- Case report
- Open access
- Published: 27 May 2024

A rare incidence of severe dermatological toxicities triggered by concomitant administration of all-trans retinoic acid and triazole antifungal in patients with acute promyelocytic leukemia: a case series and review of the literature
- Aisha Jamal ORCID: orcid.org/0000-0001-5022-7498 1 , 3 ,
- Rafia Hassam 1 ,
- Qurratulain Rizvi 1 ,
- Ali Saleem 1 ,
- Anum Khalid 2 &
- Nida Anwar 1
Journal of Medical Case Reports volume 18 , Article number: 261 ( 2024 ) Cite this article
182 Accesses
2 Altmetric
Metrics details
All-trans retinoic acid (ATRA) is an indispensable part of the treatment of acute promyelocytic leukemia (APL). Although, mild cutaneous toxicities like mucocutaneous xerosis, rash, and pruritus are well reported, ATRA associated severe dermatological toxicities are extremely rare. ATRA is primary metabolized by cytochrome P450 (CYP450) enzyme system, and triazole antifungals are notorious for their strong inhibitory effect on CYP450.
Case presentation
Three Asian APL patients experienced rare ATRA-induced severe dermatological toxicities: exfoliative dermatitis (ED) in cases 1 and 2, and necrotic scrotal ulceration in case 3. Both case 1 (33-year-old female), and case 2 (28-year-old male) landed in emergency department with dehydration, generalized skin erythema and xerosis during their induction chemotherapy. Both of these patients also developed invasive aspergillosis and required concomitant triazole antifungals during their chemotherapy. For ED, intravenous fluids and broad-spectrum antibiotics were started along with application of local emollients to prevent transdermal water loss. Although their general condition improved but skin exfoliation continued with complete desquamation of palms and soles. Dermatology was consulted, and clinical diagnosis of ED was established. Discontinuation of ATRA resulted in complete resolution of ED. Case 3 (15-year-old boy) reported two blackish mildly tender scrotal lesions during induction chemotherapy. He also had mucocutaneous candidiasis at presentation and was kept on triazole antifungal. Local bacterial & fungal cultures, and serological testing for herpes simplex virus were reported negative. Despite adequate local care and optimal antibiotic support, his lesions persisted, and improved only after temporary discontinuation of ATRA. After a thorough literature review and considering the temporal association of cutaneous toxicities with triazole antifungals, we speculate that the concomitant use of triazole antifungals inhibited the hepatic metabolism of ATRA, resulting in higher serum ATRA concentration, and markedly accentuated cutaneous toxicities in our patients.
By highlighting this crucial pharmacokinetic interaction, we want to caution the fellow oncologists to be mindful of the inhibitory effect of triazole antifungals on CYP450. We propose using a non-myelosuppressive combination of ATRA and arsenic trioxide for management of APL hence, obliterating the need of prophylactic antifungals. However, in the event of invasive fungal infection (IFI), we suggest using alternative class of antifungals.
Peer Review reports
Acute promyelocytic leukemia (APL) is a rare and potentially curable subtype of acute myeloid leukemia (AML), accounting for 5–8% of AML cases [ 1 ]. Genetically, APL is characterized by reciprocal translocation t(15:17) (q22;q11–12), with consequent fusion of promyelocytic (PML) gene on chromosome 15q22 to retinoic acid receptor-alpha (RAR-alpha) gene on chromosome 17q21. The resultant fusion oncoprotein, PML-RARA, induces transcriptional repression, chromatin condensation, maturation arrest, and accumulation of abnormal promyelocytes [ 2 ]. Advent of all-trans retinoic acid (ATRA) has revolutionized the treatment landscape of APL, and along with the backbone of anthracycline based chemotherapy, it is considered to be the standard of care for APL patients. Combination treatment with ATRA plus anthracycline based chemotherapy achieves an overall complete remission and cure rate of 95% and 80% respectively, rendering ATRA indispensable in the management of APL [ 3 ].
ATRA, an active metabolite of vitamin A, belongs to a class of retinoids. Although retinoids are well known for their dermatological side effects like xerosis, xerostomia, erythema, pruritis, and exfoliation; severe dermatological side effects of ATRA, especially in the dosage pertinent to APL (45 mg/m 2 ), are rare. So far, only a single case of exfoliative dermatitis (ED) and a few cases of scrotal ulceration have been reported in literature [ 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. We, here in, report a case series of three patients with serious and rare ATRA associated dermatological complications. We have also discussed upon the potentially precipitating pharmacokinetic interactions, as well as the detailed clinical course and management of our patients as simply withholding ATRA can jeopardize the outcome of this potentially curable malignant disorder.
In all three patients, ATRA was started as soon as abnormal promyelocytes were documented on peripheral smear/bone marrow aspirate examination (Figs. 1 , 2 , 3 ). Diagnosis was further confirmed through cytogenetic analysis as well as PML-RARA detection by polymerase chain reaction. Additionally, in all three patients, chemotherapeutic treatment was instituted according to European APL protocol, based on their risk-group classification.
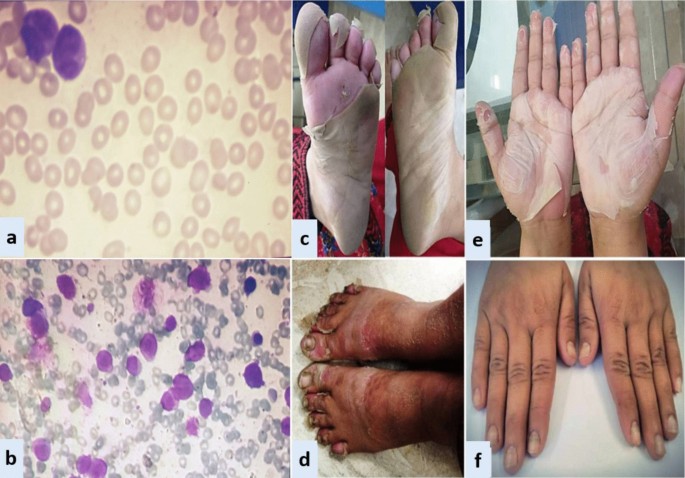
Exfoliative dermatitis &Onychomadesis (CASE 1). a Peripheral smear. b Bone marrow aspirate. c Desquamation of soles. d Desquamation of palms. e Dry exfoliation of feet and shins. f Onychomadesis
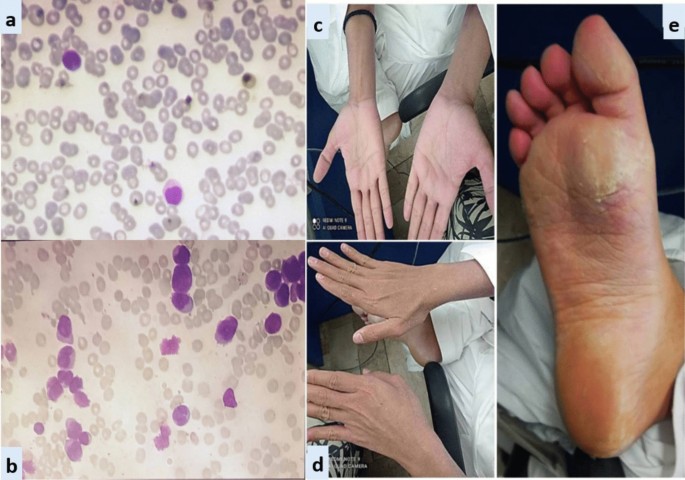
Exfoliative dermatitis (CASE 2). a Peripheral smear. b Bone marrow aspirate. c and d Erythema and scaling of hands. e Cutaneous desquamation of soles
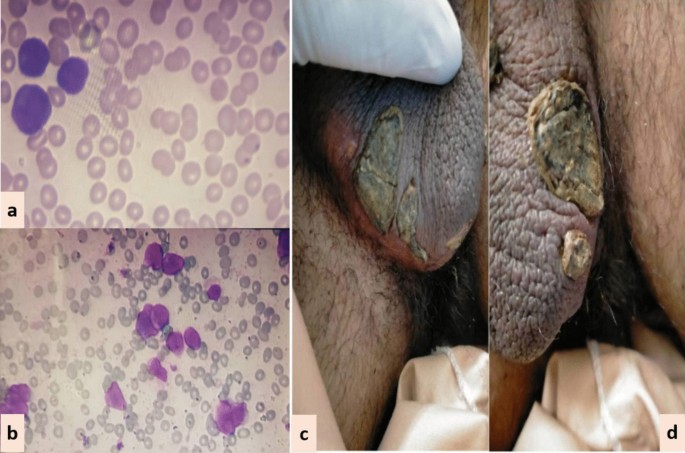
Scrotal lesions (CASE 3). a Peripheral smear. b Bone marrow aspirate. c and d Necrotic scrotal lesions with black eschar
Case 1: A 33-year-old Asian female presented in ER with history of fever, heavy menstrual bleeding and rash all over body. Induction chemotherapy and steroid prophylaxis was promptly started to prevent differentiation syndrome (DS). On Day-10 of induction chemotherapy, she developed high grade fever, cough and shortness of breath. High-resolution computerized tomography (HRCT) showed randomly scattered discrete nodular opacities with surrounding ground glass haze in both lung fields, suggestive of invasive fungal infection (IFI). Voriconazole was immediately started along with broad-spectrum antibiotics. She improved over the following 72 hour, and was discharged from hospital on Day-17. Subsequently, she landed in emergency department on Day-23 with severe dehydration, shivering, tachycardia, generalized skin erythema and discoloration of nail beds. Intravenous fluids and broad-spectrum antibiotics were started along with application of local emollients to prevent transdermal water loss. Over the next 24-36 hour, her general condition was stabilized however; skin exfoliation continued with complete desquamation of palms and soles (Fig. 1 ). Dermatology was consulted, and a clinical diagnosis of onychomadesis and exfoliative dermatitis (ED) was made. A review of her clinical case demonstrated no apparent cause for ED except for a rare association with ATRA. However, considering the curative potential of ATRA, it was continued till Day-28 as per protocol. Her skin condition gradually resolved over next 10–14 days after discontinuation of ATRA. She had recurrence of similar skin condition upon re-exposure to ATRA in her consolidation chemotherapeutic cycles, however, the exfoliation was mild and patchy that responded well to good oral hydration and local skin emollients.
Case 2: A 28-year-old Asian male presented in the out-patient clinic with the history of generalized weakness, high-grade-fever, productive cough and bruises over body. On examination, he had multiple ecchymosis and petechiae with coarse crepitations involving right-middle and left-lower lung fields. He was promptly started on broad-spectrum antibiotics. Additionally, as per protocol, induction chemotherapy and dexamethasone prophylaxis was also instituted. His fever and cough remained unresponsive despite broad-spectrum antibiotics. Voriconazole was instituted upon the identification of IFI on HRCT findings. By day-10, coagulopathy was normalized, and clearance of abnormal promyelocytes was documented by Day-18. On Day-20, he complained of skin dryness, itching and scaling; physical examination revealed generalized xerosis and erythema (Fig. 2 ). Despite aggressive skin care, generalized skin exfoliation, most pronounced on palms and soles, ensued. Clinical diagnosis of ED was established after obtaining dermatological consultation. However, in view of his clinical stability, ATRA was continued. Bone marrow aspirate on Day-28 showed morphological remission. Recurrence of erythema and exfoliation was documented during consolidation phase of chemotherapy, but the condition was responsive to local emollients and oral hydration.
Case 3: A 15-year-old Asian male presented in the out-patient clinic with complains of high-grade-fever, muco-cutaneous bleeding and pancytopenia. On presentation, patient was febrile and had oral thrush. After sending his baseline tests he was taken on broad-spectrum antibiotics and triazole antifungal (itraconazole). After completion of induction chemotherapy, patient was discharged with bi-weekly follow-ups.On Day15, he reported two blackish, mildly tender scrotal lesions with minimal serous discharge (Fig. 3 ). Antibiotic cover for soft tissue infection was commenced along with local wound care with topical steroids and antibiotics. He had no sign of systemic infection/sepsis. Local bacterial & fungal cultures and serological testing for herpes simplex virus were reported negative. Despite adequate local care and optimal antibiotic support, his lesions showed no sign of healing, and two new lesions were developed. Lesion biopsy for histopathological evaluation was declined by the patient. Keeping the rare but reported occurrence of ATRA-induced scrotal ulceration and fournier's gangrene; ATRA was transiently withheld for ten days and the lesions started to regress. However, considering the indispensable role of ATRA in APL, it was reinstituted. Scrotal lesions persisted without any worsening. ATRA was stopped after completion of protocol. Complete resolution of scrotal lesions was documented over the following two weeks. Afterwards, he received two cycles of consolidation chemotherapy, but no recurrence was reported.
Discussion and conclusion
The antineoplastic role of ATRA remains indispensable in the curative management of APL. It is considered a relatively safe drug with a well-known toxicity profile. Commonly reported adverse events include DS, pseudotumor-cerebri, hypertriglyceridemia, transaminitis, and headache. Although, mild cutaneous toxicities like muco-cutaneous xerosis, photosensitivity, rash, pruritus and sweet’s syndrome are well reported, severe dermatological toxicities are rarely reported in literature [ 18 , 19 ]. In this case series, we have discussed three cases of ATRA-induced rare dermatological complications in APL.
Case 1 and 2 developed ED during remission induction phase of chemotherapy. Literature review revealed only a single reported occurrence of ATRA-induced ED in APL by YonelIpek et al. [ 4 ]. ED is a potentially life-threatening cutaneous manifestation that is characterized by diffuse skin erythema and scaling. Various underlying disorders can trigger its onset through a complex interplay of inflammatory cytokines and phagocytes. In contrast to our cases, the case reported by Yonel Ipek et al. developed xerosis in consolidation phase, which akin to our cases started after two weeks of ATRA exposure and rapidly deteriorated to generalized erythroderma and scaling. In both cases, discontinuation of ATRA resulted in complete resolution of ED.
In case 3, we have reported ATRA-induced necrotic scrotal ulceration. Literature review revealed that over the last two decades, a total of twenty cases of ATRA-induced scrotal ulceration have been reported. Histopathological evaluation of these lesions revealed atypical granulocytic infiltration, pointing towards the possible etiological role of differentiated APL cells in the pathogenesis. Most of these cases, including ours, developed genital-lesions almost after two weeks of ATRA exposure and remained unresponsive to local and systemic antibiotics. ATRA had to be halted in most of the cases to prevent progression to fournier’s gangrene [ 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ].
Scattered over the span of three years and considered in isolation, it was not initially apparent to us that all three cases had one striking similarity: concomitant use of ATRA and triazole antifungals. ATRA is primary metabolized by cytochrome P450 enzyme system. Triazole antifungals are notorious for their strong inhibitory effect on CYP450 enzyme system, resulting in supra-therapeutic drug levels and toxicity [ 20 , 21 , 22 ].
Potentiation of serum ATRA levels by inhibition of CYP450 system was first explored by Rigas et al. [ 23 ]. This study reported 1.8 times higher serum concentration of ATRA with concomitant use of ketoconazole. Since then a number of cases have reported the augmentation of ATRA-induced toxicities due to this pharmacokinetic interaction. Concomitant use of ATRA and triazole antifungals that is voriconazole and posaconazole has been implicated to cause severe hypercalcemia [ 24 , 25 , 26 , 27 ]. Similarly, combination with fluconazole has been reported to cause severe neurotoxicity and nephrotoxicity [ 28 , 29 ].
Considering the temporal association of dermatological complications with triazole antifungals in our patients, we speculate that the concomitant use of triazole antifungals inhibited the metabolism of ATRA, resulting in higher serum concentrations and markedly accentuated cutaneous toxicities. A study further strengthening our hypothesis was conducted by Kurzrock et al. to evaluate the maximum tolerable dose of ATRA in myelodysplastic syndrome. The study reported severe dose-limiting cutaneous toxicities, such as generalized desquamation and genital ulceration, at doses > 150 mg/m 2 /day, compared to mild xerosis and erythema in the dose range of 45–100 mg/m 2 /day. Akin to our cases, the study reported complete resolution of cutaneous toxicities within 1–2 weeks of ATRA discontinuation [ 30 ].
Another important point is the recurrence of ED in both case 1 and 2 during their consolidation chemotherapy cycles, whereas recurrent scrotal ulceration was not documented in case 3. The most likely explanation is the continuation of voriconazole as secondary prophylaxis in patients with invasive fungal infections (IFI) (case 1 and 2), whereas itraconazole was discontinued after remission induction in case 3. This once again underscores the pharmacokinetic potentiation of ATRA-induced cutaneous toxicities by triazole antifungals. An important limitation of our study is that, due to the unavailability of serum voriconazole testing, we couldn’t document serum voriconazole levels, something that could provide valuable insights into the effect of serum azole levels on the severity of cutaneous manifestations.
By highlighting this crucial pharmacokinetic interaction and its potentially severe implications, we urge our fellow oncologists to remain vigilant regarding the inhibitory effects of triazole antifungals on the metabolism of ATRA. We propose the use of a non-myelosuppressive combination of ATRA and arsenic trioxide for APL, thereby eliminating the need for prophylactic antifungals. In the case of invasive fungal infections (IFI), we recommend considering alternative classes of antifungals. However, if triazole antifungals are deemed unavoidable, we suggest close monitoring for potential side effects and implementing prophylactic measures as clinically necessary.
Availability of data and materials
Data sharing is not applicable to this manuscript as no datasets were generated or analyzed during the current study.
Abbreviations
Acute promyelocytic leukemia
All-trans retinoic acid
Cytochrome P450
Differentiation syndrome
- Exfoliative dermatitis
High-resolution computerized tomography
Invasive fungal infections
Promyelocytic leukemia-retinoic acid receptor alpha
Yilmaz M, Kantarjian H, Ravandi F. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021;11:123.
Article PubMed PubMed Central Google Scholar
Lo-Coco F, Ammatuna E. The biology of acute promyelocytic leukemia and its impact on diagnosis and treatment. Hematology. 2006;2006:156–61.
Article Google Scholar
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al . Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21.
Article CAS PubMed Google Scholar
Ipek Y, Hulya D, Melih A. Disseminated exfoliative dermatitis associated with all-transretinoic Acid in the treatment of acute promyelocytic leukemia. Case Rep Med. 2012;2012:236174–236174.
Mori A, Tamura S, Katsuno T, Nishimura Y, Itoh T, Saheki K, et al . Scrotal ulcer occurring in patients with acute promyelocytic leukemia during treatment with all-trans retinoic acid. Oncol Rep. 1999;6:55–8.
CAS PubMed Google Scholar
Charles KS, Kanaa M, Winfield DA, Reilly JT. Scrotal ulceration during all-trans retinoic (ATRA) therapy for acute promyelocytic leukaemia. Clin Lab Haematol. 2000;22:171–4.
Pavithran K, Arjun R, Aruna R, Thomas M. Scrotal ulceration during induction therapy of acute promyelocytic leukemia with ATRA. Am J Hematol. 2004;75:260–1.
Article PubMed Google Scholar
Fukuno K, Tsurumi H, Goto H, Oyama M, Tanabashi S, Moriwaki H. Genital ulcers during treatment with ALL-trans retinoic acid for acute promyelocytic leukemia. Leuk Lymphoma. 2003;44:2009–13.
Mourad YA, Jabr F, Salem Z. Scrotal ulceration induced by all-trans retinoic acid in a patient with acute promyelocytic leukemia. Int J Dermatol. 2005;44:68–9.
Shimizu D, Nomura K, Matsuyama R, Matsumoto Y, Ueda K, Masuda K, et al . Scrotal ulcers arising during treatment with all-trans retinoic acid for acute promyelocytic leukemia. Internal Med (Tokyo, Japan). 2005;44:480–3.
Lee HY, Ang AL, Lim LC, Thirumoorthy T, Pang SM. All-trans retinoic acid-induced scrotal ulcer in a patient with acute promyelocytic leukaemia. Clin Exp Dermatol. 2010;35:91–2.
Tazi I, Rachid M, Quessar A, Benchekroun S. Scrotal ulceration following all-trans retinoic Acid therapy for acute promyelocytic leukemia. Indian J Dermatol. 2011;56:561–3.
Schmutz JL, Trechot P. Tretinoin and scrotal ulceration. Ann Dermatol Venereol. 2012;139:588–9.
Al Huneini M, Wasim F, Al Farsi K, Al-Khabori M, Al KS. Genital ulcer development in patients with acute promyelocytic leukaemia treated with all-trans retinoic Acid: a case series. Oman Med J. 2013;28:207–9.
Tajima K, Sagae M, Yahagi A, Akiba J, Suzuki K, Hayashi T, et al . Scrotum exfoliative dermatitis with ulcers associated with treatment of acute promyelocytic leukemia with all-trans retinoic acid [Rinsho ketsueki]. Jpn J Clin Hematol. 1998;39:48–52.
CAS Google Scholar
Drago MJ, Kim BS, Bennett D, Elder D, Rosenbach M. All-trans-retinoic acid-induced scrotal ulcers in a patient with acute promyelocytic leukemia. Cutis. 2013;91:246–7.
PubMed Google Scholar
Sutherland J, Kempton CL, Curry MA. Continuation of all-trans retinoic acid despite the development of scrotal ulcerations in a black male. J Oncol Pharmacy Pract. 2015;21:393–5.
Article CAS Google Scholar
Sun GL. Treatment of acute promyelocytic leukemia (APL) with all-trans retinoic acid (ATRA): a report of five-year experience. Zhonghua Zhong Liu Za Zhi [Chin J Oncol]. 1993;15:125–9.
BC Cancer Agency Cancer Drug Manual. Tretinoin. February 2014. Available from: http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Tretinoin_monograph_1Feb2014.pdf . Accessed 27 Sept 2023.
McSorley LC, Daly AK. Identification of human cytochrome P450 isoforms that contribute to all-trans-retinoic acid 4-hydroxylation. Biochem Pharmacol. 2000;60:517–26.
Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, et al . Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441–58.
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–79.
Article CAS PubMed PubMed Central Google Scholar
Rigas JR, Francis PA, Muindi JR, Kris MG, Huselton C, DeGrazia F, et al . Constitutive variability in the pharmacokinetics of the natural retinoid, all-trans-retinoic acid, and its modulation by ketoconazole. J Natl Cancer Inst. 1993;85:1921–6.
Bennett MT, Sirrs S, Yeung JK, Smith CA. Hypercalcemia due to all trans retinoic acid in the treatment of acute promyelocytic leukemia potentiated by voriconazole. Leuk Lymphoma. 2005;46:1829–31.
Cordoba R, Ramirez E, Lei SH, Lopezdela Guia A, Sanjurjo MJ, Carcas AJ, et al . Hypercalcemia due to an interaction of all-trans retinoic acid (ATRA) and itraconazole therapy for acute promyelocytic leukemia successfully treated with zoledronic acid. Eur J Clin Pharmacol. 2008;64:1031–2.
Yozgat AK, Akçabelen Y, Unal Y, Yaralı N. Hypercalcemia due to concomitant use of all trans retinoic acid and voriconazole. Hematol Transfusion Cell Therapy. 2020;42:73.
Afacan Ozturk HB, Albayrak M, Maral S, Reis Aras M, Yilmaz F, Akyol P, et al . Hypercalcemia associated with the interaction between all trans retinoic acid and posaconazole in an acute promyelocytic leukemia case. J Oncol Pharm Pract. 2021;27:2027–9.
Vanier KL, Mattiussi AJ, Johnston DL. Interaction of all-trans-retinoic acid with fluconazole in acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2003;25:403–4.
Yarali N, Tavil B, Kara A, Ozkasap S, Tunç B. Acute renal failure during ATRA treatment. Pediatr Hematol Oncol. 2008;25:115–8.
Kurzrock R, Estey E, Talpaz M. All-trans retinoic acid: tolerance and biologic effects in myelodysplastic syndrome. J Clin Oncol. 1993;11:1489–95.
Download references
Acknowledgements
This research did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Section of Malignant Haematology, National Institute of Blood Disease and Bone Marrow Transplantation, Karachi, Pakistan
Aisha Jamal, Rafia Hassam, Qurratulain Rizvi, Ali Saleem & Nida Anwar
Research and Development, National Institute of Blood Disease and Bone Marrow Transplantation, Karachi, Pakistan
Anum Khalid
Department of Clinical Haematology, National Institute of Blood Diseases and Bone Marrow Transplantation, Plot # Special D-3, Block-6, (Across Railway Line), P.E.C.H.S, Karachi, Pakistan
Aisha Jamal
You can also search for this author in PubMed Google Scholar
Contributions
Aisha Jamal: Conceptualization, writing-original draft, writing-review & editing. Rafia Hassam: Conceptualization, writing-original draft. Qurratulain Rizvi: Writing-review & editing. Ali Saleem: Data curation, writing—review & editing. Anum Khalid: Writing—review &editing. Nida Anwar: Writing—review & editing.
Corresponding author
Correspondence to Aisha Jamal .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee of National Institute of Blood Disease & Bone Marrow Transplantation, Karachi, Pakistan. The patient’s consent was obtained for publication of case.
Consent for publication
Written informed consent was obtained from the patients for publication of this case report and any accompanying images. Written informed consent was obtained from the patient’s legal guardian(s) for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jamal, A., Hassam, R., Rizvi, Q. et al. A rare incidence of severe dermatological toxicities triggered by concomitant administration of all-trans retinoic acid and triazole antifungal in patients with acute promyelocytic leukemia: a case series and review of the literature. J Med Case Reports 18 , 261 (2024). https://doi.org/10.1186/s13256-024-04577-1
Download citation
Received : 19 December 2023
Accepted : 01 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s13256-024-04577-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pharmacokinetic drug interaction
- Necrotic scrotal ulceration
- All-trans retinoic acid toxicity
- Triazole anti-fungal
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
CSR in times of crisis: a systematic literature review
- Published: 27 May 2024
Cite this article

- Dejan Glavas ORCID: orcid.org/0000-0002-3168-2222 1 &
- Giovanni Visentin 2
1 Altmetric
Explore all metrics
This study assesses the strategic value of corporate social responsibility (CSR) investments and their impact on corporate resilience during financial crises. We examine three main hypotheses—“Doing well by doing good,” “Delegated philanthropy,” and “Insider-initiated philanthropy”—to understand what motivates companies to participate in CSR activities in times of economic turmoil. Using a systematic literature review of peer-reviewed journal articles within social sciences, extracted from Scopus and Web of Science, we adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to screen 1022 studies, ultimately yielding a final sample of 33 key high-quality studies. Our analysis reveals that a significant number of studies support the “Doing well by doing good” hypothesis, which suggests that CSR investments not only sustain financial performance but also serve as a crucial resilience mechanism in tumultuous economic times. Our findings reveal that investment in CSR enables firms to build social capital that pays off during financial crises by fostering trust between the firm and its stakeholders.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Corporate Social Responsibility (CSR) Implementation: A Review and a Research Agenda Towards an Integrative Framework

Mandatory CSR and sustainability reporting: economic analysis and literature review
The impact of corporate governance on financial performance: a cross-sector study, data availability.
The datasets supporting the conclusions of this article are included within the article in Tables 1 , 2 , 3 . Restrictions apply to the availability of the raw data from the 1022 studies initially extracted from Scopus and Web of Science databases. Access to these raw datasets can be granted by the authors upon a reasonable request, subject to obtaining permission from Scopus and Web of Science.
The software is based on the Python language, it is used for machine learning applications and is downloadable here: https://orangedatamining.com/ .
Aerts W, Cormier D (2009) Media legitimacy and corporate environmental communication. Account Organ Soc 34:1–27. https://doi.org/10.1016/j.aos.2008.02.005
Article Google Scholar
Ahmed Haji A (2013) Corporate social responsibility disclosures over time: evidence from Malaysia. Manag Audit J 28:647–676. https://doi.org/10.1108/MAJ-07-2012-0729
Albitar K, Al-Shaer H, Elmarzouky M (2021) Do assurance and assurance providers enhance COVID-related disclosures in CSR reports? An examination in the UK context. Int J Account Inf Manag 29(3):410–428. https://doi.org/10.1108/ijaim-01-2021-0020
Albuquerque R, Koskinen Y, Yang S, Zhang C (2020) Resiliency of environmental and social stocks: an analysis of the exogenous COVID-19 market crash. Rev Corp Financ Stud 9:593–621. https://doi.org/10.1093/rcfs/cfaa011
Al-Dah B, Dah M, Jizi M (2018) Is CSR reporting always favorable? Manag Decis 56(7):1506–1525. https://doi.org/10.1108/MD-05-2017-0540
Ali W, Bekiros S, Hussain N et al (2023) Determinants and consequences of corporate social responsibility disclosure: a survey of extant literature. J Econ Surv. https://doi.org/10.1111/joes.12556
Altaf N (2021) Two decades of big data in finance: systematic literature review and future research agenda. In: Saleem TJ, Chishti MA (eds) Big data analytics for internet of things, 1st edn. Wiley, pp 351–365
Chapter Google Scholar
Ashraf Z, Afshan G, Sahibzada UF (2021) Unpacking strategic corporate social responsibility in the time of crisis: a critical review. JGR. https://doi.org/10.1108/JGR-03-2021-0030
Attig N, El Ghoul S, Guedhami O, Suh J (2013) Corporate social responsibility and credit ratings. J Bus Ethics 117:679–694. https://doi.org/10.1007/s10551-013-1714-2
Bae K-H, Ghoul SE, Gong Z, Guedhami O (2021) Does CSR matter in times of crisis? Evidence from the COVID-19 pandemic. J Corp Financ 67:101876. https://doi.org/10.1016/j.jcorpfin.2020.101876
Bannier CE, Bofinger Y, Rock B (2023) Doing safe by doing good: non-financial reporting and the risk effects of corporate social responsibility. Eur Account Rev 32:903–933. https://doi.org/10.1080/09638180.2022.2042349
Bansal P, Jiang GF, Jung JC (2015) Managing responsibly in tough economic times: strategic and tactical CSR during the 2008–2009 global recession. Long Range Plan 48:69–79. https://doi.org/10.1016/j.lrp.2014.07.002
Barauskaite G, Streimikiene D (2021) Corporate social responsibility and financial performance of companies: the puzzle of concepts, definitions and assessment methods. Corp Soc Responsib Environ Manag 28:278–287. https://doi.org/10.1002/csr.2048
Barnea A, Rubin A (2010) Corporate social responsibility as a conflict between shareholders. J Bus Ethics 97:71–86. https://doi.org/10.1007/s10551-010-0496-z
Bénabou R, Tirole J (2010) Individual and corporate social responsibility. Economica 77:1–19. https://doi.org/10.1111/j.1468-0335.2009.00843.x
Berkman H, Li M, Lu H (2021) Trust and the value of CSR during the global financial crisis. Account Financ 61(3):4955–4965. https://doi.org/10.1111/acfi.12721
Block J, Wagner M (2013) The effect of family ownership on different dimensions of corporate social responsibility : evidence from large US firms. Bus Strategy Environ 23(7):475–492. https://doi.org/10.1002/bse.1798
Broadstock DC, Chan K, Cheng LTW, Wang X (2021) The role of ESG performance during times of financial crisis: evidence from COVID-19 in China. Financ Res Lett 38:101716. https://doi.org/10.1016/j.frl.2020.101716
Buchanan B, Cao CX, Chen C (2018) Corporate social responsibility, firm value, and influential institutional ownership. J Corp Financ 52:73–95. https://doi.org/10.1016/j.jcorpfin.2018.07.004
Cao Z, Rees W (2020) Do employee-friendly firms invest more efficiently? Evidence from labor investment efficiency. J Corp Financ 65:101744. https://doi.org/10.1016/j.jcorpfin.2020.101744
Cespa G, Cestone G (2007) Corporate social responsibility and managerial entrenchment. Econ Manag Strategy 16:741–771. https://doi.org/10.1111/j.1530-9134.2007.00156.x
Chakuu S, Masi D, Godsell J (2019) Exploring the relationship between mechanisms, actors and instruments in supply chain finance: a systematic literature review. Int J Prod Econ 216:35–53. https://doi.org/10.1016/j.ijpe.2019.04.013
Chaudhry SM, Saeed A, Ahmed R (2021) Carbon neutrality: the role of banks in optimal environmental management strategies. J Environ Manag 299:113545. https://doi.org/10.1016/j.jenvman.2021.113545
Chintrakarn P, Jiraporn P, Treepongkaruna S (2021) How do independent directors view corporate social responsibility (CSR) during a stressful time? Evidence from the financial crisis. Int Rev Econ Financ 71:143–160. https://doi.org/10.1016/j.iref.2020.08.007
Daugaard D (2020) Emerging new themes in environmental, social and governance investing: a systematic literature review. Account Financ 60:1501–1530. https://doi.org/10.1111/acfi.12479
Dedrick J, Gurbaxani V, Kraemer KL (2003) Information technology and economic performance: a critical review of the empirical evidence. ACM Comput Surv 35:1–28. https://doi.org/10.1145/641865.641866
Deegan C, Rankin M (1997) The materiality of environmental information to users of annual reports. Account Audit Account J 10:562–583. https://doi.org/10.1108/09513579710367485
del Rio M, Infante J, Gil-Alana LA (2021) Gender Diversity Index. Measuring persistence. Res Int Bus Financ 58:101474. https://doi.org/10.1016/j.ribaf.2021.101474
Delle Foglie A, Panetta IC (2020) Islamic stock market versus conventional: Are islamic investing a ‘Safe Haven’ for investors? A systematic literature review. Pac Basin Financ J 64:101435. https://doi.org/10.1016/j.pacfin.2020.101435
Diemont D, Moore K, Soppe A (2016) The downside of being responsible: corporate social responsibility and tail risk. J Bus Ethics 137:213–229. https://doi.org/10.1007/s10551-015-2549-9
El Ghoul S, Guedhami O, Kwok CCY, Mishra DR (2011) Does corporate social responsibility affect the cost of capital? J Bank Financ 35:2388–2406. https://doi.org/10.1016/j.jbankfin.2011.02.007
Evans A, Tzavara D (2012) Corporate social responsibility strategies in the light of the financial crisis: the case of Milan-based global companies. EJIM 6:154. https://doi.org/10.1504/EJIM.2012.045795
Fassin Y, Gosselin D (2011) The collapse of a european bank in the financial crisis: an analysis from stakeholder and ethical perspectives. J Bus Ethics 102:169–191. https://doi.org/10.1007/s10551-011-0812-2
Flammer C, Ioannou I (2021) Strategic management during the financial crisis: how firms adjust their strategic investments in response to credit market disruptions. Strateg Manag J 42:1275–1298. https://doi.org/10.1002/smj.3265
Friede G, Busch T, Bassen A (2015) ESG and financial performance: aggregated evidence from more than 2000 empirical studies. J Sustain Financ Invest 5:210–233. https://doi.org/10.1080/20430795.2015.1118917
Gillan SL, Koch A, Starks LT (2021) Firms and social responsibility: a review of ESG and CSR research in corporate finance. J Corp Financ 66:101889. https://doi.org/10.1016/j.jcorpfin.2021.101889
Glavas D, Bancel F (2023) Does state ownership impact green bond issuance ? Int Evid Financ 44(1):62–113. https://doi.org/10.3917/fina.pr.020
Godfrey PC, Merrill CB, Hansen JM (2009) The relationship between corporate social responsibility and shareholder value: an empirical test of the risk management hypothesis. Strateg Manag J 30:425–445. https://doi.org/10.1002/smj.750
Gray R, Kouhy R, Lavers S (1995) Corporate social and environmental reporting: a review of theliterature and a longitudinal study of UK disclosure. Account Audit Account J 8:47–77. https://doi.org/10.1108/09513579510146996
Greenland S, Robins JM (1985) Confounding and misclassification. Am J Epidemiol 122:495–506. https://doi.org/10.1093/oxfordjournals.aje.a114131
Grewal R, Tansuhaj P (2001) Building organizational capabilities for managing economic crisis: the role of market orientation and strategic flexibility. J Mark 65:67–80. https://doi.org/10.1509/jmkg.65.2.67.18259
Habermann F, Fischer FB (2023) Corporate social performance and the likelihood of bankruptcy: evidence from a period of economic upswing. J Bus Ethics 182(1):243–259. https://doi.org/10.1007/s10551-021-04956-4
Hannah ST, Sayari N, Harris FHB, Cain CL (2021) The direct and moderating effects of endogenous corporate social responsibility on firm valuation: theoretical and empirical evidence from the global financial crisis. J Manag Stud 58(2):421–456. https://doi.org/10.1111/joms.12586
Havlinova A, Kukacka J (2023) Corporate social responsibility and stock prices after the financial crisis: the role of strategic CSR activities. J Bus Ethics 182:223–242. https://doi.org/10.1007/s10551-021-04935-9
Hughes A (2012) Corporate ethical trading in an economic downturn: recessionary pressures and refracted responsibilities. J Econ Geogr 12:33–54. https://doi.org/10.1093/jeg/lbr004
Hyun S, Kim JM, Han J, Anderson M (2022) Female executive leadership and corporate social responsibility. Account Financ 62:3475–3511. https://doi.org/10.1111/acfi.12894
Jahmane A, Gaies B (2020) Corporate social responsibility, financial instability and corporate financial performance: linear, non-linear and spillover effects—the case of the CAC 40 companies. Financ Res Lett 34:101483. https://doi.org/10.1016/j.frl.2020.101483
Jensen MC, Meckling WH (1976) Theory of the firm: managerial behavior, agency costs and ownership structure. J Financ Econ 3:305–360. https://doi.org/10.1016/0304-405X(76)90026-X
Jiao Y (2010) Stakeholder welfare and firm value. J Bank Financ 34:2549–2561. https://doi.org/10.1016/j.jbankfin.2010.04.013
Lins KV, Servaes H, Tamayo A (2017) Social capital, trust, and firm performance: the value of corporate social responsibility during the financial crisis. J Financ 72:1785–1824. https://doi.org/10.1111/jofi.12505
Magrizos S, Apospori E, Carrigan M, Jones R (2021) Is CSR the panacea for SMEs? A study of socially responsible SMEs during economic crisis. Eur Manag J 39(2):291–303. https://doi.org/10.1016/j.emj.2020.06.002
Malik M (2015) Value-enhancing capabilities of CSR: a brief review of contemporary literature. J Bus Ethics 127:419–438. https://doi.org/10.1007/s10551-014-2051-9
Margolis JD, Elfenbein HA, Walsh JP (2009) Does it pay to be good...and does it matter? A meta-analysis of the relationship between corporate social and financial performance. SSRN J. https://doi.org/10.2139/ssrn.1866371
Marie Lauesen L (2013) CSR in the aftermath of the financial crisis. Soc Responsib J 9:641–663. https://doi.org/10.1108/SRJ-11-2012-0140
Masulis RW, Reza SW (2015) Agency problems of corporate philanthropy. Rev Financ Stud 28:592–636. https://doi.org/10.1093/rfs/hhu082
Mathes T, Walgenbach M, Antoine S-L et al (2014) Methods for systematic reviews of health economic evaluations: a systematic review, comparison, and synthesis of method literature. Med Decis Mak 34:826–840. https://doi.org/10.1177/0272989X14526470
Matos P (2020) ESG and responsible institutional investing around the world: a critical review. SSRN J. https://doi.org/10.2139/ssrn.3668998
Metaxas T, Tsavdaridou M (2013) CSR in metallurgy sector in Greece: a content analysis. Resour Policy 38:295–309. https://doi.org/10.1016/j.resourpol.2013.03.010
Mongeon P, Paul-Hus A (2016) The journal coverage of Web of Science and Scopus: a comparative analysis. Scientometrics 106:213–228. https://doi.org/10.1007/s11192-015-1765-5
Nakai M, Yamaguchi K, Takeuchi K (2016) Can SRI funds better resist global financial crisis? Evidence from Japan. Int Rev Financ Anal 48:12–20. https://doi.org/10.1016/j.irfa.2016.09.002
Omura A, Roca E, Nakai M (2021) Does responsible investing pay during economic downturns: evidence from the COVID-19 pandemic. Finance Res Lett 42:101914. https://doi.org/10.1016/j.frl.2020.101914
Orlitzky M, Schmidt FL, Rynes SL (2003) Corporate social and financial performance: a meta-analysis. Organ Stud 24:403–441. https://doi.org/10.1177/0170840603024003910
Ossenblok TLB, Verleysen FT, Engels TCE (2014) Coauthorship of journal articles and book chapters in the social sciences and humanities (2000–2010). J Asso Info Sci Technol 65:882–897. https://doi.org/10.1002/asi.23015
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
Paul J, Lim WM, O’Cass A et al (2021) Scientific procedures and rationales for systematic literature reviews (SPAR-4-SLR). Int J Consum Stud. https://doi.org/10.1111/ijcs.12695
Petitjean M (2019) Eco-friendly policies and financial performance: was the financial crisis a game changer for large US companies? Energy Econ 80:502–511. https://doi.org/10.1016/j.eneco.2019.01.028
Raza A, Ilyas MI, Rauf R, Qamar R (2012) Relationship between corporate social responsibility (CSR) and corporate financial performance (CFP): literature review approach. Elixir Financ Manag 46:8404–8409
Google Scholar
Roehrich JK, Lewis MA, George G (2014) Are public–private partnerships a healthy option? A systematic literature review. Soc Sci Med 113:110–119. https://doi.org/10.1016/j.socscimed.2014.03.037
Sajko M, Boone C, Buyl T (2021) CEO greed, corporate social responsibility, and organizational resilience to systemic shocks. J Manag 47(4):957–992. https://doi.org/10.1177/0149206320902528
Sakunasingha B, Jiraporn P, Uyar A (2018) Which CSR activities are more consequential? Evidence from the great recession. Financ Res Lett 27:161–168. https://doi.org/10.1016/j.frl.2018.02.003
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647–g7647. https://doi.org/10.1136/bmj.g7647
Singh A (2020) COVID-19 and safer investment bets. Financ Res Lett 36:101729. https://doi.org/10.1016/j.frl.2020.101729
Tsai H-J, Wu Y (2022) Changes in corporate social responsibility and stock performance. J Bus Ethics 178:735–755. https://doi.org/10.1007/s10551-021-04772-w
Van Beurden P, Gössling T (2008) The worth of values—a literature review on the relation between corporate social and financial performance. J Bus Ethics 82:407–424. https://doi.org/10.1007/s10551-008-9894-x
Velte P (2022) Meta-analyses on Corporate Social Responsibility (CSR): a literature review. Manag Rev Q 72:627–675. https://doi.org/10.1007/s11301-021-00211-2
Xu X, Chen X, Jia F et al (2018) Supply chain finance: a systematic literature review and bibliometric analysis. Int J Prod Econ 204:160–173. https://doi.org/10.1016/j.ijpe.2018.08.003
Yu S, Lee N (2016) Financial crisis, politically connected CEOs, and the performance of state-owned enterprises: evidence from Korea. Emerg Mark Financ Trade 52:2087–2099. https://doi.org/10.1080/1540496X.2016.1186445
Zhang L, Shan YG, Chang M (2021) Can CSR disclosure protect firm reputation during financial restatements? J Bus Ethics 173(1):157–184. https://doi.org/10.1007/s10551-020-04527-z
Download references
Acknowledgements
Our deepest gratitude is extended to everyone who has contributed to this research in various capacities. We would like to acknowledge Ms. Astrid Denimal, our research assistant for this work. Authors would like to thank the EU*Asia Institute for their support. We also wish to express our appreciation to Gilles Grolleau, Anna Dimitrova, and Alice Crepin for their extensive comments and valuable insights.
Authors do not have any funding to declare for this work.
Author information
Authors and affiliations.
AI for Sustainability Institute, ESSCA School of Management, 55 Quai Alphonse Le Gallo, 92513, Boulogne-Billancourt, France
Dejan Glavas
INCAE Business School, 2P3C+468 Campus Walter Kissling Gam, La Garita, Provincia de Alajuela, Costa Rica
Giovanni Visentin
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Dejan Glavas .
Ethics declarations
Conflict of interest.
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 119 KB)
Supplementary file2 (ows 469 kb), supplementary file3 (csv 20 kb), supplementary file4 (csv 44 kb), rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Glavas, D., Visentin, G. CSR in times of crisis: a systematic literature review. Manag Rev Q (2024). https://doi.org/10.1007/s11301-024-00445-w
Download citation
Received : 04 September 2023
Accepted : 16 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1007/s11301-024-00445-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Corporate social responsibility
- Economic crises
- Systematic review
JEL Classification
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 24 May 2024
Vertebral hemangiomas: a review on diagnosis and management
- Kyle Kato 1 ,
- Nahom Teferi 2 ,
- Meron Challa 1 ,
- Kathryn Eschbacher 3 &
- Satoshi Yamaguchi 2
Journal of Orthopaedic Surgery and Research volume 19 , Article number: 310 ( 2024 ) Cite this article
188 Accesses
2 Altmetric
Metrics details
Vertebral hemangiomas (VHs) are the most common benign tumors of the spinal column and are often encountered incidentally during routine spinal imaging.
A retrospective review of the inpatient and outpatient hospital records at our institution was performed for the diagnosis of VHs from January 2005 to September 2023. Search filters included “vertebral hemangioma,” "back pain,” “weakness,” “radiculopathy,” and “focal neurological deficits.” Radiographic evaluation of these patients included plain X-rays, CT, and MRI. Following confirmation of a diagnosis of VH, these images were used to generate the figures used in this manuscript. Moreover, an extensive literature search was conducted using PubMed for the literature review portion of the manuscript.
VHs are benign vascular proliferations that cause remodeling of bony trabeculae in the vertebral body of the spinal column. Horizontal trabeculae deteriorate leading to thickening of vertical trabeculae which causes a striated appearance on sagittal magnetic resonance imaging (MRI) and computed tomography (CT), “Corduroy sign,” and a punctuated appearance on axial imaging, “Polka dot sign.” These findings are seen in “typical vertebral hemangiomas” due to a low vascular-to-fat ratio of the lesion. Contrarily, atypical vertebral hemangiomas may or may not demonstrate the “Corduroy” or “Polka-dot” signs due to lower amounts of fat and a higher vascular component. Atypical vertebral hemangiomas often mimic other neoplastic pathologies, making diagnosis challenging. Although most VHs are asymptomatic, aggressive vertebral hemangiomas can present with neurologic sequelae such as myelopathy and radiculopathy due to nerve root and/or spinal cord compression. Asymptomatic vertebral hemangiomas do not require therapy, and there are many treatment options for vertebral hemangiomas causing pain, radiculopathy, and/or myelopathy. Surgery (corpectomy, laminectomy), percutaneous techniques (vertebroplasty, sclerotherapy, embolization), and radiotherapy can be used in combination or isolation as appropriate. Specific treatment options depend on the lesion's size/location and the extent of neural element compression. There is no consensus on the optimal treatment plan for symptomatic vertebral hemangioma patients, although management algorithms have been proposed.
While typical vertebral hemangioma diagnosis is relatively straightforward, the differential diagnosis is broad for atypical and aggressive lesions. There is an ongoing debate as to the best approach for managing symptomatic cases, however, surgical resection is often considered first line treatment for patients with neurologic deficit.
Introduction
Vertebral hemangiomas (VHs) are benign vascular lesions formed from vascular proliferation in bone marrow spaces that are limited by bony trabeculae [ 1 ]. VHs are quite common and are often incidental findings on spinal computed tomography (CT) and magnetic resonance imaging (MRI) of patients presenting with back or neck pain [ 2 , 3 ]. Previous, large autopsy series such as Schmorl (1926) and Junghanns (1932) found a VH prevalence of 11% in adult specimens [ 1 , 4 ]. However, the prevalence is believed to be higher as modern imaging techniques allow for better detection of small VHs that may not be easily diagnosed on autopsy specimens [ 5 ]. They can occur at any age but are most often seen in individuals in their 5th decade of life with a slight female preponderance [ 2 , 6 , 7 ]. Most VHs are found in the thoracic or lumbar spinal column and often involve the vertebral body, though they can extend to the pedicle, lamina, or spinous process, and may span multiple spinal segments [ 5 ].
The vast majority of VHs are asymptomatic, quiescent lesions [ 3 ]. Prior studies have stated less than 5% of VHs are symptomatic [ 8 , 9 ], although the 2023 study by Teferi et. al. demonstrated 35% of their 75 VH patients presented with symptoms including localized pain, numbness, and/or paresthesia [ 1 ]. 85% of symptomatic cases in this series were found to have VHs localized in the thoracic spine [ 1 ].
Among symptomatic VHs, up to 20–45% of cases may exhibit aggressive features including damage to surrounding bone and soft tissue or demonstrate rapid growth that extends beyond the vertebral body and invades the paravertebral and/or epidural space [ 1 , 5 , 10 , 11 ]. When “aggressive”, VHs may compress the spinal cord and nerve roots causing severe symptoms [ 1 , 5 ]. 45% of symptomatic VH patients present with neurologic deficits secondary to compressive lesions, bony expansion, disrupted blood flow, or vertebral body collapse while the remaining 55% present solely with back pain [ 8 , 12 , 13 , 14 , 15 ].
VHs are primarily diagnosed with radiographs, CT, and MRI, although other studies such as angiography, nuclear medicine studies, and positron emission—computed tomography (PET-CT) have been previously utilized to a lesser extent [ 1 , 15 , 16 , 17 , 18 , 19 ]. Radiologically, these lesions can be grouped into Typical, Atypical, and Aggressive subtypes (see radiological features). Histologically, VHs are composed of varying proportions of adipocytes, blood vessels, and interstitial edema which leads to thickening of vertical trabeculae in the affected vertebra [ 5 ]. This histopathology leads to the characteristic “polka-dot” sign on axial CT/MRI and “corduroy” sign on coronal and sagittal CT/MRI [ 5 , 20 ].
In terms of management, conservative treatment with observation and pain control are the mainstay of treatment for asymptomatic VH patients and those with mild-to-moderate pain respectively [ 21 ]. Surgical decompression is indicated for patients with neurologic deficits including compressive myelopathy or radiculopathy [ 22 ]. Other symptomatic patients have a wide variety of treatment options available including sclerotherapy, embolization, radiotherapy, and/or vertebroplasty [ 1 , 5 , 23 ]. The best approach in managing an individual patient with a symptomatic VH has not been elucidated and there have been different management algorithms suggested based on varying institutional experiences [ 1 , 5 , 24 , 25 ].
This article will review what is currently known regarding VHs. Diagnostic techniques and challenges will be highlighted as well as current treatment recommendations from the literature.
A retrospective review of the inpatient and outpatient hospital records at our institution was performed for the diagnosis of VHs from January 2005 to September 2023. Search filters included “vertebral hemangioma” "back pain,” “weakness,” “radiculopathy,” and “focal neurological deficits.” Radiographic evaluation of these patients included plain X-rays, CT, and MRI. Following confirmation of a diagnosis of VH, these images were used to generate the figures used in this manuscript. Moreover, an extensive literature search was conducted using PubMed for the literature review portion of the manuscript.
68 Articles were selected from our PubMed search. This article will review what is currently known about VHs. Diagnostic techniques and challenges will be highlighted as well as current treatment recommendations from the literature.
Histopathological features
VHs are benign tumors composed of various sized blood vessels, adipocytes, smooth muscle, fibrous tissue, hemosiderin, interstitial edema, and remodeled bone [ 5 , 7 , 26 , 27 ]. Macroscopically, they appear as soft, well-demarcated, dark red masses with intralesional, sclerotic boney trabeculae and scattered blood-filled cavities lending to a honeycomb appearance [ 5 , 6 , 7 ].
Microscopically, there are four subtypes of hemangiomas based on vascular composition: capillary, cavernous, arteriovenous (AV), and venous hemangiomas [ 28 ] (Fig. 1 ). Capillary hemangiomas are composed of small, capillary-sized blood vessels while cavernous hemangiomas present with collections of larger, dilated blood vessels [ 1 ]. AV hemangiomas are composed of interconnected arterial and venous networks while an abnormal collection of veins comprises venous hemangiomas [ 1 ]. VHs are predominately capillary and cavernous subtypes with thin-walled blood vessels surrounded by edematous stroma and boney trabeculae that permeate the bone marrow space [ 1 , 7 , 27 ]. In a sample of 64 surgically treated VHs cases, Pastushyn et al. reported 50% were capillary subtype, 28% were cavernous subtype, and 22% were mixed [ 29 ]. Occasionally, secondary reactive phenomena such as fibrous and/or adipose involution of bone marrow and remodeling of bone trabeculae may be seen [ 7 , 26 ]. Symptomatic VHs can be caused by all hemangioma subtypes, and there are no distinguishing features between subtypes on imaging [ 1 ]. However, cavernous and capillary subtypes are associated with favorable postsurgical outcomes [ 29 ].
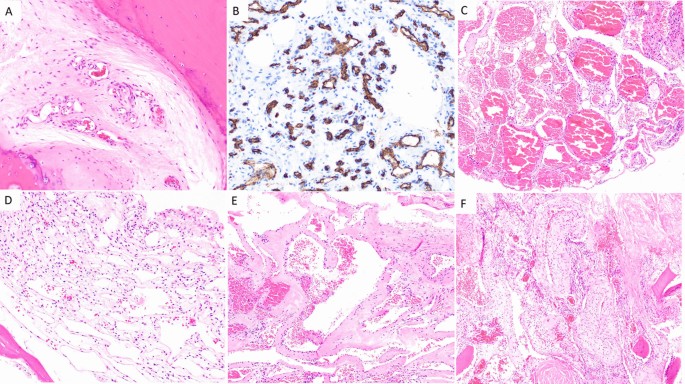
Capillary hemangioma ( A and B ): A H&E 200× magnification showing proliferation of small caliber vessels within a fibrous stroma with surrounding bone, B CD34 immunohistochemical stain, 200× magnification highlighting small caliber vascular spaces. Cavernous hemangioma ( C and D ): C H&E 100× magnification showing proliferation of thin-walled, dilated, blood filled vascular channels, D H&E 200× magnification: Thin-walled, dilated vascular channels within a loose stroma with adjacent mature bone. Venous hemangioma ( E and F ): E H&E 100 × magnification showing abnormal proliferation of thick-walled vessels with dilated lumens. F H&E 100× magnification reveals tightly packed, thick-walled vessels with adjacent fragments of mature bone
Radiographic features
The histopathology of VHs gives rise to imaging features used to classify VHs as typical, atypical, or aggressive [ 13 ]. Typical and atypical MRI findings are correlated with the intralesional ratio of fat to vascular components [ 20 ]. Lesions with a high fat content are more likely to demonstrate features of typical VHs while those with a high vascular content (atypical VHs) tend to present without these findings [ 5 , 30 , 31 ]. Aggressive VHs have features including destruction of the cortex, invasion of the epidural and paravertebral spaces, and lesions extending beyond the vertebral body [ 13 , 15 , 20 ].
Laredo et al. demonstrated that VHs with a higher fatty content are generally quiescent lesions, while those with a higher vascular content are more likely to display “active” behavior and potentially evolve into compressive lesions [ 20 ]. Therefore, asymptomatic VHs can display both typical or atypical imaging findings while symptomatic lesions are more likely to present with atypical or aggressive findings [ 1 ]. Despite radiographically typical VHs being relatively easy to diagnose, atypical and aggressive VHs are much more challenging to recognize as they do not present with classic imaging findings and often mimic other pathologies such as multiple myeloma, metastatic bone lesions, and inflammatory conditions [ 5 , 30 , 31 ]. Compressive VHs often have coinciding radiologic and clinical classifications due to the correlation between aggressive behavior and compressive symptoms [ 5 ].
While MRI, CT, and radiographs are the primary imaging modalities used in the workup of VHs, other studies have also been used. Angiography will occasionally be performed to identify feeding/draining vessels and evaluate the blood supply to the spinal cord [ 5 ]. Multiphase technetium 99-methyl diphosphonate ( 99 Tc-MDP) bone scintigraphy may show increased tracer uptake in all phases (perfusion, blood pool, and delayed) due to technetium 99-labeled red blood cell accumulation in the tumors, which occurs in all hemangiomas [ 16 ]. PET-CT has been used to classify VHs as “hot” or “cold” lesions based on the degree of 18-FDG and 68-Ga DOTATATE uptake [ 17 , 18 , 19 ]. Although angiography is useful in clarifying the vascular network of aggressive VHs primarily, nuclear medicine studies offer a much more limited contribution to diagnosis when compared to CT and MRI [ 5 ].
Typical VHs
The collection of thin-walled, blood-filled spaces that comprise VHs cause resorption of horizontal trabeculae and reinforcement of vertical trabeculae, leading to a pattern of thickened vertical trabeculae interspersed with lower density bone of the nonexpanding vertebral body [ 15 , 31 , 32 ]. This composition is responsible for the “corduroy cloth” appearance seen in typical VHs on radiographic images [ 31 ].
On unenhanced axial CT images, typical VHs are characterized by a “polka dot” appearance, termed polka-dot sign. This is caused by small, punctate areas of high attenuation from hyperdense trabeculae surrounded by hypodense stroma [ 20 , 33 ] (Fig. 2 ). Like radiographs, sagittal and coronal CT images display the “corduroy” sign caused by thickened trabeculae in a field of hypodense bone (Fig. 2 ). There is no extraosseous extension of the hemangioma in typical VHs [ 5 ].
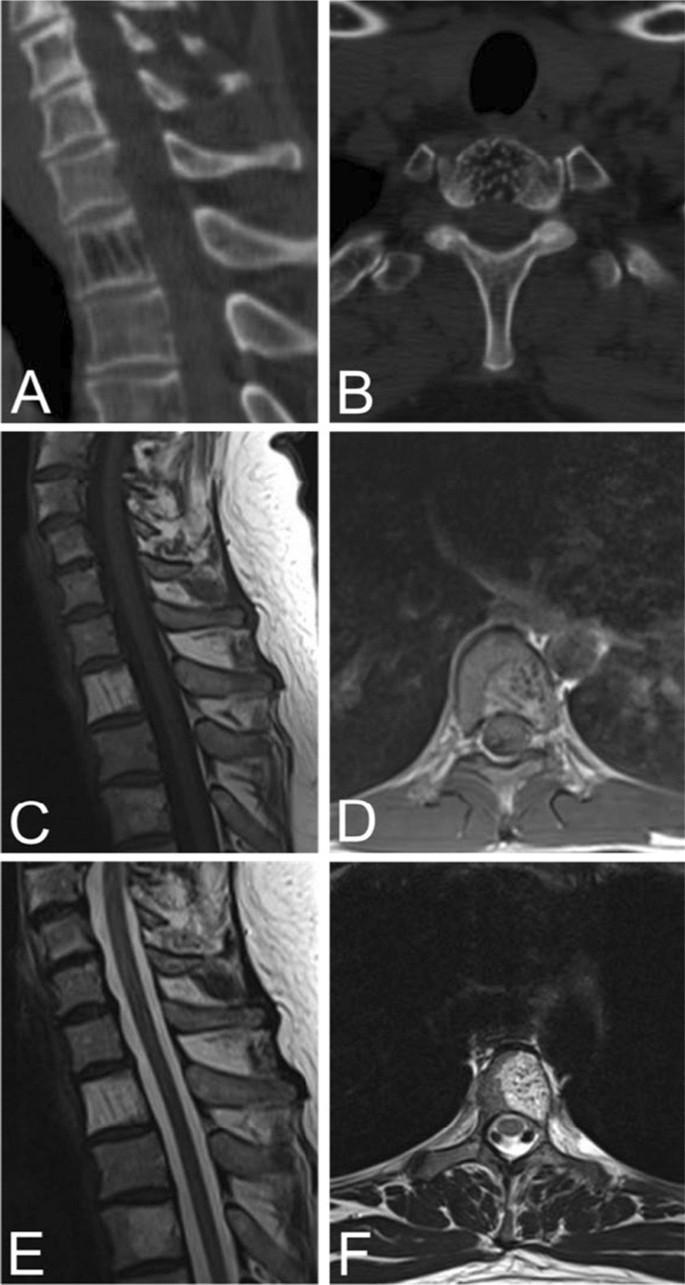
Sagittal ( A ) and axial ( B ) CT scans of a typical VH in an asymptomatic 50-year-old male demonstrating the “Corduroy” and “Polka-dot” signs respectively. Sagittal ( C ) and axial ( D ) T1-weighted MRIs of typical VHs are predominately hyperintense with areas of hypo-intensity due to thickening of vertical trabeculae. Sagittal ( E ) and axial ( F ) T2-weighted MRIs of typical VHs also appear as hyperintense lesions with areas of hypo-intensity that may demonstrate the “Corduroy” and “Polka-dot” signs as seen in CT images of typical VHs
Typical VHs tend to appear as hyperintense lesions on T1- and T2-weighted MRI sequences due to predominately fatty overgrowth with penetrating blood vessels [ 31 ] (Fig. 2 ). There are punctate areas of slight hypointensity within the lesion on axial T1-weighted MRI due to thickened vertical trabeculae which resembles the “polka-dot" sign [ 5 ] (Fig. 2 ). These trabeculae appear as linear striations on sagittal/coronal T1- and T2-weighted MRI [ 5 ] (Fig. 2 ). Fluid-sensitive sequences (i.e. short-tau inversion recovery or fat-saturated T2-weighted MRI) appear slightly hyperintense due to the vascular components of the lesion, and T1-weighted MRI with contrast demonstrates heterogenous enhancement of the lesion [ 3 ] (Fig. 3 ).
Contrast-enhanced T1 MRIs of a T8 VH in an asymptomatic fourteen-year-old female ( A ) and L3, L5 VHs in a thirty-one-year-old female with back pain ( B ), illustrating the heterogenous presentation of hemangiomas on post-contrast MRI
Atypical VHs
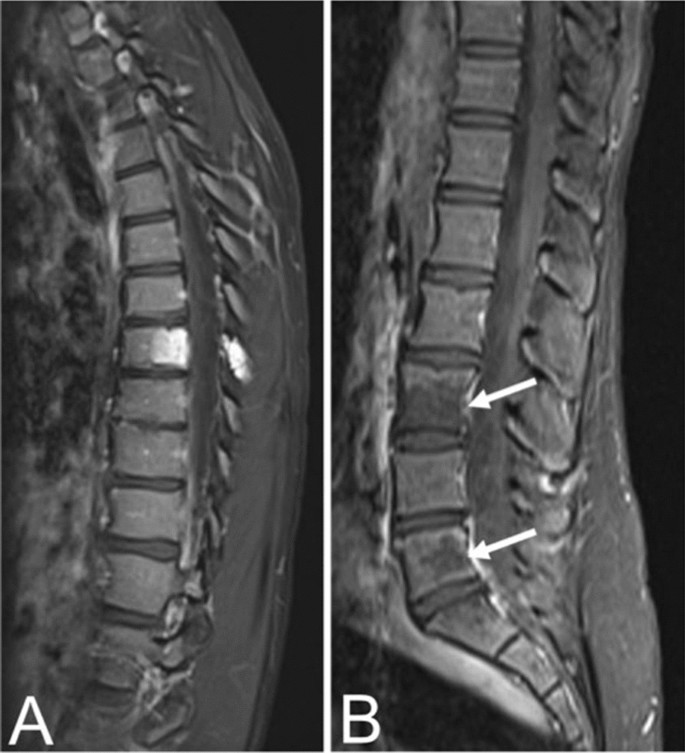
In contrast to typical VHs, atypical VHs tend to have a higher vascular component-to-fat ratio and may not demonstrate the classical imaging findings such as the “corduroy” and “polka-dot” signs [ 5 ]. This composition gives the lesion an iso- to hypointense appearance on T1-weighted MRI as well as a very high intensity appearance on T2-weighted and fluid-sensitive MRI [ 20 , 31 ] (Fig. 4 ). Atypical VHs often mimic primary bony malignancies or metastases and are more likely to demonstrate aggressive features, often making them difficult to diagnose [ 12 , 13 , 14 , 15 ].
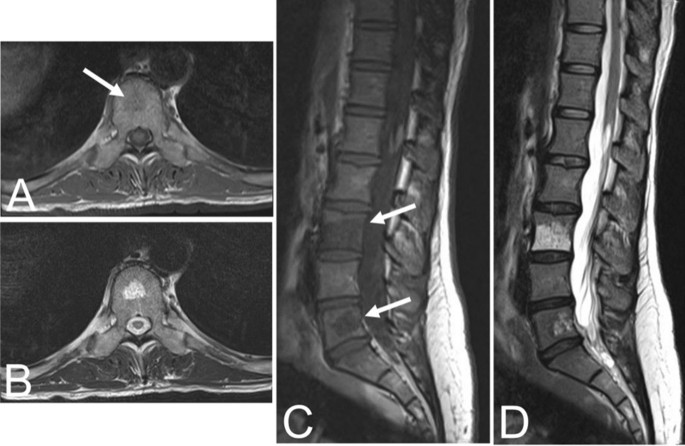
Asymptomatic fifty-six-year-old male with a T9 atypical vertebral hemangioma that appears iso- to hypointense on axial T1 MRI ( A ) and hyperintense on axial T2 MRI ( B ). Atypical vertebral hemangiomas of the L3 and L5 vertebral bodies in a thirty-one-year-old female who presented with backpain. Sagittal T1 ( C ) and T2 ( D ) demonstrate hypo- and hyperintense lesions respectively
Aggressive VHs
Aggressive VHs routinely have atypical features on any imaging modality [ 1 , 5 ]. They may appear radiographically normal or show nonspecific findings such as osteoporosis, pedicle erosion, cortex expansion, vertebral collapse, or irregular vertical trabeculae associated with lytic areas of varying size [ 13 , 15 ] (Fig. 5 ).
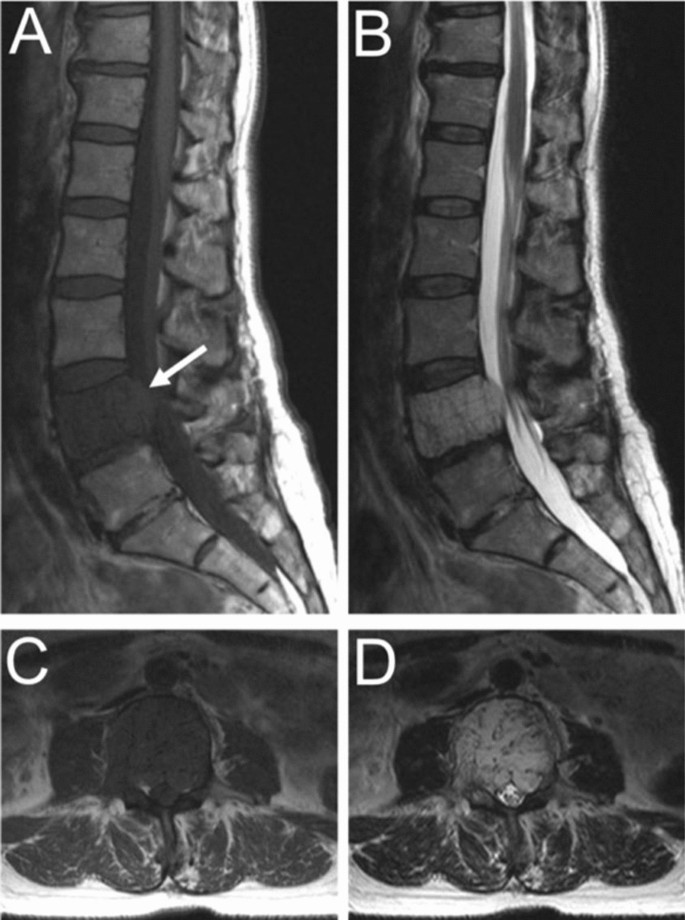
Fifty-five-year-old female with an aggressive vertebral hemangioma of the L4 vertebral body with extension into the spinal canal. A Sagittal T1 MRI shows hypo-intensity of the entire vertebral body, although vertebral height is maintained. B Sagittal T2 MRI redemonstrates the lesion but appears hyperintense due to the vascularity of the hemangioma. Axial T1 ( C ) and T2 ( D ) MRI show involvement of the pedicles bilaterally and extension of the lesion into the anterior epidural space
CT findings are often nonspecific, including features such as extraosseous soft tissue expansion, cortical ballooning, or cortical lysis [ 34 , 35 ]. As with atypical VHs, the “corduroy” and “polka-dot” signs may not be readily visualized in aggressive or destructive lesions due to the higher vascular-to-fat ratio common in these hemangiomas [ 5 ]. However, it is important to be mindful of these signs because they can guide to the correct diagnosis. Other CT features that may assist in the diagnosis of inconspicuous VHs include extension of the lesion into the neural arch, involvement of the entire vertebral body, or an irregular honeycomb pattern due to serpentine vascular channels and fatty proliferation within the network of reorganizing bony trabeculae [ 20 ]. Vertebral fractures are rare due to the reinforcement of vertical trabeculae [ 1 ].
The composition of aggressive VHs, with a hypervascular stroma and less fat, results in a hypointense lesion on T1-weighted MRI [ 20 , 31 ] (Fig. 5 ). Again, this may conceal the “corduroy” and “polka-dot” signs which remain amongst the most useful imaging findings in the diagnosis of VHs, particularly in cases where other findings are nonspecific [ 5 ]. These non-specific findings may include hyperintensity on T2-weighted MRI due to the vascular components of the lesion (Fig. 5 ), which is also seen in most neoplastic and inflammatory lesions [ 31 ]. Areas of hyperintensity on fluid-sensitive MRI and the presence of lipid-dense content within the lesion may be seen as well [ 31 , 36 ]. Other features suggestive of an aggressive VH include a maintained vertebral body height, a sharp margin with normal marrow, an intact cortex adjacent to a paraspinal mass, or enlarged paraspinal vessels, however these findings are also nonspecific and relatively uncommon [ 5 , 13 ]. Although highly unusual, there have been cases of aggressive VHs with extensive intraosseous fatty stroma and simultaneous extraosseous extension of the lesion, permitting a straightforward diagnosis [ 36 ].
Even though some aggressive VHs may be diagnosed on CT and MRI, challenging cases may warrant the use of more advanced imaging techniques for accurate diagnosis. Higher fluid content relative to cellular soft tissue gives hemangiomas a bright appearance on diffusion weighted imaging (DWI) with elevated apparent diffusion coefficient (ADC) values, distinguishing them from metastases [ 37 ]. Volume transfer constant (K trans ) and plasma volume, which reflect capillary permeability and vessel density respectively, are quantitative measures derived from dynamic contrast enhanced magnetic resonance imaging (DCE MRI) perfusion imaging that can also be used to differentiate VHs and metastases [ 38 ]. K trans and plasma volume are both low in VHs and elevated in metastatic lesions [ 38 ]. Furthermore, aggressive VHs may show a signal drop when comparing non-contrast T1-weighted MRI with and without fat suppression, as well as microscopic lipid content on chemical shift imaging [ 39 ]. Finally, characteristic findings of aggressive VHs in angiography include vertebral body arteriole dilation, multiple capillary phase blood pools, and complete vertebral body opacification [ 15 ].
Laredo et al. [ 15 ] proposed a six-point scoring system to assist in the diagnosis of aggressive VHs based on the more common features observed in radiographs and CT. One point was given for each of the following findings: a soft tissue mass, thoracic location between T3–T9, involvement of the entire vertebral body, an irregular honeycomb appearance, cortical expansion, and extension into the neural arch [ 15 ]. The authors suggest that aggressive VHs should be suspected when a patient presents with nerve root pain in association with three or more of these features [ 15 ]. However, additional studies are needed to determine the utility of this scoring system as the predictive power has not been determined [ 5 ].
Some VHs are difficult to diagnose because they can have nonspecific findings on radiographs, CT, and MRI, making characteristic findings such as the “corduroy” and “polka-dot” signs, when present, important diagnostic features. VHs may also coexist with other vertebral lesions, further complicating the diagnosis. In these cases, angiography can differentiate a VH from a nonvascular lesion [ 40 ]. Ultimately, a biopsy may be required for accurate diagnosis, especially when there is potential for a malignant lesion such as angiosarcoma or epithelioid hemangioendothelioma.
Clinical features
VHs are often noted incidentally on spinal imaging and are often observed in patients in their fifth to sixth decade of life. Studies have shown that vertebral hemangiomas exhibit a slight female preponderance, with a male-to-female ratio of 1:1.5. [ 6 ]. Clinically, most VHs are asymptomatic and quiescent lesions, which rarely demonstrate active behavior and become symptomatic [ 41 ]. VHs occur most frequently in the thoracic spine [ 42 ], followed by the lumbar spine and cervical spine; sacral involvement is very rare [ 43 ].
When symptomatic, VHs can present with localized back pain or result in neurologic symptoms that are attributable to spinal cord compression, nerve root compression, or both, leading to myelopathy and/or radiculopathy [ 1 ]. At least 4 mechanisms of spinal cord and nerve root compression have been suggested: (1) hypertrophy or ballooning of the posterior cortex of the vertebral body caused by the angioma, (2) extension of the angioma through the cortex into the epidural space, (3) compression fracture of the involved vertebra, and (4) epidural hematoma [ 44 ]. When aggressive and symptomatic with spinal cord compression, VHs tend to occur in the thoracic spine [ 42 ].
Boriani et al. classified VHs into 4 groups based on the presence of symptoms and radiographic findings [ 45 ]. These include: Type I—latent, mild bony destruction with no symptoms; Type II—active, bony destruction with pain; Type III—aggressive, asymptomatic lesion with epidural and/or soft-tissue extension; and Type IV—aggressive, neurologic deficit with epidural and/or soft tissue extension.
Management options
Most VHs are asymptomatic and do not require treatment [ 1 , 21 ]. Treatment is indicated in cases with back pain or neurological symptoms, including myelopathy and/or radiculopathy, often caused by neuronal compression or vertebral fracture [ 1 ]. Previously, surgery was the primary treatment option offered to these patients, which was associated with an increased risk of complications, particularly intraoperative bleeding [ 1 ]. New modalities such as vertebroplasty have since gained traction as adjuncts or alternatives to surgery [ 1 ]. Today, there are several management options available for the treatment of symptomatic VHs, including conservative medical therapy, surgery, percutaneous techniques, radiotherapy, or a combination of these modalities [ 1 , 46 ].
There is no consensus on the best treatment strategy, however recently Teferi et. al. proposed a treatment algorithm for VHs based on their institutional experience and literature review (Fig. 6 ) [ 1 ]. They recommend conservative management for typical, asymptomatic VHs, CT-guided biopsy and metastatic workup with PET-CT for radiographically atypical VHs, surgical intervention with or without adjuvant therapy in cases with epidural spinal cord compression or vertebral compression fracture, and radiotherapy for recurrent, asymptomatic VHs following surgery.
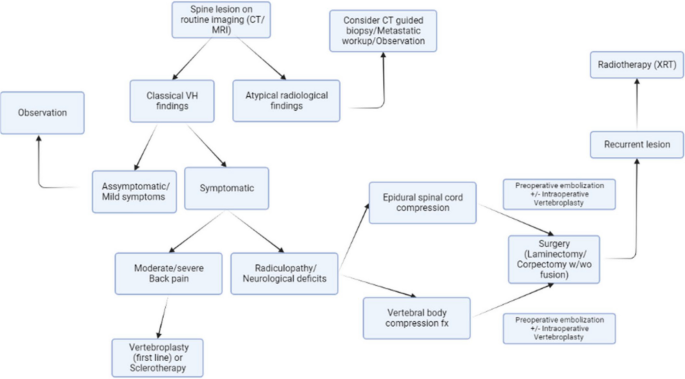
Algorithm for diagnosis and management of VHs proposed by Teferi et al. [ 1 ]
Surgical treatment of VHs is recommended in cases with rapid or progressive neurologic symptoms including compressive myelopathy or radiculopathy [ 47 ]. Baily et al. documented the first case of surgical management for VHs after they successfully resolved a patient’s paraplegia secondary to an aggressive VH [ 48 ]. Prior to the 1960s, the average neurological recovery rate was 73% (range, 43–85%) with a mortality rate of 11.7% [ 49 ]. This is consistent with a series published by Ghormley et al. in 1941 where 5 symptomatic VH patients were treated with decompressive laminectomy and postoperative radiotherapy. Although three patients achieved partial or complete resolution of neurologic deficits, the procedure resulted in the death of the remaining two patients secondary to significant blood loss [ 50 ]. There were very few cases of symptomatic VHs documented prior to the 1960s, with one literature review reporting only 64 instances of VHs with neurologic dysfunction [ 49 ]. More recent studies demonstrate improvement in surgical outcomes with neurological recovery reaching 100% and mortality as low as 0% [ 42 ].
The goal of surgery is to decompress neural elements and stabilize the spine [ 1 ]. Potential options include corpectomy, involving resection of a portion of the vertebral body containing the hemangioma, followed by anterior column reconstruction and/or laminectomy, which offers indirect decompression [ 1 ]. The selected approach depends on the size of the hemangioma and the extent of vertebral body and/or neural arch involvement due to potential weaknesses in the anterior column and the location of the epidural intrusion into the spinal canal [ 1 ]. For example, corpectomy and reconstruction could be performed in cases with ventral spinal cord compression while cases with dorsal compression could be treated with laminectomy [ 1 ].
Corpectomy has an increased risk of substantial intraoperative blood loss, up to 5 L in some cases, due to the hypervascular nature of VHs [ 1 , 51 ]. Acosta et al. reported an average blood loss of 2.1 L in their series of 10 aggressive VHs treated with corpectomy [ 51 ]. Conversely, laminectomy has a lower surgical burden and reduced risk of significant intraoperative blood loss [ 1 ]. Laminectomy blood loss can be further reduced by nearly 50% by performing vertebroplasty before laminectomy [ 8 ]. Preoperative embolization of VHs should also be considered to minimize intraoperative blood loss and reduce mortality [ 1 , 22 ].
Goldstein et al. demonstrated that en bloc resection may not be necessary, as intralesional resection produced equivalent long-term survival and prevention of recurrence in their series of 65 patients [ 47 ]. However, there have not been any large-scale studies comparing outcomes and recurrence rates of indirect decompression versus corpectomy [ 1 ].
The treatment algorithm proposed by Teferi et al. suggests dividing symptomatic VH patients with radiculopathy or neurological deficit into cohorts of epidural spinal cord compression (ESCC) versus vertebral body compression fracture to determine appropriate surgical intervention (Fig. 6 ) [ 1 ]. Patients with ESCC are encouraged to undergo preoperative embolization followed by laminectomy with or without fusion depending on spinal stability, or preoperative embolization followed by corpectomy and fusion if ESCC is accompanied by extensive anterior column compromise [ 1 ]. Conversely, the recommended treatment for symptomatic VHs secondary to vertebral body compression fracture is posterior laminectomy with decompression and fusion [ 1 ].
Whether through corpectomy or laminectomy, surgical management of VHs has a low recurrence rate [ 1 ]. Piper et al. reported complete remission in 84% of VHs treated surgically in their 2020 meta-analysis [ 52 ]. They also reported a severe complication rate, including pathological fracture, significant intraoperative blood loss, wound infection, and cerebrospinal fluid leak, of 3.5% [ 1 , 52 ].
Percutaneous techniques
Percutaneous techniques include vertebroplasty, sclerotherapy, and embolization which have been rising in popularity as treatment options for VHs in isolation or in combination with surgery [ 1 ].
Vertebroplasty is a minimally invasive procedure that improves the structural integrity of a vertebra by injecting an acrylic compound, such as polymethyl methacrylate (PMMA), into a lesion [ 1 ]. It was first utilized in the treatment of VHs by Galibert et al. in 1987 [ 53 ]. PMMA causes thrombosis and irreversible sclerosis of the hemangiomatous venous pool, shrinking the lesion and consolidating trabecular microfractures [ 1 ]. It allows for rapid recovery of mobility, enhances anterior column support, and provides vertebral stabilization, but does not induce new bone formation due to poor biological activity and absorbability [ 54 , 55 ]. Vertebroplasty is particularly effective in alleviating back pain in VH patients with intravertebral fractures by providing an immediate analgesic effect and has previously been recommended as stand-alone first line therapy for VHs with moderate to severe back pain without neurologic compromise [ 1 , 54 ]. It can also be used in combination with surgery to reduce intraoperative blood loss when given as a preoperative adjunct therapy [ 8 ]. The most common complication of vertebroplasty is extravasation of injected compound outside the vertebral body with rates of 20–35% [ 55 , 56 ]. However, some researchers suggest small amounts of extravasation should be considered a stopping point rather than a complication as the vast majority of cases are asymptomatic [ 55 , 56 ]. In a series of 673 vertebroplasty cases, Layton et al. reported extravasation in 25% of patients with only 1% developing clinical symptoms of new onset radiculopathy (5 patients) or symptomatic pulmonary embolism (1 patient) [ 56 ]. Their second most common complication was rib fracture related to lying prone on the fluoroscopy table during the procedure which occurred in 1% of cases (7 patients) [ 56 ].
Alternatively, sclerotherapy involves direct intralesional injection of ethanol under percutaneous CT-guidance which causes thrombosis and destruction of endothelium, resulting in devascularization, shrinkage of the lesion, and, consequently, decompression of the neural elements [ 46 ]. It was first described as a treatment for VHs in 1994 by Heiss et al. and is less common in the treatment of VHs [ 57 ]. CT angiography is a prerequisite to target the most hypervascular subsection of the lesion and ensure patients are candidates for the procedure without leakage of contrast media, which occurred in 25% of patients in a series of 18 cases [ 58 ]. There are reports of intraoperative sclerotherapy as an adjunct to surgery, but the sample sizes are similarly limited [ 59 , 60 ]. Complications of direct ethanol injection include neurologic deterioration (including Brown- Sequard syndrome), pathologic fractures, and VH recurrence [ 46 , 61 ].
The last option for percutaneous intervention is trans-arterial embolization of feeding vessels using particulate agents [ 1 ]. It has been used as a preoperative adjunct therapy with surgery to reduce blood loss as well as a primary treatment for VHs alone or in conjunction with vertebroplasty [ 41 , 62 , 63 , 64 ]. In a series of 26 patients, Premat et al. demonstrated embolization combined with vertebroplasty was safe and effective in treating pain associated with aggressive VHs but was less effective in resolving motor deficits [ 65 ]. The primary role for embolization in the treatment of compressive VHs is preoperative adjunct therapy to reduce the risk of procedural bleeding [ 62 ].
- Radiotherapy
Radiotherapy (XRT) is a noninvasive approach that can obliterate hemangiomas and relieve pain through vascular necrosis and/or anti-inflammatory effects [ 1 ]. It is a suitable option for VH patients with back pain and no neurologic deficits, or as postoperative adjunct therapy after suboptimal surgical decompression. Patients with neural element compromise often require prompt decompression to prevent irreversible injury that is more appropriately managed with surgery rather than the delayed response offered by XRT [ 1 , 21 , 66 ]. Neurological deficits may, in fact, be aggravated by XRT, as demonstrated in 20% of patients with aggressive VHs from a series of 29 cases by Jiang et al. [ 8 ]. Multiple studies have proclaimed a 60–80% success rate in eliminating symptoms from VHs using XRT, which increases to over 90% when including partial symptom relief [ 8 , 67 , 68 ]. This does include neurological deficits in some cases, but the response of these symptoms to XRT continues to vary [ 52 ]. A radiation dose of at least 34 Gy was recommended by Heyd et al. after their multicenter study identified significantly greater symptom relief and recurrence control compared to lower doses [ 67 ].
XRT is gaining popularity as a postoperative adjunct therapy intended to reduce local recurrence, especially in subtotal resections [ 8 , 52 , 67 ]. There is a 50% recurrence rate in partial resections without adjunct XRT [ 8 , 11 ]. The extent to which XRT can reduce recurrence has not been fully elucidated and has been suggested for future study [ 52 ]. However, these potential benefits must be weighed against the known adverse effects including nausea, fatigue, anorexia, ileus, radionecrosis, and specifically in spinal XRT, radiation myelitis [ 1 , 8 , 52 ].
VHs are often asymptomatic, incidental findings on routine spinal imaging that do not require treatment or follow-up imaging unless they become symptomatic. Most can be diagnosed with characteristic CT and MRI findings while atypical lesions may be difficult to differentiate from alternative diagnoses. Some authors suggest the utilization of emerging imaging techniques such as DWI or DCE MRI to differentiate atypical lesions from malignancies, which is a promising solution that requires further research. Other authors suggest observation with regular follow-up may be the best course of management for asymptomatic, atypical lesions while others still recommend biopsy for definitive diagnosis of atypical lesions. Regardless, there is a consensus that symptomatic lesions should be treated. Most authors recommend surgical decompression for treatment in patients with neurological deficits, but there is ongoing debate as to the optimal treatment for back pain alone. There are several treatment options which should be considered case-by-case given the properties of various lesions. Management algorithms have been suggested but additional research is required to identify the optimal treatment for the many different classifications of VHs.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- Vertebral hemangioma
Computed tomography
Magnetic resonance imaging
Arteriovenous
Positron emission-computed tomography
Technetium 99-methyl diphosphonate
Diffusion weighted imaging
Apparent diffusion coefficient
Volume transfer constant
Dynamic contrast enhanced magnetic resonance imaging
Epidural spinal cord compression
Polymethyl methacrylate
Teferi N, Chowdhury AJ, Mehdi Z, Challa M, Eschbacher K, Bathla G, Hitchon P. Surgical management of symptomatic vertebral hemangiomas: a single institution experience and literature review. Spine J. 2023;23(9):1243–54.
Article PubMed Google Scholar
Rodallec MH, Feydy A, Larousserie F, Anract P, Campagna R, Babinet A, Zins M, Drapé JL. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics. 2008;28(4):1019–41.
Baudrez V, Galant C, Vande Berg BC. Benign vertebral hemangioma: MR-histological correlation. Skelet Radiol. 2001;30:442–6.
Article CAS Google Scholar
Huvos AG. Hemangioma, lymphangioma, angiomatosis/lymphangiomatosis, glomus tumor. Bone tumors: diagnosis, treatment, and prognosis. 2nd ed. Philadelphia: Saunders. 1991;553–78.
Gaudino S, Martucci M, Colantonio R, Lozupone E, Visconti E, Leone A, Colosimo C. A systematic approach to vertebral hemangioma. Skelet Radiol. 2015;44:25–36.
Article Google Scholar
Campanacci M. Hemangioma. In: Campanacci M, editors. Bone and soft tissue tumors: clinical features, imaging, pathology and treatment. Padova: Piccin Nuova Libraria & Wien: Springer; 1999. p. 599–618.
Hameed M, Wold LE. Hemangioma. In: Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, editors. WHO classification of tumors of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. p. 332.
Google Scholar
Jiang L, Liu XG, Yuan HS, Yang SM, Li J, Wei F, Liu C, Dang L, Liu ZJ. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. Spine J. 2014;14(6):944–54.
Unni KK, Inwards CY. Dahlin's bone tumors: general aspects and data on 10,165 cases. Lippincott Williams & Wilkins; 2010.
Corniola MV, Schonauer C, Bernava G, Machi P, Yilmaz H, Lemée JM, Tessitore E. Thoracic aggressive vertebral hemangiomas: multidisciplinary management in a hybrid room. Eur Spine J. 2020;29:3179–86.
Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78(1):36–45.
Article CAS PubMed Google Scholar
Murphey MD, Fairbairn KJ, Parman LM, Baxter KG, Parsa MB, Smith WS. From the archives of the AFIP. Musculoskeletal angiomatous lesions: radiologic-pathologic correlation. Radiographics. 1995;15(4):893–917.
Cross JJ, Antoun NM, Laing RJ, Xuereb J. Imaging of compressive vertebral haemangiomas. Eur Radiol. 2000;10:997–1002.
Alexander J, Meir A, Vrodos N, Yau YH. Vertebral hemangioma: an important differential in the evaluation of locally aggressive spinal lesions. Spine. 2010;35(18):E917–20.
Laredo JD, Reizine D, Bard M, Merland JJ. Vertebral hemangiomas: radiologic evaluation. Radiology. 1986;161(1):183–9.
Elgazzar AH. Musculoskeletal system. In: Synopsis of pathophysiology in nuclear medicine. New York: Springer; 2014. p. 90–2.
Choi YY, Kim JY, Yang SO. PET/CT in benign and malignant musculoskeletal tumors and tumor-like conditions. In: Editors. Seminars in musculoskeletal radiology. Thieme Medical Publishers; 2014. pp. 133–48
Brogsitter C, Hofmockel T, Kotzerke J. 68Ga DOTATATE uptake in vertebral hemangioma. Clin Nucl Med. 2014;39(5):462–3.
Basu S, Nair N. “Cold” vertebrae on F-18 FDG PET: causes and characteristics. Clin Nucl Med. 2006;31(8):445–50.
Laredo JD, Assouline E, Gelbert F, Wybier M, Merland JJ, Tubiana JM. Vertebral hemangiomas: fat content as a sign of aggressiveness. Radiology. 1990;177(2):467–72.
Dang L, Liu C, Yang SM, Jiang L, Liu ZJ, Liu XG, Yuan HS, Wei F, Yu M. Aggressive vertebral hemangioma of the thoracic spine without typical radiological appearance. Eur Spine J. 2012;21:1994–9.
Article PubMed PubMed Central Google Scholar
Kato S, Kawahara N, Murakami H, Demura S, Yoshioka K, Okayama T, Fujita T, Tomita K. Surgical management of aggressive vertebral hemangiomas causing spinal cord compression: long-term clinical follow-up of five cases. J Orthop Sci. 2010;15:350–6.
Klekamp J, Samii M. Epidermal Tumors. In: Surgery of spinal tumors. Springer; 2007. p. 321–522.
Blecher R, Smorgick Y, Anekstein Y, Peer A, Mirovsky Y. Management of symptomatic vertebral hemangioma: follow-up of 6 patients. Clin Spine Surg. 2011;24(3):196–201.
Subramaniam MH, Moirangthem V, Venkatesan M. Management of aggressive vertebral haemangioma and assessment of differentiating pointers between aggressive vertebral haemangioma and metastases—a systematic review. Global Spine J. 2023;13(4):1120–33.
Hart JL, Edgar MA, Gardner JM. Vascular tumors of bone. In: Seminars in diagnostic pathology. WB Saunders; 2014. p. 30–8.
Dorfman HD, Czerniak B. Vascular lesions. In: Dorfman HD, Czerniak B, editors. Bone tumors. St. Louis: Mosby; 1998. p. 729–814.
Rudnick J, Stern M. Symptomatic thoracic vertebral hemangioma: a case report and literature review. Arch Phys Med Rehabil. 2004;85(9):1544–7.
Pastushyn AI, Slin’ko EI, Mirzoyeva GM. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50(6):535–47.
Blankstein A, Spiegelmann R, Shacked I, Schinder E, Chechick A. Hemangioma of the thoracic spine involving multiple adjacent levels: case report. Spinal Cord. 1988;26(3):186–91.
Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010;30(1):127–42.
Ross JS, Masaryk TJ, Modic MT, Carter JR, Mapstone T, Dengel FH. Vertebral hemangiomas: MR imaging. Radiology. 1987;165(1):165–9.
Persaud T. The polka-dot sign. Radiology. 2008;246(3):980–1.
Nguyen JP, Djindjian M, Gaston A, Gherardi R, Benhaiem N, Caron JP, Poirier J. Vertebral hemangiomas presenting with neurologic symptoms. Surg Neurol. 1987;27(4):391–7.
Gaston A, Nguyen JP, Djindjian M, Le Bras F, Gherardi R, Benhaiem N, Marsault C. Vertebral haemangioma: CT and arteriographic features in three cases. J Neuroradiol. 1985;12(1):21–33.
CAS PubMed Google Scholar
Friedman DP. Symptomatic vertebral hemangiomas: MR findings. AJR Am J Roentgenol. 1996;167(2):359–64.
Winfield JM, Poillucci G, Blackledge MD, Collins DJ, Shah V, Tunariu N, Kaiser MF, Messiou C. Apparent diffusion coefficient of vertebral haemangiomas allows differentiation from malignant focal deposits in whole-body diffusion-weighted MRI. Eur Radiol. 2018;28:1687–91.
Morales KA, Arevalo-Perez J, Peck KK, Holodny AI, Lis E, Karimi S. Differentiating atypical hemangiomas and metastatic vertebral lesions: the role of T1-weighted dynamic contrast-enhanced MRI. Am J Neuroradiol. 2018;39(5):968–73.
Article CAS PubMed PubMed Central Google Scholar
Shi YJ, Li XT, Zhang XY, Liu YL, Tang L, Sun YS. Differential diagnosis of hemangiomas from spinal osteolytic metastases using 3.0 T MRI: comparison of T1-weighted imaging, chemical-shift imaging, diffusion-weighted and contrast-enhanced imaging. Oncotarget. 2017;8(41):71095–104.
McEvoy SH, Farrell M, Brett F, Looby S. Haemangioma, an uncommon cause of an extradural or intradural extramedullary mass: case series with radiological pathological correlation. Insights Imaging. 2016;7(1):87–98.
Teferi N, Abukhiran I, Noeller J, Helland LC, Bathla G, Ryan EC, Nourski KV, Hitchon PW. Vertebral hemangiomas: diagnosis and management. A single center experience. Clin Neurol Neurosurg. 2020;190:105745.
Acosta FL Jr, Sanai N, Chi JH, Dowd CF, Chin C, Tihan T, Chou D, Weinstein PR, Ames CP. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am. 2008;19(1):17–29.
Wang B, Zhang L, Yang S, Han S, Jiang L, Wei F, Yuan H, Liu X, Liu Z. Atypical radiographic features of aggressive vertebral hemangiomas. JBJS. 2019;101(11):979–86.
Jayakumar PN, Vasudev MK, Srikanth SG. Symptomatic vertebral haemangioma: endovascular treatment of 12 patients. Spinal cord. 1997;35(9):624–8.
Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine: terminology and surgical staging. Spine. 1997;22(9):1036–44.
Doppman JL, Oldfield EH, Heiss JD. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214(2):341–8.
Goldstein CL, Varga PP, Gokaslan ZL, Boriani S, Luzzati A, Rhines L, Fisher CG, Chou D, Williams RP, Dekutoski MB, Quraishi NA. Spinal hemangiomas: results of surgical management for local recurrence and mortality in a multicenter study. Spine. 2015;40(9):656–64.
Bailey P, Bucy PC. Cavernous hemangioma of the vertebrae. J Am Med Assoc. 1929;92(21):1748–51.
Krueger EG, Sobel GL, Weinstein C. Vertebral hemangioma with compression of spinal cord. J Neurosurg. 1961;18(3):331–8.
Ghormley RK, Adson AW. Hemangioma of vertebrae. JBJS. 1941;23(4):887–95.
Acosta FL Jr, Sanai N, Cloyd J, Deviren V, Chou D, Ames CP. Treatment of Enneking stage 3 aggressive vertebral hemangiomas with intralesional spondylectomy: report of 10 cases and review of the literature. Clin Spine Surg. 2011;24(4):268–75.
Piper K, Zou L, Li D, Underberg D, Towner J, Chowdhry AK, Li YM. Surgical management and adjuvant therapy for patients with neurological deficits from vertebral hemangiomas: a meta-analysis. Spine. 2020;45(2):E99-110.
Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–8.
Guarnieri G, Ambrosanio G, Vassallo P, Pezzullo MG, Galasso R, Lavanga A, Izzo R, Muto M. Vertebroplasty as treatment of aggressive and symptomatic vertebral hemangiomas: up to 4 years of follow-up. Neuroradiology. 2009;51:471–6.
Kim BS, Hum B, Park JC, Choi IS. Retrospective review of procedural parameters and outcomes of percutaneous vertebroplasty in 673 patients. Interv Neuroradiol. 2014;20(5):564–75.
Layton KF, Thielen KR, Koch CA, Luetmer PH, Lane JI, Wald JT, Kallmes DF. Vertebroplasty, first 1000 levels of a single center: evaluation of the outcomes and complications. Am J Neuroradiol. 2007;28(4):683–9.
CAS PubMed PubMed Central Google Scholar
Heiss JD, Doppman JL, Oldfield EH. Relief of spinal cord compression from vertebral hemangioma by intralesional injection of absolute ethanol. N Engl J Med. 1994;331(8):508–11.
Bas T, Aparisi F, Bas JL. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: a mean follow-up of 2 years. Spine. 2001;26(14):1577–81.
Murugan L, Samson RS, Chandy MJ. Management of symptomatic vertebral hemangiomas: review of 13 patients. Neurol India. 2002;50(3):300.
Singh P, Mishra NK, Dash HH, Thyalling RK, Sharma BS, Sarkar C, Chandra PS. Treatment of vertebral hemangiomas with absolute alcohol (ethanol) embolization, cord decompression, and single level instrumentation: a pilot study. Neurosurgery. 2011;68(1):78–84.
Niemeyer T, McClellan J, Webb J, Jaspan T, Ramli N. Brown-Sequard syndrome after management of vertebral hemangioma with intralesional alcohol: a case report. Spine. 1999;24(17):1845.
Singh PK, Chandra PS, Vaghani G, Savarkar DP, Garg K, Kumar R, Kale SS, Sharma BS. Management of pediatric single-level vertebral hemangiomas presenting with myelopathy by three-pronged approach (ethanol embolization, laminectomy, and instrumentation): a single-institute experience. Childs Nerv Syst. 2016;32:307–14.
Yao KC, Malek AM. Transpedicular N-butyl cyanoacrylate-mediated percutaneous embolization of symptomatic vertebral hemangiomas. J Neurosurg Spine. 2013;18(5):450–5.
Kawahara N, Tomita K, Murakami H, Demura S, Yoshioka K, Kato S. Total en bloc spondylectomy of the lower lumbar spine: a surgical techniques of combined posterior-anterior approach. Spine. 2011;36(1):74–82.
Premat K, Clarençon F, Cormier É, Mahtout J, Bonaccorsi R, Degos V, Chiras J. Long-term outcome of percutaneous alcohol embolization combined with percutaneous vertebroplasty in aggressive vertebral hemangiomas with epidural extension. Eur Radiol. 2017;27:2860–7.
Yang ZY, Zhang LJ, Chen ZX, Hu HY. Hemangioma of the vertebral column: a report on twenty-three patients with special reference to functional recovery after radiation therapy. Acta Radiol Oncol. 1985;24(2):129–32.
Heyd R, Seegenschmiedt MH, Rades D, Winkler C, Eich HT, Bruns F, Gosheger G, Willich N, Micke O, German Cooperative Group on Radiotherapy for Benign Diseases. Radiotherapy for symptomatic vertebral hemangiomas: results of a multicenter study and literature review. Int J Radiat Oncol* Biol* Phys. 2010;77(1):217–25.
Asthana AK, Tandon SC, Pant GC, Srivastava A, Pradhan S. Radiation therapy for symptomatic vertebral haemangioma. Clin Oncol. 1990;2(3):159–62.
Download references
Acknowledgements
Not applicable.
The University of Iowa Hospitals and Clinics provided funding for this research.
Author information
Authors and affiliations.
University of Iowa Carver, College of Medicine, Iowa City, IA, USA
Kyle Kato & Meron Challa
Department of Neurosurgery, University of Iowa Carver, College of Medicine, Iowa City, IA, USA
Nahom Teferi & Satoshi Yamaguchi
Department of Pathology, University of Iowa Carver, College of Medicine,, Iowa City, IA, USA
Kathryn Eschbacher
You can also search for this author in PubMed Google Scholar
Contributions
KK performed a portion of the literature review and was a major contributor in writing the manuscript/generating figures. NT performed most of the literature review and was a major contributor in reviewing the manuscript. MC contributed to writing the manuscript. KE provided histological images for figures. SY was a major contributor in reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kyle Kato .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Consent was obtained to publish Fig. 6 from Teferi et al. [ 1 ].
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kato, K., Teferi, N., Challa, M. et al. Vertebral hemangiomas: a review on diagnosis and management. J Orthop Surg Res 19 , 310 (2024). https://doi.org/10.1186/s13018-024-04799-5
Download citation
Received : 02 April 2024
Accepted : 18 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s13018-024-04799-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Laminectomy
- Sclerotherapy
- Vertebroplasty
Journal of Orthopaedic Surgery and Research
ISSN: 1749-799X
- Submission enquiries: [email protected]
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

COMMENTS
Journal of Management (JOM) peer-reviewed and published bi-monthly, is committed to publishing scholarly empirical and theoretical research articles that have a high impact on the management field as a whole.JOM covers domains such as business strategy and policy, entrepreneurship, human resource management, organizational behavior, organizational theory, and research methods.
Online publication from 2024. International Journal of Management Reviews will be published in online-only format effective with the 2024 volume. This is a proactive move towards reducing the environmental impact caused by the production and distribution of printed journal copies and will allow the journal to invest in further innovation ...
This is why the literature review as a research method is more relevant than ever. Traditional literature reviews often lack thoroughness and rigor and are conducted ad hoc, rather than following a specific methodology. ... She has published in the Journal of Business Research, European Journal of Marketing, Journal of Service Management and ...
The International Journal of Management Reviews (IJMR) ... This is a rolling special section for articles that seek to advance our knowledge of literature review methods and methodologies associated with undertaking literature reviews. Submissions should make an original and innovative contribution to debates around how different types of ...
Parameters and Decision Elements of Writing Effective Literature Review Papers: Empirical Evidence From Multiple Stakeholders on POWER Framework; PRISMA for Review of Management Literature - Method, Merits, and Limitations - An Academic Review; Realist Synthesis: An Innovative Approach to Literature Review for Complex Management Phenomena
The Journal of Management Studies is a multidisciplinary business and management journal advancing the fields of management and organization. Abstract In this article, we argue that integrative literature reviews are among the most useful vehicles for advancing knowledge and furthering research in a topic domain.
The application of systematic or structured literature reviews (SLRs) has developed into an established approach in the management domain (Kraus et al. 2020), with 90% of management-related SLRs published within the last 10 years (Clark et al. 2021).Such reviews help to condense knowledge in the field and point to future research directions, thereby enabling theory development (Fink 2010 ...
The present methodological literature review (cf. Aguinis et al., 2020) addresses this void and aims to identify the dominant approaches to sample selection and provide insights into essential choices in this step of systematic reviews, with a particular focus on management research.To follow these objectives, I have critically reviewed systematic reviews published in the two most prominent ...
Literature review articles, including structured literature reviews, bibliometric analyses, and meta-analyses, are invaluable tools in the realm of management research, significantly contributing to its growth and development (Hulland and Houston 2020).They serve as cornerstones for theory development, policy formulation, and evidence-based decision-making, thus playing a pivotal role in the ...
The Academy of Management Review, now in its 26th year, is the most cited of management references.AMR ranks as one of the most influential business journals, publishing academically rigorous, conceptual papers that advance the science and practice of management.AMR is a theory development journal for management and organization scholars around the world.
Journal of Services Marketing "Review of Management Literature is a must read by all researchers and faculties. A good literature review helps in identifying gaps in the existing research and also helps in generating new ideas for future research. The book covers some of the latest frameworks and trends in
Management Review Quarterly is a double-blind, peer-reviewed journal specializing in (interpretative) literature reviews, meta-analyses, and replication studies in business and management.. covers all fields of business and management research including accounting, business information systems, corporate finance and governance/ESG, digitalization, entrepreneurship, family firms, marketing ...
The literature review co nducted for this paper covers t he broad spect rum of western pu bli cations . ... Journal of Management, 19, 2:419-459. Smith, Adam (1776) ...
The mission of Journal of Management Development is to publish academic research that tests, ... Literature review. This category should only be used if the main purpose of the paper is to annotate and/or critique the literature in a particular field. It could be a selective bibliography providing advice on information sources, or the paper may ...
A Literature Review and Overview of Performance Management: A Guide to the Field. January 2021; ... Sumerianz Journal of Business Management and Marketing, 2021, Vol. 4, No. 1, pp. 1-11.
International Journal of Management Reviews. Volume 8, Issue 2 p. 71-89. Proximity and inter-organizational collaboration: A literature review. J. Knoben, J. Knoben. ... A systematic literature review is presented in order to disentangle the dimensions of the proximity concept. Based on this literature review, three dimensions of proximity ...
Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Methods: We conducted a comprehensive literature review to identify clinical practice guidelines for cancer care delivery specific to AYAs published in PubMed or MEDLINE since 2010. MeSH and other key words were applied, using a search strategy guided by a health science librarian (EM). 795 papers were identified, with 690 excluded based on ...
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
e24078 Background: Effective pain management in cancer care is crucial for patient quality of life. Shared decision-making (SDM) interventions are increasingly recognized for their potential to improve patient outcomes by facilitating patient engagement in care decisions. This systematic review synthesizes evidence on the impact of SDM interventions in cancer pain management. Methods: This ...
Science and Technology Management Research, 38(08): 194-200. Google Scholar; Zhang Qichun, Xi Yongqin. 2017. Symbiotic network for urban waste resource utilization: concept, characteristics and system analysis. Journal of Ecology, 37(11): 3607-3618. Google Scholar; Michael Martin, Steve Harris. 2018.
e24069 Background: Managing cancer-related pain poses significant challenges, prompting research into alternative approaches such as the study of ketamine. This systematic review aims to analyze and summarize the impact of ketamine as an adjuvant to opioid therapy for cancer-related pain. Methods: We conducted a literature review in MEDLINE, EMBASE, and Scopus, spanning from January 1, 1982 ...
Literature review revealed that over the last two decades, a total of twenty cases of ATRA-induced scrotal ulceration have been reported. Histopathological evaluation of these lesions revealed atypical granulocytic infiltration, pointing towards the possible etiological role of differentiated APL cells in the pathogenesis.
The term dystocia refers to labor characterized by a slow progression with delayed rates or even pauses in the dilation of the cervix or the descent of the fetus. Dystocia describes the deviation from the limits that define a normal birth and is often used as a synonym for the term pathological birth. Shoulder dystocia, also known as the manual exit of the shoulders during vaginal delivery on ...
Management Review Quarterly - This study assesses the strategic value of corporate social responsibility (CSR) investments and their impact on corporate resilience during financial crises. ... Using a systematic literature review of peer-reviewed journal articles within social sciences, extracted from Scopus and Web of Science, we adhered to ...
The International Journal of Management Reviews (IJMR) is the leading global review journal in Organisation and Management Studies (OMS). ... It publishes literature reviews, debate essays and review methodology pieces of a multi-disciplinary, interdisciplinary, and internationally significant nature, and which are committed to making a ...
The existence of such publications as Business History and the Journal of Management History and the inclusion of history in organization studies, pioneered by Kieser (1994) and to which the Academy of Management Review dedicated a special issue (Godfrey et al., 2016), rest on this contention, broadly understood.
Background Vertebral hemangiomas (VHs) are the most common benign tumors of the spinal column and are often encountered incidentally during routine spinal imaging. Methods A retrospective review of the inpatient and outpatient hospital records at our institution was performed for the diagnosis of VHs from January 2005 to September 2023. Search filters included "vertebral hemangioma," "back ...
Start Preamble AGENCY: Federal Communications Commission. ACTION: Notice and request for comments. SUMMARY: As part of its continuing effort to reduce paperwork burdens, as required by the Paperwork Reduction Act (PRA) of 1995, the Federal Communications Commission (FCC or the Commission) invites the general public and other Federal Agencies to take this opportunity to comment on the following ...