Psych Scene Hub

The Dopamine Hypothesis of Schizophrenia – Advances in Neurobiology and Clinical Application
The dopamine hypothesis stems from early research carried out in the 1960’s and 1970’s when studies involved the use of amphetamine (increases dopamine levels) which increased psychotic symptoms while reserpine which depletes dopamine levels reduced psychotic symptoms.
The original dopamine hypothesis was put forward by Van Rossum in 1967 that stated that there was hyperactivity of dopamine transmission, which resulted in symptoms of schizophrenia and drugs that blocked dopamine reduced psychotic symptoms. [1]
DOPAMINE PRODUCTION AND METABOLISM
Dopamine is synthesised from the amino acid tyrosine. Tyrosine is converted into DOPA by the enzyme tyrosine hydroxylase.
DOPA is converted into dopamine (DA) by the enzyme DOPA decarboxylase (DOPADC).
This dopamine is packed and stored into synaptic vesicles via the vesicular monoamine transporter (VMAT2) and stored until its release into the synapse.

Dopamine Receptors:
When dopamine is released during neurotransmission, it acts on 5 types of postsynaptic receptors (D1-D5).
A negative feedback mechanism exists through the presynaptic D2 receptor which regulates the release of dopamine from the presynaptic neuron.

Dopamine Breakdown

Any excess dopamine is also ‘mopped up’ from the synapse by Dopamine transporter (DAT) and stored in the vesicles via VMAT2.
Dopamine is broken down by monoamine oxidase A (MAO-A), MAO-B and catechol-o-methyltransferase (COMT).
Learning points:
- Tyrosine hydroxylase is the rate-limiting step in the production of dopamine. Its expression is significantly increased in the substantia nigra of schizophrenia patients when compared to normal healthy subjects. [2]
- Carbidopa is a peripheral DOPA-decarboxylase inhibitor co-administered with levodopa. Carbidopa prevents the conversion of levodopa to dopamine in the periphery, thus allowing more levodopa to pass the blood-brain barrier to be converted into dopamine for its therapeutic effect.
- Methamphetamine increases extracellular dopamine by interacting at vesicular monoamine transporter-2 (VMAT2) to inhibit dopamine uptake and promote dopamine release from synaptic vesicles, increasing cytosolic dopamine available for reverse transport by the dopamine transporter (DAT).
- Valbenazine a highly selective VMAT2 inhibitor has been approved by the FDA for the treatment of tardive dyskinesia.
- There is compelling evidence that presynaptic dopamine dysfunction results in increased availability and release of dopamine and this has been shown to be associated with prodromal symptoms of schizophrenia. Furthermore, dopamine synthesis capacity has also been shown to steadily increase with the onset of severe psychotic symptoms. [3] , [Howes & Shatalina, 2022]

- Dopaminergic transmission in the prefrontal cortex is mainly mediated by D1 receptors , and D1 dysfunction has been linked to cognitive impairment and negative symptoms of schizophrenia . [4]
THE 4 DOPAMINE PATHWAYS IN THE BRAIN

1.The Mesolimbic Pathway
- The pathway projects from the ventral tegmental area (VTA) to the nucleus accumbens in the limbic system.
- Hyperactivity of dopamine in the mesolimbic pathway mediates positive psychotic symptoms. The pathway may also mediate aggression.
- The mesolimbic pathway is also the site of the rewards pathway and mediates pleasure and reward. Antipsychotics can block D2 receptors in this pathway reducing pleasure effects. This may be one explanation as to why individuals with schizophrenia have a higher incidence of smoking as nicotine enhances dopamine in the reward pathway (self-medication hypothesis)
- Antagonism of D2 receptors in the mesolimbic pathway treats positive psychotic symptoms.
- There is an occupancy requirement with the minimum threshold at 65% occupancy for treatment to be effective. Observations support this relationship between D2-receptor occupancy and clinical response that 80% of responders have D2-receptor occupancy above this threshold after treatment. [5]
2.The Mesocortical Pathway
- Projects from the VTA to the prefrontal cortex.
- Projections to the dorsolateral prefrontal cortex regulate cognition and executive functioning.
- Projections into the ventromedial prefrontal cortex regulate emotions and affect.
- Decreased dopamine in the mesocortical projection to the dorsolateral prefrontal cortex is postulated to be responsible for negative and depressive symptoms of schizophrenia.
- Nicotine releases dopamine in the mesocortical pathways alleviating negative symptoms (self-medication hypothesis).
3.The Nigrostriatal Pathway
- Projects from the dopaminergic neurons in the substantia nigra to the basal ganglia or striatum.
- The nigrostriatal pathway mediates motor movements.
- Blockade of dopamine D2 receptors in this pathway can lead to dystonia, parkinsonian symptoms and akathisia.
- Hyperactivity of dopamine in the nigrostriatal pathway is the postulated mechanism in hyperkinetic movement disorders such as chorea, tics and dyskinesias.
- Long-standing D2 blockade in the nigrostriatal pathway can lead to tardive dyskinesia.
4.The Tuberoinfundibular (TI) Pathway
- Projects from the hypothalamus to the anterior pituitary.
- The TI pathway inhibits prolactin release.
- Blockade of D2 receptors in this pathway can lead to hyperprolactinemia which clinically manifests as amenorrhoea, galactorrhoea and sexual dysfunction.
- Long-term hyperprolactinemia can be associated with osteoporosis.
Conceptualisation of Schizophrenia
Based on the above understanding, schizophrenia is best conceptualised as a complex entity which involves multiple pathways.

In clinical practice, there can be a disproportionate focus on positive psychotic symptoms.
It is however, important to recognise that affective (e.g depressive), negative and cognitive symptoms are a core part of schizophrenia and should be taken into account in treatment.
The aim of treatment, thus, is to modulate treatment creating a balance between effectiveness and reduction of side effects.
The balance is achieved by optimal dopamine blockade in the mesolimbic pathway while preserving (or enhancing) dopamine transmission in the other pathways.
DOPAMINE AND SCHIZOPHRENIA
The dopamine hypothesis of schizophrenia has moved from the dopamine receptor hypothesis (increased dopamine transmission at the postsynaptic receptors) to a focus on presynaptic striatal hyperdopaminergia.
According to Howes and Kapur-
This hypothesis accounts for the multiple environmental and genetic risk factors for schizophrenia and proposes that these interact to funnel through one final common pathway of presynaptic striatal hyperdopaminergia. In addition to funneling through dopamine dysregulation, the multiple environmental and genetic risk factors influence diagnosis by affecting other aspects of brain function that underlie negative and cognitive symptoms. Schizophrenia is thus dopamine dysregulation in the context of a compromised brain. [6]
Read more on the molecular imaging of dopamine abnormalities in schizophrenia.
Clinical Implications
The hypothesis that the final common pathway is presynaptic dopamine dysregulation has some important clinical implications. Firstly, it implies that current antipsychotic drugs are not treating the primary abnormality and are acting downstream. While antipsychotic drugs block the effect of inappropriate dopamine release, they may paradoxically worsen the primary abnormality by blocking presynaptic D2 autoreceptors, resulting in a compensatory increase in dopamine synthesis. This may explain why patients relapse rapidly on stopping their medication, and if the drugs may even worsen the primary abnormality, it also accounts for more severe relapse after discontinuing treatment. This suggests that drug development needs to focus on modulating presynaptic striatal dopamine function, either directly or through upstream effects. [6]
Concept of Salience
Usually, dopamine’s role is to mediate motivational salience and thereby gives a person the ability to determine what stimulus grabs their attention and drives the subsequent behaviour.
The salience network consists of the Anterior Cingulate Cortex (ACC), insula and the amygdala.

Schizophrenia is associated with an aberrant attribution of salience due to dysregulated striatal dopamine transmission.

Dysregulation of the dopamine system ultimately leads to irrelevant stimuli becoming more prominent which provides a basis for psychotic phenomena such as ideas of reference, where everyday occurrences may be layered with a with a heightened sense of bizarre significance. Furthermore, this misattribution of salience can lead to paranoid behaviour and persecutory delusions. [7]
A stimulus, even if initially lacking inherent salience, once paired with dopaminergic activity, maintains the ability to evoke dopaminergic activity over time. This suggests that in psychosis, once an environmental stimulus has been highlighted by aberrant dopamine signalling, it may maintain its ability to trigger dopaminergic activity, potentially cementing its position in a delusional framework, even if the system subsequently returns to normal function. [McCutcheon, et al, 2019]
LIMITATIONS OF THE DOPAMINE HYPOTHESIS OF SCHIZOPHRENIA
Current research shows that one-third of individuals with schizophrenia do not respond to non-clozapine antipsychotics despite high levels of D2-receptor occupancy.
Furthermore, a study using tetrabenazine (used as augmentation) which depletes presynaptic dopamine was not found to be effective in augmenting a clinical response in schizophrenia. [8]
Therefore, for a significant number of patients with schizophrenia, the basis of their symptoms is either unrelated to dopaminergic dysfunction or is associated with something more than just dopamine excess.
Alternatively, this could also mean that for some patients with schizophrenia there might be a non-dopaminergic sub-type of schizophrenia.
The current dopamine hypothesis of schizophrenia does not adequately explain the cognitive and negative symptoms. Current treatments which modulate dopamine transmission have only modest effects in improving these symptoms.
It has taken two decades for the dopamine hypothesis to evolve and reach its current state. More recent evidence shows another neurotransmitter, glutamate playing an essential role in schizophrenia.
The future likely holds a lot more secrets about schizophrenia which should unravel with the advances in understanding the brain.
Learn more:
Simplified Guide to Mechanisms of Action of Oral Antipsychotics
RECOMMENDED BOOKS
Howes O, et al . Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain . 2013
Howes OD, Shatalina E. Integrating the Neurodevelopmental and Dopamine Hypotheses of Schizophrenia and the Role of Cortical Excitation-Inhibition Balance. Biol Psychiatry. 2022 Sep 15;92(6):501-513.
Howes, O., McCutcheon, R., & Stone, J. (2015). Glutamate and dopamine in schizophrenia: an update for the 21st century. Journal of psychopharmacology , 29 (2), 97-115.
Kapur S, et al . Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. American Journal of Psychiatry . 2000
Howes O, Murray R. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet . 2014
McCutcheon, R. A., Abi-Dargham, A., & Howes, O. D. (2019). Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends in neurosciences , 42 (3), 205-220
Does the dopamine hypothesis explain schizophrenia?
- PMID: 23843581
- DOI: 10.1515/revneuro-2013-0011
The dopamine hypothesis has been the cornerstone in the research and clinical practice of schizophrenia. With the initial emphasis on the role of excessive dopamine, the hypothesis has evolved to a concept of combining prefrontal hypodopaminergia and striatal hyperdopaminergia, and subsequently to the present aberrant salience hypothesis. This article provides a brief overview of the development and evidence of the dopamine hypothesis. It will argue that the current model of aberrant salience explains psychosis in schizophrenia and provides a plausible linkage between the pharmacological and cognitive aspects of the disease. Despite the privileged role of dopamine hypothesis in psychosis, its pathophysiological rather than etiological basis, its limitations in defining symptoms other than psychosis, as well as the evidence of other neurotransmitters such as glutamate and adenosine, prompt us to a wider perspective of the disease. Finally, dopamine does explain the pathophysiology of schizophrenia, but not necessarily the cause per se. Rather, dopamine acts as the common final pathway of a wide variety of predisposing factors, either environmental, genetic, or both, that lead to the disease. Other neurotransmitters, such as glutamate and adenosine, may also collaborate with dopamine to give rise to the entire picture of schizophrenia.
Publication types
- Brain / diagnostic imaging
- Brain / metabolism*
- Brain / pathology
- Dopamine / genetics
- Dopamine / metabolism*
- Models, Biological*
- Neuroimaging
- Radionuclide Imaging
- Schizophrenia / etiology*
- Schizophrenia / metabolism*
- Schizophrenia / pathology
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 11 April 2023
The synaptic hypothesis of schizophrenia version III: a master mechanism
- Oliver D. Howes ORCID: orcid.org/0000-0002-2928-1972 1 , 2 , 3 &
- Ellis Chika Onwordi 1 , 2 , 3 , 4
Molecular Psychiatry volume 28 , pages 1843–1856 ( 2023 ) Cite this article
9600 Accesses
20 Citations
13 Altmetric
Metrics details
- Neuroscience
- Schizophrenia
The synaptic hypothesis of schizophrenia has been highly influential. However, new approaches mean there has been a step-change in the evidence available, and some tenets of earlier versions are not supported by recent findings. Here, we review normal synaptic development and evidence from structural and functional imaging and post-mortem studies that this is abnormal in people at risk and with schizophrenia. We then consider the mechanism that could underlie synaptic changes and update the hypothesis. Genome-wide association studies have identified a number of schizophrenia risk variants converging on pathways regulating synaptic elimination, formation and plasticity, including complement factors and microglial-mediated synaptic pruning. Induced pluripotent stem cell studies have demonstrated that patient-derived neurons show pre- and post-synaptic deficits, synaptic signalling alterations, and elevated, complement-dependent elimination of synaptic structures compared to control-derived lines. Preclinical data show that environmental risk factors linked to schizophrenia, such as stress and immune activation, can lead to synapse loss. Longitudinal MRI studies in patients, including in the prodrome, show divergent trajectories in grey matter volume and cortical thickness compared to controls, and PET imaging shows in vivo evidence for lower synaptic density in patients with schizophrenia. Based on this evidence, we propose version III of the synaptic hypothesis. This is a multi-hit model, whereby genetic and/or environmental risk factors render synapses vulnerable to excessive glia-mediated elimination triggered by stress during later neurodevelopment. We propose the loss of synapses disrupts pyramidal neuron function in the cortex to contribute to negative and cognitive symptoms and disinhibits projections to mesostriatal regions to contribute to dopamine overactivity and psychosis. It accounts for the typical onset of schizophrenia in adolescence/early adulthood, its major risk factors, and symptoms, and identifies potential synaptic, microglial and immune targets for treatment.
Similar content being viewed by others

Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes
Brain-specific deletion of GIT1 impairs cognition and alters phosphorylation of synaptic protein networks implicated in schizophrenia susceptibility
Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications
Introduction.
Schizophrenia is generally a severe mental illness with a lifetime prevalence of about 1% of the population worldwide [ 1 ], and a major cause of global disease burden [ 2 ]. Symptoms typically begin in late adolescence or early adulthood, and can be separated into three domains: (1) positive (e.g., hallucinations, delusions, paranoia and thought disorder), (2) negative (e.g., anhedonia, avolition, social withdrawal and thought poverty) and (3) cognitive (e.g., dysfunction in attention, working memory and executive function) [ 3 ]. The onset of the first psychotic episode is commonly preceded by a prodrome generally of 1–5 years and characterised by sub-clinical negative, cognitive and psychotic symptoms [ 4 , 5 ].
In up to 20% of patients with schizophrenia, their illness shows a limited response to adequate trials of two different anti-psychotic drugs and clozapine [ 6 ] and treatments for negative symptoms and cognitive deficits remain an unmet clinical need [ 7 ]. Thus, there is a need to understand the pathoaetiology of schizophrenia to help identify new treatment targets.
In 1982, Irwin Feinberg first proposed that a fault in synaptic elimination in adolescence is causal to schizophrenia [ 8 ]. The hypothesis was subsequently revised to propose that a combination of excessive pruning of cortical synapses in prefrontal circuits and insufficient pruning of subcortical synapses underlies the onset of schizophrenia [ 9 ]. Judged by the number of citations on the theme, the synaptic hypothesis of schizophrenia has stimulated substantial interest, particularly in the last few years (see Fig. 1 ).
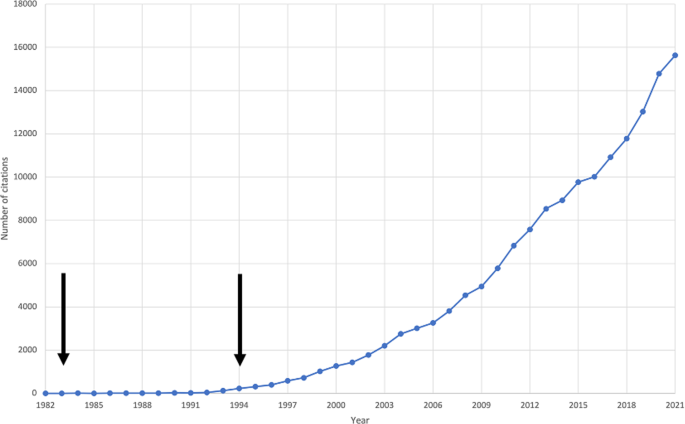
The first arrow indicates the year Feinberg’s hypothesis was published. The second arrow indicates the year Keshavan et al.’s hypothesis was published.
However, since these iterations of the synaptic hypothesis of schizophrenia, new data from novel methods, such as induced pluripotent stem cell (iPSC) and genome-wide association studies (GWAS) and in vivo synaptic imaging, have emerged. Here, we update the synaptic hypothesis in light of this new evidence.
The synaptic hypothesis version I
Feinberg proposed arguably the earliest version of the synaptic hypothesis of schizophrenia, stating that ‘too many, too few, or the wrong synapses are eliminated’ [ 8 ]. He speculated that this led to impaired neuronal integration, which resulted in auditory hallucinations, thought interference and the loss of self-boundaries observed in schizophrenia [ 8 , 10 ].
Feinberg referred to ‘reduced synaptic density’ as an umbrella term for qualitative changes in synapses, such as reorganisation, as well as a quantitative reduction in the number of synapses. He cited four main lines of evidence in support of a role for synaptic alterations in schizophrenia. First, Feinberg observed that EEG wave amplitudes markedly increase in infancy, and decline substantially in adolescence, with little variation in adulthood. Second, brain metabolic rate, as measured by CMRO 2 uptake, peaks in the first decade of life, declining rapidly through adolescence and early adulthood, before declining more slowly through the remaining adulthood [ 11 ]. Third, the degree of neuroanatomical plasticity observed in childhood, whereby the brain is capable of functional recovery from injury, is lost by adolescence. Fourth, Feinberg speculated that cognitive performance (termed ‘functional power’) peaks in adolescence. Feinberg observed that these electrophysiological, metabolic, anatomical and cognitive trajectories track trajectories for synaptic density, which peaks in childhood, before rapidly declining in late childhood and early adolescence [ 12 ]. He also observed that schizophrenia typically emerges in adolescence or early adulthood, thereby correlating temporally with the period of marked synaptic elimination. In addition, he noted that markers tracking synaptic trajectories (EEG wave amplitude and cognitive performance) are altered in schizophrenia.
Much of the evidence concerning synapses cited in version I of the hypothesis was indirect in nature, for instance, that cortical glucose metabolism is lower in schizophrenia than in controls. However, whilst lower glucose could reflect lower synaptic density, approximately 30% of cortical glucose metabolism supports non-signaling processes, unrelated to synaptic levels or activity [ 13 ]. This highlighted the need for more direct evidence of synaptic levels in schizophrenia.
The synaptic hypothesis version II
In 1994, Keshavan et al. updated the synaptic hypothesis with new evidence for structural and metabolic abnormalities in schizophrenia, and revised it to propose excessive cortical pruning and insufficient subcortical pruning [ 9 ]. They also highlighted that there could be a failure to form synapses in the first place, excessive synaptic elimination later in neurodevelopment, excess synaptic production early in development, or a combination of these processes.
Keshavan et al. synthesised new evidence regarding normal neurodevelopment, by drawing on non-human primate and human data, which indicated a peak in cortical synaptic density in normal early postnatal development, followed by a sharp decline in synaptic density through puberty, and a slower decline in adulthood [ 14 , 15 , 16 , 17 ]. Keshavan et al. noted that these trajectories were consistent with Feinberg’s hypothesised synaptic trajectory in normal human neurodevelopment. However, they noted that the locus of synaptic elimination (both spatial, in relation to synapse type, laminar location, and regional variation, and temporal) had yet to be established.
Keshavan et al. built on the neurostructural foundations of Feinberg’s hypothesis, by incorporating new evidence from CT studies indicating that grey-white matter ratios reduce from childhood during normal development. They also incorporated new evidence for neurostructural alterations in schizophrenia, including post-mortem studies showing reduced brain volume, cortical thinning and sulcal enlargement [ 18 , 19 ] and early MRI studies showing reduced frontal lobe volume [ 20 , 21 ], greater frontal sulcal size [ 22 ], and reduced cortical grey matter volume [ 23 , 24 ] in schizophrenia patients relative to healthy controls. The data were not all consistent, however, with other MRI studies failing to find significant differences in frontal or cerebral volume [ 25 ].
In addition, Feinberg speculated that synaptic elimination in adolescence may underlie reductions in cerebral metabolism and, when faulty, the onset of schizophrenia. This would suggest alterations in cerebral metabolism in schizophrenia. Keshavan et al.’s second version of the synaptic hypothesis of schizophrenia incorporated emerging evidence for frontal lobe hypometabolism, including positron emission tomography (PET), single-photon emission computed tomography, 133 Xe inhalation, 31 P-magnetic resonance spectroscopy, and cerebral blood flow studies indicating frontal hypometabolism [ 26 , 27 , 28 ], although again, these data were not unequivocal, with some studies failing to find evidence for hypofrontality [ 29 ].
Feinberg’s hypothesis lacked specificity in terms of the precise location of suspected synaptic alterations in schizophrenia. This was refined in the second synaptic hypothesis, which proposed excessive cortical pruning and insufficient subcortical pruning. The evidence for a failure of subcortical synaptic pruning was derived from individual MRI studies, which reported greater lenticular nucleus and left caudate volume in patients with schizophrenia [ 30 , 31 ]. However, this could be an effect of anti-psychotic treatment and meta-analysis of these and subsequent studies have not found consistent evidence for subcortical alterations in anti-psychotic-free patients [ 32 ].
Critically, neither this nor version I proposed a mechanism for faulty synaptic elimination, or how this is linked to genetic or environmental risk factors. To address this, we review new lines of evidence and propose an updated version of the hypothesis.
Evidence for synaptic changes in normal brain development
Multiple lines of evidence indicate that synapses undergo dramatic reorganisation through the course of life. Preclinical studies have found that normal development shows an early phase of net synaptic production followed by a phase of net synaptic elimination, and then comparatively balanced elimination and production leading to relatively stable synaptic levels in adulthood [ 15 , 16 , 33 ]. Consistent with this, human post-mortem studies show the greatest synapse density in early childhood, followed by intermediate levels during adolescence and early adulthood and lower, stable levels in adulthood (Fig. 2 ) [ 34 ]. Synaptic density reduces by approximately 40% from childhood through adolescence, with elimination particularly affecting glutamatergic synapses [ 9 , 15 , 16 ]. There was limited longitudinal in vivo imaging evidence for brain changes during human development when versions I and II of the hypothesis were proposed, but there have been a number of large studies since then. These show that cortical grey matter volumes increase and peak early in development [ 35 ], before undergoing a monotonic decrease into early adulthood [ 36 , 37 , 38 ]. Although beyond the scope of this review, it is important to recognise that findings from longitudinal imaging studies vary according to region, volumetric measure and developmental windows studied (see review [ 38 ]). The timing of these structural changes is broadly in line with the timing of synaptic changes seen in the preclinical and human post-mortem data on synaptic changes [ 36 , 39 ], although this temporal association does not mean they are causally related (discussed further in the section on evidence for structural alterations in schizophrenia).
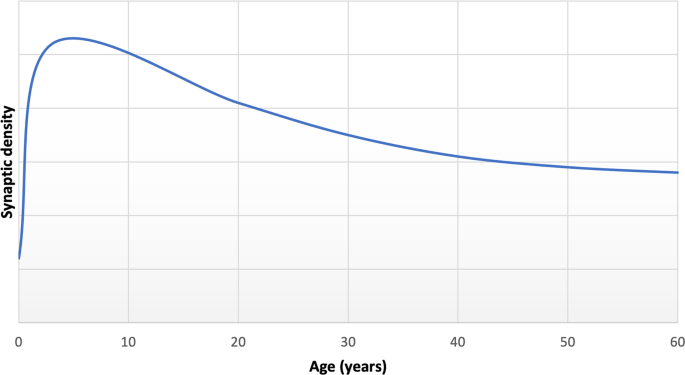
Adapted from post-mortem data reported by Petanjek et al. for basal dendritic spine density of pyramidal cells from layer IIIc [ 34 ].
The mechanisms governing synaptic elimination
Microglia are histiocytes that play a central role in synaptic elimination during normal brain maturation [ 40 , 41 ]. During neurodevelopment, redundant synapses are tagged by complement proteins such as C1q (the cascade-initiating protein) and other complement proteins including C3 and C4, in a process which triggers the phagocytosis of the synapse by microglia (Fig. 3 ) [ 41 , 42 ].
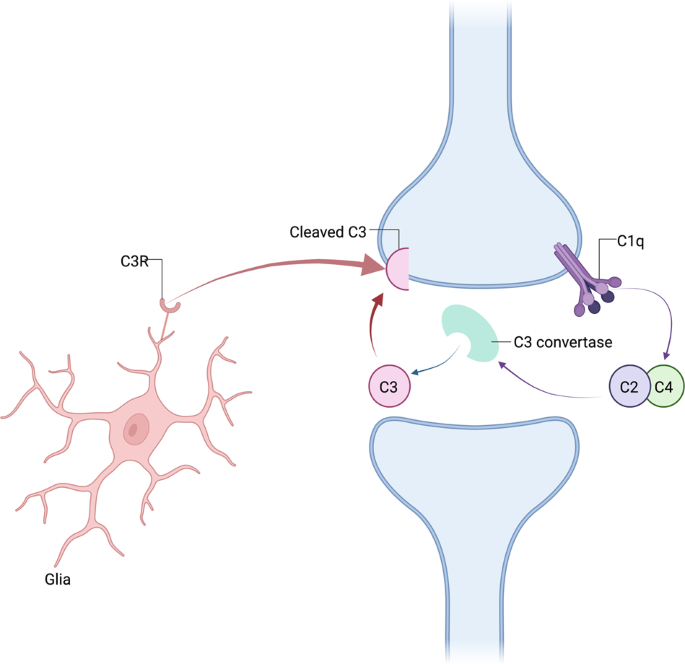
Complement component C1q interacting with binding partners triggers cleavage of the complement components C2 and C4, thereby promoting the generation of activated C3. Activated C3 induces synaptic phagocytosis by microglia. Other complement signalling pathways can also trigger phagocytosis.
Adult mice overexpressing human C4 show increased microglial engulfment of synapses and reduced synapse density in the medial prefrontal cortex [ 43 ]. Similarly, C4-overexpressing mice show reduced spine number, and decreased spine turnover in juvenile mice, as well as abnormalities in glutamatergic cells [ 44 ]. In contrast, complement-dependent pruning is reduced by repeated firing of a synapse [ 42 ]. Thus, taken together, these lines of evidence show synaptic pruning is activity dependent and can be altered by genetic variants affecting the complement system.
In addition, astrocytes play both indirect and direct roles in synaptic elimination [ 45 ]. For example, C1q mRNA expression is dependent on factors secreted by astrocytes [ 42 ]. Moreover, astrocytes directly engulf synapses through the activation of phagocytic pathways necessary for normal neural circuit refinement [ 46 ].
Non-glial-related mechanisms also play a role in the elimination of synapses. For example, activation of the transcription factor myocyte enhancer factor 2 leads to ubiquitination of post-synaptic density protein 95 (PSD-95), which is then targeted for proteasomal degradation [ 47 ]. Stimulated synapses release molecules that change the actin skeleton in neighbouring dendritic spines, ultimately leading to their elimination [ 48 ]. Other molecules, such as semaphorin 7A, are released post-synaptically, and act in a retrograde manner to promote the elimination of pre-synaptic elements [ 49 ].
Genetic and environmental risk factors for schizophrenia implicate synapses
There has been a massive expansion in the power of genetic association studies since earlier versions of the hypothesis. The latest genome wide association study (GWAS) includes 76,755 schizophrenia patients and 243,649 controls, and identified 287 common variant loci associated with schizophrenia. These associations implicate genes involved in synaptic organisation, differentiation and transmission, and post-synaptic terms, with additional enrichment of genes playing roles in synaptic transmission and signalling [ 50 ].
The most significant GWAS signal for schizophrenia in a sample predominantly of European origin lies in the major histocompatibility complex (MHC) and has been shown to link to complement expression, in particular to higher C4A brain expression levels [ 51 , 52 ]. Complement-dependent pruning is reduced by repeated firing of a synapse and increased calcium signalling, as shown in the mouse visual cortex [ 42 ]. This suggests that the refinement of neural circuits via synapses could be altered by a genetic predisposition to impairments in the complement system and/or in glutamatergic signalling. However, it should be noted that a recent GWAS in people predominantly of East Asian ancestry did not find this association [ 53 ]. This highlights the need for more genetic studies in ethnically diverse groups to test the generalisability of findings [ 54 ]. Notwithstanding this, other risk factors for schizophrenia, such as stress, affect synaptic pruning (see summary in synaptic hypothesis version III section), and may account for aberrant synaptic pruning independent of genetic effects.
Brain co-expression network analyses coupled with gene ontogeny analyses have revealed that C4A expression levels are inversely associated with expression levels of synaptic genes, and that schizophrenia risk loci occur in synaptic pathways [ 52 ]. In addition, numerous other genetic loci associated with schizophrenia are linked to genes encoding proteins that mediate synaptogenesis [ 55 ], synaptic plasticity [ 56 ], spine formation and those involved in mechanisms of refining circuitry [ 55 ]. Animal models of some of these genetic risk factors have shown that they lead to lower synaptic marker levels [ 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 ].
Genetic loci linked to increased risk for schizophrenia are also involved in synaptic pathways during development. These include VRK2 , encoding vaccinia-associated kinase 2, which is involved in neurodevelopmental microglia-mediated synaptic elimination [ 56 ], CUL3 , encoding Culin-3, which is involved in neural development, glutamate receptor turnover and maintenance of excitation-inhibition balance [ 56 , 67 , 68 ], KALRN , encoding kalirin, which mediates dendritic spine formation [ 67 , 68 ] and CLSTN3 , encoding Calsyntenin-3, which promotes inhibitory and excitatory synaptic development [ 67 , 68 ].
Early environmental insults, such as maternal infection, are risk factors for schizophrenia [ 69 , 70 ], and animal models of antenatal infection or immune challenge show that these affect synaptic development, with some effects enduring into adulthood [ 71 , 72 , 73 ]. These seem to particularly affect synaptic development in glutamatergic neurons [ 72 , 73 ].
Preclinical models of a number of other environmental risk factors for schizophrenia also show lower levels of synaptic markers. For example, studies of social isolation have identified that rats weaned on postnatal day 21 and subsequently reared in isolation for 8 weeks show lower medial prefrontal cortical and hippocampal dendritic spine density in adulthood (postnatal day 77) compared to controls reared in social groups [ 74 ]. Similarly, rats aged 28–32 days subsequently reared in individual caged environments for 30 days showed lower dorsolateral striatal dendritic spine density in adulthood compared to controls reared in complex environments containing social groups and objects [ 75 ]. A model of chronic social defeat stress has found that mice aged 8–10 weeks introduced to and attacked by unfamiliar resident mice show lower levels of prefrontal cortical pyramidal neuron apical dendritic spine density 30 days following the stress procedure compared to controls [ 76 ]. Numerous studies have identified that rodents subjected to chronic stress show changes in dendritic arborisation, with effects varying according to the brain regions studied [ 77 , 78 , 79 , 80 ]. Furthermore, studies of maternal immune activation models, which expose pregnant rodents to lipopolysaccharide [ 81 ] or polyribocytidylic acid (poly I:C) [ 82 ] on gestational day 9.5, have found lower levels of both pre-synaptic and post-synaptic markers in cortical brain regions as adults. This includes lower levels of pre-synaptic proteins such as synaptophysin, syntaxin-1 and synaptobrevin, and lower levels of post-synaptic proteins, including PSD-95 and SH3 and multiple ankyrin repeat domains proteins 1, 2 and 3, at postnatal days 52–54 [ 81 ], and also lower dendritic spine density at postnatal day 80 [ 82 ] compared to controls. These models show that risk factors acting at various developmental stages can lead to loss of synaptic markers. Similarly, lifetime stress has been linked to lower levels of dendritic spine density post-mortem in human cortical tissue [ 83 ]. It should be recognised that a range of brain and behavioural alterations are seen in these preclinical models, and it remains to be established that the synaptic changes are primary rather than secondary to other alterations. Furthermore, the effects of stress on dendritic spine density may show specificity in terms of brain region, spine type and timing of stressful events [ 76 ]. Nevertheless, taken together with the genetic studies, these findings indicate that a range of risk factors for schizophrenia affect synaptic levels to potentially increase vulnerability to the disorder.
The link between complement expression and brain structure and function has begun to be investigated. A recent imaging genetics study of a mixed sample of healthy control subjects ( n = 46), patients with psychosis ( n = 40) and individuals at clinical high risk for psychosis ( n = 43) showed that levels of genotype-predicted brain C4A expression were positively associated with brain levels of translocator protein (TSPO, a marker expressed by glia), and negatively associated with hippocampal surface area [ 84 ]. However, there was no significant effect of clinical group on these relationships, indicating it is most likely a common mechanism. Further work in a large UK Biobank sample (n > 27,000) which excluded individuals with diagnoses of neurological or mental disorders identified that predicted C4A expression levels are negatively associated with cortical thickness in a number of brain regions implicated in schizophrenia pathogenesis, including the parahippocampal, insula, entorhinal, medial orbitofrontal and parts of the cingulate cortices [ 85 ]. Other work has investigated the relationship between complement markers and phosphorous magnetic resonance spectroscopy, which enables the quantification of membrane phospholipid precursors and catabolites [ 86 ] that are considered as proxies for the degree of neuropil contraction [ 87 ]. Higher C4A gene copy number has been shown to be directly associated with higher levels of catabolites in the inferior frontal cortex, and lower levels of precursors in the inferior parietal lobule, suggestive of greater neuropil contraction in these regions, in patients with schizophrenia [ 87 ]. In addition, higher C4A expression levels have been negatively associated with middle temporal cortex activation in healthy controls during an functional MRI (fMRI) visual processing task, and with episodic memory performance in healthy controls and patients with schizophrenia [ 88 ]. The findings discussed in this section thus show associations between diminished cortical volumes and thickness and altered brain activation and complement levels, consistent with a model that higher complement levels could underlie brain structural and functional changes in schizophrenia, although, importantly, it remains to be established if complement leads to the changes. It should also be recognised that there are inconsistencies in the relationship between C4A and brain imaging measures [ 85 , 87 ]. Thus, further work is needed to investigate if complement expression underlies brain structural and functional changes, and to test if there is a link between complement expression and in vivo synaptic marker levels in patients with schizophrenia.
Post-mortem synaptic markers in schizophrenia
Since earlier iterations of the synaptic hypothesis of schizophrenia, a wealth of post-mortem evidence for lower levels of markers of synaptic density in schizophrenia has accumulated.
Post-mortem studies have reported lower levels of a number of pre-synaptic markers in schizophrenia relative to controls, with moderate-to-large effect size lower levels of synaptophysin in the frontal cortex, cingulate cortex and hippocampus on meta-analysis [ 89 ]. Furthermore, a meta-analysis of post-mortem studies found lower levels of post-synaptic elements (comprising dendritic spine density, post-synaptic density and post-synaptic density (PSD) protein expression levels) in people with schizophrenia, with a moderate effect size in cortical tissues [ 90 ]. Subgroup analysis demonstrated that levels of post-synaptic elements were lower in cortical but not subcortical tissues [ 90 ]. Thus, findings of lower pre-synaptic and post-synaptic markers in separate meta-analyses of post-mortem studies are highly suggestive of cortical synaptic alterations in schizophrenia. These findings are supported by electron microscopy studies, which provide the gold standard means for directly measuring synaptic density, showing lower levels of axospinous [ 91 ] and axodendritic [ 92 ] synaptic density in the anterior cingulate cortex, perforated (principally glutamatergic) synapses in the striatum [ 93 ], and lower total synaptic density in the substantia nigra, particularly affecting symmetric (inhibitory) synapses [ 94 ] in tissue from patients with schizophrenia compared to controls. However, there are inconsistencies in the findings, potentially relating to differences in methodological approaches and the specific markers used [ 95 ]. Post-mortem studies in schizophrenia are also subject to a number of potentially confounding factors, such as differences in lifetime anti-psychotic exposure, differences in cause of death and post-mortem interval [ 95 ]. Moreover, post-mortem studies are highly labour intensive, therefore limiting the number of subjects and regions investigated in the individual studies. These issues limit the generalisability of findings. Critically, they cannot provide conclusive evidence of synaptic density changes in the living brain, or when they occur in the illness.
Findings from neuronal cultures derived using induced pluripotent stem cells (iPSC) from patients
A significant technological advance since the earlier versions of the synaptic hypothesis has been the ability to use stem cells from patients to derive neuronal cultures. This enables neuronal development to be studied in brain tissue with the same genetic background as patients [ 96 ]. As seen in Table 1 , studies implementing these methods show evidence for both pre- and post-synaptic deficits, such as lower synaptic vesicle 2 (SV2) and synapsin I puncta density and synaptic vesicle release, and lower levels of post-synaptic markers including PSD-95 protein levels and dendritic spine density. They also show functional alterations in synaptic signalling in neurons derived from people with schizophrenia compared to controls (Table 1 ).
Importantly, recent iPSC models investigating synapse-glia interactions in vitro have demonstrated elevated, complement-dependent elimination of synaptic structures [ 97 ], highlighting a potential mechanism for excessive synaptic pruning by glia in schizophrenia [ 98 , 99 ], summarised in Fig. 4 . Thus, the evidence from patient-derived neural cultures indicates a failure to form and/or preserve synapses in early neurodevelopment in schizophrenia. Whilst beyond the scope of our review, it should be noted that in addition to synaptic alterations, iPSC models also show evidence for alterations in other aspects of neuronal development [ 100 ]. The role of these in the synaptic alterations remains to be determined.
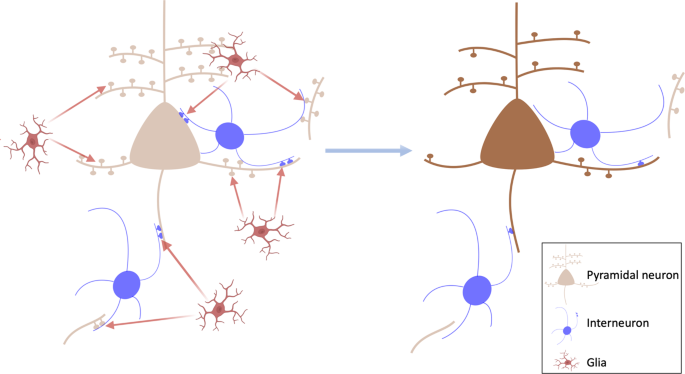
Left: potential model of glia-mediated elimination of synapses in schizophrenia. This could affect glutamatergic synapses, including dendritic spines and collaterals that synapse onto inhibitory interneurons, as well as inhibitory synapses onto pyramidal neurons. Right: loss of synapses on pyramidal neurons and inhibitory interneurons, which could disrupt pyramidal neuron function and lead to negative and cognitive symptoms.
It is also important to appreciate the limitations of evidence obtained through patient-derived neuronal lines. As these neural cells are cultured in vitro, they do not adequately reflect the impact of environmental factors on neurobiology in schizophrenia [ 101 ]. Furthermore, the limited maturity of derived neural cells, methodological variability in processes for cellular reprogramming and brain cell generation, and genetic background variations may affect findings [ 96 ]. These considerations highlight the need for in vivo studies with other techniques in patients.
Evidence for altered brain structure in schizophrenia
There has been a step-change in the in vivo imaging evidence for altered brain structure in schizophrenia since versions I and II of the synaptic hypothesis were elaborated with, now, well over a hundred studies across brain regions and phases of illness [ 32 , 102 ]. Meta-analyses of findings in patients show well-replicated evidence for lower cortical grey matter volumes (Hedges g ~0.26–0.66 [ 32 ], Cohen’s d ~0.31–1.09 [ 102 ]), and lower or unaltered subcortical volumes (Hedges g = ~0.11–0.46 [ 32 ], Cohen’s d = 0.18 [ 102 ]) relative to healthy controls across illness phases from the first episode. Moreover, there is now substantial evidence for similar patterns of lower grey matter volumes and cortical thinning in people at clinical high risk for schizophrenia, and that this is particularly marked in those who go on to develop the disorder [ 103 , 104 ]. Meta-analysis also indicates that cortical thickness in fronto-temporal brain regions is lower in schizophrenia from the onset of the disorder ( z ~1.94–3.25) and in clinical high-risk subjects ( z ~1.01) [ 105 ].
In addition, studies have compared age-related brain structure in patients with controls. Cross-sectional imaging studies suggest that there is an accelerated age-related decline of grey matter volume in schizophrenia patients compared with controls [ 106 ]. This has been directly tested in longitudinal imaging studies, with meta-analyses of these indicating greater grey matter loss over time in patients compared to controls [ 107 , 108 ]. Furthermore, cross-sectional imaging has identified that these deficits start early in disorder [ 106 ], and longitudinal studies have demonstrated that elevated rates of cortical grey matter loss are associated with conversion from clinical high risk to a psychotic illness [ 104 , 109 ]. Moreover, there is an inverse relationship between cerebral volume and symptom severity in schizophrenia [ 110 , 111 , 112 ]. These observations implicate neurostructural alteration in the development of schizophrenia, raising the question as to the cellular and molecular basis of these changes.
A key question is, thus, whether structural MR imaging changes could be due to synaptic loss. Kassem et al. addressed this key question by combining structural MRI and confocal microscopy [ 113 ]. They observed grey matter volume loss on MRI in the anterior cingulate cortex and hippocampus of stressed mice, and reduced dendritic volume and spine density, in the absence of changes in the number or volumes of neuronal soma, astrocytes or oligodendrocytes. Moreover, there was a strong linear relationship ( R 2 > 0.9) between dendritic volume loss and MRI-estimated grey matter volume loss [ 113 ]. Similarly, Keifer et al. deployed voxel-based morphometry and confocal microscopy to investigate the effects of an auditory fear conditioning paradigm in mice. They found increased grey matter voxel intensity in the conditioned mice relative to the controls in the auditory cortex, amygdala and insula; concurrent increases in dendritic spine density in the auditory cortex; a positive relationship between dendritic spine density and grey matter voxel intensity; no change in neuronal nuclei density; and no relationship between nuclei density and grey matter voxel intensity [ 114 ]. These data show that synaptic changes can lead to changes in grey matter volumes measured by MRI, and indicate that stress can contribute to this. It should be noted, however, that Keifer et al. did not identify a significant change in auditory cortical thickness, suggesting dendritic spine density is less strongly linked to cortical thickness than to grey matter density.
Whilst cortical neuronal number remains largely unchanged in schizophrenia [ 115 ], post-mortem evidence shows lower cortical neuropil [ 116 ], cortical dendritic spine density [ 90 , 117 , 118 , 119 , 120 ], spine plasticity markers and synaptic vesicle protein levels relative to controls (as discussed above; also see [ 89 , 121 , 122 , 123 , 124 ]). Thus, taken together, this preclinical and post-mortem evidence indicates that synaptic changes could contribute to the neurostructural alterations seen in schizophrenia, although it does not prove it.
There are limitations to interpreting structural MRI findings as indicative of synaptic alterations in schizophrenia. Neuronal and glial number and size, and vasculature, as well as synaptic elements, may contribute to the grey matter signal ([ 114 , 125 ], and as discussed in [ 126 ]). Factors such as anti-psychotic treatment and movement artefacts could also confound case–control differences [ 126 , 127 ]. Thus, alterations in non-synaptic factors may contribute to or even account for structural MRI findings in schizophrenia, and so there has been a pressing need to develop in vivo imaging measures that are specific to synapses. This is reviewed in the next section.
In vivo evidence for lower synaptic markers in schizophrenia
A major limitation of the synaptic hypothesis was the lack of evidence for synaptic alterations in patients with schizophrenia in vivo. However, investigating synaptic density in the living human brain has recently been made possible by the development of PET radioligands, such as [ 11 C]UCB-J, that are specific for synaptic vesicle glycoprotein 2A (SV2A) [ 128 ]. SV2A is ubiquitously expressed in pre-synaptic terminals, and is a marker of synaptic terminal density [ 128 ]. Non-human primate studies demonstrate a strong positive relationship between [ 11 C]UCB-J volumes of distribution and in vitro SV2A levels measured using western blots ( r > 0.8) and binding assays ( r > 0.9) [ 128 ]. Displacement studies using levetiracetam [ 128 , 129 ], a drug which binds selectively to SV2A [ 130 ], show the [ 11 C]UCB-J signal is largely blocked, indicating high specificity of [ 11 C]UCB-J to SV2A. This evidence indicates that [ 11 C]UCB-J is a specific marker of SV2A levels. SV2A, one of three isoforms of SV2, is expressed throughout the brain and is present in GABAergic and glutamatergic pre-synaptic nerve terminals [ 131 ]. Furthermore, SV2A levels are strongly, positively correlated with synaptophysin levels in the brain ( r > 0.95) [ 128 ], which is reduced in disorders associated with synaptic loss, and is widely used as a marker of synaptic density [ 132 ]. Moreover, SV2 shows lower variability in terms of copy number per synaptic vesicle than synaptophysin [ 133 ]. [ 11 C]UCB-J PET has demonstrated sensitivity to synaptic reductions in temporal lobe epilepsy and Alzheimer’s disease [ 128 , 134 ], showing it is able to detect alterations in disorders in which loss of synaptic density is expected.
The first [ 11 C]UCB-J PET study in patients with schizophrenia found that [ 11 C]UCB-J volume of distribution was lower in patients compared to healthy volunteers in the frontal and anterior cingulate cortices with large effect sizes, and possibly lower in the hippocampus as well [ 135 ]. This study also found evidence for lower synaptic density in subcortical regions in patients with schizophrenia, in contrast to the prediction from version II of the synaptic hypothesis. These findings have since been independently replicated [ 136 ]. To our knowledge, SV2A levels have not been studied post-mortem in schizophrenia in the frontal or anterior cingulate cortices or hippocampus, although a post-mortem study found lower SV2A transcript levels in the cerebellar cortex in schizophrenia compared to controls [ 137 ]. Both PET studies were in patients with chronic illnesses who were taking anti-psychotic drugs. However, neither study found a relationship between anti-psychotic exposure and [ 11 C]UCB-J binding, and a rodent study showed anti-psychotic drug exposure had no effect on SV2A protein or SV2A radioligand binding levels, indicating anti-psychotic treatment is unlikely to explain lower SV2A levels in schizophrenia [ 135 ].
In healthy volunteers, [ 11 C]UCB-J binding and glutamate levels are directly associated in the anterior cingulate cortex and hippocampus, consistent with the high proportion of glutamatergic synapses there [ 138 ]. However, no significant relationship is seen between [ 11 C]UCB-J and glutamate measures in schizophrenia, suggesting a loss of glutamatergic synapses and/or a lower ratio of glutamatergic to GABAergic synapses in the disorder [ 138 ].
There are a number of considerations in interpreting the [ 11 C]UCB-J signal as a marker of synaptic density. As a marker of SV2A levels, changes in [ 11 C]UCB-J binding could reflect altered SV2A levels, and/or synaptic vesicle numbers and/or pre-synaptic terminal density and/or synaptic density. However, as discussed earlier, there is post-mortem evidence showing lower levels of a number of pre- and post-synaptic elements in schizophrenia. This includes lower levels of synaptophysin and other synaptic vesicle proteins [ 89 , 121 , 122 , 123 , 124 ], lower transcript levels of SV2A [ 137 ], lower cortical dendritic spine density and other post-synaptic elements, [ 90 , 117 , 118 , 120 ] and lower spine plasticity [ 139 ], in the context of unaltered neuronal numbers in schizophrenia [ 115 ]. When this post-mortem evidence is taken with the [ 11 C]UCB-J findings, the most parsimonious explanation is, thus, that lower [ 11 C]UCB-J binding reflects lower synaptic density in schizophrenia.
Version III of the synaptic hypothesis of schizophrenia: a master mechanism
The evidence from the post-mortem and PET studies discussed above provides direct evidence for lower synaptic levels, particularly in frontal regions, in schizophrenia, whilst the iPSC studies show lower synaptic marker levels, synaptic signalling deficits and elevated microglial-mediated synaptic pruning in neurons derived from patients relative to controls. On top of this, the MRI imaging data in schizophrenia show a greater loss of grey matter than seen in normal neurodevelopment and functional dysconnectivity across brain regions, both starting early in the course of the disorder. A number of brain changes could account for these structural and functional imaging alterations. However, given the preclinical data indicating that synaptic loss can account for at least a proportion of grey matter volume reductions, and taken with the PET, iPSC and post-mortem findings, synaptic loss likely contributes to these structural and functional alterations.
Based on these lines of evidence, we propose a revised synaptic hypothesis, summarised in Fig. 5 . The GWAS data link schizophrenia to risk variants involved in synapse formation (see earlier discussion), and a variant complement protein associated with increased microglial-mediated synaptic pruning, whilst the iPSC data indicate genetic risk translates into elevated microglial-mediated pruning and aberrant signalling in neurons derived from patients with schizophrenia. Schizophrenia is also associated with a number of environmental risk factors that are immune activators, such as maternal infections and obstetric complications [ 140 ], which have been shown to activate microglia so that they show an enhanced response to subsequent activation by later stressors in a process termed priming [ 141 , 142 ]. These environmental and genetic risk factors may, thus, increase the vulnerability of the individual to an excessive response to subsequent microglial activation. Exposure to psychosocial stressors, such as physical or emotional abuse, bullying or other adverse life events, also increases the risk of schizophrenia [ 3 ]. Animal studies show that stressors that recapitulate aspects of these risk factors, such as repeated exposure to dominant animals, activate microglia and lead to synaptic pruning [ 76 , 143 , 144 , 145 , 146 ]. Thus, genetic vulnerability translates into the aberrant formation of synapses and higher levels of complement proteins that tag synapses for elimination by glia, whilst environmental risk factors for schizophrenia may prime glia early in development and reactivate them later to lead to aberrant pruning of synapses by glia. Thus, we propose a multi-hit model, with both early and late risk factors converging to lead to synapse dysfunction and aberrant glial-mediated synaptic pruning. As microglia show priming, exposure to early risk factors may affect the timing of illness onset by enhancing their response to subsequent risk factors, such as psychosocial stress. Priming could thus account for findings that people exposed to early developmental environmental risk factors, such as obstetric complications, may be at increased risk of early onset of schizophrenia [ 140 ] because primed glia show an enhanced response to subsequent activation by stress.
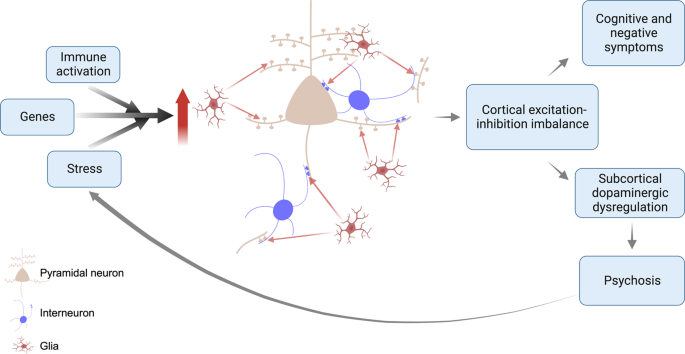
This is a multi-hit model in which genetic variants increase the vulnerability of synapses to elimination, and subsequent environmental risk factors such as stress, then induce aberrant glial-mediated pruning. Aberrant synaptic pruning leads to cortical excitation-inhibition imbalance, resulting in cognitive impairment and negative symptoms, and dysregulated projections to the striatum and midbrain. This leads to dopaminergic neuron disinhibition, and impairments in predictive learning and processing of sensory stimuli, causing psychotic symptoms. The stress of psychosis feeds back on this system to lead to further aberrant pruning.
A key question is how aberrant synaptic function and pruning could contribute to cognitive and negative symptoms. Schizophrenia is associated with lower grey matter in cortical regions, such as the frontal cortex, that play key roles in goal-directed behaviours, working memory and other processes that underlie these symptoms [ 32 ]. A balance between excitation and inhibition is critical to ensure the optimal signal-to-noise ratio in cortical neuronal arrays [ 147 ]. We propose that aberrant synaptic pruning leads to excitation-inhibition imbalance in cortical arrays and a lower signal-to-noise ratio [ 148 ]. This would impair function in cortical regions, contributing to the cognitive impairments and negative symptoms seen in schizophrenia. It could also account for neurofunctional alterations, such as findings of frontal hypometabolism [ 149 ], or fMRI studies finding altered cortical function and connectivity in schizophrenia [ 150 , 151 , 152 , 153 , 154 , 155 , 156 ]. However, it is important to note that numerous factors contribute to fMRI measures of functional connectivity, including blood flow, blood volume and cerebral metabolic rate [ 157 ], and the extent to which synaptic factors contribute to fMRI connectivity measures and cerebral glucose metabolism remains undetermined. Moreover, it remains to be established whether aberrant synaptic pruning affects particular excitatory or inhibitory synapses.
Another central question is how aberrant synaptic function and pruning could contribute to psychotic symptoms. Multiple lines of evidence indicate that overactivity in mesostriatal dopamine neurons underlies the development of psychotic symptoms by dysregulating their role in predictive learning and the assignment of confidence to the detection of sensory stimuli [ 158 ]. Preclinical evidence indicates that disrupting cortical excitation-inhibition balance, for example, using ketamine, can lead to mesostriatal dopamine overactivity [ 159 , 160 , 161 ] and result in elevated striatal dopamine synthesis capacity, as seen in patients with schizophrenia [ 161 , 162 ]. Moreover, activating inhibitory interneurons in cortical brain regions may prevent hyperdopaminergia in a sub-chronic ketamine rodent model [ 161 ]. Thus, we propose that cortical excitation-inhibition imbalance due to aberrant synaptic function and/or pruning in turn may contribute to the dysregulation of neurons that project to the striatum and midbrain to disinhibit dopaminergic neurons in these regions, impairing predictive learning and processing of sensory stimuli to lead psychotic symptoms (for review see [ 163 ]). It should also be recognised that psychosis is, itself, intensely stressful, which may feedback on the system to cause further glial-mediated synaptic pruning and, in turn, worsen symptoms; setting up a vicious cycle (Fig. 5 ). Supporting this potential effect of psychosis, there is some evidence that greater duration of untreated psychosis is associated with larger grey matter reductions, albeit this does not directly show synaptic changes and further work is needed to test this association further [ 164 , 165 ].
This model could explain why schizophrenia is rare in childhood: during this period, the net production of synapses provides a buffer against overactive pruning and synaptic dysfunction. However, the normal developmental switch to net synaptic elimination in adolescence/early adulthood makes the system much more vulnerable to overactive pruning, unmasking the vulnerability to schizophrenia, and explaining its peak onset during this period.
Version III builds on the earlier versions, and we acknowledge a great debt to the many contributors to these. It extends them by incorporating new evidence to propose a mechanism that links risk factors to synaptic changes and then to symptoms. The new hypothesis is also a multi-hit model whereby genetics increase the vulnerability of synapses to elimination, and environmental risk factors act later on this vulnerable system to cause aberrant synapse-microglia interactions, resulting in synaptic dysfunction and excess microglia-mediated pruning, contributing to symptoms in disorder. This could be considered a master mechanism, as multiple risk factors may converge to lead to synaptic dysfunction and aberrant synaptic elimination which, in turn, has the potential to underlie many other pathophysiological findings in schizophrenia, including structural and functional brain imaging findings, and dopaminergic dysfunction. The hypothesis is falsifiable by, for example, showing that synaptic alterations are not associated with the worsening of symptoms.
The evidence outlined above has implications for developing new treatments. As synaptic elimination is a dynamic process governed by the complement system and glial activity, these could be novel treatment targets to restore normal synaptic organisation by, for example, reducing the activation of microglia.
Gaps in the evidence and future directions
A key evidence gap is whether there are synaptic alterations at the first episode, or earlier in the development of schizophrenia. PET studies in clinical high risk and unmedicated, first-episode schizophrenia would be invaluable in testing this. The finding of an altered relationship between an SV2A marker and glutamate measures in patients requires replication and the development of more specific imaging probes of glutamatergic synapses to enable it to be directly tested. An evidential gap is thus the link between synaptic loss and excitation-inhibition imbalance, and whether the synaptic loss particularly affects specific synapses, such as glutamatergic synapses. This could be tested using multi-modal imaging in patients and in studies of neurons derived from patients. Furthermore, developing organoid models would permit the investigation of neurodevelopmental stages later than those captured by 2D iPSC models.
We have drawn on evidence from preclinical and clinical studies using a variety of markers related to synapses, including pre-synaptic elements, post-synaptic structures and dendritic spine densities, and considered these as indicative of synaptic levels. However, whilst changes in these could be consistent with altered synaptic levels, we cannot exclude that some could be altered in the absence of synaptic changes. This highlights the value of future studies including measures of both pre- and post-synaptic elements to confirm that differences reflect synaptic loss. A related consideration is that some lines of evidence, such as post-mortem findings of lower dendritic spine density in schizophrenia relative to controls, could be due to a failure to form synapses instead of, or in addition to, aberrant synaptic pruning. Longitudinal studies would help disambiguate these possibilities.
To our knowledge, there have not been post-mortem studies of SV2A protein levels in schizophrenia, and the study of SV2A transcript levels was in the cerebellar cortex so it remains unclear if there are SV2A protein alterations post-mortem in the regions where changes are seen in vivo [ 137 ]. Conducting post-mortem studies of SV2A would be useful to corroborate the PET findings, and to investigate the relationship between levels of SV2A and those of other pre-, as well as post-synaptic proteins. Moreover, synaptic loss alone is highly unlikely to account for all the grey matter volume changes in schizophrenia and changes in related structures, such as dendritic spine density, likely contribute as well (see [ 126 ] for discussion). Another gap in knowledge is, thus, the degree to which synaptic loss accounts for the structural and functional imaging findings in schizophrenia. Multi-modal imaging studies combining synaptic marker measures, such as [ 11 C]UCB-J, and other modalities would be invaluable in assessing this.
The hypothesis proposes that glia are activated by environmental risk factors, such as stress. There is evidence for higher levels of markers associated with an activated glial phenotype in schizophrenia relative to controls from post-mortem studies (effect size ~0.7 on meta-analysis) [ 166 ], and that greater PET signal for TSPO, a protein expressed by activated glia, is associated with greater predicted complement levels in patients [ 84 ]. However, there are inconsistencies in the TSPO PET findings in schizophrenia (reviewed in [ 167 ]). Whilst the inconsistencies could be due to methodological issues [ 167 , 168 ], inconsistencies could also be due to the timing of the scans in relation to glial activation. Supporting the latter, a mouse study found social defeat increased TSPO PET signal in cortical regions in a time-specific manner [ 169 ]. Studies using TSPO PET imaging and synaptic markers in preclinical models of schizophrenia risk factors would be useful to further test these links, whilst the development of new tools to image glia is needed to provide greater sensitivity and specificity [ 170 ].
We have highlighted a potential role of C4, and this is now supported by recent evidence that cerebrospinal fluid concentrations of C4A are elevated in two separate cohorts of people with schizophrenia, and inversely related to levels of a synaptic marker [ 171 ]. However, it is important to recognise that this is only one potential pathway and other immune pathways or alternative mechanisms may also be involved. Indeed, there may be several pathways to the abnormal synaptic formation and/or pruning and the relative importance of C4 in this is unknown. These issues need further investigation. It should also be recognised that the GWAS studies are predominantly in European samples, and a recent GWAS in a population from East Asia did not find an association between schizophrenia and the major histocompatability locus linked to C4 variants [ 53 ]. There is thus a need for studies to include more diverse populations to test generalisability. Moreover, schizophrenia shows heterogeneity in its clinical and other features [ 32 , 172 , 173 ], and may show pathophysiologically based sub-types associated with distinct clinical phenotypes, such as late onset, treatment resistance and substance dependence, and variable illness trajectories, including some that show recovery after one episode [ 174 , 175 , 176 ]. It is unlikely that anyone neurobiological hypothesis can account for all sub-types of schizophrenia. For this reason, it is a potential master mechanism, but may not be the only one. Thus, it is important to determine if the synaptic loss is seen in sub-types of schizophrenia as well as typical presentations. By the same token, studies comparing synaptic markers in patients with neurodevelopmental disorders that share some genetic and other risk factors, such as schizophrenia and bipolar disorder, would be useful to determine if they share a common underlying mechanism. Finally, the proposed relationship between synaptic alterations and striatal dopamine dysregulation remains to be tested in vivo. This aspect of the hypothesis could be falsified by showing there is no association between cortical synaptic indices and striatal dopaminergic measures.
A considerable body of new evidence for synaptic loss in patients has grown over the four decades since Feinberg first proposed the synaptic hypothesis of schizophrenia. Crucially, there is now in vivo evidence for lower synaptic terminal levels in patients, a mechanism mediated by microglia that accounts for genetic and environmental risk factors for the disorder, and new understanding on how synaptic loss could contribute to symptoms. We have revised the hypothesis to account for these new data. We call it version III, as we anticipate elements may need revision as further evidence accumulates. Notwithstanding this, it has the potential to explain a number of key aspects of the epidemiology and clinical expression of schizophrenia, including the peak age of onset and symptoms, and identifies mechanisms and new potential targets for treatment.
The views expressed are those of the authors and not necessarily those of the National Health Services, the National Institute of Health Research, or the Department of Health and Social Care of the United Kingdom.
Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141.
Article PubMed PubMed Central Google Scholar
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia – an overview. JAMA Psychiatry. 2020;77:201–10.
Article PubMed Google Scholar
Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–87.
Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37.
Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–70.
Article CAS PubMed Google Scholar
Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–7.
Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704.
Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–34.
Keshavan MS, Anderson S, Pettergrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–65.
Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–40.
Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. J Chronic Dis. 1956;3:478–86.
Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Res. 1979;163:195–205.
Yu Y, Herman P, Rothman DL, Agarwal D, Hyder F. Evaluating the gray and white matter energy budgets of human brain function. J Cereb Blood Flow Metab. 2018;38:1339–53.
Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–5.
Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–20.
Article CAS PubMed PubMed Central Google Scholar
Zecevic N, Bourgeois J-P, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50:11–32.
Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9.
CAS PubMed Google Scholar
Brown R, Colter N, Corsellis JA, Crow TJ, Frith CD, Jagoe R, et al. Postmortem evidence of structural brain changes in schizophrenia. Differences in brain weight, temporal horn area, and parahippocampal gyrus compared with affective disorder. Arch Gen Psychiatry. 1986;43:36–42.
Pakkenberg B. Post-mortem study of chronic schizophrenic brains. Br J Psychiatry. 1987;151:744–52.
Andreasen N, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, et al. Structural abnormalities in the frontal system in schizophrenia. A magnetic resonance imaging study. Arch Gen Psychiatry. 1986;43:136–44.
DeMyer MK, Gilmor RL, Hendrie HC, DeMyer WE, Augustyn GT, Jackson RK. Magnetic resonance brain images in schizophrenic and normal subjects: influence of diagnosis and education. Schizophr Bull. 1988;14:21–37.
Rubin P, Karle A, Moller-Madsen S, Hertel C, Povlsen UJ, Noring U, et al. Computerised tomography in newly diagnosed schizophrenia and schizophreniform disorder. A controlled blind study. Br J Psychiatry. 1993;163:604–12.
Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49:195–205.
Harvey I, Ron MA, Du Boulay G, Wicks D, Lewis SW, Murray RM. Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol Med. 1993;23:591–604.
Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, et al. Magnetic resonance imaging of the brain in schizophrenia. The pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry. 1990;47:35–44.
Buchsbaum MS, Haier RJ. Functional and anatomical brain imaging: impact on schizophrenia research. Schizophr Bull. 1987;13:115–32.
Buchsbaum MS. The frontal lobes, basal ganglia, and temporal lobes as sites for schizophrenia. Schizophr Bull. 1990;16:379–89.
Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, et al. Frontostriatal disorder of cerebral metabolism in never-medicated schizophrenics. Arch Gen Psychiatry. 1992;49:935–42.
Cleghorn JM, Garnett ES, Nahmias C, Firnau G, Brown GM, Kaplan R, et al. Increased frontal and reduced parietal glucose metabolism in acute untreated schizophrenia. Psychiatry Res. 1989;28:119–33.
Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:881–90.
Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992;49:921–6.
Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74:1104–11.
Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22.
Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–6.
Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 2015;25:2204–12.
Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37:3402–12.
Mills KL, Goddings AL, Herting MM, Meuwese R, Blakemore SJ, Crone EA, et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–81.
Norbom LB, Ferschmann L, Parker N, Agartz I, Andreassen OA, Paus T, et al. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: integrating macro- and microstructural MRI findings. Prog Neurobiol. 2021;204:102109.
Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol. 2011;95:275–300.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78.
Yilmaz M, Yalcin E, Presumey J, Aw E, Ma M, Whelan CW, et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci. 2021;24:214–24.
Druart M, Nosten-Bertrand M, Poll S, Crux S, Nebeling F, Delhaye C, et al. Elevated expression of complement C4 in the mouse prefrontal cortex causes schizophrenia-associated phenotypes. Mol Psychiatry. 2021;26:3489–501.
Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol. 2015;7:a020370.
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400.
Caroni P, Chowdhury A, Lahr M. Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci. 2014;37:604–14.
Stein IS, Zito K. Dendritic spine elimination: molecular mechanisms and implications. Neuroscientist. 2019;25:27–47.
Uesaka N, Kano M. Presynaptic mechanisms mediating retrograde semaphorin signals for climbing fiber synapse elimination during postnatal cerebellar development. Cerebellum. 2018;17:17–22.
Trubetskoy V, Pardinas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52.
Article PubMed Central Google Scholar
Kim M, Haney JR, Zhang P, Hernandez LM, Wang LK, Perez-Cano L, et al. Brain gene co-expression networks link complement signaling with convergent synaptic pathology in schizophrenia. Nat Neurosci. 2021;24:799–809.
Lam M, Chen CY, Li Z, Martin AR, Bryois J, Ma X, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51:1670–8.
Abdellaoui A, Dolan CV, Verweij KJH, Nivard MG. Gene-environment correlations across geographic regions affect genome-wide association studies. Nat Genet. 2022;54:1345–54.
Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469–82.e11.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–78.
Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–95.
Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–60.
Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–10.
Davenport EC, Szulc BR, Drew J, Taylor J, Morgan T, Higgs NF, et al. Autism and schizophrenia-associated CYFIP1 regulates the balance of synaptic excitation and inhibition. Cell Rep. 2019;26:2037–51.e6.
De Rubeis S, Pasciuto E, Li KW, Fernandez E, Di Marino D, Buzzi A, et al. CYFIP1 coordinates mRNA translation and cytoskeleton remodeling to ensure proper dendritic spine formation. Neuron. 2013;79:1169–82.
Papaleo F, Yang F, Paterson C, Palumbo S, Carr GV, Wang Y, et al. Behavioral, neurophysiological, and synaptic impairment in a transgenic neuregulin1 (NRG1-IV) murine schizophrenia model. J Neurosci. 2016;36:4859–75.
Jia JM, Hu Z, Nordman J, Li Z. The schizophrenia susceptibility gene dysbindin regulates dendritic spine dynamics. J Neurosci. 2014;34:13725–36.
Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, et al. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci USA. 2001;98:3477–82.
Jiang DY, Wu Z, Forsyth CT, Hu Y, Yee SP, Chen G. GABAergic deficits and schizophrenia-like behaviors in a mouse model carrying patient-derived neuroligin-2 R215H mutation. Mol Brain. 2018;11:31.
Li Z, Chen J, Yu H, He L, Xu Y, Zhang D, et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat Genet. 2017;49:1576–83.
Ikeda M, Takahashi A, Kamatani Y, Momozawa Y, Saito T, Kondo K, et al. Genome-wide association study detected novel susceptibility genes for schizophrenia and shared trans-populations/diseases genetic effect. Schizophr Bull. 2019;45:824–34.
Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–57.
Kępińska AP, Iyegbe CO, Vernon AC, Yolken R, Murray RM, Pollak TA. Schizophrenia and influenza at the centenary of the 1918-1919 Spanish Influenza pandemic: mechanisms of psychosis risk. Front Psychiatry. 2020;11:72.
Mirabella F, Desiato G, Mancinelli S, Fossati G, Rasile M, Morini R, et al. Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity. 2021;54:2611–31.e8.
Soumiya H, Fukumitsu H, Furukawa S. Prenatal immune challenge compromises development of upper-layer but not deeper-layer neurons of the mouse cerebral cortex. J Neurosci Res. 2011;89:1342–50.
Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34.
Silva-Gómez AB, Rojas DX, Juárez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–36.
Comery TA, Shah R, Greenough WT. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiol Learn Mem. 1995;63:217–9.
Colyn L, Venzala E, Marco S, Perez-Otano I, Tordera RM. Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav Brain Res. 2019;373:112079.
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8.
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4.
Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6.
Gilabert-Juan J, Bueno-Fernandez C, Castillo-Gomez E, Nacher J. Reduced interneuronal dendritic arborization in CA1 but not in CA3 region of mice subjected to chronic mild stress. Brain Behav. 2017;7:e00534.
Cieslik M, Gassowska-Dobrowolska M, Jesko H, Czapski GA, Wilkaniec A, Zawadzka A, et al. Maternal immune activation induces neuroinflammation and cortical synaptic deficits in the adolescent rat offspring. Int J Mol Sci. 2020;21:4097.
Li WY, Chang YC, Lee LJ, Lee LJ. Prenatal infection affects the neuronal architecture and cognitive function in adult mice. Dev Neurosci. 2014;36:359–70.
Kaul D, Smith CC, Stevens J, Frohlich AS, Binder EB, Mechawar N, et al. Severe childhood and adulthood stress associates with neocortical layer-specific reductions of mature spines in psychiatric disorders. Neurobiol Stress. 2020;13:100270.
Da Silva T, Guma E, Hafizi S, Koppel A, Rusjan P, Kennedy JL, et al. Genetically predicted brain C4A expression is associated with TSPO and hippocampal morphology. Biol Psychiatry. 2021;90:652–60.
O’Connell KS, Sonderby IE, Frei O, van der Meer D, Athanasiu L, Smeland OB, et al. Association between complement component 4A expression, cognitive performance and brain imaging measures in UK Biobank. Psychol Med. 2021;52:1–11.
PubMed Google Scholar
Stanley JA, Pettegrew JW, Keshavan MS. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings–part I. Biol Psychiatry. 2000;48:357–68.
Prasad KM, Chowdari KV, D’Aiuto LA, Iyengar S, Stanley JA, Nimgaonkar VL. Neuropil contraction in relation to Complement C4 gene copy numbers in independent cohorts of adolescent-onset and young adult-onset schizophrenia patients-a pilot study. Transl Psychiatry. 2018;8:134.
Donohoe G, Holland J, Mothersill D, McCarthy-Jones S, Cosgrove D, Harold D, et al. Genetically predicted complement component 4A expression: effects on memory function and middle temporal lobe activation. Psychol Med. 2018;48:1608–15.
Osimo EF, Beck K, Reis Marques T, Howes OD. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol Psychiatry. 2019;24:549–61.
Berdenis van Berlekom A, Muflihah CH, Snijders G, MacGillavry HD, Middeldorp J, Hol EM, et al. Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46:374–86.
Roberts RC, Barksdale KA, Roche JK, Lahti AC. Decreased synaptic and mitochondrial density in the postmortem anterior cingulate cortex in schizophrenia. Schizophr Res. 2015;168:543–53.
Aganova EA, Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol. 1992;22:59–65.
Kung L, Conley R, Chute DJ, Smialek J, Roberts RC. Synaptic changes in the striatum of schizophrenic cases: a controlled postmortem ultrastructural study. Synapse. 1998;28:125–39.
Mabry SJ, McCollum LA, Farmer CB, Bloom ES, Roberts RC. Evidence for altered excitatory and inhibitory tone in the post-mortem substantia nigra in schizophrenia. World J Biol Psychiatry. 2020;21:339–56.
McCullumsmith RE, Hammond JH, Shan D, Meador-Woodruff JH. Postmortem brain: an underutilized substrate for studying severe mental illness. Neuropsychopharmacology. 2014;39:65–87.
Wang M, Zhang L, Gage FH. Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell. 2020;11:45–59.
Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–85.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83.
Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173:44–52.
Noh H, Shao Z, Coyle JT, Chung S. Modeling schizophrenia pathogenesis using patient-derived induced pluripotent stem cells (iPSCs). Biochim Biophys Acta Mol Basis Dis. 2017;1863:2382–7.
Shen X, Yeung HT, Lai KO. Application of human-induced pluripotent stem cells (hiPSCs) to study synaptopathy of neurodevelopmental disorders. Dev Neurobiol. 2019;79:20–35.
Kuo SS, Pogue-Geile MF. Variation in fourteen brain structure volumes in schizophrenia: a comprehensive meta-analysis of 246 studies. Neurosci Biobehav Rev. 2019;98:85–94.
Enigma Clinical High Risk for Psychosis Working Group, Jalbrzikowski M, Hayes RA, Wood SJ, Nordholm D, Zhou JH, et al. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an ENIGMA Working Group mega-analysis. JAMA Psychiatry. 2021;78:753–66.
Article Google Scholar
Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–57.
Zhao Y, Zhang Q, Shah C, Li Q, Sweeney JA, Li F, et al. Cortical thickness abnormalities at different stages of the illness course in schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:560–70.
Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2017;174:286–95.
Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190.
Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 2015;78:403–12.
Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–30.
Padmanabhan JL, Tandon N, Haller CS, Mathew IT, Eack SM, Clementz BA, et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 2015;41:154–62.
Lui S, Deng W, Huang XQ, Jiang LJ, Ma XH, Chen HF, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205.
Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–66.
Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47:645–61.
Keifer OP Jr., Hurt RC, Gutman DA, Keilholz SD, Gourley SL, Ressler KJ. Voxel-based morphometry predicts shifts in dendritic spine density and morphology with auditory fear conditioning. Nat Commun. 2015;6:7582.
Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. image 5
Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25.
Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–53.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73.
Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107.
MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, et al. Selective loss of smaller spines in schizophrenia. Am J Psychiatry. 2017;174:586–94.
Davidsson P, Gottfries J, Bogdanovic N, Ekman R, Karlsson I, Gottfries C-G, et al. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophr Res. 1999;40:23–9.
Matosin N, Fernandez-Enright F, Lum JS, Engel M, Andrews JL, Gassen NC, et al. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2016;2:16022.
Eastwood SL, Harrison PJ. Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neuroscience. 1995;69:339–43.
Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:660–9.
May A, Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr Opin Neurol. 2006;19:407–11.
Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2023;48:151–67.
Weinberger DR, Radulescu E. Structural magnetic resonance imaging all over again. JAMA Psychiatry. 2021;78:11–2.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96.
Nabulsi NB, Mercier J, Holden D, Carre S, Najafzadeh S, Vandergeten MC, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–84.
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–6.
Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–35.
Looney MR, Dohan FC Jr, Davies KG, Seidenberg M, Hermann BP, Schweitzer JB. Synaptophysin immunoreactivity in temporal lobe epilepsy-associated hippocampal sclerosis. Acta Neuropathol. 1999;98:179–85.
Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci. 2011;31:1461–70.
Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 2018;75:1215–24.
Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel M-C, Wells L, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020;11:246.
Radhakrishnan R, Skosnik PD, Ranganathan M, Naganawa M, Toyonaga T, Finnema S, et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol Psychiatry. 2021;26:7690–8.
Mudge J, Miller NA, Khrebtukova I, Lindquist IE, May GD, Huntley JJ, et al. Genomic convergence analysis of schizophrenia: mRNA sequencing reveals altered synaptic vesicular transport in post-mortem cerebellum. PLoS One. 2008;3:e3625.
Onwordi EC, Whitehurst T, Mansur A, Statton B, Berry A, Quinlan M, et al. The relationship between synaptic density marker SV2A, glutamate and N-acetyl aspartate levels in healthy volunteers and schizophrenia: a multimodal PET and magnetic resonance spectroscopy brain imaging study. Transl Psychiatry. 2021;11:393.
Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neurosci Lett. 2015;601:46–53.
Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lonnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157:801–7.
Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7:e1024.
Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–9.
Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–68.
Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22:1442–54.
Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl). 2016;233:1637–50.
Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–505.
Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702.
Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–13.
Townsend L, Pillinger T, Selvaggi P, Veronese M, Turkheimer F, Howes O. Brain glucose metabolism in schizophrenia: a systematic review and meta-analysis of (18)FDG-PET studies in schizophrenia. Psychol Med. 2022:1–18.
Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res. 2016;176:83–94.
Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–51.
Cui LB, Liu K, Li C, Wang LX, Guo F, Tian P, et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173:13–22.
White TP, Wigton R, Joyce DW, Collier T, Fornito A, Shergill SS. Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology. 2016;41:1274–85.
Shukla DK, Chiappelli JJ, Sampath H, Kochunov P, Hare SM, Wisner K, et al. Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr Bull. 2019;45:1051–9.
O’Neill A, Mechelli A, Bhattacharyya S. Dysconnectivity of large-scale functional networks in early psychosis: a meta-analysis. Schizophr Bull. 2019;45:579–90.
Lord LD, Allen P, Expert P, Howes O, Lambiotte R, McGuire P, et al. Characterization of the anterior cingulate’s role in the at-risk mental state using graph theory. Neuroimage. 2011;56:1531–9.
Zhang K, Huang D, Shah NJ. Comparison of resting-state brain activation detected by BOLD, blood volume and blood flow. Front Hum Neurosci. 2018;12:443.
McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33.
Quiroz C, Orru M, Rea W, Ciudad-Roberts A, Yepes G, Britt JP, et al. Local control of extracellular dopamine levels in the medial nucleus accumbens by a glutamatergic projection from the infralimbic cortex. J Neurosci. 2016;36:851–9.
Kim IH, Rossi MA, Aryal DK, Racz B, Kim N, Uezu A, et al. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat Neurosci. 2015;18:883–91.
Kokkinou M, Irvine EE, Bonsall DR, Natesan S, Wells LA, Smith M, et al. Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: a translational imaging study with ketamine. Mol Psychiatry. 2021;26:2562–76.
D’Ambrosio E, Jauhar S, Kim S, Veronese M, Rogdaki M, Pepper F, et al. The relationship between grey matter volume and striatal dopamine function in psychosis: a multimodal (18)F-DOPA PET and voxel-based morphometry study. Mol Psychiatry. 2021;26:1332–45.
Howes OD, Hird EJ, Adams RA, Corlett PR, McGuire P. Aberrant salience, information processing, and dopaminergic signaling in people at clinical high risk for psychosis. Biol Psychiatry. 2020;88:304–14.
Cahn W, Rais M, Stigter FP, van Haren NE, Caspers E, Hulshoff Pol HE, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–51.
Howes OD, Whitehurst T, Shatalina E, Townsend L, Onwordi EC, Mak TLA, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20:75–95.
van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075.
Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med. 2019;49:2186–96.
Plaven-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, et al. Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: a meta-analysis using individual participant data. Biol Psychiatry. 2018;84:433–42.
Kopschina Feltes P, de Vries EF, Juarez-Orozco LE, Kurtys E, Dierckx RA, Moriguchi-Jeckel CM, et al. Repeated social defeat induces transient glial activation and brain hypometabolism: a positron emission tomography imaging study. J Cereb Blood Flow Metab. 2019;39:439–53.
McCluskey SP, Plisson C, Rabiner EA, Howes O. Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur J Nucl Med Mol Imaging. 2020;47:451–89.
Gracias J, Orhan F, Horbeck E, Holmen-Larsson J, Khanlarkani N, Malwade S, et al. Cerebrospinal fluid concentration of complement component 4A is increased in first episode schizophrenia. Nat Commun. 2022;13:6427.
Wolfers T, Doan NT, Kaufmann T, Alnaes D, Moberget T, Agartz I, et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75:1146–55.
Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer JP, Marder S, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80:18com12123.
Jauhar S, Veronese M, Nour MM, Rogdaki M, Hathway P, Turkheimer FE, et al. Determinants of treatment response in first-episode psychosis: an (18)F-DOPA PET study. Mol Psychiatry. 2019;24:1502–12.
Veronese M, Santangelo B, Jauhar S, D’Ambrosio E, Demjaha A, Salimbeni H, et al. A potential biomarker for treatment stratification in psychosis: evaluation of an [(18)F] FDOPA PET imaging approach. Neuropsychopharmacology. 2021;46:1122–32.
Jaaskelainen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39:1296–306.
Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5.
Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–8.
Shao Z, Noh H, Bin Kim W, Ni P, Nguyen C, Cote SE, et al. Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia. Nat Neurosci. 2019;22:229–42.
Kathuria A, Lopez-Lengowski K, Watmuff B, McPhie D, Cohen BM, Karmacharya R. Synaptic deficits in iPSC-derived cortical interneurons in schizophrenia are mediated by NLGN2 and rescued by N-acetylcysteine. Transl Psychiatry. 2019;9:321.
Grunwald LM, Stock R, Haag K, Buckenmaier S, Eberle MC, Wildgruber D, et al. Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with schizophrenia and autism. Transl Psychiatry. 2019;9:179.
Download references
Acknowledgements
For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. Figures were created with BioRender.com and Inkscape.
This study was funded by Medical Research Council-UK (no. MC_U120097115), Maudsley Charity (no. 666), and Wellcome Trust (no. 094849/Z/10/Z) grants to ODH and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. ECO acknowledges funding from NIHR. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Author information
Authors and affiliations.
Faculty of Medicine, Institute of Clinical Sciences (ICS), Imperial College London, London, W12 0NN, UK
Oliver D. Howes & Ellis Chika Onwordi
Psychiatric Imaging Group, Medical Research Council, London Institute of Medical Sciences, Hammersmith Hospital, London, W12 0NN, UK
Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, SE5 8AF, UK
Centre for Psychiatry and Mental Health, Wolfson Institute of Population Health, Queen Mary University of London, London, E1 2AB, UK
Ellis Chika Onwordi
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation, formal analysis, methodology, project administration, resources, supervision, validation and writing—review and editing: ODH and ECO. Funding acquisition: ODH and ECO. Data curation, investigation, software, visualisation and writing—original draft: ECO.
Corresponding authors
Correspondence to Oliver D. Howes or Ellis Chika Onwordi .
Ethics declarations
Competing interests.
ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Angelini, Autifony, Biogen, Boehringer Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Janssen, Lundbeck, Neurocrine, Otsuka, Sunovion, Recordati, Roche and Viatris/Mylan and was a part time employee of H Lundbeck A/s. ODH has a patent for the use of dopaminergic imaging. No other conflicts of interest were declared.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Howes, O.D., Onwordi, E.C. The synaptic hypothesis of schizophrenia version III: a master mechanism. Mol Psychiatry 28 , 1843–1856 (2023). https://doi.org/10.1038/s41380-023-02043-w
Download citation
Received : 30 June 2022
Revised : 16 March 2023
Accepted : 20 March 2023
Published : 11 April 2023
Issue Date : May 2023
DOI : https://doi.org/10.1038/s41380-023-02043-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Schizophrenia: from neurochemistry to circuits, symptoms and treatments.
- Oliver D. Howes
- Bernard R. Bukala
- Katherine Beck
Nature Reviews Neurology (2024)
Cortical gene expression architecture links healthy neurodevelopment to the imaging, transcriptomics and genetics of autism and schizophrenia
- Richard Dear
- Konrad Wagstyl
- Petra E. Vértes
Nature Neuroscience (2024)
Parkinson’s disease and schizophrenia interactomes contain temporally distinct gene clusters underlying comorbid mechanisms and unique disease processes
- Kalyani B. Karunakaran
- Sanjeev Jain
- Madhavi K. Ganapathiraju
Schizophrenia (2024)
Association study of the complement component C4 gene and suicide risk in schizophrenia
- Mahbod Ebrahimi
- Kowsar Teymouri
- James L. Kennedy
Psychosis and autism spectrum disorder: a special issue of Molecular Psychiatry
- Julio Licinio
Molecular Psychiatry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Search Menu
- Advance articles
- Editor's Choice
- Supplements
- Submission Site
- Author Guidelines
- Open Access
- About Schizophrenia Bulletin
- About the University of Maryland School of Medicine
- About the Maryland Psychiatric Research Center
- About the NIH Public Access Policy
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, the dopamine hypothesis: version i, the dopamine hypothesis: version ii, new evidence and the rationale for version iii, advances in neurochemical imaging of schizophrenia, advances in understanding the genetic etiology of schizophrenia, environmental risk factors for schizophrenia, multiple routes to dopamine dysfunction: interacting environmental and genetic factors, findings from the prodrome and “extended phenotype” of schizophrenia, schizophrenia or psychosis, specificity of presynaptic striatal dopamine elevation to schizophrenia or psychosis, linking dopamine abnormalities to clinical expression of schizophrenia, the dopamine hypothesis of schizophrenia: version iii, implications of the dopamine hypothesis of schizophrenia: version iii, what about the other dimensions of schizophrenia in version iii, what would lead to a rejection of the hypothesis, conclusions.
- < Previous
The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway
- Article contents
- Figures & tables
- Supplementary Data
Oliver D. Howes, Shitij Kapur, The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway, Schizophrenia Bulletin , Volume 35, Issue 3, May 2009, Pages 549–562, https://doi.org/10.1093/schbul/sbp006
- Permissions Icon Permissions
The dopamine hypothesis of schizophrenia has been one of the most enduring ideas in psychiatry. Initially, the emphasis was on a role of hyperdopaminergia in the etiology of schizophrenia (version I), but it was subsequently reconceptualized to specify subcortical hyperdopaminergia with prefrontal hypodopaminergia (version II). However, these hypotheses focused too narrowly on dopamine itself, conflated psychosis and schizophrenia, and predated advances in the genetics, molecular biology, and imaging research in schizophrenia. Since version II, there have been over 6700 articles about dopamine and schizophrenia. We selectively review these data to provide an overview of the 5 critical streams of new evidence: neurochemical imaging studies, genetic evidence, findings on environmental risk factors, research into the extended phenotype, and animal studies. We synthesize this evidence into a new dopamine hypothesis of schizophrenia—version III: the final common pathway. This hypothesis seeks to be comprehensive in providing a framework that links risk factors, including pregnancy and obstetric complications, stress and trauma, drug use, and genes, to increased presynaptic striatal dopaminergic function. It explains how a complex array of pathological, positron emission tomography, magnetic resonance imaging, and other findings, such as frontotemporal structural and functional abnormalities and cognitive impairments, may converge neurochemically to cause psychosis through aberrant salience and lead to a diagnosis of schizophrenia. The hypothesis has one major implication for treatment approaches. Current treatments are acting downstream of the critical neurotransmitter abnormality. Future drug development and research into etiopathogenesis should focus on identifying and manipulating the upstream factors that converge on the dopaminergic funnel point.
The hypothesis that dopamine and dopaminergic mechanisms are central to schizophrenia, and particularly psychosis, has been one of the most enduring ideas about the illness. Despite a relatively inauspicious start—dopamine was initially thought to be a precursor molecule of little functional significance—the idea has evolved and accommodated new evidence to provide an increasingly sophisticated account of the involvement of dopamine in schizophrenia. This review summarizes the evolution of the dopamine hypothesis, which we characterize as having 2 main prior incarnations (version I, the original incarnation, and version II, which was articulated in 1991 and has been the guiding framework since). The main effort in this article is to synthesize the evidence since version II and articulate what we call “The Dopamine Hypothesis of Schizophrenia: Version III,” which represents the most parsimonious account of the current state of knowledge. We call it version III—because we expect it to be revised. However, we highlight features of version III that we believe are sufficiently well established that they are likely to be constant in future revisions, as well as aspects that are still in evolution. Finally, we review the explanatory power of the hypothesis—indicating the known aspects of schizophrenia that it can and cannot explain.
The first version of the dopamine hypothesis could be entitled the dopamine receptor hypothesis. It emerged from the discovery of antipsychotic drugs 1 and the seminal work of Carlsson and Lindqvit who identified that these drugs increased the metabolism of dopamine when administered to animals. 2 Further evidence came from observations that reserpine, which is effective for treating psychosis, was found to block the reuptake of dopamine and other monoamines, leading to their dissipation. 3 Studies showing that amphetamine, which increases synaptic monoamine levels, can induce psychotic symptoms (reviewed in Lieberman et al 4 ) provided additional evidence. It was not until the 1970s, however, that the dopamine hypothesis was finally crystallized with the finding that the clinical effectiveness of antipsychotic drugs was directly related to their affinity for dopamine receptors. 5–7 The focus at the time was on excess transmission at dopamine receptors and blockade of these receptors to treat the psychosis (eg, Matthysse 8 and Snyder 9 ). While version I accounted for the data available then, it was seen as a hypothesis of schizophrenia as a whole without a clear articulation of its relationship to any particular dimension (eg, positive vs negative symptoms) and no link was made to genetics and neurodevelopmental deficits (understandably as little was then known about them), and there was little clear indication of where the abnormality was in the living brain—this would require the later application of in vivo imaging techniques. Additionally, dopamine was thought of in isolation, with little consideration of how it might relate to known risk factors for schizophrenia, and finally there was no framework for linking the dopaminergic abnormality to the expression of symptoms.
In 1991, Davis et al 10 published a landmark article describing what they called “a modified dopamine hypothesis of schizophrenia” that reconceptualized the dopamine hypothesis in the light of the findings available at the time. The main advance was the addition of regional specificity into the hypothesis to account for the available postmortem and metabolite findings, imaging data, and new insights from animal studies into cortical-subcortical interactions. It was clear by this stage that dopamine metabolites were not universally elevated in the cerebrospinal fluid (CSF) or serum of patients with schizophrenia. Also the focus on D2 receptors was brought into question by findings showing that clozapine had superior efficacy for patients who were refractory to other antipsychotic drugs despite having rather low affinity for and occupancy at D2 receptors. Furthermore, the postmortem studies of D2 receptors in schizophrenia could not exclude the confounds of previous antipsychotic treatment, and the early positron emission tomography (PET) studies of D2/3 receptors in drug-naive patients showed conflicting results.
Taken together, these findings were incompatible with the simple excess dopaminergic neurotransmission proposal of version I. Furthermore, there was the paradox that dopamine metabolite measures were reduced in some patients with schizophrenia while still correlating with symptom severity and response to antipsychotic drugs. Davis et al 10 drew on these inconsistencies and the emerging evidence that dopamine receptors show different brain distributions—characterized as D1 predominantly cortical and D2 predominantly subcortical—to provide a basis for suggesting that the effects of abnormalities in dopamine function could vary by brain region. However, it was PET studies showing reduced cerebral blood flow in frontal cortex that provided the best evidence of regional brain dysfunction in schizophrenia. “Hypofrontality” in these studies was directly correlated with low CSF dopamine metabolite levels. Because CSF dopamine metabolite levels reflect cortical dopamine metabolism, they argued that the relationship between hypofrontality and low CSF dopamine metabolite levels indicates low frontal dopamine levels. Thus, the major innovation in version II was the move from a one-sided dopamine hypothesis explaining all facets of schizophrenia to a regionally specific prefrontal hypodopaminergia and a subcortical hyperdopaminergia. While the evidence for this in humans was indirect, animal studies provided direct evidence of a link between hypo- and hyperdopaminergia. Lesions of dopamine neurons in the prefrontal cortex result in increased levels of dopamine and its metabolites and D2 receptor density in the striatum, 11 while the application of dopamine agonists to prefrontal areas reduced dopamine metabolite levels in the striatum. 12 This provided a mechanism to propose that schizophrenia is characterized by frontal hypodopaminergia resulting in striatal hyperdopaminergia. Furthermore, Davis et al 10 hypothesized that negative symptoms of schizophrenia resulted from frontal hypodopaminergia, based on the similarities between the behavior exhibited by animals and humans with frontal lobe lesions and the negative symptoms of schizophrenia. Positive symptoms were hypothesized to result from striatal hyperdopaminergia, based on the findings that higher dopamine metabolite levels are related to greater positive symptoms and response to antipsychotic drug treatment.
Although a substantial advance, there are a number of weaknesses in “version II” of the dopamine hypothesis, many of which the authors acknowledged at the time. Much of the evidence for the hypothesis relied on inferences from animal studies or other clinical conditions. There was no direct evidence for low dopamine levels in the frontal cortex and limited direct evidence for elevated striatal dopaminergic function. It was unclear how the dopaminergic abnormalities were linked to the clinical phenomena—there was no framework describing how striatal hyperdopaminergia translates into delusions or how frontal hypodopaminergia results into blunted affect, for example. Furthermore, it has subsequently become clear that the cortical abnormalities are more complicated that just the hypofrontality proposed at that time (eg, see reviews by Davidson and Heinrichs 13 and McGuire et al 14 ) and little clear evidence of frontal hypodopaminergia in schizophrenia has emerged (see below). But, more importantly, version II predated the studies into the neurodevelopment and prodromal aspects of schizophrenia, did not describe the etiological origins of the dopaminergic abnormality, and, beyond specifying “hyperdopaminergia” or “hypodopaminergia,” did not pinpoint which element of dopaminergic transmission was abnormal.
Much has changed since version II. There have been more than 6700 articles and 181 000 citations to the topic of “dopamine and schizophrenia” since 1991. It is not possible to provide a comprehensive review of all the new findings since then, much less try to weave them into a coherent hypothesis. So, the focus of our effort is to identify the 5 most critical streams of new evidence, briefly summarize what we see as the key findings from these, and use them to develop the most parsimonious understanding of the role of dopamine in schizophrenia—version III.
Presynaptic Dopamine Function and Synaptic Dopamine
Although it is not possible to measure dopamine levels directly in humans, techniques have been developed that provide indirect indices of dopamine synthesis and release and putative synaptic dopamine levels. Presynaptic striatal dopaminergic function can be measured using radiolabelled L -dopa, which is converted to dopamine and trapped in striatal dopamine nerve terminals ready for release. This provides an index of the synthesis and storage of dopamine in the presynaptic terminals of striatal dopaminergic neurons (see review by Moore et al 15 ). Seven out of 9 studies in patients with schizophrenia using this technique have reported elevated presynaptic striatal dopamine synthesis capacity in schizophrenia, 16–22 with effect sizes in these studies ranging from 0.63 to 1.89. 23 The other 2 studies, both in chronic patients, reported either a small but not significant elevation 24 or a small reduction in levels. 25 All the studies that investigated patients who were acutely psychotic at the time of PET scanning found elevated presynaptic striatal dopamine availability, 18–21 with effect sizes from 0.63 to 1.25. 23 This, then, is the single most widely replicated brain dopaminergic abnormality in schizophrenia, and the evidence indicates the effect size is moderate to large.
The next step in dopamine transmission is the release of dopamine. Striatal synaptic dopamine release can be assessed following a challenge that releases dopamine from the neuron using PET and single photon emission computerized tomography (SPECT). The released dopamine competes with the radioligand and leads to a reduction in radiotracer binding and is considered to be an indirect index of released dopamine. 26 , 27 All the studies using this approach have found evidence of roughly doubled radiotracer displacement in patients with schizophrenia compared with controls—an elevation that is again equivalent to a moderate to large effect size. 28–32 Finally, if dopamine synthesis is increased and is more sensitive to release in the face of challenges, one would expect heightened levels of endogenous synaptic dopamine when patients are psychotic. Evidence in line with this comes from a SPECT study using a dopamine depletion technique that found that baseline occupancy of D2 receptors by dopamine is also increased in schizophrenia. 33
Dopamine Receptors
PET and SPECT studies have used various radiotracers to image dopamine D2/3 receptors in schizophrenia. As Davis et al 10 noted, the findings of the initial studies were inconsistent, with some reporting increased D2/3 receptor binding in schizophrenia 34–36 and others no difference from controls. 37 , 38 There have now been at least 19 studies investigating striatal D2/3 receptors in patients with schizophrenia and 3 meta-analyses. 30 , 39 , 40 These meta-analyses conclude that there is at most a modest (10%–20%) elevation in striatal D2/3 receptor density in schizophrenia independent of the effects of antipsychotic drugs. This appears to be specific to D2/3 receptors—striatal D1 receptor densities are unaltered, 30 , 39 , 41 , 42 and this elevation may be regionally specific because these increases are not seen in the extrastriatal regions. If anything, there is a decrease in D2/D3 receptors in extrastriatal areas such as the thalamus and anterior cingulate. 43–46 The D2 receptor exists in 2 states, and it remains to be determined if the balance between these 2 states is altered in schizophrenia. 47 Also, because the current tracers bind to a mix of D2 and D3 receptors, it is difficult to be precise whether changes are in the D3 or the D2 subtype of the receptors—though preliminary data with a recently developed tracer, [ 11 C]-(+)-4-propyl-9-hydroxynaphthoxazine, show that there is no abnormality in high states or in D3 receptors in schizophrenia. 48
Dopaminergic transmission in the prefrontal cortex is mainly mediated by D1 receptors, and D1 dysfunction has been linked to cognitive impairment and negative symptom in schizophrenia (see reviews by Goldman-Rakic et al 49 and Tamminga 50 among others). Three studies have investigated D1 receptor levels in drug-free patients with schizophrenia and found associations with cognitive impairment and negative symptoms. One reported reduced D1 receptor density 41 another no difference from controls, 42 and a further study using a different radiotracer reported increased D1 levels. 51 This variation may be explained by different properties of the radiotracers: the effect of dopamine depletion on binding by the tracer used in the first 2 studies may obscure D1 receptor density elevation that is detectable by the tracer used in the last study. 52 The increased binding shown by the tracer used by Abi-Dargham and colleagues, which was directly correlated with cognitive impairment, is thus consistent with chronic low levels of dopamine in the prefrontal cortex underlying cognitive dysfunction in schizophrenia, assuming that there has been a compensatory D1 receptor density upregulation. 51 Further studies in patients are required to clarify this, particularly because both tracers may also bind to serotonin receptors. 53
Treatment and Dopamine Receptors
Over 120 neurochemical imaging studies have investigated the in vivo effects of antipsychotic treatments on dopamine receptors in schizophrenia (see, eg, review by Frankle and Laruelle 54 ). These show that at clinical doses all currently licensed antipsychotic drugs block striatal D2 receptors. Furthermore, a threshold striatal D2 blockade is required for antipsychotic efficacy, but this is not sufficient—some patients show little improvement despite high D2 occupancy. 55–57 A major stumbling block for the dopamine hypothesis used to be the notion that antipsychotic response was delayed for 2–3 weeks after the start of treatment (see review by Grace et al 58 ). However, there is now convincing evidence that there is no delayed response: the onset of antipsychotic action is early, 59 , 60 this response is related to striatal D2 receptor occupancy, 61 and D2 occupancy at as early as 48 hours predicts the nature of response that follows over the next 2 weeks. 62 Thus, the original tenet of version I still stands—dopamine D2 receptors continue to dominate and remain necessary for antipsychotic treatment and the imaging data has further strengthened the quantitative and temporal aspects of this relationship.
In summary, the molecular imaging studies show that presynaptic striatal dopaminergic function is elevated in patients with schizophrenia and correlates most closely with the symptom dimension of psychosis and blockade of this heightened transmission, either by decreasing dopamine levels or blocking dopamine transmission, leads to a resolution of symptoms for most patients.
The dopamine hypothesis ‘version II’ was published before the Human Genome Project and the huge advances in genetic research in schizophrenia. After over 1200 studies, it seems clear that no one gene “encodes” for schizophrenia. 63 Rather, in common with many other complex diseases, there are a number of genes each of small effect size associated with schizophrenia. 63 The gene database on the Schizophrenia Research Forum ( http://www.schizophreniaforum.org ) provides a systematic and regularly updated meta-analysis of genetic association studies. As of autumn 2008, 4 of the top 10 gene variants most strongly associated with schizophrenia are directly involved in dopaminergic pathways. The strongest association is with a gene variant affecting the vesicular monoamine transporter protein ( rs2270641, odds ratio 1.63). This protein acts to accumulate dopamine and other monoamines into vesicles, which fits with the PET studies that show elevated radiolabeled dopamine accumulation into striatal vesicles in schizophrenia. Additionally, other gene variants in the list of the strongest associations, such as in the genes for methylenetetrahydrofolate reductase and V-akt murine thymoma viral oncogene homolog 1, indirectly affect the dopaminergic system among other effects. 64 Many of the other gene variants in the top list are involved in brain development, such as the gene for dysbindin, or influence more ubiquitous brain transmitters such as glutamate or γ-aminobutyric acid (GABA). 63 , 64 While recent findings have breathed great interest in the copy number variations in schizophrenia—the early evidence there also suggests that they are rare, tend to be unique to families, and are unlikely to account for more than a few percent of schizophrenia. 63 , 65–67 It would be premature to try and synthesize these genes into a pathway leading to dopamine abnormality because the precise number, nature, function, and association of these genes to schizophrenia is evolving. The most parsimonious statement that can be made today is that while a number of genetic associations have been identified, none of them accounts for the majority of schizophrenia and most of them are likely to be susceptibilities. Of the ones that have been identified, some have already been tied to altered dopamine transmission. 68 However, the functional relevance of most of them to dopamine function is not known. 68 This view of schizophrenia genetics then reemphasizes a critical role for other interacting factors—particularly the environmental risk factors for schizophrenia.
A large number of disparate environmental factors clearly contribute to the risk for schizophrenia, yet many hypotheses of schizophrenia, including previous versions of the dopamine hypothesis, make no allowance for them. Markers of social adversity such as migration, unemployment, urban upbringing, lack of close friends, and childhood abuse are all associated with a well-established increased risk for schizophrenia that cannot readily be explained by genetic factors alone. 69 These factors either directly index social isolation/subordination or are linked to these experiences. 70 Studies in animals of social isolations 71–73 and subordination 73 , 74 find that these factors lead to dopaminergic overactivity.
Other environmental factors, such as pregnancy/obstetric complications, act in early life to increase the subsequent risk of schizophrenia (reviewed by Cannon et al, 75 Geddes and Lawrie, 76 and Kunugi et al 77 ). There is now substantial evidence from animal models that pre- and perinatal factors can lead to long-term overactivity in mesostriatal dopaminergic function (reviewed by Boksa and El-Khodor 78 and Boksa 79 ). For example, neonatal lesions affecting the hippocampus 80 , 81 or frontal cortex 82 increase dopamine-mediated behavioral responses in rats, as does prenatal stress, whether induced by corticosterone administration 83 or maternal handling. 84 Neonatal exposure to toxins also leads to increased dopamine-mediated behavioral responses 85 and elevated striatal dopamine release. 86 Prenatal and neonatal stress, such as maternal separation, also increases striatal dopamine metabolism 83 and release. 87 , 88 The latter findings parallel the increased presynaptic dopaminergic function found in schizophrenia.
A number of psychoactive substances also increase the risk of schizophrenia. The relationship between stimulants, psychosis, and their effects on dopaminergic function has already been considered (eg, Lieberman et al, 4 Angrist and Gershon, 89 and Yui et al 90 ). However, recent PET imaging work has shown that even a few doses of a stimulant may sensitize the striatal dopamine system and can lead to enduring increases in dopamine release to amphetamine even after many months of abstinence. 91 Since earlier versions of the dopamine hypothesis, cannabis use has emerged as a risk factor for schizophrenia. 92 , 93 The main psychoactive component of cannabis primarily acts at cannabinoid receptors, 94 and this as well as other cannabinoid agonists have been shown in animals to increase striatal dopamine release. 95 , 96 Initial findings indicate this is the case in man as well, 97 a result supported by observations that dopamine metabolite levels are increased in patients admitted during a first episode of psychosis associated with cannabis use. 98 Psychoactive drugs acting on other systems may also indirectly act on the dopaminergic system by potentiating dopamine release caused by other effects. This has been shown for the N -methyl- D -aspartic acid (NMDA) blocker ketamine, which has been found to increase amphetamine-induced dopamine release in healthy humans to the levels seen in schizophrenia. 99 These new data therefore indicate that even psychoactive drugs that do not directly act on the dopamine system can impact on dopamine release through indirect effects.
Genes and environmental factors do not exist in isolation. Many add to each other, and some show synergistic effects on the risk of schizophrenia or brain abnormalities associated with schizophrenia (see, eg, Cannon et al 100 and Nicodemus et al 101 and reviews by Mittal et al 102 and van Os et al 103 ). Furthermore, animal studies indicate that at least some of these factors interact in their effects on the dopamine system: social isolation rearing potentiates the later effects of stimulants 104 , 105 or of stress 106 on the dopamine system. 105 Similar effects have also been found in humans, where striatal dopamine release in response to stress was increased in people who reported low maternal care during their early childhood. 107 Additionally, there are interactions with other neurotransmitter systems: dopamine release is not seen under the influence of ketamine alone 108 but enhances the action of amphetamine, suggesting the effects of NMDA blockade, or by extension other putative causes of glutamatergic dysfunction, such as neonatal insults, are modulatory. GABA interneurons are also involved in the regulation of subcortical dopamine function and have been implicated in schizophrenia. 109
Interactions between gene variants, including those influencing dopaminergic function, and environmental risk factors are another possible route to dopaminergic dysfunction. This is illustrated by findings that variants of the catechol- O -methyltransferase gene (involved in dopamine catabolism) interact with early cannabis exposure to increase the subsequent risk of psychosis 110 and, in other studies, to increase stress reactivity and paranoid reactions to stress (see review by van et al 70 ). Family history of psychosis also interacts with environmental factors such as urbanicity to increase the risk of schizophrenia. 111 , 112 Additionally, genetic risk for schizophrenia appears to interact with obstetric complications: some “schizophrenia” genetic factors make the individual more susceptible to the effects of obstetric complications, such as frontal and temporal structural abnormalities (see review by Mittal et al 102 ). As reviewed above, animal studies indicate that frontal and temporal dysfunction can lead to increased striatal dopamine release and suggest that this is another route to dopamine dysregulation.
While further work is clearly needed to investigate the nature and extent of all these possible interactions, the evidence indicates that many disparate, direct and indirect environmental and genetic, factors may lead to dopamine dysfunction and that some occur independently while others interact. The striking empirical fact is this: the relative risks for developing schizophrenia that are accorded to migration (about 2.9 113 ), obstetric complications (about 2.0, see meta-analyses 75 , 76 ), and frequent cannabis or amphetamine use (2.09 for cannabis 93 and about 10 for amphetamine use 114 ) are considerably higher than those for any single gene variant. Thus, as the dopamine hypothesis evolves, the scientific challenge will be not just to find predisposing genes but to articulate how genes and environment interact to lead to dopamine dysfunction.
Another area of significant neurobiological research over recent years has focused on the early signs, or “prodrome,” of the illness and the subtler manifestations of symptoms within family members and the population at large. These groups are at increased risk of schizophrenia but have not yet developed the illness. Evidence from studying these groups therefore has the potential to provide information about the causal chain of events leading to the development of schizophrenia. Individuals meeting clinical criteria for a high risk of psychosis, eg, have an approximate 400-fold increased risk of developing of psychotic illnesses, predominantly schizophrenia, within the following few years. 115 , 116 They show elevated striatal [ 18 F]-dopa accumulation, which is positively associated with greater symptom severity and approaches the levels seen in patients with schizophrenia. 20 Elevated presynaptic striatal dopaminergic function is also seen in other groups with an increased risk of developing psychosis, such as schizotypy, 117 , 118 and the relatives of people with schizophrenia. 119 The latter also show a greater change in dopamine metabolite levels in response to a given stressor than healthy controls 120 and an association between greater change in dopamine metabolite levels with higher levels of psychotic-like symptoms following stress. 121 These dopaminergic abnormalities appear intermediate to those seen in patients with schizophrenia, 20 , 117 , 120 although this needs to be tested in adequately powered studies. Overall, these findings indicate that dopaminergic abnormalities are not just seen in people who are frankly psychotic but are also seen in people with risk factors for psychosis, who often have symptoms, albeit at a less severe level. Furthermore, stress in these individuals has been linked to both an increase in these symptoms and an increase in dopaminergic indices (see review by van et al 70 ). This suggests that the dopaminergic abnormalities might underlie “psychosis proneness” and shows how the environment might further impact on this to lead to frank psychosis.
A further development since version II of the dopamine hypothesis is the evidence regarding structural differences prior to the onset of schizophrenia. Individuals with prodromal signs also show brain structural deficits, quite like those in patients, although to a lesser degree (see review by Wood et al 122 ), as do the relatives of people with schizophrenia and people with schizotypal features 123 (see review by Dickey et al 124 ). These brain abnormalities are in frontotemporal regions—the same areas where lesions in animals result in striatal dopaminergic abnormalities. 80 , 82 , 125 There is also evidence of longitudinal brain structural changes in schizophrenia (eg, DeLisi 126 and van Haren et al 127 ) and people at risk of schizophrenia. 122 , 128 However, the contribution of factors such as medication 129 , 130 and cannabis use 131 to the longitudinal brain changes has yet to be fully resolved—as such these changes are not addressed in the proposed dopamine hypothesis: version III. It is not just brain structure that is altered in these individuals at risk of schizophrenia—there are functional differences as well that are generally in similar brain regions to those seen in schizophrenia (see reviews by Fusar-Poli et al 132 and Lawrie et al 133 ) and a similar pattern of neurocognitive impairments to those seen in schizophrenia, although again to a lesser degree (see review and subsequent studies by Brewer et al, 134 Eastvold et al, 135 and Simon et al 136 ).
Parsimoniously, one can conclude that striatal dopaminergic elevation is present in a compromised brain in schizophrenia and that the same appears true in the “extended phenotype.” Furthermore, there is some evidence that the 2 are connected in the prodrome as well as in schizophrenia: greater striatal dopaminergic elevation in “prodromal individuals” is directly associated with poorer neurocognitive function and altered activation in frontal cortical areas during the task. 20 There are also indications that there may be a gradation in the degree of dopaminergic elevation, although direct comparisons are required to substantiate this. Finally, recent studies in schizophrenia and its prodrome have begun to further localize the presynaptic dopamine elevation in the striatum to the parts functionally linked to associative cortical areas. 20 , 137
The diagnosis of schizophrenia encapsulates patients with markedly different clinical features and courses (see reviews by Dutta et al 138 and Peralta and Cuesta 139 ). Classification systems have attempted to deal with this categorically by proposing subtypes and intermediate syndromes. 138 , 139 On the other hand, factor analyses have identified a number of symptom dimensions: positive, negative, disorganized, affective, and cognitive, eg, Dutta et al 138 and Peralta and Cuesta. 139 The dominance and mix of the dimensions may fluctuate during the natural history of the illness. 138 , 139 Additionally, many patients meet Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) ( DSM-IV ) criteria for other psychiatric disorders as well. 140 Despite this variability, it remains the fact that the vast majority of patients with schizophrenia come to clinical attention due to their psychosis. However, psychosis itself is not unique to schizophrenia. About 8% of the general population also report psychotic experiences, and in some 4% or so this is associated with impairment and distress (see review by van Os et al 141 ). Thus, the distinction between clinical and subclinical psychosis may reflect interacting personal and sociocultural factors as much as it does biology. 141
The paragraph above underlines that it would be highly implausible that any one biological factor could deterministically “explain” a diagnosis of schizophrenia. A much more likely scenario is that a biological dysfunction may contribute to one of the major dimensions of the illness. The evidence certainly suggests that striatal dopamine function appears most elevated in people who are acutely psychotic whether in the context of schizophrenia or psychosis seen in another condition. The dopamine dysfunction is present even in subjects reflecting the extended phenotype—family members, people with schizotypy, and symptomatic individuals at high risk of psychosis. 20 , 117 , 119 Thus, the current evidence is consistent with dopamine hyperfunction being most closely linked to the dimension of psychosis. Insofar because psychosis is a hallmark of schizophrenia, dopamine abnormality is routinely seen in schizophrenia. However, we would predict that if nonpsychotic forms of schizophrenia were studied (and such a category is allowable under the DSM-IV ), they would not show similar dopamine abnormalities—thus dissociating psychosis from schizophrenia.
Striatal dopamine elevation is not seen in mania, depression, or other psychiatric disorders without psychosis 142–147 and not related to measures of anxiety or depression in people with psychotic symptoms. 20 , 148 Thus, it is not a nonspecific indicator of psychiatric morbidity. However, striatal dopamine elevation is seen in psychosis associated with psychosis in at least one disorder other than schizophrenia. 22 Furthermore, dopamine blockade with antipsychotic drugs does not respect diagnostic boundaries either—it is effective for psychosis related to mania, depression, or Parkinson disease 149 , 150 as well as for psychosis in schizophrenia. While further studies and direct comparisons are required, dopamine elevation appears specifically related more generally to psychosis proneness and not just to psychosis in schizophrenia.
If a neurochemical hypothesis (based on dopamine or any other neurotransmitter) is to explain a psychiatric illness defined by its clinical expression, it has to link the 2. A major shortcoming of the first 2 versions of the dopamine hypothesis was the total silence on the issues of how dopaminergic abnormalities led to the clinical expression of the disease. Since version II of the dopamine hypothesis, developments in neuroscience have provided increasing evidence of dopamine's role in motivational incentive salience. The experiments and syntheses of data by Berridge and Robinson, 151 Robbins and Everitt, 152 , 153 and Schultz and others 154–158 have implicated a distinct role for subcortical dopamine systems in incentive or motivational salience and reward prediction, respectively. These conceptualizations provided a framework to link neurochemical dysfunction to clinical expression using concepts of salience and reward. According to one such extension of the dopamine hypothesis, 159 , 160 the abnormal firing of dopamine neurons and the abnormal release of dopamine leads to an aberrant assignment of salience to innocuous stimuli. It is argued that psychotic symptoms, especially delusions and hallucinations, emerge over time as the individual's own explanation of the experience of aberrant salience. Psychosis is, therefore, aberrant salience driven by dopamine and filtered through the individual's existing cognitive and sociocultural schemas—thus allowing the same chemical (dopamine) to have different clinical manifestations in different cultures and different individuals. 159 , 160 Incentive salience models also provide a plausible explanation for negative symptoms: dopamine dysregulation may increase the noise in the system, “drowning out” dopaminergic signals linked to stimuli indicating reward, eg, Roiser et al 161 and Seamans and Yang. 162 The net result would be reduced motivational drive that would lead over time to negative symptoms, such as social withdrawal, and neglect of interests. As an explanation, this has face validity, and there is some evidence that schizophrenia is associated with reduced ventral striatal activation to reward, and greater reduction is related to higher levels of negative symptoms. 163 However, this proposal and the hypothesis linking low frontal dopamine levels to the cognitive impairments in schizophrenia both need to be tested by further in vivo studies of neurochemical function in patients.
We propose a revised “third version” of the dopamine hypothesis to account for the new evidence, drawing on the work of many previous reviews (eg, Laruelle and Abi-Dargham, 32 van et al, 70 Cannon et al, 164 and Howes et al 165 ). The hypothesis has 4 distinctive components.
Firstly, we hypothesize that multiple “hits” interact to result in dopamine dysregulation—the final common pathway to psychosis in schizophrenia. This is illustrated schematically in figure 1 . Second, the locus of dopamine dysregulation moves from being primarily at the D2 receptor level to being at the presynaptic dopaminergic control level. Third, dopamine dysregulation is linked to “psychosis” rather than schizophrenia, and perhaps in the fullness of time it will be about “psychosis proneness.” The exact diagnosis, however, reflects the nature of the hits coupled with sociocultural factors and not the dopamine dysfunction per se. And finally, the dopamine dysregulation is hypothesized to alter the appraisal of stimuli, perhaps through a process of aberrant salience.
Multiple hits interact to result in striatal dopamine dysregulation to alter the appraisal of stimuli and resulting in psychosis, whilst current antipsychotic drugs act downstream of the primary dopaminergic dysregulation.
The hypothesis that the final common pathway is presynaptic dopamine dysregulation has some important clinical implications. Firstly, it implies that current antipsychotic drugs are not treating the primary abnormality and are acting downstream. While antipsychotic drugs block the effect of inappropriate dopamine release, they may paradoxically worsen the primary abnormality by blocking presynaptic D2 autoreceptors, resulting in a compensatory increase in dopamine synthesis. There is some evidence from healthy volunteers that acute antipsychotic treatment does increase presynaptic dopamine synthesis capacity, 166 and while successful subacute treatment can reduce this, 167 it is nevertheless elevated in patients who have received antipsychotic treatment for many years. 17 This may explain why patients relapse rapidly on stopping their medication, and if the drugs may even worsen the primary abnormality, it also accounts for more severe relapse after discontinuing treatment. This suggests that drug development needs to focus on modulating presynaptic striatal dopamine function, either directly or through upstream effects.
An attractive feature of version II was that it proposed a dysfunction in the dopamine system as a complete explanation for schizophrenia: a prefrontal hypodopaminergia leading to a subcortical hyperdopaminergia. We depart from this parsimony in version III mainly because in the last 2 decades there has been little convincing evidence for this sequence of dopamine dysfunction. On the other hand, the last 2 decades have provided substantially more evidence about the multiple routes (genetic, neurodevelopmental, environmental, social) that lead to the striatal hyperdopaminergia, as discussed earlier. Furthermore, the appreciation of the dimensional nature of symptoms of schizophrenia also speaks for partial independence of the different features (cognitive, negative) from psychosis. 139 There is of course correlational evidence that striatal dopamine abnormalities are associated with poor performance on cognitive tasks 17 , 20 , 168 and suggestion that higher striatal dopamine synthesis capacity is linked to functional abnormalities in the cortical regions engaged by these tasks. 168 , 169 However, it should be noted that recent data suggest that these frontal/cognitive changes need not necessarily be primary but instead may arise as a consequence of striatal dysfunction. 170 Thus, in contrast to version II, which proposed a single pathway, we propose that changes in multiple transmitter/neural systems underlie the cognitive dysfunction and negative symptoms of schizophrenia, and in many cases these dysfunctions precede the onset of psychosis. It is when these pathways, in convergence with other biological or environmental influences, lead to striatal dopamine hyperfunction that psychosis becomes evident and the label of schizophrenia is assigned. Thus, rather than being a hypothesis of schizophrenia—version III is more accurately a “dopamine hypothesis of psychosis-in-schizophrenia.” It remains to be tested whether this is specific to psychosis of schizophrenia or is seen with psychosis in other disorders too.
Because so much is unknown, it is given that the hypothesis will be revised as more data become available. The more intriguing question is whether one can envisage evidence that would lead to a wholesale rejection of the hypothesis. The 2 central claims of version III are the primacy of the presynaptic abnormality and the claim that dopamine is the “final common pathway.” Two different kinds of evidence could lead to a complete rejection of the hypothesis. PET studies directly implicating presynaptic dopamine dysfunction are a major foundation of this new version of the hypothesis. PET data require to be modeled to provide estimates of L -dopa uptake or synaptic dopamine levels—and the results are inferred rather than direct measurements. Thus, if it turns out that the body of evidence based on PET imaging is a confound or an artifact of modeling and technical approaches, this would be a serious blow for version III, though the data behind versions I and II would still stand strong. While possible, we think this to be highly unlikely. What is perhaps more likely is that a new drug is found that treats psychosis without a direct effect on the dopamine system. In other words, the dopamine abnormalities continue unimpeded, and psychosis improves despite them. A good example of such a new drug might be LY2140023, an mGlu 2/3 agonist. 171 If this were to be an effective antipsychotic and it could be shown that the new pathways do not show any interaction with the dopamine system, then the fundamental claim of version III, that it is the final common pathway, would be demolished. A similar situation would arise if a pathophysiological mechanism that does not impact on the dopamine system is found to be universal to schizophrenia. Much more likely is the possibility that the hypothesis will be revised but with a stronger version IV. The next decade will provide more information on the role of dopamine, particularly how genetic and environmental factors combine to influence the common pathway, and better drugs will be developed that directly influence presynaptic dopaminergic function—both logical successors to the idea of a final common pathway.
A considerable body of new evidence has amassed in the last 2 decades that is not compatible with reconceptualization of Davis and colleagues of the dopamine hypothesis of schizophrenia. To account for these developments, we have elaborated the dopamine hypothesis of schizophrenia: version III—the final common pathway. This hypothesis accounts for the multiple environmental and genetic risk factors for schizophrenia and proposes that these interact to funnel through one final common pathway of presynaptic striatal hyperdopaminergia. Furthermore, it provides a framework linking the abnormal neurochemistry to symptoms and explains both why many disparate risk factors and functional and structural abnormalities are associated with schizophrenia but are not specific to schizophrenia. It provides an explanation for overlapping findings in people with risk factors for schizophrenia and explains eventual diagnosis not in neurochemical terms but as the result of individual factors interacting with the sociocultural milieu. In addition to funneling through dopamine dysregulation, the multiple environmental and genetic risk factors influence diagnosis by affecting other aspects of brain function that underlie negative and cognitive symptoms. Schizophrenia is thus dopamine dysregulation in the context of a compromised brain. It follows from this that future drug development should focus on the systems acting on the funnel points leading to the final common pathway.
Howes has received investigator-led charitable research funds or speaking engagements from AstraZeneca, Eli Lilly, and Janssen. Kapur has received grant support or has been a consultant/scientific advisor or had speaking engagements with AstraZeneca, Bristol Meyers Squibb, Eli Lilly, EMD—Darmstadt, Glaxo Smith Kline, Janssen (Johnson and Johnson), Neuromolecular Inc, Pfizer, Otsuka, Organon, Sanofi-Synthelabo, Servier, and Solvay Wyeth.
Google Scholar
- psychotic disorders
- schizophrenia
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations

- Online ISSN 1745-1701
- Print ISSN 0586-7614
- Copyright © 2024 Maryland Psychiatric Research Center and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
The Relationship Between Schizophrenia and Dopamine
Arlin Cuncic, MA, is the author of The Anxiety Workbook and founder of the website About Social Anxiety. She has a Master's degree in clinical psychology.
:max_bytes(150000):strip_icc():format(webp)/ArlinCuncic_1000-21af8749d2144aa0b0491c29319591c4.jpg)
Alla Bielikova / Getty Images
- Dopamine and Schizophrenia Symptoms
- Implications for Treatment
What Does This Mean for Patients?
- Causes of Schizophrenia
- High vs. Low Dopamine
- Implications

Serotonin and Schizophrenia
Experts do not fully understand what causes schizophrenia, but evidence suggests that dopamine abnormalities may play a role. High and low levels of dopamine in certain regions of the brain can also affect different symptoms of schizophrenia.
Schizophrenia is a debilitating mental disorder with a multitude of symptoms. These can range from disorganized speech and behavior to delusions and hallucinations. Some cases are far more disabling than others, but in most cases, people with this disorder require lifelong treatment and care.
Current research suggests that schizophrenia is a neurodevelopmental disorder with an important dopamine component. Four decades of research have focused on the role of dopamine in schizophrenia, and it seems clear that excesses or deficiencies in dopamine can lead to schizophrenic symptoms.
At a Glance
While other factors also play a role in the development of schizophrenia, dopamine imbalances have been identified as a key factor affecting symptoms. Too much dopamine in key areas of the brain results in delusions and hallucinations (positive symptoms) or cognitive deficits and reduced social/emotional activity (negative symptoms). Understanding the factors that contribute to dopamine symptoms can help doctors treat the condition more effectively.
What Is the Dopamine Hypothesis of Schizophrenia?
The dopamine hypothesis of schizophrenia was one of the first neurobiological theories for this disease.
Dopamine Hypothesis
This theory suggests that an imbalance of dopamine is responsible for schizophrenic symptoms. In other words, dopamine plays a role in controlling our sense of reality, and too much or too little can cause delusions and hallucinations.
The evidence for this theory comes from many sources, including post-mortem studies that have imbalances of dopamine as well as its metabolites in schizophrenic patients. In addition, drugs that block the receptors for dopamine can help control schizophrenic symptoms.
How Does Dopamine Cause Schizophrenic Symptoms?
There are two types of schizophrenia symptoms that an excess of dopamine may cause: positive and negative . Positive symptoms include delusions and hallucinations. Negative symptoms include a decrease in social activity, emotional range, and cognitive function.
Positive Symptoms
Positive symptoms are those that appear to come from outside the person. These can include delusions, hallucinations, or thought disorders.
Dopamine contributes to the development of positive symptoms through its effects on subtype-3A dopamine receptors (D3) of cortical neurons. The subtype-3A receptor is found in the prefrontal cortex, which controls planning, thinking, and other cortical areas.
When these receptors are activated by dopamine, they overstimulate neurons. This can lead to all three types of positive symptoms. Evidence for this idea comes from studies that show that patients with schizophrenia have significantly lower levels of the D3 receptor than healthy people.
Negative Symptoms
While positive symptoms appear to come from outside, negative symptoms appear to be internal. These include decreased social activity and emotional range, as well as cognitive deficits like poor problem-solving or memory deficit.
The mechanisms contributing to negative symptoms are linked to dopamine levels in the limbic system . Dopamine excess leads to an increase in the activity of dopamine receptors, creating overstimulation similar to that seen in positive symptoms.
Some researchers suggest that this overactivity decreases neuronal inhibition , leading to decreased social behavior and cognitive deficits.
Treatment Implications of the Dopamine Hypothesis
The dopamine hypothesis has important treatment implications. The vast majority of current antipsychotic medications target dopamine, and this makes sense, given that these drugs were discovered through serendipitous observations of their effect on schizophrenia.
The most important dopamine-affecting medications are the typical antipsychotics, which increase post-synaptic receptor stimulation by blocking dopamine receptors.
Unfortunately, these medications produce a number of debilitating side effects, most notably extrapyramidal symptoms (EPS) like tardive dyskinesia . Newer second-generation antipsychotics have fewer side effects, but none are perfect.
Treatment with dopamine agonists is a third possibility suggested by the dopamine hypothesis. Dopamine agonists stimulate post-synaptic dopamine receptors directly, and as such, they can be used to treat schizophrenia without producing EPS.
Being diagnosed with schizophrenia can be extremely hard on patients and their families. It's important that doctors and researchers continually investigate new treatments that could improve the lives of people living with this disorder.
However, it's also important to remember that schizophrenia is a complex disorder, and there are many ways the disease can manifest. Dopamine hyperactivity may not be the primary cause of schizophrenia in all patients. Furthermore, even if dopamine hyperactivity is the primary cause it still doesn't explain why some patients respond more strongly than others to the same treatment.
The best way for patients and their loved ones to navigate these issues is by staying informed and asking questions about any new or experimental treatments. They should also work with doctors to develop a personalized treatment plan that's appropriate for their own needs.
Does Too Much Dopamine Cause Schizophrenia?
Increased activity of the mesolimbic pathway is related to positive symptoms of schizophrenia (delusions, hallucinations, etc.). This means that increasing the activity of dopamine receptors in this brain system could theoretically reduce delusions and hallucinations.
A closely related idea is that by blocking post-synaptic dopamine receptors, scientists can reduce the psychotic symptoms of schizophrenia.
As mentioned previously, this is what most modern medications do: they block post-synaptic dopamine receptors in order to reduce psychotic symptoms. Unfortunately, when scientists block all available dopamine receptors they also produce a number of debilitating side effects such as extrapyramidal symptoms (EPS) and tardive dyskinesia.
Is Dopamine High or Low in Schizophrenia?
The most common theory about the cause of schizophrenia is that there are too many dopamine receptors in certain parts of the brain, specifically the mesolimbic pathway. This causes an increase in mesolimbic activity which results in delusions, hallucinations, and other psychotic symptoms.
Other research suggests that schizophrenia might be caused by a lack of dopamine activity in other parts of the brain. For example, scientists have discovered that the hippocampus is overactive in schizophrenia.
Schizophrenia might also be characterized by low dopamine in the prefrontal cortex, but again the evidence is inconclusive. Some studies have found that schizophrenics have elevated levels of dopamine in this region, while others suggest that there are too few dopamine receptors.
Implications of the Dopamine Hypothesis
It's important to note that schizophrenia is a complex disorder. Even if dopamine hyperactivity is the primary cause, certain types of schizophrenia might be characterized by increased activity in certain brain areas while others are characterized by reduced activity in certain brain areas.
Furthermore, it's also possible that different patients will respond to treatment differently based on how their disease manifests.
It's important for healthcare providers and researchers to continue investigating how schizophrenia works in the brain. This will help them develop better treatments for this complex disorder.
Research also implicates serotonin as a regulator of dopamine release. Antipsychotic medications, including olanzapine and clozapine , reduce serotonin activity and increase dopamine activity.
For example, olanzapine-induced reductions in serotonin metabolism were associated with significant improvements in negative and positive symptoms, but not cognitive deficits.
Schizophrenia is a severe mental disorder that can be treated. If you or someone you know was recently diagnosed with schizophrenia, you might be wondering what the future holds. Healthcare professionals can help you manage your symptoms and chart a course for the best possible outcome.
Sometimes, there may be periods of remission that allow you to live a productive life even when coping with schizophrenia. As new treatments are continually being developed, we can look forward to better options for people who experience this disorder in the future.
Murray RM, Lappin J, Di Forti M. Schizophrenia: from developmental deviance to dopamine dysregulation . Eur Neuropsychopharmacol . 2008;18 Suppl 3:S129-S134. doi:10.1016/j.euroneuro.2008.04.002
Brisch R, Saniotis A, Wolf R, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue [published correction appears in Front Psychiatry. 2014;5:110. Braun, Anna Katharina [corrected to Braun, Katharina]; Kumaritlake, Jaliya [corrected to Kumaratilake, Jaliya]]. Front Psychiatry . 2014;5:47. Published 2014 May 19. doi:10.3389/fpsyt.2014.00047
Purves-Tyson TD, Owens SJ, Rothmond DA, et al. Putative presynaptic dopamine dysregulation in schizophrenia is supported by molecular evidence from post-mortem human midbrain . Transl Psychiatry . 2017;7(1):e1003. Published 2017 Jan 17. doi:10.1038/tp.2016.257
Ceraso A, Lin JJ, Schneider-Thoma J, et al. Maintenance treatment with antipsychotic drugs for schizophrenia . Cochrane Database Syst Rev . 2020;8:CD008016. doi:10.1002/14651858.CD008016.pub3
Guma E, Rocchetti J, Devenyi GA, et al. Role of D3 dopamine receptors in modulating neuroanatomical changes in response to antipsychotic administration . Sci Rep . 2019;9(1):7850. doi:10.1038/s41598-019-43955-4
Maia TV, Frank MJ. An Integrative Perspective on the Role of Dopamine in Schizophrenia . Biol Psychiatry . 2017;81(1):52-66. doi:10.1016/j.biopsych.2016.05.021
Weiner I. The "two-headed" latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment . Psychopharmacology (Berl) . 2003;169(3-4):257-297. doi:10.1007/s00213-002-1313-x
Stępnicki P, Kondej M, Kaczor AA. Current concepts and treatments of schizophrenia . Molecules . 2018;23(8):2087. doi:10.3390/molecules23082087
Preda A, Shapiro BB. A safety evaluation of aripiprazole in the treatment of schizophrenia . Expert Opin Drug Saf . 2020;19(12):1529-1538. doi:10.1080/14740338.2020.1832990
Gomes FV, Zhu X, Grace AA. Stress during critical periods of development and risk for schizophrenia . Schizophr Res . 2019;213:107-113. doi:10.1016/j.schres.2019.01.030
McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment . World Psychiatry . 2020;19(1):15-33. doi:10.1002/wps.20693
Correll CU. Current Treatment Options and Emerging Agents for Schizophrenia . J Clin Psychiatry . 2020;81(3):MS19053BR3C. Published 2020 Apr 14. doi:10.4088/JCP.MS19053BR3C
Bever KA, Perry PJ. Olanzapine: a serotonin-dopamine-receptor antagonist for antipsychotic therapy . Am J Health Syst Pharm . 1998;55(10):1003-1016. doi:10.1093/ajhp/55.10.1003
By Arlin Cuncic, MA Arlin Cuncic, MA, is the author of The Anxiety Workbook and founder of the website About Social Anxiety. She has a Master's degree in clinical psychology.
Schizophrenia A-level Revisions Notes
Bruce Johnson
A-level Psychology Teacher
B.A., Educational Psychology, University of Exeter
Bruce Johnson is an A-level psychology teacher, and head of the sixth form at Caterham High School.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
On This Page:
What do the examiners look for?
- Accurate and detailed knowledge
- Clear, coherent, and focused answers
- Effective use of terminology (use the “technical terms”)
In application questions, examiners look for “effective application to the scenario” which means that you need to describe the theory and explain the scenario using the theory making the links between the two very clear. If there is more than one individual in the scenario you must mention all of the characters to get to the top band.
Difference between AS and A level answers
The descriptions follow the same criteria; however you have to use the issues and debates effectively in your answers. “Effectively” means that it needs to be clearly linked and explained in the context of the answer.
Read the model answers to get a clearer idea of what is needed.
Exam Advice
You MUST revise everything – because the exam board could choose any question, however, it does make sense to spend more time on those topics which have not appeared for a while.
With these particular questions there is a sizeable risk that people don’t understand the difference between the questions, and then write about the wrong thing.
Make sure you know which is which, for example do you understand the difference between “genetic explanation” and “neural correlates explanation”, and do you have a model essay for each?
Schizophrenia is a severe mental illness where contact with reality and insight are impaired, an example of psychosis.
Section 1: Diagnosis and Classification of Schizophrenia
Classification is the process of organising symptoms into categories based on which symptoms cluster together in sufferers. Psychologists use the DSM and ICD to diagnose a patient with schizophrenia.
Diagnosis refers to the assigning of a label of a disorder to a patient. The ICD-10 (only negative symptoms need to be present) is used worldwide and the DSM-5 (only positive symptoms need to be present) is used in America.
In order to diagnose Schizophrenia the Mental Health Profession developed the DSM (Diagnostic and Statistical Manual) still used today as a method of classifying mental disorders (particularly in the USA).
It is also used as a basis for the ICD (International Classification of Diseases) used by the World Health Organisation in classifying all disorders (mental and physical).
Note: you may come across the terms DSM-IV and ICD-10. These refer to the latest editions of the two classification systems.
Positive Symptoms
an excess or distortion of normal functions: including hallucinations and delusions.
Positive symptoms are an excess or distortion of normal functions, for example hallucinations, delusions and thought disturbances such as thought insertion.
• Hallucinations are usually auditory or visual perceptions of things that are not present. Imagined stimuli could involve any of the senses. Voices are usually heard coming from outside the person’s head giving instructions on how to behave. • Delusions are false beliefs. Usually the person has convinced him/herself that he/she is someone powerful or important, such as Jesus Christ, the Queen (e.g. Delusions of Grandeur). There are also delusions of being paranoid, worrying that people are out to get them. • Psychomotor Disturbances: Stereotypyical – Rocking backwards and forwards, twitches, & repetitive behaviors. Catatonia- staying in position for hours/days on end, cut off from the world.
Negative Symptoms
where normal functions are limited: including speech poverty and avolition.
Negative symptoms are a diminution or loss of normal functions such as psychomotor disturbances, avolition (the reduction of goal-directed behavior), disturbances of mood and thought disorders.
• Thought disorder in which there are breaks in the train of thought and the person appears to make illogical jumps from one topic to another (loose association). Words may become confused and sentences incoherent (so called ‘word salad). Broadcasting is a thought disorder whereby a person believes their thoughts are being broadcast to others, for example over the radio or through TV. Alogia – aka speech poverty – is a thought disorder were correct words are used but with little meaning. • Avolition: Lack of volition (i.e. desire): in which a person becomes totally apathetic and sits around waiting for things to happen. They engage in no self motivated behavior. Their get up and go has got up and gone!
Classification
Slater & Roth (1969) say that hallucinations are the least important of all the symptoms, as they are not exclusive to schizophrenic people.
Classification and diagnosis does have advantages as it allows doctors to communicate more effectively about a patient and use similar terminology when discussing them. In addition, they can then predict the outcome of the disorder and suggest related treatment to help the patient.
Scheff (1966) points out that diagnosis classification labels the individual, and this can have many adverse effects, such as a self-fulfilling prophecy (patients may begin to act how they are expected to act), and lower self-esteem.
Ethics – do the benefits of classification (care, treatment, safety) outweigh the costs (possible misdiagnosis, mistreatment, loss of rights and responsibility, prejudice due to labelling).
Reliability and Validity in Diagnosis and Classification of Schizophrenia
with reference to co-morbidity, culture and gender bias and symptom overlap.
Reliability
For the classification system to be reliable, differfent clinicians using the same system (e.g. DSM) should arrive at the same diagnosis for the same individual.
Reliability is the level of agreement on the diagnosis by different psychiatrists across time and cultures; stability of diagnosis over time given no change in symptoms.
Diagnosis of schizophrenia is difficult as the practitioner has no physical signs but only symptoms (what the patient reports) to make a decision on.
Jakobsen et al. (2005) tested the reliability of the ICD-10 classification system in diagnosing schizophrenia. A hundred Danish patients with a history of psychosis were assessed using operational criteria, and a concordance rate of 98% was obtained. This demonstrates the high reliability of the clinical diagnosis of schizophrenia using up-to-date classification.
Comorbidity describes people who suffer from two or more mental disorders. For example, schizophrenia and depression are often found together. This makes it more difficult to confidently diagnose schizophrenia. Comorbidity occurs because the symptoms of different disorders overlap. For example, major depression and schizophrenia both involve very low levels of motivation. This creates problems of reliability. Does the low motivation reflect depression or schizophrenia, or both?
Gender bias: Loring and Powell (1988) found that some behavior which was regarded as psychotic in males was not regarded as psychotic in females.
Validity – the extent to which schizophrenia is a unique syndrome with characteristics, signs and symptoms.
For the classification system to be valid it should be meaningful and classify a real pattern of symptoms, which result from a real underlying cause.
The validity of schizophrenia as a single disorder is questioned by many. This is a useful point to emphasise in any essay on the disorder. There is no such thing as a ‘normal’ schizophrenic exhibiting the usual symptoms.
Since their are problems with the validity of diagnois classification, unsuitable treatment may be administered, sometimes on an involuntary basis. This raises practical and ethical issues when selecting different types of tretment.
Problems of validity: Are we really testing what we think we are testing? In the USA only 20% of psychiatric patients were classed as having schizophrenia in the 1930s but this rose to 80% in the 1950s . In London the rate remained at 20%, suggesting neither group had a valid definition of schizophrenia.
Neuropsychologist Michael Foster Green suggests that neurocognitive deficits in basic functions such as memory, attention, central executive and problem solving skills may combine to have an outcome which we are labelling “Schizophrenia” as if it was the cause when in fact it is simply an umbrella term for a set of effects.
Predictive validity. If diagnosis leads to successful treatment, the diagnosis can be seen as valid. But in fact some Schizophrenics are successfully treated whereas others are not. Heather (1976) there is only a 50% chance of predicting what treatment a patient will receive based on diagnosis, suggesting that diagnosis is not valid.
Aetiological validity – for a diagnosis to be valid, all patients diagnosed as schizophrenic should have the same cause for their disorder. This is not the case with schizophrenia: The causes may be one of biological or psychological or both.
David Rosenhan (1973) famous experiment involving Pseudopatients led to 8 normal people being kept in hospital despite behaving normally. This suggests the doctors had no valid method for detecting schizophrenia. They assumed the bogus patients were schizophrenic with no real evidence. In a follow up study they rejected genuine patients whom they assumed were part of the deception.
Culture – One of the biggest controversies in relation to classification and diagnosis is to do with cultural relativism and variations in diagnosis. For example in some Asian countries people are not expected to show emotional expression, whereas in certain Arabic cultures public emotion is encouraged and understood. Without this knowledge a person displaying overt emotional behavior in a Western culture might be regarded as abnormal. Cochrane (1977) reported that the incidence of schizophrenia in the West Indies and the UK is 1 %, but that people of Afro-Caribbean origin are seven times more likely to be diagnosed as schizophrenic when living in the UK.
Cultural bias – African Americans and those of Afro-carribean descent are more likely to be diagnosed than their white counterparts but diagnostic rates in Africa and the West Indies is low – Western over diagnosis is a result of cultural norms and the diagnosis lacks validity.
Section 2: Biological Explanations for Schizophrenia
Family studies find individuals who have schizophrenia and determine whether their biological relatives are similarly affected more often than non-biological relatives.
There are two types of twins – identical (monozygotic) and fraternal (dizygotic). To form identical twins, one fertilised egg (ovum) splits and develops two babies with exactly the same genetic information.
• Gottesman (1991) found that MZ twins have a 48% risk of getting schizophrenia whereas DZ twins have a 17% risk rate. This is evidence that the higher the degree of genetic relativeness, the higher the risk of getting schizophrenia. • Benzel et al. (2007) three genes: COMT, DRD4, AKT1 – have all been associated with excess dopamine in specific D2 receptors, leading to acute episodes, positive symptoms which include delusions, hallucinations, strange attitudes. • Research by Miyakawa et al. (2003) studied DNA from human families affected by schizophrenia and found that those with the disease were more likely to have a defective version of a gene, called PPP3CC which is associated with the production of calcineurin which regulates the immune system. Also, research by Sherrington et al. (1988) has found a gene located on chromosome 5 which has been linked in a small number of extended families where they have the disorder. • Evidence suggests that the closer the biological relationship, the greater the risk of developing schizophrenia. Kendler (1985) has shown that first-degree relatives of those with schizophrenia are 18 times more at risk than the general population. Gottesman (1991) has found that schizophrenia is more common in the biological relatives of a schizophrenic, and that the closer the degree of genetic relatedness, the greater the risk.
Very important to note genetics are only partly responsible, otherwise identical twins would have 100% concordance rates.
One weakness of the genetic explanation of schizophrenia is that there are methodological problems. Family, twin and adoption studies must be considered cautiously because they are retrospective, and diagnosis may be biased by knowledge that other family members who may have been diagnosed. This suggests that there may be problems of demand characteristics.
A second weakness is the problem of nature-v-Nurture. It is very difficult to separate out the influence of nature-v-nurture. The fact that the concordance rates are not 100% means that schizophrenia cannot wholly be explained by genes and it could be that the individual has a pre-disposition to schizophrenia and simply makes the individual more at risk of developing the disorder. This suggests that the biological account cannot give a full explanation of the disorder.
A final weakness of the genetic explanation of schizophrenia is that it is biologically reductionist. The Genome Project has increased understanding of the complexity of the gene. Given that a much lower number of genes exist than anticipated, it is now recognised that genes have multiple functions and that many genes behavior.
Schizophrenia is a multi-factorial trait as it is the result of multiple genes and environmental factors. This suggests that the research into gene mapping is oversimplistic as schizophrenia is not due to a single gene.
The Dopamine Hypothesis
• Dopamine is a neurotransmitter. It is one of the chemicals in the brain which causes neurons to fire. The original dopamine hypothesis stated that schizophrenia suffered from an excessive amount of dopamine. This causes the neurons that use dopamine to fire too often and transmit too many messages. • High dopamine activity leads to acute episodes, and positive symptoms which include: delusions, hallucinations, confused thinking. • Evidence for this comes from that fact that amphetamines increase the amounts of dopamine . Large doses of amphetamine given to people with no history of psychological disorders produce behavior which is very similar to paranoid schizophrenia. Small doses given to people already suffering from schizophrenia tend to worsen their symptoms. • A second explanation developed, which suggests that it is not excessive dopamine but that fact that there are more dopamine receptors. More receptors lead to more firing and an over production of messages. Autopsies have found that there are generally a large number of dopamine receptors (Owen et al., 1987) and there was an increase in the amount of dopamine in the left amygdale (falkai et al. 1988) and increased dopamine in the caudate nucleus and putamen (Owen et al, 1978).
One criticism of the dopamine hypothesis is there is a problem with the chicken and egg. Is the raised dopamine levels the cause of the schizophrenia, or is it the raised dopamine level the result of schizophrenia?
It is not clear which comes first. This suggests that one needs to be careful when establishing cause and effect relationships in schizophrenic patients.
One of the biggest criticisms of the dopamine hypothesis came when Farde et al found no difference between schizophrenics’ levels of dopamine compared with ‘healthy’ individuals in 1990.
Noll (2009) also argues around one third of patients do not respond to drugs which block dopamine so other neurotransmitters may be involved.
A final weakness of the dopamine hypothesis is that it is biologically deterministic. The reason for this is because if the individual does have excessive amounts of dopamine then does it really mean that thy ey will develop schizophrenia? This suggests that the dopamine hypothesis does not account for freewill.
Neural Correlates
• Neural correlates are patterns of structure or activity in the brain that occur in conjunction with schizophrenia • People with schizophrenia have abnormally large ventricles in the brain . Ventricles are fluid filled cavities (i.e. holes) in the brain that supply nutrients and remove waste. This means that the brains of schizophrenics are lighter than normal. The ventricles of a person with schizophrenia are on average about 15% bigger than normal (Torrey, 2002).
A strength is that the research into enlarged ventricles and neurotransmitter levels have high reliability. The reason for this is because the research is carried out in highly controlled environments, which specialist, high tech equipment such as MRI and PET scans.
These machines take accurate readings of brain regions such as the frontal and pre-frontal cortex, the basil ganglia, the hippocampus and the amygdale. This suggests that if this research was tested and re-tested the same results would be achieved.
Supporting evidence for the brain structure explanation comes from further empirical support from Suddath et al. (1990). He used MRI (magnetic resonance imaging) to obtain pictures of the brain structure of MZ twins in which one twin was schizophrenic.
The schizophrenic twin generally had more enlarged ventricles and a reduced anterior hypothalamus. The differences were so large the schizophrenic twins could be easily identified from the brain images in 12 out of 15 pairs.
This suggests that there is wider academic credibility for enlarged ventricles determining the likelihood of schizophrenia developing.
A second weakness of the neuroanatomical explanations is that it is biologically deterministic. The reason for this is because if the individual does have large ventricles then does it really mean that they will develop schizophrenia? This suggests that the dopamine hypothesis does not account for freewill.
Section 3: Psychological Explanations for Schizophrenia
Family dysfunction.
Family Dysfunction refers to any forms of abnormal processes within a family such as conflict, communication problems, cold parenting, criticism, control and high levels of expressed emotions. These may be risk factors for the development and maintenance of schizophrenia.
• Laing and others rejected the medical / biological explanation of mental disorders. They did not believe that schizophrenia was a disease. They believed that schizophrenia was a result of social pressures from life. Laing believed that schizophrenia was a result of the interactions between people, especially in families. • Bateson et al. (1956) suggested the double bind theory, which suggests that children who frequently receive contradictory messages from their parents are more likely to develop schizophrenia. For example parents who say they care whilst appearing critical or who express love whilst appearing angry. They did not believe that schizophrenia was a disease. They believed that schizophrenia was a result of social pressures from life. • Prolonged exposure to such interactions prevents the development of an internally coherent construction of reality; in the long run, this manifests itself as typically schizophrenic symptoms such as flattening affect, delusions and hallucinations, incoherent thinking and speaking, and in some cases paranoia. • Another family variable associated with schizophrenia is a negative emotional climate, or more generally a high degree of expressed emotion (EE). EE is a family communication style that involves criticism, hostility and emotional over-involvement. The researchers concluded that this is more important in maintaining schizophrenia than in causing it in the first place, (Brown et al 1958). Schizophrenics returning to such a family were more likely to relapse into the disorder than those returning to a family low in EE. The rate of relapse was particularly high if returning to a high EE family was coupled with no medication.
One strength of the double bind explanation comes from further empirical support provided by Berger (1965). They found that schizophrenics reported a higher recall of double bind statements by their mothers than non-schizophrenics.
However, evidence may not be reliable as patient’s recall may be affected by their schizophrenia. This suggests that there is wider academic credibility for the idea of contradictory messages causing schizophrenia.
A second strength of the research into expressed emotion (EE) is that it has practical applications. For example Hogarty (1991) produced a type of therapy session, which reduced social conflicts between parents and their children which reduced EE and thus relapse rates.
This suggests that gaining an insight into family relationships allows psychiatric professionals to help improve the quality of patient’s lives.
Individual differences – EE is associated with relapse but not all patients who live in high EE families relapse and not all patients in low EE families avoid relapse – Family dysfunction is an incomplete explanation for schizophrenia.
A weakness of the family relationsships appraoch is that there is a problem of cause and effect. Mischler & Waxler (1968) found significant differences in the way mothers spoke to their schizophrenic daughters compared to their normal daughters, which suggests that dysfunctional communication may be a result of living with the schizophrenic rather than the cause of the disorder.
This suggests that there is a problem of the chicken and egg scenario in relation to expressed emotion causing schizophrenia.
A second weakness of the double bind theory is that there are ethical issues. There are serious ethical concerns in blaming the family, particularly as there is little evidence upon which to base this.
Gender bias is also an issue as the mother tends to be blamed the most, which means such research is highly socially sensitive. This suggests that the research therefore does not protect individuals from harm.
Cause and effect – It remains unclear whether cognitive factors cause schizophrenia or if schizophrenia causes these cognitions – Family dysfunction may not be a valid explanation for schizophrenia.
Cognitive explanations
including dysfunctional thought processing.
Cognitive approaches examine how people think, how they process information. Researchers have focused on two factors which appear to be related to some of the experiences and behaviors of people diagnosed with schizophrenia.
First, cognitive deficits which are impairments in thought processes such as perception, memory and attention. Second, cognitive biases are present when people notice, pay attention to, or remember certain types of information better than other.
Cognitive Deficits
• There is evidence that people diagnosed as schizophrenic have difficulties in processing various types of information, for example visual and auditory information. Research indicates their attention skills may be deficient – they often appear easily distracted. • A number of researchers have suggested that difficulties in understanding other people’s behavior might explain some of the experiences of those diagnosed as schizophrenic. Social behavior depends, in part, on using other people’s actions as clues for understanding what they might be thinking. Some people who have been diagnosed as schizophrenic appear to have difficulties with this skill. • Cognitive deficits have been suggested as possible explanations for a range of behaviors associated with schizophrenia. These include reduced levels of emotional expression, disorganised speech and delusions.
Cognitive Biases
• Cognitive biases refer to selective attention. The idea of cognitive biases has been used to explain some of the behaviors which have been traditionally regarded as ‘symptoms’ of ‘schizophrenia’. • Delusions: The most common delusion that people diagnosed with schizophrenia report is that others are trying to harm or kill them – delusions of persecution. Research suggests that these delusions are associated with specific biases in reasoning about and explaining social situations. Many people who experience feelings of persecution have a general tendency to assume that other people cause the things that go wrong with their lives.
A strength of the cognitive explanation is that it has practical applications. Yellowless et al. (2002) developed a machine that produced virtual hallucinations, such as hearing the television telling you to kill yourself or one person’s face morphing into another’s.
The intention is to show schizophrenics that their hallucinations are not real. This suggests that understanding the effects of cognitive deficits allows psychologists to create new initiatives for schizophrenics and improve the quality of their lives.
A final strength is that it takes on board the nurture approach to the development of schizophrenia. For example, it suggests that schizophrenic behavior is the cause of environmental factors such as cognitive factors.
One weakness of the cognitive explanation is that there are problems with cause and effect. Cognitive approaches do not explain the causes of cognitive deficits – where they come from in the first place.
Is it the cognitive deficits which causes the schizophrenic behavior or is the schizophrenia that causes the cognitive deficits? This suggests that there are problems with the chicken and egg problem.
A second weakness of the cognitive model is that it is reductionist. The reason for this is because the approach does not consider other factors such as genes.
It could be that the problems caused by low neurotransmitters creates the cognitive deficits. This suggests that the cognitive approach is oversimplistic when consider the explanation of schizophrenia.
Section 4: Drug Therapy: typical and atypical antipsychotics
Drug therapy is a biological treatment for schizophrenia. Antipsychotic drugs are used to reduce the intensity of symptoms (particularly positive symptoms).
Typical Antipsychotics
• First generation Antipsychotics are called “Typical Antipsychotics” Eg. Chlorpromazine and Haloperidol. • Typical antipsychotic drugs are used to reduce the intensity of positive symptoms, blocking dopamine receptors in the synapses of the brain and thus reducing the action of dopamine. • They arrest dopamine production by blocking the D2 receptors in synapses that absorb dopamine, in the mesolimbic pathway thus reducing positive symptoms, such as auditory hallucinations. • But they tended to block ALL types of dopamine activity, (in other parts of the brain as well) and this caused side effects and may have been harmful.
Atypical Antipsychotics
• Newer drugs, called “atypical antipsychotics” attempt to target D2 dopamine activity in the limbic system but not D3 receptors in other parts of the brain. • Atypical antipsychotics such as Clozapine bind to dopamine, serotonin and glutamate receptors. • Atypical antipsychotic drugs work on negative symptoms, improving mood, cognitive functions and reducing depression and anxiety. • They also have some effect on other neurotransmitters such as serotonin . They generally have fewer side effects eg. less effect on movement Eg. Clozapine, Olazapine and Risperidone.
Since the mid-1950s antipsychotic medications have greatly improved treatment. Medications reduce positive symptoms particularly hallucinations and delusions; and usually allow the patient to function more effectively and appropriately.
Antipsychotic drugs are highly effective as they are relatively cheap to produce, easy to administer and have a positive effect on many sufferers. However they do not “cure” schizophrenia, rather they dampen symptoms down so that patients can live fairly normal lives in the community.
Kahn et al. (2008) found that antipsychotics are generally effective for at least one year, but second- generation drugs were no more effective than first-generation ones.
Some sufferers only take a course of antipsychotics once, while others have to take a regular dose in order to prevent symptoms from reappearing.
There is a sizeable minority who do not respond to drug treatment. Pills are not as helpful with other symptoms, especially emotional problems.
Older antipsychotics like haloperidol or chlorpromazine may produce side effects Sometimes when people with schizophrenia become depressed, so it is common to prescribe anti-depressants at the same time as the anti-psychotics.
All patients are in danger of relapsing but without medication the relapses are more common and more severe which suggests the drugs are effective.
Clozapine targets multiple neurotransmitters, not just dopamine, and has been shown to be more effective than other antipsychotics, although the possibility of severe side effects – in particular, loss of the white blood cells that fight infection.
Even newer antipsychotic drugs, such as risperidone and olanzapine are safer, and they also may be better tolerated. They may or may not treat the illness as well as clozapine, however.
Meta–analysis by Crossley Et Al (2010) suggested that Atypical antipsychotics are no more effective, but do have less side effects.
Recovery may be due to psychological factors – The placebo effect is when patients’ symptoms are reduced because they believe that it should.
However, Thornley et al carried out a meta-analysis comparing the effects of Chlorpromazine to placebo conditions and found Chlorpromazine to be associated with better overall functioning – Drug therapy is an effective treatment for SZ.
RWA – Offering drugs can lead to an enhanced quality of life as patients are given independence – Positive impact on the economy as patients can return to work and no longer need to be provided with institutional care.
Ethical issues – Antipsychotics have been used in hospitals to calm patients and make them easier for staff to work with rather than for the patients’ benefit – Can lead to the abuse of the Human Rights Act (no one should be subject to degrading treatment).
Severe side effects – Long term use can result in tardive dyskinesia which manifests as involuntary facial movements such as blinking and lip smacking – While they may be effective, the severity of the side effects mean the costs outweigh the benefits therefore they are not an appropriate treatment.
In most cases the original “typical antipsychotics” have more side effects, so if the exam paper asks for two biological therapies you can write about typical anti-psychotics and emphasise the side effects, then you can write about the atypical antipsychotics and give them credit for having less side effects.
Section 5: Psychological Therapies for Schizophrenia
Family therapy.
Family therapy is a form of therapy carried out with members of the family with the aim of improving their communication and reducing the stress of living as a family.
Family Therapy aims to reduce levels of expressed emotion, and reduced the likelihood of relapse.
Aims of Family Therapy
• To educate relatives about schizophrenia. • To stabilize the social authority of the doctor and the family. • To improve how the family communicated and handled the situation. • To teach patients and carers more effective stress management techniques.
Methods used in Family Therapy
• Pharoah identified examples of how family therapy works: It helps family members achieve a balance between caring for the individual and maintaining their own lives, it reduces anger and guilt, it improves their ability to anticipate and solve problems and forms a therapeutic alliance. • Families taught to have weekly family meetings solving problems on family and individual goals, resolve conflict between members, and pinpoint stressors. • Preliminary analysis: Through interviews and observation the therapist identifies strengths and weaknesses of family members and identifies problem behaviors. • Information transfer – teaching the patient and the family the actual facts about the illness, it’s causes, the influence of drug abuse, and the effect of stress and guilt. • Communication skills training – teach family to listen, to express emotions and to discuss things. Additional communication skills are taught, such as “compromise and negotiation,” and “requesting a time out” . This is mainly aimed at lowering expressed emotion.
A study by Anderson et al. (1991) found a relapse rate of almost 40% when patients had drugs only, compared to only 20 % when Family Therapy or Social Skills training were used and the relapse rate was less than 5% when both were used together with the medication.
Pharaoh et al. (2003) meta – analysis found family interventions help the patient to understand their illness and to live with it, developing emotional strength and coping skills, thus reducing rates of relapse.
Pharoah identified examples of how family therapy works: It helps family members achieve a balance between caring for the individual and maintaining their own lives, it reduces anger and guilt, it improves their ability to anticipate and solve problems and forms a therapeutic alliance.
Economic Benefits: Family therapy is highly cost effective because it reduces relapse rates, so the patients are less likely to take up hospital beds and resources. The NICE review of family therapy studies demonstrated that it was associated with significant cost savings when offered to patients alongside the standard care – Relapse rates are also lower which suggests the savings could be even higher.
Lobban (2013) reports that other family members felt they were able to cope better thanks to family therapy. In more extreme cases the patient might be unable to cope with the pressures of having to discuss their ideas and feelings and could become stressed by the therapy, or over-fixated with the details of their illness.
Token Economy
• Token economies aim to manage schizophrenia rather than treat it. • They are a form of behavioral therapy where desirable behaviors are encouraged by the use of selective reinforcement and is based on operant conditioning. • When desired behavior is displayed eg. Getting dressed, tokens (in the form of coloured discs) are given immediately as secondary reinforcers which can be exchanged for rewards eg. Sweets and cigarettes. • This manages schizophrenia because it maintains desirable behavior and no longer reinforces undesirable behavior. • The focus of a token economy is on shaping and positively reinforcing desired behaviors and NOT on punishing undesirable behaviors. The technique alleviates negative symptoms such as poor motivation, and nurses subsequently view patients more positively, which raises staff morale and has beneficial outcomes for patients. • It can also reduce positive symptoms by not rewarding them, but rewarding desirable behavior instead. Desirable behavior includes self-care, taking medication, work skills, and treatment participation.
Paul and Lentz (1977) Token economy led to better overall patient functioning and less behavioral disturbance, More cost-effective (lower hospital costs)
Upper and Newton (1971) found that the weight gain associated with taking antipsychotics was addressed with token economy regimes. Chronic schizophrenics achieved 3lbs of weight loss a week.
McMonagle and Sultana (2000) reviewed token economy regimes over a 15-year period, finding that they did reduce negative symptoms, though it was unclear if behavioral changes were maintained beyond the treatment programme.
It is difficult to keep this treatment going once the patients are back at home in the community. Kazdin et al. Found that changes in behavior achieved through token economies do not remain when tokens are with¬drawn, suggesting that such treatments address effects of schizophrenia rather than causes. It is not a cure.
There have also been ethical concerns as such a process is seen to be dehumanising, subjecting the patient to a regime which takes away their right to make choices.
In the 1950s and 60s nurses often “rewarded” patients with cigarettes. Due to the pivotal role of dopamine in schizophrenia this led to a culture of heavy smoking an nicotine addiction in psychiatric hospitals of the era.
Ethical issues – Severely ill patients can’t get privileges because they are less able to comply with desirable behaviors than moderately ill patients – They may suffer from discrimination
Cognitive Behavioral Therapy
In CBT, patients may be taught to recognise examples of dysfunctional or delusional thinking, then may receive help on how to avoid acting on these thoughts. This will not get rid of the symptoms of schizophrenia but it can make patients better able to cope with them.
Central idea: Patients problems are based on incorrect beliefs and expectations. CBT aims to identify and alter irrational thinking including regarding:
- General beliefs.
- Self image.
- Beliefs about what others think.
- Expectations of how others will act.
- Methods of coping with problems.
In theory, when the misunderstandings have been swept away, emotional attitudes will also improve.
Assessment : The therapist encourages the patient to explain their concerns.
• describing delusions • reflecting on relationships • laying out what they hope to achieve through the therapy.
Engagement :
The therapist wins the trust of the patient, so they can work together. This requires honesty, patience and unconditional acceptance. The therapist needs to accept that the illusions may seem real to the patient at the time and should be dealt with accordingly.
ABC : Get the patients to understand what is really happening in their life:
A: Antecedent – what is triggering your problem ? B: behavior – how do you react in these situations ? C: Consequences – what impact does that have on your relationships with others?
Normalisation :
Help the patient realise it is normal to have negative thoughts in certain situations. Therefore there is no need to feel stressed or ashamed about them.
Critical Collaborative Analysis :
Carrying on a logical discussion till the patient begins to see where their ideas are going wrong and why they developed. Work out ways to recognise negative thoughts and test faulty beliefs when they arise, and then challenge and re-think them.
Developing Alternative Explanations :
Helping the patient to find logical reasons for the things which trouble them Let the patient develop their own alternatives to their previous maladaptive behavior by looking at coping strategies and alternative explanations.
Another form of CBT: Coping Strategy Enhancement (CSE)
• Tarrier (1987) used detailed interview techniques, and found that people with schizophrenia can often identify triggers to the onset of their psychotic symptoms, and then develop their own methods of coping with the distress caused. These might include things as simple as turning up the TV to drown out the voices they were hearing! • At least 73% of his sample reported that these strategies were successful in managing their symptoms. • CSE aims to teach individuals to develop and apply effective coping strategies which will reduce the frequency, intensity and duration of psychotic symptoms and alleviate the accompanying distress. There are two components: 1. Education and rapport training: therapist and client work together to improve the effectiveness of the client’s own coping strategies and develop new ones. 2. Symptom targeting: a specific symptom is selected for which a particular coping strategy can be devised Strategies are practised within a session and the client is helped through any problems in applying it. They are then given homework tasks to practice, and keep a record of how it worked.
CBT does seem to reduce relapses and readmissions to hospital (NICE 2014). However, the fact that these people were on medication and having regular meetings with doctors would be expected to have that effect anyway.
Turkington et al. (2006) CBT is highly effective and should be used as a mainstream treatment for schizophrenia wherever possible.
Tarrier (2005) reviewed trials of CBT, finding evidence of reduced symptoms, especially positive ones, and lower relapse rates.
Requires self-awareness and willingness to engage – Held back by the symptoms schizophrenics encounter – It is an ineffective treatment likely to lead to disengagement.
Lengthy – It takes months compared to drug therapy that takes weeks which leads to disengaged treatment as they don’t see immediate effects – A patient who is very distressed and perhaps suicidal may benefit better in the short term from antipsychotics.
Addington and Addington (2005) claim that CBT is of little use in the early stages of an acute schizophrenic episode, but perhaps more useful when the patient is more calm and beginning to worry about how life will be after they recover. In other words, it doesn’t cure schizophrenia, it just helps people get over it.
Research in Hampshire, by Kingdon and Kirschen (2006) found that CBT is not suitable for all patients, especially those who are too thought disorientated or agitated, who refuse medication, or who are too paranoid to form trusting alliances with practitioners.
As there is strong evidence that relapse is related to stress and expressed emotion within the family, it seems likely that CBT should be employed alongside family therapy in order to reduce the pressures on the individual patient.
Section 6: Interactionist Approach
The Interactionist approach acknowledges that there are a range of factors (including biological and psychological) which are involved in the development of schizophrenia.
The Diathesis-stress Model
• The diathesis-stress model states that both a vulnerability to SZ and a stress trigger are necessary to develop the condition. • Zubin and Spring suggest that a person may be born with a predisposition towards schizophrenia which is then triggered by stress in everyday life. But if they have a supportive environment and/or good coping skills the illness may not develop. • Concordance rates are never 100% which suggests that environmental factors must also play a role in the development of SZ. MZ twins may have the same genetic vulnerability but can be triggered by different stressors. • Tienari Et. A. (2004): Adopted children from families with schizophrenia had more chance of developing the illness than children from normal families. This supports a genetic link. However, those children from families schizophrenia were less likely to develop the illness if placed in a “good” family with kind relationships, empathy, security, etc. So environment does play a part in triggering the illness.
Holistic – Identifies that patients have different triggers, genes etc. – Patients can receive different treatments for their SZ which will be more effective.
Falloon et al (1996) stress – such as divorce or bereavement, causes the brain to be flooded with neurotransmitters which brings on the acute episode.
Brown and Birley (1968) 50% people who had an acute schizophrenic episode had experienced a major life event in 3 weeks prior.
Substance abuse: Amphetamine and Cannabis and other drugs have also been identified as triggers as they affect serotonin and glutamate levels.
Vasos (2012) Found the risk of schizophrenia was 2.37 times greater in cities than it was in the countryside, probably due to stress levels. Hickling (1999) the stress of urban living made African-Carribean immigrants in Britain 8 to 10 times more likely to experience schizophrenia.
Faris and Dunham (1939) found clear pattern of correlation between inner city environments and levels of psychosis. Pederson and Mortensen (Denmark 2001) found Scandanavian villages have very LOW levels of psychosis, but 15 years of living in a city increased risk.
Fox (1990): It is more likely that factors associated with living in poorer conditions (e.g. stress) may trigger the onset of schizophrenia, rather than individuals with schizophrenia moving down in social status.
Bentall’s meta-analysis (2012) shows that stress arising from abuse in childhood increases the risk of developing schizophrenia.
Toyokawa, Et. Al (2011) suggest many aspects of urban living – ranging from life stressors to the use of drugs, can have an effect on human epigenetics. So the stressors of modern living could cause increased schizophrenia in future generations.
From Genes To Behavior: Exploring The Pathophysiology Of Schizophrenia
Schizophrenia can be seen as a severe and chronic brain disorder that impacts how a person functions in the world. Individuals with schizophrenia may experience positive or psychotic symptoms like hallucinations or negative symptoms like social withdrawal, cognitive deficits, or loss of motivation. These symptoms may affect interpersonal relationships and can be challenging to manage without help from a mental health professional.
To manage schizophrenia symptoms, it may be useful to understand the pathophysiology of schizophrenia—in other words, how the disease develops. Several risk factors may lead to the development of schizophrenia, including genetic factors, abnormalities related to brain development, and changes in neurotransmitters. In addition to the biological risk factors, environmental factors may also play a role in the development of schizophrenia. A person may be diagnosed with schizophrenia when they meet the required symptoms as outlined by the Diagnostic and Statistical Manual, 5th Edition (DSM-V).
Genetic factors
The cause of schizophrenia is not completely understood. However, studies show that there may be a genetic link , although updated research may be needed. It can be important to note that even when a family member has schizophrenia, the disorder isn’t always passed down to others.
In addition to genetic factors, it is also possible that epigenetic factors play a role in the development of schizophrenia in some individuals. Epigenetics generally refers to changes in gene expression rather than changes in the genetic code itself. Studies have shown that changes in various genetic loci and expression s may be present in individuals with schizophrenia, but more research may be needed in this area.
Neurodevelopmental factors
In addition to genetic factors, neurodevelopmental factors may contribute to schizophrenia. Older research shows that schizophrenia may be linked to other neurodevelopmental disorders that can impact cognitive impairment, such as attention-deficit/hyperactivity disorder, intellectual disabilities, and autism spectrum disorder. This connection is thought to demonstrate that these mental disorders may exist on a spectrum linked by neurodevelopmental factors.
Neurodevelopmental factors connected to cognitive dysfunction may begin during prenatal and perinatal stages. During these stages, maternal infections, such as influenza, may lead to a higher rate of schizophrenia in children as they become adults. These infections can cause changes in cytokine-related inflammation, which may be attributed to inducing schizophrenia-like behaviors in adolescents and young adults.
Neuroimaging techniques have begun to uncover more in terms of how neurodevelopmental factors may cause psychotic disorders, including schizophrenia. Brain imaging studies have shown links between delayed maturation of connections in the nervous system and brain and schizophrenia , although updated evidence may be necessary. Additional studies and research into the neurodevelopmental factors may lay the groundwork for preventing schizophrenia in some people.
Neurotransmitter abnormalities
Neurotransmitters can be defined as chemicals that signal various functions to happen within the human body. These chemicals typically serve as messengers, sending information from one cell to another, and they allow different parts of the body to communicate. Some neurotransmitters include acetylcholine, glutamate, GABA, glycine, dopamine, norepinephrine, and serotonin.
It is believed that abnormalities in some neurotransmitters and their production levels may impact schizophrenia symptoms. Two neurotransmitters that have potential links to schizophrenia are dopamine and glutamate.
Dopamine hypothesis
The dopamine hypothesis usually refers to the belief that individuals with schizophrenia may experience abnormal production of the neurotransmitter dopamine in various brain regions, including the prefrontal cortex. Studies have shown that positive schizophrenia symptoms, such as hallucinations and delusions, may be linked to the excess production of dopamine in the brain.
In addition to excess dopamine, individuals with schizophrenia may also have a higher density of dopamine receptors, meaning that they may be more sensitive to changes in dopamine levels.
Although the dopamine hypothesis may be influential in understanding the neurobiology of schizophrenia, dopamine dysfunction may be just one of many factors contributing to the complex pathophysiology of the disorder. Other neurotransmitters, as well as environmental and structural components, may also play a role in the development of schizophrenia.
Glutamate hypothesis
Another neurotransmitter that may play a role in the development of schizophrenia is glutamate. The glutamate hypothesis generally refers to the idea that glutamate levels could be higher in individuals with schizophrenia due to various interactions and receptor blockers. The increase in glutamate could serve to over-excite or overstimulate nerve cells and may be associated with negative symptoms of schizophrenia, such as social withdrawal and lack of motivation.
As a widely varied disorder, the glutamate hypothesis may hold for some individuals with schizophrenia, but not for others.
Structural and functional brain abnormalities
Using advanced imaging techniques, scientists have discovered that several structural brain abnormalities may contribute to the development of schizophrenia. The root of these abnormalities may lie in neural connectivity disruption that can be caused by environmental or genetic risk factors during prenatal or adolescent stages. These changes or abnormalities may lead to structural changes in the cortical or other brain regions in people with schizophrenia.
New advancements in functional neuroimaging technology have also revealed changes in gray and white matter in individuals with schizophrenia. These changes may lead to abnormalities in cognitive functioning and could impact memory, focus, and motivation.
In some individuals, these changes in brain activity can continue throughout the progression of schizophrenia. Managing schizophrenia symptoms may start by continuing to understand how the disorder impacts the brain. This can help professionals identify the disorder sooner and begin treatment before symptoms progress and intensify.
Environmental factors
While genetic and structural factors likely play a role in the development of schizophrenia, environmental factors may also impact how the disorder develops. Environmental factors that may impact schizophrenia can include the following:
- Pregnancy and birth complications: Complications during pregnancy, such as hypoxia, influenza, premature birth, and malnutrition, may increase the chance of developing schizophrenia later in life.
- Stress: Anxiety and related stress may cause an increase in schizophrenia symptoms, such as psychosis. Older studies report that approximately 46% of individuals with a schizophrenia diagnosis experienced a stressful life event within three months of receiving their diagnosis.
- Substance use disorder: The use of some substances may trigger schizophrenia symptoms. For example, stimulants like cocaine and methamphetamine may induce psychosis in some individuals.
- Childhood trauma: Trauma during childhood, such as abuse*, neglect, bullying, and other traumatic events, may play a role in the development of positive schizophrenia symptoms as an adult.
- Growing up in an urban setting: There may be an association between city living and schizophrenia , and studies show that moving from a rural environment to a city environment may substantially increase the risk of developing schizophrenia. It is unclear as to the exact mechanism for the increased rate of schizophrenia in urban areas. However, it has been hypothesized that social adversity, increased substance use, and discrimination may play a role.
As with other factors related to the pathophysiology of schizophrenia, environmental factors may be only one piece of the puzzle. With this in mind, it may be beneficial to view treating and managing schizophrenia with a holistic approach.
*If you or a loved one are witnessing or experiencing any form of abuse, please know that help is available. You can call the National Domestic Violence Hotline anytime at 1-800-799-SAFE (7233).
Managing the symptoms of schizophrenia
The management of schizophrenia can be multifaceted and may require cooperation from a full team of medical and mental health professionals. In many cases, medication in the form of antipsychotics may be used in conjunction with cognitive behavioral therapy (CBT) sessions to manage symptoms and improve daily function. Always speak to your doctor before starting, stopping, or changing the way you take any form of medication.
Online cognitive behavioral therapy techniques may be effective at treating symptoms of schizophrenia . A therapist may use a variety of techniques in an online setting to identify triggers for symptoms and to provide individuals with tools and resources to manage schizophrenia. However, it can be important to note that those experiencing acute positive or psychotic symptoms may need to seek care in person.
For individuals with schizophrenia, finding a local therapist who can meet their needs can be challenging. Online therapy generally enables individuals to choose therapists who meet their needs, even if they are not located nearby. This added flexibility can help to ensure that the client and therapist are a good fit for a long-term therapeutic relationship.
Understanding the pathophysiology of schizophrenia may be important when trying to understand how the disorder develops and impacts different individuals. Common risk factors for developing schizophrenia may include genetic factors, neurodevelopmental factors, neurotransmitter abnormalities, brain structure abnormalities, and environmental factors. In-person or online therapy can effectively treat symptoms of schizophrenia and can often be combined with doctor-prescribed medication for symptom management.
- Depression And Schizophrenia: How They Are Related Medically reviewed by Nikki Ciletti , M.Ed, LPC
- When Was Schizophrenia Discovered? A Brief History Of The Disorder Medically reviewed by Andrea Brant , LMHC
- Schizophrenia
- Relationships and Relations
The Dopamine Hypothesis in Schizophrenia USHMedstudent
Thank you Angela MacFarlane, OMS III and Joshua Hansen, OMS III for developing this podcast. There is a strong review of specific types of strategies for shelf questions and some high yield content about antipsychotic medications at the start of the podcast. This podcast tackles the evolution of the role that dopamine has in both psychosis and in schizophrenia. The podcast has a number of high yield facts through the discussion. We enjoyed our discussion and hope you find it as interesting as we did! Thank you Jordan Turner for creating the perfect bumper music!
- Episode Website
- More Episodes
- Kent Roundy and Tom Rayner
Top Podcasts In Education

IMAGES
VIDEO
COMMENTS
Brief History of Dopamine Hypothesis in Schizophrenia. Dopamine, adrenaline, and noradrenaline are neurotransmitters that belong to the catecholamine family. ... This increase in D (2)-high-receptors is a necessary basic requirement for the development of a psychosis that correlates with dopamine supersensitivity . This specific increase in D ...
This review examines the history of discoveries that contributed to development of the dopamine hypothesis of schizophrenia. The origin of the hypothesis is traced to the recognition that neuroleptic drugs interfere with brain dopamine function. This insight was derived from two distinct lines of research. The first line originated from the ...
The Dopamine Hypothesis: Version II. In 1991, Davis et al 10 published a landmark article describing what they called "a modified dopamine hypothesis of schizophrenia" that reconceptualized the dopamine hypothesis in the light of the findings available at the time. The main advance was the addition of regional specificity into the hypothesis to account for the available postmortem and ...
The ' dopamine hypothesis of schizophrenia ', simply stated, postulates that certain dopaminergic pathways are overactive in schizophrenia and so cause the symptoms of an acute schizophrenic episode. Clinical studies indicate that drugs like L-dopa or amphetamine, which potentiate dopaminergic activity, may induce or exacerbate ...
The dopamine hypothesis of schizophrenia or the dopamine hypothesis of psychosis is a model that attributes the positive symptoms of schizophrenia to a disturbed and hyperactive dopaminergic signal transduction.The model draws evidence from the observation that a large number of antipsychotics have dopamine-receptor antagonistic effects. The theory, however, does not posit dopamine ...
The dopamine hypothesis of schizophrenia has moved from the dopamine receptor hypothesis (increased dopamine transmission at the postsynaptic receptors) to a focus on presynaptic striatal hyperdopaminergia. ... This suggests that drug development needs to focus on modulating presynaptic striatal dopamine function, either directly or through ...
The dopamine hypothesis, which posits that dysregulation of the dopaminergic system is etiologic for schizophrenia, is among the most enduring biological theories in psychiatry. Although variation within genes related to dopaminergic functioning has been associated with schizophrenia, an aggregate test of variation, using the largest publicly ...
The stagnation in drug development for schizophrenia highlights the need for better translation between basic and clinical research. Understanding the neurobiology of schizophrenia presents ...
Schizophrenia has long been thought to be a consequence of abnormalities in major neurotransmitter systems. The dopamine hypothesis, concerning the underlying neurochemistry of schizophrenia, originated many years ago (58, 59). The hypothesis has since gone through many refinements on the basis of new scientific evidence.
The dopamine hypothesis is the longest standing pathoetiologic theory of schizophrenia. Because it was initially based on indirect evidence and findings in patients with established schizophrenia, it was unclear what role dopamine played in the onset of the disorder. However, recent studies in people at risk of schizophrenia have found elevated ...
Abstract. The dopamine hypothesis of schizophrenia, which was formulated in the 1960s after the discovery of the antipsychotic actions of chlorpromazine, was extremely successful as a heuristic principle for interpreting aspects of the phenomenology of schizophrenia. The development of improved antipsychotic medications was guided by a search ...
The dopamine hypothesis has been the cornerstone in the research and clinical practice of schizophrenia. With the initial emphasis on the role of excessive dopamine, the hypothesis has evolved to a concept of combining prefrontal hypodopaminergia and striatal hyperdopaminergia, and subsequently to the present aberrant salience hypothesis.
Glutamate and dopamine systems play distinct roles in terms of neuronal signalling, yet both have been proposed to contribute significantly to the pathophysiology of schizophrenia. In this paper we assess research that has implicated both systems in the aetiology of this disorder. We examine evidence from post‐mortem, preclinical ...
The dopamine hypothesis is one of the widely accepted explanations for the pathophysiology underpinning schizophrenia and posits that hyperactive dopaminergic signal transduction in the ...
An exciting and relatively more recent development in the theories of psychosis is the notion that dopamine hyperactivity is actually a downstream consequence of glutamate ... The integrated model of glutamate and dopamine hypothesis for schizophrenia: Prediction and personalized medicine for prevent potential treatment-resistant patients ...
However, since these iterations of the synaptic hypothesis of schizophrenia, new data from novel methods, such as induced pluripotent stem cell (iPSC) and genome-wide association studies (GWAS ...
The dopamine hypothesis in schizophrenia was based primarily on observational results in its introduction. Since then, numerous studies pointing at a definite link between dopamine changes and ...
The Dopamine Hypothesis of Schizophrenia: Version III. We propose a revised "third version" of the dopamine hypothesis to account for the new evidence, drawing on the work of many previous reviews (eg, Laruelle and Abi-Dargham, 32 van et al, 70 Cannon et al, 164 and Howes et al 165). The hypothesis has 4 distinctive components.
Dopamine Hypothesis. This theory suggests that an imbalance of dopamine is responsible for schizophrenic symptoms. In other words, dopamine plays a role in controlling our sense of reality, and too much or too little can cause delusions and hallucinations. The evidence for this theory comes from many sources, including post-mortem studies that ...
The dopamine hypothesis of how antipsychotic drugs exert their beneficial effect in psychotic illness has an interesting history that dates back to 1950. This hypothesis is not to be confused with the dopamine hypothesis of schizophrenia; the aim of the latter is to explain the etiology of schizophrenia. The present review does not deal with ...
The original dopamine hypothesis stated that schizophrenia suffered from an excessive amount of dopamine. This causes the neurons that use dopamine to fire too often and transmit too many messages. ... Bentall's meta-analysis (2012) shows that stress arising from abuse in childhood increases the risk of developing schizophrenia. Toyokawa, Et ...
For many years, the central hypothesis of the field concerning the pathogenesis of schizophrenia focused on dopamine. This was based primarily on the fact that all drugs with antipsychotic potency block dopamine D2 receptors; conversely, drugs that stimulate dopamine release, including cocaine and amphetamine, produce short-lived psychotic ...
Other neurotransmitters, as well as environmental and structural components, may also play a role in the development of schizophrenia. Glutamate hypothesis. Another neurotransmitter that may play a role in the development of schizophrenia is glutamate. The glutamate hypothesis generally refers to the idea that glutamate levels could be higher ...
The Dopamine Hypothesis in Schizophrenia USHMedstudent ... OMS III for developing this podcast. There is a strong review of specific types of strategies for shelf questions and some high yield content about antipsychotic medications at the start of the podcast. This podcast tackles the evolution of the role that dopamine has in both psychosis ...
The pathophysiology of schizophrenia is complex, and it has been studied for years with many factors yet to be discovered. Genetic studies show that schizophrenia involves different genetic loci and is highly pleiotropic . Among all neurotransmitters involved in the pathophysiology of schizophrenia, dopamine plays a major role in psychosis.