An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Pharmacol

The Widely Used Antihelmintic Drug Albendazole is a Potent Inducer of Loss of Heterozygosity
Luiza s. e. p. will castro.
1 Division of Tumor Biology and Immunology, Netherlands Cancer Institute, Amsterdam, Netherlands
2 Department of Biochemistry, Federal University of Santa Catarina, Florianópolis, Brazil
Wietske Pieters
Mir farshid alemdehy, muhammad a. aslam.
3 Institute of Molecular Biology and Biotechnology, Bahauddin Zakariya University, Multan, Pakistan
Olimpia Alessandra Buoninfante
Jonne a. raaijmakers.
4 Division of Cell Biology, Netherlands Cancer Institute, Amsterdam, Netherlands
Bas Pilzecker
Paul c. m. van den berk, hein te riele, rené h. medema, rozangela c. pedrosa, heinz jacobs.
Jan Willem Van Der Laan , Medicines Evaluation Board, Netherlands
Associated Data
The RNA-Seq reported in this article have been deposited at the National Center for Biotechnology Information under the accession number {"type":"entrez-geo","attrs":{"text":"GSE163419","term_id":"163419"}} GSE163419 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc= {"type":"entrez-geo","attrs":{"text":"GSE163419","term_id":"163419"}} GSE163419 ).
The antihelmintic drug ABZ and its metabolites belong to the chemical family of benzimidazoles (BZM) that act as potent tubulin polymerization inhibitors, suggesting a potential re-direction of BZMs for cancer therapy. Applying UV-Vis spectrometry we here demonstrate ABZ as a DNA intercalator. This insight led us to determine the primary mode of ABZ action in mammalian cells. As revealed by RNA sequencing, ABZ did neither grossly affect replication as analyzed by survival and replication stress signaling, nor the transcriptome. Actually, unbiased transcriptome analysis revealed a marked cell cycle signature in ABZ exposed cells. Indeed, short-term exposure to ABZ arrested mammalian cells in G2/M cell cycle stages associated with frequent gains and losses of chromatin. Cellular analyses revealed ABZ as a potent mammalian spindle poison for normal and malignant cells, explaining the serious chromosome segregation defects. Since chromosomal aberrations promote both cancer development and cell death, we determined if besides its general cytotoxicity, ABZ could predispose to tumor development. As measured by loss of heterozygosity (LOH) in vitro and in vivo ABZ was found as a potent inducer of LOH and accelerator of chromosomal missegregation.
Introduction
In 1982 the United States. Food and Drug Administration (FDA) approved Albendazole (ABZ) for the treatment of helminthiasis in humans ( Dayan, 2003 ). Since then, ABZ developed as an affordable drug that is currently manufactured by 385 companies and distributed under at least 626 brand names listed in the Medindia’s database ( Medindia, 2017 ). These large numbers reflect the necessity of affordable drugs in treating soil transmitted helminth (STH) as well as lymphatic filariasis infections. Pullan et al. provided a global estimation of STH infections for the year 2010, where around 439 million people were infected with hookworm, 819 million with Ascaris lumbricoides and 465 million with Trichuris trichiura ( Pullan et al., 2014 ). According to the World Health Organization (WHO), approximately 947 million people in 54 countries worldwide are threatened by lymphatic filariasis ( World Health Organization, 2020a ) and approximately 1.5 billion people are infected with STH worldwide ( World Health Organization, 2020b ). Other WHO data report preventive chemotherapy of (pre)school-aged children with ABZ as a monotherapy or in combination with another drug, in many areas of the world. Altogether these data highlight the global challenge of STH to mankind, the current importance of ABZ chemotherapy in fighting STH infections in humans, and its extensive global use ( Supplementary Figure S1 ) (for more information we like to refer readers of this study to the WHO website ( World Health Organization, 2020c ).
The pharmacokinetics, dynamics, and mode of action of ABZ are well described ( Dayan, 2003 ). ABZ is a low aqueous solubility drug and therefore poorly absorbed from the gastrointestinal tract, with an absorption rate in humans less than 5% ( Rigter et al., 2004 ). Nevertheless, administration with enhancers like fatty meals and grapefruit has been reported to increase its solubility and bioavailability ( Rigter et al., 2004 ). ABZ and its metabolites belong to the chemical family of benzimidazoles (BZM) that act as potent tubulin polymerization inhibitors ( Ramírez et al., 2007 ). BZM based compounds inhibit microtubule dynamics, an essential process in the formation of a functional spindle apparatus and in the proper distribution of the sister chromatids during mitosis. This marked cytotoxic feature classifies BZM as effective spindle poisons ( Ramírez et al., 2007 ). These activities suggest a potential re-direction of BZMs for cancer therapy. We and others previously reported on the potential use of ABZ as an anti-cancer drug ( Pourgholami et al., 2010 ; Hanušová et al., 2015 ; Castro et al., 2016 ). While spindle poisons are effective in cancer treatment, ABZs action on the mammalian spindle apparatus is poorly understood ( Manchado et al., 2012 ). Therefore, we here followed an unbiased approach to characterize the mode of action of ABZ in transformed as well as non-transformed mammalian systems.
To ensure that during mitosis the duplicated genome is equally distributed over the daughter cells, several checkpoints are operative. Defects in the spindle apparatus predispose to checkpoint activation, chromosome segregation errors, and apoptosis. These processes form the molecular basis of cancer therapy with spindle poisons. Physiologically, these checkpoints critically contribute to genome maintenance. Inhibition of mitotic proteins like polo-like kinase 1 (PLK1), Aurora kinase A (AURKA) and Aurora kinase B (AURKB) leads to chromosome segregation errors, aneuploidy, polyploidy and micronuclei formation ( Lens et al., 2010 ). Under conditions of spindle poisoning or checkpoint inhibition, whole chromosomes can easily be gained or lost. In healthy individuals, these gain and losses usually provide a selective growth disadvantage to the daughter cells, explaining why oncogenic site effects have not been found experimentally in ABZ treated rats nor in ABZ treated patients ( Dayan, 2003 ; Sheltzer et al., 2017 ). In contrast, patients suffering from heterozygous inactivating mutations of tumor suppressor genes, such as Familial Adenomatous Polyposis-FAP (APC), Seckel Syndrome (ATR), Blooms Syndrome (BLM), Familial Breast or Ovarian Cancer Syndrome (BRCA1/BRCA2), Lynch Syndrome (MLH1/MSH2), Neurofibromatosis Type 1 (NF1), Familial Retinoblastoma (RB), and Li-Fraumeni Syndrome (TRP53) are expected to be at particular risk. Loss of the corresponding wild type allele by loss of the entire or part of a chromosome can trigger tumor development. As such, spindle poisons are expected to accelerate loss of heterozygosity (LOH) of tumor suppressors and hence cancer development, particularly in this predisposed patient group.
A PubMed search on genotoxic studies of ABZ in mammalian cells is limited to a few reports ( Mantovani, 1992 ; Oztas et al., 2007 ; Tweats et al., 2016 ). While these reports suggest an overall genotoxicity of ABZ, conclusions drawn are quite divergent. For example, ABZ gave clear negative results in the Ames test (for review see ( Dayan, 2003 )) whereas other tests noted an activity of ABZ in inducing micronuclei, which often arise as a consequence of chromosomal aberrations and missegregation ( Ramírez et al., 2007 ). Of note, ABZ was independently found to display strong teratogenicity in rats and sheep ( Dayan, 2003 ). Clearly, as pointed out by Dayan in this retrospective evaluation of ‘old’ drugs ( Dayan, 2003 ), the present experimental non-clinical data about activity, toxicity, and kinetics would be considered inadequate based on strict application of today’s professional and regulatory guidelines. Because ABZ is a relatively old, widely used drug with a potential anti-cancer activity, we decided to further analyze its drug safety by providing a systematic analysis on its potential genotoxicity. We here demonstrate ABZ as a potent inducer of chromosomal missegregation, the molecular basis of aneuploidy, a hallmark of cancer.
Materials and Methods
Ethics-statement.
All experiments were performed in accordance to Dutch and European guidelines. Protocols were approved by the local Animal Ethical Committee (IVD Instantie voor dierenwelzijn) at The Netherlands Cancer Institute, Amsterdam, The Netherlands, and the CCD (Centrale Commissie Dierproeven, the national central commission for animal experimentation) located in Den Haag, The Netherlands under 9.2.8175, where 9 is the CCD number, 2 the CCD subgroup number, and 8175 the IVD number.
Mice were housed at room temperature and a relative humidity of approximately 55% in disposable individually ventilated cages (dIVC, Innovive®). Mice were fed Transbreed (E) PL MIN (Special Diet Services) pellet nutrition and water (Aquavive®) ad libitum. Msh2 +/− and Msh2 +/+ (WT) male and female mice on the FVB background were identified by genotyping using allele specific PCR primers on DNA extracted from toe biopsies P1: 5′-CGGCCTTGAGCTAAGTCTATTATAAGG-3′, P2: 5′-GGTGGGATTAGATAATGCCTGCTCT-3′, P3: 5′-CCAAGATGACTGGTCGTACATAAG-3′ ( De Wind et al., 1995 ).
ABZ powder was brought in suspension 0.5% Sodium Carboxy-methylcellulose + 1% Tween-80. Temozolomide (TMZ) was prepared as described previously ( Wojciechowicz et al., 2014 ). Mice were exposed either to ABZ or vehicle alone by oral gavage in the morning followed by a subsequent oral treatment with TMZ in the afternoon on indicated days.
Immunohistochemistry
Two weeks after the last ABZ exposure, mice were sacrificed using CO 2 and intestines were fixed in 4% formaldehyde and embedded in paraffin for tissue sectioning. After deparaffinization, tissue sections were treated with TRIS/EDTA pH 9.0 to allow antigen retrieval. Endogenous peroxidases were inactivated using 3% H 2 O 2 in methanol. Slides were blocked with PBS containing 4% bovine serum albumin (BSA) and 5% normal goat serum (NGS). Slides were incubated overnight with a MSH2-specific mouse monoclonal IgG antibody (Calbiochem; NA27) in PBS containing 1% BSA and 1.25% NGS. After washing, slides were incubated with a rabbit-anti-mouse IgG1, IgG2a and IgG3 monoclonal antibody (Abcam; ab133469). MSH2 specific immune-complexes were visualized by Labeled Polymer-HRP Anti-Rabbit Envision (DakoCytomation; K4011) using DAB (Sigma; D-5905), and rinsed with demi water. Sections were then counterstained with haematoxylin, washed in tap water and mounted with Entallan (Sigma; 1.07960). All washing in between steps was performed using PBS containing 0.05% Tween 20, unless otherwise indicated. Slides were scanned on the ScanScope ® XT slide scanner (Aperio, Leica Biosystems) and analyzed using Aperio ImageScope software (Aperio, Leica Biosystems). The absolute number of MSH2 deficient crypts was counted along the entire length of the small intestine.
Interactions with Calf Thymic-DNA
CT-DNA binding was evaluated by UV-Vis spectrometry. Absorption scanning was done using CT-DNA (40 µM) and ABZ (10 µM). Spectra were obtained by reading the absorption from 230 to 320 nm (TECAN Infinity M200). Variations of absorption as well as the displacement of the wavelength of spectral maximum absorption were evaluated ( Kubota et al., 1999 ).
CT-DNA intercalation was evaluated by fluorescence measurements at excitation/emission wavelengths of 492 nm and 620 nm. Compounds able to intercalate into DNA compete with ethidium bromide causing fluorescence reduction. CT-DNA (10 µM) was saturated with ethidium bromide (20 µM) in 50 mM phosphate buffer containing NaCl (0.1 M), pH 7.4 and ABZ was added at 10, 20, 30 and 40 µM ( Da Silveira et al., 2011 ).
DNA damage tolerant (DDT) and DNA damage intolerant (DDinT) cells were cultured in RPMI medium supplemented with 8% of Fetal Bovine Serum (FBS), 100 U/ml Penicillin, 100 μg/ml Streptomycin, 50 µM 2-Mercaptoethanol, 200 μM L-arginine ( Buoninfante et al., 2018 ). HeLa, U2OS and RPE-1 cell lines were cultured in DMEM supplemented with 8% FBS, 100 U/ml Penicillin, 100 μg/ml Streptomycin, and 2 mM L-glutamine. All cell lines were incubated at 37°C and 5% CO 2 . Cells were exposed to increasing concentrations of ABZ and after 24 h cells were harvested and resuspended in PBS/1% BSA/0.02 µM Sodium Azide buffer containing 1 μg/ml propidium iodide (PI) and measured using FACSArray (Becton Dickinson). The data were analyzed with FlowJo™ vX 0.7 software following the gating strategy as described ( Wit et al., 2015 ). The complete procedure was repeated to generate three biological replicates. Comparison between the groups was performed by Analysis of Variance (ANOVA) comparing the groups with the control.
Sample preparation: DDT lymphoma cells were plated (10 7 cells) in Petri dishes and treated with ABZ (400 nM), EtBr (400 nM) or mock treated for 12 h. Hereafter, the cells were lysed in Trizol and frozen for subsequent processing. The complete procedure was repeated to generate three biological replicates for each treatment. Quality and quantity of the total RNA was assessed by the 2,100 Bioanalyzer using a Nano chip (Agilent). Only RNA samples having an RNA Integrity Number (RIN) > 8 were subjected to library generation.
Library preparation: Strand-specific cDNA libraries were generated using the TruSeq Stranded mRNA sample preparation kit (Illumina) according to the manufacturer’s protocol. The libraries were analyzed for size and quantity of cDNAs on a 2,100 Bioanalyzer using a DNA 7500 chip (Agilent), diluted and pooled in multiplex sequencing pools. The libraries were sequenced as 65 base single reads on a HiSeq2500 (Illumina).
Pre-processing: Strand-specific RNA reads (11–33 million reads per sample), 65 bp single-end, were aligned against the mouse reference genome (Ensembl build 38) using Tophat (version 2.1, bowtie version 1.1). Tophat was supplied with a Gene Transfer File (GTF, Ensembl version 77) and was supplied with the following parameters: “--prefilter-multihits–no-coverage-search–bowtie1–library-type fr-firststrand.” In order to count the number of reads per gene, a custom script which is based on the same ideas as HTSeq-count has been used. A list of the total number of uniquely mapped reads for each gene that is present in the provided Gene Transfer Format (GTF) file was generated.
Analysis: For checking the data quality and performing statistical analysis, we used different packages including limma, edgeR and GoSeq ( Robinson et al., 2009 ; Young et al., 2010 ). All the analyses were performed in R language (version 3.5.1). Only relevant samples were used for the differential gene expression analysis using edgeR package under default arguments with the design set to either of the two conditions form control, ABZ and EtBr treatments. Genes were considered to be differentially expressed when the False discovery rate (FDR) was below 0.001 after the Benjamini-Hochberg multiple testing correction. Sets of differentially expressed genes in indicated conditions were called ‘gene signatures. MA plots were generated after differential gene expression analysis carried by edgeR package ( Robinson et al., 2009 ; McCarthy et al., 2012 ).
DDT lymphoma cells were exposed to different concentrations of ABZ treated for 24 h, fixed in 70% ethanol, and stored at −20°C. On day of analysis, cells were re-suspended in PBS and treated with RNAse-A (100 μg/ml) for 20 min and subsequently re-suspended in PBS containing 5 μg/ml PI and measured using FACScalibur (Becton Dickinson). The data were analyzed using the FlowJo™ vX 0.7 software ( Wit et al., 2015 ).
Cell Imaging to Detect Microtubule Dynamics
HeLa (7.5 × 10 4 /well) cells were plated in 48 well-plates and after adherence were treated with ABZ (400, 800 and 1,600 nM), Paclitaxel (1 µM), Nocodazole (825 nM) or Noscapine (25 µM) for 12 h. The cells were fixed for 20 min (formaldehyde 4%, Triton X 0.5% in PBS). Cells were incubated overnight at 4°C with antibodies against α -tubulin (Sigma, 1:10,000) and crest (Cortex Biochem, 1:5,000) followed by 2 h of incubation with secondary antibody (Molecular probes, 1:500) and DAPI at room temperature. All antibodies were diluted in PBS/Tween 0.2%. The images were acquired using a Deltavision deconvolution microscope (Applied Precision) with a 60x (NA 1.42) oil objective (Olympus). Softworx (Applied Precision), ImageJ, Adobe Photoshop and Illustrator CS6 were used to process acquired images.
Loss of Heterozygosity in Vitro
LOH analyses were performed using mouse embryonic stem cells (mESC) with a heterozygous deletion of the MLH1 allele (MLH1 wt/− ) and cultured as previously described ( te Riele, 2009 ; Aarts and te Riele, 2010 ). The 6-thioguanine (6-TG, 400 nM) treatment is very toxic for mismatch repair (MMR) proficient- but not MMR-deficient cells, providing a simple measure for LOH ( De Wind et al., 1995 ). For each condition 10 6 mESC cells were plated in 10 cm Petri dishes. After 24 h, cells were exposed to 100 nM and 200 nM ABZ or 200 nM reversine, a potent inhibitor of Monopolar Spindle 1 (MPS1) for 24 h. Mock treated cells were used as negative control. After 3 days of treatment with ABZ or Reversine, the 6-TG (400 nM) treatment was started. New medium containing fresh stock of 6-TG was refreshed after every 4 th day. Colony formation was photographed after 15 days.
ABZ Interacts with DNA and Competes with EtBr
The pharmacological features of ABZ have been widely studied. While ABZ acts as a tubulin polymerization inhibitor in helminth, its effect in mammalian cells is poorly understood. Given the small planar molecular structure of ABZ we considered that ABZ might actually intercalate into DNA ( Kubota et al., 1999 ; Kubota et al., 2002 ). To test this possibility, we first used hypochromism as a read out for ABZ DNA binding properties and its potential intercalating activity. Hypochromism is generally consistent with the forces of interaction by intercalation, as this connection involves stacking interactions between an aromatic chromophore and DNA base pairs. As shown in Figure 1A , ABZ reduced the absorbance of purified DNA at defined wavelength. This hypochromic effect suggested that ABZ may in fact intercalate with DNA. To verify intercalation as potential type of DNA interaction we took advantage of the fact that intercalating DNA compounds usually induce hypochromism and bathochromic displacement ( Shahabadi and Moghadam, 2012 ). If ABZ is a DNA intercalator, one predicts that ABZ competes with the DNA intercalator ethidium bromide (EtBr), a widely applied fluorescent dye to visualize and measure DNA. Indeed, ABZ was found to compete efficiently with EtBr and thereby decreased EtBr specific fluorescence ( Figure 1B ). This bathochromic displacement effect further indicated ABZ as a DNA intercalator, a finding in accordance with its small planar molecular structure.

Albendazole interacts with DNA and competes with the DNA-intercalator EtBr. (A) Evaluation of ABZ (10 μM) binding to purified DNA (40 μM) by UV-Vis spectrometry (reading the absorption from 230 to 320 nm). (B) The fluorescence intensity of CT-DNA (10 μM) stained with EtBr (20 μM) is reduced by ABZ (10, 20, 30 and 40 μM). Fluorescence intensity was measured at excitation/emission wavelengths of 492 nm and 620 nm (n = 2).
ABZ Does not Induce Replication Stress
To determine if the non-covalent interaction of ABZ with DNA mimics DNA damage and blocks replication, we took advantage of a unique isogenic set of wild type, i.e. DNA damage tolerant (DDT) and PcnaK164R mutant, i.e. DNA damage intolerant (DDinT) thymic lymphoma cell lines established from a p53 ko ; Pcna K164R/loxP mutant mouse ( Langerak et al., 2007 ; Buoninfante et al., 2018 ). DDT and DDinT lymphoma cells were exposed to increasing concentrations of ABZ. Both cell lines were found to be equal sensitive to ABZ, suggesting that ABZ does not grossly interfere with DNA replication, at least not in a manner that sensitizes DDinT cells ( Figure 2A ). We conclude that ABZ does not interfere at the level of replication. Most likely, as intercalators interact non-covalently with DNA, they are efficiently removed during DNA unwinding.

Albendazole affects cell survival but does not induce replication stress. (A) Evaluation of DDT and DDinT lymphoma cell lines survival upon treatment with different concentrations of ABZ. (B) Survival of HeLa, U2-OS and RPE-1 cell lines upon exposure to different concentration of ABZ. Data represent the mean of three independent experiments. Values are expressed as mean ± SEM (n = 3). *, ** and *** denote statistical difference of ABZ treated cells compared to non-treated cells of the control when p < 0.1, p < 0.01 and p < 0.001, respectively. Comparison between these groups was performed by Analysis of Variance (ANOVA).
To extend our studies to the human system, we determined the toxicity of ABZ on human transformed cell lines as well as non-transformed immortalized cell lines. Similar to murine lymphoma cells, both cell lines were found hypersensitive to ABZ. The slight differential sensitivity threshold of transformed HeLa cells (400 nM) as compared to immortalized RPE-1 cells and transformed U2-OS (800 nM) likely relates to differences in the rate of proliferation ( Figure 2B ). Apparently, the toxicity of ABZ on murine and human cells is quite similar.
Transcriptome of ABZ Exposed Cells
As ABZ and EtBr intercalate with DNA, we questioned whether and in which way EtBr and ABZ affected gene expression patterns. To approach this question in a genome-wide and an unbiased manner, we exposed the previously mentioned DDT lymphoma cell line to ABZ (400 nM), EtBr (400 nM), or to DMSO, as a vehicle control. After exposure, cells were harvested, total RNA was isolated and used for RNA-Seq analysis. Subsequently, specific or generic changes in the transcriptome pertaining to ABZ or EtBr alone were defined by comparing ABZ and EtBr dataset to the DMSO control. First, we determined the ABZ signature by defining specific transcripts that are either induced or suppressed by ABZ ( Figure 3A , red and blue labeled respectively, and Supplementary Table S1 . Remarkably, the majority of transcripts that were differentially expressed in the ABZ setting remained unaffected by EtBr, except for three genes: Idi1, Ccne2 and Aacs, which were found downregulated in both conditions. Likewise, the EtBr signature appeared entirely different from ABZ ( Figure 3B ). A closer analysis of the ABZ signatures ( Figure 3C ) revealed a marked decrease of genes directly linked to mitosis. This observation suggested that critical mitotic genes are expressed prior to chromosome condensation and the overrepresentation of mitotic arrested cells in the ABZ condition result in an underrepresentation of these transcripts.

Albendazole affects genes linked to mitosis. (A) MA-plot of lymphoma cell lines exposed to ABZ or a vehicle control. Differentially up- or down-regulated genes (FDR < 0.001) are shown in red and blue, respectively. (B) ABZ and EtBr differentially affect the transcriptome. MA-plot of lymphoma cell lines exposed to EtBr or a vehicle control showed that the genes up- or down-regulated by ABZ, are not affected by EtBr. (C) Exposure to ABZ affects transcription of cyclins, cyclin dependent kinases, DNA damage response, spindle, segregation, and tubulin genes (for a list of shown genes in each subgroup, see Supplementary Table S2 ).
ABZ Affects Cell Cycle Progression and Causes Chromosomal Missegregation
The pronounced underrepresentation of mitotic genes in the ABZ exposed samples led us to analyze the impact of ABZ on the cell cycle. Increasing doses of ABZ resulted in a gradual increase of cells arrested in the G2/M phase. This effect was associated with a dose dependent increase of viable aneuploid cells ( Figure 4 ). Apart from subG1 cells, this aneuploidy was characterized by the frequent occurrence of surG2 cells, pointing to a critical role of ABZ in promoting chromosomal segregation errors. This phenomenon was not observed for EtBr.

Albendazole affects cell cycle progression and causes chromosomal aneuploidy. (A) Flow cytometric analysis of cell cycle progression in the presence of ABZ or EtBr (concentrations are indicated). Data are representative of two experiments with two independent cell lines. (B) The relative cell cycle distribution of DDT lymphoma cells in the presence of ABZ or EtBr. The averages of DDT cell line are shown (Mean ± SD).
ABZ Poisons the Mammalian Spindle Apparatus
To measure any direct effect of ABZ on the human spindle apparatus, we exposed HeLa cells to ABZ. As positive controls for spindle poisons we selected Paclitaxel as a typical microtubule stabilizer, Nocodazole as a microtubule polymerization inhibitor, and Noscapine as a spindle poison that affects microtubule dynamics ( Figure 5 ). After 12 h cells were fixed and the spindle apparatus was revealed with α-tubulin specific antibodies (green), and centromeres with CREST specific antibodies (pink). DNA was revealed by DAPI staining (blue). As visualized by wide-field fluorescence microscopy ( Figure 5 ), ABZ was found as a potent mammalian spindle poison, inducing misalignments. Mitotic figures most closely resembled those of Noscapine, with the marked notion that the ABZ effect was achieved at a molar concentration that was 60-fold lower as compared to Noscapine. These observations explain the high frequency of aneuploidy in cells treated with low doses of ABZ.

Albendazole induces spindle apparatus disruption. ABZ act as spindle poison for mammalian cells, disrupting the spindle apparatus. HeLa cells were exposed to the indicated drugs, fixed and stained with an anti-alpha tubulin antibody (green) and an anti-centromere antibody (crest, red). DAPI was used to stain the DNA. The images were acquired by wide-field fluorescence microscopy.
Taken together, ABZ acts as a strong spindle poison and potent inducer of aneuploidy, both in helminth as well as mammals, thereby inhibiting cell cycle progression in both systems. Transcriptional alterations induced by ABZ appeared largely indirect.
ABZ Strongly Stimulates Loss of Heterozygosity in vitro
The sum of these data implicated a high risk for ABZ in inducing chromosomal missegregation. As chromosomal missegregation is a hallmark of cancer, we questioned if ABZ could promote aneuploidies in general and especially in tumor-prone patients that display a haploinsufficiency for a specific tumor suppressor gene. If this is the case, one expects that ABZ exposure stimulates loss of heterozygosity (LOH). To address this critical issue, we took advantage of an in vitro approach enabling a direct measure of the LOH frequency ( De Wind et al., 1995 ). Embryonic stem (ES) cells haploinsufficient for the mismatch repair gene Mlh1 were cultured in the presence and absence of ABZ for 24 h. Three days later, LOH cells were selected by exposure to 6-thioguanine (6-TG) over a time course of fifteen days. 6-TG specifically kills mismatch repair (MMR) proficient cells while MMR deficient cells can resist low doses of 6-TG. Hereafter, surviving mismatch repair deficient colonies, i.e. those that lost the remaining functional Mlh1 allele, were determined for different concentrations of ABZ. Remarkably, a low concentration of ABZ sufficed to induce LOH ( Figure 6 ).

Albendazole stimulates loss of heterozygosity in vitro . mES cells haploinsufficient for the mismatch repair (MMR) component Mlh1 ( Mlh1 wt/− ) were plated (10 6 cells/dish) in the presence and absence of ABZ. After 24 h, ABZ was removed. The cells subsequently were exposed to 6-TG. Cells that lost MMR activity because of LOH events form 6-TG-resistant colonies. Reversine (200 nM, a potent inhibitor of MPS1) was used as positive control.
ABZ Is a Potent Stimulator of LOH in vivo
The fact that ABZ stimulates LOH in vitro , led us to address whether ABZ could also induce the same effect in vivo . To accomplish this goal, we took advantage of Msh2 heterozygous ( Msh2 +/− ) mice, a model for Lynch Syndrome (LS), in which LOH could be addressed in vivo . In LS patients, MMR deficient crypt foci are commonly found in the intestinal tract, which have the potential to progress into tumors ( De Wind et al., 1995 ; Kloor et al., 2012 ). As chromosome mis-segregation generally generates a cell cycle arrest and chromosome gains or losses of larger chromosomes commonly function as tumor suppressors ( Duijf et al., 2013 ; Santaguida et al., 2017 ; Sheltzer et al., 2017 ), and in particular a loss of MSH2 does not confer a direct proliferative advantage to the cells, we provided MSH2-deficient cells a selective advantage by exposure to the methylating agent Temozolomide (TMZ). In short, mice were exposed daily to ABZ or carrier solution only, for 32 consecutive days, while every week this treatment was followed by subsequent TMZ administrations for three consecutive days ( Figure 7A ). Hereafter, we scored MSH2-deficient crypts from immunohistochemical staining covering the entire small intestine ( Figure 7B ). Quantification of the number of MSH2 deficient crypts along the entire length of the small intestinal tract showed a significant increase in the ABZ treated group, indicating that ABZ stimulates LOH in vivo ( Figure 7C ).

Albendazole accelerates LOH in intestinal stem cells in vivo . (A) In vivo experimental set up. Msh2 +/− mice were exposed to ABZ (400 mg/kg) (n = 7) or control solution (n = 8) by oral gavage for 32 consecutive days. Every 3–4 days this was followed by subsequent administration of TMZ (100 mg/kg) by oral gavage for three consecutive days. After a two-week rest period, mice were sacrificed and intestines were fixed. (B) Immunohistochemical staining of small intestine for MSH2. (C) Quantification of B . Individual MSH2-deficient crypts along the entire length of the small intestine were counted manually. WT mice exposed to ABZ were used as negative control (n = 8). Plotted are the mean and SD, asterisk indicates p value <0.05 (One way ANOVA).
Since the FDA approval of ABZ in 1982 for the treatment of helminthiasis in humans, ABZ developed as an affordable drug that is currently manufactured and distributed by 385 companies, reflecting the necessity of affordable drugs in treating STH as well as lymphatic filariasis infections ( Dayan, 2003 ). Although teratogenicity of ABZ has been reported in sheep and rats ( Dayan, 2003 ), the precise molecular impact of ABZ on mammalian, and in particular humans remains unclear. Given this uncertainty, pregnant woman are advised not to use ABZ. However, as patients may be unaware of a pregnancy, a potential teratogenic risk of ABZ usage might occur. Strikingly, recent reports suggest a re-direction of ABZ in cancer treatment ( Hanušová et al., 2015 ; Castro et al., 2016 ; Ghasemi et al., 2017 ; Varbanov et al., 2017 ; Priotti et al., 2018 ). These new insights prompted us to investigate the molecular impact of ABZ on mammalian cells. Despite the fact that ABZ interacts with DNA and competes with EtBr, ABZ appeared to have no direct impact on replication and transcription. Yet, the remarkable sensitivity of human and murine transformed and non-transformed cells to ABZ led us to study potential alterations in the transcriptome of ABZ exposed cells.
Similar to EtBr, the effects of ABZ on the transcriptome appeared indirect. While our results and those of others ( Piechota et al., 2006 ; Lee et al., 2008 ) revealed that EtBr poisons mitochondrial genes, the transcriptional profile of ABZ pointed to a marked increase of cells arrested in G2/M. These observations led us to determine the effect of ABZ on cell cycle progression. In line with the ABZ specific transcriptome profile, cells exposed to ABZ arrested in a dose dependent manner at G2/M, which is consistent with previous findings in various cancer cell lines ( Ghasemi et al., 2017 ; Zhang et al., 2017 ). Furthermore, the ABZ induced cell cycle arrest was associated with a marked dose dependent increase in the frequency of viable cells with chromosomal aneuploidy, which is likely caused by the potent inhibitory activity of ABZ on tubulin polymerization, an essential process during mitosis ( Dawson et al., 1984 ; Chu et al., 2009 ). These results question the preferential targeting of helminth tubulin by ABZ ( Lacey, 1988 ; Hanušová et al., 2015 ). In fact, our study indicates that ABZ toxicity in helminths and mammalian cells follows the same molecular principle, as has been reported by others ( Chu et al., 2009 ; Ghasemi et al., 2017 ; Zhang et al., 2017 ).
To determine the impact of ABZ on tubulin polymerization, de-polymerization and spindle dynamics, we here set out to determine the toxicity of ABZ on the mammalian spindle apparatus in independent human cell lines. ABZ acted as a strong spindle poison, which resulted in the accumulation of cells in the G2/M phase in the cell cycle, explaining not only the typical ABZ transcriptome signature but also the effective and rapid induction of aneuploidy in vitro . While tissue specific patterns of aneuploidy can prohibit or initiate cancer development ( Albertson et al., 2003 ; Fröhling and Döhner, 2008 ; Stratton et al., 2009 ; Sack et al., 2018 ), our observations led us to perform LOH studies in the presence of ABZ. Again, consistent with the high frequency of aneuploid cells upon ABZ exposure, ABZ was found to be remarkably efficient in inducing LOH in mouse embryonic stem cells. Only 100 nM of ABZ sufficed to effectively induce LOH. Interestingly, these observations made in vitro also applied to in vivo settings, where ABZ accelerated the formation of MSH2 deficient intestinal crypts in Msh2 heterozygous mice. Though we appreciate that the dose of ABZ used in our in vivo experiments is higher than the dose that is given to humans, the poor solubility of ABZ might give rise to high local concentrations in the gut. Nevertheless, future studies should address whether our observations also apply to conditions where lower doses of ABZ are continuously administered. The fact that the tumor incidence did not rise abruptly in the general population of human ABZ users, supports the concept that a chromosomal aneuploidy is detrimental to a cell, and not oncogenic ( Sheltzer et al., 2017 ). However, our data suggests this may be different in the context of tumor suppressor gene haploinsufficiency. To examine the putative tumor promoting properties of ABZ in a predisposed genetic background, future studies may take advantage of the Apc +/ min mouse model ( Yamada and Mori, 2007 ).
Our results argue against prophylactic anti-helminth treatment of haploinsufficient carriers of tumor suppressor genes. These include patients suffering from Familial Adenomatous Polyposis-FAP (APC), Seckel Syndrome (ATR), Blooms Syndrome (BLM), Familial Breast or Ovarian Cancer Syndrome (BRCA1/BRCA2), Lynch Syndrome (MLH1/MSH2), Neurofibromatosis Type 1 (NF1), Familial Retinoblastoma (RB), and Li-Fraumeni Syndrome (TRP53). The observations made in this study are likely to apply to other tubulin poisons used to treat helminthiasis, such as Ivermectin (IVM), Praziquantel (PZQ), and Metronidazole (MNZ) ( Fennell et al., 2008 ). Of note, these drugs have recently been reconfirmed and categorized by the WHO as important, most essential medicines in the treatment of helminthiasis and specific bacteria.
The sum of these insights indicates that ABZ should be prescribed with caution to haploinsufficient carriers of tumor suppressor genes. Furthermore, it highlights the need for developing more selective drugs in treating helminthiasis.
Acknowledgments
The authors like to acknowledge Hellen Houlleberghs for help and suggestions with the LOH assay and the superb staff members off the outstanding scientific support facilities at the Netherlands Cancer Institute.
Data Availability Statement
Ethics statement.
The animal study was reviewed and approved by the Animal Ethical Committee (IVD Instantie voor Dierenwelzijn) at the Netherlands Cancer Institute, Amsterdam, the Netherlands, and the CCD (Centrale Commissie Dierproeven, the national central commission for animal experimentation) located in Den Haag, the Netherlands, under 9.2.8175, where 9 is the CCD number, 2 the CCD subgroup number, and 8175 the IVD number.
Author Contributions
LW, MFA, JR, and PB performed in vitro experiments and analyzed data. WP performed and analyzed the in vivo experiments. MFA. and MAA provided bioinformatical analyses. OB and BP provided DDT and DDinT system, HR and RM provided input and reagents, RP and HJ supervised the study. HJ, LW and MFA wrote the manuscript. All authors commented on the manuscript.
This project was executed at the NKI-AVL. LW Castro was supported by research grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-DS, Brazil) and Programa de Doutorado Sanduíche no Exterior (PDSE 99999.000252/2015-08 by CAPES Foundation). RP (Proc. 302404/2011-2) and DWF (Proc. 303234/2015-6) are recipient of research grants from the Conselho Nacional de Pesquisa (CNPq, Brazil). This project greatly profited from reagents that became available due to the generous support from the Dutch cancer foundation (KWF, grant NKI-2012-5243) and the Netherlands Organization for Scientific Research (ZonMW Top grant 91213018) to HJ and NKI/AVL facilities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.596535/full#supplementary-material .
- Aarts M., te Riele H. (2010). Parameters of oligonucleotide-mediated gene modification in mouse ES cells . J. Cell Mol. Med. 14 , 1657–1667. 10.1111/j.1582-4934.2009.00847.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Albertson D. G., Collins C., McCormick F., Gray J. W. (2003). Chromosome aberrations in solid tumors . Nat. Genet. 34 , 369–376. 10.1038/ng1215 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Buoninfante O. A., Pilzecker B., Aslam M. A., Zavrakidis I., van der Wiel R., van de Ven M., et al. (2018). Precision cancer therapy: profiting from tumor specific defects in the DNA damage tolerance system . Oncotarget 9 , 18832–18843. 10.18632/oncotarget.24777 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Castro L. S. E. P. W., Kviecinski M. R., Ourique F., Parisotto E. B., Grinevicius V. M. A. S., Correia J. F. G., et al. (2016). Albendazole as a promising molecule for tumor control . Redox Biol. 10 , 90–99. 10.1016/j.redox.2016.09.013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chu S. W. L., Badar S., Morris D. L., Pourgholami M. H. (2009). Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole . Anticancer Res. 29 , 3791–3796. . [ PubMed ] [ Google Scholar ]
- Da Silveira V. C., Benezra H., Luz J. S., Georg R. C., Oliveira C. C., Ferreira A. M. (2011). Binding of oxindole-Schiff base copper(II) complexes to DNA and its modulation by the ligand . J. Inorg. Biochem. 105 , 1692–1703. 10.1016/j.jinorgbio.2011.09.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dawson P. J., Gutteridge W. E., Gull K. (1984). A comparison of the interaction of anthelmintic benzimidazoles with tubulin isolated from mammalian tissue and the parasitic nematode Ascaridia galli . Biochem. Pharmacol. 33 , 1069–1074. 10.1016/0006-2952(84)90515-X [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dayan A. D. (2003). Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics . Acta Trop. 86 , 141–159. 10.1016/S0001-706X(03)00031-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- De Wind N., Dekker M., Berns A., Radman M., te Riele H. (1995). Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer . Cell 82 , 321–330. 10.1016/0092-8674(95)90319-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duijf P. H., Schultz N., Benezra R. (2013). Cancer cells preferentially lose small chromosomes . Int. J. Canc. 132 , 2316–2326. 10.1002/ijc.27924 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fennell B., Naughton J., Barlow J., Brennan G., Fairweather I., Hoey E., et al. (2008). Microtubules as antiparasitic drug targets . Expet Opin. Drug Discov. 3 , 501–518. 10.1517/17460441.3.5.501 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fröhling S., Döhner H. (2008). Chromosomal abnormalities in cancer . N. Engl. J. Med. 359 , 722–734. 10.1056/NEJMra0803109 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ghasemi F., Black M., Vizeacoumar F., Pinto N., Ruicci K. M., Le C. C. S. H., et al. (2017). Repurposing Albendazole: new potential as a chemotherapeutic agent with preferential activity against HPV-negative head and neck squamous cell cancer . Oncotarget 8 , 71512–71519. 10.18632/oncotarget.17292 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hanušová V., Skálová L., Králová V., Matoušková P. (2015). Potential anti-cancer drugs commonly used for other indications . Ccdt 15 , 35–52. 10.2174/1568009615666141229152812 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kloor M., Huth C., Voigt A. Y., Benner A., Schirmacher P., von Knebel Doeberitz M., et al. (2012). Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: a pathological study . Lancet Oncol. 13 , 598–606. 10.1016/S1470-2045(12)70109-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kubota Y., Tani S., Nakamura J. (2002). The interaction of 2-phenylbenzimidazole compounds with DNA: the influence of terminal substituents . Nucleic Acids Res. Suppl. 3 , 193–194. 10.1093/nass/2.1.193 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kubota Y., Iwamoto T., Seki T. (1999). The interaction of benzimidazole compounds with DNA: intercalation and groove binding modes . Nucleic Acids Symp. Ser. 42 , 53–54. 10.1093/nass/42.1.53 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lacey E. (1988). The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles . Int. J. Parasitol. 18 , 885–936. 10.1016/0020-7519(88)90175-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Langerak P., Nygren A. O., Krijger P. H., Van Den Berk P. C., Jacobs H. (2007). A/T mutagenesis in hypermutated immunoglobulin genes strongly depends on PCNAK164 modification . J. Exp. Med. 204 , 1989–1998. 10.1084/jem.20070902 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee W., Choi H. I., Kim M. J., Park S. Y. (2008). Depletion of mitochondrial DNA up-regulates the expression of MDR1 gene via an increase in mRNA stability . Exp. Mol. Med. 40 , 109–117. 10.3858/emm.2008.40.1.109 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lens S. M., Voest E. E., Medema R. H. (2010). Shared and separate functions of polo-like kinases and aurora kinases in cancer . Nat. Rev. Canc. 10 , 825–841. 10.1038/nrc2964 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Manchado E., Guillamot M., Malumbres M. (2012). Killing cells by targeting mitosis . Cell Death Differ. 19 , 369–377. 10.1038/cdd.2011.197 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mantovani A. (1992). The role of multigeneration studies in safety assessment of residues of veterinary drugs and additives . Ann. Ist. Super Sanita 28 , 429–435. . [ PubMed ] [ Google Scholar ]
- McCarthy D. J., Chen Y., Smyth G. K. (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation . Nucleic Acids Res. 40 , 4288–4297. 10.1093/nar/gks042 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Medindia (2017). ABZ price of 626 brands . Available at: https://www.medindia.net/drug-price/albendazole.htm (Accessed December 9, 2020).
- Oztas S., Salman A. B., Tatar A., Yigiter M., Yazgi H., Ertek M., et al. (2007). Genotoxic effect of albendazole in pediatric patients with hepatic hydatid disease . Int. J. Infect. Dis. 11 , 446–449. 10.1016/j.ijid.2007.01.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Piechota J., Szczęsny R., Wolanin K., Chlebowski A., Bartnik E. (2006). Nuclear and mitochondrial genome responses in HeLa cells treated with inhibitors of mitochondrial DNA expression . Acta Biochim. Pol. 53 , 485–495. 10.18388/abp.2006_3319 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pourgholami M. H., Szwajcer M., Chin M., Liauw W., Seef J., Galettis P., et al. (2010). Phase i clinical trial to determine maximum tolerated dose of oral albendazole in patients with advanced cancer . Canc. Chemother. Pharmacol. 65 , 597–605. 10.1007/s00280-009-1157-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Priotti J., Baglioni M. V., García A., Rico M. J., Leonardi D., Lamas M. C., et al. (2018). Repositioning of anti-parasitic drugs in cyclodextrin inclusion complexes for treatment of triple-negative Breast cancer . AAPS Pharm Sci. Tech. 19 , 3734–3741. 10.1208/s12249-018-1169-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pullan R. L., Smith J. L., Jasrasaria R., Brooker S. J. (2014). Global numbers of infection and disease burden of soil transmitted helminth infections in 2010 . Parasites Vectors 7 , 37–19. 10.1186/1756-3305-7-37 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramírez T., Eastmond D. A., Herrera L. A. (2007). Non-disjunction events induced by albendazole in human cells . Mutat. Res. Genet. Toxicol. Environ. Mutagen 626 , 191–195. 10.1016/j.mrgentox.2006.09.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rigter I. M., Schipper H. G., Koopmans R. P., Van Kan H. J. M., Frijlink H. W., Kager P. A., et al. (2004). Relative bioavailability of three newly developed albendazole formulations: a randomized crossover study with healthy volunteers . Antimicrob. Agents Chemother. 48 , 1051–1054. 10.1128/AAC.48.3.1051-1054.2004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2009). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data . Bioinformatics 26 , 139–140. 10.1093/bioinformatics/btp616 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sack L. M., Davoli T., Li M. Z., Li Y., Xu Q., Naxerova K., et al. (2018). Profound tissue specificity in proliferation control underlies cancer drivers and aneuploidy patterns . Cell 173 , 499–e23. 10.1016/j.cell.2018.02.037 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Santaguida S., Richardson A., Iyer D. R., M’Saad O., Zasadil L., Knouse K. A., et al. (2017). Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system . Dev. Cell 41 , 638. 10.1016/j.devcel.2017.05.022 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shahabadi N., Moghadam N. H. (2012). Determining the mode of interaction of calf thymus DNA with the drug sumatriptan using voltammetric and spectroscopic techniques . Spectrochim. Acta Mol. Biomol. Spectrosc. 99 , 18–22. 10.1016/j.saa.2012.09.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sheltzer J. M., Ko J. H., Replogle J. M., Habibe Burgos N. C., Chung E. S., Meehl C. M., et al. (2017). Single-chromosome gains commonly function as tumor suppressors . Canc. Cell 31 , 240–255. 10.1016/j.ccell.2016.12.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stratton M. R., Campbell P. J., Futreal P. A. (2009). The cancer genome . Nature 458 , 719–724. 10.1038/nature07943 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- te Riele H. (2009). Recreating stem cells: a novel entrance to the fountain of youth . Cell Stem Cell 4 , 279–280. 10.1016/j.stem.2009.03.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tweats D. J., Johnson G. E., Scandale I., Whitwell J., Evans D. B. (2016). Genotoxicity of flubendazole and its metabolites in vitro and the impact of a new formulation on in vivo aneugenicity . Mutagenesis 31 , 309–321. 10.1093/mutage/gev070 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Varbanov H. P., Kuttler F., Banfi D., Turcatti G., Dyson P. J. (2017). Repositioning approved drugs for the treatment of problematic cancers using a screening approach . PloS One 12 , e0171052. 10.1371/journal.pone.0171052 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wit N., Buoninfante O. A., Van Den Berk P. C., Jansen J. G., Hogenbirk M. A., De Wind N., et al. (2015). Roles of PCNA ubiquitination and TLS polymerases κ and η in the bypass of methyl methanesulfonate-induced DNA damage . Nucleic Acids Res. 43 , 282–294. 10.1093/nar/gku1301 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wojciechowicz K., Cantelli E., Van Gerwen B., Plug M., Van Der Wal A., Delzenne-Goette E., et al. (2014). Temozolomide increases the number of mismatch repair-deficient intestinal crypts and accelerates tumorigenesis in a mouse model of Lynch syndrome . Gastroenterology 147 , 1064. 10.1053/j.gastro.2014.07.052 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health Organization (2020a). Available at: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (Accessed August 13, 2020).
- World Health Organization (2020b). Available at: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (Accessed August 13, 2020).
- World Health Organization (2020c). Available at: https://apps.who.int/gho/cabinet/pc.jsp (Accessed August 13, 2020).
- Yamada Y., Mori H. (2007). Multistep carcinogenesis of the colon in Apc(Min/+) mouse . Canc. Sci. 98 , 6–10. 10.1111/j.1349-7006.2006.00348.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Young M. D., Wakefield M. J., Smyth G. K., Oshlack A. (2010). Gene ontology analysis for RNA-seq: accounting for selection bias . Genome Biol. 11 , R14. 10.1186/gb-2010-11-2-r14 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang X., Zhao J., Gao X., Pei D., Gao C. (2017). Anthelmintic drug albendazole arrests human gastric cancer cells at the mitotic phase and induces apoptosis . Exp. Ther. Med. 13 , 595–603. 10.3892/etm.2016.3992 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ] Retracted
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 July 2023
The effect of single dose albendazole (400 mg) treatment on the human gut microbiome of hookworm-infected Ghanaian individuals
- Francis Appiah-Twum 1 ,
- Jewelna Akorli 1 ,
- Lydia Okyere 1 , 2 ,
- Kate Sagoe 1 , 3 ,
- Dickson Osabutey 1 ,
- Michael Cappello 4 &
- Michael D. Wilson 1
Scientific Reports volume 13 , Article number: 11302 ( 2023 ) Cite this article
3914 Accesses
12 Altmetric
Metrics details
- Metagenomics
- Next-generation sequencing
- Parasitic infection
Microbes play a key role in human gut homeostasis, metabolic, immunologic and physiopathology of the body. A longitudinal study conducted during 2018–2021 in the Kintampo North Municipality in Ghana demonstrated low hookworm infection cure rates following treatment with a single dose of 400 mg albendazole in some communities. To investigate associations between hookworm infection and the gut microbiome, we examined stool samples from consented participants who were either cured or remained infected after treatment. At each time point, stool was collected prior to and 10–14 days after albendazole treatment. We used 16S rRNA amplicon sequencing of DNA extracted from stool samples to investigate the composition and diversity of the gut microbiota and to identify potential microbial biomarkers associated with treatment outcomes. Hookworm infection was associated with increased species richness ( p = 0.0093). Among treated individuals, there was also a significant variation in microbiota composition at 10–14 days following single-dose albendazole treatment. Individuals cured of hookworm infection after treatment showed a significant reduction in microbiota composition when compared to their pre-treatment state (ANOSIM; p = 0.02), whilst individuals who failed to clear the infection showed no change in microbiota composition (ANOSIM; p = 0.35). Uninfected individuals and those who were successfully treated were similar in their microbial composition and structure. We also found that the abundance of Clostridia spp. was increased in infected individuals pre- or post-treatment. Predictive functional profiling revealed the enrichment of two pyruvate ferredoxin oxidoreductase subunit pathways in individuals who remained infected after treatment ( p < 0.05), alluding to an upturn of strictly anaerobic commensal bacteria such as Clostridia spp. This study suggests a relationship between human gut microbiome dysbiosis and albendazole therapy outcomes of hookworm infection. Future studies will further characterize specific biomarkers identified within this study to establish their potential for assessment of pharmacological responses to anthelminthic therapies, as well as explore the possibility of using probiotic supplementation as an adjunct treatment to increase albendazole effectiveness against hookworm.
Introduction
The human gut microbiota is a diverse collection of microbes (i.e., bacteria, fungi, and viruses) that thrive in the gastrointestinal tract of humans 1 . They play an integral role in the competent biological functioning of the human body, due to the complex interplay that exists between enteric bacteria and human cells 2 . This symbiotic relationship allows for proper host metabolic functioning 3 , ensures adequate immune modulation 4 and provides protection against pathogenic microorganisms, among a host of other functions 5 .
In addition to microbes, other pathological organisms such as helminths can also colonize the human gut 6 . Hookworms, particularly Necator americanus and Ancylostoma duodenale , are soil-transmitted helminths (STH) responsible for approximately 472 million human infections worldwide. These infections mainly occur in disadvantaged communities situated in tropical and subtropical regions 7 . Upon host infection, hookworm larvae are carried from the site of skin penetration through the bloodstream to the lungs, after which the larvae migrate up the trachea to be swallowed. The ingested larvae settle in the lining of the duodenum where they develop to the adult, blood feeding stage 8 . Scientific evidence suggests that helminth infections dynamically alter the structure and composition of the intestinal microbiota 9 . This results from microbiota sensitivity to homeostatic imbalances within the human gastrointestinal tract 10 , a phenomenon referred to as helminth-induced human gastrointestinal dysbiosis.
Hookworm infections are routinely treated with albendazole (albendazole sulfoxide), a benzimidazole drug that causes degeneration of microtubules within intestinal and tegument cells of adult worms and larvae 11 . The World Health Organization (WHO) recommends preventive chemotherapy for populations at risk of STH infections with the oral administration of a single dose of 400 mg albendazole either annually or biannually 12 . STH resistance to benzimidazoles is well documented in animals such as livestock 13 but not in humans, partly due to limited monitoring of anthelminthic response in communities subjected to mass drug administration (MDA) 14 , 15 . The overall state of albendazole efficacy in the treatment of human hookworm infections, therefore, remains unclear. Historically, single-dose albendazole therapy has shown adequate efficacy in the treatment of human hookworm infections with moderately high cure rates 16 , 17 , 18 . However, there are recent reports of reduced cure rates especially in areas where treatment has been extensive due to MDA efforts 19 , 20 , 21 .
The gut microbiome has been shown to play a role in helminth infections, and studies to identify the associations between gut microbes and deworming treatment outcomes have gained interest 22 . The current study aimed to investigate the influence of single-dose 400 mg albendazole on the human gut microbiota of individuals in communities with reported low cure rates to gain deeper insights into the relationship between gut microbial alteration and reduced responsiveness to therapy.
Materials and methods
Study design.
This study was a nested case–control study from a larger cohort study under the NIH/NIAID-funded Tropical Medicine Research Centre (TMRC), the Noguchi Memorial Institute Initiative for NTDs Elimination (NIINE). For the NIINE study, stool samples were collected from consented individuals over a two-year period in the Kintampo North Municipality, Ghana. At each sample collection event i.e., baseline, 9 and 18 months, stool samples were prepared using the Kato-Katz technique 23 and examined for the presence of hookworm eggs with the help of a compound microscope at 10 × and 40 × objectives. Each slide was inspected independently by two experienced technologists, and a third for quality control. The remaining stool samples were stored at − 80 °C within 6 h of collection (Fig. 1 ).
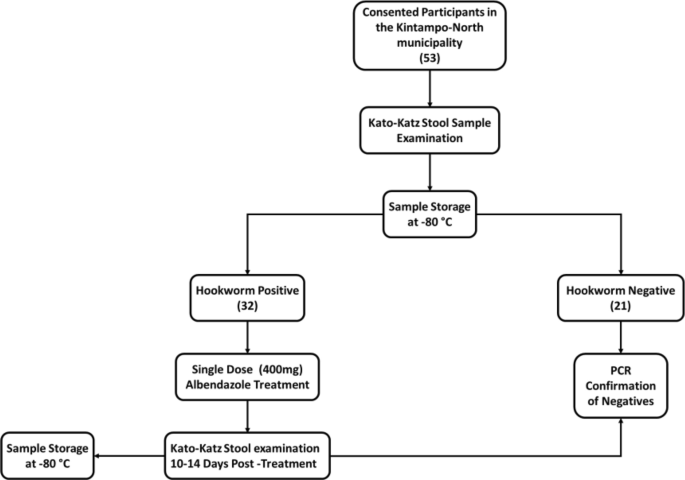
Sample collection, processing, and storage at baseline.
Hookworm-positive participants were treated with a single dose of 400 mg albendazole within 24 h of the first stool collection, and the treatment outcome was assessed 10–14 days after by examining stool samples as described. Those who remained infected were categorized as ‘treatment failures. The hookworm infection status of all microscopy-negative samples was further corroborated using polymerase chain reaction amplification (PCR) method for the identification of both N. americanus and A. duodenale 24 .
In this microbiome-related study, 97 stool samples were obtained from 53 individuals selected from archived samples. The sample selection was based on infection status and treatment outcomes. Positive samples were selected based of Kato-Katz results whilst only PCR confirmed negative samples were selected as true negatives. The availability of samples and complete stool data for both pre-treatment and post-treatment was also taken into consideration during sample selection. (Table 1 ).
16S rRNA gene amplicon sequencing
Genomic DNA was extracted using a modified extraction protocol of the Quick-DNA Faecal/Soil Microbe DNA Miniprep Kit from Zymo Research. The samples were lysed mechanically via bead beating using a high speed vortexing and chemically using lysis buffers to ensure adequate debris elimination and maximise DNA yield. To control for potential contamination in downstream analyses, two mock samples (no-template controls) were included during the extraction process. The concentrations of all DNA samples were determined with a Qubit Fluorometer 2.0 (Invitrogen).
The V3–V4 hypervariable region of the bacterial 16S rRNA gene was amplified by PCR using 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) primers. A 16S rRNA sequencing library was prepared according to the 16S Metagenomic Sequencing Library Preparation protocol (Illumina™, Inc., San Diego, CA, United States) 25 . Samples were multiplexed and individual barcode sequences were added to each DNA fragment during next-generation sequencing (NGS) library preparation and, sequenced on the NovaSeq 6000 Illumina platform following standard Illumina sequencing protocols.
Sequence filtering and taxonomic annotation
Paired-end raw reads of length 250 bp and were generated per sample after sequencing. Sequence filtering, clustering and taxonomic classifications were performed with established plugins in QIIME2 version 2020.8.0 26 . Denoising, chimeric read detection and removal were performed based on read quality 23 using the dada2 denoise-paired command 26 , 27 . Dada2 performed the read merging automatically after denoising resulting in distinct Amplicon Sequence Variants ( ASVs) samples 25 .
Operational Taxonomic Unit (OTU) clustering was achieved with closed-reference method using the vsearch plugin 26 , 28 . ASVs with ≥ 97% sequence similarity in the SILVA SSU rRNA database 138, were assigned to the same Operational Taxonomic Unit (OTU). Taxonomic annotation of OTUs to the species level was performed against the SILVA SSU rRNA database 138 to a confidence threshold of 0.8–1. Phylogenetic relationships between OTUs were established following multiple sequence alignments using the phylogeny plugin through the align-to-tree-mafft-fasttree command 26 .
Contaminant removal and data normalization
To control for biological contaminants, two mock samples (no-template controls) were processed together with the study samples. The sequence data of the mock samples were used to identify and remove ‘contaminant’ sequences in the test samples at sequence similarity threshold of 50%. This process was performed using the decontam package in R (version 4.1.3) 29 , 30 .
To enable accurate comparison of downstream statistics for different computational measurements, the clustered OTUs were normalized using rarefaction. Samples were rarefied to a depth equal to the minimum sequencing depth within the dataset. The rarefaction was executed using the rarefy_even_depth function of the Phyloseq package in R (version 4.1.3) 29 , 31 .
Diversity analyses
Species richness and, Shannon Entropy and Gini-Simpson alpha-diversity indices, which account for effective number of species, were calculated to determine the complexity of sample biodiversity among samples within the same group. This was performed using the get_alphaindex function in the MicrobiotaProcess package in R (version 4.1.3) 29 , 30 , 31 , 32 .
Beta diversity was investigated with Permutational multivariate analysis of variance (PERMANOVA) and Analysis of similarity (ANOSIM) using the adonis and anosim functions respectively on weighted Unifrac distances in the R vegan package 29 , 33 . These methods were used to test for the significance of structural and compositional differences between sample group microbial communities to determine the existence of significant dissimilarities across sample groups. Non-metric multi-dimensional scaling (NMDS) was used to graphically represent these between-group dissimilarities.
Microbial biomarker discovery
Wilcoxon rank-sum test and Kruskal Wallis test were performed using linear discriminant analysis (LDA) to establish the association between the taxonomic abundance of individual microbial taxa within sample groups based on treatment outcomes. These tests were executed using the diff_analysis function in the MicrobiotaProcess package in R (version 4.1.3) 29 , 31 . A microbial taxon is considered a potential biomarker if it had an LDA score ≥ 4 and, p -value ≤ 0.05 and ≤ 0.01 after Wilcoxon and Kruskal Wallis tests, respectively.
Predictive functional profiling
The KEGG Orthologs (KO) 34 were generated using the full pipeline.py command of PICRUSt2 (version 2.4.1) 35 . This was done to predict the abundance of key high-level pathways within sample group microbial communities using the OTUs obtained from 16S rRNA gene sequencing. KO abundances within sample groups were visualized using the STAMP software 36 .
Ethical approval
This study received ethical approvals from the Noguchi Memorial Institute for Medical Research (NMIMR/CPN#: 100/16-17), NIAID DMID (# 17-0061), Kintampo Health Research Centre (KHRCIEC2017-20) and Council for Scientific and Industrial Research (RPN 008/CSIR-IRB/2017). Written informed consent was obtained directly from all adult participants and legal guardians provided informed consent on behalf of all minors (below 18 years) within the study group. Procedures in this study were performed in accordance with the Ghana Public Health Act, 2012 (Act 851) and the Data Protection Act, 2012.
Removal of contaminant sequences
A total of 4153 unique sequences were obtained after 16s rRNA sequencing. 147 sequences of which were purged after contaminant removal representing 3.5% of unique sequences. As such, 4006 unique Operational Taxonomic Units (OTUs) were identified and adequately depicted the total microbiota composition.
Assessment of microbiome pre-treatment
We used a cross-sectional approach to first compare the microbiome of 32 hookworm positives and 21 negative individuals. Taxa with average relative abundance greater than 1% were compared between the sample groups. The hookworm positive sample group showed a higher relative abundance of both Bacillota ( p = 0.0028). and Actinomycetota ( p < 0.0001). compared to uninfected individuals, while Bacteroidota ( p = 0.0058). and Pseudomonadota ( p = 0.04). were less abundant (Fig. 2 A).
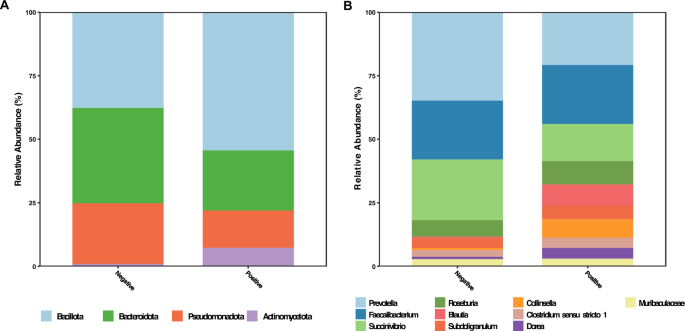
Taxonomic distribution of pre-treatment hookworm positive and negative samples with relative abundances greater than 1%. ( A ) Phylum. level. ( B ) Genus level.
Abundance distribution at the genus level revealed that Prevotella, Succinivibrio and Collinsella , accounted for the greatest proportion of taxa within the Bacteroidota , Pseudomonadota and Actinomycetota phyla, respectively. Bacillota on the other hand demonstrated a wider distribution, with six genera having relative abundances greater than 1%. Among these genera Blautia , Subdoligranulum , Dorea and Clostridium sensu stricto 1 had increased relative abundances in the positive group (Fig. 2 B).
Positive samples recorded higher median values across all alpha diversity indices estimated (Fig. 3 ). The variances in diversity within the sample groups, however, did not differ i.e., p- values for Shannon and Simpson > 0.05, although species richness showed a significant difference between positive and negative samples ( p = 0.0093).
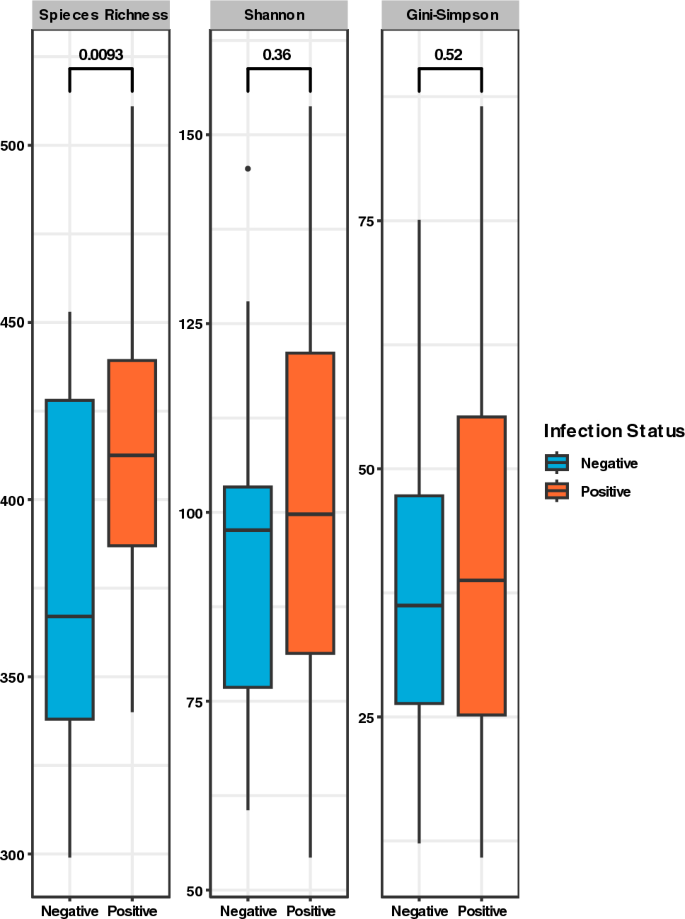
Alpha diversity plot comparing microbial diversity among negative (blue) and positive (orange) individuals pre-treatment. Both Shannon and Gini-Simpson indices were calculated to represent effective number of species.
Comparing the microbial diversity between hookworm infected and uninfected individuals revealed substantial dissimilarity (PERMANOVA: p = 0.007 , R 2 = 0.063). It was observed with non-metric multidimensional scaling (stress = 0.053) that both groups formed distinct clusters implying differences in microbiome structure and reiterating high variance in positive hookworm individuals (Fig. 4 ).
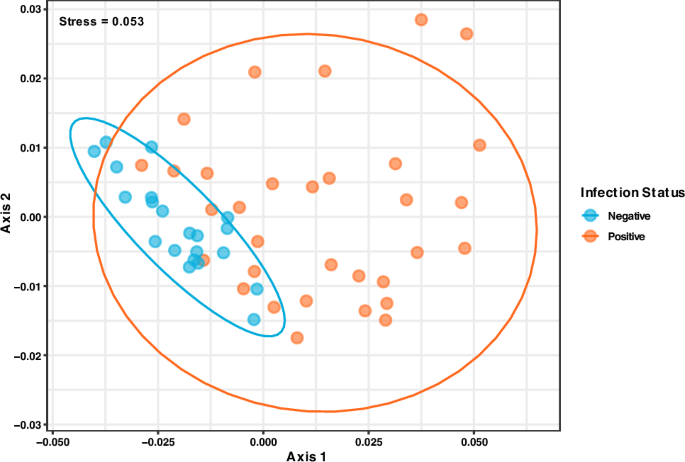
Non-metric multidimensional scaling (NMDS) plot comparing microbiome structure across negative (blue) and positive (orange) individuals pre-treatment.
Employing linear discriminant analysis (LDA), deferential microbial testing was performed to identify potential microbial taxa driving dissimilarities between the infection groups. When baseline positive and negative samples were compared, positive samples showed a significantly higher relative abundance of Clostridia and Blautia while Prevotella was significantly higher within negative samples (LDA score > 4.00) (Fig. 5 ).
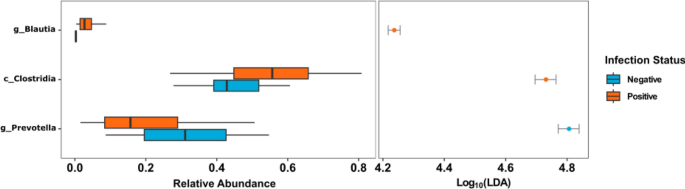
LDA plot outlining significantly associated microbial taxa within positive (orange) and negative (blue) samples before treatment.
Effect of albendazole treatment on gut microbiome
The microbiomes of individuals were assessed in stool samples collected before and 10–14 days after treatment to provide insight into the effects of deworming. Pre- and post-treatment samples for successful therapy (pre-ST vs post-ST) and the failed therapy (pre-FT vs post-FT) groups were compared separately to observe how the microbiome is altered after either successful or sub-optimal treatment. Microbiota abundance was reduced after drug administration in both successful therapy and failed therapy groups. However, this reduction was only significant after successful clearance (PERMANOVA: p = 0.01, R 2 = 0.10873) and not with the failed therapy group (PERMANOVA: p = 0.78, R 2 = 0.02024).
We further compared the microbiome structure and composition of individuals before and after treatment i.e., those that cleared infection (ST) against individuals who remained infected (FT) after treatment. Before and after treatment the microbiota composition of FT samples was represented most significantly by taxa within the class Clostridia such as Lachnospiraceae, Blautia and Ruminococcus . ST samples, on the other hand were represented by an increased abundance of other taxa such as Succinivibrio and Prevotellaceae (LDA score > 4) (Fig. 6 A,B).
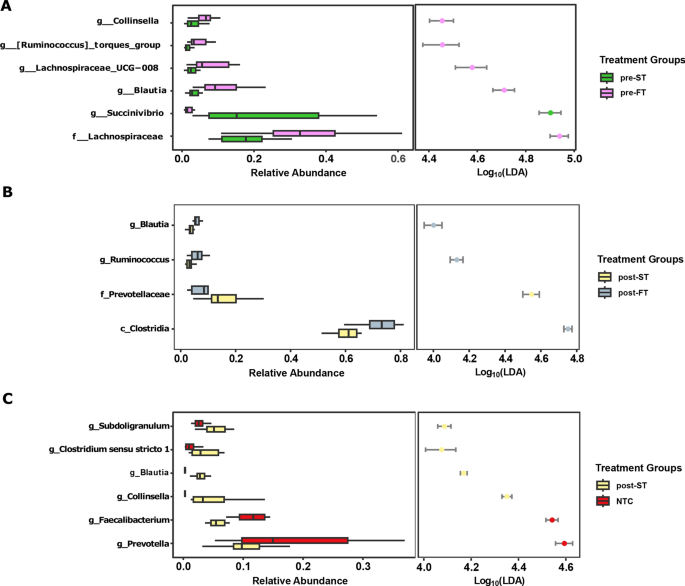
LDA plots outlining significantly associated microbial taxa based on treatment outcome. ( A ) Comparison between successful infection clearance (green) and treatment failure samples (purple) before treatment. ( B ) Comparison between successful infection clearance (yellow) and treatment failure samples (grey) after treatment. ( C ) Comparison between infection clearance (yellow) and no infection controls (red).
As part of our study, we also sought to investigate whether clearing the hookworm infection led to significant restructuring of the gut microbiome to a non-infected state. However, we noticed that even after complete clearance of the helminth infection, a significant difference in the microbiota composition existed between the two groups, implying the microbiome did not revert to a non-infected state (PERMANOVA: p = 0.0004, R 2 = 0.22612). When compared to baseline negative samples (NTC), individuals cleared after treatment had a higher abundance of Collinsella and Clostridia spp . such as Subdoligranulum , Clostridium_sensu_stricto_1 and Blautia (Fig. 6 C).
Predictive function of gut microbiome in successful and failed treatment groups
We also investigated microbiota function based on predicted metagenomes, and compared post-ST and post-FT in the Kyoto Encyclopaedia of Genes and Genomes (KEGG) orthology (KO). Among 559 affiliated KEGG pathways, 65 differed between post-ST and post-FT groups ( p < 0.05). Among these, 63 pathways were associated with the successful treatment group and 2 with the failed treatment group ( p < 0.05) (Fig. 7 ).
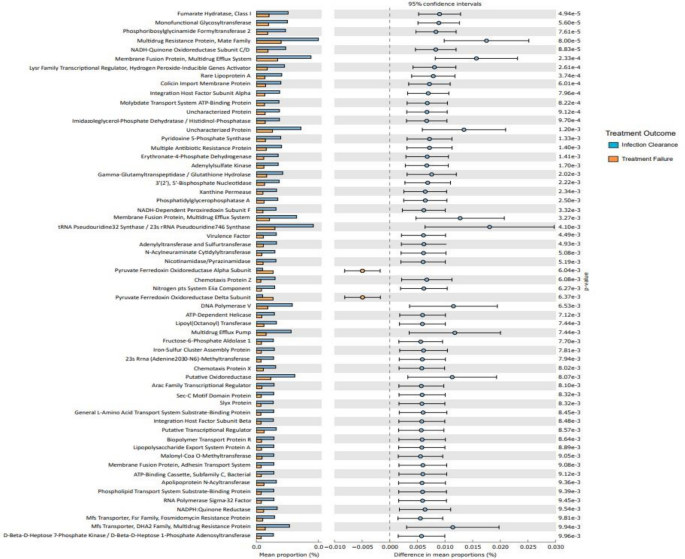
Predictive functional analysis outlining high-level KEGG pathways 34 associated with infection clearance (blue) and failed treatment (orange) groups.
Interestingly, pathways associated with metabolism, biosynthesis of cofactors and membrane transporters were enriched in the successful treatment group whilst the failed treatment group showed enrichment of pyruvate ferredoxin oxidoreductase (PFOR) subunit pathways.
Gut microbiome, chronicity and post-treatment reinfection
Individuals who demonstrated a pattern of reinfection (SR) were also analysed for association of microbial biomarkers during each time-point. These individuals included those who presented with hookworm at baseline, cleared the infection following treatment but were infected again at the 9-month follow-up study. There was no variation in microbial diversity across samples within the successive reinfection group (PERMANOVA: p = 0.17, R 2 = 0.02024), suggesting that the treatment may have resulted in an incomplete clearing leading to undetectable low levels of infections which became observable in the 9-month follow-up.
Across the larger cohort, one individual maintained a persistent hookworm infection over all sample collection events, designated hereafter as ‘consistent treatment failure’ (CTF). The microbiome state of this individual was also analysed to assess the effects of progressive treatment failure and chronicity of hookworm infection. Interestingly, the effective number of species within the microbiome of the individual reduced at every timepoint (i.e., baseline, 9 and 18 months), although not statistically significant (Fig. 8 ).
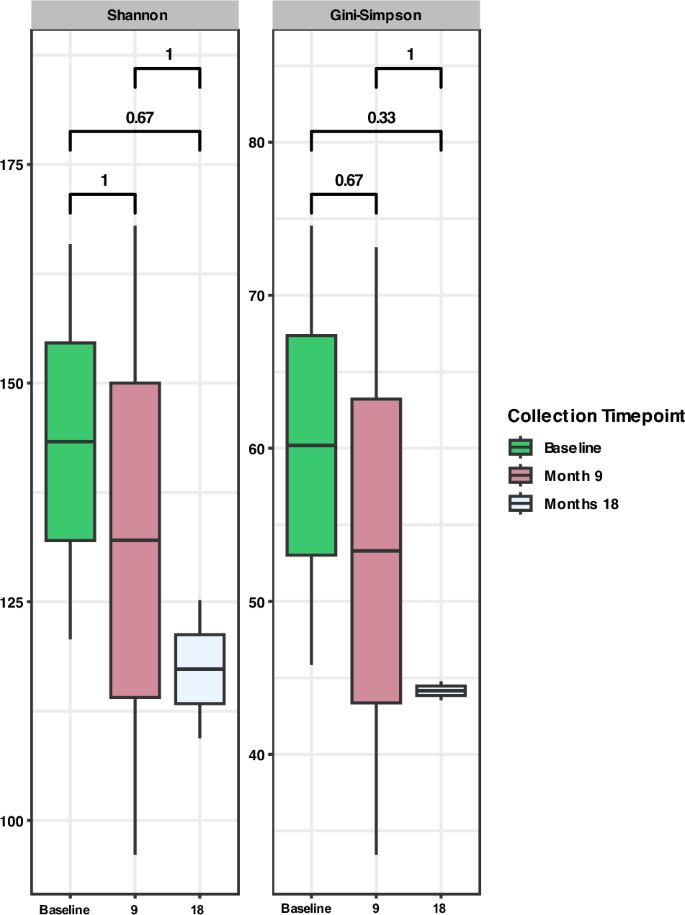
Alpha diversity plot comparing microbial diversity of CTF across all sample collection timepoints; baseline (green), 9 months (pink) and 18 months (grey). Both Shannon and Gini-Simpson indices were calculated to represent effective number of species.
The physiological tug of war between parasitic helminths and gut microbiota contributes to increased modulation of gut microbiome diversity and composition 37 . In this study, we concentrated on corroborating the association between hookworm infection, anthelminthic treatment, and the resulting effect on gut microbiome diversity. We found an association between hookworm infection and increased microbiota species richness. Studies across sub-Saharan Africa and parts of Asia have shown a similar pattern of association in both mixed and single-helminth infections 2 , 38 , 39 .
There was an increased relative abundance of the bacteria belonging to class Clostridia among infected individuals and an increase in both Prevotella and Succinivibrio among uninfected individuals. Commensal Clostridia are anaerobic gram-positive bacteria 40 . During active infection, Clostridia plays an essential role in gut defence mechanisms, both directly through colonisation resistance and indirectly through immune cell priming and modulation of immunological tolerance 41 , 42 . Studies comparing microbiome composition between rural and urban areas revealed an increased abundance of Prevotella and Succinivibrio in rural settlers 43 , 44 . Prevotella and Succinivibrio species are involved in polysaccharide metabolism and dietary fibre fermentation 44 , 45 , 46 . Their association with uninfected participants may occur because of an increased intake of plant-based diets 47 in an agrarian lifestyle cultivated by people living in the study site, Kintampo North Municipality of Ghana 48 .
Our study also showed a significant variation in microbiota composition 10–14 days after single-dose albendazole treatment. Individuals who were successfully treated showed a significant reduction in microbiota composition when compared to their pre-treatment state, while individuals who failed to clear the helminth infection after treatment showed no significant change in microbiota composition. A similar study in Western Kenya also reported changes in the abundance of microbial taxa after successful helminth clearance post albendazole treatment 49 . This in line with our findings that successful single dose albendazole treatment may result in structural and compositional change to the gut microbiome. Notwithstanding this, it must be noted that, this alteration in microbiome structure and composition could be because of microbial rearrangement in response to adult worm and egg clearance and not a direct effect of drug administration 50 .
It is known that gut microbial signatures could be used to predict patient response to antibiotic therapy 51 . Microbiota composition was different between individuals who got cured and those that did not. The abundance of Clostridia was increased in positive individuals before treatment and continued to show an increased abundance in individuals who failed treatment. The gut microbiome of individuals who failed treatment also saw an enrichment of high-level pathways associated with pyruvate ferredoxin oxidoreductase (PFOR). PFOR catalyses the oxidative decarboxylation of pyruvate to carbon dioxide and acetyl-CoA 52 and is a key enzyme in Clostridia metabolism as it is required to promote heterotrophic and lithoautotrophic growth in anaerobic bacteria 53 . The correlation between PFOR pathways and individuals who failed treatment could be associated with the increased abundance of strict anaerobes such as Clostridia spp. within the gut microbiome. These findings suggest persistent Clostridia as a potential predictive indicator for treatment failure in hookworm infection.
The gut microbiome has been demonstrated to undergo complete or partial reversion to its pre-treatment state in adults after cessation of anti-microbial therapy 54 , 55 , 56 . Prevotella was increased in individuals negative for hookworm infection and also after successful albendazole treatment. This increased representation of Prevotella in successfully treated individuals could point to a compositional shift in microbiota abundance towards a pre-infection state. However, when hookworm negative individuals were compared to individuals who successfully cleared the infection, the two groups were dissimilar. Whilst this dissimilarity implies a non-reversal of the microbiome structure after single dose albendazole therapy, we speculate that 10–14 days may not have been enough time for a significant reversion in microbiota composition to become apparent.
We recommend the application of meta-transcriptomics to ascertain microbial gene expression patterns 57 in albendazole treatment failures to better understand the correlation between Clostridia spp. and treatment failure. Metabolomics can also be used to identify metabolites produced by Clostridia spp. and explore the possibility of using probiotic supplementation 58 , 59 , 60 as an adjunct treatment in the bid to overcome treatment albendazole failure, especially when distributed in mass drug administration programs targeting hookworm infection.
Although studies have referred to bead associated mechanical lysis as the gold standard lysis technique for faecal DNA extraction 61 , newer studies have revealed that bead homogenization may lead to biases in characterising the microbiome 62 , 63 , 64 . As such, our choice to incorporate bead homogenisation within the study could serve as confounding variable. The sample size being small served as a limitation for our study. We will be performing follow up studies with significantly larger participant numbers to validate the results of this pilot study.
Conclusions
This study focused on the role the gut microbiome plays, if any, in influencing cure rates after single-dose albendazole treatment. We found that the composition and diversity of the gut microbiota change in response to treatment. We also found Clostridia to be a potential microbial indicator of albendazole treatment failure. These data corroborate the concept that specific microbial taxa or taxa assemblages can be used as significant discriminants in the assessment of the effects of helminth infection and response to treatment on the human gut microbiome.
Our study contributes to the ongoing discussion on the effects of helminth infections and anthelminthic treatment on the human gut microbiome. With the reduction in the cost of 16S rRNA sequencing and the advent of open-source computational pipelines, we look forward to conducting subsequent well-regulated studies on the impact of gut microbiome changes on human health.
Data availability
All data generated from 16s gene amplicon sequencing are available in the NCBI Sequence Read Archive (SRA) via http://www.ncbi.nlm.nih.gov/bioproject/923309 . All other original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Zhu, B., Wang, X. & Li, L. Human gut microbiome: The second genome of human body. Prot. Cell https://doi.org/10.1007/s13238-010-0093-z (2010).
Article Google Scholar
Martin, I. et al. Dynamic changes in human-gut microbiome in relation to a placebo-controlled anthelminthic trial in Indonesia. PLoS Neglected Trop. Dis. https://doi.org/10.1371/journal.pntd.0006620 (2018).
Ryan PM, Delzenne NM. Chapter 18 - Gut Microbiota and Metabolism. In: Hyland N, Stanton C, editors. The Gut-Brain Axis [Internet]. Academic Press; 2016. p. 391–401. Available from: https://www.sciencedirect.com/science/article/pii/B9780128023044000189
Cani, P. D. Human gut microbiome: Hopes, threats and promises. Gut 67 , 1716–1725 (2018).
Article CAS PubMed Google Scholar
Chen, Y., Zhou, J. & Wang, L. Role and mechanism of gut microbiota in human disease. Front. Cell Infect. Microbiol. 11 , 86 (2021).
CAS Google Scholar
Hodges, P. & Kelly, P. Intestinal parasites. In Textbook of Pediatric Gastroenterology, Hepatology and Nutrition (eds Guandalini, S. & Dhawan, A.) 219–29 (Springer, 2022).
Chapter Google Scholar
Haldeman, M. S., Nolan, M. S. & Ng’habi, K. R. N. Human hookworm infection: Is effective control possible? A review of hookworm control efforts and future directions. Acta Tropica. 201 , 105214 (2020).
Article PubMed Google Scholar
Anisuzzaman, T. N. Schistosomiasis and hookworm infection in humans: Disease burden, pathobiology and anthelmintic vaccines. Parasitol. Int. 75 , 102051 (2020).
Kupritz, J., Angelova, A., Nutman, T. B. & Gazzinelli-Guimaraes, P. H. Helminth-induced human gastrointestinal dysbiosis: A systematic review and meta-analysis reveals insights into altered taxon diversity and microbial gradient collapse. mBio Am. Soc. Microbiol. 12 , e02890 (2021).
Partney, H. & Yissachar, N. Regulation of Host Immunity by the Gut Microbiota (Springer, 2022).
Book Google Scholar
Malik, K. & Albendazole, D. A. Kucers the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs (CRC Press, 2021).
Google Scholar
World Health Organisation. Guideline: Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. (2017).
Mphahlele, M., Molefe, N., Tsotetsi-Khambule, A. & Oriel, T. Anthelmintic resistance in livestock. Helminthiasis https://doi.org/10.5772/INTECHOPEN.87124 (2019).
Krücken, J. et al. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int. J. Parasitol. Drugs Drug Resist. 7 , 262–271 (2017).
Article PubMed PubMed Central Google Scholar
Schneeberger, P. H. H. et al. Off-target effects of tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel-pamoate, and albendazole plus oxantel-pamoate on the human gut microbiota. Int. J. Parasitol. Drugs Drug Resist. 8 , 372–378 (2018).
Keiser, J. & Utzinger, J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA 299 , 1937–1948 (2008).
Norhayati, M., Oothuman, P., Azizi, O. & Fatmah, M. S. Efficacy of single dose albendazole on the prevalence and intensity of infection of soil-transmitted helminths in Orang Asli children in Malaysia. Southeast Asian J. Trop. Med. Public Health. 28 , 563–569 (1997).
CAS PubMed Google Scholar
Horton, J. Albendazole: A review of anthelmintic efficacy and safety in humans. Parasitology 121 , S113–S132 (2000).
Patel, C. et al. Efficacy and Safety of albendazole in hookworm-infected preschool-aged children, school-aged children, and adults in côte d’ivoire: A phase 2 randomized, controlled dose-finding trial. Clin. Infect. Dis. 73 , e494-502 (2021).
Humphries, D. et al. Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: Patterns of malaria coinfection, anemia, and albendazole treatment failure. Am. J. Trop. Med. Hyg. 84 , 792–800 (2011).
Humphries, D. et al. Effectiveness of albendazole for hookworm varies widely by community and correlates with nutritional factors: A cross-sectional study of school-age children in Ghana. Am. J. Trop. Med. Hyg. 96 , 347–354 (2017).
Article CAS PubMed PubMed Central Google Scholar
Schneeberger, P. H. H. et al. Different gut microbial communities correlate with efficacy of albendazole-ivermectin against soil-transmitted helminthiases. Nat. Commun. 13 , 1–12 (2022).
Magalhães, R. J. S. et al. Mapping Helminth co-infection and co-intensity: Geostatistical prediction in Ghana. PLoS Negl. Trop. Dis. 5 , e1200 (2011).
Monti, J. R., Chilton, N. B., Bao-Zhen, Q. & Gasser, R. B. Specific amplification ofNecator americanusorAncylostoma duodenaleDNA by PCR using markers in ITS-1 rDNA, and its implications. Mol. Cell. Probes. 12 , 71–78 (1998).
Illumina. 16S Metagenomic Sequencing Library Preparation. 2013 [cited 2022 May 14]; Available from: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37 , 852–857 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 13 , 581–583 (2016).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ. 4 , e2584 (2016).
R Core Team. R: A Language and Environment for Statistical Computing. 2013. http://www.R-project.org/ .
Davis, N. M., DiM, P., Holmes, S. P., Relman, D. A. & Callahan, B. J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6 , 1–14 (2018).
Xu, S., & Yu, S. MicrobiotaProcess: A comprehensive R package for managing and analyzing microbiome and other ecological data within the tidy framework. R package version 1.8.1. https://github.com/YuLab-SMU/MicrobiotaProcess/ . (2022).
Ssekagiri, A. T., Sloan, W., & Ijaz, U. Z.(2017). microbiomeSeq: an R package for analysis of microbial communities in an environmental context. In ISCB Africa ASBCB Conference, Kumasi, Ghana. https://github.com/umerijaz/microbiomeSeq. . DOI: https://doi.org/10.13140/RG.2.2.17108.71047
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14 , 927–930 (2003).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 , D587–D592 (2023).
Douglas, G. M. et al. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38 , 685–688 (2020).
Parks, D. H., Tyson, G. W., Hugenholtz, P. & Beiko, R. G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 30 , 3123–3124 (2014).
Zaiss, M. M. & Harris, N. L. Interactions between the intestinal microbiome and helminth parasites. Parasite Immunol. 38 , 5–11 (2016).
Lee, S. C., Tang, M. S., Lim, Y. A. L., Choy, S. H. & Kurtz, Z. D. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 8 , 2880 (2014).
Toro-Londono, M. A., Bedoya-Urrego, K., Garcia-Montoya, G. M., Galvan-Diaz, A. L. & Alzate, J. F. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ 2019 , e6200 (2019).
Lopetuso, L. R., Scaldaferri, F., Petito, V. & Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 5 , 1–8 (2013).
Lee, S. C. et al. Linking the effects of helminth infection, diet and the gut microbiota with human whole-blood signatures. PLoS Pathog. 15 , e1008066 (2019).
Peachey, L. E. et al. Dysbiosis associated with acute helminth infections in herbivorous youngstock – observations and implications. Sci. Rep. 9 , 1–16 (2019).
Article CAS Google Scholar
Rubel, M. A. et al. Lifestyle and the presence of helminths is associated with gut microbiome composition in Cameroonians. Genome Biol. 21 , 1–32 (2020).
de Filippo, C. et al. Diet, environments, and gut microbiota. A preliminary investigation in children living in rural and urban Burkina Faso and Italy. Front. Microbiol. 8 , 1979 (2017).
Chung, W. S. F. et al. Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides. BMC Microbiol. 20 , 1–14 (2020).
Precup, G. & Vodnar, D. C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 122 , 131–140 (2019).
de Filippis, F., Pellegrini, N., Laghi, L., Gobbetti, M. & Ercolini, D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome. 4 , 1–6 (2016).
Kintampo North Municipal. https://mofa.gov.gh/site/sports/district-directorates/brong-ahafo-region/176-kintampo-north-municipal . Accessed 29 May 2022.
Easton, A. V. et al. The Impact of anthelmintic treatment on human gut microbiota based on cross-sectional and pre- and postdeworming comparisons in western Kenya. mBio 10 , 1–14 (2019).
Midha, A., Schlosser, J. & Hartmann, S. Reciprocal interactions between nematodes and their microbial environments. Front. Cell. Infect. Microbiol. 7 , 144 (2017).
Khanna, S. et al. Gut microbiota changes as predictors of treatment failure in primaryclostridium difficileinfection: ACG category award. Off. J. Am. College Gastroenterol. ACG 110 , 578 (2015).
Xiao, L., Liu, G., Gong, F., Cai, Z. & Li, Y. The reductive carboxylation activity of heterotetrameric pyruvate synthases from hyperthermophilic archaea. Biochem. Biophys. Res. Commun. 572 , 151–156 (2021).
Katsyv, A., Schoelmerich, M. C., Basen, M. & Müller, V. The pyruvate: Ferredoxin oxidoreductase of the thermophilic acetogen, Thermoanaerobacter kivui. FEBS Open Bio. 11 , 1332–1342 (2021).
MacPherson, C. W. et al. Gut bacterial microbiota and its resistome rapidly recover to basal state levels after short-term amoxicillin-clavulanic acid treatment in healthy adults. Sci. Rep. 8 , 1–14 (2018).
Raymond, F., Déraspe, M., Boissinot, M., Bergeron, M. G. & Corbeil, J. Partial recovery of microbiomes after antibiotic treatment. Gut Microbes. 7 , 428 (2016).
Lamb, C. A. et al. O12 Reversion to baseline microbiome following successful course of exclusive enteral nutrition in paediatric Crohn’s disease. Gut 70 (Suppl 1), A6-7 (2021).
Jiang, Y., Xiong, X., Danska, J. & Parkinson, J. Metatranscriptomic analysis of diverse microbial communities reveals core metabolic pathways and microbiomespecific functionality. Microbiome 4 , 1–8 (2016).
Na, X. & Kelly, C. Probiotics in Clostridium difficile infection. J. Clin. Gastroenterol. 45 , S154 (2011).
Mills, J. P., Rao, K. & Young, V. B. Probiotics for prevention of Clostridium difficile infection. Curr. Opin. Gastroenterol. 34 , 3–10 (2018).
Al Aharaby, A., Abugoukh, T. M., Ahmed, W., Ahmed, S. & Elshaikh, A. O. Do probiotics prevent clostridium difficile-associated diarrhea?. Cureus https://doi.org/10.7759/cureus.27624 (2022).
Maghini, D. G., Moss, E. L., Vance, S. E. & Bhatt, A. S. Improved high-molecular-weight DNA extraction, nanopore sequencing and metagenomic assembly from the human gut microbiome. Nat. Protoc. 16 , 458–471 (2021).
Zhang, B. et al. Impact of bead-beating intensity on the genus- and species-level characterization of the gut microbiome using amplicon and complete 16S rRNA gene sequencing. Front. Cell. Infect. Microbiol. 11 , 585 (2021).
Gill, C., Van De Wijgert, J. H. H. M., Blow, F. & Darby, A. C. Evaluation of lysis methods for the extraction of bacterial DNA for analysis of the vaginal microbiota. PLoS ONE 11 , e0163148 (2016).
Teng, F. et al. Impact of DNA extraction method and targeted 16S-rRNA hypervariable region on oral microbiota profiling. Sci. Rep. 8 , 1–12 (2018).
Download references
Acknowledgements
We appreciate the NIINE laboratory and field work teams, along with all faculty of the Kintampo Health and Research Centre, community leaders and participants in the Kintampo-North municipality.
The study was funded by a NIH/NIAID awards to MDW (Ref. ID: U19AI129916) and MC (Ref. ID: R01AI132452). The funding agency did not play any role in the design and execution of the study.
Author information
Authors and affiliations.
Department of Parasitology, Noguchi Memorial Institute for Medical Research, University of Ghana, PO Box LG 581, Legon, Accra, Ghana
Francis Appiah-Twum, Jewelna Akorli, Lydia Okyere, Kate Sagoe, Dickson Osabutey & Michael D. Wilson
Department of Pathobiology, University of Illinois, Urbana-Champaign, 2522 Vet Med Basic Sciences Bldg., 2001 South Lincoln Avenue, Urbana, IL, 61802, USA
Lydia Okyere
Pan African University Institute for Basic Sciences, Technology, and Innovation (PAUSTI), P. O. Box 62000 00200, Nairobi, Kenya
Department of Epidemiology of Microbial Diseases, Yale School of Public Health, Yale University, 60 College St, New Haven, CT, 06520, USA
Michael Cappello
You can also search for this author in PubMed Google Scholar
Contributions
M.D.W., J.A., F.A.T. and M.C. conceived the project. F.A.T., L.O., D.O. and K.S. conducted the experiments and sample collection. F.A.T. and J.A. analysed the data. F.A.T. drafted the manuscript. All the authors read and reviewed the manuscript.
Corresponding author
Correspondence to Michael D. Wilson .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Appiah-Twum, F., Akorli, J., Okyere, L. et al. The effect of single dose albendazole (400 mg) treatment on the human gut microbiome of hookworm-infected Ghanaian individuals. Sci Rep 13 , 11302 (2023). https://doi.org/10.1038/s41598-023-38376-3
Download citation
Received : 27 December 2022
Accepted : 07 July 2023
Published : 12 July 2023
DOI : https://doi.org/10.1038/s41598-023-38376-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Study protocol
- Open access
- Published: 07 June 2022
Resistance to single dose albendazole and reinfection with intestinal helminths among children ages 2 to 11 years from the Peruvian Amazon region: a study protocol
- Greisi Curico 1 ,
- Paul García-Bardales 1 ,
- Tackeshy Pinedo 1 ,
- Wagner Shapiama 1 ,
- Miguel Moncada-Yaicate 1 ,
- Lucero Romaina 1 ,
- Pablo P. Yori 2 ,
- Maribel Paredes-Olortegui 1 ,
- Graciela Meza-Sánchez 3 ,
- Andrés G. Lescano 4 ,
- Valerie A. Paz-Soldan 5 ,
- Francesca Schiaffino 1 ,
- Richard A. Oberhelman ORCID: orcid.org/0000-0003-4166-8050 5 &
- Margaret N. Kosek 2
BMC Infectious Diseases volume 22 , Article number: 528 ( 2022 ) Cite this article
8070 Accesses
2 Citations
2 Altmetric
Metrics details
Deworming programs aimed at reducing morbidity and mortality from geohelminth infections are common in many countries where these infections are endemic, but data demonstrating increasing levels of resistance to albendazole and mebendazole are causes for concern. Studies to evaluate the clinical efficacy of deworming programs are critical to maintain high infection control goals.
We propose to assess the clinical efficacy of Peruvian national guidelines for deworming programs in a prospective observational study conducted in the Amazon River basin area near Iquitos, Peru. Major outcomes to be evaluated include (1) albendazole resistance of intestinal helminths (trichuriasis, ascariasis, hookworm), and (2) frequency of reinfection with intestinal helminths 4 months after treatment with albendazole. Children ages 2–11 years from the Belén District of Iquitos will be identified based on a community census. Following parental informed consent, demographic data, weight, and height will be recorded and a stool specimen for parasitological exam by direct observation and Kato-Katz concentration method, and helminthic egg counts will be collected prior to administration of albendazole, following Peruvian national guidelines. Follow-up stool specimens examined in the same manner will be collected at 20 days, 90 days, and 100 days following initial administration of albendazole, and based on parasites found repeat treatment will be administered in accordance with national guidelines. Real-time multiplex qPCR will be performed on helminth positive samples collected prior to initial deworming and on helminth-positive specimens detected on day 15–20. A total sample size of 380 participants was calculated based on total population in the target group and prevalence estimates of helminth infections and clinical resistance based on recent data.
Data from observational clinical efficacy studies are important to guide geohelminth infection control programs.
Trial registration https://www.researchregistry.com/ . Identification number: researchregistry7736; Registered retrospectively March 13, 2022; https://www.researchregistry.com/browse-the-registry#home/registrationdetails/622e024cf06132001e3327bf/
Peer Review reports
Worldwide, approximately 1.5 billion people or about 24% of the world’s population, are infected with soil-transmitted helminths (STH) [ 1 ]. Soil-transmitted helminth infections are widely distributed in tropical and subtropical areas, especially in sub-Saharan Africa, America, China and East Asia. More than 267 million preschool-age children and more than 568 million school-age children live in areas with intense transmission and are able to benefit from treatment and prevention strategies [ 2 ]. In low- and middle-income countries, risk factors for STH infections include poverty and malnutrition, and STH infections have been associated with delays in cognitive, motor, and social development in children under 5 years of age [ 3 , 4 ]. The presence of helminths also may cause intestinal blood loss with iron deficiency that manifests as anemia, especially in children with hookworm infection [ 5 ].
The World Health Organization and the Pan American Health Organization (WHO/PAHO) set standards for the control of soil-transmitted helminthiasis in Latin America and the Caribbean. These organizations recommend deworming strategies such as preventive chemotherapy (PC), which is an important part of a comprehensive package to eliminate morbidity due to soil-transmitted helminths in populations at risk. This strategy is applied according to the prevalence of soil-transmitted helminths in high-risk areas for preschool-age children (1 to 4 years) and school-age children (5 to 14 years) [ 6 ]. In high-risk areas with a prevalence of > 50%, the recommendation is for treatment twice a year (every 6 months), while in low-risk areas with a prevalence > 20 to < 50%, once a year (every 12 months) treatment with albendazole (400 mg) or mebendazole (500 mg) is recommended. In Peru, a universal dose is administered to children between 2 and 11 years of age twice a year in primary care health centers [ 7 ].
The efficacy of albendazole is variable against Trichuris trichiura compared to other anthelmintics; the cure rate ranges between 2.6 and 64.5% [ 8 ] and the egg reduction rate ranges between 7 and 83.1%. However, high cure rates of Trichuris trichiura are observed in some contexts [ 9 , 10 , 11 ]. In a study conducted in the Peruvian Amazon, an open-pair randomized trial was conducted in fifth-grade children from 18 primary schools (9 intervention and 9 control). Intensity of Ascaris lumbricoides infection at follow-up was 58% lower in children from intervention schools, a significant reduction as compared to children from control schools (RR = 0.42; 95% CI 0.21 to 0.85), but no significant changes were observed in the intensity of hookworm or Trichuris trichiura [ 12 ]. In another study from Peru, a total of 1193 school-age children were dewormed with a single dose of Albendazole (400 mg). Of the 909 children who tested positive for at least one STH infection, a random sample of 385 were followed up 2 weeks later with a second stool sample. The efficacy of albendazole was variable, with an egg reduction rate of 99.8% for Ascaris lumbricoides (95% CI 99.3–100); 93.6% for hookworm (95% CI 88.2–96.6), and 72.7% for Trichuris trichiura (95% CI 58.5–79, 1) [ 13 ]. In many cases treatment with albendazole alone appeared inadequate, especially for Trichuris trichiura . Other interventions such as combined therapy have been evaluated in other contexts. Ivermectin results in higher cure rates than albendazole and is well tolerated. The benefits of ivermectin for helminth infections would depend on the amount of resistance present [ 14 , 15 , 16 ].
Large-scale interventions to control soil-transmitted helminth infections at the community level with anthelmintic drugs such as albendazole have the potential to exert selective pressures which may favor the development of drug resistance, which could significantly reduce the benefits provided by deworming programs. Drug resistance in parasitic nematodes is caused by a single nucleotide polymorphism (SNP) in the β-tubulin gene at codon positions 200 (T → A), 167 (T → A) or 198 (A → C) [ 17 ].
Herein we describe the protocol for our observational study of a prospectively enrolled cohort to assess clinical resistance to albendazole and reinfection by intestinal helminths in children from the Peruvian Amazon receiving treatment according to Peruvian Ministry of Health treatment guidelines [ 7 ], to measure preventive treatment effectiveness for 6 months.
Study design (see Fig. 1 )
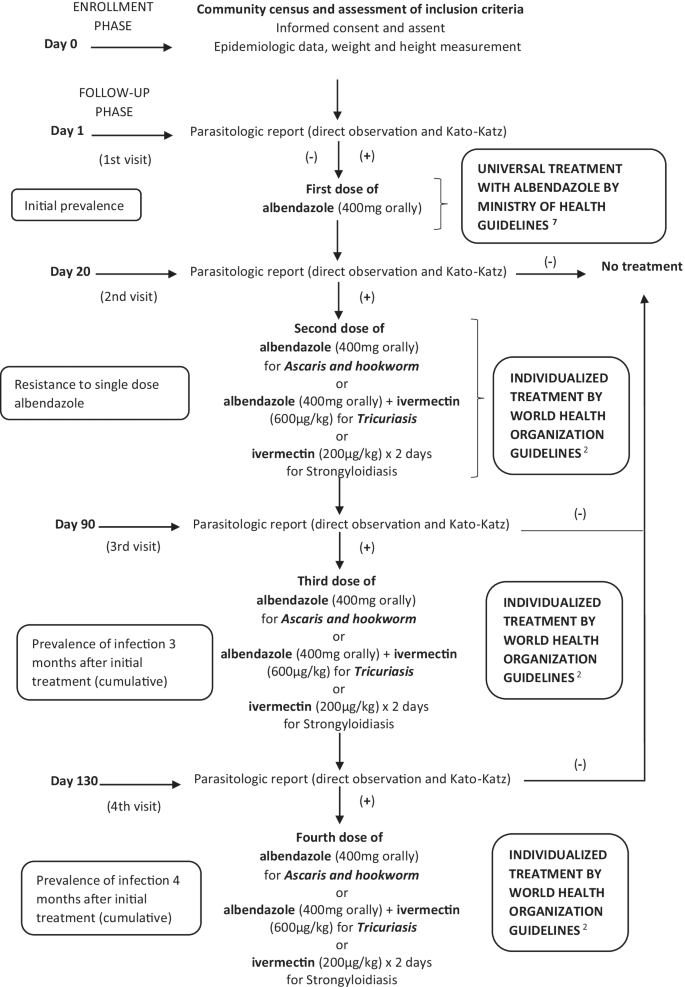
Study design
Prospective cohort study of children aged 2 to 11 years, with an individual time of participation of 4 and a half months.
Participants will be boys and girls ages 2 to 11 years who live in the catchment area of the Centro de Salud 6 de Octubre, located in the district of Belén, city of Iquitos, and department of Loreto in Peru.
Inclusion criteria:
Parental written informed consent, in addition to the verbal and written assent for children ages 6 to 11 years.
No antiparasitic treatment in the prior 3 months [ 18 ].
Permanent residence in the study community, with no plans to move in the next 6 months.
Major outcomes:
Albendazole resistance of intestinal helminths (Trichuriasis, Ascariasis, Hookworm, Strongyloides).
Reinfection with intestinal helminths 4 months after treatment with Albendazole.
Sample size
Sampling will be carried out throughout the jurisdiction of the 6 de Octubre Health Center, which includes rural, peri-urban and urban areas, with a total potential study population of 3379 children ages 2 to 11 years based on health center databases. Based on this study population of 3379 children aged 2 to 11 years, to measure clinical resistance to albendazole a sample of 380 children was needed. This calculation was made in Epi Info Version 7.2 applying a type I error of 5% and assuming 50% prevalence of helminths in children, 25% frequency of Trichuris trichiura in children with helminths, 50% resistance to albendazole based on detection of any helminth in a follow-up sample and adjusting for 10% loss to follow-up for study participants.
Recruitment of participants
To identify eligible children for the study, a community census is being conducted in the catchment area of the Centro de Salud 6 de Octubre, which includes rural, peri-urban and urban areas. Rural areas are defined as streets located on the opposite side of the Itaya river from the city of Iquitos, which is a flood prone area without basic services, electricity and drinking water. Peri-urban areas are located on the Iquitos side of the river and extend into the river, with houses on stilts or raft houses that float on the surface of the river. Many of these houses already have electricity and drinking water, and they are usually located around the health center. Another part of the study community is located in the urban area of Iquitos, with concrete houses that all basic services and access to food markets, etc.
For enrollment in rural areas, a sweep strategy will be applied, meaning that we will look for children who meet the inclusion criteria throughout the community. For the peri-urban area homes without an address (located on rafts) we will also apply the same strategy. In the peri-urban and urban area where there are well-defined maps with streets and addresses, we will recruit candidates with numerical codes from the community census based on a random number generator, starting from the first block and then moving forward clockwise to the next block.
Once the first randomly selected child of any age between 2 and 11 years has been recruited, enrollment will continue in the same way until the established strata are completed: age 2 years = 36 children; 3 years = 36 children; 4 years = 36; 5 years = 32 children; 6 years = 33 children; 7 years = 34 children; 8 years = 35 children; 9 years = 36 children; 10 years = 33 children; 11 years = 34 children. Once all the blocks of the study community have been sampled, if the target number of participants for each stratum were not completed, the process will be repeated from the beginning and thus complete the corresponding sample for each age group.
Data and specimen collection (see Fig. 1 for overview of study visits and procedures)
Following informed consent, demographic data will be collected, including housing characteristics, general caregiver information, and information on hygiene and childcare. Weight and height measurements will be performed by a member of the on-site investigation team, and a stool collection container will be provided to the caregiver [ 19 ]. Fresh feces (10–20 g) will be collected from each participant and the date and time of specimen collection will be recorded upon retrieval. At the end of the day specimens will be transported to the laboratory in a biological sample transport box with ice at the end of the day, for analyses that will be carried out within the next 24 h.
Albendazole treatment and clinical follow-up
Following enrollment and initial stool specimen collection, albendazole 400 mg will be administered orally in accordance with Peruvian Ministry of Health guidelines (RM No. 479-2017-MINSA) as part of the universal treatment policy in Peru. Stool samples for the study will be collected three times after the first stool sample is taken. This means that the same procedures will be repeated at 15 to 25 days, 90 to 100 days and at 130 to 140 days (in order to measure resistance and reinfection at 11%, 50% and 75% of the 6-month interval recommended for repeating preventive treatment). If parasites are found in samples after treatment with albendazole, individualized treatment will be administered according to World Health Organization standard of care treatment guidelines [ 2 ]. For Ascaris and hookworm, albendazole 400 mg will be administered as a single oral dose. For Trichuris trichiura , ivermectin 600 µg/kg/day will be administered with albendazole 400 mg, both as single oral doses [ 20 , 21 ]. For Strongyloides stercolaris , Ivermectin 200 µg/kg/day for 2 days will be administered [ 22 , 23 , 24 ].
Laboratory analysis
Fecal samples will be analyzed by direct observation of eggs and/or larvae by microscopy and egg counting by the Kato-Katz technique. Direct microscopy is the diagnostic standard in areas with limited resources. The Kato-Katz method is used to estimate the intensity of infection by Ascaris lumbricoides , hookworms and Trichuris trichiura . Although this method is more sensitive than direct microscopy, it requires more time and labor to detect geohelminth eggs [ 25 , 26 ]. Once the procedures have been carried out and the results obtained, the remaining stool samples will be aliquoted and stored at − 80 °C.
In the second sample, molecular detection of helminths by polymerase chain reaction (qPCR) will be performed on specimens with helminths detected (i.e., clinical resistance) in order to determine the presence of resistance markers at the genetic level.
Direct observation: In a properly labeled slide, 0.5 g of feces will be applied with a wooden applicator and 20 µL of saline solution will be added. The first observation will be made from left to right, then the procedure will be repeated with 20 µL of Lugol’s solution added. Both observations will be made with the 10× and 40× objectives in order to qualitatively identify the absence or presence of helminths ( Ascaris lumbricoides , Trichuris trichiura , hookworms, and Strongyloides stercoralis ).
Kato-Katz method: Two grams of feces will be placed on a smooth and sterile surface, covered with a piece of nylon and pressed with a flat-edge plastic applicator. The filtered feces will then be placed in the center of a 41.7 mg template on a slide until the hole is filled, after which the template will be removed and the sample will be covered with a cellophane sheet previously imbued in a glycerol-blue solution of methylene, and pressure will be applied until the sample is distributed homogeneously. After a 30-min incubation, eggs counts will be carried out using the 10× objective and a cell counter, the total number of eggs counts per species will be reported in eggs per gram (epg) and will be multiplied by 24 (41.7 mg template) [ 27 ], and the results of each parasite found will be recorded and reported quantitatively. This procedure will be performed on the 4 samples per subject collected throughout the study [ 28 ].
STH infection by qPCR: To measure the intensity of the infection, quantitative polymerase chain reaction (qPCR) will be used in such a way that the Kato-Katz variability between users is reduced and the sensitivity of the detection of low-grade parasitic infections is increased [ 29 , 30 ]. Real-time multiplex qPCR will be performed on positive samples (Day 0) for helminths that were identified by direct observation and Kato-Katz for the simultaneous detection of Ascaris lumbricoides , Trichuris trichiura , Necator americanus , Ancylostoma duodenale , and Strongyloides stercoralis [ 31 ]. If the sample from Day 0 is negative, the sample from Day 15 will be analyzed if it is positive for helminths. Amplification will be performed in a Quant Studio 7 Flex thermocycler (Applied biosystems) in a total volume of 25 µL using GoTaq® qPCR Probe Master Mix (Promega, USA) and 5 µL of fecal DNA. There will be two multiplex qPCR assays (STH1 and STH2); STH1 will be used to detect Ascaris lumbricoides , Trichuris trichiura and Strongyloides stercoralis , while STH2 will be used for the 3 species of hookworms. The cycling conditions for both cases will be the following: 1 cycle at 95 °C for 5 min, 40 cycles at 95 °C for 10 s and 1 cycle at 60 °C for 60 s. The primers will be the same ones used by Azzopardi et al. [ 32 ] which can be seen in Table 1 .
DNA extraction from STH: Nucleic acid extraction will be performed using the purification method by column centrifugation and cell disruption by bead beater, which consists of lysing the sample by mechanical disruption. The guidelines of the modified protocol of the QIAmp Fast DNA Stool mini kit (CAT 51604) will be followed, which consists of 4 phases (lysis, filtering, washing and elution), with an approximate time of 60 min for 8 samples. To evaluate the quality of the extraction and the efficiency of the amplification, extrinsic controls (MS2 and PhHV) will be added to each sample during the lysis phase. Subsequently, the DNA obtained will be stored at − 20 °C for the following processes or at − 80 °C for prolonged storage.
Statistical analysis
Data will be analyzed in Access using Stata, using chi square, Fisher’s exact, and Wilcoxon tests to compare frequencies between groups. To analyze the data from direct observation and quantification by the Kato-Katz method, descriptive statistics will be used, to report the prevalence found in percentages. For the sociodemographic and clinical data of the study population, measures of central tendency and dispersion will be used for the quantitative variables and measures of relative frequency for the qualitative variables. Comparisons will also be made between sample 1, sample 2, sample 3 and sample 4 to determine the positivity of each species of parasite found using the Yates chi-square test, setting a significance level of p < 0.05.
The sociodemographic variables will be compared with the parasite load and presence/absence of parasites, using a multiple logistic regression model (univariate t-test), to determine the variables statistically associated with infection by Ascaris lumbricoides , Trichuris trichiura and hookworms.
The quantification performed with the Kato-Katz method will be compared with the Ct value (Ct threshold) of the multiplex qPCR, by converting Log10 of the absolute egg count per stool sample, in order to estimate the absolute egg counts in eggs per gram (EPG) in the DNA extractions, using the following formula [ 33 ]:
To determine treatment efficacy, the prevalence will be measured before and after receiving treatment and compared using the Yates chi-square test. For calculations of cure rate and intensity of infection obtained from qPCR, only data from positive results before taking the first dose will be included [ 34 ].
The cure rate will be measured using the following formula:
Intensity of infection will be measured using the following formula:
And the calculation of the rate of reduction of eggs by helminth species:
In addition, a comparison of the cycle threshold (Ct) by qPCR will be made using the Student’s t-test for paired samples.
Data from observational clinical efficacy studies such as this one are vitally important to assure continued high levels of geohelminth infection control, and to permit re-evaluation of clinical guidelines when necessary to achieve desired program goals.
Availability of data and materials
Data generated from this study will be available from the corresponding author on reasonable request, or published with the final results, after all findings are available. Data will be shared after approval of a proposal by the authors for legitimate scientific purposes.
Abbreviations
Soil-transmitted helminths
World Health Organization
Pan American Health Organization
Preventive chemotherapy
Quantitative polymerase chain reaction
Two multiplex qPCR assays for detection of geohelminths
Deoxyribonucleic acid
Keller L, Welsche S, Patel C, Sayasone S, Ali SM, Ame SM, et al. Long-term outcomes of ivermectin–albendazole versus albendazole alone against soil-transmitted helminths: results from randomized controlled trials in Lao PDR and Pemba Island, Tanzania. PLoS Negl Trop Dis. 2021;15(6): e0009561.
Article CAS PubMed PubMed Central Google Scholar
Soil-transmitted helminth infections. 2022. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections . Accessed 26 Apr 2022.
Turner J, Badireddy M. Anemia—StatPearls—NCBI. Anemia. 2022. https://www.ncbi.nlm.nih.gov/books/NBK499994/ . Accessed 26 Apr 2022.
Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by intestinal parasites, stunting and anemia in school-aged children from southern Angola. PLoS ONE. 2015;10(9): e0137327.
Article PubMed PubMed Central Google Scholar
Prevalence of anaemia in children aged 6–59 months (%). World Health Organization. 2022. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-children-under-5-years-(-) . Accessed 26 Apr 2022.
Novianty S, Dimyati Y, Pasaribu S, Pasaribu AP. Risk factors for soil-transmitted helminthiasis in preschool children living in farmland, North Sumatera, Indonesia. J Trop Med. 2018;2018: e6706413.
Article Google Scholar
Ministerio de Salud del Perú. Resolución Ministerial N° 755–2017/MINSA. 2017. https://www.gob.pe/institucion/minsa/normas-legales/188666-755-2017-Minsa .
Speich B, Ame SM, Ali SM, Alles R, Huwyler J, Hattendorf J, et al. Oxantel pamoate-albendazole for Trichuris trichiura infection. N Engl J Med. 2014;370(7):610–20.
Article CAS PubMed Google Scholar
Samuel F, Degarege A, Erko B. Efficacy and side effects of albendazole currently in use against Ascaris , Trichuris and hookworm among school children in Wondo Genet, southern Ethiopia. Parasitol Int. 2014;63(2):450–5.
Krücken J, Fraundorfer K, Mugisha JC, Ramünke S, Sifft KC, Geus D, et al. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol Drugs Drug Resist. 2017;7(3):262–71.
Levecke B, Easton AV, Cools P, Albonico M, Ame S, Gilleard JS, et al. The optimal timing of post-treatment sampling for the assessment of anthelminthic drug efficacy against Ascaris infections in humans. Int J Parasitol Drugs Drug Resist. 2018;8(1):67–9.
Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis. 2013;7(9): e2397. https://doi.org/10.1371/journal.pntd.0002397 .
Gyorkos TW, Maheu-Giroux M, Blouin B, Saavedra L, Casapía M. Eficacia del Albendazol en dosis única sobre las infecciones por helmintos transmitidos por el suelo en escolares de una comunidad de Iquitos, Perú [Efficacy of a single dose of Albendazole for soil-transmitted helminth infections in school children of a village in Iquitos, Perú]. Rev Peru Med Exp Salud Publica. 2013;30(4):601–7 ( In Spanish ).
PubMed Google Scholar
Organización Panamericana de la Salud. Pautas operativas para la puesta en marcha de actividades integradas de desparasitación; 2015—OPS/OMS. https://www.paho.org/es/documentos/pautas-operativas-para-puesta-marcha-actividades-integradas-desparasitacion-2015 . Accessed 13 Jan 2022.
Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC Jr, Terashima A, Samalvides F, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD007745.pub3 .
Speich B, Ali SM, Ame S, Bogoch I, Alles R, Huwyler J, et al. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial. Lancet Infect Dis. 2015;15:277–84.
Diawara A, Halpenny CM, Churcher TS, Mwandawiro C, Kihara J, Kaplan RM, et al. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl Trop Dis. 2013;7(5): e2247.
Cabada MM, Lopez M, Arque E, Clinton WA. Prevalence of soil-transmitted helminths after mass albendazole administration in an indigenous community of the Manu jungle in Peru. Pathog Glob Health. 2014;108(4):200–5.
Molla E, Mamo H. Soil-transmitted helminth infections, anemia and undernutrition among schoolchildren in Yirgacheffee, South Ethiopia. BMC Res Notes. 2018;11:585.
Palmeirim MS, Hürlimann E, Knopp S, Speich B, Belizario V, Joseph SA, et al. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: a systematic review, meta-analysis and individual patient data analysis. PLoS Negl Trop Dis. 2018;12(4): e0006458.
Matamoros G, Sánchez A, Gabrie JA, Juárez M, Ceballos L, Escalada A, et al. Efficacy and safety of albendazole and high-dose ivermectin coadministration in school-aged children infected with Trichuris trichiura in Honduras: a randomized controlled trial. Clin Infect Dis. 2021;73(7):1203–10.
Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Terashima A, Samalvides F, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;1:CD007745.
Google Scholar
Patel C, Hürlimann E, Keller L, Hattendorf J, Sayasone S, Ali SM, et al. Efficacy and safety of ivermectin and albendazole co-administration in school-aged children and adults infected with Trichuris trichiura : study protocol for a multi-country randomized controlled double-blind trial. BMC Infect Dis. 2019;19(1):1–10.
Keller L, Hürlimann E, Patel C, Welsche S, Sayasone S, Ali SM, et al. Chapter Five—Insights gained from conducting a randomised controlled trial on ivermectin–albendazole against Trichuris trichiura in Côte d’Ivoire, Lao PDR and Pemba Island. In: Rollinson D, Stothard JR, editors., et al., Advances in parasitology. New York: Academic Press; 2021. p. 253–76.
Mejia R, Vicuña Y, Broncano N, Sandoval C, Vaca M, Chico M, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg. 2013;88(6):1041–7.
Booth M, Vounatsou P, Ngoran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Cte dIvoire. Parasitology. 2003;127(6):525–31.
World Health Organization. Bench aids for the diagnosis of intestinal parasites. 2nd ed. Geneva: World Health Organization; 2019. p. 32.
Witek-McManus S, Simwanza J, Chisambi AB, Kepha S, Kamwendo Z, Mbwinja A, et al. Epidemiology of soil-transmitted helminths following sustained implementation of routine preventive chemotherapy: demographics and baseline results of a cluster randomised trial in southern Malawi. PLoS Negl Trop Dis. 2021;15(5): e0009292.
Vaz Nery S, Qi J, Llewellyn S, Clarke NE, Traub R, Gray DJ, et al. Use of quantitative PCR to assess the efficacy of albendazole against Necator americanus and Ascaris spp. in Manufahi District, Timor-Leste. Parasites Vectors. 2018;11(1):373.
Easton AV, Oliveira RG, O’Connell EM, Kepha S, Mwandawiro CS, Njenga SM, et al. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasites Vectors. 2016;9(1):38.
Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements ACA, et al. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis. 2016;10(1): e0004380.
Azzopardi KI, Hardy M, Baker C, Bonnici R, Llewellyn S, McCarthy JS, Traub RJ, Steer AC. Detection of six soil-transmitted helminths in human stool by qPCR—a systematic workflow. PLoS ONE. 2021;16(9): e0258039. https://doi.org/10.1371/journal.pone.0258039 .
Zendejas-Heredia PA, Colella V, Hii SF, Traub RJ. Comparison of the egg recovery rates and limit of detection for soil-transmitted helminths using the Kato-Katz thick smear, faecal flotation and quantitative real-time PCR in human stool. PLoS Negl Trop Dis. 2021;15(5): e0009395. https://doi.org/10.1371/journal.pntd.0009395 .
Walker M, Cools P, Albonico M, Ame SM, Ayana M, Dana D, et al. Individual responses to a single oral dose of albendazole indicate reduced efficacy against soil-transmitted helminths in an area with high drug pressure. PLoS Negl Trop Dis. 2021;15(10): e0009888.
Download references
Acknowledgements
Not applicable.
The study protocol reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW010913 entitled “Enabling Infectious Disease Research Capacity in the Peruvian Amazon”. AGL is sponsored by Emerge, the Emerging Diseases Epidemiology Research Training grant D43 TW007393 awarded by the Fogarty International Center of the US National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder was not involved in the study protocol described here.
Author information
Authors and affiliations.
Laboratorio Satelite Iquitos, Asociación Benéfica Prisma, Área de Investigaciones Biomédicas, Calle Ramirez Hurtado Nº 622, Iquitos, Peru
Greisi Curico, Paul García-Bardales, Tackeshy Pinedo, Wagner Shapiama, Miguel Moncada-Yaicate, Lucero Romaina, Maribel Paredes-Olortegui & Francesca Schiaffino
Division of Infectious Diseases and International Health, Department of Internal Medicine, University of Virginia, MR-6 Rm 2207, 345 Crispell Dr, Charlottesville, VA, 22908, USA
Pablo P. Yori & Margaret N. Kosek
Universidad Nacional de la Amazonia Peruana, Jirón Nauta, 16002, Iquitos, Peru
Graciela Meza-Sánchez
Facultad de Salud Pública y Administración, Universidad Peruana Cayetano Heredia, Av. Honorio Delgado, 430. San Martin de Porres, Lima, Peru
Andrés G. Lescano
Department of Tropical Medicine, Tulane School of Public Health and Tropical Medicine, 1440 Canal Street, Suite 2310, New Orleans, LA, 70112, USA
Valerie A. Paz-Soldan & Richard A. Oberhelman
You can also search for this author in PubMed Google Scholar
Contributions
Study design and protocol development by GC, PGB, TP, WS, GMS, AGL, RAO, and MNK. Laboratory support by PGB, TP, WS, MMY, LR, and PPY. Subject enrollment and field work by MPO, GC, MMY, and LR. Implementation consulting by VAPS and FS. Regulatory and registration support by RAO, MNK, and GMS. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Richard A. Oberhelman .
Ethics declarations
Ethics approval and consent to participate.
Approved as protocol CE0686.21 by the PRISMA ethics committee (FWA00001219), and as protocol 2019-2142 by the Tulane University Institutional Review Board (FWA00002055). Parental written informed consent was obtained, in addition to the verbal and written assent for children ages 6 to 11 years
Consent for publication
Competing interests.
All authors declare no competing interests or conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Curico, G., García-Bardales, P., Pinedo, T. et al. Resistance to single dose albendazole and reinfection with intestinal helminths among children ages 2 to 11 years from the Peruvian Amazon region: a study protocol. BMC Infect Dis 22 , 528 (2022). https://doi.org/10.1186/s12879-022-07494-0
Download citation
Received : 29 April 2022
Accepted : 25 May 2022
Published : 07 June 2022
DOI : https://doi.org/10.1186/s12879-022-07494-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Albendazole
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 10, Issue 5
- Albendazole induces immunotherapy response by facilitating ubiquitin-mediated PD-L1 degradation
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Lin Zhu 1 , 2 ,
- Xinwei Kuang 1 ,
- Guanxiong Zhang 1 , 3 ,
- Long Liang 1 , 2 ,
- Dandan Liu 2 ,
- Zuozhong Xie 1 ,
- Hui Li 1 , 2 , 4 ,
- Hong Liu 1 , 5 ,
- Xiang Chen 1 and
- http://orcid.org/0000-0001-5588-3865 Jing Liu 2
- 1 Department of Dermatology, Hunan Key Laboratory of Skin Cancer and Psoriasis, Hunan Engineering Research Center of Skin Health and Disease, Xiangya Clinical Research Center for Cancer Immunotherapy, Xiangya Hospital , Central South University , Changsha , Hunan , China
- 2 Molecular Biology Research Center, Center for Medical Genetics, Hunan Province Key Laboratory of Basic and Applied Hematology, School of Life Sciences , Central South University , Changsha , Hunan , China
- 3 Department of Research and Development , Beijing GAP Biotechnology Co., Ltd , Beijing , China
- 4 Molecular Science and Biomedicine Laboratory, State Key Laboratory for Chemo/Biosensing and Chemometrics, College of Biology, College of Chemistry and Chemical Engineering, Collaborative Innovation Center for Chemistry and Molecular Medicine , Hunan University , Changsha , Hunan , China
- 5 Research Center of Molecular Metabolomics, Xiangya Clinical Research Center for Cancer Immunotherapy , Central South University , Changsha , Hunan , China
- Correspondence to Professor Hong Liu; hongliu1014{at}csu.edu.cn ; Professor Hui Li; lihuiscience{at}163.com ; Professor Mao Ye; goldleaf{at}hnu.edu.cn ; Professor Xiang Chen; chenxiangck{at}126.com ; Professor Jing Liu; jingliucsu{at}hotmail.com
Background Immune checkpoint inhibitors (ICIs) have been increasingly used in patients with various cancers and have shown efficient therapeutic outcomes. However, fewer than 40% of cases across multiple cancer types show a response to ICIs. Therefore, developing more efficient combinational approaches with ICIs and revealing the underlying mechanisms are important goals for achieving rapid clinical transformation and application.
Methods The effects on antitumor immunity activity of albendazole (ABZ) and the synergistic effects of ABZ with CD73 blockade were investigated in the melanoma B16F10 and the Lewis lung cancer tumor-bearing immune-competent mice models. The mechanism of ABZ reducing PD-L1 protein level through suppressing UBQLN4 was identified and validated through immunoprecipitation-mass spectrometry and molecular methods. Bioinformatics and anti-PD-1 therapy melanoma patients samples analysis were used to assess the level of UBQLN4/PD-L1 in the therapeutic efficacy of anti-PD-1 therapy.
Results ABZ induces CD8 + T cell activity and subsequent immunotherapy response associated with suppression of PD-L1 protein level. Mechanistically, we revealed that ABZ promotes ubiquitin-mediated degradation of PD-L1 via suppressing UBQLN4, which was bound to PD-L1 and stabilized PD-L1 protein. Preclinically, genetic deletion or target inhibition of CD73 showed synergistic effects with ABZ treatment in the immune-competent mice models. Significantly, UBQLN4 and PD-L1 levels were higher in the tumor region of responders versus non-responders and correlated with better progression-free survival and overall survival in anti-PD-1 therapy melanoma patients.
Conclusions Our findings revealed a previously unappreciated role of ABZ in antitumor immunity by inducing ubiquitin-mediated PD-L1 protein degradation, identified predictors for assessing the therapeutic efficacy of anti-PD-1 therapy, and provided novel therapeutic possibility by combination treatment of ABZ and CD73 blockade in cancers.
- Drug Therapy, Combination
- Immunotherapy
- Tumor Escape
Data availability statement
Data are available in a public, open access repository.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/jitc-2021-003819
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Immune checkpoint inhibitors (ICIs) have been increasingly used in patients with various cancers, including metastatic melanoma and non-small-cell lung cancer (NSCLC), and have shown promising therapeutic outcomes. 1 2 However, fewer than 40% of cases across multiple cancer types show a response to ICIs. 3 Recent studies have demonstrated that ICIs combined with other treatment strategies induce rapid and substantial tumor regression. For instance, nivolumab, a specific anti-programmed cell death protein-1 (PD-1) antibody, combined with ipilimumab induced tumor regression in ~50% of metastatic melanoma patients, with 85% of patients surviving even after 1 year of treatment. 4 Moreover, nivolumab is being investigated in combination with chemotherapies, immunotherapies, and vaccine-based therapies such as dendritic cell vaccines, NY-ESO-1 vaccines, and Tri-Mix in advanced melanoma patients. 5 6 Therefore, finding novel combinatorial approaches with ICIs may benefit patients with cancer and hold a great deal of promise in this new era of cancer immunotherapies.
Drug repositioning and repurposing is an alternative strategy to discover and develop novel combined immunotherapy regimens. By reason of the tumor PD-L1 level is a determinant and a common biomarker for the assessment of the clinical response to anti-PD-L1/PD-1 therapy, 7 we screened and found that albendazole (ABZ) could significantly reduce the expression of tumor cell membrane PD-L1 levels. ABZ is an FDA-approved broad-spectrum antiparasitic agent with low toxicity and is widely used in humans and animals. 8 Recently, ABZ has been reported to possess antitumor activity in several cancer cell types, 9 10 and one phase I clinical trial has been performed to determine the maximum tolerated dose of oral ABZ in patients with advanced cancer. 11 Moreover, ABZ can enhance immunological responses; for example, ABZ increased the counts of CD4 + and CD8 + T cells and significantly stimulated the IFN-gamma (a Th1-type cytokine) response in mice and human patients infected with Echinococcus. 12–14 However, the functions and underlying mechanism of ABZ in anti-tumor immunity remain unclear.
CD73 is a checkpoint molecule expressed on Treg cells and plays an important role in tumor immune escape, and is expected to be a next-generation target in immuno-oncology. 15 With accumulating evidence implicating CD73 involved in cancer progression and immune escape, blockade of CD73 may be a potent anticancer therapeutic approach. 16 For example, an anti-CD73 monoclonal antibody (mAb) significantly increased the antitumor effects of anti-CTLA-4 and anti-PD-1 mAbs in colon, prostate, and breast cancer models, 17 and the combination of a CD73-specific inhibitor (adenosine 5'-(α,β-methylene) diphosphate, APCP) and an anti-CTLA-4 mAb synergistically suppressed melanoma tumor growth. 18
In this study, we found that ABZ induces immunotherapy response, and promotes ubiquitin-mediated degradation of PD-L1 via suppressing UBQLN4, which is bound to PD-L1 and stabilized PD-L1 protein. We further evaluated the synergistic efficacy of ABZ in combination with CD73 blockages which is a potential strategy of combination immunotherapy for cancer treatment.
Cell culture
The human malignant melanoma cancer cell lines A375 and SK-MEL-28, Mouse melanoma cell line B16F10 and Lewis lung cancer (LLC) cells were purchased from American Type Culture Collection (ATCC). The human NSCLC cell lines A549, H460 and cell lines HEK293T come from our lab. Cells (A375, SK-MEL-28 and A549) were incubated in Dulbecco’s modified Eagle’s medium (DMEM, gibco, USA), others were cultured in RPMI1640 medium with 10% Fetal bovine serum (FBS, gibco, USA) and antibiotics (gibco, USA) at 37°C and 5% CO 2 atmosphere. Test for mycoplasma contamination was performed routinely and all cell lines were verified to be negative. A375, SK-MEL-28 and A549 were pretreated with Interferon gamma (IFN-γ, 200 ng/mL) and subjected to a complete medium with the indicated concentration of ABZ dissolved in dimethyl sulfoxide (DMSO). Cycloheximide (200 ng/mL) was added for the indicated times. MG132 (10 µM) or Bafilomycin A1 (Baf A1, 100 nM) were then added to a complete medium 6–12 hours before the cell lines were harvested. The source and identifier of the aforementioned reagents were listed in online supplemental table 1 .
Supplemental material
Clinical tissue samples.
Paraffin sections of tumor tissue from melanoma patients treated with PD-1 mAb were collected from Xiangya Hospital. All tissue samples were collected in compliance with the informed consent policy. Clinical information is summarized in online supplemental table 2 .
Animal models
All in vivo experiments were conducted in strict accordance with the approval of the Animal Care and Use Committee of the Central South University (Changsha, Hunan, China, license no. 2019sydw011). Wild-type B16F10 cells (1×10 6 ) or LLC cells (1×10 6 ) were inoculated subcutaneously into 6-week-old Wide type or CD73 Knockout C57BL/6 female mice (from the shanghai SLAC). Nearly 1 week after inoculation, mice were randomly divided into designated experimental and control groups treated daily with ABZ (50 mg/kg/2 day i.p) and DMSO, respectively. In the other part, the combination treatment effect of ABZ and APCP was also observed. APCP (400 µg/mouse) was administered to the mice by the peritumoral (p.t.) on day 4 and day 8, combined therapy with ABZ (50 mg/kg/2 day i.p). At the endpoint, tumors were harvested and analyzed by fluorescence-activated cell sorting (FACS). The removed xenografts were also snap-frozen in liquid nitrogen.
For biochemical analysis of the blood routine examination of mice, taking a blood sample from the retro-orbital venous plexus and collecting it into anticoagulation tubes. The blood samples were investigated for routine complete blood count (CBC, XN-1000-B1) analysis including white cell count, white cell classification count, red cell count, hemoglobin, and platelets), neutrophil count, neutrophil ratio, lymphocyte count and lymphocyte ratio. H&E-staining of the kidney, liver, and spleen tissue were performed and analyzed by Hunan AiFang biological.
FACS analysis of tumor immune cell
Single-cell suspension from mice B16F10 or LLC-xenograft tumor was obtained by rapid and gentle excising, physical grinding, and filter filtration. After blocking with trustain fcX anti-mouse CD16/32 (101320) antibody and getting rid of dead cells with Zombie Aqua Fixable Viability Kit (423102), enriched cells were stained using APC/CY7-CD45 (103116), CD3 (BV711-100241, APC-100236), PerCP/CY5.5-CD4 (100434), PE/CY7-CD8 (100722), APC-CD25 (17-0251-82), BV421-PD-1 (135218), BV605-CTLA4 (369610), PD-L1 (BV421-124315, APC-124312) for 25 min. After fixation and permeabilization (421402), intracellular GZMB was stained using GZMB (APC-372204, PE/Dazzle594-372216) antibody. Intranuclear FOXP3 was stained using PE/CY7-FOXP3 (25-5773-82) antibody. To detect the expression of PD-L1 on the membrane of single-cell suspension from human cell lines in culture, a PD-L1 (PE-329706) antibody is used. Cells were incubated with Human TruStain FcX (422302) block, and dead cells were gotten rid of with Zombie Aqua Fixable Viability Kit. The source and identifier of the aforementioned antibodies were listed in online supplemental table 1 . Stained cells were analyzed by FACS Dxp AthenaTM and Aurora (Cytek, USA). Furthermore, data were processed by Flow Jo V.10.0 software.
T cell-mediated tumor cell killing assay
T cell killing assay was performed as we previously described. 19 To acquire activated T cells, healthy human donors peripheral blood mononuclear cells were isolated from the whole blood and cultured in CTSTM AIIM VTM SFM (A3021002; Gibco) with Immuno Cult Human CD3/CD28/CD2 T cell activator (10970; STEMCELL Technologies) and IL-2 (1000 U/mL, 202–1 L-050, R&D) for 1 week according to the manufacturer’s protocol. Once adhering to the plates, cancer cells were then treated with IFN-γ for 24 hours, incubated with ABZ (0, 0.625 or 1.25 µM) for 24 hours, then processed with activated T cells for 24 hours. The ratio between cancer cells and activated T cells is 1–3. T cells and cancer cell debris were removed through PBS buffer, and the rest of the cells were quantified by crystal violet staining.
The 3×10 5 T cells were seeded in the 12-well plate. T cells were then treated with ABZ (0, 0.625, 1.25 µM) for 24 hours, centrifuge 300 g for 5 min to remove the medium, and the fixed cells were washed with PBS. Cells were incubated with Human TruStain FcX (422302) block, and dead cells were gotten rid of with Zombie Aqua Fixable Viability Kit. Enriched cells were stained using APC/Cya7-CD3 (300317), PE-CD8 (980902) and BV650-PD-1 (367429) antibodies at room temperature for 25 min. Stained cells were analyzed by FACS Dxp AthenaTM and Aurora (Cytek, USA). Furthermore, data were processed by Flow Jo V.10.0 software.
RNA isolation, quantitative real-time PCR
Total RNA was extracted from cultured human cancer cells with TRIzol according to the standard protocol. cDNA was generated by SuperScript III First-Strand cDNA synthesis system with 500 ng total RNA. Quantitative PCR was performed using a qPCR system (Eppendorf, Hamburg, Germany) in accordance with the manufacturer’s instructions. The sequences of human PD-L1 and GAPDH primers were listed in online supplemental table 1 . All mRNA expression levels were calculated using the comparative Ct method.
Western blotting analysis
Western blotting was performed as we previously described. 20 In brief, 30 µg content of total protein was subjected to SDS-polyacrylamide gel and subsequently transferred onto nitrocellulose membranes. After blocking with 5% skim milk resolved in TBST (Tris-buffered saline and Tween-20) for 1 hour, the membranes were then incubated with primary antibodies against PD-L1, UBQLN4, GAPDH, Ubiquitin, HA-Tag and His-Tag antibody, overnight at 4°C to detect target proteins. The source and identifier of the aforementioned antibodies were listed in online supplemental table 1 . After incubation with a Peroxidase AffiniPure Goat Anti-Mouse IgG (H+L) or Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L) for 2 hours at room temperature. The protein blot was visualized using a chemical chemiluminescence imaging system. ImageLab (Bio-Rad, California, USA) was applied to process images.
Co-immunoprecipitation
Endogenous co-immunoprecipitation (co-IP) was performed as we previously described. 21 In brief, cells were lysed in a cold IP Lysis buffer containing protease inhibitor cocktail, in addition to 5% cell extracts saved as the input, the rest of protein lysates were incubated with primary antibody overnight at 4°C and then protein A/G agarose beads for 4 hour at 4°C. After overnight incubation, the immunocomplexes were washed three times with PBST (pH7.4 PBS with 0.1% Tirton-X100). Bound proteins were eluted by boiling with 2×SDS loading buffer before being resolved by SDS-PAGE.
Pull-down assay
A375 cells with NC or HA-UBQLN4 overexpression were lysed using IP lysis buffer supplemented with protease inhibitor cocktail. Protein lysis was spun at 13,800 rpm for 15 min at 4°C and the supernatant was transferred to a new Eppendorf tube for use. After measuring the protein concentration, equal amounts of protein were extracted to incubate with Anti-HA magnetic beads-conjugated antibody against the target protein in a compatible buffer overnight at 4°C with rotation. Lysate beads were then washed with the washing buffer (10 mM Tris-HCl, pH 8.0, 1 M NaCl, 1 mM EDTA, 1% NP-40) three times. 2.5 µg purified human protein His-PD-L1 was used for incubation with the above immunocomplex overnight. After washing three times, the lysate beads with IP lysis were boiled for 10 min with 2×SDS loading buffer. Samples were loaded into SDS-PAGE gel for immunoblotting analysis following western blot protocol.
Immunofluorescence
Double fluorescence staining was performed as we previously described. 22 Briefly, 4 mm paraffin sections of patient samples were baked for 120 min at 60°C and then deparaffinized. Antigen was retrieved at EDTA antigen retrieval buffer (pH 8.0) and maintain at a sub-boiling temperature for 8 min, standing for 8 min and then followed by another sub-boiling temperature for 7 min. After spontaneous fluorescence quenching, the samples were blocked in 3% BSA, PBS with 0.25% Triton X-100 for 1 hour at room temperature. Primary antibodies targeting (PD-L1, UBQLN4) were incubated overnight at 4°C in the blocking solution and the following day for 30 min at room temperature. After extensive washing in PBS-0.25% Triton X-100, the secondary antibody including anti-rabbit Alexa Fluor 488 or Cy3 dye conjugate (Jackson) was added to the blocking solution and incubated for 2 hours. Then incubate with DAPI solution at room temperature for 10 min, kept in dark place. Images were detected and captured by Fluorescent Microscopy (Nikon, ECLIPSE Ts2R).
Cell transfection
The 2×10 5 cells per well were harvested and planted into 6-well plates and culture for 24 hours. when the cells density reaches 80%–90% confluency, UBQLN4-siRNA was transfected into cancer cells using RiboFECT CP Transfection Kit buffer (1×) following product manual protocol. The sequences of human UBQLN4-siRNAs and Control siRNA were listed in online supplemental table 1 . After 48 hours transfection, cancer cells were collected and used for total protein and mRNA expression detection.
HEK293T cells with an 80% confluency were transfected for packaging of lentiviral viruses using Turbofect according to the manufacturer’s instruction. After 48 hours and 72 hours transfection, supernatant with viruses was harvested and centrifuged at 2000 rpm for 10 min perspectively. When it was used for infecting cancer cells (30% confluency) in the presence of 8 µg/mL polybrene. After infection 48 hours, cancer cells were subjected to puromycin selection (1 µg/mL) for 3 days for obtaining the stable transfected cells.
Plasmid construction
pLV-mPuro-C-HA-UBQLN4 constructed by cloning the corresponding cDNAs into pLV-mPuro-C-HA vector was purchased from Sino Biological(HG21666-CYLP, Beijing, China). For CRISPR-mediated knockout A375 cell lines, sgRNAs were subcloned into pLenti CRISPRV2 vector (Addgene) following manufacturer instructions. The sequences of human sgUBQLN4#1 and #2 were listed in online supplemental table 1 .

Data collection and processing
Gene expression profiles (including raw read counts and FPKM) of 33 cancer types were downloaded by R package TCGA biolinks. 23 Differential expressed genes were identified using limma (|log2(fold change)|>0.58 and BH-adjusted pvalue <0.05). 24 Other published expression data for patients with ICIs were downloaded from Gene Expression Omnibus. 1 25–33 The protein expression profile for melanoma patients with ICIs was downloaded from online supplemental materials . For this cohort, the samples treated with anti-PD-1 and taken before treatment were considered, resulting in 66 samples in this cohort. 34
Gene set variation analysis was performed to calculate the score of immune cell populations. 35 The gene signature of immune cell populations was obtained from Charoentong et al . 36 Spearman’s correlation test was performed between the expression of interest genes and immune features, considering p<0.05 for statistical significance.
Statistical analysis
For the tumor growth data analysis, an overall difference at each data collection time point was tested by one-way analysis of variance (ANOVA). No experiment showed obvious bias toward a specific group in starting tumor volume. For comparisons among specific pairs of groups, statistical significance was assessed by the one-way ANOVA followed by Tukey’s multiple comparisons test. The assumption of ANOVA testing was checked to ensure the model assumption is not severely violated. Overall survival analyses were performed using the R package survival and survminer. Patients were divided into two groups by median expression or specified parameters (~45% quantile calculated by maxstat algorithm for protein profile of melanoma cohort with anti-PD-1 therapy). Log-rank tests were performed to compare overall survival between different groups.
ABZ enhances CTL activity in association with decrease of tumor PD-L1 expression
To find novel therapeutic agents to combine with ICIs for tumor therapy, and because the tumor PD-L1 level is a determinant factor for anti-PD-L1/PD-1 therapy, 22 we screened a common clinical drug library containing eight imidazoles, which have not been reported in anti-tumor immunity, to investigate their effects on tumor PD-L1 expression. We found that only ABZ significantly reduced the expression of tumor cell membrane PD-L1 levels ( online supplemental figure 1A ). To further assess whether ABZ regulates tumor PD-L1 levels in vitro, human melanoma cells A375 and SK-MEL-28 and human lung cancer cells A549 were pretreated with IFN-γ to increase PD-L1 levels, human lung cancer cell line H460 and mouse melanoma cells B16F10, which were highly expressed PD-L1 without IFN-γ treatment, followed by treatment with ABZ. Indeed, the protein levels of PD-L1 significantly decreased in an ABZ dose-dependent manner ( figure 1A , online supplemental figure 1B,C ). Flow cytometry analysis also showed that the IFN-γ-induced increases in membrane PD-L1 levels were decreased in a dose-dependent manner after treatment with ABZ in A375, SK-MEL-28 and A549 cells ( figure 1B ). Similarly, ABZ treatment significantly decreased the membrane PD-L1 levels in H460 and B16F10 cells without IFN-γ treatment ( online supplemental figure 1D ). In addition, a T cell killing assay was performed to test the effect of ABZ-associated expression level changes in tumor PD-L1 on CTL activity. As expected, ABZ pretreatment significantly enhanced T cell-mediated cancer cell death ( figure 1C , online supplemental figure 1E ). However, the expression of PD-1 on CD8 + T cells has no significant change after ABZ treatment, indicating that ABZ activates CTL by downregulating the level of tumor PD-L1 ( online supplemental figure 1F ). To investigate the role of ABZ in anti-tumor immunity, we treated the melanoma B16F10 ( figure 1D ) and the LLC ( figure 1H ) tumor-bearing immune-competent mice with ABZ and showed that ABZ treatment significantly reduced mouse tumor growth and weight ( figure 1E–G, I–K ). Administration of ABZ did not result in a significant change of mouse body weight, the blood routine examination, the morphology and the organizational structure of kidney, liver, and spleen of mice ( online supplemental figure 2A-E ), suggesting limited toxicity of ABZ treatment. Because immunity-based tumor elimination depends primarily on the activated CD8 + T cell, which exerts cytolytic capacity by secreting granzyme B (GZMB), we investigated the infiltrated CD8 + T cell abundance and activity in the tumor regions of ABZ-treated mice. Indeed, ABZ treatment significantly increased the CD8 + T cell proportion ( online supplemental figure 2F,G ) and GZMB + CD8 + T cell proportion ( figure 1L,M ) in both melanoma B16F10 and LLC tumor-bearing immune-competent mouse models. The activity of CD8 + T cells is mainly controlled by immune checkpoints. Interestingly, we found that ABZ treatment significantly decreased the tumor PD-L1 level ( figure 1N,O ) but did not affect the levels of PD-1 and CTLA-4 on CD8 + T cells ( online supplemental figure 2H,I ). Together, these data suggested that ABZ increases CTL activity by reducing tumor PD-L1 levels.
- Download figure
- Open in new tab
- Download powerpoint
ABZ enhances CTL activity in association with decrease of tumor PD-L1 expression. (A) Representative western blotting of PD-L1 protein level in A375, SK-MEL-28 and A549 cells treated with increasing concentrations of ABZ (0.625–1.25 µM) for 24 hours under IFN-γ exposure. (B) Representative profiles and quantitative analysis of membrane PD-L1 expression by flow-cytometric analysis after increasing concentrations of ABZ (0.625–1.25 µM) treated A375, SK-MEL-28 and A549 cells for 24 hours under IFN-γ exposure. (C) A375 and SK-MEL-28 cells co-cultured with activated T cells for 24 hours with or without ABZ (0.625–1.25 µM) were subjected to crystal violet staining. The tumor cell to T cell ratio, 1:3. The quantitative analysis of A375 and SK-MEL-28 cell survive rate from three independent experiments and showed as means±SD, *p<0.05, **p<0.01, ***p<0.001. (D–G). C57BL/6 mice were implanted with 1×10 6 B16F10 and received 50 mg/kg of ABZ treatment. (D) A schematic view of the treatment plan. (E) Tumor volume was measured on the indicated different time points. (F,G) Photographs of representative tumors and tumor weight were measured after ABZ treatment on day 12 in the B16F10 tumor burden mouse model. Data represent mean±SD, ***p<0.001. (H-K) C57BL/6 mice were implanted with 1×10 6 LLC and received 50 mg/kg of ABZ treatment. (H) A schematic view of the treatment plan. (I) Tumor volume was measured on the indicated different time points. (J,K) Photographs of representative tumors and tumor weight were measured after ABZ treatment on day 15 in the LLC tumor burden mouse model. Data represent mean±SD, **p<0.01. (L,M) Representative profiles and quantification of flow cytometry-based detection of the GZMB + CD8 + in B16F10 and LLC tumor mass from the different treatment groups (n=5 mice per group). Data represent mean±SD, ***p<0.001. (N,O) Representative profiles and quantification of flow cytometry-based detection of the PD-L1 in B16F10 and LLC tumor mass from the different treatment groups (n=5 mice per group). Data represent mean±SD, ***p<0.001. ABZ, albendazole; CTL, cytotoxic T lymphocyte; IFN-γ, interferon gamma; LLC, Lewis lung cancer.
ABZ induces ubiquitin-mediated PD-L1 degradation
Next, we aim to explore the molecular mechanism of ABZ-mediated regulation of PD-L1. Our results showed that the mRNA levels of PD-L1 did not significantly change after ABZ treatment ( figure 2A ), indicating that ABZ may regulate PD-L1 expression via protein-level modifications rather than at the transcriptional level. Furthermore, melanoma cells were treated with cycloheximide (CHX) to inhibit protein biosynthesis, and protein extracts obtained at the indicated time points were analyzed. We found that ABZ treatment significantly decreased the half-life of the PD-L1 protein in A375 and SK-MEL-28 cells ( figure 2B ), suggesting that ABZ regulates the stability of PD-L1 protein. Interestingly, the reduction in PD-L1 protein levels was blocked by the proteasome inhibitor MG132 but not by the lysosome inhibitor bafilomycin A1 (Baf A1, figure 2C , online supplemental figure 3A ), suggesting that ABZ induces degradation of PD-L1 protein via the ubiquitin-proteasome system (UPS) rather than via the autophagy/lysosome pathway. To further confirm that ABZ induces degradation of the PD-L1 protein via the UPS pathway, we measured the levels of polyubiquitylated PD-L1 in melanoma cells. As shown in figure 2D , ABZ treatment led to a significant increase in PD-L1 polyubiquitylation. Taken together, these data indicated that ABZ treatment induces PD-L1 ubiquitin-mediated degradation.
ABZ decreases UBQLN4 and induces PD-L1 ubiquitin-mediated degradation. (A) Bar graph presentation of PD-L1 mRNA levels as determined by qRT-PCR of increasing concentrations of ABZ (0.625–1.25 µM) treatment of A375 and SK-MEL-28 cells for 24 hours under IFN-γ exposure, each bar represents the mean±SD of three independent experiments, NS P >0.05, ***p<0.001. (B) A375 and SK-MEL-28 cells were treated with ABZ (1.25 µM) for 24 hours under IFN-γ exposure then treated with CHX (200 ng/mL), and collected at the indicated times for western blotting. Line graph presentation of quantitative analysis of PD-L1 protein expression in A375 and SK-MEL-28 cells. (C) A375 and SK-MEL-28 cells were pretreated with increasing concentrations of ABZ (0.625–1.25 µM), then treated with Baf A1 (100 nM) and MG132 (10 µM) for 6 hour and the PD-L1 protein level were detected by western blotting. (D) A375 and SK-MEL-28 cells were treated with ABZ (1.25 µM) for 24 hours under IFN-γ exposure then treated with MG132 (10 µM) for 6 hours before harvest. PD-L1 was immunoprecipitated with an anti-PD-L1 antibody, and the immunoprecipitates were probed with an anti-ubiquitin (UB) antibody. (E) Immunoprecipitates from A375 cells treated with ABZ (1.25 µM) or DMSO were separated by SDS-PAGE and the immunoprecipitates were probed with anti-PD-L1 antibody and stained with colloidal silver to visualize proteins, respectively. The proteins pointed by the arrow were analyzed by LC/MS-MS. (F) Peptide enrichment fingerprints of UBQLB4 from LC/MS-MS QSTAR analysis. (G) Kaplan-Meier estimates for overall survival; patients from TCGA SKCM cohort were stratified into two groups based on the median expression level of UBQLN4. Significance was determined by the log-rank test. (H) Significantly correlation between UBQLN4 expression level and infiltration of activated immune cell (left panel) or expression level of activated checkpoints (right panel) in TCGA SKCM cohort, respectively. Spearman’s correlation test was performed, black border, *p<0.05. (I) A375 cell lysates were subjected to immunoprecipitation with control IgG, anti-PD-L1 or anti-UBQLN4 antibodies. The immunoprecipitates were then probed with anti-UBQLN4 or anti-PD-L1 antibody, respectively. (J) Representative western blotting analysis of pull-down of purified HA-UBQLN4 with purified His-PD-L1. (K) Representative western blotting and quantitative analysis of UBQLN4 and PD-L1 proteins expression when knockdown UBQLN4 in A375 and SK-MEL-28 cells under IFN-γ exposure, each bar represents the mean±SDof three independent experiments, NS P >0.05, *p<0.05, **p<0.01, ***p<0.001. (L) A375 cells transfected with control or UBQLN4 siRNA were treated with CHX (200 ng/mL) and collected at the indicated times for western blotting. Line graph presentation of quantitative analysis of PD-L1 protein level. (M) A375 cells transfected with a vector expressing HA-UBQLN4 and an empty vector were treated with CHX (200 ng/mL) and collected at the indicated times for western blotting. Line graph presentation of quantitative analysis of PD-L1 protein level, GAPDH was used as the loading control. ABZ, albendazole; IFN-γ, Interferon gamma; CHX, cycloheximide; LC/MS-MS, liquid chromatography tandem mass spectrometry; GAPDH, glyceraldehyde-3-phosphate dehydrogenase .
UBQLN4 interacts with PD-L1 and stabilizes the PD-L1 protein
To further investigate the molecular mechanism underlying ABZ-induced ubiquitin-mediated degradation of PD-L1, we performed immunoprecipitation coupled to mass spectrometry in A375 cells with ABZ treatment ( figure 2E ). UBQLN4 was identified as the top candidate in terms of enrichment of peptides among all the candidate ubiquitylation-related proteins ( figure 2F , online supplemental table 3 ). UBQLN4 has been reported to function as a UBL-UBA protein (proteins that contain both a ubiquitin-like region and ubiquitin-associated domain) that stabilizes its target substrate, wild-type ataxin-1-(30Q). 37 More importantly, UBQLN4 was significantly correlated with worse survival ( figure 2G ) and negatively correlated with activated CTLs and immune checkpoints in melanoma ( figure 2H , online supplemental figure 3B ). To confirm the interaction between UBQLN4 and PD-L1, co-IP was performed and revealed the endogenous interaction of UBQLN4 with PD-L1 in A375 cells ( figure 2I ). Furthermore, a pull-down assay showed that purified HA-UBQLN4 protein was directly bound to His-PD-L1 protein ( figure 2J ). Given the identified interaction of UBQLN4 with PD-L1, we next investigated whether UBQLN4 affects the protein level of PD-L1. Interestingly, knockdown of UBQLN4 using two siRNA sequences significantly decreased PD-L1 protein levels in A375 and SK-MEL-28 cells ( figure 2K ). More importantly, we found that the knockdown of UBQLN4 significantly decreased the half-life of the PD-L1 protein ( figure 2L ). In contrast, overexpression of UBQLN4 significantly increased the half-life of the PD-L1 protein ( figure 2M ). Together, these data suggest that UBQLN4 interacts with PD-L1 and stabilizes the PD-L1 protein.
To validate that UBQLN4 is a functional target of ABZ and is responsible for reducing PD-L1 expression and enhancing CTL activity, we first treated A375, SK-MEL-28 and H460 cells with ABZ and found that the expression of UBQLN4 was significantly decreased, and the PD-L1 levels were also decreased ( figure 3A , online supplemental figure 3C ). Interestingly, knockout of UBQLN4 using clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) technology with the single guide RNAs (sgRNAs) sgUBQLN4 #1 and sgUBQLN4 #2 abolished the ABZ treatment-induced reduction in PD-L1 protein levels ( figure 3B ). However, overexpression of UBQLN4 strongly reversed the ABZ-mediated decrease in PD-L1 ( figure 3C ). In addition, a T cell killing assay also showed that ABZ pretreatment-induced enhancement of T cell-mediated cancer cell death was rescued by overexpression of UBQLN4 ( figure 3D ). These results suggested that UBQLN4 is a functional target of ABZ and is required for ABZ to reduce PD-L1 and enhance CTL activity.
ABZ reduces PD-L1 and enhances CTL activity by downregulating UBQLN4. (A) Representative western blotting and quantitative analysis of UBQLN4 and PD-L1 protein levels in A375 and SK-MEL-28 cells treated with increasing concentrations of ABZ (0.625–1.25 µM) for 24 hours under IFN-γ exposure, each bar represents the mean±SD of three independent experiments, *p<0.05, **p<0.01, ***p<0.001. (B) Representative western blotting and quantitative analysis of PD-L1 protein levels in A375, which were stable transfected with sgRNAs (sgUBQLN4 #1, sgUBQLN4 #2 and control (NC)) and treated with increasing concentrations of ABZ (0.625-1.25 µM), each bar represent the mean±SD of three independent experiments, NS P >0.05, *p<0.05, **p<0.01, ***p<0.001. (C) Representative western blotting and quantitative analysis of PD-L1 protein levels in A375 and SK-MEL-28 cells, which were transfected with HA-UBQLN4 and empty vector and treated with increasing concentrations of ABZ (0.625–1.25 µM), each bar represents the mean±SD of three independent experiments, *p<0.05, **p<0.01, ***p<0.001. (D) A375 and SK-MEL-28 cells were transfected with HA-UBQLN4 and empty vector and cocultured with activated T cells for 24 hours with or without ABZ (0.625-1.25 µM) were subjected to crystal violet staining. The tumor cell to T cell ratio, 1:3. The quantitative analysis of A375 and SK-MEL-28 cells survive rate from three independent experiments and showed as means±SD, NS P >0.05, *p<0.05, **p<0.01, ***p<0.001. # p<0.05, ### p<0.001, vs the DMSO group. ††† p<0.001 vs the ABZ (0.625 µM) group. SSS p <0.001 vs the ABZ (1.25 µM) group. NS∙T p >0.05, #∙T p <0.05, vs the DMSO +T group. †∙T p <0.05, †††∙T p <0.001 vs the ABZ (0.625 µM+T) group. SS∙T p <0.001, SSS∙T p<0.001 vs the ABZ (1.25 µM+T) group. ABZ, albendazole; IFN-γ, interferon gamma.
Genetic deletion of CD73 enhanced ABZ-mediated immunotherapy response in vivo
ABZ increased CD8 + T cell cytotoxicity while elevating Treg cell abundance as an additional effect in vivo ( figure 4A ). Bioinformatics analysis showed that CD73 expression was significantly positively correlated with immunosuppressive cells (Treg cells, macrophages and MDSCs) and inhibitory immune checkpoints ( figure 4B,C , online supplemental figure 4A,B ). Moreover, blocking CD73 has been shown to enhance antitumor responses in a number of tumor models. 38 Therefore, we further investigated whether deficiency of CD73, a checkpoint molecule expressed on Treg cells, 15 could synergize with the tumor elimination effect of ABZ in immune-competent mice. We examined the tumor elimination effects of ABZ in immune-competent CD73 wild-type (WT) and knockout (KO) mice bearing B16F10 ( figure 4D ) and LLC tumors ( figure 4H ). We observed that the growth of B16F10 and LLC subcutaneous tumors was significantly inhibited in ABZ-treated KO mice in comparison with that in ABZ- or DMSO-treated WT mice and CD73 KO mice ( figure 4E, I ). Moreover, ABZ-treated CD73 KO mice displayed a significantly greater reduction in tumor mass and weight than ABZ-treated or DMSO-treated WT mice and CD73 KO mice ( figure 4F,G,J,K ) without significant changes in body weight, the blood routine examination, the morphology of lung, liver, and spleen of mice in both melanoma B16F10 and LLC tumor-bearing immune-competent mouse models ( online supplemental figure 4C–H ). As expected, ABZ-treated CD73 KO mice showed further decreased PD-L1 expression levels in the B16F10 and LLC subcutaneous tumor regions ( figure 4L,M ) and increased proportions of infiltrated CD8 + and GZMB + CD8 + T cells in the tumor region ( figure 4N,O , online supplemental figure 5A-D ). More importantly, ABZ-treated CD73 KO mice exhibited a markedly decreased Treg population compared with ABZ- or DMSO-treated WT mice and CD73 KO mice ( online supplemental figure 5E ).
Genetic deletion of CD73 enhanced ABZ-mediated immunotherapy response in vivo. (A) Representative profiles and quantification of flow cytometry-based detection of the Foxp3 + CD4 + TILs in B16F10 tumor mass from the different treatment groups (n=5 mice per group, **p<0.01). (B,C) Significantly correlation for suppressive immune cell types or inhibitory immune checkpoints and CD73 expression level in TCGA SKCM cohort. Spearman’s correlation test was performed. Bars with black border, *p<0.05. Points in the scatter plot represent positive (RS >0 and pvalue <0.05, in red), negative (RS <0 and pvalue <0.05, in blue), or non-significant correlation (in gray). (D–G) CD73 WT and KO mice (n=5 mice per group) were implanted with 1×10 6 WT B16F10 and received 50 mg/kg of ABZ treatment as the chart indicated. (D) a schematic view of the treatment plan. (E) Tumor volume was measured on the indicated different time points (n=5 mice per group). Data represent mean±SD, **p<0.01, ***p<0.001. (F) Photographs of representative tumors harvested from CD73 WT and KO mice after ABZ treatment at day 12. (G) Tumor weight was measured on day 12. Data represent mean±SD, *p<0.05, ***p<0.001. (H–K) CD73 WT and KO mice (n=6 mice per group) were implanted with 1×10 6 WT LLC and received 50 mg/kg of ABZ treatment as the chart indicated. (H) A schematic view of the treatment plan. (I)Tumor volume was measured on the indicated different time points (n=6 mice per group). Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. (J) Photographs of representative tumors harvested from CD73 WT and KO mice after ABZ treatment at day 12. (K) Tumor weight was measured on day 12. Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. (L,M) representative profiles and quantification of flow cytometry-based detection of the PD-L1 from B16F10 (L) and LLC (M) tumor mass (n=5 mice per group). Data represent mean±SD, NS P >0.05, **p<0.01, ***p<0.001. (N,O) quantification of flow cytometry-based detection of the GZMB + CD8 + in CD3 + TILs from B16F10 (N) and LLC (O) tumor mass (n=5 mice per group). Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. ABZ, albendazole;TILs, tumor infiltrates lymphocytes; LLC, Lewis lung cancer.
Synergistic effect of CD73 specific inhibitor and ABZ therapy in the melanoma mouse model
We further identified whether combining ABZ with a CD73-specific inhibitor (APCP) can enhance tumor therapeutic efficacy. To test this hypothesis, B16F10 tumor-bearing mice were treated with ABZ, APCP, ABZ plus APCP, or the control ( figure 5A ). Notably, combining ABZ and APCP reduced tumor growth and tumor weight much more significantly than either treatment alone ( figure 5B–D ) without causing significant changes in body weight ( figure 5E ). Similar to the combination of ABZ and CD73 deficiency, cotreatment with ABZ and APCP synergistically decreased PD-L1 expression levels in the tumor regions ( figure 5F ). Notably, the combination of ABZ and APCP also markedly increased the population of infiltrated CD8 + and GZMB + CD8 + T cells in the tumor region ( figure 5G,H ). Moreover, cotreatment with ABZ and APCP synergistically decreased the Treg cell populations compared with ABZ or APCP treatment alone ( figure 5I ). These results indicate that ABZ reduces tumor PD-L1 levels and enhances the efficacy of CD73 blockade immunotherapy.
Synergistic effect of CD73 specific inhibitor and ABZ therapy in the melanoma mouse model. (A) Mouse melanoma cell line B16F10 cells were injected into mice (n=6 mice per group) on day −6, ABZ (50 mg/kg) and APCP (400 µg/mouse) were administered alone or together as the chart indicated. (B) Tumor volume was measured on the indicated different time points (n=6 mice per group). Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. (C) Photographs of representative tumors after ABZ and APCP treatment on day 12 in the B16F10 tumor burden mouse model. (D) Tumor weight was measured on day 12. Data represent mean±SD, ***p<0.001. (E) Mice weight was measured on the indicated different time points. (F) Representative profiles and quantification of flow cytometry-based detection of the PD-L1 from B16F10 tumor mass (n=6 mice per group). Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. (G) Representative profiles and quantification of flow cytometry-based detection of the CD8 + in CD3 + TILs in B16F10 tumor mass from the different treatment groups (n=6 mice per group). Data represent mean±SD, *p<0.05, ***p<0.001. (H) Representative profiles and quantification of flow cytometry-based detection of the GZMB + CD8 + in CD3 + TILs from the B16F10 tumor mass (n=6 mice per group). Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. (I) Representative profiles and quantification of flow cytometry-based detection of the Foxp3 + in CD4 + TILs in B16F10 tumor mass from the different treatment groups (n=6 mice per group). Data represent mean±SD, *p<0.05, **p<0.01, ***p<0.001. ABZ, albendazole; APCP, adenosine 5'-(α,β-methylene) diphosphate; TILs, tumor infiltrates lymphocytes.
UBQLN4/PD-L1 expression levels were correlated with the efficacy of PD-1 mAb therapy in melanoma patients
To investigate the clinical application potential of strategies targeting UBQLN4/PD-L1, we compared the expression levels of UBQLN4/PD-L1 between paired tumor and normal tissue samples across 16 cancer types from The Cancer Genome Atlas (TCGA). We observed that UBQLN4 and PD-L1 were upregulated in most cancer types, including esophageal carcinoma, head and neck squamous cell carcinoma, adenocarcinoma of the stomach and lung adenocarcinoma ( figure 6A ). We further performed survival analyses and demonstrated that high UBQLN4/PD-L1 scores were associated with worse survival in most cancer types from TCGA, the patients of whom were mainly treated with chemotherapy or targeted therapy ( figure 6B ). In contrast, we found that the expression levels of UBQLN4/PD-L1 were associated with better survival in most cancer types from the public ICB cohort ( figure 6C ). One reason that only a small subset of patients respond to PD-1/PD-L1 blockade is that PD-1-associated immune resistance depends on the expression of PD-L1 in the tumor. Therefore, we analyzed the levels of UBQLN4/PD-L1 in biopsy samples from two immunotherapy cohorts. We collected 19 melanoma patients treated with anti-PD-1 monotherapy in Xiangya Hospital ( figure 6D , online supplemental table 2 ). As expected, samples from responders had a relatively higher UBQLN4/PD-L1 signal than the samples from non-responders ( figure 6E,F ). Meanwhile, a strong positive correlation between the UBQLN4/PD-L1 expression was observed in patient samples ( figure 6G ). Moreover, we observed that patients with high UBQLN4 and PD-L1 expression in the tumor region had better progression-free survival (PFS; median PFS 10 months vs 2 months, log-rank test, p<0.001) after anti-PD-1 treatment ( figure 6H,I ). In addition, we further separate patients into three groups based on UBQLN4 and PD-L1 expression, and revealed that patients with status of ‘PD-L1-low and UBQLN4-low’ showed the worst survival ( online supplemental figure 6 ). We also collected published protein expression profiles for 66 melanoma patients treated with anti-PD-1 antibodies. 39 Similarly, we found that a high protein level of UBQLN4 was correlated with a better OS in Harel et al . Dataset (overall Survival, log-rank test, p=0.027, figure 6J ). Taken together, these data demonstrated that the expression of UBQLN4/PD-L1 is a promising cancer biomarker and potential predictor for assessing the efficacy of anti-PD-1 therapy in the clinic.
UBQLN4/PD-L1 expression levels were correlated with the efficacy of PD-1 mAb therapy in melanoma patients. (A) Differential expression of UBQLN4 and PD-L1 across 16 cancer types compared with paired normal samples (fold change >1.5; adjusted p<0.05). Cancer types with UBQLN4 or PD-L1 significantly differentially expressed were shown. (B) Survival analysis demonstrated the effect of UBQLN4 and PD-L1 on patient overall survival in traditional therapy strategies. (C) Survival analysis demonstrated the effect of UBQLN4 and PD-L1 on patient overall survival in anti-PD-1 immunotherapy. (D) Clinical characteristics of anti-PD-1 monotherapy cohorts. Patients were stratified into response groups based on RECIST (Response Evaluation Criteria in Solid Tumors) 1.1 criteria, patients with a complete response (CR), partial response (PR), or stable disease (SD) with progression-free survival (PFS) longer than 6 months were classified as responders, while patients with SD with PFS shorter than 6 months and PD were categorized as non-responders. TNM stage based on the eighth Edition Melanoma Stage Classification. (E) Representative fluorescent images of UBQLN4 and PD-L1 in two patients with a different response. (F) Significantly differential expression of UBQLN4 and PD-L1 between non-responder and responder. Significance was determined by the Wilcoxon rank-sum test, **p<0.01. (G) Scatterplot showed a significant correlation between UBQLN4 and PD-L1 expression. (H,I) Kaplan-Meier estimates for PFS of patients derived from Xiangya hospital patients (n=19) were stratified into two groups based on the median expression level of PD-L1 (H) and UBQLN4 (I), respectively. Significance was determined by the log-rank test. (J) Kaplan-Meier estimates for overall survival of patients derived from Harel et al , 39 (n=66).
ICIs have shown substantial clinical benefits for several malignancies, such as metastatic melanoma and NSCLC. However, the overall response rate to ICIs has been relatively low. 19 Previous preclinical studies and clinical trials have also shown that traditional chemotherapeutic drugs combined with ICIs, such as metformin plus CTLA-4 blockade, 40 sunitinib plus CTLA-4 blockade, 22 and pembrolizumab plus carboplatin plus pemetrexed (phase II of KEYNOTE-021), 41 are more effective in reversing T cell exhaustion and restoring antitumor immunity than single-agent treatment. In particular, drug repurposing and/or repositioning is a method for developing new treatments for cancers. 42 In this study, we revealed a previously unappreciated role of the antiparasitic agent ABZ: it can induce an antitumor immune response by increasing CD8 + T cell cytotoxicity. Preclinically, we showed that ABZ had a synergistic effect with CD73 blockade in the treatment of immune-competent mice, providing a novel combination strategy for cancer immunotherapy.
PD-L1 is a critical immune checkpoint protein that binds to the PD-1 receptor on T cells and activates coinhibitory signaling to suppress the function of CTLs, allowing cancer cells to escape immunosuppression. 43 It is not surprizing that PD-L1 is expressed at relatively much higher levels in many types of cancer cells and is often correlated with poor patient prognosis. 44 45 Recent studies have reported that the tumor PD-L1 expression level is a determinant and a common biomarker for the assessment of the clinical response to anti-PD-L1/PD-1 therapy. 7 However, how and when PD-L1 is upregulated during the pathogenesis of cancer remains less well understood. Therefore, it is critical to understand the molecular mechanism of tumor PD-L1 regulation, which is important for the improvement of anti-PD-L1/PD-1 therapy and its subsequent clinical effect. Previous studies have reported that the tumor PD-L1 level can be regulated at the transcriptional level (eg, via EGFR, the MAPK pathway, Janus kinase-signal transducer and activator of transcription pathway, nuclear factor kappa-B (NF-κB) pathway, hypoxia-inducible factor-1α, MYC, Anaplastic lymphoma kinase, Met, BRD4), 46 and via post-translational modification (eg, via CDK4, B3GNT3, GSK3β, CSN5, CMTM4, and CMTM6). 47 In this study, we revealed that UBQLN4 interacts with PD-L1 and stabilizes the PD-L1 protein. ABZ induced ubiquitination and degradation of PD-L1 by reducing the expression of UBQLN4. We not only uncovered the molecular mechanisms by which ABZ promotes antitumor immunity but also, more importantly, identified UBQLN4 as a novel posttranslational regulatory molecule of PD-L1.
UBQLN4 belongs to the UBL-UBA family of proteins, which are adaptor proteins that deliver ubiquitinated proteins through the UBA domain to s5a located in the proteasome through simultaneous interaction with the UBL domain and play main roles in protein degradation. 48 For instance, UBQLN4 interacts with endoplasmic reticulum-localized connexin 43 (Cx43) and stimulates its proteasomal degradation. 49 However, recent findings have contradictorily reported that UBL-UBA proteins also exert stabilizing effects on their substrates. 50 51 Recently, some reports have shown that UBQLN4 is overexpressed in neuroblastoma and hepatocellular carcinoma (HCC) tumors and that high UBQLN4 expression is associated with poor overall survival and disease-free survival rates. 52 53 Herein, we demonstrated that UBQLN4, CD73, and PD-L1 were significantly highly expressed in most cancers and that their high expression correlated with lower survival of cancer patients. These data provide potential new diagnostic and therapeutic targets in cancers. On the other hand, we observed higher UBQLN4 and PD-L1 levels in the tumor region of melanoma patients who responded to anti-PD-1 therapy than in the tumor region of nonresponders, indicating that UBQLN4 and PD-L1 might be potential biomarkers for assessing and predicting the efficacy of anti-PD-1 therapy in the clinic.
In summary, our data revealed a previously uncharacterized function of ABZ in promoting antitumor immunity by disrupting the UBQLN4-mediated stabilization of the PD-L1 protein. Our findings also suggest that ABZ can be exploited to overcome tumor immune escape in combination with current ICIs, such as CD73 inhibitors.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethical standards of the Ethics Committee of Hunan Cancer Hospital of Central South University and procedures approved by the committee (license no. SBQLL-2020-019).
Acknowledgments
We thank Xiangya Hospital of Central South University for providing melanoma patients samples.
- Zaretsky JM ,
- Sun L , et al
- Bocquet-Tremoureux S ,
- Scharbarg E ,
- Nguyen J-M , et al
- Iams W , et al
- Wolchok JD ,
- Callahan MK , et al
- Koppolu V ,
- Rekha Vasigala VK
- Kudchadkar RR ,
- Yu B , et al
- Herbst RS ,
- Soria J-C ,
- Kowanetz M , et al
- Zhang Q-L ,
- Zhu MJ , et al
- Pourgholami MH ,
- Szwajcer M ,
- Chin M , et al
- Dvoroznáková E ,
- Hrcková G ,
- Borosková Z , et al
- Ricken FJ ,
- Grüner B , et al
- Jebbawi F ,
- Bellanger A-P ,
- Lunström-Stadelmann B , et al
- Ghalamfarsa G ,
- Kazemi MH ,
- Raoofi Mohseni S , et al
- Smyth MJ , et al
- Iannone R ,
- Maiolino P , et al
- Zhang Y , et al
- Cao W , et al
- Xiao X , et al
- Liang L , et al
- Colaprico A ,
- Olsen C , et al
- Ritchie ME ,
- Phipson B ,
- Wu D , et al
- Margolis CA ,
- Gao W , et al
- Van Allen EM ,
- Schilling B , et al
- Makarov V , et al
- Auslander N ,
- Lee JS , et al
- Menzies AM , et al
- Nathanson T ,
- Funt SA , et al
- Ascierto ML ,
- McMiller TL ,
- Berger AE , et al
- Gartrell RD , et al
- Mariathasan S ,
- Turley SJ ,
- Nickles D , et al
- Ortenberg R ,
- Varanasi SK , et al
- Hänzelmann S ,
- Castelo R ,
- Charoentong P ,
- Finotello F ,
- Angelova M , et al
- Zoghbi HY , et al
- Xia W , et al
- Yang JC-H ,
- Gadgeel SM ,
- Sequist LV , et al
- Corsello SM ,
- Bittker JA ,
- Liu Z , et al
- Jin P , et al
- Qiu S-J , et al
- Xu L , et al
- Wang Q , et al
- Nakagawa R ,
- Koval M , et al
- Funakoshi M ,
- Hunt T , et al
- Jachimowicz RD ,
- Beleggia F ,
- Isensee J , et al
- Cui G , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
LZ, XK and GZ contributed equally.
Contributors JL, XC, HoL and HuL conceived the project and designed the experiments. LZ, HuL, LL, GZ, BH, DL and ZX carried out the main experiments and collected the data. XK and GZ collected the human melanoma tissues and analyzed the information of clinical samples, HuL, HoL, MY and JL analyzed the data. HuL wrote the manuscript. HoL, XC, MY and JL revised and validated the manuscript. JL responsible for the overall content as the guarantor.
Funding This work was supported by grants from the National Key Research and Development Program of China (Nos. 2018YFA0107800, 2019YFA0111600, 2019YFE0120800), the Natural Science Foundation of China for outstanding Young Scholars (No. 82022060), the National Natural Science Foundation of China (Nos. 82003286, 81874242, 31800979, 81970195, 81920108004, 82102891), the Natural Science Foundation of Hunan Province for outstanding Young Scholars (No. 2019JJ30040), the Natural Science Foundation of Hunan Province (No. 2021JJ40054), the fellowship of China Postdoctoral Science Foundation (Nos. 2020M672474, 2021T140195), the Changsha Municipal Natural Science Foundation (No. kq2014041), the Fundamental Research Funds for the Central Universities of Central South University (No. 2020zzts428).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Mix of T. saginata and T. solium Numbers small Recs PZQ>ALB unless using ALB for something else
Safety of Praziquantel and Albendazole Coadministration for the Control and Elimination of Schistosomiasis and Soil-Transmitted Helminths Among Children in Rwanda: An Active Surveillance Study
- Original Research Article
- Open access
- Published: 11 July 2022
- Volume 45 , pages 909–922, ( 2022 )
Cite this article
You have full access to this open access article
- Joseph Kabatende ORCID: orcid.org/0000-0002-3718-3213 1 , 2 ,
- Abbie Barry ORCID: orcid.org/0000-0002-3859-3660 1 ,
- Michael Mugisha 3 ,
- Lazare Ntirenganya 2 ,
- Ulf Bergman 1 ,
- Emile Bienvenu ORCID: orcid.org/0000-0002-1555-7376 2 , 3 &
- Eleni Aklillu ORCID: orcid.org/0000-0002-9788-0790 1
2893 Accesses
10 Citations
3 Altmetric
Explore all metrics
Introduction
School-based preventive chemotherapy (Deworming) with praziquantel and albendazole to control and eliminate schistosomiasis and soil-transmitted helminths as public health problems is recommended by the World Health Organization (WHO). Safety monitoring during mass drug administration (MDA) is imperative but data from sub-Saharan Africa are scarce.
The aim of this active safety surveillance study was to identify the incidence, type, severity, and risk factors for adverse events (AEs) following mass administration of praziquantel and albendazole.
Overall, 8037 school children aged 5–15 years in Rwanda were enrolled. Baseline sociodemographic, medical history and any pre-existing clinical symptoms were recorded. Participants received a single dose of praziquantel and albendazole during MDA. AEs were actively monitored on days 1, 2, and 7 post MDA.
Overall, 3196 AEs were reported by 1658 children; 91.3%, 8.4%, and 0.3% of the AEs were mild, moderate, and severe, respectively, and most resolved within 3 days. Headache (21%), dizziness or fainting (15.2 %), nausea (12.8%) and stomach pain (12.2%) were the most common AEs. The overall cumulative incidence of experiencing at least one type of AE was 20.6% (95% confidence interval [CI] 19.7–21.5%), being significantly higher ( p < 0.001) in children with pre-MDA clinical events (27.5%, 95% CI 25.4–29.6%) than those without (18.7%, 95% CI 17.7–19.7%). Females, older age, having pre-MDA events, types of food taken before MDA and taking two or more praziquantel tablets were significant predictors of AEs.
Conclusions
Praziquantel and albendazole MDA is safe and well-tolerated; however, one in five children experience transient mild to moderate, and in few cases severe, AEs. The incidence of AEs varies significantly between sex and age groups. Pharmacovigilance in the MDA program is recommended for timely detection and management of AEs.
Similar content being viewed by others
Efficacy and safety of praziquantel treatment against Schistosoma mansoni infection among pre-school age children in southern Ethiopia
Tafese Tadele, Ayalew Astatkie, … Solomon Mequanente Abay
Putting the treatment of paediatric schistosomiasis into context
Takafira Mduluza & Francisca Mutapi
Feasibility and safety of integrating mass drug administration for helminth control with seasonal malaria chemoprevention among Senegalese children: a randomized controlled, observer-blind trial
Muhammed O. Afolabi, Doudou Sow, … Jean Louis A. Ndiaye
Avoid common mistakes on your manuscript.
1 Introduction
Schistosomiasis and soil-transmitted helminths (STH) are among the most prevalent neglected tropical diseases (NTDs) and public health problems that impose a great burden in many parts of the world especially in sub-Saharan Africa (SSA) [ 1 , 2 ]. Globally, over 1.5 billion people, i.e. approximately 24% of the world’s population, are infected with STH. SSA is one of the regions with a high burden of STH infections, and more than 90% of all schistosomiasis cases are from SSA [ 1 , 2 , 3 , 4 ]. Although NTDs are both preventable and treatable, they cause significant morbidity and mortality, especially among children [ 5 , 6 , 7 , 8 ]. Schistosomiasis and STH are both endemic in Rwanda, especially along Lake Kivu where a prevalence of up to 69.5% and 77%, respectively, is reported [ 4 , 9 ].
The World Health Organization (WHO) has introduced a global intervention strategy to control and eliminate these NTDs from being public health problems [ 10 ]. The primary intervention strategy recommended by the WHO is preventive chemotherapy (deworming), a large-scale periodical mass administration of praziquantel and albendazole to all at-risk population groups irrespective of disease status [ 11 ]. Children are at a higher risk of getting these infections and are therefore the main target groups for praziquantel and albendazole mass drug administration (MDA) [ 12 ]. In Rwanda, the annual MDA of praziquantel and biannual MDA of albendazole started in 2008 and several rounds have been conducted since then. More than 1 million school-aged children and 98,017 adults received praziquantel preventive chemotherapy for schistosomiasis and over 5 million preschool and school-aged children received mass albendazole preventive chemotherapy for STH in 2019 [ 13 ].
In most SSA countries, schistosomiasis and STH are coendemic, hence praziquantel and albendazole are coadministered during MDA. Although coadministration of the two drugs targeting the two NTDs is cost effective, several factors, including risk for drug interaction and overlapping toxicities, underscores the need for safety monitoring [ 14 , 15 ]. Safety monitoring is essential to detect the number, type, and severity of AEs and identify risk factors associated with mass albendazole and praziquantel administration-related AEs for timely interventions, thereby minimizing harm and sustaining public confidence in the MDA [ 16 ].
The WHO recommends ensuring safety in public health programs implementing large-scale MDA of anthelminthics drugs, including praziquantel and albendazole [ 17 ]. MDA of praziquantel and albendazole is delivered to all at-risk populations without prior screening or diagnosis, and hence the infection status and/or intensity is unknown. Individuals with heavy infection intensity experience more AEs compared to those with light to moderate infection, partly due to immunologic reactions as a result of parasites’ treatment-induced death [ 18 , 19 , 20 ], therefore it is important to monitor public drug safety during MDA.
Every year, millions of children receive periodic MDA of praziquantel and albendazole in SSA, including Rwanda, but safety data from large-scale active cohort event monitoring (CEM) studies are scarce partly due to lack of fully functional pharmacovigilance systems [ 14 , 21 , 22 ]. The NTD programs prioritize MDA coverage and AEs are often managed and contained at the sites they happened for fear of rumors, which may negatively affect public confidence, and are not reported to the National Medicines Regulatory Authorities (NMRAs) [ 14 , 22 ]. The underreporting of AEs during MDA campaigns makes it challenging to accurately estimate the risks of medicine-related harm and inform healthcare policy and practice [ 14 ]. The WHO recommends active CEM for safety surveillance of medicines used in public health interventions, particularly in areas where passive reporting is challenging [ 23 ].
Few studies that assessed the safety profiles of albendazole and praziquantel in different settings reported transient AEs such as abdominal pain, nausea, vomiting, diarrhea, headache, dizziness, and fainting [ 18 , 20 , 24 , 25 , 26 ]. Safety surveillance studies in various target populations are important since local factors such as comorbidities, nutritional status, genetic and environment differences affect variability in drug responses [ 27 , 28 ]. Despite multiple rounds of MDA, the safety profile of praziquantel and albendazole MDA has not been investigated in Rwanda. Thus, we conducted a large-scale school-based active safety surveillance study to identify the incidence, type, severity, risk factors for AEs, and tolerability of single-dose mass administration of praziquantel and albendazole among school children in Rwanda.
2.1 Study Design, Population, and Setting
This was a prospective, observational CEM study assessing the AEs following MDA of praziquantel and albendazole. The study participants were children attending eight schools in four districts of the Western Province of Rwanda in April 2019. The public did not participate in the design of study. The study was conducted in four districts located around the belt of Lake Kivu, namely Rubavu, Rutsiro, Nyamasheke, and Rusizi in the Western Province (Fig. 1 ). The districts were selected using a purposive sampling method based on (1) schistosomiasis and STH prevalence data from previous studies [ 4 , 9 ]; and (2) inclusion in the national NTD program for distribution of praziquantel and albendazole MDA in 2019. Within each district, two schools were selected based on three criteria: (1) proximity to the lakes; (2) the number of school children attending; and (3) previous schistosomiasis and STH prevalence data. A sample proportion of each school to contribute to the whole study sample was based on each student population size. A total of 8037 children aged 5–15 years from the eight selected schools were enrolled in the safety monitoring study.
Map of Rwanda showing the study districts and selected schools [ 4 ]
The target sample size for our study was 10,000, which was guided based on event frequency according to the WHO’s estimation. The sample size to detect at least three events at a frequency of 1 per 3333 with 95% confidence (95% probability) is 10,000 [ 29 ]. Our sample size of 8037 will detect at least three events at a frequency of 1 per 2679 with 95% confidence.
2.2 Study Enrollment and Data Collection
Before starting the study, awareness creation and sensitization meetings were held with education offices at the district and the Head of district hospitals, health centers, school teachers, school administrators, and parents/guardians. The awareness meetings were held to provide information about the study, including the study objective, significance, procedures, and type of data to collect. The ultimate intention of the awareness meetings was to get permission from the community authorities and school administrators to conduct the study in the selected schools and obtain oral and written informed consent from parents/guardians.
School children aged between 5 and 15 years attending the selected eight schools whose parents or guardians gave informed consent and provided assent to participate were enrolled. They consented to the collection of personal data, including sociodemographic, comorbidities, concomitant medications, nutritional status using anthropometric data, and any medical, clinical, or physical event before and after the MDA. Children whose parents or guardians were not willing to provide informed consent and or dissent were not included in the study; however, they remained able to participate in the national MDA and received preventive chemotherapy deployed under Rwanda's NTD program, but no safety data were collected from them.
Data including sociodemographic, breakfast status, type of meal, concomitant medication, chronic medical condition, and pre-MDA symptoms were collected at baseline using a case record form before receiving MDA through in-person interviews. After receiving MDA, study participants were followed to document treatment-associated AEs on days 1, 2, and 7 post MDA through in-person interviews. Data collection was performed by trained data collectors and were entered in the electronic database using tablets. A data manager reviewed submitted data daily to cross-check and rectify for errors. The study coordinator assigned to each school supervised the study enrolment, data collection, and data entry into the database. For anthropometric measurements, children’s body weight was measured in kilograms (kg) and height was measured in centimeters (cm), and converted to body mass index (BMI)-for-age Z score (BAZ) and height-for-age Z score (HAZ), respectively, using WHO Anthro-Plus software for school-age children [ 30 ]. Children whose HAZ and BAZ scores were < 2 standard deviations were considered stunted and wasted/thin, respectively.
2.3 Exposure and Outcome Variables
2.3.1 exposure definition and measurement.
The main exposure of this study was receiving single-dose albendazole and praziquantel oral administration as preventive chemotherapy provided through MDA under the national NTD program. Study participants received praziquantel according to height for children (≥ 94 cm dose pole, designed to deliver a dose of at least 40 mg/kg) and albendazole 400 mg following the national and WHO MDA guidelines [ 31 , 32 ]. The Rwanda Ministry of Health NTD public health program provided and administered praziquantel and albendazole as preventive chemotherapy to prevent transmission of schistosomiasis and STH. MDA was administered to all children attending the eight schools as scheduled by the Rwanda Ministry of Health. The study team had no role in the MDA planning, and providing or administering the drugs.
2.3.2 Outcome Definition and Measurement
The primary study outcome was the incidence of MDA-associated AEs (post-MDA AEs), defined as any event that was not reported before albendazole and praziquantel administration but occurred after drug exposure. The secondary outcomes were the type and severity of AEs. Prior to MDA, all participants were interviewed for any pre-existing clinical symptoms (pre-MDA event), such as fever, loss of appetite, dizziness or fainting, confusion, drowsiness, headache, cough, difficulty in breathing, nausea, vomiting, diarrhea, stomach pain, itching, rash, and any other symptoms. Study participants were actively and prospectively monitored for any AEs on days 1, 2, and 7 post MDA. Between days 3 and 6, participants only reported if they experienced any AEs.
Events reported by each study participant before and after MDA were cross-checked and verified to differentiate pre-existing clinical symptoms from treatment-associated AEs following praziquantel and albendazole MDA. Events reported after MDA (post MDA) were considered as MDA-associated AEs if the same type of events were not reported before drug intake (pre-MDA). In addition, any event reported on days 2–7 was also considered an MDA-associated AE if the study participant experienced that event on any of the follow-up days but did not experience the same symptom pre MDA and on preceding days.
All reported AEs were graded based on five levels of severity grading using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [ 33 ] as follows.
Grade 1—Mild: Asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
Grade 2—Moderate: Minimal, local, or non-invasive intervention indicated; limiting age-appropriate instrumental activities of daily living (ADL).
Grade 3—Severe or medically significant but not immediately life-threatening: hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL.
Grade 4—Life-threatening consequences: urgent intervention indicated.
Grade 5—Death related to AE
2.4 Statistical Analysis
All data collected in the electronic database were imported into STATA 13 (StataCorp LLC, College Station, TX, USA) for cleaning and analysis. The outcome variable was categorized as a dichotomous variable (having any AE or not), irrespective of the type of AE. Associations between a categorical dependent variable and independent categorical variables were analyzed using the Chi-square test. Predictors of AEs were analyzed by a univariate followed by multivariate binomial logistic regression analysis. Biologically plausible predictor variables with a p value ≤0.2 in the univariate analyses were entered into the final multivariate model for analysis. We used log transformation to change coefficients into incidence risk ratios (IRR) for interpretation without changing their estimations. A p value < 0.05 was considered statistically significant.
2.5 Ethical Consideration
This study was approved by the Rwandan National Ethics Committee (Review Approval Notice No. 0064/RNEC/2019) and the National Health Research Committee of the Ministry of Health, Rwanda (NHRC/2018/PROT/042). Before initiating the study, awareness creation and sensitization meetings were held with education offices at the district and the Head of district hospitals, health centers, school teachers, school administrators, and parents/guardians. Prior to enrolment, participants and their parents or legal guardians received information about the study. For participants ≤ 12 years of age, verbal and written informed consent was obtained from their parent or guardian, and for participants > 12 years of age, verbal and written informed consent was obtained from the parent or guardian and assent was obtained from the study participant.
3.1 Characteristics of Study Participants
The study enrolled 8037 school children between 5 and 15 years of age who received albendazole and praziquantel during the MDA campaign in 2019. The sociodemographic and baseline characteristics of study participants is presented in Table 1 . Of the total 8037 children, 4224 (52.6%) were male and 5112 (63.6%) were between 10 and 15 years of age. The study participants were recruited from eight schools in four districts located along the shores of Lake Kivu in the Western Province of Rwanda. The anthropometric measurements indicated that 2805 children (34.9%) were stunted and 833 (10.4%) were wasted. Of the total study participants, 3301 (41.1%) received one praziquantel tablet, 2415 (30%) received two to three praziquantel tablets, and 2321 (28.9%) received more than three praziquantel tablets. Single-dose albendazole 400 mg was coadministered to all study participants.
3.2 Incidence of Mass Drug Administration (MDA)-Associated Adverse Events (AEs)
The study participants were followed prospectively for 7 days to record all suspected AEs following the MDA. Baseline assessment of self-reported symptoms through interviews were recorded before drug administration. A total of 1795 children (22.3%) of 8037 participants reported at least one type of clinical symptom before taking MDA (pre-MDA event).
A total of 3196 AEs were reported by 1658 children. The overall cumulative incidence of experiencing at least one type of AE among school children was 20.6% (95% confidence interval [CI] 19.7–21.5%, n = 1658) during the 7-day follow-up. The cumulative incidence of experiencing at least one type of MDA-associated AE among those who reported a pre-MDA event (27.5%, 95% CI 25.4–29.6%) was significantly higher ( p < 0.0001) than those who did not report pre-MDA events (18.7%, 95% CI 17.7–19.7%). The study flow chart and findings from the pre-MDA (baseline) and incidence of post-MDA safety monitoring are presented in Fig. 2 .
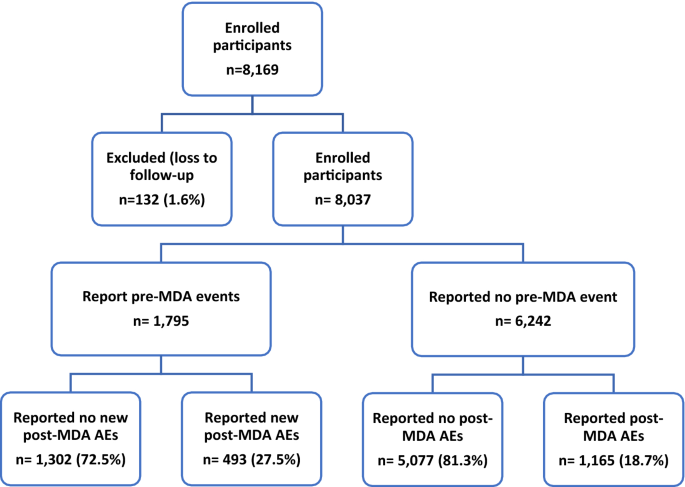
Study flowchart of enrolled participants and follow-up. AEs adverse events, MDA mass drug administration
3.3 Proportion of Various Types of AEs
Overall, 3196 AEs were reported during the 7 days of active surveillance. Headache (21%), dizziness or fainting (15.2%), nausea (12.8%), and stomach pain (12.2%) were the most common AEs following praziquantel and albendazole MDA among school children. The least reported AEs were confusion (1%), other symptoms (1.6%), and rash (1.9%). The proportion of AEs reported during a 7-day follow-up period stratified by type of AE is presented in Fig. 3 .
Proportion of adverse events post mass drug administration (7-day follow-up)
During the 7 days of AE monitoring, the highest number of AEs were observed on day 1 [2588 AEs (81%)] followed by day 2 [570 AEs (17.8%)], and 38 AEs were reported during days 3–7 post MDA. Most AEs reported on day 1 were headache [544 (7.4%)], dizziness or fainting [419 (5.6%)], nausea [352 (4.4%)], and stomach pain [327 (4.3%)]. On day 2, like day 1, headache [118 (1.5%)] and dizziness or fainting [66 (0.9%)] were the most frequently reported AEs. Between days 3 and 7, headache 8 (0.1%) and diarrhea 7 (0.1%) were the most reported AEs, as presented in Fig. 4 .
Cumulative incidence of adverse events per day over a 7-day follow-up.
3.4 Severity Grading of AEs
The severity of the reported AEs (3196) was graded as mild, moderate, or severe, following the CTCAE version 5.0 [ 33 ]. None of the participants reported potentially life-threatening or disabling AEs or death. The incidences of mild, moderate, and severe AEs were 91.3% (number of AEs = 2919), 8.4% (number of AEs = 267), and 0.3% (number of AEs = 10) respectively. The total number of AEs reported as severe included one case of headache, seven cases of cough, and two cases of stomach pain, as described in Table 2 .
3.5 Factors Associated with Post-MDA AEs
The incidence and associations of reported AEs post MDA are presented in Table 3 . Sex was significantly associated with the occurrence of AEs ( p < 0.001), and the incidence of AEs was significantly higher among female participants (23.2%) than male participants (17.7%) ( p < 0.001). Stratifying by sex, all types of reported AEs, except vomiting, cough, and itching and skin rash, were significantly higher in female participants than male participants (Fig. 5 ). Age group was significantly associated with the occurrence of AEs ( p < 0.001); participants between 10 and 15 years of age reported more AEs (24.8%) than those between 5 and 9 years of age (13.4%). The type of food taken before MDA was significantly associated with the occurrence of AEs ( p < 0.001); study participants who had a fatty meal before MDA reported more AEs (30.8%), followed by those who had a high protein meal (29.5%) and those who had a carbohydrate meal (20.4%). The number of praziquantel tablets administered was significantly associated with the occurrence of AEs ( p < 0.001). Study participants who had taken more than three tablets reported more AEs (29.6%), followed by study participants who had taken two to three tablets (21.8%) and those who had taken one tablet (13.5%). Other variables such as stunting, concomitant medication, taking any traditional medicine, and having any chronic medical condition were not significantly associated with the occurrence of AEs.
Cumulative incidence of adverse events stratified by sex. Asterisks indicate statistically significant differences. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001
3.6 Risk Factors Associated with Post-MDA AEs
We analyzed risk factors associated with developing any AE following MDA using univariate and multivariate analysis and the results are presented in Table 4 . Using log binomial regression in univariate analysis, sex, age category, wasting, type of food taken before MDA, and number of praziquantel tablets were significant predictors of developing at least one post-MDA AE. In multivariate analysis using log binomial regression for all variables from univariate analysis with a p value < 0.2, sex, age category, type of food taken, and number of praziquantel tablets administered were significant predictors of developing any post-MDA AEs.
4 Discussion
This observational active safety surveillance study investigated the incidence and associated factors of AEs following mass praziquantel and albendazole administration among children in Rwanda. Our results indicate that about one-fifth of the children who received praziquantel and albendazole as MDA experienced mild-to-moderate AEs, mostly within the first 2 days after MDA. Most AEs were transient and resolved within 1 week after MDA. The overall cumulative incidence of experiencing at least one type of AE was 20.6%. Different studies from SSA reported varying incidence rates of AEs associated with mass praziquantel and albendazole administration. Studies conducted in Kenya (25.3%), Tanzania (28.5%), Angola (55.9%), and Ethiopia (83%) reported higher incidences than our study finding [ 19 , 24 , 25 , 34 ]. Differences in the reported incidence of AEs can be due to variation in the studies, such as data collection methods, AE definition, and/or infection status of the study population (treatment in infected children versus preventive chemotherapy in the target population with unknown infection status). The lower incidence of AEs in our study could be due to our stringent definition set for MDA-associated AEs that excludes pre-existing clinical symptoms.
The incidence rates of MDA-associated AEs were significantly different among schools and the four study districts (Table 3 ). Children attending schools in the Rubavu district had the highest AE incidence, possibly due to a higher prevalence of STH (92%), compared with the other districts Rutsiro (54%), Nyamasheke (60%), and Rusizi (87%) [ 4 ]. Study participants in the Rubavu district may have also had higher infection intensity compared with participants in the other districts. Children with moderate to heavy infection intensity are more likely to experience AEs compared with those with light infection intensity [ 18 , 19 , 20 ]. AEs may be induced by drugs or immunologic reaction due to the killing of the parasites mostly in heavy infection intensity [ 25 , 35 ]. The occurrence of AEs could be influenced by other factors such as age, co-infections or comorbidities, concomitant medication, nutritional status, socioeconomic conditions, and environmental exposure [ 25 , 36 , 37 ]. A recent study from Kenya reported concomitant medications and chronic illness as significant risk factors for developing AEs following diethylcarbamazine citrate and albendazole MDA [ 38 ]. In our study, we did not find any significant association between chronic illness/comorbidity or concomitant traditional medicine use with AEs. This could be because of the small number of children who had chronic illness/comorbidity (2.7%) in our study population, and hence a lack of adequate power to detect any such association. On the other hand, we found that children who reported pre-MDA events experienced more post-MDA AEs (28%) compared with children who did not report pre-MDA events (19%). The association of having pre-MDA clinical symptoms with higher incidence of MDA-associated AEs has also been reported recently [ 38 , 39 ]. Therefore, children with underlying clinical symptoms should be closely monitored after MDA.
The incidence of AEs varies depending on the type of AEs. A meta-analysis that evaluated the clinical efficacy and tolerability of praziquantel in the treatment of intestinal and urinary schistosomiasis reported that the incidence of AEs ranged from 2.3% for urticaria to 31.1% for abdominal pain [ 40 ]. The most observed AEs in our study were headache (21%), dizziness or fainting (15.2%), nausea (12.8%), and stomach pain (12.2%), as similarly reported by other studies from Angola, Ethiopia, Kenya, and Tanzania [ 19 , 24 , 25 , 34 ]. Wide interindividual variations in plasma praziquantel concentrations partly due to pharmacogenetic variations has recently been reported [ 41 , 42 ]. Any association of praziquantel pharmacokinetics and pharmacogenetic variation with susceptibility to treatment-induced AEs remains to be investigated. Generally, the AEs occurred within days 1 and 2 of post drug administration and were transient; most resolved by day 3 (Fig. 4 ). These findings are in line with previous studies where the majority of the reported AEs (80.8%) resolved within a day or two [ 24 , 43 ].
Among the reported AEs, 91.3% were mild, 8.4% were moderate, and 0.3 % were graded as severe, which is in line with reports from other studies, including a randomized controlled trial and meta-analysis [ 19 , 24 , 44 , 45 ]. However, a recent study conducted in India reported severe and serious AEs among children who received albendazole alone [ 46 ]. The severity of AEs could potentially be due to several factors, including, but not limited to, infection intensity and nutritional and health status. Thus, safety surveillance during praziquantel and albendazole mass administration in various settings is imperative.
The current study revealed that older age, having pre-MDA events, type of food taken before MDA, and increased number of praziquantel tablets taken were significant risk factors for developing at least one AE post MDA (Table 4 ). All study participants received one tablet of albendazole (400 mg) regardless of age, but the dose of praziquantel was based on the height of the child, corresponding to 40 mg/kg as recommended by the WHO MDA guideline [ 31 ]. Thus, the observed higher incidence of AEs among older children could be due to the increased number of praziquantel tablets administered. Indeed, taking two or more praziquantel tablets was a significant predictor of AEs; however, after adjusting for the number of praziquantel tablets, the older age group still had an increased risk for AEs. This could be due to the higher STH prevalence in the older age group, which might be associated with a higher risk of AEs, as previously described [ 4 , 36 ]. Interestingly, children who had a fatty or high protein diet before MDA had a higher risk of experiencing more AEs than those who had a carbohydrate meal. In line with our findings, a recent study reported the association of having a fatty or high protein meal before MDA with a higher incidence of AEs following mass diethylcarbamazine and albendazole administration for the elimination of lymphatic filariasis in Kenya [ 38 ]. Altered drug absorption and bioavailability with a fatty or high protein diet has been previously reported [ 47 ]. Accordingly, food–drug interactions influencing drug absorption could possibly alter the susceptibility to AEs following praziquantel and albendazole MDA.
Our findings indicate that females are at a higher risk of experiencing more AEs than males. Similar to our findings, another study assessing the safety of praziquantel and albendazole in Kenya reported that females and older children (≥ 10 years) experienced more AEs [ 24 ]. A higher incidence of MDA-associated AEs in females than males who received preventive chemotherapy for the prevention of lymphatic filariasis has also been recently reported [ 38 , 39 ]. Generally, women have a higher risk of developing AEs compared with men, however the reason for this is unclear but is possibly due to physiological and sex-related hormonal differences, which can affect drug metabolism [ 38 , 48 , 49 ].
To our knowledge, this is the first and largest active CEM study to investigate the incidence, type, severity, and predictors for AEs following mass praziquantel and albendazole administration for the control and elimination of schistosomiasis and STHs in Rwanda and SSA. The strength of our study is the large sample size, thereby enhancing the ability to detect rare AEs as recommended by the WHO. Secondly, assessment of the AEs was conducted using active surveillance for up to 7 days post MDA. This provided a chance to explore the immediate AEs following drug administration and those that can occur at a later stage. However, as mass albendazole and praziquantel administration is given to all eligible school children without a prior diagnosis, we could not compare the incidence of AEs between schistosomiasis and/or STH-infected versus healthy children, and this may subsequently be considered a limitation of our study.
5 Conclusions
Single-dose praziquantel and albendazole administered during MDA for the control of schistosomiasis and soil-transmitted helminthiasis among school children is safe and tolerable. However, the finding that one in five children experience transient mild-to-moderate AEs and few cases of severe AEs underlines the need to integrate pharmacovigilance in MDA campaigns. Our study suggests being female, older age, taking two or more praziquantel tablets and having pre-existing clinical conditions or symptoms are independent risk factors for AEs following praziquantel and albendazole MDA. Our study highlights the importance of integrating pharmacovigilance into the national NTD public health program and safety monitoring during MDA for the timely detection and management of AEs.
World Health Organization. Schistosomiasis Fact Sheet. Geneva; World Health Organization; 2020. https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis . Accessed 31 Jan 2020.
World Health Organization. Soil-transmitted helminth infections. Geneva: World Health Organization; 2021. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections . Accessed 15 Jan 2022.
Gebreyesus TD, Tadele T, Mekete K, Barry A, Gashaw H, Degefe W, et al. Prevalence, intensity, and correlates of schistosomiasis and soil-transmitted helminth infections after five rounds of preventive chemotherapy among school children in Southern Ethiopia. Pathogens. 2020;9:1–14.
Article Google Scholar
Kabatende J, Mugisha M, Ntirenganya L, Barry A, Ruberanziza E, Mbonigaba JB, et al. Prevalence, intensity, and correlates of soil-transmitted helminth infections among school children after a decade of preventive chemotherapy in Western Rwanda. Pathogens. 2020;9:1–20.
Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8: e2865.
Global Atlas of Helminth Infections. Global burden; 2018. http://www.thiswormyworld.org/worms/global-burden . Accessed 21 May 2022.
Hall A, Hewitt G, Tuffrey V, De Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4:118–236.
Pabalan N, Singian E, Tabangay L, Jarjanazi H, Boivin MJ, Ezeamama AE. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1): e0005523.
Rujeni N, Morona D, Ruberanziza E, Mazigo HD. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: an update on their epidemiology and control. Infect Dis Poverty. 2017;6:1–11.
World Health Organization. Eliminating soil-transmitted helminthiases as a public health problem in children. Progress report 2001−2010 and strategic plan 2011−2020; Geneva; World Health Organization; 2012. https://www.who.int/publications/i/item/9789241503129 . Accessed 21 Jan 2021.
World Health Organization. Update on the global status of implementation of preventive chemotherapy (PC). World Health Organization; 2020. https://www.who.int/neglected_diseases/preventive_chemotherapy/PC_Update.pdf?ua =. Accessed 26 Feb 2021.
World Health Organization. Guideline: preventive chemotherapy to control soil-transmitted helminths infections in at risk population groups. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2017.
Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Rwanda: 2019. https://espen.afro.who.int/countries/rwanda . Accessed 6 Sept 2021.
Barry A, Olsson S, Khaemba C, Kabatende J, Dires T, Fimbo A, et al. Comparative assessment of the pharmacovigilance systems within the neglected tropical diseases programs in East Africa—Ethiopia, Kenya, Rwanda, and Tanzania. Int J Environ Res Public Health. 2021;18:1–13.
Minzi OM, Mnkugwe RH, Ngaimisi E, Kinung’hi S, Hansson A, Pohanka A, et al. Effect of dihydroartemisinin-piperaquine on the pharmacokinetics of praziquantel for treatment of schistosoma mansoni infection. Pharmaceuticals. 2021;14:1–12.
Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36:75–81.
World Health Organization. Assuring Safety of Preventive Chemotherapy Interventions for the Control of Neglected Tropical Diseases. Geneva: World Health Organization; 2011. Available at: https://apps.who.int/iris/handle/10665/44683 . Accessed 10 Jan 2021.
Zwang J, Olliaro P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: a meta-analysis. Parasites Vectors. 2017;10:1–16.
Mnkugwe RH, Minzi OS, Kinungʹhi SM, Kamuhabwa AA, Aklillu E. Efficacy and safety of praziquantel for treatment of schistosoma mansoni infection among school children in Tanzania. Pathogens. 2020;9:1–12.
Google Scholar
Olds GR, King C, Hewlett J, Olveda R, Wu G, Ouma J, et al. Double-blind placebo-controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. J Infect Dis. 1999;179:996–1003.
Article CAS Google Scholar
Barry A, Olsson S, Minzi O, Bienvenu E, Makonnen E, Kamuhabwa A, et al. Comparative assessment of the national pharmacovigilance systems in East Africa: Ethiopia, Kenya, Rwanda and Tanzania. Drug Saf. 2020;43:339–50.
Kiguba R, Olsson S, Waitt C. Pharmacovigilance in low- and middle-income countries: a review with particular focus on Africa. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.15193 .
Article PubMed Google Scholar
World Health Organization. The Safety of Medicines in Public health programmes: pharmacovigilance an essential tool. WHO Library Catalog Data; 2006:61. https://apps.who.int/iris/handle/10665/43384 . Accessed 20 May 2022.
Njenga SM, Ng’Ang’a PM, Mwanje MT, Bendera FS, Bockarie MJ. A school-based cross-sectional survey of adverse events following co-administration of albendazole and praziquantel for preventive chemotherapy against urogenital schistosomiasis and soil-transmitted helminthiasis in Kwale County, Kenya. PLoS One. 2014;9:6–10.
Lemos M, Pedro JM, Fançony C, Moura S, Brito M, Nery SV, et al. Schistosomiasis and soil-transmitted helminthiasis preventive chemotherapy: adverse events in children from 2 to 15 years in Bengo province, Angola. PLoS One. 2020;15:1–13.
Raso G, N’Goran EK, Toty A, Luginbühl A, Adjoua CA, Tian-Bi NT, et al. Efficacy and side effects of praziquantel against Schistosoma mansoni in a community of western Côte d’Ivoire. Trans R Soc Trop Med Hyg. 2004;98:18–27.
Yimer G, Amogne W, Habtewold A, Makonnen E, Ueda N, Suda A, et al. High plasma efavirenz level and CYP2B66 are associated with efavirenz-based HAART-induced liver injury in the treatment of naïve HIV patients from Ethiopia: a prospective cohort study. Pharmacogenomics J. 2012;12:499–506.
Mugusi S, Ngaimisi E, Janabi M, Minzi O, Bakari M, Riedel KD, et al. Liver enzyme abnormalities and associated risk factors in HIV patients on efavirenz-based HAART with or without tuberculosis co-infection in Tanzania. PLoS One. 2012;7:1–9.
World Health Organization. Cohort event monitoring (CEM) for safety signal detection after vaccination with COVID-19 vaccines. Geneva: World Health Organization; 2021. https://www.who.int/publications/i/item/10665338400 . Accessed 10 Feb 2022.
World Health Organization. WHO Anthroplus Software; Software for assessing Growth and Development of the World’s Children and Adolescents. Department of Nutrition for Health and Development. Geneva: World Health Organization; 2009.
World Health Organization. Preventive chemotherapy in human helminthiasis. Geneva: World Health Organization; 2006. http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf . Accessed 30 Jan 2021.
Montresor A, Odermatt P, Muth S, Iwata F, Raja’a YA, Assis AM, et al. The WHO dose pole for the administration of praziquantel is also accurate in non-African populations. Trans R Soc Trop Med Hyg. 2005;99:78–81.
Cancer Therapy Evaluation Program (CTEP). Common Terminology Criteria for Adverse Events (CTCAE).v.5.0 [5x7]. Cancer Therapy Evaluation Program; 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 . Accessed 11 Oct 2021.
Erko B, Degarege A, Tadesse K, Mathiwos A, Legesse M. Efficacy and side effects of praziquantel in the treatment of Schistosomiasis mansoni in schoolchildren in Shesha Kekele Elementary School, Wondo Genet, Southern Ethiopia. Asian Pac J Trop Biomed. 2012;2:235–9.
Hong S. Albendazole and praziquantel: review and safety monitoring in Korea. Infect Chemother. 2018;50:1–10.
Samuel F, Degarege A, Erko B. Efficacy and side effects of albendazole currently in use against Ascaris, Trichuris and hookworm among school children in Wondo Genet, southern Ethiopia. Parasitol Int. 2014;63:450–5.
Sousa-Figueiredo JC, Betson M, Atuhaire A, Arinaitwe M, Navaratnam AMD, Kabatereine NB, et al. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl Trop Dis. 2012;6(10): e1864. https://doi.org/10.1371/journal.pntd.0001864 .
Khaemba C, Barry A, Omondi WP, Bota K, Matendechero S, Wandera C, et al. Safety and tolerability of mass diethylcarbamazine and albendazole administration for the elimination of lymphatic filariasis in Kenya: an active surveillance study. Pharmaceuticals (Basel). 2021;14(3):264.
Fimbo AM, Minzi OM, Mmbando BP, Gurumurthy P, Kamuhabwa AAR, Aklillu E. Safety and tolerability of ivermectin and albendazole mass drug administration in lymphatic filariasis endemic communities of Tanzania: a cohort event monitoring study. Pharmaceuticals (Basel). 2022;15(5):594.
Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis: a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8(11): e3286.
Mnkugwe RH, Minzi O, Kinung’hi S, Kamuhabwa A, Aklillu E. Effect of pharmacogenetics variations on praziquantel plasma concentrations and schistosomiasis treatment outcomes among infected school-aged children in Tanzania. Front Pharmacol. 2021;12:712084.
Mnkugwe RH, Ngaimisi KE, Kinung’hi S, Kamuhabwa AAR, Minzi OM, Aklillu E. Optimal single sampling time-point for monitoring of praziquantel exposure in children. Sci Rep. 2021;11(1):17955.
Njomo DW, Tomono N, Muhoho N, Mitsui Y, Josyline KC, Mwandawiro CS. The adverse effects of albendazole and praziquantel in mass drug administration by trained schoolteachers. Afr J Health Sci. 2010;17:10–4.
Coulibaly JT, Panic G, Silué KD, Kovač J, Hattendorf J, Keiser J. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial. Lancet Glob Health. 2017;5:e688–98.
Olliaro PL, Coulibaly JT, Garba A, Halleux C, Keiser J, King CH, et al. Efficacy and safety of single 40 mg/kg oral praziquantel in the treatment of schistosomiasis in preschool-age versus school-age children: an individual participant data meta-analysis. PLoS Negl Trop Dis. 2020;14(6): e0008277.
Agrawal P, Srivastava B, Bhardwaj R, Gaur S. Adverse events of albendazole due to mass drug administration. Int J Basic Clin Pharmacol. 2017;6:1674–7.
Ayo JA, Agu H, Madaki I. Food and drug interactions: its side effects. Nutr Food Sci. 2005;35:243–52.
de Vries ST, Denig P, Ekhart C, Burgers JS, Kleefstra N, Mol PGM, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85:1507–15.
Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17: 100188.
Download references
Acknowledgements
The authors would like to extend their sincere thanks to the school directors and teachers from the schools that participated in the study for their support during the data collection. They also thank the parents/guardians and school children from all eight schools that participated in the study. The authors also appreciate the technical support from the National Reference Laboratory, and data managers from hospitals and health centers who contributed to make this study feasible.
Open access funding provided by Karolinska Institute.
Author information
Authors and affiliations.
Division of Clinical Pharmacology, Department of Laboratory Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, 14186, Stockholm, Sweden
Joseph Kabatende, Abbie Barry, Ulf Bergman & Eleni Aklillu
Rwanda Food and Drugs Authority, Nyarutarama Plaza, KG 9 Avenue, Kigali, Rwanda
Joseph Kabatende, Lazare Ntirenganya & Emile Bienvenu
College of Medicine and Health Sciences, University of Rwanda, KK 737, Kigali, Rwanda
Michael Mugisha & Emile Bienvenu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eleni Aklillu .
Ethics declarations
This study was conducted as part of the Pharmacovigilance Infrastructure and Postmarketing Surveillance System Capacity Building for Regional Medicine Regulatory Harmonization in East Africa (PROFORMA) project funded by the European and Developing Countries Clinical Trials Partnership (EDCTP) 2 program supported by the European Union (Grant number CSA2016S-1618) and the Swedish International Development Cooperation Agency (SIDA).
Author contributions
Conceptualization: AB, JK, MM, LN, EB and EA. Data curation: JK, AB, MM, LN, and EA. Formal analysis: AB, JK, LN, and MM. Investigation: JK, AB, EB, MM, and EA. Methodology: AB, JK, EB, and EA. Supervision: EB, UB, and EA. Writing—original draft: JK and AB. Writing—review and editing: AB, JK, MM, LN, EB, UB, and EA. All authors read and approved the final version.
Conflict of interest
Joseph Kabatende, Abbie Barry, Michael Mugisha, Lazare Ntirenganya, Ulf Bergman, Emile Bienvenu, and Eleni Aklillu declare no conflicts of interest.
Ethics approval
This study was approved by the Rwandan National Ethics Committee (Review Approval Notice No. 0064/RNEC/2019) and the National Health Research Committee of the Ministry of Health, Rwanda (NHRC/2018/PROT/042).
Consent to participate
Participant consent was documented on a written informed consent form, which was accompanied by written information for parents/guardians, and the informed consent forms were approved by the same Ethics Committees that approved this protocol.
Consent for publication
Not applicable.
Availability of data and material
Data are available upon reasonable request owing to privacy and ethical restrictions from the authors.
Code availability
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Kabatende, J., Barry, A., Mugisha, M. et al. Safety of Praziquantel and Albendazole Coadministration for the Control and Elimination of Schistosomiasis and Soil-Transmitted Helminths Among Children in Rwanda: An Active Surveillance Study. Drug Saf 45 , 909–922 (2022). https://doi.org/10.1007/s40264-022-01201-3
Download citation
Accepted : 06 June 2022
Published : 11 July 2022
Issue Date : August 2022
DOI : https://doi.org/10.1007/s40264-022-01201-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
Ubenimex combined with Albendazole for the treatment of Echinococcus multilocularis -induced alveolar echinococcosis in mice
Affiliations.
- 1 Research Center for High Altitude Medicine of Qinghai University, Xining, Qinghai, China.
- 2 Key Laboratory of High Altitude Medicine in Qinghai Provincial, Qinghai University, Xining, Qinghai, China.
- 3 Department of Medical Imaging Center, Qinghai University Affiliated Hospital, Xining, Qinghai, China.
- PMID: 38585297
- PMCID: PMC10995866
- DOI: 10.3389/fvets.2024.1320308
Introduction: Alveolar echinococcosis (AE) is a parasitic disease caused by E. multilocularis metacestodes and it is highly prevalent in the northern hemisphere. We have previously found that vaccination with E. multilocularis -Leucine aminopeptidase (EM-LAP) could inhibit the growth and invasion of E. multilocularis in host liver, and Ubenimex, a broad-spectrum inhibitor of LAP, could also inhibit E. multilocularis invasion but had a limited effect on the growth and development of E. multilocularis .
Methods: In this study, the therapeutic effect of Ubenimex combined with Albendazole on AE was evaluated. Mice were intraperitoneally injected with protoscoleces and imaging examination was performed at week 8 and week 16 to detect cyst change. During this period, mice were intraperitoneally injected with Ubenimex and intragastrically administered with Albendazole suspension. At last, the therapeutic effect was evaluated by morphological and pathological examination and liver function.
Results: The results revealed that the combined treatment could inhibit the growth and infiltration of cysts in BALB/c mice infected with E. multilocularis protoscoleces . The weight, number, invasion and fibrosis of cysts were reduced in mice treated with Ubenimex in combination with Albendazole. The same effect was achieved by the single Ubenimex treatment because of its inhibitory effect on LAP activity, but it was less effective in inhibiting the growth of cysts. The levels of ALT, AST, TBIL, DBIL, ALP, and γ-GT were reduced after the combined treatment, indicating that treatment with both Ubenimex and Albendazole could alleviate liver damage.
Discussion: This study suggests that the combined treatment with Ubenimex and Albendazole could be a potential therapeutic strategy for E. multilocularis infections.
Keywords: Albendazole; E. multilocularis; LAP; Ubenimex; liver; mice.
Copyright © 2024 Zhou, Huayu, Mu, Tang and Ge.
Grants and funding
Loading metrics
Open Access
Peer-reviewed
Research Article
Therapeutic efficacy of albendazole against soil-transmitted helminthiasis in children measured by five diagnostic methods
Contributed equally to this work with: Johnny Vlaminck, Piet Cools
Roles Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation Department of Virology, Parasitology and Immunology, Ghent University, Merelbeke, Belgium
Roles Conceptualization, Funding acquisition, Resources, Writing – review & editing
Affiliations Center for Tropical Diseases, Sacro Cuore Don Calabria Hospital, Negrar, Italy, Department of Life Sciences and Systems Biology, University of Turin, Turin, Italy
Roles Investigation, Resources, Writing – review & editing
Affiliation Laboratory Division, Public Health Laboratory-Ivo de Carneri, Chake Chake, United Republic of Tanzania
Roles Investigation, Methodology, Resources, Writing – review & editing
Affiliation Jimma University Institute of Health, Jimma University, Jimma, Ethiopia
Roles Resources, Writing – review & editing
Affiliation Department of Veterinary Medicine and Animal Production, University of Naples Federico II, Naples, Italy
Roles Investigation, Writing – review & editing
Roles Conceptualization, Funding acquisition, Methodology, Writing – review & editing
Affiliations Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Basel, Switzerland, University of Basel, Basel, Switzerland
Affiliations Laboratory of Molecular and Cellular Immunology, Research Center René Rachou—FIOCRUZ, Belo Horizonte, Brazil, Nursing school, Federal University of Minas Gerais, Brazil
Affiliation Department of Control of Neglected Tropical Diseases, World Health Organization, Geneva, Switzerland
Roles Resources, Software, Writing – review & editing
Affiliation Techion Group Ltd, Dunedin, New Zealand
Affiliation Laboratory of Molecular and Cellular Immunology, Research Center René Rachou—FIOCRUZ, Belo Horizonte, Brazil
Affiliation Lao Tropical and Public Health Institute, Ministry of Health, Vientiane, Lao People's Democratic Republic
Affiliation Techion Group Ltd, Aberystwyth, United Kingdom
Roles Conceptualization, Funding acquisition, Writing – review & editing
Affiliation Laboratory for Medical Microbiology and Immunology, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands
- [ ... ],
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
- [ view all ]
- [ view less ]
- Johnny Vlaminck,
- Piet Cools,
- Marco Albonico,
- Shaali Ame,
- Mio Ayana,
- Giuseppe Cringoli,
- Daniel Dana,
- Jennifer Keiser,
- Maria P. Maurelli,

- Published: August 1, 2019
- https://doi.org/10.1371/journal.pntd.0007471
- Reader Comments
Preventive chemotherapy (PC) with benzimidazole drugs is the backbone of soil-transmitted helminth (STH) control programs. Over the past decade, drug coverage has increased and with it, the possibility of developing anthelmintic resistance. It is therefore of utmost importance to monitor drug efficacy. Currently, a variety of novel diagnostic methods are available, but it remains unclear whether they can be used to monitor drug efficacy. In this study, we compared the efficacy of albendazole (ALB) measured by different diagnostic methods in a head-to-head comparison to the recommended single Kato-Katz.
An ALB efficacy trial was performed in 3 different STH-endemic countries (Ethiopia, Lao PDR and Tanzania), each with a different PC-history. During these trials, stool samples were evaluated with Kato-Katz (single and duplicate), Mini-FLOTAC, FECPAK G2 , and qPCR. The reduction rate in mean eggs per gram of stool (ERR) and mean genome equivalents / ml of DNA extract (GERR) were calculated to estimate drug efficacy.
Principal findings and conclusions
The results of the efficacy trials showed that none of the evaluated diagnostic methods could provide reduction rates that were equivalent to a single Kato-Katz for all STH. However, despite differences in clinical sensitivity and egg counts, they agreed in classifying efficacy according to World Health Organization (WHO) guidelines. This demonstrates that diagnostic methods for assessing drug efficacy should be validated with their intended-use in mind and that other factors like user-friendliness and costs will likely be important factors in driving the choice of diagnostics. In addition, ALB efficacy against STH infections was lower in sites with a longer history of PC. Yet, further research is needed to identify factors that contribute to this finding and to verify whether reduced efficacy can be associated with mutations in the β-tubulin gene that have previously been linked to anthelmintic resistance.
Trial registration
ClinicalTrials.gov NCT03465488 .
Author summary
During the last decade, the scale of deworming programs that aim to eliminate the morbidity caused by intestinal worms has increased to a level that is unprecedented in history. It is therefore of utmost importance to monitor any change in therapeutic efficacy that may arise from emerging drug resistance. Currently, a variety of novel methods have been described, but it remains unclear whether they can be used for monitoring drug efficacy. We applied different diagnostic methods to measure the efficacy of a commonly administered drug in deworming programs in 3 countries with different historical exposure to deworming programs. Compared to the standard diagnostic method, all diagnostic methods revealed good agreement in classifying the therapeutic efficacy according to World Health Organization guidelines, despite clear differences in diagnostic performance. We also noticed that the drug efficacy was lower in countries where drug pressure has been high. However, more research is necessary to identify factors that explain this variation in drug efficacy, including but not limited to the frequency in mutations in genes that are known to be linked with anthelmintic resistance.
Citation: Vlaminck J, Cools P, Albonico M, Ame S, Ayana M, Cringoli G, et al. (2019) Therapeutic efficacy of albendazole against soil-transmitted helminthiasis in children measured by five diagnostic methods. PLoS Negl Trop Dis 13(8): e0007471. https://doi.org/10.1371/journal.pntd.0007471
Editor: Cinzia Cantacessi, University of Cambridge, UNITED KINGDOM
Received: February 4, 2019; Accepted: May 16, 2019; Published: August 1, 2019
Copyright: © 2019 Vlaminck et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: BL is a postdoctoral fellow of the Research Foundation ( www.FWO.be ). JV is financially supported through an International Coordination Action of the Flemish Research Foundation. This study and PC were financially supported by a grant from the Bill and Melinda Gates foundation (OPP1120972, PI is BL, www.starworms.org ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The FECPAKG2 technology was produced and distributed by Techion Group Ltd, of which ET is an employee and GM is managing director. Both hold stocks in Techion Group Ltd. The Mini-FLOTAC device is a commercial product distributed by GC, LR and MPM through the University of Napoli Federico II. However, their affiliations did not play any role in the preparation and submission of this manuscript. All other authors declared that they have no competing interests.
Introduction
Infections with soil-transmitted helminths (STHs; Ascaris lumbricoides , Trichuris trichiura , Necator americanus and Ancylostoma duodenale ) are responsible for the highest burden among all neglected tropical diseases. Recent global estimates indicate that in 2015, more than 1.6 billion people were infected with at least one of the four STH species [ 1 ], resulting in a global burden of approximately 1.9 million disability-adjusted life years [ 2 ]. Preventive chemotherapy (PC) or the periodical administration of a single-oral dose of albendazole (ALB; 400 mg) or mebendazole (MEB; 500 mg) to preschool- (PSAC) and school-aged children (SAC) is the main strategy to control the morbidity caused by STHs [ 3 ]. In 2017, global coverage of PC in at-risk populations was nearly 70%, though the target is to reach 75% coverage by 2020, and to eventually eliminate soil-transmitted helminthiasis as a public health problem [ 4 – 6 ]. The latter target is defined by reaching less than 1% moderate and heavy intensity infections in SAC [ 5 ].
The downside of these increased control efforts is that resistance to anthelmintic drugs, such as ALB and MEB, is likely to develop. Both drugs belong to the same drug class (benzimidazole (BZ) drugs) and share the same mode of action. Moreover, they are administered in single doses that usually do not achieve 100% efficacy [ 7 – 10 ]. Should anthelmintic resistance (AR) against these BZ drugs eventually emerge and spread, it will jeopardize PC-based control of STH due to the few acceptable alternative treatment options [ 11 , 12 ]. All this reinforces the urgent need to promote accessibility of anthelmintic drugs with different modes of action, alone or in combination, and a thoroughly designed surveillance system that detects any changes in anthelmintic drug efficacy arising through the evolution of AR.
The World Health Organization advises to monitor drug efficacy in case treatment failure is suspected or–regardless of suspected drug failure–when drugs have been administered in PC-programs for at least four years [ 13 ]. To monitor the efficacy of anthelmintic drugs against STHs, WHO currently recommends measuring the reduction in number of STH eggs excreted in stool after drug administration (egg reduction rate, ERR) using either a single Kato-Katz thick smear or the McMaster method [ 13 ].
Recently, novel methods have been introduced in the field of STH diagnostics, including Mini-FLOTAC [ 14 , 15 ], FECPAK G2 [ 16 , 17 ] and the DNA-based diagnostic methods such as quantitative PCR (qPCR) [ 18 – 20 ]. Each of these methods offers one or more advantages over the recommended methods, pertaining to increased clinical sensitivity [ 15 , 18 , 21 – 23 ] and specificity (qPCR is able to differentiate different helminths at the species level) [ 24 – 27 ], quality assurance (FECPAK G2 automatically stores images of each sample which can be consulted at any time [ 17 , 28 ]; qPCR includes internal controls within each run [ 29 ]), flexibility as to when samples are examined (for both Mini-FLOTAC and qPCR, stool can be preserved for analysis at a later time point [ 23 , 25 , 30 – 32 ]). Although each of these novel methods has recently been used to evaluate drug efficacy [ 16 , 33 , 34 ], there remains a paucity of studies that perform a head-to-head comparison of the drug efficacy obtained by different diagnostic methods. Moreover, these studies tested the hypothesis that the methods provide significantly different ERR estimates. Rather, the correct hypothesis is to assess whether these differences are within the bounds of equivalence. As illustrated in supplementary information ( S1 Info ), the absence of a significant difference does not imply equivalent ERR estimates nor does the presence of a significant difference rule out equivalent ERR results.
Therefore, in this study we compared the equivalence in ALB efficacy measured by duplicate Kato-Katz thick smear, Mini-FLOTAC, FECPAK G2 and qPCR in a head-to-head comparison with a single Kato-Katz thick smear. For this, a drug efficacy trial with ALB was performed in three different countries (Ethiopia, Lao PDR and Pemba (Tanzania)) with different historical levels of drug exposure.
Ethics statement
The study protocol has been reviewed and approved by the Institutional Review Board (IRB) of the Faculty of Medicine and Health Sciences of Ghent University, Belgium (Ref. No B670201627755; 2016/0266). The trial protocol was subsequently reviewed and approved by the IRBs associated with each trial site (Ethical Review Board of Jimma University, Jimma, Ethiopia: RPGC/547/2016; National Ethics Committee for Health Research, Vientiane, Lao PDR: 018/NECHR; Zanzibar Medical Research and Ethics Committee, United Republic of Tanzania: ZAMREC/0002/February/2015 and the IRB from Centro de Pesquisas René Rachou, Belo Horizonte, Brazil: 2.037.205). The trial was retrospectively registered on Clinicaltrials.gov (ID: NCT03465488) on March 7, 2018.
Parent(s)/guardians of participants signed an informed consent document indicating that they understood the purpose and procedures of the study, and that they allowed their child to participate. If the child was ≥5 years, he or she had to orally assent in order to participate. Participants of ≥12 years of age were only included if they signed an informed consent document indicating that they understood the purpose and the procedures of the study, and were willing to participate.
Study design and population
The selection of the study sites was based on their experience in assessing drug efficacy, evaluating the performance of diagnostic methods, the availability of well-equipped diagnostic facilities and skilled personnel, and PC-history [ 35 ]. Based on the reported national coverage of drug administration to both PSAC and SAC for the last 5 years (2009–2014; Preventive Chemotherapy Database of the WHO), the site in Ethiopia was considered to have experienced a low drug exposure, the site in Lao PDR a medium drug exposure and the site in Pemba (Tanzania) a high drug exposure prior to the start of the trials [ 35 ] ( Table 1 ). Note that the initial study protocol included a study site in Brazil. However, due to the low number of cases on which not all diagnostic methods were performed, the site was excluded from this report.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pntd.0007471.t001
The trials were designed to assess an equivalence in treatment efficacy of a single oral dose of 400 mg ALB against STH infections in SAC measured by a variety of diagnostic methods. The study focused on SAC (age 5–14) since they are the major target of PC programs, and they usually represent the group with highest worm burdens for A . lumbricoides and T . trichiura [ 36 ]. Subjects were not included in the study if they could not provide a stool sample at baseline or follow-up and had active diarrhea or any other acute medical condition at baseline. Children with a known hypersensitivity to ALB or MEB, who received anthelmintic treatment within 90 days prior to the start of the trial were and did not swallow the entire drug tablet or vomited within four hours following drug ingestion were also excluded from the study.
At the start of each trial, schools were visited by the local principal investigator and a team of field officers, who explained the planned trial and sampling method to the parents and teachers and the children. At baseline, SAC were asked to provide a fresh stool sample, after which they were administered a single oral dose of 400 mg ALB under supervision. The ALB used in the different studies originated from the same production batch (GlaxoSmithKline Batch N°: 335726) and was provided by WHO. All collected stool samples were kept in a cooler with ice packs while transported to the laboratory, where they were processed on the same day of collection. Stool samples were processed to determine the fecal egg counts (FECs; expressed in eggs per gram of stool (EPG)) for each STH using Kato-Katz (single and duplicate), Mini-FLOTAC and FECPAK G2 . As FECs of the latter technique could not finalized on the day of sample collection (see section Diagnostic methods), results of the FECPAK G2 technique were not used to select individuals for inclusion at follow-up. Aliquots of a subset of the baseline samples were preserved in ethanol for molecular analysis. Preliminary data has indicated that downstream analysis of STH β-tubulin genes was very challenging when egg concentration was low (data not published). Therefore, only samples with a FEC of ≥150 EPG for at least one STH species were withheld for further molecular analysis. Fourteen to 21 days after drug administration, a second stool sample was collected from all the children that were found positive for any STH by duplicate Kato-Katz or Mini-FLOTAC at baseline. Stool samples collected at follow-up were again examined by Kato-Katz (single and duplicate), Mini-FLOTAC and FECPAK G2 . Aliquots from all follow-up samples were preserved for further molecular analysis regardless of the FECs.
Sample size calculation
A sample size was calculated to test the hypothesis that FECPAK G2 , Mini-FLOTAC and duplicate Kato-Katz provide equivalent drug efficacy results measured by ERRs compared to a single Kato-Katz. This sample size calculation did not include the qPCR method. Given the differences in drug efficacy of ALB across the STH species [ 8 , 9 ] ( A . lumbricoides : ~99%, hookworms: ~96%, T . trichiura : ~65%), a level of equivalence that is acceptable for T . trichiura may not be acceptable for A . lumbricoides . We therefore applied a level of equivalence that was tailored to the different STH species. The level of equivalence for A . lumbricoides , hookworms and T . trichiura was set arbitrarily at -/+2.5, -/+5.0 and -/+10-point percentage respectively. This means that a method provides equivalent drug efficacy estimates as single Kato-Katz if the confidence intervals surrounding the mean difference in drug efficacy does not exceed these set of values ( S1 Info ). To calculate the corresponding sample size for each of the STH species, a simulation study was performed that considered (i) the variation in ERR and baseline FECs both across and within STH species, (ii) the variation in FECs introduced by the egg counting process, (iii) the paired ERR results across diagnostic methods, and (iv) a post-hoc correction for a pair-wise comparison. Based on this simulation, at least 110, 100 and 12 complete cases are required for T . trichiura , hookworm and A . lumbricoides , respectively. A detailed description of the sample size calculation is available elsewhere [ 35 ].
Diagnostic methods
Upon arrival in the laboratory, stool samples were homogenized with a wooden spatula and subsequently subjected to microscopic examination by means of single and duplicate Kato-Katz, Mini-FLOTAC and FECPAK G2 . Two aliquots of 0.5 g stool were also preserved in an Eppendorf tube containing 1 ml of absolute ethanol for later DNA extraction and qPCR analysis. Detailed standard operating procedures (SOPs) for the different diagnostic methods were published earlier [ 35 , 37 ]. Here we briefly mention the most important steps for each of the methods.
For Kato-Katz, two slides were prepared (slide A and B) and examined for the presence of STH eggs within 30–60 min following preparation. The results of slide A represented the results of a single Kato-Katz and egg counts were multiplied by 24 to obtain the FECs (expressed as EPG). The sum of the egg counts obtained after reading slide A and B represented the results for duplicate Kato-Katz and were multiplied by 12 to obtain the FECs.
For Mini-FLOTAC, we homogenized 2 g of fresh stool with 38 ml of flotation solution (saturated salt solution, density = 1.20) in the Fill-FLOTAC recipient [ 15 ]. After transferring the suspension into the two chambers of the Mini-FLOTAC device, the device was placed on a horizontal surface for 10 min after which the reading disk was translated. Finally, both Mini-FLOTAC chambers were screened for the presence of STH eggs. The number of eggs counted were multiplied by 10 to obtain the FECs.
The FECPAK G2 method was performed as described by Ayana et al. [ 17 ]. Briefly, stool was homogenized in tap water in a Fill-FLOTAC device [ 15 ], after which it was transferred into a FECPAK G2 sedimenter to allow STH eggs to sediment. The following day, the supernatant was poured off and saturated saline solution (specific density = 1.2) was added to the remaining slurry. The whole content of the sedimenter was then poured into a FECPAK G2 filtration unit from which 2 separate aliquots were taken and transferred to 2 wells of a FECPAK G2 cassette. Following an accumulation step of at least 20 minutes, the cassettes were placed in the Micro-I device for image capture. The device automatically imaged both wells and stored the images prior to uploading them to the FECPAK G2 server. Finally, the mark-up technician identified and counted any STH eggs present in the images using specialized software. Mark-up of the images was not performed on the day of examination and hence the results were not used to select individuals for inclusion in follow-up. Results of the mark-up were saved automatically for reporting and analysis. For FECPAK G2 the eggs counted in both wells were multiplied by 34 to calculate the FECs.
For quality control purposes, a predefined, randomly selected subset of samples (10% of the total number of samples) was re-evaluated by each of the three egg count methods. To this end, a senior researcher, who was blinded to the initial FECs, re-counted STH eggs across all three egg count methods. A third examiner would re-count STH eggs in case of discrepancies. An in-depth analysis of these quality control results will be published in a separate manuscript.
In order to perform qPCR, DNA was extracted from the preserved stool samples and analyzed for the presence of DNA of STH at the Laboratory for Medical Microbiology and Immunology (Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands) as part of two multiplex qPCR assays [ 35 ]. The variation between runs was monitored by means of the Cq values of the positive controls (DNA template for each STH species). We defined the variation between runs negligible when the difference in Cq values of the positive controls between runs did not exceed 1. The inhibition of the qPCR assay was controlled by adding a known quantity of phocine herpes virus DNA in each DNA extract and by subsequently quantifying this virus’ DNA by qPCR. Inhibition was present in the sample when the difference in Cq-value between the virus’ DNA in a clinical sample and a pure virus DNA sample did not exceed 1. We did not observe a difference in Cq across controls of more than 1 Cq, nor did we observe inhibition of the qPCRs in any of the samples (quality control results will be published in a follow-up manuscript). For each target species, qPCR results were expressed as genomic equivalents per ml of DNA extract (GE/ml). The reported qPCR results for hookworms were calculated as the sum of GE/ml of both hookworm species ( Ancylostoma and Necator americanus ).
Statistical analysis
A sample was considered positive for a STH infection if it tested positive on at least one diagnostic method (duplicate Kato-Katz, Mini-FLOTAC, FECPAK G2 or qPCR). The efficacy of a single oral dose of 400 mg ALB is reported separately for each STH species and for each microscopic method by means of ERR, using the following formula: ERR (%) = 100% x [1- (arithmetic mean FEC at follow-up / arithmetic mean FEC at baseline)]. For qPCR, a similar formula was used, where FEC was replaced by DNA concentration (GE/ml), yielding the genome equivalent reduction rate (GERR): GERR (%) = 100% x [1- (arithmetic mean DNA concentration at follow-up / arithmetic mean DNA concentration at baseline)]. A bootstrap analysis was used to determine the corresponding 95% confidence intervals (95%CI) around the (G)ERR point estimate for each diagnostic method and the difference in drug efficacy compared to a single Kato-Katz across diagnostic methods. A permutation test was used to assess the equivalence in (G)ERR between single Kato-Katz thick smear and either duplicate Kato-Katz, Mini-FLOTAC, FECPAK G2 or qPCR. Bonferroni’s correction was applied for multiple comparison between methods (level of significance was set at 0.0125 = 0.05 / 4 comparisons).
The (G)ERR point estimates were used to classify drug efficacy as ‘satisfactory’, ‘doubtful’ or ‘reduced’ following the WHO criteria recommended for a single Kato-Katz [ 13 ] ( Table 2 ). In addition, the agreement between a single Kato-Katz and the other methods in the assignment of drug efficacy into ‘satisfactory’, ‘doubtful’ and ‘reduced’ was evaluated by Fleiss’ kappa statistic (κ Fleiss ). The value of this statistic indicates a slight (κ Fleiss <0.2), fair (0.2≤ κ Fleiss <0.4), moderate (0.4≤ κ Fleiss <0.6), substantial (0.6≤ κ Fleiss , <0.8) or an almost perfect agreement (κ Fleiss ≥0.8).
The efficacy is measured as the reduction in mean fecal egg counts following drug administration (ERR). These thresholds were recommended by the World Health Organization for a single Kato-Katz.
https://doi.org/10.1371/journal.pntd.0007471.t002
Finally, we also assessed the distribution of individual responses measured across the five diagnostic methods. Individual ERR (iERR) were calculated using the following formula: iERR = 100% x [1- (FEC at follow-up of individual i / FEC at baseline of individual i )]. Individual GERR (iGERR) were calculated using the following formula: iGERR = 100% x [1- (DNA concentration at follow-up of individual i / DNA concentration at baseline of individual i )]. We classified the individual response for each STH species and for each method into ‘cured’ (no eggs/DNA was found in follow-up sample), ‘satisfactory’, ‘doubtful’ or ‘reduced’ (see Table 1 ) and ‘absent’ (drug efficacy was below zero due to higher egg counts or DNA-concentration in the follow-up sample than in baseline sample). Subsequently, the agreement between a single Kato-Katz and the other methods in the assignment of individual drug efficacy was assessed by Fleiss’ kappa statistic (κ Fleiss ). All statistical analyses were performed in R [ 38 ]. Graphs were produced using R.
Demographics and STH status of the complete cases
The number of children that were withheld after recruitment and at baseline or follow-up visits, and those that were eventually incorporated in the final statistical analysis are summarized in Fig 1 . Complete data was available for 645 children across three of the four study sites (Ethiopia: 161 cases; Lao PDR: 239 cases; Pemba (Tanzania): 245 cases).
FECs: fecal egg counts expressed in eggs per gram of stool (EPG), 2x KK: duplicate Kato-Katz.
https://doi.org/10.1371/journal.pntd.0007471.g001
With the exception of Pemba (Tanzania), where more females (n = 137) were included than males (n = 108), the sex ratio (males:females) was approximately 1:1 in all study sites. The median age (25 th and 75 th quantile) of the children across the three study sites equaled 11.0 years (9.0; 12.0). The participants in Ethiopia (9.0 years [8.0; 10.0]) were slightly younger than those in Lao PDR (12.0 years [11.0; 13.0]) and Pemba (Tanzania; 11.0 years [10.0; 12.0]). In total, there were 441 complete cases for A . lumbricoides (Ethiopia: 137, Lao PDR: 111, Pemba (Tanzania): 193), 456 for T . trichiura (Ethiopia: 106, Lao PDR: 105, Pemba (Tanzania): 245) and 457 for hookworm (Ethiopia: 90, Lao PDR: 228, Pemba (Tanzania): 139). The qPCR results showed that all individuals who excreted hookworm eggs were infected with N . americanus . Both N . americanus and Ancylostoma DNA was detected in only 8 individuals from one school in Pemba (Tanzania). The qPCR results for hookworm used in our calculations represent the combined GE/ml detected for both species. Due to the nature of the school selection procedure (prioritization of schools where STH prevalence was expected to be moderate to high and premature discontinuation of recruitment in a school when the prevalence of STH was low), the number of complete cases is not equally distributed across the schools. A minority of the schools actually provide the majority of the infected children ( S2 Info ).
Equivalence in therapeutic drug efficacy
Tables 3 – 5 describe the efficacy of ALB measured by the different diagnostic methods across the three study sites for A . lumbricoides , T . trichiura and hookworm, respectively. For A . lumbricoides infections, efficacy of a single-oral dose of 400 mg ALB estimated by single Kato-Katz was high (ERR >95%) across the different study sites ( Table 3 ). This high drug efficacy was confirmed by the three microscopic methods as well as by qPCR. The absolute point percent difference in drug efficacy did not exceed 2% (duplicate Kato-Katz: 0–0.1%; Mini-FLOTAC: 0.1%– 0.6%; FECPAK G2 : 0.0%– 1.8%; qPCR: 0.0%– 0.8%). All diagnostic methods provided significantly equivalent estimates of drug efficacy compared to a single Kato-Katz (i.e. the 95%CI around the difference in drug efficacy between diagnostic methods did not include 2.5%), except for FECPAK G2 in Pemba (Tanzania) and qPCR in both Ethiopia and Pemba (Tanzania), where evidence of equivalent drug efficacy was marginal since CIs included the 2.5% bounds of equivalence (FECPAK G2 : [-5.7%; 0.8%]; qPCR: [-6.5%; 5.5%] in Pemba (Tanzania) and [-0.1%; 4.3%] in Ethiopia).
Mean intensity of infection corresponds with the mean fecal egg counts (FEC; expressed as eggs per gram of stool (EPG)) for single (1x KK) and duplicate (2x KK) Kato-Katz, Mini-FLOTAC and FECPAK G2 and with the mean DNA concentration (expressed as genome equivalents per ml of DNA (GE/ml)) for qPCR. For A . lumbricoides , there is significant evidence that a diagnostic method results in equivalent drug efficacy results compared to a 1x KK when the 95% confidence intervals (95%CI) around the difference in drug efficacy does not include +/-2.5%. Significant evidence of equal drug efficacy results is indicated by ‘*’. For A . lumbricoides , drug efficacy was classified as ‘satisfactory’ when drug efficacy ≥95%, reduced when drug efficacy was less than 85% or doubtful in all other cases.
https://doi.org/10.1371/journal.pntd.0007471.t003
Mean intensity of infection corresponds with the mean fecal egg counts (FEC; expressed as eggs per gram of stool (EPG)) for single (1x KK) and duplicate (2x KK) Kato-Katz, Mini-FLOTAC and FECPAK G2 and with the mean DNA concentration (expressed as genome equivalents per ml of DNA (GE/ml)) for qPCR. For T . trichiura , there is significant evidence that a diagnostic method results in equivalent drug efficacy results compared to a 1x KK when the 95% confidence intervals (95%CI) around the difference in drug efficacy does not include +/-10%. Significant evidence of equal drug efficacy results is indicated by ‘*’. For T . trichiura , drug efficacy was classified as ‘satisfactory’ when drug efficacy ≥50%, reduced when drug efficacy was less than 40% or doubtful in all other cases.
https://doi.org/10.1371/journal.pntd.0007471.t004
Mean intensity of infection corresponds with the mean fecal egg counts (FEC; expressed as eggs per gram of stool (EPG)) for a single (1x KK) and duplicate (2x KK) Kato-Katz, Mini-FLOTAC and FECPAK G2 and with the mean DNA concentration (expressed as genome equivalents per ml of DNA (GE/ml)) for qPCR. For hookworm, there is significant evidence that a diagnostic method results in equivalent drug efficacy results compared to a 1x KK when the 95% confidence intervals (95%CI) around the difference in drug efficacy does not include +/- 5%. Significant evidence of equal drug efficacy results is indicated by ‘*’. For hookworm, drug efficacy was classified as ‘satisfactory’ when drug efficacy ≥90%, reduced when drug efficacy was less than 80% or doubtful in all other cases.
https://doi.org/10.1371/journal.pntd.0007471.t005
For T . trichiura infections, ALB efficacy estimations varied significantly depending on the study site and the diagnostic method that was used ( Table 4 ). Estimations obtained by single Kato-Katz were 52.9% in Ethiopia, 36.7% in Lao PDR and -11.2% in Pemba (Tanzania). There was a large deviation from the drug efficacy measured by single Kato-Katz and those based on the other diagnostic methods. The absolute point percent difference between single Kato-Katz and the other methods was the smallest for duplicate Kato-Katz (4.8%– 6.3%) and the largest for qPCR (14.6%– 60.2%). None of the methods provided equal drug efficacy results (CI around the difference in drug efficacy between diagnostic methods included the 10% bounds of equivalence). Moreover, clear difference could be noted across the different methods. In contrast to the other methods, drug efficacy measured by a duplicate Kato-Katz was marginally equivalent to those obtained by single Kato-Katz with the CI just including the 10-point percent (Ethiopia: [-0.6%; 10.4%]; Lao PDR: [-12.0%; 3.1%] and Pemba (Tanzania): [-14.9%; 1.9%]).
For hookworm infections ( Table 5 ), the drug efficacy measured by single Kato-Katz was high (>95%) in both Ethiopia (96.3%) and Lao PDR (96.1%), but moderate in Pemba (Tanzania) (84.2%). Overall, these drug efficacy estimates were confirmed by the other diagnostic methods. The absolute point percent differences in drug efficacy did not exceed 7% (duplicate Kato-Katz: 0%– 0.6%; Mini-FLOTAC: 0.9%– 2.7%; FECPAK G2 : 1.3%– 6.1%; qPCR: 1.5%– 4.6%). A duplicate Kato-Katz provided equivalent drug efficacy estimates across all study sites (the CI around the difference in drug efficacy between diagnostic methods did not include the 5% bounds of equivalence, Ethiopia: [95%CI: -0.5; 0.9], Lao PDR: [95%CI: -0.6; 0.2]); Pemba (Tanzania) [95%CI: -1.5; 3.2]). The only other significant equivalent drug efficacy result was found for FECPAK G2 in Lao PDR (95%CI: -3.7; 0.9). The remaining pair-wise comparisons in both Ethiopia (Mini-FLOTAC: [95%CI: -2.1; 9.0]; FECPAK G2 : [95%CI: -8.1; 0.9]; qPCR: [95%CI: -2.4; 7.6]) and Lao PDR (Mini-FLOTAC: [95%CI: -0.6; 6.0%]; qPCR: [95%CI: -2.1; 7.7]) indicated that drug efficacies were marginally equivalent, the 95%CI just including the 5% equivalence threshold. This was in contrast to the findings from Pemba (Tanzania) where no clear evidence of equivalent drug efficacies for Mini-FLOTAC [95%CI: -11.8; 9.0%], FECPAK G2 [95%CI: -15.9; 3.0] and qPCR [95%CI: -10.8; 31.4] was observed.
In summary, a duplicate Kato-Katz provided drug efficacy significantly or marginally equivalent to a single Kato-Katz for the three STH species in all study sites. For the other methods, the equivalence of drug efficacy varied by STH species and study site.
Agreement in classifying drug efficacy
Table 6 summarizes the agreement in classifying the efficacy of ALB between single Kato-Katz and the other diagnostic methods across the three study sites for each STH species. For both duplicate Kato-Katz (κ Fleiss = 0.81, p <0.001) and qPCR (κ Fleiss = 0.84, p <0.001) there was almost a perfect agreement (κ Fleiss ≥0.8). Both these methods agreed with a single Kato-Katz in 7 out of the 9 observations. For FECPAK G2 (κ Fleiss = 0.65, p = 0.01) there was a substantial agreement (0.61 ≤ κ Fleiss <0.81), for MiniFLOTAC (κ Fleiss = 0.60, p = 0.03) there was a moderate agreement (0.41 ≤ κ Fleiss <0.61).
The cross tables represent the agreement in classifying the efficacy of albendazole as ‘satisfactory’, ‘doubtful’ or ‘reduced’, between a single Kato-Katz (1x KK) and a duplicate Kato-Katz (2x KK), Mini-FLOTAC, FECPAK G2 and qPCR across the three study sites and for each soil-transmitted helminth species (9 observations). Drug efficacy is based on egg reduction rate (ERR) for microscopic methods and by means of reduction in genome equivalents (GERR) for qPCR. World Health Organization criteria to define drug efficacy into ‘satisfactory’, ‘doubtful’ and ‘reduced’ were previously recommended for a single Kato Katz (see Table 2 ).
https://doi.org/10.1371/journal.pntd.0007471.t006
Individual drug responses
Figs 2 – 4 provide an overview of individual drug responses to ALB for the five diagnostic methods across the different sites for A . lumbricoides , T . trichiura and hookworm, respectively. The top three bar plots illustrate the different classifications of individual drug efficacy for that helminth species detected in the study population in each of the three countries by five different diagnostic methods. Based on their i(G)ERR, individuals are classified into one of seven different categories, which in turn correspond with a specific color, ranging from dark green (completely cured, i(G)ERR = 100%) to dark red (eggs/DNA detected at follow-up but not at baseline, i(G)ERR = -∞). The grey part of the bar represents the individuals for whom no efficacy could be calculated since no eggs or DNA was detected for that species at baseline and follow-up. The bottom three bar plots represent the individual drug efficacy for those 770 individuals for whom i(G)ERR was measured with each of the five diagnostic methods.
The upper three bar plots illustrate the different classifications of individual albendazole efficacy for A . lumbricoides detected in the study population (n = 441; Ethiopia: 137, Lao PDR: 111, Pemba (Tanzania): 193) in each of the 3 countries using 5 different diagnostic methods (single (1x KK) and duplicate Kato-Katz (2x KK), Mini-FLOTAC (MF), FECPAK G2 (FP) and qPCR). The bars represent the number of cases. The color of the bars reflects the individual egg or genome equivalent reduction rate (i(G)ERR). The bottom three bar plots represent the classification of the i(G)ERRs for A . lumbricoides for those individuals for which i(G)ERR data was available for all five diagnostic methods (n = 258; Ethiopia: 80, Lao PDR: 50, Pemba (Tanzania): 128).
https://doi.org/10.1371/journal.pntd.0007471.g002
The upper three bar plots illustrate the different classifications of individual albendazole efficacy for T . trichiura detected in the study population (n = 456; Ethiopia: 106, Lao PDR: 105, Pemba (Tanzania): 245) in each of the 3 countries using 5 different diagnostic methods (single (1x KK) and duplicate Kato-Katz (2x KK), Mini-FLOTAC (MF), FECPAK G2 (FP) and qPCR). The bars represent the number of cases. The color of the bars reflects the individual egg or genome equivalent reduction rate (i(G)ERR). The bottom three bar plots represent the classification of the i(G)ERRs for T . trichiura for those individuals for which i(G)ERR data was available for all five diagnostic methods (n = 256; Ethiopia: 21, Lao PDR: 29, Pemba (Tanzania): 206).
https://doi.org/10.1371/journal.pntd.0007471.g003
The upper three bar plots illustrate the different classifications of individual ALB efficacy for hookworm detected in the study population (n = 457; Ethiopia: 90, Lao PDR: 228, Pemba, Tanzania: 139) in each of the 3 countries using 5 different diagnostic methods (single (1x KK) and duplicate Kato-Katz (2x KK), Mini-FLOTAC (MF), FECPAK G2 (FP) and qPCR). The bars represent the number of cases. The color of the bars reflects the individual egg or genome equivalent reduction rate (i(G)ERR). The bottom three bar plots represent the classification of the i(G)ERRs for hookworm for those individuals for which i(G)ERR data was available for all five diagnostic methods (n = 256; Ethiopia: 34, Lao PDR: 166, Pemba, Tanzania: 56).
https://doi.org/10.1371/journal.pntd.0007471.g004
Generally, these figures highlight three important findings. First, they confirm the distinct differences in sensitivity across the diagnostic methods. FECPAK G2 was previously evaluated as being less sensitive than Kato-Katz, while qPCR was found to have superior sensitivity for all STH [ 16 , 26 , 39 ]. This is also supported by the results of our study, where we noticed high numbers of false negative test results at baseline and follow-up for FECPAK G2 . When applying FECPAK G2 , drug efficacy could not be measured in 438 (32.3%) of the 1,354 individuals with STH infection, because of false negative results at baseline ( A . lumbricoides : 148/441, T . trichiura : 135/456 and hookworms: 155/457). In contrast, when applying qPCR, individual drug efficacy could not be measured in only 56 (4.1%) of the 1,354 individuals because of false negative results at baseline (top graphs of Figs 2 – 4 : A . lumbricoides : 22/441, T . trichiura : 11/456 and hookworm: 23/456).
Second, they indicate that there are a number of cases where eggs or DNA were found at follow-up, but not at baseline (this mathematically results in an infinite increase of eggs or DNA at follow-up or an individual drug efficacy of minus infinity (dark red portion of bars in upper panels). Overall, these types of cases were observed by at least one diagnostic method in 8% of the total number of cases (n = 1,354), but differences across diagnostic methods and STH were observed. They were more prevalent when the FECPAK G2 method was used (5.5% = 75/1,354). For the other diagnostic methods, the proportion of samples that were positive at follow-up but negative at baseline did not exceed 1.3% (single Kato-Katz: 1.2%; duplicate Kato-Katz: 1.3%; Mini-FLOTAC: 1.0%; qPCR: 1.2%), the majority being T . trichiura cases (17.2% = 78/456). The cases were less frequently observed for hookworm (5.3% = 24/457) and A . lumbricoides (1.8% = 8/441).
Third, they indicate that variation in individual drug response across STH and countries is similar across diagnostic methods. This is most obvious when we focus on the cases for which an individual drug efficacy response was available for all methods (bottom panels of Figs 2 – 4 ). When employing a single Kato-Katz, the highest drug efficacy was observed for A . lumbricoides followed by hookworms and T . trichiura . For A . lumbricoides , 96.9% (= 250/258) of the individuals showed a drug response that was at least satisfactory (light green + dark green). For hookworm, this proportion equaled 79.7% (= 204/256), whereas for T . trichiura this was only 34.4% (= 88/256).
For A . lumbricoides , the proportions of individuals with at least satisfactory drug efficacy (light green + dark green) as measured by single Kato-Katz were comparable across the 3 countries (Ethiopia: 100% (= 80/80); Lao PDR: 94.0% (= 47/50) and Pemba (Tanzania): 96.1% (= 123/128)) (bottom graphs of Figs 2 – 4 ). For T . trichiura , fewer individuals showed satisfactory drug efficacy to ALB in Pemba (Tanzania) compared to Lao PDR or Ethiopia (Pemba (Tanzania): 28.6% (= 59/206) vs . Lao PDR: 51.7% (= 15/29) or Ethiopia: 66.7% (= 14/21)). When investigating the individual ALB response to hookworm, fewer individuals show satisfactory drug efficacy in Pemba (Tanzania) (66.1% (= 37/56)) compared to Lao PDR (82.5% (= 137/166)) or Ethiopia (88.2% (= 30/34)). Regardless of the diagnostic method used, the same trends in individual efficacy were apparent across STH species and countries.
Cross tables were made to gain more insights into the agreement between individual drug response across the different methods ( S 3 Info ). These tables illustrate the agreement of calculated i(G)ERR using single Kato-Katz and the four other methods for A . lumbricoides , T . trichiura and hookworms, respectively. For a duplicate Kato-Katz an almost perfect agreement (κ Fleiss ≥ 0.80) was observed for each of the STH ( A . lumbricoides : 0.96 T . trichiura : 0.91; hookworm: 0.96, p <0.001). For Mini-FLOTAC, there was an almost perfect agreement for A . lumbricoides (κ Fleiss = 0.88, p <0.001) and a substantial agreement for the other two STH ( T . trichiura : 0.62; hookworm: 0.79, p <0.001). For FECPAK G2 , there was moderate agreement for A . lumbricoides (κ Fleiss = 0.55, p <0.001) and a fair agreement for the remaining STH ( T . trichiura : 0.31; hookworm: 0.39, p <0.001). For qPCR, there was a substantial agreement for hookworms (κ Fleiss = 0.61, p < 0.001), moderate agreement for both A . lumbricoides (κ Fleiss = 0.59, p <0.001), and a fair agreement for T . trichiura (κ Fleiss = 0.36, p <0.001).
The present study evaluated the efficacy of ALB against STH infections in three different endemic study sites using five different diagnostic methods. The rationale for this study was twofold. First, we wanted to evaluate if the different diagnostic methods provide equivalent drug efficacy results compared to a single Kato-Katz (the WHO recommended method) and to ultimately make recommendations on which diagnostic methods can be used for assessing drug efficacy. The second goal was to evaluate the ALB efficacy against STH in all three study sites with varying anthelmintic drug pressure histories. The presented study is unique in a number of ways. It is the first study that performs a multi-country, standardized, head-to-head comparison of established (single and duplicate Kato-Katz) and novel microscopic (Mini-FLOTAC and FECPAK G2 ) and molecular (qPCR) diagnostic methods for assessing drug efficacy against STHs. This study was not designed to prove that ERR estimates differ across methods, rather it verified whether methods are equivalent in assessing drug efficacy, which, as illustrated in S1 Info , is a subtle, but important difference.
No single diagnostic method provides ERR that are equivalent to single Kato-Katz for all STH, but they agree in classifying drug efficacy according to the WHO guidelines
We found that none of the evaluated tests provided equivalent results to those obtained by single Kato-Katz for all three STH. However, this conclusion needs to be interpreted with some caution. First, the species-specific levels of equivalence (the predefined bounds of equivalence) are arbitrary and likely to be set too strict. For instance, setting the level of equivalence at 10% for T . trichiura might be too strict for Pemba (Tanzania) given that ALB efficacy measured by duplicate Kato-Katz was -11.2%. On the other hand, the sample size was initially determined to compare the microscopic methods only (See [ 35 ]). By adding the qPCR results to this comparison, we increased the number of comparisons from 3 to 4. Consequentially, the level at which significant equivalence could be shown was reduced (0.05/4 = 0.0125 instead of 0.05/3 = 0.0166). Moreover, the sample size calculation was performed bases on certain assumptions regarding the ERR and FECs across and within STH species, which might have resulted in an underestimation of the true variation in the population [ 40 , 41 ]. However, despite the lack of equivalence, for most methods there was relatively good agreement in classifying ALB efficacy according to WHO guidelines. This suggests that each method holds promise for the assessment of drug efficacy in the context of assessing drug efficacy within STH control programs.
Diagnostic methods for assessing drug efficacy need to be validated for their intended-use
The results of the present study highlight that the impact of the diagnostic sensitivity on ERR results is minimal ( Tables 3 – 5 and Figs 2 – 4 ). Although there were substantial differences in FECs across the different microscopic methods, this did not have a major impact on the equivalence of ERR. For example, the FECs for A . lumbricoides based on single Kato-Katz were at least double of those based on FECPAK G2 across all study sites (Ethiopia: 7,870 EPG vs . 1,622 EPG; Lao PDR: 13,029 EPG vs . 2,711 EPG; Pemba (Tanzania): 14,372 EPG vs . 6,322 EPG), yet at each study site both methods agreed that the efficacy is still satisfactory. This agreement in drug efficacy, despite the clear differences in diagnostic sensitivity and FECs, are in line with previous studies involving both animal [ 42 ] and human helminths [ 43 – 45 ], and underscore that diagnostic methods need to be to be validated for their intended-use. Moreover, it highlights that other aspects such as user-friendliness and operational costs might become pivotal factors when deciding to recommend or use any given method. Additionally, it should be noted that our findings for qPCR do not necessarily apply for other qPCR assays, given that the plethora of described qPCR assays for STHs can differ substantially in performance. It is also important to point out that these findings are based on results obtained in sites where STH prevalence and intensities of STH infections are still relatively high. It is possible that the impact of the diagnostic sensitivity of a method on ERR calculations, as illustrated in animals, will increase when working in settings with very low infection intensities [ 46 , 47 ]
The efficacy of single ALB against was lower in sites where drug pressure has been high
We strategically selected the different study sites to cover a wide range of drug pressure. In our study, the study site in Ethiopia was least exposed to BZ drugs, followed by the one in Lao PDR. On Pemba (Tanzania), BZ drugs had been most frequently administered. When focusing on the drug efficacy estimated by single Kato-Katz, there was an obvious trend between the drug pressure and drug efficacy for each of the three STH species. The ERRs dropped as a function of historic drug pressure. The declining trend was most pronounced for T . trichiura , for which ERR ranged from 52.9% in Ethiopia over 36.7% in Lao PDR to -11.2% in Pemba (Tanzania). For both A . lumbricoides and hookworm, the efficacy of ALB was highest in both Ethiopia and Lao PDR ( A . lumbricoides : ~ 99% and hookworms: ~96%), and lowest on Pemba, Tanzania ( A . lumbricoides : 96.8%; hookworms: 84.2%). Whether this reduced drug efficacy on Pemba (Tanzania) is indicative for the emergence of AR remains to be verified since it has been shown that other factors may contribute to a reduced efficacy. For example, it has been described that the efficacy of ALB against T . trichiura infections declines as a function of increasing infection intensity [ 48 ]. This also seems to be the case in the present study, where we notice a trend between average infection intensity (Pemba (Tanzania): 3,111 EPG; Lao PDR: 357 EPG; Ethiopia: 207 EPG) and reduced drug efficacy (Pemba (Tanzania): -11.2% ERR; Lao PDR: 36.7% ERR; Ethiopia: 52.9% ERR by single Kato-Katz). It is also possible that both these processes occur simultaneously and mutually enhance the noticed effects of reduced drug efficacy. Poor drug efficacy could result in increasing transmission and more subjects being infected with a large number of worms.
To further assess the emergence of AR, we will analyze the frequency of known single nucleotide polymorphisms (SNPs) in the β-tubulin gene at codons 167 (TTC to TAC), 198 (GAA to GCA) and 200 (TTC to TAC) in a subset of the baseline and follow-up samples [ 35 ]. Subsequently, individual-based drug efficacy models will be built to explore the association between the frequency of these SNPs and other factors, including, but not limited to, infection intensity [ 49 ]. The results of this analysis will be presented and discussed in detail in a follow-up paper. At present, only a few studies with small sample sizes originating from a limited number of endemic areas have been performed to assess the association between β -tubulin SNPs and reduced anthelmintic efficacy in human STH [ 33 , 50 – 55 ]. In these studies, it was noted that polymorphisms were predominantly found in codon 200 of the β -tubulin gene and that these mutations were more abundant in a T . trichiura worm population following drug administration. Nevertheless, no association could ever be proven with reduced drug efficacy in any STH species. Overall, there are limited reports of declining or poor drug efficacy [ 9 , 33 , 56 , 57 ]. Of note, some of these studies were flawed in terms of their design or analysis. For example, Krücken and colleagues reported a poor efficacy of ALB against A . lumbricoides infections in Rwandan SAC, but the study findings might have been negatively affected by the fact that follow-up sampling occurred too soon after drug administration (7–10 days), which likely led to the detection of eggs from dying or degenerating worms [ 58 ].
Differential susceptibility of hookworm species to ALB
Interestingly, Pemba was the only site where both hookworm species were detected by qPCR. In eight children, mixed infections with Ancylostoma spp. and N . americanus were identified, confirming the finding by Albonico et al. [ 59 ]. Follow-up samples of these eight individuals were all negative for Ancylostoma spp. (cure rate (CR) of 100%), while two still excreted Necator DNA (CR = 75%). Although this was observed in only eight cases, it supports the findings on the efficacy of ALB to different helminth infections presented by Horton [ 60 ] who reported a notably lower CR for Necator infections (CR = 75%, 30 studies) compared to Ancylostoma spp. infections (CR = 92%, 23 studies). Given the seemingly differential susceptibility of both hookworm genera to ALB, it is important to differentiate hookworm infections in order to have correct efficacy estimates for each species. This is of particular interest when the possible contribution of zoonotic A . ceylanicum infections from animal reservoirs to the observed drug efficacy is investigated.
The present study investigated the equivalence of five different diagnostic tools for the evaluation of anthelmintic efficacy. None of the evaluated tests provided equivalent results to those obtained by the currently recommended single Kato-Katz for all STH, but this might be due to the number of pairwise-comparisons and the strict bounds of equivalence. Overall, there was an acceptable agreement in classifying the efficacy of ALB, suggesting that each of the investigated methods holds promise to assess drug efficacy in the context of STH control programs. The results also highlight that the clinical sensitivity or the ability to accurately estimate egg counts should not be the only parameters to determine the best diagnostic tool to assess drug efficacy. Instead, there are a number of other aspects that should also be considered to make a well-founded decision on what method(s) to recommend for monitoring drug efficacy in STH control programs, like user-friendliness and operational costs per test. We observed a decreasing trend in drug efficacy as a function of increasing historic drug pressure, yet further research is needed to identify factors that are contributing to this variation and to determine whether reduced efficacy can be linked with the known β-tubulin SNPs.
Supporting information
S1 info. testing for difference vs . testing for equivalence..
https://doi.org/10.1371/journal.pntd.0007471.s001
S2 Info. Number of complete cases per school, sex and age across the four study sites.
https://doi.org/10.1371/journal.pntd.0007471.s002
S3 Info. Agreement in classifying the individual response against A. lumbricoides, T. trichiura and hookworm between a single Kato-Katz and duplicate Kato-Katz, Mini-FLOTAC, FECPAK G2 and qPCR.
https://doi.org/10.1371/journal.pntd.0007471.s003
S4 Info. Complete dataset.
https://doi.org/10.1371/journal.pntd.0007471.s004
Acknowledgments
First and foremost, the authors would like to express their gratitude towards all children, their parents, the school teachers and principals that participated in this study. Second, we wish to specifically thank all the people that provided the necessary laboratory and logistic support in each of the four different sampling sites. This work would not have been possible without their willful participation and assistance.
- View Article
- PubMed/NCBI
- Google Scholar
- 3. World Health Organization. Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. Geneva, Switzerland: World Health Organization, 2017.
- 4. World Health Organization. Crossing the billion. Lymphatic filariasis, onchocerciasis, schistosomiasis, soil-transmitted helminthiasis and trachoma: preventive chemotherapy for neglected tropical diseases. Geneva: World Health Organization, 2017
- 13. World Health Organization. Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. Geneva, Switserland: World Health Organisation, 2013.
- 37. Starworms.org. The starworms project website. Available from: https://www.starworms.org/ . [cited 2019 Jan 24]
- 38. R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Australia: URL https://www.R-project.org/ .
- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
Transformations That Work
- Michael Mankins
- Patrick Litre

More than a third of large organizations have some type of transformation program underway at any given time, and many launch one major change initiative after another. Though they kick off with a lot of fanfare, most of these efforts fail to deliver. Only 12% produce lasting results, and that figure hasn’t budged in the past two decades, despite everything we’ve learned over the years about how to lead change.
Clearly, businesses need a new model for transformation. In this article the authors present one based on research with dozens of leading companies that have defied the odds, such as Ford, Dell, Amgen, T-Mobile, Adobe, and Virgin Australia. The successful programs, the authors found, employed six critical practices: treating transformation as a continuous process; building it into the company’s operating rhythm; explicitly managing organizational energy; using aspirations, not benchmarks, to set goals; driving change from the middle of the organization out; and tapping significant external capital to fund the effort from the start.
Lessons from companies that are defying the odds
Idea in Brief
The problem.
Although companies frequently engage in transformation initiatives, few are actually transformative. Research indicates that only 12% of major change programs produce lasting results.
Why It Happens
Leaders are increasingly content with incremental improvements. As a result, they experience fewer outright failures but equally fewer real transformations.
The Solution
To deliver, change programs must treat transformation as a continuous process, build it into the company’s operating rhythm, explicitly manage organizational energy, state aspirations rather than set targets, drive change from the middle out, and be funded by serious capital investments.
Nearly every major corporation has embarked on some sort of transformation in recent years. By our estimates, at any given time more than a third of large organizations have a transformation program underway. When asked, roughly 50% of CEOs we’ve interviewed report that their company has undertaken two or more major change efforts within the past five years, with nearly 20% reporting three or more.
- Michael Mankins is a leader in Bain’s Organization and Strategy practices and is a partner based in Austin, Texas. He is a coauthor of Time, Talent, Energy: Overcome Organizational Drag and Unleash Your Team’s Productive Power (Harvard Business Review Press, 2017).
- PL Patrick Litre leads Bain’s Global Transformation and Change practice and is a partner based in Atlanta.
Partner Center
- New Terms of Use
- New Privacy Policy
- Your Privacy Choices
- Closed Caption Policy
- Accessibility Statement
This material may not be published, broadcast, rewritten, or redistributed. ©2024 FOX News Network, LLC. All rights reserved. Quotes displayed in real-time or delayed by at least 15 minutes. Market data provided by Factset . Powered and implemented by FactSet Digital Solutions . Legal Statement . Mutual Fund and ETF data provided by Refinitiv Lipper .
New data reveals voters are shifting to this major political party ahead of heated 2024 rematch
American voters are almost equally split as either republican or democrat, new data finds.

Wall Street Journal poll shows Biden losing support from Black men
Fox News political analyst Gianno Caldwell tells 'The Story' that the Democratic Party has taken the Black community for granted.
America is split in political party affiliation heading into the 2024 presidential election , seeing one of the most evenly split electorates in the past two decades, resurfaced research reveals.
A Pew Research Center analysis examined voter identification across different ages, races, religions and education levels, comparing how voters identified in 1996 to new data from 2023.
Fifty-one percent of Americans said they identified with the Republican Party in 1994, while 47% identified as Democrats. The tables turned over the years, with 5% more of American voters identifying as Democrats over Republicans in 2020. However, the Pew results from 2023 reveal a significant shift in party affiliation this cycle, reporting that 49% of voters identify as Democrat or leaning Democrat, while 48% identify as Republican or leaning Republican.
Additionally, about 33% of respondents said they identify as being conservative or moderate in 2023, while the other side of the aisle only sees 23% identifying as liberal Democrats or leaning liberal.
BLACK GEORGIA VOTERS ABANDONING BIDEN SAY THEY'RE SENDING MESSAGES ON GAZA: ‘DEMOCRATS SHOULD LISTEN’

Ranked choice voting comes in multiple forms and is used in a wide variety of states and localities around the U.S. (Paul Richards)
The poll found that while Democrats remain the party of choice for most Hispanic, Black and Asian voters, party support among non-Hispanic White Democratic voters has dropped 21 percentage points since 1996, falling from 77% to 56% in 2023.
Recent polls have found that despite their advantage, Democratic support among minority voters is shrinking. A recent Gallup poll found that 19% of Black adults said they identify as lean Republican or Republican, while 66% identify as Democrat or lean Democrat, the "smallest Gallup has recorded in its polling, dating back to 1999."
UNDECIDED BATTLEGROUND VOTERS UNANIMOUSLY BLAST BIDEN ON ECONOMY: ‘ABSOLUTELY DISASTROUS’
Among different age groups, Democrats maintain their advantage among young voters, while the majority of older individuals are Republican affiliated.

Former President Donald Trump arrives at Atlanta’s Hartsfield-Jackson Airport in Georgia on Wednesday to host a campaign fundraising event. (Robin Rayne for Fox News Digital)
Republicans have gained ground among Hispanic voters in recent years, tripling affiliation with their base in the demographic over the past two decades from 3% to 9%.
Rural voters also appear to be shifting towards the GOP, with the new poll showing the party holds a 25-point advantage over Democrats, 60% to 35%, after the parties were evenly split among voters in 2008.
President Biden and former President Donald Trump are expected to compete in a presidential election rematch in November. While Biden won the 2020 election against Trump, Pew's analysis reveals a potential shift in the political landscape that could be echoed on the ballot this fall.
CLICK HERE TO GET THE FOX NEWS APP
Pew Research Center conducted the surveys via telephone for the results dating 1994 to 2018, and via online surveys from 2019 to 2023 among registered voters.
Aubrie Spady is a Production Assistant for Fox News Digital.

Get the latest updates from the 2024 campaign trail, exclusive interviews and more Fox News politics content.
You've successfully subscribed to this newsletter!
More from Politics

Whitmer remains silent on protesters' 'Death to America' chants while weighing in on war in Israel, election

Republicans slam Biden’s ‘Don’t’ deterrence: ‘Every time he says Don’t, they do’
Sen. Kennedy advises ‘wobbly’ Biden to get tough on Iran after Israel attack: 'Go on Amazon and buy a spine'

Five key questions on how start of Trump's first criminal trial will impact presidential campaign
- Skip to main content
- Keyboard shortcuts for audio player
6 in 10 U.S. Catholics are in favor of abortion rights, Pew Research report finds

Jason DeRose

Pope Francis remains popular among U.S. Catholics, with 75% having favorable views of him, according to a Pew Research report. But many self-identified Catholics disagree with various teachings of their church. Andrew Medichini/AP hide caption
Pope Francis remains popular among U.S. Catholics, with 75% having favorable views of him, according to a Pew Research report. But many self-identified Catholics disagree with various teachings of their church.
Catholics in the U.S., one of the country's largest single Christian groups, hold far more diverse views on abortion rights than the official teaching of their church.
While the Catholic Church itself holds that abortion is wrong and should not be legal, 6 in 10 U.S. adult Catholics say abortion should be legal in all or most cases, according to a newly released profile of Catholicism by Pew Research .
Catholic opinion about abortion rights, according to the report, tends to align with political leanings: Fewer Catholic Republicans favor legal abortion than Catholic Democrats. And Pew says Hispanic Catholics, who make up one-third of the U.S. church, are slightly more in favor of legal abortion than white Catholics.

Despite church prohibitions, Catholics still choose IVF to have children
Pew found that 20% of the U.S. population identifies as Catholic, but only about 3 in 10 say they attend mass regularly. Opinions about abortion rights appear to be related to how often someone worships — just 34% of Catholics who attend mass weekly say abortion should be legal in all or most cases, whereas that number jumps to 68% among those who attend mass monthly or less.
Most U.S. Catholics are white (57%), but that number has dropped by 8 percentage points since 2007, according the new report. About 33% identify as Hispanic, 4% Asian, 2% Black, and 3% describe themselves as another race.
Pew Research also found that as of February, Pope Francis remains highly popular, with 75% of U.S. Catholics rating him favorably. However, there is a partisan divide, with Catholic Democrats more strongly supporting him.
About 4 in 10 U.S. Catholics view Francis as a major agent of change, with 3 in 10 saying he is a minor agent of change.

Catholic Church works to explain what same-sex blessings are and are not
Pew reports that many U.S. Catholics would welcome more change. Some 83% say they want the church to allow the use of contraception, 69% say priests should be allowed to get married, 64% say women should be allowed to become priests, and 54% say the Catholic Church should recognize same-sex marriage.
In December 2023, the Vatican issued guidance to priests that they may bless people in same-sex relationships. But the church insists those blessings not be construed in any way to be a form of marriage or even take place as part of a worship service.
- Pope Francis
- Abortion rights
- Catholic church
- Pew Research

IMAGES
VIDEO
COMMENTS
Albendazole is an antihelminthic medication with numerous indications such as cystic hydatid disease of the liver, lung, and peritoneum resulting from the larval form of the dog tapeworm, Echinococcus granulosus. ... More research is necessary as there are very few articles in the literature about the potential effects on pregnancy and the ...
Albendazole induces spindle apparatus disruption. ABZ act as spindle poison for mammalian cells, disrupting the spindle apparatus. HeLa cells were exposed to the indicated drugs, fixed and stained with an anti-alpha tubulin antibody (green) and an anti-centromere antibody (crest, red). DAPI was used to stain the DNA.
Hookworm-positive participants were treated with a single dose of 400 mg albendazole within 24 h of the first stool collection, and the treatment outcome was assessed 10-14 days after by ...
Albendazole's unique broad-spectrum activity is exemplified in the overall cure rates calculated from studies employing the recommended doses for hookworm (78% in 68 studies: 92%, for A. duodenale ...
Albendazole is an orally administered broad-spectrum anthelmintic agent. The use of albendazole has fewer side effects than metronidazole. ... and Cochrane Controlled Trials Register for trials published before February 2010 as well as in references of relevant research and review articles. Eight randomized clinical trials (including 900 ...
Author summary More than 500 million children worldwide receive a single oral dose of albendazole or mebendazole annually to reduce disease caused by intestinal worms (roundworm, whipworm and hookworm). However, it is unclear whether individuals respond differently to treatment. We re-analyzed 645 individual patient data from three standardized clinical trials with albendazole using a ...
Deworming programs aimed at reducing morbidity and mortality from geohelminth infections are common in many countries where these infections are endemic, but data demonstrating increasing levels of resistance to albendazole and mebendazole are causes for concern. Studies to evaluate the clinical efficacy of deworming programs are critical to maintain high infection control goals.
Background Immune checkpoint inhibitors (ICIs) have been increasingly used in patients with various cancers and have shown efficient therapeutic outcomes. However, fewer than 40% of cases across multiple cancer types show a response to ICIs. Therefore, developing more efficient combinational approaches with ICIs and revealing the underlying mechanisms are important goals for achieving rapid ...
Overall albendazole appears more effective for A. lumbricoides and hookworm, but the 3 day dosing with mebendazole is probably more effective for curing T. trichiura infections in individuals. The efficacy of albendazole was substantially better against N. americanus infections as suggested by Holzer & Frey (1987).
Author Summary Soil-transmitted helminths (STHs) infect millions of children worldwide. To fight STH, large-scale de-worming programs are implemented in which anthelmintic drugs (either albendazole (ABZ) or mebendazole (MEB)) are administered. However, there is a wide range of other brands, which are even more accessible, but for which the efficacy and quality remain poorly explored.
Abstract. This comprehensive review briefly describes the history and pharmacology of albendazole as an anthelminthic drug and presents detailed summaries of the efficacy and safety of albendazole's use as an anthelminthic in humans. Cure rates and % egg reduction rates are presented from studies published through March 1998 both for the ...
Albendazole is a broad-spectrum anthelmintic drug used for parasitic infections. In addition, due to its mechanism of action, it has been studied as an anticancer agent. However, poor and highly variable bioavailability are limiting factors for its use in systemic illnesses. The present study aimed to develop two parenteral formulations of albendazole and to compare its pharmacokinetic profile ...
Albendazole (ABZ) is an effective anthelminthic agent forming active and inactive metabolites in both humans and animals (Dayan, 2003; Rawden et al., 2000; Sanyal, 1998; Sotelo and Jung, 1998). ... Web of Science was used as a primary source to retrieve original research articles. To accommodate references mentioned in this primary search ...
Research Paper Efficacy and safety of ascending dosages of albendazole against Trichuris trichiura in preschool-aged children, school-aged children and adults: A multi-cohort randomized controlled trial Chandni Patela,b, Jean T. Coulibalya,c, Jessica D. Schulza,b, Yves N'Gbessod, Jan Hattendorfa,b, Jennifer Keisera,b,*
Albendazole is a treatment of choice for neurocysticercosis, the dosage for albendazole is 15 mg/kg per day for ten days. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C 12 H 15 N 3 O 2 S. Its molecular weight is 265.34 [7] and structural formula of albendazole is shown in Figure 1.
Mass drug administration (MDA) of single-dose albendazole to all at-risk populations as preventive chemotherapy (deworming) is recommended by WHO to halt transmission of soil-transmitted helminth (STH) in endemic countries. We assessed the effectiveness of single-dose albendazole against STH infection in the western province of Rwanda, where STH prevalence remains high despite the ...
Albendazole is a broad-spectrum antihelmintic and antiprotozoal agent of the benzimidazole type. ... Summarized research data relating to the durations of these preslaughter and early pregnancy periods when albendazole should not be administered are found in US FDA NADA 110-048 (cattle) and 140-934 (sheep). ...
Albendazole (ABZ) and mebendazole (MBZ) are two benzimidazole-derived drugs that show remarkable antihelmintic activity and are widely used in the treatment and control of helminths. ... Research Articles. A comparative study of albendazole and mebendazole-induced, time-dependent oxidative stress References; Citations Metrics; Reprints ...
Benzimidazole (BZ) anthelmintics are among the most important treatments for parasitic nematode infections in the developing world. Widespread BZ resistance in veterinary parasites and emerging resistance in human parasites raise major concerns for the continued use of BZs. Knowledge of the mechanisms of resistance is necessary to make informed treatment decisions and circumvent resistance.
Introduction School-based preventive chemotherapy (Deworming) with praziquantel and albendazole to control and eliminate schistosomiasis and soil-transmitted helminths as public health problems is recommended by the World Health Organization (WHO). Safety monitoring during mass drug administration (MDA) is imperative but data from sub-Saharan Africa are scarce. Objective The aim of this active ...
This study suggests that the combined treatment with Ubenimex and Albendazole could be a potential therapeutic strategy for E. multilocularis infections. ... The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundations ...
Bioluminescent petunias, thanks to genetics research for pharmaceuticals Research into new pharmaceuticals has produced an unanticipated by-product: Petunias that glow in the dark.
Author summary During the last decade, the scale of deworming programs that aim to eliminate the morbidity caused by intestinal worms has increased to a level that is unprecedented in history. It is therefore of utmost importance to monitor any change in therapeutic efficacy that may arise from emerging drug resistance. Currently, a variety of novel methods have been described, but it remains ...
In this article the authors present one based on research with dozens of leading companies that have defied the odds, such as Ford, Dell, Amgen, T-Mobile, Adobe, and Virgin Australia. The ...
Aging research is helping us understand the deep biological implications of this advice. Eating a variety of healthy foods in moderation can prevent the health risks of obesity.
A Pew Research Center analysis examined voter identification across different ages, races, religions and education levels, comparing how voters identified in 1996 to new data from 2023.
Physicist Peter Higgs, whose subatomic particle research changed the world, has died The Nobel-Prize-winning physicist Peter Higgs has died at age 94. He was celebrated for his work on the mass of ...
Pew Research also found that as of February, Pope Francis remains highly popular, with 75% of U.S. Catholics rating him favorably. However, there is a partisan divide, with Catholic Democrats more ...