COVID-19 vaccines: Get the facts
Looking to get the facts about COVID-19 vaccines? Here's what you need to know about the different vaccines and the benefits of getting vaccinated.
As the coronavirus disease 2019 (COVID-19) continues to cause illness, you might have questions about COVID-19 vaccines. Find out about the different types of COVID-19 vaccines, how they work, the possible side effects, and the benefits for you and your family.

COVID-19 vaccine benefits
What are the benefits of getting a covid-19 vaccine.
Staying up to date with a COVID-19 vaccine can:
- Help prevent serious illness and death due to COVID-19 for both children and adults.
- Help prevent you from needing to go to the hospital due to COVID-19 .
- Be a less risky way to protect yourself compared to getting sick with the virus that causes COVID-19.
- Lower long-term risk for cardiovascular complications after COVID-19.
Factors that can affect how well you're protected after a vaccine can include your age, if you've had COVID-19 before or if you have medical conditions such as cancer.
How well a COVID-19 vaccine protects you also depends on timing, such as when you got the shot. And your level of protection depends on how the virus that causes COVID-19 changes and what variants the vaccine protects against.
Talk to your healthcare team about how you can stay up to date with COVID-19 vaccines.
Should I get the COVID-19 vaccine even if I've already had COVID-19?
Yes. Catching the virus that causes COVID-19 or getting a COVID-19 vaccination gives you protection, also called immunity, from the virus. But over time, that protection seems to fade. The COVID-19 vaccine can boost your body's protection.
Also, the virus that causes COVID-19 can change, also called mutate. Vaccination with the most up-to-date variant that is spreading or expected to spread helps keep you from getting sick again.
Researchers continue to study what happens when someone has COVID-19 a second time. Later infections are generally milder than the first infection. But severe illness can still happen. Serious illness is more likely among people older than age 65, people with more than four medical conditions and people with weakened immune systems.
Safety and side effects of COVID-19 vaccines
What covid-19 vaccines have been authorized or approved.
The COVID-19 vaccines available in the United States are:
- 2023-2024 Pfizer-BioNTech COVID-19 vaccine, available for people age 6 months and older.
- 2023-2024 Moderna COVID-19 vaccine, available for people age 6 months and older.
- 2023-2024 Novavax COVID-19 vaccine, available for people age 12 years and older.
These vaccines have U.S. Food and Drug Administration (FDA) emergency use authorization or approval.
In December 2020, the Pfizer-BioNTech COVID-19 vaccine two-dose series was found to be both safe and effective in preventing COVID-19 infection in people age 18 and older. This data helped predict how well the vaccines would work for younger people. The effectiveness varied by age.
The Pfizer-BioNTech vaccine is approved under the name Comirnaty for people age 12 and older. The FDA authorized the vaccine for people age 6 months to 11 years. The number of shots in this vaccination series varies based on a person's age and COVID-19 vaccination history.
In December 2020, the Moderna COVID-19 vaccine was found to be both safe and effective in preventing infection and serious illness among people age 18 or older. The vaccine's ability to protect younger people was predicted based on that clinical trial data.
The FDA approved the vaccine under the name Spikevax for people age 12 and older. The FDA authorized use of the vaccine in people age 6 months to 11 years. The number of shots needed varies based on a person's age and COVID-19 vaccination history.
In July 2022, this vaccine was found to be safe and effective and became available under an emergency use authorization for people age 18 and older.
In August 2022, the FDA authorized the vaccine for people age 12 and older. The number of shots in this vaccination series varies based on a person's age and COVID-19 vaccination history.
In August 2022, the FDA authorized an update to the Moderna and the Pfizer-BioNTech COVID-19 vaccines. Both included the original and omicron variants of the virus that causes COVID-19. In June 2023, the FDA directed vaccine makers to update COVID-19 vaccines. The vaccines were changed to target a strain of the virus that causes COVID-19 called XBB.1.5. In September and October 2023, the FDA authorized the use of the updated 2023-2024 COVID-19 vaccines made by Novavax, Moderna and Pfizer-BioNTech.
How do the COVID-19 vaccines work?
COVID-19 vaccines help the body get ready to clear out infection with the virus that causes COVID-19.
Both the Pfizer-BioNTech and the Moderna COVID-19 vaccines use genetically engineered messenger RNA (mRNA). The mRNA in the vaccine tells your cells how to make a harmless piece of virus that causes COVID-19.
After you get an mRNA COVID-19 vaccine, your muscle cells begin making the protein pieces and displaying them on cell surfaces. The immune system recognizes the protein and begins building an immune response and making antibodies. After delivering instructions, the mRNA is immediately broken down. It never enters the nucleus of your cells, where your DNA is kept.
The Novavax COVID-19 adjuvanted vaccine is a protein subunit vaccine. These vaccines include only protein pieces of a virus that cause your immune system to react the most. The Novavax COVID-19 vaccine also has an ingredient called an adjuvant that helps raise your immune system response.
With a protein subunit vaccine, the body reacts to the proteins and creates antibodies and defensive white blood cells. If you later become infected with the COVID-19 virus, the antibodies will fight the virus. Protein subunit COVID-19 vaccines don't use any live virus and can't cause you to become infected with the COVID-19 virus. The protein pieces also don't enter the nucleus of your cells, where your DNA is kept.
Can a COVID-19 vaccine give you COVID-19?
No. The COVID-19 vaccines available in the U.S. don't use the live virus that causes COVID-19. Because of this, the COVID-19 vaccines can't cause you to become sick with COVID-19.
It can take a few weeks for your body to build immunity after getting a COVID-19 vaccination. As a result, it's possible that you could become infected with the virus that causes COVID-19 just before or after being vaccinated.
What are the possible general side effects of a COVID-19 vaccine?
Some people have no side effects from the COVID-19 vaccine. For those who get them, most side effects go away in a few days.
A COVID-19 vaccine can cause mild side effects after the first or second dose. Pain and swelling where people got the shot is a common side effect. That area also may look reddish on white skin. Other side effects include:
- Fever or chills.
- Muscle pain or joint pain.
- Tiredness, called fatigue.
- Upset stomach or vomiting.
- Swollen lymph nodes.
For younger children up to age 4, symptoms may include crying or fussiness, sleepiness, loss of appetite, or, less often, a fever.
In rare cases, getting a COVID-19 vaccine can cause an allergic reaction. Symptoms of a life-threatening allergic reaction can include:
- Breathing problems.
- Fast heartbeat, dizziness or weakness.
- Swelling in the throat.
If you or a person you're caring for has any life-threatening symptoms, get emergency care.
Less serious allergic reactions include a general rash other than where you got the vaccine, or swelling of the lips, face or skin other than where you got the shot. Contact your healthcare professional if you have any of these symptoms.
You may be asked to stay where you got the vaccine for about 15 minutes after the shot. This allows the healthcare team to help you if you have an allergic reaction. The healthcare team may ask you to wait for longer if you had an allergic reaction from a previous shot that wasn't serious.
Contact a healthcare professional if the area where you got the shot gets worse after 24 hours. And if you're worried about any side effects, contact your healthcare team.
Are there any long-term side effects of the COVID-19 vaccines?
The vaccines that help protect against COVID-19 are safe and effective. Clinical trials tested the vaccines to make sure of those facts. Healthcare professionals, researchers and health agencies continue to watch for rare side effects, even after hundreds of millions of doses have been given in the United States.
Side effects that don't go away after a few days are thought of as long term. Vaccines rarely cause any long-term side effects.
If you're concerned about side effects, safety data on COVID-19 vaccines is reported to a national program called the Vaccine Adverse Event Reporting System in the U.S. This data is available to the public. The U.S. Centers for Disease Control and Protection (CDC) also has created v-safe, a smartphone-based tool that allows users to report COVID-19 vaccine side effects.
If you have other questions or concerns about your symptoms, talk to your healthcare professional.
Can COVID-19 vaccines affect the heart?
In some people, COVID-19 vaccines can lead to heart complications called myocarditis and pericarditis. Myocarditis is the swelling, also called inflammation, of the heart muscle. Pericarditis is the swelling, also called inflammation, of the lining outside the heart.
Symptoms to watch for include:
- Chest pain.
- Shortness of breath.
- Feelings of having a fast-beating, fluttering or pounding heart.
If you or your child has any of these symptoms within a week of getting a COVID-19 vaccine, seek medical care.
The risk of myocarditis or pericarditis after a COVID-19 vaccine is rare. These conditions have been reported after COVID-19 vaccination with any of the vaccines offered in the United States. Most cases have been reported in males ages 12 to 39.
These conditions happened more often after the second dose of the COVID-19 vaccine and typically within one week of COVID-19 vaccination. Most of the people who got care felt better after receiving medicine and resting.
These complications are rare and also may happen after getting sick with the virus that causes COVID-19. In general, research on the effects of the most used COVID-19 vaccines in the United States suggests the vaccines lower the risk of complications such as blood clots or other types of damage to the heart.
If you have concerns, your healthcare professional can help you review the risks and benefits based on your health condition.
Things to know before a COVID-19 vaccine
Are covid-19 vaccines free.
In the U.S., COVID-19 vaccines may be offered at no cost through insurance coverage. For people whose vaccines aren't covered or for those who don't have health insurance, options are available. Anyone younger than 18 years old can get no-cost vaccines through the Vaccines for Children program. Adults can get no-cost COVID-19 vaccines through the temporary Bridges to Access program, which is scheduled to end in December 2024.
Can I get a COVID-19 vaccine if I have an existing health condition?
Yes, COVID-19 vaccines are safe for people who have existing health conditions, including conditions that have a higher risk of getting serious illness with COVID-19.
The COVID-19 vaccine can lower the risk of death or serious illness caused by COVID-19. Your healthcare team may suggest that you get added doses of a COVID-19 vaccine if you have a moderately or severely weakened immune system.
Cancer treatments and other therapies that affect some immune cells also may affect your COVID-19 vaccine. Talk to your healthcare professional about timing additional shots and getting vaccinated after immunosuppressive treatment.
Talk to your healthcare team if you have any questions about when to get a COVID-19 vaccine.
Is it OK to take an over-the-counter pain medicine before or after getting a COVID-19 vaccine?
Don't take medicine before getting a COVID-19 vaccine to prevent possible discomfort. It's not clear how these medicines might impact the effectiveness of the vaccines. It is OK to take this kind of medicine after getting a COVID-19 vaccine, as long as you have no other medical reason that would prevent you from taking it.
Allergic reactions and COVID-19 vaccines
What are the signs of an allergic reaction to a covid-19 vaccine.
Symptoms of a life-threatening allergic reaction can include:
If you or a person you're caring for has any life-threatening symptoms, get emergency care right away.
Less serious allergic reactions include a general rash other than where you got the vaccine, or swelling of the lips, face or skin other than where the shot was given. Contact your healthcare professional if you have any of these symptoms.
Tell your healthcare professional about your reaction, even if it went away on its own or you didn't get emergency care. This reaction might mean that you are allergic to the vaccine. You might not be able to get a second dose of the same vaccine. But you might be able to get a different vaccine for your second dose.
Can I get a COVID-19 vaccine if I have a history of allergic reactions?
If you have a history of severe allergic reactions not related to vaccines or injectable medicines, you may still get a COVID-19 vaccine. You're typically monitored for 30 minutes after getting the vaccine.
If you've had an immediate allergic reaction to other vaccines or injectable medicines, ask your healthcare professional about getting a COVID-19 vaccine. If you've ever had an immediate or severe allergic reaction to any ingredient in a COVID-19 vaccine, the CDC recommends not getting that specific vaccine.
If you have an immediate or severe allergic reaction after getting the first dose of a COVID-19 vaccine, don't get the second dose. But you might be able to get a different vaccine for your second dose.
Pregnancy, breastfeeding and fertility with COVID-19 vaccines
Can pregnant or breastfeeding women get the covid-19 vaccine.
The CDC recommends getting a COVID-19 vaccine if:
- You are planning to or trying to get pregnant.
- You are pregnant now.
- You are breastfeeding.
Staying up to date on your COVID-19 vaccine helps prevent severe COVID-19 illness. It also may help a newborn avoid getting COVID-19 if you are vaccinated during pregnancy.
People at higher risk of serious illness can talk to a healthcare professional about additional COVID-19 vaccines or other precautions. It also can help to ask about what to do if you get sick so that you can quickly start treatment.
Children and COVID-19 vaccines
If children don't often experience severe illness with covid-19, why do they need a covid-19 vaccine.
While rare, some children can become seriously ill with COVID-19 after getting the virus that causes COVID-19 .
A COVID-19 vaccine might prevent your child from getting the virus that causes COVID-19 . It also may prevent your child from becoming seriously ill or having to stay in the hospital due to the COVID-19 virus.
After a COVID-19 vaccine
Can i stop taking safety precautions after getting a covid-19 vaccine.
You can more safely return to activities that you might have avoided before your vaccine was up to date. You also may be able to spend time in closer contact with people who are at high risk for serious COVID-19 illness.
But vaccines are not 100% effective. So taking other action to lower your risk of getting COVID-19 still helps protect you and others from the virus. These steps are even more important when you're in an area with a high number of people with COVID-19 in the hospital. Protection also is important as time passes since your last vaccination.
If you are at higher risk for serious COVID-19 illness, basic actions to prevent COVID-19 are even more important. Some examples are:
- Avoid close contact with anyone who is sick or has symptoms, if possible.
- Use fans, open windows or doors, and use filters to move the air and keep any germs from lingering.
- Wash your hands well and often with soap and water for at least 20 seconds. Or use an alcohol-based hand sanitizer with at least 60% alcohol.
- Cough or sneeze into a tissue or your elbow. Then wash your hands.
- Clean and disinfect high-touch surfaces. For example, clean doorknobs, light switches, electronics and counters regularly.
- Spread out in crowded public areas, especially in places with poor airflow. This is important if you have a higher risk of serious illness.
- The CDC recommends that people wear a mask in indoor public spaces if COVID-19 is spreading. This means that if you're in an area with a high number of people with COVID-19 in the hospital a mask can help protect you. The CDC suggests wearing the most protective mask possible that you'll wear regularly, that fits well and is comfortable.
Can I still get COVID-19 after I'm vaccinated?
COVID-19 vaccination will protect most people from getting sick with COVID-19. But some people who are up to date with their vaccines may still get COVID-19. These are called vaccine breakthrough infections.
People with vaccine breakthrough infections can spread COVID-19 to others. However, people who are up to date with their vaccines but who have a breakthrough infection are less likely to have serious illness with COVID-19 than those who are not vaccinated. Even when people who are vaccinated get symptoms, they tend to be less severe than those felt by unvaccinated people.
Researchers continue to study what happens when someone has COVID-19 a second time. Reinfections and breakthrough infections are generally milder than the first infection. But severe illness can still happen. Serious illness is more likely among people older than age 65, people with more than four medical conditions and people with weakened immune systems.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Benefits of getting a COVID-19 vaccine. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html. Accessed April 15, 2024.
- Mercadé-Besora N, et al. The role of COVID-19 vaccines in preventing post-COVID-19 thromboembolic and cardiovascular complications. Heart. 2024; doi: 10.1136/heartjnl-2023-323483.
- Vaccine effectiveness studies. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/how-they-work.html. Accessed April 15, 2024.
- Goldman L, et al., eds. COVID-19: Epidemiology, clinical manifestations, diagnosis, community prevention, and prognosis. In: Goldman-Cecil Medicine. 27th ed. Elsevier; 2024. https://www.clinicalkey.com. Accessed April 15, 2024.
- Deng J, et al. Severity and outcomes of SARS-CoV-2 reinfection compared with primary infection: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health. 2023; doi:10.3390/ijerph20043335.
- What is COVID-19 reinfection? Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html. Accessed April 15, 2024.
- Stay up to date with COVID-19 vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html. Accessed April 15, 2024.
- Interim clinical considerations for use of COVID-19 vaccines in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed April 15, 2024.
- Comirnaty. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/Comirnaty. Accessed April 15, 2024.
- Spikevax summary basis for regulatory action. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/Spikevax. Accessed April 15, 2024.
- Spikevax package insert. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/Spikevax. Accessed April 15, 2024.
- Overview of COVID-19 Vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html. Accessed April 15, 2024.
- Novavax COVID-19 vaccine, adjuvanted. Food and Drug Administration. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted. Accessed April 15, 2024.
- Pfizer-BioNTech emergency use authorization for unapproved product review memorandum. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/Comirnaty. Accessed April 15, 2024.
- Link-Gelles, et al. Estimates of bivalent mRNA vaccine durability in preventing COVID-19-associated hospitalization and critical illness among adults with and without immunocompromising conditions — VISION network, September 2022-April 2023. MMWR Morbidity and Mortality Weekly Report. 2023; doi:10.15585/mmwr.mm7221a3.
- Updated COVID-19 vaccines for use in the United States beginning in fall 2023. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2023. Accessed April 15, 2024.
- Coronavirus (COVID-19), CBER-regulated biologics: COVID-19 vaccines. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/industry-biologics/coronavirus-covid-19-cber-regulated-biologics. Accessed April 15, 2024.
- Understanding how COVID-19 vaccines work. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html. Accessed April 15, 2024.
- Safety of COVID-19 vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/safety-of-vaccines.html. Accessed April 15, 2024.
- Getting your COVID-19 vaccine. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect.html. Accessed April 15, 2024.
- COVID-19 VIS. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/covid-19.html. Accessed April 15, 2024.
- Allergic reactions after COVID-19 vaccination. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html. Accessed April 15, 2024.
- Orenstein W, et al., eds. Vaccine safety. In: Plotkin's Vaccines. 8th ed. Elsevier; 2024. https://www.clinicalkey.com. Accessed April 15, 2024.
- Vaccine adverse event reporting system (VAERS). Vaccine Adverse Event Reporting System. https://vaers.hhs.gov/. Accessed April 15, 2024.
- V-safe. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/v-safe/index.html. Accessed April 15, 2024.
- Myocarditis and pericarditis following mRNA COVID-19 vaccination. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html. Accessed April 15, 2024.
- Vaccines for children. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/programs/vfc/index.html. Accessed April 15, 2024.
- Bridge access program. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/programs/bridge/index.html. Accessed April 15, 2024.
- COVID-19: What people with cancer should know. National Cancer Institute. https://www.cancer.gov/about-cancer/coronavirus/coronavirus-cancer-patient-information. Accessed April 15, 2024.
- COVID-19 vaccines while pregnant or breastfeeding. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Accessed April 15, 2024.
- Berghella V, et al. COVID-19: Overview of pregnancy issues. https://www.uptodate.com/contents/search. Accessed April 15, 2024.
- How to protect yourself and others. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed April 15, 2024.
- Pediatric data. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#pediatric-data. Accessed April 15, 2024.
- Hygiene and respiratory viruses prevention. Centers for Disease Control and Prevention. https://www.cdc.gov/respiratory-viruses/prevention/hygiene.html. Accessed April 15, 2024.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and coronavirus
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- Long-term effects of COVID-19
- COVID-19 tests
- COVID-19 drugs: Are there any that work?
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Fight coronavirus (COVID-19) transmission at home
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to measure your respiratory rate
- How to take your pulse
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
Related information
- Debunking COVID-19 myths - Related information Debunking COVID-19 myths
- Different types of COVID-19 vaccines: How they work - Related information Different types of COVID-19 vaccines: How they work
- COVID-19 vaccines for kids: What you need to know - Related information COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines Get the facts
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
10 facts about Americans and coronavirus vaccines
The coronavirus pandemic has claimed more than 670,000 lives in the United States as of Sept. 20, and the spread of the highly transmissible delta variant has added new urgency to the federal government’s efforts to vaccinate all Americans against the virus. As the drive to inoculate more people continues, here are 10 facts about Americans and COVID-19 vaccines, based on an August Pew Research Center survey of more than 10,000 U.S. adults.
Pew Research Center published this analysis to examine how COVID-19 vaccination patterns in the United States differ by demographic, religious and political factors, and to assess broader public attitudes on key questions related to coronavirus vaccines. The findings in this analysis are based primarily on a survey of 10,348 U.S. adults, conducted from Aug. 23 to 29, 2021.
Everyone who took part in the survey is a member of Pew Research Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way, nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology . Here are the questions asked in the August survey, along with responses, and its methodology .
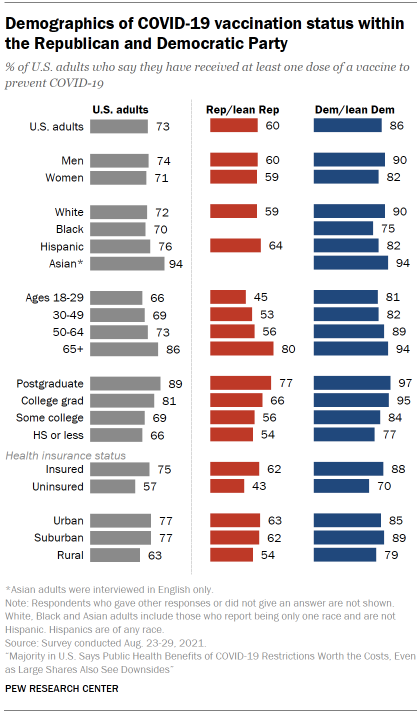
Around three-quarters of U.S. adults (73%) said in August that they had received at least one dose of a COVID-19 vaccine, with the vast majority in this group saying they were fully vaccinated. The survey findings align closely with administrative data from the Centers of Disease Control and Prevention, which, as of early September, showed that three-quarters of adults had received at least one vaccine dose. Surveys have generally aligned closely with the CDC’s vaccination data.
Democrats are far more likely than Republicans to have received at least one COVID-19 vaccine dose, though majorities in both groups say they have done so. Among Democrats and independents who lean to the Democratic Party, 86% said they were at least partially vaccinated as of August, compared with six-in-ten Republicans and GOP leaners. Factoring in ideology as well as party affiliation, nine-in-ten self-described liberal Democrats said they had received at least one dose, compared with 83% of conservative or moderate Democrats, 63% of moderate or liberal Republicans and 58% of conservative Republicans.
In both parties, older people and those with higher levels of education are more likely to have received at least one dose. As of August, 86% of Americans ages 65 and older said they were at least partially vaccinated, compared with 73% of those ages 50 to 64, 69% of those 30 to 49 and 66% of those 18 to 29. Older Americans were among the first groups to become eligible for COVID-19 vaccines due to the increased risk of severe illness they face from the virus.
Americans with more formal schooling are also more likely to be vaccinated. Around nine-in-ten of those with a postgraduate degree or more (89%) said they had received at least one dose by August, compared with smaller shares of those with a bachelor’s degree (81%), some college education (69%) or a high school diploma or less (66%).
These patterns appear in both partisan coalitions. Among Republicans, for example, 80% of those ages 65 and older said they had received at least one dose by August – far higher than the share of younger Republicans who had done so.
Unlike earlier in the pandemic, Black and White adults are about equally likely to say they have been vaccinated. Around seven-in-ten Black and White Americans (70% and 72%, respectively) said they had received at least one vaccine dose as of August. Earlier in the outbreak, Black adults were less likely than White adults to say they planned to get a COVID-19 vaccine or had already done so.
Other major racial and ethnic groups also reported high vaccination rates in the August survey. Around three-quarters of Hispanic adults (76%) said they were at least partially vaccinated, as did 94% of Asian adults. It’s important to note, however, that Asian adults were surveyed in English only and may not necessarily be representative of all Asian Americans.
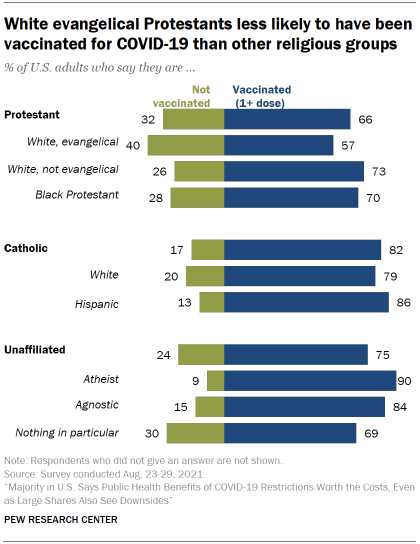
Vaccination patterns vary considerably among major U.S. religious groups. Around eight-in-ten Catholic adults (82%) said they were at least partially vaccinated as of August – a figure that included 86% of Hispanic Catholics and 79% of White Catholics. Among Protestants, 73% of White non-evangelicals and 70% of those who are Black said they had received at least dose, but the share was considerably lower among White evangelical Protestants (57%).
When it comes to religiously unaffiliated Americans, three-quarters said they were at least partially vaccinated as of August. But there were differences within this group too: Nine-in-ten atheists said they had received at least one dose, compared with somewhat smaller shares of agnostics (84%) and those who describe their religion as “nothing in particular” (69%).
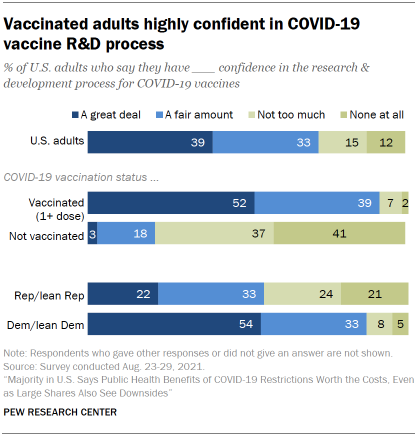
Vaccination status is strongly linked with confidence in the vaccine research and development process. Around nine-in-ten adults who were at least partially vaccinated as of August (91%) said they had a great deal or fair amount of confidence in the vaccine R&D process, compared with only 21% of those who were not vaccinated. Only 3% of unvaccinated adults said they had a great deal of confidence in the vaccine R&D process.
Americans who are not inoculated against COVID-19 express a range of concerns about the vaccines. The August survey asked Americans whether various statements about COVID-19 vaccines describe them very well, somewhat well, not too well or not at all well. At least eight-in-ten unvaccinated adults said the following statements describe them very or somewhat well: “There’s too much pressure on Americans to get a COVID-19 vaccine” (88%); “We don’t really know yet if there are serious health risks from COVID-19 vaccines” (81%); and “Public health officials are not telling us everything they know about COVID-19 vaccines” (80%). Seven-in-ten unvaccinated adults also said the following statement describes them very or somewhat well: “It’s hard to make sense of all the information about COVID-19 vaccines.”
The survey also asked Americans for their reactions to changing public health guidance around some aspects of the pandemic, including information about how the virus spreads and how effective masks are in limiting its spread. Three-quarters or more of unvaccinated Americans expressed negative reactions to the changing guidance, such as by saying it made them wonder if public health officials were holding back important information (78%) and that it made them less confident in public health officials’ recommendations (75%).
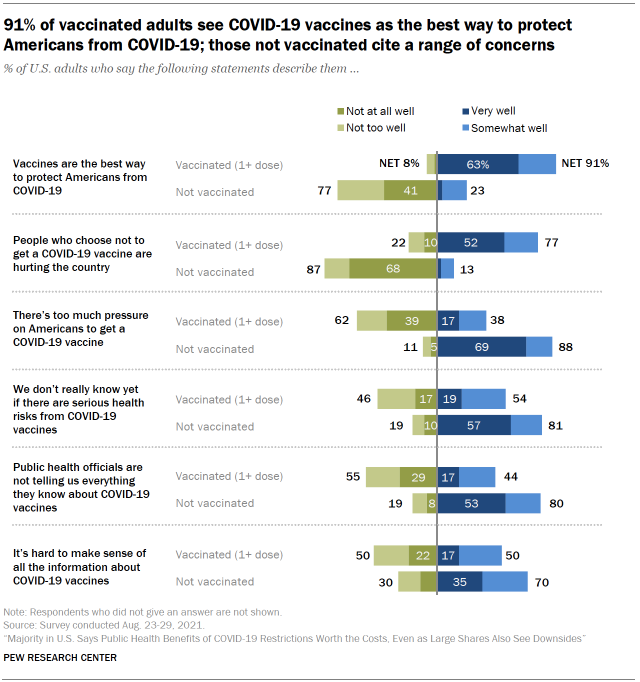
Most vaccinated Americans – but not all – say they would probably get a booster shot if recommended by public health officials. The U.S. Food and Drug Administration recently approved an additional dose of a COVID-19 vaccine for Americans with weakened immune systems, and booster shots could be in the works for a broader swath of the public, too. In August, a large majority of vaccinated adults – amounting to 62% of all U.S. adults – said they would probably get a vaccine booster if recommended. But a small segment of vaccinated adults – amounting to 10% of all adults – said they would probably not get a booster shot.
Americans have mixed views about vaccine requirements in public places. Majorities said in August that they favor requiring adults to show proof of COVID-19 vaccination to travel by airplane (61%), attend public colleges and universities in person (57%) and go to a sporting event or concert (56%). But the public was evenly divided over such a requirement for eating inside a restaurant (50% favored it, 50% opposed it), and slightly more likely to oppose than favor proof of vaccination for shopping inside stores and businesses (54% vs. 45%).
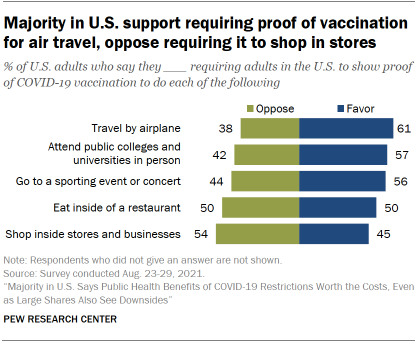
Americans’ views on these questions were closely linked to party affiliation: Majorities of Democrats favored requiring proof of vaccination in each of these five settings, while majorities of Republicans were opposed in all five cases. Public attitudes were also tied to vaccination status, with majorities of vaccinated adults favoring proof of vaccination in all five settings and unvaccinated adults overwhelmingly opposed.
The public was also divided over how U.S. businesses should approach vaccinations. Around four-in-ten adults (39%) said most businesses should require employees to get a COVID-19 vaccine, while 35% say businesses should encourage but not require it. A quarter of adults said businesses should neither require nor encourage it. Here, too, views were linked to party affiliation and vaccination status. The survey was conducted before President Joe Biden announced that employers with more than 100 workers would be required to have their employees vaccinated or tested weekly for COVID-19.
Few Americans know there is little access to COVID-19 vaccines in developing countries – and few say it should be a top priority for the U.S. to provide such nations with large numbers of vaccines. Only around a quarter of U.S. adults (26%) correctly said in August that very few people in developing countries have access to coronavirus vaccines.
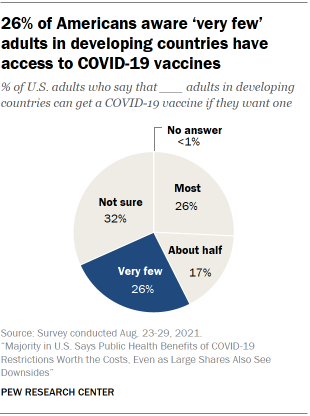
On a separate question, 26% of adults said it should be a top priority for the U.S. to provide large numbers of COVID-19 vaccines to developing countries. Around half (49%) said it should be an important priority but not a top priority, while 23% said that it should be a lower priority or that it should not be done at all. Democrats were more than three times as likely as Republicans to say it should be a top priority for the U.S. to provide large numbers of vaccines to developing countries (39% vs. 11%); Republicans, in turn, were roughly four times as likely as Democrats to say it should be a lower priority or should not be done at all (40% vs. 9%).
Note: This is an update of a post originally published March 23, 2021. Here are the questions asked in the August survey, along with responses, and its methodology .
- Coronavirus (COVID-19)
- COVID-19 & Politics
- COVID-19 & Science
- Health Policy
- Religion & Science

Cary Funk is director of science and society research at Pew Research Center .

John Gramlich is an associate director at Pew Research Center .
How Americans View the Coronavirus, COVID-19 Vaccines Amid Declining Levels of Concern
Online religious services appeal to many americans, but going in person remains more popular, about a third of u.s. workers who can work from home now do so all the time, how the pandemic has affected attendance at u.s. religious services, mental health and the pandemic: what u.s. surveys have found, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
- Contact Tracing
- Pandemic Data Initiative
- Events & News
- Primer on COVID-19 Vaccine
- COVID-19 Vaccine Matters
JHU has stopped collecting data as of
After three years of around-the-clock tracking of COVID-19 data from...
Vaccine Research & Development
How can covid-19 vaccine development be done quickly and safely, typical vaccine development timeline.
- Each clinical trial phase follows completion of the prior phase
- Can take a long time to accumulate cases to assess vaccine efficacy outside pandemic
- Manufacturing capacity is scaled-up after phase III trial and regulatory approval
Accelerated timeline in a pandemic
- Some clinical trial phases are combined
- Cases accumulate rapidly to assess vaccine efficacy because of the pandemic
- Manufacturing capacity is scaled up during the clinical trials but at financial risk
Typical Timeline
A typical vaccine development timeline takes 5 to 10 years, and sometimes longer, to assess whether the vaccine is safe and efficacious in clinical trials, complete the regulatory approval processes, and manufacture sufficient quantity of vaccine doses for widespread distribution.
to the Accelerated Timeline
Preclinical Trials
Preclinical testing of vaccine candidates typically starts in animal models, first in small mammals such as mice or rats and then non-human primates such as monkeys. Preclinical studies are important for eliminating potential vaccines that are either toxic or do not induce protective immune responses. But many vaccines that appear to be safe and induce protective immune responses in animals fail in human studies. Only vaccine candidates that are very promising in preclinical testing move forward into phase I clinical trials.
Phase I Clinical Trials to Assess Safety, Dosing, and Immune Responses
Phase I clinical trials are the first step in assessing vaccines in people. Typically involving one to several dozen healthy volunteers, phase I trials assess short-term safety (e.g., soreness at the site of injection, fever, muscle aches) and immune responses, often with different vaccine dosages. Only if a vaccine candidate is shown to be safe in phase I trials will it move to larger phase II trials.
Phase 1 trials can be completed in two to three months, allowing for two doses of a vaccine three to four weeks apart
Phase II Clinical Trials to Assess Safety and Immune Responses
Phase II clinical trials continue to assess safety and immune responses but in a larger number and more diverse group of volunteers, typically one to several hundred people. Phase II trials may include target populations of a specific age or sex, or those with underlying medical conditions. Vaccines for children start with adult volunteers and move to progressively younger groups of children. Different types of immune responses are often measured, including antibodies and cell-mediated immunity, but phase II trials do not assess how well a vaccine actually works. Only in phase III trials is vaccine efficacy assessed.
Phase 2 trials can be completed in three to four months, allowing for longer follow-up to better assess safety and immunogenicity. This timeline is shortened when phase 1 and phase 2 trials are combined.
Phase III Clinical Trials to Assess Safety and Efficacy
Phase III clinical trials are critical to understanding whether vaccines are safe and effective. Phase III trials often include tens of thousands of volunteers. Participants are chosen at random to receive the vaccine or a placebo. In Phase III, participants and most of the study investigators do not know who has received the vaccine and who received the placebo. Participants are then followed to see how many in each group get the disease. Assessing short- and long-term safety is also a major goal of phase 3 trials.
Phase 3 trials may take six to nine months to allow early assessment of safety and efficacy, particularly if conducted in areas with a high risk of infection, but with follow-up continuing for two years or more to assess long-term safety and efficacy.
Regulatory Approval Process
Each country has a regulatory approval process for vaccines. In the United States, the Food and Drug Administration (FDA) is responsible for regulating vaccines. In situations when there is good scientific reason to believe that a vaccine is safe and is likely to prevent disease, the FDA may authorize its use through an Emergency Use Authorization (EAU) even if definitive proof of the efficacy of the vaccine is not known, especially for diseases that cause high mortality.
Scaling Up Vaccine Manufacturing
Scaling up vaccine manufacturing is typically done near the end of the regulatory process because of the huge financial investment needed. In the United States, the FDA will inspect the manufacturing facilities. The cost of developing a new vaccine can be several billion U.S. dollars prior to the scale up of manufacturing facilities.
Post-Licensure Vaccine Safety Monitoring
After a vaccine is approved and in widespread use, it is critically important to continue to monitor vaccine safety. Some very rare side effects may only be detectable when large numbers of people have been vaccinated. Safety concerns that are discovered at this late stage could lead a licensed vaccine to be withdrawn from use, although this is very rare.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 May 2024
Vaccine effectiveness against emerging COVID-19 variants using digital health data
- Tanner J. Varrelman ORCID: orcid.org/0000-0002-8766-0129 1 ,
- Benjamin Rader 1 , 2 ,
- Christopher Remmel 1 ,
- Gaurav Tuli 1 ,
- Aimee R. Han ORCID: orcid.org/0000-0001-8927-3432 1 ,
- Christina M. Astley ORCID: orcid.org/0000-0002-5063-8470 1 , 3 , 4 , 5 &
- John S. Brownstein ORCID: orcid.org/0000-0001-8568-5317 1 , 4
Communications Medicine volume 4 , Article number: 81 ( 2024 ) Cite this article
514 Accesses
3 Altmetric
Metrics details
- Computational biology and bioinformatics
Participatory surveillance of self-reported symptoms and vaccination status can be used to supplement traditional public health surveillance and provide insights into vaccine effectiveness and changes in the symptoms produced by an infectious disease. The University of Maryland COVID Trends and Impact Survey provides an example of participatory surveillance that leveraged Facebook’s active user base to provide self-reported symptom and vaccination data in near real-time.
Here, we develop a methodology for identifying changes in vaccine effectiveness and COVID-19 symptomatology using the University of Maryland COVID Trends and Impact Survey data from three middle-income countries (Guatemala, Mexico, and South Africa). We implement conditional logistic regression to develop estimates of vaccine effectiveness conditioned on the prevalence of various definitions of self-reported COVID-like illness in lieu of confirmed diagnostic test results.
We highlight a reduction in vaccine effectiveness during Omicron-dominated waves of infections when compared to periods dominated by the Delta variant (median change across COVID-like illness definitions: −0.40, IQR[−0.45, −0.35]. Further, we identify a shift in COVID-19 symptomatology towards upper respiratory type symptoms (i.e., cough and sore throat) during Omicron periods of infections. Stratifying COVID-like illness by the National Institutes of Health’s (NIH) description of mild and severe COVID-19 symptoms reveals a similar level of vaccine protection across different levels of COVID-19 severity during the Omicron period.
Conclusions
Participatory surveillance data alongside methodologies described in this study are particularly useful for resource-constrained settings where diagnostic testing results may be delayed or limited.
Plain language summary
Surveys that are sent out to users of social media can be used to supplement traditional methods to monitor the spread of infectious diseases. This has the potential to be particularly useful in areas where other data is unavailable, such as areas with less surveillance of infectious disease prevalence and access to infectious disease diagnostics. We used data from a survey available to users of the social media platform Facebook to collect information about any potential symptoms of COVID-19 infection and vaccines received during the COVID-19 pandemic. We found a potential reduction in vaccine effectiveness and change in symptoms when the Omicron variant was known to be circulating compared to the earlier Delta variant. This method could be adapted to monitor the spread of COVID-19 and other infectious diseases in the future, which might enable the impact of infectious diseases to be recognized more quickly.
Similar content being viewed by others

Swift and extensive Omicron outbreak in China after sudden exit from ‘zero-COVID’ policy

Population-scale longitudinal mapping of COVID-19 symptoms, behaviour and testing
Real time monitoring of COVID-19 intervention effectiveness through contact tracing data
Introduction.
Timely identification of alterations in vaccine effectiveness (VE) with the emergence of novel COVID-19 variants, such as Omicron, is important for informing the global public health response. The attributable risk proportion of vaccine-preventable diseases is often estimated using relative risk measures obtained from cohort studies or odds ratios determined through case-control designs, which typically rely on gold-standard diagnostic testing 1 , 2 . These studies are conducted retrospectively, leading to a lag between variant emergence and VE estimates. In an effort to provide timely VE insights, monitoring systems have been developed that leverage digital health data 3 , 4 . However, even these real-time methodologies are bounded by some form of diagnostic testing data, whether it be self-reported or through other means of collection. While resource-rich locales across the world have managed to scale up diagnostic testing to inform pandemic response efforts, many low-and middle-income countries (LMICs) have struggled to establish widespread testing 5 , 6 , therefore limiting the applicability of current VE monitoring systems. Alternatively, digital health surveys of self-reported symptoms and vaccination status provide a data source that may be used in place of limited/delayed testing data 7 , 8 , 9 .
In this study, we use data from the University of Maryland Global COVID Trends and Impact Survey (UMD-CTIS) to develop a methodology to simultaneously characterize potential changes in VE and COVID-19 symptomatology for Delta and Omicron-dominated periods of infections. UMD-CTIS is a digital health survey that leveraged Facebook’s active user base, providing cross-sectional survey data in near real-time from 114 countries, starting in 2020 and ending in 2022. Our analyses utilize aggregate data from three MICs that were selected based on the quality of UMD-CTIS data and the presence of distinct Delta and Omicron periods of infections. The selected countries include Guatemala, Mexico, and South Africa. Our analyses of this data reveal reduced vaccine effectiveness against suspected COVID-19 infection during the Omicron period compared to Delta, as well as a shift towards more upper respiratory-type symptoms like cough and sore throat.

Syndromic surveillance data
The University of Maryland Global COVID Trends and Impact Survey (UMD-CTIS), in partnership with Facebook, is a cross-sectional survey that sampled Facebook’s active user base on a daily basis. Facebook users were presented an invitation at the top of their news feed, inviting them to participate in the survey. It is important to note that survey invitations did not include any type of incentive, and participation was driven purely by individuals’ willingness to contribute to digital health. If an individual decided to accept the invitation, they were navigated off of the Facebook platform to the digital health survey hosted by Qualtrics, with data collection being performed by the Joint Program in Survey Methodology at the University of Maryland. On the Qualtrics survey itself, respondents were shown the consent page explaining the purpose of the research to gain a better public understanding of where and how the coronavirus pandemic is spreading, that the survey would take 3–5 min, and that their responses would remain confidential and anonymous. After providing informed consent and confirmation of being at least 18 years of age, respondents could proceed with the survey. Survey respondents and non-respondents were entered back into the sampling pool after a duration of a few weeks or months, depending on the sample size for a given area. Survey data included self-reported information such as demographics, recent symptoms, and COVID-19 vaccination status. While Facebook acts as the survey sampling frame, the company cannot access individually identified respondent answers. Further, to work with these data, institutions must have a signed Data Use Agreement (data access and survey questions available https://covidmap.umd.edu ) 7 , 10 , which our institution signed in order to access and analyze the UMD-CTIS data. Boston Children’s Hospital Institutional Review Board (P00023700) approved this study using UMD-CTIS data. Additional details on the survey design, methodology, and validation can be found in Astley et al. (2021) 7 .
To select the study locations, we began by focusing on countries that met three criteria: they are included in the UMD-CTIS sample, have encountered distinct waves of COVID-19 infections primarily driven by the Delta and Omicron variants, and are considered a low or middle-income country as described by the Organization for Economic Co-operation and Development (OECD). Next, we visualized the time-series symptom data and ruled out countries where the UMD-CTIS data was noticeably erratic.
Using peak detection (Python (3.8.2), scipy.signal.argrelextrema (1.7.1), order parameter = 70) for all CLI time series (April 2021–February 2022), we infer 2-week consensus variant periods prior to each peak, for Delta and Omicron, respectively, for Guatemala (peak date September 13, 2021 [survey No. 4137] and peak date February 2, 2022 [survey No. 2387]), South Africa (July 22, 2021 [survey No. 7371] and December 19, 2021 [survey No. 5320]), and Mexico (August 22, 2021 [survey No. 52775] and January 26, 2022 [survey No. 71990]), that coincided with >80% variant share per public reports 11 .
Statistics and reproducibility
We utilize conditional logistic regression to estimate the attributable risk proportion (ARP) for illness in 2-dose vaccinated individuals (clogit function with method=’approximate’, R (4.1.1), survival library (3.2-13)). VE is given by VE = ARP ≈ 1−OR. We consider exposure as the vaccination status of a respondent (unvaccinated vs. 2-dose vaccinated), and the outcome as to whether a respondent reported CLI in past 14 days, with missing symptoms assumed absent. We also include strata for dichotomized age (>44 years), gender (male/female), and country of the survey respondent to limit potential confounding and differences in country-level sampling. Importantly, UMD-CTIS does not collect data on vaccine formulation. Consequently, we cannot definitively determine whether a single dose of any specific vaccine within our dataset consistently provides full protection, as seen with the Janssen COVID-19 vaccine formulation. Therefore, we have chosen not to include individuals who have received only one dose in this study. Age and gender were dichotomized in order to maintain sufficient sample sizes per stratum. We do not filter the individual vaccine effectiveness estimates by p -value, as we are interested in the group behavior of the CLI definitions and not the hypothesis of whether a single definition of CLI produces a statistically significant vaccine effectiveness estimate. Moreover, to maintain the same number of data points for each of our comparisons, we do not remove outlier data from the analyses in this study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
To estimate VE, we adapted case-control methods 1 for prevalent COVID-like illness (CLI) as a proxy for confirmed COVID-19 cases. Therefore, our estimates of VE measure a vaccine’s ability to prevent suspected symptomatic infections defined by CLI. To allow for changes in variant-specific symptomatology, we iterate across all possible CLI defined by 66 pair-wise combinations of 12 self-reported symptoms (fever, cough, difficulty breathing, fatigue, stuffy or runny nose, aches or muscle pain, sore throat, chest pain, nausea, loss of smell or taste, headache, chills). We then cluster the vaccine effectiveness estimates according to a single symptom of interest and evaluate the median vaccine effectiveness across all CLI definitions in the cluster. As an example, using a COVID-19-specific symptom (loss of smell or taste) as an anchor symptom, we evaluate VE estimates for all CLI definitions inclusive of this symptom during Delta and Omicron waves of infections, resulting in VE estimates for 11 pairwise combinations of symptoms. Consistent with previous estimates of VE that used PCR test data as the outcome 2 , our analyses reveal a median VE Delta of 0.77, IQR[0.76, 0.80] (Fig. 1 a, triangle). In comparison, analyzing the data from the Omicron period reveals a median VE Omicron of 0.47, IQR[0.41, 0.53] (Fig. 1 a, circle). Further expanding the approach to all CLI definitions reveals a median VE Delta of 0.71, IQR[0.65, 0.75] (Fig. 1 b). In contrast, the VE Omicron estimate is even lower (median 0.29, IQR[0.20, 0.38]). Notably, our findings align with those from a recent meta-analysis study focused on real-world vaccine effectiveness for fully vaccinated individuals. This study reported a VE of 70.9% (95% CI, 68.9–72.7) against Delta infections and a VE of 23.5% (95% CI, 17.0–29.5) against Omicron variant infections 12 . To understand how VE estimates for each CLI definition vary by wave, we take the difference between the two VE period estimates (VE Omicron −VE Delta ) for each CLI definition. Doing so reveals a median within-CLI definition change of −0.40, IQR[−0.45, −0.35] (Fig. 2 a), suggesting lower VE Omicron regardless of the CLI definition that is used. Additionally, we find that the pattern of change in VE across CLI definitions is similar when evaluating individual country estimates (see Supplementary Fig. 1 ).
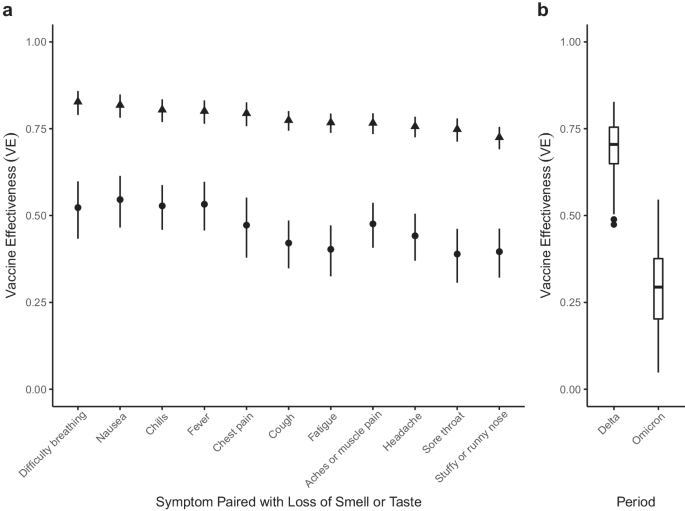
a VE estimates for symptoms paired with the loss of smell or taste for the Delta (triangle) and Omicron (circle) periods. 95% confidence intervals are calculated for each VE estimate, with Delta and Omicron period estimates derived from 64,283 and 79,697 survey responses, respectively. b Box and whisker plot of VE estimates across all 66 possible CLI defined by pairwise combinations of symptoms for Delta and Omicron periods. The box represents the interquartile range (IQR) of estimates, with the horizontal line inside the box indicating the median. The whiskers extend to the largest/smallest values up to 1.5 times the IQR. Outlier values are represented as points. The sample size for each VE estimate is consistent with the sample sizes described in panel ( a ).
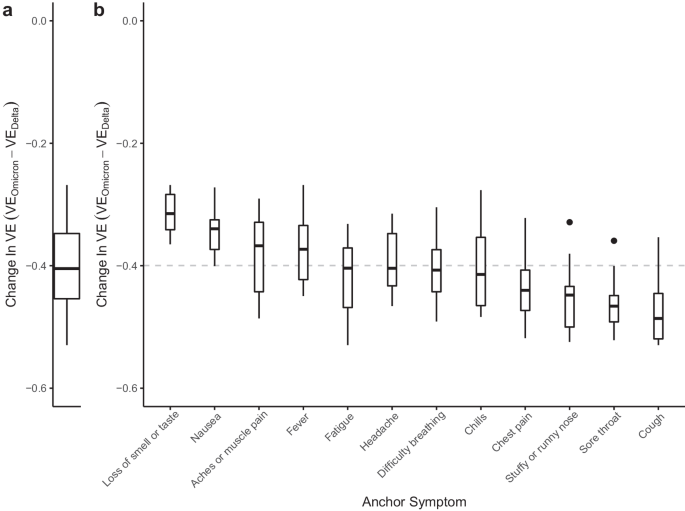
a Distribution of within-CLI change (VE Omicron −VE Delta ) across all CLI definitions. b Distributions of VE Omicron −VE Delta among CLI definitions within each anchor symptom. Each box-plot contains estimates for an anchor symptom paired with the 11 other symptoms. Box-plots are ordered according to the magnitude of the median change, with the median across all VE indicated by the gray dashed line. Each box represents the interquartile range (IQR) of estimates, with the horizontal line inside the box indicating the median. The whiskers extend to the largest/smallest values up to 1.5 times the IQR. Outlier values are represented as points. Each VE estimate from the Delta and Omicron periods is derived from 64,283 and 79,697 survey responses, respectively.
To identify potential alterations in COVID-19 symptomatology, we evaluate the change in VE estimates for CLI definitions with a single anchor symptom, like loss of smell and taste. We reason that if symptoms are similar across variants, the within-anchor median change in VE will be similar across anchor symptoms. Our analyses provide evidence for a potential change in COVID-19 symptomatology from the Delta period to the Omicron period, as we note that some symptoms have more or less decline in VE (Fig. 2 b). Specifically, we find that CLI definitions that include loss of smell or taste have the smallest median change in VE (median: −0.31, IQR[−0.34, −0.28]), while definitions with the largest median change include a cough, or sore throat (cough median: −0.49, IQR[−0.52, −0.45]; sore throat median: −0.47, IQR[−0.49, −0.45]). The observed pattern of change in VE across anchor symptoms is similar when evaluating VE estimates from individual countries (see Supplementary Fig. 2) , however, with increased uncertainty in estimates as measured by the span of anchor symptom distributions (see Supplementary Results ). Similarly, a survey-based study that used PCR testing data as the outcome demonstrated a shift away from symptomatology that includes loss of smell or taste and towards upper-respiratory type symptoms (i.e., sore throat) during the Omicron period 13 . Furthermore, a study conducted in Jalisco, Mexico, analyzed reported symptoms for confirmed infections with wild-type SARS-CoV-2, Delta, and Omicron variants, revealing that Omicron infections were linked to a higher incidence of runny nose and sore throat, aligning with the findings of our country-level analysis for Mexico (see Supplementary Fig. 3) 14 . These results corroborate our overall findings, which also identified increased reporting of sore throat during a wave of COVID-19 infections dominated by the Omicron variant. Collectively, these findings suggest a shift in symptomatology associated with the Omicron variant towards more upper respiratory-type symptoms.
In addition to providing insights into changes in COVID-19 symptomatology, the VE estimates also include information about a vaccine’s ability to protect against COVID-19 illness presenting at different levels of severity as defined by pairwise combinations of symptoms. Importantly, we do not have information about the true severity of each respondent’s reported illness, and we instead infer severity based on the presence and absence of key symptoms. For instance, all CLI definitions that include at least a fever, cough, aches or muscle pain, sore throat, nausea, loss of smell or taste, or a headache in the absence of difficulty breathing or chest pain are considered mild syndromes. However, according to the NIH, CLI definitions that include difficulty breathing or chest pain are considered more severe forms of illness 15 . To understand potential changes in VE against mild and severe COVID-19 syndromes, we partition our CLI-informed VE estimates according to the above classifications. As a result, we end up with 42 mild and 21 severe definitions of CLI. We find that severe definitions of illness were more protected than mild definitions during the Delta period (median severe VE: 0.74, IQR[0.70, 0.79], median mild VE: 0.54, IQR[0.45, 0.64]) (Fig. 3 ). However, protection against mild and severe illness was similar during Omicron (median severe VE: 0.30, IQR[0.25, 0.38], median mild VE: 0.22, IQR[0.16, 0.33]). Importantly, VE against severe illness may appear higher, as vaccines are producing milder illness when an individual is infected with COVID-19 16 , making it seem as if VE against mild illness is less effective. During the Delta wave of infections, we observed a total of 13,220 reports of mild illness and 5316 reports of severe illness. In contrast, during the Omicron wave of infections, there were 24,408 reports of mild illness and 10,234 reports of severe illness.
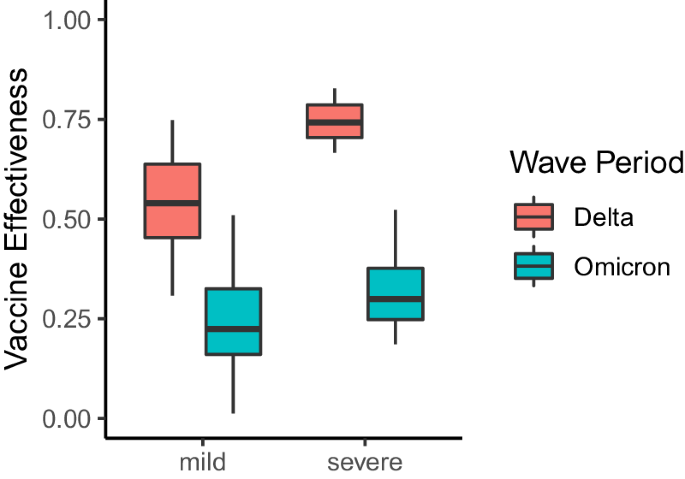
VE estimates for pairwise combinations of symptoms that include a fever, cough, aches or muscle pain, sore throat, nausea, loss of smell or taste, or a headache in the absence of difficulty breathing or chest pain (mild illness), and pairwise combinations of symptoms that include difficulty breathing or chest pain (severe illness). Each box represents the interquartile range (IQR) of estimates, with the horizontal line inside the box indicating the median. The whiskers extend to the largest/smallest values up to 1.5 times the IQR. Outlier values are represented as points. Each VE estimate from the Delta and Omicron periods is derived from 64,283 and 79,697 survey responses, respectively.
It is critical to note that our estimates of VE measure the preventable syndrome attributed to receiving 2-doses of vaccine and represent only one of many components that contribute to true vaccine effectiveness. For instance, we are unable to account for asymptomatic breakthrough infections, and we do not have information on natural immunity among the unvaccinated nor on vaccine formulation or timing for the vaccinated. Therefore, we do not have enough information to distill whether changes in VE are caused by waning vaccine immunity, or increased penetration of an emerging variant. To this end, we would suggest that future digital health surveys include information on vaccine formulation, the general timing of vaccination, as well as information on booster doses that have been administered. While quickly adapting a digital health survey is a monumental task, it would enhance the capabilities of methods such as those described in this study. Furthermore, our VE estimates are solely derived from self-reported survey data and are thus vulnerable to a range of biases 17 . For instance, self-report bias is likely influenced by the perception around COVID-19 vaccination at a given time for a given locale. Even so, a U.S.-based survey that incorporated viral testing demonstrated that self-reported vaccination is a strong predictor for true vaccination status 18 , thus providing support for self-reported measures. Further, our estimates rely on the assumption that the range of self-reported CLI definitions defined in this study is a valid proxy for incident COVID-19 infection. Consequently, our VE estimates may be an underestimation if CLI is capturing non-COVID illness. We limit this assumption by selecting time periods reflective of when COVID-19 is circulating within the unvaccinated population of survey respondents for each country.
Although the assumptions mentioned above limit the interpretation of our VE estimates, the methodology still demonstrates notable strengths that should not be discounted. For example, simple surveys that collect self-reported symptoms and vaccination status can be collected rapidly and at a fraction of the cost of traditional surveillance measures 19 . Moreover, while we performed the retrospective analysis with knowledge of specific COVID-19 variants, CLI-informed VE estimates can be derived during suspected variant spread, with careful contextualization of a country’s epidemiological situation (i.e., absence of co-circulating pathogens and sufficient geographic coverage of surveys). In the case of UMD-CTIS, there was a two-week delay between survey completion and its availability for our modeling, allowing us to use it as a valuable near-real-time dataset for VE analyses. It is critical to note that UMD-CTIS collected a substantial number of survey samples from numerous countries, enabling meaningful insights into COVID-19. However, some countries within the UMD-CTIS sample exhibited noisy data, characterized by high variability in the number of reported CLI instances between time steps, which limited the utility of these specific datasets. While UMD-CTIS has yielded valuable data from a wide range of countries, it’s important to acknowledge that the determination of survey sampling intensity, size, and other attributes of sampling can impact the reliability and applicability of findings. To truly understand the minimum number of samples required for robust statistical analyses, further research, and investigation into these sampling parameters are essential. Such efforts will not only enhance the effectiveness of syndromic surveillance but also contribute to more accurate and comprehensive insights into COVID-19 dynamics.
Historically, understanding the impact of infectious diseases, including the effectiveness of vaccination, has relied on detailed clinical data, often gathered through sentinel surveillance networks 20 . For example, the CDC’s U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) provides information about symptom prevalence for suspected flu cases across the United States over time. While an invaluable resource, ILINet is limited to individuals seeking medical care due to its reliance on sentinel providers for data collection. Therefore, individuals who lack access to such sentinel providers or those who do not seek care will not be represented in these data. Consequently, epidemiological parameters derived from these data may not be entirely representative of the population of interest. Participatory digital surveillance systems like Flu Near You, the ZOE App, and UMD-CTIS enable broader symptom tracking by collecting data directly from the public 3 , 21 . These community-based data sources can provide complementary signals to those derived through clinical data-dependent systems like ILINet 22 . Our analysis of self-reported symptoms from UMD-CTIS demonstrates how digital health data can also be rapidly utilized to infer symptomatic shifts across populations, with the advantage of timeliness and scope beyond only those seeking care. While this application does not provide the same level of clinical confirmation as traditional studies, combining evidence from both clinical and digital participatory data sources allows for earlier response guidance while gold-standard data are collected. For instance, applying our methodology of detecting potential changes in symptomatology could help direct early public health mitigation strategies.
The COVID-19 pandemic exposed vulnerabilities in health infrastructure, particularly for LMICs that struggled to establish testing facilities 8 , needed to support real-time epidemiological parameter estimation that depends on diagnostic testing results. Leveraging the power of global participatory epidemiology in the form of digital health surveys 23 has the potential to supplement these critical testing gaps. Thus, our methods of using self-reported symptom data to understand VE and changes in symptomatology is a powerful rapid response tool, that can provide the medical community with timely insights into emerging variants. Due to our agnostic approach in defining a syndrome (i.e., all pairwise symptoms), the utility of our methods goes beyond COVID-19 and can be applied to other upper-respiratory illnesses and/or locations to support response to emerging threats.
Data availability
To access the raw data used in this manuscript, a request must be submitted to the Facebook Data for Good website: https://dataforgood.facebook.com/dfg/docs/covid-19-trends-and-impact-survey-request-for-data-access . The Global UMD-CTIS Open Data API, Microdata Repository, and contingency tables are available from The University of Maryland Social Data Science Center Global COVID-19 Trends and Impact Survey website ( https://covidmap.umd.edu ). The results of the conditional logistic regression can be found in Supplemental Data 1 and Supplemental Data 2 .
Code availability
The R and Python code used to perform the analyses in this study is available at https://doi.org/10.5281/zenodo.10775701 24 .
Collie, S., Champion, J., Moultrie, H., Bekker, L.-G. & Gray, G. Effectiveness of bnt162b2 vaccine against omicron variant in South Africa. N. Engl. J. Med. 386 , 494–496 (2022).
Article PubMed Google Scholar
Andrews, N. et al. Covid-19 vaccine effectiveness against the omicron (b.1.1.529) variant. N. Engl. J. Med. 386 , 1532–1546 (2022).
Article CAS PubMed Google Scholar
Menni, C. et al. Covid-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the Zoe Covid study. Lancet Infect. Dis. 22 , 1002–1010 (2022).
Bitzegeio, J., Hemmers, L., Bartel, A. & Werber, D. Timely monitoring COVID-19 vaccine protection, Berlin, Germany, April 15 to December 15, 2021. Int. J. Public Health 67 , 1604633 (2022).
Batista, C. et al. The silent and dangerous inequity around access to COVID-19 testing: a call to action. EClinicalMedicine 43 , 101230 (2022).
Aziz, A. B. et al. Integrated control of COVID-19 in resource-poor countries. Int. J. Infect. Dis. 101 , 98–101 (2020).
Article CAS PubMed PubMed Central Google Scholar
Astley, C. M. et al. Global monitoring of the impact of the COVID-19 pandemic through online surveys sampled from the Facebook user base. Proc. Natl Acad. Sci. USA 118 , https://www.pnas.org/content/118/51/e2111455118 (2021).
Fulcher, I. R. et al. Syndromic surveillance using monthly aggregate health systems information data: methods with application to COVID-19 in Liberia. Int. J. Epidemiol. 50 , 1091–1102 (2021).
Article PubMed PubMed Central Google Scholar
Eames, K. et al. Rapid assessment of influenza vaccine effectiveness: analysis of an internet-based cohort. Epidemiol. Infect. 140 , 1309–1315 (2012).
Kreuter, F. Partnering with a global platform to inform research and public policy making. Survey Res. Methods 14 , 159–163 (2020).
Google Scholar
Hodcroft, E. B. Covariants: SARS-CoV-2 Mutations and Variants of Interest (accessed 30 March 2022). CoVariants https://covariants.org/ (2022).
Zeng, B., Gao, L., Zhou, Q., Yu, K. & Sun, F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 20 , 200 (2022).
Vihta, K.-D. et al. Omicron-associated changes in severe acute respiratory syndrome coronavirus 2 (sars-cov-2) symptoms in the United Kingdom. Clin. Infect. Dis. 76 , e133–e141 (2022).
Peña Rodríguez, M. et al. Prevalence of symptoms, comorbidities, and reinfections in individuals infected with wild-type sars-cov-2, delta, or omicron variants: a comparative study in western Mexico. Front. Public Health 11 , 1149795 (2023).
National Institutes of Health. Clinical Spectrum of SARS-COV-2 infection (accessed 12 June 2022). National Institutes of Health https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (2022).
Centers for Disease Control and Prevention. Covid-19 After Vaccination: Possible Breakthrough Infection (accessed 12 June 2022). Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html (2022).
Bradley, V. C. et al. Unrepresentative big surveys significantly overestimated us vaccine uptake. Nature https://doi.org/10.1038/s41586-021-04198-4 (2021).
Siegler, A. J. et al. Trajectory of COVID-19 vaccine hesitancy over time and association of initial vaccine hesitancy with subsequent vaccination. JAMA Netw. Open 4 , e2126882–e2126882 (2021).
Aiello, A. E., Renson, A. & Zivich, P. Social media-and Internet-based disease surveillance for public health. Annu. Rev. Public Health 41 , 101 (2020).
Torner, N. et al. Influenza vaccine effectiveness assessment through sentinel virological data in three post-pandemic seasons. Hum. Vaccines Immunother. 11 , 225–230 (2014).
Article Google Scholar
Chunara, R., Aman, S., Smolinski, M. S. & Brownstein, J. S. Flu near you: an online self-reported influenza surveillance system in the USA. Online J. Public Health Inform. 5 , https://api.semanticscholar.org/CorpusID:31097654 (2013).
Wójcik, O., Brownstein, J., Chunara, R. & Johansson, M. Public health for the people: participatory infectious disease surveillance in the digital age. Emerg. Themes Epidemiol. 11 , 7 (2014).
Chan, A. T. & Brownstein, J. S. Putting the public back in public health—surveying symptoms of COVID-19. N. Engl. J. Med. 383 , e45 (2020).
Varrelman, T. Code from: Vaccine Effectiveness Against Emerging COVID-19 Variants using Digital Health Data https://doi.org/10.5281/zenodo.10775701 https://doi.org/10.5281/zenodo.10775701 (2024).
Download references
Acknowledgements
This work was supported by a Facebook Sponsored Research Agreement (T.J.V., B.R., C.R., G.T., A.H., C.M.A., J.S.B., INB1116217). Authors report research grant funding from the Massachusetts Consortium on Pathogen Readiness (J.S.B.), the Rockefeller Foundation (J.S.B.), and the National Institutes of Health (CMA, K23 DK120899) during the conduct of the study.
Author information
Authors and affiliations.
Computational Epidemiology Lab, Boston Children’s Hospital, Boston, MA, 02115, USA
Tanner J. Varrelman, Benjamin Rader, Christopher Remmel, Gaurav Tuli, Aimee R. Han, Christina M. Astley & John S. Brownstein
Department of Epidemiology, Boston University, Boston, MA, 02118, USA
Benjamin Rader
Division of Endocrinology, Boston Children’s Hospital, Boston, MA, 02115, USA
Christina M. Astley
Harvard Medical School, Boston, MA, 02115, USA
Christina M. Astley & John S. Brownstein
Broad Institute of Harvard and MIT, Cambridge, MA, 02142, USA
You can also search for this author in PubMed Google Scholar
Contributions
T.J.V., B.R., C.M.A., and J.B. conceived the study. T.J.V. and G.T. analyzed the data. T.J.V, B.R., C.M.A., and J.B. drafted the manuscript. T.J.V., B.R., G.T., C.R., A.H., C.M.A., and J.B. reviewed and edited the manuscript. All authors approved of the final manuscript.
Corresponding author
Correspondence to Tanner J. Varrelman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Communications Medicine thanks Ifeolu David, Farrokh Habibzadeh and Nick Tsinoremas for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental material, description of additional supplementary files, supplemental data 1, supplemental data 2, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Varrelman, T.J., Rader, B., Remmel, C. et al. Vaccine effectiveness against emerging COVID-19 variants using digital health data. Commun Med 4 , 81 (2024). https://doi.org/10.1038/s43856-024-00508-9
Download citation
Received : 07 June 2023
Accepted : 24 April 2024
Published : 06 May 2024
DOI : https://doi.org/10.1038/s43856-024-00508-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
National Academies Report: COVID-19 Vaccines Don’t Cause Many Potential Harms
The COVID-19 vaccines made by Pfizer-BioNTech and Moderna do not cause such conditions as female infertility, myocardial infarction, Bell palsy, or Guillain-Barré syndrome in adults, according to a report by the National Academies of Sciences, Engineering, and Medicine that reviewed evidence for potential harms for 19 conditions associated with these vaccines. There was not enough evidence involving children to draw conclusions about possible harms.
The committee did conclude that there is evidence that these vaccines can cause myocarditis, although the group emphasized cases linked with vaccines are rare. The report follows a recent study published by the US Centers for Disease Control and Prevention showing no association between COVID-19 vaccination and sudden cardiac death among healthy young people.
The committee’s evidence also suggests that the Janssen vaccine may cause Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome. More broadly, the committee concluded that vaccinations in general might cause certain shoulder injuries from the injection itself.
The results of the report, which analyzed studies conducted soon after vaccines were available, represent a “snapshot in time,” and might not fully reflect the real-world use of the vaccines, author George Isham, MD, said in a statement . The report was sponsored by the Health Resources and Services Administration, a branch of the US Department of Health and Human Services.
Published Online: May 10, 2024. doi:10.1001/jama.2024.7724
See More About
Harris E. National Academies Report: COVID-19 Vaccines Don’t Cause Many Potential Harms. JAMA. Published online May 10, 2024. doi:10.1001/jama.2024.7724
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Coronavirus (COVID-19) Information and Updates
Covid-19 vaccine.
The CDC recommends everyone 6 months and older get an updated COVID-19 vaccine to protect against the potentially serious outcomes of COVID-19 illness this fall and winter.
The updated COVID vaccines for the fall/winter 2023-24 season are based on a variant called XBB.1.5. They are the only COVID vaccines that are available this season. The recommendation is 1 single dose of an updated COVID-19 vaccine formulation. (People who are immunocompromised or ages 6 months to 4 years may need more than one 2023-2024 vaccine.)
People 5 years and older should wait at least two months after getting the last dose of any COVID-19 vaccine before getting the updated COVID vaccine.
Learn more from Johns Hopkins about the COVID-19 vaccine .
Get more information about COVID-19 vaccine updates from the CDC .
Vaccine Resources
Johns hopkins medicine pharmacies.
The 2023-34 COVID-19 vaccine is available for Johns Hopkins Medicine patients at the following Johns Hopkins Outpatient Pharmacy locations: JHOC, Bayview, Weinberg, Monument Street, Bartlett, and Green Spring Station.
More information about pharmacy locations and hours
Schedule an appointment with the pharmacy or walk-in to receive a vaccination today!
Mobile Vaccine Clinic
Johns Hopkins Medicine operates a mobile COVID-19 and flu vaccination clinic open to everyone. We will be offering the updated 2023-24 COVID-19 vaccine to everyone age 5+, and the 2023-24 dose flu vaccination to everyone age 9+. No appointment is necessary.
Sacred Heart Vaccination Clinic
Address: Sacred Heart Church, 600 S. Conkling St. , Baltimore, Maryland.
Time: Fridays, 4-7 p.m. (except holidays)
Vaccines: The Pfizer COVID-19 vaccine is available for patients 5 years and older. The 2023-24 flu vaccination is available for patients 9 years and older.
A parent or guardian must accompany any individual under the age of 18 to the vaccine clinic and sign the COVID-19 vaccine consent form. Children ages 5–17 who do not have a signed consent form will not receive the COVID-19 vaccine or flu shot from Johns Hopkins Medicine.
- Vaccine Clinic Locator
- Maryland COVID-19 Vaccine Resources
- State of Maryland’s COVID-19 Vaccination Support Center: 1-855-MDGOVAX (1-855-634-6829)
Washington, D.C.
- Vaccination Registration
- Washington, D.C., COVID-19 Vaccine Resources
- Florida COVID-19 Vaccine Resources
Additional Resources
- Equity for COVID-19 Vaccines and Care
- For Hopkins Employees (JHED ID Required)
Genetics Played Role in Rare Blood Clots Linked to COVID-19 Vaccines, Researchers Find

R are but deadly blood clots tied to Johnson & Johnson and AstraZeneca Plc’s Covid-19 shots were caused by an autoimmune reaction that some people are predisposed to, researchers found, a discovery that they say will shape development of future vaccines.
Adenovirus-based vaccines, like the J&J and AstraZeneca shots that were later pulled from the market, contain a component that, in genetically susceptible people, can trigger the production of unusually structured antibodies against a protein involved in blood clotting, scientists said Wednesday in a letter to the New England Journal of Medicine. Researchers plan to identify the culprit and then try to remove it using genetic engineering.
Read More: How COVID-19 Vaccines and Infections Are Tweaking Our Immunity
An extremely similar deleterious antibody response occurs in susceptible patients after infection with adenoviruses , which often infect the airways and lead to cold-like symptoms, the study found. It’s not known how many people may be susceptible to the complication, said Tom Gordon, head of immunology at Flinders University in South Australia, whose molecular sleuthing led to the finding .
The immune reaction linked to the shot is “a new disease,” he said in an interview. Hematologists and intensive care specialists are likely to spot more cases as they become familiar with it, he said.
“It’s a kind of autoimmunity where we know the trigger,” said immunologist James McCluskey, assistant vice chancellor of the University of Melbourne, who wasn’t involved in the research. “That’s unusual. In most cases we never get a handle on the trigger.”
Vaccines withdrawn
Out of more than 18 million people who received the single-dose J&J vaccine, 60 cases of the clotting disorder were reported and nine people died, according to the Yale School of Medicine.
A small number of clot-related deaths tied to the AstraZeneca vaccine led to its withdrawal or restriction in Denmark, Norway and other countries in 2021. The complication occurred in about 2-3 people per 100,000 vaccinated with the Astra shot under age 60 in Australia, where it hasn’t been available since March 2023. The European Commission withdrew the marketing authorization for the immunization in March 2024.
“AstraZeneca welcomes any further examination of the possible underlying mechanism of thrombosis with thrombocytopenia syndrome (TTS), given that, despite extensive investigation, we do not yet understand the mechanism that can in very rare cases be a trigger for TTS,” a spokesperson for the company said.
J&J also said it supports research that helps guide development of safe and effective vaccines.
Read More: The Miracle Workers: Vaccine Scientists Are TIME’s 2021 Heroes of the Year
“More data are needed to fully understand potential factors that may be associated with this rare event, including its potential relationship with adeno- and other viruses, to draw appropriate conclusions about the underlying pathogenesis,” the company said in an email.
Both shots played an important role in vaccine programs during the early stages of the pandemic. One analysis found the Astra vaccine saved an estimated 6.3 million lives in 2021.
The mRNA vaccines made by the Pfizer Inc.-BioNTech SE partnership and Moderna Inc. were later found to be more effective at protecting against Covid and have been updated to tackle more recent virus variants.
More Must-Reads from TIME
- The New Face of Doctor Who
- Putin’s Enemies Are Struggling to Unite
- Women Say They Were Pressured Into Long-Term Birth Control
- Scientists Are Finding Out Just How Toxic Your Stuff Is
- Boredom Makes Us Human
- John Mulaney Has What Late Night Needs
- The 100 Most Influential People of 2024
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]

Link between COVID-19 vaccine complication and rare 'common cold' blood disease
N ew research led by Flinders University and international experts is expanding understanding of vaccine-induced immune thrombocytopenia and thrombosis (known as VITT).
At the height of the COVID-19 pandemic in 2021, VITT emerged as a new disease following adenovirus vector-based vaccines—notably the Oxford-AstraZeneca vaccine.
VITT was found to be caused by an unusually dangerous blood autoantibody directed against a protein termed platelet factor 4 (or PF4).
In separate research in 2023, researchers from Canada, North America, Germany and Italy described a virtually identical disorder with the same PF4 antibody that was fatal in some cases after natural adenovirus (common cold) infection.
Flinders University researchers Dr. Jing Jing Wang and Flinders Professor Tom Gordon, Head of Immunology at SA Pathology in South Australia, led a previous study in 2022 which cracked the molecular code of the PF4 antibody and identified a genetic risk factor related to an antibody gene termed IGLV3.21*02.
Now, the Flinders group has collaborated with this international group of researchers to find that the PF4 antibodies in both adenovirus infection-associated VITT and classic adenoviral vectored VITT share identical molecular fingerprints or signatures.
The research will also have implications for improving vaccine development, says Flinders University researcher Dr. Wang, first author on the article published in the New England Journal of Medicine and titled "Antibody Fingerprints Linking Adenoviral Anti-PF4 Disorders."
"These findings, using a completely new approach for targeting blood antibodies developed at Flinders University, indicate a common triggering factor on virus and vaccine structures that initiates the pathological pF4 antibodies," explains Professor Gordon.
"Indeed, the pathways of lethal antibody production in these disorders must be virtually identical and have similar genetic risk factors.
"Our findings have the important clinical implication that lessons learned from VITT are applicable to rare cases of blood clotting after adenovirus (a common cold) infections, as well as having implications for vaccine development," he says.
More information: Jing Jing Wang et al, Antibody Fingerprints Linking Adenoviral Anti-PF4 Disorders, New England Journal of Medicine (2024). DOI: 10.1056/NEJMc2402592
Provided by Flinders University

Advertisement
Supported by
There’s a New Covid Variant. What Will That Mean for Spring and Summer?
Experts are closely watching KP.2, now the leading variant.
- Share full article

By Dani Blum
For most of this year, the JN.1 variant of the coronavirus accounted for an overwhelming majority of Covid cases . But now, an offshoot variant called KP.2 is taking off. The variant, which made up just one percent of cases in the United States in mid-March, now makes up over a quarter.
KP.2 belongs to a subset of Covid variants that scientists have cheekily nicknamed “FLiRT,” drawn from the letters in the names of their mutations. They are descendants of JN.1, and KP.2 is “very, very close” to JN.1, said Dr. David Ho, a virologist at Columbia University. But Dr. Ho has conducted early lab tests in cells that suggest that slight differences in KP.2’s spike protein might make it better at evading our immune defenses and slightly more infectious than JN.1.
While cases currently don’t appear to be on the rise, researchers and physicians are closely watching whether the variant will drive a summer surge.
“I don’t think anybody’s expecting things to change abruptly, necessarily,” said Dr. Marc Sala, co-director of the Northwestern Medicine Comprehensive Covid-19 Center in Chicago. But KP.2 will most likely “be our new norm,’” he said. Here’s what to know.
The current spread of Covid
Experts said it would take several weeks to see whether KP.2 might lead to a rise in Covid cases, and noted that we have only a limited understanding of how the virus is spreading. Since the public health emergency ended , there is less robust data available on cases, and doctors said fewer people were using Covid tests.
But what we do know is reassuring: Despite the shift in variants, data from the C.D.C. suggests there are only “minimal ” levels of the virus circulating in wastewater nationally, and emergency department visits and hospitalizations fell between early March and late April.
“I don’t want to say that we already know everything about KP.2,” said Dr. Ziyad Al-Aly, the chief of research and development at the Veterans Affairs St. Louis Healthcare System. “But at this time, I’m not seeing any major indications of anything ominous.”
Protection from vaccines and past infections
Experts said that even if you had JN.1, you may still get reinfected with KP.2 — particularly if it’s been several months or longer since your last bout of Covid.
KP.2 could infect even people who got the most updated vaccine, Dr. Ho said, since that shot targets XBB.1.5, a variant that is notably different from JN.1 and its descendants. An early version of a paper released in April by researchers in Japan suggested that KP.2 might be more adept than JN.1 at infecting people who received the most recent Covid vaccine. (The research has not yet been peer-reviewed or published.) A spokesperson for the C.D.C. said the agency was continuing to monitor how vaccines perform against KP.2.
Still, the shot does provide some protection, especially against severe disease, doctors said, as do previous infections. At this point, there isn’t reason to believe that KP.2 would cause more severe illness than other strains, the C.D.C. spokesperson said. But people who are 65 and older, pregnant or immunocompromised remain at higher risk of serious complications from Covid.
Those groups, in particular, may want to get the updated vaccine if they haven’t yet, said Dr. Peter Chin-Hong, an infectious disease specialist at the University of California, San Francisco. The C.D.C. has recommended t hat people 65 and older who already received one dose of the updated vaccine get an additional shot at least four months later.
“Even though it’s the lowest level of deaths and hospitalizations we’ve seen, I’m still taking care of sick people with Covid,” he said. “And they all have one unifying theme, which is that they’re older and they didn’t get the latest shot.”
The latest on symptoms and long Covid
Doctors said that the symptoms of both KP.2 and JN.1 — which now makes up around 16 percent of cases — are most likely similar to those seen with other variants . These include sore throat, runny nose, coughing, head and body aches, fever, congestion, fatigue and in severe cases, shortness of breath. Fewer people lose their sense of taste and smell now than did at the start of the pandemic, but some people will still experience those symptoms.
Dr. Chin-Hong said that patients were often surprised that diarrhea, nausea and vomiting could be Covid symptoms as well, and that they sometimes confused those issues as signs that they had norovirus .
For many people who’ve already had Covid, a reinfection is often as mild or milder than their first case. While new cases of long Covid are less common now than they were at the start of the pandemic, repeat infections do raise the risk of developing long Covid, said Fikadu Tafesse, a virologist at Oregon Health & Science University. But researchers are still trying to determine by how much — one of many issues scientists are trying to untangle as the pandemic continues to evolve.
“That’s the nature of the virus,” Dr. Tafesse said. “It keeps mutating.”
Dani Blum is a health reporter for The Times. More about Dani Blum
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Efficacy of COVID-19 vaccines: From clinical trials to real life
Dominique deplanque.
a Université de Lille, Inserm, CHU Lille, CIC 1403 – Clinical Investigation Center, 59000 Lille, France.
b F-CRIN IREIVAC/COVIREIVAC, 75679 Paris, France
Odile Launay
c Université de Paris, Inserm CIC 1417, Assistance publique – Hôpitaux de Paris, hôpital Cochin, 75679 Paris, France
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread around the globe leading to the COVID-19 pandemic. To mitigate the effects of the virus on public health and the global economy, vaccines were rapidly developed. In less than one year, with respect to usual clinical development rules, several vaccines have been put on the market and mass vaccination campaigns have been deployed. During the phase I to phase III clinical trials, most of these vaccines have demonstrated both their safety and efficacy. Despite questions remain about the impact of virus variants and the duration of the immune response, messenger RNA (mRNA)-based and adenoviral vectored vaccines have demonstrated an overall efficacy from 70 to 95% in both phase III trials and real life. In addition, all these vaccines also reduce the severe forms of the disease and might strongly impact the mortality which could change the course of the pandemic.
Abbreviations
Introduction.
Since December 2019, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has rapidly spread around the globe leading to the Coronavirus Disease 2019 (COVID-19) pandemic. From January 2020 to the beginning of second quarter of 2021, the intensity and rapidity of SARS-CoV-2 transmission have led to about 150,000,000 cases and more than 3,000,000 deaths in the world putting considerable pressure on public health systems and the global economy. In the context of extraordinary scientific and technical mobilization, the genetic sequence of SARS-CoV-2 was published on January 11 th 2020, triggering intense global Research and Development (R&D) activity to develop a vaccine against this disease. To date, World Health Organization (WHO) lists about 200 vaccine candidates in preclinical development, 100 in clinical evaluation and 13 received authorization [1] .
In Europe, 4 vaccines have already been authorized by the European Medicines Agency (EMA) and several mass vaccination campaigns are also underway worldwide. These rapid developments have been made possible by the important contribution of both public and private funding, high level of volunteers’ participation in clinical trials as well as by changes in the review process of regulatory agencies [2] . On the other hand, the success of messenger RNA (mRNA) vaccine platform is probably the consequence of previous researches for more than 15 years and their previous identification as vaccine platform of choice for emerging infectious diseases [3] . Since the mechanisms of action and undesirable effects of these vaccines are addressed in other articles of this special issue, we will focus on the efficacy of coronavirus disease 2019 (COVID-19) vaccines, more particularly on the four vaccines already available in Europe.
COVID-19 vaccine development: an unprecedented timeframe
In the last decade, there was a marked evolution of vaccine platforms including the development of nucleic acid-based vaccine candidates and vectored vaccines, a number of approaches that have been used to accelerate COVID-19 vaccines elaboration. Moreover, previous preclinical data from vaccine candidates for SARS-CoV and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) enabled the initial step of exploratory vaccine design to be largely overlooked, saving a considerable amount of time. In most cases, production processes were also adapted from those of existing vaccines [2] .
As a result, a first phase I clinical trial of a vaccine candidate for SARS-CoV-2 began in March 2020 testing the mRNA 1273 (Moderna) [4] . In April 2020, another phase I trial testing different sequences of the mRNA BNT 162 (BioNTech/Pfizer) has also begun [5] then followed by adenovirus vectored vaccines, in June and July 2020, respectively ChAdOx1nCov-19 (AstraZeneca/Oxford) and Ad26COVS1 (Janssen) [6] , [7] . One important particularity of this period was that clinical phases were overlapping and trial starts were staggered, with initial phase I/II trials followed by fast progression to phase III trials mainly considering interim analysis of the phase I/II data. Most of the manufacturers had also rapidly started the large-scale commercial production of vaccines despite the absence of any result from phase III trials [2] .
Finally, both Food and Drug Administration (FDA) and EMA implemented a rolling review process, which means that a drug company can submit completed sections of its new drug application for review, rather than waiting until every section of the application is completed before the entire application can be reviewed. Beyond these exceptional adaptations, one of the key factors of the accelerated development of COVID-19 vaccines was financial risk-taking that was greatly sustained by public funds, namely in Germany, UK and USA. Nonetheless, it should be underlined that despite these financial considerations but also the pandemic-associated emergency, no concession was made on safety.
Phase I/II clinical trials: safety and immunogenicity analysis
While phase I trials are usually designed to determine the safety profile at several dosages of a drug candidate, in the field of vaccines, the immunogenicity as a marker of drug-response is also evaluated. Indeed, this evaluation constitutes an essential step in the construction of future trials. In this context, phase II trials are intended to more precisely evaluate immunogenicity in relation to dose regimen in the way to determine the final dose to use in phase III trials. Here, most of the published studies were combined phase I/II trials aiming to determine safety and immunogenicity including the effect of a second vaccine dose according to different dosages and intervals [4] , [5] , [6] , [7] , [8] .
Phase I studies usually recruited a low number of subjects but during the early development of COVID-19 vaccines at least one study reported here included about 200 volunteers [5] and according to a phase II design, another study included more than 500 subjects [6] . Such an important recruitment clearly helped to provide stronger results and has probably facilitated the design of phase III studies. This large number of subjects also allowed to strengthen the excellent results about safety profile of these vaccine candidates whatever the platform used namely mRNA or adenoviral vector [4] , [5] , [6] , [7] , [8] .
As summarized in Table 1 , all the vaccine candidates reported here were able to induce an immune response against the spike protein of the SARS-CoV-2. Thus, there was a clear dose- or time-dependent increased in both specific Ig-G and neutralizing antibody titers ( Table 1 ) which are enhanced by the second dose of vaccine [4] , [5] , [6] , [7] , [8] . Some differences regarding Ig-G and neutralizing antibody titers may be discussed between these vaccines but it is important to point out that the assays used to perform these measures vary greatly and then comparisons must be made with caution. Nevertheless, an increase in specific Ig-G and neutralizing antibody titers has been shown in 90 to 100% of subjects while cellular responses were also observed. The phase I study on Ad26COVS1 vaccine also brings more details about immune response by analyzing the binding and functional profiles of vaccine-elicited antibodies by systems serology analyses. It was then showed the induction of S- and RBD-specific IgA1, IgA2, IgG1, IgG2, IgG3, IgG4, and IgM subclasses; FcγR2a, FcγR2b, FcγR3a, and FcγR3b binding; antibody-dependent complement deposition, antibody-dependent neutrophil phagocytosis, antibody-dependent cellular phagocytosis, and antibody-dependent NK cell activation functional antiviral responses [7] .
Main data from phases I/II clinical trials of the 4 vaccines available in Europe.
Ad26: adenovirus type-26; GMT: geometric mean titer; MNA: microneutralization assay; MT: microneutralization titer; ND: not determined; PRNT: plaque reduction neutralization testing; SFCs: spot-forming cells; VNA: virus neutralizing antibody; VP: viral particles; Y: years.
Another important information provided by these phase I/II studies was related to the effect of age. Among studies shown in Table 1 , only studies on BNT 162 b2 and ChAdOx1nCov-19 vaccines included subjects over 65 years of age [5] , [6] , [8] . These studies demonstrated both antigen-binding Ig-G and virus-neutralizing responses in older adults but also a possible decrease in such responses with increasing age [5] , [6] . Nevertheless, an elderly-dedicated phase I study showed that mRNA 1273 vaccine induces a good Ig-G response against the spike-protein as well as significative neutralizing antibody titers in adults older than 70 years of age [8] . Taken as a whole, these results suggested the possibility to demonstrate COVID-19 vaccines’ efficacy in phase III trials.
Phase III studies: evidence about a high level of efficacy
One of the main issues of phase III trials, particularly in the case of a new pathology, is to define the primary criterion to be used as efficacy markers as well as to define the level of efficacy that will be considered as pertinent. Undeniably, the definition of the primary criterion strongly impacts both the conduct of the trial and the future modalities of prescription of the vaccine. On June 2020, the FDA has released a guidance document for the development and licensure of COVID-19 vaccines [9] . This document stated that symptomatic laboratory-confirmed COVID-19 might be an acceptable primary endpoint for a COVID-19 vaccine efficacy trial with an efficacy level of at least 50% required. Moreover, other secondary criteria might be used to determine the possible efficacy on asymptomatic or severe infections. As a consequence, study sample sizes and timing of interim analyses had to be based on the statistical success criteria for primary and secondary efficacy analyses and realistic, data-driven estimates of vaccine efficacy and incidence of COVID-19 for the populations and locales in which the trials are conducted [9] .
According to these conditions, phase III studies have been conducted in countries where the virus spread was extremely high and have included in a few months several tens of thousands of subjects [10] , [11] , [12] , [13] . According to these studies and except unwanted effects related to usual reactogenicity, these vaccines appear safe. As summarized in Table 2 , among the four vaccines already authorized in Europe the overall efficacy defined as reduction of symptomatic COVID-19 varies from 67% to 95% [10] , [11] , [12] , [13] . These results are largely greater than the minimum efficacy level of 50% required by the FDA [9] . Furthermore, these levels of efficacy are also similar or even higher than that the mean 60% observed with the influenza vaccines [14] . It should be underlined that mRNA vaccines [10] , [11] appear to have a higher efficacy than that of adenoviral vectored vaccines [12] , [13] . Nevertheless, a definitive conclusion about any comparisons should be done with caution. The complexity of the design of ChAdOx1nCov-19 vaccine phase III study does not facilitate neither the interpretation nor any comparisons of data. On the other hand, regarding interim analyses of trials reported in the Table 2 , the protection against severe infection appears to be close to 100% for all vaccines but not for Ad26COVS1 vaccine where data recently published show protection against severe infection with one dose of vaccine of only 77% [13] . It remains to determine how this result is only representative of this vaccine rather than may be finally observed with all other COVID-19 vaccines when long-term follow-up data will be available.
Clinical efficacy observed in phase III studies of the 4 vaccines available in Europe.
ND: not determined; VP: viral particles; Y: years.
Some other elements remain to be clarified in real life conditions, namely the effect on mortality, the level of efficacy in the elderly and the clinical efficacy of vaccines according to emerging variants and the duration of protection. Concerning mortality, the studies have not been designed to answer this question, it may then be difficult to bring clear and conclusive result. Moreover, according to the drastic reduction of severe infections observed in vaccinated-people, these studies are probably under-powered to clearly demonstrate the effect of vaccines on mortality.
Regarding elderly, not all published phase III studies had included many elderly people but in studies with a significant number of elderly participants ( Table 2 ), the efficacy level remains relatively close to the one observed in younger subjects (86.4% and 94.7% in people of 65 or over, for mRNA 1273 and BNT 162 b2 respectively) [10] , [11] . Clinical efficacy of vaccines on emerging variants remains also to be more precisely evaluated. Due to the relatively recent emergence of different virus variants’, only few clinical data are available but they show the preservation of ChAdOx1nCov-19 vaccine efficacy on B.1.1.7 variant [15] and a possible decrease in efficacy of both Ad26COVS1 and ChAdOx1nCov-19 vaccines on B.1.351 variant ( Table 2 ) [13] , [16] .
COVID-19 vaccines effectiveness in real life
When a new drug comes on the market, it takes usually several years before it could be demonstrated the reproducibility of the phase III clinical trial results’ in real life. Here, according the pandemic situation and the rapid development of mass vaccination campaigns in Israel, USA and UK, we already have many data demonstrating this achievement and also providing supplementary informations. Indeed, as summarized in Table 3 , at least 4 studies that had included 20,000 to more than 1,300,000 vaccinated people provide us impressive results on symptomatic or asymptomatic infections, severe infections as well as on mortality [17] , [18] , [19] , [20] .
Effectiveness of SARS-CoV-2 vaccination in real life.
CI: confidence interval; ND: not determined; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Israel was probably the country which developed the most rapid and extensive mass vaccination campaign in the world by using the BNT 162 b2 vaccine. All people recorded on the Clalit Health Services (53% of Israeli population) who were newly vaccinated during the period from December 20, 2020, to February 1, 2021, were matched to unvaccinated controls according to demographic and clinical characteristics leading to 2 study groups of 596,618 persons each [17] . Looking on results 7 days or more after the second dose of vaccine, symptomatic and asymptomatic as well as severe COVID-19 infections are reduced by 92%. Mortality was also reduced by 84% when regarding 21 to 27 days after the first dose [17] . Even if such results have to be strengthened by a longer-term follow-up, the present study also provides informations about a similar vaccine effectiveness across different age groups and a slightly lower effectiveness among patients with multiple coexisting pathological conditions [17] .
In another paper under review, similar results are provided with the Mayo Clinic Health Database showing a 90% decrease in the development of a symptomatic or asymptomatic COVID-19 infection after BNT 162 b2 or mRNA 1273 vaccination in 31 609 persons [18] . Same kind of results are also provided by both Scotland and England data where both BNT 162 b2 and ChAdOx1nCov-19 vaccines were largely used with evidence of 85% decrease in symptomatic and asymptomatic infections and about 90% decrease in severe infections ( Table 3 ) [19] , [20] . Interestingly, in the Scottish study, such results were observed while the vaccines were administered at only one dose to extend the number of subjects receiving at least one dose of vaccine. Besides the fact that all these data confirm the results of phase III clinical trials, they also help to discuss the question of the effect of vaccine on virus transmission. Because asymptomatic infections are also reduced, it may be that virus transmission is decreased. Moreover, a recently published paper brings supplemental arguments about an effect of vaccines on transmission. Indeed, people who develop a COVID-19 despite vaccination with the BNT 162 b2 vaccine have a clear reduction of viral load that might help to break the spread of the virus [21] . Complementary strategies, such as vaccines able to develop mucosal immunity are also under development [22] .
Several questions remain on hold
One of the first questions on hold is the duration of the protection afforded by the vaccination. According to studies follow-up duration, it might be supposed that this protection is as at least 6 to 12 months. This duration will determine the need of a new vaccine boost and possibly the need of annual boost. Some large population-based studies using test-negative designs might help to evaluate these different issues particularly the situations of vaccine failure [23] .
The question of using different vaccines than the one use initially is also crucial. New studies should be designed including studies for the evaluation of the impact of immunogenicity against adenoviral vector if such a vaccine is used for a third injection. Because phase III clinical studies provide no or only few data about immunogenicity, it remains important to also designed studies able to determine more precisely the immune response according to real life administration scheme namely regarding the role of both cellular and innate immunity. Actually, in France, 2 studies are in course to answer such questions by using BNT 162 b2 or mRNA 1273 vaccines in adults including a sub-population of elderly people over 75 years of age.
Besides older people, the question of the efficacy of vaccines in particular populations is also crucial. Several recently published studies bring some answers. To date, there is only few data about the efficacy of vaccine in young people under 18 years of age [10] . Studies evaluating COVID-19 mRNA vaccines in young people aged 12 to 16 years are actually in course but both FDA and EMA have already beenquestioned to provide an authorization of use. On the other hand, COVID-19 mRNA vaccines seem to generate robust humoral immunity in pregnant and lactating women, with immunogenicity and reactogenicity similar to that observed in non-pregnant women [24] . In this study, vaccine-induced immune responses were also significantly greater than the response to natural infection and immune transfer to neonates occurred via placenta and breastmilk [24] . Other data also suggest that a single dose of mRNA vaccine elicits rapid immune responses in SARS-CoV-2 seropositive participants, with post-vaccination antibody titers similar to or exceeding titers found in seronegative participants who received two vaccinations [25] . On the other hand, the immunization rate among kidney transplant recipients who received 2 doses of an mRNA vaccine can be as low as 48% [26] . Very low immune response is evidenced also in others immunocompromised populations. The issue of a third vaccine dose in these non-responsive patients is an intriguing one that will be usefully explored in further research. In this context, a large cohort has been opened in France (COV-POPART) to evaluate the immune response in a number of particular populations including patients with cancer, transplant recipients as well as patients with auto-immune disease receiving immune-modulator treatments.
Last, an important challenge also concerns the efficacy of vaccines regarding the emergence of viral variants [27] . We have previously described some data from clinical trials regarding the decrease or the absence of efficacy of the ChAdOx1nCov-19 namely on B.1.351 variant ( Table 2 ) [12] . Some similar concerns are discussed with the other vaccines but most of the data come from in vitro studies that are not able to consider the possible role of cellular immune response [27] , [28] . Besides to evaluate the effect of a boost particularly with new mRNA sequences, future trials should also explore more precisely the whole immune response against viral variants.
In less than one year, face to a worldwide pandemic due to an unknown virus, several vaccines have been put on the market and have been already used in several tens of millions of people. These vaccines are importantly effective and relatively safe according to the severity of the disease they prevent. Beyond this impressive efficacy of COVID-19 vaccines, notably those based on mRNA, such a technology may first contribute to modify the course of the pandemic but also could bring the world in a new era of specific treatments for numerous pathologies [3] .
Disclosure of interest
The authors declare that they have no competing interest.

COMMENTS
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
By the end of November 2021, scientists estimate that mRNA COVID-19 vaccines had prevented at least 1 million deaths, 10 million hospitalizations, and 36 million SARS-CoV-2 infections in the United States. Sometimes people who are fully vaccinated get a breakthrough infection, meaning that they test positive for SARS-CoV-2 or become ill with ...
Abstract. The coronavirus disease 2019 (COVID-19) pandemic is a global crisis, with devastating health, business and social impacts. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. By the end of August 2021, only 24.6% of the world population has received two doses of a COVID-19 vaccine.
1. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) This clinical trial is designed to assess the safety, reactogenicity, and immunogenicity of mRNA-1273. It encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2.
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Efficacy/effectiveness of current COVID-19 vaccines. A systematic review on the efficacy of vaccines covering studies from January 1 to May 14, 2021 identified 30 studies, showed 80-90 per cent vaccine efficacy against symptomatic and asymptomatic infections in fully vaccinated people in nearly all studies 20.In clinical trials, three vaccines had higher (>90%) efficacy against COVID-19 ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816 ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
Now, given the urgent need for COVID-19 vaccines, unprecedented financial investments and scientific collaborations are changing how vaccines are developed. This means that some of the steps in the research and development process have been happening in parallel, while still maintaining strict clinical and safety standards.
Two U.S. Food and Drug Administration (FDA)-approved mRNA vaccines for COVID-19 have saved millions of lives. These vaccines were developed with NIH support and research on a protein found on SARS-CoV-2, the virus that causes COVID-19. Clinical trials for the COVID-19 vaccines in people were established in what seemed like record time. But in reality, more than 50 years of public and private ...
Find COVID-19 datasets, data tools, and publications to use in research. EXPLORE COVID-19 DATA. Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines.
COVID-19 vaccines. Everyone, everywhere, should have access to COVID-19 vaccines. Major progress has been made with the COVID-19 vaccination response, and it is critical to continue the progress, particularly for those most at risk of disease. WHO recommends a simplified single-dose regime for primary immunization for most COVID-19 vaccines ...
These vaccines have U.S. Food and Drug Administration (FDA) emergency use authorization or approval. 2023-2024 Pfizer-BioNTech COVID-19 vaccine.. In December 2020, the Pfizer-BioNTech COVID-19 vaccine two-dose series was found to be both safe and effective in preventing COVID-19 infection in people age 18 and older.
The coronavirus pandemic has claimed more than 670,000 lives in the United States as of Sept. 20, and the spread of the highly transmissible delta variant has added new urgency to the federal government's efforts to vaccinate all Americans against the virus. As the drive to inoculate more people continues, here are 10 facts about Americans and COVID-19 vaccines, based on an August Pew ...
The R&D Blueprint has been activated to accelerate diagnostics, vaccines and therapeutics for this novel coronavirus. ... The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints ...
A typical vaccine development timeline takes 5 to 10 years, and sometimes longer, to assess whether the vaccine is safe and efficacious in clinical trials, complete the regulatory approval processes, and manufacture sufficient quantity of vaccine doses for widespread distribution. Accelerated Timeline. 1-2 Years In Total.
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
Varrelman et al. use participatory surveillance data to develop a method to identify changes in vaccine effectiveness and disease symptomatology. Using data from the COVID-19 pandemic, they ...
The COVID-19 vaccines made by Pfizer-BioNTech and Moderna do not cause such conditions as female infertility, myocardial infarction, Bell palsy, or Guillain-Barré syndrome in adults, according to a report by the National Academies of Sciences, Engineering, and Medicine that reviewed evidence for potential harms for 19 conditions associated with these vaccines.
The CDC recommends everyone 6 months and older get an updated COVID-19 vaccine to protect against the potentially serious outcomes of COVID-19 illness this fall and winter. The updated COVID vaccines for the fall/winter 2023-24 season are based on a variant called XBB.1.5. They are the only COVID vaccines that are available this season.
By Jason Gale / Bloomberg. May 16, 2024 12:15 AM EDT. R are but deadly blood clots tied to Johnson & Johnson and AstraZeneca Plc's Covid-19 shots were caused by an autoimmune reaction that some ...
Soon after their arrival in late December 2020, the Covid-19 vaccines turned the pandemic around and opened a path back to normalcy. They prevented about 14.4 million deaths worldwide, according ...
At the height of the COVID-19 pandemic in 2021, VITT emerged as a new disease following adenovirus vector-based vaccines—notably the Oxford-AstraZeneca vaccine. VITT was found to be caused by an ...
OHSU has committed its entire organization to deploying COVID-19 vaccines, starting with difficult-to-reach community members and underserved communities. The university has also engaged students and trainees to vaccinate Oregonians.. After federal and state authorities recommended two COVID-19 vaccines for children as young as 6 months old in June 2022, OHSU organized appointment-only ...
The latest on symptoms and long Covid. Doctors said that the symptoms of both KP.2 and JN.1 — which now makes up around 16 percent of cases — are most likely similar to those seen with other ...
COVID-19 vaccine development: an unprecedented timeframe. In the last decade, there was a marked evolution of vaccine platforms including the development of nucleic acid-based vaccine candidates and vectored vaccines, a number of approaches that have been used to accelerate COVID-19 vaccines elaboration.