- Search Menu
- Advance articles
- Themed Issues
- Author Guidelines
- Open Access
- About ILAR Journal
- About the Institute for Laboratory Animal Research
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, determining a suitable research strategy, choosing a model, principles of experimental design, designing powerful experiments: controlling variation and choosing an appropriate sample size, statistical analysis, presentation of results, appendix: a numerical example.
- < Previous
Design and Statistical Methods in Studies Using Animal Models of Development
Michael F.W. Festing, Ph.D., has retired from the MRC Toxicology Unit, University of Leicester, UK. Dr. Festing continues to lecture, publish, and consult on statistics and genetics.
- Article contents
- Figures & tables
- Supplementary Data
Michael F. W. Festing, Design and Statistical Methods in Studies Using Animal Models of Development, ILAR Journal , Volume 47, Issue 1, 2006, Pages 5–14, https://doi.org/10.1093/ilar.47.1.5
- Permissions Icon Permissions
Experiments involving neonates should follow the same basic principles as most other experiments. They should be unbiased, be powerful, have a good range of applicability, not be excessively complex, and be statistically analyzable to show the range of uncertainty in the conclusions. However, investigation of growth and development in neonatal multiparous animals poses special problems associated with the choice of “experimental unit” and differences between litters: the “litter effect.” Two main types of experiments are described, with recommendations regarding their design and statistical analysis: First, the “between litter design” is used when females or whole litters are assigned to a treatment group. In this case the litter, rather than the individuals within a litter, is the experimental unit and should be the unit for the statistical analysis. Measurements made on individual neonatal animals need to be combined within each litter. Counting each neonate as a separate observation may lead to incorrect conclusions. The number of observations for each outcome (“n”) is based on the number of treated females or whole litters. Where litter sizes vary, it may be necessary to use a weighted statistical analysis because means based on more observations are more reliable than those based on a few observations. Second, the more powerful “within-litter design” is used when neonates can be individually assigned to treatment groups so that individuals within a litter can have different treatments. In this case, the individual neonate is the experimental unit, and “n” is based on the number of individual pups, not on the number of whole litters. However, variation in litter size means that it may be difficult to perform balanced experiments with equal numbers of animals in each treatment group within each litter. This increases the complexity of the statistical analysis. A numerical example using a general linear model analysis of variance is provided in the Appendix. The use of isogenic strains should be considered in neonatal research. These strains are like immortal clones of genetically identical individuals (i.e., they are uniform, stable, and repeatable), and their use should result in more powerful experiments. Inbred females mated to males of a different inbred strain will produce F1 hybrid offspring that will be uniform, vigorous, and genetically identical. Different strains may develop at different rates and respond differently to experimental treatments.
The principles of experimental design are universal. They apply equally, for example, to experiments in the life sciences involving humans, animals, plants, and cell cultures. However, in some areas of research, the experimental subjects may have characteristics that necessitate special attention if the experiments are to be designed well and analyzed correctly. Experiments involving neonates of multiparous species are just such a special case. Investigators must identify the correct “experimental unit” (EU 1 ) and take “litter effect” into account for the experiments to afford correct results. These critical aspects of experimental design are discussed below.
There are several different types of investigation, which include but are not limited to the following: observational studies, pilot studies, exploratory experiments, confirmatory studies, and experiments that seek parameter estimates. The first example, observational studies , do not involve the imposition of an experimental treatment. The comparison of animals of two different genotypes is an observational study even though it may have the appearance of being an experiment. Because it is not possible to assign a genotype to an individual at random, it is the investigator's responsibility to ensure that the animals are, to the extent possible, identical in all other respects apart from their genotype. However, the statistical methods used for observational and experimental studies are essentially the same.
Pilot studies are usually small investigations, sometimes involving only a single animal, with the aim of testing the logistics of a proposed study, and sometimes of gaining preliminary data to be used in the design of a more definitive experiment. For example, a pilot study could be used to assess whether dose levels are appropriate, and to gain information on likely responses and variability.
Exploratory experiments look at the pattern of response to some treatment but are not based on a formal, testable hypothesis. Often many outcomes (characters) are measured, requiring multiple statistical tests. Even though one may use a correction of the p values (e.g., Bonferroni's method of dividing the chosen critical value [usually 0.05] by the number of statistical tests) ( Roberts and Russo 1999 ), exploratory experiments tend to generate more questions than they provide answers. They are usually used to generate hypotheses to be tested in a confirmatory study , where the aim is to test some formal, prestated, preferably quite simple hypothesis. Experiments may also be done to estimate parameters such as dose-response curves, means, and proportions.
There is surprisingly little discussion of the concept of “models” in biomedical research despite their extensive use ( Festing 2004 ). According to the American philosopher Marx Wartofsky, “Theories, hypotheses, models and analogies I take all to be species of a genus, and my thesis is best stated directly by characterizing this genus, as representation (although “imaging” or “mirroring” will do quite as well)” ( Wartofsky 1979 ). He goes on to say, “There is an additional trivial truth, which may strike some people as shocking: anything can be a model of anything else! This is to say no more than that between any two things in the universe there is some property they both share….”
Although the preceding statements are of little help in deciding whether or not a particular animal or in vitro system is a good model of humans, it does at least clarify the fact that models do not have to resemble the thing being modeled in every respect. Indeed, in some cases it is essential for the model to be different from the thing being modeled. Rodents are used widely as models of humans because they are small and economical. The availability of isogenic strains is also an advantage because they make it possible to do efficient experiments using fewer animals and scientific resources. The critical factor is whether the model is like humans for the specific system being modeled, such as the growth and differentiation of some organ or biochemical characteristic, or the response of neonates to xenobiotics.
The basic principles of experimental design were formulated many years ago ( Fisher 1960 ), and they remain unchanged. To understand the ensuing brief discussion of these principles, however, it is first necessary to understand the two special characteristics of neonates that strongly influence the design and statistical analysis of experiments involving them.
“Experimental Unit”
Experiments normally involve a number of subjects, or EUs, in each treatment group to afford information about interunit variation and a comparison with the variation between treatment groups. Each EU must be capable of being assigned to a different treatment group, and the data recorded on the individual EUs are subjected to the statistical analysis.
The EU in animal research is commonly the individual animal. However, in research involving neonates, if the pregnant female or the whole litter is subjected to an experimental treatment, the female or the whole litter, not the individual neonate, is the EU, because individual pups within a litter do not receive different treatments (although see below). It is incorrect to use the data from individual pups because the number of independent observations (“n”) would be too large and the results would be incorrect, potentially leading to false-positive results ( Raubertas et al. 1999 ; Zorrilla 1997 ). Values from individual neonates may be taken into account, for example, by averaging them. Such averaging could improve the precision of the litter mean, although they do not contribute as individual EUs ( Haseman and Hogan 1975 ).
Because litters vary in size, if all the neonates are measured in each litter, the averages will vary in precision according to the number of pups per litter. It may be advantageous to use a weighted statistical analysis when evaluating the results. Pups from large litters may also be smaller and less developed than those from smaller litters, so if size (e.g., crown-rump length) is an important outcome, it may be important to correct for this difference in the statistical analysis. Where the outcome is a binary variable such as “normal/abnormal,” a full statistical analysis may require advanced statistical methods ( Hunt and Bowman 2004 ; Yamamoto and Yanagimoto 1994 ).
If individual pups within a litter are subjected to different treatments either postnatally or as a result of surgical or other intervention on the pregnant female, then “n” will be based on the number of individual pups in a treatment group, and the individual pup is the EU. It is possible to have an experiment that is a mixture of a between-litter and a within-litter design. For example, if pregnant females receive one of two or more treatments (e.g., a drug treatment or a vehicle control), and then after birth the neonates within each litter receive additional individual treatments (e.g., some but not all receive a vitamin supplement), then for the drug treatment the pregnant female is the EU, while for the vitamin supplement the neonate is the EU. This design, known as a “split-plot” experimental design ( Cox 1958 ), is often useful although the statistical analysis probably requires professional advice.
“Litter Effect”
In most cases, individual neonates within a litter are more similar than individuals from different litters; in other words, litters differ in a wide range of characteristics. If genetically heterogeneous animals are being used, then individuals within a litter will be full sibs and genetically more similar than unrelated animals. Both pre- and postnatally, animals also tend to have a similar environment. For example, animals from a large litter may be relatively small and immature. There may even be inaccuracies in recording time of birth so that some litters appear to be older than they really are.
It is important to consider “litter effect” when designing an experiment that involves neonates as the EUs. Suppose, for example, that the experiment involves treating some of the neonates with a hormone, while others receive a placebo. Operationally it would be most convenient to treat whole litters, because then pups would not need to be individually identified before weaning. However, in such a case, the litter rather than the individual pups will be the EU. Each litter will be an “n” of one rather than the number of pups in the litter. In contrast, if pups within a litter can be individually identified and assigned to the treatments, then the pups will be the EUs, and each pup will be an “n” of one. However, in this case although the pups within a litter will tend to be quite similar (e.g., in a character such as weight), there may be large differences between pups having the same treatment but in different litters. It will be necessary to remove these differences between litters in the analysis because otherwise, the power of the experiment to detect treatment effects will be severely reduced.
An additional complication is that litters vary in size, so it may be difficult to obtain a balanced design with equal numbers of animals on each treatment within every litter. As a result, it may even be difficult to calculate treatment means. A numerical example of the analysis of a within-litter experiment illustrating some of these problems is given in the Appendix.
Some litter effects due to the common environment of litter mates may gradually disappear once the animals are weaned and are no longer dependent on milk supply. However, litter effects due to the genetic similarity of full sibs will remain for the life of the animals, assuming studies are performed using genetically heterogeneous animals such as Sprague-Dawley rats or any breed of rabbits.
Cross-fostering soon after birth may reduce but will not entirely eliminate litter effects. For example, cross-fostering did not eliminate a litter effect associated with susceptibility to dental caries ( Peeling and Looker 1987 ), a highly inherited character, in outbred Sprague-Dawley rats, or an effect on growth rate ( Raubertas et al. 1999 ). Standardization of postnatal litter size is a common practice and is likely to reduce, but not eliminate, between-litter variability associated with maternal effects such as limitations in milk yield. One commercial company pooled all 2-day-old Sprague-Dawley pups and made up single sex litters of 12 young. Most female pups were discarded at this age because demand was almost entirely for males. Females left without a litter were returned to the breeding colony where they soon became pregnant again without any apparent problems ( Lane-Peter et al. 1968 ). Such a procedure will reduce but not eliminate litter effects because females will still differ in milk yield. It may increase the variability within a litter because individuals will no longer be full siblings, and the procedure is likely to be practical only in breeding colonies where large numbers of females litter at the same time. Nevertheless, it may be worth investigating for neonatal research because it would be very convenient for all litters to have the same number of pups.
Requirements for a Well-designed Experiment
The principles of good experimental design have been known for many years ( Cox 1958 ). These principles are described very briefly as follows.
Absence of bias must be ensured through the use of the use of randomization and blinding. Animals must be selected and assigned to the treatment groups in such a way that there is no systematic difference among groups before starting or during the conduct of the experiment. These factors may be mistaken for the effects of the treatment. This goal is usually achieved by assigning animals (or other experimental subjects) to the treatment groups using a formal randomization system. Subsequent housing and necessary measurements should be in random order. Randomization distributes uncontrolled variation among the groups with equal probability.
The exact method of randomization depends on the design of the experiment. In the most simple “completely randomized” design (i.e., in a between-litter experiment), subjects (e.g., pregnant females) are simply assigned to treatments regardless of their characteristics. Thus, if a teratology experiment involves 20 treated and 20 control pregnant rats, 20 bits of paper could have the letter “C” and 20 the letter “T” written on them. These would be placed in a receptacle and thoroughly shaken. A piece of paper would then be withdrawn, and the first rat would be assigned to the indicated treatment. This process would be repeated with all of the remaining rats. When the neonate is to be the EU in a within-litter experiment, randomization must be done separately within each litter. Again, it is possible to use physical randomization, tables of random numbers, or random numbers generated by a computer.
Ideally, subjects should be identified by codes so that the investigator and other staff members are blind with respect to the treatment groups to the extent possible. Blinding is likely to be particularly important when there is a subjective element to recording observations (e.g., when reading and scoring histological preparations). It would be very unacceptable, for example, to score, measure, or record data from all of the controls first, and subsequently from each treatment group, because standards may change as the scorer becomes more expert. Thus, all manipulations and recording of information should be done either in random order or in such a way as to take account of any time trends with treatment groups equally represented at each time point.
A powerful experiment is one that has a high probability of detecting a difference between treatment groups, assuming that a difference exists. Power depends on the relationship between the variability of the experimental subjects, the size of the treatment effect, and the sample size (discussed in more detail below). Large experiments are likely to be expensive and may exceed the available resources of a facility, so it is worth spending some time and effort to choose uniform experimental material that is sensitive to the effects of the treatment. Thus, if the experimental subjects are adult animals (as in a teratogenesis experiment), they should be closely matched for age, weight, genotype (e.g., by using an isogenic strain where practical), and previous history.
Choosing the Strain or Breed
There are many different strains of mice ( www.informatics.jax.org ) and rats ( www.rgdb.mcw.org ) as well as several breeds of rabbits, dogs, and other species. It may be possible to choose one or more strains that are sensitive to the proposed treatments, although for the larger species it is usually necessary to use whatever is available.
Isogenic strains (inbred strains and F1 hybrids between two such strains) of mice and rats are widely available and have many useful properties ( Beck et al. 2000 ; Festing 1999a , b ; Festing and Fisher 2000 ). They resemble immortal clones of genetically identical individuals in some respects. Tissue and organ grafts between individuals of the same isogenic strain are not immunologically rejected and therefore such strains could be of particular value for studies involving such procedures.
Isogenic strains remain genetically constant for many generations and have an international distribution, so that work involving the same strains can be replicated throughout the world. A single individual can be genotyped at loci of interest, which will serve to genotype all animals of that strain. Thus, a genetic profile of the genes present in each strain can be built up by all investigators working on that strain. The genetic authenticity of the animals can be tested using a small sample of DNA. Each strain has a unique set of characteristics, which may make a particular strain valuable for a particular type of study. Some care must be taken in interpreting results if a single inbred strain is used because it represents only a single genotype. However, the interpretation of results is also not easy when using an outbred stock because generally little is known about its genotype.
One disadvantage of inbred strains for neonatal research is that they often have a poor breeding performance, which may limit their use. When the individual neonate is the EU (in a within-litter experiment), it may be worth using inbred mothers mated to a male of a different inbred strain. The pups will then be F1 hybrids, which are vigorous and uniform. Litter size is about 30% larger than when pure isogenic strains are used. When the mother is the EU, it may be worthwhile to use F1 hybrids, which breed exceptionally well as a result of hybrid vigor ( Festing 1976 ). The sire could be either another F1 hybrid of the same strain, in which case the pups will be genetically heterogeneous F2 hybrids, or the females could be backcrossed to one of her parental strains so that the pups would be backcross individuals that, although genetically heterogeneous, are less variable than F2 hybrids.
Outbred stocks such as Sprague-Dawley or Wistar rats and Swiss mice are used widely, but the scientific case for doing to is questionable ( Festing 1999b ). Animals from different breeders will be genetically different even though they may have the same name. The genotype of any individual will be unknown, the stock is subject to genetic drift over a period of time, the actual degree of genetic heterogeneity is usually unknown, and few methods of genetic quality control are available. It is not even possible to distinguish genetically between Wistar and Sprague-Dawley rats ( Festing 1999b ). Thus, it is necessary to balance the advantage of better breeding performance against these disadvantages.
Designing the Experiment
After choosing the EU (the female, and/or litter, or individual neonate), it is necessary to determine the number and types of treatment. It may be useful to perform a small pilot study to define dose levels and clarify logistics. It may be necessary to study male and female neonates separately, in which case a factorial design including both sexes in the one experiment may be appropriate (see below, Increasing the Range of Applicability). Outcomes (characters) to be measured or counted must be decided. Where measurements are possible, they are frequently more precise than a “count” (number of positive/negative), and greater precision requires fewer EUs. Each neonate may provide several numerical observations. For example, one should give thought to methods of analyzing individual growth curves within an overall analysis. A microarray experiment may result in thousands of observations from each individual, so the method of statistical analysis of the resulting data should always be considered at this design stage.
Determining Sample Size
The usual way of estimating sample size is to use a power analysis. The success of using this tool depends on a mathematical relationship between several variables, as shown in Figure 1 . However, a serious limitation of this method is that it depends critically on the estimate of the standard deviation. This value is not available because the experiment has not yet been done, so it must be estimated from a previous experiment or from the literature. Unfortunately, because standard deviations can vary substantially between different experiments, the power calculations can provide only an indication of the appropriate size of an experiment. This should be interpreted with common sense and in relation to available facilities.

The variables involved in a power analysis for a two-sample t-test. Usually the effect size of interest, the significance level, sidedness of the test, variablilty of the material and power are specified, which determines the required sample size. Alternatively, if the sample size is fixed due to resource limitations, the method can be used to assess power or effect size.
It is easiest to describe the method for a character where there is a treated and control group with a measurement outcome that can be analyzed using an unpaired t-test, such as a teratology experiment with two treatment groups, treated and control. Six variables are involved. Usually the significance level and sidedness of the test are specified, (often the significance level “α” is set at 0.05 with a two-sided test) and the variability of the material (i.e., standard deviation) is taken from a previous study or the literature. When the neonate is the EU, it is necessary to estimate the standard deviation from the pooled standard deviations within litters and treatment groups. The effect size is the minimum difference in means between the two groups the investigator considers to be of biological or clinical importance. Somewhat arbitrarily, the power (i.e., chance that the study will find a statistically significant effect of the specified size) is usually set somewhere between 80 and 95%. It is then possible to estimate the required sample size.
For the calculations, a number of dedicated computer programs such as nQuery Advisor ( Elashoff 2000 ) are available. In addition, many statistical packages such as MINITAB have routines for power analysis, and there are a number of free sites on the web (e.g., http://www.biomath.info ), where one can enter data to obtain estimates of required sample sizes. In some circumstances, such as when resources are limited, the sample size may be fixed and the power analysis can then be used to estimate the power of the proposed experiment (i.e., the chance that the specified effect is likely to be detected). The calculations are similar for a binary variable (normal/abnormal) with two groups, but the specification becomes more difficult when there are several treatment groups, or when the data are not appropriate for a parametric analysis ( Dell et al. 2002 ).
An alternative method of sample size determination is the so-called “resource equation method,” which depends on the law of diminishing returns. This method is useful for small and complex biological experiments that involve several treatment groups for which the results are to be analyzed using the analysis of variance. In such a situation, it is difficult to use a power analysis. The experiment should be of an appropriate size if the error degrees of freedom in an analysis of variance are somewhere between 10 and 20 ( Festing et al. 2002 ; Mead 1988 ). This case reduces to the very simple equation:
X = N – T – B + 1,
where N is the total number of observations, T is the number of treatments, B is the number of blocks (litters for a within-litter experiment), and X should be between approximately 10 and 20.
For a within-litter experiment with three treatments, an average litter size of six, and a proposal to use five litters,
X = (6 × 5) – 3 – 5 + 1 =23.
The limits of X being between 10 and 20 can be liberally interpreted, so this proposed experiment would be of an appropriate size, although just beyond the suggested upper limit.
The experiment described in the Appendix has X = 50, which is more than twice as large as suggested by this method. A repeated analysis of the data in the Appendix using only the first three litters gives X = 23 and a p value for treatments of 0.007 compared with p = 0.001 using six litters. Thus, if the experiment had been performed with approximately half the number of animals, the conclusions would have been about the same. Compared with the power analysis, the resource equation method is somewhat crude. Nevertheless, it often seems to work in practice, particularly when relatively large treatment effects are expected.
Increasing the Range of Applicability: Factorial Designs
It is often important to know the extent to which a response to a treatment can be generalized. Is the same response found in males and females, or in different strains of animals, or with different diets? Does the presence of some drug or chemical alter response? Factorial experimental designs allow such questions to be examined without requiring any substantial increase in resources. A typical example might be to learn whether alcohol potentiates the effect of a teratogen in rats. If, for example, the basic plan was to have 20 pregnant females as controls and 20 treated with the teratogen, then the effect of alcohol might be studied by administering alcohol to half the rats in each group. There would then be four groups of 10 pregnant females with or without alcohol and with or without the teratogen. It first seems as though the group size has been reduced from two to 10 rats, but in fact the effect of the teratogen is still determined by comparing those receiving the teratogen (20 rats) and those that do not receive it (20 rats). Similarly, the effect of the alcohol is determined by comparing the 20 rats that receive it with the 20 rats that do not receive it. Finally, any potentiating effect of alcohol is determined by seeing whether the difference in fetal weight, number of abnormalities, and other factors between the teratogen-treated and -untreated rats is greater in the group receiving alcohol than in those that do not receive it.
Factorial designs can also be used for within-litter experiments. Pups could be sexed and assigned separately at random to either a control or a treated group. There would then be four groups within each litter: male and female controls and male and female treated. The experiment could then be analyzed (probably using an analysis of variance) to determine whether the pups responded to the treatment, averaging across sexes; whether the measured outcome (e.g., weaning weight) differed between males and females, averaging across treatments; and whether the response to the treatment differed between the two sexes.
Factorial designs provide a way of obtaining more information from the same scientific resources at relatively little extra cost. Any number of factors (e.g., treatments, strain, sex, diet) can be involved, and each can have any number of levels (i.e., there can be any number of dose levels within a factor). The main extra cost is the increase in the complexity of the experiment, which could lead to mistakes, and the increased complexity of the statistical analysis. Splitting groups into a number of subgroups does not lead to any substantial loss of power, provided the experiment is not too small.
Avoiding Excessive Complexity
Complex experiments may lead to mistakes and invalid conclusions. All experiments should be planned ahead, with written protocols and standard operating procedures. It is appropriate to alter experiments while they are in progress only in exceptional cases (e.g., for ethical reasons). Animal care staff should be regarded as integral and valued members of the research team. If mistakes occur, it is vital to acknowledge them, rather than covered them up, so that staff members are not made to fear that they will be in serious trouble if they make a mistake.
No experiment should be started without the investigator having a clear idea of how the results will be analyzed statistically, although it may be necessary to modify the analysis later in the light of actual results. For example, it may be necessary to transform scales and to account for missing observations. However, the statistical analysis is a basic and integral part of the experimental design. Moreover, time (i.e., avoiding delay) is important. Normally, it is important to analyze experiments as soon as they have been completed so that the results can be used in formulating future experiments (e.g., adjusting dose levels or altering the timing of observations in subsequent experiments).
The aim of the statistical analysis is to obtain summarized results that may be easily understood and that clarify the range of uncertainty in the conclusions. Access to a good statistical textbook is highly recommended. A basic assumption is that the EUs are a random sample from a population of such units (real or hypothetical), and the aim is to make inferences about the population from the sample. The accuracy of these inferences will depend mainly on the biological variability of the EUs and the sample size, assuming that the experiment has been designed well to avoid bias. Clearly, if the sample size is very small and/or the variation is large, then only rough estimates of the population characteristics will be available.
It is essential to use a good-quality statistical package. Spread sheets such as EXCEL are adequate for storing and manipulating the raw data, but they should not be used for the main statistical analysis. The output is often not standard, and it fails to provide the range of methods available in a dedicated package. For example, the statistical analysis presented in the Appendix could not be done using EXCEL. Packages such as SPSS, MINITAB, SAS, Statistika, Graphpad, GLIM, Genstat, and BMDP are readily available and have been tested thoroughly for errors. One or more are usually available on most institutional networks.
The first step in the analysis should be to screen the data for errors. Histograms and dotplots showing individual observations (e.g., as in Figure 2 in the Appendix), possibly plotted against dose levels, or plots of two outcomes likely to be correlated will often show whether there are any serious outliers. Any outliers should be individually checked against notebooks or original printouts to ensure that they are not transcription errors, and should be corrected if necessary. Outliers that appear to be valid should not be discarded at this stage. Many outcomes of measurement data, particularly concentrations of a substance, have a log-normal distribution, with most numbers being relatively low but with a few very high. If this is the case, the data can be transformed by taking logarithms or square roots of the raw observations. This step frequently removes outliers and allows parametric statistical methods—usually a t test or an analysis of variance (ANOVA 1 )—to be used in the analysis. These parametric methods depend on the assumption that the residuals (deviations of each observation from group means) have a normal distribution and the variation is approximately the same in each group.
One way to deal with one or two persistent outliers is to perform the statistical analysis with and without them. If it makes no difference to the conclusions, then they can be retained. However, if the conclusions depend entirely on one or a few outliers, and these appear to be perfectly valid data points, the results should be treated with caution. Outliers that are more than 3 standard deviations from the mean (assuming an approximately normal distribution) are automatically rejected by some authors; but again, it may be worth seeing what effect the outliers have on the overall conclusions.
When it is not possible to normalize badly skewed data using a scale transformation, and when the aim is to compare groups, it may be necessary to analyze the data using nonparametric methods such as the Mann-Whitney or Wilcoxon test. Dose response curves are normally estimated using some form of regression analysis. A numerical example illustrating the statistical analysis of a within-litter experiment using the analysis of variance is shown in the Appendix.
Scientific papers are often written in such a way as almost to observe exactly what the investigators did. In theory, sufficient information should be given so that others can repeat the studies. Unfortunately, in a surprisingly large proportion of papers, it is difficult or impossible to determine exactly how many animals were used, or how many separate experiments were involved.
Guidelines are available for the design and statistical analysis of experiments using animals (e.g., Festing and Altman 2002 ), and they include a number of suggestions for presenting results.
Label and number each experiment;
State the number of animals used in each experiment, along with the purpose of each experiment;
Identify the species, breed, and/or strain of animals complying with agreed international nomenclature rules where these are available (e.g., for rats and mice, WWW.informatics.jax.org );
Provide details of husbandry (e.g., diet and housing) to the extent allowed by the journal editor;
Describe efforts where possible to minimize pain, distress, or lasting harm to the animals;
Describe methods of statistical analysis, with references in the case of any unusual methods used;
Identify the statistical software used;
Avoid excess decimal places where means, proportions, or differences are presented;
Include measures of variation (e.g., standard deviations, standard errors, or, preferably, confidence intervals [ Altman 1991 ; Altman et al. 2000 ]);
Identify the number of observations for every mean, including those shown graphically. It is not adequate to make statements such as “the number in each group ranged from four to 10.” Where possible, tabulate means in columns for ease of comparison.
Use graphs to illustrate points that are difficult to show in tables or in the text. Where possible, show individual observations rather than means with error bars because this presentation more clearly indicates the distribution of the observations. If error bars are used, explain clearly whether they are standard deviations, standard error, or confidence intervals.
Again, the main aim in presenting the results should be to state as clearly and succinctly as possible exactly what was done and what results were obtained.
Consider the weaning weight of 59 unsexed Sprague-Dawley rats (real data), including one that died as a missing observation ( Table 1 ). When pups were 2 days old, each litter was split, and the pups were assigned at random to a control group, a “low-dose” group, or a “high-dose” group (simulated by subtracting 0.5 g from the low-dose group and 1.0 g from the high-dose group). Within each litter, to the extent possible, the same number of pups were assigned to each treatment, and pups were individually marked for subsequent identification. The sex of the pups was not recorded. The aim of the statistical analysis is to determine whether the treatments altered weaning weight, and if so to what extent. (Note: It should be a reduction of approximately 0.5 g and 1.0 g in the low and high groups, respectively.)
Data for the numerical example. The table shows weaning weight (g) of six litters of Sprague-Dawley rats assigned to three treatments: control, low, and high doses. Weights are real data, but treatments are simulated (see text).
X, missing observation due to death of animal.
(1) Mean of litter by treatment means. These means are biased (see 4, below).
(2) Mean of all animals in a treatment group, ignoring litter. These means are biased (see 4, below).
(3) Differences between least squares means give the best unbiased estimate of the treatment differences.
(4) Numbers in parenthesis show the size of the treatment effect (control mean-dose mean) estimated from these means. The least squares means give the best unbiased estimate of the size of the treatment effect.
The first step in analyzing such data is to examine it graphically to learn whether there are any obvious outliers and to obtain a visual impression of the situation (see plot in Figure 2 ). In this case, there are no obvious outliers. However, the litter effect is very obvious and clearly there is considerable variation within each litter. Although there is a tendency for the controls to weigh more than the treated groups (e.g., in litter 6), in litter 2 the lightest pup is a control.

Weaning weight by litter number and treatment for the numerical example. Note that some random variation or “jitter” has been applied on the X-axis to avoid too much overlap between points (see text for details).
Anyone planning to make a career in animal research is strongly advised to familiarize him- or herself with the analysis of variance as it is the most appropriate statistical method for dealing with most data arising from formal experiments like this one. A good introduction to the methods is given by Roberts and Russo (1999) , and it is also described in detail in most statistical textbooks.
The data in Table 1 can be analyzed using a two-way (treatment and litter) analysis of variance “without interaction.” A t test would be entirely inappropriate because there are more than two groups, and it is necessary to account for the litter effect. The ANOVA quantifies the variation associated with treatments, litters, and the remaining “residual” or “error” variation. It is assumed that the response is the same in each litter apart from sampling variation (hence “without interaction”). However, there is a problem with these data as they stand. The usual two-way ANOVA assumes that there are equal numbers in each treatment group within each litter. In this case, there is one missing observation in litter 2 and two extra animals in litters 5 (high-dose) and 6 (control). The data could be adjusted by discarding at random two animals from groups where there are the extras, and replacing the value for the animal that died by an appropriate value. Missing values can be worked out using formulae available in most of the older textbooks (e.g., Cochran and Cox 1957 ). In situations where there is more than one animal in a litter by treatment subgroup, as in this case, it would probably be sufficiently accurate (although not strictly correct) to replace the missing value with the mean of the rest of the animals in the group. Having a balanced design used to be almost essential because otherwise the calculations were extremely tedious. However, modern statistical packages now make it possible to do a “general linear model” ANOVA, which is capable of accommodating unequal numbers in each group, so a balanced design is no longer so essential.
A general linear model ANOVA of the data in Table 1 is shown in Table 2 . Note that whereas in the normal ANOVA there is a heading labeled “Sums of Squares” (or simply SS), in this case there are two headings “Seq SS” and “Adj SS,” with the two being slightly different for the litter effect. The ANOVA shows an F value of 7.99 and a p value of 0.001 for the treatment effect (abbreviated Trt). The “least squares means” presented in Table 2 are marginally different from the simple means and weighted means presented in Table 1 (all three types of means are shown in Table 1 ) inasmuch as they take account of the unequal group sizes.
General linear model analysis of variance of the data in Table 1
Wt, weight; SS, sums of squares; DF, degrees of freedom; Seq, sequential; Adj, adjusted; F, variance ratio (a test statistic like Student's t); Trt, treatment; SE, standard error; p, probability that a difference as large as or larger than the one observed could have arisen by chance; T-value, Student's t.
It is often necessary to use a post hoc comparison to determine which means differ from which. When the aim is to compare the means of the treatment groups with the control, Dunnett's test is appropriate (shown in Table 2 ). If the aim is to compare each mean with every other mean, it is appropriate to use other available post hoc comparisons (e.g., Tukey's test [ Roberts and Russo 1999 ]). Dunnett's test subtracts the mean of the control group from each of the other groups and then either gives a 95% confidence interval (CI 1 ) for the difference, or involves a t test to resolve whether it is different from zero. Both approaches are shown in the case. Note that the differences between the three groups are larger than the simulated treatment effect of -0.5 g and -1.0 g in the low- and high-dose groups, respectively, because the groups already differed by chance.
In this case, 95% CIs for the means should be calculated by hand. The error mean square of 5.26 is the pooled within-group variance, so the standard deviation is the square root of this value, or 2.29. Standard errors are calculated by dividing 2.29 by the square root of the number in each mean (19 in the control and low-dose group, 20 in the high-dose group). The 95% CI is estimated from the formulae given below (also shown in most statistical text books):
M- SE*t 0.05,d.f. < M < M + SE*t 0.05,d.f.,
where M is the observed mean, the SE is the standard error of the mean, and t 0.05,d.f. is the value of the Student's t for the 0.05 level of significance for the degrees of used in estimating the variance, which is 50 ( Table 2 ). The means can now be presented as follows:
Control mean = 48.1 (95% CI 47.0, 49.1);
Low-dose mean = 46.2 (95% CI 45.1, 47.2);
High-dose mean = 45.2 (95% CI 44.1, 46.2).
These confidence intervals could be used as error bars in a bar diagram.
Finally, if one performed a similar experiment, but treated whole litters rather than doing a within-litter experiment, the EU would be the litter, rather than the individual pup within the litter. To determine how many litters would be needed, assume for simplicity that there would be only a control and a high-dose group. The question can be addressed using a power analysis as described above. The standard deviation of litter means in Table 1 is 5.68 g.
If one decided that a treatment effect (difference between treated and control groups) of 4 g in mean pup weight would be of scientific interest, and the experiment should have a 90% power and a significance level of 0.05, with a two-sided t-test, then using the power calculator in MINITAB, 44 litters in each group would be required to perform this experiment. Thus, the between-litter experiment would involve a total of 88 litters and at an average of 9.7 pups per litter over 850 pups, yet would only be capable of distinguishing an effect of 4.0 g compared with a resolution of 2.9 g in the within-litter experiment involving six litters and only 59 pups. Clearly, between-litter designs should only be used in situations where there is no alternative, such as in teratology experiments.
Altman DG . 1991 . Practical Statistics for Medical Research . London : Chapman and Hall .
Altman DG Machin D Bryant TN Gardiner MJ . 2000 . Statistics with Confidence . London : BMJ Press .
Beck JA Lloyd S Hafezparast M Lennon-Pierce M Eppig JT Festing MFW Fisher EMC . 2000 . Genealogies of mouse inbred strains. Nat Genet 24 : 23 – 25 .
Google Scholar
Cochran WG Cox GM . 1957 . Experimental Designs . New York : John Wiley & Sons, Inc .
Cox DR . 1958 . Planning Experiments . New York : John Wiley & Sons .
Dell R Holleran S Ramakrishnan R . 2002 . Sample size determination. ILAR J 43 : 207 – 213 .
Elashoff JD . 2000 . nQuery Advisor Version 4 .0 User's Guide. Cork : Statistical Solutions .
Festing MFW . 1976 . Effects of marginal malnutrition on the breeding performance of inbred and F1 hybrid mice-a diallel study. In: Antikatzides T ed. The Laboratory Animal in the Study of Reproduction . Stuttgart : Gustav Fischer . p 99 – 114 .
Google Preview
Festing MFW . 1999 . Introduction to laboratory animal genetics . In: Poole T ed. The UFAW Handbook on the Care and Use of Laboratory Animals . Harlow : Longman Scientific and Technical . p 61 – 94 .
Festing MFW . 1999 . Warning: The use of genetically heterogeneous mice may seriously damage your research. Neurobiol Aging 20 : 237 – 244 .
Festing MFW . 2004 . Is the use of animals in biomedical research still necessary in 2002? Unfortunately, “yes.” Altern Anim Res 32 (S1) : 733 – 739 .
Festing MFW Altman DG . 2002 . Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 43 : 233 – 243 .
Festing MFW Fisher EMC . 2000 . Mighty mice. Nature 404 : 815 .
Festing MFW Overend P Gaines Das R Cortina Borja M Berdoy M . 2002 . The Design of Animal Experiments . London : Laboratory Animals Ltd .
Fisher RA . 1960 . The Design of Experiments . New York : Hafner Publishing Company, Inc .
Haseman JK Hogan MD . 1975 . Selection of the experimental unit in teratology studies. Teratology 12 : 165 – 171 .
Hunt DL Bowman D . 2004 . A parametric model for detecting hormetic effects in developmental toxicity studies. Risk Anal 24 : 65 – 72 .
Lane-Peter W Lane-Petter ME Boutwell CW . 1968 . Intensive breeding of rats. I. Crossfostering. Lab Anim 2 : 35 – 39 .
Mead R . 1988 . The Design of Experiments . Cambridge : Cambridge University Press .
Peeling AN Looker T . 1987 . Problem of standardising growth rates for animals suckled in separate litters. Growth 51 : 165 – 169 .
Raubertas RF Davis BA Bowen WH Pearson SK Watson GE . 1999 . Litter effects on caries in rats and implications for experimental design. Caries Res 33 : 164 – 169 .
Roberts MJ Russo R . 1999 . A Student's Guide to the Analysis of Variance . London : Routledge .
Wartofsky MW . 1979 . Models: Representation and the Scientific Understanding . Dordrecht : D. Reidel Publishing Company .
Yamamoto E Yanagimoto T . 1994 . Statistical methods for the beta-binomial model in teratology. Environ Health Perspect 102 (Suppl 1) : 25 – 31 .
Zorrilla EP . 1997 . Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol 30 : 141 – 150 .
Abbreviations used in this article: ANOVA, analysis of variance; 95% CI, 95% confidence interval; EU, experimental unit.
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1930-6180
- Print ISSN 1084-2020
- Copyright © 2024 Institute for Laboratory Animal Research
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, large animal models in regenerative medicine and tissue engineering: to do or not to do.

- 1 Veterm, Department for Companion Animals and Horses, University Equine Hospital, University of Veterinary Medicine Vienna, Vienna, Austria
- 2 Laboratory of Organ Bioengineering and Regenerative Medicine, Health Research Institute of Aragon (IIS Aragon), Zaragoza, Spain
- 3 Department of Veterinary Medicine, Università degli Studi di Milano, Milan, Italy
- 4 Department of Comparative Biomedicine and Food Science, University of Padua, Padua, Italy
- 5 Clinical Unit of Small Animal Surgery, Department for Companion Animals and Horses, University of Veterinary Medicine Vienna, Vienna, Austria
- 6 Department of Clinical Sciences and Services, Royal Veterinary College, Hertfordshire, United Kingdom
- 7 Department of Clinical Sciences of Companion Animals, Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands
Rapid developments in Regenerative Medicine and Tissue Engineering has witnessed an increasing drive toward clinical translation of breakthrough technologies. However, the progression of promising preclinical data to achieve successful clinical market authorisation remains a bottleneck. One hurdle for progress to the clinic is the transition from small animal research to advanced preclinical studies in large animals to test safety and efficacy of products. Notwithstanding this, to draw meaningful and reliable conclusions from animal experiments it is critical that the species and disease model of choice is relevant to answer the research question as well as the clinical problem. Selecting the most appropriate animal model requires in-depth knowledge of specific species and breeds to ascertain the adequacy of the model and outcome measures that closely mirror the clinical situation. Traditional reductionist approaches in animal experiments, which often do not sufficiently reflect the studied disease, are still the norm and can result in a disconnect in outcomes observed between animal studies and clinical trials. To address these concerns a reconsideration in approach will be required. This should include a stepwise approach using in vitro and ex vivo experiments as well as in silico modeling to minimize the need for in vivo studies for screening and early development studies, followed by large animal models which more closely resemble human disease. Naturally occurring, or spontaneous diseases in large animals remain a largely untapped resource, and given the similarities in pathophysiology to humans they not only allow for studying new treatment strategies but also disease etiology and prevention. Naturally occurring disease models, particularly for longer lived large animal species, allow for studying disorders at an age when the disease is most prevalent. As these diseases are usually also a concern in the chosen veterinary species they would be beneficiaries of newly developed therapies. Improved awareness of the progress in animal models is mutually beneficial for animals, researchers, human and veterinary patients. In this overview we describe advantages and disadvantages of various animal models including domesticated and companion animals used in regenerative medicine and tissue engineering to provide an informed choice of disease-relevant animal models.
Introduction
The use of sentient animals for research purposes is a controversial topic, which has raised public and ethical concerns and is criticized by opponents claiming that animal models often do not generate appropriate benefit with regards to their potential risks and harm and as a consequence, are often ethically not permissible. The increasing status of pets as family members and corresponding high level of veterinary care for privately owned pets further amplifies the controversy over the use of animals for research purposes.
However, animal models are still an important and, at a regulatory level, a compulsory component of translational research, which cannot yet be replaced by in vitro experiments. Although in vitro models allow for systematic, standardized analysis of various cellular, biophysical and biochemical cues in a controlled environment, without the natural variability inherent to in vivo animal models, they can only offer an abstract insight into the pathophysiology of diseases and disorders. Therefore, while animal models cannot yet be replaced, the number of animals used should be reduced to a minimum and experiments involving animals should be optimized with regard to their translatability and the welfare of the animals.
However, to date a reductionist approach often using immature laboratory species is commonly employed ( Jackson et al., 2017 ). Small rodent animals, specifically mouse and rat, are valuable for research into mechanisms of disease and fundamental biology, but findings from such small animal models often do not translate into human clinical applications ( Prabhakar, 2012 ; Lorbach et al., 2015 ). Shanks et al. impressively illustrated the translational challenges, showing the difference in bioavailability of pharmaceuticals between humans, primates, dogs and rodents ( Shanks et al., 2009 ). However, although awareness is increasing there is still a massive disproportion between rodent studies and large animal studies.
Therefore, the European Medicines Agency (EMA), the USA Federal Food and Drug Administration (FDA) and the International Society for Stem Cell Research (ISSCR) recommend the use of large animal models to evaluate efficacy, durability, dose response, degradation and safety of advanced therapeutic medicinal products (ATMPs) 1 , 2 . For successful and timely translation from animal models to regulatory approval and clinical application, a step-wise development using laboratory animals for screening and early development work, followed by a large animal model such as the pig, sheep or horse which offers a more realistic approach for late development and pivotal studies would be more appropriate ( Hurtig et al., 2011 ).
Moreover, animals develop many naturally occurring (or spontaneous) diseases that are equivalent to human disease leading to the development of the “One Health One Medicine” concept which presumes that diseases in men and animals (mostly mammals) have similar aetiologies and pathophysiologies and require analogous therapeutic approaches. Hence, human and veterinary medicine can mutually benefit from research that applies a one health approach. Using large animal models with naturally occurring disease with a similar pathophysiology as in humans, allows study of not only new treatment strategies but also disease development and prevention at a relevant age. However, although using naturally occurring disease models best reflect disease complexity, standardization of disease grade and availability of sufficient clinical case numbers for recruitment into studies can be challenging.
In order to achieve the best output while following the three R’s principle (to r educe, r efine and r eplace animal models) of using the smallest possible number of animals, animal models need to be optimized to the greatest possible extent ( Madden et al., 2012 ). They require careful selection and design to ensure they are fit-for-purpose and address both optimal predictive validity, as well as ethical, animal-welfare and societal considerations. Species, anatomic, physiologic, biomechanical aspects and their clinical relevance need to be considered.
Furthermore, knowledge regarding the epidemiology and natural history of diseases in different animal species, disease similarities to humans, availability of diagnostics, treatment options, and outcome measures as well as criteria defining species specific quality of life and functional parameters is important but still scarce in the scientific community. Other important considerations in using large animal models include availability, handling and economic concerns.
To optimize scientific output and translational potential with animal welfare needs, tight cooperation between basic science, human and veterinary medicine is necessary. The veterinary academic environment offers unique expertise to make that goal attainable to the highest standards. This includes the veterinary knowledge required to make a rational decision for the choice of animal model rather than being based on in-house availability.
There are several research groups which have a track record of developing preclinical large animal models, some of which have managed to translate their research into clinical applications ( Kang et al., 2010 , 2013 ; Mcilwraith et al., 2011 , 2012 ; Godwin et al., 2012 ; Smith et al., 2013 ; Bach et al., 2017 ; Whitehouse et al., 2017 ; Goldberg et al., 2018 ; Tellegen et al., 2018 ; Broeckx et al., 2019 ; Tellegen et al., 2019 ). This − by no means exhaustive − list clearly demonstrates the collective efforts of the veterinary community to provide large animal models to be used in translational projects.
However, yet it is still often argued, that translational studies using large animal models are rare because they are complex, time-consuming, technically demanding, slow, and usually not suitable for mechanistic investigations. Nevertheless, because large animals better reflect the human body conformation and pathophysiology of certain naturally occurring diseases than rodent models, these studies are essential justifying the challenges and costs. Unfortunately, the added value of the clinical relevance of large animal models is often not appreciated by reviewers of manuscripts and grant applications are assigned low scores on the basis of lack of mechanistic insights and insufficient conceptual novelty. However, for a successful translation of tissue engineering and regenerative medicine research into clinical therapies, it is critical that this misperception is corrected.
It is the authors’ hope that this review, which introduces different large animal models, their naturally occurring diseases and their specificities, may stimulate biomedical researchers to look for the very best model possible for their specific research question and that it will encourage interdisciplinary cooperation to optimize the choice of disease-relevant animal models in the future. Deciding which animal model should be used in a particular study is first and foremost dependent on defining the specific question that needs to be answered. Only then can the pertinent benefits and drawbacks of individual models be considered and a decision made.
In this review, we focus on horses, sheep, dogs, cats and pigs as the most frequently used large animal models in research and do not include primates due to the ethical dimension and limited indications, which require their specific use. Using animals which are so similar to humans, raises serious ethical concerns. Therefore, the use of non-human primates is closely monitored and strictly regulated and much has been done to specifically safeguard these animals. The use of great apes has been completely prohibited. As long as non-human primates are used for medical research, the European Commission strongly advocates the “3Rs principle,” now a legal obligation embedded in the EU legislation to: Replace non-human primates with viable alternatives whenever feasible, Reduce the use of non-human primates and Refine scientific procedures and the care and treatment of the animals. Even phasing-out the use of non-human primates in Europe is discussed 3 .
Why the Choice of Animal Models Is Crucial
The most obvious and demonstrative reason why the choice of animal models is crucial, are gross anatomic differences between the human and different animals and even between animals of different species ( Figure 1 ). These differences imply that the same anatomic structures may have a different function and are subjected to different biomechanical strains. Table 1 illustrates differences of different animals to emphasize the importance of correct model selection with respect to species’ physiologic aspects.
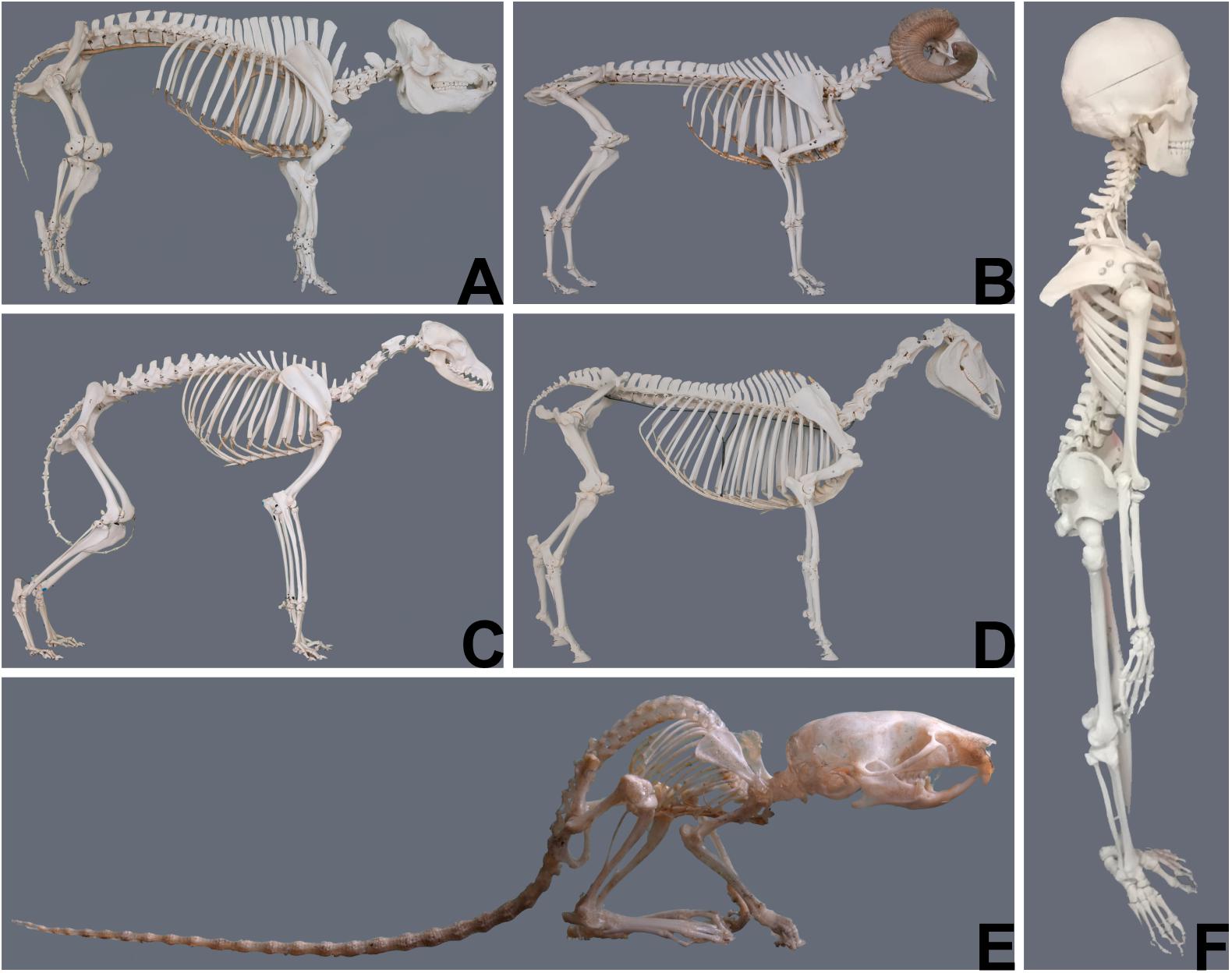
Figure 1. Gross anatomical differences between animals of different species (courtesy of Niklas Dresen, Institute of veterinary anatomy, University Leipzig) and the human (courtesy of Elfriede Cremer, Bernhard Cremer and Elisabeth Schieder). (A) Pig; (B) Sheep; (C) Dog; (D) Horse; (E) Mouse; (F) Human.
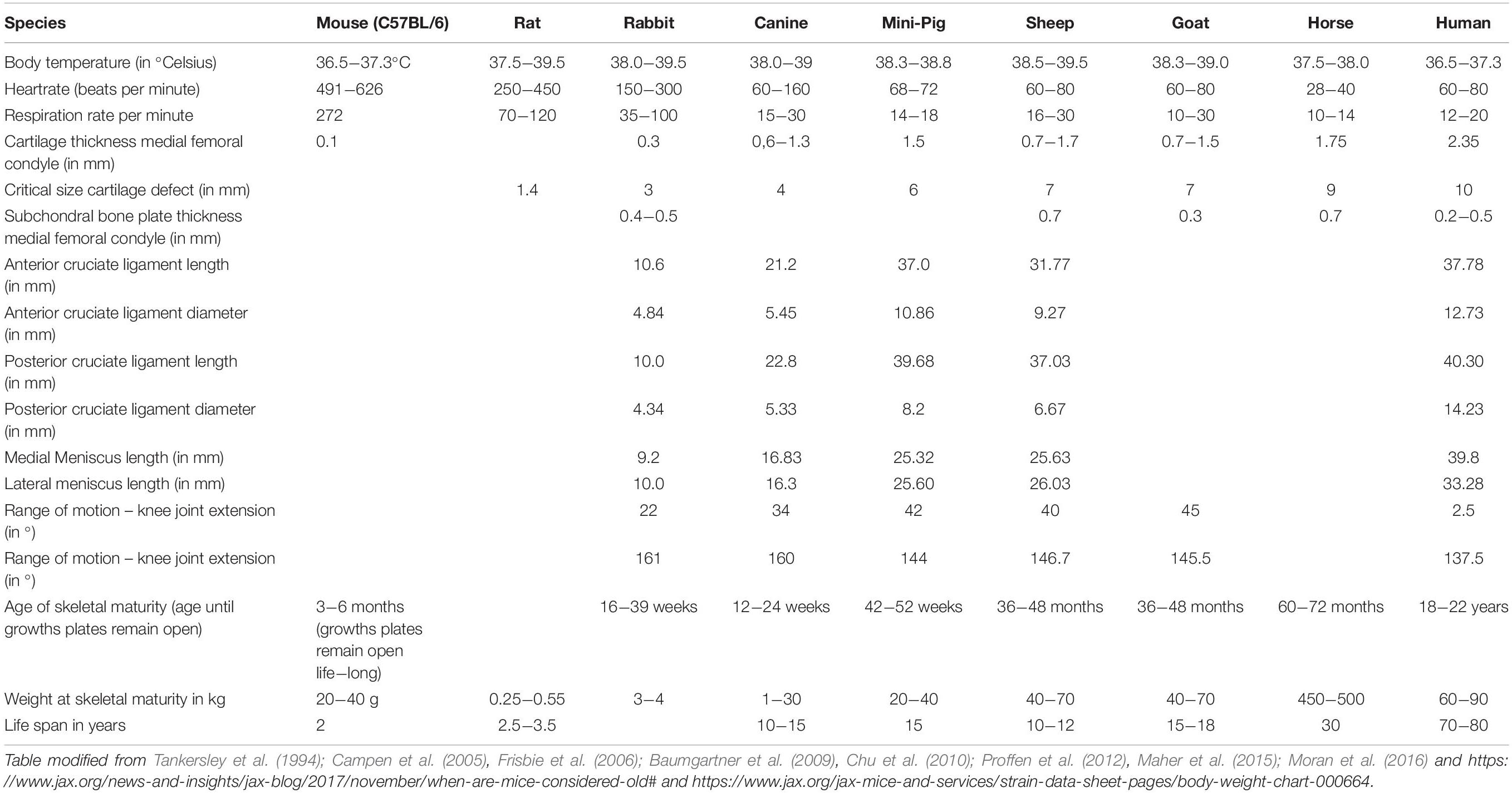
Table 1. Comparison of physiologic and biomechanical parameters of different model animals.
Age Matters
Age should be an important consideration in the choice of any animal model independent of the species used ( Jackson et al., 2017 ). For practical and organizational reasons animal trials are often carried out in juvenile or neonatal animals. However, differences in the healing potential and therefore the healing response between juvenile and adult animals can bias the outcome of such trials ( Namba et al., 1998 ; Beredjiklian et al., 2003 ; Conboy et al., 2005 ; Favata et al., 2006 ; Bos et al., 2008 ; Ansorge et al., 2012 ; Connizzo et al., 2013 ; Mienaltowski et al., 2016 ; Van Weeren and Back, 2016 ; Jackson et al., 2017 ). The use of skeletally mature animals of an appropriate age ( Table 1 ) to mimic adult disease and healing potential is therefore a critical consideration for optimal study design. To truly reflect human age-related disease, the animals used should be of comparable age. Ideally old animals would be used to study age-related diseases as for instance senile osteoporosis. However, the use of elderly compared to immature or young adult animals requires specific considerations, as aged animals are more difficult to procure and may suffer from comorbidities. Hence, potential animal loss due to other diseases needs to be accounted for in the study design and financial planning. Furthermore, the predisposition for age-related diseases varies between species.
Naturally Occurring and Generated Models of Genetic Disease
Many naturally occurring genetic diseases have been identified in companion and farm animals which are often caused by a mutation in an orthologous gene and lead to a comparable clinical phenotype as observed in human patients, including the pathological alterations at the biochemical and cellular levels ( Lairmore and Khanna, 2014 ; Kol et al., 2015 ). Most of these animal models are associated with congenital heart disease, lysosomal storage disease, hemophilia, muscular dystrophies, neurological disorders, immunodeficiencies and dwarfism ( Lairmore and Khanna, 2014 ). Information about these naturally occurring genetic diseases in animals was compiled in a comprehensive database − Online Mendelian Inheritance in Animal − created by Prof. Frank Nicholas at The University of Sydney and Australian National Genomic Information Service 4 .
Naturally Occurring and Generated Models of Musculoskeletal Disease
Osteoarthritis (OA) is a heterogeneous disease for which no single animal model perfectly recapitulates the complex etiology and clinical manifestations of the human disease ( Aigner et al., 2010 ; Cohen-Solal et al., 2012 ; Little and Zaki, 2012 ; Mccoy, 2015 ). Currently available OA models are generally grouped into spontaneous, or surgically induced models. Spontaneous models include naturally occurring disease or genetically manipulated models, whereas surgically induced models employ (i) destabilization of the joint such as partial or total meniscectomy, meniscal tear, anterior cruciate ligament or posterior cruciate ligament transection, medial and/or lateral collateral ligament transection or osteotomy (ii) physical defects of the articular cartilage such as creation of articular grooves, (iii) impact trauma including transarticular impact, and intra-articular osteochondral fragmentation (iv) chemically induced lesions using intra-articular injection of monosodium iodoacetate, collagenase, carrageenan or Freund adjuvant ( Bentley, 1975 ; Little and Zaki, 2012 ; Lampropoulou-Adamidou et al., 2014 ; Mccoy, 2015 ). Spontaneous models that develop progressive and chronic disease are likely to more closely mimic idiopathic OA. However, these models take longer to develop and tend to be more variable with respect to outcome measures ( Vincent et al., 2012 ; Teeple et al., 2013 ; Lampropoulou-Adamidou et al., 2014 ; Mccoy, 2015 ).
Surgical models have the advantage of repeatability and reproducibility as well as rapid onset and progression ( Lampropoulou-Adamidou et al., 2014 ), but for that reason are less ideal models of spontaneous OA and are often regarded as posttraumatic (secondary) OA ( Bendele, 2001 ; Little and Hunter, 2013 ; Teeple et al., 2013 ; Mccoy, 2015 ).
The validity of chemically induced models for OA has been questioned ( Poole et al., 2010 ; Teeple et al., 2013 ) due to the resulting widespread cell death and rapid joint destruction, which are not considered typical for either spontaneous or posttraumatic OA ( Little and Zaki, 2012 ).
Animal models are further widely used in osteoporosis research. They include, among others, models for disuse induced osteoporosis, glucocorticoid-induced osteoporosis and postmenopausal osteoporosis. The most popular animal models of postmenopausal osteoporosis are those generated in the mouse, rat, sheep, and nonhuman primates by ovariectomy ( Iwaniec, 2008 ).
The choice of the animal models differ markedly, depending on the objectives of the study. It has to be noted that rodents for example are of limited value for investigating intra−cortical bone remodeling, because they lack true Haversian cortical bone remodeling under physiological conditions due to their small weight ( Baron et al., 1984 ; Lelovas et al., 2008 ; Iwaniec, 2008 ). Larger animals such as dogs are more appropriate for these studies because, similar to humans, dogs have well-developed Haversian remodeling ( Iwaniec, 2008 ).
Challenges of Translating Results From Animal Models to Human Patients – An Example
To date, animal models of human asthma have included: Drosophila, rats, guinea pigs, cats, dogs, pigs, cattle, sheep, horses and primates, but the most widely used model is the mouse ( Zosky and Sly, 2007 ; Kirschvink and Reinhold, 2008 ; Shapiro, 2008 ; Blume and Davies, 2013 ). The mouse is a useful model due to the availability of specific probes and reagents for studying allergic outcomes, such as cellular and humoral responses, and the good adaptability for genetic manipulation ( Shapiro, 2008 ; Bonamichi-Santos et al., 2015 ). Nevertheless, this model has some limitations for translational medicine mainly related to the anatomical and physiological differences with respect to man. Obviously, the lung and bronchial tree, total lung capacity (6 liter for man vs. 1 ml for mouse), and the blood-gas barrier thickness (0.62 μm vs. 0.32 μm) are much smaller than in man and the bronchial artery supplies the entire lung in man but is absent in the pleura, septa and alveoli in mice. In addition, the respiratory rate, or beats per minute (10−14 vs. 250−350), is very different in people and mice. Moreover, the mouse lacks sub-mucosal glands and has limited airway smooth muscles compared to man ( Lange-Consiglio et al., 2019 ). In view of these important differences, the pre-clinical results obtained when using a mouse model for asthma should be interpreted with care. Furthermore, the mouse does not have natural inflammatory or allergic pulmonary pathologies, so airway inflammation is usually induced by exposure to ovalbumin (OVA) or other aeroallergens. In contrast to naturally occurring human asthma, which is a chronic disease characterized by persistent inflammation and remodeling due to intermittent or continuous inhalation exposure to allergens resulting in chronic eosinophilic/neutrophilic inflammation ( Aun et al., 2017 ; Bullone and Lavoie, 2019 ), the mouse model shows more acute (<3 months) inflammation and no remodeling. To circumvent this problem, systemic sensitization protocols and repeated exposures to allergens have been tried but the results obtained from different routes of systemic sensitization (subcutaneous injection, intraperitoneal injection or intranasal inhalation) and different allergens (OVA, fungi, Ascaris antigens, house dust mite, cockroach extracts), used alone or in combination, are difficult to compare and to interpret ( Aun et al., 2017 ). For example, in the mouse the induced inflammation profile, although dependent on the antigen, is mainly Th2, mirroring disease in only a subsection of human asthmatics who are Th2 and/or Th1/Th17 ( Douwes et al., 2002 ; Woodruff et al., 2009 ). Another criticism of the mouse model is that OVA does not induce asthma in human patients and the sensitization routes do not mimic the routes of exposure to allergens in human asthma ( Aun et al., 2017 ). Hence, differences in the results may be due to the different types of allergens and sensitization routes.
An appropriate animal model for translational studies should mimic the pathological changes associated with human asthma and reflect the environmental factors that determine the evolution of human asthma.
Horses as Animal Models
General considerations.
Horses ( equus caballus ) are a well-accepted, well-established and clinically relevant animal model particularly for musculoskeletal disease, which is of major interest in regenerative medicine.
An important aspect of clinical research is the precise demonstration of the initial injury, the disease progress, outcome and follow up. The validated applicability of advanced diagnostic methodologies in horses such as arthroscopy and MRI (together with scoring approaches) ( Brittberg and Winalski, 2003 ; Marlovits et al., 2006 ), ultrasound, radiographs, CT and scintigraphy, has made the horse a popular model for which non-terminal studies with thorough evaluation and monitoring are possible. Also, second-look arthroscopy and serial sampling are feasible. Moreover, the large size of horses allows for the creation of critical size defects or multiple defects and offers a high amount of material that can be sampled for analysis. This enables large and comprehensive studies which may not be possible in smaller animals. Together with well-established histologic scoring ( Mcilwraith et al., 2010 ) and pain scores ( Price et al., 2003 ; Graubner et al., 2011 ; Dalla Costa et al., 2014 ; Gleerup et al., 2015 ) or assessment of other clinical parameters for horses these methods facilitate comparability of diagnosis, follow-up and results. Controlled postoperative exercise programs and rehabilitation protocols using e.g., treadmills and horse walkers further support standardization of the results. A broad offer of modern methods to further objectify outcome measures became available including gait kinematics (e.g., lameness locators) and/or kinetics using force plate/ground reaction force analysis.
Also the lack of traceability of cells injected for cell therapies could be overcome to a certain extent by using either super paramagnetic iron oxide particle (SPIO) for MRI ( Delling et al., 2015a , b ; Julke et al., 2015 ; Berner et al., 2016 ; Burk et al., 2016 ) or nuclear labeled (Technetium 99M, GFP, Indium 111) cells for scintigraphic tracing ( Sole et al., 2012 , 2013 ; Becerra et al., 2013 ; Trela et al., 2014 ; Dudhia et al., 2015 ; Spriet et al., 2015 ; Espinosa et al., 2016 ; Geburek et al., 2016 ; Scharf et al., 2016 ).
Some disadvantages of using the horse as a model include high costs of animal of animal purchase, maintenance/handling as well as ethical concerns and lower acceptance of the horse as an experimental animal compared to small animal studies by the lay public. In addition, some key parameters building the framework used in studies applying Omics approaches are not well enough researched in horses yet. A restrictive annotation status and availability of equine specific antibodies, molecular tools and markers are limiting factors. A major challenge when using horses is that their weight precludes non-weight-bearing investigations postoperatively. Significant limitations may arise regarding biomechanical strains, which far exceed those considered physiologic in humans and other animal models, which could render the stabilization of injured structures, transplants and/or sutures ineffective. Therefore, horses are a less amenable model for meniscus or bone repair. Nonetheless, these are major challenges in equine patients and several different attempts have been made or are envisaged to support healing of these structures by regenerative medicine approaches ( Fox et al., 2010 ; Milner et al., 2011 ; Ferris et al., 2012 ; Kisiday et al., 2012 ; Mcduffee et al., 2012 ; Seo et al., 2014 ; Warnock et al., 2014 ; Govoni, 2015 ; Yu et al., 2015 ; Gonzalez-Fernandez et al., 2016 ) which may also hold valuable preclinical results for human medicine. For example, hyperextension of the stifle joint was found to lead to pathologic levels of forces and injury in the cranial horn of the equine medial meniscus, analogous to observations in the human posterior medial horn upon hyperflexion ( Drosos and Pozo, 2004 ).
Tendinopathy
Horses commonly suffer from naturally occurring tendon injuries (tendinopathy) and degenerative joint disease (osteoarthritis − OA) with similar pathophysiology to the human in terms of etiology and risk factors, which include over-exercise, age and genetic factors ( Goodship et al., 1994 ; Patterson-Kane and Firth, 2009 ; Mcilwraith et al., 2012 ; Voleti et al., 2012 ; Smith et al., 2014 ; Andarawis-Puri et al., 2015 ). As athletic individuals, horses incur idiopathic primary or sports related injuries to tendon and joint related tissue.
An example is the equine superficial digital flexor tendon (SDFT) which performs a similar function to the human Achilles tendon during high-speed locomotion. In both species, their respective tendons are one of the most frequently injured ( Jarvinen et al., 2005 ; Thorpe et al., 2010 ) with age and participation in sports as key risk factors. The SDFT supports the metacarpophalangeal (MCP) joint and functions as an energy storing elastic tissue to enable efficient locomotion. During high-speed locomotion the SDFT can experience strains of 16% as the MCP joint hyperextends. These strains are within the functional limit of the SDFT at which failure can occur ( Richardson et al., 2007 ). The Achilles tendon can experience strains of up to 8% allowing as much as 34% of the total work performed by the calf muscles to be stored in the Achilles ( Fukashiro et al., 1995 ).
Acute and chronic Achilles tendon pathology is estimated to be responsible for as many as 50% of all sports-related injuries in humans ( Fukashiro et al., 1995 ; Maffulli, 1999 ; Jarvinen et al., 2005 ). The incidence of SDFT tendinitis in horses is reported to be as high as 8−43% ( Dowling et al., 2000 ). Injuries in both often manifest within the body of the tendon as core lesions, which heal by the formation of fibrous scar tissue. This scar tissue is biomechanically inferior with significantly reduced elasticity which leads to a high risk of re-injury ( Smith, 2008 ). It is therefore essential that repair strategies are aimed at restoring function by achieving scar-free healing for which regenerative medicine holds great potential. Studies in the horse to test and improve cell and cell free therapies for tendon regeneration ( Smith et al., 2003 ; Pacini et al., 2007 ; Richardson et al., 2007 ; Schnabel et al., 2007 ; Fortier and Smith, 2008 ; Lacitignola et al., 2008 ; Smith, 2008 ; Godwin et al., 2012 ; Marfe et al., 2012 ; Carvalho Ade et al., 2013 ; Renzi et al., 2013 ; Smith et al., 2013 ; Van Loon et al., 2014 ; Geburek et al., 2015 ; Muttini et al., 2015 ) could serve as preclinical data for human medicine.
However, due to the challenges of standardization of disease grade and availability of sufficient clinical case numbers for recruitment of horses with naturally occurring disease, a number of induced equine models have been developed to investigate both tendon and joint disease.
Several surgically induced tendon injury models have been developed to try to achieve a standard lesion size, anatomical location and the ensuing inflammatory response as well as time to treatment ( Guest et al., 2008 ; Schramme et al., 2010 ; Caniglia et al., 2012 ; Cadby et al., 2013 ). While most of these are aimed at partial or full transection of the tendon, the mechanically induced model described by Schramme et al. (2010) mimics a typical tendon core lesion of spontaneous disease with similarities in healing characteristics ( Cadby et al., 2013 ). In contrast, collagenase induced tendon injury models which attempt to mimic core lesions ( Williams et al., 1984 ; Nixon et al., 2008 ; Moraes et al., 2009 ; Schnabel et al., 2009 ; Crovace et al., 2010 ; Karlin et al., 2011 ; Watts et al., 2011 , 2012 ; Carvalho Ade et al., 2013 ) lead to a strong inflammatory response and are difficult to standardize with respect to location, size, shape and volume due to leakage of collagenase through the injection sites and uncontrollable diffusion from the center of the tendon ( Schramme et al., 2010 ).
Cartilage Injuries and Osteoarthritis
Another example to illustrate “what the horse can tell the human” is Osteoarthritis, a degenerative joint disease characterized by progressive loss of articular cartilage. Adult articular cartilage has limited capacity for repair and regeneration ( Kim et al., 1991 ). Any disruption of the superficial zone, or injury to the chondrocytes that maintain the cartilage matrix and zonal architecture, affects the load-distribution of the viscoelastic hyaline cartilage and may ultimately culminate in degenerative joint disease ( Rolauffs et al., 2010 ). OA of the knee and hip joints is one of the most commonly diagnosed diseases in human general practice with 52 million people (=22.7% of adults older than 18 years) in the United States and an estimated 30 – 40 million Europeans suffering from arthritis of one or more joints ( Cheng et al., 2012 ; Johnson and Hunter, 2014 ). With age and obesity as key risk factors the prevalence of OA is expected to double by the year 2020 ( Johnson and Hunter, 2014 ). As currently no proven disease-modifying therapy capable of restoring damaged articular cartilage and function of the joint is available, there is an increasing demand for novel, safe and effective treatments, which regenerative medical research could offer. In equids as for human patients, there is an unmet need for early diagnosis and effective treatments that allow return to full performance ( Mccoy, 2015 ). In horses OA constitutes the main cause of chronic lameness with an incidence of chronic degenerative joint disease in elderly horses of up to 83.5%. Interestingly, not only is the pathophysiology of equine OA similar to the human but also the thickness of the knee cartilage is similar to the human ( Frisbie et al., 2006 ; Malda et al., 2012 ). These similarities support the horse as relevant model for studies on naturally occurring OA.
A number of surgically induced equine models of articular cartilage degeneration and healing have been developed which were reviewed by Mcilwraith et al. (2011) . As in humans, the major aims of OA research are to achieve resurfacing of the damaged cartilage with biomechanically resilience and acceptable pain control. However, for any studies on cartilage repair it is important that the duration should be at least 8 to 12 months, as failure at long-term follow-up is a common outcome in human and equine clinical trials even if short-term results look promising in animal models ( Mcilwraith et al., 2011 ).
Horses, analogous to humans, commonly suffer from asthma. Asthma is a chronic inflammatory disease characterized by airway hyper-responsiveness and airway remodeling due to increased mucus production, epithelial fibrosis, hypertrophy and hyperplasia of airways smooth muscles, and gland enlargement ( Shinagawa and Kojima, 2003 ). This remodeling can induce irreversible obstruction of airways and may be a consequence of chronic tissue inflammation and altered repair processes. Since function and structure are closely related, the hypothesis is that remodeling leads to loss of airway and lung function ( Bullone and Lavoie, 2019 ).
Around 300 million people worldwide (both adults and children) suffer from asthma and hence the societal impact is high 5 . The standard therapy is based on corticosteroid administration to reduce airway obstruction thus improving quality of life. However, about 20% of people are corticosteroid resistant and do not respond to therapy ( Panettieri, 2016 ). Corticosteroid therapy is reparative and not regenerative and does not counteract remodeling. Better therapies may be derived from a regenerative approach to asthma-induced pathology.
The gold standard species for studies into human asthma would be human patients, but such studies are ethically impossible, because of the large number of patients requiring repeated biopsies to understand the causes of remodeling. Therefore, although requiring ethical authorizations, animal models are essential to advance understanding of the disease.
Severe equine asthma (SEA), which occurs spontaneously in horses ( Herszberg et al., 2006 ; Williams and Roman, 2016 ), shares many features with human asthma. The horse has potential to be a good animal model with similar lung anatomy to man. SEA shares many features with human asthma: lower airway inflammation, completely reversible airflow obstruction, bronchial hyperresponsiveness, increased respiratory efforts at rest, coughing and exercise intolerance ( Bullone and Lavoie, 2015 ; Couetil et al., 2007 ). This condition is spontaneously triggered by exposure to environmental antigens present in horse housing, similar to exposure in man and it can become incurable like chronic asthma in people. Up to 10–15% of adult horses suffer from SEA ( Hotchkiss et al., 2007 ) with a Th-2 predominant cytokine profile (increase of IL-4), as described in human asthma ( Lavoie et al., 2001 ; Klukowska-Rotzler et al., 2012 ), and decrease of Th-1 profile (decrease of interferon-γ). The predominant cell type in bronchoalveolar lavage fluid (BALF) found in horses may be different to humans depending on the severity of asthma: horses with severe and late-onset asthma have neutrophilic inflammation ( Panettieri, 2016 ) as demonstrated in some people ( Cosmi et al., 2016 ), while increased eosinophils are frequently detected in milder forms of equine asthma ( Couetil et al., 2007 ). As in people with neutrophilic asthma, horses with SEA can show an increase in Th-17 expression ( Debrue et al., 2005 ; Cosmi et al., 2016 ).
In a good animal model, homology of genes regulating immune function is essential and the horse shares higher homology with man for IL2, IL23 , and IL17 , compared to the mouse ( Tompkins et al., 2010 ; Lange-Consiglio et al., 2019 ). However, the most interesting aspect of the horse as a model to study asthma is airway remodeling, although this is less marked and involves the bronchial tree more peripherally than in man ( Bullone and Lavoie, 2019 ). The remodeling can be completely reversed by appropriate corticosteroid treatment in both human patients and horses ( Bullone and Lavoie, 2019 ) and sequential biopsies can be collected from the same standing sedated horse without the imperative to sacrifice the animal as compared to the mouse ( Leclere et al., 2011 ).
Additional Considerations Regarding Horses
In horses’ wounds on the distal limbs show delayed healing compared to wounds located on the upper body.
Reasons for this are not fully understood. However, differences in the rate of epithelization and wound contraction, inefficient inflammatory response (resulting in chronic inflammation and hence impaired formation of healthy granulation tissue), imbalance in collagen homeostasis, profibrotic environment, tissue hypoxia and inappropriate cell apoptosis are discussed as contributing factors ( Provost, 2019 ).
Interestingly ponies heal better and faster than horses, with ponies yielding a quicker and more intense inflammatory response and an improved resistance to infection as compared to horses ( Provost, 2019 ). Some of the most important advantages and disadvantages of using horses as model animals are summarized in Table 2 .
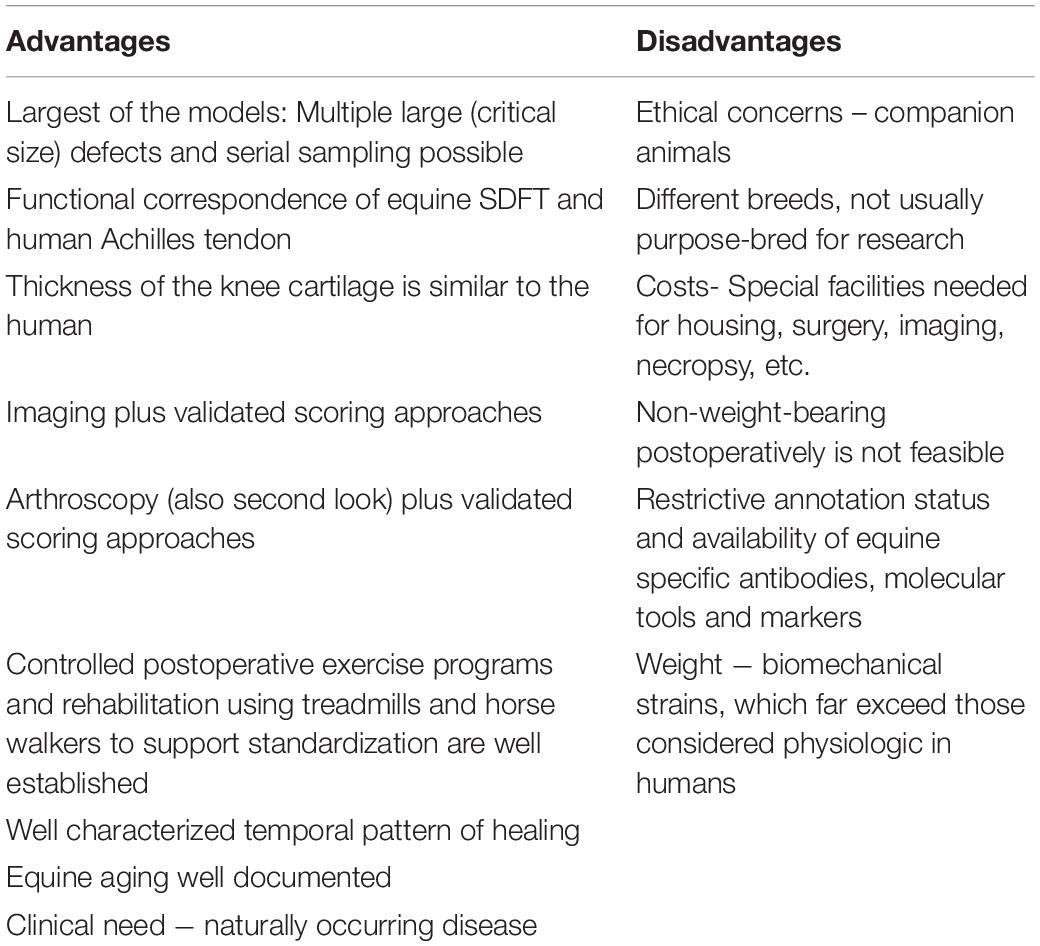
Table 2. Advantages and disadvantages of the horse as a model.
Another challenge of using horses as animal models, particularly for orthopedic disease, is so called supporting limb laminitis (SLL). Laminitis is a disorder of the tissue suspensory apparatus which suspends the distal phalanx to the inside of the horse’s hoof wall. SLL of the contralateral or supporting limb occurs when horses are forced to bear weight predominantly unilaterally (with the supporting limb) for prolonged periods, due to a severe, unilateral lameness. Mechanical loading or overloading of the supporting limb is the primary factor in its pathogenesis ( Baxter and Morrison, 2008 ; Orsini, 2012 ).
Sheep as Animal Models
Domestic sheep ( Ovis aries ) provide unique opportunities in research as an experimental and pre-clinical animal model ( Hems and Glasby, 1992 ; Glasby et al., 1993 ; Al Abri et al., 2014 ) because of their availability, low costs and acceptance by the society as a research animal ( Diogo et al., 2017 ). Sheep are docile, easy to handle and relatively inexpensive with respect to housing and feeding. Their size (50−90 kgs) is more similar to humans than small animal models, lending themselves to repeated sampling from different anatomical structures over an extended period. Their size is ideal for clinical imaging modalities designed for humans such as MRI or CT (which are limited with other large animal models like the horse). At the same time, it allows for testing surgical procedures and medical devices in animals similar to human-size (e.g., bioengineered constructs, pacemakers, stents). On the other hand, sheep housing requires more space (barns for pens) which are not widely available. The commercial availability of molecular tools (e.g., antibodies) is also more limited than for rodents although these are increasing. Nonetheless, the practical disadvantages of the sheep as an experimental model do not make it inaccessible. Based on the aim of the study, the potential benefits may compensate its technical limitations. The publication and annotation of the sheep genome ( Jiang et al., 2014 ) should improve the amount of commercially available reagents, thus facilitating the use of the ovine model in future studies. Concomitantly, the annotation of the sheep genome could support the development of useful biological tools for sheep as genetic models of human diseases (e.g., Huntington’s Disease) ( Pinnapureddy et al., 2015 ). Moreover, anesthesia and surgical equipment in sheep is more similar to humans than other large animals (like horses) and small rodents: Hence, using sheep does not require significant investment in large and specialized handling equipment, or surgical tables. At the same time, sheep can be sourced relatively easily and at low cost and they are considered as a socially acceptable animal model for research that raises fewer ethical issues than companion animals ( Entrican et al., 2015 ; Rogers, 2016 ).
Sheep are used as models for a wide range of pathologies: cardiovascular diseases ( Divincenti et al., 2014 ; Rabbani et al., 2017 ), orthopedics ( Kon et al., 2000 ; Vandeweerd et al., 2013 ; Dias et al., 2018 ; Mcgovern et al., 2018 ; Music et al., 2018 ), respiratory function ( Meeusen et al., 2009 ) and reproductive or pregnancy disorders ( Andersen et al., 2018 ; Morrison et al., 2018 ). A major reason is that ruminants, as compared to rodents, share more anatomical and physiological characteristics (with exception of the digestive tract − testing efficacy of drugs may be complicated by the 4-stomach system and uptake dynamics which defer from human gastrointestinal tract characteristics) with humans ( Scheerlinck et al., 2008 ). This makes the sheep a useful model for preclinical and translational studies in fields of Tissue Engineering and Regenerative Medicine.
Musculoskeletal Disorders
Sheep have anatomical and biomechanical features relatively similar to humans (bone composition, weight, joint structure and architecture) which allows for good simulation of healing and remodeling processes of bone or cartilage tissue ( Newman et al., 1995 ; Taylor et al., 2006 ). In addition, arthroscopic evaluation is possible in the sheep due to the size of their stifle joints. Therefore, the ovine species is the most commonly used large animal model in orthopedic research including studies on: cartilage repair ( Music et al., 2018 ), meniscal repair ( Hurtig et al., 1998 ; Tytherleigh-Strong et al., 2005 ), osteochondral tissue engineering ( Sanjurjo-Rodriguez et al., 2017 ), tendon defects ( Crovace et al., 2008 ; Martinello et al., 2013 ), osteoarthritis ( Oakley et al., 2004 ; Gugjoo et al., 2019 ), and osteoporosis ( Dias et al., 2018 ) among the others.
Sheep have been involved in studies for treating critical-sized bone defects using scaffolds with or without Mesenchymal Stem cells (MSCs). These treatments were shown to enhance bone formation and improve mechanical properties if compared to gold standard reparative methodologies like bone grafts ( Kon et al., 2000 ; Cipitria et al., 2013 ; Fernandes et al., 2014 ; Berner et al., 2015 ; Mcgovern et al., 2018 ; Pobloth et al., 2018 ).
Although the ovine knee cartilage differs in thickness to human cartilage (0.7−1.7 mm and 2.35 mm, respectively), it provides a close match regarding mechanical properties for preclinical studies ( Frisbie et al., 2006 ; Chu et al., 2010 ; Mclure et al., 2012 ). Tissue engineering approaches including different cell sources (as MSCs or chondrocytes) have been widely tested in the sheep for chondral/osteochondral defects ( Lo Monaco et al., 2018 ; Gugjoo et al., 2019 ). Cells can also be applied with scaffolds of different nature to improve and support regeneration (Chitosan, type I/III collagen, b-TCP, collagen hydrogels) ( Bernstein et al., 2013 ; Sanz-Ramos et al., 2014 ; Dias et al., 2018 ). For example, Hopper et al. (2015) used a biphasic collagen-GAG scaffold loaded with MSCs in a full-thickness osteochondral defect boosting cartilage repair while Zorzi et al. (2015) used a 1:1 chitosan-collagen scaffold seeded with human MSCs for articular cartilage regeneration ( Hopper et al., 2015 ; Zorzi et al., 2015 ). Recently, a bilayered scaffold to simulate the bone-cartilage interface (chondral and bone tissue components) has been developed and tested in sheep ( Schagemann et al., 2009 ; Fan et al., 2013 ).
Furthermore, regenerative strategies for osteoarthritis (usually induced by meniscectomy) have been investigated in sheep ( Song et al., 2014 ; Desando et al., 2016 ; Feng et al., 2018 ). Of particular interest are the studies on scaffolds for meniscal repair because of its shared characteristics with the human meniscus (cellularity, vascularity, biomechanics) ( Chevrier et al., 2009 ; Brzezinski et al., 2017 ). Gruchenberg et al. (2015) tested a silk fibroin scaffold as a meniscal implant after meniscectomy in sheep showing its biocompatibility ( Gruchenberg et al., 2015 ).
Spontaneous cartilage lesions (including osteoarthritis) have been observed in the sheep without experimental induction ( Hurtig et al., 2011 ; Vandeweerd et al., 2013 ; Kuyinu et al., 2016 ). These are especially prevalent in aging sheep and might better recapitulate the human ailment than artificially created cartilage defects.
Sheep, like horses, are ideal candidates for tendinopathy modeling, but cheaper and easier to handle and house. Martinello et al. (2013) showed the treatment efficacy of MSCs, with or without PRP (platelet rich plasma), on collagenase-induced tendinitis in the superficial digital flexor tendon, with a better structural organization of the repaired tendon ( Martinello et al., 2013 ). Deprés-Tremblay et al. (2018) tested the use of chitosan-PRP implants in an ovine acute defect model to mimic rotator cuff injuries. The implants led to an extensive bone remodeling and tissue ingrowth at the tendon-bone interface level ( Deprés-Tremblay et al., 2018 ).
Nervous System
The ovine species also serves as an adequate and effective model to study peripheral nerve regeneration, because of the similar nerve size ( Starritt et al., 2011 ) and similar regenerative behavior ( Hems et al., 1994 ; Fullarton et al., 2001 ) compared to humans ( Diogo et al., 2017 ).
Apart from conventional autografts and allografts for repairing peripheral nerve injuries in sheep ( Frey et al., 1990 ; Matsuyama et al., 2000 ), tissue engineering techniques have also been applied. Casanas et al. (2014) applied a commercially available biodegradable scaffold with MSCs or PRP to reconstruct damaged radial and tibial nerves. The addition of MSCs, with or without PRP, led to the production of myelinated nerve fibers at the distal and proximal level with fiber regeneration and functional recovery after 6 months ( Casanas et al., 2014 ). Radtke et al. (2011) compared the use of autologous nerve and acellularized vein grafts produced from spider silk. The outcomes obtained with the construct where similar to the nerve autograft results: axonal regeneration and myelination were achieved at 10 months ( Radtke et al., 2011 ).
Using sheep models, MSCs were shown to play a reparative role in intervertebral disc regeneration. Injection of MSCs led to a reduction of degeneration of the discs compared to the control group ( Freeman et al., 2016 ; Daly et al., 2018 ).
Heart Disease
Sheep have been frequently used as model for cardiovascular applications, especially for testing heart valves which have similar valve anatomy to the human and the sheep size permits access to the pulmonary and aortic valve. Kluin et al. (2017) developed an in-situ heart valve replacement for the pulmonary valve using a resorbable synthetic graft. 12 months post-implantation the tissue-engineered valve was shown to be colonized by host cells and replaced by newly formed tissue with a mature organization of the extracellular matrix without any sign of valve calcification ( Kluin et al., 2017 ).
Cell therapies with MSCs have further been applied in acute myocardial infarction models to improve myocardial function. The inoculation of cells has been demonstrated to be safe, to increase vasculature, and to reduce fibrosis in the infarcted heart ( Houtgraaf et al., 2013 ). Rabbani et al. (2017) showed that the injection of MSCs and endothelial cells (ECs) promoted angiogenesis and cardiac function, supposing that one of the mechanisms of action of the MSCs might lie in their differentiation potential toward the endothelial lineage ( Rabbani et al., 2017 ).
Also, different tissue engineering approaches for the development of preclinical vascular grafts have been tested in the sheep model ( Cummings et al., 2012 ; Aper et al., 2016 ; Fukunishi et al., 2016 ; Koobatian et al., 2016 ).
Tissue Engineering Applications in Other Systems
The ovine model has further been deployed to test regenerative approaches for treating respiratory disorders (similar airways structure and lung size to humans):
MSCs led to a reduction of inflammation and oedema and an improved oxygenation in sheep models of acute respiratory distress ( Asmussen et al., 2014 ; Kocyildirim et al., 2017 ). In an induced emphysema model, the infusion of MSCs resulted in blood reperfusion of the damaged tissue and the formation of new extracellular matrix ( Ingenito et al., 2012 ).
Recently, Kajbafzadeh et al. (2019) have tested the transplantation viability of decellularized kidneys in sheep.
The sheep model has also been described for wound healing studies because it allows for the creation of relatively large and deep wounds to mimic the typical scenario of traumatic injuries like burn injuries or decubitus ulcers. Martinello et al. (2018) used a sheep second intention wound healing model and showed how the intradermal and topical application of allogeneic MSCs led to a better re-epithelialization and dermal structure as compared to the control group at 42 days after wounding ( Martinello et al., 2018 ). The identical model was recently used by Iacopetti et al. (2020) to compare secondary intention healing of wounds, treated with a topical application of commercially available hyaluronic acid, Manuka honey or Acemannan gel ( Iacopetti et al., 2020 ).
In a similar ovine wound model, Liebsch et al. (2018) applied native spider silk as a wound dressing to test its biocompatibility and regenerative capacities ( Liebsch et al., 2018 ). Mazzone et al. (2020) used bioengineered autologous skin substitutes to treat myelomeningocele in a spina bifida repair model. The skin substitute, made of hydrogel colonized by autologous fibroblasts and keratinocytes, was transplanted in utero. The skin substitutes showed a normal histology after 1 month ( Mazzone et al., 2020 ).
Recently, Martines et al. (2020) evaluated the use of a low-temperature atmospheric pressure plasma (ionized gas) as a treatment for extensive wounds in a sheep model. The plasma stimulated cell proliferation, angiogenesis and the development of skin adnexa; concomitantly, it reduced bacterial infection and inflammation ( Martines et al., 2020 ).
A different tissue engineering approach to treat myelomeningocele was used by Watanabe et al. to treat spina bifida wounds with a gelatin/collagen sponge hybrid scaffold ( Watanabe et al., 2016 ).
Embryonic/Fetal Healing
True “scarless healing” is observed only in embryos and early fetus ( Stramer et al., 2007 ). The restitutio ad integrum in embryos ( Beredjiklian et al., 2003 ) is considered an ideal situation unmatched by any treatment regimen in adults. Therefore, an increasing amount of research studies is performed in embryos or fetal animals. To study the mechanism of fetal regeneration, relevant in vivo as well as in vitro models are required. Fetal sheep share many important physiological and developmental characteristics with humans and have hence proven themselves invaluable models for mammalian physiology ( Almeida-Porada et al., 2004 ; Jeanblanc et al., 2014 ). Sheep frequently carry twins, which allows using one twin as uninjured control on a background of low genetic variation to enable differentiation between regular fetal development and fetal response injury.
Furthermore, their long gestational period (150 days) provides sufficient temporal resolution to translate findings obtained in sheep into human parameters ( Almeida-Porada et al., 2004 ; Jeanblanc et al., 2014 ).
Fetal sheep have a fully functioning immune system by 75 days of gestation (gd) ( Emmert et al., 2013 ). They produce leukocytes by 32 gd ( Sawyer et al., 1978 ), TNF and Il-1 as early as 30−40 gd ( Dziegielewska et al., 2000 ) and obtain the capability to form significant amounts of specific antibodies in response to antigenic stimulation as early as 70 gd ( Silverstein et al., 1963 ). Fetal lambs reject orthotopic skin grafts and stem cell xenotransplants placed post 75−77 gd ( Silverstein et al., 1964 ) and mount an inflammatory response to injury by gestational day 65 ( Nitsos et al., 2006 ; Moss et al., 2008 ; Herdrich et al., 2010 ; Morris et al., 2014 ).
For all these reasons, results obtained in the fetal lamb have been directly applicable to the understanding of human fetal growth and development and are highly predictive of clinical outcome in a variety of applications including in utero stem cell transplantation ( Liechty et al., 2000 ; Almeida-Porada et al., 2004 , 2007 ; Porada et al., 2005 ; Kuypers et al., 2012 ; Kim et al., 2013 ; Jeanblanc et al., 2014 ).
Additional Considerations Regarding Sheep
Due to their special stomach system (4 stomachs: rumen, reticulum, omasum, and abomasum) bio-availability and efficacy of drugs administered orally is questionable for the human GI tract. Moreover, prolonged inappetence and application of non-steroidal anti-inflammatory drugs, antibiotics or both resulting in sustained high acidity in the abomasum may cause abomasal ulceration. Also stress, high dietary fiber and inadequate dietary fiber are believed to play a role ( Ducharme, 2004 ; Fubini and Ducharme(eds), 2004 ).
Therefore, pain management and anti-microbial management have to be planned carefully and adapted to meet the special requirements of sheep ( Lizarraga and Chambers, 2012 ; Varcoe et al., 2019 ). Sheep guidelines for pain assessment by facial expression are available ( Hager et al., 2017 ) which may help managing pain.
Some of the most important advantages and disadvantages of using sheep as model animals are summarized in Table 3 .
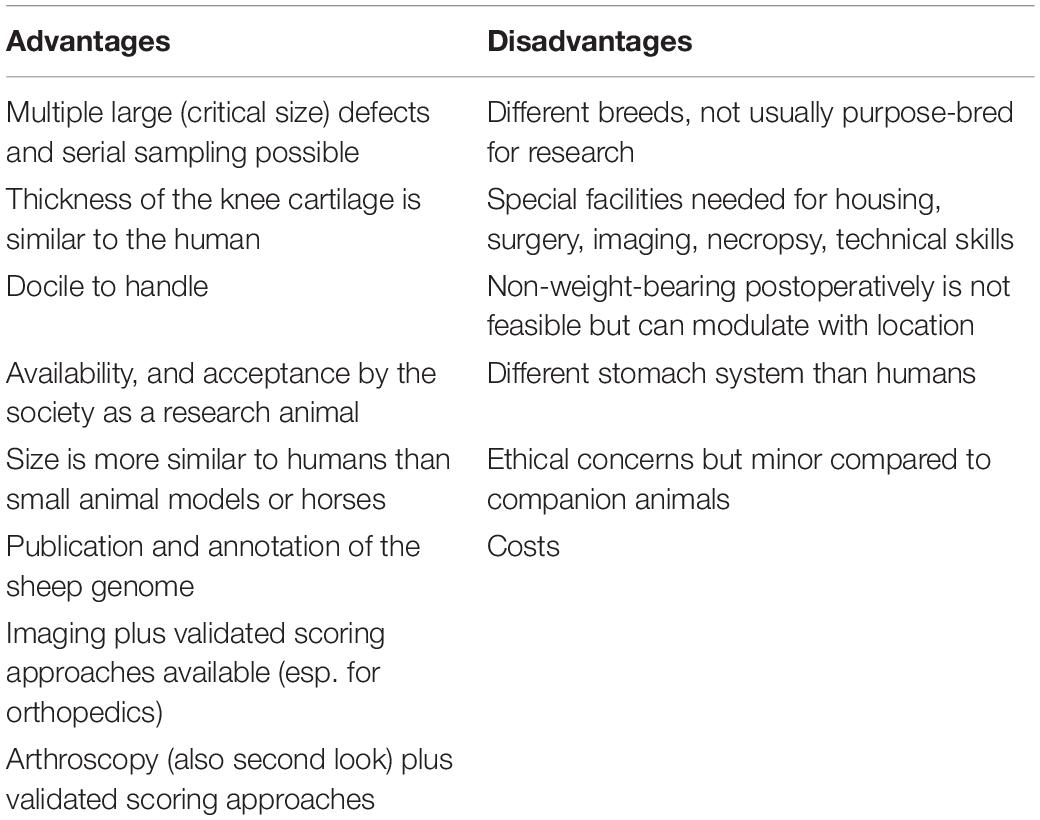
Table 3. Advantages and disadvantages of the sheep as a model.
Pigs as Animal Models
Porcine models present the advantage of having similarities with the human in terms of gastrointestinal anatomy, metabolism and physiology ( Court et al., 2004 ). When compared with other farm animals, pigs acquire early sexual maturity, sizeable litter size and have a quick reproduction time. They also breed year-round, which makes them highly suitable for biomedical research programs ( Polejaeva et al., 2016 ). Due to these characteristics and the anatomical and physiological similarities, and also their size (young pigs have a size and body weight similar to human adults), pigs are widely used as models in organ transplantation and other surgical procedures ( Kahn et al., 1988 ; Chari et al., 1994 ; Martin et al., 1999 ; He et al., 2013 ; Spetzler et al., 2015 ; Vogel et al., 2017 ), or as preclinical models in drug discovery ( Swindle et al., 2012 ; Segatto et al., 2017 ), and numerous naturally occurring and generated genetic models of human disease ( Swindle et al., 2012 ; Polejaeva et al., 2016 ). Hence, and similarly to the areas of medicine described above, the pig is gaining traction as the large animal model of choice for the study of tissue engineering and regenerative medicine products and applications, and of biomechanic studies. A good evidence of this is the steep rise in the number of publications in these broad areas in the past 30 years ( Cone et al., 2017 ).
Drug Discovery and Toxicology
Traditionally, animal models used for preclinical testing of new drugs and toxicology studies have been rodents, mainly mice and rats, for the primary screening studies. Nonetheless, because translation from rodents into humans is often not fully realized, regulatory agencies also demand the use of non-rodent models. Pigs are increasingly being used as an alternative to dogs or primates, the previous nonrodent species of choice ( Swindle et al., 2012 ). However, due to growing pressure from the public, there has been a drive for new alternatives. The pig has been favored as a suitable alternative, since they have many anatomical and physiological features valuable for translational research and are already well accepted as one of the gold standard surgical models ( Swindle et al., 2012 ). In particular, the cardiovascular system, skin and digestive tract closely mimic the human. Due to these similarities the metabolism and toxic effects of chemicals and drugs in pigs may more closely resemble the effects in man than some other laboratory animals. The minipig has been introduced recently as another alternative ( Dalgaard, 2015 ) which is frequently used due to its smaller size and easier handling for drug discovery and toxicology applications ( Mcanulty et al., 2011 ), boosted by the publication of the RETHINK project ( Forster et al., 2010 ). Furthermore, the porcine CYP450 system has been studied and partially described, and their metabolic pathways have been found to be relatively analogous to humans, with substantial overlap in substrate specificity ( Skaanild, 2006 ; Murayama et al., 2009 ).
Generated Genetic Models
With the advent of DNA recombination and gene editing technologies, modifying the pigs genome has enabled its use as a genetic model of numerous human diseases ( Flisikowska et al., 2014 ; Yao et al., 2016 ). This is reflected in the multiple pig strains developed to study, amongst others, cancers, Duchenne muscular dystrophy, autosomal polycystic kidney disease, Huntington’s disease, spinal muscular atrophy, cystic fibrosis, hemophilia A, X-linked severe combined immunodeficiency, retinitis pigmentosa, Stargardt’s Disease, Alzheimer’s disease, various forms of diabetes mellitus and cardiovascular diseases ( Flisikowska et al., 2014 ; Rogers, 2016 ; Yao et al., 2016 ; Perleberg et al., 2018 ). From these, the RAG2 or RAG2/IL2RG KO pigs are particularly relevant for biomedical research, since they can accept xenografts and/or human bioengineered tissue/organs ( Boettcher et al., 2018 ).
Transplantation Models
The pig has been used as a teaching and research animal model in surgery in the past decades. Starting in the 1990s, it became so prominent in academic and surgical training that it can be regarded as default model for non-survival surgical teaching classes, substituting the dog ( Swindle, 2007 ). Its ubiquitous presence and use in academia, enabled also its widespread adoption in multiple models of liver, lung, heart, pancreas and kidney transplantation ( Marubayashi et al., 1995 ; Martin et al., 1999 ; He et al., 2013 ; Fonouni et al., 2015 ; Mariscal et al., 2018 ). Furthermore, in transplantation medicine, the pig has also been proposed as xenograft donor, where porcine grafts have been transplanted into non-human primates with different degrees of success ( Sachs et al., 2009 ; Griesemer et al., 2014 ). This has encouraged several research groups to target the porcine genome to eliminate the major xeno-antigen(s) recognized by human natural antibodies, in a so-called effort of humanizing the pig ( Lai et al., 2002 ; Phelps et al., 2003 ; Petersen et al., 2011 ; Jeong et al., 2013 ). If ultimately realized, these procedures might enable the future xenotransplantation of porcine organs into humans as the main approach for transplantation medicine. Efforts are currently being taken to reduce the risk of viral zoonosis from porcine endogenous retrovirus (PERV), either by pharmacological treatment of PERV or by inactivating it with gene editing tools ( Denner, 2017 ; Niu et al., 2017 ). Finally, other efforts have been concentrated on porcine uterus, urethra, kidney or liver bioengineering for transplantation ( Baptista et al., 2011 ; Sullivan et al., 2012 ; Campo et al., 2017 ; Simoes et al., 2017 ). All these are an important testimony of the relevance of the pig as a vital translation research animal model.
The minipig has been used as a model in the development of dermatological products ( Mitra et al., 2015 ; Yamamoto et al., 2017 ), and more recently, as a model for microbiome studies ( Ericsson, 2019 ). As omnivores with an analogous gastrointestinal tract to humans, the well-characterized fecal microbiota of young and adult domestic pigs and other strains used in research also offers compositional resemblances to that of humans ( Pedersen et al., 2013b ; Zhao et al., 2015 ). Remarkably, many of these strains are used to investigate diet-induced obesity in genetically susceptible individuals and the same modifications (e.g., an increase in the ratio of Firmicutes to Bacteroidetes) observed between lean and obese humans are emulated in these pig models during the development of obesity ( Pedersen et al., 2013a ).
In this particular area of biomedicine, the pig is experiencing a higher increase in adoption when compared to other large animal models ( Cone et al., 2017 ) and several studies have been published assessing interspecies and interstrain differences in the anatomy and biomechanics of tissues and joints and their applicability in tissue engineering and regenerative medicine studies. Porcine models have a long history of use for studying the biomechanics of specific joints like the knee or the temporomandibular joint (TMJ), and specific tissues, including bone, cartilage, and ligaments ( Xerogeanes et al., 1998 ; Sweigart et al., 2004 ; Proffen et al., 2012 ; Murphy et al., 2013 ; O’leary et al., 2017 ). Hence, the pig has been used with success to test the efficacy of bone substitute biomaterials ( Li et al., 2015 ) and in osteochondral defect studies ( Gotterbarm et al., 2008 ; Meng et al., 2020 ). Similarly, extensive research has been conducted with the pig in tendon and ligament repair as reviewed by others ( Carpenter and Hankenson, 2004 ).
Pigs have also been used recently as a model of amyotrophic lateral sclerosis (ALS). This research has been based on the use of transgenic pigs with a mutated human copper/zinc superoxide dismutase 1 gene that mimics the human neurodegenerative disease in these pigs ( Chieppa et al., 2014 ; Yang et al., 2014 ). Similarly, a pig model of Duchenne muscular dystrophy (DMD) has been created by Klymiuk et al. by deleting DMD exon 52 in male pig cells by gene targeting. The offspring generated by nuclear transfer exhibit absence of dystrophin in skeletal muscles, progressive dystrophic changes of skeletal muscles with impaired mobility, muscle weakness and a maximum life span of 3 months due to respiratory impairment ( Klymiuk et al., 2013 ).
Additional Considerations Regarding Pigs
Pigs suffer from porcine malignant hyperthermia also known as porcine stress syndrome which is characterized by hyperthermia triggered by stress, certain anesthetic agents or intense exercise and may lead to sudden death ( Nelson, 1990 ). Some of the most important advantages and disadvantages of using pigs as model animals are summarized in Table 4 .
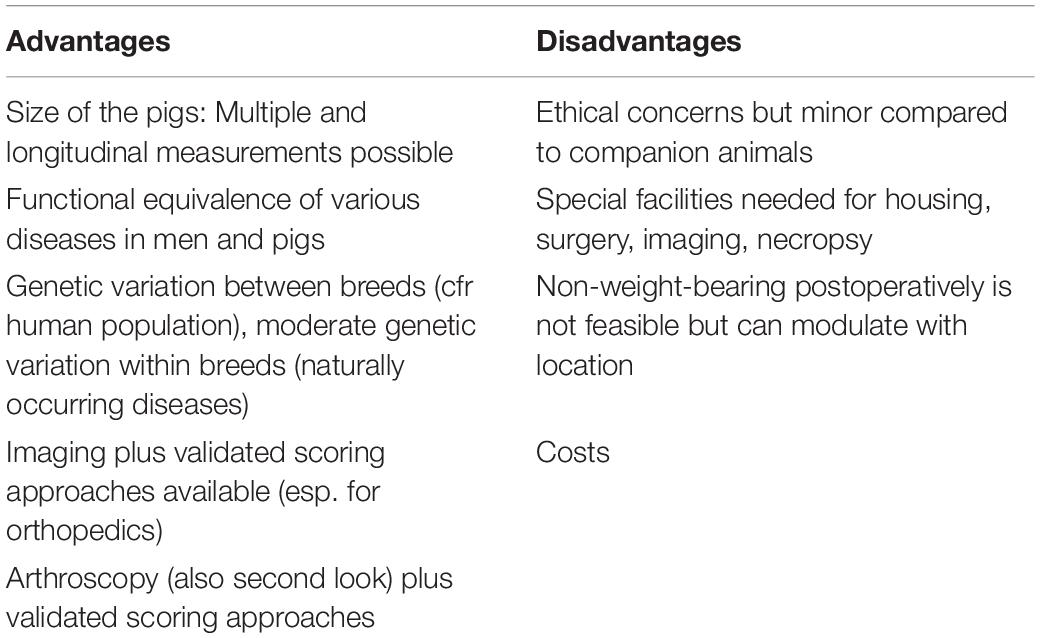
Table 4. Advantages and disadvantages of the pig as a model.
Companion Animals as Animal Models
The importance of companion animals to serve as models for human disease has received significant attention through the One Health initiative which aims to “break through the species barrier” in a drive toward a better link between medical and veterinary research for the benefit of both the human and veterinary patient ( Christopher, 2015 ).
While the definition of companion animals covers a range of animals this article extends only to the dog and cat as models, as they share remarkable similarities with the human and provide unique opportunities for developing advanced therapeutics.
One of the main reasons why dogs returned as a focus of genetic research is related to the specific population structure that has been created over the past 150−200 years.
To fully appreciate and exploit the biomedical potential of dogs (both as pets and as experimental animals), some insight into the unique canine population structure is necessary. Domesticated dogs were subjected to rigorous breeding selection, for instance for behavioral traits and/or specific morphological features such as excessive muscle formation, short limbs or a specific coat color ( Larson et al., 2012 ). Illustrative for this process is the extreme size variation, by far the largest of all mammals known, ranging from less than 1 kg for Chihuahua dogs to over 70 kg for Irish wolfhounds and Neapolitan Mastiffs. This selection process was intensified in the last two centuries and resulted in isolated genetic populations of dog breeds ( Parker et al., 2010 ). Whereas the genetic variation over the various breeds remained intact, the reduced genetic variability within breeds worked as a genetic amplifier and offers “genetic dissection microscope” for research ( Lindblad-Toh et al., 2005 ; Parker et al., 2010 ; Larson et al., 2012 ; Van Steenbeek et al., 2016 ). Together with the selection for unique traits, an increased risk for the development of specific inheritable disorders arose within breeds, providing physiologically relevant models corresponding to human conditions. To make the best out of the current situation may be to exploit the downside of inbreeding as a gene-discovery instrument for causative and modifier genes involved in complex diseases and/or rare diseases.
Canine Inherited Copper Toxicosis
The trace element copper is indispensable for critical biochemical processes such as enzyme function, for instance cytochrome c oxidase (part of the respiratory enzyme complex) or superoxide dismutase (conversion of superoxide radicals into molecular oxygen or hydrogen peroxide) ( Inesi, 2017 ). Since copper is a transition element (reduced as Cu+ and oxidized as Cu 2 +) its Jekyll and Hyde character becomes evident in the involvement in chemical reactions leading to the production of reactive oxygen species. In a Fenton reaction, Cu + catalyzes the formation of the highly reactive hydroxyl radical (OH . ). In the converse Haber-Weiss reaction Cu 2+ inactivates the damaging superoxide radical O 2 . Therefore, regulation of its intracellular free concentrations is of utmost importance and needs to be controlled within very narrow limits ( Kim et al., 2008 ). Several inherited copper-related diseases are diagnosed in men such as Menke’s Disease (copper deficiency disorder), Wilson Disease (WD, copper accumulation), and the very rare Indian childhood cirrhosis ( Tanner, 1998 ), endemic Tyrolean infantile cirrhosis ( Muller et al., 1996 ), and idiopathic copper toxicosis ( Scheinberg and Sternlieb, 1996 ). These all are rare diseases posing specific obstacles for researchers aiming to dissect molecular pathways and for rational drug design. These obstacles include limited financial resources compared to diseases affecting large numbers of patients, smaller patient cohorts for clinical phase 1−3 studies, difficulties for properly matched case-control studies in genetics and molecular signaling studies.
Copper disorders also affect sheep and dogs ( Twedt et al., 1979 ; Haywood et al., 2001 ; Fuentealba and Aburto, 2003 ). Deleteriously increased levels of hepatic copper are described in a number of dog breeds including Bedlington terriers, Skye terriers, West-Highland White terriers, Doberman, Dalmatians and Labrador retrievers ( Twedt et al., 1979 ; Haywood et al., 1988 ; Thornburg et al., 1996 ; Thornburg, 1998 ; Webb et al., 2002 ; Hoffmann et al., 2006 ). In 1999 genetic mapping studies revealed that the copper toxicosis locus within Bedlington terriers was located on canine chromosome 10. 3 years after positional cloning a 13kB deletion covering exon-2 of the murr1 gene was identified as the causative mutation for Bedlington terrier copper toxicosis ( Van De Sluis et al., 1999 , 2002 ). The causative role of murr1 mutations in WD is a matter of debate. Stuehler et al. found an association between murr1 mutations and WD, whereas two other papers did not detect a correlation between murr1 mutations and WD ( Stuehler et al., 2004 ; Lovicu et al., 2006 ; Wu et al., 2006 ). This novel gene product, currently called COMMD1 (COpper Metabolism Murr1 Domain-containing protein 1) had no known function at the time it was discovered, and the mechanism of action related to hepatic copper accumulation remained enigmatic. The discovery that COMMD1 and ATP7B interact intracellularly revealed a mechanistic link between COMMD1 protein and copper toxicosis, later confirmed for the Menkes Disease protein ATP7A ( De Bie et al., 2007 ; Vonk et al., 2012 ).
The discovery of the COMMD1 mutation and subsequent investigations into functions of COMMD1 is an intriguing example for a useful exploitation of inbred dog strains to reveal novel molecular and genetic pathways. Genetically speaking the big advantage of canine genetics to benefit human genetics is the ease to discover modifier genes. This is a needle-in-a-haystack technology in men even today, but the specific genetic population structure in inbred dogs clearly facilitates this approach.
Labrador retrievers are among the most popular breeds in the Western world.
It was already known for a long time that approximately one in every three first-line relatives of Labradors retrievers with copper toxicosis had elevated copper levels ( Hoffmann et al., 2006 ). This pushed investigations into whether or not Labrador retrievers were new model animals for WD and as a consequence propelled genetic studies ( Fieten et al., 2014 ). A SNP based genome-wide association study aiming to discover the genetic background of inherited copper toxicosis in Labrador retrievers included over 200 Labrador retrievers (154F, 81 M cases; 37F and 22 M as replication cohort) in the Netherlands that were genotyped on the 170k SNP Illumina Canine HD Bead Chip ( Fieten et al., 2016 ). For details on the mechanism of action of these mutations the readers are referred elsewhere ( Fieten et al., 2016 ). Approximately 12% of the phenotype can be explained by two mutations identified in Labrador retrievers. Since mutations in these genes were already described in copper-related disorders, it remains to be seen what other as-yet-unidentified genetic mutations will be discovered.
This genetic study clearly illustrates the power of the canine model. Explaining 12% of the phenotypic variation with an ample 250 dogs doesn’t even remotely resemble the number of human patients used to explain similar percentage for age at menarche, Inflammatory Bowel Disease (IBD) and Rheumatoid arthritis (RA) for which over 100,000 individuals were included ( Elks et al., 2010 ; Okada et al., 2014 ; Liu et al., 2015 ).
The examples prove that due to the specific population structure of inbred dog breeds, genetic studies can be successfully performed even for rare and/or complex genetic diseases.
In order to investigate COMMD1-deficient dogs as a preclinical model for liver stem cell transplantations, a breeding colony of five COMMD1 deficient dogs was created on a Beagle background and followed for over 4 years ( Favier et al., 2011 , 2012 ; Favier et al., 2015 ). This model for inherited copper toxicosis has some practical features specifically relevant for pre-clinical studies that aim to investigate surgical procedures. In contrast to mouse models, that are sacrificed for every liver measurement, the dogs’ size allowed for a true longitudinal study permitting liver biopsy sampling twice a year.
The most prevalent non ischaemic cardiomyopathies in humans are hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM), reported to affect 1 in 500 and 35 in 100,000 people, respectively (2017, Heron, 2016 ). Arrhythmogenic ventricular cardiomyoapthy (AVC) is also recognized as an important and distinct form of cardiomyopathy. Together they are associated with mechanical and/or electrical dysfunction and manifestations of the disease can range from microscopic alterations in cardiomyocytes and cardic fibroblasts to heart failure (which results in inadequate tissue perfusion and fluid retention) and arrhythmia which may cause sudden death. In veterinary species HCM is the most common feline cardiac disease affecting around 1 in 15 cats and DCM is the second most common cardiac disease in dogs and can affect a wide variety of breeds including the Doberman where its cumulative prevalance is as high as 44%. AVC has been comprehensively described in the Boxer breed at the molecular, cellular and clinical levels. All three cardiomyopathies share striking pathological and clinical similarities with the human disease. While there has been progress in the management of the symptoms associated with these cardiomyopathies in human patients, the actual disease processes remain a challenge to treat as there are few therapies that target the underlying pathology. There has therefore been an emphasis on the use of regenerative cellular therapies, although most studies have focused on ischaemic myocardial disease using mesenchymal stem cells (MSCs) derived mostly from bone marrow or adipose tissue. Stem cells derived from myocardial tissue have more recently been developed and have been tested in a number of induced disease models. A comparison of MSCs and cardiosphere derived cells (CDCs) suggests that CDCs are more efficacious in their ability to regenerate the myocardium ( Li et al., 2012 ) and phase 1 clinical trials using autologous CDCs show encouraging results ( Bolli et al., 2011 ; Makkar et al., 2012 ; Malliaras et al., 2014 ).
The development of cell-based approaches in the feline and canine clinic will have significant benefits for translation in human cardiomyopathy treatment.
Human and Feline Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is the most common cardiomyopathy in both humans and cats with a prevalence of approximately 0.1−0.2% and 16%, respectively ( Maron et al., 1995 ; Payne et al., 2010 ; Semsarian et al., 2015 ; Husser et al., 2018 ). There is increasing literature that supports the cat as an animal model of human HCM and evidence suggests it is essentially the same disease in both species ( Maron and Fox, 2015 ). HCM is characterized by left ventricular hypertrophy in the absence of systemic causes and can result in heart failure and/or sudden death. In humans genetic mutations are identified in 60% of HCM cases, mainly in genes encoding sarcomeric proteins ( Cahill et al., 2013 ). HCM in the cat is also considered to have a familial cause although only two causative mutations have so far been identified ( Maron and Fox, 2015 ), in contrast several hundred have been identified in human patients. Both of the feline mutations occur in the cardiac myosin binding protein C (MYBPC3) gene, one of which occurs in the Maine Coon breed (A31P mutation) and the other in the Ragdoll breed (R820W mutation) ( Meurs et al., 2005 , 2007 ). It is of interest to note that one specific non-truncating mutation, MYBPC3/R820W, that occurs in Ragdolls has been identified in a human family with HCM ( Ripoll Vera et al., 2010 ; Borgeat et al., 2014 ). The role sarcomeric mutations play in the development of HCM in non-pedigree cats requires further investigation.
The underlying molecular pathogenesis driving HCM remains to be elucidated although a common pathway is thought to exist in both humans and cats in which altered calcium handling within the myofilaments enhances calcium sensitivity, causing maximal force production and energy deficiency promoting mitochondrial dysfunction, cell death, fibrosis and cardiomyocyte hypertrophy ( Huke and Knollmann, 2010 ; Marston, 2011 ; Song et al., 2013 ; Robinson et al., 2018 ).
Studies using myocardial tissue from a cat homozygous for the MYBPC3/R820W mutation suggest that increased myofilament calcium sensitivity can occur in the absence of haploinsufficiency, which is common feature in human MYBPC3 mutations ( Messer et al., 2017 ). Increased myofilament calcium sensitivity was also seen in other HCM affected cats of unknown genotype but not in unaffected cats. An additional feature of the study was that the calcium sensitivity of the sarcomere is uncoupled from the phosphorylation status of troponin I, although it remains unclear how mutations outside the troponin complex cause this uncoupling phenomenon. The reasons clearly are complex but the similarities at the molecular level show the cat to be a highly relevant natural disease model for human HCM for deciphering the mechanisms. Targeting the disease with Epigallocatechin-3-gallate, for example can reverse troponin I phosphorylation uncoupling in cat HCM ( Messer et al., 2017 ) which has been replicated in human HCM samples ( Sheehan et al., 2018 ).
Such studies highlight the need to identify detailed molecular mechanisms for precise drug targeting. However, there are practical limitations with obtaining sufficient heart tissue and the survival of isolated primary cardiomyocytes is poor. Induced pluripotent stem cells (iPSC) or embryonic stem cells (ESCs) represent an alternative and robust source for preparing cardiomyocytes. The development and use of human ESCs represents an ethical dilemma and while less of an issue in veterinary species, there are only two reports of ES-like cells from cats, but these do not replicate indefinitely in culture unlike true ES cells. iPSCs on the other hand do not have the concerns associated with ESCs and can be relatively readily prepared from somatic cells.
Feline iPSCs have recently been reported for the first time by our group, the development of which represents a significant step in the generation of iPSC derived cardiomyocytes from a veterinary species ( Dutton et al., 2019 ). It paves the way for generating further cell lines from feline patients carrying the HCM causing MYBPC3/R820W mutation to test novel therapeutics for modifying the disease. iPSCs can further be manipulated with technologies such as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) to enable targeted genetic manipulation of both normal and diseased patient cell lines ( Cai et al., 2018 ; Sasaki-Honda et al., 2018 ).
iPSCs derived from patients with HCM or iPSCs with a genetic mutation inserted using CRISPR to model HCM, display characteristics of hypertrophic cardiomyocytes in culture ( Mosqueira et al., 2018 ) suggesting the suitability of the approach in establishing cell models of HCM. The availability of feline iPSC lines will enable dissecting out the molecular mechanisms of HCM enabling targeted drug screening where promising molecules can be rapidly assessed in the feline clinic with the potential of swift translation to human patients.
Human and Canine Dilated and Arrhythmogenic Ventricular Cardiomyopathy
DCM is the third most common inherited myocardial disease in humans with an estimated prevalance of 0.35% and some 2.5 million cases globally affected 6 . It is the second most common cardiac disease in dogs and accounts for 10% of canine cardiac diagnosis ( Egenvall et al., 2006 ). As with feline HCM there are remarkable similarities in the pathophysiology of DCM between human and dog. Although it is a heterogenous disease it is characterized by progressive enlargement of the left ventricle that leads to reduced systolic function, congestive heart failure and a variety of arrhythmias. Underlying causes include systemic disorders such as hypertension and atherosclerosis in humans but is also now recognized as a primary genetic disorder that may manifest with or without accompanying predisposing factors. Giant dog breeds such as the Great Dane and Newfoundlands are at risk and a genetic basis has been proposed in some dog breeds including the Doberman Pinscher and Boxer in which the disease is both common and severe with a cumulative prevalence in European Dobermans >8 years of age of 44% ( Mausberg et al., 2011 ; Simpson et al., 2015a , b ). A genetic deletion in the Pyruvate Dehydrogenase Kinase 4 (PDK4) gene has been reported. PDK4 is critical in regulating mitochondrial energy metabolism as the genetic deletion predisposes affected individuals to developing DCM as it results in chronic energy attenuation ( Meurs et al., 2012 ). More recently a missense variant in the titin gene has been reported in affected Doberman pinscher dogs negative for the PDK4 mutation. The Boxer breed has a distinct form of cardiomyopathy that closely resembles AVC in humans ( Vischer et al., 2017 ). A causative mutation in the striatin gene has been identified in Boxer dogs in the United States but this was not seen in the UK population ( Meurs et al., 2010 ; Cattanach et al., 2015 ). The role of genetics in other dog breeds with DCM remain to be better described.
Histopathological observations of the myocardium show that canine cardiomyopathy displays either an attenuated wavy fiber type and fibro-fatty infiltration type ( Tidholm and Jonsson, 2005 ) with the latter highly similar to AVC in humans. These findings emphasize the comparable pathological changes and clinical presentation between the two species ( Basso et al., 2004 ; Meurs et al., 2014 ; Vila et al., 2017 ). The pathophysiologic mechanism underlying AVC is thought to involve mechanical and electrical decoupling and cardiomyocyte apoptosis ( Wess et al., 2010 ) which with the fibro-fatty replacement of the myocardium are considered primary drivers for risk of arrhythmia and sudden cardiac death. Dogs that survive develop progressive ventricular dilation and systolic dysfunction leading to congestive heart failure ( Wess et al., 2010 ; Meurs et al., 2014 ).
There have been efforts to use stem cells for the treatment of cardiac disease in humans spurred by observations that the adult heart processes regenerative ability ( Condorelli et al., 2001 ; Nadal-Ginard et al., 2003 ). A number of clinical trials are under way or completed using adipose or bone marrow derived mesenchymal stem cells (MSC) although these are predominantly for ischaemic disease. One published study in Doberman pinchers with DCM administered allogeneic adipose derived MSCs that were virally transfected to overexpress stromal derived factor-1 to enhance homing and engrafting capabilities of endogenous MSCs to the myocardium ( Pogue et al., 2013 ). Although no significant improvements in survival rates were found at 2-year follow up, the study demonstrated that the dog model of naturally occurring DCM can be utilized to overcome a number of challenges for regenerative therapies. There is increasing interest in CDCs as they appear to possess a superior ability to regenerate the myocardium ( Li et al., 2012 ) compared to MSCs. CDCs are a heterogenous cardiac stem cell population which display features typical of stem cells such as forming clones, self-renewal and commitment to multiple lineages ( Johnston et al., 2009 ; Chimenti et al., 2010 ; Cheng et al., 2014 ; Hensley et al., 2015 ). The use of CDCs clearly is not practical because of the need to sample from the patient and also because of expansion of cells from a diseased individual which adds to patient risk, time and treatment costs. Allogeneic cells offer an alternative off-the-shelf-product but risks include immunological complications that may lead to graft versus host disease. Work in a rodent model and other induced disease models suggests allogeneic CDCs are non-immunogenic ( Malliaras et al., 2012 ). Allogeneic CDCs have been tested in a small clinical trial in dogs affected with DCM ( Hensley et al., 2017 ) and no significant adverse effects were reported. Nevertheless, the process of cryofreezing of cell stocks may potentially alter intrinsic properties of the cells as has been shown for MSCs ( Moezzi et al., 2005 ). Effects such as chromosome abnormalities resulting in abberrant cellular activity and risk of tumorigenesis may compromise their clinical use. However we have demonstrated that cryopreservation of dog CDCs does not alter their immunophenotype and cellular characteristics ( Dutton et al., 2018a ). Furthermore, we have shown at a molecular level that canine CDCs are also immune- privileged similar to the immunomodulatory function of MSCs ( Dutton et al., 2018b ) and cryopreservation retains this property suggesting they are safe to use in vivo .
Musculoskeletal Disorders in Companion Animals
Osteoarthritis.
Dog models have long been used to study joint disorders particularly osteoarthritis. The canine model for osteoarthritis has been more commonly used than the horse, sheep or goat model ( Mccoy, 2015 ). One of the reasons might be the easier post-operative management and follow up using various exercise regimes on e.g., treadmills ( Mccoy, 2015 ). While there are some similarities in cartilage anatomy between humans and dogs, the standing angle in the hindlimb in dogs is much larger. This should be considered when biomechanical aspects are compared and evaluated ( Mccoy, 2015 ). As stated previously the cartilage thickness in dogs is 0.6−1.3 mm and cartilage defects are considered to have a critical size at a minimum diameter of 4mm. Experimental OA is preferably induced in the stifle joint ( Pond and Nuki, 1973 ; Marijnissen et al., 2002 ; Kuroki et al., 2011 ), whereas naturally occurring disease is also common in the elbow or hip joint with an estimation prevalence of OA affecting 20% of adult dogs ( Mccoy, 2015 ).
With respect to osteoarthritis dogs are divided in two classes, non-chondordystrophic (NCD) and chondrodystrophic (CD) dogs. The last group presents with disproportionally short limbs, caused by aberrant endochondral ossification of long bones. Dachshunds are typical examples. The molecular mechanisms of this short limb phenotype is associated with a retrogene insertion of the FGF4 fibroblast growth factor 4 gene. This leads to elevated levels of FGF3 signaling. Interestingly, whereas CD dogs are more prone to intravertebral disc degeneration (IVDD), the insertion of the retrogene renders short-limb dogs less likely to develop OA in comparison with NC-dog ( Tellegen et al., 2019 ). These examples emphasize the need to carefully select for a specific dog breed for musculoskeletal investigations.
Intervertebral Disc Degeneration
Despite walking on four legs in contrast to men walking on two only, both species develop intervertebral disc degeneration with great similarities and similar prevalence. Link-N is a protein involved in proteoglycan stabilization (beneficial) and is highly homologous between men and dogs. However, neither human link-N nor canine link-N can protect cultured canine intervertebral disc cells form degeneration, whereas human link-N improved glycosaminoglycn deposition in human and bovine chondrocyte-like cell cultures ( Bach et al., 2017 ).
In a classcial pre-clinical study a controlled release system for the COX-2 inhibitor celecoxib (cyclooxygenase-2) was tested in a dog model for IVDD ( Tellegen et al., 2018 ). Since celecoxib prevented IVDD progression and reduced inflammation, follow-up studes will be conducted in a clincal study aiming to eliviate the chronic pain associated with low back pain.
Cranial Cruciate Disease and Meniscal Injury
Naturally occurring cranial cruciate disease has been studied extensively in veterinary medicine ( Cook, 2010 ; Bergh et al., 2014 ). It can therefore be stated, that the pathophysiology differs between injuries in humans and canines, because dogs typically suffer from degenerative ruptures ( Comerford et al., 2011 ) as compared to acute traumatic injuries seen in humans. To study new treatment approaches and validate their success, experimental models with artificially severed cruciate ligaments should be employed ( Bozynski et al., 2016 ).
Dogs also suffer from naturally occurring meniscal pathologies and hence lend themselves as potential translational models to study mechanisms of degeneration or for testing new treatment strategies ( Krupkova et al., 2018 ). The canine meniscus has comparable anatomic features (vascularization, cellularity, collagen structure) and similar permeability to the human ( Sweigart et al., 2004 ; Deponti et al., 2015 ). However, some differences between canine and human menisci especially with regard to biomechanical properties such as the aggregate- and shear-modulus should be pointed out ( Sweigart et al., 2004 ; Gupte et al., 2007 ).
Cats often serve as models to study spinal cord healing and comparative aspects in neurosurgery ( Barbeau and Rossignol, 1987 ; Bélanger et al., 1996 ; Abelew et al., 2000 ; Bouyer and Rossignol, 2003 ). Biomechanical motion analyses using treadmills and force plates as well as electromyography (EMG) are performed to evaluate spine kinematics and muscular properties following experimentally induced spinal cord or cerebral lesions.
Additional Considerations Regarding Companion Animals
Dogs and cats are companion animals and pets and as such subject of unprecedented love and care in our society. Therefore, studies involving dogs and/or cats raise more ethical debate than other animal studies. However, most studies in these animals use clinical cases seen in veterinary hospitals and clinics, which highlights the importance of this underused resource for research. Some of the most important advantages and disadvantages of using dogs as model animals are summarized in Table 5 .
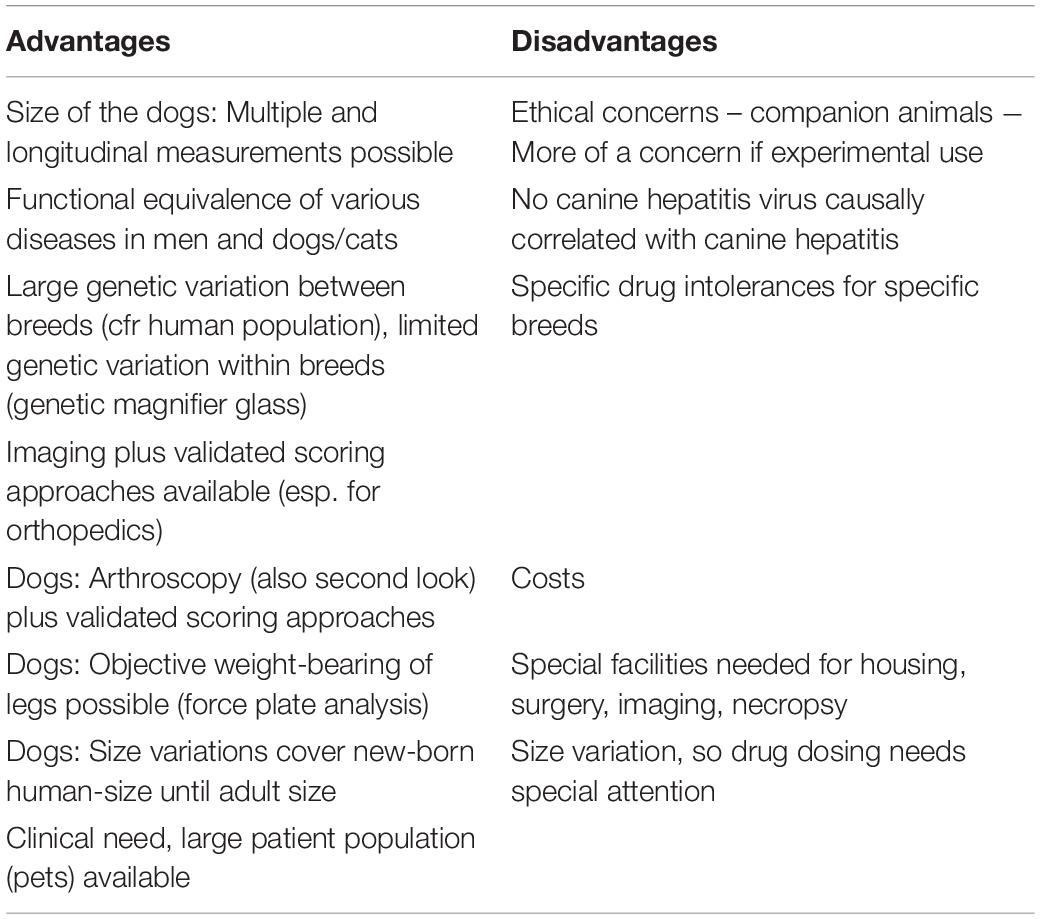
Table 5. (a) Advantages and disadvantages of canine research in general and hepatology in particular. (b) Advantages and disadvantages of companion animals as models.
Companion animal and large animal models offer realistic naturally occuring disease models that more accurately evaluate safety and efficacy of new treatments as they share the heterogeniety of the human population including genetic and physiological variations and the complex interactions of these with the environment.
There are an increasing number of studies emerging from companion animals and large animal species that demonstrate they have much to offer to the human clinic in the quest for the next generation of drug or cell-based therapies and tissue engineering. The use of large animal models will enable greater attention to key questions. These include route of administration as it is not clear as yet which route(s) allow optimal engraftment of injected cells for different diseases. It also needs to be determined whether multiple injections will be more beneficial and if so the question arises whether there is an associated increase in risk of an adverse immune reaction. Cell therapies likely function via a paracrine mechanism and as such alternative approaches such as cell-free extracellular vesicle fractions or soluble factors, need to be explored that may reduce some risks posed by cell administration particularly of allogeneic cells.
For tissue engineered constructs implantation studies using animals with similar size and weight as human patients are crucial to test the implants under relevant biomechanical conditions.
To answer these questions pre-clinical trials with patient cohorts of sufficient size are required which need to be designed robustly to measure appropriate safety and efficacy readouts. Equivalent diseases in animals makes them not only relevant models which offer a more accurate evaluation of safety and efficacy of new treatments, but at the same time are potential beneficiaries of new treatment approaches. Hence, human and veterinary medicine can mutually benefit if one appreciates the similarities.
Author Contributions
IR contributed to conceptualization, writing the manuscript, merging the parts contributed by other authors, and revision and editing of the manuscript. PB, AL-C, LM, MP, ES-F, LD, DC, and FS wrote the manuscript. FJ revised and edited the manuscript. JD wrote, revised, and edited the manuscript. LP conceived the idea and wrote, revised, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Niklas Dresen, Institute of Veterinary Anatomy, University Leipzig as well as John Breteler, Elfriede Cremer, Bernhard Cremer, and Elisabeth Schieder for the provided graphical support and Michaela Hauser for formatting the references. PB acknowledges the funded projects PI15/00563 and PI18/00529 from ISCIII, Spain.
- ^ http://www.isscr.org/docs/guidelines/isscrglclinicaltrans.pdf
- ^ https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances
- ^ https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_004.pdf
- ^ https://omia.org/home/
- ^ https://goldcopd.org/wp-content/uploads/2016/04/GOLD-2018-WMS.pdf
- ^ https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2017
Abelew, T. A., Miller, M. D., Cope, T. C., and Nichols, T. R. (2000). Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J. Neurophysiol. 84, 2709–2714. doi: 10.1152/jn.2000.84.5.2709
PubMed Abstract | CrossRef Full Text | Google Scholar
Aigner, T., Cook, J. L., Gerwin, N., Glasson, S. S., Laverty, S., Little, C. B., et al. (2010). Histopathology atlas of animal model systems - overview of guiding principles. Osteoarthritis Cartilage 18, (Suppl. 3), S2–S6.
Google Scholar
Al Abri, R., Kolethekkat, A. A., Kelleher, M. O., Myles, L. M., and Glasby, M. A. (2014). Effect of locally administered ciliary neurotrophic factor on the survival of transected and repaired adult sheep facial nerve. Oman Med. J. 29, 208–213. doi: 10.5001/omj.2014.51
Almeida-Porada, G., Porada, C., Gupta, N., Torabi, A., Thain, D., and Zanjani, E. D. (2007). The human-sheep chimeras as a model for human stem cell mobilization and evaluation of hematopoietic grafts’ potential. Exp. Hematol. 35, 1594–1600. doi: 10.1016/j.exphem.2007.07.009
Almeida-Porada, G., Porada, C., and Zanjani, E. D. (2004). Plasticity of human stem cells in the fetal sheep model of human stem cell transplantation. Int. J. Hematol. 79, 1–6. doi: 10.1007/bf02983526
Andarawis-Puri, N., Flatow, E. L., and Soslowsky, L. J. (2015). Tendon basic science: development, repair, regeneration, and healing. J. Orthop. Res. 33, 780–784. doi: 10.1002/jor.22869
Andersen, M. D., Alstrup, A. K. O., Duvald, C. S., Mikkelsen, E. F. R., Vendelbo, M. H., and Ovesen, P. G. (2018). “Animal models of fetal medicine and obstetrics,” in Experimental Animal Models of Human Diseases - An Effective Therapeutic Strategy ed. B. Ibeh (London: InTech).
Ansorge, H. L., Hsu, J. E., Edelstein, L., Adams, S., Birk, D. E., and Soslowsky, L. J. (2012). Recapitulation of the Achilles tendon mechanical properties during neonatal development: a study of differential healing during two stages of development in a mouse model. J. Orthop. Res. 30, 448–456. doi: 10.1002/jor.21542
Aper, T., Wilhelmi, M., Gebhardt, C., Hoeffler, K., Benecke, N., Hilfiker, A., et al. (2016). Novel method for the generation of tissue-engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater. 29, 21–32. doi: 10.1016/j.actbio.2015.10.012
Asmussen, S., Ito, H., Traber, D. L., Lee, J. W., Cox, R. A., Hawkins, H. K., et al. (2014). Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax 69, 819–825. doi: 10.1136/thoraxjnl-2013-204980
Aun, M. V., Bonamichi-Santos, R., Arantes-Costa, F. M., Kalil, J., and Giavina-Bianchi, P. (2017). Animal models of asthma: utility and limitations. J. Asthma Allergy 10, 293–301. doi: 10.2147/jaa.s121092
Bach, F. C., Laagland, L. T., Grant, M. P., Creemers, L. B., Ito, K., Meij, B. P., et al. (2017). Link-N: the missing link towards intervertebral disc repair is species-specific. PLoS One 12:e0187831. doi: 10.1371/journal.pone.0187831
Baptista, P. M., Siddiqui, M. M., Lozier, G., Rodriguez, S. R., Atala, A., and Soker, S. (2011). The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53, 604–617. doi: 10.1002/hep.24067
Barbeau, H., and Rossignol, S. (1987). Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95. doi: 10.1016/0006-8993(87)91442-9
CrossRef Full Text | Google Scholar
Baron, R., Tross, R., and Vignery, A. (1984). Evidence of sequential remodeling in rat trabecular bone: morphology, dynamic histomorphometry, and changes during skeletal maturation. Anat. Rec. 208, 137–145. doi: 10.1002/ar.1092080114
Basso, C., Fox, P. R., Meurs, K. M., Towbin, J. A., Spier, A. W., Calabrese, F., et al. (2004). Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation 109, 1180–1185. doi: 10.1161/01.cir.0000118494.07530.65
Baumgartner, W., Gauly, M., Schuh, M., Hildebrandt, N., Moritz, A., Christen, C., et al. (2009). Klinische Propädeutik der Haus-und Heimtiere. Stuttgart: Enke Verlag.
Baxter, G. M., and Morrison, S. (2008). Complications of unilateral weight bearing. Vet. Clin. North Am. Equine Pract. 24, 621–642. ix, doi: 10.1016/j.cveq.2008.10.006
Becerra, P., Valdes Vazquez, M. A., Dudhia, J., Fiske-Jackson, A. R., Neves, F., Hartman, N. G., et al. (2013). Distribution of injected technetium(99m)-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J. Orthop. Res. 31, 1096–1102. doi: 10.1002/jor.22338
Bélanger, M., Drew, T., Provencher, J., and Rossignol, S. (1996). A comparison of treadmill locomotion in adult cats before and after spinal transection. J. Neurophysiol. 76, 471–491. doi: 10.1152/jn.1996.76.1.471
Bendele, A. M. (2001). Animal models of osteoarthritis. J. Musculoskelet. Neuronal Interact. 1, 363–376.
Bentley, G. (1975). Articular cartilage studies and osteoarthrosis. Ann. R. Coll. Surg. Engl. 57, 86–100.
Beredjiklian, P. K., Favata, M., Cartmell, J. S., Flanagan, C. L., Crombleholme, T. M., and Soslowsky, L. J. (2003). Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann. Biomed. Eng. 31, 1143–1152. doi: 10.1114/1.1616931
Bergh, M. S., Sullivan, C., Ferrell, C. L., Troy, J., and Budsberg, S. C. (2014). Systematic review of surgical treatments for cranial cruciate ligament disease in dogs. J. Am. Anim. Hosp. Assoc. 50, 315–321. doi: 10.5326/jaaha-ms-6356
Berner, A., Henkel, J., Woodruff, M. A., Steck, R., Nerlich, M., Schuetz, M. A., et al. (2015). Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model. Stem Cells Transl. Med. 4, 503–512. doi: 10.5966/sctm.2014-0244
Berner, D., Brehm, W., Gerlach, K., Gittel, C., Offhaus, J., Paebst, F., et al. (2016). Longitudinal cell tracking and simultaneous monitoring of tissue regeneration after cell treatment of natural tendon disease by low-field magnetic resonance imaging. Stem Cells Int. 2016:1207190.
Bernstein, A., Niemeyer, P., Salzmann, G., Sudkamp, N. P., Hube, R., Klehm, J., et al. (2013). Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 9, 7490–7505. doi: 10.1016/j.actbio.2013.03.021
Blume, C., and Davies, D. E. (2013). In vitro and ex vivo models of human asthma. Eur. J. Pharm. Biopharm. 84, 394–400.
Boettcher, A. N., Loving, C. L., Cunnick, J. E., and Tuggle, C. K. (2018). Development of Severe Combined Immunodeficient (Scid) pig models for translational cancer modeling: future insights on how humanized Scid Pigs can improve preclinical cancer research. Front. Oncol. 8:559. doi: 10.3389/fonc.2018.00559
Bolli, R., Chugh, A. R., D’amario, D., Loughran, J. H., Stoddard, M. F., Ikram, S., et al. (2011). Cardiac stem cells in patients with ischaemic cardiomyopathy (Scipio): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857. doi: 10.1016/s0140-6736(11)61590-0
Bonamichi-Santos, R., Aun, M. V., Agondi, R. C., Kalil, J., and Giavina-Bianchi, P. (2015). Microbiome and Asthma: What have experimental models already taught us? J. Immunol. Res. 2015:614758.
Borgeat, K., Casamian-Sorrosal, D., Helps, C., Luis Fuentes, V., and Connolly, D. J. (2014). Association of the myosin binding protein C3 mutation (MYBPC3 R820W) with cardiac death in a survey of 236 Ragdoll cats. J. Vet. Cardiol. 16, 73–80. doi: 10.1016/j.jvc.2014.03.005
Bos, P. K., Kops, N., Verhaar, J. A., and Van Osch, G. J. (2008). Cellular origin of neocartilage formed at wound edges of articular cartilage in a tissue culture experiment. Osteoarthritis Cartilage 16, 204–211. doi: 10.1016/j.joca.2007.06.007
Bouyer, L. J., and Rossignol, S. (2003). Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J. Neurophysiol. 90, 3640–3653. doi: 10.1152/jn.00497.2003
Bozynski, C. C., Stannard, J. P., Smith, P., Hanypsiak, B. T., Kuroki, K., Stoker, A., et al. (2016). Acute management of anterior cruciate ligament injuries using novel canine models. J. Knee Surg. 29, 594–603. doi: 10.1055/s-0035-1570115
Brittberg, M., and Winalski, C. S. (2003). Evaluation of cartilage injuries and repair. J. Bone Joint. Surg. Am. 85-A, 58–69. doi: 10.2106/00004623-200300002-00008
Broeckx, S. Y., Martens, A. M., Bertone, A. L., Van Brantegem, L., Duchateau, L., Van Hecke, L., et al. (2019). The use of equine chondrogenic-induced mesenchymal stem cells as a treatment for osteoarthritis: a randomised, double-blinded, placebo-controlled proof-of-concept study. Equine Vet. J. 51, 787–794. doi: 10.1111/evj.13089
Brzezinski, A., Ghodbane, S. A., Patel, J. M., Perry, B. A., Gatt, C. J., and Dunn, M. G. (2017). ( ∗ ) The ovine model for meniscus tissue engineering: considerations of anatomy, function, implantation, and evaluation. Tissue Eng. Part C Methods 23, 829–841. doi: 10.1089/ten.tec.2017.0192
Bullone, M., and Lavoie, J. P. (2015). Asthma “of horses and men”–how can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol. Immunol. 66, 97–105. doi: 10.1016/j.molimm.2014.12.005
Bullone, M., and Lavoie, J. P. (2019). The equine asthma model of airway remodeling: from a veterinary to a human perspective. Cell Tissue Res. 380, 223–236. doi: 10.1007/s00441-019-03117-4
Burk, J., Berner, D., Brehm, W., Hillmann, A., Horstmeier, C., Josten, C., et al. (2016). Long-term cell tracking following local injection of mesenchymal stromal cells in the equine model of induced tendon disease. Cell Transplant. 25, 2199–2211. doi: 10.3727/096368916x692104
Cadby, J. A., David, F., Van De Lest, C., Bosch, G., Van Weeren, P. R., Snedeker, J. G., et al. (2013). Further characterisation of an experimental model of tendinopathy in the horse. Equine Vet. J. 45, 642–648. doi: 10.1111/evj.12035
Cahill, T. J., Ashrafian, H., and Watkins, H. (2013). Genetic cardiomyopathies causing heart failure. Circ. Res. 113, 660–675. doi: 10.1161/circresaha.113.300282
Cai, B., Sun, S., Li, Z., Zhang, X., Ke, Y., Yang, J., et al. (2018). Application of CRISPR/Cas9 technologies combined with ipscs in the study and treatment of retinal degenerative diseases. Hum. Genet. 137, 679–688. doi: 10.1007/s00439-018-1933-9
Campen, M. J., Tagaito, Y., Jenkins, T. P., Balbir, A., and O’donnell, C. P. (2005). Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J. Appl. Physiol. 99, 807–813. doi: 10.1152/japplphysiol.00039.2005
Campo, H., Baptista, P. M., Lopez-Perez, N., Faus, A., Cervello, I., and Simon, C. (2017). De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod. 96, 34–45. doi: 10.1095/biolre/bio143396
Caniglia, C. J., Schramme, M. C., and Smith, R. K. (2012). The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendonitis. Equine Vet. J. 44, 587–593. doi: 10.1111/j.2042-3306.2011.00514.x
Carpenter, J. E., and Hankenson, K. D. (2004). Animal models of tendon and ligament injuries for tissue engineering applications. Biomaterials 25, 1715–1722. doi: 10.1016/s0142-9612(03)00507-6
Carvalho Ade, M., Badial, P. R., Alvarez, L. E., Yamada, A. L., Borges, A. S., Deffune, E., et al. (2013). Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: a randomized controlled trial. Stem Cell Res. Ther. 4:85. doi: 10.1186/scrt236
Casanas, J., De La Torre, J., Soler, F., Garcia, F., Rodellar, C., Pumarola, M., et al. (2014). Peripheral nerve regeneration after experimental section in ovine radial and tibial nerves using synthetic nerve grafts, including expanded bone marrow mesenchymal cells: morphological and neurophysiological results. Injury 45, (Suppl. 4), S2–S6.
Cattanach, B. M., Dukes-Mcewan, J., Wotton, P. R., Stephenson, H. M., and Hamilton, R. M. (2015). A pedigree-based genetic appraisal of Boxer Arvc and the role of the Striatin mutation. Vet. Rec. 176:492. doi: 10.1136/vr.102821
Chari, R. S., Collins, B. H., Magee, J. C., Dimaio, J. M., Kirk, A. D., Harland, R. C., et al. (1994). Brief report: treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N. Engl. J. Med. 331, 234–237. doi: 10.1056/nejm199407283310404
Cheng, K., Ibrahim, A., Hensley, M. T., Shen, D., Sun, B., Middleton, R., et al. (2014). Relative roles of Cd90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J. Am. Heart Assoc. 3:e001260.
Cheng, Y. J., Imperatore, G., Caspersen, C. J., Gregg, E. W., Albright, A. L., and Helmick, C. G. (2012). Prevalence of diagnosed arthritis and arthritis-attributable activity limitation among adults with and without diagnosed diabetes: United States, 2008-2010. Diabetes Care 35, 1686–1691. doi: 10.2337/dc12-0046
Chevrier, A., Nelea, M., Hurtig, M. B., Hoemann, C. D., and Buschmann, M. D. (2009). Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J. Orthop. Res. 27, 1197–1203. doi: 10.1002/jor.20869
Chieppa, M. N., Perota, A., Corona, C., Grindatto, A., Lagutina, I., Vallino Costassa, E., et al. (2014). Modeling amyotrophic lateral sclerosis in hsod1 transgenic swine. Neurodegener. Dis. 13, 246–254.
Chimenti, I., Smith, R. R., Li, T. S., Gerstenblith, G., Messina, E., Giacomello, A., et al. (2010). Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 106, 971–980. doi: 10.1161/circresaha.109.210682
Christopher, M. M. (2015). One health, one literature: weaving together veterinary and medical research. Sci. Transl. Med. 7:303fs36.
Chu, C. R., Szczodry, M., and Bruno, S. (2010). Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 16, 105–115. doi: 10.1089/ten.teb.2009.0452
Cipitria, A., Reichert, J. C., Epari, D. R., Saifzadeh, S., Berner, A., Schell, H., et al. (2013). Polycaprolactone scaffold and reduced rhbmp-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 34, 9960–9968. doi: 10.1016/j.biomaterials.2013.09.011
Cohen-Solal, M., Hay, E., and Funck-Brentano, T. (2012). Animal models in OA: a means to explore bone. Osteoporos. Int. 23, (Suppl. 8), S853–S856.
Comerford, E. J., Smith, K., and Hayashi, K. (2011). Update on the aetiopathogenesis of canine cranial cruciate ligament disease. Vet. Comp. Orthop. Traumatol. 24, 91–98. doi: 10.3415/vcot-10-04-0055
Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., and Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. doi: 10.1038/nature03260
Condorelli, G., Borello, U., De Angelis, L., Latronico, M., Sirabella, D., Coletta, M., et al. (2001). Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration. Proc. Natl. Acad. Sci. U.S.A. 98, 10733–10738. doi: 10.1073/pnas.191217898
Cone, S. G., Warren, P. B., and Fisher, M. B. (2017). Rise of the pigs: utilization of the porcine model to study musculoskeletal biomechanics and tissue engineering during skeletal growth. Tissue Eng. Part C Methods 23, 763–780. doi: 10.1089/ten.tec.2017.0227
Connizzo, B. K., Yannascoli, S. M., and Soslowsky, L. J. (2013). Structure-function relationships of postnatal tendon development: a parallel to healing. Matrix Biol. 32, 106–116. doi: 10.1016/j.matbio.2013.01.007
Cook, J. L. (2010). Cranial cruciate ligament disease in dogs: biology versus biomechanics. Vet. Surg. 39, 270–277. doi: 10.1111/j.1532-950x.2010.00653.x
Cosmi, L., Liotta, F., and Annunziato, F. (2016). Th17 regulating lower airway disease. Curr. Opin. Allergy Clin. Immunol. 16, 1–6. doi: 10.1097/aci.0000000000000227
Couetil, L. L., Hoffman, A. M., Hodgson, J., Buechner-Maxwell, V., Viel, L., Wood, J. L., et al. (2007). Inflammatory airway disease of horses. J. Vet. Intern. Med. 21, 356–361. doi: 10.1111/j.1939-1676.2007.tb02975.x
Court, F. G., Laws, P. E., Morrison, C. P., Teague, B. D., Metcalfe, M. S., Wemyss-Holden, S. A., et al. (2004). Subtotal hepatectomy: a porcine model for the study of liver regeneration. J. Surg. Res. 116, 181–186. doi: 10.1016/j.jss.2003.08.007
Crovace, A., Lacitignola, L., Francioso, E., and Rossi, G. (2008). Histology and immunohistochemistry study of ovine tendon grafted with cBMSCs and BMMNCs after collagenase-induced tendinitis. Vet. Comp. Orthop. Traumatol. 21, 329–336. doi: 10.3415/vcot-07-05-0050
Crovace, A., Lacitignola, L., Rossi, G., and Francioso, E. (2010). Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet. Med. Int. 2010:250978.
Cummings, I., George, S., Kelm, J., Schmidt, D., Emmert, M. Y., Weber, B., et al. (2012). Tissue-engineered vascular graft remodeling in a growing lamb model: expression of matrix metalloproteinases. Eur. J. Cardiothorac. Surg. 41, 167–172.
Dalgaard, L. (2015). Comparison of minipig, dog, monkey and human drug metabolism and disposition. J. Pharmacol. Toxicol. Methods 74, 80–92. doi: 10.1016/j.vascn.2014.12.005
Dalla Costa, E., Minero, M., Lebelt, D., Stucke, D., Canali, E., and Leach, M. C. (2014). Development of the Horse Grimace Scale (Hgs) as a pain assessment tool in horses undergoing routine castration. PLoS One 9:e92281. doi: 10.1371/journal.pone.0092281
Daly, C. D., Ghosh, P., Zannettino, A. C. W., Badal, T., Shimmon, R., Jenkin, G., et al. (2018). Mesenchymal progenitor cells primed with pentosan polysulfate promote lumbar intervertebral disc regeneration in an ovine model of microdiscectomy. Spine J. 18, 491–506. doi: 10.1016/j.spinee.2017.10.008
De Bie, P., Van De Sluis, B., Burstein, E., Van De Berghe, P. V., Muller, P., Berger, R., et al. (2007). Distinct Wilson’s disease mutations in Atp7B are associated with enhanced binding to Commd1 and reduced stability of Atp7B. Gastroenterology 133, 1316–1326. doi: 10.1053/j.gastro.2007.07.020
Debrue, M., Hamilton, E., Joubert, P., Lajoie-Kadoch, S., and Lavoie, J. P. (2005). Chronic exacerbation of equine heaves is associated with an increased expression of interleukin-17 mRNA in bronchoalveolar lavage cells. Vet. Immunol. Immunopathol. 105, 25–31. doi: 10.1016/j.vetimm.2004.12.013
Delling, U., Brehm, W., Ludewig, E., Winter, K., and Julke, H. (2015a). Longitudinal evaluation of effects of intra-articular mesenchymal stromal cell administration for the treatment of osteoarthritis in an ovine model. Cell Transplant. 24, 2391–2407. doi: 10.3727/096368915x686193
Delling, U., Brehm, W., Metzger, M., Ludewig, E., Winter, K., and Julke, H. (2015b). In vivo tracking and fate of intra-articularly injected superparamagnetic iron oxide particle-labeled multipotent stromal cells in an ovine model of osteoarthritis. Cell Transplant. 24, 2379–2390. doi: 10.3727/096368914x685654
Denner, J. (2017). Can antiretroviral drugs be used to treat porcine endogenous retrovirus (PERV) Infection after Xenotransplantation? Viruses 9:213. doi: 10.3390/v9080213
Deponti, D., Di Giancamillo, A., Scotti, C., Peretti, G. M., and Martin, I. (2015). Animal models for meniscus repair and regeneration. J. Tissue Eng. Regen. Med. 9, 512–527. doi: 10.1002/term.1760
Deprés-Tremblay, G., Chevrier, A., Hurtig, M. B., Snow, M., Rodeo, S., and Buschmann, M. D. (2018). Freeze-dried chitosan-platelet-rich plasma implants for rotator cuff tear repair: pilot ovine studies. ACS Biomater. Sci. Eng. 4, 3737–3746. doi: 10.1021/acsbiomaterials.7b00354
Desando, G., Giavaresi, G., Cavallo, C., Bartolotti, I., Sartoni, F., Nicoli Aldini, N., et al. (2016). Autologous bone marrow concentrate in a sheep model of osteoarthritis: new perspectives for cartilage and meniscus repair. Tissue Eng. Part C Methods 22, 608–619. doi: 10.1089/ten.tec.2016.0033
Dias, I. R., Camassa, J. A., Bordelo, J. A., Babo, P. S., Viegas, C. A., Dourado, N., et al. (2018). Preclinical and translational studies in small ruminants (sheep and goat) as models for osteoporosis research. Curr. Osteoporos. Rep. 16, 182–197. doi: 10.1007/s11914-018-0431-2
Diogo, C. C., Camassa, J. A., Pereira, J. E., Costa, L. M. D., Filipe, V., Couto, P. A., et al. (2017). The use of sheep as a model for studying peripheral nerve regeneration following nerve injury: review of the literature. Neurol. Res. 39, 926–939. doi: 10.1080/01616412.2017.1331873
Divincenti, L. Jr., Westcott, R., and Lee, C. (2014). Sheep ( Ovis aries ) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J. Am. Assoc. Lab. Anim. Sci. 53, 439–448.
Douwes, J., Gibson, P., Pekkanen, J., and Pearce, N. (2002). Non-eosinophilic asthma: importance and possible mechanisms. Thorax 57, 643–648. doi: 10.1136/thorax.57.7.643
Dowling, B. A., Dart, A. J., Hodgson, D. R., and Smith, R. K. (2000). Superficial digital flexor tendonitis in the horse. Equine Vet. J. 32, 369–378. doi: 10.2746/042516400777591138
Drosos, G. I., and Pozo, J. L. (2004). The causes and mechanisms of meniscal injuries in the sporting and non-sporting environment in an unselected population. Knee 11, 143–149. doi: 10.1016/s0968-0160(03)00105-4
Ducharme, N. G. (2004). “Surgery of the bovine digestive system,” in Farm Animal Surgery , 1st Edn, ed. S. L. Fubini and N.G. Ducharme (Philadelphia, PA: Elsevier).
Dudhia, J., Becerra, P., Valdes, M. A., Neves, F., Hartman, N. G., and Smith, R. K. (2015). In vivo imaging and tracking of technetium-99m labeled bone marrow mesenchymal stem cells in equine tendinopathy. J. Vis. Exp. 106:e52748.
Dutton, L. C., Church, S. A. V., Hodgkiss-Geere, H., Catchpole, B., Huggins, A., Dudhia, J., et al. (2018a). Cryopreservation of canine cardiosphere-derived cells: implications for clinical application. Cytometry A 93, 115–124. doi: 10.1002/cyto.a.23186
Dutton, L. C., Dudhia, J., Catchpole, B., Hodgkiss-Geere, H., Werling, D., and Connolly, D. J. (2018b). Cardiosphere-derived cells suppress allogeneic lymphocytes by production of PGE2 acting via the EP4 receptor. Sci. Rep. 8:13351.
Dutton, L. C., Dudhia, J., Guest, D. J., and Connolly, D. J. (2019). Inducing pluripotency in the domestic cat ( Felis catus ). Stem Cells Dev. 28, 1299–1309. doi: 10.1089/scd.2019.0142
Dziegielewska, K. M., Moller, J. E., Potter, A. M., Ek, J., Lane, M. A., and Saunders, N. R. (2000). Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 299, 335–345. doi: 10.1007/s004419900157
Egenvall, A., Bonnett, B. N., and Haggstrom, J. (2006). Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. J. Vet. Intern. Med. 20, 894–903. doi: 10.1111/j.1939-1676.2006.tb01803.x
Elks, C. E., Perry, J. R., Sulem, P., Chasman, D. I., Franceschini, N., He, C., et al. (2010). Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 42, 1077–1085.
Emmert, M. Y., Weber, B., Wolint, P., Frauenfelder, T., Zeisberger, S. M., Behr, L., et al. (2013). Intramyocardial transplantation and tracking of human mesenchymal stem cells in a novel intra-uterine pre-immune fetal sheep myocardial infarction model: a proof of concept study. PLoS One 8:e57759. doi: 10.1371/journal.pone.0057759
Entrican, G., Wattegedera, S. R., and Griffiths, D. J. (2015). Exploiting ovine immunology to improve the relevance of biomedical models. Mol. Immunol. 66, 68–77. doi: 10.1016/j.molimm.2014.09.002
Ericsson, A. C. (2019). The use of non-rodent model species in microbiota studies. Lab. Anim. 53, 259–270. doi: 10.1177/0023677219834593
Espinosa, P., Spriet, M., Sole, A., Walker, N. J., Vaughan, B., and Galuppo, L. D. (2016). Scintigraphic tracking of allogeneic mesenchymal stem cells in the distal limb after intra-arterial injection in standing horses. Vet. Surg. 45, 619–624. doi: 10.1111/vsu.12485
Fan, W., Wu, C., Miao, X., Liu, G., Saifzadeh, S., Sugiyama, S., et al. (2013). Biomaterial scaffolds in cartilage-subchondral bone defects influencing the repair of autologous articular cartilage transplants. J. Biomater. Appl. 27, 979–989. doi: 10.1177/0885328211431310
Favata, M., Beredjiklian, P. K., Zgonis, M. H., Beason, D. P., Crombleholme, T. M., Jawad, A. F., et al. (2006). Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J. Orthop. Res. 24, 2124–2132. doi: 10.1002/jor.20271
Favier, R. P., Spee, B., Fieten, H., Van Den Ingh, T. S., Schotanus, B. A., Brinkhof, B., et al. (2015). Aberrant expression of copper associated genes after copper accumulation in Commd1-deficient dogs. J. Trace Elem. Med. Biol. 29, 347–353. doi: 10.1016/j.jtemb.2014.06.007
Favier, R. P., Spee, B., Penning, L. C., and Rothuizen, J. (2011). Copper-induced hepatitis: the Commd1 deficient dog as a translational animal model for human chronic hepatitis. Vet. Q. 31, 49–60. doi: 10.1080/01652176.2011.563146
Favier, R. P., Spee, B., Schotanus, B. A., Van Den Ingh, T. S., Fieten, H., Brinkhof, B., et al. (2012). Commd1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS One 7:e42158. doi: 10.1371/journal.pone.0042158
Feng, C., Luo, X., He, N., Xia, H., Lv, X., Zhang, X., et al. (2018). Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng. Part A 24, 219–233. doi: 10.1089/ten.tea.2017.0039
Fernandes, M. B., Guimaraes, J. A., Casado, P. L., Cavalcanti Ados, S., Goncalves, N. N., Ambrosio, C. E., et al. (2014). The effect of bone allografts combined with bone marrow stromal cells on the healing of segmental bone defects in a sheep model. BMC Vet. Res. 10:36. doi: 10.1186/1746-6148-10-36
Ferris, D., Frisbie, D., Kisiday, J., and Mcilwraith, C. W. (2012). In vivo healing of meniscal lacerations using bone marrow-derived mesenchymal stem cells and fibrin glue. Stem Cells Int. 2012:691605.
Fieten, H., Gill, Y., Martin, A. J., Concilli, M., Dirksen, K., Van Steenbeek, F. G., et al. (2016). The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: a new canine model for copper-metabolism disorders. Dis. Model. Mech. 9, 25–38. doi: 10.1242/dmm.020263
Fieten, H., Penning, L. C., Leegwater, P. A., and Rothuizen, J. (2014). New canine models of copper toxicosis: diagnosis, treatment, and genetics. Ann. N. Y. Acad. Sci. 1314, 42–48. doi: 10.1111/nyas.12442
Flisikowska, T., Kind, A., and Schnieke, A. (2014). Genetically modified pigs to model human diseases. J. Appl. Genet. 55, 53–64. doi: 10.1007/s13353-013-0182-9
Fonouni, H., Tahmasbi Rad, M., Esmaeilzadeh, M., Golriz, M., Majlesara, A., and Mehrabi, A. (2015). A simplified technique of pancreas transplantation in a porcine model. Eur. Surg. Res. 54, 24–33. doi: 10.1159/000367844
Forster, R., Bode, G., Ellegaard, L., and Van Der Laan, J. W. (2010). The RETHINK project–minipigs as models for the toxicity testing of new medicines and chemicals: an impact assessment. J. Pharmacol. Toxicol. Methods 62, 158–159.
Fortier, L. A., and Smith, R. K. (2008). Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet. Clin. North Am. Equine Pract. 24, 191–201. doi: 10.1016/j.cveq.2007.11.002
Fox, D. B., Warnock, J. J., Stoker, A. M., Luther, J. K., and Cockrell, M. (2010). Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res. Vet. Sci. 88, 326–332. doi: 10.1016/j.rvsc.2009.07.015
Freeman, B. J., Kuliwaba, J. S., Jones, C. F., Shu, C. C., Colloca, C. J., Zarrinkalam, M. R., et al. (2016). Allogeneic mesenchymal precursor cells promote healing in postero-lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model. Spine 41, 1331–1339. doi: 10.1097/brs.0000000000001528
Frey, M., Gruber, H., Happak, W., Girsch, W., Gruber, I., and Koller, R. (1990). Ipsilateral and cross-over elongation of the motor nerve by nerve grafting: an experimental study in sheep. Plast. Reconstr. Surg. 85, 77–89. doi: 10.1097/00006534-199001000-00014
Frisbie, D. D., Cross, M. W., and Mcilwraith, C. W. (2006). A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet. Comp. Orthop. Traumatol. 19, 142–146. doi: 10.1055/s-0038-1632990
Fubini, S. L., and Ducharme, N. G. (eds) (2004). “Surgical considerations,” in Farm Animal Surgery , 1st Edn (St. Louis, MO: Elsevier).
Fuentealba, I. C., and Aburto, E. M. (2003). Animal models of copper-associated liver disease. Comp. Hepatol. 2:5.
Fukashiro, S., Komi, P. V., Jarvinen, M., and Miyashita, M. (1995). In vivo Achilles tendon loading during jumping in humans. Eur. J. Appl. Physiol. Occup. Physiol. 71, 453–458. doi: 10.1007/bf00635880
Fukunishi, T., Best, C. A., Sugiura, T., Shoji, T., Yi, T., Udelsman, B., et al. (2016). Tissue-engineered small diameter arterial vascular grafts from cell-free Nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One 11:e0158555. doi: 10.1371/journal.pone.0158555
Fullarton, A. C., Myles, L. M., Lenihan, D. V., Hems, T. E., and Glasby, M. A. (2001). Obstetric brachial plexus palsy: a comparison of the degree of recovery after repair of a C6 ventral root avulsion in newborn and adult sheep. Br. J. Plast. Surg. 54, 697–704. doi: 10.1054/bjps.2001.3700
Geburek, F., Lietzau, M., Beineke, A., Rohn, K., and Stadler, P. M. (2015). Effect of a single injection of autologous conditioned serum (Acs) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res. Ther. 6:126.
Geburek, F., Mundle, K., Conrad, S., Hellige, M., Walliser, U., Van Schie, H. T., et al. (2016). Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions–a pilot study. Stem Cell Res. Ther. 7:21.
Glasby, M. A., Mountain, R. E., and Murray, J. A. (1993). Repair of the facial nerve using freeze-thawed muscle autografts. A surgical model in the sheep. Arch. Otolaryngol. Head Neck Surg. 119, 461–465. doi: 10.1001/archotol.1993.01880160109018
Gleerup, K. B., Forkman, B., Lindegaard, C., and Andersen, P. H. (2015). An equine pain face. Vet. Anaesth. Analg. 42, 103–114.
Godwin, E. E., Young, N. J., Dudhia, J., Beamish, I. C., and Smith, R. K. (2012). Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 44, 25–32. doi: 10.1111/j.2042-3306.2011.00363.x
Goldberg, A. J., Zaidi, R., Brooking, D., Kim, L., Korda, M., Masci, L., et al. (2018). Autologous Stem Cells in Achilles Tendinopathy (ASCAT): protocol for a phase IIA, single-centre, proof-of-concept study. BMJ Open 8:e021600.
Gonzalez-Fernandez, M. L., Perez-Castrillo, S., Sanchez-Lazaro, J. A., Prieto-Fernandez, J. G., Lopez-Gonzalez, M. E., Lobato-Perez, S., et al. (2016). Assessment of regeneration in meniscal lesions by use of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Am. J. Vet. Res. 77, 779–788. doi: 10.2460/ajvr.77.7.779
Goodship, A. E., Birch, H. L., and Wilson, A. M. (1994). The pathobiology and repair of tendon and ligament injury. Vet. Clin. North Am. Equine Pract. 10, 323–349. doi: 10.1016/s0749-0739(17)30359-0
Gotterbarm, T., Breusch, S. J., Schneider, U., and Jung, M. (2008). The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab. Anim. 42, 71–82. doi: 10.1258/la.2007.06029e
Govoni, K. E. (2015). HORSE SPECIES SYMPOSIUM: use of mesenchymal stem cells in fracture repair in horses. J. Anim. Sci. 93, 871–878.
Graubner, C., Gerber, V., Doherr, M., and Spadavecchia, C. (2011). Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet. J. 188, 178–183. doi: 10.1016/j.tvjl.2010.04.029
Griesemer, A., Yamada, K., and Sykes, M. (2014). Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol. Rev. 258, 241–258. doi: 10.1111/imr.12152
Gruchenberg, K., Ignatius, A., Friemert, B., Von Lubken, F., Skaer, N., Gellynck, K., et al. (2015). In vivo performance of a novel silk fibroin scaffold for partial meniscal replacement in a sheep model. Knee Surg. Sports Traumatol. Arthrosc. 23, 2218–2229. doi: 10.1007/s00167-014-3009-2
Guest, D. J., Smith, M. R., and Allen, W. R. (2008). Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet. J. 40, 178–181. doi: 10.2746/042516408x276942
Gugjoo, M. B., Fazili, M. R., Gayas, M. A., Ahmad, R. A., and Dhama, K. (2019). Animal mesenchymal stem cell research in cartilage regenerative medicine - a review. Vet. Q. 39, 95–120. doi: 10.1080/01652176.2019.1643051
Gupte, C. M., Bull, A. M., Murray, R., and Amis, A. A. (2007). Comparative anatomy of the meniscofemoral ligament in humans and some domestic mammals. Anat. Histol. Embryol. 36, 47–52. doi: 10.1111/j.1439-0264.2006.00718.x
Hager, C., Biernot, S., Buettner, M., Glage, S., Keubler, L. M., Held, N., et al. (2017). The Sheep Grimace Scale as an indicator of post-operative distress and pain in laboratory sheep. PLoS One 12:e0175839. doi: 10.1371/journal.pone.0175839
Haywood, S., Muller, T., Muller, W., Heinz-Erian, P., Tanner, M. S., and Ross, G. (2001). Copper-associated liver disease in North Ronaldsay sheep: a possible animal model for non-Wilsonian hepatic copper toxicosis of infancy and childhood. J. Pathol. 195, 264–269. doi: 10.1002/path.930
Haywood, S., Rutgers, H. C., and Christian, M. K. (1988). Hepatitis and copper accumulation in Skye terriers. Vet. Pathol. 25, 408–414. doi: 10.1177/030098588802500602
He, B., Musk, G. C., Mou, L., De Boer, B., Delriviere, L., and Hamdorf, J. (2013). Laparoscopic surgery for orthotopic kidney transplant in the pig model. J. Surg. Res. 184, 1096–1101. doi: 10.1016/j.jss.2013.03.015
Hems, T. E., Clutton, R. E., and Glasby, M. A. (1994). Repair of avulsed cervical nerve roots. An experimental study in sheep. J. Bone Joint Surg. Br. 76, 818–823. doi: 10.1302/0301-620x.76b5.8083277
Hems, T. E., and Glasby, M. A. (1992). Repair of cervical nerve roots proximal to the root ganglia. An experimental study in sheep. J. Bone Joint Surg. Br. 74, 918–922. doi: 10.1302/0301-620x.74b6.1447258
Hensley, M. T., De Andrade, J., Keene, B., Meurs, K., Tang, J., Wang, Z., et al. (2015). Cardiac regenerative potential of cardiosphere-derived cells from adult dog hearts. J. Cell Mol. Med. 19, 1805–1813. doi: 10.1111/jcmm.12585
Hensley, M. T., Tang, J., Woodruff, K., Defrancesco, T., Tou, S., Williams, C. M., et al. (2017). Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J. Cell. Mol. Med. 21, 1503–1512. doi: 10.1111/jcmm.13077
Herdrich, B. J., Danzer, E., Davey, M. G., Bermudez, D. M., Radu, A., Zhang, L., et al. (2010). Fetal tendon wound size modulates wound gene expression and subsequent wound phenotype. Wound Repair Regen. 18, 543–549. doi: 10.1111/j.1524-475x.2010.00615.x
Heron, M. (2016). Deaths: leading causes for 2014. Natl. Vital Stat. Rep. 65, 1–96.
Herszberg, B., Ramos-Barbon, D., Tamaoka, M., Martin, J. G., and Lavoie, J. P. (2006). Heaves, an asthma-like equine disease, involves airway smooth muscle remodeling. J. Allergy Clin. Immunol. 118, 382–388. doi: 10.1016/j.jaci.2006.03.044
Hoffmann, G., Van Den Ingh, T. S., Bode, P., and Rothuizen, J. (2006). Copper-associated chronic hepatitis in Labrador Retrievers. J. Vet. Intern. Med. 20, 856–861. doi: 10.1111/j.1939-1676.2006.tb01798.x
Hopper, N., Wardale, J., Brooks, R., Power, J., Rushton, N., and Henson, F. (2015). Peripheral blood mononuclear cells enhance cartilage repair in in vivo osteochondral defect model. PLoS One 10:e0133937. doi: 10.1371/journal.pone.0133937
Hotchkiss, J. W., Reid, S. W., and Christley, R. M. (2007). A survey of horse owners in Great Britain regarding horses in their care. Part 2: Risk factors for recurrent airway obstruction. Equine Vet. J. 39, 301–308. doi: 10.2746/042516407x180129
Houtgraaf, J. H., De Jong, R., Kazemi, K., De Groot, D., Van Der Spoel, T. I., Arslan, F., et al. (2013). Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ. Res. 113, 153–166. doi: 10.1161/circresaha.112.300730
Huke, S., and Knollmann, B. C. (2010). Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J. Mol. Cell. Cardiol. 48, 824–833. doi: 10.1016/j.yjmcc.2010.01.011
Hurtig, M. B., Buschmann, M. D., Fortier, L. A., Hoemann, C. D., Hunziker, E. B., Jurvelin, J. S., et al. (2011). Preclinical studies for cartilage repair: recommendations from the international cartilage repair society. Cartilage 2, 137–152. doi: 10.1177/1947603511401905
Hurtig, M. B., Novak, K., Mcpherson, R., Mcfadden, S., Mcgann, L. E., Mul Drew, K., et al. (1998). Osteochondral dowel transplantation for repair of focal defects in the knee: an outcome study using an ovine model. Vet. Surg. 27, 5–16. doi: 10.1111/j.1532-950x.1998.tb00092.x
Husser, D., Ueberham, L., Jacob, J., Heuer, D., Riedel-Heller, S., Walker, J., et al. (2018). Prevalence of clinically apparent hypertrophic cardiomyopathy in Germany-An analysis of over 5 million patients. PLoS One 13:e0196612. doi: 10.1371/journal.pone.0196612
Iacopetti, I., Perazzi, A., Martinello, T., Gemignani, F., and Patruno, M. (2020). Hyaluronic acid, Manuka honey and Acemannan gel: wound-specific applications for skin lesions. Res. Vet. Sci. 129, 82–89. doi: 10.1016/j.rvsc.2020.01.009
Inesi, G. (2017). Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life 69, 211–217. doi: 10.1002/iub.1590
Ingenito, E. P., Tsai, L., Murthy, S., Tyagi, S., Mazan, M., and Hoffman, A. (2012). Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant. 21, 175–189. doi: 10.3727/096368910x550233
Iwaniec, T. (2008). “Animal models for osteoporosis,” in Osteoporosis , 3rd Edn, eds R. Marcus, D. Feldman, D. Nelson, and C. Rosen (Amsterdam: Elsevier).
Jackson, S. J., Andrews, N., Ball, D., Bellantuono, I., Gray, J., Hachoumi, L., et al. (2017). Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 51, 160–169. doi: 10.1177/0023677216653984
Jarvinen, T. A., Kannus, P., Maffulli, N., and Khan, K. M. (2005). Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 10, 255–266. doi: 10.1016/j.fcl.2005.01.013
Jeanblanc, C., Goodrich, A. D., Colletti, E., Mokhtari, S., Porada, C. D., Zanjani, E. D., et al. (2014). Temporal definition of haematopoietic stem cell niches in a large animal model of in utero stem cell transplantation. Br. J. Haematol. 166, 268–278. doi: 10.1111/bjh.12870
Jeong, Y. H., Park, C. H., Jang, G. H., Jeong, Y. I., Hwang, I. S., Jeong, Y. W., et al. (2013). Production of multiple transgenic Yucatan miniature pigs expressing human complement regulatory factors, human CD55, CD59, and H-transferase genes. PLoS One 8:e63241. doi: 10.1371/journal.pone.0063241
Jiang, Y., Xie, M., Chen, W., Talbot, R., Maddox, J. F., Faraut, T., et al. (2014). The sheep genome illuminates biology of the rumen and lipid metabolism. Science 344, 1168–1173.
Johnson, V. L., and Hunter, D. J. (2014). The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 28, 5–15.
Johnston, P. V., Sasano, T., Mills, K., Evers, R., Lee, S. T., Smith, R. R., et al. (2009). Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120, 1075–1083. doi: 10.1161/circulationaha.108.816058
Julke, H., Veit, C., Ribitsch, I., Brehm, W., Ludewig, E., and Delling, U. (2015). Comparative labeling of equine and ovine multipotent stromal cells with superparamagnetic iron oxide particles for magnetic resonance imaging in vitro . Cell Transplant. 24, 1111–1125. doi: 10.3727/096368913x675737
Kahn, D., Hickman, R., Terblanche, J., and Von Sommoggy, S. (1988). Partial hepatectomy and liver regeneration in pigs–the response to different resection sizes. J. Surg. Res. 45, 176–180. doi: 10.1016/0022-4804(88)90062-5
Kajbafzadeh, A. M., Khorramirouz, R., Nabavizadeh, B., Ladi Seyedian, S. S., Akbarzadeh, A., Heidari, R., et al. (2019). Whole organ sheep kidney tissue engineering and in vivo transplantation: effects of perfusion-based decellularization on vascular integrity. Mater. Sci. Eng. C Mater. Biol. Appl. 98, 392–400. doi: 10.1016/j.msec.2019.01.018
Kang, N. V., Morritt, D., Pendegrass, C., and Blunn, G. (2013). Use of ITAP implants for prosthetic reconstruction of extra-oral craniofacial defects. J. Plast. Reconstr. Aesthet. Surg. 66, 497–505. doi: 10.1016/j.bjps.2012.11.036
Kang, N. V., Pendegrass, C., Marks, L., and Blunn, G. (2010). Osseocutaneous integration of an intraosseous transcutaneous amputation prosthesis implant used for reconstruction of a transhumeral amputee: case report. J. Hand Surg. Am. 35, 1130–1134. doi: 10.1016/j.jhsa.2010.03.037
Karlin, W. M., Stewart, A. A., Durgam, S. S., Naughton, J. F., O’dell-Anderson, K. J., and Stewart, M. C. (2011). Evaluation of experimentally induced injury to the superficial digital flexor tendon in horses by use of low-field magnetic resonance imaging and ultrasonography. Am. J. Vet. Res. 72, 791–798. doi: 10.2460/ajvr.72.6.791
Kim, B. E., Nevitt, T., and Thiele, D. J. (2008). Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 4, 176–185. doi: 10.1038/nchembio.72
Kim, H. K., Moran, M. E., and Salter, R. B. (1991). The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J. Bone Joint Surg. Am. 73, 1301–1315. doi: 10.2106/00004623-199173090-00004
Kim, J., Zanjani, E. D., Jeanblanc, C. M., Goodrich, A. D., and Hematti, P. (2013). Generation of CD34+ cells from human embryonic stem cells using a clinically applicable methodology and engraftment in the fetal sheep model. Exp. Hematol. 41, 749–758.e5. doi: 10.1016/j.exphem.2013.04.003
Kirschvink, N., and Reinhold, P. (2008). Use of alternative animals as asthma models. Curr. Drug Targets 9, 470–484. doi: 10.2174/138945008784533525
Kisiday, J. D., Mcilwraith, C. W., Rodkey, W. G., Frisbie, D. D., and Steadman, J. R. (2012). Effects of platelet-rich plasma composition on anabolic and catabolic activities in equine cartilage and meniscal explants. Cartilage 3, 245–254. doi: 10.1177/1947603511433181
Kluin, J., Talacua, H., Smits, A. I., Emmert, M. Y., Brugmans, M. C., Fioretta, E. S., et al. (2017). In situ heart valve tissue engineering using a bioresorbable elastomeric implant - From material design to 12 months follow-up in sheep. Biomaterials 125, 101–117. doi: 10.1016/j.biomaterials.2017.02.007
Klukowska-Rotzler, J., Swinburne, J. E., Drogemuller, C., Dolf, G., Janda, J., Leeb, T., et al. (2012). The interleukin 4 receptor gene and its role in recurrent airway obstruction in Swiss Warmblood horses. Anim. Genet. 43, 450–453. doi: 10.1111/j.1365-2052.2011.02277.x
Klymiuk, N., Blutke, A., Graf, A., Krause, S., Burkhardt, K., Wuensch, A., et al. (2013). Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum. Mol. Genet. 22, 4368–4382. doi: 10.1093/hmg/ddt287
Kocyildirim, E., Cardenes, N., Ting, A., Caceres, E., Bermudez, C., and Rojas, M. (2017). The use of GMP-produced bone marrow-derived stem cells in combination with extracorporeal membrane oxygenation in ARDS: an animal model. ASAIO J. 63, 324–332. doi: 10.1097/mat.0000000000000566
Kol, A., Arzi, B., Athanasiou, K. A., Farmer, D. L., Nolta, J. A., Rebhun, R. B., et al. (2015). Companion animals: translational scientist’s new best friends. Sci. Transl. Med. 7:308s21.
Kon, E., Muraglia, A., Corsi, A., Bianco, P., Marcacci, M., Martin, I., et al. (2000). Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. 49, 328–337. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q
Koobatian, M. T., Row, S., Smith, R. J. Jr., Koenigsknecht, C., Andreadis, S. T., and Swartz, D. D. (2016). Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials 76, 344–358. doi: 10.1016/j.biomaterials.2015.10.020
Krupkova, O., Smolders, L., Wuertz-Kozak, K., Cook, J., and Pozzi, A. (2018). The pathobiology of the meniscus: a comparison between the human and dog. Front. Vet. Sci. 5:73. doi: 10.3389/fvets.2018.00073
Kuroki, K., Cook, C. R., and Cook, J. L. (2011). Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage 19, 1142–1149. doi: 10.1016/j.joca.2011.06.007
Kuyinu, E. L., Narayanan, G., Nair, L. S., and Laurencin, C. T. (2016). Animal models of osteoarthritis: classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 11:19.
Kuypers, E., Ophelders, D., Jellema, R. K., Kunzmann, S., Gavilanes, A. W., and Kramer, B. W. (2012). White matter injury following fetal inflammatory response syndrome induced by chorioamnionitis and fetal sepsis: lessons from experimental ovine models. Early Hum. Dev. 88, 931–936. doi: 10.1016/j.earlhumdev.2012.09.011
Lacitignola, L., Crovace, A., Rossi, G., and Francioso, E. (2008). Cell therapy for tendinitis, experimental and clinical report. Vet. Res. Commun. 32, (Suppl. 1), S33–S38.
Lai, L., Kolber-Simonds, D., Park, K. W., Cheong, H. T., Greenstein, J. L., Im, G. S., et al. (2002). Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295, 1089–1092. doi: 10.1126/science.1068228
Lairmore, M. D., and Khanna, C. (2014). Naturally occurring diseases in animals: contributions to translational medicine. ILAR J. 55, 1–3. doi: 10.1093/ilar/ilu022
Lampropoulou-Adamidou, K., Lelovas, P., Karadimas, E. V., Liakou, C., Triantafillopoulos, I. K., Dontas, I., et al. (2014). Useful animal models for the research of osteoarthritis. Eur. J. Orthop. Surg. Traumatol. 24, 263–271.
Lange-Consiglio, A., Stucchi, L., Zucca, E., Lavoie, J. P., Cremonesi, F., and Ferrucci, F. (2019). Insights into animal models for cell-based therapies in translational studies of lung diseases: Is the horse with naturally occurring asthma the right choice? Cytotherapy 21, 525–534. doi: 10.1016/j.jcyt.2019.02.010
Larson, G., Karlsson, E. K., Perri, A., Webster, M. T., Ho, S. Y., Peters, J., et al. (2012). Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc. Natl. Acad. Sci. U.S.A. 109, 8878–8883. doi: 10.1073/pnas.1203005109
Lavoie, J. P., Maghni, K., Desnoyers, M., Taha, R., Martin, J. G., and Hamid, Q. A. (2001). Neutrophilic airway inflammation in horses with heaves is characterized by a Th2-type cytokine profile. Am. J. Respir. Crit. Care Med. 164, 1410–1413. doi: 10.1164/ajrccm.164.8.2012091
Leclere, M., Lavoie-Lamoureux, A., Gelinas-Lymburner, E., David, F., Martin, J. G., and Lavoie, J. P. (2011). Effect of antigenic exposure on airway smooth muscle remodeling in an equine model of chronic asthma. Am. J. Respir. Cell Mol. Biol. 45, 181–187. doi: 10.1165/rcmb.2010-0300oc
Lelovas, P. P., Xanthos, T. T., Thoma, S. E., Lyritis, G. P., and Dontas, I. A. (2008). The laboratory rat as an animal model for osteoporosis research. Comp. Med. 58, 424–430.
Li, T. S., Cheng, K., Malliaras, K., Smith, R. R., Zhang, Y., Sun, B., et al. (2012). Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 59, 942–953. doi: 10.1016/j.jacc.2011.11.029
Li, Y., Chen, S. K., Li, L., Qin, L., Wang, X. L., and Lai, Y. X. (2015). Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 3, 95–104. doi: 10.1016/j.jot.2015.05.002
Liebsch, C., Bucan, V., Menger, B., Kohne, F., Waldmann, K. H., Vaslaitis, D., et al. (2018). Preliminary investigations of spider silk in wounds in vivo - Implications for an innovative wound dressing. Burns 44, 1829–1838. doi: 10.1016/j.burns.2018.03.016
Liechty, K. W., Mackenzie, T. C., Shaaban, A. F., Radu, A., Moseley, A. M., Deans, R., et al. (2000). Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat. Med. 6, 1282–1286. doi: 10.1038/81395
Lindblad-Toh, K., Wade, C. M., Mikkelsen, T. S., Karlsson, E. K., Jaffe, D. B., Kamal, M., et al. (2005). Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438, 803–819.
Little, C. B., and Hunter, D. J. (2013). Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat. Rev. Rheumatol. 9, 485–497. doi: 10.1038/nrrheum.2013.72
Little, C. B., and Zaki, S. (2012). What constitutes an “animal model of osteoarthritis”–the need for consensus? Osteoarthritis Cartilage 20, 261–267. doi: 10.1016/j.joca.2012.01.017
Liu, J. Z., Van Sommeren, S., Huang, H., Ng, S. C., Alberts, R., Takahashi, A., et al. (2015). Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986. doi: 10.1038/ng.3359
Lizarraga, I., and Chambers, J. P. (2012). Use of analgesic drugs for pain management in sheep. N. Z. Vet. J. 60, 87–94. doi: 10.1080/00480169.2011.642772
Lo Monaco, M., Merckx, G., Ratajczak, J., Gervois, P., Hilkens, P., Clegg, P., et al. (2018). Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018:9079538.
Lorbach, O., Baums, M. H., Kostuj, T., Pauly, S., Scheibel, M., Carr, A., et al. (2015). Advances in biology and mechanics of rotator cuff repair. Knee Surg. Sports Traumatol. Arthrosc. 23, 530–541.
Lovicu, M., Dessi, V., Lepori, M. B., Zappu, A., Zancan, L., Giacchino, R., et al. (2006). The canine copper toxicosis gene Murr1 is not implicated in the pathogenesis of Wilson disease. J. Gastroenterol. 41, 582–587. doi: 10.1007/s00535-006-1807-0
Madden, J. C., Hewitt, M., Przybylak, K., Vandebriel, R. J., Piersma, A. H., and Cronin, M. T. (2012). Strategies for the optimisation of in vivo experiments in accordance with the 3Rs philosophy. Regul. Toxicol. Pharmacol. 63, 140–154. doi: 10.1016/j.yrtph.2012.03.010
Maffulli, N. (1999). Rupture of the Achilles tendon. J. Bone Joint. Surg. Am. 81, 1019–1036.
Maher, R. L., Barbash, S. M., Lynch, D. V., and Swoap, S. J. (2015). Group housing and nest building only slightly ameliorate the cold stress of typical housing in female C57bl/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R1070–R1079.
Makkar, R. R., Smith, R. R., Cheng, K., Malliaras, K., Thomson, L. E., Berman, D., et al. (2012). Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904. doi: 10.1016/s0140-6736(12)60195-0
Malda, J., Benders, K. E., Klein, T. J., De Grauw, J. C., Kik, M. J., Hutmacher, D. W., et al. (2012). Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthritis Cartilage 20, 1147–1151. doi: 10.1016/j.joca.2012.06.005
Malliaras, K., Li, T. S., Luthringer, D., Terrovitis, J., Cheng, K., Chakravarty, T., et al. (2012). Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125, 100–112. doi: 10.1161/circulationaha.111.042598
Malliaras, K., Makkar, R. R., Smith, R. R., Cheng, K., Wu, E., Bonow, R. O., et al. (2014). Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 63, 110–122.
Marfe, G., Rotta, G., De Martino, L., Tafani, M., Fiorito, F., Di Stefano, C., et al. (2012). A new clinical approach: use of blood-derived stem cells (BDSCs) for superficial digital flexor tendon injuries in horses. Life Sci. 90, 825–830. doi: 10.1016/j.lfs.2012.03.004
Marijnissen, A. C., Van Roermund, P. M., Tekoppele, J. M., Bijlsma, J. W., and Lafeber, F. P. (2002). The canine ‘groove’ model, compared with the ACLT model of osteoarthritis. Osteoarthritis Cartilage 10, 145–155. doi: 10.1053/joca.2001.0491
Mariscal, A., Caldarone, L., Tikkanen, J., Nakajima, D., Chen, M., Yeung, J., et al. (2018). Pig lung transplant survival model. Nat. Protoc. 13, 1814–1828. doi: 10.1038/s41596-018-0019-4
Marlovits, S., Singer, P., Zeller, P., Mandl, I., Haller, J., and Trattnig, S. (2006). Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 57, 16–23. doi: 10.1016/j.ejrad.2005.08.007
Maron, B. J., and Fox, P. R. (2015). Hypertrophic cardiomyopathy in man and cats. J. Vet. Cardiol. 17, (Suppl. 1), S6–S9.
Maron, B. J., Gardin, J. M., Flack, J. M., Gidding, S. S., Kurosaki, T. T., and Bild, D. E. (1995). Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 92, 785–789. doi: 10.1161/01.cir.92.4.785
Marston, S. B. (2011). How do mutations in contractile proteins cause the primary familial cardiomyopathies? J. Cardiovasc. Transl. Res. 4, 245–255. doi: 10.1007/s12265-011-9266-2
Martin, J., Sarai, K., Yoshitake, M., Haberstroh, J., Takahashi, N., Lutter, G., et al. (1999). Successful orthotopic pig heart transplantation from non-heart-beating donors. J. Heart Lung Transplant. 18, 597–606. doi: 10.1016/s1053-2498(98)00017-5
Martinello, T., Bronzini, I., Perazzi, A., Testoni, S., De Benedictis, G. M., Negro, A., et al. (2013). Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J. Orthop. Res. 31, 306–314. doi: 10.1002/jor.22205
Martinello, T., Gomiero, C., Perazzi, A., Iacopetti, I., Gemignani, F., DeBenedictis, G. M., et al. (2018). Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res . 14, 1–9. doi: 10.1186/s12917-018-1527-8
Martines, E., Brun, P., Cavazzana, R., Cordaro, L., Zuin, M., Martinello, T., et al. (2020). Wound healing improvement in large animals using an indirect helium plasma treatment. Clin. Plasma Med. 17-18:100095. doi: 10.1016/j.cpme.2020.100095
Marubayashi, S., Asahara, T., Ono, E., Tashiro, H., Okugawa, K., Okimoto, T., et al. (1995). Auxiliary heterotopic partial liver transplantation in pigs with acute liver failure. Surg. Today 25, 429–432. doi: 10.1007/bf00311820
Matsuyama, T., Midha, R., Mackinnon, S. E., Munro, C. A., Wong, P. Y., and Ang, L. C. (2000). Long nerve allografts in sheep with Cyclosporin A immunosuppression. J. Reconstr. Microsurg. 16, 219–225.
Mausberg, T. B., Wess, G., Simak, J., Keller, L., Drogemuller, M., Drogemuller, C., et al. (2011). A locus on chromosome 5 is associated with dilated cardiomyopathy in Doberman Pinschers. PLoS One 6:e20042. doi: 10.1371/journal.pone.0020042
Mazzone, L., Moehrlen, U., Ochsenbein-Kolble, N., Pontiggia, L., Biedermann, T., Reichmann, E., et al. (2020). Bioengineering and in utero transplantation of fetal skin in the sheep model: a crucial step towards clinical application in human fetal spina bifida repair. J. Tissue Eng. Regen. Med. 14, 58–65. doi: 10.1002/term.2963
Mcanulty, P., Dayan, A., Ganderup, N.-C., Hastings, K., Turk, J., and Laughlin, M. (2011). The Minipig in Biomedical Research. Boca Raton, FL: Taylor & Francis.
Mccoy, A. M. (2015). Animal models of osteoarthritis: comparisons and key considerations. Vet. Pathol. 52, 803–818. doi: 10.1177/0300985815588611
Mcduffee, L. A., Pack, L., Lores, M., Wright, G. M., Esparza-Gonzalez, B., and Masaoud, E. (2012). Osteoprogenitor cell therapy in an equine fracture model. Vet. Surg. 41, 773–783. doi: 10.1111/j.1532-950x.2012.01024.x
Mcgovern, J. A., Griffin, M., and Hutmacher, D. W. (2018). Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 11:dmm033084. doi: 10.1242/dmm.033084
Mcilwraith, C. W., Fortier, L. A., Frisbie, D. D., and Nixon, A. J. (2011). Equine Models of Articular Cartilage Repair. Cartilage 2, 317–326. doi: 10.1177/1947603511406531
Mcilwraith, C. W., Frisbie, D. D., and Kawcak, C. E. (2012). The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 1, 297–309. doi: 10.1302/2046-3758.111.2000132
Mcilwraith, C. W., Frisbie, D. D., Kawcak, C. E., Fuller, C. J., Hurtig, M., and Cruz, A. (2010). The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the horse. Osteoarthritis Cartilage 18, (Suppl. 3), S93–S105.
Mclure, S. W., Fisher, J., Conaghan, P. G., and Williams, S. (2012). Regional cartilage properties of three quadruped tibiofemoral joints used in musculoskeletal research studies. Proc. Inst. Mech. Eng. H 226, 652–656. doi: 10.1177/0954411912447158
Meeusen, E. N., Snibson, K. J., Hirst, S. J., and Bischof, R. J. (2009). Sheep as a model species for the study and treatment of human asthma and other respiratory diseases. Drug Discov. Today Dis. Model. 6, 101–106. doi: 10.1016/j.ddmod.2009.12.002
Meng, X., Ziadlou, R., Grad, S., Alini, M., Wen, C., Lai, Y., et al. (2020). Animal models of osteochondral defect for testing biomaterials. Biochem Res. Int. 2020:9659412.
Messer, A. E., Chan, J., Daley, A., Copeland, O., Marston, S. B., and Connolly, D. J. (2017). Investigations into the sarcomeric protein and Ca(2+)-regulation abnormalities underlying hypertrophic cardiomyopathy in cats ( Felix catus ). Front. Physiol. 8:348. doi: 10.3389/fphys.2017.00348
Meurs, K. M., Lahmers, S., Keene, B. W., White, S. N., Oyama, M. A., Mauceli, E., et al. (2012). A splice site mutation in a gene encoding for PDK4, a mitochondrial protein, is associated with the development of dilated cardiomyopathy in the Doberman pinscher. Hum. Genet. 131, 1319–1325. doi: 10.1007/s00439-012-1158-2
Meurs, K. M., Mauceli, E., Lahmers, S., Acland, G. M., White, S. N., and Lindblad-Toh, K. (2010). Genome-wide association identifies a deletion in the 3’ untranslated region of striatin in a canine model of arrhythmogenic right ventricular cardiomyopathy. Hum. Genet. 128, 315–324. doi: 10.1007/s00439-010-0855-y
Meurs, K. M., Norgard, M. M., Ederer, M. M., Hendrix, K. P., and Kittleson, M. D. (2007). A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 90, 261–264. doi: 10.1016/j.ygeno.2007.04.007
Meurs, K. M., Sanchez, X., David, R. M., Bowles, N. E., Towbin, J. A., Reiser, P. J., et al. (2005). A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum. Mol. Genet. 14, 3587–3593. doi: 10.1093/hmg/ddi386
Meurs, K. M., Stern, J. A., Reina-Doreste, Y., Spier, A. W., Koplitz, S. L., and Baumwart, R. D. (2014). Natural history of arrhythmogenic right ventricular cardiomyopathy in the boxer dog: a prospective study. J. Vet. Intern. Med. 28, 1214–1220. doi: 10.1111/jvim.12385
Mienaltowski, M. J., Dunkman, A. A., Buckley, M. R., Beason, D. P., Adams, S. M., Birk, D. E., et al. (2016). Injury response of geriatric mouse patellar tendons. J. Orthop. Res. 34, 1256–1263. doi: 10.1002/jor.23144
Milner, P. I., Clegg, P. D., and Stewart, M. C. (2011). Stem cell-based therapies for bone repair. Vet. Clin. North Am. Equine Pract. 27, 299–314.
Mitra, A., Leyes, A., Manser, K., Roadcap, B., Mestre, C., Tatosian, D., et al. (2015). Use of minipig skin biopsy model as an innovative tool to design topical formulation to achieve desired pharmacokinetics in humans. J. Pharm. Sci. 104, 1701–1708. doi: 10.1002/jps.24383
Moezzi, L., Pourfathollah, A. A., Alimoghaddam, K., Soleimani, M., and Ardjmand, A. R. (2005). The effect of cryopreservation on clonogenic capacity and in vitro expansion potential of umbilical cord blood progenitor cells. Transplant. Proc. 37, 4500–4503. doi: 10.1016/j.transproceed.2005.10.107
Moraes, J. R., Facco, G. G., Moraes, F. R., Engracia Filho, J. R., Miyazato, L. G., and Beretta, D. C. (2009). Effects of glycosaminoglycan polysulphate on the organisation of collagen fibres in experimentally induced tendonitis in horses. Vet. Rec. 165, 203–205. doi: 10.1136/vr.165.7.203
Moran, C. J., Ramesh, A., Brama, P. A., O’byrne, J. M., O’brien, F. J., and Levingstone, T. J. (2016). The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 3:1.
Morris, M. W. Jr., Allukian, M. III, Herdrich, B. J., Caskey, R. C., Zgheib, C., Xu, J., et al. (2014). Modulation of the inflammatory response by increasing fetal wound size or interleukin-10 overexpression determines wound phenotype and scar formation. Wound Repair Regen. 22, 406–414. doi: 10.1111/wrr.12180
Morrison, J. L., Berry, M. J., Botting, K. J., Darby, J. R. T., Frasch, M. G., Gatford, K. L., et al. (2018). Improving pregnancy outcomes in humans through studies in sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R1123–R1153.
Mosqueira, D., Mannhardt, I., Bhagwan, J. R., Lis-Slimak, K., Katili, P., Scott, E., et al. (2018). CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur. Heart J. 39, 3879–3892. doi: 10.1093/eurheartj/ehy249
Moss, T. J., Knox, C. L., Kallapur, S. G., Nitsos, I., Theodoropoulos, C., Newnham, J. P., et al. (2008). Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am. J. Obstet. Gynecol. 198, 122.e1–122.e8. doi: 10.1016/j.ajog.2007.06.065
Muller, T., Feichtinger, H., Berger, H., and Muller, W. (1996). Endemic Tyrolean infantile cirrhosis: an ecogenetic disorder. Lancet 347, 877–880. doi: 10.1016/s0140-6736(96)91351-3
Murayama, N., Kaneko, N., Horiuchi, K., Ohyama, K., Shimizu, M., Ito, K., et al. (2009). Cytochrome P450-dependent drug oxidation activity of liver microsomes from Microminipigs, a possible new animal model for humans in non-clinical studies. Drug Metab. Pharmacokinet. 24, 404–408. doi: 10.2133/dmpk.24.404
Murphy, M. K., Arzi, B., Hu, J. C., and Athanasiou, K. A. (2013). Tensile characterization of porcine temporomandibular joint disc attachments. J. Dent. Res. 92, 753–758. doi: 10.1177/0022034513494817
Music, E., Futrega, K., and Doran, M. R. (2018). Sheep as a model for evaluating mesenchymal stem/stromal cell (Msc)-based chondral defect repair. Osteoarthritis Cartilage 26, 730–740. doi: 10.1016/j.joca.2018.03.006
Muttini, A., Russo, V., Rossi, E., Mattioli, M., Barboni, B., Tosi, U., et al. (2015). Pilot experimental study on amniotic epithelial mesenchymal cell transplantation in natural occurring tendinopathy in horses. Ultrasonographic and histological comparison. Muscles Ligaments Tendons J. 5, 5–11.
Nadal-Ginard, B., Kajstura, J., Leri, A., and Anversa, P. (2003). Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ. Res. 92, 139–150. doi: 10.1161/01.res.0000053618.86362.df
Namba, R. S., Meuli, M., Sullivan, K. M., Le, A. X., and Adzick, N. S. (1998). Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J. Bone Joint Surg. Am. 80, 4–10. doi: 10.2106/00004623-199801000-00003
Nelson, T. E. (1990). Porcine malignant hyperthermia: critical temperatures for in vivo and in vitro responses. Anesthesiology 73, 449–454. doi: 10.1097/00000542-199009000-00013
Newman, E., Turner, A. S., and Wark, J. D. (1995). The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone 16, 277s–284s. doi: 10.1016/8756-3282(95)00026-a
Nitsos, I., Rees, S. M., Duncan, J., Kramer, B. W., Harding, R., Newnham, J. P., et al. (2006). Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J. Soc. Gynecol. Investig. 13, 239–247. doi: 10.1016/j.jsgi.2006.02.011
Niu, D., Wei, H. J., Lin, L., George, H., Wang, T., Lee, I. H., et al. (2017). Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 357, 1303–1307. doi: 10.1126/science.aan4187
Nixon, A. J., Dahlgren, L. A., Haupt, J. L., Yeager, A. E., and Ward, D. L. (2008). Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am. J. Vet. Res. 69, 928–937. doi: 10.2460/ajvr.69.7.928
Oakley, S. P., Lassere, M. N., Portek, I., Szomor, Z., Ghosh, P., Kirkham, B. W., et al. (2004). Biomechanical, histologic and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthritis Cartilage 12, 667–679. doi: 10.1016/j.joca.2004.05.006
Okada, Y., Wu, D., Trynka, G., Raj, T., Terao, C., Ikari, K., et al. (2014). Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381.
O’leary, S. A., Link, J. M., Klineberg, E. O., Hu, J. C., and Athanasiou, K. A. (2017). Characterization of facet joint cartilage properties in the human and interspecies comparisons. Acta Biomater. 54, 367–376. doi: 10.1016/j.actbio.2017.03.017
Orsini, J. A. (2012). Supporting limb laminitis: the four important ‘whys’. Equine Vet. J. 44, 741–745. doi: 10.1111/j.2042-3306.2012.00662.x
Pacini, S., Spinabella, S., Trombi, L., Fazzi, R., Galimberti, S., Dini, F., et al. (2007). Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 13, 2949–2955. doi: 10.1089/ten.2007.0108
Panettieri, R. A. Jr. (2016). Neutrophilic and Pauci-immune Phenotypes in Severe Asthma. Immunol. Allergy Clin. North Am. 36, 569–579. doi: 10.1016/j.iac.2016.03.007
Parker, H. G., Shearin, A. L., and Ostrander, E. A. (2010). Man’s best friend becomes biology’s best in show: genome analyses in the domestic dog. Annu. Rev. Genet. 44, 309–336. doi: 10.1146/annurev-genet-102808-115200
Patterson-Kane, J. C., and Firth, E. C. (2009). The pathobiology of exercise-induced superficial digital flexor tendon injury in Thoroughbred racehorses. Vet. J. 181, 79–89. doi: 10.1016/j.tvjl.2008.02.009
Payne, J., Luis Fuentes, V., Boswood, A., Connolly, D., Koffas, H., and Brodbelt, D. (2010). Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J. Small Anim. Pract. 51, 540–547. doi: 10.1111/j.1748-5827.2010.00989.x
Pearce, A. I., Richards, R. G., Milz, S., Schneider, E., and Pearce, S. G. (2007). Animal models for implant biomaterial research in bone: a review. Eur. Cell Mater. 13, 1–10. doi: 10.22203/ecm.v013a01
Pedersen, R., Andersen, A. D., Molbak, L., Stagsted, J., and Boye, M. (2013a). Changes in the gut microbiota of cloned and non-cloned control pigs during development of obesity: gut microbiota during development of obesity in cloned pigs. BMC Microbiol. 13:30. doi: 10.1186/1471-2180-13-30
Pedersen, R., Ingerslev, H. C., Sturek, M., Alloosh, M., Cirera, S., Christoffersen, B. O., et al. (2013b). Characterisation of gut microbiota in Ossabaw and Gottingen minipigs as models of obesity and metabolic syndrome. PLoS One 8:e56612. doi: 10.1371/journal.pone.0056612
Perleberg, C., Kind, A., and Schnieke, A. (2018). Genetically engineered pigs as models for human disease. Dis. Model. Mech. 11:dmm030783. doi: 10.1242/dmm.030783
Petersen, B., Ramackers, W., Lucas-Hahn, A., Lemme, E., Hassel, P., Queisser, A. L., et al. (2011). Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation 18, 355–368. doi: 10.1111/j.1399-3089.2011.00674.x
Phelps, C. J., Koike, C., Vaught, T. D., Boone, J., Wells, K. D., Chen, S. H., et al. (2003). Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299, 411–414. doi: 10.1126/science.1078942
Pinnapureddy, A. R., Stayner, C., Mcewan, J., Baddeley, O., Forman, J., and Eccles, M. R. (2015). Large animal models of rare genetic disorders: sheep as phenotypically relevant models of human genetic disease. Orphanet J. Rare Dis. 10:107.
Pobloth, A. M., Schell, H., Petersen, A., Beierlein, K., Kleber, C., Schmidt-Bleek, K., et al. (2018). Tubular open-porous beta-tricalcium phosphate polycaprolactone scaffolds as guiding structure for segmental bone defect regeneration in a novel sheep model. J. Tissue Eng. Regen. Med. 12, 897–911. doi: 10.1002/term.2446
Pogue, B., Estrada, A. H., Sosa-Samper, I., Maisenbacher, H. W., Lamb, K. E., Mincey, B. D., et al. (2013). Stem-cell therapy for dilated cardiomyopathy: a pilot study evaluating retrograde coronary venous delivery. J. Small Anim. Pract. 54, 361–366. doi: 10.1111/jsap.12098
Polejaeva, I. A., Rutigliano, H. M., and Wells, K. D. (2016). Livestock in biomedical research: history, current status and future prospective. Reprod. Fertil. Dev. 28, 112–124.
Pond, M. J., and Nuki, G. (1973). Experimentally-induced osteoarthritis in the dog. Ann. Rheum. Dis. 32, 387–388. doi: 10.1136/ard.32.4.387
Poole, R., Blake, S., Buschmann, M., Goldring, S., Laverty, S., Lockwood, S., et al. (2010). Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage 18, (Suppl. 3), S10–S16.
Porada, C. D., Park, P. J., Almeida-Porada, G., Liu, W., Ozturk, F., Glimp, H. A., et al. (2005). Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer. Mol. Ther. 11, 284–293. doi: 10.1016/j.ymthe.2004.09.009
Prabhakar, S. (2012). Translational research challenges: finding the right animal models. J. Investig. Med. 60, 1141–1146. doi: 10.2310/jim.0b013e318271fb3b
Price, J., Catriona, S., Welsh, E. M., and Waran, N. K. (2003). Preliminary evaluation of a behaviour-based system for assessment of post-operative pain in horses following arthroscopic surgery. Vet. Anaesth. Analg. 30, 124–137. doi: 10.1046/j.1467-2995.2003.00139.x
Proffen, B. L., Mcelfresh, M., Fleming, B. C., and Murray, M. M. (2012). A comparative anatomical study of the human knee and six animal species. Knee 19, 493–499. doi: 10.1016/j.knee.2011.07.005
Provost, P. J. (2019). “Wound healing,” in Equine Surgery , 5th Edn, eds J. A. Auer, J. A. Stick, J. M. Kümmerle, and T. Prang (Amsterdam: Elsevier).
Rabbani, S., Soleimani, M., Sahebjam, M., Imani, M., Nassiri, S. M., Atashi, A., et al. (2017). Effects of endothelial and mesenchymal stem cells on improving myocardial function in a sheep animal model. J. Tehran Heart Cent. 12, 65–71.
Radtke, C., Allmeling, C., Waldmann, K. H., Reimers, K., Thies, K., Schenk, H. C., et al. (2011). Spider silk constructs enhance axonal regeneration and remyelination in long nerve defects in sheep. PLoS One 6:e16990. doi: 10.1371/journal.pone.0016990
Renzi, S., Ricco, S., Dotti, S., Sesso, L., Grolli, S., Cornali, M., et al. (2013). Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: a clinical report. Res. Vet. Sci. 95, 272–277. doi: 10.1016/j.rvsc.2013.01.017
Richardson, L. E., Dudhia, J., Clegg, P. D., and Smith, R. (2007). Stem cells in veterinary medicine–attempts at regenerating equine tendon after injury. Trends Biotechnol. 25, 409–416. doi: 10.1016/j.tibtech.2007.07.009
Ripoll Vera, T., Monserrat Iglesias, L., Hermida Prieto, M., Ortiz, M., Rodriguez Garcia, I., and Govea Callizo, N. (2010). The R820W mutation in the MYBPC3 gene, associated with hypertrophic cardiomyopathy in cats, causes hypertrophic cardiomyopathy and left ventricular non-compaction in humans. Int. J. Cardiol. 145, 405–407. doi: 10.1016/j.ijcard.2010.04.032
Robinson, P., Liu, X., Sparrow, A., Patel, S., Zhang, Y. H., Casadei, B., et al. (2018). Hypertrophic cardiomyopathy mutations increase myofilament Ca(2+) buffering, alter intracellular Ca(2+) handling, and stimulate Ca(2+)-dependent signaling. J. Biol. Chem. 293, 10487–10499. doi: 10.1074/jbc.ra118.002081
Rogers, C. S. (2016). Genetically engineered livestock for biomedical models. Transgenic Res. 25, 345–359. doi: 10.1007/s11248-016-9928-6
Rolauffs, B., Williams, J. M., Aurich, M., Grodzinsky, A. J., Kuettner, K. E., and Cole, A. A. (2010). Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis Rheum. 62, 489–498.
Sachs, D. H., Sykes, M., and Yamada, K. (2009). Achieving tolerance in pig-to-primate xenotransplantation: reality or fantasy. Transpl. Immunol. 21, 101–105. doi: 10.1016/j.trim.2008.11.005
Sanjurjo-Rodriguez, C., Castro-Vinuelas, R., Hermida-Gomez, T., Fernandez-Vazquez, T., Fuentes-Boquete, I. M., De Toro-Santos, F. J., et al. (2017). Ovine mesenchymal stromal cells: morphologic, phenotypic and functional characterization for osteochondral tissue engineering. PLoS One 12:e0171231. doi: 10.1371/journal.pone.0171231
Sanz-Ramos, P., Duart, J., Rodriguez-Goni, M. V., Vicente-Pascual, M., Dotor, J., Mora, G., et al. (2014). Improved chondrogenic capacity of collagen hydrogel-expanded chondrocytes: in vitro and in vivo analyses. J. Bone Joint Surg. Am. 96, 1109–1117. doi: 10.2106/jbjs.m.00271
Sasaki-Honda, M., Jonouchi, T., Arai, M., Hotta, A., Mitsuhashi, S., Nishino, I., et al. (2018). A patient-derived iPSC model revealed oxidative stress increases facioscapulohumeral muscular dystrophy-causative DUX4. Hum. Mol. Genet. 27, 4024–4035. doi: 10.1093/hmg/ddy293
Sawyer, M., Moe, J., and Osburn, B. I. (1978). Ontogeny of immunity and leukocytes in the ovine fetus and elevation of immunoglobulins related to congenital infection. Am. J. Vet. Res. 39, 643–648.
Schagemann, J. C., Erggelet, C., Chung, H. W., Lahm, A., Kurz, H., and Mrosek, E. H. (2009). Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Eng. Part A 15, 75–82. doi: 10.1089/ten.tea.2008.0087
Scharf, A., Holmes, S. P., Thoresen, M., Mumaw, J., Stumpf, A., and Peroni, J. (2016). MRI-based assessment of intralesional delivery of bone marrow-derived mesenchymal stem cells in a model of equine tendonitis. Stem Cells Int. 2016:8610964.
Scheerlinck, J. P., Snibson, K. J., Bowles, V. M., and Sutton, P. (2008). Biomedical applications of sheep models: from asthma to vaccines. Trends Biotechnol. 26, 259–266. doi: 10.1016/j.tibtech.2008.02.002
Scheinberg, I. H., and Sternlieb, I. (1996). Wilson disease and idiopathic copper toxicosis. Am. J. Clin. Nutr. 63, 842s–845s.
Schnabel, L. V., Lynch, M. E., Van Der Meulen, M. C., Yeager, A. E., Kornatowski, M. A., and Nixon, A. J. (2009). Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 27, 1392–1398. doi: 10.1002/jor.20887
Schnabel, L. V., Mohammed, H. O., Miller, B. J., Mcdermott, W. G., Jacobson, M. S., Santangelo, K. S., et al. (2007). Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J. Orthop. Res. 25, 230–240. doi: 10.1002/jor.20278
Schramme, M., Hunter, S., Campbell, N., Blikslager, A., and Smith, R. (2010). A surgical tendonitis model in horses: technique, clinical, ultrasonographic and histological characterisation. Vet. Comp. Orthop. Traumatol. 23, 231–239.
Segatto, N. V., Remiao, M. H., Schachtschneider, K. M., Seixas, F. K., Schook, L. B., and Collares, T. (2017). The oncopig cancer model as a complementary tool for phenotypic drug discovery. Front. Pharmacol. 8:894. doi: 10.3389/fphar.2017.00894
Semsarian, C., Ingles, J., Maron, M. S., and Maron, B. J. (2015). New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254.
Seo, J. P., Tsuzuki, N., Haneda, S., Yamada, K., Furuoka, H., Tabata, Y., et al. (2014). Osteoinductivity of gelatin/beta-tricalcium phosphate sponges loaded with different concentrations of mesenchymal stem cells and bone morphogenetic protein-2 in an equine bone defect model. Vet. Res. Commun. 38, 73–80. doi: 10.1007/s11259-013-9587-5
Shanks, N., Greek, R., and Greek, J. (2009). Are animal models predictive for humans? Philos. Ethics Humanit. Med. 4:2.
Shapiro, S. D. (2008). The use of transgenic mice for modeling airways disease. Pulm. Pharmacol. Ther. 21, 699–701. doi: 10.1016/j.pupt.2008.01.006
Sheehan, A., Messer, A. E., Papadaki, M., Choudhry, A., Kren, V., Biedermann, D., et al. (2018). Molecular defects in cardiac Myofilament Ca(2+)-regulation due to cardiomyopathy-linked mutations can be reversed by small molecules binding to troponin. Front. Physiol. 9:243. doi: 10.3389/fphys.2018.00243
Shinagawa, K., and Kojima, M. (2003). Mouse model of airway remodeling: strain differences. Am. J. Respir. Crit. Care Med. 168, 959–967. doi: 10.1164/rccm.200210-1188oc
Silverstein, A. M., Prendergast, R. A., and Kraner, K. L. (1964). Fetal response to antigenic stimulus. IV. Rejection of skin homografts by the fetal lamb. J. Exp. Med. 119, 955–964. doi: 10.1084/jem.119.6.955
Silverstein, A. M., Uhr, J. W., Kraner, K. L., and Lukes, R. J. (1963). Fetal response to antigenic stimulus. Ii. Antibody production by the fetal lamb. J. Exp. Med. 117, 799–812. doi: 10.1084/jem.117.5.799
Simoes, I. N., Vale, P., Soker, S., Atala, A., Keller, D., Noiva, R., et al. (2017). Acellular urethra bioscaffold: decellularization of whole urethras for tissue engineering applications. Sci. Rep. 7:41934.
Simpson, S., Edwards, J., Emes, R. D., Cobb, M. A., Mongan, N. P., and Rutland, C. S. (2015a). A predictive model for canine dilated cardiomyopathy-a meta-analysis of Doberman Pinscher data. PeerJ 3:e842. doi: 10.7717/peerj.842
Simpson, S., Edwards, J., Ferguson-Mignan, T. F., Cobb, M., Mongan, N. P., and Rutland, C. S. (2015b). Genetics of human and canine dilated cardiomyopathy. Int. J. Genomics 2015:204823.
Skaanild, M. T. (2006). Porcine cytochrome P450 and metabolism. Curr. Pharm. Des. 12, 1421–1427. doi: 10.2174/138161206776361183
Smith, R., Mcilwraith, W., Schweitzer, R., Kadler, K., Cook, J., Caterson, B., et al. (2014). Advances in the understanding of tendinopathies: a report on the Second Havemeyer Workshop on equine tendon disease. Equine Vet. J. 46, 4–9. doi: 10.1111/evj.12128
Smith, R. K. (2008). Mesenchymal stem cell therapy for equine tendinopathy. Disabil. Rehabil. 30, 1752–1758. doi: 10.1080/09638280701788241
Smith, R. K., Korda, M., Blunn, G. W., and Goodship, A. E. (2003). Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 35, 99–102. doi: 10.2746/042516403775467388
Smith, R. K., Werling, N. J., Dakin, S. G., Alam, R., Goodship, A. E., and Dudhia, J. (2013). Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 8:e75697. doi: 10.1371/journal.pone.0075697
Sole, A., Spriet, M., Galuppo, L. D., Padgett, K. A., Borjesson, D. L., Wisner, E. R., et al. (2012). Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using (99m) Tc-HMPAO. Equine Vet. J. 44, 594–599. doi: 10.1111/j.2042-3306.2011.00530.x
Sole, A., Spriet, M., Padgett, K. A., Vaughan, B., Galuppo, L. D., Borjesson, D. L., et al. (2013). Distribution and persistence of technetium-99 hexamethyl propylene amine oxime-labelled bone marrow-derived mesenchymal stem cells in experimentally induced tendon lesions after intratendinous injection and regional perfusion of the equine distal limb. Equine Vet. J. 45, 726–731. doi: 10.1111/evj.12063
Song, F., Tang, J., Geng, R., Hu, H., Zhu, C., Cui, W., et al. (2014). Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int. J. Clin. Exp. Pathol. 7, 1415–1426.
Song, W., Vikhorev, P. G., Kashyap, M. N., Rowlands, C., Ferenczi, M. A., Woledge, R. C., et al. (2013). Mechanical and energetic properties of papillary muscle from ACTC E99K transgenic mouse models of hypertrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 304, H1513–H1524.
Spetzler, V. N., Goldaracena, N., Knaak, J. M., Louis, K. S., Selzner, N., and Selzner, M. (2015). Technique of porcine liver procurement and orthotopic transplantation using an active porto-caval shunt. J. Vis. Exp. 99:e52055.
Spriet, M., Buerchler, S., Trela, J. M., Hembrooke, T. A., Padgett, K. A., Rick, M. C., et al. (2015). Scintigraphic tracking of mesenchymal stem cells after intravenous regional limb perfusion and subcutaneous administration in the standing horse. Vet. Surg. 44, 273–280. doi: 10.1111/j.1532-950x.2014.12289.x
Starritt, N. E., Kettle, S. A., and Glasby, M. A. (2011). Sutureless repair of the facial nerve using biodegradable glass fabric. Laryngoscope 121, 1614–1619. doi: 10.1002/lary.21868
Stramer, B. M., Mori, R., and Martin, P. (2007). The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest. Dermatol. 127, 1009–1017. doi: 10.1038/sj.jid.5700811
Stuehler, B., Reichert, J., Stremmel, W., and Schaefer, M. (2004). Analysis of the human homologue of the canine copper toxicosis gene Murr1 in Wilson disease patients. J. Mol. Med. 82, 629–634.
Sullivan, D. C., Mirmalek-Sani, S. H., Deegan, D. B., Baptista, P. M., Aboushwareb, T., Atala, A., et al. (2012). Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33, 7756–7764. doi: 10.1016/j.biomaterials.2012.07.023
Sweigart, M. A., Zhu, C. F., Burt, D. M., Deholl, P. D., Agrawal, C. M., Clanton, T. O., et al. (2004). Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann. Biomed. Eng. 32, 1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c
Swindle, M. M. (2007). Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques , 2nd Edn. Boca Raton, FL: CRC Press.
Swindle, M. M., Makin, A., Herron, A. J., Clubb, F. J. Jr., and Frazier, K. S. (2012). Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49, 344–356. doi: 10.1177/0300985811402846
Tankersley, C. G., Fitzgerald, R. S., and Kleeberger, S. R. (1994). Differential control of ventilation among inbred strains of mice. Am. J. Physiol. 267, R1371–R1377.
Tanner, M. S. (1998). Role of copper in Indian childhood cirrhosis. Am. J. Clin. Nutr. 67, 1074s–1081s. doi: 10.1093/ajcn/67.5.1074s
Taylor, W. R., Ehrig, R. M., Heller, M. O., Schell, H., Seebeck, P., and Duda, G. N. (2006). Tibio-femoral joint contact forces in sheep. J. Biomech. 39, 791–798. doi: 10.1016/j.jbiomech.2005.02.006
Teeple, E., Jay, G. D., Elsaid, K. A., and Fleming, B. C. (2013). Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 15, 438–446. doi: 10.1208/s12248-013-9454-x
Tellegen, A. R., Dessing, A. J., Houben, K., Riemers, F. M., Creemers, L. B., Mastbergen, S. C., et al. (2019). Dog as a model for osteoarthritis: the fgf4 retrogene insertion may matter. J. Orthop. Res. 37, 2550–2560. doi: 10.1002/jor.24432
Tellegen, A. R., Rudnik-Jansen, I., Beukers, M., Miranda-Bedate, A., Bach, F. C., De Jong, W., et al. (2018). Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J. Control. Release 286, 439–450. doi: 10.1016/j.jconrel.2018.08.019
Thornburg, L. P. (1998). Histomorphological and immunohistochemical studies of chronic active hepatitis in Doberman Pinschers. Vet. Pathol. 35, 380–385. doi: 10.1177/030098589803500507
Thornburg, L. P., Rottinghaus, G., Dennis, G., and Crawford, S. (1996). The relationship between hepatic copper content and morphologic changes in the liver of West Highland White Terriers. Vet. Pathol. 33, 656–661. doi: 10.1177/030098589603300604
Thorpe, C. T., Clegg, P. D., and Birch, H. L. (2010). A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 42, 174–180. doi: 10.2746/042516409x480395
Tidholm, A., and Jonsson, L. (2005). Histologic characterization of canine dilated cardiomyopathy. Vet. Pathol. 42, 1–8. doi: 10.1354/vp.42-1-1
Tompkins, D., Hudgens, E., Horohov, D., and Baldwin, C. L. (2010). Expressed gene sequences of the equine cytokines interleukin-17 and interleukin-23. Vet. Immunol. Immunopathol. 133, 309–313. doi: 10.1016/j.vetimm.2009.08.008
Trela, J. M., Spriet, M., Padgett, K. A., Galuppo, L. D., Vaughan, B., and Vidal, M. A. (2014). Scintigraphic comparison of intra-arterial injection and distal intravenous regional limb perfusion for administration of mesenchymal stem cells to the equine foot. Equine Vet. J. 46, 479–483. doi: 10.1111/evj.12137
Twedt, D. C., Sternlieb, I., and Gilbertson, S. R. (1979). Clinical, morphologic, and chemical studies on copper toxicosis of Bedlington Terriers. J. Am. Vet. Med. Assoc. 175, 269–275.
Tytherleigh-Strong, G., Hurtig, M., and Miniaci, A. (2005). Intra-articular hyaluronan following autogenous osteochondral grafting of the knee. Arthroscopy 21, 999–1005. doi: 10.1016/j.arthro.2005.05.001
Van De Sluis, B., Rothuizen, J., Pearson, P. L., Van Oost, B. A., and Wijmenga, C. (2002). Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum. Mol. Genet. 11, 165–173. doi: 10.1093/hmg/11.2.165
Van De Sluis, B. J., Breen, M., Nanji, M., Van Wolferen, M., De Jong, P., Binns, M. M., et al. (1999). Genetic mapping of the copper toxicosis locus in Bedlington terriers to dog chromosome 10, in a region syntenic to human chromosome region 2p13-p16. Hum. Mol. Genet. 8, 501–507. doi: 10.1093/hmg/8.3.501
Van Loon, V. J., Scheffer, C. J., Genn, H. J., Hoogendoorn, A. C., and Greve, J. W. (2014). Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet. Q. 34, 92–97. doi: 10.1080/01652176.2014.949390
Van Steenbeek, F. G., Hytonen, M. K., Leegwater, P. A., and Lohi, H. (2016). The canine era: the rise of a biomedical model. Anim. Genet. 47, 519–527. doi: 10.1111/age.12460
Van Weeren, P. R., and Back, W. (2016). Musculoskeletal disease in aged horses and its management. Vet. Clin. North Am. Equine Pract. 32, 229–247. doi: 10.1016/j.cveq.2016.04.003
Vandeweerd, J. M., Hontoir, F., Kirschvink, N., Clegg, P., Nisolle, J. F., Antoine, N., et al. (2013). Prevalence of naturally occurring cartilage defects in the ovine knee. Osteoarthritis Cartilage 21, 1125–1131. doi: 10.1016/j.joca.2013.05.006
Varcoe, T. J., Gatfort, K. L., Holman, S. L., Cheung, P., Berry, M. J., Wiese, M. D., et al. (2019). Considerations in selecting postoperative analgesia for pregnant sheep following fetal instrumentation surgery. Anim. Front. 9, 60–67. doi: 10.1093/af/vfz019
Vila, J., Pariaut, R., Moise, N. S., Oxford, E. M., Fox, P. R., Reynolds, C. A., et al. (2017). Structural and molecular pathology of the atrium in boxer arrhythmogenic right ventricular cardiomyopathy. J. Vet. Cardiol. 19, 57–67. doi: 10.1016/j.jvc.2016.09.001
Vincent, T. L., Williams, R. O., Maciewicz, R., Silman, A., and Garside, P. (2012). Mapping pathogenesis of arthritis through small animal models. Rheumatology 51, 1931–1941. doi: 10.1093/rheumatology/kes035
Vischer, A. S., Connolly, D. J., Coats, C. J., Fuentes, V. L., Mckenna, W. J., Castelletti, S., et al. (2017). Arrhythmogenic right ventricular cardiomyopathy in Boxer dogs: the diagnosis as a link to the human disease. Acta Myol. 36, 135–150.
Vogel, T., Brockmann, J. G., Pigott, D., Neil, D. A. H., Muthusamy, A. S. R., Coussios, C. C., et al. (2017). Successful transplantation of porcine liver grafts following 48-hour normothermic preservation. PLoS One 12:e0188494. doi: 10.1371/journal.pone.0188494
Voleti, P. B., Buckley, M. R., and Soslowsky, L. J. (2012). Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng. 14, 47–71.
Vonk, W. I., De Bie, P., Wichers, C. G., Van Den Berghe, P. V., Van Der Plaats, R., Berger, R., et al. (2012). The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression. Cell. Mol. Life Sci. 69, 149–163. doi: 10.1007/s00018-011-0743-1
Warnock, J. J., Fox, D. B., Stoker, A. M., Beatty, M., Cockrell, M., Janicek, J. C., et al. (2014). Culture of equine fibroblast-like synoviocytes on synthetic tissue scaffolds towards meniscal tissue engineering: a preliminary cell-seeding study. PeerJ 2:e353. doi: 10.7717/peerj.353
Watanabe, M., Li, H., Kim, A. G., Weilerstein, A., Radu, A., Davey, M., et al. (2016). Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of Myelomeningocele. Biomaterials 76, 133–143. doi: 10.1016/j.biomaterials.2015.10.051
Watts, A. E., Nixon, A. J., Yeager, A. E., and Mohammed, H. O. (2012). A collagenase gel/physical defect model for controlled induction of superficial digital flexor tendonitis. Equine Vet. J. 44, 576–586. doi: 10.1111/j.2042-3306.2011.00471.x
Watts, A. E., Yeager, A. E., Kopyov, O. V., and Nixon, A. J. (2011). Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem Cell Res. Ther. 2:4. doi: 10.1186/scrt45
Webb, C. B., Twedt, D. C., and Meyer, D. J. (2002). Copper-associated liver disease in Dalmatians: a review of 10 dogs (1998-2001). J. Vet. Intern. Med. 16, 665–668. doi: 10.1111/j.1939-1676.2002.tb02405.x
Wess, G., Schulze, A., Butz, V., Simak, J., Killich, M., Keller, L. J., et al. (2010). Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J. Vet. Intern. Med. 24, 533–538. doi: 10.1111/j.1939-1676.2010.0479.x
Whitehouse, M. R., Howells, N. R., Parry, M. C., Austin, E., Kafienah, W., Brady, K., et al. (2017). Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl. Med. 6, 1237–1248. doi: 10.1002/sctm.16-0199
Williams, I. F., Mccullagh, K. G., Goodship, A. E., and Silver, I. A. (1984). Studies on the pathogenesis of equine tendonitis following collagenase injury. Res. Vet. Sci. 36, 326–338. doi: 10.1016/s0034-5288(18)31954-4
Williams, K., and Roman, J. (2016). Studying human respiratory disease in animals–role of induced and naturally occurring models. J. Pathol. 238, 220–232. doi: 10.1002/path.4658
Woodruff, P. G., Modrek, B., Choy, D. F., Jia, G., Abbas, A. R., Ellwanger, A., et al. (2009). T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 180, 388–395. doi: 10.1164/rccm.200903-0392oc
Wu, Z. Y., Zhao, G. X., Chen, W. J., Wang, N., Wan, B., Lin, M. T., et al. (2006). Mutation analysis of 218 Chinese patients with Wilson disease revealed no correlation between the canine copper toxicosis gene Murr1 and Wilson disease. J. Mol. Med. 84, 438–442. doi: 10.1007/s00109-005-0036-y
Xerogeanes, J. W., Fox, R. J., Takeda, Y., Kim, H. S., Ishibashi, Y., Carlin, G. J., et al. (1998). A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann. Biomed. Eng. 26, 345–352.
Yamamoto, S., Karashima, M., Sano, N., Fukushi, C., Tohyama, K., Arai, Y., et al. (2017). Utility of gottingen minipigs for prediction of human pharmacokinetic profiles after dermal drug application. Pharm. Res. 34, 2415–2424. doi: 10.1007/s11095-017-2247-7
Yang, H., Wang, G., Sun, H., Shu, R., Liu, T., Wang, C. E., et al. (2014). Species-dependent neuropathology in transgenic SOD1 pigs. Cell Res. 24, 464–481. doi: 10.1038/cr.2014.25
Yao, J., Huang, J., and Zhao, J. (2016). Genome editing revolutionize the creation of genetically modified pigs for modeling human diseases. Hum. Genet. 135, 1093–1105. doi: 10.1007/s00439-016-1710-6
Yu, H., Adesida, A. B., and Jomha, N. M. (2015). Meniscus repair using mesenchymal stem cells - a comprehensive review. Stem Cell Res. Ther. 6:86.
Zhao, W., Wang, Y., Liu, S., Huang, J., Zhai, Z., He, C., et al. (2015). The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One 10:e0117441. doi: 10.1371/journal.pone.0117441
Zorzi, A. R., Amstalden, E. M., Plepis, A. M., Martins, V. C., Ferretti, M., Antonioli, E., et al. (2015). Effect of human adipose tissue mesenchymal stem cells on the regeneration of ovine articular cartilage. Int. J. Mol. Sci. 16, 26813–26831. doi: 10.3390/ijms161125989
Zosky, G. R., and Sly, P. D. (2007). Animal models of asthma. Clin. Exp. Allergy 37, 973–988.
Keywords : large animal models, sheep, pig, horse, dog, regenerative medicine, tissue engineering, naturally occurring disease
Citation: Ribitsch I, Baptista PM, Lange-Consiglio A, Melotti L, Patruno M, Jenner F, Schnabl-Feichter E, Dutton LC, Connolly DJ, van Steenbeek FG, Dudhia J and Penning LC (2020) Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 8:972. doi: 10.3389/fbioe.2020.00972
Received: 12 February 2020; Accepted: 27 July 2020; Published: 13 August 2020.
Reviewed by:
Copyright © 2020 Ribitsch, Baptista, Lange-Consiglio, Melotti, Patruno, Jenner, Schnabl-Feichter, Dutton, Connolly, van Steenbeek, Dudhia and Penning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iris Ribitsch, [email protected]
This article is part of the Research Topic
Highlights from TERMIS EU 2019
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 18 January 2013
Animal models of traumatic brain injury
- Ye Xiong 1 ,
- Asim Mahmood 1 &
- Michael Chopp 2 , 3
Nature Reviews Neuroscience volume 14 , pages 128–142 ( 2013 ) Cite this article
29k Accesses
885 Citations
11 Altmetric
Metrics details
- Animal disease models
- Brain injuries
- Neurodegeneration
- Neurological disorders
- Preclinical research
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity both in civilian life and on the battlefield worldwide.
Animal models are essential for studying the biomechanical, cellular and molecular aspects of human TBI that cannot be addressed in the clinical setting, as well as for developing and characterizing novel therapeutic interventions.
Nevertheless, promising neuroprotective drugs, which were identified as being effective in animal TBI models, have all failed in Phase II or Phase III clinical trials.
This Review provides a broad overview of current knowledge of animal models of TBI, identifies the issues and challenges of therapeutic strategies in preclinical studies and highlights research strategies for improving animal models and therapeutic efficacy.
To achieve a therapeutic breakthrough in TBI, multifaceted approaches are probably required, including the development of new clinically relevant models, refinements of established models and functional tests, consideration of systemic insults and multimodality monitoring, identification of specific and sensitive biomarkers, and optimization of therapeutic dosing and timing of single and combination treatments, as well as improvement in clinical trial design and operation.
In addition, more research into the effects of age, sex and species or strain on the outcome of TBI is necessary. Additional studies in improving brain drug delivery systems and monitoring of target drug levels and drug effects are warranted in both animal models and the clinical setting.
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity both in civilian life and on the battlefield worldwide. Survivors of TBI frequently experience long-term disabling changes in cognition, sensorimotor function and personality. Over the past three decades, animal models have been developed to replicate the various aspects of human TBI, to better understand the underlying pathophysiology and to explore potential treatments. Nevertheless, promising neuroprotective drugs that were identified as being effective in animal TBI models have all failed in Phase II or Phase III clinical trials. This failure in clinical translation of preclinical studies highlights a compelling need to revisit the current status of animal models of TBI and therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Formation of memory assemblies through the DNA-sensing TLR9 pathway
Vladimir Jovasevic, Elizabeth M. Wood, … Jelena Radulovic

Predator-induced fear causes PTSD-like changes in the brains and behaviour of wild animals
Liana Y. Zanette, Emma C. Hobbs, … Michael Clinchy

Molecular and cellular mechanisms of selective vulnerability in neurodegenerative diseases
Martin Kampmann
Maas, A. I., Stocchetti, N. & Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7 , 728–741 (2008).
Article PubMed Google Scholar
Langlois, J. A., Rutland-Brown, W. & Wald, M. M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21 , 375–378 (2006).
Masel, B. E. & DeWitt, D. S. Traumatic brain injury: a disease process, not an event. J. Neurotrauma 27 , 1529–1540 (2010).
Davis, A. E. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit. Care Nurs. Q. 23 , 1–13 (2000).
Article CAS PubMed Google Scholar
Cernak, I. Animal models of head trauma. NeuroRx 2 , 410–422 (2005). This article comprehensively reviews animal models of TBI and addresses key factors in the development of animal models and neuroprotection for this condition.
Article PubMed PubMed Central Google Scholar
Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 115 , 4–18 (2004).
Bramlett, H. M. & Dietrich, W. D. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161 , 125–141 (2007).
Thompson, H. J. et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22 , 42–75 (2005). This article reviews the lateral fluid percussion model of TBI in small animal species, with a particular emphasis on its validity, clinical relevance and reliability.
Marklund, N., Bakshi, A., Castelbuono, D. J., Conte, V. & McIntosh, T. K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 12 , 1645–1680 (2006).
Povlishock, J. T. & Christman, C. W. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J. Neurotrauma 12 , 555–564 (1995). This article provides a review of factors involved in the pathogenesis of traumatically induced axonal injury in both animals and man.
Raghupathi, R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 14 , 215–222 (2004).
Raghupathi, R., Graham, D. I. & McIntosh, T. K. Apoptosis after traumatic brain injury. J. Neurotrauma 17 , 927–938 (2000).
Yakovlev, A. G. et al. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J. Neurosci. 21 , 7439–7446 (2001).
Article CAS PubMed PubMed Central Google Scholar
Niogi, S. N. et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 29 , 967–973 (2008).
Lipton, M. L. et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25 , 1335–1342 (2008).
Rubovitch, V. et al. A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 232 , 280–289 (2011).
Browne, K. D., Chen, X. H., Meaney, D. F. & Smith, D. H. Mild traumatic brain injury and diffuse axonal injury in swine. J. Neurotrauma 28 , 1747–1755 (2011).
Schouten, J. W. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr. Opin. Crit. Care 13 , 134–142 (2007).
Margulies, S. & Hicks, R. Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma 26 , 925–939 (2009).
Gurdjian, E. S., Lissner, H. R., Webster, J. E., Latimer, F. R. & Haddad, B. F. Studies on experimental concussion: relation of physiologic effect to time duration of intracranial pressure increase at impact. Neurology 4 , 674–681 (1954).
Walker, A. E. The physiological basis of concussion: 50 years later. J. Neurosurg. 81 , 493–494 (1994).
Denny-Brown, D. E. & Russell, W. R. Experimental concussion: (section of neurology). Proc. R. Soc. Med. 34 , 691–692 (1941).
CAS PubMed PubMed Central Google Scholar
Dixon, C. E. et al. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67 , 110–119 (1987). This study in rats systematically examines the effects of varying the magnitude of FPI on neurological, systemic physiological and histopathological changes.
Dixon, C. E., Clifton, G. L., Lighthall, J. W., Yaghmai, A. A. & Hayes, R. L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39 , 253–262 (1991).
Lighthall, J. W. Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma 5 , 1–15 (1988). This study introduces a new experimental model of mechanical brain injury, which was produced in the laboratory ferret using a stroke-constrained pneumatic impactor.
Marmarou, A. et al. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 80 , 291–300 (1994). This study describes the development of an experimental head injury model that is capable of producing diffuse brain injury in the rodent.
Cernak, I. et al. Involvement of the central nervous system in the general response to pulmonary blast injury. J. Trauma. 40 , S100–S104 (1996).
Leung, L. Y. et al. Blast related neurotrauma: a review of cellular injury. Mol. Cell. Biomech. 5 , 155–168 (2008).
PubMed Google Scholar
Potts, M. B., Adwanikar, H. & Noble-Haeusslein, L. J. Models of traumatic cerebellar injury. Cerebellum 8 , 211–221 (2009).
McIntosh, T. K., Noble, L., Andrews, B. & Faden, A. I. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4 , 119–134 (1987).
McIntosh, T. K. et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28 , 233–244 (1989).
Hardman, J. M. & Manoukian, A. Pathology of head trauma. Neuroimaging Clin. N. Am. 12 , 175–187 (2002).
Graham, D. I., McIntosh, T. K., Maxwell, W. L. & Nicoll, J. A. Recent advances in neurotrauma. J. Neuropathol. Exp. Neurol. 59 , 641–651 (2000).
Sanders, M. J., Dietrich, W. D. & Green, E. J. Cognitive function following traumatic brain injury: effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J. Neurotrauma 16 , 915–925 (1999).
Vink, R., Mullins, P. G., Temple, M. D., Bao, W. & Faden, A. I. Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. J. Neurotrauma 18 , 839–847 (2001).
Floyd, C. L., Golden, K. M., Black, R. T., Hamm, R. J. & Lyeth, B. G. Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J. Neurotrauma 19 , 303–316 (2002).
Hayes, R. L. et al. A new model of concussive brain injury in the cat produced by extradural fluid volume loading: II. Physiological and neuropathological observations. Brain Inj. 1 , 93–112 (1987).
Hartl, R., Medary, M., Ruge, M., Arfors, K. E. & Ghajar, J. Blood-brain barrier breakdown occurs early after traumatic brain injury and is not related to white blood cell adherence. Acta Neurochir. Suppl. 70 , 40–242 (1997).
CAS PubMed Google Scholar
Stalhammar, D. et al. A new model of concussive brain injury in the cat produced by extradural fluid volume loading: I. biomechanical properties. Brain Inj. 1 , 73–91 (1987).
Carbonell, W. S., Maris, D. O., McCall, T. & Grady, M. S. Adaptation of the fluid percussion injury model to the mouse. J. Neurotrauma 15 , 217–229 (1998).
Sullivan, H. G. et al. Fluid-percussion model of mechanical brain injury in the cat. J. Neurosurg. 45 , 521–534 (1976).
Millen, J. E., Glauser, F. L. & Fairman, R. P. A comparison of physiological responses to percussive brain trauma in dogs and sheep. J. Neurosurg. 62 , 587–591 (1985).
Dixon, C. E., Lighthall, J. W. & Anderson, T. E. Physiologic, histopathologic, and cineradiographic characterization of a new fluid-percussion model of experimental brain injury in the rat. J. Neurotrauma 5 , 91–104 (1988).
Alder, J., Fujioka, W., Lifshitz, J., Crockett, D. P. & Thakker-Varia, S. Lateral fluid percussion: model of traumatic brain injury in mice. J. Vis. Exp. 54 , e3063 (2011).
Google Scholar
Pfenninger, E. G., Reith, A., Breitig, D., Grunert, A. & Ahnefeld, F. W. Early changes of intracranial pressure, perfusion pressure, and blood flow after acute head injury. Part 1: an experimental study of the underlying pathophysiology. J. Neurosurg. 70 , 774–779 (1989).
Zink, B. J., Walsh, R. F. & Feustel, P. J. Effects of ethanol in traumatic brain injury. J. Neurotrauma 10 , 275–286 (1993).
Hicks, R., Soares, H., Smith, D. & McIntosh, T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 91 , 236–246 (1996).
Bramlett, H. M. & Dietrich, W. D. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 103 , 607–614 (2002).
Liu, Y. R. et al. Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. J. Nucl. Med. 51 , 1788–1795 (2010).
Morales, D. M. et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience 136 , 971–989 (2005).
Hamm, R. J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma 18 , 1207–1216 (2001).
Pierce, J. E., Smith, D. H., Trojanowski, J. Q. & McIntosh, T. K. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87 , 359–369 (1998).
Kabadi, S. V., Hilton, G. D., Stoica, B. A., Zapple, D. N. & Faden, A. I. Fluid-percussion-induced traumatic brain injury model in rats. Nature Protoc. 5 , 1552–1563 (2010).
Article CAS Google Scholar
Lifshitz, J., Kelley, J. B. & Povlishock, J. T. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J. Neuropathol. Exp. Neurol. 66 , 218–229 (2007).
Cao, T., Thomas, T. C., Ziebell, J. M., Pauly, J. R. & Lifshitz, J. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience 225 , 65–75 (2012).
Smith, D. H. et al. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12 , 169–178 (1995).
Lighthall, J. W., Goshgarian, H. G. & Pinderski, C. R. Characterization of axonal injury produced by controlled cortical impact. J. Neurotrauma 7 , 65–76 (1990).
Manley, G. T. et al. Controlled cortical impact in swine: pathophysiology and biomechanics. J. Neurotrauma 23 , 128–139 (2006).
King, C. et al. Brain temperature profiles during epidural cooling with the ChillerPad in a monkey model of traumatic brain injury. J. Neurotrauma 27 , 1895–1903 (2010).
Hall, E. D. et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22 , 252–265 (2005).
Mao, H., Zhang, L., Yang, K. H. & King, A. I. Application of a finite element model of the brain to study traumatic brain injury mechanisms in the rat. Stapp Car Crash J. 50 , 583–600 (2006).
Wang, H. C. & Ma, Y. B. Experimental models of traumatic axonal injury. J. Clin. Neurosci. 17 , 157–162 (2010).
Goodman, J. C., Cherian, L., Bryan, R. M. Jr & Robertson, C. S. Lateral cortical impact injury in rats: pathologic effects of varying cortical compression and impact velocity. J. Neurotrauma 11 , 587–597 (1994).
Saatman, K. E., Feeko, K. J., Pape, R. L. & Raghupathi, R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma 23 , 1241–1253 (2006).
Washington, P. M. et al. The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate and severe controlled cortical impact injury in mice. J. Neurotrauma 29 , 2283–2296 (2012).
Fox, G. B., Fan, L., Levasseur, R. A. & Faden, A. I. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 15 , 599–614 (1998).
Marklund, N. & Hillered, L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 164 , 1207–1229 (2011). This review describes the usefulness of animal models of TBI for preclinical evaluation of pharmacological compounds.
Dixon, C. E. et al. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 14 , 285–294 (1999).
Dixon, C. E. et al. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16 , 109–122 (1999).
Kochanek, P. M. et al. Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J. Neurotrauma 19 , 1029–1037 (2002).
Alessandri, B., Heimann, A., Filippi, R., Kopacz, L. & Kempski, O. Moderate controlled cortical contusion in pigs: effects on multi-parametric neuromonitoring and clinical relevance. J. Neurotrauma 20 , 1293–1305 (2003).
Williams, A. J. et al. Characterization of a new rat model of penetrating ballistic brain injury. J. Neurotrauma 22 , 313–331 (2005). This study characterizes the pathophysiology of a new rat model of penetrating brain injury that simulates the large temporary cavity caused by energy dissipation from a penetrating bullet round.
Williams, A. J., Hartings, J. A., Lu, X. C., Rolli, M. L. & Tortella, F. C. Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J. Neurotrauma 23 , 1828–1846 (2006).
Williams, A. J., Ling, G. S. & Tortella, F. C. Severity level and injury track determine outcome following a penetrating ballistic-like brain injury in the rat. Neurosci. Lett. 408 , 183–188 (2006).
Carey, M. E., Sarna, G. S. & Farrell, J. B. Brain edema following an experimental missile wound to the brain. J. Neurotrauma 7 , 13–20 (1990).
Carey, M. E., Sarna, G. S., Farrell, J. B. & Happel, L. T. Experimental missile wound to the brain. J. Neurosurg. 71 , 754–764 (1989).
Davis, A. R., Shear, D. A., Chen, Z., Lu, X. C. & Tortella, F. C. A comparison of two cognitive test paradigms in a penetrating brain injury model. J. Neurosci. Methods. 189 , 84–87 (2010).
Williams, A. J., Wei, H. H., Dave, J. R. & Tortella, F. C. Acute and delayed neuroinflammatory response following experimental penetrating ballistic brain injury in the rat. J. Neuroinflamm. 4 , 17 (2007).
Shear, D. A., Williams, A. J., Sharrow, K., Lu, X. C. & Tortella, F. C. Neuroprotective profile of dextromethorphan in an experimental model of penetrating ballistic-like brain injury. Pharmacol. Biochem. Behav. 94 , 56–62 (2009).
Chen, Z. et al. Human amnion-derived multipotent progenitor cell treatment alleviates traumatic brain injury-induced axonal degeneration. J. Neurotrauma 26 , 1987–1997 (2009).
Shear, D. A. et al. Longitudinal characterization of motor and cognitive deficits in a model of penetrating ballistic-like brain injury. J. Neurotrauma 27 , 1911–1923 (2010).
Plantman, S., Ng, K. C., Lu, J., Davidsson, J. & Risling, M. Characterization of a novel rat model of penetrating traumatic brain injury. J. Neurotrauma 29 , 1219–1232 (2012).
Wei, G., Lu, X. C., Yang, X. & Tortella, F. C. Intracranial pressure following penetrating ballistic-like brain injury in rats. J. Neurotrauma 27 , 1635–1641 (2010).
Foda, M. A. & Marmarou, A. A new model of diffuse brain injury in rats. Part II: morphological characterization. J. Neurosurg. 80 , 301–313 (1994).
Shear, D. A. et al. Severity profile of penetrating ballistic-like brain injury on neurofunctional outcome, blood-brain barrier permeability, and brain edema formation. J. Neurotrauma 28 , 2185–2195 (2011).
Feeney, D. M., Boyeson, M. G., Linn, R. T., Murray, H. M. & Dail, W. G. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 211 , 67–77 (1981).
Dail, W. G., Feeney, D. M., Murray, H. M., Linn, R. T. & Boyeson, M. G. Responses to cortical injury: II. widespread depression of the activity of an enzyme in cortex remote from a focal injury. Brain Res. 211 , 79–89 (1981).
Gasparovic, C., Arfai, N., Smid, N. & Feeney, D. M. Decrease and recovery of N -acetylaspartate/creatine in rat brain remote from focal injury. J. Neurotrauma 18 , 241–246 (2001).
Shapira, Y. et al. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 16 , 258–265 (1988). This article presents a model of CHI in rats that was developed using a calibrated weight-drop device.
Shohami, E., Shapira, Y. & Cotev, S. Experimental closed head injury in rats: prostaglandin production in a noninjured zone. Neurosurgery 22 , 859–863 (1988).
Chen, Y., Constantini, S., Trembovler, V., Weinstock, M. & Shohami, E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13 , 557–568 (1996).
Stahel, P. F. et al. Experimental closed head injury: analysis of neurological outcome, blood–brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 20 , 369–380 (2000).
Flierl, M. A. et al. Mouse closed head injury model induced by a weight-drop device. Nature Protoc. 4 , 1328–1337 (2009).
Goldstein, L. E. et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4 , 134ra60 (2012). This study develops a mouse model of blast TBI that recapitulated chronic traumatic encephalopathy-linked neuropathology 2 weeks after exposure to a single blast.
PubMed PubMed Central Google Scholar
Albert-Weissenberger, C., Varrallyay, C., Raslan, F., Kleinschnitz, C. & Siren, A. L. An experimental protocol for mimicking pathomechanisms of traumatic brain injury in mice. Exp. Transl. Stroke Med. 4 , 1 (2012).
Christman, C. W., Grady, M. S., Walker, S. A., Holloway, K. L. & Povlishock, J. T. Ultrastructural studies of diffuse axonal injury in humans. J. Neurotrauma 11 , 173–186 (1994).
Johnson, V. E., Stewart, W. & Smith, D. H. Axonal pathology in traumatic brain injury. Exp. Neurol. 20 Jan 2012 (doi:10.1016/j.expneurol.2012.01.013).
Heath, D. L. & Vink, R. Impact acceleration-induced severe diffuse axonal injury in rats: characterization of phosphate metabolism and neurologic outcome. J. Neurotrauma 12 , 1027–1034 (1995).
Schmidt, R. H., Scholten, K. J. & Maughan, P. H. Cognitive impairment and synaptosomal choline uptake in rats following impact acceleration injury. J. Neurotrauma 17 , 1129–1139 (2000).
Shohami, E., Shapira, Y., Sidi, A. & Cotev, S. Head injury induces increased prostaglandin synthesis in rat brain. J. Cereb. Blood Flow Metab. 7 , 58–63 (1987).
Kilbourne, M. et al. Novel model of frontal impact closed head injury in the rat. J. Neurotrauma 26 , 2233–2243 (2009).
Warden, D. Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21 , 398–402 (2006).
Benzinger, T. L. et al. Blast-related brain injury: imaging for clinical and research applications: report of the 2008 St. Louis workshop. J. Neurotrauma 26 , 2127–2144 (2009).
Wang, Y. et al. Tightly coupled repetitive blast-induced traumatic brain injury: development and characterization in mice. J. Neurotrauma 28 , 2171–2183 (2011).
Cheng, J. et al. Development of a rat model for studying blast-induced traumatic brain injury. J. Neurol. Sci. 294 , 23–28 (2010).
Risling, M. & Davidsson, J. Experimental animal models for studies on the mechanisms of blast-induced neurotrauma. Front. Neurol. 3 , 30 (2012).
Long, J. B. et al. Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma 26 , 827–840 (2009).
Bauman, R. A. et al. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma 26 , 841–860 (2009).
de Lanerolle, N. C. et al. Characteristics of an explosive blast-induced brain injury in an experimental model. J. Neuropathol. Exp. Neurol. 70 , 1046–1057 (2011).
DeWitt, D. S. & Prough, D. S. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J. Neurotrauma 26 , 877–887 (2009).
Reneer, D. V. et al. A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J. Neurotrauma 28 , 95–104 (2011).
Cernak, I. & Noble-Haeusslein, L. J. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 30 , 255–266 (2010).
Garman, R. H. et al. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma 28 , 947–959 (2011). This study identifies diffuse axonal injury as the most prominent feature during the initial 2 weeks following blast exposure in rats with body shields.
Kuehn, R. et al. Rodent model of direct cranial blast injury. J. Neurotrauma 28 , 2155–2169 (2011).
Saljo, A., Svensson, B., Mayorga, M., Hamberger, A. & Bolouri, H. Low-level blasts raise intracranial pressure and impair cognitive function in rats. J. Neurotrauma 26 , 1345–1352 (2009).
Cernak, I. et al. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol. Dis. 41 , 538–551 (2011).
Koliatsos, V. E. et al. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70 , 399–416 (2011).
Bhattacharjee, Y. Neuroscience. Shell shock revisited: solving the puzzle of blast trauma. Science. 319 , 406–408 (2008).
Sundaramurthy, A. et al. Blast-induced biomechanical loading of the rat: experimental and anatomically accurate computational blast injury model. J. Neurotrauma 29 , 2352–2364 (2012).
Daneshvar, D. H. et al. Long-term consequences: effects on normal development profile after concussion. Phys. Med. Rehabil. Clin. N. Am. 22 , 683–700, ix (2011).
[No authors listed.] Sports-related recurrent brain injuries — United States. Centers for disease control and prevention. Int. J. Trauma Nurs. 3 , 88–90 (1997).
Weber, J. T. Experimental models of repetitive brain injuries. Prog. Brain Res. 161 , 253–261 (2007).
Daneshvar, D. H., Nowinski, C. J., McKee, A. C. & Cantu, R. C. The epidemiology of sport-related concussion. Clin. Sports Med. 30 , 1–17 (2011).
DeFord, S. M. et al. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J. Neurotrauma 19 , 427–438 (2002).
Creeley, C. E., Wozniak, D. F., Bayly, P. V., Olney, J. W. & Lewis, L. M. Multiple episodes of mild traumatic brain injury result in impaired cognitive performance in mice. Acad. Emerg. Med. 11 , 809–819 (2004).
DeRoss, A. L. et al. Multiple head injuries in rats: effects on behavior. J. Trauma. 52 , 708–714 (2002).
Raghupathi, R. & Margulies, S. S. Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma 19 , 843–853 (2002).
Kane, M. J. et al. A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods. 203 , 41–49 (2012).
Shultz, S. R., MacFabe, D. F., Foley, K. A., Taylor, R. & Cain, D. P. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav. Brain Res. 224 , 326–335 (2011).
Shultz, S. R. et al. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma 29 , 281–294 (2012).
Shultz, S. R., MacFabe, D. F., Foley, K. A., Taylor, R. & Cain, D. P. Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav. Brain Res. 229 , 145–152 (2012).
Friess, S. H. et al. Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma 26 , 1111–1121 (2009).
Laurer, H. L. & McIntosh, T. K. Experimental models of brain trauma. Curr. Opin. Neurol. 12 , 715–721 (1999).
Povlishock, J. T., Hayes, R. L., Michel, M. E. & McIntosh, T. K. Workshop on animal models of traumatic brain injury. J. Neurotrauma 11 , 723–732 (1994).
Reid, W. M. et al. Strain-related differences after experimental traumatic brain injury in rats. J. Neurotrauma 27 , 1243–1253 (2010).
Tan, A. A., Quigley, A., Smith, D. C. & Hoane, M. R. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J. Neurotrauma 26 , 539–548 (2009).
Fox, G. B., LeVasseur, R. A. & Faden, A. I. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma 16 , 377–389 (1999).
Roof, R. L. & Hall, E. D. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma 17 , 1155–1169 (2000).
Berry, C. et al. The effect of gender on patients with moderate to severe head injuries. J. Trauma. 67 , 950–953 (2009).
Stein, D. G. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience 191 , 101–106 (2011).
Junpeng, M., Huang, S. & Qin, S. Progesterone for acute traumatic brain injury. Cochrane Database Syst. Rev. CD008409 (2011).
Farace, E. & Alves, W. M. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J. Neurosurg. 93 , 539–545 (2000).
Maas, A. I. & Lingsma, H. F. New approaches to increase statistical power in TBI trials: insights from the IMPACT study. Acta Neurochir. Suppl. 101 , 119–124 (2008).
Lu, J., Marmarou, A., Lapane, K., Turf, E. & Wilson, L. A method for reducing misclassification in the extended Glasgow Outcome Score. J. Neurotrauma 27 , 843–852 (2010).
Beit-Yannai, E., Zhang, R., Trembovler, V., Samuni, A. & Shohami, E. Cerebroprotective effect of stable nitroxide radicals in closed head injury in the rat. Brain Res. 717 , 22–28 (1996).
Markgraf, C. G. et al. Injury severity and sensitivity to treatment after controlled cortical impact in rats. J. Neurotrauma 18 , 175–186 (2001).
Jiang, Q. et al. MRI evaluation of axonal reorganization after bone marrow stromal cell treatment of traumatic brain injury. NMR Biomed. 24 , 1119–1128 (2011).
Li, L. et al. MRI measurement of angiogenesis and the therapeutic effect of acute marrow stromal cell administration on traumatic brain injury. J. Neurotrauma 32 , 2023–2032 (2012).
Kou, Z. et al. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J. Head Trauma Rehabil. 25 , 267–282 (2010).
Benson, R. R. et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J. Neurotrauma 24 , 446–459 (2007).
Li, L. et al. Transplantation of marrow stromal cells restores cerebral blood flow and reduces cerebral atrophy in rats with traumatic brain injury: in vivo MRI study. J. Neurotrauma 28 , 535–545 (2011).
Immonen, R. J., Kharatishvili, I., Grohn, H., Pitkanen, A. & Grohn, O. H. Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage 45 , 1–9 (2009).
Ehrenreich, H. et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 8 , 495–505 (2002).
Ehrenreich, H. et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40 , e647–e656 (2009).
Jia, L., Chopp, M., Zhang, L., M. Lu & Zhang, Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke 41 , 2071–2076 (2010).
Zechariah, A., ElAli, A. & Hermann, D. M. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke 41 , 1008–1012 (2010).
Loane, D. J. & Faden, A. I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 31 , 596–604 (2010). This article critically reviews strategies for developing experimental neuroprotective therapies and proposes criteria for improving the probability of successful clinical translation.
Menon, D. K. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 37 , S129–S135 (2009).
Zhang, Z. G. & Chopp, M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 8 , 491–500 (2009).
Fujimoto, S. T. et al. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 28 , 365–378 (2004). This article describes and evaluates the tests used to assess functional outcomes after TBI and provides an overview of the aspects of cognitive, sensory and motor function that may be suitable targets for therapeutic intervention.
Mahmood, A., D. Lu, C. Qu, Goussev, A. & Chopp, M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J. Neurosurg. 104 , 272–277 (2006).
Smith, D. H. et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma 14 , 715–727 (1997).
Ning, R. et al. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 1384 , 140–150 (2011).
Meng, Y. et al. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J. Neurosurg. 115 , 550–560 (2011).
Mahmood, A., Lu, D., Yi, L., Chen, J. L. & Chopp, M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J. Neurosurg. 94 , 589–595 (2001).
Xiong, Y. et al. Delayed transplantation of human marrow stromal cell-seeded scaffolds increases transcallosal neural fiber length, angiogenesis, and hippocampal neuronal survival and improves functional outcome after traumatic brain injury in rats. Brain Res. 1263 , 183–191 (2009).
Lindner, M. D. et al. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J. Neurotrauma 15 , 199–216 (1998).
Shear, D. A. et al. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res. 1026 , 11–22 (2004).
Salmond, C. H. & Sahakian, B. J. Cognitive outcome in traumatic brain injury survivors. Curr. Opin. Crit. Care 11 , 111–116 (2005).
Sinson, Voddi, G. M. & McIntosh, T. K. Nerve growth factor administration attenuates cognitive but not neurobehavioral motor dysfunction or hippocampal cell loss following fluid-percussion brain injury in rats. J. Neurochem. 65 , 2209–2216 (1995).
Hyder, A. A., Wunderlich, C. A., Puvanachandra, P., Gururaj, G. & Kobusingye, O. C. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22 , 341–353 (2007).
Hellewell, S. C., Yan, E. B., Agyapomaa, D. A., Bye, N. & Morganti-Kossmann, M. C. Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J. Neurotrauma 27 , 1997–2010 (2010).
Robertson, C. L. et al. No long-term benefit from hypothermia after severe traumatic brain injury with secondary insult in rats. Crit. Care Med. 28 , 3218–3223 (2000).
Stiefel, M. F., Tomita, Y. & Marmarou, A. Secondary ischemia impairing the restoration of ion homeostasis following traumatic brain injury. J. Neurosurg. 103 , 707–714 (2005).
Bramlett, H. M., Dietrich, W. D. & Green, E. J. Secondary hypoxia following moderate fluid percussion brain injury in rats exacerbates sensorimotor and cognitive deficits. J. Neurotrauma 16 , 1035–1047 (1999).
Matsushita, Y., Bramlett, H. M., Kuluz, J. W., Alonso, O. & Dietrich, W. D. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 21 , 847–856 (2001).
Sawauchi, S., Marmarou, A., Beaumont, A., Signoretti, S. & Fukui, S. Acute subdural hematoma associated with diffuse brain injury and hypoxemia in the rat: effect of surgical evacuation of the hematoma. J. Neurotrauma 21 , 563–573 (2004).
Keel, M. & Trentz, O. Pathophysiology of polytrauma. Injury 36 , 691–709 (2005).
Maegele, M. et al. Differential immunoresponses following experimental traumatic brain injury, bone fracture and “two-hit”-combined neurotrauma. Inflamm. Res. 56 , 318–323 (2007).
Prins, M. L. & Hovda, D. A. Developing experimental models to address traumatic brain injury in children. J. Neurotrauma 20 , 123–137 (2003).
Potts, M. B. et al. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx 3 , 143–153 (2006).
Giza, C. C., Kolb, B., Harris, N. G., Asarnow, R. F. & Prins, M. L. Hitting a moving target: basic mechanisms of recovery from acquired developmental brain injury. Dev. Neurorehabil. 12 , 255–268 (2009).
Ibrahim, N. G., Ralston, J., Smith, C. & Margulies, S. S. Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma 27 , 1021–1035 (2010).
Hoane, M. R., Lasley, L. A. & Akstulewicz, S. L. Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav. Brain Res. 153 , 189–197 (2004).
Flanagan, S. R., Hibbard, M. R. & Gordon, W. A. The impact of age on traumatic brain injury. Phys. Med. Rehabil. Clin. N. Am. 16 , 163–177 (2005).
Thompson, H. J., McCormick, W. C. & Kagan, S. H. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 54 , 1590–1595 (2006).
Depreitere, B., Meyfroidt, G., Roosen, G., Ceuppens, J. & Grandas, F. G. Traumatic brain injury in the elderly: a significant phenomenon. Acta Neurochir Suppl. 114 , 289–294 (2012).
Xiong, Y., Mahmood, A. & Chopp, M. Emerging treatments for traumatic brain injury. Expert Opin. Emerg. Drugs 14 , 67–84 (2009).
Clifton, G. L. et al. Early induction of hypothermia for evacuated intracranial hematomas: a post hoc analysis of two clinical trials. J. Neurosurg. 117 , 714–720 (2012).
Hamm, R. J. et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9 , 11–20 (1992).
Smith, D. H., Okiyama, K., Thomas, M. J., Claussen, B. & McIntosh, T. K. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J. Neurotrauma 8 , 259–269 (1991).
Richardson, R. M., Sun, D. & Bullock, M. R. Neurogenesis after traumatic brain injury. Neurosurg. Clin. N. Am. 18 , 169–181 (2007).
Bullock, M. R. et al. Outcome measures for clinical trials in neurotrauma. Neurosurg. Focus 13 , ECP1 (2002).
Maas, A. I. et al. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics 7 , 127–134 (2010).
Saatman, K. E. et al. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25 , 719–738 (2008).
Doppenberg, E. M., Choi, S. C. & Bullock, R. Clinical trials in traumatic brain injury: lessons for the future. J. Neurosurg. Anesthesiol. 16 , 87–94 (2004).
Andriessen, T. M., Jacobs, B. & Vos, P. E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 14 , 2381–2392 (2010).
Muir, K. W. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr. Opin. Pharmacol. 6 , 53–60 (2006).
Lee, L. L., Galo, E., Lyeth, B. G., Muizelaar, J. P. & Berman, R. F. Neuroprotection in the rat lateral fluid percussion model of traumatic brain injury by SNX-185, an N-type voltage-gated calcium channel blocker. Exp. Neurol. 190 , 70–78 (2004).
Xiong, Y., Q. Gu, Peterson, P. L., Muizelaar, J. P. & Lee, C. P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14 , 23–34 (1997).
McCall, J. M., Braughler, J. M. & Hall, E. D. Lipid peroxidation and the role of oxygen radicals in CNS injury. Acta Anaesthesiol. Belg. 38 , 373–379 (1987).
Bains, M. & Hall, E. D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta. 1822 , 675–684 (2012).
Raghavendra Rao, V. L., Dhodda, V. K., Song, G., Bowen, K. K. & Dempsey, R. J. Traumatic brain injury-induced acute gene expression changes in rat cerebral cortex identified by GeneChip analysis. J. Neurosci. Res. 71 , 208–219 (2003).
Ziebell, J. M. & Morganti-Kossmann, M. C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7 , 22–30 (2010).
Chen, J. et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32 , 2682–2688 (2001).
Mahmood, A. D. Lu & Chopp, M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 21 , 33–39 (2004).
Levin, H. S., Eisenberg, H. M., Wigg, N. R. & Kobayashi, K. Memory and intellectual ability after head injury in children and adolescents. Neurosurgery 11 , 668–673 (1982).
Fox, G. B. & Faden, A. I. Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J. Neurosci. Res. 53 , 718–727 (1998).
Schwarzbold, M. L. et al. Effects of traumatic brain injury of different severities on emotional, cognitive, and oxidative stress-related parameters in mice. J. Neurotrauma 27 , 1883–1893 (2010).
Pandey, D. K., Yadav, S. K., Mahesh, R. & Rajkumar, R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav. Brain Res. 205 , 436–442 (2009).
Jones, N. C. et al. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J. Neurotrauma 25 , 1367–1374 (2008).
Milman, A., Rosenberg, A., Weizman, R. & Pick, C. G. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma 22 , 1003–1010 (2005).
Friess, S. H. et al. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 204 , 234–243 (2007).
Narayan, R. K. et al. Clinical trials in head injury. J. Neurotrauma 19 , 503–557 (2002). This article provides informative and insightful views on previous and future TBI trials.
Stein, D. G. & Wright, D. W. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin. Investig. Drugs 19 , 847–857 (2010).
Beauchamp, K., Mutlak, H., Smith, W. R., Shohami, E. & Stahel, P. F. Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 14 , 731–740 (2008).
Lu, D. et al. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J. Neurotrauma 22 , 1011–1017 (2005).
Lu, D. et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma 21 , 21–32 (2004).
Zhang, Y. et al. Sprouting of corticospinal tract axons from the contralateral hemisphere into the denervated side of the spinal cord is associated with functional recovery in adult rat after traumatic brain injury and erythropoietin treatment. Brain Res. 1353 , 249–257 (2010).
Oshima, T. et al. TNF-α contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res. 1290 , 102–110 (2009).
Xiong, Y. et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 113 , 598–608 (2010).
Xiong, Y., Mahmood, A. & Chopp, M. Neurorestorative treatments for traumatic brain injury. Discov. Med. 10 , 434–442 (2010).
Xiong, Y. et al. Treatment of traumatic brain injury with thymosin β4 in rats. J. Neurosurg. 114 , 102–115 (2011).
Armstead, W. M. Vasopressin-induced protein kinase C-dependent superoxide generation contributes to atp-sensitive potassium channel but not calcium-sensitive potassium channel function impairment after brain injury. Stroke 32 , 1408–1414 (2001).
Albert-Weissenberger, C. & Siren, A. L. Experimental traumatic brain injury. Exp. Transl. Stroke Med. 2 , 16 (2010).
Download references
Acknowledgements
We thank the three anonymous referees for their excellent comments, and we apologize to those researchers whose work has not been cited owing to space limitations. This work was supported by the National Institutes of Health grants RO1 NS062002 (Y.X.), PO1 NS042345 (A.M. and M.C.) and PO1 NS023393 (M.C.).
Author information
Authors and affiliations.
Department of Neurosurgery, E&R Building, Room 3096, Henry Ford Health System, 2799 West Grand Boulevard, Detroit, 48202, Michigan, USA
Ye Xiong & Asim Mahmood
Department of Neurology, E&R Building, Room 3056, Henry Ford Health System, 2799 West Grand Boulevard, Detroit, 48202, Michigan, USA
Michael Chopp
Department of Physics, Oakland University, Rochester, 48309, Michigan, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ye Xiong .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Related links
Further information.
Ye Xiong's homepage
Asim Mahmood's homepage
Michael Chopp's homepage
(DAI). DAI is characterized by impaired axoplasmic flow that progresses to axotomy and is typically identified by the presence of the amyloid-β precursor protein, as revealed by immunohistochemical staining.
The modified NSS (neurological severity score) is a composite of motor, sensory, reflex and balance tests for use in rats.
(NSS). The NSS is a reliable tool for evaluating neurological damage in closed head trauma in mice and rats, and assesses both motor function and behaviour.
This is the accumulation of hyperphosphorylated tau protein (a highly soluble microtubule-associated protein), which causes the formation of neurofibrillary tangles. These tangles are a pathological hallmark of tauopathies, which are a group of diseases including Alzheimer's disease, frontal temporal dementia with Parkinsonism and corticobasal degeneration.
A biomarker is a specific biochemical, molecular, anatomical or physiological characteristic that is used to measure or indicate the presence or progress of disease or the effects of treatment.
(GCS). The GCS is a standardized scale that is used to measure the level of consciousness, to assess the degree of brain impairment and to identify the seriousness of injury in relation to outcome after TBI. The score is determined by summing the ratings of how the patient responds to certain standard stimuli by opening their eyes, giving a verbal response and giving a motor response. A high score of 13 to 15 indicates a mild brain injury, a score of 9 to 12 reflects a moderate brain injury and a score of 3 to 8 reflects a severe brain injury.
(GOS). The GOS is an outcome score in which individuals with TBI are assigned to one of five categories: dead, vegetative state, severe disability, moderate disability or good recovery. The extended GOS (GOSe) provides more detailed categorization into eight categories by subdividing the categories of severe disability, moderate disability and good recovery into lower and upper categories.
(EPO). EPO is a glycoprotein hormone secreted by the kidney in adult mammals and by the liver in the fetus; it acts on stem cells of the bone marrow to stimulate red blood cell production (that is, erythropoiesis).
(tPA). tPA is an enzyme that catalyses the conversion of plasminogen to plasmin and is used to dissolve blood clots rapidly and selectively, especially in the treatment of heart attacks and ischaemic stroke.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Xiong, Y., Mahmood, A. & Chopp, M. Animal models of traumatic brain injury. Nat Rev Neurosci 14 , 128–142 (2013). https://doi.org/10.1038/nrn3407
Download citation
Published : 18 January 2013
Issue Date : February 2013
DOI : https://doi.org/10.1038/nrn3407
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Engineering of an electrically charged hydrogel implanted into a traumatic brain injury model for stepwise neuronal tissue reconstruction.
- Satoshi Tanikawa
- Shinya Tanaka
Scientific Reports (2023)
Near-infrared-IIb emitting single-atom catalyst for imaging-guided therapy of blood-brain barrier breakdown after traumatic brain injury
Nature Communications (2023)
Whole-Blood Metabolomics of a Rat Model of Repetitive Concussion
- Ahmad Raza Khan
- Samiya Zehra
- Kamlesh Bhaisora
Journal of Molecular Neuroscience (2023)

Advances in the Mechanistic Study of the Control of Oxidative Stress Injury by Modulating HDAC6 Activity
- Yanfang Zhou
Cell Biochemistry and Biophysics (2023)
Genetic deletion of Krüppel-like factor 11 aggravates traumatic brain injury
Journal of Neuroinflammation (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Experimental Animal Models of Human Diseases - An Effective Therapeutic Strategy
Evaluation of Animal Models Suitable for Hair Research and Regeneration
Submitted: 21 November 2016 Reviewed: 11 May 2017 Published: 20 December 2017
DOI: 10.5772/intechopen.69698
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Experimental Animal Models of Human Diseases - An Effective Therapeutic Strategy
Edited by Ibeh Bartholomew
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
1,967 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Hair loss and regeneration are the subjects of tremendous amount of research for multiple reasons: the well-known importance of hair in individual beauty, the fact that alopecia is a frequent dermatological disease, and that there are limited treatment options. The present work focuses on the evaluation of animal models used for hair research and regeneration. Besides mentioning the option of in vitro studies, the chapter analyzes the need of an animal model of alopecia, common used study designs, hair regrowth evaluation methods, and the limitations of the animal models in hair regrowth research. This chapter also discusses the structure of hair, its chemical composition, the properties and functions of hair, consequences of hair loss, the biology of hair loss, and regeneration and existing treatment options for alopecia. By using proper and well thought-out animal models, we aim to refine our knowledge on human hair diseases and hair regrowth. Hair research provides insights into the physiopathological pathways, genetic and cell biochemical mechanisms, and remains a field intensively explored and still inexhaustible.
- animal models
- research in vivo
- hair regrowth
- hair regeneration
Author Information
Meda sandra orăsan *.
- Department of Physiopathology, University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca, Romania
Andrei Coneac
- Department of Histology, University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca, Romania
*Address all correspondence to: [email protected]
1. Introduction
Hair loss and regeneration is the subject of tremendous amount of research for multiple reasons. First of all, as hair loss or alopecia is a frequent dermatological disease; second, the treatment options are limited and generate variable rates of success. Last but not least, hair is an important component of human outlook with a strong impact on the overall beauty and attraction of an individual. As several studies have shown, hair plays an interesting part in social and sexual communication.
The chapter addresses several issues: the importance of hair from both personal and social perspective, the structure and chemical composition of hair, hair functions and properties, biology of hair loss and hair regrowth, and consequences of hair loss and treatment options. The authors aim to offer an overview of the hair regrowth in vivo and in vitro studies, focusing on the animal models, and describing the common study designs and their limitations.
The main reason for hair research on animal models relies on the similarities between human and animal skin biology. New treatments for alopecia with different hair growth-promoting agents and various administration techniques have been tested on animal models to prove efficacy and to minimize possible adverse reactions.
2. Functions of hair
Also known as “fur” in animals, hair is a defining characteristic of mammals. Besides its important thermoregulatory function, it also has a camouflage purpose and offers protection. In animals, hair follicles can modify their type and density during seasonal coat changes [ 1 ]. It is noted that in some species, hair provides sensory and defensive functions, while in others it is used for signaling and communication [ 2 , 3 ].
Although human hair has lost its main thermoregulatory function, on the scalp, it preserves a heat insulation and cooling purpose, by evaporating sweat from soaked hair [ 4 , 5 , 6 ]. It also acts as a sunscreen, offering the skin protection against ultra-violet radiation [ 7 , 8 ].
3. Structure of hair
Hair is defined as an accessory structure of the integument along with the sebaceous glands, sweat glands, and nails [ 4 ]. The shaft of the hair (hard filamentous part that extends above the skin surface) consists of three layers, starting from the outside: the cuticle (having several layers of flat, thin cells, overlapping one another), the cortex (containing the keratin bundles in rod-like cell structures), and the medulla (a disorganized and open area at the fiber’s center) [ 9 , 10 ].
In the dermis, we find the bulb of the hair, which contains the dermal papilla. It has an important role in hair formation, growth, and hair cycle [ 11 ]. Besides maintaining stem cells that regrow the hair after it falls out, it also nourishes the hair follicle (providing nutrients and oxygen to epidermal cells in the lower layer) due to the blood vessels present at the bottom of the dermal papilla [ 1 ].
4. Chemical composition of hair
Hair has a complex chemical structure, containing organic substances (glycogen, acidic polysaccharides, lipids and proteins—amino acids). About 90% of the hair structure consists of proteins, out of which keratin (a combination of 18 amino acids) is the essential component, being produced by the skin keratinocytes. The lipids represent 3% of the hair composition and are supplied by the sebaceous glands or produced in the hair bulb from sterols, fatty acids, and ceramides [ 12 , 13 ].
Hair also contains inorganic substances (carbon 45.2%, oxygen 28%, hydrogen 6.6%, nitrogen 15%, and sulfur 5.2%) and water. Other mineral components of hair consist of iron, copper, calcium, magnesium, zinc, potassium, and lead, all of them of external sources.
5. Properties of hair
The color of hair depends on the type and quantity of melanin inside the cell. The hair follicle pigmentary unit provides the hair shaft color due to the melanin components (eumelanin and pheomelanin) and the interactions between follicular melanocytes, keratinocytes, and fibroblasts (also involved in wound healing) [ 11 , 14 ]. In the case of the black hair, the pigment is also found in the extracellular compartment.
Hair is flexible and has elastic properties, being able to get longer by 20–50% under controlled traction. Under heat action, the elasticity decreases and hair can break easily. Hair is also hygroscopic, it can absorb water; a fact which decreases hair elasticity and resistance to a third of its normal value [ 4 , 10 ].
Hair resistance is mostly due to cysteine amino acid, a substance rich in sulphur, which plays an important role in hair cohesion. Hair resistance seems to be increased to physical and biological agents and decreased to chemicals. Excessive light with UV exposure, repetitive hair-dye, and hair perm generate the alteration of the hair elastic properties by the chemical and photochemical degradation of the amino acids from the keratin structure. Hair resistance equals to a force of 60 kg, but it is decreased in children and elderly people. Hair resistance also depends on the hair diameter [ 4 , 6 ].
6. Biology of the hair loss and hair regrowth
Human hair is different from hair grown by mammals due to unsynchronized growth cycles and a sensitive response to androgen.
Human hair exhibits a certain seasonal coordination, but the follicles work independently [ 15 , 16 , 17 ]. Latest research results sustain the idea that hair follicles act like neurons, being able to interconnect and generate hair loss and hair regrowth in a small region of the scalp. Human hair has a mosaic pattern as it consists of hair in different stages: the majority of the hair follicles (90%) being in growing phase (also known as anagen), 1–2% in regression (catagen phase), and 8–9% of the hair follicles are resting (in telogen phase) [ 18 , 19 ]. The cyclic changes from anagen to telogen via catagen involve rapid remodeling of both the epithelial and dermal components of hair follicles [ 20 , 21 ].
In both humans and animals, hair cycle is influenced by stimulatory and inhibitory factors, such as hormones, growth factors, cytokines, neuropeptides, and pharmaceutical products [ 18 , 22 , 23 ]. The dermal papilla supports an increased cell division and growth rate and induces the shift between anagen, catagen, and telogen [ 18 , 19 ]. In telogen phase, the old hair is lost, but the follicle will be regenerated in early anagen, when new hair grows up [ 24 , 25 ].
Current concepts of hair loss pathogenesis include genetic, genomic, hormonal, and immune contributions. Furthermore, the patient’s behavior influences the hair density and its strength. In recent years, evidence has suggested that hair loss is a multifactorial disease, and the contributing factors include the resistance to insulin, local pathologies (inflammation, hypoxia, and vascular insufficiency), predisposing physiological factors (menopause and aging), association with other diseases (polycystic ovary syndrome, hirsutism, acne, hormonal imbalances, thyroid pathologies, and other autoimmune diseases). Hair loss remains a consequence of the genotype (hereditary information of the organism)-phenotype (morphology, behavior, and development) interaction [ 25 , 26 ].
The most common form of hair loss is known as “androgenetic alopecia” (AGA), which represents almost 95%. In this case, hair loss is generated by hair cycle abnormalities, such as the shortening of the anagen, within an abnormal hair cycle, and the anagen-telogen rate shifting from 6:1 to 2:1. Also, hair loss can be due to a small-sized dermal papilla. Both situations lead to shortening of hairs, decreasing hair diameter, shaft loss, and an increased number of hairs in telogen phase. In most situations, the changes of hair diameter (hair thinning) are followed by the loss of pigment: final hair (thick and pigmented) can turn back into vellus (thin and white). Studies point out that another cause of hair loss is the fact that the scalp suffers from vasoconstriction and hypoxia [ 27 , 28 ].
Hair cycle disturbances are mainly caused by an excess of androgens, which alters the production of regulatory factors (soluble paracrine factors and extracellular matrix components) by the dermal papilla cells [ 13 ]. Some specific sites of the body (beard, axillary, and pubic hair) react differently than hair from the scalp, as they are androgen-sensitive [ 4 ]. Hair miniaturization and thinning, followed by hair fall is most common in the vertex and the crown-frontal area of the scalp [ 29 , 30 ].
The occipital part of the scalp is an androgen insensitive area that is why in alopecia, hair is still present in this region, and hair follicles are suitable to be used in hair transplants [ 31 , 32 ]. The androgen effect on hair can be summarized by the metabolization of the testosterone into 5-alpha-dihydrotestosterone by 5-alpha reductase. A good metabolization limits the hair length (in case of the beard, for example) and deficiencies of the 5-alpha reductase generate enlarged hair diameter (thicker hair in the axillary and pubic area) [ 19 , 33 , 34 , 35 ].
Another form of alopecia is Alopecia areata (AA), a cell-mediated disease directed against active growing hair follicles. It is a nonscarring alopecia, with limited alopecic patches on the scalp or the body, sometimes affecting also the nails. The pathogenesis of AA includes an autoimmune etiology, linked to human leukocyte antigen (HLA) class II alleles and to the T lymphocytic co-stimulatory cascade [ 30 ].
7. Genes associated with hair loss
Several studies including recent genome-wide association analyses concluded that a large number of single nucleotide polymorphisms (SNPs) are associated with AGA susceptibility. So far, only some of the genes involved in hair loss have been discovered: genes AR androgen receptor and EDA2R ectodysplasin A2 receptor from chromosome X, region located at 20p1 on chromosome 20, and additional loci associated with early onset baldness in Europeans, such as HDAC9 in 7p21.1, TARDBP (chr1), HDAC4 (chr2), AUTS2 (chr7), SETBP1 (chr18), q35 ( WNT10A ), chr3q25 ( SUCNR1 ), chr5q33.3 ( EBF1 ), and chr12p12.1 ( SSPN ) [ 36 ].
8. Consequences of hair loss
Alopecia can be a part of the normal aging process. Still, hair loss represents a great concern for patients. Several studies have shown that it generates anxiety and distress especially in females, affecting couple and social relationships [ 37 , 38 , 39 ].
Hair loss is defined as a stressful experience for both sexes, patients being unable to cope with the progression of the disease [ 40 , 41 ]. Stress functions not only as a cause, a risk factor, but also as a consequence of hair loss. Alopecia determines a poor quality of life by the physical and psychological sequelae: low self-esteem, depression, distorted social perception, and psychosocial functioning [ 42 , 43 , 44 , 45 ].
9. Hair regrowth treatment
Up to the present, although many treatments have been tested, hair loss continues to be a frequent dermatological condition [ 46 ].
Two FDA-approved hair loss treatment drugs: Finasteride (acting on the hormonal cause of alopecia—the excess of androgens) and Minoxidil (acting on the physical cause—the hypoxia due to vasoconstriction), are commonly used in clinical practice in order to treat androgenetic alopecia, which represents 95% of all hair loss causes [ 38 , 47 , 48 , 49 ]. Minoxidil (1 mg per day) is a topical formulation available in 2 and 5% concentration. It stops hair loss and promotes hair growth as it is a vasodilator and potassium channel opener, allowing more oxygen, blood, and nutrients to reach the follicle [ 50 , 51 , 52 , 53 ]. It has no therapeutic action on the hormonal and genetic causes of hair loss; therefore, it must be used as a continuous support for the hair follicles, otherwise the hair regrowth will cease and hair loss will begin again in 1–2 months [ 54 , 55 , 56 , 57 ]. Finasteride is a dihydrotestosterone-suppressing 5-alpha-reductase inhibitor, recommended for male use only, decreasing the serum levels of dihydrotestosterone, stopping hair fall (in 48% of the cases), and stimulating hair regrowth (in 51% of the cases). Studies have shown that 1 mg of finasteride oral treatment has an efficacy similar to daily topical application of minoxidil [ 58 , 59 , 60 ]. Given the temporary efficacy of finasteride and minoxidil and the limited number of treatments available in alopecia, new therapies are needed to prevent hair loss and enhance hair regrowth [ 61 , 62 ].
Pharmaceutical hair loss management also includes different substances (arginine, aminexil, caffeine, and taurine), different peptides, B spectrum vitamins, zinc, or different procedures (application of stem cells or plasma-rich platelets and low-level laser therapy), even if clinical studies in this respect are lacking. A large variety of over-the-counter products claim to treat hair loss pathology: hair tonics, hair balms, hair masks, shampoos, leave in conditioners, topical solutions, or foams function as potential anti-hair loss agents [ 43 , 44 , 63 , 64 , 65 , 66 , 67 , 68 ].
Alternatives to traditional treatment are laser (low-level laser therapy) and platelet-rich plasma (PRP) injections [ 47 , 69 ].
10. Hair follicle regrowth using gene therapy
Gene therapy aims to deliver genetic material (DNA) into the patients’ cells with either a prevention or therapeutic purpose. The therapeutic effect could theoretically be obtained by replacing the mutant gene that causes the disease with a healthy gene, inactivating a mutated gene that causes an imbalance in the organism or introducing a new gene that could fight a particular disease. For the introduction of the gene, a carrier called vector is used, and it usually consists of a modified virus (retrovirus) that will not produce a disease in the organism, but will deliver the gene by integrating the genetic material into the chromosome of a particular cell. The delivery pathway may consist of a direct injection into the tissue or it can be given intravenously, to reach the blood flow [ 36 ].
As new evidence shows that 80% of the baldness is genetic, gene therapy could be the solution, although it encounters technical problems that have not been solved up to the present [ 69 ]. Most of the hair loss complains in both female and male patients are due to the presence of androgenetic alopecia, caused by hyperandrogenism and sensitivity to dihydrotestosterone (DHT). It has been noticed that people naturally lacking from birth the 5-alpha reductase enzyme (which converts the testosterone to DHT) never develop androgenetic alopecia [ 50 ].
Human scalp has DHT-resistant follicles in the occipital area, this location being used to extract the hair follicles for transplant into the vertex or to the fronto-parietal area [ 53 ]. Gene therapy may be a solution in this case, if it can trigger the hair follicles with DHT-sensitive cells and change them into DHT-resistant follicles that could regrow hair without being affected by androgen hormones [ 70 ]. Another option would consist of the ribonucleic acid (RNA) interference to block the genes responsible for hair loss. Messenger ribonucleic acid (mRNA) represents the carrier of genetic information from the DNA out of the cell nucleus into the cytoplasm, where it is translated into specific proteins, such as receptors, enzymes. Small fragments of nucleic acids, such as small interfering RNAs (siRNAs), can target a specific gene and block the production of any type of protein in a cell. In hair loss, this technology could be used in order to inhibit the androgen receptor (AR) and the 5-alpha reductase enzymes.
Up to the present, an attempt to effectively control delivery of small interfering RNA using biodegradable cationized gelatin microspheres in an animal model of disease was first performed in 2008. Researchers administered local injections of interleukin-4 and neutralizing anti-interferon-γ antibody in C3H/HeJ mice. They concluded that alopecia areata was effectively treated as the treatment suppressed CD8 T cell infiltrates around the hair follicles and repressed enhanced interferon-γ mRNA expression in alopecic skin. Also, restoration of hair shaft elongation occurred due to Th1 transcription factor T-box 21 small interfering RNAs conjugated to cationized gelatin [ 71 ]. Another recent study showed that the sonic hedgehog ( shh ) gene stimulated the hair shaft production and anagen phase in C57BL/6 mice, after being delivered with an adenovirus vector [ 72 ].
Gene therapy is currently available only in research settings. It represents a promising therapeutic option for several diseases (especially those with no cure for the moment), but this procedure needs more research and improvement that need to be considered safe and to prove its effectiveness. So far, scientists have encountered difficulties in finding proper delivery pathways of the genes to the body, targeting them to particular cells, controlling the new gene(s) and their effect after they have been inserted into the body [ 73 ].
11. Hair regrowth studies in vitro
Human hair follicles as research material for hair loss and regeneration involve ethical problems, an invasive collection method and a limited quantity of follicles available for extraction and testing [ 60 , 61 ].
The first methods of isolation and maintenance of hair follicles in cell cultures go back to 1990, when several researchers used this method in order to study the biology of the hair cycle [ 74 , 75 ]. Follicles were usually taken during face lifting surgery, but only a third were suitable for the isolation phase of the hair transplant, due to improper collection procedures. The follicles needed to be isolated from human scalp in a few hours, maintained at 2–6°C, in an Earl medium, combined with phosphate buffered saline solution, with calcium and magnesium added. Only the follicles that seemed intact were used.
In vitro hair research was supported by the identification of growth factor function in the process of hair regrowth and differentiation [ 76 , 77 , 78 , 79 ]. Philpott et al. have reported that in the absence of insulin, follicles prematurely enter the catagen stage [ 80 ]. Subsequent in vitro and in vivo studies, in murine and human models of hair follicles, have demonstrated that IGF-1 level is a regulation factor of hair growth and together with IGF-1 receptor influence hair growth cycle.
Other studies performed in 1990 have shown that transforming growth factor beta 2 (TGF-ß2) promotes anagen to catagen transition. Several inhibitors of hair follicle growth in vitro have been identified such as interleukins (IL-1 alpha and beta) and tumor necrosis factor (TNF-alpha). Researchers concluded that these cytokines play a significant part in the pathophysiology of hair inflammatory diseases. Although the factors that perform the transition in vitro from anagen to catagen have been discovered, inducing a full hair growth cycle has not been made possible yet. Murine models of hair follicles, isolated at different growth stages in vitro seem to maintain their cyclic activity and to illustrate their status in vivo [ 81 ].
On the other hand, healthy human dermal papilla cells, isolated from hair follicle, lose the ability to produce hair growth when being outside the body. Also, cycling hair follicles cannot be maintained in culture for any length of time [ 82 ].
The data that we now possess about the life and function of the hair follicle in health and disease rely on the successful research performed in vivo (experiments on natural animals and genetically manipulated models) and in vitro (cultures of a cell type—dermal papilla or organ culture of isolated cell follicles). The preference for one of the two experimental alternatives depends on several factors: the purpose of the research and the advantages and disadvantages involved.
12. Hair regrowth studies in vivo
12.1. the need of animal models.
Animals and humans are remarkably similar at physiological and anatomical levels. Also, genetically speaking, we share 67% of our DNA with earthworms and 99% with mice. Almost 90% of the veterinary medicines used to treat animals are similar to the ones developed for human use. Animal models can mimic human responses, but the differences in species and even in individual animals must be taken into consideration [ 83 ]. By recreating human diseases in animal models, we can study and understand the physiopathological processes involved in the disease and maybe find an efficient cure. The first Nobel Prize was awarded in 1901 and other 94 prizes were directly dependent on animal research [ 84 ].
Laboratory animals are used when human testing is not available for practical or ethical reasons. Animals represent good research subjects as they have a shorter life cycle that enables scientists to observe the animal throughout the entire life and across several generations. Also, animal models can be easily influenced by the environment, which is controlled by the researcher as far as the diet, temperature, lighting, and other factors are concerned.
Researchers use animal models for short-term objectives (to determine how the animal model responds to a stimuli or a treatment) and long-term purposes (development of a new drug, evaluation of bioavailability or toxicity, genetic study). The animal model should be sensitive, appropriate for the studied condition either by using specific evidence of previous studies or using a new animal model with the risk of generating inaccurate results [ 69 ]. Besides the similarity with the human response, other key features of the biomedical research on animal models are specificity to the study purpose, validation of the animal model, and improvement for further research. Animal research has brought many benefits not only to humans but also to animals in disease prevention and treatment [ 47 , 48 , 69 ].
For more than a 100 years, almost all the information obtained in the human and animal health research has been the result of studies performed on animal models. The most common aim of animal models use is the development of new methods for the diagnosis and treatment of diseases, through an understanding of the biology and the physiopathological processes involved [ 47 , 69 ].
Even though animal models remain a necessity, alternatives consist of computer models, tissue and cell cultures, and other nonanimal-related research methods. In order to minimize the harmful effect of research performed on animal models, scientists tend to reduce the number of animals used to obtain valid results, to refine the experimental technique, or replace it with nonanimal research methods.
12.2. Animal models used in hair loss and regrowth
A large variety of animals (mice, rats, hamsters, rabbits, sheep, and even stump-tailed macaque) provide useful models for the in vivo study of hair loss and regrowth, but 95% of the animals bred for research purposes are rats and mice [ 85 , 86 , 87 , 88 , 89 ].
Mice represent an excellent model to study the hair cycle for several reasons: the first two cycles of the mouse hair follicle are synchronized; the mouse hair cycle is short, lasting for 3 weeks; hair follicles can be easily harvested and examined at specific time points in the cycle. Most importantly, the stages of the hair cycle have been well characterized in the mouse: anagen being morphologically subdivided into six stages and catagen into eight [ 22 ]. The periodic intervals of rodent hair cycles (especially the anagen-growing phase) seem to be less susceptible to iatrogenic influences [ 90 ]. The mouse hair cycle does not differ structurally from the human hair follicle cycle, except for the fact that during catagen the hair bulb is remodeled, but the vibrissae follicles do not retract. Scientists have recently discovered that a certain progenitor cell population in mice is analogous to the human cells, encouraging research on this particular animal model.
Besides studying the normal hair cycle on mice, scientists also focussed on the growth waves and hormonal control [ 91 ]. Significant differences between species regarding the follicular function and limited androgen-sensitive models were noticed [ 92 ]. Spontaneous mutations have been discovered and studied on hairless, nude, and tabby mutants, waved and angora animals, leading to the identification of new genes involved in hair loss and opening the path for transgenic technology research [ 93 , 94 ].
Transgenic mice, also known as “knockout mice,” are mice with altered genome through the use of genetic engineering. This gene-targeting technique has revolutionized the biomedical research by offering researchers the ability to create a specific animal model for the most common human diseases. In order to select the most appropriate immunodeficient mouse models for research purpose, scientists also take into consideration: background strain, behavior, husbandry, disease susceptibility, life span, breeding performance, radiosensitivity, functionality of various endogenous immune system components, and leakiness (tendency to produce functional B and T cells as they age).
Up to the present, immunodeficient mice (with T and B cells deficiencies) were used as models for autoimmune disease mechanisms and androgenetic alopecia studies. The androgen action upon the hair follicles has been studied on spontaneous and genetically engineered nude mutant mice [ 95 ].
The C57BL/6 mouse is the most popular laboratory rodent, widely used and studied, having its entire genome published. Research applications using this particular type of mice include immunology, cancer, neurodegerative disease, age-related hearing loss, bone density, diabetes, obesity, and biomarker studies. This black coat mouse has been used for the skin-free pigment and early visible pigmented tips of new anagen regrowth [ 88 ]. C57BL/6 represents one of the most well-characterized models available, with a minimum risk of genetic drift. It is also a convenient model for creating transgenic mice, which are recognized by the mixed coat colors.
The C3H/HeJ mouse model was used in a large range of studies: immunology, cancer (especially mammary tumors), inflammation, sensorineural, and cardiovascular disease. This animal model was the most widely reported for hair growth promotion, most possibly due to the fact that C3H/HeJ mice can spontaneously develop alopecia areata (AA) from 6 to 18 months of age. Also, alopecia areata can be surgically induced by skin-grafting from a donor animal with AA onto an isogenic C3H/HeJ recipient (normal haired mice of the same strain) [ 90 , 96 ].
In 2010, researchers created the first rodent model of AGA, taking into consideration its relationship to androgen metabolism and androgen signaling, mediated by the androgen receptor (AR). They used transgenic mice overexpressing human AR in the skin under control of the keratin 5 promoter and exposed them to high levels of 5-alpha dihydrotestosterone, which led to delayed hair regeneration, mimicking AGA. The scientists concluded that androgen-mediated hair loss is AR-dependent and suggested that AR and beta-catenin mediate this effect [ 97 ].
There are many rat strains raised for research purposes, but the albino Wistar Bratislava rat is the most commonly used. Gene knockout techniques are relatively difficult to be applied and successfully achieved in rats. For hair loss and regeneration experiments, the Wistar rats and the Dundee Experimental Bald Rat (DEBR) strain were commonly used. The latter has the ability to spontaneously develop adult onset alopecia areata (AA) at a higher frequency than in the mouse model [ 98 ].
In the research field of hair loss and regeneration two major achievements must be mentioned on the rat animal model: coaxing human stem cells to become dermal papilla and producing new hair follicles when transplanted on rat skin [ 98 ]. Also, by inhibiting the rejection of foreign skin, human skin grafts were applied and even rat dermal papillae continued to produce hair after reimplantation in vivo on a rat model [ 99 , 100 ].
Research performed on a rabbit animal model, added important data to the field, proving that full thickness transplants, made with full pedicle graft (separated from their original nervous and vascular supply) retain their original intrinsec activity and are not modified by the action of the surrounding tissue [ 101 ]. Furthermore, the rabbit represents a common animal model used to screen compounds potentially efficient in treating alopecia.
The Golden Syrian hamster ( Mesocricetus auratus ) has been previously used for research purposes, even though it is a very common pet. The hamster flank organ has served as a model to study the effect of testosterone (T) upon the hair follicle, the sebaceous glands, and the dermal pigment. This hamster is known to be useful for the specific and quantitative assessment of different substances on hair growth, being also useful for therapy testing in hirsutism. Macroscopic (hair density evaluation) and microscopic (hair diameter analysis) hair growth assessments have been performed on Golden hamsters [ 102 ].
12.3. Common study designs in hair loss and regrowth
12.3.1. housing conditions.
In vivo hair regrowth studies usually use animals of either sex and weight, kept in experimental rooms that are free of pathogens and opportunistic agents. For 7–14 days prior to the experiment, the animals are housed under specific conditions: room temperature of 23°C, controlled humidity, a 12:12 h light, and dark cycle. In order to avoid licking, individual housing is preferred or a maximum of two animals per cage. Standard laboratory diet and water ad libitum are provided. After completing the experiment, animals are euthanized according to the current regulations. For accurate results, most of the studies on animal models are performed in triplicate [ 47 , 69 ].
12.3.2. Depilation methods
Experimental designs may include one of the depilation methods: shaving, the use of a raisin mixture, or a hair removal cream [ 91 , 103 ]. The most commonly used is the shaving of a larger skin area (the whole back or body) or of several smaller areas that are denuded for testing. For animal immobilization during procedures, general anesthesia is commonly performed with a combination of ketamine (i.p. 50 mg/kg b.w.) and xylazine (20 mg/kg b.w.) [ 47 , 48 , 69 ].
Some study designs, such as that of Mester et al., required before each successive hair treatment, the shaving of the skin. This procedure can induce mechanic stimulation of hair growth, as previously reported in the literature, and influence the study results. Other experiments done on adult rats point out that after the fur was dyed and shaved, the regrowing hairs formed a system of linear loops that were closely correlated with the shaving process [ 66 , 67 ].
In order to avoid this effect, it is recommended not to shave the skin of the animal model before each session of therapy. Other factors which influence the hair regrowth are physical factors such as low temperature, which triggers fast regrowth after shaving.
Depilation-induced hair cycle has been studied, and it follows a strict course: nine days after depilation, the hair follicles enter the final stage of the growth cycle (anagen VI). On day 17 after depilation, the follicles enter the regression stage (catagen), while on day 20 follicles get to the resting stage (telogen) [ 22 ].
12.3.3. Evaluation of hair loss and hair regrowth
Efficacy of the treatment is screened by observing the presence, rate, and cosmetic acceptability of hair regrowth. More sophisticated assays include determining how the drug induced hair regrowth and exploring the pathogenesis of AA.
Researchers do not possess standardized methods for in vivo hair regrowth assessment. New, accurate, and minimally invasive procedures are still needed as the most commonly used tools are qualitative assessments, limited in number. They include macroscopic assessment with the naked eye (visualization and photographs of the area of interest) based on scales that assess the percentage of hair regrowth on the interest area and tricoscopic evaluation (with a hand-held dermatoscope, with polarized light and magnification abilities) [ 41 ]. Trichoscopy allows a correct hair regrowth evaluation, as it can detect decrease of hair diameter up to ten times or diameter variations. Both macroscopic and microscopic methods assess hair regrowth with the help of personalized hair growth scales or standardized, already published scales.
Usually, the dorsal part of the animals is used for the testing. After being depilated and treated, the animal skin is observed and photographed at specific time intervals (day 1, 7, 14, and 21) to record the start of the hair regrowth period and the pattern of hair regrowth, compared to controls. Several hair regrowth potential scores are mentioned by literature. The one described by Matsuda et al., for instance, ranges from 0 to 5: 0 = no hair growth, 1 = less than 20% of hair growth, 2 = 20–39% of hair growth, 3 = 40–59% of hair regrowth, 4 = 60–79% of hair regrowth, 5 = 80–100% of hair regrowth [ 54 ]. Researchers also use self-designed scales of hair regrowth that consider: Type IV (high hair density, full, thick fur), Type III (moderate hair density with no visible skin area), Type II (low hair density, with the visualization of the skin), Type I (uneven hair growth on the test area, skin easily seen) [ 47 , 69 ].
The hair regrowth potential scores can be applied for both macroscopic and microscopic assessments ( Figures 1 and 2 ).

Figure 1.
Classification of the hair regrowth effect (type I, type II, type III, type IV) for macroscopic and microscopic assessments—personal study performed on Wistar Bratislava rats. The control area is marked with red (left side of the picture), the test area with blue (right side).

Figure 2.
Classification of the hair regrowth effect (type 0, type 1, type 2, type 3, type 4, type 5) for macroscopic and microscopic assessments—personal study performed on New Zealand Rabbits.
On the other hand, quantitative methods, such as hair weight determinations, hair density measurements, or histopathological examination offer more accurate results. For hair weight determination, the regrown hair from an area of 1 cm 2 of skin is cut and weighed with an analytical balance [ 48 ].
In order to analyze the histological features at the end of the treatment period, the animals are sacrificed and a skin biopsy is isolated for histopathological examination. The thickness of the skin and the location of hair follicles in the dermis can be assessed by microscopic photography.
Also the hair cycle can be assessed, as the anagen induction can be calculated with the formula: (number of follicles in hypodermis) × 100/(number of follicles in dermis). Literature data showed an association of increasing skin thickness, follicle count, and macroscopic development of skin pigmentation with anagen induction [ 18 , 23 ]. The study by Liu et al. found that in the anagen phase the bulb of the hair follicles was enlarged and deeply inserted into the dermis. The research also revealed that the hair follicles in the shaved, bare areas were short, small, and in the phases of telogen, anagen, or catagen [ 69 ].
The hair growth cycle, consisting of three phases (anagen, catagen, and telogen) is used by both practitioners and researchers to diagnose the hair growth condition and to decide on the hair growth-promoting agent. In human subjects, digital trichoscopy is available, with automatic assessment of the number of follicles in each hair growth phase.
Several studies focused on the validation of Minoxidil 2% treatment on the animal model used, as this topical treatment is thought to be the gold standard treatment for hair loss. This substance affected the normal hair cycle by shortening telogen, causing premature entry of the resting follicles into anagen phase [ 103 , 104 ].
12.4. Limitations of animal models regarding hair regrowth
The limitation of using an animal model while studying hair regeneration can be briefly summarized as follows. First, synchronized hair cycles generate waves of new hair regrowth, which make the interpretation of result a hard task. Second, the lack of independence of the hair follicles, since they have coordinated regrowth pattern on a precise time scale, as described by Muller-Rover et al. [ 22 ]. Third, young mice present the drawback of patchy growth after the second wave of hair growth is completed. Lastly, the increased hair density on an animal model leads to difficulties of assessment by densitometry or cross-section trichometer [ 69 ].
The results of our hair growth research performed on Wistar rats showed, besides a normal hair growth in the majority of the animal models, a lack of hair regrowth on the tested area. Other studies performed on black-and-white mice reported that no further hair growth was observed on half of the control animals.
We also noticed a diffuse hair regrowth in some study groups, while in others, the hair appeared to make some specific linear loops that were observed macroscopically. Literature date confirmed our findings. In similar situations, researchers experienced a diffuse hair growth in some animals with an uncharacteristic, diagonal strip [ 66 ]. Li-Yaun Liu et al. described four main linear hair regrowth patterns noticed on a rat model: the dorsal loop and the lateral dorsal loop (running along the dorsum and hind limb) and the ventral loop and lateral ventral loop (traveling along the thorax, abdomen, and forelimb). These hair-loop-lines create cranio-caudally-oriented waves of regrowth 2–15 mm wide, symmetrically on both sides of the body, running from the head through the torso to the limbs [ 105 ]. Li-Yaun Liu et al. concluded that after shaving the skin, the hair follicles from these new hair lines were always in an anagen phase [ 106 ].
Also, the behavior of the animals should be taken into consideration, as it can create issues and interfere with the research results [ 107 ]. For example, the C57BL/6 mice show barbering behavior, the dominant mouse in a cage selectively removing hair from its subordinate cage mates. Mice that have been barbered have large bald patches on their bodies, especially around the head, snout, and shoulders [ 108 ].
Regardless of the shortcomings of either animal model, most of them validate their usefulness for drug efficacy and safety testing for humans.
13. Conclusion
Although the studies performed on animal models encounter both technical and objective issues, further scientific research is not impeded and continues to remain an intensively explored field. By using proper and well-thought out animal models, we aim to refine our knowledge on human hair diseases and hair regrowth. Hair research provides further insights into the physiopathological pathways and genetic and cell biochemical mechanisms that could promise the cure of hair loss.
- 1. Krause K, Foitzik K. Biology of the hair follicles: The basics. Seminars in Cutaneous Medicine and Surgery. 2006; 25 :2‐10
- 2. Sabah NH. Controlled stimulation of hair follicle receptors. Journal of Applied Physiology. 1974; 36 (2):256‐257
- 3. Ruben JA, Jones TD. Selective factors associated with the origin of fur and feathers. American Zoologist. 2000; 40 (4):585‐596
- 4. Randall VA, Sundberg JP, Philpott MP. Animal and in vitro models for the study of hair follicles. Journal of Investigative Dermatology Symposium Proceedings. 2003; 8 :39‐45
- 5. Montagna W. The evolution of human skin. Journal of Human Evolution. 1985; 14 :3‐10
- 6. Wheeler PE. The loss of functional body hair in man: The influence of thermal environment, body form and bipedality. Journal of Human Evolution. 1985; 14 :23‐28
- 7. Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proceedings of the National Academy of Sciences of the United States of America. 2010; 107 (Suppl 2):8962‐8968
- 8. Iyengar B. The hair follicle: a specialised UV receptor in the human skin? Biological Signals and Receptors. 1998; 7 (3):188‐194
- 9. Feughelman M. Mechanical Properties and Structure of Alpha-keratin Fibers: Wool, Human Hair and Related Fibers. Sydney: UNSW Press; 1996
- 10. Goodier M, Hordinsky M. Normal and aging hair biology and structure. Current Problems in Dermatology. 2015; 47 :1‐9
- 11. Randall VA, Hibberts NA, Thornton MJ, Merrick AE, Hamada K, Kato S, et al. Do androgens influence hair growth by altering the paracrine factors secreted by dermal papilla cells. European Journal of Dermatology. 2001; 11 :315‐320
- 12. Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. Journal of Investigative Dermatology. 2005; 124 (1):13‐21
- 13. Lin C, Li Y, Ji YC, Keng H, Cai XN, Zhang JK. Micro-encapsulated human hair dermal papilla cells: A substitute for dermal papilla. Archives of Dermatological Research. 2008; 300 (9):531‐535
- 14. Liao YH, Kuo WC, Chou SY, Tsai CS, Lin GL, Tsai MR, et al. Quantitative analysis of intrinsic skin aging in dermal papillae by in vivo harmonic generation microscopy. Biomedical Optics Express. 2014; 5 (9):885‐889
- 15. Randall VA, Ebling FJG. Seasonal changes in human hair growth. British Journal of Dermatology. 1991; 124 :146‐151
- 16. Orentreich N. Biology of the skin. In: Montagna W, Dobson RL, editors. Hair Growth. Vol. IX. Oxford: Pergamon; 1969. pp. 99‐108
- 17. Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Reviews in Molecular Medicine. 2002; 4 (22):1‐11
- 18. Dry E. The coat of the mouse ( Mus musculus ). Journal of Genetics. 1926; 1 :287‐340
- 19. Randall VA. Androgens are the main regulator of human hair growth. In: Camacho F, Randall VA, Price V, editors. Hair and its Disorders: Biology, Pathology and Management. London: Martin Dunitz; 2000. pp. 121‐136
- 20. Paus R. Therapeutic strategies for treating hair loss. Drug Discovery Today: Therapeutic Strategies. 2006; 3 (1):101‐110
- 21. Angelelli L, Cavina G, Morewtti G, Siniscalchi P. Quantitative analysis of phospholipids of biochemical and pharmaceutical interest by means of thin layer chromatography: Evaluation of the precision of the method. Il Farmaco; Edizione Pratica. 1966; 21 (9):493‐507
- 22. Muller-Rover S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. Journal of Investigative Dermatology. 2001; 117 (1):3‐15
- 23. Datta K, Singh AT, Mukherjee A, Bhat B, Ramesh B, Burman AC. Eclipta alba extract with potential for hair growth promoting activity. Journal of Ethnopharmacology. 2009; 124 (3):450‐456
- 24. Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992; 115 (2):587‐593
- 25. Courtois M, Loussouarn G, Hourseau C, Grollier JF. Ageing and hair cycles. British Journal of Dermatology. 1995; 132 (1):86‐93
- 26. Courtois M, Loussouarn G, Hourseau C, Grollier JF. Hair cycle and alopecia. Skin Pharmacology. 1994; 7 :84‐89
- 27. Whiting DA. Scalp biopsy as a diagnostic and prognostic tool in androgenetic alopecia. Dermatologic Therapy. 1998; 8 :24‐33
- 28. Whiting DA, Waldstreicher J, Sanchez M, Kaufman KD. Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts in horizontal sections of serial scalp biopsies: results of finasteride 1 mg treatment of men and postmenopausal women. Journal of Investigative Dermatology. Symposium Proceedings. 1999; 4 :282‐284
- 29. Yip L, Rufaut N, Sinclair R. Role of genetics and sex steroid hormones in male androgenetic alopecia and female pattern hair loss: An update of what we now know. Australasian Journal of Dermatology. 2011; 52 (2):81‐88
- 30. Goodheart HP. Photoguide to Common Skin Disorders. Diagnostic and Management. Philadelphia: Lippincott Williams & Wilkins; 2009
- 31. Wilson JD, Griffin JE, Russell DW. Steroid 5-alpha reductase 2 deficiency. Endocrine Reviews. 1993; 14 :577‐593
- 32. Itami S, Kurata S, Sonoda T, Takayasu S. Characterization of 5 alpha-reductase in cultured human dermal papilla cells from beard and occipital scalp hair. Journal of Investigative Dermatology. 1991; 96 :57‐60
- 33. Thornton MJ, Laing I, Hamada K, Messenger AG, Randall VA. Differences in testosterone metabolism by beard and scalp dermal papilla. Clinical Endocrinology. 1993; 39 :633‐639
- 34. Hamada K, Thornton MJ, Laing I, Messenger AG, Randall VA. The metabolism of testosterone by dermal papilla cells cultured from human pubic and axillary hair follicles concurs with hair growth in 5 alpha-reductase deficiency. Journal of Investigative Dermatology. 1996; 106 :1017‐1022
- 35. Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. Journal of Endocrinology. 1992; 133 :141‐147
- 36. Gene Therapy. Available at: https://americanhairloss.org/hair_loss_research/gene_tehrapy.asp [Accessed: 02 Jan 2017]
- 37. Hadshiew IM, Foizik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. Journal of Investigative Dermatology. 2004; 123 (3):455‐457
- 38. Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: Comparison with balding men and with female control subjects. Journal of the American Academy of Dermatology. 1993; 29 (4):568‐575
- 39. Hunt N, McHale S. Understanding Alopecia. London: Sheldon; 2004
- 40. Cash TF. The psychological effects of androgenetic alopecia in men. Journal of the American Academy of Dermatology. 1992; 26 :926‐931
- 41. Alfonso M, Richter-Appelt H, Tosti A, Viera MS, García M. The psychosocial impact of hair loss among men: A multinational European study. Current Medical Research and Opinion. 2005; 21 :1829‐1837
- 42. Hunt N, McHale S. The psychosocial impact of alopecia. BMJ. 2005; 331 :951‐955
- 43. Yoon JI, Al-Reza SM, Kang SC. Hair growth promoting effect of Zizyphus jujuba essential oil. Food and Chemical Toxicology. 2010; 48 (5):1350‐1354
- 44. Bandaranayake I, Mirmirani P. Hair loss remedies–separating fact from fiction. Cutis. 2004; 73 (2):107‐114
- 45. Chartier MB, Hoss DM, Grant-Kels JM. Approach to the adult female patient with diffuse nonscarring alopecia. Journal of the American Academy of Dermatology. 2002; 47 (6):809‐818
- 46. Nandeesh R, Kumar BS, Lakshman K, et al. Evaluation of hair growth activity of Buxus wallichiana Baill extract in rats. Iranian Journal of Basic Medical Sciences. 2009; 11 (4):236‐241
- 47. Orasan MS, Coneac A, Mihu CM, Mare C, Muresan A. Minoxidil and neoptide topical application reinforced by low-level laser therapy on an animal model of alopecia. Studia UBB Chemia. 2015; 60 (2):295‐308
- 48. Orasan MS, Bolfa P, Coneac A, Mitrea RD, Moldovan R, Mihu C, et al. Stimulation of hair regrowth using low level laser treatment in a rat model of alopecia. Digest Journal of Nanomaterials and Biostructures. 2013; 8 (4):1571‐1580
- 49. Cash TF. The psychology of hair loss and its implications for patient care. Clinics in Dermatology. 2001; 19 (2):161‐166
- 50. Otoberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinology and Metabolism Clinics of North America. 2007; 36 (2):379‐398
- 51. Price VH. Treatment of hair loss. The New England Journal of Medicine. 1999; 341 (13):964‐973
- 52. Lucky A, Picquadio D, Ditre C. A randomized, placebo-control trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. Journal of the American Academy of Dermatology. 2004; 50 :541‐553
- 53. Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. Journal of Drugs in Dermatology. 2010; 9 (11):1412‐1419
- 54. Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clinic Proceedings. 2005; 80 (10):1316‐1322
- 55. Olsen EA, DeLong ER, Weiner MS. Long term follow up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 1987; 16 :688‐695
- 56. Tsuboi R, Tanaka T, Nishikawa T. A randomized placebo-control trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. European Journal of Dermatology. 2007; 17 :37‐44
- 57. Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. Journal of the American Academy of Dermatology. 2008; 59 (4):547‐566
- 58. Tosti A, Duque-Estrada B. Treatment strategies for alopecia. Expert Opinion on Pharmacotherapy. 2009; 10 :1017‐1026
- 59. Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G. Efficacy and safety of finasteride therapy for androgenetic alopecia: A systematic review. Archives of Dermatology. 2010; 146 :1141‐1150
- 60. Burgdorf W, Plewig G, Wolff HH, Landthaler M. Braun-Falco’s Dermatology. 3rd ed. Heidelberg: Springer; 2009
- 61. McPhee SJ, Papadakis MA, Tierney LM. Current Medical Diagnosis and Treatment. 46th ed. Lange. New York: McGraw Hill Companies; 2007
- 62. Kondo S, Hozumi Y, Aso K. Organ culture of human scalp hair follicles: Effect of testosterone and oestrogen on hair growth. Archives of Dermatological Research. 1990; 282 :442‐445
- 63. Desmet KD, Paz DA, Corry JJ, Eells JT, Wong-Riley MT, Henry MM, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomedicine and Laser Surgery. 2006; 24 :121‐128
- 64. Hamblin MR, Demidova TN. Mechanisms of low level light therapy. Proceedings of SPIE. 2006; 6140 :1‐12
- 65. Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochemistry and Photobiology. 2008; 84 (5):1091‐1099
- 66. Mester E, Szende B, Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiologia, Radiotherapia (Berlin). 1968; 9 :621‐625
- 67. Hamblin MR. Mechanism of Laser-Induced Hair Regrowth, Dose-Response (Prepress) Formerly Nonlinearity in Biology, Toxicology and Medicine. University of Massachusetts; 2009
- 68. Avram MR, Leonard RT Jr, Epstein ES, Williams JL, Bauman AJ. The current role of laser/light sources in the treatment of male and female pattern hair loss. Journal of Cosmetic and Laser Therapy. 2007; 1 :27‐28
- 69. Orăsan MS. Factorii fiziopatologici si optiunile terapeutice in alopecia androgenetica la femei [Doctoral dissertation]; 2013
- 70. Ustuner ET. Cause of androgenic alopecia: Crux of the matter. Plastic and Reconstructive Surgery Global Open. 2013; 1 (7):e64. DOI: 10.1097/GOX.0000000000000005
- 71. Nakamura M, Jo J, Tabata Y, Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (Alopecia Areata) in a C3H/HeJ mouse model. The American Journal of Pathology. 2008; 172 (3):650‐658
- 72. Hoffman RM. The hair follicle as a gene therapy target. Nature Biotechnology. 2000; 18 :20‐22
- 73. How Does Gene Therapy Work? Genetics Home Reference. Available at: https://ghr.nlm.nih.gov/primer/therapy/procedures [Accessed: 20 Apr 2017]
- 74. Soma T, Ogo M, Suzuki J, Takahasi T, Hibino T. Analysis of apoptotic cell death in human hair follicles. Journal of Investigative Dermatology. 1998; 112 :518‐526
- 75. Cotsarelis M. Male pattern balding may be due to stem cell inactivation. University of Pennsylvania School of Medicine. Science Daily; 6 Jan 2011
- 76. Sundber PJ, King EL. Mouse models for study of human hair loss. Dermatologic Clinics. 1996; 14 (4):619‐632
- 77. Tarlow JK, Clay FE, Cork MJ, Blakemore AIF, McDonagh AJG, Messenger AG, et al. Severity of alopecia areata is associated with a polymorphism in the interleukin-1 receptor antagonist gene. Journal of Investigative Dermatology. 1994; 96 :673‐681
- 78. Williams R, Philpott MP, Kealey T. Metabolism of freshly isolated human hair follicles capable of hair elongation: a glutaminolytic, aerobic glycolytic tissue. Journal of Investigative Dermatology. 1993; 100 :834‐840
- 79. Philpott MP, Sanders DA, Bowen J, Kealey T. Effects of interleukins, colony stimulating factor and tumour necrosis factor on human hair follicle growth in vitro : A possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. British Journal of Dermatology. 1996; 135 :942‐948
- 80. Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin-like growth factors on human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. Journal of Investigative Dermatology. 1994; 102 :857‐861
- 81. Philpott MP, Sanders DA, Kealey T. Whole human hair follicle culture. In: Whiting DA, editor. Dermatologic Clinics. Philadelphia: W.B. Saunders Press; 1996. pp. 595‐607
- 82. Sanders DA, Philpott MP, Nicolle FV, Kealey T. The isolation and maintenance of the human pilosebaceous unit. British Journal of Dermatology. 1994; 131 :166‐176
- 83. Philpott MP, Green MR, Kealey T. Human hair growth in vitro. Journal of Cell Science. 1990; 97 :463‐471
- 84. Ebling FJ. The biology of hair. Dermatologic Clinics. 1987; 5 (3):467‐481
- 85. Hamilton JB. Age, sex and genetic factors in the regulation of hair growth in man: A comparison of Caucasian and Japanese populations. In: Montagna W, Ellis RA, editors. The Biology of Hair Growth. New York: Academic Press; 1958. pp. 399‐433
- 86. Hamilton JB. Patterned loss of hair in man; types and incidence. Annals of the New York Academy of Sciences. 1951; 53 :708‐728
- 87. Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. British Journal of Dermatology. 1977; 97 :247‐254
- 88. Chase HB, Rauch H, Smith VW. Critical stages of hair development and pigmentation in the mouse. Physiological Zoology. 1951; 24 :1‐8
- 89. Uno H, Imamura K, Pan H. Androgenetic alopecia in the stump-tailed macaque. An important model for investigating the pathology and antiandrogenetic therapy of male pattern baldness. In: Camacho F, Randall VA, Price V, editors. Hair and its Disorders: Biology, Pathology and Management. London: Martin Dunitz; 2000. pp. 137‐151
- 90. Van Neste DB, de Brouwer. Human hair follicle grafts in nude mice. An important in vivo model for investigating the control of hair growth. In: Camacho F, Randall VA, Price V, editors. Hair and its Disorders: Biology, Pathology and Management. London: Martin Dunitz; 2000. pp. 115‐119
- 91. Ebling FJG, Hale P, Randall VA. Hormones and hair growth. In: Goldsmith L, editor. Biochemistry and Physiology of the Skin. 2nd ed. New York: Oxford University Press; 1991
- 92. Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. The Journal of Dermatology. 1990; 17 (5):276‐281
- 93. Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, et al. Cathepsin L deficiency as molecular defect of furless: Hyperproliferation of keratinocytes and perturbation of hair follicle cycling. FASEB Journal. 2000; 14 :2075‐2086
- 94. Porter RM. Mouse models for human hair loss disorders. Journal of Anatomy. 2003; 202 (1):125‐131
- 95. Matias JR, Malloy V, Orentreich N. Animal models of androgen-dependent disorders of the pilosebaceous apparatus. Archives of Dermatological Research. 1989; 281 :247-253
- 96. Gilhar A, Shalaginov R, Assay B, Serafimovich S, Kalish RS. Alopecia areata is a T-lymphocyte mediated autoimmune disease: Lesional human T-lymphocytes transfer alopecia areata to human skin grafts on SCID mice. Journal of Investigative Dermatology Symposium Proceedings. 1999; 4 :207‐210
- 97. Crabtree JS, Kilbourne EJ, Peano BJ, Chippari S, Kenney T, McNally C, et al. A mouse model of androgenetic alopecia. Endocrinology. 2010 May; 151 (5):2373‐2380
- 98. Sun J, Silva KA, McElwee KJ, King LE, Sundberg JP. The C3H/HeJ mouse and DEBR rat models for alopecia areata: Review of preclinical drug screening approaches and results. Experimental Dermatology. 2008; 17 (10):793‐805. DOI: 10.1111/j.1600-0625.2008.00773.x
- 99. Wikramanayake TC, Rodriguez R, Choudhary S, Mauro LM, Nouri K, Schachner LA, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers in Medical Science. 2012; 27 (2):431‐436
- 100. Sundberg JP, King LE Jr, Bascom C. Animal models for male pattern (androgenetic) alopecia. European Journal of Dermatology. 2001; 11 :321‐325
- 101. Whiteley HJ. Studies on hair regrowth in the rabbit. Journal of Anatomy. 1958; 92 (Pt 4):563‐567
- 102. Gnann LA, Castro RF, Azzalis LA, Feder D, Perazzo FF, Pereira EC, et al. Hematological and hepatic effects of vascular epidermal growth factor used to stimulate hair regrowth in an animal model. BMC Dermatology. 2013; 13 :15‐21
- 103. Messenger AG, Rundegren J. Minoxidil: Mechanisms of action on hair growth. British Journal of Dermatology. 2004; 150 :186‐194
- 104. Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB Journal. 2008; 22 (6):1725‐1736
- 105. Liu LY, Zhang H, Pan J, Pen A. The existence of a linear system consisted of sympathetic endings in the rat’s skin. Anat Embryol (Berl). 2005; 210 :91-100
- 106. Liu LY, Guo DS, Xin XY, Fang J. Observation of a system of linear loops formed by re-growing hairs on rat skin. Anatomical Record (Hoboken). 2008; 291 (7):858‐868
- 107. Randall VA. Hormonal regulation of hair follicles exhibits a biological paradox. Seminars in Cell and Developmental Biology. 2007; 18 :274‐285
- 108. Sarna JR, Dyck RH, Whishaw IQ. The Dalila effect: C57BL6 mice barber whiskers by plucking. Behavioural Brain Research. 2000; 108 (1):39‐45
© 2017 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Experimental animal models of human diseases.
Edited by Bartholomew Ibeh
Published: 23 May 2018
By María Eugenia Castañeda-Lopez, Idalia Garza-Veloz,...
2179 downloads
By Ljubica Gavrilović, Vesna Stojiljković, Nataša Pop...
1378 downloads
By Josephine T. Tauer, Bernadette A. S. Jäger, Anna U...
1322 downloads

Can We Move Away from Animal Models of Human Disease Research?
The research modernization deal 2021 outlines a move away from animal research..
Posted December 18, 2021 | Reviewed by Gary Drevitch
I recently read a highly-detailed, comprehensive, science-based report called The Research Modernization Deal 2021. It unambiguously shows that numerous experiments on nonhuman animals (animals) fail to lead to effective treatments and cures for human diseases, including the top killers in the U.S. Advertisements for a wide variety of prescription drugs clearly acknowledge this lack of efficacy in their disclaimers and other material. 1 Reliance on animal models is diverting funds away from more promising areas of research and delaying the development of effective drugs and treatments. The plethora of data speaks for itself.
One of the leaders in this movement is neuroscientist Emily Trunnell, who answered a few questions for me about the goals of the project. 2
Marc Bekoff: Why have you gotten involved in the movement to reform biomedical research and move away from work done on nonhuman animals that generates animal models of disease?
ET: While obtaining my doctorate in neuroscience, I used mice and rats in experiments aimed at understanding how diet may affect learning and memory . At the time, I was under the impression that the use of animals in biomedical research was necessary and that animals were well cared for. The experiences I had during the four years it took me to complete my Ph.D. convinced me otherwise.
During that time, I became increasingly disturbed by how easy it was for me, a graduate student, to design and conduct invasive experiments on mice and rats with very little supervision and after providing only specious justifications to our university oversight committee. When writing my dissertation, I was faced with the task of explaining how the animal experiments I conducted were relevant to human health. I felt like I was really stretching to describe how my work translated to humans and it was then that I began to realize that not only was what I was doing cruel to animals and poorly regulated, but it had little, if any, scientific justification.
After graduating, I decided to look at the science and more seriously question this system that prioritizes grant funding, publications, and outdated tradition over research ethics , animal welfare, and societal good. The Research Modernization Deal presents steps for wider policy changes that would automatically eliminate the use of animals where there is the greatest harm and the smallest benefit and provide a framework to transition toward more human-relevant research methods that don’t use other animals. If I had to go back and do graduate school over, I certainly would not use animals for my research projects. However, I’m glad that I can now use my experience to advocate on behalf of animals and better science.
MB: What are some of the topics you consider in the Research Modernization Deal 2021?
ET: In the Research Modernization Deal, we present a wealth of scientific data that challenge the notion that using animals in biomedical research protects human health. For example, studies over the past decade show:
- 81% of the time, animal tests fail to detect the potential side effects of drugs in humans.
- 89% of experiments cannot even be reproduced—a critical research step—resulting in $28 billion annual waste.
- 90% of basic research, most of which involves animals, has failed to lead to any human therapies.
- 95% of new drugs that test safe and effective in laboratory animals fail in human clinical trials.
The failure rates in specific diseases are even worse:
- 100% of treatments for stroke and sepsis tested in animals have failed in humans.
- 99.6% of Alzheimer’s disease treatments developed in animals have failed in humans.
- Only 3.4% of oncology drugs tested on animals succeed in humans.
- There is no effective vaccine for HIV, despite decades of experiments on monkeys .
In some cases, animal research misleads scientists and the results for humans are debilitating and deadly. There are other factors to consider, such as waning public support for the use of animals in laboratories and the economic advantages of investing in superior technology that is more humane and human-relevant.

The Research Modernization Deal provides a commonsense roadmap for how we can transition away from the use of animals in terms of biomedical research funding:
- Stop doing what doesn’t work, and cease funding for animal experiments in the areas where they have shown to be the poorest predictors of humans.
- Assess additional fields of research to determine where else the use of animals is not proving to be fit for the purpose. This can be done through systematic reviews and meta-analyses.
- Prioritize funding for non-animal methods and decrease funding for animal experiments, to incentivize researchers to choose animal-free methods.
- Implement an ethical harm-benefit analysis system so that cumulative harms to animals are assessed and weighed against an evidence-based calculation of the potential benefit of the experiment, taking into account past failures.
- Globally harmonize which non-animal tests are accepted for regulatory toxicity testing.
MB: How does your work differ from others concerned with the same general topics?
ET: Many of the efforts underway to spare or improve the lives of animals used in laboratories focus on a specific animal experiment or species. These types of campaigns are vitally important, but without an overarching plan to change the paradigm, they can feel like emptying the ocean with a spoon. If the Research Modernization Deal were adopted, many of these more specific campaigns would become unnecessary, because the most cruel or scientifically unjustified experiments would be eliminated.
MB: Are you hopeful that as people learn more about what you want to do they will be more open to your ideas?
ET: It often feels like members of the scientific community think people involved in animal protection are anti-science, but nothing could be farther from the truth. Scientific evidence is what shows that 1) other animals are intelligent, feel complex emotions, and suffer in captivity; and that 2) experiments on animals continuously fail to lead to treatments and cures for humans. I feel confident that the more that we can demonstrate, through reason and scholarship, that the logical conclusion is to take steps to move away from animal experimentation, our message will get through to those who need to hear it.
In conversation with Dr. Emily Trunnell.
1) For further discussions about the failure of animal models of human diseases to produce desired results, including commentary from researchers themselves, click here .
2) Dr. Emily Trunnell graduated magna cum laude from the University of Georgia with a degree in nutrition science and earned a doctorate in neuroscience from the University of Georgia in 2016. Dr. Trunnell is currently a senior scientist in Science Advancement and Outreach for People for the Ethical Treatment of Animals (PETA). She works with policy makers and other scientists to replace the use of animals with superior research methods. Her peer-reviewed papers, letters, opinion pieces have appeared in numerous publications, including Drug Discovery Today and Scientific American.
Bekoff, Marc It's Time to Move on From Nonhuman Animal Models .
_____. A New Report Details Why Numerous Animal Models Fail Human s.
_____. "Mice are lousy models for clinical studies": Animal models in biomedical research .'
_____. Citizens for Alternatives to Animal Research (CAARE) .
_____. We're Being Bombarded by Ads for Drugs. (They're filled with unreadable warnings, unintelligible talk, and actors.)
Dr. Aysha Akhtar on the End of Animal Testing . Sentient Media, December 15, 2021.

Marc Bekoff, Ph.D. , is professor emeritus of ecology and evolutionary biology at the University of Colorado, Boulder.
- Find a Therapist
- Find a Treatment Center
- Find a Support Group
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience
Ethical care for research animals
WHY ANIMAL RESEARCH?
The use of animals in some forms of biomedical research remains essential to the discovery of the causes, diagnoses, and treatment of disease and suffering in humans and in animals., stanford shares the public's concern for laboratory research animals..
Many people have questions about animal testing ethics and the animal testing debate. We take our responsibility for the ethical treatment of animals in medical research very seriously. At Stanford, we emphasize that the humane care of laboratory animals is essential, both ethically and scientifically. Poor animal care is not good science. If animals are not well-treated, the science and knowledge they produce is not trustworthy and cannot be replicated, an important hallmark of the scientific method .
There are several reasons why the use of animals is critical for biomedical research:
• Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us!
• Animals are susceptible to many of the same health problems as humans – cancer, diabetes, heart disease, etc.
• With a shorter life cycle than humans, animal models can be studied throughout their whole life span and across several generations, a critical element in understanding how a disease processes and how it interacts with a whole, living biological system.
The ethics of animal experimentation
Nothing so far has been discovered that can be a substitute for the complex functions of a living, breathing, whole-organ system with pulmonary and circulatory structures like those in humans. Until such a discovery, animals must continue to play a critical role in helping researchers test potential new drugs and medical treatments for effectiveness and safety, and in identifying any undesired or dangerous side effects, such as infertility, birth defects, liver damage, toxicity, or cancer-causing potential.
U.S. federal laws require that non-human animal research occur to show the safety and efficacy of new treatments before any human research will be allowed to be conducted. Not only do we humans benefit from this research and testing, but hundreds of drugs and treatments developed for human use are now routinely used in veterinary clinics as well, helping animals live longer, healthier lives.
It is important to stress that 95% of all animals necessary for biomedical research in the United States are rodents – rats and mice especially bred for laboratory use – and that animals are only one part of the larger process of biomedical research.
Our researchers are strong supporters of animal welfare and view their work with animals in biomedical research as a privilege.
Stanford researchers are obligated to ensure the well-being of all animals in their care..
Stanford researchers are obligated to ensure the well-being of animals in their care, in strict adherence to the highest standards, and in accordance with federal and state laws, regulatory guidelines, and humane principles. They are also obligated to continuously update their animal-care practices based on the newest information and findings in the fields of laboratory animal care and husbandry.
Researchers requesting use of animal models at Stanford must have their research proposals reviewed by a federally mandated committee that includes two independent community members. It is only with this committee’s approval that research can begin. We at Stanford are dedicated to refining, reducing, and replacing animals in research whenever possible, and to using alternative methods (cell and tissue cultures, computer simulations, etc.) instead of or before animal studies are ever conducted.

Organizations and Resources
There are many outreach and advocacy organizations in the field of biomedical research.
- Learn more about outreach and advocacy organizations

Stanford Discoveries
What are the benefits of using animals in research? Stanford researchers have made many important human and animal life-saving discoveries through their work.
- Learn more about research discoveries at Stanford


Sustainable Food Production pp 89–116 Cite as
Animal Breeding, Modeling in
- Lawrence R. Schaeffer 7
- Reference work entry
3417 Accesses
Definition of the Subject
Modeling in animal breeding involves describing the major factors that influence the performance ability or production level of animals in order to predict the genetic merit of future progeny for that ability. Successful modeling depends on good record collection systems, accurate pedigree records, and sophisticated statistical models. Models have evolved over time as computer technology has advanced. Genetic evaluation of dairy bulls began in the early 1930s using simple daughter averages for milk production in selection index procedures of Lush and his students [ 1 ]. Genetic evaluation systems spread to all livestock and to many countries due to Lush. Henderson [ 2 ] introduced best linear unbiased prediction (BLUP) around 1950, and this methodology is still widely used in animal breeding except that the models are more detailed and complex. Gianola and others [ 3 , 4 ] taught animal breeders how to use Bayesian methods...
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Abbreviations
A group of animals of approximately the same age living in the same environment and being treated by the same management practices during the same interval of time.
An estimate of the total additive genetic merit of an individual, the effects that are directly passed to offspring.
The particular set of alleles at all gene loci that influence the phenotypes.
The proportion of alleles at gene loci that are identical due to being inherited from a common ancestor.
A genetic model that assumes there are an infinite number of gene loci affecting a trait each with a small and equal effect.
Proposed by Henderson in 1949 for the estimation of breeding values and other nongenetic effects from phenotypes.
The observable characteristics of an animal that can be measured, scored, or recorded.
Bibliography
Lush JL (1945) Animal breeding plans. Iowa State University Press, Ames
Google Scholar
Henderson CR (1984) Applications of linear models in animal breeding. University of Guelph, Ontario
Gianola D, Fernando RL (1986) Bayesian methods in animal breeding. J Anim Sci 63:217–244
Sorensen D, Gianola D (2002) Likelihood , Bayesian and MCMC methods in quantitative genetics. Springer, New York
Johannsen WJ (1909) Elemente der Exakten Erblichkeitslehre. Gustav Fischer, Jena
Wright SE (1933) Evolution and the genetics of populations, vol I. University of Chicago Press, Chicago
Henderson CR (1976) A simple method for computing the inverse of a numerator relationship matrix used for prediction of breeding values. Biometrics 32:69–79
Article Google Scholar
Meuwissen THE, Luo Z (1992) Computing inbreeding coefficients in large populations. Genet Sel Evol 24:305–313
Quaas RL (1988) Additive genetic model with groups and relationships. J Dairy Sci 71:1338–1345
Robinson GK (1986) Group effects and computing strategies for models for estimating breeding values. J Dairy Sci 69:3106–3111
Patterson HD, Thompson R (1971) Recovery of interblock information when block sizes are unequal. Biometrika 58:545–554
Graser H-U, Smith SP, Tier B (1987) A derivative free approach for estimating variance components in animal models by restricted maximum likelihood. J Anim Sci 64:1362–1370
Meyer K, Smith SP (1996) Restricted maximum likelihood estimation for animal models using derivatives of the likelihood. Genet Sel Evol 28:23–49
Wang CS, Rutledge JJ, Gianola D (1993) Marginal inferences about variance components in a mixed linear model using Gibbs sampling. Genet Sel Evol 25:41–62
Bijma P (2006) Estimating maternal genetic effects in livestock. J Anim Sci 84:800–806
PubMed CAS Google Scholar
Thompson R (1976) The estimation of maternal genetic variances. Biometrics 32:903–918
Article PubMed CAS Google Scholar
Schaeffer LR, Kennedy BW (1989) Effects of embryo transfer in beef cattle on genetic evaluation methodology. J Anim Sci 67:2536–2543
Heydarpour M, Schaeffer LR, Yazdi MH (2008) Influence of population structure on estimates of direct and maternal parameters. J Anim Breed Genet 125:89–99
Meyer K (2005) Random regression analyses using B-splines to model growth of Australian Angus cattle. Genet Sel Evol 37:473–500
Article PubMed Google Scholar
Schaeffer LR, Dekkers JCM (1994) Random regressions in animal models for test-day production in dairy cattle. In: Proceedings of the 5th world congress of genetics applied to livestock production, Guelph, 7–12 Aug 1994, vol 18 p 443
Schaeffer LR (2004) Application of random regression models in animal breeding. Livest Prod Sci 86:35–45
Misztal I (2006) Properties of random regression models using linear splines. J Anim Breed Genet 123:74–80
Henderson CR, Quaas RL (1976) Multiple trait evaluation using relatives’ records. J Anim Sci 43:1188–1197
Pollak EJ, Quaas RL (1981) Monte Carlo study of within-herd multiple trait evaluation of beef cattle growth traits. J Anim Sci 52:248–256
Schaeffer LR (1984) Sire and cow evaluation under multiple trait models. J Dairy Sci 67:1567–1580
Pollak EJ, van der Werf J, Quaas RL (1984) Selection bias and multiple trait evaluation. J Dairy Sci 67:1590–1596
Gianola D (1982) Theory and analysis of threshold characters. J Anim Sci 54:1079–1096
Harville DA, Mee RW (1984) A mixed model procedure for analyzing ordered categorical data. Biometrics 40:393–408
Foulley JL, Gianola D (1984) Estimation of genetic merit from bivariate all or none responses. Genet Sel Evol 16:285–306
Gianola D, Foulley JL (1983) Sire evaluation for ordered categorical data with a threshold model. Genet Sel Evol 15:201–223
Meijering A, Gianola D (1985) Linear versus nonlinear methods of sire evaluation for categorical traits: a simulation study. Genet Sel Evol 17:115–132
Snell EJ (1964) A scaling procedure for ordered categorical data. Biometrics 20:592–607
Foulley JL, Gianola D (1986) Sire evaluation for multiple binary responses when information is missing on some traits. J Dairy Sci 69:2681–2695
Simianer H, Schaeffer LR (1989) Estimation of covariance components between one continuous and one binary trait. Genet Sel Evol 21:303–315
Veerkamp RF, Brotherstone S, Engel B, Meuwissen THE (2001) Analysis of censored survival data using random regression models. Anim Sci 72:1–10
Meuwissen THE, Veerkamp RF, Engel B, Brotherstone S (2002) Single and multitrait estimates of breeding values for survival using sire and animal models. Anim Sci 75:15–24
Jamrozik J, Fatehi J, Schaeffer LR (2008) Comparison of models for genetic evaluation of survival traits in dairy cattle: a simulation study. J Anim Breed Genet 125:75–83
Ducrocq VP, Solkner J (1994) “The survival kit”, a FORTRAN package for the analysis of survival data. In: Proceedings of the fifth world congress on genetics applied to livestock production, Guelph, 7–12 Aug 1994, vol 22, pp 51–52
Wu XL, Heringstad B, Gianola D (2010) Bayesian structural equation models for inferring relationships between phenotypes: a review of methodology, identifiability, and applications. J Anim Breed Genet 127:3–15
Download references
Author information
Authors and affiliations.
Department of Animal and Poultry Science, Centre for Genetic Improvement of Livestock, University of Guelph, Guelph, ON, Canada
Dr. Lawrence R. Schaeffer
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Lawrence R. Schaeffer .
Editor information
Editors and affiliations.
Department de Produccio Vegetal i Ciencia Forestal, Universitat de Lleida/ICREA, Lleida, Spain
Paul Christou
Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain
Department of Crop and Forest Sciences, University of Lleida, Lleida, Spain
Roxana Savin
University of New England, Marine Science Center, Biddeford, ME, USA
Barry A. Costa-Pierce
Department of Animal and Dairy Science Breeding and Genetics, University of Georgia, Athens, GA, USA
Ignacy Misztal
The Roslin Institute and Royal (Dick) School of Veterinary Studies, Division of Developmental Biology, University of Edinburgh, Midlothian, Scotland, UK
C. Bruce A. Whitelaw
Rights and permissions
Reprints and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this entry
Cite this entry.
Schaeffer, L.R. (2013). Animal Breeding, Modeling in. In: Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A. (eds) Sustainable Food Production. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5797-8_335
Download citation
DOI : https://doi.org/10.1007/978-1-4614-5797-8_335
Publisher Name : Springer, New York, NY
Print ISBN : 978-1-4614-5796-1
Online ISBN : 978-1-4614-5797-8
eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Bibliography
- More Referencing guides Blog Automated transliteration Relevant bibliographies by topics
- Automated transliteration
- Relevant bibliographies by topics
- Referencing guides
Dissertations / Theses on the topic 'Animal model'
Create a spot-on reference in apa, mla, chicago, harvard, and other styles.
Consult the top 50 dissertations / theses for your research on the topic 'Animal model.'
Next to every source in the list of references, there is an 'Add to bibliography' button. Press on it, and we will generate automatically the bibliographic reference to the chosen work in the citation style you need: APA, MLA, Harvard, Chicago, Vancouver, etc.
You can also download the full text of the academic publication as pdf and read online its abstract whenever available in the metadata.
Browse dissertations / theses on a wide variety of disciplines and organise your bibliography correctly.
Brännström, Åke. "Modelling animal populations." Doctoral thesis, Umeå universitet, Matematik och matematisk statistik, 2004. http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-205.
Camus, Sandrine. "Etho-Psychiatry : animal model to model animal : Identification of a « spontaneous » non-human primate model of depressive symptoms." Thesis, Bordeaux 2, 2013. http://www.theses.fr/2013BOR22032/document.
Cullen, J. R. "Sudden hearing loss : an animal model." Thesis, Queen's University Belfast, 1997. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.326426.
Stewart, Richard James. "Aspects of unilateral cryptorchidism : an animal model." Thesis, Queen's University Belfast, 1988. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.336195.
Godinho, Sofia Isabel Henriques. "An animal model of acute lung injury." Thesis, University of Bristol, 2005. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.424508.
Parsons, Sven David Charles. "Natural animal model systems to study tuberculosis." Thesis, Stellenbosch : University of Stellenbosch, 2010. http://hdl.handle.net/10019.1/4505.
Friedenberg, Steven Gene. "The role of mitochondrial DAMPs on the inflammatory response in an in vitro model of canine SIRS." The Ohio State University, 2013. http://rave.ohiolink.edu/etdc/view?acc_num=osu1365174635.
Faig, Martí Jordi. "Nou model animal experimental per a l'artrosi precoç." Doctoral thesis, Universitat de Barcelona, 2003. http://hdl.handle.net/10803/1207.
Mercer, David Frederick. "Development of an animal model of hepatitis C." Thesis, National Library of Canada = Bibliothèque nationale du Canada, 2000. http://www.collectionscanada.ca/obj/s4/f2/dsk1/tape4/PQDD_0009/NQ60004.pdf.
Munguia, Raymundo. "CiprofloxacinDexamethasone ototoxicity in an animal and human model." Thesis, McGill University, 2005. http://digitool.Library.McGill.CA:80/R/?func=dbin-jump-full&object_id=97975.
Qasim, Faieza Jabeen. "Study of an animal model of experimental vasculitis." Thesis, University of Cambridge, 1995. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.361670.
Hallam, B. "Simulating animal conditioning : investigating Halperin's neuro-connector model." Thesis, University of Edinburgh, 2001. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.651976.
Leung, Wai-hin, and 梁瑋軒. "Neurogenesis in animal model of systemic lupus erythematosus." Thesis, The University of Hong Kong (Pokfulam, Hong Kong), 2013. http://hdl.handle.net/10722/209497.
Chaniary, Kunal. "Electrophysiological Analysis in an Animal Model of Dystonia." VCU Scholars Compass, 2010. http://scholarscompass.vcu.edu/etd/93.
Chaniary, Kunal Dilip. "Electromyographic Characterization in an Animal model of Dystonia." VCU Scholars Compass, 2008. http://scholarscompass.vcu.edu/etd/648.
Bellwald, Dominik. "The avascular talus : revascularization in an animal model /." Bern : [s.n.], 2004. http://www.ub.unibe.ch/content/bibliotheken_sammlungen/sondersammlungen/dissen_bestellformular/index_ger.html.
DuBose, Candis Schrelle. "An animal model for discogenic low back pain." Diss., University of Iowa, 2010. https://ir.uiowa.edu/etd/794.
Albertí, i. Fitó Glòria. "Model animal en laminectomia lumbar: factors quirúrgics i variabilitat individual." Doctoral thesis, Universitat Autònoma de Barcelona, 2016. http://hdl.handle.net/10803/381247.
Cully, Louise. "Biomechanical and physiological investigations in the IBMPFD animal model." Thesis, University of East Anglia, 2016. https://ueaeprints.uea.ac.uk/59209/.
Colantonio, David A. "Troponin modifications, from animal model to the Emergency Department." Thesis, National Library of Canada = Bibliothèque nationale du Canada, 2002. http://www.collectionscanada.ca/obj/s4/f2/dsk3/ftp04/MQ65612.pdf.
Richards, Sonja. "An animal model of autism : remediation with tactile stimulation." Thesis, Lethbridge, Alta. : University of Lethbridge, Dept. of Neuroscience, 2011, 2011. http://hdl.handle.net/10133/3126.
Tucker, Lawrence Maskew. "Neural transplantation in an animal model for Huntington's disease." Thesis, University of Cambridge, 1994. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.362863.
Najjar, Deborah Anne. "Towards a more ethical animal model in biomedical research." Thesis, Massachusetts Institute of Technology, 2018. http://hdl.handle.net/1721.1/120675.
Maglennon, G. A. "Study of papillomavirus latent infection in an animal model." Thesis, University College London (University of London), 2011. http://discovery.ucl.ac.uk/1306763/.
Pomeroy, Ian. "Neocortical lesions in an animal model of multiple sclerosis." Thesis, University of Oxford, 2006. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.670186.
Alexander, Kathleen Shannon. "Elevated Kynurenic Acid as an Animal Model of Schizophrenia." The Ohio State University, 2011. http://rave.ohiolink.edu/etdc/view?acc_num=osu1304691359.
Remus, Jennifer Lynn. "Neuroimmune Mechanisms of an Animal Model of Recurrent Depression." Kent State University / OhioLINK, 2015. http://rave.ohiolink.edu/etdc/view?acc_num=kent1429197762.
Tse, Yiu Chung. "Glutamate receptors in an animal model of Parkinson's disease." HKBU Institutional Repository, 1999. http://repository.hkbu.edu.hk/etd_ra/226.
Saijo(Kim), Misa. "Generation of transgenic animal model of hyperthyroid Graves' disease." Kyoto University, 2004. http://hdl.handle.net/2433/147457.
Galuppo, Andrea Giannotti. "Avaliação da sensibilidade de zigotos murinos à Brucella abortus para o estabelecimento de um modelo experimental em estudos de interações embriões-patógenos." Universidade de São Paulo, 2005. http://www.teses.usp.br/teses/disponiveis/10/10134/tde-23082007-141648/.
Glennie, Richard. "Incorporating animal movement with distance sampling and spatial capture-recapture." Thesis, University of St Andrews, 2018. http://hdl.handle.net/10023/16467.
Muehlmann, Amber M. "Pharmacological challenges of an animal model of self-injurious behavior." [Gainesville, Fla.] : University of Florida, 2005. http://purl.fcla.edu/fcla/etd/UFE0011875.
Diller, James W. "Development of an animal model of choice between aversive events." Morgantown, W. Va. : [West Virginia University Libraries], 2006. https://eidr.wvu.edu/etd/documentdata.eTD?documentid=4916.
Rudissaar, Ruth. "Neuropharmacology of atypical antipsychotics and an animal model of psychosis /." Online version, 2006. http://dspace.utlib.ee/dspace/bitstream/10062/1294/5/rudissaarruth.pdf.
Schiel, Thomas. "Arthroscopic transfer of osteochondral allografts in a bovine animal model." Thesis, National Library of Canada = Bibliothèque nationale du Canada, 1998. http://www.collectionscanada.ca/obj/s4/f2/dsk3/ftp04/nq31898.pdf.
Poirier, Denise Marie. "Nutrient absorption from liquid therapeutic diets in an animal model." Thesis, McGill University, 1988. http://digitool.Library.McGill.CA:80/R/?func=dbin-jump-full&object_id=61694.
Waters, C. M. "A cytochemical evaluation of an animal model of Parkinson's disease." Thesis, University of Cambridge, 1986. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.377231.
Hawkins, C. A. "Some studies on an animal model of temporal lobe epilepsy." Thesis, University of Oxford, 1985. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.375246.
Haberzettl, Robert [Verfasser]. "A validated animal model for the Serotonin Syndrome / Robert Haberzettl." Berlin : Freie Universität Berlin, 2015. http://d-nb.info/1077211929/34.
Omar, Rumana Zareen. "A multistate model for the analysis of animal tumourigenicity experiments." Thesis, University of Reading, 1991. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.303167.
Stapley, Sarah A. "Experimental studies of resuscitation following hypovolaemia in an animal model." Thesis, University of Southampton, 2004. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.403821.
Best, Nicholas James. "Paravalbumin-immunoreactive hippocampal neurons in an animal model of epilepsy." Thesis, University of Southampton, 1994. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.296242.
Binello, Emanuela. "Efficacy of boron neutron capture synovectomy in an animal model." Thesis, Massachusetts Institute of Technology, 1999. http://hdl.handle.net/1721.1/85314.
Resende, Ana Paula Simões Nunes de. "Study on erythropoietin subconjunctival administration in a glaucoma animal model." Doctoral thesis, Universidade de Lisboa, Faculdade de Medicina Veterinária, 2018. http://hdl.handle.net/10400.5/16041.
Man, James K. C. "Characterisation of a novel animal model for obsessive-compulsive disorder." Thesis, University of Bristol, 2005. http://hdl.handle.net/1983/6cec00ce-f3c1-4d07-ba98-54c137b7524a.
Schultes, Klaus. "Ultrastructural characterization of ultraviolet induced corneal disease : an animal model." Master's thesis, University of Cape Town, 1994. http://hdl.handle.net/11427/27046.
Monreal, Gretel. "Ventricular Remodeling in a Large Animal Model of Heart Failure." The Ohio State University, 2008. http://rave.ohiolink.edu/etdc/view?acc_num=osu1210007937.
Roland, Jessica Justine. "Septohippocampal system modulation in an animal model of diencephalic amnesia." Diss., Online access via UMI:, 2008.
Anzalone, Steven J. "Cholinergic cortical dysfunction in an animal model of diencephalic amnesia." Diss., Online access via UMI:, 2009.
TROVATELLI, MARCO. "SHEEP AS ANIMAL MODEL IN MINIMALLY INVASIVE NEUROSURGERY IN EDEN2020." Doctoral thesis, Università degli Studi di Milano, 2020. http://hdl.handle.net/2434/707384.
DigitalCommons@University of Nebraska - Lincoln
Home > Animal Science > ANIMALSCIDISS
Department of Animal Science
Department of animal science: dissertations, theses, and student research.
Forages and Technology Management in Growing and Finishing Beef Cattle Systems , Kelton Adair
Predicting Body Weight of Cattle and Nutrient Digestion of Individual Sweet Bran Components to Improve Beef Cattle Production Efficiency , Dalton J. Anderson
Factors Affecting Forage Quality and the Subsequent Response in Production and Energy Metabolism in Lactating Jersey Cows , Kassidy Kate Buse
Supplemental Table 1. Influence of the count of positive days on DMI, milk yield, milk fat, milk protein, and pregnancy rate , Addison Carroll and Paul J. Kononoff
The Evaluation of Feed Additives on Reducing Enteric Methane Production from Cattle , Reba L. Colin
Methods to Reduce Nitrogen and Carbon Losses from Finishing Beef Cattle , Hanna Cronk
Dry-Aged Beef Flavor Development, and the Effect of High Levels of Vitamin-E on Beef Color Stability , Nicolas Herrera
Mid-Gestation Maternofetal Inflammation Impacts Growth, Skeletal Muscle Glucose Metabolism, and Inflammatory Tone in the Ovine Fetus During Late Gestation , Zena Hicks
The Evaluation of Encapsulated Megasphaera elsdenii in an Accelerated Beef Step-Up Program and an Acidosis Challenge Model and the Evaluation of RAMP Versus a Traditional Forage Grain Adaptation Strategy on Methane and Respired Carbon Dioxide , Cindy D. Mansfield
The Impact of Silage Inclusion in Diets with Different Corn Processing and the Effect of Natural and Conventional Feeding Programs on Finishing Cattle Performance , Jessica L. Miller
Identification and Assessment of Lameness in Sows Through the Utilization of NUtrack AND GAITFour Systems , Lexi M. Ostrand
Students' Attitudes Towards Animals Influences Youth Development Constructs Based on Interactions with Different Animal Species Prior to College , Allison K. Pachunka
Winter Hardy Small Cereals for Grazing or Silage in Eastern Nebraska , Abigail Sartin
Consequences of cow-calf production with limited perennial forage grazing , Hannah Speer
Impact of Distillers Removal and Impact of Lowering Inclusions of Distillers Grains Plus Solubles and Different Roughage Quality on Finishing Cattle Performance , Sofia Suarez Lorences
Effect of Supplementation Prior to Artificial Insemination and During Gestation in Beef Females , Landon Tadich
Investigation of Breeding Objectives and Indexes-in-Retrospect , Hunter F. Valasek
Feed Value and Utilization of Corn Residue: Implications for Cow Performance and Grazing Strategies , Kaylee E. Wheeler
Early-Life Supplementation of Omega-3 Polyunsaturated Fatty Acids Improved Growth and Skeletal Muscle Glucose Metabolism in the Heat Stress-Induced IUGR Neonatal Lamb , Melanie Ryann White
ENVIRONMENTAL DETERMINATION OF GASTROINTESTINAL PARASITISM IN KATAHDIN SHEEP , Brian Arisman
Annotating Gene Expression and Regulatory Elements in Tissues from Healthy Thoroughbred Horses and Identifying Candidate Mutations Associated with Perosomus Elumbis in an Angus Calf , Alexa Barber
Impact of Increasing Level of Milk Production on Cow and Calf Behavior and Performance in the Nebraska Sandhills , Selby Boerman
Molecular Mechanisms Underlying Aberrant Circulating Steroid Hormones In GnRHR-II Knockdown Boars and Their Control Littermates , Dorothy Elsken
The Invisible Meat Microcosmos - Investigations of Processed Meats' Specific Spoilage Organisms , REBECCA FURBECK
Students' Perceptions of Online Equine Courses and Their Impacts on Learning Outcomes , Blaire (Gibbens) Speck
Impacts of Feeding Biochar to Beef Cattle on Greenhouse Gas Emissions and Performance and Characterizing Yearling Steers Grazing Smooth Bromegrass Pasture Using GPS , Holly Heil
The Nebraska 4-H Equine Advancement Level Program’s Role in Positive Youth Development Using the Five Cs Model: An Exploratory Study , Eunhye McCarthy
Identifying Early-Life Behavior to Predict Mothering Ability in Swine Utilizing NUtrack System , Savannah Millburn
Targeting Inflammation in Heat-Stressed Wethers Improves Growth and Efficiency and Alters Body Composition; A Brief Exploration and Application of Extension Principles , Micah Most
Evaluation of the Feeding Value of Proso Millet in Growing-Finishing Diets for Pigs and Effects of Feed Ingredients and Medium-Chain Fatty Acids on Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Survivability , Khang Nguyen
Variance Component Estimates for Growth Traits in Beef Cattle Using Selected Variants from Imputed Low-Pass Sequence Data , Chad A. Russell
Investigating Bacterial Community Composition and Antimicrobial Resistance Genes in Beef Cattle , Arena See
Evaluation of corn processing method and Sweet Bran inclusion on beef cattle performance and nutrient digestion and individual Sweet Bran components on nutrient digestion , Rebecca L. Sjostrand
The impact of plant cell wall lignin on energy utilization in lactating Jersey cows. , Jason Stypinski
The Impact of Steam-flaked Rye Replacing Steam-flaked Corn on Performance and Carcass Characteristics of Yearling Steers and The Effect of Exogenous Megasphaera elsdenii Administration Techniques on Ruminal Fermentation and Blood Parameters in an Acidosis Challenge Model , Samantha K. Wagner
Evaluation of Nutritional Factors Affecting Sow Reproductive Longevity , J'Nan E. Wittler
Using Strategic Supplementation to Enhance Cow/Calf Productivity in Summer Calving Herds in the Nebraska Sandhills , Nicole M. Woita
Host Genetics and Phenotype Associations Within the Bovine Rumen Microbiome , Waseem Abbas
Small Grain Cereal Cover Crops for Late Fall or Early Spring Grazing , Kallie Calus
Feeding a New Corn Milling Coproduct to Lactating Dairy Cattle; Examination of Whole Animal Energy and Nitrogen Balance , Addison Carroll
Evaluation of Grain Type and Processing Method on Steer Performance, Carcass Characteristics, and Nutrient Digestion , Caitlin Coulson
The Effects of Different Feed Supplements on Performance Parameters, Egg Measurements, and Eggshell Integrity in Older White Leghorn Laying Hens , Josephine Foley
Evaluation of Condensed Algal Residue Solubles as an Ingredient in Cattle Finishing Diets and Its Effects on Digestibility and Fatty Acid Flow and a Comparison of Single and Dual Implant Strategies in Finishing Heifers , John Gibbons
Evaluation of Cattle Management for Systems with Limited Perennial Pasture , Morgan Grabau
Management of Late Summer Planted Annual Forages for Grazing and the Impacts of Novel Sweet Bran Plus Products on Performance and Carcass Characteristics of Beef Finishing Steers , Devin Jakub
Changes in Whole Blood Parameters in Beef Heifers May Contribute to Delayed Pubertal Attainment , Jessica A. Keane
UNDERSTANDING RUMEN MICROBIAL COMMUNITY STRUCTURE AND FUNCTION TOWARDS DECREASING METHANE EMISSIONS , Allison L. Knoell
The Effects of Omega-3 PUFA Infusions During Late Gestation on Developmental Pathologies in the Intrauterine Growth Restricted Fetus , Taylor Lacey
Greenhouse Gas Emissions From Two Contrasting Beef Systems from Birth to Slaughter in Eastern Nebraska , Levi McPhillips
The Role of Fatty Acids in Ruminant Diets and Novel Feed Ingredients High in Omega– 3 Fatty Acids Fed in Feedlot Diets , Mitchell M. Norman
Utilizing Online Resources to Enhance Distribution of Competitive Animal Evaluation Knowledge and Benefits , Brooke L. Parrish
The Effect of Heat Stress and Beta-Adrenergic Agonists on Fatty Acid Mobilization and Their Individual and Interacting Impact on the Adipose Transcriptome of Ruminant Livestock , Rachel R. Reith
Variation in the Genome and Transcriptome Associated with Beef Cattle Production and Investigation of the Metabolic Consequences of Beta-adrenergic Agonist Supplementation , Renae L. Sieck
Low-Oxygen Dry Aging of Beef , Joseph Sonderman
Impact of Wood-Sourced Biochar on Carbon and Nitrogen Capture in Beef Feedlot Systems , Jessica L. Sperber
QUANTIFICATION AND REPEATED MEASUREMENTS OF CONFORMATION TRAITS IN REPLACEMENT FEMALES TO OPTIMIZE SOW LONGEVITY , Melanie D. Trenhaile Grannemann
ESTIMATION OF BREED EFFECTS AND GENETIC PARAMETERS FOR AGE AT SLAUGHTER AND DAYS TO FINISH IN A MULTIBREED BEEF CATTLE POPULATION , Lindsay Upperman
Enhancement of Dry-Aged Beef Quality by Dietary Supplementation of High Levels of Vitamin E , David Velazco
Advancing the Science of Dry-Aged Beef , Felipe Azevedo Ribeiro
Improving the Accuracy of Genomic Predictions: Investigation of Training Methods and Data Pooling , Johnna Baller
Evaluation of Maternal Diet and its Effect on Milk Composition and Piglet Health and Growth Performance , Shana Barnett
Investigating microbiomes and developing direct-fed microbials to improve cattle health , Alison Bartenslager
Comparison of comprehensive health score in North American housed giraffe and free-ranging giraffe from South Africa , Haley Beer
Impact of Diet and Quality Grade on Meat Quality Characteristics and Their Relationship to Oxidative Stress , Nicolas Bland
Characterization of protein and fat in dairy feeds and implications on digestibility and milk composition , Kassidy Buse
The Role of Postnatal Adrenergic Manipulation in the Mediation of Fetal Programming Adaptions in Growth and Skeletal Muscle Glucose Metabolism That Persist in the Juvenile IUGR-born Lamb , Rachel L. Gibbs
The Impact of Oxidative Stress on Postmortem Meat Quality , Nicolas J. Herrera
Influence of Strategic Supplementation and Genetic Potential for Milk Yield on Forage Digestibility, Amino Acid Utilization, and Livestock Production , Tasha M. King
Effects of NDF digestibility on Lactating Jersey Cows: Observed and Modeled Performance , Kirby Krogstad
Interaction of Urea with Frequency and Amount of Distillers Grains Supplementation for Growing Steers on a High Forage Diet , Haley F. Linder
ENERGY AND AMINO ACID METABOLISM IN LACTATING JERSEY COWS CONSUMING FEED BYPRODUCTS , Kyle McLain
Energy metabolism in Jersey cows: Improving our understanding of energy requirements and utilization , Dennis Morris
Evaluation of Protein Sources and Holstein Finishing Systems for Organic Beef Production and a Comparison of Single and Dual Implant Strategies in Finishing Heifers , Elizabeth Schumacher
Heat stress-induced deficits in growth, metabolic efficiency, and cardiovascular function coincided with chronic systemic inflammation and hypercatecholaminemia in ractopamine-supplemented feedlot lambs; Atypical cyclicity at puberty in beef cows is associated with early deficits in muscling, metabolic indicators, and myoblast function in offspring but does not impact feedlot performance. , Rebecca M. Swanson
Translational Genomics For Improving Sow Fertility , Hiruni R. Wijesena
EVALUATION OF NOVEL ADDITIVES AND CORN SILAGE AS NATURAL ALTERNATIVES TO ANTIBIOTICS FOR THE PREVENTION OF RUMINAL ACIDOSIS AND LIVER ABSCESSES IN BEEF FINISHING CATTLE , Hannah C. Wilson
IMPACT OF COW SIZE AND VALIDATION OF AN ELECTRONIC FEEDER TO OPTIMIZE RESOURCES IN BEEF PRODUCTION SYSTEMS , Rob Ziegler
Evaluation of Microbial Community Dynamics Impacting the Shelf-Life of Processed Meats , Chad G. Bower
Evaluation of Alpha Amylase Containing Corn on Beef Cattle Performance and Digestibility and Double-Cropped Annual Forages Following Corn Harvest , McKenna Brinton
Effects of Late Gestation Supplementation and Creep Feeding on Spring Calving Beef Cows in the Nebraska Sandhills , Devin Broadhead
Impact of Feeding Distillers Grains or Isolated Components in Distillers Grains to Growing and Finishing Cattle, and the Comparison of Protein Content and In-Situ Digestibility of Feeds Commonly Used in Feedlot Diets , Brianna B. Conroy
Selective harvest methods and chemical treatment of baled corn residue for utilization in growing calf and dry cow diets , Ashley C. Conway
Impact of Beta-Adrenergic Agonist Supplementation and Heat Stress on the Microbiome and Gastrointestinal Transcriptome of Sheep , Erin M. Duffy
Beef Production Systems in the Nebraska Sandhills , McKay Erickson
The Effects of Butyric Acid on Performance Parameters, Egg Quality and Nutrient Utilization in Young White Leghorn Hens , Dani-el Hanna
Impact of Myoglobin Oxygenation State on Color Stability of Beef Steaks During Frozen Storage and Thawed Retail Display , Morgan Lee Henriott
Effects of Alternative Carbohydrate Sources and Soybean-Derived Isoflavones on the Microbiome and Immune System of Nursery Piglets and Intestinal Porcine Epithelial Cell Line J-2 , Sydney Kinstler
Undergraduate Success in Animal Science Courses Based on Demographics, Motivation, and Online Courses , Haylee Anne Lavoie
Attainment and Maintenance of Pubertal Cyclicity May Predict High A4 Cows with Reduced Fertility , Sarah Nafziger
The Utilization of Brown Midrib Corn Silage Hybrids and Kernel Processing to Improve Corn Silage Value and the Use of High Protein Distillers Grains to Evaluate Starch Digestion , Lauren A. Ovinge
The Role of Inflammatory Pathways in Development, Growth, and Metabolism of Skeletal Muscle in IUGR Offspring; Blood Gene Expression of Inflammatory Factors as Novel Biomarkers for Assessing Stress and Wellbeing in Exotic Species. , Robert J. Posont
Evaluation of Protein Utilization in Low and High Protein Forage Sources and the Economic Value of Supplementing Field Peas (pisum sativum) to Growing Cattle Grazing Crested Wheatgrass Pastures , Braden C. Troyer
SURVEILLANCE AND EVALUATION OF MANURE TREATMENT PRACTICES FOR MITIGATION OF THE PORCINE EPIDEMIC DIARRHEA VIRUS (PEDV) IN A COMMERCIAL SWINE FARM SETTING , Erin Boyles
Corn Residue Grazing as a Component of Semi-Confined Cow-Calf Production and the Effects of Post-weaning Management on Feedlot Performance , Shelby E. Gardine
Impact of ethanol process changes on distillers grains for beef cattle , Shelby Garland
GENOME-WIDE ASSOCIATION STUDY FOR THE RELATIONSHIP BETWEEN TEMPERATURE AND FEED INTAKE IN BEEF CATTLE , Robel Ghebrewold
Quality Effects on Beef from Cattle Fed High-Protein Corn Distillers Grains or Other Ethanol By-Products , Kellen B. Hart
Effect of corn silage harvest, hybrid, and concentration on performance in growing and finishing beef cattle , Fred H. Hilscher Jr
EVALUATION OF ALPHA AMYLASE CONTAINING CORN ON FINISHING CATTLE PERFORMANCE AND DIGESTIBILTY , Melissa L. Jolly-Breithaupt
Evaluation of the Interaction of Beta-Adrenergic Agonists Supplementation and Heat Stress on Growth Performance and Carcass Composition in Feeder Lambs , Lauren Elisabeth Kett
Advanced Search
Search Help
- Notify me via email or RSS
- Administrator Resources
- How to Cite Items From This Repository
- Copyright Information
- Collections
- Disciplines
Author Corner
- Guide to Submitting
- Submit your paper or article
- Animal Science Website
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Asian-Australas J Anim Sci
- v.31(9); 2018 Sep
Guidelines for experimental design and statistical analyses in animal studies submitted for publication in the Asian-Australasian Journal of Animal Sciences
Seongwon seo.
1 Division of Animal and Dairy Science, Chungnam National University, Daejeon 34134, Korea
Seoyoung Jeon
2 Asian-Australasian Journal of Animal Sciences, Seoul 08776, Korea
3 Department of Agricultural Biotechnology, College of Agriculture and Life Science, Seoul National University, Seoul 08826, Korea
Animal experiments are essential to the study of animal nutrition. Because of the large variations among individual animals and ethical and economic constraints, experimental designs and statistical analyses are particularly important in animal experiments. To increase the scientific validity of the results and maximize the knowledge gained from animal experiments, each experiment should be appropriately designed, and the observations need to be correctly analyzed and transparently reported. There are many experimental designs and statistical methods. This editorial does not aim to review and present particular experimental designs and statistical methods. Instead, we discuss some essential elements when designing an animal experiment and conducting statistical analyses in animal nutritional studies and provide guidelines for submitting a manuscript to the Asian-Australasian Journal of Animal Sciences for consideration for publication.
INTRODUCTION
For scientific, ethical, and economic reasons, experiments involving animals should be appropriately designed, correctly analyzed, and transparently reported. This increases the scientific validity of the results and maximizes the knowledge gained from each experiment. Nonetheless, biologists, on average, feel uncomfortable with mathematics and statistics, and they often design experiments and analyze data in inappropriate ways [ 1 ]. Therefore, in some fields of research where animal experiments are essential, the editorial board regularly reviews the statistical methodologies reported in the papers and presents their suitability [ 2 – 5 ]. Some fields of research have set up consortia and provide guidelines for animal experiments [ 6 , 7 ], and some scientific journals have guidelines for their authors to follow for publication [ 8 , 9 ]. For example, in the animal science field, the Journal of Dairy Science provides detailed guidance on statistical methodology in the instructions to authors [ 10 ]. Animal Feed Science and Technology has published two editorials that discuss proper experimental design and statistical analyses to guide authors who are submitting manuscripts to the journal [ 11 , 12 ].
The Asian-Australasian Journal of Animal Sciences (AJAS) published the first issue in January 1988, and its contribution and influence to the animal science fields have continuously expanded over the past three decades. In particular, a total of 102 nutritional studies were published in AJAS in 2017, which included 84 in vivo trials. In these studies, statistical methods are essential, and authors should strive to employ an appropriate experimental design and statistical analyses to provide the reader with scientifically relevant and valid knowledge.
This editorial will discuss some of the principles of experimental design and statistical analysis and provide guidelines when submitting nutritional studies to AJAS for consideration for publication.
EXPERIMENTAL DESIGN
Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Details include animal characteristics (e.g., species, breed, gender, weight), number of treatments, number of experimental and sampling units, arrangement of treatments (e.g., factorial, change-over), and consideration for known variation (e.g., blocking, covariates). Only properly designed experiments can yield valid and reliable results that lead to correct and appropriate interpretations and conclusions in the study.
The experimental unit and the number of replicates
Treatments, the set of circumstances created for an experiment based on research hypotheses, are the effects to be measured and compared in the experiment [ 13 ]. The treatment is applied to the experimental unit, and one experimental unit corresponds to a single replication; Kuehl [ 14 ] defines the experimental unit as “the physical entity” or subject exposed to the treatment independently of other units. The number of replicates (i.e., sample size) is the number of experimental units per each treatment. Defining the experimental unit correctly is crucial for proper experimental design and statistical analysis. However, correctly defining the experiment unit is sometimes not easy. This is especially true in the cases where a group of animals are fed together in a pen, there is debate as to the most appropriate experimental unit between statisticians and biologists [ 11 ].
Like most other biostatisticians [ 14 , 15 ], editors of AJAS have a more conservative view regarding the determination of the experimental unit. For many nutritional studies, the purpose of the experiment is to infer population means. For example, in a feeding trial in which different dietary treatments are applied to different groups of animals, the ultimate goal of the experiment is not to observe the treatment effect within the experimental animals but to investigate its effect on independent animals in the real world. The role of replication is to provide measures of how much the results are reliable and reproducible, and thus replicates are to be independent observations and experimental units must be independent of each other. If a treatment is applied to a group of animals in a single pen, the individual animals are not independent; thus, the pen is considered the experimental unit even when measurements are made individually. The treatment effect is confounded by the effect of the pen in this case, and it is obvious that the pen should be the experimental unit because it is unknown whether the results of the experiment were caused by the treatment of the pen. On the other hand, if treatments are randomly assigned to individual animals within a group of animals in a pen, the individual animal can be considered the experimental unit even though they are in the same pen.
A sufficient number of replicates are needed to obtain a reliable outcome from an experiment. Because the number of replicates is related with the power of a test, more experimental replicates can provide greater statistical power to detect a desired difference among treatments. The cost of replicates, however, is high in animal experiments, and the smallest number of replicates is preferred, as long as it is sufficient to detect a difference. For this purpose, power tests are performed prior to initiating an experiment to determine the required sample size based on expected variation in means and the size of the difference between means that needs to be detected.
Power tests are also useful for supporting the validity of an experiment when no significant difference is observed between the treatment means. It is not uncommon to fail to detect a significant difference between treatments, and in this case, one can argue that significance was not observed simply because the sample size was small. The result from the power test can provide supportive evidence that the reason for the failure to detect a difference between treatments was not because the sample size was small, rather the difference between the treatment means was not great enough to be considered significant.
Therefore, AJAS encourages authors to provide the results of power tests. The results of power tests can be used to justify that the experiment was appropriately designed.
Consideration for known variations
To properly test for treatment effects, factors other than the main treatment that may affect the response of the animals should be minimized or at least accounted for. In this regard, the use of a block or covariate is recommended.
Blocking is a practice wherein the experimental units are placed into groups of similar units based on one or more factors that are known or expected to affect the responses to be measured and tested. Physical and physiological characteristics, such as sex, litter, and initial body weight, are commonly used for blocking in the animal science field. Blocking controls the variability of experimental units and reduces experimental error.
Covariates are variables that are known or expected to be related to the response variables of interest. The primary difference between blocks and covariates is that covariates are continuous variables, whereas blocks are categorical variables. For example, animals can be grouped or blocked as high, medium, and low groups according to their body weight. Conversely, individual body weight can be used as a covariate to reduce the estimates of experimental error in the statistical model. Blocking is applied at the experimental design stage, whereas the use of covariates is applied when conducting statistical analysis.
The use of a block and covariate is a sound and logical way to account for known errors and reduce unexplained errors. The AJAS editorial board thus encourages authors to use blocks and covariates if there are known or expected variables that could have a significant effect on the response to be tested for in the experimental treatments.
When a limited number of animals are available or when individual animal variation is to be removed, crossover (i.e., changeover) designs are often used in animal nutritional studies. In this case, it can be an issue if a carryover effect from a treatment given in a previous period influences the response in the following treatment. It should be noted that crossover designs should be avoided when significant carryover effects are expected [ 16 ]. Even if a significant carryover effect is not expected, the potential for a carryover effect should not be ignored in crossover designs. A sufficient rest or wash-out period between two treatment periods is one of the practical ways to minimize carryover effects. More importantly, the order of treatments for each animal should be balanced to avoid confounding of treatment and period effects and to minimize the influence of carryover effects. In a balanced crossover design, each treatment follows each of the other treatments an equal number of times, and each treatment is assigned once for each animal and the same number of times in each period. When a carryover effect is suspected, its significance also needs to be tested by statistical analysis. The AJAS editorial board recommends authors describe the procedure used to minimize possible carryover effects and show that carryover effects are not significant in their study when using a crossover design.
Randomization
Randomization is an essential procedure to ensure the reliability of the experiment and the validity of the statistical analysis. The purpose of an experiment is to make inferences about the population mean and variance, and the statistical analysis assumes the observations are from a random sample from a normally distributed population. This assumption can be valid only through randomization. In animal nutritional studies, two randomization processes are required: random sampling of experimental units and random allocation of treatments to experimental animals.
Theoretically, experimental animals represent the animal population of interest; thus, they need to be randomly selected from the population. However, this is usually not feasible, if not impossible, in the real world and whether experimental animals can be considered a random sample is questionable. Nevertheless, whenever possible, randomization must be practiced in selecting experimental animals to eliminate biases and to obtain valid estimates of experimental error variance. For example, when a deep analysis is performed on selected animals (e.g., blood analysis for selected animals from a group of animals in each treatment), random selection should be conducted.
Random allocation of treatments to experimental units is the most important and critical step to justify and establish the validity of statistical inferences for the parameters of the population and tests of hypothesis. The experimental errors are assumed to be independently and normally distributed. Estimation of parameters and statistical inferences can be possible if and only if this assumption is valid. Random assignment of treatments to experimental animals is the only method that guarantees the independence of observations and permits us to proceed with the analysis as if the observations are independent and normally distributed. The authors are required to describe the randomization procedure used for their animal trials.
STATISTICAL ANALYSIS
Statistical analysis is conducted to test the hypotheses and significance of tests in a study. There are many methods for conducting statistical analysis and various methods yield different results and conclusions. Proper statistical methods should be applied when conducting an experiment, and details of statistical methods should be provided in the statistical methods section of a manuscript to allow reviewers and readers to assess the quality of statistical methods used in the study.
Statistical models
When submitting a manuscript for publication in AJAS, authors should clearly define their statistical models used for the statistical analysis. Statistical models are usually expressed as linear models with the overall mean of the response variable, fixed or random variables that are known to influence the response variable, and unexplained experimental random error. The statistical model should be consistent with the experimental design and be appropriate to analyze the observations from the experiment. A clear description of the statistical model as an equation, as well as in words, is useful to understand the analytical procedure and the meaning of statistical implications and to evaluate the correctness and relevance of the statistical methods used in the study. Thus, the statistical model is often used as a criterion for the recommendation of manuscript rejection by reviewers and editors [ 11 ].
Statistical methods
Various statistical methods are available, and the choice of method depends on the data type of observations, research questions to answer, and the statistical model.
If observations of the response variables are binary (i.e., yes or no) or categorical, the logistic model or other categorical analysis needs to be used. Sometimes research questions are not about means but seek to understand the quantitative relationship between response variables or between the response variable and treatment (e.g., dose-response analysis). The linear or non-linear regression analysis is the method to be used in this case.
When the response variable of interest is a continuous variable and the research question is about means or an interval of the value, either parametric or non-parametric statistical methods can be applied. The most famous parametric statistical methods are the t-test and analysis of variance (ANOVA). A t-test is used for comparing two samples or treatments, whereas the ANOVA is used when there are more than two treatments. Different methods can be used within a t-test and an ANOVA. For example, if two samples are paired (e.g., blood samples collected before and after treatment in the same animal), a paired t-test is most appropriate. Additionally, because different levels of complexity can exist in statistical models (e.g., the existence of both fixed and random effects and their interactions, repeated measures over time), the most appropriate method may vary by the statistical models when conducting an ANOVA. Parametric methods assume that the observations are independent and normally distributed around their mean. This assumption is generally true in animal nutritional studies as long as randomization is practiced. However, it is always a good practice to test this assumption, especially if variables are expected not to follow it. For example, particle size normally has a log-normal distribution [ 17 ], and thus statistical tests need to be performed on transformed values.
If the observations are not normally distributed or the sample size is not large enough, non-parametric analyses (e.g., Mann-Whitney U test instead of a t-test and Kruskal-Wallis H test instead of a one-way ANOVA) would be the methods of choice. Non-parametric methods do not assume a normal distribution of experimental errors and more powerful to detect differences among treatments than parametric methods (e.g., t-test and ANOVA). Because non-parametric methods have more statistical power, they can exaggerate the significance of the difference between treatments. A parametric method is thus preferred when it is applicable.
Comparing the means of interest
When an ANOVA reveals that the probability that treatment means are all equal is sufficiently small enough to conclude that at least one of the treatment means is different from the others, we may ask further questions, such as which ones are different from each other? Before conducting further analyses, two things are to be considered.
First, we need to determine how small is sufficiently small. This is called the level of significance, and it is normally assumed that the probability of less than 5% (i.e., p<0.05) is statistically significant in animal nutritional studies. The level of significance is also called type I error or α, which is the probability of rejecting a null hypothesis when it is true. If α = 0.05, the test can mistakenly find treatment effects in a maximum of one out of 20 trials. When the p-value obtained using an ANOVA test is less than the level of significance, the results may be meaningful and need to be discussed; thus, comparing the means becomes interesting. If the obtained p-value is larger than the predetermined level of significance, we need to conclude that the null hypothesis is plausible, and we do not have enough evidence to reject the null hypothesis and accept the alternative hypothesis. It should be pointed out that we must not accept the null hypothesis. It is logically impossible to test whether the null hypothesis is true and to prove all the means are the same. We cannot ensure that the null hypothesis would remain plausible if the number of replicates was larger. The authors are thus required to state the level of statistical significance in the statistical analysis section.
Next, we need to determine which techniques are most appropriate for the post hoc analysis on the basis that there is a significant difference among the treatments using an ANOVA. One of the most intuitive and simplest methods to compare the means of interest is linear contrasts. If the number of treatments is t, then a set of t – 1 orthogonal contrasts can be tested. Sets of orthogonal contrasts are not unique for a given experiment; there may be many such sets. Finding an appropriate set of orthogonal contrasts lies in the structure of the treatments. For example, suppose there is an experiment of testing two feed additives as alternatives to antibiotics, and it has four treatments: without feed additives (CONT), antibiotics (ANTI), feed additive A (ADTA), and feed additive B (ADTB). A set of 3 (4 – 1) orthogonal contrasts that can be made, and logical and obvious contrasts are i) CONT vs the others, ii) ANTI vs (ADTA and ADTB), and iii) ADTA vs ADTB.
In addition to linear contrasts, there are many methods available for multiple comparisons of means; the most widely used methods include Dunnett’s test [ 18 ], Tukey’s test [ 19 ], Scheffe’s test [ 20 ], the least significant difference (LSD) [ 21 ], and Duncan’s multiple range test [ 22 ]. Among these, Duncan’s test is the most popular method in the animal nutritional studies. Approximately 37% of animal nutrition papers that conducted pair-wise comparisons in 2017 in AJAS used Duncan’s test. The second most used tests were the LSD and Tukey’s test; each accounted for 14% of multiple comparison tests.
The AJAS editorial board does not take a position on which test is more desirable under certain circumstances and leaves the decision to authors as long as the test can properly test logical questions according to the experimental design. For example, for a dose-response experiment with increasing inclusion levels, testing the significance of differences between particular means is inappropriate. Instead, linear and curve-linear regression for testing the dose-response relationship would be a better choice. A pairwise comparison procedure is appropriate to use when there is no structure among a series of treatments.
Statistical software packages and statistical procedures
There are several software packages available for statistical analysis. Even using the data analysis add-in of Microsoft Excel allows for the t-test, analysis for correlation, linear regression analysis, and one-way ANOVA to be performed. More complicated statistical models, however, require software with statistical packages, which include the statistical analysis system (SAS), general statistics (GENSTAT), statistical program for the social sciences (SPSS), Minitab, and R. The most commonly used statistical software in animal nutritional studies is SAS. Fifty-five percent of animal nutrition papers published in 2017 in AJAS used SAS. The second most popular statistical software was SPSS (27.5%), and more than 83% of the papers used one of them. Like other journals, AJAS takes no position on which of these statistical software packages is more desirable in any particular circumstances and leaves that decision to authors. However, it is required for authors to report which software is used for the statistical analysis.
Even within each statistical software package, there are different procedures that can be used for analyzing data. For example, when conducting an ANOVA in SAS, any procedures that can solve a general linear model, such as the ANOVA, GLM, and MIXED procedures, can be used. However, each procedure may have different features and work better for a specific circumstance. For example, in SAS, compared with the GLM procedure (PROC GLM) which is designed to analyze a general linear model with fixed effects, the MIXED procedure can better handle statistical models having random effects. For the analysis of binary or categorical variables with fixed effects, the GENMOD procedure that uses a generalized linear model should be used instead of PROC GLM. A more recent procedure, PROC GLIMMIX, can analyze statistical models with fixed and random effects for both categorical and continuous variables. AJAS does not take a position on which procedures are more desirable under certain circumstances and leaves the decision to authors as long as the procedure can properly handle the data type. However, when the observations are repeatedly measured or random effects are included in the statistical model, PROC MIXED or PROC GLIMMIX in SAS or similar procedures in other statistical packages are preferred.
Reporting all relevant information is important in scientific papers to increase the transparency and validity of the results and provide information for confidence and limitations of scientific knowledge gained from experiments. Not only the probability value (p-value) but also error measures (e.g., standard error of means [SEM]) should be reported in tables. Likewise, error measures should be present as error bars in figures. Error measures can be expressed in several ways: standard deviation (SD), SEM, and the standard error of the difference (SED). AJAS recommends the presentation of the pooled SEM because the objective of animal nutritional studies is usually to provide inferences about the population. If the sample sizes are different among treatments, the sample sizes are to be reported, as well as pooled SEM. However, the use of SD is also allowed when it is used for descriptive statistics.
When there are outliers or missing data, they need to be clearly reported in the Materials and Methods section or the Results section of the manuscript where it is more appropriate. In particular, the methods and their rationale for identifying outliers should be provided, and the results from the statistical analysis of the data with and without outliers should be compared and discussed in the manuscript.
SUMMARY OF RECOMMENDATIONS
The AJAS editorial board takes no position on which experimental designs and statistical methods are more desirable in certain circumstances and leaves that decision to the authors. Nevertheless, a summary of the recommendations of the AJAS editorial board is as follows:
- Provide details of experimental design and statistical methods in the Materials and Methods section.
- Define the experimental unit and report the number of replicates. Replicates are to be independent observations and experimental units must be independent of each other.
- Conduct power tests and provide their results to justify the experiment was appropriately designed.
- Use blocks or covariates whenever applicable to reduce unexplained experimental errors.
- Describe the procedure used to minimize possible carryover effects and to show carryover effects are not significant when using a crossover design.
- Ensure the implementation of randomization when sampling experimental units and allocating treatments to experimental units.
- Describe the statistical models used for the statistical analysis as equations, as well as in words.
- Use appropriate statistical methods depending on the data type of observations, research questions to be answered, and the statistical model.
- Test if the observations are normally distributed around their mean. If not or the sample size is not large enough, use non-parametric analyses instead; otherwise, use parametric methods.
- State the level of statistical significance in the statistical analysis section.
- Conduct post hoc analysis on the basis that there is a significant difference among the treatments and use appropriate methods according to the structure of the treatments.
- Perform pair-wise comparisons (e.g., Duncan’s multiple range test) only when there is no structure among a series of treatments.
- Report which software and procedures are used for the statistical analysis.
- Use appropriate statistical methods and procedures if observations are repeatedly measured or random effects are expected.
- Present both probability value (p-value) and pooled SEM as error measures. The standard deviation can only be used for descriptive statistics.
- Report outliers or missing data in the Materials and Methods section or the Results section where it is more appropriate.
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.

IMAGES
VIDEO
COMMENTS
Abstract. The animal model deals with the species other than the human, as it can imitate the disease progression, its' diagnosis as well as a treatment similar to human. Discovery of a drug and/or component, equipment, their toxicological studies, dose, side effects are in vivo studied for future use in humans considering its' ethical issues.
TYPES OF ANIMAL MODELS. According to different pathological and pathophysiological factors, models can be divided into spontaneous, interventional, and genetically modified models (Neha et al., 2014).Aβ accumulation and tau hyperphosphorylation may occur spontaneously in non-human primates (NHPs), but only one of these phenomena appear to occur in a given species (Braidy et al., 2012).
When examining the literature as a whole, however, it is clear that animal models continue to divert scarce research funding, provide limited advancement in clinical studies, and are heavily biased without proper analysis and reporting [36].In the United Kingdom (UK), while the overall number of animals used in research decreased by 7% from 2017 to 2018, the number of dogs and non-human ...
Simple Summary. The present review highlights and examines the importance of animal models in relevant topics concerning current human and animal health. Over the past five years, different animal species have been used to study pandemics, such as the 2019 Coronavirus, diabetes, and obesity. Through murine, primate, porcine, and even aquatic ...
Animal models have provided invaluable information in the pursuit of medical knowledge and alleviation of human suffering. The foundations of our basic understanding of disease pathophysiology and human anatomy can largely be attributed to preclinical investigations using various animal models. Recently, however, the scientific community ...
According to the American philosopher Marx Wartofsky, "Theories, hypotheses, models and analogies I take all to be species of a genus, and my thesis is best stated directly by characterizing this genus, as representation (although "imaging" or "mirroring" will do quite as well)" ( Wartofsky 1979). He goes on to say, "There is an ...
Rapid developments in Regenerative Medicine and Tissue Engineering has witnessed an increasing drive toward clinical translation of breakthrough technologies. However, the progression of promising preclinical data to achieve successful clinical market authorisation remains a bottleneck. One hurdle for progress to the clinic is the transition from small animal research to advanced preclinical ...
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity in humans, but the therapeutic options for TBI are limited. Xionget al. critically review animal models of TBI and ...
However, despite the considerable social and scientific relevance of the use of animal models in biomedical research, there is a scarcity of scholarly literature exploring the topic from a pedagogical standpoint. The case study presented in this paper aims to investigate the effectiveness of role play simulation in promoting critical ...
Hair loss and regeneration are the subjects of tremendous amount of research for multiple reasons: the well-known importance of hair in individual beauty, the fact that alopecia is a frequent dermatological disease, and that there are limited treatment options. The present work focuses on the evaluation of animal models used for hair research and regeneration. Besides mentioning the option of ...
FIGURE 2 Operative technique using pigs as an animal model. A nimAl experimentAtion: A look into ethics, welfAre And A lternAtive methods. r eV assoc Med bras 2017; 63 (11):923-928 925 ...
A transgenic animal model in which tau protein aggregation is expected to produce behavioural symptoms similar to those observed in certain tauopathies in humans has been examined in this thesis. The transgenic mouse lines, when tested in a heterozygous genetic background, showed no cognitive deficit although non-associative learning was impaired.
Animal models have been used to address a variety of scientific questions, from basic science to the development and assessment of novel vaccines, or therapies. The use of animals is not only based on the vast commonalities in the biology of most mammals, but also on the fact that human diseases often affect other animal species. ...
81% of the time, animal tests fail to detect the potential side effects of drugs in humans. 89% of experiments cannot even be reproduced—a critical research step—resulting in $28 billion ...
Researchers requesting use of animal models at Stanford must have their research proposals reviewed by a federally mandated committee that includes two independent community members. It is only with this committee's approval that research can begin. We at Stanford are dedicated to refining, reducing, and replacing animals in research whenever ...
Reviewed by Emily Henderson, B.Sc. Mar 23 2022. Indiana University School of Medicine researchers found that niacin limits Alzheimer's disease progression when used in models in the lab, a ...
The common model in animal breeding is called an animal model, based on the underlying infinitesimal genetic model, containing factors to account for time trends, contemporary groups, additive genetic effects, residual, and other factors, that depend on the species and trait of interest.Figure 1 depicts a typical animal model in a diagrammatic format.
List of dissertations / theses on the topic 'Animal model'. Scholarly publications with full text pdf download. Related research topic ideas.
Abstract: Nonhuman animal ("animal") experimentation is typically defended by arguments that it is reliable, that animals provide sufficiently good models of human biology and diseases to yield relevant information, and that, consequently, its use provides major human health benefits. I demonstrate that a growing body of scientific ...
a continuous space model for animal movement, the animal's movement step is a velocity vector pointing from the animal's location at a given time to the animal's next (in time) observed location. In a discrete space model for animal movement, the movement step is the change from one location to another (e.g., Turchin, 1998). For ...
Department of Animal Science: Dissertations, Theses, and Student Research. ... Deposit of your thesis or project is required. (If an embargo ... The Nebraska 4-H Equine Advancement Level Program's Role in Positive Youth Development Using the Five Cs Model: An Exploratory Study, ...
The Asian-Australasian Journal of Animal Sciences (AJAS) published the first issue in January 1988, and its contribution and influence to the animal science fields have continuously expanded over the past three decades. In particular, a total of 102 nutritional studies were published in AJAS in 2017, which included 84 in vivo trials. In these ...