
By: Ahiskalioglu et al.
By: Marim et al.
By: Yuksel et al.
By: Bayraktar et al.
By: Ozcura and Irgat.
By: Ugan and Un.
By: Sarioglu et al.
By: Sertkaya et al.
By: Gulhan et al.
By: Gunaydin, Caner; Bilge, S. Sirri
.png)
- Pre-Submission Check
- Early View Articles
- Manuscript Submission
- ICMJE Recommendations
- Copyright Agreement
- Abstracting and Indexing
- Permissions
- MANUSCRIPT SUBMISSION
- EARLY VIEW ARTICLES
- EDITORIAL BOARD
- INSTRUCTIONS TO AUTHORS

Send To Friend
Eurasian Journal of Medicine

Subject Area and Category
- Medicine (miscellaneous)
Publication type
13088734, 13088742
2012, 2014-2023
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Identifiers
Linking ISSN (ISSN-L): 2795-7624
URL https://geniusjournals.org/index.php/emrp
Google https://www.google.com/search?q=ISSN+%222795-7624%22
Bing https://www.bing.com/search?q=ISSN+%222795-7624%22
Yahoo https://search.yahoo.com/search?p=ISSN%20%222795-7624%22
ISSN Belgium https://opac.kbr.be/Library/search.aspx?SC=KBR_UNIFIED&QUERY=sys_base:SYRACUSE%20AND%20Issn_idx:2795-7624
Resource information
Title proper: Eurasian research medical periodical.
Country: Belgium
Medium: Online
Record information
Last modification date: 24/11/2021
Type of record: Confirmed
ISSN Center responsible of the record: ISSN National Centre for Belgium
downloads requested
Discover all the features of the complete ISSN records
Display mode x.
Labelled view
MARC21 view
UNIMARC view
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Eurasian J Med
- EJMI Home |

Volume: 8 Issue: 1 - 2024

Quick Search

Copyright � 2021 Eurasian Journal of Medical and Investigation, EJMI

Publication Categories
Plan for joint implementation of the tapi of the gas pipeline.

Pakistan and Turkmenistan signed a joint plan to implement the project for the construction of the Turkmenistan-Afghanistan-Pakistan-India (TAPI) transnational gas pipeline on June 8, 2023. This decision is aimed at accelerating the completion of the feasibility study of the project and moving it towards the construction phase. The ceremony of […]
March 9, 2024
The Concept of the National Vision and its Priorities in Uzbekistan and Kazakhstan

A National Vision is a concept that determines the mid-term and long-term development goals of a country and provides the basis for the development of national strategies and implementation plans. The national political leadership usually shapes the concept of the National Vision. The strength and credibility of such a vision […]
Can the EAEU and Iran FTA Boost Eurasian Trade?

On December 25, 2023, members of the Eurasian Economic Union (EAEU) signed a Free Trade Agreement (FTA) with Iran on the sidelines of the Supreme Eurasian Economic Council’s meeting. The FTA will eliminate import tariffs on almost 90% of the product range, which accounts for more than 95% of bilateral […]
Expanding China’s Education Diplomacy in Central Asia

With the post-pandemic resurgence of diplomatic activities, China has been strengthening its education diplomacy. In Central Asia, where China is an established strategic partner, its efforts to widen educational cooperation have been gaining new impetus. In late December 2023, China’s Vice Minister of Education, Sun Yao, visited Kazakhstan, Uzbekistan, and […]
Outcomes of the 16th Summit of the Organization for Economic Cooperation

The 16th Summit of the Economic Cooperation Organization (ECO) was held in Tashkent, Uzbekistan, on 8-9 November 2023. The ECO Summit, hosted by President of Uzbekistan Shavkat Mirziyoyev, was attended by President of Türkiye Recep Tayyip Erdoğan, President of Iran Ibrahim Reisi, Interim Government Prime Minister of Pakistan Anwaar-ul Haq […]
January 9, 2024
The Growing Importance of Xinjiang in Kazakhstan-China Relations

On October 18, 2023, Kazakh President Kassym-Jomart Tokayev visited Urumqi and met with Ma Xingrui, a member of the Political Bureau of the Central Committee of the Chinese Communist Party and Communist Party Secretary of Xinjiang Uyghur Autonomous Region (XUAR). Prior to that, in May 2023, Kazakh Minister of Agriculture […]
Tags: Foreign Policy , International Relations , Politics
A Spatial Analysis of Internal Migration in Kazakhstan

The 2021 census became the first census conducted since 2009, and the recent publication of its final results unveils valuable insights into various demographic aspects of Kazakhstan over the twelve-year intercensal period. A particularly interesting addition to the census of 2021 is that it places a significant emphasis on migration, […]
Trade in Agricultural Products between Kazakhstan and Russia

In November 2023, Kazakhstan and Russia conducted the 19th Interregional Cooperation Forum, which was devoted to agricultural cooperation between the two countries with the participation of Presidents Kassym-Jomart Tokayev and Vladimir Putin. Kazakhstan’s Kostanay city hosted the forum. However, the Presidents participated online from Kazakhstan’s capital Astana. The governments of […]
Eurasian Journal of Medical and Biological Sciences
About the journal.
Eurasian Journal of Medical and Biological Sciences (Eurasian J Med Biol Sci) is an international, open access periodical published in accordance with independent, unbiased, and double-blinded peer-review principles.
The journal is published as two issues per year (June and December). The journal will consider submissions from all over the world, on research works not being published or submitted for publication towards publication as full paper, review article and short communication. The publication language of the journal is English
Announcements
Publication frequency.
Eurasian Journal of Medical and Biological Sciences publish as two issues per year in June and December.
Early Pub Issues
Publication fee, current issue, review article, physical activities guidelines for healthy life style, some medicinal poisonous plants found in antalya, blood-based biomarkers for prognosis and diagnosis of colorectal cancer, thyroid gland and vocational exposure to low dose ionizing radiation in health, medical science, enhanced heterologous immuno-boost (ehib) covid-19 vaccines: a novel concept, measuring the awareness of university students educated in the field of health about microplastics and its effects on human health, investigation of various antibiotics in the structure of sars cov-2 mpro by molecular docking and molecular dynamics simulation method, biological science, silver nanoparticles entrapped chitosan:poly(vinyl alcohol) thin films via in situ synthesis, information.
- For Readers
- For Authors
- For Librarians
Make a Submission
Indexing and abstracting.

ISSN:2757-8453

Quick Search

- Open access
- Published: 18 April 2024
Research ethics and artificial intelligence for global health: perspectives from the global forum on bioethics in research
- James Shaw 1 , 13 ,
- Joseph Ali 2 , 3 ,
- Caesar A. Atuire 4 , 5 ,
- Phaik Yeong Cheah 6 ,
- Armando Guio Español 7 ,
- Judy Wawira Gichoya 8 ,
- Adrienne Hunt 9 ,
- Daudi Jjingo 10 ,
- Katherine Littler 9 ,
- Daniela Paolotti 11 &
- Effy Vayena 12
BMC Medical Ethics volume 25 , Article number: 46 ( 2024 ) Cite this article
1145 Accesses
6 Altmetric
Metrics details
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice. In this paper we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town, South Africa in November 2022.
The GFBR is an annual meeting organized by the World Health Organization and supported by the Wellcome Trust, the US National Institutes of Health, the UK Medical Research Council (MRC) and the South African MRC. The forum aims to bring together ethicists, researchers, policymakers, research ethics committee members and other actors to engage with challenges and opportunities specifically related to research ethics. In 2022 the focus of the GFBR was “Ethics of AI in Global Health Research”. The forum consisted of 6 case study presentations, 16 governance presentations, and a series of small group and large group discussions. A total of 87 participants attended the forum from 31 countries around the world, representing disciplines of bioethics, AI, health policy, health professional practice, research funding, and bioinformatics. In this paper, we highlight central insights arising from GFBR 2022.
We describe the significance of four thematic insights arising from the forum: (1) Appropriateness of building AI, (2) Transferability of AI systems, (3) Accountability for AI decision-making and outcomes, and (4) Individual consent. We then describe eight recommendations for governance leaders to enhance the ethical governance of AI in global health research, addressing issues such as AI impact assessments, environmental values, and fair partnerships.
Conclusions
The 2022 Global Forum on Bioethics in Research illustrated several innovations in ethical governance of AI for global health research, as well as several areas in need of urgent attention internationally. This summary is intended to inform international and domestic efforts to strengthen research ethics and support the evolution of governance leadership to meet the demands of AI in global health research.
Peer Review reports
Introduction
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice [ 1 , 2 , 3 ]. Beyond the growing number of AI applications being implemented in health care, capabilities of AI models such as Large Language Models (LLMs) expand the potential reach and significance of AI technologies across health-related fields [ 4 , 5 ]. Discussion about effective, ethical governance of AI technologies has spanned a range of governance approaches, including government regulation, organizational decision-making, professional self-regulation, and research ethics review [ 6 , 7 , 8 ]. In this paper, we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health research, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town, South Africa in November 2022. Although applications of AI for research, health care, and public health are diverse and advancing rapidly, the insights generated at the forum remain highly relevant from a global health perspective. After summarizing important context for work in this domain, we highlight categories of ethical issues emphasized at the forum for attention from a research ethics perspective internationally. We then outline strategies proposed for research, innovation, and governance to support more ethical AI for global health.
In this paper, we adopt the definition of AI systems provided by the Organization for Economic Cooperation and Development (OECD) as our starting point. Their definition states that an AI system is “a machine-based system that can, for a given set of human-defined objectives, make predictions, recommendations, or decisions influencing real or virtual environments. AI systems are designed to operate with varying levels of autonomy” [ 9 ]. The conceptualization of an algorithm as helping to constitute an AI system, along with hardware, other elements of software, and a particular context of use, illustrates the wide variety of ways in which AI can be applied. We have found it useful to differentiate applications of AI in research as those classified as “AI systems for discovery” and “AI systems for intervention”. An AI system for discovery is one that is intended to generate new knowledge, for example in drug discovery or public health research in which researchers are seeking potential targets for intervention, innovation, or further research. An AI system for intervention is one that directly contributes to enacting an intervention in a particular context, for example informing decision-making at the point of care or assisting with accuracy in a surgical procedure.
The mandate of the GFBR is to take a broad view of what constitutes research and its regulation in global health, with special attention to bioethics in Low- and Middle- Income Countries. AI as a group of technologies demands such a broad view. AI development for health occurs in a variety of environments, including universities and academic health sciences centers where research ethics review remains an important element of the governance of science and innovation internationally [ 10 , 11 ]. In these settings, research ethics committees (RECs; also known by different names such as Institutional Review Boards or IRBs) make decisions about the ethical appropriateness of projects proposed by researchers and other institutional members, ultimately determining whether a given project is allowed to proceed on ethical grounds [ 12 ].
However, research involving AI for health also takes place in large corporations and smaller scale start-ups, which in some jurisdictions fall outside the scope of research ethics regulation. In the domain of AI, the question of what constitutes research also becomes blurred. For example, is the development of an algorithm itself considered a part of the research process? Or only when that algorithm is tested under the formal constraints of a systematic research methodology? In this paper we take an inclusive view, in which AI development is included in the definition of research activity and within scope for our inquiry, regardless of the setting in which it takes place. This broad perspective characterizes the approach to “research ethics” we take in this paper, extending beyond the work of RECs to include the ethical analysis of the wide range of activities that constitute research as the generation of new knowledge and intervention in the world.
Ethical governance of AI in global health
The ethical governance of AI for global health has been widely discussed in recent years. The World Health Organization (WHO) released its guidelines on ethics and governance of AI for health in 2021, endorsing a set of six ethical principles and exploring the relevance of those principles through a variety of use cases. The WHO guidelines also provided an overview of AI governance, defining governance as covering “a range of steering and rule-making functions of governments and other decision-makers, including international health agencies, for the achievement of national health policy objectives conducive to universal health coverage.” (p. 81) The report usefully provided a series of recommendations related to governance of seven domains pertaining to AI for health: data, benefit sharing, the private sector, the public sector, regulation, policy observatories/model legislation, and global governance. The report acknowledges that much work is yet to be done to advance international cooperation on AI governance, especially related to prioritizing voices from Low- and Middle-Income Countries (LMICs) in global dialogue.
One important point emphasized in the WHO report that reinforces the broader literature on global governance of AI is the distribution of responsibility across a wide range of actors in the AI ecosystem. This is especially important to highlight when focused on research for global health, which is specifically about work that transcends national borders. Alami et al. (2020) discussed the unique risks raised by AI research in global health, ranging from the unavailability of data in many LMICs required to train locally relevant AI models to the capacity of health systems to absorb new AI technologies that demand the use of resources from elsewhere in the system. These observations illustrate the need to identify the unique issues posed by AI research for global health specifically, and the strategies that can be employed by all those implicated in AI governance to promote ethically responsible use of AI in global health research.
RECs and the regulation of research involving AI
RECs represent an important element of the governance of AI for global health research, and thus warrant further commentary as background to our paper. Despite the importance of RECs, foundational questions have been raised about their capabilities to accurately understand and address ethical issues raised by studies involving AI. Rahimzadeh et al. (2023) outlined how RECs in the United States are under-prepared to align with recent federal policy requiring that RECs review data sharing and management plans with attention to the unique ethical issues raised in AI research for health [ 13 ]. Similar research in South Africa identified variability in understanding of existing regulations and ethical issues associated with health-related big data sharing and management among research ethics committee members [ 14 , 15 ]. The effort to address harms accruing to groups or communities as opposed to individuals whose data are included in AI research has also been identified as a unique challenge for RECs [ 16 , 17 ]. Doerr and Meeder (2022) suggested that current regulatory frameworks for research ethics might actually prevent RECs from adequately addressing such issues, as they are deemed out of scope of REC review [ 16 ]. Furthermore, research in the United Kingdom and Canada has suggested that researchers using AI methods for health tend to distinguish between ethical issues and social impact of their research, adopting an overly narrow view of what constitutes ethical issues in their work [ 18 ].
The challenges for RECs in adequately addressing ethical issues in AI research for health care and public health exceed a straightforward survey of ethical considerations. As Ferretti et al. (2021) contend, some capabilities of RECs adequately cover certain issues in AI-based health research, such as the common occurrence of conflicts of interest where researchers who accept funds from commercial technology providers are implicitly incentivized to produce results that align with commercial interests [ 12 ]. However, some features of REC review require reform to adequately meet ethical needs. Ferretti et al. outlined weaknesses of RECs that are longstanding and those that are novel to AI-related projects, proposing a series of directions for development that are regulatory, procedural, and complementary to REC functionality. The work required on a global scale to update the REC function in response to the demands of research involving AI is substantial.
These issues take greater urgency in the context of global health [ 19 ]. Teixeira da Silva (2022) described the global practice of “ethics dumping”, where researchers from high income countries bring ethically contentious practices to RECs in low-income countries as a strategy to gain approval and move projects forward [ 20 ]. Although not yet systematically documented in AI research for health, risk of ethics dumping in AI research is high. Evidence is already emerging of practices of “health data colonialism”, in which AI researchers and developers from large organizations in high-income countries acquire data to build algorithms in LMICs to avoid stricter regulations [ 21 ]. This specific practice is part of a larger collection of practices that characterize health data colonialism, involving the broader exploitation of data and the populations they represent primarily for commercial gain [ 21 , 22 ]. As an additional complication, AI algorithms trained on data from high-income contexts are unlikely to apply in straightforward ways to LMIC settings [ 21 , 23 ]. In the context of global health, there is widespread acknowledgement about the need to not only enhance the knowledge base of REC members about AI-based methods internationally, but to acknowledge the broader shifts required to encourage their capabilities to more fully address these and other ethical issues associated with AI research for health [ 8 ].
Although RECs are an important part of the story of the ethical governance of AI for global health research, they are not the only part. The responsibilities of supra-national entities such as the World Health Organization, national governments, organizational leaders, commercial AI technology providers, health care professionals, and other groups continue to be worked out internationally. In this context of ongoing work, examining issues that demand attention and strategies to address them remains an urgent and valuable task.
The GFBR is an annual meeting organized by the World Health Organization and supported by the Wellcome Trust, the US National Institutes of Health, the UK Medical Research Council (MRC) and the South African MRC. The forum aims to bring together ethicists, researchers, policymakers, REC members and other actors to engage with challenges and opportunities specifically related to research ethics. Each year the GFBR meeting includes a series of case studies and keynotes presented in plenary format to an audience of approximately 100 people who have applied and been competitively selected to attend, along with small-group breakout discussions to advance thinking on related issues. The specific topic of the forum changes each year, with past topics including ethical issues in research with people living with mental health conditions (2021), genome editing (2019), and biobanking/data sharing (2018). The forum is intended to remain grounded in the practical challenges of engaging in research ethics, with special interest in low resource settings from a global health perspective. A post-meeting fellowship scheme is open to all LMIC participants, providing a unique opportunity to apply for funding to further explore and address the ethical challenges that are identified during the meeting.
In 2022, the focus of the GFBR was “Ethics of AI in Global Health Research”. The forum consisted of 6 case study presentations (both short and long form) reporting on specific initiatives related to research ethics and AI for health, and 16 governance presentations (both short and long form) reporting on actual approaches to governing AI in different country settings. A keynote presentation from Professor Effy Vayena addressed the topic of the broader context for AI ethics in a rapidly evolving field. A total of 87 participants attended the forum from 31 countries around the world, representing disciplines of bioethics, AI, health policy, health professional practice, research funding, and bioinformatics. The 2-day forum addressed a wide range of themes. The conference report provides a detailed overview of each of the specific topics addressed while a policy paper outlines the cross-cutting themes (both documents are available at the GFBR website: https://www.gfbr.global/past-meetings/16th-forum-cape-town-south-africa-29-30-november-2022/ ). As opposed to providing a detailed summary in this paper, we aim to briefly highlight central issues raised, solutions proposed, and the challenges facing the research ethics community in the years to come.
In this way, our primary aim in this paper is to present a synthesis of the challenges and opportunities raised at the GFBR meeting and in the planning process, followed by our reflections as a group of authors on their significance for governance leaders in the coming years. We acknowledge that the views represented at the meeting and in our results are a partial representation of the universe of views on this topic; however, the GFBR leadership invested a great deal of resources in convening a deeply diverse and thoughtful group of researchers and practitioners working on themes of bioethics related to AI for global health including those based in LMICs. We contend that it remains rare to convene such a strong group for an extended time and believe that many of the challenges and opportunities raised demand attention for more ethical futures of AI for health. Nonetheless, our results are primarily descriptive and are thus not explicitly grounded in a normative argument. We make effort in the Discussion section to contextualize our results by describing their significance and connecting them to broader efforts to reform global health research and practice.
Uniquely important ethical issues for AI in global health research
Presentations and group dialogue over the course of the forum raised several issues for consideration, and here we describe four overarching themes for the ethical governance of AI in global health research. Brief descriptions of each issue can be found in Table 1 . Reports referred to throughout the paper are available at the GFBR website provided above.
The first overarching thematic issue relates to the appropriateness of building AI technologies in response to health-related challenges in the first place. Case study presentations referred to initiatives where AI technologies were highly appropriate, such as in ear shape biometric identification to more accurately link electronic health care records to individual patients in Zambia (Alinani Simukanga). Although important ethical issues were raised with respect to privacy, trust, and community engagement in this initiative, the AI-based solution was appropriately matched to the challenge of accurately linking electronic records to specific patient identities. In contrast, forum participants raised questions about the appropriateness of an initiative using AI to improve the quality of handwashing practices in an acute care hospital in India (Niyoshi Shah), which led to gaming the algorithm. Overall, participants acknowledged the dangers of techno-solutionism, in which AI researchers and developers treat AI technologies as the most obvious solutions to problems that in actuality demand much more complex strategies to address [ 24 ]. However, forum participants agreed that RECs in different contexts have differing degrees of power to raise issues of the appropriateness of an AI-based intervention.
The second overarching thematic issue related to whether and how AI-based systems transfer from one national health context to another. One central issue raised by a number of case study presentations related to the challenges of validating an algorithm with data collected in a local environment. For example, one case study presentation described a project that would involve the collection of personally identifiable data for sensitive group identities, such as tribe, clan, or religion, in the jurisdictions involved (South Africa, Nigeria, Tanzania, Uganda and the US; Gakii Masunga). Doing so would enable the team to ensure that those groups were adequately represented in the dataset to ensure the resulting algorithm was not biased against specific community groups when deployed in that context. However, some members of these communities might desire to be represented in the dataset, whereas others might not, illustrating the need to balance autonomy and inclusivity. It was also widely recognized that collecting these data is an immense challenge, particularly when historically oppressive practices have led to a low-trust environment for international organizations and the technologies they produce. It is important to note that in some countries such as South Africa and Rwanda, it is illegal to collect information such as race and tribal identities, re-emphasizing the importance for cultural awareness and avoiding “one size fits all” solutions.
The third overarching thematic issue is related to understanding accountabilities for both the impacts of AI technologies and governance decision-making regarding their use. Where global health research involving AI leads to longer-term harms that might fall outside the usual scope of issues considered by a REC, who is to be held accountable, and how? This question was raised as one that requires much further attention, with law being mixed internationally regarding the mechanisms available to hold researchers, innovators, and their institutions accountable over the longer term. However, it was recognized in breakout group discussion that many jurisdictions are developing strong data protection regimes related specifically to international collaboration for research involving health data. For example, Kenya’s Data Protection Act requires that any internationally funded projects have a local principal investigator who will hold accountability for how data are shared and used [ 25 ]. The issue of research partnerships with commercial entities was raised by many participants in the context of accountability, pointing toward the urgent need for clear principles related to strategies for engagement with commercial technology companies in global health research.
The fourth and final overarching thematic issue raised here is that of consent. The issue of consent was framed by the widely shared recognition that models of individual, explicit consent might not produce a supportive environment for AI innovation that relies on the secondary uses of health-related datasets to build AI algorithms. Given this recognition, approaches such as community oversight of health data uses were suggested as a potential solution. However, the details of implementing such community oversight mechanisms require much further attention, particularly given the unique perspectives on health data in different country settings in global health research. Furthermore, some uses of health data do continue to require consent. One case study of South Africa, Nigeria, Kenya, Ethiopia and Uganda suggested that when health data are shared across borders, individual consent remains necessary when data is transferred from certain countries (Nezerith Cengiz). Broader clarity is necessary to support the ethical governance of health data uses for AI in global health research.
Recommendations for ethical governance of AI in global health research
Dialogue at the forum led to a range of suggestions for promoting ethical conduct of AI research for global health, related to the various roles of actors involved in the governance of AI research broadly defined. The strategies are written for actors we refer to as “governance leaders”, those people distributed throughout the AI for global health research ecosystem who are responsible for ensuring the ethical and socially responsible conduct of global health research involving AI (including researchers themselves). These include RECs, government regulators, health care leaders, health professionals, corporate social accountability officers, and others. Enacting these strategies would bolster the ethical governance of AI for global health more generally, enabling multiple actors to fulfill their roles related to governing research and development activities carried out across multiple organizations, including universities, academic health sciences centers, start-ups, and technology corporations. Specific suggestions are summarized in Table 2 .
First, forum participants suggested that governance leaders including RECs, should remain up to date on recent advances in the regulation of AI for health. Regulation of AI for health advances rapidly and takes on different forms in jurisdictions around the world. RECs play an important role in governance, but only a partial role; it was deemed important for RECs to acknowledge how they fit within a broader governance ecosystem in order to more effectively address the issues within their scope. Not only RECs but organizational leaders responsible for procurement, researchers, and commercial actors should all commit to efforts to remain up to date about the relevant approaches to regulating AI for health care and public health in jurisdictions internationally. In this way, governance can more adequately remain up to date with advances in regulation.
Second, forum participants suggested that governance leaders should focus on ethical governance of health data as a basis for ethical global health AI research. Health data are considered the foundation of AI development, being used to train AI algorithms for various uses [ 26 ]. By focusing on ethical governance of health data generation, sharing, and use, multiple actors will help to build an ethical foundation for AI development among global health researchers.
Third, forum participants believed that governance processes should incorporate AI impact assessments where appropriate. An AI impact assessment is the process of evaluating the potential effects, both positive and negative, of implementing an AI algorithm on individuals, society, and various stakeholders, generally over time frames specified in advance of implementation [ 27 ]. Although not all types of AI research in global health would warrant an AI impact assessment, this is especially relevant for those studies aiming to implement an AI system for intervention into health care or public health. Organizations such as RECs can use AI impact assessments to boost understanding of potential harms at the outset of a research project, encouraging researchers to more deeply consider potential harms in the development of their study.
Fourth, forum participants suggested that governance decisions should incorporate the use of environmental impact assessments, or at least the incorporation of environment values when assessing the potential impact of an AI system. An environmental impact assessment involves evaluating and anticipating the potential environmental effects of a proposed project to inform ethical decision-making that supports sustainability [ 28 ]. Although a relatively new consideration in research ethics conversations [ 29 ], the environmental impact of building technologies is a crucial consideration for the public health commitment to environmental sustainability. Governance leaders can use environmental impact assessments to boost understanding of potential environmental harms linked to AI research projects in global health over both the shorter and longer terms.
Fifth, forum participants suggested that governance leaders should require stronger transparency in the development of AI algorithms in global health research. Transparency was considered essential in the design and development of AI algorithms for global health to ensure ethical and accountable decision-making throughout the process. Furthermore, whether and how researchers have considered the unique contexts into which such algorithms may be deployed can be surfaced through stronger transparency, for example in describing what primary considerations were made at the outset of the project and which stakeholders were consulted along the way. Sharing information about data provenance and methods used in AI development will also enhance the trustworthiness of the AI-based research process.
Sixth, forum participants suggested that governance leaders can encourage or require community engagement at various points throughout an AI project. It was considered that engaging patients and communities is crucial in AI algorithm development to ensure that the technology aligns with community needs and values. However, participants acknowledged that this is not a straightforward process. Effective community engagement requires lengthy commitments to meeting with and hearing from diverse communities in a given setting, and demands a particular set of skills in communication and dialogue that are not possessed by all researchers. Encouraging AI researchers to begin this process early and build long-term partnerships with community members is a promising strategy to deepen community engagement in AI research for global health. One notable recommendation was that research funders have an opportunity to incentivize and enable community engagement with funds dedicated to these activities in AI research in global health.
Seventh, forum participants suggested that governance leaders can encourage researchers to build strong, fair partnerships between institutions and individuals across country settings. In a context of longstanding imbalances in geopolitical and economic power, fair partnerships in global health demand a priori commitments to share benefits related to advances in medical technologies, knowledge, and financial gains. Although enforcement of this point might be beyond the remit of RECs, commentary will encourage researchers to consider stronger, fairer partnerships in global health in the longer term.
Eighth, it became evident that it is necessary to explore new forms of regulatory experimentation given the complexity of regulating a technology of this nature. In addition, the health sector has a series of particularities that make it especially complicated to generate rules that have not been previously tested. Several participants highlighted the desire to promote spaces for experimentation such as regulatory sandboxes or innovation hubs in health. These spaces can have several benefits for addressing issues surrounding the regulation of AI in the health sector, such as: (i) increasing the capacities and knowledge of health authorities about this technology; (ii) identifying the major problems surrounding AI regulation in the health sector; (iii) establishing possibilities for exchange and learning with other authorities; (iv) promoting innovation and entrepreneurship in AI in health; and (vi) identifying the need to regulate AI in this sector and update other existing regulations.
Ninth and finally, forum participants believed that the capabilities of governance leaders need to evolve to better incorporate expertise related to AI in ways that make sense within a given jurisdiction. With respect to RECs, for example, it might not make sense for every REC to recruit a member with expertise in AI methods. Rather, it will make more sense in some jurisdictions to consult with members of the scientific community with expertise in AI when research protocols are submitted that demand such expertise. Furthermore, RECs and other approaches to research governance in jurisdictions around the world will need to evolve in order to adopt the suggestions outlined above, developing processes that apply specifically to the ethical governance of research using AI methods in global health.
Research involving the development and implementation of AI technologies continues to grow in global health, posing important challenges for ethical governance of AI in global health research around the world. In this paper we have summarized insights from the 2022 GFBR, focused specifically on issues in research ethics related to AI for global health research. We summarized four thematic challenges for governance related to AI in global health research and nine suggestions arising from presentations and dialogue at the forum. In this brief discussion section, we present an overarching observation about power imbalances that frames efforts to evolve the role of governance in global health research, and then outline two important opportunity areas as the field develops to meet the challenges of AI in global health research.
Dialogue about power is not unfamiliar in global health, especially given recent contributions exploring what it would mean to de-colonize global health research, funding, and practice [ 30 , 31 ]. Discussions of research ethics applied to AI research in global health contexts are deeply infused with power imbalances. The existing context of global health is one in which high-income countries primarily located in the “Global North” charitably invest in projects taking place primarily in the “Global South” while recouping knowledge, financial, and reputational benefits [ 32 ]. With respect to AI development in particular, recent examples of digital colonialism frame dialogue about global partnerships, raising attention to the role of large commercial entities and global financial capitalism in global health research [ 21 , 22 ]. Furthermore, the power of governance organizations such as RECs to intervene in the process of AI research in global health varies widely around the world, depending on the authorities assigned to them by domestic research governance policies. These observations frame the challenges outlined in our paper, highlighting the difficulties associated with making meaningful change in this field.
Despite these overarching challenges of the global health research context, there are clear strategies for progress in this domain. Firstly, AI innovation is rapidly evolving, which means approaches to the governance of AI for health are rapidly evolving too. Such rapid evolution presents an important opportunity for governance leaders to clarify their vision and influence over AI innovation in global health research, boosting the expertise, structure, and functionality required to meet the demands of research involving AI. Secondly, the research ethics community has strong international ties, linked to a global scholarly community that is committed to sharing insights and best practices around the world. This global community can be leveraged to coordinate efforts to produce advances in the capabilities and authorities of governance leaders to meaningfully govern AI research for global health given the challenges summarized in our paper.
Limitations
Our paper includes two specific limitations that we address explicitly here. First, it is still early in the lifetime of the development of applications of AI for use in global health, and as such, the global community has had limited opportunity to learn from experience. For example, there were many fewer case studies, which detail experiences with the actual implementation of an AI technology, submitted to GFBR 2022 for consideration than was expected. In contrast, there were many more governance reports submitted, which detail the processes and outputs of governance processes that anticipate the development and dissemination of AI technologies. This observation represents both a success and a challenge. It is a success that so many groups are engaging in anticipatory governance of AI technologies, exploring evidence of their likely impacts and governing technologies in novel and well-designed ways. It is a challenge that there is little experience to build upon of the successful implementation of AI technologies in ways that have limited harms while promoting innovation. Further experience with AI technologies in global health will contribute to revising and enhancing the challenges and recommendations we have outlined in our paper.
Second, global trends in the politics and economics of AI technologies are evolving rapidly. Although some nations are advancing detailed policy approaches to regulating AI more generally, including for uses in health care and public health, the impacts of corporate investments in AI and political responses related to governance remain to be seen. The excitement around large language models (LLMs) and large multimodal models (LMMs) has drawn deeper attention to the challenges of regulating AI in any general sense, opening dialogue about health sector-specific regulations. The direction of this global dialogue, strongly linked to high-profile corporate actors and multi-national governance institutions, will strongly influence the development of boundaries around what is possible for the ethical governance of AI for global health. We have written this paper at a point when these developments are proceeding rapidly, and as such, we acknowledge that our recommendations will need updating as the broader field evolves.
Ultimately, coordination and collaboration between many stakeholders in the research ethics ecosystem will be necessary to strengthen the ethical governance of AI in global health research. The 2022 GFBR illustrated several innovations in ethical governance of AI for global health research, as well as several areas in need of urgent attention internationally. This summary is intended to inform international and domestic efforts to strengthen research ethics and support the evolution of governance leadership to meet the demands of AI in global health research.
Data availability
All data and materials analyzed to produce this paper are available on the GFBR website: https://www.gfbr.global/past-meetings/16th-forum-cape-town-south-africa-29-30-november-2022/ .
Clark P, Kim J, Aphinyanaphongs Y, Marketing, Food US. Drug Administration Clearance of Artificial Intelligence and Machine Learning Enabled Software in and as Medical devices: a systematic review. JAMA Netw Open. 2023;6(7):e2321792–2321792.
Article Google Scholar
Potnis KC, Ross JS, Aneja S, Gross CP, Richman IB. Artificial intelligence in breast cancer screening: evaluation of FDA device regulation and future recommendations. JAMA Intern Med. 2022;182(12):1306–12.
Siala H, Wang Y. SHIFTing artificial intelligence to be responsible in healthcare: a systematic review. Soc Sci Med. 2022;296:114782.
Yang X, Chen A, PourNejatian N, Shin HC, Smith KE, Parisien C, et al. A large language model for electronic health records. NPJ Digit Med. 2022;5(1):194.
Meskó B, Topol EJ. The imperative for regulatory oversight of large language models (or generative AI) in healthcare. NPJ Digit Med. 2023;6(1):120.
Jobin A, Ienca M, Vayena E. The global landscape of AI ethics guidelines. Nat Mach Intell. 2019;1(9):389–99.
Minssen T, Vayena E, Cohen IG. The challenges for Regulating Medical Use of ChatGPT and other large Language models. JAMA. 2023.
Ho CWL, Malpani R. Scaling up the research ethics framework for healthcare machine learning as global health ethics and governance. Am J Bioeth. 2022;22(5):36–8.
Yeung K. Recommendation of the council on artificial intelligence (OECD). Int Leg Mater. 2020;59(1):27–34.
Maddox TM, Rumsfeld JS, Payne PR. Questions for artificial intelligence in health care. JAMA. 2019;321(1):31–2.
Dzau VJ, Balatbat CA, Ellaissi WF. Revisiting academic health sciences systems a decade later: discovery to health to population to society. Lancet. 2021;398(10318):2300–4.
Ferretti A, Ienca M, Sheehan M, Blasimme A, Dove ES, Farsides B, et al. Ethics review of big data research: what should stay and what should be reformed? BMC Med Ethics. 2021;22(1):1–13.
Rahimzadeh V, Serpico K, Gelinas L. Institutional review boards need new skills to review data sharing and management plans. Nat Med. 2023;1–3.
Kling S, Singh S, Burgess TL, Nair G. The role of an ethics advisory committee in data science research in sub-saharan Africa. South Afr J Sci. 2023;119(5–6):1–3.
Google Scholar
Cengiz N, Kabanda SM, Esterhuizen TM, Moodley K. Exploring perspectives of research ethics committee members on the governance of big data in sub-saharan Africa. South Afr J Sci. 2023;119(5–6):1–9.
Doerr M, Meeder S. Big health data research and group harm: the scope of IRB review. Ethics Hum Res. 2022;44(4):34–8.
Ballantyne A, Stewart C. Big data and public-private partnerships in healthcare and research: the application of an ethics framework for big data in health and research. Asian Bioeth Rev. 2019;11(3):315–26.
Samuel G, Chubb J, Derrick G. Boundaries between research ethics and ethical research use in artificial intelligence health research. J Empir Res Hum Res Ethics. 2021;16(3):325–37.
Murphy K, Di Ruggiero E, Upshur R, Willison DJ, Malhotra N, Cai JC, et al. Artificial intelligence for good health: a scoping review of the ethics literature. BMC Med Ethics. 2021;22(1):1–17.
Teixeira da Silva JA. Handling ethics dumping and neo-colonial research: from the laboratory to the academic literature. J Bioethical Inq. 2022;19(3):433–43.
Ferryman K. The dangers of data colonialism in precision public health. Glob Policy. 2021;12:90–2.
Couldry N, Mejias UA. Data colonialism: rethinking big data’s relation to the contemporary subject. Telev New Media. 2019;20(4):336–49.
Organization WH. Ethics and governance of artificial intelligence for health: WHO guidance. 2021.
Metcalf J, Moss E. Owning ethics: corporate logics, silicon valley, and the institutionalization of ethics. Soc Res Int Q. 2019;86(2):449–76.
Data Protection Act - OFFICE OF THE DATA PROTECTION COMMISSIONER KENYA [Internet]. 2021 [cited 2023 Sep 30]. https://www.odpc.go.ke/dpa-act/ .
Sharon T, Lucivero F. Introduction to the special theme: the expansion of the health data ecosystem–rethinking data ethics and governance. Big Data & Society. Volume 6. London, England: SAGE Publications Sage UK; 2019. p. 2053951719852969.
Reisman D, Schultz J, Crawford K, Whittaker M. Algorithmic impact assessments: a practical Framework for Public Agency. AI Now. 2018.
Morgan RK. Environmental impact assessment: the state of the art. Impact Assess Proj Apprais. 2012;30(1):5–14.
Samuel G, Richie C. Reimagining research ethics to include environmental sustainability: a principled approach, including a case study of data-driven health research. J Med Ethics. 2023;49(6):428–33.
Kwete X, Tang K, Chen L, Ren R, Chen Q, Wu Z, et al. Decolonizing global health: what should be the target of this movement and where does it lead us? Glob Health Res Policy. 2022;7(1):3.
Abimbola S, Asthana S, Montenegro C, Guinto RR, Jumbam DT, Louskieter L, et al. Addressing power asymmetries in global health: imperatives in the wake of the COVID-19 pandemic. PLoS Med. 2021;18(4):e1003604.
Benatar S. Politics, power, poverty and global health: systems and frames. Int J Health Policy Manag. 2016;5(10):599.
Download references
Acknowledgements
We would like to acknowledge the outstanding contributions of the attendees of GFBR 2022 in Cape Town, South Africa. This paper is authored by members of the GFBR 2022 Planning Committee. We would like to acknowledge additional members Tamra Lysaght, National University of Singapore, and Niresh Bhagwandin, South African Medical Research Council, for their input during the planning stages and as reviewers of the applications to attend the Forum.
This work was supported by Wellcome [222525/Z/21/Z], the US National Institutes of Health, the UK Medical Research Council (part of UK Research and Innovation), and the South African Medical Research Council through funding to the Global Forum on Bioethics in Research.
Author information
Authors and affiliations.
Department of Physical Therapy, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada
Berman Institute of Bioethics, Johns Hopkins University, Baltimore, MD, USA
Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Department of Philosophy and Classics, University of Ghana, Legon-Accra, Ghana
Caesar A. Atuire
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Phaik Yeong Cheah
Berkman Klein Center, Harvard University, Bogotá, Colombia
Armando Guio Español
Department of Radiology and Informatics, Emory University School of Medicine, Atlanta, GA, USA
Judy Wawira Gichoya
Health Ethics & Governance Unit, Research for Health Department, Science Division, World Health Organization, Geneva, Switzerland
Adrienne Hunt & Katherine Littler
African Center of Excellence in Bioinformatics and Data Intensive Science, Infectious Diseases Institute, Makerere University, Kampala, Uganda
Daudi Jjingo
ISI Foundation, Turin, Italy
Daniela Paolotti
Department of Health Sciences and Technology, ETH Zurich, Zürich, Switzerland
Effy Vayena
Joint Centre for Bioethics, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada
You can also search for this author in PubMed Google Scholar
Contributions
JS led the writing, contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. JA contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. CA contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. PYC contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. AE contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. JWG contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. AH contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. DJ contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. KL contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. DP contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. EV contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper.
Corresponding author
Correspondence to James Shaw .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shaw, J., Ali, J., Atuire, C.A. et al. Research ethics and artificial intelligence for global health: perspectives from the global forum on bioethics in research. BMC Med Ethics 25 , 46 (2024). https://doi.org/10.1186/s12910-024-01044-w
Download citation
Received : 31 October 2023
Accepted : 01 April 2024
Published : 18 April 2024
DOI : https://doi.org/10.1186/s12910-024-01044-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Artificial intelligence
- Machine learning
- Research ethics
- Global health
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
Eurasian Research Publishing team is exclusively inviting you to submit your research work at the Eurasian Research Publishing database which can be found at ww.erepub.com. We are an open-access publication and our main aim is to publish content frequently every month without any delay. Eurasian Research Publishing covers full-length research articles, review articles, case studies, short communication, thesis papers, e-Books, book chapters, etc.
Eurasian Research Publishing covers all types of research fields including Sciences, Engineering, Arts, Business, and Commerce-related topics.
Eurasian Research Publishing also covering such as Information Science, Information Science & Technology, Computational Intelligence in Bioinformatics, Information Science & Education, Database Theory & Application, Information Technology & Library Science, Information & Communications Technology, Administration Science, Tourism & Business, Hospitality Administration & Management, Accounting & Taxation, Business Development, Business Administration, Business & Technology, Management & Information Technology, Economics & Finance, Engineering & Management, Organizational Behavior, Agriculture, Agricultural Processing, Sustainable Agriculture, Horticulture & Forestry, Agriculture & Food Sciences, Advanced Bioengineering, Agriculture & Management, Advanced Biotechnology, Applied Engineering in Agriculture, Agricultural Engineering & Biotechnology, Agricultural Biotechnology & Sustainable Development, Renewable Energy & Smart Grid, Energy Conversion & Management, Renewable Energy, Energy & Power Engineering, Environmental Sciences, Waste Management, Oceanography & Marine Science, Earth Science & Engineering, Environment & Development Economics, Resources & Environment, Biodiversity & Conservation, Environmental Forensics, Waste Management & Technology, Environmental Science & Water Resources, Environmental Protection & Management Science, Metal & Steel, Polymer Degradation & Stability, Globalization & Industrial Technology, Carbon Nanotechnology, Water Science & Technology, Colloid & Interface Science, Industrial & Technology Management, Alternative Energy & Petroleum Technology, Patent, International Relations, Media & Mass Communication, Applied Anthropology, Political Science, Social Science & Geography, Music & Dance, Developing Country Education, Educational Sustainability, Management & Leadership, Ocean Engineering, Design Engineering, Ceramic Engineering, Earthquake Engineering, Computers & Mechanical Engineering, Traffic & Transportation Engineering, Construction Engineering & Management, Automotive Engineering, Coal Science & Engineering, Engineering Studies, Control Science & Engineering, Mining & Mineral Engineering, Transportation Engineering, Computers & Electrical Engineering, Wireless & Mobile Communications Engineering, Analytic Geometry, Statistics & Mathematics, Biochemistry, Circuits & Systems, Inorganic Chemistry, Mathematical Finance, Theoretical Physics, Statistical Design & Analysis, Entomology, Polymer Science, Quantum Mechanics, Animal Physiology, Astronomy, Molecular Catalysis, Optics Science, Wildlife Ecology, Microscopy & Cytometry, Electrochemical Science, Advanced Biochemistry & Microbiology, Behavioural Sciences, Psychology, Public Health, Pediatric Science, Behavioral Health & Neuroscience, Medical & Diagnostic Biochemistry, Addiction, Health Science, Diabetes & Endocrinology, Medicinal Plant, Obstetrics & Gynecology, Medical Practice & Reviews, Metabolomics & Systems Biology, Environmental Health Sciences & Toxicology,
Eurasian Research Publishing's main aim is to publish articles every month. Academic and Scientific Publishing serves standard quality publications for all authors and readers. All authors and readers can copy or download articles from www.erepub.com for free.
Call for papers or Manuscripts
Submit your manuscript e-mail: [email protected].

Policy Notice
Terms & Conditions
Follow us on social media.
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 7—July 2024

Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024
Suggested citation for this article
We report highly pathogenic avian influenza A(H5N1) virus in dairy cattle and cats in Kansas and Texas, United States, which reflects the continued spread of clade 2.3.4.4b viruses that entered the country in late 2021. Infected cattle experienced nonspecific illness, reduced feed intake and rumination, and an abrupt drop in milk production, but fatal systemic influenza infection developed in domestic cats fed raw (unpasteurized) colostrum and milk from affected cows. Cow-to-cow transmission appears to have occurred because infections were observed in cattle on Michigan, Idaho, and Ohio farms where avian influenza virus–infected cows were transported. Although the US Food and Drug Administration has indicated the commercial milk supply remains safe, the detection of influenza virus in unpasteurized bovine milk is a concern because of potential cross-species transmission. Continued surveillance of highly pathogenic avian influenza viruses in domestic production animals is needed to prevent cross-species and mammal-to-mammal transmission.
Highly pathogenic avian influenza (HPAI) viruses pose a threat to wild birds and poultry globally, and HPAI H5N1 viruses are of even greater concern because of their frequent spillover into mammals. In late 2021, the Eurasian strain of H5N1 (clade 2.3.4.4b) was detected in North America ( 1 , 2 ) and initiated an outbreak that continued into 2024. Spillover detections and deaths from this clade have been reported in both terrestrial and marine mammals in the United States ( 3 , 4 ). The detection of HPAI H5N1 clade 2.3.4.4b virus in severe cases of human disease in Ecuador ( 5 ) and Chile ( 6 ) raises further concerns regarding the pandemic potential of specific HPAI viruses.
In February 2024, veterinarians were alerted to a syndrome occurring in lactating dairy cattle in the panhandle region of northern Texas. Nonspecific illness accompanied by reduced feed intake and rumination and an abrupt drop in milk production developed in affected animals. The milk from most affected cows had a thickened, creamy yellow appearance similar to colostrum. On affected farms, incidence appeared to peak 4–6 days after the first animals were affected and then tapered off within 10–14 days; afterward, most animals were slowly returned to regular milking. Clinical signs were commonly reported in multiparous cows during middle to late lactation; ≈10%–15% illness and minimal death of cattle were observed on affected farms. Initial submissions of blood, urine, feces, milk, and nasal swab samples and postmortem tissues to regional diagnostic laboratories did not reveal a consistent, specific cause for reduced milk production. Milk cultures were often negative, and serum chemistry testing showed mildly increased aspartate aminotransferase, gamma-glutamyl transferase, creatinine kinase, and bilirubin values, whereas complete blood counts showed variable anemia and leukocytopenia.
In early March 2024, similar clinical cases were reported in dairy cattle in southwestern Kansas and northeastern New Mexico; deaths of wild birds and domestic cats were also observed within affected sites in the Texas panhandle. In > 1 dairy farms in Texas, deaths occurred in domestic cats fed raw colostrum and milk from sick cows that were in the hospital parlor. Antemortem clinical signs in affected cats were depressed mental state, stiff body movements, ataxia, blindness, circling, and copious oculonasal discharge. Neurologic exams of affected cats revealed the absence of menace reflexes and pupillary light responses with a weak blink response.
On March 21, 2024, milk, serum, and fresh and fixed tissue samples from cattle located in affected dairies in Texas and 2 deceased cats from an affected Texas dairy farm were received at the Iowa State University Veterinary Diagnostic Laboratory (ISUVDL; Ames, IA, USA). The next day, similar sets of samples were received from cattle located in affected dairies in Kansas. Milk and tissue samples from cattle and tissue samples from the cats tested positive for influenza A virus (IAV) by screening PCR, which was confirmed and characterized as HPAI H5N1 virus by the US Department of Agriculture National Veterinary Services Laboratory. Detection led to an initial press release by the US Department of Agriculture Animal and Plant Health Inspection Service on March 25, 2024, confirming HPAI virus in dairy cattle ( 7 ). We report the characterizations performed at the ISUVDL for HPAI H5N1 viruses infecting cattle and cats in Kansas and Texas.
Materials and Methods
Milk samples (cases 2–5) and fresh and formalin-fixed tissues (cases 1, 3–5) from dairy cattle were received at the ISUVDL from Texas on March 21 and from Kansas on March 22, 2024. The cattle exhibited nonspecific illness and reduced lactation, as described previously. The tissue samples for diagnostic testing came from 3 cows that were euthanized and 3 that died naturally; all postmortem examinations were performed on the premises of affected farms.
The bodies of 2 adult domestic shorthaired cats from a north Texas dairy farm were received at the ISUVDL for a complete postmortem examination on March 21, 2024. The cats were found dead with no apparent signs of injury and were from a resident population of ≈24 domestic cats that had been fed milk from sick cows. Clinical disease in cows on that farm was first noted on March 16; the cats became sick on March 17, and several cats died in a cluster during March 19–20. In total, >50% of the cats at that dairy became ill and died. We collected cerebrum, cerebellum, eye, lung, heart, spleen, liver, lymph node, and kidney tissue samples from the cats and placed them in 10% neutral-buffered formalin for histopathology.
At ISUVDL, we trimmed, embedded in paraffin, and processed formalin-fixed tissues from affected cattle and cats for hematoxylin/eosin staining and histologic evaluation. For immunohistochemistry (IHC), we prepared 4-µm–thick sections from paraffin-embedded tissues, placed them on Superfrost Plus slides (VWR, https://www.vwr.com ), and dried them for 20 minutes at 60°C. We used a Ventana Discovery Ultra IHC/ISH research platform (Roche, https://www.roche.com ) for deparaffinization until and including counterstaining. We obtained all products except the primary antibody from Roche. Automated deparaffination was followed by enzymatic digestion with protease 1 for 8 minutes at 37°C and endogenous peroxidase blocking. We obtained the primary influenza A virus antibody from the hybridoma cell line H16-L10–4R5 (ATCC, https://www.atcc.org ) and diluted at 1:100 in Discovery PSS diluent; we incubated sections with antibody for 32 minutes at room temperature. Next, we incubated the sections with a hapten-labeled conjugate, Discovery anti-mouse HQ, for 16 minutes at 37°C followed by a 16-minute incubation with the horse radish peroxidase conjugate, Discovery anti-HQ HRP. We used a ChromoMap DAB kit for antigen visualization, followed by counterstaining with hematoxylin and then bluing. Positive controls were sections of IAV-positive swine lung. Negative controls were sections of brain, lung, and eyes from cats not infected with IAV.
We diluted milk samples 1:3 vol/vol in phosphate buffered saline, pH 7.4 (Gibco/Thermo Fisher Scientific, https://www.thermofisher.com ) by mixing 1 unit volume of milk and 3 unit volumes of phosphate buffered saline. We prepared 10% homogenates of mammary glands, brains, lungs, spleens, and lymph nodes in Earle’s balanced salt solution (Sigma-Aldrich, https://www.sigmaaldrich.com ). Processing was not necessary for ocular fluid, rumen content, or serum samples. After processing, we extracted samples according to a National Animal Health Laboratory Network (NAHLN) protocol that had 2 NAHLN-approved deviations for ISUVDL consisting of the MagMax Viral RNA Isolation Kit for 100 µL sample volumes and a Kingfisher Flex instrument (both Thermo Fisher Scientific).
We performed real-time reverse transcription PCR (rRT-PCR) by using an NAHLN-approved assay with 1 deviation, which was the VetMAX-Gold SIV Detection kit (Thermo Fisher Scientific), to screen for the presence of IAV RNA. We tested samples along with the VetMAX XENO Internal Positive Control to monitor the possible presence of PCR inhibitors. Each rRT-PCR 96-well plate had 2 positive amplification controls, 2 negative amplification controls, 1 positive extraction control, and 1 negative extraction control. We ran the rRT-PCR on an ABI 7500 Fast thermocycler and analyzed data with Design and Analysis Software 2.7.0 (both Thermo Fisher Scientific). We considered samples with cycle threshold (Ct) values <40.0 to be positive for virus.
After the screening rRT-PCR, we analyzed IAV RNA–positive samples for the H5 subtype and H5 clade 2.3.4.4b by using the same RNA extraction and NAHLN-approved rRT-PCR protocols as described previously, according to standard operating procedures. We performed PCR on the ABI 7500 Fast thermocycler by using appropriate controls to detect H5-specific IAV. We considered samples with Ct values <40.0 to be positive for the IAV H5 subtype.
We conducted genomic sequencing of 2 milk samples from infected dairy cattle from Texas and 2 tissue samples (lung and brain) from cats that died at a different Texas dairy. We subjected the whole-genome sequencing data to bioinformatics analysis to assemble the 8 different IAV segment sequences according to previously described methods ( 8 ). We used the hemagglutinin (HA) and neuraminidase (NA) sequences for phylogenetic analysis. We obtained reference sequences for the HA and NA segments of IAV H5 clade 2.3.4.4 from publicly available databases, including GISAID ( https://www.gisaid.org ) and GenBank. We aligned the sequences by using MAFFT version 7.520 software ( https://mafft.cbrc.jp/alignment/server/index.html ) to create multiple sequence alignments for subsequent phylogenetic analysis. We used IQTree2 ( https://github.com/iqtree/iqtree2 ) to construct the phylogenetic tree from the aligned sequences. The software was configured to automatically identify the optimal substitution model by using the ModelFinder Plus option, ensuring the selection of the most suitable model for the dataset and, thereby, improving the accuracy of the reconstructed tree. We visualized the resulting phylogenetic tree by using iTOL ( https://itol.embl.de ), a web-based platform for interactive tree exploration and annotation.
Gross Lesions in Cows and Cats
All cows were in good body condition with adequate rumen fill and no external indications of disease. Postmortem examinations of the affected dairy cows revealed firm mammary glands typical of mastitis; however, mammary gland lesions were not consistent. Two cows that were acutely ill before postmortem examination had grossly normal milk and no abnormal mammary gland lesions. The gastrointestinal tract of some cows had small abomasal ulcers and shallow linear erosions of the intestines, but those observations were also not consistent in all animals. The colon contents were brown and sticky, suggesting moderate dehydration. The feces contained feed particles that appeared to have undergone minimal ruminal fermentation. The rumen contents had normal color and appearance but appeared to have undergone minimal fermentation.
The 2 adult cats (1 intact male, 1 intact female) received at the ISUVDL were in adequate body and postmortem condition. External examination was unremarkable. Mild hemorrhages were observed in the subcutaneous tissues over the dorsal skull, and multifocal meningeal hemorrhages were observed in the cerebrums of both cats. The gastrointestinal tracts were empty, and no other gross lesions were observed.
Microscopic Lesions in Cows and Cats
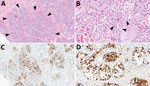
Figure 1 . Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary gland...
The chief microscopic lesion observed in affected cows was moderate acute multifocal neutrophilic mastitis ( Figure 1 ); however, mammary glands were not received from every cow. Three cows had mild neutrophilic or lymphocytic hepatitis. Because they were adult cattle, other observed microscopic lesions (e.g., mild lymphoplasmacytic interstitial nephritis and mild to moderate lymphocytic abomasitis) were presumed to be nonspecific, age-related changes. We did not observe major lesions in the other evaluated tissues. We performed IHC for IAV antigen on all evaluated tissues; the only tissues with positive immunoreactivity were mastitic mammary glands from 2 cows that showed nuclear and cytoplasmic labeling of alveolar epithelial cells and cells within lumina ( Figure 1 ) and multifocal germinal centers within a lymph node from 1 cow ( Table 1 ).
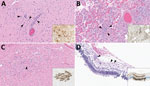
Figure 2 . Lesions in cat tissues in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Tissue sections were stained with...
Both cats had microscopic lesions consistent with severe systemic virus infection, including severe subacute multifocal necrotizing and lymphocytic meningoencephalitis with vasculitis and neuronal necrosis, moderate subacute multifocal necrotizing and lymphocytic interstitial pneumonia, moderate to severe subacute multifocal necrotizing and lymphohistiocytic myocarditis, and moderate subacute multifocal lymphoplasmacytic chorioretinitis with ganglion cell necrosis and attenuation of the internal plexiform and nuclear layers ( Table 2 ; Figure 2 ). We performed IHC for IAV antigen on multiple tissues (brain, eye, lung, heart, spleen, liver, and kidney). We detected positive IAV immunoreactivity in brain (intracytoplasmic, intranuclear, and axonal immunolabeling of neurons), lung, and heart, and multifocal and segmental immunoreactivity within all layers of the retina ( Figure 2 ).
PCR Data from Cows and Cats
We tested various samples from 8 clinically affected mature dairy cows by IAV screening and H5 subtype-specific PCR ( Table 3 ). Milk and mammary gland homogenates consistently showed low Ct values: 12.3–16.9 by IAV screening PCR, 17.6–23.1 by H5 subtype PCR, and 14.7–20.0 by H5 2.3.4.4 clade PCR (case 1, cow 1; case 2, cows 1 and 2; case 3, cow 1; and case 4, cow 1). We forwarded the samples to the National Veterinary Services Laboratory, which confirmed the virus was an HPAI H5N1 virus strain.
When available, we also tested tissue homogenates (e.g., lung, spleen, and lymph nodes), ocular fluid, and rumen contents from 6 cows by IAV and H5 subtype-specific PCR ( Table 3 ). However, the PCR findings were not consistent. For example, the tissue homogenates and ocular fluid tested positive in some but not all cows. In case 5, cow 1, the milk sample tested negative by IAV screening PCR, but the spleen homogenate tested positive by IAV screening, H5 subtype, and H5 2.3.4.4 PCR. For 2 cows (case 3, cow 1; and case 4, cow 1) that had both milk and rumen contents available, both samples tested positive for IAV. Nevertheless, all IAV-positive nonmammary gland tissue homogenates, ocular fluid, and rumen contents had markedly elevated Ct values in contrast to the low Ct values for milk and mammary gland homogenate samples.
We tested brain and lung samples from the 2 cats (case 6, cats 1 and 2) by IAV screening and H5 subtype-specific PCR ( Table 3 ). Both sample types were positive by IAV screening PCR; Ct values were 9.9–13.5 for brain and 17.4–24.4 for lung samples, indicating high amounts of virus nucleic acid in those samples. The H5 subtype and H5 2.3.4.4 PCR results were also positive for the brain and lung samples; Ct values were consistent with the IAV screening PCR ( Table 3 ).
Phylogenetic Analyses
We assembled the sequences of all 8 segments of the HPAI viruses from both cow milk and cat tissue samples. We used the hemagglutinin (HA) and neuraminidase (NA) sequences specifically for phylogenetic analysis to delineate the clade of the HA gene and subtype of the NA gene.

Figure 3 . Phylogenetic analysis of hemagglutinin gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate different...
For HA gene analysis, both HA sequences derived from cow milk samples exhibited a high degree of similarity, sharing 99.88% nucleotide identity, whereas the 2 HA sequences from cat tissue samples showed complete identity at 100%. The HA sequences from the milk samples had 99.94% nucleotide identities with HA sequences from the cat tissues, resulting in a distinct subcluster comprising all 4 HA sequences, which clustered together with other H5N1 viruses belonging to clade 2.3.4.4b ( Figure 3 ). The HA sequences were deposited in GenBank (accession nos. PP599465 [case 2, cow 1], PP599473 [case 2, cow 2], PP692142 [case 6, cat 1], and PP692195 [case 6, cat 2]).

Figure 4 . Phylogenetic analysis of neuraminidase gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate different...
For NA gene analysis, the 2 NA sequences obtained from cow milk samples showed 99.93% nucleotide identity. Moreover, the NA sequences derived from the milk samples exhibited complete nucleotide identities (100%) with those from the cat tissues. The 4 NA sequences were grouped within the N1 subtype of HPAI viruses ( Figure 4 ). The NA sequences were deposited in GenBank (accession nos. PP599467 [case 2, cow 1], PP599475 [case 2, cow 2], PP692144 [case 6, cat 1], and PP692197 [case 6, cat 2]).
This case series differs from most previous reports of IAV infection in bovids, which indicated cattle were inapparently infected or resistant to infection ( 9 ). We describe an H5N1 strain of IAV in dairy cattle that resulted in apparent systemic illness, reduced milk production, and abundant virus shedding in milk. The magnitude of this finding is further emphasized by the high death rate (≈50%) of cats on farm premises that were fed raw colostrum and milk from affected cows; clinical disease and lesions developed that were consistent with previous reports of H5N1 infection in cats presumably derived from consuming infected wild birds ( 10 – 12 ). Although exposure to and consumption of dead wild birds cannot be completely ruled out for the cats described in this report, the known consumption of unpasteurized milk and colostrum from infected cows and the high amount of virus nucleic acid within the milk make milk and colostrum consumption a likely route of exposure. Therefore, our findings suggest cross-species mammal-to-mammal transmission of HPAI H5N1 virus and raise new concerns regarding the potential for virus spread within mammal populations. Horizontal transmission of HPAI H5N1 virus has been previously demonstrated in experimentally infected cats ( 13 ) and ferrets ( 14 ) and is suspected to account for large dieoffs observed during natural outbreaks in mink ( 15 ) and sea lions ( 16 ). Future experimental studies of HPAI H5N1 virus in dairy cattle should seek to confirm cross-species transmission to cats and potentially other mammals.
Clinical IAV infection in cattle has been infrequently reported in the published literature. The first report occurred in Japan in 1949, where a short course of disease with pyrexia, anorexia, nasal discharge, pneumonia, and decreased lactation developed in cattle ( 17 ). In 1997, a similar condition occurred in dairy cows in southwest England leading to a sporadic drop in milk production ( 18 ), and IAV seroconversion was later associated with reduced milk yield and respiratory disease ( 19 – 21 ). Rising antibody titers against human-origin influenza A viruses (H1N1 and H3N2) were later again reported in dairy cattle in England, which led to an acute fall in milk production during October 2005–March 2006 ( 22 ). Limited reports of IAV isolation from cattle exist; most reports occurred during the 1960s and 1970s in Hungary and in the former Soviet Union, where H3N2 was recovered from cattle experiencing respiratory disease ( 9 , 23 ). Direct detection of IAV in milk and the potential transmission from cattle to cats through feeding of unpasteurized milk has not been previously reported.
An IAV-associated drop in milk production in dairy cattle appears to have occurred during > 4 distinct periods and within 3 widely separated geographic areas: 1949 in Japan ( 17 ), 1997–1998 and 2005–2006 in Europe ( 19 , 21 ), and 2024 in the United States (this report). The sporadic occurrence of clinical disease in dairy cattle worldwide might be the result of changes in subclinical infection rates and the presence or absence of sufficient baseline IAV antibodies in cattle to prevent infection. Milk IgG, lactoferrin, and conglutinin have also been suggested as host factors that might reduce susceptibility of bovids to IAV infection ( 9 ). Contemporary estimates of the seroprevalence of IAV antibodies in US cattle are not well described in the published literature. One retrospective serologic survey in the United States in the late 1990s showed 27% of serum samples had positive antibody titers and 31% had low-positive titers for IAV H1 subtype-specific antigen in cattle with no evidence of clinical infections ( 24 ). Antibody titers for H5 subtype-specific antigen have not been reported in US cattle.
The susceptibility of domestic cats to HPAI H5N1 is well-documented globally ( 10 – 12 , 25 – 28 ), and infection often results in neurologic signs in affected felids and other terrestrial mammals ( 4 ). Most cases in cats result from consuming infected wild birds or contaminated poultry products ( 12 , 27 ). The incubation period in cats is short; clinical disease is often observed 2–3 days after infection ( 28 ). Brain tissue has been suggested as the best diagnostic sample to confirm HPAI virus infection in cats ( 10 ), and our results support that finding. One unique finding in the cats from this report is the presence of blindness and microscopic lesions of chorioretinitis. Those results suggest that further investigation into potential ocular manifestations of HPAI H5N1 virus infection in cats might be warranted.
The genomic sequencing and subsequent analysis of clinical samples from both bovine and feline sources provided considerable insights. The HA and NA sequences derived from both bovine milk and cat tissue samples from different Texas farms had a notable degree of similarity. Those findings strongly suggest a shared origin for the viruses detected in the dairy cattle and cat tissues. Further research, case series investigations, and surveillance data are needed to better understand and inform measures to curtail the clinical effects, shedding, and spread of HPAI viruses among mammals. Although pasteurization of commercial milk mitigates risks for transmission to humans, a 2019 US consumer study showed that 4.4% of adults consumed raw milk > 1 time during the previous year ( 29 ), indicating a need for public awareness of the potential presence of HPAI H5N1 viruses in raw milk.
Ingestion of feed contaminated with feces from wild birds infected with HPAI virus is presumed to be the most likely initial source of infection in the dairy farms. Although the exact source of the virus is unknown, migratory birds (Anseriformes and Charadriiformes) are likely sources because the Texas panhandle region lies in the Central Flyway, and those birds are the main natural reservoir for avian influenza viruses ( 30 ). HPAI H5N1 viruses are well adapted to domestic ducks and geese, and ducks appear to be a major reservoir ( 31 ); however, terns have also emerged as an important source of virus spread ( 32 ). The mode of transmission among infected cattle is also unknown; however, horizontal transmission has been suggested because disease developed in resident cattle herds in Michigan, Idaho, and Ohio farms that received infected cattle from the affected regions, and those cattle tested positive for HPAI H5N1 ( 33 ). Experimental studies are needed to decipher the transmission routes and pathogenesis (e.g., replication sites and movement) of the virus within infected cattle.
In conclusion, we showed that dairy cattle are susceptible to infection with HPAI H5N1 virus and can shed virus in milk and, therefore, might potentially transmit infection to other mammals via unpasteurized milk. A reduction in milk production and vague systemic illness were the most commonly reported clinical signs in affected cows, but neurologic signs and death rapidly developed in affected domestic cats. HPAI virus infection should be considered in dairy cattle when an unexpected and unexplained abrupt drop in feed intake and milk production occurs and for cats when rapid onset of neurologic signs and blindness develop. The recurring nature of global HPAI H5N1 virus outbreaks and detection of spillover events in a broad host range is concerning and suggests increasing virus adaptation in mammals. Surveillance of HPAI viruses in domestic production animals, including cattle, is needed to elucidate influenza virus evolution and ecology and prevent cross-species transmission.
Dr. Burrough is a professor and diagnostic pathologist at the Iowa State University College of Veterinary Medicine and Veterinary Diagnostic Laboratory. His research focuses on infectious diseases of livestock with an emphasis on swine.
Acknowledgment
We thank the faculty and staff at the ISUVDL who contributed to the processing and analysis of clinical samples in this investigation, the veterinarians involved with clinical assessments at affected dairies and various conference calls in the days before diagnostic submissions that ultimately led to the detection of HPAI virus in the cattle, and the US Department of Agriculture National Veterinary Services Laboratory and NAHLN for their roles and assistance in providing their expertise, confirmatory diagnostic support, and communications surrounding the HPAI virus cases impacting lactating dairy cattle.
- Caliendo V , Lewis NS , Pohlmann A , Baillie SR , Banyard AC , Beer M , et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci Rep . 2022 ; 12 : 11729 . DOI PubMed Google Scholar
- Bevins SN , Shriner SA , Cumbee JC Jr , Dilione KE , Douglass KE , Ellis JW , et al. Intercontinental movement of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4 virus to the United States, 2021. Emerg Infect Dis . 2022 ; 28 : 1006 – 11 . DOI PubMed Google Scholar
- Puryear W , Sawatzki K , Hill N , Foss A , Stone JJ , Doughty L , et al. Highly pathogenic avian influenza A(H5N1) virus outbreak in New England seals, United States. Emerg Infect Dis . 2023 ; 29 : 786 – 91 . DOI PubMed Google Scholar
- Elsmo EJ , Wünschmann A , Beckmen KB , Broughton-Neiswanger LE , Buckles EL , Ellis J , et al. Highly pathogenic avian influenza A(H5N1) virus clade 2.3.4.4b infections in wild terrestrial mammals, United States, 2022. Emerg Infect Dis . 2023 ; 29 : 2451 – 60 . DOI PubMed Google Scholar
- Bruno A , Alfaro-Núñez A , de Mora D , Armas R , Olmedo M , Garcés J , et al. First case of human infection with highly pathogenic H5 avian influenza A virus in South America: a new zoonotic pandemic threat for 2023? J Travel Med. 2023 ;30:taad032.
- Pulit-Penaloza JA , Brock N , Belser JA , Sun X , Pappas C , Kieran TJ , et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg Microbes Infect . 2024 ; 2332667 . DOI PubMed Google Scholar
- United States Department of Agriculture Animal and Plant Health Inspection Service . Federal and state veterinary, public health agencies share update on HPAI detection in Kansas, Texas dairy herds. 2024 [ cited 2024 Mar 29 ]. https://www.aphis.usda.gov/news/agency-announcements/federal-state-veterinary-public-health-agencies-share-update-hpai
- Sharma A , Zeller MA , Souza CK , Anderson TK , Vincent AL , Harmon K , et al. Characterization of a 2016–2017 human seasonal H3 influenza A virus spillover now endemic to U.S. swine. MSphere . 2022 ; 7 : e0080921 . DOI PubMed Google Scholar
- Sreenivasan CC , Thomas M , Kaushik RS , Wang D , Li F . Influenza A in bovine species: a narrative literature review. Viruses . 2019 ; 11 : 561 . DOI PubMed Google Scholar
- Sillman SJ , Drozd M , Loy D , Harris SP . Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J Comp Pathol . 2023 ; 205 : 17 – 23 . DOI PubMed Google Scholar
- Klopfleisch R , Wolf PU , Uhl W , Gerst S , Harder T , Starick E , et al. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet Pathol . 2007 ; 44 : 261 – 8 . DOI PubMed Google Scholar
- Keawcharoen J , Oraveerakul K , Kuiken T , Fouchier RAM , Amonsin A , Payungporn S , et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis . 2004 ; 10 : 2189 – 91 . DOI PubMed Google Scholar
- Kuiken T , Rimmelzwaan G , van Riel D , van Amerongen G , Baars M , Fouchier R , et al. Avian H5N1 influenza in cats. Science . 2004 ; 306 : 241 . DOI PubMed Google Scholar
- Herfst S , Schrauwen EJA , Linster M , Chutinimitkul S , de Wit E , Munster VJ , et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science . 2012 ; 336 : 1534 – 41 . DOI PubMed Google Scholar
- Agüero M , Monne I , Sánchez A , Zecchin B , Fusaro A , Ruano MJ , et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill . 2023 ; 28 : 2300001 . DOI PubMed Google Scholar
- Leguia M , Garcia-Glaessner A , Muñoz-Saavedra B , Juarez D , Barrera P , Calvo-Mac C , et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun . 2023 ; 14 : 5489 . DOI PubMed Google Scholar
- Saito K . An outbreak of cattle influenza in Japan in the fall of 1949. J Am Vet Med Assoc . 1951 ; 118 : 316 – 9 . PubMed Google Scholar
- Gunning RF , Pritchard GC . Unexplained sporadic milk drop in dairy cows. Vet Rec . 1997 ; 140 : 488 . PubMed Google Scholar
- Brown IH , Crawshaw TR , Harris PA , Alexander DJ . Detection of antibodies to influenza A virus in cattle in association with respiratory disease and reduced milk yield. Vet Rec . 1998 ; 143 : 637 – 8 . PubMed Google Scholar
- Crawshaw TR , Brown I . Bovine influenza. Vet Rec . 1998 ; 143 : 372 . PubMed Google Scholar
- Gunning RF , Brown IH , Crawshaw TR . Evidence of influenza A virus infection in dairy cows with sporadic milk drop syndrome. Vet Rec . 1999 ; 145 : 556 – 7 . DOI PubMed Google Scholar
- Crawshaw TR , Brown IH , Essen SC , Young SCL . Significant rising antibody titres to influenza A are associated with an acute reduction in milk yield in cattle. Vet J . 2008 ; 178 : 98 – 102 . DOI PubMed Google Scholar
- Lopez JW , Woods GT . Influenza virus in ruminants: a review. Res Commun Chem Pathol Pharmacol . 1984 ; 45 : 445 – 62 . PubMed Google Scholar
- Jones-Lang K , Ernst-Larson M , Lee B , Goyal SM , Bey R . Prevalence of influenza A virus (H1N1) antibodies in bovine sera. New Microbiol . 1998 ; 21 : 153 – 60 . PubMed Google Scholar
- Briand FX , Souchaud F , Pierre I , Beven V , Hirchaud E , Hérault F , et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in domestic cat, France, 2022. Emerg Infect Dis . 2023 ; 29 : 1696 – 8 . DOI PubMed Google Scholar
- Frymus T , Belák S , Egberink H , Hofmann-Lehmann R , Marsilio F , Addie DD , et al. Influenza virus infections in cats. Viruses . 2021 ; 13 : 1435 . DOI PubMed Google Scholar
- Songserm T , Amonsin A , Jam-on R , Sae-Heng N , Meemak N , Pariyothorn N , et al. Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis . 2006 ; 12 : 681 – 3 . DOI PubMed Google Scholar
- Thiry E , Zicola A , Addie D , Egberink H , Hartmann K , Lutz H , et al. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol . 2007 ; 122 : 25 – 31 . DOI PubMed Google Scholar
- Lando AM , Bazaco MC , Parker CC , Ferguson M . Characteristics of U.S. consumers reporting past year intake of raw (unpasteurized) milk: results from the 2016 Food Safety Survey and 2019 Food Safety and Nutrition Survey. J Food Prot . 2022 ; 85 : 1036 – 43 . DOI PubMed Google Scholar
- Fourment M , Darling AE , Holmes EC . The impact of migratory flyways on the spread of avian influenza virus in North America. BMC Evol Biol . 2017 ; 17 : 118 . DOI PubMed Google Scholar
- Guan Y , Smith GJD . The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res . 2013 ; 178 : 35 – 43 . DOI PubMed Google Scholar
- de Araújo AC , Silva LMN , Cho AY , Repenning M , Amgarten D , de Moraes AP , et al. Incursion of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus, Brazil, 2023. Emerg Infect Dis . 2024 ; 30 : 619 – 21 . DOI PubMed Google Scholar
- American Veterinary Medical Association . States with HPAI-infected dairy cows grows to six. USDA provides guidance for veterinarians, producers on protecting cattle from the virus. 2024 [ cited 2024 Apr 10 ]. https://www.avma.org/news/states-hpai-infected-dairy-cows-grows-six
- Figure 1 . Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary...
- Figure 2 . Lesions in cat tissues in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Tissue sections were stained...
- Figure 3 . Phylogenetic analysis of hemagglutinin gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate...
- Figure 4 . Phylogenetic analysis of neuraminidase gene sequences in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Colors indicate...
- Table 1 . Microscopic lesions observed in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
- Table 2 . Microscopic lesions observed in cats in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
- Table 3 . PCR results from various specimens in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024
Suggested citation for this article : Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis. 2024 Jul [ date cited ]. https://doi.org/10.3201/eid3007.240508
DOI: 10.3201/eid3007.240508
Original Publication Date: April 29, 2024
Table of Contents – Volume 30, Number 7—July 2024
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eric R. Burrough, Iowa State University Veterinary Diagnostic Laboratory, 1937 Christensen Dr, Ames, IA 50011, USA
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Metric Details
What is the altmetric attention score.
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 1, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Brief anger may impair blood vessel function, says new research
by American Heart Association

A brief episode of anger triggered by remembering past experiences may negatively impact the blood vessels' ability to relax, which is essential for proper blood flow, according to new research published in the Journal of the American Heart Association .
Previous research has found that impairment of blood vessels' ability to relax may increase the risk of developing atherosclerosis, which may in turn increase the risk of heart disease and stroke.
"Impaired vascular function is linked to an increased risk of heart attack and stroke," said lead study author Daichi Shimbo, M.D., a professor of medicine at the Columbia University Irving Medical Center in New York City. "Observational studies have linked feelings of negative emotions with having a heart attack or other cardiovascular disease events. The most common negative emotion studied is anger, and there are fewer studies on anxiety and sadness, which have also been linked to heart attack risk."
In this study, the researchers investigated whether negative emotions—anger, sadness and anxiety—may have an adverse impact on blood vessel function compared to a neutral emotion. The 280 adults in the study were randomly assigned to one of four emotional tasks for 8 minutes: recalling a personal memory that made them angry; recalling a personal memory of anxiety; reading a series of depressing sentences that evoked sadness; or repeatedly counting to 100 to induce an emotionally neutral state.
This protocol, "Putative mechanisms Underlying Myocardial infarction onset and Emotions (PUME)," was described by the researchers in a previous paper .
Researchers assessed the cells lining each study participant's blood vessels before the tasks and at several points after, looking for evidence of impaired blood vessel dilation, increased cell injury and/or reduced cell repair capacity. The measurements taken before the emotional tasks were repeated after tasks were completed.
Measurements were taken for each participant at baseline (0 minutes) and at four different timepoints after experiencing the assigned emotional task: 3 minutes, 40 minutes, 70 minutes and 100 minutes. The analysis found:
- Tasks that recalled past events causing anger led to an impairment in blood vessel dilation, from zero to 40 minutes after the task. The impairment was no longer present after the 40-minute mark.
- There were no statistically significant changes to participants' blood vessel linings at any time points after experiencing the anxiety and sadness emotional tasks.
"We saw that evoking an angered state led to blood vessel dysfunction, though we don't yet understand what may cause these changes," Shimbo said. "Investigation into the underlying links between anger and blood vessel dysfunction may help identify effective intervention targets for people at increased risk of cardiovascular events."
According to an American Heart Association 2021 scientific statement, " Psychological Health, Well-Being, and the Mind-Heart-Body Connection ," mental well-being can positively or negatively impact a person's health and risk factors for heart disease and stroke.
"This study adds nicely to the growing evidence base that mental well-being can affect cardiovascular health, and that intense acute emotional states, such as anger or stress, may lead to cardiovascular events," said Glenn Levine, M.D., FAHA, writing committee chair of the scientific statement, and master clinician and professor of medicine at Baylor College of Medicine, and chief of the cardiology section at the Michael E. DeBakey VA Medical Center, both in Houston.
"For instance, we know that intense sadness or similar emotions are a common trigger for Takatsubo cardiomyopathy , and events such as earthquakes or even as a fan watching a world soccer match, which provoke stress, may lead to myocardial infarction and/or to arrhythmias.
"This current study very eloquently shows how anger can negatively impact vascular endothelial health and function, and we know the vascular endothelium, the lining of blood vessels, is a key player in myocardial ischemia and atherosclerotic heart disease. While not all the mechanisms on how psychological states and health impact cardiovascular health have been elucidated, this study clearly takes us one step closer to defining such mechanisms."
Study background and details:
- The Putative mechanisms Underlying Myocardial infarction onset and Emotions (PUME) study is a randomized controlled experimental study conducted from August 2013 to May 2017.
- Participants were recruited from the community surrounding Columbia University Irving Medical Center in New York City.
- Participants were ages 18 or older and healthy. In this study, healthy was defined as no history of heart disease, stroke, bypass surgery or stents, transient ischemic attack, peripheral arterial disease, heart failure, high blood pressure , high cholesterol, Type 2 diabetes or self-reported diagnosis of a mental health disorder; not taking any prescription medications or dietary supplements; and not currently smoking.
- The average age of study participants was 26 years. Approximately 50% of participants self-identified as women. About 40% of participants self-identified as white adults; 29% as Hispanic/Latino adults; 19% as Asian adults; and 14% as Black adults.
- Participants' blood vessel health was assessed before and after completing the emotional tasks with finger probes that detect changes in blood flow in arteries.
- Before completing the emotional tasks, participants were seated in a comfortable chair in a temperature-controlled room and instructed to relax for 30 minutes, during which time they were not allowed to talk, use their phones, read any documents or sleep.
- After the participants had relaxed for 30 minutes, researchers measured participants' blood pressure with a cuff and corresponding heart rate. Two blood pressure measurements were taken one minute apart, then the dilation of participants' blood vessels was measured, and blood samples were collected for testing. Repeat measurements of blood pressure and dilation were conducted, and blood samples were collected again after the assigned emotional task was completed.
- Researchers determined the extent to which participants' blood vessels were unable to dilate by measuring the blood flow in the participants' non-dominant forearm. They assessed the injury to participants' blood vessels by counting the number of circulating blood vessel lining biomarkers in the blood and assessed the regenerative ability of participants' vascular cells by measuring their circulating levels of bone-marrow-derived cells, which are essential for repair.
The study's limitations included that participants were young and apparently healthy, "making it unclear whether the results would apply to older adults with other health conditions, who would most likely be taking medications," Shimbo noted. In addition, participants were observed in a health care setting, rather than in real-world situations, and the study only assessed the short-term effects of evoked emotions.
Explore further
Feedback to editors

Study elucidates how energy metabolism is regulated at cellular level
15 minutes ago

Study finds metformin reduces COVID-19 viral load, viral rebound
23 minutes ago

Adding AI to artificial pancreas enhances efficiency, study finds
31 minutes ago

Researchers discover how immune B cells hunt down cancer around the body
50 minutes ago

Sea slugs inspire highly stretchable biomedical sensor
56 minutes ago

Study in women shows significant link between regular exercise during middle-age and physical health in later life

Synchronization between central and circadian clocks of tissues found to preserve their functioning, prevent aging
International study compares rapid antigen tests and highlights poor performance in some
2 hours ago

AI can tell if a patient battling cancer needs mental health support

Analysis of flour and rice shows high levels of harmful fungal toxins
Related stories.

Cultivating spirituality can lower blood pressure
Apr 8, 2024
Researchers identify potential 'missing link' for why South Asians have higher rates of heart disease
Feb 15, 2024
Impaired blood vessel and kidney function underlie heart disease risk in people with HIV
Dec 17, 2020

High blood pressure while lying down linked to higher risk of heart health complications
Sep 7, 2023

Can drinking cocoa protect your heart when you're stressed?
Mar 31, 2021

Reducing systolic blood pressure to less than 120 mm Hg found to reduce cardiovascular event risk
Nov 13, 2023
Recommended for you

Global study reveals stark differences between females and males in disease burden causes
20 hours ago

Long-term follow-up reports key findings and clinical messages from largest study of women's health in the US

Study finds ChatGPT fails at heart risk assessment

Cardiologists train large AI model to assess heart structure, function
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Spatial Variations of the Activity of 137 Cs and the Contents of Heavy Metals and Petroleum Products in the Polluted Soils of the City of Elektrostal
- DEGRADATION, REHABILITATION, AND CONSERVATION OF SOILS
- Open access
- Published: 15 June 2022
- Volume 55 , pages 840–848, ( 2022 )
Cite this article
You have full access to this open access article

- D. N. Lipatov 1 ,
- V. A. Varachenkov 1 ,
- D. V. Manakhov 1 ,
- M. M. Karpukhin 1 &
- S. V. Mamikhin 1
1463 Accesses
2 Citations
Explore all metrics
The levels of specific activity of 137 Cs and the contents of mobile forms (1 M ammonium acetate extraction) of heavy metals (Zn, Cu, Ni, Co, Cr, Pb) and petroleum products were studied in the upper soil horizon of urban landscapes of the city of Elektrostal under conditions of local radioactive and chemical contamination were studied. In the soils within a short radius (0–100 m) around the heavy engineering plant, the specific activity of 137 Cs and the contents of mobile forms of Pb, Cu, and Zn were increased. The lognormal distribution law of 137 Cs was found in the upper (0–10 cm) soil layer; five years after the radiation accident, the specific activity of 137 Cs varied from 6 to 4238 Bq/kg. The coefficients of variation increased with an increase in the degree of soil contamination in the following sequence: Co < Ni < petroleum products < Cr < 137 Cs < Zn < Pb < Cu ranging from 50 to 435%. Statistically significant direct correlation was found between the specific activity of 137 Cs and the contents of mobile forms of Pb, Cu, and Zn in the upper horizon of urban soils, and this fact indicated the spatial conjugacy of local spots of radioactive and polymetallic contamination in the studied area. It was shown that the specific activity of 137 Cs, as well as the content of heavy metals and petroleum products in the upper layer (0–10 cm) of the soils disturbed in the course of decontamination, earthwork and reclamation is reduced.
Similar content being viewed by others
Accumulation and migration of heavy metals in soils of the rostov region, south of russia.

Geographical Features of Pollution of the Territory of Yakutia With Cesium-137

Activity Concentration of Natural Radionuclides and Total Heavy Metals Content in Soils of Urban Agglomeration
Avoid common mistakes on your manuscript.
INTRODUCTION
Contaminants migrate and accumulate in urban ecosystems under the impact of both natural and technogenic factors. The processes of technogenic migration of 137 Cs are most pronounced in radioactively contaminated territories. It was found in urboecological studies that the intensity of sedimentation of aerosol particles containing radionuclides and heavy metals is determined by the types of the surfaces of roofs, walls, roads, lawns, and parks and by their position within the urban wind field [ 12 , 26 ]. Traffic in the cities results in significant transport of dust and associated contaminants and radionuclides [ 15 , 24 ]. During decontamination measures in the areas of Chernobyl radioactive trace, not only the decrease in the level of contamination but also the possibility of secondary radioactive contamination because of the transportation of contaminated soil particles by wind or water, or anthropogenic transfer of transferring of ground were observed [ 5 , 6 ]. Rainstorm runoff and hydrological transport of dissolved and colloidal forms of 137 Cs can result in the accumulation of this radionuclide in meso- and microdepressions, where sedimentation takes place [ 10 , 16 ]. Different spatial distribution patterns of 137 Cs in soils of particular urban landscapes were found in the city of Ozersk near the nuclear fuel cycle works [ 17 ]. Natural character of 137 Cs migration in soils of Moscow forest-parks and a decrease in its specific activity in industrial areas have been revealed [ 10 ]. Determination of the mean level and parameters of spatial variations of 137 Cs in soils is one of primary tasks of radioecological monitoring of cities, including both unpolluted (background) and contaminated territories.
Emissions and discharges from numerous sources of contamination can cause the accumulation of a wide range of toxicants in urban soils: heavy metals (HMs), oil products (OPs), polycyclic aromatic hydrocarbons (PAHs), and other chemical substances. Soil contamination by several groups of toxicants is often observed in urban landscapes [ 20 , 23 ] because of the common contamination source or close pathways of the migration of different contaminants. A comprehensive analysis of contamination of urban soils by radionuclides and heavy metals has been performed in some studies [ 21 , 25 ]. The determination of possible spatial interrelationships between radioactive and chemical contaminations in urban soils is an important problem in urban ecology.
A radiation accident took place in the Elektrostal heavy engineering works (EHEW) in April 2013: a capacious source of 137 Cs entered the smelt furnace, and emission of radioactive aerosols from the aerating duct into the urban environment took place. The activity of molten source was estimated at about 1000–7000 Ci [ 14 ]. The area of contamination in the territory of the plant reached 7500 m 2 . However, radioactive aerosols affected a much larger area around the EHEW, including Krasnaya and Pervomaiskaya streets, and reached Lenin Prospect.
Geochemical evaluation of contamination of the upper soil horizon in the city of Elektrostal was carried out in 1989–1991. This survey indicated the anomalies of concentrations of wolfram, nickel, molybdenum, chromium, and other heavy metals related to accumulation of alloying constituent and impurities of non-ferrous metals in the emissions of steelmaking works [ 19 ].
The aim of our work was to determine the levels of specific activity of 137 Cs, concentrations of mobile forms of heavy metals (Zn, Cu, Ni, Co, Cr, and Pb) and oil products in the upper soil horizons in different urban landscapes of the city of Elektrostal under the conditions of local radioactive and chemical contamination.
Author information
Authors and affiliations.
Lomonosov Moscow State University, 119991, Moscow, Russia
D. N. Lipatov, V. A. Varachenkov, D. V. Manakhov, M. M. Karpukhin & S. V. Mamikhin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to D. N. Lipatov .
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Chicheva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Lipatov, D.N., Varachenkov, V.A., Manakhov, D.V. et al. Spatial Variations of the Activity of 137 Cs and the Contents of Heavy Metals and Petroleum Products in the Polluted Soils of the City of Elektrostal. Eurasian Soil Sc. 55 , 840–848 (2022). https://doi.org/10.1134/S1064229322060072
Download citation
Received : 21 October 2021
Revised : 22 December 2021
Accepted : 30 December 2021
Published : 15 June 2022
Issue Date : June 2022
DOI : https://doi.org/10.1134/S1064229322060072
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- urban soils
- urban ecosystems
- radiation monitoring
- decontamination
- Urban Technosols
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Eurasian Medical Research Periodical (EMRP) SJIF 2024: 7.667 Area: Multidisciplinary Evaluated version: online Previous evaluation SJIF. 2023: 6.611 2022: 5.629 2021: Not indexed 2020: Not indexed. The journal is indexed in: SJIFactor.com Basic information ...
Mitochondrial Deoxyribonucleic Acid Copy Number Elevation As a Predictor for Extended Survival and Favorable Outcomes in High-Grade Brain Tumor Patients: A Malaysian Study. (Eurasian J Med 2024; 56: 7-14) DOI: 10.5152/eurasianjmed.2024.23172.
Eurasian Journal of Medicine (Eurasian J Med) is an international, scientific, open access periodical published by independent, unbiased, and triple-blinded peer-review principles. ... The aim of the Eurasian Journal of Medicine is to publish original research papers of the highest scientific and clinical value in all medical fields. The ...
Eurasian Medical Research Periodical www.geniusjour nals.org. P a g e | 43. time higher than maternal mortality related to . normal vaginal birth (26). Conclusion . A cesarean delivery is a surgical .
Discover all the features of the complete ISSN records Access the full version of the ISSN Portal
Eurasian Medical Research Periodical www.geniusjournals.org Page | 4 Figure 4: Patient infected with CCHF [28]. Prevention and Control The key measure to achieving prevention for ...
Eurasian Medical Research Periodical. Pseudomonas aeruginosa and t he. multifactorial antibiotic resistance. Sarah Ahmed Hasan. Microbiologist, University of Kirkuk, Kirkuk, Iraq. Author email ...
Eurasian Journal of Medicine. 1308-8734 (Print) / 1308-8742 (Online) Website. ISSN Portal. About. Articles.
Articles from The Eurasian Journal of Medicine are provided here courtesy of Ataturk University School of Medicine. Follow NCBI. Connect with NLM. National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894. Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. NLM; NIH; HHS; USA.gov ...
0. Prognostic Significance of C-Reactive Protein and Neutrophil- Lymphocyte Ratio in Lung Adenocarcinoma Patients. Akin Ozturk, Ozlem Oruc, Merve Hormet Igde, Ozgur Bilgin Topcuoglu, Murat Kavas, Cansel Atinkaya Baydemir, Mahmut Gumus. doi: 10.14744/ejmi.2023.42951 Pages 8 - 12.
Eurasia Outlook 2021: Economic, Social and Political Perspectives in Eurasia. As the Eurasian Research Institute (ERI), we have been presenting our weekly E-bulletin publications to our readers, in which we have evaluated the issues in the Eurasian region within an academic framework since 2015. We discussed the economy, security, international ...
A Spatial Analysis of Internal Migration in Kazakhstan. The 2021 census became the first census conducted since 2009, and the recent publication of its final results unveils valuable insights into various demographic aspects of Kazakhstan over the twelve-year intercensal period. A particularly interesting addition to the census of 2021 is that ...
About the Journal. Eurasian Journal of Medical and Biological Sciences (Eurasian J Med Biol Sci) is an international, open access periodical published in accordance with independent, unbiased, and double-blinded peer-review principles. ... The journal will consider submissions from all over the world, on research works not being published or ...
RESEARCH ARTICLE: 5. Histomorphological Changes in Campanacci Grade III Giant Cell Tumors After Use of Denosumab in a Neoadjuvant Setting Rahma Rashid, Shaheera Malik, Sheikh Muhammad Ebad Ali, Badaruddin Sahito, Sarush Siddiqui, Muhammed Mubarak doi: 10.14744/ejma.2023.52523 Pages 120 - 126
Eurasian Medical Research Periodical www.geniusjournals.org Page | 36 Introduction Cronobacter sakazakii, recently known as Enterobacter sakazakii, is a Gram-negative, pole formed, pathogenic microscopic organisms that can get by in amazingly dry conditions (). The majority of Cronobacter sakazakii cases occur in ...
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice. In this paper we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town ...
Eurasian Medical Research Periodical www.geniusjournals.org. Page | 11. Figure (3) Effect of vitamin B 12 on the values of high-density lipoproteins (HDL) On the other hand , Table (1) A significant .
Eurasian Research Publishing covers all types of research fields including Sciences, Engineering, Arts, Business, and Commerce-related topics. Eurasian Research Publishing's main aim is to publish articles every month. Academic and Scientific Publishing serves standard quality publications for all authors and readers.
An article published in The FASEB Journal describes a Brazilian study analyzing the correlation between two key energy metabolism regulation processes: the absorption and release of calcium ions ...
Features of the macrostructure and microstructure of uranium dioxide powders are considered. Assumptions are made on the mechanisms of the behavior of powders of various natures during pelletizing. Experimental data that reflect the effect of these powders on the quality of fuel pellets, which is evaluated by modern procedures, are presented. To investigate the structure of the powders, modern ...
Research Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024 ... In late 2021, the Eurasian strain of H5N1 (clade 2.3.4.4b) was detected in North America (1,2) and initiated an outbreak that continued into 2024. ... Original Publication Date: April 29, 2024. Table of ...
Previous research has found that impairment of blood vessels' ability to relax may increase the risk of developing atherosclerosis, which may in turn increase the risk of heart disease and stroke. ...
Eurasian Medical Research Periodical www.geniusjournals.org Page | 172 regeneration by releasing biological micro molecules at the injection site [3, 4] The use of the PRP in reproduction seems to ...
Eurasian Soil Science is a peer-reviewed journal dedicated to original research on global and regional soil studies. Publishes research discussing genesis, ...
Field research was carried out in the city of Elektrostal, Moscow oblast, in July 2018, i.e. 5 years and 3 months after the local fallout of 137 Cs in the result of radiation accident. The emission of 137 Cs from the chimney of steel melting plant spread to the west of heavy engineering works in April 2013 in the day of radiation accident [].The surveyed part of the city near the plant ...
IUBMB Life is the flagship journal of the International Union of Biochemistry and Molecular Biology and is devoted to the rapid publication of the most novel and significant short articles, ... Special Issue on Environmental Management and Biomedical Research. Submission deadline: Tuesday, 10 September 2024 ... Approaches for medical imaging ...
Eurasian Medical Research Periodical www.geniusjournals.org. Page | 40. taken from someone who should be . experiencing symptoms, or from an oral . glucose tolerance test, with a value in the .
See other industries within the Information sector: All Other Telecommunications , Media Streaming Distribution Services, Social Networks, and Other Media Networks and Content Providers , Motion Picture and Video Industries , Newspaper, Periodical, Book, and Directory Publishers , Radio and Television Broadcasting Stations , Satellite Telecommunications , Software Publishers , Sound Recording ...