An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Beneficial effects of green tea: A literature review
Sabu m chacko.
1 NPO International Laboratory of Biochemistry, 1-166 Uchide, Nakagawa-ku, Nagoya, 454-0926, Japan
Priya T Thambi
Ramadasan kuttan.
2 Amala Cancer Research Center, Amala Nagar, Thrissur, Kerala, 680 555, India
Ikuo Nishigaki
The health benefits of green tea for a wide variety of ailments, including different types of cancer, heart disease, and liver disease, were reported. Many of these beneficial effects of green tea are related to its catechin, particularly (-)-epigallocatechin-3-gallate, content. There is evidence from in vitro and animal studies on the underlying mechanisms of green tea catechins and their biological actions. There are also human studies on using green tea catechins to treat metabolic syndrome, such as obesity, type II diabetes, and cardiovascular risk factors.
Long-term consumption of tea catechins could be beneficial against high-fat diet-induced obesity and type II diabetes and could reduce the risk of coronary disease. Further research that conforms to international standards should be performed to monitor the pharmacological and clinical effects of green tea and to elucidate its mechanisms of action.
In recent years, the health benefits [ 1 ] of consuming green tea, including the prevention of cancer [ 2 ] and cardiovascular diseases [ 3 ], the anti-inflammatory [ 4 ], antiarthritic [ 5 ], antibacterial [ 6 ], antiangiogenic [ 7 ], antioxidative [ 8 ], antiviral [ 9 ], neuroprotective [ 10 ], and cholesterol-lowering effects [ 11 ] of green tea and isolated green tea constituents are under investigation. However, adding green tea to the diet may cause other serious health concerns.
The health-promoting effects of green tea are mainly attributed to its polyphenol content [ 12 ], particularly flavanols and flavonols, which represent 30% of fresh leaf dry weight [ 1 ]. Recently, many of the aforementioned beneficial effects of green tea were attributed to its most abundant catechin, (-)-epigallocatechin-3-gallate (EGCG) [ 13 - 15 ]. Green tea extracts are more stable than pure epigallocatechin gallate, one of the major constituents of green tea, because of the presence of other antioxidant constituents in the extract [ 8 ]. In general, herbal medicines are complex mixtures of different compounds that often act in a synergistic fashion to exert their full beneficial effect [ 11 ]. However, relatively few herbal medicines have been well characterized and their efficacy demonstrated in systematic clinical trials as compared to Western drugs. This review article highlights the recent research on the efficacy, action mechanisms, and side effects of green tea and its catechins in in vitro , in vivo , and ex vivo systems [ 16 ].
The review on green tea and its catechins focused on language literature in English. The literature search was conducted in the following databases: Pubmed (1980-2009), EMBASE (1980-2009), Allied and complementary Medicine Database (AMED, 1985-2009) and China Journals Full Text Database (1975-2009). The keywords used were selected from the following terms: green tea, catechins, anticancer, diabetes, polyphenols, in vivo studies, general pharmacology and toxicology. The health benefits and adverse effects of green tea and its catechins were reviewed.
The authors read full articles and reached consensus after discussion. Articles included in the study covered the following effects of green tea: (1) the health benefits in humans and animals, (2) absorption of metal ions and drug-metabolizing enzymes, (3) antioxidation and inhibition of oxidative stress, (4) carbohydrate metabolism and diabetes mellitus, and (5) adverse effects. A total of 105 peer-reviewed papers in English were selected for this review.
Tea is one of the most popular beverages consumed worldwide. Tea, from the plant Camellia sinensis , is consumed in different parts of the world as green, black, or Oolong tea. Among all of these, however, the most significant effects on human health have been observed with the consumption of green tea [ 17 ]. The first green tea was exported from India to Japan during the 17th century. It is estimated that about 2.5 million tons of tea leaves are produced each year throughout the world, with 20% produced as green tea, which is mainly consumed in Asia, some parts of North Africa, the United States, and Europe [ 18 ]. The association between tea consumption, especially green tea, and human health has long been appreciated [ 19 , 20 ]. Green tea and black tea are processed differently during manufacturing. To produce green tea, freshly harvested leaves are immediately steamed to prevent fermentation, yielding a dry, stable product. This steaming process destroys the enzymes responsible for breaking down the color pigments in the leaves and allows the tea to maintain its green color during the subsequent rolling and drying processes. These processes preserve natural polyphenols with respect to the health-promoting properties. As green tea is fermented to Oolong and then to black tea, polyphenol compounds (catechins) in green tea are dimerized to form a variety of theaflavins, such that these teas may have different biological activities.
Green tea composition
The chemical composition of green tea is complex: proteins (15-20% dry weight), whose enzymes constitute an important fraction; amino acids (1-4% dry weight) such as theanine or 5- N- ethylglutamine, glutamic acid, tryptophan, glycine, serine, aspartic acid, tyrosine, valine, leucine, threonine, arginine, and lysine; carbohydrates (5-7% dry weight) such as cellulose, pectins, glucose, fructose, and sucrose; minerals and trace elements (5% dry weight) such as calcium, magnesium, chromium, manganese, iron, copper, zinc, molybdenum, selenium, sodium, phosphorus, cobalt, strontium, nickel, potassium, fluorine, and aluminum; and trace amounts of lipids (linoleic and α-linolenic acids), sterols (stigmasterol), vitamins (B, C, E), xanthic bases (caffeine, theophylline), pigments (chlorophyll, carotenoids), and volatile compounds (aldehydes, alcohols, esters, lactones, hydrocarbons). Due to the great importance of the mineral presence in tea, many studies have determined their levels in tea leaves and their infusions (Table (Table1) 1 ) [ 21 ]. Fresh leaves contain, on average, 3-4% of alkaloids known as methylxanthines, such as caffeine, theobromine, and theophylline [ 22 ]. In addition, there are phenolic acids such as gallic acids and characteristic amino acid such as theanine present [ 22 ].
Composition (%) of green tea, black tea, and black tea infusion [ 21 ]
* Data refer to dry weight of tea leaves.
† Infusion time: 3 minutes
‡ Especially flavonoids
§ Especially thearubigins and theaflavins
Green tea contains polyphenols, which include flavanols, flavandiols, flavonoids, and phenolic acids; these compounds may account for up to 30% of the dry weight. Most of the green tea polyphenols (GTPs) are flavonols, commonly known as catechins. Products derived from green tea are mainly extracts of green tea in liquid or powder form that vary in the proportion of polyphenols (45-90%) and caffeine content (0.4-10%). The major flavonoids of green tea are various catechins, which are found in greater amounts in green tea than in black or Oolong tea [ 23 ]. There are four kinds of catechins mainly find in green tea: epicatechin, epigallocatechin, epicatechin-3-gallate, and EGCG [ 24 ]. The preparation methods influence the catechins both quantitatively and qualitatively; the amount of catechins also varies in the original tea leaves due to differences in variety, origin, and growing conditions [ 25 ]. The preparation of fresh green tea cannot totally extract catechins from the leaves; therefore, the concentration found differs from the absolute values determined through the complete extraction of leaves [ 26 ]. Moreover, catechins are relatively unstable and could be quantitatively and qualitatively modified during the time frame of an experiment [ 27 , 28 ]. Thus, comparison of ingested doses in animal studies is not possible because the catechin quantification before administration is often not known.
Health benefits of green tea in humans and animals
Studies using animal models show that green tea catechins provide some protection against degenerative diseases [ 29 ]. Some studies indicated that green tea has an antiproliferative activity on hepatoma cells and a hypolipidemic activity in hepatoma-treated rats, as well as the prevention of hepatoxicity [ 29 ] and as a preventive agent against mammary cancer post-initiation [ 29 ]. Green tea catechins could also act as antitumorigenic agents [ 30 ] and as immune modulators in immunodysfunction caused by transplanted tumors or by carcinogen treatment [ 29 ]. Moreover, green tea, its extract, and its isolated constituents were also found to be effective in preventing oxidative stress [ 31 ] and neurological problems [ 32 ].
Green tea consumption has also been linked to the prevention of many types of cancer, including lung, colon, esophagus, mouth, stomach, small intestine, kidney, pancreas, and mammary glands [ 33 ]. Several epidemiological studies and clinical trials showed that green tea (and black and Oolong teas to a lesser extent) may reduce the risk of many chronic diseases [ 34 ]. This beneficial effect has been attributed to the presence of high amounts of polyphenols, which are potent antioxidants. In particular, green tea may lower blood pressure and thus reduce the risk of stroke and coronary heart disease. Some animal's studies suggested that green tea might protect against the development of coronary heart disease by reducing blood glucose levels and body weight [ 35 ]. However, all these data are based on middle-aged animals' populations, not the elderly populations, which nutritional status tends to be more adversely influenced by age-related biological and socioeconomic factors [ 36 ].
Tea components possess antioxidant, antimutagenic, and anticarcinogenic effects and could protect humans against the risk of cancer by environmental agents [ 37 ]. Sano et al . [ 38 ] reported the inhibitory effects of green tea leaves against tert-butyl hydroperoxide-induced lipid peroxidation, and a similar antioxidant effect on the kidney was observed after oral administration of the major tea polyphenol EGCG. The antioxidative potency of crude catechin powder and individual catechins was tested in experiments using the active oxygen method. Crude catechins reduced the formation of peroxides far more effectively than dl-α-tocopherol [ 39 ]. Shim et al . [ 40 ] studied the chemopreventive effect of green tea among cigarette smokers and found that it can block the cigarette-induced increase in sister chromatid exchange frequency.
The effectiveness of green tea in treating any type of diarrhea and typhoid has been known in Asia since ancient times [ 41 - 43 ]. Green tea catechins have an inhibitory effect on Helicobacter pylori infection [ 44 , 45 ]. Effects of green tea against the influenza virus, especially in its earliest stage, as well as against the Herpes simplex virus have also been demonstrated [ 46 - 48 ]. Furthermore, Weber et al . [ 9 ] observed that adenovirus infection is inhibited in vitro by green tea catechins.
In humans, Hirasawa and Takada [ 49 ] studied the antifungal activity of green tea catechins against Candida albicans and the convenience of a combined treatment with catechins and lower doses of antimycotics, which may help to avoid the side effects of antimycotics. Green tea consumption has also been associated with increased bone mineral density, and it has been identified as an independent factor protecting against the risk of hip fractures; this effect was considered independent of smoking status, hormone replacement therapy, coffee drinking, and the addition of milk to tea [ 50 ]. Park et al . [ 51 ] observed the positive effects of green tea extracts and GTPs on the proliferation and activity of bone cells. The proliferation of hepatic stellate cells is closely related to the progression of liver fibrosis in chronic liver diseases, and EGCG has a potential inhibitory effect on the proliferation of these cells [ 52 , 53 ]. Green tea strengthens the immune system action because it protects it against oxidants and radicals. Recent studies suggested that GTPs might protect against Parkinson's and Alzheimer's diseases and other neurodegenerative diseases [ 10 , 54 ]. Studies have demonstrated GTP neuroprotectant activity in cell cultures and animal models, such as the prevention of neurotoxin-induced cell injury [ 54 ]. Green tea is considered to be useful for insect stings due mainly to its anti-inflammatory effects and its capacity to stop bleeding [ 55 , 56 ]. Some studies have suggested an inverse association between green tea consumption and the risk of kidney stone formation [ 41 , 57 ]. In an experimental cataractogenesis system, green tea acted by preserving the antioxidant defense system of the lens [ 58 ]. Skrzydlewska et al . [ 59 ] indicated a beneficial effect of green tea in alcohol intoxication. In addition to all of these reported properties, which have helped the recognition of green tea as functional food by some authors [ 60 ], green tea is also currently used in the preparation of a variety of foods, pharmaceutical preparations, dentifrices, and cosmetics [ 61 ].
Tea has been shown anticarcinogenic effects against breast cancer in experimental studies [ 62 ]. However, epidemiologic evidence that tea protects against breast cancer has been inconsistent [ 62 ]. A case-control study was conducted in southeastern China between 2004 and 2005 [ 63 ]. The incidence cases were 1009 female patients aged 20-87 years with histologically confirmed breast cancer, and the 1009 age-matched controls were healthy women randomly recruited from breast disease clinics. Information on duration, frequency, quantity, preparation, and type of tea consumption as well as diet and lifestyle were collected by face-to-face interviews using a validated and reliable questionnaire. In comparison with non-tea drinkers, green tea drinkers tended to reside in urban settings, to have more education, and to consume more coffee, alcohol, soy, vegetables, and fruits. After adjusting established and potential confounding factors, green tea consumption was associated with a reduced risk of breast cancer. Similar dose-response relationships were observed for duration of drinking green tea, number of cups consumed, and new batches prepared per day.
Hsu et al . [ 64 ] demonstrated the effects of supplementation with decaffeinated green tea extract (catechins) on hemodialysis-induced reactive oxygen species, atherosclerotic disease risk factors, and proinflammatory cytokines. The pharmacokinetics of one oral dose of catechins was compared between healthy subjects and hemodialysis patients. The authors compared the antioxidant effects of three different doses (0, 455, and 910 mg) of oral catechins with that of oral vitamin C (500 mg) during a hemodialysis session. In patients, catechin supplementation reduced hemodialysis-enhanced plasma hypochlorous acid activity more effectively than did placebo or vitamin C. Between the treatments with 455 and 910 mg catechins, no significant difference was found in the reduction of plasma hypochlorous acid activity. Catechins also significantly reduced proinflammatory cytokine expression enhanced by hemodialysis.
Effects on absorption of metal ions
Tea catechins can affect iron absorption, particularly in groups at risk of iron deficiency [ 65 , 66 ], but their effects on other ions are poorly understood. Green tea ingestion over a long period does not affect the apparent absorption of copper, whereas it decreases that of zinc and increases that of manganese [ 67 ]. However, catechin intake does not affect the plasma concentration of these ions [ 68 ]. Green tea catechins have the potential to affect absorption and metabolism of ions because flavonoids interact with a variety of metal ions [ 69 ].
Effects on drug-metabolizing enzymes
Long-term ingestion of green tea increases UDP-glucuronosyl transferase activity in rats [ 66 , 70 , 71 ], and after being absorbed, catechins are metabolized by drug-metabolizing enzymes in various organs [ 72 , 73 ]. Thus, the increased glucuronidation through UDP-glucuronosyl transferase induction is postulated to contribute to the anticarcinogenic effect of green tea by facilitating the metabolism of chemical carcinogens into inactive products that are readily excreted. The interaction between 2-amino-3-methylimidazol (4,5-f)quinoline (IQ) and green tea catechin metabolism was examined [ 74 ]. IQ is a precarcinogen that was originally detected in an extract of fried meat. The major route of IQ biotransformation in rats is cytochrome P450 in the first step, followed by conjugation to a sulfate and a glucuronide conjugate. Green tea modifies IQ metabolism in rats, increasing the formation of IQ glucuronides, which are then excreted in the urine. Moreover, protection against cancers induced by polycyclic aromatic hydrocarbons by green tea catechins may be due to the inhibition of their cytochrome P450 metabolism, but the effect of green tea on cytochrome P450 enzymes depends on the particular form. The long-term consumption of green tea increases cytochrome P450 1A1 and 1A2 activities, but not 2B1 and 2E1 activities, in normal rats. However, it is difficult to draw conclusions about a beneficial effect of green tea against carcinogens involving only modulation of this metabolic pathway.
Effects on antioxidant markers and oxidative stress
Green tea is a popular neutraceutical as an antioxidant. Antioxidants are compounds that protect cells against the damaging effects of reactive oxygen species, such as singlet oxygen, superoxide, peroxyl radicals, hydroxyl radicals, and peroxynitrite. An imbalance between antioxidants and reactive oxygen species results in oxidative stress, leading to cellular damage [ 75 ]. Catechins are hypothesized to help protect against these diseases by contributing, along with antioxidant vitamins (i.e., vitamins C and E) and enzymes (i.e., superoxide dismutase and catalase), to the total antioxidant defense system [ 76 ].
In vivo studies showed that green tea catechins increase total plasma antioxidant activity [ 77 , 78 ]. Intake of green tea extracts also increases the activity of superoxide dismutase in serum and the expression of catalase in the aorta; these enzymes are implicated in cellular protection against reactive oxygen species [ 78 , 79 ]. This action is combined with direct action on oxygen species by a decrease in the nitric oxide plasma concentration [ 80 ]. Malondialdehyde, a marker of oxidative stress, also decreases after green tea intake [ 77 , 80 ]. These results suggest that catechins could have a direct (antioxidant) or indirect (increase of activity or expression) effect. Since catechins can act as antioxidants in vitro , they might prevent the oxidation of other antioxidants, such as vitamin E. However, ingestion of green tea catechins does not modify the plasma status of vitamins E and C in vivo [ 78 , 81 , 82 ]. Nevertheless, one study reported that catechins increase vitamin E concentration in low-density lipoprotein [ 81 ] and in this way could protect low-density lipoprotein against peroxidation [ 77 ].
Pilipenko et al . [ 83 ] assessed the tolerance of tableted green tea and its effect on the antioxidant status indices. Twenty-five patients with different gastrointestinal pathologies were included in the study and divided into treatment and control groups. The tolerance of tableted green tea was good in the treatment group, who showed better dynamics of quality-of-life indices, especially in scales of body pain and social functioning. There were no significant differences in biochemical analysis between the groups, which may indicate the safety of this product. Analysis revealed that the treatment group showed a decreased level of all antioxidant status indices, as reflected in a significant decreasing of the lipid peroxidation index from 4.63 to 4.14.
Effects on carbohydrate metabolism
Type II diabetes is a heterogeneous disorder that involves resistance of glucose and lipid metabolism in peripheral tissues to the biological activity of insulin and inadequate insulin secretion by pancreatic β cells [ 84 ]. Animal models of diabetes are available: Zucker rats, which are genetically obese; injection of streptozotocin or alloxan, which destroys pancreatic β cells; and treatment with sucrose-rich diets, which induces obesity and insulin resistance.
In a study by Sabu et al . [ 85 ], administration of GTPs (500 mg/kg) to normal rats increased glucose tolerance significantly at 60 minutes. GTPs were also found to reduce significantly serum glucose levels in alloxan diabetic rats at a dose of 100 mg/kg. Continued daily administration (15 days) of the extract at 50 or 100 mg/kg produced 29% and 44% reduction, respectively, in the elevated serum glucose level produced by alloxan administration. Elevated hepatic and renal enzymes produced by alloxan were found to be reduced significantly by GTPs. The serum lipid peroxidation level was increased by alloxan and reduced significantly by the administration of 100 mg/kg of GTPs. Decreased liver glycogen resulting from alloxan administration showed a significant increase after GTP treatment. The GTP-treated group showed increased antioxidant potential, as seen from improvements in superoxide dismutase and glutathione levels. However, catalase, lipid peroxidation, and glutathione peroxidase levels were unchanged. These results indicate that alterations in the glucose utilizing system and oxidation status in rats that were increased by alloxan were partially reversed by the administration of GTPs [ 85 ].
Catechins also reduced plasma triglyceride levels in an oral glucose-tolerance test in normal rats [ 86 ]. Green tea extract intake reduced these values in both Zucker rats and rats fed a sucrose-rich diet [ 87 , 88 ]. Several human- and animal-based studies suggested that green tea and its flavonoids have antidiabetic effects [ 86 , 89 , 90 ]. Green tea flavonoids were also shown to have insulin-like activities [ 91 ] as well as insulin-enhancing activity [ 92 ].
The antihyperglycemic effect of black tea was reported by Gomes et al . [ 93 ]. EGCG was found to inhibit intestinal glucose uptake by the sodium-dependent glucose transporter SGLT1, indicating its increase in controlling blood sugar [ 94 ]. Streptozotocin diabetic rats showed increased sensitivity to platelet aggregation and thrombosis, and this abnormality could be improved by dietary catechins from green tea [ 95 , 96 ]. Alloxan produces oxygen radicals in the body, which cause pancreatic injury [ 75 ] and are responsible for increased blood sugar.
Under in vivo conditions, glutathione acts as an antioxidant, and its decrease was reported in a diabetes mellitus model [ 97 ]. The increased glutathione content in the liver of the rats treated with GTPs may be one of the factors responsible for the inhibition of lipid peroxidation. Superoxide dismutase and catalase are the two major scavenging enzymes that remove the toxic free radicals in vivo . Vucic et al . [ 98 ] reported that the activity of superoxide dismutase is low in diabetes mellitus.
The Mediterranean Islands (MEDIS) epidemiological study is a cross-sectional health and nutrition survey that aims to evaluate the association between various sociodemographic, bioclinical, dietary, and other lifestyle habits and the prevalence of the common cardiovascular disease risk factors (i.e., hypertension, dyslipidemia, diabetes, and obesity) among elderly people without a history of any chronic disease and living in the Mediterranean islands. Because data relating tea consumption with clinical characteristics are lacking in elderly populations, in the context of the MEDIS study, the authors sought to evaluate whether green tea consumption is independently associated with fasting blood glucose levels and the prevalence of type II diabetes mellitus [ 99 ]. An earlier study was aimed at providing evidence of improvement in glucose metabolism in diabetic mice and healthy humans upon green tea consumption [ 35 ]. Green tea promoted glucose metabolism in healthy human volunteers at 1.5 g/kg as shown in oral glucose-tolerance tests. Green tea also lowered blood glucose levels in diabetic db+/db+ mice and streptozotocin-diabetic mice two to six hours after administration at 300 mg/kg without affecting serum insulin level, whereas no effect was observed in control mice (+m/+m and normal ddY mice).
Effect of EGCG on diabetes
A study by Waltner-Law et al . [ 91 ] provided compelling in vitro evidence that EGCG decreases glucose production of H4IIE rat hepatoma cells. The investigators showed that EGCG mimics insulin, increases tyrosine phosphorylation of the insulin receptor and the insulin receptor substrate, and reduces gene expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase. Recently, green tea and green tea extracts were demonstrated to modify glucose metabolism beneficially in experimental models of type II diabetes mellitus [ 35 , 100 ]. In addition, EGCG ameliorates cytokine-induced β cell damage in vitro [ 101 ] and prevents the decrease of islet mass induced by treatment with multiple low doses of streptozotocin in vivo [ 102 ].
Lambert et al . [ 103 ] showed that intragastric administration of EGCG at a dose of 75 mg/kg resulted in a Cmax of 128 mg/l total plasma EGCG and a terminal half-life of 83 minutes. Furthermore, in humans an oral intake of EGCG at a dose of 50 mg (0.7 mg/kg) resulted in a Cmax of 130 mg/l total plasma EGCG and a terminal half-life of 112 minutes [ 104 ]. These results indicate that rodents must be orally administered 100- to 600-fold more EGCG (depending on whether they are administered by gavage or by feed admixture) to achieve similar plasma concentrations as those found in humans. Total plasma EGCG concentrations shown to be efficacious in mice and rats can be reached by an intake of low to moderate doses of EGCG in humans.
Effect on obesity
The effects of tea on obesity and diabetes have received increasing attention. Tea catechins, especially EGCG, appear to have antiobesity and antidiabetic effects [ 105 ]. African black tea extract has been shown to suppress the elevation of blood glucose during food intake and reduce the body weight in KK-A(y)/TaJcl diabetic mice [ 106 ]. Although few epidemiological and clinical studies have shown the health benefits of EGCG on obesity and diabetes, the mechanisms of its actions are emerging based on various laboratory data. These mechanisms may be related to certain pathways, such as through the modulations of energy balance, endocrine systems, food intake, lipid and carbohydrate metabolism, and redox status [ 88 ].
A double-blind, placebo-controlled, cross-over design study showed that consumption of a beverage containing green tea catechins, caffeine, and calcium increases 24-h energy expenditure by 4.6%, but the contribution of the individual ingredients could not be distinguished. It was suggested that such modifications were sufficient to prevent weight gain. It has been reported that the body weights of rats and their plasma triglyceride, cholesterol, and low-density lipoprotein cholesterol were significantly reduced by feedings of Oolong, black, and green tea leaves to the animals. In addition, the inhibition of growth and suppression of lipogenesis in MCF-7 breast cancer cells may be through down-regulation of fatty acid synthase gene expression in the nucleus and stimulation of cell energy expenditure in the mitochondria [ 107 , 108 ]. When fed to mice, EGCG purified from green tea decreased diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation [ 109 ]. The increased and prolonged sympathetic stimulation of thermogenesis by the interaction between polyphenols and caffeine could be of value in assisting the management of obesity [ 110 ].
Recent data from human studies indicate that the consumption of green tea and green tea extracts may help reduce body weight, mainly body fat, by increasing postprandial thermogenesis and fat oxidation. In a randomized, double-blind, placebo-controlled, cross-over pilot study, six overweight men were given 300 mg EGCG per day for two days. Fasting and postprandial changes in energy expenditure and substrate oxidation were assessed. Resting energy expenditure did not differ significantly between EGCG and placebo treatments, although during the first postprandial monitoring phase, respiratory quotient values were significantly lower with EGCG treatment compared to the placebo. These findings suggest that EGCG alone has the potential to increase fat oxidation in men and may thereby contribute to the antiobesity effects of green tea. However, more studies with a greater sample size and a broader range of age and body mass index are needed to define the optimal dose [ 111 ].
Adverse effects of green tea
Although green tea has several beneficial effects on health, the effects of green tea and its constituents may be beneficial up to a certain dose yet higher doses may cause some unknown adverse effects. Moreover, the effects of green tea catechins may not be similar in all individuals. EGCG of green tea extract is cytotoxic, and higher consumption of green tea can exert acute cytotoxicity in liver cells, a major metabolic organ in the body [ 112 ]. Another study found that higher intake of green tea might cause oxidative DNA damage of hamster pancreas and liver [ 113 ]. Yun et al . [ 114 ] clarified that EGCG acts as a pro-oxidant, rather than an antioxidant, in pancreatic β cells in vivo . Therefore, high intake of green tea may be detrimental for diabetic animals to control hyperglycemia. At a high dose (5% of diet for 13 wk), green tea extract induced a thyroid enlargement (goiter) in normal rats [ 115 , 116 ]. This high-level treatment modified the plasma concentrations of the thyroid hormones. However, drinking even a very high dietary amount of green tea would be unlikely to cause these adverse effects in humans.
Harmful effects of tea overconsumption (black or green) are due to three main factors: (1) its caffeine content, (2) the presence of aluminum, and (3) the effects of tea polyphenols on iron bioavailability. Green tea should not be taken by patients suffering from heart conditions or major cardiovascular problems. Pregnant and breast-feeding women should drink no more than one or two cups per day, because caffeine can cause an increase in heart rhythm. It is also important to control the concomitant consumption of green tea and some drugs, due to caffeine's diuretic effects [ 117 ]. Some studies revealed the capacity of tea plants to accumulate high levels of aluminum. This aspect is important for patients with renal failure because aluminum can be accumulated by the body, resulting in neurological diseases; it is therefore necessary to control the intake of food with high amounts of this metal [ 118 ]. Likewise, green tea catechins may have an affinity for iron, and green tea infusions can cause a significant decrease of the iron bioavailability from the diet [ 119 ].
Conclusions
Laboratory studies showed the health effects of green tea. As the human clinical evidence is still limited, future research needs to define the actual magnitude of health benefits, establishes the safe range of tea consumption associated with these benefits, and elucidates the mechanisms of action. Development of more specific and sensitive methods with more representative models along with the development of good predictive biomarkers will give a better understanding of how green tea interacts with endogenous systems and other exogenous factors. Definitive conclusions concerning the protective effect of green tea have to come from well-designed observational epidemiological studies and intervention trials. The development of biomarkers for green tea consumption, as well as molecular markers for its biological effects, will facilitate future research in this area.
Abbreviations
EGCG: epigallocatechin-3-gallate; GTPs: green tea polyphenols; UDP: Uridine di-phospatase; IQ: 2-amino-3-methylimidazol (4,5-f)quinoline; MEDIS: Mediterranean Islands; SDLT: Sodium dependent glucose transporter; AMED: Allied and complementary Medicine Database.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SMC and PTT did the literature search and drafted the manuscript. RK and IN critically reviewed the literature and revised the manuscript. All authors read and approved the final version of the manuscript.
- McKay DL, Blumberg JB. The role of tea in human health: An update. J Am Coll Nutr. 2002; 21 :1–13. [ PubMed ] [ Google Scholar ]
- Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001; 82 :387–398. doi: 10.1002/jcb.1164. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sueoka N, Suganuma M, Sueoka E, Okabe S, Matsuyama S, Imai K, Nakachi K, Fujiki H. A new function of green tea: prevention of lifestyle-related diseases. Ann N Y Acad Sci. 2001; 928 :274–280. [ PubMed ] [ Google Scholar ]
- Dona M, Dell'Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003; 170 :4335–4341. [ PubMed ] [ Google Scholar ]
- Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci USA. 1999; 96 :4524–4529. doi: 10.1073/pnas.96.8.4524. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sudano Roccaro A, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother. 2004; 48 :1968–1973. doi: 10.1128/AAC.48.6.1968-1973.2004. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002; 132 :2307–2311. [ PubMed ] [ Google Scholar ]
- Osada K, Takahashi M, Hoshina S, Nakamura M, Nakamura S, Sugano M. Tea catechins inhibit cholesterol oxidation accompanying oxidation of low density lipoprotein in vitro . Comp Biochem Physiol Part C Toxicol Pharmacol. 2001; 128 :153–164. doi: 10.1016/S1532-0456(00)00192-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003; 58 :167–173. doi: 10.1016/S0166-3542(02)00212-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Weinreb O, Mandel S, Amit T, Youdim MBH. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J Nutr Biochem. 2004; 15 :506–516. doi: 10.1016/j.jnutbio.2004.05.002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003; 14 :326–332. doi: 10.1016/S0955-2863(03)00054-8. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007; 81 :519–533. doi: 10.1016/j.lfs.2007.06.011. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004; 62 :204–211. doi: 10.1111/j.1753-4887.2004.tb00041.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol(-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004; 88 :1555–1569. [ PubMed ] [ Google Scholar ]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003; 43 :89–143. doi: 10.1080/10408690390826464. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008; 22 (7):851–858. doi: 10.1002/ptr.2384. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea: a review. J Am Coll Nutr. 2006; 25 :79–99. [ PubMed ] [ Google Scholar ]
- Japanese Green Tea Online.com. http://www.japanesegreenteaonline.com
- Weisburger JH. Approaches for chronic disease prevention based on current understanding of underlying mechanisms. Am J Clin Nutr. 2000; 71 (6):1710S–1714S. [ PubMed ] [ Google Scholar ]
- Sato T, Miyata G. The nutraceutical benefit, part I: green tea. Nutrition. 2000; 16 :315–317. doi: 10.1016/S0899-9007(99)00301-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Belitz DH, Grosch W. Quı'mica de los Alimentos. Zaragoza: Acribia; 1997. [ Google Scholar ]
- Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992; 21 :334–350. doi: 10.1016/0091-7435(92)90041-F. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vinson JA. Black and green tea and heart disease: a review. Biofactors. 2000; 13 :127–132. doi: 10.1002/biof.5520130121. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sano M, Tabata M, Suzuki M, Degawa M, Miyase T, Maeda-Yamamoto M. Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst. 2001; 126 :816–820. doi: 10.1039/b102541b. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khokhar S, Magnusdottir SGM. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 2002; 50 :565–570. doi: 10.1021/jf010153l. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fernandez PL, Martin MJ, Gonzalez AG, Pablos F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst. 2000; 125 :421–425. doi: 10.1039/a909219f. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen ZY, Zhu QY, Wong YF, Zhang Z, Chung HY. Stabilizing effect of ascorbic acid on green tea catechins. J Agr Food Chem. 1998; 46 :2512–2516. doi: 10.1021/jf971022g. [ CrossRef ] [ Google Scholar ]
- Chen ZY, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agr Food Chem. 2001; 49 :477–482. doi: 10.1021/jf000877h. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vanessa C, Gary W. A Review of the Health Effects of Green Tea Catechins in In Vivo Animal Models. J Nutr. 2004; 134 :3431S–3440S. [ PubMed ] [ Google Scholar ]
- Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, RathIn M. In vitro and in vivo antitumorigenic activity of a mixture of lysine, proline, ascorbic acid, and green tea extract on human breast cancer lines MDA-MB-231 and MCF-7. Medical Oncol. 2007; 22 (2):129–138. doi: 10.1385/MO:22:2:129. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Babu PV, Sabitha KE, Shyamaladevi CS. Therapeutic effect of green tea extract on oxidative stress in aorta and heart of streptozotocin diabetic rats. Chem Biol Interact. 2006; 162 :114–120. doi: 10.1016/j.cbi.2006.04.009. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Unno K, Takabayashi F, Yoshida H, Choba D, Fukutomi R, Kikunaga N, Kishido T, Oku N, Hoshino M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology. 2007; 8 (2):89–95. doi: 10.1007/s10522-006-9036-8. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koo MWL, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004; 500 :177–185. doi: 10.1016/j.ejphar.2004.07.023. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006; 78 :2073–2080. doi: 10.1016/j.lfs.2005.12.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004; 4 :18–21. doi: 10.1186/1471-2210-4-18. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meydani M. Nutrition interventions in aging and age associated disease. Ann N Y Acad Sci. 2001; 928 :226–235. [ PubMed ] [ Google Scholar ]
- Mukhtar H, Wang ZY, Katlya SK, Agarwal R. Tea components: antimutagenic and anticarcinogenic effects. Prev Med. 1992; 21 :351–360. doi: 10.1016/0091-7435(92)90042-G. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sano M, Takahashi Y, Yoshino K, Shimoi K, Nakamura Y, Tomita I, Oguni I, Konomoto H. Effect of tea ( Camellia sinensis L.) on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol Pharm Bull. 1995; 18 :1006–1008. [ PubMed ] [ Google Scholar ]
- Hara Y. Nippon Shokuhin Kogyo. Tokyo: Gakkai: Korin; 1990. Advances in Food Science and Technology. [ Google Scholar ]
- Shim JS, Kang MH, Kim YH, Roh JK, Roberts C, Lee IP. Chemopreventive effect of green tea ( Camellia sinensis ) among cigarette smokers. Cancer Epidemiol Biomarkers. 1995; 4 :387–391. [ PubMed ] [ Google Scholar ]
- McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002; 21 :1–13. [ PubMed ] [ Google Scholar ]
- Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003; 31 :452–461. doi: 10.1124/dmd.31.4.452. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res. 2003; 11 :1088–1095. doi: 10.1038/oby.2003.149. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takabayashi F, Harada N, Yamada M, Murohisa B, Oguni I. Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 2004; 39 :61–63. doi: 10.1007/s00535-003-1246-0. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yee YK, Koo MWL, Szeto ML. Chinese tea consumption and lower risk of Helicobacter infection. J Gastroenterol Hepatol. 2002; 17 :552–555. doi: 10.1046/j.1440-1746.2002.02718.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Toda M, Okubo S, Ohnishi R, Shimamura T. Antibacterial and bactericidal activities of Japanese green tea. Nippon Saikingaku Zasshi. 1989; 44 :669–672. [ PubMed ] [ Google Scholar ]
- Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, Shimamura T. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn J Med Sci Biol. 1991; 44 :181–186. [ PubMed ] [ Google Scholar ]
- Yama TS, Shaha S, Hamilton-Millera JMT. Microbiological activity of whole and fractionated crude extracts of tea ( Camellia sinensis ), and of tea components. FEMS Microbiol Lett. 1997; 152 :169–174. doi: 10.1111/j.1574-6968.1997.tb10424.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hirasawa M, Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans . J Antimicrob Chemother. 2004; 53 :225–229. doi: 10.1093/jac/dkh046. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Muraki S, Yamamoto S, Ishibashi H, Horiuchi T, Hosoi T, Suzuki T, Orimo H, Nakamura K. Green tea drinking is associated with increased bone mineral density. J Bone Miner Res. 2003; 18 :S241. [ Google Scholar ]
- Park H, Ko S, Kim J, Kim S. Effects of green tea extracts and polyphenols on the proliferation and activity of bone cells. J Bone Miner Res. 2003; 18 :S342. [ Google Scholar ]
- Dorchies OM, Wagner S, Waldhauser KM, Buetler TM, Ruegg UT. Anti-fibrotic properties of green tea catechins on mouse muscle cell cultures. Neuromuscul Disord. 2003; 13 :639. [ Google Scholar ]
- Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M. Green tea polyphenols epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol. 2004; 40 :52–59. doi: 10.1016/S0168-8278(03)00477-X. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pan TH, Jankovic J, Le WD. Potential therapeutic properties of green tea polyphenols in Parkinson's disease. Drugs Aging. 2003; 20 :711–721. doi: 10.2165/00002512-200320100-00001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sagesaka-Mitane Y, Miwa M, Okada S. Platelet aggregation inhibitors in middle aged Japanese men and women. Ann Epidemiol. 1998; 7 :280–284. [ Google Scholar ]
- Dvorakova K, Dorr RT, Valcic S, Timmermann B, Alberts DS. Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother Pharmacol. 1999; 43 :331–335. doi: 10.1007/s002800050903. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ishizuk H, Eguchi H, Oda T, Ogawa S, Nakagawa K, Honjo S, Kono S. Relation of coffee, green tea, and caffeine intake to gallstone disease in middle-age Japanese men. Eur J Epidemiol. 2003; 18 :401–405. doi: 10.1023/A:1024237927985. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gupta SK, Halder N, Srivastava S, Trivedi D, Joshi S, Varma SD. Green tea ( Camellia sinensis ) protects against selenite-induced oxidative stress in experimental cataractogenesis. Ophthalmic Res. 2002; 34 :258–263. doi: 10.1159/000063881. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Skrzydlewska E, Ostrowska J, Stankiewicz A, Farbiszewski R. Green tea as a potent antioxidant in alcohol intoxication. Addict Biol. 2002; 7 :307–314. doi: 10.1080/13556210220139523. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ferrari CKB, Torres EAFS. Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed Pharmacother. 2003; 57 :251–260. doi: 10.1016/S0753-3322(03)00032-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003; 17 :987–1000. doi: 10.1002/ptr.1363. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Min Zhang C, D'Arcy JH, Jiang-ping H, Xing X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2005; 28 (5):1074–1078. doi: 10.1093/carcin/bgl252. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang M, Holman CDAJ, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in southeast China. Carcinogenesis. 2008; 29 (8):1594–1600. doi: 10.1093/carcin/bgn129. [ CrossRef ] [ Google Scholar ]
- Hsu SP, Wu MS, Yang CC, Huang KC, Liou SY, Hsu SM, Chien CT. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007; 86 (5):1539–1547. [ PubMed ] [ Google Scholar ]
- Samman S, Sandstrom B, Toft MB, Bukhave K, Jensen M, Sorensen SS, Hansen M. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001; 73 :607–612. [ PubMed ] [ Google Scholar ]
- Nelson M, Poulter J. Impact of tea drinking on iron status in the UK: a review. J Hum Nutr Diet. 2004; 17 :43–54. doi: 10.1046/j.1365-277X.2003.00497.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deng Z, Tao B, Li X, He J, Chen Y. Effect of green tea and black tea on the metabolisms of mineral elements in old rats. Biol Trace Elem Res. 1998; 65 :75–86. doi: 10.1007/BF02784115. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Record IR, McInerney JK, Dreosti IE. Black tea, green tea, and tea polyphenols: effects on trace element status in weanling rats. Biol Trace Elem Res. 1996; 53 :27–43. doi: 10.1007/BF02784542. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mira L, Fernandez MT, Santos M, Rocha R, Florencio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002; 36 :1199–1208. doi: 10.1080/1071576021000016463. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maliakal PP, Coville PF, Wanwimolruk S. Tea consumption modulates hepatic drug metabolizing enzymes in Wistar rats. J Pharm Pharmacol. 2001; 53 :569–577. doi: 10.1211/0022357011775695. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sohn OS, Surace A, Fiala ES, Richie JP Jr, Colosimo S, Zang E, Weisburger JH. Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994; 24 :119–127. doi: 10.3109/00498259409043226. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donovan JL, Crespy V, Manach C, Morand C, Besson C, Scalbert A, Remesy C. Catechin is metabolized by both the small intestine and liver of rats. J Nutr. 2001; 131 :1753–1757. [ PubMed ] [ Google Scholar ]
- Okushio K, Suzuki M, Matsumoto N, Nanjo F, Hara Y. Methylation of tea catechins by rat liver homogenates. Biosci Biotechnol Biochem. 1999; 63 :430–432. doi: 10.1271/bbb.63.430. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Embola CW, Weisburger JH, Weisburger MC. Urinary excretion of N-OH-2-amino-3-methylimidazo [4,5-f]quinoline-N-glucuronide in F344 rats is enhanced by green tea. Carcinogenesis. 2001; 22 :1095–1098. doi: 10.1093/carcin/22.7.1095. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Clarendon Press; 1985. [ PubMed ] [ Google Scholar ]
- Abdel-Raheim MAM, Enas AH, Khaled AE. Effect of green tea extract and vitamin c on oxidant or antioxidant. Indian J Clin Biochem. 2009; 24 (3):280–287. doi: 10.1007/s12291-009-0053-7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002; 50 :3549–3552. doi: 10.1021/jf020029h. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002; 9 :232–238. doi: 10.1078/0944-7113-00119. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr. 2004; 134 :38–42. [ PubMed ] [ Google Scholar ]
- Yokozawa T, Nakagawa T, Lee KI, Cho EJ, Terasawa K, Takeuchi S. Effects of green tea tannin on cisplatin-induced nephropathy in LLC-PK1 cells and rats. J Pharm Pharmacol. 1999; 51 :1325–1331. doi: 10.1211/0022357991776912. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tijburg LBM, Wiseman SA, Meijer GW, Weststrate JA. Effects of green tea, black tea and dietary lipophilic antioxidants on LDL oxidizability and atherosclerosis in hypercholesterolaemic rabbits. Atherosclerosis. 1997; 135 :37–47. doi: 10.1016/S0021-9150(97)00139-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alessio HM, Hagerman AE, Romanello M, Carando S, Threlkeld MS, Rogers J, Dimitrova Y, Muhammed S, Wiley RL. Consumption of green tea protects rats from exercise-induced oxidative stress in kidney and liver. Nutr Res. 2003; 22 :1177–1188. doi: 10.1016/S0271-5317(02)00421-9. [ CrossRef ] [ Google Scholar ]
- Pilipenko VI, Shakhovskaia AK, Mal'tsev GIU, Isakov VA. Influence of tableted green tea on index the antioxidant status patients with disease digestion organs. Vopr Pitan. 2008; 77 (4):58–62. [ PubMed ] [ Google Scholar ]
- Del Prato S, Piero M, Riccardo CB. Phasic Insulin Release and Metabolic Regulation in Type 2 Diabetes. Diabetes. 2007; 51 :S109. doi: 10.2337/diabetes.51.2007.S109. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002; 83 :109–116. doi: 10.1016/S0378-8741(02)00217-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004; 52 :643–648. doi: 10.1021/jf030365d. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hasegawa N, Yamda N, Mori M. Powdered green tea has antilipogenic effect on Zucker rats fed a high-fat diet. Phytother Res. 2003; 17 :477–480. doi: 10.1002/ptr.1177. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang MH, Wang CH, Chen HL. Green, Oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem. 2001; 12 :14–20. doi: 10.1016/S0955-2863(00)00140-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144 :554–562. [ PubMed ] [ Google Scholar ]
- Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006; 136 :3512–3518. [ PubMed ] [ Google Scholar ]
- Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatecin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002; 277 :34933–34940. doi: 10.1074/jbc.M204672200. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson RA, Polansky MM. Tea enhances insulin activity. J Agric Food Chem. 2002; 50 :7182–7186. doi: 10.1021/jf020514c. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gomes A, Vedasiromoni JR, Das M, Sharma RM, Ganguly DK. Anti-hyperglycemic effect of black tea ( Camellia sinensis ) in rat. J Ethnopharmacol. 1995; 45 :223–226. doi: 10.1016/0378-8741(95)01223-Z. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, Miyamoto Y, Shimizu M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000; 48 :5618–5623. doi: 10.1021/jf0006832. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang JA, Choi JH, Rhee SJ. Effects of green tea catechin on phospholipase A2 activity and antithrombus in streptozotocin diabetes rats. J Nutr Sci Vitaminol (Tokyo) 1999; 45 :337–346. [ PubMed ] [ Google Scholar ]
- Choi JH, Cha BK, Rhee SJ. Effect of green tea catechin on hepatic microsomal phospholipase A2 activities and changes of hepatic phospholipid species in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo) 1998; 44 :673–683. [ PubMed ] [ Google Scholar ]
- Illing EKB, Gray CH, Lawrence RD. Blood glutathione and non-glucose reducing substances in diabetes. J Biochem. 1951; 48 :637–640. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vucic M, Gavell M, Bozikov V, Ashcroft JH, Rocic B. Superoxide dismutase activity in lymphocytes and polymorphonuclear cells of diabetic patients. Eur J Clin Chem Biochem. 1997; 35 :517–521. [ PubMed ] [ Google Scholar ]
- Polychronopoulos E, Panagiotakos DB, Polystipioti A. Diet, lifestyle factors and hypercholesterolemia in elderly men and women from Cyprus. Lipids Health Dis. 2005; 4 :17–21. doi: 10.1186/1476-511X-4-17. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu LY, Juan CC, Hwang LS, Hsu YP, Ho PH, Ho LT. Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur J Nutr. 2004; 43 :116–124. doi: 10.1007/s00394-004-0450-x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Han MK. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med. 2003; 35 :136–139. [ PubMed ] [ Google Scholar ]
- Song EK, Hur H, Han MK. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch Pharm Res. 2003; 26 :559–563. doi: 10.1007/BF02976881. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lambert JD, Lee MJ, Lu H, Meng X, Hong JJJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003; 133 :4172–4177. [ PubMed ] [ Google Scholar ]
- Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003; 31 :88–101. [ PubMed ] [ Google Scholar ]
- Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006; 50 (2):188–210. doi: 10.1002/mnfr.200500109. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shoji Y, Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Arch Pharmacol Res. 2006; 29 (9):786–794. doi: 10.1007/BF02974080. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rudelle S, Ferruzzi MG, Cristiani I, Moulin J, Mace K, Acheson KJ, Tappy L. Effect of a thermogenic beverage on 24-hour energy metabolism in humans. Obesity. 2007; 15 (2):349–355. doi: 10.1038/oby.2007.529. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006; 50 (2):211–217. doi: 10.1002/mnfr.200500138. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes. 2005; 29 (6):615–623. doi: 10.1038/sj.ijo.0802926. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000; 24 (2):252–258. doi: 10.1038/sj.ijo.0801101. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boschmann M, Thielecke F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. J Am Coll Nutr. 2007; 26 (4):389S–395S. [ PubMed ] [ Google Scholar ]
- Schmidt M, Schmitz HJ, Baumgart A, Guedon D, Netsch MI, Kreuter MH, Schmidlin CB, Schrenk D. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem Toxicol. 2005; 43 :307–314. doi: 10.1016/j.fct.2004.11.001. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takabayashi F, Tahara S, Kanerko T, Harada N. Effect of green tea catechins on oxidative DNA damage of hamster pancreas and liver induced by N-nitrosobis (2-oxopropyl) amine and/or oxidized soybean oil. Biofactors. 2004; 21 :335–337. doi: 10.1002/biof.552210165. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yun SY, Kim SP, Song DK. Effects of ( - )-epigallocatechin-3-gallate on pancreatic beta-cell damage in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006; 541 :115–121. doi: 10.1016/j.ejphar.2006.04.040. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sakamoto Y, Mikuriya H, Tayama K, Takahashi H, Nagasawa A, Yano N, Yuzawa K, Ogata A, Aoki N. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch Toxicol. 2001; 75 :591–596. doi: 10.1007/s00204-001-0286-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, Yamada K, Aoki N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. 2002; 40 :925–933. doi: 10.1016/S0278-6915(02)00066-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bruneton J. Pharmacognosie. Phytochimie. Plantes Me'dicinales. Paris: Technique Documentation-Lavoisier; 2001. [ Google Scholar ]
- Costa LM, Gouveia ST, Nobrega JA. Comparison of heating extraction procedures for Al, Ca, Mg and Mn in tea samples. Ann Sci. 2002; 18 :313–318. doi: 10.2116/analsci.18.313. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamdaoui MH, Chabchob S, Heidhili A. Iron bioavailability and weight gains to iron-deficient rats fed a commonly consumed Tunisian meal "bean seeds ragout" with or without beef and with green or black tea decoction. J Trace Elem Med Biol. 2003; 17 :159–164. doi: 10.1016/S0946-672X(03)80020-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]

Green Tea 101: A Complete Guide
Water is the most-consumed beverage in the world, but occupying the No. 2 spot is tea, according to 2019–2020 data from the Tea Association of the USA . More than 159 million Americans have this brewed beverage every day, and no wonder: Tea can be enjoyed hot or cold, and comes in different varieties for different tastes. Plus, tea has been well studied for its health benefits, particularly green tea. This guide explores all the reasons why green tea is good for you, as well as ways to drink more.
What Exactly Is Green Tea?
All tea comes from the same plant, Camellia sinensis , but the leaves are processed differently to make green, black, and oolong tea, according to the National Center for Complementary and Integrative Health (NCCIH) . Green tea leaves are steamed, which accounts for their fresh, almost grassy flavor.
Tea-drinking is a ritual that people have been practicing for centuries, dating back to B.C. 2737 in Asia, according to some accounts . It is known for its high content of antioxidants called catechins (more on those later) and beloved for its crash- and jitter-free dose of caffeine, which is thanks to its generous supply of L-theanine, an amino acid that research has found to have a calming effect on the nervous system. Melissa Salazar, an International Tea Master Association–certified tea master, says that green tea has the highest quantity of L-theanine compared with other teas. “It helps to increase brain waves, which induces deep relaxation and increases focus,” she adds. “This makes green tea a very special plant indeed.”
Some small studies have observed this as well, finding that L-theanine increased alpha wave emission in people with anxiety and improved mental alertness. A study of 69 Japanese men and women published in 2021 in the Journal of Medicinal Food found it to improve attention and memory-related tasks.
With that being said, some green tea blends have more caffeine than others. Matcha , a popular powdered form of green tea, has the most. That’s because it’s made by grinding the entire Camellia sinensis leaf, explains Salazar, and is delivered to the body in its entirety, as opposed to tea leaves that are steeped in water and then removed prior to serving. Matcha’s unique preparation also makes it more plentiful in the good stuff, like antioxidants.
Today, green tea is still most commonly sipped in drink form, but it’s also finding its way into supplements, skin care, and more.
Common Questions & Answers
Matcha two ways: latte and tea.

Next up video playing in 10 seconds
What’s in green tea a look at its nutrition facts.
Brewed green tea is primarily water based, which means it’s free of the usual macronutrients found in other foods and drinks. It doesn’t contain any fat, carbohydrates, or protein, and there aren’t any calories in unsweetened tea. It gets its healthy reputation from compounds called catechins, specifically epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG). These catechins have anti-inflammatory and anti-cancer effects, according to a review published in March 2020 in the International Journal of Molecular Sciences . They’re also believed to have probiotic benefits, per a study published in Nutrients .
A cup of green tea has around 28 milligrams (mg) of caffeine, which puts it slightly behind black tea’s 47 mg, according to the Mayo Clinic . However, there can be a lot of variation in caffeine content depending on how tea is processed and brewed.
The amount of catechins per cup also varies, with a systematic review stating a range of between 25 and 750 mg per cup.
What Are the Possible Health Benefits of Drinking Green Tea?
Green tea's benefits may include:
- Increased Mental Alertness A review published in Current Pharmaceutical Design found that caffeine, particularly the amount in matcha, improved alertness, arousal, and vigor during long, demanding cognitive tasks.
- Protection Against Heart Disease Not many long-term studies have been done, but the ones that have been completed suggest that green tea’s antioxidants may help lower high blood pressure (hypertension) and keep cholesterol in check, reducing the risk of developing heart disease. A Japanese study found that people who consumed five or more cups of green tea each day had a 26 percent lower risk of dying of cardiovascular disease during a seven-year period compared with people who drank only a cup per day. A article published in December 2020 in the European Journal of Preventive Cardiology surveyed health data from 100,000 participants and found that those who frequently drank tea were 20 percent less likely to suffer from heart disease or stroke — and green tea had the strongest impact.
- Lower Cholesterol A systematic review and meta-analysis published in 2020 in Nutrition Journal concluded that green tea consumption lowers levels of LDL ("bad") cholesterol in people of all body weights.
- Cancer Prevention Some researchers suspect that catechins have the ability to block cancer-causing free radicals. Research has been inconsistent, though, and according to the National Cancer Institute , drinking green tea isn’t a proven way to protect against cancer.
- Reduced Risk of Diabetes In a study of a half million Chinese adults published in July 2021 in The American Journal of Clinical Nutrition , daily green tea consumption was associated with a lower risk of type 2 diabetes and a lower risk of all causes of mortality in patients with diabetes.
Can Green Tea Aid Weight Loss? What the Science Says
You’ve probably heard that sipping green tea can turn your body into a fat-burning machine. The thinking is that the caffeine and catechins in tea work together to send the metabolism into overdrive, which helps the body burn calories and, as a result, drop pounds.
It sounds too good to be true — and it is. These claims come from studies that presented green tea as the secret to weight loss , but most of them were small and short term, and often involved green tea extract rather than cups of brewed tea. Unfortunately, expecting green tea to produce a significant change in your waistline isn’t realistic.
“Evidence from clinical trials is mixed in their findings of weight loss associated with green tea consumption,” says Caroline West Passerrello, RDN , a licensed dietitian nutritionist and spokesperson for the Academy of Nutrition and Dietetics. “Extracts that are rich in ECGC may increase calorie and fat metabolism (maybe because of the catechins, caffeine, and theanine), and it might suppress appetite in animal research. Impact remains undetermined with well-designed studies.”
A study published in BMC Complementary and Alternative Medicine analyzed the effects of green tea extract on overweight women with high LDL levels compared with those taking a placebo. After six weeks, there were no significant changes in participants’ weight. Additionally, a review of 15 clinical trials published in Nutrición Hospitalaria found that green tea was effective for weight loss only when combined with 80 to 300 mg of caffeine per day.
On its own, however, plain, unsweetened green tea is a low-calorie beverage that is part of a sensible diet and will save you calories when swapped for sugary soda, juice, or high-calorie coffee drinks.
How to Select Green Tea
The type of green tea you choose will depend on the benefits you’re seeking, explains Salazar. If you’re looking for max caffeine and antioxidants, she says that matcha is your best bet. You can also steep the leaves of your favorite Chinese variety, she adds. It’s all a matter of trial and error to find what you like.
Like other teas, green tea is available loose, which requires a tea infuser or strainer to brew, or in sachets and tea bags.
You can also find green tea in dozens of products:
- Bottled beverages
- Supplements
- Weight loss products
Experts caution not to overdo the supplements and weight loss products because the U.S. Food and Drug Administration (FDA) doesn’t regulate them. Everything else, though, is relatively safe.
If weight loss is your goal, be sure to check the ingredient label of green tea beverages. Regular, unsweetened tea is always a better bet than the sweetened bottled versions, which may have loads of added sugars. Unsweetened Green Tea from Pure Leaf, for instance, has 0 calories and 0 grams of (g) sugar in 18.5 ounces, while Arizona Green Tea packs 130 calories and 34 g of sugar into 8 ounces. Keep in mind that sweetened teas often come in larger portions than the unsweetened variety, so they have a greater potential to lead to weight gain.
How to Brew Green Tea
Preparation varies slightly depending on the tea type, as well as taste preference. But it’s important to know that overall green tea is sensitive to high temperatures and can get bitter if boiled, per Salazar. “The general rule of thumb is that you use a lower temperature than boiling,” she explains, which is anywhere from 150 to 175 degrees Fahrenheit.
“I like to brew my matcha at 165 degrees,” she adds. “How delicate the leaves are will determine the actual steeping time.” She recommends measuring out 1 teaspoon of leaves per 6 ounces of water, then steeping for one to two minutes. If you want to enjoy your cup cold, no need to worry about sacrificing its benefits. Salazar says that they don’t really wane from hot to iced. “For iced tea, you would simply steep the tea as you would and then pour over ice,” she adds.
What Are the Possible Side Effects of Drinking Too Much Green Tea?
Although green tea is generally considered safe and healthy, thanks to its many proven benefits, as with any food or drink, there can be too much of a good thing. Because green tea contains caffeine, drinking too much of it can lead to classic signs of caffeine overconsumption, such as feeling jittery and having trouble with sleep, per the NCCIH.
Consuming green tea in the form of concentrated extracts can end up damaging the liver. A study published in Cancer Prevention Research found that women who took a high dose of green tea extract (equivalent to five cups of brewed tea) daily developed high levels of liver enzymes, which could indicate that the cells within the liver have been damaged.
The take-home message? Approach green tea extracts with caution because they’re not regulated by the FDA. But if you decide you want to try them, experts recommend taking them only with meals and stop taking them and see a doctor if you notice signs that your liver’s in trouble, such as if you have especially dark urine or experience abdominal pain .
Also, stay away from green tea if you have a heart condition or other cardiovascular problems, or renal failure. For anyone who is pregnant or breastfeeding, up to six cups of green tea per day has been found to be safe, according to the NCCIH . It’s a good idea to consult your healthcare provider for individual recommendations.
Other Uses for Green Tea
You may spot green tea as an active ingredient in skin-care products because its anti-inflammatory properties and antioxidants make it an effective multitasker, according to Marisa Garshick, MD, a board-certified dermatologist at Manhattan Dermatology and Cosmetic Surgery and adviser to BioRepublic . Some research has found that the polyphenolic compound EGCG in green tea has antimicrobial activity, which may help inhibit the growth of bacteria that cause some difficult to treat skin infections.
The polyphenols in green tea may also prevent sun damage with their anti-inflammatory and antibacterial properties, a study found. And specific polyphenols called catechins have been found to have a moisturizing effect on skin, which may help reduce the appearance of fine lines, age spots, and wrinkles. “It’s a powerful antibacterial agent for treating acne and unclogging pores ,” Garshick adds, although only small, limited studies have been done on this topic, and further research is necessary.“Green tea is chock-full of vitamin B2 and vitamin E, both essential for skin health.”
Green tea is a plant-based beverage that has been used medicinally for centuries and has plenty of research to back up its health-boosting properties. Its high concentration of antioxidants and versatility make it a popular drink as well as an ingredient to add to other foods and wellness products. Because it contains caffeine, it is important to use caution with supplements and extracts, and only use them in the recommended amounts.
Additional reporting by Kayla Blanton .
Editorial Sources and Fact-Checking
Everyday Health follows strict sourcing guidelines to ensure the accuracy of its content, outlined in our editorial policy . We use only trustworthy sources, including peer-reviewed studies, board-certified medical experts, patients with lived experience, and information from top institutions.
- Fernando CD et al. Extraction Kinetics of Phytochemicals and Antioxidant Activity During Black Tea ( Camellia sinensis L. ) Brewing. Nutrition Journal . July 2015.
- Tea Fact Sheet — 2019–2020. Tea Association of the USA .
- Green Tea. National Center for Complementary and Integrative Health . October 2020.
- The Health Benefits of Tea. Academy of Nutrition and Dietetics . January 10, 2023.
- Hidese S et al. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized, Controlled Trial [PDF]. Nutrients . October 2019.
- Musial C et al. Beneficial Properties of Green Tea Catechins. International Journal of Molecular Sciences . March 2020.
- Caffeine Content for Coffee, Tea, Soda, and More. Mayo Clinic . April 26, 2022.
- Dietz C et al. Effect of Green Tea Phytochemicals on Mood and Cognition. Current Pharmaceutical Design . 2017.
- Kuriyama S et al. Green Tea Consumption and Mortality Due to Cardiovascular Disease, Cancer, and All Causes in Japan. JAMA . September 2006.
- Wang X et al. Tea Consumption and the Risk of Atherosclerotic Cardiovascular Disease and All-Cause Mortality: The China-PAR Project. European Journal of Preventive Cardiology . January 2020.
- Tea and Cancer Prevention. National Cancer Institute . November 17, 2010.
- Nie J et al. Tea Consumption and Long-Term Risk of Type 2 Diabetes and Diabetic Complications: A Cohort Study of 0.5 Million Chinese Adults. The American Journal of Clinical Nutrition . July 2021.
- Huang LH et al. Effects of Green Tea Extract on Overweight and Obese Women With High Levels of Low-Density Lipoprotein Cholesterol (LDL-C): A Randomized, Double-Blind, and Cross-Over Placebo-Controlled Clinical Trial. BMC Complementary and Alternative Medicine . November 2018.
- Vázquez Cisneros LC et al. Effects of Green Tea and Its Epigallocatechin (EGCG) Content on Body Weight and Fat Mass in Humans: A Systematic Review. Nutrición Hospitalaria . June 2017.
- Yu Z et al. Effect of Green Tea Supplements on Liver Enzyme Elevation: Results From a Randomized Intervention Study in the United States. Cancer Prevention Research . October 2017.
- Chacko S et al. Beneficial Effects of Green Tea: A Literature Review. Chinese Medicine . 2010.
- Kobayashi K et al. Effects of L-Theanine on the Release of Alpha-Brain Waves in Human Volunteers. Journal of the Agricultural Chemical Society of Japan (PDF). 1998.
- Baba Y et al. Effects of L-Theanine on Cognitive Function in Middle-Aged and Older Subjects: A Randomized, Placebo-Controlled Study. Journal of Medicinal Food . April 2021.
- Jeon J et al. The Antimicrobial Activity of (-)-Epigallocatehin-3-Gallate and Green Tea Extracts Against Pseudomonas Aeruginosa and Escherichia coli Isolated From Skin Wounds. Annals of Dermatology . October 2014.
- OyetakinWhite P et al. Protective Mechanisms of Green Tea Polyphenols in Skin. Oxidative Medicine and Cellular Longevity . June 2012.
- Hsu S. Green Tea and the Skin. Journal of the American Academy of Dermatology . July 2005.
- Saric S et al. Green Tea and Other Tea Polyphenols: Effects on Sebum Production and Acne Vulgaris. Antioxidants . March 2017.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 April 2024
Effects of green tea and roasted green tea on human responses
- Chie Kurosaka ORCID: orcid.org/0000-0002-0363-0433 1 ,
- Chika Tagata 2 ,
- Sae Nakagawa 2 ,
- Makoto Kobayashi 2 &
- Shinji Miyake 3
Scientific Reports volume 14 , Article number: 8588 ( 2024 ) Cite this article
510 Accesses
23 Altmetric
Metrics details
- Biomedical engineering
- Occupational health
- Quality of life
Our objective was to elucidate the effects of tea consumption on refreshment and stress reduction/recovery through examining the multiple associations among factors such as various physiological responses and task performance. Participants included 20 healthy young men who performed a mental arithmetic task while 11 physiological responses were measured. The experiments were conducted twice under different beverage consumption conditions on separate days. The mental arithmetic task was executed six times in 1 day; participants ingested hot water, green tea, or roasted green tea (hojicha) before each task. Several subjective assessments: subjective fatigue, stress, mental workload, and flow were evaluated after each task. The R–R intervals, heart rate variability spectral components, the Poincaré plot indices (SD1 and SD2) and plethysmogram amplitude tended to decrease during task periods compared to resting periods. Tissue blood volume/flow (TBV, TBF) and near-infrared spectroscopy responses (NIRS) were lower in the tea condition than in the hot water condition. By scrutinizing various indicators, we found that aromatic stimulation of Japanese tea beverages has the potential to induce positive effects, enhance mental task performance, promote refreshment, and alleviate feelings of fatigue. These positive effects were observed even in small quantities and within a short duration, mirroring responses observed in daily consumption.
Similar content being viewed by others

Autonomic adaptations mediate the effect of hydration on brain functioning and mood: Evidence from two randomized controlled trials
Hayley A. Young, Alecia Cousins, … David Benton
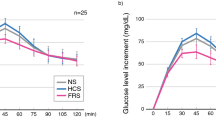
Positive effect of inaudible high-frequency components of sounds on glucose tolerance: a quasi-experimental crossover study
Norie Kawai, Manabu Honda, … Tsutomu Oohashi
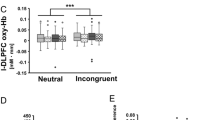
Groove rhythm stimulates prefrontal cortex function in groove enjoyers
Takemune Fukuie, Kazuya Suwabe, … Hideaki Soya
Introduction
Japanese tea has become increasingly popular with the rise in health consciousness. Green tea, in particular, is consumed worldwide as a healthy drink because of its characteristic inhibition of cholesterol absorption by the body through tea catechins (gallated bioactive compounds found in green tea), as well as its antioxidant and cavity-preventing (antibacterial) effects 1 . Various types of green tea (sencha, roasted green tea (hojicha), gyokuro, and bancha) currently exist, differing based on the production method. Sencha is the most popular green tea. Sencha is tea leaves grown without blocking sunlight and is produced using a method that involves rolling and drying the tea leaves in several stages. Gyokuro is produced from tea leaves grown with blocking sunlight, therefore it is rich in theanine and poor in catechins. Bancha is mainly produced from tea leaves harvested after summer and less expensive than Sencha. Rosted green tea (hojicha) is a tea made by roasting sencha or bancha at 160–180 °C and has a unique aroma and light astringency. In a study of elderly people in Japan, higher green tea consumption has been reported to lower mortality 2 and reduce the risk of developing diabetes 3 , colorectal cancer and gastric cancer 2 , 4 , 5 . Hursel et al. investigated how catechin- and caffeine-rich teas (CCRTs), such as green tea and oolong tea, maintained and promoted energy expenditure and increased fat oxidation 6 . They suggest that CCRTs’ thermogenic effects may have significant effects on metabolism, fat absorption, and energy intake. Furthermore, green tea was found to lower blood pressure 7 ; people who drank green tea for 10 years had smaller waists and lower body fat levels 8 , suggesting that green tea may prevent lifestyle-related diseases.
Green tea contains not only catechins and caffeine but also theanine and vitamins. Catechins have thermogenic and body fat-reducing effects 9 , and long-term continuous consumption has been shown to reduce body weight 10 . In addition, l -theanine, which is abundant in green tea, is known to suppress caffeine-induced excitation 11 . In a study using mice, theanine intake was shown to reduce brain atrophy, learning disabilities, and depressive-like behaviors, as well as stress 12 . Another study showed that l -theanine orally ingested by female university students produced alpha waves in the occipital and parietal regions of the brain, resulting in a possible relaxation effect 13 . Relatedly, a study on the effects of l -theanine at more realistic dietary levels revealed that alpha wave increases were induced in the l -theanine condition regardless of gender 14 .
The aroma of green tea has been further reported to induce positive emotions, increase vitality scores and task performance after stress, and induce positive physiological responses such as electroencephalogram (EEG) activity and salivary chromogranin A (CgA) 15 , 16 . An odor administration study using two types of Japanese tea with different manufacturing processes, which evaluated electroencephalogram, subjective assessment, and task performance revealed that smelling Japanese tea induced an increase in the band power of alpha and beta waves and enhanced task performance and relaxation scores 15 . In a related experiment, participants consumed warm water and two types of green tea, and the result showed that green tea suppressed the increase in salivary CgA concentration and improved mood after mental workload, indicating that the aroma of green tea could be involved in this effect 16 . Another study investigated autonomic nervous system activity (heart rate, blood pressure, and electrodermal activity) and endocrine salivary cortisol responses to the inhalation of linalool, a component of green tea, demonstrating that this aroma component regulates salivary cortisol levels 17 . The authors examined the results of autonomic activity and concluded that chirality was related to the physiological effects of aromatic substances and that aromatic substances might have different effects on certain physiological indices. Compared with green tea and water, hojicha has been shown to enhance feelings of refreshment and motivation and to peak earlier in the EEG P300 wave (a measure of concentration), suggesting a positive correlation with improved concentration and cognitive ability 18 . Although roasted green tea contains less catechin, theanine, and vitamins than non-roasted green tea, roasting reduces the amount of caffeine, bitterness, and astringency compared to non-roasted green tea, making it easier for pregnant women and children to drink it regardless of the time of day. In addition, hojicha is rich in pyrazine, a component that lends a distinctive aroma and is expected to improve blood circulation, reduce sensitivity to cold, relieve fatigue, and relax the body.
Green tea is ingested in various daily situations such as eating, quenching thirst, alleviating fatigue, want to change mood. Several studies have been conducted on the relationship between the constituents of Japanese tea and disease prevention and health maintenance, and on the psychological effects of aromatic constituents. However, most of these studies are long-term observations or examinations of the effects of consuming large amounts of specific ingredients on the body; few studies have objectively evaluated the physiological responses and subjective amounts of tea consumed in daily life. Furthermore, reports of green tea on multiple physiological responses, task performance, and subjective evaluations are scarce. A variety of elements, such as caffeine, theanine, aroma, taste, are likely to contribute to these effects. Investigating the responses regarding the two types of Japanese tea (green tea and roasted green tea), each with different taste, aroma, and ratios of caffeine and theanine components, would clarify their effects. Therefore, this study measured multiple physiological responses, task performance, and subjective evaluations of the taste and aroma of water, green tea, and roasted green tea (hojicha), examining their interrelationships from various perspectives. By analyzing the relationships among these beverages, we aimed to objectively evaluate the effects of tea consumption on refreshment and stress reduction/recovery. We hypothesize that the consumption of Japanese tea during a mental task will likely have positive effects such as enhancing task performance and alleviating fatigue and stress, and these effects are observed even with the same small amount consumed in daily life.
Materials and methods
Participants.
Twenty healthy men aged 18–30 years (mean ± SD = 22.8 ± 2.54 years) participated in this study, which eliminated the need to consider female hormonal cycle effects 19 , 20 . The altered functioning of the autonomic nervous system in the late luteal phase could be associated with diverse psychosomatic and behavioral symptoms appearing premenstrually; specifically, in the premenstrual syndrome group, which is experienced by many women, high frequency component power of heart rate variability and total power were significantly decreased in the late luteal phase compared to the follicular phase 19 . Furthermore, the menstrual cycle has been shown to affect task performance, with a decline in performance observed premenstrually 20 . The sample size was determined based on our previous experiment (not published) and with reference to Cohen’s literature 21 . Participants were excluded from the experiment if they (1) had circulatory problems, such as arrhythmia or were taking medication, (2) smoked, or (3) had dry skin, such as atopic dermatitis. The following conditions were imposed on participants: (1) no heavy drinking and sufficient sleep on the night before the experiment, (2) no strenuous exercise on the day of the experiment, (3) no caffeine consumption until the end of the experiment, and (4) maintain usual daily living conditions.
The experiment started at 9:00 AM or 1:00 PM, and AM and PM were counterbalanced among the participants. All participants were examined twice, under different beverage intake conditions and on different experimental days, with the interval between the first and second experimental days being within one month. All participants provided written informed consent. This study was approved by the Ethics Committee of the University of Occupational and Environmental Health, Japan (Approval No. R2-066), and performed in accordance with relevant guidelines and regulations.
First, after checking the experimental procedure and instructions for the mental task, participants practiced the mental task. Electrodes and transducers were then attached, and the experiment started after approximately 10 min of acclimation time. The experimental procedure is illustrated in Fig. 1 . First, the Subjective Fatigue Feelings (SFF) 22 questionnaire was administered; subsequently, the subjects were asked to respond to 18 psychological stress reaction items (items #18–35) of the Brief Job Stress Questionnaire (BJSQ; Japan Industrial Safety and Health Association, n.d., Retrieved November 10, 2023) regarding their current state of stress. After these subjective assessments, participants were asked to stay calm (for 5 min), consume a beverage (hot water or green tea), evaluate the beverage in terms of its taste and aroma by semantic differential (SD) method, and perform a mental task (5 min).
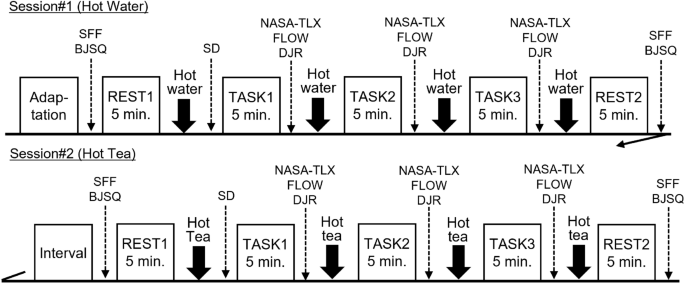
Experimental procedure. SD evaluation of drink by semantic differential method, SFF subjective fatigue feelings, BJSQ brief job stress questionnaire, NASA-TLX NASA task load index, FLOW flow check list, DJR duration judgment ratio.
The NASA Task Load Index (NASA-TLX) 23 , 24 , the Flow Experience Checklist (FLOW; 12 questions, simplified version) 25 , 26 , and the Duration Judgment Ratio (DJR) 27 tests were then administered. NASA Task Load Index (NASA-TLX) consists of six subscales: Mental demand (MD), Physical demand (PD), Temporal demand (TD), Own Performance (OP), Effort (EF), and Frustration level (FR). All participants were required to answer each subscale using the Visual Analog Scale (VAS) method, ranging from 0 to 100, to indicate low/high levels (own performance scale being poor/good). The FLOW was rated on a 7-point scale (1: strongly disagree, 2: disagree, 3: slightly disagree, 4: undecide, 5: slightly agree, 6: agree, 7: strongly agree) for 12 questions. Regarding the DJR, participants were asked to rate their perception of mental task compared to actual time, 5 min, based on the VAS method (from 0: shortest to 100: longest).
The procedure from beverage intake to DJR was repeated three times; rest recordings (5 min), SFF, and BJSQ were recorded each time. The beverage was evaluated only during the first time, although all participants consumed a beverage four times per each session. After the hot water consumption session, a 10-min break was taken, the beverage type was changed to tea, and the same procedure was repeated. After all experiments were completed, participants reported their daily beverage intake and preferences.
All participants performed the two conditions (hot water + green tea and hot water + roasted green tea) on two separate days. Each condition was provided to the participants in an offsetting order; however, the beverages were administered in the order of hot water, followed by tea. Participants were alone in the sound-proof room and followed instructions from the adjacent monitoring room. Subjective evaluation and mental tasks were displayed on a 27-inch PC display on a desk in front of the participant, and all subjective evaluations and tasks were performed using mouse operation only.
Procedure for beverage administration
The beverages used included water, green tea, and roasted green tea. The beverages were warmed in a microwave oven, divided into four paper cups (50 ml each), and kept at a temperature of 55 ± 1 °C in a warming cabinet. Temperatures were continuously measured using thermocouples at two locations in the warming cabinet and at the bottom of each paper cup. The results of the beverages’ component analysis by the Japan Food Research Laboratories and Showa Denko Materials Techno-Service Corporation are shown in Supplementary Table 1 . Analysis of aroma components, such as 2-Ethyl-3, 5-dimethylpyrazine, tetramethylpyrazine, and 2,3-diethyl-5-methylpyrazine were analyzed by dynamic headspace-gas chromatography (DHS-GC)-/MS using Tenax TA trapping system (GERSTEL GmbH & Co.KG, Germany). The DHS-GC-MS systems used were the GERSTEL MPS autosampler (GERSTEL GmbH & Co.KG), Agilent 7890B GC system (Agilent Technologies, Santa Clara, CA), and Agilent 5977A mass spectrometric degradation system (Agilent). The analytical column was DB-WAX (60 m × 0.25 mm i.d.; d f 0.25 μm, Agilent Technologies).
Mental task
The mental task is based on the MATH algorithm proposed by Turner et al. 28 . In this task, an addition or subtraction problem and answer are shown on a computer screen. A numerical problem is displayed for 2 s, after which the target number is revealed following the word “EQUALS”.
Participants were required to press the left mouse button if the target number was correct and click the right mouse button if not. The task was performed in machine-paced conditions, and the next problem automatically appeared every 5 s, regardless of whether the participants responded. The task contained five levels of difficulty, as shown in Table 1 . However, these formulae differed from those used by Turner et al. The initial question was always at level 3. The next question level increased if the prior answer was correct and decreased otherwise. Participants were instructed to respond with the mouse within 1.5 s of the number being presented and to continue with the next mental arithmetic task even if they could not respond in time. Participants were not informed of their level and were not given feedback on whether their answers were correct.
Physiological measurement
The physiological measurement indices and positions are shown in Table 2 . All physiological indices were measured continuously throughout the experimental sessions and recorded at a sampling rate of 1 kHz data logger (KEYENCE NR-600/NR2000). To prevent numbness or discomfort during extended noninvasive continuous blood pressure measurements, the blood pressure sensor was temporarily paused after the first half of the session. In the second half of the session, the measurement finger was changed before resuming the blood pressure measurements. Tissue blood volume and blood flow based on the fluctuation of the scattered laser light were measured by using the Laser Doppler Blood Flowmeter. NIRS data were obtained using a two-channel wireless system (Pocket NIRS HM, DynaSence, Hamamatsu, Japan). This device measured changes in levels of oxygenated hemoglobin (oxyhemoglobin–hemoglobin) and deoxygenated hemoglobin (deoxyhemoglobin) at three wavelengths of 735, 810, and 850 nm from the baseline, which is the level when sensors were attached. Concentrations of oxyhemoglobin–hemoglobin and deoxyhemoglobin levels were based on the modified Beer–Lambert law. Data were recorded at a sampling interval of 16 ms and converted to analog data to obtain them simultaneously with other physiological signals at a sampling rate of 1 ms. NIRS sensors were placed on both the left and right sides of the frontopolar (prefront cortex).
Analysis methods
Task performance and subjective assessments.
The response rate (percentage of questions answered within the time limit) and correct response rate (number of correct answers/total number of questions) for each task, as well as the average level of difficulty, were calculated for task performance. For NASA-TLX, the mean of the adaptive weighting workload (AWWL) and six subscales were calculated; for the FLOW score, the mean of all items and four subscales (AIM, TIME, CHALLENGE, and FEEDBACK) were determined; and for the DJR, the mean of each trial score was obtained. The weighted mean of six subscales of NASA-TLX was calculated without the paired comparisons. The weight coefficient for each subscale was decided by the subscale value itself. For example, the weight coefficients of the maximum and minimum subscales are six and one, respectively. The weighted mean of this method is called the Adaptive Weighted Workload (AWWL) score and indicates a high correlation with the original weighted workload (WWL) score 24 .
Task performance and NASA-TLX data were incomplete for three participants, whereas FLOW and DJR data were missing for one participant.
For all indices except FLOW, standardized scores were calculated for six blocks (3 task blocks × 2 sessions) for each participant per condition; since the FLOW score is an index with meaning in absolute values, we used the rating value. Multiple comparisons were conducted for all measures using the repeated two-factor analyses of variance and Ryan–Einot–Gabriel–Welsch F test (R–E–G–W F test) for the two drinking sessions and three task blocks. Regarding the SFF score, five factors (feeling of drowsiness: Factor I; feeling of instability: Factor II; feeling of uneasiness: Factor III; feeling of local pain or dullness: Factor IV; and feeling of eyestrain: Factor V) were calculated. The SFF total score was derived from the sum of these five factors. The BJSQ and SFF scores were compared before and after each session by separately calculating the standardized scores of the four blocks for each participant (paired t-test). Cohen’s d was applied for effect sizes 21 , 29 . A detailed relationship between task performance and subjective evaluation has been reported by Tagata et al. 30 .
Physiological signals
The R–R interval (RRI) was obtained from the ECG waveform by detecting the R wave at each beat. We then calculated the mean values of the RRI, heart rate variability (HRV) such as low frequency (LF) component, high frequency (HF) component, LF/HF ratio, and Poincaré plot indices (SD1, SD2, Cardiac Vagal Index: CVI, and Cardiac Sympathetic Index: CSI) 31 . SD2 is the length of the longitudinal axis of the ellipse which is parallel with the line RRI k = RRI ( k +1) and SD1 is the length of the vertical axis to this line. These indices mean the standard deviation of the Poincaré plot perpendicular/parallel to each axis. For continuous blood pressure, mean blood pressure (MBP) was calculated from systolic blood pressure (SBP) and diastolic blood pressure (DBP. MBP = DBP + (SBP-DBP/3)) to obtain the total peripheral resistance (TPR = MBP/CO: CO is cardiac output). Baroreceptor reflex sensitivity (BRS) was simply calculated using the square root of the ratio of LF RR and LF SBP (0.05–0.15 Hz) components in the spectral analysis of RRI and SBP 32 . The PTG amplitudes were obtained by simply calculating them based on the knowledge of a significant correlation between PTG amplitude and the standard deviation of PTG waveform 33 .
For all 24 indicators, the mean value for each block and standardized scores for each participant by condition (by date of administration) were calculated. Repeated two-factor analysis of variance (condition 2 × block 5) and the R–E–G–W F tests were conducted when the main effect was significant. When a significant interaction was found, multiple comparisons were made for all pairs using the Holm method. The significance level was set at p < 0.05 and p < 0.10 was considered to be marginally significant.
Correlation coefficients among the HRV indices, BRS, and TPR were calculated for each session, and then mean correlation coefficients were obtained by inverse z-transformation of the mean values after Fisher’s z-transformation.
One participant with a large RRI variability (SDNN = 146.6 ms) and a large deviation from the normal heart rate (SDNN = 50 ± 16 ms) was excluded from the HRV analysis (Smirnov-Grubbs test: p = 0.006). Two participants had missing blood pressure data owing to incomplete measurements. Therefore, HRV analysis was conducted for 19 participants, and blood pressure data analysis was performed for 18 participants.
Ethics approval
All participants provided written informed consent. This study was approved by the Ethics Committee of the University of Occupational and Environmental Health, Japan (Approval No. R2-066), and performed in accordance with relevant guidelines and regulations.
In all trials, participants consumed water in the first half session (session#1) and green tea or roasted green tea in the second half session (session#2). Within each session, 5 min blocks were denoted as REST1 and REST2 to indicate resting periods and TASK1 to TASK3 to indicate mental tasks.
In the green tea condition, the average problem level was significantly higher in the tea session than in the water session ( F (1, 16) = 9.95, p = 0.006, 1 − β = 0.84, partial η 2 = 0.38), but no significant trend was observed in the other indicators. However, in the roasted green tea condition, the correct response rate and the response rate were significantly higher in the tea session than in the water session (response rate: F (1, 16) = 4.55, p = 0.049, 1 − β = 0.52, partial η 2 = 0.22; correct response rate: F (1, 16) = 8.99, p = 0.008, 1 − β = 0.80, partial η 2 = 0.36). These task performance indicators also showed main effects between blocks, with significantly higher values in TASK2 and TASK3 compared to TASK1 (response rate: F (2, 32) = 8.93, p = 0.001, 1 − β = 0.95, partial η 2 = 0.36; correct response rate: F (2, 32) = 5.41, p = 0.011, 1 − β = 0.78, partial η 2 = 0.25). Across sessions, task performance was higher in the tea condition than in the hot water condition, and across blocks, task performance was better in TASK2 and TASK3 than in TASK1 in the roasted green tea condition (Fig. 2 ).
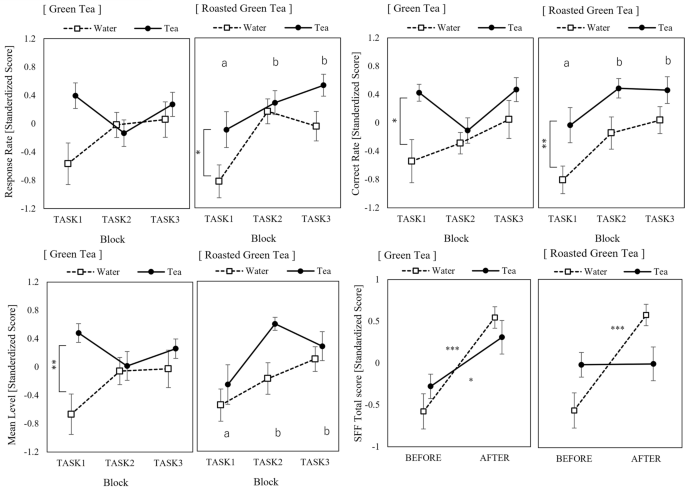
Task performance and SFF total score. Bars indicate standard errors of the mean. The alphabets indicate homogenous subset results based on the REGW F-test.
The results in NASA-TLX, FLOW, and DJR showed no obvious trends. Supplementary Table 2 shows the analysis of variance results for all measures. There were no significant differences in BJSQ scores between sessions, and the SFF total score increased significantly after the task in all conditions except the second half session of the roasted green tea condition (Fig. 2 ). session#1 of the green tea: p < 0.001, 95% confidence intervals (CI) 0.53–1.60. session#2 of the green tea-tea: p = 0.036. session#1 of the roasted green tea: p = 0.001, 95% CI 0.54–1.72). For each item of the SFF, feelings of uneasiness (Factor III) and local pain or dullness (Factor IV) showed a similar trend to the SFF total score; these scores did not increase after the tasks only in the roasted green tea condition. The feeling of drowsiness (Factor I) showed an increasing trend after the task only in the W condition (session#1 of the green tea: p = 0.002, 95% CI 0.26–1.46. session#1 of the roasted green tea: p = 0.077, 95% CI − 0.10 to 1.19).
The physiological responses under each condition are presented in Table 3 . The physiological responses that exhibited distinct trends in each session are shown in Fig. 3 . The statistical analysis results for all physiological responses are provided in Supplementary Table 3 .
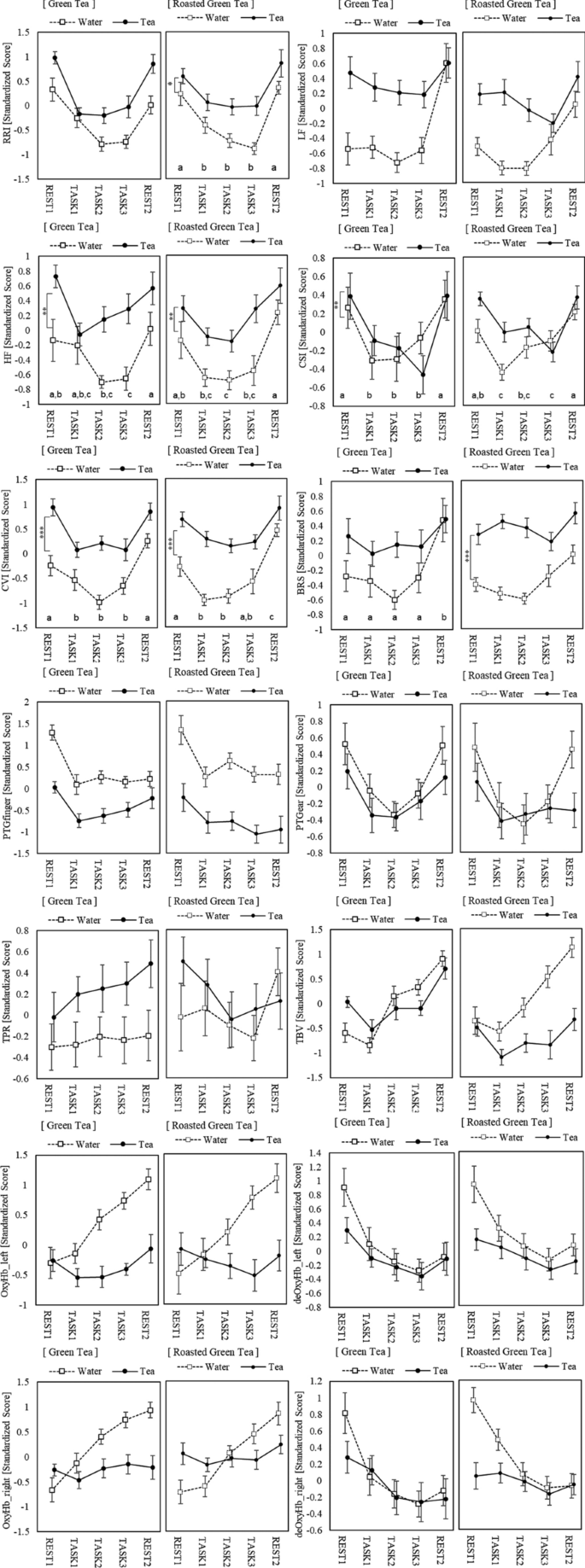
Physiological responses. Bars indicate standard errors of the mean. The alphabets ( a–d ) indicate homogenous subset results based on the REGW F-test. Asterisks indicate the significant differences between sessions (***p < 0.001, **p < 0.01).
In the green tea condition, the RRI, HRV spectral indices, and PTGfinger amplitude showed interaction effects. Analysis of the differences between blocks in each condition showed that, compared with resting periods, all indices tended to decrease during task periods. For the Poincaré plot indices, both SD1 and SD2 showed significant differences in both Session and Block. They were smaller in the hot water condition than in the tea condition and tended to decrease during task periods compared with resting periods. The CVI showed a similar tendency to SD1 and SD2, but the CSI did not show a consistent trend. Blood pressure was significantly higher during the task periods than during the resting periods, and there was no clear trend in TPR, and skin potential level (SPL). The tissue blood volume (TBV), tissue blood flow (TBF), and near infrared spectroscopy (NIRS) responses were significantly lower in the tea condition than in the hot water condition. The EEG results are not included in this study, as the study is specifically on autonomic nervous system activity. The EEG results will be reported separately in a future study.
Table 4 shows the correlation coefficients of the HRV spectral analysis indices, which are reported to sympathetic activity (LF/HF, CSI) and parasympathetic activity (HF, CVI, and BRS) indices. High correlations were observed between parasympathetic indices.
Our study results demonstrate that tea intake improved mental task performance, such as the correct response rate of machine-paced mental arithmetic tasks compared to the hot water condition. Particularly in the roasted tea condition, the response rate also improved and the task performance increased with the number of trial repetitions. Considering the ingestion of tea ingredients, it was not possible to counterbalance the order of the hot water and tea conditions in this study. Therefore, fatigue was expected to accumulate in the later tea condition. As shown in Fig. 2 , task performance was not stable; a slight sluggishness in the green tea condition was shown in TASK2. However, the SFF results indicated that fatigue did not increase after the task in the roasted green tea condition; moreover, task performance increased with the number of task repetitions. Subjective assessments such as the NASA-TLX and stress scores showed no significant changes in either condition. These results suggest that only the roasted green tea condition might have anti-fatigue properties, even though the mental workload demands and stress levels were consistent across all conditions. It is entirely possible that the level of arousal obtained with drowsiness (SFF factor I) is related to the anti-fatigue effect. One factor that contributes to maintaining wakefulness under tea conditions is the tea components’ arousal effects, specifically caffeine 34 . It is also reported that caffeine (40 mg) in combination with l -theanine (97 mg) helps to improve task performance, subjective arousal level, and self-reported tiredness 35 . The half-life of caffeine in the bloodstream is relatively broad, ranging from 2 to 8 h (average of approximately 4 h), indicating a gradual decline 36 . If the results of this study were influenced by oral caffeine intake, the effects could be expected to persist for an extended period. However, most components, including caffeine and l -theanine, are reduced in roasted green tea compared to green tea. Further, as discussed later, the amount of caffeine consumed in this study was very small compared with that in previous research. Considering the rate of ingredient absorption into the body, the potential impact of oral caffeine intake in this study is considered low. In contrast, the only components that are more prevalent in roasted green tea than in green tea are pyrazines and aromatic components (Supplementary Table 1 ). Although Murao et al. 15 have reported that aroma of green tea increases feeling of relaxation and vitality through the odor stimuli experiment, a direct relationship between pyrazines and arousal level or anti-fatigue has not been reported. Our findings could be the first report of such a relationship, and further studies on the aroma components of tea should be conducted in more detail.
RRI is well-known as a physiological index sensitive to mental stress, typically resulting in an increase in heart rate (decrease in RRI) during periods of mental stress 37 . A similar tendency was observed in this study. Although the LF component has been reported to decrease during mental tasks in HRV spectral analysis 38 , the same change was not observed in this study. This might be attributed to effects such as participants’ before-task tension. The LF responses during mental tasks are not consistently uniform. Therefore, both LF and LF/HF ratios using these values may not serve as reliable indicators for assessing mental workload. After the task, the LF component appeared to have recovered to its resting level, and in the REST2 block, comparable levels were observed in hot water and tea conditions. Indeed, the LF component also decreased during the task block in the tea condition; however, the magnitude of this decrease was smaller than that observed in the hot water condition. The familiarity effect on the task may have influenced the LF component results. However, considering that the same task was repeated three times in the hot water condition, and that task performance did not necessarily correlate with the number of task repetitions, we could not rule out that the tea might have influenced LF component responses. HF is a component that reflects respiratory sinus arrhythmia (RSA) and is known to be eliminated by vagal blockade 39 , 40 . There were significant differences between sessions in the HF component; thus, it is possible that tea ingredients affect vagal activity. However, LF/HF ratio did not exhibit a consistent trend. Although the LF/HF ratio has been reported as an indicator of the balance between—and ratio of—sympathetic and parasympathetic activity 41 , many researchers have questioned this point 38 , 42 , 43 , 44 . Computation of the autonomic balance as the LF/HF ratio is based on a hypothesis that LF is mainly mediated by the sympathetic nervous system and the physiological assumption of autonomic reciprocity 38 . However, LF reflects baroreflex function, originating from vagal activity 42 . Therefore, LF does not reflect sympathetic nervous system activity 43 . Furthermore, the autonomic reciprocity is not supported by current research and fractional transformation such as LF/HF distorts the data, making the applied indices dubious 38 . Billman 44 claims that LF/HF cannot accurately quantify sympatho-vagal balance. Therefore, HRV spectral analysis requires careful discussion considering other related physiological responses. Regarding the Poincaré plot indices, both SD1 and SD2 decreased during work because of RRI reduction (heart rate increased), and because the Poincaré plot ellipse became smaller. SD1 and Root Mean Square of Successive Differences (RMSSD) have been reported to be mathematically equivalent HRV indices 45 , and the CVI obtained as the product of SD1 and SD2 has been reported to be associated with parasympathetic nervous system activity 31 . The BRS is a reflex vagal activity that is reduced during mental arithmetic tasks and is related to task performance 46 , 47 . del Paso et al. propose that the association between mental arithmetic tasks and BRS may be modulated by gender and blood pressure. In this study, BRS was obtained using a noninvasive method proposed by DeBoer et al. 32 . Guzik et al. 48 suggest that CVI may be related to parasympathetic indices such as HF, RMSSD, and BRS. On the other hand, in a review that investigated the Poincaré plot indices and other indices during resting and mental arithmetic tasks, Allen et al. 49 showed that CVI is unlikely to produce results similar to those of other indices. Studies conducted on HRV analysis indicate its interest and importance as a measure of autonomic venous system activity, despite that it is not being widely used in clinical practice 48 , 50 . Therefore, Nunan et al. 50 identified various factors such as the analysis method, duration of electrocardiogram recording, assessment conditions, and participant characteristics. In our previous experiments, we have concurrently measured these indices and investigated their correlations under standardized measurement conditions and analytical methods. However, these indices rarely showed consistently high correlations. Interestingly, in this study, indices commonly reported as parasympathetic nervous system activity, namely HF, CVI, and BRS, showed a high correlation. Nevertheless, as mentioned above, these indices have been subject to considerable debate, and it is difficult to interpret them uniquely. We believe that a multifaceted analysis of data obtained through simultaneous measurement of various indices, as in our study, will deepen this discussion. Future studies should explore composite analyses, including blood pressure, pulse waves, and EDA activity, to further enhance our understanding. In addition, our previous experiments (not published) suggest that fingertip and earlobe pulse waves exhibit different reactivity and the amplitude of the plethysmogram on fingertip may show a characteristic tendency in the recovery process. The difference in responses between two sites in this study appears to support this tendency. The details will be published in future research.
The physiological responses of crucial interest in this study were changes in the TBV, TBF of the nose tip, and NIRS indices. NIRS values were measured as the amount of relative change from the starting point of measurement 51 . These indices showed significant differences between the blocks in the hot water condition, but there were few changes in the tea condition. Clear differences in the physiological response trends between the hot water and tea conditions indicated that the tea ingredients had some effect on capillary blood flow. Notably, both hot water and tea were provided at the same temperature; therefore, the beverage temperature effects were not considered in this study. Previous studies report that brain activation changes depending on the complexity of calculations and anxiety levels, with differences in the left–right trend 52 , 53 . However, we observed no clear differences in the changes between the left and right sides in this study. Deoxygenated hemoglobin (deOxyHb) significantly decreased immediately after the start of the task, suggesting activation of brain function. Caffeine is known to constrict cerebral blood vessels and decrease cerebrate blood flow throughout the brain 54 , 55 , 56 , but the time to reach maximum blood concentration is 30–120 min after oral intake 57 . In previous studies, the caffeine intake ranged from 200 to 250 mg, approximately ten times higher than the caffeine intake in this study (green tea: 28 mg/roasted green tea: 26 mg). Regarding green tea ingredients such as catechins and theanine, one study reported a decrease in error rates and an improvement in simple reaction time in the Cognitrax Continuous Performance Test 58 . Theanine has been shown to directly affect the brain by increasing alpha waves without inducing drowsiness 59 , suggesting potential benefits such as improved working memory and relaxation 58 , 60 . However, the amounts of catechins (green tea: 94.4 mg/roasted green tea: 37.4 mg) and theanine (green tea: 6 mg/roasted green tea: not detected) ingested by participants in this study were considerably lower than those ingested by participants in previous studies (catechins 336.4 mg; theanine 97-200 mg). Other tea ingredients, such as the green tea polyphenol epigallocatechin-3-gallate (EGCG), have been shown to have physiologically neuroprotective effects 61 . EGCG at a low dose (135 mg) has been shown to decrease OxyHb and totalHb, with no change in deOxyHb 62 . Caffeine has been shown to decrease OxyHb; however, a previous study 63 found that when caffeine was combined with l -theanine, the effect of caffeine on OxyHb disappeared, with no change in deOxyHb. Although we found similar results to those of previous studies, specific indicators related to acute cognitive performance due to tea components such as caffeine and theanine have not been definitively identified 64 , 65 , 66 . Considering the absorption time and intake amount, it is difficult to conclude that the ingestion of tea ingredients, such as caffeine and theanine, affected the capillary blood flow response in our study.
Other factors that can influence physiological indices over a short duration and in small quantities include aroma components. Aromatic compounds such as those found in plant-derived essential oils are known to have relaxation effects. For instance, orange essential oil has been reported to alleviate anxiety 67 , whereas rose oil is associated with an increased sense of happiness 68 , 69 . Igarashi et al. 70 conducted psychological and physiological measurement experiments on female university students, exposing them to odor stimuli from rose and orange oils. Their results indicated that these odor stimuli induced an increase in psychological states such as “comfortable”, “relaxed”, and “natural” feelings, accompanied by a significant decrease in OxyHb concentration in the prefrontal cortex. Thus, olfactory stimuli such as aromatic oils have long been known to have mental relaxation effects. Regarding the aroma components of green tea, animal and human experiments have shown that green tea’s odor and linalool have anti-stress effects 71 , 72 , 73 , 74 . Furthermore, an experiment investigating the effects of odor stimuli from water and two types of green tea shows that the aroma of green tea increases feelings of relaxation and vitality following exposure to stress 15 , 16 . These results indicate that different types of green tea have varied effects on brain activity, task performance, and mood; the results of roasted green tea in this study support these conclusions. In addition, Höferl et al. 17 examined autonomic nervous system activity (heart rate, blood pressure, and cutaneous electrical activity) through the inhalation of linalool, showing that the odorant relieved stress.
Ohata et al. 75 investigated the effects of 2,3-dimethylylalazine (3DP), which has a nutty or cooked rice-like aroma, and 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF), known for its caramel-like and sweet flavor, on mood and physiological responses by the Maillard reaction. They reported that odor stimulations significantly increased miosis rate by the pupillary light reflex and fingertip temperature and significantly decreased OxyHb (for 2 min), especially in the frontal region. 3DP has the same basis as 2-ethyl-3,5-dimethylpyrazine, which is contained in the green tea and roasted green tea used in this study. Naturally, these are different chemical substances, but 2-ethyl-3,5-dimethylpyrazine is described as having an almond-like aroma; from an organic chemistry standpoint, it is highly likely that when inhaled, it will react similarly to 3DP. In addition to 3DP and DMHF, this study used Maillard reaction reagents and found similar physiological responses to all three chemicals, leading to the conclusion that odorant stimuli produced by the Maillard reaction can induce physiological relaxation by promoting mood and inhibiting sympathetic nerve activity. The Maillard reaction is a chemical reaction in which reducing sugars (carbohydrates) and amino compounds (proteins) are heated to generate brown substances such as melanoidin. In the production of roasted green tea, the Maillard reaction occurs during the roasting process, resulting in a brown color and distinctive aroma. Pyrazines are known to be components of the unique aroma of roasted green tea. Green tea also contains 2-ethyl-3,5-dimethylpyrazine, albeit to a lesser extent than roasted green tea. Therefore, we suggest that the physiological response in OxyHb observed in this study was due to a change in mood induced by aromatic stimulation of pyrazines produced by the Maillard reaction.
It has been reported that TBV responds to stress, correlates with the difficulty level of mental tasks 76 , 77 , and decreases during mental tasks compared with resting conditions. However, the physiological responses were extremely small in this study and hardly changed under the tea conditions. In the hot water condition, as in the other physiological responses, TBV started at a low level, likely associated with tension related to participation in the experiment, but increased during the repeated mental tasks, which is consistent with previous research. In contrast, under the tea condition, it is possible that the aromatic stimulation of the tea aroma caused peripheral vasoconstriction measured at the nose tip rather than a stress response to the mental arithmetic task. As Ohata et al. reported, if these odorant stimuli inhibited sympathetic nervous system activity and promoted both physiological and subjective relaxation, they might also be related to the unified response across multiple indicators of parasympathetic nervous system activity observed in this study. This study was limited to a number of young male participants. Moreover, considering the residual components of tea, it was not possible to counterbalance the order of water and tea, and to conduct with different tea conditions on the same day. Consequently, it was not possible to adequately confirm the changes associated with habituation to the experiment and the mental tasks, and directly compare the differences between types of tea. In the future, it will be necessary to expand the range of participants and explore a more robust experimental procedure or indicators to confirm the effects of habituation and types of tea. It is also desirable to investigate the effects of olfactory stimulation alone, rather than oral ingestion, on the aroma components suggested as factors for positive effects.
This study comprehensively assessed various physiological responses, task performance, and subjective evaluations of the flavor and aroma of water, green tea, and roasted green tea (hojicha). Tea intake improved mental arithmetic task performance compared to the hot water condition. Furthermore, the results revealed tea consumption effects in promoting refreshment and facilitating subjective feelings of fatigue reduction and recovery. Through examining the multiple indicators, aromatic stimulation from tea drinks suggests the potential to exert positive physiological and subjective effects in a short duration and in small quantities, similar to amounts consumed daily. It is important to note that a limited number of young male participants and the effects of participation in physiological experiments and familiarity with tasks may have influenced the results. Further investigations are warranted to explore these aspects. It is also necessary to investigate the effects of aroma stimulation alone, without oral ingestion.
Data availability
All data are maintained by the administrator in the university laboratory repository. The data sets generated during the current study are available from the corresponding author on reasonable request.
Kobayashi, M. et al. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. J. Agric. Food Chem. 62 , 2881–2890 (2014).
Article CAS PubMed Google Scholar
Suzuki, E. et al. Green tea consumption and mortality among Japanese elderly people: The prospective Shizuoka elderly cohort. Ann. Epidemiol. 19 , 732–739 (2009).
Article PubMed Google Scholar
Kim, H. M. & Kim, J. The effects of green tea on obesity and type 2 diabetes. Diabetes Metab. J. 37 , 173–175 (2013).
Article PubMed PubMed Central Google Scholar
Yamane, T. et al. Inhibition of azoxymethane-induced colon carcinogenesis in rat by green tea polyphenol fraction. Jpn. J. Cancer Res. 82 , 1336–1339 (1991).
Article CAS PubMed PubMed Central Google Scholar
Hou, I.-C., Amarnani, S., Chong, M. T. & Bishayee, A. Green tea and the risk of gastric cancer: Epidemiological evidence. World J. Gastroenterol. 19 , 3713–3722 (2013).
Hursel, R. & Westerterp-Plantenga, M. S. Catechin- and caffeine-rich teas for control of body weight in humans. Am. J. Clin. Nutr. 98 , 1682–1693 (2013).
Article Google Scholar
Peng, X. et al. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 4 , 6251 (2014).
Wu, C.-H. et al. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes. Res. 11 , 1088–1095 (2003).
Dulloo, A. G. et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 70 , 1040–1045 (1999).
Wang, H. et al. Effects of catechin enriched green tea on body composition. Obesity 18 , 773–779 (2010).
Kakuda, T., Nozawa, A., Unno, T., Okamura, N. & Okai, O. Inhibiting effects of theanine on caffeine stimulation evaluated by EEG in the rat. Biosci. Biotechnol. Biochem. 64 , 287–293 (2000).
Unno, K. et al. Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic. Res. 45 , 966–974 (2011).
Kobayashi, K. et al. Effects of L-theanine on the release of alpha-brain waves in human volunteers. J. Agric. Chem. Soc. Jpn. 72 , 153–157 (1998).
CAS Google Scholar
Nobre, A. C., Rao, A. & Owen, G. L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac. J. Clin. Nutr. 17 (suppl), 167–168 (2008).
CAS PubMed Google Scholar
Murao, S., Yoto, A. & Yokogoshi, H. Effect of smelling green tea on mental status and EEG activity. Int. J. Affect. Eng. 12 , 37–43 (2013).
Yoto, A., Murao, S., Nakamura, Y. & Yokogoshi, H. Intake of green tea inhibited increase of salivary chromogranin A after mental task stress loads. J. Physiol. Anthropol. 33 , 20 (2014).
Höferl, M., Krist, S. & Buchbauer, G. Chirality influences the effects of Linalool on physiological parameters of stress. Planta Med. 72 , 1188–1192 (2006).
Neo Marketing, Inc. A Study of the Drinking Effect of Roasted Green Tea (Hojicha): A Questionnaire Survey and EEG Measurements . https://prtimes.jp/main/html/rd/p/000000120.000003149.html (2019).
Matsumoto, T., Ushiroyama, T., Kimura, T., Hayashi, T. & Moritani, T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. Biopsychosoc. Med. 1 , 1–8 (2007).
Jensen, B. K. Menstrual cycle effects on task performance examined in the context of stress research. Acta Psychol. 50 , 159–178 (1982).
Article CAS Google Scholar
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Lawrence Erlbaum Associates, 1988).
Google Scholar
Working Group for Occupational Fatigue. Jikaku-sho shirabe [Subjective Fatigue Feelings] . http://square.umin.ac.jp/of/service.html (2012).
Hart, S. G. & Staveland, L. E. Development of NASA-TLX (task load index): Results of empirical and theoretical research. In Human Mental Workload (eds Hancock, P. A. & Meshkati, N.) 139–183 (North-Holland Press, 1988).
Chapter Google Scholar
Miyake, S. & Kumashiro, M. Subjective mental workload assessment technique—An introduction to NASA-TLX and SWAT and a proposal of simple scoring methods. Jpn. J. Ergon. 29 , 399–408 (1993).
Jackson, S. A. & Marsh, H. W. Development and validation of a scale to measure optimal experience: The flow state scale. J. Sport Exerc. Psychol. 18 , 17–35 (1996).
Kawabata, M. & Harimoto, F. Evaluation of the Japanese version of the flow state scale: Part 1. Jpn. Soc. Phys. Educ. Health Sport Sci. 51 , 183 (2000).
Zakay, D. & Block, R. A. Prospective and retrospective duration judgments: An executive-control perspective. Acta Neurobiol. Exp. (Wars.) 64 , 319–328 (2004).
Turner, J. R. et al. Graded mental arithmetic as an active psychological challenge. Int. J. Psychophysiol. 3 , 307–309 (1986).
Article ADS CAS PubMed Google Scholar
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 4 , 1–12 (2013).
Tagata, C. et al. Effects of green tea and roasted green tea intake on mental task performance and subjective evaluation. Jpn. Pharmacol. Ther. 51 , 1377–1388 (2023).
Toichi, M., Sugiura, T., Murai, T. & Sengoku, A. A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R-R interval. J. Auton. Nerv. Syst. 62 , 79–84 (1997).
DeBoer, R. W., Karemaker, J. M. & Strackee, J. Hemodynamic fluctuations and baroreflex sensitivity in humans: A beat-to-beat model. Am. J. Physiol. 253 , H680–H689 (1987).
Miyake, S., Kurosaka, C. & Kuraoka, H. Plethysmogram amplitude can be simply measured by the standard deviation of its waveform. In Proc. Japan Human Factors and Ergonomics Society Hokkaido Branch 2022 Annual Meeting 23–26 (2022).
Nehlig, A., Daval, J.-L. & Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 17 , 139–170 (1992).
Giesbrecht, T., Rycroft, J. A., Rowson, M. J. & De Bruin, E. A. The combination of L-theanine and caffeine improves cognitive performance and increases subjective alertness. Nutr. Neurosci. 13 , 283–290 (2010).
Knutti, R., Rothweiler, H. & Schlatter, C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur. J. Clin. Pharmacol. 21 , 121–126 (1981).
Boucsein, W. & Backs, R. W. Engineering psychophysiology as a discipline: Historical and theoretical aspects. In Engineering Psychophysiology: Issues and Applications (eds Backs, R. W. & Boucsein, W.) 3–30 (Lawrence Erlbaum Associates Publishers, 2000).
del Paso, G. A. R., Langewitz, W., Mulder, L. J. M., van Roon, A. & Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 50 , 477–487 (2013).
Hayano, J. et al. Spectral component of heart rate variability as an index of autonomic nervous function. Seibutsu Butsuri 28 , 198–202 (1988).
Pomeranz, B. et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. Heart Circ. Physiol. 248 , H151–H153 (1985).
Pagani, M. et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59 , 178–193 (1986).
del Paso, G. A. R., Langewitz, W., Robles, H. & Pérez, N. A between-subjects comparison of respiratory sinus arrhythmia and baroreceptor cardiac reflex sensitivity as non-invasive measures of tonic parasympathetic cardiac control. Int. J. Psychophysiol. 22 , 163–171 (1996).
Goldstein, D. S., Bentho, O., Mee-Yeong Park, M.-Y. & Yehonatan Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 96 , 1255–1261 (2011).
Billman, G. E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4 , 1–5 (2013).
Ciccone, A. B. et al. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 56 , 674–678 (2017).
Yasumasu, T., del Paso, G. A. R., Takahara, K. & Nakashima, Y. Reduced baroreflex cardiac sensitivity predicts increased cognitive performance. Psychophysiology 43 , 41–45 (2006).
del Paso, G. A. R., Mata, J. L. & Martín-Vázquez, M. Relationships between baroreceptor cardiac reflex sensitivity and cognitive performance: Modulations by gender and blood pressure. Psychophysiology 49 , 138–144 (2012).
Guzik, P. et al. Correlations between the Poincaré plot and conventional heart rate variability parameters assessed during paced breathing. J. Physiol. Sci. 57 , 63–71 (2007).
Allen, J. J. B., Chambers, A. S. & Towers, D. N. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biol. Psychol. 74 , 243–262 (2007).
Nunan, D., Sandercock, G. R. H. & Brodie, D. A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 33 , 1407–1417 (2010).
Watanabe, T., Sekine, R., Mizuno, T. & Miwa, M. Development of portable, wireless and smartphone controllable near-infrared spectroscopy system. Adv. Exp. Med. Biol. 923 , 385–392 (2016).
Verner, M., Herrmann, M. J., Troche, S. J., Roebers, C. M. & Rammsayer, T. H. Cortical oxygen consumption in mental arithmetic as a function of task difficulty: A near-infrared spectroscopy approach. Front. Hum. Neurosci. 7 , 217 (2013).
Horiuchi-Hirose, M. & Sawada, K. Rightward shift of two-channel NIRS-defined prefrontal cortex activity during mental arithmetic tasks with increasing levels of state anxiety. Symmetry 12 , 538 (2020).
Article ADS CAS Google Scholar
Mathew, R. J. Caffeine induced changes in cerebral circulation. Stroke 17 , 1337–1337 (1986).
Addicott, M. A. et al. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum. Brain Mapp. 30 , 3102–3114 (2009).
Chang, D. et al. Caffeine caused a widespread increase of resting brain entropy. Sci. Rep. 8 , 2700 (2018).
Article ADS PubMed PubMed Central Google Scholar
Blanchard, J. & Sawers, S. J. A. The absolute bioavailability of caffeine in man. Eur. J. Clin. Pharmacol. 24 , 93–98 (1983).
Baba, Y. et al. Effects of l-theanine on cognitive function in middle-aged and older subjects: A randomized placebo-controlled study. J. Med. Food 24 , 333–341 (2021).
Gomez-Ramirez, M., Kelly, S. P., Montesi, J. L. & Foxe, J. J. The effects of L-theanine on alpha-band oscillatory brain activity during a visuo-spatial attention task. Brain Topogr. 22 , 44–51 (2009).
Kimura, K., Ozeki, M., Juneja, L. R. & Ohira, H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 74 , 39–45 (2007).
Scholey, A. et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite 58 , 767–770 (2012).
Wightman, E. L., Haskell, C. F., Forster, J. S., Veasey, R. C. & Kennedy, D. O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Hum. Psychopharmacol. Clin. Exp. 27 , 177–186 (2012).
Dodd, F. L., Kennedy, D. O., Riby, L. M. & Haskell-Ramsay, C. F. A double-blind, placebo-controlled study evaluating the effects of caffeine and L-theanine both alone and in combination on cerebral blood flow, cognition and mood. Psychopharmacology 232 , 2563–2576 (2015).
Einöther, S. J. & Martens, V. E. Acute effects of tea consumption on attention and mood. Am. J. Clin. Nutr. 98 , 1700–1708 (2013).
Baba, Y. et al. Effect of daily intake of green tea catechins on cognitive function in middle-aged and older subjects: A randomized, placebo-controlled study. Molecules 25 , 4265 (2020).
Wightman, E. L., Haskell, C. F., Forster, J. S., Veasey, R. C. & Kennedy, D. O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Hum. Psychopharmacol. 27 , 177–186 (2012).
Lehrner, J., Marwinski, G., Lehr, S., Johren, P. & Deecke, L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol. Behav. 86 , 92–95 (2005).
Fitzgerald, M. et al. The effect of gender and ethnicity on children’s attitudes and preferences for essential oils: A pilot study. Explore 3 , 378–385 (2007).
Fitzgerald, M., Culbert, T., Finkelstein, M., Green, M. & Liu, M. The effect of gender and ethnicity on children’s attitudes and preferences for essential oils: A follow up study. Explore 6 , 172 (2010).
Igarashi, M., Ikei, H., Song, C. & Miyazaki, Y. Effects of olfactory stimulation with rose and orange oil on prefrontal cortex activity. Complement. Ther. Med. 22 , 1027–1031 (2014).
Watanabe, T. et al. Green odor and depressive-like state in rats: Toward an evidence-based alternative medicine? Behav. Brain Res. 224 , 290–296 (2011).
Souto-Maior, F. N. et al. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 100 , 259–263 (2011).
Linck, V. M. et al. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 17 , 679–683 (2010).
Kako, H., Fukumoto, S., Kobayashi, Y. & Yokogoshi, H. Effects of direct exposure of green odour components on dopamine release from rat brain striatal slices and PC12 cells. Brain Res. Bull. 75 , 706–712 (2008).
Ohata, M., Zhou, L., Yada, Y., Yokoyama, I. & Arihara, K. 2,3-Dimethylpyrazine (3DP) and 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF) generated by the Maillard reaction in foods affect autonomic nervous activity and central nervous activity in human. Biosci. Biotechnol. Biochem. 84 , 1894–1902 (2020).
Miyake, S., Hashimoto, M., Watanabe, T., Takae, Y. & Shiraishi, Y. Human responses to a workload change. In Proc. Japan Human Factors and Ergonomics Society 48th Annual Meeting , Vol. 48, 2344–2348 (2004).
Miyake, S., Hashimoto, M., Miao, T. & Shimizu, T. Effects of a subsidiary task on a multi-attribute task—Individual differences in responses. In Proc. 10th World Congr. Exhib. Intell. Transp. Syst. Serv. CD-ROM #4084 (2003).
Download references
This study was funded by ITOEN, Ltd. under a joint research contract (PI: Chie Kurosaka). ITOEN, Ltd. funded the study and provided support in the form of salaries for author C.T., S.N. and M.K.
Author information
Authors and affiliations.
Department of Human, Information and Life Sciences, School of Health Sciences, University of Occupational and Environmental Health, Japan, Kitakyushu, Fukuoka, Japan
Chie Kurosaka
Central Research Institute, ITOEN, Ltd., Makinohara, Shizuoka, Japan
Chika Tagata, Sae Nakagawa & Makoto Kobayashi
Graduate School of Science and Technology, Chitose Institute of Science and Technology, Chitose, Hokkaido, Japan
Shinji Miyake
You can also search for this author in PubMed Google Scholar
Contributions
C.K. oversaw the research project. C.K. performed the experiment and data collection and wrote the original draft. C.K. and S.M. analyzed the data and validation. All authors designed the research plan and reviewed the manuscript.
Corresponding author
Correspondence to Chie Kurosaka .
Ethics declarations
Competing interests.
Author S.M. has received consultant honoraria from the funding company. This competing interest has been approved by the Conflict of Interest Committee of Occupational and Environmental Health, Japan, ensuring the fairness and impartiality of the research. Other authors declare that there are no competing interests.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary tables., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kurosaka, C., Tagata, C., Nakagawa, S. et al. Effects of green tea and roasted green tea on human responses. Sci Rep 14 , 8588 (2024). https://doi.org/10.1038/s41598-024-59383-y
Download citation
Received : 19 January 2024
Accepted : 10 April 2024
Published : 13 April 2024
DOI : https://doi.org/10.1038/s41598-024-59383-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Tea is the most popular beverage in the world after water. It’s a simple preparation of pouring hot water over cured leaves of the Camellia sinensis plant. The first recording of tea described it as a medicinal beverage in China in the 3 rd century AD. Merchants helped its popularity to spread quickly across continents. In the early 19 th century, Great Britain popularized the concept of afternoon tea, a break from one’s routine in which tea is served alongside sandwiches and baked goods such as scones. The flavor of tea varies by where the tea leaves are harvested and how they are grown and processed. Black tea is the most popular worldwide, followed by green, oolong, and white tea. [1]
Herbal teas are not made from the Camellia plant but from dried herbs, spices, flowers, fruit, seeds, roots, or leaves of other plants; they do not typically contain caffeine as do traditional teas.
- Caffeine (traditional teas, not herbal)
- Flavonols – myricetin, quercetin, kaempferol
- Theaflavins – formed when black tea leaves are oxidized
- Catechins – found in green tea; epigallocatechin-3 gallate (EGCG) is the main form
Most traditional teas do not contain a significant amount of nutrients, but are rich in polyphenols. These are plant chemicals that give teas their distinct flavor and aroma and may have health-promoting properties.
Tea and Health
Animal studies suggest potential health benefits of tea due to its high polyphenol content. Human studies have generally been less conclusive, yet show promise. Observational research has found that tea consumption of 2-3 cups daily is associated with a reduced risk of premature death, heart disease, stroke, and type 2 diabetes. [2] However, there may be an increased risk of esophageal and stomach cancers from drinking tea that is too hot (more than 131-140° F [55-60° C]). [2,3] Randomized controlled trials are needed to confirm if these healthful and harmful associations are causal. In the meantime, there appears to be little risk associated with drinking tea except for frequent consumption of very hot tea. So pick a color, let it cool, and enjoy a cup!

Spotlight on tea and antioxidants
Indeed, one reason for conflicting results in observational studies may be the wide variations in tea types with varying flavonoid content. [4] Where the tea leaves are grown, the specific blend of tea leaves, type of processing, and addition of ingredients such as milk, honey, and lemon can alter specific flavonoid content. How accurately people report their tea intake (e.g., type, amount, brew strength) and their overall diet (e.g., do they eat other foods rich in flavonoids?) are other factors that need to be clarified as they can affect study results. For example:
- Some research suggests that the protein and possibly the fat in milk may reduce the antioxidant capacity of tea. [5] Flavonoids are known to “deactivate” when binding to proteins so this theory makes scientific sense. [6]
- One study that analyzed the effects of adding skimmed, semi-skimmed, and whole milk to tea concluded that skimmed milk significantly reduced the antioxidant capacity of tea. Higher-fat milks also reduced the antioxidant capacity of tea, but to a lesser degree. [7] All said, in practice it’s important to keep in mind that tea—even tea with a splash of milk—can be a healthful drink.
Learn more about some of the research on tea on health:
A Cochrane review found very few large, long-term studies that examined green or black tea for the primary prevention of cardiovascular disease . The authors noted that tea appears to show favorable effects on cardiovascular risk factors based on the available evidence, but this is based on only a modest number of small, short-term clinical trials so firm conclusions cannot be made. [8]
- Stroke and mortality —Polyphenols, the antioxidants abundant in tea, have been shown to reduce the risk of death due to cardiovascular disease, [9] including stroke. [7,10] In one study of 77,000 Japanese men and women, green tea and oolong tea consumption was linked with lower risk of death from cardiovascular disease. [11] Another cohort of 82,369 Japanese men and women followed for an average of 13 years found that those who drank 2-3 cups of green tea daily had a 14% reduced risk of stroke, and drinking 4 or more cups daily was associated with a 20% reduced risk. [12] Other large-scale studies show that black tea may contribute to heart health, [13] with research suggesting that drinking at least 3 cups of either black or green tea a day appears to reduce the risk of stroke by 21%. [14]
- High blood pressure —Tea flavonoids may help to keep the lining of blood vessels smooth and elastic. In a study of green and oolong tea consumption, regular consumption for one year reduced the risk of developing hypertension. [15] Long-term regular consumption of black tea has also been shown to lower blood pressure. [16] A meta-analysis that combined the results of 14 randomized controlled trials found that green tea extracts produced a small reduction in blood pressure in overweight and obese adults, though the authors noted that the trials included a small number of participants and therefore strong conclusions could not be drawn. [17]
- Cholesterol —Tea flavonoids have antioxidant properties that may prevent the oxidation of LDL cholesterol particles that could lead to inflammation and hardening of arteries. However, there is still a lack of consistent evidence in human studies showing a benefit. A meta-analysis of 15 randomized controlled trials found no significant effect of black tea on cholesterol levels (including total cholesterol, LDL and cholesterol) in both healthy subjects and people with coronary artery disease. [18] Two separate meta-analyses of randomized controlled trials looking at tea intake and cholesterol levels found that both black and green tea lowered LDL blood cholesterol levels (as measured in milligrams per decileter [mg/dL]). With green tea consumption, fasting total cholesterol and LDL cholesterol was significantly lowered by 7 mg/dL and 2 mg/dL, respectively. Black tea reduced LDL cholesterol by almost 5 mg/dL. However, the authors acknowledged that most of the studies included were of low quality, with short study durations and a small number of participants. [19,20]
Epigallocatechin-3-gallate (EGCG) in green tea has been shown in animal and cell studies to prevent the growth of cancer cells and cause them to die. [1] Green and black tea extracts have been shown in animal studies to reduce the risk or delay the progression of cancer. [21] Green tea might also have a positive effect in reducing risk of breast, ovarian, prostate, and endometrial cancers, though evidence is limited. [22] A meta-analysis of 41 prospective studies found no decreased risk of five cancers (breast, colorectal, liver, prostate, stomach) with black tea intake (about 3 cups daily). [21] Overall, human studies about tea and cancer are limited and results are inconsistent. Learn more about cancer and antioxidants.
A positive association has been found between drinking hot tea and a greater risk of esophageal cancer. It is believed that very hot beverages may cause cell injury that could lead to cancer. [3] Studies showing this association with tea have been largely in Asia and the Middle East. There is less evidence in Western populations, where beverages including coffee and tea are usually consumed at more moderate temperatures. Westerners also may add milk or cream to very hot beverages, immediately lowering the temperature. A meta-analysis of 16 case-control studies conducted in China, India, Iran and other countries in Europe and South America found an association of increased risk of esophageal cancer with higher consumption of both very hot beverages and foods. [23] It appears that cancer risk of this type is directly linked to temperature, rather than a specific component of the food or beverage.
A large prospective study of 50,045 Iranian men and women followed for about 10 years found a 90% increased risk of esophageal cancer when comparing those drinking “very hot” versus “cold/lukewarm” tea. It also found that the shorter the time from pouring the tea into a cup to drinking it was associated with increased risk. In May 2016, after a review of available research, the International Agency for Research on Cancer (IARC) concluded that “drinking very hot beverages above 65 C (140 F)” is “probably carcinogenic.” [3] They acknowledged certain limitations of existing studies that precluded listing a “carcinogenic” label, one being that data on the actual temperature of tea are self-reported, in which the perception of “hot” may vary among ethnicities and populations.
In 2018, a large cohort study of 456,155 men and women in China followed for a median of nine years found that participants who drank burning-hot tea daily along with excessive alcohol had five times the risk of developing esophageal cancer than those who drank less of both beverages. Those who drank burning-hot tea daily and smoked tobacco had double the risk of developing this cancer. [24]

Is decaffeinated tea healthy?
To decaffeinate tea, there are different methods. One process uses an organic chemical solvent (either ethyl acetate or methylene chloride) that also removes most of tea’s polyphenols. The residual amount of the chemical after processing is minimal to none, and no research has shown negative health effects. Another method called “effervescence” uses water and carbon dioxide, which retains the majority of polyphenols. Both methods apply the chemical or gas onto moistened tea leaves, which bonds to the caffeine; when the leaves are dried, the caffeine evaporates along with the solvent/gas. If you wish to know which processing method is used, check the package label or contact the manufacturer.
Herbal teas are naturally caffeine-free and do not undergo a decaffeination process.
Most research looks at the health effects of traditional teas, not decaffeinated. Decaffeinated tea may lose polyphenols that are associated with health benefits, depending on the processing method. Polyphenol content varies widely among teas even before the decaffeinated process, so it is hard to know the exact amount that remains. Regardless of decaffeination type, tea is still considered a healthful beverage choice.
If you visit a tea shop, you may be surprised and overwhelmed by just how many different teas exist! Traditional teas originating from the Camellia sinensis plant include black, white, green, yellow, and oolong, all of which contain caffeine. Black tea is made by crushing and drying fresh tea leaves and allowing them to ferment, which oxidizes the leaves and changes their color and flavor. Oolong tea is partly fermented, and green tea undergoes no fermentation. Matcha is a special form of green tea in which the dried leaves are ground into a fine powder.
Decaffeinated teas have been processed to remove most of the naturally occurring caffeine from the leaves. They may still contain trace amounts of caffeine. This is done by using carbon dioxide, ethyl acetate, methylene chloride, or water processing.

Teas are packaged in tea bags, tea sachets, or as loose-leaf. Loose-leaf teas sold in tin canisters or sacks allow you to control how much tea to use, using more to create a stronger flavor or less for more mellowness. Tea bags and sachets hold a standard amount of leaves for optimum flavor and are portable.
There are five elements to avoid to keep tea as fresh as possible: light, heat, moisture, odor, and air. Tea bags should be stored in their original container or placed in a sealed plastic bin. Loose-leaf teas should be stored in an airtight container. Place all teas in a dark cupboard at a consistent room temperature. Tea tends to absorb odors from food and even other strongly scented teas, so keep them separate. Freezing and refrigerating is not recommended as the moisture introduced can degrade the tea.
If unopened, tea will last about one year beyond the “best by” date. After opening, packaged and loose-leaf teas last about one year. However, some black and oolong teas can last up to two years, and more delicate teas may last only 6 months. The flavor is your best guide to determining how long to keep a tea in your cupboard.
Avoid purchasing expensive bottled teas or teas from shops that contain added sweeteners. To enjoy the maximum benefits of drinking tea, consider brewing your own at home. You can serve it hot, or make a pitcher of home-brewed iced tea during warmer months.
Black and oolong teas are generally steeped in hot or boiling water (about 210º F) and brewed for about 4-5 minutes. Green tea is steeped at a slightly lower temperature 180º F from 4-15 minutes. The longer tea steeps, the stronger the flavor with bitter notes.
Additives of sugar, cream, or milk can reduce the polyphenol content of tea. For the greatest health benefits, try serving tea plain or without too many additives. A dash of vanilla or cinnamon can mimic sweetness. Some fruit-flavored herbal teas taste naturally sweet to the palate without added sweeteners.

Sparkling Iced Tea with Lemon, Cucumber, and Mint
Did you know.
- What western coffeehouses commonly refer to as “chai” is more accurately called “masala chai” or “spiced tea” (“chai” is simply the Hindi word for “tea”). The recipe for this beverage has varied widely across time and place, but today is often made from black tea mixed with spices like cinnamon, cardamom, cloves, ginger, and peppercorns, and served with milk and sweetener.
- Japan has a rich tea culture, best known for the ceremonial preparation and drinking of green tea. A traditional Japanese tea ceremony, also called “the way of tea” (茶道 [sadō or chadō] or 茶の湯 [chanoyu]) can take up to multiple hours!
- Some advertisements claim that tea can speed weight loss, but research on the effects of green tea and fat reduction have shown little promise of weight loss benefits. [25,26] Moreover, it’s best to skip any so-called “weight loss” teas that may contain potentially harmful substances such as laxatives.
- Yerba mate, or mate, is a popular herbal tea in South America made from the dried leaves of the Ilex paraguariensis tree. It shares traits with coffee in that they both contain the antioxidant polyphenol chlorogenic acid and similar amounts of caffeine. There has been concern that certain processing methods of mate, such as drying the leaves with smoke, may introduce polycyclic aromatic hydrocarbons (the carcinogenic substances also found in grilled or smoked meats). [27] Some research links drinking large amounts of mate over time with increased risk of certain cancers, including head and neck, stomach, bladder, and lung, but it is not clear if the risk is due to drinking it very hot or the presence of polycyclic aromatic hydrocarbons. Therefore, mate fans may choose to drink it at moderate temperatures or to purchase brands that process it by air-drying rather than smoking.
- Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients . 2019 Jan;11(1):39.
- Yi M, Wu X, Zhuang W, Xia L, Chen Y, Zhao R, Wan Q, Du L, Zhou Y. Tea Consumption and Health Outcomes: Umbrella Review of Meta‐Analyses of Observational Studies in Humans. Molecular Nutrition & Food Research . 2019 Jun 19:1900389.
- Loomis D, Guyton KZ, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K. Carcinogenicity of drinking coffee, mate, and very hot beverages. The Lancet Oncology . 2016 Jul 1;17(7):877-8.
- Dwyer JT, Peterson J. Tea and flavonoids: where we are, where to go next. The American journal of clinical nutrition . 2013 Oct 30;98(6):1611S-8S.
- Ryan L, Petit S. Addition of whole, semiskimmed, and skimmed bovine milk reduces the total antioxidant capacity of black tea. Nutr Res . 2010;30:14-20.
- Arts MJ, Haenen GR, Wilms LC, et al. Interactions between flavonoids and proteins: effect on the total antioxidant capacity. J Agric Food Chem . 2002;50:1184-7.
- Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr . 2010;140:600-4.
- Hartley L, Flowers N, Holmes J, Clarke A, Stranges S, Hooper L, Rees K. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews . 2013(6).
- Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA . 2006;296:1255-65.
- Larsson SC, Virtamo J, Wolk A. Black tea consumption and risk of stroke in women and men. Ann Epidemiol . 2013;23:157-60.
- Mineharu Y, Koizumi A, Wada Y, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health . 2011;65:230-40.
- Kokubo Y, Iso H, Saito I, Yamagishi K, Yatsuya H, Ishihara J, Inoue M, Tsugane S. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: the Japan public health center-based study cohort. Stroke . 2013 May;44(5):1369-74.
- Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke . 2009;40:1786-92.
- Nechuta S, Shu XO, Li HL, et al. Prospective cohort study of tea consumption and risk of digestive system cancers: results from the Shanghai Women’s Health Study. Am J Clin Nutr . 2012;96:1056-63.
- Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ. The protective effect of habitual tea consumption on hypertension. Arch Intern Med . 2004;164:1534-40.
- Hodgson JM, Puddey IB, Woodman RJ, et al. Effects of black tea on blood pressure: a randomized controlled trial. Arch Intern Med . 2012;172:186-8.
- Li G, Zhang Y, Thabane L, Mbuagbaw L, Liu A, Levine MA, Holbrook A. Effect of green tea supplementation on blood pressure among overweight and obese adults: a systematic review and meta-analysis. Journal of hypertension . 2015 Feb 1;33(2):243-54.
- Wang D, Chen C, Wang Y, Liu J, Lin R. Effect of black tea consumption on blood cholesterol: a meta-analysis of 15 randomized controlled trials. PLoS One . 2014 Sep 19;9(9):e107711.
- Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. The American journal of clinical nutrition . 2011 Jun 27;94(2):601-10.
- Zhao Y, Asimi S, Wu K, Zheng J, Li D. Black tea consumption and serum cholesterol concentration: Systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition . 2015 Aug 1;34(4):612-9.
- Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, He J. Tea consumption and the risk of five major cancers: a dose–response meta-analysis of prospective studies. BMC Cancer . 2014 Dec;14(1):197.
- Johnson R, Bryant S, Huntley AL. Green tea and green tea catechin extracts: an overview of the clinical evidence. Maturitas . 2012;73:280-7.
- Andrici J, Eslick GD. Hot food and beverage consumption and the risk of esophageal cancer: a meta-analysis. American journal of preventive medicine . 2015 Dec 1;49(6):952-60.
- Yu C, Tang H, Guo Y, Bian Z, Yang L, Chen Y, Tang A, Zhou X, Yang X, Chen J, Chen Z. Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: a population-based cohort study. Annals of internal medicine . 2018 Apr 3;168(7):489-97.
- Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst Rev . 2012;12:CD008650.
- Wharton S, Bonder R, Jeffery A, Christensen RA. The safety and effectiveness of commonly-marketed natural supplements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016. Critical reviews in food science and nutrition . 2019 Mar 14:1-7.
- Oranuba E, Deng H, Peng J, Dawsey SM, Kamangar F. Polycyclic aromatic hydrocarbons as a potential source of carcinogenicity of mate. Journal of Environmental Science and Health , Part C. 2019 Jan 2;37(1):26-41.
Last reviewed April 2023
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
- Open access
- Published: 06 April 2010
Beneficial effects of green tea: A literature review
- Sabu M Chacko 1 ,
- Priya T Thambi 1 ,
- Ramadasan Kuttan 2 &
- Ikuo Nishigaki 1
Chinese Medicine volume 5 , Article number: 13 ( 2010 ) Cite this article
124k Accesses
582 Citations
946 Altmetric
Metrics details
The health benefits of green tea for a wide variety of ailments, including different types of cancer, heart disease, and liver disease, were reported. Many of these beneficial effects of green tea are related to its catechin, particularly (-)-epigallocatechin-3-gallate, content. There is evidence from in vitro and animal studies on the underlying mechanisms of green tea catechins and their biological actions. There are also human studies on using green tea catechins to treat metabolic syndrome, such as obesity, type II diabetes, and cardiovascular risk factors.
Long-term consumption of tea catechins could be beneficial against high-fat diet-induced obesity and type II diabetes and could reduce the risk of coronary disease. Further research that conforms to international standards should be performed to monitor the pharmacological and clinical effects of green tea and to elucidate its mechanisms of action.
In recent years, the health benefits [ 1 ] of consuming green tea, including the prevention of cancer [ 2 ] and cardiovascular diseases [ 3 ], the anti-inflammatory [ 4 ], antiarthritic [ 5 ], antibacterial [ 6 ], antiangiogenic [ 7 ], antioxidative [ 8 ], antiviral [ 9 ], neuroprotective [ 10 ], and cholesterol-lowering effects [ 11 ] of green tea and isolated green tea constituents are under investigation. However, adding green tea to the diet may cause other serious health concerns.
The health-promoting effects of green tea are mainly attributed to its polyphenol content [ 12 ], particularly flavanols and flavonols, which represent 30% of fresh leaf dry weight [ 1 ]. Recently, many of the aforementioned beneficial effects of green tea were attributed to its most abundant catechin, (-)-epigallocatechin-3-gallate (EGCG) [ 13 – 15 ]. Green tea extracts are more stable than pure epigallocatechin gallate, one of the major constituents of green tea, because of the presence of other antioxidant constituents in the extract [ 8 ]. In general, herbal medicines are complex mixtures of different compounds that often act in a synergistic fashion to exert their full beneficial effect [ 11 ]. However, relatively few herbal medicines have been well characterized and their efficacy demonstrated in systematic clinical trials as compared to Western drugs. This review article highlights the recent research on the efficacy, action mechanisms, and side effects of green tea and its catechins in in vitro , in vivo , and ex vivo systems [ 16 ].
The review on green tea and its catechins focused on language literature in English. The literature search was conducted in the following databases: Pubmed (1980-2009), EMBASE (1980-2009), Allied and complementary Medicine Database (AMED, 1985-2009) and China Journals Full Text Database (1975-2009). The keywords used were selected from the following terms: green tea, catechins, anticancer, diabetes, polyphenols, in vivo studies, general pharmacology and toxicology. The health benefits and adverse effects of green tea and its catechins were reviewed.
The authors read full articles and reached consensus after discussion. Articles included in the study covered the following effects of green tea: (1) the health benefits in humans and animals, (2) absorption of metal ions and drug-metabolizing enzymes, (3) antioxidation and inhibition of oxidative stress, (4) carbohydrate metabolism and diabetes mellitus, and (5) adverse effects. A total of 105 peer-reviewed papers in English were selected for this review.
Tea is one of the most popular beverages consumed worldwide. Tea, from the plant Camellia sinensis , is consumed in different parts of the world as green, black, or Oolong tea. Among all of these, however, the most significant effects on human health have been observed with the consumption of green tea [ 17 ]. The first green tea was exported from India to Japan during the 17th century. It is estimated that about 2.5 million tons of tea leaves are produced each year throughout the world, with 20% produced as green tea, which is mainly consumed in Asia, some parts of North Africa, the United States, and Europe [ 18 ]. The association between tea consumption, especially green tea, and human health has long been appreciated [ 19 , 20 ]. Green tea and black tea are processed differently during manufacturing. To produce green tea, freshly harvested leaves are immediately steamed to prevent fermentation, yielding a dry, stable product. This steaming process destroys the enzymes responsible for breaking down the color pigments in the leaves and allows the tea to maintain its green color during the subsequent rolling and drying processes. These processes preserve natural polyphenols with respect to the health-promoting properties. As green tea is fermented to Oolong and then to black tea, polyphenol compounds (catechins) in green tea are dimerized to form a variety of theaflavins, such that these teas may have different biological activities.
Green tea composition
The chemical composition of green tea is complex: proteins (15-20% dry weight), whose enzymes constitute an important fraction; amino acids (1-4% dry weight) such as theanine or 5- N- ethylglutamine, glutamic acid, tryptophan, glycine, serine, aspartic acid, tyrosine, valine, leucine, threonine, arginine, and lysine; carbohydrates (5-7% dry weight) such as cellulose, pectins, glucose, fructose, and sucrose; minerals and trace elements (5% dry weight) such as calcium, magnesium, chromium, manganese, iron, copper, zinc, molybdenum, selenium, sodium, phosphorus, cobalt, strontium, nickel, potassium, fluorine, and aluminum; and trace amounts of lipids (linoleic and α-linolenic acids), sterols (stigmasterol), vitamins (B, C, E), xanthic bases (caffeine, theophylline), pigments (chlorophyll, carotenoids), and volatile compounds (aldehydes, alcohols, esters, lactones, hydrocarbons). Due to the great importance of the mineral presence in tea, many studies have determined their levels in tea leaves and their infusions (Table 1 ) [ 21 ]. Fresh leaves contain, on average, 3-4% of alkaloids known as methylxanthines, such as caffeine, theobromine, and theophylline [ 22 ]. In addition, there are phenolic acids such as gallic acids and characteristic amino acid such as theanine present [ 22 ].
Green tea contains polyphenols, which include flavanols, flavandiols, flavonoids, and phenolic acids; these compounds may account for up to 30% of the dry weight. Most of the green tea polyphenols (GTPs) are flavonols, commonly known as catechins. Products derived from green tea are mainly extracts of green tea in liquid or powder form that vary in the proportion of polyphenols (45-90%) and caffeine content (0.4-10%). The major flavonoids of green tea are various catechins, which are found in greater amounts in green tea than in black or Oolong tea [ 23 ]. There are four kinds of catechins mainly find in green tea: epicatechin, epigallocatechin, epicatechin-3-gallate, and EGCG [ 24 ]. The preparation methods influence the catechins both quantitatively and qualitatively; the amount of catechins also varies in the original tea leaves due to differences in variety, origin, and growing conditions [ 25 ]. The preparation of fresh green tea cannot totally extract catechins from the leaves; therefore, the concentration found differs from the absolute values determined through the complete extraction of leaves [ 26 ]. Moreover, catechins are relatively unstable and could be quantitatively and qualitatively modified during the time frame of an experiment [ 27 , 28 ]. Thus, comparison of ingested doses in animal studies is not possible because the catechin quantification before administration is often not known.
Health benefits of green tea in humans and animals
Studies using animal models show that green tea catechins provide some protection against degenerative diseases [ 29 ]. Some studies indicated that green tea has an antiproliferative activity on hepatoma cells and a hypolipidemic activity in hepatoma-treated rats, as well as the prevention of hepatoxicity [ 29 ] and as a preventive agent against mammary cancer post-initiation [ 29 ]. Green tea catechins could also act as antitumorigenic agents [ 30 ] and as immune modulators in immunodysfunction caused by transplanted tumors or by carcinogen treatment [ 29 ]. Moreover, green tea, its extract, and its isolated constituents were also found to be effective in preventing oxidative stress [ 31 ] and neurological problems [ 32 ].
Green tea consumption has also been linked to the prevention of many types of cancer, including lung, colon, esophagus, mouth, stomach, small intestine, kidney, pancreas, and mammary glands [ 33 ]. Several epidemiological studies and clinical trials showed that green tea (and black and Oolong teas to a lesser extent) may reduce the risk of many chronic diseases [ 34 ]. This beneficial effect has been attributed to the presence of high amounts of polyphenols, which are potent antioxidants. In particular, green tea may lower blood pressure and thus reduce the risk of stroke and coronary heart disease. Some animal's studies suggested that green tea might protect against the development of coronary heart disease by reducing blood glucose levels and body weight [ 35 ]. However, all these data are based on middle-aged animals' populations, not the elderly populations, which nutritional status tends to be more adversely influenced by age-related biological and socioeconomic factors [ 36 ].
Tea components possess antioxidant, antimutagenic, and anticarcinogenic effects and could protect humans against the risk of cancer by environmental agents [ 37 ]. Sano et al . [ 38 ] reported the inhibitory effects of green tea leaves against tert-butyl hydroperoxide-induced lipid peroxidation, and a similar antioxidant effect on the kidney was observed after oral administration of the major tea polyphenol EGCG. The antioxidative potency of crude catechin powder and individual catechins was tested in experiments using the active oxygen method. Crude catechins reduced the formation of peroxides far more effectively than dl-α-tocopherol [ 39 ]. Shim et al . [ 40 ] studied the chemopreventive effect of green tea among cigarette smokers and found that it can block the cigarette-induced increase in sister chromatid exchange frequency.
The effectiveness of green tea in treating any type of diarrhea and typhoid has been known in Asia since ancient times [ 41 – 43 ]. Green tea catechins have an inhibitory effect on Helicobacter pylori infection [ 44 , 45 ]. Effects of green tea against the influenza virus, especially in its earliest stage, as well as against the Herpes simplex virus have also been demonstrated [ 46 – 48 ]. Furthermore, Weber et al . [ 9 ] observed that adenovirus infection is inhibited in vitro by green tea catechins.
In humans, Hirasawa and Takada [ 49 ] studied the antifungal activity of green tea catechins against Candida albicans and the convenience of a combined treatment with catechins and lower doses of antimycotics, which may help to avoid the side effects of antimycotics. Green tea consumption has also been associated with increased bone mineral density, and it has been identified as an independent factor protecting against the risk of hip fractures; this effect was considered independent of smoking status, hormone replacement therapy, coffee drinking, and the addition of milk to tea [ 50 ]. Park et al . [ 51 ] observed the positive effects of green tea extracts and GTPs on the proliferation and activity of bone cells. The proliferation of hepatic stellate cells is closely related to the progression of liver fibrosis in chronic liver diseases, and EGCG has a potential inhibitory effect on the proliferation of these cells [ 52 , 53 ]. Green tea strengthens the immune system action because it protects it against oxidants and radicals. Recent studies suggested that GTPs might protect against Parkinson's and Alzheimer's diseases and other neurodegenerative diseases [ 10 , 54 ]. Studies have demonstrated GTP neuroprotectant activity in cell cultures and animal models, such as the prevention of neurotoxin-induced cell injury [ 54 ]. Green tea is considered to be useful for insect stings due mainly to its anti-inflammatory effects and its capacity to stop bleeding [ 55 , 56 ]. Some studies have suggested an inverse association between green tea consumption and the risk of kidney stone formation [ 41 , 57 ]. In an experimental cataractogenesis system, green tea acted by preserving the antioxidant defense system of the lens [ 58 ]. Skrzydlewska et al . [ 59 ] indicated a beneficial effect of green tea in alcohol intoxication. In addition to all of these reported properties, which have helped the recognition of green tea as functional food by some authors [ 60 ], green tea is also currently used in the preparation of a variety of foods, pharmaceutical preparations, dentifrices, and cosmetics [ 61 ].
Tea has been shown anticarcinogenic effects against breast cancer in experimental studies [ 62 ]. However, epidemiologic evidence that tea protects against breast cancer has been inconsistent [ 62 ]. A case-control study was conducted in southeastern China between 2004 and 2005 [ 63 ]. The incidence cases were 1009 female patients aged 20-87 years with histologically confirmed breast cancer, and the 1009 age-matched controls were healthy women randomly recruited from breast disease clinics. Information on duration, frequency, quantity, preparation, and type of tea consumption as well as diet and lifestyle were collected by face-to-face interviews using a validated and reliable questionnaire. In comparison with non-tea drinkers, green tea drinkers tended to reside in urban settings, to have more education, and to consume more coffee, alcohol, soy, vegetables, and fruits. After adjusting established and potential confounding factors, green tea consumption was associated with a reduced risk of breast cancer. Similar dose-response relationships were observed for duration of drinking green tea, number of cups consumed, and new batches prepared per day.
Hsu et al . [ 64 ] demonstrated the effects of supplementation with decaffeinated green tea extract (catechins) on hemodialysis-induced reactive oxygen species, atherosclerotic disease risk factors, and proinflammatory cytokines. The pharmacokinetics of one oral dose of catechins was compared between healthy subjects and hemodialysis patients. The authors compared the antioxidant effects of three different doses (0, 455, and 910 mg) of oral catechins with that of oral vitamin C (500 mg) during a hemodialysis session. In patients, catechin supplementation reduced hemodialysis-enhanced plasma hypochlorous acid activity more effectively than did placebo or vitamin C. Between the treatments with 455 and 910 mg catechins, no significant difference was found in the reduction of plasma hypochlorous acid activity. Catechins also significantly reduced proinflammatory cytokine expression enhanced by hemodialysis.
Effects on absorption of metal ions
Tea catechins can affect iron absorption, particularly in groups at risk of iron deficiency [ 65 , 66 ], but their effects on other ions are poorly understood. Green tea ingestion over a long period does not affect the apparent absorption of copper, whereas it decreases that of zinc and increases that of manganese [ 67 ]. However, catechin intake does not affect the plasma concentration of these ions [ 68 ]. Green tea catechins have the potential to affect absorption and metabolism of ions because flavonoids interact with a variety of metal ions [ 69 ].
Effects on drug-metabolizing enzymes
Long-term ingestion of green tea increases UDP-glucuronosyl transferase activity in rats [ 66 , 70 , 71 ], and after being absorbed, catechins are metabolized by drug-metabolizing enzymes in various organs [ 72 , 73 ]. Thus, the increased glucuronidation through UDP-glucuronosyl transferase induction is postulated to contribute to the anticarcinogenic effect of green tea by facilitating the metabolism of chemical carcinogens into inactive products that are readily excreted. The interaction between 2-amino-3-methylimidazol (4,5-f)quinoline (IQ) and green tea catechin metabolism was examined [ 74 ]. IQ is a precarcinogen that was originally detected in an extract of fried meat. The major route of IQ biotransformation in rats is cytochrome P450 in the first step, followed by conjugation to a sulfate and a glucuronide conjugate. Green tea modifies IQ metabolism in rats, increasing the formation of IQ glucuronides, which are then excreted in the urine. Moreover, protection against cancers induced by polycyclic aromatic hydrocarbons by green tea catechins may be due to the inhibition of their cytochrome P450 metabolism, but the effect of green tea on cytochrome P450 enzymes depends on the particular form. The long-term consumption of green tea increases cytochrome P450 1A1 and 1A2 activities, but not 2B1 and 2E1 activities, in normal rats. However, it is difficult to draw conclusions about a beneficial effect of green tea against carcinogens involving only modulation of this metabolic pathway.
Effects on antioxidant markers and oxidative stress
Green tea is a popular neutraceutical as an antioxidant. Antioxidants are compounds that protect cells against the damaging effects of reactive oxygen species, such as singlet oxygen, superoxide, peroxyl radicals, hydroxyl radicals, and peroxynitrite. An imbalance between antioxidants and reactive oxygen species results in oxidative stress, leading to cellular damage [ 75 ]. Catechins are hypothesized to help protect against these diseases by contributing, along with antioxidant vitamins (i.e., vitamins C and E) and enzymes (i.e., superoxide dismutase and catalase), to the total antioxidant defense system [ 76 ].
In vivo studies showed that green tea catechins increase total plasma antioxidant activity [ 77 , 78 ]. Intake of green tea extracts also increases the activity of superoxide dismutase in serum and the expression of catalase in the aorta; these enzymes are implicated in cellular protection against reactive oxygen species [ 78 , 79 ]. This action is combined with direct action on oxygen species by a decrease in the nitric oxide plasma concentration [ 80 ]. Malondialdehyde, a marker of oxidative stress, also decreases after green tea intake [ 77 , 80 ]. These results suggest that catechins could have a direct (antioxidant) or indirect (increase of activity or expression) effect. Since catechins can act as antioxidants in vitro , they might prevent the oxidation of other antioxidants, such as vitamin E. However, ingestion of green tea catechins does not modify the plasma status of vitamins E and C in vivo [ 78 , 81 , 82 ]. Nevertheless, one study reported that catechins increase vitamin E concentration in low-density lipoprotein [ 81 ] and in this way could protect low-density lipoprotein against peroxidation [ 77 ].
Pilipenko et al . [ 83 ] assessed the tolerance of tableted green tea and its effect on the antioxidant status indices. Twenty-five patients with different gastrointestinal pathologies were included in the study and divided into treatment and control groups. The tolerance of tableted green tea was good in the treatment group, who showed better dynamics of quality-of-life indices, especially in scales of body pain and social functioning. There were no significant differences in biochemical analysis between the groups, which may indicate the safety of this product. Analysis revealed that the treatment group showed a decreased level of all antioxidant status indices, as reflected in a significant decreasing of the lipid peroxidation index from 4.63 to 4.14.
Effects on carbohydrate metabolism
Type II diabetes is a heterogeneous disorder that involves resistance of glucose and lipid metabolism in peripheral tissues to the biological activity of insulin and inadequate insulin secretion by pancreatic β cells [ 84 ]. Animal models of diabetes are available: Zucker rats, which are genetically obese; injection of streptozotocin or alloxan, which destroys pancreatic β cells; and treatment with sucrose-rich diets, which induces obesity and insulin resistance.
In a study by Sabu et al . [ 85 ], administration of GTPs (500 mg/kg) to normal rats increased glucose tolerance significantly at 60 minutes. GTPs were also found to reduce significantly serum glucose levels in alloxan diabetic rats at a dose of 100 mg/kg. Continued daily administration (15 days) of the extract at 50 or 100 mg/kg produced 29% and 44% reduction, respectively, in the elevated serum glucose level produced by alloxan administration. Elevated hepatic and renal enzymes produced by alloxan were found to be reduced significantly by GTPs. The serum lipid peroxidation level was increased by alloxan and reduced significantly by the administration of 100 mg/kg of GTPs. Decreased liver glycogen resulting from alloxan administration showed a significant increase after GTP treatment. The GTP-treated group showed increased antioxidant potential, as seen from improvements in superoxide dismutase and glutathione levels. However, catalase, lipid peroxidation, and glutathione peroxidase levels were unchanged. These results indicate that alterations in the glucose utilizing system and oxidation status in rats that were increased by alloxan were partially reversed by the administration of GTPs [ 85 ].
Catechins also reduced plasma triglyceride levels in an oral glucose-tolerance test in normal rats [ 86 ]. Green tea extract intake reduced these values in both Zucker rats and rats fed a sucrose-rich diet [ 87 , 88 ]. Several human- and animal-based studies suggested that green tea and its flavonoids have antidiabetic effects [ 86 , 89 , 90 ]. Green tea flavonoids were also shown to have insulin-like activities [ 91 ] as well as insulin-enhancing activity [ 92 ].
The antihyperglycemic effect of black tea was reported by Gomes et al . [ 93 ]. EGCG was found to inhibit intestinal glucose uptake by the sodium-dependent glucose transporter SGLT1, indicating its increase in controlling blood sugar [ 94 ]. Streptozotocin diabetic rats showed increased sensitivity to platelet aggregation and thrombosis, and this abnormality could be improved by dietary catechins from green tea [ 95 , 96 ]. Alloxan produces oxygen radicals in the body, which cause pancreatic injury [ 75 ] and are responsible for increased blood sugar.
Under in vivo conditions, glutathione acts as an antioxidant, and its decrease was reported in a diabetes mellitus model [ 97 ]. The increased glutathione content in the liver of the rats treated with GTPs may be one of the factors responsible for the inhibition of lipid peroxidation. Superoxide dismutase and catalase are the two major scavenging enzymes that remove the toxic free radicals in vivo . Vucic et al . [ 98 ] reported that the activity of superoxide dismutase is low in diabetes mellitus.
The Mediterranean Islands (MEDIS) epidemiological study is a cross-sectional health and nutrition survey that aims to evaluate the association between various sociodemographic, bioclinical, dietary, and other lifestyle habits and the prevalence of the common cardiovascular disease risk factors (i.e., hypertension, dyslipidemia, diabetes, and obesity) among elderly people without a history of any chronic disease and living in the Mediterranean islands. Because data relating tea consumption with clinical characteristics are lacking in elderly populations, in the context of the MEDIS study, the authors sought to evaluate whether green tea consumption is independently associated with fasting blood glucose levels and the prevalence of type II diabetes mellitus [ 99 ]. An earlier study was aimed at providing evidence of improvement in glucose metabolism in diabetic mice and healthy humans upon green tea consumption [ 35 ]. Green tea promoted glucose metabolism in healthy human volunteers at 1.5 g/kg as shown in oral glucose-tolerance tests. Green tea also lowered blood glucose levels in diabetic db+/db+ mice and streptozotocin-diabetic mice two to six hours after administration at 300 mg/kg without affecting serum insulin level, whereas no effect was observed in control mice (+m/+m and normal ddY mice).
Effect of EGCG on diabetes
A study by Waltner-Law et al . [ 91 ] provided compelling in vitro evidence that EGCG decreases glucose production of H4IIE rat hepatoma cells. The investigators showed that EGCG mimics insulin, increases tyrosine phosphorylation of the insulin receptor and the insulin receptor substrate, and reduces gene expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase. Recently, green tea and green tea extracts were demonstrated to modify glucose metabolism beneficially in experimental models of type II diabetes mellitus [ 35 , 100 ]. In addition, EGCG ameliorates cytokine-induced β cell damage in vitro [ 101 ] and prevents the decrease of islet mass induced by treatment with multiple low doses of streptozotocin in vivo [ 102 ].
Lambert et al . [ 103 ] showed that intragastric administration of EGCG at a dose of 75 mg/kg resulted in a Cmax of 128 mg/l total plasma EGCG and a terminal half-life of 83 minutes. Furthermore, in humans an oral intake of EGCG at a dose of 50 mg (0.7 mg/kg) resulted in a Cmax of 130 mg/l total plasma EGCG and a terminal half-life of 112 minutes [ 104 ]. These results indicate that rodents must be orally administered 100- to 600-fold more EGCG (depending on whether they are administered by gavage or by feed admixture) to achieve similar plasma concentrations as those found in humans. Total plasma EGCG concentrations shown to be efficacious in mice and rats can be reached by an intake of low to moderate doses of EGCG in humans.
Effect on obesity
The effects of tea on obesity and diabetes have received increasing attention. Tea catechins, especially EGCG, appear to have antiobesity and antidiabetic effects [ 105 ]. African black tea extract has been shown to suppress the elevation of blood glucose during food intake and reduce the body weight in KK-A(y)/TaJcl diabetic mice [ 106 ]. Although few epidemiological and clinical studies have shown the health benefits of EGCG on obesity and diabetes, the mechanisms of its actions are emerging based on various laboratory data. These mechanisms may be related to certain pathways, such as through the modulations of energy balance, endocrine systems, food intake, lipid and carbohydrate metabolism, and redox status [ 88 ].
A double-blind, placebo-controlled, cross-over design study showed that consumption of a beverage containing green tea catechins, caffeine, and calcium increases 24-h energy expenditure by 4.6%, but the contribution of the individual ingredients could not be distinguished. It was suggested that such modifications were sufficient to prevent weight gain. It has been reported that the body weights of rats and their plasma triglyceride, cholesterol, and low-density lipoprotein cholesterol were significantly reduced by feedings of Oolong, black, and green tea leaves to the animals. In addition, the inhibition of growth and suppression of lipogenesis in MCF-7 breast cancer cells may be through down-regulation of fatty acid synthase gene expression in the nucleus and stimulation of cell energy expenditure in the mitochondria [ 107 , 108 ]. When fed to mice, EGCG purified from green tea decreased diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation [ 109 ]. The increased and prolonged sympathetic stimulation of thermogenesis by the interaction between polyphenols and caffeine could be of value in assisting the management of obesity [ 110 ].
Recent data from human studies indicate that the consumption of green tea and green tea extracts may help reduce body weight, mainly body fat, by increasing postprandial thermogenesis and fat oxidation. In a randomized, double-blind, placebo-controlled, cross-over pilot study, six overweight men were given 300 mg EGCG per day for two days. Fasting and postprandial changes in energy expenditure and substrate oxidation were assessed. Resting energy expenditure did not differ significantly between EGCG and placebo treatments, although during the first postprandial monitoring phase, respiratory quotient values were significantly lower with EGCG treatment compared to the placebo. These findings suggest that EGCG alone has the potential to increase fat oxidation in men and may thereby contribute to the antiobesity effects of green tea. However, more studies with a greater sample size and a broader range of age and body mass index are needed to define the optimal dose [ 111 ].
Adverse effects of green tea
Although green tea has several beneficial effects on health, the effects of green tea and its constituents may be beneficial up to a certain dose yet higher doses may cause some unknown adverse effects. Moreover, the effects of green tea catechins may not be similar in all individuals. EGCG of green tea extract is cytotoxic, and higher consumption of green tea can exert acute cytotoxicity in liver cells, a major metabolic organ in the body [ 112 ]. Another study found that higher intake of green tea might cause oxidative DNA damage of hamster pancreas and liver [ 113 ]. Yun et al . [ 114 ] clarified that EGCG acts as a pro-oxidant, rather than an antioxidant, in pancreatic β cells in vivo . Therefore, high intake of green tea may be detrimental for diabetic animals to control hyperglycemia. At a high dose (5% of diet for 13 wk), green tea extract induced a thyroid enlargement (goiter) in normal rats [ 115 , 116 ]. This high-level treatment modified the plasma concentrations of the thyroid hormones. However, drinking even a very high dietary amount of green tea would be unlikely to cause these adverse effects in humans.
Harmful effects of tea overconsumption (black or green) are due to three main factors: (1) its caffeine content, (2) the presence of aluminum, and (3) the effects of tea polyphenols on iron bioavailability. Green tea should not be taken by patients suffering from heart conditions or major cardiovascular problems. Pregnant and breast-feeding women should drink no more than one or two cups per day, because caffeine can cause an increase in heart rhythm. It is also important to control the concomitant consumption of green tea and some drugs, due to caffeine's diuretic effects [ 117 ]. Some studies revealed the capacity of tea plants to accumulate high levels of aluminum. This aspect is important for patients with renal failure because aluminum can be accumulated by the body, resulting in neurological diseases; it is therefore necessary to control the intake of food with high amounts of this metal [ 118 ]. Likewise, green tea catechins may have an affinity for iron, and green tea infusions can cause a significant decrease of the iron bioavailability from the diet [ 119 ].
Conclusions
Laboratory studies showed the health effects of green tea. As the human clinical evidence is still limited, future research needs to define the actual magnitude of health benefits, establishes the safe range of tea consumption associated with these benefits, and elucidates the mechanisms of action. Development of more specific and sensitive methods with more representative models along with the development of good predictive biomarkers will give a better understanding of how green tea interacts with endogenous systems and other exogenous factors. Definitive conclusions concerning the protective effect of green tea have to come from well-designed observational epidemiological studies and intervention trials. The development of biomarkers for green tea consumption, as well as molecular markers for its biological effects, will facilitate future research in this area.
Abbreviations
epigallocatechin-3-gallate
green tea polyphenols
Uridine di-phospatase
2-amino-3-methylimidazol (4,5-f)quinoline
Mediterranean Islands
Sodium dependent glucose transporter
Allied and complementary Medicine Database.
McKay DL, Blumberg JB: The role of tea in human health: An update. J Am Coll Nutr. 2002, 21: 1-13.
Article CAS PubMed Google Scholar
Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE: Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001, 82: 387-398. 10.1002/jcb.1164.
Sueoka N, Suganuma M, Sueoka E, Okabe S, Matsuyama S, Imai K, Nakachi K, Fujiki H: A new function of green tea: prevention of lifestyle-related diseases. Ann N Y Acad Sci. 2001, 928: 274-280.
Dona M, Dell'Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S: Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003, 170: 4335-4341.
Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H: Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci USA. 1999, 96: 4524-4529. 10.1073/pnas.96.8.4524.
Article PubMed Central CAS PubMed Google Scholar
Sudano Roccaro A, Blanco AR, Giuliano F, Rusciano D, Enea V: Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother. 2004, 48: 1968-1973. 10.1128/AAC.48.6.1968-1973.2004.
Article PubMed CAS Google Scholar
Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN: Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002, 132: 2307-2311.
CAS PubMed Google Scholar
Osada K, Takahashi M, Hoshina S, Nakamura M, Nakamura S, Sugano M: Tea catechins inhibit cholesterol oxidation accompanying oxidation of low density lipoprotein in vitro . Comp Biochem Physiol Part C Toxicol Pharmacol. 2001, 128: 153-164. 10.1016/S1532-0456(00)00192-7.
Article CAS Google Scholar
Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S: Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003, 58: 167-173. 10.1016/S0166-3542(02)00212-7.
Weinreb O, Mandel S, Amit T, Youdim MBH: Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J Nutr Biochem. 2004, 15: 506-516. 10.1016/j.jnutbio.2004.05.002.
Raederstorff DG, Schlachter MF, Elste V, Weber P: Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003, 14: 326-332. 10.1016/S0955-2863(03)00054-8.
Naghma K, Hasan M: Tea polyphenols for health promotion. Life Sciences. 2007, 81: 519-533. 10.1016/j.lfs.2007.06.011.
Moyers SB, Kumar NB: Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004, 62: 204-211. 10.1111/j.1753-4887.2004.tb00041.x.
Article PubMed Google Scholar
Mandel S, Weinreb O, Amit T, Youdim MB: Cell signaling pathways in the neuroprotective actions of the green tea polyphenol(-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004, 88: 1555-1569.
Higdon JV, Frei B: Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003, 43: 89-143. 10.1080/10408690390826464.
Xiang YZ, Shang HC, Gao XM, Zhang BL: A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008, 22 (7): 851-858. 10.1002/ptr.2384.
Cabrera C, Artacho R, Giménez R: Beneficial effects of green tea: a review. J Am Coll Nutr. 2006, 25: 79-99.
Japanese Green Tea Online.com. [ http://www.japanesegreenteaonline.com ]
Weisburger JH: Approaches for chronic disease prevention based on current understanding of underlying mechanisms. Am J Clin Nutr. 2000, 71 (6): 1710S-1714S.
Sato T, Miyata G: The nutraceutical benefit, part I: green tea. Nutrition. 2000, 16: 315-317. 10.1016/S0899-9007(99)00301-9.
Belitz DH, Grosch W: Quı'mica de los Alimentos. 1997, Zaragoza: Acribia
Google Scholar
Graham HN: Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992, 21: 334-350. 10.1016/0091-7435(92)90041-F.
Vinson JA: Black and green tea and heart disease: a review. Biofactors. 2000, 13: 127-132. 10.1002/biof.5520130121.
Sano M, Tabata M, Suzuki M, Degawa M, Miyase T, Maeda-Yamamoto M: Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst. 2001, 126: 816-820. 10.1039/b102541b.
Khokhar S, Magnusdottir SGM: Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 2002, 50: 565-570. 10.1021/jf010153l.
Fernandez PL, Martin MJ, Gonzalez AG, Pablos F: HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst. 2000, 125: 421-425. 10.1039/a909219f.
Chen ZY, Zhu QY, Wong YF, Zhang Z, Chung HY: Stabilizing effect of ascorbic acid on green tea catechins. J Agr Food Chem. 1998, 46: 2512-2516. 10.1021/jf971022g.
Chen ZY, Zhu QY, Tsang D, Huang Y: Degradation of green tea catechins in tea drinks. J Agr Food Chem. 2001, 49: 477-482. 10.1021/jf000877h.
Vanessa C, Gary W: A Review of the Health Effects of Green Tea Catechins in In Vivo Animal Models. J Nutr. 2004, 134: 3431S-3440S.
Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, RathIn M: In vitro and in vivo antitumorigenic activity of a mixture of lysine, proline, ascorbic acid, and green tea extract on human breast cancer lines MDA-MB-231 and MCF-7. Medical Oncol. 2007, 22 (2): 129-138. 10.1385/MO:22:2:129.
Article Google Scholar
Babu PV, Sabitha KE, Shyamaladevi CS: Therapeutic effect of green tea extract on oxidative stress in aorta and heart of streptozotocin diabetic rats. Chem Biol Interact. 2006, 162: 114-120. 10.1016/j.cbi.2006.04.009.
Unno K, Takabayashi F, Yoshida H, Choba D, Fukutomi R, Kikunaga N, Kishido T, Oku N, Hoshino M: Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology. 2007, 8 (2): 89-95. 10.1007/s10522-006-9036-8.
Koo MWL, Cho CH: Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004, 500: 177-185. 10.1016/j.ejphar.2004.07.023.
Zaveri NT: Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78: 2073-2080. 10.1016/j.lfs.2005.12.006.
Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I: Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004, 4: 18-21. 10.1186/1471-2210-4-18.
Article PubMed Central PubMed CAS Google Scholar
Meydani M: Nutrition interventions in aging and age associated disease. Ann N Y Acad Sci. 2001, 928: 226-235.
Mukhtar H, Wang ZY, Katlya SK, Agarwal R: Tea components: antimutagenic and anticarcinogenic effects. Prev Med. 1992, 21: 351-360. 10.1016/0091-7435(92)90042-G.
Sano M, Takahashi Y, Yoshino K, Shimoi K, Nakamura Y, Tomita I, Oguni I, Konomoto H: Effect of tea ( Camellia sinensis L.) on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol Pharm Bull. 1995, 18: 1006-1008.
Hara Y: Advances in Food Science and Technology. Nippon Shokuhin Kogyo. 1990, Tokyo: Gakkai: Korin
Shim JS, Kang MH, Kim YH, Roh JK, Roberts C, Lee IP: Chemopreventive effect of green tea ( Camellia sinensis ) among cigarette smokers. Cancer Epidemiol Biomarkers. 1995, 4: 387-391.
CAS Google Scholar
McKay DL, Blumberg JB: The role of tea in human health: an update. J Am Coll Nutr. 2002, 21: 1-13.
Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS: Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003, 31: 452-461. 10.1124/dmd.31.4.452.
Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ: Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res. 2003, 11: 1088-1095. 10.1038/oby.2003.149.
Takabayashi F, Harada N, Yamada M, Murohisa B, Oguni I: Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 2004, 39: 61-63. 10.1007/s00535-003-1246-0.
Yee YK, Koo MWL, Szeto ML: Chinese tea consumption and lower risk of Helicobacter infection. J Gastroenterol Hepatol. 2002, 17: 552-555. 10.1046/j.1440-1746.2002.02718.x.
Toda M, Okubo S, Ohnishi R, Shimamura T: Antibacterial and bactericidal activities of Japanese green tea. Nippon Saikingaku Zasshi. 1989, 44: 669-672.
Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, Shimamura T: Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn J Med Sci Biol. 1991, 44: 181-186.
Yama TS, Shaha S, Hamilton-Millera JMT: Microbiological activity of whole and fractionated crude extracts of tea ( Camellia sinensis ), and of tea components. FEMS Microbiol Lett. 1997, 152: 169-174. 10.1111/j.1574-6968.1997.tb10424.x.
Hirasawa M, Takada K: Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans . J Antimicrob Chemother. 2004, 53: 225-229. 10.1093/jac/dkh046.
Muraki S, Yamamoto S, Ishibashi H, Horiuchi T, Hosoi T, Suzuki T, Orimo H, Nakamura K: Green tea drinking is associated with increased bone mineral density. J Bone Miner Res. 2003, 18: S241-
Park H, Ko S, Kim J, Kim S: Effects of green tea extracts and polyphenols on the proliferation and activity of bone cells. J Bone Miner Res. 2003, 18: S342-
Dorchies OM, Wagner S, Waldhauser KM, Buetler TM, Ruegg UT: Anti-fibrotic properties of green tea catechins on mouse muscle cell cultures. Neuromuscul Disord. 2003, 13: 639-
Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M: Green tea polyphenols epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol. 2004, 40: 52-59. 10.1016/S0168-8278(03)00477-X.
Pan TH, Jankovic J, Le WD: Potential therapeutic properties of green tea polyphenols in Parkinson's disease. Drugs Aging. 2003, 20: 711-721. 10.2165/00002512-200320100-00001.
Sagesaka-Mitane Y, Miwa M, Okada S: Platelet aggregation inhibitors in middle aged Japanese men and women. Ann Epidemiol. 1998, 7: 280-284.
Dvorakova K, Dorr RT, Valcic S, Timmermann B, Alberts DS: Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother Pharmacol. 1999, 43: 331-335. 10.1007/s002800050903.
Ishizuk H, Eguchi H, Oda T, Ogawa S, Nakagawa K, Honjo S, Kono S: Relation of coffee, green tea, and caffeine intake to gallstone disease in middle-age Japanese men. Eur J Epidemiol. 2003, 18: 401-405. 10.1023/A:1024237927985.
Gupta SK, Halder N, Srivastava S, Trivedi D, Joshi S, Varma SD: Green tea ( Camellia sinensis ) protects against selenite-induced oxidative stress in experimental cataractogenesis. Ophthalmic Res. 2002, 34: 258-263. 10.1159/000063881.
Skrzydlewska E, Ostrowska J, Stankiewicz A, Farbiszewski R: Green tea as a potent antioxidant in alcohol intoxication. Addict Biol. 2002, 7: 307-314. 10.1080/13556210220139523.
Ferrari CKB, Torres EAFS: Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed Pharmacother. 2003, 57: 251-260. 10.1016/S0753-3322(03)00032-5.
Arburjai T, Natsheh FM: Plants used in cosmetics. Phytother Res. 2003, 17: 987-1000. 10.1002/ptr.1363.
Min Zhang C, D'Arcy JH, Jiang-ping H, Xing X: Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2005, 28 (5): 1074-1078. 10.1093/carcin/bgl252.
Zhang M, Holman CDAJ, Huang JP, Xie X: Green tea and the prevention of breast cancer: a case-control study in southeast China. Carcinogenesis. 2008, 29 (8): 1594-1600. 10.1093/carcin/bgn129.
Hsu SP, Wu MS, Yang CC, Huang KC, Liou SY, Hsu SM, Chien CT: Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007, 86 (5): 1539-1547.
Samman S, Sandstrom B, Toft MB, Bukhave K, Jensen M, Sorensen SS, Hansen M: Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001, 73: 607-612.
Nelson M, Poulter J: Impact of tea drinking on iron status in the UK: a review. J Hum Nutr Diet. 2004, 17: 43-54. 10.1046/j.1365-277X.2003.00497.x.
Deng Z, Tao B, Li X, He J, Chen Y: Effect of green tea and black tea on the metabolisms of mineral elements in old rats. Biol Trace Elem Res. 1998, 65: 75-86. 10.1007/BF02784115.
Record IR, McInerney JK, Dreosti IE: Black tea, green tea, and tea polyphenols: effects on trace element status in weanling rats. Biol Trace Elem Res. 1996, 53: 27-43. 10.1007/BF02784542.
Mira L, Fernandez MT, Santos M, Rocha R, Florencio MH, Jennings KR: Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002, 36: 1199-1208. 10.1080/1071576021000016463.
Maliakal PP, Coville PF, Wanwimolruk S: Tea consumption modulates hepatic drug metabolizing enzymes in Wistar rats. J Pharm Pharmacol. 2001, 53: 569-577. 10.1211/0022357011775695.
Sohn OS, Surace A, Fiala ES, Richie JP, Colosimo S, Zang E, Weisburger JH: Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994, 24: 119-127. 10.3109/00498259409043226.
Donovan JL, Crespy V, Manach C, Morand C, Besson C, Scalbert A, Remesy C: Catechin is metabolized by both the small intestine and liver of rats. J Nutr. 2001, 131: 1753-1757.
Okushio K, Suzuki M, Matsumoto N, Nanjo F, Hara Y: Methylation of tea catechins by rat liver homogenates. Biosci Biotechnol Biochem. 1999, 63: 430-432. 10.1271/bbb.63.430.
Embola CW, Weisburger JH, Weisburger MC: Urinary excretion of N-OH-2-amino-3-methylimidazo [4,5-f]quinoline-N-glucuronide in F344 rats is enhanced by green tea. Carcinogenesis. 2001, 22: 1095-1098. 10.1093/carcin/22.7.1095.
Halliwell B, Gutteridge JMC: Free Radicals in Biology and Medicine. 1985, Oxford: Clarendon Press
Abdel-Raheim MAM, Enas AH, Khaled AE: Effect of green tea extract and vitamin c on oxidant or antioxidant. Indian J Clin Biochem. 2009, 24 (3): 280-287. 10.1007/s12291-009-0053-7.
Yokozawa T, Nakagawa T, Kitani K: Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002, 50: 3549-3552. 10.1021/jf020029h.
Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K: Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002, 9: 232-238. 10.1078/0944-7113-00119.
Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y: Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr. 2004, 134: 38-42.
Yokozawa T, Nakagawa T, Lee KI, Cho EJ, Terasawa K, Takeuchi S: Effects of green tea tannin on cisplatin-induced nephropathy in LLC-PK1 cells and rats. J Pharm Pharmacol. 1999, 51: 1325-1331. 10.1211/0022357991776912.
Tijburg LBM, Wiseman SA, Meijer GW, Weststrate JA: Effects of green tea, black tea and dietary lipophilic antioxidants on LDL oxidizability and atherosclerosis in hypercholesterolaemic rabbits. Atherosclerosis. 1997, 135: 37-47. 10.1016/S0021-9150(97)00139-1.
Alessio HM, Hagerman AE, Romanello M, Carando S, Threlkeld MS, Rogers J, Dimitrova Y, Muhammed S, Wiley RL: Consumption of green tea protects rats from exercise-induced oxidative stress in kidney and liver. Nutr Res. 2003, 22: 1177-1188. 10.1016/S0271-5317(02)00421-9.
Pilipenko VI, Shakhovskaia AK, Mal'tsev GIU, Isakov VA: Influence of tableted green tea on index the antioxidant status patients with disease digestion organs. Vopr Pitan. 2008, 77 (4): 58-62.
Del Prato S, Piero M, Riccardo CB: Phasic Insulin Release and Metabolic Regulation in Type 2 Diabetes. Diabetes. 2007, 51: S109-10.2337/diabetes.51.2007.S109.
Sabu MC, Smitha K, Kuttan R: Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002, 83: 109-116. 10.1016/S0378-8741(02)00217-9.
Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS: Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004, 52: 643-648. 10.1021/jf030365d.
Hasegawa N, Yamda N, Mori M: Powdered green tea has antilipogenic effect on Zucker rats fed a high-fat diet. Phytother Res. 2003, 17: 477-480. 10.1002/ptr.1177.
Yang MH, Wang CH, Chen HL: Green, Oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem. 2001, 12: 14-20. 10.1016/S0955-2863(00)00140-6.
Iso H, Date C, Wakai K, Fukui M, Tamakoshi A: The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006, 144: 554-562.
Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P: Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006, 136: 3512-3518.
Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK: Epigallocatecin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002, 277: 34933-34940. 10.1074/jbc.M204672200.
Anderson RA, Polansky MM: Tea enhances insulin activity. J Agric Food Chem. 2002, 50: 7182-7186. 10.1021/jf020514c.
Gomes A, Vedasiromoni JR, Das M, Sharma RM, Ganguly DK: Anti-hyperglycemic effect of black tea ( Camellia sinensis ) in rat. J Ethnopharmacol. 1995, 45: 223-226. 10.1016/0378-8741(95)01223-Z.
Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, Miyamoto Y, Shimizu M: Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000, 48: 5618-5623. 10.1021/jf0006832.
Yang JA, Choi JH, Rhee SJ: Effects of green tea catechin on phospholipase A2 activity and antithrombus in streptozotocin diabetes rats. J Nutr Sci Vitaminol (Tokyo). 1999, 45: 337-346.
Choi JH, Cha BK, Rhee SJ: Effect of green tea catechin on hepatic microsomal phospholipase A2 activities and changes of hepatic phospholipid species in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo). 1998, 44: 673-683.
Illing EKB, Gray CH, Lawrence RD: Blood glutathione and non-glucose reducing substances in diabetes. J Biochem. 1951, 48: 637-640.
Vucic M, Gavell M, Bozikov V, Ashcroft JH, Rocic B: Superoxide dismutase activity in lymphocytes and polymorphonuclear cells of diabetic patients. Eur J Clin Chem Biochem. 1997, 35: 517-521.
Polychronopoulos E, Panagiotakos DB, Polystipioti A: Diet, lifestyle factors and hypercholesterolemia in elderly men and women from Cyprus. Lipids Health Dis. 2005, 4: 17-21. 10.1186/1476-511X-4-17.
Article PubMed Central PubMed Google Scholar
Wu LY, Juan CC, Hwang LS, Hsu YP, Ho PH, Ho LT: Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur J Nutr. 2004, 43: 116-124. 10.1007/s00394-004-0450-x.
Han MK: Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med. 2003, 35: 136-139.
Song EK, Hur H, Han MK: Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch Pharm Res. 2003, 26: 559-563. 10.1007/BF02976881.
Lambert JD, Lee MJ, Lu H, Meng X, Hong JJJ, Seril DN, Sturgill MG, Yang CS: Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003, 133: 4172-4177.
Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P: A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003, 31: 88-101.
Kao YH, Chang HH, Lee MJ, Chen CL: Tea, obesity, and diabetes. Mol Nutr Food Res. 2006, 50 (2): 188-210. 10.1002/mnfr.200500109.
Shoji Y, Nakashima H: Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Arch Pharmacol Res. 2006, 29 (9): 786-794. 10.1007/BF02974080.
Rudelle S, Ferruzzi MG, Cristiani I, Moulin J, Mace K, Acheson KJ, Tappy L: Effect of a thermogenic beverage on 24-hour energy metabolism in humans. Obesity. 2007, 15 (2): 349-355. 10.1038/oby.2007.529.
Lin JK, Lin-Shiau SY: Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006, 50 (2): 211-217. 10.1002/mnfr.200500138.
Klaus S, Pultz S, Thone-Reineke C, Wolfram S: Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes. 2005, 29 (6): 615-623. 10.1038/sj.ijo.0802926.
Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J: Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000, 24 (2): 252-258. 10.1038/sj.ijo.0801101.
Boschmann M, Thielecke F: The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. J Am Coll Nutr. 2007, 26 (4): 389S-395S.
Schmidt M, Schmitz HJ, Baumgart A, Guedon D, Netsch MI, Kreuter MH, Schmidlin CB, Schrenk D: Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem Toxicol. 2005, 43: 307-314. 10.1016/j.fct.2004.11.001.
Takabayashi F, Tahara S, Kanerko T, Harada N: Effect of green tea catechins on oxidative DNA damage of hamster pancreas and liver induced by N-nitrosobis (2-oxopropyl) amine and/or oxidized soybean oil. Biofactors. 2004, 21: 335-337. 10.1002/biof.552210165.
Yun SY, Kim SP, Song DK: Effects of ( - )-epigallocatechin-3-gallate on pancreatic beta-cell damage in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006, 541: 115-121. 10.1016/j.ejphar.2006.04.040.
Sakamoto Y, Mikuriya H, Tayama K, Takahashi H, Nagasawa A, Yano N, Yuzawa K, Ogata A, Aoki N: Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch Toxicol. 2001, 75: 591-596. 10.1007/s00204-001-0286-6.
Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, Yamada K, Aoki N: Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. 2002, 40: 925-933. 10.1016/S0278-6915(02)00066-2.
Bruneton J: Pharmacognosie. Phytochimie. Plantes Me'dicinales. 2001, Paris: Technique Documentation-Lavoisier
Costa LM, Gouveia ST, Nobrega JA: Comparison of heating extraction procedures for Al, Ca, Mg and Mn in tea samples. Ann Sci. 2002, 18: 313-318. 10.2116/analsci.18.313.
Hamdaoui MH, Chabchob S, Heidhili A: Iron bioavailability and weight gains to iron-deficient rats fed a commonly consumed Tunisian meal "bean seeds ragout" with or without beef and with green or black tea decoction. J Trace Elem Med Biol. 2003, 17: 159-164. 10.1016/S0946-672X(03)80020-2.
Download references
Author information
Authors and affiliations.
NPO International Laboratory of Biochemistry, 1-166 Uchide, Nakagawa-ku, Nagoya, 454-0926, Japan
Sabu M Chacko, Priya T Thambi & Ikuo Nishigaki
Amala Cancer Research Center, Amala Nagar, Thrissur, Kerala, 680 555, India
Ramadasan Kuttan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sabu M Chacko .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
SMC and PTT did the literature search and drafted the manuscript. RK and IN critically reviewed the literature and revised the manuscript. All authors read and approved the final version of the manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Chacko, S.M., Thambi, P.T., Kuttan, R. et al. Beneficial effects of green tea: A literature review. Chin Med 5 , 13 (2010). https://doi.org/10.1186/1749-8546-5-13
Download citation
Received : 24 July 2009
Accepted : 06 April 2010
Published : 06 April 2010
DOI : https://doi.org/10.1186/1749-8546-5-13
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Alloxan Administration
- Total Plasma Antioxidant Activity
- High Dietary Amount
- Plasma EGCG Concentration
Chinese Medicine
ISSN: 1749-8546
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Home — Essay Samples — Life — Drink — Review on the green tea
Review on The Green Tea
- Categories: Drink Tea
About this sample

Words: 946 |
Published: Dec 5, 2018
Words: 946 | Pages: 2 | 5 min read
A New Way of Life
- The link between tea (tea polyphenols) and premalignant lesion. The first study evaluated the effects of tea polyphenols on premalignant oral lesions. But, it was a double-blind intervention trial. It involved 59 participants with leukoplakia (a putative precursor lesion for oral cancer). The participants were divided into two groups. The first group was assigned to receive 3 grams of a mixed tea product orally and topically. In contrast the other group was assigned to receive 3 grams of placebo. After 6 months, 38 percent of the participants in the first group had partial regression of the oral lesions compared with only 10 % of the participants in the placebo group.
- The link between tea and 8-OHDGAdditionally, there were researchers held trials to examine the effect of tea on the level of of 8-hydroxydeoxyguanosine (8-OHdG) in urine.8-HODG is a biomarker of oxidative DNA damage that may be a predictor of increased cancer risk. They found that the amount of 8-OHdG in urine is higher in persons with many types of cancer than in healthy subjects. Also, there was a higher content of 8-OHdG in many tissues than adjacent nontumor tissue. There were about 130 heavy smokers. In the trial, they had to receive 4 cups of one of the following beverages every day for 4 months: decaffeinated green tea, black tea, or water. After the 4 months, those who drank green tea had a significant decrease (31%) in urinary levels of 8-OHdG. in contrast, the other 2 groups had no change in urinary 8-OHdG levels. Although this trial indicates that green tea polyphenols can sufficiently reduce urinary 8-OHdG levels, it is unclear if reduced 8-OHdG levels are associated with reduced cancer risk.
- The effect of tea polyphenols on the level of pepsinogen in serum. Another trial involved about 160 individuals with high serum pepsinogen levels to examine the effect of polyphenols in green tea on serum pepsinogen levels. Serum pepsinogen is considered to be an indicator of increased risk for stomach cancer because it is a biomarker of gastric atrophy. Half of The participants received one capsule of tea polyphenols, and the others received six capsules daily for 1 year. Each capsule was the equivalent of about 2 cups of tea. The results was that there are no changes in serum pepsinogen levels in either two group. Eventually, we can say that the evidences regarding the benefits of green tea consumption in relation to cancer is not enough at present.
The Adverse Effect of Green Tea

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Prof. Kifaru
Verified writer
- Expert in: Life

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 829 words
2 pages / 705 words
1 pages / 388 words
2 pages / 780 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Alcohol is a widely consumed substance around the world, with a long history of use in various cultures and societies. While alcohol can be enjoyed responsibly by many individuals, it also has the potential to cause significant [...]
Keeping hydrated is one of the most essential aspects of sport and physical activity, whether you are an athlete or someone who does sport for fitness. It plays so many roles to keep your body functioning properly that without [...]
Wine can be made from many kinds of fruits and berries that contain natural sugar. The largest amount of wine is produced from grapes (white, rose, black, or red). The grapes are composed of: stem (2.5-8%), skin (6-10%), pulp, [...]
Of course, we still do not want to give away the positive effect of the ginger drink and have therefore brought along not just one, but three cheaper and homemade versions of the ginger shot for you. In addition, you will learn [...]
Beverage can consist mostly of aluminum, but it contains small amounts of other metals as well. These are typically 1% magnesium, 1% manganese, 0.4% iron, 0.2% silicon, and 0.15% copper. Before understanding the manufacturing [...]
Brandy is the alcoholic beverage distilled from wine or a fermented fruit mash. The term used alone generally refers to the grape product; brandies made from the wines or fermented mashes of other fruits are commonly identified [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- EssayBasics.com
- Pay For Essay
- Write My Essay
- Homework Writing Help
- Essay Editing Service
- Thesis Writing Help
- Write My College Essay
- Do My Essay
- Term Paper Writing Service
- Coursework Writing Service
- Write My Research Paper
- Assignment Writing Help
- Essay Writing Help
- Call Now! (USA) Login Order now
- EssayBasics.com Call Now! (USA) Order now
- Writing Guides
Green Tea Positive Effects (Essay Sample)
Table of Contents
Green Tea Positive Effects
Introduction.
Green tea is a kind of tea that is prepared from Camellia sinensis leaves that have not experience the same oxidation and withering process used in making black tea and colong. Green tea stemmed from China but its production has been spreading in many nations in Asia. Several types of Green Tea are existing which are differing substantially because of the breed of C. sinensis used, flourishing conditions, horticultural techniques, time of harvesting, and production processing. The consumption of tea has its famous origins in china during the reigning of emperor Shennong. A book composed by Lu Yu in 600-900 AD is pondered important in the history of green tea. The book of tea known as Kissa Yokoji was written by Zen priest Eisai in 1191. The book is describing how drinking of tea may be affecting the five vital organs, flowers and leaves, the forming of tea plants, and how to be growing and processing tea leaves. The process of making tea is known as brewing or steeping and generally this is done by using two grams of tea for every 100ml of water or approximately one teaspoon of green tea for every 150ml cup. Higher grade teas, like gyokuro utilize more tea leaves and are soaked numerous times for short periods. Green tea contains extracts which have numerous health benefits to human body. This paper thrives to describe green tea and its positive effects to the human body.
History of Green Tea
It is widely believed that green tea was first soaked during the reigning of emperor Shennong in 2737 BC. The Emperor was a popular figure and a mythical sage in the mythology of Chinese medicine and agriculture. It is indicated that during one of his journeys, when Shennong and his caravan were stopping to rest, few tea leaves were falling into his cup of hot water from a burned tea twig that was lying nearby. The water turned black in color, but the emperor did not notice. When he was consuming this water he discovered it to be remarkably refreshing and was requesting the members of his caravan to be preparing it for him thereafter. This incident is counted important in the history of teas, specifically the history of green tea. Some folk historians however are tracing back the history of green tea as far as 3000 years ago when raw tea leaves were eaten or chewed for leisure by people who were growing it all over southern Asia. It was much later that raw removed leaves were undergoing any form of processing before soaking in hot water. During the reigning of the Tang dynasty in 15th century, tea drinking was becoming a societal custom of all over China. Formalized “tea ceremonies” were taking shape and tea drinking was becoming an integral part of the social life of natives of China. It was during this period that the process of ‘steaming ‘the tea leaves was started and processed over the coming years.
Production of Green Tea
The green tea is grown and processed in different ways, and it depends on the kind of green tea wanted. As resulting from these ways, topmost quantity of volatile organic compounds and polyphenols are preserved, to affect taste and aroma. The growing state can be split down into fundamental types-those that are growing in the sun and those that are growing under the shade. The green tea vegetations are planted in line that are clipped to be producing shoots in an orderly manner, and commonly are reaped three times a year. The initial flushing is taking place between the late April and early May. The next reaping is usually taking place from June through July, and the third picking is generally taking place between late July and early August. Normally it is the first flushing in the spring that is producing the best quality leaves with higher pricing to compete. Green tea is processed by using either modern or artisanal processes. Charcoal firing, pan firing, or sun drying, are some of the common artisanal processes. Steaming, tumbling, and oven drying are common modern processes.
Positive Effects of Green Tea
Human research studies are confirming that there are numerous health benefits of green tea and it is the most popular beverage globally (Yashin, Yashin, & Nemzer, 2013). Green tea comprises bioactive compounds that are boosting health. These compounds are catechins and flavonoids which are functioning as powerful antioxidants. The antioxidants reduce the free radicals which are forming in the body, and protecting molecules and cells from any damaging (Cooper, 2012). According to one study, the compounds in the green tea can boost brain functioning and making one smarter because it is containing caffeine which is a known stimulating ingredient (Okello, Abadi, abadi, 2016). What caffeine is doing in the brain is blocking an inhibitory neurotransmitter known as Adenosine. This way it is actually increasing the firing of neurons and clustering of neurotransmitters. The green tea boosts burning of fat and improving physical performance. Caffeine in green tea is shown to be improving physical performance deploying fatty acids from the fat tissues and availing them for use as energy. Antioxidants in the green tea may be lowering the risk of different types of cancer. Cancer is one the globe’s leading cause of deaths and it is caused by uncontrolled growing of cells. The antioxidants can be having a protective effect against oxidative damage which is known to be contributing to development of prostate cancer among men (Lassed et al, 2016). Green tea can destroy bacteria, which is improving dental health and lowering the risk of infection. Some researchers are showing that the catechins in green tea are killing and inhibiting viruses such as influenza and potentially lowering the risk of infections. Green tea may decrease one’s risk of cardiovascular disease. This is achieved by the antioxidants in the green tea that are protecting the LDL cholesterol from oxidation which is one path leading to heart attack and strokes.
In conclusion, green tea is a kind of tea that is prepared from Camellia sinensis leaves that have not experience the same oxidation and withering process used in making black tea and colong. It stemmed from China but its production has been spreading in many nations in Asia. It is grown and processed in different ways, and it depends on the kind of green tea wanted. The green tea was originally consumed throughout Asia for recreational and medicinal purposes and today it is still contributing its health effects to the rest of the world.
- Yashin A. Yashin Y. Nemzer B. (2013). Beneficial Effect of Tea on Human Health. American Journal Biomedical Sciences, 5(4), p226-241
- Cooper R. (2012). Green Tea and Theanine: Health Benefits. International Journal of Food Sciences & Nutrition, 63, p90-97
- Okello E. Abadi A. Abadi S. (2016). Effects of green and black tea consumption on brain wave activities in healthy volunteers as measured by a simplified Electroencephalogram (EEG): A feasibility study. Nutritional Neuroscience, 19(5), p196-205.
- Lassed S. Deus C. Lourenco N. Dahdouh A. Rizvanov A. Oliveira P. Zama D. (2016). Diet, Lifestyles, Family History, and Prostate Cancer Incidence in an East Algerian Patient Group. BioMed Research International, p1-9.

Essay Mills | Most Trusted Academic Help Site
- Testimonials
- Terms & Condition
- Annotated Bibliography
- Assignment Writing Services
- Capstone Project
- Creative Writing
- Critical Thinking
- Dissertation Writing Service
- Essay Writing Service
- Grant Proposal By Professional Grant Writer
- Presentation
- Project Help
- Research Paper Writing Service
- Report Writing Help
- Review Writing
- Speech Writing
- Term Paper Writing
- Thesis Writing Service
- Business Studies
- Environment
- Health and Medicine
- Other Sample Writing
Essay: Green Tea Benefits
Sample essay – green tea.
Beverages are extremely common all over the world and tea is one beverage that in its various forms is one of the most consumed beverages in the world. Be it hot or iced tea, the popularity is the same. Rising awareness of its medicinal value is also giving rise to the usage of green tea all over the world. It is the best form of tea considered for usage and is extremely beneficial for health.
Green tea has several advantages. People use it to lose weight; it has a great extent of anti-oxidants in it and also imparts glowing fresh skin. Green tea is also very effective in usage against damage caused to the skin by the sun. This can be achieved by boiling green tea leaves in water, cooling it, and applying it to the skin before applying sunscreen.
Green tea is also very beneficial to the skin and its masks are very popular. They help replenish the skin and give it a glow. A cup of green tea each day helps the metabolic rate to rise which in turn helps lose weight. Maximum benefits can be attained by consuming tea brewed from fresh green tea leaves.
People nowadays are tending more and more towards medicine. They should realize that it’s in the natural ingredients that all benefits lay and if they are consumed adequately, no need for medicines would arise.
Place your custom writing order at an affordable price, written by professional writer.


IMAGES
VIDEO
COMMENTS
Green tea. Tea is one of the most popular beverages consumed worldwide. Tea, from the plant Camellia sinensis, is consumed in different parts of the world as green, black, or Oolong tea.Among all of these, however, the most significant effects on human health have been observed with the consumption of green tea [].The first green tea was exported from India to Japan during the 17th century.
Green Tea 101: A Complete Guide. Antioxidant-packed green tea may help prevent cancer and aid in weight loss, among other potential health benefits. Dejan Beokovic/Stocksy. Water is the most ...
Tea, which originated in China, has spread to every part of the world and become the most popular beverage, second to water. This review aims to broaden existing knowledge offering insights into the nutritional value of green tea, and its pharmacological effects on humans. Additionally, its nutraceutical and pharmaceutical formulations, patents ...
Green tea contains a relatively small amount of caffeine, approximately 29 milligrams (mg) per 8-ounce cup, compared with black tea, which has around 47 mg per cup, and coffee, which has about 95 ...
A total of 105 peer-reviewed papers in English were selected for this . review. ... semen should be stored in an extender. Green tea leaf extract (GTLE) and skim milk-goat egg yolk (SM-GEY) may be ...
A couple of papers have reported that green tea catechins, especially with a galloyl moiety, could significantly inhibit the gastrointestinal absorption of dietary nutrition and increase fecal ...
838 Words4 Pages. GREEN TEA: THE NEW HEALTH MANTRA Green tea is made from the leaves from Camellia sinensis that have undergone minimal oxidation during processing. Green tea, originally from China, has now become associated with many Asian cultures. In recent times, green tea has become relatively wide spread even in some western countries.
However, the amounts of catechins (green tea: 94.4 mg/roasted green tea: 37.4 mg) and theanine (green tea: 6 mg/roasted green tea: not detected) ingested by participants in this study were ...
Source Of. Caffeine (traditional teas, not herbal) Polyphenols. Flavonols - myricetin, quercetin, kaempferol. Theaflavins - formed when black tea leaves are oxidized. Catechins - found in green tea; epigallocatechin-3 gallate (EGCG) is the main form. Most traditional teas do not contain a significant amount of nutrients, but are rich in ...
Green tea has about 28 milligrams of caffeine in 8 ounces of brewed liquid or 19 milligrams in the same amount of a ready-to-drink bottled form, per the Mayo Clinic. That brewed amount is ...
Tea is the most common beverage consumed after water. It is brewed from the leaves of Camellia sinensis (family: Theaceae). Different types of tea manufactured are: oolong, green, black and Ilex ...
Studies have shown that green tea extracts can help increase energy expenditure and fat oxidation, potentially contributing to weight loss and improved metabolic health [11]. 3. Cinnamon ...
The health benefits of green tea for a wide variety of ailments, including different types of cancer, heart disease, and liver disease, were reported. Many of these beneficial effects of green tea are related to its catechin, particularly (-)-epigallocatechin-3-gallate, content. There is evidence from in vitro and animal studies on the underlying mechanisms of green tea catechins and their ...
1501 Words. 7 Pages. Open Document. Green tea is one of the drinks which know by around the world. It is not hard to find it so it is very convenience for human's life. Green tea becomes many people favorite drink. Some people have misunderstanding about green tea. They think it can cause bad effect for health.
Green tea contains a little chemical antioxidant called "catechin" which can destroy bacteria and viruses that cause throat infections and other dental problems. A study published by the European Journal of Nutrition found that consumption of one or more cups of green tea a day was associated with decreasing the risk of tooth loss.
The Adverse Effect of Green Tea. Tea is recognized as safe by the Food and Drug Administration (FDA). The adverse effects reported in some studies included, dizziness, headache, excess intestinal gas, nausea, heartburn, stomach ache, abdominal pain, and muscle pain. Many scientists think that The tea as any other caffeinated beverages (such as ...
Green tea contains extracts which have numerous health benefits to human body. This paper thrives to describe green tea and its positive effects to the human body. History of Green Tea. It is widely believed that green tea was first soaked during the reigning of emperor Shennong in 2737 BC.
1137 Words5 Pages. Green tea's earliest reference was 2737 BC, a beverage predating even modern time (Lenda 1). Green tea has been rumored to cause weight loss, cure cancer, lower cholesterol, and reduce risk of stroke. A scientific study was done on green tea, by Yashi Mi and his colleagues to find the effects of green tea on the mind and body.
It has been proven that green tea does affect a person's weight. It contains caffeine which known to aid in fat burning. It also contains antioxidants that help boost metabolism. The caffeine will help to boost energy as well as metabolism. With the energy absorbed from the tea a person will want to exercise which will burn even more fat than ...
A study was conducted that showed evidence that simply drinking a single cup of green tea on a daily basis can provide people with a 10 percent less chance of developing cardiovascular disease. The antioxidants contained in green tea are thought to be helpful in preventing the growth of colorectal, stomach, breast, pancreatic, lung and bladder ...
Sample Essay - Green Tea. Beverages are extremely common all over the world and tea is one beverage that in its various forms is one of the most consumed beverages in the world. Be it hot or iced tea, the popularity is the same. Rising awareness of its medicinal value is also giving rise to the usage of green tea all over the world.
Green Tea Essay. Register to read the introduction…. Especially sencha and other delicate green tea. Instead, bring water to a boil then let it sit for 2-3 minutes. Water temp should be around 60-80 C. The optimum brewing temperature for Gyokuro is 50 -60 C and Sencha is 70-90 C. For Bancha, Genmaicha and Houjicha is 100 C.