- Education Home
- Medical Education Technology Support
- Graduate Medical Education
- Medical Scientist Training Program
- Public Health Sciences Program
- Continuing Medical Education
- Clinical Performance Education Center
- Center for Excellence in Education
- Research Home
- Biochemistry & Molecular Genetics
- Biomedical Engineering
- Cell Biology
- Microbiology, Immunology, & Cancer Biology (MIC)
- Molecular Physiology & Biological Physics
- Neuroscience
- Pharmacology
- Public Health Sciences
- Office for Research
- Clinical Research
- Clinical Trials Office
- Funding Opportunities
- Grants & Contracts
- Research Faculty Directory
- Cancer Center
- Cardiovascular Research Center
- Carter Immunology Center
- Center for Behavioral Health & Technology
- Center for Brain Immunology & Glia
- Center for Diabetes Technology
- Center for Immunity, Inflammation & Regenerative Medicine
- Center for Public Health Genomics
- Center for Membrane & Cell Physiology
- Center for Research in Reproduction
- Myles H. Thaler Center for AIDS & Human Retrovirus Research
- Child Health Research Center (Pediatrics)
- Division of Perceptual Studies
- Research News: The Making of Medicine
- Core Facilities
- Virginia Research Resources Consortium
- Center for Advanced Vision Science
- Charles O. Strickler Transplant Center
- Keck Center for Cellular Imaging
- Institute of Law, Psychiatry & Public Policy
- Translational Health Research Institute of Virginia
- Clinical Home
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Neurosurgery
- Obstetrics & Gynecology
- Ophthalmology
- Orthopaedic Surgery
- Otolaryngology
- Physical Medicine & Rehabilitation
- Plastic Surgery, Maxillofacial, & Oral Health
- Psychiatry & Neurobehavioral Sciences
- Radiation Oncology
- Radiology & Medical Imaging
- UVA Health: Patient Care
- Diversity Home
- Diversity Overview
- Student Resources
- GME Trainee Resources
- Faculty Resources
- Community Resources
- Roles and responsibilities in research administration

The award process at UVA
All grant and contract proposals must be approved by the University before submission to the sponsor. At the SOM, proposals must be endorsed by the Principal Investigator, Chair, and the Office of Grants and Contracts.
The fiscal/administrative description of the proposal and approval signatures are collected on a four-page proposal approval sheet (also known as “the goldenrod,” after its former color). Grants and Contracts has posted a version for NIH e-SNAP proposals and a generic SOM version . Your research administrator should help you complete this form, though you, as Principal Investigator, are responsible for reviewing and approving its contents. The approval sheet, technical proposal, budget, justification, and other supporting documents are forwarded to your Chair and then to Grants and Contracts (who require at least five working days before the submission deadline). Grants and Contracts may ask additional questions concerning the project or information on the approval form before it is signed and ready for submission to the funding agency. Grants and Contracts performs all electronic submissions of proposals to grants.gov (see notes and reminders for submissions to grants.gov at their site). See detailed information on the proposal submission process and how to avoid pitfalls .
If your NIH proposal is likely to be funded, you will be asked to submit just-in-time documentation of other grant support, IRB or IACUC approvals, etc. You can send these directly to NIH, or via Grants and Contracts.
Sponsors generally notify both the PI and either Grants and Contracts or Sponsored Programs. Sponsored Programs then creates an internal account in the Oracle system, which may take a few weeks to complete. To avoid delays in initiating your project, ask your department administrator to request a preliminary Oracle account as soon as it is likely that your project will be funded. This will allow you to encumber and spend funds in a timely manner.
Faculty research roles and responsibilities
Faculty have the freedom to choose the nature and direction of their research program and to disseminate the results of that research to the public, within any constraints placed by the funding agency. Non-PI faculty have similar responsibilities to the PI, with the exception of overall responsibility for project direction and reporting. Collaborating investigators must:
- Ensure that their expenditures are in accordance with sponsor and university regulations, policies, and procedures
- Coordinate with the PI any approvals for restricted expenditures (e.g., equipment)
- Maintain knowledge of and compliance with University procedures related to sponsored research
- Disclose financial conflicts of interest to the PI
- Obtain and maintain applicable IACUC, IRB, IBC, and EHS approvals before initiating a research project
- Disclose prior disbarment/suspension or proposed disbarment
- Verify that their project staff have signed University Patent Agreements
Faculty conducting human subjects research should also refer to the section below on the roles/responsibilities of clinical research coordinators.
Trainee research roles and responsibilities
Postdocs and graduate students must:
- Seek and follow faculty guidance on scientific and other procedures (e.g., allowable uses of grant funds)
- Maintain knowledge of and compliance with University procedures and policies related to sponsored research
- Obtain applicable training in and practice responsible conduct of research
- Obtain applicable IACUC, IRB, IBC, and EHS approvals/training
Clinical research coordinator research roles and responsibilities
- Manage all aspects of conducting clinical trials under the direction of the PI
- Maintain in-depth knowledge of protocol requirements and Good Clinical Practice (GCP) as set described in FDA regulations
- Provide sound conduct of the clinical trial (including recruitment, screening, enrollment, and follow-up of eligible subjects per protocol)
- Maintain accurate and complete documentation (e.g., regulatory documents, signed consent forms, IRB approvals, source documents, drug dispensing and subject logs, and study-related communication)
- Provide organizational management of all aspects of the trial (e.g., timeliness in completing case report forms, data entry, reporting adverse drug experiences [ADEs], and managing caseload and study files)
- Communicate protocol-related problems to the management staff (e.g., questions regarding the conduct of the clinical trial, possible ADEs, or subject compliance)
- Maintain professional conduct in the presence of subjects, research staff, sponsors, monitors, auditors, etc.
Department research administrator research roles and responsibilities
*adapted from the Sponsored Programs web site
- Support project investigators in the development of proposals and related financial narratives and budgets
- Thoroughly understand unallowable, direct, and facilities and administrative (F&A) costs.
- Thoroughly understand and properly follow Cost Accounting Standards
- Show consistency in charging sponsored award costs
- Ensure monthly review of project costs and obtain PI approval of same in a timely manner
- Post-award Administration
- Contracts and Clinical Trials Agreements
- Intellectual Property (IP) and Entrepreneurial Activities
- Getting Started in Clinical Research
- Glossary of Research Terms
- Material Transfer Agreements
- Medical Student Research Programs at UVA
- Medical Student Research Symposium
- MSSRP On-Line Preceptor Form
- MSSRP On-Line Student Match Form
- On-line Systems
- Other Medical Student Research Opportunities
- Research: Financial Interest of Faculty
- Review of Proposed Consulting Agreements
- Transfers of NIH/Public Health Service awards
- Unmatched MSSRP projects – 2024
- Research Centers and Programs
- Other Offices Supporting Research
- SBIR and STTR Awards
- Information for students and postdoctoral trainees
- For Research Administrators
- For New Research Faculty
- Funding Programs for Junior Faculty
- NIH for new faculty
- School of Medicine surplus equipment site
- Forms and Documents
- FAQs – SOM offices supporting research
- 2024 Faculty Research Retreat
- Anderson Lecture and Symposia
- Definition of a Clinical Research Professional
With her passion for uncovering the latest innovations and trends, Kimmy Gustafson has provided valuable insights and has interviewed experts to provide readers with the latest information in the rapidly evolving field of medical technology since 2019. Kimmy has been a freelance writer for more than a decade, writing hundreds of articles on a wide variety of topics such as startups, nonprofits, healthcare, kiteboarding, the outdoors, and higher education. She is passionate about seeing the world and has traveled to over 27 countries. She holds a bachelor’s degree in journalism from the University of Oregon. When not working she can be found outdoors, parenting, kiteboarding, or cooking.
Related Articles
- Upskilling in the Allied Health Professions
- Single-use Plastics in Medicine Raise Concerns About Sustainability
- How to Become a Clinical Research Associate
Related Programs
- Top Courses
- Online Degrees
- Find your New Career
- Join for Free
The Career Path of a Clinical Research Coordinator
Learn about the clinical research coordinator role, sometimes called a clinical trials manager. Discover pathways to work in clinical research, salaries, and typical employers.
![clinical research administrator meaning [Featured Image]: Clinical Research Coordinators wearing lab coats and gloves, sitting in a lab, working on a desktop computer, and using a microscope, to analyze data conducting a clinical trial.](https://d3njjcbhbojbot.cloudfront.net/api/utilities/v1/imageproxy/https://images.ctfassets.net/wp1lcwdav1p1/1EknjKuQM15Jx6T9xBMj3f/d6661990a29d32d26b10023ff44340b1/GettyImages-563374209.jpg?w=1500&h=680&q=60&fit=fill&f=faces&fm=jpg&fl=progressive&auto=format%2Ccompress&dpr=1&w=1000)
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private companies, clinical research coordinators can seek various positions, and salary is above average compared to similar professions.
What is a clinical research coordinator?
A clinical research coordinator—sometimes known as a clinical trial manager—supervises clinical research and drug trials, such as interventions involving drugs, devices, or procedures promoting behavior changes. In this position, you would recruit and screen participants, coordinate the day-to-day running of the trials, collect data, and produce reports.
A clinical research coordinator is also responsible for ensuring a trial's safety and keeping the materials used safe during and after the study. You ensure meeting certain regulations and ethical standards and track patient health and progress.
Where does a clinical research coordinator sit in a clinical research team?
A clinical research coordinator reports to a principal investigator, who is responsible for the overall design and management of the study. In contrast, a clinical researcher organizes the day-to-day running of the trials. As a clinical research coordinator, you will also work closely with sponsors and staff within their department responsible for finance, personnel, and compliance. You will typically oversee a clinical research team, including doctors, nurses, other medical staff, and assistants.
What are the primary duties and responsibilities of a clinical research coordinator?
Clinical research coordinators work in various workplaces, from hospitals to private businesses, and your duties will vary according to where you work and what you are trialing. However, typical responsibilities include:
Coordinating and overseeing the running of clinical trials
Recruiting and screening participants for trials
Managing documents and records of participants and trials
Ensuring trials are delivered following regulatory, government, and ethical regulations and requirements
Ensuring the safety of participants, supplies, and materials
Performing cost analysis and preparing budgets
Designing and delivering training to staff and participants
Collecting data and maintaining detailed records
Acting as a point of contact for study participants to ask any questions or express concerns
Promoting the study through meetings, events, and seminars
Overseeing the research team
Career pathway steps
To work in clinical research, you need to hold a degree and have a certain amount of experience. You can pursue certifications and graduate qualifications to further your chances of a position.
Bachelor's degree
A bachelor’s degree is generally step one for anyone who wants to work as a clinical research coordinator. A Bachelor of Science in a relevant subject, such as clinical research administration or a matter under the umbrella of health sciences, public health, or microbiology, is recommended.
Read more: Bachelor of Science (BS) Degree: What It Is and How to Earn One
Work experience
Experience in health care or clinical research is essential for most positions. Some professionals come from a background in nursing or health care. If you have limited experience, getting some through an internship, voluntary assignment, or an entry-level role in a hospital or research position is a good idea. Experience is essential to be able to work towards certain industry-specific certifications.
Master's degree or graduate certificate
All you need to work as a clinical research coordinator is a bachelor’s degree and experience. However, some employers prefer a graduate degree, and having a graduate certificate or a master’s degree will help set you apart from other applicants without this education level.
You have several options to consider, and there is no set course. Still, it is most advantageous to select a subject like clinical research, clinical administration, or clinical research management to combine the elements of clinical research and a level of leadership. Specializations like medical technology are also options, as are more general course subjects like life sciences or health sciences.
When choosing a course, essential factors to consider are whether there is a good mix of theory and practical experience and whether the course covers clinical and managerial subjects to give you a broad range of expertise. If you are already working and gaining experience, consider the flexibility of studying part-time or online to achieve your educational goals.
In addition to a master’s degree, you can also study for a graduate certificate in a relevant subject. These courses tend to be part of the curriculum for a master’s degree, and you can transfer credits, so it’s a great way to gain a certificate while building towards a master’s degree.
Read more: How to Get a Master's Degree
Certification
It isn’t essential but recommended that you get a clinical research certification if you want to advance your career in clinical research. To become certified, you need to be working in the field. Certification verifies your skills and competence, potentially boosting career prospects and salary. The Association of Clinical Research Professionals (ACRP-CP) provides a variety of certifications for clinical research professionals:
ACRP Certified Professional (ACRP-CP): To validate your knowledge and skills as a clinical research professional
Clinical Research Coordinator (CCRC): To validate your knowledge and skills as a clinical research coordinator
Clinical Research Associate (CCRA): To validate your knowledge and skills as a clinical research associate
Principal Investigator (CPI): To validate your knowledge and skills as a principal investigator
ACRP Medical Device Professional Subspecialty (ACRP-MDP): To validate your knowledge of medical device trials
ACRP Project Manager Subspecialty: To validate your knowledge of project management within clinical research
The Society of Clinical Research Associates (SOCRA) also provides its Certified Clinical Research Professional credential to validate your experience, education, and knowledge as a clinical research professional.
How much do clinical research coordinators get paid?
The average clinical research coordinator's salary is $56,504, according to Glassdoor [ 1 ]. This can reach over $89,000 [ 1 ] for more senior positions. Salaries vary according to employer, industry, and type of trial.
Who are typical employers of clinical research coordinators?
A clinical research coordinator can work in various places, including health care organizations, pharmaceutical companies, research hospitals, and government and private companies. You may be employed to research new medicines for market, genetic diseases, illness prevention, and behavioral prevention.
If you’re ready to take your next steps towards a career as a clinical research coordinator, you can start with a short course on Design and Interpretation of Clinical Trials delivered by John Hopkins University on Coursera. This can give you a good grounding in understanding clinical trials and research.
Related articles
Clinical Managers: What They Do + How to Become One
Is Health Care a Good Career Path? Outlook, Jobs, and More
How to Become a Clinical Research Associate
Article sources
Glassdoor. “ Clinical Research Coordinator Salaries , https://www.glassdoor.com/Salaries/us-clinical-research-coordinator-salary-SRCH_IL.0,2_IN1_KO3,32.htm.” Accessed March 9, 2023.
Keep reading
Coursera staff.
Editorial Team
Coursera’s editorial team is comprised of highly experienced professional editors, writers, and fact...
This content has been made available for informational purposes only. Learners are advised to conduct additional research to ensure that courses and other credentials pursued meet their personal, professional, and financial goals.
What Can a Master’s in Research Administration Do for You?

Fill out the form below and we'll email you more information about UCF's online healthcare programs.
- Name * First Last
- Degree * Autism Spectrum Disorders Executive Master of Health Administration, EMHA Forensic Science, MS Fundraising Gender Studies Health Informatics and Information Management, BS Health Services Administration, BS Healthcare Simulation Healthcare Systems Engineering Certificate Healthcare Systems Engineering, MS Integrative General Studies, BGS Interdisciplinary Studies – Diversity Studies Leadership Track, BA Interdisciplinary Studies, BA/BS Master of Public Administration, MPA Master of Science in Healthcare Informatics Master of Social Work Online Nonprofit Management Nonprofit Management, MNM Nursing Education Nursing Practice, DNP, Advanced Track Nursing Practice, DNP, Executive Track Nursing, BS Nursing, BSN to PhD Nursing, MSN Nursing, PhD Project Engineering Psychology, BS Public Administration Research Administration Certificate Research Administration, MRA Systems Engineering
Privacy Notice
As the research administration field continues to grow, more training courses and certifications have become available. So, what is the benefit of pursuing a master’s degree over a professional certification?
Professional certifications focus on teaching and testing you on the processes involved in research administration. A master’s degree program prepares you for those processes and teaches you how to prepare and adjust for the little things. A master’s in research administration provides an in-depth education that enables graduates to further research by answering complex questions and mitigating future issues in a systematic way.
Pursuing a master’s degree provides students the opportunity to grow their network with other research administrators for future professional development. Some programs also offer training across a variety of research administration subjects rather than just one specific area, which can broaden employment opportunities.
What is Research Administration?
At its most basic definition, research administration is all administrative work and management performed for sponsored or funded programs.
Some people think research administration is only available at the university level, but there are research administrators across all work sectors. Research administrators work for governmental offices, non-profits, hospitals, clinical offices, and joint ventures. Anyone who manages a research project associated with a funder or financial sponsor is a research administrator.
Research Administrator Skills
The ultimate role of the research administrator is to make sure the administrative side of the project flows smoothly so that the researchers, investigators, or faculty members can focus on the research. As a result, many qualities make for a good research administrator.
Most people in the field typically possess a high level of organization and attention to detail. These are critical skills because there are many rules and regulations to follow. Time management is another valuable skill within the field as most research administrators juggle multiple tasks that need to flow smoothly to move a project along. It’s also crucial for research administrators to be personable and able to manage interpersonal relationships of the team members, which sometimes means addressing different areas to ensure that policies and guidelines are followed to keep the work flowing.
Career Opportunities
There are several different aspects of research administration. Research administrators specialize in contract negotiation, compliance management, project management, grant writing and more.
Some job titles within the field include:
- Associate Research Administrator
- Contract Administrator
- Contract Specialist
- Grants Manager
- Grants Specialist
- Compliance Manager
- Project Manager
This is not an exhaustive list of job titles in the field as there are multiple pathways into research administration, and different organizations use different titles for similar positions.
Growth Opportunities
The research administration field is rapidly growing and there are many opportunities for growth within the space. Having a master’s in research administration creates pathways to becoming a vice president or associate vice president of research/research development. There are opportunities to move from departmental offices into central offices, grow in grant development, move into the private sector, and much more. A master’s degree is also beneficial for students interested in additional graduate education.
UCF Online’s Master of Research Administration
UCF Online offers a fully online Master’s of Research Administration designed to accommodate the needs of working professionals. The program’s flexible structure prepares graduates to work in various settings, including research institutes, universities, hospitals and more. Students who complete the program will have the skills necessary to guide contract negotiation and proposal development and a deep understanding of legal, ethical, and regulatory compliance.
UCF Online’s Master’s of Research Administration also serves as a pathway to the Certified Research Administrator (CRA) exam, which is professionally recognized and can further enhance employment opportunities. Get started today to launch your career as a research administrator.
UCF’s Online Healthcare Degrees
- Autism Spectrum Disorders
- Executive Master of Health Administration, EMHA
- Forensic Science, MS
- Fundraising
- Gender Studies
- Health Informatics and Information Management, BS
- Health Services Administration, BS
- Healthcare Simulation
- Healthcare Systems Engineering Certificate
- Healthcare Systems Engineering, MS
- Integrative General Studies, BGS
- Interdisciplinary Studies – Diversity Studies Leadership Track, BA
- Interdisciplinary Studies, BA/BS
- Master of Public Administration, MPA
- Master of Science in Healthcare Informatics
- Master of Social Work Online
- Nonprofit Management
- Nonprofit Management, MNM
- Nursing Education
- Nursing Practice, DNP, Advanced Track
- Nursing Practice, DNP, Executive Track
- Nursing, BS
- Nursing, BSN to PhD
- Nursing, MSN
- Nursing, PhD
- Project Engineering
- Psychology, BS
- Public Administration
- Research Administration Certificate
- Research Administration, MRA
- Systems Engineering
You May Also Enjoy

What They Do
What does a Research Administrator do?

A research administrator oversees the progress of research programs, ensuring efficiency and smooth workflow. Their responsibilities mostly revolve around devising strategies to optimize processes, coordinating different departments, setting goals and objectives, managing the schedule and budgets, and maintaining records of all transactions. There are also instances when a research administrator must produce progress reports, participate in gathering surveys and feedbacks, and resolve issues promptly and professionally. Furthermore, as a research administrator, it is essential to lead and encourage team members to reach goals while implementing the company's policies and regulations.
- Responsibilities
- Skills And Traits
- Comparisions
- Types of Research Administrator

Research administrator responsibilities
Research administrators play pivotal roles in managing research funds, ensuring compliance with regulations, and supporting researchers. They are responsible for tasks such as budget management, overseeing grant submissions, and providing financial reports. As stated by Lee Penn Ph.D. , Director of Undergraduate Studies - Chemistry Department, Merck Professor, and Professor at the University of Minnesota, "I hear over and over again from recruiters - they want candidates with strong backgrounds in their majors PLUS two things: 1 - experience with data science, statistics, or some kind of computer science 2 - soft skills (communication, playing well with others, collaboration, etc.
On a resume, these responsibilities might appear as follows: "Managed all budgets across a variety of grants, contracts, and cooperative agreements," or "Provided post-award financial management for research funds in accordance with campus policy and agency requirements." Other examples include "Analyzed monthly expenses, executed budget and expense reconciliation, payroll analysis, and headcount management," and "Developed and edited scholarly papers for peer-review, executed data collection and experiment procedures, and performed statistical analyses."
Here are examples of responsibilities from real research administrator resumes:
- Create and manage multiple computer databases using SAS and SPSS, ensuring data up-to-date and accurate.
- Collect, manage and analyze preliminary data using SPSS statistical software.
- Prepare complex NIH clinical research proposals involving coordination with several clinical research sites.
- Assist with new patient eligibility evaluation, registration, and coordinating protocol relate care of patients enroll in clinical trials.
- Train in GCP, ICH, FDA, and local regulations for drug and device trials.
- Attend HIV treatment information meetings in Washington, D.C.
- Complete the EMR (electronic medical record) transition.
- Maintain patient data and phone encounters in the EMR.
- Coordinate with Pre-Award administrators for grant submissions, JIT and progress reports.
- Maintain study databases and oversee data collection per GCP and HIPAA guidelines.
- Download Sag3DMPRAGE structural MRI imaging sequence obtain from participants, and scull strip images Prepmr using MEDx.
- Develop a new consenting protocol for coordinators, nurses and patient educators to follow to increase patient satisfaction.
- Provide direction to others engage in the interviewing process and maintain record keeping for the HIV testing project.
- Supervise, hire and train staff in accordance with sponsor protocol, hospital standards, federal and GCP guidelines.
- Assist with the preparation of IRB applications, including protocol and inform consents and obtain approval to conduct the proposed study.
Research administrator skills and personality traits
We calculated that 10 % of Research Administrators are proficient in Research Administration , Customer Service , and Principal Investigators . They’re also known for soft skills such as Communication skills , Interpersonal skills , and Leadership skills .
We break down the percentage of Research Administrators that have these skills listed on their resume here:
Ensured effective operations of Research Administration by articulating professionally and utilizing sound judgment and time management to get the job done.
Established and developed a culture of customer service excellence and satisfaction to include measurement and continuous improvement.
Collaborate with Principal Investigators to assess the most effective means of addressing issues pertaining to institutional and/or federal regulations.
Contribute in the development of long-range plans and funding strategies for activities of assigned PIs.
Coordinated with Pre-Award administrators for grant submissions, JIT and progress reports.
Analyzed monthly expenses, executed budget and expense reconciliation, payroll analysis, and headcount management.
"research administration," "customer service," and "principal investigators" are among the most common skills that research administrators use at work. You can find even more research administrator responsibilities below, including:
Communication skills. The most essential soft skill for a research administrator to carry out their responsibilities is communication skills. This skill is important for the role because "natural sciences managers must be able to communicate clearly with a variety of audiences, such as scientists, policymakers, and the public." Additionally, a research administrator resume shows how their duties depend on communication skills: "school of public health program manager for five grant-funded research projects focused on 911 dispatcher-caller emergency communication. "
Interpersonal skills. Many research administrator duties rely on interpersonal skills. "natural sciences managers lead research teams and therefore need to work well with others in order to reach common goals," so a research administrator will need this skill often in their role. This resume example is just one of many ways research administrator responsibilities rely on interpersonal skills: "displayed exceptional interpersonal skills including ability to work effectively with team members, irb members, senior administrators, and researchers. "
Leadership skills. research administrators are also known for leadership skills, which are critical to their duties. You can see how this skill relates to research administrator responsibilities, because "natural sciences managers must be able to organize, direct, and motivate others." A research administrator resume example shows how leadership skills is used in the workplace: "administer broad range of customer service activities and scheduling functions in support of multiple leadership members and several key customer accounts. "
Problem-solving skills. A big part of what research administrators do relies on "problem-solving skills." You can see how essential it is to research administrator responsibilities because "natural sciences managers use scientific observation and analysis to find answers to complex technical questions." Here's an example of how this skill is used from a resume that represents typical research administrator tasks: "analyzed processes and recommended technical solutions, along with opportunities for process improvement. "
Time-management skills. Another crucial skill for a research administrator to carry out their responsibilities is "time-management skills." A big part of what research administrators relies on this skill, since "natural sciences managers must be able to perform multiple administrative, supervisory, and technical tasks while ensuring that projects remain on schedule." How this skill relates to research administrator duties can be seen in an example from a research administrator resume snippet: "job responsibilities also include all pre-award procedures for timely submission to grant administration and sponsor deadlines. "
All research administrator skills
The three companies that hire the most research administrators are:
- Kronos Incorporated 24 research administrators jobs
- Stanford University 23 research administrators jobs
- Harvard University 14 research administrators jobs
Choose from 10+ customizable research administrator resume templates

Compare different research administrators
Research administrator vs. clinical project manager.
A clinical project manager specializes in developing and organizing clinical trials. Their responsibilities revolve around planning and coordinating with all necessary personnel and experts, scheduling meetings, and preparing necessary documentation, ensuring compliance with all laws and regulations. Moreover, a clinical project manager must devise strategies and train all staff, develop protocols and guidelines, coordinate with vendors and suppliers, evaluate staff and verify documentation, and assist in all activities to ensure that every process aligns with the project's agenda. Should there be any issues or concerns, a clinical project manager must conduct corrective measures right away.
There are some key differences in the responsibilities of each position. For example, research administrator responsibilities require skills like "research administration," "customer service," "principal investigators," and "pis." Meanwhile a typical clinical project manager has skills in areas such as "manage cross," "patients," "oversight," and "cro." This difference in skills reveals the differences in what each career does.
Research administrator vs. Clinical trials associate
A clinical trial associate is in charge of coordinating and executing clinical trial operations, ensuring to meet all goals within budgets and deadlines. Their responsibilities revolve around preparing and processing necessary documentation and certifications, submitting requirements to government agencies, distributing essential materials within the clinical teams, and reviewing study sheets, ensuring every paperwork is complete and accurate. Furthermore, as a clinical trial associate, it is vital to lead while implementing the company's policies and regulations, including its vision and mission.
Each career also uses different skills, according to real research administrator resumes. While research administrator responsibilities can utilize skills like "research administration," "customer service," "principal investigators," and "pis," clinical trials associates use skills like "patients," "clinical operations," "consent forms," and "tmf."
Research administrator vs. Clinical trial manager
A clinical trial manager is primarily responsible for organizing and supervising clinical trials, ensuring to meet all goals while maintaining smooth operations. They are also responsible for collaborating with managers to set targets, hiring staff and participants for studies, and arranging the event. This planning includes its location and schedule. A clinical trial manager must also maintain documentation of all processes, liaise with scientists and key personnel, acquire necessary legal paperwork and certifications, and gather data accurately. Furthermore, as a manager, it is essential to implement the company's policies and regulations at all times.
The required skills of the two careers differ considerably. For example, research administrators are more likely to have skills like "research administration," "customer service," "pis," and "pre-award." But a clinical trial manager is more likely to have skills like "patients," "clinical operations," "oversight," and "ich."
Research administrator vs. Study director
Technically, a study director carries out scientific responsibilities for protocol design or study plan and approval. Study directors supervise the gathering, analysis, interpretation, documentation, and reporting of data results. They handle the matriculation of students with regard to data management system development. Working with the computer systems team is part of their duties so they will be able to establish a data management system in tracking the study participants. They also support the toxicology team or group on different project teams.
Even though a few skill sets overlap between research administrators and study directors, there are some differences that are important to note. For one, a research administrator might have more use for skills like "research administration," "customer service," "principal investigators," and "pis." Meanwhile, some responsibilities of study directors require skills like "toxicology," "study design," "data interpretation," and "laboratory practices. "
Types of research administrator
- Administrator
- Clinical Coordinator
- Clinical Research Coordinator
- Research Coordinator
Study Coordinator
- Research Project Coordinator
Updated April 25, 2024
Editorial Staff
The Zippia Research Team has spent countless hours reviewing resumes, job postings, and government data to determine what goes into getting a job in each phase of life. Professional writers and data scientists comprise the Zippia Research Team.
What Similar Roles Do
- What an Administrator Does
- What a Clinical Associate Does
- What a Clinical Coordinator Does
- What a Clinical Project Manager Does
- What a Clinical Research Assistant Does
- What a Clinical Research Associate Does
- What a Clinical Research Coordinator Does
- What a Clinical Research Manager Does
- What a Clinical Trial Coordinator Does
- What a Clinical Trial Manager Does
- What a Clinical Trials Associate Does
- What a Coordinator And Research Assistant Does
- What a Research Coordinator Does
- What a Research Nurse Does
- What a Research Project Coordinator Does
Research Administrator Related Careers
- Clinical Associate
- Clinical Project Manager
- Clinical Research Assistant
- Clinical Research Associate
- Clinical Research Manager
- Clinical Trial Coordinator
- Clinical Trial Manager
- Clinical Trials Associate
- Coordinator And Research Assistant
- Research Nurse
Research Administrator Related Jobs
Resume for related jobs.
- Administrator Resume
- Clinical Associate Resume
- Clinical Coordinator Resume
- Clinical Research Assistant Resume
- Clinical Research Associate Resume
- Clinical Research Coordinator Resume
- Research Coordinator Resume
- Research Nurse Resume
- Research Project Coordinator Resume
- Senior Clinical Research Associate Resume
- Senior Research Associate Resume
- Study Coordinator Resume
- Zippia Careers
- Executive Management Industry
- Research Administrator
- What Does A Research Administrator Do
Browse executive management jobs
- RACC Mission and Purpose
- RACC Diversity, Equity, and Inclusion Statement
- RACC Board of Directors
- RACC Board of Directors Requirements
- Quarterly Newsletter Archive
- Introduction
- Qualifications and Credentials
- What is the Body Of Knowledge?
- How Do I Prepare for an Examination?
- Certification Exam Changes
- Certification Exam Changes FAQs
- NEW CERTIFICANT INFORMATION
- Recertification Information & Applications
- Certification Term and Contact Hours
- How to Earn Contact Hours
- Candidate Handbook for Research Administrators
- CRA Body of Knowledge
- CRA Application
- CRA Petition to Waive
- Candidate Handbook for Pre-Award Research Administrators
- CPRA Body of Knowledge
- CPRA Application
- CPRA Petition to Waive
- Candidate Handbook for Financial Research Administrators
- CFRA Body of Knowledge
- CFRA Application
- CFRA Petition to Waive

Online Master’s in Clinical Research Administration (CRA) Degree Programs
Clinical research refers to the process by which new drugs, medical devices, and treatment protocols are developed and integrated into the healthcare system. This process is a complex one that typically involves scientific research, clinical trials, peer reviews, and formal approval procedures. The research and development phase often requires the coordination of multi-disciplinary teams that may include laboratory scientists, healthcare clinicians, chemical and biological engineers, computer programmers, data analysts, regulatory affairs specialists, pharmacologists, bioethicists, and medical experts. Once clinical trials are completed, the results must be compiled and presented to industry experts and government regulators according to strict protocols for official approval. Managing this multi-tiered, time-intensive, and expensive process requires training in organizational leadership and communication, a familiarity with various research methodologies, and an understanding of the laws and regulations pertaining to medical research. This is the skill set that defines the field of clinical research administration, or CRA.
What Is a Master’s in Clinical Research Administration Degree?
A master’s degree in CRA is typically a Master of Science degree program that prepares students to manage clinical research and trials that yield new medical devices, biologics, and pharmaceuticals. Students in a CRA program learn about the clinical procedures, regulatory constraints, ethical concerns, and business imperatives that apply to medical research. They study experimental design, clinical trials, and oversight protocols for studies that may involve human and/or animal subjects. And they cultivate the healthcare systems management and technical communication skills needed to align the needs of scientific researchers with those of non-technical administrators and executives at hospitals and medical centers, government agencies, pharmaceutical companies, medical device manufacturers, and other private and public medical research organizations.
Online Master’s in CRA Programs
As an alternative to traditional, campus-based master’s programs that prepare students for careers in clinical research administration, there are online programs that provide the same training and instruction through distance learning technologies. These programs utilize learning management systems (LMSs) to deliver lectures and other course materials to students anywhere with an Internet connection. Students in an online master’s in CRA program receive all or most of their instruction online. They interact with instructors, participate in discussion forums, submit assignments, and can often access library resources and other supplemental materials by logging on to the program’s LMS. Online CRA programs offer what is often a more convenient and flexible pathway to earning an advanced degree in clinical research administration for students who want formal training in this field without having to relocate or commute to a college or university campus.
How OnlineEducation.com Identifies and Classifies Online Master’s in Clinical Research Administration Degree Programs
OnlineEducation.com research online master’s programs, identifies programs that offer training in clinical research administration, and classifies these programs based on several relevant criteria. Programs must be offered by regionally accredited, non-profit colleges and universities. All or most of the required coursework in these programs must be delivered online. Programs that require students to attend more than two campus visits per year are not included on the site. Common designations for master’s in clinical research administration programs include:
- Master of Science in Clinical Research Administration
- Master of Science in Health Sciences in Clinical Research Administration
- Master of Science in Clinical Research Management
- Master of Science in Clinical Research Organization and Management
- Master of Science in Clinical Research and Product Development
What Students Learn in an Online Master’s in Clinical Research Administration Program
While there is not currently a formal accrediting agency for master’s in CRA programs, the Consortium of Academic Programs in Clinical Research (CoAPCR) maintains Core Competency Domains for professional training in the field. As outlined by CoAPCR, training in clinical research administration integrates technical knowledge of research methodologies, regulatory compliance, and medical science with what are referred to as the soft skills of organizational management, leadership, and communication. Online master’s in CRA programs offer a curriculum that addresses topics in the eight competency domains defined by the CoAPCR:
- Scientific Concepts & Principles of Research Design
- Medical Product Development
- Ethical Considerations & Responsible Conduct of Clinical Research
- Clinical Study Operations & Regulatory Compliance
- Study & Site Management
- Data Management & Informatics
- Communication of Scientific Data
- Professionalism, Teamwork, & Leadership
Students in these programs learn principles of epidemiology, biostatistical analytics, and data management. They are introduced to internal review board (IRB) processes common in medical research, FDA oversight and approval procedures, federally mandated provisions for the treatment of animals in clinical research, and relevant aspects of HIPAA data security provisions. And they study management practices in healthcare organizations with a focus on quality control, risk reduction, and regulatory compliance. Finally, many programs challenge students to apply what they have learned in a capstone project related to clinical research administration.
Online Master’s in CRA Courses
The table below offers an overview of courses commonly offered in a master’s in CRA program. The names and descriptions are drawn from actual programs.
Admissions to Online Master’s in Clinical Research Administration Programs
Baseline eligibility for admissions to online master’s in CRA programs is extended to applicants who hold a bachelor’s degree from an accredited college or university. In addition, some programs require applicants to demonstrate that they have completed one or two years of experience in a healthcare or medical field and/or several undergraduate prerequisite courses that cover anatomy, physiology, medical terminology, and/or statistical reasoning. In addition to college transcripts, programs may require applicants to submit GRE test scores, two or more letters of recommendation, and/or a personal goals statement. Selective programs may consider an applicant’s undergraduate GPA as part of the admissions process, and some programs require applicants to have a GPA of 3.0 or higher. It is important to note that admissions criteria vary by program, and that the requirements for applicants who hold an advanced degree are often different from those for applicants who hold a bachelor’s degree.
Online Master’s in Clinical Research Administration Program Formats
There are several variations in how online programs are structured and formatted that potential applicants should be aware of and may want to consider prior to submitting an application. These variations can impact the relative convenience and/or flexibility of an online program. The three areas of primary concern are: the method of online instruction (synchronous vs. asynchronous instruction); enrollment options (part-time vs. full-time enrollment); and whether or not the program requires students to attend campus-based instructional sessions.
Synchronous vs. Asynchronous Instruction : Online courses that utilize synchronous instruction hold lectures and other class activities at regularly scheduled times. Students in these courses must be prepared to log on to the program’s LMS at specified times in order to participate in these instructional sessions. Online courses that utilize asynchronous instruction do include a real-time component. Students in these courses can access lectures and other course materials 24-7. While synchronous instruction provides more structure than asynchronous instruction, it may create scheduling conflicts for some students. Asynchronous instruction poses fewer potential scheduling conflicts, but it requires students to be more vigilant and self-motivated in order to ensure that they complete assignments on time.
Part-Time vs. Full-Time Enrollment : Online master’s in clinical research administration programs are typically designed to accommodate working professionals who may not be able to manage a full-time course load. Many programs provide students with a range of enrollment options, which determine how many courses a student takes per term and how long it will take to complete the program. Enrolling full-time in a master’s in clinical research administration program typically means taking three or four courses per semester and graduating in roughly two years (four traditional semesters). Enrolling part-time allows students to take fewer classes per semester but can extend the time to completion by a year or more. Some programs offer courses year round, which allows full-time students to graduate in less than two years and part-time students to complete their requirements in two years provided they are willing to attend summer sessions.
Campus Visits : Many online master’s in clinical research programs offer all of their coursework online and do not require students to attend campus-based sessions. However, campus visits are an integral component of some online programs, which require students to come to the school’s campus or another location for seminars, workshops, and other instructional activities. These on-campus sessions, often referred to as intensives or immersions, can be a valuable part of the online educational experience, particularly for students who would like an opportunity to meet face-to-face with instructors. But they require travel and students may incur expenses in addition to base tuition and fees while attending campus sessions. Prospective applicants should inquire with programs to determine whether or not there are campus visit requirements. OnlineEducation.com does not include programs that require more than two campus visits per year.
- Clinical Research Coordinator Roles and Responsibilities
Position Role Sponsored Program Administration Financial Management Effort Reporting Conflicts of Interest Human Research Participant Protection Environmental Health and Safety Human Gene Transfer Export Controls
Position Role
The Clinical Research Coordinator (CRC) is a specialized research professional working with and under the direction of the clinical Principal Investigator (PI). While the Principal Investigator is primarily responsible for the overall design, conduct, and management of the clinical trial, the CRC supports, facilitates and coordinates the daily clinical trial activities and plays a critical role in the conduct of the study. By performing these duties, the CRC works with the PI, department, sponsor, and institution to support and provide guidance on the administration of the compliance, financial, personnel and other related aspects of the clinical study.
The clinical research coordinator reports primarily to the Principal Investigator with associated responsibilities to the department head, division administrator or program administrator.
Sponsored Program Administration
General administrative.
- Coordinates with Principal Investigator and school, department, and central administration to help ensure that clinical research and related activities are performed in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan. Maintains documentation of training.
- Assists Principal Investigator to assure that all key personnel or persons ‘engaged’ in the research project have met training requirements in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Cooperates with university compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office. Coordinates and facilitates monitoring and auditing visits. Notifies appropriate institutional officials of external audits by FDA and sponsors.
- Collaborates with PI and institution to respond to any audit findings and implement approved recommendations.
- Cooperates with university and sponsoring agency compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office.
Preparation of Scientific Proposal
- Assists the PI in study feasibility assessments as requested.
Proposal Budget
- Collaborates with the PI and department to prepare a categorized budget and justification. Confirms accuracy and completeness of budgeted costs.

Protocol Preparation & Review
- Reviews and comprehends the protocol.
- Attends investigator meetings as required or requested by the PI.
- Collaborates with the PI to prepare IRB/HRPO and any other regulatory submission documents as required by the protocol.
- Prepares other study materials as requested by the PI. These study materials include, but are not limited to, the informed consent document, case report forms (CRFs), enrollment logs, and drug/device accountability logs.
- Establishes and organizes study files, including but not limited to, regulatory binders, study specific source documentation and other materials.
Award Acceptance (Terms & Conditions)
- Reviews and develops a familiarity with the contract or award terms and conditions. Works with the PI to assure that the study is in compliance with all terms and conditions, including but not limited to education, IRB (HRPO) approval, conflict of interest disclosure, health and safety protections for participants and staff and any financial terms or conditions.
Conduct of Research
- Reviews and develops a familiarity with the protocol, e.g., study proceedings and timelines, inclusion and exclusion criteria, confidentiality, privacy protections.
- Assists PI in communication of study requirements to all individuals involved in the study. Provides appropriate training and tools for study team members. Documents date of training and signatures of study personnel trained on study specific training log.
- Collects documents needed to initiate the study and submit to the sponsor (e.g., FDA Forms 1572, CVs, etc.).
- Works with the PI to develop and implement recruitment strategies in accordance with HRPO (IRB) requirements and approvals.
- Conducts or participates in the informed consent process including interactions with the HRPO (IRB) and discussions with research participants, including answering any questions related to the study. Obtains appropriate signatures and dates on forms in appropriate places. Assures that amended consent forms are appropriately implemented and signed.
- Screens subjects for eligibility using protocol specific inclusion and exclusion criteria, documenting each potential participant’s eligibility or exclusion.
- Registers participants to the appropriate coordinating center (if multi-site study).
- Registers each participant in the billing matrix to ensure billing of study procedures to the appropriate funding source.
- Coordinates participant tests and procedures.
- Collects data as required by the protocol. Assures timely completion of Case Report Forms.
- Maintains study timelines.
- Maintains adequate inventory of study supplies. If handling investigational drugs/devices, follows the sponsor protocol and/or Washington University Policy on Investigational Drug/Device Accountability.
- Completes study documentation and maintains study files in accordance with sponsor requirements and University policies and procedures including, but not limited to, consent forms, source documentation, narrative notes if applicable, case report forms, and investigational material accountability forms.
- Retains all study records in accordance with sponsor requirements and university policies and procedures.
- Maintains effective and ongoing communication with sponsor, research participants and PI during the course of the study.
- Assists PI in preparation of any modifications to the scientific protocol in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Works with the PI to manage the day to day activities of the study including problem solving, communication and protocol management.
- Promotes the ethical conduct of research by reporting good faith suspicions of misconduct in research as defined within Washington University’s Research Integrity Policy and other misconduct as described in Washington University’s Code of Conduct.
- Assists Principal Investigator with scientific and compliance reporting requirements in accordance with Federal regulations and University and sponsoring agency policies and procedures. Assists in the registration (if required) of the study at ClinicalTrials.gov and maintains current information on the site.
Project Closeout
- Assists the Principal Investigator in submission of accurate and timely closeout documents to applicable federal agencies, university entities, and the sponsoring agency in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Arranges secure storage of study documents that will be maintained according to university policy or for the contracted length of time, whichever is longer.
Financial Management
- Reviews and accepts/corrects the billing matrix as set up by the Center for Applied Research Science (CARS) to facilitate billing of study procedures to the appropriate research fund.
- Coordinates appropriate and timely payments to participants (if applicable) in accordance with university policies and procedures.
Effort Reporting
- Reviews, adjusts and legally certifies personnel activity reports if applicable. Completes effort reporting certification within the timeframe specified by Sponsored Project Accounting.
Conflicts of Interest
- Takes appropriate steps to avoid conflicts of interest, or the appearance of conflicts of interest, between financial or other personal interests and the goals and policies of the university.
- Complies with applicable school, university, and sponsoring agency conflict of interest policies and procedures. Discloses all financial conflicts of interest to the appropriate supervisor.
- Cooperates with university compliance and monitoring efforts related to conflicts of interest and reports instances of noncompliance to the appropriate compliance office.
Human Research Participant Protection
- Assists Principal Investigator in protection of the rights and welfare of all human research participants involved in research in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Assists Principal Investigator in assuring that all key personnel involved in human research have completed the required education for the protection of human research participants in accordance with federal regulations and university and sponsoring agency policies and procedures. Maintains proof of all such education for all engaged members of the study team. Coordinates with Principal Investigator and school, department, and central administration to help ensure that clinical research and related activities are performed in accordance with Federal regulations and University and sponsoring agency policies and procedures.
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan. Maintains documentation of training.
- Cooperates with university compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office. Coordinates and facilitates monitoring and auditing visits. Notifies appropriate institutional officials of external audits by FDA and/or sponsors.
- Collaborates with PI and institution to respond to any audit findings and implement approved recommendations. Cooperates with university and sponsoring agency compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office.
- Collaborates with the PI to prepare IRB/HRPO and any other regulatory submission documents as required by the protocol. Prepares other study materials as requested by the PI. These study materials include but are not limited to the informed consent document, case report forms (CRFs), enrollment logs, and drug/device accountability logs.
- Establishes and organizes study files, including but not limited to, regulatory binders, case report forms, study specific source documentation.
Informed Consent
- Assists in preparation of all documents related to the informed consent process.
- Assists Principal Investigator in preparation and submission of informed consent documents to HRPO for review and approval.
- Collects documents needed to initiate the study for submission to the sponsor (e.g., FDA Forms 1572, CVs, etc.).
- Conducts or participates in the informed consent process including interactions with the HRPO (IRB), discussions with research participants, including answering any questions related to the protocol. Obtains appropriate signatures and dates on forms in appropriate places. Assures that amended consent forms are appropriately implemented and signed.
- Collects data as required by the protocol. Assures timely completion of Case Report Forms.
- Completes study documentation and maintains study files in accordance with sponsor requirements and University policies and procedures including, but not limited to, consent forms, source documentation, narrative notes if applicable, case report forms, and investigational material accountability forms, etc.
- Retains all study records in accordance with sponsor requirements and University policies and procedures.
- Assists PI in preparation and submission of any modifications to the scientific protocol in accordance with federal regulations and university and sponsoring agency policies and procedures.
Protected Health Information
- Adheres to and supports all Federal regulations and University policies and procedures instituted to safeguard protected health information (PHI).
- Completes the appropriate level of training regarding the access, use, and disclosure of PHI in accordance with Federal regulations and University and sponsoring agency policies and procedures. Assists PI to assure that all personnel complete appropriate training.
- Cooperates with University compliance and monitoring efforts regarding the access, use, and disclosure of PHI and reports instances of noncompliance to the appropriate compliance office.
Unanticipated Problems
- Assists the Principal Investigator in promptly reporting any unanticipated problems involving risks to research participants or others to the HRPO (Washington University’s IRB).
- Assists Principal Investigator with scientific and compliance reporting requirements in accordance with federal regulations and university and sponsoring agency policies and procedures.
- Assists in the registration (if required) of the study at ClinicalTrials.gov and maintains current information on the site.
- Arranges secure storage of study documents that will be maintained according to University policy or for the contracted length of time, whichever is longer.
Environmental Health and Safety
- Assists Principal Investigator in assuring that individuals handling hazardous or regulated materials are well trained in proper safety procedures and have completed required environmental health and safety training in accordance with federal, state, and local regulations and university and sponsoring agency policies and procedures.
- Works with Environmental Health and Safety to ensure that all facilities used are in compliance with all applicable regulations. Maintains copies of any applicable facility audits and equipment inspection/service reports.
Human Gene Transfer
- Assists Principal Investigator in assuring that all key personnel involved in human research have completed the required education for the protection of human research participants in accordance with Federal regulations and University and sponsoring agency policies and procedures. Maintains proof of all such education for all engaged members of the study team. Coordinates with Principal Investigator and school, department, and central administration to help ensure that clinical research and related activities are performed in accordance with Federal regulations and University and sponsoring agency policies and procedures.
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan. Maintains documentation of training.
- Cooperates with University compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office. Coordinates and facilitates monitoring and auditing visits. Notifies appropriate institutional officials of external audits by FDA and/or sponsors.
- Cooperates with University and sponsoring agency compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office.
- Collaborates with the PI to prepare IRB/HRPO and any other regulatory submission documents as required by the protocol. Prepares other study materials as requested by the PI. These study materials include but are not limited to the informed consent documents, case report forms (CRFs), enrollment logs, and drug/device accountability logs.
- Engages participants in the informed consent process according to the HRPO approved process.
Award Acceptance (Terms & Conditions)
- Reviews and develops a familiarity with the contract or award terms and conditions. Works with the PI to assure that the study is in compliance with all terms and conditions, including but not limited to education, IRB (HRPO) approval, conflict of interest disclosure, health and safety protections for participants and staff and any financial terms or conditions.
- Maintains adequate inventory of study supplies. If handling investigational drugs/devices, follows the sponsor and/or Washington University Policy on Investigational Drug/Device Accountability.
- Completes study documentation and maintains study files in accordance with sponsor requirements and university policies and procedures including, but not limited to, consent forms, source documentation, narrative notes if applicable, case report forms, and investigational material accountability forms, etc.
- Adheres to and supports all federal regulations and university policies and procedures instituted to safeguard protected health information (PHI).
- Completes the appropriate level of training regarding the access, use, and disclosure of PHI in accordance with federal regulations and university and sponsoring agency policies and procedures. Assists PI to assure that all personnel complete appropriate training.
- Cooperates with university compliance and monitoring efforts regarding the access, use, and disclosure of PHI and reports instances of noncompliance to the appropriate compliance office.
- Assists Principal Investigator with scientific and compliance reporting requirements in accordance with Federal regulations and University and sponsoring agency policies and procedures.
Export Controls
- Develops awareness of export control regulations and complies as appropriate.
Revised January 2009 | Created 2007
- Vice Chancellor for Research
- Annual Report & Metrics
- Faculty Resources
- Institutional Data
- Chancellor Roles and Responsibilities
- Dean Roles and Responsibilities
- Department Administrator Roles and Responsibilities
- Department Head/Chair Roles and Responsibilities
- Principal Investigator Roles and Responsibilities
- Vice Chancellor for Finance Roles and Responsibilities
- Vice Chancellor for Research Roles and Responsibilities
- Center for Applied Research Services Roles and Responsibilities
- Committee on Research Integrity Roles and Responsibilities
- Conflict of Interest Review Committee Roles and Responsibilities
- Division of Comparative Medicine Roles and Responsibilities
- Environmental Health and Safety Roles and Responsibilities
- Export Control Roles and Responsibilities
- HIPAA Roles and Responsibilities
- Human Research Protection Office Roles and Responsibilities
- Human Research QA/QI Roles and Responsibilities
- Institutional Animal Care and Use Committee Roles and Responsibilities
- Office of General Counsel Roles and Responsibilities
- Office of Sponsored Research Services Roles and Responsibilities
- Office of Technology Management Roles and Responsibilities
- Sponsored Projects Accounting Roles and Responsibilities
- University Compliance Office Roles and Responsibilities
- Ongoing Projects

- Upcoming Events
- Career Center

- Certificate Programs
- Clinical Trials Research Administration
About Clinical Trials Research Administration
The Clinical Trials Research Administration (CTRA) Certificate delivers intensive training sessions specifically designed to provide an understanding of the critical elements of successful administration of a clinical trials research program. The program has been redesigned to cover the critical elements of clinical trials management for research administrators and to more effectively integrate with other SRAI certificate offerings. The redesign maintains the required curriculum which introduces the student to the body of knowledge required to perform as an accomplished clinical trials research administrator. This certificate covers broad topics that allows for its content to be well integrated with the Research Integrity certificate also offered by SRAI.
The CTRA curriculum examines issues relevant to both National Institutes of Health-sponsored and industry-sponsored clinical trials. Much of the material is explored through experiential learning activities from seasoned research administrators including case studies. Elements of the curriculum include protocol review, recruitment, negotiation of agreements, development and negotiation of budgets, compliance, billing, international studies, and risk management and analysis. These elements, along with other relevant issues, will be presented in a combination of one full-day workshop and seven sessions for completion of the program.
Certificate Program Requirements
CTRA is comprised of one full-day workshop, five required sessions and two elective sessions. The required courses are listed below; the electives will vary meeting-to-meeting.
Required Workshop
A guide to clinical trials administration.
Clinical trials are a rapidly changing field in research administration. Many of the skills needed to administer more traditional investigator-initiated, grant-funded research projects can be adapted to managing clinical trials. This teaching workshop will examine the basics of clinical trial administration from recruiting projects to archiving records, explore the differences between industry and federally funded studies, highlight special concerns and discuss best practices for managing clinical trials.
Learning Objectives:
- Identify three milestones in the development of clinical research regulations and describe the importance of each.
- Identify three important regulatory differences between clinical research conducted in the US and in non-US/International regions.
Prerequisites: None
Required Sessions
Must take five (5).
Clinical Trials: The Industry Perspective
This is a presentation of the process of drug development from the pharmaceutical industry’s perspective Timelines, quality and cost will be driving factors for discussion. The pharmaceutical industry’s general organizational structure will be introduced. The clinical trial protocol development process and clinical study conduct will be presented. Pharmaceutical drivers for study site selection will be highlighted. Site-based contracts, including budgets, publication rights and intellectual property will also be discussed.
- Understand the drug development process including pharmaceutical industry drivers.
- Understand how sites are selected and contracts are negotiated for clinical trials.
Negotiating Clinical Trial Agreements with For-Profit Companies
Tips, traps and tricks of negotiating clinical trial agreements with the industry including a discussion of conducting negotiations via email versus teleconference discussions. Presenters will role play to illustrate tactics and strategies.
- Analyze negotiation strategies, identify their individual strengths and weakness and list common mistakes made by negotiators on each side of the table.
- Describe the benefits of compromise and tools to help finding it.
Determining Study Feasibility at Your Site
This is applicable for all studies (i.e., investigator initiated, industry funded, foundation funded or federally funded). The goal is to ensuring sufficient interest in the study design and research questions. Not just doing a study to do a study. The next steps are ensure that there are proper resources (i.e., study staffing including coordinators, investigators, research assistants; Institutional Review Board (IRB) and contract review teams, statisticians, etc.) to conduct the study; proper facilities (access to investigational pharmacy; inpatient or ambulatory facilities, etc.) and equipment (centrifuge, freezers, access to MRI or CT equipment); and sufficient and proper patient populations. Determining the proper mechanism for scientific review to ensure validity of the research questions. Proper financial review of direct and indirect costs and access to legal review through the grants and contracts team.
- Is the study answering relevant research questions through rigorous methodologies?
- The ability to analyze the legal, fiscal, ethical and scientific review of clinical trials to ensure that you are choosing the right studies for your site.
- Is this study helpful to your patient populations (i.e. are you meeting the clinical goals important to your medical environment).
Biorepository and Data Management Considerations for Clinical Trials
Changes of the common rule generated much attention around the management of biorepositories and related data in clinical research. This session will address the following topics for research administrators that are involved in clinical trial activity related to biorepository and data management: 1) Discussion of various repository models; 2) Ideas for repository management considerations; 3) Review of multiple regulatory considerations (Health and Human Services, Food and Drug Administration, and Health Insurance Portability and Accountability Act); 4) Impact on informed consent and privacy and confidentiality of data; and 5) Bio-specimen research scenarios for both current and future regulatory considerations.
- Identify different approaches and models for biorepository and data management for clinical research trials.
- Understand the impact of different regulations on bio-specimen and related data management clinical research.
Prerequisites: None
Clinical Trials: Rules and More Rules
Clinical trials must comply with the Good Clinical Practice guidelines (GCP). Unfortunately, the GCP is not well defined. This session will review the Department of Health and Human Services, Food and Drug Administration, and major international regulations and guidelines, as well as other institutions governing clinical research. Participants will learn how the actions of regulatory agencies affect budget development, contract negotiations and the way studies are conducted. Topics will include the ethical origins of the GCP, historic milestones and their regulatory significance, and discussion of best practices to enhance regulatory compliance.
Learning Objectives:
- Identify three important documents that form the core ethical basis for the regulatory environment.
- Identify three best practices for improving regulatory compliance in clinical trials.
Clinical Trials Budget Negotiation
Budgets for clinical trials should be based on sound accounting principles and an accurate analysis of the protocol. Often, establishing the costs for procedures is relatively straightforward, but accurately estimating hidden costs, such as investigator time and start-up costs, can be more difficult. A systematic process for analyzing protocols, determining costs and negotiating budgets will be discussed, along with relevant aspects of contract negotiations and establishing payment schedules. Tips to optimize budgeting for clinical trials will also be presented.
- Learn the four components of a clinical trial budget.
- Explain the differences between budgeting for clinical trials funded by industry versus federal sources.
Research Integrity: The Institutional Perspective
Academic and other research institutions are becoming more and more aware that many faculty, researchers, staff and students are not properly trained in ethics, professional standards and responsible conduct of research. This session provides a brief summary of the overall issues encountered and approaches to enhance the training and competency in research integrity and professional standards across several different types of organizations.
- Identify the institutional and cultural-discipline-specific challenges to Responsible Conduct of Research (RCR) training.
- List strategies to address the challenges in gaining "buy in" and implementing RCR training.
Prerequisites: None
Elective Sessions
Must take two.
Electives vary from meeting to meeting.
Certificates
- Financial Management
- Introduction to Research Administration and Management
- Leadership
- National Institutes of Health Grants Fundamentals
- Practice of Research Administration and Management
- Research Development
- Research Integrity
- Research Law

Society of Research Administrators International 1560 Wilson Blvd, Suite 310 Arlington, VA 22209
Phone: +1 703-741-0140
Partner with SRAI Donate to SRAI Career Center Privacy Statement Online Community Rules & Etiquette
Copyright ©2024 SRA International. All rights reserved.

Clinical Research Associate vs Coordinator (CRA vs CRC)
What is a cra/crc.
Use this guide to get a detailed side-by-side comparison of two similar acronyms with 2 very different roles.
The clinical research coordinator or CRC helps conduct the trial as one specific site and will archive all the documents at the site when the verification by CRA is complete.
The clinical research associate or CRA will review and verify documents from multiple sites conducting the same trial and do multiple visits to ensure quality and ethical conduct of the clinical trial .
CRA vs CRC (Clinical Research Coordinator vs Associate)
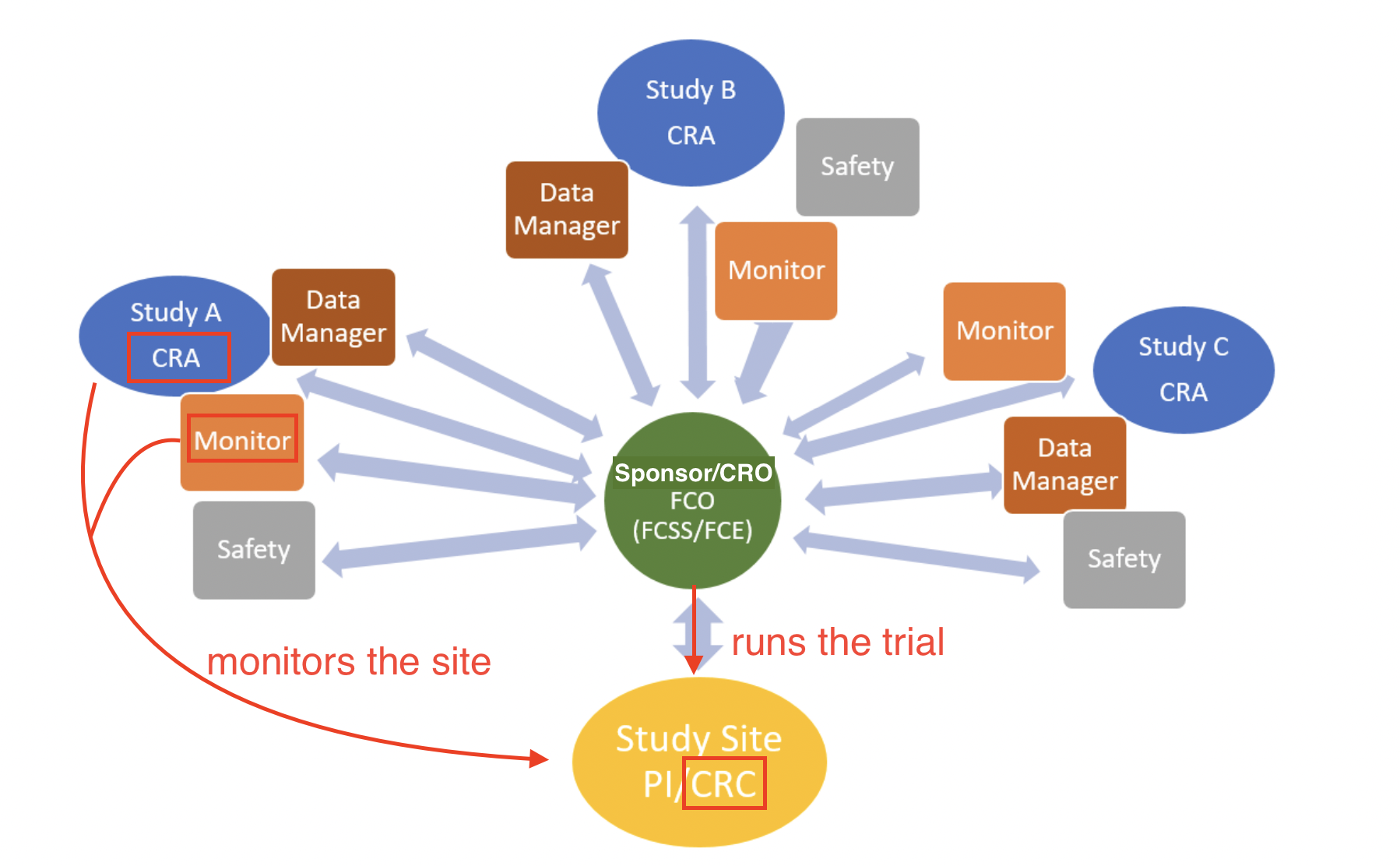
Clinical Research Associate vs Coordinator
Difference between clinical research associate and coordinator:
A clinical research associate ( CRA ), also called a clinical monitor or trial monitor , is a research professional with a minimum of a bachelors degree (usually nurses!) who works under contracts or hired by sponsors, CROs, or freelancing (by biopharma and research institutes) to perform roles listed in ICH GCP guidelines for monitoring clinical trials.
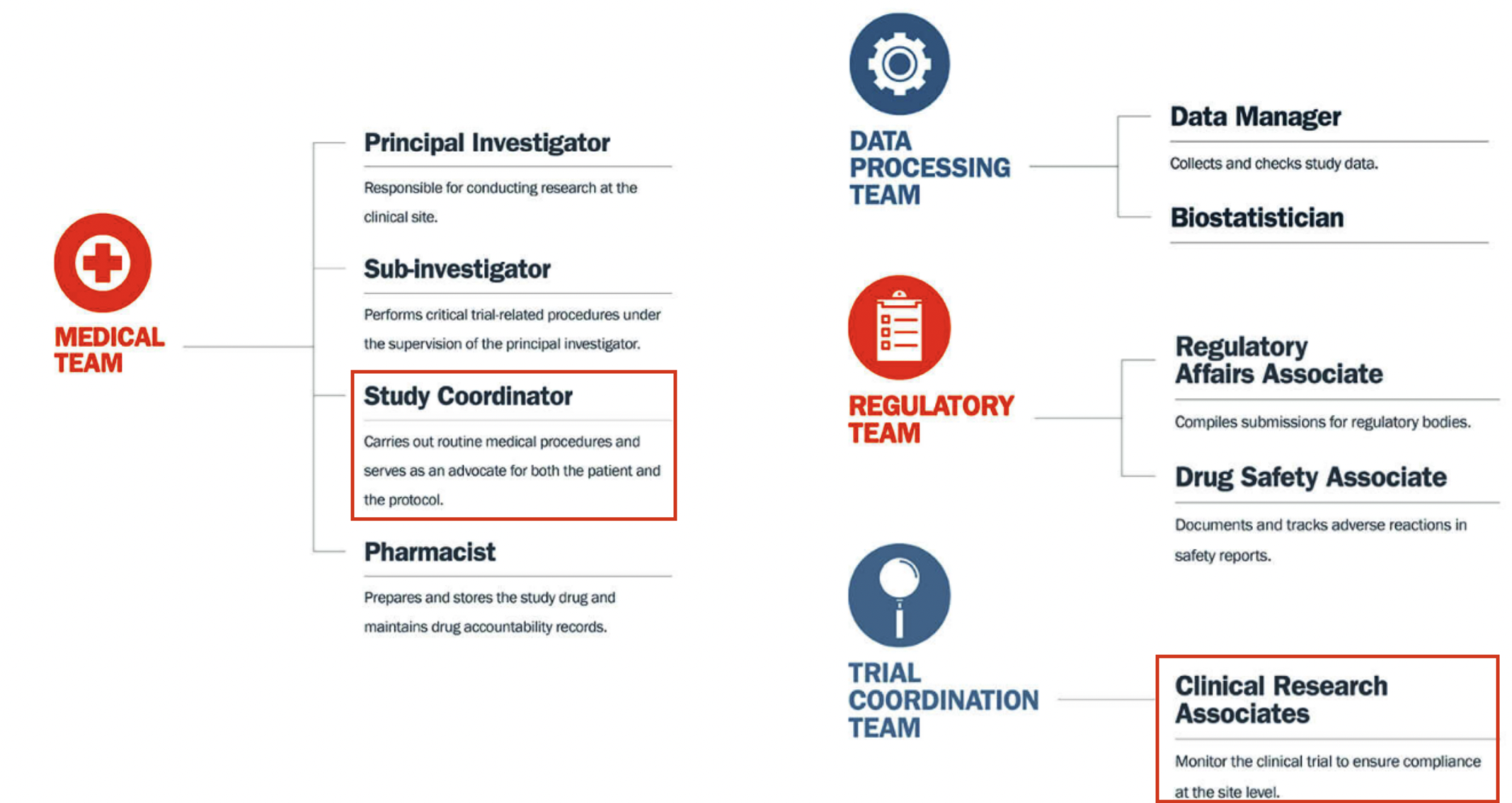
Who makes up the research team?
A CRA ensures compliance with ICH GCP and the clinical trial protocol by checking clinical site activities, making on-site visits (selection, initiation, routine, close-out), verifying “trial” case report forms (CRFs) are accurate by comparing to medical records, and speaking with the site’s CRC.
CRAs protect the ethical safety of human subjects and ensure the scientific integrity of the data collected through these processes.
Difference between clinical research coordinator and clinical research associate:
When a PI (principal investigator, i.e. often a busy, working physician running a trial on the side) is chosen to conduct a trial at their site, clinical research coordinators often take over part of the essential responsibilities of PI. This includes making sure the trial is 1) conducted and 2) in compliance with the protocol and federal or international regulations.
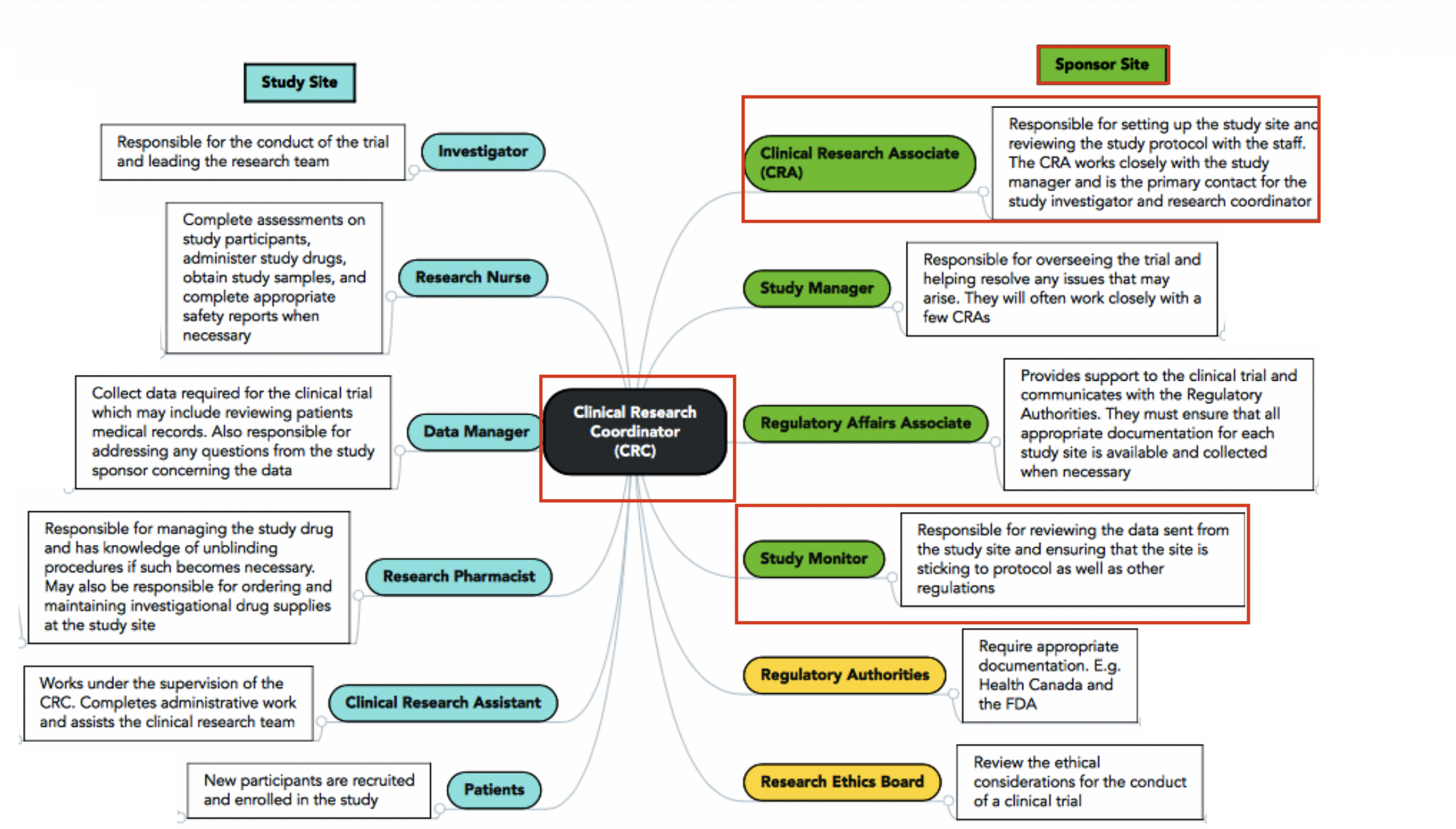
Site (CRC) vs. Sponsor (CRA)
CRC vs CRA: CRC responsibilities include writing the IRB/Ethics Committee application (specific to each site unless trial is under a single IRB/sIRB), making/performing informed consent (IC) process, developing a budget for the site, subject recruitment, patient care, adverse event reporting (a CRA simply audits and ensures that no AEs were missed!), preparing the case report form (CRF) for the CRA to review against medical records, and submitting tons of data and records to the CRA/Sponsor at each site visit.
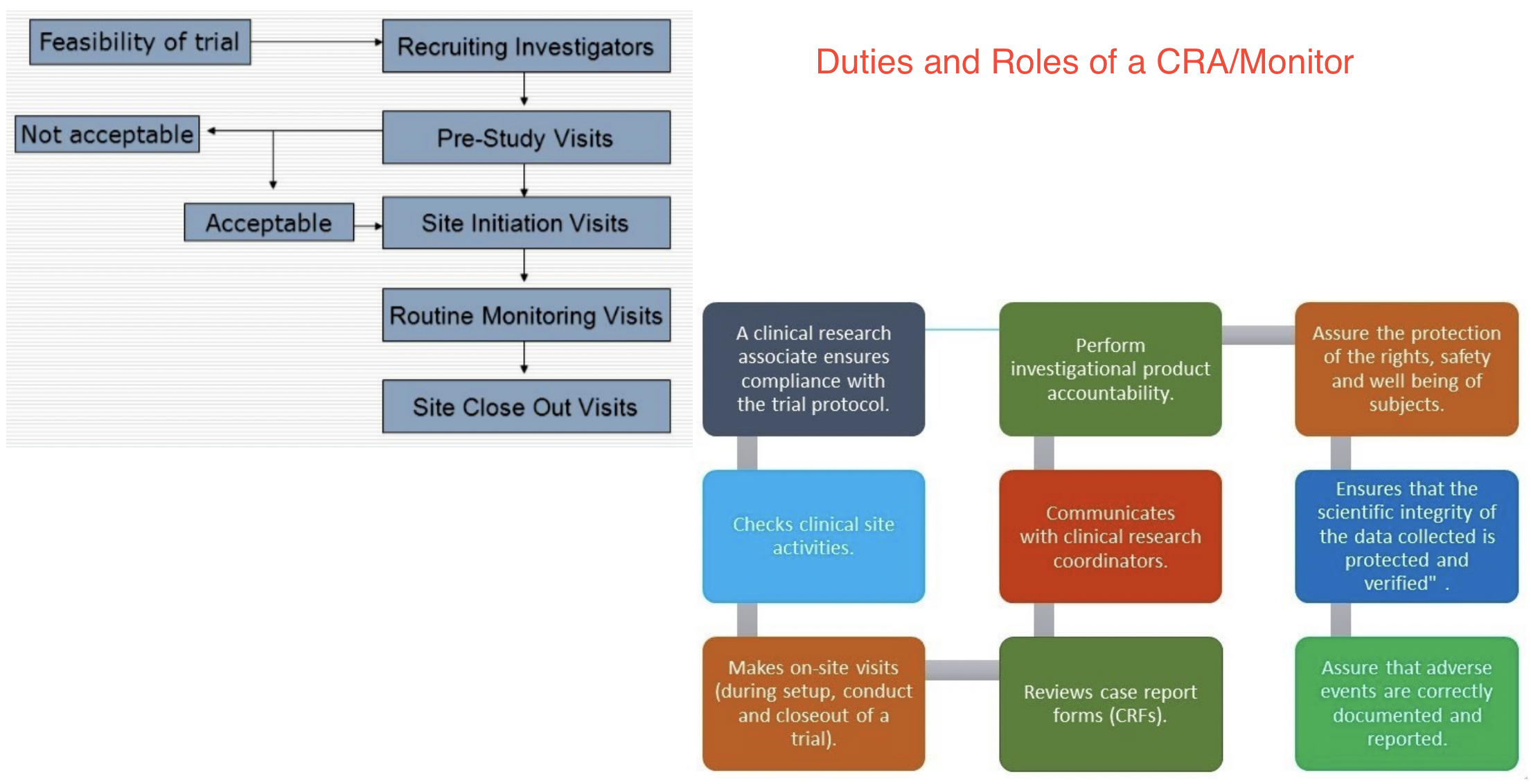
Roles of a CRA
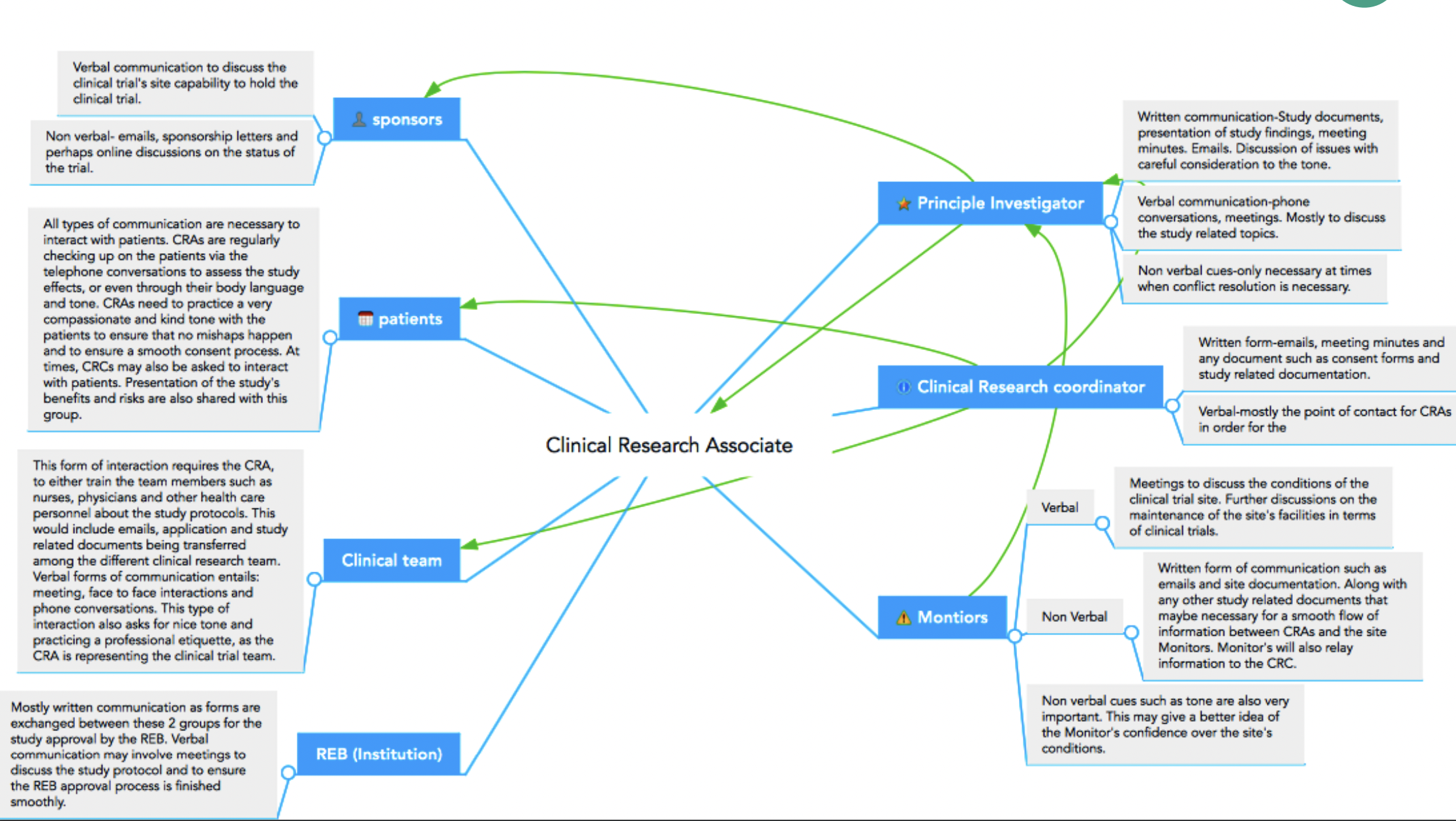
CRA interactions with other fields

Roles of a CRC
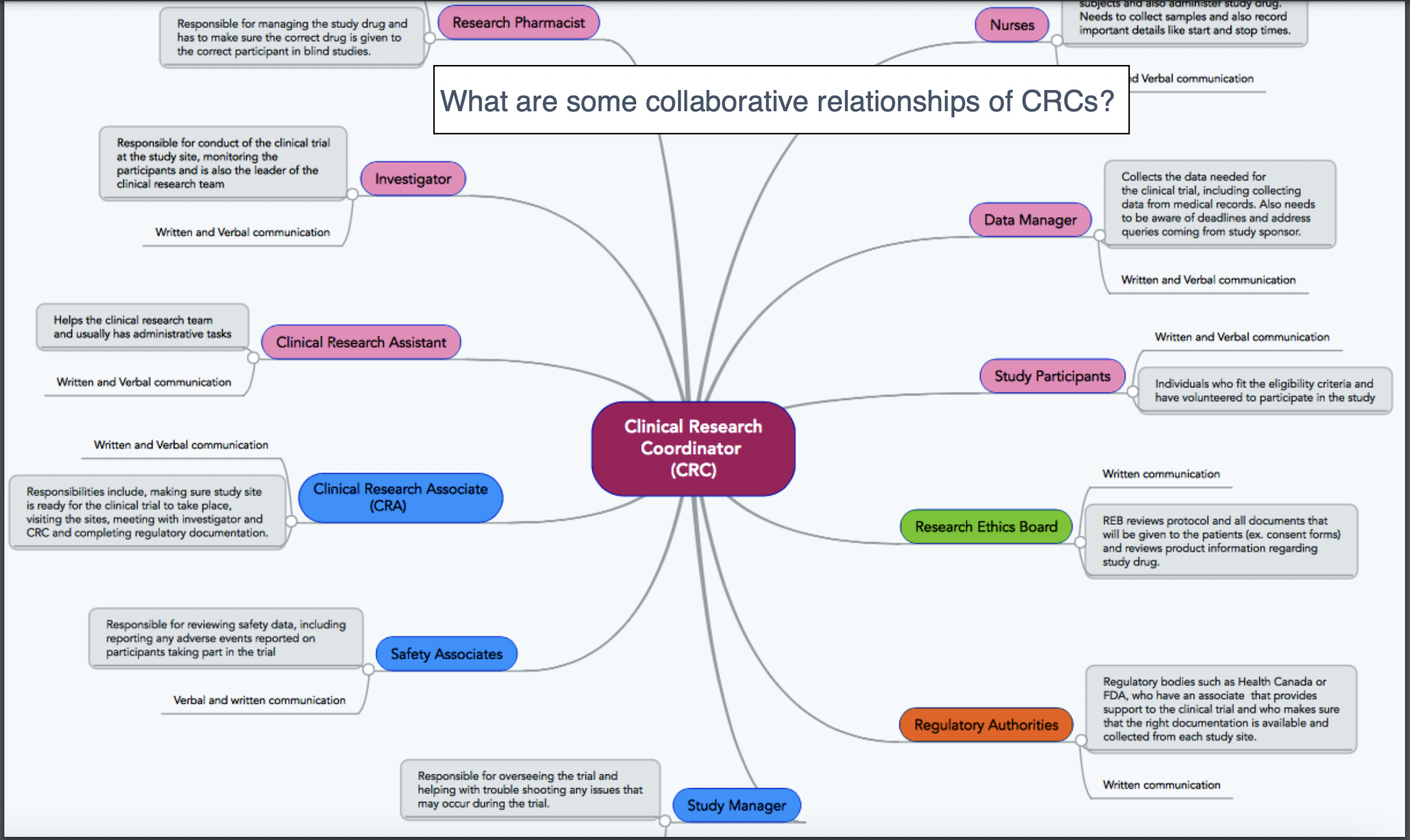
CRC interactions with other fields
Clinical Research Associate vs Coordinator Salary
Because CRAs manage multiple trial sites at one time, have a bachelors degree (minimum), and produce outcomes that are cost-effective for improving the efficiency of a trial; clinical research associates usually get paid more than coordinators. Unfortunately, clinical research coordinators are really doing the brunt of the “front-line” work and are the reason the trial occurs at that site all together. CRCs take a huge responsibility in both starting the trial and then presenting the trial documents to the CRAs as well as being the “middle-man” of the entire Site vs. Sponsor/CRO communication line. While CRCs deserve to get paid more, of course, it is not cost-effective as there are usually multiple sites and thus budgets are not capable of expanding upon the CRC’s pay-range. Luckily, CRC’s with experience can bridge to becoming a CRA through certifications and exams.
List of Relevant Courses:
Clinical Research Coordinator Certification: Enroll Here
Pharmacovigilance Certification: Enroll Here
Clinical Research Associate (CRA) Certification: Enroll Here
ICH-GCP Certification: Enroll Here
Clinical Trials Assistant Training: Enroll Here
Advanced Clinical Research Project Manager Certification: Enroll Here
Advanced Principal Investigator Physician Certification: Enroll Here
Medical Monitor Certification: Enroll Here
CRC to CRA bridge program
You can bridge into being a CRA in your own company or apply for jobs to be a CRA by completing CRA certification and trying to get experience with any on-site “in house” CRAs your site may have. CCRPS provides advanced “senior”-level CRA certification for CRCs so that:
1) on resumes you can prove knowledge competency of CRA tasks up to an advanced level (easier for in-job promotion)
2) during interviews you can prove your application of knowledge
3) during the job itself you can be efficient and diligent in preventing errors.
Clinical Research Coordinator Salary
Clinical research associate salary - what's the pay for a clinic research associate.
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
Essentials of Research Administration
This course provides an overview of research administration.
About this Course
The Essentials of Research Administration course explains concepts regarding research administration including the varying structures of sponsored programs, research advancement and development responsibilities, and award process specifics (pre-award, award negotiation and acceptance, and post-award). It also assists individuals working or seeking employment in research administration by exploring professional opportunities, various available resources, professional associations/organizations, advanced learning opportunities, and more.
It is intended for research administration professionals (sponsored programs professionals and managers), principal investigators (PIs), project directors, and others who are responsible for research administration. It may also help individuals determine whether a career in research administration is right for them.
This course was authored by Sandra M. Nordahl, CRA at Nordahl Worldwide Consulting and peer-reviewed by experts.
Language Availability: English
Suggested Audiences: Principal Investigators (PIs), Project Directors, Research Administration Professionals
Organizational Subscription Price: $675 per year/per site for government and non-profit organizations; $750 per year/per site for for-profit organizations Independent Learner Price: $139 per person
Course Content
Elements of research administration.
Describes the different areas within research administration including the varying structures of sponsored programs. It also explains the breadth of professional opportunities, resource associations/organizations, and advanced educational opportunities.
Recommended Use: Required ID (Language): 16967 (English) Author(s): Sandra M. Nordahl, CRA - Nordahl Worldwide Consulting
Elements of Research Development
Provides an overview of the different aspects of research development as related to sponsored programs. It discusses the research administration professional’s responsibility for identifying new funding opportunities, creating collaborative partnerships and interdisciplinary teams, and assisting in proposal development and communications with federal agencies.
Recommended Use: Required ID (Language): 16968 (English) Author(s): Sandra M. Nordahl, CRA - Nordahl Worldwide Consulting
Elements of Pre-Award
Discusses pre-award activities related to sponsored programs that occur during the proposal development and submission phases. It identifies elements of a good proposal, understand proposal budgeting, and explain Just In Time (JIT) submissions, while pointing out award mechanisms and compliance items.
Recommended Use: Required ID (Language): 16969 (English) Author(s): Sandra M. Nordahl, CRA - Nordahl Worldwide Consulting
Elements of Award Negotiation and Acceptance
Discusses the importance of award review, the principles of negotiation, compliance, and acceptance. This information also helps to explain the requirements for issuing timely sub-awards.
Recommended Use: Required ID (Language): 16970 (English) Author(s): Sandra M. Nordahl, CRA - Nordahl Worldwide Consulting
Elements of Post-Award
Describes activities that occur after the award of a proposal. The primary focus is on the administration of the sponsored project, from fund management, liaising with sponsors, documentation and submission of reports, and project closeout.
Recommended Use: Required ID (Language): 16971 (English) Author(s): Sandra M. Nordahl, CRA - Nordahl Worldwide Consulting
Additional Modules of Interest
Overview of the clinical trial agreement (cta).
Discusses the general purpose of a CTA, roles and responsibilities of parties to the CTA, and how the CTA fits into the research enterprise. It also compares and contrasts clinical trials involving drugs, biologics, and devices from a CTA perspective.
Note: This module is part of the CITI Program’s Human Subjects Research (HSR) series, but is recommended as part of this course. For organizations with a “Make Your Own” custom subscription, use of this module requires adding Human Subjects Research (HSR) to your organization’s subscription.
Recommended Use: Supplemental ID (Language): 17356 (English) Author(s): Dex Bilkic, HBSc, MBA - Bayer Inc.; JoAnn Pfeiffer, DrSC, RAC, CCRA - Arizona State University
Understanding the Terms of the Clinical Trial Agreement (CTA)
Provides an overview of the context behind certain CTA terms and sections, types of language used for CTA sections, and some key elements of each section. It also outlines what should be addressed in the key sections of the CTA and the aim for each section.
Recommended Use: Supplemental ID (Language): 17357 (English) Author(s): Dex Bilkic, HBSc, MBA - Bayer Inc.; JoAnn Pfeiffer, DrSC, RAC, CCRA - Arizona State University
Role of the Researcher and Site in Managing the Clinical Trial Agreement (CTA)
Discusses key roles of the researcher and site in managing the CTA, including initial assessment, review, and implementation. It also describes how the CTA is linked to site policies, the protocol, and the informed consent form, and identifies key sections of the CTA that could present risk to the site.
Recommended Use: Supplemental ID (Language): 17358 (English) Author(s): Dex Bilkic, HBSc, MBA - Bayer Inc.; JoAnn Pfeiffer, DrSC, RAC, CCRA - Arizona State University
Clinical Trial Agreement (CTA) Negotiation for Researchers and Sites
Addresses strategies and preparation for CTA and study budget negotiations. It also identifies terminology and alternative wording options to ensure a fair and balanced CTA.
Recommended Use: Supplemental ID (Language): 17359 (English) Author(s): Dex Bilkic, HBSc, MBA - Bayer Inc.; JoAnn Pfeiffer, DrSC, RAC, CCRA - Arizona State University
CME/CEU Credits
To purchase CE credits and units, you need to be affiliated with an organization that subscribes to this course or buy it as an independent learner first. Learn more about CE/CME Credits.
What is the importance of this training?
Research administration is vital to any research organization since professionals working in this field play a key role in research development, proposal development and submission, award negotiation and acceptance, and the administration of a sponsored project. Concise training on these topics helps to ensure that research efforts continue to move forward in a compliant, efficient manner.
When should someone consider taking this course?
This course is suitable for learners seeking an educational resource on research administration, as well as a discussion of professional and personal development in the industry. There is no uniform standard for how frequently this training should occur. For a retraining (refresher) cycle, organizations and independent learners should designate the frequency of retraining. Unlike other CITI Program courses, there is no “refresher” version available at this time, but learners can retake the course or complete whatever subset of content their organization has selected for them.
How is this course structured?
This course consists of five modules that provide comprehensive training on the different areas of the sponsored programs administration profession. The modules contain detailed content, images, supplemental materials (such as, case studies), and a quiz. Learners may complete the modules at their own pace.
What topics does this course cover?
It discusses the elements of research administration, research development, pre-award, award negotiation and acceptance, and post-award. It also covers professional and personal development in research administration, such as professional responsibilities, opportunities, and educational resources.
What are the standard recommendations for learner groups?
CITI Program allows organizations to customize their learner groups, which means they can choose the content modules their learners need to complete. We will work with your CITI Program designated admin to determine the learner groups that best fit your organizational needs.
The standard recommendation for this course is to designate each module as required in a learner group. This helps to ensure a complete training for the learner. However, organizations may also elect to present certain modules as supplemental, particularly when the organizations provide specific training on the topic(s).
What are the advantages of CITI Program's Research Administration training?
It was developed and reviewed by industry experts to equip organizations and individuals with a resource that will enhance research administration processes and individual success in the field. The author has over 28 years of experience in all aspects of research administration. Along with CITI Program's advantages, including our experience, customization options, cost effectiveness, and focus on organizational and learner needs, this makes it an excellent choice for this type of training.
Related Content
Explore key ethical, export, security, intellectual property, and transparency requirements related to international engagement.
A focused discussion for individuals who work with federally sponsored awards, PIs, and other members of the research team.
This course provides a step-by-step guide to help simplify the grant writing process.
Provides an overview of health disparities while raising awareness of this issue in research design and conduct.
This course provides an introduction to the False Claims Act ideally suited for faculty, researchers, and staff at research organizations.
Provides research administrators with strategies to build, improve, and retain employees.
Provides an overview of conflict, types of conflict, conflict styles, communication styles, and intervention strategies.
Provides clinical research professionals with basic and advanced training tailored to the CRC’s critical role in the conduct of clinical trials.
This course provides an expansive review of human subjects research topics for biomedical researchers.
This course provides basic CRC training.
Privacy Overview
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Learn About Drug and Device Approvals
- The Drug Development Process
Step 3: Clinical Research
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
On this page you will find information on:
Designing Clinical Trials
Clinical Research Phase Studies
The Investigational New Drug Process
Asking for FDA Assistance
FDA IND Review Team
Researchers design clinical trials to answer specific research questions related to a medical product. These trials follow a specific study plan, called a protocol , that is developed by the researcher or manufacturer. Before a clinical trial begins, researchers review prior information about the drug to develop research questions and objectives. Then, they decide:
Who qualifies to participate (selection criteria)
How many people will be part of the study
How long the study will last
Whether there will be a control group and other ways to limit research bias
How the drug will be given to patients and at what dosage
What assessments will be conducted, when, and what data will be collected
How the data will be reviewed and analyzed
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies.
What are the Clinical Trial Phases?
Watch this video to learn about the three phases of clinical trials.
Study Participants: 20 to 100 healthy volunteers or people with the disease/condition.
Length of Study: Several months
Purpose: Safety and dosage
During Phase 1 studies, researchers test a new drug in normal volunteers (healthy people). In most cases, 20 to 80 healthy volunteers or people with the disease/condition participate in Phase 1. However, if a new drug is intended for use in cancer patients, researchers conduct Phase 1 studies in patients with that type of cancer.
Phase 1 studies are closely monitored and gather information about how a drug interacts with the human body. Researchers adjust dosing schemes based on animal data to find out how much of a drug the body can tolerate and what its acute side effects are.
As a Phase 1 trial continues, researchers answer research questions related to how it works in the body, the side effects associated with increased dosage, and early information about how effective it is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Approximately 70% of drugs move to the next phase
Study Participants: Up to several hundred people with the disease/condition.
Length of Study: Several months to 2 years
Purpose: Efficacy and side effects
In Phase 2 studies, researchers administer the drug to a group of patients with the disease or condition for which the drug is being developed. Typically involving a few hundred patients, these studies aren't large enough to show whether the drug will be beneficial.
Instead, Phase 2 studies provide researchers with additional safety data. Researchers use these data to refine research questions, develop research methods, and design new Phase 3 research protocols.
Approximately 33% of drugs move to the next phase
Study Participants: 300 to 3,000 volunteers who have the disease or condition
Length of Study: 1 to 4 years
Purpose: Efficacy and monitoring of adverse reactions
Researchers design Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants.
Phase 3 studies provide most of the safety data. In previous studies, it is possible that less common side effects might have gone undetected. Because these studies are larger and longer in duration, the results are more likely to show long-term or rare side effects
Approximately 25-30% of drugs move to the next phase
Study Participants: Several thousand volunteers who have the disease/condition
Purpose: Safety and efficacy
Phase 4 trials are carried out once the drug or device has been approved by FDA during the Post-Market Safety Monitoring
Learn more about Clinical Trials .
Drug developers, or sponsors , must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.
In the IND application, developers must include:
Animal study data and toxicity (side effects that cause great harm) data
Manufacturing information
Clinical protocols (study plans) for studies to be conducted
Data from any prior human research
Information about the investigator
Drug developers are free to ask for help from FDA at any point in the drug development process, including:
Pre-IND application, to review FDA guidance documents and get answers to questions that may help enhance their research
After Phase 2, to obtain guidance on the design of large Phase 3 studies
Any time during the process, to obtain an assessment of the IND application
Even though FDA offers extensive technical assistance, drug developers are not required to take FDA’s suggestions. As long as clinical trials are thoughtfully designed, reflect what developers know about a product, safeguard participants, and otherwise meet Federal standards, FDA allows wide latitude in clinical trial design.
The review team consists of a group of specialists in different scientific fields. Each member has different responsibilities.
Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
Pharmacologist: Reviews preclinical studies.
Pharmakineticist: Focuses on the drug’s absorption, distribution, metabolism, and excretion processes.Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. FDA responds to IND applications in one of two ways:
Approval to begin clinical trials.
Clinical hold to delay or stop the investigation. FDA can place a clinical hold for specific reasons, including:
Participants are exposed to unreasonable or significant risk.
Investigators are not qualified.
Materials for the volunteer participants are misleading.
The IND application does not include enough information about the trial’s risks.
A clinical hold is rare; instead, FDA often provides comments intended to improve the quality of a clinical trial. In most cases, if FDA is satisfied that the trial meets Federal standards, the applicant is allowed to proceed with the proposed study.
The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports.
This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
- Make a Gift
- Request More Info
- Faculty & Staff
- Current Students
- Coronavirus Info

Search form
You are here.
- Graduate Programs
Master of Science in Research Administration and Compliance
- Academic Calendar
- Undergraduate Programs
- Certificate Programs
- Non-Degree Programs
- Textbooks and Course Materials
- Zero Textbook Cost Courses
- Credit for Prior Learning
- Office of Academic Affairs
- Summer Session
- Winter Session
- Fall 2024 Priority Deadline: April 4, 2024
- Fall 2024 Regular Deadline: May 16, 2024
Research administration and compliance expertise is needed across a diverse group of organizations, including academic institutions, research institutes and centers, hospitals, clinics, and the pharmaceutical industry. Each step within the life cycle of research is governed by complex regulations that overlap in their functions, and require high-level expertise to integrate concepts, policy requirements, and ethical considerations to assess, improve, and develop research programs.
The MS in Research Administration and Compliance online degree program creates a clear, attainable career path for research administration and compliance staff to become highly skilled leaders. The program guides professionals to make effective business decisions for their research enterprises by focusing on the following areas:
- Financial stewardship of research funds
- Ethical and compliant conduct of research
- Clinical research administration and compliance
- Intellectual property issues
- Policy development and implementation
- Organizational behavior and leadership
Frequently Asked Questions
What do Research Administration and Compliance professionals Do?
Read the article, ‘We’re problem solvers’: research administrators offer guidance to working scientists featuring our own faculty member, Debra Schaller-Demers.
Meet Some of Our Recent Faculty
- James Casey
- Rosemarie Gagliardi
- Nicholas Grosskopf
- Debra Schaller-Demers
- Henry Silverman
- Kristin Sommer
- Anthony Sterns
Career Prospects
According to PayScale.com, typical salaries for research administration managers across the U.S. range between $75,875 and $137,189 per year. Similarly, compliance managers can earn between $75,420 and $115,467 per year. The U.S. Bureau of Labor Statistics projects employment for research administrators and compliance officers to grow by 9.9% and 8.2%, respectively, by 2026.
Students graduating from the MS in Research Administration and Compliance program can expect to become administrators at academic institutions, healthcare organizations, government agencies, and non-profit organizations. With sufficient experience, graduates can aim to become leaders holding senior executive positions within these areas. Numerous and diverse career positions within these sectors include, but are not limited to: Contracts and Compliance Manager, Director of Sponsored Programs, Executive Director of Clinical Research Administration, Vice President or Vice Provost for Research Administration and/or Compliance, Research Program Manager, Grants Administrator, Clinical Research Quality Assurance Director, Research Finance and Post Award Administrator, Research Compliance Officer.
NCURA Education Scholarship Fund
The Education Scholarship Fund awards $2,500 scholarships to research administrators who have been National Council of University Research Administration members for at least one year and who are currently enrolled in a graduate degree program in research administration.
Academic Program Manager

Jenna Coplin
Academic Program Manager, Museum Studies/Research Administration and Compliance
Admissions Criteria
Applicants must possess a bachelor’s degree from a regionally accredited institution, with a GPA of 3.0 or higher on a 4.0 scale. Applicants are required to write a personal statement, upload a resume, and provide two letters of recommendation. Letters of recommendation may be submitted before or after submitting an application. Please note that an individual interview may be necessary.
Application Deadlines
Recent news about master of science in research administration and compliance, cuny office of research awards $400,000 in seed funding to 10 cross-disciplinary research projects.
April 03, 2023
CUNY Office of Research

Why Privacy Matters
March 23, 2023
CUNY SPS Blog
Nudges, Algorithms, and Human Choice: What Does the Future Hold?
March 10, 2022
Wisconsin Lawyer
2022 Florida Research Administration Conference Presentations
February 16, 2022
Florida Research Administration Conference
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
What marijuana reclassification means for the United States
The U.S. Drug Enforcement Administration will move to reclassify marijuana as a less dangerous drug, a historic shift to generations of American drug policy that could have wide ripple effects across the country.
FILE - Marijuana plants are seen at a secured growing facility in Washington County, N.Y., May 12, 2023. The U.S. Drug Enforcement Administration will move to reclassify marijuana as a less dangerous drug, a historic shift to generations of American drug policy that could have wide ripple effects across the country. (AP Photo/Hans Pennink, File)
- Copy Link copied
Budtender Rey Cruz weighs cannabis for a customer at the Marijuana Paradise on Friday, April 19, 2024, in Portland, Ore. (AP Photo/Jenny Kane)
Cloud 9 Cannabis employee Beau McQueen, right, helps a customer, Saturday, April 13, 2024, in Arlington, Wash. The shop is one of the first dispensaries to open under the Washington Liquor and Cannabis Board’s social equity program, established in efforts to remedy some of the disproportionate effects marijuana prohibition had on communities of color. (AP Photo/Lindsey Wasson)
WASHINGTON (AP) — The U.S. Drug Enforcement Administration is moving toward reclassifying marijuana as a less dangerous drug. The Justice Department proposal would recognize the medical uses of cannabis , but wouldn’t legalize it for recreational use.
The proposal would move marijuana from the “Schedule I” group to the less tightly regulated “Schedule III.”
So what does that mean, and what are the implications?
WHAT HAS ACTUALLY CHANGED? WHAT HAPPENS NEXT?
Technically, nothing yet. The proposal must be reviewed by the White House Office of Management and Budget, and then undergo a public-comment period and review from an administrative judge, a potentially lengthy process.
Still, the switch is considered “paradigm-shifting, and it’s very exciting,” Vince Sliwoski, a Portland, Oregon-based cannabis and psychedelics attorney who runs well-known legal blogs on those topics, told The Associated Press when the federal Health and Human Services Department recommended the change.
“I can’t emphasize enough how big of news it is,” he said.
It came after President Joe Biden asked both HHS and the attorney general, who oversees the DEA, last year to review how marijuana was classified. Schedule I put it on par, legally, with heroin, LSD, quaaludes and ecstasy, among others.
Biden, a Democrat, supports legalizing medical marijuana for use “where appropriate, consistent with medical and scientific evidence,” White House press secretary Karine Jean-Pierre said Thursday. “That is why it is important for this independent review to go through.”
Cloud 9 Cannabis employee Beau McQueen, right, helps a customer, Saturday, April 13, 2024, in Arlington, Wash. (AP Photo/Lindsey Wasson)
IF MARIJUANA GETS RECLASSIFIED, WOULD IT LEGALIZE RECREATIONAL CANNABIS NATIONWIDE?
Ap audio: what marijuana reclassification means for the united states.
AP correspondent Haya Panjwani reports on a proposal for the federal government to reclassify marijuana in what would be a historic shift that could have wide ripple effects across the country.
No. Schedule III drugs — which include ketamine, anabolic steroids and some acetaminophen-codeine combinations — are still controlled substances.
They’re subject to various rules that allow for some medical uses, and for federal criminal prosecution of anyone who traffics in the drugs without permission.
No changes are expected to the medical marijuana programs now licensed in 38 states or the legal recreational cannabis markets in 23 states, but it’s unlikely they would meet the federal production, record-keeping, prescribing and other requirements for Schedule III drugs.
There haven’t been many federal prosecutions for simply possessing marijuana in recent years, even under marijuana’s current Schedule I status, but the reclassification wouldn’t have an immediate impact on people already in the criminal justice system.
“Put simple, this move from Schedule I to Schedule III is not getting people out of jail,” said David Culver, senior vice president of public affairs at the U.S. Cannabis Council.
But rescheduling in itself would have some impact, particularly on research and marijuana business taxes.
WHAT WOULD THIS MEAN FOR RESEARCH?
Because marijuana is on Schedule I, it’s been very difficult to conduct authorized clinical studies that involve administering the drug. That has created something of a Catch-22: calls for more research, but barriers to doing it. (Scientists sometimes rely instead on people’s own reports of their marijuana use.)
Marijuana plants are seen at a secured growing facility in Washington County, N.Y., May 12, 2023. (AP Photo/Hans Pennink, File)
Schedule III drugs are easier to study, though the reclassification wouldn’t immediately reverse all barriers to study.
“It’s going to be really confusing for a long time,” said Ziva Cooper, director of the University of California, Los Angeles Center for Cannabis and Cannabinoids. “When the dust has settled, I don’t know how many years from now, research will be easier.”
Among the unknowns: whether researchers will be able to study marijuana from state-licensed dispensaries and how the federal Food and Drug Administration might oversee that.
Some researchers are optimistic.
“Reducing the schedule to schedule 3 will open up the door for us to be able to conduct research with human subjects with cannabis,” said Susan Ferguson, director of University of Washington’s Addictions, Drug & Alcohol Institute in Seattle.
WHAT ABOUT TAXES (AND BANKING)?
Under the federal tax code, businesses involved in “trafficking” in marijuana or any other Schedule I or II drug can’t deduct rent, payroll or various other expenses that other businesses can write off. (Yes, at least some cannabis businesses, particularly state-licensed ones, do pay taxes to the federal government, despite its prohibition on marijuana.) Industry groups say the tax rate often ends up at 70% or more.
The deduction rule doesn’t apply to Schedule III drugs, so the proposed change would cut cannabis companies’ taxes substantially.
They say it would treat them like other industries and help them compete against illegal competitors that are frustrating licensees and officials in places such as New York .
“You’re going to make these state-legal programs stronger,” says Adam Goers, of The Cannabist Company, formerly Columbia Care. He co-chairs a coalition of corporate and other players that’s pushing for rescheduling.
It could also mean more cannabis promotion and advertising if those costs could be deducted, according to Beau Kilmer, co-director of the RAND Drug Policy Center.
Rescheduling wouldn’t directly affect another marijuana business problem: difficulty accessing banks, particularly for loans, because the federally regulated institutions are wary of the drug’s legal status. The industry has been looking instead to a measure called the SAFE Banking Act . It has repeatedly passed the House but stalled in the Senate.
ARE THERE CRITICS? WHAT DO THEY SAY?
Indeed, there are, including the national anti-legalization group Smart Approaches to Marijuana. President Kevin Sabet, a former Obama administration drug policy official, said the HHS recommendation “flies in the face of science, reeks of politics” and gives a regrettable nod to an industry “desperately looking for legitimacy.”
Some legalization advocates say rescheduling weed is too incremental. They want to keep the focus on removing it completely from the controlled substances list, which doesn’t include such items as alcohol or tobacco (they’re regulated, but that’s not the same).
Paul Armentano, the deputy director of the National Organization for the Reform of Marijuana Laws, said that simply reclassifying marijuana would be “perpetuating the existing divide between state and federal marijuana policies.” Kaliko Castille, a past president of the Minority Cannabis Business Association, said rescheduling just “re-brands prohibition,” rather than giving an all-clear to state licensees and putting a definitive close to decades of arrests that disproportionately pulled in people of color.
“Schedule III is going to leave it in this kind of amorphous, mucky middle where people are not going to understand the danger of it still being federally illegal,” he said.
This story has been corrected to show that Kaliko Castille is a past president, not president, of the Minority Cannabis Business Association and that Columbia Care is now The Cannabist Company.
___ Peltz reported from New York. Associated Press writers Colleen Long in Washington and Carla K. Johnson in Seattle contributed to this report.

IMAGES
VIDEO
COMMENTS
Trainee research roles and responsibilities. Postdocs and graduate students must: Seek and follow faculty guidance on scientific and other procedures (e.g., allowable uses of grant funds) Maintain knowledge of and compliance with University procedures and policies related to sponsored research. Disclose financial conflicts of interest to the PI.
It typically takes 4-5 years to become a clinical research administrator: Years 1-4: Obtaining a Bachelor's degree in a relevant field, such as health sciences, public health, or a related field. Years 4-5: Accumulating the necessary work experience, such as coordinating clinical trials, managing budgets, and ensuring compliance with regulations.
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
Definition of a Clinical Research Professional. A clinical research professional's (CRP) practice is guided by one or more aspects of the principles of Good Clinical Practice (GCP). CRPs may have backgrounds in nursing, pharmacy, medical technology, business administration, health record management, statistics, science, education, or other areas.
The committee was composed of active researchers and research administrators with expertise in nursing, dentistry, evaluative clinical sciences, surgery, epidemiology, and various medical subspecialties. ... The first item on the committee's agenda was to derive a working definition of clinical research. Various definitions have been used to ...
Renewal Process: Certification is valid for three years and must be renewed by application. CCRPs must complete 45 hours/credits of continuing education during this period, 22 of which must be related to clinical research. The other 23 hours may concern the professional's specific career path. The fee to renew is $350.
What does a Research Administrator do? Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure ...
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private ...
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness ( efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. [1] [2] These research procedures are designed for the prevention, treatment, diagnosis or understanding ...
A master's in research administration provides an in-depth education that enables graduates to further research by answering complex questions and mitigating future issues in a systematic way. Pursuing a master's degree provides students the opportunity to grow their network with other research administrators for future professional ...
About CTRA 101. The Clinical Trials Research Administration ("CTRA") series delivers intensive training sessions specifically designed to provide an understanding of the critical elements of successful administration of a clinical trials research program. The program currently has two levels of progressively complex training that must be taken ...
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
On the other hand, research administrators are paid more in the health care industry with an average salary of $57,602.clinical project managers tend to reach similar levels of education than research administrators. In fact, clinical project managers are 4.1% more likely to graduate with a Master's Degree and 1.0% more likely to have a ...
Welcome to RACC. The Research Administrators Certification Council (RACC) formed in 1993 as an independent non-profit organization. RACC certifies that an individual, through experience and testing, has the fundamental knowledge necessary to be a professional research or sponsored programs administrator.
Ideal for someone new to the profession or as a refresher for a more seasoned research administrator, The Introduction to Research Administration and Management (IRAM) certificate explores the broad scope of the multi-faceted profession of research administration. The comprehensive curriculum - developed by some of the "best of the best ...
A master's degree in CRA is typically a Master of Science degree program that prepares students to manage clinical research and trials that yield new medical devices, biologics, and pharmaceuticals. Students in a CRA program learn about the clinical procedures, regulatory constraints, ethical concerns, and business imperatives that apply to ...
The Clinical Research Coordinator (CRC) is a specialized research professional working with and under the direction of the clinical Principal Investigator (PI). ... and central administration to help ensure that clinical research and related activities are performed in accordance with federal regulations and university and sponsoring agency ...
Clinical trials are a rapidly changing field in research administration. Many of the skills needed to administer more traditional investigator-initiated, grant-funded research projects can be adapted to managing clinical trials. This teaching workshop will examine the basics of clinical trial administration from recruiting projects to archiving ...
The clinical research coordinator or CRC helps conduct the trial as one specific site and will archive all the documents at the site when the verification by CRA is complete. The clinical research associate or CRA will review and verify documents from multiple sites conducting the same trial and do multiple visits to ensure quality and ethical ...
The Essentials of Research Administration course explains concepts regarding research administration including the varying structures of sponsored programs, research advancement and development responsibilities, and award process specifics (pre-award, award negotiation and acceptance, and post-award). It also assists individuals working or ...
Watch this video to learn about the three phases of clinical trials. Clinical Research Phase Studies. Phase 1. Study Participants: 20 to 100 healthy volunteers or people with the disease/condition ...
Research administration and compliance expertise is needed across a diverse group of organizations, including academic institutions, research institutes and centers, hospitals, clinics, and the pharmaceutical industry. Each step within the life cycle of research is governed by complex regulations that overlap in their functions, and require high-level expertise to integrate concepts, policy ...
NIA's Division of Geriatrics and Clinical Gerontology (DGCG) is accepting applications for Health Science Administrators (Program Officers) and Health Specialists in multiple branches within the Division. DGCG supports clinical and translational research on health and disease in the aged, and research on aging over the human life span, including its relationships to health outcomes.
DN supports research and training to further the understanding of neural and behavioral processes associated with the aging brain. Areas of special emphasis include brain-behavior relationships and how the processes of aging and age-related cognitive decline intersect with the development of AD and other dementias of aging.
What does a Research Administrator do? Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure ...
WHAT WOULD THIS MEAN FOR RESEARCH? Because marijuana is on Schedule I, it's been very difficult to conduct authorized clinical studies that involve administering the drug. That has created something of a Catch-22: calls for more research, but barriers to doing it. (Scientists sometimes rely instead on people's own reports of their marijuana ...
"Institutional support services" shall mean as defined at N.J.S.A. 52:9E-2c. "New Jersey Spinal Cord Research Fund" or "Fund" means the fund established pursuant to 52:9E-9. "Funds" as used in this chapter shall mean monies belonging to or drawn from the Fund. "Qualifying research institution" shall mean as defined at N.J.S.A. 52:9E-2d.