- Research Paper
- Book Report
- Book Review
- Movie Review
- Dissertation
- Thesis Proposal
- Research Proposal
- Dissertation - Abstract
- Dissertation Introduction
- Dissertation Review
- Dissertat. Methodology
- Dissertation - Results
- Admission Essay
- Scholarship Essay
- Personal Statement
- Proofreading
- Speech Presentation
- Math Problem
- Article Critique
- Annotated Bibliography
- Reaction Paper
- PowerPoint Presentation
- Statistics Project
- Multiple Choice Questions
- Resume Writing
- Other (Not Listed)

Genetic Engineering and its Dangers (Essay Sample)
THE ESSAY DISCUSSES THE CONS OF GENETIC ENGINEERING. APART FROM THE POSITIVE CONTRIBUTIONS OF GENETIC ENGINEERING, IT ALSO HAS SOME NEGATIVE IMPACTS WHICH EITHER HARM THE ENVIRONMENT OR THE CONSUMERS LIKE HUMANS AND ANIMALS. In genetic engineering, the final product can end up endangering the lives of humans, animals, or plants. genetic engineering has more positive than negative impacts
Surname Course Number and Name Institution Professor Date Genetic engineering and its dangers. Genetic engineering is the artificial manipulation, modification, and recombination of DNA or RNA molecules to modify an organism or population of organisms. It is generally used to refer to methods of recombinant DNA technology. Recombinant DNA technology is the joining of DNA from two different species that are inserted into a host organism to produce a new genetic combination. A hybrid organism that expresses both the characteristics of parent organisms is developed. It is majorly applied in agriculture to boost crop yield, in pharmaceuticals to manufacture vaccines, and also in gene therapy to cure genetic disorders. In genetic engineering, the final product can end up endangering the lives of humans, animals, or plants. The dangers of genetically engineered products include: Unknown harms to the environment. Although no exact amount of harm that can be caused by genetically engineered organisms/products is known, many authors have speculated that genetically engineered products are dangerous to the environment. Another possible danger of genetic engineering is that the products may be a possible
Other Topics:
- Methicillin-Resistant Staphylococcus Aureus (MRSA) Description: MRSA entail causative staph infection which is complicated to treat since it is resistant to antibiotics. The bacterium is dangerous because it leads infection to various parts of the body. To understand the Methicillin-Resistant Staphylococcus Aureus (MRSA), an essay has been prepared to analyze its... 4 pages/≈1100 words | 6 Sources | MLA | Biological & Biomedical Sciences | Essay |
- My Journey Journal Description: On Thursday, the 21st of April, I visited the Belle Isle during a biology trip. The weather forecast showed that the area's humidity was as high as 70 degrees humid. The first location where I first stopped was high-pitched with a small dark waxing. The great blue heron was standing on a rock, and there was... 1 page/≈275 words | 1 Source | MLA | Biological & Biomedical Sciences | Essay |
- To What Extent is the Use of Animals in Scientific Research Acceptable Description: Animals, ranging from mice to fruit flies, are commonly used in scientific research. They are critical in enabling scientists to create novel cosmetics and medications and understand human health and biochemistry more. Animals, on the other hand, are sentient entities who experience pain, stress, and ... 1 page/≈275 words | 2 Sources | MLA | Biological & Biomedical Sciences | Essay |
- Exchange your samples for free Unlocks.
- 24/7 Support
Genetic Engineering: Dangers and Opportunities
Introduction, genetic engineering pros and cons, works cited.
As of today, the practice of genetic engineering continues to remain highly controversial. In its turn, this can be explained by the fact that there are a number of the clearly defined ethical undertones to the very idea of inducing ‘beneficial’ genetic mutations to a living organism.
After all, this idea presupposes the eventual possibility for people to realize themselves being the masters of their own biological destiny, in the evolutionary sense of this word.
Nevertheless, even though the practice in question indeed appears utterly debatable, the very objective laws of history/evolution leave only a few doubts that, as time goes on, more and more people will perceive it as being thoroughly appropriate. This paper will explore the validity of the above-stated at length.
In general, genetic engineering can be defined as: “An artificial modification of the genetic code of an organism. It changes the physical nature of the being in question radically, often in ways that would never occur in nature” (Cyriac 65).
Thus, it is most properly discussed as an umbrella term for the biotech practices that aim to alter the molecular basis of the DNA strand for a variety of different purposes, mostly concerned with allowing people to be able to enhance their lives.
As of now, we can identify three major directions, in which the ongoing progress in the field of the genetic engineering technologies (GET) has attained an exponential momentum: a) Deciphering the structure of the human genome, b) Transferring genes from the representatives of one species to another, c) Cloning. Even though GET became available since not long ago, these technologies proved thoroughly capable of benefiting humanity in a variety of different ways.
Among the most notable of them can be well mentioned:
a) Making possible the production of genetically modified foods. As Coker noted: “In the United States and elsewhere, more than 90% of soybeans, cotton, corn, and certain other crops are already genetically engineered” (24). The reason behind the growing popularity of this type of food is quite apparent – the application of getting increases the efficiency of agriculture rather drastically, which in turn contributes to solving the problem of ‘world’s hunger.’
b) Establishing the objective preconditions for the creation of drugs that could be used for treating diseases that are now being assumed incurable, such as AIDS and cancer. This, of course, presupposes that, as a result of GET being increasingly used by pharmacologists, the lifespan of an average individual should be substantially extended. The validity of this suggestion can be illustrated, in regards to the effects of such a widely used genetically modified drug as insulin, prescribed to those who suffer from diabetes.
c) Providing people with the opportunity to have their children (or pets) being ‘genetically tailored,’ in accordance with what happened to be the concerned individual’s personal wishes, in this respect. What it means is that, due to the rise of getting, the concept of eugenics became thoroughly sound once again: “Besides ensuring that our children are born without genetic defects, we will soon be able to give them genetic enhancements: they will become taller, stronger, smarter” (Anderson 23). Consequently, this will allow the biological betterment of human societies.
Nevertheless, even though there are many reasons to consider genetic engineering utterly beneficial to the well-being of humanity, some people cannot help deeming it utterly ‘wicked’ – this especially appears to be the case among religious citizens.
The reason for this is quite apparent – one’s ability to meddle with the structure of DNA, which in turn results in the emergence of the ‘tailored’ life-forms, implies that the individual in question is nothing short of God.
In the eyes of a religious individual, however, this idea appears clearly sacrilegious: “Humans must show respect for God’s dominion through attentive obedience to the immanent laws of creation” (Clague 140). There are also a number of secular (non-religious) objections to genetic engineering.
The most commonly heard one is concerned with the fact that the effects of the consumption of genetically modified foods on humans have not been thoroughly researched. This, of course, establishes a hypothetical possibility for those individuals who consume these foods to end up suffering from a number of yet unexplored side effects.
It is also often mentioned that, because GET provides married couples with the hypothetical possibility to conceive and to give birth to ‘ideal’ babies, it may eventually result in the emergence of the previously unheard forms of social discrimination against people, whose genome happened to be unmodified.
Moreover, there is a growing concern about the fact that being artificially created, the genetically altered forms of life may bring much disbalance to the surrounding natural environment, which is supposed to evolve in accordance with the Darwinian laws of natural selection.
Out of these objections, however, only the second one can be defined as being more or less plausible. After all, the availability of getting is indeed a comparatively recent phenomenon, which in turn implies that there may be some unforeseen aspects to it.
The rest of them, however, do not appear to hold much water – this especially happened to be the case with the religious one. The reason for this is that the process of just about any organism coming to life, which religion refers to as the ‘miracle of creation,’ biologists have long ago learned to perceive as nothing but the consequence of the essentially ‘blind’ flow of molecular reactions in the concerned DNA.
As Chapman pointed out: “What causes the differentiation in the genetic code? The mechanism for this – the genetic software, if you will – comes through the epigenetic markers that surround the genome” (170). In other words, the ‘miracle of creation’ is ultimately about the chain of self-inducing genetic mutations, which presupposes that there is nothing intelligent or consciously purposeful to it in the first place.
Genetic engineering, on the other hand, makes possible the thoroughly rational manipulation with the structure of DNA – hence, allowing biologists to not only remain in full control of the process of a particular genetic mutation taking place but also to define its course.
It is understood, of course, that the practice in question does undermine the epistemological integrity of the world’s monotheistic religions, but this state of affairs has been predetermined by the laws of history and not by the practice’s ‘wickedness.’
Apparently, the fact that many people continue to refer to genetic engineering with suspicion, reflected by their irrational fear of genetically modified foods, once again proves the validity of the specifically evolutionary paradigm of life.
The reason for this is that, as we are well aware of, throughout the course of history, the implementation of technological innovations always been met with much resistance. In its turn, this can be explained by the fact that due to being ‘hairless apes’, people are naturally predisposed to cling to specifically those behavioral patterns, on their part, which proved ‘luck-inducing’ in the past.
Nevertheless, as time goes on, their ‘fear of the new’ grows progressively weakened – the direct consequence of people’s endowment with intellect. We can speculate that before deciding to become ‘stock herders,’ ‘hunter-gatherers’ used to experience a great deal of emotional discomfort, as well – yet, there was simply no way to avoid the mentioned transformation, on their part.
The reason for this is that it was dialectically predetermined. In the mentioned earlier article, Coker states: “Eventually, humans took more control of animals and plants through agriculture, and then civilization took off. Today, we can hardly imagine how harsh the pre-agricultural existence must have been” (27).
The same line of reasoning will apply when it comes to assessing what would be people’s attitudes towards genetic engineering in the future. In all probability, our descendants will look down on us in the same manner that we look down on the members of some primeval indigenous tribe, who were never able to evolve beyond the Stone Age. After all, in the future, leaving the formation of one’s genome up to a chance will be considered barbaric.
Nevertheless, it is not only the laws of historical progress that presuppose the full legitimation of genetic engineering but the evolutionary ones, as well – something the exposes the sheer erroneousness of the claim that the concerned practice is ‘unnatural.’
In this respect, one may well mention the most important principle of evolution – the likelihood for a particular quantitative process to attain a new qualitative subtlety, positively relates to how long it remained active.
This principle, of course, suggests that for as long as the representatives of a particular species continue to expand the boundaries of their environmental niche (as it happened to be the case with humans), they will be experiencing the so-called ‘evolutionary jumps.’
The emergence of getting suggests that we, as humans, are about to experience such a ‘jump’ – after having undergone the GET-induced transformation, we will instantly attain the status of ‘trans-humans’ (or ‘demi-gods’). As Bostrom pointed out: “Human nature is a work-in-progress…
Current humanity need not be the endpoint of evolution… (through) Technology and other rational means we shall eventually manage to become posthuman beings with vastly greater capacities than present human beings have” (493).
Thus, even though the practice of genetic engineering continues to spark controversies, it is highly unlikely that this will also be ceased in 10-20 years from now – those proven much too slow, taking full advantage of genetic engineering, will simply be no longer around to debate its usefulness.
The earlier provided line of argumentation, in defense of the idea that genetic engineering indeed represents the way of the future, appears fully consistent with the paper’s initial thesis. Thus, it will be fully appropriate to conclude this paper by reinstating that the sooner people grow thoroughly comfortable with getting, the better.
In this respect, it will prove rather helpful for them to become aware that the emergence of genetic engineering is yet another indication that humanity remains on the pass of progress, and there is indeed nothing ‘unnatural’ about the practice in question. This paper is expected to come as an asset within the context of just about anyone gaining such awareness.
Anderson, Clifton. “Genetic Engineering: Dangers and Opportunities.” The Futurist 34.2 (2000): 20-22. Print.
Bostrom, Nick. “Human Genetic Enhancements: A Transhumanist Perspective.” Journal of Value Inquiry 37.4 (2003): 493-506. Print.
Chapman, Davd. “Beyond Genetic Determinism.” Ethics & Medicine 29.3 (2013): 167-171. Print.
Clague, Julie. “Some Christian Responses to the Genetic Revolution.” Ethics & Medicine 19.3 (2003): 135-142. Print.
Coker, Jeffrey. “Crossing the Species Boundary: Genetic Engineering as Conscious Evolution.” Futurist 46.1 (2012): 23-27. Print.
Cyriac, Kar. “Biotech Research: Moral Permissibility vs. Technical Feasibility.” IIMB Management Review 16.2 (2004): 64-68. Print.
Cite this paper
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2020, January 3). Genetic Engineering: Dangers and Opportunities. https://studycorgi.com/genetic-engineering-dangers-and-opportunities/
"Genetic Engineering: Dangers and Opportunities." StudyCorgi , 3 Jan. 2020, studycorgi.com/genetic-engineering-dangers-and-opportunities/.
StudyCorgi . (2020) 'Genetic Engineering: Dangers and Opportunities'. 3 January.
1. StudyCorgi . "Genetic Engineering: Dangers and Opportunities." January 3, 2020. https://studycorgi.com/genetic-engineering-dangers-and-opportunities/.
Bibliography
StudyCorgi . "Genetic Engineering: Dangers and Opportunities." January 3, 2020. https://studycorgi.com/genetic-engineering-dangers-and-opportunities/.
StudyCorgi . 2020. "Genetic Engineering: Dangers and Opportunities." January 3, 2020. https://studycorgi.com/genetic-engineering-dangers-and-opportunities/.
This paper, “Genetic Engineering: Dangers and Opportunities”, was written and voluntary submitted to our free essay database by a straight-A student. Please ensure you properly reference the paper if you're using it to write your assignment.
Before publication, the StudyCorgi editorial team proofread and checked the paper to make sure it meets the highest standards in terms of grammar, punctuation, style, fact accuracy, copyright issues, and inclusive language. Last updated: September 18, 2020 .
If you are the author of this paper and no longer wish to have it published on StudyCorgi, request the removal . Please use the “ Donate your paper ” form to submit an essay.
Genetic Engineering: A Serious Threat to Human Society
Scientists have been trying to create synthetic life, life created in lab, for many years. The first breakthrough in this process happened about thirty years ago when genetic engineers began to genetically modify organisms (Savulescu). These engineers physically move genes across species in order to improve an organism or to cause an organism to function differently. Even though this process sounds as if it happens only in fantasy games, genetically modified organisms are common. For example, genetically modified crops are used every day in the world’s food supply and genetically modified bacteria have been used in medicine, chemical manufacturing, and bio warfare (Pickrell). Slowly, genetic engineering has become a powerful tool in many different fields. Recently, genetic engineering’s potential power increased when Craig Venter, a famous geneticist and entrepreneurs, recreated a living organism out of synthetic chemicals. His success proved to genetic engineers that functioning genomes can be made purely of synthetic chemicals. This power would allow genetic engineers to build new artificial genomes instead of having to modify naturally existing genomes. Genetic engineers now have the chance to broaden their fields’ applications. However, genetic engineering is unpredictable and dangerous, and broadening the application of genetic engineering only furthers the risks. Genetically engineered organisms pose lethal and economic risks to human society.
The availability of genomic information and genetic engineering technology creates a lethal threat to humanity because terrorists can use both the information and technology to recreate deadly pathogens, such as the poliovirus. The naturally occurring poliovirus killed and paralyzed millions of people for many years. In 1988, a worldwide vaccination campaign against the virus nearly exterminated it from the environment, and this solved the poliovirus epidemic. However, in 2002, well intentioned scientists decided to recreate the poliovirus for research means. Using the genomic sequence of the poliovirus found on a public database and commercially available machines, these scientists synthesized fragments of viral genomes into a functional poliovirus (Avise 7). These scientists proved that deadly pathogens can be recreated from genetic engineering techniques. Also, the information and technology used in genetic engineering is readily available and relativity cheap (Kuzma and Tanji 3). Mixing the power to recreate a deadly pathogen with the public availability of genetic engineering information and technology creates a lethal risk to humanity when terrorist exist in society. Terrorist could use genetic engineering to reinstate the poliovirus into the environment, and the virus would kill and paralyze more people. Luckily, these scientists were filled with good intent; however, there is nothing to prevent terrorists from harming innocent lives. Recreating deadly pathogens makes genetic engineering dangerous enough; however, genetic engineers also have the potential to improve the effectiveness of deadly pathogens, such as Y. pestis.
Genetic engineers can make deadly pathogens, such as Y. pestis, resistant to modern antibiotics, and these pathogens could kill innocent people if used as a weapon. Y. pestis, also known as the black plague, wreaked havoc on humanity during the Middle Ages by killing millions of people. In response to a Y. pestis threat during the 20th century, scientists developed an effective vaccine for the pathogen. However, genetic engineers at Biopreparat, a Russian biological warfare agency, engineered a new Y. pestis strain with genetic resistance to modern antibiotics and natural human immunity (Avise 6). The genetically engineered Y. pestis was more deadly and effective than the natural Y. pestis that killed millions of people during the Middle Ages. Biopreparat’s research proved that deadly pathogens can be genetically engineered into superior forms that are resistant to modern medicine. If this strain of Y. pestis was released, a black plague would devastate current human society. Militaries could use the same genetic engineering techniques that Biopreparat used to create deadly biological weapons. With this ability to make deadly pathogens resistant to modern medicine, genetically engineered organisms become lethal weapons that cannot be stopped. Other than lethal weapons, genetically engineered organisms can produce lethal chemical compounds when they are used as a manufacturing tool in the chemical industry.
Showa Denko’s genetically modified bacteria produced a lethal L-tryptophan amino acid that killed and disabled people who took the company’s food supplements. In 1989, an epidemic of eosinophilia myalgia syndrome, a syndrome that is characterized by a high eosinophil count and severe muscle pain, struck the United States (Genetic Engineering: Too Good to Go Wrong 9). This epidemic killed a hundred people and physically disabled ten thousand patients, some of which were paralyzed. Doctors eventually discovered that L-tryptophan, an amino acid used as a food supplement, was causing the epidemic. In 1990, the Journal of the American Medical Association reported that only people who took the L-tryptophan supplement made by Showa Denko, a Japanese biotech company, came down with EMS. Showa Denko’s genetically engineered organisms produced corrupted forms of L-tryptophan that were dangerous to human health (Smith 4).
Many chemical companies want to use genetically engineered organisms to produce chemicals because it is cheaper than normal manufacturing methods. If chemical companies begin to rely on genetically engineered organisms to produce food and medical chemicals, the public could be at risk for another dangerous outbreak of lethal chemicals. Using genetically engineered organisms to cutting down manufacturing costs seems as if it will help the economy; however, genetically engineered organisms, specifically anti-material organisms, can hurt economies more than help them.
Genetic engineers possess the ability to create anti-material organisms that can degrade infrastructure and man-made materials, and malicious people can use these organisms to tear down society’s infrastructures and economies. In nature, there are many organisms with the ability to degrade infrastructure and man-made materials. These microbes cost governments and industries millions of dollars in biodeterioration and biodegradation damages. For instance, bacteria are the leading cause of road and runway deterioration. In Houston, Texas, microbes have been known to degrade the concrete in the city’s sewage systems, and the city has spent millions of dollars trying to contain the problem. High-tech companies, such as airlines and fuel companies, constantly have their facilities and machinery being degraded away by anti-material organisms. These natural organisms cause enough damage to infrastructure, and fixing the damage is expensive and time consuming (Sunshine Project 2). Similarly to the artificially made poliovirus, genetic engineers have the potential to recreate or improve these naturally occurring anti-material organisms. In theory, malicious people could unleash genetically engineered anti-material organisms on infrastructures worldwide, and this would create an expensive cleanup project for governments and companies. With these expensive damages, genetically engineered organisms can destroy economies. The same economic and environmental dangers of anti-material organisms can also be seen in genetically modified crops.
Genetically modified crops will negatively impact the economy and environment because engineered genetic resistance is ineffective at stopping natural parasites in the long term. Farmers use genetically modified crops because these crops contain a genetic resistance to parasites, such as insect pests and microbes. In evolution, two organisms that are in a parasitic relationship evolve in a balance with each other. When genetically modified plants are placed into a natural environment, parasites will evolve in a direction that allows them to bypass the genetic resistance engineered into the crops. Since the majority of crop parasites go through successive generations at a fast pace, these parasites will quickly evolve into a population that can surpass the genetic resistance. This evolutionary process makes the benefits of genetically modified crops short lived. Farmers, who pay more for genetically modified seed than natural seed, then have to pay for harmful and expensive pesticides to protect their crops. In the end, farmers will lose money due to the increased costs of buying genetically modified crops and dangerous pesticides. Also, dangerous chemicals, such as DDT, will be reintroduced into the environment (Avise 73). The ineffectiveness of genetically modified crops creates an economic and environmental risk to human society in the long run since farmers will be losing more money and introducing dangerous chemicals into the environment.
Genetically engineered organisms pose an enormous risk to human society on a lethal and economic front. Natural lethal pathogens, such as the poliovirus and Y. pestis, can be recreated or improved, and malicious people could use these genetically engineered pathogens to kill millions of people. Chemicals manufactured by genetically modified bacteria have proven to be harmful to human health, which was the case during the EMS epidemic in the United States. On an economic front, genetically engineered organisms increase costs instead of minimizing them, and they harm the environment. Anti-material organisms can be created to deteriorate infrastructures, and this would cost governments and industries millions of dollars in repair costs. Also, genetically modified crops in the long term will cost farmers more money than they save because the advantages of the genetically modified crops will be nullified by evolving parasites. Genetically engineered organisms have a huge potential to harm society. However, researching new methods and applications of genetic engineering will not stop because scientists believe in the vast opportunities of the field. In order to keep human society safe, scientists must exhaust all options before turning to the power of genetic engineering. It is an unwise idea to rely on genetic engineering since it is unpredictable and imprecise form of engineering.
Bibliography
Avise, John C. The Hope, Hype, & Reality of Genetic Engineering . Oxford: Oxford UP, 2004. Web. 14 Nov. 2010.
Benner, Steven. "Q&A: Life, Synthetic Biology and Risk." BMC Biology 8 (2010): 77. Biomed Central . 2010. Web. 13 Oct. 2010. <http://www.biomedcentral.com/1741-7007/8/77>.
“Debate: Artificial life.” http://debatepedia.idebate.org/en/index.php/Debate:_Artificial_life. N.p., 2010. Web. 20 Sept. 2010. <http://debatepedia.idebate.org/en/index.php/Debate:_Artificial_life>.
"Genetic Engineering: Too Good to Go Wrong?" Green Peace . 2000. Web. 14 Nov. 2010. <http://archive.greenpeace.org/comms/97/geneng/getoogoo.html#3gen>.
Hurlbert, R. E. "Biological Weapons; Malignant Biology." Washington State University. 2000. Web. 13 Oct. 2010.
Institute for Responsible Technology. "State of the Science on the Health Risks of GM Foods." Responsible Technology . 2007. Web. 13 Oct. 2010. <http://www.saynotogmos.org/paper.pdf>.
Kuzma, Jennifer, and Todd Tanji. "Unpackaging Synthetic Biology." Regulation & Governance 4.1 (2010): 92-112. Wiley Online Library. 2010. Web. 13 Oct. 2010. <http://onlinelibrary.wiley.com/doi/10.1111/j.1748-5991.2010.01071.x/full>.
Pickrell, John. “Introduction: GM Organisms.” New Scientist . N.p., 2010. Web. 20 Sept. 2010. <http://www.newscientist.com/article/dn9921-instant-expert-gm-organisms.html>.
Savulescu, Julian. “A matter of synthetic life and death: Venter’s artificial organism invention is fraught with peril.” New York Daily News . N.p., 2010. Web. 20 Sept. 2010. <http://www.nydailynews.com/>.
Smith, Jeffrey M. "Scrambling and Gambling with the Genome." Say No To GMOs! Aug. 2005. Web. 13 Oct. 2010. <http://www.saynotogmos.org>.
Sunshine Project. "Non-Lethal Weapons Research in the US." Sunshine Project . Mar. 2002. Web. 13 Oct. 2010. <http://www.sunshine-project.org/publications/bk/bk9en.html>.
Union of Concerned Scientists. "Risks of Genetic Engineering." Union of Concerned Scientists . 2010. Web. 14 Nov. 2010. <http://www.ucsusa.org/food_and_agriculture/science_and_impacts/impacts_genetic_engineering/risks_of_genetic_engineeringeering.html#New_Allergens_in_the_Food_Supply>.
Articles copyright © 2024 the original authors. No part of the contents of this Web journal may be reproduced or transmitted in any form without permission from the author or the Academic Writing Program of the University of Maryland. The views expressed in these essays do not represent the views of the Academic Writing Program or the University of Maryland.

Arguing For and Against Genetic Engineering
Harvard philosopher Michael Sandel recently spoke at Stanford on the subject of his new book, The Case against Perfection: Ethics in the Age of Genetic Engineering. He focused on the “ethical problems of using biomedical technologies to determine and choose from the genetic material of human embryos,” an issue that has inspired much debate.
Having followed Sandel’s writings on genetic enhancement for several years, I think that this issue deserves special thought. For many years, the specter of human genetic engineering has haunted conservatives and liberals alike. Generally, their main criticisms run thus:
First, genetic engineering limits children’s autonomy to shape their own destinies. Writer Dinesh D’Souza articulates this position in a 2001 National Review Online article: “If parents are able to remake a child’s genetic makeup, they are in a sense writing the genetic instructions that shape his entire life. If my parents give me blue eyes instead of brown eyes, if they make me tall instead of medium height, if they choose a passive over an aggressive personality, their choices will have a direct, lifelong effect on me.” In other words, genetic enhancement is immoral because it artificially molds people’s lives, often pointing their destinies in directions that they themselves would not freely choose. Therefore, it represents a fundamental violation of their rights as human beings.
Second, some fear that genetic engineering will lead to eugenics. In a 2006 column, writer Charles Colson laments: “British medical researchers recently announced plans to use cutting-edge science to eliminate a condition my family is familiar with: autism. Actually, they are not ‘curing’ autism or even making life better for autistic people. Their plan is to eliminate autism by eliminating autistic people. There is no in utero test for autism as there is for Down syndrome…[Prenatal] testing, combined with abortion-on-demand, has made people with Down syndrome an endangered population…This utilitarian view of life inevitably leads us exactly where the Nazis were creating a master race. Can’t we see it?” The logic behind this argument is that human genetic enhancement perpetuates discrimination against the disabled and the “genetically unfit,” and that this sort of discrimination is similar to the sort that inspired the eugenics of the Third Reich.
A third argument is that genetic engineering will lead to vast social inequalities. This idea is expressed in the 1997 cult film Gattaca, which portrays a society where the rich enjoy genetic enhancements—perfect eyesight, improved height, higher intelligence—that the poor cannot afford. Therefore, the main character Vincent, a man from a poor background who aspires to be an astronaut, finds it difficult to achieve his goal because he is short-sighted and has a “weak heart.” This discrepancy is exacerbated by the fact that his brother, who is genetically-engineered, enjoys perfect health and is better able to achieve his dreams. To many, Gattaca is a dystopia where vast gaps between the haves and have-nots will become intolerable, due to the existence of not just material, but also genetic inequalities.
The critics are right that a world with genetic engineering will contain inequalities. On the other hand, it is arguable that a world without genetic engineering, like this one, is even more unequal. In Gattaca, a genetically “fit” majority of people can aspire to be astronauts, but an unfortunate “unfit” minority cannot. In the real world, the situation is the other way round: the majority of people don’t have the genes to become astronauts, and only a small minority with perfect eyesight and perfect physical fitness—the Neil Armstrong types—would qualify.
The only difference is that in the real world, we try to be polite about the unpleasant realities of life by insisting that the Average Joe has, at least theoretically, a Rocky-esque chance of becoming an astronaut. In that sense, our covert discrimination is much more polite than the overt discrimination of the Gattaca variety. But it seems that our world, where genetic privilege exists naturally among a tiny minority, could conceivably be less equal (and less socially mobile) than a world with genetic engineering, where genetic enhancements would be potentially available to the majority of people, giving them a chance to create better futures for themselves. Supporters of human genetic engineering thus ask the fair question: Are natural genetic inequalities, doled out randomly and sometimes unfairly by nature, more just than engineered ones, which might be earned through good old fashioned American values like hard work, determination, and effort?
“But,” the critics ask, “wouldn’t genetic engineering lead us to eugenics?” The pro-genetic engineering crowd thinks not. They suggest that genetic engineering, if done on a purely decentralized basis by free individuals and couples, will not involve any form of coercion. Unlike the Nazi eugenics program of the 1930s, which involved the forced, widespread killing of “unfit” peoples and disabled babies, the de facto effect of genetic engineering is to cure disabilities, not kill the disabled. This is a key moral difference. As pointed out by biologist Robert Sinsheimer, genetic engineering would “permit in principle the conversion of all the ‘unfit’ to the highest genetic level.” Too often, women choose to abort babies because pre-natal testing shows that they have Down syndrome or some other ailment. If anything, genetic engineering should be welcomed by pro-life groups because by converting otherwise-disabled babies into normal, healthy ones, it would reduce the number of abortions.
In addition, the world of Gattaca, for all its faults, features a world that, far from being defined along Hitler-esque racial lines, has in fact transcended racism. Being blond-haired and blue-eyed loses its racially elitist undertones because such traits are easily available on the genetic supermarket. Hair color, skin color, and eye color become a subjective matter of choice, no more significant than the color of one’s clothes. If anything, genetic engineering will probably encourage, not discourage, racial harmony and diversity.
It is true that genetic engineering may limit children’s autonomy to shape their own destinies. But it is equally true that all people’s destinies are already limited by their natural genetic makeup, a makeup that they are born with and cannot change. A short person, for example, would be unlikely to join the basketball team because his height makes it difficult for him to compete with his tall peers. An ugly person would be unable to achieve her dream of becoming a famous actress because the lead roles are reserved for the beautiful. A myopic kid who wears glasses will find it difficult to become a pilot. A student with an IQ of 75 will be unlikely to get into Harvard however hard he tries. In some way or another, our destinies are limited by the genes we are born with.
In this sense, it is arguable that genetic engineering might help to level the playing field. Genetic engineering could give people greater innate capacity to fulfill their dreams and pursue their own happiness. Rather than allow peoples’ choices to be limited by their genetic makeup, why not give each person the capability of becoming whatever he or she wants to, and let his or her eventual success be determined by effort, willpower, and perseverance? America has long represented the idea that people can shape their own destinies. To paraphrase Dr. King, why not have a society where people are judged not by the genes they inherit, but by the content of their character?
Looking at both sides, the genetic engineering controversy does raise questions that should be answered, not shouted down. Like all major scientific advances, it probably has some negative effects, and steps must be taken to ameliorate these outcomes. For example, measures should also be taken to ensure that genetic engineering’s benefits are, at least to some extent, available to the poor. As ethicists Maxwell Mehlman and Jeffrey Botkin suggest in their book Access to the Genome: The Challenge to Equality, the rich could be taxed on genetic enhancements, and the revenue from these taxes could be used to help pay for the genetic enhancement of the poor. To some extent, this will help to ameliorate the unequal effects of genetic engineering, allowing its benefits to be more equitably distributed. In addition, caution must be taken in other areas, such as ensuring that the sanctity of human life is respected at all times. In this respect, pro-life groups like Focus on the Family can take a leading role in ensuring that scientific advances do not come at the expense of moral ethics.
At the same time, we should not allow our fear of change to prevent our society from exploring this promising new field of science, one that promises so many medical and social benefits. A strategy that defines itself against the core idea of scientific progress cannot succeed. Instead of attempting to bury our heads in the sand, we should seek to harness genetic engineering for its positive benefits, even as we take careful steps to ameliorate its potential downsides.
Home — Essay Samples — Science — Genetic Engineering — The Dangers of Genetic Engineering to Humanity
The Dangers of Genetic Engineering to Humanity
- Categories: Genetic Engineering
About this sample

Words: 308 |
Published: Aug 10, 2018
Words: 308 | Page: 1 | 2 min read

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Prof Ernest (PhD)
Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
4 pages / 1848 words
2 pages / 1086 words
4 pages / 2020 words
3 pages / 1470 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
As genetic engineering continues to advance, the controversy surrounding its potential benefits and ethical considerations has become increasingly prominent. This essay aims to provide a comprehensive analysis of genetic [...]
Introduction: In the realm of scientific innovation, genetic engineering stands at the forefront, offering unprecedented possibilities for enhancing human capabilities. The ethical implications of manipulating the very essence [...]
Genetic engineering is the process of altering the genetic makeup of an organism by adding, deleting or changing its DNA. The process involves the manipulation of genes and their transfer from one organism to another, thereby [...]
In Gattaca's dystopia, the dangers of a society obsessed with genetic perfection are exposed. The film challenges viewers to reflect on the implications of genetic determinism, the erosion of personal identity, and the ethical [...]
Genetics is the most interesting part of science because it explains how certain traits are passed down by parents to their offspring. Gregor Mendel is considered the pioneer in explaining this theory of the genetics within [...]
Genetic Engineering is a powerful and potentially very dangerous too. To change the sequence of nucleotides of the DNA that code for the structure of a complex living organism, can have extremely ill effects although the [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- General Information
- Ethical and Religious Questions
- Technical Information
"What is Genetic Engineering?" by Dr. Ricarda Steinbrecher
"The Rest Of The Story Behind Genetic Engineering: An Interview with Brian Tokar" by Mark Oshinskie
"The Biotech Century - A Second Opinion : The Marriage of the Genetic Sciences and the Technologies Reshaping Our World" by Jeremy Rifkin
"PR for the 'Book of Life'" by Jackie Stevens
"Unraveling the DNA Myth: The Spurious Foundation of Genetic Engineering" by Barry Commoner
"Rebellious Bodies Dim the Glow of `Natural' Biotech Drugs" by Andrew Pollack
"Klebsiella planticola--The Gene-Altered Monster That Almost Got Away" by Elaine Ingham
"Biohazards: The Next Generation?" by Brian Tokar
"Biotech at 25--Too Soon to Celebrate"
"Scientists Are Starting to Add Letters to Life's Alphabet" by Andrew Pollack
"Scientists Admit Frankencrops Pollution is Inevitable" by Brian Tokar
"Genetically-Modified Superweeds 'Not Uncommon'" by James Randerson
"Pharm Crops - A Food Accident Waiting to Happen"
"GM Potatoes Deter One Pest But Attract Another"
"Sterile Harvest: New Crop of Terminator Patents Threatens Food Sovereignty"
"Biotechnology in Crops: Issues for the Developing World" compiled by Laura Spinney
" GM Crop DNA Found in Human Gut Bugs" by Andy Coghlin
"Genetically Modified Foods: Are They a Risk to Human/Animal Health?" By Arpad Pusztai, Ph.D.
"50 Harmful Effects of Genetically Modified Foods" by Nathan Batalion
"Biotech--The Basics" by Rachel Massey
"GM corn set to stop man spreading his seed" by Robin McKie
"GM Trials to Find Medicine Raise New Ethical Fears; Human Gene Crop Fury" by John Ingham and Toby Moore
"NASA's Earth plants could invade Mars" by David Perlman
USDA Says Yes to Terminator
GENETIC ENGINEERING AND BIOWARFARE
"The Demon in the Freezer" by Richard Preston
"Ebola Virus Could Be Synthesised" by Sylvia Pag�n Westphal
"Scientists Create a Live Polio Virus" by Andrew Pollack
"Bioterror And Biosafety" by Vandana Shiva
"US Non-lethal Weapon Reports Suppressed" by Debora MacKenzie
"Single Gene Leap Led to Flea-Borne Transmission of Plague Bacterium" by Laurie K. Doepel
"A Terrifying Power" by Philip Cohen
"With Biotechnology, a Potential to Harm" by Andrew Pollack
"Scientists Fear Miracle of Biotech Could Also Breed a Monster "
Project Censored: Human Genome Project Opens the Door to Ethnically Specific Bioweapons
"U.S. Germ Warfare Research Pushes Treaty Limits" by Judith Miller, Stephen Engelberg and William J. Broad
"Battlefield uses of biotech proposed in report to Army" by Carl T. Hall
GENETICALLY ENGINEERING HUMAN BEINGS
"Engineering Humans" by Rachel Massey
"The New Eugenics: The Case Against Genetically Modified Humans" by Marcy Darnovsky
"Scientists Raise Spectre of Gene-Modified Athletes" by James Randerson
"The Threshold Challenge of the New Human Genetic Technologies"
"Governing the Genome" by Ralph Brave
"The Human Genome Map: The Death of Genetic Determinism and Beyond" by Mae-Wan Ho
Boy's DNA implanted in rabbit eggs" By Roger Highfield
132 Genetic Engineering Essay Topic Ideas & Examples
Welcome to our list of genetic engineering essay topics! Here, you will find everything from trending research titles to the most interesting genetic engineering topics for presentation. Get inspired with our writing ideas and bonus samples!
🔝 Top 10 Genetic Engineering Topics for 2024
🏆 best genetic engineering topic ideas & essay examples, ⭐ good genetic engineering research topics, 👍 simple & easy genetic engineering essay topics, ❓ genetic engineering discussion questions, 🔎 genetic engineering research topics, ✅ genetic engineering project ideas.
- Ethical Issues of Synthetic Biology
- CRISPR-Cas9 and Its Applications
- Progress and Challenges in Gene Therapy
- Applications of Gene Editing in Animals
- The Process of Genetic Engineering in Plants
- Genetic Engineering for Human Enhancement
- Genetic Engineering for Improving Crop Yield
- Regulatory Issues of Genetic Editing of Embryos
- Gene Silencing in Humans through RNA Interference
- Gene Drive Technology for Controlling Invasive Species
- The Film “Gattaca” and Genetic Engineering In the film, it is convincing that in the near future, science and technology at the back of genetic engineering shall be developed up to the level which makes the film a reality.
- The Ethical Issues of Genetic Engineering Many people have questioned the health risks that arise from genetically modified crops, thus it is the politicians who have to ensure that the interests of the people are met and their safety is assured. […]
- A Major Milestone in the Field of Science and Technology: Should Genetic Engineering Be Allowed? The most controversial and complicated aspect of this expertise is Human Genetic Engineering- whereby the genotype of a fetus can be altered to produce desired results.
- Changing the world: Genetic Engineering Effects Genes used in genetic engineering have a high impact on health and disease, therefore the inclusion of the genetic process alters the genes that influence human behavior and traits.
- Religious vs Scientific Views on Genetic Engineering With the need to increase the global economy, the field of agriculture is one among the many that have been used to improve the commercial production to take care of the global needs for food […]
- The Dangers of Genetic Engineering and the Issue of Human Genes’ Modification In this case, the ethics of human cloning and human genes’ alteration are at the center of the most heated debates. The first reason to oppose the idea of manipulation of human genes lies in […]
- Is Genetic Engineering an Environmentally Sound Way to Increase Food Production? According to Thomas & Earl and Barry, genetic engineering is environmentally unsound method of increasing food production because it threatens the indigenous species.
- Human Genetic Engineering: Key Principles and Issues There are many options for the development of events in the field of genetic engineering, and not all of them have been studied. To conclude, human genetic engineering is one of the major medical breakthroughs, […]
- Mitochondrial Diseases Treatment Through Genetic Engineering Any disorders and abnormalities in the development of mitochondrial genetic information can lead to the dysfunction of these organelles, which in turn affects the efficiency of intracellular ATP production during the process of cellular respiration.
- Genetic Engineering: Is It Ethical to Manipulate Life? In the case of more complex operations, genetic engineering can edit existing genes to turn on or off the synthesis of a particular protein in the organism from which the gene was taken.
- Biotechnology and Genetic Engineering Apart from that, there are some experiments that cannot be ethically justified, at least in my opinion, for example, the cloning of human being or the attempts to find the gene for genius.
- Genetic Engineering in the Movie “Gattaca” by Niccol This would not be right at all since a person should be responsible for their own life and not have it dictated to them as a result of a societal construct created on the basis […]
- Genetic Engineering Using a Pglo Plasmid The objective of this experiment is to understand the process and importance of the genetic transformation of bacteria in real time with the aid of extrachromosomal DNA, alternatively referred to as plasmids.
- Managing Diabetes Through Genetic Engineering Genetic engineering refers to the alteration of genetic make-up of an organism through the use of techniques to introduce a new DNA or eliminate a given hereditable material. What is the role of genetic engineering […]
- The Role of Plant Genetic Engineering in Global Security Although it can be conveniently stated that the adequacy, abundance and reliability of the global food supply has a major role to play in the enhancement of human life, in the long run, they influence […]
- Significance of Human Genetic Engineering The gene alteration strategy enables replacing the specific unwanted genes with the new ones, which are more resistant and freer of the particular ailment, hence an essential assurance of a healthy generation in the future.
- Is the World Ready for Genetic Engineering? The process of manipulating genes has brought scientists to important discoveries, among which is the technology of the production of new kinds of crops and plants with selected characteristics. The problem of the advantages and […]
- Genome: Bioethics and Genetic Engineering Additionally, towards the end of the documentary, the narrator and some of the interviewed individuals explain the problem of anonymity that is also related to genetic manipulations.
- Gattaca: Ethical Issues of Genetic Engineering Although the world he lives in has determined that the only measure of a man is his genetic profile, Vincent discovers another element of man that science and society have forgotten.
- Genetic Engineering Is Ethically Unacceptable However, the current application of genetic engineering is in the field of medicine particularly to treat various genetic conditions. However, this method of treatment has various consequences to the individual and the society in general.
- Designer Genes: Different Types and Use of Genetic Engineering McKibben speaks of Somatic Gene Therapy as it is used to modify the gene and cell structure of human beings so that the cells are able to produce certain chemicals that would help the body […]
- A Technique for Controlling Plant Characteristics: Genetic Engineering in the Agriculture A cautious investigation of genetic engineering is required to make sure it is safe for humans and the environment. The benefit credited to genetic manipulation is influenced through the utilization of herbicide-tolerant and pest-safe traits.
- Genetically Engineered Food Against World Hunger I support the production of GMFs in large quality; I hold the opinion that they can offer a lasting solution to food problems facing the world.
- Genetic Engineering in Food: Development and Risks Genetic engineering refers to the manipulation of the gene composition of organisms, to come up with organisms, which have different characteristics from the organic ones.
- Genetic Engineering in the Workplace The main purpose of the paper is to evaluate and critically discuss the ethical concerns regarding the implementation of genetic testing in the workplace and to provide potential resolutions to the dilemmas.
- Designer Babies Creation in Genetic Engineering The creation of designer babies is an outcome of advancements in technology hence the debate should be on the extent to which technology can be applied in changing the way human beings live and the […]
- Genetic Engineering and Eugenics Comparison The main idea in genetic engineering is to manipulate the genetic make-up of human beings in order to shackle their inferior traits. The concept of socially independent reproduction is replicated in both eugenics and genetic […]
- Future of Genetic Engineering and the Concept of “Franken-Foods” This is not limited to cows alone but extends to pigs, sheep, and poultry, the justification for the development of genetically modified food is based on the need to feed an ever growing population which […]
- Ecological Effects of the Release of Genetically Engineered Organisms Beneficial soil organisms such as earthworms, mites, nematodes, woodlice among others are some of the soil living organisms that are adversely affected by introduction of genetically engineered organisms in the ecosystem since they introduce toxins […]
- Proposition 37 and Genetically Engineered Foods The discussion of Proposition 37 by the public is based on the obvious gap between the “law on the books” and the “law in action” because Food Safety Law which is associated with the Proposition […]
- Is Genetically Engineered Food the Solution to the World’s Hunger Problems? However, the acceptance of GMO’s as the solution to the world’s food problem is not unanimously and there is still a multitude of opposition and suspicion of their use.
- Benefits of Genetic Engineering as a Huge Part of People’s Lives Genetic Engineering is said to question whether man has the right to manipulate the course and laws of nature and thus is in constant collision with religion and the beliefs held by it regarding life.
- Perfect Society: The Effects of Human Genetic Engineering
- Genetic Engineering and Forensic Criminal Investigations
- Biotechnology Assignment and Genetic Engineering
- Genetic Engineering and Genetically Modified Organisms
- Bio-Ethics and the Controversy of Genetic Engineering
- Health and Environmental Risks of Genetic Engineering in Food
- Genetic Engineering and the Risks of Enforcing Changes on Organisms
- Genetic Engineering and How It Affects Globel Warming
- Cloning and Genetic Engineering in the Food Animal Industry
- Genetic Engineering and Its Impact on Society
- Embryonic Research, Genetic Engineering, & Cloning
- Genetic Engineering: Associated Risks and Possibilities
- Issues Concerning Genetic Engineering in Food Production
- Genetic Engineering, DNA Fingerprinting, Gene Therapy
- Cloning: The Benefits and Dangers of Genetic Engineering
- Genetic Engineering, History, and Future: Altering the Face of Science
- Islamic and Catholic Views on Genetic Engineering
- Gene Therapy and Genetic Engineering: Should It Be Approved in the US
- Exploring the Real Benefits of Genetic Engineering in the Modern World
- Genetic Engineering and Food Security: A Welfare Economics Perspective
- Identify the Potential Impact of Genetic Engineering on the Future Course of Human Immunodeficiency Virus
- Genetic Engineering and DNA Technology in Agricultural Productivity
- Human Genetic Engineering: Designing the Future
- Genetic Engineering and the Politics Behind It
- The Potential and Consequences of Genetic Engineering
- Genetic Engineering and Its Effect on Human Health
- The Moral and Ethical Controversies, Benefits, and Future of Genetic Engineering
- Gene Therapy and Genetic Engineering for Curing Disorders
- Genetic Engineering and the Human Genome Project
- Ethical Standards for Genetic Engineering
- Genetic Engineering and Cryonic Freezing: A Modern Frankenstein
- The Perfect Child: Genetic Engineering
- Genetic Engineering and Its Effects on Future Generations
- Agricultural Genetic Engineering: Genetically Modified Foods
- Genetic Engineering: The Manipulation or Alteration of the Genetic Structure of a Single Cell or Organism
- Analysing Genetic Engineering Regarding Plato Philosophy
- The Dangers and Benefits of Human Cloning and Genetic Engineering
- Genetic Engineering: Arguments of Both Proponents and Opponents and a Mediated Solution
- Genetic and How Genetic Engineering Is Diffusing Individualism
- Finding Genetic Harmony With Genetic Engineering
- What Is Genetic Engineering?
- Do You Think Genetically Modified Food Could Harm the Ecosystems of the Areas in Which They Grow?
- How Agricultural Research Systems Shape a Technological Regime That Develops Genetic Engineering?
- Can Genetic Engineering for the Poor Pay Off?
- How Does Genetic Engineering Affect Agriculture?
- Do You Think It’s Essential to Modify Genes to Create New Medicines?
- How Can Genetic Engineering Stop Human Suffering?
- Can Genetic Engineering Cure HIV/AIDS in Humans?
- How Has Genetic Engineering Revolutionized Science and the World?
- Do You Think Genetic Engineering Is Playing God and That We Should Leave Life as It Was Created?
- What Are Some Advantages and Disadvantages of Genetic Engineering?
- How Will Genetic Engineering Affect the Human Race?
- When Does Genetic Engineering Go Bad?
- What Are the Benefits of Human Genetic Engineering?
- Does Genetic Engineering Affect the Entire World?
- How Does the Christian Faith Contend With Genetic Engineering?
- What Are the Ethical and Social Implications of Genetic Engineering?
- How Will Genetic Engineering Impact Our Lives?
- Why Should Genetic Engineering Be Extended?
- Will Genetic Engineering Permanently Change Our Society?
- What Are People Worried About Who Oppose Genetic Engineering?
- Do You Worry About Eating GM (Genetically Modified) Food?
- What Do You Think of the Idea of Genetically Engineering New Bodily Organs to Replace Yours When You Are Old?
- Should Genetic Engineering Go Ahead to Eliminate Human Flaws, Such as Violence, Jealousy, Hate, Etc?
- Does the Government Have the Right to Limit How Far We Modify Ourselves?
- Why Is Genetic Food Not Well Accepted?
- What Is the Best in the Genetic Modification of Plants, Plant Cell, or Chloroplasts and Why?
- How Do You Feel About Human Gene Editing?
- Does Climate Change Make the Genetic Engineering of Crops Inevitable?
- What Do You Think About Plant Genetic Modification?
- Gene Drives and Pest Control
- The Benefits of Genetically Modified Organisms
- Challenges of Gene Editing for Rare Genetic Diseases
- The Use of Genetic Engineering to Treat Human Diseases
- Ethical Considerations and Possibilities of Designer Babies
- How Genetic Engineering Can Help Restore Ecosystems
- Basic Techniques and Tools for Gene Manipulation
- Latest Advancements in Genetic Engineering and Genome Editing
- Will Engineering Resilient Organisms Help Mitigate Climate Change?
- Creation of Renewable Resources through Genetic Engineering
- Genetic Engineering Approach to Drought and Pest Resistance
- Genetic Engineering Use in DNA Analysis and Identification
- Synthetic Microorganisms and Biofactories for Sustainable Bioproduction
- Stem Cells’ Potential for Regenerative Medicine
- The Role of Genetic Modification in Vaccine Development
- Can Genetic Engineering Help Eradicate Invasive Species Responsibly?
- Genetic Engineering for Enhancing the Body’s Defense Mechanisms
- Advancements in Transplantation Medicine and Creating Bioengineered Organs
- Genetic Editing of Microbes for Environmental Cleanup
- Is It Possible to Develop Living Detection Systems?
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 26). 132 Genetic Engineering Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/
"132 Genetic Engineering Essay Topic Ideas & Examples." IvyPanda , 26 Feb. 2024, ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
IvyPanda . (2024) '132 Genetic Engineering Essay Topic Ideas & Examples'. 26 February.
IvyPanda . 2024. "132 Genetic Engineering Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
1. IvyPanda . "132 Genetic Engineering Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
Bibliography
IvyPanda . "132 Genetic Engineering Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
- Animal Ethics Research Ideas
- Cloning Questions
- DNA Essay Ideas
- Eugenics Questions
- Extinction Research Topics
- Gene Titles
- Infertility Essay Topics
- Bioethics Titles
- Genetics Research Ideas
- Epigenetics Essay Titles
- Morality Research Ideas
- Stem Cell Essay Titles
- Biochemistry Research Topics
- Evolution Topics
studyacademia.com
Buy this domain.
The domain studyacademia.com may be for sale by its owner!
This webpage was generated by the domain owner using Sedo Domain Parking . Disclaimer: Sedo maintains no relationship with third party advertisers. Reference to any specific service or trade mark is not controlled by Sedo nor does it constitute or imply its association, endorsement or recommendation.
- Search Menu
- Volume 12, Issue 1, 2024 (In Progress)
- Volume 11, Issue 1, 2023
- Advance articles
- Editor's Choice
- Virtual Issues
- Clinical Briefs
- Author Guidelines
- Submission Site
- Open Access
- Calls for Papers
- Why submit?
- About Evolution, Medicine, and Public Health
- About the International Society for Evolution, Medicine and Public Health
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- For Reviewers
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, human enhancement, genetic engineering, conclusions.
- < Previous
Human enhancement: Genetic engineering and evolution
- Article contents
- Figures & tables
- Supplementary Data
Mara Almeida, Rui Diogo, Human enhancement: Genetic engineering and evolution, Evolution, Medicine, and Public Health , Volume 2019, Issue 1, 2019, Pages 183–189, https://doi.org/10.1093/emph/eoz026
- Permissions Icon Permissions
Genetic engineering opens new possibilities for biomedical enhancement requiring ethical, societal and practical considerations to evaluate its implications for human biology, human evolution and our natural environment. In this Commentary, we consider human enhancement, and in particular, we explore genetic enhancement in an evolutionary context. In summarizing key open questions, we highlight the importance of acknowledging multiple effects (pleiotropy) and complex epigenetic interactions among genotype, phenotype and ecology, and the need to consider the unit of impact not only to the human body but also to human populations and their natural environment (systems biology). We also propose that a practicable distinction between ‘therapy’ and ‘enhancement’ may need to be drawn and effectively implemented in future regulations. Overall, we suggest that it is essential for ethical, philosophical and policy discussions on human enhancement to consider the empirical evidence provided by evolutionary biology, developmental biology and other disciplines.
Lay Summary: This Commentary explores genetic enhancement in an evolutionary context. We highlight the multiple effects associated with germline heritable genetic intervention, the need to consider the unit of impact to human populations and their natural environment, and propose that a practicable distinction between ‘therapy’ and ‘enhancement’ is needed.
There are countless examples where technology has contributed to ameliorate the lives of people by improving their inherent or acquired capabilities. For example, over time, there have been biomedical interventions attempting to restore functions that are deficient, such as vision, hearing or mobility. If we consider human vision, substantial advances started from the time spectacles were developed (possibly in the 13th century), continuing in the last few years, with researchers implanting artificial retinas to give blind patients partial sight [ 1–3 ]. Recently, scientists have also successfully linked the brain of a paralysed man to a computer chip, which helped restore partial movement of limbs previously non-responsive [ 4 , 5 ]. In addition, synthetic blood substitutes have been created, which could be used in human patients in the future [ 6–8 ].
The progress being made by technology in a restorative and therapeutic context could in theory be applied in other contexts to treat non-pathological conditions. Many of the technologies and pharmaceutical products developed in a medical context to treat patients are already being used by humans to ‘enhance’ some aspect of their bodies, for example drugs to boost brain power, nutritional supplements, brain stimulating technologies to control mood or growth hormones for children of short stature. Assistive technology for disabled people, reproductive medicine and pharmacology, beside their therapeutic and restorative use, have a greater potential for human ‘enhancement’ than currently thought. There are also dual outcomes as some therapies can have effects that amount to an enhancement as for example, the artificial legs used by the South African sprinter Oscar Pistorius providing him with a competitive advantage.
This commentary will provide general ethical considerations on human enhancement, and within the several forms of so-called human biomedical enhancement, it will focus on genetic engineering, particularly on germline (heritable) genetic interventions and on the insights evolutionary biology can provide in rationalizing its likely impact. These insights are a subject often limited in discussions on genetic engineering and human enhancement in general, and its links to ethical, philosophical and policy discussions, in particular [ 9 ]. The rapid advances in genetic technology make this debate very topical. Moreover, genes are thought to play a very substantial role in biological evolution and development of the human species, thus making this a topic requiring due consideration. With this commentary, we explore how concepts based in evolutionary biology could contribute to better assess the implications of human germline modifications, assuming they were widely employed. We conclude our brief analysis by summarizing key issues requiring resolution and potential approaches to progress them. Overall, the aim is to contribute to the debate on human genetic enhancement by looking not only at the future, as it is so often done, but also at our evolutionary past.
The noun ‘enhancement’ comes from the verb ‘enhance’, meaning ‘to increase or improve’. The verb enhance can be traced back to the vulgar Latin inaltiare and late Latin inaltare (‘raise, exalt’), from ‘ altare ’ (‘make high’) and altus (‘high’), literally ‘grown tall’. For centuries human enhancement has populated our imagination outlined by stories ranging from the myths of supernormal strengths and eternal life to the superpowers illustrated by the 20th century comic books superheroes. The desire of overcoming normal human capacities and the transformation to an almost ‘perfect’ form has been part of the history of civilization, extending from arts and religion to philosophy. The goal of improving the human condition and health has always been a driver for innovation and biomedical developments.
In the broadest sense, the process of human enhancement can be considered as an improvement of the ‘limitations’ of a ‘natural version’ of the human species with respect to a specific reference in time, and to different environments, which can vary depending on factors such as, for example, climate change. The limitations of the human condition can be physical and/or mental/cognitive (e.g. vision, strength or memory). This poses relevant questions of what a real or perceived human limitation is in the environment and times in which we are living and how it can be shifted over time considering social norms and cultural values of modern societies. Besides, the impact that overcoming these limitations will have on us humans, and the environment, should also be considered. For example, if we boost the immune system of specific people, this may contribute to the development/evolution of more resistant viruses and bacteria or/and lead to new viruses and bacteria to emerge. In environmental terms, enhancing the longevity of humans could contribute to a massive increase in global population, creating additional pressures on ecosystems already under human pressure.
Two decades ago, the practices of human enhancement have been described as ‘biomedical interventions that are used to improve human form or functioning beyond what is necessary to restore or sustain health’ [ 10 ]. The range of these practices has now increased with technological development, and they are ‘any kind of genetic, biomedical, or pharmaceutical intervention aimed at improving human dispositions, capacities, or well-being, even if there is no pathology to be treated’ [ 11 ]. Practices of human enhancement could be visualized as upgrading a ‘system’, where interventions take place for a better performance of the original system. This is far from being a hypothetical situation. The rapid progress within the fields of nanotechnology, biotechnology, information technology and cognitive science has brought back discussions about the evolutionary trajectory of the human species by the promise of new applications which could provide abilities beyond current ones [ 12 , 13 ]. If such a possibility was consciously embraced and actively pursued, technology could be expected to have a revolutionary interference with human life, not just helping humans in achieving general health and capabilities commensurate with our current ones but helping to overcome human limitations far beyond of what is currently possible for human beings. The emergence of new technologies has provided a broader range of potential human interventions and the possibility of transitioning from external changes to our bodies (e.g. external prosthesis) to internal ones, especially when considering genetic manipulation, whose changes can be permanent and transmissible.
The advocates of a far-reaching human enhancement have been referred to as ‘transhumanists’. In their vision, so far, humans have largely worked to control and shape their exterior environments (niche construction) but with new technologies (e.g. biotechnology, information technology and nanotechnology) they will soon be able to control and fundamentally change their own bodies. Supporters of these technologies agree with the possibility of a more radical interference in human life by using technology to overcome human limitations [ 14–16 ], that could allow us to live longer, healthier and even happier lives [ 17 ]. On the other side, and against this position, are the so-called ‘bioconservatives’, arguing for the conservation and protection of some kind of ‘human essence’, with the argument that it exists something intrinsically valuable in human life that should be preserved [ 18 , 19 ].
There is an ongoing debate between transhumanists [ 20–22 ] and bioconservatives [ 18 , 19 , 23 ] on the ethical issues regarding the use of technologies in humans. The focus of this commentary is not centred on this debate, particularly because the discussion of these extreme, divergent positions is already very prominent in the public debate. In fact, it is interesting to notice that the ‘moderate’ discourses around this topic are much less known. In a more moderate view, perhaps one of the crucial questions to consider, independently of the moral views on human enhancement, is whether human enhancement (especially if considering germline heritable genetic interventions) is a necessary development, and represents an appropriate use of time, funding and resources compared to other pressing societal issues. It is crucial to build space for these more moderate, and perhaps less polarized voices, allowing the consideration of other positions and visions beyond those being more strongly projected so far.
Ethical and societal discussions on what constitutes human enhancement will be fundamental to support the development of policy frameworks and regulations on new technological developments. When considering the ethical implications of human enhancement that technology will be available to offer now and in the future, it could be useful to group the different kinds of human enhancements in the phenotypic and genetic categories: (i) strictly phenotypic intervention (e.g. ranging from infrared vision spectacles to exoskeletons and bionic limbs); (ii) somatic, non-heritable genetic intervention (e.g. editing of muscle cells for stronger muscles) and (iii) germline, heritable genetic intervention (e.g. editing of the C–C chemokine receptor type 5 (CCR5) gene in the Chinese baby twins, discussed later on). These categories of enhancement raise different considerations and concerns and currently present different levels of acceptance by our society. The degree of ethical, societal and environmental impacts is likely to be more limited for phenotypic interventions (i) but higher for genetic interventions (ii and iii), especially for the ones which are transmissible to future generations (iii).
The rapid advances in technology seen in the last decades, have raised the possibility of ‘radical enhancement’, defined by Nicholas Agar, ‘as the improvement of human attributes and abilities to levels that greatly exceed what is currently possible for human beings’ [ 24 ]. Genetic engineering offers the possibility of such an enhancement by providing humans a profound control over their own biology. Among other technologies, genetic engineering comprises genome editing (also called gene editing), a group of technologies with the ability to directly modify an organism’s DNA through a targeted intervention in the genome (e.g. insertion, deletion or replacement of specific genetic material) [ 25 ]. Genome editing is considered to achieve much greater precision than pre-existing forms of genetic engineering. It has been argued to be a revolutionary tool due to its efficiency, reducing cost and time. This technology is considered to have many applications for human health, in both preventing and tackling disease. Much of the ethical debate associated with this technology concerns the possible application of genome editing in the human germline, i.e. the genome that can be transmitted to following generations, be it from gametes, a fertilized egg or from first embryo divisions [ 26–28 ]. There has been concern as well as enthusiasm on the potential of the technology to modify human germline genome to provide us with traits considered positive or useful (e.g. muscle strength, memory and intelligence) in the current and future environments.
Genetic engineering: therapy or enhancement and predictability of outcomes
To explore some of the possible implications of heritable interventions we will take as an example the editing (more specifically ‘deletion’ using CRISPR genome editing technology) of several base pairs of the CCR5 gene. Such intervention was practised in 2018 in two non-identical twin girls born in China. Loss of function mutations of the CCR5 had been previously shown to provide resistance to HIV. Therefore, the gene deletion would be expected to protect the twin baby girls from risk of transmission of HIV which could have occurred from their father (HIV-positive). However, the father had the infection kept under control and the titre of HIV virus was undetectable, which means that risk of transmission of HIV infection to the babies was negligible [ 29 ].
From an ethical ground, based on current acceptable practices, this case has been widely criticized by the scientific community beside being considered by many a case of human enhancement intervention rather than therapy [ 29 , 30 ]. One of the questions this example helps illustrate is that the ethical boundary between a therapy that ‘corrects’ a disorder by restoring performance to a ‘normal’ scope, and an intervention that ‘enhances’ human ability outside the accepted ‘normal’ scope, is not always easy to draw. For the sake of argument, it could be assumed that therapy involves attempts to restore a certain condition of health, normality or sanity of the ‘natural’ condition of a specific individual. If we take this approach, the question is how health, normality and sanity, as well as natural per se, are defined, as the meaning of these concepts shift over time to accommodate social norms and cultural values of modern societies. It could be said that the difficulty of developing a conceptual distinction between therapy and enhancement has always been present. However, the potential significance of such distinction is only now, with the acceleration and impact of technological developments, becoming more evident.
Beyond ethical questions, a major problem of this intervention is that we do not (yet?) know exactly the totality of the effects that the artificial mutation of the CCR5 may have, at both the genetic and phenotypic levels. This is because we now know that, contrary to the idea of ‘one gene-one trait’ accepted some decades ago, a gene—or its absence—can affect numerous traits, many of them being apparently unrelated (a phenomenon also known as pleiotropy). That is, due to constrained developmental interactions, mechanisms and genetic networks, a change in a single gene can result in a cascade of multiple effects [ 31 ]. In the case of CCR5, we currently know that the mutation offers protection against HIV infection, and also seems to increase the risk of severe or fatal reactions to some infectious diseases, such as the influenza virus [ 32 ]. It has also been observed that among people with multiple sclerosis, the ones with CCR5 mutation are twice as likely to die early than are people without the mutation [ 33 ]. Some studies have also shown that defective CCR5 can have a positive effect in cognition to enhance learning and memory in mice [ 34 ]. However, it’s not clear if this effect would be translated into humans. The example serves to illustrate that, even if human enhancement with gene editing methods was considered ethically sound, assessing the totality of its implications on solid grounds may be difficult to achieve.
Genetic engineering and human evolution: large-scale impacts
Beyond providing the opportunity of enhancing human capabilities in specific individuals, intervening in the germline is likely to have an impact on the evolutionary processes of the human species raising questions on the scale and type of impacts. In fact, the use of large-scale genetic engineering might exponentially increase the force of ‘niche construction’ in human evolution, and therefore raise ethical and practical questions never faced by our species before. It has been argued that natural selection is a mechanism of lesser importance in the case of current human evolution, as compared to other organisms, because of advances in medicine and healthcare [ 35 ]. According to such a view, among many others advances, natural selection has been conditioned by our ‘niche-construction’ ability to improve healthcare and access to clean water and food, thus changing the landscape of pressures that humans have been facing for survival. An underlying assumption or position of the current debate is that, within our human species, the force of natural selection became minimized and that we are somehow at the ‘end-point’ of our evolution [ 36 ]. If this premise holds true, one could argue that evolution is no longer a force in human history and hence that any human enhancement would not be substituting itself to human evolution as a key driver for future changes.
However, it is useful to remember that, as defined by Darwin in his book ‘On the Origin of the Species’, natural selection is a process in which organisms that happen to be ‘better’ adapted to a certain environment tend to have higher survival and/or reproductive rates than other organisms [ 37 ]. When comparing human evolution to human genetic enhancement, an acceptable position could be to consider ethically sound those interventions that could be replicated naturally by evolution, as in the case of the CCR5 gene. Even if this approach was taken, however, it is important to bear in mind that human evolution acts on human traits sometimes increasing and sometimes decreasing our biological fitness, in a constant evolutionary trade-off and in a contingent and/or neutral—in the sense of not ‘progressive’—process. In other worlds, differently from genetic human enhancement, natural selection does not ‘ aim ’ at improving human traits [ 38 ]. Human evolution and the so-called genetic human enhancement would seem therefore to involve different underlying processes, raising several questions regarding the implications and risks of the latter.
But using genetic engineering to treat humans has been proposed far beyond the therapeutic case or to introduce genetic modifications known to already occur in nature. In particular, when looking into the views expressed on the balance between human evolution and genetic engineering, some argue that it may be appropriate to use genetic interventions to go beyond what natural selection has contributed to our species when it comes to eradicate vulnerabilities [ 17 ]. Furthermore, when considering the environmental, ecological and social issues of contemporary times, some suggest that genetic technologies could be crucial tools to contribute to human survival and well-being [ 20–22 ]. The possible need to ‘engineer’ human traits to ensure our survival could include the ability to allow our species to adapt rapidly to the rate of environmental change caused by human activity, for which Darwinian evolution may be too slow [ 39 ]. Or, for instance, to support long-distance space travel by engineering resistance to radiation and osteoporosis, along with other conditions which would be highly advantageous in space [ 40 ].
When considering the ethical and societal merits of these propositions, it is useful to consider how proto-forms of enhancement has been approached by past human societies. In particular, it can be argued that humans have already employed—as part of our domestication/‘selective breeding’ of other animals—techniques of indirect manipulation of genomes on a relatively large scale over many millennia, albeit not on humans. The large-scale selective breeding of plants and animals over prehistoric and historic periods could be claimed to have already shaped some of our natural environment. Selective breeding has been used to obtain specific characteristics considered useful at a given time in plants and animals. Therefore, their evolutionary processes have been altered with the aim to produce lineages with advantageous traits, which contributed to the evolution of different domesticated species. However, differently from genetic engineering, domestication possesses inherent limitations in its ability to produce major transformations in the created lineages, in contrast with the many open possibilities provided by genetic engineering.
When considering the impact of genetic engineering on human evolution, one of questions to be considered concerns the effects, if any, that genetic technology could have on the genetic pool of the human population and any implication on its resilience to unforeseen circumstances. This underlines a relevant question associated with the difference between ‘health’ and biological fitness. For example, a certain group of animals can be more ‘healthy’—as domesticated dogs—but be less biologically ‘fit’ according to Darwin’s definition. Specifically, if such group of animals are less genetically diverse than their ancestors, they could be less ‘adaptable’ to environmental changes. Assuming that, the human germline modification is undertaken at a global scale, this could be expected to have an effect, on the distribution of genetically heritable traits on the human population over time. Considering that gene and trait distributions have been changing under the processes of evolution for billions of years, the impact on evolution will need to be assessed by analysing which genetic alterations have been eventually associated with specific changes within the recent evolutionary history of humans. On this front, a key study has analysed the implications of genetic engineering on the evolutionary biology of human populations, including the possibility of reducing human genetic diversity, for instance creating a ‘biological monoculture’ [ 41 ]. The study argued that genetic engineering will have an insignificant impact on human diversity, while it would likely safeguard the capacity of human populations to deal with disease and new environmental challenges and therefore, ensure the health and longevity of our species [ 41 ]. If the findings of this study were considered consistent with other knowledge and encompassing, the impact of human genetic enhancements on the human genetic pool and associated impacts could be considered secondary aspects. However, data available from studies on domestication strongly suggests that domestication of both animals and plans might lead to not only decreased genetic diversity per se, but even affect patterns of variation in gene expression throughout the genome and generally decreased gene expression diversity across species [ 42–44 ]. Given that, according to recent studies within the field of biological anthropology recent human evolution has been in fact a process of ‘self-domestication’ [ 45 ], one could argue that studies on domestication could contribute to understanding the impacts of genetic engineering.
Beyond such considerations, it is useful to reflect on the fact that human genetic enhancement could occur on different geographical scales, regardless of the specific environment and geological periods in which humans are living and much more rapidly than in the case of evolution, in which changes are very slow. If this was to occur routinely and on a large scale, the implications of the resulting radical and abrupt changes may be difficult to predict and its impacts difficult to manage. This is currently highlighted by results of epigenetics studies, and also of the microbiome and of the effects of pollutants in the environment and their cumulative effect on the development of human and non-human organisms alike. Increasingly new evidence indicates a greater interdependence between humans and their environments (including other microorganisms), indicating that modifying the environment can have direct and unpredictable consequences on humans as well. This highlight the need of a ‘systems level’ approach. An approach in which the ‘bounded body’ of the individual human as a basic unit of biological or social action would need to be questioned in favour of a more encompassing and holistic unit. In fact, within biology, there is a new field, Systems Biology, which stresses the need to understand the role that pleiotropy, and thus networks at multiple levels—e.g. genetic, cellular, among individuals and among different taxa—play within biological systems and their evolution [ 46 ]. Currently, much still needs to be understood about gene function, its role in human biological systems and the interaction between genes and external factors such as environment, diet and so on. In the future if we do choose to genetically enhance human traits to levels unlikely to be achieved by human evolution, it would be crucial to consider if and how our understanding of human evolution enable us to better understand the implications of genetic interventions.
New forms of human enhancement are increasingly coming to play due to technological development. If phenotypic and somatic interventions for human enhancement pose already significant ethical and societal challenges, germline heritable genetic intervention, require much broader and complex considerations at the level of the individual, society and human species as a whole. Germline interventions associated with modern technologies are capable of much more rapid, large-scale impacts and seem capable of radically altering the balance of humans with the environment. We know now that beside the role genes play on biological evolution and development, genetic interventions can induce multiple effects (pleiotropy) and complex epigenetics interactions among genotype, phenotype and ecology of a certain environment. As a result of the rapidity and scale with which such impact could be realized, it is essential for ethical and societal debates, as well as underlying scientific studies, to consider the unit of impact not only to the human body but also to human populations and their natural environment (systems biology). An important practicable distinction between ‘therapy’ and ‘enhancement’ may need to be drawn and effectively implemented in future regulations, although a distinct line between the two may be difficult to draw.
In the future if we do choose to genetically enhance human traits to levels unlikely to be achieved by human evolution, it would be crucial to consider if and how our understanding of humans and other organisms, including domesticated ones, enable us to better understand the implications of genetic interventions. In particular, effective regulation of genetic engineering may need to be based on a deep knowledge of the exact links between phenotype and genotype, as well the interaction of the human species with the environment and vice versa .
For a broader and consistent debate, it will be essential for technological, philosophical, ethical and policy discussions on human enhancement to consider the empirical evidence provided by evolutionary biology, developmental biology and other disciplines.
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) of Portugal [CFCUL/FIL/00678/2019 to M.A.].
Conflict of interest : None declared.
Pham P , Roux S , Matonti F et al. Post-implantation impedance spectroscopy of subretinal micro-electrode arrays, OCT imaging and numerical simulation: towards a more precise neuroprosthesis monitoring tool . J Neural Eng 2013 ; 10 : 046002 .
Google Scholar
Maghami MH , Sodagar AM , Lashay A et al. Visual prostheses: the enabling technology to give sight to the blind . J Ophthal Vis Res 2014 ; 9 : 494 – 505 .
Weitz AC , Nanduri D , Behrend MR et al. Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration . Sci Transl Med 2015 ; 7 : 318ra203.
Bouton CE , Shaikhouni A , Annetta NV et al. Restoring cortical control of functional movement in a human with quadriplegia . Nature 2016 ; 533 : 247 – 50 .
Geddes L. First paralysed person to be ‘reanimated’ offers neuroscience insights. Technique moves man’s arm by decoding his thoughts and electrically stimulating his own muscles . Nat News 2016 ; 533 .
Squires JE. Artificial blood . Science 2002 ; 295 : 1002 – 5 .
Lowe KC. Blood substitutes: from chemistry to clinic . J Mater Chem 2006 ; 16 : 4189 – 96 .
Moradi S , Jahanian-Najafabadi A , Roudkenar MH. Artificial blood substitutes: first steps on the long route to clinical utility . Clin Med Insights Blood Disord 2016 ; 9 : 33 – 41 .
Powell R , Kahane G , Savulescu J. Evolution, genetic engineering, and human enhancement . Philos Technol 2012 ; 25 : 439 – 58 .
Parens E (ed.). Enhancing Human Traits: Ethical and Social Implications . Washington, DC : Georgetown University Press , 1998 .
Google Preview
Giubilini A , Sanyal S. Challenging human enhancement. In: Clarke S , Savulescu J , Coady T et al. (eds). The Ethics of Human Enhancement: Understanding the Debate . Oxford : Oxford University Press , 2016 .
Elliott C. Better Than Well: American Medicine Meets the American Dream . New York, NY : WWW Norton & Company, Inc ., 2003 .
Kramer P. Listening to Prozac . London : Fourth Estate , 1994 .
Moravec H. Mind Children: The Future of Robot and Human Intelligence . Cambridge : Harvard University Press , 1990 .
Bostrom N. Human genetic enhancements: a transhumanist perspective . J Value Inq 2003 ; 37 : 493 – 506 .
Kurzweil R. The Singularity is Near: When Humans Transcend Biology . New York, NY : Viking , 2005 .
Harris J. Enhancing Evolution: The Ethical Case for Making Better People . Princeton, NJ : Princeton University Press , 2010 .
Fukuyama F. Our Posthuman Future: Consequences of the Biotechnology Revolution . New York, NY : Picador , 2002 .
Sandel M. The Case Against Perfection: Ethics in the Age of Genetic Engineering . Cambridge : The Belknap Press of Harvard University Press , 2007 .
Savulescu J , Persson I. The perils of cognitive enhancement and the urgent imperative to enhance the moral character of humanity . J Appl Philos 2008 ; 25 : 162 – 77 .
Buchanan A. Beyond Humanity . Oxford : Oxford University Press , 2011 .
Persson I , Savulescu J. Moral enhancement, freedom, and the god machine . Monist 2012 ; 95 : 399 – 421 .
Leon K. Ageless bodies, happy souls: biotechnology and the pursuit of perfection . New Atlantis 2003 ; 1 : 9 – 28 .
Agar N. Humanity’s End: Why We Should Reject Radical Enhancement . Cambridge : MIT Press , 2010 .
Gaj T , Gersbach CA , Barbas CF III ,. ZFN, TALEN, and CRISPR/Cas based methods for genome engineering . Trends Biotechnol 2013 ; 3 : 397 – 405 .
Baltimore D , Berg P , Botchan M et al. Biotechnology. A prudent path forward for genomic engineering and germline gene modification . Science 2015 ; 348 : 36 – 8 .
Otieno MO. CRISPR/Cas9 human genome editing: challenges, ethical concerns and implications . J Clin Res Bioeth 2015 ; 6 : 253 .
Ishii T. Germline genome-editing research and its socio-ethical implications . Trends Mol Med 2015 ; 21 : 473 – 81 .
Bionews.org.uk. First Genome-edited Babies: A Very Different Perception of Ethics , 2018 . https://www.bionews.org.uk/page_140060 (27 August 2019, date last accessed).
Cyranoski D. CRISPR-baby scientist fails to satisfy his critics . Nat News 2018 ; 564 : 13 – 4 .
Galis F , Metz JA. Evolutionary novelties: the making and breaking of pleiotropic constraints . Integr Comp Biol 2007 ; 47 : 409 – 19 .
Falcon A , Cuevas MT , Rodriguez-Frandsen A et al. CCR5 deficiency predisposes to fatal outcome in influenza virus infection . J Gen Virol 2015 ; 96 : 2074 – 8 .
Gade-Andavolu R , Comings DE , MacMurray J et al. Association of CCR5 Δ32 deletion with early death in multiple sclerosis . Genet Med 2004 ; 6 : 126 – 31 .
Zhou M , Greenhill S , Huang S et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory . eLife 2016 ; 5 : e20985 .
Tibayrenc M , Ayala FJ (eds). On Human Nature: Biology, Psychology, Ethics, Politics, and Religion . London : Academic Press , 2017 .
Baldi P. The Shattered Self: The End of Natural Evolution . Cambridge : MIT Press , 2001 .
Darwin C. On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life . London : J. Murray , 1859 .
Gould SJ. The Structure of Evolutionary Theory . Belknap, NY : Harvard University Press , 2002 .
Rees M. Our Final Century: Will the Humans Race Survive the Twenty-first Century? Eastbourne : Gardners Books , 2003 .
Nuffield Council on Bioethics. Genome Editing: An Ethical Review . London : Nuffield Council on Bioethics , 2016 .
Powell R. The evolutionary biological implications of human genetic engineering . J Med Philos 2012 ; 37 : 204 – 26 .
Liu W , Chen L , Zhang S et al. Decrease of gene expression diversity during domestication of animals and plants . BMC Evol Biol 2019 ; 19 : 1 – 11 .
Fages A , Hanghøj K , Khan N et al. Tracking five millennia of horse management with extensive ancient genome time series . Cell 2019 ; 177 : 1419 – 35 .
Zhang J , Wang X , Yao J et al. Effect of domestication on the genetic diversity and structure of Saccharina japonica populations in China . Sci Rep 2017 ; 7 : 42158 .
Theofanopoulou C , Gastaldon S , O’Rourke T et al. Self-domestication in Homo sapiens : insights from comparative genomics . PLoS One 2018 ; 13 : e0196700 .
Capra F , Luisi PL. The Systems View of Life . Cambridge : Cambridge University Press , 2014
- genetic engineering
Email alerts
Citing articles via, affiliations.
- Online ISSN 2050-6201
- Copyright © 2024 International Society for Evolution, Medicine, and Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 20 April 2022
Beyond safety: mapping the ethical debate on heritable genome editing interventions
- Mara Almeida ORCID: orcid.org/0000-0002-0435-6296 1 &
- Robert Ranisch ORCID: orcid.org/0000-0002-1676-1694 2 , 3
Humanities and Social Sciences Communications volume 9 , Article number: 139 ( 2022 ) Cite this article
18k Accesses
10 Citations
8 Altmetric
Metrics details
- Medical humanities
- Science, technology and society
Genetic engineering has provided humans the ability to transform organisms by direct manipulation of genomes within a broad range of applications including agriculture (e.g., GM crops), and the pharmaceutical industry (e.g., insulin production). Developments within the last 10 years have produced new tools for genome editing (e.g., CRISPR/Cas9) that can achieve much greater precision than previous forms of genetic engineering. Moreover, these tools could offer the potential for interventions on humans and for both clinical and non-clinical purposes, resulting in a broad scope of applicability. However, their promising abilities and potential uses (including their applicability in humans for either somatic or heritable genome editing interventions) greatly increase their potential societal impacts and, as such, have brought an urgency to ethical and regulatory discussions about the application of such technology in our society. In this article, we explore different arguments (pragmatic, sociopolitical and categorical) that have been made in support of or in opposition to the new technologies of genome editing and their impact on the debate of the permissibility or otherwise of human heritable genome editing interventions in the future. For this purpose, reference is made to discussions on genetic engineering that have taken place in the field of bioethics since the 1980s. Our analysis shows that the dominance of categorical arguments has been reversed in favour of pragmatic arguments such as safety concerns. However, when it comes to involving the public in ethical discourse, we consider it crucial widening the debate beyond such pragmatic considerations. In this article, we explore some of the key categorical as well sociopolitical considerations raised by the potential uses of heritable genome editing interventions, as these considerations underline many of the societal concerns and values crucial for public engagement. We also highlight how pragmatic considerations, despite their increasing importance in the work of recent authoritative sources, are unlikely to be the result of progress on outstanding categorical issues, but rather reflect the limited progress on these aspects and/or pressures in regulating the use of the technology.
Similar content being viewed by others
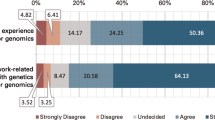
Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public
Abbie Jedwab, Danya F. Vears, … Christopher Gyngell

Between desire and fear: a qualitative interview study exploring the perspectives of carriers of a genetic condition on human genome editing
Wendy Geuverink, Carla van El, … Linda Martin
The interplay of ethics and genetic technologies in balancing the social valuation of the human genome in UNESCO declarations
Hristina Gaydarska, Kayo Takashima, … Jusaku Minari
Introduction
The ability to alter a sequence of genetic material was initially developed in microorganisms during the 1970s and 1980s (for an overview: Walters et al., 2021 ). Since then, technological advances have allowed researchers to alter DNA in different organisms by introducing a new gene or by modifying the sequence of bases in the genome. The manipulation of the genome of living organisms (typically plants) continues a course that science embraced more than 40 years ago, and may ultimately allow, if not deliberately curtailed by societal decisions, the possibility of manipulating and controlling genetic material of other living species, including humans.
Genetic engineering can be used in a diverse range of contexts, including research (e.g., to build model organisms), pharmacology (e.g., for insulin production) and agriculture (e.g., to improve crop resistance to environmental pressures such as diseases, or to increase yield). Beyond these applications, modern genetic engineering techniques such as genome editing technologies have the potential to be an innovative tool in clinical interventions but also outside the clinical realm. In the clinical context, genome editing techniques are expected to help in both disease prevention and in treatment (Porteus, 2019 ; Zhang, 2019 ). Nevertheless, genome editing technology raises several questions, including the implications of its use for human germline cells or embryos, since the technology’s use could facilitate heritable genome editing interventions (Lea and Niakan, 2019 ). This possible use has fuelled a heated debate and fierce opposition, as illustrated by the moratoriums proposed by researchers and international institutions on the use of the technology (Lander et al., 2019 ; Baltimore et al., 2015 ; Lanphier et al., 2015 ). Heritable human germline modifications are currently prohibited under various legislations (Baylis et al., 2020 ; Ledford, 2015 ; Isasi et al., 2016 ; König, 2017 ) and surveys show public concerns about such applications, especially without clear medical justification (e.g., Gaskell et al., 2017 ; Jedwab et al., 2020 ; Scheufele et al., 2017 ; Blendon et al., 2016 ).
To analyse some implications of allowing heritable genome editing interventions in humans, it is relevant to explore underlying values and associated ethical considerations. Building on previous work by other authors (e.g., Coller, 2019 ; de Wert et al., 2018 ; van Dijke et al., 2018 ; Mulvihill et al., 2017 ; Ishii, 2015 ), this article aims to provide context to the debates taking place and critically analyse some of the major pragmatic, categorical and sociopolitical considerations raised to date in relation to human heritable genome editing. Specifically, we explore some key categorical and sociopolitical considerations to underline some of the possible barriers to societal acceptance, key outstanding questions requiring consideration, and possible implications at the individual and collective level. In doing so, we hope to highlight the predominance of pragmatic arguments in the scientific debate regarding the permissible use of heritable genome editing interventions compared to categorical arguments relevant to broader societal debate.
Human genome editing: a brief history of CRISPR/Cas9
Human genome editing is an all-encompassing term for technologies that are aimed at making specific changes to the human genome. In humans, these technologies can be used in embryos or germline cells as well as somatic cells (Box 1 ). Concerning human embryos or germline cells, the intervention could introduce heritable changes to the human genome (Lea and Niakan, 2019 ; Vassena et al., 2016 ; Wolf et al., 2019 ). In contrast, an intervention in somatic cells is not intended to result in changes to the genome of subsequent generations. It is worth noting that intergenerational effects occur only when the modified cells are used to establish a pregnancy which is carried to term. Thus, a distinction has been made between germline genome editing (GGE), which may only affect in vitro embryos in research activity, and heritable genome editing (HGE), which is used in reproductive medicine (e.g., Baylis et al., 2020 ). HGE could be used to prevent the transmission of serious genetic disease; however, other applications could be imagined, e.g., creating genetic resistance or even augmenting human functions.
In the last decade, prominent technical advances in genome engineering methods have taken place, including the zinc-finger nucleases (ZFNs) and TAL effector nucleases (TALENs), making human genome modification a tangible possibility (Gaj et al., 2013 ; Li et al., 2020 ; Gupta and Musunuru, 2014 ). In 2012, a study showed that the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), combined with an enzyme called Cas9, could be used as a genome‐editing tool in human cell culture (Jinek et al., 2012 ). In 2013, the use of CRISPR/Cas9 in mammalian cells was described, demonstrating the application of this tool in the genome of living human cells (Cong et al., 2013 ). In 2014, CRISPR/Cas9 germline modifications were first used in non-human primates, resulting in the birth of gene-edited cynomolgus monkeys (Niu et al., 2014 ). This was followed in 2015 by the first-ever public reported case of genome modification in non-viable human embryos (tripronuclear zygotes) (Liang et al., 2015 ). This study has caused broad concerns in the scientific community (Bosley et al., 2015 ) with leading journals rejecting publication for ethical reasons. Five years after these initial experiments were conducted, more than 10 papers have been published reporting the use of genome editing tools on human preimplantation embryos (for an overview: Niemiec and Howard, 2020 ).
Compared to counterpart genome technologies (e.g., ZFNs and TALENs), CRISPR/Cas9 is considered by many a revolutionary tool due to its efficiency and reduced cost. More specifically, CRISPR/Cas9 seems to provide the possibility of a more targeted and effective intervention in the genome involving the insertion, deletion, or replacement of genetic material (Dance, 2015 ). The potential applicability of CRISPR/Cas9 technique is considered immense, since it can be used on all type of organisms, from bacteria to plants, non-human cells, and human cells (Barrangou and Horvath, 2017 ; Hsu et al., 2014 ; Doudna and Charpentier, 2014 ; Zhang, 2019 ).
Box 1 Difference associated with germline cells and somatic cells.
For the purposes of the analysis presented in this article, one of the main differences is the heritability of genes associated with either type of cell. Germline cells include spermatozoa, oocytes, and their progenitors (e.g., embryonic cells in early development), which can give rise to a new baby carrying a genetic heritage coming from the parents. Thus, germline are those cells in an organism which are involved in the transfer of genetic information from one generation to the next. Somatic cells, conversely, constitute many of the tissues that form the body of living organisms, and do not pass on genetic traits to their progeny.
Germline interventions: the international debate
As a reaction to the 2015 study with CRISPR/Cas9, several commentaries by scientists were published regarding the future use of the technology (e.g., Bosley et al., 2015 ; Lanphier et al., 2015 ; Baltimore et al., 2015 ). Many of them focused on germline applications, due to the possibility of permanent, heritable changes to the human genome and its implications for both individuals and future generations. These commentaries included position statements calling for great caution in the use of genome editing techniques for heritable interventions in humans and suggested a voluntary moratorium on clinical germline applications of CRISPR/Cas9, at least until a broad societal understanding and consensus on their use could be reached (Brokowski, 2018 ; Baltimore et al., 2015 ; Lander, 2015 ). Such calls for a temporary ban were often seen as reminiscent of the “Asilomar ban” on recombinant DNA technology in the mid-1970s (Guttinger, 2017 ). Other commentaries asked for research to be discouraged or halted all together (Lanphier et al., 2015 ). More firmly, the United States (US) National Institutes of Health (NIH) released a statement indicating that the NIH would not fund research using genome editing technologies on human embryos (Collins, 2015 ).
In December 2015, the first International Summit on Human Gene Editing took place, hosted by the US National Academy of Sciences, the US National Academy of Medicine, the UK Royal Society, and the Chinese Academy of Sciences (NASEM). The organizing committee issued a statement about appropriate uses of the technology that included the following: “It would be irresponsible to proceed with any clinical use of germline editing unless and until (i) the relevant safety and efficacy issues have been resolved, based on appropriate understanding and balancing of risks, potential benefits, and alternatives, and (ii) there is broad societal consensus about the appropriateness of the proposed application” (NASEM, 2015 ).
Following this meeting, initiatives from different national bodies were organized to promote debate on the ethical issues raised by the new genome editing technologies and to work towards a common framework governing the development and permissibility of their use in humans. This included an ethical review published in 2016 by the Nuffield Council on Bioethics, addressing conceptual and descriptive questions concerning genome editing, and considering key ethical questions arising from the use of the technology in both human health and other contexts (Nuffield Council on Bioethics, 2016 ). In 2017, a committee on human genome editing set up by the US National Academy of Sciences (NAS) and the National Academy of Medicine (NAM) carried out a so-called consensus study “Human Genome Editing: Science, Ethics, and Governance” (NASEM, 2017 ). This study put forward a series of recommendations on policies and procedures to govern human applications of genome editing. Specifically, the study concluded that HGE could be justified under specific conditions: “In some situations, heritable genome editing would provide the only or the most acceptable option for parents who desire to have genetically related children while minimizing the risk of serious disease or disability in a prospective child” (NASEM, 2017 ). The report stimulated much public debate and was met with support and opposition since it was seen as moving forward on the permissibility of germline editing in the clinical context (Ranisch and Ehni, 2020 ; Hyun and Osborn, 2017 ).
Following the report in 2016, the Nuffield Council on Bioethics published a second report in 2018. Similar to the NASEM 2017 report, this report emphasizes the value of procreative freedom and stresses that in some cases HGE might be the only option for couples to conceive genetically related, healthy offspring. In this document, the Nuffield Council on Bioethics maintains that there are no categorical reasons to prohibit HGE. However, it highlights three kinds of interests that should be recognized when discussing prospective HGE. They are related to individuals directly affected by HGE (parents or children), other parts of society, and future generations of humanity. In this context, two ethical principles are highlighted as important to guide future evaluations of the HGE use in specific interventions: “(...) to influence the characteristics of future generations could be ethically acceptable, provided if, and only if, two principles are satisfied: first, that such interventions are intended to secure, and are consistent with, the welfare of a person who may be born as a consequence, and second, that any such interventions would uphold principles of social justice and solidarity (…)” (Nuffield Council on Bioethics, 2018 ). This report was met with criticism for (implicitly) advocating genetic heritable interventions might be acceptable even beyond the boundaries of therapeutic uses. This is particularly controversial and goes well beyond the position previously reached by the NASEM report (which limited permissible uses of genome editing at preventing the transmission of genetic variants associated to diseases) (Drabiak, 2020 ). On the other hand, others have welcomed the report and, within it, the identification of explicit guiding ethical principles helpful in moving forward the debate on HGE (Gyngell et al., 2019 ).
As a follow-up to the 2015 conference, a second International Summit on Human Gene Editing was scheduled for November 2018 in Hong Kong (National Academies of Sciences, Engineering, and Medicine, 2019 ). The event, convened by the Hong Kong Academy of Sciences, the UK Royal Society, the US National Academy of Sciences and the US National Academy of Medicine, was supposed to focus on the prospects of HGE. Just before the Summit began, news broke that He Jiankui, a Chinese researcher and invited speaker at the Summit, created the world’s first genetically edited babies resulting from the use of CRISPR/Cas9 in embryos (Regalado, 2018 ; Lovell-Badge, 2019 ). Although an independent investigation of the case is still pending, his experiments have now been reviewed in detail by some scholars (e.g., Greely, 2019 , 2021 ; Kirksey, 2020 ; Davies, 2020 ; Musunuru, 2019 ). These experiments were globally criticized, since they did not follow suitable safety procedures or ethical guidelines (Wang and Yang, 2019 ; Lovell-Badge, 2019 ; Krimsky, 2019 ), nor considered the recommendations previously put forward by international reports (NASEM, 2017 ; Nuffield Council on Bioethics, 2018 ) and legal frameworks (Araki and Ishii, 2014 ; Isasi et al., 2016 ). Different reactions were triggered, including another call by scientists for a global moratorium on clinical human genome editing, to allow time for international discussions to take place on its appropriate uses (Lander et al., 2019 ) or an outright ban on the technology (Botkin, 2019 ). There were also calls for a measured analysis of the possible clinical applications of human genome editing, without the imposition of a moratorium (Daley et al., 2019 ; Dzau et al., 2018 ).
Most countries currently have legal frameworks to ban or severely restrict the use of heritable genome editing technologies (Araki and Ishii, 2014 ; Isasi et al., 2016 ; Baylis et al., 2020 ). However, since He’s experiment, the possibility that researchers might still attempt (with some likelihood of success) to use the technology in human embryos, became a growing concern, particularly since some scientists have already announced their interest in further clinical experiments (Cyranoski, 2019 ). For many, He’s experiments highlighted the ongoing risks associated with the use of modern genome editing technology without proper safety protocols and regulatory frameworks at an international level (Ranisch et al., 2020 ). This has triggered the need to develop clear and strict regulations to be implemented if these tools are to be used in the future. This incident also led to the formation of several working groups, including the establishment of an international commission on the Clinical Use of Human Germline Genome Editing set up by the US National Academy of Medicine, the US National Academy of Sciences, and the UK’s Royal Society. In 2020, the commission published a comprehensive report on HGE, proposing a translational pathway from research to clinical use (National Academy of Medicine, National Academy of Sciences, and the Royal Society, 2020 ). Likewise, a global expert Advisory Committee was established by the World Health Organization (WHO) with the goal of developing recommendations on governance mechanisms for human genome editing. Although the committee insisted in an interim recommendation that “it would be irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing” (WHO, 2019 ), it did not express fundamental concerns on the possibility that some forms of HGE will one day become a reality. In 2021, the WHO’s Advisory Committee issued some publications, including a “Framework for governance” report and a “Recommendations” report (WHO, 2021 ). Building on a set of procedural and substantive values and principles, the “Framework for Governance” report discusses a variety of tools and institutions necessary for developing appropriate national, transnational, and international governance and oversight mechanisms for HGE. Specifically, the report considers the full spectrum of possible applications of human genome editing (including epigenetic editing and human enhancement) and addresses specific challenges associated with current, possible and speculative scenarios. These range from somatic gene therapy for the prevention of serious hereditary diseases to potentially more controversial applications reminiscent of the He Jiankui case (e.g., the use of HGE in reproductive medicine outside regulatory controls and oversight mechanisms). Additionally, the “Recommendations” report proposes among other things whistleblowing mechanisms to report illegal or unethical research. It also highlights the need for a global human genome editing registry, that should also cover basic and preclinical research on different applications of genetic manipulation, including HGE. The report also emphasises the need of making possible benefits of human genome editing widely accessible.
The idea of a human genome editing registry has also been supported by the European Group on Ethics in Science and New Technologies (EGE), an advisory board to the President of the European Commission. After an initial statement on genome editing published in 2016, still calling for a moratorium on editing of human embryos (EGE, 2016 ), the EGE published a comprehensive Opinion in 2021 (EGE, 2021 ). Although the focus of this report is on the moral issues surrounding genome editing in animals and plants, HGE is also discussed. Similar to the WHO Advisory Committee, the EGE recommends for HGE not to be introduced prematurely into clinical application and that measures should be taken to prevent HGE’s use for human enhancement.
Overall, when reviewing reports and initiatives produced since 2015, common themes and trajectories can be identified. A key development is the observation that the acceptance of the fundamental permissibility of such interventions appears to be increasing. This constitutes an important change from previous positions, reflecting the fact that human germline interventions have long been considered a ‘red line’ or at least viewed with deep scepticism (Ranisch and Ehni, 2020 ). In particular, while there is agreement that it would be premature to bring HGE into a clinical context, key concerns expressed by authoritative international bodies and committees are now associated with acceptable uses of the technology, rather than its use per se. Consideration is now being given to the conditions and objectives under which germline interventions could be permissible, instead of addressing the fundamental question of whether HGE may be performed at all. The question of permissibility is often linked to the stage of technological development. These developments are remarkable, since the key ethical aspects of genome editing are now frequently confined to questions of safety or cost–benefit ratios, rather than categorical considerations.
Another common issue can also be found in recent reports: the question of involving society in the debate. There is consensus on the fact that the legitimacy and governance of HGE should not be left solely to scientists and other experts but should involve society more broadly. Since germline interventions could profoundly change the human condition, the need for a broad and inclusive public debate is frequently emphasized (Iltis et al., 2021 ; Scheufele et al., 2021 ). The most striking expression of the need for public engagement and a “broad societal consensus” can be found in the final statement by the 2015 International Summit on Human Gene Editing organizing committee, as previously quoted (NASEM, 2015 ). Furthermore, the EGE and others also stresses the need for an inclusive societal debate before HGE can be considered permissible.
The pleas for public engagement are, however, not free of tension. For example, the NASEM’s 2017 report was criticised for supporting HGE bypassing the commitment for the broad societal consensus (Baylis, 2017 ). Regarding HGE, some argue that only a “small but vocal group of scientists and bioethicists now endorse moving forward” (Andorno et al., 2020 ). Serious efforts to engage the public on the permissibility and uses of HGE have yet to be made. This issue not only lacks elaboration on approaches to how successful public participation can occur, but also how stop short of presenting views on how to translate the public’s views into ethical considerations and policy (Baylis, 2019 ).
Potential uses of heritable genome editing technology
HGE is expected to allow a range of critical interventions: (i) preventing the transmission of genetic variants associated with severe genetic conditions (mostly single gene disorders); (ii) reducing the risk of common diseases (mostly polygenic diseases), with the promise of improving human health; and (iii) enhancing human capabilities far beyond what is currently possible for human beings, thereby overcoming human limitations. The identification of different classes of potential interventions has shifted the debate to the applications considered morally permissible beyond the acceptable use of HGE (Dzau et al., 2018 ). Specifically, there are differences in the limits of applicability suggested by some of the key cornerstone publications discussed above. For example, the NASEM ( 2017 ) report suggests limiting the use of HGE to the transmission of genetic variants linked to severe conditions, although in a very regulated context. In a very similar way, the 2020 report from the International Commission on the Clinical Use of Human Germline Genome Editing suggests that the initial clinical use of HGE should be limited to the prevention of serious monogenic diseases. By contrast, the 2018 Nuffield Council on Bioethics Report does not seem to limit the uses of genome editing to specific applications, though suggests that applications should be aligned with fundamental guiding ethical principles and need to have followed public debate (Savulescu et al., 2015 ). The same report also discusses far-reaching and speculative uses of HGE that might achieve “other outcomes of positive value” (Nuffield Council on Bioethics, 2018 ). Some of these more speculative scenarios include “built-in genetic resistance or immunity to endemic disease”; “tolerance for adverse environmental conditions” and “supersenses or superabilities” (Nuffield Council on Bioethics, 2018 , p. 47).
There have been different views on the value of HGE technology. Some consider that HGE should be permissible in the context of therapeutic applications, since it can provide the opportunity to treat and cure diseases (Gyngell et al., 2017 ). For example, intervention in severe genetic disorders is considered as therapeutic and hence morally permissible, or even obligatory. Others consider HGE to be more like a public health measure, which could be used to reduce the prevalence of a disease (Schaefer, 2020 ). However, others maintain that reproductive uses of HGE are not therapeutic because there is no individual in a current state of disease which needs to be treated, rather a prospective individual to be born with a specific set of negative prospective traits (Rulli, 2019 ).
Below, HGE is discussed in the context of reproductive uses and conditions of clinical advantage over existent reproductive technologies. The HGE applications are explored regarding their potential for modifying one or more disease-related genes relevant to the clinical context. Other uses associated with enhancement of physical and mental characteristics, which are considered non-clinical (although the distinction is sometimes blurred), are also discussed.
Single gene disorders
An obvious application of HGE interventions is to prevent the inheritance of genetic variants known to be associated with a serious disease or condition. Its potential use for this purpose could be typically envisaged through assisted reproduction, i.e., as a process to provide reproductive options to couples or individuals at risk of transmitting genetic conditions to their offspring. Critics of this approach often argue that other assisted reproductive technologies (ARTs) and preimplantation screening technologies e.g., preimplantation genetic diagnosis (PGD), not involving the introduction of genetic modifications to germline cells, are already available for preventing the transmission of severe genetic conditions (Lander, 2015 ; Lanphier et al., 2015 ). These existent technologies aim to support prospective parents in conceiving genetically related children without the condition that affect them. In particular, PGD involves the creation of several embryos by in vitro fertilization (IVF) treatment that will be tested for genetic anomalies before being transferred to the uterine cavity (Sermon et al., 2004 ). In Europe, there is a range in the regulation of the PGD technology with most countries having restrictions of some sorts (Soini, 2007 ). The eligibility criteria for the use of PGD also vary across countries, depending on the range of heritable genetic diseases for which it can be used (Bayefsky, 2016 ).
When considering its effectiveness, PGD presents specific limitations, which include the rare cases in which either both prospective parents are homozygous carriers of a recessive genetic disease, or one of the parents is homozygous for a dominant genetic disease (Ranisch, 2020 ). In these cases, all embryos produced by the prospective parents will be affected by the genetic defect, and therefore it will not be possible to select an unaffected embryo after PGD. Currently, beyond adoption of course, the options available for these prospective parents include the use of a third-party egg or sperm donors.
Overall, given the rarity of cases in which it is not applicable, PGD is thought to provide a reliable option to most prospective parents for preventing severe genetic diseases to be transmitted to their offspring, except in very specific cases. HGE interventions have been suggested to be an alternative method to avoid single gene disorders in the rare cases in which selection techniques such as PGD cannot be used (Ranisch, 2020 ). It has also been proposed to use tools such as CRISPR/Cas9 to edit morphologically suitable but genetically affected embryos, and thus increase the number of embryos available for transfer (de Wert et al., 2018 ; Steffann et al., 2018 ). Moreover, HGE interventions are considered by some as a suitable alternative to PGD, even when the use of PGD could be possible. One argument in this respect is that, although not leading to the manifestation of the disease, the selected embryos can still be carriers of it. In this respect, differently from PGD, HGE interventions can be used to eliminate unwanted, potential future consequences of genetic diseases (i.e., by eliminating the critical mutation carried out in the selected embryo), with the advantage of reducing the risks of further propagation of the disease in subsequent future generations (Gyngell et al., 2017 ).
Overall, HGE interventions are thought to offer a benefit over PGD in some situations by providing a broader range of possible interventions, as well as by providing a larger number of suitable embryos. The latter effect is usually important in the cases where unaffected embryos are small in number, making PGD ineffective (Steffann et al., 2018 ). Whether these cases provide a reasonable ground to justify research and development on the clinical use of HGE remain potentially contentious. Some authors have suggested that the number of cases in which PGD cannot be effectively used to prevent transmission of genetic disorders is so marginal that clinical application of HGE could hardly be justified (Mertes and Pennings, 2015 ). Particularly when analyzing economic considerations (i.e., the allocation of already scarce resources towards clinical research involving expensive techniques with limited applicability) and additional risks associated with direct interventions. In either case of HGE being used as an alternative or a complementary tool to PGD, PGD will most likely still be used to identify those embryos that would manifest the disease and would hence require subsequent HGE.
The PGD technique, however, is not itself free of criticism and possible moral advantages of HGE over PGD have also been explored (Hammerstein et al., 2019 ; Ranisch, 2020 ). PGD remains ethically controversial since, identifying an unaffected embryo from the remaining embryos (which will not be used and ultimately discarded) amounts to the selection of ‘healthy’ embryos rather than ‘curing’ embryos affected by the genetic conditions. On the other hand, given a safe and effective application of the technology, the use of HGE is considered by many morally permissible to prevent the transmission of genetic variants known to be associated with serious illness or disability (de Miguel Beriain, 2020 ). One question that remains is whether HGE and PGD have a differing or equal moral permissibility or, at least, comparable. On issues including human dignity and autonomy, it was argued that HGE and PGD interventions can be considered as equally morally acceptable (Hammerstein et al., 2019 ). This equal moral status was, however, only valid if HGE is used under the conditions of existent gene variants in the human gene pool and to promote the child health’s best interest in the context of severe genetic diseases (Hammerstein et al., 2019 ). Because of selection and ‘therapy’, moral assessments resulted in HGE interventions being considered to some extent preferable to PGD, once safety is carefully assessed (Gyngell et al., 2017 ; Cavaliere, 2018 ). Specifically, PGD’s aim is selective and not ‘therapeutic’, which could be said to contradict the aims of traditional medicine (MacKellar and Bechtel, 2014 ). In contrast to PGD’s selectivity, HGE interventions are seen as ‘pre-emptively therapeutic’, and therefore closer to therapy than PGD (Cavaliere, 2018 ). However, it is also argued that HGE does not have curative aims, and thus it is not a therapeutic application, as there is no patient involved in the procedure to be cured (Rulli, 2019 ). On balance, there appears to be no consensus on which of the approaches, HGE and PGD, is morally a better strategy to prevent the transmission of single gene disorders, with a vast amount of literature expressing diverse positions when considering different scenarios (Delaney, 2011 ; Gyngell et al., 2017 ; Cavaliere, 2018 ; Ranisch, 2020 ; Rehmann-Sutter, 2018 ; Sparrow, 2021 ).
Polygenetic conditions
HGE is also argued to have the potential to be used in other disorders which have a polygenic disposition and operate in combination with environmental influences (Gyngell et al., 2017 , 2019 ). Many common diseases, which result from the involvement of several genes and environmental factors, fall into this category. Examples of common diseases of this type includes diabetes, coronary artery disease and different types of cancers, for which many of the genes involved were identified by studies of genome wide association (e.g., Wheeler and Barroso, 2011 ; Peden and Farral, 2011 ). These diseases affect the lives of millions of people globally, severely impacting health and often leading to death. Furthermore, these diseases have a considerable burden on national health systems. Currently, many of these diseases are controlled through pharmaceutical products, although making healthier life choices about diet and exercise can also contribute to preventing and managing some of them. Despite the interest, the use of PGD in polygenic conditions would hardly be feasible, due to the number of embryos needed to select the preferred genotype and available polygenic predictors (Karavani et al., 2019 ; Shulman and Bostrom, 2014 ).
In theory, HGE could be a potentially useful tool to target different genes and decrease the susceptibility to multifactorial conditions in current and future generations. The application of HGE to polygenic conditions is often argued by noting that the range of applicability of the technique (well beyond single gene disorders) would justify and outweigh the cost needed to develop it. However, to do so, a more profound knowledge of genetic interactions, of the role of genes and environmental factors in diverse processes would be needed to be able to modify such interconnected systems with limited risk to the individual (Lander, 2015 ). Besides, it is now understood that, depending on the genetic background, individuals will have different risks of developing polygenetic diseases (risk-associated variants), but hardly any certainty of it. In other words, although at the population level there would most likely be an incidence of the disease, it is not possible to be certain of the manifestation of the disease in any specific individual. As a result, the benefits of targeting a group of genes associated to a disease in a specific individual would have to be assessed in respect to the probability of incidence of the disease. The risk-benefit ratio for HGE is considerably increased for polygenic conditions compared to monogenic disorders. Additionally, the risks of adverse effects, e.g., off-target effects, increases with the number of genes targeted for editing. The latter effects make the potential benefits of HGE in polygenic diseases more uncertain than in single gene disorders.
Genetic enhancement
A widespread concern regarding the use of HGE is that such interventions could be used not only to prevent serious diseases, but also to enhance desirable genetic traits. Currently, our knowledge on how to genetically translate information into specific phenotypes is very limited and some argue that it might never be technically feasible to achieve comprehensive genetic enhancements using current gene editing technologies (Janssens, 2016 ; Ranisch, 2021 ). Similar to many diseases, in which different genetic and other factors are involved, many of the desirable traits to be targeted by any enhancement will most likely be the result of a combination of several different genes influenced by environment and context. Moreover, the implications for future generations of widespread genetic interventions in the human population and its potential impact on our evolutionary path are difficult to assess (Almeida and Diogo, 2019 ). Nevertheless, others argue that genetic enhancement through HGE could be possible in the near future (de Araujo, 2017 ).
There has been much discussion regarding the meaning of the terms and the conceptual or normative difference between ‘therapy’ and ‘enhancement’ (for an early discussion: Juengst, 1997 ; Parens, 1998 ). There are mainly three different meanings of ‘enhancement’ used in the literature. First, ‘enhancement’ is sometimes used to refer to measures that go beyond therapy or prevention of diseases, i.e., that transcend goals of medicine. Second, ‘enhancement’ is used to refer to measures that equip a human with traits or capacities that they typically do not possess. In both cases, the term points to equally controversial and contrasting concepts: on the one hand, those of ‘health’, ‘disease’ or ‘therapy’, and on the other, those of ‘normality’ or ‘naturalness’. Third, ‘enhancement’ is sometimes also used as an umbrella-term describing all measures that have a positive effect on a person’s well-being. According to this definition, the cure, or prevention of a disease is then also not opposed to an enhancement. Here again, this use refers to the controversial concept of ‘well-being’ or a ‘good life’.
It is beyond the scope of this article to provide a detailed review of the complex debate about enhancement (for an overview: Juengst and Moseley, 2019 ). However, three important remarks can be made: first, although drawing a clear line between ‘enhancement’ and ‘therapy’ (or ‘normality’, etc.) will always be controversial, some cases can be clearly seen as human enhancement. This could include modifications to augment human cognition, like having a greater memory, or increasing muscle mass to increase strength, which are not considered essential for human health (de Araujo, 2017 ).
Second, it is far from clear whether a plausible account of human enhancement would, in fact, be an objectivist account. While authors suggest that there is some objectivity regarding the conditions that constitute a serious disease (Habermas, 2003 ), the same might not be true for what constitutes an improvement of human functioning. It may rather turn out that an enhancement for some might be seen as a dis-enhancement for others. Furthermore, the use of the HGE for enhancement purposes can be considered at both an individual and a collective level (Gyngell and Douglas, 2015 ; Almeida and Diogo, 2019 ), with a range of ethical and biological implications. If HGE is to be used for human enhancement, this use will be in constant dependence on what we perceive as ‘normal’ functioning or as ‘health’. Therefore, factors such as cultural and societal norms will have an impact on where such boundaries are drawn (Almeida and Diogo, 2019 ).
Third, it should be noted that from an ethical perspective the conceptual question of what enhancement is, and what distinguishes it from therapy, is less important than whether this distinction is ethically significant in the first place. In this context, it was pointed out that liberal positions in bioethics often doubt that the distinction between therapy and enhancement could play a meaningful role in determining the limits of HGE (Agar, 1998 ). The consideration of genetic intervention for improving or adding traits considered positive by individuals have raised extreme positions. Some welcome the possibility to ameliorate the human condition, whilst others consider it an alarming attempt to erase aspects of our common human ‘nature’. More specifically, some authors consider HGE a positive step towards allowing humans the opportunity to obtain beneficial traits that otherwise would not be achievable through human reproduction, thus providing a more radical interference in human life to overcome human limitations (de Araujo, 2017 ; Sorgner, 2018 ). The advocates of this position are referred to as ‘bioliberals’ or ‘transhumanists’ (Ranisch and Sorgner, 2014 ), and its opponents are referred to as ‘bioconservatives’ (Fukuyama, 2002 ; Leon, 2003 ; Sandel, 2007 ). Transhumanism supports the possibility of humans taking control of their biology and interfering in their evolution with the use of technology. Bioconservatism defends the preservation and protection of ‘human essence’ and expresses strong concerns about the impact of advanced technologies on the human condition (Ranisch and Sorgner, 2014 ).
For the general public, HGE used in a clinical context seems to be less contentious compared when used as a possible human enhancement tool. Specifically, some surveys indicate that the general-public typically exhibits a reduced support for the use of genome editing interventions for enhancement purposes compared to therapeutic purposes (Gaskell et al., 2017 ; Scheufele et al., 2017 ). In contrast, many technologies and pharmaceutical products developed in the medical context to treat patients are already being used by individuals to ‘enhance’ some aspect of their bodies. Some examples include drugs to boost brain power, nutritional supplements, and brain-stimulating technologies to control mood, even though their efficiency and safety is not clear. This could suggest that views on enhancement may vary depending on the context and on what is perceived as an enhancement by individuals. It may be informative to carry out detailed population studies to explore whether real ethical boundaries and concerns exist, or whether these are purely the result of the way information is processed and perceived.
Heritable genome editing: Mapping the ethical debate
Even though genome editing methods have only been developed in the last decade, the normative implication of interventions into the human germline have been discussed since the second half of the 20th century (Walters et al., 2021 ). Some even argue that, virtually, all the ethical issues raised by genetic engineering were already being debated at that time (Paul, 2005 ). This includes questions about the distinction between somatic and germline interventions, as well as between therapy and enhancement (e.g., Anderson, 1985 ). Nevertheless, as it has been widely noted, it is difficult to draw clear lines between these two categories (e.g., McGee, 2020 ; Juengst, 1997 ), and alternative frameworks have been proposed, particularly in the context of HGE (Cwik, 2020 ). Other questions include the normative status of human nature (e.g., Ramsey, 1970 ), the impossibility of consent from future generations (e.g., Lappe, 1991 ), possible slippery slopes towards eugenics (e.g., Howard and Rifkin, 1977 ), or implications for justice and equality (e.g., Resnik, 1994 ).
When discussing the ethics of HGE, roughly three types of considerations can be distinguished: (i) pragmatic, (ii) sociopolitical, and iii) categorical (Richter and Bacchetta, 1998 ; cf. Carter, 2002 ). Pragmatic considerations focus on medical or technological aspects of HGE, such as the safety or efficacy of interventions, risk–benefit ratio, possible alternatives or the feasibility of responsible translational research. Such considerations largely depend on the state of science and are thus always provisional. For example, if high-risk technologies one day evolve into safe and reliable technologies, some former pragmatic considerations may become obsolete. Sociopolitical aspects, on the other hand, are concerned with the possible societal impact of technologies, e.g., how they can promote or reduce inequalities, support or undermine power asymmetries, strengthen, or threaten democracy. Similar to pragmatic considerations, sociopolitical reasons depend on specific contexts and empirical factors. However, these are in a certain sense ‘outside’ the technology—even though technologies and social realities often have a symbiotic relationship. While sociopolitical considerations can generate strong reasons against (or in favour of) implementing certain technologies, most often these concerns could be mitigated by policies or good governance. Categorical considerations are different and more akin to deontic reasons. They emphasise categorical barriers to conduct certain deeds. It could be argued, for instance, that the integrity of the human genome or the impossibility to obtain consent from future generation simply rule out certain options to modify human nature. Such categorical considerations may persist despite technological advances or changing sociopolitical conditions.
Comparing the bioethical literature on genetic engineering from the last century with the ongoing discussions shows a remarkable shift in the ethical deliberation. In the past, scholars from the field of medical ethics, as well as policy reports, used to focus on possible categorical boundaries for germline interventions and on possible sociopolitical consequences of such scenarios. For instance, the influential 1982 report “Splicing Life” from the US President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioural Research prominently discussed concerns about ‘playing God’ against the prospects of genetically engineering human beings, as well as possible adverse consequences of such interventions. Although this study addresses potential harms, pragmatic arguments played only a minor role, possibly due to the technical limitations at the time.
With the upcoming availability of effective genome editing techniques, the focus on the moral perspective seems to have been reversed. Increasingly, the analysis of the permissibility of germline interventions is confined to questions of safety and efficacy. This is demonstrated by the 2020 consensus study report produced by an international commission convened by the US National Academy of Medicine, the US National Academy of Sciences, and the UK’s Royal Society, which aimed at defining a translational pathway for HGE. Although the report recognizes that HGE interventions does not only raise pragmatic questions, ethical aspects were not explicitly addressed (National Academy of Medicine, National Academy of Sciences, and the Royal Society, 2020 ).
Similarly, in 2019, a report on germline interventions published by the German Ethics Council (an advisory body to the German government and parliament) emphasizes that the “previous categorical rejection of germline interventions” could not be maintained (Deutscher Ethikrat, 2019 , p. 5). The German Ethics Council continues to address ethical values and societal consequences of HGE. However, technical progress and the development of CRISPR/Cas9 tools seem to have changed the moral compass in the discussion about germline interventions.
For a comprehensive analysis of HGE to focus primarily on pragmatic arguments such as safety or efficacy would be inadequate. In recent years, developments in the field of genome editing have occurred at an incredibly fast pace. At the same time, there are still many uncertainties about the efficacy of the various gene editing methods and unexpected effects in embryo editing persist (Ledford, 2015 ). Social and political implication also remain largely unknown. To date, it has been virtually impossible to estimate how deliberate interventions into the human germline could shape future societies and to conduct a complete analysis of the safety aspects of germline interventions.
Moreover, as the EGE notes, we should be cautious not to limit the complex process of ethical decision-making to pragmatic aspects such as safety. The “‘safe enough’ narrative purports that it is enough for a given level of safety to be reached in order for a technology to be rolled out unhindered, and limits reflections on ethics and governance to considerations about safety” (EGE, 2021 , p. 20). Consequently, the EGE has highlighted the need to engage with value-laden concepts such as ‘humanness’, ‘naturalness’ or ‘human diversity’ when determining the conditions under which HGE could be justified. Even if a technology has a high level of safety, its application may still contradict ethical values or lead to undesirable societal consequences. Efficacy does not guarantee compatibility with well-established ethical values or cultural norms.
While concepts such as ‘safety’ or ‘risk’ are often defined in scientific terms, this does not take away the decision of what is ethically desirable given the technical possibilities. As Hurlbut and colleagues put it in the context of genome editing: “Limiting early deliberation to narrowly technical constructions of risk permits science to define the harms and benefits of interest, leaving little opportunity for publics to deliberate on which imaginations need widening, and which patterns of winning and losing must be brought into view” (Hurlbut et al., 2015 ). Therefore, if public engagement is to be taken seriously, cultural norms and values of those affected by technologies must also be considered (Klingler et al., 2022 ). This, however, means broadening the narrow focus on pragmatic reasons and allowing categorical as well as sociopolitical concerns in the discourse. Given the current attention on pragmatic reasons in current debates on HGE, it is therefore beneficial to revisit the categorical and sociopolitical concerns that remain unresolved. The following sections provide an overview of relevant considerations that can arise in the context of HGE and that underline many of the societal concerns and values crucial for public engagement.
Human genome ‘integrity’
Heritability seems to be one of the foremost considerations regarding germline genome editing, as it raises relevant questions on a ‘natural’ human genome and its role in ‘human nature’ (Bayertz, 2003 ). This follows an ongoing philosophical debate on ‘human nature’, at least as defined by the human genome. This has ensued a long debate on the value of the human genome and normative implications associated with its modification (e.g., Habermas, 2003 ). Although a comprehensive discussion of these topics goes beyond the scope of this paper, the human genome is viewed by many as playing an important role in defining ‘human nature’ and providing a basis for the unity of the human species (for discussion: Primc, 2019 ). Considering the implications for the individual and the collective, some affirm the right of all humans to inherit an unmodified human genome. For some authors, germline modification is considered unethical, e.g., a “line that should not be crossed” (Collins, 2015 ) or a “crime against humanity” (Annas et al., 2002 ).
The Universal Declaration on the Human Genome and Human Rights (UDHGHR) states that “the human genome underlies the fundamental unity of all members of the human family, as well as the recognition of their inherent dignity and diversity. In a symbolic sense, it is the heritage of humanity” (Article 1, UNESCO, 1997 ). The human genome is viewed as our uniquely human collective ‘heritage’ that needs to be preserved and protected. Critics of heritable genetic interventions argue that germline manipulation would disrupt this natural heritage and therefore would threaten human rights and human equality (Annas, 2005 ). Heritable human genome editing creates changes that can be heritable to future generations. For many, this can represent a threat to the unity and identity of the human species, as these modifications could have an impact on the human’s gene pool. Any alterations would then affect the evolutionary trajectory of the human species and, thus, its unity and identity.
However, the view of the human genome as a common heritage is confronted with observations of the intrinsic dynamism of the genome (Scally, 2016 ). Preservation of the human genome, at least in its current form, would imply that the genome is static. However, the human genome is dynamic and, at least in specific periods of environmental pressure, must have naturally undergone change, as illustrated by human evolution (Fu and Akey, 2013 ). The genome of any individual includes mutations that have occurred naturally. Most of them seem to be neither beneficial nor detrimental to the ability of an individual to live or to his/her health. Others can be detrimental and limiting to their wellbeing. It has been shown that, on average, each human genome has 60 new mutations compared to their parents (Conrad et al., 2011 ). At the human population level, a human genome can have in average 4.1–5 million variants compared to the ‘reference’ genome (Li and Sadler, 1991 ; Genomes Project C, 2015 ). The reference genome itself is thus a statistical entity, representing the statistic distribution of the probability of different gene variants in the whole genome. Human genomic variation is at the basis of the differences in the various physical traits present in humans (e.g., eye colour, height, etc.), as well as specific genetic diseases. Thus, the human population is comprised of genomes with a pattern of variants and not of ‘one’ human genome that needs to be preserved (Venter et al. 2001 ). The human genome has naturally been undergoing changes throughout human history. An essentialist view of nature seems to be the basis for calling for the preservation of genome integrity. However, in many ways, this view is intrinsically challenged by the interpretation portrayed by evolutionary biology of our genetic history already more than a century ago. Nevertheless, despite the dynamic state of the human genome, this in itself cannot justify the possibility of modifying the human genome. It is also worth considering that the integrity of the human genome could also be perceived in a ‘symbolic’ rather than biological literal meaning. Such an interpretation would not require a literally static genome over time, but instead suggest a boundary between ‘naturally’ occurring variation and ‘artificially’ induced change. This is rather a version of the ‘natural’/unnatural argument, rather than an argument for a literally unchanged genetic sequence.
The modification of the human genome raises complex questions about the characterization of the human species genome and if there should be limits on interfering with it. The options to modify the human genome could range from modifying only the genes that are part of the human gene pool (e.g., those genes involved in severe genetic diseases such as Huntington’s disease) to adding new variants to the human genome. Regarding variants which are part of the common range of variation found in the human population (although it is not possible to know all the existent variations), the question becomes whether HGE could also be used in any of them (e.g., even the ones providing some form of enhancement) or only in disease-associated variants and thus be restricted to the prevention of severe genetic diseases. In both cases, the integrity of the human genome is expected to be maintained with no disruption to human lineage. However, it could be argued that this type of modification is defending a somewhat conservative human nature argument, since it is considering that a particular genetic make-up is ‘safe’ or would not involve any relevant trade-offs. In contrast, a different conclusion could be drawn on the integrity of the human genome when introducing genotypical and phenotypical traits that do not lie within the common range of variation found in the population (Cwik, 2020 ). In all cases, since the implications of the technology are intergenerational and consequently, it will be important to carry out an assessment of the risks that we, as a species, are willing to take when dealing with disease and promoting health. For this, we will need to explore societal views, values and cultural norms associated with the human genome, as well as possibly existing perceptions of technology tampering with ‘nature’. To support such an assessment, it would be useful to draw on a firm concept of human nature and the values it implies, beyond what is implied by genetic aspects.
Human dignity
In several of the legally binding and non-binding documents addressing human rights in the biomedical field, human dignity is one of the key values emphasized. There are concerns that heritable genome interventions might conflict with the value of human dignity (Calo, 2012 ; Melillo, 2017 ). The concerns are considered in the context of preserving the human genome (Nordberg et al. 2020 ). More specifically, the recommendation on Genetic Engineering by the Council of Europe (1982) states that “ the rights to life and to human dignity protected by Articles 2 and 3 of the European Convention on Human Rights imply the right to inherit a genetic pattern which has not been artificially changed” (Assembly, 1982 ). This is supported by the Oviedo Convention on Human Rights and Biomedicine (1997), where Article 13 prohibits any genetic intervention with the aim of introducing a modification in the genome of any descendants. The Convention is the only international legally binding instrument that covers human germline modifications among the countries which have ratified it (Council of Europe, 1997 ). However, there have been some authors disputing the continued ban proposed by the Oviedo Convention (Nordberg et al. 2020 ). Such authors have focused on the improvements of safety and efficacy of the technology in contrast to authors focusing on its value for human dignity (Baylis and Ikemoto, 2017 ; Sykora and Caplan, 2017 ). The latter authors seem to highlight the concept of human dignity to challenge heritable interventions to the human genome.
But a question in debate has been to demonstrate how ‘human dignity’, described in such norms, relates to heritable genome interventions. The concept of the human genome as common genetic heritage, distinguishing humans from other species seems one of the main principles implied by such norms. In this view, the human genome determines who belongs to the human species and who does not, and thus confers an individual the dignity of being a human by association. This creates an inherent and strong link between the concept of human genome and the concept of human dignity and its associated legal rights (Annas, 2005 ). It could be argued that a genetic modification to an individual may make it difficult for him/her to be recognized as a human being and therefore, preservation of the human genome being important for human dignity to be maintained. This simple approach, or at least interpretation, however, ignores the fact that the human genome is not a fixed or immutable entity, as exemplified by human evolution (as discussed in the previous section). As a result, the view that HGE interventions are inherently inadmissible based on the need to preserve human dignity is contested (Beriain, 2018 ; Raposo, 2019 ). More broadly, the idea that biological traits are the basis for equality and dignity, supporting the need for the human genome to be preserved, is often challenged (Fenton, 2008 ).
It is argued that to fully assess the impact of the HGE interventions on human dignity, it will be necessary to have a better understanding of the concept of human dignity in the first place (Häyry, 2003 ; Cutas, 2005 ). For some, however, human dignity is a value that underlies questions of equality and justice. Thus, the dignity-based arguments could uncover relevant questions in the discussion of ethical implications on modifying the human genome (Segers and Mertes, 2020 ). In the Nuffield Council on Bioethics Report (2018) principles of social justice and solidarity, as well as welfare, are used to guide the debate on managing HGE interventions. Similarly, the concept of human dignity could, therefore, provide the platform upon which consideration of specific values could be discussed, broaden the debate on HGE to values shared by society.
Right of the child: informed consent
In many modern societies, every individual, including children, have the rights to autonomy and self-determination. Therefore, each person is entitled to decide for themselves in decisions relating to their body. These rights are important for protecting the physical integrity of a person. When assessing the implication of allowing individuals to take (informed) decisions relative to the use of heritable genetic interventions on someone else’s body, it is useful to reflect on the maturity of existing medical practices and, more broadly, on the additional complexities associated with the heritability of any such intervention.
In modern health-care systems, informed consent provides the opportunity for an individual to exercise autonomy and make an informed decision about a medical procedure, based on their understanding of the benefits and risks of such procedure. Informed consent is thus a fundamental principle in medical (research) ethics when dealing with human subjects (Beauchamp and Childress, 2019 ).
Heritable genome interventions present an ethical constraint on the impossibility of future generations of providing consent to an intervention on their genome (Smolenski, 2015 ). In other words, future generations cannot be involved in a decision which could limit their autonomy, since medical or health-related decisions affecting them are placed on the present generation (and, in the case of a child to be born, more specifically, on his/her parents). However, many other actions taken by parents of young children also intentionally influence the lives of those children and have been doing so for millennia (Ranisch, 2017 ). Although these actions may not involve altering their genes, many of such actions can have a long-lasting impact on a child’s life (e.g., education and diet). However, it could be argued that they do not have the irreversible effect that HGE will have in the child and future generations. In cases where parents act to expand the life choices of their children by eliminating disease (e.g., severe genetic diseases), this would normally be thought to outweigh any possible restriction on autonomy. In these cases, if assuming HGE benefits will outweigh risks regarding safety and efficacy, the use of HGE could be expected to contribute to the autonomy of the child, as him/her would be able in the future to have a better life, not constrained by the limitations of the disease. As a result, even if it is accepted that these technologies may in one way reduce the autonomy of future generations, some believe that this will often be outweighed by other effects increasing autonomy (Gyngell et al., 2017 ). In other words, it is reasonable to suppose that, when taken by parents based on good information and understanding of risks and impacts, the limitation in the autonomy of unborn children associated with heritable genetic interventions would be compensated by the beneficial effects of increasing their autonomy when born (Gyngell et al., 2017 ).
It has often been emphasized that possible genetic interventions must not curtail the future possibilities of offspring to live their lives according to their own idea of a good life. This view originated in the liberal tradition and is associated with the “right to an open future”, defended by Joel Feinberg ( 1992 ). That is an anticipatory autonomy right that parents can violate, even though the offspring could exercise it only in the future. Feinberg has discussed the right to an open future in the context of religious education. However, various authors have applied this argument to the question of permissible and desirable genetic interventions (Buchanan et al., 2000 ; Glover, 2006 ; Agar, 1998 ). Accordingly, germline modifications or selection would have to allow the offspring to have a self-determined choice of life plans. It would therefore be necessary to provide offspring with genetic endowments that represent the so-called all-purpose goods. These goods are “useful and valuable in carrying out nearly any plan of life or set of aims that humans typically have” (Buchanan et al., 2000 , p. 167). While this claim is certainly appealing, in reality it will be difficult to identify phenotypes that will only broaden and do not narrow the spectrum of life plans. Take, for example, body size: a physique favourable for a basketball player would at the same time be less favourable in successfully riding horses as a professional jockey and vice versa. Increasing some opportunities often means reducing other ones.
The arguments of informed consent and open future need to be explored outside the realm of severe genetic diseases by considering other scenarios (including scenarios of genetic enhancement). Hereby, the effects of the interventions on the autonomy of future generations can be assessed more comprehensively. As for enhancement, decisions outside the realm of health can be more controversial, as the traits that parents see fit to generate enhancement may inadvertently condition a child’s choices in the future in an undesirable way.
If HGE is to be used, questions on how the consent and information should be provided to parents to fully equip them to decide in the best interests of the child will need to be assessed (Evitt et al., 2015 ). This is evident if considering the informed consent used in the study conducted by He Jiankui. One of the many criticisms of the study was the inadequacy of the informed consent process provided to the parents, which did not meet regulatory or ethical standards (Krimsky, 2019 ; Kirksey, 2020 ). This raises questions on how best to achieve ethical and regulatory compliance regarding informed consent in applications of HGE (Jonlin, 2020 ).
Discrimination of people with disabilities
For many years, there has been an effort to develop selective reproduction technologies to prevent genetic diseases or conditions leading to severe disabilities. These forms of reproductive genetic disease prevention are based on effectively filtering and eradicating embryos or foetuses affected by genetic diseases. There are divergent views regarding the use of these technologies. For example, the disability rights movement argues that the use of technologies such as prenatal testing (PNT) and PGD discriminates against people living with a disability (Scully, 2008 ; Asch and Barlevy, 2012 ). The key arguments presented supporting this view are: (i) the limited value of a genetic trait in respect to the life of an embryo (Parens and Asch, 2000 ) and (ii) the ‘expressivist’ argument (Buchanan, 1996 ; Shakespeare, 2006 ). The first argument is based on the critique that a disabling trait is viewed as being more significant than the life of an embryo/foetus. This argument was initially used in the context of prenatal testing and selective termination, and has also been applied in the context of new technologies like PGD (Parens and Asch, 2000 ). The second, the ‘expressivist’ argument, argues that the use of these technologies expresses negative or discriminatory views on the disabling conditions they are targeting and subsequently on the people living with these conditions (Asch and Wasserman, 2015 ). The expressivist argument, however, has been challenged by stressing the importance of differentiating between the disability itself and the people living with disability (Savulescu, 2001 ). The technology’s use is aimed at reducing the incidence of disability, and it does not have a position of value on the people that have a specific condition.
When applying the same arguments to the use of HGE in comparison with other forms of preventing heritable genetic diseases, some important considerations can be made. Regarding the first argument, in contrast to selective reproduction technologies, HGE may allow the removal of the disabled trait with the aim of ensuring survival of the affected embryo. However, most likely, PGD would be used before and after the editing of the embryos to help the identification of the ones requiring intervention and verifying the efficiency of the genetic intervention (de Miguel Beriain, 2018 ; Ranisch, 2020 ). Similarly, the expressivist argument continues to be challenged if the application of human HGE is envisaged in the context of severe genetic diseases (e.g., Tay-Sachs and Huntington’s disease). It has been argued that the choice to live without a specific genotype neither implies discriminating people living with a respective condition nor considering the life of people living with the disease not worth living or less valuable (Savulescu, 2001 ). In other words, the expressivist argument is not a valid or a sufficiently strong ethical argument for prospective parents not to have the option to have a future child without a genetic disease.
It is worth noting that the debate on the use of reproduction technologies for the prevention of genetic diseases is not at all new, and that modern HGE techniques only serve to highlight ethical concerns that have been expressed for a long time. In the case of preventing genetic diseases, the application of both arguments to HGE intervention could be considered not to provide sufficiently strong ethical arguments to limit the use of the technology in the future. However, it is worth exploring whether scientific innovations like HGE are either ameliorating or reinvigorating ethical concerns expressed so far, for example in creating a future that respects or devalues disability as a part of the human condition. Perhaps even more importantly, given their potential spectrum of possible intervention and efficacy, it is important to reflect on whether the broad use of HGE could have an impact on concepts of disability and ‘normality’ as a whole distorting an already unclear ethical line between clinical and non-clinical interventions. Moreover, research work exploring the relationship between disability and identity indicated that personhood with disability can be an important component to people’s identity and interaction with the world. In the case of heritable human genome editing, it is not yet known how this technology will impact the notions of identity and personhood in people who had their germline genome modified (Boardman and Hale, 2018 ). For further progress on these issues public engagement might be important to gather different views and perceptions on the issue.
Justice and equality
Beside the limits of applicability, another common ethical concern associated with the use of genome editing technologies, as with many new technologies, is the question of accessibility (Baumann, 2016 ). Due to the large investments that will need to be made for continuing development of the technology, there is a (perceived) risk of it becoming an expensive technology that only a few wealthy individuals in any population (and/or only citizens in comparatively rich countries) can access. In addition, there is concern that patenting of genome editing technologies will delay widespread access or lead to unequal distribution of corresponding benefits (Feeney et al., 2018 ). This may, consequently, contribute to further increases in existing disparities, since individuals or countries with the means of accessing better health treatments may have economic advantages (Bosley et al., 2015 ). This could enhance inequality at different levels, depending on the limits of applicability of the technology. Taken to its extreme, the use of the technology could allow germline editing to create and distinguish classes of individuals that could be defined by the quality of their manipulated genome.
The concern that the possibility of germline interventions in humans could entrench or even increase inequalities has accompanied the discussion about ethics of genetic interventions from the very beginning until today (e.g. Resnik, 1994 ). In ‘Remaking Eden’ Lee Silver envisioned a divided future society, consisting of a genetically enhanced class, the “genRich”, and a genetic underclass, the “naturals” (Silver, 1997 ). Françoise Baylis recently echoed such concerns regarding future HGE interventions, namely that “unequal access to genome-editing technologies will both accentuate the vagaries of the natural lottery and introduce an unjust genetic divide that mirrors the current unjust economic and social divide between rich and poor individuals” (Baylis, 2019 , p. 67). At the same time, the possibility to genetically intervene in the ‘natural lottery’ has also been associated with the hope of countering natural inequalities and increase equality of opportunities. Robert Sinsheimer may be among the first to envision such a ‘new’ individualistic type of ‘eugenics’ that “would permit in principle the conversion of all of the unfit to the highest genetic level” (Sinsheimer, 1969 , p. 13). More recently, in the book ‘From chance to choice: Genetics and justice’ (2000) it is argued that “equality of opportunity will sometimes require genetic interventions and that the required interventions may not always be limited to the cure or prevention of disease” (Buchanan et al., 2000 , p. 102). When discussing issues related to justice and equality, it will be important to involve a broad spectrum of stakeholders to better evaluate the economic effects of the commercialization of the technology.
Conclusions
With ongoing technological developments and progress with guiding and regulating its acceptable use, the possibility of HGE interventions in the human genome is closer than ever to becoming a reality. The range of HGE applicability can go from preventing the transmission of genetic variants associated with severe genetic conditions (mostly single gene disorders but also, to a lesser extent, polygenic diseases) to genetic enhancements. The permissibility of HGE has often been considered on the basis of possible uses, with therapeutic uses generally considered more acceptable than non-therapeutic ones (including human enhancement). When compared with other technologies with similar therapeutic uses (e.g., PGD) already in use, HGE presents similarities and differences. However, from an ethical acceptability perspective, there is currently no consensus on whether HGE is more or less acceptable than PGD.
An important conclusion of this study is that, along with the technological development of genome germline editing techniques, a shift in the focus of analyses on its applicability has been observed. More specifically, the emphasis on pragmatic considerations seems to have increased substantially compared with the previous emphasis on categorical and sociopolitical arguments. Many of the most recent publications from authoritative advisory committees and institutions discuss the permissibility of HGE interventions primarily on the basis of pragmatic arguments, in which safety and efficacy are the main focus. Since germline interventions could profoundly change the human condition, the need for a broad and inclusive public debate on this topic has also been frequently emphasized. However, limited consideration has been given to approaches to carry out such action effectively, and on how to consider their outcomes in relevant policies and regulations.
It is currently not entirely clear whether: (i) the pragmatic position championed by such authoritative sources builds on the premise that the ethical debate has reached sufficient maturity to allow a turning point; (ii) the lack of progress has somewhat hampered further consideration of issues still considered controversial; (iii) regulatory pressure is somewhat de facto pushing forward the introduction of such technologies despite critical, unresolved ethical issues. Based on the analysis presented in this paper, a combination of the latter factors (ii and iii) seems more likely. In engaging the public in societal debates on the acceptability of such technologies, unresolved questions are likely to re-emerge. Specifically, it is possible that categorical and sociopolitical considerations will gain renewed focus during public engagement. In other words, when involving the public in discussions on HGE, it is possible that cultural values and norms, not only questions of safety and efficacy, will re-emerge as crucial to the acceptance of the technology (What is meant by natural? What is understood by humanity? etc.).
HGE interventions put into question specific biological and moral views of individuals, including views on the value of the human genome, on human dignity, on informed consent, on disability and on societal equality and justice. The range of ethical issues affected by the introduction of such technology, often still characterised by non-convergent, and at times conflicting, positions, illustrate the importance of further consideration of these issues in future studies and public engagement activities. As a result, society’s moral uncertainties will need to be assessed further to support the regulation of HGE technologies and form a well-informed and holistic view on how they can serve society’s common goals and values.
Data availability
This statement is not applicable.
Agar N (1998) Liberal eugenics. Public Aff Q 12(2):137–155
PubMed Google Scholar
Almeida M, Diogo R (2019) Human enhancement: genetic engineering and evolution. Evol Med Public Health 1:183–189. https://doi.org/10.1093/emph/eoz026
Article Google Scholar
Anderson WF (1985) Human Gene Therapy: scientific and ethical considerations. J Med Philos 10(3):275–291. https://doi.org/10.1093/jmp/10.3.275
Article CAS PubMed Google Scholar
Andorno R, Baylis F, Darnovsky M, Dickenson D, Haker H, Hasson K et al. (2020) Geneva statement on heritable human genome editing: the need for course correction. Trends Biotechnol 38(4):351–354
Annas GJ (2005) Bioethics: crossing human rights and health law boundaries. Oxford University Press, New York, NY
Google Scholar
Annas GJ, Andrews LB, Isasi RM (2002) Protecting the endangered human: toward an international treaty prohibiting cloning and inheritable alterations. Am J Law Med 28(2–3):151–178
Article PubMed Google Scholar
Araki M, Ishii T (2014) International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol 12(1):108–120
Article CAS PubMed PubMed Central Google Scholar
Asch A, Barlevy D (2012) Disability and genetics: a disability critique of pre-natal testing and pre-implantation genetic diagnosis. eLS. Wiley, Chichester
Asch A, Wasserman D (2015) Reproductive testing for disability. In: Arras JD, Fenton E, Kukla R (eds.) Routledge companion to bioethics. Routledge, London
Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G et al. (2015) A prudent path forward for genomic engineering and germline gene modification. Science 348(6230):36. https://doi.org/10.1126/science.aab1028
Article ADS CAS PubMed PubMed Central Google Scholar
Baltimore D, Baylis F, Berg P et al. (2015) On human gene editing: international summit statement. News release, December 3, International summit on human gene editing. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a
Barrangou R, Horvath P (2017) A decade of discovery: CRISPR functions and applications. Nat Microbiol 2(17092):1–9. https://doi.org/10.1038/nmicrobiol.2017.92
Article CAS Google Scholar
Baumann M (2016) CRISPR/Cas9 genome editing: new and old ethical issues arising from a revolutionary technology. Nanoethics 10:139–59
Bayefsky MJ (2016) Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod Biomed Soc Online 3:41–47
Bayertz K (2003) Human nature: how normative might it be? J Med Philos 28(2):131–150. https://doi.org/10.1076/jmep.28.2.131.14210
Baylis F (2017) Human germline genome editing and broad societal consensus. Nat Hum Behav 1:0103
Baylis F (2019) Human genome editing: our future belongs to all of us. Issues Sci Technol 35:42–44
Baylis F, Ikemoto L (2017) The Council of Europe and the prohibition on human germline genome editing. EMBO Rep 8(12):2084–2085. https://doi.org/10.15252/embr.201745343
Baylis F, Darnovsky M, Hasson K, Krahn TM (2020) Human Germ Line and Heritable Genome Editing: the global policy landscape. CRISPR J 3(5):365–377. https://doi.org/10.1089/crispr.2020.0082 . PMID: 33095042
Beauchamp TL, Childress JF (2019) Principles of biomedical ethics. Oxford University Press, USA
Blendon RJ, Gorski MT, Benson JM (2016) The public and the gene-editing revolution. New Engl J Med 374(15):1406–1411. https://doi.org/10.1056/NEJMp1602010
Boardman FK, Hale R (2018) How do genetically disabled adults view selective reproduction? Impairment, identity, and genetic screening. Mol Genet Genom Med 6(6):941–956
Bosley KS, Botchan M, Bredenoord AL, Carroll D, Charo RA, Charpentier E et al. (2015) CRISPR germline engineering: the community speaks. Nat Biotechnol 33(5):478–486. https://doi.org/10.1038/nbt.3227
Botkin JR (2019) The case for banning heritable genome editing. Genet Med 22:487–489
Brokowski C (2018) Do CRISPR germline ethics statements cut it? CRISPR J 1(2):115–125. https://doi.org/10.1089/crispr.2017.0024
Article PubMed PubMed Central Google Scholar
Buchanan A (1996) Choosing who will be disabled: genetic intervention and the morality of inclusion. Soc Philos Policy 13:18–46
Buchanan A, Brock DW, Daniels N, Wikler D (2000) From chance to choice: genetics and justice. Cambridge University Press
Calo Z (2012) Human dignity and health law: personhood in recent bioethical debates. Notre Dame J Law Ethics Public Policy 26:473–499
Carter L (2002) The ethics of germ line gene manipulation—a five dimensional debate. Monash Bioeth Rev 21(4):S66–S81. https://doi.org/10.1007/BF03351288
Cavaliere G (2018) Genome editing and assisted reproduction: curing embryos, society or prospective parents? Med Health Care Phil 21(2):215–25
Coller BS (2019) Ethics of human genome editing. Annu Rev Med 27(70):289–305. https://doi.org/10.1146/annurev-med-112717-094629
Council of Europe (1997) Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: convention on human rights and biomedicine. COE, Oviedo
Collins, F. (2015) Director, National Institutes of Health, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologieshuman-embryos
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F et al. (2011) Variation in genome-wide mutation rates within and between human families. Nat Genet 43(7):712–4. https://doi.org/10.1038/ng.862
Cutas DE (2005) Looking for the meaning of dignity in the bioethics convention and the cloning protocol. Health Care Anal 13(4):303–313
Cwik B (2020) Revising, correcting, and transferring genes. Am J Bioeth 20(8):7–18
Cyranoski D (2019) Russian biologist plans more CRISPR-edited babies. Nature 570(7760):145–147
Article ADS CAS PubMed Google Scholar
Daley GQ, Lovell-Badge R, Steffann J (2019) After the storm—a responsible path for genome editing. N Engl J Med 380:897–899
de Araujo M (2017) Editing the genome of human beings: CRISPR-Cas9 and the ethics of genetic enhancement. J Evol Technol 27(1):24–42. http://jetpress.org/v27.1/araujo.pdf
Dance A (2015) Core concept: CRISPR gene editing. Proc Natl Acad Sci USA 112:6245–6246
Davies K (2020) Editing humanity: the CRISPR revolution and the new era of genome editing. Pegasus Books, New York, NY
Delaney JJ (2011) Possible people, complaints, and the distinction between genetic planning and genetic engineering. J Med Ethics 37(7):410–414
Deutscher Ethikrat, (2019) Intervening in the Human Germline. https://www.ethikrat.org/en/publications/publication-details/?tx_wwt3shop_detail%5Bproduct%5D=119&tx_wwt3shop_detail%5Baction%5D=index&tx_wwt3shop_detail%5Bcontroller%5D=Products&cHash=25e88ad52f8b75d311510a9bf7a8dc86
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096. https://doi.org/10.1126/science.1258096
Drabiak K (2020) The Nuffield Council’s green light for genome editing human embryos defies fundamental human rights law. Bioethics 34:223–227
Dzau VJ, McNutt M, Bai C (2018) Wake-up call from Hong Kong. Science 362(6420):1215. https://doi.org/10.1126/science.aaw3127
de Miguel Beriain I (2020) Gene editing and disabled people: a response to Felicity Boardman. J Community Genet 11(3):241–243
de Miguel Beriain I (2018) Human dignity and gene editing. EMBO Rep 19:e46789
de Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, van El CG (2018) Responsible innovation in human germline gene editing: background document to the recommendations of ESHG and ESHRE. Eur J Hum Genet 26(4):450–470. https://doi.org/10.1038/s41431-017-0077-z
EGE (2016) Statement on gene editing. https://ec.europa.eu/info/sites/default/files/research_and_innovation/ege/gene_editing_ege_statement.pdf
EGE (2021) Ethics of genome editing. https://ec.europa.eu/info/sites/default/files/research_and_innovation/ege/ege_ethics_of_genome_editing-opinion_publication.pdf
Evitt NH, Mascharak S, Altman RB (2015) Human germline crispr-cas modification: toward a regulatory framework. Am J Bioeth 15(12):25–29
Feeney O, Cockbain J, Morrison M, Diependaele L, Van Assche K, Sterckx S (2018) Patenting foundational technologies: lessons from CRISPR and other core biotechnologies. Am J Bioeth 18(12):36–48
Fenton E (2008) Genetic enhancement – a threat to human rights? Bioethics 22(1):1–7 https://doi.org/10.1111/j.1467-8519.2007.00564.x
Feinberg J (1992) The child’s right to an open future. In: Feinberg J (ed.) Freedom and fulfillment: philosophical essays. Princeton University Press, Princeton, pp. 6–97
Fu W, Akey JM (2013) Selection and adaptation in the human genome. Annu Rev Genom Hum Genet 14:467–89
Fukuyama F (2002) Our posthuman future: consequences of the biotechnology revolution. Picador, New York, NY
Gaj T, Gersbach CA, Barbas III CF (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31(7):397–405
Gaskell G, Bard I, Allansdottir A, da Cunha RV, Eduard P, Hampel J et al. (2017) Public views on gene editing and its uses. Nat Biotechnol 35(11):1021–1023. https://doi.org/10.1038/nbt.3958
Genomes Project C et al. (2015) A global reference for human genetic variation. Nature 526(7571):68–74
Article ADS CAS Google Scholar
Greely HT (2019) Human germline genome editing: an assessment. CRISPR J 2(5):253–265. https://doi.org/10.1089/crispr.2019.0038
Glover J (2006) Choosing children: genes, disability, and design. Oxford University Press, Oxford
Book Google Scholar
Greely HT (2021) CRISPR people: the science and ethics of editing humans. MIT Press, Cambridge, MA; London, UK
Gupta RM, Musunuru K (2014) Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Investig 124(10):4154–4161. https://doi.org/10.1172/JCI72992
Gyngell C, Douglas T, Savulescu J (2017) The ethics of germline gene editing. J Appl Philosy 34(4):498–513
Gyngell C, Bowman-Smart H, Savulescu J (2019) Moral reasons to edit the human genome: picking up from the Nuffield report. J Med Ethics 0:1–10. https://doi.org/10.1136/medethics-2018-105084
Gyngell C, Douglas T (2015) Stocking the genetic supermarket: reproductive genetic technologies and collective action problems. Bioethics 29(4):241–250
Guttinger S (2017) Trust in science: CRISPR-Cas9 and the ban on human germline editing. Sci Eng Eth 1–20. https://doi.org/10.1007/s11948-017-9931-1
Habermas J (2003) The future of human nature. Polity Press, Cambridge
Hammerstein AL, Eggel M, Biller-Andorno N (2019) Is selecting better than modifying? An investigation of arguments against germline gene editing as compared to preimplantation genetic diagnosis. BMC Med Eth 83:20
Häyry M (2003) Philosophical arguments for and against human reproductive cloning. Bioethics 17(5–6):447–460
Howard T, Rifkin J (1977) Who should play God? The artificial creation of life and what it means for the future of the human race. Dell Publ. Co
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157(6):1262–1278. https://doi.org/10.1016/j.cell.2014.05.010
Hurlbut JB, Saha K, Jasanoff S (2015) CRISPR democracy: gene editing and the need for inclusive deliberation. Issues Sci Technol 32(1):25–32
Hyun I, Osborn C (2017) Query the merits of embryo editing for reproductive research now. Nat Biotechnol 35(11):1023–1025. https://doi.org/10.1038/nbt.4000 . PMID: 29121025
Iltis AS, Hoover S, Matthews KRW (2021) Public and stakeholder engagement in developing human heritable genome editing policies: what does it mean and what should it mean? Front Political Sci 3. https://www.frontiersin.org/article/10.3389/fpos.2021.730869
Isasi R, Kleiderman E, Knoppers BM (2016) Genetic technology regulation: editing policy to fit the genome? Science 351(6271):337–339. https://doi.org/10.1126/science.aad6778
Ishii T (2015) Germline genome-editing research and its socio-ethical implications. Trends Mol Med 21(8):473–481. https://doi.org/10.1016/j.molmed.2015.05.006
Janssens AC (2016) Designing babies through gene editing: science or science fiction? Genet Med 18(12):1186–1187. https://doi.org/10.1038/gim.2016.28
Jedwab A, Vears DF, Tse C, Gyngell C (2020) Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public. J Hum Genetics65(12):1055–1065
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) Programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jonlin EC (2020) Informed consent for human embryo genome editing. Stem Cell Rep 14(4):530–537. https://doi.org/10.1016/j.stemcr.2020.03.010
Juengst ET (1997) Can enhancement be distinguished from prevention in genetic medicine? J Med Philos 22(2):125–142
Juengst, ET, Moseley D (2019) Human enhancement. In: Zalta EN (ed.) The Stanford encyclopedia of philosophy (Summer 2019 Edition), Metaphysics Research Lab, Philosophy Department, Stanford University, Stanford
Karavani E, Zuk O, Zeevi D, Barzilai N, Stefanis NC, Hatzimanolis A et al. (2019) Screening human embryos for polygenic traits has limited utility. Cell 179(6):1424–1435
Kirksey E (2020) The Mutant Project: inside the global race to genetically modify humans. St. Martin’s Press
Klingler C, Wiese L, Arnason G, Ranisch R (2022) Public engagement with brain organoid research and application: lessons from genome editing. Am J Bioeth Neurosci 13(2):98–100. https://doi.org/10.1080/21507740.2022.2048733
König H (2017) The illusion of control in germline-engineering policy. Nat Biotechnol 35(6):502–506. https://doi.org/10.1038/nbt.3884
Krimsky S (2019) Ten ways in which He Jiankui violated ethics. Nat Biotechnol 37(1):19–20. https://doi.org/10.1038/nbt.4337
Lander ES (2015) Brave new genome. N Engl J Med 373(1):5–8
Lander ES, Baylis F, Zhang F, Charpentier E, Berg P, Bourgain C et al. (2019) Adopt a moratorium on heritable genome editing. Nature 567(7747):165–168. https://doi.org/10.1038/d41586-019-00726-5
Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J (2015) Don’t edit the human germ line. Nature 519(7544):410–1. https://doi.org/10.1038/519410a
Lappe M (1991) Ethical issues in manipulating the human germ line. J Med Philos 16(6):621–639. https://doi.org/10.1093/jmp/16.6.621
Lea RA, Niakan KK (2019) Human germline genome editing. Nat Cell Biol 21(12):1479–1489. https://doi.org/10.1038/s41556-019-0424-0
Ledford H (2015) The landscape for human genome editing. Nature 526(7573):310–311
Leon K (2003) Ageless bodies, happy souls: biotechnology and the pursuit of perfection. New Atlantis 1:9–28
Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X (2020) Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther 5(1):1–23
Li WH, Sadler LA (1991) Low nucleotide diversity in man. Genetics 129(2):513–23
Liang P, Xu Y, Zhang X et al. (2015) CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6(5):363–372
Lovell-Badge R (2019) CRISPR babies: a view from the centre of the storm. Development 146(3):dev175778
MacKellar C, Bechtel C eds. (2014) The ethics of the new eugenics. Berghahn Books, New York, Oxford
McGee A (2020) Using the therapy and enhancement distinction in law and policy. Bioethics 34(1):70–80
Melillo TR (2017) Gene editing and the rise of designer babies. Vand J Trans Law 50:757–790
Mertes H, Pennings G (2015) Modification of the embryo’s genome: more useful in research than in the clinic. Am J Bioeth 15(12):52–53. https://doi.org/10.1080/15265161.2015.1103813
Mulvihill JJ, Capps B, Joly Y, Lysaght T, Zwart HAE, Chadwick R, International Human Genome Organisation Committee of Ethics Law and Society (2017) Ethical issues of CRISPR technology and gene editing through the lens of solidarity. Br Med Bull 122(1):17–29. https://doi.org/10.1093/bmb/ldx002
Musunuru K (2019) The CRISPR generation: the story of the world’s first gene-edited babies. BookBaby.
National Academies of Sciences, Engineering, and Medicine (2015) International summit on human gene editing: a global discussion. The National Academies Press, Washington
National Academy of Sciences, National Academy of Medicine (2017) Human genome editing: science, ethics and governance. The National Academies Press, Washington, DC
National Academy of Medicine, National Academy of Sciences, and the Royal Society (2020) Heritable human genome editing. The National Academies Press, Washington, DC
Niemiec E, Howard HC (2020) Ethical issues related to research on genome editing in human embryos. Comput Struct Biotechnol J 18:887–896
National Academies of Sciences, Engineering, and Medicine (2019) Second International Summit on human genome editing: continuing the global discussion: proceedings of a workshop in brief. The National Academies Press, Washington
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L et al. (2014) Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156(4):836–843
Nordberg A, Minssen T, Feeney O, de Miguel Beriain I, Galvagni L, Wartiovaara K (2020) Regulating germline editing in assisted reproductive technology: an EU cross‐disciplinary perspective. Bioethics 34(1):16–32
Nuffield Council on Bioethics (2016) Genome Editing: an ethical review. https://www.nuffieldbioethics.org/publications/genome-editing-an-ethical-review
Nuffield Council on Bioethics (2018) Genome editing and human reproduction: social and ethical issues. http://nuffieldbioethics.org/project/genome-editing-human-reproduction
Parens E, Asch A (2000) The disability rights critique of prenatal testing: reflections and recommendations. In: Parens E, Asch A (eds.) Prenatal testing and disability rights. Georgetown University Press, Washington
Parliamentary Assembly (1982) Recommendation on genetic engineering. In Recommendation 934. Council of Europe
Paul D (2005) Genetic engineering and eugenics: the uses of history. In: Baillie HW, Casey TK (eds.) Is human nature obsolete? Genetics, bioengineering, and the future of the human condition. MIT Press, Cambridge Mass
Peden JF, Farrall M (2011) Thirty-five common variants for coronary artery disease: the fruits of much collaborative labour. Hum Mol Genet 20:R198–205
Porteus MH (2019) A new class of medicines through DNA editing. New Engl J Med 380(10):947–959. https://doi.org/10.1056/NEJMra1800729
Parens ET (Ed.) (1998) Enhancing human traits: ethical and social implications. Georgetown University Press, Washington, DC
Primc N (2020) Do we have a right to an unmanipulated genome? The human genome as the common heritage of mankind Bioethics 34(1):41–48
Article MathSciNet PubMed Google Scholar
Ramsey P (1970) Fabricated man: the ethics of genetic control. Yale University Press, New Haven
Ranisch R (2017) Germline genome editing and the functions of consent. Am J Bioeth 17(12):27–29
Ranisch R (2020) Germline genome editing versus preimplantation genetic diagnosis: Is there a case in favour of germline interventions? Bioethics 34:60–69. https://doi.org/10.1111/bioe.12635
Ranisch R, Ehni HJ (2020) Fading red lines? Bioethics of germline genome editing. Bioethics 34(1):3–6. https://doi.org/10.1111/bioe.12709
Ranisch R, Rudolph T, Cremer HJ, Knoepffler N (2020) Ordo-responsibility for germline gene editing. CRISPR J 3(1):37–43
Ranisch R (2021) When CRISPR meets fantasy: transhumanism and the military in the age of gene editing. In: Transhumanism: the proper guide to a posthuman condition or a dangerous idea?. Springer, Cham, pp. 111–120
Ranisch R, Sorgner SL (2014) Introducing post-and transhumanism. In: Ranisch & Sorgner (eds.) Post-and transhumanism: an introduction. pp. 7–27. Peter Lang Group AG, Switzerland
Raposo VL (2019) Gene editing, the mystic threat to human dignity. Bioeth Inq 16:249–257. https://doi.org/10.1007/s11673-019-09906-4
Regalado A (2018) EXCLUSIVE: Chinese scientists are creating CRISPR babies. MIT Technol Rev. https://www.technologyreview.com/s/612458/exclusive-chinese-scientists-are-creating-crispr-babies/ . Accessed 4 Aug 2021
Rehmann-Sutter C (2018) Why human germline editing is more problematic than selecting between embryos: ethically considering intergenerational relationships. New Bioeth 24(1):9–25. https://doi.org/10.1080/20502877.2018.1441669
Resnik D (1994) Debunking the slippery slope argument against human germ-line gene therapy. J Med Philos 19(1):23–40. https://doi.org/10.1093/jmp/19.1.23
Richter G, Bacchetta MD (1998) Interventions in the human genome: some moral and ethical considerations. J Med Philos 23(3):303–317. https://doi.org/10.1076/jmep.23.3.303.2581
Rulli T (2019) Reproductive CRISPR does not cure disease. Bioethics 33:1072–1082
Sandel M (2007) The case against perfection: ethics in the age of genetic engineering. The Belknap Press of Harvard University Press, Cambridge
Savulescu J (2001) Procreative beneficence: why we should select the best children. Bioethics 15(5–6):413–26
Savulescu J, Pugh J, Douglas T, Gyngell C (2015) The moral imperative to continue gene editing research on human embryos. Protein Cell 6:476–479
Scally A (2016) The mutation rate in human evolution and demographic inference. Curr Opin Genet Dev 41:36–43
Schaefer GO (2020) Can reproductive genetic manipulation save lives? Med Health Care Philos 23(3):381–386
Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, Hardy BW (2017) U.S. attitudes on human genome editing. Science 357(6351):553–554. https://doi.org/10.1126/science.aan3708
Scheufele DA, Krause NM, Freiling I, Brossard D (2021) What we know about effective public engagement on CRISPR and beyond. Proc Natl Acad Sci USA 118(22). https://doi.org/10.1073/pnas.2004835117
Scully JL (2008) Disability and genetics in the era of genomic medicine. Nat Rev Genet 9(10):797–802
Segers S, Mertes H (2020) Does human genome editing reinforce or violate human dignity? Bioethics 34(1):33–40
Sermon K, Van Steirteghem A, Liebaers I (2004) Preimplantation genetic diagnosis. Lancet 363(9421):1633–1641. https://doi.org/10.1016/S0140-6736(04)16209-0
Shakespeare T (2006) Disability rights and wrongs. Routledge, London
Shulman C, Bostrom N (2014) Embryo selection for cognitive enhancement: curiosity or game-changer? Glob Policy 5(1):85–92. https://doi.org/10.1111/1758-5899.12123
Silver LM (1997) Remaking Eden: cloning and beyond in a brave new world. William Morrow, New York, NY
Sinsheimer RL (1969). The prospect for designed genetic change. Am Sci, 57(1):134–142. http://www.jstor.org/stable/27828443
Smolenski J (2015) Crispr/cas9 and germline modification: new difficulties in obtaining informed consent. Am J Bioeth 15(12):35–37
Soini S (2007) Preimplantation genetic diagnosis (PGD) in Europe: diversity of legislation a challenge to the community and its citizens. Med Law 26(2):309–323
ADS CAS PubMed Google Scholar
Sorgner SL (2018) Genes, CRISPR/Cas 9, and posthumans. In: Sinaci M and Sorgner SL (eds.) Ethics of emerging biotechnologies, pp. 5–17. Trivent Publishing
Sparrow R (2021) Human germline genome editing: on the nature of our reasons to genome edit Am J Bioeth 19:1–12
Steffann J, Jouannet P, Bonnefont JP, Chneiweiss H, Frydman N (2018) Could failure in preimplantation genetic diagnosis justify editing the human embryo genome? Cell Stem Cell 22(4):481–482. https://doi.org/10.1016/j.stem.2018.01.004
Sykora P, Caplan A (2017) The Council of Europe should not reaffirm the ban on germline genome editing in humans. EMBO Rep 18:1871–1872. https://doi.org/10.15252/embr.201745246
UNESCO (1997) Universal declaration on the human genome and human rights. UNESCO, Paris
van Dijke I, Bosch L, Bredenoord AL, Cornel M, Repping S, Hendriks S (2018) The ethics of clinical applications of germline genome modification: a systematic review of reasons Hum Reprod 33(9):1777–1796
Vassena R, Heindryckx B, Peco R, Pennings G, Raya A, Sermon K, Veiga A (2016) Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update 22(4):411–419. https://doi.org/10.1093/humupd/dmw005
Venter JC et al. (2001) The sequence of the human genome. Science 291(5507):1304–51
Walters L, Cook-Deegan RM, Adashi EY (2021) Governing heritable human genome editing: a textual history and a proposal for the future. CRISPR J 4(4):469–476
Wang H, Yang H (2019) Gene-edited babies: what went wrong and what could go wrong. PLoS Biol 17(4). https://doi.org/10.1371/journal.pbio.3000224
Wheeler E, Barroso I (2011) Genome-wide association studies and type 2 diabetes. Brief Funct Genom 10:52–60
WHO (2019) Statement on governance and oversight of human genome editing. https://www.who.int/news/item/26-07-2019-statement-on-governance-and-oversight-of-human-genome-editing
WHO (2021) Human genome editing: position paper. https://www.who.int/publications/i/item/9789240030404
Wolf DP, Mitalipov PA, Mitalipov SM (2019) Principles of and strategies for germline gene therapy. Nat Med 25(6):890–897
Zhang F (2019) Development of CRISPR-Cas systems for genome editing and beyond. Q Rev Biophys 52. https://doi.org/10.1017/s0033583519000052
Download references
Acknowledgements
This work was supported by Fundação para Ciência e a Tecnologia (FCT) of Portugal [UIDP/00678/2020 to M.A]. We thank Dr. Michael Morrison for his comments and Dr. Gustav Preller for his proofreading of this manuscript.
Author information
Authors and affiliations.
Centro de Filosofia das Ciências da Universidade de Lisboa, Campo Grande, 1749-016, Lisbon, Portugal
Mara Almeida
Faculty of Health Sciences Brandenburg, University of Potsdam, Karl-Liebknecht-Str. 24-25, House 16, 14476, Potsdam, Golm, Germany
- Robert Ranisch
Research Unit “Ethics of Genome Editing”, Institute for Ethics and History of Medicine, University of Tübingen, Gartenstr. 47, 72074, Tübingen, Germany
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mara Almeida .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
An ethical approval is not applicable.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.

Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Almeida, M., Ranisch, R. Beyond safety: mapping the ethical debate on heritable genome editing interventions. Humanit Soc Sci Commun 9 , 139 (2022). https://doi.org/10.1057/s41599-022-01147-y
Download citation
Received : 06 August 2021
Accepted : 28 March 2022
Published : 20 April 2022
DOI : https://doi.org/10.1057/s41599-022-01147-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Clinical translation of wireless soft robotic medical devices.
- Tianlu Wang
- Metin Sitti
Nature Reviews Bioengineering (2024)
An analysis of different concepts of “identity” in the heritable genome editing debate
- Ying-Qi Liaw
Medicine, Health Care and Philosophy (2024)
Initial heritable genome editing: mapping a responsible pathway from basic research to the clinic
- Katharina Trettenbach
- Gardar Arnason
Medicine, Health Care and Philosophy (2023)
“What if” should precede “whether” and “how” in the social conversation around human germline gene editing
- Diewertje Houtman
- Wendy Geuverink
- Sam Riedijk
Journal of Community Genetics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neurol Med Chir (Tokyo)
- v.60(10); 2020 Oct
Historic Overview of Genetic Engineering Technologies for Human Gene Therapy
Ryota tamura1.
1 Department of Neurosurgery, Keio University School of Medicine, Tokyo, Japan
Masahiro TODA
The concepts of gene therapy were initially introduced during the 1960s. Since the early 1990s, more than 1900 clinical trials have been conducted for the treatment of genetic diseases and cancers mainly using viral vectors. Although a variety of methods have also been performed for the treatment of malignant gliomas, it has been difficult to target invasive glioma cells. To overcome this problem, immortalized neural stem cell (NSC) and a nonlytic, amphotropic retroviral replicating vector (RRV) have attracted attention for gene delivery to invasive glioma. Recently, genome editing technology targeting insertions at site-specific locations has advanced; in particular, the clustered regularly interspaced palindromic repeats/CRISPR-associated-9 (CRISPR/Cas9) has been developed. Since 2015, more than 30 clinical trials have been conducted using genome editing technologies, and the results have shown the potential to achieve positive patient outcomes. Gene therapy using CRISPR technologies for the treatment of a wide range of diseases is expected to continuously advance well into the future.
Introduction
Gene therapy is a therapeutic strategy using genetic engineering techniques to treat various diseases. 1 , 2) In the early 1960s, gene therapy first progressed with the development of recombinant DNA (rDNA) technology, 1) and was further developed using various genetic engineering tools, such as viral vectors. 3 – 5) More than 1900 clinical trials have been conducted with gene therapeutic approaches since the early 1990s. In these procedures, DNA is randomly inserted into the host genome using conventional genetic engineering tools. In the 2000s, genome editing tootls, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the recently established clustered regularly interspaced palindromic repeats/CRISPR-associated-9 (CRISPR/Cas9) technologies, were developed, which induce genome modifications at specific target sites. 5) Genome editing tools are efficient for intentional genetic engineering, which has led to the development of novel treatment strategies for a wide range of diseases, such as genetic diseases and cancers. Therefore, gene therapy has again became a major focus of medical research. However, because gene therapy involves changing the genetic background, it raises important ethical concerns. In this article, we review the brief history of gene therapy and the development of genetic engineering technologies.
History of Genetic Engineering Technologies
Ethical issues.
In 1968, the initial proof-of-concept of virus- mediated gene transfer was made by Rogers et al. 6) who showed that foreign genetic material could be transferred into cells by viruses. In the first human gene therapy experiment, Shope papilloma virus was transduced into two patients with genetic arginase deficiency, because Rogers et al. hypothesized that the Shope papilloma virus genome contained a gene that encodes arginase. However, this gene therapy produced little improvement in the arginase levels in the patients. 7) Sequencing of the Shope papilloma virus genome revealed that the virus genome did not contain an arginase gene. 7)
This experiment prompted public concerns about the risks and ethical issues of gene therapy. In 1972, Friedman et al. 8) proposed ethical standards for the clinical application of gene therapy to prevent premature application in human. However, in 1980, genetic engineering was unethically performed in patients with thalassemia without the approval of the institutional review board. 9) The patients’ bone marrow cells were harvested and returned into their bone marrow after transduction with the plasmid DNA containing an integrated b-globin gene. 9) This treatment showed no effects, and the experiments were regarded as morally dubious. The gene therapy report of the President's Commission in the United States, Splicing Life , emphasized the distinction between somatic and germline genome editing in humans, and between medical treatment and non-medical enhancement. 10) An altered gene inserted into sperm or egg cells (germ cells) would lead to changes not only in the individual receiving the treatment but also in their future offspring. Interventions aimed at enhancing “normal” people also are problematic because they might lead to attempts to make “perfect” human beings.
Beginning of gene therapy using viral vector
In 1980, only nonviral methods, such as microinjection and calcium-phosphate precipitation, were used for gene delivery. Nonviral methods showed some advantages compared with viral methods, such as large-scale production and low host immunogenicity. However, nonviral methods yielded lower levels of transfection and gene expression, resulting in limited therapeutic efficacy. 11) In 1989, the rDNA Advisory Committee of the National Institutes of Health proposed the first guidelines for the clinical trials of gene therapy. In 1990, retroviral infection, which is highly dependent on host cell cycle status, was first performed for the transduction of the neomycin resistance marker gene into tumor-infiltrating lymphocytes that were obtained from patients with metastatic melanoma. 3 , 4) Then, the lymphocytes were cultured in vitro and returned to the patients’ bodies. 3 , 4) The first Food and Drug Administration (FDA)- approved gene therapy using a retroviral vector was performed by Anderson et al. in 1990; the adenosine deaminase (ADA) gene was transduced into the white blood cells of a patient with ADA deficiency, resulting in temporary improvements in her immunity. 2 , 12)
First severe complications
A recombinant adenoviral (AV) vector was developed after advances in the use of the retroviral vector. In 1999, a clinical trial was performed for ornithine transcarbamylase (OTC) deficiency. A ubiquitous DNA AV vector (Ad5) containing the OTC gene was delivered into the patient. Four days after administration, the patient died from multiple organ failure that was caused by a cytokine storm. 13 , 14) In 1999, of the 20 patients enrolled in two trials for severe combined immunodeficiency (SCID)-X1, T-cell leukemia was observed in five patients at 2–5.5 years after the treatment. Hematopoietic stem cells with a conventional, amphotropic, murine leukemia virus-based vector and a gibbon-ape leukemia virus-pseudotyped retrovirus were used for gene transduction in those trials. 15 , 16) Although four patients fully recovered after the treatment, one patient died 15 , 16) because oncogene activation was mediated by viral insertion. 15 , 16)
Development of viral vectors
Viral vectors continued to be crucial components in the manufacture of cell and gene therapy. Adeno- associated viral (AAV) vectors were applied for many genetic diseases including Leber’s Congenital Amaurosis (LCA), and reverse lipoprotein lipase deficiency (LPLD). In 2008, remarkable success was reported for LCA type II in phase I/II clinical trials. 17) LCA is a rare hereditary retinal degeneration disorder caused by mutations in the RPE65 gene (Retinoid Isomerohydrolase RPE65), which is highly expressed in the retinal pigment epithelium and encodes retinoid isomerase. 17) These trials confirm that RPE65 could be delivered into retinal pigment epithelial cells using recombinant AAV2/2 vectors, resulting in clinical benefits without adverse events. 17) Recently, the FDA approved voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics, Philadelphia, PA, USA) for patients with LCA type II. Alipogene tiparvovec Glybera (uniQure, Lexington, MA, USA) is the first gene-therapy-based drug to reverse LPLD to be approved in Europe in 2012. The AAV1 vector delivers an intact LPL gene to the muscle cells. 18) To date, more than 200 clinical trials have been performed using AAV vectors for several genetic diseases, including spinal muscular atrophy, 19) retinal dystrophy, 20) and hemophilia. 21)
Retrovirus is still one of the mainstays of gene therapeutic approaches. Strimvelis (GlaxoSmithKline, London, UK) is an FDA-approved drug consisting of an autologous CD34 (+)-enriched cell population that includes a gammaretrovirus containing the ADA gene that was used as the first ex-vivo stem cell gene therapy in patients with SCID because of ADA deficiency. 22) Subsequently, retroviral vectors were often used for other genetic diseases, including X-SCID. 23)
Lentivirus belongs to a family of viruses that are responsible for diseases, such as aquired immunodeficiency syndrome caused by the human immunodeficiency virus (HIV) that causes infection by inserting DNA into the genome of their host cells. 24) The lentivirus can infect non-dividing cells; therefore, it has a wider range of potential applications. Successful treatment of the patients with X-linked adrenoleukodystrophy was demonstrated using a lentiviral vector with the deficient peroxisomal adenosine triphosphate–binding cassette D1. 25) Despite the use of a lentiviral vector with an internal viral long terminal repeat, no oncogene activation was observed. 25)
A timeline showing the history of scientific progress in gene therapy is highlighted in Table 1 .
ADA: adenosine deaminase, ALD: adrenoleukodystrophy, B-ALL: B cell acute lymphoblastic leukemia, CAR: chimeric antigen receptor-modified, DLBCL: diffuse large B-cell lymphoma, GT: gene therapy, LCA: Leber’s congenital amaurosis, LPL: lipoprotein lipase deficiency, OTC: ornithine transcarbamylase, SCID: severe combined immunodeficiency, SMA: spinal muscular atrophy, TCR: T cell receptor, TIL: tumor infiltrating lymphocyte
Gene Therapeutic Strategies for Brain Tumor
A variety of studies were performed to apply gene therapy to malignant tumors. The concept of gene therapy for tumors is different from that for genetic diseases, in which new genes are added to a patient's cells to replace missing or malfunctioning genes. In malignant tumors, the breakthrough in gene therapeutic strategy involved designing suicide gene therapy, 26) which was first applied for malignant glioma in 1992. 26 , 27) The first clinical study was performed on 15 patients with malignant gliomas by Ram et al (phase I/II). 27) Stereotactic intratumoral injections of murine fibroblasts producing a replication-deficient retrovirus vector with a suicide gene (herpes simplex virus-thymidine kinase [HSV-TK]) achieved anti-tumor activity in four patients through bystander killing effects. 27) Subsequently, various types of therapeutic genes have been used to treat malignant glioma. Suicide genes (cytosine deaminase [CD]), genes for immunomodulatory cytokines (interferon [IFN]-β, interleukin [IL]-12, granulocyte- macrophage colony-stimulating factor [GM-CSF]), and genes for reprogramming (p53, and phosphatase and tensin homolog deleted from chromosome [PTEN]) have been applied to the treatment of malignant glioma using viral vectors. 28 , 29)
Recently, a nonlytic, amphotropic retroviral replicating vector (RRV) and immortalized human neural stem cell (NSC) line were used for gene delivery to invasive glioma. 30 – 32) In 2012, a nonlytic, amphotropic RRV called Toca 511 was developed for the delivery of a suicide gene (CD) to tumors. 32) A tumor-selective Toca 511 combined with a prodrug (Toca FC) was evaluated in patients with recurrent high-grade glioma in phase I clinical trial. 30) The complete response rate was 11.3% in 53 patients. 30) In addition, the sub-analysis of this clinical trial revealed that the objective response was 21.7% in the 23-patient phase III eligible subgroup. 33) However, in the recent phase III trial, treatment with Toca 511 and Toca FC did not improve overall survival compared with standard therapy in patients with recurrent high-grade glioma. A further combinational treatment strategy using programmed cell-death ligand 1 (PD-L1) checkpoint blockade delivered by TOCA-511 was evaluated in experimental models, which may lead to future clinical application. 34) Since 2010, intracranial administration of allogeneic NSCs containing CD gene (HB1.F3. CD) has been performed by a team at City of Hope. Autopsy specimens indicate the HB1.F3. CD migrates toward invaded tumor areas, suggesting a high tumor-trophic migratory capacity of NSCs. 31) No severe toxicities were observed in the trial. Generally, it is difficult to obtain NSCs derived from human embryonic or fetal tissue. The use of human embryos for research on embryonic stem cells is ethically controversial because it involves the destruction of human embryos, and the use of fetal tissue associated with abortion also raises ethical considerations. 35) Recently, the tumor-trophic migratory activity of NSCs derived from human-induced pluripotent stem cells (hiPSCs) was shown using organotypic brain slice culture. 36) Moreover, hiPSC-derived NSCs with the HSV-TK suicide gene system demonstrated considerable therapeutic potential for the treatment of experimental glioma models. 36) Furthermore, iPSCs have the ability to overcome ethical and practical issues of NSCs in clinical application.
New Genetic Engineering Technologies for Gene Therapy
Genetic engineering technologies using viral vectors to randomly insert therapeutic genes into a host genome raised concerns about insertional mutagenesis and oncogene activation. Therefore, new technology to intentionally insert genes at site-specific locations was needed. Genome editing is a genetic engineering method that uses nucleases or molecular scissors to intentionally introduce alterations into the genome of living organisms. 6) As of 2015, three types of engineered nucleases have been used: ZFNs, TALENs, and CRISPR/Cas ( Table 2 ). 6)
CRISPR/Cas9: clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins 9, PAM: protospacer adjacent motif, TALENs: transcription activator-like effector nucleases, ZFNs: zinc finger nucleases
Genome editing tools
ZFNs are fusions of the nonspecific DNA cleavage domain of the Fok I restriction endonuclease and zinc-finger proteins that lead to DNA double-strand breaks (DSBs). Zinc-finger domains recognize a trinucleotide DNA sequence ( Fig. 1 ). However, design and selection of zinc-finber arrays is difficult and time-consuming. 37)

Genome editing tools. Three types of genome editing tools including ZFNs, TALENs, and CRISPR/Cas9 are shown. ZFNs are hybrid proteins using zinc-finger arrays and the catalytic domain of FokI endonuclease. TALENs are hybrid proteins containing the TAL effector backbone and the catalytic domain of FokI endonuclease. The CRISPR/Cas9 system is composed of Cas9 endonuclease and sgRNA. Cas9: CRISPR-associated-9, CRISPR: clustered regularly interspaced palindromic repeats, sgRNA: single-guide RNA, TALENs: transcription activator-like effector nucleases, ZFNs: zinc-finger nucleases.
TALENs are fusions of the Fok I cleavage domain and DNA-binding domains derived from TALE proteins. TALEs have multiple 33–35 amino acid repeat domains that recognizes a single base pair, leading to the targeted DSBs, similar to ZFNs ( Fig. 1 ). 38)
The CRISPR/Cas9 system consists of Cas9 nuclease and two RNAs (CRISPR RNA [crRNA] and trans- activating CRISPR RNA [tracrRNA]). 39) The crRNA/tracrRNA complex (gRNA) induces the Cas9 nuclease and cleaves DNA upstream of a protospacer-adjacent motif (PAM, 5’-NGG-3’ for S. pyogenes ) ( Fig. 1 ). 40) Currently, Cas9 from S. pyogenes (SpCas9) is the most popular tool for genome editing. 40)
Critical issues in geneome editing
Several studies have demonstrated the off-target effects of Cas9/gRNA complexes. 41) It is important to select unique target sites without closely homologous sequences, resulting in minimum off-target effects. 42) Additionally, other CRISPR/Cas9 gene editing tools were developed to mitigate off-target effects, including gRNA modifications (slightly truncated gRNAs with shorter regions of target complementarity <20 nucleotides) 43) and SpCas9 variants, such as Cas9 paired nickases (a Cas9 nickase mutant or dimeric Cas9 proteins combined with pairs of gRNAs). 44) The type I CRISPR-mediated distinct DNA cleavage (CRISPR/Cas3 system) was developed recently in Japan to decrease the risk of off-target effets. Cas3 triggered long-range deletions upstream of the PAM (5'-ARG). 45)
A confirmatory screening of off-target effects is necessary for ensuring the safe application of genome editing technologies. 46) Although off-target mutations in the genome, including the noncoding region, can be evaluated using whole genome sequencing, this method is expensive and time-consuming. With the development of unbiased genome-wide cell-based methods, GUIDE-seq (genome-wide, unbiased identification of DSBs enabled by sequencing) 47) and BLESS (direct in situ breaks labeling, enrichment on streptavidin; next-generation sequencing) 48) were developed to detect off-target cleavage sites, and these methods do not require high sequencing read counts.
Applications of Genome Editing Technologies
Gene therapy has in- vivo and ex- vivo strategies. For the in- vivo strategy, vectors containing therapeutic genes are directly delivered into the patients, and genetic modification occurs in situ . For the ex- vivo strategy, the harvested cells are modified by the appropriate gene delivery tools in vitro (e.g., recombinant viruses and genome editing technologies). The modified cells are then delivered back to the patient via autologous or allogeneic transplantation after the evaluation of off-target effects ( Fig. 2 ).

In- vivo and ex- vivo strategies of gene therapy. In- vivo and ex- vivo gene transfer strategies are shown. For in- vivo gene transfer, genetic materials containing therapeutic genes, such as viral vectors, nanoparticles, and ribosomes, are delivered directly to the patient, and genetic modification occurs in situ . For ex- vivo gene transfer, the harvested cells are modified by the appropriate gene delivery tools in vitro (e.g., recombinant viruses genome editing technologies). The modified cells are then delivered back to the patient via autologous or allogeneic transplantation after the evaluation of off-target effects.
HIV-resistant T cells were established by ZFN- mediated disruption of the C-C chemokine receptor (CCR) 5 coreceptor for HIV-I, which is being evaluated as an ex- vivo modification in early-stage clinical trials. 49 , 50) Disruption of CCR5 using ZFNs was the first-in-human application of a genome editing tool. Regarding hematologic disorders, since 2016, clinical trials have attempted the knock-in of the factor IX gene using AAV/ZFN-mediated genome editing approach for patients with hemophilia B. 51)
In addition to these promising ongoing clinical trials for genetic diseases, CRISPR/Cas9 and TALEN technologies have improved the effect of cancer immunotherapy using genome-engineered T cells. Engineered T cells express synthetic receptors (chimeric antigen receptors, CARs) that can recognize epitopes on tumor cells. The FDA approved two CD19-targeting CAR-T-cell products for B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma. 52 , 53) Engineered CARs target many other antigens of blood cancers, including CD30 in Hodgkin's lymphoma as well as CD33, CD123, and FLT3 of acute myeloid leukemia. 54) Recent research has shown that Cas9-mediated PD-1 disruption in the CAR-T cells improved the anti-tumor effect observed in in- vitro and in- vivo experimental models, leading to the performance of a clinical trial. 55 , 56) All other ongoing clinical trials using genome-editing technologies are highlighted in Table 3 .
AAV: adeno-associated virus, CAR: chimeric antigen receptor, CRISPR/Cas9: clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 proteins, HIV: human immunodeficiency virus, HPV: human papillovirus, MPS: mucopolysaccharidosis, N/A: not available, PD-1: programmed cell death-1, TALEN: transcription activator-like effector nucleases, ZFN: zinc finger nucleases
Future Direction
Gene therapy has advanced treatments for patients with congenital diseases and cancers throughout recent decades by optimizating various types of vectors and the introduction of new techniques including genome editing tools. The CRISPR/Cas9 system is considered one of the most powerful tools for genetic engineering because of its high efficiency, low cost, and ease of use. CRISPR technologies have progressed and are expected to continuously advance. Although there are still many challenging obstacles to overcome to achieve safe clinical application, these methods provide the possibility of treatment for a wide variety of human diseases.
Acknowledgement
We thank Lisa Kreiner, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Conflicts of Interest Disclosure
The authors declare no conflicts of interest associated with this manuscript. This work was supported in part by grants from the Japan Society for the Promotion of Science (JSPS) (18K19622 to M.T.). All authors have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

14.4: Genetic Engineering - Risks, Benefits, and Perceptions
- Last updated
- Save as PDF
- Page ID 77491

Learning Objectives
- Summarize the mechanisms, risks, and potential benefits of gene therapy
- Identify ethical issues involving gene therapy and the regulatory agencies that provide oversight for clinical trials
- Compare somatic-cell and germ-line gene therapy
Many types of genetic engineering have yielded clear benefits with few apparent risks. Few would question, for example, the value of our now abundant supply of human insulin produced by genetically engineered bacteria. However, many emerging applications of genetic engineering are much more controversial, often because their potential benefits are pitted against significant risks, real or perceived. This is certainly the case for gene therapy, a clinical application of genetic engineering that may one day provide a cure for many diseases but is still largely an experimental approach to treatment.
Mechanisms and Risks of Gene Therapy
Human diseases that result from genetic mutations are often difficult to treat with drugs or other traditional forms of therapy because the signs and symptoms of disease result from abnormalities in a patient’s genome. For example, a patient may have a genetic mutation that prevents the expression of a specific protein required for the normal function of a particular cell type. This is the case in patients with Severe Combined Immunodeficiency (SCID), a genetic disease that impairs the function of certain white blood cells essential to the immune system.
Gene therapy attempts to correct genetic abnormalities by introducing a nonmutated, functional gene into the patient’s genome. The nonmutated gene encodes a functional protein that the patient would otherwise be unable to produce. Viral vectors such as adenovirus are sometimes used to introduce the functional gene; part of the viral genome is removed and replaced with the desired gene (Figure \(\PageIndex{1}\)). More advanced forms of gene therapy attempt to correct the mutation at the original site in the genome, such as is the case with treatment of SCID.

So far, gene therapies have proven relatively ineffective, with the possible exceptions of treatments for cystic fibrosisand adenosine deaminase deficiency, a type of SCID. Other trials have shown the clear hazards of attempting genetic manipulation in complex multicellular organisms like humans. In some patients, the use of an adenovirus vector can trigger an unanticipated inflammatory response from the immune system, which may lead to organ failure. Moreover, because viruses can often target multiple cell types, the virus vector may infect cells not targeted for the therapy, damaging these other cells and possibly leading to illnesses such as cancer. Another potential risk is that the modified virus could revert to being infectious and cause disease in the patient. Lastly, there is a risk that the inserted gene could unintentionally inactivate another important gene in the patient’s genome, disrupting normal cell cycling and possibly leading to tumor formation and cancer. Because gene therapy involves so many risks, candidates for gene therapy need to be fully informed of these risks before providing informed consent to undergo the therapy.
Gene Therapy Gone Wrong
The risks of gene therapy were realized in the 1999 case of Jesse Gelsinger, an 18-year-old patient who received gene therapy as part of a clinical trial at the University of Pennsylvania. Jesse received gene therapy for a condition called ornithine transcarbamylase (OTC) deficiency, which leads to ammonia accumulation in the blood due to deficient ammonia processing. Four days after the treatment, Jesse died after a massive immune response to the adenovirus vector. 1
Until that point, researchers had not really considered an immune response to the vector to be a legitimate risk, but on investigation, it appears that the researchers had some evidence suggesting that this was a possible outcome. Prior to Jesse’s treatment, several other human patients had suffered side effects of the treatment, and three monkeys used in a trial had died as a result of inflammation and clotting disorders. Despite this information, it appears that neither Jesse nor his family were made aware of these outcomes when they consented to the therapy. Jesse’s death was the first patient death due to a gene therapy treatment and resulted in the immediate halting of the clinical trial in which he was involved, the subsequent halting of all other gene therapy trials at the University of Pennsylvania, and the investigation of all other gene therapy trials in the United States. As a result, the regulation and oversight of gene therapy overall was reexamined, resulting in new regulatory protocols that are still in place today.
Exercise \(\PageIndex{1}\)
- Explain how gene therapy works in theory.
- Identify some risks of gene therapy.
Oversight of Gene Therapy
Presently, there is significant oversight of gene therapy clinical trials. At the federal level, three agencies regulate gene therapy in parallel: the Food and Drug Administration (FDA), the Office of Human Research Protection (OHRP), and the Recombinant DNA Advisory Committee (RAC) at the National Institutes of Health (NIH). Along with several local agencies, these federal agencies interact with the institutional review board to ensure that protocols are in place to protect patient safety during clinical trials. Compliance with these protocols is enforced mostly on the local level in cooperation with the federal agencies. Gene therapies are currently under the most extensive federal and local review compared to other types of therapies, which are more typically only under the review of the FDA. Some researchers believe that these extensive regulations actually inhibit progress in gene therapy research. In 2013, the Institute of Medicine (now the National Academy of Medicine) called upon the NIH to relax its review of gene therapy trials in most cases. 2 However, ensuring patient safety continues to be of utmost concern.
Ethical Concerns
Beyond the health risks of gene therapy, the ability to genetically modify humans poses a number of ethical issues related to the limits of such “therapy.” While current research is focused on gene therapy for genetic diseases, scientists might one day apply these methods to manipulate other genetic traits not perceived as desirable. This raises questions such as:
Exercise \(\PageIndex{2}\)
- Which genetic traits are worthy of being “corrected”?
- Should gene therapy be used for cosmetic reasons or to enhance human abilities?
- Should genetic manipulation be used to impart desirable traits to the unborn?
- Is everyone entitled to gene therapy, or could the cost of gene therapy create new forms of social inequality?
- Who should be responsible for regulating and policing inappropriate use of gene therapies?
The ability to alter reproductive cells using gene therapy could also generate new ethical dilemmas. To date, the various types of gene therapies have been targeted to somatic cells, the non-reproductive cells within the body. Because somatic cell traits are not inherited, any genetic changes accomplished by somatic-cell gene therapy would not be passed on to offspring. However, should scientists successfully introduce new genes to germ cells (eggs or sperm), the resulting traits could be passed on to offspring. This approach, called germ-line gene therapy, could potentially be used to combat heritable diseases, but it could also lead to unintended consequences for future generations. Moreover, there is the question of informed consent, because those impacted by germ-line gene therapy are unborn and therefore unable to choose whether they receive the therapy. For these reasons, the U.S. government does not currently fund research projects investigating germ-line gene therapies in humans.
Risky Gene Therapies
While there are currently no gene therapies on the market in the United States, many are in the pipeline and it is likely that some will eventually be approved. With recent advances in gene therapies targeting p53, a gene whose somatic cell mutations have been implicated in over 50% of human cancers, 3 cancer treatments through gene therapies could become much more widespread once they reach the commercial market.
Bringing any new therapy to market poses ethical questions that pit the expected benefits against the risks. How quickly should new therapies be brought to the market? How can we ensure that new therapies have been sufficiently tested for safety and effectiveness before they are marketed to the public? The process by which new therapies are developed and approved complicates such questions, as those involved in the approval process are often under significant pressure to get a new therapy approved even in the face of significant risks.
To receive FDA approval for a new therapy, researchers must collect significant laboratory data from animal trials and submit an Investigational New Drug (IND) application to the FDA’s Center for Drug Evaluation and Research (CDER). Following a 30-day waiting period during which the FDA reviews the IND, clinical trials involving human subjects may begin. If the FDA perceives a problem prior to or during the clinical trial, the FDA can order a “clinical hold” until any problems are addressed. During clinical trials, researchers collect and analyze data on the therapy’s effectiveness and safety, including any side effects observed. Once the therapy meets FDA standards for effectiveness and safety, the developers can submit a New Drug Application (NDA) that details how the therapy will be manufactured, packaged, monitored, and administered.
Because new gene therapies are frequently the result of many years (even decades) of laboratory and clinical research, they require a significant financial investment. By the time a therapy has reached the clinical trials stage, the financial stakes are high for pharmaceutical companies and their shareholders. This creates potential conflicts of interest that can sometimes affect the objective judgment of researchers, their funders, and even trial participants. The Jesse Gelsinger case (see Case in Point: Gene Therapy Gone Wrong ) is a classic example. Faced with a life-threatening disease and no reasonable treatments available, it is easy to see why a patient might be eager to participate in a clinical trial no matter the risks. It is also easy to see how a researcher might view the short-term risks for a small group of study participants as a small price to pay for the potential benefits of a game-changing new treatment.
Gelsinger’s death led to increased scrutiny of gene therapy, and subsequent negative outcomes of gene therapy have resulted in the temporary halting of clinical trials pending further investigation. For example, when children in France treated with gene therapy for SCID began to develop leukemia several years after treatment, the FDA temporarily stopped clinical trials of similar types of gene therapy occurring in the United States. 4 Cases like these highlight the need for researchers and health professionals not only to value human well-being and patients’ rights over profitability, but also to maintain scientific objectivity when evaluating the risks and benefits of new therapies.
Exercise \(\PageIndex{3}\)
- Why is gene therapy research so tightly regulated?
- What is the main ethical concern associated with germ-line gene therapy?
Key Concepts and Summary
- While gene therapy shows great promise for the treatment of genetic diseases, there are also significant risks involved.
- There is considerable federal and local regulation of the development of gene therapies by pharmaceutical companies for use in humans.
- Before gene therapy use can increase dramatically, there are many ethical issues that need to be addressed by the medical and research communities, politicians, and society at large.
- 1 Barbara Sibbald. “Death but One Unintended Consequence of Gene-Therapy Trial.” Canadian Medical Association Journal 164 no. 11 (2001): 1612–1612.
- 2 Kerry Grens. “Report: Ease Gene Therapy Reviews.” The Scientist , December 9, 2013. http://www.the-scientist.com/?articl...erapy-Reviews/ . Accessed May 27, 2016.
- 3 Zhen Wang and Yi Sun. “Targeting p53 for Novel Anticancer Therapy.” Translational Oncology 3 , no. 1 (2010): 1–12.
- 4 Erika Check. “Gene Therapy: A Tragic Setback.” Nature 420 no. 6912 (2002): 116–118.

Viewpoint: ‘Enemy of Science’ — Friends of the Earth renews its organic industry-funded campaign against GM crops
All while claiming to be “evidence-based.” Redefining “evidence-based” as only embracing “studies” – which means computer simulations, dead mice papers, and suspect epidemiological claims by political insiders – that match their ideological agenda is why Friends of The Earth is lying to poor Mexican people. The group is claiming that genetic engineering – but only genetic engineering that came after Mutagenesis, which is used by the “organic food” corporations that fund Friends of the Earth – “poses serious health risks” to Mexicans.
How? Does Friends of the Earth think Mexicans are a different species? A trillion animals and 2 billion people have eaten “GMOs” (the term they use for all post-mutagenesis food despite GMOs being only one off-patent technology) and there are no health issues. Here is their latest headline:
High Levels of Genetically Engineered Toxins and Glyphosate in GMO Corn Pose Serious Health Risks in Mexico
Do you believe plants are little people and that every scientist and Democratic President since Roosevelt has been colluding with corporations to poison you? Then you’re ready to donate to Friends of the Earth or be on a San Francisco jury!
Their newest assault on science manipulates the process of science for emotional effect.
1. FOE says safety studies are “industry assertions”, not science
This conspiracy theory actually works with coastal progressives and Whole Foods shoppers nationwide but like all conspiracy theories has trouble making real-world sense. FDA and EPA employees are 90 percent Democrats and the left-wing are the only people who donate to Friends of the Earth so we have to believe the left-wing is duped by corporations – if they are scientists in government.
Science is science, the belief system of anti-science hippies does not change reality. But it can impact people in Mexico who are poor. Like in the Philippines with Golden Rice or Sri Lanka with all organic food, they target the least-educated in their war on science and do not care how many people they harm or kill.
In the real world, scientists in government agencies are exceedingly over-cautious, but activists have begun to get their allied epidemiologists hired into the Biden administration, and believe their war of extinction on science will succeed if biology, toxicology and chemistry are ignored and statistical ‘correlation’ makes science policy.
2. Companies are required by law to pay for studies to prove science is safe, but FOE claims that is “industry-funded .”
Should a company be allowed to create a product and hand it off to the government and declare that taxpayers have to pay to prove it does not work?
Of course not, but Friends of the Earth claims this stuff and gullible Mother Jones and Washington Post journalists repeat it as if it were a real argument. Those studies have to pass by the most critical government scientists out there, many of whom have been in three of four administrations by now.
All studies are “industry-funded” by law, and that is a good thing. The government should force companies to prove products are safe.
3. The “scientist” they quote is just a PhD in environmental policy
From Berkeley , the university that also claimed weedkillers turns frogs gay. There is nothing at all wrong with the humanities, and FOE can give a “scientist” title to anyone they want, but let’s not pretend that FOE isn’t as sexist as every other environmental group and hiring young progressive women because they are true believers who will therefore accept a lower salary. American Association of University Women data found that environmentalists are the most sexist group in Science, Technology, Engineering and Math (STEM) fields, paying women only 79% of what men get .
Their policy expert has a few op-eds in pay-to-publish outlets like the Guardian claiming predictable anti-science fluff like that “neonics” are toxic to humans and that if a hand-picked lab can’t ‘detect’ organic pesticides in urine then the companies that fund FOE must be better for health.
4. The co-author on this claim is discredited organic industry flunky Chuck Benbrook
Benbrook is famous for writing papers like that organic strawberries are healthier – but looking at his methodology his only data was they have better ‘mouth feel’ according to organic shoppers on surveys . He is derided in science circles because he is a known liar. For example, he claimed to be a “professor” at Washington State University but was only an Adjunct and the school terminated his contract when we exposed that an organic food company was paying the university to pay him.
The U.S. government has not presented an ‘appropriate’ risk assessment to the tribunal as called for in the USMCA dispute because such an assessment has never been done in the U.S. or anywhere in the world. — Co-author Dr. Charles Benbrook
Like vaccines, cell phones, and Israel defending itself against terrorists, anti-science progressives use ‘needs more study’ to block any product made by any company that isn’t giving them money. Benbrook understands none of the science in how products are approved, he wants ‘risk assessment’ because it instead only using food surveys – like organic food shoppers who claim they get a rash if they don’t use non-GMO rock salt.
Now Benbrook is a professional “pesticide litigation consultant” for lawyers hoping to sue companies using papers like this FOE conspiracy screed.
5. Their only data consists of “emerging evidence.”
Like “suggests”, this is a word that statisticians use when they want to create an appearance of legitimacy, but it means nothing except that the finding is not science.
While much of the focus of the health harms of GMO corn rightfully center on glyphosate and other hazardous herbicides that the crops have been engineered to withstand, emerging evidence on these toxins is concerning. Data show the potential for risk of adverse impacts on the human microbiome and GI tract , risks of allergenicity stimulating an immune system response “as potent as that elicited by cholera toxin ,” and presence of antibodies against Cry toxins in at least 8% of Americans, clear evidence that the toxin remains mostly intact after passing through the human GI tract.
Illiterate 17th century peasants who had no fertilizers or pesticides could still not get a job at FOE – because they knew ‘the dose makes the poison’ while FOE believes 1 part per quadrillion is killing people, despite science knowing that is impossible.
Environmental groups are famous for soliciting an author to ‘write’ an epidemiology paper – basically, get a grad student to look at other hand-picked epidemiology papers and create enough correlation to claim the scientifically meaningless “statistical significance” – where the conclusion will be a chemical they want to sue over is bad. Then they get other authors to write supporting papers citing the first, and then they use that pool of papers to claim their entirely manufactured claim is “emerging evidence.”
Hank Campbell is founder of Science 2.0 and author of Science Left Behind . Follow Hank on X @HankCampbell
A version of this article was originally posted at Science 2.0 and is reposted here with permission. Any reposting should credit both the GLP and original article. Find Science 2.0 on X @science2_0

GLP Podcasts & Podcast Videos More...

GLP podcast: Dangers of ‘diet weed’; Making insulin in cow’s milk; The conservative case for genetic enhancement
Glp podcast: ge crops have lived up to the hype; growing ‘mini’ organs from stem cells; how do we solve right-wing vaccine hesitancy, videos more....

Video: Why does love make us feel so good? Examining its effect on our brains
Bees & pollinators more....

Dissecting claims about Monsanto suing farmers for accidentally planting patented seeds

Analysis: Do neonicotinoid and glyphosate pesticides threaten bees? A reassessment

How effective and safe are current-generation pesticides?
Infographics more....

Are pesticide residues on food something to worry about?
Gmo faqs more....

Why is there controversy over GMO foods but not GMO drugs?

How are GMOs labeled around the world?

How does genetic engineering differ from conventional breeding?

Alex Jones: Right-wing conspiracy theorist stokes fear of GMOs, pesticides to sell ‘health supplements’

IARC (International Agency for Research on Cancer): Glyphosate cancer determination challenged by world consensus
Most popular.

Newsletter Subscription
- Weekly Newsletter (Wed)
- Daily Digest (Mon, Tue, Thu, Fri)
- Weekly Top Six (Sun)
- Featured Articles Only
- Human Articles Only
- Agriculture Articles Only
- All Types of Content
Get news on human & agricultural genetics and biotechnology delivered to your inbox.

IMAGES
VIDEO
COMMENTS
Genetic engineering and its dangers. Genetic engineering is the artificial manipulation, modification, and recombination of DNA or RNA molecules to modify an organism or population of organisms. It is generally used to refer to methods of recombinant DNA technology. Recombinant DNA technology is the joining of DNA from two different species ...
Genetic engineering is a technology of genetic modification. It allows scientists to alter the arrangement of genes by manipulating the protein sequence of the gene. This technology started to gain momentum in the middle of the twentieth century, although the concept first appeared in the 1920s. With the structure of DNA being revealed in 1953 ...
a) Making possible the production of genetically modified foods. As Coker noted: "In the United States and elsewhere, more than 90% of soybeans, cotton, corn, and certain other crops are already genetically engineered" (24). The reason behind the growing popularity of this type of food is quite apparent - the application of getting ...
Genetically engineered organisms pose lethal and economic risks to human society. The availability of genomic information and genetic engineering technology creates a lethal threat to humanity because terrorists can use both the information and technology to recreate deadly pathogens, such as the poliovirus. The naturally occurring poliovirus ...
technique, known by its acronym CRISPR, has revolutionized the process of decoding and precisely editing genetic information of 3 A gene drive is a genetic engineering technology—adding ...
Learning Objectives. Summarize the mechanisms, risks, and potential benefits of gene therapy. Identify ethical issues involving gene therapy and the regulatory agencies that provide oversight for clinical trials. Compare somatic-cell and germ-line gene therapy. Many types of genetic engineering have yielded clear benefits with few apparent risks.
The risks involved in biotechnology. Risk can be defined as the possibility or probability of harm — that is, of a loss, an injury, an unwanted outcome or an undesired result. The main risks involved in genetic engineering are the following. The release of genetically altered organisms in the environment can increase human suffering (when ...
Genetic engineering will have an effect on our world, not only on food production but will cause effects on humans and the society. From the cultural perspective, human beings are inviolable and live free according to their rights in the society. The society accepts them the way they are and the type of life they live, therefore introducing ...
The logic behind this argument is that human genetic enhancement perpetuates discrimination against the disabled and the "genetically unfit," and that this sort of discrimination is similar to the sort that inspired the eugenics of the Third Reich. A third argument is that genetic engineering will lead to vast social inequalities.
Essay on The Dangers of Genetic Engineering. Decent Essays. 619 Words. 3 Pages. Open Document. Genetic engineering has a fine line to when it becomes unethical. Ethically new research has offered to help people with disabilities and prevent them to better a persons life. The line is drawn when parents have the choice to modify their child ...
Genetic Engineering. 2 pages / 835 words. Genetic engineering, also known as genetic modification, is the direct manipulation of DNA to alter an organism's characteristics (phenotype) in a particular way. It is a set of technologies used to change the genetic makeup of cells to produce improved or novel organisms.
Dangers And Disadvantages Of Genetic Engineering. This essay sample was donated by a student to help the academic community. Papers provided by EduBirdie writers usually outdo students' samples. 360,000 babies are born every single day. Now imagine each one of them the exact same.
Get original essay. Conclusively, genetic engineering is not safe to humanity because of the many complicated issues it has brought to the society. It is one of the primary causes of cancer and its related diseases. Genetic engineering involves the use of chemicals, and most of these chemicals are carcinogenic.
Genetic Engineering and Its Dangers. Compiled by Professor Ron Epstein. [email protected]. TENTH ANNIVERSARY YEAR SITE FOUNDED IN 1996. ESSAYS ABOUT GENETIC ENGINEERING. GENETICALLY ENGINEERING HUMAN BEINGS. GENETICALLY ENGINEERED PLANTS AND FOOD. INTERNET LINKS.
Genetic Engineering Using a Pglo Plasmid. The objective of this experiment is to understand the process and importance of the genetic transformation of bacteria in real time with the aid of extrachromosomal DNA, alternatively referred to as plasmids. Managing Diabetes Through Genetic Engineering.
Show more. Genetic Engineering and its Dangers Genetic Engineering and its Dangers Genetic engineering (GE) is defined as the process of designing and altering genes to alter the DNA via laboratory based technologies for enhancing certain characteristics, such as industrial or agricultural production, disease resistance, etc. (www.genome.gov).
Human evolution and the so-called genetic human enhancement would seem therefore to involve different underlying processes, raising several questions regarding the implications and risks of the latter. But using genetic engineering to treat humans has been proposed far beyond the therapeutic case or to introduce genetic modifications known to ...
Genetic engineering has provided humans the ability to transform organisms by direct manipulation of genomes within a broad range of applications including agriculture (e.g., GM crops), and the ...
The genetic engineering of animals has increased significantly in recent years, and the use of this technology brings with it ethical issues, some of which relate to animal welfare — defined by the World Organisation for Animal Health as "the state of the animal…how an animal is coping with the conditions in which it lives" ().These issues need to be considered by all stakeholders ...
Introduction. Gene therapy is a therapeutic strategy using genetic engineering techniques to treat various diseases. 1, 2) In the early 1960s, gene therapy first progressed with the development of recombinant DNA (rDNA) technology, 1) and was further developed using various genetic engineering tools, such as viral vectors. 3 - 5) More than ...
This page titled 14.4: Genetic Engineering - Risks, Benefits, and Perceptions is shared under a CC BY license and was authored, remixed, and/or curated by OpenStax. Many types of genetic engineering have yielded clear benefits with few apparent risks. However, many emerging applications of genetic engineering are much more controversial, often ...
Here's my sample essay for the question below. Genetic engineering is an important issue in society today. Some people think that it will improve people's lives in many ways. Others feel that it may be a threat to life on earth. Discuss both these views and give your own opinion. It is true that genetic engineering is a key area of modern scientific research, with broad implications for all ...
genetic engineering, the artificial manipulation, modification, and recombination of DNA or other nucleic acid molecules in order to modify an organism or population of organisms. The term genetic engineering is generally used to refer to methods of recombinant DNA technology, which emerged from basic research in microbial genetics.
The group is claiming that genetic engineering - but only genetic engineering that came after Mutagenesis, which is used by the "organic food" corporations that fund Friends of the Earth ...