Improvement of Soybean; A Way Forward Transition from Genetic Engineering to New Plant Breeding Technologies
- Published: 04 February 2022
- Volume 65 , pages 162–180, ( 2023 )

Cite this article
- Saleem Ur Rahman 1 , 2 ,
- Evan McCoy 3 ,
- Ghulam Raza 1 , 2 ,
- Zahir Ali 4 ,
- Shahid Mansoor 1 , 2 &
- Imran Amin ORCID: orcid.org/0000-0003-3063-4103 1 , 2
4508 Accesses
23 Citations
2 Altmetric
Explore all metrics
Soybean is considered one of the important crops among legumes. Due to high nutritional contents in seed (proteins, sugars, oil, fatty acids, and amino acids), soybean is used globally for food, feed, and fuel. The primary consumption of soybean is vegetable oil and feed for chickens and livestock. Apart from this, soybean benefits soil fertility by fixing atmospheric nitrogen through root nodular bacteria. While conventional breeding is practiced for soybean improvement, with the advent of new biotechnological methods scientists have also engineered soybean to improve different traits (herbicide, insect, and disease resistance) to fulfill consumer requirements and to meet the global food deficiency. Genetic engineering (GE) techniques such as transgenesis and gene silencing help to minimize the risks and increase the adaptability of soybean. Recently, new plant breeding technologies (NPBTs) emerged such as zinc-finger nucleases, transcription activator‐like effector nucleases, and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9), which paved the way for enhanced genetic modification of soybean. These NPBTs have the potential to improve soybean via gene functional characterization precision genome engineering for trait improvement. Importantly, these NPBTs address the ethical and public acceptance issues related to genetic modifications and transgenesis in soybean. In the present review, we summarized the improvement of soybean through GE and NPBTs. The valuable traits that have been improved through GE for different constraints have been discussed. Moreover, the traits that have been improved through NPBTs and potential targets for soybean improvements via NPBTs and solutions for ethical and public acceptance are also presented.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
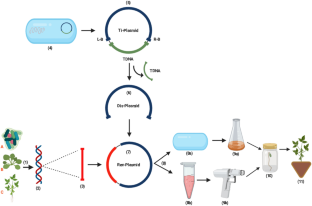
source organism; ( 3 ) amplified gene/DNA fragment from source genomic DNA also known as gene of interest for different trait(s) improvement; ( 4 ) Agrobacterium tumefaciens ; ( 5 ) tumor inducing (Ti) plasmid with T-DNA isolated from A. tumefaciens ; ( 6 ) Disarmed Plasmid; ( 7 ) recombinant DNA with transgene showed in red color; ( 8a ) Agrobacterium transformed by electroporation method; ( 8b ) loading of recombinant DNA onto gold particles; ( 9a ) transformed Agrobacterium culture ready for soybean infection; ( 9b ) biolistic/Gene gun transformation of recombinant plasmid; ( 10 ) regeneration of putative transgenic soybean on selection media, and ( 11 ) acclimatization of transgenic soybean with desired trait(s)
Similar content being viewed by others

Translational Genomics and Breeding in Soybean

Soybean [Glycine max (L.) Merr.] Breeding: History, Improvement, Production and Future Opportunities

Genomic Designing for Abiotic Stress Tolerant Soybean
Baianu, I., You, T., Costescu, D., Lozano, P., Prisecaru, V., & Nelson, R. (2012). Determination of soybean oil, protein and amino acid residues in soybean seeds by high resolution nuclear magnetic resonance (NMRS) and near infrared (NIRS). Nature Precedings, 7 , 1–1.
Google Scholar
Kanchana, P., Santha, M. L., & Raja, K. D. (2015). A review on Glycine max (L.) Merr. (soybean). World Journal of Pharmacy and Pharmaceutical Sciences, 5 (1), 356–371.
Nandakishor, H., Kumar, P., & Mane, S. (2017). Transmission studies of soybean mosaic virus. International Journal of Current Microbiology and Applied Science., 6 (4), 867–869.
Article Google Scholar
Cahoon, E. B. (2003). Genetic enhancement of soybean oil for industrial uses: Prospects and challenges. AgBioforum, 6 (1), 11–13.
Messina, M. (1995). Modern applications for an ancient bean: soybeans and the prevention and treatment of chronic disease. The Journal of Nutrition, 125 (3), 567S-569S.
CAS Google Scholar
Youseif, S. H., El-Megeed, F. H. A., Ageez, A., Mohamed, Z. K., Shamseldin, A., & Saleh, S. A. (2014). Phenotypic characteristics and genetic diversity of rhizobia nodulating soybean in Egyptian soils. European Journal of Soil Biology, 60 , 34–43.
Article CAS Google Scholar
Ogoke, I., Carsky, R., Togun, A., & Dashiell, K. (2003). Maturity class and P effects on soya bean grain yield in the moist savanna of West Africa. Journal of Agronomy and Crop Science, 189 (6), 422–427.
Slavin, J. (1991). Nutritional benefits of soy protein and soy fiber. Journal of the American Dietetic Association., 91 (7), 816–819.
Yaklich, R., Vinyard, B., Camp, M., & Douglass, S. (2002). Analysis of seed protein and oil from soybean northern and southern region uniform tests. Crop Science, 42 (5), 1504–1515.
Wilson, I., & DP, M., & HE, S. (1978). Isolation and characterization of starch from mature soybeans. Cereal Chemistry, 55 (5), 661–670.
Weaver, C. M., & Plawecki, K. L. (1994). Dietary calcium: Adequacy of a vegetarian diet. The American Journal of Clinical Nutrition., 59 (5), 1238S-1241S.
Tepavčević, V., Cvejić, J., Poša, M., & Popović, J. (2011). Isoflavone content and composition in soybean. Soybeanbiochemistry, chemistry, and physiology. Croatia: InTech. (pp. 281–294)
Lee, S. J., Ahn, J. K., Khanh, T. D., Chun, S. C., Kim, S. L., Ro, H. M., et al. (2007). Comparison of isoflavone concentrations in soybean ( Glycine max (L.) Merrill.) sprouts grown under two different light conditions. Journal of Agricultural and Food Chemistry, 55 (23), 9415–9421.
Messina, M. J., & Loprinzi, C. L. (2001). Soy for breast cancer survivors: A critical review of the literature. The Journal of Nutrition, 131 (11), 3095S-3108S.
Carrao-Panizzi, M. C., & Erhan, S. Z. (2007). Environmental and genetic variation of soybean tocopherol content under Brazilian growing conditions. Journal of the American Oil Chemists Society, 84 (10), 921–928.
Luckmann, W. (1971). The insect pests of soybean. World Farm, 13 (5), 18–19.
Gaur, N., & Mogalapu, S. (2018). Pests of Soybean. Pests and Their Management (pp. 137–162). Springer.
Ghosh, L. K. (2008). Handbook on Hemipteran pests in India . Zoological Survey of India.
Mohammad, A. (1981). The groundnut leafminer, Aproaerema modicella Deventer (= Stomopteryx subsecivella Zeller)(Lepidoptera: Gelechiidae). A Review of World Literature., 14 , 33.
Panizzi, A. R., McPherson, J., James, D. G., Javahery, M., & McPherson, R. M. (2000). Stink bugs (Pentatomidae). Heteroptera of Economic Importance, 828 .
Perring, T. M. (2001). The Bemisia tabaci species complex. Crop Protection, 20 (9), 725–737.
Singh, S., Ballal, C., & Poorani, J. (2002). Old world bollworm Helicoverpa armigera , associated Heliothinae and their natural enemies. Bangalore, India, Project Directorate of Biological Control, Technical Bulletin. 31 .
Hodgson, E. (2010). Metabolism of pesticides. Hayes' Handbook of Pesticide Toxicology (pp. 893–921). Elsevier.
Bass, C., Denholm, I., Williamson, M. S., & Nauen, R. (2015). The global status of insect resistance to neonicotinoid insecticides. Pesticide Biochemistry and Physiology., 121 , 78–87.
Hanson, A. A., Menger-Anderson, J., Silverstein, C., Potter, B. D., MacRae, I. V., Hodgson, E. W., & Koch, R. L. (2017). Evidence for soybean aphid (Hemiptera: Aphididae) resistance to pyrethroid insecticides in the upper midwestern United States. Journal of Economic Entomology, 110 (5), 2235–2246.
Oerke, E. (2006). Crop losses to pests. The Journal of Agricultural Science, 144 , 31.
Lal, S., Rana, V., Sapra, R., & Singh, K. (2005). Screening and utilization of soybean germplasm for breeding resistance against Mungbean Yellow Mosaic Virus. Soybean Genet News Letter, 1 , 32.
Hajimorad, M., Domier, L. L., Tolin, S., Whitham, S., & Saghai Maroof, M. (2018). Soybean mosaic virus: A successful potyvirus with a wide distribution but restricted natural host range. Molecular Plant Pathology., 19 (7), 1563–1579.
Buttle, L., Burrells, A., Good, J., Williams, P., Southgate, P., & Burrells, C. (2001). The binding of soybean agglutinin (SBA) to the intestinal epithelium of Atlantic salmon, Salmo salar and Rainbow trout, Oncorhynchus mykiss , fed high levels of soybean meal. Veterinary Immunology and Immunopathology, 80 (3–4), 237–244.
Grant, G. (1989). Anti-nutritional effects of soyabean: A review. Progress in Food & Nutrition Science., 13 (3–4), 317–348.
Liener, I. E. (1994). Implications of antinutritional components in soybean foods. Critical Reviews in Food Science & Nutrition., 34 (1), 31–67.
Potter, L. & Potchanakorn, M. (1985). Digestibility of the carbohydrate fraction of soybean meal by poultry.
Jaffe, G. (1981). Phytic acid in soybeans. Journal of the American Oil Chemists’ Society., 58 (3), 493–495.
Wang, Y.-C., Klein, T. M., Fromm, M., Cao, J., Sanford, J. C., & Wu, R. (1988). Transient expression of foreign genes in rice, wheat and soybean cells following particle bombardment. Plant Molecular Biology, 11 (4), 433–439.
Hinchee, M. A., Connor-Ward, D. V., Newell, C. A., McDonnell, R. E., Sato, S. J., Gasser, C. S., et al. (1988). Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Nature Biotechnology., 6 (8), 915.
Carpenter, J. E., & Gianessi, L. P. (2001). Agricultural biotechnology: Updated benefit estimates . Washington, DC: National Center for Food and Agricultural Policy.
James, C. (2003). Global review of commercialized transgenic crops. Current Science., 84 (3), 303–309.
Scheitrum, D., Schaefer, K. A., & Nes, K. (2020). Realized and potential global production effects from genetic engineering. Food Policy, 93 , 101882.
Green, J. M., Hazel, C. B., Forney, D. R., & Pugh, L. M. (2008). New multiple-herbicide crop resistance and formulation technology to augment the utility of glyphosate. Pest Management Science., 64 (4), 332–339.
Waltz, E. (2010). Food firms test fry Pioneer’s trans fat-free soybean oil. Nature Biotechnology, 28 , 769.
Pham, A. T., Lee, J.-D., Shannon, J. G., & Bilyeu, K. D. (2010). Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biology, 10 , 195–195.
Pham, A. T., Shannon, J. G., & Bilyeu, K. D. (2012). Combinations of mutant FAD2 and FAD3 genes to produce high oleic acid and low linolenic acid soybean oil. Theoretical and Applied Genetics, 125 (3), 503–515.
Demorest, Z. L., Coffman, A., Baltes, N. J., Stoddard, T. J., Clasen, B. M., Luo, S., et al. (2016). Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biology., 16 (1), 225.
Benbrook, C. (1999). Evidence of the magnitude and consequences of the Roundup Ready soybean yield drag from university-based varietal trials in 1998 (Vol. 1): Citeseer.
Phillips, M. (2011). The cost and time involved in the discovery, development and authorization of a new plant biotechnology derived trait. Crop Life International 1–24.
Hesler, L. S. (2013). Resistance to soybean aphid among wild soybean lines under controlled conditions. Crop Protection., 53 , 139–146.
Bales, C., Zhang, G., Liu, M., Mensah, C., Gu, C., Song, Q., et al. (2013). Mapping soybean aphid resistance genes in PI 567598B. Theoretical and Applied Genetics., 126 (8), 2081–2091.
Hill, C. B., Li, Y., & Hartman, G. L. (2006). A single dominant gene for resistance to the soybean aphid in the soybean cultivar Dowling. Crop Science., 46 (4), 1601–1605.
Jun, T., Mian, M. R., & Michel, A. (2013). Genetic mapping of three quantitative trait loci for soybean aphid resistance in PI 567324. Heredity, 111 (1), 16–22.
Li, Y., Hill, C. B., Carlson, S. R., Diers, B. W., & Hartman, G. L. (2007). Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group M. Molecular Breeding, 19 (1), 25–34.
Mian, M. R., Kang, S.-T., Beil, S. E., & Hammond, R. B. (2008). Genetic linkage mapping of the soybean aphid resistance gene in PI 243540. Theoretical and Applied Genetics., 117 (6), 955–962.
Zhang, G., Gu, C., & Wang, D. (2009). Molecular mapping of soybean aphid resistance genes in PI 567541B. Theoretical and Applied Genetics, 118 (3), 473–482.
Kim, K.-S., Hill, C. B., Hartman, G. L., Hyten, D. L., Hudson, M. E., & Diers, B. W. (2010). Fine mapping of the soybean aphid-resistance gene Rag2 in soybean PI 200538. Theoretical and Applied Genetics., 121 (3), 599–610.
Zhang, F., Maeder, M. L., Unger-Wallace, E., Hoshaw, J. P., Reyon, D., Christian, M., et al. (2010). High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proceedings of the National Academy of Sciences, 107 (26), 12028–12033.
Kim, K.-S., Hill, C. B., Hartman, G. L., Mian, M., & Diers, B. W. (2008). Discovery of soybean aphid biotypes. Crop Science., 48 (3), 923–928.
Stewart, C. N., Jr., Adang, M. J., All, J. N., Boerma, H. R., Cardineau, G., & D. Tucker & Parrott, W. A. (1996). Genetic transformation, recovery, and characterization of fertile soybean transgenic for a synthetic Bacillus thuringiensis cryIAc gene. Plant Physiology, 112 (1), 121–129.
Furutani, N., Hidaka, S., Kosaka, Y., Shizukawa, Y., & Kanematsu, S. (2006). Coat protein gene-mediated resistance to soybean mosaic virus in transgenic soybean. Breeding Science., 56 (2), 119–124.
Kim, H. J., Kim, M.-J., Pak, J. H., Im, H. H., Lee, D. H., Kim, K.-H., Lee, J.-H., Kim, D.-H., Choi, H. K., & Jung, H. W. (2016). RNAi-mediated Soybean mosaic virus (SMV) resistance of a Korean Soybean cultivar . Springer.
Book Google Scholar
Yang, J., Xing, G., Niu, L., He, H., Guo, D., Du, Q., et al. (2018). Improved oil quality in transgenic soybean seeds by RNAi-mediated knockdown of GmFAD2-1B . Transgenic Research, 27 (2), 155–166.
Kumari, A., Hada, A., Subramanyam, K., Theboral, J., Misra, S., Ganapathi, A., & Malathi, V. G. (2018). RNAi-mediated resistance to yellow mosaic viruses in soybean targeting coat protein gene. Acta Physiologiae Plantarum, 40 (2), 32.
Singh, V. B., Haq, Q., & Malathi, V. (2013). Antisense RNA approach targeting Rep gene of Mungbean yellow mosaic India virus to develop resistance in soybean. Archives of Phytopathology and Plant Protection, 46 (18), 2191–2207.
Lund, M. E., Mourtzinis, S., Conley, S. P., & Ané, J. M. (2018). Soybean cyst nematode control with Pasteuria nishizawae under different management practices. Agronomy Journal, 110 (6), 2534–2540.
Cook, D. E., Lee, T. G., Guo, X., Melito, S., Wang, K., Bayless, A. M., et al. (2012). Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science, 338 (6111), 1206–1209.
Lin, J., Mazarei, M., Zhao, N., Hatcher, C. N., Wuddineh, W. A., Rudis, M., et al. (2016). Transgenic soybean overexpressing Gm SAMT 1 exhibits resistance to multiple-HG types of soybean cyst nematode Heterodera glycines . Plant Biotechnology Journal., 14 (11), 2100–2109.
Lu, L., Dong, C., Liu, R., Zhou, B., Wang, C., & Shou, H. (2018). Roles of soybean plasma membrane intrinsic protein GmPIP2; 9 in drought tolerance and seed development. Frontiers in Plant Science., 9 , 530.
Bhatnagar-Mathur, P., Devi, M. J., Reddy, D. S., Lavanya, M., Vadez, V., Serraj, R., et al. (2007). Stress-inducible expression of AtDREB1A in transgenic peanut ( Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Reports, 26 (12), 2071–2082.
Shinozaki, K., & Yamaguchi-Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany., 58 (2), 221–227.
Fuganti-Pagliarini, R., Ferreira, L. C., Rodrigues, F. A., Molinari, H. B., Marin, S. R., Molinari, M. D., et al. (2017). Characterization of soybean genetically modified for drought tolerance in field conditions. Frontiers in Plant Science., 8 , 448.
Polizel, A., Medri, M., Nakashima, K., Yamanaka, N., Farias, J., de Oliveira, M., Marin, S., Abdelnoor, R., Marcelino, F., & Fuganti, R. (2011). Molecular, anatomical and physiological properties of a genetically modified soybean line transformed with rd29A: AtDREB1A for the improvement of drought tolerance. Genetics and Molecular Research, 10 (4), 3641–3656.
Hamwieh, A., Tuyen, D., Cong, H., Benitez, E., Takahashi, R., & Xu, D. (2011). Identification and validation of a major QTL for salt tolerance in soybean. Euphytica, 179 (3), 451–459.
He, Y., Yang, X., Xu, C., Guo, D., Niu, L., Wang, Y., et al. (2018). Overexpression of a novel transcriptional repressor GmMYB3a negatively regulates salt–alkali tolerance and stress-related genes in soybean. Biochemical and Biophysical Research Communications., 498 (3), 586–591.
An, J., Cheng, C., Hu, Z., Chen, H., Cai, W., & Yu, B. (2018). The Panax ginseng PgTIP1 gene confers enhanced salt and drought tolerance to transgenic soybean plants by maintaining homeostasis of water, salt ions and ROS. Environmental and Experimental Botany., 155 , 45–55.
Cao, D., Hou, W., Liu, W., Yao, W., Wu, C., Liu, X., & Han, T. (2011). Overexpression of TaNHX2 enhances salt tolerance of ‘composite’and whole transgenic soybean plants. Plant Cell, Tissue and Organ Culture., 107 (3), 541–552.
Li, T. Y., Zhang, Y., Liu, H., Wu, Y., Li, W., & Zhang, H. (2010). Stable expression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1, and salt tolerance in transgenic soybean for over six generations. Chinese Science Bulletin, 55 (12), 1127–1134.
Wang, Y., Jiang, L., Chen, J., Tao, L., An, Y., Cai, H., & Guo, C. (2018). Overexpression of the alfalfa WRKY11 gene enhances salt tolerance in soybean. PLoS ONE, 13 (2), e092382.
Cheng, C., Li, C., Wang, D., Zhai, L., & Cai, Z. (2018). The soybean gmNARK affects ABA and salt responses in transgenic Arabidopsis thaliana . Frontiers in Plant Science., 9 , 514.
Ahmed, F., Rafii, M., Ismail, M. R., Juraimi, A. S., Rahim, H., Asfaliza, R., & Latif, M. A. (2013). Waterlogging tolerance of crops: Breeding, mechanism of tolerance, molecular approaches, and future prospects. BioMedical Research International, 1 , 10. https://doi.org/10.1155/2013/963525
Sullivan, M., VanToai, T., Fausey, N., Beuerlein, J., Parkinson, R., & Soboyejo, A. (2001). Evaluating on-farm flooding impacts on soybean. Crop Science, 41 (1), 93–100.
Zhao, T., Aleem, M., & Sharmin, R. A. (2018). Adaptation to water stress in soybean: morphology to genetics. Plant, abiotic stress and responses to climate change. Intech Open, London , pp. 33–68.
Lu, Y., An, Y., Lv, C., Ma, W., Xi, Y., & Xiao, R. (2018). Dietary soybean isoflavones in Alzheimer’s disease prevention. Asia Pacific Journal of Clinical Nutrition, 27 (5), 946–954.
Subramanian, S., Graham, M. Y., Yu, O., & Graham, T. L. (2005). RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae . Plant Physiology, 137 (4), 1345–1353.
Funaki, A., Waki, T., Noguchi, A., Kawai, Y., Yamashita, S., Takahashi, S., & Nakayama, T. (2015). Identification of a highly specific isoflavone 7- O -glucosyltransferase in the soybean ( Glycine max (L.) Merr.). Plant and Cell Physiology, 56 (8), 1512–1520.
Zhao, M., Wang, T., Wu, P., Guo, W., Su, L., Wang, Y., et al. (2017). Isolation and characterization of GmMYBJ3, an R2R3-MYB transcription factor that affects isoflavonoids biosynthesis in soybean. PLoS ONE, 12 (6), e0179990.
Chu, S., Wang, J., Zhu, Y., Liu, S., Zhou, X., Zhang, H., et al. (2017). An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genetics., 13 (5), 100–6770.
Cheng, Q., Li, N., Dong, L., Zhang, D., Fan, S., Jiang, L., et al. (2015). Overexpression of soybean isoflavone reductase (GmIFR) enhances resistance to Phytophthora sojae in soybean. Frontiers in Plant Science., 6 , 1024.
Veremeichik, G., Grigorchuk, V., Silanteva, S., Shkryl, Y., Bulgakov, D., Brodovskaya, E., & Bulgakov, V. (2019). Increase in isoflavonoid content in Glycine max cells transformed by the constitutively active Ca 2+ independent form of the AtCPK1 gene. Phytochemistry, 157 , 111–120.
Kim, M. J., Kim, J. K., Kim, H. J., Pak, J. H., Lee, J. H., Kim, D. H., et al. (2012). Genetic modification of the soybean to enhance the β-carotene content through seed-specific expression. PLoS ONE, 7 (10), e48287.
Zimmermann, R., & Qaim, M. (2004). Potential health benefits of Golden Rice: A Philippine case study. Food Policy, 29 (2), 147–168.
Kim, W.-S., Chronis, D., Juergens, M., Schroeder, A. C., Hyun, S. W., Jez, J. M., & Krishnan, H. B. (2012). Transgenic soybean plants overexpressing O -acetylserine sulfhydrylase accumulate enhanced levels of cysteine and Bowman-Birk protease inhibitor in seeds. Planta, 235 (1), 13–23.
Karunanandaa, B., Qi, Q., Hao, M., Baszis, S. R., Jensen, P. K., Wong, Y.-H.H., et al. (2005). Metabolically engineered oilseed crops with enhanced seed tocopherol. Metabolic Engineering, 7 (5–6), 384–400.
Van Eenennaam, A. L., Lincoln, K., Durrett, T. P., Valentin, H. E., Shewmaker, C. K., Thorne, G. M., et al. (2003). Engineering vitamin E content: From Arabidopsis mutant to soy oil. The Plant Cell, 15 (12), 3007–3019.
Tavva, V. S., Kim, Y.-H., Kagan, I. A., Dinkins, R. D., Kim, K.-H., & Collins, G. B. (2007). Increased α-tocopherol content in soybean seed overexpressing the Perilla frutescens γ-tocopherol methyltransferase gene. Plant Cell Reports, 26 (1), 61–70.
Krishnan, H. B., & Jez, J. M. (2018). The promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Plant Science, 272 , 14–21.
El-Shemy, H., Khalafalla, M., Fujita, K., & Ishimoto, M. (2007). Improvement of protein quality in transgenic soybean plants. Biologia Plantarum., 51 (2), 277–284.
Koshiyama, I. (1968). Chemical and physical properties of a 7S protein in soybean globulins. Cereal Chemistry, 45 , 394–404.
Falco, S., Guida, T., Locke, M., Mauvais, J., Sanders, C., Ward, R., & Webber, P. (1995). Transgenic canola and soybean seeds with increased lysine. Biotechnology, 13 (6), 577–582.
Flores, T., Karpova, O., Su, X., Zeng, P., Bilyeu, K., Sleper, D. A., et al. (2008). Silencing of Gm FAD3 gene by siRNA leads to low α-linolenic acids (18: 3) of fad3-mutant phenotype in soybean [ Glycine max (Merr.)]. Transgenic Research., 17 (5), 839–850.
Chen, W., Song, K., Cai, Y., Li, W., Liu, B., & Liu, L. (2011). Genetic modification of soybean with a novel grafting technique: Downregulating the FAD2-1 gene increases oleic acid content. Plant Molecular Biology Reporter., 29 (4), 866–874.
Bilyeu, K., Škrabišová, M., Allen, D., Rajcan, I., Palmquist, D. E., Gillen, A., et al. (2018). The interaction of the soybean seed high oleic acid oil trait with other fatty acid modifications. Journal of the American Oil Chemists’ Society., 95 (1), 39–49.
Valentine, M. F., De Tar, J. R., Mookkan, M., Firman, J. D., & Zhang, Z. J. (2017). Silencing of soybean raffinose synthase gene reduced raffinose family oligosaccharides and increased true metabolizable energy of poultry feed. Frontiers in Plant Science., 8 , 692.
Krishnan, H. B., Kim, W.-S., Jang, S., & Kerley, M. S. (2009). All three subunits of soybean β-conglycinin are potential food allergens. Journal of Agricultural and Food Chemistry, 57 (3), 938–943.
Herman, E. M., Helm, R. M., Jung, R., & Kinney, A. J. (2003). Genetic modification removes an immunodominant allergen from soybean. Plant Physiology., 132 (1), 36–43.
Watanabe, D., Lošák, T., & Vollmann, J. (2018). From proteomics to ionomics: Soybean genetic improvement for better food safety. Genetika, 50 (1), 333–350.
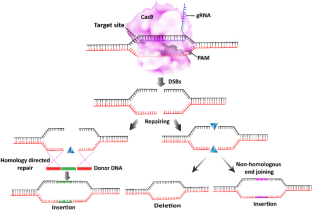
Liu, X., Wu, S., Xu, J., Sui, C., & Wei, J. (2017). Application of CRISPR/Cas9 in plant biology. Acta Pharmaceutica Sinica B., 7 (3), 292–302.
Zaidi, S., & S.-e.-A., Vanderschuren, H., Qaim, M., Mahfouz, M. M., Kohli, A., Mansoor, S., & Tester, M. (2019). New plant breeding technologies for food security. Science, 363 (6434), 1390–1391.
Osakabe, K., Osakabe, Y., & Toki, S. (2010). Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proceedings of the National Academy of Sciences, 107 (26), 12034–12039.
Du, H., Zeng, X., Zhao, M., Cui, X., Wang, Q., Yang, H., et al. (2016). Efficient targeted mutagenesis in soybean by TALENs and CRISPR/Cas9. Journal of Biotechnology., 217 , 90–97.
Gao, J., Wang, G., Ma, S., Xie, X., Wu, X., Zhang, X., et al. (2015). CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum . Plant Molecular Biology., 87 (1–2), 99–110.
Mao, Y., Zhang, H., Xu, N., Zhang, B., Gou, F., & Zhu, J.-K. (2013). Application of the CRISPR–Cas system for efficient genome engineering in plants. Molecular Plant, 6 (6), 2008–2011.
Schiml, S., Fauser, F., & Puchta, H. (2014). The CRISPR/C as system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in A rabidopsis resulting in heritable progeny. The Plant Journal, 80 (6), 1139–1150.
Upadhyay, S. K., Kumar, J., Alok, A., & Tuli, R. (2013). RNA-guided genome editing for target gene mutations in wheat. G3: Genes, Genomes, Genetics, 3 (12), 2233–2238.
Curtin, S. J., Voytas, D. F., & Stupar, R. M. (2012). Genome engineering of crops with designer nucleases. The Plant Genome, 5 (2), 42–50.
Mohanta, T. K., Bashir, T., Hashem, A., Abd Allah, E. F., & Bae, H. (2017). Genome editing tools in plants. Genes., 8 (12), 399.
Sánchez-Rivera, F. J., & Jacks, T. (2015). Applications of the CRISPR–Cas9 system in cancer biology. Nature Reviews Cancer, 15 (7), 387–395.
Weinthal, D., Tovkach, A., Zeevi, V., & Tzfira, T. (2010). Genome editing in plant cells by zinc finger nucleases. Trends in Plant Science, 15 (6), 308–321.
Boch, J., Scholze, H., Schornack, S., Landgraf, A., Hahn, S., Kay, S., et al. (2009). Breaking the code of DNA binding specificity of TAL-type III effectors. Science, 326 (5959), 1509–1512.
Deveau, H., Garneau, J. E., & Moineau, S. (2010). CRISPR/Cas system and its role in phage-bacteria interactions. Annual Review of Microbiology., 64 , 475–493.
Joung, J. K., & Sander, J. D. (2013). TALENs: A widely applicable technology for targeted genome editing. Nature Reviews Molecular Cell Biology, 14 (1), 49–55.
Sorek, R., Lawrence, C. M., & Wiedenheft, B. (2013). CRISPR-mediated adaptive immune systems in bacteria and archaea. Annual Review of Biochemistry., 82 , 237–266.
Sun, X., Hu, Z., Chen, R., Jiang, Q., Song, G., Zhang, H., & Xi, Y. (2015). Targeted mutagenesis in soybean using the CRISPR-Cas9 system. Scientific Reports, 5 (1), 1–10.
Gao, H., Wu, X., Chai, J., & Han, Z. (2012). Crystal structure of a TALE protein reveals an extended N-terminal DNA binding region. Cell Research, 22 (12), 1716–1720.
El-Mounadi, K., Morales-Floriano, M. L., & Garcia-Ruiz, H. (2020). Principles, applications, and biosafety of plant genome editing using CRISPR-Cas9. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2020.00056
Shan, Q., Wang, Y., Li, J., Zhang, Y., Chen, K., Liang, Z., Zhang, K., Liu, J., Xi, J. J., & Qiu, J. L. (2013). Targeted genome modification of crop plants using a CRISPR-Cas system. Nature Biotechnology, 31 (8), 686–688.
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., Romero, D. A., & Horvath, P. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science, 315 (5819), 1709–1712.
Hille, F., & Charpentier, E. (2016). CRISPR-Cas: Biology, mechanisms and relevance. Philosophical Transactions of the Royal Society B: Biological Sciences, 371 (1707), 20150496.
Murovec, J., Pirc, Z., & Yang, B. (2017). New variants of CRISPR RNA-guided genome editing enzymes. Plant Biotechnology Journal., 15 (8), 917–926.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337 (6096), 816–821.
Sonoda, E., Hochegger, H., Saberi, A., Taniguchi, Y., & Takeda, S. (2006). Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair, 5 (9–10), 1021–1029.
Barnes, D. E. (2001). Non-homologous end joining as a mechanism of DNA repair. Current Biology, 11 (12), R455–R457.
Čermák, T., Curtin, S. J., Gil-Humanes, J., Cegan, R., Kono, T. J., Konečná, E., et al. (2017). A multipurpose toolkit to enable advanced genome engineering in plants. The Plant Cell, 29 (6), 1196–1217.
Curtin, S. J., Xiong, Y., Michno, J. M., Campbell, B. W., Stec, A. O., Čermák, T., et al. (2018). Crispr/cas9 and talen s generate heritable mutations for genes involved in small RNA processing of glycine max and medicago truncatula. Plant Biotechnology Journal., 16 (6), 1125–1137.
Osakabe, Y., & Osakabe, K. (2015). Genome editing with engineered nucleases in plants. Plant and Cell Physiology., 56 (3), 389–400.
Schaeffer, S. M., & Nakata, P. A. (2015). CRISPR/Cas9-mediated genome editing and gene replacement in plants: Transitioning from lab to field. Plant Science, 240 , 130–142.
Liu, X., Xie, C., Si, H., & Yang, J. (2017). CRISPR/Cas9-mediated genome editing in plants. Methods, 121 , 94–102.
Butt, H., Rao, G. S., Sedeek, K., Aman, R., Kamel, R., & Mahfouz, M. (2020). Engineering herbicide resistance via prime editing in rice. Plant Biotechnology Journal, 18 (12), 2370.
Soda, N., Verma, L., & Giri, J. (2018). CRISPR-Cas9 based plant genome editing: Significance, opportunities and recent advances. Plant Physiology and Biochemistry., 131 , 2–11.
Katayose, Y., Kanamori, H., Shimomura, M., Ohyanagi, H., Ikawa, H., Minami, H., et al. (2012). DaizuBase, an integrated soybean genome database including BAC-based physical maps. Breeding Science, 61 (5), 661–664.
Liu, Y., Du, H., Li, P., Shen, Y., Peng, H., Liu, S., et al. (2020). Pan-genome of wild and cultivated soybeans. Cell, 182 (1), 162–176.
Schmutz, J., Cannon, S. B., Schlueter, J., Ma, J., Mitros, T., Nelson, W., et al. (2010). Genome sequence of the palaeopolyploid soybean. Nature, 463 (7278), 178–183.
Haun, W., Coffman, A., Clasen, B. M., Demorest, Z. L., Lowy, A., Ray, E., et al. (2014). Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnology Journal., 12 (7), 934–940.
Cai, Y., Chen, L., Liu, X., Sun, S., Wu, C., Jiang, B., et al. (2015). CRISPR/Cas9-mediated genome editing in soybean hairy roots. PLoS ONE, 10 (8), e0136064.
Jacobs, T. B., LaFayette, P. R., Schmitz, R. J., & Parrott, W. A. (2015). Targeted genome modifications in soybean with CRISPR/Cas9. BMC Biotechnology, 15 (1), 16.
Elvira-Torales, L. I., García-Alonso, J., & Periago-Castón, M. J. (2019). Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants., 8 (7), 229.
Li, Z., Liu, Z.-B., Xing, A., Moon, B. P., Koellhoffer, J. P., Huang, L., et al. (2015). Cas9-guide RNA directed genome editing in soybean. Plant Physiology, 169 (2), 960–970.
Bao, A., Chen, H., Chen, L., Chen, S., Hao, Q., Guo, W., et al. (2019). CRISPR/Cas9-mediated targeted mutagenesis of GmSPL9 genes alters plant architecture in soybean. BMC Plant Biology, 19 (1), 131.
Tang, F., Yang, S., Liu, J., & Zhu, H. (2016). Rj4, a gene controlling nodulation specificity in soybeans, encodes a thaumatin-like protein but not the one previously reported. Plant Physiology, 170 (1), 26–32.
Michno, J.-M., Wang, X., Liu, J., Curtin, S. J., Kono, T. J., & Stupar, R. M. (2015). CRISPR/Cas mutagenesis of soybean and Medicago truncatula using a new web-tool and a modified Cas9 enzyme. GM Crops & Food, 6 (4), 243–252.
Curtin, S. J., Zhang, F., Sander, J. D., Haun, W. J., Starker, C., Baltes, N. J., et al. (2011). Targeted mutagenesis of duplicated genes in soybean with zinc-finger nucleases. Plant Physiology., 156 (2), 466–473.
Cai, Y., Chen, L., Liu, X., Guo, C., Sun, S., Wu, C., et al. (2018). CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnology Journal, 16 (1), 176–185.
Cai, Y., Wang, L., Chen, L., Wu, T., Liu, L., Sun, S., et al. (2020). Mutagenesis of GmFT2a and GmFT5a mediated by CRISPR/Cas9 contributes for expanding the regional adaptability of soybean. Plant Biotechnology Journal., 18 (1), 298–309.
Wu, N., Lu, Q., Wang, P., Zhang, Q., Zhang, J., Qu, J., & Wang, N. (2020). Construction and analysis of GmFAD2-1A and GmFAD2-2A soybean fatty acid desaturase mutants based on CRISPR/Cas9 technology. International Journal of Molecular Sciences., 21 (3), 1104.
Bonawitz, N. D., Ainley, W. M., Itaya, A., Chennareddy, S. R., Cicak, T., Effinger, K., Jiang, K., Mall, T. K., Marri, P. R., & Samuel, J. P. (2019). Zinc finger nuclease-mediated targeting of multiple transgenes to an endogenous soybean genomic locus via non-homologous end joining. Plant Biotechnology Journal, 17 (4), 750–761.
Kanazashi, Y., Hirose, A., Takahashi, I., Mikami, M., Endo, M., Hirose, S., et al. (2018). Simultaneous site-directed mutagenesis of duplicated loci in soybean using a single guide RNA. Plant Cell Reports, 37 (3), 553–563.
Di, Y.-H., Sun, X.-J., Hu, Z., Jiang, Q.-Y., Song, G.-H., Zhang, B., et al. (2019). Enhancing the CRISPR/Cas9 system based on multiple GmU6 promoters in soybean. Biochemical and Biophysical Research Communications, 519 (4), 819–823.
Do, P. T., Nguyen, C. X., Bui, H. T., Tran, L. T., Stacey, G., Gillman, J. D., et al. (2019). Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2–1A and GmFAD2–1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biology., 19 (1), 311.
Li, C., Nguyen, V., Liu, J., Fu, W., Chen, C., Yu, K., & Cui, Y. (2019). Mutagenesis of seed storage protein genes in Soybean using CRISPR/Cas9. BMC Research Notes., 12 (1), 176.
Al Amin, N., Ahmad, N., Nan, W., Xiuming, F., Nan, W., Xiaoxue, B., et al. (2018). An efficient transient assay for CRISPR CAS9 system delivering targeted mutation using synthetic oligo SgRNA in soybean (Glycine max). Pakistan Journal of Botany, 50 (6), 2223–2230.
Bai, M., Yuan, J., Kuang, H., Gong, P., Li, S., Zhang, Z., et al. (2020). Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnology Journal., 18 (3), 721–731.
Sander, J. D., Dahlborg, E. J., Goodwin, M. J., Cade, L., Zhang, F., Cifuentes, D., et al. (2011). Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nature Methods, 8 (1), 67–69.
Kim, H., Kim, S. T., Ryu, J., Kang, B. C., Kim, J. S., & Kim, S. G. (2017). CRISPR/Cpf1-mediated DNA-free plant genome editing. Nature Communications, 8 (1), 1–7.
Wang, J., Kuang, H., Zhang, Z., Yang, Y., Yan, L., Zhang, M., et al. (2019). Generation of seed lipoxygenase-free soybean using CRISPR-Cas9. The Crop Journal, 8 (3), 432–439.
Campbell, B. W., Hoyle, J. W., Bucciarelli, B., Stec, A. O., Samac, D. A., Parrott, W. A., & Stupar, R. M. (2019). Functional analysis and development of a CRISPR/Cas9 allelic series for a CPR5 ortholog necessary for proper growth of soybean trichomes. Scientific Reports, 9 (1), 1–11.
Wang, L., Sun, S., Wu, T., Liu, L., Sun, X., Cai, Y., et al. (2020). Natural variation and CRISPR/Cas9-mediated mutation in GmPRR37 affect photoperiodic flowering and contribute to regional adaptation of soybean. Plant Biotechnology Journal, 18 , 1869–1881.
Cai, Y., Chen, L., Zhang, Y., Yuan, S., Su, Q., Sun, S., et al. (2020). Target base editing in soybean using a modified CRISPR/Cas9 system. Plant Biotechnology Journal., 18 (10), 1996–1998.
Zhang, P., Du, H., Wang, J., Pu, Y., Yang, C., Yan, R., et al. (2020). Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnology Journal, 18 (6), 1384–1395.
Cheng, Q., Dong, L., Su, T., Li, T., Gan, Z., Nan, H., et al. (2019). CRISPR/Cas9-mediated targeted mutagenesis of GmLHY genes alters plant height and internode length in soybean. BMC Plant Biology, 19 (1), 1–11.
Yang, C., Huang, Y., Lv, W., Zhang, Y., Bhat, J. A., Kong, J., et al. (2020). GmNAC8 acts as a positive regulator in soybean drought stress. Plant Science, 293 , 110442.
Li, C., Li, Y.-H., Li, Y., Lu, H., Hong, H., Tian, Y., et al. (2020). A domestication-associated gene GmPRR3b regulates the circadian clock and flowering time in Soybean. Molecular Plant, 13 (5), 745–759.
Wang, Y., Yuan, L., Su, T., Wang, Q., Gao, Y., Zhang, S., et al. (2020). Light-and temperature-entrainable circadian clock in soybean development. Plant, Cell & Environment., 43 (3), 637–648.
Zheng, N., Li, T., Dittman, J. D., Su, J., Li, R., Gassmann, W., et al. (2020). CRISPR/Cas9-based gene editing using egg cell-specific promoters in Arabidopsis and soybean. Frontiers in Plant Science, 11 , 800.
Ge, L., Yu, J., Wang, H., Luth, D., Bai, G., Wang, K., & Chen, R. (2016). Increasing seed size and quality by manipulating BIG SEEDS1 in legume species. Proceedings of the National Academy of Sciences., 113 (44), 12414–12419.
Stacey, M. G., Cahoon, R. E., Nguyen, H. T., Cui, Y., Sato, S., Nguyen, C. T., et al. (2016). Identification of homogentisate dioxygenase as a target for vitamin E biofortification in oilseeds. Plant Physiology, 172 (3), 1506–1518.
Tang, X., Su, T., Han, M., Wei, L., Wang, W., Yu, Z., et al. (2017). Suppression of extracellular invertase inhibitor gene expression improves seed weight in soybean (Glycine max). Journal of Experimental Botany, 68 (3), 469–482.
Ping, J., Liu, Y., Sun, L., Zhao, M., Li, Y., She, M., et al. (2014). Dt2 is a gain-of-function MADS-domain factor gene that specifies semideterminacy in soybean. The Plant Cell, 26 (7), 2831–2842.
Xu, J., Kang, B. C., Naing, A. H., Bae, S. J., Kim, J. S., Kim, H., & Kim, C. K. (2020). CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnology Journal, 18 (1), 287–297.
Liu, W., Jiang, B., Ma, L., Zhang, S., Zhai, H., Xu, X., et al. (2018). Functional diversification of Flowering Locus T homologs in soybean: GmFT1a and GmFT2a/5a have opposite roles in controlling flowering and maturation. New Phytologist, 217 (3), 1335–1345.
Guo, W., Chen, L., Chen, H., Yang, H., You, Q., Bao, A., et al. (2020). Overexpression of GmWRI1b in soybean stably improves plant architecture and associated yield parameters, and increases total seed oil production under field conditions. Plant Biotechnology Journal., 18 (8), 1639–1641.
Zhang, G., Bahn, S.-C., Wang, G., Zhang, Y., Chen, B., Zhang, Y., et al. (2019). PLDα1-knockdown soybean seeds display higher unsaturated glycerolipid contents and seed vigor in high temperature and humidity environments. Biotechnology for Biofuels, 12 (1), 1–23.
Li, J., Meng, X., Zong, Y., Chen, K., Zhang, H., Liu, J., et al. (2016). Gene replacements and insertions in rice by intron targeting using CRISPR–Cas9. Nature Plants, 2 (10), 1–6.
Chandrasekaran, J., Brumin, M., Wolf, D., Leibman, D., Klap, C., Pearlsman, M., et al. (2016). Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Molecular Plant Pathology, 17 (7), 1140–1153.
Shan, Q., & Voytas, D. F. (2018). Editing plant genes one base at a time. Nature Plants, 4 (7), 412–413.
Zong, Y., Wang, Y., Li, C., Zhang, R., Chen, K., Ran, Y., et al. (2017). Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nature Biotechnology., 35 (5), 438.
Butt, H., Eid, A., Ali, Z., Atia, M. A., Mokhtar, M. M., Hassan, N., et al. (2017). Efficient CRISPR/Cas9-mediated genome editing using a chimeric single-guide RNA molecule. Frontiers in Plant Science, 8 , 1441.
Doudna, J. A., & Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science . https://doi.org/10.1126/science.1258096
Wang, M., Lu, Y., Botella, J. R., Mao, Y., Hua, K., & Zhu, J.-K. (2017). Gene targeting by homology-directed repair in rice using a geminivirus-based CRISPR/Cas9 system. Molecular Plant, 10 (7), 1007–1010.
Csörgő, B., León, L. M., Chau-Ly, I. J., Vasquez-Rifo, A., Berry, J. D., Mahendra, C., et al. (2020). A compact Cascade–Cas3 system for targeted genome engineering. Nature Methods , 1–8.
Ali, Z., Shami, A., Sedeek, K., Kamel, R., Alhabsi, A., Tehseen, M., et al. (2020). Fusion of the Cas9 endonuclease and the VirD2 relaxase facilitates homology-directed repair for precise genome engineering in rice. Communications Biology, 3 (1), 1–13.
Araki, M., & Ishii, T. (2015). Towards social acceptance of plant breeding by genome editing. Trends in Plant Science, 20 (3), 145–149.
Nakajima, O., Nishimaki-Mogami, T., & Kondo, K. (2016). Cas9 in genetically modified food is unlikely to cause food allergy. Biological and Pharmaceutical Bulletin, 39 (11), 1876–1880.
Servick, K. (2015). US to review agricultural biotech regulations. American Association for the Advancement of Science . 131.
Gao, C. (2018). The future of CRISPR technologies in agriculture. Nature Reviews Molecular Cell Biology., 19 (5), 275–276.
Jones, H. D. (2015). Regulatory uncertainty over genome editing. Nature Plants, 1 (1), 1–3.
Seyran, E., & Craig, W. (2018). New breeding techniques and their possible regulation. AgBioforum, 21 (1), 1–12.
Duensing, N., Sprink, T., Parrott, W. A., Fedorova, M., Lema, M. A., Wolt, J. D., & Bartsch, D. (2018). Novel features and considerations for ERA and regulation of crops produced by genome editing. Frontiers in Bioengineering and Biotechnology., 6 , 79.
Jones, H. D. (2015). Future of breeding by genome editing is in the hands of regulators. GM Crops & Food., 6 (4), 223–232.
Parrott, W. (2018). Outlaws, old laws and no laws: The prospects of gene editing for agriculture in United States. Physiologia Plantarum, 164 (4), 406–411.
Mackelprang, R., & Lemaux, P. G. (2020). Genetic engineering and editing of plants: an analysis of new and persisting questions. Annual Review of Plant Biology, 71 , 659–687.
Khandelwal, R., & Jain, M. (2018). Genome engineering tools for functional genomics and crop improvement in legumes. Pulse Improvement (pp. 219–234). Springer.
Zhang, D., Hussain, A., Manghwar, H., Xie, K., Xie, S., Zhao, S., et al. (2020). Genome editing with the CRISPR-Cas system: An art, ethics and global regulatory perspective. Plant Biotechnology Journal., 18 (8), 1651–1669.
Ahmad, S., Wei, X., Sheng, Z., Hu, P., & Tang, S. (2020). CRISPR/Cas9 for development of disease resistance in plants: Recent progress, limitations and future prospects. Briefings in Functional Genomics, 19 (1), 26–39.
Mao, Y., Zhang, Z., Feng, Z., Wei, P., Zhang, H., Botella, J. R., & Zhu, J. K. (2016). Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnology Journal, 14 (2), 519–532.
Ali, Z., Ali, S., Tashkandi, M., Zaidi, S., & S.-e.-A., & Mahfouz, M. M. (2016). CRISPR/Cas9-mediated immunity to geminiviruses: Differential interference and evasion. Scientific Reports, 6 (1), 1–13.
Pyott, D. E., Sheehan, E., & Molnar, A. (2016). Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Molecular Plant Pathology, 17 (8), 1276–1288.
Ali, Z., Abulfaraj, A., Idris, A., Ali, S., Tashkandi, M., & Mahfouz, M. M. (2015). CRISPR/Cas9-mediated viral interference in plants. Genome Biology, 16 (1), 238.
Iqbal, Z., Sattar, M. N., & Shafiq, M. (2016). CRISPR/Cas9: A tool to circumscribe cotton leaf curl disease. Frontiers in Plant Science, 7 , 475.
Cunningham, F. J., Goh, N. S., Demirer, G. S., Matos, J. L., & Landry, M. P. (2018). Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends in Biotechnology., 36 (9), 882–897.
Deng, H., Huang, W., & Zhang, Z. (2019). Nanotechnology based CRISPR/Cas9 system delivery for genome editing: Progress and prospect. Nano Research, 12 , 2437.
Download references
The work presented received no funding.
Author information
Authors and affiliations.
National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan
Saleem Ur Rahman, Ghulam Raza, Shahid Mansoor & Imran Amin
Constituent College Pakistan Institute of Engineering and Applied Sciences, Nilore, Islamabad, Pakistan
Center for Applied Genetic Technologies (CAGT), University of Georgia, Athens, USA
Laboratory for Genome Engineering, Center for Desert Agriculture and Division of Biological Sciences, 4700 King Abdullah University of Science and Technology, Thuwal, 23955-6900, Saudi Arabia
You can also search for this author in PubMed Google Scholar
Contributions
SM and ZA conceived the manuscript. SR and EM retrieved the literature and drafted the manuscript. SM, ZA, IA, and GR reviewed and drafted the manuscript.
Corresponding author
Correspondence to Imran Amin .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Rahman, S.U., McCoy, E., Raza, G. et al. Improvement of Soybean; A Way Forward Transition from Genetic Engineering to New Plant Breeding Technologies. Mol Biotechnol 65 , 162–180 (2023). https://doi.org/10.1007/s12033-022-00456-6
Download citation
Received : 23 August 2021
Accepted : 21 January 2022
Published : 04 February 2022
Issue Date : February 2023
DOI : https://doi.org/10.1007/s12033-022-00456-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Conventional breeding
- Genetic engineering
- New plant breeding technologies
Advertisement
- Find a journal
- Publish with us
- Track your research
ORIGINAL RESEARCH article
Characterization of soybean genetically modified for drought tolerance in field conditions.

- 1 Embrapa Soybean, Coordination for the Improvement of Higher Education Personnel (CAPES), Londrina, Brazil
- 2 Embrapa Soybean, National Council for Scientific and Technological Development (CNPq), Londrina, Brazil
- 3 Embrapa Agroenergy, Brasília, Brazil
- 4 Embrapa Soybean, Londrina, Brazil
- 5 Biological Sciences Center, Londrina State University, Londrina, Brazil
- 6 Japan International Research Center for Agricultural Sciences, Tsukuba, Japan
- 7 Laboratory of Plant Molecular Physiology, Tokyo University, Tokyo, Japan
Drought is one of the most stressful environmental factor causing yield and economic losses in many soybean-producing regions. In the last decades, transcription factors (TFs) are being used to develop genetically modified plants more tolerant to abiotic stresses. Dehydration responsive element binding (DREB) and ABA-responsive element-binding (AREB) TFs were introduced in soybean showing improved drought tolerance, under controlled conditions. However, these results may not be representative of the way in which plants behave over the entire season in the real field situation. Thus, the objectives of this study were to analyze agronomical traits and physiological parameters of AtDREB1A (1Ab58), AtDREB2CA (1Bb2193), and AtAREB1 (1Ea2939) GM lines under irrigated (IRR) and non-irrigated (NIRR) conditions in a field experiment, over two crop seasons and quantify transgene and drought-responsive genes expression. Results from season 2013/2014 revealed that line 1Ea2939 showed higher intrinsic water use and leaf area index. Lines 1Ab58 and 1Bb2193 showed a similar behavior to wild-type plants in relation to chlorophyll content. Oil and protein contents were not affected in transgenic lines in NIRR conditions. Lodging, due to plentiful rain, impaired yield from the 1Ea2939 line in IRR conditions. qPCR results confirmed the expression of the inserted TFs and drought-responsive endogenous genes. No differences were identified in the field experiment performed in crop season 2014/2015, probably due to the optimum rainfall volume during the cycle. These field screenings showed promising results for drought tolerance. However, additional studies are needed in further crop seasons and other sites to better characterize how these plants may outperform the WT under field water deficit.
Introduction
Drought is currently one of the most stressful environmental factor to economic crops. As a consequence, yield reductions are constant and economic and financial losses inevitable. In soybean, an important worldwide commodity, problems arising from water deficit impaired crop yield in the entire world. In Brazil, which is the second highest soybean producer worldwide and one of the few countries that could considerably increase its production in the next decades, water deficit also compromise productivity. Losses due to drought events during the period of 2003/2004 and 2014/2015 crop seasons are estimated to be in the US$46.6 billion range (Personal communication). In the crop season 2013/2014, although Brazilian production numbers increased, some regions from the South and Southeast registered significant losses ( Companhia Nacional de Abastecimento [Conab], 2014 ).
As drought tolerance is a multigenic and quantitative trait, some difficulties arise when attempting to breed for tolerance using conventional approaches. Furthermore, time, intensity, duration and frequency of the water deficit as well as diverse plant–soil–atmosphere interactions are factors that influence plant responses ( Bhatnagar-Mathur et al., 2007 ). As one of the strategies to cope with water deficit periods, biotechnological tools currently allow the development of genetically modified plants using gene constructs that confer drought tolerance. Among the stress-tolerant genes currently used, transcription factors (TFs) show great potential as they recognize and bind to specific DNA sequences in the regulatory regions of target genes, activating and regulating the expression of downstream genes responsible for cellular protection processes under dehydration ( Shinozaki and Yamaguchi-Shinozaki, 2007 ).
Since existing evidence demonstrates that drought response pathways can be both abscisic acid (ABA)-independent and -dependent, TFs also acts through these two systems that govern drought-inducible gene expression. Among these TFs, dehydration responsive element binding (DREB) proteins interact with DRE/CRT by their AP2 DNA-binding domain, thus mediating downstream gene expression in the stress-responsive pathway. In contrast, the ABA-responsive element (ABRE) mainly mediates downstream gene expression in the ABA-signaling pathway ( Yamaguchi-Shinozaki and Shinozaki, 2005 ). Insertion of the TF AtDREB1A , under the control of the stress-inducible rd29A promoter, successfully improved the drought tolerance responses in Arabidopsis thaliana ( Gilmour et al., 1998 ; Jaglo-Ottosen et al., 1998 ; Liu et al., 1998 ), tobacco ( Kasuga et al., 2004 ), rice ( Dubouzet et al., 2003 ; Oh et al., 2005 ; Ito et al., 2006 ), maize ( Qin et al., 2004 , 2007 ), wheat ( Pellegrineschi et al., 2004 ; Gao et al., 2009 ) and peanut ( Bhatnagar-Mathur et al., 2004 , 2007 ; Devi et al., 2011 ; Vadez et al., 2013 ). Particularly in soybean, in experiments under greenhouse conditions, transgenic lines containing TF AtDREB1A , presented both a higher survival rate after a severe water deficit and important physiological responses to water deprivation, such as higher stomatal conductance and the maintenance of photosynthesis and photosynthetic efficiency ( Polizel et al., 2011 ; Rolla et al., 2013 ). Furthermore, data suggested that the higher survival rates of DREB plants are because of lower water use due to lower transpiration rates under well-watered conditions. In addition to physiological studies, molecular analysis revealed that drought-response genes were highly expressed in DREB1A plants subjected to severe water deficit ( Polizel et al., 2011 ).
Another member of the DREB family, DREB2A protein, has also been used to develop genetically modified drought-tolerant plants. In Arabidopsis , the overexpression of a constitutively active (CA) DREB2A form resulted in significant tolerance to drought and heat stress ( Sakuma et al., 2006a , b ). AtDREB2A homologous genes were studied in maize ( Qin et al., 2007 ), rice ( Dubouzet et al., 2003 ), sunflower ( Almogueva et al., 2009 ), wheat ( Terashima and Takumi, 2009 ) and chrysanthemum ( Liu et al., 2008 ). AtDREB2A was also successfully introduced in soybean. Molecular analysis conducted under hydroponic experiments showed that transgenic plants exhibited high expression of the transgene, with roots showing the highest expression levels during water deficit. Recently, Mizoi et al. (2013) identified a soybean DREB2 gene, GmDREB2A , and showed that its heterologous expression in Arabidopsis induced stress-inducible genes such as RD29A, RD29B, HsfA3 , and HSP70 and improved stress tolerance. These findings indicate that plants overexpressing AtDREB2A and DREB2Alike proteins have increased tolerance to abiotic stress, drought and heat, which often occur together under field conditions ( Engels et al., 2013 ).
Considering ABA-dependent TFs, the AREB (ABA-responsive element-binding) protein family has showed interesting results conferring drought tolerance. In Arabidopsis , AREB acts as the major TF under abiotic stress ( Kobayashi et al., 2008 ; Lee et al., 2010 ) and has been reported to regulate environmental stress responses and ABA signaling during the vegetative stage ( Jakoby et al., 2002 ; Fujita et al., 2005 ; Côrrea et al., 2008 ; Yoshida et al., 2010 ). The overexpression of AREB1 in A. thaliana resulted in hypersensitivity to ABA, the induction of drought-responsive genes such as RD29B and improved water deficit tolerance. In soybean, the AREB1 gene was introduced and overexpressing AtAREB1FL lines showed the ability to survive for a period of 5 days without water under greenhouse conditions, exhibiting no leaf damage. These lines also displayed better growth and physiological performance under water-deficit (higher relative rate of shoot length, stomatal conductance, and photosynthesis) when compared to the wild type ( Barbosa et al., 2012 ). Particularly, line 1Ea2939 showed AtAREB1FL expression and a greater total number of pods and seeds and increased dry matter of seeds. The best performance of line 1Ea2939 relative to BR16 plants (wild type) might be related to the mechanisms of drought prevention through reduced stomatal conductance and leaf transpiration under control conditions (no water restriction). Such results suggest that the constitutive overexpression of the TF AtAREB1 leads to an improved capacity of the soybean crop to cope with drought with no yield losses ( Marinho et al., 2016 ).
Although all previously obtained data in greenhouses show the potential use of the TFs DREB and AREB to develop genetically modified soybean lines for drought-tolerance, these information were generated under monitored and controlled conditions of light, temperature, water, weeds, insects and diseases. According to Passioura (2012) , the results obtained under controlled conditions in greenhouses may not be representative of the way in which plants behave over the entire season in the real field situation. In particular, in the vegetative stage, in the flowering-pod-filling phase, plants have a daily demand of 7–8 mm of water; thus, water deficit in these periods implies greater losses ( Berlato et al., 1986 ; Embrapa, 2015 ).
Additionally, as in other countries in the world, tests in the field are a legal requirement of the Brazilian National Technical Biosafety Commission prior to approving a commercial product. Thus, it is important to test GM plants in the field to accurately gauge whether the technology is successful. As a result, considering that few studies have reported results from genetically modified crops under realistic field conditions and the fact that there is a lack of understanding with respect to the mechanisms of tolerance of DREB and AREB transgenic plants performing under a real crop season, the objectives of this study were to analyze gene expression, physiological parameters and agronomic traits of AtDREB1A, AtDREB2CA , and AtAREB1FL GM lines under irrigated and non-irrigated conditions in the reproductive stage, in a field experiment, for two crop seasons. This knowledge would provide new insights into the mechanism of drought tolerance of the DREBs and AREB plants and help soybean breeders to choose the best performing lines in real crop situations to introduce into the breeding program, aiming to develop a cultivar to be released to soybean producers.
Materials and Methods
Biological material.
Soybean genetically modified with rd29A:AtDREB1A (Patent Nos. 3183458) (line 1Ab58), rd29A:AtDREB2CA (Patent Nos. 3178672/PCT/JP2004/01003) (line 1Bb2193), 35S:AtAREB1FL (Patent Nos. US-2009-0089899-A1) (line 1Ea2939) constructs and the conventional cultivar BR16 (genetic background), considered drought-sensitive [39], were sown in a field experiment, carried out during 2013/2014 and 2014/2015 crop seasons, at Embrapa Soybean (Londrina, PR, 23°18′36″S 51°09′46″O). Soil chemical corrections and cultivations were performed according to recommendations for the crop ( Embrapa, 2013 ). All of the necessary documentation to test GM lines in field conditions were submitted and approved by The National Technical Biosafety Commission (CTNBio) (Process n° 01200.003078/2013-15 published in the Brazilian Official Journal on August 21th, 2013 by the number 3.721/2013 and Process n° 01200.003132/2014-11 published in the Brazilian Official Journal on September 09th, 2014 by the number 4.188/2014).
Experimental Design
The experiment was carried out in the field area located in the National Soybean Research Center (23°11′ S, 51°11′ W, 630 m altitude) (Embrapa Soybean, Londrina, PR, Brazil) a branch of the Brazilian Agricultural Research Corporation during the crop seasons 2013/2014 and 2014/2015. A completely randomized split-plot design was used, with four blocks. Plots corresponded to two water conditions – irrigated (IRR, water from precipitation + irrigation when needed) and non-irrigated (NIRR, water from precipitation). Subplots corresponded to the conventional Brazilian soybean cultivar BR 16, considered drought-sensitive ( Oya et al., 2004 ) and three transgenic isolines – 1Ab58 ( rd29A:AtDREB1A ), 1Bb2193 ( rd29A:AtDREB2CA ), 1Ea2939 ( 35S:AtAREB1FL ). The area of each subplot was 220 m 2 in IRR and NIRR conditions. Seeds were sown on November 5th, 2013 with 0.5 m spacing between rows and maintenance of 16 plants m -1 . Cultivation conditions followed the procedures routinely adopted at Embrapa Soybean. Plants of the soybean cultivar BRS 295RR were used as a 10 m isolation border, following Brazilian legislation. Air temperature and relative air humidity were monitored daily by a weather station located close to the experimental area.
Physiological and Agronomic Evaluations
Net CO 2 assimilation rate ( A ), transpiration rate ( E ) and stomatal conductance ( gs ) were measured in the central leaflet of the third fully expanded trifoliate leaf (apex-to-base direction) of one plant located in the middle portion of each subplot through a portable infrared gas analyzer (LCpro-SD, ADC BioScientific) fitted for 1000 μmol m -2 s -1 photosynthetically active radiation (PAR) under sunny sky conditions between 9 and 11 a.m. (Brazilian daylight saving time). After gas exchange measurements, the instantaneous ( A / E ) and the intrinsic ( A / gs ) water use efficiency (WUE) were calculated. Chlorophyll index (SPAD) was measured in one lateral leaflet from the same above-mentioned trifoliate leaf using a portable chlorophyll meter (SPAD-502, Minolta). Then, SPAD index was converted into chlorophyll content (mg cm -2 ) through an 80% acetone standard curve ( Fritschi and Ray, 2007 ). Plant height was the mean distance between the cotyledonary node and the stem apex from five plants per subplot. Mean length of internodes corresponded to the ratio between height per plant and number of nodes per plant. Leaf area index (LAI) corresponded to the ratio between the total leaf area, obtained through an area meter (LI-3100C, LI-COR), and the soil area occupied by the plants. Total dry matter of pods and seeds per plant and grain yield were evaluated (10 plants per subplot) in the harvest period. These measurements were carried out in all four experimental blocks, at the reproductive developmental stage.
The percentage contents of protein and oil in the samples of soybean grains at harvest were determined in whole seeds and grains using the reflectance technique of Near InfraRed (NIR) according to Heil (2010) .
Statistical Analysis
All residuals showed normal distribution and met the other assumptions of the analysis of variance (ANOVA). Thus, data were submitted to ANOVA and means compared by the Tukey’s test ( p ≤ 0.05).
Molecular Analysis
Leaf samples from GM soybean 1Ab58, 1Bb2193, 1Ea2939 lines and the conventional cultivar BR 16 were collected from field experiments in the irrigated (IRR) and non-irrigated (NIRR) treatments. Three samples from three different blocks were individually collected, based on physiological results. Samples were immediately placed into liquid nitrogen and stored in freezer at -80°C until the moment of RNA extraction.
Total RNA was extracted from 1Ab58, 1Bb2193, 1Ea2939, and BR16 leaf samples using Trizol ® reagent. Following RNA extraction, samples were treated with DNAse I (Invitrogen cat n° 18047-019). To verify the presence of remaining genomic DNA, a conventional PCR was performed. cDNA synthesis was carried out using Super Script III First Strand kit (Invitrogen cat 18080-051) according to manufacturer’s instruction.
Expression level of transgenes AtDREB1A, AtDREB2CA , and AtAREB1FL was assessed using qPCR. Also, based on a search of the available literature, some genes related to drought responses were selected. The expression level of these genes was quantified under IRR and NIRR conditions. Genes related to drought response such as stomata overture/closure and osmotic adjustment, photosynthesis, metabolic and hormone pathways such as nitrogen assimilation, proteins related to drought such as dehydrins and heat shock proteins and water channels were chosen. Thus, selected genes were phosphatase GmPP2C (Glyma14G195200), alanine aminotransferase GmAlaAT (Glyma01G026700, and Glyma07G045900), Δ- 1-pyrroline-5-carboxylate synthetase - P5CS (Glyma18G034300), galactinol/Gols (Glyma10G145300), late embryogenesis abundant/LEA18 (Glyma17G164200), dehydrins (Glyma09G185500), heat shock proteins (Glyma17G072400), putative soybean aquaporin pip1/UDP galactose transporter (Glyma12G066800), putative soybean aquaporin pip2/aquaporin transporter/glycerol uptake facilitator (Glyma12G172500), ribulose-1,5-bisphosphate carboxylase/oxygenase – small chain (Glyma13G046200) and chlorophyll a/b binding protein – Cab21 (Glyma16G165800).
Using gene sequences obtained from Phytozome, sets of primers for each gene were designed using Primer3Plus platform available online 1 (Additional File S1 ). To verify homo- and heterodimers and hairpin formation, the Multiple primer analyzer software was used 2 . Forward and reverse primers and amplified fragment size are described in the Additional File S1 . PCR reactions were carried out in biological and technical triplicate using the kit Platinum ® SYBR Green ® qPCRSuperMix-UDG with ROX according to the manufacturer’s instructions in a 7900HT Fast Real-Time PCR System with 384-Well Block (Applied Biosystems cat n° 4329001). The β-actin gene (No Access: GMU60500) was used as the reference gene ( Stolf-Moreira et al., 2011 ).
The efficiency of the amplification reaction was estimated using five serial dilutions of cDNA (1, 5, 25, 125, and 625×). To compute the efficiency of the reaction, the equation: E = [10-1/slope]-1 was applied, in which only primers with efficiency above 90% were used. The cycling parameters for the reactions were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. To check the specificity of the amplified products, a dissociation curve was generated after the end of each reaction. The relative expression was determined by normalization to the reference gene β-actin. The expression was calculated by the 2 -ΔΔCt method ( Bustin, 2002 ).
An in silico research for putative cis -elements was also performed using gene sequences obtained from Phytozome and software Genomatix 3 aiming to identify possible TF sites in the promoter regions and also other sites related to drought tolerance mechanisms.
Physiological and Agronomical Evaluations
Results from crop season 2013/2014, for instantaneous ( A / E ) and intrinsic ( A / gs ) water use efficiency, and LAI (Figures 1A–C ) did not show any significant interaction between water conditions and plant materials. In each water condition, there were no differences between plant materials regarding A / E (Figure 1A ). However, the line 1Ea2939 showed higher A / gs than the other plant materials under NIRR treatment (Figure 1B ). Furthermore, in each water condition, the line 1Ea2939 presented higher values for LAI than those of other plant materials in general. The lines 1Ab58 and 1Bb2193 showed a similar behavior than WT plants in relation to chlorophyll content (Figure 1D ) regardless of water condition. Conversely, the line 1Ea2939 had lower chlorophyll content relative to WT plants under NIRR conditions (Figure 1D ).
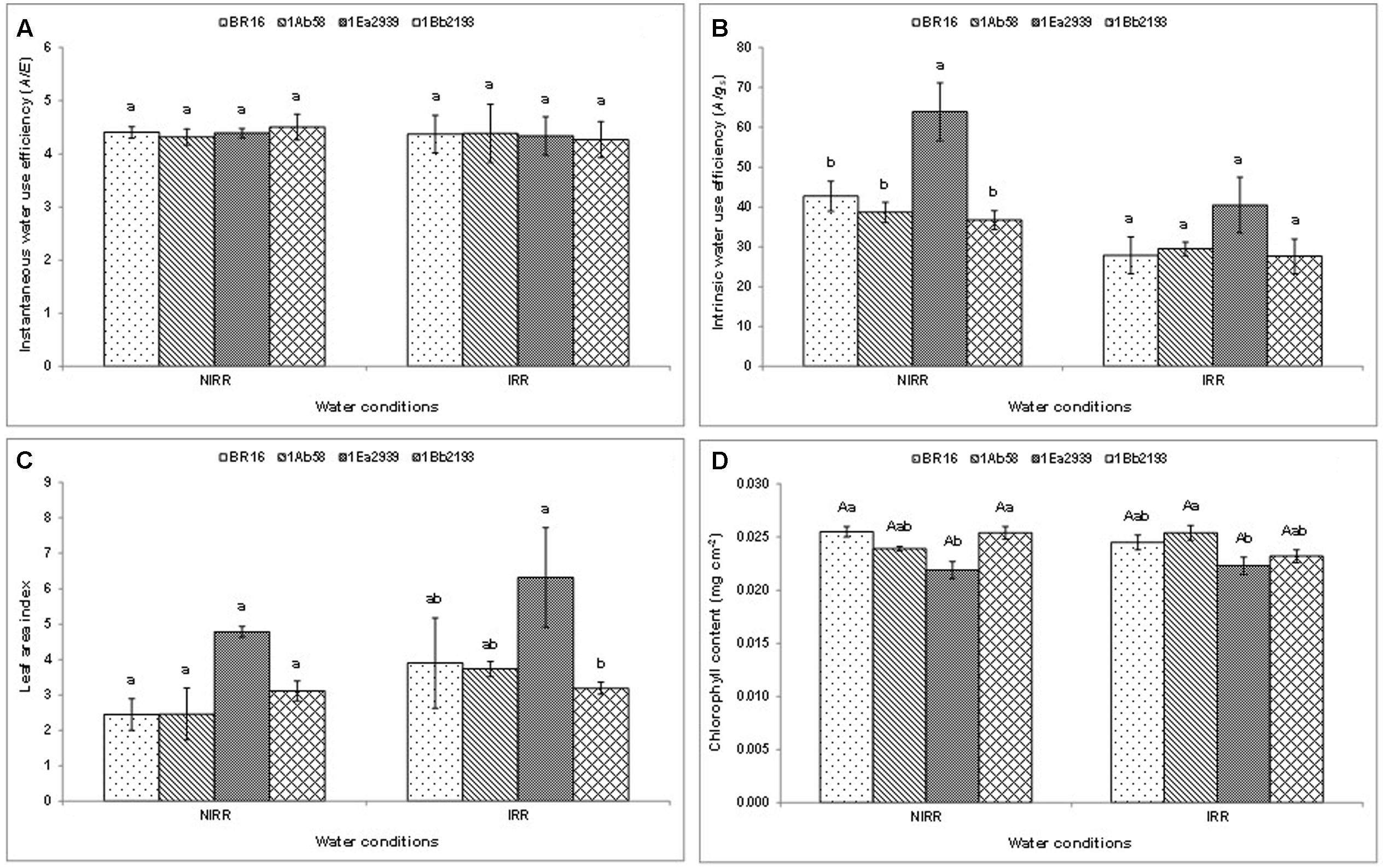
FIGURE 1. Instantaneous (A) , intrinsic (B) water use efficiency (WUE), leaf area index (LAI) (C) and chlorophyll content (D) of the transgenic lines 1Ab58, 1Ea2939, and 1Bb2193, and WT plants (BR 16 cultivar) subjected to non-irrigated (NIRR) and irrigated (IRR) treatments under field conditions. For (A–C) , in each water condition, means ± standard error followed by the same letter did not differ according to the Tukey’s test ( p ≤ 0.05). For (D) , means ± standard error followed by the same uppercase letters (between water conditions) and lowercase letters (among plant materials) did not differ according to the Tukey’s test ( p ≤ 0.05). n = 4 blocks.
With regard to plant height (Figure 2A ) and the mean length of internodes (Figure 2B ), there was no significant interaction between water conditions and plant materials. Thus, in each water condition, the line 1Ea2939 presented higher values for plant height than those of other plant materials in general. Furthermore, in both agronomic traits, the lines 1Ab58 and 1Bb2193 showed similar values relative to those of WT genotype under IRR and NIRR treatments. Plant materials did not show differences as to mean length of internodes in each condition (Figure 2B ). Moreover, plants showed similar results between IRR and NIRR treatments regarding total dry matter of pods and seeds per plant, except for the line 1Bb2193, which showed lower values under NIRR for both agronomical traits (Figures 2C,D ). In both traits, transgenic lines showed similar results to those of WT plants (BR 16 cultivar) regardless of water conditions. Furthermore, the line 1Ea2939 had lower values of both traits than those of the line 1Bb2193 under IRR treatment.
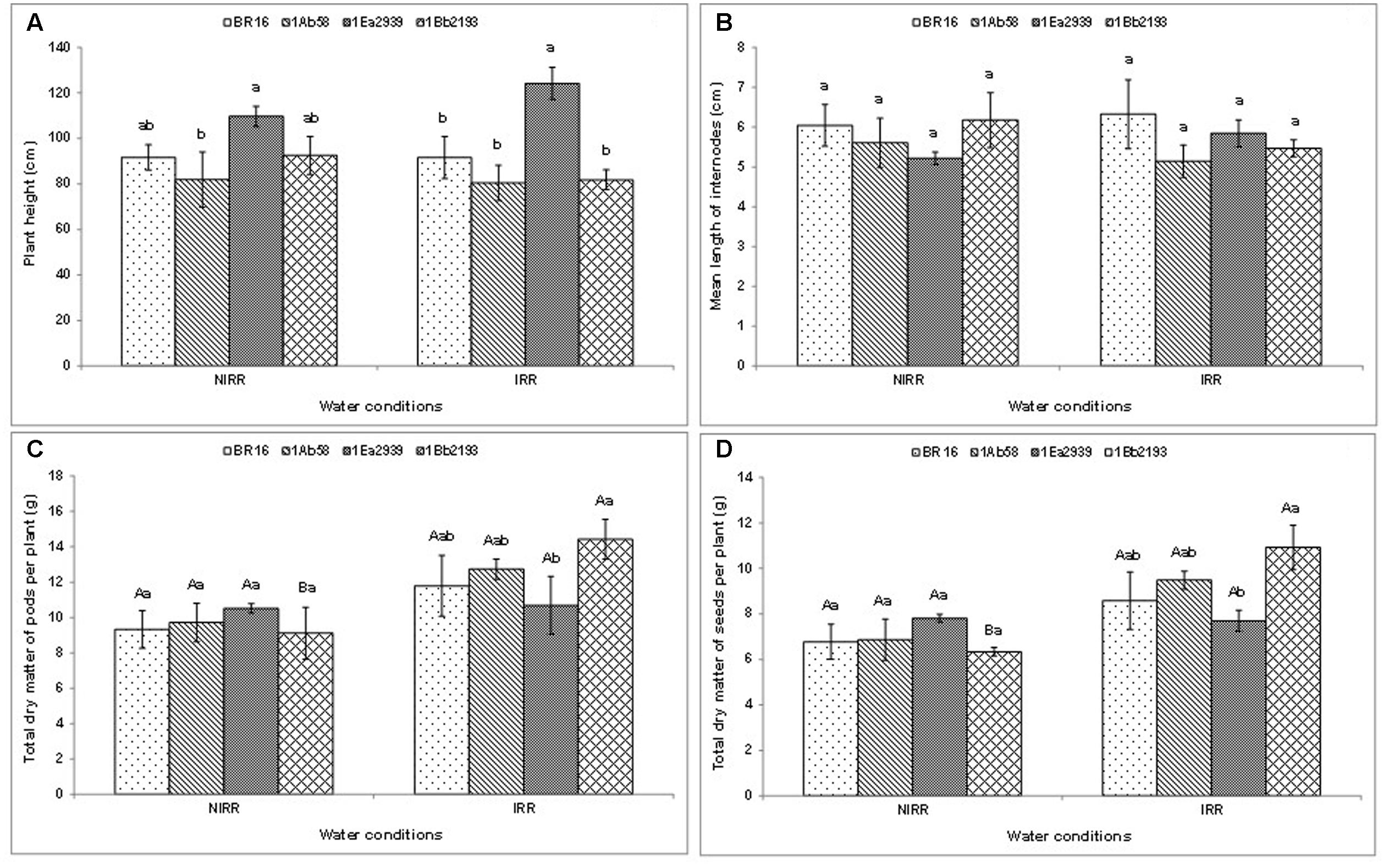
FIGURE 2. Plant height (A) , mean length of internodes (B) , and total dry matter of pods (C) and seeds (D) per plant of the transgenic lines 1Ab58, 1Ea2939, and 1Bb2193, and WT plants (BR 16 cultivar) subjected to non-irrigated (NIRR) and irrigated (IRR) treatments under field conditions. For (A,B) , in each water condition, means ± standard error followed by the same letter did not differ according to the Tukey’s test ( p ≤ 0.05). For (C,D) , means ± standard error followed by the same uppercase letters (between water conditions) and lowercase letters (among plant materials) did not differ according to the Tukey’s test ( p ≤ 0.05). n = 4 blocks.
Yield results showed significant interactions between water conditions and plant materials (Figure 3 ). No differences were identified between transgenic lines and WT plants at NIRR conditions; however, line 1Ea2939 presented the highest yield value reaching 2.153 kg ha -1 , showing no differences with the final yield obtained for this line at IRR treatment (2.012 kg ha -1 ). Nevertheless, in this water condition, line 1Ea2939 presented lower yield values when compared to other transgenic lines and WT plants (BR 16 cultivar), due to a severe lodging that occurred after a plentiful rain (341.4 mm), decreasing productivity and final potential yield numbers (Additional Files S2 , S3 ). Before the abundant rain, line 1Ea2939 showed a higher number of nodes (five plants average) and higher number of pods per plant, thus being more impaired in the harvest due to lodging (Additional File S4 ).
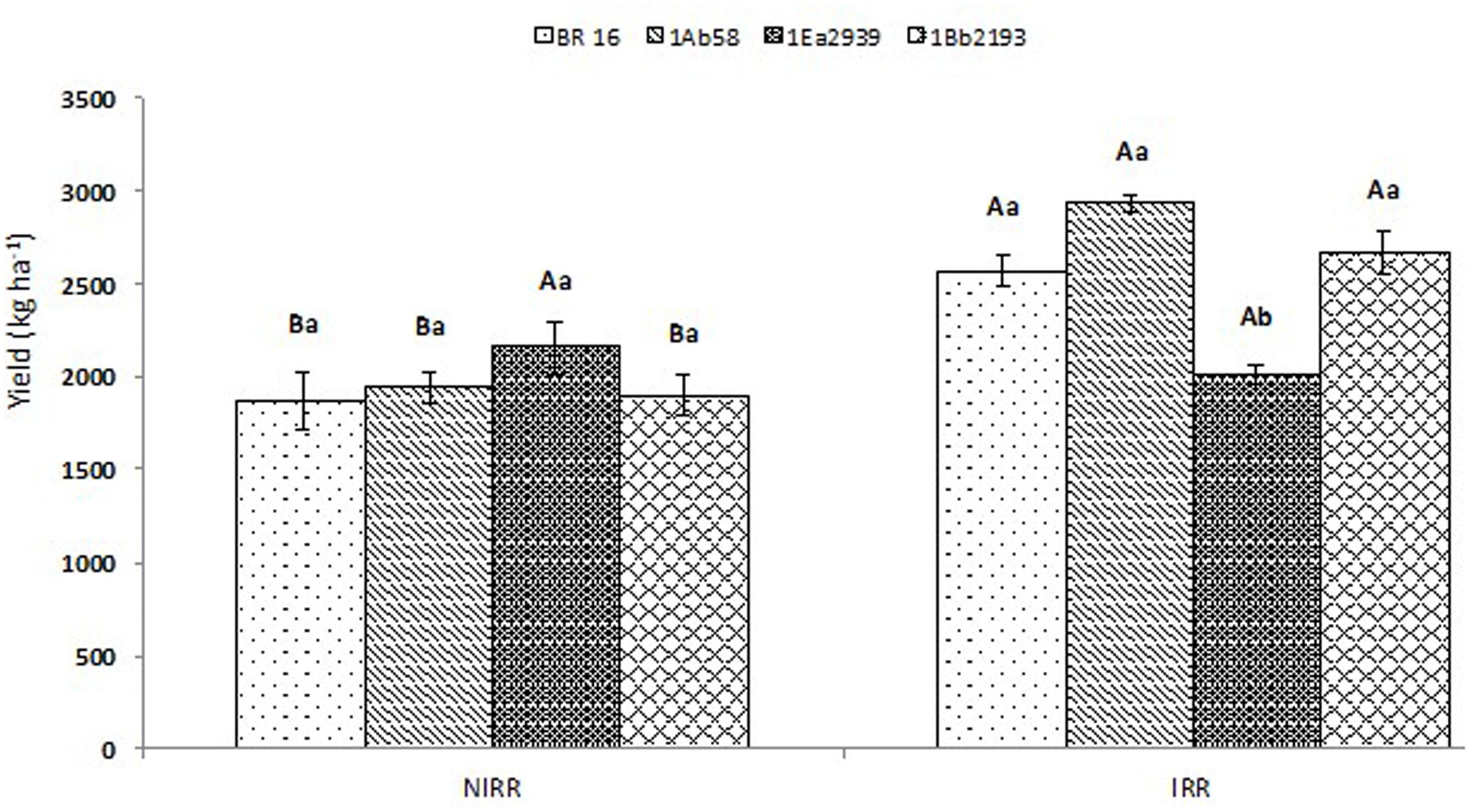
FIGURE 3. Yield of the transgenic lines 1Ab58, 1Ea2939, and 1Bb2193, and WT plants (BR 16 cultivar) subjected to non-irrigated (NIRR) and irrigated (IRR) treatments under field conditions. Means ± standard error followed by the same uppercase letters (between water conditions) and lowercase letters (among plant materials) did not differ according to the Tukey’s test ( p ≤ 0.05). n = 4 blocks.
Protein and oil contents in soybean seeds were not affected by insertion of the FTs DREB1A, DREB2A, and AREB1 (Figures 4A–D ). In crop season 2013/2014, for protein content under IRR conditions, final values ranged from 37.07% (1Bb2193) to 40.50% (1Ea2939) and for NIRR, values varied from 38.01% (BR 16 cultivar) to 39.77% (1Ea2939) (Figure 4A ). Oil content in the seeds ranged from 20.11% (1Ea2939) to 21.76% (1Bb2193) for IRR conditions and from 20.31% (BR 16 cultivar) to 21.08% (1Bb2193) for the NIRR treatment (Figure 4C ). In crop season 2014/2015, overall, values for protein content were higher and for oil content lower when compared to crop season 2013/2014, with line 1Ea2939 reaching values for protein content of around 41–42% and between 15 and 17% for oil content (Figures 4B,D ). However, it must be emphasized that, in both crop seasons, line 1Ea2939 showed the highest protein content and the lowest oil content, both in IRR and NIRR conditions, relative to the other plant materials.
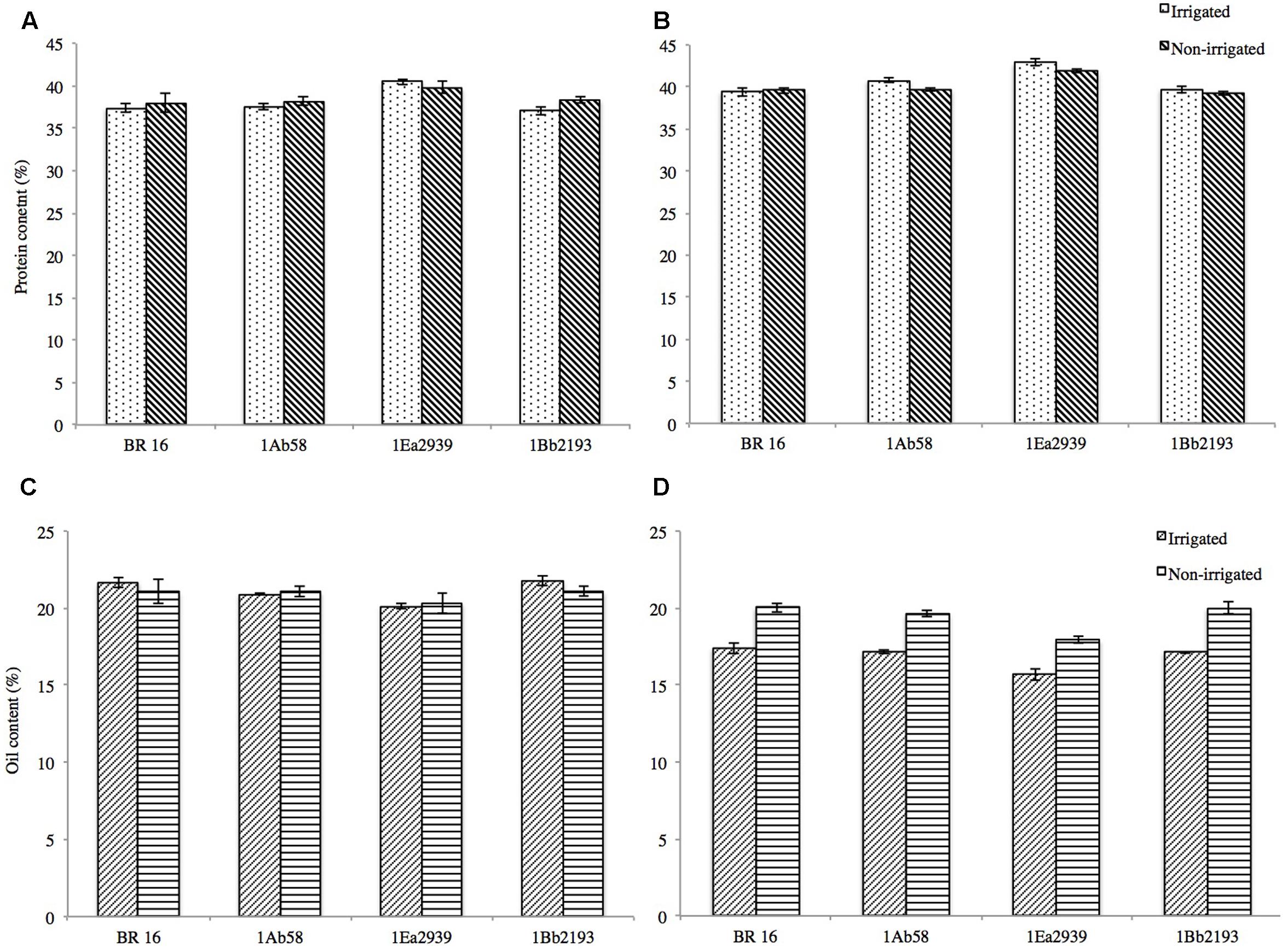
FIGURE 4. Protein (%) and oil (%) content in soybean GM lines 1Ab58, 1Bb2193, and 1Ea2939 and WT plants (BR 16 cultivar) subjected to irrigated (IRR) and non-irrigated (NIRR) treatments under field conditions. (A,C) Data from crop season 2013/2014. (B,D) Data from crop season 2014/2015. Values represent mean ± standard error; n = 4 replicates.
No differences were identified for physiological and agronomic parameters in the field experiment performed in crop season 2014/2015, probably due to the optimum rainfall volume during the whole cycle, thus resulting in scarce water stress in plants. According to the data collected by the weather station located in the experiment spot, a total rainfall of 790.8 mm was registered (Additional File S5 ). The recommendations for soybean crop for water requirements vary between 450 and 800 mm/cycle, depending on weather conditions, crop management and cycle duration ( Embrapa, 2013 ). Although a short period of water deficit occurred in October/2014, the experiment was sown on November 6th. Thus, as no significant water deficit period occurred during the cycle, no differences were shown between GM lines and BR16 cultivar. As no differences were identified, no molecular analyses were performed.
Gene expression analysis, performed in samples collected in crop season 2013/2014 showed that transgenes AtDREB1A, AtDREB2CA , and AtAREB1FL were induced under NIRR conditions in each respective transgenic line. Among TFs, the higher expression was identified for AtDREB1A gene (line 1Ab58) with expression value reaching 4.806x. Transgenes AtDREB2CA and AtAREB1FL gene expression were 2.91x and 1.34x. No expression was identified for BR16 soybean conventional cultivar.
All of the analyzed endogenous drought-responsive genes showed statistical differences between plant materials, although pattern expression varied depending on the GM line. However, some expression behaviors, regardless of being up or down, were identified (Figure 5 ).
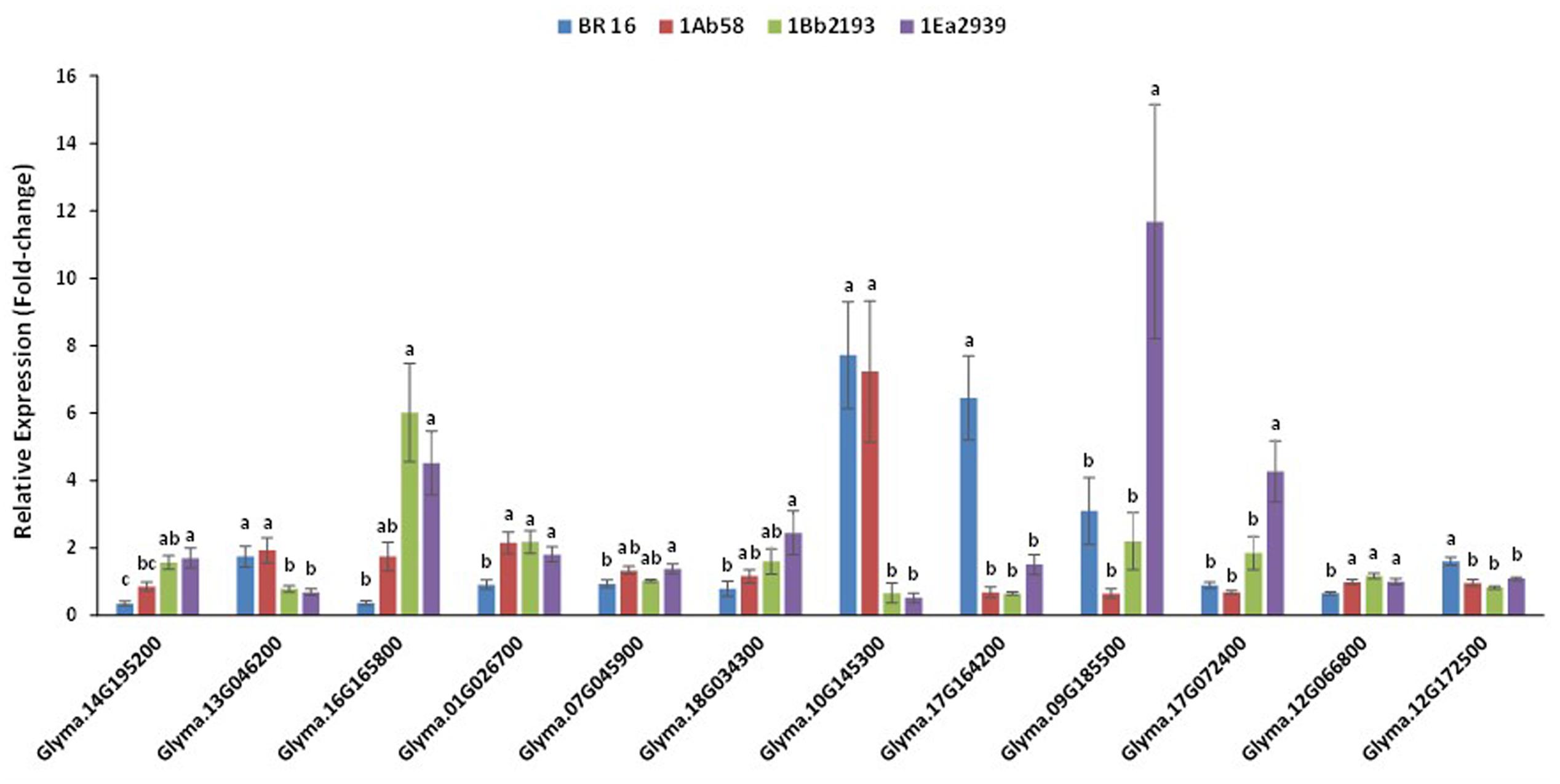
FIGURE 5. Relative expression level of phosphatase GmPP2C (Glyma14g195200), ribulose-1,5-bisphosphate carboxylase/oxygenase – small chain (Glyma13g046200), chlorophyll a/b binding protein – Cab21 (Glyma16g165800), alanine aminotransferase GmAlaAT (Glyma01g026700 and Glyma07g045900), Δ- 1-pyrroline-5-carboxylate synthetase – P5CS (Glyma18g034300), galactinol/Gols (Glyma10g145300), late embryogenesis abundant/LEA18 (Glyma17g164200), dehydrins (Glyma09g185500), heat shock proteins (Glyma17g072400), putative soybean aquaporin pip1/UDP galactose transporter (Glyma12g066800), putative soybean aquaporin pip2/aquaporin transporter/glycerol uptake facilitator (Glyma12g172500) genes, in plants of transgenic lines 1Ab58, 1Bb2193, and 1EA2939 and WT plants (BR 16 cultivar) subjected to non-irrigated (NIRR) and irrigated (IRR) treatments under field conditions. Expression was normalized with the reference gene Gmβ-actin. On each gene, means ± standard error followed by the same letter do not differ according to the Tukey test ( p ≤ 0.05); n = 9.
Thus, for Glyma01G026700 (alanine aminotransferase GmAlaAT ), Glyma17G164200 (late embryogenesis abundant – LEA), Glyma12G066800 (putative soybean aquaporin pip1/UDP galactose transporter) and Glyma12G172500 (putative soybean aquaporin pip2/aquaporin transporter/glycerol uptake facilitator), no statistical difference was found between GM lines, but they were different from BR 16 (WT plants). For Glyma13G046200 (ribulose-1,5-bisphosphate carboxylase/oxygenase-small chain) and Glyma10G145300 (galactinol), GM lines 1Ea2939 and 1Bb2193 showed similar expression to each other, but different expression to that of BR 16 and 1Ab58. Furthermore, the GM line 1Ea2939 presented higher expression than other plant materials for Glyma09g185500 (dehydrins) and Glyma17G072400 (heat shock protein) (Figure 5 ).
For the phosphatase GmPP2C (Glyma14G195200) gene, the GM lines 1Ea2939 and 1Bb2193 showed higher expression than 1Ab58 and the conventional cultivar BR 16. However, 1Ab58 and 1Bb2193 also shared similar expression profile. Considering Glyma13G046200, GM lines 1Ea2939 and 1Bb2193 showed similar expression each other, but lower expression than BR 16 and the line 1Ab58. For chlorophyll a/b binding protein – Cab21 (Glyma16G165800) gene, the GM lines 1Ea2939 and 1Bb2193 showed similar expression to each other, and higher expression than BR 16 and 1Ab58, which also presented expression that was strongly similar to the conventional cultivar. For genes involved in the nitrogen assimilation process, results showed that no significant difference was identified between transgenic lines (Glyma01G026700), although lines 1Ab58 and 1Bb2193 were statistically similar to BR16 for Glyma07G045900. However, for both genes, the AREB1 line (1Ea2939) showed different expression when compared to the conventional cultivar. This expression pattern was also found for the Δ-1-pyrroline-5-carboxylate synthetase (P5CS) gene (Glyma18G034300) (Figure 5 ).
Considering genes involved in osmotic adjustment, such as galactinol/Gols (Glyma10G145300), higher expression was detected for the GM line 1Ab58 and BR 16 plants. For the LEA protein gene (Glyma17G164200), no significant difference was identified between transgenic lines; however, they showed lower expression than BR16 plants. For the dehydrin gene (Glyma09G185500) and heat shock protein gene (Glyma17G072400), the GM line 1Ea2939 showed higher expression than the other plant materials. Water channel-related genes such as Glyma12G066800 and Glyma12G172500 showed similar expression between GM lines, but their expression was higher and lower than that of the conventional cultivar BR16, respectively (Figure 5 ).
Besides standard plant promoters motifs such as the TATA-box and CCAAT-box and the DRE (conserved motif sequence A/G)CCGACNT) and ABRE (conserved motif sequence PyACGTGG/TC) cis -elements, which are specific binding sites for DREB and AREB TFs, other putative cis -elements related to drought response mechanisms in plants were identified in the promoter regions of the endogenous genes analyzed. Circadian cycle control and light response element motifs such as evening element, circadian clock associated 1, late elongated hypocotyl, GAP-box, and time-of-day-specific elements were identified. Motifs related to sugar-responsive genes and heat shock responses were also present in the genes’ promoter regions (Additional File 6 ).
As the world’s weather is altering, probably due to climate change, the development of drought-tolerant crops is gaining prominence. In general, plant drought resistance involves four major mechanisms: drought avoidance (DA), drought tolerance (DT), drought escape (DE), and drought recovery. DA is mainly characterized by the maintenance of high plant water potentials in the presence of water shortage, and is accomplished through three basic general strategies: (1) reducing water loss via rapid stomatal closure, leaf rolling and increasing wax accumulation on the leaf surface; (2) enhancing the water uptake ability through a well-developed root system and enhancing the water storage abilities in specific organs; and (3) accelerating or decelerating the conversion from vegetative to reproductive growth to avoid complete abortion at the severe drought stress stage. DT refers to the ability of plants to sustain a certain level of physiological activities under severe drought conditions through the regulation of thousands of genes and series of metabolic pathways to reduce or repair the resulting stress damage ( Fang and Xiong, 2015 ). The different expression pattern identified for endogenous soybeans genes related to drought response (Figure 5 ) illustrates how one or more of these mechanisms can be activated and interact within and between them to cope with water deficit periods.
The strategy to improve drought tolerance by inserting TFs that regulate the expression of several drought-responsive genes, from either the ABA (ABA)-independent or -dependent response pathway has already shown promising results for model plants such as Arabidopsis ( Gilmour et al., 1998 ; Jaglo-Ottosen et al., 1998 ; Liu et al., 1998 ; Kasuga et al., 1999 ; Fujita et al., 2005 ), as well as for important economic crops like potato ( Behnam et al., 2006 ), tobacco ( Kasuga et al., 2004 ), rice ( Dubouzet et al., 2003 ; Oh et al., 2005 ; Ito et al., 2006 ), wheat ( Pellegrineschi et al., 2004 ; Gao et al., 2009 ), maize ( Qin et al., 2004 , 2007 ), peanut ( Bhatnagar-Mathur et al., 2007 ; Devi et al., 2011 ; Vadez et al., 2013 ), and soybean ( Polizel et al., 2011 ; Barbosa et al., 2012 ; Rolla et al., 2013 ; Leite et al., 2014 ; Marinho et al., 2016 ).
Using soybean lines GM with FTs DREB and AREB, most of the previous molecular and physiological characterizations were performed in greenhouses under controlled conditions of light, temperature, water, weeds, insects, and diseases. In this contained environment, results for “concept proof” were promising ( Polizel et al., 2011 ; Barbosa et al., 2012 ; Rolla et al., 2013 ; Leite et al., 2014 ; Marinho et al., 2016 ); however, when the main objective is a commercial cultivar release, real field condition screenings are necessary, as, according to Passioura (2012) , the results obtained under controlled conditions in greenhouses may not be representative of the way in which plants behave over the entire season in the real field situation. Still, according to our observations, in containment conditions, plants are not able to express their total potential, as limitations due to pot size and controlled water amount, temperature fluctuations, diseases and pests do not challenge the organism as a whole, but reduce environmental real situations.
Under drought, photosynthesis is among the primary processes which are down-regulated. Molecularly, in response to the stress condition, mRNA levels of the light and dark reaction genes (such as RbcS , Glyma13G046200, in lines 1Bb2193 and 1Ea2939, Figure 5 ) rapidly reduced, a phenomenon referred to as stress-induced mRNA decay (SMD) ( Park et al., 2012 ). Physiologically, as observed, in NIRR conditions line 1Ea2939 decreased gas exchanges (data not presented), by stomatal closure (lower gs ) which probably occurs as a mechanism to keep water in the cell, which, however, did not imply losses in the use of the free CO 2 , as instantaneous water use efficiency ( A / E ) was equal to that of the other transgenic lines and WT plants and intrinsic water use efficiency ( A / g s ) was higher (Figure 1 ). The explanation for stomatal closure during water stress in seed plants relies on the phytohormone ABA, which is seen as a cornerstone of stomatal function, because it has been shown to trigger responses in guard cell membrane channels and transporters that cause a reduction in guard cell turgor, thereby closing stomata ( Bauer et al., 2013 ). In the field, few studies show a strong correlation between the level of ABA and gs during water deficit. In field-grown grapevine ( Vitis vinifera L. cv Cabernet Sauvignon), a correlation between ABA abundance in the xylem sap and gs strongly supported the involvement of ABA in stomatal regulation under field conditions. The different irrigation levels significantly altered the Ψ leaf and gs of the vines across two crop seasons ( Speirs et al., 2013 ).
In the ABA-mediated stomatal closure, the light-harvesting chlorophyll a/b-binding (LHCB) proteins can also be involved ( Xu et al., 2012 ). In the present study, Glyma16G165800, a LHCB protein was up-regulated in lines 1Bb2193 and 1Ea2939 (Figure 5 ) and ABRE cis -elements were found in the promoter region (Additional File S6 ). In higher plants, the superfamily of these proteins is composed of more than 20 members associated with photosystem I (PSI) or photosystem II (PSII). As observed here for soybean, in Arabidopsis , LHCBs positively regulate plant drought tolerance by functioning to positively control stomatal movement, through guard cell signaling, in response to ABA ( Xu et al., 2012 ). In date palm ( Phoenix dactylifera L.) cultivar “Sagie” subjected to drought, LHCBs were up-regulated. The accumulation of these proteins in PSI exposed to salt and drought stress might represent one of the strategies to prevent or lower light stress-induced damage. It was proposed that these proteins might have a protective function within PSII under stress conditions, either by binding free chlorophyll molecules and preventing the formation of free radicals and/or by acting as sinks for excitation energy, because under stress conditions, a mobile pool of these proteins moves from PSII to PSI due to the reversible phosphorylation of these proteins by a thylakoid bound kinase ( El Rabey et al., 2015 ).
Furthermore, in response to water deficit, ABA binds directly to the PYR/PYL/RCAR family of ABA receptors, thus resulting in the inhibition of type-C protein phosphatases 2C (PP2C). In the absence of protein phosphatase PP2C activity, SnRK2 protein kinases are free to be activated by autophosphorylation, and activate downstream target genes as a result ( Klingler et al., 2010 ; Boneh et al., 2012 ). In the cytoplasm, kinases may also phosphorylate anionic slow channels (SLAC1) or potassium channels (kat1) to induce stomatal closure in response to ABA ( Umezawa et al., 2010 ).
Thus, stomata closure exhibited by AtAREB1FL line 1Ea2939 was probably triggered by the combination of different physiological (Figures 1 , 2 ) and molecular mechanisms, as Glyma14G195200 (phosphatase GmPP2C ) was up-regulated in this line (but also in line 1Bb2193) (Figure 5 ).
Considering the obtained molecular and physiological data together, it suggests that GMs lines 1Bb2193 and 1Ea2939 share some similarities in the drought response behavior, targeting more than one mechanism to cope with water deficit periods combining modulation of the gene expression profile and physiological responses, as a strategy to conserve more water and protect cells during water starvation.
However, since drought response is a complex mechanism and plants, as sessile organisms, cannot avoid abiotic stresses by sheltering, they have evolved a series of mechanisms that alone or combined together overcome drought conditions. Thus, many other molecules are synthesized to cope with water deficit. Considering alanine aminotransferase (AlaAT) (Glyma01G026700 and Glyma07G045900) genes, which are evolved in nitrogen metabolism, no differences were identified among the GM lines, which presented slight up-regulation when compared to BR16. This expression profile also occurs for proline (P5CS – Glyma18G034300). In general, AlaAT plays a key role in plant metabolism by linking primary carbon metabolism with the synthesis of amino acids. If considered that under drought, a decrease in protein synthesis, protein levels and activity of enzymes occurs, thus, in GM lines, AlaATs might be ensuring amino acid and protein synthesis during drought, which explains a higher proline accumulation in transgenic lines when compared to BR16. This amino acid, under stress conditions, as well as acting as an excellent osmolyte, plays three major roles, as a metal chelator, an antioxidative defense molecule and a signaling molecule. Overproduction of proline in plants imparts stress tolerance by maintaining cell turgor or osmotic balance; stabilizing membranes to prevent electrolyte leakage; and bringing concentrations of reactive oxygen species (ROS) within normal ranges, thus preventing oxidative burst in plants ( Hayat et al., 2012 ). In wheat, drought stress tolerance at a cellular level was associated with the ability to accumulate proline and high water level conservation ( Sultan et al., 2012 ). In sugarcane, proline accumulation in transgenic plants under water-deficit stress acts in the cytoplasmatic osmotic adjustment but also as a component of the antioxidative defense system ( Molinari et al., 2007 ). In soybean, proline content increased in the leaves and nodules of plants subjected to water deficit during the flowering stage ( Silvente et al., 2012 ). Also, a positive and significant correlation among the activity of antioxidant enzymes, ABA and proline content with seed and oil yield in water deficit stress was observed ( Masoumi et al., 2011 ). Higher accumulation of proline was still associated with less injury and a greater amount of water retention in a stress-tolerant soybean genotype ( Angra et al., 2010 ).
Dehydrins (DHNs are the group II, D11 family of LEAs – Late Embryogenesis Abundant) (Glyma09G185500) and heat shock proteins (HSPs) (Glyma17G072400) were also induced under drought treatment, in line AtAREB1FL 1Ea2939, which presented the higher expression among transgenic and BR16 plants (Figure 5 ). Dehydrins are exclusively found in plants and accumulate in the late stages of embryogenesis, when water content in seeds declines, or in response to various stressors. The presence of these proteins has been observed in several independent studies on drought and salinity stresses as well as on cold acclimation and after ABA treatment ( Rorat et al., 2006 ; Tripepi et al., 2011 ). DHNs play critical roles in desiccation tolerance by capturing water, stabilizing and protecting the structure and function of proteins and membranes, as well as acting as molecular chaperons (as heat shock proteins) and hydrophilic solutes to protect cells from the damage of water stress ( Hand et al., 2011 ). In Olea europaea L. Subsp. europaea , var. sylvestris , the wild plant of olive, the expression OesDHN was induced under mild drought stress. In addition, Arabidopsis transgenic plants showed a better tolerance to osmotic stress suggesting that OesDHN expression is induced by drought stress and is able to confer osmotic stress tolerance ( Chiappetta et al., 2015 ). In grapevine, DHN1 and DHN2 genes were induced by drought, cold, heat, embryogenesis, as well as the application of ABA, salicylic acid (SA), and methyl jasmonate (MeJA) and a higher number of putative ABRE cis -elements was located in DHN1 and DHN2 promoters ( Yang et al., 2012 ), which were also identified here in soybean (Glyma09G185500, Additional File S5 ).
It has also been suggested that some dehydrins probably play a role in antioxidative defense response directly by their radical scavenging activity ( Hara et al., 2004 ), or indirectly by their capability of binding toxic metals and preventing the production of ROS ( Hara et al., 2005 ). There are likewise some indications about the relationship between the response to oxidative stress and the response to heat stress, as both stresses induce the pathways leading to the expression/accumulation of Hsps ( Al-Whaibi, 2011 ). Thus, dehydrin and heat shock proteins might be acting combined in line 1Ea2939 to protect cells against drought but also with potential to guard from other different stresses. The expression of other drought-responsive molecules suggests that line 1Ea2939 displays yet more mechanisms to cope with water deficit periods, keeping water in the cell to maintain plant metabolism aiming to sustain yield.
As observed here, lines 1Bb2193 and 1Ea2939, containing ABA-independent and -dependent TFs (DREB2A and AREB1), respectively, showed a similar expression pattern under drought conditions for some endogenous genes (Figure 5 ). Interestingly, increasing evidence shows that DRE/CRT can act as a coupling element of the ABRE cis -element to regulate downstream gene expression ( Narusaka et al., 2003 ). Thus, there exists a comprehensive connection between stress-responsive and ABA-signaling pathways ( Shinozaki and Yamaguchi-Shinozaki, 2000 ; Shinozaki et al., 2003 ). A few of the DREB-type TFs were found to be involved in the ABA-dependent pathway ( Egawa et al., 2006 ). In maize, ZmABI4, a DREB protein that shows ABA-induced expression, binds to CE1 and acts as a coupling element of the ABRE ( Niu et al., 2002 ). Consistently, overexpression of DREB1D/CBF4, which is an ABA-responsive gene of Arabidopsis , activates the expression of drought and cold-related downstream genes that contain the DRE/CRT cis -element ( Knight et al., 2004 ). In rice, three ABRE elements were identified in the promoter region of ARAG1 gene, which encodes a DREB-like protein containing the characterized AP2 DNA binding domain ( Zhao et al., 2010 ). In Arabidopsis , evidence shows that AREB/ABF proteins physically interact with DREB/CBFs including DREB1A, DREB2A, and DREB2C ( Lee et al., 2010 ). In Setaria italica , the transcript level of SiARDP , an abscisic-responsive DREB-binding protein, increased not only after drought, high salt and low temperature stresses, but also after an ABA treatment in foxtail millet seedlings ( Li et al., 2014 ). Furthermore, transient-expression analyses coupled with ChIP (Chromatin Immunoprecipitation) assays have shown AREB/ABFs such as AREB1, AREB2, and ABF3 can bind to the promoter of DREB2A and thereby induce them in an ABRE-dependent manner ( Kim et al., 2011 ).
As a drought-sensitive genotype, WT BR16 also targets molecular mechanisms in response to water deficit. Thus, expression profile showed that LEA protein (Glyma17G164200) was highly induced (Figure 5 ). In the conventional cultivar, this protein, acting as molecular chaperone, might be associated with the osmotic adjustment (OA) mechanism, as galactinol ( Gols – Glyma10G145300) and aquaporin (Glyma12G172500) also presented higher expression levels. In the OA, the accumulation of a variety of organic and inorganic substances increases the concentration in the cytochylema, reducing the osmotic potential and improving cell water retention in response to water stress. In overexpressing Arabidopsis , a wheat ( Triticum durum ) group 2 LEA protein (DHN-5) improved tolerance to salt and drought stress through osmotic adjustment. The water potential was more negative in transgenic than in wild type plants and, in addition, these plants have lower water loss rate under water stress ( Brini et al., 2007 ). In Arabidopsis , sugar analysis showed that drought, high salinity and cold treated plants accumulate a large amount of raffinose and galactinol, functioning as osmoprotectants in drought-stress tolerance ( Taji et al., 2002 ). In poplar ( Populus spp.), the osmolytes raffinose and galactinol exhibited increased abundance under drought stress ( Barchet et al., 2013 ). Also, in Coffea arabica and C. canephora, GolS genes were highly induced under water deficit periods ( Dos Santos et al., 2011 , 2015 ). It must be emphasized that, although BR16 (WT) plants seemed to have presented OA mechanism under drought stress, such a response was not more efficient than the mechanisms shown by the transgenic lines, based on physiological, growth and agronomical results (Figures 1 – 3 ).
Besides these molecules, aquaporins (AQP), which were differentially expressed in BR16 (Glyma12G066800 and Gyma12G172500) (Figure 5 ), have also been demonstrated to have crucial roles in OA in plant cells. AQPs participate in the rapid transmembrane water flow and when plants are subjected to drought or salt conditions, increased transport of water across membranes is crucial to maintain a healthy physiological status. In banana ( Musa acuminata L.), the aquaporin gene MaPIP1;1 was induced in leaves and roots after salinity stress and simulated drought treatments, and overexpressing Arabidopsis displayed better growth, more green leaves, higher survival rates and lower water loss rate compared to WT under drought conditions. Transgenic lines also experimented less lipid peroxidation and membrane injury, and improved osmotic adjustment under drought treatment ( Xu et al., 2014 ). Once more, these data suggest that soybean conventional cultivar BR16 might be targeting, mainly, the OA mechanism to cope with water deficit, as GM line 1Ab58.
Finally, when yield parameters were assessed in crop season 2013/2014, results showed lower productivity for 1Ea2939 plants under IRR conditions (2.0212 kg ha -1 ) when compared to NIRR (2.153 kg ha -1 ). This difference of approximately 140 kg ha -1 was due to a lodging that occurred in the IRR treatment (Additional File S2 ). As observed in the climatologic water balance (Additional File S3 ), after a severe drought, plentiful rain occurred (341.4 mm) in a short period. Since 1Ea2939 plants were higher (Figure 2A ), exhibiting greater LAI (Figure 1C ), and a higher number of nodes and total pod number per plant (Additional File S4 ), lodging implications were more austere for this GM line, decreasing the final production in this experimental plot. However, based on the number of nodes and number of pods per plant on 17th February 2014 (after water deficit and before rainfall – Additional File S4 ), we can consider that the potential productivity for this line is presumably greater. Line 1Ea2939 showed 21 nodes/plant (average of five plants) in both IRR and NIRR conditions, while other transgenic lines and WT plants presented an average ranging from 14 to 15 nodes in both treatments. A positive relationship between pods, nodes and yield or nodes and pods and seeds has been reported ( Kahlon et al., 2011 ; Egli, 2013 ). This association is also related to environmental conditions and other plant characteristics such as photosynthesis, crop growth rate and maturity, since later maturing cultivars have longer vegetative growth periods and more nodes than earlier cultivars (88). Line 1Ea2939 showed longer cycle (150 and 153 days in IRR and NIRR, respectively) than other transgenic lines and WT plants (ranging from 128 and 132 days in both water conditions). In general, data demonstrated that this line was able to cope with drought through some plant defense mechanisms and invest in growth and productivity, in association with a higher intrinsic WUE ( A / gs ) (Figure 1B ).
Besides increased yields, soybean producers and agricultural biotechnology worldwide are searching for grain quality to benefit health, growth and/or nutrition of animals, as this grain remains the most important and preferred source of high quality vegetable protein for animal feed manufacture. The chemical composition of soybeans may vary somewhat according to variety (genetic component) and growing conditions (environmental component). Through plant breeding, it has been possible to obtain protein levels between 40 and 45%, and lipid levels between 18 and 20%. Overall, GM lines presented protein and oil content values accepted by crushing industry, meting quality references, and commercial specifications ( Embrapa, 2015 ). The maintenance of these parameters is essential to be considered in the development of GM lines, as it adds value to the grain and ensures the competitiveness of soy in the world market. Transgenic DREB and AREB lines evaluated here under water deficit conditions presented acceptable oil and protein percentages, enabling cultivar obtained from these lines to enter in feed market for poultry, pork, cattle, other farm animals and pets.
Transcription factors introduced in soybean conventional cultivar BR16 and endogenous genes related to water deficit-responses showed higher expression in drought condition, showing that plants can modulate the metabolism in response to this adverse environmental circumstance by targeting different mechanisms, aiming to survival and keep productivity. Although these soybean transgenic lines expressing TFs DREB in general did not present a better performance when compared to WT plants (BR16 conventional cultivar), in field conditions under water deficit condition, a better performance was observed for 1Ea2939 AREB line which showed a higher performance than WT and other GM lines. Oil and protein content in the transgenic lines were not affected by the introduction of TFs, ensuring possible commercial cultivars derived form these lines competitiveness in livestock market.
Author Contributions
RF-P and LF contributed equally for the manuscript, being responsible for experiments installation, data collect and analysis, and manuscript write. FR helped in molecular analysis and manuscript review. HM helped with manuscript writing and editing. SM helped in the laboratory procedures and MM was involved with experiments performed in the greenhouse. JM-G and LM-H helped with manuscript review. JF and NN gave important support on physiological data analysis and MdO performed all statistical analysis. NK, YF, and JM developed the genetic construction used to obtain GM soybean lines. KN and KY-S participated in the experimental and molecular characterization design and reviewed the manuscript. AN is the coordinator of the research group and was directly involved in all stages of the research described in this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by Japan International Research Center for Agricultural Sciences (JIRCAS) and Embrapa Soybean. We are grateful to the Science and Technology Research Partnership for Sustainable Development (SATREPS) of the Japan Science and Technology Agency/Japan International Cooperation Agency (JST/JICA), which supported our study. We are also grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) for granting the scholarship to the authors RF-P and FR (Process #23038004021201133) and the National Council for Scientific and Technological Development (CNPq) for granting the scholarship to JM-G (Process #312433/2015-8) and LF (Process #503569/2012-7). The authors are also grateful to staff and students from the laboratories of Agrometeorology, Ecophysiology, and Plant Biotechnology of Embrapa Soybean for their support with all evaluations.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00448/full#supplementary-material
FILE S1 | Genes, Glymas, primers sequences, and fragment sizes of all endogenous genes analyzed.
FILE S2 | Photo from lodging and pod details from 1Ea2939 line. Lodging occurred after plentiful rain (341.4 mm) in a short period.
FILE S3 | Climatologic water balance from crop season 2013/2014, showing rainfall (mm), water deficit, water withdrawal and maximum temperature (°C). Graphic is scaled in a 10-day period from October 2013 to April 2014.
FILE S4 | Number of nodes and total pod number per plant for GM lines 1Ab58, 1Ea2939, and 1Bb2193 and WT plants (BR 16 cultivar) on February 17th, 2014 and on harvest (April, 2014), in non-irrigated (NIRR) and irrigated (IRR) treatments under field conditions.
FILE S5 | Climatologic water balance from crop season 2014/2015, showing rainfall (mm), deficit, and water withdrawal, scaled in a 10-day period from October 2014 to April 2015.
FILE S6 | Putative cis -elements present in the positive strand of the endogenous genes analyzed.
- ^ http://primer3plus.com/cgi-bin/dev/primer3plus.cgi
- ^ http://www.thermoscientificbio.com/webtools/multipleprimer/
- ^ http://www.genomatix.de
Almogueva, C., Prieto-Dapena, P., Díaz-Martín, J., Espinosa, J. M., Carranco, R., and Jordano, R. (2009). The HaDREB2 transcription factor enhances basal thermotolerance and longevity of seeds through functional interaction with HaHSFA9. BMC Plant Biol. 9:75. doi: 10.1186/1471-2229-9-75
PubMed Abstract | CrossRef Full Text | Google Scholar
Al-Whaibi, M. H. (2011). Plant heat-shock proteins: a mini review. J. King Saud Univ. Sci. 23, 139–150. doi: 10.1016/j.jksus.2010.06.022
Angra, S., Kaur, S., Singh, K., Pathania, D., Kaur, N., Sharma, S., et al. (2010). Water-deficit stress during seed filling in contrasting soybean genotypes: association of stress sensitivity with profiles of osmolytes and antioxidants. Int. Agric. Res. 5, 328–345. doi: 10.3923/ijar.2010.328.345
CrossRef Full Text | Google Scholar
Barbosa, E. G. G., Leite, J. P., Marin, S. R. R., Marinho, J. P., Carvalho, J. F. C., Fuganti-Pagliarini, R., et al. (2012). Overexpression of the ABA-dependent AREB1 transcription factor from Arabidopsis thaliana improves soybean tolerance to water deficit. Plant Mol. Biol. Rep. 31, 719–730. doi: 10.1007/s11105-012-0541-4
Barchet, G. L. H., Dauwe, R., Guy, R. D., Schroeder, W. R., Soolanayakanahally, R. Y., Campbell, M. M., et al. (2013). Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol. 34, 1203–1219. doi: 10.1093/treephys/tpt080
Bauer, H., Ache, P., Lautner, S., Fromm, J., Hartung, W., Al-Rasheid Khaled, A. S., et al. (2013). The stomatal response to reduced relative humidity requires guard cell autonomous ABA synthesis. Curr. Biol. 1, 53–57. doi: 10.1016/j.cub.2012.11.022.
Behnam, B., Kikuchi, A., Celebi-Toprak, F., Yamanaka, S., Kasuga, M., Yamaguchi-Shinozaki, K., et al. (2006). The Arabidopsis DREB1A gene driven by the stress-inducible rd29A promoter increases salt-stress tolerance in proportion to its copy number in tetrasomic tetraploid potato ( Solanum tuberosum ). Plant Biotechnol. J. 23, 169–177. doi: 10.5511/plantbiotechnology.23.169
Berlato, M. A., Matzenauer, R., and Bergamaschi, H. (1986). Evapotranspiração máxima da soja e relações com a evapotranspiração calculada pela equação de Penman, evaporação do tanque “classe A” e radiação solar global. Agron. Sulriograndense Porto Alegre 22, 251–259.
Bhatnagar-Mathur, P., Devi, M. J., Reddy, D. S., Lavanya, M., Vadez, V., Serraj, R., et al. (2007). Stress-inducible expression of AtDREB1A in transgenic peanut ( Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Rep. 26, 2071–2082. doi: 10.1007/s00299-007-0406-8
Bhatnagar-Mathur, P., Devi, M. J., Serraj, R., Yamaguchi-Shinozaki, K., Vadez, V., and Sharma, K. K. (2004). Evaluation of transgenic groundnut lines under water limited conditions. Int Arch. Newsl. 24, 33–34.
Google Scholar
Boneh, U., Biton, I., Schwartz, A., and Ben-Ari, G. (2012). Characterization of the ABA signal transduction pathway in Vitis vinifera . Plant Sci. 187, 89–96. doi: 10.1016/j.plantsci.2012.01.015
Brini, F., Hanin, M., Lumbreras, V., Amara, I., Khoudi, H., Hassairi, A., et al. (2007). Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana . Plant Cell Rep. 26, 2017–2026. doi: 10.1007/s00299-007-0412-x
Bustin, S. A. (2002). Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 29, 23–39. doi: 10.1677/jme.0.0290023
Chiappetta, A., Muto, A., Bruno, L., Woloszynska, M., MLijsebettens, M. V., and Bitonti, B. M. (2015). A dehydrin gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants. Front. Plant Sci. 6:392. doi: 10.3389/fpls.2015.00392
Companhia Nacional de Abastecimento [Conab] (2014). Acompanhamento da Safra Brasileira- Grãos. Minneapolis, MN: Agosto.
Côrrea, L. T., Riano-Pachon, D. M., Schrago, C. G., Dos Santos, R. V., Mueller- Roeber, B., and Vincentz, M. (2008). The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PLoS ONE 3:e2944. doi: 10.1371/journal.pone.0002944
Devi, J. M., Bhatnagar-Mathur, P., Sharma, K. K., Serraj, R., Anwar, S. Y., and Vadez, V. (2011). Relationships between transpiration efficiency (TE) and its surrogate traits in the rd29A:DREB1A transgenic groundnut. J. Agron. Crop Sci. 197, 272–283. doi: 10.1111/j.1439-037X.2011.00464.x
Dos Santos, T. B., Budzinski, I. G., Marur, C. J., Petkowicz, C. L., Pereira, L. F., and Vieira, L. G. (2011). Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiol. Biochem. 49, 441–448. doi: 10.1016/j.plaphy.2011.01.023
Dos Santos, T. B., de Lima, R. B., Nagashima, G. T., Petkowicz, C. L. O., Carpentieri-Piìpolo, V., Pereira, L. F. P., et al. (2015). Galactinol synthase transcriptional profile in two genotypes of Coffea canephora with contrasting tolerance to drought. Genet. Mol. Biol. 38, 182–190. doi: 10.1590/S1415-475738220140171
Dubouzet, J. G., Sakuma, Y., Ito, Y., Kasuga, M., Dubouzet, E. D., Miura, S., et al. (2003). Os-DREB genes in rice, Oryza sativa L., encoded transcription activators that function in drought, high-salt and cold-responsive gene expression. Plant J. 33, 751–763. doi: 10.1046/j.1365-313X.2003.01661.x
Egawa, C., Kobayashi, F., Ishibashi, M., Nakamura, T., Nakamura, C., and Takumi, S. (2006). Differential regulation of transcript accumulation and alternative splicing of a DREB2 homologue under abiotic stress conditions in common wheat. Biochem. Biophys. Res. Commun. 9, 299–305.
Egli, D. B. (2013). The relationship between the number of nodes and nods in soybean communities. Crop Sci. 53, 1668–1676. doi: 10.2135/cropsci2012.11.0663
El Rabey, H. A., Al-Malki, A. L., Abulnaja, K. O., and Rohde, W. (2015). Proteome analysis for understanding abiotic stress (salinity and drought) tolerance in date palm ( Phoenix dactylifera L.). Int. J. Genom. doi: 10.1155/2015/407165
Embrapa (2013). Empresa Brasileira de Pesquisa Agropecuária Available at: http:// www.cnpso.embrapa.br
Embrapa (2015). Comunicado Tecnico 86. Teores de Óleo e Proteína em Soja: Fatores Envolvidos e Qualidade Para a Industria. Londrina: Embrapa.
Engels, C., Fuganti-Pagliarini, R., Marin, S. R. R., Marcelino-Guimarães, F. C., Oliveira, M. C. N., Kanamori, N., et al. (2013). Introduction of the rd29A:AtDREB2A CA gene into soybean ( Glycine max L. Merril) and its molecular characterization in leaves and roots during dehydration. Genet. Mol. Biol. 36, 556–565. doi: 10.1590/S1415-47572013000400015
Fang, Y., and Xiong, L. (2015). General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 72, 673–689. doi: 10.1007/s00018-014-1767-0
Fritschi, F. B., and Ray, J. D. (2007). Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 45, 92–98. doi: 10.1007/s11099-007-0014-4
Fujita, Y., Fujita, M., Satoh, R., Maruyama, K., Parvez, M. M., Seki, M., et al. (2005). AREB1 is a transcription activator of novel ABRE dependent ABA signaling that enhances drought stress tolerance in Arabidopsis . Plant Cell 17, 3470–3488. doi: 10.1105/tpc.105.035659
Gao, S. Q., Chen, M., Xia, L. Q., Xiu, H. J., Xu, Z. S., Li, L. C., et al. (2009). A cotton ( Gossypium hirsutum ) DRE binding transcription factor gene, GhDREB, confers enhanced tolerance to drought, high salt, and freezing stresses in transgenic wheat. Plant Cell Rep. 28, 301–311. doi: 10.1007/s00299-008-0623-9.
Gilmour, S. J., Zarka, D. G., Stockinger, E. J., Salazar, M. P., Houghton, J. M., and Thomashow, M. F. (1998). Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 16, 433–443. doi: 10.1046/j.1365-313x.1998.00310.x
Hand, S. C., Menze, M. A., Toner, M., Boswell, L., and Moore, D. (2011). LEA proteins during water stress: not just for plants anymore. Annu. Rev. Physiol. 73, 115–134. doi: 10.1146/annurev-physiol-012110-142203.
Hara, M., Fujinaga, M., and Kuboi, T. (2004). Radical scavenging activity and oxidative modification of citrus dehydrin. Plant Physiol. Biochem. 42, 657–662. doi: 10.1016/j.plaphy.2004.06.004
Hara, M., Fujinaga, M., and Kuboi, T. (2005). Metal binding by citrus dehydrin with histidine-rich domains. J. Exp. Bot. 56, 2695–2703. doi: 10.1093/jxb/eri262
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., and Ahmad, A. (2012). Role of proline under changing environments–A review. Plant Signal. Behav. 7, 1456–1466. doi: 10.4161/psb.21949.
Heil, C. (2010). Rapid, Multi-Component Analysis of Soybeans by FT-NIR Spectroscopy . Madison: Thermo Fisher Scientific.
Ito, Y., Katsura, K., Maruyama, K., Taji, T., Kobayashi, M., Seki, M., et al. (2006). Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 47, 141–153. doi: 10.1093/pcp/pci230
Jaglo-Ottosen, K. R., Gilmour, S. J., Zarka, D. G., Schabenberger, O., and Thomashow, M. F. (1998). Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280, 104–106. doi: 10.1126/science.280.5360.104
Jakoby, M., Weisshaar, B., Dröge-Laser, W., Vicente-Carbajosa, J., Tiedemann, J., Kroj, T., et al. (2002). bZIP transcription factors in Arabidopsis . Trends Plant Sci. 7, 106–111. doi: 10.1016/S1360-1385(01)02223-3
Kahlon, C. S., Board, J. E., and Kang, M. S. (2011). An analysis of yield component changes for new and old cultivars. Agron. J. 103, 13–22. doi: 10.2134/agronj2010.0300
Kasuga, M., Liu, Q., Miura, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999). Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress inducible transcription factor. Nat Biotechnol. 17, 287–291. doi: 10.1038/7036
Kasuga, M., Miura, S., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2004). A Combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol. 45, 346–350. doi: 10.1093/pcp/pch037
Kim, J. S., Mizoi, J., Yoshida, T., Fujita, Y., Nakajima, Y., Ohori, T., et al. (2011). An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis . Plant Cell. Physiol. 52, 2136–2146. doi: 10.1093/pcp/pcr143
Klingler, J. P., Batelli, G., and Zhu, J. K. (2010). ABA receptors: the START of a new paradigm in phytohormone signaling. J. Exp. Bot. 60, 3199–3210. doi: 10.1093/jxb/erq151
PubMed Abstract | CrossRef Full Text
Knight, K., Zarka, D. G., Okamoto, H., Thomashow, M. F., and Knight, M. R. (2004). Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiology 135:1710–1717. doi: 10.1104/pp.104.043562
Kobayashi, F., Maeta, E., Terashima, A., and Takumi, S. (2008). Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiol. Plant. 134, 74–86. doi: 10.1111/j.1399-3054.2008.01107.x
Lee, J. S., Kang, J. Y., Park, H. J., Kim, M. D., Bae, M. S., Choi, H. I., et al. (2010). DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol. 153, 716–727. doi: 10.1104/pp.110.154617
Leite, J. P., Barbosa, E. G. G., Marin, S. R. R., Marinho, J. P., Carvalho, J. F. C., Fuganti-Pagliarini, R., et al. (2014). Overexpression of the activated form of the AtAREB1 gene (AtAREB1ΔQT) improves soybean responses to water deficit. Genet. Mol. Res. 13, 6272–6286. doi: 10.4238/2014.August.15.10
Li, C., Yue, J., Wu, X., Xu, C., and Yu, Y. (2014). An ABA-responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, plays a critical role under drought stress. J. Exp. Bot. 65, 5415–5427. doi: 10.1093/jxb/eru302
Liu, L., Zhu, K., Yang, Y., Wu, J., Chen, F., and YU, D. (2008). Molecular cloning, expression profiling and trans-activation property studies of a DREB2-like gene from chrysanthemum ( Dendranthema vestitum ). J. Plant Res. 121, 215–226. doi: 10.1007/s10265-007-0140-x
Liu, Q., Sakuma, Y., Abe, H., Kasuga, M., Miura, S., Yamaguchi- Shinozaki, K., et al. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain, separate two cellular signal transduction pathways in drought- and low temperature-responsive gene expression, respectively, in Arabidopsis . Plant Cell 10, 1491–1406. doi: 10.1105/tpc.10.8.1391
Marinho, J. P., Kanamori, N., Ferreira, L. C., Fuganti-Pagliarini, R., Carvalho, J. F. C., Freitas, R. S., et al. (2016). Characterization of molecular and physiological responses under water deficit of genetically modified soybean plants overexpressing the AtAREB1 transcription factor. Plant Mol. Biol. Rep. 34, 410–426. doi: 10.1007/s11105-015-0928-0
Masoumi, H., Darvish, F., Daneshian, J., Nourmohammadi, G., and Habibi, D. (2011). Chemical and biochemical responses of soybean ( Glycine max L.) cultivars to water deficit stress. Aust. J. Crop Sci. 5, 544–553.
Mizoi, J., Ohori, T., Moriwaki, T., Kidokoro, S., Todaka, D., Maruyama, K., et al. (2013). GmDREB2A;2, a canonical dehydration- responsive element binding protein2-type transcription factor in soybean, is post-translationally regulated and mediates dehydration-responsive element-dependent gene expression. Plant Physiol. 161, 346–361. doi: 10.1104/pp.112.204875
Molinari, H. B. C., Marur, C. J., Daros, E., de Campos, M. K. F., Portela de Carvalho, J. F. R., Bespalhok Filho, J. C., et al. (2007). Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol. Plant. 130, 218–229. doi: 10.1111/j.1399-3054.2007.00909.x
Narusaka, Y., Nakashima, K., Shinwari, Z. K., Sakuma, Y., Furihata, T., Abe, H., et al. (2003). Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 34, 137–148. doi: 10.1046/j.1365-313X.2003.01708.x
Niu, X., Helentjaris, T., and Bate, N. J. (2002). Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell 14: 2565–2575. doi: 10.1105/tpc.003400
Oh, S. J., Song, S. I., Kim, Y. S., Jang, H. J., Kim, S. Y., Kim, M., et al. (2005). Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol. 138, 341–351. doi: 10.1104/pp.104.059147
Oya, T., Nepomuceno, A. L., Neumaier, N., Farias, J. R. B., Tobita, S., and Ito, O. (2004). Drought tolerance characteristics of Brazilian soybean cultivars–evaluation and characterization of drought tolerance of various Brazilian soybean cultivars in the field. Plant Prod. Sci. 7, 129–137. doi: 10.1626/pps.7.129
Park, S. H., Chung, P. J., Juntawong, P., Bailey-Serres, J., Kim, Y. S., Jung, H., et al. (2012). Posttranscriptional control of photosynthetic mRNA decay under stress conditions requires untranslated regions and correlates with differential polysome association in rice. Plant Physiol. 159, 1111–1124. doi: 10.1104/pp.112.194928
Passioura, J. B. (2012). Phenotyping for drought tolerance in grain crops: when is it useful to breeders? Funct. Plant Biol. 39, 851–859. doi: 10.1071/FP12079
Pellegrineschi, A., Reynolds, M., Pacheco, M., Brito, R. M., Almeraya, R. Yamaguchi-Shinozaki, K., et al. (2004). Stress induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47, 493–500. doi: 10.1139/g03-140
Polizel, A., Medri, M. E. K., Nakashima Yamanaka, N., Farias, J. R., de Oliveira, M. C., Marin, S. R. R., et al. (2011). Molecular, anatomical and physiological properties of a genetically modified soybean line transformed with rd29A:AtDREB1A for the improvement of drought tolerance. Genet. Mol. Res. 10, 3641–3656. doi: 10.4238/2011
Qin, F., Kakimoto, M., Maruyama, K., Osakabe, Y., Tran, L. S., Shinozaki, K., et al. (2007). Regulation and functional analysis of ZMDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 50, 54–69. doi: 10.1111/j.1365-313X.2007.03034.x
Qin, F., Sakuma, Y., Li, J., Liu, Q., Li, Y. Q., Shinozaki, K., et al. (2004). Cloning and functional analysis of a novel DREB1/ CBF transcription factor involved in cold responsive gene expression in Zea mays L. Plant Cell Physiol. 45, 1042–1052. doi: 10.1093/pcp/pch118
Rolla, A. A., Carvalho, J. F. C., Fuganti-Pagliarini, R., Engels, C., Rio, A., Marin, S. R. R., et al. (2013). Phenotyping soybean plants transformed with rd29A:AtDREB1A for drought tolerance in the greenhouse and field. Transgenic Res. 23, 75–87. doi: 10.1007/s11248-013-9723-6
Rorat, T., Szabala, B. M., Grygorowicz, W. J., Wojtowicz, B., Yin, Z., and Rey, P. (2006). Expression of SK3-type dehydrin in transporting organs is associated with cold acclimation in Solanum species. Planta 224, 205–221. doi: 10.1007/s00425-005-0200-1
Sakuma, Y., Maruyama, K., Qin, F., Osakabe, Y., Qin, F., Seki, M., et al. (2006a). Functional analysis of an Arabidopsis transcription factor DREB2A, involved in drought-responsive gene expression. Plant Cell 18, 1292–1309. doi: 10.1105/tpc.105.035881
Sakuma, Y., Maruyama, K., Qin, F., Osakabe, Y., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2006b). Dual function of an Arabidopsis transcription factor DREB2A in water- stress-responsive and heat-stress–responsive gene expression. Proc. Natl. Acad. Sci. U.S.A. 103, 18822–18827. doi: 10.1073/pnas.0605639103
Shinozaki, K., and Yamaguchi-Shinozaki, K. (2000). Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 3, 217–223. doi: 10.1016/S1369-5266(00)00067-4
Shinozaki, K., and Yamaguchi-Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 58, 221–227. doi: 10.1093/jxb/erl164
Shinozaki, K., Yamaguchi-Shinozaki, K., and Seki, M. (2003), Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6, 410–417. doi: 10.1016/S1369-5266(03)00092-X
CrossRef Full Text
Silvente, S., Sobolev, A. P., and Lara, M. (2012). Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 7:e38554. doi: 10.1371/journal.pone.0038554
Speirs, J., Binney, A., Collins, M., Edwards, E., and Loveys, B. (2013). Expression of ABA synthesis and metabolism genes under different irrigation strategies and atmospheric VPDs is associated with stomatal conductance in grapevine ( Vitis vinifera L. cv Cabernet Sauvignon). J. Exp. Bot. 64, 1907–1916. doi: 10.1093/jxb/ert052
Stolf-Moreira, R., Lemos, E. G. M., Abdelnoor, R. V., Beneventi, M. A., Rolla, A. A. P., Pereira, S. S., et al. (2011). Identification of reference genes for expression analysis by real-time quantitative PCR in drought-stressed soybean. Pesqui. Agropecu. Bras. 46, 58–65. doi: 10.1590/S0100-204X2011000100008
Sultan, M. A. R. F., Hui, L., Yang, L. J., and Xian, Z. H. (2012). Assessment of drought tolerance of some triticum L. species through physiological indices czech. J. Genet. Plant Breed. 48, 178–184.
Taji, T., Ohsumi, C., Iuchi, S., Seki, M., Kasuga, M., Kobayashi, M., et al. (2002). Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana . Plant J. 29, 417–426. doi: 10.1046/j.0960-7412.2001.01227.x
Terashima, A., and Takumi, S. (2009). Allopolyploidization reduces alternative splicing efficiency for transcripts of the wheat DREB2 homolog, WDREB2. Genome 52, 100–105. doi: 10.1139/G08-101
Tripepi, M., Pöhlschroder, M., and Bitonti, M. B. (2011). Diversity of dehydrins in Olea europaea plants exposed to stress. Open Plant Sci. J. 5, 9–13. doi: 10.2174/1874294701105010009
Umezawa, T., Nakashima, K., Miyakawa, T., Kuromori, T., Tanokura, M., Shinozaki, K., et al. (2010). Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol. 51, 1821–1839. doi: 10.1093/pcp/pcq156
Vadez, V., Rao, J. S., Bhatnagar-Mathur, P., and Sharma, K. K. (2013). DREB1A promotes root development in deep soil layers and increases water extraction under water stress in groundnut. Plant Biol. 15, 45–52. doi: 10.1111/j.1438-8677.2012.00588.x
Xu, Y., Hu, W., Liu, J., Zhang, J., Jia, C., Miao, H., et al. (2014). A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 14:59. doi: 10.1186/1471-2229-14-59
Xu, Y. H., Liu, R., Yan, J., Liu, Z. Q., Jiang, S. C., Shen, Y. Y., et al. (2012). Light harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis . J. Exp. Bot. 63, 1095–1106. doi: 10.1093/jxb/err315
Yamaguchi-Shinozaki, K., and Shinozaki, K. (2005). Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 10, 88–94. doi: 10.1016/j.tplants.2004.12.012
Yang, Y., He, M., Zhu, Z., Li, S., Xu, Y., Zhang, C., et al. (2012). Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 12:140. doi: 10.1186/1471-2229-12-140
Yoshida, T., Fujita, Y., Sayama, H., Kidokoro, S., Maruyama, K., Mizoi, J., et al. (2010). AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 61, 672–685. doi: 10.1111/j.1365-313X.2009.04092.x
Zhao, L., Hu, Y., Chong, K., and Wang, T. (2010). ARAG1, an ABA-responsive DREB gene, plays a role in seed germination and drought tolerance of rice. Ann. Bot. 105, 401–409. doi: 10.1093/aob/mcp303
Keywords : Glycine max , transcription factors, transgene, ABA, water deficit, yield
Citation: Fuganti-Pagliarini R, Ferreira LC, Rodrigues FA, Molinari HBC, Marin SRR, Molinari MDC, Marcolino-Gomes J, Mertz-Henning LM, Farias JRB, de Oliveira MCN, Neumaier N, Kanamori N, Fujita Y, Mizoi J, Nakashima K, Yamaguchi-Shinozaki K and Nepomuceno AL (2017) Characterization of Soybean Genetically Modified for Drought Tolerance in Field Conditions. Front. Plant Sci. 8:448. doi: 10.3389/fpls.2017.00448
Received: 28 July 2016; Accepted: 15 March 2017; Published: 11 April 2017.
Reviewed by:
Copyright © 2017 Fuganti-Pagliarini, Ferreira, Rodrigues, Molinari, Marin, Molinari, Marcolino-Gomes, Mertz-Henning, Farias, de Oliveira, Neumaier, Kanamori, Fujita, Mizoi, Nakashima, Yamaguchi-Shinozaki and Nepomuceno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre L. Nepomuceno, [email protected]
† These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 October 2019
Detection and identification of transgenic events by next generation sequencing combined with enrichment technologies
- Frédéric Debode 1 na1 ,
- Julie Hulin ORCID: orcid.org/0000-0003-3271-0157 1 na1 ,
- Benoît Charloteaux 2 ,
- Wouter Coppieters 2 ,
- Marc Hanikenne 3 ,
- Latifa Karim 2 &
- Gilbert Berben 1
Scientific Reports volume 9 , Article number: 15595 ( 2019 ) Cite this article
8410 Accesses
24 Citations
1 Altmetric
Metrics details
- Molecular biology
Next generation sequencing (NGS) is a promising tool for analysing the quality and safety of food and feed products. The detection and identification of genetically modified organisms (GMOs) is complex, as the diversity of transgenic events and types of structural elements introduced in plants continue to increase. In this paper, we show how a strategy that combines enrichment technologies with NGS can be used to detect a large panel of structural elements and partially or completely reconstruct the new sequence inserted into the plant genome in a single analysis, even at low GMO percentages. The strategy of enriching sequences of interest makes the approach applicable even to mixed products, which was not possible before due to insufficient coverage of the different genomes present. This approach is also the first step towards a more complete characterisation of agrifood products in a single analysis.
Similar content being viewed by others

Improved sampling and DNA extraction procedures for microbiome analysis in food-processing environments
Coral Barcenilla, José F. Cobo-Díaz, … Avelino Alvarez-Ordóñez
Simultaneous identification of animal-derived components in meats using high-throughput sequencing in combination with a custom-built mitochondrial genome database
Yinan Zhang, Qinfeng Qu, … Fei Tao
Identification of an unauthorized genetically modified bacteria in food enzyme through whole-genome sequencing
Marie-Alice Fraiture, Bert Bogaerts, … Nancy H. C. Roosens
Introduction
The number and diversity of GMOs have greatly increased in recent years. Currently, the reference method for GMO detection is real-time PCR. The main problem of real-time PCR is that it can only be used to detect targeted sequences, which means that searches are somewhat limited since they can only find what is being looked for. Moreover, new solutions need to be found for the characterisation of authorised and unauthorised GMOs. NGS approaches may address the problem of identifying all GMOs in a sample. High-throughput sequencing can sequence several million fragments in parallel and is able to provide the whole sequence of plant genomes 1 . NGS has already been used to help molecularly characterise a genetically modified (GM) soybean without the need for Southern Blot analysis 2 . Several approaches have been developed that use the potential of high-throughput sequencing for the detection of GMOs or GMO-derived products 3 , 4 , 5 , 6 . However, NGS is still not frequently used for GMO detection due to important challenges, such as uneven coverage of the genome 7 . This problem can be reinforced as a function of the genome size of the plant considered, e.g., the soybean genome is ~1.1 gigabases (Gb) 8 while the wheat genome is ~17 Gb 9 , and genetic diversity is even greater in complex food products containing several plant species.
Several approaches for GMO detection have already been developed. First, pilot studies have shown that NGS using whole genome sequencing approaches is able to detect GMOs 10 , 11 , 12 . NGS became a method for checking for inserted sequences 2 , 3 , 4 . However, these methods have only been tested on pure GM material, while a large number of sequencing runs would be required to gain sufficient coverage to allow the detection of low GM contents 7 . To detect GMOs present at low levels, sequencing of a large number of targeted amplicons by NGS was proposed 13 . This method was able to detect numerous structural elements but was not suitable for reconstructing the inserted sequence. The sensitivity of this method was not evaluated in depth, but its performance was poorer than real-time PCR 13 . Only techniques combining NGS with SiteFinding PCR 5 and DNA walking strategies 14 , 15 have been able to provide information on the junction sequence between a plant and GM construct at low percentages. The method using genome walking with ALF (amplification of linearly enriched fragment) could detect a level as low as 1% 15 . This method starts with two structural elements, p35S and tNOS. DNA walking method using anchored PCR followed by two semi-nested PCRs was able to detect a level of 0.1% of Bt rice 14 . This method is now capable of starting from five structural elements (p35S, t35S pCambia, tNOS and cry) 14 , 16 . However, these strategies, based on the sequencing of amplicons by NGS, are time-consuming, cannot cover large fragments of GM constructs and are dependent on a starting point linked to the presence of a precise structural element.
We developed an approach combining NGS with a strategy of enriching the regions of interest that differs from the eleven enrichment strategies listed by Arulandhu et al . 17 . The regions of interest correspond to a series of structural elements frequently introduced into transgenic constructs. We checked the capacity of the method for GMO detection, even in flour containing low percentages of transgenic plants, and developed a bioinformatic pipeline for the detection and characterisation of GM events.
Results and Discussion
Our work started with the development of a database of sequences that could be used for enrichment. The present version of the database gathers the sequences of 10 promoters, 6 terminators and 23 genes or miscellaneous elements that are found in transgenic constructs (Table 1 ). The total size of the enrichment sequences in the database is ~53 kb, but the database is still far from its limit as the methodology can be scaled up to 24 Mb. The covering a large number of GM events is possible, as the database includes the sequences of the structural elements most commonly used in genetically modified plants 18 , 19 . Sequences corresponding to antibiotic resistance or other selection markers were not included in the database, as they could generate unexpected signals linked to the presence of traces of DNA from the bacteria and recombinant plasmids used for the production of the enzymes employed for PCR amplification and sequencing. If we compare the potential of detection with the 328 GM events listed in the GMOseek matrix 19 and in relation to 23 plant species, only 3 GM events (AR9 Azuki bean, LY038 maize and BPS-CV127-9 soybean) would not be detected because they do not contain any of the 40 structural elements used to design the enrichment. AR9 Azuki bean, LY038 maize and BPS-CV127-9 soybean contain structural elements that are particular to these transgenic events. The sequences of these structural elements are not currently available but could be added in the future. However, the AR9 Azuki bean also contains npt II, providing tolerance to antibiotics 20 . This example shows the importance of not excluding selection markers from the enrichment database in the future and is why the pros and cons of the presence of such sequences should be evaluated in the next version of the enrichment database.
The developed database was then used to create capture probes focusing on the elements listed in Table 1 . Two types of methodologies were tested for sequence enrichment through capture probes. The first methodology used numerous probes of 50-80 bp that had a high level of overlap (SeqCapEZ technology, Roche Diagnostics/NimbleGen, Madison, WI), in which each base is generally covered by at least 7 probes. The second methodology used larger probes (~120 bp) with a low level of overlap (SureSelect technology, Agilent Technologies, Santa Clara, CA). No degeneracy was introduced in the probes. The probes are supposed to be able to catch fragments of up to 500 bp in size, which would allow the captured fragments to include junctions between structural elements or junctions between the plant and inserted sequence.
The enrichment principle is presented in Fig. 1 . From a theoretical point of view, both methodologies have advantages: shorter and more numerous probes should be better at capturing degraded DNA or sequences of structural elements that slightly vary from what is expected, while longer probes should lead to increased specificity of sequence capture. The comparison of the SureSelect and NimbleGen technologies has already been discussed for several medical applications with results favouring either the NimbleGen approach 21 , 22 , 23 or SureSelect technology 24 or indicating comparable performances 25 . The comparisons show that both methodologies have pros and cons depending on the objectives of the project 26 and indicate that the balance in favour of one method can change as a function of the evolution of kits and protocols 26 .
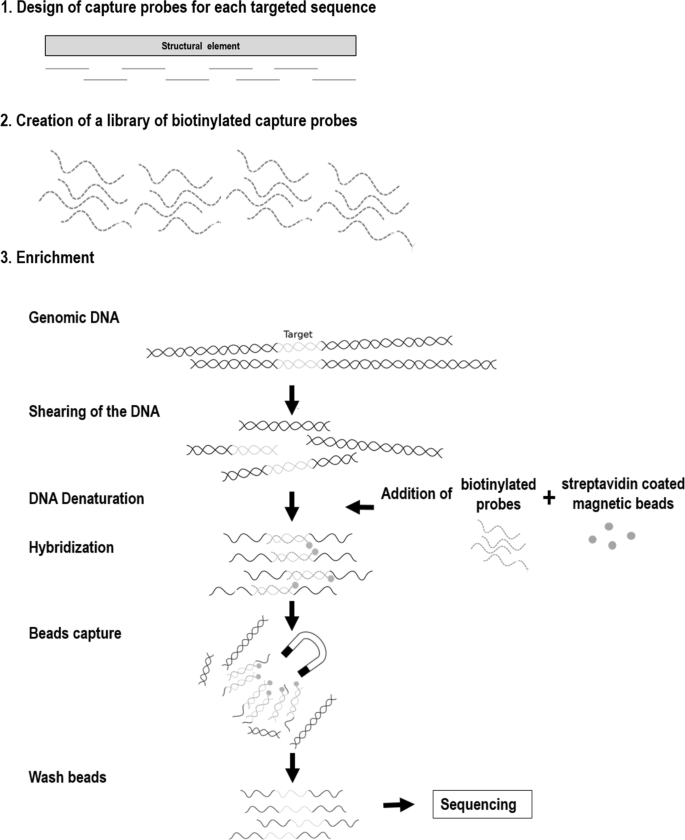
Workflow of the enrichment technology prior to sequencing.
In this study, after analysing sequencing runs on Illumina devices, better enrichments with fewer unexpected assignations were observed when using SureSelect technology. This paper focuses on the best results obtained with this technology. After enrichment, the DNA libraries were sequenced on an Illumina MiSeq system (Illumina, San Diego, CA).
To analyse the large amount of read data, a bioinformatic workflow was created. The workflow was divided into two parts. In the first part, which was aimed at GMO detection, reads were aligned onto the sequences used for enrichment and filtered according to their alignment scores. Statistical analysis was then performed to determine whether the reads could be distinguished from noise and assimilated to positive results. The objective of the second part of the workflow was to characterise the GMO through the creation of contigs in an attempt to reconstruct the whole transgene, possibly including the plant-construct junction specific to the event. The bioinformatics workflow used different scripts and programs, as presented in Fig. 2 .
Bioinformatic workflow developed for detecting and identifying GMOs. The bioinformatic packages used are indicated in grey.
The analysed samples included five species, eight transgenic events and variable fractions (0.1%, 1%, 10% and 100%) of GMOs (Table 2 in the methods section). Concerning GMO detection, the structural elements listed in the enrichment database and present in the GM events tested were all detected (Figs 3 and 4 ).
Detection and characterisation of GMOs by NGS. The structures of the inserts of seven GMOs are presented. The 281 × 3006 cotton has two GM inserts. The mixed sample contains 50% A2704 soybean and 50% LL62 rice. The structural elements in grey were present in the database used for enrichment and were detected by NGS. The reads associated with these structural elements were used to create contigs. Only larger contigs covering several structural elements are shown here. Larger structural elements not covered by the capture probes created gaps, making it impossible to reconstruct the entire sequence of the transgenic cassette. Junction regions covering the plant and transgenic insert were also obtained.
Sequence of GTS 40-3-2 soybean and alignments of the contigs obtained in this research. The structural elements in grey shown in the database were used for enrichment and were detected by NGS. ( A ) Expected sequences of the GTS-40-3-2 soybean, as announced by Monsanto and as described by Windels et al . 28 . Additional sequence corresponds to a duplication of part of the EPSPS gene and an unknown rearranged sequence. ( B ) Positions of the contigs created for the samples containing GTS-40-3-2 soybean at 10%, 1% and 0.1%.
Logically, the percentage of sequenced reads assigned to the structural element present depended on the GM percentage. An example is given in Table 3 for GTS-40-3-2 soybean, in which the percentage of reads aligned with p35S, tNOS and EPSPS increased as a function of the GM percentage. The absolute number of reads is linked to the length of the structural elements (this point can, however, be normalised) and to the DNA quantities introduced in the experiments. The number of reads cannot be used for quantitative approaches, and sequencing will not replace real-time PCR or digital PCR for GMO quantification. However, once the system is updated with taxon-specific genes (preliminary experiments are underway), the system may be able to provide an indication of the GM percentage. This information would, however, remain semi-quantitative.
In the bioinformatics workflow, a threshold level was set for considering an element beyond background noise and thus as being detected. In the SureSelect experiments, this threshold was based on the mean number of reads, standardised in RPKM (reads per kilobase per million mapped reads), obtained for non-GM plants plus five times the calculated standard deviation, giving a probability of differentiation between positive and negative results greater to 99% 27 . Structural elements were clearly distinguished from each other except for cry gene sequences, as its variants showed similarities in their sequences. However, the highest number of assignations was attributed to the correct cry gene. Non-GM plants were also tested to check for unspecific mappings. With a threshold of 25 reads (standardised in RPKM), no problems were encountered with soybean, cotton or rapeseed. For maize, positive signals were observed with pUbi, pMTL and hsp70, as maize is the donor organism of these structural elements. Some similarities were also identified in maize for the EPSPS1 structural element (GACGAGGAAGCTCATGGCGATGCGGTGATCGAGATGGGTGGCGACG), as this element showed similarity with a 46-bp fragment of the maize genome. Information concerning the donor organism of the structural element and its potential presence in the sample must be taken into account to interpret the results, but the element could also be a target of interest for implementation of the detection system for the identification of plants.
For GMO characterisation, positive reads were assembled to create “blind” contigs to prevent influence from a previously known sequence. This process is important for detecting differences between the announced and the real sequence of a GMO and to mimic results that could be obtained in the presence of an unknown GMO. Contigs made it possible to partially (100% 59122 maize and 10% 281 × 3006 cotton) or totally (10% GTS-40-3-2 soybean, 100% GT73 rapeseed, 100% MS8 rapeseed, 10% MON89034 maize) reconstruct the sequence of inserts. For 281 × 3006 cotton containing three times the pUbi promoter, it was possible to propose contigs for each repetition of the promoter with its respective structural element (Fig. 3 ), which shows that the method is capable of proposing solutions to help to characterise complex sequences introduced into plants or even mixed GMOs. A sample containing 50% of A2704 soybean (construct: 35 S promoter – pat gene – 35 S terminator) and 50% of LL62 rice (construct: 35 S promoter – bar gene –35 S terminator) was also tested (Fig. 3 ). The bioinformatic pipeline was able to propose a sequence for the inserts introduced in each GM plant. The two sequences were clearly distinguishable even though the sequence of the pat and bar genes showed approximately 60% similarity when aligned. The sequences of the inserts introduced into A2704 soybean and LL62 rice are not publicly available. Therefore, no comparison between the obtained sequences with the announced sequences was possible. However, the percentage of similarity between known the pat and bar sequences falls into the same range.
Disruptions in the contigs were mainly due to the presence of structural elements that were not originally considered for enrichment and therefore constituted gaps, preventing reassembly of the whole sequence. Adding these elements to future enrichment steps would be an interesting recommendation. A definite advantage of this technology is that fragments caught by the capture probes covered junction regions as well, so it was not only possible to create contigs including junctions between structural elements but also between plant DNA and the GM construct (Figs 3 and 4 ).
The length of the contigs also depended on the fraction of the GMO in the analysed flour. An example is presented for GTS-40-3-2 soybean (Fig. 3 ), for which it was possible to assemble contigs even at a percentage as low as 0.1% of an event, proving that the methodology is very sensitive, as it still succeeded in characterising GMOs at low percentages. For GTS-40-3-2, at a level as low as 1% of GM, it was possible to recreate the transgenic construct and determine the left border (plant-GM construct junction) and the rearranged sequence as described by Windels et al . 28 on the right side. This rearrangement corresponds to a portion of the EPSPS gene and a part of the plasmid vector used for transformation. The contig for GTS40-3-2 soybean at 1% was somewhat shorter than the contig obtained for GTS40-3-2 at 10%. At 0.1%, it was possible to create two contigs, with one of them covering the left junction (plant - DNA construct). The lower number of reads available in this last case made it impossible to reconstruct the whole sequence of the transgenic cassette.
DNA enrichment has a cost of 300 euros/sample and sequencing adds additional 300 euros/sample. This price is high for an analysis in the field of agrofood products, but since the first experiments, conducted 3 years ago, the estimated cost of the approach has already been halved. If the time required to perform enrichment (2 days), sequence the libraries (2 days) and complete the bioinformatics analysis (3 hours/sample) is reasonable for a routine analysis, access to a sequencing machine - if outsourced – generally takes at least one month and remains a very limiting factor when a fast answer is needed. Therefore, the use of affordable machines (e.g., minion, Oxford Nanopore technologies, Oxford, UK) must be tested in future approaches 29 .
The sequencing approach can be used: (i) alone, as a new detection and characterisation technique that has a good coverage because of the large number of structural elements tested; (ii) as a complement to real-time PCR to characterise the GM construct(s) or event(s) initially detected by real-time PCR tests; and (iii) prior to the development of an event-specific real-time PCR test because of the characterisation of the GM insert and its border regions.
Approaches to GMO detection using NGS have been proposed before, but this is the first time that such a methodology (i) enables the detection of GMOs at low levels, (ii) can be used on products containing several plant species, (iii) focuses on a large panel of screening elements, and (iv) makes it possible to partially or completely reconstruct a GMO, thereby providing a mechanism to detect unknown events. In the case of a laboratory equipped with NGS technology, this methodology could also be applied in a time frame that is more suitable for routine analysis.
Moreover, this is the first step towards a more informative analysis, as the enrichment can be extended to sequences corresponding to additional structural elements, plant species, allergens and contaminants. Specific sequences for these elements can be added to the database for the design of capture probes, leading to a technology not only focused on GMO detection but also extendable to the determination of other interesting food and feed product features. The strategy described in this study is only valid for GMOs obtained through classical recombinant DNA technology that give rise to transgene constructs. This study is not aimed at gene editing techniques (e.g., CRISPR/Cas9).
The certified transgenic reference materials (CRMs) were obtained from the Institute for Reference Materials and Measurements (JRC, Geel, Belgium) and the American Oil Chemists’ Society (AOCS, Urbana, Illinois, USA). Commercial organic grains were collected for non-GM plant species. Tests performed using real-time PCR 30 , 31 confirmed the absence of GM material from commercial organic grains. The origin of the material is presented in the supplementary material (Table 2 ).
The samples were considered individually for sequencing (with the exception of the 50% LL62 rice/ 50% A2704-12 soybean mix), and some of the samples (maize 0% GM, soybean 0% GM, maize MON89034 100%) were repeated to observe background noise.
DNA extraction
Genomic DNA was extracted and purified from all samples following the CTAB-based method described in Annex A.3.1 of the ISO 21571:2005 international standard 32 . The quality of DNA extracted from samples was estimated using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). DNA samples were quantified by Picogreen (Quant-iT™ PicoGreen™ dsDNA Assay Kit, Invitrogen, Carlsbad, CA); 3 µg of DNA was used for library preparation.
Next generation sequencing
DNA was sheared on a Picoruptor (Diagenode, Liège, Belgium) to produce fragments of ~150–200 bp. The SureSelect XT Target Enrichment system (Agilent technologies) was used to capture sequences of interest prior to sequencing. The design includes 458 enrichment probes. The sequences of the probes are available in supplementary material (Table S1 ). Via the online tool “Suredesign” on the Agilent Technologies website and through the option “collaboration space” with reference to design ID 3045501, probes were ordered from Agilent. No degeneracy was introduced in the sequences of the probes. Sequencing was performed on an Illumina MiSeq instrument with MiSeq Reagent Kit v3 (2 × 75 bp) at the GIGA Genomics platform at the University of Liège.
The pipeline for analysing results was perfected, as shown in Fig. 2 , by the use of free access programs (with their default settings): bwa mem version 0.7.16-r1180 33 , Samtools version 0.1.19-96b5f2294a 34 , R ggplot version 2_2.2.1 35 , Seqtk version 1.2 36 , Velvet version 1.2.10 37 , Mira version 4.0.2 38 , Spades version 3.12 39 , CISA version 1.3 40 and Blast version 2.7.1+ 41 , 42 . The commands calling the different packages and an example (manifest file) are given in Supplementary Material S2 .
The assembled contigs were compared to the sequences of the inserts introduced in plants: GTS 40-3-2 soybean (Windels et al ., 2001), GT73 rapeseed (patent US 6248876), MS8 (structure of the plasmid pTHW101 as described in notification C/BE/96/01), 59122 maize (NCBI accession HW057200), 281 × 3006 cotton (patents EP2333082 and EP2862934) and MON89034 maize (NCBI accession FV532179).
Michael, T. P. & Jackson, S. The first 50 plant genomes. Plant Genome 6 (2013).
Kovalic, D. et al . The use of next generation sequencing and junction sequence analysis bioinformatics to achieve molecular characterization of crops improved through modern biotechnology. Plant Genome 5 , 149–163 (2012).
CAS Google Scholar
Wahler, D. et al . Next-generation sequencing as a tool for detailed molecular characterisation of genomic insertions and flanking regions in genetically modified plants: a pilot study using a rice event unauthorised in the EU. Food Anal. Meth. 6 , 1718–1727 (2013).
Article Google Scholar
Yang, L. et al . Characterization of GM events by insert knowledge adapted re-sequencing approaches. Sci . Rep . 3 (2013).
Liang, C. et al . Detecting authorized and unauthorized genetically modified organisms containing vip3A by real-time PCR and next-generation sequencing. Anal. Bioanal. Chem. 406 , 2603–2611 (2014).
Article CAS Google Scholar
Holst-Jensen, A. et al . Application of whole genome shotgun sequencing for detection and characterization of genetically modified organisms and derived products. Anal. Bioanal. Chem. 408 , 4595–4614 (2016).
Willems, S. et al . Statistical framework for detection of genetically modified organisms based on Next Generation Sequencing. Food Chem. 192 , 788–798 (2016).
Schmutz, J. et al . Genome sequence of the palaeopolyploid soybean. Nature 463 , 178–183 (2010).
Article CAS ADS Google Scholar
Shi, X. & Ling, H. Q. Current advances in genome sequencing of common wheat and its ancestral species. Crop J. 6 , 15–21 (2018).
Tengs, T. et al . Microarray-based method for detection of unknown genetic modifications. BMC biotechnol. 7 , 91 (2007).
Tengs, T. et al . Characterization of unknown genetic modifications using high throughput sequencing and computational subtraction. BMC biotechnol. 9 , 87 (2009).
Tengs, T. et al . Non-prejudiced detection and characterization of genetic modifications. Food Anal. Methods 3 , 120–128 (2010).
Arulandhu, A. J. et al . NGS-based amplicon sequencing approach; towards a new era in GMO screening and detection. Food Control 93 , 201–210 (2018).
Fraiture, M. A. et al . Validation of a sensitive DNA walking strategy to characterise unauthorised GMOs using model food matrices mimicking common rice products. Food Chem. 173 , 1259–1265 (2015).
Košir, A. B. et al . ALF: a strategy for identification of unauthorized GMOs in complex mixtures by a GW-NGS method and dedicated bioinformatics analysis. Sci. Rep. 8 , 17645 (2018).
Article ADS Google Scholar
Fraiture, M. A. et al . Development and validation of an integrated DNA walking strategy to detect GMO expressing cry genes. BMC biotechnol. 18 , 40 (2018).
Arulandhu, A. J. et al . DNA enrichment approaches to identify unauthorised genetically modified organisms (GMOs). Anal. Bioanal. Chem. 408 , 4575–4593 (2016).
Block, A. et al . The GMOseek matrix: a decision support tool for optimizing the detection of genetically modified plants. BMC Bioinformatics 14 , 256 (2013).
Debode, F. Développement de méthodologies pour la détection des plantes génétiquement modifiées. Phd Thesis, AGRO, UCL, 367/2017, 391 p., http://hdl.handle.net/2078.1/186329 (2017).
Angenon, G. et al . Antibiotic resistance markers for plant transformation. In Plant molecular biology manual, Springer, Dordrecht, 125–137 (1994).
Sulonen, A. M. et al . Comparison of solution-based exome capture methods for next generation sequencing. Genome Biol. 12 , 94 (2011).
Teer, J. K. Systematic comparison of three genomic enrichment methods for massively parallel DNA sequencing. Genome Res. 20 , 1420–1431 (2010).
Bodi, K. et al . Comparison of commercially available target enrichment methods for next-generation sequencing. J. Biomol. Tech. 24 , 73 (2013).
Meienberg, J. et al . New insights into the performance of human whole-exome capture platforms. Nucleic Acids Res. 43 , 1–14 (2015).
Chilamakuri, C. S. R. et al . Performance comparison of four exome capture systems for deep sequencing. BMC genomics 15 , 449 (2014).
García-García, G. et al . Assessment of the latest NGS enrichment capture methods in clinical context. Sci. Rep. 6 , 20948 (2016).
IUPAC Compendium of Chemical Terminology, 2nd ed. (Compiled by McNaught, A. D. & Wilkinson A.). Blackwell Scientific Publications, Oxford., 464 pages. ISBN 0-9678550-9-8 (1997).
Windels, P. et al . Characterisation of the Roundup Ready soybean insert. Eur. Food Res. Technol. 213 , 107–112 (2001).
Fraiture, M. A. et al . Nanopore sequencing technology: a new route for the fast detection of unauthorized GMO. Sci. Rep. 8 , 7903 (2018).
Debode, F. et al . Development of 10 new screening PCR assays for GMO detection targeting promoters (pFMV, pNOS, pSSuAra, pTA29, pUbi, pRice actin) and terminators (t35S, tE9, tOCS, tg7). Eur. Food Res. Technol. 236 , 659–669 (2013).
Debode, F. et al . Development of PCR screening assays focused on gene-coding sequences for GMO detection. Biotechnol. Agron. Soc. Environ. 22 , 230–241 (2018).
Google Scholar
ISO 21571. Foodstuffs. Methods of analysis for the detection of genetically modified organisms and derived products. Nucleic acid extraction. International Organization for Standardization, Geneva (2005).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25 , 1754–1760 (2009).
Li, H. et al . The sequence alignment/map format and SAMtools. Bioinformatics 25 , 2078–2079 (2009).
Wickham, H. ggplot2: elegant graphics for data analysis. J. Stat. Softw. 35 , 65–88 (2010).
MATH Google Scholar
Li, H. Seqtk Toolkit for processing sequences in FASTA/Q formats, https://github.com/lh3/Seqtk (2012).
Zerbino, D. R. & Birney, E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18 , 821–829 (2008).
Chevreux, B. et al . Genome Sequence Assembly Using Trace Signals and Additional Sequence Information. Computer Science and Biology. In: Proceedings of the German Conference on Bioinformatics, 45–56 (1999).
Bankevich, A. et al . SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 , 455–477 (2012).
Article CAS MathSciNet Google Scholar
Lin, S. H. & Liao, Y. C. CISA: contig integrator for sequence assembly of bacterial genomes. PloS one 8 , e60843 (2013).
Morgulis, A. et al . Database indexing for production MegaBLAST searches. Bioinformatics 24 , 1757–1764 (2008).
Camacho, C. et al . BLAST+: architecture and applications. BMC bioinformatics 10 , 421 (2009).
Download references
Acknowledgements
The first strategy of this research using NimbleGen technology was designed within a Belgian research project (Convention RF 11/6242 UGMMONITOR) financed by the Belgian Federal Public Service for Public Health, Food Chain Safety and Environment. The second strategy, using SureSelect technology, was financed by CRA-W in the framework of the NGS project (Moerman funds). We thank Cécile Ancion, Denis Roulez, Gaëlle Antoine and Eric Janssen from the GMO team of CRA-W for their help in the preparation of DNA. We thank the GIGA Genomics Platform for technical assistance with NGS data generation and analysis.

Author information
These authors contributed equally: Frédéric Debode and Julie Hulin.
Authors and Affiliations
Walloon Agricultural Research Center (CRA-W), Unit Traceability and Authentication, chaussée de Namur 24, 5030, Gembloux, Belgium
Frédéric Debode, Julie Hulin & Gilbert Berben
University of Liège, GIGA - Genomics Platform, B34, 4000, Liège (Sart Tilman), Belgium
Benoît Charloteaux, Wouter Coppieters & Latifa Karim
University of Liège, InBioS - PhytoSystems, Functional Genomics and Plant Molecular Imaging, Chemin de la Vallée, 4, B22, 4000, Liège (Sart Tilman), Belgium
Marc Hanikenne
You can also search for this author in PubMed Google Scholar
Contributions
F.D. designed the experiments and strategies; J.H. completed the bioinformatic pipeline for the analysis of the results with advice from B.C., W.C. and M.H.; B.C., L.K. and W.C. supervised the sequencing; G.B. supervised the work and funding; F.D. wrote the manuscript with comments from all the authors.
Corresponding author
Correspondence to Frédéric Debode .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Bioinformatic commands, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Debode, F., Hulin, J., Charloteaux, B. et al. Detection and identification of transgenic events by next generation sequencing combined with enrichment technologies. Sci Rep 9 , 15595 (2019). https://doi.org/10.1038/s41598-019-51668-x
Download citation
Received : 20 August 2018
Accepted : 20 September 2019
Published : 30 October 2019
DOI : https://doi.org/10.1038/s41598-019-51668-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Next-generation sequencing technology: a boon to agriculture.
- Balakrishnan Marudamuthu
- Tamanna Sharma
- Ch. Srinivasa Rao
Genetic Resources and Crop Evolution (2023)
Strategies to produce T-DNA free CRISPRed fruit trees via Agrobacterium tumefaciens stable gene transfer
- Lorenza Dalla Costa
- Stefano Piazza
- Mickael Malnoy
Scientific Reports (2020)
Targeted MinION sequencing of transgenes
- Anne-Laure Boutigny
- Florent Fioriti
- Mathieu Rolland
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

Message placeholder

1 Introduction
2 controversial issues surrounding gm crops, 3 cases concerning the safety of gm foods, 4 food safety guarantee system, 5 discussion and conclusion.
- List of tables
Research Article
The study of the impact of genetically modified soybean imports on China's food safety management
YinTong Yu *
College of Science & Technology, Ningbo University, Ningbo, Zhejiang 315300, China
* Corresponding author: [email protected]
Received: 4 February 2021 Accepted: 9 June 2021
With the widespread application of genetically modified technology, the proportion of genetically modified crops in the food sector has gradually increased. Of all of China's imported crops, genetically modified soybeans account for more than 75%. However, the safety issue associated with daily consumption, the contamination issue related to planting, as well as the attendant scientific and ethical issues have posed new challenges to the regulatory system of food safety of China. By examining the judicial and administrative management cases concerned, this author finds that the power to exercise effective safety control in regard to Genetically Modified Organism (GMO) rests with the low tier of government under the current system. In addition, the managerial measures are not well defined and targeted. The rules and regulations of China apparently fall short of the standards required of by international treaties. As a result, it is imperative for the higher tier of government to be empowered to handle the management of GMO and fine-tune the management and make some useful improvements. It is also necessary for Chinese authorities to devise a targeted system and make Chinese rules and regulations move closer to international treaties.
Key words: Genetically modified (GM) soybean imports / the food safety act / the foreign trade act / regulations on the safety control of GMO in agriculture
© Y.T. Yu, Published by EDP Sciences, 2021

Genetically Modified (GM) technology refers to biotechnology technology that transfer organism's to another organism. Since the beginning of trials of genetically modified planting technology in the United States in the 1970s, genetically modified agriculture has been developing for nearly 50 years. In the mid-1990s, the GM planting started the process of commercialization. After that, GM planting has been seeing a rapid development. A report titled Global Status of Commercialized Biotech/GM Crops: 2018 released by The International Service for the Acquisition of Agri-biotech Applications (ISAAA) 1 pointed out that by the end of 2018, the world had planted 190 million hectares of biotech crops, 113 times as many as the hectares of 1996. A total of 26 countries and regions planted biotech crops and another 44 countries and regions imported biotech crops [ 1 ]. As far as China is concerned, genetically modified crops have been increasingly becoming an important part of its people's food intake. Taking soybean oil as an example, we find that in 2019, China consumed a total of 16.67 million tons of soybean oil and much of which relied on GM soybeans for raw material, according to the departments handling commercial affairs [ 2 ]. To China, the safety of GM crops is not only a matter of agricultural safety, but also a matter of food safety. This paper is aims to find out the problems of GM soybean imports on China's food safety management, and try to give suggestion for improvement.
As a matter of fact, the rapid development of GM planting has never been free of controversies, particularly in the fields of safety and supervision. The first doubt concerning the safety issue of GM crops was raised by Losey et al., professor of Cornell University. In his research paper titled “Transgenic Pollen Harms Monarch Larvae” published by Nature in 1999, he pointed out that larvae of the monarch butterfly, Danaus plexippus , reared on milkweed leaves dusted with pollen from Bacillus thuringiensis ( Bt ) corn, grew more slowly and suffered a mortality as high as 44% than larvae reared on leaves dusted with untransformed corn pollen or on leaves without pollen [ 3 ]. The study on the potential threats of GM crops in the field of biology also captured the attention of scholars in other fields, particularly the scholars in the field of law. On Jan 29, 2000, the contracting countries of The Convention on Biodiversity passed The Cartagena Protocol on Biodiversity , establishing the “precautionary principle” based on Item 15 of The Rio Declaration and aiming to help member countries to achieve the safe handling, transport and use of living modified organisms (LMOs) resulting from modern biotech that may have adverse effects on biodiversity and human health [ 4 ]. Thereafter, the issue of regulation of GM crops based on this principle was starting to get noticed. Schnier, professor of Law School of Georgetown University elaborated on the advantages and disadvantages of GM crops in Genetically Modified Organisms and the Cartagena Protocol . He also conducted insightful analysis of the precautionary system established by The Cartagena Protocol on Biodiversity . According to Schnier, although The Cartagena Protocol on Biodiversity may not be applied to all bio safety events, it was effective in information sharing and import regulation under the precautionary system of the protocol as well. He believed that this protocol may indeed play an active role in tackling the controversies related to GM crops should the protocol can be strictly enforced [ 5 ]. Since then, precautionary principle has formed the main theoretical basis for regulating research into GM crops. Later, the application of this principle in judicial practice concerning GM crops was taken into research category. Lamping and Matthias conducted in-depth analysis of the verdicts concerning GM crops given by the European Court of Justice in Shackles for Bees? The ECJ's Judgment on GMO-Contaminated Honey [ 6 ].
GM crops have constantly been plagued with various controversies since the advent of this technology. The controversies surrounding food safety center on the following aspects.
2.1 The safety issue of food intake
Although there is no direct evidence to prove that consuming GM crops that have been approved for commercial use can cause harm to human body, GM foods may indeed have potential safety risks. Apart from American scholars, Jonathan Latham from Italy published his study in 2015, claiming that compared with goats fed with non-genetically-engineered soybeans, the pregnant goats fed with genetically engineered soybeans had offspring growing more slowly and consequently were smaller in size [ 7 ]. This finding was further supported by studies of other Italian scholars. For example, the experiment study of Tudisco and some others in the University of Naples found that the weight of internal organs of the offspring of goats fed with oat hay containing GM elements slaughtered at 60 ± 7 days of age was evidently lighter than those of the offspring of goats fed with oat hay without any GM elements [ 8 ]. Table 1 presents the detailed data.
Although the data have not received widespread attention from academia worldwide and the validity of method used for this study is yet to be tested, the findings of this study do show that consumption of GM soybeans by humans might have some risks.
The situation of pregnant goats fed with GM soybeans and fed with non-GM soybeans.
2.2 The safety issue of planting

This formula provides a quantitative description of the diversity ( D sh ) and uniformity ( J sh ) of the bacterial community in the rhizosphere soil based on the ratio of the peak area of the band to the total area of all peaks. By cluster analyzing the experimental data, the authors found that the diversity and uniformity of the bacterial community in the rhizosphere soil of the glyphosate-resistant genetically modified soybean (RRS) is significantly lower than that of the parental non-transgenic soybean (RRSS) and Dongnong 42 (D-42), Dongnong 46 (D-46) and Glycine soja (YS). The data are presented in Table 2 .
It should be noted that although research shows that glyphosate-resistant GM soybean (RRS) may be conducive to the growth of fungi in rhizosphere soil [ 10 ]. However, the impact of GM crops on the original planting environment is also a factor needed to be considered should GM foods be imported.
The situation of soil with GM corps and Non-GM corps with GM soybeans and fed with non-GM soybeans.
2.3 The genetic contamination issue
The so-called genetic contamination refers to the uncontrolled transfer of genes of GM crops and as a result the genes of GM crops spread to other non-GM crops. The earliest report of such case happened in the early 21st century when a farm producing non-GM crops in Texas of America detected the genes of GM corns grown in nearby fields. It was later confirmed that it was caused by the cross-pollination of bees between the two areas [ 11 ]. Furthermore, some research institutions have found that the insect-resistant rice straw containing the Bacillus Thuringiensis gene (also referred to as BT gene) can maintain the exogenous protein, which is the BT gene, for a long time after straw incorporation. Through the observation of the specific radioactivity of the BT gene in the insect-resistant rice accounted for the amount of initial introduction, it can be found that the BT gene in the insect-resistant rice can at least be kept in several common types of soils after the straw is returned to the field for more than one month. The Table 3 presents the detailed data [ 12 ].
According to the authoritative media, there is no evidence shows that genetic contamination will affect the human body directly [ 13 ]. However, it remains to be uncertain whether or not the mechanism of genetic contamination is due to cross-pollination or some other reproduction activities.
Specific radioactivity of Bt gene in soil accounted for amount of initial introduction (%).
2.4 Ethical issue
In the process of configuring GM crops, scientists can not only embed various types of plant genes in the crops, they can also embed animal or even human genes in the crops. While these operations could provide scientists with many new ways of thinking, they provoke bitter controversies, particularly in the field of ethics. According to media reports, a Canadian bio-pharmaceutical company added human genes to plants through GM technology and produced a safflower that could generate insulin [ 14 ]. This type of study had the potential to blur the boundaries between humans and non-humans. According to scientists, if it is acceptable to produce medicines by altering plant traits through human genes, then there will be no ethical obstacles to clone humans. If we make human genes as the sole criterion to distinguish human and non-human, then consuming crops containing human genes is no different than cannibalism. Although ethical issues do not belong to the category of food safety in the general sense, ethical issues are still the important factors to be considered when the safety issue is under evaluation given that food safety is irretrievably linked to nationality, religion as well as social traditions.
As a matter of fact, the problems associated with the regulatory system for the safety of GM soybeans does not necessarily exist in artificial environments or in other countries. In recent years, cases related to the safety of GM foods have indeed happened now and then.
In fact, the problem of the regulatory system for the safety of genetically modified soybean food does not only exist in the experimental environment or in other countries. In recent years, domestic cases related to the safety of genetically modified foods have also occurred from time to time.
3.1 The case of Yuqan v. Lotte Mart of Jiangsu, China National Foodstuffs Marketing Co, Ltd, China Oil & Foodstuff Co, Ltd Donghai Branch
In May 2015, Wang Quan, a lawyer of Huai'an of Jiangsu Province filed a lawsuit against Lott Mart of Jiangsu, China National Foodstuffs Marketing Co, Ltd, and China Oil & Foodstuff Co, Ltd Donghai Branch for producing and selling GM oil with fonts of label much smaller than the requirements set by national standards, thus violating Regulations on the Safety Management of Agricultural GMOs [ 15 ] and The Measures for the Administration of Labeling of GMOs [ 16 ] and demanding compensation for economic losses. Wang Quan further requested the court to rule that the label of the GM foods involved in this case should be redesigned before they could be marketed. However, the court of second instance rejected Wang Quan's plea, arguing that the labeling of GM foods in this case met the standard of the industry [ 17 ].
3.2 Illegal planting of GM crops in Hainan Province
In March 2014, a number of companies and research institutions were exposed online to illegally conduct genetically modified crop experiments in Hainan. The Department of Agriculture of Hainan Province subsequently claimed that at the end of 2013, enforcement of law on illegal experiments on genetically modified crops was carried out and illegal crops were destroyed, and no contamination arising from the GMOs was detected. However, the Department of Agriculture of Hainan did not publish the names of the organizations engaging in GM crop experiments, nor did it provide any detail of the law enforcement. In April 2014, Beijing Evening News and some media provided news reports on this case, but no official response was received [ 18 ].
3.3 The case of golden rice in a primary school at Hengnan County in Hunan Province
Golden rice was a genetically modified rice developed by Syngenta in Switzerland. Technicians used transgenic technology to transfer carotene-converting enzyme into rice endosperm to increase the vitamin A content of rice. As carotene could make the rice golden yellow, the rice was thus called golden rice. On August 1, 2012, the American Journal of Clinical Medicine published a study titled “β-carotene in golden rice is as good as β-carotene in oil at providing vitamin A to children”, claiming that the experiment study was conducted in an elementary school of Jiangkou Town at Hengnan County of Hunan Province in 2008. Dozens of elementary school students aged between 6 and 8 were required to eat this kind of rice. In doing so, researchers could well observe the effects of supplementation of vitamin A [ 19 ]. The publication of this research report received widespread attention. Having been investigated by the Center for Disease Control (CDC) of China, Zhejiang Academy of Medical Sciences as well as the CDC of Hunan province, this experiment study was found to have violated the relevant rules. However, no crime was involved. Members of the research team were disciplined for wrongdoing [ 20 ]. In September 2013, Tufts University made its apologies for the mishap.
3.4 China's refusal to accept American GM corn
In May 2014, the General Administration of Quality Supervision, Inspection and Quarantine of China published data, claiming that more than 1 million tons of American corn had been returned due to the detection of MIR162, a type of genetically modified ingredient not approved by the Ministry of Agriculture [ 21 ]. Although this act complied with the relevant rules and regulations of China, this act was interpreted as a political consideration. Terry Reilly, a senior commodity analyst at Futures International, argued that “if China is still importing (American corn), we have to feel that they are more like playing a political game” [ 22 ].
Having reviewed relevant cases of GM food safety guarantee system, we argue that the following issues deserve further discussion.
4.1 The development of the safety system of GM soybeans
At present, the rules and regulations concerning the safety and risk of GM soybeans and foods exist in The Food Safety Act [ 23 ], The Import and Export Trade Act [ 24 ] as well as Regulations on the Management of Genetically Modified Organisms and some others. However, these rules and regulations failed to achieve a perfect coordination and cooperation between different law enforcement agencies. Specifically, coordination should be improved between the office of food safety and risk monitoring, the office of quality supervision and risk detection as well as the office of public health and the administrative offices of agriculture, etc. It should be noted that The Measures for the Administration of the Inspection and Quarantine of the Genetically Modified Products Entering [ 25 ] and Exiting the Territory and The Labeling of Agricultural Genetically Modified Organisms of the No. 869 Announcement of the Ministry of Agriculture-1-2007 [ 26 ] are normative guidelines at best.
4.2 The effectiveness of risk management of GM soybeans and foods
In the field of safety management, China imports GM soybeans for forage and cooking oil. As a result, China requires the export countries to submit the data and methods used for GM products. However, the production tests of plant seeds may well be dispensed with according to Regulations on the Safety and Risks of Management of Genetically Modified Organisms . Currently, the bulk of imported GM soybeans are from the US and Brazil. It can be argued that making decisions by relying solely on the data submitted by export countries without initiating any well-designed studies by import country might be riddled with political and technological risks.
In terms of product labeling, although The Food Safety Act of China explicitly includes the requirement of labeling GM foods, it is not effectively implemented. Taking the most widely used blended cooking oil in China's market as an example, we find that many brands of the blended cooking oil relied on GM soybeans as raw materials. According to a study conducted by China Business Journal in 2018, many brands of blended oil used GM soybeans as raw materials and the fonts of label were small. In some cases, some brands claimed to be non-GM oil but actually used GM soybeans as raw materials. In June 2018, Fujian Wanglongshun Oil & Foodstuff Co., Ltd. was fined RMB 1 million by the Market Supervision Office of Tingping Township at Minhou County, Fuzhou of Fujian Province for labeling genetically modified soybean oil as non-transgenic products. Consequently, 3,086 bottles of blended oil were confiscated [ 27 ].
4.3 The system of risk detection and evaluation of GM soybeans and foods
Although The Food Safety Act and The National Food Safety and Risk Monitoring and Management System [Effective] [ 28 ] and some others have made the detection of risks related to GM soybeans the top priority — the public spotlight, these rules and regulations fail to single the GM foods out for special treatment. Quite different than conventional food risks, the risks arising from GM foods are widely believed to be long-term and latent. Consequently, it is very hard for the conventional risk management system to produce any monitoring effect.
4.4 The interconnectedness between the risk management of GM soybeans and foods and international treaties
The Agreement on the Implementation of Sanitary and Phytosanitary Measures within the framework of WTO aimed to strike a balance between protecting the human, animal and plant health and promoting the healthy development of international trade in agricultural products. The purpose of this agreement was to protect the health of the people of each member state and in the meantime, prevent its member states from creating new trade barriers. To comply with the requirements of the agreement, the European Union formed the European Food Safety Authority (EFSA), carrying out independent food risks evaluation for member countries of the EU. The US, however, delegated its trade representatives to communicate regularly with WTO and its member states over the GM-related issues. But in China, no such a special agency exists. The only mechanism for dealing with GMO-related issues is the inter-ministerial joint conference system created by the Ministry of Agriculture and Rural Affairs. Since the system of joint conference did not create a government entity and the primary function of which was to coordinate policy on food safety between ministries of this country, the effectiveness of the system paled in comparison with that of European countries and America. Certainly, it could not facilitate effective coordination between the safety management of GM soybeans and foods of China and international treaties. Without a doubt, it was not conducive to the prevention of international trade disputes.
In view of the problems in current regulatory system of GM soybean imports and the root causes for these problems, the governments may improve the following aspects.
5.1 Greater legal power should be vested in higher tier of government agencies and coordination between different tiers of agencies should be strengthened
The legal system concerned should be streamlined and the power of law enforcement should be vested in higher tier of government agencies. By making The Biosafety Act as a benchmark, the legislative body should further streamline The Regulations on the Safety Management of Genetically Modified Organisms and make parts of them become the law. In the meantime, The Measures for the Administration of the Inspection and Quarantine of the Genetically Modified Products Entering and Exiting the Territory and The National Food Safety and Risk Monitoring and Management System [Effective] should be re-issued as rules and stipulations of government agencies. We should further establish the national mandatory standards based on The Labeling of Agricultural Genetically Modified Organisms of the No. 869 Announcement of the Ministry of Agriculture-1-2007. Meanwhile, we should strengthen the coordination between different agencies and systems and make the inspection and quarantine of GM product imports, product labeling and risk detection and assessment an organic unity.
5.2 The supervision should be intensified and the standard of supervision should continue to be refined
First of all, supervision of imported GM soybeans for forage and cooking oil should be strengthened. Even if production tests of plant seeds may well be dispensed with, the safety certificates granted by export countries as supportive evidence should be provided as well. Secondly, the standard of labeling GM foods based on GM crops should further be refined and the supervision of the implementation should be strengthened. The crackdown on non-labeling and false labeling should be intensified. It is expected that the GM products consumers' “right-to-know” should be protected.
5.3 Developing a system aimed at detecting and evaluating risks related to GM products
On the basis of existing system of food risk detection and evaluation, a special mechanism for detecting and evaluating the risks related to GM products should be developed. Under such a mechanism, special attention should be given to long-term and latent risk detection and evaluation. Meanwhile, it is advisable to invite the third party or social organizations to do the detection and evaluation in order to minimize the use of administrative resources. In this endeavor, the public are expected to achieve a deeper understanding of GM technology and GM products. The public are not to be alarmed when they are exposed to the information related to GM technology.
5.4 International cooperation should be strengthened and trade conflicts should be avoided under the principle of improving food safety
As a matter of fact, the supervision of the safety of imported GM soybeans is not purely an internal affair, it also involves the trade issue between nations. As a result, how to exercise effective food safety control within the existing framework of international trade is still an important issue that needs to be seriously considered. However, the agenda set by current inter-ministerial joint conference system for the management of agricultural GM organisms does not incorporate the international trade issue concerning GM foods. Given the circumstances, it is imperative that the status of the Ministry of Commerce be elevated in the mechanism of the joint conference system. In doing so, it may facilitate the solution of trade disputes arising from food safety control measures.
- Xinhua News Agency, The planting area of GM crops continues to increase, Retrieved from http://www.81.cn/gjzx/2019-09/02/content-9608468.htm (published on Sep 1, 2019; accessed on Aug 8, 2020.) (in Chinese) [Google Scholar]
- China news Agency, Ministry of agriculture and rural areas: China's soybean import is expected to exceed 100 million tons in 2020, Retrieved from https://www.chinanews.com/cj/2020/12-24/9370281.shtml (published on Dec 24, 2020; accessed on Apr 20, 2021.) (in Chinese) [Google Scholar]
- J.E. Losey, L.S. Rayor, M.E. Carter, Transgenic pollen harms monarch larvae, Nature 399 , 214 (1999) [Google Scholar]
- Cartagena Protocol on Biosafety 2000. Retrieved from https://bch.cbd.int/protocol/ (enters into force on Sep 11, 2003) [Google Scholar]
- D.J. Schnier, Genetically Modified Organisms and the Cartagena Protocol. Fordham Environ, Law Review 12 , 377–415 (2000) [Google Scholar]
- M. Lamping, Shackles for Bees? The ECJ's Judgment on GMO-Contaminated Honey, Eur. J. Risk Regul. 3 , 123–129 (2012) [Google Scholar]
- L. Jonathan, GE Soybeans Give Altered Milk and Stunted Offspring, Researchers Find. Independent Science News. Retrieved from https://www.independentsciencenews.org/news/ge-soybeans-give-altered-milk-and-stunted-offspring-researchers-find/ (2015) [Google Scholar]
- R. Tudisco, M. Mastellone, M. I. Cutrignelli, P. Lombardi, F. Bovera, N. Mirabella, G. Piccolo, S. Cababro, L. Avallone, F. Infascelli, Fate of transgenic DNA and evaluation of metabolic effects in goats fed genetically modified soybean and in their offsprings, Animal 4 , 1662–1671 (2010) [PubMed] [Google Scholar]
- G.H. Xu, H.Y. Wang, J. Liu, Effects of RRS on the amount and diversity of bacteria in rhizospheric soil, J. Biol. 29 , 4535–4541 (2009) [Google Scholar]
- Z.H. Liu, G.H. Xu, H.Y. Wang, J. Liu, Effects of Roundup Ready Soybean (RRS) on Microorganisms and Nitrogen Transformation in the Rhizospheric Soil, J. Agro-Environ. Sci. 29 , 1341–1345 (2010) [Google Scholar]
- F.S. Cui, A panorama of genetic contamination, Environ. Guide 45–46 (2001) [Google Scholar]
- Y.F. Zhang, Study on environmental persistence of exogenous genes of BT rice and expressed proteins. Zhejiang University. (Unpublished Doctoral Dissertation) [Google Scholar]
- Science & Technology Daily, People Republic of China (PRC), The genes commonly seen in GM crops are detected in human bodies? Rumor!, Retrieved from http://news.southcn.com/shouyeyaowen/content/2016-04/13/content_ 145893405_2.htm (published on Sep 1, 2019; accessed on Aug 29, 2020) (in Chinese) [Google Scholar]
- Science & Technology Daily, People Republic of China (PRC), Striving to cultivate the GM crops that can generate insulin, Science & Technology Daily, Retrieved from http://news.southcn.com/shouyeyaowen/content/2016-04/13/content_145893405_2.htm (published on Apr 4, 2016; accessed on Aug 29, 2020) (in Chinese) [Google Scholar]
- China's State Council, Regulations on Administration of Agricultural Genetically Modified Organisms Safety, Available online: http://www.gov.cn/gongbao/content/2019/content_5468924.htm (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
- Ministry of agriculture, People Republic of China (PRC), Administrative Measures for Labeling Agricultural GMO Marks, Available online: http://www.moa.gov.cn/ztzl/zjyqwgz/zcfg/201007/t20100717_1601302.htm (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
- Civil Judgment of the Intermediate Court of Huai' an, Jiangsu Province (2015) No. 02573 [Google Scholar]
- W.Y. Su, Who planted illegal GM crops in Hainan?. Beijing Evening News, April 2, 2014 [Google Scholar]
- G.W. Tang, Y.M. Hu, S.A. Yin, Y. Wang, G.E. Dallal, M.A. Grusak, R.M. Russell, β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children, Am. J. Clin. Nutr. 3 , 658–664 (2012) [Google Scholar]
- The Center for Disease Control (CDC) of China, Zhejiang Academy of Medical Sciences, the CDC of Hunan province. Announcement of the investigation result of the study on “β-carotene in golden rice is as good as β-carotene in oil at providing vitamin A to children”, Retrieved from http://www.chinacdc.cn/zxdt/201212/t20121206_72794.html (published on Dec 6, 2012; accessed on Aug 20, 2020.) (in Chinese) [Google Scholar]
- Xinhua News Agency, China's refusal to import America GM corn captured world's attention, Retrieved from http://news.southcn.com/shouyeyaowen/content/2016-04/13/content_145893405_2.htm (published on Apr 13, 2016; accessed on Aug 20, 2020.) (in Chinese) [Google Scholar]
- Hexun Net, China refused to import American GM corn, trade dispute continued, Retrieved from http://news.bioon.com/article/6639806.html (published on Dec 16, 2013; accessed on Aug 25, 2020.) (in Chinese) [Google Scholar]
- Chinese People's Congress, Food Safety Law of the People's Republic of China, Available online: http://www.npc.gov.cn/npc/c30834/201901/c6d064de8295489288ec1383b33212ee.shtml (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
- Chinese People's Congress, Foreign Trade Law of the People's Republic of China, Available online: http://www.npc.gov.cn/wxzl/gongbao/2017-02/21/content_2007588.htm (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
- State General Administration of the People's Republic of China for Quality Supervision and Inspection and Quarantine, Measures for the Administration on the Inspection and Quarantine of the Genetically Modified Products Entering and Exiting the Territory, Available online: http://www.gov.cn/gongbao/content/2005/content_ 63203.htm (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
- Ministry of agriculture, People Republic of China (PRC), Announcement No. 869 of the Ministry of Agriculture and Rural Affairs, Available online: http://www.moa.gov.cn/nybgb/2007/dqq/201806/t20180614_6151996.htm (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
- The Beijing News, GM soybean oil were found to be posing as non-GM blended oil, Wanglongshun Oil & Foodstuff Co., Ltd of Fujian Province was fined more than 1 million yuan, 21 June 2018 [Google Scholar]
- National Medical Products Administration, People Republic of China (PRC), Notice on Issuing “Food Safety Risk Monitoring and Management Regulations (Trial)”, Available online: http://www.nhc.gov.cn/sps/s5853/201002/df6c5689c712424fadfbbe87045663c5.shtml (accessed on Apr 20, 2021) (in Chinese) [Google Scholar]
A small, nonprofit international organization aimed at promoting the application of biotechnology, the major providers of operation costs include the Department of Agriculture, the Grain Council of America, the German Industrial Corporation and Monsanto of the US, etc.
Cite this article as : YinTong Yu, The study of the impact of genetically modified soybean imports on China's food safety management, Int. J. Metrol. Qual. Eng. 12 , 18 (2021)
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.65(1); 2021 Mar

Prevalence of Genetically Modified Soybean in Animal Feedingstuffs in Poland
Zbigniew sieradzki.
1 Department of Hygiene of Animal Feedingstuffs National Veterinary Research Institute, 24-100, Puławy, Poland
Małgorzata Mazur
Beata król, krzysztof kwiatek, introduction.
Globally, genetically modified (GM) crops were grown on 191.7 million hectares in 2018, which were mostly sown with soybean, maize, cotton, oilseed rape, and rice. The most popular traits introduced through genetic modification include herbicide and pest insect resistance. The aim of this study was to identify and quantify genetically modified soybean used in animal feed in Poland.
Material and methods
This research was based on the real-time PCR technique. All methods for GM soybean events were adopted from the EURL GMFF database of methods and previously verified to meet the minimum criteria of acceptance. Over 15 years of research, 665 samples were examined in total.
The most common GM soybean event was MON40-3-2, tested for from the beginning of the investigation. Next, in decreasing order of frequency, were MON89788, MON87701, and A2704-12. In the majority of samples (606; 91%) GM soybeans were identified at a content level above the 0.9% GM content threshold for mandatory labelling. Only 59 soybean samples (9%) were identified as GM negative. GM negative results were mainly identified during the analyses in the last three years of the study, from 2017 to 2019.
Our data clearly indicate that the majority of soybean used in Poland for animal feeding was genetically modified.
The use of genetically modified (GM) plant seeds for food and feed production has been continuously increasing in the world. The latest data indicated that GM crops were grown on 191.7 million hectares around the world in 2018 ( 17 ). Most of these are commodity crops such as soybean, maize, cotton, oilseed rape, and rice, into which desirable traits are introduced through genetic modification including herbicide and pest insect resistance as the most popular. In 2018, more diverse crop seeds with various enhancements became available on the market. The produce from these seeds includes reduced acrylamide potatoes with non-bruising, non-browning, and late blight-resistant traits; insect-resistant and drought-tolerant sugarcane; non-browning apples; and high oleic acid canola and safflower. Genetically modified soybeans are currently the most important source of feed protein within the European Union and supply a significant proportion of it in other countries around the world. These soybeans are planted on an especially large scale in the USA, Brazil and Argentina, the three main GM crop producers.
In 2018, GM soybean occupied 50% of the global area under modified crops ( 17 ). GM soybeans have remained the main such crop since 1996, when the first commercialised genetically modified crop seeds came to notice. Throughout the 23 years since, soybeans have held the top position as regards area covered by GM crop production. The meat and bone meal (MBM) ban in the EU was the starting point of an increasing demand for soybean meal. The unique composition of amino acids and the content of primarily lysine, arginine and tryptophan, especially important in the feeding of poultry and pigs, recommend it. So far, soybean meal has no competition except the banned MBM as a protein source for these animal species in the EU. The other protein source produced in high volume in Europe, rapeseed meal ( 5 ), has critical limitations as a feedingstuff for poultry and pigs. Rapeseed oil used to have a poor reputation due to the presence of erucic acid, which has a bitter taste and was later found to cause health problems. Other characteristics recommending against the use of rapeseed meal as animal feed were the content of antinutritional and performance-detrimental glucosinolates and the poorer digestibility of rapeseed protein. Although low-erucic and low-glucosinolate rapeseed varieties are now the main types grown worldwide, it is not used in feed production to the extent it could be.
GMO-free production of food of animal origin forces feed manufacturers to look more closely at rapeseed meal as a means of achieving an appropriate content of protein-containing meals in their feed recipes. However, it is necessary to adhere to species rules for feeding animals with rapeseed. Contemporarily with GM soybean importation, many European countries have started to invest in the development of new non-GMO lines of soya which will have the ability to grow and yield highly in European climates. In countries like Austria and Poland, soybean acclimatisation and commercialisation is encouraged strongly by governments and is very palatable to public opinion. From the European point of view, the argument for the use of GM technology for feed and food production is questioned, and specifically from the continent’s citizens point of view, its use is unacceptable ( 2 , 19 ). The main reason for the lack of enthusiasm is fear concerning GM food safety and the yet-unknown consequences of its cultivation and/or consumption. Voices from within the bioscience professions and many pieces of evidence from scientific GM feed trials on animals have no power to tear down the wall of stereotypes in Europe.
Particular attention has been directed towards herbicide-tolerant crops in recent years, and specifically towards glyphosate, which has been blamed for cancer cases in humans. Glyphosate is widely used around the world, not only for GM plant production but as a good total herbicide for all kinds of plant production. That it is widely used also means that it is widely dispersed into the environment; the most recent data shows that almost all beer and wine contains glyphosate contamination in the USA, as do all popular brands of beer in Germany. Several genes afford resistance to herbicides. The EPSPS gene from the soil bacterium Agrobacterium tumefaciens L. determines the synthesis of a glyphosate-impervious protein (CP4EPSPS), and the pat gene from Streptomyces viridochromogenes imparts tolerance of herbicides containing glufosinate as the active ingredient. In accordance with the law in force in the European Union, a product containing more than 0.9% GMO must be appropriately labelled ( 7 ). Authorised EU agencies enforce Union labelling regulations by detecting contraventions. Allied work is the monitoring of the presence of GMOs in food or feed by the appropriate authorities. Molecular analytical techniques have been developed and brought into use for GMO detection such as protein-based and nucleic acid-based methods. In routine analysis of food and feed PCR, and particularly quantitative real-time PCR, has become the method of choice for the determination of the GMO content of samples. Event-specific methods are used in the detection and determination of GMO quantities that depend upon genetic material characteristic only of a specific GMO line. They target a unique site comprising a junction between the transgenic insert and the host genome ( 7 , 14 , 21 ).
The aim of this study was to detect, identify and quantify genetically modified soybean by DNA analyses in animal feed in Poland. Samples were collected under the National Control Plan for Feed. This surveillance research was based on the real-time PCR technique and was applied in GM feed analyses in the National Veterinary Research Institute (NVRI) in Puławy, Poland.
Material and Methods
Samples . Samples of compound feed and animal-feed-derived soybean meal and soybean were gathered from eastern and central Poland by Veterinary Inspectorate officers from January 2004 to July 2019. The material was taken for GM soybean content determination in execution of the National Control Plan for Feed. Certified reference materials (CRM) from the American Oil Chemists’ Society (MON89788, MON87705, MON87701 and A2704-12) and the European Commission’s Joint Research Centre (MON40-3-2) were used as calibrators to determine GMO amount in %. From 2004 to 2017, only MON40-3-2 was analysed. In 2018, MON 89788 and in January 2019 MON 87701, MON 87705 and A2704-12 were introduced into the investigation.
DNA extraction . The extraction of DNA from samples and certified reference GM soybean materials was carried out by the CTAB method described in ISO 21571 ( 16 ). After extraction, the quality and quantity of DNA was measured in a UV spectrometer (Nicolet Evolution 300, Thermo Fisher Scientific, Madison, WI, USA). The purity of the extracted DNA was determined in two steps, by the ratios of the absorbance at 260/280 nm and at 260/230 nm, with compensation for the absorbance at 320 nm.
Real-time PCR . All methods for GM soybean event determination used in this study and enumerated above are listed in the database of the European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). The sequences of PCR primers and probes used for GM soybean determination are listed in Table 1 with the corresponding EURL GMFF database record. All primers and probes were synthesised by Genomed (Warsaw, Poland), with the HPLC purification step also being performed by that supplier. Detection and determination of GM soybeans were carried out on a 7500 real-time PCR system (Applied Biosystems, Middletown, CT, USA) in a 25 μL volume containing 1x TaqMan Universal Master Mix, 75 nM of each primer, 12.5 nM of TaqMan probe and 5 μL of DNA. The amplification profile comprised a first step at 50°C for 2 min to activate the Uracil N-glycosylase and then initial denaturation at 95°C for 10 min and 45 cycles at 95°C for 10 s and 60°C for 60 s.
Primers and probes used in real-time PCR
In order to assess the GM soybean content, standard curves made in 5 dilutions in two replicates were prepared using the CRM GM soybean DNA. Each dilution had a known number of copies of a reference gene for the soybean genome (lectin) and transgene sequence (a sequence containing part of the soybean genome and transfection cassette). The quantification of GM soybeans was achieved by the amplification of the lectin gene and transgene sequences, and the GM content was a relative measure of the amount of genetically modified material in the total soybean material.
In 2004–2019, as part of the official control plan for GM feed in Poland, 665 samples were examined for the presence and amount of genetically modified soybean. GM soybean DNA was found at a content level above the 0.9% threshold requiring labelling of feed as containing GMO in 606 samples, duly noted as positive, which was 91% of the tested total. Samples totalling 59 (9%) were identified as containing either none or ≤ 0.9% of the analysed GM soybean events, and were therefore recorded as negative. Analysis of the results showed that since 2017, the level of GM soybean-negative samples has been consistently and markedly higher than in the preceding period ( Fig. 1 ). Over the first 12 years of the investigation (2004– 2016), 4% samples (22 out of 553 analysed) were negative, but in 2017 that proportion rose to 29.4% (10 out of 34), in 2018 it was 32.5% (13 out of 40), and finally in 2019, 26% (10 out of 38) of samples with soybean ingredients were GM-negative.

Percentage of positive and negative samples in the total pool of samples
As was stated previously, the negative samples were those with GMO amounts ≤ 0.9%. Closer investigation of the amount of GMO in these samples in 2018 and 2019 showed that in 2018, out of 13 negative samples, 4 (31%) did not contain detectable MON40-3-2 or MON89788 events, 6 (46%) had GMO below the limit of quantification (LOQ), and 3 (23%) revealed GMO below 0.9%. In 2019, out of 10 negative samples, 4 (40%) did not give any positive result for the presence of MON40-3-2, MON89788, MON87701, MON87705 or A2704-12 events, 4 (40%) yielded modified soybean content below the LOQ, and the last 2 (20%) harboured GMO at a level lower than 0.9%.
Analysis of positive samples showed that in the majority of them, the GM soybean event MON40-3-2 was present ( Fig. 2 ). In 2004-2017, all positive samples contained this GM event, in 2018 its presence was at the 93% level in positive samples, and in 2019 MON40-3-2 was again identified in all GM samples. Results from 2018 and 2019 showed that the second most commonly used GM event was MON89788, which was present in 100% and 90% of positive samples, respectively. Since 2019, after a major expansion of the methods’ event references, we could see that the range of GM events present in animal feedingstuffs in Poland was broader. MON40-3-2 was present in 96% of GM-positive samples, MON89788 in 96%, MON87701 in 79%, and A2704-12 in 54%. The MON87705 soybean event was not detected. Many samples contained more than one GM event, and the most common combination was MON40-3-2/MON89788, followed by MON40-3-2/ MON89788/MON87701 and MON 40-3-2/MON89788/ MON87701/A2704-12. In contrast to these findings, we also identified two samples containing only the MON40-3-2 GM event.

Percentage of GM events in the total pool of GM soybean-positive samples in three stages of the study
Just as many other countries in the EU, Poland depends on GM soybean meal as a major source of feed protein and imports it mainly from South America and the United States. Official data from the European Commission stated that in 2014 the EU was 70% dependent on imports of protein-rich crops ( 5 , 8 ). Imports of soybean meal between 2000 and 2009 ranged from 1.5 to about 1.8 million tons, and currently Poland imports around 2.3–2.5 million tons of soybean meal per year, a figure that has remained constant over the past few years. For now, relevant EU arable production cannot meet the EU feed protein demand. Production of soybean, rape and sunflower seeds as well as pulses and other legume crops offsets the EU dependence on soybean and soymeal imports to a limited extent ( 8 ). A rough estimate derived from the same European Commission data was that around 85% of imported soybean was GM. This was confirmed by a wide interlaboratory study on 135 samples (116 of them containing soybean) providing an insight into the profile of the GM events found across the EU in 2014 ( 23 ). A total of 5 soybean GM events were identified, and among them MON40-3-2 (89/116, 77%) was first on the list, followed by MON89788 (46/116, 40%) and A2704-12 (27/116, 23%). More than 10 samples also contained MON87701 and a few samples were positive for the DP-356043 GM soybean event. Kleter et al .( 18 ) stated that at the time of writing in 2018, an estimated over 90% of feed materials in EU were labelled as containing GMOs or GMO-derived materials. The widespread use of GM soybean in Poland is confirmed by research results obtained over the last 15 years. In the majority of analysed samples (92%), genetically modified soybean was determined at above the 0.9% level. Since 2004, it has been evident that the MON40-3-2 soybean variety is a major GM crop used in Poland. This should not come as a surprise, taking into account that this variety was and still is the most-grown GM soybean event globally. The presence of MON40-3-2 is common in food and feed in many countries, according to reports of other authors ( 1 , 3 , 6 , 10 , 13 , 15 , 20 , 22 , 24 , 25 , 26 , 28 , 31 , 32 ). Its presence in feed on the Polish market may also be traced back to member states of the EU, other European countries, and third countries like Ukraine, which export soybean within and to the EU. Deliberate cultivation of GM soybean was identified in Romania after its accession to the EU in 2007, which was likelylinked to the pre-accession cultivation of Roundup Ready soybean (MON40-3-2) ( 22 , 31 ). This same variety was detected in 96 out of 111 soybean samples collected from six administrative regions in Ukraine. The authors concluded that GM crops were grown and sold there ( 10 ).
From the documentation provided with samples and from the results from the last three years of the study, it can be clearly seen that the soybean meal submitted for analysis is labelled as GMO-free and is actually free of it. In the majority of these samples, the presence of genetically modified soybean is still detectable, but at very low levels, near or below the limit of quantification of real-time PCR methods. Only 6 out of 645 meal samples were totally free of GM soybean, which is less than 1%. The presence of GM soybean at low levels (usually less than 0.1%) is probably a consequence of contamination of the sample with genetically modified soybean raw material. It bears emphasising that the increasing presence of non-GM soybean on the feed market stems from the necessity to adapt to the requirements of consumers, food producers and retailers. GMO-free claims on labelling nowadays seem to betoken higher quality. Moreover, a general issue with GMO-free labelling is that the label itself may signal to consumers that GMOs are unsafe ( 4 ). Due to this, in the last five years many producers of food of animal origin have started to maintain GMO-free systems of production, even though there have been no legal regulations in force to mandate such for the Polish market.
The situation in Poland is very similar to the way the GMO-free market developed in Germany ( 27 ). Data gathered from Germany in April 2017 presented 6,170 products labelled as GMO-free, and the Verband Lebensmittel ohne Gentechnik (VLOG), the German Industry Association for Food without Genetic Engineering, estimated revenue of 4.4 million euros was generated with GMO-free-labelled products in the same year ( 30 ). Venus et al . ( 29 ) reported that 76% of those German products were from livestock farming and each of the three main animal-origin product groups (dairy, poultry, and eggs) accounts for about a quarter of the share of the total products carrying a GMO-free mark. Other product categories (comprising the remainder of the total) are pasta and cereal, beverages, honey, and others. Although consumers demand clear GMO-containing and GMO-free labelling, this can lead to new misinterpretations connected with food quality. Even with perfect information, while some consumers gain by having an opportunity to choose GMO-free products, others may lose by paying increased prices through retailers’ product differentiation ( 29 ). In Poland, where the food market is closely connected to that of Germany, GMO-free labelling also started with eggs, poultry meat and a wide range of dairy products from many manufacturers. The difference is that in Poland GMO-free labelling was started and maintained by food producers without clear applicable legislation in place. Each company implemented it in their own way, taking into account GMO food and feed provisions and the strictures of EU regulations ( 9 ). This very substantial industry movement was finally given legislative treatment by the Ministry of Agriculture and Rural Development in the Act on the labelling of products produced without the use of genetically modified organisms as free from those organisms, which was entered law in June 2019 ( 12 ).
Article 15 of the Polish Act on animal feedstuffs of 2006 was to forbid the use of genetically modified feed, but it has not come into effect ( 11 ). In 2019 a new amendment was introduced, and it once more shifts the date of entry into force of the prohibition on the manufacture, placing on the market and use in animal nutrition of genetically modified feed and genetically modified organisms. According to its provisions, this ban will apply from 1 January, 2021. GM-free soybean may already come from Brazil, Ukraine or domestic Polish cultivation, which is gradually increasing from year to year. Brazil is a good example of a country in which GM and non-GM soybeans are grown ( 18 ), the labelling of GM food is mandatory, and the food industry has adjusted to the legislation with respect to consumer requirements ( 3 ). Although Brazilian GM soybean is exported to the EU or used for food and feed production on a major commodity scale, non-GMO food in Brazil is available and food is properly labelled for GMO presence ( 6 ).
Substitution of genetically modified feedingstuffs in animal nutrition is possible; however, it involves importation of non-GMO materials for higher prices or research into the acquisition of feed protein from domestic sources. Achieving total alleviation of consumer concern and completely liberating the Polish market from GMOs is and will be extremely difficult, because there are no substitutes for soybean flour so far. Conducting monitoring for GMOs is therefore an indispensable element of exercising restrictive control. The production, storage or transport of GM-free feed material must take place under appropriate conditions that prevent contact between it and genetically modified material. In the event of non-compliance with these rules, it is easy for GMO products to contaminate GMO-free ones and for them to be used outside of controlled production and supply processes. In response to these developments in the market’s regulatory environment, some retailers and processors have begun to impose GMO-free requirements on the primary stage of production.
Despite the widespread presence of GM soybean in animal feed on the Polish market, there is a lack of publicly accessible data that provide detailed information regarding the trade in and use of GM feed materials counter posed with GMO-free equivalents. A trend fuelled by this towards marking products as GMO-free is possible to observe, which could make the entire GMO labelling system uncongenial and distorted.
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement : This study was supported by the Polish Ministry of Science and Higher Education within the statutory activity of the National Veterinary Research Institute.
Animal Rights Statement : Not applicable.
Kellogg School of Management at Northwestern University
Economics Jun 4, 2018
How a genetically modified soybean helped modernize an economy, as brazil’s farms became more efficient, workers shifted to manufacturing..
Paula Bustos
Bruno Caprettini
Jacopo Ponticelli
Gabriel Garber
Michael Meier
As Brazil grew richer in the 2000s, its agricultural workers left their farms in droves and headed to work in the rapidly growing industrial sector.
But what exactly was happening? Did new economic opportunities lure workers off farms or did changes in farming lead to industrialization? Jacopo Ponticelli , an associate professor of finance at the Kellogg School, along with University of Zurich economist Bruno Caprettini and Paula Bustos of Spain’s Center for Monetary and Financial Studies, suspected the answer had something to do with soybeans.
In 2003, Brazil legalized the revolutionary Monsanto’s Roundup Ready soybean seed. The seed (called “Maradona soy” in South America after a famously agile soccer player) had been genetically engineered to be herbicide resistant.
Up until this point, farmers had not been able to control weeds by broadly applying herbicides without also killing their crops. Instead, at the start of each planting season, they tilled their fields—a laborious process—to remove weeds. An herbicide resistant seed meant farmers could plant without having to till each year, allowing them to produce the same amount of soy with less than half of the work. This, in turn, meant that farms needed many fewer workers to get the job done.
To Ponticelli, the story of the soy seed presented an opportunity to dissect the way that countries develop from agrarian economies into more industrialized ones.
“We wanted to test the theory that an increase in agricultural productivity can get this process started,” he says.
In a pair of papers, Ponticelli and coauthors trace how businesses across Brazil reaped the benefits of this revolutionary seed. In the first paper , the researchers find that the seed freed up farm laborers to find other jobs, allowing Brazil’s industrial sector to grow. In the second, the researchers find that the seed helped farmers put more money in the bank, which led to urban centers getting access to cheaper credit, allowing banks to finance more manufacturing and services firms.
The new research counters that traditional viewpoint by showing that agricultural productivity can push both new workers and new capital towards more innovative industries.
Ponticelli says the research not only sheds new light on how economies develop, but also challenges the widespread belief that funneling resources into farming stifles growth and innovation. Indeed, the story of Brazil suggests bumps in agricultural productivity can ripple through an entire economy, not only bolstering the manufacturing sector, but exporting fresh capital to the urban centers where new industries tend to grow.
“If we think the manufacturing sector plays a key role for economic growth in the long run—because most of the patents, the R&D, the innovation happens there—then new agricultural technologies are not bad news, necessarily,” he says.
Pushing or Pulling the Workforce?
Brazil’s shift from agricultural labor to industry is not unique; it’s a pattern that has played out in growing economies from England during the Industrial Revolution to modern-day China. Economists offer two competing explanations for why—what Ponticelli calls the “pull” and “push” theories.
In the “pull” theory, a growing economy increases incomes, meaning consumers can afford to buy more manufactured goods. Additional industrial labor is needed to meet this new demand, so the industrial sector “pulls” workers away from agriculture with the promise of higher wages.
In the “push” theory, however, change begins when a new technology makes agriculture workers more productive. Since less work is required to produce the same amount of food, farm laborers are “pushed” out of agriculture and need to find jobs in the industrial sector.
The case of Brazil presented an excellent opportunity to distinguish between which theory was at work. Brazil’s economy grew by more than 40 percent between 2000 and 2010, thanks largely to rapid growth in the manufacturing sector. If increases in agricultural productivity had led to industrialization, then soy-producing regions should have seen higher fractions of their labor force move into other sectors after the introduction of Roundup Ready seeds, as soy-farm workers were freed up to do other work. If instead industrialization “pulled” workers away from other industries, there should be no meaningful differences between migration patterns in soy-producing regions and elsewhere.
The researchers used data on weather and soil characteristics to determine how much additional soy output each region of Brazil stood to gain from the Roundup Ready seed. Then, using census data, they analyzed how each region’s workforce changed in the seven years after the seed was approved.
What they found seems to support the “push” theory.
“Areas that are more likely to adopt this technology experience a decrease in the share of people working in agriculture, and an increase in the share of people working in manufacturing,” Ponticelli says, “which suggests people are moving from one sector to the other.”
Following the Money
Roundup Ready soy not only slashed farmers’ overhead, it also increased their land values. The upshot: “A lot of these farmers got richer,” Ponticelli says.
As farmers deposited their newfound wealth into savings and checking accounts, banks suddenly had more cash on hand, allowing them to extend more loans to help businesses grow—“another way in which agricultural productivity can generate development,” Ponticelli explains.
But unlike labor, money can easily move long distances, and Ponticelli wondered where those new reserves of capital were going. To find out, he teamed up with Bustos and Gabriel Garber, an economist at the Central Bank of Brazil.
They obtained detailed data from the central bank on deposits at every bank branch in Brazil, transfers of money between bank branches, and the borrowing histories of all Brazilian firms. With this rich data, the researchers were able to carefully track the profits from soy-growing regions as they flowed to different businesses around the country.
Only a tiny portion of loans remained in rural communities, they found. For every additional Brazilian real in soy profits that farmers deposited, only 0.5 percent was loaned back to agricultural businesses. Meanwhile, 48 percent of each real went to companies in the service sector, and 40 percent went to manufacturing businesses, both of which are more likely to be located in urban areas.
The nuanced research comes at a pivotal time, as Roundup Ready soy and other genetically modified seeds continue to explode worldwide.
Ponticelli explains the logic behind this pattern: “You can think of a branch in a rural area that has more deposits because there are all these rich farmers, but there are not that many investment opportunities,” he says. Meanwhile, a branch in downtown Sao Paolo may be surrounded by tech companies and manufacturing plants but lacks the cash to finance them sufficiently. “So you can see how there could be a flow of capital from the rural areas to the urban areas.”
But this uninhibited flow of capital between places (what economists call “financial integration”) did not benefit everyone equally. Ponticelli points out that rural businesses would have liked cheap credit too.
“Financial integration can be great if you’re a destination,” he says. “It can be not so great if you’re a place that mostly funnels resources into the system but doesn’t receive that much back.”
Agriculture Yields Mixed Results
Economists have long believed that specializing in agriculture tends to prevent a country from reaching its full potential.
“We know that a lot of knowledge spillovers, a lot of innovation, doesn’t come from the agricultural sector,” Ponticelli says. “It comes from manufacturing. I mean, Apple is a manufacturing company.”
However, Ponticelli notes an important caveat: in a yet-unpublished follow-up paper with Bustos and Joan Monras of Spain’s Center for Monetary and Financial Studies and Juan Manuel Castro Vincenzi from Princeton, he takes a closer look at where displaced soy-farm workers end up, and finds that many end up in relatively low-paying, unskilled manufacturing jobs, not the cutting-edge industries where research and innovation generally occur.
Furthermore, Ponticelli warns that not every increase in agricultural productivity will lead to industrialization.
For example, in the 1980s, Brazilian maize farmers found ways to add a second planting season to the year. Yet Ponticelli, Caprettini, and Bustos find that because the method was very labor intensive, maize farmers were not pushed into industrial jobs. Maize-producing areas “actually experienced an increase in the share of people working in agriculture, and a decrease in the share of people working in manufacturing,” Ponticelli says.
To Ponticelli, the contrasting stories of maize and soy demonstrate an important point: “There’s not one single answer that, ‘More agricultural productivity is bad because it locks you into this one, less innovative sector,’” he says. “It really depends on the kind of new technology that you adopt.”
The nuanced research comes at a pivotal time, as Roundup Ready soy and other genetically modified seeds continue to explode worldwide. Despite controversy over the seeds’ environmental impact, the use of biotech crops has been approved across South America, China, and India.
“The next frontier is likely to be Sub-Saharan Africa,” he says. “Trying to understand, ‘What are the consequences of these new technologies on industrialization?’ is going to be really important looking forward.”
Associate Professor of Finance
About the Writer Jake J. Smith is a writer and radio producer in Chicago.
About the Research Bustos, Paula, Bruno Caprettini, and Jacopo Ponticelli. 2016. “Agricultural Productivity and Structural Transformation. Evidence from Brazil.” Working paper. Bustos, Paula, Gabriel Garber, and Jacopo Ponticelli. 2017. “Capital Accumulation and Structural Transformation.” Working paper.

IMAGES
VIDEO
COMMENTS
Abstract. Soybean is one of the important food, feed, and biofuel crops in the world. Soybean genome modification by genetic transformation has been carried out for trait improvement for more than 4 decades. However, compared to other major crops such as rice, soybean is still recalcitrant to genetic transformation, and transgenic soybean ...
The CV127 soybean has been genetically modified by researchers from Embrapa (The Brazilian Agricultural Research Corporation) to express an altered csr1-2 gene from Arabidopsis thaliana, supplied by the German company BASF (Rech et al., 2008).
Double mutation events, knockouts in two soybean galactinol synthase (GOLS) genes, GmGOLS1A and its homeolog GmGOLS1B, showed a reduction in the total RFO content of soybean seeds from 64.7 to 41.95 mg/g dry weight, a 35.2% decrease ( Le et al., 2020 ). This product improved the soybean nutrition quality.
Although genetically modified soybean was introduced commercially as early as 1996 and various genetic ... (Gamborg et al. 1968) covered with a filter paper, ... ZJ (2012) An overview of genetic transformation of soybean. In: Board J (ed) A comprehensive survey of international soybean research—genetics, physiology, agronomy and nitrogen ...
ABSTRACT. This paper estimates the global value of using genetically modified (GM) crop technology in agriculture at the farm level. It follows and updates earlier studies which examined impacts on yields, key variable costs of production, direct farm (gross) income, and impacts on the production base of the four main crops of soybeans, corn, cotton, and canola.
Seeds from 49 conventional soybean lines (Supplemental Table 1) were obtained from the USDA National Soybean Research Center at University of Illinois and Syngenta Biotechnology, Inc. Seed for the ...
Genetically modified soybean lines exhibit less transcriptomic variation compared to natural varieties. ... including antibiotic-resistant petunia and tobacco, were created independently by three research groups in 1983. ... Resources and Regional Planning, Chinese Academy of Agricultural Sciences) for suggestions and revisions of the paper.
Soybean is considered one of the important crops among legumes. Due to high nutritional contents in seed (proteins, sugars, oil, fatty acids, and amino acids), soybean is used globally for food, feed, and fuel. The primary consumption of soybean is vegetable oil and feed for chickens and livestock. Apart from this, soybean benefits soil fertility by fixing atmospheric nitrogen through root ...
In 2019, genetically modified (GM) soy occupied over 91.9 million hectares of agricultural land and was the world's third most internationally traded plant by weight 1,2. ... Research articles
The term "genetic modified organisms (GMO)" has become a controversial topic as its benefits for both food producers and consumers are companied by potential biomedical risks and environmental side effects. Increasing concerns from the public about GMO, particularly in the form of genetic modified (GM) foods, are aimed at the short- and ...
Soybean genetically modified with rd29A:AtDREB1A (Patent Nos. 3183458) (line 1Ab58), ... The experiment was carried out in the field area located in the National Soybean Research Center (23°11′ S, 51°11′ W, 630 m altitude) (Embrapa Soybean, Londrina, PR, Brazil) a branch of the Brazilian Agricultural Research Corporation during the crop ...
Next generation sequencing (NGS) is a promising tool for analysing the quality and safety of food and feed products. The detection and identification of genetically modified organisms (GMOs) is ...
Soybean oil, which has high abundance of linoleic acid (LA, 18:2ω-6), is the most commonly consumed edible oil. Recent studies support that a high dietary intake of LA is linked with increased risks of developing colonic inflammation and colon cancer. Here we studied the effects of the genetically modified Plenish® soybean oil, which has low abundance of LA as well as α-linolenic acid (ALA ...
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgement. This research was supported by grants from the National Major Special Project of Breeding for Genetically Modified Organisms in China (No. 2016ZX08012-005 ...
The GM positive samples containing Roundup Ready Soybean were analyzed by the TaqMan® genetically modified organism (GMO) 35S Soy Detection Kit (Applied Biosystems). Data indicated that 22 positive samples contained RR soybean of more than 1%; 23 samples contained Roundup Ready soybean of less than 0.1% ( Fig. 5 ).
A number of studies show the economic benefits of using genetically modified products. Between 1996 and 2011, farmers' income worldwide increased by $92 million from the use of genetically modified crops. Part of the revenue is due to the more efficient treatment of weeds and insects, while another part is due to lower overall production costs.
Citation 187 Yield of both maize and soybean in China are much lower than the global average and China therefore has become increasingly reliant on imports of both crops, much of which will be genetically modified. China is the world's largest importer of soybean seed (60% of world imported soybean) and second largest importer of maize grain ...
In his research paper titled "Transgenic Pollen Harms Monarch Larvae" published by Nature in 1999, he pointed out that larvae of the monarch butterfly, ... In fact, the problem of the regulatory system for the safety of genetically modified soybean food does not only exist in the experimental environment or in other countries. In recent ...
However, consumers' risk perception may erode these benefits. This study examines the effect of risk perception on the choice of soybean oil using machine learning (recursive decision trees). Using 23,168 respondents, we find that about 62.02% of the respondents are willing to purchase GMO (regular) soybean oil at the expense of manual ...
The widespread use of GM soybean in Poland is confirmed by research results obtained over the last 15 years. In the majority of analysed samples (92%), genetically modified soybean was determined at above the 0.9% level. Since 2004, it has been evident that the MON40-3-2 soybean variety is a major GM crop used in Poland.
This paper summarizes the molecular mechanism of Agrobacterium-mediated genetic transformation of soybean, the factors impacting soybean genetic transformation, the latest research progress of improving transformation efficiency and the present status of genetically modified soybean. Transferring target gene into receptor cell and making target gene express in plants,the objection of rapid ...
The nuanced research comes at a pivotal time, as Roundup Ready soy and other genetically modified seeds continue to explode worldwide. Despite controversy over the seeds' environmental impact, the use of biotech crops has been approved across South America, China, and India. "The next frontier is likely to be Sub-Saharan Africa," he says.