Enter search terms to find related medical topics, multimedia and more.

Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Herpes Zoster Ophthalmicus
(herpes zoster virus ophthalmicus; ophthalmic herpes zoster; varicella-zoster virus ophthalmicus).
, MD, FACS, Sidney Kimmel Medical College at Thomas Jefferson University
- 3D Models (0)
- Calculators (0)

- Lab Test (0)

Symptoms include pain and tingling of the forehead, blisters on the forehead and nose, eye ache and redness, light sensitivity, and eyelid swelling.
Doctors diagnose herpes zoster ophthalmicus based on evidence of a shingles rash and involvement of the eye.
The shingles vaccine can help prevent reactivation of the varicella-zoster virus.
People with herpes zoster ophthalmicus are treated with antiviral drugs.
Symptoms of Herpes Zoster Ophthalmicus
Pain or tingling of the forehead may occur before any other symptoms (called a prodrome).
The skin of the forehead and sometimes the tip of the nose are covered with small, extremely painful, red blisters.
Infection of the eye causes ache, redness, light sensitivity, and eyelid swelling. The cornea (the clear layer in front of the iris and pupil) can get infected and inflamed. Months and years later, the cornea can become swollen, severely damaged, and scarred. The structures behind the cornea can become inflamed ( uveitis Uveitis Uveitis is inflammation anywhere in the pigmented inside lining of the eye, known as the uvea or uveal tract. The uveal tract may become inflamed because of infection, injury, a bodywide autoimmune... read more ), the pressure in the eye can increase ( glaucoma Glaucoma Glaucomas are a group of eye disorders characterized by progressive optic nerve damage (often, but not always, associated with increased eye pressure) that can lead to irreversible loss of vision... read more ), and the cornea can become numb, which leaves it vulnerable to injuries.
An Inside Look at the Eye
Diagnosis of herpes zoster ophthalmicus.
A doctor's evaluation
Prevention of Herpes Zoster Ophthalmicus
A recombinant shingles vaccine Shingles Vaccine The herpes zoster virus that causes shingles is the same virus that causes chickenpox. After chickenpox resolves, the virus remains in the body. It can be reactivated years later and cause shingles... read more is recommended for healthy people aged 50 or over, regardless of whether they have had chickenpox or shingles or been given the older herpes zoster vaccine. The recombinant vaccine is effective in more than 90% of people, whereas the older vaccine was effective in 50% of people.
Treatment of Herpes Zoster Ophthalmicus
Antiviral drugs taken by mouth
Corticosteroid eye drops
Eye drops to keep the pupil dilated
As with shingles anywhere in the body, early treatment with an antiviral drug such as acyclovir , valacyclovir , or famciclovir (which are taken by mouth) can reduce the duration of the painful rash. When herpes zoster infects the face and threatens the eye, treatment with an antiviral drug reduces the risk of eye complications.
Corticosteroids, usually in eye drops, may also be needed if the eye is inflamed.
Eye drops, such as cyclopentolate or atropine , are used to keep the pupil dilated, to help prevent a severe form of glaucoma, and to relieve pain.
Drugs Mentioned In This Article

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada)—dedicated to using leading-edge science to save and improve lives around the world. Learn more about the Merck Manuals and our commitment to Global Medical Knowledge .
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Edition
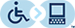
- IN THIS TOPIC
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Herpes zoster...
Herpes zoster ophthalmicus
- Related content
- Peer review
- Darren Shu Jeng Ting , clinical research fellow in ophthalmology 1 2 ,
- Niru Ghosh , consultant general physician 3 ,
- Saurabh Ghosh , consultant ophthalmologist 1
- 1 Sunderland Eye Infirmary, Sunderland, UK
- 2 Academic Ophthalmology, Division of Clinical Neuroscience, School of Medicine, University of Nottingham, Nottingham, UK
- 3 Mayfield Medical Group, Jarrow, UK
- Correspondence to Darren Shu Jeng Ting ting.darren{at}gmail.com
- Accepted 2 November 2018
What you need to know
Offer patients systemic antiviral medication to reduce complications, notably corneal complications and potentially post-herpetic neuralgia
Herpes zoster ophthalmicus may directly involve the eye and/or the skin around the eye, or may occur without ocular involvement, where only the skin of the V1 dermatomal region is affected
Refer to ophthalmology if a patient has ocular symptoms or signs
A 70 year old man attended with a two day history of painful vesicular rash affecting the left forehead accompanied by a red, painful left eye. Three days before the onset of the rash, he had experienced a tingling sensation at the left forehead. A clinical diagnosis of herpes zoster ophthalmicus with ocular involvement was made.
Herpes zoster, or shingles, is a common infection caused by the reactivation of varicella zoster virus that lies dormant in the dorsal root nerve ganglion following primary chickenpox infection. Herpes zoster ophthalmicus accounts for 10-20% of cases of herpes zoster infection. 1 Patients usually present with painful, vesicular, dermatomal rashes affecting the ophthalmic division of the trigeminal nerve (V1). The diagnosis is usually made on clinical grounds but a viral swab can confirm the diagnosis.
Herpes zoster ophthalmicus may present with ocular involvement such as conjunctivitis, keratitis, iritis, and uveitis. It can also present without ocular involvement (where only the skin of the V 1 dermatomal region is affected). Herpes zoster infection may rarely present without any cutaneous manifestation, also known as “zoster sine herpete,” with or without ocular involvement, rendering the diagnosis more difficult. 2
This article aims to discuss the key points to cover in a history, examination, and initial management plan for a person attending primary care with a likely diagnosis of herpes zoster ophthalmicus.
What you should cover
Presenting complaint.
When did the rash start? Starting oral antiviral treatment within 72 hours of the onset of rash substantially reduces the risk of long term ocular complications such as corneal pseudo-dendrites, stromal keratitis, and uveitis. 3 However, a recent Cochrane review has shown that starting oral antiviral treatment within 72 hours may reduce the severity but not the incidence of post-herpetic neuralgia. 4 5
Is there any pain affecting the eye or the periocular skin? Many patients find it hard to distinguish between the pain affecting the eye and the pain around the eye. Eye pain, but not periocular pain, suggests ocular involvement.
Patients with “zoster sine herpete” describe neuropathic pain affecting the V1 dermatome without any rash.
Are there other ocular symptoms such as photophobia, discharge, visual loss/disturbance, floaters, flashing light, or diplopia?
Medical/ocular history
Is there any recent systemic illness? Active systemic illness can impair immunity increasing the risk of developing herpes zoster.
Is there any history of chickenpox or herpes zoster infection? Recurrent episodes should prompt investigation for any underlying immunosuppression.
Is the patient immunosuppressed, for instance, any history of HIV, organ transplantation, or malignancy? Immunosuppressed patients may present with a more aggressive clinical course that requires intravenous antiviral treatment.
Drug history
Is the patient on any immunosuppressive drug?
Has the patient received any shingles vaccination recently? Studies have shown that shingles vaccination, which contains live attenuated varicella zoster virus, may rarely result in reactivation of herpes zoster ophthalmicus. 6
Social history
Is there any recent contact with patients affected by chickenpox or herpes zoster infection? Is there any close contact with children, pregnant women, or immunosuppressed individuals? If the answer is yes, those who have been in contact with the patient are advised to look out for symptoms and signs of chickenpox or shingles and seek medical attention if affected.
Examination
General examination.
Pattern of rash—whether the vesicular rash follows the V1 dermatomal distribution and does not cross the midline of the face ( figs 1 and 2 ).

A patient with left herpes zoster ophthalmicus affecting the forehead and side of the nose (positive Hutchinson’s sign; yellow arrows ). The crusted skin rashes follow the V1 dermatomal distribution and do not cross the vertical midline
- Download figure
- Open in new tab
- Download powerpoint

A patient with left herpes zoster ophthalmicus affecting the forehead but not the nose (negative Hutchinson’s sign). The crusted skin rashes follow the V1 dermatomal distribution and do not cross the vertical midline
Presence of Hutchinson’s sign ( fig 1 ) —rash involving the tip, side, or root of the nose. This sign indicates the involvement of the nasociliary branch of the trigeminal nerve, and is a strong predictor of ocular inflammation and permanent corneal denervation in herpes zoster ophthalmicus (relative risk of 3-4 times). 7 This is because the eyes and the skin of the nose are supplied by the ciliary nerves and the anterior ethmoidal nerve, respectively, which are branches of the nasociliary nerve.
Unilateral or bilateral periorbital swelling—bilateral involvement is usually due to gravitational oedema, rather than because of spread of infection to the contralateral side of the face.
Signs of secondary bacterial infection purulent discharge or worsening, high grade fever. Secondary bacterial infection is usually restricted to the side affected by herpes zoster ophthalmicus.
General wellbeing—if the patient is confused, consider the possibility of coexisting encephalitis.
Ocular examination
Formally examine visual acuity using, for example, a Snellen chart. Examine the external eye for conjunctival redness. Consider instilling a drop of fluorescein 1% to check for corneal pseudo-dendrites using a blue light. Presence of fluorescein stained corneal changes requires a more urgent referral to ophthalmology
Consider viral swab cultures for herpes simplex virus and varicella zoster virus if there is diagnostic uncertainty about whether it is shingles.
Consider other causes of a rash around the eye:
Herpes simplex virus infection— typically presents as multiple vesicles on a raised, erythematous base, followed by ulceration at a later stage. Vesicles usually occur in clusters and do not follow the dermatomal pattern and cross the midline. A viral swab culture for herpes simplex virus and varicella zoster from fresh vesicles helps distinguish between the two infections.
Impetigo— a bacterial skin infection caused by staphylococcus or streptococcus, characterised by a cluster of small blisters or yellow golden crust that do not follow a dermatomal pattern and cross the midline. More common in children than in adults.
Contact dermatitis —an inflammatory skin condition that is caused by contact with either allergens or irritants. The diagnosis can usually be made through careful history taking.
Vaccinia dermatitis —an infective, blistering skin condition that occurs in patients with atopic dermatitis after receiving the smallpox vaccine. This vaccine became obsolete after the eradication of smallpox virus.
What you should do
The diagnosis is usually made on clinical grounds (ie, dermatomal rashes affecting the V1 region and stopping at the midline of the face). Take a viral swab from active vesicles if there is any uncertainty.
Initial stage
Start all patients with herpes zoster ophthalmicus, with or without ocular involvement, on systemic antiviral treatment within 72 hours of the onset of rashes. 8 Systemic antiviral treatment can be offered beyond 72 hours after the onset of rashes (if there are new blisters forming), because the risk of side effects of treatment is low. The first line treatment in the UK is oral aciclovir 800 mg five times a day for 7-10 days. Alternatively, oral famciclovir or valaciclovir may be used as a second line treatment. 8
If superimposed bacterial infection is suspected start an oral antibiotic.
Consider prescribing analgesia such as topical capsaicin cream, amitriptyline, or gabapentin, for neuropathic pain. Explain to the patients that there is a risk of post-herpetic neuralgia.
Consider prescribing lubricating eye drops for comfort if there are lesions near the eyelid. Topical aciclovir or antibiotic eye drops are usually not recommended acutely.
Stromal keratitis or uveitis requires topical steroids to treat the disease and alleviate the pain. Topical anaesthesia is not used as it prevents corneal healing and may worsen corneal denervation.
Advise patients to avoid contact with children, pregnant women, and immunosuppressed individuals, until the vesicles have crusted over (this usually takes 1-2 weeks).
When to refer
Refer to ophthalmology— refer patients with ocular symptoms such as eye pain and blurred vision, and/or signs, including red eye and positive corneal staining with fluorescein, to the ophthalmology team for further examination. This will include a comprehensive external eye examination and a dilated fundus examination.
Potential periocular, ocular, and extraocular complications of herpes zoster ophthalmicus
- View inline
Potential periocular, ocular, and extraocular complications of herpes zoster ophthalmicus are summarised in table 1. The commonest complications are conjunctivitis, corneal pseudo-dendrites, disciform keratitis, and uveitis. The most important complications that must not be missed are uveitis and acute retinal necrosis. 9 Uveitis causes pain and photophobia without any discharge. Acute retinal necrosis causes pain with loss of vision and/or floaters. oth conditions can only be confirmed on slit-lamp examination.
Most of the ocular complications can be managed in the outpatient setting, except for acute retinal necrosis —the most serious and blinding complication—which requires hospital admission for immediate intravenous and intravitreal antiviral treatment.
Acute medicine/infectious disease team— consider referring patients to secondary care in hospital for assessment and intravenous antiviral treatment in the following circumstances 8 :
involvement of central nervous system (eg, reduced mental status)
elderly patients with severe disease (eg, multi-dermatomal involvement)
immunosuppressed patients
those who cannot take oral medication.
those with acute retinal necrosis.
Pain team— refer patients to the pain team if post-herpetic neuralgia is not controlled by simple neuropathic pain killers.
Currently there is a shingles vaccination programme available in the UK for people over 70. 13 It has been shown to reduce the incidence rate of shingles and post- herpetic neuralgia. 10
Longer term complications — include corneal denervation, recurrent uveitis, and post-herpetic neuralgia. Patients with herpes zoster ophthalmicus have a substantially increased risk of developing a cardiac event, stroke, or dementia over periods of three months to more than a year after the onset. 11 12
Education into practice
Are you aware how to obtain ophthalmology advice for a patient with suspected herpes zoster ophthalmicus and ocular involvement?
How do you counsel the patients on the risk of post-herpetic complications, particularly post-herpetic neuralgia?
Do you routinely provide shingles vaccination to people in their 70s?
How this article was created
This article was written with the aim of improving the assessment and management of herpes zoster ophthalmicus in the primary care setting. A literature search was conducted in the electronic database PubMed to identify important evidence concerning herpes zoster infection, particularly herpes zoster ophthalmicus.
How patients were involved in the creation of this article
We consulted a patient who presented to the eye emergency department with left keratouveitis following a recent herpes zoster ophthalmicus infection. He was affected by ocular pain and photophobia as well as neuropathic pain at the V1 dermatome. His clinical problem has been taken into account during the writing of the “History” and “When to refer” sections. We highlighted the symptoms and signs of uveitis, and the importance of recognising and managing post-herpetic neuralgia in patients with herpes zoster ophthalmicus.
Facial photographs were obtained from two patients with written consent for illustrative purpose.
This is part of a series of occasional articles on common problems in primary care. The BMJ welcomes contributions from GPs.
Provenance and peer review: Commissioned, based on an idea from the author; externally peer reviewed.
Competing interests The BMJ has judged that there are no disqualifying financial ties to commercial companies. The authors declare the following other interests: None
Further details of BMJ policy on financial interests is here: https://www.bmj.com/about-bmj/resources-authors/forms-policies-and-checklists/declaration-competing-interests
- Liesegang TJ
- Foulks GN ,
- Liesegang T ,
- Barbarash RA ,
- Nahlik JE ,
- Collaborative Famciclovir Herpes Zoster Study Group
- Chouliaras G ,
- Spoulou V ,
- Quinlivan M ,
- Theodoridou M
- Völker-Dieben HJ ,
- Werner RN ,
- Nikkels AF ,
- Marinović B ,
- Matthews I ,
- Erskine N ,
- Vaccinations NHS
- Guidance for Professional Practice
Clinical Management Guidelines
- Optometrists' Formulary
- Clinical advice service
- Clinical files
- Guidance for therapeutics
- Supplementary guidance
- Using Evidence in Practice
- Position statements
- Ethical scenarios
- Introduction to CPD
- Online learning
- Peer discussion and peer review
- Docet distance learning
- Independent prescribing (IP) qualification
- Higher qualifications
- Ophthalmic and Physiological Optics (OPO)
- Optometry In Practice
- Career pathways in optometry
- Research Excellence Awards
- Financial support for College members undertaking research
- Library and information services
- Fellowship by Portfolio
- Scheme for Registration
- New route to qualification
- Assessors, examiners and supervisors
- Optometry students
- International pre-registration trainees
- Sector Partnership for Optical Knowledge and Education (SPOKE)
- A career in optometry
- Join us today
- Membership categories and fees
- Request a change of membership category
- Full range of member benefits
- Reclaim your tax
- Member resources
- Locum resources
- Life and Honorary Fellowship (FCOptom)
- Taking a career break
- Member Code of Conduct
- The Benevolent Fund
- Shaping eye care
- Advancing optometry
- Championing the profession
- Patient leaflets and resources
- Get the College app
- Expert witnesses
- Compassion in practice
The CMGs offer information on the diagnosis and management of a range of conditions that present with varying frequency in primary and first contact care.
- Abnormalities of the Pupil
- Atopic Keratoconjunctivitis (AKC)
- Basal cell carcinoma (BCC) (periocular)
- Blepharitis (Lid Margin Disease)
- CL-associated Papillary Conjunctivitis (CLAPC), Giant Papillary Conjunctivitis (GPC)
- Cellulitis, preseptal and orbital
- Chalazion (Meibomian cyst)
- Concretions
- Conjunctival pigmented lesions
- Conjunctival scarring
- Conjunctivitis (Acute Allergic)
- Conjunctivitis (bacterial)
- Conjunctivitis (viral, non-herpetic)
- Conjunctivitis (seasonal & perennial allergic)
- Conjunctivitis, Chlamydial
- Conjunctivitis medicamentosa (also Dermatoconjunctivitis medicamentosa)
- Corneal (or other superficial ocular) foreign body
- Corneal Transplant Rejection
- Corneal abrasion
- Corneal hydrops
- Dacryocystitis (acute)
- Dacryocystitis (chronic)
- Dry Eye (Keratoconjunctivitis Sicca, KCS)
- Endophthalmitis (post-operative) (Exogenous endophthalmitis)
- Episcleritis
- Facial palsy (Bell's Palsy)
- Fuchs Endothelial Corneal Dystrophy (FECD)
- Glaucoma (chronic open angle) (COAG)
- Herpes Simplex Keratitis (HSK)
- Herpes Zoster Ophthalmicus (HZO)
- Keratitis (marginal)
- Keratitis, CL-associated infiltrative
- Microbial keratitis (Acanthamoeba sp.)
- Microbial keratitis (bacterial, fungal)
- Molluscum contagiosum
- Nasolacrimal duct obstruction (nasolacrimal drainage dysfunction)
- Ocular hypertension (OHT)
- Ocular rosacea
- Ophthalmia neonatorum
- Photokeratitis (Ultraviolet [UV] burn, Arc eye, Snow Blindness)
- Phthiriasis (pediculosis ciliaris)
- Pigmented fundus lesions
- Post-operative suture breakage
- Primary Angle Closure / Primary Angle Closure Glaucoma (PAC / PACG)
- Recurrent corneal epithelial erosion syndrome
- Retinal Vein Occlusion
- Steroid-related Ocular Hypertension and Glaucoma
- Sub-conjunctival haemorrhage
- Sub-tarsal foreign body (STFB)
- Trauma (blunt)
- Trauma (chemical)
- Trauma (penetrating)
- Uveitis (anterior)
- Vernal Keratoconjunctivitis
- Vitreomacular Traction and Macular Hole
- How to use the Clinical Management Guidelines

Please login to view this content.
Predisposing factors
Symptoms of herpes zoster ophthalmicus, signs of herpes zoster ophthalmicus, differential diagnosis, management by optometrist, management category, possible management by ophthalmologist, evidence base.
Herpes zoster ophthalmicus (HZO), also known as ophthalmic shingles, is caused by a localized reactivation of the varicella zoster virus (VZV) in the ophthalmic division of the trigeminal nerve. VZV is also known as human herpesvirus-3 (HHV-3). The features of herpes zoster in general are:
- previous systemic infection, typically in childhood (varicella, i.e. chickenpox)
- virus lies dormant (sometimes for decades) in dorsal root and cranial nerve sensory ganglia
- reactivation leads to herpes zoster (shingles)
- herpes zoster ophthalmicus (HZO) is defined by zoster involvement in the ophthalmic division of the trigeminal nerve
- herpes zoster affects 20-30% of the population at some point in their lifetime; 10-20% of these will develop HZO through involvement of the ophthalmic division of the trigeminal nerve. This represents a lifetime incidence of one in 100 individuals
- ocular involvement occurs in about 50% of HZO cases
- the most common sites of ocular involvement include conjunctivitis, followed by keratitis and uveitis.
- most cases of ocular involvement develop within three to four weeks of the initial primary care diagnosis
- HZO can result in moderate-to-severe loss of vision in a significant proportion of patients with ocular involvement, even with timely and appropriate management.
- Vaccination: the NHS offers routine herpes zoster vaccination for people aged 70-79 years. Vaccination has been shown to reduce the incidence rate of shingles and post-herpetic neuralgia. Two vaccines are available: - Zostavax, a live vaccine given as 1 dose - Shingrix, a non-live vaccine given as 2 doses, 2 months apart
Age: the peak incidence in healthy individuals is in the 5th to 7th decades, but one in three cases occur in people under the age of 50 Immune compromise: HIV infection, medical immunosuppression e.g. corticosteroids/chemotherapy
Pain and altered sensation (often described as “tingling“, “burning” or “shooting”) of the forehead on one side Rash affecting forehead and upper eyelid appears a day to a week later General malaise, headache, fever
Ocular symptoms in acute phase:
- photophobia
Skin features
- unilateral painful, red, vesicular rash on the forehead and upper eyelid, progressing to crusting after 2-3 weeks; resolution often involves scarring
- involvement of the skin supplied by the ophthalmic division of the trigeminal nerve (V1 dermatome). Does not cross the midline
- skin lesions on the side of the tip of the nose (Hutchinson’s sign, indicating nasociliary nerve involvement) indicates three to four times the usual risk of ocular complications, but these may also occur in one in three patients without the sign
- Zoster sine herpete is a rare variant which has no cutaneous manifestations
- periorbital oedema (may close the eyelids and spread to opposite side)
- lymphadenopathy (swollen regional lymph nodes)
Ocular lesions (variable in scope and severity, may be chronic or recurrent):
- ocular lesions may occur early or develop within one month after the onset of the skin rash and therefore patients may need to be monitored even after the rash starts to improve
- mucopurulent conjunctivitis (common), associated with vesicles on the lid margin; usually resolves within 1 week
- punctate epithelial – early sign, within 2 days (50% of cases)
- pseudodendrites – fine, multiple stellate lesions (around 4-6 days)
- nummular – fine granular deposits under Bowman’s layer
- disciform – 3 weeks after the rash (occurs in 5% of cases)
- reduced corneal sensation (neurotrophic keratitis), occurring in approximately 13% of HZ keratitis cases
- endothelial changes and KP
- episcleritis : occurs in around one third of cases
- scleritis : less common; usually develops after 1 week
- anterior uveitis
- secondary glaucoma (check IOP)
- rarely, posterior segment involvement: retinitis, acute retinal necrosis, choroiditis, optic neuritis, optic atrophy
- rarely, neurological complications: cranial nerve palsies, encephalitis
- post-herpetic neuralgia is defined as pain and/or itch lasting beyond 90 days after the onset of zoster. This affects around 25% of patients and is chronic and severe in about 7%
Complications can occur months or years after the acute phase
Ocular lesions: herpes simplex keratitis Cutaneous lesions: cellulitis, contact dermatitis, atopic eczema, impetigo
Practitioners should recognise their limitations and where necessary seek further advice or refer the patient elsewhere
Non pharmacological
Exclude uveitis, and posterior segment complications (following pupil dilatation, e.g. retinal necrosis ( GRADE*: Level of evidence=low, Strength of recommendation=strong)
Advise rest and general supportive measures (reassurance, support at home, good diet, plenty of fluids) (GRADE*: Level of evidence=low, Strength of recommendation=strong)
Advise avoidance of contact with elderly or pregnant individuals, also babies and children not previously exposed to VZV (who are non-immune) or immunodeficient patients (GRADE*: Level of evidence=low, Strength of recommendation=strong)
Pharmacological
Topical lubricants for relief of ocular symptoms (drops used during the day ± unmedicated ointment for use at bedtime) Pain relief: aspirin , paracetamol or ibuprofen (check history for contraindications). Stronger analgesics (e.g. opiates) may be indicated (co-manage with GP) (GRADE*: Level of evidence=low, Strength of recommendation=strong)
A1: for acute skin lesions: emergency referral (same day) to GP for systemic anti-viral treatment Early treatment with oral aciclovir (within 72 hours after rash onset) reduces the percentage of eye disorders in ophthalmic zoster patients from 50% to 20-30%. This early treatment also lessens acute pain.
(NB this recommendation, though representing conventional practice, is currently not evidence based. The on-going Zoster Eye Disease Study [ZEDS] may resolve this matter)
A3: first aid measures and urgent referral (within one week) to ophthalmologist if:
- untreated disciform keratitis can lead to scarring
- neurotrophic ulceration can lead to perforation
- anterior uveitis present
B3: management to resolution if co-managed with GP and keratitis mild and limited to epithelium Requires careful monitoring. Maintain low threshold for referral since HZO is associated with chronic and recurrent complications that may be sight threatening
Systemic anti-virals e.g. aciclovir, famciclovir, valaciclovir Topical anti-virals (off-licence use) Topical steroids Immunosuppressive therapy for scleritis Botulinum toxin-induced ptosis or surgical tarsorrhaphy for neurotrophic corneal ulceration Treat other ocular complications
*GRADE: Grading of Recommendations Assessment, Development and Evaluation ( www.gradingworkinggroup.org )
Sources of evidence
Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia . Cochrane Database Syst Rev 2014;2:CD006866
Cohen EJ, Jeng BH. Herpes Zoster: A Brief Definitive Review . Cornea. 2021;40(8):943-949.
Civen R et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Ped Infect Dis J 2009;28:954-9
Cohen EJ. Management and prevention of herpes zoster ocular disease. Cornea. 2015;34 Suppl 10:S3-8
Davis AR, Sheppard J. Herpes Zoster Ophthalmicus Review and Prevention . Eye Contact Lens. 2019 ;45(5):286-291.
Gelb LD. Preventing herpes zoster through vaccination. Ophthalmology. 2008;115(2 Suppl):S35-8
Li JY. Herpes zoster ophthalmicus: acute keratitis. Curr Opin Ophthalmol. 2018;29(4):328-333
McDonald EM, de Kock J, Ram FS. Antivirals for management of herpes zoster including ophthalmicus: a systematic review of high-quality randomized controlled trials. Antivir Ther. 2012;17(2):255-64
Niederer RL, Meyer JJ, Liu K, Danesh-Meyer HV. Herpes Zoster Ophthalmicus Clinical Presentation and Risk Factors for Loss of Vision . Am J Ophthalmol. 2021;226:83-89.
Opstelten W, Zaal M. Managing ophthalmic herpes zoster in primary care. BMJ 2005;331:147–51
Ting DSJ, Ghosh N, Ghosh S. Herpes zoster ophthalmicus . BMJ. 2019;364;k5234
Vadoothker S, Jeng BH. Management of chronic complications associated with herpes zoster ophthalmicus. Curr Opin Ophthalmol. 2018;29(4):334
What is Herpes Zoster Ophthalmicus?
Herpes Zoster Ophthalmicus (HZO) is a viral infection of the nerve that supplies sensation (touch and pain) to the eye surface, eyelids, skin of the forehead and nose (trigeminal nerve). The virus that affects it (Varicella Zoster Virus [VZV]) also causes chickenpox. Patients who develop HZO, like most people, have usually been exposed to chickenpox by the age of 16 and though they recover from that infection, the virus lies dormant in parts of the brain and spinal cord, with its activity suppressed by the body’s immune system. If, for some reason, that suppression weakens, viruses can become reactivated and travel down the trigeminal nerve, reaching the tissues that it supplies and causing inflammation. When the skin is involved, the condition is known as shingles. Shingles occurs more often and is likely to be more severe in older people whose immunity to VZV is weakening, and in people whose immune system is not functioning normally, as for example in HIV/AIDS, or is suppressed by medical treatment.
In HZO the skin of one side of the forehead and scalp is affected, along with the eye on the same side. Any part of the eye can be involved, but most commonly it is the eye surface, including the conjunctiva (the white of the eye) and the cornea (the clear window of the eye). The cornea reacts in various ways; the most serious long-term effects result from damage to the corneal nerves, causing loss of sensation.
How is Herpes Zoster Ophthalmicus managed?
When HZO first appears, patients benefit from anti-viral tablets prescribed as soon as possible, usually by the GP. Mild cases can be co-managed by the optometrist and the GP but more severe cases need to be referred to the ophthalmologist.
Public Health England has introduced shingles vaccination for certain people aged between 70 and 80. This is given once and provides a good measure of protection against the condition.
Herpes Zoster Ophthalmicus (HZO) Version 16 Date of search 04.05.23 Date of revision 29.06.23 Date of publication 01.09.23 Date for review 03.05.25 © College of Optometrists
Create a private prescription
IP qualified members can create a private prescription for their patients using our exclusive member only form.
Optometrists' Formulary
Our Optometrists' Formulary provides prescribing information on drugs currently available to treat eye conditions.
Drugs shortages
Find out the latest information on drug shortages and discontinuations across the UK.

SAAD SHAIKH, M.D., AND CHRISTOPHER N. TA, M.D.
Am Fam Physician. 2002;66(9):1723-1730
A patient education handout on HZO, written by the authors of this article, is provided on page 1732 .
Herpes zoster ophthalmicus occurs when the varicella-zoster virus is reactivated in the ophthalmic division of the trigeminal nerve. Herpes zoster ophthalmicus represents up to one fourth of all cases of herpes zoster. Most patients with herpes zoster ophthalmicus present with a periorbital vesicular rash distributed according to the affected dermatome. A minority of patients may also develop conjunctivitis, keratitis, uveitis, and ocular cranial-nerve palsies. Permanent sequelae of ophthalmic zoster infection may include chronic ocular inflammation, loss of vision, and debilitating pain. Antiviral medications such as acyclovir, valacyclovir, and famciclovir remain the mainstay of therapy and are most effective in preventing ocular involvement when begun within 72 hours after the onset of the rash. Timely diagnosis and management of herpes zoster ophthalmicus, with referral to an ophthalmologist when ophthalmic involvement is present, are critical in limiting visual morbidity.
Herpes zoster is a common infection caused by the human herpesvirus 3, the same virus that causes varicella (i.e., chickenpox). It is a member of the same family (Herpesviridae) as herpes simplex virus, Epstein-Barr virus, and cytomegalovirus. Reactivation of the latent virus in neurosensory ganglia produces the characteristic manifestations of herpes zoster, commonly known as shingles. Normal aging, poor nutrition, and immunocompromised status correlate with outbreaks of herpes zoster, and certain factors such as physical or emotional stress and fatigue may precipitate an episode.
Herpes zoster ophthalmicus occurs when reactivation of the latent virus in the trigeminal ganglia involves the ophthalmic division of the nerve. The virus damages the eye and surrounding structures by secondary perineural and intraneural inflammation of sensory nerves. 1 Herpes zoster ophthalmicus represents approximately 10 to 25 percent of all cases of herpes zoster. 2 Although herpes zoster ophthalmicus most often produces a classic dermatomal rash, a minority of patients may have only ophthalmic findings, limited mainly to the cornea. Direct ocular involvement is not specifically correlated with age, gender, or severity of disease. Serious sequelae include chronic ocular inflammation, vision loss, and disabling pain.
Extraocular Manifestations of Herpes Zoster Ophthalmicus
The prodromal phase of herpes zoster ophthalmicus includes an influenza-like illness with fatigue, malaise, and low-grade fever that lasts up to one week before the rash over the forehead appears. 3 About 60 percent of patients have varying degrees of dermatomal pain in the distribution of the ophthalmic nerve. 4 Subsequently, erythematous macules appear along the involved dermatome, rapidly progressing over several days to papules and vesicles containing clear serous fluid and, later, pustules. These lesions rupture and typically crust over, requiring several weeks to heal completely. 5
Immunocompromised persons, particularly those with human immunodeficiency virus infection, have a much higher risk of developing herpes zoster ophthalmicus than the normal population. 6 These patients may have a generalized vesicular rash and become severely ill one to two weeks after disease onset. In addition, such patients develop more serious visual sequelae. 7
Viral transmission from patients with herpes zoster can occur, but it is less frequent than transmission from patients with chick-enpox. 7 Virus particles can be transmitted through direct contact with secretions from vesicles and secretion-contaminated articles.
Ocular Manifestations of Herpes Zoster Ophthalmicus
The skin manifestations of herpes zoster ophthalmicus strictly obey the midline with involvement of one or more branches of the ophthalmic division of the trigeminal nerve, namely the supraorbital, lacrimal, and nasociliary branches ( Figure 1 ) . Because the nasociliary branch innervates the globe, the most serious ocular involvement develops if this branch is affected. Classically, involvement of the tip of the nose (Hutchinson's sign) has been thought to be a clinical predictor of ocular involvement. Although patients with a positive Hutchinson's sign have twice the incidence of ocular involvement, one third of patients without the sign develop ocular manifestations. 8 A summary of ocular findings in patients with herpes zoster ophthalmicus is presented in Table 1 .
BLEPHARITIS AND CONJUNCTIVITIS
The eyelids are commonly involved in herpes zoster ophthalmicus ( Figure 1 , part b). Patients may develop blepharitis and present with ptosis secondary to edema and inflammation. A vast majority of patients will have vesicular lesions on the eyelids that resolve with minimal scarring.
Conjunctivitis is one of the most common complications of herpes zoster ophthalmicus. The conjunctiva appears injected and edematous, often with petechial hemorrhages. 9 The findings usually resolve within one week. However, secondary infection, usually Staphylococcus aureus , may develop and should be treated with broad-spectrum topical and/or systemic antibiotics.
CORNEAL DISEASE
Unlike eyelid or conjunctival involvement, corneal involvement can result in significant vision loss. The clinical features of corneal disease include direct viral infection, antigen-antibody reactions, delayed cell-mediated hypersensitivity reactions, and neurotrophic damage. 7 Patients with corneal disease present with varying degrees of decreased vision, pain, and light sensitivity. Corneal complications occur in approximately 65 percent of cases of herpes zoster ophthalmicus. 7
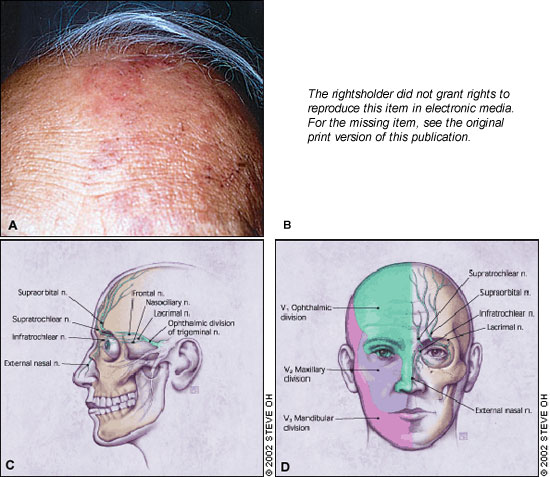
Epithelial Keratitis . The earliest corneal finding is punctate epithelial keratitis. 10 On slit lamp examination, this appears as multiple, focal, swollen lesions that stain with rose bengal or fluorescein dye. These lesions probably contain live virus and may either resolve or progress to dendrite formation. Punctate epithelial keratitis may present as early as one or two days after the initial skin rash, while dendrites often present at four to six days but can appear many weeks later. 11
Herpes zoster virus dendrites appear as elevated plaques and consist of swollen epithelial cells. They form branching or “medusa-like” patterns and have tapered ends ( Figure 2 , part a) in contrast to herpes simplex virus dendrites, which often have terminal bulbs. Dendrites also stain with rose bengal and fluorescein dye ( Figure 2 , part b) and can be viewed by Wood's lamp or slit lamp examination. Punctate and dendritic lesions can lead to anterior stromal corneal infiltrates. 11 , 12
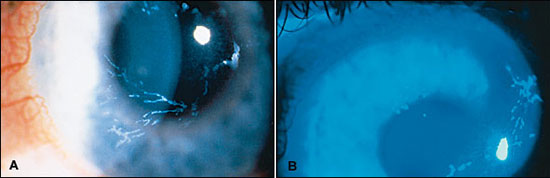
Stromal Keratitis — Anterior Stromal Keratitis . The earliest finding of corneal stromal involvement presents during the second week of disease, occurring in 25 to 30 percent of patients with herpes zoster ophthalmicus. 13 The condition, known as anterior stromal keratitis or nummular keratitis, is characterized by multiple fine granular infiltrates in the anterior corneal stroma below the epithelial layer ( Figure 3 ) . Most of the infiltrates lie directly beneath pre-existing dendrites or areas of punctate epithelial keratitis. The infiltrates are thought to arise from antigen-antibody interaction resulting from viral proliferation in the overlying epithelium. 10 , 12 Anterior stromal keratitis may be prolonged and recurrent.
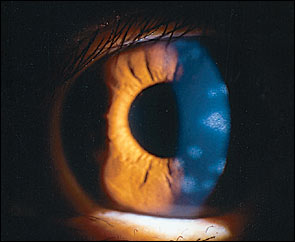
Stromal Keratitis—Deep Stromal Keratitis . This later stage of stromal keratitis is relatively uncommon and typically develops three to four months after the initial acute episode, but development can range from one month to many years later. 7 It is usually central and preceded by anterior stromal keratitis. The keratitis may present as a lesion consisting of a localized area of inflammation affecting all levels of the stroma, or as peripheral infiltrates that may have a surrounding immune ring. Corneal edema may be a prominent feature at this stage, usually with associated anterior chamber inflammation. A rare necrotizing form can also occur. A chronic relapsing course is not unusual, especially without timely and adequate treatment. Corneal neovascularization and lipid infiltrates may occur in patients with uncontrolled chronic disease. The pathogenesis of stromal disease probably involves a delayed cell-mediated hypersensitivity reaction.
Neurotrophic Keratopathy . Neurotrophic keratitis is the end result of decreased corneal sensation from herpes zoster virus-mediated destruction, including susceptibility to mechanical trauma, decreased lacrimation, and delayed epithelial healing. 7 Corneal thinning is a serious complication that may lead to corneal perforation. Such patients are at high risk for developing a secondary bacterial infection. Using preservative-free lubricating drops and ointment can prevent the development of epithelial defects.
Anterior uveitis, which is diagnosed by slit lamp examination, refers to inflammation of the iris and ciliary body and occurs frequently with herpes zoster ophthalmicus. It may be isolated or associated with keratitis. The inflammation is generally mild and transient, but it frequently causes a mild elevation in intraocular pressure. Zoster uveitis can result in iris atrophy and an irregular pupil. As with stromal keratitis, the course of disease may be prolonged, especially without timely, adequate treatment. Herpes zoster uveitis may cause glaucoma and cataract formation. Chronic inflammation can lead to endothelial cell injury, resulting in corneal edema.
EPISCLERITIS AND SCLERITIS
Findings of episcleritis include localized or diffuse redness, as well as pain and swelling of the conjunctiva and episclera. Scleritis is a more serious condition with involvement of the sclera. Both conditions may be accompanied by localized stromal keratitis.
ACUTE RETINAL NECROSIS AND PROGRESSIVE OUTER RETINAL NECROSIS SYNDROMES
Herpes zoster virus is considered the offending agent in most cases of acute retinal necrosis and progressive outer retinal necrosis syndromes. Compared with acute retinal necrosis, progressive outer retinal necrosis is a more severe viral retinitis observed in immunocompromised persons, often in patients with acquired immunodeficiency syndrome.
Symptoms include blurred vision and/or pain in one or both eyes. Acute retinal necrosis is characterized by peripheral patches of retinal necrosis that rapidly coalesce ( Figure 4 ) , occlusive vasculitis, and vitreous inflammation. Conversely, immunocompromised patients with progressive outer retinal necrosis are unable to mount a vitreous inflammatory response, leading to rapid involvement of the macula. Both conditions commonly cause retinal detachment. The prognosis is extremely poor in patients with progressive outer retinal necrosis; most patients have no light perception vision. 14 The visual prognosis in patients with acute retinal necrosis is better, with many patients achieving a visual acuity of 20/40. 15 Bilateral involvement in both forms is observed in one third of patients but may be as high as 70 percent in patients with untreated disease. 16 Treatment includes long courses of oral and intravenous acyclovir (Zovirax), and corticosteroids.
Postherpetic Neuralgia and Other Neurologic Complications
Postherpetic neuralgia affects about 7 percent of patients and is characterized by varying degrees of constant or intermittent pain in the distribution of the affected dermatome. 17 Increased age and prodromal symptoms are associated with a higher prevalence of post-herpetic neuralgia. It generally improves with time but may last for months to years. In severe cases, patients may be depressed and suicidal. Treatment includes topical capsaicin cream, over-the-counter analgesics, tricyclic antidepressants, and anticonvulsants. 18
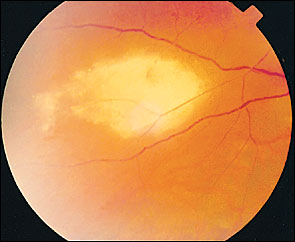
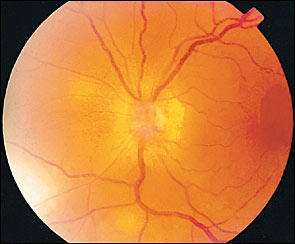
Cranial nerve palsies involving the third (most common), fourth, and sixth nerves may occur rarely ( Figure 5 ) . A majority of the cases will have spontaneous resolution within six months. Optic neuritis has been noted in about one in 400 cases and may precede retinal disease or follow acute herpes zoster ophthalmicus infection ( Figure 6 ) . 17 , 19 , 20
Treatment of Herpes Zoster Ophthalmicus
Patients with herpes zoster ophthalmicus are treated with oral acyclovir (800 mg, five times daily) for seven to 10 days. Studies report alleviation of pain with oral acyclovir during the initial stages of the disease, especially if the drug is taken within the first three days of symptoms, and it may have a favorable effect on postherpetic neuralgia. 21 – 23 [Reference 22—Evidence level A, randomized controlled tiral (RCT). Reference 23—Evidence level A, RCT] Additionally, acyclovir administered within 72 hours of onset has been found to speed resolution of skin lesions, reduce viral shedding, and decrease the incidence of dendritic and stromal keratitis as well as anterior uveitis. 24 , 25
Valacyclovir (Valtrex) has higher bioavail-ability and has been shown to be equally safe and effective for the treatment of herpes zoster at a dosage of 1,000 mg three times daily for seven or 14 days. 26 [Evidence level A, RCT] Valacyclovir in a seven-day dosage regimen was recently shown to prevent ocular complications of herpes zoster ophthalmicus, including conjunctivitis, superficial and stromal keratitis, and pain. 27 [Evidence level A, RCT] Famciclovir (Famvir), 500 mg orally three times a day for seven days, may also be used. 28 Intravenous acyclovir is recommended in immunocompromised patients. 29 , 30 [Reference 29—Evidence level A, RCT] Acute pain control is achieved by local care and oral analgesics. Topical anesthetics should never be prescribed because of their corneal toxicity. A summary of treatment and management options for various ocular manifestations of herpes zoster ophthalmicus is presented in Table 2 .
Naumann G, Gass JD, Font RL. Histopathology of herpes zoster ophthalmicus. Am J Ophthalmol. 1968;65:533-41.
Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310-6.
Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-72.
Cobo M, Foulks GN, Liesegang T, Lass J, Sutphin J, Wilhelmus K, et al. Observations on the natural history of herpes zoster ophthalmicus. Curr Eye Res. 1987;6:195-9.
Burgoon CF, Burgoon JS, Baldridge GD. The natural history of herpes zoster. JAMA. 1957;174:265.
Sandor EV, Millman A, Croxson TS, Mildvan D. Herpes zoster ophthalmicus in patients at risk for the acquired immune deficiency syndrome (AIDS). Am J Ophthalmol. 1986;101:153-5.
Baratz KH, Goins K, Cobo M. Varicella-zoster viral infections. In: Kaufman HE, ed. The cornea. New York: Churchill Livingstone, 1988.
Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-8.
Arffa RC. Viral diseases. In: Arffa RC, Grayson M, eds. Grayson's Diseases of the cornea. 4th ed. St. Louis: Mosby, 1997.
Liesegang TJ. Corneal complications from herpes zoster ophthalmicus. Ophthalmology. 1985;92:316-24.
Jones DB. Herpes zoster ophthalmicus. In: Golden B, ed. Symposium on ocular inflammatory disease. Springfield, Ill.: Thomas, 1974.
Marsh RJ. Herpes zoster keratitis. Trans Ophthalmol Soc U K. 1973;93:181-92.
Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-5.
Engstrom RE, Holland GN, Margolis TP, Muccioli C, Lindley JI, Belfort R, et al. The progressive outer retinal necrosis syndrome. A variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994;101:1488-502.
Blumenkranz M, Clarkson J, Culbertson WW, Flynn HW, Lewis ML, Young GA. Vitrectomy for retinal detachment associated with acute retinal necrosis. Am J Ophthalmol. 1988;106:426-9.
Palay DA, Sternberg P, Davis J, Lewis H, Holland GN, Mieler WF, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112:250-5.
Kanski JJ. Herpes zoster ophthalmicus. In: Kanski JJ, Nischal KK, Milewski SA, eds. Ophthalmology: clinical signs and differential diagnosis. Philadelphia: Mosby, 1999.
Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Physician. 2000;61:2437-48.
Lee MS, Cooney EL, Stoessel KM, Gariano RF. Varicella zoster virus retrobulbar optic neuritis preceding retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1998;105:467-71.
Gunduz K, Ozdemir O. Bilateral retrobulbar neuritis following unilateral herpes zoster ophthalmicus. Ophthalmologica. 1994;208:61-4.
Peterslund NA. Management of varicella zoster infections in immunocompetent hosts. Am J Med. 1988;85:74-8.
Morton P, Thomson AN. Oral acyclovir in the treatment of herpes zoster in general practice. N Z Med J. 1989;102:93-5.
Huff JC, Bean B, Balfour HH, Laskin OL, Connor JD, Corey L, et al. Therapy of herpes zoster with oral acyclovir. Am J Med. 1988;85:84-9.
Liesegang TJ. Herpes zoster keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. St. Louis: Mosby, 1997.
McGill J, Chapman C, Mahakasingam M. Acyclovir therapy in herpes zoster infection. A practical guide. Trans Ophthalmol Soc U K. 1983;103(pt 1):111-4.
Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-53.
Colin J, Prisant O, Cochener B, Lescale O, Rolland B, Hoang-Xuan T. Comparison of the efficacy and safety of valaciclovir and acyclovir for the treatment of herpes zoster ophthalmicus. Ophthalmology. 2000;107:1507-11.
Tyring SK. Efficacy of famciclovir in the treatment of herpes zoster. Semin Dermatol. 1996;15(2 suppl 1):27-31.
Balfour HH, Bean B, Laskin OL, Ambinder RF, Meyers JD, Wade JC, et al. Acyclovir halts progression of herpes zoster in immunocompromised patients. N Engl J Med. 1983;308:1448-53.
Balfour HH. Varicella zoster virus infections in immunocompromised hosts. A review of the natural history and management. Am J Med. 1988;85:68-73.
Continue Reading
More in afp, more in pubmed.
Copyright © 2002 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

2-22: Herpes Zoster Ophthalmicus
Manpreet Singh; Denise Whitfield
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Clinical summary, management and disposition.
- Full Chapter
- Supplementary Content
Reactivation of endogenous latent varicella-zoster virus within the trigeminal ganglion with neuronal spread through the ophthalmic branch results in crops of grouped vesicles on the forehead and periocularly.
Patients typically present with periocular rash and an injected eye, along with a watery discharge. The most common corneal lesion is punctate epithelial keratitis, in which the cornea has a ground-glass appearance because of stromal edema. Pseudodendrites, also very common, form from mucous deposition, are usually peripheral, and stain moderate to poorly with fluorescein. These may be differentiated from the dendrites of HSV in that the pseudodendrites lack the rounded terminal bulbs at the end of the branches and are broader and more plaquelike. Anterior stromal infiltrates may be seen in the 2nd or 3rd week after the acute infection. Follicles (hyperplastic lymphoid tissue that appears as gray or white lobular elevations, particularly in the inferior cul-de-sac) and regional adenopathy may or may not be present. Iritis is seen in approximately 40% of patients.
FIGURE 2.54
Herpes Zoster Ophthalmicus. A vesicular rash in the distribution of the ophthalmic division (V1) of the trigeminal nerve is seen. The presence of the lesion near the tip of the nose (Hutchinson sign) increases the risk of ocular involvement. (Photo contributor: Lawrence B. Stack, MD.)

Treat patients with epithelial defects with topical broad-spectrum antibiotics to prevent secondary infection. Initiate oral antivirals within 72 hours of onset, and treat for 7 to 10 days. Use cycloplegics if an iritis is present. Artificial tears or ointment may be helpful, and narcotic analgesics may be required. Ophthalmologic consultation is indicated.
Ocular complications may follow the rash by many months to years. These complications have a highly variable presentation that can mimic almost any ophthalmic condition.
Recurrence is more common in the immunocompromised host.
Perform a careful eye exam with corneal staining. Nearly two-thirds of patients will develop ocular lesions.
Corneal hypesthesia and the appearance of dendrites with fluorescein staining are seen in both herpes zoster ophthalmicus and herpes simplex keratitis.
Patients with skin lesions on the tip of the nose (Hutchinson sign) are at high risk for ocular involvement. However, the eye may be involved without a nasal lesion.
FIGURE 2.55
Herpes Zoster Ophthalmicus. A large circular dendrite is seen in this patient with ocular involvement from herpes zoster virus. (Photo contributor: Alexandra Bingnear, RN.)

FIGURE 2.56
Herpes Zoster Ophthalmicus. Grouped vesicles on the forehead and eyebrow are seen with conjunctival injection indicating ocular involvement. (Photo contributor: Lawrence E. Heiskell, MD, FACEP, FAAFP.)

FIGURE 2.57
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Clinical Overview
Clinical features, complications, vaccination, transmission, epidemiology, herpes zoster in people who received varicella vaccine.
Herpes zoster, also known as shingles, is caused by reactivation of varicella-zoster virus (VZV), the same virus that causes varicella (chickenpox).
Primary infection with VZV causes varicella. After a person has varicella, the virus remains latent in the dorsal root ganglia. VZV can reactivate later in a person’s life and cause herpes zoster, a painful maculopapular and then vesicular rash.
People with herpes zoster most commonly have a rash in one or two adjacent dermatomes. The rash most commonly appears on the trunk along a thoracic dermatome or on the face and it usually does not cross the body’s midline.
The rash is usually painful, itchy, or tingly. A person can experience the following symptoms several days before the rash appears:
- Photophobia (sensitivity to bright light)
The rash develops into clusters of vesicles. New vesicles continue to form over 3 to 5 days, and the rash progressively dries and scabs over. The rash usually heals in 2 to 4 weeks. Permanent skin discoloration and scarring can occur.
Postherpetic neuralgia (PHN)
PHN is the most common complication of herpes zoster. PHN is pain that persists in the area where the rash once was located and continues more than 90 days after rash onset. PHN can last for months or even years.
A person’s risk of having PHN after herpes zoster increases with age. Older adults are more likely to have longer lasting, more severe pain. Approximately 10% to 18% of people with herpes zoster will have PHN. PHN is rare in people younger than 40 years old. The likelihood of PHN is also higher in people who experience more pain with the rash or have a large rash.
Herpes zoster ophthalmicus
Herpes zoster that affects the ophthalmic division of the trigeminal nerve is called herpes zoster ophthalmicus. This can result in acute or chronic ocular sequelae, including vision loss.

Disseminated zoster
Disseminated zoster can include generalized skin eruptions where the lesions occur outside of the primary or adjacent dermatomes. It can be difficult to distinguish from varicella. Visceral involvement of the central nervous system (meningoencephalitis), lungs (pneumonitis), and liver (hepatitis) can also occur. Disseminated zoster generally occurs in people with compromised or suppressed immune systems.
Other complications of herpes zoster include:
- Bacterial superinfection of the lesions, usually due to Staphylococcus aureus and, less commonly, due to group A beta hemolytic streptococcus
- Cranial and peripheral nerve palsies
People with compromised or suppressed immune systems are more likely to have a severe, long-lasting rash and experience more severe complications from herpes zoster.
Recombinant zoster vaccine (RZV, Shingrix) is the recommended vaccine to prevent shingles and related complications. For information about vaccination recommendations see Shingles Vaccination .
People with active herpes zoster lesions can spread VZV , which causes varicella in people who never had varicella or never received varicella vaccine. Once varicella resolves, these people would be at risk for herpes zoster.
Active herpes zoster lesions are infectious through direct contact with vesicular fluid or through breathing in virus particles from the blisters until they dry and scab over. People with active herpes zoster lesions should cover their lesions and avoid contact with susceptible people in their household and in occupational settings until their lesions are dry and scabbed.
Also see Managing People at High Risk for Severe Varicella and Preventing VZV Transmission from Herpes Zoster in Healthcare Settings
Top of Page
Risk Factors
Anyone who had varicella can develop herpes zoster. Approximately 99.5% of people born before 1980 in the United States were infected with wild-type VZV. Children who receive varicella vaccine have a lower risk of herpes zoster compared with children who were infected with wild-type VZV.
Approximately 1 in 3 people in the United States will develop herpes zoster during their lifetime. Most people have only one episode; however, herpes zoster can recur.
A person’s risk for herpes zoster and related complications sharply increases after 50 years of age. The reasons why VZV reactivates and causes herpes zoster are not well understood. However, a person’s risk for herpes zoster increases as their VZV-specific cell-mediated immunity declines. This decline in immunity can result from increasing age and medical conditions or medications that suppress a person’s immune system. People with the following conditions that compromise or suppress their immune system have an increased risk for herpes zoster:
- Bone marrow or solid organ (renal, cardiac, liver, and lung) transplant recipients
- Cancer, especially leukemia and lymphoma
- Human immunodeficiency virus (HIV)
- Taking immunosuppressive medications, including steroids, such as for treatment of autoimmune diseases and other immune system deficiencies
Other potential risk factors for herpes zoster have been identified, but the findings are either inconsistent or unexplained. For example:
- More women than men develop herpes zoster.
- Herpes zoster is less common in Blacks than in Whites.
Disease Rates
An estimated one million cases of herpes zoster occur annually in the United States.
- The incidence of herpes zoster varies by age and is approximately 2–9 cases per 1,000 US population annually.
The precise incidence of recurrence is not known.
- Approximately 10% to 18% of people with herpes zoster will have PHN.
- Approximately 1% to 4% of people with herpes zoster are hospitalized for complications.
- Older adults and people with compromised or suppressed immune systems are more likely to be hospitalized. About 30% of people hospitalized with herpes zoster have compromised or suppressed immune systems.
One study estimated 96 deaths occur each year where herpes zoster was the underlying cause (0.28 to 0.69 per 1 million population). Almost all the deaths occurred in older adults or those with compromised or suppressed immune systems.
Herpes zoster rates among adults in the United States gradually increased over a long period of time. We do not know the reason for this increase. However, the rates across age groups have recently plateaued or declined.
CDC studies have found that herpes zoster rates started increasing before varicella vaccine was introduced in the U.S. and did not accelerate after the routine varicella vaccination program started.
Varicella vaccines contain live attenuated VZV, which results in latent infection. Although herpes zoster has always been uncommon among children, the rate of herpes zoster in U.S. children has declined since the routine varicella vaccination program started in 1996.
- Vaccinated children are less likely to become infected with wild-type VZV.
- The risk of reactivation of vaccine-strain VZV in children is lower compared with reactivation of wild-type VZV.
- Few older adults have received the varicella vaccine since it was licensed in 1995. There is very little information on the risk of herpes zoster in people who got varicella vaccine as adults.
CDC continues to monitor the impacts of the U.S. varicella and herpes zoster vaccination programs among adults and children.
- CDC. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022 . MMWR Recomm Rep . 2022;71(3):80-84.
- Leung et al. The Impact of Universal Varicella Vaccination on Herpes Zoster Incidence in the United States: Comparison of Birth Cohorts Preceding and Following Varicella Vaccination Program Launch . Journal of Infection Diseases . 2022.
- Harpaz and Leung. The Epidemiology of Herpes Zoster in the United States During the Era of Varicella and Herpes Zoster Vaccines: Changing Patterns Among Older Adults . Clin Infect Dis .2019;69(2):341-344.
- CDC. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) Recommendations for use of Herpes Zoster Vaccines . MMWR Recomm Rep . 2018;67(03):103-108.
- Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis . 2004;4(1):26-33.
- Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease . JAMA . 2011;305(2):160-6.
- Mahamud A, Marin M, Nickell SP, et al. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979-2007 . Clin Infect Dis .2012;55(7):960-6.
- Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination . Clinical Infectious Diseases . 2011;52(3):332-340.
- Yih W, Brooks D, Lett S, et al. The Incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage . BMC Public Health . 2005;5(68).
- Jumaan AO, Yu O, Jackson LA, et al. Incidence of herpes zoster, before and after varicella vaccination-associated decreases in the incidence of varicella . Journal of Infectious Diseases . 2005;191:2002-7.
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination . Annals of Internal Medicine . 2013;159(11):739-45.
- Russell ML, Dover DC, Simmonds KA, Svenson LW. Shingles in Alberta: before and after publicly funded varicella vaccination . Vaccine . 2014;32(47):6319-24.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009 . Journal of Infection Diseases . 2013;208(11):1859-68.
- Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group . N Engl J Med . 1991;325(22):1545-50.
- Information for Healthcare Professionals about Shingles (Herpes Zoster) Vaccines
- Provider Education CDC shingles podcast, courses, broadcasts, webcasts, and slide sets
- Medline Plus
- AgePage on Shingles
- Immunize.org
- Chickenpox Q&As

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,896,487 articles, preprints and more)
- Free full text
- Similar Articles
Herpes Zoster Ophthalmicus Clinical Presentation and Risk Factors for Lesion Recovery.
Author information, affiliations, orcids linked to this article.
- Xiao Z | 0000-0002-6633-8392
Clinical, Cosmetic and Investigational Dermatology , 29 Dec 2023 , 16: 3767-3773 https://doi.org/10.2147/ccid.s444766 PMID: 38170070 PMCID: PMC10759815
Abstract
Patients and methods, free full text , herpes zoster ophthalmicus clinical presentation and risk factors for lesion recovery, zupeng xiao.
1 Department of Dermatology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Xiaoli Chen
Herpes zoster ophthalmicus (HZO) causes trouble in patients’ daily life and work. In severe cases, it may even lead to a decrease or loss of vision. To understand the demographic information and ocular symptoms of hospitalized patients with HZO, and to find potential factors related to improvement time of skin rash and duration of ocular symptoms at discharge, we design this study.
Patients and Methods
This is a retrospective study. All patients diagnosed with HZO who were hospitalized in the Department of Dermatology of a hospital in Chongqing, China from January 1, 2015 to December 30, 2021 were included in this study. A total of 189 patients were included in this study. Clinical manifestations of the disease during hospitalization, improvement time of ocular skin lesions, and whether ocular skin lesions disappeared completely at discharge were recorded.
The most common ocular symptom was eyelid swelling (92.6%), followed by eye pain (48.7%). The most common ocular sign was conjunctivitis (78.3%), followed by keratitis (15.9%). There were 149 cases without residual ocular symptoms and 40 cases with residual ocular symptoms. There was no statistically significant difference in demographic characteristics between the two groups (P>0.05). Age ≥70 years (B=0.381, −0.061~0.022, P=0.005), use of glucocorticoids (B=0.260, 0.024~0.496, P=0.031), and use of topical antiviral drugs (B=0.380, 0.054~0.705, P=0.023) were factors affecting the time interval from admission to improvement of skin rash. Tearing (HR, OR=4.827, 1.956~11.909, P<0.001) and blood urea nitrogen (OR=0.787, 0.620–1.000, P=0.050) were factors influencing residual ocular symptoms.
This study could help clinicians gain a deeper understanding of the clinical manifestations and partial influencing factors of HZO patients, which may contribute to future clinical work.
- Introduction
Herpes zoster (HZ), also known as shingles, is a contagious skin disease caused by the reactivation of the varicella-zoster virus (VZV), which lies dormant in spinal or cranial nerve ganglia after a previous infection with chickenpox. 1 With the accelerated aging process and increased stress in daily work and life, as well as decreased immunity, the incidence of HZ has shown a significant upward trend in China. 2 Herpes zoster ophthalmicus (HZO) is a special type of HZ, accounting for 10–20% of HZ cases, which involves the first division of the trigeminal nerve (ophthalmic division). 3 It often presents as unilateral eyelid swelling, erythema and blisters along nerve roots, tearing, photophobia, and ipsilateral headache, and can involve the eye, including conjunctivitis, keratitis, scleritis, uveitis, etc. 3 , 4 In recent decades, the incidence and related eye complications of HZO has increased according to some previous reports. 5 The most common complication of HZO is postherpetic neuralgia (PHN), which had a significant impact on patients’ physical, psychological, functional and social health. 6 HZO can also lead to severe complications such as depression secondary to postherpetic neuralgia, persistent pain severe vision loss and even permanent vision loss. 5 , 6 The main risk factors include advancing age, pain in the affected skin area, rash condition and eye involvement. 7 However, there are few studies on herpes zoster ophthalmicus.
This study aims to investigate the clinical manifestations and risk factors of rash recovery and eye symptoms after leaving hospital in patients with HZO.
- Materials and Methods
Inclusion Criteria for Participants
This study is a retrospective study approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (ID: K2023-564). And it has the informed consent of the patient. To identify participants, this search was conducted in the discharge records of subjects reviewed at the Department of Dermatology, First Affiliated Hospital of Chongqing Medical University, between January 1, 2015, and December 31, 2021, using keywords “herpes zoster” or “herpes zoster ophthalmicus”. And they would generally be considered for discharge when the rash darkens in color, blisters dry up and form scabs without new skin lesions developing. Patients with HZO were selected according to the guideline of Werner RN if they had a typical rash of herpes zoster with primary diagnosis of HZO. 8 Patients without typical skin rash or non-v1 HZ were excluded. We identified 1105 participants through the search, of which 916 were excluded as they either did not have V1 nerve involvement or did not exhibit typical skin rash. Ultimately, a total of 189 participants were included in this study.
All hospitalized patients will receive standardized, sufficient dosage and duration of antiviral treatment. The dermatologists in the inpatient department will select appropriate antiviral medications and administration methods after evaluating the condition of patients. Topical ocular medication will be administered under the guidance of ophthalmologists.
Outcome Measures
We defined the improvement of rash as the relief of eye swelling, pain, tearing, vision problems after admission. Then we recorded the time interval from admission to the improvement of rash as the primary outcome. We defined patients who still had eye symptoms at discharge, such as pain, swelling, tearing, and photophobia, as having residual eye symptoms and used it as a secondary outcome. Patients were divided into two groups based on whether they had residual eye symptoms at discharge.
Methods Used in Statistical Analysis
Demographic data, medical history, clinical presentation, ocular signs, treatment, and outcomes of enrolled patients were reviewed and analyzed. Statistical analysis was performed using IBM/SPSS software version 23. Statistical analysis included independent sample t -tests and Chi-square test to compare baseline demographics and clinical characteristics between patients with residual eye symptoms and patients without residual eye symptoms at discharge. A linear regression model was established to evaluate the risk factors of the improvement time of ocular symptoms. Binary logistic regression model was used to analyze the factors influencing the presence of residual ocular symptoms. A two-sided P value <0.05 was considered statistically significant.
Demographic Characteristics and Underlying Diseases of Patients
A total of 189 patients with HZO were included in the analysis, and their demographic characteristics are shown in Table 1 . The median age at presentation was 61 years (mean 59.9±14.5 years), ranging from 14 to 93 years. 85 subjects (45.0%) were over 60 years old, while 19 cases (10.1%) were under 40 years old. The male-to-female ratio was 1.59. There were 47 current smokers (24.9%), 23 former smokers (12.1%), and 119 never smokers (63.0%). Fifty-four cases (28.6%) had hypertension or coronary heart disease, 34 cases (18.0%) had diabetes, 16 cases (8.5%) had hepatitis, 11 cases (5.8%) had cancer, 11 cases (5.8%) had kidney disease, and 4 cases (2.1%) had autoimmune diseases. None of the subjects had a record of previous vaccination against herpes zoster or HIV infection.
Patient Demographics
Abbreviations : CardiovasDis, Chronic cardiovascular disease; ChronKidnDis, chronic kidney disease; ChronInfecDis, Chronic infectious ocular signs and symptoms disease; COPD, chronic obstructive pulmonary disease; AutoimmuneDis, autoimmune disease.
Ocular Signs and Symptoms of Patients
The clinical manifestations of the study population are shown in Table 2 . The median time from the rash onset to ocular symptoms was 2 days (IQR 1–4 days), and the median time from rash onset to antiviral therapy was 4 days (IQR 3–6 days). The ratio of involvement between left and right sides was 1.84. All subjects presented with rashes on the head and face, with 164 cases (86.8%) having ocular symptoms, followed by 126 cases (66.7%) on the forehead, 103 cases (54.5%) on the cheeks, 82 cases (43.4%) on the scalp, and 32 cases (17.0%) at the nose and lips. Most subjects had eyelid edema (92.6%), 92 cases (48.7%) had eye pain, 35 cases (18.5%) had tearing, 25 cases (13.2%) had blurred vision, 19 cases (10.1%) had difficulty closing their eyes, 10 cases (5.3%) had photophobia, and 6 cases (3.2%) had a foreign body sensation in the eyes. The most common ocular presentation was conjunctivitis (78.3%), followed by keratitis (15.9%), scleritis (4.7%), and uveitis (1.1%). There were no cases of acute retinal necrosis or blindness among all patients.
Clinical Presentation of Individuals with Herpes Zoster Ophthalmicus
Types of Antiviral Drugs
The antiviral treatment status of this study is shown in Table 3 . All subjects received adequate antiviral therapy, with 109 cases receiving only oral administration, 44 cases receiving only intravenous administration, and 36 cases receiving intravenous treatment before oral administration. Among them, 148 patients used acyclovir or famciclovir, 29 cases used foscarnet sodium injection, and 12 cases used brivudine. Topical antiviral drugs were used by 161 patients. The median time from the rash onset to antiviral therapy was 4 days (IQR 3–6 days). The median duration of antiviral therapy was 7 days (IQR 5–8 days). There was no significant difference between the two groups in terms of antiviral treatment.
Treatment Profile of Patients with HZO
Data Analysis of Outcome Measures
Table 4 and Table 5 display the influencing factors for the gap from admin to rash recover and eye symptom leave. A linear regression model was used to examine the risk of time interval from hospital admission to resolution of rash caused by HZO. In the multivariate analysis, age (β=0.381, P=0.005), glucocorticoids (β=0.260, P=0.031) and topical antiviral (β=0.380, P=0.023) were found to be significant factors affecting the time interval from admission to resolution of rash. As age increases, the use of glucocorticoids and local antiviral drugs can increase the time interval. A logistic regression model was used to identify risk factors for residual ocular symptoms in HZO patients at discharge. In multivariate analysis, tearing (OR=4.827, P<0.001) and BUN (OR=0.787, P=0.050) were identified as significant factors affecting residual ocular symptoms at discharge.
Factors Associated with the Gap from Admin to Rash Recover
Note : *Indicates statistically significant.
Abbreviations : WBC, white blood cell; PLT, platelet; CRP, C-reactive protein.
Factors Associated with Eye Symptom Leave
Abbreviations : LOS, Length of Stay; BUN, Blood Urea Nitrogen.
HZO is a serious condition characterized by various symptoms and complications, including postherpetic neuralgia, conjunctivitis, keratitis, uveitis, and even retinal vasculitis and necrosis. 9 , 10 All HZO patients exhibited ocular manifestations in this study. Previous studies have reported an incidence rate of ocular symptoms in HZO patients ranging from around 50% to 80%. 7 , 11 , 12 This may be attributed to the fact that hospitalized patients generally have more severe symptoms. Patients with more pronounced and severe ocular involvement are more likely to be advised for hospitalization by doctors. Hence, the incidence rate of ocular symptoms among our patients is expected to be higher. Conjunctivitis, keratitis, and uveitis have been reported as the most common ocular manifestations of HZO. 4 , 7 In our study, conjunctivitis was the most frequent manifestation, followed by keratitis and scleritis. However, some of our patients did not undergo professional ophthalmic examinations, which may have resulted in certain ophthalmic symptoms being undetected in a timely manner.
Aging is a known risk factor for HZO. 7 This study identified advanced age as an independent risk factor for delayed skin lesion recovery. Emma et al’s study 13 analyzed HZO patients at the Massachusetts Eye and Ear Infirmary (MEEI), dividing the cases into two groups: 71 cases in 2007 vs 195 cases in 2013, with the average age of the latter group dropping significantly by almost six years. Carlos et al’s recent study 14 also analyzed the incidence rate of HZO in Columbia and found that its proportion increases with age in a total of 100,000 residents. We speculate that herpes zoster is showing a trend of getting younger. Additionally, the older a person is, the higher the likelihood of contracting the disease. More importantly, previous studies have shown that the specific cellular-mediated immunity to VZV may decrease with age, leading to an increasing incidence and severity of HZO. 15 And Anthony and Himal found that the herpes zoster vaccine can reduce the risk of developing herpes zoster and postherpetic neuralgia in individuals aged 50 and older, as well as those aged 70 and older. 16 , 17 Therefore, we believe that older adults with normal immune function should receive vaccination against herpes zoster.
Currently, there is a debate whether corticosteroids should be used in the treatment plan for HZO. Whitley and Han 18 , 19 analyzed 5 RCTs and conducted a Meta-analysis suggesting that early use of corticosteroids is ineffective for preventing HZO but can inhibit inflammation, alleviate acute phase pain, and accelerate skin lesion healing. Li et al’s study 20 suggested that prolonged steroid use may help with eye muscle paralysis recovery. And Langston’s study 21 indicated a noticeable acceleration in the recovery of rash recovery and acute pain relief for patients undergoing prednisone treatment, leading to an improvement in the quality of life. In our study, steroids were found to be meaningful in alleviating ocular skin lesions. Additionally, topical application of corticosteroid eye drops or ointments recommended by an ophthalmologist can be used. 1 The specific indicators for the use of corticosteroids, the selection of specific types, and the method of use still require further research.
It has been reported that topical acyclovir cream or penciclovir cream is ineffective in the treatment of HZ. 1 However, in our study, it was found that topical antiviral had a shortening effect on the improvement time of rash (P=0.005). This may be because the topical antiviral drug used in our hospital is interferon gel, which has been proved by Miyoshi et al 22 in eight cancer patients that while it did not relieve pain, it reduced the time it took for herpes to disappear. However, the sample size of this study was small and no statistical analysis was conducted. And it only studied the therapeutic effects of interferon gel in HZ, not in HZO. Whether external interferon gel can be effective in the treatment of HZO needs to be further designed and analyzed.
In addition, tearing (OR=4.827, 1.956–11.909, P< 0.001) and blood urea nitrogen (OR=0.787, 0.620–1.000, P=0.050) were the remaining influencing factors of ocular symptoms in our study. However, there is no literature on the relationship between tearing and urea nitrogen and herpes zoster. We speculate that tearing may be related to the involvement of the ophthalmic branch of the trigeminal nerve, affecting the lacrimal gland. Additionally, impaired BUN may suggest that it is not only the facial nerve that is affected. We should pay more attention to patients with renal impairment who have herpes zoster ophthalmicus. We hope to further validate this in the future.
The limitations of this study are retrospective analysis bias, as it only included HZO patients admitted to a hospital in Chongqing, China with relatively limited data collection, and some HZO patients might not have had their eye symptoms fully recognized. Additionally, some patients did not undergo complete ophthalmologic examinations, lack of relevant eye data, nor were they followed up afterwards. The main advantage of this study was that all patients were examined by at least one associate chief physician and chief physician, with diagnoses accurately determined beyond doubt. Patients’ basic information, clinical symptoms and laboratory indicators were recorded in an electronic medical record system, making comprehensive data and inflammatory indicators analysis of influencing factors for HZO possible.
- Conclusions
This study provides the demographics, clinical manifestations and some risk factors of HZO patients. HZO can lead to severe sequelae, most unfavorably involving the eyes, which may need more aggressive treatment for patients with severe rashes on the face. For those whose symptoms persist even after discharge, further studies and follow-up are necessary. This will help to gain a more comprehensive understanding of the long-term effects on patients with herpes zoster ophthalmicus and potential treatment options.
- Acknowledgments
The patients in this manuscript have given written informed consent to publication of their case details. The authors would like to thank the patients who participated in this study.
- Funding Statement
This study was supported by Special Foundation for Postdoctoral Research Projects of Chongqing (2021XM3080), Natural Science Foundation General Program of Chongqing (cstc2021jcyj-msxmX0182) and Natural Science Foundation General Program of China (81874238).
- Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the First Affiliated Hospital, Chongqing Medical University (ID: K2023-564). This article don’t involve the disclosure of patient privacy. Therefore, the ethics committee don’t require patient consent to review their medical records.
The authors declare that there is no conflict of interest regarding the publication of this paper.
Full text links
Read article at publisher's site: https://doi.org/10.2147/ccid.s444766
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence of Ocular Manifestations and Visual Outcomes in Patients With Herpes Zoster Ophthalmicus.
Szeto SK , Chan TC , Wong RL , Ng AL , Li EY , Jhanji V
Cornea , 36(3):338-342, 01 Mar 2017
Cited by: 19 articles | PMID: 27741018
Herpes Zoster Ophthalmicus Clinical Presentation and Risk Factors for Loss of Vision.
Niederer RL , Meyer JJ , Liu K , Danesh-Meyer HV
Am J Ophthalmol , 226:83-89, 08 Feb 2021
Cited by: 10 articles | PMID: 33571476
Pediatric herpes zoster ophthalmicus: a systematic review.
Hakim FE , Riaz K , Farooq A
Graefes Arch Clin Exp Ophthalmol , 261(8):2169-2179, 23 Mar 2023
Cited by: 0 articles | PMID: 36949170 | PMCID: PMC10033303
Ocular complications and loss of vision due to herpes zoster ophthalmicus in patients with HIV infection and a comparison with HIV-negative patients.
Nithyanandam S , Joseph M , Stephen J
Int J STD AIDS , 24(2):106-109, 01 Feb 2013
Cited by: 7 articles | PMID: 23512510
Herpes zoster ophthalmicus: acute keratitis.
Curr Opin Ophthalmol , 29(4):328-333, 01 Jul 2018
Cited by: 13 articles | PMID: 29794881
Europe PMC is part of the ELIXIR infrastructure
Bilateral Pattern Electroretinogram Abnormalities in Patients with Herpes Zoster Keratitis and Conjunctivitis
- ORIGINAL RESEARCH
- Open access
- Published: 08 April 2024
Cite this article
You have full access to this open access article
- Jingyi Li 1 na1 ,
- Yuexin Wang 1 na1 ,
- Xin Xie 1 na1 ,
- Weizhen Zeng 1 ,
- Shiying Li 2 , 3 ,
- Rupesh Agrawal 4 , 5 , 6 &
- Yun Feng ORCID: orcid.org/0000-0002-9453-3259 1
2 Altmetric
Explore all metrics
Introduction
Herpes zoster ophthalmicus (HZO) results from the reactivation of varicella zoster virus (VZV) in the ophthalmic branch of the trigeminal nerve. The inflammation caused by VZV involves multiple tissues in the eyes. Our goal is to evaluate pattern electroretinogram (PERG) changes and their relationship with corneal sub-basal nerve changes in patients with HZO.
Twenty-two patients with herpes zoster keratitis or conjunctivitis and 20 healthy volunteers were recruited for this cross-sectional study. A PERG test was performed on both eyes of HZO patients and one eye of the healthy controls. In vivo confocal microscopy (IVCM) was also performed on both eyes of the HZO patients to detect corneal nerve damage.
Our results showed changes in the PERG parameters in both eyes of HZO patients compared to the healthy controls. Affected eyes showed delayed N95 peak time and decreased P50 and N95 amplitude compared to the unaffected eyes ( p < 0.05, respectively). Both affected and unaffected eyes in HZO patients showed delayed P50 peak time and decreased N95 amplitude ( p < 0.05, respectively) compared to controls. In HZO patients, no significant differences in each PERG parameter were found between eyes with and without corneal lesions or between eyes with and without increased Langham’s cells in the corneal epithelial sub-basal layer. The IVCM images showed decreased total nerve length and number at the sub-basal layer of the epithelial cornea in affected eyes compared to unaffected eyes ( p < 0.05). No significant correlation was found between total nerve length and PERG changes.
Conclusions
Our results showed that VZV-affected eyes without central cornea involvement displayed reduced N95 amplitude and prolonged P50 peak time in bilateral eyes compared to the healthy controls. Larger studies are needed to further explore the effect of HZO on the electrophysiological response of the eye and the posterior segment.
Avoid common mistakes on your manuscript.
Herpes zoster ophthalmicus (HZO) is secondary to reactivation of varicella zoster virus (VZV) in the ophthalmic division of the trigeminal nerve, which accounts for 10–20% of all herpes zoster cases [ 15 , 19 ]. As VZV replicates, the inflammation along the ophthalmic nerve branch encompasses the cornea, uvea, iris, sclera, optic nerve, and retina [ 1 ]. Almost two-thirds of HZO patients develop corneal sensation loss due to corneal nerve damage, and there are recent publications about corneal nerve involvement in the fellow unaffected eyes [ 9 , 14 ]. It has been suggested that reciprocal interaction between the nervous and immune systems underlies the mechanism of bilateral involvement [ 5 ].
The incidence rate of posterior segment involvement in HZO is relatively low, but the consequences can be devastating. VZV-related uveitis involving the posterior segment varies from acute retinal necrosis (ARN) to relatively milder non-necrotizing uveitis [ 4 , 7 , 11 , 21 ]. To date, there is limited evidence reporting posterior segment involvement in herpes zoster keratitis or conjunctivitis. Considering the fact that the inflammation caused by VZV involves multiple tissues in the eyes, it is necessary to evaluate whether retinal layers are involved and the severity of retinal involvement. Pattern electroretinogram (PERG) serves as an objective and valuable tool for evaluating the function of macular and retinal ganglion cells (RGCs) [ 12 ]. Several studies have proved its usefulness in detecting glaucomatous changes and its relationship with other glaucoma-related findings, such as visual field and average thickness of retinal nerve fiber layer, as measured by optical coherence tomography [ 3 , 6 , 20 ].
The aim of this study is to explore whether electro-functional changes exist in the involved and fellow eye of patients with HZO and their association with corneal sub-basal nerve changes, as measured by an in vivo confocal microscope (IVCM). We obtained PERG responses, which were recorded for both eyes of HZO patients without central cornea involvement as well as the eyes of healthy controls.
The present retrospective cross-sectional study was performed according to the considerations of the tenets of the Declaration of Helsinki with approval by the institutional review board of Peking University Third Hospital (S2019341). All the participants signed the informed consent. A total of 22 HZO patients and 20 healthy controls were enrolled in this study. Inclusion criteria were clinical diagnosis of herpes zoster with facial or forehead skin involvement and herpes zoster keratitis or conjunctivitis and patients aged between 25 and 80 years old. Exclusion criteria were as follows: (1) central cornea involvement (within 6-mm diameter) that occluded the pupillary area; (2) the existence of other ophthalmic complications caused by VZV, such as iridocyclitis, scleritis, neuritis, retinitis, or secondary glaucoma; (3) the presence of any other retinopathy (for example, age-related macular degeneration, diabetic retinopathy) and glaucoma; (4) refractive media opacity like central cortical or posterior subcapsular cataracts; and (5) systematic diseases that may have an effect on the outcome of PERG, such as diabetes mellitus, Parkinson’s disease, or multiple sclerosis. Based on the existence of corneal lesions in affected eyes, we further divided HZO patients into lesion ( n = 14) and non-lesion groups ( n = 8), and corneal lesion was defined as the presence of VZV-related peripheral corneal infiltration or corneal nebula. According to the IVCM images, we divided HZO patients into the Langham group (eyes with activated Langham’s cells in the sub-basal layer, n = 11) and the non-Langham group (eyes without activated Langham’s cells in the sub-basal layer, n = 11). The images were observed independently by two ophthalmologists. If there was a disagreement, then it was defined by another senior doctor with more than 10 years of experience. The healthy controls were 20 volunteers aged 25–80 years who came to the ophthalmic outpatient clinic for a regular eye examination with no serious eye diseases or other systematic problems. All HZO patients underwent comprehensive eye examination performed by one ophthalmologist, including best-corrected visual acuity (BCVA) using LogMAR visual chart, non-mydriatic subjective refraction at a distance and near, intraocular pressure (IOP) with a non-contact tonometer, dilated fundus examination, posterior segment optical coherence tomography (OCT), and IVCM. A detailed medical history and ocular history were recorded. The control group did not receive the IVCM test.
PERG was recorded in accordance with the guidelines of the International Society for Clinical Electrophysiology of Vision (ISCEV) using the RETiscan System (Roland Consult, Wiesbaden, Germany) [ 2 ]. Optimal correction of each participant’s refraction was acquired before starting. The reference electrodes and ground electrodes were respectively placed on the skin of the ipsilateral outer canthus and the skin of the forehead, and the active electrodes (Dawson-Trick-Litzkow, DTL) were placed in the lower conjunctiva sac after one drop of topical anesthesia (benoxyl, 0.5% procaine hydrochloride). A black-and-white flat screen monitor (LCD color monitor; Roland Consult) was put from 1-m distance in front of each participant in a soundproof and semi-dark room. The visual angle was 23° (horizontal) × 17° (vertical). The checkerboard’s mean luminance was 80 cd/m 2 and the contrast was 97%. The reversal rate of the transient PERG measurement was 4.3 reversals per second (2.15 Hz), and at least 200 artifact-free reversals were recorded and averaged for each trial. Both eyes of every participant were recorded for at least two trials without dilation of the pupils. The P50 peak time, N95 peak time, P50 amplitude, N95 amplitude, and the N95/P50 amplitude ratios were measured.
In Vivo Confocal Microscopy
The sub-basal nerve plexus and epithelial Langham’s cells in the central cornea of bilateral eyes were imaged using in vivo confocal microscopy (Rostock Cornea Module/HRT II, Heidelberg, Germany). After one drop of topical anesthesia (0.5% proparacaine) applied to both eyes, 0.3% hypromellose was placed on the tip of the objective lens as a coupling medium. The operator manually moved the lens forward until it touched the surface of the central cornea. After a full-thickness confocal scan, 350 images were obtained at a speed of 25 frames per second. The scanning area of each image was 460 × 350 μm with a 500× magnification of and a lateral resolution of 1 μm. The sub-basal nerve plexus was analyzed using NeuronJ, a plug-in semiautomated tracing program of ImageJ, which is free online software distributed by the National Institutes of Health. The total nerve length and total nerve number were calculated after analyzing three representative images of the sub-basal nerve plexus. Three representative images at the level of basal epithelial layers were chosen to analyze Langham’s cells, which were morphologically identified as individual dendriform structures, easily differentiated from the nerve plexus. Images featuring increasing Langham’s cell density were identified manually by two observers. The IVCM test was performed in both eyes of every HZO patients.
Statistical Analysis
Statistical analysis was performed using SPSS version 26.0 (Statistical Package for Social Sciences, Chicago, IL, USA). The Anderson–Darling test confirmed the normality of the distribution. All quantitative variables were expressed by the mean and SEM (standard error of mean). Student’s t test and Fisher’s exact test were used to assess the differences in age and gender between the HZO and control groups, respectively. An analysis of variance (ANOVA) was performed to compare statistically the PERG parameters among the three groups. Student’s t test was used to assess the differences in corneal sub-basal nerve damage in bilateral eyes of HZO patients. Pearson’s correlation analysis was used to address the relationship between PERG parameters and corneal sub-basal nerve changes. Differences were considered statistically significant for p < 0.05.
The mean age of the HZO patients enrolled in this study was 54.36 ± 13.14 years (range, 29–76 years, n = 22), and the mean age of the healthy controls was 47.70 ± 10.07 years (range, 32–65 years, n = 20; p = 0.075). A total of 13 males and nine females were in the HZO group, and eight males and 12 females were in the healthy control group ( p = 0.217). Mean duration of HZO patients is 3.4 ± 2.1 months. Representative images of slit-lamp photographs, IVCM images, OCTA images, and PERG traces of a single patient with HZO are presented in Fig. 1 .
Six representative images from A to D are all from the same patient with herpes zoster ophthalmicus (HZO). A Slit-lamp photograph of his cornea and B shows the picture under cobalt blue light after corneal fluorescein staining. C , D Two consecutive pictures of in vivo confocal microscopy (IVCM) in the sub-basal layer of the epithelial cornea, showing a mass of activated Langham’s cells. E The pattern electroretinogram (PERG) trace of his affected eye. F A PERG trace of a healthy control
PERG Parameters
As shown in Fig. 2 , a statistically significant difference was found in the P50 peak time between HZO affected eyes and healthy controls as well as between unaffected eyes and healthy controls ( p < 0.0001). Both affected eyes and contralateral eyes showed delayed P50 compared to the normal controls. The P50 amplitude was significantly decreased in HZO affected eyes as compared to healthy controls ( p < 0.0001) and as compared to contralateral unaffected eyes ( p = 0.019). When comparing of affected eyes and healthy controls as well as contralateral eyes and healthy controls, the N95 amplitude for both showed a statistically significant decrease ( p < 0.0001, and p = 0.013, respectively). Additionally, the N95 amplitude also showed a decrease in contralateral unaffected eyes compared to affected eyes ( p = 0.021). To our surprise, the N95 peak time in affected eyes was significantly shorter than that in contralateral eyes and that in healthy controls ( p = 0.039 and 0.027 respectively). No significant differences among the groups were found for the N95/P50 ratio. All the PERG parameters are listed in Table 1 .
Comparison of pattern electroretinogram (PERG) parameters in herpes zoster ophthalmicus (HZO) affected eyes, unaffected eyes and healthy controls. ( A P50 peak time; B N95 peak time; C P50 amplitude; D N95 amplitude; E N95/P50 ratio; * p < 0.05; **** p < 0.0001)
Table 2 shows the differences in PERG parameters between eyes with or without corneal lesions. All the five parameters showed no statistically significant differences between these two groups, though eyes with corneal lesions had a tendency toward prolonged P50 and N95 peak time and decreased P50 and N95 amplitude.
Table 3 illustrates the differences in PERG parameters between eyes with and without increased Langham cells. Similarly, no statistically significant differences were found between the two group.
IVCM Parameters
A significant decrease in sub-basal nerve plexus was found in HZO eyes compared to unaffected eyes, including total nerve length (903.11 ± 977.08 vs. 2545.96 ± 1066.98 μm/frame, p < 0.005), and total nerve number (4.45 ± 3.70 vs. 10.53 ± 3.89, p < 0.005). The healthy control group did not receive the IVCM test.
Correlation Between Corneal Sub-basal Nerve and PERG Parameters
We performed Pearson’s correlation to analyze the relationship between PERG parameters and total corneal nerve length, and the results are given in Fig. 3 . There appears to be a trend in which total nerve length decreases as P50 peak time delays, though it is not statistically significant (Fig. 3 ). The associations between total nerve length and other PERG parameters (P50 peak time, N95 peak time, and N95 amplitude) were not statistically significant neither (Fig. 3 ).
Scatterplots showing the linear association between pattern electroretinogram (PERG) parameters and total nerve length ( A P50 peak time, R 2 = 0.0168, p = 0.6086; B N95 peak time, R 2 = 0.0127, p = 0.6558; C P50 amplitude, R 2 = 0.0054, p = 0.7726; D N95 amplitude, R 2 = 0.0007, p = 0.9723)
The current index study was designed to investigate the presence of PERG abnormalities in eyes affected by HZO and their seemingly unaffected counterparts. Additionally, we sought to establish a correlation between these PERG abnormalities and corneal subbasal nerve damage. Our primary goal was to delineate the interplay between ocular surface inflammation and ocular fundus pathology in the context of HZO. The findings from this study uncovered significant alterations in PERG parameters not only in the eyes directly affected by HZO but also in the contralateral unaffected eyes. Specifically, we observed notable changes in the P50 amplitude, N95 amplitude, and P50 peak time. As a result of these observations, we hypothesize a potential association between even mild cases of herpes zoster keratitis or conjunctivitis and retinal electrofunctional changes, as indicated by the detected PERG alterations. These findings offer a glimpse into the intricate connections between superficial ocular afflictions and underlying retinal responses in the context of HZO.
To our knowledge, this is the first study to describe electroretinographic changes in HZO patients. The reduction of the amplitude in PERG N95 reflects the abnormality of RGCs, which may be due to the lack of activities of dead RGCs or reduced activity of viable RGCs [ 8 ]. Many studies have confirmed that the dysfunction of RGCs can precede structural changes. Ventura et al. [ 18 ] conducted a cross-sectional observational study on patients with glaucoma suspect (GS) and early manifest glaucoma (EMG) and found the PERG was abnormal in 52% of GS patients and 69% of EMG patients. The research demonstrated that abnormal PERG can serve as a predictive technique for monitoring the initial onset of glaucoma by detecting RGC dysfunction. Moreover, in inflammatory diseases such as Fuchs’ heterochromic cyclitis (FHC), changes in RGCs may be among the earliest detectable signs. Murray et al. [ 13 ] found subclinical retinal damage in eyes with FHC, as their results suggested reduced amplitude and delayed peak time of both the P50 and N95 components of PERG in FHC eyes with good vision compared to normal eyes. In particular, the reduction and delay of the P50 component indicated preganglionic retinal changes in the macular area. Therefore, we hypothesize that the macular RGCs are involved and damaged in mild HZO patients. Further study is needed to explore full-scale electroretinographic changes using different measurement instruments such as multi-focal ERG and full-field ERG.
Furthermore, our results suggest that unaffected eyes also exhibit PERG abnormalities. This is consistent with previous literature. Hamrah et al. [ 9 ] demonstrated corneal nerve diminishment in HZO-affected and unaffected eyes, suggesting bilateral changes in clinically unilateral disease. Cavalcanti et al. [ 5 ] found a bilateral increase of dendritic cell (DC) density in unilateral HZO patients, and that the DC density was negatively correlated with all corneal nerve parameters, including total nerve length, the total number of nerves, and the number of nerve branches measured by IVCM. The authors postulated that a coordinated interaction between the nervous system and the immune system occurred in clinically unilateral diseases. The bilateral elevation of pro-inflammatory cytokines was confirmed in patients with unilateral bacterial keratitis and its strong correlation with nerve alteration [ 22 ]. Our finding, related to sub-basal nerve changes in bilateral eyes, is consistent with the existing literature. Thus, it is plausible to postulate that bilateral PERG abnormalities can be induced by the synergic interaction between the nervous system and immune response and that retinal ganglion cells may be involved in this process. The involvement of the posterior segment is uncommonly seen in HZO patients, and there are limited data concerning the retinal pathological changes with corneal inflammation. Roberts et al. [ 16 ] once reported two cases of herpes zoster chorioretinopathy in the retinal pigment epithelium and choroid after 8 and 5 months of the first onset. The retinal lesions of both patients remained unchanged and they ended up with good vision. Acute retinal necrosis (ARN) syndrome presents a distinctive clinical process characterized by occlusive retinal arteritis, necrotizing retinitis, and uveitis [ 17 ], whereas the clinical characteristics of patients with or without immune suppression differ greatly [ 10 ]. Thus, it is imperative to establish diagnostic tests of early retinopathy of VZV infection and to improve the visual prognosis of ARN patients.
Moreover, our results support that damage to the anterior segment does not necessarily influence damage to the posterior segment, as no significant changes were found between eyes with and without corneal lesions. The absence of corneal inflammation does not exclude posterior retinopathy, as no significant changes between eyes with and without the activated Langham cells in the corneal sub-basal layer were detected. This can be induced by direct virus infection or the secondary immune response targeted at the retinal ganglion cells. Future research needs to be conducted to elucidate the underlying mechanism of this pathogenesis.
Our study has certain limitations. First, our sample size was small. Furthermore, fundus measurement instruments other than PERG, such as full-field ERG and multifocal ERG, are not applied in this study, thus rendering the evaluation of electroretinographic changes incomplete. We speculate that the full-field ERG may be normal, as it reflects the function of the global retina. PhNR may induce certain changes in HZO patients. Future research will benefit from performing more participants and performing a more comprehensive evaluation. Moreover, the IVCM test was not performed on the control group; therefore, we failed to analyze the association between IVCM changes and PERG parameters in the control group. Many confounding factors such as patient compliance or manual counting of the total nerve length and dendritic cells using ImageJ software, might also have caused inaccuracy. Due to the nature of the retrospective study, we failed to conduct a follow-up period recording and prospectively analyze the PERG change in different HZO duration. We will improve the study design in future research.
In summary, our results show that HZO patients display diminished N95 amplitude and prolonged P50 peak time in bilateral eyes. No significant connection was established between corneal nerve changes and PERG parameters changes in bilateral eyes. The research provides evidence of retinal functional abnormality in HZO keratitis and conjunctivitis and facilitates further investigation of the multi-tissue VZV infection relationship in the future.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–81. https://doi.org/10.1128/cmr.9.3.361 .
Article CAS PubMed PubMed Central Google Scholar
Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. 2013;126:1–7. https://doi.org/10.1007/s10633-012-9353-y .
Article PubMed Google Scholar
Bach M, Hoffmann MB. Update on the pattern electroretinogram in glaucoma. Optom Vis Sci. 2008;85:386–95. https://doi.org/10.1097/OPX.0b013e318177ebf3 .
Bodaghi B, Rozenberg F, Cassoux N, Fardeau C, LeHoang P. Nonnecrotizing herpetic retinopathies masquerading as severe posterior uveitis. Ophthalmology. 2003;110:1737–43. https://doi.org/10.1016/s0161-6420(03)00580-3 .
Cavalcanti BM, Cruzat A, Sahin A, Pavan-Langston D, Samayoa E, Hamrah P. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul Surf. 2018;16:101–11. https://doi.org/10.1016/j.jtos.2017.09.004 .
Elgohary AM, Elbedewy HA, Saad HA, Eid TM. Pattern electroretinogram changes in patients with primary open-angle glaucoma in correlation with visual field and optical coherence tomography changes. Eur J Ophthalmol. 2020;30:1362–9. https://doi.org/10.1177/1120672119872606 .
Engstrom RE Jr, Holland GN, Margolis TP, Muccioli C, Lindley JI, Belfort R Jr, Holland SP, Johnston WH, Wolitz RA, Kreiger AE. The progressive outer retinal necrosis syndrome. A variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994;101:1488–502. https://doi.org/10.1016/s0161-6420(94)31142-0 .
Fiorentini A, Maffei L, Pirchio M, Spinelli D, Porciatti V. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest Ophthalmol Vis Sci. 1981;21:490–3.
CAS PubMed Google Scholar
Hamrah P, Cruzat A, Dastjerdi MH, Prüss H, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120:40–7. https://doi.org/10.1016/j.ophtha.2012.07.036 .
Helbig H, Bornfeld N, Bechrakis NE, Kellner U, Foerster MH. Varicella zoster virus infections of the retina in patients with and without immune suppression. Klin Monbl Augenheilkd. 1994;205:103–8. https://doi.org/10.1055/s-2008-1045500 .
Article CAS PubMed Google Scholar
Kim JY, Lee JH, Lee CS, Lee SC. Varicella zoster virus-associated Chorioretinitis: a case report. BMC Ophthalmol. 2018;18:28. https://doi.org/10.1186/s12886-018-0696-3 .
Article PubMed PubMed Central Google Scholar
Maffei L, Fiorentini A, Bisti S, Holländer H. Pattern ERG in the monkey after section of the optic nerve. Exp Brain Res. 1985;59:423–5. https://doi.org/10.1007/bf00230925 .
Murray DC, Stavrou P, Good PA, Murray PI. Electroretinographic findings in Fuchs’ heterochromic cyclitis. Eye (Lond). 1997;11(Pt 1):102–8. https://doi.org/10.1038/eye.1997.20 .
Pavan-Langston D. Herpes zoster ophthalmicus. Neurology. 1995;45:S50-51. https://doi.org/10.1212/wnl.45.12_suppl_8.s50 .
Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982;61:310–6. https://doi.org/10.1097/00005792-198209000-00003 .
Roberts TV, Francis IC, Kappagoda MB, Dick AD. Herpes zoster chorioretinopathy. Eye (Lond). 1995;9(Pt 5):594–8. https://doi.org/10.1038/eye.1995.146 .
Usui Y, Goto H. Overview and diagnosis of acute retinal necrosis syndrome. Semin Ophthalmol. 2008;23:275–83. https://doi.org/10.1080/08820530802111325 .
Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK 2nd. Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–9. https://doi.org/10.1016/j.ophtha.2004.07.018 .
Vrcek I, Choudhury E, Durairaj V. Herpes Zoster ophthalmicus: a review for the internist. Am J Med. 2017;130(1):21–26. https://doi.org/10.1016/j.amjmed.2016.08.039
Wanger P, Persson HE. Pattern-reversal electroretinograms in unilateral glaucoma. Investig Ophthalmol Vis Sci. 1983;24:749–53.
CAS Google Scholar
Wensing B, de Groot-Mijnes JD, Rothova A. Necrotizing and nonnecrotizing variants of herpetic uveitis with posterior segment involvement. Arch Ophthalmol. 2011;129:403–8. https://doi.org/10.1001/archophthalmol.2010.313 .
Yamaguchi T, Calvacanti BM, Cruzat A, Qazi Y, Ishikawa S, Osuka A, Lederer J, Hamrah P. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Investig Ophthalmol Vis Sci. 2014;55:7457–66. https://doi.org/10.1167/iovs.14-15411 .
Article CAS Google Scholar
Download references
Acknowledgements
We thank the participants of the study.
This study was supported by National Natural Science Foundation of China Grants (No. 81700799; 82070926). The authors funded the journal’s Rapid Service Fee.
Author information
Jingyi Li, Yuexin Wang, and Xin Xie contributed equally to this work.
Authors and Affiliations
Department of Ophthalmology, Peking University Third Hospital, No. 49, North Garden Street, Haidian District, Beijing, China
Jingyi Li, Yuexin Wang, Xin Xie, Weizhen Zeng & Yun Feng
Department of Ophthalmology, Xiang’an Hospital of Xiamen University, No. 2000, Xiang’an East Road, Xiang’an District, Xiamen, Fujian, China
Eye Institute of Xiamen University, Xiamen, China
National Healthcare Group Eye Institute, Tan Tock Seng Hospital, Singapore, Singapore
Rupesh Agrawal
Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
Singapore Eye Research Institute, Singapore, Singapore
You can also search for this author in PubMed Google Scholar
Contributions
Research design: Yun Feng; Jingyi Li; Yuexin Wang; Xin Xie; data acquisition: Xin Xie; Jingyi Li; Weizhen Zeng; Data analysis and interpretation: Jingyi Li; Yun Feng; Shiying Li; Manuscript preparation: Jingyi Li; Yuexin Wang; Yun Feng; Shiying Li; Rupesh Agrawal; Yun Feng.
Corresponding authors
Correspondence to Shiying Li or Yun Feng .
Ethics declarations
Conflict of interest.
Jingyi Li, Yuexin Wang, Xin Xie, Weizhen Zeng, Shiying Li, Rupesh Agrawal, and Yun Feng do not have any conflicts of interest to declare.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki principles. The Human Ethics Committees of Peking University Third Hospital approved the protocol numbered S2019341. All the subjects gave their written informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Li, J., Wang, Y., Xie, X. et al. Bilateral Pattern Electroretinogram Abnormalities in Patients with Herpes Zoster Keratitis and Conjunctivitis. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-00928-9
Download citation
Received : 11 January 2024
Accepted : 05 March 2024
Published : 08 April 2024
DOI : https://doi.org/10.1007/s40123-024-00928-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Herpes zoster ophthalmicus
- Pattern electroretinogram
- Varicella virus affection
- Retinal ganglion cells
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Case Rep Ophthalmol Med
- v.2012; 2012

An Acute Case of Herpes Zoster Ophthalmicus with Ophthalmoplegia
Wasim hakim.
1 The Postgraduate Education Centre, Basildon University Hospital, Essex SS16 5NL, UK
Rosalie Sherman
Kanwar pannu.
2 Department of Respiratory Medicine, The London Chest Hospital, Bonner Road, London E2 9JX, UK
Herpes zoster ophthalmicus (HZO) with oculomotor nerve involvement is rare, even rarer as an acute presentation rather than sequelae of HZO. In this paper we present a case of cutaneous HZO in which our patient's initial presentation was one of complete ophthalmoplegia.
1. Introduction
Herpes zoster (or shingles) refers to a typically vesicular rash caused by reactivation of the latent varicella zoster virus (chickenpox) from the dorsal root ganglia neurons. It usually presents in thoracic or cranial dermatoms. The lifetime risk of herpes zoster is estimated to be 10% to 20%, but in patients over the age of 80 years this risk rises to 50%. Reactivation can occur for a number of different reasons including trauma, ageing, or immune deficiency [ 1 ].
HZO is a rare form of shingles, reported in 15–25% of cases [ 2 ], that presents with a rash in the distribution of the trigeminal nerve dermatomes (mainly ophthalmic and maxillary divisions). It is often reported to be associated with a variety of complications, including episcleritis, keratitis, glaucoma, and cataracts [ 1 ], but there are very few reports of complete ophthalmoplegia being one of those.
2. Case Presentation
An 87-year-old lady who lives alone presented to the medical admissions unit with an inability to open her right eye. She has a past history of mild dementia and depression and was previous to this otherwise fit and well. She describes a 6-day history of blister formation surrounding the eye that extends to her right forehead and scalp. Her family noticed her eye was becoming increasingly droopy, red, and swollen culminating in it being permanently shut for twenty-four hours prior to admission. This visual impairment was most likely responsible for her falling before attending hospital, during which she sustained a left elbow laceration. Of note, she had been started two days previously on flucloxacillin and phenoxymethylpenicillin by her general practitioner.
On examination she had a vesicular rash covering her right scalp, forehead, eye, and upper cheek. It was erythematous, swollen, and tender. She had a complete ophthalmoplegia, and her pupil was fixed and dilated. Her visual acuity in that eye was reduced to counting fingers. The remainder of her neurological and other systems examinations were normal. She was commenced on oral and topical acyclovir, dexamethasone, and cyclopentolate subsequent to ophthalmology review. There was no evidence of vasculitis on slit-lamp examination (see Figures Figures1 1 and and2 2 ).

Oculomotor nerve palsy-pupil fixed and dilated in a position of lateral and downward gaze.

To show the rash in the distribution of the ophthalmic branch of the trigeminal nerve.
The vesicular rash resolved some two weeks later after admission, and she was discharged. She had regained some of her eye movements partially but the ptosis remained.
3. Discussion
In the limited literature, that does report opthalmoplegia as part of the sequelae of HZO, this has typically been described as a late complication, often up to 2 months after the initial herpetic rash [ 3 ] and is seen in only 11–29% of patients with HZO. However, in our case it has developed as part of the acute viral infection. The most commonly affected nerve is the third cranial nerve and, less commonly, the fourth nerve [ 4 ]. With a third nerve palsy it has been reported as being partial or complete but there is always ptosis. Isolated ptosis and isolated paralysis of the pupil have also been seen [ 5 ]. In our case, there was third cranial nerve (oculomotor) palsy causing complete external ophthalmoplegia, with her pupil fixed and middilated.
Several hypotheses have been proposed for the mechanism behind which HZO can result in ophthalmoplegia [ 5 – 8 ] although it is most likely that there are many contributing factors. It is known that the reactivated virus causes inflammation of the axons that supply the dermatomes in question. Edgerton suggested that the inflammation of the trigeminal nerve could actually spread via the cavernous sinus to affect the oculomotor nerve [ 5 ]. In addition, Naumann et al. found chronic inflammatory cells suggesting an occlusive vasculitis [ 6 ]. Carrol thought that due to the onset and rate of recovery, this pathology would suggest a demyelinating disease, and Lavin et al. were in agreement with this hypothesis based on autopsy reports [ 7 , 8 ].
Diagnosis of this condition is essentially a clinical one based on history and examination findings. Once the diagnosis is made, it is reported that the earlier treatment is initiated, the better the prognosis is, preferably within 72 hours, although beneficial effects have been reported with treatment started as late as 7 days after onset [ 9 ]. Acyclovir, famciclovir or valacyclovir have all been used, and they act by resolving skin lesions, decreasing viral shedding and decreasing the risk of ocular involvement. Some report that famciclovir and valacyclovir are better at resolving pain associated with HZO than acyclovir [ 9 ]. In addition to the antivirals it is important to treat any further complications detected. For example, if keratitis or episcleritis has developed, then topical steroids can be used. The effectiveness of steroids and antivirals alone or in combination does not, however, appear to have been formally studied, and treatment options are limited partly due to the poorly defined mechanism of HZO.
The prognosis for full recovery after complete opthalmoplegia following HZO is good. In one literature review, of 16 total cases, 9 were followed up, and all of those cases showed significant improvement in symptoms after 2 months and almost complete resolution by 18 months [ 10 ].
4. Conclusion
Ophthalmoplegia is a rare complication of HZO. Furthermore, the cases in the literature describe it as relatively late sequelae, unlike the acute presentation we have reported.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Authors' Contribution
W. Hakim, R. Sherman, T. Rezk and K. Pannu were all involved in the care of the patient on the ward and contributed to writing up the case and performing a literature review. All authors read and approved the final paper.
Conflict of Interests
The authors declare that they have no conflict of interestes.
All doctors were at Basildon Univeristy Hospital at the time when this case presented; however the instiutions listed above show their current place of work.
Abbreviation
Bilateral Pattern Electroretinogram Abnormalities in Patients with Herpes Zoster Keratitis and Conjunctivitis
Affiliations.
- 1 Department of Ophthalmology, Peking University Third Hospital, No. 49, North Garden Street, Haidian District, Beijing, China.
- 2 Department of Ophthalmology, Xiang'an Hospital of Xiamen University, No. 2000, Xiang'an East Road, Xiang'an District, Xiamen, Fujian, China. [email protected].
- 3 Eye Institute of Xiamen University, Xiamen, China. [email protected].
- 4 National Healthcare Group Eye Institute, Tan Tock Seng Hospital, Singapore, Singapore.
- 5 Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
- 6 Singapore Eye Research Institute, Singapore, Singapore.
- 7 Department of Ophthalmology, Peking University Third Hospital, No. 49, North Garden Street, Haidian District, Beijing, China. [email protected].
- PMID: 38587772
- DOI: 10.1007/s40123-024-00928-9
Introduction: Herpes zoster ophthalmicus (HZO) results from the reactivation of varicella zoster virus (VZV) in the ophthalmic branch of the trigeminal nerve. The inflammation caused by VZV involves multiple tissues in the eyes. Our goal is to evaluate pattern electroretinogram (PERG) changes and their relationship with corneal sub-basal nerve changes in patients with HZO.
Methods: Twenty-two patients with herpes zoster keratitis or conjunctivitis and 20 healthy volunteers were recruited for this cross-sectional study. A PERG test was performed on both eyes of HZO patients and one eye of the healthy controls. In vivo confocal microscopy (IVCM) was also performed on both eyes of the HZO patients to detect corneal nerve damage.
Results: Our results showed changes in the PERG parameters in both eyes of HZO patients compared to the healthy controls. Affected eyes showed delayed N95 peak time and decreased P50 and N95 amplitude compared to the unaffected eyes (p < 0.05, respectively). Both affected and unaffected eyes in HZO patients showed delayed P50 peak time and decreased N95 amplitude (p < 0.05, respectively) compared to controls. In HZO patients, no significant differences in each PERG parameter were found between eyes with and without corneal lesions or between eyes with and without increased Langham's cells in the corneal epithelial sub-basal layer. The IVCM images showed decreased total nerve length and number at the sub-basal layer of the epithelial cornea in affected eyes compared to unaffected eyes (p < 0.05). No significant correlation was found between total nerve length and PERG changes.
Conclusions: Our results showed that VZV-affected eyes without central cornea involvement displayed reduced N95 amplitude and prolonged P50 peak time in bilateral eyes compared to the healthy controls. Larger studies are needed to further explore the effect of HZO on the electrophysiological response of the eye and the posterior segment.
Keywords: Herpes zoster ophthalmicus; Pattern electroretinogram; Retinal ganglion cells; Varicella virus affection.
© 2024. The Author(s).

IMAGES
VIDEO
COMMENTS
Herpes zoster (HZ), or shingles, results from reactivation of latent infection with varicella- zoster virus, which also causes chickenpox. Anyone who has had chickenpox, even in subclinical form, is at risk for developing HZ. It is estimated that the lifetime risk of HZ is 30%, and 1 million new cases are reported annually in the United ...
Infection from the varicella-zoster virus (VZV) most commonly occurs in childhood and is spread by airborne, droplet, and contact transmission. Herpes zoster results from reactivation of the latent VZV within a sensory nerve ganglion, often presenting decades after the initial infection. The disease typically presents as a unilateral maculopapular or vesicular rash in a single dermatomal ...
Introduction. Herpes zoster (HZ), also known as shingles, is a contagious skin disease caused by the reactivation of the varicella-zoster virus (VZV), which lies dormant in spinal or cranial nerve ganglia after a previous infection with chickenpox. 1 With the accelerated aging process and increased stress in daily work and life, as well as decreased immunity, the incidence of HZ has shown a ...
Herpez zoster ophthalmicus is a severe variant of shingles (herpes zoster), which occurs when the immune system is weakened and the virus responsible for chickenpox reactivates. ... Clinical presentation. Herpes zoster most frequently involves the chest, abdomen, face or genitals. The distribution corresponds to the distribution of the nerve ...
Herpes zoster ophthalmicus is a reactivated latent varicella-zoster virus (VZV) infection ( shingles ) involving the eye. Symptoms and signs, which may be severe, include unilateral dermatomal forehead rash and painful inflammation of all the tissues of the anterior and, rarely, posterior structures of the eye.
Herpes Zoster Ophthalmicus. Herpes zoster ophthalmicus is a reactivated infection of the eye caused by the varicella-zoster virus, the virus that causes chickenpox and shingles . Symptoms include pain and tingling of the forehead, blisters on the forehead and nose, eye ache and redness, light sensitivity, and eyelid swelling.
In the United States, 30.9 out of every 100,000 people will develop herpes zoster ophthalmicus. The shingles vaccine reduces the risk of varicella zoster reactivation, and current guidelines recommend vaccinating at age 60. ... Herpes zoster ophthalmicus: declining age at presentation.
Herpes zoster, or shingles, is a common infection caused by the reactivation of varicella zoster virus that lies dormant in the dorsal root nerve ganglion following primary chickenpox infection. Herpes zoster ophthalmicus accounts for 10-20% of cases of herpes zoster infection. 1 Patients usually present with painful, vesicular, dermatomal ...
Herpes zoster ophthalmicus (HZO), also known as ophthalmic shingles, is caused by a localized reactivation of the varicella zoster virus (VZV) in the ophthalmic division of the trigeminal nerve. ... Herpes Zoster Ophthalmicus Clinical Presentation and Risk Factors for Loss of Vision. Am J Ophthalmol. 2021;226:83-89.
Herpes zoster ophthalmicus occurs when reactivation of the latent virus in the trigeminal ganglia involves the ophthalmic division of the nerve. The virus damages the eye and surrounding ...
Purpose: To determine the rate of moderate and severe vision loss following herpes zoster ophthalmicus (HZO) and to identify associated factors. Design: Retrospective cohort study. Methods: All subjects with acute HZO seen at a single center from 2006 to 2016 were included in the study. The primary outcome measure was the proportion of individuals with moderate and/or severe loss of vision ...
Herpes zoster ophthalmicus (HZO), also known as ophthalmic zoster, is shingles involving the eye or the surrounding area. Common signs include a rash of the forehead with swelling of the eyelid.There may also be eye pain and redness, inflammation of the conjunctiva, cornea or uvea, and sensitivity to light.Fever and tingling of the skin and allodynia near the eye may precede the rash.
Herpes Zoster Ophthalmicus. A vesicular rash in the distribution of the ophthalmic division (V1) of the trigeminal nerve is seen. The presence of the lesion near the tip of the nose (Hutchinson sign) increases the risk of ocular involvement. ... These complications have a highly variable presentation that can mimic almost any ophthalmic ...
is possible, resulting in herpes zoster ophthalmicus (HZO). The estimated rate of HZO is approximately 10% of all HZ cases.1,2 In recent years, the incidence of both HZ and HZO has almost tripled, possibly related to the larger aging population.2,3 HZO is a serious and vision-threatening disease. However, with proper treatment, it is
The epidemiology, pathophysiology, and clinical presentation of herpes zoster ophthalmicus in the emergency department is discussed with an emphasis on the identification of the numerous potential ocular complications. Emergency physicians need to be able to recognize the clinical features of herpes zoster ophthalmicus and initiate appropriate ...
Herpes zoster, also known as shingles, is caused by reactivation of varicella-zoster virus (VZV), the same virus that causes varicella (chickenpox). Primary infection with VZV causes varicella. After a person has varicella, the virus remains latent in the dorsal root ganglia. VZV can reactivate later in a person's life and cause herpes zoster ...
Goh and Khoo [6] found the mean age of herpes zoster in their study to be 48.8y. Herpes zoster usually occurs in advancing of age due to lowering of the immunity power reactivating the long-term harbouring the varicella virus in the sensory ganglion. Brănişteanu et al [7] found that 35% of patients were in the 70-80 years age.
Purpose: Herpes zoster ophthalmicus (HZO) is traditionally considered as an unilateral disease. However, subclinical involvements in the contralateral eye structures are evidence, giving rise to a broader understanding of varicella-zoster virus (VZV) infection. Methods: We enrolled 20 eyes of 10 patients with HZO and 12 eyes of healthy controls ...
A total of 189 patients with HZO were included in the analysis, and their demographic characteristics are shown in Table 1. The median age at presentation was 61 years (mean 59.9±14.5 years), ranging from 14 to 93 years. 85 subjects (45.0%) were over 60 years old, while 19 cases (10.1%) were under 40 years old. The male-to-female ratio was 1.59.
Varicella-zoster virus is part of the Herpesviridae family. The primary infection is usually self-limited. The virus remains dormant in the neurosensory ganglia and can be reactivated, resulting in the cutaneous presentation affecting V1 distribution of trigeminal nerve, labelled as Herpes Zoster Ophthalmicus (HZO).
Introduction Herpes zoster ophthalmicus (HZO) results from the reactivation of varicella zoster virus (VZV) in the ophthalmic branch of the trigeminal nerve. The inflammation caused by VZV involves multiple tissues in the eyes. Our goal is to evaluate pattern electroretinogram (PERG) changes and their relationship with corneal sub-basal nerve changes in patients with HZO. Methods Twenty-two ...
The aims of this study were to examine the trends in the initial management of herpes zoster ophthalmicus (HZO) in the United States from 2010 to 2018 and compare them with the treatment preferences of corneal specialists. Methods:
While typically affecting older adults and immunocompromised individuals, herpes zoster ophthalmicus (HZO) has been reported with varying manifestations and complications in children. In this review, we evaluate reported cases of pediatric HZO in the literature and discuss the epidemiology, risk factors, clinical presentation, treatment and ...
Objective: To investigate the anatomical, pathogenetic, and pharmacological characteristics of herpes zoster ophthalmicus (HZO)- related ophthalmoplegia. Methods: Case report-based systematic review was performed. Results: This study included 96 patients (54 [56.25%] women and 42 [43.75%] men [P = 0.221]). The mean age at presentation was 64.32 ± 17.48 years.
Abstract. Herpes zoster ophthalmicus (HZO) with oculomotor nerve involvement is rare, even rarer as an acute presentation rather than sequelae of HZO. In this paper we present a case of cutaneous HZO in which our patient's initial presentation was one of complete ophthalmoplegia. Go to: 1. Introduction. Herpes zoster (or shingles) refers to a ...
Shingles (herpes zoster) is caused by the reactivation of a latent varicella zoster virus (VZV) infection, generally decades after the primary infection. Primary VZV infection typically occurs during childhood and causes chickenpox (varicella); further information on this can be found in Chapter 34. Following primary VZV infection, the virus ...
Introduction: Herpes zoster ophthalmicus (HZO) results from the reactivation of varicella zoster virus (VZV) in the ophthalmic branch of the trigeminal nerve. The inflammation caused by VZV involves multiple tissues in the eyes. Our goal is to evaluate pattern electroretinogram (PERG) changes and their relationship with corneal sub-basal nerve changes in patients with HZO.