An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Open Access


Advanced body composition assessment: from body mass index to body composition profiling
Magnus borga.
1 Department of Biomedical Engineering, Linköping University, Linköping, Sweden
2 Center for Medical Image Science and Visualization (CMIV), Linköping University, Linköping, Sweden
3 Advanced MR Analytics AB, Linköping, Sweden
4 Department of Medical and Health Sciences, Linköping University, Linköping, Sweden
Jimmy D Bell
5 Research Centre for Optimal Health, University of Westminster, London, UK
Nicholas C Harvey
6 MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK
7 NIHR Southampton Biomedical Research Centre, University of Southampton, University Hospital Southampton NHS Foundation Trust, Southampton, UK
Thobias Romu
Steven b heymsfield.
8 Pennington Biomedical Research Center, Baton Rouge, Louisiana, USA
Olof Dahlqvist Leinhard
Associated data.
jim-2018-000722supp001.pdf
jim-2018-000722supp002.pdf
This paper gives a brief overview of common non-invasive techniques for body composition analysis and a more in-depth review of a body composition assessment method based on fat-referenced quantitative MRI. Earlier published studies of this method are summarized, and a previously unpublished validation study, based on 4753 subjects from the UK Biobank imaging cohort, comparing the quantitative MRI method with dual-energy X-ray absorptiometry (DXA) is presented. For whole-body measurements of adipose tissue (AT) or fat and lean tissue (LT), DXA and quantitative MRIs show excellent agreement with linear correlation of 0.99 and 0.97, and coefficient of variation (CV) of 4.5 and 4.6 per cent for fat (computed from AT) and LT, respectively, but the agreement was found significantly lower for visceral adipose tissue, with a CV of >20 per cent. The additional ability of MRI to also measure muscle volumes, muscle AT infiltration and ectopic fat, in combination with rapid scanning protocols and efficient image analysis tools, makes quantitative MRI a powerful tool for advanced body composition assessment.
INTRODUCTION
The human body—as well as the body of every other animal—is mainly composed of four molecular-level components: water, fat, proteins and minerals, usually in that order of decreasing amounts. 1 The substance that has attracted the most attention, from laypeople to medical professionals, is fat. This is, of course, motivated by the well-established fact that an excessive amount of body fat is related to increased morbidity and mortality. But also because adipose tissue (AT) is, by far, the most varying compartment—between individuals, but also within an individual over time. The most widely used way to estimate body fat is the body mass index (BMI)—body weight normalized by height squared (kg/m 2 ). Being a very simple and inexpensive method, it is the basis for WHO’s definition of overweight (25≤ BMI <30) and obesity (BMI ≥30). However, for a given BMI, the body fat percentage changes with age, and the rate of this change is different depending on sex, ethnicity and individual differences. 2 And while BMI correlates with fat accumulation and metabolic health in large populations, it is insensitive to the actual distribution of body fat. 3
When comparing methods for body composition analysis, it is important to distinguish fat (triglyceride) from AT, 4 which contains approximately 80 per cent fat, the rest being water, protein and minerals. 5 While most of the body fat is stored in AT, fat is also present in organs such as liver and skeletal muscle. Today, it is well known that the metabolic risk related to fat accumulation is strongly dependent on its distribution. Central obesity and, in particular, ectopic fat accumulation are important metabolic risk factors. 6–8 Large amounts of visceral AT (VAT) are related to increased cardiac risk, 8 9 type 2 diabetes, 10 11 liver disease 12 and cancer. 13 14 High levels of liver fat increase the risk for liver disease and type 2 diabetes, 15 and increased muscle fat has been associated with increased risk for insulin resistance and type 2 diabetes 16 and reduced mobility. 17 While there are other anthropometric measures, such as waist circumference and waist-to-hip ratio, which more strongly correlate with metabolic risk, 18 19 it is now well recognized that BMI and other anthropometric surrogate measures are poor predictors for individual fat distribution and metabolic risk. 3 20 21
Besides fat, acting as the body’s long-term energy storage, skeletal muscles are of great interest to study, and the balance between the energy-consuming muscles and the energy-storing fat compartments is, of course, highly relevant in order to understand the metabolic balance of the body. Cachexia, involuntary loss of body weight, usually with disproportionate muscle wasting, is a life-threatening condition, often related to the progression of an underlying serious disease (eg, cancer 22 ). In cancer, cachexia is defined as weight loss of >5 per cent over 6 months, BMI <20 kg/m 2 or appendicular muscle mass normalized by body height squared of <7.26 kg/m 2 or 5.45 kg/m 2 for males and females, respectively. 23 Sarcopenia, which can be related to cachexia, but is also associated with aging, is often defined as reduced physical performance following loss of muscle mass, usually accompanied by increased fat infiltration of the muscles. 24 When diagnosing sarcopenia, muscle strength tests combined with muscle volume measurements are needed. 25 Furthermore, Willis et al showed that muscle pathology progression over 1 year could be detected by quantitative MRI but not by assessing muscle strength or function. 26 These examples illustrate the need for more sophisticated body composition analysis tools that go beyond simple anthropometric measures.
Since the early part of the last century, scientists have tried to determine the body composition in different ways, with a wide range of different physical principles and devices, and using different models and assumptions. Today, local in vivo measurements of different fat depots and fat infiltration in organs can be made using tomographic imaging techniques such as CT and MRI that were not even invented when the first scientific studies on body composition were published. These techniques are now recognized as golden standard for body composition analysis. 25 27
The purpose of this paper is to give a brief introduction to the most commonly used methods for body composition analysis and a review of an MRI-based body composition analysis technique, comparing its performance to other methods. This includes a previously unpublished validation study of the agreement between this method and dual-energy X-ray absorptiometry (DXA).
TECHNOLOGY OVERVIEW
A number of different techniques for body composition assessment have been developed, from very simple indirect measures such as waist-to-hip ratio and calipers to sophisticated direct volumetric measurements based on three-dimensional imaging techniques. There are also a range of invasive or in vitro methods for body composition analysis such as inhalation or injection of water-accumulating or fat-accumulating agents, or dissection and chemical analysis of cadavers. This overview will, however, focus solely on non-invasive in vivo measurement techniques.
Hydrostatic weighing (densitometry)
Hydrostatic weighing (underwater weighing), or densitometry, is based on Archimedes’ principle. The difference of the body weight in air and water is used to compute the body’s density. Assuming a two-component model with different densities for fat mass and fat-free mass and correcting for the air volume in the lungs, the total body fat percentage can be estimated. Obviously, this technique cannot give any measurements of the distribution of AT or lean tissue (LT).
Air displacement plethysmography (ADP)
ADP is perhaps better known under its commercial brand name BOD POD (Life Measurement, Concord, California, USA). Similar to hydrostatic weighing, ADP measures the overall body density and hence total body fat and LT but not their distributions. By putting the body in an enclosed chamber and changing the chamber’s volume, the volume of the displaced air (ie, the volume of the body) can be determined from the changes in air pressure. Since ADP is based on the same two-component model as hydrostatic weighing, it is also affected by the same confounders, mainly variations in bone mineral content (BMC) and hydration. Due to the limitations of the two-component model used in densitometry and ADP, a four-component (4C) model is often recommended. 28 29 In addition to fat and LT, the 4C model also takes BMC and total body water (TBW) into account. However, these two additional components have to be measured by other techniques (eg, DXA for the BMC and deuterium oxide dilution for TBW 30 ) The repeatability (coefficient of variation (CV)) of ADP for body fat has been reported to be between 1.7 and 4.5 per cent when measured within 1 day. 31 Obviously, ADP, as well as hydrostatic weighing, is limited to gross body composition analysis, not making any estimates of regional fat or muscles.
Bioelectrical impedance analysis (BIA)
BIA uses the electrical properties of the body to estimate the TBW and from that the body fat mass. 32 33 The body is modeled as five cylindrical LT compartments; the trunk and the four limbs, while fat is considered to be an insulator. The impedance is assumed to be proportional to the height and inversely proportional to the cross-sectional area of each compartment, and the electrical equivalent is a resistor (extracellular water) in parallel with a capacitor and a resistor in series (intracellular water). The model of uniform distribution of fat and water fits better to the extremities than the trunk, 34 and while there are BIA measurements that correlate well with total abdominal AT, BIA cannot be used for measuring VAT. 35 Potential error sources are variations in limb length (usually estimated from body height), recent physical activity, nutrition status, tissue temperature and hydration, blood chemistry, ovulation and electrode placement. 32 BIA requires different model parameters to be used depending on age, gender, level of physical activity, amount of body fat and ethnicity in order to be reliable. 36 37
Dual-energy X-ray absorptiometry
DXA is a two-dimensional imaging technique that uses X-rays with two different energies. The attenuation of an X-ray is dependent on the thickness of the tissue and the tissue’s attenuation coefficient, which is dependent on the X-ray energy. By using two different energy levels, the images can be separated into two components (eg, bone and soft tissue). DXA is mainly used for bone mineral density measurements, where it is considered as the gold standard, 38 but it can also be used to estimate total and regional body fat and LT mass. Pixels, where the ratio between attenuations of the two energies falls below a certain threshold, are classified as soft tissue (ie, without bone), and in those pixels, the attenuation is linearly dependent on the fat fraction of the soft tissue. Pixels above the threshold contain a mixture of bone and soft tissue, and there the soft tissue properties need to be interpolated from surrounding soft tissue pixels. 39 Approximately one-third of the pixels of the projected body contains bone. 40
DXA has been found to be more accurate than density-based methods for estimating total body fat. 41 A possible confounder is that the DXA analysis assumes a constant hydration of lean soft tissue, which is not always true as hydration varies with age, gender and disease. 42 Excellent repeatability (CV) in the range 1–2 per cent for body fat and 0.5–2 per cent for LT has been reported for DXA.
Since DXA only gives a two-dimensional (coronal) projection, it is not possible to obtain direct compartmental volumetric measurements, so regional volume estimates are obtained indirectly using anatomical models. For example, VAT and parts of the subcutaneous adipose tissue (SAT) are mixed and cannot be separated in the DXA image. The distribution between VAT and SAT then needs to be estimated from an anatomical model predicting the SAT thickness. Furthermore, the physical properties of the technology do not allow for measurements of ectopic fat in organs such as liver fat or muscle fat infiltration. However, due to its ability to estimate regional fat and measure LT, in combination with relatively high availability, DXA has been used for body composition analysis in a wide range of clinical applications. 43
CT gives a three-dimensional high-resolution image volume of the complete or selected parts of the body, computed from a large number of X-ray projections of the body from different angles. The known differences in attenuations of X-rays between lean soft tissue and AT can then be used to separate these tissues, as well as to determine mixtures between them. As opposed to the previously described techniques, CT can accurately determine fat in skeletal muscle tissue 16 and in the liver. 44 It is, however, significantly less accurate for liver fat <5 per cent which limits its use to diagnose low-grade steatosis. 44 Being a three-dimensional imaging technique, CT has the potential of giving direct volumetric measurements of organs and different AT depots. In practice, however, CT-based body composition analysis is in most cases limited to two-dimensional analysis of one or a limited number of axial slices of the body, leading to the utilization of the area measured as a proxy for the volume. There are two reasons for this limitation: first, it is important to keep the part of the body being scanned to a minimum in order to minimize the ionizing radiation dose. 45 This is particularly important in the ethical considerations of research studies on healthy subjects. Second, manual segmentation of different compartments in the images is a very labor intensive task, which can be reduced by limiting the analysis to a few slices rather than a complete three-dimensional volume. This approach, however, limits its precision since the exact locations of slices, in relation to internal organs, cannot be determined a priory and will therefore vary between scans. Nevertheless, CT, together with MRI, is today considered the gold standard for body composition analysis, in particular regional.
MRI uses the different magnetic properties of the nuclei of certain chemical elements (normally hydrogen in water and fat) in the cells to produce images of soft tissue in the body. A number of MRI-based methods for quantification of AT (eg, see the review by Hu et al 46 ) and muscles 47–52 have been developed and implemented in the past.
By using so-called ‘quantitative fat water imaging’, precise measurements of regional AT and LT, as well as diffuse fat infiltration in other organs, can be obtained. The basis for quantitative fat water imaging is fat water separated, or Dixon, imaging, 53 where the different magnetic resonance frequencies of protons in fat and water are used for separating the two signals into a fat image and a water image. Due to a number of undeterminable factors affecting the MR signal, an MR image is not calibrated on an absolute scale and therefore not quantitative in itself. But by using different postprocessing techniques, the image can be calibrated to quantitatively measure fat or AT. Examples of such methods are proton density fat fraction (PDFF) 54 measuring the fraction of fat in MR-visible soft tissue and fat-referenced MRI 55–57 measuring the amount of AT in each voxel.
As opposed to CT and DXA, MRI does not use ionizing radiation, which enables true volumetric three-dimensional imaging even in healthy volunteers and infants. Still, many studies using MRI for body composition analysis have used one or a limited set of two-dimensional slices, mostly due to the lack of efficient image analysis tools for handling three-dimensional image segmentation. However, since there is no ionizing radiation limiting the image acquisition, the slices can be selected from a complete image volume, thereby reducing the uncertainty in their locations. Still, using a sparse set of slices as a proxy for the complete volume will inevitably negatively affect accuracy and precision as only a fraction of the data is used. It has, for example, been shown that single-slice MRI is poor at predicting VAT and SAT changes during weight loss. 58 59
BODY COMPOSITION PROFILING USING FAT-REFERENCED MRI
Body composition profiling implies the simultaneous collection and analysis of a number of body composition parameters, including subcutaneous and visceral AT, ectopic fat such as liver and skeletal muscle fat and muscle volumes. Fat-referenced MRI is a methodology that enables all such measurements in one single rapid examination. This section gives a brief introduction to body composition profiling using fat-referenced MRI, together with a review of published validation results of the method. Finally, a previously unpublished validation study of the agreement between this method and DXA for measurements of body fat/AT, body LT and VAT is presented.
The body composition profiling methodology combines fat-referenced MRI with automated image segmentation of different compartments and was first described by Dahlqvist Leinhard et al. 55 Different aspects of the method have been further described in other publications. 47 60–62 The two key features of this method are that it produces quantitative fat-referenced images and that it uses a supervised automated segmentation tool.
In a quantitative fat-referenced image, the value in each image volume element (voxel) represents the amount of fat in that voxel in relation to the amount of fat in pure AT. Hence, a voxel in pure AT has a value of one and a voxel without any fat has the value zero. This means that the following can be measured: the total amount of AT in any given region by summation of the voxel values in that region, AT-free volume by removal of amount of AT from volume measurements of regional LT (eg, muscles) and fractions of fat in specific internal organs, such as the liver.
The supervised automated segmentation tool enables an efficient way of segmenting different AT compartments, as well as different muscle groups, reducing the manual work to a few minutes, rather than hours, for analyzing a whole-body data set. Anatomical compartments, such as the visceral compartment and different muscle groups, are automatically segmented using predefined anatomical atlases and the operator can then adjust the segmentations if needed. An example of such segmentations is illustrated in figure 1 .

Example of segmentation of abdominal subcutaneous AT (ASAT), visceral AT (VAT) and 10 muscle groups from fat water separated MRI using fat-referenced MRI and multi-atlas image segmentation. To the left is the fat image with ASAT (blue) and VAT (red), and to the right is the water image with the different muscle groups colored. Reproduced with permission from AMRA Medical AB.
See online supplementary appendix 1 for a summary of how fat-referenced MRI is implemented in AMRA Profiler (AMRA Medical AB, Linköping, Sweden), which is the tool for body composition profiling that was used in the validation studies of fat-referenced MRI.
Supplementary data
Precision and accuracy.
In a previous study, 61 the accuracy of body composition profiling using fat-referenced MRI, in terms of agreement with manual quantification of T1-weighted MR images, was evaluated on 23 (11 females, 12 males) subjects with an average BMI of 31.7±5.1 kg/m 2 (range 22–46 kg/m 2 ); age 36–66 years. There was no significant difference in the measured amount of VAT (4.73±1.99 vs 4.73±1.75 L, P=0.97). Furthermore, the agreement between the methods was excellent for both VAT (95 per cent limits of agreement (LoA) −1.06 to 1.07 L) and abdominal subcutaneous AT (ASAT) (−0.36 to 1.60 L). However, a very small yet statistically significant difference in ASAT was observed (10.39±5.38 vs 9.78±5.36 L, P<0.001). Clearly this small difference has no clinical significance.
Test–retest repeatability and agreement with manual quantification for VAT was evaluated by Newman et al . 63 The study included 30 subjects with five subjects from each gender for each of the following categories of BMI: 18–25 kg/m 2 , 25–30 kg/m 2 and >30 kg/m 2 . Each subject was scanned twice with at least 20 min interval, during which the subject left the scanner room. There was no significant difference between the evaluated method and manual quantification of VAT (P=0.73). Bland-Altman analysis of the test–retest repeatability showed a bias of −0.04 L (95 per cent LoA −0.12 to 0.13 L) for VAT and 0.05 L (95 per cent LoA −0.55 to 0.64 L) for ASAT. The CV was 1.80 per cent for VAT and 2.98 per cent for ASAT using the method above. The CV for manual quantification of VAT was 6.33 per cent as a comparison.
Middleton et al evaluated the accuracy and repeatability of VAT, ASAT and thigh muscle quantification by comparing with manual segmentation on 20 subjects. 64 Due to the laborious work with manual segmentation, 15 two-dimensional axial slices were manually segmented in the abdominal region for VAT and ASAT and 5 slices over the thigh muscles. For repeatability assessment, the subjects were scanned three times, with the subject remaining in the same position on the scan table between scans 1 and 2 and with the subject removed from the table between scans 2 and 3. The intraexamination (scans 1–2) repeatability test obtained a CV of 3.3 per cent for VAT, 2.2 per cent for ASAT and 1.5 per cent for total thigh muscle volume. For the inter-examination test (scans 2–3), the CVs were 3.6, 2. 6 and 1.5 per cent for VAT, ASAT and thigh muscle volume, respectively. Good agreement with the manual measurements in the 20 slices was observed for all measurements. Neither the slopes nor the intercepts of the regression lines were significantly different from those of the identity lines.
Test–retest repeatability of muscle quantification of left and right abdominal muscles, left and right, anterior and posterior thigh muscles and left and right lower limb muscles, as well as accuracy of lower leg muscle quantification, were evaluated by Thomas et al 65 comparing the method above with manual segmentation. The study included 15 subjects of each gender, ranging from normal weight to obese. Each subject was scanned twice with at least 20 min interval, during which the subject left the scanner room. The intraclass correlation (ICC) between the first and second scan was almost perfect (between 0.99 and 1.0) for all muscle groups. The 95 per cent LoA ranged from −0.04 to 0.02 L for the posterior thigh muscles to −0.15 to 0.08 L for the left lower limb. The lowest accuracy for the lower limbs was a bias of −0.08 L with 95 per cent LoA of −0.25 to 0.09 L.
Test–retest repeatability of measurements of VAT and ASAT volumes and volumes and fat infiltration of left and right posterior and anterior thigh muscles, lower leg muscles and abdominal muscles were evaluated by West et al on 36 sedentary postmenopausal women. 66 Each subject was scanned twice, and the subjects were removed from the scanner room between the acquisitions. The intraexamination CV was 1.54 per cent for VAT, 1.06 per cent for ASAT, 0.8–1.9 per cent for volumes of muscle groups (thigh, lower leg and abdomen) and 2.3–7.0 per cent for individual muscle volumes. The 95 per cent LoA was −0.13 to 0.10 L for VAT, −0.38 to 0.29 L for ASAT. The LoA for liver PDFF were within ±1.9 per cent, and for muscle fat infiltration, they were within ±2.06 per cent for muscle groups and within ±5.13 per cent for individual muscles.
The method’s reproducibility of fat-free muscle volume quantification between 1.5 T and 3 T MR scanners, as well as the agreement with manual segmentation, was investigated on 11 different muscle groups. 47 The ICC between the automated method and manual measurements was at least 0.97 for all muscle groups except in the arms. Except for the arms, the ICC between 1.5 T and 3 T data ranged from 0.97 (left lower leg) to 1.00 (left posterior thigh) with a mean difference volume ranging from 0.39 L (95 per cent LoA 0.01 to 0.77 L) (left abdomen) to 0.0 L (95 per cent LoA −0.10 to 0.09 L) (right lower leg). The muscles of the arms had worse accuracy and reproducibility due to difficulties to include the arms in the field of view.
Agreement with ADP
A previous study 67 compared AT measured using fat-referenced MRI with total body fat measured by ADP. The ICC was 0.984. After converting the ADP body fat measures to AT volume (assuming that most of the fat resided in AT and a density of 0.9 kg/L for AT), a Bland-Altman analysis showed that ADP underestimated AT by 0.78 L on average, but the bias was strongly dependent on the level of adiposity with significant underestimation for lean subjects and significant overestimation for subjects with higher amounts of AT. Similar bias dependence has been observed when ADP has been compared with DXA 31 and MRI. 68
Agreement with BIA
Ulbrich et al 69 investigated the agreement between fat-referenced MRI and BIA on 80 subjects between 20 and 62 years with a BMI range from 17.5 to 26.2 kg/m 2 . The linear correlation between body fat mass measured by BIA and AT volume measured by MRI was 0.75 and 0.81 for females and males, respectively. The total AT measured by MRI was converted to total fat mass (again assuming that most of the fat resided in AT and using a constant density of 0.94 kg/L). Compared with MRI, the BIA underestimated the total fat with approximately 5 kg (±7 kg LoA) on average, this despite the fact that the MRI-based measurements of total body fat excluded the arms and lower legs. The highest linear correlation found between BIA and MRI-derived measures was 0.75 and 0.81 for females and males, respectively. These correlations were found between BIA-derived body mass percentage and the MRI-derived ‘total AT index’ (total AT divided by body height squared).
Agreement with DXA
Methods and materials.
The agreement between DXA and the fat-referenced MRI technique was assessed using data from the UK Biobank study, 70 approved by the North West Multicenter Research Ethics Committee, UK, and with written informed consent obtained from all subjects prior to study entry. The age range for inclusion was 40–69 years of age. For the present analysis, participants were selected, out of the first 6214 scanned, who had both DXA and MRI scans. One subject with obviously erroneous DXA values (2.7 kg total fat and 6.8 kg LT) was excluded, yielding a total 4753 subjects (2502 females and 2251 males). All included MRI images were analyzable for VAT, ASAT and both thigh muscles according the predefined quality criteria. 62 The BMI range was 16.4–54.3 with a mean of 26.2 kg/m 2 .
The MR images were acquired using a Siemens Aera 1.5 T scanner (Syngo MR D13) (Siemens, Erlangen, Germany) with the dual-echo Dixon Vibe protocol, covering neck to knees as previously described. 62 The MR images were analyzed using AMRA Profiler. The body AT and LT were measured from the bottom of the thigh muscles to level of the top of vertebrae T9 ( figure 2 ). The LT was defined as the volume of soft tissue subtracted by the volume of AT. 47

(A) The definition of lean and adipose tissue measured by MRI from the bottom of the thigh muscles to top of vertebrae T9 marked in blue color in the water (left) and fat (right) image. (B) An example of a dual-energy X-ray absorptiometry (DXA) image from the study cohort. DXA image copyright UK Biobank. Reprinted with permission.
Whole-body DXA data were acquired using a GE-Lunar iDXA (GE Healthcare, Madison, Wisconsin, USA) with the subjects in supine position. 71 The images were analyzed using the GE enCORE software by the radiographer at, or soon after, the scan. The GE iDXA estimates VAT within an automatically segmented region with the lower border at the top of the iliac crest and its height is set to 20 per cent of the distance from the top of the iliac crest to the base of the skull. 72
Since the DXA and MRI analyses measure different entities (fat and LT mass vs AT and LT volume, respectively) and they do not cover the same part of the body, a linear model was estimated by linear regression between the MRI and DXA measurements using a training data set of 2376 randomly selected subjects. The remaining 2377 subjects were then used for estimating the agreement between the techniques after linear transformation using the linear model (ie, validating the linear model). The MRI-based measurements (L) were transformed to predict the DXA measurements (kg) using the linear regression coefficients from the training data, and a Bland-Altman analysis was performed to investigate the agreement between MRI-derived and DXA-derived measurements in the validation data. To investigate the agreement between DXA and MRI-derived VAT measurements, a linear model was estimated between the DXA and MRI measurements. Of the 4669 subjects with available DXA VAT measurements, 2334 cases were used to estimate the model and the remaining 2335 subjects were used to validate the agreement between VAT measured by MRI and the transformed DXA measurements using Bland-Altman analysis.
The linear regression between MRI and DXA was 1.23 x – 0.12 (kg/L) for body fat/AT and 1.88 x + 1.82 (kg/L) for body LT. The linear correlation coefficient, r, between DXA and the transformed MRI measurements was 0.99 for body fat and 0.97 for LT. The 95 per cent LoA from the Bland-Altman analysis were −2.25 to 2.31 kg for fat and −4.33 to 4.31 kg for LT ( figure 3 ). The prediction error SD relative to the mean (CV) was 4.5 per cent for body fat and 4.6 per cent for LT. The correlation between VAT measured by MRI and VAT as predicted by DXA was 0.97 and the LoA were −1.02 to 1.05 L, with CV=21 per cent ( figure 4 ).

Correlation plots (upper row) between dual-energy X-ray absorptiometry (DXA) and corresponding measurement predicted from MRI using a linear transformation for body fat (left) and body lean tissue (right). The bottom row shows Bland-Altman plots of the agreement between DXA and corresponding measures predicted from MRI.

Correlation between visceral adipose tissue (VAT) predicted by dual-energy X-ray absorptiometry (DXA) and VAT measured by MRI (left) and Bland-Altman plot showing the agreement (liters) between the methods (right).
Densitometry, including ADP, shows relatively good precision and high correlation with MRI-based measurements of whole-body AT, but with a significant volume-dependent bias. Since these methods only measure the volume or density of the body, they cannot be used for regional measurements and body composition profiling.
BIA is highly available and its relatively low cost is an advantage, which also makes it useful for consumer products. Furthermore, it can differentiate intracellular water from extracellular water, which is a unique capability of BIA. BIA can also, in principle, be used for regional measurements, but it is severely limited when it comes to measuring VAT or ectopic fat in internal organs.
DXA techniques have shown good accuracy when evaluated against MRI for whole-body measurements and very good repeatability. The prediction of whole-body fat and LT from MRI agrees well with DXA after a linear transformation, but less so for VAT. While the correlation between DXA and MRI-derived VAT was high (r=0.97), the agreement after a linear transformation was, however, much lower than for total body fat and body LT, with a CV >20 per cent. The high linear correlation, despite a modest agreement, can be explained by the very wide range of measured VAT volumes, ranging from almost 0 to >14 L. The CV for VAT is in line with the results by Kaul et al with a CV of 15.6 per cent for females and 25.9 per cent for males when comparing the same DXA model with CT. 72 Park et al found a linear correlation of 0.85 between VAT measured by DXA and MRI in a study including 90 non-obese men. 73 However, Kamel et al found that the correlation was much lower (r=0.46) for obese men. 74 The fact that the agreement is lower for obese subjects can also be observed in figure 4 where the prediction error increases with increased VAT volume. Silver et al found an excellent correlation without significant bias between fat water MRI and DXA for ‘gross body adipose tissue’ but with a significant negative bias (MRI – DXA) for ‘total trunk adipose tissue’ as well as total and trunk LT. 75 Interestingly, for DXA, the lowest precision is for fat in the arms, with reported CV up to 11 per cent. 76 This is the same compartment that is difficult to measure with MRI due to signal loss in the outer parts of the field of view. A strength with DXA, compared with MRI, is the simultaneous assessment of bone mineral density and mass.
When comparing different technologies, both accuracy and precision are important. Accuracy, however, can be rather difficult to compare between technologies for several reasons. First, there is no ground truth available. Even though there is a growing consensus that tomographic methods are the gold standard that can be used to assess accuracy for other methods, they differ between themselves and are difficult to compare in terms of accuracy. Using physical phantoms is one way to assess accuracy, but they miss the difficulties caused by anatomical variations that we know can lead to different measurement errors. Automated tomographic imaging methods can be evaluated against manual methods, but this addresses only one of several important components in the measurement system—the segmentation of different compartments. Second, not all methods measure the same thing, so even if two technologies correlate strongly, there may be a significant bias if they measure different physical entities. For example, AT is not equivalent to fat—besides fat AT also contains water, protein and minerals. When comparing a method that measures AT in volume units, such as MRI, to a method that measures fat in weight units (eg, DXA), we have to convert one unit to the other using a density that is assumed to be constant, which again may not be always accurate.
Although this review has not focused on measurements of ectopic fat, this is an important component in body composition profiling, especially for understanding metabolic status and assessing risk. Among the techniques discussed here, CT and quantitative MRI are the only methods that can quantify local diffuse infiltration of AT and ectopic fat. (Non-invasive measurements of ectopic fat, in particular liver fat, are commonly done by MR spectroscopy (MRS), but since MRS only measures local substance concentrations and not absolute amounts of fat, AT or LT, this technology was not included in this study.) While it is possible—and sometimes necessary—to use different equipment for different measurements in a study, it is often desirable to keep the number of different examinations and modalities to a minimum in order to optimize the work flow. By using quantitative MRI, or CT if the radiation dose is not a concern, a large number of metabolically relevant body composition parameters can be measured with high accuracy and precision in a single examination.
A comparison of the capabilities of different measurements of the techniques discussed above is summarized in table 1 .
Comparison of the capabilities of different techniques for body composition analysis
ADP, air displacement plethysmography; BIA, bioelectrical impedance analysis; DXA, dual-energy X-ray absorptiometry; VAT, visceral adipose tissue.
There are several methods available that can measure whole-body AT or fat and LT. In terms of precision and accuracy, DXA and MRI are comparable as they show excellent agreement after a linear transformation. However, the agreement is much lower for compartmental measurements such as VAT. Moreover, MRI gives access to accurate and direct measurements of diffuse infiltration of AT in muscles and ectopic fat (eg, liver fat). Rapid MRI scanning protocols, in combination with efficient image analysis methods, have promoted MRI to a competitive option for advanced body composition assessment, thus enabling a more complete description of a person’s body composition profile from a single examination.
Acknowledgments
This research has been conducted using the UK Biobank Resource under Data Access Application 6569. For full acknowledgements, see online supplementary appendix 2 .
Contributors: MB, JW and ODL planned the work. MB, TR and ODL developed and applied the MR analysis methods used. JDB was responsible for the UK Biobank body MRI and NCH for the UK Biobank DXA scans. MB and JW performed the statistical analyses. SBH contributed with expertise on body composition. MB drafted the manuscript. All authors contributed to editing the text.
Funding: Funding support for analysis of UK Biobank data was provided by Pfizer.
Competing interests: MB, JW, TR and ODL are employees and stockholders of AMRA Medical AB.
Patient consent: Obtained.
Ethics approval: North West Multicenter Research Ethics Committee (MREC), UK.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: The underlying data used in this study are available though the UK Biobank resource. All bonafide researchers can apply to use the UK Biobank resource for health-related research that is of public interest by applying in the Access Management System (AMS). For details, see: www.ukbiobank.ac.uk/
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 August 2023
Nutrition and Health (including climate and ecological aspects)
The bioelectrical impedance analysis (BIA) international database: aims, scope, and call for data
- Analiza M. Silva ORCID: orcid.org/0000-0002-8984-8600 1 ,
- Francesco Campa ORCID: orcid.org/0000-0002-3028-7802 2 ,
- Silvia Stagi ORCID: orcid.org/0000-0002-6469-4334 3 ,
- Luís A. Gobbo 4 ,
- Roberto Buffa 3 ,
- Stefania Toselli 5 ,
- Diego Augusto Santos Silva 6 ,
- Ezequiel M. Gonçalves 7 ,
- Raquel D. Langer ORCID: orcid.org/0000-0002-9098-1863 7 ,
- Gil Guerra-Júnior 7 ,
- Dalmo R. L. Machado 8 ,
- Emi Kondo ORCID: orcid.org/0000-0001-9145-0549 9 ,
- Hiroyuki Sagayama ORCID: orcid.org/0000-0002-9040-7650 9 ,
- Naomi Omi 9 ,
- Yosuke Yamada ORCID: orcid.org/0000-0002-4284-6317 10 ,
- Tsukasa Yoshida 10 ,
- Wataru Fukuda 11 ,
- Maria Cristina Gonzalez ORCID: orcid.org/0000-0002-3901-8182 12 ,
- Silvana P. Orlandi 13 ,
- Josely C. Koury ORCID: orcid.org/0000-0002-3189-9261 14 ,
- Tatiana Moro 2 ,
- Antonio Paoli ORCID: orcid.org/0000-0003-0474-4229 2 ,
- Salome Kruger 15 ,
- Aletta E. Schutte ORCID: orcid.org/0000-0001-9217-4937 16 ,
- Angela Andreolli 17 ,
- Carrie P. Earthman ORCID: orcid.org/0000-0002-7783-7437 18 ,
- Vanessa Fuchs-Tarlovsky 19 ,
- Alfredo Irurtia ORCID: orcid.org/0000-0002-3463-6643 20 ,
- Jorge Castizo-Olier 21 ,
- Gabriele Mascherini ORCID: orcid.org/0000-0002-8842-0354 22 ,
- Cristian Petri 23 ,
- Laura K. Busert 24 ,
- Mario Cortina-Borja ORCID: orcid.org/0000-0003-0627-2624 24 ,
- Jeanette Bailey 25 ,
- Zachary Tausanovitch 25 ,
- Natasha Lelijveld 26 ,
- Hadeel Ali Ghazzawi 27 ,
- Adam Tawfiq Amawi ORCID: orcid.org/0000-0001-7810-748X 28 ,
- Grant Tinsley ORCID: orcid.org/0000-0002-0230-6586 29 ,
- Suvi T. Kangas 25 ,
- Cécile Salpéteur 30 ,
- Adriana Vázquez-Vázquez 24 ,
- Mary Fewtrell ORCID: orcid.org/0000-0001-9783-3444 24 ,
- Chiara Ceolin 31 ,
- Giuseppe Sergi 31 ,
- Leigh C. Ward ORCID: orcid.org/0000-0003-2378-279X 32 ,
- Berit L. Heitmann ORCID: orcid.org/0000-0002-6809-4504 33 nAff34 ,
- Roberto Fernandes da Costa ORCID: orcid.org/0000-0002-8789-1744 35 ,
- German Vicente-Rodriguez 36 ,
- Margherita Micheletti Cremasco ORCID: orcid.org/0000-0002-5948-7584 37 ,
- Alessia Moroni ORCID: orcid.org/0000-0003-3780-2931 37 ,
- John Shepherd 38 ,
- Jordan Moon 39 ,
- Tzachi Knaan ORCID: orcid.org/0000-0002-6697-9894 40 ,
- Manfred J. Müller ORCID: orcid.org/0000-0002-7280-2411 41 ,
- Wiebke Braun 41 ,
- José M. García‐Almeida 42 ,
- António L. Palmeira 43 ,
- Inês Santos 44 ,
- Sofus C. Larsen 45 , 46 ,
- Xueying Zhang ORCID: orcid.org/0000-0001-5746-2191 47 ,
- John R. Speakman ORCID: orcid.org/0000-0002-2457-1823 47 , 48 ,
- Lindsay D. Plank ORCID: orcid.org/0000-0003-2737-0151 49 ,
- Boyd A. Swinburn 50 ,
- Jude Thaddeus Ssensamba 51 , 52 ,
- Keisuke Shiose 53 ,
- Edilson S. Cyrino ORCID: orcid.org/0000-0001-9016-8779 54 ,
- Anja Bosy-Westphal 41 ,
- Steven B. Heymsfield ORCID: orcid.org/0000-0003-1127-9425 55 ,
- Henry Lukaski ORCID: orcid.org/0000-0002-5418-5851 56 ,
- Luís B. Sardinha ORCID: orcid.org/0000-0002-6230-6027 1 ,
- Jonathan C. Wells ORCID: orcid.org/0000-0003-0411-8025 24 &
- Elisabetta Marini ORCID: orcid.org/0000-0001-8779-8745 3
European Journal of Clinical Nutrition volume 77 , pages 1143–1150 ( 2023 ) Cite this article
1861 Accesses
2 Citations
25 Altmetric
Metrics details
- Scientific community
Bioelectrical impedance analysis (BIA) is a technique widely used for estimating body composition and health-related parameters. The technology is relatively simple, quick, and non-invasive, and is currently used globally in diverse settings, including private clinicians’ offices, sports and health clubs, and hospitals, and across a spectrum of age, body weight, and disease states. BIA parameters can be used to estimate body composition (fat, fat-free mass, total-body water and its compartments). Moreover, raw measurements including resistance, reactance, phase angle, and impedance vector length can also be used to track health-related markers, including hydration and malnutrition, and disease-prognostic, athletic and general health status. Body composition shows profound variability in association with age, sex, race and ethnicity, geographic ancestry, lifestyle, and health status. To advance understanding of this variability, we propose to develop a large and diverse multi-country dataset of BIA raw measures and derived body components. The aim of this paper is to describe the ‘BIA International Database’ project and encourage researchers to join the consortium.
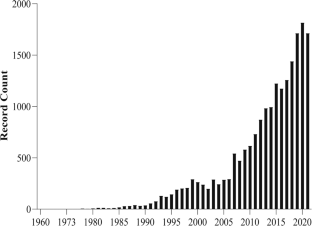
The Exercise and Health Laboratory of the Faculty of Human Kinetics, University of Lisbon has agreed to host the database using an online portal. At present, the database contains 277,922 measures from individuals ranging from 11 months to 102 years, along with additional data on these participants.
The BIA International Database represents a key resource for research on body composition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
Purchase on Springer Link
Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Life expectancy can increase by up to 10 years following sustained shifts towards healthier diets in the United Kingdom
Lars T. Fadnes, Carlos Celis-Morales, … John C. Mathers

Genome-wide characterization of circulating metabolic biomarkers
Minna K. Karjalainen, Savita Karthikeyan, … Johannes Kettunen
Effect of gut microbiome modulation on muscle function and cognition: the PROMOTe randomised controlled trial
Mary Ni Lochlainn, Ruth C. E. Bowyer, … Claire J. Steves
Data availability
The data sets generated and/or analyzed during the current project are not publicly available due to the data confidentiality requirements of the ethics committee for each study but are available from the corresponding author on reasonable request and approval from the ethics committee.
Aleman-Mateo H, Rush E, Esparza-Romero J, Ferriolli E, Ramirez-Zea M, Bour A. et al. Prediction of fat-free mass by bioelectrical impedance analysis in older adults from developing countries: a cross-validation study using the deuterium dilution method. J Nutr Health Aging. 2010;14:418–26. https://doi.org/10.1007/s12603-010-0031-z .
Article CAS PubMed Google Scholar
Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pr. 2004;19:433–46. https://doi.org/10.1177/0115426504019005433 .
Article Google Scholar
Earthman C, Traughber D, Dobratz J, Howell W. Bioimpedance spectroscopy for clinical assessment of fluid distribution and body cell mass. Nutr Clin Pr. 2007;22:389–405. https://doi.org/10.1177/0115426507022004389 .
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM. et al. Bioelectrical impedance analysis–part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. https://doi.org/10.1016/j.clnu.2004.06.004 .
Article PubMed Google Scholar
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J. et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–53. https://doi.org/10.1016/j.clnu.2004.09.012 .
Campa F, Gobbo LA, Stagi S, Cyrino LT, Toselli S, Marini E, et al. Bioelectrical impedance analysis versus reference methods in the assessment of body composition in athletes. Eur J Appl Physiol. 2022;122:561–89. https://doi.org/10.1007/s00421-021-04879-y .
Lukaski HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr. 2013;67:S2–9. https://doi.org/10.1038/ejcn.2012.149 .
Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20:330–9. https://doi.org/10.1097/MCO.0000000000000387 .
Heitmann BL. Prediction of body water and fat in adult Danes from measurement of electrical impedance. A validation study. Int J Obes. 1990;14:789–802.
CAS PubMed Google Scholar
Bedogni G, Grugni G, Tringali G, Agosti F, Sartorio A. Assessment of fat-free mass from bioelectrical impedance analysis in obese women with Prader-Willi syndrome. Ann Hum Biol. 2015;42:538–42. https://doi.org/10.3109/03014460.2014.990922 .
Cleary J, Daniells S, Okely AD, Batterham M, Nicholls J. Predictive validity of four bioelectrical impedance equations in determining percent fat mass in overweight and obese children. J Am Diet Assoc. 2008;108:136–9. https://doi.org/10.1016/j.jada.2007.10.004 .
Costa RFD, Masset K, Silva AM, Cabral B, Dantas PMS Development and cross-validation of predictive equations for fat-free mass and lean soft tissue mass by bioelectrical impedance in Brazilian women. Eur J Clin Nutr. 2021. https://doi.org/10.1038/s41430-021-00946-x
Deurenberg P, van der Kooy K, Leenen R, Weststrate JA, Seidell JC. Sex and age specific prediction formulas for estimating body composition from bioelectrical impedance: a cross-validation study. Int J Obes. 1991;15:17–25.
Deurenberg P, van der Kooy K, Paling A, Withagen P. Assessment of body composition in 8-11 year old children by bioelectrical impedance. Eur J Clin Nutr. 1989;43:623–9.
Dey DK, Bosaeus I, Lissner L, Steen B. Body composition estimated by bioelectrical impedance in the Swedish elderly. Development of population-based prediction equation and reference values of fat-free mass and body fat for 70- and 75-y olds. Eur J Clin Nutr. 2003;57:909–16. https://doi.org/10.1038/sj.ejcn.1601625 .
Gonzalez MC, Orlandi SP, Santos LP, Barros AJD. Body composition using bioelectrical impedance: Development and validation of a predictive equation for fat-free mass in a middle-income country. Clin Nutr. 2019;38:2175–9. https://doi.org/10.1016/j.clnu.2018.09.012 .
Goran MI, Kaskoun MC, Carpenter WH, Poehlman ET, Ravussin E, Fontvieille AM. Estimating body composition of young children by using bioelectrical resistance. J Appl Physiol. 1993;75:1776–80. https://doi.org/10.1152/jappl.1993.75.4.1776 .
Kanellakis S, Skoufas E, Karaglani E, Ziogos G, Koutroulaki A, Loukianou F. et al. Development and validation of a bioelectrical impedance prediction equation estimating fat free mass in Greek - Caucasian adult population. Clin Nutr ESPEN. 2020;36:166–70. https://doi.org/10.1016/j.clnesp.2020.01.003 .
Kotler DP, Burastero S, Wang J, Pierson RN,Jr. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996;64:489S–97S. https://doi.org/10.1093/ajcn/64.3.489S .
Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition. 2001;17:248–53. https://doi.org/10.1016/s0899-9007(00)00553-0 .
Luke A, Bovet P, Forrester TE, Lambert EV, Plange-Rhule J, Dugas LR. et al. Prediction of fat-free mass using bioelectrical impedance analysis in young adults from five populations of African origin. Eur J Clin Nutr. 2013;67:956–60. https://doi.org/10.1038/ejcn.2013.123 .
Article CAS PubMed PubMed Central Google Scholar
Matias CN, Campa F, Santos DA, Lukaski H, Sardinha LB, Silva AM. Fat-free Mass Bioelectrical Impedance Analysis Predictive Equation for Athletes using a 4-Compartment Model. Int J Sports Med. 2021;42:27–32. https://doi.org/10.1055/a-1179-6236 .
Steinberg A, Manlhiot C, Li P, Metivier E, Pencharz PB, McCrindle BW. et al. Development and Validation of Bioelectrical Impedance Analysis Equations in Adolescents with Severe Obesity. J Nutr. 2019;149:1288–93. https://doi.org/10.1093/jn/nxz063 .
Stolarczyk LM, Heyward VH, Goodman JA, Grant DJ, Kessler KL, Kocina PS, et al. Predictive accuracy of bioimpedance equations in estimating fat-free mass of Hispanic women. Med Sci Sports Exerc. 1995;27:1450–6.
Stolarczyk LM, Heyward VH, Hicks VL, Baumgartner RN. Predictive accuracy of bioelectrical impedance in estimating body composition of Native American women. Am J Clin Nutr. 1994;59:964–70. https://doi.org/10.1093/ajcn/59.5.964 .
Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–40. https://doi.org/10.1093/ajcn/77.2.331 .
Tint MT, Ward LC, Soh SE, Aris IM, Chinnadurai A, Saw SM, et al. Estimation of fat-free mass in Asian neonates using bioelectrical impedance analysis. Br J Nutr. 2016;115:1033–42. https://doi.org/10.1017/s0007114515005486 .
da Costa RF, Silva AM, Masset K, Cesário TM, Cabral B, Ferrari G, et al. Development and Cross-Validation of a Predictive Equation for Fat-Free Mass in Brazilian Adolescents by Bioelectrical Impedance. Front Nutr. 2022;9:820736. https://doi.org/10.3389/fnut.2022.820736 .
Article PubMed PubMed Central Google Scholar
Wang L, Hui SS, Wong SH. Validity of bioelectrical impedance measurement in predicting fat-free mass of Chinese children and adolescents. Med Sci Monit. 2014;20:2298–310. https://doi.org/10.12659/msm.890696 .
Nightingale CM, Rudnicka AR, Owen CG, Donin AS, Newton SL, Furness CA, et al. Are ethnic and gender specific equations needed to derive fat free mass from bioelectrical impedance in children of South asian, black african-Caribbean and white European origin? Results of the assessment of body composition in children study. PLoS One. 2013;8:e76426. https://doi.org/10.1371/journal.pone.0076426 .
Essa’a VJ, Dimodi HT, Ntsama PM, Medoua GN. Validation of anthropometric and bioelectrical impedance analysis (BIA) equations to predict total body water in a group of Cameroonian preschool children using deuterium dilution method. Nutrire. 2017;42:20. https://doi.org/10.1186/s41110-017-0045-y .
Article CAS Google Scholar
van Zyl A, White Z, Ferreira J, Wenhold FAM. Developing an Impedance Based Equation for Fat-Free Mass of Black Preadolescent South African Children. Nutrients 2019;11. https://doi.org/10.3390/nu11092021
Nigam P, Misra A, Colles SL. Comparison of DEXA-derived body fat measurement to two race-specific bioelectrical impedance equations in healthy Indians. Diabetes Metab Syndr. 2013;7:72–7. https://doi.org/10.1016/j.dsx.2013.02.031 .
Beaudart C, Bruyère O, Geerinck A, Hajaoui M, Scafoglieri A, Perkisas S, et al. Equation models developed with bioelectric impedance analysis tools to assess muscle mass: A systematic review. Clin Nutr ESPEN. 2020;35:47–62. https://doi.org/10.1016/j.clnesp.2019.09.012 .
Matias CN, Santos DA, Judice PB, Magalhaes JP, Minderico CS, Fields DA. et al. Estimation of total body water and extracellular water with bioimpedance in athletes: A need for athlete-specific prediction models. Clin Nutr. 2016;35:468–74. https://doi.org/10.1016/j.clnu.2015.03.013 .
Sergi G, Bussolotto M, Perini P, Calliari I, Giantin V, Ceccon A, et al. Accuracy of bioelectrical impedance analysis in estimation of extracellular space in healthy subjects and in fluid retention states. Ann Nutr Metab. 1994;38:158–65. https://doi.org/10.1159/000177806 .
Dittmar M, Reber H. Validation of different bioimpedance analyzers for predicting cell mass against whole-body counting of potassium (40 K) as a reference method. Am J Hum Biol. 2004;16:697–703. https://doi.org/10.1002/ajhb.20078 .
Flury S, Trachsler J, Schwarz A, Ambuhl PM. Quantification of excretory renal function and urinary protein excretion by determination of body cell mass using bioimpedance analysis. BMC Nephrol. 2015;16:174 https://doi.org/10.1186/s12882-015-0171-9 .
Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–71. https://doi.org/10.1152/jappl.2000.89.2.465
Silva AM, Fields DA, Heymsfield SB, Sardinha LB. Body composition and power changes in elite judo athletes. Int J Sports Med. 2010;31:737–41. https://doi.org/10.1055/s-0030-1255115 .
Knudsen NN, Kjærulff TM, Ward LC, S‘bye D, Holst C, Heitmann BL. Body water distribution and risk of cardiovascular morbidity and mortality in a healthy population: A prospective cohort study. PLoS One. 2014;9:e87466. https://doi.org/10.1371/journal.pone.0087466 .
Silva AM, Fields DA, Heymsfield SB, Sardinha LB. Relationship between changes in total-body water and fluid distribution with maximal forearm strength in elite judo athletes. J Strength Cond Res. 2011;25:2488–95. https://doi.org/10.1519/JSC.0b013e3181fb3dfb
Silva AM, Matias CN, Santos DA, Rocha PM, Minderico CS, Sardinha LB. Increases in intracellular water explain strength and power improvements over a season. Int J Sports Med. 2014;35:1101–5. https://doi.org/10.1055/s-0034-1371839 .
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. https://doi.org/10.1016/j.metabol.2018.09.005
Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206. https://doi.org/10.1186/cc12901
Soares MN, Eggelbusch M, Naddaf E, Gerrits KHL, van der Schaaf M, van den Borst B, et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J Cachexia Sarcopenia Muscle. 2022;3:11–22. https://doi.org/10.1002/jcsm.12896
Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12 https://doi.org/10.1186/cc13189
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169
Buffa R, Floris G, Marini E. Assessment of nutritional status in free-living elderly individuals by bioelectrical impedance vector analysis. Nutrition. 2009;25:3–5. https://doi.org/10.1016/j.nut.2008.07.014
Langer RD, Larsen SC, Ward LC, Heitmann BL. Phase angle measured by bioelectrical impedance analysis and the risk of cardiovascular disease among adult Danes. Nutrition. 2021;89:111280 https://doi.org/10.1016/j.nut.2021.111280 .
Campa F, Matias CN, Marini E, Heymsfield SB, Toselli S, Sardinha LB, et al. Identifying Athlete Body Fluid Changes During a Competitive Season With Bioelectrical Impedance Vector Analysis. Int J Sports Physiol Perform. 2019;1-7. https://doi.org/10.1123/ijspp.2019-0285
Castizo-Olier J, Irurtia A, Jemni M, Carrasco-Marginet M, Fernandez-Garcia R, Rodriguez FA. Bioelectrical impedance vector analysis (BIVA) in sport and exercise: Systematic review and future perspectives. PLoS One. 2018;13:e0197957 https://doi.org/10.1371/journal.pone.0197957
Girma T, Hother Nielsen AL, Kaestel P, Abdissa A, Michaelsen KF, Friis H, et al. Biochemical and anthropometric correlates of bio-electrical impedance parameters in severely malnourished children: A cross-sectional study. Clin Nutr. 2018;37:701–5. https://doi.org/10.1016/j.clnu.2017.02.017
Girma T, Kaestel P, Molgaard C, Ritz C, Andersen GS, Michaelsen KF, et al. Utility of bio-electrical impedance vector analysis for monitoring treatment of severe acute malnutrition in children. Clin Nutr. 2021;40:624–31. https://doi.org/10.1016/j.clnu.2020.06.012
Lee S, Bountziouka V, Lum S, Stocks J, Bonner R, Naik M, et al. Ethnic variability in body size, proportions and composition in children aged 5 to 11 years: is ethnic-specific calibration of bioelectrical impedance required? PLoS One. 2014;9:e113883 https://doi.org/10.1371/journal.pone.0113883
Marini E, Campa F, Buffa R, Stagi S, Matias CN, Toselli S, et al. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin Nutr. 2020;39:447–54. https://doi.org/10.1016/j.clnu.2019.02.016
Moroni A, Varde C, Giustetto A, Stagi S, Marini E, Micheletti Cremasco M. Bioelectrical Impedance Vector Analysis (BIVA) for the monitoring of body composition in pregnancy. Eur J Clin Nutr. 2022;76:604–9. https://doi.org/10.1038/s41430-021-00990-7 .
Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854–61. https://doi.org/10.1016/j.clnu.2012.05.008 .
Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF, et al. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer. 2008;8:249 https://doi.org/10.1186/1471-2407-8-249
Langer RD, Ward LC, Larsen SC, Heitmann BL. Can change in phase angle predict the risk of morbidity and mortality during an 18-year follow-up period? A cohort study among adults. Front Nutr. 2023;10:1157531 https://doi.org/10.3389/fnut.2023.1157531 .
Sardinha LB. Physiology of exercise and phase angle: another look at BIA. Eur J Clin Nutr. 2018;72:1323–7. https://doi.org/10.1038/s41430-018-0215-x .
Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92:957–62. https://doi.org/10.1079/bjn20041292
Kyle UG, Genton L, Pichard C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin Nutr. 2013;32:294–9. https://doi.org/10.1016/j.clnu.2012.08.001
Kyle UG, Soundar EP, Genton L, Pichard C. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin Nutr. 2012;31:875–81. https://doi.org/10.1016/j.clnu.2012.04.002 .
Schwenk A, Beisenherz A, Romer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr. 2000;72:496–501. https://doi.org/10.1093/ajcn/72.2.496
Valdespino-Trejo A, Orea-Tejeda A, Castillo-Martinez L, Keirns-Davis C, Montanez-Orozco A, Ortiz-Suarez G, et al. Low albumin levels and high impedance ratio as risk factors for worsening kidney function during hospitalization of decompensated heart failure patients. Exp Clin Cardiol. 2013;18:113–7.
PubMed PubMed Central Google Scholar
Brantlov S, Jødal L, Andersen RF, Lange A, Rittig S, Ward LC. An evaluation of phase angle, bioelectrical impedance vector analysis and impedance ratio for the assessment of disease status in children with nephrotic syndrome. BMC Nephrol. 2019;20:331 https://doi.org/10.1186/s12882-019-1511-y .
Oh JH, Song S, Rhee H, Lee SH, Kim DY, Choe JC, et al. Normal Reference Plots for the Bioelectrical Impedance Vector in Healthy Korean Adults. J Korean Med Sci. 2019;34:e198 https://doi.org/10.3346/jkms.2019.34.e198 .
Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. https://doi.org/10.1093/ajcn.82.1.49
Kuchnia AJ, Teigen LM, Cole AJ, Mulasi U, Gonzalez MC, Heymsfield SB, et al. Phase Angle and Impedance Ratio: Reference Cut-Points From the United States National Health and Nutrition Examination Survey 1999-2004 From Bioimpedance Spectroscopy Data. JPEN J Parenter Enter Nutr. 2017;41:1310–5. https://doi.org/10.1177/0148607116670378
Bosy-Westphal A, Danielzik S, Dorhofer RP, Later W, Wiese S, Muller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enter Nutr. 2006;30:309–16. https://doi.org/10.1177/0148607106030004309
Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17:534–41. https://doi.org/10.1016/s0899-9007(01)00555-x
Campa F, Thomas DM, Watts K, Clark N, Baller D, Morin T, et al. Reference Percentiles for Bioelectrical Phase Angle in Athletes. Biology. 2022;11:264. https://doi.org/10.3390/biology11020264
Wells JCK, Williams JE, Quek RY, Fewtrell MS. Bio-electrical impedance vector analysis: testing Piccoli’s model against objective body composition data in children and adolescents. Eur J Clin Nutr. 2019;73:887–95. https://doi.org/10.1038/s41430-018-0292-x
Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int. 1994;46:534–9. https://doi.org/10.1038/ki.1994.305 .
Marini E, Sergi G, Succa V, Saragat B, Sarti S, Coin A, et al. Efficacy of specific bioelectrical impedance vector analysis (BIVA) for assessing body composition in the elderly. J Nutr Health Aging. 2013;17:515–21. https://doi.org/10.1007/s12603-012-0411-7
Buffa R, Saragat B, Cabras S, Rinaldi AC, Marini E. Accuracy of specific BIVA for the assessment of body composition in the United States population. PLoS One. 2013;8:e58533. https://doi.org/10.1371/journal.pone.0058533
Stagi S, Silva AM, Jesus F, Campa F, Cabras S, Earthman CP, et al. Usability of classic and specific bioelectrical impedance vector analysis in measuring body composition of children. Clin Nutr. 2022;41:673–9. https://doi.org/10.1016/j.clnu.2022.01.021 .
Wells JC, Williams JE, Ward LC, Fewtrell MS. Utility of specific bioelectrical impedance vector analysis for the assessment of body composition in children. Clin Nutr. 2021;40:1147–54. https://doi.org/10.1016/j.clnu.2020.07.022 .
De Palo T, Messina G, Edefonti A, Perfumo F, Pisanello L, Peruzzi L, et al. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutrition. 2000;16:417–24. https://doi.org/10.1016/s0899-9007(00)00269-0
Ibanez ME, Mereu E, Buffa R, Gualdi-Russo E, Zaccagni L, Cossu S, et al. New specific bioelectrical impedance vector reference values for assessing body composition in the Italian-Spanish young adult population. Am J Hum Biol. 2015;27:871–6. https://doi.org/10.1002/ajhb.22728
Piccoli A, Nigrelli S, Caberlotto A, Bottazzo S, Rossi B, Pillon L, et al. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am J Clin Nutr. 1995;61:269–70. https://doi.org/10.1093/ajcn/61.2.269
Piccoli A, Pillon L, Dumler F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate Z scores. Nutrition. 2002;18:153–67. https://doi.org/10.1016/s0899-9007(01)00665-7
Ward LC, Heitmann BL, Craig P, Stroud D, Azinge EC, Jebb S, et al. Association between ethnicity, body mass index, and bioelectrical impedance. Implications for the population specificity of prediction equations. Ann N. Y Acad Sci. 2000;904:199–202. https://doi.org/10.1111/j.1749-6632.2000.tb06449.x .
Heitmann BL, Swinburn BA, Carmichael H, Rowley K, Plank L, McDermott R, et al. Are there ethnic differences in the association between body weight and resistance, measured by bioelectrical impedance? Int J Obes Relat Metab Disord. 1997;21:1085–92. https://doi.org/10.1038/sj.ijo.0800477 .
Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. https://doi.org/10.1002/j.1550-8528.1995.tb00124.x
Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond). 2009;6:17 https://doi.org/10.1186/1743-7075-6-17 .
Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22:76–82. https://doi.org/10.1002/ajhb.20956 .
Ward LC. Electrical Bioimpedance: From the Past to the Future. J Electr Bioimpedance. 2021;12:1–2. https://doi.org/10.2478/joeb-2021-0001 .
Marini E, Buffa R, Saragat B, Coin A, Toffanello ED, Berton L. et al. The potential of classic and specific bioelectrical impedance vector analysis for the assessment of sarcopenia and sarcopenic obesity. Clin Inter Aging. 2012;7:585–91. https://doi.org/10.2147/CIA.S38488 .
Toselli S, Marini E, Maietta Latessa P, Benedetti L, Campa F. Maturity related differences in body composition assessed by classic and specific bioimpedance vector analysis among male elite youth soccer players. Int J Environ Res Public Health. 2020;17:729. https://doi.org/10.3390/ijerph17030729 .
Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99. https://doi.org/10.1038/nrclinonc.2012.209 .
World Health Organization. Social determinants of health. Geneva, Switzerland: World Health Organization; 2009.
Google Scholar
Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, et al. The double burden of malnutrition: aetiological pathways and consequences for health. Lancet. 2020;395:75–88. https://doi.org/10.1016/s0140-6736(19)32472-9 .
Download references
Acknowledgements
Faculdade Motricidade Humana-Universidade de Lisboa kindly hosted the BIA database in the website for which we are thankful. Management group of the BIA International Database: AMS, LCW, ESC, AB-W, SBH, HL, LBS, JCW, EM.
Author information
Berit L. Heitmann
Present address: Section for general Practice, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Authors and Affiliations
Exercise and Health Laboratory, CIPER, Faculdade de Motricidade Humana, Universidade de Lisboa, 1499-002, Lisbon, Portugal
Analiza M. Silva & Luís B. Sardinha
Department of Biomedical Science, University of Padova, 35100, Padova, Italy
Francesco Campa, Tatiana Moro & Antonio Paoli
Department of Life and Environmental Sciences, University of Cagliari, Cittadella Universitaria, Monserrato, 09042, Cagliari, Italy
Silvia Stagi, Roberto Buffa & Elisabetta Marini
Skeletal Muscle Assessment Laboratory, Physical Education Department, School of Technology and Science, São Paulo State University, Presidente Prudente, 19060-900, Brazil
Luís A. Gobbo
Department for Life Quality Studies, University of Bologna, 47921, Rimini, Italy
Stefania Toselli
Research Center of Kinanthropometry and Human Performance, Sports Center, Universidade Federal de Santa Catarina, Florianópolis, Brazil
Diego Augusto Santos Silva
Growth and Development Laboratory, Center for Investigation in Pediatrics (CIPED), School of Medical Sciences, University of Campinas (UNICAMP), Campinas, 13083-887, Brazil
Ezequiel M. Gonçalves, Raquel D. Langer & Gil Guerra-Júnior
Laboratory of Kinanthropometry and Human Performance, School of Physical Education and Sport of Ribeirão Preto, University of São Paulo, 05508-030, São Paulo, Brazil
Dalmo R. L. Machado
Faculty of Health and Sport Sciences, University of Tsukuba, Ibaraki, 305-8574, Japan
Emi Kondo, Hiroyuki Sagayama & Naomi Omi
National Institute of Health and Nutrition, National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, 566-0002, Japan
Yosuke Yamada & Tsukasa Yoshida
Yokohama Sports Medical Center, Yokohama Sport Association, Kanagawa, 222-0036, Japan
Wataru Fukuda
Postgraduate Program in Nutrition and Food, Federal University of Pelotas, 96010-610 Pelotas, Brazil
Maria Cristina Gonzalez
Nutrition Department, Federal University of Pelotas, 96010-610, Pelotas, Brazil
Silvana P. Orlandi
Nutrition Institute, State University of Rio de Janeiro, 20550-013, Rio de Janeiro, Brazil
Josely C. Koury
Centre of Excellence for Nutrition, North-West University, Potchefstroom, 2520, South Africa
Salome Kruger
School of Population Health, University of New South Wales, The George Institute for Global Health, Sydney, NSW, Australia
Aletta E. Schutte
University of Rome Tor Vergata, Rome, Italy
Angela Andreolli
University of Delaware, Newark, DE, USA
Carrie P. Earthman
Hospital General de México, Dr. Eduardo Liceaga, Ciudad de México, Mexico
Vanessa Fuchs-Tarlovsky
National Institute of Physical Education of Catalonia (INEFC), University of Barcelona (UB), Barcelona, Spain
Alfredo Irurtia
School of Health Sciences, TecnoCampus, Pompeu Fabra University, Barcelona, Spain
Jorge Castizo-Olier
Department of Experimental and Clinical Medicine, University of Florence, Firenze, Italy
Gabriele Mascherini
Department of Sports and Computer Science, Section of Physical Education and Sports, Universidad Pablo de Olavide, Seville, Spain
Cristian Petri
Population, Policy & Practice Research and Teaching Department, UCL Great Ormond Street Institute of Child Health, London, UK
Laura K. Busert, Mario Cortina-Borja, Adriana Vázquez-Vázquez, Mary Fewtrell & Jonathan C. Wells
International Rescue Committee, New York, NY, 10168, USA
Jeanette Bailey, Zachary Tausanovitch & Suvi T. Kangas
Emergency Nutrition Network (ENN), OX5 2DN, Kiddlington, UK
Natasha Lelijveld
Department of Nutrition and Food Technology, School of Agriculture, The University of Jordan, Amman, Jordan
Hadeel Ali Ghazzawi
Department of Physical and Health Education, Faculty of Educational Sciences, Al-Ahliyya Amman University, Al-Salt, Jordan
Adam Tawfiq Amawi
Energy Balance & Body Composition Laboratory, Department of Kinesiology & Sport Management, Texas Tech University, Lubbock, TX, 79409, USA
Grant Tinsley
Department of Expertise and Advocacy, Action contre la Faim, 93358, Montreuil, France
Cécile Salpéteur
Department of Medicine (DIMED), Geriatrics Division, University of Padova, Padova, 35128, Italy
Chiara Ceolin & Giuseppe Sergi
School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, QLD, 4072, Australia
Leigh C. Ward
Research Unit for Dietary Studies, The Parker Institute, Frederiksberg and Bispebjerg Hospital, Copenhagen, Denmark
Department of Physical Education, Research Group in Physical Activity and Health, Federal University of Rio Grande do Norte, Natal, Brazil
Roberto Fernandes da Costa
Faculty of Health and Sport Science FCSD, Department of Physiatry and Nursing, University of Zaragoza, 50009, Zaragoza, Spain
German Vicente-Rodriguez
Laboratory of Anthropology, Anthropometry and Ergonomics, Department of Life Sciences and Systems Biology, University of Torino, 10123, Torino, Italy
Margherita Micheletti Cremasco & Alessia Moroni
University of Hawaii Cancer Center, Honolulu, HI, USA
John Shepherd
United States Sports Academy, Daphne, AL, 36526, USA
Jordan Moon
Weight Management, Metabolism & Sports Nutrition Clinic, Metabolic Lab, Tel-Aviv, Tel Aviv-Yafo, Israel
Tzachi Knaan
Department of Human Nutrition, Institute of Human Nutrition and Food Sciences, Christian-Albrechts University, 24105, Kiel, Germany
Manfred J. Müller, Wiebke Braun & Anja Bosy-Westphal
Department of Endocrinology and Nutrition, Virgen de la Victoria Hospital, Malaga University, 29010, Malaga, Spain
José M. García‐Almeida
CIDEFES, Universidade Lusófona, Lisboa, Portugal
António L. Palmeira
Laboratório de Nutrição, Faculdade de Medicina, Centro Académico de Medicina de Lisboa, Universidade de Lisboa, Lisboa, Portugal
Inês Santos
Research Unit for Dietary Studies at the Parker Institute, Bispebjerg and Frederiksberg Hospital, The Capital Region, Frederiksberg, Denmark
Sofus C. Larsen
The Research Unit for General Practice and Section of General Practice, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Shenzhen Key Laboratory of Metabolic Health, Center for Energy Metabolism and Reproduction, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Xueying Zhang & John R. Speakman
School of Biological Sciences, University of Aberdeen, Aberdeen, UK
John R. Speakman
Department of Surgery, University of Auckland, Auckland, New Zealand
Lindsay D. Plank
School of Population Health, University of Auckland, Auckland, New Zealand
Boyd A. Swinburn
Center for Innovations in Health Africa (CIHA Uganda), Kampala, Uganda
Jude Thaddeus Ssensamba
Makerere University Walter Reed Project, Kampala, Uganda
Faculty of Education, University of Miyazaki, Miyazaki, Japan
Keisuke Shiose
Metabolism, Nutrition, and Exercise Laboratory. Physical Education and Sport Center, State University of Londrina, Rod. Celso Garcia Cid, Km 380, 86057-970, Londrina-PR, Brazil
Edilson S. Cyrino
Pennington Biomedical Research Center, Baton Rouge, LA, 70808, USA
Steven B. Heymsfield
Department of Kinesiology and Public Health Education, Hyslop Sports Center, University of North Dakota Grand Forks, Grand Forks, ND, 58202, USA
Henry Lukaski
You can also search for this author in PubMed Google Scholar

Contributions
All authors contributed to the drafting and editing of the manuscript and to construction of the BIA International database.
Corresponding author
Correspondence to Analiza M. Silva .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Silva, A.M., Campa, F., Stagi, S. et al. The bioelectrical impedance analysis (BIA) international database: aims, scope, and call for data. Eur J Clin Nutr 77 , 1143–1150 (2023). https://doi.org/10.1038/s41430-023-01310-x
Download citation
Received : 22 November 2022
Revised : 10 July 2023
Accepted : 12 July 2023
Published : 02 August 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s41430-023-01310-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Using physical activity to advance a career in clinical nutrition.
- Henry C. Lukaski
European Journal of Clinical Nutrition (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 6, Issue 1
- Assessment of adult body composition using bioelectrical impedance: comparison of researcher calculated to machine outputted values
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Maria Franco-Villoria 1 ,
- Charlotte M Wright 2 ,
- John H McColl 1 ,
- Andrea Sherriff 2 ,
- Mark S Pearce 3 ,
- and the Gateshead Millennium Study core team
- 1 School of Mathematics and Statistics, University of Glasgow , Glasgow , UK
- 2 PEACH Unit , School of Medicine, University of Glasgow , Glasgow , UK
- 3 Institute of Health and Society, Newcastle University , Newcastle upon Tyne , UK
- Correspondence to Professor Charlotte M Wright; charlotte.wright{at}glasgow.ac.uk
Objectives To explore the usefulness of Bioelectrical Impedance Analysis (BIA) for general use by identifying best-evidenced formulae to calculate lean and fat mass, comparing these to historical gold standard data and comparing these results with machine-generated output. In addition, we explored how to best to adjust lean and fat estimates for height and how these overlapped with body mass index (BMI).
Design Cross-sectional observational study within population representative cohort study.
Setting Urban community, North East England
Participants Sample of 506 mothers of children aged 7–8 years, mean age 36.3 years.
Methods Participants were measured at a home visit using a portable height measure and leg-to-leg BIA machine (Tanita TBF-300MA).
Measures Height, weight, bioelectrical impedance (BIA).
Outcome measures Lean and fat mass calculated using best-evidenced published formulae as well as machine-calculated lean and fat mass data.
Results Estimates of lean mass were similar to historical results using gold standard methods. When compared with the machine-generated values, there were wide limits of agreement for fat mass and a large relative bias for lean that varied with size. Lean and fat residuals adjusted for height differed little from indices of lean (or fat)/height 2 . Of 112 women with BMI >30 kg/m 2 , 100 (91%) also had high fat, but of the 16 with low BMI (<19 kg/m 2 ) only 5 (31%) also had low fat.
Conclusions Lean and fat mass calculated from BIA using published formulae produces plausible values and demonstrate good concordance between high BMI and high fat, but these differ substantially from the machine-generated values. Bioelectrical impedance can supply a robust and useful field measure of body composition, so long as the machine-generated output is not used.
- measurement
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
https://doi.org/10.1136/bmjopen-2015-008922
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
Population-based cohort, but female only and restricted to parents.
Explicit, well-evidenced computational approach.
Validated against published gold standard data.
Compared with widely used commercial methods.
Introduction
The WHO defines obesity as “the disease in which excess body fat has accumulated to such an extent that health may be adversely affected”. 1 Although prevention is the first step, being able to reliably identify people with excess fat is essential if the problem is to be recognised and appropriate measures taken. Body mass index (BMI) (weight/height 2 ) is only an indirect measure of fatness, so reliable methods of assessing body composition are also needed. Hydrodensitometry is usually regarded as the nearest to a gold standard, 2 but is impractical for most studies. For this reason, alternative less direct techniques have been developed. These include stable isotope methods and X-ray densitometry (DXA), but isotope methods require costly materials and processing while DXA equipment is non-portable. Thus, for ambulatory assessment, a cheaper and portable method such as Bioelectrical Impedance Analysis (BIA) is valuable. The equipment necessary is portable, relatively inexpensive and the procedure simple and painless, making it a suitable method for studying large groups of participants. 3 , 4 Measurements are taken by using four surface electrodes at different sites which send an imperceptible electrical current through the body (50 kHz alternating current of 800 μA between electrodes). Although there are also whole body machines, the most commonly used field method has been the four electrode leg-to-leg (eg, Tanita), where the participant stands with bare feet on the analyser’s footpads. The impedance value (Z) reflects the resistance and reactance that the electrical signal encounters when passing through the body; the ionised fluid in lean tissue acts as a conductor, and the current passes only through these fluids. 4 The objective physical reading of impedance cannot be interpreted without further statistical manipulation, but assuming that LM∝TBW∝height 2 /Z, lean mass (LM) and total body water (TBW) can then be estimated, from which fat mass (FM) can be calculated. 3 , 4
Although BIA is already widely used in practice and some body composition research, there remain doubts about its accuracy and precision. 5 In fact, the measurement of impedance itself is reasonably precise and repeatable as long as it is performed in healthy individuals using the same method. 6 However, the problems begin with the transformation of the impedance data. As described above, impedance has to be mathematically transformed to create meaningful estimates of TBW and thus LM. However, the prediction equations used to convert impedance measurements into measures of body fatness seem to vary between BIA machine manufacturers and incorporate elements other than the key components of height, impedance and the resistivity and hydration constants. Most manufacturers do not publish their formulae for commercial reasons, but the formulae used for a Tanita leg-to-leg machine have been published and these reveal that they incorporate weight as well as height 2 /Z. 7 It is not clear what impact this would have on the results.
A further problem is that lean and FM values are difficult to interpret in isolation, as they differ systematically depending on the participant’s height, 6 , 7 so in estimates of adiposity, FM is usually adjusted for body size by expressing it as a percentage of total mass. However, this then renders LM invisible, which is inappropriate, in individuals where LM varies markedly, since this will create differences in percentage fat (%fat), despite identical FM. 6 This thus risks misclassifying individuals with low LM as having excess body fat and underestimating FM in very muscular individuals. It has been proposed as an alternative that lean and fat should simply be expressed as indices by dividing each by height 2 , 6 but we have shown in children that this still leaves considerable unadjusted confounding by height. 6 We have previously described an alternative approach in children which produces lean and fat residuals that fully adjust both lean and FM for height and compares them to a large population reference. 6 , 8 We have now further applied these to children from the Gateshead Millennium cohort. 9 As part of the same study, we wished to compare these children to similar measures collected in their parents, but there is no generally recognised method of doing this for adults.
Finally, it is widely believed in the lay population that BMI is a poor predictor of actually fatness. Published information on this suggests generally that BMI has high specificity, but low sensitivity to identify high %fat, 10 but as described above, %fat may not be the best way to identify excess FM. We have already explored the concordance between BMI and fat residuals in children and found good concordance in the upper ranges of both, with very weak concordance for low BMI. 11
We thus set out to:
Identify best-evidenced formulae to calculate lean and FM and compare these to historical gold standard data;
Compare these results with machine-generated output;
Explore how to best adjust estimates for height and how these overlap with BMI.
Participants and methods
Participants.
The impedance data were obtained from mothers of participants in the Gateshead Millennium Study (GMS). 12 This study set out to recruit all babies born to Gateshead residents between 1 June 1999 and 31 May 2000 in prespecified recruiting weeks. A wide range of information relating to feeding, growth and latterly obesity were collected on both children and parents and they have now been followed up to beyond age 9 years. 12 The work presented here is based on data collected on the children's mothers in 2007, when the children were aged around 7 years. Written informed consent was obtained from all participants.
The data were collected on the children's parents at a home visit. While it was possible to study mothers at most of these visits, participation by fathers was minimal, so the paternal data were not used further. Impedance was measured using a single frequency (50 kHz) leg-to-leg BIA machine (Tanita TBF-300MA, Tokyo, Japan). The participants were measured wearing light clothing and bare feet after being asked to empty their bladders. The raw impedance and the machine calculated values for LM, FM and %fat were recorded. Height was measured without shoes and socks using a portable scale (Leicester height measure) to 0.1 cm with the head in the Frankfort plane. Weight was measured to 0.1 kg using the Tanita TBF-300MA. BMI was calculated as weight (kg)/height (m) 2 .
Analytical methods
The analysis was carried out using the software package R (V.2.2.0). We used the measured impedance to arrive at our own estimates of TBW and thus lean and FM using best published estimates of various constants. We assumed the hydration constant to be equal to 0.732 in adults, supported by previous studies 13–15 which gives the equation LM=TBW/0.732. Values for the resistivity constant from various papers differ, but we used those of Bell, 15 the only study where impedance was measured using leg-to-leg techniques. This gives a resistivity constant for adults ρ=0.66, that is, TBW=0.66 (height 2 /Z). Combining these two formulas, we obtained the following, simple prediction equation for adult women: LM=0.66/0.732 (height 2 /Z) or LM=0.898 (height 2 /Z). FM was then obtained as weight minus LM. To check whether the values we obtained for TBW, LM and FM using this approach were reasonable, we compared them to reference values from the two previous studies which had used gold standard measurement methods and published separate values for women. 16 , 17 The first 16 estimated TBW using 2 H 2 O dilution, body density using underwater weighting and a three-component model to estimate %fat and LM. The second 17 estimated TBW using either 2 H 2 O or 3 H 2 O dilution and FM and LM using dual-energy X-ray absorptiometry (DXA).
LM residual and FM residual
In order to produce estimates of FM and LM adjusted for height, a regression method to obtain lean and fat residuals for children 8 was adapted to produce lean and fat residuals for their mothers, using their height as a covariate. A range of transformations of raw LM and raw FM were explored in order to achieve approximate normality and constant variance of residuals when regressed on height. The residuals from regression were then standardised (subtracting the mean and dividing by the SD) to get the so-called lean and fat standardised residuals. In addition, lean and fat indices were calculated (LM or FM divided by height 2 ).
The Bland-Altman method 18 was used to compare the Tanita-generated values of FM and LM and the ones produced following the equations presented in this paper. The Bland-Altman plot is widely used in the literature to evaluate the agreement between two methods that are measuring the same thing. This involves calculating the mean difference (bias) between the two measures for each individual and the limits of agreement. In addition, the difference is then plotted against the mean of the two measures, which supplies a visual presentation of how the spread and pattern of the points varies with the reading (variable bias). Linear regression was then used to test for a significant degree of variable bias.
When the cohort was formed in 1999–2000, 1009 (81%) eligible mothers agreed to join the study and impedance and growth data were collected on 498 mothers in 2007, with mean (SD) age 36.3 (5.6) years (age range 23.6–53.1 years). Sixteen (3.2%) women were underweight (BMI <19), 141 women (28%) were overweight (BMI 25–30) and 112 (22%) were obese (BMI >30).
Total body water, lean mass and fat mass
Descriptive statistics for the anthropometric measurements are summarised in table 1 . Values for TBW, LM, FM and %fat were produced according to the predictive equations discussed in the previous section. Summary statistics were then calculated using both this method and that of Tanita ( table 1 ).
- View inline
Descriptive statistics for the anthropometric measurements
Our results are compared with the results from the two historical papers in table 2 , 16 , 17 with the GMS mothers stratified by age to allow direct comparability. Compared with the historical papers, the GMS values for weight BMI and %fat were much higher, with differences of the order of 1 SD in the youngest women. In contrast, the LM differed by no more than ¼ SD and were actually lower in the youngest GMS group.
Values (only females) reported by Hewitt 1993 and Chumlea 2001 (means±SD) compared with GMS values
Using our method, 22 (4%) women had fat <20%, and 180 (36%) had fat >40%. A majority of women with BMI >30 kg/m 2 (88, 79%) also had greater than 40% fat, but only a minority of women with BMI <19 kg/m 2 (4, 25%) had less than 20% fat.
How well do the manufactures algorithms describe body composition?
The machine-calculated values were also available for all but eight mothers. The sample mean of the Tanita LM values was lower than our calculated values (mean (SD) difference −1.19 (3.33) kg, 95% CI −1.49 to −0.90) while they were higher for FM and %fat (1.19 (3.33) kg, 95% CI 0.90 to 1.49 and 1.97 (4.86) %, 95% CI 1.54% to 2.40%, respectively). The two sets of results were compared using the Bland-Altman method, 18 and major discrepancies were found between the two methods. The relative bias in LM calculated by Tanita varied from a mean of −4.68 for all participants in the lowest quintile for LM to +2.55 for the highest quintile ( figure 1 A). Using regression, this revealed a statistically significant slope (B=0.387 p<0.001). No equivalent relationship was seen for FM or %fat ( figure 1 B, C).
- Download figure
- Open in new tab
- Download powerpoint
Bland-Altman plots for (A) lean mass (LM), (B) fat mass (FM) and (C) percentage fat (%fat) comparing our own calculated values to the machine output values (Tanita).
Calculation of lean residual and fat residual
These residuals were normally distributed with mean 0 and variance 1. Fourteen women (2.4%) had fat residuals <−2 SD (roughly the 2.5th centile for the normal distribution) and 124 (25%) had fat residuals >0.68 SD (roughly the 75th centile) as expected.
Relationship of the FM and LM residuals to other measurements
As would be expected, there was no association between height and the lean and fat residuals (Spearman correlation (95% CI) of height with lean residual −0.02 (−0.11 to 0.07); with fat residual 0.01 (−0.07 to 0.10)), but nor was there any significant correlation of height with the lean index (LM/height 2 : −0.05 (−0.14 to 0.04)) or the fat index (FM/height 2 : −0.03 (−0.12 to 0.06)). Of the 112 women with BMI >30 kg/m 2 , 100 (91%) also had fat residuals >75th centile, while a BMI of >30 kg/m 2 identified 81% of all with high fat residual. In contrast, of the 16 with BMI <19 kg/m 2 only 5 (31%) also had fat residual <2nd centile ( figure 2 ).
Scatter plot of lean mass adjusted for height (lean mass residual) against fat mass adjusted for height (fat mass residual) per body mass index (BMI) category (underweight (<19) and obese (≥30). The vertical lines denote the cut-off for low (<2nd centile) and high (>75th centile) fat residual.
In this analysis, we set out first to identify the best published constants to use for estimating lean and FM from BIA. The use of different devices and methods, under different conditions and on different populations, can make it difficult to extrapolate formulas from one study to another, but when we compared our estimated values for FM, LM and TBW to historical data, these revealed that results for LM were similar, while in contrast there were striking increases in average fat for the youngest, though not in the oldest category, who were already relatively more adipose even in the earlier cohorts. 16 , 17 Overall, the participants had a high median %fat (34.5) and nearly a quarter had BMI in the obese range. The results thus vividly reflect the well-recognised secular trend to increased fatness in the population of young to middle-aged women. They also illustrate good concordance between high BMI and high adiposity.
Although based on a simplified mathematical model of the human body’s shape and composition, BIA has been shown to be a reliable method in population studies, though likely to have less accuracy in individuals. 3 , 18 The large number of different published equations reflects differences in the reference methods, instrument used or the characteristics of the sample, but the constants used here seem to be the best ones currently in the public domain. The resulting prediction equation is strikingly simple in comparison to many others proposed in the literature, since it expresses LM as directly proportional to height 2 /Z with no involvement of other variables.
There are limitations to the study. We were not able to directly compare the results to a gold standard method and had to rely instead on published data. However, we were able to show how similar our results were to these, when using this simple parsimonious computational approach. The age range of the women was relatively narrow, but while there are major changes in body composition, hydration and body proportions during infancy and again in old age, 16 in young adults body composition is fairly settled, making the model reported here valid for most adult women. We have data only on women as there were insufficient data on fathers in the GMS for useful analysis. The equations published here may well also be valid for use in men, but ideally this approach should also be extended in future, using a data set of adult men. While the hydration constant (relating TBW to LM) is fairly well established in adults, the resistivity constant (which relates impedance to TBW) was particularly difficult to find. A range of different values were found in the literature, but they were based on unusual samples, 19 only males, 13 , 20 or samples covering a wide age range. 21 Only one study 15 used the now more common leg-to-leg method and this is the value we used.
The results we obtained were very different from those automatically produced by the Tanita machine. It is important to understand the distinction between these essential mathematical transformation and factors that then correlate with or influence LM and FM, such as weight, sex and age. Manufacturers may seek to include these other variables in their output to contextualise their final estimates of adiposity. However, this then is no longer the true estimate of actual LM for that individual, derived solely from the impedance reading.
The equations used by different manufacturers and for different models are not made generally available, but have been published for a machine similar to the one used in our study. 7 These show that the prediction equation used by Tanita for LM in adult women relies on weight as well as height 2 /Z and that the relative contribution of impedance to the final value is tiny relative to that of weight. For example, within our study, a decrease in impedance by 1SD (75 Ω) changes the Tanita-generated FFM estimate by <1 g while an increase in weight by 1SD (16 kg) increases it by 10% (2.8 kg). Thus, the machine estimate of FFM at least is actually largely based on weight rather than impedance.
Our results are in substantial agreement with the findings of Jebb et al , 7 that Tanita underestimates LM in adult women by between 1 and 2 kg on average, and adds the new conclusion that the relative bias in the Tanita estimate of LM varies with size. The size of the positive biases in FM and %fat obtained using Tanita, relative to our method, are very similar to the bias relative to the four-compartment model reported in Jebb et al 23 and our results also confirm poor agreement in individual cases.
Meanwhile, BIA technology has been moving on and there are now multifrequency devices and eight electrode techniques which aim to estimate different body segments and intracellular and extracellular fluids separately. However, the underpinning assumption and prediction formulae for these machines are likely to be even more complex and difficult to assess objectively.
We also considered the most robust way to adjust measures of fat and lean for height. A method that expresses lean and FM separately adjusted for height is much more informative than raw LM and FM estimates. We have shown previously in children that lean and fat residuals are effective in fully adjusting for height as well as allowing the data to be expressed as SD scores compared with a reference population. 8 , 9 However, with adult women, simply dividing LM and FM by height 2 also fully adjusted for height, suggesting that this would be equally valid and simpler. This adds further weight to Well's proposal 22 that 1/Z could be used as a simple height-adjusted lean index, since lean index=LM/Ht 2 =(H 2 /Z)/Ht 2 =1/Z. Ideally, any reference should be validated against a more direct measure of body composition, but such studies seem only to have been done in children.
We have also shown that, as in children, 11 the correspondence between high fat index and BMI is strong, with BMI >30 kg/m 2 showing 90% specific and 80% sensitivity for fat index above the internal 75th centile. This is generally a much better correspondence than was found in a systematic review of the use of various BMI thresholds to detect high %fat measured, using different methods. 10 However, most reviewed studies used much less stringent thresholds for both BMI and %fat, making comparison difficult.
In conclusion, these data demonstrate that using BIA in models with published constants produces estimates of LM that are, on average, very similar to earlier studies using more direct methods, while the larger FM values are entirely plausible given the secular trends in obesity. These suggest that the physical measurement of impedance can produce useful estimates when appropriately transformed. However, the machine-generated estimates are likely to vary between machines and manufacturers and usually do not only reflect the physical measurement of impedance. They cannot therefore be used to validate or verify other measures of adiposity such as BMI. We would recommend that researchers using BIA in future should not rely on machine-generated estimates and should instead express lean and fat indices, divided by height 2 in order to adjust for height.
Acknowledgments
The authors are grateful for the participation and advice of all those involved with The Gateshead Millennium Study—the research team, the families and children who took part, the External Reference Group, Gateshead Health NHS Foundation Trust, Gateshead Education Authority and local schools.
- ↵ [No authors listed ]. Obesity: preventing and managing the global epidemic. Report of a WHO consultation . World Health Organ Tech Rep Ser 2000 ; 894 : i – xii , 1–253 . OpenUrl PubMed
- Moscrip V ,
- Houtkooper LB ,
- Lohman TG ,
- Going SB , et al
- Bosaeus I ,
- De Lorenzo AD , et al
- Reilly JJ ,
- Slater C , et al
- Wright CM ,
- Sherriff A ,
- Ward SC , et al
- Doman D , et al
- Reilly JJ , et al
- Sherriff A , et al
- Okorodudu DO ,
- Jumean MF ,
- Montori VM , et al
- Parkinson KN ,
- Pearce MS ,
- Dale A , et al
- Lukaski HC ,
- Johnson PE ,
- Bolonchuk WW , et al
- Deurenberg P ,
- Wang W , et al
- Hewitt MJ ,
- Williams DP , et al
- Chumlea WC ,
- Zeller CM , et al
- Kushner RF ,
- Schoeller DA
- Hoffer EC ,
- Meador CK ,
- Schoeller DA ,
- Fjeld CR , et al
- Williams JE ,
- Fewtrell M , et al
Collaborators Gateshead Millennium Study core team: Ashley Adamson, Anne Dale, Robert Drewett, Ann Le Couteur, Paul McArdle, Kathryn Parkinson, John J Reilly.
Contributors MSP and the Gateshead Millennium Study core team designed the research and supervised the data collection and data entry. MF-V analysed the data, performed the statistical analysis and initially drafted the paper. JHM and AS supervised the analysis and commented on successive drafts of the paper. CMW designed the research study, supervised the analysis, edited the paper and has primary responsibility for final content. All authors read and approved the final manuscript.
Funding Gateshead Millennium Study was first established with funding from the Henry Smith Charity and Sport Aiding Research in Kids (SPARKS) and followed up with grants from Gateshead NHS Trust R&D, Northern and Yorkshire NHS R&D, and Northumberland, Tyne and Wear NHS Trust. This study wave was supported by a grant from the National Prevention Research Initiative (incorporating funding from British Heart Foundation; Cancer Research UK; Department of Health; Diabetes UK; Economic and Social Research Council; Food Standards Agency; Medical Research Council; Research and Development Office for the Northern Ireland Health and Social Services; Chief Scientist Office, Scottish Government Health Directorates; Welsh Assembly Government and World Cancer Research Fund).
Competing interests None declared.
Ethics approval Gateshead Local Research Ethics Committee (LREC).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.
Read the full text or download the PDF:
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Body Composition
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- Strength & Conditioning Follow Following
- Philosophy of Neuroscience Follow Following
- Insulin Resistance Follow Following
- Resistance Training Follow Following
- Medicine and Science In Sports and Exercise Follow Following
- Infection Control Follow Following
- Mycobacterium tuberculosis Follow Following
- Human Sciences Follow Following
- Human Follow Following
- Anthropometry Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
The five-level model: a new approach to organizing body-composition research
Affiliation.
- 1 Obesity Research Center, St Luke's-Roosevelt Hospital, Columbia University, College of Physicians and Surgeons, New York, NY.
- PMID: 1609756
- DOI: 10.1093/ajcn/56.1.19
Body-composition research is a branch of human biology that has three interconnecting areas: body-composition levels and their organizational rules, measurement techniques, and biological factors that influence body composition. In the first area, which is inadequately formulated at present, five levels of increasing complexity are proposed: I, atomic; II, molecular; III, cellular; IV, tissue-system; and V, whole body. Although each level and its multiple compartments are distinct, biochemical and physiological connections exist such that the model is consistent and functions as a whole. The model also provides the opportunity to clearly define the concept of a body composition steady state in which quantitative associations exist over a specified time interval between compartments at the same or different levels. Finally, the five-level model provides a matrix for creating explicit body-composition equations, reveals gaps in the study of human body composition, and suggests important new research areas.
- Adipose Tissue / anatomy & histology
- Body Composition*
- Bone and Bones / anatomy & histology
- Extracellular Matrix
- Extracellular Space
- Glycogen / analysis
- Lipids / analysis
- Minerals / analysis
- Models, Biological*
- Muscles / anatomy & histology
- Proteins / analysis
Body Composition and Wages
This paper examines the effect of body composition on wages. We develop measures of body composition - body fat (BF) and fat-free mass (FFM) - using data on bioelectrical impedance analysis (BIA) that are available in the National Health and Nutrition Examination Survey III and estimate wage models for white respondents in the National Longitudinal Survey of Youth 1979. Previous research used body size or BMI for measuring obesity despite the growing concern in the medical literature that BMI-based measures do not distinguish between body fat and fat-free body mass and that BMI does not adequately control for non-homogeneity inside human body. Therefore, measures used in this paper represent a useful alternative to BMI-based proxies of obesity. This paper also contributes to the growing literature on the role of non-cognitive skills on wage determination. Our results indicate that calculated BF is unambiguously associated with decreased wages for both males and females among whites We also present evidence indicating that FFM is consistently associated with increased wages. We show that these results are not the artifacts of unobserved heterogeneity. Finally, our findings are robust to numerous specification checks and to a large number of alternative BIA prediction equations from which the body composition measures are derived.
Roy Wada is an AHRQ postdoctoral fellow in Health Service Research at UCLA and RAND. Alexander Brumlik and Jason Delaney provided excellent research assistance. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research.
MARC RIS BibTeΧ
Download Citation Data
- November 9, 2007
Published Versions
More from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .


IMAGES
VIDEO
COMMENTS
These techniques are now recognized as golden standard for body composition analysis. 25 27. The purpose of this paper is to give a brief introduction to the most commonly used methods for body composition analysis and a review of an MRI-based body composition analysis technique, comparing its performance to other methods.
In addition, research has shown that the combination of both RT and aerobic exercise (i.e., concurrent training) can be an effective approach to optimize body recomposition ( 5,57 ). Thus, practitioners, coaches, and trainers commonly recommend concurrent training for individuals aiming to gain muscle and lose fat ( 24 ).
This simple two-compartment (2C) model of the human body has become the mainstay of body composition assessment in clinical nutrition. A little later, in 1940s and 1950s, the 2C model was ...
Increased training duration improves muscle gain and strength. Furthermore, time spent in training influences the oxidation of fatty acids and carbohydrates [39].Barakat et al. emphasized that the ...
Body composition is a key component for maintaining good general health and longevity. It can be influenced by a variety of factors, including genetics, environment, and lifestyle choices. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial ...
This paper gives a brief overview of common non-invasive techniques for body composition analysis and a more in-depth review of a body composition assessment method based on fat-referenced ...
The BIA International Database represents a key resource for research on body composition. Bioelectrical impedance analysis (BIA) is a technique widely used for estimating body composition and ...
Change in body composition due to dietary, exercise, and lifestyle interventions is of great importance to the decision-making Models of body composition with 2 (2-C), 3 (3-C), or 4 (4-C ...
Bioelectrical impedance can supply a robust and useful field measure of body composition, so long as the machine-generated output is not used. ... JHM and AS supervised the analysis and commented on successive drafts of the paper. CMW designed the research study, supervised the analysis, edited the paper and has primary responsibility for final ...
In our study, body fat in men and women were 18.33 and 19.82. The body fat percentage in men and women were 25.74% and 34.01%. Visceral fat area in men and women were 91.98 and 77 cm 2. And, with the increase of age, body fat, body fat percentages and visceral fat area also increased, both in men and in women.
Abstract —Bioelectrical impedance (BIA) is a painless, non-invasive, and easily portable technique that may. clarify how th e human body is operational. Body. composition (BC) assessment is ...
The Effect of 8-week CrossFit®-based Training on Body Composition in Healthy Adults. The purpose of this study was to evaluate the 8-week training influence according to CrossFit® concept on the healthy adults' body composition and to determine the diversity the training outcomes by gender.
Body-composition research is a branch of human biology that has three interconnecting areas: body-composition levels and their organizational rules, measurement techniques, and biological factors that influence body composition. In the first area, which is inadequately formulated at present, five levels of increasing complexity are proposed: I ...
By Carey Phillips March 10, 2023. NATICK, Mass. — The U.S. Army Research Institute of Environmental Medicine (USARIEM) cross-divisional team recently completed the U.S. Army Center for Initial ...
This paper examines the effect of body composition on wages. We develop measures of body composition - body fat (BF) and fat-free mass (FFM) - using data on bioelectrical impedance analysis (BIA) that are available in the National Health and Nutrition Examination Survey III and estimate wage models for white respondents in the National Longitudinal Survey of Youth 1979.