An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Educ Health Promot

Impact of knowledge, attitude, and practices of Type 2 diabetic patients: A study in the locality in Vietnam
Nghiep ke le.
Department of Public Health, Faculty of Public Health, Mahasarakham University, Mahasarakham, Thailand
Niruwan Turnbull
Cuong van dam.
1 University Administrators, Faculty of Medicine, Can Tho University of Medicine and Pharmacy, Can Tho Province, Vietnam
Santisith Khiewkhern
Surasak thiabrithi, background:.
Disease knowledge, appropriate attitude, and proper practices play an important role in disease control and reduction of diabetes-related complications and deaths. This study aims to investigate the impact of knowledge, attitude, and practices (KAPs) of Type 2 diabetic patients' outcomes.
MATERIALS AND METHODS:
A cross-sectional research was conducted on a group of 102 Type 2 diabetic participants in 17 communities in Tam Binh District, Vinh Long Province, Vietnam. The research tool employed the KAP questionnaire using IBM SPSS 22 to analyze the data.
The participants' average age was 57.02 ± 6.323 years. The proportion of women was 76.5% (three times higher than men). The knowledge score of the participants was low (30.04 ± 12.823), the attitude toward score of diabetics was moderate (61.544 ± 29.99), and the practice of self-care score was low (50.59 ± 14.881). There were also some significant relationships between KAPs with ethnicity, marital status, diabetic duration, location, employment status, and treatment method. In addition, there were only significant differences between the self-care practice groups and patients' attitude toward Type 2 diabetes.
CONCLUSION:
There is a significant relationship between KAP with some participants' characteristics. The KAPs of the diabetic patients in Tam Binh district are still low. This result showed that although the patient's attitude towards disease was good, it was not enough for them to practice good self-control due to poor knowledge.
Introduction
Type 2 diabetes mellitus (T2DM) is a long-term metabolic confusion disease that is related to a high rate of complication and mortality in a population.[ 1 , 2 ] The worldwide prevalence of diabetes was 177 million in 2000,[ 3 ] which increased to 422 million in 2014,[ 4 ] and it will be reaching 592 million by 2035.[ 5 ] In 2015, there were over 3.5 million Vietnamese adults living with diabetes. Particularly, T2DM is the most common type, with the incidence doubling in the previous decade (2.7% in 2002–5.4% in 2012).[ 6 , 7 ]
Diabetic treatment is a lifelong process, so self-motivation of the patient is needed. Therefore, patients need a basic knowledge of diabetes, and if they have knowledge about the disease, they will be more positive about the attitude and better practice.[ 8 , 9 ] It can help early disease detection and complication reduction.[ 10 , 11 ] Some authors have assessed the knowledge, attitude, and practice (KAP) of diabetes using the KAP questionnaire and promoted them for better cognizance of how to manage risk factors including program intervention of the diabetes.[ 12 ] They also indicated that diabetes knowledge, attitudes toward disease, and practices of the diabetic self-management are associated with a greater understanding of the prevention, diagnosis, and control of risk factors.[ 13 ] This study assessed the impact of knowledge, attitude toward diabetes, and practice of self-care management of T2DM patients. In spite of that, the knowledge related to diabetic control has globally been realized to be scanty.[ 9 ] Especially, no studies have been conducted on the general population in Tam Binh district, Vinh Long province, Vietnam, to assess the KAP of T2DM.
Therefore, this study aims to ascertain the impact of the knowledge, attitude toward diabetes, and practices of T2DM in Tam Binh district, Vinh Long province, Vietnam, which will further identify the relationship between KAPs in participants.
Materials and Methods
The participants.
This cross-sectional research was conducted on one group including 102 participants at 17 communes (six participants per commune) in Tam Binh district, Vinh Long province, Vietnam, from July to August 2019. The participants were randomly selected based on each local diabetic management list. Sampling criteria were patients aged 35–65 years with T2DM; diabetic duration from 6 months or more; those who were not hospitalized in the past 3 months; and those who did not have neurological abnormalities and malformations.
The knowledge, attitude, practice questionnaire
The KAP questionnaire was created by the researcher in both Vietnamese and English to suit Vietnamese culture [ Supplement Table 1 ]. The KAP questionnaire consists of four parts including (1) the demographic of the participants, (2) the knowledge of individuals with diabetes, (3) participants' attitude toward diabetics, and (4) participants' self-care management of diabetes. The knowledge part contains ten multiple choices with 1 score for each correct answer.

The practice of self-care section has ten questions about diabetic self-management. For a question that is divided into several subtleties, if the participant gives an incorrect answer any of details, the question was considered wrong. Each correct answer is scored “1;” on the other hand, an incorrect answer is scored “0.”
The scores are divided into three levels, namely, low level (<60% of the total points), moderate level (60%–79% of the total points), and high level (≥80% of the total points).[ 16 ]
Data collection
The questionnaire was reviewed by five experts with a doctoral or higher degree in Can Tho University of Medicine and Pharmacy, with an item objective congruence = 1 [ Supplement Table 2 ]. Then, the questionnaire was administered to ten participants in Tam Binh District Health Centre center with Cronbach's alpha = 0.738 [ Supplement Table 3 ]. The questionnaire was sent directly to each patient. The staffs would guide how to answer but they had absolutely no hint of the answer.
Supplement Table 2
The item objective congruence index
IOCI=Item objective congruence index
Supplement Table 3
The reliability and validity of the knowledge, attitude, and practice questionnaire
Statistical analysis
All collected data were coded before they were analyzed by IBM SPSS software version 22, IBM corporation. The descriptive statistics including frequency, mean, and standard deviation were used for evaluating participant characteristics and KAP score. Correlation between variables was assessed using Pearson's correlation coefficients. The relation between knowledge, practice, and attitude sections was analyzed by regression correlation. The significance level for all tests was fixed at α < 0.05.
Besides, age was separated into two groups as Group 1 from 35 to 49 years and Group 2 from 50 to 65 years. In addition, the duration of T2DM was divided into four groups as Group 1 under 10 years, Group 2 from 10 to 20 years, Group 3 from 20 to 30 years, and Group 4 over 30 years. Furthermore, the glycemic levels diverged into three groups such as group 1 under 3.9 mmol/L, Group 2 from 3.9 to 6.4 mmol/L, and Group 3 above 6.4 mmol/L. In addition, the HbA1c levels were divided into three groups as Group 1 below 4%, Group 2 from 4% to 6%, and Group 3 above 6%.
Participant demographic data
All the study patients (102) had an average age of 57.02 ± 6.32 years. The proportion of women accounted for 76.5% (more than three times of men, 23.5%). The ethnicity was Kinh who suffered the most from diabetes, 96.1%; 101 participants (99%) were married and are living with small families for 1–2 generations (73.5%), while 26.5% of the participants are living in large families over three generations. Most of the participants had primary to higher education (94.1%); only 5.9% of them were illiterate. Nearly 76.5% of the patients had jobs, both part time and full time, and the remaining (23.5%) did not work including retirement and unemployment. The majority of participants had a high monthly income of 82.4% (84 participants). The average duration of the diabetics was 4.33 ± 4.56 years, the longest was 22 years, the shortest was 0.5 years. The blood glucose level and HbA1c level of the participants were 9.60 ± 3.77 mmol/L and 7.40 ± 2.46%, respectively [ Table 1 ].
The demographic data and knowledge, attitudes, and practices of the participants
SD=Standard deviation, KAP=Knowledge, attitude, and practice
The participants' knowledge, attitudes, and practices
All patients completed the KAP questionnaire, in which the score was low (50.057 ± 10.644). Specifically, their knowledge score was low (30.04 ± 12.823). In particular, the majority of participants (97 people) had a low knowledge level of 95.1% [ Table 1 ]. Despite this, some knowledge had a quite high patient rate such as: “how many types of diabetes” were 71.6%; “the concept of type 2 diabetes” had 53.9%; “the symptoms of hypoglycemic” occupied 66.7%. However, their attitude score was moderate (61.544 ± 29.99). Among them, those with low attitudes accounted for more than half of the 52% (53 people), followed by those with an average attitude of 25.5% (26 patients), and those with high attitude22.5% (23 participants) [ Table 1 ]. In addition, the practice score was low at 50.59 ± 14.881. In this section, the practice was recorded as an average with 8.8% (14 people), six times lower than patients with a low level of practice of 86.3% (88 people). However, only 5.9% of the people with diabetes practiced high level of practice [ Table 1 ].
Regarding diabetic self-management practice, the highest percentage of patients treated with oral medication constituted 77.5% (77 participants), followed by insulin injections with 6.9% (7 patients) and diet therapy with 5.9% (6 participants); in addition, patients without treatment accounted for 11.8% (12 patients). The majority of patients using one type of drug to treat diabetes each day accounted for 56.9%. Two patients (2%) used six tables of diabetic drug per day. Patients in the study injected the insulin into the abdomen and shoulders [ Table 2 ].
The proportion of the components of practice section
The relation between participants' characteristics and knowledge, attitude, and practice
Table 3 describes the relation between patients' KAP and their characteristics such as age, gender, ethnicity, location, marital status, type of family, education level, employment status, monthly income, diabetic duration, diabetic information, glycemic level, HbA1c status, glycemic checking place, other disease, treatment method, hypoglycemia, smoking history, and drinking history. It showed a significant relationship in diabetic knowledge between Kinh and Khmer ethnic groups, as well as between groups of patients with different diabetic duration ( P = 0.000 and 0.043) [ Table 3 ]. Moreover, the results also described a statistically significant relationship between the patients' attitude to diabetes and different patient groups in terms of location ( P = 0.003) [ Table 3 ], employment status ( P = 0.000), treatment method, hypo-glycemia and diabetic duration. On the other hand, the research results also found a significant association between marital status and diabetic duration with patients' daily disease self-management practices [ Table 3 ].
The relation between patients’ characteristics and knowledge, attitudes, and practices by one-way ANOVA
The relation between knowledge, attitude, and practice
Table 4 shows the difference in knowledge and attitude of Type 2 diabetic patients between the different practice groups. In this relationship, only the difference in the practice of the attitude groups was statistically significant ( P = 0.014). There were also differences in knowledge between practice groups, but this was not statistically significant.
The relation between patients’ knowledge, attitude, and practice
Diabetes is a chronic metabolic disorder with many different complications.[ 5 ] Therefore, in order to control the disease effectively, patients need to have the right KAP about diabetes.[ 9 ] This study assessed diabetic patients' KAP of diabetes management. It also explored the relationship between KAPs of Type 2 diabetic patients.
The study was conducted on individuals aged between 35 and 65 years because at this age diabetes had been seem to be highly prevalent in Vietnam according to the 2002 National Statistical Survey ,[7 ] and it is also an age group of cognitive maturity. The median age of the patients in this study was 57.02 years, which is consistent with the study of Ng et al .[ 1 ] and Le Roux et al .[ 9 ] Like many other studies, this study had a higher proportion of women with Type 2 diabetes than men.[ 3 , 6 , 9 ] However, some studies report that diabetes is more common in men than in women,[ 5 , 17 ] but the difference was not significant.
Furthermore, Salem et al . also reported that the patients in their study were highly educated from high school and above.[ 13 ] Simultaneously, the study of Saengtipbovorn et al . reported that 76.5% of their participants had completed primary school education.[ 2 ] Similarly, this study found that most patients had primary or higher level of education (93%). Nevertheless, a study in Iran by Mohammadi et al . found that nearly 27 illiterate patients, but the majority (41%) of the study participants, were not attending primary school.[ 18 ] The low levels of education were also found in the study by Al-Maskari et al . with 46% illiteracy.[ 19 ]
Most patients had a job, so their income was high. Concurrently, a study by Saengtipbovorn et al . showed that 37.1% of the study participants earned <1500 baht per month.[ 2 ] In addition, a study by Mohammadi et al . found that only 27% of the patients had jobs and their monthly income was <8,000,000 Rials.[ 18 ] The average duration of diabetes in the study by Al-Maskari et al . was 9 years.[ 19 ] Rahaman et al . also showed that the average duration of diabetes was 9.16 ± 6.03 years.[ 20 ] However, patients in the current study had a significantly lower duration of Type 2 diabetes than the previous two studies (4.33 ± 4.56 years). More than half of the patients have received information about diabetes. However, Rahaman et al . reported that only 38.6% of the patients participated in a diabetes-related education program.[ 20 ] About one-quarter (26%) of the patients in the study by Magbanua and Lim-Alba participated in the diabetes education.[ 21 ]
Most patients had at least one other condition related to diabetes (95.1%) such as hypertension, hypercholesterolemia, heart disease, vision problems, neurological problems, poor sexual desire, and kidney problems. These issues were also found in the study by Mohammadi et al . in Iran.[ 18 ] Participants' blood sugar and HbA1c levels were quite high. High levels of HbA1c were also found in the study by Al-Maskari et al .[ 19 ] and Rahaman et al .[ 20 ] Rahaman et al . also showed that blood glucose levels were also high, although participants tested their own blood glucose levels at home and in the hospital.[ 20 ] However, patients in this study did not self-test their blood glucose and HbA1c level; most of them checked it at government hospitals and a few did at private clinics. Moreover, the results of this study showed that patients with poor glycemic control have a relatively high rate of hypoglycemia (59.8%).
Similar to the research by Karaoui et al .,[ 22 ] most patients in the present study have used oral medications to control the disease. In addition, this result was similar to those of Salem et al .,[ 13 ] with high smoking denial rates. Similar results were found in the study of Saengtipbovorn et al . with the rate of never smokers up to 87.1%.[ 2 ] In contrast, Karaoui et al . reported that more than half of the smoking patients participated in the study.[ 22 ] Correspondingly, the alcohol consumption rate in this study was low.
The related of knowledge within people with diabetes
The analysis showed that participants' knowledge of diabetes was still low. This was because patients had not been provided with basic information about Type 2 diabetes. This problem had also been reported by Cao My Phuong et al .[ 23 ] Nhung and Dao showed that knowledge about diabetes treatment and complications of the patients was low.[ 24 ] In addition, a research by Karaoui et al . showed that the knowledge base of diabetes in the research population was still low.[ 22 ] Indeed, Rahaman et al . reported a lack of diabetic knowledge in the research community.[ 20 ] Indeed, the study by Quang et al . also indicated that the number of participants without knowledge about diabetes was quite high.[ 7 ]
Attitude toward diabetes in Vietnamese culture
Al-Maskari et al . concluded that although patients have poor knowledge, a positive attitude was an important issue in the care and practice of diabetes.[ 19 ] Meanwhile, Salem et al . stated that, although most patients have the knowledge of diabetes, it was not at a high level, and their attitude and practice were not satisfactory.[ 13 ] Similarly, this study also showed that participants had an average attitude level toward diabetes.
Practice of self-care management
The participants' diabetes management practices were generally poor. This showed that a medium attitude score is not enough; it requires good knowledge to lead to the right practices to control diabetes. Ng et al . concluded that factors of proper knowledge and attitude led to good disease control practices.[ 1 ] Saadia et al . also confirmed that the participants' knowledge of diabetes in research was good, but their attitude and practice were poor.[ 25 ]
The relation of participants' components and knowledge, attitude, and practice
Our research shows that most of the relationships between participants' characteristics and their KAPs had a negligible difference. However, there were some significant relational characteristics, such as race and blood sugar that differed significantly in knowledge about Type 2 diabetes; marital status and family type were statistically significantly related to the patient's attitude toward the disease. Moreover, gender, marital status, education, and monthly income were significantly related to diabetes control practices. Similarly, Ghannadi et al . also showed that the relationship between sex and marital status with KAP was not statistically significant.[ 17 ] However, Salem et al . reported that there was a significant relationship between KAP scores and different categories such as location, gender, and education.[ 13 ] Moreover, Ng et al . showed a significant inverse correlation between KAP scores and HbA1c.[ 1 ]
The relation of knowledge and attitude with practice
The results of this study showed that the relationship between patient attitude groups and practical components was statistically significant. However, this was not found in the relationship between knowledge and attitude of diabetic patients. This was due to the culture of the Vietnamese people. Indeed, the study of Al-Maskari et al . also found that there was a significant relationship between practice and attitude of patients, but the authors also reported more meaningful results between attitude and knowledge.[ 19 ] Meanwhile, the study by Ghannadi et al . showed that higher knowledge was significantly correlated with higher attitudes and practices.[ 17 ]
Although KAP of self-control in diabetes are important contributions to the good treatment of the disease, patients in the study had low scores for these issues. Despite the average attitude about Type 2 diabetes, limited knowledge about the disease is not sufficient, the lack of which leads to poor practices of care and control. However, the results showed that there was only significant difference between attitude and practice in patients with Type 2 diabetes. Furthermore, the relationship between KAP with patients' characteristics had different significance.
Financial support and sponsorship
This article is a part of my thesis “The development of health-related quality of life programme among type 2 diabetic patients in Tam Binh District, Vinh Long Province, Vietnam,” which is accepted by the ethical committee for the fieldwork of Mahasarakham University; with the certificate of approval number of 071/2019.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the participants and the local Government from Tam Binh District, Vinh Long Province, Vietnam, and Dr. Ngo Van Truyen PhD, MD, Dean of Faculty of Medicine; Dr. Le Van Minh PhD, MD, Vice Dean of Faculty of Medicine and Deputy Head of the Department of Interventional Cardiology-Neurology; Dr. Tran Kim Son PhD, MD, Department of Internal Medicine; Dr. Vo Pham Minh Thu PhD, MD, Head of the Personal Department and Dean of Department of General Medicine; Dr. Nguyen Thi Diem PhD, MD, Faculty of Medicine and ethics committee and public health faculty of Mahasarakham University, Thailand, who had made the study possible, and the health commune staffs and the research sampling groups.
Supplement Table 1: Knowledge, Attitude, and Practice Questionnaire

MAHASARAKHAM UNIVERSITY
DIABETIC KNOWLEDGE, ATTITUDE, PRACTICE
Participant Number (Office use): ___________
Date: ___________________
A. PARTICIPANT INFORMATION
- Full name: ______________________________________________________
- Birth year: __________________
- Gender: □ Male □ Female
- Address: _______________________________________________________
- Glycemia: ______________ mmol/L
- HbA 1 C: ________ %
B. DIABETIC KNOWLEDGE
Please circle in the letter that you think is the best.
- Diabetes is a chronic metabolic disorder characterized by hyperglycemia
- Diabetes is a chronic metabolic disorder with a manifestation of hypoglycemia
- Diabetes is a disease spread in the community
- Because the body produces lack or does not produce insulin
- Because the body is resistant to insulin (usually occurs in obese people and >40 years old)
- Occurs in pregnant women (no previous diabetes)
- People who are obese, sedentary, eat a lot of fat, sweet, starch, alcohol, tobacco, family history of diabetes
- Muscular people, exercise regularly, eat well, do not smoke, do not drink alcohol
- Thin people, eat normally, have no family history of diabetes
- Eat a lot, drink a lot, lose weight a lot, urinate a lot
- Eating normally, losing little weight, moderate urination
- Eat less, lose weight, urinate often
- One type: acute complications
- Two types: acute complications and chronic complications
- Three types: acute complication, subacute complication and chronic complication
- Hyperglycemia and foot ulcer
- Insomnia, anxiety and weight loss
- Hypoglycemia and coma due to hyperglycemia, ketoacidosis and lactic infections
- Hypoglycemia and coma
- Cardiovascular complications, decreased vision, kidney failure, impotence, foot ulcers
- Insomnia, anxiety, difficulty breathing
- Routine blood glucose testing, prescription medication, reasonable eating, proper exercise
- There is no need for routine blood glucose testing, no need for food, no medication, and limited movement
- Test whenever you want, just taking the medicine is enough without don't need the well eating and exercise
- High fever, cold shaking
- Uncomfortable, sweating, dizziness
- Abdominal pain, difficulty breathing
C. DIABETIC ATTITUDE
Please circle the answer you choose
1. Do you agree that blood glucose testing for you and your family is necessary?

2. Do you agree that diabetes can be well controlled?
3. Do you agree that blood sugar can be controlled by exercise, sports and medicine?
4. Do you agree with a reasonable diet that can control blood sugar?
5. Do you agree with the need to have regular medical checkups and blood sugar checks?
6. Do you agree that complications of diabetes are a very serious problem?
7. Do you agree that prevention of complications is important in treating diabetes?
8. Do you agree that daily exercise can control diabetes complications?
9. Do you agree about worrying about hypoglycemic complications?
10. Do you agree with taking care of your feet while treating diabetes?
D. DIABETIC PRACTICE
Please answer all the questions below
1. Which method do you treat diabetes with?
□ Oral medicine. How many tablets per day? ____ tablets. How many times
per day? ____ times
□ Insulin injection. How many times of injection? ____________ times.
Injection site? ___________________
2. Do you have regular blood sugar tests? ___ yes ___ no
Where do you check? ______________________ How often? ____________
3. Do you have an HbA1C test? _____ has _____ no
Where do you check? ______________________ How often? _________
4. Do you exercise regularly? ______ yes _______ no
How long is a day? ___________ How many days per week? ____________
Which method do you exercise? ___________________________________
Do you know exercise can lower blood sugar? ___ yes ___ no
5. How many meals do you eat a day? _______________________________
Should you skip meals? ______ yes _______ no
6. What kind of foods do you need to limit or reduce?
______________________________________________________________
7. Do you smoke cigarettes? _______ has ________ no
How many cigarettes per day? _________________ cigarettes
How long have you smoked? __________________________
8. Do you drink alcohol? ________ yes ________ no
If yes, what is the level of drinking? _______________________________
9. Have you ever had hypoglycemia? _____ has _______ not yet
If so, how did you handle it? __________________________________
10. How do you take care of your feet?
THANK YOU FOR YOUR ANSWERS!
- Search Menu
- Sign in through your institution
- Cytogenetics
- Cytotechnology
- Histotechnology
- Management/Administration
- Microbiology
- Molecular Biology
- Molecular Pathology
- Transfusion Medicine
- Advance articles
- COVID-19 articles
- Current Virtual Issue
- Cover Archive
- Author Guidelines
- Open Access
- Submission Site
- Why publish?
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Laboratory Medicine
- About the American Society for Clinical Pathology
- Editorial Board
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
- Introduction
- Acknowledgments
- Conflict of Interest Disclosure
- Data Availability
A positive correlation of serum SFRP1 levels with the risk of developing type 2 diabetes mellitus: a case-control study
- Article contents
- Figures & tables
- Supplementary Data
Ahmed Salim Najm Alhilfi, Reza Afrisham, Alireza Monadi Sefidan, Reza Fadaei, Nariman Moradi, Lotfollah Saed, Nahid Einollahi, A positive correlation of serum SFRP1 levels with the risk of developing type 2 diabetes mellitus: a case-control study, Laboratory Medicine , 2024;, lmae030, https://doi.org/10.1093/labmed/lmae030
- Permissions Icon Permissions
Secreted frizzled-related protein 1 (SFRP1) is an adipokine whose production is significantly altered in metabolic disorders. Considering the relationship between dysfunction of Wnt/β-catenin signaling and metabolic disorders as well as the inhibitory effects of SFRP1 on this signaling pathway, the present work aimed to investigate the correlation between serum SFRP1 levels and type 2 diabetes mellitus (T2DM) and its developing risk factors for the first time.
This case-control study measured serum levels of SFRP1, tumor necrosis factor (TNF)-α, interleukin (IL)-6, adiponectin, and fasting insulin using enzyme-linked immunosorbent assay kits in 80 T2DM patients and 80 healthy individuals. Biochemical parameters were determined using the AutoAnalyzer instrument.
The T2DM group had higher levels of SFRP1 compared with the controls (146.8100 ± 43.61416 vs 81.9531 ± 32.78545 pg/mL; P < .001). There was a positive correlation between SFRP1 and insulin ( r = 0.327, P = .003), TNF-α ( r = 0.420, P < .001) as well as homeostatic model assessment for insulin resistance ( r = 0.328, P = .003) in the T2DM group. In addition, 10-unit changes in SFRP1 levels showed the risk of T2DM in both the unadjusted (odds ratio [OR] [95% CI] = 1.564 [1.359-1.800]) and adjusted models accounting for age, gender, and body mass index (OR [95% CI] = 1.564 [1.361-1.799]; P < .001). A cut-off value of SFRP1 (105.83 pg/mL) was identified to distinguish between the T2DM patients and the healthy subjects, with sensitivity of 75.0% and specificity of 80.0%.
According to our research, there was a significant and positive link between the amount of SFRP1 and the likelihood of developing T2DM as well as the related factors like insulin resistance index and TNF-α. These results indicated that SFRP1 might have a potential role in the development of T2DM.
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1943-7730
- Print ISSN 0007-5027
- Copyright © 2024 American Society for Clinical Pathology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 26 May 2024
An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory
- Tongyue Yang 1 ,
- Feng Qi 2 , 3 ,
- Feng Guo 1 ,
- Mingwei Shao 1 ,
- Yi Song 1 ,
- Gaofei Ren 1 ,
- Zhao Linlin 1 ,
- Guijun Qin 1 &
- Yanyan Zhao ORCID: orcid.org/0000-0001-6294-9447 1
Molecular Medicine volume 30 , Article number: 71 ( 2024 ) Cite this article
168 Accesses
Metrics details
Diabetes mellitus, a chronic metabolic disease, often leads to numerous chronic complications, significantly contributing to global morbidity and mortality rates. High glucose levels trigger epigenetic modifications linked to pathophysiological processes like inflammation, immunity, oxidative stress, mitochondrial dysfunction, senescence and various kinds of cell death. Despite glycemic control, transient hyperglycemia can persistently harm organs, tissues, and cells, a latent effect termed "metabolic memory" that contributes to chronic diabetic complications. Understanding metabolic memory's mechanisms could offer a new approach to mitigating these complications. However, key molecules and networks underlying metabolic memory remain incompletely understood. This review traces the history of metabolic memory research, highlights its key features, discusses recent molecules involved in its mechanisms, and summarizes confirmed and potential therapeutic compounds. Additionally, we outline in vitro and in vivo models of metabolic memory. We hope this work will inform future research on metabolic memory's regulatory mechanisms and facilitate the development of effective therapeutic compounds to prevent diabetic complications.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by elevated blood glucose caused by deficiency or resistance to insulin (Joslin 1946 ). Chronic hyperglycemia can lead to multiple organ injury, thereby causing various complications, such as diabetic retinopathy (DR), diabetic kidney disease (DKD), and diabetic cardiovascular disorders (Zheng et al. 2018 ). Epidemiological studies have revealed that DM has emerged as a significant threat to human mortality. At present, the International Diabetes Federation (IDF) estimates that DM affects approximately 536.6 million adults worldwide, and that number is expected to increase to 783.2 million by 2045 (Sun et al. 2022 ).
In addition to its high incidence, the pathogenesis of diabetic complications is also very complex. In the early stages of DM, hyperglycemia induces oxidative stress and excessive advanced glycation end product (AGE) formation (Domingueti et al. 2016 ). As the disease progresses, protein glycation and mitochondrial DNA (mtDNA) damage to respiratory chain components can in turn exacerbate oxidative stress injury (Bhatti et al. 2022 ). Metabolic imbalance then promotes inflammation through binding receptors for glycation products to cause senescence or cell death (Takahashi et al. 2022 ; Phoenix et al. 2022 ; Teodoro et al. 2018 ). These structural changes can lead to various diabetes-related vascular complications (Teodoro et al. 2018 ). To improve the mechanisms described above, multiple novel hypoglycemic agents, such as sodium glucose co-transporter 2 inhibitor (SGLT2i), dipeptidyl peptidase 4 inhibitors (DPP4i) and glucagon-like peptide 1 receptor agonists (GLP-1RAs), have been applied in clinical practice (Mouhayyar et al. 2020 ; Nathan et al. 2013 ; Zhang and Wu 2014 ) (Mostafa et al. 2016 ; Mostafa et al. 2015 ). However, early hyperglycemia can still lead to a variety of diabetic complications. Fortunately, the novel concept of “metabolic memory” may explain this phenomenon. Metabolic memory, also known as hyperglycemic memory, arises from the enduring presence of an underlying driver. The persistence of cellular changes and characteristics represents the organism's recovery of a prior metabolic state, potentially playing a pivotal role in the etiology of DM and its chronic complications (Reddy et al. 2015 ).
In this comprehensive review, we aim to delve into the research chronology and distinct characteristics of metabolic memory. Additionally, we present a summary of the diverse molecular mechanisms that govern its regulation. By emphasizing its prevalence and profound implications, we highlight the significance of metabolic memory in various chronic diabetes complications. Furthermore, we delve into potential mechanisms and pharmacological advancements related to metabolic memory. Additionally, we consolidate information on various in vitro and in vivo models of metabolic memory. We hope that this review can offer valuable insights into the intricacies of metabolic memory, thereby paving the way for novel therapeutic strategies for the treatment of DM and its complications.
Overview of metabolic memory
The metabolic memory of diabetes refers to the observation that patients are vulnerable to developing diabetic complications due to early hyperglycemia, even if effective hypoglycemic agents are taken to maintain blood glucose within normal levels in the later stage of DM. As shown in Fig. 1 A, in 1987, Engerman et al. (Engerman and Kern 1987 ) first described the phenomenon of metabolic memory that decreased hyperglycemia to normal levels after 2.5 years of exposure in diabetic dogs, and the incidence of DR was still high (Engerman and Kern 1987 ). In addition, high glucose caused an increase in fibronectin and collagen IV expression that could not be reversed even after restoration to normal levels in diabetic rats in 1990 (Roy et al. 1990 ). In 1993, Hammes et al. (Hammes et al. 1993 ) further described the exposure time more accurately. Their research indicated that islet transplantation to diabetic rats could prevent the occurrence of DR within 6 weeks after onset. However, at 12 weeks after onset, DR still occurred. Later, in 2003, the Diabetes Control and Complications Trial (DCCT) with further follow-up in the Epidemiology of Diabetes Interventions and Complications (EDIC) study (DCCT/EDIC), where the concept of "metabolic memory" was first proposed, demonstrated that initial hyperglycemia still increased the risk of long-term diabetic complications, although the HbA1c of the intensive treatment group and conventional treatment group was maintained at similar levels (Writing Team 2003 ). In 2008, the United Kingdom Prospective Diabetes Study (UKPDS) again demonstrated the term “legacy effect”, in which early intensive glucose lowering can lead to long-term benefits in patients with newly diagnosed type 2 diabetes (Holman et al. 2008 ; Ranjit Unnikrishnan et al. 2011 ). Both "metabolic memory" and the “legacy effect” refer to the long-term effects of blood glucose on macrovascular and microvascular complications of diabetes. However, the concept of metabolic memory may focus on the negative effect of hyperglycemia impairment, while the legacy effect mainly focuses on the positive influence of effective treatments.
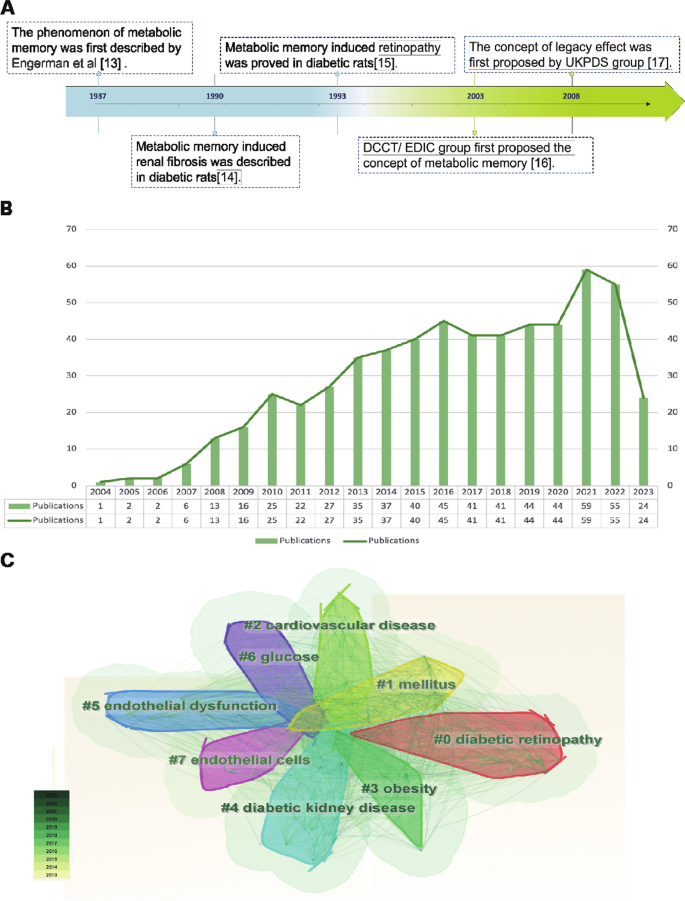
Overview of metabolic memory. A Chronological depiction of key events in the development of metabolic memory. B , C Bibliometric analysis exploring the intersection of metabolic memory and diabetic complications. Search criteria were set as follows: TS = ((“metabolic memory” OR “hyperglycemic memory”) AND (“diabetes” OR “diabetic”)) with a date range of DOP = (2013–08-01/2023–08-01). B Illustration of the annual trend in the number of published articles. C Clustered view of the key terms and concepts emerging from the literature
The bibliometric analysis of the research published on metabolic memory in the decade following its formal designation in 2004. Based on the information provided by the Web of Science (webofscience.com), we analyzed the scientific output related to metabolic memory and diabetes from 2000 to 2022. In total, 579 articles were identified. The trend of research related to metabolic memory and diabetes is displayed in Fig. 1 B, which shows a steady upward trend since its official naming in 2004, particularly in 2021–2022. Cluster analysis of high-frequency keywords related to metabolic memory and diabetes was performed using CiteSpace (Fig. 1 C). The clustering outcomes revealed a preponderance of research centering on the interplay between metabolic memory and diabetes, with a focus on DR, cardiovascular disease, endothelial dysfunction, DKD and obesity. Notably, obesity is intricately intertwined with glycemic and metabolic homeostasis, as evident in previous studies (El-Mesallamy et al. 2013 ; Aboouf et al. 2015 ; Khella et al. 2017 ). However, Zapata et al. ( 2022 ) also observed that obesity elicits a persistent metabolic imprint that persists despite weight loss, phenotypically resembling metabolic memory. Despite this, the existing literature consistently associates metabolic memory with glycemic fluctuations. This preponderance of findings can be partially attributed to the inherent constraints of bibliometric analysis, including the challenges associated with the precision and breadth of bibliographic databases, the absence of contextual understanding, and potential biases towards high-impact journals or specific research domains. Consequently, there is a pressing need for further exploration in this realm to clarify the intricate relationships among obesity, metabolic memory, and glycemic fluctuations. Our review primarily centered on metabolic memory and the potential long-term health implications of transient abnormalities in glucose metabolism.
The main molecular mechanisms of metabolic memory
The underlying mechanisms of metabolic memory and diabetic complications include inflammation and immunity, oxidative stress and mitochondrial dysfunction, senescence and various kinds of cell death. In fact, these mechanisms involve crosstalk with each other (Galicia-Garcia, et al. 2020 ; Berezin 2016 ). Epigenetic modifications can lead to inflammation, oxidative stress, and senescence, which in turn can be regulated by these mechanisms (Fig. 2 ).
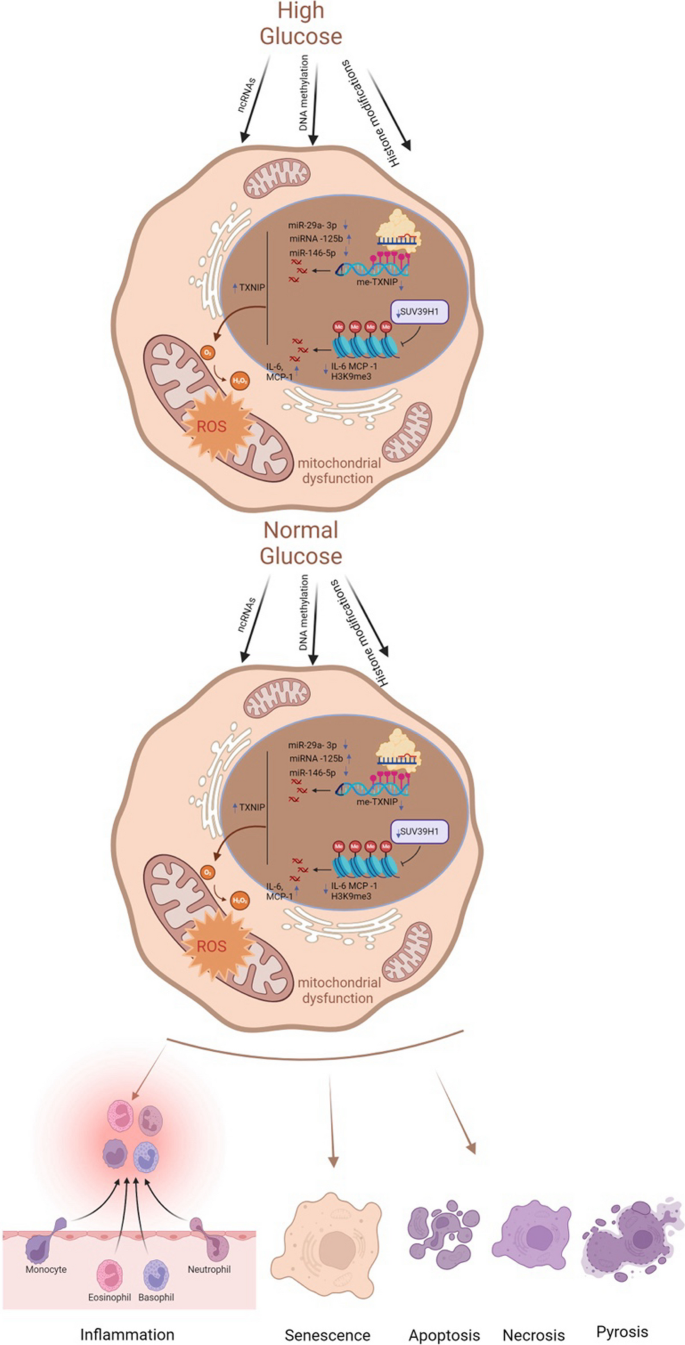
Key molecular mechanisms of metabolic memory. Despite the normalization of glucose levels, epigenetic modifications, inflammatory and immune responses, oxidative stress, mitochondrial dysfunction, cellular senescence, and apoptosis persist. These processes constitute the core molecular mechanisms underlying metabolic memory. ncRNAs noncoding RNAs, TXNIP thioredoxin-interacting protein, me-TXNIP thioredoxin-interacting protein, IL-6 interleukin-6, MCP-1 monocyte chemotactic protein 1, H3K9me3 trimethylated histone H3 at lysine 9, ROS reactive oxygen species
Epigenetic mechanisms involved in metabolic memory
Epigenetic mechanisms, including DNA methylation, histone modifications and noncoding RNAs (ncRNAs), can influence transcription activity and the generation of a heritable phenotype without changing DNA sequences (Goldberg et al. 2007 ). Emerging studies have indicated a key role for epigenetic modifications in the regulation of physiological and pathological processes associated with diabetic complications and metabolic memory (Chen and Natarajan 2022 ). Thus, this section mainly focuses on various modifications involved in hyperglycemic memory.
DNA methylation
DNA methylation, the most stable and widely reported epigenetic mechanism, is considered the primary transcriptional regulator. To investigate the relationship between hyperglycemic memory and DNA methylation, Chen et al. ( 2016 ) selected patients with type 1 diabetes mellitus (T1DM) from DCCT and EDIC studies. They discovered twelve distinctively annotated differentially methylated loci that exhibited a strong association with hyperglycemia and were intricately linked to diabetic complications. Notably, among these loci, thioredoxin-interacting protein ( TXNIP ) is a pivotal gene in the pathogenesis of diabetic complications. Transient hyperglycemic episodes were found to trigger hypomethylation at the 3’ untranslated region (3′ UTR) of TXNIP, leading to persistently elevated expression of this protein in peripheral blood cells (Thielen and Shalev 2018 ). This, in turn, triggered oxidative stress and triggered apoptotic and pyroptotic processes (Choi and Park 2023 ). Moreover, Park et al. ( 2014 ) derived foot fibroblasts from patients with diabetes with or without ulcers and from nondiabetic subjects without foot ulcers. Then, foot fibroblasts from patients with DM were cultured for four passages under normoglycemic conditions, and global and genome-wide DNA methylation profiles were used to identify alterations in DNA methylation. Their results illustrated that DNA methylation and metabolic memory were associated with poor wound healing outcomes in patients with diabetic foot ulceration. Similarly, proximal tubular epithelial cells (PTECs) derived from patients with or without diabetes were cultured via normoglycemic culture for four passages. After integrative omics analysis, multiple changes in DNA methylation sites were detected; among these changes, HNF4A may regulate epigenetic and hyperglycemic memory in DKD (Bansal et al. 2020 ).
In summary, these studies suggest that DNA methylation plays a vital role in metabolic memory and diabetic complications. In addition, as DNA methylation is involved in hyperglycemic memory, a review speculated that emerging m6A RNA methylation may also be a potential mechanism (Kumari et al. 2021 ). However, this theory remains to be confirmed in the future.
Histone modifications
Histones, including the corehistones H2A, H2B, H3, and H4 and the linker histone H1, can bind tightly to DNA to form nucleosome structures. Histone posttranslational modifications (HPTMs) refer to covalent modifications in which different modifications are added to one or several amino acid residues on the tails of histones. The modified histones change the loose or tight binding state between histones and DNA to effectively regulate gene transcription. The most common HPTMs are acetylation (Kac) and methylation (Kme) (Jin and Jeong 2023 ; Sun et al. 2023 ). Filgueiras et al. ( 2017 ) demonstrated that STAT1/MyD88 mRNA and protein levels remained elevated for a minimum of six days in macrophages from diabetic mice. This upregulation could be attenuated by the histone acetyltransferase (HAT) inhibitor anacardic acid. Furthermore, in the skeletal muscle tissue of diabetic mice, persistent enhanced Ped/Pea-15 expression was related to histone H3 lysine 4 monomethylation (H3K4me1) but not histone H3 Lys27 acetylation (H3K27Ac). The high expression of H3K4me1 remained stable even after re-exposure to 5 mM glucose-containing medium. However, there was a prompt loss of acetylation at K27 on histone H3 and a reduction in p300 recruitment at Ped/Pea-15 (Vastolo et al. 2018 ). In addition to H3K4me1, H3K9me3, a crucial repressive and relatively stable epigenetic chromatin mark, also contributes to metabolic memory in vascular smooth muscle cells (VSMCs) derived from db/db mice. The persistent downregulation of H3K9me3 and the inflammatory phenotype could be reversed by overexpressing suppressor of variegation 3–9 homolog 1 (Suv39h1), which is a histone methyltransferase (Sun et al. 2023 ). H3K4me1 and H3K9me3 also regulate metabolic memory in CMs and vascular endothelial cells, respectively (Yu et al. 2012 ; Okabe et al. 2012 ; Mao et al. 2019 ). Regrettably, relatively few studies on HPTMs and metabolic memory, especially some emerging HPTMs, such as lactylation, ubiquitination and glycosylation.
ncRNAs, which mainly include microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), play a vital role in diabetes and its complications as well as multidrug resistance (Li et al. 2022a ; Mahmoud et al. 2021 ). As another major mechanism of epigenetic regulation, ncRNAs can regulate gene expression by modulating protein synthesis at the posttranscriptional and translational levels (Taft et al. 2010 ). miRNAs, a class of endogenous single-stranded RNAs composed of 20–22 nucleotides, can participate in regulating posttranscriptional gene expression by binding to target mRNAs (Krol et al. 2010 ). Currently, various miRNAs have been reported to participate in metabolic memory and diabetic complications. To identify hyperglycemic memory-related miRNAs in human aortic endothelial cells, Zhong et al. ( 2015 ) used a miRCURY LNA array to screen for transcriptional changes in the normal glucose, high glucose and metabolic memory groups. After validation in vitro and in vivo, miR-125b, miR-29a-3p, and miR-146a-5p were shown to potentially be important for metabolic memory. Notably, miR-125b was the only miRNA confirmed to be related to metabolic memory, specifically targeting Suv39h1 to promote inflammation in VSMCs from diabetic mice (Villeneuve et al. 2010 ). Subsequently, Costantino et al. ( 2016 ) screened 268 miRNAs that remained significantly altered after 3 weeks of intensive glycemic control with insulin from heart samples. The majority of miRNAs related to metabolic memory effects, according to an ingenuity pathway analysis, regulate the myocardial pathways of apoptosis, autophagy, oxidative stress, fibrosis, hypertrophy and heart failure. Regrettably, they verified miRNA expression in left ventricular samples from controls, diabetic mice, and diabetic mice treated with insulin without further exploring the underlying mechanisms involved. In addition, miR-23b-3p has been proven to regulate high glucose-induced metabolic memory via the SIRT1-dependent signaling pathway in DR (Zhao et al. 2016 ). However, in-depth studies of the links between key lncRNAs and the crosstalk between lncRNAs and miRNAs in metabolic memory and diabetic complications still need further exploration.
Inflammation, immunity, oxidative stress and mitochondrial dysfunction
High blood glucose can induce chronic metabolic inflammation, which contributes to the development of various complications (Nedosugova et al. 2022 ). Monocytes and macrophages, crucial components of immunity, participate in inflammation in diabetic complications. The proinflammatory activation of macrophages within the liver and adipose tissue can initiate the recruitment and promotion of macrophage polarization, thereby inducing these cells to secrete inflammatory cytokines, including IL-1β, IL-6, and TNF-α. This, in turn, results in immune imbalance, highlighting the critical role of macrophage activation in the pathogenesis of inflammatory conditions (Bleriot et al. 2023 ; Ding et al. 2022 ). To further investigate the intricate relationships among inflammation, immunity, and metabolic memory, Mossel et al. ( 2020 ) investigated metabolic memory in primary human macrophages. Their findings revealed that even after normalizing glucose levels, the expression of S100A9 and S100A12 remained elevated, potentially due to transient hyperglycemia-induced histone methylation at the promoters of these genes. In addition, innate immune cells, which are integral to diabetes-related complications, can establish nonspecific immunological memory (trained immunity) through epigenetic regulation. Thiem et al. ( 2021 ) established both in vitro and in vivo trained immunity models using bone marrow cell transplantation and monocyte isolation. Their study demonstrated that glucose modulation of innate immune cell histone methylation levels can persist, leading to increased glycolysis and exacerbated inflammatory responses even after glucose normalization. Given these insights, diabetes and its complications related to oxidative stress and inflammation, as well as immunity, can significantly benefit from vitamin E intake (Hamdy et al. 2009 ).
In addition to the aforementioned factors, oxidative stress and mitochondrial dysfunction play essential roles in metabolic memory (Peng, et al. 2020 ). An imbalance between oxidative and antioxidative processes gives rise to oxidative stress, which can trigger lipid accumulation, inflammation, and fibrosis in diabetic complications (Zhang et al. 2020 ). Reactive oxygen species (ROS), a hallmark of oxidative stress, encompass a range of free radicals, including superoxide anions, hydroxyl and peroxyl radicals, and other compounds capable of generating free radicals (Halliwell 2006 ). Since mitochondria are key intracellular sources of ROS, mitochondrial dysfunction is intimately linked to oxidative stress (Cojocaru, et al. 2023 ). Multiple studies have established that oxidative stress and mitochondrial dysfunction are integral to the mechanism of metabolic memory in diabetic complications, particularly in the progression of DR (Wang et al. 2018 ; Zhong and Kowluru 2013 ; Voronova et al. 2017 ; Drzewoski et al. 2009 ). Sirtuin-1 (SIRT-1) functions as a modulator of antioxidant defense, energy metabolism, and organelle homeostasis, making it a key player in oxidative stress and mitochondrial dysfunction in various diseases (Kung, et al. 2021 ; Li et al. 2022b ). Lee et al. ( 2022 ) demonstrated that SIRT-1 was a link between hyperglycemic memory and oxidative stress and mitochondrial dysfunction in DR. Additionally, Kowluru et al. ( 2023 ) provided evidence that transient hyperglycemia results in a persistent imbalance in mitochondrial fission, mitophagy, and new mitochondrial formation, ultimately leading to oxidative stress in DR. Beyond mitochondrial dysfunction, oxidative stress intersects with other organelle dysfunctions, including endoplasmic reticulum (ER) stress, Golgi apparatus stress, and lysosomal homeostasis (Maamoun et al. 2019 ; Gong et al. 2022 ; Jiang et al. 2011 ). However, the intricate relationships between these processes remain largely unexplored and require further investigation.
Senescence and cell death
Cellular senescence, a type of permanent proliferative arrest without cell death, is divided into epigenetically induced senescence, oxidative stress-induced senescence and DNA damage-induced senescence (Hernandez-Segura et al. 2018 ). The process of senescence is closely related to programmed cell death (PCD) (Galluzzi and Myint 2023 ). When cellular damage cannot be efficiently repaired, irreversible dysfunction of cells can lead to PCD, including apoptosis, autophagy, pyroptosis and ferroptosis (Moujalled et al. 2021 ). Recently, a p21 -dependent pathway was identified that contributes to senescence and hyperglycemic memory in DKD (Al-Dabet et al. 2022 ). Furthermore, Mansour et al. ( 2023 ) demonstrated that overexpressed p21 can lead to senescence and increase the expression of BAX, a pro-apoptotic gene, to alleviate apoptosis. These results indirectly illustrate that p21, a key gene in metabolic memory, also participates in senescence and apoptosis and may be a promising target. Moreover, in DR, temporary high glucose could lead to consistent upregulation of miR-195 to decrease the expression of its target gene Bcl-2 , which is an antiapoptotic gene (Liu et al. 2019a ). This research suggested that epigenetic mechanisms, as representative ncRNAs, may interact with senescence and cell death in hyperglycemic memory. Nevertheless, how do other types of cell death regulate metabolic memory in diabetic complications? This question is still unanswered.
Metabolic memory and chronic complications of DM
Multiple large-scale clinical trials have verified that early intensive glycemic control can reduce the incidence and progression of macrovascular and microvascular complications of diabetes, including diabetic cardiovascular disorders, DKD, DR, and diabetic foot disease (DF) (C., I. 2003 ; Cuore et al. 2023 ; Brown et al. 2010 ; Nathan et al. 2014 ; Aiello et al. 2014 ), which is basically consistent with the results of our bibliometric analysis (Fig. 1 C). Numerous studies have also used experiments to elucidate the mechanisms underlying this clinical phenomenon in diabetic complications (Yamagishi et al. 2017 ; Zhong et al. 2023 ; Kato and Natarajan 2019 ). Thus, in this section, we will discuss the relationship between metabolic memory and chronic complications of DM (Fig. 3 ).
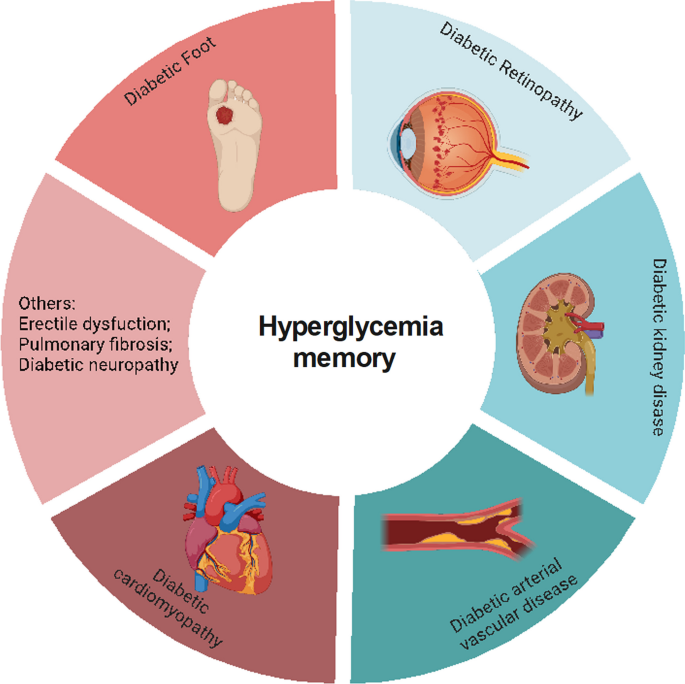
Metabolic memory and chronic complications of diabetes. Hyperglycemia can trigger a range of diabetic complications, including diabetic cardiomyopathy, diabetic arterial vascular disease, diabetic kidney disease, diabetic retinopathy, and diabetic foot. This figure illustrates the intricate relationship between metabolic memory and these chronic conditions
Diabetic cardiovascular disorders and metabolic memory
Diabetic cardiovascular disorders, including diabetic cardiomyopathy (DCM) and arterial vasculopathy, are the leading causes of death among patients with diabetes (Fang et al. 2004 ). Elevated blood glucose stimulates inflammation, regulates immune cells, and promotes the production of cytotoxic free radicals, thereby attacking myocardial cells and vascular endothelial cells (Johnson et al. 2022 ; Xie et al. 2022 ). Under the action of these mechanisms triggered by high glucose, damaged cells further secrete harmful irritants, which promote the transdifferentiation of other cell types into cardiac fibroblasts (Cheng et al. 2023 ). Subsequently, various adhesion molecules and adipokines, such as adiponectin, influence these fibroblasts, activating them to migrate and aggregate, thus exacerbating myocardial and vascular injury (El-Mesallamy et al. 2011 ). However, evidence from clinical trials has indicated that even with intensified blood glucose control, patients with diabetes are still at risk for diabetic cardiovascular diseases due to metabolic memory. This section discusses the relationship between diabetic cardiovascular diseases and metabolic memory.
DCM and metabolic memory
DCM is a cardiovascular complication that arises from DM and causes alterations in cardiac structure and function, independent of hypertension, coronary atherosclerotic heart disease, or any other known cardiac risk factors (Jia et al. 2018 ). Previous studies have established that metabolic dysfunction in cardiomyocytes, myocardial interstitial fibrosis, abnormal calcium transients in cardiomyocytes, and cardiac autonomic neuropathy play pivotal roles in the pathogenesis of DCM (Palomer et al. 2018 ; Marwick et al. 2018 ). Roy et al. ( 1990 ) demonstrated that fibronectin mRNA expression increased even after blood glucose returned to normal in streptozotocin (STZ)-induced diabetic rats. Given the mechanisms and manifestations of DCM, metabolic memory may play a key role in its development and progression (Zhan et al. 2022 ). Additionally, previous studies have shown that miR-320 mediates apoptosis in DCM (Su et al. 2020 ). Moreover, multiple studies have suggested that cluster of differentiation 36 (CD36) regulates free fatty acid uptake in DCM, and CD36-deficient patients and CD36 knockout mice exhibit a significant reduction in the myocardial uptake of long-chain fatty acids (LCFAs) (Zhang et al. 2021 ). A recent study revealed a connection between these factors, revealing that miR-320 serves as a central ncRNA in metabolic memory and positively interacts with CD36 to alleviate diastolic dysfunction caused by hyperglycemic memory in cardiomyocytes (Zhan et al. 2023 ). This finding offers novel insights into the pathogenesis of DCM and its molecular functions.
Diabetic arterial vasculopathy and metabolic memory
Elevated blood glucose can inflict substantial harm on both the microvascular and macrovascular systems, ultimately leading to endothelial dysfunction, atherosclerosis, and various vascular complications (Li et al. 2023 ). Observations from studies such as the EDIC and UKPDS revealed that individuals in the intensive treatment group developed fewer microvascular and macrovascular diseases (C., I. 2003 ; Retnakaran et al. 2006 ). Jax et al. ( 2010 ) argued that structural alterations, including perivascular fibrosis of microvessels, can exert a direct impact on upstream arteries, gradually leading to endothelial dysfunction and, subsequently, the development of atherosclerosis. The endothelium, the largest organ of the body, plays a pivotal role in regulating the functionality of blood vessels. Persistent hyperglycemia leads to oxidative stress, inflammation, and abnormal mitochondrial metabolism, all of which contribute to endothelial dysfunction (Wang et al. 2022 ). Remarkably, even when transient hyperglycemic conditions revert to normal glycemic levels, oxidative stress and inflammatory factors persist within aortic endothelial cells (El-Osta et al. 2008 ). Damaged endothelial cells lose their functionality and undergo a process known as endothelial-to-mesenchymal transition (EndMT), during which they transform into mesenchymal cells or myofibroblasts, thereby contributing to pathological fibrosis (Xu and Kovacic 2023 ; Bischoff 2019 ). Previous research has shown that hyperglycemic memory can also trigger EndMT and fibrosis (Al-Dabet et al. 2022 ). In this context, miR-27a, a ncRNA closely associated with EndMT and fibrosis, has been further implicated in the NF-κB/miR-27a-3p/NRF2/ROS/TGF-β/EndMT feedback loop, which regulates metabolic memory in endothelial cells (Liu et al. 2019b ; Yao et al. 2022 ). Reddy et al. ( 2016 ) demonstrated that the expression of miR-504 remains persistently high in diabetic VSMCs even after several passages of in vitro culture, enhancing ERK1/2 activation and VSMC dysfunction in atherosclerosis and restenosis.
In summary, metabolic memory is intricately linked to oxidative stress, inflammation, and fibrosis and plays a pivotal role in the pathogenesis of DCM and diabetic arterial vasculopathy. The involvement of ncRNAs, such as miR-320 and miR-27a, points to complex regulatory mechanisms underlying these processes. Nevertheless, other miRNAs, such as miR-423, miR-499, and miR-199a, have been implicated in metabolic memory and the diabetic heart, but further investigation is needed to fully elucidate their roles (Costantino et al. 2016 ).
DKD and metabolic memory
DKD is one of the most common and severe complications of DM and is also the leading cause of end-stage kidney disease (ESKD) in the general population (Novak et al. 2016 ; Collins, et al. 2011 ). The minimal functional unit of the kidney is the nephron, which consists of the glomerulus and renal tubule. Hyperglycemia can cause or exacerbate injuries in both the glomerulus and renal tubule to induce renal dysfunction.
Glomerular injury and metabolic memory
The glomeruli are composed of glomerular endothelial cells (GECs), mesangial cells, podocytes and parietal epithelial cells. As GECs serve as the primary barrier to exposure to high glucose conditions, they can initiate crosstalk between mesangial cells and podocytes. Hyperglycemia can increase the permeability of GECs, alter the glycocalyx and induce GEC apoptosis (Dou and Jourde-Chiche 2019 ). On the one hand, damaged GECs regulate the expression and secretion of endothelin-1 (ET-1), nitric oxide (NO), endothelial nitric oxide synthase (eNOS) and VEGF family members, thereby aggravating the dysfunction of other cell types, including mesangial cells and podocytes (Thomas and Ford Versypt 2022 ; Mahtal et al. 2021 ; Zou et al. 2019 ). Conversely, dysfunction in mesangial cells and podocytes can also deleteriously affect GECs through the regulation of VEGF expression (Fu, et al. 2022 ; Bartlett et al. 2016 ). This intricate crosstalk among glomerular cells plays a pivotal role in the pathogenesis and progression of glomerular injury. Notably, even after the restoration of normoglycemia, the damage to these cells persists. Li et al. ( 2022c ) demonstrated that Sirt7 cooperates with ELK1 to participate in metabolic memory and DKD through the modulation of DAPK3 expression and endothelial inflammation both in vitro and in vivo. Similarly, for podocytes, the expression of SHP-1 remains elevated despite the reduction in blood glucose levels achieved by insulin treatment for the last two months in diabetic mice (Lizotte et al. 2016 ). Additionally, free fatty acids, such as palmitate, contribute significantly to the development of insulin resistance. Thus, Novak et al. ( 2016 ) further demonstrated that a high-fat diet or palmitate can alter H3K36me2 and H3K27me3 on the promoter region of the FOXO1 gene, thereby regulating metabolic memory in podocytes. This comprehensive understanding of the interactions and responses among glomerular cells highlights the complexity and persistence of glomerular injury in patients with diabetes.
Tubular injury and metabolic memory
The injury of tubular epithelial cells (TECs), which account for the largest proportion of all cell types in the kidney, is an essential link in the pathogenesis of DKD (Vallon and Thomson 2020 ). On the one hand, hyperglycemia can cause structural alterations in renal tubules, including renal tubule atrophy, tubular cell hypertrophy, thickening of the tubular basement membrane and tubulointerstitial fibrosis (Slyne et al. 2015 ; Pourghasem et al. 2015 ). On the other hand, high glucose conditions can also lead to inflammation, programmed cell death, senescence and mitochondrial dysfunction in TECs (Zhou et al. 2023 ; Shen et al. 2022 ; Chang et al. 2021 ). Among them, cellular senescence in TECs is related to epigenetic modifications, which are the core mechanism of metabolic memory (Shen et al. 2022 ; Tonna et al. 2010 ). Recent research identified p21 as a key hyperglycemic memory-related gene that regulates TEC senescence in DKD, and activated protein C (aPC), an enzyme that epigenetically inhibits redox p66Shc, could inhibit p21 methylation to ameliorate metabolic memory and senescence (Al-Dabet et al. 2022 ).
In conclusion, metabolic memory is an emerging mechanism in glomerular and tubular injury. Regrettably, studies on the role of metabolic memory in DKD are rare, especially studies on mesangial cells and the crosstalk between different cell types in the kidney. A recent study on the multimodal integration of single nucleus RNA (snRNA-seq) and an assay for transposase-accessible chromatin sequencing (snATAC-seq) in DKD may provide more information on the epigenetic regulation of chromatin accessibility, which could contribute to the long-term expression of DKD and metabolic memory-related genes (Wilson et al. 2022 ). However, further studies are still needed.
DR and metabolic memory
DR, characterized as a neurodegenerative and microangiopathic disease, is the major cause of visual impairment in patients with diabetes, accounting for approximately 30 to 40% of cases (Ting et al. 2016 ; Altmann and Schmidt 2018 ). Hyperglycemia remains the major factor that contributes to the development and progression of DR (Cheung et al. 2010 ). The pathophysiological mechanisms underlying DR are complex and include oxidative stress, inflammation, autophagy, cellular dysfunction and cell death. The inflammatory cascades are primarily triggered by oxidative stress. Both inflammation and oxidative stress stimulate retinal autophagy, which leads to cellular dysfunction and cell death in nerve cells, endothelial cells and pericytes. All these factors may interact with each other, ultimately contributing to the development of DR (Wei et al. 2022 ; Madsen-Bouterse and Kowluru 2008 ).
Coincidentally, multiple studies have shown that the mechanisms mentioned above regulate hyperglycemic memory to affect DR pathogenesis (Liu et al. 2023 ). Metabolic memory-induced retinopathy was initially observed in diabetic dogs, which indicated that DR was not improved by good glycemic control (Engerman and Kern 1987 ). Tewari et al. ( 2012 ) reported that despite the restoration of normoglycemia in retinal endothelial cells, hypermethylation of POLG1 promoters did not change, which resulted in mtDNA replication dysfunction. Liu et al. ( 2019a ) demonstrated that miR-195 remained upregulated in human retinal pigment epithelial cells (RPEs) following three days of culture under high glucose conditions and subsequent normalization to normal glucose levels for another three days, leading to mitochondrial dysfunction-induced apoptosis. Furthermore, Astragalus polysaccharide (APS) attenuated the expression of miR-195 in a dose-dependent manner. Recent studies have demonstrated that the pathogenesis of metabolic memory-induced microvascular dysfunction in DR is regulated by mitochondrial dysfunction, which can be ameliorated by dopamine, mdivi-1 and leflunomide (Lee et al. 2022 ; Kowluru and Alka 2023 ; Mohammad and Kowluru 2022 ). Therefore, mitochondrial dysfunction may be the core mechanism of metabolic memory in DR. Both DKD and DR are microvascular complications of diabetes, and the kidneys and eyes are mitochondria-rich organs. However, studies on the relationship between mitochondrial dysfunction and hyperglycemia in DKD are limited and may be worthy of further research.
Epigenetic modifications also play a vital role in the progression of DR. The high glucose-induced histone 3 lysine 4 (H3K4) hypomethylation status of retinal Sod2 remains persistent even after reversing hyperglycemia (Zhong and Kowluru 2013 ). Mishra et al. ( 2014 ) also proved that as termination of hyperglycemia injury cannot change H3K4 methylation, the binding activity of the transcription factor Nrf2 remains compromised, which leads to oxidative stress. Furthermore, numerous miRNAs also participate in metabolic memory in DR. Apart from miR-195 mentioned above, miR-23b-3p regulates the miR-23b-3p/SIRT1/NF-κB feedback loop to maintain metabolic memory in DR (Zhao et al. 2016 ). Nevertheless, the mechanisms of DNA methylation, lncRNA or other epigenetic modifications are still relatively unknown.
DF and metabolic memory
DF, a common and severe complication of DM, is a major cause of extremity amputation, and the worldwide prevalence of DF is 6.3% (Zhang et al. 2017 ; Afonso, et al. 2021 ). The risk factors involved in the progression of DF are diabetic neuropathy, vascular insufficiency and immunological dysfunction (Noor et al. 2015 ). As there were no obvious improvements in wound healing even when glycemic control was achieved in patients with DM, metabolic memory may participate in DF (Zhao et al. 2021 ; Berlanga-Acosta et al. 2023 ). Del Cuore et al. ( 2023 ) used single nucleotide polymorphism (SNP) analysis in a population with diabetic foot disease. Their results indicated that patients with DF showed predominant expression of the VEGF C2578A CC polymorphism and reduced expression of the AC allele. They also found that miR-217-5p and miR-503-5p may be involved in regulating hyperglycemic memory in DF. Inflammation and DNA methylation are involved in metabolic memory, which is also a key mechanism in DR (Acosta et al. 2008 ; Deng et al. 2023 ). The genome-wide DNA methylation profiles of foot fibroblasts indicated that the change in DNA methylation was associated with metabolic memory, especially in patients with poor wound healing outcomes of diabetic foot ulceration (Park et al. 2014 ). Zhao et al. ( 2021 ) further demonstrated that transient hyperglycemia upregulated DNA methyltransferase 1 (DNMT1) expression, leading to the persistent hypermethylation of Ang-1 during subsequent normoglycemia, which induced inflammation and endothelial dysfunction in vitro and in vivo. These findings implied that epigenetic modifications are a hub contributor to metabolic memory in DR, although the present research is still limited.
Other diabetic complications and metabolic memory
As high blood glucose can injure multiple tissues and organs, hyperglycemic memory is also associated with other chronic complications of DM. Erectile dysfunction (ED) is a common complication of DM, with an approximate prevalence of 35–90% (Malavige and Levy 2009 ). A retrospective case‒control study showed that early hyperglycemia exposure could have long-term effects on erectile function in patients with DM, which could be sustained even after good glycemic control (Hui et al. 2021 ). A previous study showed that hyperglycemia could induce endothelial cell injury to cause microvascular leakage in the lung, which can further lead to pulmonary fibrosis (Lee et al. 2022 ). Jeon et al. ( 2023 ) further illustrated that high glucose-induced microvascular leakage and fibrosis in the lung could not be alleviated even after good blood glucose control. Furthermore, the pathophysiological mechanisms of diabetic neuropathy (DN) are epigenetic modifications, inflammation, oxidative stress and mitochondrial dysfunction, which are similar to the mechanisms of metabolic memory (Jankovic, et al. 2021 ). Thus, a review suggested that metabolic memory may also take part in the development of DN (Jankovic, et al. 2021 ). Regrettably, directly relevant research is rare, so more solid evidence is needed.
In summary, numerous studies have demonstrated that metabolic memory plays an important role in the progression of multiple chronic complications in patients with DM.
Potential therapeutic drugs for metabolic memory
Numerous molecular compounds have been proven to act on the key mechanisms of hyperglycemic memory, such as epigenetic modifications, inflammation, and senescence (Table 1 ). In addition, some molecular compounds may also regulate metabolism, but there is a lack of clear evidence supporting this possibility. Thus, in this section, we summarize the progress of current studies on metabolic memory-related potential drugs for treating diabetes and its complications.
SGI-1027, a highly lipophilic small-molecule inhibitor of DNMT1 based on its quinoline structure, potently inhibits DNA methylation, thereby suppressing senescence, apoptosis, and fibrosis (Sun et al. 2018 ; Gao et al. 2022 ; Wang et al. 2019 ). In DKD, DNMT1 regulates senescence and fibrosis by modulating the DNA methylation status of p21 (Al-Dabet et al. 2022 ). Given these findings, we hypothesize that SGI-1027 may hold promise for mitigating hyperglycemia memory and hyperglycemic memory-induced senescence, apoptosis, and fibrosis.
Chaetocin, a small-molecule natural product isolated from Chaetomium fungi, can regulate several mechanisms of metabolic memory, such as apoptosis, oxidative stress, autophagy and immune function (Jiang et al. 2021 ). SUV39H1 regulated sustained inflammation in vascular cells that were transiently cultured in high glucose through the modification of H3K9me3 (Villeneuve et al. 2008 ). Moreover, chaetocin can also decrease histone H3K9me3 levels at the promoter of the p21 WAF1 gene, which has also been proven to be a hyperglycemic memory-related gene in DKD (Al-Dabet et al. 2022 ; Lin et al. 2016 ). Interestingly, miR-125b , a key ncRNA that regulates hyperglycemic memory, plays an upstream role in the regulation of inflammatory genes in diabetic mice by downregulating SUV39H1 (Villeneuve et al. 2010 ; Wang and Chang 2011 ). These results further support the notion that chaetocin or a miR-125b inhibitor may be effective inhibitors of metabolic memory.
Research on these drugs is currently only at the experimental stage due to safety and other reasons, so their clinical use is still limited. Targeting the site of metabolic processes without interfering with regular metabolic processes is still a challenge. However, understanding the mechanisms of metabolic memory in diabetic complications is benefit in exploring new therapeutic approaches.
- Models of metabolic memory
The concept of metabolic memory was proposed in 2003, with studies involving insulin treatment groups and an average follow-up duration of 6.5 years (Pop-Busui et al. 2009 ). More recently, Al-Dabet et al. ( 2022 ) used SGLT2i for 7.2 ± 0.8 months to manage hyperglycemia and evaluated urinary P21 expression as a marker of persistent tubular damage in DKD. Li et al. ( 2022c ) tested DAPK3 in kidney tissue from DKD patients with poor HbA1c (10.2 ± 3.9) and those with good glycemic control (HbA1c 5.4 ± 0.5). However, crucial details such as the hypoglycemic medications used and the duration of glycemic control were omitted from their study. Hui et al. ( 2021 ) divided participants into three groups: a glycemic control group (regular treatment with normal glycemic levels in the past 5 years), a glycemic non-control group (non-regular treatment with poor glycemic control in the past 5 years), and a metabolic memory group (regular treatment and normal glycemic levels in the past year but non-regular treatment with poor glycemic control a year ago). Nevertheless, they also did not describe the hypoglycemic medications used in detail. Given the inherent challenges in controlling variables in clinical research, the majority of studies have resorted to animal and cell models to investigate the mechanisms underlying metabolic memory. Regrettably, there is a lack of consistency in the models of metabolic memory, both in vitro and in vivo. Thus, Table 2 lists different models used in different studies.
For in vitro models of metabolic memory, most researchers used high glucose conditions in cultured cells and then changed the glucose concentration to a normal level to simulate intensive treatment in DCCT/EDIC studies. In the majority of studies exploring diabetes and its chronic complications, a hyperglycemic exposure period of 48–72 h is typically considered representative of chronic hyperglycemia with long-term deleterious effects. This holds true for conditions such as DKD, DF, and diabetic cardiomyopathy (Hu et al. 2024 ; Wang et al. 2024 ; Li, et al. 2024 ; Song et al. 2023 ; Feng et al. 2023 ). However, it is worth noting that the duration of exposure to high glucose, as well as the periods of exposure to normal glucose, vary significantly across studies examining metabolic memory. In DKD, Al-Dabet et al. ( 2022 ) Mouse primary tubular cells were cultured under high-glucose conditions for 24 h and then at normal levels for another 24 h. However, Li et al. ( 2022c ) Human glomerular endothelial cells were exposed to high levels of glucose for 3 days, followed by 3 days under normal conditions. Although these studies cultured cells for different durations, they equally distributed the time to high and normal glucose levels. Zhong et al. ( 2021 ) compared different in vitro models of metabolic memory in DF. Interestingly, different models performed similarly, and they used cultured human aortic endothelial cells for 1 day under high glucose and 6 days under normal glucose conditions. Another uncommon method involves deriving human proximal tubular epithelial cells from people with type 2 diabetes and culturing them under normal glucose conditions for 4 passages to establish a metabolic memory model (Bansal et al. 2020 ). Regrettably, there are no accepted standards for metabolic memory models in vitro , and most studies have chosen these models directly. However, for different cell lines, comparing different culture times might be more accurate.
For in vivo models of metabolic memory, the majority of studies have utilized insulin to lower blood glucose levels in diabetic mice or rats. Notably, in 2022, Al-Dabet et al. ( 2022 ) reported a novel attempt to employ SGLT2i in the construction of a metabolic memory model. Although they presumably considered the renal benefits of SGLT2i, they did not compare its effectiveness with that of insulin in model establishment. The duration of glycemic control in these studies varied significantly, ranging from a minimum of 3 weeks in rats with DKD treated with insulin to a maximum of 4 months in rats with DR also treated with insulin (Li et al. 2022c ; Mohammad and Kowluru 2022 ). This wide range raises the following question: what is the optimal duration for the construction of in vivo models of metabolic memory? Furthermore, does this duration differ across various diabetic complications?
In this review, we explored the regulatory mechanism of metabolic memory, including inflammation and immunity, oxidative stress and mitochondrial dysfunction, senescence and various kinds of cell death. Then, we discuss the function of metabolic memory in diabetic complications. In addition, we analyzed confirmed and potential metabolic memory-related inhibitors. Finally, we also summarized the in vitro and in vivo models of metabolic memory. In conclusion, metabolic memory might be a vital mechanism in the occurrence and development of various diabetic complications and is a promising therapeutic target for preventing the progression of complications.
There have been extensive studies on the essential mechanisms and regulators of metabolic memory, oxidative stress, mitochondrial dysfunction and apoptosis, which are the most studied and are mainly focused on DR. On the one hand, these traditional mechanisms may be the core mechanisms of metabolic memory and need to be validated more widely for the treatment of different diabetic complications. However, emerging mechanisms, such as senescence, ferroptosis, and pyroptosis, also deserve further exploration. In addition, animal and cellular models of metabolic memory are still controversial. Thus, we speculated that for different cell lines and methods for generating diabetic models, preexperiments may be necessary. Furthermore, numerous key molecules, including miR-320 , p21 and Sod2 , have been identified. However, there are currently no well-recognized markers that can represent metabolic memory in vitro or in vivo, such as ColI and fibronectin for fibrosis, let alone in vitro diagnostic markers used in clinical practice. In addition, small molecule drugs, such as polysaccharide, dopamine and aPC, have also been found to alleviate hyperglycemic memory to mitigate diabetic complications. We also speculated that SGI-1027 and chaetocin may be potential molecular compounds against metabolic memory. Regrettably, these drugs have been limited to animal and cellular models.
In the future, further studies of metabolic memory may start from the following perspectives: (1) develop explicit animal and cellular models of metabolic memory; (2) elucidate the mechanisms of metabolic memory and further uncover more hub molecules that regulate metabolic memory to obtain representative markers; (3) transform small molecule compounds that can be used to regulate metabolic memory in clinical practice; and (4) extensively study the crosstalk between lncRNAs and miRNAs; however, regrettably, pertinent research on the intricate network of lncRNAs and other ncRNAs remains to be conducted.
Although there have been certain studies that have summarized the relationship between metabolic memory and DKD, DR or epigenetic modification, studies on the connection between metabolic memory and chronic complications of DM, along with potential therapeutic drugs, remain scarce. Notably, our review is the first to comprehensively summarize various models of metabolic memory, given that the exact duration and severity of high-glucose toxicity are still elusive. Despite our efforts to comprehensively review the pathogenesis of metabolic memory in diverse chronic complications of DM, we acknowledge certain limitations, including the constraints of our research perspectives. Nevertheless, with further in-depth exploration of metabolic memory in chronic DM complications and elucidation of its underlying mechanisms, we are confident that this study will pave the way for reliable and innovative therapeutic targets that can retard or even arrest the progression of DM and its associated complications. Additionally, we intend to conduct regular systematic summaries in this field of research.
Availability of data and materials
The data of this study are included within the paper.
Abbreviations
Diabetes mellitus
Diabetic retinopathy
Diabetic kidney disease
International Diabetes Federation
Advanced glycation end product
Mitochondrial DNA
Sodium glucose co-transporter 2 inhibitor
Dipeptidyl peptidase 4 inhibitors
Glucagon-like peptide 1 receptor agonists
Diabetes Control and Complications Trial
Epidemiology of Diabetes Interventions and Complications
United Kingdom Prospective Diabetes Study
Noncoding RNAs
Thioredoxin-interacting protein
3’ Untranslated region
Histone posttranslational modifications
Type 2 diabetes mellitus
Suppressor of variegation 3–9 homolog 1
Histone H3 lysine 4 monomethylation
Long noncoding RNAs
Diabetic foot
Diabetic cardiomyopathy
Streptozotocin
Cluster of differentiation 36
Endothelial-to-mesenchymal transition
Vascular smooth muscle cells
End-stage kidney disease
Glomerular endothelial cells
Endothelin-1
Tubular epithelial cells
Activated protein C
Single-nucleus RNA
Assay for transposase-accessible chromatin sequencing
Retinal pigment epithelial cells
Histone 3 lysine 4
DNA methyltransferase 1
Diabetic neuropathy
Endoplasmic reticulum
Reactive oxygen species
Transglutaminase
Human embryonic kidney cells
Boston University mouse proximal tubular cells
Nonobese diabetic
Tert-butylhydroquinone
Glutathione
Adeno-associated virus
5-Aza-deoxycytidine
Proximal tubular cells
Human retinal endothelial cells
Bovine retinal endothelial cells
Aboouf MA, et al. Genotype screening of APLN rs3115757 variant in Egyptian women population reveals an association with obesity and insulin resistance. Diabetes Res Clin Pract. 2015;109(1):40–7.
Article CAS PubMed Google Scholar
Acosta JB, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5(4):530–9.
Article PubMed PubMed Central Google Scholar
Afonso AC, et al. Biofilms in diabetic foot ulcers: impact, risk factors and control strategies. Int J Mol Sci 2021;22(15).
Aiello LP, D.E.R. Group (2014) Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 37(1): 17–23
Al-Dabet MM, et al. Reversal of the renal hyperglycemic memory in diabetic kidney disease by targeting sustained tubular p21 expression. Nat Commun. 2022;13(1):5062.
Article CAS PubMed PubMed Central Google Scholar
Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci 2018;19(1).
Bansal A, et al. Integrative omics analyses reveal epigenetic memory in diabetic renal cells regulating genes associated with kidney dysfunction. Diabetes. 2020;69(11):2490–502.
Bartlett CS, Jeansson M, Quaggin SE. Vascular growth factors and glomerular disease. Annu Rev Physiol. 2016;78:437–61.
Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr. 2016;10(2 Suppl 1):S176–83.
Article PubMed Google Scholar
Berlanga-Acosta J, et al. Endogenous biological drivers in diabetic lower limb wounds recurrence: hypothetical reflections. Int J Mol Sci 2023;24(12).
Bhatti JS, et al. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022;184:114–34.
Bischoff J. Endothelial-to-mesenchymal transition. Circ Res. 2019;124(8):1163–5.
Bleriot C, et al. Inflammatory and immune etiology of type 2 diabetes. Trends Immunol. 2023;44(2):101–9.
Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7(7):369–75.
Chang J, et al. Update on the mechanisms of tubular cell injury in diabetic kidney disease. Front Med (lausanne). 2021;8: 661076.
Chen Z, Natarajan R. Epigenetic modifications in metabolic memory: what are the memories, and can we erase them? Am J Physiol Cell Physiol. 2022;323(2):C570–82.
Chen Z, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci U S A. 2016;113(21):E3002–11.
Cheng Y, et al. Central role of cardiac fibroblasts in myocardial fibrosis of diabetic cardiomyopathy. Front Endocrinol (lausanne). 2023;14:1162754.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36.
Choi EH, Park SJ. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp Mol Med. 2023;55(7):1348–56.
Cojocaru KA, et al. Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants (Basel), 2023;12(3).
Collins AJ, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl):A7, e1-420.
PubMed Google Scholar
Costantino S, et al. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37(6):572–6.
Del Cuore A, et al. Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms (SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction. Cardiovasc Diabetol. 2023;22(1):148.
Deng JY, et al. Targeting DNA methylation and demethylation in diabetic foot ulcers. J Adv Res 2023.
Ding Q, et al. Inflammation-related epigenetic modification: the bridge between immune and metabolism in type 2 diabetes. Front Immunol. 2022;13: 883410.
Domingueti CP, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–45.
Dou L, Jourde-Chiche N. Endothelial toxicity of high glucose and its by-products in diabetic kidney disease. Toxins (Basel), 2019;11(10).
Drzewoski J, Kasznicki J, Trojanowski Z. The role of “metabolic memory” in the natural history of diabetes mellitus. Pol Arch Med Wewn. 2009;119(7–8):493–500.
El Mouhayyar C, et al. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol. 2020;2020:1762164.
El-Mesallamy HO, et al. Adiponectin and E-selectin concentrations in relation to inflammation in obese type 2 diabetic patients with coronary heart disease(s). Minerva Endocrinol. 2011;36(3):163–70.
CAS PubMed Google Scholar
El-Mesallamy HO, Hamdy NM, Sallam AA. Effect of obesity and glycemic control on serum lipocalins and insulin-like growth factor axis in type 2 diabetic patients. Acta Diabetol. 2013;50(5):679–85.
El-Osta A, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–17.
Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–12.
Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–67.
Feng Q, et al. Quercetin ameliorates diabetic kidney injury by inhibiting ferroptosis via activating Nrf2/HO-1 signaling pathway. Am J Chin Med. 2023;51(4):997–1018.
Filgueiras LR, et al. Imbalance between HDAC and HAT activities drives aberrant STAT1/MyD88 expression in macrophages from type 1 diabetic mice. J Diabetes Complications. 2017;31(2):334–9.
Fu J, et al. Regeneration of glomerular metabolism and function by podocyte pyruvate kinase M2 in diabetic nephropathy. JCI Insight, 2022;7(5).
Galicia-Garcia U. et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci, 2020. 21(17).
Galluzzi L, Myint M. Cell death and senescence. J Transl Med. 2023;21(1):425.
Gao Q, et al. Inhibition of DNA methyltransferase aberrations reinstates antioxidant aging suppressors and ameliorates renal aging. Aging Cell. 2022;21(1): e13526.
Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–8.
Gong ZG, et al. Epigenetic regulator BRD4 is involved in cadmium-induced acute kidney injury via contributing to lysosomal dysfunction, autophagy blockade and oxidative stress. J Hazard Mater. 2022;423(Pt A): 127110.
Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–22.
Hamdy NM, Suwailem SM, El-Mesallamy HO. Influence of vitamin E supplementation on endothelial complications in type 2 diabetes mellitus patients who underwent coronary artery bypass graft. J Diabetes Complications. 2009;23(3):167–73.
Hammes HP, et al. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol vis Sci. 1993;34(6):2092–6.
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–53.
Holman RR, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Hu K, et al. MicroRNA-221-3p inhibits the inflammatory response of keratinocytes by regulating the DYRK1A/STAT3 signaling pathway to promote wound healing in diabetes. Commun Biol. 2024;7(1):300.
Hui J, et al. Effects of “metabolic memory” on erectile function in diabetic men: a retrospective case-control study. Andrology. 2021;9(1):288–96.
Jankovic M, et al, Genetic and epigenomic modifiers of diabetic neuropathy. Int J Mol Sci 2021;22(9).
Jax TW. Metabolic memory: a vascular perspective. Cardiovasc Diabetol. 2010;9:51.
Jeon HY, et al. Simultaneous attenuation of hyperglycemic memory-induced retinal, pulmonary, and glomerular dysfunctions by proinsulin C-peptide in diabetes. BMC Med. 2023;21(1):49.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Jiang Z, et al. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic Biol Med. 2011;50(8):907–17.
Jiang H, et al. Chaetocin: a review of its anticancer potentials and mechanisms. Eur J Pharmacol. 2021;910: 174459.
Jin ML, Jeong KW. Histone modifications in drug-resistant cancers: from a cancer stem cell and immune evasion perspective. Exp Mol Med. 2023;55(7):1333–47.
Johnson J, et al. Oxidative stress in neutrophils: implications for diabetic cardiovascular complications. Antioxid Redox Signal. 2022;36(10–12):652–66.
Joslin EP. Diabetes mellitus. N Engl J Med. 1946;234:476.
Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15(6):327–45.
Khella MS, et al. The (FTO) gene polymorphism is associated with metabolic syndrome risk in Egyptian females: a case-control study. BMC Med Genet. 2017;18(1):101.
Kowluru RA, Alka K. Mitochondrial quality control and metabolic memory phenomenon associated with continued progression of diabetic retinopathy. Int J Mol Sci 2023;24(9).
Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610.
Kumari N, et al. The potential role of m6A RNA methylation in diabetic retinopathy. Exp Eye Res. 2021;208: 108616.
Kung HC, et al. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson's disease. Biomedicines 2021;9(8).
Lee YJ, et al. Dopamine ameliorates hyperglycemic memory-induced microvascular dysfunction in diabetic retinopathy. FASEB J. 2022;36(12): e22643.
Li C, et al. Non-coding RNAs in diabetes mellitus and diabetic cardiovascular disease. Front Endocrinol (lausanne). 2022a;13: 961802.
Li Q, et al. Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1alpha-mediated oxidative stress and mitochondrial dysfunction. Front Pharmacol. 2022b;13: 859723.
Li X, et al. Sirt7 associates with ELK1 to participate in hyperglycemia memory and diabetic nephropathy via modulation of DAPK3 expression and endothelial inflammation. Transl Res. 2022c;247:99–116.
Li Y, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8(1):152.
Li SS, et al. Diabetes promotes myocardial fibrosis via AMPK/EZH2/PPAR-gamma signaling pathway. Diabetes Metab J 2024.
Lin SH, et al. Histone methyltransferase Suv39h1 attenuates high glucose-induced fibronectin and p21(WAF1) in mesangial cells. Int J Biochem Cell Biol. 2016;78:96–105.
Liu P, et al. Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol Med. 2019a;25(1):21.
Liu T, et al. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019b;227:64–73.
Liu DD, et al. Epigenetic modifications and metabolic memory in diabetic retinopathy: beyond the surface. Neural Regen Res. 2023;18(7):1441–9.
Lizotte F, et al. Persistent insulin resistance in podocytes caused by epigenetic changes of SHP-1 in diabetes. Diabetes. 2016;65(12):3705–17.
Maamoun H, et al. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Front Physiol. 2019;10:70.
Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9(4):315–27.
Mahmoud MM, Sanad EF, Hamdy NM. MicroRNAs’ role in the environment-related non-communicable diseases and link to multidrug resistance, regulation, or alteration. Environ Sci Pollut Res Int. 2021;28(28):36984–7000.
Mahtal N, Lenoir O, Tharaux PL. Glomerular endothelial cell crosstalk with podocytes in diabetic kidney disease. Front Med (lausanne). 2021;8: 659013.
Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–47.
Mansour MA, et al. P21 overexpression promotes cell death and induces senescence in human glioblastoma. Cancers (Basel), 2023;15(4).
Mao R, et al. Enhancer RNAs: a missing regulatory layer in gene transcription. Sci China Life Sci. 2019;62(7):905–12.
Marwick TH, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51.
Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic Biol Med. 2014;75:129–39.
Mohammad G, Kowluru RA. Mitochondrial dynamics in the metabolic memory of diabetic retinopathy. J Diabetes Res. 2022;2022:3555889.
Mossel DM, et al. Epigenetic regulation of S100A9 and S100A12 expression in monocyte-macrophage system in hyperglycemic conditions. Front Immunol. 2020;11:1071.
Mostafa AM, et al. Glucagon-like peptide 1 (GLP-1)-based therapy upregulates LXR-ABCA1/ABCG1 cascade in adipocytes. Biochem Biophys Res Commun. 2015;468(4):900–5.
Mostafa AM, et al. Effect of vildagliptin and pravastatin combination on cholesterol efflux in adipocytes. IUBMB Life. 2016;68(7):535–43.
Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029–44.
Nathan DM, DER Group, The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37(1):9-16.
Nathan DM, D.E.R. Group (2014) The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37(1): 9–16
Nedosugova LV, et al. Inflammatory mechanisms of diabetes and its vascular complications. Biomedicines 2022;10(5).
Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192–9.
Novak M, et al. Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in patients with diabetes with comorbid depression. Diabetes Care. 2016;39(11):1940–7.
Okabe J, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res. 2012;110(8):1067–76.
Palomer X, Pizarro-Delgado J, Vazquez-Carrera M. Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci. 2018;39(5):452–67.
Park LK, et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014;9(10):1339–49.
Peng QH, et al. Astragalus polysaccharide attenuates metabolic memory-triggered ER stress and apoptosis via regulation of miR-204/SIRT1 axis in retinal pigment epithelial cells. Biosci Rep 2020;40(1).
Phoenix A, Chandran R, Ergul A. Cerebral microvascular senescence and inflammation in diabetes. Front Physiol. 2022;13: 864758.
Pop-Busui R, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119(22):2886–93.
Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med. 2015;6(3):120–7.
PubMed PubMed Central Google Scholar
Ranjit Unnikrishnan I, Anjana RM, Mohan V (2011) Importance of controlling diabetes early—the concept of metabolic memory, legacy effect and the case for early insulinisation. J Assoc Physicians India. 59(Suppl): 8–12
Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–55.
Reddy MA, et al. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36(5):864–73.
Retnakaran R et al (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55(6): 1832–9
Roy S, et al. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990;87(1):404–8.
Shen S, Ji C, Wei K. Cellular senescence and regulated cell death of tubular epithelial cells in diabetic kidney disease. Front Endocrinol (lausanne). 2022;13: 924299.
Slyne J, et al. New developments concerning the proximal tubule in diabetic nephropathy: in vitro models and mechanisms. Nephrol Dial Transplant. 2015;30(Suppl 4):60–7.
Article Google Scholar
Song Y, et al. Novel lncRNA-prader willi/angelman region RNA, SNRPN neighbour (PWARSN) aggravates tubular epithelial cell pyroptosis by regulating TXNIP via dual way in diabetic kidney disease. Cell Prolif. 2023;56(2): e13349.
Su D, et al. Tcf3-activated lncRNA Gas5 regulates newborn mouse cardiomyocyte apoptosis in diabetic cardiomyopathy. J Cell Biochem. 2020;121(11):4337–46.
Sun N, et al. DNMTs inhibitor SGI-1027 induces apoptosis in Huh7 human hepatocellular carcinoma cells. Oncol Lett. 2018;16(5):5799–806.
CAS PubMed PubMed Central Google Scholar
Sun H, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119.
Sun Y, et al. Epigenetic regulation of mesenchymal stem cell aging through histone modifications. Genes Dis. 2023;10(6):2443–56.
Taft RJ, et al. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–39.
Takahashi P, et al. Transcript expression profiles and microRNA regulation indicate an upregulation of processes linked to oxidative stress, DNA repair, cell death, and inflammation in type 1 diabetes mellitus patients. J Diabetes Res. 2022;2022:3511329.
Teodoro JS, et al. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol. 2018;9:1857.
Tewari S, et al. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest Ophthalmol vis Sci. 2012;53(8):4881–8.
Thielen L, Shalev A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr Opin Endocrinol Diabetes Obes. 2018;25(2):75–80.
Thiem K, et al. Hyperglycemic memory of innate immune cells promotes in vitro proinflammatory responses of human monocytes and murine macrophages. J Immunol. 2021;206(4):807–13.
Thomas HY, Ford Versypt AN. Pathophysiology of mesangial expansion in diabetic nephropathy: mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J Biol Eng. 2022;16(1):19.
Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–77.
Tonna S, et al. Metabolic memory and diabetic nephropathy: potential role for epigenetic mechanisms. Nat Rev Nephrol. 2010;6(6):332–41.
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16(6):317–36.
Vastolo V, et al. High-fat diet unveils an enhancer element at the Ped/Pea-15 gene responsible for epigenetic memory in skeletal muscle. Metabolism. 2018;87:70–9.
Villeneuve LM, et al. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105(26):9047–52.
Villeneuve LM, et al. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes. 2010;59(11):2904–15.
Voronova V, et al. Interpretation of metabolic memory phenomenon using a physiological systems model: what drives oxidative stress following glucose normalization? PLoS ONE. 2017;12(2): e0171781.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–14.
Wang Z, et al. Metabolic memory in mitochondrial oxidative damage triggers diabetic retinopathy. BMC Ophthalmol. 2018;18(1):258.
Wang JY, et al. Suppressing microRNA-29c promotes biliary atresia-related fibrosis by targeting DNMT3A and DNMT3B. Cell Mol Biol Lett. 2019;24:10.
Wang M, et al. Endothelial dysfunction and diabetic cardiomyopathy. Front Endocrinol (lausanne). 2022;13: 851941.
Wang H, et al. VDR activation attenuates renal tubular epithelial cell ferroptosis by regulating Nrf2/HO-1 signaling pathway in diabetic nephropathy. Adv Sci (weinh). 2024;11(10): e2305563.
Wei L, et al. The pathophysiological mechanisms underlying diabetic retinopathy. Front Cell Dev Biol. 2022;10: 963615.
Wilson PC, et al. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat Commun. 2022;13(1):5253.
Writing Team for the Diabetes C, I. Complications Trial/Epidemiology of Diabetes, and G. Complications Research (200) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290(16): 2159–67
Xian Y, et al. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(10):2009–17.
Xie L, et al. Emerging roles of sodium glucose cotransporter 2 (SGLT-2) inhibitors in diabetic cardiovascular diseases: focusing on immunity inflammation and metabolism. Front Pharmacol. 2022;13: 836849.
Xu Y, Kovacic JC. Endothelial to mesenchymal transition in health and disease. Annu Rev Physiol. 2023;85:245–67.
Yamagishi SI, Nakamura N, Matsui T. Glycation and cardiovascular disease in diabetes: a perspective on the concept of metabolic memory. J Diabetes. 2017;9(2):141–8.
Yao Y, et al. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc Res. 2022;118(1):196–211.
Yu XY, et al. High levels of glucose induce “metabolic memory” in cardiomyocyte via epigenetic histone H3 lysine 9 methylation. Mol Biol Rep. 2012;39(9):8891–8.
Zapata RC, et al. Adipocytes control food intake and weight regain via Vacuolar-type H(+) ATPase. Nat Commun. 2022;13(1):5092.
Zhan J, et al. Hyperglycemic memory in diabetic cardiomyopathy. Front Med. 2022;16(1):25–38.
Zhan J, et al. Positive feedback loop of miR-320 and CD36 regulates the hyperglycemic memory-induced diabetic diastolic cardiac dysfunction. Mol Ther Nucleic Acids. 2023;31:122–38.
Zhang E, Wu Y. Metabolic memory: mechanisms and implications for diabetic vasculopathies. Sci China Life Sci. 2014;57(8):845–51.
Zhang P, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med. 2017;49(2):106–16.
Zhang P, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583–600.
Zhang X, et al. CD36 signaling in diabetic cardiomyopathy. Aging Dis. 2021;12(3):826–40.
Zhao S, et al. miR-23b-3p induces the cellular metabolic memory of high glucose in diabetic retinopathy through a SIRT1-dependent signalling pathway. Diabetologia. 2016;59(3):644–54.
Zhao J, et al. Transient high glucose causes persistent vascular dysfunction and delayed wound healing by the DNMT1-mediated Ang-1/NF-kappaB pathway. J Invest Dermatol. 2021;141(6):1573–84.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Zhong Q, Kowluru RA. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest Ophthalmol vis Sci. 2013;54(1):244–50.
Zhong X, et al. The microRNAs in the pathogenesis of metabolic memory. Endocrinology. 2015;156(9):3157–68.
Zhong Y, et al. Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: a narrative review. Int J Biol Macromol. 2023;259(Pt 1): 128182.
Zhou X, et al. Role of renal tubular programed cell death in diabetic kidney disease. Diabetes Metab Res Rev. 2023;39(2): e3596.
Zou HH, et al. Endothelial cells secreted endothelin-1 augments diabetic nephropathy via inducing extracellular matrix accumulation of mesangial cells in ETBR(-/-) mice. Aging (albany NY). 2019;11(6):1804–20.
Download references
Acknowledgements
We thank the Translational Medicine Center, the First Affiliated Hospital of Zhengzhou University, for their support.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (U200410394), The Research and Innovation Team Project of the First Affiliated Hospital of Zhengzhou University (QNCXTD2023015) and 2021 Henan Province Health Young and Middle-aged Discipline Leader Training Project.
Author information
Authors and affiliations.
Division of Endocrinology, Department of Internal Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China
Tongyue Yang, Feng Guo, Mingwei Shao, Yi Song, Gaofei Ren, Zhao Linlin, Guijun Qin & Yanyan Zhao
Traditional Chinese Medicine Integrated Department of Nephrology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China
Research Institute of Nephrology, Zhengzhou University, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
TY, YZ and QF conceptualized and wrote the manuscript. TY, FG, MS, YS, GR, LZ and YZ contributed to manuscript writing. QF, GQ and YZ reviewed and revised the manuscript. All authors have read and approved the final version of the manuscript being submitted.
Corresponding author
Correspondence to Yanyan Zhao .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Authors, hereby consent to the publication of my paper "An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory" in Molecular Medicine. I confirm that the content is accurate and that I am aware of any potential disclosures. I waive any rights to claim authorship and hold the publisher harmless from any liabilities arising from publication.
Competing interests
The authors declare no conflicts of interest that pertain to this work.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yang, T., Qi, F., Guo, F. et al. An update on chronic complications of diabetes mellitus: from molecular mechanisms to therapeutic strategies with a focus on metabolic memory. Mol Med 30 , 71 (2024). https://doi.org/10.1186/s10020-024-00824-9
Download citation
Received : 25 January 2024
Accepted : 29 April 2024
Published : 26 May 2024
DOI : https://doi.org/10.1186/s10020-024-00824-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Metabolic memory
- Diabetic complications
- Molecular mechanisms
- Treatment progress
Molecular Medicine
ISSN: 1528-3658
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

- Previous Article
- Next Article
Research Design and Methods
Article information, literature review of type 2 diabetes management and health literacy.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Rulla Alsaedi , Kimberly McKeirnan; Literature Review of Type 2 Diabetes Management and Health Literacy. Diabetes Spectr 1 November 2021; 34 (4): 399–406. https://doi.org/10.2337/ds21-0014
Download citation file:
- Ris (Zotero)
- Reference Manager
The purpose of this literature review was to identify educational approaches addressing low health literacy for people with type 2 diabetes. Low health literacy can lead to poor management of diabetes, low engagement with health care providers, increased hospitalization rates, and higher health care costs. These challenges can be even more profound among minority populations and non-English speakers in the United States.
A literature search and standard data extraction were performed using PubMed, Medline, and EMBASE databases. A total of 1,914 articles were identified, of which 1,858 were excluded based on the inclusion criteria, and 46 were excluded because of a lack of relevance to both diabetes management and health literacy. The remaining 10 articles were reviewed in detail.
Patients, including ethnic minorities and non-English speakers, who are engaged in diabetes education and health literacy improvement initiatives and ongoing follow-up showed significant improvement in A1C, medication adherence, medication knowledge, and treatment satisfaction. Clinicians considering implementing new interventions to address diabetes care for patients with low health literacy can use culturally tailored approaches, consider ways to create materials for different learning styles and in different languages, engage community health workers and pharmacists to help with patient education, use patient-centered medication labels, and engage instructors who share cultural and linguistic similarities with patients to provide educational sessions.
This literature review identified a variety of interventions that had a positive impact on provider-patient communication, medication adherence, and glycemic control by promoting diabetes self-management through educational efforts to address low health literacy.
Diabetes is the seventh leading cause of death in the United States, and 30.3 million Americans, or 9.4% of the U.S. population, are living with diabetes ( 1 , 2 ). For successful management of a complicated condition such as diabetes, health literacy may play an important role. Low health literacy is a well-documented barrier to diabetes management and can lead to poor management of medical conditions, low engagement with health care providers (HCPs), increased hospitalizations, and, consequently, higher health care costs ( 3 – 5 ).
The Healthy People 2010 report ( 6 ) defined health literacy as the “degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.” Diabetes health literacy also encompasses a wide range of skills, including basic knowledge of the disease state, self-efficacy, glycemic control, and self-care behaviors, which are all important components of diabetes management ( 3 – 5 , 7 ). According to the Institute of Medicine’s Committee on Health Literacy, patients with poor health literacy are twice as likely to have poor glycemic control and were found to be twice as likely to be hospitalized as those with adequate health literacy ( 8 ). Associations between health literacy and health outcomes have been reported in many studies, the first of which was conducted in 1995 in two public hospitals and found that many patients had inadequate health literacy and could not perform the basic reading tasks necessary to understand their treatments and diagnoses ( 9 ).
Evaluation of health literacy is vital to the management and understanding of diabetes. Several tools for assessing health literacy have been evaluated, and the choice of which to use depends on the length of the patient encounter and the desired depth of the assessment. One widely used literacy assessment tool, the Test of Functional Health Literacy in Adults (TOFHLA), consists of 36 comprehension questions and four numeric calculations ( 10 ). Additional tools that assess patients’ reading ability include the Rapid Estimate of Adult Literacy in Medicine (REALM) and the Literacy Assessment for Diabetes. Tests that assess diabetes numeracy skills include the Diabetes Numeracy Test, the Newest Vital Sign (NVS), and the Single-Item Literacy Screener (SILS) ( 11 ).
Rates of both diabetes and low health literacy are higher in populations from low socioeconomic backgrounds ( 5 , 7 , 12 ). People living in disadvantaged communities face many barriers when seeking health care, including inconsistent housing, lack of transportation, financial difficulties, differing cultural beliefs about health care, and mistrust of the medical professions ( 13 , 14 ). People with high rates of medical mistrust tend to be less engaged in their care and to have poor communication with HCPs, which is another factor HCPs need to address when working with their patients with diabetes ( 15 ).
The cost of medical care for people with diabetes was $327 billion in 2017, a 26% increase since 2012 ( 1 , 16 ). Many of these medical expenditures are related to hospitalization and inpatient care, which accounts for 30% of total medical costs for people with diabetes ( 16 ).
People with diabetes also may neglect self-management tasks for various reasons, including low health literacy, lack of diabetes knowledge, and mistrust between patients and HCPs ( 7 , 15 ).
These challenges can be even more pronounced in vulnerable populations because of language barriers and patient-provider mistrust ( 17 – 19 ). Rates of diabetes are higher among racial and ethnic minority groups; 15.1% of American Indians and Alaskan Natives, 12.7% of Non-Hispanic Blacks, 12.1% of Hispanics, and 8% of Asian Americans have diagnosed diabetes, compared with 7.4% of non-Hispanic Whites ( 1 ). Additionally, patient-provider relationship deficits can be attributed to challenges with communication, including HCPs’ lack of attention to speaking slowly and clearly and checking for patients’ understanding when providing education or gathering information from people who speak English as a second language ( 15 ). White et al. ( 15 ) demonstrated that patients with higher provider mistrust felt that their provider’s communication style was less interpersonal and did not feel welcome as part of the decision-making process.
To the authors’ knowledge, there is no current literature review evaluating interventions focused on health literacy and diabetes management. There is a pressing need for such a comprehensive review to provide a framework for future intervention design. The objective of this literature review was to gather and summarize studies of health literacy–based diabetes management interventions and their effects on overall diabetes management. Medication adherence and glycemic control were considered secondary outcomes.
Search Strategy
A literature review was conducted using the PubMed, Medline, and EMBASE databases. Search criteria included articles published between 2015 and 2020 to identify the most recent studies on this topic. The search included the phrases “diabetes” and “health literacy” to specifically focus on health literacy and diabetes management interventions and was limited to original research conducted in humans and published in English within the defined 5-year period. Search results were exported to Microsoft Excel for evaluation.
Study Selection
Initial screening of the articles’ abstracts was conducted using the selection criteria to determine which articles to include or exclude ( Figure 1 ). The initial search results were reviewed for the following inclusion criteria: original research (clinical trials, cohort studies, and cross-sectional studies) conducted in human subjects with type 2 diabetes in the United States, and published in English between 2015 and 2020. Articles were considered to be relevant if diabetes was included as a medical condition in the study and an intervention was made to assess or improve health literacy. Studies involving type 1 diabetes or gestational diabetes and articles that were viewpoints, population surveys, commentaries, case reports, reviews, or reports of interventions conducted outside of the United States were excluded from further review. The criteria requiring articles to be from the past 5 years and from the United States were used because of the unique and quickly evolving nature of the U.S. health care system. Articles published more than 5 years ago or from other health care systems may have contributed information that was not applicable to or no longer relevant for HCPs in the United States. Articles were screened and reviewed independently by both authors. Disagreements were resolved through discussion to create the final list of articles for inclusion.
PRISMA diagram of the article selection process.
Data Extraction
A standard data extraction was performed for each included article to obtain information including author names, year of publication, journal, study design, type of intervention, primary outcome, tools used to assess health literacy or type 2 diabetes knowledge, and effects of intervention on overall diabetes management, glycemic control, and medication adherence.
A total of 1,914 articles were collected from a search of the PubMed, MEDLINE, and EMBASE databases, of which 1,858 were excluded based on the inclusion and exclusion criteria. Of the 56 articles that met criteria for abstract review, 46 were excluded because of a lack of relevance to both diabetes management and health literacy. The remaining 10 studies identified various diabetes management interventions, including diabetes education tools such as electronic medication instructions and text message–based interventions, technology-based education videos, enhanced prescription labels, learner-based education materials, and culturally tailored interventions ( 15 , 20 – 28 ). Figure 1 shows the PRISMA diagram of the article selection process, and Table 1 summarizes the findings of the article reviews ( 15 , 20 – 28 ).
Findings of the Article Reviews (15,20–28)
SAHLSA, Short Assessment of Health Literacy for Spanish Adults.
Medical mistrust and poor communication are challenging variables in diabetes education. White et al. ( 15 ) examined the association between communication quality and medical mistrust in patients with type 2 diabetes. HCPs at five health department clinics received training in effective health communication and use of the PRIDE (Partnership to Improve Diabetes Education) toolkit in both English and Spanish, whereas control sites were only exposed to National Diabetes Education Program materials without training in effective communication. The study evaluated participant communication using several tools, including the Communication Assessment Tool (CAT), Interpersonal Processes of Care (IPC-18), and the Short Test of Functional Health Literacy in Adults (s-TOFHLA). The authors found that higher levels of mistrust were associated with lower CAT and IPC-18 scores.
Patients with type 2 diabetes are also likely to benefit from personalized education delivery tools such as patient-centered labeling (PCL) of prescription drugs, learning style–based education materials, and tailored text messages ( 24 , 25 , 27 ). Wolf et al. ( 27 ) investigated the use of PCL in patients with type 2 diabetes and found that patients with low health literacy who take medication two or more times per day have higher rates of proper medication use when using PCL (85.9 vs. 77.4%, P = 0.03). The objective of the PCL intervention was to make medication instructions and other information on the labels easier to read to improve medication use and adherence rates. The labels incorporated best-practice strategies introduced by the Institute of Medicine for the Universal Medication Schedule. These strategies prioritize medication information, use of larger font sizes, and increased white space. Of note, the benefits of PCL were largely seen with English speakers. Spanish speakers did not have substantial improvement in medication use or adherence, which could be attributed to language barriers ( 27 ).
Nelson et al. ( 25 ) analyzed patients’ engagement with an automated text message approach to supporting diabetes self-care activities in a 12-month randomized controlled trial (RCT) called REACH (Rapid Education/Encouragement and Communications for Health) ( 25 ). Messages were tailored based on patients’ medication adherence, the Information-Motivation-Behavioral Skills model of health behavior change, and self-care behaviors such as diet, exercise, and self-monitoring of blood glucose. Patients in this trial were native English speakers, so further research to evaluate the impact of the text message intervention in patients with limited English language skills is still needed. However, participants in the intervention group reported higher engagement with the text messages over the 12-month period ( 25 ).
Patients who receive educational materials based on their learning style also show significant improvement in their diabetes knowledge and health literacy. Koonce et al. ( 24 ) developed and evaluated educational materials based on patients’ learning style to improve health literacy in both English and Spanish languages. The materials were made available in multiple formats to target four different learning styles, including materials for visual learners, read/write learners, auditory learners, and kinesthetic learners. Spanish-language versions were also available. Researchers were primarily interested in measuring patients’ health literacy and knowledge of diabetes. The intervention group received materials in their preferred learning style and language, whereas the control group received standard of care education materials. The intervention group showed significant improvement in diabetes knowledge and health literacy, as indicated by Diabetes Knowledge Test (DKT) scores. More participants in the intervention group reported looking up information about their condition during week 2 of the intervention and showed an overall improvement in understanding symptoms of nerve damage and types of food used to treat hypoglycemic events. However, the study had limited enrollment of Spanish speakers, making the applicability of the results to Spanish-speaking patients highly variable.
Additionally, findings by Hofer et al. ( 22 ) suggest that patients with high A1C levels may benefit from interventions led by community health workers (CHWs) to bridge gaps in health literacy and equip patients with the tools to make health decisions. In this study, Hispanic and African American patients with low health literacy and diabetes not controlled by oral therapy benefited from education sessions led by CHWs. The CHWs led culturally tailored support groups to compare the effects of educational materials provided in an electronic format (via iDecide) and printed format on medication adherence and self-efficacy. The study found increased adherence with both formats, and women, specifically, had a significant increase in medication adherence and self-efficacy. One of the important aspects of this study was that the CHWs shared cultural and linguistic characteristics with the patients and HCPs, leading to increased trust and satisfaction with the information presented ( 22 ).
Kim et al. ( 23 ) found that Korean-American participants benefited greatly from group education sessions that provided integrated counseling led by a team of nurses and CHW educators. The intervention also had a health literacy component that focused on enhancing skills such as reading food package labels, understanding medical terminology, and accessing health care services. This intervention led to a significant reduction of 1–1.3% in A1C levels in the intervention group. The intervention established the value of collaboration between CHW educators and nurses to improve health information delivery and disease management.
A collaboration between CHW educators and pharmacists was also shown to reinforce diabetes knowledge and improve health literacy. Sharp et al. ( 26 ) conducted a cross-over study in four primary care ambulatory clinics that provided care for low-income patients. The study found that patients with low health literacy had more visits with pharmacists and CHWs than those with high health literacy. The CHWs provided individualized support to reinforce diabetes self-management education and referrals to resources such as food, shelter, and translation services. The translation services in this study were especially important for building trust with non-English speakers and helping patients understand their therapy. Similar to other studies, the CHWs shared cultural and linguistic characteristics with their populations, which helped to overcome communication-related and cultural barriers ( 23 , 26 ).
The use of electronic tools or educational videos yielded inconclusive results with regard to medication adherence. Graumlich et al. ( 20 ) implemented a new medication planning tool called Medtable within an electronic medical record system in several outpatient clinics serving patients with type 2 diabetes. The tool was designed to organize medication review and patient education. Providers can use this tool to search for medication instructions and actionable language that are appropriate for each patient’s health literacy level. The authors found no changes in medication knowledge or adherence, but the intervention group reported higher satisfaction. On the other hand, Yeung et al. ( 28 ) showed that pharmacist-led online education videos accessed using QR codes affixed to the patients’ medication bottles and health literacy flashcards increased patients’ medication adherence in an academic medical hospital.
Goessl et al. ( 21 ) found that patients with low health literacy had significantly higher retention of information when receiving evidence-based diabetes education through a DVD recording than through an in-person group class. This 18-month RCT randomized participants to either the DVD or in-person group education and assessed their information retention through a teach-back strategy. The curriculum consisted of diabetes prevention topics such as physical exercise, food portions, and food choices. Participants in the DVD group had significantly higher retention of information than those in the control (in-person) group. The authors suggested this may have been because participants in the DVD group have multiple opportunities to review the education material.
Management of type 2 diabetes remains a challenge for HCPs and patients, in part because of the challenges discussed in this review, including communication barriers between patients and HCPs and knowledge deficits about medications and disease states ( 29 ). HCPs can have a positive impact on the health outcomes of their patients with diabetes by improving patients’ disease state and medication knowledge.
One of the common themes identified in this literature review was the prevalence of culturally tailored diabetes education interventions. This is an important strategy that could improve diabetes outcomes and provide an alternative approach to diabetes self-management education when working with patients from culturally diverse backgrounds. HCPs might benefit from using culturally tailored educational approaches to improve communication with patients and overcome the medical mistrust many patients feel. Although such mistrust was not directly correlated with diabetes management, it was noted that patients who feel mistrustful tend to have poor communication with HCPs ( 20 ). Additionally, Latino/Hispanic patients who have language barriers tend to have poor glycemic control ( 19 ). Having CHWs work with HCPs might mitigate some patient-provider communication barriers. As noted earlier, CHWs who share cultural and linguistic characteristics with their patient populations have ongoing interactions and more frequent one-on-one encounters ( 12 ).
Medication adherence and glycemic control are important components of diabetes self-management, and we noted that the integration of CHWs into the diabetes health care team and the use of simplified medication label interventions were both successful in improving medication adherence ( 23 , 24 ). The use of culturally tailored education sessions and the integration of pharmacists and CHWs into the management of diabetes appear to be successful in reducing A1C levels ( 12 , 26 ). Electronic education tools and educational videos alone did not have an impact on medication knowledge or information retention in patients with low health literacy, but a combination of education tools and individualized sessions has the potential to improve diabetes medication knowledge and overall self-management ( 20 , 22 , 30 ).
There were several limitations to our literature review. We restricted our search criteria to articles published in English and studies conducted within the United States to ensure that the results would be relevant to U.S. HCPs. However, these limitations may have excluded important work on this topic. Additional research expanding this search beyond the United States and including articles published in other languages may demonstrate different outcomes. Additionally, this literature review did not focus on A1C as the primary outcome, although A1C is an important indicator of diabetes self-management. A1C was chosen as the method of evaluating the impact of health literacy interventions in patients with diabetes, but other considerations such as medication adherence, impact on comorbid conditions, and quality of life are also important factors.
The results of this work show that implementing health literacy interventions to help patients manage type 2 diabetes can have beneficial results. However, such interventions can have significant time and monetary costs. The potential financial and time costs of diabetes education interventions were not evaluated in this review and should be taken into account when designing interventions. The American Diabetes Association estimated the cost of medical care for people with diabetes to be $327 billion in 2017, with the majority of the expenditure related to hospitalizations and nursing home facilities ( 16 ). Another substantial cost of diabetes that can be difficult to measure is treatment for comorbid conditions and complications such as cardiovascular and renal diseases.
Interventions designed to address low health literacy and provide education about type 2 diabetes could be a valuable asset in preventing complications and reducing medical expenditures. Results of this work show that clinicians who are considering implementing new interventions may benefit from the following strategies: using culturally tailored approaches, creating materials for different learning styles and in patients’ languages, engaging CHWs and pharmacists to help with patient education, using PCLs for medications, and engaging education session instructors who share patients’ cultural and linguistic characteristics.
Diabetes self-management is crucial to improving health outcomes and reducing medical costs. This literature review identified interventions that had a positive impact on provider-patient communication, medication adherence, and glycemic control by promoting diabetes self-management through educational efforts to address low health literacy. Clinicians seeking to implement diabetes care and education interventions for patients with low health literacy may want to consider drawing on the strategies described in this article. Providing culturally sensitive education that is tailored to patients’ individual learning styles, spoken language, and individual needs can improve patient outcomes and build patients’ trust.

Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors conceptualized the literature review, developed the methodology, analyzed the data, and wrote, reviewed, and edited the manuscript. R.A. collected the data. K.M. supervised the review. K.M. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Portions of this research were presented at the Washington State University College of Pharmacy and Pharmaceutical Sciences Honors Research Day in April 2019.
Email alerts
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 May 2024
A digital twin model incorporating generalized metabolic fluxes to identify and predict chronic kidney disease in type 2 diabetes mellitus
- Naveenah Udaya Surian 1 ,
- Arsen Batagov ORCID: orcid.org/0000-0002-9620-079X 1 ,
- Andrew Wu 1 ,
- Wen Bin Lai 1 ,
- Yan Sun 2 ,
- Yong Mong Bee ORCID: orcid.org/0000-0002-5482-2646 3 &
- Rinkoo Dalan ORCID: orcid.org/0000-0001-9769-2696 4 , 5
npj Digital Medicine volume 7 , Article number: 140 ( 2024 ) Cite this article
476 Accesses
1 Altmetric
Metrics details
- Diabetes complications
- Outcomes research
We have developed a digital twin-based CKD identification and prediction model that leverages generalized metabolic fluxes (GMF) for patients with Type 2 Diabetes Mellitus (T2DM). GMF digital twins utilized basic clinical and physiological biomarkers as inputs for identification and prediction of CKD. We employed four diverse multi-ethnic cohorts ( n = 7072): a Singaporean cohort (EVAS, n = 289) and a North American cohort (NHANES, n = 1044) for baseline CKD identification, and two multi-center Singaporean cohorts (CDMD, n = 2119 and SDR, n = 3627) for 3-year CKD prediction and risk stratification. We subsequently conducted a comprehensive study utilizing a single dataset to evaluate the clinical utility of GMF for CKD prediction. The GMF-based identification model performed strongly, achieving an AUC between 0.80 and 0.82. In prediction, the GMF generated with complete parameters attained high performance with an AUC of 0.86, while with incomplete parameters, it achieved an AUC of 0.75. The GMF-based prediction model utilizing complete inputs is the standard implementation of our algorithm: HealthVector Diabetes®. We have established the GMF digital twin-based model as a robust clinical tool capable of predicting and stratifying the risk of future CKD within a 3-year time horizon. We report the correlation of GMF with basic input parameters, their ability to differentiate between future health states and medication status at baseline, and their capability to quantify CKD progression rates. This holistic methodology provides insights into patients’ health states and CKD progression rates based on GMF metabolic profile differences, enabling personalized care plans.
Similar content being viewed by others

Identifying proteomic risk factors for cancer using prospective and exome analyses of 1463 circulating proteins and risk of 19 cancers in the UK Biobank

Genome-wide association studies

Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial
Introduction.
Diabetes has emerged as a prominent global health crisis in the 21st century. The lifespan of patients with diabetes is estimated to be reduced by 12 years due to vascular complications 1 . In a multi-ethnic population of Singapore, the prevalence of diabetes has doubled over the past four decades, resulting in the highest global prevalence of diabetic kidney failure 2 . Among non-fatal complications of diabetes, end-stage renal disease requiring dialysis stands out as a significant driver of healthcare costs 3 . It is therefore crucial to detect chronic kidney disease (CKD) early in its course, before reaching a non-reversible state.
A digital twin can be defined as a direct digital representation of an individual based on the individuals own comprehensive biological data 4 . Using this concept, a virtual replica of the vascular system in the individual can be represented based on a mechanistic model 5 . These digital twins can then be used to simulate progression of chronic diseases and to predict probable trajectories of disease development 6 . Moreover, they can inform clinicians how altering specific mechanistic components could change disease trajectories, thereby leading patients towards less severe disease states.
In recent years, digital twin technology has surged in popularity within the healthcare sector. A PubMed trend search for the keyword ‘digital twin’ unveiled a tenfold increase in mentions over the past five years. Different techniques, measurements and applications can be used to build a patient’s digital twin. In this paper, we utilize generalized metabolic fluxes (GMF) to construct personalized digital twins of patients and study the trajectory of microvascular complications leading to chronic CKD 6 .
The GMF digital twin analysis allows us to represent the long-term changes in the rates of metabolic processes in the body as a mathematical model of personalized metabolic rates. The components of the GMF (outputs) are obtained based on a best fit approach from the observed biochemical and physiological measurements (inputs) of an individual patient. Our GMF-based digital twin is used here to identify the CKD disease states of patients at baseline and predict the occurrence of CKD within a three-year period. We also comprehensively demonstrate the utility of GMF digital twins as a clinical application tool for CKD in individuals with Type 2 Diabetes Mellitus (T2DM).
Dataset characteristics
A detailed summary of the main characteristics of each dataset is outlined in Table 1 . We utilized four main datasets (EVAS, NHANES, SDR, and CDMD), two for the identification (EVAS and NHANES) and two for prediction (SDR and CDMD) of CKD. The EVAS, SDR and CDMD, datasets represented Asian-Singaporean cohorts, while the NHANES dataset represented a North America cohort. These varied datasets were chosen to showcase the versatility of GMF digital twins for analyses across different populations (Fig. 1 ). The EVAS dataset comprised 289 patients, of which 98 had CKD at baseline. In the NHANES dataset, there were 1044 patients, with 360 having CKD at baseline. The SDR dataset included 3627 patients, with 1420 diagnosed with CKD within 3 years, while the CDMD dataset involved 2112 patients, with 719 diagnosed with CKD in 3 years.

The scheme illustrates the datasets employed and how the generalized metabolic fluxes (GMF) digital twins were created and subsequently analyzed in this study. From known biological relationships, the metabolic GMF processes are constructed to form the GMF digital twins. The performance and capabilities of GMF were tested in a few analyses. We first assess its capabilities in the identification and prediction of chronic kidney disease (CKD) as well as CKD risk stratification. Subsequently, we performed a comprehensive characterization of GMF digital twins as a clinical tool for CKD.
The four datasets exhibited mostly similar characteristics, with some observed differences. Notably, the EVAS dataset included younger patients (54 [11.1]) with higher levels of glycated hemoglobin (HbA1c) (8.6 [1.8]) compared to those in the CDMD dataset (57 [12.4], 8.0 [1.8]), NHANES dataset (59 [11.9], 7.6 [1.9]), and SDR dataset (61 [11.0], 7.4 [1.6]). The NHANES dataset had slightly lower systolic blood pressure (SBP) (130.3 [18.6]), higher serum creatinine (85.0 [56.3]), and body mass index (BMI) (32.5 [7.5]) values compared to the EVAS dataset (133.2 [14.8], 27.7 [5.0], 74.2 [26.9]), the CDMD dataset (133.8 [17.9], 26.8 [5.6], 74.0 [22.7]) and the SDR dataset (132.1 [15.1], 26.6 [5.5], 70.8 [23.3]).
GMF digital twins for the identification and prediction of CKD
In the NHANES and the EVAS cohorts, we used baseline observations of biochemical and physiological measurements of patients (Table 2 ) to build individual GMF digital twins using the method described earlier 6 . Each digital twin represented the state of metabolic dynamic variables, GMF, which quantify the rates of biochemical pathways and physiological processes. Since the inputs did not include the diagnostic indicators of CKD, the estimated glomerular filtration rate (eGFR) and the urinary albumin to creatinine ratio (ACR), we first sought to test if the digital twin-based models could reflect the CKD condition, based on the evaluation of the holistic metabolic state of the patient (Fig. 1) . In other terms, this analysis involved identifying the CKD state based on a set of estimated metabolic rates. For the identification of CKD, our GMF-based model achieved an AUC of 0.80 (confidence interval (CI): 0.74–0.85) in the EVAS dataset and an AUC of 0.82 (CI: 0.79–0.84) in the NHANES dataset (Fig. 2 a, b).

a The identification of CKD in EVAS at baseline yielded an AUC of 0.80. b The identification of CKD in NHANES at baseline yielded an AUC of 0.82. c The prediction of CKD within 3 years in SDR with complete inputs yielded an AUC of 0.86. d The prediction of CKD in CDMD with incomplete inputs yielded an AUC of 0.75. The LR models for the AUC-ROC curve generations utilized the GMF values, age and gender as input parameters. The CI represents the 95% confidence interval of the AUC-ROC curve in the EVAS, NHANES and CDMD datasets. The SDR AUC-ROC curve is depicted for the Testing-2 subset of the SDR dataset, generated by the LR model trained on the SDR training set. e The correlation between biochemical and physiological input parameters with GMF in the CDMD dataset. The numbers in each box represent Kendall’s τ correlation value between two variables, the input parameters vs the GMF values. Stronger positive correlation is indicated by values closer to +1 and depicted in a deeper red hue. Conversely, stronger negative correlation is denoted by values closer to -1 and depicted in a deeper blue hue. These relationships are illustrated in the legend located on the right. Serum creatinine is positively correlated with a number of individual GMF, particularly the GMF related to the respiration-circulation pathways, reactive oxygen species and HbA1c production pathways. It is also negatively correlated to the individual GMF related to the albumin-ACR pathways. LDL, Cholesterol, BMI, glucose and HbA1c cluster together, whereby LDL, Cholesterol and BMI are strongly correlated to the individual GMF related to the lipid metabolism pathways. CKD chronic kidney disease, GMF generalized metabolic fluxes, AUC-ROC area under the curve receiver operating characteristic, LR logistic regression, CI confidence interval, SN Sensitivity, SP Specificity, HbA1c glycated hemoglobin, ACR albumin-creatinine ratio, LDL low density lipoprotein, BMI body mass index.
Having showed that GMF digital twins could reflect and distinguish between non-CKD and CKD states among patients at the baseline time point, we examined whether the estimated metabolic rates from the GMF digital twins could predict the progression from the non-CKD to the CKD state in the future. We evaluated two logistic regression (LR) prediction models: the complete input and the incomplete input models. In the SDR dataset, where patient input values were complete, the LR model was trained on the training set and tested on two equally divided testing sets, Testing-1 and Testing-2, for evaluation. Performance metrics, including AUC, Sensitivity (SN), Specificity (SP), Negative Predictive Value (NPV), and Positive Predictive Value (PPV) for the SDR dataset, are presented in Table 3 . Notably, we achieved identical AUCs of 0.86 and nearly identical values for SP, NPV, and PPV in both the testing sets, Testing-1 and Testing-2 (Fig. 2 c, Table 3) . We subsequently evaluated the performance of GMF digital twins in the CDMD dataset, which had incomplete patient input values (Fig. 2 d). The LR prediction model here achieved an AUC of 0.75 (CI: 0.73–0.77). This underscores the robustness of GMF digital twins for the prediction of CKD.
Next, we explored how our predictive model stratifies patients into three different risk groups: high, moderate, and low. The distribution of the SDR and CDMD patients across the risk groups is presented in Table 4 . Most patients who developed CKD in 3 years came from the high-risk group. In the SDR dataset, 62.9% of patients developed CKD in the high-risk group, whereas this fraction was 19.3% in the moderate-risk group and 5.4% in the low-risk group. The distribution of CKD cases in the CDMD dataset was overall similar: 53.3%, 17.3%, and 9.8% in the high-, moderate- and low-risk groups, respectively (Table 4) . The observed frequencies in both datasets align with the expected risk frequencies, as well as the values reported in previous studies 7 , 8 . We also assessed the distribution of future CKD patients across CKD stages and their corresponding risk categories determined by the GMF-based prediction model in the CDMD dataset. Microalbuminuria (3.3 mg/mmol < ACR < 30 mg/mmol) was the prevailing condition among future CKD cases, constituting 93%, of which 73% were categorized as high risk (Supplementary Table 1) .
Correlation between the observable biochemical and physiological inputs and GMF
Utilizing the CDMD dataset, we investigated the clinical applications of GMF digital twins, extending their utility for risk prediction. We first looked at the correlation between the input parameters (biochemical and physiological) used to obtain GMF with the GMF values itself. The input parameters show strong correlation with the relevant individual GMF (Fig. 2 e). Serum creatinine, which is an indicator for CKD, has a strong positive correlation with the individual GMF related to the circulation-respiratory pathways, the reactive oxygen species and HbA1c production pathways and negative correlation to the individual GMF related to the albumin-ACR pathways. Furthermore, the parameters LDL, Cholesterol and BMI show strong positive correlation to the individual GMF related to the lipid metabolism pathways.
The influence of individual GMF impact on CKD prediction
After observing the correlations between the input parameters and GMF, we studied the individual GMF that were significantly impacting the CKD predictive performance based on the coefficient weights from the LR model in CDMD. Out of the 14 individual GMF and two demographic variables (age and gender) used as predictor variables for CKD prediction, the LR model revealed that two individual GMF along with gender emerged as the most statistically significant predictor variables (Table 5 , p < 0.05). The two individual GMF were associated with HbA1c production 9 and kidney function 10 : Hb+ROM → HbA1c and HbA1c → ACR. Removing these three predictor variables led to a drop in prediction accuracy, yielding an AUC of 0.62 (CI: 0.60-0.65, Fig. 3a ). Interestingly, using only these three predictor variables in the LR model also resulted in much lower prediction accuracy for CKD, with an AUC of 0.61 (CI: 0.59-0.63, Fig. 3b ). This highlights that reducing the GMF digital twin to only a subset of statistically significant individual GMF strongly reduces predictive performance and, consequently, clinical utility.
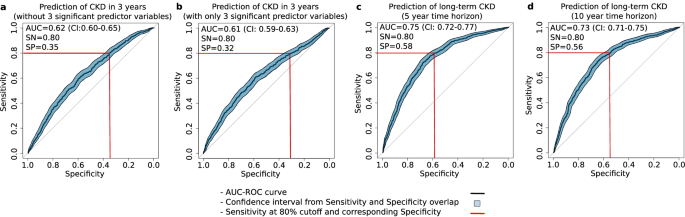
a The prediction of CKD without the top 3 significant predictors yielded an AUC of 0.62. b The prediction of CKD with only the top three significant predictors yielded an AUC of 0.61. c The prediction of CKD in 5 years yielded an AUC of 0.75. d The prediction of CKD in 10 years yielded an AUC of 0.73. The LR models for the AUC-ROC curve generations utilized the non-significant predictor variables (a) or the significant predictor variables (b) or the GMF values, age and gender (c and d) as input parameters depending on the analysis in the CDMD dataset. The figure displays the CI values for Sn and Sp as filled polygons, overlapping the AUC-ROC curve along the vertical (Sn) and horizontal (Sp) axes.
Patient health states and medication effects with GMF
To identify predictive factors for future categorization into CKD-positive or negative groups, we analyzed baseline GMF profiles. In the CDMD dataset, a subgroup analysis comparing the GMF metabolic profiles of future CKD and future non-CKD patients revealed that the future CKD group exhibited elevated individual GMF associated with circulation, blood pressure, glucose metabolism, and kidney function (Fig. 4a , Supplementary Table 2) . This clearly shows that future CKD patients display a more deteriorated health state (GMF metabolic profile) compared to future non-CKD patients.

a The GMF profile from a subgroup analysis of future CKD patients vs future non-CKD patients in the CDMD dataset. The GMF profile of patients who develop CKD in the future shows a poorer health profile than the GMF profile of patients who do not develop CKD in the future. This subgroup had elevated individual GMF associated with circulation, blood pressure, glucose metabolism, and kidney function. b The time to CKD event analysis in CDMD. Risk group 0 represents patients classified as low/moderate-risk patients whereas risk group 1 represents patients classified as high-risk patients by the GMF digital twin based logistic regression model. There is a significant difference in the event rate between the high-risk vs moderate/low- risk as evidenced by the elevated hazard ratio (HR = 3.57, p < 2.0 e-16). c Clustering pattern of patients in the CDMD dataset using GMF together with demographics. N represents the number of patients within the specific cluster, while CKD positive outcomes are calculated as the ratio of patients developing future CKD (within 3 years) to the total number of patients in that cluster. The cluster with the highest CKD outcomes rate has a distinct pattern with elevated individual GMF values related to the respiration-circulation pathways, reactive oxygen species and HbA1c production pathways and reduced individual GMF values related to the albumin-ACR pathways. The colors in each cell of the heatmap correspond to normalized values for each individual GMF or demographic within each cluster, where elevated values compared to the median are depicted in a deeper red hue, while reduced values compared to the median are shown in a deeper blue hue. This color scale is represented on the right side of the heatmap. d The relationship between the cluster distances and CKD outcomes rate in CDMD. There is a significant correlation (τ = 0.6, p = 0.017) between cluster distance as measured by the distance metric and CKD outcomes rate where the cluster with the highest CKD outcomes rate is furthest away from the cluster with the lowest CKD outcomes rate. Standard error (SE) is denoted by the gray shaded area.
Medications, such as sodium-glucose transport protein 2 inhibitors (SGLT2i), can influence metabolic pathways, and SGLT2i is known for its role in reducing CKD risk. In this study, we investigated the impact of SGLT2i on GMF in two patient subgroups: the future CKD group and the future non-CKD group based on their baseline GMF profiles. Significant differences in individual GMF related to lipid metabolism (Fat.lipids ↔ FFA and FFA → BMI) were observed in the future non-CKD group ( p < 0.05, Table 6 ). Although the effects in the future CKD group were not statistically significant but near significance ( p < 0.1, Table 6) , there was a noticeable trend involving the same lipid metabolism GMF. Our GMF-based analysis highlights the impact of medication on metabolic profiles, specifically the influence of SGLT2i on lipid metabolism, aligning with findings from prior studies 11 .
GMF as a metric for CKD progression rate
Having observed that GMF profiles provide insights into CKD health states as well as medication effects, our next objective was to quantify CKD progression rates in different risk groups within the CDMD dataset. To assess the rate of CKD progression in high-risk versus moderate/low-risk groups, we conducted a Kaplan–Meier time-to-event analysis and calculated the hazard ratios from the Cox-proportional hazard model. The highly significant hazard ratio of 3.6 ( p < 0.01) indicates a markedly faster progression rate in the high-risk group compared to the moderate/low-risk group (Fig. 4b ). After confirming the heightened progression rate in the high-risk patient group, we assessed the capability of GMF based digital twins to predict CKD beyond 3 years. The initial count of CKD-positive patients within 3 years was 719. Extending the prediction timeframe to 5 and 10 years resulted in a 10% and 24% increase, totaling 793 patients in 5 years and 889 patients in 10 years. The LR model’s predictive performance with GMF digital twins for long-term CKD within a 5-year and 10-year horizon yielded AUC values of 0.75 (CI: 0.72-0.77) and 0.73 (CI: 0.71-0.75), respectively. (Fig. 3 c, d). This suggests that GMF digital twins is proficient in predicting CKD over extended periods, achieving AUCs similar to those obtained originally within 3 years.
Among CKD-negative patients at baseline, some profiles resembled CKD patients while others did not. As we have clearly established that GMF can be used to measure CKD progression rates as well as predict longer term CKD, we sought a metric to distinguish the profiles of patients clustered using GMF. We conducted patient clustering using two sets of variables: the first set consisted of GMF values, while the second set utilized observable inputs (biochemical and physiological). When clustering patients based on GMF values, we observed that the cluster with the highest fraction of future CKD outcomes was markedly distinct from all other clusters (Fig. 4 c, a). In this cluster, the individual GMF related to circulation-respiratory pathways and the reactive oxygen, creatinine and HbA1c production pathways were increased whereas the individual GMF related to the albumin-ACR pathways were decreased. To analyze the relationship between GMF profiles and CKD development, we ordered the clusters by increasing CKD outcomes rate. We picked the cluster with the lowest fraction of positive CKD outcomes as the starting point. For all other clusters, we computed the Euclidean distance from their centers to the starting point. We examined the dependence of the CKD outcomes rate in each cluster on this distance metric. There is a significant positive correlation ( τ = 0.6, p < 0.05) between the distance metric and CKD outcomes rate when we used GMF to cluster patients (Fig. 4 d, b). The cluster with the highest fraction of positive CKD outcomes was the furthest away from the cluster with the lowest fraction of positive CKD outcomes. By contrast, we found no distinct cluster profiles and no significant associations between the distance metric and CKD outcomes rate when we used input parameters for clustering (Supplementary Fig. 1) .
Current clinical guidelines recommend regular monitoring for diabetes-related complications, including cardio-metabolic and renal screenings every 4–6 months (e.g., lipid panel, HbA1c, urine ACR, eGFR), along with annual retinal exams and foot assessments for peripheral vascular disease 12 . However, these recommendations are not always followed, resulting in selective sparsity in patient data and delayed detection of complications. Current clinical indicators for kidney function, such as ACR and eGFR, are used to diagnose and stage patients with CKD 12 . Without these indicators, detecting CKD becomes challenging. Moreover, these indicators are unreliable for prognostication; ACR accurately predicts future progression in only 30% of cases, and eGFR exhibits high variability due to age and gender effects, which is also compounded by the absence of a standardized equation 13 , 14 . These observations underscore the current unmet clinical need for a reliable CKD identification and prediction tool in T2DM patients 15 .
To address these needs, we applied the GMF digital twin method 6 to (i) obtain the best-fit approximation of patient health states using complete and incomplete inputs, (ii) identify CKD development in patients without relying on ACR and eGFR values, (iii) predict CKD progression, and (iv) elucidate the metabolic traits associated with high CKD progression risk as predictor variables. GMF digital twins is made up of multiple dynamic variables (from the observable inputs) characterizing the present metabolic state of a patient and mapping their future health trajectory. The principle of GMF digital twins is derived from biological information and pathways (Supplementary Table 3) . To evaluate the performance of the GMF digital twins in identification of CKD at baseline (characterizing the present health state) or predicting CKD progression in the future (mapping the future health trajectory), we utilized a logistic regression (LR) model and generated corresponding AUC-ROC curves.
In retrospective clinical datasets, GMF digital twins could identify and characterize the metabolic states that corresponded to CKD (AUC = 0.80–0.82, Fig. 2 a, b). Various machine learning models have been devised for CKD detection, ranging from simple methods utilizing common screening parameters to sophisticated deep learning techniques incorporating retinal imaging 16 , 17 . However, these approaches often suffer from either reduced identification accuracy or high complexity, necessitating additional expertise, time, and cost. GMF digital twins could accurately predict CKD progression within a 3-year period and stratify patients based on their CKD progression risk levels. When utilizing the complete set of 14 input parameters, they demonstrated high performance in predicting CKD achieving an AUC of 0.86 (Fig. 2 c). Overall, the predictive performance of our model was higher and more robust than most published models that use single-time point readings (biochemical or otherwise) as their inputs 8 , 18 , 19 , 20 , 21 , 22 (Supplementary Table 4) . Moreover, our GMF digital twins successfully stratified patients based on their CKD risk scores, with the highest proportion of patients developing CKD found in the high-risk group (53.9–62.9%), consistent with findings from previous studies 7 , 8 . We also confirmed a significant association between ethnic diversity and future CKD prevalence and risk stratification (Supplementary Table 5 and Supplementary Table 6) , which was consistent with prior observations 23 . Our GMF-based digital twin prediction model was evaluated on multiple time scales in diverse cross-country multi-center and multi-ethnic cohorts suggesting its wide applicability. These findings lay the groundwork for future clinical applications of GMF digital twins in assessing CKD risk and planning personalized care for diverse populations with T2DM.
To address the common limitation of data sparsity in clinical databases, we developed the incomplete parameter prediction model using the CDMD dataset where we utilized 11 common input parameters with missing values to construct the GMF digital twins. Despite the presence of missing parameters, this GMF-based prediction model exhibited reasonable performance with an AUC of 0.75 (Fig. 2 d). We further assessed the predictive performance of GMF digital twins for CKD over extended durations within the same dataset, achieving consistent AUCs of 0.75 at 5 years and 0.73 at 10 years (Fig. 3 c, d). This underscores the capability of GMF digital twins to effectively handle missing input parameters by employing a best-fit solution across multiple parameters, which remains applicable over extended time periods.
While reporting the GMF digital twins predictive capabilities, it remains crucial to identify the most significant individual GMF as predictor variables and assess their impact on the predictive performance. By analyzing the LR model, we identified three key predictor variables (two individual GMF and gender) (Table 6 ). Removal of these predictors from the LR model resulted in a significant drop in AUC (AUC = 0.62, Fig. 3 a). Notably, the two significant individual GMF (Hb+ROM → HbA1c and HbA1c → ACR) are involved in glucose/HbA1c metabolism and kidney function, providing explainable insights into affected pathways in future CKD states. While these pathways are known to deteriorate in T2DM patients with microvascular complications, utilizing them as sole predictor variables is insufficient, as evidenced again by a substantial drop in AUC (AUC=0.61, Fig. 3 b). The unique strength of GMF lies in its holistic nature, where each individual GMF is interconnected, mirroring the functions of the biological metabolic network.
Further exploration reveals additional insights from GMF. GMF digital twins inform the long-term rates of metabolic changes and predict the evolution of metabolic characteristics of microvascular complications of diabetes 6 . It can identify early indicators of disease progression to predict future health states. Our analysis reveals that patients who will develop CKD in the future exhibit an overall deteriorated metabolic profile at baseline, as indicated by elevated individual GMF or metabolic rates related to glucose production and kidney function compared to patients who do not go on to develop CKD (Fig. 4 a, Supplementary Table 2) . We also observed distinct GMF profile differences between patients taking SGLT2i and patients not taking SGLT2i. There were significant differences in individual lipid metabolism GMF within the future non-CKD group ( p < 0.05, Table 6 ). The difference was more evident in the future non-CKD group compared to the future CKD group. We attribute this to the relatively smaller sample size of patients taking SGLT2i in the future CKD group compared to the future non-CKD group ( n = 16 vs n = 23), causing the effects within the future CKD group to be non-significant but approaching significance ( p < 0.1). This finding was consistent with earlier studies, where SGLT2i was recognized for its influence on lipid metabolism both directly 11 and indirectly 24 . Further studies involving cohorts with a more extensive representation of patients taking SGLT2i could provide additional clarity.
GMF digital twins not only elucidate future health states but also quantify the rates of health state progression, reflecting the evolution of health. Using the time-to-event analysis, we found highly significant differences in CKD progression rates between high- and moderate/low-risk patients (HR = 3.57, p < 0.01, Fig. 4 b). These metabolic changes in the GMF profiles may be associated with microvascular deterioration driving the increased rate of future CKD outcomes. At the initial baseline state, GMF profiles are able to cluster patients according to their metabolic characteristics which in turn reflect the degree of health deterioration. The individual GMF related to respiratory circulation, glucose metabolism and kidney function were distinctly elevated in the cluster with the highest CKD outcomes rate (Fig. 4 c). Measuring the distance between cluster centers reveals a positive correlation between cluster distance and CKD outcomes rate ( p < 0.05, Fig. 4 d). The cluster with the highest positive CKD outcomes is farthest from the cluster with the lowest positive CKD outcomes. This highlights the ability of GMF to map baseline health states to patient health trajectories. In comparison, basic clinical and physiological input parameters failed to demonstrate distinct patient cluster profiles and exhibited no correlation with cluster distances and CKD outcomes rate (Supplementary Fig. 1) . Overall, we could demonstrate that the explainability of our predictive model follows from the structure of GMF digital twins as personalized representations of the metabolic network. It opens opportunities for clinicians to obtain insights into key metabolic pathways leading to future dysfunctions in a given patient. This property sets our method apart from black-box machine learning approaches.
Our study has demonstrated promising results; however, it is important to acknowledge the limitations that exist. In the CDMD and SDR real-world data sets, single time-point readings for all 11 or 14 input parameters used to generate the GMF digital twins were unavailable. Consequently, we applied data aggregation to derive input parameter values which involved taking the median values of each parameter with repeated measurements over a single year. We agree that some parameters, like ACR, have high intra-individual variability 25 . In our complete model (SDR), the inclusion of ACR led to a drop in the predictive model’s performance (Supplementary Fig. 2) . Due to the lack of single-day measurements for each of the parameters, stringent control over input parameters was compromised, introducing non-uniformity. Therefore, ACR was excluded from our complete prediction model (SDR) (Table 2) . In our incomplete model (CDMD), we opted to include ACR. Due to the presence of missing data in the CDMD dataset, excluding an additional parameter would further reduce the model’s accuracy. Consequently, we retained ACR as an input in this model, achieving reasonable predictive performance. Secondly, our comprehensive analysis demonstrated robust results regarding the utility of GMF digital twins as a clinical application tool for CKD prediction. However, this analysis was conducted solely on the CDMD dataset, which had incomplete parameters. Additionally, certain subgroup analyses, particularly those examining medication effects, were constrained by limited sample sizes. Specialized studies on larger cohorts with complete parameters are warranted to address these limitations. Thirdly, the impact of other data forms such as omics (metabolomics, genomics, proteomics, transcriptomics), imaging, and social determinants of health on the generation of our GMF digital twins was not considered. Incorporating these aspects would enrich our GMF digital twins, enhancing their predictive accuracy and providing deeper insights into CKD pathology. Fourthly, while we compiled a comparison table of our complete inputs GMF-based model against other published models for CKD prediction (Supplementary Table 5) , we did not conduct a comparative analysis with our dataset for benchmarking purposes. This was because validated open algorithms with matching endpoints and input structures were not available. As more models with similar outcomes become accessible, future studies could facilitate direct comparisons. These limitations underscore areas for future research and refinement of our approach. We seek to explore the enhanced capabilities of GMF as a metabolic digital twin application in our upcoming studies. Our future investigations aim to explore the longitudinal effects of GMF derived from multiple time points and their implications for enhancing CKD prediction accuracy. Additionally, to evaluate our GMF digital twins predictive performance in real-world settings, we plan to assess the impact of GMF-based prediction and risk stratification on patient outcomes in prospective clinical studies. These analyses were beyond the scope and objectives of the current study. Therefore, we plan to explore them in future research with an expanded study design and additional objectives.
In conclusion, we have developed a GMF-based digital twin application that successfully identifies existing metabolic pathways dysfunctional in CKD patients, and predicts future CKD progression within a 3-year time horizon. The standard implementation of our algorithm (HealthVector Diabetes®), utilizes the GMF digital twin with complete inputs. This positions our algorithm as a promising candidate for adoption in clinical settings as an identification and predictive tool for preventing CKD among T2DM patients.
Study design and dataset characteristics
Our study included four populations: three multi-ethnic T2DM cohorts from Singapore and one multi-ethnic T2DM North American cohort. Our first dataset (EVAS) was a multi-ethnic cohort of T2DM patients from Singapore’s Tan Tock Seng Hospital (TTSH) that were part of a clinical study and were followed-up for 5 years between 2015 to 2020 26 . The second dataset (NHANES) was obtained by selecting T2DM patients from data collected in the National Health and Nutrition Examination Survey (NHANES) that were recruited between 1999 to 2018 as part of the major National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC) program 27 . The third dataset (SDR) was a group of T2DM patients extracted from a health database registry of Singapore Health Services (SingHealth), the SingHealth Diabetes Registry (SDR) between 2013 and 2020 28 . The fourth dataset (CDMD), the National Healthcare Group Chronic Disease Management Datamart (NHG-CDMD) was obtained from the electronic medical records (EMR) of TTSH, Singapore between 2008 to 2021. All patients used in the four datasets were aged between 20 and 80 years and their baseline characteristics are outlined below (Table 1) . Baseline GMF metabolic profiles were calculated for each individual from the selected list of clinical and physiological parameters relevant to T2DM and CKD (Table 2) . We conducted the following analyses (Fig. 1) : i) identification of CKD at baseline ii) prediction of future CKD within three years iii) CKD risk stratification, iv) correlation between observable input parameters and GMF, v) impact of individual GMF on CKD prediction, vi) health states and medication effects with GMF, vii) GMF as a metric for CKD progression rate. For the identification of CKD, we used the EVAS and NHANES datasets and for the prediction of CKD and risk stratification, we used the SDR and CDMD datasets. Subsequently, for all the other analyses, we used the CDMD dataset. Ethics approval was obtained from the Singaporean (NHG and SingHealth) Institutional Review Board (IRB), EVAS (DSRB Ref: 2014/00236), CDMD (DSRB Ref: 2022/00163), and SDR (CIRB Ref: 2022/2164). No informed consent was obtained as waiver of consent was granted by DSRB and CIRB. For the NHANES study, IRB approval and informed consent were duly obtained by the NCHS-CDC (IRB: Protocol 98-12, 2005-06, 2011-17, and 2018-01). This study was conducted in accordance to the principles of the Declaration of Helsinki 29 .
Generalized metabolic fluxes (GMF) digital twin generation and development
In this study, we utilized GMF to create personalized digital twins for each patient 6 . GMF digital twins comprise a network of dynamic variables, which represent the metabolic rates of the patient at a specific health state. We distinguished two reference health states: A) T2DM without CKD and B) T2DM with CKD. At the baseline time point, patients were either in state A or state B and using GMF, we identified and described their health state at that time point. On the other hand, during the course of our prediction study, some patients progressed from state A to state B within a defined period of time (i.e., 3 years), while others remained in state A. For each patient, the progression occurred along a single health state progression scale, which we termed a generalized extent, spanning between the two basic states: A and B. This scale depicts a continuous change in the patient’s metabolic profile. As a dynamic variable, a single individual GMF measures the rate of change of a specific metabolite concentration or a physiological reading at any given state along the progression scale. The collection of individual GMF for a particular patient at a particular time point represents that patient’s metabolic digital twin (Fig. 1 , Supplementary Table 3) . The GMF methodology allows us to use both complete and incomplete inputs to produce digital twins of patients and best fit models. We first evaluated the ability of GMF-based digital twins to identify CKD at baseline in two datasets (EVAS and NHANES). We then investigated the performance of GMF (complete and incomplete) in two separate datasets (SDR and CDMD) for the prediction of CKD. The completeness of inputs in each dataset is characterized in the Supplementary Materials (Supplementary Table 7) . Our GMF methodology was capable of handling missing parameters without the need for imputation. We verified this by conducting the multiple imputation by chained equation (MICE) analysis with the CDMD dataset. Multiple imputation using Fully Conditional Specification (FCS) implemented by the MICE algorithm was used to impute data that were missing in this dataset. Each variable has its own imputation model and can therefore be extrapolated to fit into the other missing variables. Five imputed datasets were used to produce five sets of corresponding GMF values and this was subsequently used in the LR prediction model. The AUC obtained from these five imputed sets were then compared to the original AUC with missing parameters (Supplementary Table 8) . We confirmed that using data imputation does not provide additional benefits and our GMF-based prediction model from incomplete inputs was robust enough in handling missing values.
The detailed method of producing patients GMF digital twins has been explained in our earlier technical paper 6 . For the identification of CKD, the GMF values were obtained from 10 biochemical and physiological parameters as inputs and produced 21 informative individual GMF as outputs. The outputs were combined with the patient’s age and gender (demographics) to build the LR model for the identification of CKD cases in the EVAS and NHANES datasets. Similarly, for the prediction of CKD, there were two LR models: (i) the model with complete inputs and ii) the model with incomplete inputs. For the complete model, the GMF values were obtained from 14 parameters as inputs and was tested in the SDR dataset whereas for the incomplete model, the GMF values were obtained from 11 parameters as inputs and was tested in the CDMD dataset. Both produced 21 informative individual GMF as outputs. The comprehensive list of input parameters utilized in the analysis of each dataset is shown in Table 2 .
Clinical definitions and parameter selection
Clinical measurements were taken at their point of recruitment (all datasets) and follow-up (CDMD and SDR). In the EVAS, NHANES, and CDMD datasets, patients were identified to have CKD if they fell under one of the following two categories: i) they had an Albumin to Creatinine Ratio (ACR) value of more than 3.3 mg/mmol (equivalent to 30 mg/g) ii) they had an estimated glomerular filtration rate (eGFR) value of less than 60 mL/min/1.73 m 2 12 . For the SDR dataset, we used only the second criterion: the eGFR value of less than 60 mL/min/1.73 m 2 . This was due to the SDR dataset being primarily composed of primary care patients whereas the EVAS and CDMD datasets were primarily composed of tertiary care patients (Supplementary Fig. 1) . For the EVAS and CDMD datasets, the eGFR value was calculated using the New Asian Modified CKD-EPI formula because this was the equation used by the clinician in charge of these datasets in TTSH 30 . Whereas for the NHANES and the SDR datasets, the standard Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used 31 . A complete list of abbreviations of the terms used in this study is detailed in the Supplementary Materials (Supplementary Table 9) .
Logistic regression (LR) and risk stratification analysis
We first performed the analysis on the identification of CKD disease state at baseline to determine the present health state of patients using two datasets, the EVAS and the NHANES datasets. Subsequently, we predicted the CKD disease state within three years using two separate LR models, the model with complete inputs in the SDR dataset and the model with incomplete inputs in the CDMD dataset to assess the performance of our GMF digital twins in predicting the health state of patients within 3 years. We developed the LR model using GMF derived from basic inputs (Table 2) , age, and gender as predictor variables, where we plotted the receiver operating characteristic (ROC) curve and estimated the area under the curve (AUC). The final AUC was determined by calculating the median value from 2000 bootstrapping iterations for both the identification (EVAS and NHANES) and the incomplete input (CDMD) prediction models. The range of AUC values obtained from this iteration is depicted as the confidence interval (CI). In the complete parameter prediction model, the SDR dataset was randomly split into the training set (50% of the population) and two testing sets, Testing-1 (25%) and Testing-2 (25%). This randomization procedure aimed to balance key population characteristics (age, gender, hypertension status), which are significantly associated with CKD outcomes, across the training set and the two testing sets (Testing-1 and Testing-2). This was conducted to evaluate the robustness of these characteristics and to ensure the reliability of the complete parameter model’s prediction of CKD. We trained on the training set to obtain the parameters of the LR model, which was then used to predict the probability of CKD (AUC value) in the Testing-1 and Testing-2 sets.
To evaluate the efficacy of our model in stratifying patients into high-risk, moderate-risk, and low-risk groups for CKD, we compared the observed frequency of future CKD positive outcomes in each risk group to the expected frequency of CKD positive outcomes investigated in the SDR and CDMD datasets. The expected frequency of the risk groups was based on previously published risk intervals 7 , 8 . High-risk is classified as having > 30% CKD patients, moderate-risk as having 10–30% CKD patients and low-risk as having < 10% CKD patients.
The correlation between observable input parameters and GMF
We examined the correlation between the input parameters and GMF together with demographic values in the CDMD dataset. Kendall’s τ correlation method was employed for this assessment. In cases of missing values, we substituted them with the median values from the CDMD dataset and all values were normalized before running the correlation analysis. To identify groups of correlated parameters, we utilized hierarchical clustering, using the Euclidean distance metric and the complete linkage method on the resultant correlation matrix (Fig. 2 e). This was visualized using a heatmap. The actual correlation values between each input and individual GMF were presented in each cell. Stronger positive correlations were depicted by values above 0 and closer to +1 with the cells appearing more red. On the other hand, stronger negative correlations were represented by values below 0 and closer to -1 with cells appearing more blue.
Impact of individual GMF on CKD prediction
Out of the 21 informative GMF values and the two demographic values used as predictor variables in the LR model, we identified the most significant predictors by considering the LR model coefficient weights with significant p-values. Table 5 provides a list of these significant predictor variables.
Health states and medication effects with GMF
We then performed a metabolic profile subgroup analysis to explain the variation in the GMF metabolic profiles in different groups of patients in the CDMD dataset, based on inputs at baseline. Here, we investigated the GMF differences seen in patients who develop CKD in the future with respect to patients who do not develop CKD in the future. The grouped median values of each individual GMF within the entire GMF profile were compared in each of the subgroup analyses. The significant difference of individual GMF between the groups were evaluated using the Wilcoxon-Mann-Whitney U test with the two-sided null hypothesis that no significant difference was present (Supplementary Table 4) . The GMF profiles were visualized in a standard graphical form, wherein each individual GMF within the entire GMF profile is represented as colored directed edges (Fig. 4a) . The color on the map corresponds to the ratio between the median value of each individual GMF in a specific group and its median in the reference group. Elevated and reduced individual GMF that were significant in one subgroup vs the other subgroup are shown in red and blue, respectively. The individual GMF that do not vary are shown in black.
We also performed another subgroup analysis in the CDMD dataset to explain the difference in the GMF metabolic profiles of patients based on their medication status. Patient medication information was referred to identify patients taking SGLT2i. Patients had to have taken SGLT2i prior to the baseline measurement period in order to identify the effects of SGLT2i on GMF metabolic profiles. We performed a subgroup analysis to elucidate variations in GMF profiles between patients on SGLT2i and those not on SGLT2i in the future non-CKD group, using the same method explained earlier. This analysis was subsequently repeated in the future CKD group (SGLT2i vs no SGLT2i).
We performed the time-to-first CKD event analysis in the CDMD dataset. We first expressed the parameter measurement events as a function of time using the Kaplan-Meier survival model. The GMF, along with demographic factors, was incorporated into an LR model to predict high-risk and moderate/low-risk CKD groups of patients. To compare the survival models of the patient groups, we used the log-rank statistical test. The Cox proportional hazard model was then used to predict the time of the first CKD event and the probability for a given patient to develop CKD at a given time point.
We also extended our CKD prediction to longer time windows of 5 and 10 years, utilizing the same predictive methodology in the CDMD dataset. This extension identified additional patients who would develop future CKD, thereby increasing the pool of future CKD-positive patients.
We next investigated the relationship between future CKD outcomes and clusters of patients in the CDMD dataset. To reveal associations between the groups of patients and their metabolic features, we applied k-means clustering. To determine the optimal number of clusters (k), we assessed the Between Sum of Squares (BSS) metric over a range of k values from 5 to 20. The BSS with 50% was achieved with k = 10 and this k was selected as the optimal choice for our analysis. After applying the k-means algorithm, patient clustering patterns were visualized using heatmaps. The cells in the heatmap displayed normalized values for each individual GMF or demographics (age and gender) within clusters, with elevated values appearing more red and reduced values appearing more blue. The clusters were then assigned numerical indices and ordered in ascending order of CKD outcomes rate. The cluster with the lowest fraction of future CKD-positive patients was designated as index 1. For all indices, we computed the Euclidean distance between the centroid of the i-th index and the centroid of the cluster with index 1. These distances were then plotted against the fraction of future CKD positives in each cluster, and their correlation was evaluated using Kendall’s τ with the two-sided null hypothesis that no correlation is present.
Statistical analysis and computational tools
All statistical analyses were performed using R version 3.6.3. For logistic regression analysis, we utilized the glm.fit function within the core stats package in R 32 . The significant predictor variable model coefficients were obtained from the glm summary function. The ROC curves were generated using the pROC R package 33 , 34 . The AUC confidence interval values were also obtained from the pROC package. The Wilcoxon-Mann-Whitney U test from the core stats package in R was used to determine the significant difference of the grouped median fluxes in the different patient subgroups 33 . For the Cox proportional hazard analysis and the Kaplan–Meier survival/event estimator analysis, we used the survival R package 35 . For the correlation analysis, the cor function was used within the core stats package 33 . Subsequently, the pheatmap package was used to visualize correlation outputs 36 . The pheatmap package was also used to cluster patients with k-means and to visualize the clusters 33 , 36 .
Data availability
The authors agree to provide the data and materials supporting the results or analyses presented in this paper upon reasonable request. Access to the SingHealth Diabetes Registry (SDR) dataset and other datasets in this paper can be granted upon reasonable request to the corresponding authors, under restrictions subject to obtaining ethics approval from institutional boards and an appropriate data-use and/or research agreement.
Code availability
The code in this study is available from the authors upon reasonable request.
Sikdar, K. C., Wang, P. P., MacDonald, D. & Gadag, V. G. Diabetes and its impact on health-related quality of life: a life table analysis. Qual. Life Res. 19 , 781–787 (2010).
Article PubMed Google Scholar
Bee, Y. M., Tai, E. S. & Wong, T. Y. Singapore’s “war on diabetes”. Lancet Diabetes Endocrinol. 10 , 391–392 (2022).
Chen, H.-Y., Kuo, S., Su, P.-F., Wu, J.-S. & Ou, H.-T. Health care costs associated with macrovascular, microvascular, and metabolic complications of type 2 diabetes across time: Estimates from a population-based cohort of more than 0.8 million individuals with up to 15 years of follow-up. Diabetes Care 43 , 1732–1740 (2020).
Article PubMed PubMed Central Google Scholar
Bruynseels, K., de Sio, F. S. & van den Hoven, J. Digital twins in health care: Ethical implications of an emerging engineering paradigm. Front. Genet. 9 (2018).
Venkatesh, K. P., Raza, M. M. & Kvedar, J. C. Health digital twins as tools for precision medicine: Considerations for computation, implementation, and regulation. npj Digital Medicine 5 (2022).
Batagov, A. et al. Generalized metabolic flux analysis framework provides mechanism-based predictions of ophthalmic complications in type 2 diabetes patients. Health Inform. Sci. Syst. 11 (2023).
Jiang, W. et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care 43 , 925–933 (2020).
Article CAS PubMed Google Scholar
Chan, L. et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia 64 , 1504–1515 (2021).
Article CAS PubMed PubMed Central Google Scholar
Pundir, C. S. & Chawla, S. Determination of glycated hemoglobin with special emphasis on biosensing methods. Anal. Biochem. 444 , 47–56 (2014).
Mukherjee, B., Patra, S. & Das, A. K. Glycated albumin and glycated hemoglobin—a comparison. Int. J. Biomed. Res. 4 , 381 (2013).
Article Google Scholar
Szekeres, Z., Toth, K. & Szabados, E. The effects of sglt2 inhibitors on lipid metabolism. Metabolites 11 , 87 (2021).
Goh, S. et al. Ministry of health clinical practice guidelines: Diabetes mellitus. Singapore Med. J. 55 (2014).
Currie, G. Biomarkers in diabetic nephropathy: present and future. World J. Diabetes 5 , 763 (2014).
Glassock, R. J. & Winearls, C. Screening for CKD with eGFR: Doubts and dangers. Clin. J. Am. Soc. Nephrol. 3 , 1563–1568 (2008).
Dennis, J. & Witting, P. Protective role for antioxidants in acute kidney disease. Nutrients 9 , 718 (2017).
Bradshaw, C. et al. Early detection of chronic kidney disease in low-income and middle-income countries: development and validation of a point-of-care screening strategy for india. BMJ Glob. Health 4 , e001644 (2019).
Sabanayagam, C. et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet Digital Health 2 , e295–e302 (2020).
Dong, W. et al. Prediction models and nomograms for 10-year risk of end-stage renal disease in Chinese type 2 diabetes mellitus patients in primary care. Diabetes Obes. Metab. 23 , 897–909 (2021).
Zhang, K. et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat. Biomed. Eng. 5 , 533–545 (2021).
Lin, C.-C. et al. Development and validation of a risk prediction model for chronic kidney disease among individuals with type 2 diabetes. Sci. Rep. 12 (2022).
Wu, M. et al. A non-laboratory-based risk score for predicting diabetic kidney disease in Chinese patients with type 2 diabetes. Oncotarget 8 , 102550–102558 (2017).
Sheng Qian, Y. & Moy, F.-M. Predicting the risk of chronic kidney disease among type 2 diabetes mellitus patients in a primary care setting: an evaluation of the qkidney model. Malays. J. Med. Health Sci. 15 , 67–73 (2019).
Google Scholar
Loh, P. T., Toh, M. P. H. S., Molina, J. A. & Vathsala, A. Ethnic disparity in prevalence of diabetic kidney disease in an Asian primary healthcare cluster. Nephrology 20 , 216–223 (2015).
Lazarte, J., Kanagalingam, T. & Hegele, R. A. Lipid effects of sodium-glucose cotransporter 2 inhibitors. Curr. Opin. Lipidol. 32 , 183–190 (2021).
Hoogeveen, E. K. The epidemiology of diabetic kidney disease. Kidney Dialysis 2 , 433–442 (2022).
Dalan, R. et al. Impact of vitamin e supplementation on vascular function in haptoglobin genotype stratified diabetes patients (EVAS trial): a randomised controlled trial. Nutrition Diabetes 10 (2020).
Centers for disease control and prevention. about the national health and nutrition examination survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (Accessed 11 Nov 2022).
Lim, D. Y. Z., Chia, S. Y., Kadir, H. A., Salim, N. N. M. & Bee, Y. M. Establishment of the SingHealth diabetes registry. Clin. Epidemiol. 13 , 215–223 (2021).
World Medical Association. World Medical Association Declaration of Helsinki. JAMA 310 , 2191 (2013).
Wang, J. et al. The new Asian modified CKD-EPI equation leads to more accurate FR estimation in Chinese patients with CKD. Int. Urol. Nephrol. 48 , 2077–2081 (2016).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150 , 604 (2009).
Müller, M. Generalized Linear Models. In: XploRe — Learning Guide (Springer, Berlin, Heidelberg, 2000).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013). ISBN 3-900051-07-0.
Robin, X. et al. proc: an open-source package for r and s+ to analyze and compare roc curves. BMC Bioinform. 12 , 77 (2011).
Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model (Springer New York, 2000).
Kolde, R. pheatmap: Pretty Heatmaps https://CRAN.R-project.org/package=pheatmap (2019). R package version 1.0.12.
Download references
Acknowledgements
The authors would like to acknowledge Prof. Frank Eisenhaber for insights and fruitful discussions on the biological and mathematical aspects of the work. We would also like to acknowledge Dr. Jonathan Wei Xiong Ng for his exploratory analysis and insights that increased the clarity of the scope of the study. The work was supported by Enterprise SG, under the Startup SGTech POC and POV grant and also by A*STAR, Singapore under its Industry Alignment Pre-Positioning Fund (Grant No. H19/01/a0/023—Diabetes Clinic of the Future). R.D. is supported by Ministry of Health, Clinician Scientist Award (MOH-000014), Ng Teng Fong Foundation Grant and the National Healthcare Group. The funding bodies played no role in the design of the study, the collection, analysis, interpretation of data or in writing the manuscript.
Author information
Authors and affiliations.
Mesh Bio Pte. Ltd., 10 Anson Rd, #22-02, 079903, Singapore, Singapore
Naveenah Udaya Surian, Arsen Batagov, Andrew Wu & Wen Bin Lai
Health Services and Outcomes Research, National Healthcare Group, 3 Fusionopolis Link, #03-08, 138543, Singapore, Singapore
Department of Endocrinology, Singapore General Hospital, Outram Road, 169608, Singapore, Singapore
Yong Mong Bee
Department of Endocrinology, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, 308433, Singapore, Singapore
Rinkoo Dalan
Lee Kong Chian School of Medicine, Nanyang Technological University, 11 Mandalay Road, 308232, Singapore, Singapore
You can also search for this author in PubMed Google Scholar
Contributions
This study was designed and planned by A.B., N.U.S., R.D. and Y.M.B. N.U.S. and A.B. analyzed the data. The clinical datasets were provided by R.D., S.Y. and Y.M.B. along with clinical and methodological expertise. A.W. and W.L. provided strategic guidance and oversight. N.U.S. and A.B. drafted the manuscript. Writing, review and editing were performed by all authors. The final version of the paper has been seen and approved by all the authors. Funding acquisition for this study was by R.D., A.W., A.B. and W.L.
Corresponding authors
Correspondence to Yong Mong Bee or Rinkoo Dalan .
Ethics declarations
Competing interests.
Y.M.B., R.D. and S.Y. have no specific conflict of interest with regards to this manuscript. A.B. and A.W. are the co-founders of Mesh Bio Pte. Ltd. and have filed a patent (application number: WO2022225460A1) related to this work. W.L. and N.U.S. are employees of Mesh Bio Pte. Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary materials, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Surian, N.U., Batagov, A., Wu, A. et al. A digital twin model incorporating generalized metabolic fluxes to identify and predict chronic kidney disease in type 2 diabetes mellitus. npj Digit. Med. 7 , 140 (2024). https://doi.org/10.1038/s41746-024-01108-6
Download citation
Received : 29 September 2023
Accepted : 12 April 2024
Published : 24 May 2024
DOI : https://doi.org/10.1038/s41746-024-01108-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 29 May 2024
An ensemble-based machine learning model for predicting type 2 diabetes and its effect on bone health
- Belqes Alsadi 1 ,
- Saleh Musleh 1 ,
- Hamada R. H. Al-Absi 1 ,
- Mahmoud Refaee 2 ,
- Rizwan Qureshi 3 ,
- Nady El Hajj 1 , 4 &
- Tanvir Alam 1
BMC Medical Informatics and Decision Making volume 24 , Article number: 144 ( 2024 ) Cite this article
Metrics details
Diabetes is a chronic condition that can result in many long-term physiological, metabolic, and neurological complications. Therefore, early detection of diabetes would help to determine a proper diagnosis and treatment plan.
In this study, we employed machine learning (ML) based case-control study on a diabetic cohort size of 1000 participants form Qatar Biobank to predict diabetes using clinical and bone health indicators from Dual Energy X-ray Absorptiometry (DXA) machines. ML models were utilized to distinguish diabetes groups from non-diabetes controls. Recursive feature elimination (RFE) was leveraged to identify a subset of features to improve the performance of model. SHAP based analysis was used for the importance of features and support the explainability of the proposed model.
Ensemble based models XGboost and RF achieved over 84% accuracy for detecting diabetes. After applying RFE, we selected only 20 features which improved the model accuracy to 87.2%. From a clinical standpoint, higher HDL-Cholesterol and Neutrophil levels were observed in the diabetic group, along with lower vitamin B12 and testosterone levels. Lower sodium levels were found in diabetics, potentially stemming from clinical factors including specific medications, hormonal imbalances, unmanaged diabetes. We believe Dapagliflozin prescriptions in Qatar were associated with decreased Gamma Glutamyltransferase and Aspartate Aminotransferase enzyme levels, confirming prior research. We observed that bone area, bone mineral content, and bone mineral density were slightly lower in the Diabetes group across almost all body parts, but the difference against the control group was not statistically significant except in T12, troch and trunk area. No significant negative impact of diabetes progression on bone health was observed over a period of 5-15 yrs in the cohort.
This study recommends the inclusion of ML model which combines both DXA and clinical data for the early diagnosis of diabetes.
Peer Review reports
Introduction
Diabetes mellitus is a metabolic disorder characterized by excessive glucose (sugar) levels in the blood that can be controlled with proper diet, exercise, or medications. Diabetes is a common and increasing non-communicable disease with high prevalence rates worldwide. It may also increase the risk of kidney disease, heart disease, blindness, amputation, osteoporosis, etc. [ 1 ]. Type 1 diabetes (T1D) is when beta cells in the pancreas stop producing insulin, while Type 2 diabetes (T2D), previously referred to as adult-onset diabetes, occurs when muscle, liver, and fat cells develop resistance to insulin [ 2 ]. The number of diagnosed diabetic patients is currently on the rise, and it is one of the most common conditions affecting people of all ages [ 3 ]. According to a World Health Organization (WHO), \(\sim\) 393 million people were living with diabetes in 2011 [ 4 ]. Diabetes statistics from 2013 showed an increase to 415 million diabetic patients worldwide, which indicates that diabetes is rapidly expanding from a widespread health problem to a worldwide epidemic [ 5 ]. Diabetes in the leading cause of death in most developed countries, and mounting evidence suggests that it is becoming more common in several developing countries. According to the International Diabetes Federation (IDF), the population with diabetes is projected to increase to 629 million by 2045 [ 6 ].
As reported by the Ministry of Public Health in Qatar, diabetes is the leading cause of death in the country causing an economic burden on the healthcare sector. The prevalence of diabetes in Qatar is among the highest in the world and is rising dramatically when compared to regional and international averages. In 2008, the WHO projected that the global prevalence of diabetes among persons aged 25 and older was approximately 10%, with the greatest rates in the Middle East and the Americas (11% for both sexes) [ 7 ]. Moreover, The IDF report highlighted that the prevalence of diabetes among adults in Qatar increased from 3% in 1991 to more than 12% in 2000 and later to 17.5% in 2006. The largest increase in diabetes rate was observed for women, with an increase from 4% to 18% [ 8 ]. As shown in Fig. 1 , the number of people with diabetes in Qatar has been steadily increasing over the past decade, and this increase is expected to continue in the coming years [ 9 ].
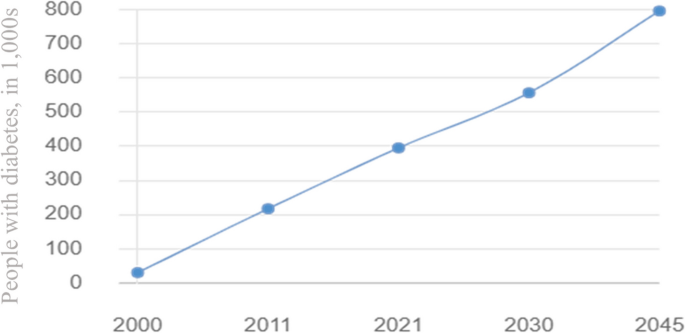
Diabetes status and expected progression report in Qatar 2000 - 2045 [ 9 ]
Multiple factors can affect diabetes, including diet and exercise. The relationship between these two is of particular interest. A study by Hassan et al., compared diabetics vs non-diabetics to understand how physical activity may influence bone health in the Qatari population [ 10 ]. Nazeemudeen et al. conducted a study on Qatari diabetic cohort of 500 person to evaluate their food habit and physical activity level [ 11 ]. Only a limited number of studies have been conducted in Qatar to predict diabetes using ML techniques. Abbas et al. [ 12 ] conducted a study on 7268 Qatari citizens, and their objective was to identify significant risk factors for prediabetes in the Middle East. The results showed great promise in detecting prediabetes early on and, as a result, reducing the incidence of diabetes in the region. Using 2,590 individuals from Qatar Biobank (QBB), Sadek et al. [ 13 ] developed two scoring models to identify individuals at risk of developing impaired glucose metabolism (IGM) or type two diabetes mellitus (T2DM). This study evaluated and compared several scoring models for T2DM screening, which lead to the development of a Qatari-specific diabetes and IGM risk scores to identify high-risk individuals and can thus help establish a nationwide primary prevention program [ 13 ]. Furthermore, Musleh et al. developed machine learning (ML) models to classify diabetic patients from non-diabetic participants of the QBB [ 14 ]. A total of 25 potential risk factors were identified in this study which could be used to distinguish diabetics from non-diabetics. Based on the identified risk factors, HbA1c, Glucose, and LDL-cholesterol were found to be the most influential risk factors [ 14 ]. Recently, Islam et al. proposed a deep learning model DiaNet to diagnose diabetes from retinal images only [ 15 ]. The proposed model achieved over 84% accuracy in diagnosing Qatari population in the QBB cohort [ 15 ]. An update of DiaNet model is recently been published with hither accuracy of 92% [ 16 ]. Recently Wachinger et al. proposed a deep learning model for the detection of T2D based on MRI images only [ 17 ]. Based on the MRI images the authors achieved an accuracy of 78.7%. Sadek et al. used demographics and anthropometic metasurements for the early detection of diabetes [ 18 ]. UK Biobank collection of accelerometer traces from 103712 was used for the T2D detection [ 19 ] The proposed model achieved F1-score of around 0.80 for positive class and 0.73 for negative class. Interested readers are referred to this article for a quick review on the existing ML models for controlling diabetes [ 20 , 21 ]. A summary of the ML based studies for diabetes detection is presented in Table 1 .
Diabetes can have lifelong consequences on your physical health, including influencing the bone health. Bone mineral density provides one measure of how well the bones are working and lower bone mineral density may be associated with a higher risk for fractures when patients become older [ 22 ]. Dual X-ray Absorptiometry (DXA) measures body composition in a non-invasive and fast manner [ 23 ] in terms of mass, fat, bone, and muscle composition. Because of its reliability and accuracy, DXA has become the gold standard for measuring bone mass and overall body composition [ 23 ]. Recently Musleh et al. used DXA data to analyze the bone health of the QBB diabetic cohort and build a model on early onset of osteoporosis or osteopenia [ 24 ]. ML-based technique has recently been proposed to find the link between DXA and cardiovascular disease [ 23 ]. This study aims to develop ML for identifying diabetic and non-diabetic patients in Qatar using two different types of datasets collected from the QBB dataset. The first dataset focuses on the bone health indicators derived from full-body DXA scan measurements, whereas the second dataset includes the clinical lab results based on the blood samples. The contribution of this thesis can be summarized as follows:
We proposed an ML-based model based on DXA and clinical data for the early detection of diabetes in a cohort size of 1000 from QBB.
The proposed model achieved over 87% accuracy in identifying diabetes patients from normal participants even without considering the known biomarkers such as glucose and HbA1c leading towards the discovery of potential novel biomarker for diabetes. Moreover, we showed that combination DXA with clinical data improved the performance of ML model.
Our study revealed that the control group exhibited greater bone area, BMC, lean mass, fat mass, and bone mass for almost all body parts in comparison to the target group. But we could not observe any deteriorating effect of diabetes progression on bone health of diabetic patients over a period of 5-15yrs of time.
The article is organized in following sections. In Material and methods section, we have provided a high-level summary of overall method with a schematic diagram. Then we provided details of the dataset used in the study. We also provided details of statistical analysis and machine learning (ML) model development workflow. In Results section, we have provided the results from statistical analysis as well as the performance of ML models. In Discussion section, we highlighted the principal findings of the work, compared the performance of the proposed ML model against other existing models, and limitation of the study. Then in the Conclusion and future works section, we conclude with the future works and final remarks of this work.
Material and methods
In this case-control study, we first collected clinical information from the QBB participants. Then data preprocessing steps were applied to clean the dataset. ML models were developed to distinguish diabetes patients from the control group highlighting that there exists significant difference in the clinical profile of these two groups. To understand the difference of their profile and identify key biomarkers that distinguish the groups, we used statistical technique, RFE based feature subset selection. Moreover, we used SHAP to quantify the relative importance of the proposed markers for detecting diabetes from normal cases. Figure 2 highlights the schematic diagram of the workflow adopted for this study.
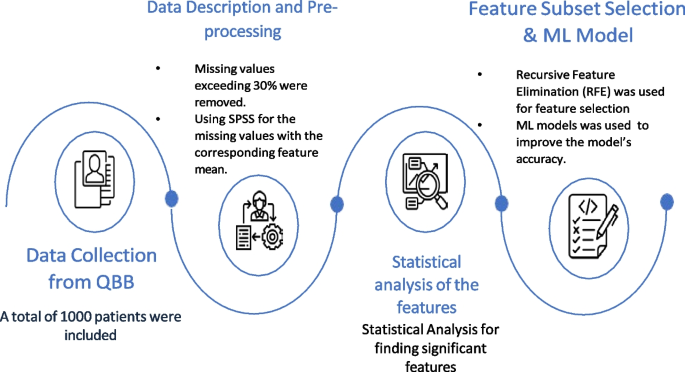
Overall summary of the workflow for this study
Data collection from QBB
In this study, we collected deidentified data from QBB for a cohort of 500 participants with the type 2 diabetes (T2D) having HbA1c >6.5. As part of our study, we had a group of 500 non-diabetic participants (HbA1c \(\le\) 6.5) who were free from diabetes. A total of 1000 participants from QBB were included in the study, of which 541 were males and 459 were females. In the diabetic group there were 209 males and 291 females. The study protocol was approved by IRB committee of QBB (according to the guidelines of the Ministry of Public Health, Qatar) and only de-identified dataset was obtained from QBB.
Data description and pre-processing
The dataset contained 163 different measurements from DXA. In DXA machines, different body parts are scanned for densitometry and composition. Densitometry measures bone Area, weight, height, bone mineral content (BMC), and bone mineral density (BMD). DXA composition measurement measures bone mass, fat mass, and lean mass. The dataset also includes lab results for QBB participants based on their blood samples. Measurements having missing values exceeding 30% of total records were removed. For the remaining measurements, we replaced the missing values by the corresponding feature mean using PASW Statistics 18 (SPSS Inc.). Finally, 129 features from DXA and 77 features from clinical data were obtained for analysis. It is important to emphasize that we dropped measurements like glucose level, HbA1c for building ML models as these known biomarkers would bias the outcome of ML model.
Statistical analysis of the features
Statistics were analysed using JASP software. Both the target and control groups were analysed by descriptive statistics. Moreover, all data were subjected to a normality test to ensure that they were distributed normally. We used the student t-test and Mann-Whitney U (MU) test to determine the significance level for the target and control groups.
Feature subset selection
As part of the development of ML models with highly relevant features, feature subset selection (FSS) technique was employed to select a subset of key features. In the FSS technique, information is eliminated without significant loss by eliminating redundant or highly correlated features from the dataset [ 25 ]. In this study, we applied Recursive Feature Elimination (RFE) to enhance the generalization capability of the model by decreasing its variance. Due to its simplicity and effectiveness, this algorithm selects the features (columns) in a training dataset that have greater or lesser relevance to predicting the target variable within a training dataset [ 25 ].
Machine learning model development, evaluation and explnation
Our research objective was to develop ML models to distinguish diabetic patients from non-diabetic people using clinical measurements from blood sample and DXA scan measurements. The following ML algorithms were used: Logistic Regression (LR), Support Vector Machine (SVM), Decision Tree (DT), Random Forest (RF), Naive Bayes (NB), k-Nearest Neighbor (KNN), Artificial Neural Network (ANN), XGBoost and CatBoost. A five-fold cross validation was applied to the model to evaluate its performance. For the evaluation of the proposed ML models, we carried out 5 fold cross validation (CV) using 80% of the data as a training dataset and 20% as a testing dataset. The models were evaluated on different testing datasets for every fold. Subsequently, the performance metrics were averaged across all folds to derive the final results. Multiple evaluation metrics (Eqs. 1 - 5 ) were applied: (1) Accuracy, (2) Sensitivity (Recall), (3) Specificity, (4) Precision, and (5) Matthew’s Correlation Coefficient (MCC) when analysing the performance of ML models:
Here, TP stands for true positive, FN stands for false negative, FP stands for false positive, while TN stands for true negative. Since the dataset was balanced (500:500 for diabetics and non-diabetics), accuracy was used as the evaluation metric to select the final model. All hyperparameters of the models were optimized using GridSearchCV of Scikit-Learn package of Python. For explaining the relative importance of the selected features on the performance of ML models we used PCA Biplot and SHAP [ 26 ] analysis.
Features with statistical significance
There was a total of 206 features for each participant of the QBB dataset including 129 DXA measurements from seven different body parts and 77 clinical features. The results of analysing all 206 features are shown in Table 2 . A total of 31 features were considered as statistically significant ( based on p -value \(\le\) 0.05) while 173 features were not statistically significant. A detailed analyses of all the features is presented in the Supplementary Table S1 along with their mean, standard deviation, and p -values. Out of these 31 features, 4 features were from DXA, 27 features were from clinical measurements (Table 2 ).
An ablation study based on different types of features used in ML model
Our aim was to assess the effectiveness of the two diverse types of features proposed for developing ML models. An ablation study was conducted on the combination of two types of features, and then we evaluated how ML performed in this combination. Table 3 compares the performance of ML model on different types of features, 129 features are from DXA data, and 77 features are from to clinical data. This study indicates that the LR-based model is accurate in calculating bone area by 69%, whereas the kNN model reaches a score of 56% for Anthropometric measurements, SVM scores 57% for BMC, kNN scores 54% for BMD, KNN scores 55% for bone mass, NB scores 54% for fat mass, and kNN scores 52.6% for lean mass. RF-based and XGBoost models achieved 84.4% accuracy based on all DXA measurements (129 features). The CatBoost model achieved 84.8% accuracy for all 77 features of the Clinical Data.
In Fig. 3 , we compared different types of DXA measurements by feeding them into ML models as different feature groups. We can observe the same performance in different ML models on DXA measurement and bone area with 28 features having the highest performance across all ML algorithms. As further step we combined the features of DXA (129) and clinical data (77), where SVM model had the highest accuracy of 84.8% (Table 4 ). Most of the models gave better results for clinical data than DXA as shown in Fig. 4 , with the exception of RF model in which DXA had better results than clinical data. In addition, the models performed better when clinical data and DXA data were combined.
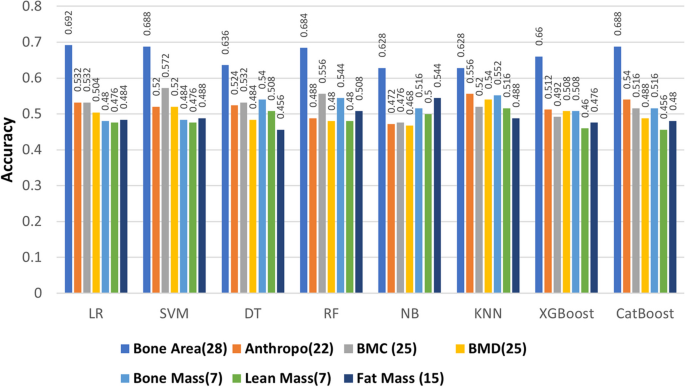
Performance of different ML models on DXA measurements
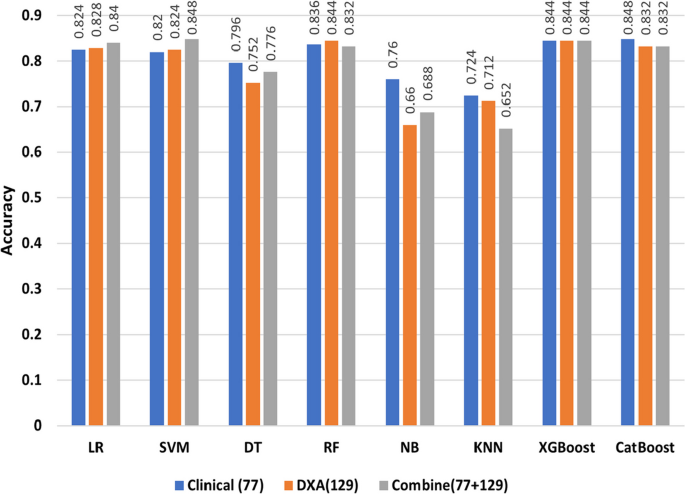
Performance of different ML models on DXA measurements, Clinical data and their combination
Performance of the model after RFE based feature subset selection
To distinguish diabetic patients from non-diabetic participants, we built different classifiers based on the selected features after RFE. There were 16 features selected from LR and 11 features selected from SVM. We then selected the union of these features. Then RFE based 20 features were used again to run the models. Based on the selected features we found that accuracy levels have increased, with CatBoost achieving the highest accuracy at 87.2% (Table 5 ).
Bone health in the QBB diabetic cohort vs. control
Bone area, bone mass, lean mass, and fat mass were measured in both the diabetic (target) and control groups. Almost everywhere on the body, the control group had slightly greater bone area than the target group (Supplementary Table S1). Similarly, we noticed that the control group had slightly higher bone mass, lean mass, fat mass than the diabetes group in all body areas but none of the variables were not statistically significant (Supplementary Table S1). Bone area, bone mass, lean mass, and fat mass were measured in both the diabetic (target) and control groups. Almost everywhere on the body, the control group had slightly greater bone area than the target group (Supplementary Table S1). Similarly, we noticed that the control group had slightly greater bone mass, lean mass, and fat mass than the diabetes group in all body areas but none of the variables were not statistically significant (Supplementary Table S1).
In addition, we noticed a similar trend in other bone health parameters between the diabetes and control groups. We found only three variables representing bone health which are statistically significant while comparing diabetes vs. the control group. Average width of T12 bone, which sits above the lumbar spine, is lower in diabetic group compared to the control group (diab: control = 10.474±1.532: 10.669± 1.546, p -value=0.046). The other two significant variables were the area of troch and trunk. And in both of these areas the average area of troch (diab:control = 13.543±2.567: 14.001±2.664, p -value=0.006) and trunk (diab:control = 738.456±100.509: 749.37±89.511, p -value=0.049) were lower in the diabetic group compared to the control group.
Impact of diabetes progression on bone health
Figure 5 shows the distribution on total BMD among diabetes patients who are having diabetes for 5, 10, or 15 yrs. We could not observe any major deteriorating effect of diabetes progression on total BMD over the period of time for diabetic patients (Fig. 5 ). Rather, in all cases (n=5,10 and 15) we found that the mean value of total BMD was higher for patients having diabetes for a longer period of time ( p -value = 0.005, 0.012, 0.019 for 5, 10, 15 yrs, respectively).
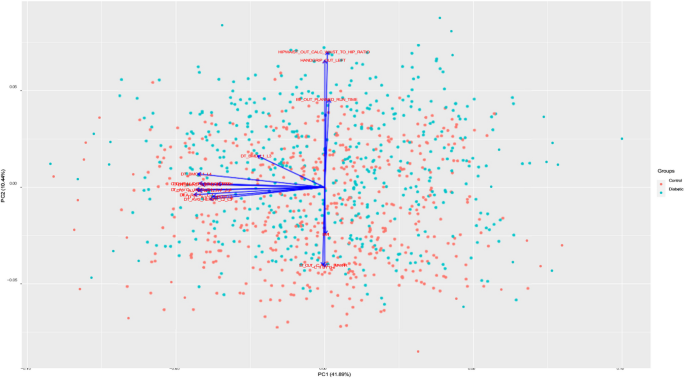
Distribution of Total BMD in participants having diabetes for less than n yrs vs. more than n yrs (n=5,10,15)
Clinical implications
We observed that among the clinical markers HDL-Cholesterol (diab:control = 1.37 ± 0.395 : 1.3 \(\pm\) 0.378; p -value=0.002) and Neutrophil (diab : control= 54.045 ± 9.206: 52.557 ± 9.985; p -value=0.044) were having higher values in the diabetic vs. control group in the QBB cohort (Supplementary Table S1). HDL-cholesterol supports to have a better heart health and Neutrophil support to boost the immune system in human. Therefore, these two markers indicating better cardiac health and immune system for the diabetic cohort in Qatar. Higher value of HDL might be due to the fact that diabetic patients in Qatar were taking lipid lowering agent that may contribute to increasing HDL level whis is part of their mechanism of action. These agents lower LDL cholesterol levels, but raise HDL levels [ 27 ]. In addition, we observed that vitamin B12 (diab:control= 284.527±148.163: 320.606±307.276; p value= 0.018) was lower in the diabetic group since many diabetic patient are on Metformin for controlling blood sugar and this medication may lower vitamin B12 [ 28 ]. We also observed lower testosterone levels (diab:control= 9.421±8.363: 10.721±9.169; p value= 0.019) in the diabetic group. Many studies have reported a possible link between low testosterone levels and T2D [ 29 ].
From the other statistically significant clinical variables, we found Sodium (diab : control= 139.59 ± 2.529 : 140.12 ± 2.306; p value= 0.002), Bilirubin (diab : control= 7.931 ± 4.536: 8.468±4.715; p value= 0.044), AST (diab:control= 19.084 ± 9.832: 19.43 ± 7.978; p value= 0.039), GGT (diab:control = 31.403 ± 27.771 : 35.13 ± 41.018; p value= 0.048), etc. we slightly lower in the diabetes group compared to the control group. Low sodium levels, also known as hyponatremia, may result from various factors such as excessive fluid intake, certain medications, hormonal imbalances, and underlying medical conditions. Severe cases of hyponatremia can be seen in people with uncontrolled diabetes who are also experiencing other health complications [ 30 ]. Gamma glutamyltransferase (GGT), aspartate aminotransferase (AST), are common liver enzymes and abnormal levels of these enzymes may signal liver function disorder [ 31 ]. In Qatar, as many diabetic patients are prescribed dapagliflozin, the decreased levels of these enzymes validate the findings from earlier studies conducted on the Qatari cohort [ 32 ].
Figure 6 shows the PCA Biplot for the selected features by RFE. From biplot we can observe that the first two components of the selected features cover over 40% of the variance in the dataset. The direction of vector in Fig. 6 indicates the high correlation between BMI, Chloride and hip circumference. We also observed a nearly opposite direction between chloride and Exercise Test Planned run time. From SHAP analysis of the selected features (Fig. 7 ), we can observe that BMI, Waist to hip ratio were among the top two important variables for the detection of diabetes. This indicates that obesity plays a big role in diabetes. Lower values of exercise test (“ \(ER\_OUT\_CALC\_MAXHR\) ”) for diabetic group indicates that this group need to improve their physical level. From SHAP plot, we also observed the importance of bone densitometry in lumber spines region i.e., L1,L2,L3 and L4,in diagnosing the diabetic patients and their bone health.
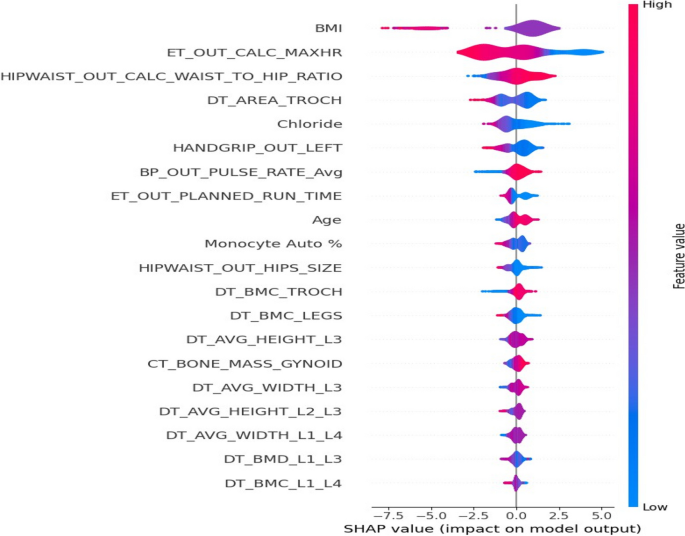
PCA biplot for the selected features by RFE
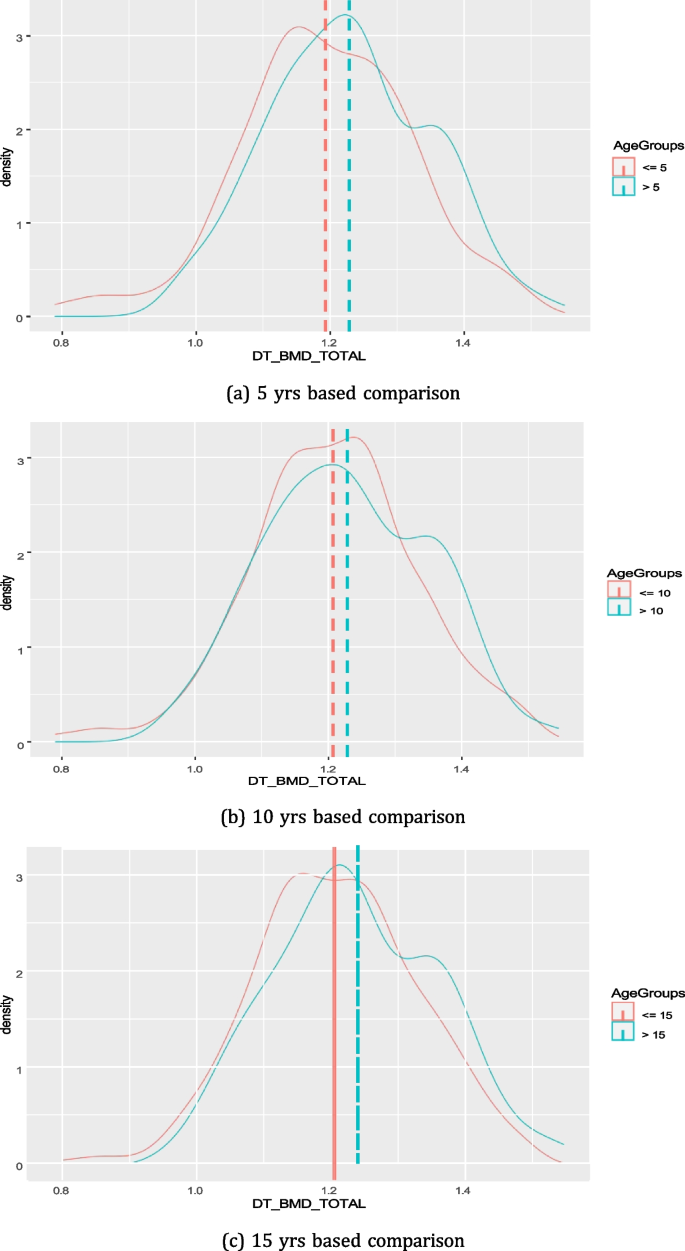
SHAP plot for the selected features by RFE
In this article, we propose a ML-based approach to predict diabetics from non-diabetics based on a dataset collected from QBB. To develop this model, we used DXA measurements and clinical data. In the following section, we will highlight and discuss the principal findings, compare our methods against other methods, and articulate the usefulness, implications, and limitations of our models.
Principal findings on ML modelling
In this work, an accuracy of \(\ge\) 87% achieved with the proposed ML model for distinguishing diabetic patients from non-diabetic participants. We found that DXA and clinical data can be used to identify diabetics at an early stage. We analysed eight distinct ML models to develop a classifier to differentiate the target group from the control group. Different types of DXA measures were fed into ML models as individual feature groups in an ablation study to determine which ones were most effective. As indicated in Fig. 3 , ablation study on different types of DXA measurements showed relatively low accuracy, however bone area showed relatively better accuracy in classifying the diabetes group from the control group with nearly 70% accuracy. When we combined all types of DXA measurements (129 features) in the models, the performance of the models improved to reach \(\ge\) 84% accuracy. Among all the models, RF and XGBoost attained the highest accuracy of \(\ge\) 84.4%. For 77 clinical data features, the performance of the models was better compared to the individual type of DXA features (Figs. 3 and 4 ). Boosting-based algorithms such as XGBoost and CatBoost were among the top-performing algorithms. With an accuracy of 84.8%, CatBoost achieved the best performance among all the models we evaluated. Finally, when all the DXA features and clinical data were combined to build ML models, it achieved the best performing model (Fig. 4 ). As shown in Fig. 4 , the performance of the models based on the combination of DXA and clinical features achieved the best performance accuracy for SVM (84.8%), XGBoost (84.4%) and CatBoost (83.2%). It is important to emphasize that introducing complex model such as ANN than simpler model i.e., LR does not guarantee a higher performing results as evident in Tables 3 , 4 , and 5 . The performance of model depends upon the dataset we are working on and the underlying pattern that model can discover out of this approach. After applying RFE, we obtained a shorter list of selected features, which were used to re-run the models. The results indicated that 16 features were selected from LR and 11 features from SVM, and all the unique features from the two runs were used to build the models. With an accuracy of 87.2%, CatBoost achieved the highest score (Table 5 ) for the selected features. It is worth mentioning that we selected 20 unique variables based on RFE, where most of these variables, were statistically significant ( p -value \(\le\) 0.05).
Comparison against other methods
Our present study puts forward ML models to differentiate between the diabetic and non-diabetic groups in a cohort from Qatar. Prior research has highlighted the widespread application of ML in healthcare. For instance, in a study of 68,994 individuals with diabetes and healthy individuals from China, the random forest method demonstrated the highest accuracy (ACC = 80.84%) after identifying appropriate features [ 33 ]. Another study [ 34 ] involving 768 patient records of Pima Indian women with nine attributes showed that SVM and KNN provide the highest degree of accuracy in predicting diabetes. Compared to the other algorithms used in that paper, both algorithms provide 77% accuracy [ 34 ]. It is plausible that ML can be used to predict diabetes, but it will require finding appropriate attributes, classifiers, and data mining methods. According to a study [ 15 ] conducted in Qatar, retinal images can be used to determine whether a patient has diabetes or not. An accuracy level of over 84% was achieved using a multi-stage convolutional neural network (CNN)-based model DiaNet [ 15 ]. There was another study [ 14 ] in Qatar which used QBB data to develop machine-learning models to differentiate diabetic patients from non-diabetic participants. Several hundred measurements were analyzed to identify 25 potential risk factors that might help distinguish diabetic patients from non-diabetics. According to the results, HbA1c, Glucose, and LDL-Cholesterol were the most influential risk factors. Classifiers perform nearly the same, with SVM slightly outperforming linear regression (LR) and quadratic discriminant analysis (QDA) at accuracy (0.881) [ 14 ]. However, they were able to achieve this accuracy because they include both HbA1c and Glucose measurements as features in ML model, while we did not use these known biomarkers to build ML models since they are already known markers for diabetes and inclusion of those features would improve the prediction accuracy.
It is crucial to highlight that the impact of diabetes on the bone health of patients within the realm of clinical epidemiology remains a subject of debate. While certain studies have shown a potential connection between diabetes and reduced BMD, others have reported BMD levels within the normal range or even increased BMD [ 31 ]. In our research, we observed lower BMC and BMD in various anatomical regions among individuals with diabetes when compared to the control group, although these differences did not reach statistical significance. A recent systematic review has also drawn similar conclusions, suggesting a lack of a definitive link between diabetes and the deterioration of bone health [ 35 ]. Our study reaffirms these findings, based on the QBB cohort. However, it is imperative to conduct further investigations in clinical settings to delve deeper into the potential connections between diabetes and bone health decline.
Limitations
This research is limited by the size of the dataset and the number of missing attribute values. Our cohort covered only 500 diabetic patients and 500 control individuals. In addition, we focused exclusively on Qatari nationals, hence the results of this study may not be applicable to other cohorts from different ethnicity without validation. Nevertheless, we expect the results of this study to be applicable to other GCC nations since lifestyle and behavioral characteristics of Qatari nationals are comparable among GCC nationals.
Conclusion and future works
Diabetes prediction at an early stage is one of the key research areas in healthcare. Clinicians could detect diabetes earlier with the help of a ML-based approach. In this study, ML models were utilized to determine whether an individual will get diabetes at an early stage. ML models predicted more accurate results when combining DXA measurements and clinical data, which indicates the importance of incorporating DXA scan with existing clinical data for the early diabetes detection. Our study highlighted key factors i.e., cholesterol, neutrophil, sodium, chloride, bilirubin, AST, GGT, etc. for the early detection of diabetes . We also showed that the effect of diabetes on bone health over time is not significant. These results showed great promise in detecting prediabetes early on and, as a result, reducing the incidence of diabetes in the region. Our future work will focus on integrating other methods i.e., ensemble-based methods to improve the performance of models for better accuracy. Testing the models on larger datasets may reveal more insights and better prediction accuracy. Considering the clinical significance of HbA1c levels in diabetes management and the heterogeneity within Type 2 diabetes conditions, a regression model predicting HbA1c values could offer a more detailed and clinically relevant outcome which we will focus as part of our near future endeavor.
Availability of data and materials
Data used in this research can be accessed upon the approval from QBB. Please contact [email protected] for data access.
Mohsin S, Baniyas MM, AlDarmaki RS, Tekes K, Kalász H, Adeghate EA. An update on therapies for the treatment of diabetes-induced osteoporosis. Exp Opin Biol Ther. 2019;19(9):937–48.
Article CAS Google Scholar
Patel R. Studies on Genotype-Phenotype Correlation in Type II Diabetics and Evaluation of Melatonin and DPP-IV inhibitor on Experimental Diabetic Models. India: Maharaja Sayajirao University of Baroda; 2021.
Google Scholar
Egan AM, Dinneen SF. What is diabetes? Medicine. 2019;47(1):1–4.
Article Google Scholar
World Health Organization. Global diffusion of eHealth: making universal health coverage achievable: report of the third global survey on eHealth. World Health Organization; 2017.
Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(4):209–22.
Article PubMed Google Scholar
Hernandez C. The diabetes epidemic prediction in 2045. 2019. https://wordofhealth.com/2018/11/30/the-diabetes-epidemic-prediction-in-2045/ . Accessed 15 Dec 2023.
Ministry of Public Health Q. Ministry of Public Health - Qatar Public Health Strategy 2022 - 2017. 2017. https://moph.gov.qa/english/strategies/supporting-strategies-and-frameworks/qatarpublichealthstrategy/pages/default.aspx . Accessed 03 Feb 2023.
Lefèbvre P, Pierson A. The global challenge of diabetes. World Hosp Health Serv. 2004;40(3):37–9.
PubMed Google Scholar
International Diabetes Federation. IDF Diabetes Atlas 10th Edition. 2021. https://www.diabetesatlas.org/data/en/ . Accessed 04 Feb 2023.
Hassan A. Trends in nutrition related chronic diseases in Qatar: a call for action. Emirates J Food Agric. 1994;6:128–40.
Nazeemudeen A, Al-Absi HR, Refaee MA, Househ M, Shah Z, Alam T. Understanding the Food Habits and Physical Activities of Diabetes Cohort in Qatar. In: The Importance of Health Informatics in Public Health during a Pandemic. IOS Press; 2020. pp. 453–6.
Abbas M, Mall R, Errafii K, Lattab A, Ullah E, Bensmail H, et al. Simple risk score to screen for prediabetes: A cross-sectional study from the Qatar Biobank cohort. J Diabetes Investig. 2021;12(6):988–97.
Sadek KW, Abdelhafez I, Al-Hashimi I, Al-Shafi W, Tarmizi F, Al-Marri H, et al. Diabetes risk score in Qatar: Model development, validation, and external validation of several models. medRxiv. 2021;2021–04.
Musleh S, Alam T, Bouzerdoum A, Belhaouari SB, Baali H. Identification of potential risk factors of diabetes for the qatari population. In: 2020 IEEE International Conference on Informatics, IoT, and Enabling Technologies (ICIoT). IEEE; 2020. pp. 243–6.
Islam MT, Al-Absi HR, Ruagh EA, Alam T. DiaNet: A deep learning based architecture to diagnose diabetes using retinal images only. IEEE Access. 2021;9:15686–95.
Al-Absi HR, Pai A, Naeem U, Mohamed FK, Arya S, Sbeit RA, et al. DiaNet v2 deep learning based method for diabetes diagnosis using retinal images. Sci Rep. 2024;14(1):1595.
Article CAS PubMed PubMed Central Google Scholar
Wachinger C, Wolf TN, Pölsterl S. Deep learning for the prediction of type 2 diabetes mellitus from neck-to-knee Dixon MRI in the UK biobank. Heliyon. 2023;9(11).
Sadek K, Abdelhafez I, Al-Hashimi I, Al-Shafi W, Tarmizi F, Al-Marri H, et al. Screening for diabetes and impaired glucose metabolism in Qatar: Models’ development and validation. Prim Care Diabetes. 2022;16(1):69–77.
Article CAS PubMed Google Scholar
Lam B, Catt M, Cassidy S, Bacardit J, Darke P, Butterfield S, et al. Using wearable activity trackers to predict type 2 diabetes: machine learning-based cross-sectional study of the UK Biobank accelerometer cohort. JMIR Diabetes. 2021;6(1):e23364.
Article PubMed PubMed Central Google Scholar
Jacobs PG, Herrero P, Facchinetti A, Vehi J, Kovatchev B, Breton M, et al. Artificial intelligence and machine learning for improving glycemic control in diabetes: best practices, pitfalls and opportunities. IEEE Rev Biomed Eng. 2023;17:19-41.
D’Antoni F, Petrosino L, Marchetti A, Bacco L, Pieralice S, Vollero L, et al. Layered meta-learning algorithm for predicting adverse events in type 1 diabetes. IEEE Access. 2023;11:9074–94.
Piepkorn B, Kann P, Forst T, Andreas J, Pfützner A, Beyer J. Bone mineral density and bone metabolism in diabetes mellitus. Horm Metab Res. 1997;29(11):584–91.
Refaee MA, Al-Absi HR, Islam MT, Househ M, Shah Z, Rahman MS, et al. The Linkage Between Bone Densitometry and Cardiovascular Disease. In: Informatics and Technology in Clinical Care and Public Health. IOS Press; 2022. pp. 244–7.
Musleh S, Nazeemudeen A, Islam MT, El Hajj N, Alam T. A machine learning based study to assess bone health in a diabetic cohort. Inform Med Unlocked. 2022;33:101079.
Remeseiro B, Bolon-Canedo V. A review of feature selection methods in medical applications. Comput Biol Med. 2019;112:103375.
Lundberg SM, Lee SI. A unified approach to interpreting model predictions. Adv Neural Inf Process Syst. 2017;30:4765-74.
Niemeijer-Kanters S, Banga J, Erkelens D. Lipid-lowering therapy in diabetes mellitus. Neth J Med. 2001;58(5):214–22.
Sparre Hermann L, Nilsson B, Wettre S. Vitamin B12 status of patients treated with metformin: a cross-sectional cohort study. Brit J Diabetes Vasc Dis. 2004;4(6):401–6.
Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96(8):2341–53.
Khan RN, Saba F, Kausar SF, Siddiqui MH. Pattern of electrolyte imbalance in Type 2 diabetes patients: Experience from a tertiary care hospital. Pak J Medical Sci. 2019;35(3):797.
Kunutsor SK, Abbasi A, Apekey TA. Aspartate aminotransferase-risk marker for type-2 diabetes mellitus or red herring? Front Endocrinol. 2014;5:189.
Benjamin S, Ramanjaneya M, Butler AE, Janjua I, Paramba F, Palaki J, et al. Dapagliflozin, as Add-on Therapy in Type 2 Diabetes Patients, Is Associated With a Reduction in Albuminuria and Serum Transaminase Levels. Front Clin Diabetes Healthc. 2021;2:733693.
Zou Q, Qu K, Luo Y, Yin D, Ju Y, Tang H. Predicting diabetes mellitus with machine learning techniques. Front Genet. 2018;9:515.
Sarwar MA, Kamal N, Hamid W, Shah MA. Prediction of diabetes using machine learning algorithms in healthcare. In: 2018 24th international conference on automation and computing (ICAC). IEEE; 2018. pp. 1–6.
Qiu J, Li C, Dong Z, Wang J. Is diabetes mellitus a risk factor for low bone density: a systematic review and meta-analysis. BMC Endocr Disord. 2021;21(1):1–11.
Download references
Acknowledgements
This work was supported by the College of Science and Engineering, Hamad Bin Khalifa University, Doha, Qatar. Authors would like to thank the College of Science and Engineering at Hamad Bin Khalifa University, Qatar.
The open access publication of this article was funded by the College of Science and Engineering, Hamad Bin Khalifa University (HBKU), Doha, Qatar. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
College of Science and Engineering, Hamad Bin Khalifa University, Doha, Qatar
Belqes Alsadi, Saleh Musleh, Hamada R. H. Al-Absi, Nady El Hajj & Tanvir Alam
Hamad Medical Corporation, Doha, Qatar
Mahmoud Refaee
Department of Imaging Physics, MD Anderson Cancer Center, The University of Texas, Houston, USA
Rizwan Qureshi
College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar
Nady El Hajj
You can also search for this author in PubMed Google Scholar
Contributions
Conceived and design: TA. Initial Draft: BA, TA. Experiments: BA, SM, HRHA. Analysis: MR,RZ,NEH,TA. Writing: All authors.
Corresponding author
Correspondence to Tanvir Alam .
Ethics declarations
Ethics approval and consent to participate.
The ethical aspect of study protocol was approved by IRB committee of QBB according to the guidelines of the Ministry of Public Health (MoPH), Qatar. For all the adult participants informed consent was obtained from all subjects by QBB.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alsadi, B., Musleh, S., Al-Absi, H.R.H. et al. An ensemble-based machine learning model for predicting type 2 diabetes and its effect on bone health. BMC Med Inform Decis Mak 24 , 144 (2024). https://doi.org/10.1186/s12911-024-02540-0
Download citation
Received : 01 October 2023
Accepted : 17 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1186/s12911-024-02540-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Machine learning
- Bone health
- Dual Energy X-ray Absorptiometry
- Qatar Biobank (QBB)
BMC Medical Informatics and Decision Making
ISSN: 1472-6947
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Introduction: Type 2 diabetes mellitus (T2DM) is a serious and chronic metabolic disorder in which the pancreas is unable to properly break down glucose in the cell, leaving excess glucose ... This dissertation would not have been possible without the financial support of the
The International Diabetes Federation has estimated that there were 3.72 million diagnosed. cases of T2DM in the Philippines with a 6.2% prevalence rate in adults in 2017 (104) and also it. is reported that around 1.76 million (2%) people with T2DM remain undiagnosed in 2014 (105). 3.
This Dissertation is brought to you for free and open access by the Walden Dissertations and Doctoral Studies Collection at ScholarWorks. It has been ... In the United States, diabetes mellitus (DM) Type 2 is considered a health care dilemma, generating a massive national and global economic disparity. The Center for Disease Control and ...
2.1.1 Classification and symptoms of diabetes 23 2.1.2 Global disease burden 23 2.1.3 Risk factors for developing type II diabetes 24 2.1.4 Complications that can result from type II diabetes 25 2.1.5 Treatment of type II diabetes 25 2.1.6 Why is it important to study type II diabetes? 27 2.2 Study background: Collaboration for Leadership in ...
Type 2 Diabetes Mellitus (T2DM) is a chronic condition wherein the beta cells. in the body do not produce enough insulin - a hormone that regulates blood. sugar - or the body does not use insulin well enough (also called insulin. resistance), or there is complete absence of insulin production.
DEVELOPING TYPE 2 DIABETES MELLITUS IN A RURAL POPULATION Amanda Culp-Roche University of Kentucky, [email protected] Author ORCID Identifier: ... This Doctoral Dissertation is brought to you for free and open access by the College of Nursing at UKnowledge. It
This Dissertation is brought to you for free and open access by the Walden Dissertations and Doctoral Studies Collection at ScholarWorks. It has been ... Type 2 diabetes mellitus (T2DM) is a disease that affects the body's ability to metabolize glucose effectively. The disease is predicted to be prevalent in over 300 million people
Chapter one sets the context for the thesis by providing an overview of type 2 diabetes, IGR, and GDM, and discusses the evidence for interventions to prevent progression to ... impaired glucose regulation and progression to type 2 diabetes mellitus in the Tayside region of Scotland. Diabetes Research and Clinical Practice, 104 (1), pp. e16-e19 ...
Specifically, individuals without a. high school degree exhibited a 12.6% prevalence of diagnosed diabetes cases, compared to. 9.5% for high school graduates, and 7.2% for those with a post-high school education. In. terms of modifiable risk factors of T2DM, diet and exercise are the two most commonly.
1. Introduction. Diabetes Mellitus (DM) commonly referred to as diabetes, is a chronic disease that affects how the body turns food into energy .It is one of the top 10 causes of death worldwide causing 4 million deaths in 2017 , .According to a report by the International Diabetes Federation (IDF) , the total number of adults (20-79 years) with diabetes in 2045 will be 629 million from 425 ...
defects in insulin production, insulin action, or both.1,2 Globally, rates of type 2 diabetes were 15.1 million in 2000,3 the number of people with diabetes worldwide is projected to increase to 36.6 million by 2030.4 In 2007, 23.6 million people, or 7.8% of the United States population had type 2 diabetes. Of these,
The major type of diabetes is Type 2 DM (T2DM), which is caused due to insufficient production of insulin or desensitization of insulin receptors that precludes the entry of glucose into the cell [7,8]. The type is predominantly seen in 90-95% of cases. There is another type of diabetes called gestational diabetes mellitus (GDM) that occurs ...
The objective of this thesis is to investigate and describe early patterns and risk indicators of type 2 diabetes. The focus is on type 2 diabetes as one component in metabolic syndrome, i.e. ... Key words: Type 2 diabetes mellitus, metabolic syndrome, risk, obesity, lifestyle, ...
Type 2 diabetes mellitus (T2DM) is a global epidemic, impacting the economy and quality of life of affected individuals. The treatment and management of the disease often rely heavily on self-efficacy and provider guidance. Adherence to provider treatment regimens can help to prevent T2DM-related complications. However, non-adherence to
Type 2 diabetes mellitus (T2DM) is a long-term metabolic confusion disease that is related to a high rate of complication and mortality in a population. ... This article is a part of my thesis "The development of health-related quality of life programme among type 2 diabetic patients in Tam Binh District, Vinh Long Province, Vietnam," which ...
Primary prevention of type 2 diabetes mellitus Descriptive literature review Name of Degree Bachelor of Nursing Abstract The current prevalence of type 2 diabetes mellitus is critical and a vast number of peo-ple have a high risk for its onset. One of the main reasons for disease are poor nutrition and sedentary lifestyle.
Type 2 diabetes mellitus (T2DM) and its related health complications are linked to untimely deaths, thereby posing a significant global health care challenge. 1 The development of T2DM is the result of a mix of genetic factors that affect insulin secretion and resistance as well as lifestyle, such as overeating, obesity, and insufficient ...
Diabetes mellitus, a chronic metabolic disease, often leads to numerous chronic complications, significantly contributing to global morbidity and mortality rates. High glucose levels trigger epigenetic modifications linked to pathophysiological processes like inflammation, immunity, oxidative stress, mitochondrial dysfunction, senescence and various kinds of cell death. Despite glycemic ...
Diabetes is the seventh leading cause of death in the United States, and 30.3 million Americans, or 9.4% of the U.S. population, are living with diabetes (1,2).For successful management of a complicated condition such as diabetes, health literacy may play an important role.
Diabetes mellitus type 2 (DM2) is a hypercoagulable state with enhanced platelet (PLT) activation and increased clotting factor production. Simultaneously, the fibrinolytic cell system is inhibited due to the formation of clots with high fibrinolysis resistance. The stages of PLT "activation" have been well characterized microscopically, morphometrically, and nanomechanically using a light ...
Dong, W. et al. Prediction models and nomograms for 10-year risk of end-stage renal disease in Chinese type 2 diabetes mellitus patients in primary care. Diabetes Obes. Metab. 23 , 897-909 (2021).
According to Abu-Qamar (2014) diabetes self-. management education was a key strategy in the prevention of foot ulceration, which was. one of the most common causes for hospitalization for patients with Type 2 diabetes. Patients with lower limb amputations are faced with numerous challenges and experts.
To assess clinical-causal validity evidence of the nursing diagnosis, risk for unstable blood glucose level (00179), in individuals with type 2 diabetes mellitus. Methods. A case-control study was conducted in 5 primary healthcare units, involving 107 subjects with type 2 diabetes mellitus, 60 in the case group and 47 in the control group.
e22523 Background: The relationship between pancreatic cancer (PC) and type 2 diabetes mellitus (T2DM) is complex and bidirectional. While T2DM can be a risk factor for PC, pancreatic cancer can also lead to the onset or exacerbation of T2DM. Especially when these conditions coexist, individuals confront heightened mortality risks due to the compounded effects of both. Thus, our CDC analysis ...
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) is estimated to affect upto 70-80% of people with type 2 diabetes mellitus (T2DM). Although several anti-hyperglycemic drugs have shown to be effective in such patients, there remain an unmet need for newer drugs.
Objective: To investigate the impact of the Nutrition and Culinary in the Kitchen (NCK) Program on the cooking skills of Brazilian individuals with type 2 diabetes mellitus (T2DM). Methods: A randomized controlled intervention study was performed, with intervention and control groups. The intervention group participated in weekly sessions of the NCK Program for six weeks (including two in ...
More so, in terms of rural and urban divides, type 2 diabetes mellitus was found predominantly among urban dwellers. Conclusion: Findings from the study call for mass sensitisation and awareness creation in respect of diabetes, to ensure that people are well informed on the dynamics of the disease in the region and, by extension, the country at ...
Diabetes is a chronic condition that can result in many long-term physiological, metabolic, and neurological complications. Therefore, early detection of diabetes would help to determine a proper diagnosis and treatment plan. In this study, we employed machine learning (ML) based case-control study on a diabetic cohort size of 1000 participants form Qatar Biobank to predict diabetes using ...
Staff nurses, nurse practitioners, and certified diabetes nurse educators can use the toolkit for patients' first visit and on. each follow-up visit to assess and evaluate patients' and families' knowledge related to. type 2 diabetes prevention and management. Nurses play a significant role in educating.