An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ther Adv Psychopharmacol


How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding
Michael p. hengartner.
Department of Applied Psychology, Zurich University of Applied Sciences (ZHAW), PO Box 707, Zurich, CH-8037, Switzerland
Medical Faculty, University of Zurich, Zurich, Switzerland
The aim of this article is to discuss the validity of relapse prevention trials and the issue of withdrawal confounding in these trials. Recommendations for long-term antidepressant treatment are based almost exclusively on discontinuation trials. In these relapse prevention trials, participants with remitted depression are randomised either to have the antidepressant abruptly discontinued and replaced by inert placebo or to continue active treatment. The drug–placebo difference in relapse rates at the end of the maintenance phase is then interpreted as a prophylactic drug effect. These trials consistently produce remarkable benefits for maintenance treatment. However, the internal validity of this trial protocol is compromised, as research has shown that abruptly stopping antidepressants can cause severe withdrawal reactions that lead to (or manifest as) depression relapses. That is, there is substantial withdrawal confounding in discontinuation trials, which renders their findings uninterpretable. It is not clear to what degree the drug–placebo separation in relapse prevention (discontinuation) trials is due to withdrawal reactions, but various estimations suggest that it is presumably the majority. A review of findings based on other methodologies, including real-world long-term effectiveness trials like STAR*D and various naturalistic cohort studies, do not indicate that antidepressants have considerable prophylactic effects. As absence of evidence does not imply evidence of absence, no definitive conclusions can be drawn from the literature. To enable a thorough risk–benefit evaluation, real-world effectiveness trials should not only focus on relapse prevention, but also assess antidepressants’ long-term effects on social functioning and quality of life. Thus far, reliable long-term data on these outcome domains are lacking.
Introduction
Treatment guidelines like those published by National Institute for Health and Care Excellence (NICE) or the American Psychiatric Association (APA) strongly recommend long-term maintenance treatment in people with (or at risk of) recurrent depression to prevent relapses. 1 , 2 In accordance with these recommendations, the rate and duration of antidepressant use is steadily increasing in the general population, 3 – 6 but this trend has stirred considerable controversy. 7 , 8 It has been suggested that long-term antidepressant treatment should be revisited, 9 – 11 and research indicates that many patients in receipt of long-term antidepressant medication do not necessarily require maintenance treatment. 12 – 14 Some authors cautioned that long-term antidepressant use may be largely ineffective, or even harmful. 10 , 11 , 15 , 16 One possible driver of unnecessary long-term prescriptions could be the propensity of antidepressants to cause dependence and withdrawal reactions. 17 – 22 This notion is often met with disbelief, and sometimes it is fiercely dismissed by leading academics as it stands in sharp contrast to the consistently positive findings from dozens of relapse prevention trials. 23 – 27 In this article, I will ponder these seemingly contradictory findings and critically discuss major issues that may resolve the conflicting literature on the benefits of long-term antidepressant treatment. To that end, I will focus mostly on antidepressants’ prophylactic effects, as relapse prevention is the main indication for long-term antidepressant use in people with (recurrent) depressive disorders. A critical discussion of potential adverse effects of long-term use is important to consider but beyond the scope of the present article. For tolerability and safety issues, interested readers are referred to the pertinent literature. 28 – 30
Relapse prevention trials: too good to be true?
The scientific evidence in support of long-term maintenance antidepressant treatment is based almost exclusively on relapse prevention trials. 1 , 2 , 31 These long-term studies are basically discontinuation trials, where antidepressant users in (stable) remission are randomised to either have the antidepressant abruptly stopped and replaced by inert placebo or to continue active treatment. The difference in relapse rates between the antidepressant and the placebo arm at the end of the maintenance phase is then assumed to reflect a prophylactic drug effect. As stated above, the results of these trials are unequivocally positive and consistently show that, after about 12 months, the relapse rate is roughly 40% for those participants who were abruptly switched to placebo and 20% for those maintained on active treatment, which results in a relative risk of 2 and a number needed to treat (NNT) of 5. 25 – 27
This, in short, is the scientific evidence on which treatment guidelines largely base their recommendation for long-term antidepressant treatment. 1 , 2 , 31 At first glance, this evidence base indeed appears impressive, and, without a critical look at the methodology of these trials, which number in dozens, one is understandably tempted to conclude that antidepressants have ‘remarkable’ long-term efficacy. 32 Based on evidence from relapse prevention (discontinuation) trials, it was even claimed that antidepressants are ‘one of the most effective of all drugs’. 23 However, as I already pointed out in previous articles, 10 , 33 the validity of these trials, and hence the interpretation of their findings, cannot be accepted at face value. As researchers, we should not be seduced into believing that a drug is highly effective simply because a specific trial protocol has consistently produced impressive treatment effects, as these effects could be the result of a flawed trial protocol. 34 Such systematic bias in clinical trials is also referred to as ‘hard-wired bias’. 35
The persistent superiority of antidepressants over placebo in relapse prevention (discontinuation) trials is a peculiar finding, given that only about 50% of acute treatment trials are positive, 36 , 37 which results in a disappointingly small average treatment effect, 38 , 39 and a NNT of about 9. 40 , 41 This recently led researchers from the Nordic Cochrane Center to state that ‘Taken together, the evidence does not support definitive conclusions regarding the efficacy of antidepressants for depression in adults, including whether they are more efficacious than placebo for depression’ (p. 8). 39 Moreover, it is important to note that trial protocols other than discontinuation trials failed to find reliable evidence of remarkable long-term benefits. 42 – 44 This prompted SN Ghaemi, a leading psychiatric researcher from Tufts Medical Center in Boston, MA, to conclude that ‘(Antidepressants’) long-term prophylactic effectiveness in recurrent unipolar major depression remains uncertain’ (p. 957). 16 In this respect, the evidence from relapse prevention (discontinuation) trials indeed appears too good to be true. 34 How could a drug that has very limited efficacy in the acute and long-term treatment of depression symptoms possibly have such impressive prophylactic effects? We therefore need to consider that the strong and consistent effects produced in relapse prevention (discontinuation) trials are possibly a methodological artefact. I will now explain how this impressive drug-placebo separation could come about.
Withdrawal confounding in relapse prevention trials
Relapse prevention (discontinuation) trials are very popular in psychiatry but have a bad reputation among critics. According to various authors, their validity is poor and findings hence difficult to interpret. 34 , 42 , 45 , 46 Issues discussed in the literature include, among others, poor representativity and generalisability of results (findings apply only to a subset of users who responded particularly well to the drugs), inflated effect size estimates (treatment responders are assessed for treatment response, which is tautological) and unblinding effects (participants who have their active treatment abruptly discontinued may notice it). Here, I will focus on one particular issue, that is, withdrawal confounding. 46
Various authors have stressed that prolonged antidepressant use can cause neurochemical adaptations (physical dependence) and corresponding withdrawal reactions upon dose reduction or discontinuation comparable with other central nervous system (CNS) drugs like benzodiazepines, stimulants or opioides. 18 , 22 , 47 , 48 There is now compelling evidence from clinical trials, observational studies and user surveys that stopping antidepressants can cause severe and persistent withdrawal reactions in a substantial portion of users. 49 , 50 Withdrawal symptoms include, among others, anxiety, panic, irritability, aggression, lethargy, flu-like symptoms, electric-shock sensations (brain zaps), fatigue, dizziness, tremor, dysphoria, bouts of crying, suicidality, insomnia, anorexia and nausea. Many of these symptoms are, therefore, easily misdiagnosed as a depression relapse when relapses are assessed via symptom rating scales such as the Hamilton Depression Rating Scale that cannot differentiate withdrawal from relapse. 51 , 52
Withdrawal reactions can be so severe that they classify as a depression relapse in up to 27% of users within 5–8 days of double-blind placebo-controlled treatment interruption. 53 That is, abrupt discontinuation of antidepressants relates to significantly higher rate of new depression episodes. 53 , 54 This increased risk is not necessarily due to misclassification of acute withdrawal symptoms, yet is likely caused by withdrawal reactions, for example, neurochemical adaptations suddenly unopposed. 55 , 56 These types of withdrawal reactions are commonly defined as rebound disorders (rapid return of original symptoms at greater intensity) and persistent (protracted) post-acute withdrawal disorders (return of persistent original symptoms at greater intensity and/or symptoms related to new emerging disorders). 50 While rebound disorders usually occur within a few days after drug discontinuation, and resolve spontaneously within up to 6 weeks, persistent post-acute withdrawal disorders may also have a delayed onset and last for several months or, occasionally, even years. 47 , 57 , 58 Rebound disorders and persistent post-acute withdrawal disorders have also been described with various other CNS drugs, including opioids, benzodiazepines, stimulants, antipsychotics and lithium. 48 , 59
According to two placebo-controlled trials, abrupt discontinuation of antidepressants can lead to a significant decline in social functioning within a few days, with further progression of impairments very likely. 60 , 61 These functional impairments that come along with withdrawal symptoms may cause stress that can trigger or precipitate a depression relapse. 62 , 63 The link between withdrawal-related functional impairments and depression relapse has never been examined directly, 60 , 61 but is indirectly supported by robust epidemiological findings that social functioning deficits, for example, due to job strain, 64 , 65 relate prospectively to increased risk of depression. 66 Finally, there is evidence that the more users had previously been exposed to and the longer they had been on antidepressants, the higher the risk of severe withdrawal reactions. 17 , 50 , 67 , 68 Thus, as cumulative exposure to antidepressants appears to influence the incidence and severity of withdrawal reactions, 50 , 67 discontinuation trials with a longer pre-randomization (stabilization) phase may thus have more confounded results. Moreover, it is important to note that a majority of participants who enter a relapse prevention (discontinuation) trial had already been on antidepressants and other psychotropic drugs for a long time. In the lead-in (washout) phase, these participants may thus already undergo withdrawal, and then again in the space of a few weeks if randomised to the discontinuation (placebo) arm. For someone who has been on prescribed psychotropics for years, this may cause no small degree of disturbance both psychologically and physiologically. 45 , 62
In sum, abruptly stopping antidepressants can cause various types of withdrawal reactions that meet diagnostic criteria of a new depression episode, including rebound disorders and persistent post-acute withdrawal disorders. 47 , 48 , 50 Moreover, acute withdrawal symptoms can be misdiagnosed as depression relapse or may trigger a relapse due to withdrawal-related functional impairments. 51 , 52 , 62 It follows that a significant portion (possibly even a majority) of events recorded as depression relapses in the discontinuation arm of maintenance studies are in fact due to withdrawal reactions. 69 , 70 When we examine the survival curves in relapse prevention (discontinuation) trials, we easily see that the drug–placebo separation occurs almost completely within the first 12 weeks (see for instance the graphs presented in the FDA review 25 ). That is, antidepressants appear to exert a ‘prophylactic’ effect for the first 12 weeks only; thereafter, the drugs do not protect any better against relapse than a placebo pill. This has been noted by various authors and is empirically well established. 10 , 70 – 72 The findings detailed above hence indeed question the validity of relapse prevention (discontinuation) trials, of which the vast majority, noteworthy, does not attempt to differentiate relapse from withdrawal. 46 , 69 Of course genuine depression relapses also occur in the discontinuation (placebo) arm, but this is not the point. The fundamental issue is that events recorded as relapses could very well be, and in many cases certainly are, the result of withdrawal reactions. Therefore, the internal validity of relapse prevention (discontinuation) trials is compromised. 34 , 46 , 73 Given that the outcome in these maintenance studies is confounded, we must acknowledge that they are uninterpretable and cannot serve as a valid evidence base for long-term maintenance treatment. The next question hence is whether there is evidence of prophylactic effects from studies with other methodologies that would support long-term antidepressant treatment.
Extension trials and longitudinal observational studies: do they concur with relapse prevention trials?
Extension trials start as double-blind acute phase trials with a placebo and antidepressant arm. After the acute treatment phase, treatment responders continue on the same treatment they were initially randomised to. The advantage of extension trials over discontinuation trials is thus that they avoid withdrawal confounding, as acute treatment responders continue with the same treatment they were already on (i.e. the placebo arm is not a discontinuation arm). Unfortunately, there are only very few placebo-controlled extension trials. A systematic review and meta-analysis by Zimmerman et al. found only five small trials of 6–12 months duration. 74 They report an average relapse rate of 8% for active treatment and 25% for placebo. However, there are flaws in this meta-analysis. For instance, in one trial the reported relapse rate for placebo was not from the extension arm (that is, from participants who were treated with placebo during the acute phase), but from participants re-randomised from antidepressant to placebo (hence a typical discontinuation arm affected by withdrawal confounding). 75 In another trial, 76 the rates reported by Zimmerman et al. were actually not for relapses (new depression episodes; not reported in the target article), but for loss of response (<30% symptom reduction from baseline), 74 which is a different outcome. Due to these flaws, the results reported by Zimmerman et al. must be interpreted with caution. 74
The National Institute of Mental Health (NIMH)-sponsored real-world effectiveness trial STAR*D also included a 12-month extension phase for treatment responders, but unfortunately it was not placebo-controlled. 77 Nevertheless, the results show that, when prophylactic effects are assessed via long-term follow up of continuously treated acute-phase responders (rather than via abrupt treatment discontinuation after the acute phase), then sustained remission with antidepressants is a rare event. 16 , 43 According to the intent-to-treat re-analysis by Pigott et al. , 43 the rate of sustained remission for participants who entered the extension phase in remission was only 6% at the final 12-month assessment. A similarly very low rate of sustained remission (only 11% over 12 months of treatment) was also reported in another NIMH-sponsored real-world effectiveness trial. 44 These publicly funded real-world trials based on representative outpatient samples indicate that the long-term benefits of antidepressants appear disappointingly poor once their prophylactic effects are assessed with protocols other than discontinuation trials. These findings are largely confirmed by the meta-analysis of classic long-term trials conducted by Deshauer et al. , 42 according to which there is no significant drug–placebo difference in remission rates after 6–8 months of treatment (drug: 45%, placebo: 38%).
I will now turn to a brief discussion of observational studies on relapse prevention. Eli Lilly, manufacturer of fluoxetine, published evidence from observational studies suggesting that short-term antidepressant use, relative to continued use, relates to higher relapse rates. 78 , 79 This was seen as a confirmation that long-term treatment is often necessary and beneficial. However, it was later demonstrated that these studies sponsored by Eli Lilly applied a flawed statistical method that systematically biases the results against short-term use. 80 In fact, when the observational data are analysed with an unbiased statistical method, then short-term antidepressant use is associated with lower relapse rates than continued use. 80 – 82 Systematic reviews of longitudinal cohort studies likewise do not indicate that antidepressant treatment prevents relapses, chronicity or clinical progression of depression. 83 – 85 Noteworthy, in the most recent review of primary care and community studies, the authors stated that antidepressant use typically relates to similar or even worse outcomes than non-use. 86 Indeed, many observational studies point to the possibility that (long-term) antidepressant use may increase the risk of recurrent or persistent depression. 87 – 89 These findings are also supported by research on the pharmacodynamic mechanisms of tolerance and tachyphylaxis, which suggests that the more and the longer a person has been treated with antidepressants, the larger the risk of non-response, relapse and chronicity; 77 , 90 , 91 for a comprehensive review, see Fava and Offidani. 56
Finally, the average rate of sustained recovery in patients with mood disorders was higher in the pre-treatment era (that is, before the widespread use of antidepressants) than in psychiatry’s modern drug-centred treatment era, despite today’s patients diagnosed with mood disorders being, on average, less severely ill. 92 , 93 Although the aim of this article is not to provide a comprehensive review of observational studies, it can be concluded from previous systematic reviews that antidepressant use does not, on average, relate to less relapses or sustained recovery in people with depression. 83 , 85 If anything, observational studies hint at increased risk of relapses and chronicity with long-term antidepressant use. 10 , 83 , 86 , 93 It must be borne in mind that the validity of observational studies is limited due to confounding by indication, so these studies cannot prove that long-term use is ineffective or harmful. However, taken together the findings from observational studies certainly do not indicate that long-term antidepressant use has remarkable benefits.
Summary and conclusion
Relapse prevention (discontinuation) trials have produced strong and consistent evidence of drug–placebo separation during the first 12 weeks of treatment; thereafter, treatment effects remain constant for at least 12 months. 26 , 27 , 72 The common interpretation of these findings is that antidepressants have strong prophylactic effects, and that they effectively prevent depression relapses. 1 , 2 , 23 , 31 This interpretation is challenged by research on antidepressant withdrawal reactions, which also emerge within days or a few weeks after treatment discontinuation (or dose reduction), and which can be severe and persistent. 21 , 50 , 94 Clinical trials and observational studies have shown that when antidepressants are abruptly (or rapidly) stopped, patients are at increased risk of relapse. 53 , 54 Severe withdrawal symptoms and related functional impairments may develop within a few days in patients who were in stable remission, 53 , 61 but late onset and slow but persistent progression of symptoms is also possible. 47 , 48 , 51 Withdrawal reactions comprise not only acute withdrawal symptoms, but also rebound disorders and persistent post-acute withdrawal disorders. 47 , 48 , 50 This makes the differentiation between withdrawal and relapse even more challenging for an assessor in a clinical trial. For the vivid personal account of a psychiatrist with lived experience, see Stockmann. 95 Hundreds of individual case reports are posted on SurvivingAntidepressants.org .
It is difficult to quantify the extent to which events recorded as depression relapse in maintenance studies are related to withdrawal reactions, but different estimations suggest that it is presumably the majority. 46 , 69 , 70 These findings indicate that there is substantial withdrawal confounding in relapse prevention (discontinuation) trials and that the internal validity of these studies is compromised. It follows that the results of these trials are uninterpretable. Publicly funded real-world long-term effectiveness trials like STAR*D showed that the benefits of continued antidepressant use are disappointingly poor. 16 , 43 , 77 The results of longitudinal observational studies likewise do not indicate that (long-term) antidepressant use prevents relapses or chronicity. 83 – 85 If anything, it appears that long-term antidepressant treatment, compared with short-term use or non-use, relates to worse outcomes. 10 , 15 , 81 More research is urgently needed to explain how such findings come about, but the pharmacodynamic mechanisms of tolerance and tachyphylaxis are probably a good starting point. 56 , 96
This article concurs with a growing number of physicians and researchers who caution against indiscriminate long-term antidepressant treatment. 8 – 11 , 55 Currently, there is no reliable evidence that long-term antidepressant treatment is beneficial and there are legitimate concerns that it may be largely ineffective or even harmful in a substantial portion of users. 10 , 11 , 16 , 55 , 96 It is particularly problematic that we have almost no data on antidepressants’ long-term effects on objective measures of social functioning (e.g. employment and disability rates) and patient-oriented outcomes such as quality of life. A critical reappraisal of current treatment guidelines along these lines is required. However, in keeping with the logical principle of ‘absence of evidence is not evidence of absence’ we must remain mindful that long-term antidepressant use may be useful to some patients. 97 It is therefore important to conduct large real-world effectiveness trials that can adequately evaluate antidepressants’ long-term effects on depression symptoms, social functioning and quality of life. Classic long-term parallel-arm placebo-controlled trials are the preferred methodology. Discontinuation trials should be avoided unless they apply very slow and individually tailored tapers and carefully discriminate withdrawal reactions from genuine depression relapses. Finally, it would also be worthwhile to focus more generally on influences of industry-sponsorship and authors’ conflicts of interest, 10 , 98 as these may systematically bias the literature on the risks and benefits of antidepressants. 36 , 99 – 102
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author declares that there is no conflict of interest.
Ethics statement: No approval was required for this work

Contributor Information
Michael P. Hengartner, Department of Applied Psychology, Zurich University of Applied Sciences (ZHAW), PO Box 707, Zurich, CH-8037, Switzerland. Medical Faculty, University of Zurich, Zurich, Switzerland.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 17 October 2022
Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: a systematic review and network meta-analysis
- Taro Kishi ORCID: orcid.org/0000-0002-9237-2236 1 ,
- Toshikazu Ikuta 2 ,
- Kenji Sakuma 1 ,
- Makoto Okuya 1 ,
- Masakazu Hatano 1 , 3 ,
- Yuki Matsuda 4 &
- Nakao Iwata ORCID: orcid.org/0000-0003-3189-6076 1
Molecular Psychiatry volume 28 , pages 402–409 ( 2023 ) Cite this article
29k Accesses
28 Citations
103 Altmetric
Metrics details
- Drug discovery
A systematic review and random-effects model network meta-analysis were conducted to compare the efficacy, acceptability, tolerability, and safety of antidepressants to treat adults with major depressive disorder (MDD) in the maintenance phase. This study searched the PubMed, Cochrane Library, and Embase databases and included only double-blind, randomized, placebo-controlled trials with an enrichment design: patients were stabilized on the antidepressant of interest during the open-label study and then randomized to receive the same antidepressant or placebo. The outcomes were the 6-month relapse rate (primary outcome, efficacy), all-cause discontinuation (acceptability), discontinuation due to adverse events (tolerability), and the incidence of individual adverse events. The risk ratio with a 95% credible interval was calculated. The meta-analysis comprised 34 studies ( n = 9384, mean age = 43.80 years, and %females = 68.10%) on 20 antidepressants (agomelatine, amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, fluvoxamine, levomilnacipran, milnacipran, mirtazapine, nefazodone, paroxetine, reboxetine, sertraline, tianeptine, venlafaxine, vilazodone, and vortioxetine) and a placebo. In terms of the 6-month relapse rate, amitriptyline, citalopram, desvenlafaxine, duloxetine, fluoxetine, fluvoxamine, mirtazapine, nefazodone, paroxetine, reboxetine, sertraline, tianeptine, venlafaxine, and vortioxetine outperformed placebo. Compared to placebo, desvenlafaxine, paroxetine, sertraline, venlafaxine, and vortioxetine had lower all-cause discontinuation; however, sertraline had a higher discontinuation rate due to adverse events. Compared to placebo, venlafaxine was associated with a lower incidence of dizziness, while desvenlafaxine, sertraline, and vortioxetine were associated with a higher incidence of nausea/vomiting. In conclusion, desvenlafaxine, paroxetine, venlafaxine, and vortioxetine had reasonable efficacy, acceptability, and tolerability in the treatment of adults with stable MDD.
Similar content being viewed by others
Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis

Item-based analysis of the effects of duloxetine in depression: a patient-level post hoc study

A phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial to verify the efficacy and safety of ansofaxine (LY03005) for major depressive disorder
Introduction.
Major depressive disorder (MDD) is a common mental illness [ 1 ], with a 12-month prevalence of 4.4% worldwide [ 2 ]. Individuals with MDD in the acute phase undergo pharmacotherapy (e.g., antidepressant therapy) [ 3 ] or non-pharmacotherapy (e.g., psychotherapy [ 4 ] and electroconvulsive therapy) [ 5 ]. Relapse/recurrence rate of these patients is >85% within a decade of an index depressive episode and an average of ≥50% within 6 months of apparent clinical remission if the initially effective treatment is not continued [ 6 ]. Therefore, maintenance therapy is necessary to avoid relapse/recurrence [ 1 ].
Kato and colleagues recently conducted an important pairwise meta-analysis that included only double-blind, randomized placebo-controlled trials (DBRPCTs) with an enrichment design in which individuals with MDD were stabilized on the antidepressant of interest during the open-label study and then randomized to receive the same antidepressant or a placebo (40 studies, n = 8890) [ 7 ]. According to this meta-analysis, the antidepressant maintenance group had a significantly lower relapse rate than the antidepressant discontinuation group (odds ratio = 0.38, 95% confidence interval = 0.33–0.43, p < 0.00001). As the relapse rate remained unchanged in both the maintenance and discontinuation groups from 6 months to 1 year, Kato et al. concluded that antidepressant maintenance treatment for at least 6 months after remission is recommended to prevent relapse, with special attention to relapses and treatment failure during this 6-month period. Thanks to this excellent study, we conceived the new clinical question of which antidepressants were better in terms of efficacy, acceptability, tolerability, and safety for adult individuals with MDD as a maintenance treatment. A network meta-analysis on individuals with MDD in the acute phase demonstrated although some antidepressants (e.g., agomelatine, escitalopram, mirtazapine, paroxetine, and sertraline) have a relatively higher response rate and lower dropout rate than the others, fluvoxamine, reboxetine, and trazodone have been reported to have generally inferior efficacy and acceptability profiles compared with the other antidepressants [ 8 ]. This suggests that not all antidepressants have similar efficacies and acceptability in individuals with MDD in the acute phase. A network meta-analysis is a technique to compare three or more interventions simultaneously in a single analysis by combining both direct and indirect evidence across a network of studies [ 9 ]. A network meta-analysis also produces estimates of the relative effects between any pair of interventions in the network and usually yields more precise estimates than a single direct or indirect estimate, thereby allowing estimation of the ranking and hierarchy of interventions [ 9 ]. Results from a network meta-analysis cannot be obtained by a pairwise meta-analysis. Moreover, the previous pairwise meta-analyses for individuals with MDD in the maintenance phase did not evaluate the risk of individual adverse events of antidepressants [ 7 , 10 , 11 ]. To answer our clinical question, we conducted a systematic review and network meta-analysis on the 13 outcomes related to the efficacy, acceptability, tolerability, and safety of 20 antidepressants for the treatment of adults in the maintenance phase of MDD.
Materials and methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 12 ] (Table S1 ) and was registered on the Open Science Framework ( https://osf.io/xwezp ). At least two authors double-checked the accuracy of the literature search, data transfer, and calculations.
Search strategy and inclusion criteria
A systematic literature review was conducted in accordance with the Population, Intervention, Comparison, Outcome strategy: the population comprised adults in the maintenance phase of MDD, the intervention was monotherapy with antidepressants, the comparator medication was a placebo, and the outcomes were described in the following section. The inclusion criteria were as follows: (1) DBRPCTs with a minimum duration of 12 weeks and (2) DBRPCTs with an enrichment design in which patients were stabilized on the antidepressant of interest during the open-label study and then randomized to receive the same antidepressant or a placebo. The following studies were excluded: (1) studies focusing on specific generations (e.g., children and/or adolescents or older individuals) because the efficacy and safety of antidepressants in children and older individuals differ from those in the general adult population [ 1 ]; (2) studies including individuals with a dual diagnosis of MDD and other disorders because these studies could lead to heterogeneity [ 1 ]; and (3) continuation studies in which individuals with acute symptoms were randomly assigned to treatment groups (i.e., the target population for a continuation study was individuals with MDD in the acute phase). In the present systematic review and meta-analysis, among adults with MDD who benefited symptomatically from antidepressant treatment (i.e., the target population for our systematic review and meta-analysis was individuals with MDD in the maintenance phase), the differences in relapse rates were compared between those who continued with the same antidepressant and those who discontinued the antidepressant. Information on the literature search is displayed in Fig. S1 .
Data synthesis, outcome measures, and data extraction
The primary outcome was the 6-month relapse rate (efficacy), and the secondary outcome was all-cause discontinuation (acceptability). Other outcomes included discontinuation due to adverse events (tolerability) and the incidence of individual adverse events (safety). If at least five studies have data sufficient to perform a network meta-analysis for a specific safety outcome, a network meta-analysis was conducted for the safety outcome. In the International Classification of Diseases 11th Revision [ 13 ], recurrent depressive disorder is defined by a history of at least two depressive episodes with an interval of several months without substantial mood disturbance. In the present study, the term “relapse” is used for convenience rather than “recurrence” similar to the previous study [ 7 ], because few studies in this meta-analysis included cases in which worsening of symptoms during the study period was considered a recurrence. The definitions of relapse for each included study are presented in Table S2 , and the data synthesis results are shown in Table S3 . To avoid unit-of-analysis errors in studies involving two or more treatment arms of the same drug at different doses, data from the treatment arms were pooled for analysis [ 9 ]. The extracted data were analyzed based on intention-to-treat or modified intention-to-treat principles. If necessary data were missing from the studies, we searched for them in published systematic review articles; we also attempted to contact the original investigators in order to obtain previously unpublished data.
Meta-analysis methods
Both pairwise [ 14 ] and Bayesian network meta-analyses [ 15 ] were performed using the random-effects model [ 16 ]. Because all of the outcomes in our study were dichotomous, risk ratios (RRs) with 95% credible intervals (CrIs) were calculated as effect sizes. Network heterogeneity was assessed using τ ² statistics. In pairwise meta-analyses, heterogeneity was assessed using I 2 statistics. A statistical evaluation of incoherence was not possible because there was no head-to-head study comparing different antidepressants. The treatments for each outcome were ranked using the surface under the curve cumulative ranking probabilities. The methodological quality of the included studies was evaluated using the Cochrane risk of bias tool for randomized trials (ROB2) ( https://www.riskofbias.info/welcome/rob-2-0-tool ). The assumption of transitivity was tested by extracting potential effect modifiers such as sample size, duration of study, and mean age and comparing their distribution across comparisons in the network. We determined whether the distribution differences were large enough to threaten the validity of the analysis by comparing the distribution of these possible effect modifiers across treatments included in the network meta-analysis using the Kruskal–Wallis test (continuous variables), the Pearson chi-squared test or the Fisher exact test (categorical variables) and by assessing their actual impact on the treatment effect through meta-regression analyses [ 17 , 18 ]. A meta-regression analysis was performed to determine the relationship of potentially confounding factors (e.g., mean age, proportion of females, number of episodes, total number of participants, patient status, publication year, sponsorship, duration of preliminary phase, country, discontinuation methods, risk of bias, antidepressant class, dosage schedule, and antidepressant dose) to the magnitude of the effect on the primary outcome. Funnel plots were created to investigate potential publication bias. Finally, to assess the credibility of the findings of each network meta-analysis, the findings were incorporated into the Confidence in Network Meta-Analysis (CINeMA) application, which is an adaptation of the Grading of Recommendations Assessment, Development, and Evaluation approach [ 19 , 20 , 21 ].
Study characteristics
The literature search and selection strategy are depicted in Fig. S1 . The initial search retrieved 148 articles, 50 of which were excluded as duplicates, 95 were excluded based on a review of the abstract and/or title, and three were included in our study [ 22 , 23 , 24 ]. In addition, 31 studies were retrieved [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 ] by manually searching the reference lists of previous review article [ 7 ]. There were no additional studies found in the clinical trial registers. Finally, the present review included a total of 34 DBRPCTs comprising 9384 patients with MDD (mean age = 43.80 years and %females = 68.10%). The characteristics of the 34 DBRPCTs included are summarized in Table S4 . The average length of the study was 40.94 ± 16.27 weeks. Adults in the maintenance group were administered agomelatine ( K = 2), amitriptyline ( K = 1), bupropion ( K = 1), citalopram ( K = 3), desvenlafaxine ( K = 2), duloxetine ( K = 2), escitalopram ( K = 1), fluoxetine ( K = 4), fluvoxamine ( K = 1), levomilnacipran ( K = 2), milnacipran ( K = 1), mirtazapine ( K = 1), nefazodone ( K = 1), paroxetine ( K = 2), reboxetine ( K = 1), sertraline ( K = 2), tianeptine ( K = 1), venlafaxine ( K = 3), vilazodone ( K = 1), and vortioxetine ( K = 2). In 32 studies, participants in the acute study were required to have a scale-derived minimum of symptoms at baseline. However, one study lacked such a criterion, while another lacked detailed information on the criterion. Although 20 of the studies included only outpatients, six included both inpatients and outpatients, and the remaining eight did not report the status. All studies employed operationalized criteria such as those found in Diagnostic and Statistical Manual of Mental Disorders [ 56 ]. For the placebo group, the drug was discontinued abruptly (7 studies) and gradually (12 studies), and the remaining 15 studies did not report the detailed method of drug discontinuation. In addition, 31 studies were sponsored by the industry. The distribution of potential effect modifiers was similar across the comparisons in the network (Table S5 ). In at least one domain of the ROB2 tool, no studies were determined to be at high risk of bias (Table S6 ).
Network meta-analysis results
The network meta-analysis results are shown in Appendices S1 – S13 .
In terms of the 6-month relapse rate, amitriptyline, citalopram, desvenlafaxine, duloxetine, fluoxetine, fluvoxamine, mirtazapine, nefazodone, paroxetine, reboxetine, sertraline, tianeptine, venlafaxine, and vortioxetine outperformed the placebo (Fig. 1 , Appendix S1 ), with RRs (95% CrIs) ranging from 0.149 (0.018–0.610) for nefazodone to 0.583 (0.410–0.789) for fluoxetine. In addition, citalopram, fluvoxamine, and tianeptine outperformed vilazodone. Moreover, nefazodone outperformed agomelatine, bupropion, and vilazodone. Furthermore, sertraline outperformed agomelatine, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, fluoxetine, levomilnacipran, milnacipran, paroxetine, reboxetine, venlafaxine, vilazodone, and vortioxetine. Global heterogeneity was moderate. A funnel plot for this outcome, although no comparisons included at least 10 studies, is displayed in Appendix S1 . On meta-regression analyses, no potentially confounding factors were associated with the RR of the primary outcome (Appendix S1 ). Heterogeneity was not strongly reduced despite adjustments for any potentially confounding factors in a meta-regression (Appendix S1 ). Thus, no clear evidence of violations of the transitivity assumption for any of the potential effect modifiers analyzed was found (Table S5 and Appendix S1 ).
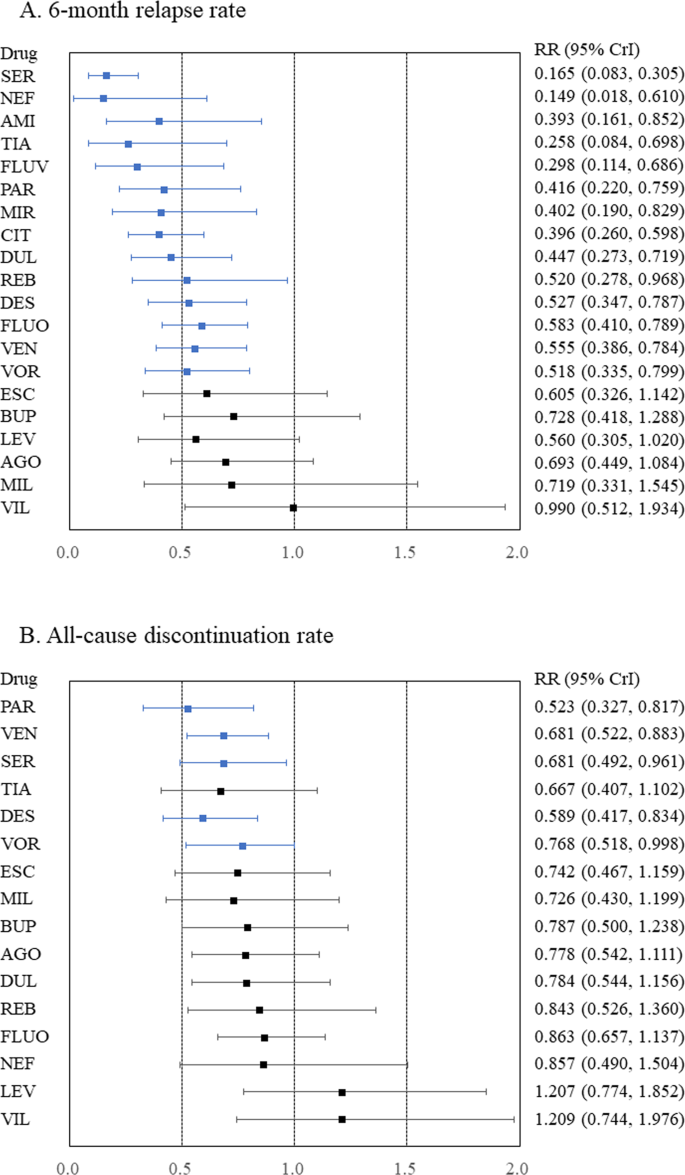
A 6-month relapse rate and B all-cause discontinuation rate. Medications were compared to a placebo. Colors indicate the presence or absence of a statistically significant difference, with blue indicating that the drug was superior to the placebo and black indicating that the drug was comparable to the placebo. 95% CrI 95% credible interval, AGO agomelatine, AMI amitriptyline, BUP bupropion, CIT citalopram, DES desvenlafaxine, DUL duloxetine, ESC escitalopram, FLUO fluoxetine, FLUV fluvoxamine, LEV levomilnacipran, MIL milnacipran, MIR mirtazapine, NEF nefazodone, PAR paroxetine, REB reboxetine, RR risk ratio, SER sertraline, TIA tianeptine, VEN venlafaxine, VIL vilazodone, VOR vortioxetine.
Acceptability
Compared to placebo, desvenlafaxine, paroxetine, sertraline, venlafaxine, and vortioxetine had lower all-cause discontinuation (Fig. 1 , Appendix S2 ), with RRs (95% CrIs) ranging from 0.523 (0.327–0.817) for paroxetine to 0.768 (0.518–0.998) for vortioxetine. Desvenlafaxine, paroxetine, and venlafaxine outperformed levomilnacipran and vilazodone. Sertraline also outperformed levomilnacipran. Global heterogeneity was moderate.
Tolerability and safety outcomes
Compared to placebo, sertraline was associated with a higher rate of discontinuation due to adverse events (Fig. 2 and Appendix S3 ). Compared to placebo, although desvenlafaxine, sertraline, and vortioxetine were associated with a higher incidence of nausea/vomiting (Fig. 2 and Appendix S4 ), venlafaxine was associated with a lower incidence of dizziness (Appendix S5 ). Compared to placebo, any antidepressants were not associated with an increased incidence of headache, somnolence, insomnia, dry mouth, constipation, sweating, weight gain, or sexual dysfunction (Appendices S6 – 13 ).
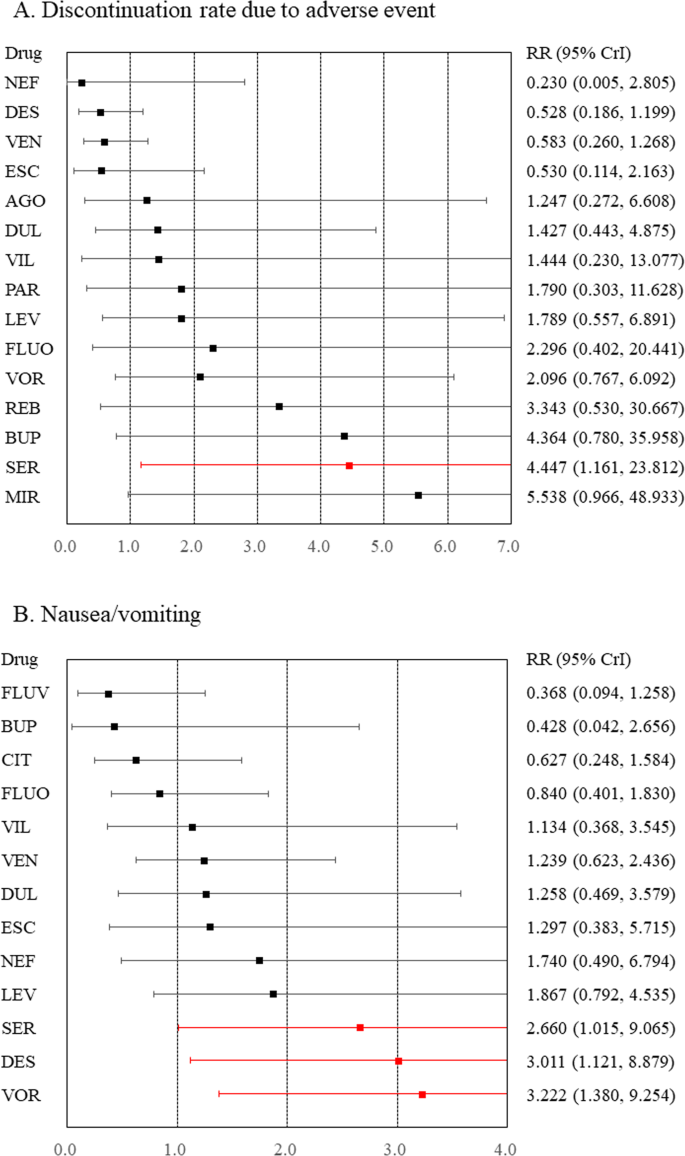
A Discontinuation rate due to adverse events and B nausea/vomiting. Medications were compared with a placebo. Colors indicate the presence or absence of a statistically significant difference, with red indicating that the drug was inferior to the placebo and black indicating that the drug was comparable to the placebo. 95% CrI 95% credible interval, AGO agomelatine, BUP bupropion, CIT citalopram, DES desvenlafaxine, DUL duloxetine, ESC escitalopram, FLUO fluoxetine, FLUV fluvoxamine, LEV levomilnacipran, MIR mirtazapine, NEF nefazodone, PAR paroxetine, REB reboxetine, RR risk ratio, SER sertraline, VEN venlafaxine, VIL vilazodone, VOR vortioxetine.
Heterogeneity, inconsistency, and network meta-analysis results graded using the CINeMA application
Global heterogeneity was rated as moderate for all outcomes, except for constipation and sexual dysfunction, for which global heterogeneity was rated as high (Appendices S1 – 13 ). A considerable local heterogeneity was observed for the majority of outcomes in specific comparisons. Statistical evaluation of incoherence was impossible due to the absence of a head-to-head study comparing various antidepressants. Between network meta-analysis and pairwise meta-analysis, results showed differences in the following in comparison to placebo: agomelatine and levomilnacipran for the 6-month relapse rates, tianeptine for all-cause discontinuation rate, desvenlafaxine and mirtazapine for discontinuation rates due to adverse events, sertraline for nausea/vomiting, desvenlafaxine for dizziness, duloxetine for dry mouth, citalopram for constipation, and sertraline for sexual dysfunction. The within-study bias was rated as “some concerns” for all comparisons. Because funnel plots with fewer than 10 studies were not meaningful [ 9 ], all comparisons for publication bias were rated as “suspected,” and any inconsistency could not be evaluated. Furthermore, the comparison was downgraded one level if it was based only on indirect evidence. Therefore, the confidence in the evidence for all comparisons other than vortioxetine versus placebo (low) in terms of the primary outcome was rated as “very low (Appendix S1 ).”
To the best of our knowledge, this is the first systematic review and network meta-analysis to investigate which antidepressant has the best balance of efficacy and acceptability for the treatment of adult individuals with MDD in the maintenance phase. Although desvenlafaxine, paroxetine, sertraline, venlafaxine, and vortioxetine had the best balance, sertraline was not well tolerated due to its association with nausea/vomiting. Therefore, desvenlafaxine, paroxetine, venlafaxine, and vortioxetine may be beneficial to individuals with MDD in the maintenance phase. However, desvenlafaxine and vortioxetine were associated with a risk of nausea/vomiting in adults with MDD in the maintenance phase as well as in the acute phase [ 57 ]. The efficacy, acceptability, tolerability, and safety of the treatment of MDD in the maintenance phase should be carefully considered as treatments prescribed for an acute depressive episode are typically continued into maintenance. Results of a network meta-analysis of adults with acute MDD also revealed that desvenlafaxine, paroxetine, venlafaxine, and vortioxetine had good efficacy and acceptability [ 8 ].
In contrast, the findings of the present network meta-analysis suggest that agomelatine, bupropion, escitalopram, levomilnacipran, milnacipran, and vilazodone did not outperform the placebo in terms of 6-month relapse rate. The original DBRPCTs reported that although vilazodone did not differ from placebo in terms of relapse rate at the study-endpoint [ 23 ], escitalopram and bupropion were superior to placebo [ 43 , 55 ]. Two DBRPCTs on agomelatine had inconsistent results [ 32 , 33 ]. One DBRPCT reported that levomilnacipran outperformed placebo in terms of relapse rate at the study-endpoint [ 22 ], while another DBRPCT did not report the statistical result of the outcome [ 49 ]; one trial investigating milnacipran also did not report the statistical results [ 47 ]. Our pairwise meta-analysis showed that agomelatine and levomilnacipran outperformed the placebo (Appendix S1 ). Due to the small number of individuals in these antidepressant trials, the 95% CrIs for the primary outcome in the network meta-analysis might be wider. As a result, our network meta-analysis might not be able to detect the significant differences between these antidepressants and placebo.
A previous meta-regression analysis based on a pairwise meta-analysis showed that the effect size of the relapse rates was greater for tricyclics, selective serotonin reuptake inhibitors, and other newer agents, in that order, compared with the placebo [ 7 ]. However, our study did not demonstrate this trend (Appendix S1 ). Through a network meta-analysis, the relative effects can be estimated using any pair of interventions in the network simultaneously as well as the ranking and hierarchy of the interventions based on effectiveness [ 9 , 58 ]. Thus, when comparing the efficacy of individual antidepressants, a network meta-analysis is likely to yield more robust results than a pairwise meta-analysis.
There are some limitations to this study. First, the number of participants and DBRPCTs for some antidepressants, especially for tricyclic antidepressants, is small. The results of the present meta-analysis for some antidepressants were based on only one study. Second, important clinical issues regarding treatment decision-making in routine clinical practice (e.g., monotherapy or combination of antidepressants with nonpharmacological treatments) were not covered. A Finnish nationwide cohort study of individuals with severe MDD requiring hospitalization (mean follow-up time, 7.9 ± 5.3 years) found that lithium treatment was associated with the lowest risk of hospital readmission in patients with severe unipolar depression compared with other pharmacological treatments such as antidepressant and antipsychotics [ 59 , 60 ]. Sim and colleagues also reported that psychotherapy may have long-term benefits, particularly for patients with at least three previous major depressive episodes [ 10 ]. However, because there were no DBRPCT with an enrichment design for those treatments, our study did not evaluate these treatments for individuals with MDD. Third, due to a lack of available data, our study did not include some important antidepressant side effects such as agitation.
In conclusion, antidepressants such as desvenlafaxine, paroxetine, venlafaxine, and vortioxetine had balanced efficacy, acceptability, and tolerability in the treatment of adults with MDD in the maintenance phase. However, desvenlafaxine and vortioxetine had a risk of nausea/vomiting in adults with MDD in both the maintenance and acute phases.
Data availability
The current study data were reported in articles cited in this paper.
Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, et al. Time for united action on depression: a Lancet-World Psychiatric Association Commission. Lancet. 2022;399:957–1022.
Google Scholar
WHO. Depression and other common mental disorders: global health estimates. World Health Organization 2017; Geneva.
Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540–60.
Parikh SV, Quilty LC, Ravitz P, Rosenbluth M, Pavlova B, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 2. Psychological treatments. Can J Psychiatry. 2016;61:524–39.
Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561–75.
Baldessarin IR Chemotherapy in Psychiatry, 3rd edition. Springer Press 2013; New York.
Kato M, Hori H, Inoue T, Iga J, Iwata M, Inagaki T, et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2021;26:118–33.
CAS Google Scholar
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. wwwtrainingcochraneorg/handbook 2021.
Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol 2015;19:pyv076.
Glue P, Donovan MR, Kolluri S, Emir B. Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Aust N. Z J Psychiatry. 2010;44:697–705.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
WHO. International Statistical Classification of Diseases and Related Health Problems 11th. World Health Organization.
Rücker G, Schwarzer G, Krahn U, König J netmeta: Network Meta-Analysis using Frequentist Methods (R package version 0.9-5). https://CRANR-projectorg/package=netmeta 2017; (accessed March 14, 2020).
van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3:285–99.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials. 1986;7:177–88.
Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159:130–7.
Ostuzzi G, Bertolini F, Tedeschi F, Vita G, Brambilla P, Del Fabro L, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21:295–307.
Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9:e99682.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082.
Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16:e1080.
Durgam S, Chen C, Migliore R, Prakash C, Thase ME. Relapse prevention with levomilnacipran ER in adults with major depressive disorder: A multicenter, randomized, double-blind, placebo-controlled study. Depress Anxiety. 2019;36:225–34.
Durgam S, Gommoll C, Migliore R, Chen C, Chang CT, Aguirre M, et al. Relapse prevention in adults with major depressive disorder treated with vilazodone: a randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2018;33:304–11.
Thase ME, Jacobsen PL, Hanson E, Xu R, Tolkoff M, Murthy NV. Vortioxetine 5, 10, and 20 mg significantly reduces the risk of relapse compared with placebo in patients with remitted major depressive disorder: The RESET study. J Affect Disord. 2022;303:123–30.
Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26:1408–16.
Dalery J, Dagens-Lafont V, De, Bodinat C. Efficacy of tianeptine vs placebo in the long-term treatment (16.5 months) of unipolar major recurrent depression*. Hum Psychopharmacol. 2001;16:S39–47.
Dekker J, Jonghe F, Tuynman H. The use of anti-depressants after recovery from depression. Eur J Psychiatry. 2000;14:207–12.
Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468–77.
Doogan DP, Caillard V. Sertraline in the prevention of depression. Br J Psychiatry. 1992;160:217–22.
Feiger AD, Bielski RJ, Bremner J, Heiser JF, Trivedi M, Wilcox CS, et al. Double-blind, placebo-substitution study of nefazodone in the prevention of relapse during continuation treatment of outpatients with major depression. Int Clin Psychopharmacol. 1999;14:19–28.
Gilaberte I, Montejo AL, de la Gandara J, Perez-Sola V, Bernardo M, Massana J, et al. Fluoxetine in the prevention of depressive recurrences: a double-blind study. J Clin Psychopharmacol. 2001;21:417–24.
Goodwin GM, Boyer P, Emsley R, Rouillon F, de Bodinat C. Is it time to shift to better characterization of patients in trials assessing novel antidepressants? An example of two relapse prevention studies with agomelatine. Int Clin Psychopharmacol. 2013;28:20–8.
Goodwin GM, Emsley R, Rembry S, Rouillon F. Agomelatine Study G. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:1128–37.
Hochstrasser B, Isaksen PM, Koponen H, Lauritzen L, Mahnert FA, Rouillon F, et al. Prophylactic effect of citalopram in unipolar, recurrent depression: placebo-controlled study of maintenance therapy. Br J Psychiatry. 2001;178:304–10.
Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, et al. Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA. 1998;280:1665–72.
Kocsis JH, Thase ME, Trivedi MH, Shelton RC, Kornstein SG, Nemeroff CB, et al. Prevention of recurrent episodes of depression with venlafaxine ER in a 1-year maintenance phase from the PREVENT Study. J Clin Psychiatry. 2007;68:1014–23.
McGrath PJ, Stewart JW, Quitkin FM, Chen Y, Alpert JE, Nierenberg AA, et al. Predictors of relapse in a prospective study of fluoxetine treatment of major depression. Am J Psychiatry. 2006;163:1542–8.
Montgomery SA, Dunbar G. Paroxetine is better than placebo in relapse prevention and the prophylaxis of recurrent depression. Int Clin Psychopharmacol. 1993;8:189–95.
Montgomery SA, Entsuah R, Hackett D, Kunz NR, Rudolph RL. Venlafaxine 335 Study G. Venlafaxine versus placebo in the preventive treatment of recurrent major depression. J Clin Psychiatry. 2004;65:328–36.
Montgomery SA, Rasmussen JG, Tanghoj P. A 24-week study of 20 mg citalopram, 40 mg citalopram, and placebo in the prevention of relapse of major depression. Int Clin Psychopharmacol. 1993;8:181–8.
Perahia DG, Gilaberte I, Wang F, Wiltse CG, Huckins SA, Clemens JW, et al. Duloxetine in the prevention of relapse of major depressive disorder: double-blind placebo-controlled study. Br J Psychiatry. 2006;188:346–53.
Perahia DG, Maina G, Thase ME, Spann ME, Wang F, Walker DJ, et al. Duloxetine in the prevention of depressive recurrences: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:706–16.
Rapaport MH, Bose A, Zheng H. Escitalopram continuation treatment prevents relapse of depressive episodes. J Clin Psychiatry. 2004;65:44–9.
Rickels K, Montgomery SA, Tourian KA, Guelfi JD, Pitrosky B, Padmanabhan SK, et al. Desvenlafaxine for the prevention of relapse in major depressive disorder: results of a randomized trial. J Clin Psychopharmacol. 2010;30:18–24.
Robert P, Montgomery SA. Citalopram in doses of 20-60 mg is effective in depression relapse prevention: a placebo-controlled 6 month study. Int Clin Psychopharmacol. 1995;10:29–35.
Rosenthal JZ, Boyer P, Vialet C, Hwang E, Tourian KA. Efficacy and safety of desvenlafaxine 50 mg/d for prevention of relapse in major depressive disorder:a randomized controlled trial. J Clin Psychiatry. 2013;74:158–66.
Rouillon F, Warner B, Pezous N, Bisserbe JC. Milnacipran efficacy in the prevention of recurrent depression: a 12-month placebo-controlled study. Milnacipran recurrence prevention study group. Int Clin Psychopharmacol. 2000;15:133–40.
Schmidt ME, Fava M, Robinson JM, Judge R. The efficacy and safety of a new enteric-coated formulation of fluoxetine given once weekly during the continuation treatment of major depressive disorder. J Clin Psychiatry. 2000;61:851–7.
Shiovitz T, Greenberg WM, Chen C, Forero G, Gommoll CP. A randomized, double-blind, placebo-controlled trial of the efficacy and safety of levomilnacipran ER 40-120mg/day for prevention of relapse in patients with major depressive disorder. Innov Clin Neurosci. 2014;11:10–22.
Simon JS, Aguiar LM, Kunz NR, Lei D. Extended-release venlafaxine in relapse prevention for patients with major depressive disorder. J Psychiatr Res. 2004;38:249–57.
Stein MK, Rickels K, Weise CC. Maintenance therapy with amitriptyline: a controlled trial. Am J Psychiatry. 1980;137:370–1.
Terra JL, Montgomery SA. Fluvoxamine prevents recurrence of depression: results of a long-term, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 1998;13:55–62.
Thase ME, Nierenberg AA, Keller MB, Panagides J. Relapse Prevention Study G. Efficacy of mirtazapine for prevention of depressive relapse: a placebo-controlled double-blind trial of recently remitted high-risk patients. J Clin Psychiatry. 2001;62:782–8.
Versiani M, Mehilane L, Gaszner P, Arnaud-Castiglioni R. Reboxetine, a unique selective NRI, prevents relapse and recurrence in long-term treatment of major depressive disorder. J Clin Psychiatry. 1999;60:400–6.
Weihs KL, Houser TL, Batey SR, Ascher JA, Bolden-Watson C, Donahue RM, et al. Continuation phase treatment with bupropion SR effectively decreases the risk for relapse of depression. Biol Psychiatry. 2002;51:753–61.
APA. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D. C.
Oliva V, Lippi M, Paci R, Del Fabro L, Delvecchio G, Brambilla P, et al. Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110266.
Leucht S, Chaimani A, Cipriani AS, Davis JM, Furukawa TA, Salanti G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. 2016;266:477–80.
Tiihonen J. Use of lithium in patients with unipolar depression - Author’s reply. Lancet Psychiatry. 2017;4:663.
Tiihonen J, Tanskanen A, Hoti F, Vattulainen P, Taipale H, Mehtala J, et al. Pharmacological treatments and risk of readmission to hospital for unipolar depression in Finland: a nationwide cohort study. Lancet Psychiatry. 2017;4:547–53.
Download references
Acknowledgements
We would like to thank Dr. Masaki Kato (Department of Neuropsychiatry, Kansai Medical University) for providing the article reprint that we were unable to obtain. We would also like to thank MARUZEN-YUSHODO Co., Ltd. ( https://kw.maruzen.co.jp/kousei-honyaku/ ) for the English language editing.
The present study was funded by a Grant-in-Aid for Young Scientists (21K15738) and a Research Grant for Early-Career Scientists from Fujita Health University’s School of Medicine.
Author information
Authors and affiliations.
Department of Psychiatry, Fujita Health University School of Medicine, Toyoake, Aichi, 470–1192, Japan
Taro Kishi, Kenji Sakuma, Makoto Okuya, Masakazu Hatano & Nakao Iwata
Department of Communication Sciences and Disorders, School of Applied Sciences, University of Mississippi, University, Oxford, MS, 38677, USA
Toshikazu Ikuta
Department of Clinical Pharmacy, Fujita Health University School of Medicine, Toyoake, Aichi, 470–1192, Japan
Masakazu Hatano
Department of Psychiatry, The Jikei University School of Medicine, Minato-ku, Tokyo, 105–8461, Japan
Yuki Matsuda
You can also search for this author in PubMed Google Scholar
Contributions
TK had full access to all the study data and assumes responsibility for the data integrity, as well as the analysis accuracy. TK contributed to the study’s conception and design. TK and TI performed the statistical analysis. All authors were responsible for data acquisition, interpretation, and manuscript writing. NI supervised the review.
Corresponding author
Correspondence to Taro Kishi .
Ethics declarations
Competing interests.
The interests from the past 3 years are as follows: TK received a speaker’s honoraria from Sumitomo, Eisai, Takeda, Janssen, Otsuka, Meiji, Viatris, MSD, and Tanabe-Mitsubishi, in addition to a research grant from the Japanese Ministry of Health, Labor and Welfare, a Grant-in-Aid for Scientific Research C, the Japan Agency for Medical Research and Development, and Fujita Health University School of Medicine. TI has nothing to disclose. KS received a speaker’s honoraria from Sumitomo, Eisai, Kissei, Meiji, and Otsuka, in addition to a research grant from a Grant-in-Aid for Young Scientists, the Japan Agency for Medical Research and Development, and the Fujita Health University School of Medicine Research Grant for Early-Career Scientists. MH received a speaker’s honoraria from Sumitomo, Janssen, Kyowa, Otsuka, Tanabe-Mitsubishi, and Yoshitomi. MO received speaker’s honoraria from Sumitomo, Eisai, Kissei, Meiji, and Otsuka, in addition to a research grant from a Grant-in-Aid for Young Scientists (21K15738) and the Fujita Health University School of Medicine Research Grant for Early-Career Scientists. YM received a speaker’s honoraria from Sumitomo, Janssen, Kyowa, Otsuka, Tanabe-Mitsubishi, and Yoshitomi, in addition to a research grant from the Japan Agency for Medical Research and Development. NI received a speaker’s honoraria from Sumitomo, Eisai, Takeda, Eli Lilly, Viatris, Janssen, Otsuka, Meiji, Shionogi, and Tanabe-Mitsubishi, in addition to research grants from Eisai, Takeda, Sumitomo, and Otsuka. The authors declare that they have no conflicts of interest regarding the subject of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kishi, T., Ikuta, T., Sakuma, K. et al. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: a systematic review and network meta-analysis. Mol Psychiatry 28 , 402–409 (2023). https://doi.org/10.1038/s41380-022-01824-z
Download citation
Received : 27 July 2022
Revised : 12 September 2022
Accepted : 27 September 2022
Published : 17 October 2022
Issue Date : January 2023
DOI : https://doi.org/10.1038/s41380-022-01824-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Polypharmacology: promises and new drugs in 2022.
- Piotr Ryszkiewicz
- Barbara Malinowska
- Eberhard Schlicker
Pharmacological Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- The trouble with...
The trouble with antidepressants: why the evidence overplays benefits and underplays risks—an essay by John B Warren
- Related content
- Peer review
- John B Warren , director
- Medicines Assessment, Ipswich, Suffolk, UK
- jbwarren5{at}gmail.com
Widespread prescribing has not reduced mental disability or suicide, raising questions about the assessment of evidence on effectiveness and safety of antidepressants, writes John Warren
Depression can be severe and reduce life expectancy. Antidepressant prescribing has increased substantially in recent years so that one in eight UK adults, some 7.3 million people, now receive a prescription for antidepressants each year, and many take them long term. 1 More than 60% of US residents taking antidepressants do so for more than two years. 2
Although meta-analyses seem to support widespread use, concerns have been raised about the effectiveness and safety of the drugs. The conclusions of meta-analyses have been criticised because of manufacturers’ influence on trials, 3 4 under-recognition of the placebo effect, inadequate attention to negative data, different methods used to assess risk and benefit, and lack of benefit on suicide. There are also concerns about limited safety databases and the huge commercial promotion of these drugs.
Analysis of the benefits and risks of drugs in psychiatry differs from other therapeutic areas. There are no reliable biomarkers of psychiatric disease and no primary endpoint to summarise safety and efficacy (an equivalent to mortality in cardiovascular or oncology trials). Psychiatry therefore depends on composite scales for diagnosis and to assess drug efficacy. As composite measures are rarely used for adverse events, trials are likely to overestimate benefit and underestimate risks, with serious implications for public health. Although prescribers will often see patients improve over time, questions remain about how much antidepressants contribute to this and whether long term treatment is safe.
Unclear mechanism of action
A common justification for using antidepressants is that they correct a chemical deficiency in the brain. The monoamine hypothesis, over 50 years old, implicates serotonergic, noradrenergic, and dopaminergic neurotransmission in the pathogenesis of depression.
Deficiency of the neurotransmitter dopamine explains Parkinson’s disease, but no similar chemical deficiency has been shown in the human brain for depression, the biochemistry of which remains complex and unexplained. 5 6 Depression has no subclassification depending on which of the three amines is deficient, even though each amine differs in its pharmacology and physiology.
The limitations of the monoamine hypothesis are widely accepted in terms of drug efficacy, though altered monoamine neurotransmission remains relevant to much of the safety profile of antidepressants. But composite endpoint safety data from long term trials does not have sufficient sensitivity to fully document the effect of these alterations in brain biochemistry on the psyche. This includes quantifying neurophysiological adaptation to long term treatment.
Promotion of small effects
Symptom severity fluctuates spontaneously during depression, and antidepressants started during exacerbations can appear to be more successful than they are. In a typical 6-12 week trial, scores among participants in the placebo arm fall from a mean of roughly 25 to 12-15 on the widely used Hamilton Depression Rating Scale. Any additional effect of active treatment is usually of questionable clinical importance.
A cycle of enthusiasm for the latest drug, big pharma’s large promotional budgets, and the delayed recognition of risk recurs throughout the history of pharmacology. Past examples in psychiatry include morphine, heroin, insulin, metronidazole, chloral hydrate, bromides, hyoscine, barbiturates, amphetamines, and major tranquilisers.
Esketamine, although not a typical antidepressant, is a recent example of how limited evidence for a new drug can attract favourable publicity. 7 The trials used the Montgomery-Åsberg Depression Rating Scale (score range 0-60), which is more sensitive to changes induced by antidepressants than the Hamilton scale. The US Food and Drug Administration approved esketamine in 2019 8 based on a finding of a 20 points reduction with esketamine compared with 16 points with placebo in the first 28 days of treatment. 8 Most of the reduction in the esketamine arm was also seen with placebo. The four point difference between drug and placebo reached a significance of P<0.05 in some trials only if a one sided P value was used. 9 Despite these small changes and no evidence of a persistent, clinically relevant, benefit, the FDA approval was accompanied by press coverage 7 and the drug heralded as a “first in class” treatment in the New England Journal of Medicine . 9
The small effect sizes reported for antidepressants are often further reduced after a drug is marketed. This was the fate of reboxetine, 10 11 authorised in the UK in 1997. The effectiveness of reboxetine was analysed by remission and responder rates, which do not translate directly into clinical significance. Reboxetine was ineffective in mild or moderate depression. A post-hoc analysis showed a statistically significant treatment effect on response rate for severe depression, though not a clinically significant benefit in symptom rating scales. 10 A former FDA employee noted the full set of data, on which the FDA had based a negative opinion for reboxetine, is not publicly available. 12
Meta-analyses and mean differences
Two recent systematic reviews and meta-analyses—one examining 21 common antidepressants 13 and the other selective serotonin reuptake inhibitors (SSRIs) 14 —found statistically, but not clinically, significant effects. Both attracted publicity that promoted antidepressant use 15 despite criticisms of the analyses. 16 17 18 19 20
Unblinding from adverse events may have contributed to the 0.3 standardised mean difference in effect size seen with antidepressants 13 and the mean difference of <2 on the Hamilton scale with SSRIs. 14 Standardised mean difference compensates for different rating scales, and a minimum of difference of 0.5 has been recommended for clinical significance. 21 A 2004 guideline from the National Institute for Health and Care Excellence (NICE) proposed a minimum of three points for a clinically significant difference on the 52 point Hamilton scale. 22 Even a three point change may be too low a threshold, 23 because it is undetectable by clinicians. 24
The spontaneous variability of the disease within the study population means that both placebo and active treatment patients can sometimes be classified as responders. It is the mean difference between the two groups that defines the treatment effect; it is not valid to say some individuals have responded to treatment, as this cannot be distinguished from background fluctuation in symptoms.
No evidence shows that increasing antidepressant dose increases the response in severe depression (standardised mean difference 0.05; 95% confidence interval −0.14 to 0.25). 25 Higher doses have been linked to violence, suicidality, homicide, mania, and psychosis. 26
The Oxford meta-analysis of 21 antidepressants did not sufficiently account for bias, selective outcome reporting, or reasons for attrition. 16 17 18 19 20 Efficacy analysis was restricted to 8-12 weeks’ treatment, though treatment for years is common. For the SSRI meta-analysis, there were almost no data on suicidal behaviour, quality of life, and long-term effects. 14 A reasonable conclusion of systematic meta-analyses is that antidepressants do not cause clinically significant improvements in depression.
Missing negative data
Meta-analyses depend on data from systematic reviews, 27 but to be reliable they need to dig into regulatory datasets to find the data. 3 21
The more compounds that are developed, the greater the chance of a false positive result. Less than half of antidepressant trials submitted to the FDA have positive results, 3 28 but many more trials with negative findings are not submitted. Journals rarely check protocol endpoints, and published claims of efficacy are often greater than the effects observed on the protocol specified primary endpoint. 29 When antidepressants are approved, negative data can be overlooked, as with vilazodone in 2011, when two positive trials were mentioned in the FDA label and five negative trials omitted. 29 Negative results must be included in risk-benefit analyses, as shown by the case of reboxetine. 10 11
It is a challenge to ensure meta-analyses consider all available data. Many negative trials of antidepressants are not publicly available, 25 26 28 and about half of trials do not comply with EU requirements to register their results. 30
Negative trials may be submitted to regulators under conditions of confidentiality. Trials from failed developments may never be submitted. 17 Some drugs developed for depression, such as sibutramine or varenicline, have been approved for other indications after trials in depression failed. Meta-analyses consider the influence of multiplicity on statistical significance; this is important as more than 20 P values can be reported for a single trial. 31 But meta-analyses cannot consider the influence of multiplicity for trials whose results are not available, or for drugs where a failed development means they are no longer classified as antidepressants, even though their results may cast doubt on a class benefit.
Unbalanced methods for assessing benefit and risk
Psychiatry depends on composite endpoints for the diagnosis and monitoring of mental illness. The Montgomery-Åsberg scale measures 10 symptoms, and the Hamilton scale a minimum of 17. Were these symptoms assessed separately, no trial of efficacy would reach statistical significance. By contrast, adverse events are categorised separately and rarely made into a composite.
The safety database submitted for esketamine approval was unusual as it included a composite to assess dissociation, the Clinician-Administered Dissociative Symptoms Scale. The assessment showed that 61%-75% of patients with current treatment developed dissociative or perceptual changes. Other safety indicators, including misuse, suicidal thoughts and behaviours, increased blood pressure, cognitive impairment, and cystitis were measured separately and not summarised in a composite. An incidence of at least 5% and at least twice that of placebo was reported for dissociation, dizziness, nausea, sedation, vertigo, hypoaesthesia, anxiety, lethargy, blood pressure increase, vomiting, and feeling drunk. 8 Whereas one primary endpoint was used to summarise benefit, safety was analysed as a collection of symptoms with no single endpoint, mitigating against finding statistical significance 32 and leading to the asymmetrical analysis of risk and benefit. 33
Limited safety data
Concerns raised by patient representatives about dependence, safety, and the level of prescribing led the UK All-Party Parliamentary Group for Prescribed Drug Dependence to examine the evidence on withdrawal problems with antidepressants. 34 35 More than half of people who attempt to stop taking antidepressants reported withdrawal effects; almost half of the reported effects were severe and some lasted for weeks or months. Many patients have made individual reports of withdrawal adverse events, though such reports have been criticised for a lack methodological rigour. 36 Nonetheless, there is concern that patients’ experiences of withdrawal problems are not fully reflected in drug information labels. 37 38
Data from the US, UK, Australia, Denmark, Iceland, and Sweden suggest a correlation between the use of antidepressants and the increasing prevalence of mental disability. Antidepressants might increase the risk of chronic depression, the incidence of conversion from a unipolar to bipolar disorder, and the chance of being classified disabled. 39
Long term use may propel the illness to a more malignant and unresponsive course, 40 possibly triggered by changes in brain biochemistry. 41 Since 1966 there has been concern that antidepressants might shorten the intervals between depressive episodes; about 6% of patients are in remission after 12 months’ treatment compared with about 85% in control groups. 39 The involvement of the cholinergic system in several types of dementia raises concern about the long term anticholinergic effects of many antidepressants. 42
An observational study of antidepressant use among older people found increases in serious adverse events such as death, falls, fractures, strokes and seizures. 43 Despite the study’s weakness, serious morbidity and mortality cannot be excluded as a consequence of antidepressant treatment of older people.
Inconsistencies
Many prescribers consider their accumulated clinical observations of individual cases proves the efficacy of antidepressants. Given the long established use of these drugs, it might be expected that the level of prescribing would now be stable. Yet the use of antidepressants in the UK has doubled in the past decade, and most are taken for more than a year. This has not reduced the rate of mental disability, or suicide—the most serious outcome in depression. If antidepressants were effective it might be expected that treatment would reduce the suicide rate, or at least suicide ideation.
A review of esketamine describes how a third of patients with treatment resistant depression attempt suicide 9 but does not discuss the effect of the drug on this endpoint. This is despite the fact that three patients who received the drug died by suicide during trials, compared with none in the control group. 7 The FDA label describes a non-significant increase in suicidality, recommending that patients who experience emergent suicidal thoughts or behaviours should stop treatment. 8
Public health concern
Given limited efficacy and long term safety concerns, the current level of UK prescribing is a major public health concern. Widespread prescribing is supported by short duration trials using composite endpoints designed to detect minor changes in efficacy, enthusiastic interpretation of meta-analyses, 13 and large promotion budgets.
The antidepressant market globally has exceeded $10bn (£7.6bn; €8.5bn) a year for several years. Fluoxetine (Prozac) reached blockbuster status, and esketamine is also predicted to reach sales over $1bn a year. Substantial sums are spent on marketing, advertising, public relations, and support of medical education and academia. Direct-to-consumer advertising for SSRIs has cited the monoamine hypothesis, even though it has no scientific support. 44
Money for independent reviews is negligible by comparison. The case to reduce prescribing is driven largely by patient advocates and a safety database devoid of the composites capable of summarising long term safety or identifying dependence. Considerable expertise is needed to select patients with more severe depression who will benefit from treatment and to determine how best to wean off patients for whom the long term risk-benefit balance is not favourable.
Teaching prescribers which drug to choose, when to prescribe it, and to which patient, needs to be expanded to include when not to prescribe and how to deprescribe. Prescribers in busy practice with limited consultation time should be cautious about starting patients on antidepressants for mild or moderate depression.
John Warren is a clinical pharmacologist whose interest in antidepressants developed during 16 years spent as an expert medical assessor at the Medicines and Healthcare Products Regulatory Agency. He represented the UK on the scientific advice working party of the European Medicines Agency, which highlighted the limitations of data used to justify new antidepressant authorisations, particularly an imbalance in the use of risk-benefit composite endpoints.
Acknowledgments
I thank S Dimmitt, R Ferner, Y Looke, J Ritter, H Stamfer, and P Feldschreiber for their help.
Competing interests: I have read and understood BMJ policy on declaration of interests and declare I am paid dividends from the medical consultancy Medicines Assessment. I am a member of the joint specialty committee for clinical pharmacology and therapeutics at the Royal College of Physicians and acted as an RCP representative at the APPG for Prescribed Drug Dependence, Westminster. I advise the Cure Parkinson’s Trust.
Provenance and peer review: Commissioned; externally peer reviewed.
- ↵ Taylor S, Annand F, Burkinshaw P, et al. Dependence and withdrawal associated with some prescribed medicines: an evidence review. Public Health England, 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/829777/PHE_PMR_report.pdf
- Turner EH ,
- Matthews AM ,
- Linardatos E ,
- Rosenthal R
- Ebrahim S ,
- Malachowski C ,
- Ioannidis JP
- Chávez-Castillo M ,
- ↵ Huetteman E. FDA Overlooked red flags in drugmaker’s testing of new depression medicine. Kaiser Health News 2019. https://khn.org/news/fdas-approval-of-new-depression-drug-overlooked-red-flags-in-its-testing/
- ↵ FDA. Esketamine—full prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf
- Farchione T ,
- ↵ Medicines and Healthcare Products Regulatory Authority. Public assessment report—reboxetine: a review of the benefits and risks. 2011. http://www.mhra.gov.uk/home/groups/s-par/documents/websiteresources/con129107.pdf
- Lelgemann M ,
- Grouven U ,
- Cipriani A ,
- Furukawa TA ,
- Salanti G ,
- Jakobsen JC ,
- Katakam KK ,
- Adlington K
- Irving Kirsch I ,
- Jakobsen JC
- Paludan-Müller AS ,
- Moncrieff J ,
- Gøtzche P ,
- ↵ National Institute for Clinical Excellence. Depression: management of depression. primary and secondary care clinical practice guideline no 23. 2004. https://www.nice.org.uk/guidance/CG23 .
- Fennema H ,
- Kaspers-Janssen M ,
- Lepping P ,
- Henssler J ,
- Franklin J ,
- Liberati A ,
- Altman DG ,
- Tetzlaff J ,
- Laughren TP
- Goldacre B ,
- DeVito NJ ,
- Heneghan C ,
- Harrington D ,
- D’Agostino RB Sr . ,
- Gatsonis C ,
- Warren JB ,
- Feldschreiber P
- ↵ Davies J, Pauli R, Montagu L. Antidepressant withdrawal. Survey of patients’ experience by the all-party parliamentary group for prescribed drug dependence. 2018. http://prescribeddrug.org/wp-content/uploads/2018/09/APPG-PDD-Antidepressant-Withdrawal-Patient-Survey.pdf
- Andrews PW ,
- Thomson JA Jr . ,
- Amstadter A ,
- ↵ Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times 2018 Apr 7. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html
- El-Mallakh RS ,
- Coupland CAC ,
- Morriss R ,
- Hippisley-Cox J
- Coupland C ,
- Lacasse JR ,

Antidepressants: A Research Update and a Case Example
What experiences do people have if they take antidepressants.
Posted December 20, 2018
- Find a therapist to overcome depression or anxiety

This post briefly reviews what researchers have been finding about the effectiveness and also the downsides of antidepressant medications — Zoloft, Prozac, and the like. The post then adds a fascinating case example, a self-description submitted to me by a reader who has experienced both the ups and the downs of antidepressant drugs. First, though, a word about my personal bias : Antidepressant pills definitely do help some people. At the same time, I regard them as vastly over-prescribed for mild to moderate depression and also for anxiety . Other treatment strategies for these situations can be equally or more effective, and without the downsides. In my TEDx talk on lifting depression , for instance, I demonstrate an antidepressant visualization exercise that I have used effectively in my clinical practice for decades. See also the techniques here .
What does the latest research suggest about whether you should take antidepressant medications?
For a particularly comprehensive review of the established medical risks of antidepressants , this article from Harvard Medical School is especially informative. You can find many similar articles with a web search of antidepressant risks.
In addition, a recent comprehensive review article of 522 trials and more than 116,000 patients — a meta-analysis combining the findings of all the available studies — reported on findings regarding 21 antidepressant drugs. This review was described as the most comprehensive analysis of the evidence ever undertaken. The Lancet Psychiatry , which reported the study, also then published a further analysis by the study’s authors. Here are their conclusions:
- Antidepressants can be, on average, an effective treatment for adults with moderate-to-severe major depression in the acute phase of illness.
- Effective as defined in this study means that there was a 50 percent or more reduction of depressive symptoms over an eight-week period. “Effective” did not imply complete remission (removal) of the depression.
- Some patients experienced great benefit from the medication ; others gained little or no benefit. In general, the more severe the depression, the more benefits from the antidepressant.
- The average response to a placebo (a sugar pill disguised as medication) was 35 percent. The average response to antidepressants ranged between 42 percent (reboxetine) and 53 percent (amitriptyline).
- For between 47 and 58 percent of subjects, depending on the specific drug, the medication was not effective. That is, they did not experience at least a 50 percent reduction in their depression symptoms.
Note that the depressed clients who did receive relief from taking an antidepressant medication definitely felt better — and yet not necessarily fully healed. Again, "effective" is defined as a 50 percent improvement in symptoms. This definition raises a number of questions:
- What about the remaining effects of depression, if only 50 percent of the symptoms have been relieved by the medication?
- Are antidepressant drugs appropriate to prescribe for milder depression? Or are non-medication therapy techniques just as or more effective? The research deals with only moderate to severe depression. Yet most prescriptions for antidepressants are given for milder to moderate depressive reactions.
- Earlier studies have concluded that the combination of both drugs and psychotherapy has the highest response rate. Both show about equal effectiveness on their own, except that psychotherapy has longer-lasting positive impacts, because it teaches skills and understandings that have long-term benefits. And what about the European research which has found that after people have taken an antidepressant, they become more likely to have subsequent depressive episodes?
- Because of the addictive potential of anti-anxiety drugs, like Xanax and Librium (xxx), antidepressants with sedating side-effect profiles now are prescribed to keep anxiety at bay. What are the effectiveness rates of antidepressants for treating anxiety?
- What about the negative side effects of antidepressants? The Lancet Psychiatry summary article says nothing about these, the most significant of which is drug dependency. Drug dependency means that once people have taken an antidepressant over a significant time period, their body begins to depend on it. The result is that when they try to discontinue taking the medication, their body has a rebound reaction of depression. That depression does not mean that they needed the antidepressant all along. It just means that the drug has caused their body to no longer produce the chemistry of well-being on its own.
A Medications Case Example: Despair, Delight, Disaster, and More
Many thanks to LC, for sharing her antidepressant experiences.
LC: It all started one late afternoon. I was in my car with my toddler-aged son, driving home through typical late afternoon traffic.
Suddenly I smelled the distinct scent of burning. Ahead of me, just five cars away, a plume of neon orange fire was climbing higher and higher. It was so out of place and so sudden that I didn't feel panicked or scared, I just stared for a few seconds, mouth wide open, my brain calibrating a fire on the highway.
Then I saw the people starting to run. And the panic set in. People all around me were jumping out of their cars and running down the highway, away from the gas truck that was literally on fire in front of us. The truck was still mostly intact, and it dawned on me all at once that a larger explosion might be imminent.
I jumped out of the car, pried open the car-seat straps, and then, flinging my son over my shoulder, ran to get as far away from the gasoline truck as possible. There was a BOOM sound, but I didn't look back. I just kept running and saying, "It's OK. We just need to move away from the fire," both to my son, and to myself.
The sirens started. Police and fire-trucks and ambulances somehow made their way through the maze of stopped cars.
A tragic gas leak had killed the driver of the truck. I texted my husband. I called and apologized profusely to my one-year-old's sitter for being so late.
Three hours later, I was on my way home. I had to run to the grocery store, pick up my 4-year-old from preschool, and make dinner. With three young children, I didn't have time to panic, process, or recover. I had to just keep going.

It was only later that night, after 11:00 p.m., that I felt the effects of that experience. My husband tried to calm me down. I was inconsolable. I wanted to scream or cry or run, but I was paralyzed and terrified.
The next day, I couldn't do anything. My anxiety was telling me that I was in danger. I wasn't, but the panic was still there. I was dreading trying to sleep again.
My sister told me to go immediately to a psychiatrist. I did. The psychiatrist talked to me for about 1 minute and then handed me a Xanax (an anti-anxiety pill) and a cup of water: "You are having a panic attack, and you've been in it for almost 24 hours. We need to get you calmed down."
Having a doctor hand me something I could swallow immediately soothed me. I was able finally to speak enough to tell the psychiatrist that I had seen a terrifying accident, and that I had never really suffered from anxiety or panic attacks before. I begged her to please make the anxiety stop.
The psychiatrist prescribed Xanax for a couple of weeks and then Cipralex, a commonly-used SSRI antidepressant that treats both depression and anxiety, to take long-term. She also said that it was imperative that I find a therapist and explore what was going on in my mind. I guess she assumed the trigger was deeper than just seeing a gasoline truck in flames.
Dr. Heitler: Traumatic events can trigger intense panic either during or at some point after the dangerous event has concluded. Eventually, especially with a chance to talk about what happened, the anxiety calms down. In LC’s case however, the parasympathetic nervous system , whose job it is to calm feelings of fear , was not functioning.
Fortunately, the anti-anxiety pill, Xanax, is fast-acting and effective.
Fortunately also, the psychiatrist had suggested that Lia speak with a talk therapist. Talking about the thoughts that were barraging her would enable Lia to digest her thoughts and feelings, both from the recent trauma and from prior events that had troubled her for some time.
Unfortunately, the psychiatrist did not offer non-pill options to calm the intense anxiety reaction. As the saying goes, to a man with a hammer, the world is a nail.
In this case, the hammer was in fact effective. Xanax brought Lia immediate relief. There are, however, non-pill options that can produce the same immediate calming effect. Both acupoint tapping and a visualization called the spinning technique would probably have done the job equally well. In addition, Lia easily could learn to do these techniques on her own at home should the anxiety return.
LC: The thing is . . . I knew that I needed therapy. It had been a long time coming. An unspoken trauma from the past was finding its way out, visiting me in dreams , and violating random moments in my life. I had been doing my best to silence it, shushing it desperately, hoping that it would just go away. So I started therapy. And I started the antidepressant drugs. And I was able to breathe. For a while.
Therapy opened my mind to myself. I had closed it years before. Re-opening it was as if a door had been kicked down. The halls and rooms of my mind were inviting me to explore, to wander, and to get reacquainted with my inner-world.
The SSRI seemed to be working too. I was more calm. I was more at ease. I wasn't barking at my husband about crumbs on the counter or scrubbing toys with bleach every night. I was laughing a little more, yelling a little less, more balanced.
What was from therapy, and what was from the SSRI? I didn’t care. I was just relieved to be breathing normally.
Dr. Heitler: Multiple studies of the treatment of serious emotional distress conclude that the combination of medication and psychotherapy is more potent than either alone. Lia’s case exemplifies this principle. Pills and talk therapy can potentiate each other, that is, cause each other to be more effective than either treatment alone could be.
At the same time, newer therapy techniques, such as the Body Code and Emotion Code, enable a therapist to radically shorten the time and intensity of talk therapy. Within one session or several, an Emotion Code therapist can pinpoint the earlier problem and immediately release trapped negative emotions so that they cease to have impact. With the underground spring that had been feeding anxious, angry and/or depressed feelings turned off, the feelings of vitality and well-being that we call mental health can emerge.
Marriage therapy also might well have helped Lia. My policy is when anyone who is married seeks therapy with me, I encourage them to bring their spouse. In almost all cases, underlying marital issues have been fanning the flames of negative emotions.
The spouse also can have a significant role in fostering a return to mental health. For instance, an anxious or depressed person may have an impulse to spend his evenings isolating and ruminating, saying troubling thoughts over and over to himself. Rumination exacerbates anxiety and depression. If husband and wife enjoy activities together in the evening, they are likely to be able to replace the rumination with pleasant interactions.
LC: I don't regret starting the antidepressant, the Cipralex. I truly feel like that drug saved my mind. It also probably held my marriage together for several more years. But by a year later, I knew that something was off. I knew that it was the medication.
Dr. Heitler: An antidepressant, especially in combination with good talk therapy, can work miracles in enabling people to get back to functioning in a normal emotional zone. The difficulties tend to come with the duration of use.
By prescribing an antidepressant medication and then keeping her on it for more than an initial several months, LC’s psychiatrist had inadvertently invited increasingly negative side effects. The negative side effects which had begun while Lia was taking the pills became even worse when she tried to get off the pills.
LC: At about a year, I started feeling fuzzy, numb, and detached. I would have several-minute episodes of not knowing what I was doing or how I got there. Then the confusion would dissipate, and I would be left thinking that I was just imagining it. But it would happen again. Fleeting, but tangible. Almost leaving a taste in my mouth.
I shared this with my husband, but he was worried about the anxiety returning if I messed with my medication. I waited.
Dr. Heitler: LC’s husband’s concerns had some genuine validity. The difficulty is that after a year of taking antidepressants, anyone who attempts to stop taking them must end their use very slowly. Otherwise, removal of the drugs can precipitate serious depression and/or anxiety.
It’s not that these emotions had been lurking there all along. Rather, antidepressants create drug dependency. The body forgets how to produce the chemicals that sustain well-being when they are being provided artificially by pills.
LC: The side effects worsened. I had no sex drive. I stopped feeling motivated to hang out with friends. I stopped caring about how I looked or what I was wearing. I was sinking. I had been saved from anxiety, and was now slipping into depression.
I made a unilateral decision to go off my meds. It wasn't a wise one. Looking back, I see that it was very much a desperate stand against the many factors in my life that I wasn't in control of — my devastation over my marriage that was quietly but quickly ending, my loss of focus on my passions and hobbies, my overweight and exhausted body, too strict in my religious life . . . the list goes on.
To simply argue that the SSRIs were ruining my life would be short-sighted and most likely wrong. I was ruining my life. But I was absolutely clear that the drug I was putting into my body every day was dragging me down and making it much harder to move forward. I felt very much alone — and for the first time in a while, very clear in my mission.
Dr. Heitler: In addition to creating drug dependency when used for more than several months at a stretch, antidepressants can produce a number of further negative side effects. Weight gain, loss of sexual feelings, emotional numbness, and "brain zapping" are among the most common. LC experienced these, and more.
LC: Going off SSRIs cold-turkey is nothing short of a ride through hell. The physical and emotional effects of suddenly depriving your brain of serotonin is horrific.
I was tormented by anxiety. I experienced electric pulses starting in my head and traveling down my entire body. I found myself in tears over everything. I had so much guilt over the decision. But I couldn't put that pill back in my mouth.
I pushed. It was raw without the drug. My husband and I separated. I said goodbye to God on a park bench and said hello to myself. I sabotaged a friendship — not something I'm proud of. I lost 35 pounds. I started singing out loud. I started running.
I told the psychiatrist what I had done. Even though so many things were better, I was on the verge of another breakdown, and I didn't know what to do.
The psychiatrist prescribed a different drug, this time an SNRI (two chemicals for the brain's "happy" place instead of one). She explained that since I was in the middle of a divorce — a major life-crisis for anyone — it probably wasn't the best time to go off psychiatric drugs.
That night I sat with the new pill in my hand. It took a serious pep talk to swallow it, but I did. I felt like I needed all the help I could get. I had three young children depending on me to keep it together, and I couldn't afford to let emotions destroy me. I had delved extensively into my past and had finally put to rest the lurking earlier trauma. I told myself I would take the drug, and when life settled down, I would get off.
Fast-forward a year and a half. A very similar cycle ensued. At first the SNRI filled me with renewed calm. It was like a rosy tint on life was just a pill away. And then . . . the fog set in. Again, about a year in, I felt that familiar detachment. I stopped caring about the little things. I started to feel like I was being numbed. Like I was underwater. Watching the world from below, too slow to stay actively involved in my own life. My sex drive started dying, and with it, my drive for life deteriorated.
With this new and more powerful drug, I again started feeling physical side effects. If I took the pill a few hours later than usual, I would get extremely nauseous. But if I took it in the morning, I would also get nausea and throw up. On the drug, I was more prone to migraines , I fainted several times that year, and I started gaining weight quite rapidly — despite my strictly healthy lifestyle.
This time around, I was determined to get off the drug safely. I checked in with a doctor. I started by taking off just one-quarter of the dose and did so every four weeks, allowing my brain to adapt each time.
Nonetheless, again it was hard, even painful. Each time I weaned down a dose, I had a week of horrible brain zaps. Even worse, I was much more reactive and impatient with my children. The weaning process took four months.
At the same time, I truly feel like this time around I experienced a beautiful and inspiring rebirth of myself. My senses feel heightened. My experiences are fully my own again.
Dr. Heitler: Paradoxically, ending her use of antidepressants turned out for LC to be the ultimate cure. With the pills no longer compromising her body’s chemistry, LC’s natural vitality eventually returned. So did her sexual feelings, ability to lose weight, eventual loss of the brain zapping, and a return of her former good-humored self.
LC’s conclusions: I'm still forming an objective opinion on the use of SSRIs. The power of these drugs, for better and for worse, is something that shouldn't be taken lightly. Off them now though, for me, heading away from antidepressants is heading in the right direction.
Dr. Heitler’s conclusions: Again, as I said at the outset, for a severe or suicidal depressive episode, antidepressant medications can relieve the intensity of dark thoughts and desperate feelings.
At the same time, Lia’s case illustrates well that antidepressants may:
- Have limited or no effectiveness for almost half of users
- Help somewhat, while many aspects of the depression remain
- Produce problematic side effects, like weight gain, decreased sexual feelings, brain zapping, nausea, clouded thinking, and numbing of feelings of joy as well as of negative emotions
- Create drug dependence when used for longer than a few months, and therefore difficult withdrawal symptoms, including withdrawal-induced depression
- Be prescribed for usages for which they are not intended (i.e., mild depressive reactions and anxiety) and for which non-drug options may be equally effective
- Be prescribed at length, for years rather than months, increasing the difficulties of eventual withdrawal

Susan Heitler, Ph.D ., is the author of many books, including From Conflict to Resolution and The Power of Two . She is a graduate of Harvard University and New York University.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
- Research article
- Open access
- Published: 13 May 2024
Deprescribing of antidepressants: development of indicators of high-risk and overprescribing using the RAND/UCLA Appropriateness Method
- Vita Brisnik 1 , 2 ,
- Jochen Vukas 1 , 2 ,
- Caroline Jung-Sievers 2 , 3 , 4 ,
- Karoline Lukaschek 1 , 2 ,
- G Caleb Alexander 1 , 5 , 6 ,
- Ulrich Thiem 7 , 8 ,
- Petra Thürmann 10 , 9 ,
- Cornelius Schüle 11 ,
- Sebastian Fischer 1 , 12 ,
- Erika Baum 13 ,
- Michael Drey 14 ,
- Sebastian Harder 15 ,
- Wilhelm Niebling 16 ,
- Ulrike Janka 17 ,
- Olaf Krause 18 ,
- Jochen Gensichen 1 , 2 &
- Tobias Dreischulte 1 , 2
for the POKAL-Group
BMC Medicine volume 22 , Article number: 193 ( 2024 ) Cite this article
203 Accesses
2 Altmetric
Metrics details
Antidepressants are first-line medications for many psychiatric disorders. However, their widespread long-term use in some indications (e.g., mild depression and insomnia) is concerning. Particularly in older adults with comorbidities and polypharmacy, who are more susceptible to adverse drug reactions, the risks and benefits of treatment should be regularly reviewed. The aim of this consensus process was to identify explicit criteria of potentially inappropriate antidepressant use (indicators) in order to support primary care clinicians in identifying situations, where deprescribing of antidepressants should be considered.
We used the RAND/UCLA Appropriateness Method to identify the indicators of high-risk and overprescribing of antidepressants. We combined a structured literature review with a 3-round expert panel, with results discussed in moderated meetings in between rounds. Each of the 282 candidate indicators was scored on a 9-point Likert scale representing the necessity of a critical review of antidepressant continuation (1–3 = not necessary; 4–6 = uncertain; 7–9 = clearly necessary). Experts rated the indicators for the necessity of review, since decisions to deprescribe require considerations of patient risk/benefit balance and preferences. Indicators with a median necessity rating of ≥ 7 without disagreement after 3 rating rounds were accepted.
The expert panel comprised 2 general practitioners, 2 clinical pharmacologists, 1 gerontopsychiatrist, 2 psychiatrists, and 3 internists/geriatricians (total N = 10). After 3 assessment rounds, there was consensus for 37 indicators of high-risk and 25 indicators of overprescribing, where critical reviews were felt to be necessary. High-risk prescribing indicators included settings posing risks of drug-drug, drug-disease, and drug-age interactions or the occurrence of adverse drug reactions. Indicators with the highest ratings included those suggesting the possibility of cardiovascular risks (QTc prolongation), delirium, gastrointestinal bleeding, and liver injury in specific patient subgroups with additional risk factors. Overprescribing indicators target patients with long treatment durations for depression, anxiety, and insomnia as well as high doses for pain and insomnia.
Conclusions
Explicit indicators of antidepressant high-risk and overprescribing may be used directly by patients and health care providers, and integrated within clinical decision support tools, in order to improve the overall risk/benefit balance of this commonly prescribed class of prescription drugs.
Peer Review reports
Antidepressants are first-line medications for many psychiatric disorders (including depression, anxiety disorders, and obsessive–compulsive disorder) and have proven to have substantial benefits particularly in patients with moderate to severe symptoms of depression or anxiety disorders [ 1 ]. Antidepressants are also some of the most commonly prescribed prescription drugs globally, and their use has increased over time. For example, according to one cross-sectional study in the USA, the proportion of persons aged ≥ 18 years using antidepressants increased by 60% from 6.5 to 10.4% between 1999 and 2010 [ 2 ]. More recently, the volume of antidepressant prescribing increased by 97% in England between 2008 and 2018 [ 3 ] and by 30% in Germany between 2012 and 2021 [ 4 ]. Increased use is desirable if this reflects increased awareness and diagnoses of mental health conditions and reduced stigma associated with affective disorders. However, the increasing use of antidepressants for longer durations than recommended by the guidelines has also been identified as a key driver [ 5 ]. General practitioners typically manage maintenance treatment with antidepressants and are therefore often faced with decisions around continuing or deprescribing antidepressants.
While antidepressants play an important role in the pharmacologic management of common and debilitating psychiatric illnesses as well as neuropathic pain and migraine, medication review interventions show they are also used in situations where they may have an unfavorable risk/benefit balance. For example, in one prospective cohort study, antidepressant use could be stopped, reduced, or switched (deprescribed) in almost one-quarter (23.2%) of antidepressant users [ 6 ]. Potential indications for stopping antidepressants in primary care include their use in mild forms of depression (where benefits are limited [ 1 , 7 , 8 ]), their long-term use for non-psychiatric illnesses such as primary sleep disorders [ 9 , 10 ], and excessive treatment durations [ 5 , 11 , 12 , 13 ]. Newer generation antidepressants (e.g., selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SNRIs)) are generally considered safer than traditional ones (e.g., tricyclic antidepressants (TCAs)) [ 14 ]. However, even SSRIs and SNRIs are not risk-free, especially among vulnerable older people, where long treatment durations are particularly common [ 15 , 16 , 17 ] and where comorbidity and comedication may increase the risk of adverse effects, such as falls and fractures, gastrointestinal bleeding, electrolyte imbalances, and cardiovascular events [ 18 , 19 , 20 , 21 ]. For example, a recent systematic review shows that antidepressants as a group are associated with a significantly increased risk of falls (odds ratio 1.57 [95% confidence interval (CI) 1.43–1.74]) [ 20 ], and in one observational study, the 1-year numbers needed to harm for fractures were 247 (for SSRIs) and 308 (for TCAs) among 65 to 74-year-olds, and 53 and 81 for people 75 years or older, respectively, while mirtazapine only significantly increased fracture risk among the older age group [ 22 ].
Despite the opportunities to improve the overall risk/benefit balance of antidepressant use in clinical practice, such opportunities may easily be overlooked by primary care clinicians due to competing priorities. The explicit criteria could help alert prescribers to consider deprescribing where indicated, even when decisions to deprescribe require considerations of patient-specific balance of benefits and risks as well as patient preferences. In addition to discontinuing antidepressants, deprescribing may also encompass dose reduction or switching to a safer agent, which may be the preferred option if antidepressant therapy continues to be necessary to control symptoms. Although existing generic lists of potentially inappropriate medication (PIM) generally advise caution in the use of antidepressants in the elderly [ 23 ], more specific advice as to when deprescribing of antidepressants should be considered is desirable to guide the identification of deprescribing opportunities. As an aid to encourage antidepressant deprescribing where indicated, the aim of this study was to establish evidence-based expert consensus on situations, where a critical review of antidepressant continuation would be warranted in primary care. We envisioned that by prompting earlier and proactive reviews of antidepressant use, the resulting set of explicit criteria could help prevent antidepressant-related harm, especially in vulnerable older people.
Study design
We used a consensus process based on the RAND/UCLA (University of California) Appropriateness Method (RAM) [ 24 ] to develop our indicators. First, we assembled a list of candidate indicators based on a structured literature review including primary and secondary English and German literature sources. The candidate indicators were subjected to a three-round expert consensus process, with feedback and synchronous discussion of first and second round ratings before second and third round ratings were placed, respectively.
Selection of the expert panel
We recruited a diverse set of experts with clinical or scientific experience in the use of antidepressants from different fields of professional practice in order to achieve a broad range of perspectives and expertise. We therefore recruited general practitioners, psychiatrists, geriatricians, a gerontopsychiatrist, and clinical pharmacologists from Germany. We identified an initial set of 20 potential experts using our professional networks, planning for the ultimate inclusion of approximately 12 participants. Experts participating in the consensus process did not receive any compensation for their participation.
Identification of candidate indicators
Definitions.
For the purposes of this study, we distinguished between two types of settings, where antidepressant deprescribing should be considered. We defined high-risk prescribing as the use of antidepressants in the presence of risk factors increasing the likelihood of an adverse drug reaction (ADR), whether comedication (drug-drug interactions), comorbidities (drug-disease interactions), or advanced age (drug-age interactions). We defined overprescribing as the use of antidepressants for longer periods than indicated or for indications without evidence of relevant benefit or at higher doses than indicated. We included SSRIs, SNRIs, TCAs, monoamine oxidase inhibitors (MAOIs), and atypical antidepressants such as mirtazapine, trazodone, bupropion, agomelatine, and opipramol in this study. Structurally, opipramol belongs to the class of TCAs and is widely prescribed in Germany for insomnia.
- High-risk prescribing
In order to identify candidate indicators of high-risk prescribing of antidepressants, we initially searched for previously developed indicators targeting potentially inappropriate antidepressant prescribing [ 25 , 26 , 27 , 28 , 29 ]. We also considered systematic and clinical reviews of adverse antidepressant effects as well as clinical practice guidelines in English and German language. Based on consensus among a subset of co-authors (T.D. and V.B.), we prioritized ADRs for which a continuation of antidepressant use could either lead to serious harm, such as hospital admission, or severely affect patients’ quality of life. We conducted further searches in PubMed/MEDLINE and EMBASE to identify candidate indicators linked to each ADR of interest. To this end, we conducted searches including carefully selected (MeSH and non-Mesh) terms for each specific adverse drug reaction of interest and combined these with terms for each group of antidepressants (e.g., SSRIs). We initially searched for recent systematic reviews and meta-analyses but also considered primary literature where reviews were not available or required updating. If applicable, we also examined the reference lists of important reviews for additional studies. We provide more details of the literature search and the search terms used in Additional file 1 .
- Overprescribing
In order to identify candidate indicators of overprescribing of antidepressants, we considered clinical practice guidelines in English and German languages for depression, anxiety and panic disorders, insomnia, and pain [ 30 , 31 , 32 , 33 ]. We searched for recommendations concerning treatment duration and the recommended doses when prescribed for insomnia and pain. In addition, we also searched for clinical guideline recommendations (e.g., for dementia) specifically not recommending antidepressants for a first depressive episode.
Design of the rating form and supporting materials
Members of the expert panel were sent the following materials: the rating form, a summary of clinical evidence summary, and rating instructions. The rating form included the candidate indicators, which were organized into 2 sections (high-risk and overprescribing), and each section was divided into chapters. In the high-risk prescribing section, there were 23 chapters for candidate indicators relating to each ADR (e.g., fall, GI bleeding), while in the overprescribing section, there was 1 chapter for candidate indicators relating to each indication (depression, anxiety, insomnia, pain). The indicators followed a standardized format and were designed as variations around the same topic in order to determine thresholds beyond which a critical review would be considered necessary (1 example is provided in Table 1 ). For each chapter, we developed a summary of clinical evidence supporting the candidate indicators to be considered by the expert panel as part of the rating process. The rating instructions defined rating constructs and assumptions and provided guidance on how the rating form was to be completed and returned.
We piloted the rating form, the summary of clinical evidence, and the supporting instructional materials with one psychiatrist, one clinical pharmacologist, and one general practitioner, using their feedback to optimize the final version of the first round survey. All materials are available from the authors upon request.
Rating constructs and scales
Each expert rated each candidate indicator based on a 9-point Likert scale representing the necessity of a critical review of that particular clinical instance (1 to 3 = not necessary; 4 to 6 = might be necessary; 7 to 9 = clearly necessary). We also asked experts to rate the subset of indicators reflecting high-risk prescribing for “likelihood of harm,” and each linked ADR was additionally rated for “severity of harm.” For all candidate indicators, the necessity to review was the decisive criterion for the acceptance of indicators, and we used these latter ratings to inform discussion in case of disagreements.
Necessity of review
We asked for the necessity of review rather than the necessity of deprescribing since deprescribing decisions may depend on a patient-specific balance of benefits and risks as well as patient preferences, which are unfeasible to pre-specify. We defined “critical review” as a critical assessment of the balance of benefits and risks of antidepressant use to be conducted within 3 months, which would involve patient empowerment and shared decision-making and take at least 30 min to conduct. A critical review may result in dose reduction, switching, or discontinuation of an antidepressant (deprescribing). Consistent with RAM, we defined “necessary” to mean that omitting the review would be considered improper care, that conducting the review would have a reasonable chance of benefitting the patient and that the benefit is not small (Table 2 ).
Likelihood and severity of harm
We defined likelihood of harm as the likelihood of the adverse drug reaction happening if the clinical situation was to be continued for another year and severity of harm as the severity of the harm if the adverse drug events happened as a result of antidepressant use.
Rating scales
We used ordinal scales of 1 to 9 for all ratings. We pre-specified that an indicator would be accepted as necessary , when the median across all expert assessments was ≥ 7, and there was no disagreement. Disagreement was pre-specified to mean that at least 30% of the experts rated items 1–3, and at least 30% rated items 7–9. Candidate indicators with a median of < 7 or disagreement were rejected.
RAM process
The RAM process comprised two virtual discussions and three rating rounds. All expert panel members were sent the first RAM survey by e-mail (on 01/08/2022), together with a one-page overview of the project, rating instructions, and the summarized clinical evidence for each overarching topic. Experts were instructed to place their ratings based on both the evidence report and clinical judgment. The experts were instructed to place their ratings in relation to an average patient on antidepressants treated in primary care. The panel members were given 4 weeks to complete the first round of the RAM survey.
The experts met in a moderated videoconference (moderated by TD) on 01/09/2022. The first round assessments were summarized and presented to the experts, highlighting the median and distribution of ratings as well as the presence of disagreement. The focus of the videoconference was the discussion of indicators with disagreement for the necessity ratings after the first round assessment. After discussing the candidate indicators relating to each ADR (in case of high-risk prescribing indicators) or each indication (in case of overprescribing indicators), the panel members had time to complete the second round assessment.
Indicators reaching a median of ≥ 7 after the second round of assessment were summarized, and the redundant indicators were removed (see Table 1 for an example). The pre-final list of indicators was sent to expert panel members on 24/02/2023. The experts met on 16/03/2023 for a second virtual discussion. The summarized list of indicators allowed the experts to discuss the remaining indicators in more detail and if necessary optimize them for implementation in primary care. Requests for changes in the indicators were implemented and put to a final vote in a third rating round using the same rating constructs and scales as before.
Expert panel composition
The first round RAM survey was sent to 11 expert panel members. All 11 experts participated in the moderated videoconference, and 10 (90.9%) members successfully completed the second and third round survey (general practitioners ( n = 2), clinical pharmacologists ( n = 2), psychiatrists ( n = 2), geriatricians ( n = 3), and a gerontopsychiatrist ( n = 1)). All 10 experts were clinically trained physicians (with an average [range] of 30 [13 to 46] years since training) with regular patient care experience, and 9 (90.0%) also had current research experience.
Candidate indicators
The literature search identifying potential candidate indicators yielded a recent systematic review that contained an extensive list of potential prescribing safety indicators related to mental health [ 34 ]. Antidepressant-associated indicators from this review were combined with those included in commonly used PIM lists [ 25 , 26 , 27 , 28 , 29 ]. Further high-risk prescribing candidate indicators were identified from clinical practice guidelines, such as those for depression or chronic heart failure [ 30 , 35 ], literature reviews of adverse events associated with antidepressant drugs [ 14 , 36 , 37 , 38 ], and further reviews from searches for selected ADRs (detailed in Additional file 1 ). The first round of the survey included 212 variations of potential candidate indicators for high-risk prescribing. It should be noted that many indicators were highly dose-specific, e.g., experts were asked to differentiate between the risk of different dose levels of TCAs per day and also between the risk of synergistic pharmacological effects combining 2 or more drugs (e.g., with anticholinergic properties). This allowed for a very fine differentiation between potentially high-risk constellations.
For depression and anxiety, the indicators of overprescribing focused mainly on the duration of treatment without symptom improvement or on the total duration of treatment. With the exception of doxepin, antidepressants are not officially approved for insomnia, and guidelines are not clear on dose recommendations or duration of treatment for antidepressants as a sedative [ 32 ]. Dose recommendations were also considered for pain [ 33 ]. The first round of the survey included 70 variations of potential candidate indicators for overprescribing.
Figure 1 shows that after round 1, 121 (57.1%) of 212 candidate indicators were accepted as “clearly necessary to review.” Six indicators (2.8%) were consented as “not necessary” and 81 indicators (38.2%) as “might be necessary to review.” There was disagreement for 4 indicators (1.9%). Changes after the first round assessment and during the moderated videoconference resulted in 222 potential high-risk prescribing indicators being rated in the second round, of which 129 candidate indicators (58.1%) were accepted as “clearly necessary,” 6 indicators (2.7%) as “not necessary,” and 86 indicators (38.7%) as “might be necessary to review.” There was disagreement for 1 indicator (0.5%). We provide the expert ratings of round 2 in Additional file 2 . Removing redundant candidate criteria yielded 50 indicators for high-risk prescribing. After the second moderated videoconference, 37 remaining indicators were validated in the third round of assessment, and all were agreed to be “clearly necessary to review.” Changes to the indicators after the second round of assessment and the rationale for the changes are detailed in Additional file 3 . Table 3 reports the consented indicators after the third round of assessment. Prioritized indicators target patients who are particularly vulnerable to (risk factors: drug-drug, drug-disease, or drug-age interactions) or who have developed adverse drug reactions. High-risk prescribing indicators included constellations of known anticholinergic (e.g., cognitive decline, delirium, constipation, voiding disorders, and glaucoma) and cardiovascular (e.g., QTc prolongation) risks but also falls, orthostatic hypotension/dizziness, bleeding, serotonin syndrome, hyponatremia, hepatic injury, sleep disturbances, and sexual dysfunction. Some of these constellations could lead to serious harm, if antidepressants are continued, particularly in older adults with comedication and comorbidities (e.g., cardiovascular adverse effects, fall-related injuries, delirium, gastrointestinal and intracranial bleeding, hyponatremia). The remaining constellations with the corresponding adverse drug reactions can severely affect patients’ quality of life (constipation, sleep disturbances, and sexual dysfunction). Indicators with the highest ratings (median = 9) included those suggesting the possibility of cardiovascular risks such as QTc prolongation associated with citalopram and escitalopram, delirium associated with anticholinergic antidepressants, gastrointestinal bleeding associated with SSRIs and SNRIs, and liver injury associated with agomelatine.
Flow chart showing the RAM process. *Not clearly necessary: might be necessary 4 to 6 or not necessary 1 to 3
Fig. 1 shows that after round 1, 52 (74.3%) of 70 candidate indicators were accepted as “clearly necessary to review.” One indicator (1.4%) was consented as “not necessary” and 6 indicators (8.6%) as “might be necessary to review.” There was disagreement for eleven indicators (15.7%). A total of 53 candidate indicators (75.7%) were accepted as “clearly necessary,” 0 indicators (0%) as “not necessary,” and 12 indicators (17.1%) as “might be necessary to review” in the second round of assessment. There was disagreement for 5 indicators (7.1%). We provide the expert ratings of round 2 in Additional file 4 . Removing redundant candidate criteria yielded 27 indicators for overprescribing. After the second moderated videoconference, 25 remaining indicators were validated in the third round of assessment, and all were agreed to be “clearly necessary to review.” Table 4 reports the consented indicators after the third round of assessment. Prioritized indicators target patients who have a high medication burden potentially associated with antidepressants due to long treatment durations, inappropriate indications, or high doses.
Summary of findings
Antidepressants are some of the most commonly prescribed drugs in the world. Despite their value, there are instances where they may have an unfavorable risk/benefit balance. We performed a structured literature review and expert consensus process (RAM) in order to synthesize and reach consensus on a set of 62 explicit indicators (37 indicators of high-risk prescribing and 25 indicators of overprescribing of antidepressants) that should prompt a critical review of antidepressant continuation. Indicators with the highest ratings included those suggesting the possibility of cardiovascular risks such as QTc prolongation, delirium, gastrointestinal bleeding, and liver injury associated with certain antidepressants in specific patient subgroups with additional risk factors.
Comparison to literature
To the best of our knowledge, this is the first consensus study focused on identifying indicators for high-risk and overprescribing of antidepressants. Compared to more generic lists of potentially inappropriate medications [ 23 , 39 ], our focus on a specific class of drugs allowed for the development of a comprehensive set of indicators specifically related to antidepressants. For example, STOPP (Screening Tool of Older Person’s Prescriptions/START (Screening Tool to Alert doctors to Right Treatment) version 3 includes 10 indicators related to antidepressants (7.5%) [ 23 ], while FORTA (Fit fOR The Aged) identifies individual antidepressants for 6 indications [ 39 ]. In comparison, this study identified 37 high-risk prescribing indicators related to a broad spectrum of adverse outcomes. Our findings also include certain risks that are inconsistently listed in clinical guidelines, such as bleeding and fall risks associated with SSRIs, despite systematic reviews supporting these risks [ 18 , 20 ].
Although broadly consistent with previously published tools for identifying PIMs [ 23 ], some differences are worth highlighting. First, the indicator set developed here is likely to identify more patients at risk of bleeding. For example, in contrast to the STOPP criteria, our set also considers the bleeding risk associated with SNRIs [ 40 , 41 ] as well as co-prescription with nonsteroidal anti-inflammatory drugs (NSAIDs) and/or antiplatelets [ 40 , 42 ]. Second, in contrast to STOPP Fall, our expert panel did not confirm a higher fall risk for tricyclic antidepressants than other antidepressants [ 43 ], and our set identifies additional patients at risk for falls, such as those with cognitive impairment or dementia. Third, our set identifies a particular need to review antidepressants in patients with hyponatremia who are not co-prescribed diuretics (which would then primarily require review) and also accounts for the co-prescription of antidepressants with other hyponatremia-inducing drugs. Fourth, unlike previously published lists [ 23 ], our indicator set considers the risk of insomnia with activating antidepressants (such as SSRIs, SNRIs, MAOIs, or bupropion). Fifth, our indicators also identify antidepressant risks related to serotonin syndrome, hepatic injury, and sexual dysfunction, which are usually not included in PIM lists as they are not unique to older adults. Several factors may contribute to these differences, including our focus on identifying patients in need of a review specifically targeting antidepressants, the composition of our expert panel, and the evolution of clinical evidence.
Strengths and limitations
Our study has several strengths. First, an important advantage of the RAM compared to the commonly used Delphi process is that panelists have the opportunity to exchange perspectives in between rounds and for moderators to ensure that rating constructs are understood correctly and applied consistently. Second, our expert panel included generalists and specialists that promoted informed discussions regarding how to optimally balance comprehensiveness, relevance, and feasibility of implementation in primary care. Third, our indicators present a more holistic view of the patient and his or her individual situation combining patient-specific risk factors (e.g., certain comorbidities, co-prescribed medications). Moreover, pharmacological features such as dose-related and synergistic effects were taken into account. While the experts practiced in Germany, our literature review and supporting evidence base were comprehensive and international in scope. Although we cannot exclude that the selection and wording of candidate indicators may have influenced our findings, all experts were given an opportunity to suggest additional indicators and clarify ambiguous wording during panel meetings. Our indicator set focuses on a broad set of adverse effects and common indications for antidepressant use, but it is important to note that it cannot cover all instances of overprescribing or sources of antidepressant-related adverse events.
Implications for clinical practice and research
The indicators consented in this study may be used to inform clinical practice as well as clinical surveillance and research. Clinical practice guidelines typically focus on the appropriate use of antidepressants but do not explicitly state when their use may require caution or review with a view to deprescribing. This set of indicators may therefore complement such guidelines and could be used in conjunction with other established PIM lists [ 23 , 39 , 44 ]. Decision aids, ideally implemented in practice management systems, can trigger a process of shared decision-making, thereby strengthening the physician–patient interaction, ensuring desired effects, and preventing adverse effects of antidepressants before they occur. Indicators could also be used as a decision aid prior to starting antidepressants, but this may not be sufficient given that patients’ clinical circumstances may change during treatment. The indicators could also be used to monitor antidepressant use at the population level and as endpoints to evaluate the impact of interventions to enhance the appropriate use of antidepressants in primary care. The indicators may also be useful in informing and empowering patients, which may be particularly relevant in disjointed health care systems, where changes in comorbidity and comedication that could unfavorably affect the benefit/risk ratio of antidepressant use may remain unnoticed by the antidepressant prescriber. However, providing detailed information about potential risks must be balanced against the risk of adversely affecting patient adherence.
In addition, it is important to note that despite its potential benefits, deprescribing antidepressants implies a risk of disease recurrence and withdrawal symptoms. The risk of the latter can be reduced by close monitoring and timely adaptation of tapering schemes, but their implementation may be time-consuming to clinicians and patients alike. The indicators developed here may therefore only serve as a prompt to consider deprescribing, but whether deprescribing should be attempted (or whether alternative measures to reduce the risk of adverse effects are preferable or suffice) requires clinicians to consider individual patient circumstances and also patient preferences. In cases where an adverse drug reaction from antidepressants is suspected (e.g., sexual dysfunction or insomnia), it is also important to carefully consider whether there may be alternative causes prior to changing treatment. In addition, whether and to which extent the implementation of the indicators developed here produces a net benefit to patients and/or health care systems requires evaluation in prospective studies.
This study has identified a comprehensive set of clinical situations that require a timely critical review of the continuation or deprescribing of antidepressants. It thereby closes an important gap in the current clinical guidelines, which has the potential to counterbalance the use of antidepressants in situations, where they have no relevant benefit, no longer have relevant benefit, or are associated with a high risk of harm. While antidepressants have an irreplaceable role in the treatment of moderate to severe forms of depression and anxiety disorders, in some cases (e.g., in combination with comedication, comorbidity, or age), the risks may outweigh the benefits of therapy, particularly in cases involving milder symptoms as frequently observed in primary care. If the use of the indicators developed here leads to a negative benefit-risk assessment, decisions to deprescribe antidepressant treatment should also take into account the potential harms of deprescribing, including withdrawal symptoms and a potential relapse of symptoms (which may occur with some latency), particularly in those with a history of severe psychiatric disorders. It is also important to note that in some cases, dose reduction or switching to a safer antidepressant may be a better alternative than discontinuation. The explicit indicators of high-risk and overprescribing of antidepressants developed here may be used directly by patients and health care providers in primary care, as well as integrated within clinical decision support tools, in order to improve the overall risk/benefit balance of this commonly prescribed class of prescription drugs. Further research is underway (as part of the POKAL project [ 45 ]) to examine the prevalence and longitudinal time trends of the developed indicators using claims data, to examine their acceptability among primary care clinicians, and to evaluate the performance (sensitivity and specificity) of the indicator set in identifying actual opportunities for antidepressant deprescribing.
Availability of data and materials
The data generated during this study are included in this published article in Tables 3 and 4 [and in Additional files 2 and 4 ]. Further supporting materials relating to the RAM process described in this article are available upon request.
Abbreviations
Adverse drug reaction
Fit fOR The Aged
Monoamine oxidase inhibitors
Nonsteroidal anti-inflammatory drugs
Potentially inappropriate medication
RAND/UCLA Appropriateness Method
Selective serotonin-norepinephrine reuptake inhibitors
Selective serotonin reuptake inhibitors
Screening Tool of Older Person’s Prescriptions/Screening Tool to Alert doctors to Right Treatment
Tricyclic antidepressant
Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53.
Article CAS PubMed PubMed Central Google Scholar
Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75(2):169–77.
Article CAS PubMed Google Scholar
Iacobucci G. NHS prescribed record number of antidepressants last year. BMJ. 2019;364:l1508.
Article PubMed Google Scholar
Ludwig W, Mühlbauer B, Seifert R. Arzneiverordnungs-Report 2022. Berlin/Heidelberg: Springer; 2023, p. 347, pp 387–409.
Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ. 2009;339: b3999.
Article PubMed PubMed Central Google Scholar
Johnson CF, Macdonald HJ, Atkinson P, Buchanan AI, Downes N, Dougall N. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. Br J Gen Pract. 2012;62(604):e773–9.
Arroll B, Roskvist R, Moir F, Harwood M, Eggleton K, Dowrick C, et al. Antidepressants in primary care: limited value at the first visit. World Psychiatry. 2023;22(2):340.
Barbui C, Cipriani A, Patel V, Ayuso-Mateos JL, van Ommeren M. Efficacy of antidepressants and benzodiazepines in minor depression: systematic review and meta-analysis. Br J Psychiatry. 2011;198(1):11–6 (sup 1).
Schäfer W, Reinders T, Riedel O, Haug U. How often are antidepressants prescribed off-label among older adults in Germany? A claims data analysis. Br J Clin Pharmacol. 2021;87(4):1778–89.
Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356: j603.
Lunghi C, Antonazzo IC, Burato S, Raschi E, Zoffoli V, Forcesi E, et al. Prevalence and determinants of long-term utilization of antidepressant drugs: a retrospective cohort study. Neuropsychiatr Dis Treat. 2020;16:1157–70.
Mars B, Heron J, Kessler D, Davies NM, Martin RM, Thomas KH, et al. Influences on antidepressant prescribing trends in the UK: 1995–2011. Soc Psychiatry Psychiatr Epidemiol. 2017;52(2):193–200.
McCool A, Lukas K, Hayes P, Kelly D. Antidepressant medication prescribing patterns in Irish general practice from 2016 to 2020 to assess for long-term use. Ir J Med Sci. 2022;191(5):2239–46.
Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–88.
Huijbregts KM, Hoogendoorn A, Slottje P, van Balkom A, Batelaan NM. Long-term and short-term antidepressant use in general practice: data from a large cohort in the Netherlands. Psychother Psychosom. 2017;86(6):362–9.
Dörks M, Hoffmann F, Jobski K. Antidepressant drug use and regional prescribing patterns in Germany: results from a large population-based study. International Clinical Psychopharmacology. 2022;37(5):185–92.
O’Neill A, McFarland J, Kelly D. Long-term antidepressant use in a cohort of older people. Int J Geriatr Psychiatry. 2021;36(8):1241–51.
Jiang HY, Chen HZ, Hu XJ, Yu ZH, Yang W, Deng M, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13(1):42-50.e3.
Bansal N, Hudda M, Payne RA, Smith DJ, Kessler D, Wiles N. Antidepressant use and risk of adverse outcomes: population-based cohort study. BJPsych Open. 2022;8(5): e164.
Seppala LJ, Wermelink A, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics J Am Med Dir Assoc. 2018;19(4):371 e11-e17.
Google Scholar
De Picker L, Van Den Eede F, Dumont G, Moorkens G, Sabbe BG. Antidepressants and the risk of hyponatremia: a class-by-class review of literature. Psychosomatics. 2014;55(6):536–47.
Hauff J, Rottenkolber M, Oehler P, Fischer S, Gensichen J, Drey M, et al. Single and combined use of fall-risk-increasing drugs and fracture risk: a population-based case-control study. Age Ageing. 2023;52(6):afad079.
O’Mahony D, Cherubini A, Guiteras AR, Denkinger M, Beuscart JB, Onder G, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625–32.
Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA Appropriateness Method user’s manual. Santa Monica, CA: RAND Corporation; 2001.
Pazan F, Weiss C, Wehling M. The EURO-FORTA (Fit fOR The Aged) List: international consensus validation of a clinical tool for improved drug treatment in older people. Drugs Aging. 2018;35(1):61–71.
American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94.
Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107(31–32):543–51.
PubMed PubMed Central Google Scholar
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
Khawagi WY, Steinke DT, Nguyen J, Pontefract S, Keers RN. Development of prescribing safety indicators related to mental health disorders and medications: modified e-Delphi study. Br J Clin Pharmacol. 2021;87(1):189–209.
DGPPN B, KBV A, AkdÄ B, BApK D, DEGAM D, DGPs D. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression-Langfassung. Auflage Version. 2015;5:2015.
Bandelow B, Aden I, Alpers GW, Benecke A, Benecke C, Beutel ME, Deckert J, Domschke K, Eckhardt-Henn A, Geiser F, Gerlach AL, Harfst TH, Hoffmann S, Hoyer J, Hunger-Schoppe C, Kellner M, Köllner V, Kopp I, Langs G, Liebeck H, Matzat J, Ohly M, Rüddel HP, Rudolf S, Scheufele E, Simon R, Staats H, Ströhle A, Waldherr B, Wedekind D, Werner AM, Wiltink J, Wolters JP. Deutsche s3-leitlinie behandlung von angststörungen, version 2. AWMF. 2021.
Riemann D, Baum E, Cohrs S, Crönlein T, Hajak G, Hertenstein E, et al. S3-Leitlinie nicht erholsamer schlaf/schlafstörungen. Somnologie. 2017;21(1):2–44.
Article Google Scholar
Schlereth T. S2k-leitlinie: diagnose und nicht interventionelle Therapie neuropathischer Schmerzen. DGNeurologie. 2020;3(1):21–40.
Khawagi WY, Steinke DT, Nguyen J, Keers RN. Identifying potential prescribing safety indicators related to mental health disorders and medications: a systematic review. PLoS ONE. 2019;14(5):e0217406.
Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz – Langfassung, 3. Auflage. Version 3. 2019 [cited: 2023-09-10]. https://doi.org/10.6101/AZQ/000482 .
Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–62.
Whiskey E, Taylor D. A review of the adverse effects and safety of noradrenergic antidepressants. J Psychopharmacol. 2013;27(8):732–9.
Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, et al. Selective serotonin reuptake inhibitors and adverse effects: a narrative review. Neurol Int. 2021;13(3):387–401.
Pazan F, Weiss C, Wehling M. The EURO-FORTA (Fit fOR The Aged) List version 2: consensus validation of a clinical tool for improved pharmacotherapy in older adults. Drugs Aging. 2023;40(5):417–26.
Nochaiwong S, Ruengorn C, Awiphan R, Chai-Adisaksopha C, Tantraworasin A, Phosuya C, et al. Use of serotonin reuptake inhibitor antidepressants and the risk of bleeding complications in patients on anticoagulant or antiplatelet agents: a systematic review and meta-analysis. Ann Med. 2022;54(1):80–97.
Mawardi G, Markman TM, Muslem R, Sobhanian M, Converse M, Meadows HB, et al. SSRI/SNRI therapy is associated with a higher risk of gastrointestinal bleeding in LVAD patients. Heart Lung Circ. 2020;29(8):1241–6.
Alam SM, Qasswal M, Ahsan MJ, Walters RW, Chandra S. Selective serotonin reuptake inhibitors increase risk of upper gastrointestinal bleeding when used with NSAIDs: a systemic review and meta-analysis. Sci Rep. 2022;12(1):14452.
Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbińska K, et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing. 2021;50(4):1189–99.
Mann NK, Mathes T, Sönnichsen A, Pieper D, Klager E, Moussa M, et al. Potentially inadequate medications in the elderly: PRISCUS 2.0. Dtsch Arztebl Int. 2023;120(1–2):3–10.
Gensichen J, Lukaschek K, Jung-Sievers C, Falkai P, Schmitt A, Henningsen P, et al. Predictors and outcomes in primary depression care (POKAL) – a research training group develops an innovative approach to collaborative care. BMC Primary Care. 2022;23(1):309.
Download references
Acknowledgements
The POKAL-Group (PrädiktOren und Klinische Ergebnisse bei depressiven ErkrAnkungen in der hausärztLichen Versorgung (POKAL, DFG-GrK 2621)) consists of the following principle investigators: Tobias Dreischulte, Peter Falkai, Jochen Gensichen, Peter Henningsen, Markus Bühner, Caroline Jung-Sievers, Helmut Krcmar, Karoline Lukaschek, Gabriele Pitschel-Walz, and Antonius Schneider Kirsten Lochbuhler, Barbara Prommegger, Andrea Schmitt. The following doctoral students are members of the POKAL-Group: Katharina Biersack, Constantin Brand, Christopher Ebert, Julia Eder, Feyza Gökce, Carolin Haas, Lisa Hattenkofer, Lukas Kaupe, Jonas Raub, Philipp Reindl-Spanner, Hannah Schillok, Petra Schönweger, Clara Teusen, Marie Vogel, Victoria von Schrottenberg, Jochen Vukas, and Puya Younesi Vita Brisnik. We also want to thank all the experts for their participation in the RAM survey.
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the German Research Foundation (Deutsche Forschungsgesellschaft, https://www.dfg.de/ ) (grant no. GrK 2621). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.

Author information
Authors and affiliations.
Institute of General Practice and Family Medicine, LMU University Hospital, LMU Munich, Munich, Germany
Vita Brisnik, Jochen Vukas, Karoline Lukaschek, G Caleb Alexander, Sebastian Fischer, Jochen Gensichen & Tobias Dreischulte
Graduate Program “POKAL - Predictors and Outcomes in Primary Care Depression Care”, (DFG - GrK 2621), Munich, Germany
Vita Brisnik, Jochen Vukas, Caroline Jung-Sievers, Karoline Lukaschek, Jochen Gensichen & Tobias Dreischulte
Institute of Medical Data Processing, Biometrics and Epidemiology (IBE), Faculty of Medicine, LMU Munich, Munich, Germany
Caroline Jung-Sievers
Pettenkofer School of Public Health, Munich, Germany
Center for Drug Safety and Effectiveness, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
G Caleb Alexander
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Ulrich Thiem
Department of Geriatrics, Albertinen-Haus, Hamburg, Germany
Chair of Clinical Pharmacology, Faculty of Health, Department of Medicine, University Witten/Herdecke, Witten, Germany
Petra Thürmann
Philipp Klee-Institute of Clinical Pharmacology, Helios University Hospital Wuppertal, Wuppertal, Germany
Department of Psychiatry and Psychotherapy, LMU University Hospital, LMU Munich, Munich, Germany
Cornelius Schüle
Psychiatric Services Lucerne, Lucerne, Switzerland
Sebastian Fischer
Institute of General Practice and Family Medicine, Philipps University Marburg, Marburg, Germany
Department of Medicine IV, Geriatrics, LMU University Hospital, LMU Munich, Munich, Germany
Michael Drey
Institute for Clinical Pharmacology, University Hospital, Goethe University Frankfurt, Frankfurt, Germany
Sebastian Harder
Department of Medicine, Division of General Practice, Medical Center, University of Freiburg, Freiburg, Germany
Wilhelm Niebling
Department of Psychiatry and Psychotherapy, Paracelsus Medical University Nuremberg, Nuremberg, Germany
Ulrike Janka
Institute of General Practice and Palliative Medicine, Medical School Hannover, Hannover, Germany
Olaf Krause
You can also search for this author in PubMed Google Scholar
- Tobias Dreischulte
- , Peter Falkai
- , Jochen Gensichen
- , Peter Henningsen
- , Markus Bühner
- , Caroline Jung-Sievers
- , Helmut Krcmar
- , Karoline Lukaschek
- , Gabriele Pitschel-Walz
- , Antonius Schneider
- , Katharina Biersack
- , Constantin Brand
- , Christopher Ebert
- , Julia Eder
- , Feyza Gökce
- , Carolin Haas
- , Lisa Hattenkofer
- , Lukas Kaupe
- , Jonas Raub
- , Philipp Reindl-Spanner
- , Hannah Schillok
- , Petra Schönweger
- , Clara Teusen
- , Marie Vogel
- , Victoria von Schrottenberg
- , Jochen Vukas
- & Puya Younesi
Contributions
Conceptualization: T.D. and V.B. Methodology: T.D. and V.B. Formal analysis: V.B. Investigation: V.B. Data curation: V.B. Writing—original draft: V.B. Writing—review and editing: V.B., T.D., J.V., C.J.-S., K.L., G.C.A., U.T., P.T., C.S., S.F., E.B., M.D., S.H., W.N., U.J., O.K., and J.G. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tobias Dreischulte .
Ethics declarations
Ethics approval and consent to participate.
This study did not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Search strategy examples.
Additional file 2.
Expert ratings of round two of the RAM-Survey for high-risk prescribing.
Additional file 3.
Table with corresponding references and comments in the event of changes between round 2 and round 3 of the RAM-assessment.
Additional file 4.
Expert ratings of round two of the RAM-Survey for overprescribing.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Brisnik, V., Vukas, J., Jung-Sievers, C. et al. Deprescribing of antidepressants: development of indicators of high-risk and overprescribing using the RAND/UCLA Appropriateness Method. BMC Med 22 , 193 (2024). https://doi.org/10.1186/s12916-024-03397-w
Download citation
Received : 12 October 2023
Accepted : 18 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12916-024-03397-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antidepressants
- Deprescribing
- Adverse drug events
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Dimerization and antidepressant recognition at noradrenaline transporter
Affiliations.
- 1 State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.
- 2 Lingang Laboratory, Shanghai, China.
- 3 State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.
- 4 National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.
- 5 School of Life Science and Technology, ShanghaiTech University, Shanghai, China.
- 6 School of Pharmacy, Fudan University, Shanghai, China.
- 7 Shanghai Advanced Electron Microscope Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.
- 8 University of Chinese Academy of Sciences, Beijing, China.
- 9 Research Center for Deepsea Bioresources, Sanya, China.
- 10 Department of Pharmacology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
- 11 School of Pharmacy, Hainan Medical University, Haikou, China.
- 12 State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. [email protected].
- 13 National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. [email protected].
- 14 University of Chinese Academy of Sciences, Beijing, China. [email protected].
- 15 State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. [email protected].
- 16 School of Life Science and Technology, ShanghaiTech University, Shanghai, China. [email protected].
- 17 Shanghai Advanced Electron Microscope Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. [email protected].
- 18 Lingang Laboratory, Shanghai, China. [email protected].
- 19 School of Life Science and Technology, ShanghaiTech University, Shanghai, China. [email protected].
- PMID: 38750358
- DOI: 10.1038/s41586-024-07437-6
The noradrenaline transporter has a pivotal role in regulating neurotransmitter balance and is crucial for normal physiology and neurobiology 1 . Dysfunction of noradrenaline transporter has been implicated in numerous neuropsychiatric diseases, including depression and attention deficit hyperactivity disorder 2 . Here we report cryo-electron microscopy structures of noradrenaline transporter in apo and substrate-bound forms, and as complexes with six antidepressants. The structures reveal a noradrenaline transporter dimer interface that is mediated predominantly by cholesterol and lipid molecules. The substrate noradrenaline binds deep in the central binding pocket, and its amine group interacts with a conserved aspartate residue. Our structures also provide insight into antidepressant recognition and monoamine transporter selectivity. Together, these findings advance our understanding of noradrenaline transporter regulation and inhibition, and provide templates for designing improved antidepressants to treat neuropsychiatric disorders.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Rapid Antidepressant-Like Potential of Chaihu Shugan San Depends on Suppressing Glutamate Neurotransmission and Activating Synaptic Proteins in Hippocampus of Female Mice
- Original Article
- Published: 11 May 2024
Cite this article

- Chao Lu 1 ,
- Zi-wei Gao 1 ,
- Shan Xing 1 , 2 ,
- Hui-hui Wang 1 ,
- Yun-ke Huang 3 ,
- Hang Zhou 1 &
32 Accesses
Explore all metrics
To explore the rapid antidepressant potential and the underlying mechanism of Chaihu Shugan San (CSS) in female mice.
Liquid chromatography mass spectrometry (LC-MS)/MS was used to determine the content of main components in CSS to determine its stability. Female C57BL/6J mice were randomly divided into 4 groups, including control (saline), vehicle (saline), CSS (4 g/kg) and ketamine (30 mg/kg) groups. Mice were subjected to irregular stress stimulation for 4 weeks to establish the chronic mild stress (CMS) model, then received a single administration of drugs. Two hours later, the behavioral tests were performed, including open field test, tail suspension test (TST), forced swimming test (FST), novelty suppression feeding test (NSF), and sucrose preference test (SPT). Western blot analysis was used to detect the expression levels of N-methyl-D-aspartate receptor (NMDA) subtypes [N-methyl-D-aspartate receptor 1 (NR1), NR2A, NR2B], synaptic proteins [synapsin1 and post synaptic density protein 95 (PSD95)], and brain-derived neurotrophic factor (BDNF). Moreover, the rapid antidepressant effect of CSS was tested by pharmacological technologies and optogenetic interventions that activated glutamate receptors, NMDA.
Compared with the vehicle group, a single administration of CSS (4 g/kg) reversed all behavioral defects in TST, FST, SPT and NSF caused by CMS ( P <0.05 or P <0.01). CSS also significantly decreased the expressions of NMDA subtypes (NR1, NR2A, NR2B) at 2 h in hippocampus of mice (all P <0.01). In addition, similar to ketamine, CSS increased levels of synaptic proteins and BDNF ( P <0.05 or P <0.01). Furthermore, the rapid antidepressant effects of CSS were blocked by transient activation of NMDA receptors in the hippocampus (all P <0.01).
Rapid antidepressant effects of CSS by improving behavioral deficits in female CMS mice depended on rapid suppression of NMDA receptors and activation of synaptic proteins.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Antidepressant effects of the ginsenoside metabolite compound k, assessed by behavioral despair test and chronic unpredictable mild stress model.

Antidepressant-Like Effects of Chaihu Shugan Powder (柴胡疏肝散) on Rats Exposed to Chronic Unpredictable Mild Stress through Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis
Saikosaponin D exerts antidepressant effect by regulating Homer1-mGluR5 and mTOR signaling in a rat model of chronic unpredictable mild stress
Ely BA, Nguyen TNB, Tobe RH, Walker AM, Gabbay V. Multimodal investigations of reward circuitry and anhedonia in adolescent depression. Front Psychiatry 2021;12:678709.
Article PubMed PubMed Central Google Scholar
Wang Z, Cheng YT, Lu Y, Sun GQ, Pei L. Baicalin ameliorates corticosterone-induced depression by promoting neurodevelopment of hippocampal via mTOR/GSK3 β pathway. Chin J Integr Med 2023;29:405–412.
Article CAS PubMed Google Scholar
Kleih TS, Entringer S, Scholaske L, Kathmann N, DePunder K, Heim CM, et al. Exposure to childhood maltreatment and systemic inflammation across pregnancy: the moderating role of depressive symptomatology. Brain Behav Immun 2022;101:397–409.
Kanezawa S, Zhu YB, Wang Q. Correlation between Chinese medicine constitution and skin types: a study on 187 Japanese women. Chin J Integr Med 2020;26:174–179.
Article PubMed Google Scholar
Wang J, Cosci F. Neonatal withdrawal syndrome following late in utero exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis of observational studies. Psychother Psychosom 2021;90:299–307.
Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med 2019;381:1–4.
Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 2019;24:606–615.
McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry 2021;178:383–399.
Shi X, Zhang Q, Li J, Liu X, Zhang Y, Huang M, et al. Disrupting phosphorylation of Tyr-1070 at GluN2B selectively produces resilience to depression-like behaviors. Cell Rep 2021;36:109612.
Akil H, Nestler EJ. The neurobiology of stress: vulnerability, resilience, and major depression. Proc Natl Acad Sci U S A 2023;120:e2312662120.
Article CAS PubMed PubMed Central Google Scholar
Huang S, Deng Q, Zhao Y, Chen G, Geng A, Wang X. l-Glutamate seed priming enhances 2-acetyl-1-pyrroline formation in fragrant rice seedlings in response to arsenite stress. J Agric Food Chem 2023;71:18443–18453.
Natarajan S, Abass G, Kim L, Wells C, Rezvani AH, Levin ED. Acute and chronic glutamate NMDA antagonist treatment attenuates dopamine D 1 antagonist-induced reduction of nicotine self-administration in female rats. Pharmacol Biochem Behav 2023;234:173678.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010;329:959–964.
Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 2012;15:746–753.
Xia B, Zhang H, Xue W, Tao W, Chen C, Wu R, et al. Instant and lasting down-regulation of NR1 expression in the hippocampus is associated temporally with antidepressant activity after acute Yueju. Cell Mol Neurobiol 2016;36:1189–1196.
Zhang H, Sun Y, Qian S, Ge R, Guo X, Shen Q, et al. Yueju-Ganmaidazao Decoction confers rapid antidepressant-like effects and the involvement of suppression of NMDA/NO/cGMP signaling. J Ethnopharmacol 2020;250:112380.
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 2014;3:e03581.
Burgdorf JS, Zhang XL, Stanton PK, Moskal JR, Donello JE. Zelquistinel is an orally bioavailable novel NMDA receptor allosteric modulator that exhibits rapid and sustained antidepressant-like effects. Int J Neuropsychopharmacol 2022;25:979–991.
Camargo A, Dalmagro AP, Alte GA, Zeni ALB, Tasca CI, Rodrigues ALS. NMDA receptor-mediated modulation on glutamine synthetase and glial glutamate transporter GLT-1 is involved in the antidepressant-like and neuroprotective effects of guanosine. Chem Biol Interact 2023;375:110440.
Araujo JRC, Junior J, Damasceno M, Santos S, Vieira-Neto AE, Lobo MDP, et al. Neuropharmacological characterization of frutalin in mice: evidence of an antidepressant-like effect mediated by the NMDA receptor/NO/cGMP pathway. Int J Biol Macromol 2018;112:548–554.
Gordillo-Salas M, Pilar-Cuellar F, Auberson YP, Adell A. Signaling pathways responsible for the rapid antidepressant-like effects of a GluN2A-preferring NMDA receptor antagonist. Transl Psychiatry 2018;8:84.
Shi ZW, Kang LP, Peng HS, Yang SH, Zhang LX, Jing ZX, et al. Exploring the clinical characters of Shugan Jieyu Capsule through text mining. China J Chin Mater Med (Chin) 2017;42:3435–3442.
CAS Google Scholar
Xie W, Qiu X, Huang X, Xie Y, Wu K, Wang Y, et al. Comparison between the pharmacokinetics of meranzin hydrate in a rat model of chronic depression and in controls following the oral administration of Chaihu-Shugan-San. Exp Ther Med 2013;6:913–918.
Jia KK, Pan SM, Ding H, Liu JH, Zheng YJ, Wang SJ, et al. Chaihu-shugan San inhibits inflammatory response to improve insulin signaling in liver and prefrontal cortex of CUMS rats with glucose intolerance. Biomed Pharmacother 2018;103:1415–1428.
Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 2017;6:78–93.
Weinstock M. Prenatal stressors in rodents: effects on behavior. Neurobiol Stress 2017;6:3–13.
Liu YL, Xu JJ, Han LR, Liu XF, Lin MH, Wang Y, et al. Meranzin hydrate improves depression-like behaviors and hypomotility via ghrelin and neurocircuitry. Chin J Integr Med 2023;29:490–499.
Wu L, Zhang T, Chen K, Lu C, Liu XF, Zhou JL, et al. Rapid antidepressant-like effect of Fructus Aurantii depends on cAMP-response element binding protein/brain-derived neurotrophic facto by mediating synaptic transmission. Phytother Res 2021;35:404–414.
Shi X, Bai H, Wang J, Wang J, Huang L, He M, et al. Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Front Neurol 2021;12:667511.
Camargo A, Pazini FL, Rosa JM, Wolin IAV, Moretti M, Rosa PB, et al. Augmentation effect of ketamine by guanosine in the novelty-suppressed feeding test is dependent on mTOR signaling pathway. J Psychiatr Res 2019;115:103–112.
Pesarico AP, Stangherlin EC, Rosa SG, Mantovani AC, Zeni G, Nogueira CW. Contribution of NMDA, GABAA and GABAB receptors and l-arginine-NO-cGMP, MEK1/2 and CaMK-II pathways in the antidepressant-like effect of 7-fluoro-1,3-diphenylisoquinoline-1-amine in mice. Eur J Pharmacol 2016;782:6–13.
Han SK, Kim JK, Park HS, Shin YJ, Kim DH. Chaihu-Shugan-San (Shihosogansan) alleviates restraint stress-generated anxiety and depression in mice by regulating NF-κ B-mediated BDNF expression through the modulation of gut microbiota. Chin Med 2021;16:77.
Zhang S, Lu Y, Chen W, Shi W, Zhao Q, Zhao J, et al. Network pharmacology and experimental evidence: PI3K/AKT signaling pathway is involved in the antidepressive roles of Chaihu Shugan San. Drug Des Devel Ther 2021;15:3425–3441.
Zeng Q, Li L, Siu W, Jin Y, Cao M, Li W, et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed Pharmacother 2019;120:109370.
Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest 2020;130:1336–1349.
Xue W, Zhou X, Yi N, Jiang L, Tao W, Wu R, et al. Yueju Pill rapidly induces antidepressant-like effects and acutely enhances BDNF expression in mouse brain. Evid Based Complement Alternat Med 2013;2013:184367.
Dogra S, Kumar A, Umrao D, Sahasrabuddhe AA, Yadav PN. Chronic kappa opioid receptor activation modulates NR2B: implication in treatment resistant depression. Sci Rep 2016;6:33401.
Jiang J, Zheng Y, Chen Y, Zahra A, Long C, Yang L. Exposure to prenatal antidepressant alters medial prefrontalstriatal synchronization in mice. Brain Res 2019;1717:27–34.
Murphy N, Lijffijt M, Ramakrishnan N, Vo-Le B, Vo-Le B, Iqbal S, et al. Does mismatch negativity have utility for NMDA receptor drug development in depression? Braz J Psychiatry 2022;44:61–73.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016;22:238–249.
Tang XH, Zhang GF, Xu N, Duan GF, Jia M, Liu R Z, et al. Extrasynaptic CaMK II α is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model. J Neuroinflammation 2020;17:181.
Viana GSB, Vale EMD, Araujo ARA, Coelho NC, Andrade SM, Costa ROD, et al. Rapid and long-lasting antidepressant-like effects of ketamine and their relationship with the expression of brain enzymes, BDNF, and astrocytes. Braz J Med Biol Res 2020;54:e10107.
Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 2021;42:929–942.
Chen MH, Wu HJ, Li CT, Lin WC, Tsai SJ, Hong CJ, et al. Low-dose ketamine infusion for treating subjective cognitive, somatic, and affective depression symptoms of treatment-resistant depression. Asian J Psychiatr 2021;66:102869.
Download references
Author information
Authors and affiliations.
Department of Chinese Medicine Preparations, Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Province Hospital of Chinese Medicine, Nanjing, 210029, China
Chao Lu, Zi-wei Gao, Shan Xing, Hui-hui Wang, Hang Zhou & Lei Wu
College of Chinese Medicine, Nanjing University of Chinese Medicine, Nanjing, 210046, China
Department of Chinese Medicine, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, 310000, China
Yun-ke Huang
You can also search for this author in PubMed Google Scholar
Contributions
Lu C, Gao ZW and Wu L conceived and designed the experiments. Wu L, Gao ZW, Huang YK, Lu C, and Wang HH performed the experiments. Wu L, Lu C and Zhou H analyzed the data. Lu C, Xing S and Wu L contributed to the writing of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Lei Wu .
Ethics declarations
The authors declare no competing financial interest.
Additional information
Supported by the National Natural Science Foundation of China (No. 82174107, 82304898), Jiangsu Provincial Administration of Traditional Chinese Medicine Fund (No. YB2020014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 035062005001)
Electronic Supplementary Material
Supplementary material, approximately 277 kb., rights and permissions.
Reprints and permissions
About this article
Lu, C., Gao, Zw., Xing, S. et al. Rapid Antidepressant-Like Potential of Chaihu Shugan San Depends on Suppressing Glutamate Neurotransmission and Activating Synaptic Proteins in Hippocampus of Female Mice. Chin. J. Integr. Med. (2024). https://doi.org/10.1007/s11655-024-3906-2
Download citation
Accepted : 01 February 2023
Published : 11 May 2024
DOI : https://doi.org/10.1007/s11655-024-3906-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chaihu Shugan San
- rapid antidepressant potential
- N-methyl-D-aspartate receptor
- synaptic protein
- optogenetic
- Chinese medicine
- Find a journal
- Publish with us
- Track your research
Is ketamine the answer to treatment-resistant depression?
Uc expert joins wvxu's cincinnati edition roundtable discussion.

While once considered a counterculture drug, ketamine is now giving hope for patients with treatment-resistant depression.
The University of Cincinnati's Stephen Rush, MD, treats patients with intranasal ketamine and esketamine in the Treatment Resistant Depression Clinic and joined WVXU's Cincinnati Edition to discuss the drugs' benefits and the importance of administering the drug in a safe and controlled setting.
Rush explained ketamine was initially developed as an anesthetic, and its "mirror image" molecule esketamine has been approved by the FDA for treatment-resistant depression.
"Ketamine or esketamine impact an entirely different molecule in the brain (than traditional antidepressants)," said Rush, associate professor of clinical psychiatry in UC's College of Medicine and medical director of ambulatory services. "Ketamine increases the neuroplasticity of the brain. That is, it increases the ability of our nervous system to change its activity by reorganizing its structure, functions and connections with other parts of the brain, going from a function causing depression to a function in which depression is no longer present."
Rush said administering intranasal ketamine and esketamine in the clinic is structured with safety in mind, as vital signs like heart rate and blood pressure are monitored, side effects are reported and patients stay for 2-4 hours after treatment to confirm their vital signs are in normal range and they are safe to be discharged.
"My personal opinion is that the data on safety around home-use ketamine is not there yet, and I would generally advise against it," Rush said. "Certainly someone who is participating in treatment in our clinics cannot also be getting home treatment through another service."
Since the use of ketamine or esketamine is focused on people who have failed traditional treatments, Rush said it is exciting to see patients entering remission from depression through this treatment. Meta-analyses of studies have found likelihood of remission with esketamine treatment is about 30-45%, while likelihood of having at least a 50% reduction in symptoms is about 40-55%.
"I often hear about patients telling me about how life has changed, whether that means going back to work and coming off of disability, or going back to school, engaging in relationships in ways they haven’t for years and generally functioning," Rush said. "Not only are we talking about the improvement in mood, we’re talking about the increase in functioning and the person’s ability to contribute to society and be productive, which is a huge factor in determining someone's sense of wellness."
Listen to the Cincinnati Edition segment.
Featured photo at top of ketamine vial. Photo/Jennifer Fontan/iStock.
- Faculty Staff
- In The News
- College of Medicine
- UC Gardner Neuroscience Institute
- Academic Health Center
- Psychiatry & Behavioral Neuroscience
Related Stories
Enquirer: experts discuss reasons for drop in overdose deaths.
April 12, 2023
The University of Cincinnati's Christine Wilder, MD, spoke with the Cincinnati Enquirer about Hamilton County data that overdose deaths in 2022 dropped for the first time in six years.
U.S. News & World Report: Metformin may help young patients with bipolar disorder avoid weight gain
October 31, 2023
U.S. News & World Report highlighted recent research led by the University of Cincinnati and Northwell Health that found the drug metformin can help prevent or reduce weight gain in youth taking medication to treat bipolar disorder.
Cape Girardeau, Missouri news highlights UC clinical trial
February 20, 2024
KFVS-TV in Cape Girardeau, Missouri highlighted a University of Cincinnati clinical trial testing a wearable neurostimulation device to help patients with opioid use disorder and post-traumatic stress disorder stick with medication treatment while finding the right dose.

BHealthy Blog
Transcranial Magnetic Stimulation, an Innovative Treatment for Depression
- May 16, 2024

By Dr. Siva Koppolu , psychiatrist at Baptist Health Behavioral Clinic-North Little Rock
Transcranial Magnetic Stimulation (TMS) is an innovative and effective non-invasive treatment for people suffering from major depression who have responded poorly to antidepressant medications. TMS is an FDA-approved treatment available by prescription only and delivered under the supervision of a board-certified psychiatrist, like Dr. Siva Koppolu at Baptist Health Behavioral Clinic-North Little Rock .
What is TMS and How Does it Work?
TMS targets the prefrontal cortex, a vital area of the brain that controls mood. This area is assumed to be underactive when someone is suffering from major depression. Using electric current field pulses to stimulate that area, TMS may restore appropriate function and reduce the symptoms of depression.
Who qualifies for TMS?
People who qualify for TMS are those who have not responded well to antidepressant medications. It is also an option for those who cannot tolerate the side effects of antidepressant medications or those who are seeking an alternative to electroconvulsive therapy (ECT).
Are there any side effects?
One of the most significant benefits of TMS treatment is the lack of side effects typically experienced with antidepressant medications. The most common side effect of TMS is mild scalp or a slight headache, which typically goes away after the sessions or after the first session.
Each TMS treatment takes less than 45 minutes and is conducted in a doctor’s office five days a week for approximately four to six weeks. During treatment, the person is awake and alert and can speak with the doctor or clinician at any time. After a TMS treatment, people can return to usual activities.
During TMS, a magnetic coil device is placed on the head while the patient sits in a comfortable treatment chair. The patient may hear a clicking sound and feel a tapping sensation. However, the treatment is typically well-tolerated and not painful.
Clinical trials have proved the effectiveness of TMS in treating patients with major depression. In fact, one out of two patients improved significantly, and one out of three patients was free of depression symptoms completely.
Is TMS covered by insurance?
TMS is covered by some insurance plans, and many people find it to be a valuable alternative to traditional antidepressant medications. If you or a loved one is struggling with major depression, contact Dr. Siva Koppolu with Baptist Health Behavioral Clinic-North Little Rock to see if TMS may be right for you.
Search Blog Posts
Explore by topic.
- Considering Pregnancy (19)
- COVID-19 (28)
- Eat Well (103)
- Español (251)
- Get Fit (63)
- Healthy Living (166)
- Inspiring Stories (47)
- Labor and Delivery (7)
- Postpartum and Newborn Care (41)
- Pregnancy (28)
- Safety & Prevention (153)
- Treatment & Wellbeing (153)
- Women's Health (32)
Find More Ways To Be Healthy
- Classes & Resources
Discover More
Follow on social media.
- Work & Careers
- Life & Arts
Become an FT subscriber
Try unlimited access Only $1 for 4 weeks
Then $75 per month. Complete digital access to quality FT journalism on any device. Cancel anytime during your trial.
- Global news & analysis
- Expert opinion
- Special features
- FirstFT newsletter
- Videos & Podcasts
- Android & iOS app
- FT Edit app
- 10 gift articles per month
Explore more offers.
Standard digital.
- FT Digital Edition
Premium Digital
Print + premium digital, ft professional, weekend print + standard digital, weekend print + premium digital.
Essential digital access to quality FT journalism on any device. Pay a year upfront and save 20%.
- Global news & analysis
- Exclusive FT analysis
- FT App on Android & iOS
- FirstFT: the day's biggest stories
- 20+ curated newsletters
- Follow topics & set alerts with myFT
- FT Videos & Podcasts
- 20 monthly gift articles to share
- Lex: FT's flagship investment column
- 15+ Premium newsletters by leading experts
- FT Digital Edition: our digitised print edition
- Weekday Print Edition
- Videos & Podcasts
- Premium newsletters
- 10 additional gift articles per month
- FT Weekend Print delivery
- Everything in Standard Digital
- Everything in Premium Digital
Complete digital access to quality FT journalism with expert analysis from industry leaders. Pay a year upfront and save 20%.
- 10 monthly gift articles to share
- Everything in Print
- Make and share highlights
- FT Workspace
- Markets data widget
- Subscription Manager
- Workflow integrations
- Occasional readers go free
- Volume discount
Terms & Conditions apply
Explore our full range of subscriptions.
Why the ft.
See why over a million readers pay to read the Financial Times.
International Edition
Mental Health & Substance Use Disorders
Medicare covers certain screenings, services, and programs that aid in the treatment and recovery of mental health and substance use disorders.
If you or someone you know is struggling or in crisis, call or text 988, the free and confidential Suicide Crisis Lifeline. You can call and speak with a trained crisis counselor 24 hours a day, 7 days a week. You can also connect with a counselor through web chat at 988lifeline.org . Call 911 if you're in immediate medical crisis.

Services & programs Medicare covers
Medicare covers a wide range of behavioral health services, including inpatient, outpatient, and more.
If you’re eligible for Medicare and Medicaid you may have even more coverage than what’s listed here. Call your State Medical Assistance (Medicaid) office to find out what other health services may be covered in your state.
Types of mental health care
Outpatient mental health care.
Services to help diagnose and treat mental health conditions (often called counseling or psychotherapy).
Intensive Outpatient Program Services
Part-time mental health care for people who need at least 9 hours of services per week.
Partial Hospitalization
Full-day mental health care for people who need at least 20 hours of services per week.
Inpatient Care
Mental health care services when you’re admitted to a general or psychiatric hospital.
Preventive screenings & counseling
Depression screenings.
Assesses signs and symptoms of depression.
Opioid Use Disorder Treatments
Counseling, therapy, assessments, and more to help recover from opioid use disorder.
Tobacco Use Counseling
Counseling for smoking and tobacco use cessation.
Alcohol Misuse Screenings
To identify unhealthy drinking habits and counseling needs.
Find a mental health care provider
Find and compare providers for mental health and substance use disorder services near you. These providers can help to help treat conditions like depression, anxiety, or substance use disorders. Some providers may offer these services via telehealth, which allows you to communicate in real-time with your health care provider without going to the doctor’s office. Get information about covered telehealth services.
Find Providers
Helpful links
FindSupport.gov - Explore care, support, and treatment options.
Mental Health America – Find help for you or someone else.
National Institute of Mental Health – Get care tips and resources.
National Council on Aging – Learn about mental health issues impacting older adults.
Tribal behavioral health coverage – Explore resources for American Indian and Alaska Native people.
National Alliance on Mental Illness – Find resources for people living with mental illness.
Findtreatment.gov – Find state-licensed treatment options in your area for addiction and substance use disorder.
Centers for Disease Control and Prevention – Learn how to manage pain without opioids.
Clinically administered ketamine shows positive outcomes for Angela's treatment-resistant depression
Once a week, Angela Neale pops on some headphones and plays her favourite tracks by singer-songwriter Enya.
But before that she takes a ketamine nasal spray — a drug she says helps manage her treatment-resistant depression.
"You feel quite funny, sort of out of your body for a little while," Ms Neale said.
She said the benefits really started when the effects of the drug wore off.
"You start to think in different ways. The sad thoughts that go round and round tend to stop. You're more flexible in your mind," Ms Neale said.
She is part of a small group who are now regularly treated with ketamine in a clinical setting at the Gold Coast University Hospital.
After years of studies with some promising outcomes, it is one of a few publicly funded clinics nationwide offering the drug.
Why does this matter?
One in seven Australians will experience depression in their lifetime, according to the Black Dog Institute.
It is also a leading cause of ill health and disability globally according to the World Health Organization.
Not everyone is responsive to their first treatment for depression, according to Brett Emmerson, Queensland chair of the Royal Australian New Zealand College of Psychiatrists (RANZCP).
He said new treatments were needed, and ketamine could be an addition to the arsenal of treatments used by mental health professionals in Australia.
But he said ketamine was still "slightly experimental" because research was ongoing into the best way to deliver the drug.
The RANZCP says there is enough evidence only for using ketamine for treatment-resistant depression .
Where's the proof ketamine works?
Ketamine has delivered promising results in research over the years .
It caught the eye of Associate Professor Shanthi Sarma, who runs the clinic Ms Neale visits for treatment.
She contributed to research published in the British Journal of Psychiatry that found that one in five people who took part in a trial had relief from hard-to-treat depression .
While some people did not feel relief, those who participated were 10 times more likely to see their symptoms improve.
"These are people who've had really, really difficult-to-treat depression that haven't responded to the normal treatments," Dr Sarma said.
She said there was still a lot that learn about the drug, including longer-term side effects.
It was not a silver bullet either.
"It's just one part of the treatment strategy," Dr Sarma said.
"The other problem can be relapse when the treatment stops, so it's used in combination with a number of treatments. Things like talking therapy are very important."
It is important to note that the drug is used illegally as a hallucinogen.
The Drug and Alcohol Foundation said the risk of death by ketamine overdose is low, but mixing ketamine with other drugs could increase the risk .
A festival pill testing trial last year found only 53 per cent of substances sold illegally as ketamine were actually ketamine .
Experts stress that ketamine should only be administered by trained medical professionals.
So, can I get treated with ketamine?
Do not expect your doctor to prescribe the drug to treat depression at your next appointment.
For example, Dr Sarma said about 40 patients have had their depression treated with ketamine in the past five years at the Gold Coast's specialist mood disorders unit.
It is the only location in Queensland that offers the treatment publicly, and existing patients are part of a special program.
This, along with the fact that patients need to be closely monitored, makes the drug very expensive for people outside of special programs and clinical trial s .
But Professor Emmerson said ketamine treatments could be scaled up once clearer protocols had been determined.
He said it could eventually get the support of the RANZCP to be covered by Medicare.
"I can see that happening over the next couple of years … and ultimately the way to make this more widely available is to get it covered by Medicare," he said.
"If the reports continue to show positive results then I think we would be much more likely to support having ketamine registered as an accepted treatment under Medicare."
How's Angela doing now?
Under the care of professionals, Ms Neale said the regular treatments had helped her a lot.
"I have quite dramatic seasonal depression, so lots of winters where I'd need to be admitted to hospital. I've had two great winters now," she said.
She said she had more motivation to get outdoors.
"I love going out in nature and used to do a lot of hiking … whereas previously I wouldn't be interested and just go back to bed," she said.
She was also working with her psychologist on how to look to the future.
"I'm working on goals, how I see the future. It's really hard, but it's a lot easier on this medication at least. It's different, but exciting," she said.
- X (formerly Twitter)
Related Stories
One-in-five people with severe depression see results from ketamine in landmark australia-nz study.
I have 'treatment-resistant' depression. Doctors hoped ketamine would help me
Thousands sign up for ketamine trial to wipe out chronic surgical pain
- Maroochydore
- Medical Procedures
- Medical Research
- Mental Health
- Pharmaceuticals

IMAGES
VIDEO
COMMENTS
1. Introduction. Major depression disorder (MDD) is the prominent cause of disability-adjusted life years lost in 10-19-year-olds, with a global prevalence of 4-25% [1,2,3].Since COVID-19 was declared an international public health emergency, youth around the world have experienced dramatic disruptions to their everyday lives, which has increased this prevalence and the associated ...
Introduction. Treatment guidelines like those published by National Institute for Health and Care Excellence (NICE) or the American Psychiatric Association (APA) strongly recommend long-term maintenance treatment in people with (or at risk of) recurrent depression to prevent relapses. 1,2 In accordance with these recommendations, the rate and duration of antidepressant use is steadily ...
However, compared with their efficacy for adult patients , the efficacy of antidepressant drugs for the treatment of young people with MDD is controversial, as shown in our recent network meta ...
All antidepressants were more efficacious than placebo in adults with major depressive disorder. Smaller differences between active drugs were found when placebo-controlled trials were included in the analysis, whereas there was more variability in efficacy and acceptability in head-to-head trials. These results should serve evidence-based practice and inform patients, physicians, guideline ...
Most antidepressant drugs prescribed today have been available for decades. Nonetheless, their mechanism of action in treating depression has remained elusive. On the basis of neurochemical studies in laboratory animals, hypotheses explaining their therapeutic effects have been formulated. The most attractive of these theories involves antidepressant-induced changes in the sensitivity of ...
The average AROC for predicting propensity of medications was 82%. 15 models were constructed to predict remission for 15 different antidepressants. The average prevalence of remission ranged from 3.1% to 49.3%. The cross-validated AROC ranged from 69.2% to 78.5%, with an average of 72.0%.
The magnitude of benefit of antidepressant medication compared with placebo increases with severity of depression symptoms and may be minimal or nonexistent, on average, in patients with mild or moderate symptoms. CONTEXT Antidepressant medications represent the best established treatment for major depressive disorder, but there is little evidence that they have a specific pharmacological ...
Antidepressants are the mainstay of depression treatment and rank among the top three most commonly used therapeutic drug classes in the USA - in the 2011-2014 period, 12.7% of persons in the USA aged 12 and over reported antidepressant use in the past month (Pratt et al. 2017). Antidepressants are prescribed for a wide range of conditions ...
A systematic review and random-effects model network meta-analysis were conducted to compare the efficacy, acceptability, tolerability, and safety of antidepressants to treat adults with major ...
Antidepressants are widely used in the general population, primarily to treat depression but also commonly in conditions such as anxiety disorders and neuropathic pain. This article considers the significant pharmacodynamic and pharmacokinetic drug interactions of different antidepressants, whilst remembering that individual patient ...
The present study elucidates the need for effective interventions to facilitate antidepressant adherence and enhance doctor-patient communication, benefiting both the individuals and the healthcare system and leading to better clinical outcomes and reduction of relapse-related costs. Background: Medication adherence is a prerequisite to achieving beneficial treatment outcomes. In major ...
The national crises of depression, suicide, and opioid-related harms such as overdose are closely linked ().Pain and depression are among the most common medical conditions (2, 3) and are frequently comorbid.In primary care settings, 27% of patients with pain have concurrent major depression, and its prevalence increases to 52% among patients in specialty pain care settings ().
This family of antidepressant drugs have their origins in the hydrazines and the research carried out by Emil Fischer in the 1870s. This father of organic chemistry discovered phenylhydrazine in 1874, accidentally, while he was working in the laboratory of Adolf von Baeyer in Strasbourg [].From hydrazine hydrate, a powerful reducing agent, Hans Meyer and Josef Malley, of the German Charles ...
Widespread prescribing has not reduced mental disability or suicide, raising questions about the assessment of evidence on effectiveness and safety of antidepressants, writes John Warren Depression can be severe and reduce life expectancy. Antidepressant prescribing has increased substantially in recent years so that one in eight UK adults, some 7.3 million people, now receive a prescription ...
A Medications Case Example: Despair, Delight, Disaster, and More. Many thanks to LC, for sharing her antidepressant experiences. LC: It all started one late afternoon. I was in my car with my ...
This Open Access Thesis is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Theses by an authorized administrator of DigitalCommons@WayneState. Recommended Citation Kay, Rachel, "Antidepressant Medications And Cognitive Functioning In Major Depressive Disorder ...
Background Antidepressants are first-line medications for many psychiatric disorders. However, their widespread long-term use in some indications (e.g., mild depression and insomnia) is concerning. Particularly in older adults with comorbidities and polypharmacy, who are more susceptible to adverse drug reactions, the risks and benefits of treatment should be regularly reviewed. The aim of ...
Several systematic reviews and meta-analyses on the topic have also indicated that antidepressant use is associated with an increased risk of dementia. 6, 20-22 Contrarily, a study using large health insurance claims data in over 100 million records identified mirtazapine (a TCA antidepressant) as one of the medications associated with a ...
Antidepressants are often present in combination with other drugs in suicides and drug-related deaths, so a sensitive and specific method to detect and quantify antidepressants is necessary. We developed a method for the detection and quantification of 18 different antidepressants in whole blood, with a range of 2.5-900 ng/mL and LOQ of 2.5 ng/mL.
The noradrenaline transporter has a pivotal role in regulating neurotransmitter balance and is crucial for normal physiology and neurobiology 1.Dysfunction of noradrenaline transporter has been implicated in numerous neuropsychiatric diseases, including depression and attention deficit hyperactivity disorder 2.Here we report cryo-electron microscopy structures of noradrenaline transporter in ...
Objective To explore the rapid antidepressant potential and the underlying mechanism of Chaihu Shugan San (CSS) in female mice. Methods Liquid chromatography mass spectrometry (LC-MS)/MS was used to determine the content of main components in CSS to determine its stability. Female C57BL/6J mice were randomly divided into 4 groups, including control (saline), vehicle (saline), CSS (4 g/kg) and ...
While once considered a counterculture drug, ketamine is now giving hope for patients with treatment-resistant depression. The University of Cincinnati's Stephen Rush, MD, treats patients with intranasal ketamine and esketamine in the Treatment Resistant Depression Clinic and joined WVXU's Cincinnati Edition to discuss the drugs' benefits and the importance of administering the drug in a safe ...
Transcranial Magnetic Stimulation (TMS) is an innovative and effective non-invasive treatment for people suffering from major depression who have responded poorly to antidepressant medications. TMS is an FDA-approved treatment available by prescription only and delivered under the supervision of a board-certified psychiatrist,
Ketamine has been approved by the US Food and Drug Administration for use as an anaesthetic since the 1970s. But more recently, specialist clinics have begun touting it as a mental health treatment.
These providers can help to help treat conditions like depression, anxiety, or substance use disorders. Some providers may offer these services via telehealth, which allows you to communicate in real-time with your health care provider without going to the doctor's office. Get information about covered telehealth services. Find Providers
In short: Researchers say ketamine has been shown to help with treatment-resistant depression for some patients where other drugs have failed. A small group of patients are now regularly treated ...