Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 29 July 2020

Molecular mechanisms and clinical management of cancer bone metastasis
- Manni Wang 1 na1 ,
- Fan Xia 2 na1 ,
- Yuquan Wei 1 &
- Xiawei Wei 1
Bone Research volume 8 , Article number: 30 ( 2020 ) Cite this article
15k Accesses
75 Citations
1 Altmetric
Metrics details
- Bone cancer
- Pathogenesis
As one of the most common metastatic sites of malignancies, bone has a unique microenvironment that allows metastatic tumor cells to grow and flourish. The fenestrated capillaries in the bone, bone matrix, and bone cells, including osteoblasts and osteoclasts, together maintain the homeostasis of the bone microenvironment. In contrast, tumor-derived factors act on bone components, leading to subsequent bone resorption or excessive bone formation. The various pathways involved also provide multiple targets for therapeutic strategies against bone metastases. In this review, we summarize the current understanding of the mechanism of bone metastases. Based on the general process of bone metastases, we specifically highlight the complex crosstalk between tumor cells and the bone microenvironment and the current management of cancer bone metastases.
Similar content being viewed by others
Novel approaches to target the microenvironment of bone metastasis
Lorenz C. Hofbauer, Aline Bozec, … Klaus Pantel
Evolving cancer–niche interactions and therapeutic targets during bone metastasis
Robert L. Satcher & Xiang H.-F. Zhang
Bone metastases
Robert E. Coleman, Peter I. Croucher, … Luis Costa
Introduction
The distant metastasis of cancer cells has long been known to have characteristic preferences. 1 , 2 Bone is one of the most common metastatic sites for malignancies, such as breast, prostate, and lung cancer. 3 Bone metastases can be categorized into osteolytic metastases with bone resorption, osteoblastic metastases with excessive bone formation, and a mixed phenotype of both. 4 , 5 According to the “seed and soil” hypothesis, bone metastasis is dependent on the interactions between tumor cells and the bone microenvironment. The preferential colonization of tumor cells to bone partly relies on the fenestrated capillaries in bone, bone matrix, and cells in the bone marrow (BM) stroma, such as osteoblasts and osteoclasts. 6 , 7 , 8 , 9 , 10 These components together maintain the homeostasis of the bone microenvironment. Several deleterious complications, such as ostealgia, fractures, serious hypercalcemia, and nerve compression syndromes, occur in bone metastasis. 11 As recent advances in bone metastasis research have revealed various pathways involved in this process, both in the primary tumor site and in the resident bone microenvironment, we herein describe the current understanding of the mechanism for bone metastases. Based on the general process of bone metastases, we specifically highlight the complex crosstalk between tumor cells and the bone microenvironment.
Bone microenvironment
Tumor metastasis is a complex process that involves the reciprocal interactions between tumor cells and the bone microenvironment. The preferential tumor metastasis to bone is therefore probably attributed to the bone microenvironment, which corresponds to the “seed and soil” hypothesis described below. 12 The bone matrix, BM sinusoid capillaries with a fenestrated structure that provides an abundant blood supply, and the cells in the BM stroma such as osteoblasts and osteoclasts all contribute to the bone microenvironment. 7 , 10 Therefore, elucidation of the composition of the bone microenvironment and its interaction with cancer cells can help clarify the underlying mechanisms of metastatic organotropism. The constant remodeling of the bone microenvironment is another potential reason for the preference of circulating tumor cells to colonize bones, and the regulation of cytokines and hormones during this process will be discussed herein.
The premetastatic niche
The premetastatic niche refers to the supportive environment of potential metastatic sites before the arrival of cancer cells, providing a fertile “soil” to facilitate the invasion, localization, survival, and proliferation of the “seeds,” namely, metastatic tumor cells. 12 , 13 In recent decades, studies on the selective colonization of cancer cells to bone have been primarily based on the “seed and soil” theory. 14 , 15 , 16 First proposed by Steven Paget, this theory was based on the autopsy analysis of 735 breast cancer patients and was published in the Lance t in 1889. Intrinsic differences, such as the genomic composition of tumor cells, are primarily attributed to the proliferative phenotype and metastatic potential of the “seeds,” but the microenvironmental condition of the host tissues, or the “soil,” is equally important. The premetastatic niche can be formed even before tumor dissemination due to the tumor-derived factors released from primary tumors. 17 These factors include several growth factors, such as vascular endothelial growth factor (VEGF). Moreover, the formation of a premetastatic niche relies on a suppressive immune system. Primary tumors recruit myeloid cells, which then allow tumor cells to evade immune surveillance, leading to metastasis. 18 , 19 Chemokines facilitate the recruitment of BM-derived cells (BMDCs) and the related immune evasion of primary tumor cells.
BM is a complex system consisting of multifunctional cells, including hematopoietic and mesenchymal stem cells (MSCs). 20 The mobilized and recruited BMDCs, as well as other stromal cells, together create a premetastatic niche by releasing various growth factors, inflammatory cytokines and chemokines, and proangiogenic molecules to support tumor cell colonization. 21 , 22 Recent studies have identified a subset of nonmalignant BM-derived hematopoietic progenitor cells (HPCs) that express VEGF receptor-1 (VEGFR-1) and promote the arrival of metastatic tumor cells at distant sites. 23 In response to primary tumor-derived chemokines, VEGFR-1 + BMDCs proliferate in the bloodstream and then preferentially localize to fibronectin-rich areas. At distant premetastatic sites, VEGFR-1 + HPCs upregulate the expression of the very late antigen-4 ligand, which specifically adheres to the newly synthesized fibronectin to establish a fibronectin-rich local microenvironment for cellular cluster formation. 13 These studies revealed that BM-derived HPCs could help prepare the premetastatic site for tumor metastasis. VEGFR-1 + BMDCs, fibronectin, and associated stromal cells also promote the secretion of other chemokines, such as stromal cell-derived factor-1 (SDF-1), and altogether reshape the bone microenvironment for the colonization, survival, and growth of metastatic tumor cells. 23 , 24
In addition to osteoblasts and osteoclasts, which we will describe later in this review, osteocytes are a major cell type involved in the regulation of bone modeling and remodeling. 25 Osteocytes are abundant in the calcified bone matrix and have a unique structure that allows them to interconnect with each other, osteoclasts, and BM cells through dendritic processes. 26 This highly interconnected network allows the exchange of nutrients and metabolites of bone cells and the transport of assorted factors from osteocytes. Factors produced by osteocytes include sclerostin (SOST) 27 as well as receptor activator of nuclear factor-kappa B ligand (RANKL), 28 , 29 dentin matrix acidic phosphoprotein 1, 30 and β-catenin, 31 which modulate bone formation and resorption, especially in response to mechanical stimuli. Osteocytes also act as an endocrine organ that releases soluble growth factors to regulate the physiological functions of distant organs such as the kidney 32 for the maintenance of phosphate and vitamin D equilibrium. 33
MSCs are multifunctional non-HPCs. BM-derived MSCs (BMSCs) are a crucial MSC subgroup for osteogenesis and chondrogenesis. 34 Due to their potent capacity for differentiation, BMSCs develop into cells such as BM stromal cells, skeletal myocytes, and osteoblasts. 35 , 36 , 37 Recently, BMSCs have been shown to display immunoregulatory properties. 38 , 39 The BM microenvironment is potentially conducive to the development of T cells in the absence of the thymus. 40 , 41 However, prior studies have indicated that BMSCs can inhibit the proliferation of mature T cells and natural killer cells. 42 , 43
Regulatory factors in the premetastatic niche
Compelling preclinical data have indicated that even before tumor cell migration, primary tumors can release soluble molecules into the circulation and prepare the soil for disseminating tumor cells. Exosomes are small vesicles of ~100 nm in diameter that are released by cells. 44 Exosomes secreted by cancer cells have recently been found to express integrins, a group of membrane receptors that allow the targeted movement of exosomes toward distant organs. 45 For example, exosomes expressing integrin αvβ5 preferentially move to the liver, while those expressing α6β4 target the lungs. 45 The secreted exosomes are then internalized by the host organ cells, as α6β4-expressing exosomes colocalize with S100A4-positive fibroblasts in the lung and integrin αvβ5-expressing exosomes colocalize with S100P-positive Kupffer cells in the liver. 45 S100A4 and S100P belong to the S100 family, a group of acidic Ca 2+ -binding proteins that interact with various intracellular effector proteins and mediate downstream protein phosphorylation and cytoskeleton dynamics. 46 , 47 The expression of S100A8/A9 is substantially elevated in human breast cancer cells, resulting in a migratory phenotype in cancer cells. 48
The best-characterized function of lysyl oxidase (LOX) is extracellular matrix (ECM) remodeling, which by strengthening the crosslinking of collagen and elastin, thereby improving the structural integrity of the ECM. 49 LOX expression can be induced under hypoxia, which is frequently observed in almost all solid tumors, and LOX can thus be used as a biomarker for premalignant changes during tumorigenesis. 50 Moreover, LOX can prepare the premetastatic niche by activating bone resorption. 51 , 52 Bone homeostasis is partly regulated by LOX activity, either directly through an RANKL-independent pathway BM stromal cells such as osteoblasts and osteoclasts 52 or indirectly through RANKL-dependent mechanisms. 53 In these ways, LOX disrupts bone homeostasis and facilitates premetastatic lesion formation. The elevated collagen fiber reticulation, 54 which facilitates the anchorage and colonization of neoplasms in metastatic sites, 51 , 54 is probably the cause of the protumoral effects of LOX. A recent investigation indicated that LOX silencing in primary tumors or antagonism of tumor-secreted LOX can prevent the formation of focal premetastatic lesions and the subsequent metastatic burden in bones. 55 Prior studies have also found the existence of exosomes secreted by bone cells in the bone microenvironment. 56 , 57 , 58 Exosomes can either be derived from osteoclasts to regulate osteoclastogenesis 59 or released from osteoblasts to stimulate RANK signaling in osteoclast precursors, ultimately inducing osteoclast formation. 60 One such example is exosomal-derived osteoclastic miR-214-3p, which is significantly correlated with bone formation in elderly female individuals with bone fractures. 61
The hypoxic bone microenvironment could promote cancer cell metastasis and growth. A key mediator of hypoxic signaling is hypoxia-inducible factor-1 (HIF-1), which initiates the transcription of hypoxia-response-related genes. 62 Many of these genes are prometastatic and are essential for angiogenesis, tumor cell apoptosis, and growth factor/cytokine activities. 63 Tumor cells that can survive in the hypoxic bone microenvironment then colonize and thrive in bones, leading to a vicious cycle of bone metastases. HIF-1α interacts with several growth factors and cytokines, such as VEGF, insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), and epidermal growth factor; the expression of these molecules on cancer cells can further enhance cancer cell proliferation and metastasis.
Extracellular pH in bones is associated with osteoblast and osteoclast function and with bone acidification leading to enhanced osteoclast resorption. 64 Metastatic tumor cells in localized bone regions produce lactic acid, resulting in acidosis of the bone microenvironment. 64 Tumor acidosis, in turn, increases the secretion of proteins that degrade the ECM and thus facilitate metastases, such as cathepsin B and matrix metallopeptidases (MMPs). 65
Osteolytic and osteoblastic bone metastasis
The two major categories of bone metastases are osteolytic and osteoblastic, based on which type of cells exhibit the predominant activities, 66 and the impaired balance between bone formation and resorption is frequently observed in both types of metastasis. Despite the excess occurrence of bone resorption and formation, growing evidence has suggested the coexistence of osteolytic and osteoblastic metastases, leading to mixed-type bone metastases. 67 Based on the distinct cytokine profile detected, lung cancer-derived bone metastases are preponderantly osteolytic, 68 whereas prostate cancer shows preferential osteoblastic bone metastases. 69 The development of osteoclastic and osteoblastic bone metastases is shown in Fig. 1 .
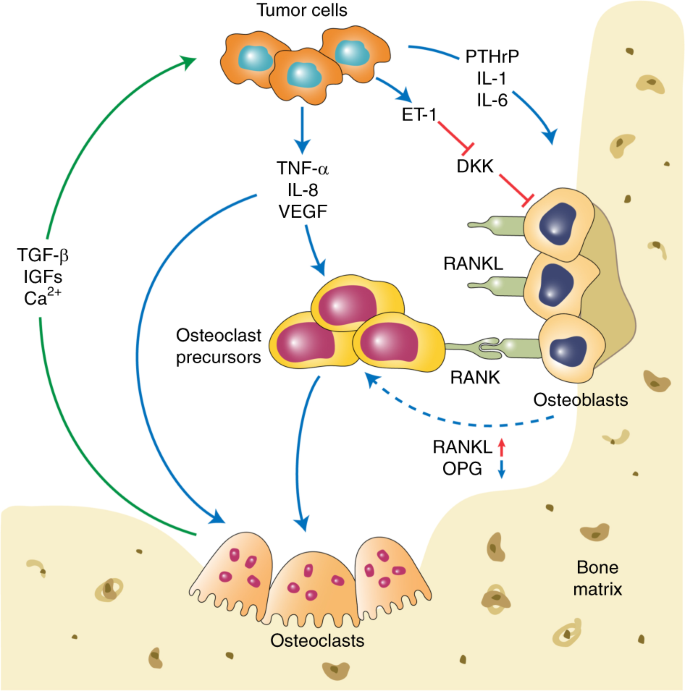
The development of osteoclastic and osteoblastic bone metastases. Tumor cells interact with both osteoclasts and osteoblasts in the bone microenvironment, which leads to a local increase in tumor-derived factors to promote osteoclastogenesis and osteoblastogenesis. Mature osteoclasts, in turn, release survival factors, such as insulin-like growth factor 1 (IGF-1) and transforming growth factor beta (TGF-β), which promote the survival and proliferation of tumor cells
Osteolytic bone metastasis
One prerequisite for the occurrence of osteolytic bone metastases is osteoclast activation. Osteoclasts are multinucleated cells that differentiate from their mononuclear macrophage/monocyte-lineage hematopoietic precursors and are involved in regulating intracellular calcium and inorganic phosphate levels. In the BM, multiple osteoclastogenic factors induce the differentiation of mononuclear macrophage/monocyte-lineage hematopoietic precursors into osteoclast precursors, which enter the bloodstream and localize to the remodeling sites of the bone. 70 The differentiation of osteoclast precursors is initiated after exposure to the two main regulatory factors, macrophage colony-stimulating factor and RANKL. RANKL, produced by osteoblasts, binds to its receptor RANK on the osteoclast precursor surface, which stimulates downstream signaling molecules, including mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3K)/Akt, and promotes the maturation of osteoclast precursors into functional osteoclasts. 71 In addition to RANKL, osteoprotegerin (OPG), a decoy receptor that is produced by osteoblasts, eliminates RANKL and thus inhibits the RANK–RANKL signaling pathway. Therefore, the activation of osteoclasts is attributed to the delicate balance between RANKL and OPG. 72 , 73
RANK is a surface receptor of the tumor necrosis factor (TNF) family. 74 This receptor is crucial for the formation, activation, and function of osteoclasts and also regulates calcium metabolism. 75 Although RANK is considered to be primarily expressed on osteoclasts and their progenitors, recent studies have also detected its expression on tumor cells, indicating the potential participation of RANK in tumor metastasis. 76 , 77 RANKL is a polypeptide that belongs to type II transmembrane proteins. 78 RANKL, the ligand of RANK, can be expressed on the surface of osteoblasts and bone stromal cells and exists within the bone microenvironment in a soluble form. Recent investigations have also detected high expression of RANKL in osteocytes, suggesting an important role in bone remodeling. 28 , 29 Moreover, RANKL has been found on both T and B lymphocytes. However, this molecule is not involved in bone remodeling under normal conditions. 28 Several osteotropic factors, including parathyroid hormone (PTH), vitamin D3, TNF-α, Wnt Family Member 5A, and IL-6, can stimulate RANKL expression, thus promoting osteoclastogenesis. 79 , 80 In osteoclast precursor cells, RANKL enhances the production of mature osteoclasts through stimulation of M-CSF at low levels. 81 , 82
RANK/RANKL signaling involves many transcription factors. The recruitment of TNF receptor-associated factors (TRAFs) is crucial for RANK/RANKL-mediated osteoclastogenesis, which activates various transcription factors, such as nuclear factor kappa beta and AP-1, and prevents the apoptosis of mature osteoclasts. 83 , 84 RANKL also induces the phosphorylation of another transcription factor, microphthalmia transcription factor (MITF), and activates downstream MAPK. 85 The stimulated transcription factor complex has a functional role in the expression of osteoclast-specific genes. The cytoplasmic domain of RANK recruits the adapter proteins Gab2 and PLCγ2 and thus activates calcium signaling in osteoclastogenesis. 86 PLCγ2 then acts synergistically with costimulatory signals to activate NFATc1, a transcription factor regulated by calcium signaling. 87 , 88 Furthermore, NFATc1 participates in the transcription of vacuolar ATPase and dendritic cell-specific transmembrane protein, which are closely related to the multinucleation of osteoclasts. 89 , 90 , 91
In a breast cancer model, RANKL exerts its promigratory effect on cancer cells and thus promotes their metastasis to bone. 92 Although breast cancer cells do not produce RANKL, they produce parathyroid hormone-related protein (PTHrP), which stimulates RANKL production in bone cells. 93 , 94 , 95 PTHrP regulates the activation of osteoclasts as a specific mediator of osteolysis in breast cancer metastases. 93 , 96 The expression of PTH-rP was significantly higher in breast cancer cells that metastasize to the bone than in those in nonbone soft tissues. 97 , 98 , 99 Moreover, monoclonal antibodies (mAb) that target PTH-rP potently inhibited the progression of bone metastases. 100 Hence, PTH-rP can promote bone metastases by activating the bone resorption activity of osteoclasts.
Conceptually, the balance between RANKL and OPG activities primarily determines the level of osteoclastogenesis, with a relatively higher OPG level leading to decreased bone resorption. 101 , 102 OPG is a member of the small integrin-binding ligand N-linked glycoprotein family and is a soluble receptor specific for RANKL. 103 In this way, OPG competes with RANK for RANKL and thus hinders RANKL–RANK communication on the osteoclast cell membrane and disrupts osteoclastogenesis and subsequent bone resorption. 104 However, some ECM components within bone microenvironments, such as glycosaminoglycans, may inhibit the RANKL–OPG interaction. 105 , 106
In prostate cancers, the overexpression of OPG could inhibit the development of bone metastatic tumor cells, with no impact on the proliferation of tumor cells. 107 Researchers have hypothesized that the essential role of osteolysis in tumor-bone metastases is to release growth factors from bones and to maintain the space needed for tumor growth in bone. In this indirect manner, OPG prevents bone lysis and thus reduces metastatic bone lesions. Previous reports have emphasized that the OPG produced by BM stromal cells was associated with the survival capacity of malignant prostate cells. 108 As described earlier, OPG can also be produced by cancer cells and protects cancer cells from TNF-related apoptosis-inducing ligand-induced apoptosis in a prostate cancer model. 109 However, a recent study found that the serum level of OPG was increased in patients with prostate cancer bone metastasis compared with nonmetastatic patients. 110 These contradictory results indicate the need for further studies on the role of serum OPG level as a bone metastatic marker of prostate cancers.
Calcium-sensing receptor (CaSR)
Extracellular calcium released from the bone matrix plays an active role in the vicious cycle of cancer bone metastasis. Under physiological conditions, the calcium balance is delicately maintained within a physiologic range in the bone microenvironment. The extracellular calcium of cancer cells is recognized through the CaSR or the P2X receptor, which manipulates calcium influx and efflux through ion channels or transporters. Therefore, once cancer cells reach bones, exposure to high calcium concentrations in the microenvironment, in turn, activates the CaSR. Although some evidence indicates that CaSR plays a tumor-suppressive role in gastric and colon cancers, 111 , 112 this receptor has also been shown to promote bone metastasis of some other cancer types, such as renal cell carcinoma (RCC), as CaSR is widely detected in both normal and malignant renal tissues. 113 In a clinical study, higher expression of CaSR was observed in RCC patients with bone metastases than in primary cancer patients 5 years after surgery. 114 Due to the osteolytic activities in RCC bone metastases, the high serum calcium concentrations may potentially increase the activities of CaSR-expressing tumor cells. 115 A recent study found that CaSR overexpression increased the adhesion, migration, and proliferation of RCC cells in a calcium-dependent manner, indicating that cellular calcium might enhance the metastatic behavior of RCC via CaSR. 116 Similar findings were reported in breast cancer cells in which the overexpression of CaSR was correlated with an increase in osteolytic potential. 117
TNF-α, a proinflammatory cytokine, is frequently observed in the tumor microenvironment and is mainly produced by tumor-associated macrophages and tumor cells. 118 , 119 , 120 TNF-α was reported to accelerate tumor cell apoptosis at a high dose. 121 However, when released into the tumor microenvironment, TNF-α also promotes cancer metastases at a low dose. 122 The proinflammatory cytokine TNF-α is one of the strongest inducers of bone resorption. 123 This molecule can stimulate the expression of RANKL and M-CSF in stromal cells, osteoblasts, and activated T cells 124 , 125 , 126 , 127 and directly promote the formation of TRAP + multinucleated osteoclasts in the presence of M-CSF and the absence of RANKL by activating NF-κB and AP-1 signaling. 128 , 129 , 130 , 131 Moreover, a recent investigation reported the correlation between RANKL and TNF-α in osteoclastogenesis. 132 TNF-α induces osteoclast differentiation from TRAF6 −/− osteoclast precursors through the RANKL-mediated degradation of TRAF3, suggesting that RANKL could enhance TNF-α-induced osteoclastogenesis in a TRAF6-independent manner. 132 In RA patients, TNF-α upregulates the IL-34 level via the NF-κB and JNK signaling pathways, 133 and TNF-α inhibitors, such as infliximab, adalimumab, certolizumab, and golimumab, have all shown clinical success. 134
Interleukins (ILs)
IL-1, either directly or indirectly, acts on the differentiation of osteoclasts depending on the levels of other growth factors in the bone microenvironment. 135 , 136 For example, IL-1α has been reported to directly induce osteoclast differentiation through MITF in BM macrophages in an RANKL-independent mechanism. 137 However, the activation of osteoclastic markers, such as TRAP, cathepsin K, and MMP-9, by IL-1α can also be associated with RANKL levels. 138 IL-1β, a proinflammatory cytokine, can potently stimulate osteoclast differentiation and subsequent bone resorption. 139 Likewise, IL-1β either indirectly stimulates TNF-α-induced osteoclastogenesis by inducing RANKL expression or directly promotes p38 MAPK-regulated osteoclastogenesis in the presence of sufficient RANKL. 140 Treatment strategies targeting IL-1, such as IL-1 receptor inhibitors (e.g., anakinra) and IL-1 antagonists (e.g., rilonacept and canakinumab), are now in clinical use for RA patients. 141
The IL-6 cytokine family members share a common subunit, gp130, in their signaling receptor complex. 142 IL-6 promotes osteoclastogenesis via interaction with the IL-6 receptor (IL-6R), which induces RANKL expression in osteoblasts and stromal cells. 143 Interestingly, OPG treatment failed to prevent osteoclastogenesis induced by IL-6 in the presence of M-CSF, which could be inhibited by a gp130 antibody. 144 Likewise, in an animal model, anti-human IL-6R antibodies could also prevent bone metastases. 145 Thus, IL-6 may promote osteoclastogenesis in an RANKL-independent manner. 146 One study, however, has reported the suppressive function of IL-6 in osteoclast differentiation; IL-6 inhibited RANK-mediated NF-κB and JNK activation. 143 This result was supported by a recent study showing that the IL-6 and IL-6R interaction could differentially manipulate RANKL-induced osteoclastogenesis via the NF-κB, ERK, and JNK signaling pathways. 147 In the presence of low-level RANKL, IL-6/IL-6R enhanced osteoclastogenesis, which was significantly suppressed by high-level RANKL. Thus, IL-6 can act either as an osteoclastogenic factor or bone protector, depending on the level of RANKL in the bone microenvironment. Oncostatin M (OSM), a member of the IL-6 family, exhibits multiple effects in physiological processes, including hematopoiesis, neurogenesis, and bone homeostasis. 148 OSM was shown to promote epithelial to mesenchymal transition (EMT) 149 and tumor cell invasion, 150 , 151 as well as upregulate proteases to degrade the local ECM in breast cancer. 151 , 152 In an in vitro experiment, breast cancer cell lines cocultured with OSM showed increases in osteoclastogenesis, which could be reversed by treatment with amphiregulin, an antibody targeting a previously uncharacterized OSM-regulated bone metastatic factor. 153
By increasing the release of RANKL and TNF-α in T cells, IL-7 has previously been identified as an indirect stimulator of osteoclast formation. 154 , 155 , 156 , 157 TNF-α, in turn, stimulates IL-7 production, which promotes the expansion of IL-17-producing T helper 17 (Th17) cells. 126 The Th17 cytokine IL-17 is an RANKL inducer known to disturb the RANKL/OPG balance and ultimately lead to bone resorption. 158 A recent investigation, however, indicated that IL-7 could directly induce osteoclastogenesis via STAT5 signaling, which was independent of RANKL. 159 Other pathways were then identified by the neutralization of IL-17A, which blocked C-X-C chemokine receptor type 4 (CXCR4)/SDF-1 signaling in metastatic microenvironments and substantially decreased bone metastases. 160 However, the inhibitory effect of IL-17 on osteoclastogenesis, which mainly involves the induction of granulocyte-macrophage colony-stimulating factor (GM-CSF) in osteoblasts, has been observed. 161 GM-CSF is secreted by cells, including activated T cells, fibroblasts, and macrophages, and then stimulates osteoclastogenesis via Ras/ERK signaling. 162 , 163 GM-CSF was shown to increase the number of osteoclast precursors within the bone microenvironment, 164 which is contradictory to another study suggesting that the expression of TNF-α-induced GM-CSF suppresses hematopoietic precursors of osteoclasts. 165 Therefore, the role of GM-CSF in osteoclastogenesis remains unclear.
IL-11 has long been identified as a functionally pleiotropic member of the IL-6 cytokine family due to its capacity to stimulate IL-6-dependent cell proliferation. 166 This molecule is initially produced in a BM-derived stromal cell line and can be released by mature osteoblasts to enhance osteoclastogenesis. 167 An early study demonstrated that various osteotropic factors, including IL-1, TNFα, PGE2, PTH, and 1 alpha, 25-dihydroxy vitamin D3 [1 alpha, 25(OH) 2 D3], could promote the production of IL-11 by osteoblasts, whereas IL-6, IL-4, and TGF-β could not. 168 , 169 In cocultures of both osteoblasts and BM cells, IL-11 induced the formation of osteoclast-like multinucleated cells in a dose-dependent manner, but this process was strongly inhibited by anti-IL-11 antibodies. 170 A recent clinical study found that the serum levels and mRNA expression of IL-11 in breast cancer patients were significantly elevated in the metastatic group compared with the nonmetastatic group, suggesting that IL-11 has predictive value in breast cancer bone metastasis. 171
IL-8 is another potential stimulator of osteoclastogenesis and bone destruction in bone metastases. 172 IL-8 may bind to the IL-8 receptor (IL-8R) on the osteoclast surface independent of RANK–RANKL signaling, 173 and IL-8 antibodies or IL-8R inhibitors significantly suppressed osteoclast differentiation in vitro. 174 A recent study suggested that the serum level of IL-8 was elevated in patients with anti-citrullinated protein antibody (ACPA)-positive RA and that ACPA-induced osteoclastogenesis can be inhibited by IL-8 neutralizing antibodies. 175
Osteoblastic bone metastasis
Despite recent research efforts on osteoclastic bone metastases, little is known about this condition, which mainly occurs in advanced prostate cancers and less frequently occurs in breast cancers. Osteoblasts are stimulated by metastatic tumor cell-derived factors, including FGFs, urokinase-type plasminogen activator (uPA), endothelin-1 (ET-1), prostate-specific antigen (PSA), IGFs, bone morphogenic proteins (BMPs), and VEGF. 176 , 177 , 178 , 179 , 180 ET-1 plays a vital role in the osteoblastic response to cancer bone metastasis. 181 The binding of ET-1 to the endothelin A receptor (ETAR) downregulates the autocrine production of a Wnt antagonist, Dickkopf-1 (Dkk-1). 182 , 183 The subsequent Wnt pathway activation is crucial for the differentiation and function of osteoblasts. Moreover, a previous study reported that the inverse correlation between the level of Dkk-1 and osteoblastogenesis is independent of osteoclastogenesis. 184
ET-1, acknowledged as a vasoactive peptide, is actively involved in the formation of new bone in the context of osteoblastic bone metastasis. 185 , 186 In a mouse tumor model, the osteoblastic metastasis of breast cancers was closely associated with the secretion of ET-1, the activity of which relied on its binding to ETAR. 181 The study also demonstrated that ETAR blockade strongly suppressed osteoblastic bone metastasis and reduced the tumor burden in bone, suggesting the potential value of ETAR inhibitors for bone metastatic cancer patients. 181 However, no precise molecular mechanisms for ET-1-regulated bone metastases have been established. Previous research has indicated that ET-1 can suppress osteoblast apoptosis by stimulating the calcineurin/NFAT pathway. 187 This process may also involve E-cadherin augmentation, which correlates with tumor cell adhesion, as well as upregulated Runx2 activity and SPARC expression, which is related to osteomimicry. 188 , 189 The inductive effect of ET-1 on IL-18 expression has been identified in osteoblasts at the gene promoter/transcriptional level through a p38 MAPK-dependent pathway. 190 IL-18, as discussed above, acts as a regulator of osteoblast proliferation. A recent study also evaluated the impact of ETS proto-oncogene 1 (ETS-1) and HIFs on ET-1 signaling and found that the balance between ETS-1 and HIF might affect downstream signals represented by ET-1. 191
As discussed earlier in the review, bone metastases usually have a mix of osteolytic and osteoblastic metastases. Accumulating evidence has suggested that osteolysis is the first step, even in the osteoblastic metastasis setting. In prostate cancer bone metastasis, DKK-1 acts as a molecular switch that converts osteolytic metastasis to osteoblastic metastasis. 192 DKK-1 also enhances the bone metastasis of breast cancers through the regulation of canonical WNT pathways in osteoblasts. However, targeting canonical WNT alone may fail to prevent cancer metastases, whereas combinational inhibition of JNK and TGF-β signaling could effectively treat cancer metastases to the lung and bone. 193 Cancer cells themselves can also secrete DKK-1 and regulate DKK-1 levels in the local microenvironment independently of ET-1. For example, PC3, a prostate cancer cell line, could produce DKK-1 and was converted from an osteolytic to an osteoblastic phenotype when transfected with Dkk-1 siRNA. 184 In a clinical setting, the serum levels of DKK-1 were robustly elevated in breast cancer patients with bone metastases compared with healthy controls or nonmetastatic patients. 194 Another inhibitor of WNT signaling is SOST, the functional loss of which may lead to multiple bone disorders due to dysregulation of bone remodeling. 195 , 196 Sclerostin, known as the protein product of the SOST gene, can inhibit canonical WNT signaling 197 , 198 and thus promote bone formation. 199
Other factors regulating osteoblastic bone metastasis
The role of PTHrP in osteoblastic metastases, particularly in prostate cancers, has long been disputed. 200 The expression of PTHrP correlates with increased malignancy and incidence of skeletal metastasis in multiple cancers, including prostate cancers. 201 , 202 Tumor-derived PTHrP not only plays an important role in the bone remodeling process but also directly facilitates the proliferation, adhesion, and survival of tumor cells. 203 , 204 , 205 PTHrP was shown to potently stimulate osteoclastogenesis by increasing the production of RANKL by osteoblasts. 206 However, PTHrP also facilitates osteoblastic alterations. 207 PSA, a serine proteinase, can cleave PTHrP into fragments at residue 23, 208 impairing PTH/PTHrP-mediated activation of its receptor. 209 Given the structural similarity between PTHrP-1-16 and ET-1 in the N-terminus, the inactive fragment PTHrP-1–16 can bind to and thus activate ETAR. 210 In addition to its cleavage of PTHrP, PSA may stimulate osteoblasts by preventing IGF from its binding protein and transforming latent TGF-β into its active form. 211 , 212 In vivo studies are needed to demonstrate PSA-mediated bone metastasis, as most of the evidence thus far is based on in vitro studies. 213 Prostate cancer cells also secrete the paracrine factor BMP4, an activator of osteoblast differentiation. 214 A recent study suggested that BMP4 might potentially mediate the endothelial-to-osteoblast (EC-to-OSB) conversion of endothelial cells into osteoblasts (28586644), which was consistent with previous reports that treatment with a BMP receptor inhibitor in mice with prostate cancer significantly prevented tumor-induced bone formation (21670081). Furthermore, prostate cancer cells produce FGF-9, which promoted the osteoblastic phenotype of MDA PCa-118 xenografts. 215
The multistep process of bone metastases
Bone metastasis does not occur randomly. This process is a well-organized procedure that involves a vicious cycle between the tumor and bone, where one promotes the other, disrupting the bone matrix and leading to bone metastasis. 216 , 217 The general bone metastatic process can be divided into cancer cell escape and dissemination, adhesion and invasion to the bone, and metastasis in bone. The general multistep process of bone metastasis and its regulating factors are presented in Fig. 2 and Table 1 .
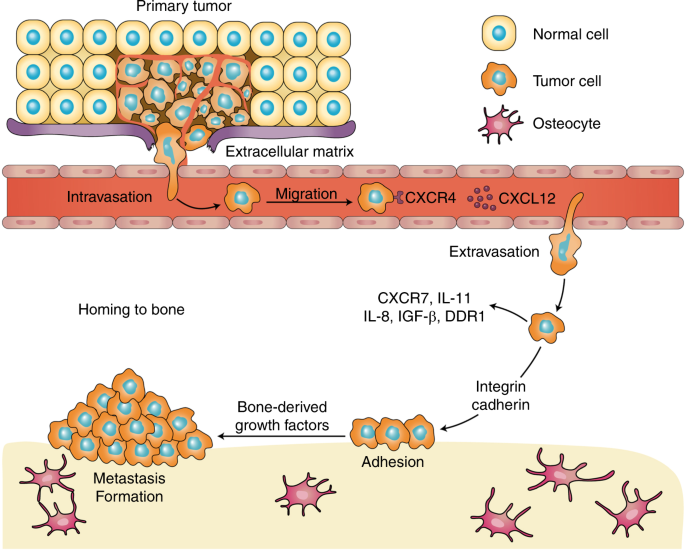
The general multistep process of bone metastasis and its correlated regulatory factors
Cancer cell escape and dissemination
The ability of tumor cells to escape their local microenvironment and degrade ECM proteins is an integral part of the malignancy of cancers. To intravasate into the bloodstream and colonize the metastatic site, tumor cells must pass through the basement membrane and the ECM. MMPs are a superfamily of multiple zinc-dependent proteinases that degrade ECM proteins. 218 High MMP levels have been observed in various malignancies, including prostate, bladder, lung, and breast cancers, as well as head and neck squamous cell carcinomas, 219 , 220 , 221 , 222 and are correlated with poor clinical outcome. 223 , 224
The MMP family is closely correlated with angiogenesis. Both in vitro and in vivo investigations have reported the antiangiogenic effect of MMP inhibitors. 225 , 226 , 227 The angiogenic response was shown to be significantly reduced in MMP-deficient mice. 228 , 229 Of all the MMP members, MMP-2 is the best-studied protein due to its function in angiogenesis. The addition of exogenous pro-MMP-2 to endothelial cell culture could lead to morphologic changes that indicate angiogenesis. 230 Furthermore, MMP-2 acts synergistically with adhesion molecules (e.g., E-cadherin). 231 High expression of both MMP-2 and MMP-9 (an MMP family member closely related to MMP-2) was linked to a poor prognosis in breast cancer. 224 In support of this hypothesis, MMP-2 positivity indicated an increase in the risk of death in the first 10-year follow-up. 232 Furthermore, MMP-2 was substantially elevated in patients with HER2/neu gene-amplified tumors, known as an aggressive tumor phenotype. A previous investigation evaluating the association between MMP-2 and clinicopathological parameters found that MMP-2 was an indicator of more invasive phenotypes and was related to lymph node metastasis. 233 MMP-2 also induces angiogenesis through the regulation of VEGF and the cleavage of ECM molecules (e.g., type IV collagen) 234 , 235 and therefore facilitates angiogenesis in the tumor microenvironment. 236 However, previous studies have found that MMP-2 promotes the release of bioactive fragments of ECM, such as endostatin, 237 restin, 238 and arrestin, 239 which inhibits angiogenesis. This inhibitory effect is related to the dormancy of breast cancer, where MMP-2 induces disseminated breast tumor cells to enter dormancy by promoting the expression of the dormancy promoter TGF-β2 in the BM. 240 A recent report found that thrombospondin-2 could promote the migration of prostate cancer cells by enhancing MMP-2 expression. 241
Osteolytic bone metastasis was significantly reduced in an MMP-7-deficient prostate cancer model, which had low levels of osteolysis due to defects in RANKL processing and osteoclast activation. 242 MMP-7, producing a soluble form of RANKL from membrane-bound RANKL, promotes osteolytic bone metastases in prostate cancer. 242 In prostate cancers, tumor growth in the bone microenvironment can be stimulated by osteoclast-derived MMP-9, which enhances angiogenesis without altering the osteolytic or osteogenic properties of tumors. 243 However, BMP-6, a member of the TGF-β superfamily, suppresses the paracrine secretion of MMP-9 in breast cancer cells via MAPK/p38/AP-1 signaling. 244 MMP-13 overexpression was first detected in breast carcinomas and was potentially induced by IL-1α and IL-1β. 245 , 246 In squamous cell carcinomas, MMP-13 is predominantly expressed on cancer cells and the stromal fibroblasts surrounding the cancer cells. In addition, MMP-13 is strongly indicative of the invasive and metastatic capacity of tumors. 247 , 248 The specific role of MMP-13 has not yet been elucidated in breast cancer. A recent investigation revealed that the upregulation of MMP-13 in the tumor-stromal interaction, especially at the tumor-bone interface, is crucial to tumor-induced osteolysis, suggesting the potential value of MMP-13 in the treatment of breast cancers with bone metastasis. 249
Cancer cell adhesion and invasion
Among all the chemokines, SDF1a (also known as CXCL12) is particularly involved in bone metastasis 250 and is often expressed in common metastatic sites such as BM. CXCR4 and CXCR7 represent two cognate receptors for CXCL12. 251 , 252 , 253 Both CXCR7 and CXCL12 are highly expressed on certain cancer cells. 254 , 255 CXCL12 can also be detected in normal tissues such as blood vascular endothelial cells, 255 , 256 and fibroblasts are probably a major source of CXCL12 secretion in tumor tissue. 257 , 258 Initially shown to facilitate the mobilization of hematopoietic stem cells and create a microenvironment for cancer stem cells, 259 CXCL12/CXCR4 pathway signaling also plays an important role in cancer cell proliferation and angiogenesis. 259 , 260
The inhibition of the CXCL12/CXCR4 interaction with CXCR4 mAb or CXCR4 blocking peptides prevents the migration of bone metastases of prostate cancer cells 261 and reduces the in vivo metastatic load. 260 Experimental evidence suggests that the CXCL12/CXCR4 signaling axis participates in prostate cancer cell adhesion to BM endothelial cells. 262 , 263 , 264 Consistent with this hypothesis, antagonists of αvβ3, an adhesion-related integrin induced by CXCL12, significantly decreased the adhesion of prostate cancer cells to endothelial cells. 265 CXCL12/CXCR4 is not the only signaling pathway involved in the adhesion of prostate cancer cells to endothelial cells but is instead part of a sequence of other interactions, such as those involving CD164. 266
HER2/CXCR4/AKT signaling has been investigated in the bone metastasis of breast cancers. 267 High expression of CXCR4 is found in breast cancer cells, promoting tumor cell homing and bone metastasis, with a highly osteolytic subclone observed in a breast cancer cell line. 268 Addition of an anti-CXCR4 antibody or gene silencing of CXCR4 significantly decreased the migration of breast cancer cells to regional lymph nodes and the lung. 269 Multiple preclinical studies have assessed the effectiveness of CXCR4 in blocking bone metastases in breast cancers. 269 , 270 However, the CXCL12/CXCR4 axis can facilitate bone invasion processes by inducing MMP-9 and downregulating the expression of tissue inhibitor of metalloproteinases 2 in prostate cancer cells by this pathway. 271 , 272 MMP family members are not only involved in cancer cell escape but also promote the extravasation of cancer cells from the ECM before proliferation in bones. 265 Given that broad-spectrum MMP inhibitors fail to demonstrate clinical efficacy, more individualized targeting of proteinases may be a promising strategy to prevent bone metastases in cancer patients.
In addition to the CXCR4/CXCL12-induced CD164 and αvβ3 integrins mentioned above, a vast majority of adhesion molecules have been discovered in the interaction of cancer cells with BM endothelium. These molecules include galectin-3/Thomsen–Friedenreich antigen 273 , 274 as well as CD44/hyaluronan, 275 the inhibition of which impairs the adhesion of cancer cells to BM endothelial monolayers. Another member of the chemokine superfamily, CCL5, is produced by BMDCs and local stem cells in the bone microenvironment, and together with its receptor CCR5, CCL5 enhances cancer bone metastases. 276 , 277 A recent report identified an increase in CCL5 secretion from bone stromal cells in the metastatic microenvironment, which induced prostate cancer cell migration involving androgen receptor signaling. 278 The lymphotactin receptor (XCR1) is also a member of the chemokine receptor family, and its interaction with the ligand XCL1 substantially promotes the proliferation and migration of cancer cells. 279 , 280 , 281 Other cancer cell-derived proteinases, such as ADAM 282 and uPA82, 271 are also implicated in the degradation of the bone matrix, promoting the effective invasion of cancer cells into bones. A recent study demonstrated the positive effect of cyclooxygenase-2 (COX-2) on cell adhesion and proliferation in bones. 283 Human melanomas frequently overexpress the COX-2 gene, 284 which exerts its regulatory effect on melanoma cell adhesion to proliferation in BMSCs in response to BMSC-derived VEGF. 283
Metastasis formation in bone
In addition to the processes by which cancer cells escape from original sites and invade bones, another fundamental step toward bone metastasis is the maintenance of cell proliferation as well as the consequent formation of metastases. As discussed above, the proposed mechanism for bone metastasis is the disruption of normal bone remodeling, leading to imbalanced bone resorption and bone formation. For cancer cell survival and growth, multiple growth factors produced by osteoblasts are embedded within the bone matrix and released during osteoclastic bone resorption. 285
TGF-β participates in various normal physiological procedures, including immune responses and bone remodeling, 286 and is also an important growth factor for osteoclastic bone resorption. Enhanced TGF-β signaling was detected in both preclinical 287 , 288 , 289 , 290 and clinical breast cancer models. 288 The TGF-β1 level was significantly elevated in the plasma of breast or prostate cancer patients with bone metastases. 291 Smad-dependent TGF-β signaling was also observed in samples of the bone metastatic sites of breast cancer patients. 292 However, TGF-β can play a paradoxical role in cancer, where it suppresses tumor growth at an early stage and promotes invasion and metastasis to bones at later stages. 293 Various genes referred to as bone metastasis stimulators, including CXCR4, MMP-1, IL-11, JAG1, and PTHRP, were shown to be regulated by TGF-β, 288 , 294 , 295 and anti-TGF-β therapies showed strong efficacy in controlling cancer-related bone metastases in mice. 289 , 296
The IGF family is essential for bone growth, 297 as all skeletal cells express IGF-1 and its receptor IGF-1R to maintain physiological functions. 297 Moreover, IGF-1 promotes bone colonization of metastasizing tumor cells and facilitates their expansion inside bones. One such example is breast cancer metastasis stimulated by bone-derived IGFs through the activation of AKT and NF-κB to increase the proliferation and survival of cancer cells. 298 In addition to the survival of cancer cells in bone, IGFs participate in the homing process. In triple-negative breast cancers, cancer-associated fibroblasts release IGF-1, which primes cells to home to the IGF-1-rich bone microenvironment. 299 In prostate cancer with bone metastases, IGF-1 causes resistance-related genetic alterations of cancer cells through its binding to IGF-R, which has been identified in several proliferative and antiapoptotic mechanisms. 300 This protective effect of IGF-1 on prostate cancer cells can be antagonized by agents that downregulate both local and systemic IGF-1 production, which is used to improve symptoms among prostate cancer patients with bone metastases. 301 , 302 , 303
MicroRNA (miRNA)
To better adapt to the bone microenvironment, cancer cells undergo osteomimicry, which involves gene expression that is normally found on bone cells. The well-ordered expression of metastasis-related molecules during adaptation suggests that miRNAs are crucial regulators of osteomimicry. By binding to corresponding sequences in downstream target genes, miRNAs degrade and inhibit the translation of mRNAs. 304 Accumulating evidence suggests that the aberrant expression of miRNAs indicates the invasive and metastatic phenotypes of tumor cells, 305 , 306 , 307 and several miRNAs have been identified to mediate bone metastases, especially those of prostate cancer. 308 , 309 , 310 MiR-141-3p is one of the earliest studied miRNAs. Previous studies found that the dysregulation of miR-141-3p is involved in the metastatic behavior of cancer cells. 311 , 312 The expression of miR-141-3p disrupts NF-κB signaling by targeting TRAF5 and TRAF6, and the silencing of miR-141-3p promotes EMT, leading to cancer cell invasion and migration. 313 Likewise, the downregulation of miR-145 caused by loss of wild-type p53 could promote bone metastasis through enhanced EMT. 314 , 315 A growing number of miRNAs, such as miR-133b and miR-19a-3p, which directly target the activity of TGF-β signaling, have recently been shown to negatively regulate bone metastases of prostate cancers. 316 , 317
Furthermore, the decreased expression of several miRNAs (e.g., miR-335, miR-126, and miR-206) was detected in human breast cancer cells metastasizing to the bone, and the restoration of their expression prevented bone metastatic progression. 318 MiR-135 and miR-203 have been reported to downregulate the expression of Runx2 in breast cancer cells, thus reducing metastasis formation in bone. 319 MiRNAs do not always exert negative effects on bone metastases, and some of them act as onco-miRNAs. The stimulation of miR-10b and miR-21, caused by the transcription factor Twist-1 and lysophosphatidic acid, respectively, promoted the invasion of breast cancer cells in BM. 320
Autotaxin (ATX or ENPP2) is a member of the nucleotide pyrophosphate–pyrophosphatase family. The expression of ATX in human primary breast tumor biopsies does not impact overall survival (OS), indicating that its expression at the primary tumor sites is not a prognostic indicator. 321 However, a recent study showed that nontumoral ATX directs the early stage of tumor cell colonization in bones. 322
Management of cancer bone metastases
The optimal treatment of cancer bone metastases involves a multidisciplinary approach, including medical oncology, radiation oncology, and surgical oncology. Based on the basic biology of bone metastasis discussed above, concomitantly preventing new metastases and the growth of established metastases is theoretically an effective therapeutic strategy. 323 The main treatment strategies for cancer bone metastasis are summarized in Table 2 .
Hormone therapies
Endocrine therapies are considered a first-line treatment for hormone-responsive cancer patients. For many years, tamoxifen, a selective estrogen receptor modulator (SERM), has been regarded as the standard endocrine therapy for patients with estrogen receptor-positive breast cancer. Based on clinical research, postsurgical tamoxifen treatment decreases breast cancer mortality by 34%. 324 Other SERMs, including raloxifene and toremifene, have also been found to efficaciously block cell growth in patients with estrogen-responsive breast cancers. 325 , 326 , 327 , 328 Aromatase is a key enzyme implicated in estrogen biosynthesis and converts androgens to estrogens. Recently, multiple large randomized trials have compared third-generation aromatase inhibitors (AIs), such as anastrozole, exemestane, and letrozole, with tamoxifen and found that AI therapies are more efficacious and tolerable in patients with breast cancer. 329 , 330 , 331 , 332 Thus, AIs have now replaced SERMs in female cancer patients, especially postmenopausal women. 333 , 334 , 335 , 336 Clinical treatment regimens include the upfront AI monotherapy, 329 , 337 2–3 years of tamoxifen prior to AI treatment, 338 , 339 , 340 and extended adjuvant AI treatment. 341 , 342 However, the AI-induced decrease in estrogen levels contributed to increased risks of bone resorption 343 and fractures. 329 , 332 , 344
Androgen deprivation therapy (ADT), a standard treatment for prostate cancer patients with distant metastases, 345 , 346 can be achieved by antiandrogens, orchiectomy, and agonists or antagonists of gonadotropin-releasing hormone. 347 The majority of ADT-treated patients experience symptomatic relief, metastasis regression, and decreased serum levels of PSAs. Similar to estrogen deprivation, ADT is also related to skeletal complications such as decreased bone mineral density and fracture risk. 348 , 349 , 350 , 351 Abiraterone acetate is known to target CYP17A1, an essential enzyme for androgen synthesis, and substantially improved OS in prostate cancer patients with bone metastases. 352 , 353 Similar results were obtained from another androgen receptor antagonist, enzalutamide, which is used as a first-line intervention for bone metastatic prostate cancer patients after castration and docetaxel treatment. 354 , 355 However, most patients with prostate cancer eventually develop therapeutic resistance to androgen blockade, and it has recently been suggested that patients should receive at least three different lines of treatment. 356
Radioisotopes
Therapeutic radioisotopes with high affinity for bones such as phosphorus-32 have long been used to treat metastatic breast and prostate cancers. 357 These radioisotopes can emit α- or β-particles and deliver detrimental radiation to cancer cells. The vast majority of radioisotopes harbor different physical properties, allowing them to address different clinical implications. The most commonly used β-emitting radioisotopes to treat bone metastases are strontium-89 ( 89 Sr) and samarium-135 ( 135 Sm). In one study, prostate cancer patients with bone metastases randomly received either 89 Sr, a high-energy β-emitting radioisotope, or external beam radiation. Both types of treatment were effective, whereas the 89 Sr treatment showed more effective relief of bone pain. 358 Compared with 89 Sr, 135 Sm has a shorter half-life, allowing it to be delivered at larger doses with the same treatment time. A meta-analysis suggested that β-emitting radioisotope therapy alleviates bone pain over 1–6 months but frequently caused severe side effects, including leukopenia and thrombocytopenia. 359 Moreover, 89 Sr and 153 Sm are renally excreted, which reduces their efficacy in patients with genitourinary cancers. Radium-223 ( 223 Ra) is an α-emitting radioisotope abundant in the bone matrix in the area of osteoblast-induced mineralization. In a phase III trial, 223 Ra prolonged OS in patients with castration-resistant prostate cancer and symptomatic bone metastases and was thus approved by the Food and Drug Administration in 2013. 360 Ongoing clinical trials strive to optimize the treatment of bone metastases by evaluating the combination of radioisotopes with chemotherapy. 361 , 362 , 363 , 364
External beam radiation therapy (EBRT)
EBRT is a conventional palliative treatment for cancer bone metastases to prevent potential bone fractures and can function synergistically with surgical treatments. This treatment also provides timely control for focal bone pain, with ~50% of ERBT-treated patients reporting pain relief in 2 weeks. 200 EBRT is effective even in radioresistant tumors, such as those originating from metastatic sarcoma or RCC. 365 In the past few decades, multiple studies have compared the efficacy of high-dose, short-fraction radiation with radiation at lower doses and more fractions. Based on these trials, meta-analyses suggest no significant difference in either complete or partial responses between patients treated with hypofractionation or multifractionation EBRT. 366 , 367 , 368 For example, in a recent study, similar pain-control effects were achieved with a single 8 Gy fraction and 30 Gy administered in ten fractions. 369
One common complication of bone metastases is the large area of osteolytic lesions leading to high fracture risks, especially in breast and renal cancer metastases. 370 In addition to bone pain as a result of fracture at any location, fracture of long bones such as the femur is more likely to cause disability and may decrease the quality of life and negatively affect prognosis. 371 , 372 , 373 , 374 , 375 Prophylactic surgery for potential fracture includes plate osteosynthesis and prosthetic implant insertion. 376 For maximum efficacy, radiation therapy and embolization are usually combined with surgeries as a local adjuvant treatment. 377 , 378 Due to the limited guidelines for cancer bone metastases, 379 , 380 many surgeons currently make clinical decisions based on the standard practice for fractures or according to their experience. A recent study provides an algorithm for the treatment of patients with long bone metastatic diseases, suggesting that the characteristics of individual bone lesions should be considered when performing surgical fixations or prosthetic reconstructions. 381 Percutaneous cementoplasty is frequently adopted to treat bone metastases or to prevent impending fractures in weight-bearing bones. However, the single use of percutaneous cementoplasty is not necessarily curative and is suggested to be preceded by ablative treatment. 382 Eisenberg et al. described a cervical cancer patient who received magnetic resonance-guided focused ultrasound surgery (MRgFUS) for iliac bone metastasis. 383 In this case, MRgFUS led to a dramatic reduction in pain, and percutaneous cementoplasty was thus not considered. MRgFUS is a noninvasive surgical procedure that is effective in controlling bone metastasis-related pain in multiple clinical trials. 384 , 385 , 386
Bisphosphonates
Bisphosphonates are well known for their high affinity for the surface of bones that undergo bone resorption. Bisphosphonate therapy is commonly used for the long-term treatment of osteolytic and metastatic bone diseases. One classification of bisphosphonates is nitrogen-containing bisphosphonate (N-BP); for example, alendronate (ALN) and zoledronic acid (ZOL) are robust inhibitors of protein isoprenylation, which promotes osteoclast apoptosis. Examples of non-N-BPs include clodronate and etidronate, which induce osteoclast apoptosis by impairing mitochondrial function. 387 , 388
In addition to the antiresorptive activity, the antitumor characteristics of bisphosphonates, including the inhibition of tumor cell adhesion and invasion, 390 , 391 as well as induction of tumor apoptosis, 392 have been extensively investigated both in vitro and in vivo. 389 The antiangiogenic effect of bisphosphonates has been studied in several animal models. 393 , 394 , 395 , 396 Pamidronate and clodronate can both abrogate angiogenesis in breast cancers, potentially by suppressing the expression of VEGF and the accumulation of IGF-1-induced HIF-1a protein. 397 A recent study investigated connexin (Cx) 43 hemichannels, describing a self-defense mechanism of osteocytes against metastatic breast cancer cells. 398 Treatment with either ALN or ZOL opens the Cx43 molecular passage between osteocytes and extracellular environments. In vivo studies also suggest that bisphosphonates could reduce tumor burden and bone metastasis formation in a dose-dependent manner. 399
Randomized clinical trials evaluated the safety profile and the efficacy of pamidronate to prevent bone metastases in patients with breast cancer. 400 , 401 The first skeletal-related event (SRE) was chosen as the primary outcome, and the number of SREs per year was recorded. The pamidronate-treated patients had fewer SREs per year and a longer time to the first SRE than those in the control group. In a phase II/III clinical study, patients with osteolytic lesions secondary to multiple myeloma or metastatic breast cancer were treated with pamidronate and ZOL to define the optimal dose of these two agents. 402 , 403 The oral administration of clodronate prevented SREs. 404 In female patients with primary breast cancer, the treatment of clodronate significantly reduced bone metastases during the 5-year follow-up. 405 The oral administration of clodronate has also been reported to reduce both symptomatic progression and death in male patients with hormone-responsive diseases. 406 ADT, as described above, may decrease the bone mineral density in prostate cancer patients and thus requires the application of bisphosphonates. 407 Although osteoblastic bone metastases are frequently observed in patients with prostate cancer, the potent efficacy of bisphosphonates in these patients demonstrates the increased osteoclast activities in osteoblastic bone metastases. 408 One of the side effects of bisphosphonates is osteonecrosis of the jaw (ONJ), which is associated with drug-exposed bone in the oral cavity. 409 Due to the high dose of bisphosphonates in the treatment, more than 95% of patients with bone metastases present ONJ. 410 In the case of ONJ, conservative treatments, including antibiotics and mouth rinses, are recommended. 411
The combination of bisphosphonates and chemotherapy has also been studied. ZOL increased tumor cell apoptosis in vitro when administered after doxorubicin, possibly due to increased uptake of bisphosphonates caused by chemotherapy. 412 A randomized phase II trial is currently testing the synergy of ZOL and 5-fluorouracil-epirubicin-cyclophosphamide in a neoadjuvant setting. 413 Combined treatment with third-generation NBP (YM529) and IFN-α inhibited the aggravation of established bone metastases in the RCC model, which is probably mediated by the inhibition of YM529 on osteoclast activation and the antiangiogenic effect of IFN-α. 414
Novel therapies
Following the identification of the vicious cycle of bone metastases, novel agents that specifically target the complex pathways in bone metastases have been developed, many of which are currently under clinical evaluation. The target inhibition of pathways involved in bone metastases is presented in Fig. 3 .
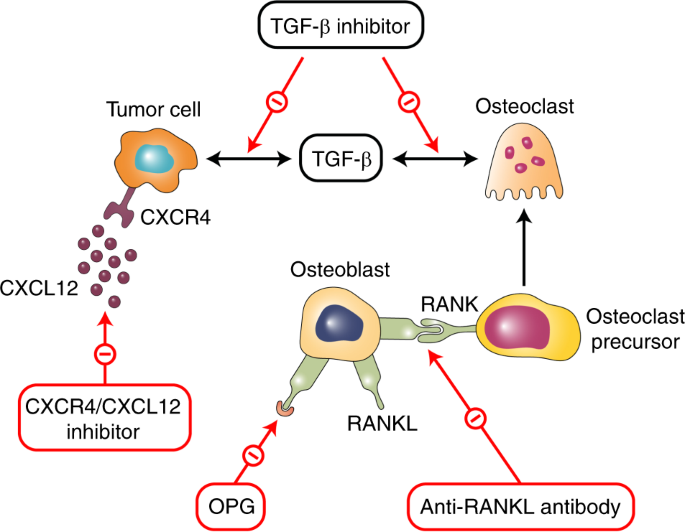
The target inhibition of pathways involved in bone metastases
RANK/RANKL signaling is an essential strategy for blocking targets for osteolytic bone diseases. Denosumab, a humanized RANKL antibody and the first drug of this class, has demonstrated superiority over ZOL in preventing bone diseases in both breast and prostate cancers with bone metastases. 415 , 416 Denosumab prevents the RANKL/RANK interaction by mimicking the action of OPG and thus reduces the survival and bone resorption activity of these osteoclasts. 417 , 418 Based on the overall satisfactory results of clinical trials, denosumab has now been approved for SRE prevention in patients with cancer. A recent study found that AMG161, an equivalent of denosumab, could block RANKL signaling and the formation of micrometastases in BM. 419
As previously discussed, the CXCL12 pathway is an important regulator of metastases in prostate, colorectal, and breast cancers. Blockade of the CXCL12 pathway has been found to substantially delay both primary tumor growth and distant metastasis in multiple preclinical studies. 137 , 138 , 139 , 140 , 141 Anti-CXCL12 agents work especially well in the prophylactic setting when treatments start early and are less effective in established tumors. 420 A previous report showed that the pan-VEGFR tyrosine kinase inhibitor cediranib could upregulate circulating CXCL12 concentrations, 421 , 422 , 423 , 424 and genetic testing results revealed that CXCL12/CXCR4 pathway activation could lead to the specific inhibition of VEGFR activity in BMDCs. 425 Therefore, anti-CXCL12 therapy can be used in combination with anti-VEGFR therapies to reach maximum clinical efficacy. Other anticancer treatments are also promising candidates for anti-CXCL12 agents. Several chemotherapeutics, such as paclitaxel or vascular-disrupting agents, can lead to an increased level of circulating CXCL12 and mobilization of BMDCs. 426 , 427 Moreover, irradiation upregulates CXCL12 expression, both directly and indirectly, through hypoxia and the related HIF-1α activation. 428 , 429 , 430 These results indicate that when used in synergy with other treatment options, anti-CXCL12 therapy demonstrates promising efficacy compared with monotherapy.
TGF-β inhibitors represent another class of novel therapies to prevent cancer bone metastases, as more than half of breast cancers display increased TGF-β activities. 431 The inhibition of TGF-β has been found in preclinical research to suppress tumor growth and distant metastases, including those to bones, and is highly potent in triple-negative breast cancers. 432 Many TGF-β antagonists, most of which remain at the preclinical stage, are currently under clinical development. These TGF-β antagonists include TGF-β antibodies (1D11), 433 , 434 , 435 receptor kinase inhibitors such as LY2109761, 249 , 389 , 390 and other antagonizing agents such as BMP7. 389 , 390
HMG-CoA reductase (HMGR) inhibitors have been examined in different cancers (e.g., lung cancer). 436 HMGR is an important enzyme for cholesterol biosynthesis, 437 the inhibition of which has demonstrated antitumor effects in multiple tumor types. Simvastatin is an HMGR antagonist and can reduce osteolytic bone metastases of lung cancers, potentially through the downregulation of CD44, P53, and MMPs 438 or the antagonistic interaction between p53 and CD44. 439 Furthermore, a thrombin inhibitor, argatroban, could reduce the bone metastasis of breast cancer cells by suppressing the activation of tissue factors and VEGF secretion. 440 The ET axis is another promising therapeutic target for the treatment of prostate cancer bone metastases. In clinical studies, the ETAR inhibitor atrasentan successfully decreased PSA in male patients with hormone-refractory disease 185 and markers of bone turnover in prostate cancer patients with bone metastases. 441
Biological intracontrol treatment (BICT) is an herbal medicine-based therapy involving herbal extracts and palliative care. In a case report, a 59-year-old lung cancer patient who failed first‑line chemotherapy treatment and presented with multiple bone metastases was concomitantly treated with BICT and bisphosphonates, which inhibited tumor growth and simultaneously promoted bone repair. 442 Fish oil has been shown to have a new function, targeting the prometastatic molecule CD44 on the cell surface to suppress the migration and invasion of tumor cells. 443 Tasquinimod is an experimental drug that has been proven effective in controlling both tumor growth and distant metastases of prostate cancer. 444 , 445 , 446 , 447 , 448 The methyl group donor is another drug under experimental evaluation. S-adenosylmethionine has also been found to reduce skeletal metastases both in vitro and in vivo, which probably correlates with increased bone density. 449 Since bone loss is a severe complication of cancer patients with bone metastases, various bone-anabolic agents that stimulate the synthetic activities of osteoblasts, such as PTH agents, are commonly used in clinical practice. Examples of anabolic agents to prevent bone loss include CaSR antagonists 450 , 451 , 452 and PTH/PTHrP. 453 , 454
Hypoxic signaling contributes to the preparation of the bone microenvironment for cancer metastases and is therefore an attractive therapeutic target. The inhibition of the hypoxia pathway impairs the development of chemotherapeutic resistance mediated by HIF. A wide variety of hypoxic signaling inhibitors, including the small molecule inhibitor (SMI) 2-methoxyestradiol, 455 which downregulates HIF-1α levels and VEGF expression in tumor cells, are under preclinical investigation. 456 , 457 Clinical evaluations have also been initiated to assess 2-methoxyestradiol and analogs to treat multiple cancers, with the analogs exhibiting more potent antiangiogenic and antitumor effects. 458 Other examples of SMIs that target hypoxic signaling include inhibitors of topoisomerases I and II as well as PI3K inhibitors, which negatively act on HIF-mediated gene transcription. 459 Based on the interaction between HIF-1 and many other signaling pathways, inhibitors of hypoxic signaling may be used in combination with other therapies to induce sufficient suppression of tumor growth and spread. 460
Conclusion and perspective
Bone metastasis is one of the most lethal complications of cancer, and further elucidation of this process should provide new insights into the bone tropism of cancer cells and novel therapies that reduce mortality in cancer patients. Three steps contributing to bone metastases include (1) cancer cell escape and dissemination, (2) adhesion and invasion to bone, and (3) colonization and metastasis in bone. The first step is similar to metastases to nonbone organs, such as the lung and liver, whereas the second and third steps are specific to bone metastases, owing to their distinct cellular and molecular profiles. In particular, bone metastasis involves complex interactions between tumor cells and the bone microenvironment. Thus, the various pathways in the bone microenvironment, such as RANK/RANKL signaling, can be specific therapeutic targets for bone metastases.
In addition to traditional treatments such as hormone therapies, radioisotopes, and bisphosphonates, novel inhibitors that block these pathways, such as the RANKL inhibitors that block osteoclast differentiation, have demonstrated significant antitumor effects in bone metastasis models. It is expected that the concomitant use of novel inhibitors with conventional therapies will provide optimal treatment for bone metastases, but long-term clinical studies are needed to evaluate whether these combinations lead to survival benefits in patients. An in-depth elucidation of the premetastatic niche in bone is also essential for the early intervention of bone metastases. However, the majority of studies on the premetastatic bone niche are based on animal models, which may not fully represent the bone microenvironment in humans. Developing animal models that can mimic the general bone metastatic process in human cancers is thus essential. Moreover, elucidating the specific mechanisms for bone metastases in diverse tumors will promote the development of tumor-type-specific treatments for bone metastases. Hopefully, the current knowledge and ongoing studies will provide additional alternatives for the treatment of cancer patients with bone metastases.
Ruoslahti, E. How cancer spreads. Sci. Am. 275 , 72–77 (1996).
CAS PubMed Google Scholar
Rusciano, D. & Burger, M. M. Why do cancer cells metastasize into particular organs? Bioessays 14 , 185–194 (1992).
Coleman, R. E. Skeletal complications of malignancy. Cancer 80 , 1588–1594 (1997).
Kraljevic Pavelic, S., Sedic, M., Bosnjak, H., Spaventi, S. & Pavelic, K. Metastasis: new perspectives on an old problem. Mol. Cancer 10 , 22 (2011).
PubMed PubMed Central Google Scholar
Guise, T. A. The vicious cycle of bone metastases. J. Musculoskelet. Neuronal Interact. 2 , 570–572 (2002).
Nguyen, D. X., Bos, P. D. & Massague, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9 , 274–284 (2009).
Roodman, G. D. Mechanisms of bone metastasis. N. Engl. J. Med 350 , 1655–1664 (2004).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127 , 679–695 (2006).
Saki, N., Abroun, S., Farshdousti Hagh, M. & Asgharei, F. Neoplastic bone marrow niche: hematopoietic and mesenchymal stem cells. Cell J. 13 , 131–136 (2011).
CAS PubMed PubMed Central Google Scholar
Chiang, A. C. & Massague, J. Molecular basis of metastasis. N. Engl. J. Med 359 , 2814–2823 (2008).
Theriault, R. L. & Theriault, R. L. Biology of bone metastases. Cancer Control 19 , 92–101 (2012).
PubMed Google Scholar
Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 8 , 98–101 (1989).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438 , 820–827 (2005).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3 , 453–458 (2003).
Langley, R. R. & Fidler, I. J. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128 , 2527–2535 (2011).
Mathot, L. & Stenninger, J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer Sci. 103 , 626–631 (2012).
Peinado, H., Lavotshkin, S. & Lyden, D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 21 , 139–146 (2011).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17 , 302–317 (2017).
Kitamura, T., Qian, B. Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15 , 73–86 (2015).
Kawai, H. et al. Characterization and potential roles of bone marrow-derived stromal cells in cancer development and metastasis. Int. J. Med. Sci. 15 , 1406–1414 (2018).
Kaplan, R. N., Psaila, B. & Lyden, D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 25 , 521–529 (2006).
Psaila, B. & Lyden, D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 9 , 285–293 (2009).
Sahoo, M. et al. Hematopoietic stem cell specific V-ATPase controls breast cancer progression and metastasis via cytotoxic T cells. Oncotarget 9 , 33215–33231 (2018).
Kaplan, R. N., Rafii, S. & Lyden, D. Preparing the “soil”: the premetastatic niche. Cancer Res. 66 , 11089–11093 (2006).
Atkins, G. J. & Findlay, D. M. Osteocyte regulation of bone mineral: a little give and take. Osteoporos. Int. 23 , 2067–2079 (2012).
Bonewald, L. F. The amazing osteocyte. J. Bone Miner. Res. 26 , 229–238 (2011).
Winkler, D. G. et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 22 , 6267–6276 (2003).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17 , 1231–1234 (2011).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17 , 1235–1241 (2011).
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38 , 1310–1315 (2006).
Kramer, I. et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 30 , 3071–3085 (2010).
Bonewald, L. F. Osteocytes as dynamic multifunctional cells. Ann. N. Y. Acad. Sci. 1116 , 281–290 (2007).
Quarles, L. D. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat. Rev. Endocrinol. 8 , 276–286 (2012).
Lin, H., Sohn, J., Shen, H., Langhans, M. T. & Tuan, R. S. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials 203 , 96–110 (2019).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284 , 143–147 (1999).
Wakitani, S., Saito, T. & Caplan, A. I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18 , 1417–1426 (1995).
Reyes, M. et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 98 , 2615–2625 (2001).
Gao, F. et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 7 , e2062 (2016).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105 , 1815–1822 (2005).
Dejbakhsh-Jones, S., Jerabek, L., Weissman, I. L. & Strober, S. Extrathymic maturation of alpha beta T cells from hemopoietic stem cells. J. Immunol. 155 , 3338–3344 (1995).
Barda-Saad, M., Rozenszajn, L. A., Globerson, A., Zhang, A. S. & Zipori, D. Selective adhesion of immature thymocytes to bone marrow stromal cells: relevance to T cell lymphopoiesis. Exp. Hematol. 24 , 386–391 (1996).
Di Nicola, M. et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99 , 3838–3843 (2002).
Krampera, M. et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24 , 386–398 (2006).
Vlassov, A. V., Magdaleno, S., Setterquist, R. & Conrad, R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 1820 , 940–948 (2012).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527 , 329–335 (2015).
Bresnick, A. R., Weber, D. J. & Zimmer, D. B. S100 proteins in cancer. Nat. Rev. Cancer 15 , 96–109 (2015).
Zimmer, D. B., Eubanks, J. O., Ramakrishnan, D. & Criscitiello, M. F. Evolution of the S100 family of calcium sensor proteins. Cell Calcium 53 , 170–179 (2013).
Moon, A. et al. Global gene expression profiling unveils S100A8/A9 as candidate markers in H-ras-mediated human breast epithelial cell invasion. Mol. Cancer Res. 6 , 1544–1553 (2008).
Kagan, H. M. & Trackman, P. C. Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 5 , 206–210 (1991).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139 , 891–906 (2009).
Reynaud, C. et al. Lysyl oxidase is a strong determinant of tumor cell colonization in bone. Cancer Res. 77 , 268–278 (2017).
Cox, T. R. et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522 , 106–110 (2015).
Monteiro, A. C. et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 8 , e68171 (2013).
Barker, H. E., Cox, T. R. & Erler, J. T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 12 , 540–552 (2012).
Cox, T. R., Gartland, A. & Erler, J. T. Lysyl Oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 76 , 188–192 (2016).
Sun, W. et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2 , 16015 (2016).
Deng, L. et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79 , 37–42 (2015).
Zhang, H. C. et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 21 , 3289–3297 (2012).
Huynh, N. et al. Characterization of regulatory extracellular vesicles from osteoclasts. J. Dent. Res. 95 , 673–679 (2016).
Yuan, F. L. et al. Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast-osteoblasts communication in bone remodeling. Front. Physiol. 9 , 628 (2018).
Li, D. et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 7 , 10872 (2016).
Harris, A. L. Hypoxia–a key regulatory factor in tumour growth. Nat. Rev. Cancer 2 , 38–47 (2002).
Le, Q. T., Denko, N. C. & Giaccia, A. J. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 23 , 293–310 (2004).
Arnett, T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 62 , 511–520 (2003).
Gatenby, R. A., Gawlinski, E. T., Gmitro, A. F., Kaylor, B. & Gillies, R. J. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 66 , 5216–5223 (2006).
Hamaoka, T., Madewell, J. E., Podoloff, D. A., Hortobagyi, G. N. & Ueno, N. T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 22 , 2942–2953 (2004).
Taichman, R. S. et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE 8 , e61873 (2013).
Roato, I. Bone metastases: when and how lung cancer interacts with bone. World J. Clin. Oncol. 5 , 149–155 (2014).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20 , 701–714 (2011).
Muto, A. et al. Lineage-committed osteoclast precursors circulate in blood and settle down into bone. J. Bone Miner. Res. 26 , 2978–2990 (2011).
Lee, A. W. & States, D. J. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol. Cell. Biol. 20 , 6779–6798 (2000).
Dougall, W. C. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 18 , 326–335 (2012).
Dougall, W. C., Holen, I. & Gonzalez Suarez, E. Targeting RANKL in metastasis. Bonekey Rep. 3 , 519 (2014).
Anderson, D. M. et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390 , 175–179 (1997).
Li, J. et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl Acad. Sci. USA 97 , 1566–1571 (2000).
Santini, D. et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE 6 , e19234 (2011).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440 , 692–696 (2006).
Suda, T. et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20 , 345–357 (1999).
Takahashi, N., Udagawa, N. & Suda, T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 256 , 449–455 (1999).
Maeda, K. et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 18 , 405–412 (2012).
Karst, M., Gorny, G., Galvin, R. J. & Oursler, M. J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell. Physiol. 200 , 99–106 (2004).
Hodge, J. M., Collier, F. M., Pavlos, N. J., Kirkland, M. A. & Nicholson, G. C. M-CSF potently augments RANKL-induced resorption activation in mature human osteoclasts. PLoS ONE 6 , e21462 (2011).
Naito, A. et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4 , 353–362 (1999).
Armstrong, A. P. et al. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 277 , 44347–44356 (2002).
Hu, R. et al. Eos, MITF, and PU.1 recruit corepressors to osteoclast-specific genes in committed myeloid progenitors. Mol. Cell. Biol. 27 , 4018–4027 (2007).
Taguchi, Y., Gohda, J., Koga, T., Takayanagi, H. & Inoue, J. A unique domain in RANK is required for Gab2 and PLCgamma2 binding to establish osteoclastogenic signals. Genes Cells 14 , 1331–1345 (2009).
Negishi-Koga, T. & Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 231 , 241–256 (2009).
Mao, D., Epple, H., Uthgenannt, B., Novack, D. V. & Faccio, R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J. Clin. Investig. 116 , 2869–2879 (2006).
Lee, S. H. et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 12 , 1403–1409 (2006).
Kukita, T. et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 200 , 941–946 (2004).
Yagi, M. et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202 , 345–351 (2005).
Campbell, J. P. et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 10 , e1001363 (2012).
Kakonen, S. M. & Mundy, G. R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 97 , 834–839 (2003).
Park, H. R. et al. Expression of osteoprotegerin and RANK ligand in breast cancer bone metastasis. J. Korean Med. Sci. 18 , 541–546 (2003).
Thomas, R. J. et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140 , 4451–4458 (1999).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2 , 584–593 (2002).
Powell, G. J. et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 51 , 3059–3061 (1991).
Vargas, S. J. et al. Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. J. Bone Miner. Res. 7 , 971–979 (1992).
Kohno, N. et al. The expression of parathyroid hormone-related protein in human breast cancer with skeletal metastases. Surg. Today 24 , 215–220 (1994).
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Investig. 98 , 1544–1549 (1996).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423 , 337–342 (2003).
Lacey, D. L. et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11 , 401–419 (2012).
Yasuda, H. et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139 , 1329–1337 (1998).
Roodman, G. D. Biology of osteoclast activation in cancer. J. Clin. Oncol. 19 , 3562–3571 (2001).
Irie, A. et al. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone 41 , 165–174 (2007).
Lamoureux, F. et al. Glycosaminoglycans as potential regulators of osteoprotegerin therapeutic activity in osteosarcoma. Cancer Res. 69 , 526–536 (2009).
Corey, E. et al. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 65 , 1710–1718 (2005).
Nyambo, R. et al. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J. Bone Miner. Res. 19 , 1712–1721 (2004).
Holen, I., Croucher, P. I., Hamdy, F. C., Eaton, C. L. & Osteoprotegerin, O. P. G. is a survival factor for human prostate cancer cells. Cancer Res. 62 , 1619–1623 (2002).
Brown, J. M. et al. Osteoprotegerin and rank ligand expression in prostate cancer. Urology 57 , 611–616 (2001).
Tae, C. H. et al. Significance of calcium-sensing receptor expression in gastric cancer. Scand. J. Gastroenterol. 51 , 67–72 (2016).
Singh, N., Aslam, M. N., Varani, J. & Chakrabarty, S. Induction of calcium sensing receptor in human colon cancer cells by calcium, vitamin D and aquamin: promotion of a more differentiated, less malignant and indolent phenotype. Mol. Carcinog. 54 , 543–553 (2015).
Diez-Fraile, A., Lammens, T., Benoit, Y. & D’Herde, K. G. The calcium-sensing receptor as a regulator of cellular fate in normal and pathological conditions. Curr. Mol. Med. 13 , 282–295 (2013).
Joeckel, E. et al. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol. Cancer 13 , 42 (2014).
Zekri, J., Ahmed, N., Coleman, R. E. & Hancock, B. W. The skeletal metastatic complications of renal cell carcinoma. Int. J. Oncol. 19 , 379–382 (2001).
Frees, S. et al. Calcium-sensing receptor (CaSR) promotes development of bone metastasis in renal cell carcinoma. Oncotarget 9 , 15766–15779 (2018).
Boudot, C. et al. Overexpression of a functional calcium-sensing receptor dramatically increases osteolytic potential of MDA-MB-231 cells in a mouse model of bone metastasis through epiregulin-mediated osteoprotegerin downregulation. Oncotarget 8 , 56460–56472 (2017).
Wu, Y. & Zhou, B. P. TNF-alpha/NF-kappaB/snail pathway in cancer cell migration and invasion. Br. J. Cancer 102 , 639–644 (2010).
Ji, H. et al. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat. Commun. 5 , 4944 (2014).
Calcinotto, A. et al. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J. Immunol. 188 , 2687–2694 (2012).
Ikemoto, S. et al. TNF alpha, IL-1 beta and IL-6 production by peripheral blood monocytes in patients with renal cell carcinoma. Anticancer Res. 20 , 317–321 (2000).
Balkwill, F. & Joffroy, C. TNF: a tumor-suppressing factor or a tumor-promoting factor? Future Oncol. 6 , 1833–1836 (2010).
Walsh, M. C. et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 24 , 33–63 (2006).
Kitaura, H. et al. Immunological reaction in TNF-alpha-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013 , 181849 (2013).
Kitaura, H. et al. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Investig. 115 , 3418–3427 (2005).
Weitzmann, M. N., Cenci, S., Rifas, L., Brown, C. & Pacifici, R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood 96 , 1873–1878 (2000).
Komine, M. et al. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone 28 , 474–483 (2001).
Azuma, Y., Kaji, K., Katogi, R., Takeshita, S. & Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 275 , 4858–4864 (2000).
Kanazawa, K., Azuma, Y., Nakano, H. & Kudo, A. TRAF5 functions in both RANKL- and TNFalpha-induced osteoclastogenesis. J. Bone Miner. Res. 18 , 443–450 (2003).
Kanazawa, K. & Kudo, A. TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J. Bone Miner. Res. 20 , 840–847 (2005).
Zhang, Y. H., Heulsmann, A., Tondravi, M. M., Mukherjee, A. & Abu-Amer, Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 276 , 563–568 (2001).
Yao, Z. et al. RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors. J. Biol. Chem. 292 , 10169–10179 (2017).
Hwang, S. J. et al. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 14 , R14 (2012).
Corrado, A., Neve, A., Maruotti, N. & Cantatore, F. P. Bone effects of biologic drugs in rheumatoid arthritis. Clin. Dev. Immunol. 2013 , 945945 (2013).
Tani-Ishii, N., Tsunoda, A., Teranaka, T. & Umemoto, T. Autocrine regulation of osteoclast formation and bone resorption by IL-1 alpha and TNF alpha. J. Dent. Res. 78 , 1617–1623 (1999).
Sunyer, T., Lewis, J., Collin-Osdoby, P. & Osdoby, P. Estrogen’s bone-protective effects may involve differential IL-1 receptor regulation in human osteoclast-like cells. J. Clin. Investig. 103 , 1409–1418 (1999).
Kim, J. H. et al. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 183 , 1862–1870 (2009).
Jules, J. et al. Molecular basis of requirement of receptor activator of nuclear factor kappaB signaling for interleukin 1-mediated osteoclastogenesis. J. Biol. Chem. 287 , 15728–15738 (2012).
Ruscitti, P. et al. The role of IL-1beta in the bone loss during rheumatic diseases. Mediators Inflamm. 2015 , 782382 (2015).
Wei, S., Kitaura, H., Zhou, P., Ross, F. P. & Teitelbaum, S. L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 115 , 282–290 (2005).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11 , 633–652 (2012).
Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol . 10 , a028415 (2018).
Yoshitake, F., Itoh, S., Narita, H., Ishihara, K. & Ebisu, S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J. Biol. Chem. 283 , 11535–11540 (2008).
Kudo, O. et al. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32 , 1–7 (2003).
Wakabayashi, H. et al. Interleukin-6 receptor inhibitor suppresses bone metastases in a breast cancer cell line. Breast Cancer 25 , 566–574 (2018).
Amarasekara, D. S. et al. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 18 , e8 (2018).
Feng, W. et al. Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-kappaB, ERK and JNK signaling pathways. Sci. Rep. 7 , 41411 (2017).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374 , 1–20 (2003).
Argast, G. M. et al. Cooperative signaling between oncostatin M, hepatocyte growth factor and transforming growth factor-beta enhances epithelial to mesenchymal transition in lung and pancreatic tumor models. Cells Tissues Organs 193 , 114–132 (2011).
Holzer, R. G., Ryan, R. E., Tommack, M., Schlekeway, E. & Jorcyk, C. L. Oncostatin M stimulates the detachment of a reservoir of invasive mammary carcinoma cells: role of cyclooxygenase-2. Clin. Exp. Metastasis 21 , 167–176 (2004).
Jorcyk, C. L., Holzer, R. G. & Ryan, R. E. Oncostatin M induces cell detachment and enhances the metastatic capacity of T-47D human breast carcinoma cells. Cytokine 33 , 323–336 (2006).
Hui, W., Rowan, A. D., Richards, C. D. & Cawston, T. E. Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 48 , 3404–3418 (2003).
Bolin, C. et al. Oncostatin M promotes mammary tumor metastasis to bone and osteolytic bone degradation. Genes Cancer 3 , 117–130 (2012).
Aguila, H. L. et al. Osteoblast-specific overexpression of human interleukin-7 rescues the bone mass phenotype of interleukin-7-deficient female mice. J. Bone Miner. Res. 27 , 1030–1042 (2012).
Toraldo, G., Roggia, C., Qian, W. P., Pacifici, R. & Weitzmann, M. N. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc. Natl Acad. Sci. USA 100 , 125–130 (2003).
Yu, J. et al. Generation of an osteoblast-based artificial niche that supports in vitro B lymphopoiesis. Exp. Mol. Med. 49 , e400 (2017).
Roato, I. et al. IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor. PLoS ONE 1 , e124 (2006).
Lubberts, E. et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J. Immunol. 170 , 2655–2662 (2003).
Kim, J. H. et al. Interleukin-7 induces osteoclast formation via STAT5, independent of receptor activator of NF-kappaB ligand. Front. Immunol. 8 , 1376 (2017).
Roy, L. D. et al. Systemic neutralization of IL-17A significantly reduces breast cancer associated metastasis in arthritic mice by reducing CXCL12/SDF-1 expression in the metastatic niches. BMC Cancer 14 , 225 (2014).
Balani, D., Aeberli, D., Hofstetter, W. & Seitz, M. Interleukin-17A stimulates granulocyte-macrophage colony-stimulating factor release by murine osteoblasts in the presence of 1,25-dihydroxyvitamin D(3) and inhibits murine osteoclast development in vitro. Arthritis Rheum. 65 , 436–446 (2013).
Shi, Y. et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 16 , 126–133 (2006).
Lee, M. S. et al. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J. Immunol. 183 , 3390–3399 (2009).
Ruef, N. et al. Granulocyte-macrophage colony-stimulating factor-dependent CD11c-positive cells differentiate into active osteoclasts. Bone 97 , 267–277 (2017).
Atanga, E., Dolder, S., Dauwalder, T., Wetterwald, A. & Hofstetter, W. TNFalpha inhibits the development of osteoclasts through osteoblast-derived GM-CSF. Bone 49 , 1090–1100 (2011).
Paul, S. R. et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc. Natl Acad. Sci. USA 87 , 7512–7516 (1990).
Zhang, Y. et al. Production of interleukin-11 in bone-derived endothelial cells and its role in the formation of osteolytic bone metastasis. Oncogene 16 , 693–703 (1998).
Romas, E. et al. The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J. Exp. Med. 183 , 2581–2591 (1996).
Elias, J. A., Tang, W. & Horowitz, M. C. Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endocrinology 136 , 489–498 (1995).
Girasole, G., Passeri, G., Jilka, R. L. & Manolagas, S. C. Interleukin-11: a new cytokine critical for osteoclast development. J. Clin. Investig. 93 , 1516–1524 (1994).
Ren, L., Wang, X., Dong, Z., Liu, J. & Zhang, S. Bone metastasis from breast cancer involves elevated IL-11 expression and the gp130/STAT3 pathway. Med. Oncol. 30 , 634 (2013).
Bendre, M. S. et al. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33 , 28–37 (2003).
Bendre, M. S. et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 65 , 11001–11009 (2005).
Kopesky, P. et al. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol. Open 3 , 767–776 (2014).
Liu, Y. et al. Role of IL-8 and its receptor in anti-citrullinated protein antibody mediated osteoclastogenesis in RA. Ann. Rheum. Dis. 75 , AB0078 (2016).
Google Scholar
Bhattacharyya, R. S. & Stern, P. H. IGF-I and MAP kinase involvement in the stimulatory effects of LNCaP prostate cancer cell conditioned media on cell proliferation and protein synthesis in MC3T3-E1 osteoblastic cells. J. Cell. Biochem. 90 , 925–937 (2003).
Feeley, B. T. et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J. Bone Miner. Res. 20 , 2189–2199 (2005).
Kitagawa, Y. et al. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res. 65 , 10921–10929 (2005).
Guise, T. A., Yin, J. J. & Mohammad, K. S. Role of endothelin-1 in osteoblastic bone metastases. Cancer 97 , 779–784 (2003).
Smollich, M. & Wulfing, P. The endothelin axis: a novel target for pharmacotherapy of female malignancies. Curr. Vasc. Pharm. 5 , 239–248 (2007).
CAS Google Scholar
Yin, J. J. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl Acad. Sci. USA 100 , 10954–10959 (2003).
Clines, G. A. et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol. Endocrinol. 21 , 486–498 (2007).
Guise, T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 37 , S2–S14 (2010).
Hall, C. L., Bafico, A., Dai, J., Aaronson, S. A. & Keller, E. T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 65 , 7554–7560 (2005).
Carducci, M. A. et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J. Clin. Oncol. 21 , 679–689 (2003).
Nelson, J. B., Udan, M. S., Guruli, G. & Pflug, B. R. Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia 7 , 631–637 (2005).
Van Sant, C. et al. Endothelin signaling in osteoblasts: global genome view and implication of the calcineurin/NFAT pathway. Mol. Cancer Ther. 6 , 253–261 (2007).
Bendinelli, P. et al. Microenvironmental stimuli affect Endothelin-1 signaling responsible for invasiveness and osteomimicry of bone metastasis from breast cancer. Biochim. Biophys. Acta 1843 , 815–826 (2014).
Maroni, P. et al. High SPARC expression starting from dysplasia, associated with breast carcinoma, is predictive for bone metastasis without enhancement of plasma levels. Int. J. Mol. Sci. 16 , 28108–28122 (2015).
Zhong, X., Wang, H. & Huang, S. Endothelin-1 induces interleukin-18 expression in human osteoblasts. Arch. Oral. Biol. 59 , 289–296 (2014).
Matteucci, E. et al. Microenvironment stimuli HGF and hypoxia differently affected miR-125b and Ets-1 function with opposite effects on the invasiveness of bone metastatic cells: a comparison with breast carcinoma cells. Int. J. Mol. Sci. 19 , 258 (2018).
Hall, C. L. & Keller, E. T. The role of Wnts in bone metastases. Cancer Metastasis Rev. 25 , 551–558 (2006).
Zhuang, X. et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 19 , 1274–1285 (2017).
Kasoha, M. et al. Dickkopf-1 (Dkk1) protein expression in breast cancer with special reference to bone metastases. Clin. Exp. Metastasis 35 , 763–775 (2018).
Balemans, W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10 , 537–543 (2001).
Brunkow, M. E. et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68 , 577–589 (2001).
Li, X. et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 280 , 19883–19887 (2005).
Glinka, A. et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 12 , 1055–1061 (2011).
ten Dijke, P., Krause, C., de Gorter, D. J., Lowik, C. W. & van Bezooijen, R. L. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J. Bone Joint Surg. Am. 90 , 31–35 (2008).
Clines, G. A. & Guise, T. A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 10 , e7 (2008).
Liao, J. & McCauley, L. K. Skeletal metastasis: established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer Metastasis Rev. 25 , 559–571 (2006).
Deftos, L. J., Barken, I., Burton, D. W., Hoffman, R. M. & Geller, J. Direct evidence that PTHrP expression promotes prostate cancer progression in bone. Biochem. Biophys. Res. Commun. 327 , 468–472 (2005).
Hoey, R. P. et al. The parathyroid hormone-related protein receptor is expressed in breast cancer bone metastases and promotes autocrine proliferation in breast carcinoma cells. Br. J. Cancer 88 , 567–573 (2003).
Shen, X. & Falzon, M. PTH-related protein enhances LoVo colon cancer cell proliferation, adhesion, and integrin expression. Regul. Pept. 125 , 17–27 (2005).
Massfelder, T. et al. Parathyroid hormone-related protein is an essential growth factor for human clear cell renal carcinoma and a target for the von Hippel-Lindau tumor suppressor gene. Cancer Res. 64 , 180–188 (2004).
Ma, Y. L. et al. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 142 , 4047–4405 (2001).
Liao, J. et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int. J. Cancer 123 , 2267–2278 (2008).
LeBeau, A. M., Kostova, M., Craik, C. S. & Denmeade, S. R. Prostate-specific antigen: an overlooked candidate for the targeted treatment and selective imaging of prostate cancer. Biol. Chem. 391 , 333–343 (2010).
Cramer, S. D., Chen, Z. & Peehl, D. M. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J. Urol. 156 , 526–531 (1996).
Schluter, K. D., Katzer, C. & Piper, H. M. A N-terminal PTHrP peptide fragment void of a PTH/PTHrP-receptor binding domain activates cardiac ET(A) receptors. Br. J. Pharmacol. 132 , 427–432 (2001).
Fielder, P. J. et al. Biochemical analysis of prostate specific antigen-proteolyzed insulin-like growth factor binding protein-3. Growth Regul. 4 , 164–172 (1994).
Killian, C. S., Corral, D. A., Kawinski, E. & Constantine, R. I. Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem. Biophys. Res. Commun. 192 , 940–947 (1993).
Williams, S. A., Singh, P., Isaacs, J. T. & Denmeade, S. R. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate 67 , 312–329 (2007).
Lee, Y. C. et al. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 71 , 5194–5203 (2011).
Li, Z. G. et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J. Clin. Investig. 118 , 2697–2710 (2008).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2 , 563–572 (2002).
Johnson, R. W. & Suva, L. J. Hallmarks of bone metastasis. Calcif. Tissue Int. 102 , 141–151 (2018).
Egeblad, M. & Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2 , 161–174 (2002).
Djonov, V. et al. Tumor cell specific expression of MMP-2 correlates with tumor vascularisation in breast cancer. Int. J. Oncol. 21 , 25–30 (2002).
Ruokolainen, H., Paakko, P. & Turpeenniemi-Hujanen, T. Expression of matrix metalloproteinase-9 in head and neck squamous cell carcinoma: a potential marker for prognosis. Clin. Cancer Res. 10 , 3110–3116 (2004).
Bachmeier, B. E., Nerlich, A. G., Lichtinghagen, R. & Sommerhoff, C. P. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 21 , 3821–3828 (2001).
Upadhyay, J. et al. Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin. Cancer Res. 5 , 4105–4110 (1999).
Nakopoulou, L. et al. MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res. Treat. 77 , 145–155 (2003).
Ranuncolo, S. M., Armanasco, E., Cresta, C., Bal De Kier Joffe, E. & Puricelli, L. Plasma MMP-9 (92 kDa-MMP) activity is useful in the follow-up and in the assessment of prognosis in breast cancer patients. Int. J. Cancer 106 , 745–751 (2003).
Benelli, R. et al. Inhibition of AIDS-Kaposi’s sarcoma cell induced endothelial cell invasion by TIMP-2 and a synthetic peptide from the metalloproteinase propeptide: implications for an anti-angiogenic therapy. Oncol. Res. 6 , 251–257 (1994).
Anand-Apte, B. et al. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Investig. Ophthalmol. Vis. Sci. 38 , 817–823 (1997).
Hiraoka, N., Allen, E., Apel, I. J., Gyetko, M. R. & Weiss, S. J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95 , 365–377 (1998).
Vu, T. H. et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93 , 411–422 (1998).
Itoh, T. et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 58 , 1048–1051 (1998).
Schnaper, H. W. et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J. Cell. Physiol. 156 , 235–246 (1993).
Jezierska, A. & Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med. Sci. Monit. 15 , RA32-40 (2009).
Talvensaari-Mattila, A., Paakko, P. & Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer 89 , 1270–1275 (2003).
Chen, Y., Wang, X., Chen, G., Dong, C. & Zhang, D. The impact of matrix metalloproteinase 2 on prognosis and clinicopathology of breast cancer patients: a systematic meta-analysis. PLoS ONE 10 , e0121404 (2015).
Hashimoto, G. et al. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J. Biol. Chem. 277 , 36288–36295 (2002).
Chetty, C., Lakka, S. S., Bhoopathi, P. & Rao, J. S. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int. J. Cancer 127 , 1081–1095 (2010).
Heroult, M. et al. Heparin affin regulatory peptide binds to vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Oncogene 23 , 1745–1753 (2004).
O’Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88 , 277–285 (1997).
Ramchandran, R. et al. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem. Biophys. Res. Commun. 255 , 735–739 (1999).
Colorado, P. C. et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 60 , 2520–2526 (2000).
Tauro, M. & Lynch, C. C. Cutting to the chase: how matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers 10 , 185 (2018).
Chen, P. C. et al. Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. J. Hematol. Oncol. 10 , 33 (2017).
Lynch, C. C. et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 7 , 485–496 (2005).
Bruni-Cardoso, A., Johnson, L. C., Vessella, R. L., Peterson, T. E. & Lynch, C. C. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol. Cancer Res. 8 , 459–470 (2010).
Wang, C. et al. BMP-6 inhibits MMP-9 expression by regulating heme oxygenase-1 in MCF-7 breast cancer cells. J. Cancer Res. Clin. Oncol. 137 , 985–995 (2011).
Freije, J. M. et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J. Biol. Chem. 269 , 16766–16773 (1994).
Mauviel, A. Cytokine regulation of metalloproteinase gene expression. J. Cell. Biochem. 53 , 288–295 (1993).
Johansson, N. et al. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am. J. Pathol. 151 , 499–508 (1997).
Cazorla, M. et al. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J. Pathol. 186 , 144–150 (1998).
Ren, X.-F., Mu, L.-P., Jiang, Y.-S., Wang, L. & Ma, J.-F. LY2109761 inhibits metastasis and enhances chemosensitivity in osteosarcoma MG-63 cells. Eur. Rev. Med. Pharmacol. 19 (7), 1182–1190 (2015).
Wang, J., Loberg, R. & Taichman, R. S. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 25 , 573–587 (2006).
Sun, X. et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 29 , 709–722 (2010).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410 , 50–56 (2001).
Kojima, Y. et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl Acad. Sci. USA 107 , 20009–20014 (2010).
Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 4 , 540–550 (2004).
Hattermann, K. et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 70 , 3299–3308 (2010).
Miao, Z. et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl Acad. Sci. USA 104 , 15735–15740 (2007).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121 , 335–348 (2005).
Kishimoto, H. et al. The p160 family coactivators regulate breast cancer cell proliferation and invasion through autocrine/paracrine activity of SDF-1alpha/CXCL12. Carcinogenesis 26 , 1706–1715 (2005).
Burger, J. A. & Kipps, T. J. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 107 , 1761–1767 (2006).
Sun, Y. X. et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Miner. Res. 20 , 318–329 (2005).
Taichman, R. S. et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 62 , 1832–1837 (2002).
Singh, S., Srivastava, S. K., Bhardwaj, A., Owen, L. B. & Singh, A. P. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br. J. Cancer 103 , 1671–1679 (2010).
Maderna, E., Salmaggi, A., Calatozzolo, C., Limido, L. & Pollo, B. Nestin, PDGFRbeta, CXCL12 and VEGF in glioma patients: different profiles of (pro-angiogenic) molecule expression are related with tumor grade and may provide prognostic information. Cancer Biol. Ther. 6 , 1018–1024 (2007).
Xu, L. et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 69 , 7905–7910 (2009).
Engl, T. et al. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia 8 , 290–301 (2006).
Havens, A. M. et al. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer 6 , 195 (2006).
Cabioglu, N. et al. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 65 , 6493–6497 (2005).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3 , 537–549 (2003).
Liang, Z. et al. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 65 , 967–971 (2005).
Richert, M. M. et al. Inhibition of CXCR4 by CTCE-9908 inhibits breast cancer metastasis to lung and bone. Oncol. Rep. 21 , 761–767 (2009).
Chinni, S. R. et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 66 , 32–48 (2006).
Wang, J. et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 17 , 1578–1592 (2005).
Glinskii, O. V. et al. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia 7 , 522–527 (2005).
Glinsky, V. V. et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 61 , 4851–4857 (2001).
Hill, A., McFarlane, S., Johnston, P. G. & Waugh, D. J. The emerging role of CD44 in regulating skeletal micrometastasis. Cancer Lett. 237 , 1–9 (2006).
Pinilla, S. et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 284 , 80–85 (2009).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449 , 557–563 (2007).
Urata, S. et al. C-C motif ligand 5 promotes migration of prostate cancer cells in the prostate cancer bone metastasis microenvironment. Cancer Sci. 109 , 724–731 (2018).
Kim, M. et al. The lymphotactin receptor is expressed in epithelial ovarian carcinoma and contributes to cell migration and proliferation. Mol. Cancer Res. 10 , 1419–1429 (2012).
Khurram, S. A. et al. Functional expression of the chemokine receptor XCR1 on oral epithelial cells. J. Pathol. 221 , 153–163 (2010).
Gantsev, S. K. et al. The role of inflammatory chemokines in lymphoid neoorganogenesis in breast cancer. Biomed. Pharmacother. 67 , 363–366 (2013).
Sun, Y. X. et al. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate 67 , 61–73 (2007).
Valcarcel, M. et al. Vascular endothelial growth factor regulates melanoma cell adhesion and growth in the bone marrow microenvironment via tumor cyclooxygenase-2. J. Transl. Med. 9 , 142 (2011).
Denkert, C. et al. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 61 , 303–308 (2001).
Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer 11 , 411–425 (2011).
Mohammad, K. S. et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE 4 , e5275 (2009).
Korpal, M. et al. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat. Med. 15 , 960–966 (2009).
Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl Acad. Sci. USA 102 , 13909–13914 (2005).
Juarez, P. & Guise, T. A. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone 48 , 23–29 (2011).
Serganova, I. et al. Multimodality imaging of TGFbeta signaling in breast cancer metastases. FASEB J. 23 , 2662–2672 (2009).
Baselga, J. et al. TGF-beta signalling-related markers in cancer patients with bone metastasis. Biomarkers 13 , 217–236 (2008).
Buijs, J. T. et al. TGF-beta and BMP7 interactions in tumour progression and bone metastasis. Clin. Exp. Metastasis 24 , 609–617 (2007).
Pickup, M., Novitskiy, S. & Moses, H. L. The roles of TGFbeta in the tumour microenvironment. Nat. Rev. Cancer 13 , 788–799 (2013).
Yin, J. J. et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 103 , 197–206 (1999).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19 , 192–205 (2011).
Wan, X. et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 50 , 695–703 (2012).
Kawai, M. & Rosen, C. J. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol. Metab. Clin. North Am. 41 , 323–333 (2012).
Hiraga, T. et al. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res. 72 , 4238–4249 (2012).
Zhang, X. H. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154 , 1060–1073 (2013).
Mitsiades, C. S. & Koutsilieris, M. Molecular biology and cellular physiology of refractoriness to androgen ablation therapy in advanced prostate cancer. Expert Opin. Investig. Drugs 10 , 1099–1115 (2001).
Koutsilieris, M. et al. A combination therapy of dexamethasone and somatostatin analog reintroduces objective clinical responses to LHRH analog in androgen ablation-refractory prostate cancer patients. J. Clin. Endocrinol. Metab. 86 , 5729–5736 (2001).
Koutsilieris, M. et al. Combination of dexamethasone and a somatostatin analogue in the treatment of advanced prostate cancer. Expert Opin. Investig. Drugs 11 , 283–293 (2002).
Koutsilieris, M. et al. Combination of somatostatin analog, dexamethasone, and standard androgen ablation therapy in stage D3 prostate cancer patients with bone metastases. Clin. Cancer Res. 10 , 4398–4405 (2004).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136 , 215–233 (2009).
Khew-Goodall, Y. & Goodall, G. J. Myc-modulated miR-9 makes more metastases. Nat. Cell Biol. 12 , 209–211 (2010).
Ma, L. et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 12 , 247–256 (2010).
Ren, D. et al. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR145. Int. J. Oncol. 42 , 1473–1481 (2013).
Ren, D. et al. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-kappaB signaling pathway. Mol. Cancer 16 , 117 (2017).
Colden, M. et al. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 8 , e2572 (2017).
Siu, M. K. et al. Transforming growth factor-beta promotes prostate bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. Oncogene 34 , 4767–4776 (2015).
Lou, G. et al. Direct targeting sperm-associated antigen 9 by miR-141 influences hepatocellular carcinoma cell growth and metastasis via JNK pathway. J. Exp. Clin. Cancer Res. : C 35 , 14 (2016).
Liu, C. et al. MicroRNA-141 suppresses prostate cancer stem cells and metastasis by targeting a cohort of pro-metastasis genes. Nat. Commun. 8 , 14270 (2017).
Huang, S. et al. Downregulation of miR-141-3p promotes bone metastasis via activating NF-kappaB signaling in prostate cancer. J. Exp. Clin. Cancer Res. 36 , 173 (2017).
Ren, D. et al. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res. 358 , 763–778 (2014).
Guo, W. et al. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J. Cell. Biochem. 114 , 1606–1615 (2013).
Huang, S. et al. Transcriptional downregulation of miR-133b by REST promotes prostate cancer metastasis to bone via activating TGF-beta signaling. Cell Death Dis. 9 , 779 (2018).
Wa, Q. et al. Downregulation of miR19a3p promotes invasion, migration and bone metastasis via activating TGFbeta signaling in prostate cancer. Oncol. Rep. 39 , 81–90 (2018).
Croset, M., Kan, C. & Clezardin, P. Tumour-derived miRNAs and bone metastasis. Bonekey Rep. 4 , 688 (2015).
Taipaleenmaki, H. et al. Targeting of Runx2 by miR-135 and miR-203 Impairs Progression of Breast Cancer and Metastatic Bone Disease. Cancer Res. 75 , 1433–1444 (2015).
Croset, M. et al. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J. Bone Miner. Res. 29 , 1886–1899 (2014).
David, M. et al. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS ONE 5 , e9741 (2010).
Leblanc, R. et al. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood 124 , 3141–3150 (2014).
D'Oronzo, S., Coleman, R., Brown, J. & Silvestris, F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J. Bone Oncol. 15 , 004–4 (2019).
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365 , 1687–1717 (2005).
Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N. Engl. J. Med . 319 , 1681–1692 (1988).
Pyrhonen, S. et al. Meta-analysis of trials comparing toremifene with tamoxifen and factors predicting outcome of antiestrogen therapy in postmenopausal women with breast cancer. Breast Cancer Res. Treat. 56 , 133–143 (1999).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 90 , 1371–1388 (1998).
Cauley, J. A. et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res. Treat. 65 , 125–134 (2001).
Howell, A. et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365 , 60–62 (2005).
Coates, A. S. et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J. Clin. Oncol. 25 , 486–492 (2007).
Coombes, R. C. et al. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369 , 559–570 (2007).
Arimidex,Tamoxifen, Alone or in Combination (ATAC) Trialists' Group et al.Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 9 , 45–53 (2008).
Buzdar, A. et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J. Clin. Oncol. 19 , 3357–3366 (2001).
Buzdar, A. U. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the international letrozole breast cancer group. J. Clin. Oncol. 22 , 3199–3200 (2004).
Hong, N. et al. Different patterns in the risk of newly developed fatty liver and lipid changes with tamoxifen versus aromatase inhibitors in postmenopausal women with early breast cancer: a propensity score-matched cohort study. Eur. J. Cancer 82 , 103–114 (2017).
De Placido, S. et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol. 19 , 474–485 (2018).
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365 , 1687–1717 (2005).
Coombes, R. C. et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N. Engl. J. Med. 350 , 1081–1092 (2004).
Jakesz, R. et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366 , 455–462 (2005).
Boccardo, F. et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J. Clin. Oncol. 23 , 5138–5147 (2005).
Goss, P. E. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl Cancer Inst. 97 , 1262–1271 (2005).
Mamounas, E. P. Adjuvant exemestane therapy after 5 years of tamoxifen: rationale for the NSABP B-33 trial. Oncology 15 , 35–39 (2001).
Heshmati, H. M. et al. Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J. Bone Miner. Res. 17 , 172–178 (2002).
Bouvard, B. et al. Fracture incidence after 3 years of aromatase inhibitor therapy. Ann. Oncol. 25 , 843–847 (2014).
Heidenreich, A. et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 65 , 467–479 (2014).
Huggins, C., Stevens, R. E. Jr. & Hodges, C. V. Studies on prostatic cancer: ii. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 43 , 209–223 (1941).
Lepor, H. & Shore, N. D. LHRH agonists for the treatment of prostate cancer: 2012. Rev. Urol. 14 , 1–12 (2012).
Hatano, T., Oishi, Y., Furuta, A., Iwamuro, S. & Tashiro, K. Incidence of bone fracture in patients receiving luteinizing hormone-releasing hormone agonists for prostate cancer. BJU Int. 86 , 449–452 (2000).
Smith, M. R. et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med 345 , 948–955 (2001).
Oefelein, M. G. et al. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J. Urol. 166 , 1724–1728 (2001).
Melton, L. J. III et al. Fracture risk following bilateral orchiectomy. J. Urol. 169 , 1747–1750 (2003).
Fizazi, K. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 13 , 983–992 (2012).
Ryan, C. J. et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 16 , 152–160 (2015).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367 , 1187–1197 (2012).
Beer, T. M. & Tombal, B. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371 , 1755–1756 (2014).
Sonpavde, G. et al. Sequencing of cabazitaxel and abiraterone acetate after docetaxel in metastatic castration-resistant prostate cancer: treatment patterns and clinical outcomes in multicenter community-based US oncology practices. Clin. Genitourin. Cancer 13 , 309–318 (2015).
Serafini, A. N. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases. Cancer 88 , 2934–2939 (2000).
Quilty, P. M. et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother. Oncol. 31 , 33–40 (1994).
Roque, I. F. M., Martinez-Zapata, M. J., Scott-Brown, M. & Alonso-Coello, P. WITHDRAWN: radioisotopes for metastatic bone pain. Cochrane Database Syst. Rev. 3 , CD003347 (2017).
Hoskin, P. et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 15 , 1397–1406 (2014).
Morris, M. J. et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J. Clin. Oncol. 27 , 2436–2442 (2009).
Tu, S. M. et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 357 , 336–341 (2001).
Sciuto, R. et al. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J. Nucl. Med 43 , 79–86 (2002).
Fizazi, K. et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J. Clin. Oncol. 27 , 2429–2435 (2009).
Chow, E., Harris, K., Fan, G., Tsao, M. & Sze, W. M. Palliative radiotherapy trials for bone metastases: a systematic review. J. Clin. Oncol. 25 , 1423–1436 (2007).
McQuay, H. J., Carroll, D. & Moore, R. A. Radiotherapy for painful bone metastases: a systematic review. Clin. Oncol. 9 , 150–154 (1997).
Wu, J. S. et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J. Radiat. Oncol. Biol. Phys. 55 , 594–605 (2003).
Sze, W. M., Shelley, M. D., Held, I., Wilt, T. J. & Mason, M. D. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy–a systematic review of randomised trials. Clin. Oncol. 15 , 345–352 (2003).
Hartsell, W. F. et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl Cancer Inst. 97 , 798–804 (2005).
Higinbotham, N. L. & Marcove, R. C. The management of pathological fractures. J. Trauma 5 , 792–798 (1965).
Coleman, R. E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12 , 6243s–6249s (2006).
Schulman, K. L. & Kohles, J. Economic burden of metastatic bone disease in the U.S. Cancer 109 , 2334–2342 (2007).
Forsberg, J. A., Eberhardt, J., Boland, P. J., Wedin, R. & Healey, J. H. Estimating survival in patients with operable skeletal metastases: an application of a bayesian belief network. PLoS ONE 6 , e19956 (2011).
Ratasvuori, M. et al. Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J. Surg. Oncol. 110 , 360–365 (2014).
Harvey, N., Ahlmann, E. R., Allison, D. C., Wang, L. & Menendez, L. R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin. Orthop. Relat. Res. 470 , 684–691 (2012).
Harrington, K. D. Orthopedic surgical management of skeletal complications of malignancy. Cancer 80 , 1614–1627 (1997).
Facchini, G. et al. Palliative embolization for metastases of the spine. Eur. J. Orthop. Surg. Traumatol. 26 , 247–252 (2016).
Rossi, G. et al. Embolisation of bone metastases from renal cancer. Radio. Med. 118 , 291–302 (2013).
Cheung, F. H. The practicing orthopedic surgeon's guide to managing long bone metastases. Orthop. Clin. North Am. 45 , 109–119 (2014).
Willeumier, J. J., van der Linden, Y. M., van de Sande, M. A. J. & Dijkstra, P. D. S. Treatment of pathological fractures of the long bones. EFORT Open Rev. 1 , 136–145 (2016).
Errani, C. et al. Treatment for long bone metastases based on a systematic literature review. Eur. J. Orthop. Surg. Traumatol. 27 , 205–211 (2017).
Sun, G., Jin, P., Liu, X. W., Li, M. & Li, L. Cementoplasty for managing painful bone metastases outside the spine. Eur. Radio. 24 , 731–737 (2014).
Eisenberg, E., Shay, L., Keidar, Z., Amit, A. & Militianu, D. Magnetic resonance-guided focused ultrasound surgery for bone metastasis: from pain palliation to biological ablation? J. Pain. Symptom Manag. 56 , 158–162 (2018).
Hurwitz, M. D. et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J. Natl Cancer Inst. 106 , dju082 (2014).
Liberman, B. et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann. Surg. Oncol. 16 , 140–146 (2009).
Gianfelice, D. et al. Palliative treatment of painful bone metastases with MR imaging–guided focused ultrasound. Radiology 249 , 355–363 (2008).
Roelofs, A. J., Thompson, K., Gordon, S. & Rogers, M. J. Molecular mechanisms of action of bisphosphonates: current status. Clin. Cancer Res. 12 , 6222s–6230s (2006).
Lehenkari, P. P. et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharm. 61 , 1255–1262 (2002).
Pickup, M. W., Owens, P. & Moses, H. L. TGF-β, bone morphogenetic protein, and activin signaling and the tumor microenvironment. Cold Spring Harb Perspect Biol. 9 (5), a022285, https://doi.org/10.1101/cshperspect.a022285 (2017).
Article CAS PubMed PubMed Central Google Scholar
Buijs, J. T., Stayrook, K. R. & Guise, T. A. The role of TGF-β in bone metastasis: Novel therapeutic perspectives. Bonekey Rep. 1 , 96–705, https://doi.org/10.1038/bonekey.2012.96 (2012).
Article PubMed PubMed Central Google Scholar
Dalle Carbonare, L. et al. Bisphosphonates decrease telomerase activity and hTERT expression in MCF-7 breast cancer cells. Mol. Cell. Endocrinol. 240 , 23–31 (2005).
Van Poznak, C. et al. Expression of osteoprotegerin (OPG), TNF related apoptosis inducing ligand (TRAIL), and receptor activator of nuclear factor kappaB ligand (RANKL) in human breast tumours. J. Clin. Pathol. 59 , 56–63 (2006).
Soltau, J. et al. Antitumoral and antiangiogenic efficacy of bisphosphonates in vitro and in a murine RENCA model. Anticancer Res. 28 , 933–941 (2008).
Backman, U., Svensson, A., Christofferson, R. H. & Azarbayjani, F. The bisphosphonate, zoledronic acid reduces experimental neuroblastoma growth by interfering with tumor angiogenesis. Anticancer Res. 28 , 1551–1557 (2008).
Ribatti, D. et al. Clodronate inhibits angiogenesis in vitro and in vivo. Oncol. Rep. 19 , 1109–1112 (2008).
Hashimoto, K. et al. Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem. Biophys. Res. Commun. 354 , 478–484 (2007).
Tang, X. et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling pathways in human breast cancer cells. Int. J. Cancer 126 , 90–103 (2010).
Zhou, J. Z. et al. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene 35 , 5597–5607 (2016).
Mundy, G. R., Yoneda, T. & Hiraga, T. Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Semin Oncol. 28 , 35–44 (2001).
Kokufu, I., Kohno, N., Yamamoto, M. & Takao, S. Adjuvant pamidronate therapy prevents the development of bone metastases in breast cancer patients with four or more positive nodes. Oncol. Lett. 1 , 247–252 (2010).
Lipton, A. et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 88 , 1082–1090 (2000).
Berenson, J. R. et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91 , 1191–1200 (2001).
Rosen, L. S. et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 7 , 377–387 (2001).
Atula, S. et al. Extended safety profile of oral clodronate after long-term use in primary breast cancer patients. Drug Saf. 26 , 661–671 (2003).
Powles, T. et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 8 , R13 (2006).
Dearnaley, D. P. et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J. Natl Cancer Inst. 95 , 1300–1311 (2003).
Saad, F. et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 94 , 1458–1468 (2002).
Higano, C. S. Understanding treatments for bone loss and bone metastases in patients with prostate cancer: a practical review and guide for the clinician. Urol. Clin. North Am. 31 , 331–352 (2004).
Khosla, S. et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 22 , 1479–1491 (2007).
Woo, S. B., Hellstein, J. W. & Kalmar, J. R. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann. Intern. Med. 144 , 753–761 (2006).
Rodriguez-Lozano, F. J. & Onate-Sanchez, R. E. Treatment of osteonecrosis of the jaw related to bisphosphonates and other antiresorptive agents. Med. Oral Patol. Oral Cir. Bucal 21 , e595–e600 (2016).
Neville-Webbe, H. L., Rostami-Hodjegan, A., Evans, C. A., Coleman, R. E. & Holen, I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int. J. Cancer 113 , 364–371 (2005).
Winter, M. C., Holen, I. & Coleman, R. E. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat. Rev. 34 , 453–475 (2008).
Kurabayashi, A. et al. Combination with third-generation bisphosphonate (YM529) and interferon-alpha can inhibit the progression of established bone renal cell carcinoma. Cancer Sci. 106 , 1092–1099 (2015).
Stopeck, A. T. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 28 , 5132–5139 (2010).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377 , 813–822 (2011).
Lipton, A. & Goessl, C. Clinical development of anti-RANKL therapies for treatment and prevention of bone metastasis. Bone 48 , 96–99 (2011).
Schieferdecker, A. et al. Denosumab mimics the natural decoy receptor osteoprotegerin by interacting with its major binding site on RANKL. Oncotarget 5 , 6647–6653 (2014).
Liu, Y. et al. AB0078|Role of IL-8 and Its Receptor in Anti-Citrullinated Protein Antibody Mediated Osteoclastogenesis in RA. Ann. Rheum. Dis. 75 , 923 (2016).
Duda, D. G. et al. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin. Cancer Res. 17 , 2074–2080 (2011).
Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11 , 83–95 (2007).
Batchelor, T. T. et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 28 , 2817–2823 (2010).
di Tomaso, E. et al. Glioblastoma recurrence after cediranib therapy in patients: lack of "rebound" revascularization as mode of escape. Cancer Res. 71 , 19–28 (2011).
Kamoun, W. S. et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J. Clin. Oncol. 27 , 2542–2552 (2009).
Hiratsuka, S. et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc. Natl Acad. Sci. USA 108 , 302–307 (2011).
Shaked, Y. et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14 , 263–273 (2008).
Shaked, Y. et al. Contribution of granulocyte colony-stimulating factor to the acute mobilization of endothelial precursor cells by vascular disrupting agents. Cancer Res. 69 , 7524–7528 (2009).
Kozin, S. V. et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 70 , 5679–5685 (2010).
Kioi, M. et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Investig. 120 , 694–705 (2010).
Chang, C. C. et al. Dose-dependent effect of radiation on angiogenic and angiostatic CXC chemokine expression in human endothelial cells. Cytokine 48 , 295–302 (2009).
Padua, D. et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133 , 66–77 (2008).
Tan, A. R., Alexe, G. & Reiss, M. Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer? Breast Cancer Res. Treat. 115 , 453–495 (2009).
Biswas, S. et al. Anti-transforming growth factor ss antibody treatment rescues bone loss and prevents breast cancer metastasis to bone. PLoS ONE 6 , e27090 (2011).
Ganapathy, V. et al. Targeting the transforming growth factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer 9 , 122 (2010).
Dasch, J. R., Pace, D. R., Waegell, W., Inenaga, D. & Ellingsworth, L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J. Immunol. 142 , 1536–1541 (1989).
Andrade, S. E., Donahue, J. G., Chan, K. A., Watson, D. J. & Platt, R. Liver function testing in patients on HMG-CoA reductase inhibitors. Pharmacoepidemiol. Drug Saf. 12 , 307–313 (2003).
Wong, W. W., Dimitroulakos, J., Minden, M. D. & Penn, L. Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16 , 508–519 (2002).
Liu, C. M. et al. In vivo targeting of ADAM9 gene expression using lentivirus-delivered shRNA suppresses prostate cancer growth by regulating REG4 dependent cell cycle progression. PLoS ONE 8 , e53795 (2013).
Mandal, C. C., Ghosh-Choudhury, N., Yoneda, T., Choudhury, G. G. & Ghosh-Choudhury, N. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J. Biol. Chem. 286 , 11314–11327 (2011).
Asanuma, K. et al. The thrombin inhibitor, argatroban, inhibits breast cancer metastasis to bone. Breast Cancer 20 , 241–246 (2013).
Nelson, J. B. et al. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J. Urol. 169 , 1143–1149 (2003).
Pu, R. et al. Rapid bone repair in a patient with lung cancer metastases to the spine using a novel herbal medicine: A case report. Oncol. Lett. 12 , 2023–2027 (2016).
Mandal, C. C., Ghosh-Choudhury, T., Yoneda, T., Choudhury, G. G. & Ghosh-Choudhury, N. Fish oil prevents breast cancer cell metastasis to bone. Biochem. Biophys. Res. Commun. 402 , 602–607 (2010).
Dalrymple, S. L., Becker, R. E. & Isaacs, J. T. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate 67 , 790–797 (2007).
Isaacs, J. T. et al. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate 66 , 1768–1778 (2006).
Isaacs, J. T. The long and winding road for the development of tasquinimod as an oral second-generation quinoline-3-carboxamide antiangiogenic drug for the treatment of prostate cancer. Expert Opin. Investig. Drugs 19 , 1235–1243 (2010).
Bratt, O. et al. Open-label, clinical phase I studies of tasquinimod in patients with castration-resistant prostate cancer. Br. J. Cancer 101 , 1233–1240 (2009).
Jennbacken, K. et al. Inhibition of metastasis in a castration resistant prostate cancer model by the quinoline-3-carboxamide tasquinimod (ABR-215050). Prostate 72 , 913–924 (2012).
Shukeir, N. et al. Pharmacological methyl group donors block skeletal metastasis in vitro and in vivo. Br. J. Pharmacol. 172 , 2769–2781 (2015).
Petrel, C. et al. Positive and negative allosteric modulators of the Ca 2+ -sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J. Biol. Chem. 279 , 18990–18997 (2004).
Chang, W. et al. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J. Biol. Chem. 282 , 25030–25040 (2007).
Kumar, S. et al. An orally active calcium-sensing receptor antagonist that transiently increases plasma concentrations of PTH and stimulates bone formation. Bone 46 , 534–542 (2010).
Henry, J. G., Mitnick, M., Dann, P. R. & Stewart, A. F. Parathyroid hormone-related protein-(1-36) is biologically active when administered subcutaneously to humans. J. Clin. Endocrinol. Metab. 82 , 900–906 (1997).
Horwitz, M. J. et al. Parathyroid hormone-related protein for the treatment of postmenopausal osteoporosis: defining the maximal tolerable dose. J. Clin. Endocrinol. Metab. 95 , 1279–1287 (2010).
Mooberry, S. L. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr. Opin. Oncol. 15 , 425–430 (2003).
Mabjeesh, N. J. et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3 , 363–375 (2003).
Mooberry, S. L. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist. Updat. 6 , 355–361 (2003).
Tinley, T. L. et al. Novel 2-methoxyestradiol analogues with antitumor activity. Cancer Res. 63 , 1538–1549 (2003).
Powis, G. & Kirkpatrick, L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol. Cancer Ther. 3 , 647–654 (2004).
Melillo, G. Inhibiting hypoxia-inducible factor 1 for cancer therapy. Mol. Cancer Res. 4 , 601–605 (2006).
Download references
Acknowledgements
This work is supported by the National Natural Science Foundation of China (reference number: 81602492), the National Key Research and Development Program of China (reference number: 2016YFA0201402), and the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (reference number: 2018ZX09733001).
Author information
These authors contributed equally: Manni Wang and Fan Xia
Authors and Affiliations
Laboratory of Aging Research and Cancer Drug Targets, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, No. 17, Block 3, Southern Renmin Road, Chengdu, 610041, Sichuan, P.R. China
Manni Wang, Yuquan Wei & Xiawei Wei
Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, 610041, Sichuan, P.R. China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Xiawei Wei .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wang, M., Xia, F., Wei, Y. et al. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res 8 , 30 (2020). https://doi.org/10.1038/s41413-020-00105-1
Download citation
Received : 30 May 2019
Revised : 03 September 2019
Accepted : 23 October 2019
Published : 29 July 2020
DOI : https://doi.org/10.1038/s41413-020-00105-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Macrophages in immunoregulation and therapeutics.
- Shanze Chen
- Abdullah F.U.H. Saeed
- Yanhong Duo
Signal Transduction and Targeted Therapy (2023)
Neuropeptide Y and its receptors in prostate cancer: associations with cancer invasiveness and perineural spread
- Dawid Sigorski
- Wojciech Wesołowski
- Ewa Iżycka-Świeszewska
Journal of Cancer Research and Clinical Oncology (2023)
Bone-specific response according to MDA criteria predicts immunotherapy efficacy among advanced non-small cell lung cancer (NSCLC) patients
- Andrea De Giglio
- Chiara Deiana
- Alessandro Di Federico
Uncarboxylated osteocalcin promotes proliferation and metastasis of MDA-MB-231 cells through TGF-β/SMAD3 signaling pathway
- Jiaojiao Xu
- Jianhong Yang
BMC Molecular and Cell Biology (2022)

Comparison of 68Ga-PSMA PET/CT with fluoride PET/CT for detection of bone metastatic disease in prostate cancer
- Naresh Regula
- Vasileios Kostaras
- Jens Sörensen
European Journal of Hybrid Imaging (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
REVIEW article
A comprehensive review of the role of bone marrow biopsy and pet-ct in the evaluation of bone marrow involvement in adults newly diagnosed with dlbcl.

- 1 Department of Hematology, Stem Cell Transplant and Cellular Therapy, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 2 Division of Hematology-Oncology, Blood and Marrow Transplantation Program, Mayo Clinic, Jacksonville, FL, United States
Diffuse large B cell lymphoma (DLBCL) is one of the most prevalent subtypes of non-Hodgkin lymphoma (NHL) and is known for commonly infiltrating extra-nodal sites. The involvement of the bone marrow by lymphoma cells significantly impacts the staging, treatment, and prognosis among the extra-nodal sites in DLBCL. Bone marrow biopsy has been considered the standard diagnostic procedure for detecting bone marrow involvement. However, advancements in imaging techniques, such as positron emission tomography-computed tomography (PET-CT), have shown an improved ability to detect bone marrow involvement, making the need for bone marrow biopsy debatable. This review aims to emphasize the importance of bone marrow evaluation in adult patients newly diagnosed with DLBCL and suggest an optimal diagnostic approach to identify bone marrow involvement in these patients.
1 Introduction
Non-Hodgkin lymphoma (NHL) constitutes approximately 4% of annual cancer diagnoses. It ranks as the sixth most common cause of cancer and accounts for almost 6% of malignancy-related mortality in Europe and the United States ( 1 – 3 ). Within the spectrum of NHL, diffuse large B cell lymphoma (DLBCL) emerges as the most prevalent subtype, accounting for 30 – 40% of aggressive NHL cases in adults worldwide ( 3 – 7 ). The diagnosis of DLBCL depends on histological confirmation, complemented by clinical and radiological findings ( 2 ). DLBCL is recognized for its variable biological and clinical features, along with frequent extra-nodal site infiltration, which has a momentous impact on the staging and, consequently, management and prognosis ( 2 , 7 , 8 ). Accurate staging of DLBCL is crucial for optimal management, with the Ann Arbor staging system being one of the most recognized systems, initially relying on physical examination and bone marrow biopsy ( 2 ). Other staging systems used in NHL include the Lugano and LYRIC criteria ( 9 , 10 ). The advancements in diagnostic medicine and the development of positron emission tomography and computed tomography (PET-CT) have revolutionized disease assessment and treatment response evaluation. PET-CT has significantly influenced the initial staging and subsequent disease re-assessment following therapy in DLBCL. Its impact has become increasingly evident since immunotherapy agents have become widely available and PET-specific response criteria have been developed ( 11 ). These advancements have increased the diagnostic and prognostic value of PET-CT in managing DLBCL. Despite the progression in imaging techniques, the value of PET-CT in detecting bone marrow involvement in DLBCL and its ability to replace bone marrow biopsy remains controversial ( 2 ).
The International Prognostic Index score (IPI score) has been widely accepted as a prognostication tool for risk-stratifying patients with DLBCL. In the pre-rituximab era, the IPI score had limitations in identifying higher-risk patients, which urged its revision by the National Comprehensive Cancer Network (NCCN) guidelines ( 12 ). This adjustment aimed to improve the ability of the IPI score to discriminate low-risk from high-risk patients, particularly in DLBCL patients treated with rituximab, with a focus on overall survival (OS) ( Table 1 ) ( 13 , 14 ).
Table 1 International Prognostic Index (IPI) Score Components ( 13 ).
2 Bone marrow involvement in DLBCL
In diffuse large B-cell lymphoma (DLBCL), the involvement of extra-nodal tissue often signifies a more advanced disease, correlating with poorer outcomes ( 2 ). Bone marrow (BM) involvement is reported in 10-30% of DLBCL cases, making it a critical aspect of the initial evaluation of DLBCL as it holds prognostic and therapeutic implications ( 6 ). Specifically, BM involvement has been linked to suboptimal prognosis in DLBCL patients with advanced Ann Arbor staging and higher IPI scores ( 3 , 4 , 7 , 12 , 15 – 17 ). In cases with limited stages, bone marrow involvement leads to upstaging and necessitates adjustments in the management plan ( 18 ). Individuals with BM involvement in DLBCL face an increased risk of primary refractory disease reported at a rate of 10-15%, a 20-30% chance of relapse ( 8 ), and, notably, as reported in one study, a higher incidence of rituximab infusion-related reactions ( 19 ). Consequently, a more aggressive therapeutic approach may be warranted for patients with bone marrow involvement. Notably, the standard iliac crest bone marrow biopsy demonstrates a limited ability to detect BM involvement in DLBCL, identifying only 27% of patients with confirmed bone marrow involvement ( 4 , 7 , 8 , 20 ). The inclusion of other diagnostic techniques, including flow cytometry, enhances the biopsy’s sensitivity in detecting bone marrow involvement ( 2 , 7 ). PET-CT frequently identifies bone marrow involvement in a focal or diffuse uptake distribution, while bone marrow biopsy relies on the morphological examination of slides, immunohistochemistry stains, and flow cytometry to detect lymphoma cells and specific clusters of differentiation (CD) markers ( 8 ).
3 Methods for bone marrow involvement detection
The two most commonly used techniques for determining bone marrow involvement in DLBCL are bone marrow biopsy and PET-CT scan.
3.1 Bone marrow aspirate and biopsy
The gold standard for assessing bone marrow involvement in lymphomas has been a random unilateral, occasionally bilateral, posterior iliac crest trephine bone marrow biopsy and aspirate. Previously, this procedure was a crucial part of the staging process ( 2 – 4 , 8 , 21 ). This procedure is usually performed blindly (i.e., not directed towards a lesion) at the bedside under local anesthesia and aseptic precautions. While there is no universally accepted definition for an adequate sample size, consensus suggests an acceptable range of 0.5 – 1 cm, but optimally 2 – 3 cm ( 8 , 17 , 22 ). Although bone marrow biopsy is considered relatively safe, it has its risks and limitations ( Table 2 ) ( 2 , 3 , 8 ). Complication rates, as reported in one study, were approximately 0.07%, with bleeding, most frequently retroperitoneal hemorrhage, being a critical and severe complication, particularly associated with certain risk factors such as myeloproliferative neoplasms, platelet dysfunction, anticoagulation use, disseminated intravascular coagulation (DIC), renal impairment, and obesity ( 24 – 28 ). It is worth noting that the operator’s years of experience did not show a clear correlation with bleeding rates ( 27 ).
Table 2 Advantages and Disadvantages of Bone Marrow Biopsy.
The necessity of bone marrow aspirate and biopsy has recently been debatable for evaluating bone marrow involvement in DLBCL ( 16 ). Some experts continue to support the usage of bone marrow biopsy by citing studies showing that patients with histologically confirmed bone marrow involvement had inferior overall survival (OS), event-free survival (EFS), and progression-free survival (PFS) results ( 12 , 14 , 20 ). Furthermore, histological examination provides insight into the specific cell types involved, as bone marrow could be infiltrated by an unrelated lymphoma, such as indolent lymphoma, which is known as discordant bone marrow involvement ( 4 ). Histological examination of the bone marrow continues to be essential in patients with early-stage disease based on imaging, as it may significantly impact disease upstaging and subsequent treatment decisions ( 23 ).
3.2 Positron emission tomography and computed tomography
PET-CT, a radiological technique used in staging aggressive lymphomas, provides a comprehensive 3-dimensional whole-body image for evaluating cellular metabolic activity and function using radioactive agents ( 17 ). Several types of PET-CT use different tracers, including but not limited to fluorine-18-deoxyglucose, sodium fluoride, and oxygen-15 ( 29 – 33 ). The most commonly used radiopharmaceutical agent in oncology is Fluorine-18-deoxyglucose (18F-FDG), a glucose analog that facilitates the detection of metabolically active sites. Following the administration of the FDG agent, images are captured using a full-ring detector PET scanner combined with a multidetector helical CT machine. While PET combined with CT yields superior results compared to PET alone, the inclusion of CT introduces higher radiation exposure. However, low-dose (80 mAs) PET-CT options are available for staging, minimizing radiation exposure ( 34 ). Standardized uptake values (SUV), the most frequently used parameter, express the ratio of radioactivity concentration within the region of interest (ROI) to the decay-corrected amount of injected radio-labeled FDG (kBq) per patient’s weight (kg), are used to report metabolic activity, presented as kBq/ml ( 8 , 35 ). SUV is corrected in patients of extreme body weights, using the lean body weight (SUL) in obese patients and body surface area in patients of small body weight ( 36 ). Other less commonly used parameters in the assessment of tumor volume and metabolic activity include metabolic tumor volume (MTV), total lesion glycolysis (TLG), and tumor-to-blood ratio (TBR) ( 37 – 39 ). PET-CT is considered the standard tool for lymphoma staging, exhibiting high sensitivity and specificity in identifying lymphoma activity sites, particularly in Hodgkin’s lymphoma and aggressive subtypes of NHL like DLBCL, owing to their heightened FDG avidity ( 2 , 21 , 40 ). Notably, PET-CT scans accurately identify both nodal and extra-nodal involvement, facilitating precise staging and prognosis quantification ( 4 ). This positions PET-CT as the preferred modality for pre-treatment disease assessment and post-therapy response evaluation ( 2 ). Achieving greater consistency in data is crucial for evaluating the efficacy of PET-CT in detecting bone marrow involvement, particularly in instances of discordant bone marrow involvement by a different lymphoma subtype ( 21 ). Across the majority of published literature, PET-CT demonstrates excellent precision in detecting concordant bone marrow involvement. In a study by Khan et al., PET-CT exhibited exceptional accuracy, with only two cases showing negative PET-CT results but positive bone marrow biopsy results, both consistent with low-volume disease (i.e., 10% large B cells) and already labeled as stage IV based on uptake elsewhere ( 4 ). Another study supports the accuracy of PET-CT in detecting bone marrow involvement, at least as effectively as bone marrow biopsy ( 1 ). PET-CT’s remarkable ability to detect bone marrow involvement is particularly evident in concordant cases with aggressive B-cell lymphomas like DLBCL, which usually shows increased FDG avidity and higher SUV on imaging with a high Deauville score, which is an ordinal 5-point scale that relies on the visual comparison between the glucose uptake of the tumor and that of the liver or mediastinum ( 34 , 41 – 43 ). However, PET-CT’s accuracy weakens in discordant cases where the marrow may be involved by a lymphoma other than DLBCL ( 1 , 40 , 44 ). PET-CT surpasses CT with bone marrow biopsy in identifying occult lymphoma sites ( 40 ). For optimal results, PET-CT should be performed in adherence to standardized procedures and be interpreted by highly experienced radiologists and nuclear medicine specialist with focused training in PET-CT evaluation for lymphomas. Less experienced interpretation may lead to overanalysis of FDG uptake in the bone marrow and increased false-positive rates ( 7 , 12 , 17 , 45 ). To enhance the accuracy of PET-CT and avoid incorrect results, a unified definition of bone marrow involvement is essential. Positive bone marrow involvement by PET-CT can be defined by several features:
1. The mean SUV max, measured by FDG uptake, should be higher than that of the liver, quantifying at more than 3.8 with a Deauville score of 4 or 5 ( 4 , 7 , 8 , 15 , 16 , 46 ). Higher SUV is often associated with positive bone marrow involvement by lymphoma ( 4 ).
2. Bone marrow involvement should not be a contiguous spread from nearby disease involving soft tissue ( 4 ).
3. No anatomical changes should suggest an alternative underlying benign abnormality ( 4 , 21 ).
4. Increased FDG activity at sites of previous bone marrow biopsy or fractures is considered negative ( 15 ).
Some references do not consider diffuse bone marrow uptake on PET-CT as positive for bone marrow involvement unless proven by histopathology review ( 12 , 21 ).
Additional methods to confirm disease-related bone marrow uptake on PET-CT include:
1. Confirmation through MRI imaging on bone lesions ( 18 , 40 ).
2. Repeat PET-CT after treatment to assess if uptake resolves with the resolution of involved nodal sites ( 18 ).
3. Tissue biopsy of the enhanced lesion (i.e., directed biopsy) ( 18 , 40 ).
It is crucial to acknowledge that not all lesions on PET-CT can be confirmed by the mentioned methods ( 4 ). While PET-CT offers user-friendly convenience, it is not without its inherent limitations ( Table 3 ).
Table 3 Advantages and Disadvantages of PET-CT.
PET-CT exhibits an enhanced utility in the context of DLBCL therapeutic advancements, particularly with the increased use of immunotherapy. The use of PET-CT in DLBCL has added significant value in initial diagnosis, staging, assessment of extra-nodal uptake, response assessment, and has further extended to the ability to detect signs of toxicities related to immunotherapy, such as inflammatory reactions, reactive changes, and tumor flare reactions ( 51 ). PET-CT has also had an important role in assessment of response to therapy, using several metrics to distinguish between different types of response, pseudo-progression, and progression ( 51 ). These facilities of PET-CT reinforce its diagnostic capabilities in aggressive lymphomas.
4 Patterns of bone marrow involvement in DLBCL
Bone marrow involvement can be categorized based on the detection method, the infiltrative cell type, and the imaging distribution.
4.1 Classification based on infiltrative cell type
Histopathological findings play a crucial role in classifying bone marrow involvement into two main categories: concordant bone marrow involvement, where features align with DLBCL characteristics in the bone marrow, and discordant bone marrow involvement, which exhibits features of lymphomas other than DLBCL, including indolent lymphomas such as follicular lymphoma, marginal zone lymphoma, and mantle cell lymphoma ( 20 , 21 ). The majority of cases presenting bone marrow involvement fall under the concordant category ( 20 ). Discordant bone marrow involvement can be further classified into clonally-related and clonally-unrelated lymphomas based on immunoglobulin gene rearrangements ( 20 ). This distinction aids in discriminating between actual discordance, where two different pathologies coexist, and transformation of indolent lymphoma to DLBCL. When discordant bone marrow lymphoma cells and nodal DLBCL cells exhibit clonal similarity, there is an increased probability that the DLBCL arises as a transformation from the original indolent lymphoma ( 20 ). Notably, most cases of DLBCL with discordant bone marrow involvement that experience disease relapse show progression of the more aggressive DLBCL rather than transformation of their indolent lymphoma (discordant bone marrow involvement) ( 13 ). Prognostic data on biopsy-proven concordant bone marrow involvement is scattered. Sehn et al. reported that cases with concordant bone marrow involvement, histologically proven DLBCL, demonstrated an inferior outcome in terms of OS and PFS ( 14 ). Discordant bone marrow involvement was associated with lower PFS, supporting the presumed prognosis based on the IPI score in these patients ( 14 ). Additional studies supported the findings of poor OS in patients with concordant bone marrow involvement ( 52 – 54 ). It is worth noting that discordant bone marrow involvement did not independently correlate with inferior PFS or OS ( 16 , 52 – 54 ).
4.2 Classification based on uptake distribution on imaging
Bone marrow uptake observed in PET-CT scans manifests in various patterns, including focal, diffuse, or a combination of both, with focal areas exhibiting higher FDG uptake ( 16 ). Focal uptake can involve a single site (unifocal), two sites (bifocal), or multiple sites (multifocal) ( Figure 1 ) ( 1 , 12 , 21 , 55 ). There has been controversy regarding the interpretation of PET positivity for bone marrow involvement in cases with diffuse bone marrow uptake on scans. Al-Sabbagh et al. found that none of the patients displaying diffuse bone marrow changes on PET-CT had a positive bone marrow biopsy, and conversely, none of the patients with a positive bone marrow biopsy exhibited diffuse bone marrow uptake on PET without a focal lesion ( 18 ). In contrast, another study reported that diffuse bone marrow uptake on PET-CT scans, conducted for patients with aggressive NHL, was frequently associated with a positive bone marrow biopsy ( 56 ). The pattern of bone marrow involvement in DLBCL is more commonly reported in a focal pattern on PET-CT as opposed to diffusely increased bone marrow uptake ( 12 ). The FDG uptake on PET-CT scans can vary in bone marrow involvement by different types of lymphomas, other than DLBCL ( 4 , 40 ).
Figure 1 Patterns of bone marrow involvement on PET-CT. (A) Diffuse bone marrow FDG uptake on PET-CT. (B) Multifocal, scattered, bone marrow FDG uptake on PET-CT.
5 PET-CT versus bone marrow biopsy for detecting bone marrow involvement in DLBCL
In recent years, an ongoing debate has been ongoing regarding the potential replacement of bone marrow biopsy by PET-CT for evaluating bone marrow involvement in DLBCL. Several studies have examined the accuracy of each modality ( Table 4 ).
Table 4 Comparing the ability of PET-CT and bone marrow biopsy in bone marrow involvement detection.
5.1 Precision of PET-CT vs. bone marrow biopsy in detecting bone marrow involvement
Numerous studies, including a meta-analysis, consistently report high sensitivity, specificity, and accuracy of PET-CT in detecting bone marrow involvement when compared to bone marrow biopsy ( 1 , 3 , 4 , 8 , 12 , 16 , 18 , 59 , 60 ). The negative predictive value and specificity of PET-CT for bone marrow involvement detection are exceptionally high, ranging between 85-98% and nearly 100%, respectively ( 3 , 16 , 34 , 40 ). Rare instances have been documented where PET-CT produced negative results for bone marrow involvement, while bone marrow biopsy came back positive ( 18 , 57 , 61 ). These compelling findings have encouraged some groups to advocate for omitting routine bone marrow biopsy if PET-CT scan is positive ( 12 , 18 ). One study reported no false-positive results for bone marrow involvement detected by PET-CT ( 18 ). Kaddu-Mulindwa et al. highlighted the superior accuracy and sensitivity of PET-CT over bone marrow biopsy for detecting bone marrow involvement, with rates reaching approximately 84% versus 38%, respectively ( 15 ). Bone marrow biopsy may generate false-negative results, particularly in cases of focal disease distant from the iliac crest ( 4 ). Adams et al. reinforced the inferior sensitivity of bone marrow biopsy for bone marrow involvement detection, citing a histologically proven bone marrow involvement rate of around 13-17% in newly diagnosed DLBCL cases ( 23 ). Some argue that patients with negative or limited focal bone marrow involvement away from the iliac crest on PET-CT may not benefit from bone marrow biopsy, suggesting its elimination in such cases ( 4 ).Nevertheless, certain groups advocate for the continued use of bone marrow biopsy before initiating treatment due to the potential for a worse prognosis with biopsy-proven bone marrow involvement and the ability to detect involvement missed by PET-CT ( 14 , 16 , 21 ). In the majority of studies, the percentage of bone marrow involvement missed by PET-CT, which was concordant DLBCL in the bone marrow, was minimal. Alzahrani et al. reported only 1% of patients with negative PET-CT had a positive concordant bone marrow biopsy ( 21 ). Another study confirmed that PET-CT successfully detected all cases with bone marrow involvement by DLBCL ( 4 ). However, a separate study reported a false negativity rate of PET-CT scans reaching almost 15% compared to the standard bone marrow biopsy, with most cases attributed to microscopic disease ( 40 ). Substantial evidence supports the complementary role of PET-CT with bone marrow biopsy for detecting bone marrow involvement, particularly in cases displaying diffuse bone marrow uptake on PET-CT ( 4 , 59 ).
5.2 Impact of bone marrow involvement detected by PET-CT or bone marrow biopsy on staging, management, and outcome
Detection of bone marrow involvement through PET-CT in the literature has led to upstaging the disease to stage IV in nearly 25% of cases, whereas bone marrow biopsy has not been commonly associated with significant changes in the disease stage ( 4 , 12 , 18 ). In a limited number of cases, an elevation in the NCCN-IPI risk score has been reported, but there is insufficient evidence to establish a substantial difference in the actual disease stage for most patients ( 23 ). Management adjustments based on PET-CT positivity were observed in cases that were upstaged. However, in various studies, positive bone marrow involvement detected by biopsy rarely resulted in changes to the treatment plan ( 52 , 53 ). Histologically discordant bone marrow involvement, particularly with low-grade lymphoma, typically does not lead to major alterations in the therapy plan, with treatment often directed toward the more aggressive lymphoma type ( 20 , 52 , 53 ). Nevertheless, it may prompt modifications in the management plan, including the potential use of maintenance rituximab and lifelong follow-up appointments ( 21 ). The prognostic impact of bone marrow involvement, whether detected by PET-CT or bone marrow biopsy, remains argumentative. Some studies suggest that histologically proven concordant bone marrow involvement by DLBCL is associated with an inferior prognosis, particularly in terms of OS and/or PFS, along with an increased risk of CNS involvement by DLBCL and CNS relapse ( 2 , 4 , 18 , 20 , 21 , 61 , 62 ). Critics argue that bone marrow biopsy typically identifies extensive disease involvement, contributing to the inferior outcomes in these patients ( 12 , 49 , 63 , 64 ). Other studies report minimal or no impact of biopsy-proven bone marrow involvement on prognosis or the risk of CNS involvement, especially in cases with discordant bone marrow involvement ( 62 , 65 ). Regarding bone marrow involvement detected by PET-CT as an independent adverse risk factor in DLBCL patients, most studies do not support this claim, particularly in patients already categorized as stage IV DLBCL for non-bone marrow involvement related criteria, where bone marrow involvement detection by PET-CT does not contribute to their inferior prognosis ( 4 , 18 , 23 ). However, a small study by Berthet et al. suggests that PET-CT positivity for bone marrow involvement serves as an independent risk factor for poor prognosis in DLBCL cases ( 41 ). Bone marrow involvement detected by both PET-CT and bone marrow biopsy together has been associated with a worse outcome than involvement detected by either method alone ( 1 , 7 , 12 ). A study by Cerci et al. reported an event-free survival (EFS) of around 78% (95% CI: 63-88%) and OS of 87% (95% CI: 73-94%) in DLBCL patients with focal FDG uptake in the bone marrow on PET-CT ( 12 ). In contrast, significantly inferior outcomes were reported in patients with both focal bone marrow involvement on PET-CT and positive bone marrow biopsy, with EFS and OS at 46% and 57%, respectively ( 12 ). This finding is supported by other studies, suggesting that combining both biopsy and PET-CT for detecting bone marrow involvement may be justified ( 1 , 7 ). One study highlighted that bone marrow involvement in DLBCL detected by any method (i.e., bone marrow biopsy or PET-CT) influences staging, IPI score, and prognosis adversely ( 8 ). Despite the increased accuracy achieved by combining both biopsy and PET-CT for bone marrow involvement detection, a false-negative result does not completely rule out the presence of disease in the bone marrow ( 15 ).
5.3 Indicators elevating pretest probability for bone marrow involvement in DLBCL
Characteristic findings are frequently seen in DLBCL patients with bone marrow involvement. These findings can be used as markers to suggest the presence of bone marrow involvement and help guide the selection of diagnostic tools for the assessment of bone marrow involvement. Noteworthy features include the presence of cytopenia (i.e., Hemoglobin less than 10 g/dL, WBC less than 4 x 10^9/L) and/or bulky disease ( 66 ). In cases where anemia, leukopenia, and bulky disease are absent, the negative predictive value (NPV) approaches almost 99.2% ( 66 ). Additionally, an elevated LDH level above the upper limit of normal and the presence of adverse factors in the IPI score, excluding bone marrow involvement, should be considered as factors suggestive of bone marrow involvement ( 15 ). We suggest an algorithm designed to improve the accuracy of detecting bone marrow involvement in newly diagnosed DLBCL, as illustrated in Figure 2 . However, the reliability and efficiency of this algorithm need to be thoroughly assessed through further studies.
Figure 2 Suggested algorithm on the approach to bone marrow involvement assessment to identify the need for bone marrow biopsy in DLBCL staging.
6 Future insights
The absence of a definitive “gold standard” for bone marrow involvement detection in lymphoma persists. Efforts to enhance non-invasive techniques in identifying bone marrow involvement in aggressive lymphomas have been ongoing. PET-based radiomics is one intriguing technique being investigated; it combines PET imaging with radiomics, an approach that focuses on data extraction and the analysis of features from a large volume of images to uncover radiological patterns seen in the disease of interest that are frequently overlooked by conventional techniques, forecast treatment response, and acquire a deeper understanding of disease characteristics ( 67 , 68 ). In a study by Filippi et al., 17 papers were reviewed, with 9 focusing on Non-Hodgkin lymphomas. These studies used multiple radiomic characteristics from baseline PET-CT scans to create machine learning-derived models. The models showed excellent results in predicting outcomes, especially the 2-year EFS in lymphomas. These results contribute to prognostication by highlighting the biological diversity and three-dimensional nature of lesions. Nevertheless, additional investigation, including validated prospective studies, is necessary to confirm the utility of PET-radiomics in the clinical practice ( 67 ). An alternative approach showing promise involves combining PET with MRI instead of CT. This innovative method holds potential as a tool that could replace the need for bone marrow biopsy, offering patients relief from the discomfort and pain that accompanies the biopsy ( 3 , 16 , 34 ).
7 Conclusion
The presence of bone marrow involvement has a significant impact on the prognosis and treatment of patients with DLBCL. While bone marrow biopsy has traditionally been considered the gold standard for evaluating such involvement, its use is accompanied by limitations and complications. The development and advancements in PET-CT and its precise capability to detect both nodal and extra-nodal involvement in DLBCL raise the possibility of precluding the need for bone marrow biopsy in this context. Although the histological examination of the bone marrow in DLBCL can have an impact on prognostication and can differentiate between concordant and discordant lymphoma cells in the bone marrow, yet the impact on the management of these patients is generally minimal, especially in patients classified as advanced stage by imaging. Both tools possess value but may not be universally necessary. Further cohort studies are needed to assess the validity of this statement and the applicability of the new advancements in the field of nuclear medicine to be standardized as the diagnostic tool for bone marrow involvement detection in DLBCL.
Author contributions
RA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. RF: Writing – review & editing. AhA: Writing – review & editing. AbA: Supervision, Writing – review & editing. MK-D: Writing – review & editing. MA: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Teagle AR, Barton H, Charles-Edwards E, Dizdarevic S, Chevassut T. Use of FDG PET/CT in identification of bone marrow involvement in diffuse large B cell lymphoma and follicular lymphoma: comparison with iliac crest bone marrow biopsy. Acta Radiol . (2017) 58:1476–84. doi: 10.1177/0284185117701305
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Vishnu P, Wingerson A, Lee M, Mandelson MT, Aboulafia DM. Utility of bone marrow biopsy and aspirate for staging of diffuse large B cell lymphoma in the era of positron emission tomography with 2-deoxy-2-[Fluorine-18]fluoro-deoxyglucose integrated with computed tomography. Clin Lymphoma Myeloma Leuk . (2017) 17:631–6. doi: 10.1016/j.clml.2017.06.010
3. Almaimani J, Tsoumpas C, Feltbower R, Polycarpou I. FDG PET/CT versus bone marrow biopsy for diagnosis of bone marrow involvement in non-hodgkin lymphoma: A systematic review. Appl Sci . (2022) 12:540. doi: 10.3390/app12020540
CrossRef Full Text | Google Scholar
4. Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood . (2013) 122:61–7. doi: 10.1182/blood-2012-12-473389
5. Armitage JO. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin Proc . (2012) 87:161–71. doi: 10.1016/j.mayocp.2011.11.007
6. Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology . (2011) 2011:498–505. doi: 10.1182/asheducation-2011.1.498
7. Han EJ, JH O, Yoon H, Ha S, Yoo IR, Min JW, et al. Comparison of FDG PET/CT and bone marrow biopsy results in patients with diffuse large B cell lymphoma with subgroup analysis of PET radiomics. Diagnostics . (2022) 12:222. doi: 10.3390/diagnostics12010222
8. Asif H, Zubair R, Siddiqui IA, Tariq Mahmood M, Jamil A, Tahir A. The diagnostic accuracy of bone marrow biopsy versus PET/CT scan in identifying bone marrow involvement in diffuse large B cell lymphoma patients at a cancer hospital. Cureus (2023) 15(2). doi: 10.7759/cureus.34901
9. Al Tabaa Y, Casasnovas RO, Baillet C, Bachy E, Nicolas-Virelizier E, De colella JMS, et al. Prospective evaluation of lymphoma response to immunomodulatory therapy criteria in GATA trial from the LYSA group. Blood Adv . (2023) 7:3735–8. doi: 10.1182/bloodadvances.2023009911
10. Armitage JO. Staging non-hodgkin lymphoma. CA Cancer J Clin . (2005) 55:368–76. doi: 10.3322/canjclin.55.6.368
11. Lang D, Wahl G, Poier N, Graf S, Kiesl D, Lamprecht B, et al. Impact of PET/CT for assessing response to immunotherapy—A clinical perspective. J Clin Med . (2020) 9:3483. doi: 10.3390/jcm9113483
12. Cerci JJ, Györke T, Fanti S, Paez D, Meneghetti JC, Redondo F, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med . (2014) 55:1591–7. doi: 10.2967/jnumed.113.134486
13. Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood . (2007) 109:1857–61. doi: 10.1182/blood-2006-08-038257
14. Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol . (2011) 29:1452–7. doi: 10.1200/JCO.2010.33.3419
15. Kaddu-Mulindwa D, Altmann B, Held G, Angel S, Stilgenbauer S, Thurner L, et al. FDG PET/CT to detect bone marrow involvement in the initial staging of patients with aggressive non-Hodgkin lymphoma: results from the prospective, multicenter PETAL and OPTIMAL<60 trials. Eur J Nucl Med Mol Imaging . (2021) 48:3550–9. doi: 10.1007/s00259-021-05348-6
16. El Karak F, Bou-Orm IR, Ghosn M, Kattan J, Farhat F, Ibrahim T, et al. PET/CT scanner and bone marrow biopsy in detection of bone marrow involvement in diffuse large B-cell lymphoma. PloS One . (2017) 12:e0170299. doi: 10.1371/journal.pone.0170299
17. Yilmaz F, Soyer N, Kiper D, Özsan N, Şahin F, Saydam G, et al. The role of PET/CT in evaluation of bone marrow involvement in lymphoma patients at the initial staging. Marmara Med J . (2017) 30:1–1. doi: 10.5472/marumj.299374
18. Al-Sabbagh A, Ibrahim F, Szabados L, Soliman DS, Taha RY, Fernyhough LJ. The role of integrated positron emission tomography/computed tomography (PET/CT) and bone marrow examination in staging large B-cell lymphoma. Clin Med Insights Oncol . (2020) 14:117955492095309. doi: 10.1177/1179554920953091
19. Hong J, Kim JY, Ahn HK, Lee S-M, Sym SJ, Park J, et al. Bone marrow involvement is predictive of infusion-related reaction during rituximab administration in patients with B cell lymphoma. Supp Care Cancer . (2013) 21:1145–52. doi: 10.1007/s00520-012-1639-9
20. Brudno J, Tadmor T, Pittaluga S, Nicolae A, Polliack A, Dunleavy K. Discordant bone marrow involvement in non-Hodgkin lymphoma. Blood . (2016) 127:965–70. doi: 10.1182/blood-2015-06-651968
21. Alzahrani M, El-Galaly TC, Hutchings M, Clasen-Linde E, Brown P, Villa D, et al. The value of routine bone marrow biopsy in patients with diffuse large B-cell lymphoma staged with PET/CT: a Danish-Canadian study. Ann Oncol . (2016) 27:1095–9. doi: 10.1093/annonc/mdw137
22. Campbell JK, Matthews JP, Seymour JF, Wolf MM, Juneja SK. Optimum trephine length in the assessment of bone marrow involvement in patients with diffuse large cell lymphoma. Ann Oncol . (2003) 14:273–6. doi: 10.1093/annonc/mdg055
23. Alzahrani M, El-Galaly T. C, Hutchings M, Clasen-Linde E, Brown P, Villa D, et al. Bone marrow biopsy in diffuse large B-cell lymphoma: Useful or redundant test? Acta Oncol (Madr) . (2015) 54:67–72. doi: 10.3109/0284186X.2014.958531
24. Bain BJ. Bone marrow biopsy morbidity and mortality. Br J Haematol . (2003) 121:949–51. doi: 10.1046/j.1365-2141.2003.04329.x
25. Bain BJ. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol . (2004) 26:315–8. doi: 10.1111/j.1365-2257.2004.00630.x
26. Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol . (2005) 58:406–8. doi: 10.1136/jcp.2004.022178
27. Bain BJ. Morbidity associated with bone marrow aspiration and trephine biopsy - a review of UK data for 2004. Haematologica . (2006) 91:1293–4.
PubMed Abstract | Google Scholar
28. Arellano-Rodrigo E, Real MI, Muntañola A, Burrel M, Rozman M, Fraire G. V, et al. Successful treatment by selective arterial embolization of severe retroperitoneal hemorrhage secondary to bone marrow biopsy in post-polycythemic myelofibrosis. Ann Hematol . (2004) 83:67–70. doi: 10.1007/s00277-003-0683-4
29. Notes for guidance on the clinical administration of radiopharmaceuticals and use of sealed radioactive sources. Administration of Radioactive Substances Advisory Committee. Nucl Med Commun . (2000) 21 Suppl:S1–93.
30. Wang M, Xu L, Gao M, Miller KD, Sledge GW, Zheng QH. [11C]Enzastaurin, the first design and radiosynthesis of a new potential PET agent for imaging of protein kinase C. Bioorg Med Chem Lett . (2011) 21:1649–53. doi: 10.1016/j.bmcl.2011.01.100
31. Takalkar A, Mavi A, Alavi A, Araujo L. PET in cardiology. Radiol Clin North Am . (2005) 43:107–19. doi: 10.1016/j.rcl.2004.09.007
32. Weinstein EA, Ordonez AA, DeMarco VP, Murawski AM, Pokkali S, Macdonald EM, et al. Imaging Enterobacteriaceae infection in vivo with 18 F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med . (2014) 6. doi: 10.1126/scitranslmed.3009815
33. Azad GK, Siddique M, Taylor B, Green A, O’Doherty J, Gariani J, et al. Is response assessment of breast cancer bone metastases better with measurement of 18 F-fluoride metabolic flux than with measurement of 18 F-fluoride PET/CT SUV? J Nucl Med . (2019) 60:322–7. doi: 10.2967/jnumed.118.208710
34. Xiao-Xue W, Xinyue H, Lijun Z. Whole body FDG-PET/CT for the assessment of bone marrow infiltration in patients with newly diagnosed lymphoma. Med Clin (Barc) . (2020) 154:61–5. doi: 10.1016/j.medcli.2019.07.022
35. Lucignani G. SUV. and segmentation: pressing challenges in tumour assessment and treatment. Eur J Nucl Med Mol Imaging . (2009) 36:715–20. doi: 10.1007/s00259-009-1085-1
36. Sarikaya I, Sarikaya A. Assessing PET parameters in oncologic 18F-FDG studies. J Nucl Med Technol . (2020) 48:278–82. doi: 10.2967/jnmt.119.236109
37. Pak K, Cheon GJ, Nam HY, Kim S-J, Kang KW, Chung J-K, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: A systematic review and meta-analysis. J Nucl Med . (2014) 55:884–90. doi: 10.2967/jnumed.113.133801
38. Pak K, Kim SJ. What do we measure in oncology PET? Nucl Med Mol Imaging . (2017) 51:212–6. doi: 10.1007/s13139-016-0416-y
39. Song Y, Meng X, Cao Z, Zhao W, Zhang Y, Guo R, et al. Harmonization of standard uptake values across different positron emission tomography/computed tomography systems and different reconstruction algorithms: validation in oncology patients. EJNMMI Phys . (2023) 10:19. doi: 10.1186/s40658-023-00540-z
40. Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood . (2007) 110:3507–16. doi: 10.1182/blood-2007-06-097238
41. Chen YK, Yeh CL, Tsui CC, Liang JA, Chen JH, Kao CH. F-18 FDG PET for evaluation of bone marrow involvement in non-hodgkin lymphoma. Clin Nucl Med . (2011) 36:553–9. doi: 10.1097/RLU.0b013e318217aeff
42. Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymph . (2009) 50:1257–60. doi: 10.1080/10428190903040048
43. Rekowski J, Hüttmann A, Schmitz C, Müller SP, Kurch L, Kotzerke J, et al. Interim PET evaluation in diffuse large B-cell lymphoma using published recommendations: comparison of the deauville 5-point scale and the ΔSUVmax method. J Nucl Med . (2021) 62:37–42. doi: 10.2967/jnumed.120.244145
44. Pakos EE, Fotopoulos AD, Ioannidis JPA. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med . (2005) 46:958–63.
45. Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, et al. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med . (2001) 28:696–703. doi: 10.1007/s002590100537
46. Saiki Y, Tomita N, Uchida A, Uemura Y, Suzuki Y, Hirakawa T, et al. Biopsy remains indispensable for evaluating bone marrow involvement in DLBCL patients despite the use of positron emission tomography. Int J Hematol . (2021) 113:675–81. doi: 10.1007/s12185-021-03080-3
47. Fan C, Hernandez-Pampaloni M, Houseni M, Chamroonrat W, Basu S, Kumar R, et al. Age-related changes in the metabolic activity and distribution of the red marrow as demonstrated by 2-deoxy-2-[F-18]fluoro-d-glucose-positron emission tomography. Mol Imaging Biol . (2007) 9:300. doi: 10.1007/s11307-007-0100-9
48. Aras M, Dede F, Ones T, Inanır S, Erdıl TY, Turoglu HT. Evaluation of physiological FDG uptake in the skeleton in adults: Is it uniformly distributed? Rev Esp Med Nucl Imagen Mol . (2014) 33:286–9. doi: 10.1016/j.remn.2014.03.007
49. Campbell J, Seymour JF, Matthews J, Wolf M, Stone J, Juneja S. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol . (2006) 76:473–80. doi: 10.1111/j.1600-0609.2006.00644.x
50. Pelosi E, Penna D, Douroukas A, Bellò M, Amati A, Arena V, et al. Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of Malignant lymphoma: results from a large multicentre study. Q J Nucl Med Mol imaging: Off Publ Ital Assoc Nucl Med (AIMN) [and] Int Assoc Radiopharmacology (IAR) [and] Section Soc of . (2011) 55:469–75.
Google Scholar
51. Al-Ibraheem A, Abdlkadir AS, Juweid ME, Al-Rabi K, Abdel-Razeq H, Mansour A, et al. FDG-PET/CT in the monitoring of lymphoma immunotherapy response: current status and future prospects. Cancers (Basel) . (2023) 15:1063. doi: 10.3390/cancers15041063
52. Hodges GF, Lenhardt TM, Cotelingam JD. Bone marrow involvement in large-cell lymphoma: prognostic implications of discordant disease. Am J Clin Pathol . (1994) 101:305–11. doi: 10.1093/ajcp/101.3.305
53. Conlan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin’s lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska Lymphoma Study Group. J Clin Oncol . (1990) 8:1163–72. doi: 10.1200/JCO.1990.8.7.1163
54. Fisher DE, Jacobson JO, Ault KA, Harris NL. Diffuse large cell lymphoma with discordant bone marrow histology. Clin features Biol implications Cancer . (1989) 64:1879–87. doi: 10.1002/1097-0142(19891101)64:9<1879::aid-cncr2820640921>3.0.co;2-d
55. Carr R, Barrington SF, Madan B, O'Doherty MJ, Saunders CA, van der Walt J, et al. Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood . (1998) 91:3340–6.
56. Adams HJA, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RAJ, de Klerk JMH. Diffusely increased bone marrow FDG uptake in recently untreated lymphoma: incidence and relevance. Eur J Haematol . (2015) 95:83–9. doi: 10.1111/ejh.12483
57. Adams HJA, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RAJ, de Klerk JMH. Bone marrow 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol . (2014) 89:726–31. doi: 10.1002/ajh.23730
58. Cortés-Romera M, Sabaté-Llobera A, Mercadal-Vilchez S, Climent-Esteller F, Serrano-Maestro A, Gámez-Cenzano C, et al. Bone marrow evaluation in initial staging of lymphoma. Clin Nucl Med . (2014) 39:e46–52. doi: 10.1097/RLU.0b013e31828e9504
59. Adams HJA, Kwee TC, de Keizer B, Fijnheer R, de Klerk JMH, Nievelstein RAJ. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging . (2014) 41:565–74. doi: 10.1007/s00259-013-2623-4
60. Ujjani CS, Hill EM, Wang H, Nassif S, Esposito G, Ozdemirli M, et al. 18F-FDG PET-CT and trephine biopsy assessment of bone marrow involvement in lymphoma. Br J Haematol . (2016) 174:410–6. doi: 10.1111/bjh.14071
61. Hong J, Lee Y, Park Y, Kim SG, Hwang KH, Park SH, et al. Role of FDG-PET/CT in detecting lymphomatous bone marrow involvement in patients with newly diagnosed diffuse large B-cell lymphoma. Ann Hematol . (2012) 91:687–95. doi: 10.1007/s00277-011-1353-6
62. Chigrinova E, Mian M, Scandurra M, Greiner TC, Chan WC, Vose JM, et al. Diffuse large B-cell lymphoma with concordant bone marrow involvement has peculiar genomic profile and poor clinical outcome. Hematol Oncol . (2011) 29:38–41. doi: 10.1002/hon.953
63. Morra E, Lazzarino M, Castello A, Inverardi D, Coci A, Pagnucco G, et al. Bone marrow and blood involvement by non-Hodgkin’s lymphoma: A study of clinicopathologic correlations and prognostic significance in relationship to the Working Formulation. Eur J Haematol . (1989) 42:445–53. doi: 10.1111/j.1600-0609.1989.tb01469.x
64. Yan Y, Chan WC, Weisenburger DD, Anderson JR, Bast MA, Vose JM, et al. Clinical and prognostic significance of bone marrow involvement in patients with diffuse aggressive B-cell lymphoma. J Clin Oncol . (1995) 13:1336–42. doi: 10.1200/JCO.1995.13.6.1336
65. Kumar A, Vanderplas A, LaCasce AS, Rodriguez MA, Crosby AL, Lepisto E, et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era. Cancer . (2012) 118:2944–51. doi: 10.1002/cncr.26588
66. Lim ST, Tao M, Cheung YB, Rajan S, Mann B. Can patients with early-stage diffuse large B-cell lymphoma be treated without bone marrow biopsy? Ann Oncol . (2005) 16:215–8. doi: 10.1093/annonc/mdi050
67. Filippi L, Ferrari C, Nuvoli S, Bianconi F, Donner D, Marongiu A, et al. Pet-radiomics in lymphoma and multiple myeloma: update of current literature. Clin Transl Imaging (2023). doi: 10.1007/s40336-023-00604-1
68. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, Jong EEC, Timmeren van J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol . (2017) 14:749–62. doi: 10.1038/nrclinonc.2017.141
Keywords: diffuse large B-cell lymphoma, bone marrow involvement, bone marrow biopsy, positron emission tomography/computed tomography (PET-CT), lymphoma
Citation: Alyamany R, El Fakih R, Alnughmush A, Albabtain A, Kharfan-Dabaja MA and Aljurf M (2024) A comprehensive review of the role of bone marrow biopsy and PET-CT in the evaluation of bone marrow involvement in adults newly diagnosed with DLBCL. Front. Oncol. 14:1301979. doi: 10.3389/fonc.2024.1301979
Received: 25 September 2023; Accepted: 12 March 2024; Published: 21 March 2024.
Reviewed by:
Copyright © 2024 Alyamany, El Fakih, Alnughmush, Albabtain, Kharfan-Dabaja and Aljurf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruah Alyamany, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Effects of bone marrow sparing radiotherapy on acute hematologic toxicity for patients with locoregionally advanced cervical cancer: a prospective phase II randomized controlled study
Affiliations.
- 1 Department of Obstetrics and Gynecology, West China University Hospital 2, Sichuan University, 610041, Chengdu, China.
- 2 Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry Education, Sichuan University, 610041, Chengdu, China.
- 3 Department of Radiation Oncology, The Second Affiliated Hospital of Xi 'an Jiaotong University, Xi' An Jiao Tong University, 710004, Xi'An, China.
- 4 Department of Radiation Oncology, The Second Affiliated Hospital of Xi 'an Jiaotong University, Xi' An Jiao Tong University, 710004, Xi'An, China. [email protected].
- 5 Department of Obstetrics and Gynecology, West China University Hospital 2, Sichuan University, 610041, Chengdu, China. [email protected].
- 6 Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry Education, Sichuan University, 610041, Chengdu, China. [email protected].
- PMID: 38594678
- PMCID: PMC11005132
- DOI: 10.1186/s13014-024-02432-7
Objective: To evaluate effects of bone marrow sparing (BMS) radiotherapy on decreasing the incidence of acute hematologic toxicity (HT) for locoregionally advanced cervical cancer (LACC) patients treated by pelvic irradiation.
Materials and methods: LACC patients were recruited prospectively from May 2021 to May 2022 at a single center and were evenly randomized into the BMS group and the control group. All patients received pelvic irradiation with concurrent cisplatin (40 mg/m2 weekly), followed by brachytherapy and BM V40 < 25% in the BMS group was additionally prescribed. Acute HT was assessed weekly. Binary logistic regression model and receiver operating characteristic (ROC) curve were used for predictive value analysis. The trial was registered with Chinese clinical trial registry (ChiCTR2200066485).
Results: A total of 242 patients were included in the analysis. Baseline demographic, disease and treatment characteristics were balanced between the two groups. In the intention-to-treat population, BMS was associated with a lower incidence of grade ≥ 2 and grade ≥ 3 acute HT, leukopenia and neutropenia s(72.70% v 90.90%, P < 0.001*; 16.50% vs. 65.30%, P < 0.001*; 66.10% vs. 85.10%, P = 0.001*; 13.20% vs. 54.50%, P < 0.001*; 37.20% vs. 66.10%, P < 0.001*; 10.70% vs. 43.80%, P < 0.001*). BMS also resulted in decreased dose delivered to the organs at risk (OARs) including rectum, bladder and left and right femoral head. Univariate and multivariate analyses showed that BM V40 was an independent risk factor for grade ≥ 3 acute HT (odds ratio [OR] = 2.734, 95% confidence interval [CI] = 1.959-3.815, P < 0.001*). Cutoff value was 25.036% and area under the curve (AUC) was 0.786. The nomogram was constructed, which was rigorously evaluated and internally cross-validated, showing good predictive performance.
Conclusions: Receiving BMS pelvic irradiation could reduce the incidence of acute HT in LACC patients, and BM V40 < 25% may be a significant factor in reducing the risks of acute HT.
Keywords: Bone marrow sparing (BMS); Cervical cancer; Hematologic toxicity (HT); Pelvic irradiation.
© 2024. The Author(s).
Publication types
- Randomized Controlled Trial
- Clinical Trial, Phase II
- Bone Marrow / radiation effects
- Chemoradiotherapy / adverse effects
- Leukopenia* / etiology
- Prospective Studies
- Radiation Injuries* / etiology
- Radiotherapy Dosage
- Radiotherapy, Intensity-Modulated* / methods
- Uterine Cervical Neoplasms* / radiotherapy
Grants and funding
- No. 2022SF-128/Key Research and Development Projects of Shaanxi Province
- No. 2021SF-013/Key Research and Development Project of Shaanxi Province
UVA Blood Cancer Research Points to New Treatment for Bone Marrow Cancer

Research by UVA School of Medicine faculty member Golam Mohi, PhD, has identified a promising treatment approach for myelofibrosis, a potentially deadly bone marrow cancer.

Pioneering research into the chronic inflammation often seen in certain blood cancers has identified a promising treatment approach for myelofibrosis, a potentially deadly bone marrow cancer.
The new research from UVA Cancer Center pinpoints an important contributor to the unrelenting inflammation associated with a group of blood cancers called myeloproliferative neoplasms. These cancers cause the bone marrow to produce too many blood cells. This leads to symptoms such as headache, fever, fatigue, weakness, bone pain, bleeding and enlarged spleen.
The research from UVA’s Golam Mohi, PhD, and colleagues provides new understanding on how cancerous bone marrow cells promote the development of myelofibrosis. They have identified a cytokine, called interleukin-1, that contributes to the progression of myelofibrosis. Targeting this cytokine could prevent myelofibrosis from progressing, the scientists report. This could spare bone marrow the harmful scarring that is the hallmark of the disease.
“The JAK2 inhibitors, ruxolitinib and fedratinib, are currently approved therapies for myelofibrosis but they do not significantly reduce bone marrow fibrosis. So, we believe that other factors in addition to JAK2 activation might be involved in the development of myelofibrosis,” said Mohi, of UVA Cancer Center and the University of Virginia School of Medicine. “Our research provides new understanding on how inflammatory signaling mediated by interleukin-1 contributes to the development of bone marrow fibrosis and could lead to new therapeutic approach for this fatal bone marrow cancer.”
About Myelofibrosis
A bone marrow transplant, to physically replace the diseased bone marrow, is the only potential cure now available for myelofibrosis. But that procedure is very taxing on the body and associated with many complications, making it risky for older patients (the group most likely to develop the cancer). Because not all patients are eligible for bone marrow transplants, new treatment options are needed badly.
UVA’s new discovery not only could provide a new treatment approach, it also sheds light on the fundamental mechanisms of progression of myeloproliferative neoplasms. Mohi and his team found that interleukin-1 (commonly called IL-1) is crucial to the development of myelofibrosis. Increasing it in lab mice accelerated bone marrow scarring and fueled the excess production of blood cells. Reducing it, on the other hand, had the opposite effect.
The researchers also looked at the levels of interleukin-1 in human patients. They found that these patients exhibited elevated levels of two forms of IL-1, bolstering the case for IL-1 or IL-1 receptor as a promising treatment target.
The researchers believe that IL-1 sends signals that amplify inflammation in the body and promote harmful changes in the bone marrow. They were able to block that process in lab mice using an antibody, reducing marrow scarring dramatically. Scientists may be able to adapt this approach or use other means to block IL-1 and stimulate similar benefits in human patients, though much more research and testing will be needed.
“Based on the findings from this study, we suggest that combination therapies targeting both JAK2 and IL-1 could be useful for treatment of myelofibrosis,” said Mohi, of UVA’s Department of Biochemistry and Molecular Genetics. “We hope that our exciting laboratory finding will translate into clinical trials and make significant improvement in the treatment of patients with myelofibrosis.”
Finding new and better ways to treat even the most challenging cancers is a key mission of UVA Cancer Center. Earlier this year, UVA became one of only 53 cancer centers in the country to be designated a Comprehensive Cancer Center by the National Cancer Institute. The designation recognizes elite cancer centers with the most outstanding cancer programs in the nation. Comprehensive Cancer Centers must meet rigorous standards for innovative research and leading-edge clinical trials.
UVA’s many efforts to improve care for rare blood cancers were bolstered this summer by a generous $5.75 million anonymous gift , allowing the Cancer Center to accelerate research and provide more patients access to cutting-edge clinical trials.
Findings Published
Mohi and his collaborators have published their findings in the scientific journal Nature Communications . (The article is open access, meaning it is free to read.) The research team consisted of Mohammed Ferdous-Ur Rahman, Yue Yang, Bao T. Le, Avik Dutta, Julia Posyniak, Patrick Faughnan, Mohammad A. Sayem, Nadine S. Aguilera and Mohi.
The research was supported by National Institutes of Health grants R01 HL095685 and R01 HL149893. In addition, Mohi disclosed that he has received funding for unrelated research from oncology company Erasca Inc.
To keep up with the latest medical research news from UVA, subscribe to the Making of Medicine blog at http://makingofmedicine.virginia.edu .
Categories: All Releases , Oncology

Public Information Officer
Email | [email protected]
Phone | 434.924.5770
Latest News

Advertisement
- Previous Article
- Next Article
Introduction
Materials and methods, acknowledgments, human bone marrow–derived mesenchymal stem cells in the treatment of gliomas.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record April 15 2005
Akira Nakamizo , Frank Marini , Toshiyuki Amano , Asadullah Khan , Matus Studeny , Joy Gumin , Julianne Chen , Stephen Hentschel , Giacomo Vecil , Jennifer Dembinski , Michael Andreeff , Frederick F. Lang; Human Bone Marrow–Derived Mesenchymal Stem Cells in the Treatment of Gliomas. Cancer Res 15 April 2005; 65 (8): 3307–3318. https://doi.org/10.1158/0008-5472.CAN-04-1874
Download citation file:
- Ris (Zotero)
- Reference Manager
The poor survival of patients with human malignant gliomas relates partly to the inability to deliver therapeutic agents to the tumor. Because it has been suggested that circulating bone marrow–derived stem cells can be recruited into solid organs in response to tissue stresses, we hypothesized that human bone marrow–derived mesenchymal stem cells (hMSC) may have a tropism for brain tumors and thus could be used as delivery vehicles for glioma therapy. To test this, we isolated hMSCs from bone marrow of normal volunteers, fluorescently labeled the cells, and injected them into the carotid artery of mice bearing human glioma intracranial xenografts (U87, U251, and LN229). hMSCs were seen exclusively within the brain tumors regardless of whether the cells were injected into the ipsilateral or contralateral carotid artery. In contrast, intracarotid injections of fibroblasts or U87 glioma cells resulted in widespread distribution of delivered cells without tumor specificity. To assess the potential of hMSCs to track human gliomas, we injected hMSCs directly into the cerebral hemisphere opposite an established human glioma and showed that the hMSCs were capable of migrating into the xenograft in vivo . Likewise, in vitro Matrigel invasion assays showed that conditioned medium from gliomas, but not from fibroblasts or astrocytes, supported the migration of hMSCs and that platelet-derived growth factor, epidermal growth factor, or stromal cell–derived factor-1α, but not basic fibroblast growth factor or vascular endothelial growth factor, enhanced hMSC migration. To test the potential of hMSCs to deliver a therapeutic agent, hMSCs were engineered to release IFN-β (hMSC-IFN-β). In vitro coculture and Transwell experiments showed the efficacy of hMSC-IFN-β against human gliomas. In vivo experiments showed that treatment of human U87 intracranial glioma xenografts with hMSC-IFN-β significantly increase animal survival compared with controls ( P < 0.05). We conclude that hMSCs can integrate into human gliomas after intravascular or local delivery, that this engraftment may be mediated by growth factors, and that this tropism of hMSCs for human gliomas can be exploited to therapeutic advantage.
There is currently no optimal treatment for glioblastoma multiforme, the most common malignant brain tumor in adults, and patients typical survive <1 year ( 1, 2 ). This poor outcome relates at least in part to the inability to deliver therapeutic agents to the tumor ( 3 ). Delivery problems have especially slowed the development of novel gene therapy strategies ( 4 ). Indeed, intratumoral injection of viral vectors has proven incapable of delivering therapeutic genes to many tumor cells ( 4 ), and systemic i.v. or intra-arterial administration has been limited by the neutralizing effects of antibodies and by immune-mediated organ toxicity ( 5, 6 ). Methods for achieving widespread distribution of therapeutic agents throughout infiltrative gliomas would substantially improve brain tumor therapy.
Recent evidence suggests that stem cells are useful delivery vehicles for brain tumor therapy. Several laboratories have shown the potential of neural stem cells to function as delivery vehicles for brain tumor therapy ( 7 – 10 ). Aboody et al. were among the first to show that after intracranial injection neural stem cells have a tropism for brain tumors that could be exploited therapeutically ( 7 ). Likewise, Ehtesham et al. have shown that locally injected neural stem cells engineered to deliver interleukin-12 or tumor necrosis factor–related apoptosis-inducing ligand could slow the growth of brain tumors ( 9, 10 ). However, the clinical application of neural stem cells will be limited undoubtedly by logistic and ethical problems associated with their isolation and by potential immunologic incompatibility due to the requirement for allogenic transplantation. Because of the significant limitations associated with isolating human neural stem cells, to date, only murine neural stem cells have been evaluated in experimental settings. These inherent problems with neural stem cells led us to ask whether other types of stem cells that are more readily accessible (and thus more clinically applicable) may be used as vehicles for delivering therapeutic agents to brain tumors.
Bone marrow is an alternative source of stem cells ( 11 – 14 ). Human bone marrow–derived stem cells are well suited for clinical application because they are easily obtained from patients and because autologous transplantation, which obviates immunologic incompatibilities, is possible ( 15 ). Of the various progenitor cells that exist within bone marrow, human mesenchymal stem cells (hMSC) are particularly attractive for clinical use because they are easily isolated, can be expanded in culture, and can be genetically manipulated using currently available molecular techniques ( 14, 16 – 23 ). hMSCs are precursors that cause bone marrow stroma by differentiating into adipocytes, chondrocytes, and osteoblasts ( 11, 20, 24 ). However, MSCs have also been shown to be capable of differentiating into nonmesodermal tissues, including neurons and astrocytes ( 25, 26 ).
The rationale for using bone marrow–derived stem cells for delivering therapies to brain tumors is based on the developing current concept that bone marrow is a source of circulating stem cells that are recruited from the blood into peripheral solid organs in times of tissue stress or injury ( 27 – 31 ). Because the microenvironments of solid tumors is similar to the environment of injured/stressed tissue ( 32, 33 ), it is logical to hypothesize that solid tumors may provide a permissive environment for the engraftment of exogenously given hMSCs ( 27 ). In this context, we have shown previously that systemically delivered hMSCs are capable of integrating into human tumors grown within the lungs of nude mice ( 27 ). However, the unique features of the microenvironment of the brain and gliomas, including their highly specialized vasculature and glia-derived stroma, led us to evaluate whether brain tumors would also provide a permissive environment for the selective engraftment of hMSCs. Using an intracranial model of gliomas, we now show that hMSCs have a tropism for human gliomas after intravascular and local delivery and that this tropism can be exploited therapeutically by engineering hMSCs to release a soluble antiglioma factor.
Mesenchymal stem cell isolation and culture. Human MSCs were isolated as described previously ( 27 ) from the bone marrow of normal individuals undergoing bone marrow harvest for allogeneic bone marrow transplantation after informed consent according to institutional guidelines under an approved protocol. Briefly, mononuclear cells were separated by centrifugation over a Ficoll-Hypaque gradient (Sigma Chemical Co., St. Louis, MO) and suspended in α-MEM containing 20% fetal bovine serum (FBS; Life Technologies, Inc., Rockville, MD), l -glutamine, and penicillin-streptomycin mixture (Flow Laboratories, Rockville, MD) followed by plating at an initial seeding density of 1 × 10 6 cells/cm 2 . After 3 days, the nonadherent cells were removed by washing with PBS, and monolayers of adherent cells were cultured until they reached confluence. Cells were then trypsinized (0.25% trypsin with 0.1% EDTA), subcultured at densities of 5,000 to 6,000 cells/cm 2 , and used for experiments during passages 3 to 4.
Mouse MSCs (mMSC) were isolated from long bones of C57BL/6-TgN(ACTbEGFP)1Osb(GFPtg) mice (The Jackson Laboratory, Bar Harbor, ME) using the methods described by Peister et al. ( 34 ). Briefly, cells from each long bone were plated in 40 mL complete isolation medium (see ref. 34 ). After 24 hours, nonadherent cells were removed and adherent cells were washed with PBS, and fresh complete isolation medium was added every 3 to 4 days for 4 weeks. Cells were collected by trypsinization and replated in 30 mL complete isolation medium in 175 cm 2 flasks. After 1 to 2 weeks, cells were trypsinized and plated in complete expansion medium (see ref. 34 ). After another 1 to 2 weeks, passage 3 cells were either frozen or expanded further by plating at 50 cells/cm 2 and incubating in complete expansion medium.
Cell lines. Glioblastoma multiforme cell lines U87 and LN229 were obtained from the American Type Culture Collection (Manassas, VA). U251 cells were obtained from W.K. Alfred Yung (M.D. Anderson Cancer Center, Houston, TX). U87 and U251 cells were maintained in α-MEM supplemented with 10% FBS, and LN229 cells were maintained in α-MEM supplemented with 20% FBS and l -glutamine. Normal human astrocytes (NHA) were obtained from Cambrex (Walkersville, MD). The fibroblast line C29 was obtained from Juan Fueyo (M.D. Anderson Cancer Center). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO 2 /95% air.
Animal subjects. Male athymic nude mice ( nu/nu ) were purchased from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). All animal manipulations were done in accordance with institutional guidelines under approved protocols.
Intracranial xenografting of human glioma cells. Monolayers of human glioma cell lines were detached by trypsinization, washed, and resuspended in PBS at a concentration of 1 × 10 5 cells in 5 μL. Cells were injected into the right frontal lobe of nude mice using a guide-screw system implanted within the skull as described previously ( 35 ). To increase uniformity of xenograft take and growth, cells were injected into 10 animals simultaneously using a multiport Microinfusion Syringe Pump (Harvard Apparatus, Holliston, MA). Animals were anesthetized with xylazine/ketamine during the procedure.
Internal carotid artery injection of human mesenchymal stem cells. hMSCs were injected into the internal carotid artery of xenograft-bearing nude mice according to the previously described method ( 36 ). Briefly, animals were anesthetized with ketamine/xylazine and the internal carotid artery was surgically identified under microscopic visualization. Monolayer cultures of hMSCs were trypsinized and suspended in 100 μL α-MEM plus 10% FBS and injected into the internal carotid artery using a prefabricated injection cannula. Injections were done manually over 3 to 5 minutes. Animals were monitored continuously until awaking and then daily for neurologic deterioration related to the injection.
Human mesenchymal stem cell labeling with SP-DiI. The fluorescent dye SP-DiI (Molecular Probes, Eugene, OR) was dissolved in dimethylformamide (Sigma Chemical) to the concentration of 2.5 mg/mL as described previously ( 27 ). SP-DiI dye was then added directly to culture medium to a final concentration of 10 μg/mL. hMSCs were incubated with 25 mL medium with SP-DiI in T175 flask for 48 hours, after which time cells were washed with PBS, incubated with dye-free medium for 4 hours, and used for experiments.
Brain tissue/tumor preparation. At indicated time points, animals were sacrificed by CO 2 inhalation, and 6 μm serial coronal cryosections from frozen brains were processed for light and fluorescent microscopy. Adjacent sections were stained with H&E for visualization of the tumor mass. In some sections, nuclei were stained with fluorescence-conjugated antibody [4′,6-diamidino-2-phenylindole (DAPI), Molecular Probes]. Imaging was done with a Nikon microscope equipped with a CCD camera. Images were merged using Adobe Photoshop software version 7.0 (Adobe Systems, Inc., San Jose, CA).
In vivo migration assay. The ability of hMSCs to migrate toward gliomas was assessed in vivo by implanting U87 glioma cells [10 5 cells, stably transfected with plasmid containing green fluorescent protein ( GFP ) gene, gift of Charles Conrad, M.D. Anderson Cancer Center] into the frontal lobe of nude mice as described above. Seven days later, SP-DiI-labeled hMSCs (10 5 cells) were implanted in the opposite hemisphere. Migration toward the tumor was assessed at 14 days by direct visualization using fluorescent microscopy.
In vitro migration assay. The tropism of hMSCs for tumor cells and growth factors was determined using an in vitro migration assay according to previously described methods ( 37 ). hMSCs in serum-free medium were placed in the upper well of 24 mm tissue culture Transwell plates (12 μm, Nunc, Naperville, IL) coated with polylysine and Matrigel (1 mg/mL in α-MEM). U87 cells, fibroblasts, or NHAs were incubated in serum-free medium for 48 hours, and the resulting conditioned medium was aspirated and placed in the lower well of the Transwell plates. In selected experiments, growth factor [epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), or stromal cell–derived factor-1α (SDF-1α), 100 mg/mL] was added to the lower compartment. In other experiments, a cocktail of antibodies that blocked the activity of specific growth factors [i.e., anti-PDGF-BB (Sigma Chemical), anti-EGF (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-SDF-1α (Chemicon, Temecula, CA) each at 0.8 μg/mL] was added to the conditioned medium from gliomas cells. hMSCs were incubated for 48 hours at 37°C, and the migration ratio was determined using colorimetric assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] as described previously ( 38 ) or by fixing the membrane, staining the cells using the Hema3 staining kit (Fisher Diagnostics, Pittsburgh, PA), directly counting the number of migrated cells in 10 high-power fields, and calculating the mean. All experiments were done in triplicate.
Adenoviral vectors and human mesenchymal stem cell transfection. Adenoviruses carrying the IFN-β gene or the β-galactosidase (β-gal) were engineered using the bacterial plasmid recombination AdEasy system (Qbiogene, Irvine, CA) as described previously ( 27 ). To transfect hMSCs, cells were incubated with adenoviruses at specified multiplicity of infection for 2 hours, cells were washed and used as described in each experiment. IFN-β expression was detected by ELISA (R&D Systems, Minneapolis, MN).
In vitro efficacy experiments of hMSC-IFN-β. For coculture experiments, U87 glioblastoma cells (10 5 cells) were plated onto 6 cm dish along with increasing ratios of IFN-β-secreting hMSCs. As a control, MSCs-β-gal were used. Cells were trypsinized, stained with 0.04% trypan blue solution, and counted using a hematocytometer. Growth curves were generated by combining data from three independent experiments.
For efficacy experiments involving Transwell plates, U87 cells were grown as monolayers in the lower well of 24 mm tissue culture Transwell plates on porous inserts (12 μm). hMSCs, hMSC-β-gal, or hMSC-IFN-β were plated in the upper well of the Transwell plates. U87 cells were trypsinized and viable cells were counted using a hemocytometer after trypan blue staining. All experiments were done in triplicate.
In vivo efficacy experiments of hMSC-IFN-β. To evaluate the effects of hMSCs in vivo , direct intratumoral injection of hMSC-IFN-β was undertaken. U87 cells (5 × 10 5 cells in 5 μL PBS) were implanted into the right frontal lobes of nude mice as described above. After 10 days, when tumors where well established, hMSC-IFN-β were injected directly into the tumor using the permanently implanted guide-screw system or into the ipsilateral carotid artery as described above. Animals were followed until moribund at which time they were sacrificed by CO 2 inhalation. In all cases, brains were removed to verify the presence of tumor as the cause of death.
Statistical methods. For migration assays, differences between groups were determined using Fischer's exact test. For efficacy experiments, differences in survival among groups were determined by a log-rank test.
Isolation and expansion of human mesenchymal stem cells. Bone marrow aspirates were obtained from normal human donors, isolated, and expanded according to previously described methods ( 27 ). Cells had a typical spindle shape, consistent with the morphology reported by others ( 11, 39 – 42 ). Although hMSCs do not have a specific antigen profile, for each culture, we verified that isolated cells were negative for typical hematopoietic antigens CD45, CD34, and CD38 and were positive for CD44 and CD105 (data not shown). The doubling time of our cultures varied between 30 and 40 hours and cells could be expanded to 10 to 11 passages before developing a large flat morphology. Thus, based on available criteria, the cells used in our experiments had the properties of hMSCs as described previously ( 11, 39 – 42 ).
Localization of human mesenchymal stem cells in human glioma xenografts after regional intravascular administration. To determine the extent to which hMSCs are capable of selectively integrating into human gliomas after systemic delivery in vivo , intracranial xenografts of the human glioma cell line U87 were established in the frontal lobes of nude mice using a guide-screw system as described previously ( 35 ). Seven days after tumor inoculation when xenografts were established, hMSCs (10 6 suspended in 200 μL α-MEM with 10% FBS) were injected into the carotid artery ipsilateral to the implanted tumor ( n = 8). To visualize them on postmortem histologic sections, hMSCs were stained before injection with a fluorescent vital dye SP-DiI as described in Materials and Methods. Animals were sacrificed 1 and 7 days after injection, the brains were removed, and frozen sections were analyzed by light and fluorescent microscopy. SP-DiI-labeled hMSCs were seen exclusively within the U87 tumor mass both 1 and 7 days after injection ( Fig. 1 ). Essentially no hMSCs were seen in the peritumoral normal brain or in the hemisphere opposite to the implanted tumor in all animals assessed, indicating that hMSCs specifically localize in the tumor but not in the normal brain. The specificity of hMSCs for U87 xenografts was seen best in whole mounts of the entire brain in which SP-DiI-labeled hMSCs were confined specifically within the borders of the irregularly shaped tumor but were not seen in the surrounding brain ( Fig. 2 ).

Photomicrograph of brain sections showing hMSCs in U87 intracranial xenograft. Section was acquired 7 days after hMSCs were injected intra-arterially. Top, light microscopy of tumor, T ( left ), adjacent to tumor ( center ), and contralateral brain ( right ). Middle, corresponding fluorescent microscopy view. Fluorescently labeled hMSCs ( red ) are seen only in the tumor. Bottom, high-power view (×100) of the tumor.

Photomicrograph of a section of the entire brain showing selectivity of hMSC engraftment for U87 glioma after intracarotid injection of SP-DiI-labeled hMSCs (10 6 cells). Light microscopy ( center ) and H&E staining ( bottom left ) reveals tumor in right hemisphere. Fluorescent microscopy ( bottom right ) show fluorescently labeled cells conforming to tumor shape.
The selectivity of hMSCs for U87 gliomas was further supported by experiments in which SP-DiI-labeled hMSCs were injected into the internal carotid artery contralateral to the hemisphere bearing the U87 xenograft ( n = 3). Similar to the ipsilateral injection, contralateral injection resulted in the localization of SP-DiI-labeled hMSCs exclusively within the tumor, including (in one example) within small tumor nodules away from the main mass but not in the normal brain ( Fig. 3 ). In addition to supporting the concept that hMSCs are selective for gliomas compared with normal brain tissue, these results also indicate that the localization within the xenograft was not merely the result of preferential blood flow to the tumor mass.

Photomicrograph of section of entire brain showing selectivity of hMSC engraftment for right hemisphere U87 glioma after SP-DiI–labeled hMSCs (10 6 cells) were injected into the contralateral ( left ) carotid artery. U87 cells were stably transfected with GFP and appear green under fluorescent microscopy ( insets ). SP-DiI–labeled hMSCs ( red ) are located within the tumor ( inset ) as well as in a smaller tumor nodule ( smaller inset ) but not in brain ( left lower inset ).
To show that injected hMSCs remained as whole cells within the xenograft, engrafted SP-DiI-labeled hMSCs were counterstained with DAPI, a nuclear-specific probe. Dual DAPI-positive and SP-DiI-labeled cells were seen exclusively within the xenografts, indicating that engrafted hMSCs were morphologically intact ( Fig. 4 ).

Photomicrograph of U87 tumor treated with intravascularly given SP-DiI-labeled hMSCs. Sections were also stained with DAPI nuclear stain. Top, H&E-stained section of tumor in brain; bottom, fluorescent microscopic views. Red, SP-DiI-labeled hMSCs; blue, DAPI nuclear stain. Merged images showed SP-DiI labeling in the membrane and a DAPI-positive nuclei, indicating that the cells are intact inside the tumor.
To determine whether hMSCs could localize to gliomas other than U87, xenografts were established in the frontal lobe of nude mice using the U251 ( n = 5) or LN229 ( n = 4) glioma cell lines. After injection into the carotid artery ipsilateral to the implanted tumor, SP-DiI-labeled hMSCs were identified within the LN229 and U251 xenografts ( Fig. 5 ), suggesting that the ability to support the integration of hMSCs was not a unique property of U87 cells.

Photomicrograph of brain section showing hMSCs in U251 intracranial xenograft. Section was acquired 7 days after hMSCs were injected intra-arterially. Top, light microscopy of tumor ( left ), adjacent to tumor ( center ), and contralateral brain ( right ). Middle, corresponding fluorescent microscopy view. Fluorescently labeled hMSCs ( red ) are seen only in the tumor. Bottom, high-power view (×100) of tumor.
To show that the tropism of hMSCs for gliomas was a unique property of these stem cells and not a property of other human cells, SP-DiI-labeled human fibroblasts were injected into the ipsilateral carotid artery of mice bearing U87 gliomas. Fibroblasts were chosen because hMSCs were originally described as fibroblast colony-forming cells and because fibroblasts are morphologically similar to hMSCs ( 14 ). However, injections of fibroblasts into the carotid artery consistently resulted in the death of animals ( n = 8) presumably due to intravascular cellular embolization and secondary brain ischemia, a finding suggestive of widespread distribution of fibroblasts in the brain without tumor specificity. As an alternative approach, U87 tumor cells were used. In contrast to hMSCs, ipsilateral carotid injection of SP-DiI-labeled U87 tumor cells into the carotid artery of animals bearing U87 xenografts resulted in widespread distribution of injected cells throughout the brain with no cells colocalizing to the tumor ( Fig. 6A ). These studies show that not all human cells are capable of localizing to human gliomas after intracarotid injection; thus, the capacity of hMSCs to integrate into gliomas is a specific property of these stem cells. These studies also indicate that the observed localization of hMSCs within human glioma xenografts was not due to the fact that human cells were injected into mice (i.e., that the results were due to a species-specific interaction between human xenografts and human stem cells in a mouse brain background), because not all injected human cells have the capacity to localize to human xenografts in this model system.

A, photomicrograph of section of entire brain showing lack of tropism of intravascularly given U87 cells for established U87 xenografts. U87 cells (containing GFP) were implanted in the frontal lobe of nude mice and SP-DiI-labeled U87 cells (10 6 cells) were injected into the ipsilateral carotid artery. SP-DiI-labeled U87 cells ( red ) are not found within the U87 xenograft (GFP-labeled; right inset ). However, SP-DiI-labeled U87 cells ( red ) are found in the brain within the opposite hemisphere ( left inset ). B, photomicrograph of U87 xenograft after treatment with MSCs obtained from mouse bone marrow (mMSCs). U87 cells were implanted into the frontal lobe, and after 7 days, SP-DiI-labeled mMSCs (10 6 ) were injected into the carotid artery. One week later, brains were sectioned. Left, light microscopic view of representative unstained frozen section showing area of tumor (×100); right, higher-power fluorescent microscopic view (×400) of area in black box. C, photomicrograph of brain showing migration of hMSCs toward glioma. U87 cells (GFP-labeled) were implanted in the right frontal lobe (day 0), and after 7 days, SP-DiI-labeled hMSCs were implanted in the left lobe. Left, injection site and tumor position. Middle, high-power view of area within the rectangle; left, view within the tumor. After 14 days, hMSCs ( red ) were seen between injection site and tumor ( middle ) as well as in the GFP-labeled tumor ( right ).
To further show that the observed localization of hMSCs within gliomas was not a function of the model system (i.e., a species-specific interaction), mMSCs were harvested from the bone marrow of C57 mice (see Materials and Methods). mMSCs were labeled with SP-DiI and injected into the ipsilateral carotid artery of nude mice bearing established U87 xenografts ( n = 3). Similar to the hMSCs, mMSCs were found exclusively within the xenografts 7 days after injection ( Fig. 6B ).
Human mesenchymal stem cells migrate toward gliomas after intracranial injection. Although the above studies indicate that hMSCs can localize to human gliomas after intravascular delivery, it is also of interest to determine the extent to which hMSCs have the capacity to migrate toward gliomas once within the brain. To better define the extent to which hMSCs are capable of migrating toward human gliomas in vivo , local delivery experiments were carried out. Specifically, intracranial xenografts of the human glioma cell line U87 were established in the right frontal lobe in mice using a guide-screw ( 35 ). Seven days after tumor inoculation, SP-DiI-labeled hMSCs (10 5 cells in 10 μL medium/FBS) were injected directly into the opposite cerebral lobe ( n = 5). As a control, a group of tumor-bearing animals received intracranial injections of human fibroblasts (10 5 cells in 10 μL medium/FBS). Animals were sacrificed 14 days after injection, their brains were removed, and frozen sections were analyzed by light and fluorescent microscopy. By 14 days after administration, SP-DiI-labeled hMSCs were seen extending from the site of injection, across the brain between the tumor and the injection site, and within the tumor ( Fig. 6C ). In contrast, fibroblasts remained within the injection site. Thus, hMSCs have an intrinsic attraction for gliomas and are capable of migrating between hemispheres toward gliomas.
Factors mediating tropism of human mesenchymal stem cell for gliomas. We hypothesized that a factor released by the glioma cells may be a potential mediator of the tropism of hMSCs for human gliomas. To test this hypothesis, in vitro Matrigel invasion assays using Transwell plates were done as a surrogate assay for the tropism of hMSCs for gliomas. We first investigated if human glioma U87 cell lines were capable of stimulating the migration of hMSCs. Thus, hMSCs were placed in the upper wells on Matrigel, and conditioned medium from U87 gliomas and C29 fibroblasts grown in serum-free medium were placed in the lower wells. Conditioned medium from NHAs was used as another control to better mimic the normal brain milieu. A semiporous membrane (8 μm pores) separated the wells. Cell-free medium without and with 20% FCS was also used as controls. Migration was quantified by directly visualizing and counting migrated cells under the microscope after cell staining. Whereas exposure to cell-free medium or to conditioned medium from fibroblasts or NHAs resulted in low levels of migrating hMSCs, exposure to conditioned medium from U87 cells produced significant hMSC migration ( Fig. 7A ). The observed differences in migration were not due to increases in hMSC proliferation because the total number of hMSCs (invading plus noninvading) was the same for each condition.

A, hMSC invasion in response to conditioned medium of the glioma cell line U87 compared with fibroblasts (C29 cells; CCD ) and NHAs. Whereas medium alone and conditioned medium from fibroblasts or NHAs did not promote hMSC invasion into Matrigel, conditioned medium from U87 cells ( P < 0.05) and FCS ( P < 0.001) enhanced hMSC invasion. B, effects of growth factors on hMSC invasion. Increased invasion is seen with PDGF-BB and EGF but not FGF and VEGF. α-MEM is negative control and α-MEM + FCS is positive control. C, effect of SDF-1α on hMSC invasion. SDF-1α and EGF had similar effects on promoting hMSC invasion of Matrigel. PDGF is positive control and α-MEM is negative control. D, effects on hMSC invasion after of inhibiting PDGF-BB, EGF, and SDF-1α. Conditioned medium form U87 cells was mixed with a cocktail containing anti-PDGF, anti-EGF, and anti-SDF-1α antibodies. Treatment with medium alone (α-MEM) was a negative control and treatment with 20% FCS was the positive control.
Because exposure to 20% FCS stimulated significant hMSC migration ( Fig. 7A ), we analyzed the effects on hMSC migration of several growth factors (specifically PDGF-BB, EGF, basic FGF, and VEGF) that are commonly present in serum and that have been implicated in glioma growth. Maximal hMSC migration occurred with exposure to PDGF-BB (100 ng/mL). Intermediate levels of migration were observed after exposure to EGF (100 ng/mL) and SDF-1α (100 ng/mL), whereas basic FGF (100 ng/mL) and VEGF (100 ng/mL) had no significant effect compared with serum-free medium ( Fig. 7B and C ).
To document that the increase in migration of hMSCs that was seen after exposure to conditioned medium from U87 cells was due to the presence of PDGF-BB, EGF, or SDF-1, conditioned medium from U87 cells was treated with a cocktail containing anti-PDGF-BB, anti-EGF, and anti-SDF-1 antibodies (see Materials and Methods) that are capable of blocking the activity of each growth factor. Whereas conditioned medium from U87 cells resulted in a significant increase in hMSC migration, treatment with the blocking antibody cocktail significantly attenuated the migration of hMSCs through Matrigel ( Fig. 7D ). These results suggest that specific growth factors may at least in part mediate the tropism of hMSCs for gliomas.
Therapeutic potential of genetically engineered human mesenchymal stem cells on human gliomas: in vitro studies. As a proof of principle that hMSCs are capable of delivering a therapeutic agent to brain tumors, we transfected hMSCs with an adenoviral vector containing the cDNA of the IFN-β gene (Ad-IFN-β) as described previously ( 27 ). A quantified ELISA assay revealed that monolayers of these engineered hMSCs (designated hMSC-IFN-β) released IFN-β into the medium dependent on the number of viral particles used to transfect the cells ( Fig. 8A ). Based on these results, hMSCs were typically infected with 3,000 multiplicities of infection for all subsequent experiments.
A, expression of IFN-β in the medium of Ad-IFN-β-transfected hMSCs. Monolayers of hMSCs were plated and infected at the doses (multiplicity of infection) shown. After 24 hours, cells were assayed using a quantitative ELISA assay for IFN-β. A dose-dependent release of IFN-β into the medium is seen. B, effects of hMSC-IFN-β on survival of U87 gliomas based on in vitro coculture experiments. U87 cells (10 5 ) were cocultured with the indicated percentage of hMSC-IFN-β. As a control, U87 was cocultured with hMSC-β-gal (50%). Cells were counted 3, 5, and 7 days after plating. A dose-dependent decrease in U87 survival is evident after treatment with hMSCs capable of producing IFN-β. Dark circle, 50% hMSC-β-gal; dark square, 0.1% hMSC-IFN-β; small circle, 1% hMSC-IFN-β; triangle, 10% hMSC-IFN-β; open square, 50% hMSC-IFN-β. C, effects of hMSC-IFN-β on survival of U87 gliomas based on in vitro Transwell experiments. U87 cells (3 × 10 5 ) were plated on the lower well (day −2), and after 48 hours, hMSC-IFN-β were plated in the upper well at indicated cell numbers (day 0). U87 cells were assayed for viability 5 and 7 days after hMSCs were plated. hMSC-IFN-β resulted in a significant decrease in U87 cell survival. D, amount of IFN-β detected in the medium of cells treated under the protocol and conditions described in ( C ). Medium was collected on day 7 before cell counting. The concentration of IFN-β was determined for each condition using an ELISA assay.
To determine whether hMSC-IFN-β are of therapeutic benefit, U87 glioma cells were cocultured with increasing ratios (0.1-50%) of hMSC-IFN-β. As a control, hMSCs transfected with adenovirus containing the β-gal cDNA (hMSC-β-gal) were cocultured with U87 cells at a ratio of 2:1 (U87:hMSC). hMSC-IFN-β significantly inhibited the growth of human gliomas even when the ratio of U87 to hMSC-IFN-β was 1,000:1 (0.1%; Fig. 8B ).
To prove that this growth inhibition was specifically due to the release of soluble IFN-β, hMSCs (untransfected), hMSC-β-gal, or hMSC-IFN-β were grown in the upper well of Transwell plates in increasing numbers and U87 glioma cells were grown in the lower well. A semiporous membrane (8 μm pores) separated the cells. Whereas treatment with hMSCs or hMSC-β-gal resulted in progressive increase in cell numbers, exposure to hMSC-IFN-β resulted in a dose-dependent growth inhibition of U87 glioma cells ( Fig. 8C ). To verify that this effect was due to release of soluble IFN-β, the concentration of IFN-β in the medium of the lower well was determined at each time point ( Fig. 8D ). There was a dose-dependent increase in the amount of soluble IFN-β that directly correlated with the inhibition of tumor cell growth.
Therapeutic potential of genetically engineered human mesenchymal stem cells on human gliomas: in vivo studies. To determine if hMSC-IFN-β-induced growth inhibition also occurs in vivo , U87 cells (5 × 10 5 ) were implanted into the frontal lobe of nude mice, and after 10 days, animals were treated with a single intratumoral injection of PBS, hMSC-β-gal (2.5 × 10 5 cells), or hMSC-IFN-β (2.5 × 10 4 or 2.5 × 10 5 cells). In a group of animals, hMSC-IFN-β (2.5 × 10 5 cells) were given s.c. Compared with animals treated with PBS or with hMSC-β-gal, treatment with 2.5 × 10 5 hMSC-IFN-β resulted in a significant ( P < 0.05) increase in animal survival ( Fig. 9A ). Interestingly, injection of 2.5 × 10 4 hMSC-IFN-β did not prolong survival, suggesting that in this model >2.5 × 10 4 hMSC-IFN-β must be present within the tumor for growth inhibition to occur. Furthermore, s.c. administration of hMSC-IFN-β had no effect on survival compared with controls, suggesting that local (intratumoral) release of IFN-β is required for an antitumoral effect.
A, survival of mice after intracranial intratumoral injection of hMSC-IFN-β into established U87 gliomas. U87 cells (10 5 ) were implanted into the frontal lobe of nude mice. After 10 days, tumors were injected with a single dose of hMSC-IFN-β, hMSC-β-gal, or PBS ( n = 5/group). A significant increase in survival is evident with treatment with 2 × 10 5 hMSC-IFN-β ( star ). Diamond, PBS; triangle, 2 × 10 5 hMSC-β-gal; cross, 2 × 10 5 hMSC-IFN-β s.c.; square, 2 × 10 4 hMSC-IFN-β; star, 2 × 10 5 hMSC-IFN-β. B, survival of mice after intra-arterial treatment with hMSC-IFN-β. U87 cells (10 5 ) were implanted into the frontal lobe of nude mice ( n = 6/group). After 10 days, animals were treated with hMSC-IFN-β or controls by injection of 10 6 cells into the carotid artery. Treatment with hMSC-IFN-β significantly increased survival. Circle, PBS; triangle, IFN-β i.v.; diamond, hMSC-IFN-β i.v.; square, hMSC-IFN-β flank; cross, hMSC-β-gal intra-arterial; dark circle, hMSC-IFN-β intra-arterial.
To determine whether regional delivery of hMSC-IFN-β is an effective antiglioma approach, U87 cells were implanted into the frontal lobe of nude mice ( n = 6/group). Ten days later, animals were treated with PBS, hMSC-β-gal (10 6 cells), or hMSC-IFN-β (10 6 cells) by injection into the internal carotid artery ( Fig. 9B ). A group of animals were also treated with human IFN-β (50,000 IU) given i.v. and another group received a s.c. injection of hMSC-IFN-β (10 6 cells). Only intra-arterial treatment with hMSC-IFN-β significantly extended the survival of the animals ( P < 0.05). Neither systemic treatment with IFN-β (not carried by hMSCs) nor s.c. injection (i.e., distant from the tumor) of hMSC-IFN-β altered animal survival compared with controls. Thus, hMSC-IFN-β are effective against intracranial gliomas when delivered regionally.
In this study, we provide evidence that human bone marrow–derived MSCs can localize to human gliomas after regional intra-arterial delivery and can migrate toward human gliomas after local intracranial delivery. We also show that the tropism of hMSCs for gliomas may be mediated at least in part by specific growth factors/chemokines. Most importantly, in vitro and in vivo efficacy studies show that hMSCs can be engineered to release a soluble factor (e.g., IFN-β) and that these engineered hMSCs can be exploited to therapeutic advantage against gliomas.
The finding that hMSCs localize to human gliomas is of interest because it suggests that the capacity for integration into tumors is an intrinsic property of these stem cells. This observation is consistent with the hypothesis that the intratumoral integration of exogenously delivered hMSCs is a recapitulation of the natural recruitment of endogenous, circulating hMSCs to aid in the process of stroma formation and tissue remodeling and suggests that hMSCs may contribute to the stroma of tumors ( 27, 30 ). Initial work from our group showed that bone marrow–derived hMSCs are capable of integrating into the stroma of metastatic melanoma grown either s.c. or in the lungs of nude mice ( 27 ). We now show that human gliomas grown in the brain of nude mice also support the engraftment of hMSCs delivered by an intravascular route. This finding in brain tumors is surprising because the stroma of primary brain tumors is composed of glial/astrocytic cells (ectodermal origin) and is thus distinct from the fibroblast-based (mesenchymal) stroma of most systemic (extracerebral) cancers. However, it has been shown that MSCs are capable of differentiating into glial cells ( 25, 26 ), including astrocytes, and it is thus possible that this property may explain the intrinsic capacity of hMSCs to integrate into the stroma of gliomas. Whether hMSCs that have localized to gliomas differentiate into astrocytes in our system is currently under investigation. Alternatively, human gliomas, similar to other cancers, require the elaboration of mesodermal elements, specifically endothelial cells and pericytes. It has been suggested that MSCs are a main source of pericytes within the bone marrow stroma ( 11, 16, 17, 40, 43 ); thus, hMSCs may integrate into gliomas to contribute to the mesenchymal elements of the tumor. In support of this concept is the observation that animals bearing U87 xenografts that received hMSC-β-gal (i.e., nonsecreting hMSCs) survived for shorter times than did animals who received saline treatments in our experiments (see Fig. 9 ). Thus, hMSCs may localize to tumor under physiologic conditions to assist with tissue repair and in so doing provide a microenvironment conducive to improved tumor growth. Regardless of their physiologic role within tumors, this present study plus our previous work ( 27 ) suggest that hMSCs seem to have the capacity to engraft themselves into a variety of histologically disparate tumors, including gliomas, and thus may be a cellular vehicle that is universally applicable for delivery of therapeutic agents to most tumor types.
Several lines of evidence suggest that the localization of hMSCs to gliomas was not merely a consequence of the in vivo model system used in our studies. First, the integration of hMSCs is seen with several xenografts, including the U87, U251, and LN229 cell lines. These cell lines are genetically and phenotypically distinct. In addition, the LN229 cell line is fairly invasive and grows phenotypically similar to invasive gliomas in situ . Second, the engraftment of gliomas by hMSCs was seen after injecting the stem cells into the carotid artery either ipsilateral or contralateral to the hemisphere harboring the tumor. Thus, the results are not merely a fortuitous outcome of cerebral blood flow patterns but rather are due to a selectivity of hMSCs for the tumor compared with the normal brain. Third, intra-arterial injection of human fibroblasts resulted in death of most animals presumably due to widespread arterial occlusion. Furthermore, intracarotid injection of U87 tumor cells did not result in localization of delivered U87 cells within established U87 xenografts. Thus, the capacity to localize to a glioma after intravascular delivery seems to be a specific property of hMSCs and does not seem to be a function of all human cells. Fourth, similar to hMSCs, mMSCs that were delivered intravascularly also were capable of localizing to human gliomas. Taking these findings together, it is unlikely that the localization of hMSCs within human gliomas grown in nude mice was merely the result of a species-specific (human cell-human cell) tropism. Instead, hMSCs seem to have an intrinsic, cell-specific capacity to localize to human gliomas.
The isolation of MSCs from human subjects is an important aspect of our study. In fact, to our knowledge, this is the first demonstration that MSCs obtained from the bone marrow of human subjects can engraft themselves into human gliomas. Although Nakanura et al. reported recently the use of MSC in glioma therapy, these investigators isolated the stem cells from rat species ( 44 ). This is important because differences between nonhuman (i.e., murine or rat MSCs) and human MSCs have been noted ( 42 ). In addition, all studies using neural stem cells have by necessity relied on murine-derived neural stem cells ( 7 – 10 ). Because the MSCs used in our studies were derived from human subjects, our results support the application of these cells in an autologous transplantation setting in patients.
Although we show that hMSCs can migrate toward gliomas after intracranial delivery, an important aspect of our study from the perspective of clinical application is the use of intravascular delivery. Although direct intratumoral (intracranial) delivery has been reported for rat MSCs ( 44 ) and mouse neural stem cells ( 4, 7 – 10 ), intravascular delivery has the advantage that it obviates invasive surgical interventions and that repeated injections over an extended period are clinically feasible. To our knowledge, no other report has shown the propensity of hMSCs to localize to brain tumors after intravascular delivery. It should be noted, however, that we originally sought to deliver hMSCs systemically by tail vein injection but found that the majority of cells were filtered by the lung and only rare hMSCs were integrated into the tumor (at least when examined 7 days after injection; data not shown). Aboody et al. reported that after tail vein injection murine neural stem cells localized to tumors, albeit with low efficiency ( 45 ). In our studies, filtering of hMSCs within the lungs of nude mice may have been due at least in part to species incompatibilities. Whether trapped pulmonary hMSCs eventually recirculate and localize to intracerebral tumors is currently under investigation. Whether similar intrapulmonary entrapment will also occur in patients treated with hMSCs will ultimately require studies in human subjects.
Our results indicate that the tropism of hMSCs for gliomas may be mediated at least in part by growth factors/chemokines. This observation is consistent with the concept that hMSCs are attracted to the tumor milieu because tumors mimic tissue injury ( 31 – 33 ). Similar to damaged tissue, human gliomas express EGF, PDGF, VEGF, and FGF as well as the chemokine SDF-1α (see refs. 46, 47 for review). Despite this wide array of growth factors within tumors, however, we show that there is selectivity of hMSCs for specific factors. Whereas FGF and VEGF had little effect on hMSC migration, PDGF, EGF, and SDF-1α enhanced hMSC tropism. Moreover, a cocktail of antibodies that block PDGF-BB, EGF, and SDF-1α was able to attenuate the migration of hMSCs toward conditioned medium derived from U87 cells. Indeed, hMSCs are known to express EGF and PDGF receptors on their surface ( 43 ). It should be noted, however, that the in vitro invasion assay employed in this study may not directly mimic the in vivo conditions necessary for migration of hMSCs from the vasculature to the tumor. In vivo models that better recapitulate this process are currently under development. Further elucidation of the mechanism underlying the tropism of hMSCs for gliomas may provide insights into methods for increasing the efficiency of the engraftment process.
Our studies of IFN-β indicate that hMSCs can be used to deliver a diffusible molecule that can be released form hMSCs to achieve tumoricidal effects. IFN-β is a particularly good choice to show the proof of principle of this approach because phase I clinical trials of recombinant human IFN-β have shown that although responses do occur, systemic delivery of high doses of IFN-β is associated with toxicity and a narrow therapeutic index that limits the overall efficacy ( 48 – 52 ). Because IFN-β functions physiologically as a paracrine factor, its antitumoral effects can be enhanced and its toxicity is reduced if it is given locally ( 53, 54 ). We reasoned that hMSCs engineered to produce IFN-β would provide a high degree of local intratumoral delivery, with reduced systemic toxicity. In this context, we used an adenoviral vector to transfer the IFN-β gene into hMSCs and found that these engineered hMSCs (hMSC-IFN-β) released high levels of IFN-β and were capable of directly killing human glioma cell lines grown in vitro . Indeed, in Transwell experiments in which hMSC-IFN-β were physically separated from the glioma cells, there was a dose-dependent tumoricidal effect, the amount directly correlated with the concentration of soluble IFN-β released by the engineered hMSCs. Most importantly, regional delivery of hMSC-IFN-β by injection into the internal carotid artery in vivo significantly extended the survival of animals harboring established intracranial gliomas. These results were due to the local delivery of IFN-β by the engrafted hMSCs because IFN-β given i.v. did not extend animal survival compared with saline-treated controls, and hMSC-IFN-β implanted s.c. (i.e., at a site distant from the tumor) also had no effect. Thus, these studies provide the proof of principle that hMSCs can be engineered to release a soluble factor into brain tumors. Although our studies suggest that IFN-β is itself a good therapeutic agent worthy of assessment in patients with glioma, the same approach can be exploited in the delivery of other agents with antitumor activity.
Comparing the experiments in which hMSC-IFN-β were directly injected in the tumor with those in which hMSC-IFN-β were delivered intravascularly provides insight into the number of hMSCs that may integrate into a tumor after intravascular delivery. Specifically, we found that a significant increase in animal survival required a direct intratumoral injection of at least 2.5 × 10 5 hMSC-IFN-β; intratumoral injection of 2.5 × 10 4 did not extend animal survival ( Fig. 9A ). In this context, it is reasonable to assume the at least 2.5 × 10 5 cells integrated into the tumor after intravascular delivery; otherwise, we would not have observed the reported increase in survival that occurred after administering hMSC-IFN-β by this route. Because we saw a significant increase in survival after intra-arterial injection of 10 6 hMSCs, we estimate that at least 25% of the cells (2.5 × 10 5 ) must have integrated into the tumor.
As mentioned above, these in vivo studies revealed that animals bearing U87 xenografts that received hMSC-β-gal (i.e., nonsecreting hMSCs) had shorter survival than did animals who received saline injections. Although this observation implies that hMSCs may have a role in tumor development under physiologic conditions (see above), the fact that animal survival was increased when hMSCs were engineered to secrete IFN-β indicates that this physiologic role can be exploited and tumor growth is reversed when a protein with antiglioma effects is released by the hMSCs. From a therapeutic perspective, these observations imply that relatively pure populations of stem cells engineered to carry a therapeutic gene (i.e., populations free of untransfected cells) will be needed to achieve maximal antitumoral effects. Methods for maximizing transfection of therapeutic genes to hMSCs and for separating transfected from nontransfected cells are challenges for the ultimate application of this and other stem cell approaches to tumors.
Although our studies have focused on bone marrow–derived hMSCs, recent work has suggested that other cells in the bone marrow may also be useful as delivery vehicles for brain tumors ( 55 ). Specifically, Fine et al. reported recently that a neural stem-like cell could be isolated from the bone marrow and that these bone marrow–derived stem cells can be used to deliver therapeutic biological agents to brain tumors ( 55 ). Interestingly, using cDNA microarray technology, these investigators showed that the profile of expressed genes of bone marrow–derived hMSCs (i.e., the same cells used in our studies) is distinct from the expression profile of bone marrow–derived neural stem-like cells used in their studies, indicating that the two cell populations are unique. Thus, the bone marrow may be a rich source of several stem cell populations that may be useful in the treatment of brain tumors. Future studies comparing the functional properties of these distinct bone marrow–derived stem cell population will be of great interest for the clinical application of this type of stem cell therapy.
Grant support: Anthony Bullock III Foundation and Elias Family Fund for Brain Tumor Research (F.F. Lang).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Citing articles via
Email alerts, related content.
- Online First
- Collections
- Online ISSN 1538-7445
- Print ISSN 0008-5472
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Translational research for bone marrow failure patients
Camille malouf.
a Centre for Regenerative Medicine, University of Edinburgh, Edinburgh, UK
Stephen J. Loughran
b Wellcome–MRC Cambridge Stem Cell Institute, University of Cambridge, Cambridge, UK
Adam C. Wilkinson
c MRC Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, UK
Akiko Shimamura
d Bone Marrow Failure and Myelodysplastic Syndrome Program, Dana–Farber/Boston Children's Cancer and Blood Disorders Center, Harvard Medical School, Boston, MA
Paula Río
e Division of Hematopoietic Innovative Therapies, Centro de Investigaciones Energéticas Medioambientales y Tecnológicas (CIEMAT), Madrid, Spain
f Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER-ISCIII), Madrid, Spain
g Advanced Therapies Unit, Instituto de Investigación Sanitaria Fundación Jiménez Díaz (IIS-FJD/UAM), Madrid, Spain
All authors contributed to the drafting and editing of the article.
Bone marrow failure syndromes encompass a range of inherited and acquired hematological diseases that result in insufficient blood cell production, which leads to severe complications including anemia, weakening of the immune system, impaired coagulation, and increased risk of cancer. Within inherited bone marrow failure syndromes, a number of genetically distinct diseases have been described including Shwachman–Diamond syndrome and Fanconi anemia. Given the genetic complexity and poor prognosis of these inherited bone marrow failure syndromes, there is increasing interest in both characterizing the genetic landscapes of these diseases and developing novel gene therapies to effectively monitor and cure patients. These topics were the focus of the winter 2021 International Society for Experimental Hematology New Investigator Webinar, which featured presentations by Dr. Akiko Shimamura and Dr. Paula Río. Here, we review the topics covered within this webinar.
Lifelong function of the blood and immune systems are essential for health. Continuous blood cell production from hematopoietic stem and progenitor cells (HSPCs) within the bone marrow and other hematopoietic organs, such as the spleen, sustains hematopoietic system homeostasis [ 1 - 4 ]. Aberrations in hematopoiesis can result in over- or underproduction of blood cells that causes a range of diseases such as leukemia, lymphoma, cytopenias, and anemias. One group of particularly severe hematological diseases is the bone marrow failure syndromes [ 5 , 6 ].
Bone marrow failure describes the loss of homeostatic hematopoiesis, resulting in insufficient production of platelets, red blood cells, and white blood cells [ 5 , 6 ]. It occurs as part of a heterogeneous group of inherited and acquired syndromes and usually presents clinically as pancytopenia, which can progress to a more severe disease such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). A number of genetic mutations responsible for inherited bone marrow failure syndromes have been identified [ 5 , 7 ]. Among the most prevalent syndromes are Fanconi anemia (FA), dyskeratosis congenita/telomere biology disorders, Diamond–Blackfan anemia, and Shwachman–Diamond syndrome (SDS). Acquired bone marrow failure may be caused by autoimmunity, lymphoma, chemotherapy, or radiotherapy [ 8 ]. People of all ages can be affected with severe inherited bone marrow failure. The study of inherited bone marrow failure syndromes has provided several important insights into the biology of hematopoiesis and has become an active area for developing novel therapeutic paradigms to monitor and treat patients.
The severity of inherited bone marrow failure can vary significantly between patients. At present, the only curative therapy is allogeneic hematopoietic stem cell transplantation (alloHSCT). However, this treatment can have significant adverse side effects, often caused by the conditioning regimens or subsequent development of graft-versus-host disease [ 9 ]. It is therefore crucial to accurately identify which patients will most benefit from alloHSCT while minimizing the risk of serious complications. Furthermore, the development of new therapies that circumvent the complications of alloHSCT would greatly benefit patients. Inherited bone marrow failures have therefore been identified as targets for ex vivo hematopoietic stem cell (HSC) gene therapy followed by autologous transplantation.
The International Society for Experimental Hematology (ISEH) New Investigators Committee Webinar, “Bone Marrow Failure: Lab to Patient,” was moderated by Camille Malouf from the University of Edinburgh in January 2021 (available to watch via the ISEH website, www.ISEH.org/page/ISEHwebinars ). The first speaker was Dr. Akiko Shimamura from the Boston Children’s Hospital (Boston, MA), who described her laboratory’s recent research findings in risk stratification for patients affected with SDS. The second session was presented by Dr. Paula Río from CIEMAT (Madrid, Spain), who described her latest work developing gene therapies to treat patients with FA. This review presents a synthesis of the topics discussed during this webinar and aims to place the research findings presented within the wider context of the bone marrow failure field.
NEW MOLECULAR AND GENETIC INSIGHTS INTO SHWACHMAN–DIAMOND SYNDROME—DR. AKIKO SHIMAMURA
SDS was first described in 1964 as a syndrome of pancreatic insufficiency and bone marrow dysfunction [ 10 ]. SDS is a rare autosomalrecessive multisystem disease that manifests by 4–6 months of age. It is characterized by impaired hematopoiesis linked to a low number of white blood cells, immunological disorders, growth problems linked to a difficulty to absorb food as well as skeletal, cardiac, and hepatic abnormalities [ 11 ]. The majority of SDS cases are caused by biallelic mutations in the ribosome assembly factor SBDS gene [ 12 - 16 ].
Patients with SDS have a significant risk of progressing to MDS or AML (~20%) [ 17 ]. Myeloid malignancies carry a poor prognosis because of both treatment-related toxicities and resistant disease [ 18 ], so strategies to identify patients at high risk of clonal evolution offer an opportunity to intervene prior to malignancy development. As such, SDS patients can require an alloHSCT in their therapeutic regimen. Given the significant risks and hematopoietic stress linked to this procedure for patients, it is important to identify which patients will have a higher risk of developing severe complications. Therefore, one of the critical aspects in SDS is the need to improve patient surveillance for risk stratification [ 18 ]. This can be achieved by gaining a better understanding of the genetic mutations that cooperate in driving SDS pathogenesis to a more severe form (MDS/AML).
Highlighting how better understanding patients’ mutational burden can help predict SDS-related complications, an elegant retrospective study published in 2017 on a cohort of MDS patients who underwent alloHSCT reported that TP53 mutations are present in a significant proportion of MDS patients (19%) and are associated with a shorter survival and shorter time to relapse compared with MDS patients who do not carry TP53 mutations [ 19 ]. One year later, another group reported that clonal hematopoiesis caused by TP53 mutations are prevalent in SDS patients, raising questions about the implications of this finding for clonal evolution in SDS [ 20 , 21 ]. TP53 mutations were not found in healthy donors or patients with severe congenital neutropenia (SCN) who also have a high risk of developing leukemia, suggesting that they could be more specific to SDS patients.
Using clinically annotated paired marrow and fibroblast samples collected longitudinally through the SDS registry, genomic studies and functional validation to elucidate the mechanisms driving clonal evolution in SDS were led by Dr. Akiko Shimamura and Dr. R. Coleman Lindsley from the Boston Children’s Hospital and the Dana–Farber Cancer Institute (Boston, MA) [ 22 ]. Clonal hematopoiesis can be identified by quantifying somatic mutation allele frequencies in hematopoietic cells [ 23 - 27 ]. To uncover mutations that cooperated with SBDS mutations to drive clonal hematopoiesis, as well as those that contribute to the development of MDS/AML, whole-genome sequencing was performed on bone marrow aspirates and paired fibroblast from SDS patients. In this study by Kennedy et al. [ 22 ], genetic mutations in eukaryotic translation initiation factor 6 ( EIF6) were found to be common in both SDS patients that progressed to MDS/AML and SDS patients who did not progress to severe bone marrow failures. They identified 256 EIF6 mutations that were specific to SDS patients given the absence of EIF6 mutations in control cohorts (SDS-like patients, patients with other leukemia predisposition and AML) and control cells.
During ribosome maturation, SBDS plays a key role in the release of EIF6 from the pre-60S ribosome, which is an essential step to ribosome activation [ 15 , 16 ]. Most of the prevalent EIF6 mutations identified in SDS patients led to EIF6 protein destabilization. However, the most common EIF6 mutation ( EIF6 N106S ) impaired EIF6 interaction with the 60S ribosomal subunit 22. This reduced EIF6 function in SDS patients improved assembly of the 80S subunit, partially relieved the SDS-associated impairment of translation, and alleviated p53 activation. Hematopoietic colony-forming assays showed that EIF6 I13N and EIF6 A194T (EIF6 destabilization) and EIF6 N106S (disrupt EIF6 −60S ribosomal protein RPL23 interaction) mutations expressed in SBDS-deficient human CD34 + cells increased their myeloid and erythroid colony output, suggesting that EIF6 mutations increased their hematopoietic function. A subsequent study also reported similar loss of function EIF6 mutations in patients with SDS [ 28 ].
Kennedy et al. [ 22 ] found that TP53 mutations were more frequent in SDS patients that progressed to MDS/AML. Differently from EIF6 mutations, TP53 mutations did not lead to ribosome joining defects. TP53 and EIF6 mutations were both detected in some SDS patients, but single-cell DNA sequencing in patients with clonal hematopoiesis confirmed that these mutations were mutually exclusive. Both TP53 and EIF6 mutations in SDS patients could lead to clonal hematopoiesis for years without initiating the development of MDS and/or AML. None of the leukemic clones were observed to harbor EIF6 mutations. Of note, deletion of 20q is a common cytogenetic abnormality in SDS, which results in heterozygous deletion of EIF6 [ 29 ]. The del20q clones have not been associated with clonal evolution to malignancy.
This study has uncovered two distinct pathways in SDS patients by which different cooperating somatic mutations increase the fitness of SDS HSCs, allowing their clonal expansion. Firstly, heterozygous EIF6 inactivation partially corrects the ribosome maturation defect caused by germline SBDS mutations. Crucially, this enhances the fitness of the HSC but has not been associated with progression to leukemia. Secondly, TP53 inactivation gives rise to a maladaptive pathway by subverting normal tumor suppressor checkpoints without correcting the ribosome defect. The transition from SDS to MDS/AML was seen in patients who gained biallelic alterations of the TP53 locus via deletion, CN-LOH, or point mutation. These data can potentially inform the monitoring of SDS patients for high risk of transformation, but further studies are needed to address this prospectively. These data also identified EIF6 as a potential therapeutic target to both improve hematopoiesis and reduce leukemia risk. The identification of SDS patients with high risk of developing leukemia may improve clinical outcomes by enabling preemptive intervention with alloHSCT [ 22 ].
NOVEL THERAPEUTIC OPPORTUNITIES IN FANCONI ANEMIA—DR PAULA RíO
The second webinar presentation focused on a different type of inherited bone marrow failure, FA, which is caused by biallelic mutations in one of 23 genes (FA and FA-like genes such as FANCA ) involved in a molecular pathway responsible for the repair of DNA interstrand crosslinks. Individuals with FA manifest a serious defect in blood cell production, as well as serious complications such as a predisposition to cancer, congenital abnormalities (~70%), and earlyonset bone marrow failure (~80%) [ 30 ].
The only currently available curative therapy for the hematologic abnormalities in FA patients is alloHSCT. However, the conditioning regimens required for this treatment and subsequent graft-versus-host disease are associated with acute toxicity and an amplified incidence of squamous cell carcinoma [ 31 ]. Transplantation of autologous HSCs corrected by gene therapy may potentially circumvent these allogeneic transplant-related complications. Several groups have therefore worked to develop strategies involving autologous HSCs corrected by gene therapy to prevent FA-associated BMF.
The safety and efficacy of autologous HSC gene therapy for FA were recently studied in a phase I/II clinical trial ( ClinicalTrials.gov , {"type":"clinical-trial","attrs":{"text":"NCT03157804","term_id":"NCT03157804"}} NCT03157804 ; European Clinical Trials Database, 2011- 006100-12) [ 32 ]. CD34 + cells were purified from the mobilized blood of FA patients, transduced in a very short protocol with a lentiviral vector encoding the FANCA gene, and then infused back into the patients without any conditioning. The gene-corrected HSCs provided sustained multilineage and polyclonal engraftment and contributed to progressively increasing proportions of gene-corrected cells in the peripheral blood over several years, indicating that they have a proliferative advantage. This is consistent with previous studies reporting that gene-corrected FA patient HSCs had a proliferative advantage over uncorrected HSCs after transplantation into NSG mice, and by the observed expansion of HSC clones in mosaic patients in whom the pathogenic allele is reverted to wild type by a naturally occurring secondary somatic mutation [ 33 , 34 ]. No serious adverse effects were observed in the trial with a maximum follow-up of 30 months in one of the patients; gene therapy stabilized neutrophil counts and hemoglobin levels in patient FA-02002 after 6 months postinfusion, and a similar trend was noted in patients FA-02005 and FA-02006 [ 32 ].
The description of efficient strategies to target HSCs by gene editing and the possibility to specifically correct the gene of interest using designed nucleases prompted us to test the potential of gene editing to correct FA-HSCs [ 35 , 36 ]. Although gene editing mediated by homology-directed repair (HDR) is an ideal strategy to correct the different mutations described in a specific gene, HDR is relatively infrequently active in HSCs and is partially hampered in FA cells, making this approach challenging for FA gene therapy [ 37 - 39 ]. On the contrary, FA HSCs more frequently undergo non-homologous end joining (NHEj) repair of strand breaks [ 40 , 41 ]. NHEJ repair is error-prone and generates a variety of insertions and/or deletions (indels) in the DNA. These indels may be therapeutically exploited to restore FA gene function, mimicking the secondary mutations observed in mosaic patients, by deleting a premature stop codon or correcting a frameshift mutation to restore the open reading frame (ORF).
The restoration of gene function in FA HSPCs by CRISPR Cas9 targeting and NHEj-mediated repair was reported recently [ 42 ]. The first experiments aimed to correct a frameshift mutation in FANCA (c.3558insG, p.R1187EfsX28) that causes a premature stop codon. Naturally occurring secondary deletions near this site have been observed in somatic mosaic patients [ 34 ]. FA cells were electroporated with a plasmid encoding Cas9 and a gRNA targeting the mutation site. Therapeutic, ORF-restoring indels were detected in 7.62% of FANCA alleles 5 days later. After maintaining the cells 25 more days in culture, the proportion of therapeutic indels had increased to 16.45% of alleles. This competitive advantage indicated that the therapeutic indel had restored the function of FANCA and the FA repair pathway, which was confirmed by demonstrating restored FANCA protein levels, resistance to DNA crosslinking agents, and the absence of chromosomal abnormalities.
The same NHEJ-mediated repair strategy was extended to successfully restore FA pathway function in cells carrying a more common mutation in FANCA (c.295C>T) that causes a premature stop codon. Frameshift mutations in FANCB, FANCC, FANCD2, and FANCD1/BRCA2 were similarly targeted, this time by electroporating Cas9/gRNA RNPs; in all cases, therapeutic indel sequences were observed at high frequency, and the recovery of FA pathway function was confirmed by proliferative advantage in culture and resistance to a DNA crosslinker. After Cas9/gRNA treatment, NHEJ-mediated repair was observed in the most primitive CD34 + CD133 + CD90 + HSCs at the same high levels as the overall CD34 + population, and the cells fully retained their ability to engraft in NSG mice after transplantation [ 42 ].
These studies demonstrate the potential of gene therapy to restore HSC function in FA patients and to improve clinical outcomes for patients; they also highlight gene editing by NHEJ repair of CRISPR/Cas9-induced DNA breaks as an efficient, safe approach to the treatment of FA, which could also be applied to other monogenic disorders affecting the hematopoietic system. It is also worth noting that the original idea for using the NHEJ pathway to repair loss-of-function gene mutations in FA came from the careful molecular characterization of the somatic mutations in FA patients displaying mosaicism-associated clonal hematopoiesis [ 34 ].
CONCLUSIONS
Together, these recent studies highlight how the study of bone marrow failure provides an important biological context in which to understand hematopoiesis and to develop novel therapeutic paradigms. Basic and translational research can uncover molecular features that offer better risk stratification for patients, which will facilitate their monitoring for severe complications. Improvements in gene therapies are also affording new and safer approaches to repair defective genes and pathways in inherited bone marrow failure syndromes. These are some of the many ways how laboratory research can translate directly to patients. We are confident that these important advances in the bone marrow failure and gene therapy fields will improve the treatment and cure of these inherited hematological diseases and offer patients a better quality of life.
- Recent developments in bone marrow failure research are explored.
- Distinct mechanisms of clonal evolution and implications for surveillance for patients affected with Shwachman–Diamond syndrome are discussed.
- Recent advances in gene therapies for Fanconi anemia using patients’ own hematopoietic stem cells are reviewed.
Acknowledgments
We thank ISEH staff and the New Investigators Committee for their support. ACW gratefully acknowledges funding support from the Edward P. Evans Foundation, the Leukemia and Lymphoma Society, and the Kay Kendall Leukaemia Fund. AS was funded by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases RC2DK122533.
Conflict of interest disclosure
PR has licensed medicinal products and receives research funding and equity from Rocket Pharmaceuticals, Inc., Patents & Royalties, Research Funding. The remaining authors have no conflicts to declare.

IMAGES
VIDEO
COMMENTS
Bone Research - Molecular mechanisms and clinical management of cancer bone metastasis ... Kawai, H. et al. Characterization and potential roles of bone marrow-derived stromal cells in cancer ...
The bone marrow is the main site for hematopoiesis. It contains a unique microenvironment that provides niches that support self-renewal and differentiation of hematopoietic stem cells (HSC), multipotent progenitors (MPP), and lineage committed progenitors to produce the large number of blood cells required to sustain life. The bone marrow is ...
The current differentiation protocol was optimized primarily to study myeloid malignancies and cancer-associated bone marrow fibrosis, and refinement of the growth factor supplements may improve the maintenance of lymphoid malignancies. ... MRC Weatherall Institute of Molecular Medicine). B. Psaila receives funding from a Cancer Research UK ...
This review seeks to provide an update on the current state of the science in bone metastasis research and give a snap shot of therapies in clinical trials for bone metastatic cancer. ... It has been proposed that adipocytes play a supporting role for cancer cell survival in the bone marrow as an energy source [66, 67]. Bone marrow adipocytes ...
Multiple myeloma (MM), a cancer of bone marrow plasma cells, is the second-most common hematological malignancy. However, despite immunotherapies like chimeric antigen receptor (CAR)-T cells, relapse is nearly universal. The bone marrow (BM) microenvironment influences how MM cells survive, proliferate, and resist treatment. Yet, it is unclear which BM niches give rise to MM pathophysiology ...
Combined, this work presents eBM as a cellular construct that mimics the complex bone marrow environment that is useful for mechanistic bone cancer research and drug screening. Osteosarcoma (OS) is the most common primary, malignant bone tumor in children and adolescents ( 1 ).
Clinical trial requirements can be burdensome and deter patients from participation. Initiatives through ASCO focus on simplifying such protocols in a patient-centered approach to encourage enrollment. 1-3 In clinical practice of follicular lymphoma (FL), utility of bone marrow biopsies (BMB) is controversial. The National Comprehensive Cancer Network (NCCN) guidelines for FL recommend BMB and ...
Advances are revolutionizing research and treatment for osteosarcoma, the most common malignant bone cancer in children. A groundbreaking material — engineered bone marrow (eBM) — has the potential to improve treatment for osteosarcoma, a malignant bone cancer with low survival rates. A new study involving UC Davis researchers published in ...
The presence of adiponectin and IL-6 in the bone marrow microenvironment of lung cancer bone metastasis suggests that BMAs may promote EC growth and new blood vessel formation, providing additional nutrients and pathways for cancer metastasis . 4.2.4 The role of inflammatory factors secreted by BMAs in lung cancer bone metastasis
1 Department of Hematology, Stem Cell Transplant and Cellular Therapy, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia; 2 Division of Hematology-Oncology, Blood and Marrow Transplantation Program, Mayo Clinic, Jacksonville, FL, United States; Diffuse large B cell lymphoma (DLBCL) is one of the most prevalent subtypes of non-Hodgkin lymphoma (NHL) and is known for ...
Objective: To evaluate effects of bone marrow sparing (BMS) radiotherapy on decreasing the incidence of acute hematologic toxicity (HT) for locoregionally advanced cervical cancer (LACC) patients treated by pelvic irradiation. Materials and methods: LACC patients were recruited prospectively from May 2021 to May 2022 at a single center and were evenly randomized into the BMS group and the ...
The new research from UVA Cancer Center pinpoints an important contributor to the unrelenting inflammation associated with a group of blood cancers called myeloproliferative neoplasms. These cancers cause the bone marrow to produce too many blood cells. This leads to symptoms such as headache, fever, fatigue, weakness, bone pain, bleeding and ...
Osteosarcoma (OS) is the most common primary malignant bone cancer in children and adolescents. While many other cancers now have promising therapeutic advances, treatment options for OS have remained unchanged since the introduction of standard chemotherapeutics and offer less than a 25% five-year survival rate for those with metastatic disease.
The relationship between bone and cancer has undergone profound changes in recent years and oncology has to manage with an increase of bone metastases incidence with a radical change of epidemiological data and a strong clinical impact. ... bone marrow suppression and decline of Performance Status. Pain is the most frequent symptoms ...
The poor survival of patients with human malignant gliomas relates partly to the inability to deliver therapeutic agents to the tumor. Because it has been suggested that circulating bone marrow-derived stem cells can be recruited into solid organs in response to tissue stresses, we hypothesized that human bone marrow-derived mesenchymal stem cells (hMSC) may have a tropism for brain tumors ...
A great deal of research is being done to learn more about the genetic changes inside bone cancer cells. Doctors are using what they learn to develop new targeted drugs for some types of bone cancer, as well as to test and use existing targeted drugs that focus on some of these gene changes. These drugs might change the cancer's ability to grow ...
Objective The evaluation of bone marrow (BM) status is an integral part of the initial workup of patients diagnosed with lymphoma as it plays an important role in staging and predicting prognosis in these patients. This article determines the incidence and pattern of BM involvement in lymphoma cases and distinguishes benign from malignant lymphoid aggregates in BM biopsies.
Osteosarcoma (OS) is the most common primary bone malignancy that affects children and young adults. OS is characterized by a high degree of malignancy, strong invasiveness, rapid disease progression, and extremely high mortality rate; it is considered as a serious threat to the human health globally. The incidence of OS is common in the ...
Owl Radio Reading Service for the Blind · 8h · Shared with Public. Follow. Comments
Basic and translational research can uncover molecular features that offer better risk stratification for patients, which will facilitate their monitoring for severe complications. Improvements in gene therapies are also affording new and safer approaches to repair defective genes and pathways in inherited bone marrow failure syndromes.