An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10780254


Colorectal Cancer: Current Updates and Future Perspectives
Rosa marcellinaro.
1 Department of General Surgery, S. Eugenio Hospital, 00144 Rome, Italy; [email protected] (D.S.); [email protected] (M.G.); ti.liamtoh@82-far (R.T.); ti.oohay@60samiloig (G.L.); ti.ilacsit@inilracxam (M.C.)
Domenico Spoletini
Michele grieco, pasquale avella.
2 Department of Clinical Medicine and Surgery, University of Naples “Federico II”, 80138 Naples, Italy; moc.liamg@79allevaelauqsap (P.A.); moc.liamg@42oiccuppacaleacim (M.C.)
3 Hepatobiliary and Pancreatic Surgery Unit, Pineta Grande Hospital, Castel Volturno, 81030 Caserta, Italy
Micaela Cappuccio
Raffaele troiano, giorgio lisi, giovanni m. garbarino, massimo carlini.
Colorectal cancer is a frequent neoplasm in western countries, mainly due to dietary and behavioral factors. Its incidence is growing in developing countries for the westernization of foods and lifestyles. An increased incidence rate is observed in patients under 45 years of age. In recent years, the mortality for CRC is decreased, but this trend is slowing. The mortality rate is reducing in those countries where prevention and treatments have been implemented. The survival is increased to over 65%. This trend reflects earlier detection of CRC through routine clinical examinations and screening, more accurate staging through advances in imaging, improvements in surgical techniques, and advances in chemotherapy and radiation. The most important predictor of survival is the stage at diagnosis. The screening programs are able to reduce incidence and mortality rates of CRC. The aim of this paper is to provide a comprehensive overview of incidence, mortality, and survival rate for CRC.
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignant neoplasm and the second cause of death due to cancer worldwide [ 1 , 2 , 3 , 4 ]. There are significant variations in CRC incidence and mortality rates among different countries of the world which are based on different factors: gender [ 5 ], age [ 6 , 7 , 8 ], and ethnicity [ 6 ]. It imposes a considerable global load in terms of its complications, mortality, side effects of treatment, health care services utilization, and medical costs [ 5 , 9 ]. The higher rates of incidence are registered in most developed countries, but the diffusion of Western behaviors and lifestyle is responsible for the increase of colorectal cancer cases in less developed countries [ 10 ]. The different possibilities of access to care still play a fundamental role in survival and mortality for this neoplasm [ 11 ].
In recent years, artificial intelligence has also played a key role in early diagnosis and prediction of cancers [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ], including CRC [ 19 , 20 ], as described by numerous studies in the literature. In this clinical context, knowledge of epidemiological data is of great importance to analyze the trend of incidence and prevalence of pathology and to develop new predictive oncological techniques.
Nowadays, the most commonly used CRC serum biomarker is carcinoembryonic antigen (CEA) [ 21 , 22 , 23 ]. CEA is a protein that is produced by the developing fetus and by some types of cancer cells. CEA levels are typically low in healthy people, but they can be elevated in patients with CRC. CEA is not a specific biomarker for CRC, as it can also be elevated in patients with other diseases [ 24 ], such as inflammation of the colon. However, it is a useful biomarker for monitoring patients with CRC for recurrence or progression of the disease [ 25 ]. Another CRC serum biomarker is carbohydrate antigen 19-9 (CA 19-9) [ 22 , 26 , 27 ]. CA 19-9 is a protein that is produced by the pancreas and by some types of cancer cells, including cholangiocarcinoma [ 28 , 29 , 30 , 31 ]. CA 19-9 levels are typically low in healthy people, but they can be elevated in patients with CRC, especially those with advanced disease [ 22 , 32 ]. CA 19-9 is not a specific biomarker for CRC, as it can also be elevated in patients with other diseases, such as pancreatitis. However, it can be a useful biomarker for monitoring patients with CRC for recurrence or progression of the disease. SEPT9 [ 33 ], methylated SEPT9 [ 33 , 34 ], microRNAs, and circulating tumor DNA (ctDNA), [ 35 ] and urine-based biomarkers, as volatile organic compounds (VOCs) [ 36 ], microRNAs [ 37 ], and DNA methylation markers, showed promising results in early-stage CRC detection.
The aim of this paper is to provide a comprehensive overview of incidence, mortality and survival rate for CRC. Furthermore, we analyze the current screening program available and the future perspectives of serological tests to early detect CRC.
2. Biological Background
It is currently estimated that 75–80% of CRCs are sporadic cancers due to the successive accumulation of mutations in genes involved in growth, differentiation, and proliferation of epithelial cells [ 1 ]. This multistep carcinogenesis mechanism, called adenoma–carcinoma sequence , is due to mutations of at least 15 cancer-related genes. Considered from a molecular perspective, CRC is not a unique pathology, but can be classified into different subtypes characterized by specific genetic and morphological alterations. Chromosomal instability (CIN) is the most common genetic alteration that accounts for 84% of all sporadic CRC; it is characterized by many changes in chromosome numbers and their mutations like deletions, gains, translocations, loss of heterozygosity for a specific genomic regions, and other chromosomal rearrangements. These show a frequent alteration of the number of DNA somatic copies, which are a feature of most tumors that originate by the adenoma–carcinoma sequence. Another subtype of CRC (around ~13–16% of sporadic CRC) is hypermutated and shows microsatellite instability (MSI); this alteration is related to defects in DNA mismatch repair (MMR) system, often associated with wild-type TP53 and a near-diploid pattern of chromosomal instability [ 1 , 33 ]. In addition to the CIN and MSI mechanism, in almost 15% of CRC is evident a hypermethylation of CpG islands at gene promoters with consequent epigenetic silencing of the adjacent genes. This modification of the normal DNA methylation pattern is defined as CpG islands hypermethylation phenotype (CIMP) and contributes to the global deregulation of the expression of genes involved in cell differentiation. Moreover, in almost 40% of sporadic CRC are described mutation in KRAS, HRAS, and NRAS which are responsible for the signal transduction from different growth factor receptors; other common alterations involve PI3K (15–25% of cases) and BRAF (5–10%). Inactivating mutations are also very frequent and the most common involve PTEN phosphatase (10% of cases), TGF-β signaling pathway, and p53 (70% of CRC) [ 6 ].
Hereditary forms contribute to about 15–30% of all colorectal cancers [ 38 ]. In these conditions, important tumor suppressor or DNA repair genes are silenced by mono allelic gene expression in the germline, and a somatic event (second hit) abolishing the functionality of the residual wildtype allele causes the carcinogenesis. Among the hereditary colorectal cancers, the most common forms are hereditary non-polyposis colon cancer (Lynch syndrome, LS) and familial adenomatous polyposis coli (FAP). Both syndromes are autosomal dominant disorders. Lynch syndrome-associated cancers show signs of mismatch repair deficiency and a faster adenoma–carcinoma transition which takes 3–5 years compared to about 20 years of sporadic CRC. LS also confer an increased risk for extra-colonic cancers such as those of the gynecological, gastrointestinal, hepatobiliary, urological, and nervous system [ 1 , 5 ]. FAP accounts for 1% of all CRCs and its distribution is the same for men and women; it is characterized by hundreds to thousands of adenoma developing in the colon and the rectum due to the germline mutation in adenomatous polyposis coli (APC) gene and shows the classic adenoma–carcinoma sequence. Polyps generally rise in the early teens and lifetime risk of CRC reaches up to 100% if prophylactic colectomy is not performed. Moreover, there is a small potential risk for extra-colonic cancers including that of the duodenum, thyroid, hepatoblastoma, osteomas, stomach, pancreas, and desmoid tumors [ 1 , 5 ].
3. Incidence
CRC is the third most commonly diagnosed malignancy after breast and lung cancer with more than 1.9 million of new cases; 72% of these develop in the colon and only 28% originate in the rectum. It accounts for about 10% of all cancer incidence worldwide [ 39 , 40 ]. It is estimated that the incidence of CRC can increase by 60% in 2030 [ 41 ], and the patients affected by this neoplasm reach 3.2 million by 2040 [ 42 ]. Based on the sex, it is the third most common cancer in men after lung and prostate cancers, and the second most frequent in females after lung cancer [ 38 ]. In general, the CRC is more frequent in males than in females. Furthermore, CRC develops in different sites, depending on the sex of the patient: females have cancer in the right colon while males have it in the left colon. Additionally, males have a greater tendency to develop metastatic cancer of the colon while females are more likely to develop metastatic rectal cancer as they age [ 43 ].
The global incidence of CRC is not uniformly distributed among regions of the world but varies substantially up to 8- and 6-fold for colon and rectal cancers, respectively [ 39 ].
The highest CRC rate is documented in Asia, where are documented 52.3% of all global CCR in 2020; China alone accounts for 28.8% of CRC cases worldwide [ 43 ]. Considering rectal cancer, the East Asian regions have the highest incidence rate, particularly Korean males and Macedonian females ranked first [ 39 ]. In Europe the incidence rate is 26.9% of all global cases of CCR in 2020 with the highest age-standardized incidence rate (ASIR) reported for Hungary (45.3 per 100,000). The ASIRs in most European countries exceeded 40 per 100,000—higher than the world average rate [ 44 ]. Norway ranks first for CRC in females while Hungary ranks first for CRC cases reported in males [ 45 ]. In Italy, CCR accounts for 12.7% of all cancers with 48.576 new cases diagnosed in 2020 (25,588 males and 22,988 females). The Italian incidence rate is decreasing in all regions, for both males and females [ 38 ]. The incidence of CCR in the United States accounts for 25.6 per 100,000 persons. Colon and rectal cancers incidences are low in Africa and Southern Asia [ 39 , 45 ].
Colorectal cancer can be considered an index of socio-economic development, and its incidence rates tend to rise uniformly with increasing human development index (HDI). The HDI is a statistical index composed of three variables: life expectancy, education (mean years of schooling completed and expected years of schooling upon entering the education system), and per capita income indicators. A country scores a higher level of HDI when its population lives long, is highly educated, and has a high per capita income. Countries undergoing economic growth and westernization (medium HDI nations, such as Brazil, Russia, China, Latin America, the Philippines, and the Baltics) are experiencing increasing incidence of CRC. This trend reflects changes in lifestyle factors and diet: the economic development is responsible of increased consumption of red meat, fat, sugar, animal-source foods, and energy-dense food, which is associated with reduced physical activity and rising of being overweight and obese [ 46 , 47 , 48 , 49 , 50 ]. Most high-HDI nations (such as Canada, the UK, Denmark, and Singapore) have seen an increase in incidence but lowering in mortality, probably due to improved therapies. Highest HDI nations such as the US, Iceland, Japan, and France have witnessed a reduction in both mortality and incidence due to improvement in prevention and treatment [ 39 ].
However, the decline in CRC incidence slowed from 3–4% annually during the 2000s to 1% annually during 2011–2019, due partly to an increase in patients younger than 55 years of 1–2% annually since the mid-1990s [ 38 ]. The early incidence of CCR in the United States has increased approximately to 45% in adult ages 20–49 years, from 8.6 per 100,000 in 1992 to 13.1 per 100,000 in 2016 [ 51 ]. A similar trend is evident among the populations of New Zealand, Australia, Canada, and Northern-Central Europe [ 43 ], but not in Italy [ 52 ]. Incidence in individuals younger than 50 years increased by about 2% per year for rectal cancer vs. 0.5% per year for tumors in the proximal colon. Early onset patients are also more often diagnosed with advanced disease (27% vs. 21% of older patients) [ 53 ].
Several studies reported racial disparity in CRC incidence [ 39 , 44 , 46 , 51 , 52 ]. Siegel et al. [ 53 ] have demonstrated a different incidence of mortality depending on racial group in the US during the period 2012–2016; the African Americans showed the highest incidence rate (45.7 per 100,000 persons); it was 38.6 per 100,000 people in non-Hispanic Caucasians and 34.1 per 100,000 persons in Hispanics, the lowest incidence rate was registered in Asian Americans/Pacific Islanders (30.0 per 100,000 people). Several studies focused on the racial difference in genetic susceptibly to CRC found no racial disparity, but the difference in incidence among the ethnic groups seems to be linked to inequality in health care access and exposure to risk factors.
4. Mortality
CRC is the second cause of death due to cancer with 935,173 deaths estimated worldwide in 2020 [ 38 ], which accounted for about 9% of all cancer-related mortality [ 5 ]. The mortality rate for CRC seems to be decreasing in recent years, but this trend has slowed from about 4% annually during the early 2000s to about 2% from 2012 through 2020. Although mortality is decreasing in the majority of developed countries, the number of deaths is estimated to increase by 60.0% for colon cancer and 71.5% for rectal cancer until 2035 [ 54 ]. Nevertheless, it is important to underline the increase in surgical interventions in frailer elderly affected by concomitant chronic diseases [ 55 , 56 ].
A disparity in CRC mortality rate and trend for gender has been noticed worldwide: the overall mortality rate is 43% higher in men than in women. A further difference in CRC mortality rate is related to age. The mortality rate all over the world is higher in patients of 65 years and older [ 57 ]. However, it is evident an increase in mortality rate among younger compared to older population [ 53 ]. According to the authors, this trend could be explained by two different factors: on the one hand, there could be an earlier exposure to the known risk factors of the new generations compared to the previous ones, while on the other hand, it is known that neoplasms appearing at a younger age often show greater biological aggressiveness [ 6 ]. According to the data, in the US in 2020, 68% of CRC mortality was registered in patients ≥ 65 years old, 25% in the group 50–64 years, and 7% in patients < 49 years old [ 5 ]. In Europe, during the period 1990 and 2016, the CRC mortality rate increased by 1.1% in patients of age group 30–39, while in patients of 40–49 years old, the mortality rate diminished by 2.4% between 1990 and 2009, but raised by 1.1% between 2009 and 2016 [ 5 ].
The differences in terms of mortality rate are also related to the racial group. In the US, the African American population showed the highest mortality rate (19.0 per 100,000 persons) while the lowest rate was registered in Asian Americans/Pacific Islanders (9.5 per 100,000 persons). The mortality rate in non-Hispanic whites was 13.8 per 100,000 persons and 11.1 per 100,000 persons in Hispanics [ 53 ].
CRC mortality rates change globally, with an attenuated pattern compared to that of the incidence. About 60% of all deaths occur in countries with high or very high HDI [ 39 ]. The age-standardized rate of CRC mortality per 100,000 people was 27.1 in very high HDI countries compared to 2.75 in low HDI countries, with a direct proportionality between the CRC mortality index and HDI [ 5 ]. Decreasing trends were observed in central European countries (Austria, the Czech Republic, and Germany) and in the United States and Canada [ 5 ]. Colorectal cancer-related deaths are increasing in countries with low–medium HDI like countries of Eastern Europe, Asia, and South America. The lowest mortality rates were registered in Ecuador whilst the steepest fall in mortality was in Denmark [ 53 ]. In Italy, CCR ranks second in terms of mortality after lung cancer; if only rectal cancer is considered, it ranks ninth in terms of mortality while colon cancer alone ranks eleventh. In 2020, there were an estimated 21,789 deaths with a huge prevalence in males. The Southern regions show a higher mortality rate than Northern regions [ 38 ].
5. Survival
The five-year relative survival rate for CRC increased by 15%, from 50% in the mid-1970s to 65% during 2012–2018. This trend reflects earlier detection of CRC through routine clinical examinations and screening, more accurate staging through advances in imaging, improvements in surgical techniques, and advances in chemotherapy and radiation [ 5 , 58 , 59 , 60 , 61 , 62 ]. Stage at diagnosis is the most important predictor of survival, with five-year relative survival ranging from 91% for initial disease to 14% for metastatic one. Approximately 10% of survivors live with metastases, 44% of whom were initially diagnosed with early-stage disease. The largest survival incomes are for metastatic rectal cancer, with 30% of patients diagnosed during 2016–2018 surviving three years compared with 25% only a decade earlier [ 63 ].
Liver represents the leading metastase site [ 3 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 ]. Up to 25% of patients simultaneously experienced primary tumour and colorectal liver metastases (CRLM) diagnosis [ 73 ], while 20% will progress to stage IV. Although the availability of chemotherapy regimen progress in many primary tumours [ 15 ], surgical resection is considered the gold standard treatment for CRLM, with a five-year survival rate from 30% to 60% [ 9 , 40 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 ]. However, about 80% of patients are affected by unresectable CRLM, due to bilobar multiple liver metastases and/or extrahepatic disease. Nowadays, systemic chemotherapy regimens [ 64 , 76 , 79 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 ] are proposed to convert patients with initially unresectable CRLM to obtaining and improving long-term surgical and oncological outcomes [ 23 , 82 , 90 , 91 , 92 , 93 ]. Nevertheless, some patients will progress during neoadjuvant chemotherapy with a debated role of liver resection in this subgroup.
Lung metastases occur in 5–15% of CRC patients; if not treated, metastatic CRC carries a very poor prognosis with a five-year survival rate of less than 5% [ 63 ]. During the last two decades, new chemotherapeutics agents have been developed, such as irinotecan, oxaliplatin, and monoclonal antibodies against EGFR and VEGF. These allow prolonged progression-free survival and overall survival in metastatic colorectal cancer [ 21 , 23 ].
Stage at diagnosis reflects the different socio-economic status of racial groups. Black patients are most likely to be diagnosed with metastatic disease than the white ones (25% vs. 21%), likely caused by unequal access to care. However, the five-year relative survival rate for localized disease is quite similar (89–91%) among racial and ethnic groups [ 63 ].
Men have a slightly lower five-year relative survival rate (64%) compared with females (65%) despite a more favorable tumor site. In particular, 35% of males vs. 44% of females develop tumors in the proximal colon, which have a higher risk of death compared with left-sided cancers independent of histological and molecular characteristics. However, the largest sex difference in five-year survival is also for left-sided tumors at 66% in men vs. 68% in women for distal colon cancer and 67% vs. 70%, respectively, for rectal cancer [ 63 ]. Survival of patients with left-sided tumors was overall higher than that of patients with proximal colon cancer. This observation may be explained by a more favourable stage of cancers located in the distal than in the proximal colon, as well by distinct molecular features between subsites [ 94 ].
Survival rates for patients with screen-detected cancer were higher than those found for patients with non-screen-detected cancer within each disease stage. This evidence is probably due to higher adherence to therapy and more healthy behaviour of patients undergoing screening tests compared to non-screen-detected cancers, which contributes to the observed disparities in survival, particularly for patients with stage III and IV cancers.
The CRC survival rate varies among geographic regions. CONCORD-3 study [ 95 ] shows the survival for CRC in 71 countries. Five-year survival for colon cancer was higher than 70% in Israel, Jordan, Korea, and Australia. Survival was in the range of 50–69% in 26 countries: Mauritius; Costa Rica and Puerto Rico; Canada and the US; Japan, Singapore, and Taiwan; in 17 European countries (Denmark, Finland, Iceland, Ireland, Norway, Sweden, and the UK; Italy, Portugal, Slovenia, and Spain; Austria, Belgium, France, Germany, the Netherlands, and Switzerland); and in New Zealand. As for colon, five-year net survival for rectal cancer varied widely. Survival was higher than 70% in Jordan (73%), Korea (71%), and Australia (71%). Survival was in the range 60–69% in 24 countries: in Canada and the US; in 4 Asian countries, in 17 European countries: (Denmark, Finland, Iceland, Ireland, and Norway; Sweden and the UK; Italy, Portugal, Slovenia, and Spain; Austria; Belgium; France, Germany, the Netherlands, and Switzerland); and in New Zealand [ 95 ].
In Italy, the five-year survival for CRC is 62%, and the ten-year survival rate is 58% both in men and women. The southern regions have survival approximately 5–8% lower than in the Centre–North regions [ 52 ].
6. Screening and Prevention
Several screening modalities for CRC are currently available: a yearly or two-year fecal occult blood tests (FOBT) or fecal immunochemical test (FIT), sigmoidoscopy every 5 years, or colonoscopy every 10 years [ 96 ].
The CRC screening program is recommended for people aged between 50 and 75 years. However, in recent years, the American Cancer Society and the United States Preventive Service Task Force (USPSTF) have lowered the recommended start age for screening in average-risk populations to 45 years old due to the early onset of CRC [ 5 ]. Among the European countries The Netherlands reported the highest participation rate of 71.3% to the screening programs, twelve countries reported participation rates of over 50%, while the participation rates in Poland (16.7%) and Belgium (4.5%) were less than 20%. In general, the participation to the screening programs is higher in Northern Europe than in South and Central Europe. In Italy, the participation rate amounts to 45.7%, with higher adherence in the northern regions than in the southern ones.
Over the last decades, screening programs have deeply influenced the incidence and mortality rates of CRC. In the US, a CRC incidence has been reduced by 20% for annual FOBT screening and by 18% for biennial regimen [ 44 ]. In Japan, a 60% risk reduction in CRC incidence was achieved among subjects who underwent FOBT screening in comparison to the non-screened population [ 44 ]. In the UK, a randomized trial reported that flexible sigmoidoscopy screening resulted in a 26% reduction of CRC incidence [ 44 ]. With the implementation of a screening program in Italy, a reduction of cumulative incidence by 10% among the people aged between 50–69 years of age has been registered [ 44 ].
In US, FOBT caused reduction in mortality by 32% for the yearly screening and an 18% reduction thanks to the two-yearly screening. FOBT screening caused CRC mortality reduction by 15% in the UK, by 18% in Denmark, and by 16% in France and Sweden [ 5 ], by 30% in Japan, and by 31.7% in China [ 5 ]. In Italy, the effects of the screening program are similar to other European countries with a reduction of 13% in CRC mortality.
Screening colonoscopy, in addition to diagnosing early lesions or polyps, allows their excision. Complete polypectomy is essential to reduce the risk of early recurrence and the development of interval cancers, defined as the occurrence of CRC following a colonoscopy prior to the next scheduled surveillance procedure. The European society of gastrointestinal endoscopy (ESGE) clinical guideline strongly recommends that all polyps must be resected, except for diminutive rectal and recto-sigmoid polyps which can be predicted with high confidence to be hyperplastic [ 97 , 98 ]. Moreover, adenoma removal significantly reduced the risk of death from colorectal cancer, as compared with that in the general population. Zauber et al. recorded a 53% reduction in mortality from colorectal cancer in patients undergoing polypectomy compared to a control group corresponding [ 99 ].
Magnification endoscopy and chromoendoscopy represent useful techniques that enhance lesion demarcation improving adenoma resections. It is linked with a higher sensitivity rate when compared to classical endoscopy [ 98 ]. Moreover, the number of artificial intelligence (AI) systems developed or in development for gastrointestinal endoscopy has grown exponentially in recent years. Computer-aided detection (CADe) systems recognise characteristic features in order to discern the presence of a polyp within a still image or a video. Computer-aided diagnosis (CADx) systems are able to distinguish between polyp types and degrees of dysplasia, from benign hyperplastic polyps to advanced cancers, providing a real-time diagnosis to the proceduralist. AI’s ability to assist in precise polyp characterisation can help mitigate the risk of misdiagnoses, reducing unnecessary treatments, and enhancing patient care quality [ 100 ].
Despite surgical and endoscopic innovations, many procedures have been proposed but not included in routine clinical examinations: virtual colonoscopy, serum proteomics, and molecular blood tests represent promising tools for the early detection of colorectal lesions. For example, CIMP is already evident in early polyps and in the US is available as an assay able to detect the presence of methylated CpG upon PCR amplification of promoter regions of specific genes starting from DNA extracted from stool or plasma [ 33 , 97 ]. Although these tests are still being evaluated, the sensitivity is 83% and the specificity is 82%; also, this sensitivity is the same for stage I to III of CRC [ 97 ]. Further studies are needed to evaluate the sensitivity and specificity of these techniques, but it is reasonable to hope that these tests can overcome current invasive screening methods.
Several risk factors have been identified in the last decades as possible tumorigenesis causes: tobacco use, physical inactivity, obesity, and alcohol.
Even though clinical trials with dietary interventions (e.g., increases in fibre, fruits, and vegetables, and reductions in fat and alcohol) have shown little effect, several observational studies support a role of dietary modifications. Many drugs are being investigated for chemoprevention of this cancer [ 97 , 98 , 101 , 102 ]. Several drugs (e.g., aspirin and nonsteroidal anti-inflammatory drugs) are responsible for a significant risk reduction for colorectal cancer or adenomas, but the role of chemoprevention needs to be further defined.
Surgical prevention is established for FAP and ulcerative colitis and the surgical procedure recommended as a gold standard therapy for these patients is restorative proctocolectomy with ileoanal J-pouch [ 102 , 103 , 104 , 105 ]. For HNPCC, the role of prophylactic surgery is less well defined, but some authors suggest prophylactic colectomy [ 102 , 103 ]. Because prophylactic surgery is mostly on young, apparently healthy people, morbidity and mortality from surgery has to be kept to a minimum.
7. Conclusions
CRC is one of the cancers whose incidence and mortality are modifiable by following healthy lifestyles. However, the burden of CRC is expected to increase due to the aging of the population and to the westernization of less developed countries. Screening programs are able to reduce incidence and mortality rates of CRC. More studies are required to explain the reasons for the increasing burden of CRC in young adults. More efforts are needed to implement screening programs and to control risk factors of CRC to reduce its burden.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization, R.M., D.S., P.A. and M.C. (Massimo Carlini); investigation, R.M., R.T. and D.S.; writing—review and editing, R.M, D.S., P.A., M.G. and M.C. (Micaela Cappuccio); visualization, G.L. and G.M.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 August 2023
The effect of time before diagnosis and treatment on colorectal cancer outcomes: systematic review and dose–response meta-analysis
- Allison Drosdowsky ORCID: orcid.org/0000-0001-5346-3277 1 ,
- Karen E. Lamb 2 ,
- Amalia Karahalios 2 ,
- Rebecca J. Bergin 1 , 3 ,
- Kristi Milley 1 , 4 ,
- Lucy Boyd 1 ,
- Maarten J. IJzerman 2 &
- Jon D. Emery ORCID: orcid.org/0000-0002-5274-6336 1 , 4
British Journal of Cancer volume 129 , pages 993–1006 ( 2023 ) Cite this article
415 Accesses
2 Altmetric
Metrics details
- Cancer epidemiology
- Health services
This systematic review and meta-analysis aimed to evaluate existing evidence on the relationship between diagnostic and treatment intervals and outcomes for colorectal cancer.
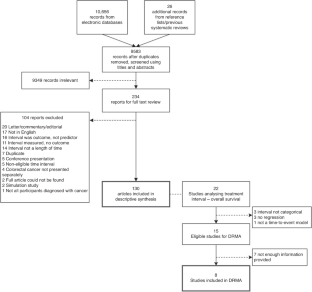
Four databases were searched for English language articles assessing the role of time before initial treatment in colorectal cancer on any outcome, including stage and survival. Two reviewers independently screened articles for inclusion and data were synthesised narratively. A dose–response meta-analysis was performed to examine the association between treatment interval and survival.
One hundred and thirty papers were included in the systematic review, eight were included in the meta-analysis. Forty-five different intervals were considered in the time from first symptom to treatment. The most common finding was of no association between the length of intervals on any outcome. The dose–response meta-analysis showed a U-shaped association between the treatment interval and overall survival with the nadir at 45 days.
The review found inconsistent, but mostly a lack of, association between interval length and colorectal cancer outcomes, but study design and quality were heterogeneous. Meta-analysis suggests survival becomes increasingly poorer for those commencing treatment more than 45 days after diagnosis.
Registration
This review was registered, and the protocol is available, in PROSPERO, the international database of systematic reviews, with the registration ID CRD42021255864.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
251,40 € per year
only 10,48 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Data availability
Data used in this review is provided in Supplementary Appendices; any additional data are available upon request to the corresponding author.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Article PubMed Google Scholar
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174.
Article PubMed PubMed Central Google Scholar
Emery J, Brown G, Macrae F, Bell C, Tse J, Skinner I, et al. Guidelines: colorectal cancer/the symptomatic patient. Sydney: Cancer Council Australia; 2017.
Neal RD. Do diagnostic delays in cancer matter? Br J Cancer. 2009;101:S9–s12.
Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106:1262–7.
Article CAS PubMed PubMed Central Google Scholar
Aslam MI, Chaudhri S, Singh B, Jameson JS. The "two-week wait" referral pathway is not associated with improved survival for patients with colorectal cancer. Int J Surg. 2017;43:181–5.
Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101:S5–8.
Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17:110–8.
Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguiló A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur J Cancer. 2007;43:2467–78.
Ramos M, Esteva M, Cabeza E, Llobera J, Ruiz A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer. 2008;44:510–21.
Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087.
Whittaker TM, Abdelrazek MEG, Fitzpatrick AJ, Froud JLJ, Kelly JR, Williamson JS, et al. Delay to elective colorectal cancer surgery and implications for survival: a systematic review and meta-analysis. Colorectal Dis. 2021;23:1699–711.
Franssen RFW, Strous MTA, Bongers BC, Vogelaar FJ, Janssen-Heijnen MLG. The association between treatment interval and survival in patients with colon or rectal cancer: a systematic review. World J Surg. 2021;45:2924–37.
Hangaard Hansen C, Gögenur M, Tvilling Madsen M, Gögenur I. The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur J Surg Oncol. 2018;44:1479–85.
Article CAS PubMed Google Scholar
Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–107.
Castelo M, Sue-Chue-Lam C, Paszat L, Kishibe T, Scheer AS, Hansen BE, et al. Time to diagnosis and treatment in younger adults with colorectal cancer: a systematic review. PLoS ONE. 2022;17:e0273396.
Tørring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65:669–78.
Rupassara KS, Ponnusamy S, Withanage N, Milewski PJ. A paradox explained? Patients with delayed diagnosis of symptomatic colorectal cancer have good prognosis. Colorectal Dis. 2006;8:423–9.
Crawford SC, Davis JA, Siddiqui NA, de Caestecker L, Gillis CR, Hole D, et al. The waiting time paradox: population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ. 2002;325:196.
Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–96.
Orsini N, Spiegelman D Meta-analysis of dose-response relationships. Handbook of meta-analysis. Boca Raton, FL: Chapman and Hall/CRC; 2020. p. 395-428.
Li Y, Gu M, Jing F, Cai S, Bao C, Wang J, et al. Association between physical activity and all cancer mortality: dose-response meta-analysis of cohort studies. Int J Cancer. 2016;138:818–32.
Wang F, Yeung KL, Chan WC, Kwok CC, Leung SL, Wu C, et al. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann Oncol. 2013;24:2724–32.
Drosdowsky A, Lamb KE, Bergin RJ, Boyd L, Milley K, IJ MJ, et al. A systematic review of methodological considerations in time to diagnosis and treatment in colorectal cancer research. Cancer Epidemiol. 2023;83:102323.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Veritas Health Innovation. Covidence systematic review software, Melbourne, Australia, 2023.
The Joanna Briggs Institute. Critical Appraisal Tools. 2023. https://joannabriggs.org/critical-appraisal-tools .
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16.
PlotDigitizer. 2022. https://plotdigitizer.com/ .
Il'yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C. Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control. 2005;16:383–8.
Taylor K. Wanting a particular reference category in categorical risk data. University of Oxford; 2022. https://www.cebm.ox.ac.uk/resources/data-extraction-tips-meta-analysis/reference-category-risk-data .
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2021.
Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R package. J Stat Softw. 2016;72:1–15.
Article Google Scholar
Gleason F, Chu DI, Kennedy GD, Kenzik KM. Early elective surgery after colon cancer diagnosis has higher risk of readmission and death. Ann Surg. 2021;273:188–94.
Kaltenmeier C, Shen C, Medich DS, Geller DA, Bartlett DL, Tsung A, et al. Time to surgery and colon cancer survival in the United States. Ann Surg. 2021;274:1025–31.
Lo BD, Caturegli G, Stem M, Biju K, Safar B, Efron JE, et al. The impact of surgical delays on short- and long-term survival among colon cancer patients. Am Surg. 2021;87:1783–92.
Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Berlin: Springer; 2001.
Abdulaal A, Arhi C, Ziprin P. Effect of health care provider delays on short-term outcomes in patients with colorectal cancer: multicenter population-based observational study. Interact J Med Res. 2020;9:e15911.
Ahmed RN, Rai L, Samo KA, Saeed S, Salam A, Khan H, et al. Factors affecting delay in diagnosis of colorectal cancer: a cross‐sectional study from a tertiary care hospital of Karachi, Pakistan. Int J Clin Pract. 2021;75:e14529.
Amri R, Bordeianou LG, Sylla P, Berger DL. Treatment delay in surgically-treated colon cancer: does it affect outcomes? Ann Surg Oncol. 2014;21:3909–16.
Arbman G, Nilsson E, Störgren-Fordell V, Sjödahl R. A short diagnostic delay is more important for rectal cancer than for colonic cancer. Eur J Surg. 1996;162:899–904.
CAS PubMed Google Scholar
Arhi CS, Burns EM, Bottle A, Bouras G, Aylin P, Ziprin P, et al. Delays in referral from primary care worsen survival for patients with colorectal cancer: a retrospective cohort study. Br J Gen Pract. 2020;70:e463–e71.
Auvinen A. Social class and colon cancer survival in Finland. Cancer 1992;70:402–9.
Bagaria SP, Heckman MG, Diehl NN, Parker A, Wasif N. Delay to colectomy and survival for patients diagnosed with colon cancer. J Invest. Surg. 2019;32:350–7.
Bako G, Hill G, Ferenczi L, Hanson J. Factors influencing the survival of patients with cancer of the colon or rectum. Chronic Dis Can. 1988;9:101–4.
Google Scholar
Barillari P, De Angelis R, Valabrega S, Indinnimeo M, Gozzo P, Ramacciato G, et al. Relationship of symptom duration and survival in patients with colorectal carcinoma. Eur J Surg Oncol. 1989;15:441–5.
Bassett ML, Bennett SA, Goulston KJ. Colorectal cancer. A study of 230 patients. Med J Aust. 1979;1:589–92.
Ben-Ishay O, Brauner E, Peled Z, Othman A, Person B, Kluger Y. Diagnosis of colon cancer differs in younger versus older patients despite similar complaints. Isr Med Assoc J. 2013;15:284–7.
PubMed Google Scholar
Bharucha S, Hughes S, Kenyon V, Anderson ID, Carlson GL, Scott NA. Targets and elective colorectal cancer: outcome and symptom delay at surgical resection. Colorectal Dis. 2005;7:169–71.
Bjerkeset T, Søreide O. Symptoms in colorectal adenocarcinomas and their relation to tumor characteristics and survival. Dig Surg. 1988;5:61–5.
Cerdán-Santacruz C, Cano-Valderrama O, Cárdenas-Crespo S, Torres-García AJ, Cerdán-Miguel J. Colorectal cancer and its delayed diagnosis: have we improved in the past 25 years? Rev Esp Enferm Dig. 2011;103:458–63.
Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985;72:698–702.
Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol. 2017;15:728–37.e3.
Clarke AM, Jones IS. Diagnostic accuracy and diagnostic delay in carcinoma of the large bowel. NZ Med J. 1970;71:341–7.
CAS Google Scholar
Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98:238–9.
Cubiella J, Lorenzo M, Baiocchi F, Tejido C, Conde A, Sande-Meijide M, et al. Impact of a colorectal cancer screening program implantation on delays and prognosis of non-screening detected colorectal cancer. World J Gastroenterol. 2021;27:6689–700.
Curtis NJ, West MA, Salib E, Ockrim J, Allison AS, Dalton R, et al. Time from colorectal cancer diagnosis to laparoscopic curative surgery-is there a safe window for prehabilitation? Int J Colorectal Dis. 2018;33:979–83.
Davidson JTT, Abelson JS, Glasgow SC, Hunt SR, Mutch MG, Wise PE, et al. Delaying definitive resection in early stage (I/II) colon cancer appears safe up to 6 weeks. Am J Surg. 2021;222:402–7.
de Roos MAJ, Hugen N, Hazebroek EJ, Spillenaar Bilgen EJ. Delayed surgical resection of primary left-sided obstructing colon cancer is associated with improved short- and long-term outcomes. J Surg Oncol. 2021;124:1146–53.
de Sousa JB, Souza CS, Fernandes MB, de Castro Durães L, de Almeida RM, Dos Santos AC, et al. Do young patients have different clinical presentation of colorectal cancer causing delay in diagnosis? Int J Colorectal Dis. 2014;29:519–27.
Delisle M, Helewa RM, Ward MA, Hochman DJ, Park J, McKay A. The association between wait times for colorectal cancer treatment and health care costs: a population-based analysis. Dis Colon Rectum. 2020;63:160–71.
Deng SX, An W, Gao J, Yin J, Cai QC, Yang M, et al. Factors influencing diagnosis of colorectal cancer: a hospital-based survey in China. J Dig Dis. 2012;13:517–24.
Di Girolamo C, Walters S, Gildea C, Benitez Majano S, Rachet B, Morris M. Can we assess cancer waiting time targets with cancer survival? A population-based study of individually linked data from the National Cancer Waiting Times monitoring dataset in England, 2009-2013. PLoS ONE. 2018;13:e0201288.
Dregan A, Møller H, Charlton J, Gulliford MC. Are alarm symptoms predictive of cancer survival?: population-based cohort study. Br J Gen Pract. 2013;63:e807–12.
Duff SE, Wood C, McCredie V, Levine E, Saunders MP, O'Dwyer ST. Waiting times for treatment of rectal cancer in North West England. J R Soc Med. 2004;97:117–8.
Edwards GC, Gamboa AC, Feng MP, Muldoon RL, Hopkins MB, Abdel-Misih S, et al. What’s the magic number? Impact of time to initiation of treatment for rectal cancer. Surgery. 2022;171:1185–92.
Eldar S, Kemeny MM, Terz JJ. Extended resections for carcinoma of the colon and rectum. Surg Gynecol Obstet. 1985;161:319–22.
Fegiz G, Barillari P, Ramacciato G, De Angelis R, Gozzo P, Indinnimeo M, et al. Right colon cancer: long-term results after curative surgery and prognostic significance of duration of symptoms. J Surg Oncol. 1989;41:250–5.
Fernández-de Castro JD, Baiocchi Ureta F, Fernández González R, Pin Vieito N, Cubiella Fernández J. The effect of diagnostic delay attributable to the healthcare system on the prognosis of colorectal cancer. Gastroenterol Hepatol. 2019;42:527–33.
Fisher DA, Zullig LL, Grambow SC, Abbott DH, Sandler RS, Fletcher RH, et al. Determinants of medical system delay in the diagnosis of colorectal cancer within the Veteran Affairs Health System. Dig Dis Sci. 2010;55:1434–41.
Flemming JA, Nanji S, Wei X, Webber C, Groome P, Booth CM. Association between the time to surgery and survival among patients with colon cancer: a population-based study. Eur J Surg Oncol. 2017;43:1447–55.
Garcia-Botello S, Martín-Arevalo J, Cozar-Lozano C, Benitez-Riesco A, Moro-Valdezate D, Pla-Martí V, et al. Does delaying curative surgery for colorectal cancer influence long-term disease-free survival? A cohort study. Langenbeck’s Arch Surg. 2021;406:2383–90.
Gómez-Domínguez E, Trapero-Marugán M, del Pozo AJ, Cantero J, Gisbert JP, Maté J. The colorectal carcinoma prognosis factors. Significance of diagnosis delay. Rev Esp Enferm Dig. 2006;98:322–9.
Gonzalez-Hermoso F, Perez-Palma J, Marchena-Gomez J, Lorenzo-Rocha N, Medina-Arana V. Can early diagnosis of symptomatic colorectal cancer improve the prognosis? World J Surg. 2004;28:716–20.
Goodman D, Irvin TT. Delay in the diagnosis and prognosis of carcinoma of the right colon. Br J Surg. 1993;80:1327–9.
Gort M, Otter R, Plukker JT, Broekhuis M, Klazinga NS. Actionable indicators for short and long term outcomes in rectal cancer. Eur J Cancer. 2010;46:1808–14.
Graffner H, Olsson SA. Patient’s and doctor’s delay in carcinoma of the colon and rectum. J Surg Oncol. 1986;31:188–90.
Grass F, Behm KT, Duchalais E, Crippa J, Spears GM, Harmsen WS, et al. Impact of delay to surgery on survival in stage I-III colon cancer. Eur J Surg Oncol. 2020;46:455–61.
Guzmán Laura KP, Bolíbar Ribas I, Alepuz MT, González D, Martín M. Impact on patient care time and tumor stage of a program for fast diagnostic and treatment of colorectal cancer. Rev Esp Enferm Dig. 2011;103:13–9.
Hafström L, Johansson H, Ahlberg J. Does diagnostic delay of colorectal cancer result in malpractice claims? A retrospective analysis of the Swedish board of malpractice from 1995-2008. Patient Saf Surg. 2012;6:13.
Helewa RM, Turner D, Park J, Wirtzfeld D, Czaykowski P, Hochman D, et al. Longer waiting times for patients undergoing colorectal cancer surgery are not associated with decreased survival. J Surg Oncol. 2013;108:378–84.
Holliday HW, Hardcastle JD. Delay in diagnosis and treatment of symptomatic colorectal cancer. Lancet. 1979;1:309–11.
Irvin TT, Greaney MG. Duration of symptoms and prognosis of carcinoma of the colon and rectum. Surg, Gynecol Obstet. 1977;144:883–6.
Iversen LH, Antonsen S, Laurberg S, Lautrup MD. Therapeutic delay reduces survival of rectal cancer but not of colonic cancer. Br J Surg. 2009;96:1183–9.
Janssen RM, Takach O, Nap-Hill E, Enns RA. Time to endoscopy in patients with colorectal cancer: analysis of wait-times. Can J Gastroenterol Hepatol. 2016;2016:8714587.
Järvinen HJ, Turunen MJ. Colorectal carcinoma before 40 years of age: prognosis and predisposing conditions. Scand J Gastroenterol. 1984;19:634–8.
Jolly KD, Scott JP, MacKinnon MJ, Clarke AM. Diagnosis and survival in carcinoma of the large bowel. Aust NZ J Surg. 1982;52:12–6.
Article CAS Google Scholar
Jullumstrø E, Lydersen S, Møller B, Dahl O, Edna TH. Duration of symptoms, stage at diagnosis and relative survival in colon and rectal cancer. Eur J Cancer. 2009;45:2383–90.
Keddie N, Hargreaves A. Symptoms of carcinoma of the colon and rectum. Lancet. 1968;2:749–50.
Khattak I, Eardley NJ, Rooney PS. Colorectal cancer–a prospective evaluation of symptom duration and GP referral patterns in an inner city teaching hospital. Colorectal Dis. 2006;8:518–21.
Khorana AA, Tullio K, Elson P, Pennell NA, Grobmyer SR, Kalady MF, et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS ONE. 2019;14:e0213209.
Khubchandani M. Relationship of symptom duration and survival in patients with carcinoma of the colon and rectum. Dis Colon Rectum. 1985;28:585–7.
Kim TJ, Kim ER, Hong SN, Chang DK, Kim YH. Long-term outcome and prognostic factors of sporadic colorectal cancer in young patients: a large institutional-based retrospective study. Medicine 2016;95:e3641.
Kiran PR, Glass RE. Duration of symptoms and spread of colorectal cancer: a short history does not mean early disease. Ann R Coll Surg Engl. 2002;84:381–5.
Korsgaard M, Pedersen L, Sørensen HT, Laurberg S. Delay of treatment is associated with advanced stage of rectal cancer but not of colon cancer. Cancer Detect Prev. 2006;30:341–6.
Kucejko RJ, Holleran TJ, Stein DE, Poggio JL. How soon should patients with colon cancer undergo definitive resection? Dis Colon Rectum. 2020;63:172–82.
Kyle SM, Isbister WH, Yeong ML. Presentation, duration of symptoms and staging of colorectal carcinoma. Aust NZ J Surg. 1991;61:137–40.
Langenbach MR, Sauerland S, Kröbel KW, Zirngibl H. Why so late?!–delay in treatment of colorectal cancer is socially determined. Langenbeck’s Arch Surg. 2010;395:1017–24.
Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003;27:304–8.
Law CW, Roslani AC, Ng LL. Treatment delay in rectal cancer. Med J Malays. 2009;64:163–5.
Lee YH, Kung PT, Wang YH, Kuo WY, Kao SL, Tsai WC. Effect of length of time from diagnosis to treatment on colorectal cancer survival: a population-based study. PLoS ONE. 2019;14:e0210465.
Leiva A, Esteva M, Llobera J, Macià F, Pita-Fernández S, González-Luján L, et al. Time to diagnosis and stage of symptomatic colorectal cancer determined by three different sources of information: a population based retrospective study. Cancer Epidemiol. 2017;47:48–55.
Lim BS, Dennis CR, Gardner B, Newman J. Analysis of survival versus patient and doctor delay of treatment in gastrointestinal cancer. Am J Surg. 1974;127:210–4.
Lino-Silva LS, Guzmán-López JC, Zepeda-Najar C, Salcedo-Hernández RA, Meneses-García A. Overall survival of patients with colon cancer and a prolonged time to surgery. J Surg Oncol. 2019;119:503–9.
MacArthur C, Smith A. Factors associated with speed of diagnosis, referral, and treatment in colorectal cancer. J Epidemiol Commun Health 1984;38:122–6.
Maguire A, Porta M, Malats N, Gallén M, Piñol JL, Fernandez E. Cancer survival and the duration of symptoms. An analysis of possible forms of the risk function. ISDS II Project Investigators. Eur J Cancer. 1994;30a:785–92.
Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol. 1999;94:3039–45.
Marble K, Banerjee S, Greenwald L. Colorectal carcinoma in young patients. J Surg Oncol. 1992;51:179–82.
McDermott F, Hughes E, Pihl E, Milne BJ, Price A. Symptom duration and survival prospects in carcinoma of the rectum. Surg Gynecol Obstet. 1981;153:321–6.
McDermott FT, Hughes ES, Pihl E, Milne BJ, Price AB. Prognosis in relation to symptom duration in colon cancer. Br J Surg. 1981;68:846–9.
Mulcahy H, O’Donoghue D. Duration of colorectal cancer symptoms and survival: the effect of confounding clinical and pathological variables. Eur J Cancer. 1997;33:1461–7.
Murchie P, Raja EA, Brewster DH, Campbell NC, Ritchie LD, Robertson R, et al. Time from first presentation in primary care to treatment of symptomatic colorectal cancer: effect on disease stage and survival. Br J Cancer. 2014;111:461–9.
Nilsson E, Bolin S, Sjödahl R. Carcinoma of the colon and rectum. Delay in diagnosis. Acta Chir Scand. 1982;148:617–22.
Olsson L, Bergkvist L, Ekbom A. Symptom duration versus survival in non-emergency colorectal cancer. Scand J Gastroenterol. 2004;39:252–8.
Ortiz-Ortiz KJ, Ríos-Motta R, Marín-Centeno H, Cruz-Correa M, Ortiz AP. Factors associated with late stage at diagnosis among Puerto Rico’s government health plan colorectal cancer patients: a cross-sectional study. BMC Health Serv Res. 2016;16:1–10.
Öztürk E, Kuzu MA, Öztuna D, Işık Ö, Canda AE, Balık E, et al. Fall of another myth for colon cancer: duration of symptoms does not differ between right- or left-sided colon cancers. Turk J Gastroenterol. 2019;30:686–94.
Påhlman L, Glimelius B, Enblad P. Clinical characteristics and their relation to surgical curability in adenocarcinoma of the rectum and rectosigmoid. A population-based study on 279 consecutive patients. Acta Chir Scand. 1985;151:685–93.
Patel R, Anderson JE, McKenzie C, Simpson M, Singh N, Ruzvidzo F, et al. Compliance with the 62-day target does not improve long-term survival. Int J Colorectal Dis. 2018;33:65–9.
Pearson C, Fraser J, Peake M, Valori R, Poirier V, Coupland VH, et al. Establishing population-based surveillance of diagnostic timeliness using linked cancer registry and administrative data for patients with colorectal and lung cancer. Cancer Epidemiol. 2019;61:111–8.
Pescatori M, Maria G, Beltrani B, Mattana C. Site, emergency, and duration of symptoms in the prognosis of colorectal cancer. Dis Colon Rectum. 1982;25:33–40.
Pita-Fernández S, González-Sáez L, López-Calviño B, Seoane-Pillado T, Rodríguez-Camacho E, Pazos-Sierra A, et al. Effect of diagnostic delay on survival in patients with colorectal cancer: a retrospective cohort study. BMC Cancer. 2016;16:664.
Polissar L, Sim D, Francis A. Survival of colorectal cancer patients in relation to duration of symptoms and other prognostic factors. Dis Colon Rectum. 1981;24:364–9.
Porta M, Gallén M, Malats N, Planas J. Influence of "diagnostic delay" upon cancer survival: an analysis of five tumour sites. J Epidemiol Commun Health 1991;45:225–30.
Porter GA, Inglis KM, Wood LA, Veugelers PJ. Access to care and satisfaction in colorectal cancer patients. World J Surg. 2005;29:1444–51.
Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control. 2013;24:961–77.
Ratcliffe R, Kiff RS, Hoare EM, Kingston RD, Walsh SH, Jeacock J. Early diagnosis in colorectal cancer still no benefit? Ann Chir. 1989;43:570–4.
Redaniel MT, Martin RM, Ridd MJ, Wade J, Jeffreys M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: historical cohort study using the Clinical Practice Research Datalink. PLoS ONE. 2015;10:e0126608.
Ristvedt SL, Birnbaum EH, Dietz DW, Fleshman JW, Kodner IJ, Read TE. Delayed treatment for rectal cancer. Dis colon rectum. 2005;48:1736–41.
Robinson E, Mohilever J, Zidan J, Sapir D. Colorectal cancer: incidence, delay in diagnosis and stage of disease. Eur J Cancer Clin Oncol. 1986;22:157–61.
Roder D, Karapetis CS, Olver I, Keefe D, Padbury R, Moore J, et al. Time from diagnosis to treatment of colorectal cancer in a South Australian clinical registry cohort: how it varies and relates to survival. BMJ Open. 2019;9:e031421.
Roland CL, Schwarz RE, Tong L, Ahn C, Balch GC, Yopp AC, et al. Is timing to delivery of treatment a reliable measure of quality of care for patients with colorectal adenocarcinoma? Surgery. 2013;154:421–8.
Roncoroni L, Pietra N, Violi V, Sarli L, Choua O, Peracchia A. Delay in the diagnosis and outcome of colorectal cancer: a prospective study. Eur J Surg Oncol. 1999;25:173–8.
Rowejones DC, Aylett SO. Delay in treatment in carcinoma of colon and rectum. Lancet. 1965;2:973–6.
Rubin M, Zer M, Dintsman M. Factors influencing delay in treatment of cancer of rectum and colon in Israel. Isr J Med Sci. 1980;16:641–5.
Sandar M, Hsiang LG, Yew CK, Guat LB. Use of population-based cancer registry data to determine the effect of timely treatment on the survival of colorectal cancer patients. J Registry Manag. 2015;42:130–8.
Sey MS, Gregor J, Adams P, Khanna N, Vinden C, Driman D, et al. Wait times for diagnostic colonoscopy among outpatients with colorectal cancer: a comparison with Canadian Association of Gastroenterology targets. Can J Gastroenterol. 2012;26:894–6.
Shin DW, Cho J, Kim SY, Guallar E, Hwang SS, Cho B, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20:2468–76.
Simunovic M, Rempel E, Thériault ME, Baxter NN, Virnig BA, Meropol NJ, et al. Influence of delays to nonemergent colon cancer surgery on operative mortality, disease-specific survival and overall survival. Can J Surg. 2009;52:E79–e86.
PubMed PubMed Central Google Scholar
Singh H, Shu E, Demers A, Bernstein CN, Griffith J, Fradette K. Trends in time to diagnosis of colon cancer and impact on clinical outcomes. Can J Gastroenterol. 2012;26:877–80.
Stapley S, Peters TJ, Sharp D, Hamilton W. The mortality of colorectal cancer in relation to the initial symptom at presentation to primary care and to the duration of symptoms: a cohort study using medical records. Br J Cancer. 2006;95:1321–5.
Strous MTA, Janssen-Heijnen MLG, Vogelaar FJ. Impact of therapeutic delay in colorectal cancer on overall survival and cancer recurrence - is there a safe timeframe for prehabilitation? Eur J Surg Oncol. 2019;45:2295–301.
Stubbs RS, Long MG. Symptom duration and pathologic staging of colorectal cancer. Eur J Surg Oncol. 1986;12:127–30.
Terhaar sive Droste JS, Oort FA, van der Hulst RW, Coupé VM, Craanen ME, Meijer GA, et al. Does delay in diagnosing colorectal cancer in symptomatic patients affect tumor stage and survival? A population-based observational study. BMC Cancer. 2010;10:332.
Thompson MR, Asiimwe A, Flashman K, Tsavellas G. Is earlier referral and investigation of bowel cancer patients presenting with rectal bleeding associated with better survival? Colorectal Dis. 2011;13:1242–8.
Tiong J, Gray A, Jackson C, Thompson-Fawcett M, Schultz M. Audit of the association between length of time spent on diagnostic work-up and tumour stage in patients with symptomatic colon cancer. ANZ J Surg. 2017;87:138–42.
Tørring ML, Falborg AZ, Jensen H, Neal RD, Weller D, Reguilon I, et al. Advanced-stage cancer and time to diagnosis: an International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur J Cancer Care. 2019;28:e13100.
Tørring ML, Frydenberg M, Hansen RP, Olesen F, Hamilton W, Vedsted P. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer. 2011;104:934–40.
Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49:2187–98.
Tørring ML, Murchie P, Hamilton W, Vedsted P, Esteva M, Lautrup M, et al. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11720 patients in five countries. Br J Cancer. 2017;117:888–97.
Trepanier M, Paradis T, Kouyoumdjian A, Dumitra T, Charlebois P, Stein BS, et al. The impact of delays to definitive surgical care on survival in colorectal cancer patients. J Gastrointest Surg. 2020;24:115–22.
Turnbull PR, Isbister WH. Colorectal cancer in New Zealand: a Wellington study. Aust NZ J Surg. 1979;49:45–8.
Turunen MJ, Peltokallio P. Delay in the diagnosis of colorectal cancer. Ann Chir Gynaecol. 1982;71:277–82.
Van Hout AM, de Wit NJ, Rutten FH, Peeters PH. Determinants of patient’s and doctor’s delay in diagnosis and treatment of colorectal cancer. Eur J Gastroenterol Hepatol. 2011;23:1056–63.
Viiala CH, Tang KW, Lawrance IC, Murray K, Olynyk JK. Waiting times for colonoscopy and colorectal cancer diagnosis. Med J Aust. 2007;186:282–5.
Walming S, Block M, Bock D, Angenete E. Timely access to care in the treatment of rectal cancer and the effect on quality of life. Colorectal Dis. 2018;20:126–33.
Wanis KN, Patel SVB, Brackstone M. Do moderate surgical treatment delays influence survival in colon cancer? Dis Colon Rectum. 2017;60:1241–9.
Wattacheril J, Kramer JR, Richardson P, Havemann BD, Green LK, Le A, et al. Lagtimes in diagnosis and treatment of colorectal cancer: determinants and association with cancer stage and survival. Aliment Pharmacol Ther. 2008;28:1166–74.
Wheeler, Wakefield, Mortensen NJMcC, Kettlewell. Delays experienced by patients with symptomatic colorectal cancer. Colorectal Dis. 1999;1:174–6.
Young CJ, Sweeney JL, Hunter A. Implications of delayed diagnosis in colorectal cancer. Aust NZ J Surg. 2000;70:635–8.
Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731–7.
Zhang JK, Fang LL, Wu XM, Liu JC, Zhang CD, Dai DQ. Factors associated with delaying medical assessment of patients and impacting the prognosis of rectal cancer. Eur J Cancer Prev 2015;24:391–9.
Burke JR, Brown P, Quyn A, Lambie H, Tolan D, Sagar P. Tumour growth rate of carcinoma of the colon and rectum: retrospective cohort study. BJS Open. 2020;4:1200–7.
Ng J, Stovezky YR, Brenner DJ, Formenti SC, Shuryak I. Development of a model to estimate the association between delay in cancer treatment and local tumor control and risk of metastases. JAMA Netw Open. 2021;4:e2034065.
Cohen J. The cost of dichotomization. Appl Psychol Meas. 1983;7:249–53.
Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–41.
Petrova D, Špacírová Z, Fernández-Martínez NF, Ching-López A, Garrido D, Rodríguez-Barranco M, et al. The patient, diagnostic, and treatment intervals in adult patients with cancer from high- and lower-income countries: a systematic review and meta-analysis. PLoS Med. 2022;19:e1004110.
Siciliani L, Moran V, Borowitz M. What works? Waiting policies in the health sector. Eurohealth Observer. 2015;21:14–7.
Australian Government Department of Health. Optimal cancer care pathways. Canberra: Commonwealth of Australia; 2016.
Download references
AD is supported by a NHMRC Postgraduate Scholarship. RJB is supported by a Victorian Cancer Agency, Early Career Research Fellowship (ECRF20015).
Author information
Authors and affiliations.
Department of General Practice and Centre for Cancer Research, The University of Melbourne, Parkville, VIC, Australia
Allison Drosdowsky, Rebecca J. Bergin, Kristi Milley, Lucy Boyd & Jon D. Emery
Melbourne School of Population and Global Health, The University of Melbourne, Parkville, VIC, Australia
Karen E. Lamb, Amalia Karahalios & Maarten J. IJzerman
Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, VIC, Australia
Rebecca J. Bergin
Primary Care Collaborative Cancer Clinical Trials Group (PC4), Carlton, VIC, Australia
Kristi Milley & Jon D. Emery
You can also search for this author in PubMed Google Scholar
Contributions
All authors have significantly contributed to the conceptualisation, design, conduct and reporting (writing and reviewing) of this systematic review.

Corresponding author
Correspondence to Allison Drosdowsky .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval was not required for this review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary table 1, supplementary figure 1, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Drosdowsky, A., Lamb, K.E., Karahalios, A. et al. The effect of time before diagnosis and treatment on colorectal cancer outcomes: systematic review and dose–response meta-analysis. Br J Cancer 129 , 993–1006 (2023). https://doi.org/10.1038/s41416-023-02377-w
Download citation
Received : 19 December 2022
Revised : 28 June 2023
Accepted : 24 July 2023
Published : 01 August 2023
Issue Date : 05 October 2023
DOI : https://doi.org/10.1038/s41416-023-02377-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Advances in Colorectal Cancer Research
Colorectal cells grown into organoids, stem cell-derived human 'mini-organs' that are used to study human development and disease.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat colorectal cancer. They are also looking at what factors influence screening behaviors, how to address disparities, and the rising rates of colorectal cancer in younger people.
This page highlights some of the latest colorectal cancer research, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and findings from recent studies.
Prevention and Early Detection
Screening can prevent colorectal cancer through detection of precancerous growths, or polyps , which can be removed before they become cancerous. It can also allow colorectal cancers to be detected early, before they cause symptoms and when treatment may be more effective.
Colorectal cancer screening tests. These include colonoscopy , sigmoidoscopy , stool-based tests to detect hidden blood ( fecal immunochemical test ing (FIT) or fecal occult blood testing (FOBT)), and virtual colonoscopy . (See Screening Tests to Detect Colorectal Cancer and Polyps for more information.)
Despite the availability of effective colorectal cancer screening tests, some people choose not to get screened. Some reasons may be because of the personal nature of the procedures, a lack of recommendation by their doctor, perceived costs or lack of insurance, or the preparation involved for a colonoscopy.
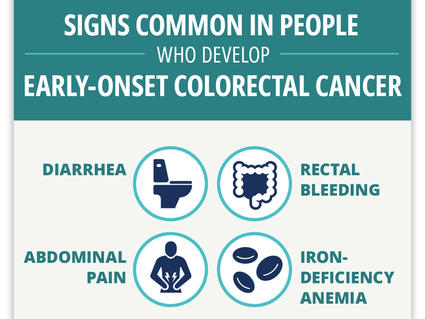
Can Colorectal Cancer in Younger Adults Be Found Early?
Researchers find four “red flag” signs that may identify colorectal cancer early in younger adults.
Although not currently recommended for screening, there are new techniques under development such as:
- finding technologies that improve the genetic analysis of stool samples, which may reveal the presence of tumor DNA
- looking at changes in the gut microbiome and trying to identify specific bacteria that could potentially help identify patients at risk for colorectal cancer
Repeat screening or follow-up . The guideline for getting a screening colonoscopy is every 10 years. However, people who have noncancerous polyps detected at colonoscopy are generally asked to return for a repeat colonoscopy earlier than that.
NCI’s FORTE Colorectal Cancer Prevention Trial , is now looking at whether some people with one or two small polyps can wait 10 years before returning for another colonoscopy. By comparing two study groups, one with repeat colonoscopy after 5 years, and one with repeat colonoscopy after 10 years, researchers hope to learn whether waiting 10 years is as good at preventing colorectal cancer as follow-up exams after 5 years.
For colorectal cancer screening to be effective, people need to follow up on abnormal test results. In one study, researchers found that people who had a positive at-home stool test to screen for colorectal cancer, but did not have a follow-up colonoscopy , were twice as likely to die from colorectal cancer as those who did have a follow-up colonoscopy.
NCI is funding research to better understand the many factors that can contribute to why a person may not have a follow-up test and how to increase repeat screening and follow-up colonoscopy after abnormal stool tests. Researchers are also studying how the many levels of the healthcare delivery system affect the decision to get screened.
Treatment for Colorectal Cancer
Surgically removing the cancer is the most common treatment for many stages of colorectal cancer. Chemotherapy, radiation , targeted therapy , radiofrequency ablation , and cryosurgery are other treatments that may be used to treat colorectal cancer, depending on the stage.
Because of an increased risk of recurrence, differences in anatomy, and poorer prognosis , the treatment of rectal cancer may differ from that of colon cancer. Although surgery remains a common type of treatment for local and locally advanced rectal cancer, people with some stages may be treated with radiation, chemotherapy, and/or targeted therapy with or without surgery.
In addition to these standard treatments for rectal cancer, researchers continue to study both new treatments, such as immunotherapies, and new combinations of existing treatments in clinical trials.
One trial is comparing a standard treatment (chemoradiation followed by combination chemotherapy) with chemoradiation followed by combination chemotherapy that includes an additional chemotherapy drug. The goal is to find out whether the additional chemotherapy drug may increase the likelihood of the cancer responding and possibly avoid the need for surgery.
Immunotherapy for patients with Lynch syndrome or MSI-H colorectal cancer
Approximately 5% of colorectal cancer cases are due to Lynch syndrome , an inherited DNA repair disorder . People with this disorder have an increased risk of developing colorectal cancer, typically before they reach the age of 50. Lynch syndrome colorectal cancer tumors have many mutations, which may make them more susceptible to immunotherapies.
A genetic feature known as microsatellite instability-high (MSI-H) is seen in about 15% of patients with stages II and III colorectal cancer and about 5% with stage IV. MSI-H means that there are mistakes in the way the DNA is copied in cancer cells, which can make them grow out of control.
The immune checkpoint inhibitors nivolumab (Opdivo) , ipilimumab (Yervoy) , and pembrolizumab (Keytruda) have all been approved for the treatment of metastatic colorectal cancer in patients with Lynch syndrome and in patients with MSI-H cancers.
The NCI-sponsored COMMIT study is testing the addition of atezolizumab (Tecentriq) to the combination of chemotherapy and the targeted therapy bevacizumab (Avastin) , for treating patients that have defective DNA mismatch repair. The hope is that combining drugs that work in different ways will improve treatment results in patients with colorectal cancer.
Another NCI-sponsored trial is studying whether atezolizumab will improve outcomes in people with earlier-stage disease (specifically, stage III colon cancer) that is deficient in DNA mismatch repair. This trial will compare combination chemotherapy with or without atezolizumab.
For people with locally advanced rectal cancer who have MSI-H cancer, one trial is studying the effects of nivolumab and ipilimumab when given together with short-course radiation therapy .
Combining immunotherapy with other treatments for patients without Lynch syndrome
Immune checkpoint inhibitors have been less effective in colorectal cancer patients without Lynch syndrome and whose cancers don't have mismatch repair deficiency . Scientists are currently testing various agents, such as chemotherapy drugs, targeted therapies and viruses, in combination with immune-based therapy to determine whether combining treatments would be effective in killing cancer cells.
Using targeted therapies for metastatic colorectal cancer
Using targeted therapies against genetic mutations that may drive tumor growth is another key area of research for metastatic colorectal cancer. The goal is to find agents that can block the activity of the abnormal proteins produced by these mutations. For example:
- The drug encorafenib (Braftovi ), which targets the BRAF protein, is approved for the treatment of some patients with colorectal cancer . This drug is used in combination with cetuximab (Erbitux) in adults with metastatic colorectal cancer whose tumors have a certain mutation in the BRAF gene and who have already undergone treatment.
- An NCI-supported trial showed that colorectal cancer that contains mutations in the BRAF gene responds to treatment with the drug vemurafenib (Zelboraf) in combination with cetuximab and irinotecan (Camptosar) . Vermurafentib targets mutant B-Raf proteins when combined with these two drugs.
- The NCI-sponsored SOLARIS trial is testing the addition of vitamin D3 to the combination of chemotherapy and bevacizumab for treating patients with metastatic colorectal cancer.
- In January 2023, the Food and Drug Administration (FDA) approved the combination of two targeted drugs, tucatinib (Tukysa) and trastuzumab (Herceptin) for people with advanced colorectal cancer that produces an excess amount of a protein called HER2. (3% or less of people with advanced colorectal cancer have tumors that overexpress this protein.) In the clinical trial that led to the approval, called MOUNTAINEER, more than one third of people who received the drug combination had their tumors shrink or disappear. For another third, tumors stopped growing for some time.
Testing liquid biopsies
Liquid biopsies are a promising new approach being explored to detect, analyze, and track DNA, cells, and other substances shed from tumors into bodily fluids, such as blood and urine. Scientists are testing this method to detect colorectal cancer early, measure treatment responses, identify treatment resistance, and monitor for disease recurrence.
One example is the COBRA trial, which found that testing blood for fragments of genetic material (DNA) shed by tumors , known as circulating tumor DNA (ctDNA), could identify patients with stage IIA colon cancer who might benefit from additional treatment with chemotherapy after surgery.
An ongoing trial is studying ctDNA in people with stage II or III colon cancer. The goal is to determine whether and what type of chemotherapy will benefit patients who have had surgery for their colon cancer based on the presence or absence of ctDNA.
NCI-Supported Research Programs
Many NCI-funded researchers at the NIH campus, and across the United States and world, are seeking ways to address colorectal cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts for colorectal cancer.
- The NCI-supported genetic study, ENLACE, aims to learn more about colorectal cancer in people of Hispanic and Latino descent , with the ultimate goal of improving treatments for this population group. To achieve this, scientists are also testing ways to engage more people from this group in cancer research.
- The Population-based Research to Optimize the Screening PRocess (PROSPR) is an NCI-supported network conducting research to better understand how to improve the entire cancer screening process (recruitment, screening, diagnosis, referral for treatment) for lung, colorectal, and cervical cancer in community healthcare settings.
- Accelerating Colorectal Cancer Screening and Follow-Up Through Implementation Science (ACCIS) is intended to promote research in colorectal cancer screening, follow-up, referral-to-care and best practices for how multilevel interventions can be scaled-up in regions of the United States where screening rates are below national standards.
- Approaches to Identify and Care for Individuals with Inherited Cancer Syndromes are studies designed to increase screening, prevention, and early treatment of people at high risk of cancer due to an inherited genetic susceptibility .
- The NCI-funded Colon Cancer Family Registry (CCFR) has established an international cohort of thousands of colorectal cancer patients, their relatives, and individuals at increased risk of colorectal and other cancers. Over 10,000 families from the United States, Canada, Australia, and New Zealand have been registered. The database includes more than 2,000 individuals with Lynch syndrome, from 781 families.
- The goal of the Screen to Save Initiative , funded by NCI’s Center to Reduce Cancer Health Disparities , is to increase colorectal cancer screening in areas that need it most. Through community health educators, the program provides education and outreach to increase access to resources for those who may be affected by colorectal cancer.
- Dissemination of a Colorectal Cancer Screening Program Across American Indian Communities in the Southern Plains and Southwest United States is an effort to increase the use of colorectal cancer screening tests in American Indians. This project supports research on system-level changes and culturally appropriate media to promote screening, with the goal of closing the gap in colorectal cancer outcomes between the American Indian population and the general US population.
- NCI's Gastrointestinal (GI) SPOREs focus on translational research in the gastrointestinal system. Currently, GI SPOREs focus on cancers of the colon, rectum, esophagus, liver, gastrointestinal stromal tumors (GIST), and pancreas, which account for the majority of new diagnoses.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for colorectal cancer screening , to prevent colon and rectal cancer , and treatment for colon cancer and rectal cancer.
Colorectal Cancer Research Results
The following are some of our latest news articles on colorectal cancer research:
- ctDNA May Guide Who Needs Chemo After Colorectal Cancer Surgery
- ENLACE Study Explores Colorectal Cancer in Hispanic and Latino People
- Is AI Ready to Play a Leading Role in Colorectal Cancer Screening?
- Some People with Rectal Cancer Can Skip Radiation Before Surgery
- How Fatty Liver Disease Helps Cancer Thrive in the Liver
- Study Identifies Potential Warning Signs of Colorectal Cancer in Younger Adults
View the full list of Colorectal Cancer Research Results and Study Updates .

IMAGES
VIDEO
COMMENTS
1. Introduction. Colorectal cancer (CRC), which comprises colon and/or rectum cancer, represents a significant health problem as the world's third most commonly diagnosed and second most fatal cancer globally [].Approximately 9.4% of cancer-related deaths were due to CRC in 2020 [].However, in light of the significant increase in the number of identified cases in the older population, it is ...
Vol. 7 (4): 20-24, Oct-Dec, 2020. Abstract. Colorectal cancer is define by partial suppression of apoptosis. This can g ive advantages to. survival in t umo urs but cause t he ineffective approach ...
1. Introduction. Colorectal cancer (CRC) is the third most commonly diagnosed malignant neoplasm and the second cause of death due to cancer worldwide [1,2,3,4].There are significant variations in CRC incidence and mortality rates among different countries of the world which are based on different factors: gender [], age [6,7,8], and ethnicity [].It imposes a considerable global load in terms ...
cases), followed by breast cancer in women (11.6%) and prostate cancer in men (7.1%). Colorectal cancer (CRC) is third in terms of recognition (6.1%) and second in terms of mortality (9.2%). It is estimated that by the year 2035, the total number of deaths from rectal and colon cancer will increase by 60% and 71.5%, respectively [1]. These figures
A comprehensive framework for early-onset colorectal cancer research. Cathy Eng, Alexandre A Jácome, Rajiv Agarwal, Muhammad Hashim Hayat, Mariana X Byndloss, Andreana N Holowatyj, Christina Bailey, Christopher H Lieu. Sporadic colorectal cancer has traditionally been viewed as a malignancy of older individuals.
Colorectal cancer accounts for approximately 10% of all annually diagnosed cancers and cancer-related deaths worldwide.1 It is the second most common cancer diag-nosed in women and third most in men. In women, incidence and mortality are approximately 25% lower than in men. These rates also vary geographically, with the highest rates seen in ...
The risk of death from colorectal cancer was 0.15% in the invited group and 0.30% in the usual-care group. The estimated risk ratio was 0.50 (95% CI, 0.27 to 0.77) (Fig. S4). In the sen-sitivity ...
Although colonoscopy is widely used as a screening test to detect colorectal cancer, ... (nejmoa2208375_research-summary.pdf) Download; 193.41 KB; Protocol (nejmoa2208375_protocol.pdf) Download;
Colorectal cancer is the third most common cancer worldwide. About 1.4 million new cases of colorectal cancer were recorded globally in 2012, accounting for 10 per cent of all new cases of cancer [2]. Colorectal cancer is the fourth most common cause of death from cancer, estimated to be responsible for almost 700,000 cancer deaths.
Colorectal cancer is one of the most common cancers worldwide. 1 In the United States, 147,000 individuals received a diagnosis of the disease in 2020, and 53,200 died from it. 2 Most patients with colorectal cancer are older than 50 years of age at diagnosis. 2 Men have a higher risk than do women and are on average 5 to 10 years younger than women when they receive the diagnosis. 3,4
Colorectal cancer (CCR) is the third most common cancer worldwide in men and women, the seco nd largest cause of. death related to cancer, and the main cause of death in gastrointestinal cancer ...
Early detection. In a systematic review of 55 studies in a total of 6425 patients younger than 40 years, O'Connell and colleagues, 8. reported that most common presenting symptoms in early-onset colorectal cancer were abdominal pain (55%), rectal bleeding (46%), weight loss (35%), and change in bowel habits (32%).
A total of 89.6% of the participants without any advanced colorectal neoplasia (colorectal cancer or advanced precancerous lesions) identified on colonoscopy had a negative cfDNA blood-based test ...
Despite a significant volume of research in this area it remains uncertain how important time to diagnosis of colorectal cancer is on stage or survival. Our meta-analysis suggests an upper limit ...
Colorectal cancer is the second leading cause of cancer-related mortality worldwide. Numerous pathophysiological mechanisms, such as abnormal cell proliferation, cell differentiation, resistance to apoptosis, invasion of structures adjacent to colorectal tumor cells, and distant metastasis, are involved in colorectal carcinogenesis. These processes are initiated by the complex interaction of a ...
Advances in Colorectal Cancer Research. Colorectal cells grown into organoids, stem cell-derived human 'mini-organs' that are used to study human development and disease. NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat colorectal cancer. They are also looking at what factors influence ...
Abstract Colorectal cancer (CRC) is the second most common cause of cancer death in the United States. ... Surveillance Research, American Cancer Society, 3380 Chastain Meadows Parkway NW, Suite 200, Kennesaw, GA 30144, USA. Email: [email protected] Search for more papers by this author. Nikita Sandeep Wagle MBBS, MHA, PhD, Nikita Sandeep Wagle ...
@article{Alshehri2024MucinousDI, title={Mucinous Differentiation in Colorectal Cancer: A 10-Year Experience Audit at King Faisal Specialist Hospital and Research Centre, Jeddah}, author={Khalid A Alshehri and Naif Alsulaimani and Wejdan A Alghamdi and Zuhoor Almansouri and Syed A Zubair and Jamal Zekri and Haitham Saimeh and Sufian Sultan ...
Colorectal cancer (CRC), which comprises colon and/or rectum cancer, represents a significant health problem as the world's third most commonly diagnosed and second most fatal cancer globally [1]. Approximately 9.4% of cancer-related deaths were due to CRC in 2020 [2]. However, in light of the significant increase in the number of ...
This review article contains a concise consideration of genetic and environmental risk factors for colorectal cancer. Known risk factors associated with colorectal cancer include familial and hereditary factors and lifestyle-related and ecological factors. Lifestyle factors are significant because of the potential for improving our understanding of the disease. Physical inactivity, obesity ...
Colorectal cancer (CRC) stands as a major cause of cancer-related mortality globally, accounting for approximately 881,000 deaths each year. Traditional approaches such as chemotherapy and surgery have been the primary treatment modalities, yet the outcomes for patients with metastatic CRC are often unsatisfactory. Recent research has focused on targeting the pathways involved in oxidative ...
Research Paper on Colon Cancer - Free download as PDF File (.pdf), Text File (.txt) or read online for free. research paper on colon cancer