
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.

2.2 Introduction to Seed Germination
Learning objectives
By the end of this section you will be able to:
- Describe the differences between epigeal and hypogeal seedling emergence.
- Understand the terms that are used to describe different parts of the seedling as it emerges.
Seeds and their importance

A seed , in botanical terms, is an embryonic plant enclosed inside its seed coat . Typically, the seed also has stored energy (proteins and carbohydrates) that are used by the seed during germination to establish itself when environmental conditions are favorable for growth. The stored energy is what makes seeds valuable for humans, too. Seeds are important in our daily lives because they feed us (food), feed livestock (aka, feed), and provide us with fuel and fiber for personal, home, and industrial purposes.
Seeds are by far the most common mode by which plants reproduce, and most people are familiar with their role in plant propagation and reproduction. The evolutionary advantage of reproduction by seed is the mixing of genetic material through meiotic recombination and the transfer of gametes (pollen) from one parent to another. This mixing of male and female parent genetics results in seeds, and thus seedlings, that are unique from one another and from the parents. The seeds may be dispersed locally or distributed far away through many mechanisms, such as wind, animals, insects, and water. Seedlings will germinate and grow, and those that are most fit in the environment will reproduce and pass on their genes to the next generation. This ability of plants to adapt to local environments and to pass on their genes is evolution in action, as new variations and even new species emerge and disappear from the landscape.
One advantage of seed production is that plants generally produce copious amounts of seed. Each seed may have slightly different germination requirements, a reflection of the diversity resulting from sexual recombination and an evolutionary strategy that allows seeds to germinate at different times. Seeds are able to remain dormant until the conditions are suitable for plant growth and survival, and have mechanisms that prevent germination before winter, during droughts, or in low-light environments. Some weedy species are excellent at interpreting these signals and may lie dormant for years in the soil “seed bank,” only germinating when the seed has been exposed.
Seedling emergence
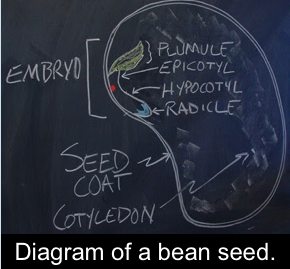
Most seeds have a very slow metabolism when they are mature, which puts them in a state of quiescence: alive, but not growing and not physiologically active. At germination, the seed’s metabolic pathways are activated, leading to embryo growth and emergence of a new seedling. Germination begins with activation by water uptake. We call this imbibition , and sometimes the seed or fruit requires special treatment for water to get into the seed and start this process. We often use the emergence of the radicle (the embryonic root) from the seed coat as a measure of successful germination. Water uptake alone is not an indication that the seed is alive and growing, despite the expansion of seed tissues.
Cell division is taking place in the epicotyl , and the hypocotyl and the shoot and root are beginning to break through the seed coat. The new plant is beginning to grow and emerge from the soil.
Two types of seedling emergence
Epigeal and hypogeal.
Epigeal and hypogeal are terms used to describe the position of the cotyledonary node during germination, indicating whether the node is above or below ground once the seedling has become established.
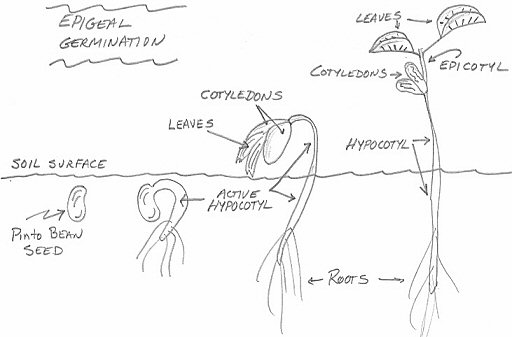
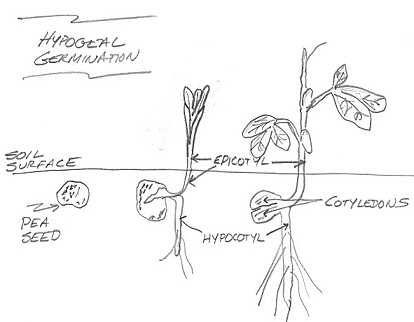
Epi means above while Hypo means below . The location of the cotyledonary node following seedling emergence is a characteristic used as a first step to differentiate plant species. The position of the cotyledon is affected by the rapidity of cell division in the hypocotyl region of the plant during germination and early seedling growth. The epicotyl is the embryonic shoot region above the attachment point of the cotyledons, and the hypocotyl the embryonic region below the cotyledon attachment point and extending down to where the root begins.
In this type of seedling emergence, cell division in the hypocotyl is initially more active and rapid than cell division in epicotyl. The actively dividing meristem in the hypocotyl causes cell growth and elongation that pushes some of the hypocotyl, as well as the cotyledonary node and epicotyl, above the soil surface. The cotyledonary node is above the ground — epigeal. The drawing shows four stages in the emergence of a pinto bean ( Phaseolus vulgaris L.) which exhibits epigeal germination.
This video shows germination of a bean seed over a 10-day time span.
In this type of seedling emergence, the apical meristem at the tip of the epicotyl is more active than the hypocotyl. This cell division and elongation pushes the epicotyl above the soil while the cotyledons and all of the hypocotyl remain below the soil surface. The cotyledonary node is below the ground — it is hypogeal. The example above is a pea ( Pisum sativum L.) which exhibits hypogeal germination.
This video shows hypogeal germination of pea, where the cotyledonary node stays below the soil, and this video shows epigeal germination of bean, where the cotyledonary node is pushed above the soil.
Review questions
- Does “epi” mean above or below? Above or below what?
- Diagram the seedling with the following: hypocotyl, cotyledons, epicotyl, and leaves.
Outer layer of the seed.
Activation of metabolic pathways of the embryo leading to the emergence of a new seedling.
Germination, when the embryo becomes active and the radicle grows through the seed coat.
Embryonic root that breaks through the seed coat during germination and develops into the seedling's root system.
Portion of the stem of a seedling or embryo located between the cotyledons and the first true leaves.
Embryonic shoot below the cotyledons.
Type of seedling emergence where cell division in the hypocotyl is initially more active and rapid than cell division in the epicotyl. Cotyledons are brought above the soil surface as the hypocotyl expands.
Type of seedling emergence where the cotyledons remain below the surface of the ground.
Food storage structure used in germination.
The Science of Plants Copyright © 2022 by The Authors is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
REVIEW article
Seed dormancy and germination—emerging mechanisms and new hypotheses.

- Department of Horticulture, Oregon State University, Corvallis, OR, USA
Seed dormancy has played a significant role in adaptation and evolution of seed plants. While its biological significance is clear, molecular mechanisms underlying seed dormancy induction, maintenance and alleviation still remain elusive. Intensive efforts have been made to investigate gibberellin and abscisic acid metabolism in seeds, which greatly contributed to the current understanding of seed dormancy mechanisms. Other mechanisms, which might be independent of hormones, or specific to the seed dormancy pathway, are also emerging from genetic analysis of “seed dormancy mutants.” These studies suggest that chromatin remodeling through histone ubiquitination, methylation and acetylation, which could lead to transcription elongation or gene silencing, may play a significant role in seed dormancy regulation. Small interfering RNA and/or long non-coding RNA might be a trigger of epigenetic changes at the seed dormancy or germination loci, such as DELAY OF GERMINATION1 . While new mechanisms are emerging from genetic studies of seed dormancy, novel hypotheses are also generated from seed germination studies with high throughput gene expression analysis. Recent studies on tissue-specific gene expression in tomato and Arabidopsis seeds, which suggested possible “mechanosensing” in the regulatory mechanisms, advanced our understanding of embryo-endosperm interaction and have potential to re-draw the traditional hypotheses or integrate them into a comprehensive scheme. The progress in basic seed science will enable knowledge translation, another frontier of research to be expanded for food and fuel production.
Introduction
The ultimate role of seeds is to produce offspring and maintain species. Therefore, plants have evolved diverse strategies to ensure successful germination of this genetic delivery system. Proper distribution of seed germination, in both temporal and spatial manners, is critical for survival and proliferation of seed plants. Spatial distribution of germination is generally controlled through seed and fruit morphology, which enhances dispersal of the offspring from the maternal habitat. In contrast, temporal distribution of germination is controlled mainly by the physiological status of seeds. A variation among individual seeds in a population, in terms of physiological status, allows each seed to germinate at a different timing, which is an important strategy for seeds to avoid competition with their siblings or extinction of all individuals due to a disastrous condition. Plants have evolved seed dormancy, temporal suppression of germination under the conditions favorable to germination. Induction of seed dormancy during the maturation stage and its release at a dry state after a certain period of time, which is called “after-ripening,” are widespread phenomena observed in diverse species of seed plants ( Bewley et al., 2013 ). There may be a universal mechanism of seed dormancy as well as a species-specific variation in the regulatory mechanisms.
Hormonal regulation may be a highly conserved mechanism of seed dormancy among seed plants. Induction and maintenance of seed dormancy by abscisic acid (ABA) and dormancy release by gibberellin (GA) are observed in many species. The molecular mechanism of antagonistic function of these two hormones was unclear for many years. However, identification of the rate-limiting hormone metabolism genes, such as nine- cis -epoxycarotenoid dioxygenase ( NCED ), an ABA biosynthesis gene and GA2ox , a GA deactivation gene, and intensive analysis of their regulatory mechanisms in the last decade, have provided a comprehensive picture of ABA and GA involvement in the seed dormancy mechanisms ( Seo et al., 2009 ). Now, we understand that seed response to light, which varies depending on species, is also controlled through hormone metabolism and signal transduction ( Seo et al., 2009 ). Progress in seed dormancy and germination research is well summarized in recent review articles and textbooks ( Graeber et al., 2012 ; Arc et al., 2013 ; Bewley et al., 2013 ). In this review, the main focus will be placed on the most recent discoveries from on-going research of seed dormancy and germination. Therefore, the contents of this review are not meant to be comprehensive but will highlight the “emerging” mechanisms and new hypotheses at the frontier of research.
Emerging Mechanisms of Seed Dormancy
Previously unknown seed dormancy-associated factors are emerging from on-going research, some of which enhance seed dormancy while others negatively affect it. The positive and negative regulators of seed dormancy, which will be discussed in this section, are summarized in Table 1 . There is a risk of over-simplifying gene function with the categorization of positive and negative regulators, because there are complex regulatory mechanisms of seed dormancy, in which a single gene product could exert both positive and negative effects, including negative feedback from a positive regulator. However, to highlight the discoveries of gene function in the original research, this categorization will be used for the discussion in this section.
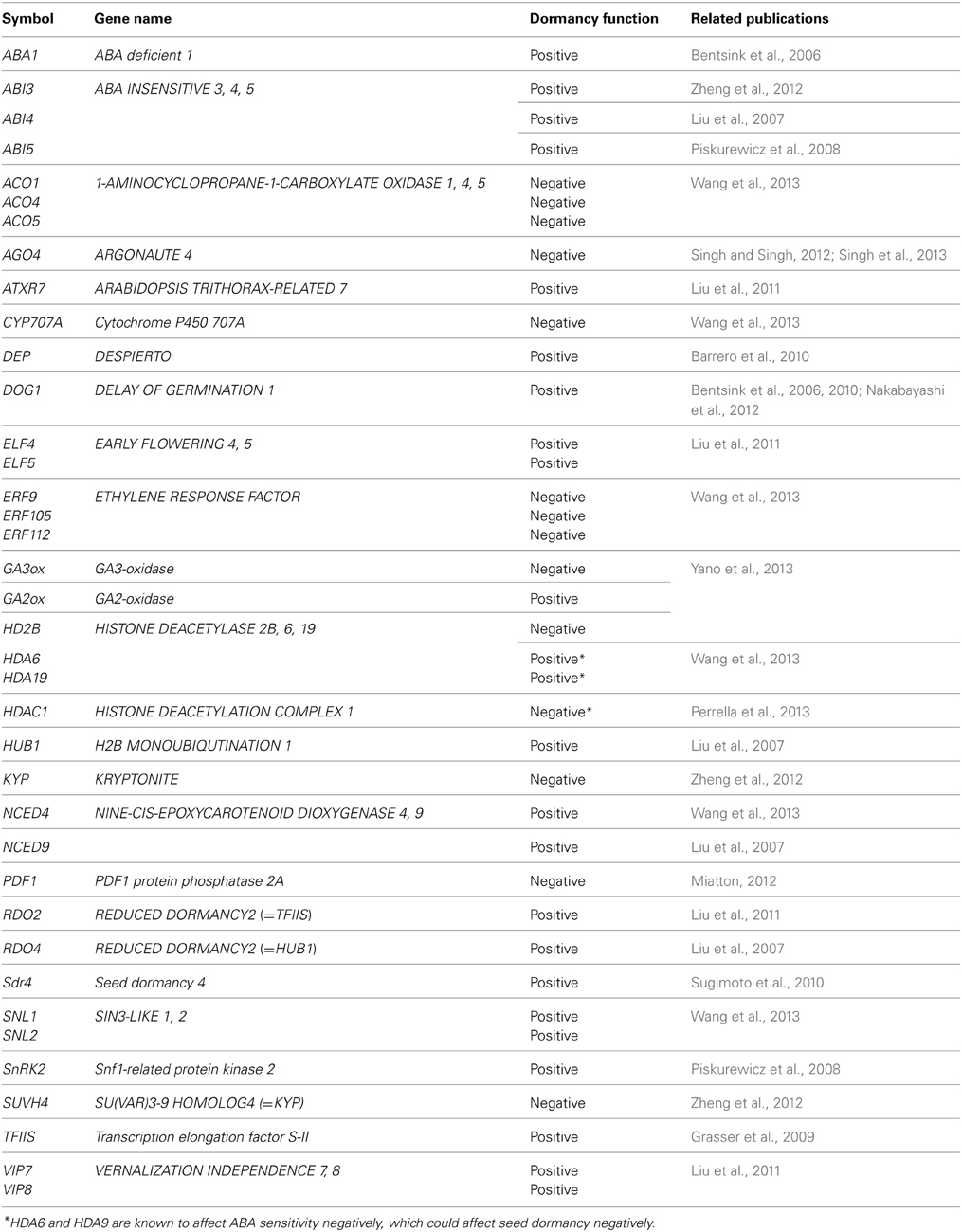
Table 1. Seed dormancy associated genes described in this article .
Positive Regulation
Dog1 –central to seed dormancy but unknown for biochemical function.
Quantitative trait locus (QTL) analysis using natural variation in Arabidopsis has identified the “seed dormancy-specific” loci, including the DELAY OF GERMINATION ( DOG ) genes ( Alonso-Blanco et al., 2003 ; Bentsink et al., 2006 , 2010 ), although some of them might not be strictly specific to dormancy ( Chiang et al., 2013 ). One of them, DOG1 has been characterized in detail. DOG1 is expressed in seeds during the maturation stage. Loss of function of DOG1 results in no dormancy ( Bentsink et al., 2006 ). The genetic role of DOG1 in seed dormancy and the significance of its expression in environment sensing and adaptation have been well documented ( Kronholm et al., 2012 ; Footitt et al., 2013 , 2014 ).
In contrast, the biochemical and molecular function of DOG1 is still a mystery. DOG1 encodes an unknown protein, for which only limited information is available. The DOG1 cDNA shows highest similarity with a Brassica napus EST from an embryo library, however this gene also is not annotated. The protein with a known function that shows the highest similarity with DOG1 is the wheat transcription factor Histone gene Binding Protein-1b (HBP-1b) ( Bentsink et al., 2006 ). HBP-1b is a leucine zipper class transcription factor, which binds to the H3 hexamer motif ACGTCA in the promoter regions of wheat histone H3 genes ( Mikami et al., 1989 ). This motif is required for transcription of the wheat H3 histone gene ( Nakayama et al., 1989 ). DOG1 has also been suggested to be a transcription factor, which is supported by its localization in the nucleus ( Nakabayashi et al., 2012 ). However, the identity between DOG1 and HBP-1b is not very high especially in the basic motifs and the heptad-repeat leucines in the leucine zipper structure ( Tabata et al., 1991 ), which are conserved in HBP-1b and other H3 hexamer-binding proteins, such as tobacco Activation Sequence Factor-1 (ASF-1) ( Lam et al., 1989 ) (Figure 1 ). Therefore, the biochemical function of DOG1 is hardly predicted from its moderate similarity to HBP-1b. So far, direct target genes of DOG1 that are clearly linked to the seed dormancy mechanisms have not been identified, although some dormancy up-(Dup) regulated genes [e.g., At5g43580 ( PR peptide ), At5g45540 ( unknown protein ), At5g45830 ( DOG1 ), At5g47160 ( YDG/SRA domain-containing protein )] or dormancy down-(Ddown) regulated genes [At4g19700 ( E3 ubiquitin ligase ), At5g04220 ( SYNAPTOTAGMIN3 ), At5g46160 ( ribosomal protein )] in the DOG1 near isogenic line (NIL) have been identified ( Bentsink et al., 2010 ).
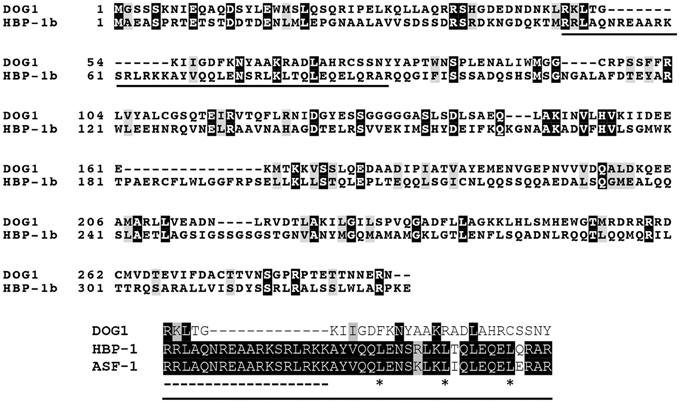
Figure 1. Alignment of Arabidopsis DOG1 , wheat HBP-1b and tobacco ASF-1. Arabidopsis DOG1 ( DELAY OF GERMINATION1 ) encodes an unknown protein, which shows some similarity to the wheat HBP-1b (Histone gene Binding Protein-1b), a leucine zipper class transcription factor ( Bentsink et al., 2006 ). However, the DOG1 protein does not show high identity to the leucine zipper domain in the HBP-1b (solid underline in the top panel). This region (extracted as the bottom panel) contains the basic motifs (dashed underline) and the heptad-repeat leucines (asterisks) in the leucine zipper structure ( Tabata et al., 1991 ), which are conserved among the wheat HBP-1b, tobacco ASF-1 (Activation Sequence Factor-1) and other leucine zipper transcription factors. Sequences were aligned using the ClustralW and boxshade programs ( http://www.expasy.org/genomics/sequence_alignment ).
Possible Modification and Partners of DOG1
DOG1 transcript accumulates during the seed maturation stage with its peak around 14–16 days after pollination (DAP) ( Bentsink et al., 2006 ), is reduced to about 20% in freshly harvested seeds, and disappears during imbibition ( Nakabayashi et al., 2012 ). DOG1 protein also accumulates during the maturation stage, however the protein level does not decrease toward the completion of seed maturation. As a consequence, freshly harvested seeds contain a relatively high level of DOG1 protein. The protein level still remains relatively high even after 13 weeks of after-ripening when seed dormancy is already released ( Nakabayashi et al., 2012 ). Thus, a correlation is lacking between the amount of DOG1 protein and dormancy levels in after-ripened seeds. It has been proposed that the chemical property of DOG1 protein, rather than its amount, is critical for DOG1 to maintain seed dormancy and that its alteration to a non-functional form during after-ripening allows seed germination ( Nakabayashi et al., 2012 ). In fact, there is a shift in the pI (isoelectric point) of the DOG1 peptides prior to and following after-ripening ( Nakabayashi et al., 2012 ).
Induction of DOG1 in imbibed dog1 mutant seeds with a heat-shock inducible system does not cause dormancy and allows 100% germination ( Nakabayashi et al., 2012 ). This can be explained by the lack of protein modification discussed above. When ABI5 , another key dormancy gene was overexpressed in Arabidopsis seeds, it was not sufficient to suppress germination. Only when the SnRK2 (Snf1-related protein kinase2), which activates ABI5, was induced in imbibed seeds, ABI5 was able to suppress seed germination ( Piskurewicz et al., 2008 ). Therefore, it is possible that the DOG1 protein induced by the heat-shock system was missing necessary modification in the ectopic induction experiment.
Recently, a search for possible DOG1 partners was conducted through a yeast two-hybrid screen, which identified multiple proteins, including the PDF1 protein phosphatase 2A ( Miatton, 2012 ). PDF1 expression is enriched in the vascular system of the embryo ( Miatton, 2012 ), which mimics the DOG1 localization ( Nakabayashi et al., 2012 ). PDF expression has its peak around 16 DAP during the maturation stage and is reduced in mature seeds, which is similar to the DOG1 expression mentioned above. Unlike the dog1 mutant, the pdf1 loss of function mutant exhibits an enhanced seed dormancy phenotype ( Miatton, 2012 ), suggesting that PDF1 is a negative regulator of seed dormancy and antagonizes DOG1 . It is hypothesized that DOG1 requires phosphorylation to be active, in terms of its function in seed dormancy induction and maintenance, and is dephosphorylated by PDF1, which could inactivate DOG1 ( Miatton, 2012 ). More analysis of PDF1 and other DOG1 -interacting proteins will potentially provide a breakthrough in seed dormancy research.
Regardless of posttranslational modification, an alternative hypothesis to explain the lack of seed dormancy in DOG1 -induced dog1 seeds is that DOG1 functions mainly during the maturation stage and the DOG1 protein contained in mature seeds might be residual. It is possible that DOG1 affects seed dormancy through its effects on ABA levels during maturation ( Nakabayashi et al., 2012 ). DOG1 has been proposed to function in a pathway independent of plant hormones. However, DOG1 is not able to impose seed dormancy in aba1-1 , an ABA-deficient mutant ( Bentsink et al., 2006 ), indicating that DOG1 function is dependent on ABA. ABA levels are reduced in dog1 mutants while GA levels are enhanced ( Bentsink et al., 2006 ; Nakabayashi et al., 2012 ), supporting the idea of possible links between the DOG1 and hormone pathways in seed dormancy. More information is necessary to obtain a clear picture about the hormone dependent and independent pathways of seed dormancy. To date, induction of DOG1 specifically at the right timing during seed maturation (14–16 DAP) has not been experimentally examined. Investigation of molecular consequences upon DOG1 induction at the right timing, including gene expression, protein phosphorylation and epigenetic changes (discussed below), will provide useful information. It should be noted that there are other dormancy(-specific) genes recently discovered, such as Seed dormancy 4 ( Sdr4 ) in rice ( Sugimoto et al., 2010 ) and DESPIERTO in Arabidopsis ( Barrero et al., 2010 ), which were not discussed here. Those genes also appear to be central to the dormancy mechanisms and are important targets of seed dormancy research.
Transcription Elongation of Seed Dormancy Genes
There is emerging evidence to suggest that regulation of transcriptional efficiency may be one of the core mechanisms of seed dormancy. Transcriptional efficiency is determined by recruitment of RNA polymerase II (Pol II) to the DNA template and the rate of transcription elongation after its binding to DNA. The efficiency of transcription elongation is influenced by an arrest of Pol II and its recovery from the arrest ( Saunders et al., 2006 ). Transcription elongation factor S-II (TFIIS) assists Pol II to overcome the temporal arrest during elongation and enhances RNA synthesis ( Kim et al., 2010 ) (Figure 2 ). A mutagenesis screen for seed dormancy in Arabidopsis yielded reduced dormancy ( rdo ) mutants ( Leon-Kloosterziel et al., 1996 ; Peeters et al., 2002 ). RDO2 , one of the genes identified from this screening, encoded TFIIS ( Liu et al., 2011 ). Another independent study also found that a mutation in TFIIS resulted in reduced seed dormancy ( Grasser et al., 2009 ). These results suggest that transcription elongation may be a critical part of the dormancy mechanisms.
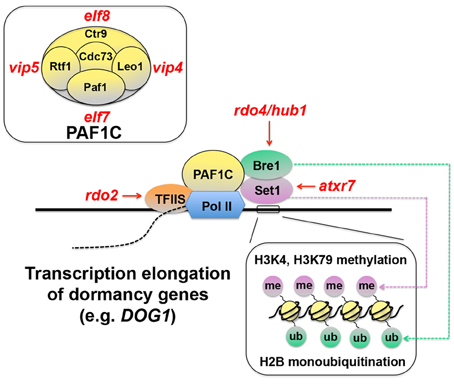
Figure 2. Schematic representation of transcription elongation of seed dormancy genes. Transcription elongation factor S-II (TFIIS) assists RNA polymerase II (Pol II) and promotes transcription elongation ( Saunders et al., 2006 ; Kim et al., 2010 ). Pol II-Associated Factor 1 Complex (PAF1C), which consists of Paf1, Rtf1, Ctr9, Leo1, and Cdc73 (top-left inset) in yeast ( Porter et al., 2005 ), also functions in this process through its interaction with Bre1, which monoubiquitinates (ub) histone 2B (H2B), and Set1, which methylates (me) histone H3 lysine 4 (H3K4), and lysine 79 (H3K79) (bottom-right inset) ( Sun and Allis, 2002 ; Zhu et al., 2005 ; Kim et al., 2009 ). These chromatin-remodeling events and their positive effects on transcription elongation are thought to be critical for induction of seed dormancy genes, because mutants in many of these components ( rdo2, rdo4, atxr7, elf 7, elf8, vip4 , and vip5 ) exhibit reduced seed dormancy ( Liu et al., 2011 ). Red italic symbols indicate Arabidopsis mutants corresponding to the yeast protein components. atxr7, arabidopsis trithorax-related 7; elf, early flowering ; hub1, h2b monoubiquitination1 ; rdo, reduced dormancy ; vip, vernalization independence .
The phenotypes of other mutants also support this contention. TFIIS and Pol II interact with the Pol II-Associated Factor 1 Complex (PAF1C) ( Kim et al., 2010 ) (Figure 2 ). In yeast, PAF1C consists of Paf1, Rtf1, Ctr9, Leo1, and Cdc73 ( Penheiter et al., 2005 ; Porter et al., 2005 ) (Figure 2 , top-left inset). The Arabidopsis orthologs of these yeast proteins EARLY FLOWERING7 (ELF7) (= Paf1), ELF8 (= Ctr9), VERNALIZATION INDEPENDENCE4 (VIP4) (=Leo1), VIP5 (= Rtf1) and PLANT HOMOLOGOUS TO PARAFIBROMIN (PHP) (= Cdc73) have been identified ( Zhang and Van Nocker, 2002 ; He et al., 2004 ; Oh et al., 2004 ; Yu and Michaels, 2010 ). Seeds of the elf7, elf8, vip4 , and vip5 mutants all exhibit reduced dormancy ( Liu et al., 2011 ), suggesting the importance of PAF1C and transcription elongation for seed dormancy.
Histone Ubiquitination and Methylation Associated with Transcription Elongation
PAF1C interacts with Bre1, a protein involved in histone 2B (H2B) monoubiquitination ( Kim et al., 2009 ) (Figure 2 ). Interestingly, rdo4 , another reduced dormancy mutant in Arabidopsis, which was isolated from the same mutagenesis screening as mentioned above, has a mutation in H2B MONOUBIQUITINATION1 ( HUB1 ) gene, an Arabidopsis ortholog of Bre1 ( Liu et al., 2007 ). Bre1 interacts with Set1, which methylates histone 3 lysine 4 and lysine 79 (H3K4, H3K79) ( Sun and Allis, 2002 ; Zhu et al., 2005 ) and promotes gene expression (Figure 2 ). A mutation in the Set1 ortholog ARABIDOPSIS TRITHORAX-RELATED 7 ( ATXR7 ) also causes reduced dormancy in seeds ( Liu et al., 2011 ). These results reinforce the idea that regulation of transcription elongation efficiency is an essential part of seed dormancy and suggest the significance of chromatin remodeling in the regulatory mechanisms.
H2B monoubiquitination and H3K4 and H3K79 methylation, which is dependent on H2B monoubiquitination ( Nakanishi et al., 2009 ), are thought to activate gene expression ( Henry et al., 2003 ). Since hub1 (= bre1 ) seeds exhibit reduced dormancy, genes down-regulated in the hub1 mutant are good candidates for seed dormancy-imposing genes, the expression of which is promoted through transcriptional elongation. ABA INSENSITIIVE4 ( ABI4 ), DOG1, NINE-CIS-EPOXYCAROTENOID DIOXYGENASE9 ( NCED9 ) and other genes have been identified as possible targets of HUB1/RDO4 ( Liu et al., 2007 ). RDO2 ( TFIIS ) and RDO4 ( HUB1 ), two positive regulators of transcription are induced during the same stages of seed maturation (~18–19 DAP). There is a significant overlap between rdo2 and rdo4 , in terms of differentially expressed genes in the mutants. These results suggest that RDO2 and RDO4 might share common targets. Intriguingly, DOG1 is one of the genes commonly down-regulated in the two mutants ( Liu et al., 2011 ). Activation of DOG1 through chromatin remodeling and transcriptional elongation might be an important mechanism of seed dormancy.
The hypothesis that seed dormancy is regulated by the efficiency of transcription elongation of DOG1 is also supported by the recent analysis of the tfIIs mutant, in which seed dormancy is reduced but reverted to the wild-type level by an extra copy of DOG1 ( Mortensen and Grasser, 2014 ). However, when the hub1/rdo4 mutant is crossed with the NIL carrying DOG1 -Cvi, which causes deep seed dormancy, the resulting seeds still show dormancy at a level between hub1 and DOG1 -Cvi NIL. Similar results are observed when the hub1/rdo4 was transformed with the Cvi DOG1 genomic fragment. The incomplete alleviation of dormancy from NIL DOG1 by hub1/rdo4 mutation in both cases suggests that HUB1 is not epistatic to DOG1. In contrast, the combination of hub1 and DOG3 -Cvi resulted in no seed dormancy, suggesting that HUB1 functions in the same pathway as DOG3 to affect seed dormancy ( Liu et al., 2007 ). More analyses of the specific targets of epigenetic modification and transcriptional elongation will be necessary to draw a clear picture about seed dormancy regulation through these processes.
Repression of Seed Germination Genes Through Histone Deacetylation
While activation of dormancy genes through transcription elongation appears to be critical for dormancy induction, continuous repression of seed germination-associated genes is also probably an essential part of dormancy maintenance. There is evidence that histone deacetylation is imperative for repression of genes positively affecting seed germination. In yeast and mammals, histone deacetylase (HDAC) interacts with SWI-INDEPENDENT3 (SIN3), an amphipathic helix repeat protein, removes acetyl groups from lysine in the histone tails, and creates a transcriptionally inactive state of chromatin ( Kadosh and Struhl, 1998 ; Lai et al., 2001 ; Grzenda et al., 2009 ) (Figure 3 ). In Arabidopsis, SIN3-LIKE1 (SNL1) physically interacts with HDA19, an Arabidopsis HDAC ortholog, both in vitro and in planta ( Wang et al., 2013 ). The Arabidopsis genome contains SNL2 , which is partially redundant to SNL1 . Seeds of the snl1 snl2 double mutant exhibit reduced dormancy. A reduced dormancy phenotype is also observed in hda19 mutant seeds ( Wang et al., 2013 ). These results indicate that SNLs and HDA19 are positive regulators of seed dormancy. It appears that proper repression of the SNL-HDA19 targets, which are most likely germination-inducing genes, through histone deacetylation is essential for normal seed dormancy. Acetylation of H3K9/18 and H3K14 is increased in the snl1 snl2 double mutant ( Wang et al., 2013 ), which confirms that in wild-type seeds the SIN3-HDAC complex deacetylates histones and puts repressive marks on the chromatin ( Richon and O'Brien, 2002 ) (Figure 3 ).
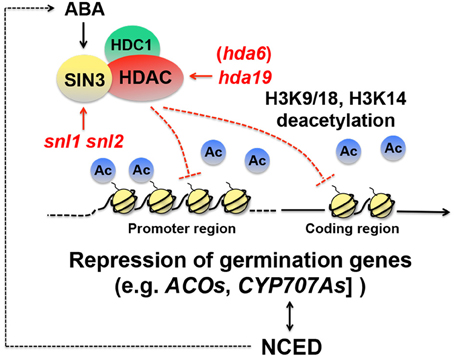
Figure 3. Schematic representation of repression of seed germination genes through histone deacetylation. In yeast, a histone deacetylase (HDAC) interacts with SWI-INDEPENDENT3 (SIN3) ( Kadosh and Struhl, 1998 ; Lai et al., 2001 ; Grzenda et al., 2009 ). HDA19, an HDAC ortholog in Arabidopsis, interacts with SIN3-LIKEs (SNLs) ( Wang et al., 2013 ) and HDC1 (Histone Deacetylation Complex1) ( Perrella et al., 2013 ), and removes acetyl groups (Ac) from histone 3 lysine9/18 (H3K9/18) and lysine14 (H3K14) and represses genes positively affecting germination, such as 1-AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASEs ( ACOs ) and CYP707As , ABA deactivation genes ( Wang et al., 2013 ). Deacetylation occurs in both the promoter and coding regions. Both snl and hda19 mutations cause reduced dormancy ( Wang et al., 2013 ). Expression of NCED4 , an ABA biosynthesis gene, is reduced in the snl1 snl 2 double mutant, suggesting that the SNL-HDA19 complex imposes seed dormancy also through the promotion of ABA biosynthesis. SNL expression is promoted by ABA ( Wang et al., 2013 ), which suggests that there is a positive feedback loop to maintain high ABA levels through the SNL-HDA19 pathway.
Global gene expression analysis between the snl1 snl2 double mutant and wild-type seeds with RNA sequencing identified possible targets of SNL-HDA19. Ethylene biosynthesis genes, such as 1-AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASE1 ( ACO1 ), ACO4 , and ACO5 and ethylene response genes, such as ETHYLENE RESPONSE FACTOR9 ( ERF9 ), ERF105 , and ERF112 , were up-regulated in the mutant ( Wang et al., 2013 ). Quantitative PCR combined with chromatin immunoprecipitation with the H3K9/18 acetylation-specific antibodies showed that the ACOs and ERFs genes were indeed hyperacetylated in the mutant, which mainly occurred in the promoter region but were also found in the coding region ( Wang et al., 2013 ). These results suggest that SNL-HDA19 causes seed dormancy by suppressing the ethylene pathway, which positively affects seed germination in Arabidopsis ( Chiwocha et al., 2005 ; Arc et al., 2013 ).
In contrast, the same study suggests that SNL-HDA19 increases ABA levels and thereby enhances seed dormancy. CYP707A1 and CYP707A2 , ABA deactivation genes, which reduce ABA levels, were up-regulated in the snl1 snl2 double mutants. Consistently, NCED4 , an ABA biosynthesis gene, was down-regulated in the same mutant ( Wang et al., 2013 ). These results suggest that SNL-HDA19 suppresses CYP707As and activates NCED4 in wild type, both of which increase ABA levels and enhance seed dormancy. Interestingly, ABA stimulates SNL1 and SNL2 expression ( Wang et al., 2013 ), which suggests that there is positive feedback regulation to maintain high levels of ABA through the histone deacetylation pathway (Figure 3 ). While this study suggests that ABA levels are positively affected by SNL-HDA19, other studies suggest that ABA sensitivity is negatively regulated by HDA19 (and HDA6). Mutations in HDA6 and HDA19 cause ABA hypersensitivity during germination ( Chen et al., 2010 ; Chen and Wu, 2010 ). Loss of function in Histone Deacetylation Complex1 (HDC1), another component of the SNL- and HDA19-containing complex, which physically interacts with HDA6 and HDA19 (Figure 3 ), also causes ABA hypersensitivity in seedlings. HDC1 overexpression promotes seedling emergence ( Perrella et al., 2013 ), although detailed information about sensu stricto germination and a dormancy phenotype of the mutant seeds is not available. The significance of the opposite effects of the HDAC multiprotein complex to ABA levels (positive) and sensitivity (negative) in the regulatory mechanisms of seed dormancy is not known. It is possible that the seemingly counterintuitive effects are associated with negative feedback regulation.
Negative Regulation
Repression of dormancy genes and activation of germination genes through histone deacetylation.
HISTONE DEACETYLASE 2B ( HD2B ), another HDAC gene, is also involved in seed dormancy. In this case, it negatively affects seed dormancy ( Yano et al., 2013 ). This discovery was made through a combination of genome-wide association mapping (GWA) ( Atwell et al., 2010 ) and transcriptomics. The efficiency of QTL analysis using different accessions of Arabidopsis, such as Cvi, L er , and Col, for seed dormancy is well exemplified by the successful identification and characterization of the DOG genes ( Alonso-Blanco et al., 2003 ; Bentsink et al., 2006 , 2010 ). Since the comparison of a few different Arabidopsis accessions is so powerful, multiplying this approach using many accessions with natural variations in seed dormancy is expected to produce fruitful outcomes in seed dormancy research, especially when it is combined with GWA, which identified a number of single nucleotide polymorphisms (SNPs) likely associated with various phenotypes ( Atwell et al., 2010 ). Based on this concept, 113 accessions were analyzed to identify SNPs associated with natural variation in seed dormancy using GWA and transcriptomics, which identified HD2B as a strong candidate of a seed dormancy-associated gene. HD2B expression levels are significantly lower in 24 dormant accessions than 28 less-dormant accessions, although there are some exceptions. When the highly dormant Cvi line was transformed with the genomic fragment of Col HD2B (termed Col HD2B /Cvi), mature seeds of Col HD2B /Cvi exhibited reduced dormancy, which was not evident immediately after harvest without cold stratification but became clear when seeds were stratified or partially after-ripened ( Yano et al., 2013 ).
Cold stratification releases seed dormancy through an increase in GA levels. GA3ox1 , a rate-limiting GA biosynthesis gene, is induced by cold stratification ( Yamauchi et al., 2004 ), which triggers expansion of cortex cells in the radicle/hypocotyl region and then generates growth potential of the embryo for germination ( Ogawa et al., 2003 ). Evidence suggests that HD2B mediates this dormancy-releasing process. In Col HD2B /Cvi seeds, expression of GA3ox1 and GA3ox2 and GA 4 levels are increased, while expression of GA2ox2 , a GA deactivation gene, is reduced compared to wild-type Cvi seeds ( Yano et al., 2013 ). Since HDAC represses gene expression through histone deacetylation, GA2ox2 repression could be a direct effect of HD2B. In contrast, the up-regulation of GA3ox genes may be through repression of their upstream regulators or some other mechanisms. It is interesting that the three separate hormone pathways (ethylene, ABA, and GA) associated with seed dormancy are regulated by histone deacetylation. These results demonstrate that epigenetic regulation through chromatin remodeling is a robust mechanism to alter hormone levels in seeds.
Silencing of Seed Dormancy Genes Through Histone and DNA Methylation
The studies mentioned above showed that HDAC could affect seed dormancy either positively (HDA19) or negatively (HD2B), depending on the target genes. Histone methylation also affects seed dormancy in both ways. While H3K4 and H3K79 methylation activates gene expression and causes seed dormancy as mentioned above (Set1 or ATXR7), dimethylation of H3K9 (H3K9me2), a repressive mark, occurs on the chromatin associated with seed dormancy genes. Analysis of gene silencing at the Arabidopsis SUPERMAN ( SUP ) locus identified the KRYPTONITE (KYP) methyltransferase, which causes H3K9me2 (Figure 4 ). The methylated histone recruits the DNA methyltrasferase CHROMOMETHYLASE3 (CMT3) through its interaction with HETEROCHROMATIN PROTEIN1 (HP1) and triggers the methylation of cytosine nucleotides of DNA and silences the gene ( Jackson et al., 2002 ; Johnson et al., 2007 ) (Figure 4 ). KYP is SU(VAR)3-9 ( Rea et al., 2000 ) HOMOLOG 4 and is also called SUVH4. The kyp-2 mutant seeds show enhanced dormancy, suggesting that KYP/SUVH4 suppresses seed dormancy genes. Interestingly, again, DOG1 is one of the up-regulated genes in the mutant, as well as ABI3 ( Zheng et al., 2012 ). These results suggest that histone methylation caused by KYP/SUVH4 induces silencing of DOG1 and ABI3 through DNA methylation and negatively affects seed dormancy.
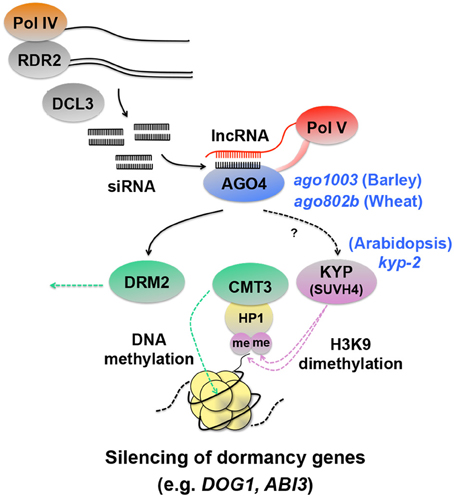
Figure 4. Schematic representation of silencing of dormancy genes through histone and DNA methylation. RNA polymerase IV (Pol IV) transcripts are converted to double-stranded RNA by RNA-Dependent RNA polymerase 2 (RDR2), which are then processed into 24-nt siRNAs by DICER-LIKE3 (DCL3) ( Xie et al., 2004 ; Herr et al., 2005 ; Onodera et al., 2005 ; Law et al., 2011 ). siRNAs are loaded onto ARGONAUTE4 (AGO4) ( Qi et al., 2006 ) and interact with long non-coding RNAs (lncRNAs) produced by Pol V, which are thought to function as scaffold transcripts to guide siRNAs to specific loci to be silenced ( Wierzbicki et al., 2008 , 2009 ; Wierzbicki, 2012 ). In this way, the AGO4 complex containing siRNAs and lncRNAs triggers RNA-directed DNA methylation (RdDM) ( Wierzbicki, 2012 ). A possible event downstream of AGO4 is histone 3 lysine 9 dimethylation (H3K9me2) by the KRYPTONITE (KYP), which causes HETEROCHROMATIN PROTEIN1 (HP1) to bind to the modified histone and recruit CHROMOMETHYLASE3 (CMT3), a DNA methyltransferase that induces gene silencing ( Jackson et al., 2002 ; Zilberman et al., 2004 ; Tran et al., 2005 ; Johnson et al., 2007 ). A mutation in KYP in Arabidopsis causes enhanced dormancy and up-regulation of DOG1 and ABI3 ( Zheng et al., 2012 ), suggesting that the seed dormancy genes are silenced by the KYP-CMT3 pathway. The AGO4 complex is also involved in gene silencing by DOMAINS REARRANGED METHYLTRANSFERASE2 (DRM2) ( Zilberman et al., 2004 ; Wierzbicki, 2012 ), although DRM2 involvement in seed dormancy regulation is not known. Direct evidence for siRNAs and lncRNAs involvement in DOG1 and ABI3 regulation is lacking, however AGO4 has been shown to be a negative regulator of dormancy in barley and wheat seeds ( Singh and Singh, 2012 ; Singh et al., 2013 ). The Arabidopsis, barley and wheat seed dormancy mutants corresponding to the protein components in the RdDM pathway are indicated by blue italic symbols.
The KYP-CMT3 gene-silencing pathway mediates RNA-directed DNA methylation (RdDM), which is triggered by small interfering RNAs (siRNAs) produced by DICER-LIKE3 (DCL3) and their loading onto ARGONAUTE4 (AGO4) ( Zilberman et al., 2004 ; Tran et al., 2005 ) (Figure 4 ). AGO proteins are components of the RNA-induced silencing complex (RISC) and are involved in gene silencing. While AGO1 and AGO10 proteins function mainly in posttranscriptional gene silencing (PTGS) through the MIR (microRNA) and TAS (trans-acting siRNA) pathways, the AGO4/AGO6/AGO9 clade proteins are associated with transcriptional gene silencing (TGS) through RdDM ( Mallory and Vaucheret, 2010 ). Little information is available for silencing of seed dormancy genes through RdDM, however a possible involvement of AGO4 in seed dormancy regulation has been suggested from studies of cereal seed dormancy. AGO1003 , an ARGONAUTE ( AGO ) 4_9 gene in barley, is expressed differentially in the embryos of dormant and non-dormant grains and is thought to function as a negative regulator of seed dormancy through RdDM ( Singh and Singh, 2012 ). A separate study in wheat supports this hypothesis. AGO802B , a wheat ortholog of AGO4_9 gene is expressed during grain development (5–20 DAP). AGO802B expression is significantly lower in preharvest sprouting (PHS)-resistant (i.e., more dormant) varieties than in susceptible ones ( Singh et al., 2013 ). This result also suggests that AGO4 is a negative regulator of dormancy. It is not known whether specific coding genes are subjected to silencing through RdDM in wheat seeds. However, analysis of 5S ribosomal DNA from PHS-resistant and susceptible varieties with the methylation-sensitive restriction enzyme Msp I suggested that ribosomal DNA methylation was reduced in PHS-resistant varieties ( Singh et al., 2013 ), supporting the hypothesis that AGO4 enhances histone and DNA methylation and acts as a negative regulator of seed dormancy.
The chromatin-remodeling factors mentioned above include both positive and negative regulators of seed dormancy, which could be considered as negative and positive regulators of seed germination, respectively. The description “activation of seed dormancy genes” or “repression of seed germination genes,” which could mean the same consequence (dormancy or no germination), is confusing. It is even more confusing when the description is combined with different terminology of histone modification, such as histone (de)acetylation, monoubiquitination or (de)methylation, because they could be either repressive or active marks depending on the position of residues in the histone tail. To avoid the confusion, the positive and negative regulators of seed dormancy, their roles in chromatin and DNA modification, and possible consequences in gene expression downstream are summarized in Figure 5 .
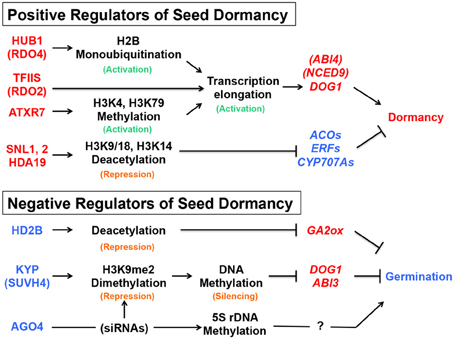
Figure 5. Summary of the seed dormancy or germination pathways described in this article. The positive (red) and negative (blue) regulators of seed dormancy and their roles in the chromatin remodeling, DNA modification or siRNA pathways are indicated, together with promotive (arrows) or suppressive (blocked arrows) effects on the downstream genes (italics). Active (green) or repressive (orange) marks on histones or DNA are also indicated. See text for gene and protein symbols and references.
New Hypotheses for Germination Events
Remaining barriers of seed germination.
A quiescent state of the embryo is changed when molecular repression on seed germination genes is removed, which is probably orchestrated with silencing of dormancy genes. However, an active embryo is still unable to complete germination when the suppressive force, or mechanical resistance, of the covering tissues, such as the testa and endosperm, exceeds embryo growth potential. When the embryo is not dormant, it is the mechanical resistance of the covering tissues that mainly determines whether the embryo emerges from imbibed seeds. In fact, the embryos in dormant seeds in many species are able to grow when they are excised from seeds, which is called coat-imposed dormancy ( Bewley et al., 2013 ). While further increase in embryo growth potential may still be necessary, alteration of the properties of covering tissues plays a significant role in germination. The testa in a mature seed is generally a non-living tissue, therefore the major reduction in the mechanical resistance of the covering tissues depends on physiological changes in the living endosperm. Changes in the properties of the endosperm significantly affect timing of radicle emergence in non-dormant seeds also. Therefore, the mechanisms of endosperm weakening have been a focal point in seed germination research.
Basic information about endosperm weakening is summarized in other literature ( Linkies et al., 2010 ; Bewley et al., 2013 ). Briefly, the micropylar region of endosperm (ME) surrounds the radicle tip and provides an opposing force to it (Figure 6 ), which is reduced during germination through weakening. The mechanical resistance of ME is mainly due to the thick and rigid cell walls in this tissue. Therefore, cell wall modification is thought to play an essential role in ME weakening ( Bewley et al., 2013 ). In fact, genes encoding cell wall-modifying proteins, such as xyloglucan endotransglycosylase/hydrolases (XTHs) and expansins (EXPs), are expressed exclusively in ME of Arabidopsis ( Dekkers et al., 2013 ), Lepidium sativum ( Voegele et al., 2011 ) and tomato ( Chen and Bradford, 2000 ; Chen et al., 2002 ) seeds during germination. While distinct cell wall architecture is observed in ME of seeds depending on plant species and family ( Lee et al., 2012a ), ME weakening by cell wall modifying proteins seems to be a widely conserved mechanism of germination.
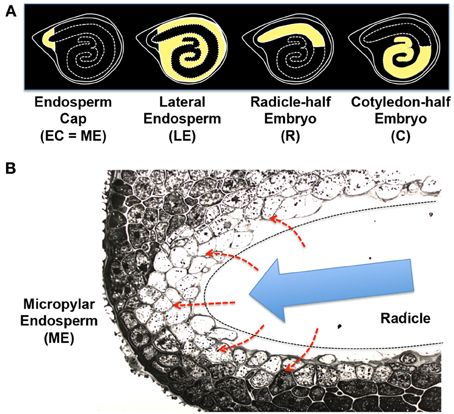
Figure 6. Tomato seed tissues and regulatory mechanisms of endosperm weakening. (A) Schematic representation of the four different tissue parts of tomato seeds used for the transcriptome analysis ( Martinez-Andujar et al., 2012 ). Endosperm cap [EC = ME (micropylar endosperm)], lateral endosperm (LE), radicle-half embryo (R), and cotyledon-half embryo (C) are highlighted by yellow filling. (B) Photograph of micropylar endosperm (ME) cells. The radicle tip (outlined by dashed line) has been removed. Note that cell walls are fuzzy and protein storage vacuoles and lipid bodies are disappearing in the inner layers of ME cells facing the embryo ( Nonogaki et al., 1998 ). Possible secretion of GA or peptides from the embryo to the endosperm (red dashed arrows) and the growth potential generated by the embryo (blue arrow), which provides pressure onto the endosperm, are indicated in the scheme.
Embryo-Endosperm Interaction in Tomato Seeds
A high throughput transcriptome analysis of germinating tomato seeds showed enrichment of cell wall-associated genes in ME ( Martinez-Andujar et al., 2012 ), supporting the hypothesis discussed above. In this study, tomato seeds were dissected into the endosperm cap (EC, equivalent to ME), lateral endosperm (LE), radicle-half embryo (R), and cotyledon-half embryo (C) (Figure 6A ). In addition to the cell wall-associated genes, PR (pathogenesis-related) or wound-response genes were detected as another major group of ME-enriched genes. The 5' upstream sequences of the ME-enriched PR genes contain the conserved sequences, including the DNA motifs targeted by ethylene response factors (ERFs). Interestingly, Tomato ERF1 ( TERF1 ), an experimentally validated upstream regulator of the PR genes, was also one of the ME-enriched genes in tomato seeds ( Martinez-Andujar et al., 2012 ). These results suggest that TERF1 is a major upstream regulator in ME and induces other ME genes, such as PR- or wound response genes and possibly cell wall-associated genes also.
The degradation of cell wall in ME of tomato seeds, which is accompanied by disappearance of storage vacuoles and lipid bodies from the cells, is initiated at the inner cells adjacent to the radicle tips (Figure 6B ), suggesting that ME activation is under the control of the embryo. A traditional view of the mechanism of ME gene induction is that diffusible signals, such as GA, or non-diffusible signals, such as peptide ligands, are secreted from the embryo to ME (Figure 6B , red dashed arrows) and then stimulate gene expression in this tissue. However, the new finding about the TERF1 cascade and possible involvement of a PR- or wounding response in ME gene expression generated a new hypothesis of “mechanosensing.” In this hypothesis, pressure, rather than chemical molecules, which is generated by the embryo and placed onto ME cells (Figure 6B , blue arrow), triggers a wound response, TERF1 expression, and then induction of the downstream genes in ME.
The “Touch” Genes in Arabidopsis Seeds
A similar but more comprehensive and dynamic transcriptomic analysis in Arabidopsis seeds provided supporting evidence for the mechanosensing hypothesis. It is technically difficult to dissect ME from Arabidopsis seeds. Therefore, in this study gene expression was compared for the micropylar and charazal endosperm (MCE), peripheral endosperm (PE, similar to LE), radicle (RAD), and cotyledons (COT) ( Dekkers et al., 2013 ) (Figure 7A ). The high-resolution data set included many time points including those before and after testa rupture (TR) and endosperm rupture (ER), which are the signature events during germination and at the completion of germination, respectively (Figure 7B ). This study demonstrated that TR was marked by activation of the specific genes in MCE, such as TOUCH3 and TOUCH4 , which are known to be induced by touch or thigmotropism ( Braam, 2005 ). The comparison of MCE genes at TR in Arabidopsis seeds with the genes up-regulated by touching the aerial part of Arabidopsis plants ( Lee et al., 2005 ) showed significant overlaps. These results suggest that ME gene induction in Arabidopsis seeds is also caused by touch or mechanosensing ( Dekkers et al., 2013 ).
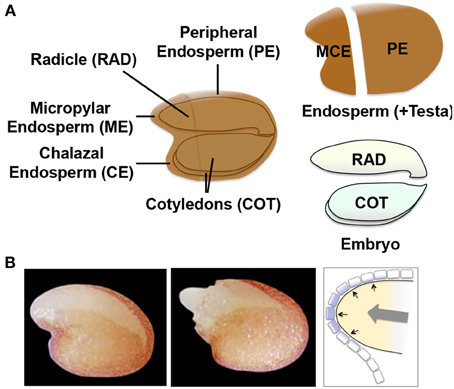
Figure 7. Arabidopsis seed tissues and regulatory mechanisms of endosperm weakening. (A) Schematic representation of the four different tissue parts of Arabidopsis seeds used for the transcriptome analysis ( Dekkers et al., 2013 ). The micropylar (ME) plus charazal (CE) endosperm (MCE), peripheral endosperm (PE, similar to LE), radicle (RAD) and cotyledons (COT) are indicated in the scheme. (B) Photographs of Arabidopsis seeds at testa rupture (TR, left) and endosperm rupture (ER, right). Schematic representation of ME is shown to the right with the growth potential of the embryo (gray arrow) and pressure (black arrows) placed onto the single cell layer of endosperm (with purple filling).
No conclusive evidence has been obtained to date for the mechanosensing or touch hypothesis. However, the new findings have great potential to re-draw the traditional view of ME gene regulation, which is a core mechanism of germination. It is well known that GA stimulates ME gene expression in the GA-deficient gib-1 tomato seeds, which absolutely require GA for radicle emergence ( Groot and Karssen, 1987 ; Nonogaki et al., 2000 ). The GA requirement for ME gene expression can be substituted by co-incubation of ME with the embryonic axes, suggesting that the embryo produces GA and secretes it to the endosperm ( Groot and Karssen, 1987 ). There seems to be no doubt that ME gene expression is under the control of GA and the embryo. However, it should be noted that exogenous GA stimulates gene expression in both ME and LE when tomato seeds are dissected, while only ME is responsive when GA is applied to intact seeds ( Martinez-Andujar et al., 2012 ). This raises the question as to why LE in an intact seed remains unaffected by GA or why only ME is responsive to it? The new hypothesis (mechanosensing or touch) could answer these questions. If the GA-dependent embryonic effects on ME gene expression are not directly exerted through chemical secretion but are indirectly mediated by the pressure provided by the radicle tip, the highly localized gene expression in ME, which is in close contact with the radicle tip, could be explained. Since generation of embryo growth potential, which causes the pressure onto ME, is dependent on GA ( Ni and Bradford, 1993 ; Yamaguchi et al., 2001 ), the concept of pressure-triggered stimulation of ME gene expression is well integrated with the traditional concept (and evidence) of GA- and embryo dependency of ME gene expression. While the possibility of direct stimulation of ME by GA or insoluble secondary messengers should not be excluded, the recent data sets provided the new concept for embryo-endosperm interaction and opened the next phase of seed germination research.
Perspectives for Basic Research and Knowledge Translation
More discoveries expected through epigenetic study.
A number of discoveries were made in the recent studies of seed dormancy and germination. More significant discoveries will probably be made from epigenetic studies of seed dormancy and germination over the next few years. While bioinformatics and systems biology could generate new hypotheses, the exciting discoveries happening from characterization of seed dormancy mutants look very convincing and promising. Exploring these emerging mechanisms with forward genetics and biochemical and molecular approaches will result in more progress in seed dormancy research. The information obtained from individual mutants of chromatin remodeling was assembled into several schemes in this article to provide an overview of the frontier of this field. However, information to connect each component precisely in the schemes is still missing. For example, while histone methylation and subsequent silencing of DOG1 by DNA methylation seems likely, contribution of DCL3, AGO4, and RdDM to the DOG1 -dependent dormancy pathway is not clear (Figure 4 ). It is possible that siRNAs and long non-coding RNAs (lncRNAs), including antisense transcripts ( Yamada et al., 2003 ; Liu et al., 2010 ; Sun et al., 2013 ), are involved in repression of key dormancy genes. Recent studies suggest that the Polycomb Repressive Complex (PRC), which is involved in histone methylation and gene silencing, also targets DOG1 ( Bouyer et al., 2011 ; Muller et al., 2012 ; Molitor et al., 2014 ). This is very interesting because PRC is known to mediate gene silencing triggered by expression of long non-coding RNA, at least in the case of the flowering gene FLOWERING LOCUS C ( Swiezewski et al., 2009 ; De Lucia and Dean, 2011 ; Heo and Sung, 2011 ). It is possible that some dormancy genes are regulated through the lncRNA-PRC pathway (Figure 8 ), which could maintain dormancy genes “dormant.” Missing information in the current schemes of regulatory mechanisms of seed dormancy and germination genes might already be emerging from other epigenetic studies. In addition, the current schemes, which seem to be separate pathways, could be combined with each other and integrated into a single comprehensive scheme, through more discoveries. The crosstalk between the histone deacetylation and DNA methylation pathways is known ( To et al., 2011 ; Kim et al., 2012 ), however little is known about their interaction directly linked to the seed dormancy mechanisms. This might be one of the areas in which the major discoveries could be made in the future.
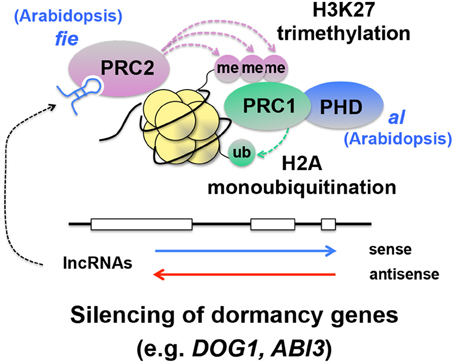
Figure 8. Hypothetical integration of the known lncRNA-PRC pathway into the silencing mechanisms of seed dormancy genes. In this scheme, long non-coding RNAs (lncRNAs) ( Swiezewski et al., 2009 ; Heo and Sung, 2011 ), interact with Polycomb Repressive Complex 2 (PRC2), which causes histone 3 lysine 27 trimethylation (H3K27me3) ( Simon and Kingston, 2009 ; De Lucia and Dean, 2011 ). This histone modification recruits PRC1, which monoubiquitinates H2A ( Simon and Kingston, 2009 ). While H2B monoubiquitination promotes transcription elongation (see Figure 2 ), H2A monoubiquitination is thought to be a repressive mark and silence genes ( Simon and Kingston, 2009 ). A mutation in FERTILIZATION INDEPENDENT ENDOSPERM ( FIE ), an essential component of PRC2, causes enhanced dormancy ( Bouyer et al., 2011 ), supporting the idea that PRC suppresses dormancy genes and promotes germination. A mutation in ALFIN1-like (AL), a Plant Homeo Domain (PHD) finger that interacts with PRC1, also promotes dormancy ( Molitor et al., 2014 ). Evidence has not been obtained for the involvement of specific lncRNAs in suppression of dormancy genes through PRC.
Knowledge Translation of Seed Hormone Biology
The topic of hormonal regulation of seed dormancy, such as the regulation of ABA or GA biosynthesis and deactivation enzymes by the environmental signals (e.g., light and temperature), was minimized in the discussion above, because it is well summarized elsewhere ( Finkelstein et al., 2008 ; Seo et al., 2009 ) and this article focuses on emerging mechanisms and new hypotheses. Nonetheless, this is probably the area of seed biology that has been most advanced in the last decade, and from a knowledge translation point of view, this area has the greatest potential for agricultural application. For example, identification of the rate-limiting ABA biosynthesis gene NCED advanced our understanding of thermoinhibition of lettuce seed germination, which is a critical issue in agriculture. Now, we understand that thermoinhibition of germination at high temperature, which could induce secondary dormancy, is caused by NCED expression ( Argyris et al., 2008 , 2011 ). Likewise, screening of wheat populations for mutations in ABA 8'-hydroxyase, an ABA deactivation enzyme, has successfully identified the genetic lines, which are potentially resistant to PHS, another serious issue in agriculture ( Chono et al., 2013 ). A separate screen for a mutation in the ENHANCED RESPONSE to ABA ( ERA ) gene also isolated PHS-resistant wheat lines ( Schramm et al., 2013 ). The information about MOTHER OF FT AND TFL1 ( MFT ) gene, which is a recently identified member of the ABA and GA signaling pathways in Arabidopsis ( Xi et al., 2010 ), has already been translated into wheat ( Nakamura et al., 2011 ; Lei et al., 2013 ; Liu et al., 2013 ).
More progressive efforts are being made to translate seed hormone biology. It has been demonstrated that direct manipulation of the rate-limiting enzymes in the hormone metabolism pathways can successfully be used to alter seed performance. Silencing NCED with RNA interference can promote germination in lettuce seeds ( Huo et al., 2013 ). In contrast, chemical induction of NCED , a single gene, was sufficient to suppress precocious germination in Arabidopsis, which can also be applied to PHS prevention in cereal crops ( Martinez-Andujar et al., 2011 ). While the latter approach was tested in the model system Arabidopsis, the gene induction experiments in this study were performed with the chemical ligand that has been approved for field application by the U.S. Environmental Protection Agency, making the principle applicable to agriculture. Even more advanced system of NECD enhancement, which does not require chemical application, has been established recently, using a positive feedback mechanism. In this system, a chimeric NCED gene, which is designed to trigger positive feedback regulation, amplifies ABA biosynthesis and signaling in seeds and causes hyperdormancy in a spontaneous manner ( Nonogaki et al., 2014 ). This positive feedback system was created based on the mechanisms emerged from, and the comprehensive understanding established by, the past research on the ABA metabolism and signaling pathway in seeds. The translational research unexpectedly revealed that a positive feedback mechanism is also present in the native system of NCED expression in seeds ( Nonogaki et al., 2014 ), demonstrating the synergy between basic and translational research. Other positive feedback mechanisms in the hormonal regulation of seed dormancy and germination are also emerging from on-going discoveries (summarized in Figure 9 ). More findings and understanding of elegant pathways in nature will provide greater opportunities of knowledge translation, another frontier of research that should be expanded in the future.
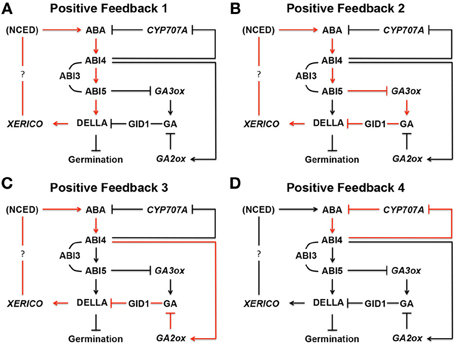
Figure 9. Positive feedback loops in ABA biosynthesis in seeds. (A) In Positive Feedback 1, ABA produced by NCED, a rate-limiting ABA biosynthesis enzyme, induces ABIs. ABI3, and ABI5 interacts with each other while ABI4 induces ABI5 by binding its promoter region. ABI5 binds to the promoter region of a DELLA gene, such as RGL2 , and up-regulates its expression. DELLA then promotes expression of XERICO , which increases ABA biosynthesis through unknown mechanism(s). In this way, the originally produced ABA in seeds enhances ABA biosynthesis through positive feedback. (B) In Positive Feedback 2, ABI5 down-regulates GA3ox , a GA biosynthesis gene, and reduces GA and GA response by GID1, a GA receptor. Reduced GA levels stabilize DELLA protein, such as RGL2, and increases ABA biosynthesis through XERICO , as described above. (C) In Positive Feedback 3, ABI4 up-regulates GA2ox , a GA deactivation gene, resulting in the same outcome as Positive Feedback 2. (D) ABI4 down regulates CYP707A , an ABA deactivation gene. Therefore, ABA starts to accumulate in seeds, which further enhances the same pathway through positive feedback. In these schemes, many other components, which may be participating in the pathways, and negative feedback loops are omitted. ABI, ABA INSENSITIVE; CYP707A, CYTOCHROME P450 707A ; DELLA, D (aspartic acid) E (glutamic acid) L (leucine) L (leucine) A (alanine) protein; GA, gibberellin; GA2ox; GA 2-oxidase; GA3ox, GA 3-oxidase; GID1, GA INSENSITIVE DWARF; NCED, nine- cis -epoxycarotenoid dioxygenase; RGA, REPRESSOR OF GAI; RGL2, RGA-LIKE 2; XERICO, “XERICO” (Greek for drought tolerant). The schemes are based on Ko et al. (2006) , Zentella et al. (2007) , Ariizumi et al. (2008) , Piskurewicz et al. (2008) , Bossi et al. (2009) , Lee et al. (2012b) , Cantoro et al. (2013) , Kong et al. (2013) , Lim et al. (2013) , and Shu et al. (2013) .
Conflict of Interest Statement
A patent application has been filed for a technology described in this article. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I am grateful to Roger Beachy, World Food Center, University of California, Davis, USA, for collaboration and continuous support in the translational biology projects described in this article, and Khadidiatou Sall and Mariko Nonogaki for critical reading of and helpful suggestions for the manuscript.
Alonso-Blanco, C., Bentsink, L., Hanhart, C. J., Vries, H. B.-D., and Koornneef, M. (2003). Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana . Genetics 164, 711–729.
Pubmed Abstract | Pubmed Full Text
Arc, E., Sechet, J., Corbineau, F., Rajjou, L., and Marion-Poll, A. (2013). ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci . 4:63. doi: 10.3389/fpls.2013.00063
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Argyris, J., Dahal, P., Hayashi, E., Still, D. W., and Bradford, K. J. (2008). Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol . 148, 926–947. doi: 10.1104/pp.108.125807
Argyris, J., Truco, M., Ochoa, O., McHale, L., Dahal, P., Van Deynze, A., et al. (2011). A gene encoding an abscisic acid biosynthetic enzyme ( LsNCED4 ) collocates with the high temperature germination locus Htg6.1 in lettuce ( Lactuca sp .). Theor. Appl. Genet . 122, 95–108. doi: 10.1007/s00122-010-1425-3
Ariizumi, T., Murase, K., Sun, T.-P., and Steber, C. M. (2008). Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell 20, 2447–2459. doi: 10.1105/tpc.108.058487
Atwell, S., Huang, Y. S., Vilhjalmsson, B. J., Willems, G., Horton, M., Li, Y., et al. (2010). Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature] 465, 627–631. doi: 10.1038/nature08800
Barrero, J. M., Millar, A. A., Griffiths, J., Czechowski, T., Scheible, W. R., Udvardi, M., et al. (2010). Gene expression profiling identifies two regulatory genes controlling dormancy and ABA sensitivity in Arabidopsis seeds. Plant J . 61, 611–622. doi: 10.1111/j.1365-313X.2009.04088.x
Bentsink, L., Hanson, J., Hanhart, C. J., Blankestijn-de Vries, H., Coltrane, C., Keizer, P., et al. (2010). Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proc. Natl. Acad. Sci. U.S.A . 107, 4264–4269. doi: 10.1073/pnas.1000410107
Bentsink, L., Jowett, J., Hanhart, C. J., and Koornneef, M. (2006). Cloning of DOG1 , a quantitative trait locus controlling seed dormancy in Arabidopsis . Proc. Natl. Acad. Sci. U.S.A . 103, 17042–17047. doi: 10.1073/pnas.0607877103
Bewley, J. D., Bradford, K. J., Hilhorst, H. W. M., and Nonogaki, H. (2013). Seeds: Physiology of Development, Germination and Dormancy . New York, NY: Springer. doi: 10.1007/978-1-4614-4693-4
CrossRef Full Text
Bossi, F., Cordoba, E., Dupre, P., Mendoza, M. S., Roman, C. S., and Leon, P. (2009). The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J . 59, 359–374. doi: 10.1111/j.1365-313X.2009.03877.x
Bouyer, D., Roudier, F., Heese, M., Andersen, E. D., Gey, D., Nowack, M. K., et al. (2011). Polycomb repressive complex 2 controls the embryo-to-seedling phase transition. PLoS Genet . 7:e1002014. doi: 10.1371/journal.pgen.1002014
Braam, J. (2005). In touch: plant responses to mechanical stimuli. New Phytol . 165, 373–389. doi: 10.1111/j.1469-8137.2004.01263.x
Cantoro, R., Crocco, C. D., Benech-Arnold, R. L., and Rodríguez, M. V. (2013). In vitro binding of Sorghum bicolor transcription factors ABI4 and ABI5 to a conserved region of a GA 2-OXIDASE promoter: possible role of this interaction in the expression of seed dormancy. J. Exp. Bot . 64, 5721–5735. doi: 10.1093/jxb/ert347
Chen, F., and Bradford, K. J. (2000). Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol . 124, 1265–1274. doi: 10.1104/pp.124.3.1265
Chen, F., Nonogaki, H., and Bradford, K. J. (2002). A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J. Exp. Bot . 53, 215–223. doi: 10.1093/jexbot/53.367.215
Chen, L.-T., Luo, M., Wang, Y.-Y., and Wu, K. (2010). Involvement of Arabidopsis histone deacetylase HDA6 in ABA and salt stress response. J. Exp. Bot . 61, 3345–3353. doi: 10.1093/jxb/erq154
Chen, L.-T., and Wu, K. (2010). Role of histone deacetylases HDA6 and HDA19 in ABA and abiotic stress response. Plant Signal. Behav . 5, 1318–1320. doi: 10.4161/psb.5.10.13168
Chiang, G. C., Barua, D., Dittmar, E., Kramer, E. M., de Casas, R. R., and Donohue, K. (2013). Pleiotropy in the wild: the dormancy gene DOG1 exerts cascading control on life cycles. Evolution 67, 883–893. doi: 10.1111/j.1558-5646.2012.01828.x
Chiwocha, S. D., Cutler, A. J., Abrams, S. R., Ambrose, S. J., Yang, J., Ross, A. R., et al. (2005). The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J . 42, 35–48. doi: 10.1111/j.1365-313X.2005.02359.x
Chono, M., Matsunaka, H., Seki, M., Fujita, M., Kiribuchi-Otobe, C., Oda, S., et al. (2013). Isolation of a wheat ( Triticum aestivum L.) mutant in ABA 8'-hydroxylase gene: effect of reduced ABA catabolism on germination inhibition under field condition. Breed. Sci . 63, 104–115. doi: 10.1270/jsbbs.63.104
Dekkers, B. J., Pearce, S., van Bolderen-Veldkamp, R. P., Marshall, A., Widera, P., Gilbert, J., et al. (2013). Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiol . 163, 205–215. doi: 10.1104/pp.113.223511
De Lucia, F., and Dean, C. (2011). Long non-coding RNAs and chromatin regulation. Curr. Opin. Plant Biol . 14, 168–173. doi: 10.1016/j.pbi.2010.11.006
Finkelstein, R., Reeves, W., Ariizumi, T., and Steber, C. (2008). Molecular aspects of seed dormancy. Annu. Rev. Plant Biol . 59, 387–415. doi: 10.1146/annurev.arplant.59.032607.092740
Footitt, S., Clay, H. A., Dent, K., and Finch-Savage, W. E. (2014). Environment sensing in spring-dispersed seeds of a winter annual Arabidopsis influences the regulation of dormancy to align germination potential with seasonal changes. New Phytol . 202, 929–939. doi: 10.1111/nph.12694
Footitt, S., Huang, Z., Clay, H. A., Mead, A., and Finch-Savage, W. E. (2013). Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. Plant J . 74, 1003–1015. doi: 10.1111/tpj.12186
Graeber, K. A. I., Nakabayashi, K., Miatton, E., Leubner-Metzger, G., and Soppe, W. J. J. (2012). Molecular mechanisms of seed dormancy. Plant Cell Environ . 35, 1769–1786. doi: 10.1111/j.1365-3040.2012.02542.x
Grasser, M., Kane, C. M., Merkle, T., Melzer, M., Emmersen, J., and Grasser, K. D. (2009). Transcript elongation factor TFIIS is involved in Arabidopsis seed dormancy. J. Mol. Biol . 386, 598–611. doi: 10.1016/j.jmb.2008.12.066
Groot, S. P. C., and Karssen, C. M. (1987). Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta 171, 525–531. doi: 10.1007/BF00392302
Grzenda, A., Lomberk, G., Zhang, J.-S., and Urrutia, R. (2009). Sin3: master scaffold and transcriptional corepressor. Biochim. Biophys. Acta . 1789, 443–450. doi: 10.1016/j.bbagrm.2009.05.007
He, Y., Doyle, M. R., and Amasino, R. M. (2004). PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis . Genes Dev . 18, 2774–2784. doi: 10.1101/gad.1244504
Henry, K. W., Wyce, A., Lo, W. S., Duggan, L. J., Emre, N. C., Kao, C. F., et al. (2003). Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev . 17, 2648–2663. doi: 10.1101/gad.1144003
Heo, J. B., and Sung, S. (2011). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331, 76–79. doi: 10.1126/science.1197349
Herr, A. J., Jensen, M. B., Dalmay, T., and Baulcombe, D. C. (2005). RNA polymerase IV directs silencing of endogenous DNA. Science 308, 118–120. doi: 10.1126/science.1106910
Huo, H., Dahal, P., Kunusoth, K., McCallum, C. M., and Bradford, K. J. (2013). Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance. Plant Cell 25, 884–900. doi: 10.1105/tpc.112.108902
Jackson, J. P., Lindroth, A. M., Cao, X., and Jacobsen, S. E. (2002). Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416, 556–560. doi: 10.1038/nature731
Johnson, L. M., Bostick, M., Zhang, X., Kraft, E., Henderson, I., Callis, J., et al. (2007). The SRA methyl-cytosine-binding domain links DNA and histone methylation. Curr. Biol . 17, 379–384. doi: 10.1016/j.cub.2007.01.009
Kadosh, D., and Struhl, K. (1998). Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo . Genes Dev . 12, 797–805. doi: 10.1101/gad.12.6.797
Kim, J., Guermah, M., McGinty, R. K., Lee, J.-S., Tang, Z., Milne, T. A., et al. (2009). RAD6-mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell 137, 459–471. doi: 10.1016/j.cell.2009.02.027
Kim, J., Guermah, M., and Roeder, R. G. (2010). The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 140, 491–503. doi: 10.1016/j.cell.2009.12.050
Kim, J.-M., To, T. K., and Seki, M. (2012). An epigenetic integrator: new insights into genome regulation, environmental stress responses and developmental controls by HISTONE DEACETYLASE 6. Plant Cell Physiol . 53, 794–800. doi: 10.1093/pcp/pcs004
Ko, J.-H., Yang, S. H., and Han, K.-H. (2006). Upregulation of an Arabidopsis RING-H2 gene, XERICO , confers drought tolerance through increased abscisic acid biosynthesis. Plant J . 47, 343–355. doi: 10.1111/j.1365-313X.2006.02782.x
Kong, Y., Chen, S., Yang, Y., and An, C. (2013). ABA-insensitive (ABI) 4 and ABI5 synergistically regulate DGAT1 expression in Arabidopsis seedlings under stress. FEBS Lett . 587, 3076–3082. doi: 10.1016/j.febslet.2013.07.045
Kronholm, I., Pico, F. X., Alonso-Blanco, C., Goudet, J., and de Meaux, J. (2012). Genetic basis of adaptation in Arabidopsis thaliana : local adaptation at the seed dormancy QTL DOG1 . Evolution 2287–2302. doi: 10.1111/j.1558-5646.2012.01590.x
Lai, A., Kennedy, B. K., Barbie, D. A., Bertos, N. R., Yang, X. J., Theberge, M.-C., et al. (2001). RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol. Cell. Biol . 21, 2918–2932. doi: 10.1128/MCB.21.8.2918-2932.2001
Lam, E., Benfey, P. N., Gilmartin, P. M., Fang, R. X., and Chua, N. H. (1989). Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc. Natl. Acad. Sci. U.S.A . 86, 7890–7894. doi: 10.1073/pnas.86.20.7890
Law, J. A., Vashisht, A. A., Wohlschlegel, J. A., and Jacobsen, S. E. (2011). SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV. PLoS Genet . 7:e1002195. doi: 10.1371/journal.pgen.1002195
Lee, D., Polisensky, D. H., and Braam, J. (2005). Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol . 165, 429–444. doi: 10.1111/j.1469-8137.2004.01238.x
Lee, K. J., Dekkers, B. J., Steinbrecher, T., Walsh, C. T., Bacic, A., Bentsink, L., et al. (2012a). Distinct cell wall architectures in seed endosperms in representatives of the Brassicaceae and Solanaceae. Plant Physiol . 160, 1551–1566. doi: 10.1104/pp.112.203661
Lee, K. P., Piskurewicz, U., Tureckova, V., Carat, S., Chappuis, R., Strnad, M., et al. (2012b). Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes Dev . 26, 1984–1996. doi: 10.1101/gad.194266.112
Lei, L., Zhu, X., Wang, S., Zhu, M., Carver, B. F., and Yan, L. (2013). TaMFT-A1 is associated with seed germination sensitive to temperature in winter wheat. PLoS ONE 8:e73330. doi: 10.1371/journal.pone.0073330
Leon-Kloosterziel, K. M., van de Bunt, G. A., Zeevaart, J. A., and Koornneef, M. (1996). Arabidopsis mutants with a reduced seed dormancy. Plant Physiol . 110, 233–240. doi: 10.1104/pp.110.1.233
Lim, S., Park, J., Lee, N., Jeong, J., Toh, S., Watanabe, A., et al. (2013). ABA-INSENSITIVE3, ABA-INSENSITIVE5, and DELLAs interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis . Plant Cell 25, 4863–4878. doi: 10.1105/tpc.113.118604
Linkies, A., Graeber, K., Knight, C., and Leubner-Metzger, G. (2010). The evolution of seeds. New Phytol . 186, 817–831. doi: 10.1111/j.1469-8137.2010.03249.x
Liu, F., Marquardt, S., Lister, C., Swiezewski, S., and Dean, C. (2010). Targeted 3' processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327, 94–97. doi: 10.1126/science.1180278
Liu, S., Sehgal, S. K., Li, J., Lin, M., Trick, H. N., Yu, J., et al. (2013). Cloning and characterization of a critical regulator for pre-harvest sprouting in wheat. Genetics 195, 263–273. doi: 10.1534/genetics.113.152330
Liu, Y., Geyer, R., van Zanten, M., Carles, A., Li, Y., Hörold, A., et al. (2011). Identification of the Arabidopsis REDUCED DORMANCY 2 gene uncovers a role for the polymerase associated factor 1 complex in seed dormancy. PLoS ONE 6:e22241. doi: 10.1371/journal.pone.0022241
Liu, Y., Koornneef, M., and Soppe, W. J. J. (2007). The absence of histone H2B monoubiquitination in the Arabidopsis hub1 ( rdo4 ) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell 19, 433–444. doi: 10.1105/tpc.106.049221
Mallory, A., and Vaucheret, H. (2010). Form, function, and regulation of ARGONAUTE proteins. Plant Cell 22, 3879–3889. doi: 10.1105/tpc.110.080671
Martinez-Andujar, C., Ordiz, M. I., Huang, Z., Nonogaki, M., Beachy, R. N., and Nonogaki, H. (2011). Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc. Natl. Acad. Sci. U.S.A . 108, 17225–17229. doi: 10.1073/pnas.1112151108
Martinez-Andujar, C., Pluskota, W. E., Bassel, G. W., Asahina, M., Pupel, P., Nguyen, T. T., et al. (2012). Mechanisms of hormonal regulation of endosperm cap-specific gene expression in tomato seeds. Plant J . 71, 575–586. doi: 10.1111/j.1365-313X.2012.05010.x
Miatton, E. (2012). Characterization of PDF1 and Its Interaction With DELAY OF GERMINATION1 (DOG1) in the Control of Seed Dormancy in Arabidopsis thaliana . Köln: University of Köln.
Mikami, K., Takase, H., Tabata, T., and Iwabuchi, M. (1989). Multiplicity of the DNA-binding protein HBP-1 specific to the conserved hexameric sequence ACGTCA in various plant gene promoters. FEBS Lett . 256, 67–70. doi: 10.1016/0014-5793(89)81719-3
Molitor, A. M., Bu, Z., Yu, Y., and Shen, W. H. (2014). Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes. PLoS Genet . 10:e1004091. doi: 10.1371/journal.pgen.1004091
Mortensen, S. A., and Grasser, K. D. (2014). The seed dormancy defect of Arabidopsis mutants lacking the transcript elongation factor TFIIS is caused by reduced expression of the DOG1 gene. FEBS Lett . 588, 47–51. doi: 10.1016/j.febslet.2013.10.047
Muller, K., Bouyer, D., Schnittger, A., and Kermode, A. R. (2012). Evolutionarily conserved histone methylation dynamics during seed life-cycle transitions. PLoS ONE 7:e51532. doi: 10.1371/journal.pone.0051532
Nakabayashi, K., Bartsch, M., Xiang, Y., Miatton, E., Pellengahr, S., Yano, R., et al. (2012). The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 24, 2826–2838. doi: 10.1105/tpc.112.100214
Nakamura, S., Abe, F., Kawahigashi, H., Nakazono, K., Tagiri, A., Matsumoto, T., et al. (2011). A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell 23, 3215–3229. doi: 10.1105/tpc.111.088492
Nakanishi, S., Lee, J. S., Gardner, K. E., Gardner, J. M., Takahashi, Y. H., Chandrasekharan, M. B., et al. (2009). Histone H2BK123 monoubiquitination is the critical determinant for H3K4 and H3K79 trimethylation by COMPASS and Dot1. J. Cell Biol . 186, 371–377. doi: 10.1083/jcb.200906005
Nakayama, T., Ohtsubo, N., Mikami, K., Kawata, T., Tabata, T., Kanazawa, H., et al. (1989). Cisacting sequences that modulate transcription of wheat histone H3 and 3'processing of H3 premature mRNA. Plant Cell Physiol . 30, 825–832.
Ni, B. R., and Bradford, K. J. (1993). Germination and dormancy of abscisic acid- and gibberellin-deficient mutant tomato ( Lycopersicon esculentum ) seeds (sensitivity of germination to abscisic acid, gibberellin, and water potential). Plant Physiol . 101, 607–617.
Nonogaki, H., Gee, O. H., and Bradford, K. J. (2000). A germination-specific endo-beta -mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol . 123, 1235–1246. doi: 10.1104/pp.123.4.1235
Nonogaki, H., Nomaguchi, M., Okumoto, N., Kaneko, Y., Matsushima, H., and Morohashi, M. (1998). Temporal and spatial pattern of the biochemical activation of the endosperm during and following imbibition of tomato seeds. Physiol. Plant . 102, 236–242. doi: 10.1034/j.1399-3054.1998.1020211.x
Nonogaki, M., Sall, K., Nambara, E., and Nonogaki, H. (2014). Amplification of ABA biosynthesis and signaling through a positive feedback mechanism in seeds. Plant J . 78, 527–539. doi: 10.1111/tpj.12472
Ogawa, M., Hanada, A., Yamauchi, Y., Kuwahara, A., Kamiya, Y., and Yamaguchi, S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15, 1591–1604. doi: 10.1105/tpc.011650
Oh, S., Zhang, H., Ludwig, P., and van Nocker, S. (2004). A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell 16, 2940–2953. doi: 10.1105/tpc.104.026062
Onodera, Y., Haag, J. R., Ream, T., Costa Nunes, P., Pontes, O., and Pikaard, C. S. (2005). Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120, 613–622. doi: 10.1016/j.cell.2005.02.007
Peeters, A. J. M., Blankestijn-de Vries, H., Hanhart, C. J., Léon-Kloosterziel, K. M., Zeevaart, J. A. D., and Koornneef, M. (2002). Characterization of mutants with reduced seed dormancy at two novel rdo loci and a further characterization of rdo1 and rdo2 in Arabidopsis. Physiol. Plant . 115, 604–612. doi: 10.1034/j.1399-3054.2002.1150415.x
Penheiter, K. L., Washburn, T. M., Porter, S. E., Hoffman, M. G., and Jaehning, J. A. (2005). A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol. Cell 20, 213–223. doi: 10.1016/j.molcel.2005.08.023
Perrella, G., Lopez-Vernaza, M. A., Carr, C., Sani, E., Gossele, V., Verduyn, C., et al. (2013). Histone deacetylase complex1 expression level titrates plant growth and abscisic acid sensitivity in Arabidopsis. Plant Cell 25, 3491–3505. doi: 10.1105/tpc.113.114835
Piskurewicz, U., Jikumaru, Y., Kinoshita, N., Nambara, E., Kamiya, Y., and Lopez-Molina, L. (2008). The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20, 2729–2745. doi: 10.1105/tpc.108.061515
Porter, S. E., Penheiter, K. L., and Jaehning, J. A. (2005). Separation of the Saccharomyces cerevisiae Paf1 complex from RNA polymerase II results in changes in its subnuclear localization. Eukaryotic Cell 4, 209–220. doi: 10.1128/EC.4.1.209-220.2005
Qi, Y., He, X., Wang, X.-J., Kohany, O., Jurka, J., and Hannon, G. J. (2006). Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature 443, 1008–1012. doi: 10.1038/nature05198
Rea, S., Eisenhaber, F., O'Carroll, D., Strahl, B. D., Sun, Z.-W., Schmid, M., et al. (2000). Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–599. doi: 10.1038/35020506
Richon, V. M., and O'Brien, J. P. (2002). Histone deacetylase inhibitors: a new class of potential therapeutic agents for cancer treatment. Clin. Cancer Res . 8, 718–728, 662–664.
Saunders, A., Core, L. J., and Lis, J. T. (2006). Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell Biol . 7, 557–567. doi: 10.1038/nrm1981
Schramm, E., Nelson, S., Kidwell, K., and Steber, C. (2013). Increased ABA sensitivity results in higher seed dormancy in soft white spring wheat cultivar ‘Zak.’ Theor. Appl. Genet . 126, 791–803. doi: 10.1007/s00122-012-2018-0
Seo, M., Nambara, E., Choi, G., and Yamaguchi, S. (2009). Interaction of light and hormone signals in germinating seeds. Plant Mol. Biol . 69, 463–472. doi: 10.1007/s11103-008-9429-y
Shu, K., Zhang, H., Wang, S., Chen, M., Wu, Y., Tang, S., et al. (2013). ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet . 9:e1003577. doi: 10.1371/journal.pgen.1003577
Simon, J. A., and Kingston, R. E. (2009). Mechanisms of Polycomb gene silencing: knowns and unknowns. Nat. Rev. Mol. Cell Biol . 10, 697–708. doi: 10.1038/nrm2763
Singh, M., and Singh, J. (2012). Seed development-related expression of ARGONAUTE4_9 class of genes in barley: possible role in seed dormancy. Euphytica 188, 123–129. doi: 10.1007/s10681-012-0624-1
Singh, M., Singh, S., Randhawa, H., and Singh, J. (2013). Polymorphic homoeolog of key gene of RdDM pathway, ARGONAUTE4_9 class is associated with pre-harvest sprouting in wheat ( Triticum aestivum L.). PLoS ONE 8:e77009. doi: 10.1371/journal.pone.0077009
Sugimoto, K., Takeuchi, Y., Ebana, K., Miyao, A., Hirochika, H., Hara, N., et al. (2010). Molecular cloning of Sdr4 , a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. U.S.A . 107, 5792–5797. doi: 10.1073/pnas.0911965107
Sun, Q., Csorba, T., Skourti-Stathaki, K., Proudfoot, N. J., and Dean, C. (2013). R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340, 619–621. doi: 10.1126/science.1234848
Sun, Z.-W., and Allis, C. D. (2002). Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418, 104–108. doi: 10.1038/nature00883
Swiezewski, S., Liu, F., Magusin, A., and Dean, C. (2009). Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462, 799–802. doi: 10.1038/nature08618
Tabata, T., Nakayama, T., Mikami, K., and Iwabuchi, M. (1991). HBP-1a and HBP-1b: leucine zipper-type transcription factors of wheat. EMBO J . 10, 1459–1467.
To, T. K., Kim, J.-M., Matsui, A., Kurihara, Y., Morosawa, T., Ishida, J., et al. (2011). Arabidopsis HDA6 regulates locus-directed heterochromatin silencing in cooperation with MET1. PLoS Genet . 7:e1002055. doi: 10.1371/journal.pgen.1002055
Tran, R. K., Zilberman, D., de Bustos, C., Ditt, R. F., Henikoff, J. G., Lindroth, A. M., et al. (2005). Chromatin and siRNA pathways cooperate to maintain DNA methylation of small transposable elements in Arabidopsis . Genome Biol . 6, R90. doi: 10.1186/gb-2005-6-11-r90
Voegele, A., Linkies, A., Muller, K., and Leubner-Metzger, G. (2011). Members of the gibberellin receptor gene family GID1 ( GIBBERELLIN INSENSITIVE DWARF1 ) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J. Exp. Bot . 62, 5131–5147. doi: 10.1093/jxb/err214
Wang, Z., Cao, H., Sun, Y., Li, X., Chen, F., Carles, A., et al. (2013). Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid-ethylene antagonism mediated by histone deacetylation. Plant Cell 25, 149–166. doi: 10.1105/tpc.112.108191
Wierzbicki, A. T. (2012). The role of long non-coding RNA in transcriptional gene silencing. Curr. Opin. Plant Biol . 15, 517–522. doi: 10.1016/j.pbi.2012.08.008
Wierzbicki, A. T., Haag, J. R., and Pikaard, C. S. (2008). Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135, 635–648. doi: 10.1016/j.cell.2008.09.035
Wierzbicki, A. T., Ream, T. S., Haag, J. R., and Pikaard, C. S. (2009). RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nat. Genet . 41, 630–634. doi: 10.1038/ng.365
Xi, W., Liu, C., Hou, X., and Yu, H. (2010). MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis . Plant Cell 22, 1733–1748. doi: 10.1105/tpc.109.073072
Xie, Z., Johansen, L. K., Gustafson, A. M., Kasschau, K. D., Lellis, A. D., Zilberman, D., et al. (2004). Genetic and functional diversification of small RNA pathways in plants. PLoS Biol . 2:E104. doi: 10.1371/journal.pbio.0020104
Yamada, K., Lim, J., Dale, J. M., Chen, H., Shinn, P., Palm, C. J., et al. (2003). Empirical analysis of transcriptional activity in the Arabidopsis genome. Science 302, 842–846. doi: 10.1126/science.1088305
Yamaguchi, S., Kamiya, Y., and Sun, T. (2001). Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J . 28, 443–453. doi: 10.1046/j.1365-313X.2001.01168.x
Yamauchi, Y., Ogawa, M., Kuwahara, A., Hanada, A., Kamiya, Y., and Yamaguchi, S. (2004). Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16, 367–378. doi: 10.1105/tpc.018143
Yano, R., Takebayashi, Y., Nambara, E., Kamiya, Y., and Seo, M. (2013). Combining association mapping and transcriptomics identify HD2B histone deacetylase as a genetic factor associated with seed dormancy in Arabidopsis thaliana . Plant J . 74, 815–828. doi: 10.1111/tpj.12167
Yu, X., and Michaels, S. D. (2010). The Arabidopsis Paf1c complex component CDC73 participates in the modification of FLOWERING LOCUS C chromatin. Plant Physiol . 153, 1074–1084. doi: 10.1104/pp.110.158386
Zentella, R., Zhang, Z.-L., Park, M., Thomas, S. G., Endo, A., Murase, K., et al. (2007). Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis . Plant Cell 19, 3037–3057. doi: 10.1105/tpc.107.054999
Zhang, H., and Van Nocker, S. (2002). The VERNALIZATION INDEPENDENCE 4 gene encodes a novel regulator of FLOWERING LOCUS C . Plant J . 31, 663–673. doi: 10.1046/j.1365-313X.2002.01380.x
Zheng, J., Chen, F., Wang, Z., Cao, H., Li, X., Deng, X., et al. (2012). A novel role for histone methyltransferase KYP/SUVH4 in the control of Arabidopsis primary seed dormancy. New Phytol . 193, 605–616. doi: 10.1111/j.1469-8137.2011.03969.x
Zhu, B., Zheng, Y., Pham, A.-D., Mandal, S. S., Erdjument-Bromage, H., Tempst, P., et al. (2005). Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol. Cell 20, 601–611. doi: 10.1016/j.molcel.2005.09.025
Zilberman, D., Cao, X., Johansen, L. K., Xie, Z., Carrington, J. C., and Jacobsen, S. E. (2004). Role of Arabidopsis ARGONAUTE4 in RNA-directed DNA methylation triggered by inverted repeats. Curr. Biol . 14, 1214–1220. doi: 10.1016/j.cub.2004.06.055
Keywords: chromatin remodeling, dormancy, embryo, endosperm, germination, hormone
Citation: Nonogaki H (2014) Seed dormancy and germination—emerging mechanisms and new hypotheses. Front. Plant Sci . 5 :233. doi: 10.3389/fpls.2014.00233
Received: 21 March 2014; Accepted: 10 May 2014; Published online: 28 May 2014.
Reviewed by:
Copyright © 2014 Nonogaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyuki Nonogaki, Department of Horticulture, Oregon State University, 4017 ALS Bldg., Corvallis OR 97331, USA e-mail: [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Search Menu
- Volume 16, Issue 3, June 2024 (In Progress)
- Volume 16, Issue 2, February 2024
- Advance articles
- Editor's Choice
- Special Issues
- Author Guidelines
- Article Metrics
- Review Procedures and Criteria
- Open Access Policy
- Publication Charges
- Submission Site
- Call for Papers
- Why Publish?
- About AoB PLANTS
- Impact Factor
- Editorial Board
- About the Annals of Botany Company
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, conclusions, sources of funding, contributions by the authors, conflicts of interest statement, acknowledgements, literature cited.
- < Previous
Effect of saline water on seed germination and early seedling growth of the halophyte quinoa
Guest Editor: Tim J. Flowers
- Article contents
- Figures & tables
- Supplementary Data
M. R. Panuccio, S. E. Jacobsen, S. S. Akhtar, A. Muscolo, Effect of saline water on seed germination and early seedling growth of the halophyte quinoa, AoB PLANTS , Volume 6, 2014, plu047, https://doi.org/10.1093/aobpla/plu047
- Permissions Icon Permissions
Salinization is increasing on a global scale, decreasing average yields for most major crop plants. Investigations into salt resistance have, unfortunately, mainly been focused on conventional crops, with few studies screening the potential of available halophytes as new crops. This study has been carried out to investigate the mechanisms used by quinoa, a facultative halophytic species, in order to cope with high salt levels at various stages of its development. Quinoa is regarded as one of the crops that might sustain food security in this century, grown primarily for its edible seeds with their high protein content and unique amino acid composition. Although the species has been described as a facultative halophyte, and its tolerance to salt stress has been investigated, its physiological and molecular responses to seawater (SW) and other salts have not been studied. We evaluated the effects of SW and different salts on seed germination, seedling emergence and the antioxidative pathway of quinoa. Seeds were germinated in Petri dishes and seedlings grown in pots with SW solutions (25, 50, 75 and 100 %) and NaCl, CaCl 2 , KCl and MgCl 2 individually, at the concentrations in which they are present in SW. Our results demonstrated that all salts, at lower concentrations, increased the germination rate but not the germination percentages, compared with control (pure water). Conversely, seedlings were differently affected by treatments in respect to salt type and concentration. Growth parameters affected were root and shoot length, root morphology, fresh and dry weight, and water content. An efficient antioxidant mechanism was present in quinoa, activated by salts during germination and early seedling growth, as shown by the activities of antioxidant enzymes. Total antioxidant capacity was always higher under salt stress than in water. Moreover, osmotic and ionic stress factors had different degrees of influence on germination and development.
Soil salinity and sodicity cause severe problems in agriculture worldwide, and salt tolerance in crops is an extremely important trait and a major focus of research. Detrimental effects of high salinity on crops are multifaceted and affect plants in several ways: drought stress, ion toxicity, nutritional disorders, oxidative stress, alteration of metabolic processes, membrane disorganization and reduction of cell division and expansion ( Hasegawa et al. 2000 ; Munns 2002 ; Muscolo et al. 2007 , 2013 ; Zhu 2007 ; Sidari et al. 2008 ). As a result, plant growth, development and survival are reduced ( Muscolo et al. 2011 ; Schleiff and Muscolo 2011 ). Two major stresses affecting plants under salinity are osmotic and ionic stresses. Osmotic stress, occurring immediately in the root medium on exposure to salts, can result in inhibition of water uptake, cell expansion and lateral bud development ( Munns and Tester 2008 ). Ionic stress develops when toxic ions (e.g. Na + ) accumulate in cells causing increase in leaf mortality, chlorosis, necrosis and decrease in the activity of cellular metabolism including photosynthesis ( Yeo and Flowers 1986 ; Glenn and Brown 1999 ). In fact, excess Na + and Cl − have the potential to affect plant enzymes, resulting in reduced energy production and other physiological processes ( Larcher 1980 ; Morais et al. 2012 a , b ). Ionic stress results in premature senescence of older leaves and in toxicity symptoms (chlorosis, necrosis) in mature leaves due to high Na + and Cl − which affect plants by disrupting protein synthesis and by interfering with enzyme activity ( Munns and Termaat 1986 ; Hasegawa et al. 2000 ; Munns 2002 ).
In order to counteract the detrimental effects of salinity on agricultural production, extensive research on plant screening for salt tolerance has been conducted, with the aim of providing more tolerant cultivars. However, these studies have mainly focused on conventional crops, screening criteria and investigating how plants tolerate salts ( Shannon and Noble 1990 ; Chen et al. 2005 ; Sevengor et al. 2011 ). Unfortunately, there are few investigations on screening of available halophytes and their responses to saline conditions ( Flowers et al. 2010 ). The seed crop quinoa is a facultative halophyte native to the Andean region of Bolivia and Peru, and a member of the Amaranthaceae: quinoa is traditionally cultivated across a range of extreme environments. Due to its huge genetic variability, the species can be grown under unfavourable soil and climatic conditions ( Ruiz-Carrasco et al. 2011 ), showing a diverse tolerance to a wide range of abiotic stresses such as frost, salinity and drought, as well as an ability to grow on marginal soils ( Jacobsen et al. 2005 , 2007 , 2009 ; Maughan et al. 2009 ; Sun et al. 2014 ). Some varieties can grow in salt concentrations similar to those found in seawater (SW, 40 dS m −1 ) and even higher ( Jacobsen et al. 2001 ; Adolf et al. 2012 , 2013 ; Shabala et al. 2012 , 2013 ), well above the threshold for any known crop species.
Quinoa is considered a major alternative crop to meet food shortages in this century ( Jensen et al. 2000 ; Jacobsen et al. 2003 ; Sanchez et al. 2003 ; Trognitz 2003 ; Ruiz et al. 2014 ), for its gluten-free seeds and also as its grains provide a rich source of a wide range of minerals (Ca, P, Mg, Fe and Zn), vitamins (B 1 , B 9 , C and E), linolenate, natural antioxidants and high-quality protein, containing ample amounts of essential amino acids such as lysine and methionine ( Abugoch et al. 2008 ; Koyro and Eisa 2008 ). Quinoa's tolerance to high salinity at the primary stages of seed germination is based upon alterations in the levels of primary metabolites and enzyme activity ( González and Prado 1992 ; Adolf et al. 2013 ). Most of the studies on the effect of salinity on seed germination of halophytes have, however, been conducted using NaCl solutions. Such investigations may not provide information on germination under field conditions, because soils contain different salts, which may collectively influence germination in different ways from their individual effects ( Ungar 1996 ). Sea salt mimics the composition of saline soil solutions and can be used to study the synergistic effect of different salts on seed germination ( Liu et al. 2006 ). Therefore, the work presented here was carried out to examine the effects of SW and its component salts on seed germination, seedling emergence and the antioxidative pathway of quinoa cv. Titicaca, as well as the relative importance of two components (ionic and osmotic) of salinity stress.
Quinoa cultivars have been shown to differ in salt tolerance ( Bonales-Alatorre et al. 2013 ). In general, varieties originating from salt-affected areas are adapted to saline conditions and hence are less affected by salinity ( Adolf et al. 2012 ; Shabala et al. 2013 ) than those from non-saline areas. In this study, we used the Danish-bred quinoa cv. Titicaca ( Jacobsen et al. 2010 ; Adolf et al. 2012 ) to verify the salinity tolerance of a variety well adapted to European climatic conditions. Quinoa production may be a viable option for farmers interested in a high-value crop with regional production and local markets in Mediterranean countries where saline water and soil salinity are major risks for the future of agricultural development. Here fresh water resources are limited, while food requirements and pressure from climate change are still growing. The use of saline water resources may constitute a remedy for the current water scarcity. For these reasons, quinoa offers the possibility of an alternative, promising, cash crop to be cultivated in arid and semiarid environments that are prohibitive for other species and so may be able to utilize saline soils in a sustainable and productive way.
Plant material
Mature seeds of the Danish-bred quinoa ( Chenopodium quinoa cv. Titicaca) (provided by Department of Plant Environmental Science, University of Copenhagen) were stored at 5 °C until the start of experiments. Two different experiments were carried out in a growth chamber (Green line WRS 96-85, KW, Scientific Equipment, Italy) (temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %) to characterize the responses of quinoa to salt stress. Seed germination and biochemical responses were studied in the first experiment, while morphological, physiological and biochemical responses of seedlings were studied in the second experiment.
Experiment 1: seed germination
Germination conditions and experimental design.
Seeds were surface-sterilized for 20 min in 20 % (v/v) sodium hypochlorite, rinsed and soaked for 1 h in distilled water. The sterilization procedure is required to eliminate saponine from seeds and to avoid contamination by microorganisms during the germination process. The entire sterilization procedure, including soaking, took 1 h and did not affect the germination process ( Ruiz-Carrasco et al. 2011 ; Burrieza et al. 2012 ). For the germination tests, five 50-seed replicates were used with either Mediterranean SW collected from the Tirreno sea (Calabria Southern Italy) with a salinity of 38 % ( Cotruvo 2005 ) or solutions of NaCl, CaCl 2 , KCl or MgCl 2 at the concentration in which they were in the SW and at various dilutions. In the experiment, five different concentrations of NaCl (0, 100, 200, 300 and 400 mM); KCl (0, 2.54, 5.08, 7.62 and 10.2 mM); CaCl 2 (0, 2.54, 5.08, 7.62 and 10.2 mM) and MgCl 2 (0, 13.4, 26.7, 40.1 and 53.5 mM) were used to test whether the various ions differently affected germination indexes and to verify possible antagonistic or synergic ion effects on seed germination. Seeds were placed on filter paper in 9 cm diameter Petri dishes containing 3 mL of each solution. The Petri dishes were hermetically sealed with Parafilm to prevent evaporation and kept in the growth chamber at a temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %. Seeds were considered germinated when the radicle had extended at least 2 mm.
Germination indexes
Determination of ionic and osmotic effect, determination of enzyme activities.
Seeds (0.5 g) that had been soaked for 3 days in the test solutions were ground using a chilled mortar and pestle and homogenized in 0.1 M phosphate buffer solution (pH 7.0) containing 100 mg soluble polyvinylpolypyrrolidone and 0.1 mM ethylenediamine tetra acetic acid (EDTA). The homogenate was filtered through two layers of muslin cloth and centrifuged at 15 000 g for 15 min at 4 °C. The resulting supernatant was used to evaluate the activity of catalase (CAT, EC 1.11.1.6), peroxidase (POX, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.11) and superoxide dismutase (SOD EC 1.15.1.1). All enzyme activities were measured at 25 °C by a UV–visible light spectrophotometer (UV-1800 CE, Shimadzu, Japan).
Catalase activity was determined by monitoring the disappearance of H 2 O 2 at 240 nm, calculated using its extinction coefficient ( ε ) = 0.036 mM −1 cm −1 . The reaction mixture contained 1 mL of potassium phosphate buffer (50 mM, pH 7.0), 40 μL of enzyme extract and 5 μL of H 2 O 2 ( Beaumont et al. 1990 ).
Ascorbate peroxidase activity was assayed according to Nakano and Asada (1981) . The reaction mixture (1.5 mL) contained 50 mM phosphate buffer (pH 6.0), 0.1 μM EDTA, 0.5 mM ascorbate, 1.0 mM H 2 O 2 and 50 μL enzyme extract. The reaction was started by the addition of H 2 O 2 and ascorbate oxidation measured at 290 nm for 1 min. Enzyme activity was quantified using the molar extinction coefficient for ascorbate (2.8 mM −1 cm −1 ).
Peroxidase activity was measured on the basis of determination of guaiacol oxidation at 436 nm for 90 s ( Panda et al. 2003 ). The reaction mixture contained 1 mL of potassium phosphate buffer (0.1 M, pH 7.0), 20 μL of guaiacol, 40 μL of enzyme extract and 15 μL of H 2 O 2 . Peroxidase activity was quantified by the amount of tetraguaiacol formed using its extinction coefficient ( ε ) = 25.5 mM −1 cm −1 .
Superoxide dismutase activity was estimated by recording the decrease in the absorbance of superoxide nitro-blue tetrazolium complex by the enzyme ( Gupta et al. 1993 ). The reaction mixture (3 mL) contained 0.1 mL of 200 mM methionine, 01 mL of 2.25 mM nitro-blue tetrazolium, 0.1 mL of 3 mM EDTA, 1.5 mL of 100 mM potassium phosphate buffer, 1 mL of distilled water and 0.05 mL of enzyme extract. The assay was performed in duplicate for each sample. Two tubes without enzyme extract were used as a background control. The reaction was started by adding 0.1 mL of riboflavin (60 μM) and placing the tubes below a light source of two 15 W florescent lamps for 15 min. The reaction was stopped by switching off the light and covering the tubes with black cloth. Tubes without enzyme developed maximum colour. A non-irradiated complete reaction mixture which did not develop colour served as the blank. Absorbance was recorded at 560 nm and one unit of enzyme activity was taken as the quantity of enzyme which reduced the absorbance of samples to 50 % in comparison with tubes lacking enzymes.
For CAT, APX, SOD and POX activities, the results were expressed as enzyme units (U) per mg fresh weight. One unit of enzyme was defined as the amount of enzyme necessary to decompose 1 μmol of substrate per min at 25 °C.
Determination of total antioxidant capacity
Seeds (treated with different salt solutions for 3 days) were homogenized in a chilled mortar with distilled water at a ratio of 1 : 4 (seeds/water; w/v) and centrifuged at 14 000 g for 30 min. All steps were performed at 4 °C. The supernatants were filtered through two layers of muslin cloth and were used to determine the total antioxidant capacity by the spectrophotometric method of Prieto et al. (1999) . Aqueous extracts of the seeds were mixed in Eppendorf tubes with 1 mL of reagent solution (0.6 M H 2 SO 4 , 28 mM sodium phosphate, 4 mM ammonium molybdate mixture). The tubes were incubated for 90 min at 95 °C, then cooled to room temperature, and the absorbance read at 695 nm against a blank (mixture without seed extract). The assay was conducted in triplicate and the total antioxidant activity expressed as the absorbance of the sample at 695 nm. The higher the absorbance value, the higher the antioxidant activity ( Prasad et al. 2009 ).
Determination of total phenolic content
Total phenolic content was determined with the Folin–Ciocalteu reagent according to a modified procedure described by Singleton and Rossi (1965) . Briefly, 0.50 mL of the aqueous extract of the seeds was reacted with 2.5 mL of Folin–Ciocalteu reagent (1 : 10 diluted with distilled water) for 4 min, and then 2 mL of saturated sodium carbonate solution (∼75 g/L) was added to the reaction mixture. The absorbance readings were taken at 760 nm after incubation at room temperature for 2 h. Tannic acid was used as a reference standard, and the results were expressed as milligram tannic acid equivalent (mg TAET/g fresh weight).
Experiment 2: morphological, physiological and biochemical responses of seedlings
Plantlet growth in pots.
Seeds were germinated in Petri dishes. After 3 days from the beginning of germination, germinated seeds were grown for 21 days in plastic pots (10 cm diameter × 7 cm height), in a growth chamber (Green line WRS 96-85, KW apparecchi scientifici, Italy), under white light (80 W m −2 , Osram HQI halogen vapor W lamp, PAR 1055 μmol m −2 s−1) in a 16/8-h photoperiod, 70 % relative humidity and at 21 °C. All pots were filled with Perlite that had been equilibrated, before transplanting the germinated seeds, with one of the different salts or SW solutions at the desired concentration. All reagents used were of the highest analytical grade and were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All pots were watered with a one-fourth strength Murashige and Skoog medium (MS /4 ) containing macro and micronutrients at pH 5.8: the pots were weighed daily, and watered when their weight decreased by 30 % (corresponding to water that was lost by evapotranspiration). The control pots were watered with MS /4 alone. Leaf and root length were evaluated 21 days after the beginning of the stress, using six plants for each treatment.
Measurement of enzyme activities
After 21 days in pots under different salinity treatments, plantlet material was ground with a mortar and pestle in 100 mM HEPES–NaOH (pH 7.5), 5 mM MgCl 2 and 1 mM dithiothreitol . The ratio of plant material to buffer was 1 : 3. The extract was filtered through two layers of muslin and clarified by centrifugation at 15 000 g for 15 min. The supernatant was used for CAT, APX, POX, SOD analyses and total antioxidant capacity as described above. All steps were performed at 4 °C.
Cations (Na + , K + , Ca 2+ Mg 2+ and NH 4 + ) and anions (Cl − and SO 4 2− ) were determined in the water extracts of treated seedlings by ion chromatography (DIONEX ICS-1100).
Measurement of root morphology
Seedlings were harvested and root weight was recorded. Roots were scanned using an Epson Expression/STD 1600 scanner and personal computer with Intel Pentium III/500 CPU, 128 MB RAM, optimized for root analyses by Regent Instrument, Inc., and their length was analysed using the WinRHIZO image analysis system (Regent Instruments, Quebec, Canada). When scanning, each root sample was placed in a rectangular glass dish (300 × 200 mm) with ∼4–5 mm of water to untangle the roots and minimize root overlap. Three replicated roots were analysed for each treatment.
Statistical analysis
All data were analysed by one-way analysis of variance (ANOVA) with the salt concentration as the grouping factor. Separate ANOVAs were performed for each of four salt types and concentrations: NaCl (0, 100, 200, 300, 400 mM); KCl (0, 2.54, 5.08, 7.62, 10.16 mM); CaCl 2 (0, 2.54, 5.08, 7.62, 10.16 mM) and MgCl 2 (0, 13.36, 26.72, 40.09, 53.46 mM). The response variables for these ANOVAs were: seed germination, seedling growth, enzyme activities, ion contents and root morphology. Since salt concentration had five levels, on all significant ANOVAs we performed Tukey's multiple comparison tests to compare all pairs of means. The germination percentage data were previously subjected to arcsine transformation but are reported in tables as untransformed values. All data collected were statistically analysed using SYSTAT 8.0 software (SPSS Inc.).
Experiment 1: Germination under saline conditions
In water, all (100 %) seeds germinated (Table 1 ). At the lower concentrations, individual salts (NaCl, CaCl 2 , KCl and MgCl 2 ) did not have any significant effects on the germination percentage of quinoa seeds. Conversely, dilute SW significantly lowered germination (Table 1 ). With increasing salt concentration, the germination percentage decreased, irrespective of the treatment, except for MgCl 2 . The strongest reduction of germination was observed in the presence of 75 and 100 % SW in comparison to the other salts. The inhibition of different salt solutions on seed germination was in the order of SW > NaCl > KCl > CaCl 2 > MgCl 2 (Table 1 ). There were no significant differences among the treatments in germination rapidity (CVG), except in the SW (Table 1 : with increasing SW concentration, the CVG decreased, with a reduction of 53 % at 75 % SW). The GRI, reflecting the percentage of germination on each day of the germination period, decreased under NaCl and SW. The strongest decrease was observed in SW. No significant differences were observed among NaCl, CaCl 2 , KCl and MgCl 2 and the control, in terms of MGT (MGT, Table 1 ). Conversely, with increasing SW percentage, the MGT increased, reaching values 10 times greater than the control and of the other treatments. The strong significant inverse relationship between SW concentrations and germination indexes confirmed the detrimental effects of the SW on seed germination (Table 1 ).
Germination indices: total germination; CVG, GRI and MGT determined for quinoa seeds after 7 days of germination in the presence of NaCl, CaCl 2 , KCl, MgCl 2 and SW at different concentrations. Data are expressed as percentage in respect to control. Data are the means of five replicates. *** P < 0.001; ** P < 0.01: * P < 0.05.
Separation of ionic and osmotic components
Calculating the relative importance of the osmotic and ionic component stresses showed that the two stressful factors made a different contribution to the deterioration of germination depending on the salts used. In the presence of MgCl 2 , the two stressful factors (ionic and osmotic) had a proportional effect on the reduction of seed germination as shown by the value of the IE/OE ratio (1.0, Table 2 ). Regarding NaCl, the osmotic effect prevailed (IE/OE ratio = 0.53). In CaCl 2 and KCl, at LD 50 concentrations, seed germination decreased, mainly due to osmotic factors, as suggested by the IE/OE ratios that were always <1.0 and by IE values that were under 50 (Table 2 ). Seawater (the most toxic) affected seed germination mainly through its IE as evidenced by the IE/OE ratio >1.0 (Table 2 ).
Influence of osmotic and ionic factors on seed germination of Titicaca quinoa seeds in the presence of NaCl, KCl, MgCl 2 , CaCl 2 and SW at LD 50max concentration. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). The values correspond to the average of five replicates.
Enzyme activities, phenols and antioxidants
With increasing salt concentrations, POX activity decreased, with respect to the control in the presence of NaCl, CaCl 2 and SW. Conversely, an increase in POX activity was observed with MgCl 2 and particularly KCl (Fig. 1 A). Ascorbate peroxidase, CAT and SOD activities were always lower in control seeds compared with treated seeds; the highest concentrations of KCl and SW increased APX activity five and four times, respectively, compared with control. In NaCl and MgCl 2 , APX activity was higher at the lower, than at the higher, concentrations, and it was unaffected by CaCl 2 treatment (Fig. 1 B). Catalase activity increased with increasing concentration of CaCl 2 and SW. In contrast, in the presence of KCl and MgCl 2 , CAT activity decreased when the concentration increased (Fig. 1 C). Superoxide dismutase activity decreased as the concentrations of NaCl and CaCl 2 increased. Conversely, in the presence of increasing concentrations of KCl, MgCl 2 and SW, SOD activity increased, but to different extents. The highest values of SOD were observed in the presence of SW and KCl (Fig. 1 D).

Effect of different salts on POX, APX, CAT, SOD of quinoa seeds 3 days after sowing. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
The amount of total phenols and the total antioxidant capacity of seeds varied with the salt used. Total phenols increased in seeds treated with NaCl and SW, but the greatest increase was observed in the presence of SW (Table 3 ). Increasing the concentrations of KCl and MgCl 2 decreased total phenols; no significant differences were instead observed with increasing the concentration of CaCl 2 with respect to control and the other treatments. Total antioxidant capacity increased in all treated seeds compared with control. The highest antioxidant capacity was detected in the presence of SW (Table 3 ).
Total antioxidant activity and total phenol content in quinoa seeds 3 days after sowing with different salt treatments: A= control; B= 100 mM NaCl, 2.54 mM KCl, 2.54 mM CaCl 2 , 13.38 mM MgCl 2 , 25 % SW; C= 200 mM NaCl, 5.08 mM KCl, 5.08 mM CaCl 2 , 26.76 mM MgCl 2 , 50 % SW; D = 300 mM NaCl, 7.62 mM KCl, 7.62 mM CaCl 2 , 40.1 mM MgCl 2 , 75 % SW; E = 400 mM NaCl, 10.16 mM KCl, 10.16 mM CaCl 2 , 53.52 mM MgCl 2 , 100 % SW. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
Ion contents
In seeds 3 days after sowing, the total quantity of ions increased with increasing concentration of NaCl. A similar response was observed in the presence of SW, the only exception being at the higher concentrations (mainly ungerminated seeds) (Fig. 2 ). In the presence of KCl and CaCl 2 , the total ionic concentration gradually decreased with increasing concentrations of salts due to the increased number of non-germinated seeds (Fig. 2 ). On increasing MgCl 2 concentrations, the reduction in total ion concentration compared with control is likely due to the greater seed dry weight observed (+20 %). The ratio of cations/anions was unchanged in CaCl 2 and MgCl 2 and in NaCl up to a concentration of 400 mM. Increasing the concentration of KCl caused an increase in cations and a concomitant decrease in anion percentage (Fig. 2 ). Seawater, at the lowest concentrations (25 and 50 %), increased the total ions, lowering the amount of cations (33 %) with respect to the anions. Conversely, at the highest concentrations (75 and 100 %), SW decreased the number of germinated seeds and consequently the quantity of total ions but did not affect the cation–anion ratio (Fig. 2 ).

Total ion content, cation and anion percentages in seeds of quinoa after 3 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
The ratio of Na + to cations and of Cl − to anions changed significantly depending on the salts used (Table 4 ). The ratio of Na + /cations increased significantly in comparison to the control with increasing the concentration of NaCl and SW. No differences were observed in the presence of MgCl 2 , while with CaCl 2 a slight decline was observed with respect to the control. The greatest significant decrease in Na + /cations ratio (ranging from 30 to 22 %) was observed in seeds under KCl treatment. For the Cl − /anions ratio, the lowest values were observed in the presence of KCl and the highest with NaCl. Increasing the concentration of SW and NaCl, increased the Na + /Cl − ratio with respect to the control, while this ratio decreased in the presence of other salts when their concentrations increased (Table 4 ). The greatest decrease in K + /Cl − ratio was observed in the presence of NaCl with a reduction ranging from 49 to 87 %. Mg 2+ /Cl − and NH 4 + /Cl − ratios decreased with respect to the control, mainly with increasing salt concentrations (Table 4 ). The Ca 2+ /Cl − ratio decreased in each treatment except for CaCl 2 and KCl. The PO 4 3− /Cl − ratio was significantly reduced compared with control in the presence of SW, NaCl and MgCl 2 (Table 4 ). The highest SO 4 2− /Cl − ratios were observed in the presence of SW and the lowest under NaCl treatment.
Cation and anion content against chloride, in seeds of quinoa treated with different salts, expressed as percentages. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
Growth parameters
Seawater and NaCl, at the highest concentrations, affected the dry weights of the whole seedlings, as shown by the highest fresh weight/dry weight (FW/DW) ratio (Table 5 ), and additionally they reduced the root mass ratio (RMR). These findings suggest that the reduction of root mass may be the cause of the decrease in the total dry matter of the seedlings (Table 5 ). Investigating the root morphology showed that the total root length in all treatments was the most affected root parameter, as shown by F -ratios (Table 6 ). The plants irrigated with SW (50 %) had root lengths, surface areas and root volumes significantly lower than control (Table 6 ).
Total FW/DW ratio, LMR (leaf mass ratio = leaf dry weight/plant dry weight) and RMR (root mass ratio = root dry weight/plant dry weight) of quinoa seedlings after 21 days under different salt treatments. Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
Analysis of variance of the effect of different salt treatments on root morphology parameters of quinoa seedlings 21 days old. *** P < 0.001; ** P < 0.01; * P < 0.05.
Root parameters
Root length to mass ratio (SRL) and root fineness (RF), under SW, were not different from control while the ratio of root mass to volume (RTD) was lower. In seedlings irrigated with 400 mM NaCl, a higher SRL value indicated longer roots per unit root mass, while RTD and RF ratios were significantly reduced (Table 7 ), suggesting a decrease in root length and dry weight of seedlings treated with NaCl (200 mM) or MgCl 2 (26, 76 mM). Root morphology parameters were significantly changed by CaCl 2 and KCl compared with control but to different extents, depending on salt type (Table 7 ). NaCl, MgCl 2 and CaCl 2 , at lower concentrations, significantly increased RTD and RF ratios. No differences were observed when CaCl 2 and NaCl concentrations increased (Table 7 ). KCl, at all concentrations, significantly increased RTD and RF ratios, inducing a root system with thinner roots in comparison with control.
Specific root length (SRL = root length/root DW), root tissue density (RTD = root DW/root volume), root fineness (RF = root length/root volume) of quinoa seedlings after 21 days of different salt treatments. *** P < 0.001; ** P < 0.01; * P < 0.05.
In 21-day-old seedlings, total percentage of ions increased in the presence of NaCl, SW and KCl at all concentrations (Fig. 3 ) and at the highest concentrations of CaCl 2 and MgCl 2 . Total cations (Fig. 3 ) decreased in the presence of NaCl at all concentrations and at the highest concentrations of SW, MgCl 2 and CaCl 2 , with a concomitant increase in anion percentages (Fig. 3 ). No significant differences, in comparison to control, were observed in the presence of KCl.

Total ion content, cation and anion percentages in quinoa seedlings after 21 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
Different salts caused a different distribution of cations and anions between root and shoot (Fig. 4 ). More cations were accumulated in shoots than in roots, decreasing in shoots when NaCl and MgCl 2 concentrations increased, while roots accumulated more anions than cations. The highest accumulation of anions was observed with CaCl 2 and KCl but with a different trend. In CaCl 2 , the anions increased in a concentration-dependent manner; in contrast increasing KCl concentrations lowered the anion percentage (Fig. 4 ). NaCl and MgCl 2 increased the cation concentration in roots as their external concentrations increased (Fig. 4 ).

Cation and anion percentages in root and shoot of quinoa seedlings after 21 days of different salt treatments.
The ratios of Na + /total cations and of Cl − /anions changed significantly depending on the salts used (Table 8 ). The Na + /cations ratio increased in comparison to the control with increasing the concentration of NaCl and SW. In contrast, Na + /cations ratio decreased with increasing the concentration of KCl, MgCl 2 and CaCl 2 . Cl − /anions ratios increased in the different salts at all concentrations, the highest value being observed with NaCl treatment. Increasing the concentration of SW and NaCl increased the Na + /Cl − ratio, while it was lowered in the other salts as their concentration increased. The K + /Cl − ratio decreased in the presence of all salts except for KCl, the greatest decrease being observed in NaCl. The Mg 2+ /Cl − ratio decreased with increasing concentrations of salts, other than for MgCl 2 . A similar situation was seen for the Ca 2+ /Cl − ratio, which decreased in each treatment except for CaCl 2 . The NH 4 + /Cl − ratio decreased in all situations as did SO 4 2− /Cl − ratios, where the highest values were detected in SW (Table 8 ).
Cation and anion content against chloride, in seedlings of quinoa treated with different salts, expressed as percentages. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). The values correspond to the average of five replicates.
The activity of the antioxidant enzymes depended on the salt and on the concentrations used (Fig. 5 ). Ascorbate peroxidase activity significantly decreased in the presence of MgCl 2 and KCl. In contrast, it increased in CaCl 2 -, SW- and NaCl-treated seedlings compared with control. POX activity increased in all treatments except for MgCl 2 and KCl. The most significant increase in catalase activity was in NaCl and SW. The same trend was observed for the SOD activity, with the highest values seen in the presence of SW and NaCl.

Effect of different salts on antioxidant enzymatic activities of quinoa seedlings after 21 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
A significant increase in total phenols was observed in seedlings grown with NaCl and SW (Table 9 ). The SW was the most damaging agent, causing a 2-fold increase in the concentration of phenols. The total antioxidant capacity was doubled by NaCl and tripled by SW in respect to the control (Table 9 ).
Total antioxidant activity and total phenol content in quinoa seedlings after 21 days with different salt treatments: A = 200 mM NaCl, 5.08 mM KCl, 5.08 mM CaCl 2 , 26.76 mM MgCl 2 , 50 % SW; B = 400 mM NaCl, 10.16 mM KCl, 10.16 mM CaCl 2 , 53.52 mM MgCl 2 , 100 % SW. Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
In the Mediterranean region, besides water scarcity or high coastal soil salinity, it is mainly where saline water is used for irrigation that adverse effects are seen on crops, delaying or preventing germination and seedling growth ( Hegarty 1978 ; Almodares et al. 2007 ). Utilization of halophytes as crops would help in highly salinized zones, where only poor quality water, unsuitable for most agriculture, is available ( Rozema and Flowers 2008 ).
In this context, quinoa a facultative halophyte with exceptional nutritional quality could be useful to recover salinized land and to increase the poor agricultural economy of semiarid regions of the Mediterranean area. Our study focused on germination and seedling growth, because crop establishment depends on a successful germination and seedling emergence. Optimal germination for most halophytes has been reported in non-saline conditions ( Khan et al. 2002 ; Gul et al. 2013 ), and our data conform to these findings, showing toxicity of different salts. Results provided evidence for the existence of both ionic and osmotic effects by different treatments on seeds, depending on the salts used.
Our data clearly demonstrated that SW was the most detrimental solution affecting seed germination and seedling emergence of quinoa, mainly through its IE, confirming previous work showing that germination of halophytes was inhibited more by SW than different chlorides of Na, K, Mg ( Joshi et al. 1995 ). There is little information available on comparative influence of single salts and SW on seed germination of other halophytes ( Joshi et al. 1995 ; Baskin and Baskin 1998 ; Houle et al. 2001 ; Zia and Khan 2002 ; Atia et al. 2006 ; Liu et al. 2006 ). Some authors found NaCl more detrimental than SW and others the opposite ( Tirmizi et al. 1993 ; Zia and Khan 2002 ; Duan et al. 2003 ). Our data showed that the inhibition of different salt solutions on seed germination was in the order of SW > NaCl > KCl > CaCl 2 > MgCl 2 with no significant differences among the treatments in germination rapidity, except for the SW. The greatest negative effects of SW may be due to ion toxicity on germination, as a consequence of a coincident increase in cations and anions. Ion toxicity during germination has been previously demonstrated by Zehra et al. (2013) for the halophytic reed Phragmites karka : the inhibitory effect of different salts was interpreted mainly as an IE.
Although NaCl is the predominant salt in SW, its effects on seed germination and seedling growth were less detrimental than SW itself. The negative effects of SW on seedling growth may be ascribed to the induced accumulation of SO 4 2− (7.67 mmol g −1 DW, at least five times more than the other treatments) in leaves and of SO 4 2− (0.88 mmol g −1 DW) and Cl − (47.97 mmol g −1 DW) in roots. Sulfate is one of the components of sulfur-containing amino acids (cysteine and methionine) and many other compounds (e.g. glutathione or ferredoxin), which play important physiological functions, but when SO 4 2− is present in high concentration, it may affect plant development and crop yield, becoming injurious to plants ( Lianes et al. 2013 ). Lianes et al. (2013) previously showed that when the SO 4 2− is present in the medium, the capacities for ion compartmentalization and osmotic adjustment were reduced in the halophyte Prosopis strombulifera , resulting in water imbalance and symptoms of toxicity due to altered carbon metabolism (e.g. synthesis of sorbitol instead of mannitol, reduced sucrose production and protein content). This inhibition was partially mitigated when SO 4 2− and Cl − were present together in the solution, demonstrating a detrimental effect of the sulphate ion on plant growth ( Reginato et al. 2013 ).
According to Munns (2002) , the time scales for the osmotic and specific ionic component of salinity stress differ significantly, with the osmotic component dominating the first several days. Interestingly, however, comparing seed germination and seedling growth in the different salts, the results suggest that most probably ion toxicity is more detrimental to seedlings compared with the osmotic component of salt stress, as evidenced by the effect of SW treatment. This high salinity tolerance of quinoa, during germination and early seedling growth, may be explained by the existence of a significant gradient in the accumulation of potentially toxic (Na and Cl) and non-toxic essential (K, Mg, Ca, P and S) elements in seeds and also in the different distribution between shoot and root in salt-treated seedlings, as already demonstrated by Koyro and Eisa (2008) . Hence, we suggest that, once the seed's ability to exclude toxic Na + from the developing embryo fails, ion toxicity occurs, and seeds become unviable. The details of the distributions of ions between root and shoot showed differences among treatments; specifically with NaCl in shoot, we observed a significant accumulation of Na + , and little Cl − . In accordance with previous investigations ( Eisa et al. 2000 ), Na + was shown to be preferentially accumulated in shoots thereby the plants avoid excessive ion accumulation in the root tissues ( Koyro 2000 ; Ashraf et al. 2006 ).
Seawater caused an accumulation of Na + and SO 4 2− both in roots and in shoots, and an accumulation of Cl − in roots. Excessive accumulation of ions in halophytes (salt includers) under high substrate salinities (such a full strength SW) can lead to toxic effects in plants ( Munns 2005 ). The cause of injury is probably the salt load exceeding the ability of cells to compartmentalize salts in the vacuole. Salts might then build up rapidly in the cytoplasm inhibiting enzyme activity or alternatively, they might build up in cell walls, dehydrating the cell.
Considering the high energy cost of de novo synthesis of organic osmolytes ( Raven 1985 ), we can suppose that the seedlings tend to use Na + for osmotic adjustment. Hariadi et al. (2011) previously showed in quinoa that accumulation of Na + and K + was responsible for >95 % of cell turgor in old leaves and between 80 and 100 % in young leaves. A further role in the maintenance of turgor was also attributed to Cl − accumulated in roots ( James et al. 2006 ). Our results showed that the Cl − concentration was more than enough to contribute to osmotic adjustment maintaining root turgor as previously demonstrated in seedling of Stylosanthes guianensis by Veraplakorn et al. (2013) . Thus, it appears that the better germination and growth of cv. Titicaca observed in NaCl with respect to the other salts and SW may be achieved by the accumulation of inorganic osmolytes, particularly of Na + in shoots, and of Cl − in roots. The differences in ion uptake and distribution may be ascribed to properties of the roots. Roots have a high degree of plasticity, enabling plants to cope with a wide range of soil constraints ( Ho et al. 2005 ; Panuccio et al. 2011 ). Root morphology is a compromise among costs of resource capture, transport and efficiency ( Malamy 2005 ). Some morphological modifications at the individual root level can affect the structural and physiological characteristics of the entire root system and this can change water uptake and nutrient supply by plants. Specific root length, indicating root functionality ( Ryser 2006 ), characterizes the economic aspects of a root system, defining the cost-benefit ratio. Generally, under high salinity the costs per root length is minimized because of the growth limiting conditions. SW (50 %) reduced root growth and elongation, suggesting a decrease in photosynthate supply from the shoot. At the highest NaCl concentration, the greatest SRL ratio suggests the plants maximized the effectiveness of roots in water and nutrient uptake ( Fitter 1991 ). At the lowest concentrations of NaCl, KCl, CaCl 2 and MgCl 2 , the high root tissue density and root fineness ratios indicated that the seedlings explored a larger soil volume per unit of root surface area under stress than in its absence. In short, our data suggest that root morphology modifications should not be considered as a simple growth reduction, but rather as an induced reorientation of growth to avoid stress.
The results of this study clearly indicated that salt tolerance in this variety of quinoa is largely conferred by a delicate balance between osmotic adjustment and ion accumulation, showing differences in the ion compartmentalization between root and shoot. The greater negative effect of SW compared with NaCl, MgCl 2 CaCl 2 and KCl used separately suggests an additive and/or an interactive effect of these salts which cause an accumulation of ions in excess or leading to ion toxicity.
In conclusion, the present findings allow us to speculate that quinoa cv. Titicaca is a NaCl-tolerant cultivar of quinoa. Osmotic adjustment to NaCl salinity is largely conferred by inorganic ions, especially Na + , the main osmoregulatory material in the seedlings. The high SRL contributed to a high relative NaCl salinity tolerance in Titicaca, maintaining water and nutrient uptake. Higher SW toxicity may have been caused by SO 4 2− accumulation in seedlings that affected Titicaca germination and growth more than Cl − . Even if salinity reduced the productivity in terms of biomass, there was an increase in the antioxidant compounds, important health-protecting factors in food. On the basis of salt soil classifications currently used in all countries of the world, our results suggest that saline-sodic soils may be suitable for the cultivation of quinoa.
The research in the Mediterranea University laboratory and travelling was funded by Fattoria della Piana Company and by COST (STSM FA0901).
S.S.A. participated in the experiments, M.R.P. and A.M. did the experiments, analysed the data and wrote the manuscript, S.E.J. participated in the writing of the manuscript, acquired the funds for S.S.A. through the COST action ‘Putting Halophytes to Work’, and provided quinoa seed material for the study.
None declared.
The authors thank Carmelo Mallamaci for technical assistance and for taking care of the plants.
Google Scholar
Google Preview
Author notes
Email alerts, citing articles via, affiliations.
- Online ISSN 2041-2851
- Copyright © 2024 Annals of Botany Company
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.6.4: Germination
- Last updated
- Save as PDF
- Page ID 32044

- Melissa Ha, Maria Morrow, & Kammy Algiers
- Yuba College, College of the Redwoods, & Ventura College via ASCCC Open Educational Resources Initiative
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Identify the environmental factors that stimulate germination.
- Distinguish between epigeous and hypogeous germination.
- Compare germination in eudicots versus monocots.
Many mature seeds enter a period of inactivity, or extremely low metabolic activity: a process known as dormancy , which may last for months, years or even centuries. Dormancy helps keep seeds viable during unfavorable conditions. Germination occurs when the embryo, which is dormant within a mature seed, resumes growth upon a return to favorable conditions. The embryo becomes a young seedling that is no longer confined within the seed coat.
In many seeds, the presence of a thick seed coat can inhibit germination through several mechanisms: (1) the embryo may not be able to break through the thick seed coat; (2) the seed coat may contain chemicals inhibitors; and (3) the seed coat prevents the embryo from accessing water and oxygen. Dormancy is also maintained by the relative hormone concentrations in the embryo itself.
Environmental Requirements for Germination
The requirements for germination depend on the species. Common environmental requirements include light, the proper temperature, presence of oxygen, and presence of water. Seeds of small-seeded species usually require light as a germination cue. This ensures the seeds only germinate at or near the soil surface (where the light is greatest). If they were to germinate too far underneath the surface, the developing seedling would not have enough food reserves to reach the sunlight. (Recall from 14.5 Dormancy that red light induces germination by converting the inactive form of phytochrome (Pr) to the active form (Pfr), which leads to the production of amylase. This enzyme breaks down the limited food reserves in the seed, facilitating germination.)
Not only do some species require a specific temperature to germinate, but they may also require a prolonged cold period prior to germination. In this case, cold conditions gradually break down a chemical germination inhibitor. This mechanism prevents seeds from germinating during an unseasonably warm spell in the autumn or winter in temperate climates. Similarly, plants growing in hot climates may have seeds that need a hot period in order to germinate, an adaptation to avoid germination in the hot, dry summers.
Water is always needed to allow vigorous metabolism to begin. Additionally, water can leach away inhibitors in the seed coat. This is especially common among desert annuals. Seeds that are dispersed by animals may need to pass through an animal digestive tract to remove inhibitors prior to germination. Similarly, some species require mechanical abrasion of the seed coat, which could be achieved by water dispersal. Other species are fire adapted, requiring fire to break dormancy (Figure \(\PageIndex{1}\)).
The Mechanism of Germination
The first step in germination and starts with the uptake of water, also known as imbibition . After imbibition, enzymes are activated that start to break down starch into sugars consumed by embryo. The first indication that germination has begun is a swelling in the radicle.
Depending on seed size, the time taken for a seedling to emerge may vary. Species with large seeds have enough food reserves to germinate deep below ground, and still extend their epicotyl all the way to the soil surface while the seedlings of small-seeded species emerge more quickly (and can only germinate close to the surface of the soil).
During epigeous germination , the hypocotyl elongates, and the cotyledons extend above ground. During hypogeous germination , the epicotyl elongates, and the cotyledon(s) remain belowground (Figure \(\PageIndex{2}\)). Some species (like beans and onions) have epigeous germination while others (like peas and corn) have hypogeous germination. In many epigeous species, the cotyledons not only transfer their food stores to the developing plant but also turn green and make more food by photosynthesis until they drop off.
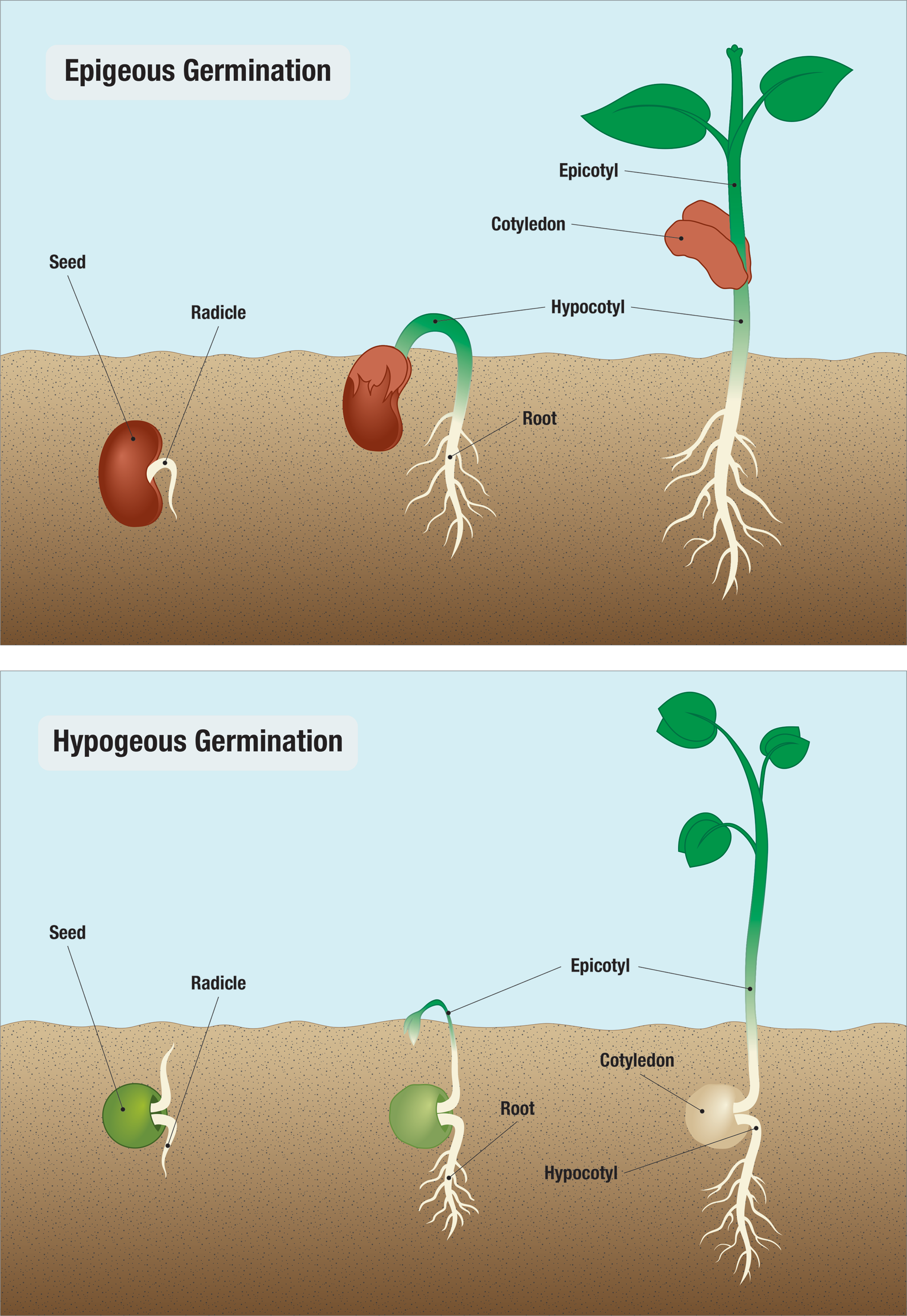
Germination in Eudicots
Upon germination in eudicot seeds, the radicle emerges from the seed coat while the seed is still buried in the soil.
For epigeous eudicots (like beans), the hypocotyl is shaped like a hook with the plumule pointing downwards. This shape is called the plumule hook, and it persists as long as germination proceeds in the dark. Therefore, as the hypocotyl pushes through the tough and abrasive soil, the plumule is protected from damage. Additionally, the two cotyledons additionally protect the from mechanical damage. Upon exposure to light, the hypocotyl hook straightens out, the young foliage leaves face the sun and expand, and the epicotyl elongates (Figure \(\PageIndex{3}\)).
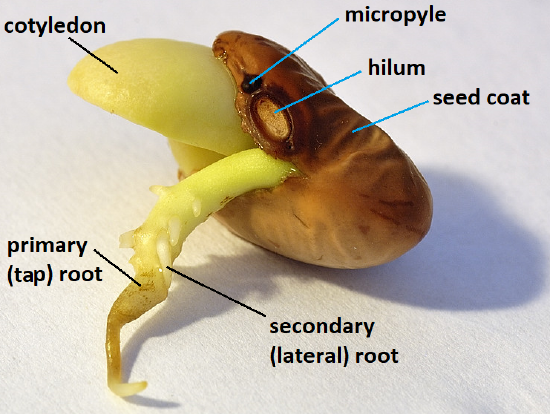
In hypogeous eudicots (like peas), the epicotyl rather than the hypocotyl forms a hook, and the cotyledons and hypocotyl thus remain underground. When the epicotyl emerges from the soil, the young foliage leaves expand. The epicotyl continues to elongate (Figure \(\PageIndex{4}\)).
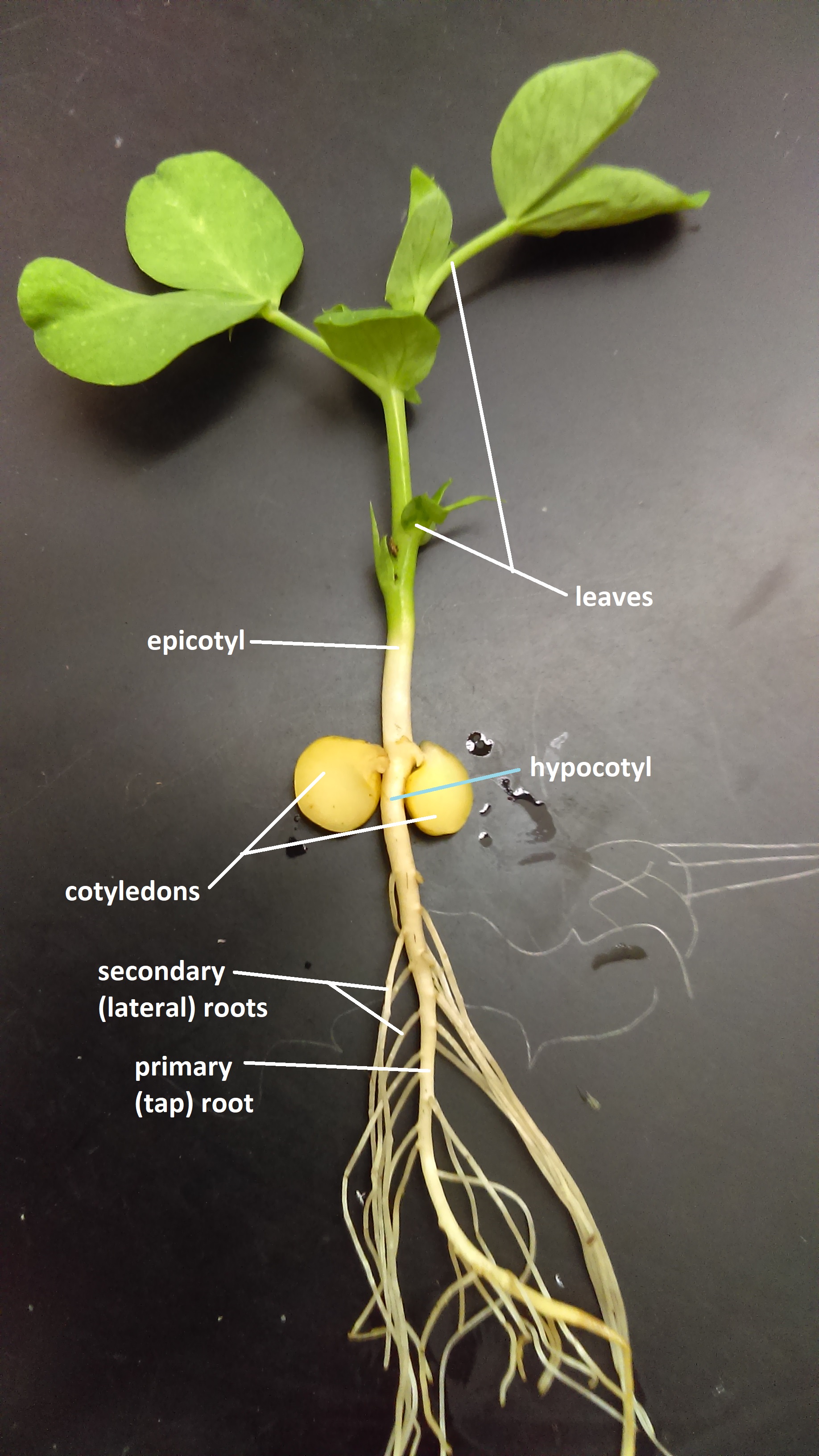
The radicle continues to grown downwards and ultimately produces the tap root. Lateral roots then branch off to all sides, producing the typical eudicot tap root system.
Germination in Monocots
As the seed germinates, the radicle emerges and forms the first root. In epigeous monocots (such as onion), the single cotyledon will bend, forming a hook and emerge before the coleoptile (Figure \(\PageIndex{5}\)). In hypogeous monocots (such as corn), the cotyledon remains belowground, and the coleoptile emerges first. In either case, once the coleoptile has exited the soil and is exposed to light, it stops growing. The first leaf of the plumule then pieces the coleoptile (Figure \(\PageIndex{6}\)), and additional leaves expand and unfold. At the other end of the embryonic axis, the first root soon dies while adventitious roots (roots that arise directly from the shoot system) emerge from the base of the stem (Figure \(\PageIndex{7}\)). This gives the monocot a fibrous root system.

Attributions
Curated and authored by Melissa Ha using the following sources:
- 16.4B Germination of Seeds from Biology by John W. Kimball ( CC-BY )
- 32.2 Pollination and Fertilization from Biology 2e by OpenStax (licensed CC-BY ). Access for free at openstax.org .
- 7.5 Origin of the Seed from Introduction to Botany by Alexey Shipunov (public domain)

Investigation: What Factors Affect Seed Germination?

Introduction
In the spring, flowers begin to bloom and you may see sprouts in the garden. How do plants know when it's spring and when to grow? If a seed grew too early, it might be exposed to a harsh cold environment. If it grew too late, it might not have enough water to survive. The process by which an organism grows from a seed into a plant is called germination . The seed of a plant is the embryo, and it contains enough energy for the plant to survive until it is time to start growing. In some cases, these seeds can survive for years.
What triggers the seed to start growing? It varies by plant and by the environmental conditions where the plant grows. The giant sequoia trees of California require fire to germinate. This adaptation ensures that new trees will grow when there is an opening created by the death of other trees. If the seeds germinated without fire, the seedlings would be too shaded to grow.
Not all plants need fire to start growing, but most do take the cues from the environment. In this activity, you will investigate factors that can affect the germination of a seedling.
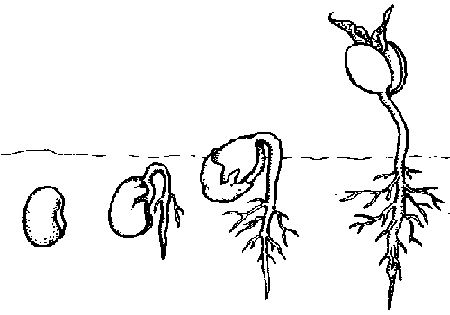
Part 1: Consider a Question / Hypothesis
Several variables might affect the germination, these variables include: light, temperature, water, soil type, air quality.
Choose ONE variable to investigate. Write a hypothesis below. Remember, that the hypothesis should be a complete sentence that can be tested.
Part 2: Design an Experiment
With your lab group, determine how you could test the variable.
What kind of data will you gather? Will you have a control group? What kind of materials or equipment will you need? How many seeds will you use?
Available Materials ( other materials may be available, check with your instructor ): radish or bean seeds, ziploc bags, paper towels, window/sunlight, water, vinegar, soil, refrigerator
Sketch or describe your experiment below. Your instructor will approve your plan before you set it up.
Part 3: Gather Data
Most seeds will germinate in a few days. Plan to make observations over the next few days and organize your observations below. Be as detailed as you can about what you see.
Part 4: Publish Your Results
Create a lab report or infographic that includes the following information from this investigation. You have written things down on this paper, now it is time to organize the information into a formal report. This report should include four detailed sections.
1. Introduction : includes background information about the lab and your hypothesis
2. Experimental Design : include a drawing and/or description of how you set up your experiment
3. Data : Organize observations into a data table
4. Conclusions : Use your data to answer the experimental question. Be specific in how you write this, your conclusions must follow your data, even if they didn't turn out as you were expecting.
5. Reflection : In this section, discuss how your results may provide insights into how the environment can affect plants in general and how this information can be useful to humans. For example why would farmers need to consider germination factors when planting crops?
Other Lessons on Plants
Investigation – Rate of Photosynthesis – using baking soda, elodea and light, measure the bubbles to observe how fast a plant photosynthesizes and releases oxygen
Investigation – Algae Beads and Photosynthesis – use algae cultures and sodium alginate, measure photosynthesis by changes in color of indicator
Investigation: Photosynthesis – this lab uses leaf disks that float to indicate photosynthesis. Students investigate factors that affect photosynthesis. (AP Lab)
Investigation: Photosynthesis and Plant Growth (virtual) – use a virtual app to show how plant growth changes in response to light color and light intensity
Investigation: Separation of Plant Pigments – use chromatography to show how leaves contain pigments that separate

- Free Resources
- Project Search
- Featured Projects
- Member Benefits
1059 Main Avenue, Clifton, NJ 07011
The most valuable resources for teachers and students

(973) 777 - 3113
1059 Main Avenue
Clifton, NJ 07011
07:30 - 19:00
Monday to Friday
123 456 789
Goldsmith Hall
New York, NY 90210

- Why We’re Unique
Factors affecting seed germination (e.g. soil, temperature, pH)
Introduction: (initial observation).
If you have ever planted some seeds, you may have noticed that some seeds do not germinate.
A good quality seed is the first step toward producing a good crop. If the seed does not germinate, the cost of seed and all related plantation and irrigation costs will be a loss for the farmer. Scientists and farmers continuously try to identify any possible factor that may affect seed germination in order to have a higher rate of germination. The rate of germination is one of the key elements in determining the quality and value of any seed.
Seeds that do not germinate are wasted and will gradually become a part of other organic maters in the soil.
This project guide contains information that you need in order to start your project. If you have any questions or need more support about this project, click on the “Ask Question” button on the top of this page to send me a message.
If you are new in doing science project, click on “How to Start” in the main page. There you will find helpful links that describe different types of science projects, scientific method, variables, hypothesis, graph, abstract and all other general basics that you need to know.
Project advisor
Please note that you will only select one of the above factors to research on. You will need to adjust this project guide and it’s experiments based on the factor that you choose to study. So, the actual title of your project will be one of the following:
The effect of pH on seed germination or
The effect of temperature on seed germination or
The effect of soil on seed germination
If you decide to study on more than one factor, you need to repeat your experiments for each factor that you study. You will also have a separate hypothesis for each factor.
Other similar research topics that can be performed using the steps provided in this project guide:
- The effect of light on seed germination
- The effect of UV on seed germination
- The effect of fertilizers on seed germination
- The effect of mold on seed germination
- The effect of salt (or any other chemical that you choose) on seed germination.
Information Gathering:
Find out about what you want to investigate. Read books, magazines or ask professionals who might know in order to learn about the effect or area of study. Keep track of where you got your information from.
Review the definition of seed germination. Learn about any other research performed by others on seed germination. Also learn about the seed that you want to study. The result of your experiments is only valid for the type of seed that you choose to study. You can not generalize the results and propose a conclusion that covers all different types of seeds. You may also want to modify the title of your project to cover the name of seed that you study. For example if you choose to study on lettuce seed, you may change the title of your project to one of the following:
The optimum pH for germination of lettuce seed or
The optimum temperature for germination of lettuce seed
If you don’t have much time for your project, you should select one of the fast germination seeds. Following are some fast germinating seeds:
Rapid Germination Seeds (assuming ideal moisture and temperature)
Where to find more information:
http://forest.wisc.edu/forestry415/TreeStructure/flowers/germ.htm
http://www.marijuanasignpost.com/guides/seedgerm.html
Seed Germination and Fruit Types
The Seed Biology Place
Question/ Purpose:
What do you want to find out? Write a statement that describes what you want to do. Use your observations and questions to write the statement. Depending on your final choice of project title, following are some sample questions:
- The purpose of this investigation is to identify the best pH for the germination of lettuce seed.
- The purpose of this investigation is to know the best temperature for germination of lettuce seed.
Identify Variables:
When you think you know what variables may be involved, think about ways to change one at a time. If you change more than one at a time, you will not know what variable is causing your observation. Sometimes variables are linked and work together to cause something. At first, try to choose variables that you think act independently of each other. Depending on the question/purpose of your project following are two sample of identifying variables:
- The independent variable is the pH. Dependent variable is the rate of germination. Controlled variables are light, temperature, soil, moisture, seed specification such as moisture, size, etc..
- The independent variable is the temperature. Dependent variable is the rate of germination. Controlled variables are light, pH, soil, moisture, seed specification such as moisture, size, etc..
Hypothesis:
Based on your gathered information, make an educated guess about what types of things affect the system you are working with. Identifying variables is necessary before you can make a hypothesis. Following are some sample hypothesis:
- I think the neutral pH is the best pH for seed germination. This hypothesis is based on my study of seed and the fact that a large part of seed is food that plant needs during germination period. So I think if the seed has it’s own food to germinate, it must also have all necessary minerals and other chemicals needed at a proper pH. Another hypothesis for pH effect: I think a slightly acidic pH such as pH 4 is the best pH. Slightly acidic pH can prevent growth of mold and other fungus that may damage seed. Also slightly acidic pH will dissolve more minerals from the soil and make them available to the plant.
- I think that temperature around 72º F is the best temperature for seed germination because this is the average weather temperature in the spring when most plants emerge. Another hypothesis for temperature effect: I think a temperature around 80º F up to 90º F is best temperature for seed germination. My hypothesis is based on my gathered information that indicates chemical and biochemical reactions will accelerate by heat. Also excess heat can be harmful to live organisms, so the temperature range of 80º F to 90º F which is almost the summer time temperature can be the best temperature for seed germination.
Experiment Design:
Design an experiment to test each hypothesis. Make a step-by-step list of what you will do to answer each question. This list is called an experimental procedure. For an experiment to give answers you can trust, it must have a “control.” A control is an additional experimental trial or run. It is a separate experiment, done exactly like the others. The only difference is that no experimental variables are changed. A control is a neutral “reference point” for comparison that allows you to see what changing a variable does by comparing it to not changing anything. Dependable controls are sometimes very hard to develop. They can be the hardest part of a project. Without a control you cannot be sure that changing the variable causes your observations. A series of experiments that includes a control is called a “controlled experiment.”
Experiment 1:
Seed Observation experiment (this is just a warm-up experiment)
Soil is an environment that provides moisture and oxygen to the seed. If the seed is under a layer of soil, we will not be able to observe the progress of seed germination. That is why we need to use other methods of seed plantation for our experiments. In this experiment we use a plastic sandwich bag and paper towel for seed germination. We expect that plastic bag will keep moisture and oxygen in the environment. Paper towel will keep moisture around the seed, otherwise seeds may get fully submerged or be in dry section of the bag.
Materials needed:
- a resealable plastic sandwich bag
- paper towel or napkin
- a cup of water
- a packet of pea or bean seeds
Soak seeds in a cup of water overnight.
Sprinkle water on the paper towel or napkin so it’s wet but not dripping.
Put the wet paper towel and the seeds in the sandwich bag, make sure you can see the seeds without opening the bag.
Seal the bag. Place the bag in a warm safe place away from direct sunlight (so it doesn’t get too hot). Check it several times a day, open it for a few seconds to give the seeds air. Then seal it to keep the moisture in. If the paper looks dry, open the bag and sprinkle more water, then make sure it’s sealed.
Soon, you’ll see the baby plant start growing and developing!!

One way to keep track of what happens to your seed is to draw it once a day. Make your first drawing of the seed before you soak it.
You can also try another experiment. Repeat the above experiment with seeds that you didn’t soak. When you do the experiment, think like a scientist. Scientists ask themselves questions. Here are some questions you could ask yourself when you do one of these experiments: Do you think the two kinds of seeds will germinate differently? How will they be different?
After you do one experiment, some other questions may come up. They might be answered by another experiment. Some suggested experiments:
- prepare two identical bags, place one in the dark and one in the light.
- prepare two identical bags. In one of the bags, place the seeds so the radicle faces toward the ceiling. In the other, place them so the radicle faces the floor.
- prepare two identical bags. Once a day, rotate the seeds in one of the bags, let the other bag sit still.
Feel free to try different experiments. Just remember, test one thing at a time and always prepare a control bag (one that you don’t change anything). That way you can compare the two bags to see if you made a difference.
Experiment 2:
The effect of UV radiation on seed germination:
In this project we want to see the effect of exposure to UV radiation in seed germination. We think that UV light might have some sterilization effect on the seed and prevent growth of harmful bacteria and mold on the seed, resulting a higher rate of germination. We are also worried that UV exposure may cause biological damage to seed that can prevent seed germination. (Note that this introduction also serves as hypothesis for this experiment.)
- Expose 10 of the selected seeds to the UV light for a period of 5 minutes. CAUTION!!! UV light can damage both your eyes and skin. Use all recommended safety precautions. We recommend adult supervision for this step.
- Take one piece of paper towel and fold it in half, and then in half again. Place the folded paper towel in the bottom of the plastic container.
- Pour approximately 15 milliliters of water over the paper towel. The entire paper towel should be wet, but without extra water in the bottom of the container. Should you need more water, use it, but measure and record so that you can use similar amounts with all other seed containers.
- Use your tweezers to place the 10 seeds on the paper towel. Make 2 rows of seeds with five seeds in each row.
- Put the lid on the container and snap it on tight to preserve the moisture.
- Label the container using masking tape. List the length of time the seeds were exposed to UV light, the date, and type of seeds (if using more than one type).
- Place the container in an out of the way place where it will not be disturbed. A warm, dark location such as a closet or under the bed would be ideal. However, handle the container with care so the seeds don’t slide all over.
- Repeat steps 1 through 7 with the only variation being an increased time of exposure to UV light. The first exposure was 5 minutes so we recommend trying 10, 15, 30, 60 and 120 minutes.
- Repeat steps 2 through 7 with 10 seeds to be your control. DO NOT expose these last seeds to UV radiation.
Making Your Observations
- Take a daily look at your seeds and check for any sign of a “sprout” or “emerging radicle” coming out of the seed. Seeds have germinated when they get such “sprouts”. Some of the sprouts might grow so fast that you can see the seed’s stem and roots with tiny hairs. Use a magnifying glass and enter illustrations of these sprouts in your log book as soon as you see evidence of them.
- By the seventh day any seeds that are going to sprout will have done so. At this time you should COUNT the number of seeds for which you can see the sprout coming out of the seed, even if it’s very small. A broken seed coating does not count if there is no sprout.
- Record the number of seeds germinated in each container.
- Measure the combined stem and root length of each “sprout” with a metric ruler and record their lengths in millimeters. Construct a table for this data for each container.
Your data/results table may look like this:
Make a graph:
You can make two different bar graphs to visually present your results.
For the germination ratio graph make one vertical bar for each exposure time starting 0 or no exposure up to 120 minute exposure. The height of each bar will represent the ratio of seeds germinated in that group.
For the speed of germination and growth graph make one vertical bar for each exposure time. The height of each bar will represent the average length of seedling (combined stem and root length) in the group.
Experiment 3:
The effect of pH on seed germination
In this experiment you use solutions of different pH from 2 to 11 instead of pure water. Handling low pH and high pH solutions requires goggles and other safety precautions as well as adult supervision.
- Prepare 10 solution with 10 different pH in 10 different bottles. Label all bottles with the pH of the solution in that bottle. Use acetic acid to lower the pH and use ammonia to increase the pH. It is good if you use pHs of 2 to 11. Use pH meter or pH paper to adjust the pH in each bottle.
- Pour approximately 15 milliliters of first solution (pH=2) over the paper towel. The entire paper towel should be wet, but without extra water in the bottom of the container. Should you need more water, use the same solution, but measure and record so that you can use similar amounts with all other seed containers.
- Put the lid on the container and snap it on tight to preserve the moisture. Label the container using masking tape. List the pH of the solution, the date, and type of seeds (if using more than one type).
- Repeat steps 1 through 7 with the only variation being the pH of water solution.
- Repeat steps 2 through 7 with 10 seeds to be your control. DO NOT adjust the pH of this last group. Use regular tap water or distilled water.
For the germination ratio graph make one vertical bar for each pH, starting the control and then 1 to 11. The height of each bar will represent the ratio of seeds germinated in that group.
For the speed of germination and growth graph make one vertical bar for each pH, starting with the control and then 1 to 11. The height of each bar will represent the average length of seedling (combined stem and root length) in that group.
Experiment 4:
The effect of temperature on seed germination
This experiment is similar to experiment 2. The difference is that you will not expose any seeds to UV radiation, instead you place your containers in locations with different temperatures. The challenge for this investigation is how to create different temperatures and keep them constant for up to 7 days.
In laboratories incubators are used for temperatures higher than room temperature and refrigerators are used for temperatures lower than room temperature. Incubators usually are not available for students who want to perform such experiments at home. However other places can be found at home that have higher or lower temperature than room temperature.
Get a thermometer and check the temperature in different locations inside your refrigerator and different locations in your basement or backyard or any other place that may have a relatively constant temperature. Decide which of these locations you want to use and place your samples in these locations. Label each container with the temperature of location that you choose to place.
In all of the above experiments you can use petri dishes instead of plastic bags and plastic containers. Petri dish cap will keep moisture inside while you can observe the seeds without removing the caps.
The picture on the right shows different bean seeds in before and after germination.
Recording Data:
Count the total number of seeds in each group and the number of seeds germinated on that group. Enter them in the the table. Divide the number of germinated seeds by the total number of seeds in each group and write the result in the Germination Ratio column.
You can use a bar graph to visually present your results. Make one vertical bar for each group. The height of the bar will show that ratio of the germination.
Variations of this experiment:
Instead of the rate of germination (the germinated ratio) you may want to measure and record the speed of germination. In this case you will measure the overall height of seedlings (from root to the shoot) in each group after a certain number of days (usually 7 days or 10 days). Then you take an average of the results in each group and write that in your results table and use that to make a graph.
Materials and Equipment:
Material and equipment that you may need for projects in this page are:
- Plastic containers with lids.
- Masking tape and marker for labeling containers.
- Paper towels and tap tap water.
- Zip lock plastic bags
- Graduated cylinder or other measurement device for water measurement.
- Latex Gloves or tweezers for seed handling (Don’t touch the seeds to avoid infection of seeds by the bacteria of your hand)
- A supply of 100 or more radish seeds (or other fast germinating seeds of your choice)
- Access to a UV light (if a UV light cannot utilized from your school’s science lab, check both the local hardware store and local flower/garden shops. Borrow or rent if possible as UV lights could cost $35-$40 or more. UV lights are also known as black light)
- Acetic acid and ammonia solution.
Depending on the subject and experiments that you choose you may not need all the above.
Results of Experiment (Observation):
Experiments are often done in series. A series of experiments can be done by changing one variable a different amount each time. A series of experiments is made up of separate experimental “runs.” During each run you make a measurement of how much the variable affected the system under study. For each run, a different amount of change in the variable is used. This produces a different amount of response in the system. You measure this response, or record data, in a table for this purpose. This is considered “raw data” since it has not been processed or interpreted yet. When raw data gets processed mathematically, for example, it becomes results.
Record the results of your experiments in tables like this:
Effect of pH on the rate of germination of lettuce seeds: (Just an example)
Comments in the table cells is what you observe on a daily bases. Last row shows the rate of germination
Calculations:
You will need to calculate the rate of germination by dividing the number of germinated seeds by the total number of seeds in each test container.
Summary of Results:
Summarize what happened. This can be in the form of a table of processed numerical data, or graphs. It could also be a written statement of what occurred during experiments.
It is from calculations using recorded data that tables and graphs are made. Studying tables and graphs, we can see trends that tell us how different variables cause our observations. Based on these trends, we can draw conclusions about the system under study. These conclusions help us confirm or deny our original hypothesis. Often, mathematical equations can be made from graphs. These equations allow us to predict how a change will affect the system without the need to do additional experiments. Advanced levels of experimental science rely heavily on graphical and mathematical analysis of data. At this level, science becomes even more interesting and powerful.
Can you make a better display?
When you report the result of your experiments, you may also create a chart or graph to provide a visual representation of the final results. Following is a sample that shows the rate of germination of different seeds. (So dependent variable has been the type of seed instead of pH or temperature)

Conclusion:
Using the trends in your experimental data and your experimental observations, try to answer your original questions. Is your hypothesis correct? Now is the time to pull together what happened, and assess the experiments you did.
You may try soil instead of paper towel in your experiments. You may also use the germinated seeds or fully grown plants as a part of your display.

Related Questions & Answers:
What you have learned may allow you to answer other questions. Many questions are related. Several new questions may have occurred to you while doing experiments. You may now be able to understand or verify things that you discovered when gathering information for the project. Questions lead to more questions, which lead to additional hypothesis that need to be tested.
Possible Errors:
If you did not observe anything different than what happened with your control, the variable you changed may not affect the system you are investigating. If you did not observe a consistent, reproducible trend in your series of experimental runs there may be experimental errors affecting your results. The first thing to check is how you are making your measurements. Is the measurement method questionable or unreliable? Maybe you are reading a scale incorrectly, or maybe the measuring instrument is working erratically.
If you determine that experimental errors are influencing your results, carefully rethink the design of your experiments. Review each step of the procedure to find sources of potential errors. If possible, have a scientist review the procedure with you. Sometimes the designer of an experiment can miss the obvious.
References:
List of References
It is always important for students, parents and teachers to know a good source for science related equipment and supplies they need for their science activities. Please note that many online stores for science supplies are managed by MiniScience.
Testimonials
" I called School Time and my husband and son came with me for the tour. We felt the magic immediately."
- Robby Robinson
" My husband and son came with me for the tour. We felt the magic immediately."
- Zoe Ranson
Contact Info
Our address, working hours.
Week Days: 07:00-19:00
Saturday: 09:00-15:00
Sunday: Closed
Science Project
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Plants (Basel)

The Effects of Temperature and Water on the Seed Germination and Seedling Development of Rapeseed ( Brassica napus L.)
Associated data.
All data, tables, and figures in this manuscript are original.
The seed germination and seedling growth of rapeseed are crucial stages in plant life, especially when facing abiotic stresses. In the present work, the effects of water and temperature on seed germination and seedling growth were investigated in a rapeseed crop ( Brassica napus L.). The plants were examined under different temperature levels (5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C) and water levels (twenty-nine levels based on either one-milliliter intervals or as a percentage of the thousand-kernel weight (TKW)). Moreover, planting densities and antifungal application techniques were investigated in the study. The findings demonstrated substantial variations between all the growth parameters investigated at all the tested temperatures, and 20 °C was considered the optimum within a broad range of 15–25 °C. Water availability plays a significant role in germination, which can be initiated at 0.65 mL, corresponding to 500% of the TKW. The method of TKW is a more accurate aspect of water application because of the consideration of the seed weight and size. The optimal water range for the accumulation of dry weight, 3.85–5.9 mL (2900–4400% of TKW), was greater than that required for seedling growth, 1.45–3.05 mL (1100–2300% of TKW). Twenty to twenty-five seeds per 9 cm Petri dish exhibited the most outstanding values compared to the others, which provides an advantage in breeding programs, especially when there are seed limitations. Seed priming is a more effective antifungal application strategy. These data can be incorporated into future rapeseed germination in vitro studies, breeding programs, and sowing date predictions.
1. Introduction
Rapeseed ( Brassica napus L.), generally known as oilseed rape, is one of the world’s most essential and prolific oilseed crops [ 1 ]. In 2020, rapeseed was cultivated on 35.49 million hectares globally, yielding 72.37 million tons of seeds [ 2 ]. It is the second most crucial oil crop [ 3 , 4 ]. Rapeseed is used on a small scale for animal feed and lubricants and in paint industries and the bioenergy industry, and it is primarily grown for its edible oil. Due to its high oleic acid (approximately 60%) and linolenic acid (omega 3, ca. 10%) contents, rapeseed oil provides more health advantages to humans than any other oilseed crop [ 4 , 5 ]. Furthermore, because of its high protein content, oilseed rape meal is used as animal feed [ 6 ].
Seed germination is the initial stage of a plant’s life. It is a three-phase process. The first phase is imbibition. Dormant seeds take up water, and the hydrolysis process takes place. Seed imbibition results from the interaction of proteins, carbohydrates, and lipids, and variations in their contents can affect the process. Protein and oil bodies are the primary reserves in oilseed crops that provide energy, carbon, and nitrogen to seedlings during their establishment [ 7 ]. The second phase is the regulation of germination, characterized by the activation of ATP synthesis in glycolysis, the Krebs cycle, respiratory chain, and the translation of stored mRNA. However, the third phase represents the completion of germination, when the radicle protrudes from the seed coat and forms a root, and the plumules form a shoot system capable of utilizing inorganic matter, water, and light energy for healthy growth [ 8 , 9 ]. Germination is a complicated process from a physiological standpoint, involving multiple signals, and it is influenced by both intrinsic and extrinsic factors [ 10 ]. Intrinsic factors include seed dormancy and available food stores, and extrinsic factors include water, temperature, oxygen, light, and relative humidity [ 11 , 12 , 13 ].
Water is considered the primary germination regulator, as germination begins with seed imbibition. Sufficient moisture must be present for germination to take place. Some research studies stated that a lack of water availability is the primary limitation affecting seed germination [ 14 , 15 ]. It is necessary for enzymatic reactions and the mobilization of the seed storage reserves, including lipids, carbohydrates, and proteins [ 9 ]. Therefore, the depletion of the soil water content influences seed imbibition. Slow water absorption by a germinating seed might threaten its emergence and the subsequent crop stand [ 16 ]. Therefore, a water shortage inhibits the enzymes responsible for hydrolyzing endosperm starch, which supplies energy for plant development by metabolizing sugar [ 17 ]. Water stress, in general, is followed by oxidative stress in seeds during germination, which is characterized by an increase in the production of reactive oxygen species [ 18 , 19 , 20 , 21 ]. Therefore, a combination of the antioxidant activity defense system and the internal content of these substances are essential for successful germination under limited water conditions [ 22 , 23 , 24 ].
Temperature is a critical environmental factor in seed germination [ 25 , 26 ]. The pace and rate of germination, which govern water absorption, may be affected by temperatures above or below the optimal range. Under optimal conditions, the absorption process is fast. Some research studies showed that the number of germinated seeds increases linearly as the temperature rises to an optimal level and then decreases linearly as the temperature exceeds the limit [ 27 , 28 ]. In addition, the temperature has a substantial impact on both biochemical and physiological metabolic processes. The latter can regulate enzymatic activity and biochemical reactions during the germination initiation process. Low temperatures reduce the activity of enzymes and slow down food mobilization, limiting the metabolic processes necessary for germination and development [ 9 ].
A good performance of seed germination results in increased plant density, which is critical for the rapeseed yield and stability in direct sowing plantations [ 29 ]. Generally, increasing the plant density increases seed production. The recommended plant density for hybrid rapeseed is approximately 60–70 plants m −2 in Europe. However, the average seed production can be obtained within a wide range of plant densities, varying from 8 to 90 plants m −2 [ 29 ]. The establishment of a high-quality crop and the subsequent crop stand performance are primary objectives of farmers in ensuring a high crop productivity and profit. In addition, the abiotic stressors, including drought, extreme heat, and salinity, can all have detrimental effects on plant growth, resulting in yield loss and a reduced crop quality [ 30 ], which can have severe economic consequences for farmers. Water and temperature stresses are the primary constraints on successful crop establishment and the subsequent crop performance. Therefore, unfavorable circumstances might severely affect the establishment of the crop and subsequent yield [ 31 , 32 , 33 ].
The southern and central parts of Europe have become more prone to drought and temperature extremes over the last two decades. Resistant crops cannot withstand prolonged periods of abiotic stress. Although winter rapeseed crops are typically sown between mid-August and early September, their emergence may be delayed and the yield may be reduced if precipitation does not occur within 10 to 14 days of sowing [ 34 , 35 ]. Extreme temperatures, combined with water scarcity, may contribute to the development of high-temperature stress. In addition, rapeseed has tiny seeds and is susceptible to desiccation when subjected to severe abiotic stress. As a cool season winter crop, oilseed rape is temperature- and water-sensitive [ 34 , 35 ].
In scientific studies aimed at enhancing seed germination and crop establishment, pre-sowing seed treatments and agronomic practices have garnered increased attention [ 36 , 37 , 38 ]. To our knowledge, the effects of water, temperature factors, the seedling density, and pre-treatments on seeds are limited in the case of in vitro rapeseed germination. Due to the importance of rapeseed cultivation for the social and economic life of Hungary, as well as the importance of this crop as an oilseed crop with significant nutritional value, it is essential to determine the optimal conditions and agronomic practices for growing rapeseed in vitro and under different climatic conditions. In this context, the objectives of this study were: (i) to assess the effect of temperature on rapeseed germination and seedling growth and determine the optimal temperature for germination; and (ii) to determine the amount of water required for seed germination using a volume of water in one-milliliter intervals and the thousand-kernel weight (TKW). This technique was recently used to optimize the amount of water required for seed germination in wheat and maize crops [ 39 , 40 ]. It was proved that the water requirements of the seeds differed depending on their size and weight. In the literature, seed size variation may result in variability in the seed water relations and the ability to emerge from a variety of sowing depths [ 41 ], as well as resistance to moisture stress [ 42 , 43 , 44 ]. Our last aim was (iii) to determine the effects of the seed number and density of the seedlings, as well as the effect of pre-sowing seed technology, used to control fungal growth, on seed germination and seedling growth. Germination tests using varying water and temperature levels and seed numbers can establish all the conditions for successful rapeseed germination regardless of the environmental factors, sowing date, or plant density in vitro. Therefore, understanding the optimal temperature and amount of water required for oilseed rape seed germination could aid in the development of an efficient germination program and production technologies.
2.1. Temperature Trial
The germination of the oilseed rape was conducted at temperatures ranging from 5 °C to 35 °C across the germination time course, with 5 degrees Celsius intervals ( Figure 1 ). Germination was detected approximately three days after the experiment began, and successful germination occurred on average after four days at temperatures of 15 °C, 20 °C, 25 °C, and 30 °C. The oilseed rapeseed appeared to germinate within a range of temperatures, with 20 °C being the most suitable and optimal temperature for obtaining a high germination percentage. At 5 °C and 10 °C, the oilseed rape seeds germinated slowly, taking approximately 9 and 13 days, respectively, and germination was poor at 35 °C.

Recorded data on the germination time of rapeseed crop at different levels of temperature.
The recorded data of the radicle and shoot growth under different temperature levels were measured when 80% of the seedlings reached 1 cm in length. Radicles and shoots can grow at a variety of temperatures but are primarily raised at 10 °C, 15 °C, 20 °C, and 25 °C ( Figure 2 and Figure 3 ). The radicle grew optimally at a temperature of 20 °C, while the shoot grew optimally at around 10–20 °C ( Figure 2 ). Given that the radicle grows in soil, it requires a higher temperature than the shoot. As a result, the optimal temperature range for their growth is between 15 and 20 °C. Although the radicle could grow at a suboptimal temperature of 15 °C, it was significantly smaller than it was when it was maintained at 20 °C. Beyond the optimal range, the growth of the two organs accelerated until they reached their maximum height, at which point they stabilized. The shoot and radicle do not require prolonged exposure to elevated temperatures ( Figure 2 and Figure 3 ). In fact, their growth rate was slow for the first four days at 30 °C, and then decreased sharply. Additionally, minimal growth was recorded under 5 °C and an absence of growth was noted at 35 °C.

Growth response of radicle to temperature levels.

Growth response of shoot to temperature levels.
The biological effects of various temperatures, ranging from 5 °C to 35 °C, on the growth of the seedlings were recorded daily during the experimental period ( Figure 4 ). The results indicated that the highest rate of growth occurred at 20 °C, followed by 15 °C, 10 °C, and 25 °C ( Figure 4 ). A temperature of 20 °C was suitable for seedling growth. Similar growth patterns were observed at 10 and 25 °C, but the seeds germinated earlier under 25 °C than at 10 °C ( Figure 1 ). At 5 °C, the seedling growth was stable but slightly inhibited, necessitating additional time for development. Additional temperature elevation beyond the optimal range was found to be detrimental to seedling growth, as evidenced by the decreased length value at 35 °C. Indeed, the seedlings grew more rapidly during the first few days at 30 °C, but their growth pattern deteriorated after a few days when they were exposed to prolonged periods of high temperatures ( Figure 4 ).

Growth response of seedlings to temperature levels.
2.2. Water Trial
The summarized results for the growth indices and dry weight accumulation in response to two water levels, based on a single milliliter and the TKW (thousand-kernel weight) percentage, revealed significant differences in the length of the seedlings’ radicles and shoots between different water levels ( Figure 5 and Figure 6 ; Table 1 and Table 2 ).

Response of seedlings, plumules, and radicles to different amounts of water tested. ( a ) Growth vs. different amounts of water based on one-milliliter intervals. ( b ) Dry weight accumulation vs. different amounts of water based on one ml intervals.

Response of seedlings, plumules, and radicles to different amounts of water at 20 °C. ( a ) Growth vs. water based on TKW%. ( b ) Dry weight accumulation vs. water based on TKW%.
Mean data on germination and seedling growth traits for the different amounts of water based on one-milliliter intervals.
Different letters indicate a significant difference between treatments at p < 0.05, starting sequentially, with the letter (a) being the most significant. Data presented as mean ± SD.
Mean germination and seedling growth values for the different amounts of water based on TKW%.
Different letters indicate a significant difference between treatments at p < 0.05. They start sequentially, with the letter (a) being the most significant. Data presented as mean ± SD. 1 Dry weight estimated for radicle, plumule, and entire seedling.
Oilseed rape can be germinated at low water levels of 0.65 mL or 500% of the TKW ( Table 2 ). The optimal range for the germination of twenty seeds per Petri dish was approximately 1.45–3.45 mL, using 500 and 4400% of TKW ( Table 2 ).
The radicle growth increased significantly as the water quantity increased to the optimal level but decreased significantly as the water quantity increased beyond the optimal level ( Figure 1 and Figure 2 ). The optimal water range for radicle growth was 1.45–1.85 mL based on the TKW, which was within the optimal range determined using the milliliter-based method ( Table 1 and Table 2 ). The plumule exhibited a similar growth pattern to the radicle but with a more extensive optimal growth range of 3.45–5.1 mL, representing 2600–3800% of the TKW.
As a result, the measurement of the water requirement for the whole seedling is more accurate. Thus, using the TKW percent method, the optimal range for seedling growth was approximately 1.45–3.05 mL, representing 1100–2300 percent of the TKW ( Table 2 ). In addition, it fell within the optimal range established by the one-milliliter (1–4 mL) water-based method ( Table 1 ). Therefore, it can be stated that the TKW method is more precise in optimizing the water requirements for germination.
The dry weight, or dry matter, is a critical parameter that is primarily used to describe water use efficiency, estimate yields, and select drought-tolerant plants. An analysis of variance was performed based on the dry weights of the shoot, radicle, and total seedling, as well as the corrected dry weight of the total seedling, which was calculated by subtracting the number of non-germinated seeds ( Table 1 and Table 2 ). The results showed significant differences in all the tested parameters across the various water levels. The dry weight of all the radicles and shoots increased significantly as the water volume increased, peaked at the optimal level, and then decreased slightly despite the increased water volume ( Figure 5 and Figure 6 ; Table 1 and Table 2 ). The optimal water range for the accumulation of dry weight by the seedlings was evaluated to be 3.85 to 5.9 mL, corresponding to 2900 to 4400% of the TKW, which was greater than the optimal range required for seedling growth (1.45–3.05 mL or 1100–2300% of the TKW). The shoot (3.85–5.5 mL) accumulated more dry weight than the radicle (1.05–1.85 mL), given the radicle’s delicate structure and lower weight. The radicle length and dry weight were reduced at levels of more than 6 mL ( Figure 5 and Figure 6 ), indicating that the plant was susceptible to waterlogging.
2.3. Seed Number Trial
The results indicated a significant difference only in the subdivision of seedlings with healthy shoot growth among which the aggregated values of 5, 20, and 25 seeds per Petri dish were used for the seed number test ( Table 3 ). However, this significantly affected the final aggregated value. The seedlings with short plumule growth and with radicle growth alone, non-germinated seeds, and seeds that were initially germinated did not vary significantly as the seed number increased ( Table 3 ). However, a higher aggregated value was observed when using 25 seeds per Petri dish. Therefore, densities of 20–25 seeds moistened with 5 mL of water appeared to be the optimal density for growing an oilseed rape crop in vitro.
Germination ratio and seedling characteristics of rapeseed as a response to the number of seeds per Petri dish.
Different letters indicate a significant difference between treatments at p < 0.05. NS means non-significant difference between the means. Data presented as mean ±SD. 1 Number of seeds tested; 2 percentage of non-germinated seeds; 3 seeds that started germination; 4 percentage of seeds that germinated with obvious roots; 5 percentage of seedlings with short shoots; 6 percentage of seedlings with healthy shoots; 7 aggregated value.
2.4. Antifungal Trial
The recorded data and comparative findings for germinating seeds pre-treated in two growth solutions containing antifungal Amistar Xtra and Hypo solution (10% Sodium hypochlorite (NaClO)) showed a significant effect on the growth parameters compared to the control ( Figure 7 ). The germination of seeds primed with Hypo significantly affected the radicle and seedling growth compared to the control, whereas the antifungal Amistar Xtra growth media inhibited fungal growth as well as the radicle, shoot, and seedling growth. The antifungal Amistar Xtra used in the growth media had a negative impact on the radicle, shoot, and seedling growth ( Figure 8 ). In fact, all the growth parameters measured here decreased proportionately as the antifungal concentration increased, even at a low concentration.

Response of the radicles, plumules, and seedlings to media amended with anti-fungicide (Amistar Xtra) and Hypo solution SP. Values denoted with different letters are significantly different at p < 0.05.

Response of radicles, plumules, and seedlings to different concentrations of antifungal. Values denoted with different letters are significantly different at p < 0.05.
The techniques of seed priming with fungi sterilizer solution and growing of seeds in growth media containing the fungicide Amistar Xtra were compared in this experiment ( Figure 9 ). The priming technique showed a significantly more positive effect on all the growth parameters than the amendment of seeds in the growth media. Seed pre-treatment or priming can offer a viable alternative for the prevention of fungal growth during seed germination in vitro.

Response of radicles, shoots, and seedlings to two different methods of fungal (sterilization and growth media). Values denoted with different letters are significantly different at p < 0.05.
3. Discussion
Temperature is critical for regulating plant growth and development [ 26 ]. The rate of the rapeseed germination potential increases linearly as the temperature rises and then decreases linearly until reaching the optimal level, followed by a reduction beyond that level [ 28 , 45 ]. In the current study, oilseed rape germinated at temperatures ranging from 5 °C to 30 °C, and 20 °C was the optimal temperature for germination within a broad range of 15–25 °C ( Figure 3 ). This finding is consistent with another study [ 46 ]. A temperature above or below the optimum caused the germination potential to drop. High temperatures of 35 °C greatly hindered seed germination and subsequent emergence owing to the inhibitory impact of high temperatures. However, low temperatures of 5 °C or below caused delayed germination, so that the process required a longer time ( Figure 1 ). This finding is consistent with previous findings [ 47 , 48 ].
The optimal temperature of 20 degrees Celsius led to the most rapid and complete germination of seeds after four days of incubation ( Figure 1 ). The increased temperature accelerated the germination rate [ 49 , 50 ]. However, germination was sluggish when the temperature was reduced to 5 °C. Metabolic reactions and enzyme activity cause variation in the germination time during the germination process. Indeed, lower temperatures cause seeds to have a slower metabolism, resulting in slower growth, whereas higher temperatures cause plants to have a faster metabolism, dissipating the seed energy required for growth. According to enthalpy approaches, as the temperature rises, the energy in the water increases, resulting in an increase in diffusion pressure, which simultaneously increases metabolic and enzyme activity and decreases the internal potential of a seed, thereby promoting increased water absorption. Thus, hydration occurs more rapidly at higher temperatures, a physical process that may accelerate germination [ 51 ]. At a super-optimal temperature, the available energy in the seed’s cellular members remains unfavorable for embryonic growth and quickly dissipates [ 49 ].
Soil temperature is a critical environmental factor affecting the growth and development of roots. When the soil temperature reaches the optimal level, root growth increases. However, root growth declines when it exceeds the optimal level [ 52 ]. At 20 °C, a rapid growth pattern with a higher growth value was observed; therefore, this was considered the optimal temperature for radicle growth. The same pattern was observed at 10 °C and 15 °C, but with lower values ( Figure 2 ). Additional temperature elevation was detrimental to radicle growth, as evidenced by the low average value at 35 °C. At 25 °C, the radicle grew slightly for a few days before ceasing to grow and stabilizing. At 30 °C, the initial growth of the radicles was more significant compared to the subsequent decrease after a few days ( Figure 2 ). The radicle’s sensitivity to high temperatures results from the cumulative temperature requirement for each growth stage. Surpassing the optimal temperature results in quicker germination, but this is not the case throughout the development and growth period ( Figure 1 ). These findings corroborate previous research [ 39 , 40 ]. The radicle requires different cumulative temperatures throughout its growth stage. Not only does germination require a specific temperature, but each stage has its own. As a result of the complexity of the germination process, the temperature response may vary throughout the germination period [ 53 ]. In addition, as oilseed rape seeds are susceptible to damage under prolonged high temperatures, prior research has often focused on heat shock and gradual temperature rises from the ideal to a higher value [ 54 , 55 ]. They concluded that the alternated heat accumulation potential stimulated by gradual temperature stress might be more important than heat tolerance induced by constant or unexpected heat exposure [ 54 ]. The radicle cannot grow for an extended period of time at constant higher temperatures, a finding that is comparable to that which we observed at 30 °C ( Figure 2 ).
The shoot can grow in a temperature range of 10–25 °C, with a minor difference between different temperature levels ( Figure 3 ). Compared to the radicle, the shoot grew in a different growth pattern ( Figure 3 ). The radicle’s optimal temperature was 20 °C, while the shoot’s optimal temperature was between 15 °C and 20 °C regardless of its growth stage ( Figure 3 ). The radicle requires a higher temperature than the shoot due to its internal organs, which grow into the soil. During the very late stage of the germination process, the steady growth of the shoot was observed at temperatures of 20 °C and 25 °C ( Figure 3 ). The seedling growth was significantly influenced by temperature. The temperature of 20 °C was proven to be the optimum temperature for seedling development ( Figure 4 ). Most enzymes become inactive at temperatures over 35 °C, negatively impacting germination and seedling development [ 56 ].
Water intake is a prerequisite for germination. It is required for seed imbibition, enzymatic activation, degradation, translocation, and the utilization of reserve storage material. The germination capacity increased significantly as the water volume increased until it reached the optimal level, and then it decreased slightly as the water level increased ( Figure 5 and Figure 6 ). Under conditions of limited water availability, seed imbibition is insufficient for initiating germination. However, increased water availability results in waterlogging, which inhibits seed germination due to oxygen depletion. The dormant seed requires water, oxygen, and an appropriate temperature to complete its life. According to the TKW method, the seeds started germinating at a water content of 0.65 mL, corresponding to 500% of the TKW ( Table 2 ). This amount of water may be very close to the moisture content required for germination demands.
Most seeds require a critical moisture content in order to germinate. This value was estimated to be 30% in maize, 40% in wheat, and 50% in soybeans [ 53 ]. Once the critical seed moisture content is reached, sufficient moisture is present to initiate germination. Moreover, numerous studies have documented the minimum water potential required to induce seed imbibition [ 15 , 57 ]. Seed imbibition is a three-part process, commencing with an initial phase of water intake, followed by a plateau period with a minor change in the water content, and concluding with an increase in the water content, as evidenced by radicle development [ 58 ]. Generally, seed germination regulation occurs during the plateau period, as germination is complete when the embryo begins to expand. The duration of this plateau phase is dependent on the water potential. Therefore, the duration of this phase is determined by water availability and the species. It can be extended at water potentials close to −0.03–1.00 MPa in some crops [ 15 , 57 ].
According to the TKW method ( Table 4 ), seeds can be germinated over a range of water levels, starting at the wilting moisture point (0.65 mL), demonstrating the TKW method’s accuracy in detecting and optimizing the water requirement for seed germination, as previous studies have reported [ 39 , 40 ]. Indeed, seeds require a hydric range within which they can either not germinate or germinate poorly or slowly [ 59 ]. Waterlogging, which occurs because of an increased water volume, depletes the oxygen available to seeds, which is a critical factor in seed germination [ 60 ].
Water amount levels based on one-milliliter intervals and TKW%.
1 TN: the number of the treatment based on the milliliter method; 2 WA: the water quantity based on a single milliliter; 3 TN: treatment number based on the TKW method; 4 PW: the suggested percentage for the water quantity application in ml based on the TKW method; 5 WA: the water quantity equivalent to the suggested percentage of water volume based on TKW; 6 RAW: rounded quantity of water in ml to the nearest measurable digit on the pipette.
The ideal level of water for germination has a significant impact on the subsequent development of seedlings. The seedling length increased significantly with increasing water availability until it reached its maximum value and decreased significantly with increasing water availability. The shoot and radicle structures exhibited distinct optimal water ranges for growth and development. Under non-limiting hydric circumstances, the radicle expanded slowly and encouraged shoot development. As a result, the measurement of the total seedling length appeared to be more accurate in determining the optimal water demand for the entire plant, which was determined to be 1.45–3.05 mL or 1100–2300% of the TKW ( Table 2 ). Under ideal circumstances, seedlings developed rapidly, which is consistent with previous research [ 57 ]. Radicle growth is the most critical factor in early seedling survival because it enables the seedling to exploit water deep in the soil via rapid root extension [ 61 ].
The dry weight accumulation at various water levels revealed a significant difference in the dry weight of the radicles and shoots ( Table 1 and Table 2 ). The optimal water range for the seedling’s dry matter was 3.85–5.9 mL, corresponding to 2900–4400 % of the TKW. Increased water availability had a detrimental effect on dry matter accumulation. The radicle is more sensitive to high water availability, requiring less water to build up a unit of dry matter. Under optimal conditions, rapid radicle growth contributed significantly to the shoots’ dry matter accumulation. According to functional balance theory, the plant will respond to increased water availability by increasing the flow and assimilating the shoot in order to increase its dry matter [ 62 ]. In conclusion, given the effect of the seed size on the internal seed moisture requirements, the TKW water application method enables the determination of the minimum and optimal water requirements for germination and seedling establishment. The current findings are supported by previous research [ 39 , 40 ].
A significant difference between seedlings with healthy shoot growth subjected to the seed number treatment of 15, 20, and 25 seeds per Petri dish was found ( Table 3 ). As the number of seeds per Petri dish increased, the subdivision of seedlings with healthy shoot growth and the aggregated values increased. Under controlled conditions, a seed density of 20–25 seeds per Petri dish is optimal for growing oilseed rape in vitro. Although the density of 15 seeds stimulated germination, the seedlings grew slower. At a low seed number, the lack of seedlings could be attributed to the scarcity of a critical resource, such as water, due to competition [ 63 ]. Additionally, dense seedlings are frequently more susceptible to lodging, which increases the rate of disease incidence and, as a result, the seedling emerging percentage [ 64 ]. Therefore, optimizing the seed number per Petri dish in germination is a critical consideration during germination, along with other environmental factors.
Seed treatment with fungicides is widely used to improve the seed emergence and resistance to seed- and soilborne fungal pathogens [ 38 ], and it has received considerable attention in several recent publications [ 65 , 66 ]. Given that the antifungal Amistar Xtra’s primary function is to inhibit fungal growth, it had a detrimental effect on the radicle, plumule, and seedling growth ( Figure 7 ). Additionally, as the antifungal concentration in the growth media increased, the inhibitory effect became more pronounced ( Figure 8 ). This is possibly because no fungi were present in our experiment rather than because of the fungicide’s phytotoxicity, as many authors explained in their works [ 65 , 67 , 68 ]. They observed a recovery of seedling growth following fungi exposure and inoculation. Amistar Xtra fungicide, which contains the active ingredient azoxystrobin, inhibits electron transport in the mitochondrial respiratory chain of fungi, thereby decreasing aerobic energy production and inhibiting fungus growth [ 69 ]. As a control, seed treatment with Hypo solution had a beneficial effect on the seedling growth. Priming seeds with Amistar Xtra or Hypo rather than the amendment of seeds in antifungal growth media was found to inhibit fungal growth in vitro ( Figure 7 and Figure 9 ).
4. Materials and Methods
This study examined the effects of abiotic stressors (water and temperature), seedling density, and fungal growth control on seed germination and seedling growth in vitro. The experiment was undertaken in 2022 at the Hungarian University of Agriculture and Life Science/Institute of Agronomy. Rapeseed ( Brassica napus L. Allison ) was chosen for this study. “ Allison ” is a highly productive hybrid with a high oil and glucosinolate content. In 2015, this hybrid was registered in France. It is a hybrid resistant to Turnip Yellowing Virus (TUYV) and has a tall stem, although it is susceptible to lodging and Cylindrosporium .
4.1. Temperature Stress Trail
The germination ability was tested at seven different temperature levels (5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C). Twenty seeds of rapeseed were placed in a standard 9 cm Petri dish with a single filter paper moistened with 5 mL of distilled water in four replications. The Petri dish was sealed with parafilm and subjected to the tested constant temperature in the incubation chambers. The measurement was taken when around 80% of the seedlings in the Petri dishes reached a length of 1 cm ( Figure 10 ). Daily, four Petri dishes were taken out of the chambers at each temperature level for the physical measurement of the length of the radicles and shoots. Germination percentages were also recorded.

Photos of seed germination in a Petri dish at 20 °C. High values of germination and seedling growth were recorded at this temperature.
4.2. Water Stress Trail
The germination ability of oilseed rape under water stress was examined at 29 different water levels. In total, 10 different amounts of distilled water were used based on a milliliter interval of 0–10, and 19 different amounts of distilled water were used on the basis of the TKW ( Table 4 ). At each water level, 100 seeds were placed in five Petri dishes (20 seeds per plate) using filter paper saturated with various amounts of distilled water ( Table 1 ). The following equation (1) was used to compute the amount of water based on the TKW:
The TKW of the oilseed rape was 6.68 g. Additional information on the calculation of the water quantity based on the TKW is available in two previous studies [ 39 , 40 ]. The Petri dishes were sealed with parafilm to prevent water evaporation and incubated at 20 °C for ten days in a growth chamber. After ten days of incubation, the radicle and shoot lengths and the number of non-germinated seeds were measured. The radicles and shoots were then separated and dried at 65 °C until they reached a constant weight (48 h).
4.3. Seed Number Trial
This section of the experiment examined the effects of the seed number on germination and seedling growth using the same volume of water (5 mL) and incubation at a temperature of 20 °C. For the amplification, 15, 20, and 25 seeds were planted in the Petri dishes. Morphological measurements were conducted ten days after incubation, and the measured parameters were divided into five categories. The aggregated germination value (AGG) was calculated using the measured parameters, as described below (Equation (2)) [ 39 , 40 ]:
where NO g is the non-germinated seed number, S is the number of seeds in which germination started, R is the number of germinated seeds with radicles only, SP is the number of seedlings with a short plumule, NS is the number of normal seedlings, and N is the total number of the tested seeds.
4.4. Antifungal Trial
Two different techniques of antifungal application were used to assess the fungicide’s potential to inhibit fungal growth. For the first technique, we added five different concentrations of Amistar Xtra, specifically 0, 20, 200, 2000, and 20,000 ppm, to the growth media. In addition, two different seed sterilization methods were tested, the first based on soaking the seeds for 3 min in a solution of 2000 ppm Amistar Xtra and the second based on a Hypo solution (10% Sodium hypochlorite (NaClO)). After sterilization, the seeds were rinsed with distilled water and incubated for 10 days at 25 °C in a growth chamber. After ten days of incubation, the radicle and shoot lengths were measured, and the germinated seeds were counted.
4.5. Statistical Analysis
Analysis of variance (ANOVA) and Fisher’s test of least significant differences (LSD) were conducted for the obtained data. Kolmogorov–Smirnov–Wilk and Shapiro–Wilk tests conducted in SPSS V27 IBM (New York, NY, USA) were used for the data normality verifications. The effects of the water level, seed number, and antifungal treatment on the germination percentage, radicle length, shoot length, and seedling growth were analyzed using a computing program (GenStat twelfth edition, GenSat procedure library release PL20.1m, and MS Excel 365). A sigmoid curve model was applied using a statistical program (JMP Pro 13,2,1 of SAS Institute, Cary, NC, USA, and MS Excel 365) to fit the data and plot the appropriate temperature levels.

5. Conclusions
The findings of this study highlighted the critical factors affecting oilseed rape germination and established the optimal range for successful germination and seedling growth. The optimal temperature for oilseed rape germination and seedling growth was 20 °C within a more comprehensive range from 10 °C to 25 °C. Between the optimal and suboptimal ranges, the germination potential decreased. This information could be used to determine planting times depending on the optimal temperature for seed germination. According to the TKW method, oilseed rape seeds can be germinated starting at a volume of 0.65 mL, representing 500% of the TKW and being close to the critical moisture content required for germination. The optimal water range for the accumulation of dry weight, 3.85–5.9 mL (2900–4400% of the TKW), was greater than that required for seedling growth, 1.45–3.05 mL (1100–2300% of the TKW). Therefore, optimizing the water availability for seeds based on the TKW percentage is a more precise strategy, since it considers the seed’s weight and size when estimating the water required to develop optimally.
Additionally, optimizing the seedling density is critical for avoiding water factor limitation and competition between seedlings and minimizing disease problems. Thus, it was determined that a seed density of 20–25 seeds per Petri dish was optimal for growing oilseed rape in vitro. Finally, seed priming with an anti-fungicide to inhibit fungal growth is recommended for the purpose of antifungal control. These findings have the potential to be beneficial in future research and breeding programs.
Acknowledgments
This study was supported by the framework of the Thematic Excellence Program 2021, under the National Defense and National Security Subprogram (TKP2021-NVA-22). The authors express their sincere thanks to all the department members of Crop Production for their support in this research.
Funding Statement
This research was funded by the Hungarian University of Agriculture and Life Sciences. Stipendium Hungaricum Foundation supported it.
Author Contributions
Conceptualization, Á.T. and Z.K.; methodology, writing—original draft preparation A.H.S.; software and investigation, H.K.; writing—review and editing, H.K.; funding acquisition, C.G. and G.P.K. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
JavaScript seems to be disabled in your browser. For the best experience on our site, be sure to turn on Javascript in your browser.
Seed and Seedling Biology

Photo credit: bigstockphoto.com
Choosing the Right Seed
Before exploring how to best grow your seeds and seedlings, start with the right seed. If you intend to run your operation as certified organic, you are required to use certified organic seed and seedlings with only a few exceptions (see the "Organic Requirements").
What Do Seeds Need to Germinate?
Viable seeds are living entities. They must contain living, healthy embryonic tissue in order to germinate. All fully developed seeds contain an embryo and, in most plant species, a store of food reserves, wrapped in a seed coat. Seeds generally "wake up" and germinate when soil moisture and temperature conditions are correct for them to grow (Miles and Brown 2007). Each seed type has individual needs--take a minute and read about their specific germination requirements.
Seeds Need the Right Environment to Germinate
Temperature, moisture, air, and light conditions must be correct for seeds to germinate. All seeds have optimal temperature ranges for germination (Table 1). The minimum temperature is the lowest temperature at which seeds can germinate effectively. The maximum is the highest temperature at which seeds can germinate. Anything above or below this temperature can damage seeds or make them go into dormancy. At optimal temperatures, germination is rapid and uniform.
All seeds need correct moisture to initiate internal processes leading up to germination. In field soil this is generally about 50-75 percent of field capacity. A fine-textured seedbed and good seed-to-soil contact are necessary for optimal germination. Aeration in the soil media allows for good gas exchange between the germinating embryo and the soil. Seeds respire just like any other living organism. They need oxygen and produce carbon dioxide (CO 2 ). This carbon dioxide needs to be able to move away from the seed. If the soil or media is not well aerated due to overwatering or compaction, the CO 2 will not dissipate and seeds can suffocate.

Not all seeds have the same light requirements. Most seeds germinate best under dark conditions and might even be inhibited by light (e.g., Phacelia and Allium spp.). However, some species (e.g., Begonia, Primula, Coleus) need light to germinate (Miles and Brown 2007). Don't confuse seed light requirements with what seedlings need. All seedlings require sunlight. Seedlings will become leggy and fragile and will not produce to their potential if they do not have sufficient light.
Table 1. Soil temperature conditions for vegetable crop germination.
Soil temperatures should be taken by inserting a soil thermometer 3-4 inches deep into the soil surface and noting temperature. Adapted from Kemble and Musgrove (2006).
Seed Dormancy
Some viable seeds might not germinate. Many seeds have developed a dormancy (or sleep) period. Seed dormancy is a condition that prevents germination even under optimal environmental conditions. Why would it benefit seeds to not all germinate when conditions are right? In nature, staggering germination keeps some seedlings safe from possible bursts of bad weather or herbivores that might eat them. Seeds of plants that grow best in the spring have self-selected to germinate only after cold winter temperatures have passed.
For seeds to come out of dormancy, we have to break their physical or chemical dormancy factors. Seeds might have a hard or thick seed coat (physical dormancy). This can be broken by soaking or scarifying (scratching the surface) the seed. Other seeds have internal chemical or metabolic conditions that prevent germination (chemical dormancy). Factors affecting seed dormancy include the presence of certain plant hormones--notably, abscisic acid, which inhibits germination, and gibberellin, which ends seed dormancy. To break chemical dormancy, you might have to leach the seed or use cold/moist stratification or fire scarification. For example, the membrane within the seed coat of some seeds forms a barrier that is permeable to water but not to oxygen. Cold temperatures (50-59°F) allow oxygen to get into the seed, while warm temperatures prevent oxygen uptake. Cool temperatures also allow the seed to digest some of its food reserve, giving it energy. For these seeds, putting them in the refrigerator for a specific period of time allows them to gain sufficient oxygen and energy to germinate (Colorado Seed Laboratory 2009).
Steps of Seed Germination
- Imbibition. The seed rapidly takes up water and the seed coat swells and softens. Think of a pea seed that you have soaked--the outer seed coat becomes soft and wrinkly with water.
- Interim or lag phase. During this phase the seed activates its internal physiology, cells respire, and the seed starts to make proteins and metabolize its stores of food (MacKean n.d.).
- Radicle and root emergence. The cells start to elongate and divide, bringing the root and radicle out of the seed.

If you save your seed from the year before, think about this: the life of a seed can be cut in half by an increase of just 1 percent in seed moisture or by an increase in storage temperature of just a few degrees. A simple rule of thumb is that the sum of the storage temperature (in degrees Fahrenheit) and percent relative humidity should not be greater than 100.
Early Seedling Development
Dicots (two-seed leaves).
The primary root, called the radicle, is the first thing to emerge from the seed. The primary root anchors the plant to the ground and allows it to start absorbing water. After the root absorbs water, the shoot emerges from the seed. In dicots, the shoot has three main parts: the cotyledons (seed leaves), the section of shoot below the cotyledons (hypocotyl), and the section of shoot above the cotyledons (epicotyl). The way the shoot emerges from soil or growing media follows two main patterns. In some plants, the section of the shoot below the cotyledons elongates and forms a hook, pulling the cotyledons and the growing tip through the soil. Once it reaches the surface, it straightens and pulls the cotyledons and shoot tip of the growing seedlings into the air. For example, beans germinate this way. This is called epigeous germination. In other plants, only the section above the cotyledons expands, leaving the cotyledons underground where they soon decompose. This is called hypogeous germination. Peas, for example, germinate this way (Raven, Ray, and Eichhorn 2005).
Monocots (One-seed Leaves)
In monocot seeds, the primary root is protected by a sheath (coleorhiza), which pushes its way out of the seed first. Then the seedling leaves emerge covered in a protective sheath called a coleoptile (Raven, Ray, and Eichhorn 2005).
Dicots and Monocots
After the shoot emerges, the seedling grows slowly while the storage tissue of the seed diminishes. Soon, the plant develops a branched root system or taproot. Then, true leaves that look like the leaves of the mature plant appear. These leaves, unlike cotyledons, photosynthesize light into energy, allowing the plant to grow and develop.
Managing for Optimal Germination and Seedling Development
Optimizing germination.

We know that seeds need optimal amounts of water, oxygen, temperature, and light to germinate. If we don't create the most optimal environment possible, then plants tend to germinate slowly and unevenly. Generally, greenhouse space is limited, so we want plants to germinate as quickly as possible. Uneven germination can also cause problems. If you have ever had to transplant out a flat of seedlings where half are ready to plant and the other half are too small with root balls that don't slide easily out of their cells, you will understand why.

One common option to achieve optimal germination temperature in growing media is to use germination mats. These mats allow you to set the temperature according to seed requirements. For example, peppers will germinate in 8 days at 86°F, but take more than 13 days to germinate at 58°F (Pennsylvania Heirloom Seed Savers Club n.d.).
Make sure you maintain optimal temperatures for your crop (see Table 1 above). It is also critical to promote air circulation to mitigate fungal pathogens such as those causing damping off.
Seedling Development
The optimal temperature for growing seedlings may be different from that for seeds (Table 2). Remember, optimal temperature will stimulate optimal growth. You can control temperature to control plant height. Cooler temperatures generally slow down growth, and warmer ones speed up growth.
Table 2. Temperature and time required for growing field transplants.
From Maynard and Hochmuth (2007).

It is still critical to maintain good air circulation and sufficient moisture. Generally, watering should be deeper to accommodate developing root systems. You may need to use different wand or hose heads to water seeds and seedlings because each use different amounts of water. Remember to carefully monitor and water the plants at the edges of flats. They dry out faster than those in the middle. However, overwatering can increase the probability of plants developing damping off.
Seeding Maturation and Hardening Off
This final step before seedlings are planted in the field gradually exposes them to the conditions they will have in the field. This process stimulates the plants to accumulate carbohydrate and nutrient reserves and strong cell walls by exposing the plants to day and night temperature fluctuations, increased air movement and wind, reduced watering, and full light.
Hardening off transplants is important, especially if they are to be planted under stressful early season conditions. Most transplants may be hardened off by reducing the temperature in the greenhouse through ventilation. Reduced watering will also provide some hardening effect. Do not let plants wilt excessively. Do not harden off transplants by reducing fertilizer application, as this often results in stunted plants that do not establish well in the field. Some growers will put plants outside for 5-7 days prior to planting. This allows the plant to become acclimated to outside conditions while still in the flat. Plants hardened off in this manner often have improved field performance as compared to those planted directly from the greenhouse (Garton, Sikkema, Tomecek 1997).
Organic Requirements
The National Organic Standards require that producers use organically grown seeds, annual seedlings, and planting stock. Nonorganically produced, untreated seeds and planting stock may be used to produce an organic crop when an equivalent organically produced variety is not commercially available.
There is no allowance for seed treated with prohibited materials. Captan, thimet, and similar chemical fungicides are not on the national list and are not permitted. Please take this seriously. If your seed is covered in a pink or orange powder, it is probably prohibited. We may not be able to certify your crop if you use seed treated with prohibited materials.
Seeds used for edible sprout production must be organic--no exceptions.
Commercial Availability
The first step is to determine whether an equivalent organically produced variety is available. By equivalent variety, look for comparable growing habits, days to maturity, insect and disease resistance, flavor, and other important qualities. If a suitable organic equivalent variety is not available, document where you tried to look for organic seed, as that is important for your certification records. Once you have found a source for a specific equivalent organic seed, the next step in determining commercial availability is to see if it is of the appropriate form, quality, and quantity.
- Form: such as sized, graded, pelleted, hot water treated
- Quality: try a small quantity the first year to make sure it does well under your particular conditions; if the only organic seed available is of inferior quality, then buying nonorganic may be acceptable
- Quantity: for example, if you want to plant 1 acre of pumpkins and the only organic seed available is in 1-ounce packets, then buying nonorganic may be acceptable
Documentation and Good Faith Efforts
Prior approval by Pennsylvania Certified Organic for using nonorganic seeds/planting stock is not required. Compliance is reviewed in the context of the organic system plan, which is verified during the annual inspection. A pattern of inadequate documentation and lack of good faith effort to obtain organically grown seeds and planting stock may be considered noncompliance and might result in Pennsylvania Certified Organic requiring prior approval regarding commercial availability issues in future planting cycles. Documenting your good faith efforts to find suitable organic seeds/planting stock is crucial.
Organic Seed Sources
Listed below are a number of sources for organic seed provided by Pennsylvania Certified Organic (2011). A list is also maintained by the Organic Materials Review Institute (OMRI) . For a more complete listing including forage, field crop, and cover crop seed and transplants, go to the Pennsylvania Certified Organic website.
Abundant Life Seeds PO Box 157 Saginaw, OR 97472 Phone: 541-767-9606 Web: www.abundantlifeseeds.com E-mail: [email protected] 100 percent organic, all open-pollinated; vegetable, flower, and herb seed; garlic and potatoes
The Cook's Garden PO Box C5030 Warminster, PA 18974 Phone: 800-457-9703 Web: www.cooksgarden.com E-mail: [email protected] Organic vegetables, beans, flowers, and herbs
Environmental Seed Producers PO Box 947 Albany, OR 97321-0354 Phone: 541-928-5868 Web: www.espseeds.com Organic vegetables, herbs, and flowers
Fedco Seeds PO Box 520 Waterville, ME 04903 Phone: 207-873-7333 Web: www.fedcoseeds.com Organic vegetables and flowers
Filaree Farm 182 Conconully Hwy Okanogan, WA 98840 Phone: 509-422-6940 Web: www.filareefarm.com Extensive collection of organic garlic varieties
Fred C. Gloeckner and Co. 600 Mamaroneck Avenue Harrison, NY 10528-1631 Phone: 800-345-3787 Fax: 914-698-2857 Web: www.fredgloeckner.com Organic vegetable, herb, and flower seeds
Gardens Alive! 500 Schenley Place Lawrenceburg, IN 47025 Phone: 513-354-1482 Web: www.gardensalive.com Organic garden and sprout seeds, plus insect and disease control and soil care products
Harris Seeds 355 Paul Road PO Box 24966 Rochester, NY 14624-0966 Phone: 800-544-7938 Web: www.harrisseeds.com Some organic vegetables and herbs
High Mowing Organic Seeds 76 Quarry Road Wolcott, VT 05680 Phone: 802-472-6174 Web: www.highmowingseeds.com High-quality organic seed for more than 500 varieties of heirloom, open-pollinated, and hybrid vegetables, flowers, herbs, potatoes, garlic, and cover crops
Johnny's Selected Seeds 955 Benton Avenue Winslow, ME 04901 Phone: 877-JOHNNYS (877-564-6697) Web: www.johnnyseeds.com Organic vegetables, flowers, and herbs
The Maine Potato Lady PO Box 65 Guilford, ME 04443 Phone: 207-343-2270 Web: www.mainepotatolady.com Organic seed potatoes, shallots, onion sets, garlic, and cover crops, plus fertilizer, soil, and seed inoculants
Rohrer Seeds PO Box 250 Smoketown, PA 17576 Phone: 717-299-2571 Web: www.rohrerseeds.com Organic vegetable seeds
Seeds of Change 3209 Richards Lane Santa Fe, NM 87507 Phone: 888-762-7333 Web: www.seedsofchange.com Organic flowers, herbs, vegetables, and cover crops and strawberry plants
Seed Savers Exchange 3094 North Winn Road Decorah, IA 52101 Phone: 563-382-5990 Web: www.seedsavers.org Some organic vegetables, garlic, herbs, potatoes, and heirloom varieties
Seedway 99 Industrial Road Elizabethtown, PA 17022 Phone: 800-952-7333 Web: www.seedway.com Some organic vegetables and herbs
Snow Seed Organic 21855 Rosehart Way Salinas, CA 93908 Phone: 831-758-9869 Web: www.snowseedco.com Many organic vegetables, including lettuces
Southern Exposure Seed Exchange PO Box 460 Mineral, VA 23117 Phone: 540-894-9480 Web: www.southernexposure.com More than 400 varieties of certified organic heirloom and open-pollinated vegetable, herb, and flower seeds, as well as garlic and perennial onion bulbs
Territorial Seed Company PO Box 158 Cottage Grove, OR 97424 Phone: 800-626-0866 Web: www.territorialseed.com Organic flowers, herbs, vegetables, garlic, and cover crop seeds, plus OMRI-listed fertilizers and soil amendments
Vitalis Organic Seeds 7 Harris Place Salinas, CA 93901 Phone: 831-262-7635 Web: www.vitalisorganic.com Organic vegetable and herb seeds, with emphasis on lettuce, spinach, tomato, pepper, cucumber, squash, and melons
Wood Prairie Farm 49 Kinney Road Bridgewater, ME 04735 Phone: 800-829-9765 Web: www.woodprairie.com Organic garden seed, seed potatoes, and cover crop seed
Garton, R. W., P. H. Sikkema, and E. J. Tomecek. Plug Transplants for Processing Tomatoes: Production, Handling and Stand Establishment. Ontario, Canada: Ministry of Agriculture and Rural Affairs, 1997.
Kemble, J., and M. Musgrove. Soil Temperature Conditions for Vegetable Seed Germination. Alabama Cooperative Extension, 2006.
Mackean, D. G. "Biology of Plants: Seeds and Germination," from "Resources for Biology Teaching." 2010.
Maynard, D., and G. Hochmuth. Knott's Handbook for Vegetable Growers. Vol. 5. Hoboken, N.J.: John Wiley and Sons, 2007.
Miles, A., and M. Brown. Teaching Organic Farming and Gardening: Resources for Instructors. Santa Cruz: University of California Farm and Garden, 2007.
Pennsylvania Certified Organic. "2011 Organic Seed Suppliers."
------. "Requirements for Seeds, Planting Stock, and Seedlings under the USDA Regulations."
Pennsylvania Heirloom Seed Savers Club. "Seed Germination and Temperature."
Raven, P. H., F. E. Ray, and S. E. Eichhorn. Biology of Plants. 7th ed. New York: W. H. Freeman, 2005.
Seed Technology Educational Programs. "Physiological Dormancy." Fort Collins: Colorado Seed Laboratory, 2009.
Prepared by S. Tianna DuPont, former sustainable agriculture educator, Penn State Extension. Reviewed by Elsa Sanchez, Penn State Department of Horticulture, and Debra Brubaker, Pennsylvania Certified Organic.
This publication was supported in part by funding from the Beginning Farmer and Rancher Development Program of the National Institute of Food and Agriculture, USDA, Grant #2009-49400-05869.
You may also be interested in ...

The Penn State Agronomy Guide
Starting at $15.00

Pennsylvania Certified Crop Adviser Study Guide

Agronomy Scout School: Crop Scouting Fundamentals

CBD Hemp: Research, Production, Harvest, and Processing

Sweet Sorghum Production Basics

Broadcasting Cover Crops into Soybeans: Encouraging but Elusive

Corn Growth Stages

Reducing the Risk of Nitrate and Prussic Acid Poisoning in Livestock

Early Season Herbicide Injury to Corn
Personalize your experience with penn state extension and stay informed of the latest in agriculture..

- TOP CATEGORIES
- AS and A Level
- University Degree
- International Baccalaureate
- Uncategorised
- 5 Star Essays
- Study Tools
- Study Guides
- Meet the Team
- Living Things in their Environment
Investigating Seed Germination. Hypothesis If there is water, oxygen and a suitable temperature in the surroundings, then the seed will germinate.
SSIS MYP Programme
Science Investigation Report
Investigating Seed Germination
Name: Kimberly Hoong Yearn Yi
Class: S4 Ruby
To investigate how the following conditions: presence of water, presence of oxygen, and the temperature would affect the germination of a seed.
If there is water, oxygen and a suitable temperature in the surroundings, then the seed will germinate.
A seed is an embryonic plant in a resting condition after the development of the embryo in the ovule of the plant stops. Water is then lost and the seed enters a state of dormancy, where its metabolic activities are suspended until germination takes place. In order for germination to occur, water, oxygen and a suitable temperature must be present.
Water must be present, because it is what sets off the metabolic activity inside the seed. Water is taken up by a process called imbibition and is needed for the metabolism of the seed as well as for breaking the seed coat by making the seed swell. With water and all other conditions (oxygen, suitable temperature) present, germination commences with the uptake of water by imbibition of the dry seed, followed by embryo expansion. The water absorbed dissolves a chemical made inside the embryo. Hydrolytic enzymes are then activated, breaking down the stored nutrients into nourishments for the embryo.
Oxygen is another important condition for germination. Before the seed can photosynthesize through its leaves, the seed has to undergo respiration in order to provide energy for germination. The seed needs oxygen so that it can respire aerobically to convert its energy stores into energy that it can use for growing. Water serves as a medium for transport of soluble food molecules, but without oxygen, there will be no transport of nutrients at all. With oxygen, the seed will be able to use the starch and oxygen to produce energy (glucose) until it produces green leaves for photosynthesis.
The suitable temperature varies for different type of seeds. The temperature range for seeds to germinate can range from 5°C to 30°C. In this case, the green bean seed that is being planted would be germinating at room temperature (15°C to 25°C). The suitable temperature is important for germination because it would affect cellular metabolic and growth rates. If the temperature is not suitable, the seed will not break dormancy. This is partly due to the hydrolytic enzymes in the seed embryo. One particular characteristic of enzymes is that at low temperature, the enzyme activity would be almost none if not zero. There is a range of temperature which enzymes work best in. Therefore, the range of temperature required for the hydrolytic enzymes in the seeds to perform most efficiently for the germination of the seed is important.
Independent Variable:
→ Conditions of surroundings – The conditions of the surroundings for the seed germination will be altered through changing the different conditions needed for germination: water, oxygen and temperature. There will be four different set ups. The four set ups would have the conditions:

Set up 1) – Normal amount of water; normal amount of oxygen; room temperature
Set up 2) – Normal amount of water; normal amount of oxygen; 4°C (stored in the fridge)
Set up 3) – Normal about of water; no oxygen; room temperature
Set up 4) – Almost no water (2 cm 3 per day); normal amount of oxygen; room temperature
Set up No. 1 is the set up with all conditions present for germination. The next three are set ups with one condition missing. When any of the conditions are not present, germination is not expected.
This is a preview of the whole essay
Dependent Variable:
→ Length of seedlings – The rate of germination, in this case the length of the seedlings, will depend on the conditions present for each of the set up. As the conditions for each set up varies, the rate of germination or length of seedling will increase, decrease or stay the same. The length of each seedling will be measured using a vernier caliper, with units in cm.
Controlled Variables:
→ Volume of Water – The volume of water poured per day onto the seeds each day should remain the same, as if the volume changes, the volume of water imbibed by the seed would change, causing the rate of germination to be affected. This in turns affect the length of the seedling. All set ups except Set up 4 should receive the same amount of water per day, which will be maintained at 10cm 3 . Set up 4 shall receive 2cm 3 of water per day. The volume of water will be measured using a 25cm 3 syringe.
→ Temperature of surroundings – The temperature of the surroundings must remain the same, as if the temperature changes, the activity of hydrolytic enzymes in the seed embryo will be affected, as enzymes are temperature-sensitive. This will then affect the rate of germination in the seed, which will in turn affect the length of the seedling. All set ups except Set up 2 will be placed in a place with room temperature, with the room temperature ranging from 22°C to 25°C. Set up 2 will be placed in the refrigerator where the temperature will be maintained at 4°C.
→ Amount of oxygen – The amount of oxygen available for germination must remain the same or else the metabolism rate of the seed embryo will be affected, which will in turn affect the rate of germination. This would then lead to the lengths of the seedlings being invalid as more than one condition is not present. All set ups will receive the same amount of oxygen available in the room, except set up 3. Set up 3 will be covered with a layer of oil where oxygen will not get through to the seed.
→ Overall time taken for germination – The amount of time given for each seed to germinate must remain the same, as if the time given for the seeds to germinate varied, some seeds would be able to have more time to grow than the others, which in turns affect the length of the seedlings. The time given for each seed in all the set ups to germinate will be maintained at 1 week.
Apparatus List
Apparatus Setup Diagram
Altogether, there will be four different set ups as mentioned above.
Set Up (1) – Normal Conditions
This set up will have all the conditions needed for the germination of the seed. Like the other set ups, it will have 3 green bean seeds on top of 4 cotton pads. It will receive 10cm 3 of water for 7 days, and be placed in an area at room temperature with oxygen. This is so that the seed can germinate as all conditions are present.
Set Up (2) – Unsuitable Temperature
This set up will have all the conditions needed for the germination of the seed except a suitable temperature. Like the other set ups, it will have 3 green bean seeds on top of 4 cotton pads. It will receive 10cm 3 of water each day for 7 days, and be placed in the refrigerator with a temperature of 4°C, with oxygen. As the condition for a suitable temperature is not present, the hydrolytic enzymes in the seed would not be able to work as well as when in an optimum temperature; the seed will not be able to germinate.
Set Up (3) – No Oxygen
This set up will have all the conditions needed for the germination of the seed except for a supply of oxygen. Like the other set ups, it will have 3 green bean seeds on top of 4 cotton pads. It will receive 10cm 3 of water each day for 7 days, and be placed in an area at room temperature. However, the seeds will be covered in a layer of oil, which will deprive the seeds of the oxygen in the air, hence drowning the green bean seeds. As oxygen is not present, the embryo will be not able to respire in order to provide energy for germination. Without energy, the seed will not be able to germinate.
Set Up (4) – No Water
This set up will have all the conditions needed for the germination of the seed except for a supply of water. Like the other set ups, it will have 3 green bean seeds on top of 4 cotton pads. It will be placed in an area at room temperature with oxygen. However, the seeds will only receive 2cm 3 of water each day for 7 days, which will not be sufficient enough for the seeds to germinate. As the supply of water is not sufficient, imbibition will not occur and the metabolism of the seed will remain dormant. Without the metabolic activity of the seed, it will not germinate.
- Lay out all apparatus, clean all of them thoroughly.
- Set up the Petri dishes with 4 cotton pads in each Petri dish. Put three green bean seeds in each Petri dish. Make sure the seeds are of equal distance away from one another.
- Label the set ups 1, 2, 3 and 4. Set up 1) will be the seeds with normal conditions, 2) will be the set up with no suitable temperature, 3) will be the set up with no oxygen and 4) will be the set up with no water.
- Using a 25cm 3 syringe, pour 10cm 3 of water into Set up 1). Try to make sure each seed gets approximately the same amount of water.
- Repeat step 4 for Set ups 2) and 3).
- Using a 25cm 3 syringe, pour 2cm 3 of water into Set up 4). Try to make sure each seed gets approximately the same amount of water.
- Using a 50cm 3 measuring cylinder, pour 50cm 3 of oil into set up 3). The layer of oil should submerge the seeds completely so that there will be no oxygen.
- Place Set up 2) into the refrigerator, which will have a temperature of 4°C. This is so that the seed will be deprived of a suitable temperature.
- Place Set ups 1), 3) and 4) near a window.
- For the next 6 days, using the 25cm 3 syringe, pour 10cm 3 of water into Set ups 1), 2) and 3). For Set up 4), using the 25cm 3 syringe, pour 2cm 3 of water.
- At the end of the week, making sure there are no zero errors, measure the length of the germinated seedlings using vernier calipers.
- Tabulate the results.
The table shows the length of seedlings in cm in each of the different set ups after one week.
Analysis of Results
After conducting the experiment, it is found that when all three conditions, presence of water, presence of oxygen and a suitable temperature were present, the seed was able to germinate. However, without any one of these conditions, the seed was unable to germinate, as can be seen from the table of results. Set up (2) was placed in an area with very low temperatures, and was not able to germinate. This meant that without a suitable temperature, the seed would not be able to germinate. Set up (3) was covered with a layer of oil, preventing the seeds from taking in any oxygen from the air, and was unable to germinate as well. This meant that without oxygen, the seed would not be able to germinate. Set up (4) was deprived of a sufficient supply of water, and the seeds were also not able to germinate. Therefore, it can be seen that if any one of these conditions are not present, the seed cannot germinate, and that all conditions must be present for germination to occur.
The results from the experiment showed that only when there was the presence of water, presence of oxygen and a suitable temperature, would the seed germinate. Therefore, I can say that my hypothesis is supported and correct.
This is because:
The seed needs water to undergo imbibition, which would then allow the water to set off the metabolic activity in the seed. The water also dissolves crucial chemicals inside the seed embryo which activates the hydrolytic enzymes. With the hydrolytic enzymes activated, nutrients are then supplied to the seed embryo. Therefore, without water, the seed will not be able to germinate as the embryo will have no nutrients.
The seed also needs oxygen to germinate, as a seed that has yet undergone germination does not have leaves. Leaves contain chlorophyll, which is vital for photosynthesis. The absence of leaves means the absence of chlorophyll, which means that the seed cannot make food to supply energy for itself before it grows leaves. Therefore, oxygen allows the seed to undergo aerobic respiration, providing energy for germination.
Lastly, the seed will also need a suitable temperature before it can germinate. As there are hydrolytic enzymes in the seed that needs to be activated before nutrients can be supplied to the seed, the enzymes will need a suitable temperature to perform its best in. If the temperature is too low or too high, the enzyme activity will be too low or close to none, or the enzyme structure will be denatured, hence not able to even function at all.
Therefore, it is only under all conditions that the seed can germinate.
Reliability
I can say that my experiment is reliable because I relied on three seeds instead of just trying to germinate one seed. This enables my results to be more accurate. There were no problems during the experiment and the lengths of the seedlings were similar to each other. The apparatus used were all reliable and in good condition. The method was as precise as possible, taking note of all the details, especially the apparatus set up diagram, where everything was listed and drawn out. For example, I tried to make sure the amount of water given to each seed was approximately the same so that each seed would receive the same amount of water. In Set up (3), I also made sure that the seeds were fully submerged in the layer of oil so that absolutely no oxygen in the air would be available to the seeds. The method described and explained every step, and provided the results needed. The results analysis were described and explained with as much details as possible, and there were no miscalculation and major errors during the experiment.
The experiment was valid, as the apparatus used measured everything needed, for example, I used a 25cm 3 syringe to measure 2cm 3 and 10cm 3 of water for the seeds. I also used a 50cm 3 measuring cylinder to measure 50cm 3 of oil to pour onto the seeds. In addition, a vernier caliper was used to measure the length of the seedlings, which is used to measure lengths and thicknesses up to 0.01cm. It was also checked for any zero errors before it was used. The refrigerator in which Set up (2) was placed in was thermostatically controlled, so the temperature was constant throughout, and there was no need for a use of a thermometer. The method was valid as the dependent and independent variable were measured and results were obtained. The hypothesis was valid as the outcome of the experiment supported it.
Limitations and Improvements
If I could do the experiment again,
→ One or two more seeds could be used so that I could get a better reading on the trends. In addition, I only took the final results, which is the final length of the seedlings. I could have observed the growth of all the seeds closely so that I would be able to further explain and analyze my results.
→ Over the weekend, the seeds were not tended to, so during those two days there was a lack of water for the seeds. I could have brought the set up home with me so that I could tend to them with full attention instead of leaving them in school.
→ During the night and day, the room temperature can vary by 4-5°C. In order to keep the temperature constant in the future, I could put it in a thermostatically controlled room or container. This would ensure that the temperature will remain constant throughout, hence not affecting the seeds’ germination.
| Page

Document Details
- Word Count 2808
- Page Count 9
- Subject Science
Related Essays

Literature Review on Germination of Orchid Seeds.

In this experiment, mung bean seedlings and Brine shrimp eggs were used to...

I will be working in a freshwater environment measuring certain variables i...

cellular respiration
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 22 March 2024
Effect of different salinity on seed germination, growth parameters and biochemical contents of pumpkin ( Cucurbita pepo L .) seeds cultivars
- Hasan Ali Irik 1 &
- Gülsah Bikmaz 2
Scientific Reports volume 14 , Article number: 6929 ( 2024 ) Cite this article
769 Accesses
Metrics details
- Plant sciences
Soil and water salinity is an important limiting factor affecting yield and production levels in arid and semi-arid areas. Salt tolerance during germination is an important parameter that also affects the other plant development stages. In this respect, this study was designed to determine the responses of pumpkin seed varieties (Develi, Ürgüp, Hybrid) to different NaCl salinities. The study was carried out in 2022 in the laboratory of Biosystems Engineering Department of Erciyes University in randomized plots design with 3 replications. Experiments were conducted with 5 different water salinity. Germination percentage (GP), germination index (GI), mean germination time (MGT), seedling vigor index (SVI), ion leakage (Il), radicula length (RL) and plumule length (PL), root and shoot fresh and dry weights and some mineral composition (Na, K, Ca) were examined. Proline, antioxidant capacity, total phenolic and DPPH content were significantly affected by salinity. In scatter plot correlation analysis SVI a positive correlation was observed between GP (r 2 = 0.774), GI (r 2 = 0.745), RL (r 2 = 0.929), FRW (r 2 = 0.837), FSW (r 2 = 0.836), DRW (r 2 = 0.894), AC (r 2 = 0.747), TP (r 2 = 0.640) and DPPH (r 2 = 0.635). It was determined that there were negative correlations between SVI and MGT (r 2 = − 0.902), II (r 2 = − 0.588), DSW (r 2 = − 0.682) and PR (r 2 = − 0.344). Present findings revealed that investigated parameters were significantly affected by increasing salinity levels. While Hybrid cultivar was the most affected by salinity, Develi cultivar was found to be resistant to saline conditions.
Similar content being viewed by others

Effects of different salt sources and salinity levels on emergence and seedling growth of faba bean genotypes
Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum

Triacontanol modulates salt stress tolerance in cucumber by altering the physiological and biochemical status of plant cells
Introduction.
Salt stress is an important abiotic stress factor that limits crop productivity through negative impacts on plant growth and development especially in arid and semi-arid regions. It was reported that approximately 19.5% of irrigated lands and 2.1% of dry lands were affected by salt stress. In addition, saline lands are continuously increasing mainly due to improper irrigation management practices 1 , 2 , 3 . Salinity-induced osmotic and ion stress negatively influence plant growth and development and such negative impacts largely depend on type of salt, level and duration of salt stress, genotype and developmental stage of the plant exposed to salt stress 4 . Salinity alters various metabolic processes and especially photosynthetic activity of the plants, then reduce the chance of survival. While some plants are sensitive to saline conditions, some survive by tolerance mechanisms induced by various physiological, biochemical and molecular responses. Plants provide tolerance mechanisms to salinity as physiological and biochemical responses. Selective accumulation or excretion of ions, control of ion uptake in roots and transmission to shoots, and accumulation of these ions in certain parts of the plant and cells. Additionally, antioxidant systems are activated with the synthesis of osmotic regulators. These molecular responses provide activation or inactivation of various genes via signal transduction pathways. Resultant physiological, biochemical and molecular responses provide the maintenance of salt regulation in plants 5 .
Seed germination and seedling growth stages are the most important and most vulnerable stages in the life cycle of plants. Therefore, salinity studies have focused on these two main stages and these stages are taken into account when determining the salt resistance of plants 6 . Previous studies have also reported that salinity stress has a negative effect on germination and growth parameters in plants (Ref. 7 in lettuce; Ref. 8 in squash; Ref. 9 in pepper). Increasing salinity concentration reduces the osmotic potential, limiting germination percentage, germination rate and root development. It also causes ion toxicity and oxidative stress 10 , 11 . Plants can combat oxidative stress through enzymatic (such as catalase, ascorbate peroxidase, and superoxide dismutase) and non-enzymatic (such as carotenoids, proline, α-tocopherol, and ascorbic acid) antioxidants 12 , 13 .
Majority of pumpkin species of Cucurbitaceae family can be grown without any problems in Turkey. Some pumpkin species are consumed fresh, while others are consumed as snacks. Majority of seed pumpkins grown in Turkey belongs to Cucurbita pepo L. species and a small number of them belongs to Cucurbita moschata species 14 . Seed pumpkin production has a significant place in income sources of Central Anatolian farmers. Of about 57,184 tons of seed pumpkin production of Turkey in 2020, 16,920 tons were produced in Kayseri province 15 . Such a number corresponds to 29.65 of country production. Pumpkin seeds are mostly consumed as appetizer. However, with a rich composition, they are also used in cosmetics, pharmaceutical, health and food industries 16 . Just because of insufficient or deficit water resources, seed pumpkin cultivation is practiced under dry (rain-fed) conditions in arid and semi-arid regions. Seed pumpkin cultivation is increasing its attractiveness in these regions day by day due to its profitability as compared to cereals, its ease of storage and marketing. This study was conducted to determine the germination response of pumpkin seeds against salinity.
Material and method
This study was carried out in the laboratories of Biosystems Engineering Department of Erciyes University in February 2022. Pumpkin seeds to be used were obtained from local farmers. Develi, Ürgüp and Hybrid (Ukrainian type) cultivars, commonly used genotypes of the region, were used as the primary materials of the study. The study was conducted in accordance with the guidelines specified by the International Seed Testing Association (ISTA).
Saline waters were obtained with the use of NaCl salt. Five different salt concentrations (S 1 , 0.3 dS/m (control); S 2 , 2.5 dS/m; S 3 , 5 dS/m; S 4 , 7.5 dS/m and S 5 , 10 dS/m) were prepared.
Before the initiation of germination tests, pumpkin seeds were sterilized with 10% sodium hypochlorite for 10 min and sterilized seeds were passed through distilled water 5 times for disinfection. Disinfected seeds were placed on 20 × 20 cm filter papers with 25 seeds on each. Experiments were conducted in randomized plots design with 3 replications. Solutions of 20 ml were added to each treatment and germination papers were placed in ziplock bags to prevent evaporation. Germination was carried out in a completely dark incubator at 20 °C. Seeds were counted at the same time each day and seeds with a rootlet length of 2 mm were considered as germinated. To prevent dry out of filter papers, 10 ml of solution was added to each treatment every other day. The total germinated seeds were counted on the eighth day 17 .
The following parameters were studied:
Seedling shoot and root length of ten randomly selected seedlings from each replication were measured at the time of harvest. Shoot dry weight and root dry weight were recorded after drying at 65 °C for 72 h.
Germination percentage (GP),
Germination index (GI),
where G t is the number of seeds germinated on day t , T t is the number of days.
Mean germination time (MGT),
where Ni is the number of newly germinated seeds at time Ti.
Seedling vigor index (SVI),
Mineral composition
The K, Ca, Na contents of the plant samples were analyzed with nitric acid-hydrogen peroxide (2:3) acid in 3 different steps (1st step; 5 min at 75% microwave power at 145 ºC, 2nd step; 90% microwave power at 180 ºC). 10 min and the 3rd step (10 min at 100 ºC at 40% microwave power) after being exposed to a 40 bar pressure resistant microwave wet combustion unit (Anton paar microvawe) 18 (P, K, Ca, Mg, Na, Fe, Mn, Zn, Cu and B) were determined by reading on the ICP OES spectrophotometer (Inductively Couple Plasma spectrophotometer) (Agilent,5110 Optima, ICP/OES) 19 .
Prolin content
In order to determine the amount of Proline in pumpkin seed 20 , by applying partial modifications to the method. 0.1 g of dried plant tissue was crushed with a 3% solution of 5 ml of sulfosalicylic acid using a mortar. The extract was centrifuged at 15000× g for 10 min. 2 ml of supernatant was added to each tube in duplicates and then 2 ml of glacial acetic acid and 2 ml of acetic acid, phosphoric acid, ninhydrin solution were added to the tubes and mixed. The tubes were boiled for 1 h, at the end of this period, instant cooling was done and 4 ml of toluene was added to the samples and mixed. The toluene portion was taken into a glass cuvette and read at 520 nm.
Antioxidant capacity
Reference 21 by applying partial modifications to the method. In this context, the reagent solution was first prepared. Combine 0.6 M sulfuric acid (30 ml), 28 mM sodium phosphate (28 ml) and 4 mM ammonium molybdate (40 ml) and make up to 100 ml with water. The solution must be prepared fresh. Afterwards, 0.4 ml of sample was mixed with 4 ml of reagent solution and after vortexing the test tubes, they were incubated in a water bath at 95 °C for 90 min. After rapid cooling in cold water, the absorbance values of the samples were measured at 695 nm with a UV–vis spectrophotometer.
Total phenolic content
Determination of the total phenolic content of the samples was carried out by applying partial modifications to the method proposed by Ref. 21 . In this context, 0.2 ml of the liquid extract was taken, 1.8 ml of distilled water and 1 ml of diluted (1:10) Folin Ciocalteu reagent were added to it. After 5 min, 2 ml of 2% Na 2 CO 3 was added to the samples and after the tubes were tightly closed and vortexed, they were left to incubate in the dark for 2 h. At the end of the incubation, the absorbance values of the samples were read with a spectrophotometer (UV-1700, Shimadzu, Japan).
DPPH radical scavenging activity
The antiradical activity of the samples was carried out by applying partial modifications to the method proposed by Ref. 22 . For this purpose, 0.1 ml of the samples were added to the test tubes and mixed with 3.9 ml of DPPH (Sigma, USA) solution (prepared in 0.1 mM and methanol), then covered with aluminum foil and left in a dark environment for 30 min. At the end of the period, the absorbance values of the test tubes were determined at 517 nm in the UV–Vis spectrophotometer zeroed with ethanol.
where A c is the control absorbance nad A s is the sample absorbance. Radical scavenging activity values were given as mg AAE/kg using the ascorbic acid calibration curve.
Experimental data were subjected to analysis of variance with the use of Jump 17 pro statistical software. Significant means were compared with the use of Duncan’s test. In addition to, principal component analysis and correlation analysis were performed.
Results and discussion
Effect of salinity on germination parameters.
Germination percentage (GP) was statistically affected by both salinity and cultivar. Salinity × cultivar interaction did not have any significant effects on GP. It was observed that GP was affected by salinity for all seed pumpkin cultivars (Table 1 ). With increasing salinity levels, GP decreased in all cultivars. Among the cultivars, the highest GP (88%) was obtained from Develi cultivar and the lowest (66%) from Hybrid cultivar. In Ürgüp cultivar, GP was 71%. For salt doses, the highest GP (81%) was seen in S 1 and lowest (68%) in S 5 treatments. In terms of interactions, the highest GP (96%) was obtained from S 1 of Develi cultivar and the lowest (61%) from S 5 of Hybrid cultivar. Reference 23 reported that GP was severely affected with increasing salinity levels in paddy. It was reported in previous studies that GP values decreased with increasing salinity levels in lettuce cultivars 24 , Tunisian squash 8 and watermelon cultivars 6 .
While the germination index (GI) values were not affected by salinity and salinity × cultivar interaction, the effect of cultivars was found to be significant (p < 0.01) (Table 1 ). GI, an indicator of resistance, varied significantly with the cultivars. In terms of cultivars, GI value was found to be 26.03 in Develi cultivar, 20.26 in Ürgüp cultivar and 16.35 in Hybrid cultivar. Present data revealed that Develi cultivar had a higher salt resistance than the others.
While salinity and cultivar had significant effects on mean germination time (MGT) (p < 0.01), salinity × cultivar interaction did not have any significant effects on MGT. It was observed that MGT increased with increasing salt doses in all cultivars (Table 1 ). MGT values were found as 3.40, 3.35 and 3.83 in Develi, Ürgüp and Hybrid cultivars, respectively. There was no significant difference between Develi and Ürgüp cultivars. In all varieties, the lowest MGT value was obtained from S 1 treatments, while the highest values were obtained from S 5 treatments. Present findings on MGT comply with the results of previous studies 25 , 26 .
Seedling vigor is a complex agronomic trait with various indicators such as germination rate, final germination percentage and germination index during the seed germination stage, root length during early seedling growth, shoot length, fresh weight and dry weight 26 . Seedling vigor index (SVI) is defined as the seed characteristics that designate the rapid and uniform emergence and development potential of normal seedlings 27 . Both salinity and cultivars had significant effects on SVI (p < 0.01), while salinity × cultivar interaction did not have any significant effects on SVI (Table 1 ). SVI decreased in all cultivars with increasing salt doses. Especially after 2.5 EC salinity, serious decreases were observed. SVI value was calculated as 982.97 for Develi cultivar, 897.17 for Ürgüp cultivar and 652.82 for Hybrid cultivar. In terms of salt doses, the highest value (1130.12) was seen in control treatment and the lowest (599.78) in 10 EC treatment. SVI values varied between 1425.7 and 620 in Develi cultivar, between 1099.6 and 656.8 in Ürgüp cultivar and between 865 and 522.5 in Hybrid cultivar. Reference 9 in their study on peppers, they obtained the highest SVI value from the control (0 nM) treatment and the lowest SVI value from the treatment with 200 mM NaCl salinity. In another study conducted on medicinal pumpkin, it was reported that the SVI value decreased as salinity stress increased 28 .
Ion leakage is an indicator of stability and integrity of cell membrane and is used as an important parameter that reveals stress tolerance of plants 29 . Ion leakage is determined to reveal the relationship of membrane integrity with environmental stresses, growth, development and genotypic changes. Stress-induced leakage allows the detection of tissue damage 30 . Cultivar, salinity and salinity × cultivar interaction generated significant differences in ion leakage values (p < 0.01) (Table 1 ). In terms of cultivars, the highest ion leakage (79.68%) was observed in Hybrid cultivar and the lowest (24.22%) in Ürgüp cultivar. Ion leakage in Develi cultivar was determined as 53.93%. In terms of salinity levels, the highest (64.97%) was obtained from S 5 treatments and the lowest (42.96%) from S 1 treatments. In terms of salinity × cultivar interaction, the greatest value was obtained from S 5 of Hybrid cultivar and the lowest from S 1 of Ürgüp cultivar. In previous studies on different plants, increased ion leakages were reported under abiotic stress conditions. In a study conducted on snake melon, ion leakage also increased as salinity stress increased 31 . In another study conducted in lettuce, increasing salinity also increased the ion leakage value 32 .
While salinity and cultivars had significant effects on root length (p < 0.01), salinity × cultivar interaction did not have any significant effects on root lengths. In terms of cultivars, the longest root length (9.41 cm) was obtained from Develi cultivar and the shortest (6.23 cm) from Hybrid cultivar. Root length was measured as 8.57 in Ürgüp cultivar. In terms of salinity, root lengths decreased with increasing salt doses. The longest root length (9.74 cm) was obtained from S 1 treatment and the shortest (6.74 cm) from S 5 treatments (Table 2 ). Reduction of root lengths and seedling shoots under saline conditions is a common phenomenon in many plants. Roots are the first organs to be exposed to salinity. They are in direct contact with the soil, they absorb water from the soil and transfer it to shoots 33 . Since salinity prevents the maintenance of nutrient levels necessary for plant growth through osmotic and specific ion toxicity, it also limits root development and seedling growth 34 , 35 . It was reported in previous studies that increasing salt doses decreased root lengths in beans 36 and sunflowers 37 .
Salt doses and cultivars had significant effects on plumule length (p < 0.01), but salinity × cultivar interaction did not have any significant effects. In terms of cultivars, the lowest plumule length (1.57 cm) was obtained from Develi cultivar and the highest plumule length was obtained from Ürgüp cultivar (4.05 cm). In terms of salt doses, the highest plumule length (4.24 cm) was obtained from S 1 treatments and the lowest from S 5 treatments (2.16 cm). Plumule lengths decreased in all cultivars with increasing salt doses. In a study conducted on wheat, it was reported that the plumule length was affected by increasing salt doses 38 . Likewise, decreasing plumule lengths were reported with increasing salinity levels in pea 39 and chili pepper 40 . The decrease in plumule length with increasing salinity can be explained as follows: Salinity, which is a result of osmotic pressure, causes a decrease in water absorption, thus reducing cell division and differentiation.
Salinity, cultivar and salinity × cultivar interaction had significant effects on root fresh and dry weights (p < 0.01) (Table 2 ). Both root fresh and dry weights decreased in all cultivars with increasing salinity levels. In terms of cultivars, the highest fresh and dry root weights were obtained from Develi cultivar (0.3063 and 0.0254 g), while the lowest values were obtained from Hybrid cultivar (0.0768 and 0.0094 g). In terms of salinity, the highest root fresh weight (0.2410 g) was obtained from control treatments and the lowest from S 5 treatments (0.0990 g). For root dry weights, S 1 and S 2 treatments were placed into the same statistical group. The highest root dry weight was obtained from 0 EC treatments as 0.0238 g, while the lowest was obtained from 10 EC treatments as 0.0120 g. In terms of salinity x cultivar interaction, Develi cultivar was superior to other cultivars in terms of both fresh and dry root weight (Table 2 ). In terms of both root fresh and dry weights, the highest values were obtained from EC of Develi cultivar and the lowest values from 10 EC of Hybrid cultivar. In previous studies, decreasing root fresh and dry weights were reported with increasing salinity levels 3 , 36 , 41 .
Salinity and cultivar had significant effects on shoot fresh weights at p < 0.01 significance level, while salinity × cultivar interaction had significant effects at p < 0.05 significance level. In terms of cultivars, the lowest shoot fresh weight was obtained from Hybrid cultivar (0.4720 g), while the highest was obtained from Develi cultivar (0.6185 g). In terms of salinity levels, the lowest shoot fresh weight was obtained from S 5 treatments (0.4037 g), while the highest was obtained from S 1 treatments (0.6570 g). In terms of interaction, the highest value was obtained from S 1 of Develi cultivar (0.7833 g) and the lowest from S 5 of Develi cultivar (0.3773 g). Present findings comply with the results of earlier studies indicating decreasing shoot fresh weights with increasing salinity levels 30 , 36 , 42 .
Salinity, cultivar and salinity × cultivar interaction had no significant effects on shoot dry weights. In terms of cultivars, shoot dry weights varied between 0.2070 and 0.2245 g, while in terms of salinity, shoot dry weights varied between 0.1936 and 0.2863 g. Reference 43 indicated that salt doses did not generate significant differences in shoot dry weights of peas. Reference 44 reported that different salt concentrations did not make any significant differences on shoot dry weights of rosemary.
Effect of salinity on mineral composition
While the effects of salinity and cultivars on root Ca contents were found to be significant at p < 0.01 significance level, they didn’t have any significant effects on shoot Ca contents. Salinity × cultivar interaction had no effect on both root and shoot Ca contents (Tables 3 , 4 ). In terms of root Ca content of the cultivars, the highest value was obtained from Ürgüp cultivar (688.12 mg/kg) and the lowest from Hybrid cultivar (366.99 mg/kg). In terms of salinity levels, the highest value was obtained from S 1 treatments (742.20 mg/kg) and the lowest from S 5 treatments (397.37 mg/kg). For shoot Ca contents, the highest value was obtained from Hybrid cultivar (291.72 mg/kg) and the lowest from Develi cultivar (255.06 mg/kg). In terms of salinity levels, the greatest shoot Ca content was obtained from S 1 treatments (309.46 mg/kg) and the lowest from S 5 treatments (229.61 mg/kg). Calcium has significant effects on various structural and physiological processes such as cell walls, membrane structure, cell division and photomorphogenesis 45 . Seeds contain all essential plant mineral nutrients, but their availability is inhibited under stress conditions such as cold, drought and salinity 46 . In such cases, Ca 2+ becomes important because it provides protection from stress by regulating many physiological and cellular events. However, the increase in Na ratio with the increase in NaCl dose reduces their binding by competing with Ca at the binding sites to plasma membranes 47 .
Salinity has significant effects on root and shoot K contents. However, salinity x cultivar interaction did not have any significant effects on root and shoot K contents. On the other hand, root K contents were significantly influenced by both cultivar and salinity (p < 0.01). Shoot K contents were significantly influenced by salinity at p < 0.01 level and cultivar at p < 0.05 level (Tables 3 , 4 ). Both root and shoot K contents were negatively affected by increasing salinity. K contents decreased with increasing salinity levels. The highest root K content was obtained from Ürgüp cultivar (14,553.95 mg/kg), while the lowest was obtained from Hybrid cultivar (6710.79 mg/kg). For shoot K contents, the highest was obtained from Ürgüp cultivar (3804.85 mg/kg), while the lowest was obtained from Develi cultivar (2680 mg/kg). In terms of salinity levels, the highest root and shoot K contents were obtained from control treatments (117,081.11 mg/kg and 4010.39 mg/kg), while the lowest values were obtained from 10 EC treatments (7610.95 mg/kg and 2767.83 mg/kg). K participates into many cellular functions such as activation of enzymatic reactions, load balancing and osmoregulation 48 . Therefore, K plays an important role in salinity stress tolerance of the plants. Salinity may result in plant nutritional disorders such as suppression of K absorption 49 . Decreasing K contents were also reported in sunflowers with increasing salinity levels 50 . Salinity stress decreases total K accumulation in plants and has negative effects on plant growth and development 51 . Reference 52 represented that increasing NaCl levels caused an increase in K leakage from the seeds.
Salinity and cultivar had significant effects on root Na contents at p < 0.01 significance level, but only salinity had significant effects on shoot Na contents (p < 0.01). The salinity × cultivar interaction did not have any significant effects on both root and shoot Na contents (Tables 3 , 4 ). Root and shoot Na contents increased with increasing salinity levels. The highest Na content was obtained from Ürgüp cultivar (9177.78 mg/kg) and the lowest from Hybrid cultivar (3891.14 mg/kg). For shoot Na contents, the lowest (1041.06 mg/kg) value was obtained from Develi cultivar, while the highest value (1388.27 mg/kg) was obtained from Hybrid cultivar. In terms of salinity levels, the lowest root and shoot Na contents were obtained from S 1 treatments (3745.04 and 694.61 mg/kg) and the highest values were obtained from S 5 treatments (8993.41 and 2060.78 mg/kg). Plant roots had greater Na contents than the shoots. High root Na levels can maintain the normal osmotic potential and prevent the transport of this ion, thus preventing the accumulation of Na in the other organs 53 . Increasing root and shoot Na contents were reported in previous studies with increasing salinity levels 50 , 54 , 55 . Under high salinity levels, Nam ay reduce N-compounds and thus slow down transport rate of essential ions, which ultimately inhibiting plant growth and biomass accumulation 56 , 57 .
It is important to determine the Na, K and Na:K ratio in order to understand the salinity tolerance mechanisms 58 . The change in Na:K ratio is presented in Fig. 1 . In our study, it was observed that there was a significant difference between varieties under salinity stress. The Na:K ratios increased with increasing NaCl doses. While this increase was lower in Ürgüp cultivar, it was observed to be almost the same in Develi and Hybrid cultivars.
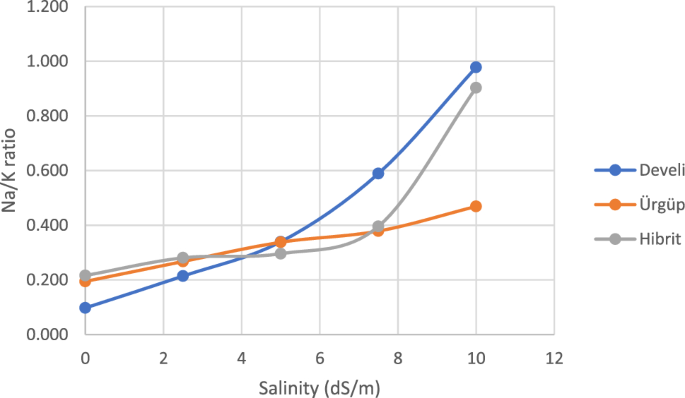
Seedling Na:K ratio of seed pumpkin cultivars.
The increase in Na ion content and decrease in K ion uptake cause ionic imbalance, inhibition of K transport process of Na in vascular tissues and Na-induced K flux from the roots as there is direct competition between these two ions. Reference 59 explained that ion antagonism occurred when nutritional imbalance was encountered due to salinity. Na increased proportionally with different salinity levels in both root and shoot, but the rate of increase was higher in root. Therefore, ionic ratios are important keys for determining relative toxicities that can provide relative biological process rates under certain ionic antagonisms. In fact, in many species, it is vital to maintain a high K/Na rather than a low Na concentration. Parallel results were obtained in studies conducted on the other plants 50 , 60 .
Effect of salinity on antioxidant capacity, prolin, total phenolic and DPPH
The effects of different salinity levels on antioxidant capacity, proline, total phenolic and DPPH content in pumpkin seed varieties are given in Table 5 .
Different salinity levels had statistically significant effects on the proline content (Table 5 ). While the difference between the cultivars was nonsignificant, the salinity × cultivar interaction was significant at p < 0.05 level. Proline content increased with increasing salt doses. In Develi cultivar, a decrease occurred after the salinity dose of 5 dS/m. The highest proline content was obtained from the S 4 and S 5 treatment, while the lowest was from the S 1 treatment.
Proline is one of the common osmolytes that maintains fluid balance in plants and is up-regulated in stress situations and provides protection against damage 61 . Salt stress disrupts the composition of cellular ions, causing ion toxicity and osmotic stress 62 . To cope with osmotic stress and consequent damage under salt stress, plants begin to produce and accumulate non-enzymatic antioxidant solutes such as proline and ascorbate as well as other enzymatic antioxidants 62 , 63 . In previous studies, it has been reported that there is an increase in the amount of proline in parallel with the increase in the salinity level 64 , 65 , 66 .
While cultivar and salinity were effective on total phenolic content at p < 0.01 significance level, salinity × cultivar interaction was effective at p < 0.05 significance level. The highest total phenolic content among the cultivars was found in Develi (5.13 mg GAE/g), there was no statistical difference between Hybrid cultivar (4.40 mg GAE/g). The total phenolic content of Urgüp cultivar was found to be 3.11 mg GAE/mg. In terms of salinity, the highest total phenolic content was taken from S 1 and S 2 treatments, while the lowest was from S 5 . According to the results of the study, increasing salinity levels caused significant decreases in total phenolic content. While the total phenolic content of Develi cultivar increased up to 2.5 dS/m salinity level, significant decreases occurred after this level. In Urgüp and Hybrid varieties, the total phenolic content decreased in all treatments after control treatment. In terms of interaction, the highest total phenolic content was obtained from Hybrid S 1 and Develi S 2 subjects, while the lowest was from Urgüp S 5 .
It is well known that abiotic stresses, including salinity, cause oxidative damage mainly by generating excess ROS (reactive oxygen species) that can attack lipids, proteins, DNA and carbohydrates. ROS consistent of both non-radical (O 2 ve H 2 O 2 ) and free radical forms (OH, O 2 − , RO ve HO 2 ) 67 . To scavenge ROS, antioxidants such as phenolic compounds are produced by plants, and thus the biosynthesis of such compounds is often stimulated in plants exposed to salt 68 . Findings parallel to the results of the study have also been reported in studies on different plants 69 , 70 , 71 .
Salinity, cultivar and salinity × cultivar interaction had significant effects on DPPH (Table 5 ). Among the cultivars, the highest DPPH content was obtained in Develi (21.04%) and the lowest in Ürgüp (11.22%). Decreases in DPPH content occurred with increasing salinity level in pumkin seed. The highest DPPH content was obtained from the control treatment (29.47%), while the lowest was obtained from the salinity level of 10dS/m. There was an 87.7% reduction in DPPH content compared to the control treatment. Compared to the control treatment, a reduction of 47.9% occurred at the salinity level of 2.5 dS/m in the hybrid cultivar. Reference 72 in cotton, Ref. 73 in coriander reported that DPPH content decreased with increasing salinity level in their studies. According to the results of the study, it was determined that the total phenolic content and DPPH results were similar. These antioxidant capacities may be directly related to the amounts of phenolic compounds due to their free radical scavenging capacity 74 .
The effect of different salinity applications on antioxidant capacity content in pumpkin seed, the effect of cultivar, salinity and salinity × cultivar interaction created a difference at p < 0.01 significance level. Among the cultivars, Develi was the variety with high antioxidant capacity, followed by Hybrid and Urgüp, respectively. (Table 5 ). With increasing salt doses, the content of antioxidant capacity decreased. The highest is taken from the S 1 subject, while the lowest is from the S 5 treatments. In terms of interaction, the highest S 1 treatment (15.33 and 14.72 mg AAE/gr) was obtained from Develi and Hybrid cultivars, while the lowest was obtained from Urgüp S 5 treatment (1.88 mg AAE/gr).
Phenolic compounds show antioxidant activity by inactivating lipid free radicals or preventing the decomposition of hydroperoxides into free radicals 75 . The degree of cellular oxidative damage in plants exposed to abiotic stress is controlled by the plants' capacity to produce antioxidant agents. However, its accumulation under salinity conditions varies considerably among plants. According to Ref. 76 Salvia mirzayanii , Ref. 77 Carthamus tinctorius L. increased with increasing salinity, Ref. 78 reported the opposite results in their study on lettuce.
Axes, eigenvalues, variance and total variance values obtained with the biplot analysis output of the parameters examined at different salinity levels of three pumpkin seed genotypes are given in Fig. 2 .
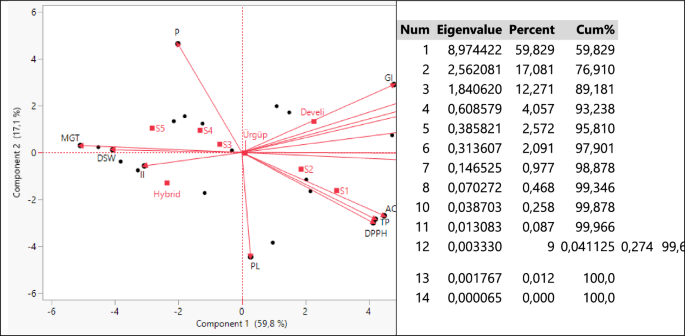
Biplot analysis on genotypes, applications and parameters studied and their values.
When Fig. 2 is examined, 3 principal component axes with eigenvalues higher than 1 have formed a total of 14 principal component axes that are independent of each other. PC1 and PC2 defined 76.91% of the total variation, while their eigenvalues were recorded as 8.97 and 2.56, respectively. These outputs show that the biplot analysis can be interpreted successfully 79 , 80 . When the lengths of the axes, their angles with each other and the regions where they are clustered are examined, the GI, FRW, GP, DRW and RL parameters are; AC, TP and DPPH parameters; MGT, DSW and II parameters were highly correlated with each other. The Develi genotype was a pioneer especially in GI, FRW and GP parameters. Hybrid genotype was separated from other genotypes in terms of II parameter and had the highest value. Since Urgup genotype is located close to the origin, it has been a genotype with average values in terms of all applications and parameters examined.
Scatter plot matrix for overview of correlations and fit lines
The correlation relationship between the data obtained as a result of the study is shown in Fig. 3 .
Scatter plot and matrix for overview of correlations and fit lines. GP germination percentage, GI germination index, MGT mean germination time, SVI seedling vigor index, Il ıon leakage, RL radicule lenght, PL plumule lenght, FRW fresh root weight, FSW fresh shoot weight, DRW dry root weight, DSW dry shoot weight, Pr prolin, AC antioxidant capacity, TP total phenolic.
While red circles show the positive relationship between the parameters examined, blue circles show the negative relationship. In addition, the size of the diameter of the circle indicates the degree of the relationship. Likewise, the distribution of colored genotypes obtained from the correlation server around the fitline lines obtained as a result of the analysis output can be seen in Fig. 3 . Among the traits examined by seedling vigor index (SVI), which is an important parameter in seed germination, a positive correlation was observed between GP (r 2 = 0.774), GI (r 2 = 0.745), RL (r 2 = 0.929), FRW (r 2 = 0.837), FSW (r 2 = 0.836), DRW (r 2 = 0.894), AC (r 2 = 0.747), TP (r 2 = 0.640) and DPPH (r 2 = 0.635). It was determined that there were negative correlations between SVI and MGT (r 2 = − 0.902), II (r 2 = − 0.588), DSW (r 2 = − 0.682) and PR (r 2 = -0.344). While all germination parameters except MGT had a positive correlation with growth parameters, a positive correlation was found only between MGT and DWS (r 2 = 0.661). While there was only a weak positive correlation between MGT and biochemical properties with Pr (r 2 = 0.361), negative correlations were found between AC (r 2 = − 0.605), TP (r 2 = − 0.499) and DPPH (r 2 = − 0.521).
This study was carried out to determine the responses of pumpkin seed varieties to different salinity stress. In the study, GP, MGT, ion leakage and SVI were affected by both salinity and variety. The increase in salinity level caused an average decrease of 16.1% in GP, a 15.5% increase in MGT, a 33.9% increase in ion leakage and a 46.9% decrease in SVI. The GI value was affected only by the variety and the highest value was obtained from the Develi variety. When the growth parameters were examined, radicule, plumule, fresh root and shoot weight, dry root weight were affected by both variety and salinity. The increase in salinity had negative effects on growth parameters. Ca, K and Na were examined as mineral composition, and both salinity and variety created a statistical difference. While the increase in salinity caused a decrease in Ca and K content, it caused an increase in Na content. When the Na/K ratio was examined, it was seen that Develi variety differed from other varieties. In this study, proline, antioxidant capacity, total phenolic and DPPH content were examined as biochemical content. As a result of the experiment, while proline level increased in parallel with the increase in salinity, other parameters decreased with the increase in salinity. It was seen that especially in semi-arid climate regions, Develi cultivar may be advantageous as compared to other cultivars in saline lands.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Kirnak, H., Dogan, E., Copur, O. & Gokalp, Z. Irrigation and yield parameters of soybean as affected by irrigation management, soil compaction and nitrogen fertilizations. J. Agric. Sci. 19 (4), 297–309 (2013).
Google Scholar
Hafsi, C. et al. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environ. Exp. Bot. 69 , 129–136 (2010).
Article CAS Google Scholar
Denhavi, A. R., Zahedi, M., Ludwiczak, A., Perez, S. C. & Piernik, A. Effect of salinity on seed germination and seedling development of sorghum ( Sorghum bicolor (L.) Moench) genotypes. Agronomy https://doi.org/10.3390/agronomy10060859 (2020).
Article Google Scholar
Cemek, B., Unlukara, A., Karaman, S. & Gokalp, Z. Effects of evapotranspiration and soil salinity on some growth parameters and yield of lettuce ( Lactuca saliva var . crispa ). Zemdirbyste 98 (2), 139–148 (2011).
Culha, S. & Cakırlar, H. The effect of salinity on plants and salt tolerance mechanisms. AKU-J. Sci. Eng. 11 , 11–34 (2011).
Aydinsakir, K., Buyuktas, D., Dinc, N. & Karaca, C. Impact os salinity stress on growing, seedling development and water consumption of peanut ( Arachis hypogaea cv. NC-7). Mediterr. Agric. Sci. 28 (2), 77–84 (2015).
Pavli, O. I. et al. Efect of salinity on seed germination and seedling development of soybean genotypes. Int. J. Environ. Sci. Nat. Resour. 27 , 556210. https://doi.org/10.19080/IJESNR.2021.27.556210 (2021).
Tarchoun, N. et al. The effects of salt stress on germination, seedling growth and biochemical responses of Tunisian Squash ( Cucurbita maxima Duchesne) germplasm. Plants 11 (6), 800. https://doi.org/10.3390/plants11060800 (2022).
Article CAS PubMed PubMed Central Google Scholar
Sarkar, A. K., Oraon, S., Mondal, S. & Sadhukhan, S. Effect of salinity on seed germination and seedling growth of bullet cultivar of chilli ( Capsicum annum L.). Braz. J. Bot. 46 , 513–525 (2023).
Acosta-Motos, J. R. et al. A Plant responses to salt stress: Adaptive mechanisms. Agronomy 7 , 18 (2017).
Mahlooji, M., Sharifi, R. S., Razmjoo, J., Sabzalian, M. R. & Sedghi, M. Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 56 (2), 549–556 (2018).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012 , 217037. https://doi.org/10.1155/2012/217037 (2012).
Singh, M. & Tiwari, N. Microbial amelioration of salinity stress in HD 2967 wheat cultivar by up-regulating antioxidant defense. Commun. Integr. Biol. 14 (1), 136–150. https://doi.org/10.1080/19420889.2021.1937839 (2021).
Yanmaz, R. Pumpkin seed cultivation. Agricultural engineers training seminar notes (1995).
TUIK. Turkish Statistical Institute. http://www.turkstat.gov.tr/Start.do (Accessed 18 September 2020) (2020).
Ermis, S. & Yanmaz, R. Effects of roasting on nutritional composition of seven lines of pumpkin (Cucurbita pepo L.) seeds. In Proc. of the Xth Eucarpia Meeting on Genetics and Breeding of Cucurbitaceae , 707–717 (2012)
Ista. International rules for seed testing. International seed testing association edition (2003).
Mertens, D. AOAC Official Method 922.02. Plants Preparation of Laboratuary Sample. Official Methods of Analysis, 18th edn. Horwitz, W., and G.W. Latimer, (Eds). Chapter 3, pp1–2, AOAC-International Suite 500, 481. North Frederick Avenue, Gaitherburg, Maryland 20877–2417, USA. (2005).
Mertens, D. AOAC Official Method 975.03. Metal in Plants and Pet Foods. Official Methods of Analysis, 18th edn. Horwitz, W., and G.W. Latimer, (Eds). Chapter 3, pp 3–4, AOAC-International Suite 500, 481. North Frederick Avenue, Gaitherburg, Maryland 20877–2417, USA (2005).
Karabal, E., Yücel, M. & Öktem, H. A. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 164 , 925–933 (2003).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 269 (2), 337–341 (1999).
Article CAS PubMed Google Scholar
Faller, A. L. K. & Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 42 (1), 210–215 (2009).
Hakim, M. A. et al. Effect of salt stress on germination and early seedling growth of rice ( Oryza sativa L.). Afr. J. Biotechnol. 9 (13), 1911–1918 (2010).
Hosein, M. & Keshavarzi, B. Studying the effects of different levels of salinity which caused by NaCl on early growth ang germination of Lactuca Sativa L. seedling. J. Stress Physiol. Biochem. 8 (1), 203–208 (2012).
Panuccio, M. R., Jacobsen, S. E., Akhtar, S. S. & Muscolo, A. Effect os saline water on seed germination and early seedling growth of the halophyte quinoa. Aob Plants https://doi.org/10.1093/aobpla/plu047 (2014).
Article PubMed PubMed Central Google Scholar
Wang, Z. F., Wang, J. F., Bao, Y. M., Wang, F. H. & Zhang, H. S. Quantitative trait loci analysis for rice seed vigor during the germination stage. J. Zhejiang Univ. Sci. B 11 , 958–964 (2010).
Zhang, A. et al. Genetic analysis for rice seedling vigor and fine mapping of a major QTL qSSL1b for seedling shoot length. Breed. Sci. 67 (3), 307–315 (2017).
Farsaraei, S., Mehdizadeh, L. & Moghaddam, M. Seed priming with putrescine alleviated salinity stress during germination and seedling growth of medicinal pumpkin. J. Soil Sci. Plant Nutr. 21 , 1782–1792 (2021).
Kocheva, K., Lambrev, P., Georgiev, G., Goltsev, V. & Karabaliev, M. Evaluation of chlorophyll fluorescence and membrane ınjury in the leaves of barley cultivars under osmotic stress. Bioelectrochemistry 63 (1), 121–124 (2004).
Yolci, M. S., Tuncturk, R. & Tuncturk, M. Effect of salt stress on some growth and physiological parameters of peanut ( Arachis hypogea L.). Yyu. J. Agric. Sci. https://doi.org/10.29133/yyutbd.768736 (2021).
Shahi-Gharahlar, A., Farhoudi, R. & Teixeira da Silva, J. A. Influence of snake melon ( Cucumis melo var. flexuosus) seed primining on seedling emergence and seedling electrolyte leakage under salinity. Seed Sci. Technol. 4 (1), 15–18 (2010).
Adetunji, A. E., Sershen Varghese, B. & Pammenter, N. W. Effects of ınorganic salt solutions on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. Plants 9 (9), 1164. https://doi.org/10.3390/plants9091164 (2020).
Asaadi, A. M. Investigation of salinity stress on seed germination of Trigonella foenum-graecum. Res. J. Biol. Sci. 4 , 1152–1155 (2009).
Abari, A. K., Nasr, M. H., Hojjati, M. & Bayat, D. Salt effects on seed germination and seedling emergence of two Acacia species. Afr. J. Plant Sci. 5 , 52–56 (2011).
Bilgili, U., Carpici, E. B., Asik, B. B. & Celik, N. Root and shoot response of comman vetch ( Vicia sativa L.); forage pea ( Pisum sativum L.) and canola ( Brassica napus L.) to salt stress during early seedling growth stages. Turk. J. Field Crop 16 , 33–38 (2011).
Oral, E., Tuncturk, R., Tuncturk, M. & Kulaz, H. Effect of silicium on reducing salt (NaCl) stress in beans ( Phaseolus vulgaris L.). Ksu J. Agric. Nat. 23 (6), 1616–1625 (2020).
Delgado, I. C. & Sanchez-Raya, A. J. Effects of sodium chloride and mineral nutrients on initial stages of development of sunflower life. Commun. Soil Sci. Plant Anal. 38 , 2013–2027 (2007).
Gupta, S. C. & Srivastava, C. P. Effect of salt stress on morphophysiological parameters in wheat ( Triticum aestivum L.). Indian J. Plant Physiol. 32 (2), 169–171 (1989).
Khan, M. A. H. et al. Salinity-induced physiological changes in pea ( Pisum sativum L.): Germination rate, biomass accumulation, relative water content, seedling vigor and salt tolerance index. Plants 11 (24), 3493. https://doi.org/10.3390/plants11243493 (2022).
Kausir, Z., Mariem, B. F., Fardaous, M. & Cherif, H. Impact of salt stress (NaCl) on growth, chlorophyll content and fluorescence of Tunisian cultivars of chili pepper ( Capsicum frutescens L.). J. Stress Physiol. Biochem. 8 , 236–252 (2012).
Asfaw, K. G. Effects of salinity on seedling biomass production and relative water content of twenty sorghum ( Sorghum biolor L.Moench) accessions. Res. J. Agron. 4 , 24–30 (2010).
Gong, H., Chen, G., Chen, G., Wang, S. & Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 169 , 313–321 (2005).
Acari, R., Yorgancılar, M., Atalay, E. & Yaman, C. The effect of different salt concentrations relative water content, chlorophyll content and plant growth in pea ( Pisum sativum L.). Selcuk J. Agric. Food Sci. 25 (3), 42–46 (2011).
Hassanpouraghdam, M. B., Mehrabani, L. V. & Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr. 20 , 335–345 (2019).
Nayyar, H. Calcium as environmental sensors in plants. Curr. Sci. India 84 , 7–10 (2003).
Knight, H. & Knight, M. R. Abiotic stress signaling pathways specificity and cross-talk. Trends Plant Sci. 6 , 262–267 (2001).
Zehra, A., Gul, B., Ansari, R. & Khan, M. A. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S. Afr. J. Bot. 78 , 122–128 (2012).
Wakeel, A., Farooq, M., Qadir, M. & Schubert, S. Potassium substitution by sodium in plants. Crit. Rev. Plant Sci. 30 , 401–413 (2011).
Shabala, S. & Cuin, T. A. Potassium transport and plant salt tolerance. Physiol. Plant. 133 , 651–669 (2008).
Wu, G. Q., Jiao, Q. & Shui, Q. Z. Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower ( Helianthus annuus L.). Plant Soil Environ. 5 , 220–226 (2015).
Al-Karaki, G. N. & Al-Raddad, A. Effects of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycorrhiza 7 (2), 83–88 (1997).
Hussain, M. I., Lyra, D. A., Farooq, M., Nikoloudakis, N. & Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 36 , 4 (2016).
Xue, Z., Zhao, S., Gao, H. & Sun, S. The salt resistance of wild soybean (Glycine soja Sieb. et Zucc.ZYD 03262) under NaCl stress is mainly determined by Na + distribution in the plant. Acta Physiol. Plant. 36 , 61–70 (2014).
Atak, M., Kaya, M. D., Kaya, G., Kıllı, Y. & Ciftci, C. Y. Effects of NaCl on the germination, seedling growth and water uptake of Triticale. Turk. J. Agric. For. 30 , 39–47 (2006).
CAS Google Scholar
Kalhori, N. et al. Effect of four different salts on seed germination and morphological characteristics of Oryza sativa L. cv. MR219. Int. J. Adv. Res. Bot. https://doi.org/10.20431/2455-4316.040005 (2018).
Hamid, M., Ashraf, M. Y., Rehman, K. U. & Arshad, M. Influence o salicylic acid seed priming on growth and some biochemical attributes on wheat growth under saline conditions. Pak. J. Bot. 40 , 361–367 (2008).
Chien, S. C., Liao, J., Wang, M. & Mannepalli, M. R. Effects og Cl – ; SO 4 2– ; and fulvate anions and Cd 2+ free ion concentrations in simulated rhizosphere soil solutions. J. Hazard. Mater. 172 , 809–817 (2009).
Zeeshan, M., Lu, M., Sehar, S., Holford, P. & Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 10 (1), 127. https://doi.org/10.3390/agronomy10010127 (2020).
Hakim, M. et al. The effect of salinity on growth, ion accumulation and yield of rice varieties. J. Anim. Plant Sci. 24 (3), 874–885 (2014).
Kaya, M. D., Akdogan, G., Kulan, E. G., Daghan, H. & Sarı, A. Salinity tolerance classification of sunflower ( Helianthus annus L.) and safflower ( Carthamus tinctorius L.) by cluster and principal component analysis. Appl. Ecol. Environ. Res. 17 (2), 3849–3857 (2019).
Hare, P. D. & Cress, W. A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21 , 79–102 (1997).
Nounjan, N., Nghia, P. T. & Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 169 , 596–604 (2012).
Gharsallah, C., Fakhfakh, H., Grubb, D. & Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants https://doi.org/10.1093/aobpla/plw055 (2016).
Yilmaz, S., Temizgul, R., Yürürdurmaz, C. & Kaplan, M. Oxidant and antioxidant enzyme response of redbine sweet sorghum under NaCl salinity stress. Bioagro 32 (1), 31–38 (2020).
Zhang, W. & Du, T. Fresh/brackish watering at growth period provided a trade-off between lettuce growth and resistance to NaCl-induced damage. Sci. Hortic. 304 , 111283. https://doi.org/10.1016/j.scienta.2022.111283 (2022).
Yavuz, D., Rashid, B. A. R. & Seymen, M. The influence of NaCl salinity on evapotranspiration, yield traits, antioxidant status, and mineral composition of lettuce grown under deficit irrigation. Sci. Hortic-Amst. 310 , 111776 (2023).
Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48 , 909–930 (2010).
Navarro, J. M., Flores, P., Garrido, C. & Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 96 , 66–73 (2006).
Lim, J. H., Park, K. J., Kim, B. K., Jeong, J. W. & Kim, H. J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat ( Fagopyrum esculentum M.) sprout. Food Chem. 135 , 1065–1070 (2012).
Rezazadeh, A., Ghasemnezhad, A., Barani, M. & Telmadarrehei, T. Effect of salinity on phenolic composition and antioxidant activity of artichoke ( Cynara scolymus L.) leaves. Res. J. Med. Plant. 6 (3), 245–252 (2012).
Zhou, Y. et al. Effects of salt stress on plant growth, antioxidant capacity, glandular trichme density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 19 , 252. https://doi.org/10.3390/ijms19010252 (2018).
Xie, Z. et al. Coronatine alleviates salinity stress in cotton by improving the antioxidative defence system and radical-scavenging activity. J. Plant Physiol. 165 , 375–384 (2008).
Neffati, M., Sriti, J., Hamdaoui, G., Kchouk, M. E. & Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 124 , 221–225 (2011).
Huang, Y. C., Chang, Y. H. & Shao, Y. Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 98 , 529–538 (2006).
Pokorny, J., Yanishlieva, N. & Gordon, M. Antioxidants in Food: Practical Applications 114–115 (CRC Press, 2001).
Book Google Scholar
Valifard, M., Mohsenzadeh, S., Kholdebarin, B. & Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 93 , 92–97 (2014).
Salem, N., Msaada, K., Dhifi, W., Limam, F. & Marzouk, B. Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol. Plant. 36 (433), 445 (2014).
Chisari, M., Todaro, A., Barbagallo, R. N. & Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce ( Lactuca sativa L. cv. Duende). Food Chem. 119 , 1502–1506 (2010).
Ozaktan, H. Technological characteristics of chickpea ( Cicer arietinum L.) cultivars grown under natural conditions. Turk. J. Field Crop 26 (2), 235–243. https://doi.org/10.17557/tjfc.1018627 (2021).
Çetin, N., Özaktan, H., Uzun, S., Uzun, O. & Ciftci, C. Y. Machine learning based mass prediction and discrimination of chickpea ( Cicer arietinum L.) cultivars. Euphytica https://doi.org/10.1007/s10681-022-03150-5 (2023).
Download references
Acknowledgements
This study was prepared from Gülsah Bikmaz’s master’s thesis.
Author information
Authors and affiliations.
Department of Biosystems Engineering, Faculty of Agriculture, University of Erciyes, Kayseri, Turkey
Hasan Ali Irik
Department of Biosystems Engineering, Institute of Graduate School of Natural and Applied Sciences, University of Erciyes, Kayseri, Turkey
Gülsah Bikmaz
You can also search for this author in PubMed Google Scholar
Contributions
H.A.I. writing, data curation, investigation, methodology. G.B. Formal analysis, data curation.
Corresponding author
Correspondence to Hasan Ali Irik .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Irik, H.A., Bikmaz, G. Effect of different salinity on seed germination, growth parameters and biochemical contents of pumpkin ( Cucurbita pepo L .) seeds cultivars. Sci Rep 14 , 6929 (2024). https://doi.org/10.1038/s41598-024-55325-w
Download citation
Received : 14 November 2023
Accepted : 22 February 2024
Published : 22 March 2024
DOI : https://doi.org/10.1038/s41598-024-55325-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pumpkin seed
- Germination
- Seedling vigour index
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Science Lab Report: Seed Germination Experiment

Related Papers
Ege Seferoglu
angel mae cabaylo
The aim of the study was to determine the effects of coco gum wastewater applied at different concentrations (100% concentration and 50% concentration) on the seed germination of pechay seeds, mungbean seeds, and corn seeds grown in petri dishes. The pH level was also measured in the three treatments used. The results obtained showed that seed germination of pechay seeds, mungbean seeds, and corn seeds was significantly reduced as the concentration of coco gum wastewater increased. As observed in the experiment, white spots were present on the seeds applied with coco gum wastewater. Moreover, the pH of the coco gum wastewater was observed to be acidic. This could likely hinder seed germination of the tested seeds.
Freshwater Biology
Gerard van der Velde
kiran kumar
Aiming to identify the simple and effective techniques for enhancement of seed germination of Acacia nilotica L., The seeds were subjected to various pre-germination treatments, which includes
Annals of Botany
alma Orozco-Segovia
Kevin Gribbins
Abstract This study examined seed ultrastructure in relation to germination of North American dandelion seeds. Based on laboratory rearing observations, it was thought that the design of the pappus acts as a conduit facilitating water entry into the seed. It was hypothesized that seeds without a pappus would yield fewer seedlings and require more time to germinate than seeds with an intact pappus.
Weed Science
Alison Hale
mozhgan sabet
Seed germination is important to know the germination pattern of a plant, more particularly the medicinal ones that might need to bring under cultivation for the primary healthcare system. The significance of the seedling in plant population ecology has long been recognized. Fabaceae family includes important medicinal plant. In this family many seeds have thick seed coat and physical dormancy. In this experiment, we chose 6 species of fabaceae family that included Abrus precatorius L., Latyrus cicera, Latyrus sativus, Securigeria securidaca, Trigonella foenigraeicum, Wisteria sinensis. These species grow and reproduce under stress conditions; it was found that the seeds require scarification to germination. Same as other legumes, these seed eco-types have a tough seed coat, which influences germination, and susceptibility to predication. Treatment of breaking dormancy method were included, scarification (by 4 sandpaper number (60, 80, 100, 150 and 220)), Piercing seed coat (by Scalpel) and Sodium hypochlorite treatment. The results of this study contribute to an understanding of the percentage of germination and the Mean germination time of these seeds in water and scarification treatments.
Global Science Books
Uraria picta (Jacq.) DC. (Pithvan), Fabaceae, is an important medicinal woody herb used in an Ayurvedic preparation " Dashmula ". The utilization of roots by uprooting the entire plants and the problems associated with the seed germination leads to shortage of root material for Ayurvedic preparation. The aim of the present study was to assess seed viability and the influence of presowing treatments on the seed germination and seedling development in U. picta. Application of 2,3,5-triphenyl tetrazolium chloride test showed that 100% of embryos were viable. The treatments applied singly and in combination include acid scarification (H 2 SO 4), cold, hot and boiling water, rubbing with sand paper, presoaking in gibberellic acid (GA 3) and application of cut to the seed coat followed by presoaking in distilled water. Significantly highest (100%) rate of seed germination and higher proportion of strong seedlings were observed in the seeds soaked in distilled water for 12 hr after application of cut to the seed coat. This was evident by the significantly higher emergence index, germination speed, germination value and vigor index when compared with the control and other treatments. The protocol developed for the seed germination and seedling development can be applied for raising a large number of plants of U. picta which will help in the conservation of plant and availability of root material for medicinal purposes.
How to Germinate Seeds: All the Basics of Seed Starting Indoors
Y ou can give your flower and vegetable garden a head start and save money by knowing how to germinate seeds. With the right amount of heat and moisture, starting seeds indoors is simple.
When the seedlings emerge, be sure that they have a good light source and a warm spot to continue growing. Soon, you'll have plants ready to transfer to the garden or a container.
Learn all the basics of germination and seed starting indoors below.
What Is Germination?
Germination of seed begins when moisture uptake activates the metabolic pathways stored in the seed to produce a plant. Dormant seeds need water, oxygen, and proper temperatures to germinate and allow the seed coat to break open and the root to emerge.
Once the root is established, a shoot that contains the stem and leaves will appear and require sunlight to thrive.
How to Get Seeds to Germinate the Quickest Way
If you are in a hurry to see if the seeds you saved from last year will germinate or if you want to teach others about germination, all you need is a paper towel, water, a sealable plastic bag or container, water, a permanent marker, and seeds.
Here's how:
- Make sure the paper towel is damp. Wet the paper towel, wringing out excessive water, and lay it flat.
- Lay the seeds on the towel. Place several seeds in neat rows on half of the paper towel and fold the other half over the seeds.
- Place the paper towel in the plastic bag. Seal it to prevent the towel from drying out.
- Label the bag. Write down the plant name and the date.
- Keep the bag warm. Be sure to place the bag in a warm area with a steady temperature of around 70 degrees.
- Check in on the seeds periodically. After three or four days, check the seeds to see if they need more moisture. Most vegetable and flower seeds germinate in seven to 10 days. If you have purchased seeds, the packet often lists how many days it takes for seeds to germinate.
Additional Method for Germinating Seeds
While using the paper towel method to germinate seeds is interesting and easy to do, it is usually not the best method for producing strong seedlings. If the seeds are left too long in the paper towel, the tiny, hair-like roots grow into the paper towel fibers and are damaged during transplanting slowing or preventing a seed from maturing into a strong seedling.
The quickest way to germinate seeds in soil is to be sure they have good contact with the seed-starting mixture, sufficient humidity, and lots of warmth. Heat mats placed under the seed starting tray are an excellent way to give the seeds a quick start.
- Prepare the seed starting medium and container. Choose a commercial seed-starting mix and moisten it well. Fill the container to within 3/4-inch from the top. The mixture should be level and firm without air pockets.
- Sow the seeds. If you are using a seed-starting tray with individual cells or small individual containers, place two or three seeds in each cell or pot. If the container is a large flat, sow the seeds uniformly in rows about two inches apart.
- Label the seeds. This is especially important if you are sowing different types of seeds in the flat or seed-starting tray.
- Lightly cover the seeds. Cover the seeds with enough dry vermiculite or seed-starting mix to equal two times the diameter of the seed. Very tiny, fine seeds can be left uncovered. Lightly pat down the soil.
- Moisten the surface. Use a mister or spray bottle to lightly moisten the surface of the seed tray or containers with water.
- Capture the moisture. Place the lid on the seed starter tray or cover the container with plastic wrap or a plastic bag to create a mini-greenhouse. Capturing the moisture means that no additional watering should be needed until after the germination of the seeds.
- Keep the seeds warm. Place the covered container in a warm place (65–75° F.) or a heated seed-starting mat.
- Watch for seedlings to emerge. At the first sign of green shoots, remove the lid or plastic covering and place the container in a bright south-facing window. The seedlings need lots of light to flourish. You can also use a grow light.
- Maintain moisture levels. Allow the seed-starting mixture to dry out slightly between waterings but do not allow the seedlings to wilt. Do not overwater and allow water to stand in the containers or the seedlings can dampen off (rot).
- Thin the seedlings. If every seed germinated and the seedlings are crowded, use garden snips to remove the excess at the soil line. Most seedlings should be transplanted to individual/larger containers when there are three sets of leaves on the stem.
What Seeds Can You Start Indoors?
Vegetables and herbs.
- Brussels sprouts
- Cauliflower
- Head lettuce
- Sweet potatoes
- Ageratum ( Ageratum houstonianum )
- Snapdragon ( Antirrhinum majus )
- Wax Begonia ( Begonia x semperflorens-cultorum )
- China Aster ( Callistephus chinensis )
- Vinca ( Cathranthus roseus )
- Cockscomb ( Cleosia spp. )
- Bachelor's Button ( Centaurea cyanus )
- Cosmos ( Cosmos spp. )
- Lisianthus ( Eustoma grandiflorum )
- Globe Amaranth ( Gomphrena globosa )
- Strawflower ( Helichrysum bracteatum )
- Impatien s ( Impatiens wallerana )
- Annual Statice ( Limonium sinuatum )
- Melampodium ( Melampodium paludosum )
- Four O'Clock ( Mirabilis jalapa )
- Flowering Tobacco ( Nicotiana alata )
- Geranium ( Pelargonium x hortorum )
- Petunia ( Petunia x hybrida )
- Moss Rose ( Portulaca grandiflora )
- Black-Eyed Susan ( Rudbeckia spp. )
- Red Salvia ( Salvia splendens )
- Mealycup Sage ( Salvia farinacea )
- Creeping Zinnia ( Sanvitalia procumbens )
- Coleus ( Plectranthus scutellarioides )
- Dahlberg Daisy ( Thymophylla tenuiloba )
- Zinnia ( Zinnia elegans )
- Verbena ( Verbena x hybrida)
- Bells of Ireland ( Moluccella laevis)
- Candytuft ( Iberis sempervirens)
- Cleome ( Cleome)
- Dianthus /pinks ( D. plumarius)
- Hollyhock ( Alcea spp.)
- Phlox ( Phlox )
- African marigold ( Tagetes erecta)
- Morning Glory ( Ipomoea purpurea)
- Sweet Peas ( Lathyrus odoratus)
What Seeds Should Not Be Started Indoors?
Some plants mature quickly enough that starting seeds indoors is not necessary. Beans, corn, peas, spinach, leaf lettuce, kale, pumpkins, melons, and squash can be direct-seeded in the garden or outdoor container.
Root crops do not transplant well from seed-starting trays. Direct sow carrot, radish, kohlrabi, beet, and turnip seeds. Seed potatoes (potato eyes) should be planted directly into the garden or a container.
Nasturtium ( Tropaeolum majus ) and Sunflower ( Helianthus annuus ) do not transplant well from a seed-starting tray. If germinating the seeds indoors, they should be started in biodegradable pots that can be directly planted into garden soil.
Additional Tips for Successful Seed Germination
- If reusing containers to germinate seeds, be sure they are cleaned and sterilized before using them. Wash the containers in warm, soapy water and then dip them in a solution of one part chlorine bleach and 10 parts water. Allow them to air dry completely before adding the seed-starting mix.
- Do not use garden soil for seed germination. It is too heavy and the seeds will have a difficult time sending down roots and sending up green shoots.
Read Next: Do Seeds Always Need Light to Germinate?
Read the original article on The Spruce .

- Digital Dale
- Change of Address/Cancellation
- Disclosure Policy
- Contest Rules
Ohio Ag Net | Ohio's Country Journal Ohio Ag Net | Ohio's Country Journal

Seed Quality Effects Germination
May 14, 2024 Crops , Ohio Field Leader , Top Headlines Leave a comment
By James Hoorman, Hoorman Soil Health Services
As spring planting gets underway, farm stress is high. When seeds germinate quickly that farm stress goes away. Getting new seeds and plants off to a vigorous start increases the potential for a healthy crop with abundant yields. However, when seeds germinate slowly because of challenging soil or weather conditions, early stress on young seedlings is likely to produce a yield drag.
When seeds germinate quickly, corn seed maggot feeding decreases. When root systems develop quickly, wireworm or rootworm larvae is greatly reduced. When seedlings grow very rapidly, and have balanced seed nutrition, they can resistant slugs and flea beetles feeding. However, none of these positive effects occur when seeds germinate slowly or when seeds are of poor quality.
Planting conditions are not always ideal. Poor weather conditions mean that often planting occurs under less-than-ideal conditions. Farmers typically have only about 9-10 days or less to get most crops planted on time. It is even probable that weather and soil conditions will be less than ideal more frequently in the future. Weather variability this year may even impact germination next year by producing poor quality seed.
When farmers purchase seed, the quality may not always be the best. Many seeds may germinate but lack vigor, and may germinate slowly, even when planted in ideal conditions. This is especially true of commodity grain crop seeds, but also for many vegetable seeds. A researcher tested several hundred seed corn samples from seed suppliers and planted them in seedling trays to test germination and seed vigor. While most of the seed samples reached the germination percentage on the label, many germinated quite slowly, emerging only 5-7 days after being planted. Some emerged 10 days after being planted, despite being maintained in perfect moisture and temperature conditions for rapid germination.
In seed corn production, small seed size seems to be ideal. Hybrid seed corn has a lower yield and often plant nutrition is adjusted for lower yields. Before pollination, the plants are “detasseled” by cutting off the plant above the ear, which removes up to 50% of the plant’s photosynthetic capacity. To keep seed size small, plants are desiccated as soon as the seeds reach maturity. The corn seed is smaller, lower in stored carbohydrate energy, and may have less mineral nutrition; resulting in lower quality seed, especially if weather conditions were poor. Seeds with lower nutrition may germinate slowly and are particularly susceptible to insects and disease. Seeds with high seed vigor and good nutrition generally germinate the fastest and get that crop off to a fast start.
The best fast germinating seeds contain abundant nutrition, mineral nutrition as well as carbohydrates, proteins and fats. Seed with generous nutrition will be heavy, have fewer seeds per pound, and have a high-test weight. In addition, the best seed also carries a population of beneficial microbes on the seed surface that immediately colonizes emerging roots and leaves. The inherited microbes from the parent seed are very important. Over time, overusing harsh chemicals on your seed may reduce the viability of these beneficial microbes that help a plant get off to a fast start.
Fast root microbial colonization helps reduce root diseases. Without beneficial microbes on the seed, new seedlings now need to recruit beneficial soil microbes. This process takes time (up to two weeks) and more energy. Diseases like fusarium, rhizoctonia, pythium, anthracnose, phytophthora and many other root-rot diseases may take over when disease repressing microbes are not present. Seeds that do not carry healthy microbes predispose young seedlings to disease susceptibility. Fungicide seed treatments may even make this process worse.
Some of these beneficial microbes are called plant growth promoting rhizobacteria or PGPR’s. These bacteria produce phytohormones that influence plant growth and development, particularly root branching. Robust root systems, established immediately after germination, are critical to producing large crop yields. PGPR’s also increase stem size and create a larger vascular system to increase plant nutrition. Plants have the genes to produce more grain and fruit, but often the pipeline is not large enough to supply the water and nutritional requirements to support higher yields
Seeds with abundant trace minerals (manganese, zinc, copper and boron) germinate quicker than those without. Some farmers apply a combination of chelated trace minerals (25 to 100 ounces each) of manganese, iron, zinc, cobalt and copper per ton of seed. When selecting seed, look for high test-weights and healthy seed to increase crop yields. Adding the right biologicals (inoculants) that contain beneficial microbes may be helpful if they are missing in your seed. Overall, seed health and plant health are important for producing high quality food and higher yields.
Related Articles

H2Ohio: What is a Voluntary Nutrient Management Plan?
May 16, 2024

Planting progress and field scouting

Feeding Farmers at Buettner Farms in Allen County
May 14, 2024

Lake Erie Harmful Algal Bloom Forecast for 2024
By Dusty Sonnenberg, CCA, Field Leader, a project of the Ohio Soybean Council and Soybean …
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Correction: Seed dispersal function of the brown bear Ursus arctos on Hokkaido Island in northern Japan: gut passage time, dispersal distance, germination, and effects of remaining pulp
- Published: 17 May 2024
Cite this article

- Yoshihiro Tsunamoto ORCID: orcid.org/0009-0000-7762-3191 1 ,
- Hifumi Tsuruga 1 ,
- Konomi Kobayashi 2 ,
- Takeshi Sukegawa 2 &
- Takuya Asakura 2
The Original Article was published on 24 January 2024
Avoid common mistakes on your manuscript.
Correction: Oecologia (2024) 204:505–515 https://doi.org/10.1007/s00442-024-05510-5
In the sentence beginning ‘Each plant’s average simulated seed dispersal distance was 202–512 m’ of the abstract of this article, the seed dispersal distance value was displayed incorrectly as ‘202–512 m’. Instead it should have been ‘181–345 m’.
Author information
Authors and affiliations.
Hokkaido Research Organization, Research Institute of Energy, Environment and Geology, Kita 19 Nishi 12, Kita-ku, Sapporo, Hokkaido, 060-0819, Japan
Yoshihiro Tsunamoto & Hifumi Tsuruga
Sapporo Maruyama Zoo, 3-1 Miyagaoka, Chuo-ku, Sapporo, Hokkaido, Japan
Konomi Kobayashi, Takeshi Sukegawa & Takuya Asakura
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yoshihiro Tsunamoto .
Rights and permissions
Reprints and permissions
About this article
Tsunamoto, Y., Tsuruga, H., Kobayashi, K. et al. Correction: Seed dispersal function of the brown bear Ursus arctos on Hokkaido Island in northern Japan: gut passage time, dispersal distance, germination, and effects of remaining pulp. Oecologia (2024). https://doi.org/10.1007/s00442-024-05565-4
Download citation
Published : 17 May 2024
DOI : https://doi.org/10.1007/s00442-024-05565-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Seeds and their importance "Germinating bean seed" by Jose Bañuelos, CC BY-NC 2.0. A seed, in botanical terms, is an embryonic plant enclosed inside its seed coat.Typically, the seed also has stored energy (proteins and carbohydrates) that are used by the seed during germination to establish itself when environmental conditions are favorable for growth.
create a hypothesis concerning the germination of seeds and their growth in the presence or absence of light. This hypothesis is generated only from prior knowledge. Many students will choose a hypothesis along the lines of "the seeds placed in the light will grow better," or "the radish plants in the light will be healthier."
Several variables might affect the germination, these variables include light, temperature, water, soil type, and air quality. Choose one variable to investigate. Write a hypothesis below. Remember, that the hypothesis should be a complete sentence that can be tested.
These findings support the hypothesis that small seed mass and light requirements have coevolved as an adaptation to ensure germination. One adult-globose cactus species, ... We evaluated seed germination under two conditions: a 12-h daily photoperiod (hereafter 'light') and one in continuous darkness (hereafter 'dark') in a germination ...
Proper distribution of seed germination, in both temporal and spatial manners, is critical for survival and proliferation of seed plants. ... Regardless of posttranslational modification, an alternative hypothesis to explain the lack of seed dormancy in DOG1-induced dog1 seeds is that DOG1 functions mainly during the maturation stage and the ...
To resolve this equation, the osmotic components (OE) were determined by germinating seeds in distilled water (zero osmolality) and in solutions of polyethylene glycol (PEG 8000) with an osmolality equivalent to the concentrations of the various salts that reduced germination by 50 % (LD 50max).Consequently, OE corresponds to the difference between the germination values obtained in pure water ...
Seeds and their importance "Germinating bean seed" by Jose Bañuelos, CC BY-NC 2.0. A seed, in botanical terms, is an embryonic plant enclosed inside its seed coat.Typically, the seed also has stored energy (proteins and carbohydrates) that are used by the seed during germination to establish itself when environmental conditions are favorable for growth.
Germination in Monocots. As the seed germinates, the radicle emerges and forms the first root. In epigeous monocots (such as onion), the single cotyledon will bend, forming a hook and emerge before the coleoptile (Figure 4.6.4.5 4.6.4. 5 ). In hypogeous monocots (such as corn), the cotyledon remains belowground, and the coleoptile emerges first.
Elevated temperature during early seed development can decrease seed size, number, and fertility, delay germination and reduce seed vigor in crops such as cereals, legumes, and vegetable crops ...
The Role of ABA. ABA is a phytohormone involved in a range of developmental and physiological processes in a plant's life cycle (Finkelstein, 2013).ABA is the dominating hormone involved in the induction of seed dormancy and control of germination (Gubler et al., 2005; Finkelstein et al., 2008; Yan & Chen, 2017).During seed development, ABA is gradually accumulated in the seed, leading to ...
Part 1: Consider a Question / Hypothesis. Several variables might affect the germination, these variables include: light, temperature, water, soil type, air quality. Choose ONE variable to investigate. Write a hypothesis below. Remember, that the hypothesis should be a complete sentence that can be tested. Part 2: Design an Experiment
Seeds constitute a key physiological stage in plants life cycle, and play a critical role in plant communities 1.At the molecular and genetic level, seed germination in wild species is a field ...
Rapid Germination Seeds (assuming ideal moisture and temperature) Days to Germinate. Types of Plants. 3. ... I think the neutral pH is the best pH for seed germination. This hypothesis is based on my study of seed and the fact that a large part of seed is food that plant needs during germination period. So I think if the seed has it's own ...
Understanding the relationships between seed traits and germination responses is crucial for assessing natural regeneration, particularly in threatened ecosystems like the seasonally dry tropical forest (SDTF). This study explored links between seed traits (mass, volume, moisture content, and dispersal type), germination responses (germinability, germination speed (v¯), time to 50% of ...
Germination and seedling development are essential stages in a plant's life cycle, greatly influenced by temperature and moisture conditions. The aim of this study was to determine maize (Zea mays L.) seeds' germination and seedling development under various abiotic stresses. Eight different temperature levels, 5, 10, 15, 20, 25, 30, 35, and 40 °C, were used. Drought and waterlogging ...
This work corroborates the hypothesis in which the seed exposure to nutrients, alone or in combination, and different pH levels (4.8 and 6.5) are important factors controlling seed germination ...
The seed germination and seedling growth of rapeseed are crucial stages in plant life, especially when facing abiotic stresses. In the present work, the effects of water and temperature on seed germination and seedling growth were investigated in a rapeseed crop (Brassica napus L.).The plants were examined under different temperature levels (5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and ...
The ability of a seed to germinate and establish a plant at the right time of year is of vital importance from an ecological and economical point of view. Due to the fragility of these early growth stages, their swiftness and robustness will impact later developmental stages and crop yield. These traits are modulated by a continuous interaction between the genetic makeup of the plant and the ...
Try sprouting seeds in different environments to test the effect of different environmental variables on seed germination. Try testing variables like temperature, soil acidity, water content, light or the presence of insects, worms or other plants. You can also cut open seeds to learn about the parts of a seed. By counting the number of ...
Sample hypothesis: The more salt added to the water, the fewer seeds will germinate. The radish seeds will not germinate at all in a solution with more than 3 teaspoons of salt in 8 oz. of water. When soil has too much salt, crops won't grow well. This experiment studies how salt affects seed germination. Klinkow CCO Public domain via Pixaby.
Wrap seeds in a moist paper towel, wait 5-10 days, and count how many seeds germinate. Illustration 1: Steps of seed germination. If you save your seed from the year before, think about this: the life of a seed can be cut in half by an increase of just 1 percent in seed moisture or by an increase in storage temperature of just a few degrees.
Investigating Seed Germination. Name: Kimberly Hoong Yearn Yi. Class: S4 Ruby . Aim. To investigate how the following conditions: presence of water, presence of oxygen, and the temperature would affect the germination of a seed. Hypothesis. If there is water, oxygen and a suitable temperature in the surroundings, then the seed will germinate.
Before the initiation of germination tests, pumpkin seeds were sterilized with 10% sodium hypochlorite for 10 min and sterilized seeds were passed through distilled water 5 times for disinfection.
2. Research: This section should be a minimum of two paragraphs and include information about your particular seed, what seeds germination is, what the requirements for germination are, etc. 3. Hypothesis: If the seeds are treated with an acidic solution with a pH of 5, then 50% of the seeds will germinate. 4.
Seeds of atg5 and atg7 mutants germinate significantly slower than Col-0, especially in the presence of ABA. Transcriptomic analyses comparing imbibed atg7 and Col-0 seeds reveal differences in gene expression associated with lipid storage and seed maturation ontology categories., Germinating seeds of atg mutants show histochemical alterations ...
The minute 'dust seeds' of some terrestrial orchids preferentially germinate and develop as mycoheterotrophic protocorms near conspecific adult plants. Here we test the hypothesis that mycorrhizal mycelial connections provide a direct pathway for transfer of recent photosynthate from conspecific green orchids to achlorophyllous protocorms.
Here's how: Make sure the paper towel is damp. Wet the paper towel, wringing out excessive water, and lay it flat. Lay the seeds on the towel. Place several seeds in neat rows on half of the paper ...
Many seeds may germinate but lack vigor, and may germinate slowly, even when planted in ideal conditions. This is especially true of commodity grain crop seeds, but also for many vegetable seeds. A researcher tested several hundred seed corn samples from seed suppliers and planted them in seedling trays to test germination and seed vigor.
In the sentence beginning 'Each plant's average simulated seed dispersal distance was 202-512 m' of the abstract of this article, the seed dispersal distance value was displayed incorrectly as '202-512 m'. Instead it should have been '181-345 m'.