causes excess unstable α chains to provoke within erythroid progenitors, leading to cell membrane decline and cell lysis. This triggers an alteration in the mycomedial environment of the bone marrow due to an imbalance of cytokines that causes the erythroid progenitors to proliferate but with inadequate maturation, which is called ineffective erythropoiesis. This cytokine imbalance together with bone marrow hyperplasia causes extramedullary erythropoiesis and subsequently the associate bone deformations. Because of anemia/chronic hypoxia, infective erythropoiesis retroelements, is maintained and perpetuates over time ( 3 , 6 ).
Ineffective Erythropoiesis
Erythropoiesis is a finely regulated process in which every stage is highly regulated by different signal transduction pathways and proteins. Erythropoiesis process in humans is divided into two parts: the early stage of erythropoiesis and the late stage. The first stage is EPO-dependent whereas the second stage is iron-dependent. Erythropoietin (EPO-dependent stage) is the main regulator of early-stage erythropoiesis whereas erythrocyte differentiation and maturation are negatively regulated by the transforming growth factor (TGF-family), the late-stage erythropoiesis or iron-dependent stage of erythropoiesis ( Figure 2 ).
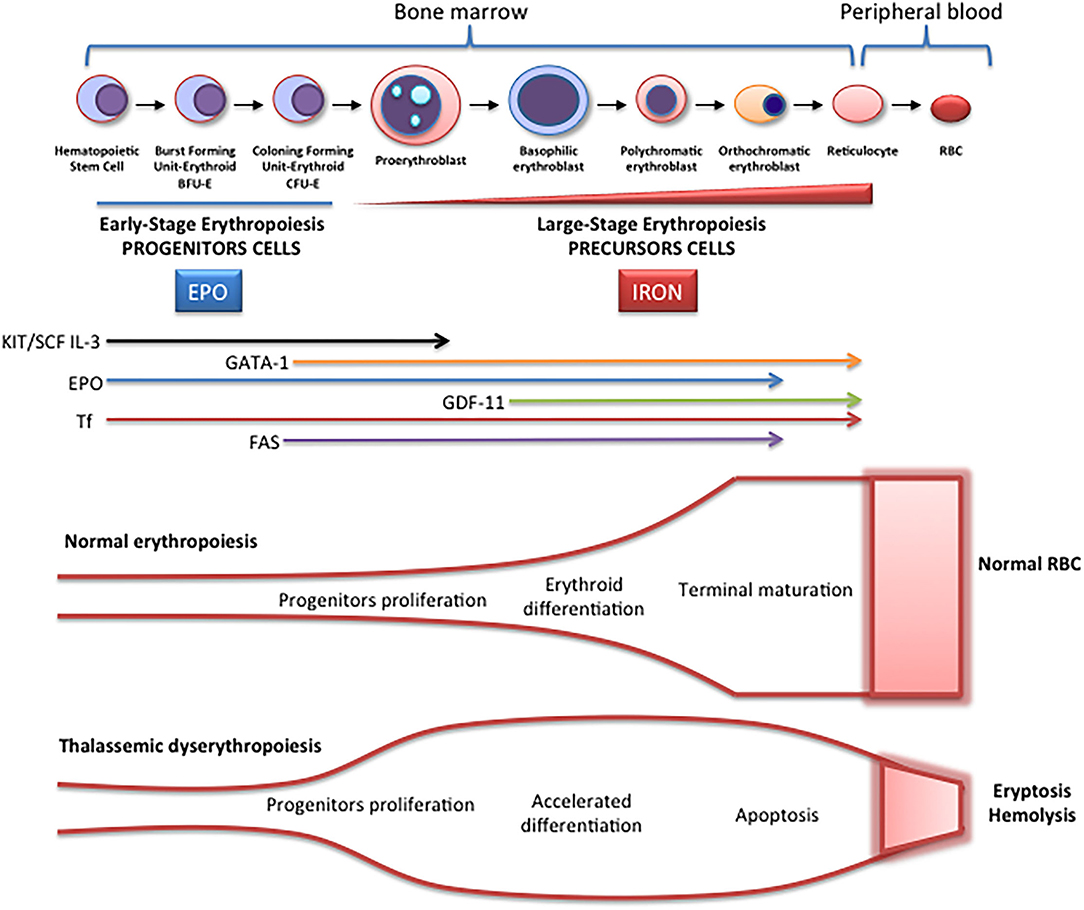
Figure 2 . Schematic representation of erythropoiesis. During erythroid development, several stages occur in which a complex network of molecules are expressed (EPO, iron, transcription factors) are involved. In early-stage erythropoiesis, EPO is the main regulator after BFU-E formation. In this stage, GATA-1 promotes erythropoiesis and increases the EPO receptor expression. In large-stage erythropoiesis molecules such as transferrin and growth/differentiating factor 11 (GDF11) are involved. Erythroid expansion is negatively regulated by association of FAS to FAS ligand, which has as a consequence the apoptosis on immature erythroid cells, and GDF11 and other members of the TGF-β family, which negatively regulate erythrocyte differentiation and maturation from the early to the late stages.
EPO-Dependent Erythropoiesis
In this stage, EPO is fundamental for the proliferation of erythroid progenitors. The recognition of EPO by EPO-R on the surface of these precursors induces JAK2 activation activating STAT5 phosphorylation, with associated induction of erythroid antiapoptotic genes and increase of erythroid progenitors proliferation and survival ( 7 ). The production of EPO and the expansion of erythropoiesis is regulated according to demand (hypoxia, hemorrhage, hemolysis). On the contrary, if the production of erythrocytes is adequate, down-regulation by a mechanism of apoptosis is performed. The predisposition of EPO-dependent erythroid progenitors to apoptosis is associated with different levels of FAS protein (CD95) expression and the FAS ligand (FASL), which belong to the family of TNF receptors, the binding of which activates the caspase cascade and consequently, the immature cell apoptosis limiting erythropoiesis expansion. On the other hand, if the need for erythrocytes increases, EPO production increases, and apoptosis is reduced because EPO stimulates the production of heat shock protein 70 (HSP70) that protects GATA1 from cleavage by FAS/FASL and the activation of the caspase cascade ( 8 ).
Fe-Dependent Erythropoiesis
In the large stage, the presence of iron is essential for the synthesis of hemoglobin, and the integrity of the erythroferrone (ERFE)-hepcidin-ferroportin axis is essential for iron homeostasis ( 9 , 10 ). ERFE is a potent negative regulator of hepcidin. When it is chronically elevated, such as in situations of ineffective erythropoiesis, low plasma iron availability occurs. Transferrin (and its cellular receptor) is also involved in this stage as well as growth differentiation factors like GDF11, a member of the TGF-β superfamily, which negatively regulate erythrocyte maturation and differentiation ( 11 ).
TGF-β receptor ligands are a group of cytokines that include TGF, activins, bone morphogenetic proteins (BMPs), and GDF-11 and play an important role in the regulation of erythropoiesis within the hematopoietic stem cell niche. Various activins, and in particular GDF-11, exert inhibitory activity at the late stage of erythropoiesis.
In the TGF signaling pathway, ligand binding to the type II receptor leads to the recruitment and phosphorylation of the type I receptor and phosphorylation of regulatory SMADs (R-SMADs), SMAD2 and 3, to form the R-SMAD/SMAD4 complex, which modulates the expression of target genes inducing an inhibitory activity on erythroid differentiation by inducing apoptosis in erythroblasts.
During normal erythroid maturation, reduced GDF11 expression with consequent TGF- β signaling suppression, and EPO stimulation occur in parallel, and both are essential for the differentiation of hematopoietic erythroid progenitor cells ( 12 ).
In β-thalassemia, ineffective erythropoiesis is triggered by two main pathogenic mechanisms ( 7 ). On the one hand, α-globin chains aggregates sequester cytosolic heat shock protein 70 (HSP70). This inhibits its nuclear translocation and protects the erythroid transcription factor GATA-factor 1 (GATA1) from cleavage. On the other hand, these toxic aggregates of α-globin chains stimulate the formation of radical oxygen species (ROS) (whose formation is also produced by other mechanisms such as iron overload), which activate GDF11, which in turn activates the inhibitory pathway of SMAD2/3, and as a consequence, erythroid differentiation is inhibited ( 13 ).
Iron Overload
Iron overload is one of the main pathogenic events in β-thalassemia. Apart from the transfusion-dependent iron overload in patients with TDT, there is a mechanism by which an inappropriate increase in intestinal iron absorption occurs, both in patients with TDT and in NTDT.
This mechanism is triggered by ineffective erythropoiesis. The accumulation of erythroid precursors during ineffective erythropoiesis increases the production of erythropherrone, potent negative regulator of hepcidin secreted by bone marrow erythroblasts ( 10 ), which negatively regulates the expression of hepcidin. The decrease in hepcidin, the main negative ferroportin modulator (an iron transporter protein in the basolateral membrane of the enterocyte), increases iron absorption (hepcidin inhibits iron absorption and recycling ferroportin) and release of iron from the reticuloendothelial in situations of iron overload and iron sequestration in erythropoiesis. It has been described that iron availability during stress erythropoiesis is produced by increase of ERFE ( 13 ).
In NTDT, growth differentiation factor 15 (GDF-15) can induce hepcidin downregulation. This is a member of the transforming growth factor- (TGF-β) family which use to be upregulated during ineffective erythropoiesis, causing the down regulation of hepcidin ( 14 ).
Bone Disease in β-Thalassemia
Recently, several advances in the understanding of the pathophysiology of bone disease in β-thalassemia have been done. Classically, it was directly attributed to ineffective erythropoiesis and secondary bone expansion, but recently it has been shown that there is an imbalance of cytokines that can directly alter bone metabolism, although the mechanisms involved are not yet well-established.
Both patients with TDT and NTDT show marked decreases in bone mineral density (BMD), despite optimization of transfusions, and low BMD continues to be a frequent complication in these patients.
The mechanisms that have been postulated to explain the loss of bone mineral density in patients with β-thalassemia include explicit effects of abnormal erythroid proliferation with bone expansion, increased circulating erythropoietin (EPO), iron bone deposit with iron toxicity, and oxidative stress with endocrine secondary disorders (hypogonadism, deficit GH-IGF-1, vitamin D deficiency) that which in turn affect the bone mineral loss and secondary osteoporosis ( 15 ).
In recent years, there is growing evidence of the relationship between erythropoiesis, bone mineral metabolism and iron homeostasis. Recently, a mechanism responsible for the activation of osteoclasts in thalassemic patients has been described that could be associated to cytokine dysregulation and, in particular, to the modification of the RANK/RANKL/OPG axis, which is essential for the regulation of osteoclastogenesis ( 16 , 17 ). The OPG/RANK/RANKL system is essential for the regulation of osteoclastogenesis. Osteoprotegerin (OPG) or osteoclastogenesis inhibition factor (OCIF or TNFRSF11B), is a member of the superfamily of tumor necrosis factor (TNFR) receptors that is expressed and secreted in numerous tissues (lung, heart, kidneys, liver, intestine, stomach, brain, thyroid gland and spinal cord) as well as in bone in which its main function is to inhibit the maturation and activation of osteoclasts.
Recently, it has been shown ERFE binds and sequesters some members of the bone morphogenetic protein (BMP) family, primarily BMP2, BMP6, and the BMP2/6 what suppresses hepcidin by inhibiting hepatic BMP/SMAD signaling. Therefore, iron availability by stimulated erythropoiesis can be regulated by ERFE ( 9 ). Bone formation by osteoblasts during skeletal development, modeling, and ongoing remodeling can be stimulated by BMPs. Therefore, ERFE seems to be key in the recently described erythropoiesis-iron-bone circuit, by modifying the availability of BMP. Therefore, ERFE appears to be an important link between abnormal erythropoiesis, iron metabolism alteration, and loss of BMD in β-thalassemia ( 16 , 17 ).
The mechanism of action of ERFE, through the sequestration of BMP, could be that the loss of ERFE, by improving the availability of BMP, stimulates the formation of osteoblastic bone ( 18 ). However, high ERFE levels are osteoprotective and prevent bone loss in β-thalassemia when erythropoiesis is extended. Therefore, there is a paradoxical effect that has not yet been fully explained. Although in TDT patients, in whom ERFE is inhibited post-transfusion, and is lower than in NTDT patients, could explain the more severe bone alterations in these patients despite transfusions and ERFE has a protective function decreasing bone loss phenotype in b-thalassemia.
In addition, other data indicate that the loss of BMP signaling (high ERFE), increases bone mass through direct inhibition of osteoclasts and activation of the Wnt pathway, predicting that the loss of ERFE would lead to a decrease in bone mass ( 16 , 17 ) by increased expression of RANKL and sclerostin.
In summary, to date the only known function of ERFE was hepcidin regulation expression through BMP sequestration, contributing to iron overload ( 9 ). However, ERFE seems to have bone metabolisms implications and a new role in bone protection has been described ( 16 ). In conditions of elevated ERFE, such as β-thalassemia and others ineffective erythropoiesis situations, BMP2 and BMP6 proteins are sequestered, decreasing signaling through the BMP/Smad and ERK pathways. This would result in decreased SOST and RANKL expression (Rankl/OPG) with decrease osteoclastogenesis and bone resorption. On the other hand, when level ERFE is low, increased BMP2, and BMP6 proteins, lead to stimulate osteoclastogenesis (RANKL/Opg), bone resorption and increased sclerostin osteocytes synthesis (expression SOST gene), with a consequent decrease in bone formation by inhibition of osteoblastic function.
GATA1 Levels Regulation in β-Thalassemia
As mentioned above, erythropoiesis starts from hematopoietic stem cells (HPSCs), in a finely regulated process, that involves various factors, and it is controlled at different molecular levels by growth factors and hormones such as erythropoietin, that activate different signaling pathways that end up activating erythroid transcription factors. The essential transcription factors for erythropoiesis are GATA-1, SCL, TAL1, LMO2, LDB1, KLF-1, and GFI-1B, although many more participate. These factors are organized in a complex called CEN (“Core ErythroidNetwork”) ( 19 ), whose operation is finely regulated by SCF and EPO between others, to ensure adequate erythrocyte development. GATA1 is considered the “master regulator” of this process, GATA1 is a DNA-binding zinc finger transcription factor that plays an essential role in the normal development of hematopoietic lines. The protein contains 413 amino acids, with an N-terminal region where its transcriptional activity resides and a C-terminal region that mediates the binding of GATA1 to DNA and other proteins. In 1995, two isoforms of GATA1 resulting from alternative splicing were identified. GATA1 encodes a 47 kDa protein and GATA1s, a shorter 40 kDa protein that lacks the transactivation domain at the N-terminus. Both proteins are capable of binding to DNA and could form dimers or heterodimers, although the shorter GATA1s isoform is less active than the long ( 20 ). In 1999 cleavage of GATA1 by Caspase 3 was reported ( 21 ). More recently, it has been linked the stabilization of GATA1 to inhibition of Caspase-1 Activity ( 22 ).
β-thalassemia shares several common elements with other forms of anemia including a deficiency of GATA1 the ‘master regulator' in erythropoiesis ( 23 ), GATA1 levels are finely regulated during erythropoiesis to develop sufficient functional erythrocytes ( 24 ). This transcription factor is necessary for normal early erythroid progenitors' differentiation [i.e., colony-forming unit-erythroid (CFU-E) and burst-forming unit erythroid (BFU-E) cells]. In β-thalassemia, α-hemoglobin chains accumulate in the cytosol due to the non-functionality of β-hemoglobin chains and sequester heat shock protein 70 (HSP70). Subsequently, HSP70 cannot be translocated into the nucleus thereby impairing GATA1 stabilization through caspase 3 cleavage, resulting in altered GATA1 levels with disturbed erythropoiesis and accumulation of unfunctional erythroid progenitors ( 23 ).
Importantly, GATA1 is highly expressed during these early stages of differentiation but GATA1 protein expression is shown to decline toward terminal erythroid differentiation ( 25 ). Recent data from Tyrkalska et al. demonstrated the role of the inflammasome, a complex of innate immune system which receptors and sensors playing important roles in infection and inflammation, in GATA1 regulation, and subsequent erythroid differentiation. Pharmacological inhibition of the inflammasome was shown to stimulate GATA1 expression and promote erythroid differentiation ( 22 ).
On the other hand, Fetal hemoglobin (HbF) increase has revealed as a promising results to treat β-hemoglobinopathies ( 26 ) with a recent implication of MiR-486-3p and miR-15a in Fetal hemoglobin induction ( 27 ). Several groups reported that increase of miR-210 levels is high in erythroid precursors from β-thalassemia patients what have an impact on fetal hemoglobin (HbF) levels ( 28 – 31 ).
Finally, other genetic studies indicate a relationship between variants in the gene BCL11A and HbF levels ( 29 ). There they described that reduced BCL11A expression induce HbF. The data published by Gasparello et al. are consistent with a globin gene regulation by BCL11A and therefore postulated BCL11A as a therapeutic candidate to reactivate HbF in disorders associated to beta-hemoglobin ( 29 ). Aligned with this, Bauer et al. published that GWAS-marked BCL11A enhancer represents another potential optional treatment in this disease ( 32 ).
All the processes described above are reflected and connected in Figure 3 .
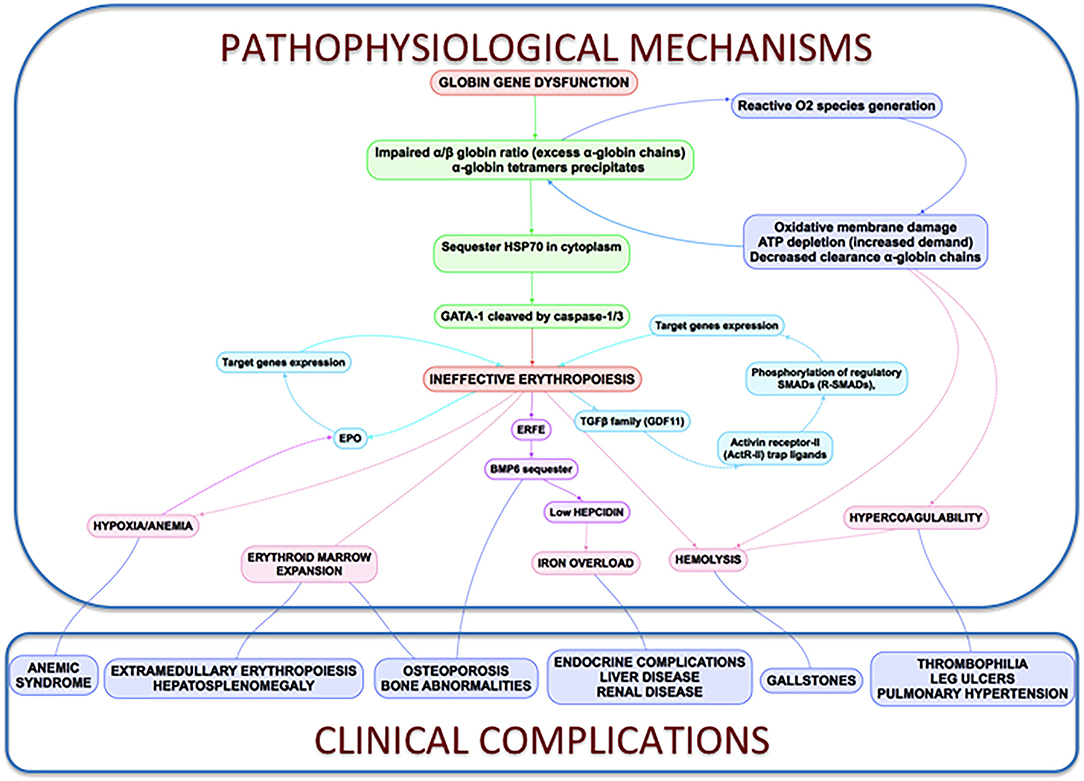
Figure 3 . Clinical complications and pathophysiological mechanisms of β-Thalassemia. In thalassemia, the imbalance α/β-globin synthesis is the fundamental initial pathogenic event. Excess α-globin chains precipitate in the cytoplasm, sequester HSP70 and GATA1 is cleaved by Caspase 3/1 which result in dysfunctional erythropoiesis and imposes metabolic stress on the erythrocytes, specifically in the form of excess generation of reactive oxygen species and increased demand on adenosine triphosphate (ATP)-dependent proteolytic mechanisms to clear excess globin chains. These pathophysiological changes lead to the characteristics of this disease: ineffective erythropoiesis, peripheral hemolysis, and subsequent anemia. Clinical implications of the α- and β-globin imbalance include lack of sufficient RBCs and Hb for effective oxygen transport, and ineffective erythropoiesis and hemolysis, which can lead to splenomegaly, bone marrow expansion (extramedullary hematopoiesis), concomitant bone deformities, and iron overload.
Discussion: Perspectives and Future New Potential β-Thalassemia Treatments
Despite the high social and economic impact of β-thalassemia, there is no curative treatment available, except for bone marrow transplant for the few pediatric patients who have an identical HLA donor, and who assume the high morbidity and mortality of transplant that is sometimes not acceptable for non-malignant disease. Currently no approved treatments to handle anemia in NTDT and, even though it has recently been approved, the first drug that improves anemia in these patients (luspatercept), has limited efficacy. Therefore, there are no effective treatments to improve anemia or to reduce red blood cell transfusions and chronic complications. However, in recent years there is a growing interest in studying these diseases with an increasing number of clinical trials directed against various therapeutic targets (gene therapy, erythroid maturation agents, pyruvate kinase activators, JAK kinase 2 inhibitors, targeting iron dysregulation). All of them are being developed and in the future may change the quality of life of these patients and we aim to be part of this scenario. All of them summarized in a succinct way by Musallam et al. ( 33 ).
Although these targets may be effective, efficacy is partial, not all patients respond, and therefore further research and treatments are required considering its multifactorial pathophysiology. Perhaps the correct approach in the future is combined treatment and, in this scenario, it will be essential to explore new therapeutic targets. In recent years, inflammasomes have emerged as a new potential therapeutic target for these types of diseases as well as the HSP70 nuclei regulation.
Inflammasome as a Potential Target Treatment in β-Thalassemia
Inflammasomes are multiprotein complexes firstly described a decade ago by Tschopp and colleagues. Inflammasomes are multiprotein complexes usually composed of sensor proteins, mainly from the NLR family, adaptor proteins such as ASC, and an effector cysteine-protease enzyme, usually caspase-1 ( 34 ). Gasdermin D (GSDMD) a pore-forming protein is cleavage and activated by caspase-1 ( 35 ), what induce cytokine release and pyroptosis ( 36 ). Several inflammasome receptors have been described with different roles including inflammatory diseases, sepsis protection or host defense ( 37 – 39 ).
Inflammasomes are broadly expressed in hematopoietic and non-hematopoietic cells and can induce different responses including production of IL-18, IL-1β, eicosanoids, and pyroptosis. Since this first description, research within the inflammasome field has been one of the most studied fields in immunology leading to huge advances.
In the innate immune context, the activation of inflammasomes is essential in the clearance of pathogens or damaged cells. On the other hand, uncontrolled inflammasome activation induces metabolic and autoimmune disorders, indicating the importance of these complexes. The recent role of the inflammasome in erythropoiesis brings the focus to these complexes to treat anemia and neutrophilia ( 22 ).
The effector protein of the inflammasomes are caspases and previous data indicated a role of Caspase 3 in GATA1 stabilization ( 21 ). More recently, inflammasome has been postulated as a potential treatment of Diamond-Blackfan anemia, this type of anemia curses by a deficiency in GATA1 levels due to a ribosomopathy that produces an inefficient translation of GATA1 ( 22 , 40 ). Tyrskalska et al. ( 22 )demonstrated an increase of GATA1 levels in human and zebrafish larvae with caspase-1 inhibitor. Therefore, potentially inhibition of Caspase-1 or Caspase-3 could be considered as a potential treatment to stabilize GATA1 levels to increase red blood cells formation. In this scenario, the identification of the inflammasome type that mediates this process will be essential to translate these results with higher specificity to the clinic ( Figure 4 ).
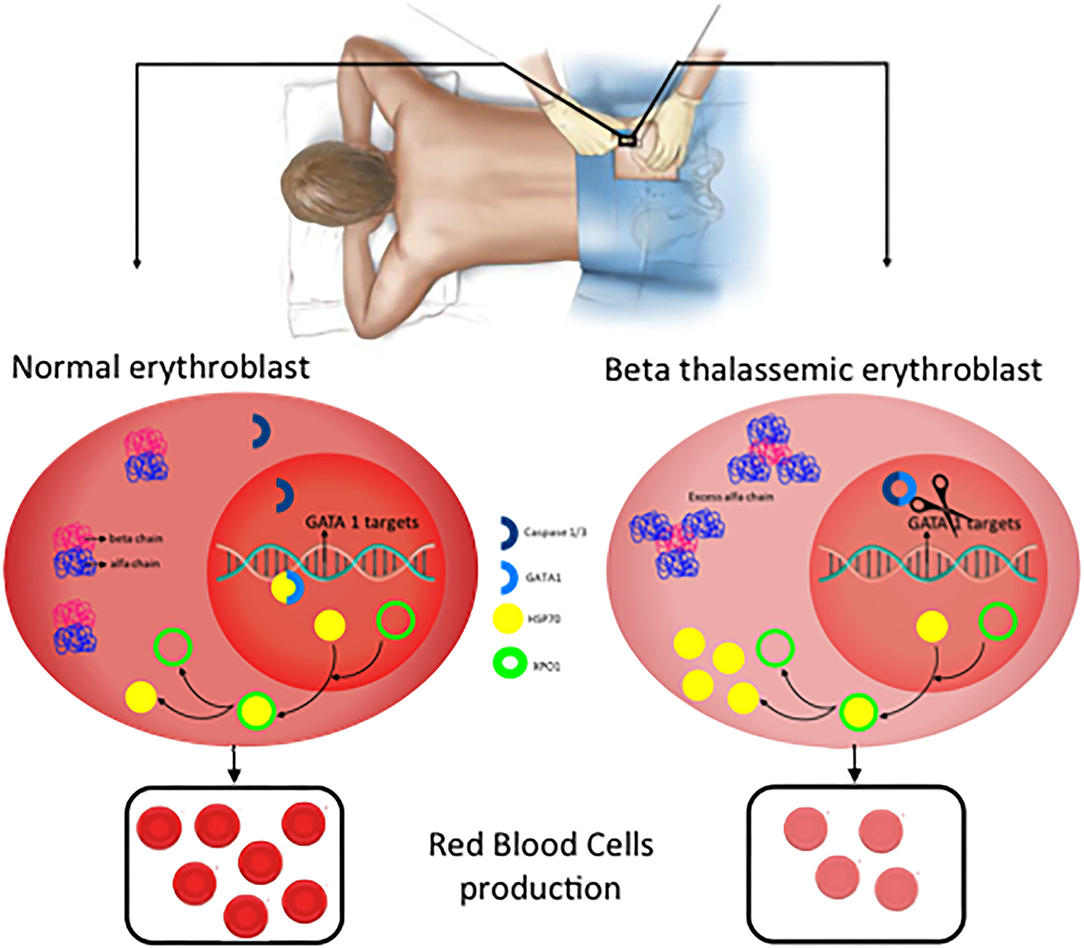
Figure 4 . New potential treatments for β-thalassemia. In normal erythroblast, GATA1 levels are regulated through the balance Caspase 3/1 cleavage and HSP70 protecting function in the nucleus. As a consequence, normal and functional erythrocytes are produced. In contrast, in β-thalassemic erythroblast, the lack of functional β-globin chains induces accumulation of free α-globin chains which restrict HSP70 distribution to the cytoplasm and therefore GATA1 is cleaved by Caspase 3/1 which result in fewer functional matured erythrocytes.
Inhibition of HSP70 Export From the Nucleus
As described above, GATA1 levels are essential in erythroid differentiation, and in this plays an important role the Heat Shock Protein 70 (HSP70) a chaperone, which is translocated to the nucleus to protect GATA1 transcription factor of caspase-3 cleavage ( 41 ). In β-thalassemia, the accumulation of free α-globin chains sequestered HSP70 in the cytosol which avoids the protective role of GATA1 into the nucleus. A recent publication demonstrated that HSP70 localization is regulated by the exportin-1 (XPO1) and inhibition of XPO1 increase HSP70 levels in normal erythroid progenitors what increase, which have as a consequence an increase in GATA1 levels ( 42 ). This introducesXPO1 inhibitors as a new therapeutic option to treat β-thalassemia ( 42 , 43 ).
For many years, management of β-Thalassemia patients has been limited to blood transfusion and iron chelation. However, β-Thalassemia is now the focus of a flourishing research field that has already offered new treatments with the potential to modify the natural history of the disease and the quality of life of the patients. In this review we have summarized the present knowledge of the pathophysiology of the disease and proposed future possible new, and more directed approaches, to its treatment ( Figure 4 ).
Conclusions
β-thalassemia (transfusion and non-transfusion dependent), is an inherited hemoglobinopathy caused by a quantitative defect in the synthesis of β-globin chains of hemoglobin, leading to the accumulation of free α-globin chains aggregates that cause ineffective erythropoiesis. The only curative treatment for these patients is hematopoietic stem cell transplantation, but this option only is feasible in a few patients with HLA-matched sibling donors. In most patients, the development of chelation and support treatments has improved survival, however, chronic complications have increased (iron overload, osteoporosis, extramedullary hematopoiesis, etc.) that limit their quality of life.
Despite increasing knowledge of the pathophysiology of β-thalassemia, only luspatercept has been recently approved in TDT patients, reducing transfusion needs but of limited effectiveness. NTDT patients, which do not require regular transfusions, lack an approved treatment and have the same (if not more) complications as TDT patients. Therefore, new treatments are needed that, alone or in combination with existing ones, can improve the expectations of these patients.
In human erythroblast, terminal erythroid maturation is altered due to HSP70 sequestration in the cytoplasm by free α-globin chains preventing its accumulation in the nucleus to protect GATA1 transcription factor from Caspase-3 cleavage, resulting in maturation arrest and apoptosis. ERFE and BMP play an important role in bone disease but are not well-established yet.
The knowledge of these critical new pathophysiological approaches can help develop new therapeutic options such as XPO1 inhibitors or inflammasome inhibitors that could rescue GATA-1 expression, improved erythroid terminal differentiation, and represent a new therapeutic option to ameliorate ineffective erythropoiesis, iron overload, and decreased mineral bone mass of β-thalassemia patients.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
MS-V: writing—original draft preparation. AP-O, ES, JM, and MB: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Institute of Health Carlos III (ISCIII) through a Miguel Servet Contract Program and the Associated Budget CP20/00028 to AP-O partially funded by European Funding. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.

Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Origa R. beta-Thalassemia. Genet Med. (2017) 19:609–19. doi: 10.1038/gim.2016.173
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Kattamis A, Forni GL, Aydinok Y, Viprakasit V. Changing patterns in the epidemiology of beta-thalassemia. Eur J Haematol. (2020) 105:692–703. doi: 10.1111/ejh.13512
3. Asadov C, Alimirzoeva Z, Mammadova T, Aliyeva G, Gafarova S, Mammadov J. beta-Thalassemia intermedia: a comprehensive overview and novel approaches. Int J Hematol. (2018) 108:5–21. doi: 10.1007/s12185-018-2411-9
4. Viprakasit V, Ekwattanakit S. Clinical classification, screening and diagnosis for thalassemia. Hematol Oncol Clin North Am. (2018) 32:193–211. doi: 10.1016/j.hoc.2017.11.006
5. Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V,. (eds.). Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT) . Nicosia: Thalassaemia International Federation (2014).
Google Scholar
6. Taher A, Vichinsky E, Musallam K, Cappellini MD, Viprakasit V. Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT) . Nicosia: Thalassaemia International Federation (2013).
PubMed Abstract | Google Scholar
7. Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. (2008) 36:1573–84. doi: 10.1016/j.exphem.2008.08.003
8. Valent P, Busche G, Theurl I, Uras IZ, Germing U, Stauder R, et al. Normal and pathological erythropoiesis in adults: from gene regulation to targeted treatment concepts. Haematologica. (2018) 103:1593–603. doi: 10.3324/haematol.2018.192518
9. Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. (2018) 132:1473–7. doi: 10.1182/blood-2018-06-857995
10. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. (2014) 46:678–84. doi: 10.1038/ng.2996
11. Blank U, Karlsson S. TGF-beta signaling in the control of hematopoietic stem cells. Blood. (2015) 125:3542–50. doi: 10.1182/blood-2014-12-618090
12. Xie Y, Bai H, Liu Y, Hoyle DL, Cheng T, Wang ZZ. Cooperative effect of erythropoietin and TGF-beta inhibition on erythroid development in human pluripotent stem cells. J Cell Biochem. (2015) 116:2735–43. doi: 10.1002/jcb.25233
13. Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. (2016) 172:512–23. doi: 10.1111/bjh.13820
14. Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. (2007) 13:1096–101. doi: 10.1038/nm1629
15. Gaudio A, Xourafa A, Rapisarda R, Zanoli L, Signorelli SS, Castellino P. Hematological diseases and osteoporosis. Int J Mol Sci. (2020) 21:3538. doi: 10.3390/ijms21103538
16. Castro-Mollo M, Gera S, Ruiz-Martinez M, Feola M, Gumerova A, Planoutene M, et al. The hepcidin regulator erythroferrone is a new member of the erythropoiesis-iron-bone circuitry. Elife. (2021) 10:e68217. doi: 10.7554/eLife.68217
17. Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, et al. Neurocircuitry of illness-induced hyperalgesia. Brain Res. (1994) 639:283–99. doi: 10.1016/0006-8993(94)91742-6
18. Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. (2016) 12:203–21. doi: 10.1038/nrendo.2016.12
19. Wontakal SN, Guo X, Smith C, MacCarthy T, Bresnick EH, Bergman A, et al. A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc Natl Acad Sci U S A. (2012) 109:3832–7. doi: 10.1073/pnas.1121019109
20. Calligaris R, Bottardi S, Cogoi S, Apezteguia I, Santoro C. Alternative translation initiation site usage results in two functionally distinct forms of the GATA-1 transcription factor. Proc Natl Acad Sci U S A. (1995) 92:11598–602. doi: 10.1073/pnas.92.25.11598
21. De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. (1999) 401:489–93. doi: 10.1038/46809
22. Tyrkalska SD, Perez-Oliva AB, Rodriguez-Ruiz L, Martinez-Morcillo FJ, Alcaraz-Perez F, Martinez-Navarro FJ, et al. Inflammasome regulates hematopoiesis through cleavage of the master erythroid transcription factor GATA1. Immunity. (2019) 51:50–63.e55. doi: 10.1016/j.immuni.2019.05.005
23. Arlet JB, Ribeil JA, Guillem F, Negre O, Hazoume A, Marcion G, et al. HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in beta-thalassaemia. Nature. (2014) 514:242–6. doi: 10.1038/nature13614
24. Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. (2005) 25:1215–27. doi: 10.1128/MCB.25.4.1215-1227.2005
25. Gutierrez L, Caballero N, Fernandez-Calleja L, Karkoulia E, Strouboulis J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life. (2020) 72:89–105. doi: 10.1002/iub.2192
26. Salinas Cisneros G, Thein SL. Research in sickle cell disease: from bedside to bench to bedside. Hemasphere. (2021) 5:e584. doi: 10.1097/HS9.0000000000000584
27. Eltaweel NH, ElKamah GY, Khairat R, Atia HAE, Amr KS. Epigenetic effects toward new insights as potential therapeutic target in B-thalassemia. J Genet Eng Biotechnol. (2021) 19:51. doi: 10.1186/s43141-021-00138-x
28. Bavelloni A, Poli A, Fiume R, Blalock W, Matteucci A, Ramazzotti G, et al. PLC-beta 1 regulates the expression of miR-210 during mithramycin-mediated erythroid differentiation in K562 cells. Oncotarget. (2014) 5:4222–31. doi: 10.18632/oncotarget.1972
29. Gasparello J, Fabbri E, Bianchi N, Breveglieri G, Zuccato C, Borgatti M, et al. BCL11A mRNA targeting by miR-210: A possible network regulating γ-globin gene expression. Int J Mol Sci . (2017) 18:2530. doi: 10.3390/ijms18122530
30. Sarakul O, Vattanaviboon P, Tanaka Y, Fucharoen S, Abe Y, Svasti S, et al. Enhanced erythroid cell differentiation in hypoxic condition is in part contributed by miR-210. Blood Cells Mol Dis. (2013) 51:98–103. doi: 10.1016/j.bcmd.2013.03.005
31. Sawant M, Chandrakala S, Colah R, Ghosh K, Nadkarni A. Does HbF induction by hydroxycarbamide work through MIR210 in sickle cell anaemia patients? Br J Haematol. (2016) 173:801–3. doi: 10.1111/bjh.13642
32. Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, Lin C, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. (2013) 342:253–7. doi: 10.1126/science.1242088
33. Musallam KM, Bou-Fakhredin R, Cappellini MD, Taher AT. 2021 update on clinical trials in beta-thalassemia. Am J Hematol. (2021) 96:1518–31. doi: 10.1002/ajh.26316
34. Schroder K, Tschopp J. The inflammasomes. Cell. (2010) 140:821–32. doi: 10.1016/j.cell.2010.01.040
35. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. (2015) 526:660–5. doi: 10.1038/nature15514
36. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. (2002) 10:417–26. doi: 10.1016/S1097-2765(02)00599-3
37. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. (2004) 20:319–25. doi: 10.1016/S1074-7613(04)00046-9
38. Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. (2005) 73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005
39. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. (2004) 430:213–8. doi: 10.1038/nature02664
40. Danilova N, Gazda HT. Ribosomopathies: how a common root can cause a tree of pathologies. Dis Model Mech. (2015) 8:1013–26. doi: 10.1242/dmm.020529
41. Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. (2007) 445:102–5. doi: 10.1038/nature05378
42. Guillem F, Dussiot M, Colin E, Suriyun T, Arlet JB, Goudin N, et al. XPO1 regulates erythroid differentiation and is a new target for the treatment of beta-thalassemia. Haematologica. (2020) 105:2240–9. doi: 10.3324/haematol.2018.210054
43. Modepalli S, Hattangadi SM. Novel use for selective inhibitors of nuclear export in beta-thalassemia: block of HSP70 export from the nucleus via exportin Xpo1 improves ineffective erythropoiesis. Haematologica. (2020) 105:2188–9. doi: 10.3324/haematol.2020.254474
Keywords: anemia, thalassemia, β-globin, GATA1, inflammasome
Citation: Sanchez-Villalobos M, Blanquer M, Moraleda JM, Salido EJ and Perez-Oliva AB (2022) New Insights Into Pathophysiology of β-Thalassemia. Front. Med. 9:880752. doi: 10.3389/fmed.2022.880752
Received: 21 February 2022; Accepted: 22 March 2022; Published: 12 April 2022.
Reviewed by:
Copyright © 2022 Sanchez-Villalobos, Blanquer, Moraleda, Salido and Perez-Oliva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo J. Salido, eduardoj.salido@carm.es ; Ana B. Perez-Oliva, anab.perez@imib.es
This article is part of the Research Topic
Constructing New Motifs in Hematology
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Gen Med
- PMC11070558
Long-Term Follow-Up of Patients Undergoing Thalidomide Therapy for Transfusion-Dependent β-Thalassaemia: A Single-Center Experience
Weijian zhu.
1 Department of Hematology, Zhuhai Clinical Medical College of Jinan University (Zhuhai People’s Hospital), Zhuahai, 519050, People’s Republic of China
Mufang Huang
Xiaoqi wang, xiaoliang li, jiangming chen.
2 Department of Haematology, Wuzhou Gongren Hospital, Wuzhou, 543001, People’s Republic of China
3 Institute of Hematology, School of Medicine, Jinan University, Guangzhou, 10632, People’s Republic of China
We evaluated the long-term safety and efficacy of thalidomide in the treatment of transfusion-dependent β-thalassemia (TDT).
Fifty patients with TDT were treated with thalidomide and followed-up for 5 years. Thalidomide at a 50 mg dose was administered once a day after dinner. The dose was increased to 150 mg/d after 3 d if well tolerated. After 1 year of treatment, the hemoglobin (Hb) level was stabilized at its maximum, and thalidomide was gradually reduced and maintained at the minimum dose. The hematological response, transfusion dependence, and haemolytic indicators were assessed.
At 9 month of follow-up, 38 (76%) patients achieved an excellent response, 1 (2%) a good response, 4(8%) a minor response, and 7(14%) did not show a response. The overall response rate was 86%. At 9 months, the Hb level increased from 79.0 ± 13.2 g/L at baseline to 99.0 ± 13.7g/L ( P <0.001). Patients who achieved excellent response continued to show an increase in Hb levels during follow-up. At 48 months, the mean Hb level was 98.99 ± 10.3g/L; 21 patients (84.0%) became transfusion independent. Thalidomide was reduced and maintained to 25 mg/d in three of these patients. Moreover, five patients completed 60 months of follow-up, and with a mean Hb level of 99.8 ± 6.7g/L. During follow-up, grade 1–2 adverse drug reactions were noted; however, no grade 3 or higher adverse event was reported. However, no decrease in hemolytic indicators was observed.
Thalidomide was well tolerated in the long term, while it significantly improved Hb levels and reduced the transfusion burden.
Introduction
Beta-thalassemia is one of the most common single gene inherited diseases. The World Health Organization reported that approximately 150 million people worldwide carry the hemoglobinopathy gene, representing a substantial global health burden. The incidence of hemolytic anemia is mainly concentrated in tropical and subtropical areas, primarily on the Mediterranean coast, African America, North Africa, Southeast Asia, the Indian subcontinent, and Melanesia in the Pacific Islands. 1 In these areas, the high incidence of hemolytic anemia caused by hemoglobinopathies is a serious public health problem. 2 , 3 Beta-thalassemia is mainly caused by point mutation of the β-globin gene or loss or insertion of individual nucleotides. This results in accumulation of excess, unbound α-globin chains that precipitate in erythroid precursors in the bone marrow and mature erythrocytes, leading to ineffective erythropoiesis and peripheral hemolysis. 4 Beta-thalassemia was classified clinically as transfusion dependent or non-transfusion dependent. 5 Patients with transfusion-dependent β-thalassemia need regular red blood cell transfusions and iron chelation therapy to long-term survival; however, blood transfusion is associated with iron overload, which causes end-organ damage, thus, increasing morbidity and mortality. Currently, curative options for these diseases include allogeneic bone marrow transplantation and gene therapy or gene editing; 6–8 however these methods are costly, and safety challenges limit their use worldwide, especially in developing countries.
Fetal hemoglobin (HbF) inducers activate γ-globin genes that are largely turned off in patients with β-thalassaemia. The synthesized γ-chain can replace the defective β-chain, and the HbF formed by the relative excess of α-chain can be compensated by an increase in the production of the β-like globin molecule, the γ-globin. Reducing the number of unbound α-globin chains in red blood cells may ameliorate its detrimental effects and increase total (Hb) levels. 9 Hydroxyurea is the most widely use HbF inducer used in sickle cell disease; however, it is less effective for patients with β-thalassemia. 10 Other inducers such as 5-azacytidine and decitabine have not been widely use in clinical practice due to their poor efficacy and potential carcinogenic effects.
Thalidomide, an immunomodulatory drug and strong HbF inducer, reduces dependence on transfusions by inducing γ-globin gene expression and significantly increasing in Hb concentration. 11–14 In a Phase 2, multicenter, randomized, double-blind clinical trial, thalidomide effectively improved Hb levels in patients with transfusion-dependent β-thalassaemia (TDT) with few adverse events (AEs). 15 All the clinical trials were short-term, and the long-term efficacy of thalidomide in patients with TDT remains unknown. Therefore, we aimed to evaluate the long-term safety and clinical response to thalidomide and its relationship with hemolytic markers, such as bilirubin.
This prospective study enrolled patients aged ≥14 years who were diagnosed with TDT from May 2018 to December 2020. Transfusion dependence was defined when as the need of at least eight transfusions or 100 mL per kilogram of body weight of leukoreduced packed RBCs per year, or frequent transfusion to maintain Hb > 70 g/L in the 2 years before enrolment. The inclusion and exclusion criteria is available in Supplementary Material 1 . This study recruited patients from Guangdong Province, South China. All patients provided written informed consent prior to enrolment. This consent was obtained from the parents or guardians of the adolescent participants. The protocol and consent form were approved by the independent ethics committee of the Zhuhai People’s Hospital (ethics number: 2018(4)/ZY[2019](28)), and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
Patients received thalidomide at a dose of 50mg/d, administered after dinner. For those who could tolerate the drug, the dose was increased to 150 mg/d after 3 d. If necessary, the dose could be adjusted according to the adverse reactions. All patients were advised to take folic acid (5 mg/d) orally, and aspirin (100 mg/d) was prescribed to prevent thrombosis. Patients with aspirin contraindications (G6PD deficiency) were administered clopidogrel as prophylaxis. Transfusion was recommended to maintain the Hb level at > 90.0 g/L, based on local blood transfusion standards, during treatment.
Considering that thalidomide has the potential risk of teratogenicity and lethality to fetuses, pregnancy was forbidden during the period of taking thalidomide administration. Fertile females must took a medically supervised pregnancy test before starting the trial and during each clinic visit, and were engaged to take reliable contraceptive measures during treatment. The dosage of thalidomide is tapered after 12 months based on the hemoglobin level and maintained to keep the hemoglobin at an appropriate level.
Treatment Response Criteria
Response to thalidomide was defined as follows: excellent response, free of blood transfusion for at least 6 weeks, reaching a final total Hb level of 90 g/L, or an elevation in total Hb level of ≥ 20 g/L; good response, a reduction in the transfusion burden of at least 50% from baseline (the 3-month period before the first of thalidomide); minor response, reduction of at least 33% and <50% from baseline; and no response, reduction in transfusion burden of <33%. 16 , 17
Patients were followed up every 3 months for 1 year and every 6 months thereafter. Follow-up included monitoring for thrombosis, nervous system toxicity, and other possible AEs as well as routine blood, serum alanine transaminase (ALT), aspartate transferase (AST), total bilirubin (TBIL), indirect bilirubin (DBIL), indirect bilirubin (IBIL), blood urea nitrogen(BUN), creatinine(Cr), and lactate dehydrogenase (LDH) levels. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. 18
Statistical methods
Statistical analysis was performed using SPSS (version 24.0; SPSS Institute, Cary, NC), with a P- value ≤ 0.05 indicating statistical significance. Continuous variables that approximate a normal distribution are represented by mean ± standard deviation. Median (interquartile range) was used for continuous variables that did not follow a normal distribution. Paired t -tests were used for pre- and post-treatment comparisons. Analysis of the measurement indicators at each monitoring time point was performed using a repeated-measures analysis of variance. Pearson’s correlation analysis was used for correlation analysis.
Safety data were used to describe the occurrence of blood or non-blood toxic reactions in detail, calculate the incidence rates of different events, and determine the composition ratios of the severity of each event.
Fifty patients were included in the study and followed-up for at least 9 months until October 2023. Mean follow-up was 4.2 years (standard deviation, 0.74) and the median value was 4.21 years. The longest follow-up time was 5.5 years. Of these patients, 29 were enrolled in the randomized controlled trial 15 and were followed up after the extension study ( Figure 1 ). Moreover, 24 men and 26 women, with a median age of 16.5 years (range, 14–30 years) were included. Nineteen patients underwent splenectomy. Eleven (22%) patients had β0/β0, 26 (52.0%) β0/β+, including a case of a merger - SEA/α-gene, and 13 (26.0%) β+/β+ genotype ( Supplementary Table 1 ). The baseline patient characteristics are shown in Table 1 .
Baseline Demographic Characteristics of Patients

Treatment, and follow-up. Twenty-nine patients were enrolled in this randomized controlled trial. The placebo-controlled period included data from month 0 (baseline) to 12. Additional 21 patients who meet the inclusion criteria were enrolled in this open-label study. The study population comprised of 50 patients who received at least one dose of thalidomide. (allo-HSCT: allogeneic hematopoietic stem cell transplantation).
Primary Efficacy Analysis
Fifty patients were administered thalidomide, and its efficacy was evaluated at 3, 6, 9, 12, 18, 24, 36, 48, and 60 months. The primary endpoints were changes in Hb levels and transfusion volume in patients who received thalidomide. After 3 months of treatment, 24 (48.0%), 11 (22.0%), 11 (22.0%) and 4 (8.0%) patients had excellent, good, minor, and no response respectively. The overall response rate (ORR) was 92%, with excellent, good and minor rates accounting for 48.0%,22.0% and 22.0%, respectively. At 6 months, 35 patients (70.0%) showed an excellent response. The effectiveness rate was higher than in the third month. Four (8.0%) patients showed a good response, four (8.0%) a minor response, and seven (14.0%) no response. The ORR was 86.0%. At 9 months, 38 (76.0%), 1 (2.0%), 4 (8.0%), and 7(14.0%) patients had excellent, good, minor, no responses, respectively. The ORR was 86.0%.
A significant increase was observed in Hb levels after 3 months. The Hb levels increased from 79. 0±13.2 g/L at baseline to 92.5 ± 14.4g/L ( P <0.001) after thalidomide therapy. At month 6, Hb level increased to 97.2 ± 15.7g/L ( P <0.001), presenting a significant increase of 18.2 ± 20.1g/L. At month 9, Hb increased to 99.0 ± 13.7g/L ( P <0.001), with a rise of 20.1 ± 19.8 g/L from baseline, After 12 months, thalidomide was discontinued in four patients who failed to respond, and a total of 46 patients completed 12 months of follow-up, with an average Hb level of 101.4 ± 13.7g/L. After 12 months, the minimum dose was maintained according to Hb levels. A total of 42 patients were followed up for 24 months; 7 patients discontinued treatment due to ineffectiveness, and 1 patient due to nephrotic syndrome. The mean Hb level was 102.6 ± 10.2g/L, which persisted throughout the follow-up period ( Table 2 , and Figure 2 ).
Change in Haemoglobin Levels

The mean change in haemoglobin levels from baseline to 24 months after thalidomide treatment. The effective group includes excellent responder, good and minor responders. The level of Hb was expressed as mean ± standard deviation (SD) and P - values were determined by Student’s t -test.
Analysis of Long-Term Efficacy After Drug Reduction
Thalidomide dose was reduced after 12 months and maintained to keep the hemoglobin at an appropriate level at 30 months (Hb level remained stable at ≥90g/L). A total of 38 patients receiving oral thalidomide for 36 months had a mean Hb level of 100.8 ± 8.8 g/L, of whom 31 (81.5%) had an excellent response (free of blood transfusion and maintaining Hb level at >90g/L), 3 (7.9%) had a good response, and 3 (7.9%) had a minor response. The patient who achieved a reduction in transfusion burden of ≤33% continued to receive thalidomide. Two patients with excellent responses were proposed to receive gene therapy; one patient stopped using thalidomide after 2 years and the other at 42 months. One patient discontinued treatment at 42 months due to receive allogeneic hematopoietic stem cell transplantation. A total of 25 patients received oral thalidomide for 48 months. The mean Hb level was 98.99 ± 10.3 g/L, 21 (84.0%),1 (4.0%), and 3 (12.0%) patients had an excellent, good, and minor responses respectively. From the 21 patients who had an excellent response, in 3 the dose was reduced and maintained to 25 mg/d, whereas the dose was 50 mg/d, 75 mg/d, 100 mg/d, 125 mg/d, and 150mg/d in 1,2,9,3 and 3 patients respectively. Of the 21 patients with and excellent response, 15 (71.4%) had a −28 (HBB:c-78A>G) locus mutation, suggesting that this mutation may be associated with a high thalidomide response. However, Hb level did not correlate with HbF levels at month 48 ( r =−0.364, P >0.05; Table 3 ). Furthermore, five patients were followed for 60 months, with a mean Hb level of 99.8 ± 6.7g/L.
Genotype of Patients Who Had an Excellent Response and Thalidomide Maintenance Dose
Secondary Study Endpoints
Laboratory assessments were performed to evaluate changes in hemolysis indices (TBIL, DBIL, IBIL, LDH, reticulocyte count, and peripheral blood nucleated red cell count), iron load, liver and kidney function, and HbF level. At 3 months of treatment, HbF significantly increased from 16.09± 12.79% to 51.72± 25.59% ( P < 0.001). Serum ferritin levels presented a reduction of 4046.42± 4098.12 ng/mL from baseline ( P >0.05). At 4 years, HbF increased to 74.40± 20.10%. Serum ferritin levels were reduced to 1651.45± 1247.60 ng/mL ( P < 0.05). Thirty patients completed the 4-year follow-up period, and complete data were obtained from 20 patients. Univariate repeated measurement ANOVA was performed for WBC, PLT, TBIL, IBIL, LDH, reticulocyte count, liver and kidney function and other indicators at 0, 12, 24, 36 and 48 months: WBC (F[2.24,20.20]) = 0.585, P =0.585>0.05), PLT (F[2.10,18.86] = 0.152 P =0.538>0.05), reticulocyte percentage (F[1.81,16.31] = 0.548, P =0.572>0.05), TBIL (F[2.49,47.24]=0.40, P =0.714>0.05), IBIL (F[2.32,44.11]=0.29, P =0.781>0.05), LDH (F[1.63,13.05]=0.915, P =0.406>0.05), Cr (F[5,60]= 0.725, P =0.725>0.05). No significant differences were observed any time point. ALT (F [2.85,56.90] = 4.54, P =0.007 P < 0.05), and AST (F[2.36,39.91]=3.14, P =0.045< 0.05) levels decreased significantly at each time point ( Figure 3 ).

( A )Changes in liver function from baseline to 48 months. ( B ) Changes in LDH and CR from baseline to 48 months. ( C ) Changes in RET and WBC from baseline to 48 months. ( D ) Changes in PLT from baseline to 48 months.
Thalidomide was administered for a median of 46 months. During follow-up, all AEs were grade 1–2, and no grade 3 or higher AE was noted. The incidence of AEs is shown in Table 4 . The most common AE was drowsiness, with an incidence rate of 42%, all of which were grade 1. Drowsiness occurred more often after starting medication and gradually decreased or becomes tolerable after approximately 1 week. Constipation (26%) was also commonly observed but generally mild. Rashes and allergies were noted in 12% and 8% of the patients, respectively, and manifested as maculopapular lesions, rubella, or blushing. They mostly occurred within 1 week of drug administration and were tolerated without increasing or decreasing the dose. The incidence rate of edema was 10%, and manifesting as edema of the foot, ankle, or face, and relieved by the short-term use of diuretics. Other common AEs included dizziness (2%), fatigue (6%), and limb numbness (2%), while the AEs of grade 2 were relieved after temporary drug discontinuation or drug dose reduction. Abdominal pain or thrombosis was not observed. One patient was free of transfusion 1 year after taking the drug, while the Hb level remained above 90g/L. This patient was diagnosed with nephrotic syndrome; the pathology of kidney biopsy showed stage III membranous nephropathy, accompanied by decreased Hb, and was withdrawn from the study to receive symptomatic treatment.
Incidence of Adverse Events (AEs)
This long-term follow-up study investigated the efficacy and safety of thalidomide for TDT. In this clinical trial, 50 patients with TDT showed a high response rate to thalidomide, with sustained increases in Hb levels, while more than 70% of the patients with TDT did not require blood transfusion. Thalidomide was well tolerated and did not cause serious AEs in 5 years of follow-up. However, in this study, no decrease in hemolysis-related indices (TBIL, DBIL, IBIL, LDH, and reticulocyte count) was noted, despite the improvement of anemia.
Previous studies showed dramatic erythroid response to thalidomide in transfusions dependent β-thalassemia. In an Indian report including 21 patients with transfusion dependent E-β thalassemia, who have failed a trial of hydroxyurea, 15 (71.4%) attained transfusion independence (complete responders) and 1 (4.7%) attained partial response (50% decrease in transfusion requirement), while 5 (23.9%) were non-responders. 19 In a multicenter study in a southern Chinese population, among 23 patients with TDT, transfusions were terminated in 43.5% (10/23) of the patients and decreased by more than 50% in 52.2% (12/23), whereas the average Hb level increased. 20 In our study, at 9 months, 38 (76.0%), 1(2.0%), 4(8.0%), and 7(14.0%) patients had excellent, good, minor and no responses, respectively. The ORR was 86.0%. The most previous studies on thalidomide the patients were followed-up for 1–2 years. In the current study, the response was sustained over a longer duration of approximately 5 years (range: 35–65 months). Moreover, a significant decrease in serum ferritin levels was observed in the responders. These levels were reduced to 1651.45± 1247.60 ng/mL from baseline after 4 years of treatment.
Recently, luspatercept (a erythroid maturation agent) was studied in the double-blind Phase III BELIEVE trial ( {"type":"clinical-trial","attrs":{"text":"NCT02604433","term_id":"NCT02604433"}} NCT02604433 ) and was associated with a significant reductions in the RBC transfusion burden in adults with TDT. The percentage of patients who achieved the primary endpoint of ≥33% reduction from baseline in transfusion burden during weeks 13–24 plus a reduction of ≥2 RBC units during the same period was 21.4%. 16 In our study, thalidomide was more effective in reducing the transfusion burden and increasing Hb levels. Regarding the degree of transfusion independence as the primary endpoint, the volume of transfusion requirement was significantly decreased in most enrolled patients, the majority of which were from Guangdong Province. However, the transfusion criteria were inconsistent across different regions. The previous blood transfusion volume at the same interval was used as baseline, which may be clinically feasible for all patients and reflects real-world clinical practice.
The unbalanced synthesis of α- and β-globin chains is the core mechanism of the pathogenesis of β-thalassemia, while thalidomide acts as an anti-angiogenic and immunomodulatory agent with an unknown mechanism of action in the treatment of β-thalassemia. Previous studies confirmed that thalidomide promotes erythroid differentiation and induces γ-globin and HbF production. In the current trial, we also observed a sustained increase in HbF levers after 4 years of treatment. HbF increased to 73.49± 21.0%; however, it was not associated with elevated Hb levels. Simultaneously, the most frequent β-globin gene mutation in our patients was −28 (−78A>G) locus mutation, which may have contributed to the high rates of thalidomide response. We also analyzed the levels of several hemolytic markers (TBIL, DBIL, IBIL, LDH, and reticulocyte counts); however, we did not observe a decline after treatment. This may be due to the fact that most of the patients in this group came from Guangdong Province and had adequate medical follow-up. Most patients had received adequate blood transfusions before admission, and bone marrow hematopoiesis was suppressed. Thalidomide corrects some of the imbalance in α- and β-globin chain synthesis and inhibits ineffective hematopoiesis. In the absence of transfusion, the destruction of immature red blood cells provokes a compensatory hyperplasia of the erythroid marrow.
The optimal dose of thalidomide for ß-thalassemia has not yet been determined. We found that efficacy was dose-dependent. In the current study, the patients received a dose of 150 mg/d for 1 year and achieved a maximum stable Hb level. AEs associated with thalidomide were mild in this patient group. During follow-up, grade 1–2 AEs occurred; no grade 3 or higher AEs were observed, and there was no clinically evident incidence of thrombosis or secondary malignancies in any patient. The risk of AEs is related to the daily dose and duration of treatment. 21 As patients need to take thalidomide for a long time to maintain its efficacy, and considering the safety of the drug, we reduced the dose and administered maintenance therapy at a relatively low dose, accompanied by an acceptable decrease in Hb levels. In the subsequent follow-up, no greater than Grade 1 AEs occurred. During the treatment, the women were not pregnant; however, the teratogenic effects of the drug remain a concern, and although there were no pregnancy-related problems during follow-up, it is necessary to increase awareness and medication guidance in case of pregnancy in patients receiving thalidomide.
However, the limitations of this study were that the sample size was relatively small and we used serum ferritin instead of liver R2* magnetic resonance imaging for to assess iron overload. As thalidomide is a lifelong drug for patients with thalassemia, its AEs remain a matter of concern, and further clinical trials with extended follow-up periods are required to comprehensively drug efficacy and safety.
Conclusions
Thalidomide can effectively improve Hb levels and reduce the need for blood transfusions in patients undergoing TDT. This drug causes mild adverse reactions, while it is a convenient, effective, and affordable treatment for patients with thalassemia who cannot undergo allogeneic stem cell transplantation. Thalidomide can also act as a bridge between transplantation and gene therapy, thereby reducing the need for blood transfusions.
No studies have been conducted to determine the optimal therapeutic dose of thalidomide for TDT. An oral thalidomide dose of 150 mg/d was used in this trial, and a preliminary exploration was conducted to determine the optimal therapeutic dose. However, to further validate these finding, designing randomized controlled studies using different treatment doses is necessary.
Acknowledgments
The authors thank the patients with transfusion-dependent β-thalassaemia for their participation and cooperation.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82260026), the Zhuhai Medical Health Science and Technology Plan project (ZH2202200041HJL), and the Guangxi Natural Science Foundation (2020GXNSFAA159097).
Data Sharing Statement
The original data sets are also available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
The authors have no competing interests to declare that are relevant to the content of this article.

IMAGES
VIDEO
COMMENTS
Thalassemia is an inherited disease, meaning that at least one of the parents must be a carrier for the disease. It is caused by either a genetic mutation or a deletion of certain key gene fragments. Alpha thalassemia is caused by alpha-globin gene deletion which results in reduced or absent production of alpha-globin chains. Alpha globin gene ...
Molecular Pathogenesis. β-Thalassemia is caused by mutations resulting in a single nucleotide substitution, small deletions or insertions within the β-globin gene or its immediate flanking ...
DOI: 10.1056/NEJMra1404415. The thalassemias are the most common human monogenic diseases. 1 These inherited disorders of hemoglobin synthesis are characterized by a reduced production of globin ...
Thalassaemia is a diverse group of genetic disorders with a worldwide distribution affecting globin chain synthesis. The pathogenesis of thalassaemia lies in the unbalanced globin chain production, leading to ineffective erythropoiesis, increased haemolysis, and deranged iron homoeostasis. The clinical phenotype shows heterogeneity, ranging from close to normal without complications to severe ...
β-thalassemia is a disease caused by genetic mutations including a nucleotide change, small insertions or deletions in the β-globin gene, or in rare cases, gross deletions into the β-globin gene. These mutations affect globin-chain subunits within the hemoglobin tetramer what induces an imbalance in the α/β-globin chain ratio, with an excess of free α-globin chains that triggers the most ...
To evaluate the prevalence and the performance of routine surveillance for thalassemia related complications during 2 periods; before and after published CPGs (2012-2014 vs 2015-2017), data ...
The global burden of thalassemia, reflected in its prevalence, incidence, mortality, and DALYs, exhibits significant disparities. Geographic and demographic shifts in disease distribution have been observed from 1990 to 2021, with an overall decrease in burden, yet an increase in cases among the elderly population. Analysis of epidemiological trends over time highlights the influence of health ...
VOL. 353 NO. 11. Thalassemia is a hereditary anemia resulting from defects in hemoglobin production. 1 β-Thalassemia, which is caused by a decrease in the production of β-globin chains ( Figure ...
Credit: toeytoey2530/ iStock / Getty Images Plus. Adding a functional gene to defective blood stem cells is a successful therapy for patients with severe beta thalassemia, according to an ...
In the time period considered in the research (1995-2016) 127 patients were transplanted (60 matched unrelated and 67 sibling HSCT) with 81% of Overall Survival (OS) and 77% of Thalassemia Free ...
This narrative review was performed by collecting clinical trials, primary research, and reviews on molecular genetics and prospects for β-thalassemia therapy. Articles published in peer-reviewed scientific journals were also included. Articles were excluded if they were not written in English or published in peer-reviewed scientific journals.
Review Article. ISSN No. 2394-3971. Abstract. Thalassemia's ar e genetic disorders inherited from a person's parents. Thalassemia's are. prevalent worldwide with 25,000 deaths in 2013 ...
The increase in number of patients with thalassemia living in California highlights the importance of provider knowledge about thalassemia in order to effectively serve these patients in their communities. Featured Articles. Thalassemia Awareness By staying committed to long-term treatment, people with thalassemia can enjoy a full life.
Research article. First published online May 9, 2024. Development of a nomogram to predict the risk of secondary failure of platelet recovery in patients with β-thalassemia major after hematopoietic stem cell transplantation: a retrospective study ... NHC Key Laboratory of Thalassemia Medicine and Guangxi Key Laboratory of Thalassemia Research
728 n engl j med 384;8 nejm.org February 25, 2021 The new england journal of medicine β-thalassemia are closely tied to the degree of im-balance between α-globin and β-globin chains. Deficient ...
Abstract. β-Thalassemia results from insufficient production of the hemoglobin subunit β-globin (β +) or from the absence of β-globin (β 0 ). Low levels of adult hemoglobin (HbA, or α 2 β 2 ...
β-thalassemia include regular blood transfusion and iron chelation. However, chronic blood transfusion poses a signicant risk of iron overload and subsequent multi-organ damage, as well as an ...
Google Scholar produces about 278 hits for the term "inertial propulsion". If patents are also included, the number of hits increases to 536. This paper discusses, in a critical way, some characteristic aspects of this controversial topic. The review starts with the halteres of athletes in the Olympic games of ancient times and then continues with some typical devices which have been ...
Beta-thalassemia is one of the most common single gene inherited diseases. The World Health Organization reported that approximately 150 million people worldwide carry the hemoglobinopathy gene, representing a substantial global health burden. ... took part in drafting, revising or critically reviewing the article; gave final approval of the ...