

Testing a Hypothesis—Plant Growth
Charles Darwin believed that there were hereditary advantages in having two sexes for both the plant and animal kingdoms. Some time after he wrote Origin of Species , he performed an experiment in his garden. He raised two large beds of snapdragons, one from cross-pollinated seeds, the other from self-pollinated seeds. He observed, “To my surprise, the crossed plants when fully grown were plainly taller and more vigorous than the self-fertilized ones.” This led him to another, more time-consuming experiment in which he raised pairs of plants, one of each type, in the same pot and measured the differences in their heights. He had a rather small sample and was not sure that he could safely conclude that the mean of the differences was greater than 0. His data for these plants were used by statistical pioneer R. A. Fisher to illustrate the use of a t -test.
Looking at Darwin’s Data
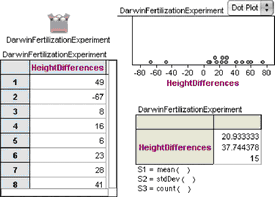
1. Open Darwin.ftm from the Tutorial Starters folder in the Sample Documents folder. This document contains the data for the experiment described above: 1 attribute, 15 cases.
2. Make a case table, a dot plot, and a summary table similar to those shown here.
We see that most of the measurements are greater than 0, meaning that the cross-pollinated plants grew bigger. But two of the measurements are less than 0. Darwin did not feel justified in tossing out these two values and was faced with a very real statistical question.
Formulating a Hypothesis
Darwin’s theory—that cross-pollination produced bigger plants than self-pollination—predicts that, on average, the difference between the two heights should be greater than 0. On the other hand, it might be that his 15 pairs of plants have a mean difference as great as they do (21-eigths of an inch) merely by chance. You can write out these two hypotheses in Fathom in a text object to be stored with your document.
3. From the shelf, drag a text object into the document.

4. Write the null hypothesis and the alternative hypothesis. At right you can see one way to phrase the hypotheses.
You can choose Edit | Show Text Palette to bring up a full suite of tools for formatting text and creating mathematical expressions.
Deciding on a Test Statistic
At the time of Darwin’s experiment, there was no very good theory for dealing with a small sample from a population whose standard deviation is not known. It was not until some years later that William Gosset, a student of Karl Pearson, developed a statistic and its distribution. Gosset published his result under the pseudonym Student, and the statistic became known as Student’s t . When the null hypothesis is that the mean is 0, the t -statistic is simply, x ̄/( s /√ n ), where x ̄ is the observed mean, s is the sample standard deviation, and n is the number of observations.
Let’s compute this statistic for Darwin’s data using one of Fathom’s built-in statistics objects.
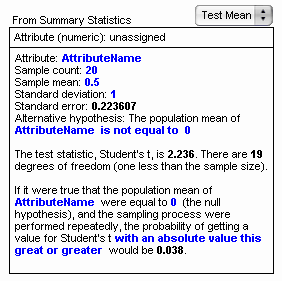
5. Drag a test object from the shelf. An empty test appears.
6. From the pop-up menu, choose Test Mean . As shown at right, the Test Mean test allows us to type in summary statistics. The blue text is editable. This is very useful when you don’t have raw data.
7. Try editing the blue text. You can, for example, enter the summary statistics for Darwin’s data.
Here are some things to notice.
- Changing something in one part of the test may affect other parts. For example, editing the AttributeName field in the first line also changes it in the hypothesis line and in the last paragraph.

- In the hypothesis line, clicking on the “is not equal to” phrase brings up a pop-up menu from which we can choose one of three options. For Darwin’s experiment, we want the third option because his hypothesis is that the true mean difference is greater than 0 . Notice that making this change alters the phrasing of the last line of the test as well.
- In addition to simple editing of numbers, we can also determine their value with a formula. For example, we might want to tie the sample count to a slider named n so that we could investigate the effect of different sample sizes. To show the formula editor, choose Edit | Edit Formula with the text cursor in the number whose value you wish to determine. These computed values display in gray instead of blue. Editing the value itself deletes the formula.
Checking Assumptions
Gosset’s work with the t -statistic relied on an assumption about the population from which measurements would be drawn, namely, that the values in the population are normally distributed. Is this a reasonable assumption for Darwin’s data?
Height measurements of living things, both plants and animals, are usually normally distributed, and so are differences between heights. But we might worry, because the two negative values give a decidedly skewed appearance to the distribution.
Fathom can help us determine qualitatively whether this amount of skew is unusual. We’ll generate measurements randomly from a normal distribution and compare the results with the original data.
8. Make a new attribute in the collection. Call it simHeight for simulated height.
9. Select simHeight and choose Edit | Edit Formula . Enter the formula shown below.
This formula tells Fathom to generate random numbers from a normal distribution whose mean and standard deviation are the same as in our original data. We want to compare the distribution of these simulated heights with the distribution of the original data. We can do that directly in the dot plot that already shows HeightDifferences .

10. Drop simHeight on the plus sign to add it to the horizontal axis. The graph now shows the original data on top and the simulated data on the bottom.
One set of simulated data doesn’t tell the whole story. We need to look at a bunch.
11. Choose Collection | Rerandomize .
Each time you rerandomize, you get a new set of 15 values from a population with the same mean and standard deviation as the original 15 measurements. Three examples are shown below.
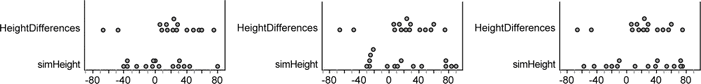
A bit of subjectivity is called for here. Does it appear that the original distribution is very unusual, or does it fit in with the simulated distributions?
Testing the Hypothesis

Once we have decided that the assumption of normality is met, we can go on to determine whether the t -statistic for Darwin’s data is large enough to allow us to reject the null hypothesis.
In step 7, we typed the summary values into the test as though we didn’t have the raw data. But we are in the fortunate position of having the raw data, so we can ask Fathom to figure out all the statistics using that data.
12. Drag HeightDifferences from the case table to the top pane of the test where it says “Attribute (numeric): unassigned.”
13. If the hypothesis line does not already say “is greater than,” then select that choice from the pop-up menu.
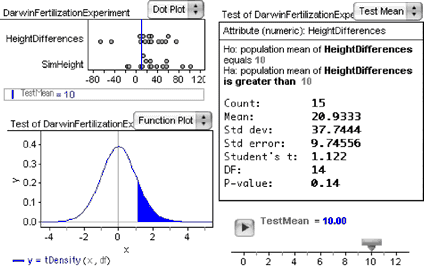
The last paragraph of the test describes the results. If the null hypothesis were true and the experiment were performed repeatedly, the probability of getting a value for Student’s t this great or greater would be 0.025. This is a pretty low P -value, so we can safely reject the null hypothesis and, with Darwin, pursue the theory that cross-pollination increases a plant’s height compared with self-pollination.
Looking at the t -Distribution
It is helpful to be able to visualize the P -value as an area under a distribution.
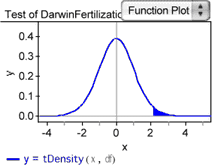
14. With the test selected, choose Test | Show Test Statistic Distribution . The curve shows the probability density for the t -statistic with 14 degrees of freedom. The shaded area shows the portion of the area under the curve to the right of the test statistic for Darwin’s data. We’ve set this up as a one-tailed test; we’re only interested in the mean difference being greater than zero. The total area under the curve is 1, so the area of the shaded portion corresponds to the P -value for Darwin’s experiment.
Let’s investigate how the P -value depends on the test mean, which is currently set to 0.

15. Drag a slider from the shelf into the document.
16. Edit the name of the slider from V1 to TestMean .
17. Select the 0 in the statement of the hypothesis in the test. Choose Edit | Edit Formula .
18. In the formula editor, enter the slider name TestMe an and click OK .
Now the value of the null hypothesis mean in the test and the shaded area under the t -distribution change to reflect the new hypothesis.
19. Drag the slider slowly and observe the changes that take place.
For what value of the slider is half the area under the curve shaded? Explain why it should be this particular value.
The illustration below shows something similar to what you probably have. Note that the test has been switched to “nonverbose” (choose Test | Verbose ).
Going Further
- Play around with changing the data and observing the effect on the P -value. How much closer to 0 can the experimental mean be (without changing the standard deviation) and still have a P -value greater than 0.05? If you make the standard deviation smaller, what happens to the P -value (and why)?
- Make a Test Mean object that tests the mean of simHeight instead of HeightDifferences . Notice that each time you rerandomize, you get a new P -value. Think about what it means when the P -value is greater than 0.05. Would you call this a “false positive” or a “false negative”? By repeatedly rerandomizing, estimate the proportion of the time that the P -value is greater than 0.05. What practical significance would that have in planning an experiment?
ORIGINAL RESEARCH article
Testing the growth rate hypothesis in two wetland macrophytes under different water level and sediment type conditions.

- 1 Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
- 2 School of Environment and Life Science, Nanning Normal University, Nanning, China
- 3 Dongting Lake Station for Wetland Ecosystem Research, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
- 4 College of Architecture and Urban Planning, Hunan City University, Yiyang, China
The growth rate hypothesis (GRH) states that a negative correlation exists between the growth rate and N:P and C:P ratios, because fast-growing organisms need relatively more phosphorus-rich RNA to support their high rates of protein synthesis. However, it is still uncertain whether the GRH is applicable in freshwater wetlands. Several studies have shown that water level and sediment type are key factors influencing plant growth and plant C:N:P characteristics in freshwater wetlands. Thus, this study aimed to elucidate the influence of these factors on plant growth and test the GRH under varying water levels and sediment conditions. We designed a controlled experiment at three water levels and under three sediment types using the two dominant plants ( Carex brevicuspis and Polygonum hydropiper ) in the East Dongting Lake wetland, and we further investigated the relative growth rate (RGR); concentrations of total carbon (TC), total nitrogen (TN), and total phosphorus (TP); and plant stoichiometry (ratios of C:N, C:P, and N:P) in the aboveground and belowground parts and whole plants in both species. Results demonstrated that the RGR and TC of both species decreased significantly with decreasing sediment nutrient supply and increasing water level. However, TN and TP of both species were markedly higher at high water levels than at low water levels; furthermore, these were significantly higher on clay than on the other two sediment types at each water level. The C:N and C:P ratios of both species decreased with increasing sediment nutrient supply and water level, whereas N:P decreased in both species with increasing sediment nutrient supply. The aboveground part of C. brevicuspis as well as the aboveground part and whole plant of P. hydropiper were negatively correlated with N:P, which is consistent with the GRH. However, the relationship between the belowground RGR and N:P of these species was inconsistent with GRH. Therefore, the water level and sediment type and their interaction significantly influenced plant RGR and C:N:P characteristics. The RGR and plant stoichiometry differed significantly between plant organs, indicating that the GRH needs refinement when applied to wetland macrophytes.
Introduction
The growth rate hypothesis (GRH) proposes that fast-growing organisms have low N:P and C:P ratios due to the relatively high demand for phosphorus-rich RNA to support rapid protein synthesis ( Acharya et al., 2004 ). Various comprehensive reviews confirmed that nutrient-rich plants tend to have low N:P ratios, and supported the validity of GRH in the realm of vascular plants, as N concentration in vascular plants tends to increase less than P concentration ( Wright et al., 2005 ; Kerkhoff and Enquist, 2006 ; Yu et al., 2012 ). However, opposite results were also reported ( Peng et al., 2010 ; Loladze and Elser, 2011 ). For instance, Matzek and Vitousek (2009) found that there was no link between growth rate and leaf N:P for pine species, because RNA comprises only a small proportion of total P (TP) to strongly influence leaf P concentration. To date, the GRH hypothesis has been tested in a variety of ecosystems, and at relatively large scales ( Güsewell, 2004 ; McGroddy et al., 2004 ; Lovelock et al., 2007 ); however, it is still uncertain whether it is applicable in freshwater wetlands.
Water level is the dominant factor influencing nutrient cycling and the structure of wetland plant communities ( Lowe et al., 2010 ; Sardans et al., 2012 ; Saaltink et al., 2018 ). It can constrain the growth and nutrient availability to wetland macrophytes mainly by limiting oxygen ( Casanova and Brock, 2000 ) and light ( Cronin and Lodge, 2003 ; Miao and Zou, 2012 ) availabilities and by changing soil nutrient cycling ( Steinman et al., 2012 ; Wang et al., 2015a ). For example, Carex brevicuspis , which has a relatively low growth rate, was reported to have high N:P ratio and high N and P concentrations at high water levels, both probably caused by anoxic stress ( Li et al., 2018a ). On the contrary, Li et al. (2013) found that increasing water level decreased the relative growth rate (RGR) of Potamogeton malaianu without affecting its N:P ratio and concentrations of N and P. This inconsistency indicates that the relationship between RGR and N:P ratio at different water levels and for different plant species is far from clear. Moreover, high water levels significantly affect soil nutrient availability by changing its geochemical cycle as well as the activity of soil microorganisms ( Niedermeier and Robinson, 2007 ; González Mace et al., 2016 ), thereby determining plant stoichiometry. For example, the soil mineralization process of organic N results in the accumulation of ammonium under anaerobic conditions, further affecting the N cycle of plants in wetlands ( Hefting et al., 2004 ). Soil P availability also increases due to the reduction of iron, which releases soluble P into the soil ( Bridgham et al., 1998 ; Saaltink et al., 2018 ). To date, many studies have focused on the effects of water level on plant growth and distribution ( Madsen et al., 2001 ; Li et al., 2012 ). However, the response of plant stoichiometry to varying water levels is still uncertain ( Cao et al., 2011 ; Yuan et al., 2013 ). Results from the few studies conducted so far are also inconsistent ( Miao and Zou, 2012 ; Li et al., 2013 ), indicating that changes in plant stoichiometry in response to water level might be species-specific and needs to be further studied.
Sediment type substantially affects plant growth rate and stoichiometry ( Luo et al., 2010 ; Li et al., 2018a ). Plants with high nutrient concentrations are able to extend their roots and enhance root uptake rate, thereby enhancing nutrient absorption abilities ( Fransen et al., 2001 ). For instance, plant RGR and concentrations of N and P in sandy sediments are lower than that in clay sediments due to the limited nutrient availability ( Li et al., 2015 ). However, the nutrient-rich sediment had no significant effect on the relative growth rates of Elodea canadensis and Callitriche cophocarpa possibly due to their low nutrient requirements ( Madsen and Cedergreen, 2002 ). Indeed, the relationship between sediment type and plant stoichiometry is often affected by water level in wetlands ( Xie et al., 2009 ; Li et al., 2017a ). The roots of wetland plants usually display contrasting properties to adjust to infertile or flooded environments, and higher water levels commonly further limit plant nutrient absorption ( Xie et al., 2009 ). Therefore, it is difficult to predict the effects of water level and sediment type on plant stoichiometry based on single factors. Although the changes in plant stoichiometry in different sediment types have been widely studied ( Morse et al., 2004 ; Li et al., 2018a ), few studies have focused on their interaction with plant C:N:P stoichiometry.
Carex brevicuspis and Polygonum hydropiper are dominant species in the vegetated zone of the East Dongting Lake wetland. C. brevicuspis is a perennial rhizomatous clonal plant widely distributed at low elevations (23–30 m). The belowground meristems of C. brevicuspis can produce long rhizomes (2–25 cm long), which are more capable of obtaining resources under stressful conditions, and short rhizomes (< 1 cm long), which are better at using resources in favorable patches. P. hydropiper is an annual herb forming patches embedded in stands of C. brevicuspis , generally sensitive to flooding stress and inhabiting elevated sites over shallow flooded habitats. Compared to P. hydropiper , C. brevicuspis has a wider optimal hydrological niche in the East Dongting Lake wetland ( Chen et al., 2014 ; Li et al., 2018a ). In this study, we investigated the interactive effects of water level and sediment type on the growth performance and stoichiometry of C. brevicuspis and P. hydropiper. These two dominant species were planted under three water levels (-30 cm, 0 cm, and 30 cm relative to the soil surface) and three sediment types (clay, sand, and a mixture of sand and clay at a 1:1 volume ratio) in a factorial design with five replicates. The RGR, total C (TC), total N (TN), TP, and C:N, C:P, and N:P ratios in the aboveground and belowground parts and in the whole plant of both species were measured for exploring the relationship between RGR and plant stoichiometry. As so, the present study aimed to (1) elucidate how differences in water level and sediment type affect plant growth and plant C:N:P characteristics; and (2) test whether the relationship between RGR and plant C:N:P stoichiometry is consistent with GRH under different water level and sediment type conditions.
Materials and Methods
Study site and plant materials.
Dongting Lake (28°30′–30°20′ N, 111°40′–113°10′ E) is the second-largest freshwater lake and the most typical river-connected lake in China; it is characterized by large seasonal fluctuations of the water level and sediment heterogeneity ( Xie et al., 2007a ). The wetlands are usually completely flooded from May to October, while being susceptible to drought from November to April. The mean annual temperature is 16.8°C, with hot summers (June–August, 27.3°C) and cold winters (December–February, 5.8°C). The mean annual precipitation is 1,382 mm, with more than 60% of the rain falling from April to August ( Li et al., 2017b ).
Carex brevicuspis (Cyperaceae) is a typical perennial rhizomatous sedge distributed in eastern mainland China. The plant is usually 20–55 cm in height, and it flowers and bears fruit from April to May, before flooding occurs in the Dongting Lake wetland ( Chen et al., 2011 ). Polygonum hydropiper (Polygonaceae) is an annual herb 40–70 cm in height. Both species experience periodic flooding that normally occurs between May and October ( Chen et al., 2014 ).
C. brevicuspis was collected in Xiaoxihu and P. hydropiper was collected in Dingzidi, both in East Dongting Lake, during March 2016. New ramets were dug up and transported to the Dongting Lake Station for Wetland Ecosystem Research, Chinese Academy of Sciences. The new ramets (about 15 cm in height) were placed in plastic basins (55 cm in length, 33 cm in width, 21 cm in height) filled to a depth of 15 cm with soil (4.01 mg g -1 soil organic carbon, 0.48 mg g -1 soil TN, and 0.57 mg g -1 soil TP) that was collected from a C. brevicuspis and P. hydropiper mixed community in the East Dongting Lake. After one month, similar-sized plants (4–5 leaves, about 25 cm in height) were selected for the experiment.
Experimental Design
Before the experiment, ten seedlings of C. brevicuspis and ten seedlings of P. hydropiper were divided into aboveground and belowground parts, oven-dried, and weighed for the calculation of plant RGR ( Li et al., 2016 ). The experiment combined three water levels (-30 cm, 0 cm, and 30 cm relative to the soil surface) and three sediment types (clay, sand, 1:1 clay–sand mixture) with the two species in a factorial design with five replicates ( Table 1 ). Clay was collected from the location described above for ramet germination, and sand was collected from the local river. In the Dongting Lake wetland, most roots of both species are distributed in the top 0–20 cm soil layer ( Chen et al., 2014 ). Therefore, the -30 cm water level was considered the drought treatment, the 0 cm water level was considered the control, and the 30 cm water level was considered the submerged treatment ( Figure 1 ). The three sediment types used in the experiment are the main sediment types present in the natural habitat of C. brevicuspis and P. hydropiper in Dongting Lake. We sampled the clay soil from the same location as plant samples while the sand was collected from the local Xiang River ( Table 1 ). On April 2, 2016, the 1,350 similar-sized ramets collected (675 for each species) were transplanted into PVC tubes (30 cm in height and 12 cm in diameter, bottoms enclosed with a nylon netting to prevent soil loss) filled with sediment. Thirty tubes (3 water levels × 2 plant species × 5 tubes) were placed into each of 15 cement pools (1 × 1 × 1 m, five pools per sediment). Three seedlings were planted into each tube for both species, and the experiment started 7 days after planting. Tap water (containing 0.51 μg L -1 NH 4 -N, 1.76 μg L -1 NO 3 -N, and 0.53 μg L -1 PO 4 3+ -P, pH = 7.2) was completely replaced every two weeks to prevent algal growth ( Figure 1 ).
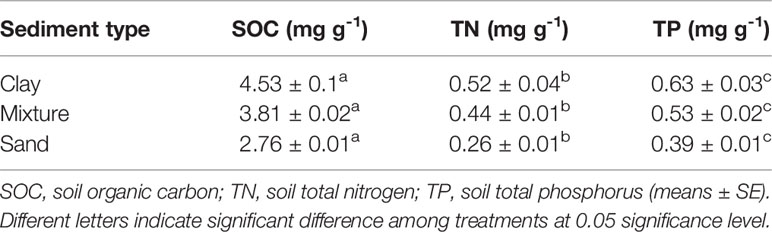
Table 1 Soil nutrient concentrations of each sediment type.
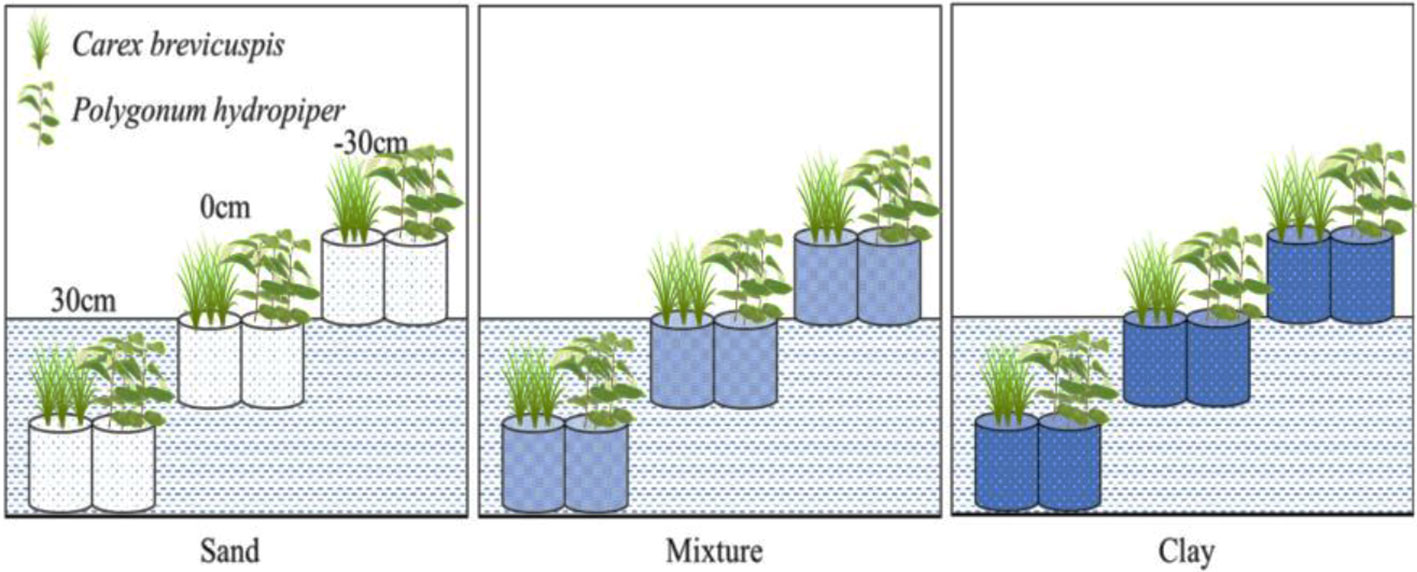
Figure 1 Experimental scheme, showing two plant species ( Carex brevicuspis and Polygonum hydropiper ), three sediment types (clay; mixture; sand) and three water levels (-30 cm; 0 cm; 30 cm). Five replicates were made of each treatment.
Harvest and Measurements
All plants were harvested after 4 months of treatment. The roots of each plant were carefully excavated from the PVC tubes, cleaned with tap water, and transported to the laboratory for measurements. Plants in each tube were divided into aboveground and belowground parts, oven-dried at 80°C for 48 h, and weighed.
The RGR (relative growth rate) of the aboveground and belowground parts and of the whole plant were calculated for each species using the following formula:
where X 1 and X 2 are the biomass of the aboveground or belowground parts or of the whole plant at the end and start of the experiment, respectively, and T is the duration of the experiment ( Yuan et al., 2016 ).
Total C, N, and P Concentrations
The aboveground and belowground parts and the whole plant of each species in each PVC tube were ground into powder and analyzed for TC and TN using an elemental analyzer (Vario EL III; Elementar, Hanau, Germany). Total P was measured with colorimetric analysis on a TU-1901 spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China) after being pretreated by H 2 SO 4 –H 2 O 2 digestion ( Xie et al., 2007b ). Three replicates were used to determine plant C, N, and P concentrations.
Statistical Analyses
The mean values of the five replicates for each treatment in each pool were used for data analysis. The effect of water level and sediment type on RGR, TC, TN, and TP concentrations and the stoichiometry of the aboveground and belowground parts and whole plant of each species were assessed using a general linear model (GLM). Multiple comparisons of the means were performed using Tukey ’ s test at the 0.05 significance level. All statistical analyses were performed in SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
RGRs of C. brevicuspis and P. hydropiper
The RGR of the aboveground and belowground parts and whole plants of C. brevicuspis and P. hydropiper were significantly affected by water level, sediment type, and their interaction ( Table 2 ; Figure 2 ). The RGR decreased significantly with increasing water levels in all sediment types, and the highest values of both species were found in the -30 cm water level + clay treatment while the lowest values were found in the 30 cm water level + sand treatment.
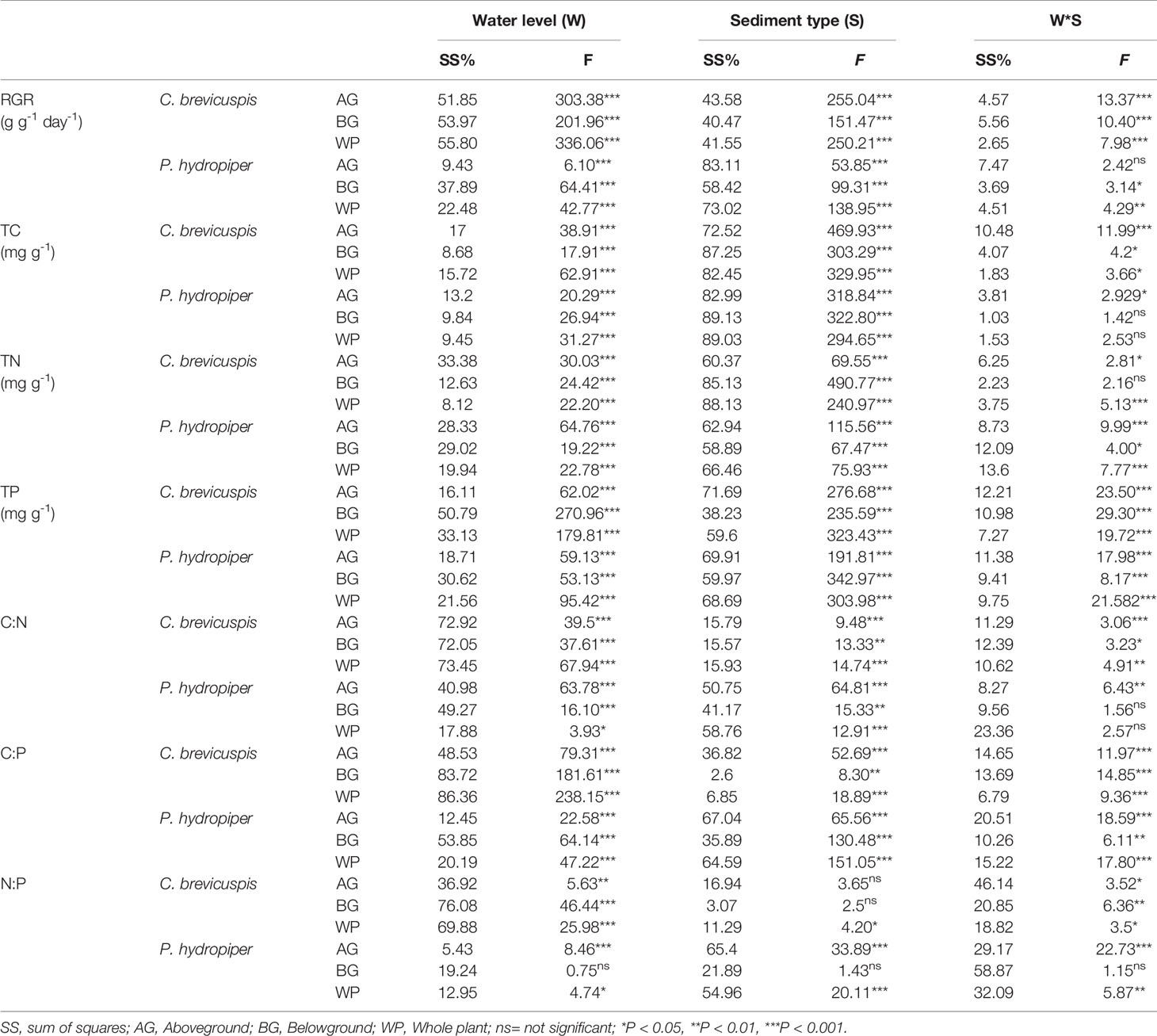
Table 2 Summary of general linear model (GLM) on plant relative growth rate (RGR), concentrations of TC, TN, and TP, and ratios of C:N, C:P, and N:P in C. brevicuspis and P. hydropiper growing in three water levels and three sediment types ( F -values).
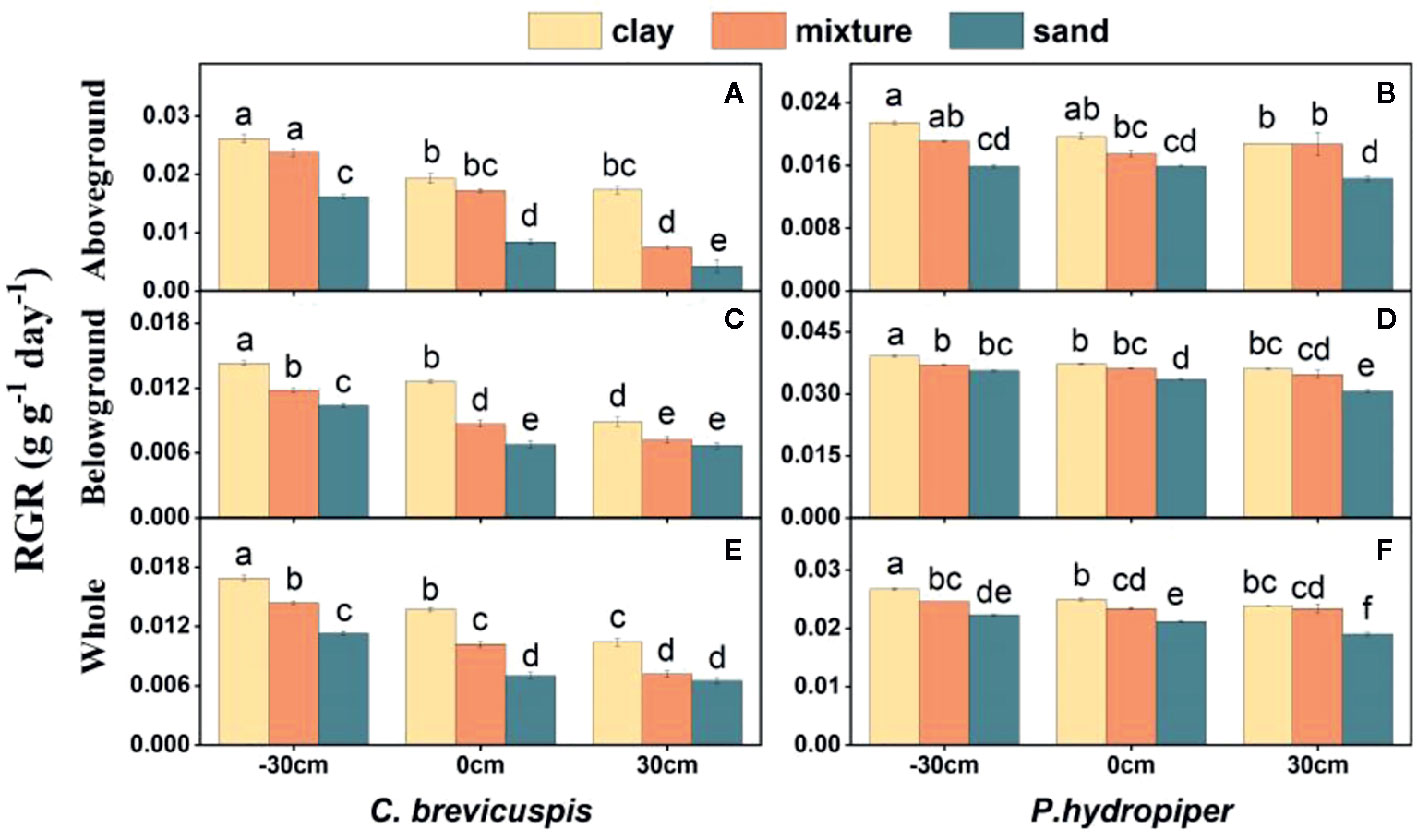
Figure 2 Relative growth rate (RGR) in aboveground part, belowground parts and whole plants of C. brevicuspis (A, C, E) and P. hydropiper (B, D, F) in treatments with three sediment types (clay; mixture; sand) and three water levels (-30 cm; 0 cm; 30 cm). Values are means ± SE, with five replications. Different letters indicate significant difference among treatments at 0.05 significance level.
Both water level and sediment type had significant effects on TC, TN, and TP concentrations in the aboveground and belowground parts and whole plants of both species ( P < 0.001) ( Table 2 ). The highest TC concentrations in the aboveground and belowground parts and whole plants of both species were found in the -30 cm water level + clay treatment and they decreased significantly with decreasing sediment nutrient concentration and increasing water level. The TN and TP concentrations in aboveground and belowground parts and whole plants of both species were highest in the 30 cm water level + clay treatment, and they decreased significantly with decreasing sediment nutrient concentration and water level ( Figure 3 ).
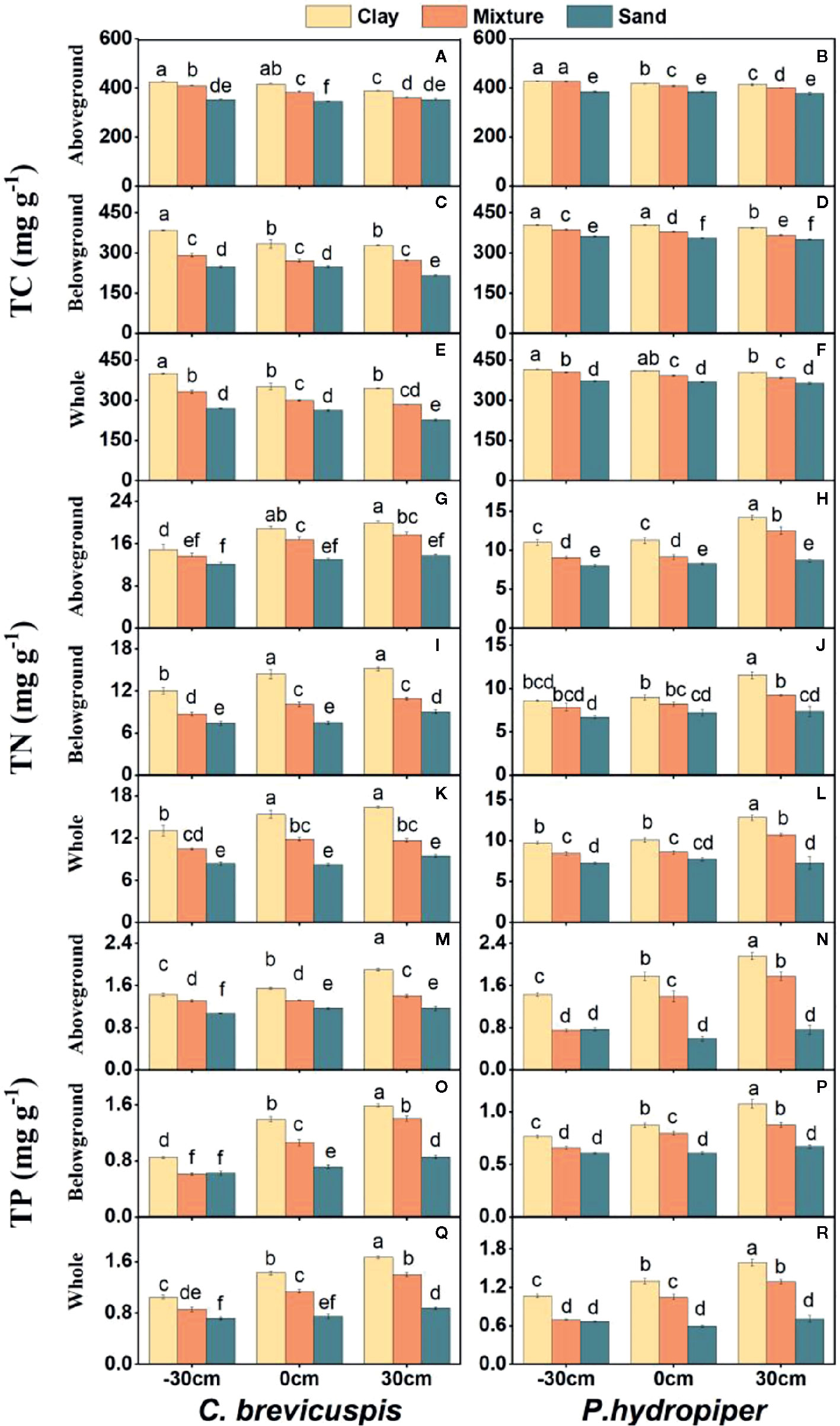
Figure 3 Concentrations of TC (A–F) , TN (G–L) , and TP (M–R) (means ± SE) in aboveground part, belowground parts and whole plants of C. brevicuspis and P. hydropiper growing in three sediment types (clay; mixture; sand) and three water levels (-30 cm; 0 cm; 30 cm). Different letters indicate significant differences among treatments ( P < 0.05).
C, N, and P Stoichiometry Ratios
Water level and sediment type significantly affected C:N and C:P ratios in the aboveground and belowground parts and whole plants of C. brevicuspis and P. hydropiper ( Table 2 ). The C:N and C:P ratios in the aboveground and belowground parts and whole plants of both species decreased with increasing sediment nutrient supply and water level. The highest N:P ratios in the aboveground and belowground parts and whole plants of P. hydropiper were found in the 0 cm + sand treatment. The highest N:P ratio in the aboveground part of P. hydropiper was found in the 0 cm + mixture treatment and in the belowground part and whole plant were found in the -30 cm + mixture treatment ( Figure 4 ).
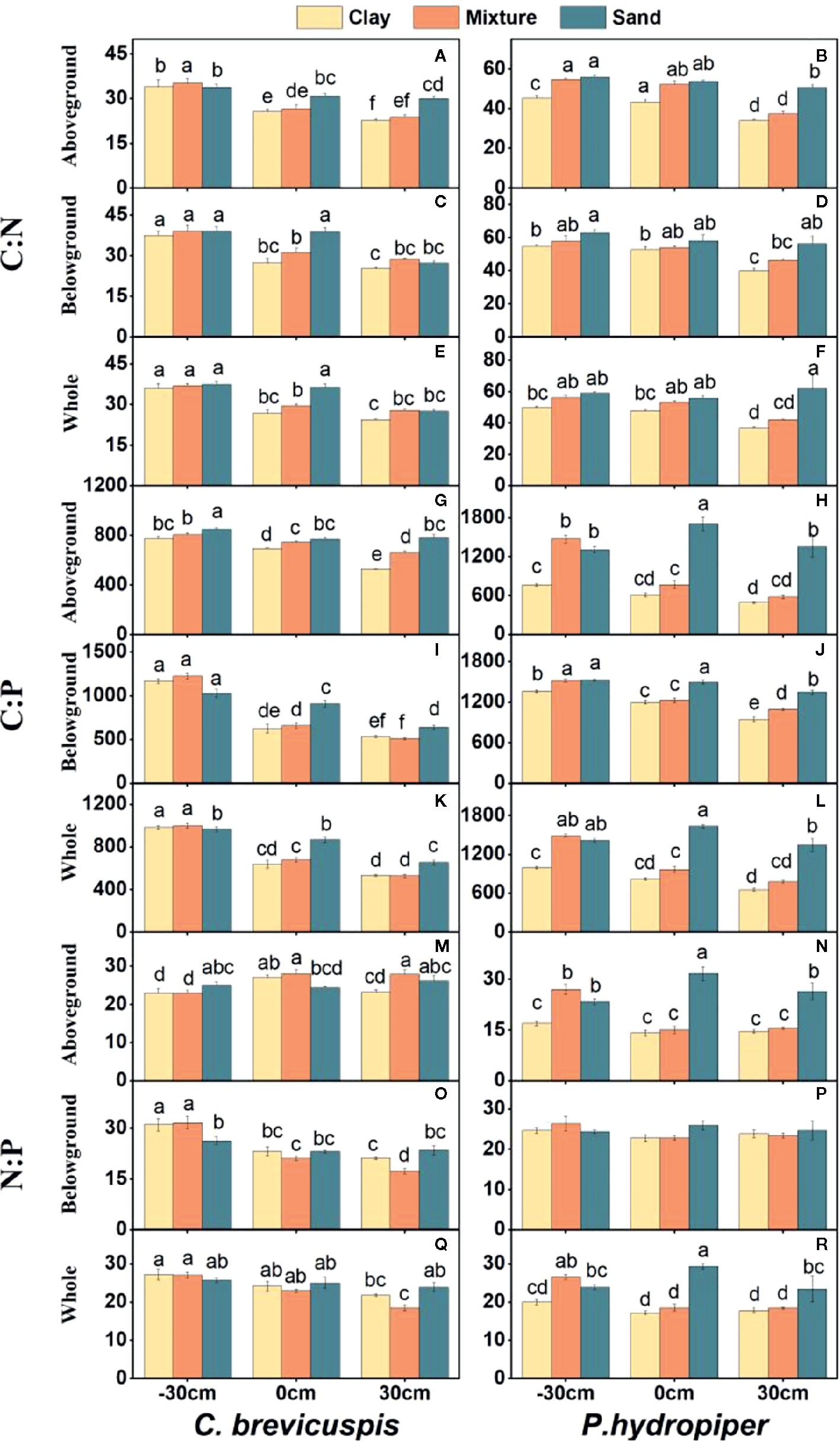
Figure 4 Ratios of C:N (A–F) , C:P (G–L) , N:P (M–R) (means ± SE) in aboveground and belowground parts and the whole plants of C. brevicuspis and P. hydropiper growing in three sediment types (clay; mixture; sand) and three water levels (-30 cm; 0 cm; 30 cm). Different letters indicate significant differences among treatments ( P < 0.05).
Relationships of RGR With C, N, and P Stoichiometry
In C. brevicuspis , the RGR of the aboveground part was positively correlated with TC and TP concentrations and negatively correlated with N:P ratio, while the RGR of the belowground part and whole plant were positively correlated with TC and TN concentrations and with C:P and N:P ratios ( Figure 5 ).
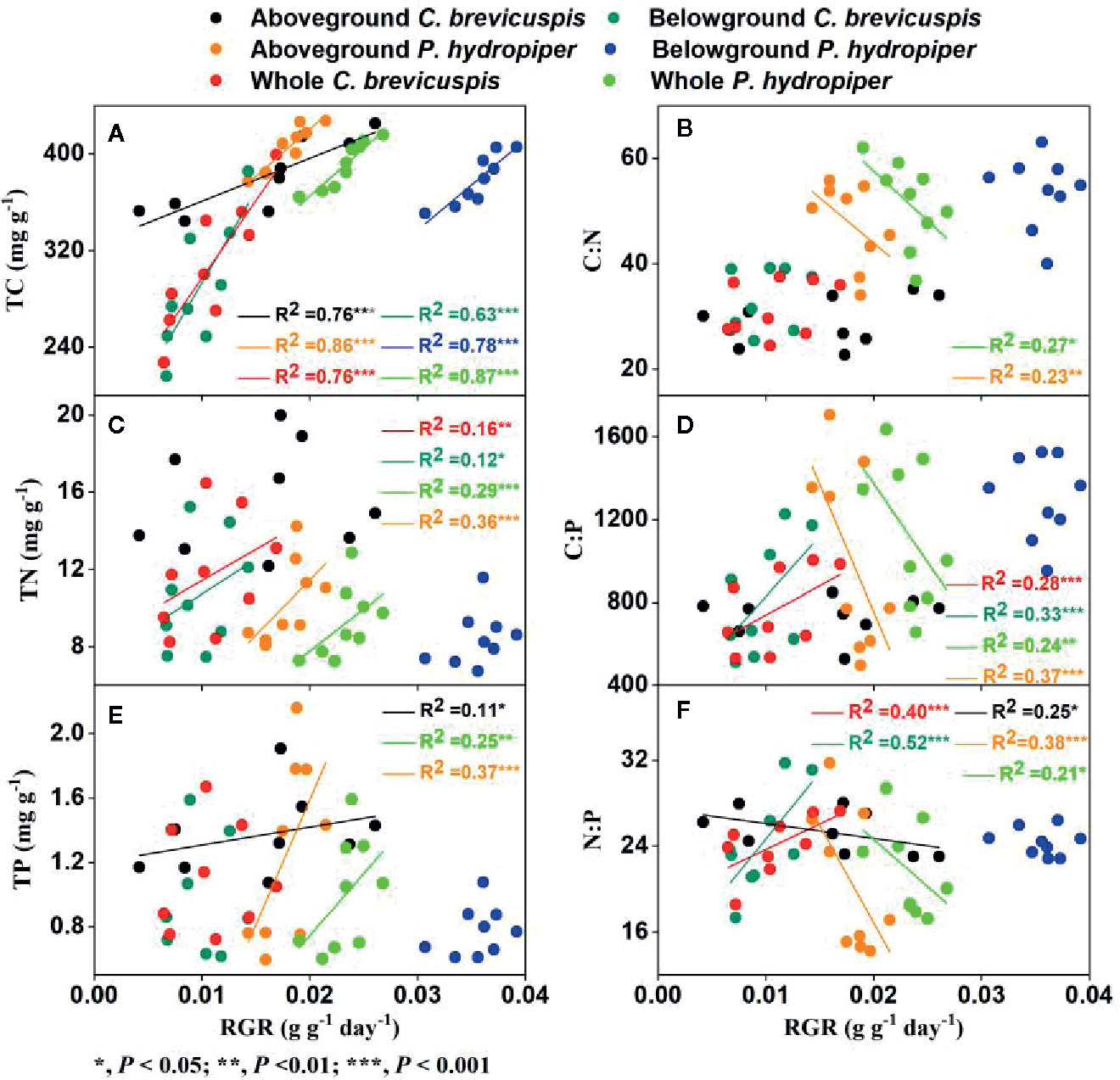
Figure 5 Relationships between relative growth rate (RGR) and concentrations of TC (A) , TN (C) , TP (E) , and ratios of C:N (B) , C:P (D) , N:P (F) (means ± SE) in aboveground and belowground parts and the whole plants of C. brevicuspis and P. hydropiper .
In P. hydropiper , the RGR of the aboveground part and whole plant were positively correlated with the TC, TN, and TP concentrations and negatively correlated with the C:N, C:P, and N:P ratios. The RGR of the belowground part was only positively correlated with TC concentration ( Figure 5 ).
The RGR of the aboveground and belowground parts and whole plants of both species decreased significantly with decreasing sediment nutrient concentrations and increasing water levels, indicating that water level, sediment type, and their interaction had a significant effect on plant growth performance ( Emery et al., 2001 ; Xie et al., 2009 ; Luo et al., 2010 ). The negative effect of high-water levels on plant growth has been reported in many studies, and it has been mainly attributed to the anaerobic environment and reduced soil redox potential, Eh ( Sorrell et al., 2000 ; Steinman et al., 2012 ). In some of the treatments conducted in the present study, e.g., 0 cm water level + mixture and 30 cm water level + clay, the similar growth performance of the aboveground parts of C. brevicuspis indicated that the negative influence of water level on plant growth could be ameliorated in nutrient-rich conditions, as supported by other studies ( Wheeler, 1999 ; Xie et al., 2009 ). Nutrient availability may increase plant root respiration and root diameter and help plants to acclimate to high water level conditions ( Xie et al., 2009 ; Chen et al., 2016 ).
The TC concentrations in the aboveground and belowground parts and whole plants of both species decreased significantly with increasing water levels, which was consistent with previous studies ( Li et al., 2013 ; Yuan et al., 2016 ). High water levels decrease plant photosynthesis, thus leading to a reduction in the synthesis of non-structural carbohydrates in plant tissues ( Cao et al., 2009 ; Su et al., 2016 ). Plant C balance can be characterized by tissue concentrations of non-structural carbohydrates. When C supply from photosynthesis exceeds the plant’s demand for growth, a large amount of non-structural carbohydrates will accumulate to support future growth. By contrast, when C demand exceeds the C supply, non-structural carbohydrates will only slightly accumulate ( Wang et al., 2018 ). Similar to RGR, plant C concentrations in both species were also higher in the clay treatment than in other sediment types, as soil nutrients are the main determinants of plant nutrient concentrations and therefore influence plant growth ( Li et al., 2017b ). Wang et al. (2015b) and Zeng et al. (2017) also reported that nutrient-rich sediment conditions result in high C concentration.
The TN and TP concentrations in the aboveground parts of both species were higher compared with those in the belowground parts and whole plants. As described in previous studies ( Li et al., 2013 ; Jing et al., 2017 ), this phenomenon can be explained by the presence of large amounts of rubisco in the photosynthetic organs ( Reich et al., 2004 ). The TN and TP concentrations in the aboveground and belowground parts and whole plants of both species increased, while C:N and C:P ratios decreased with increasing water level, which was consistent with previous studies ( Cronin and Lodge, 2003 ; Li et al., 2013 ). For example, TN and TP concentrations of Cladium jamaicense increased significantly when water levels increased from 20 to 60 cm ( Miao and Zou, 2012 ). In this study, plants were submerged in 30 cm of water, where light availability was low. The light conditions at the -30 cm water level lead to lower leaf N, probably due to the dilution of available N by increased amounts of fixed C ( Cronin and Lodge, 2003 ). Therefore, lower N and P availability for plant photosynthesis will lead to high plant N and P concentrations. Another study also confirmed that the biomass accumulation of C. brevicuspis increased with increasing elevation, while plant TN and TP concentrations decreased, which might have accounted for the dilution effect by which fast-growing plants allocate more N and P to their photosynthetic tissues to support high carbon dioxide assimilation ( Yan et al., 2006 ; Li et al., 2018b ). Water level can also influence plant nutrient absorption by changing soil biogeochemical processes ( Steinman et al., 2012 ; Recha et al., 2013 ). For instance, ammonification is the dominant process at high water levels ( Hefting et al., 2004 ), and it enhances the concentration of available N, promoting plant N absorption ( Kaštovská and Šantrůčková, 2011 ). In addition, soil anoxia can reduce iron plaque formation on roots at high water levels, and thus promote plant P uptake ( Saaltink et al., 2018 ).
At the same water level, the higher TN and TP concentrations and lower C:N, C:P, and N:P ratios in the aboveground and belowground parts and whole plants of both species on the clay sediment indicated that sediment nutrients mainly affect plant nutrients, which could further influence plant stoichiometry ( Garbey et al., 2004 ; Chen et al., 2013 ; Li et al., 2014 ). In this study, sediment N and P concentrations in the clay sediment were 2.0 and 1.6 times higher than those in the sand sediment, leading to higher plant N and P concentrations. Moreover, it has been reported that high sediment nutrient levels can promote plant growth and enhance plant nutrient concentrations ( Fraser and Feinstein, 2005 ; Güsewell, 2005 ). A high clay content would therefore promote soil N mineralization and plant N absorption, while a high sand content allows a higher rate of P leaching ( Cross and Schlesinger, 2001 ).
The N:P ratio in the aboveground parts of both species and whole plant of P. hydropiper were negatively correlated with their corresponding RGR, thus supporting the GRH and being consistent with previous studies ( Niklas et al., 2005 ; Niklas, 2006 ; Ågren, 2008 ; Cernusak et al., 2010 ). Ågren (2004) reported that P limited Betula pendula seedlings, which displayed decreased N:P at high RGR, supporting the GRH. As a possible explanation, Sterner and Elser (2002) proposed that organisms have to make a relatively large investment in P-rich ribosomes and rRNA to support the rapid protein synthesis associated with fast growth. However, opposite results were found in other studies ( Cernusak et al., 2010 ; Peng et al., 2010 ). One possible reason for these inconsistent results might be that some plants can store extra nutrients and thus change the relationship between the RGR and the N:P ratio ( Jing et al., 2017 ). Matzek and Vitousek (2009) also showed that plant protein:RNA ratio, but not leaf N:P ratio, was significantly negatively correlated with plant growth rate.
The relationship between RGR and plant stoichiometry in the belowground parts of both species and whole plant of C. brevicuspis suggests that the GRH is not valid in these cases, indicating that the applicability of this hypothesis might depend on plant organ and species. In fact, another study reported that the GRH was not consistent with the growth of various organs ( Jing et al., 2017 ). One probable reason might be that a change in environmental factors may lead to the allometric growth of different organs, and the stoichiometry of roots is more sensitive to environmental changes than that of leaves ( Minden and Kleyer, 2014 ; Schreeg et al., 2014 ). For instance, Jing et al. (2017) confirmed that N addition significantly increased the N:P ratio and RGR of Pinus tabuliformis roots in N-limited regions, resulting in a positive relationship between the RGR and N:P ratio of roots. Another reason might be that plants have developed survival strategies other than growth (e.g., storage and defense) that require N and P, in which case a decreasing N:P ratio with increasing growth rate should not necessarily be expected ( Matzek and Vitousek, 2009 ). In addition, plants can store P in vacuoles, allocate N to the production of chemical defenses, or invest different N:P ratios in different organs, all of possibly explaining why P concentration is not greater in fast-growing plants ( Méndez and Karlsson, 2005 ; Peñuelas and Sardans, 2009 ). However, our results were inconsistent with previous studies ( Ågren, 2004 ; Yu et al., 2012 ). For instance, Yu et al. (2012) confirmed that the GRH was valid for the roots of three grass plants in the grasslands of Inner Mongolia, and they also proposed that analysis of the relationship between RGR and N:P ratio should consider the N in ribosomes of vascular plants.
In addition, the RGR of the aboveground and belowground parts and whole plant of C. brevicuspis were lower than that of P. hydropiper , while the N:P ratios in the aboveground and belowground parts and whole plant of C. brevicuspis were relatively higher compared with those of P. hydropiper . These differences between the two species might be related to the higher tolerance of C. brevicuspis to water stress and drought stress compared with P. hydropiper ( Chen et al., 2014 ). Namely, stress tolerant plants (characterized by slow growth) have consistently higher N:P ratios than fast-growing plants in wetlands, as the former can focus on the uptake of nitrate while maintaining P reserves due to low internal P demands and efficient conservation ( Willby et al., 2001 ).
This study confirmed that water level, sediment type, and their interaction significantly influence plant growth and plant stoichiometry. Furthermore, we also established that the GRH is valid for the whole plant of P. hydropiper and the aboveground parts of both species, but not for whole plant of C. brevicuspis and the belowground parts of both species. These results indicate that the GRH needs to be refined for application to macrophytes. However, our study was primarily based on controlled incubation conditions with a relative short duration. Therefore, further studies are still needed to test this hypothesis under long-term natural conditions. In recent years, the area of C. brevicuspis and P. hydropiper communities in Dongting Lake wetland were seriously reduced due to reduced water levels and anthropogenic disturbances. Therefore, understanding plant growth and stoichiometry characteristics would contribute to the better understanding of macrophytes ecological processes and to establish effective measures for macrophytes’ protection and biodiversity maintenance.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Author Contributions
CH and FL wrote the manuscript and conducted the technical assays and statistical analyses. NY and Y-HX designed the experiment and edited the manuscript. X-SC and Z-MD contributed to data collection and interpretation. All authors contributed to the article and approved the submitted version.
This study was supported by the Joint Fund for Regional Innovation and Development of NSFC (U19A2051), the Youth Innovation Promotion Association of CAS (201861), Key R & D Projects in Hunan Province (2019SK2336) and Changsha Science and Technology Project (kq1907072), the Youth Innovation Development Program of Changsha (kq1802026), and the National Natural Science Foundation of China (31570431).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer [X-TL] declared a shared affiliation, though no other collaboration, with several of the authors [FL, Y-HX, X-SC, Z-MD] to the handling Editor.
Acharya, K., Kyle, M., Elser, J. J. (2004). Biological stoichiometry of Daphnia growth: An ecophysiological test of the growth rate hypothesis. Limnol. Oceanogr. 49, 656–665. doi: 10.4319/lo.2004.49.3.0656
CrossRef Full Text | Google Scholar
Ågren, G., II (2004). The C: N: P stoichiometry of autotrophs – theory and observations. Ecol. Lett. 7, 185–191. doi: 10.1111/j.1461-0248.2004.00567.x
Ågren, G., II (2008). Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 39, 153–170. doi: 10.1146/annurev.ecolsys.39.110707.173515
Bridgham, S. D., Updegraff, K., Pastor, J. (1998). Carbon, nitrogen, and phosphorus mineralization in northern wetlands. Ecology 79, 1545–1561. doi: 10.2307/176775
Cao, T., Xie, P., Ni, L., Zhang, M., Xu, J. (2009). Carbon and nitrogen metabolism of an eutrophication tolerative macrophyte, Potamogeton crispus , under NH 4 + stress and low light availability. Environ. Exp. Bot. 66, 74–78. doi: 10.1016/j.envexpbot.2008.10.004
Cao, T., Ni, L., Xie, P., Xu, J., Zhang, M. (2011). Effects of moderate ammonium enrichment on three submersed macrophytes under contrasting light availability. Freshwater Biol. 56, 1620–1629. doi: 10.1111/j.1365-2427.2011.02601.x
Casanova, M. T., Brock, M. A. (2000). How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecol. 147, 237–250. doi: 10.1023/A:1009875226637
Cernusak, L. A., Winter, K., Turner, B. L. (2010). Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytol. 185, 770–779. doi: 10.1111/j.1469-8137.2009.03106.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, X. S., Xie, Y. H., Deng, Z. M., Li, F., Hou, Z. Y. (2011). A change from phalanx to guerrilla growth form is an effective strategy to acclimate to sedimentation in a wetland sedge species Carex brevicuspis ( Cyperaceae ). Flora 206, 347–350. doi: 10.1016/j.flora.2010.07.006
Chen, Y. H., Han, W. X., Tang, L. Y., Tang, Z. Y., Fang, J. Y. (2013). Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 36, 178–184. doi: 10.1111/j.1600-0587.2011.06833.x
Chen, X. S., Deng, Z. ,. M., Xie, Y. H., Li, F., Li, X. (2014). Differential growth and vegetative reproduction of two co-occurring emergent macrophytes along a water table gradient. Pak. J. Bot. 46, 881–886.
Google Scholar
Chen, G. T., Tu, L. H., Peng, Y., Hu, H. L., Hu, T. X., Xu, Z. F., et al. (2016). Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 412, 441–451. doi: 10.1007/s11104-016-3074-z
Cronin, G., Lodge, D. M. (2003). Effects of light and nutrient availability on the growth, allocation, carbon/nitrogen balance, phenolic chemistry, and resistance to herbivory of two freshwater macrophytes. Oecologia 137, 32–41. doi: 10.1007/s00442-003-1315-3
Cross, A. F., Schlesinger, W. H. (2001). Biological and geochemical controls on phosphorus fractions in semiarid soils. Biogeochemistry 52, 155–172. doi: 10.2307/1469449
Emery, N. C., Ewanchuk, P. J., Bertness, M. D. (2001). Competition and salt - marsh plant zonation: stress tolerators may be dominant competitors. Ecology 82, 2471–2485. doi: 10.1890/0012-9658(2001)082[2471:CASMPZ]2.0.CO;2
Fransen, B., Kroon, H. D., Berendse, F. (2001). Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 82, 2534–2546. doi: 10.1890/0012-9658(2001)082[2534:SNHACB]2.0.CO;2
Fraser, L. H., Feinstein, L. M. (2005). Effects of mycorrhizal inoculant, N:P supply ratio, and water depth on the growth and biomass allocation of three wetland plant species. Can. J. Bot. 83, 1117–1125. doi: 10.1139/b05-084
Garbey, C., Murphy, K., Thiébaut, J. G., Muller, S. (2004). Variation in P - content in aquatic plant tissues offers an efficient tool for determining plant growth strategies along a resource gradient. Freshw. Biol. 49, 346–356. doi: 10.1111/j.1365-2427.2004.01188.x
González Mace, O., Steinauer, K., Jousset, A., Eisenhauer, N., Scheu, S. (2016). Flood - induced changes in soil microbial functions as modified by plant diversity. PLoS One 11, 1–15. doi: 10.1371/journal.pone.0166349
Güsewell, S. (2004). N:P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266. doi: 10.1111/j.1469-8137.2004.01192.x
Güsewell, S. (2005). Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct. Ecol. 19, 344–354. doi: 10.1111/j.0269-8463.2005.00967.x
Hefting, M., Clément, J. C., Dowrick, D., Cosandey, A. C., Bernal, S., Cimpian, C., et al. (2004). Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 67, 113–134. doi: 10.2307/1469781
Jing, H., Zhou, H. X., Wang, G. L., Xue, S., Liu, G. B., Duan, M. C. (2017). Nitrogen addition changes the stoichiometry and growth Rate of different organs in pinus tabuliformis seedlings. Front. Plant Sci. 8:1922. doi: 10.3389/fpls.2017.01922
Kaštovská, E., Šantrůčková, H. (2011). Comparison of uptake of different N forms by soil microorganisms and two wet - grassland plants: A pot study. Soil Biol. Biochem. 43, 1285–1291. doi: 10.1016/j.soilbio.2011.02.021
Kerkhoff, A. J., Enquist, B. J. (2006). Ecosystem allometry: the scaling of nutrient stocks and primary productivity across plant communities. Ecol. Lett. 9, 419–427. doi: 10.1111/j.1461-0248.2006.00888.x
Li, F., Qin, X. Y., Xie, Y. H., Chen, X. S., Hu, J. Y., Liu, Y. Y., et al. (2012). Physiological mechanisms for plant distribution pattern: responses to flooding and drought in three wetland plants from Dongting Lake, China. Limnology 14, 71–76. doi: 10.1007/s10201-012-0386-4
Li, W., Cao, T., Ni, L., Zhang, X., Zhu, G., Xie, P. (2013). Effects of water depth on carbon, nitrogen and phosphorus stoichiometry of five submersed macrophytes in an in situ experiment. Ecol. Eng. 61, 358–365. doi: 10.1016/j.ecoleng.2013.09.028
Li, L. P., Zerbe, S., Han, W. X., Thevs, N., Li, W. P., He, P., et al. (2014). Nitrogen and phosphorus stoichiometry of common reed ( Phragmites australis ) and its relationship to nutrient availability in northern China. Aquat. Bot. 112, 84–90. doi: 10.1016/j.aquabot.2013.08.002
Li, F., Zhu, L. L., Xie, Y. H., Jiang, L., Chen, X. S., Deng, Z. M. (2015). Colonization by fragments of the submerged macrophyte Myriophyllum spicatum under different sediment type and density conditions. Sci. Rep. 5, 1–9. doi: 10.1038/srep11821
Li, F., Zhu, L. L., Xie, Y. H., Liang, S. C., Hu, C., Chen, X. S., et al. (2016). Fragment growth performance of the invasive submerged macrophyte Myriophyllum spicatum under conditions of different water depths and sediment types. Aquat. Ecol. 50, 727–734. doi: 10.1007/s10452-016-9589-9
Li, F., Xie, Y. H., Yang, G. S., Zhu, L. L., Hu, C., Chen, X. S., et al. (2017a). Interactive influence of water level, sediment heterogeneity, and plant density on the growth performance and root characteristics of Carex brevicuspis . Limnologica 62, 111–117. doi: 10.1016/j.limno.2016.11.007
Li, F., Gao, H., Zhu, L. L., Xie, Y. H., Yang, G. S., Hu, C., et al. (2017b). Foliar nitrogen and phosphorus stoichiometry of three wetland plants distributed along an elevation gradient in Dongting Lake, China. Sci. Rep. 7, 1–9. doi: 10.1038/s41598-017-03126-9
Li, F., Yang, N., Zhu, L. L., Xie, Y. H., Yang, G. S., Hu, C., et al. (2018a). Competition and facilitation of two wetland macrophytes under different water levels and nutrient-heterogeneous conditions. Freshw. Sci. 37, 296–306. doi: 10.1086/697964
Li, F., Hu, J. Y., Xie, Y. H., Yang, G. S., Hu, C., Chen, X. S., et al. (2018b). Foliar stoichiometry of carbon, nitrogen, and phosphorus in wetland sedge Carex brevicuspis along a small-scale elevation gradient. Ecol. Indic. 92, 322–329. doi: 10.1016/j.ecolind.2017.04.059
Loladze, I., Elser, J. J. (2011). The origins of the Redfield nitrogen - to - phosphorus ratio are in a homoeostatic protein - to - rRNA ratio. Ecol. Lett. 14, 244–250. doi: 10.1111/j.1461-0248.2010.01577.x
Lovelock, C. E., Feller, I. C., Ball, M. C., Ellis, J., Sorrell, B. (2007). Testing the growth rate vs. geochemical hypothesis for latitudinal variation in plant nutrients. Ecol. Lett. 10, 1154–1163. doi: 10.1111/j.1461-0248.2007.01112.x
Lowe, B. J., Watts, R. J., Roberts, J., Robertson, A. (2010). The effect of experimental inundation and sediment deposition on the survival and growth of two herbaceous riverbank plant species. Plant Ecol. 209, 57–69. doi: 10.1007/s11258-010-9721-1
Luo, W., Xie, Y., Chen, X., Li, F., Qin, X. (2010). Competition and facilitation in three marsh plants in response to a water - level gradient. Wetlands 30, 525–530. doi: 10.1007/s13157-010-0064-4
Madsen, T. V., Cedergreen, N. (2002). Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich river. Freshw. Biol. 47, 283–291. doi: 10.1046/j.1365-2427.2002.00802.x
Madsen, J. D., Chambers, P. A., James, W. F., Koch, E. W., Westlake, D. F. (2001). The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444, 71–84. doi: 10.1023/A:1017520800568
Matzek, V., Vitousek, P. M. (2009). N:P stoichiometry and protein : RNA ratios in vascular plants: an evaluation of the growth - rate hypothesis. Ecol. Lett. 12, 765–771. doi: 10.1111/j.1461-0248.2009.01310.x
McGroddy, M. E., Daufresne, T., Hedin, L. O. (2004). Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield - type ratios. Ecology 85, 2390–2401. doi: 10.1890/03-0351
Méndez, M., Karlsson, P. S. (2005). Nutrient stoichiometry in Pinguicula vulgaris nutrient availability, plant size, and reproductive status. Ecology 86, 982–991. doi: 10.1890/04-0354
Miao, S. L., Zou, C. B. (2012). Effects of inundation on growth and nutrient allocation of six major macrophytes in the Florida Everglades. Ecol. Eng. 42, 10–18. doi: 10.1016/j.ecoleng.2012.01.009
Minden, V., Kleyer, M. (2014). Internal and external regulation of plant organ stoichiometry. Plant Biol. 16, 897–907. doi: 10.1111/plb.12155
Morse, J. L., Megonigal, J. P., Walbridge, M. R. (2004). Sediment nutrient accumulation and nutrient availability in two tidal freshwater marshes along the Mattaponi River, Virginia, USA. Biogeochemistry 69, 175–206. doi: 10.1023/B:BIOG.0000031077.28527.a2
Niedermeier, A., Robinson, J. S. (2007). Hydrological controls on soil redox dynamics in a peat-based, restored wetland. Geoderma 137, 318–326. doi: 10.1016/j.geoderma.2006.08.027
Niklas, K. J., Owens, T., Reich, P. B., Cobb, E. D. (2005). Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 8, 636–642. doi: 10.1111/j.1461-0248.2005.00759.x
Niklas, K. J. (2006). Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Ann. Bot. 97, 155–163. doi: 10.1093/aob/mcj021
Peng, Y. H., Niklas, K. J., Sun, S. C. (2010). The relationship between relative growth rate and whole-plant C: N: P stoichiometry in plant seedlings grown under nutrient-enriched conditions. J. Plant Ecol. 4, 147–156. doi: 10.1093/jpe/rtq026
Peñuelas, J., Sardans, J. (2009). Ecology: Elementary factors. Nature 460, 803–804. doi: 10.1038/460803a
Recha, J. W., Lehmann, J., Walter, M. T., Pell, A., Verchot, L., Johnson, M. (2013). Stream water nutrient and organic carbon exports from tropical headwater catchments at a soil degradation gradient. Nutr. Cycl. Agroecos. 95, 145–158. doi: 10.1007/s10705-013-9554-0
Reich, P. B., Oleksyn, J., Tilman, G. D. (2004). Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. U. S. A. 101, 11001–11006. doi: 10.1073/pnas.0403588101
Saaltink, R. M., Dekker, S. C., Griffioen, J., Wassen, M. J. (2018). Vegetation growth and sediment dynamics in a created freshwater wetland. Ecol. Eng. 111, 11–21. doi: 10.1016/j.ecoleng.2017.11.020
Sardans, J., Rivas-Ubach, A., Penuelas, J. (2012). The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect. Plant Ecol. 14, 33–47. doi: 10.1016/j.ppees.2011.08.002
Schreeg, L. A., Santiago, L. S., Wright, S. J., Turner, B. L. (2014). Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 95, 2062–2068. doi: 10.1890/13-1671.1
Sorrell, B. K., Mendelssohn, I. A., Mckee, K. L., Woods, R. A. (2000). Ecophysiology of wetland plant roots: a modelling comparison of aeration in relation to species distribution. Ann. Bot. 86, 675–685. doi: 10.1006/anbo.2000.1173
Steinman, A. D., Ogdahl, M. E., Weinert, M., Thompson, K., Cooper, M. J., Uzarski, D. G. (2012). Water level fluctuation and sediment - water nutrient exchange in Great Lakes coastal wetlands. J. Great Lakes Res. 38, 766–775. doi: 10.1016/j.jglr.2012.09.020
Sterner, R. W., Elser, J. J. (2002). Ecological stoichiometry: the biology of elements from molecules to the biosphere (Princeton and Oxford: Princeton University Press).
Su, H. J., Wu, Y., Xie, P., Chen, J., Cao, T., Xia, W. L. (2016). Effects of taxonomy, sediment, and water column on C:N:P stoichiometry of submerged macrophytes in Yangtze floodplain shallow lakes, China. Environ. Sci. Pollut. R. 23, 22577–22585. doi: 10.1007/s11356-016-7435-1
Wang, W. Q., Wang, C., Sardans, J., Tong, C., Jia, R. X., Zeng, C. S., et al. (2015a). Flood regime affects soil stoichiometry and the distribution of the invasive plants in subtropical estuarine wetlands in China. Catena 128, 144–154. doi: 10.1016/j.catena.2015.01.017
Wang, W. Q., Sardans, J., Wang, C., Zeng, C. S., Tong, C., Asensio, D., et al. (2015b). Ecological stoichiometry of C, N, and P of invasive Phragmites australis and native Cyperus malaccensis species in the Minjiang River tidal estuarine wetlands of China. Plant Ecol. 216, 809–822. doi: 10.1007/11258-015-0469-5
Wang, A., Wang, X., Tognetti, R., Lei, J., P. Pan, H. L., Liu, X. L., et al. (2018). Elevation alters carbon and nutrient concentrations and stoichiometry in Quercus aquifolioides in southwestern China. Sci. Total Environ. 622–623, 1463–1475. doi: 10.1016/j.scitotenv.2017.12.070
Wheeler, B. D. (1999). “Water and plants in freshwater wetlands,” in Eco-hydrology: Plants and water in terrestrial and aquatic environments (London: Routledge).
Willby, N. J., Pulford, I. D., Flowers, T. H. (2001). Tissue nutrient signatures predict herbaceous-wetland community responses to nutrient availability. New Phytol. 152, 463–481. doi: 10.1046/j.0028-646X.2001.00274.x
Wright, I. J., Reich, P. B., Cornelissen, J. H. C., Falster, D. S., Garnier, E., Hikosaka, K., et al. (2005). Assessing the generality of global leaf trait relationships. New Phytol. 166, 485–496. doi: 10.1111/j.1469-8137.2005.01349.x
Xie, Y. H., Deng, W., Wang, J. D. (2007a). Growth and root distribution of Vallisneria natans in heterogeneous sediment environments. Aquat. Bot. 86, 9–13. doi: 10.1016/j.aquabot.2006.08.002
Xie, Y. H., Luo, W. B., Ren, B., Li, F. (2007b). Morphological and physiological responses to sediment type and light availability in roots of the submerged plant Myriophyllum spicatum . Ann. Bot. 100, 1517–1523. doi: 10.1093/aob/mcm236
Xie, Y. H., Ren, B., Li, F. (2009). Increased nutrient supply facilitates acclimation to high-water level in the marsh plant Deyeuxia angustifolia : The response of root morphology. Aquat. Bot. 91, 1–5. doi: 10.1016/j.aquabot.2008.12.004
Yan, X., Yu, D., Li, Y. K. (2006). The effects of elevated CO 2 on clonal growth and nutrient content of submerge plant Vallisneria spinulosa . Chemosphere 62, 595–601. doi: 10.1016/j.chemosphere.2005.06.018
Yu, Q., Wu, H. H., He, N. P., Lu, X. T., Wang, Z. P., Elser, J. J., et al. (2012). Testing the growth rate hypothesis in vascular plants with above- and below-ground biomass. PLoS One 7, 1–9. doi: 10.1371/journal.pone.0032162
Yuan, G. X., Cao, T., Fu, H., Ni, L. Y., Zhang, X. L., Li, W., et al. (2013). Linking carbon and nitrogen metabolism to depth distribution of submersed macrophytes using high ammonium dosing tests and a lake survey. Freshw. Biol. 58, 2532–2540. doi: 10.1111/fwb.12230
Yuan, G., Fu, H., Zhong, J., Lou, Q., Ni, L., Cao, T. (2016). Growth and C/N metabolism of three submersed macrophytes in response to water depths. Environ. Exp. Bot. 122, 94–99. doi: 10.1016/j.envexpbot.2015.09.009
Zeng, Q. C., Lal, R., Chen, Y. N., An, S. S. (2017). Soil, leaf and root ecological stoichiometry of Caragana korshinskii on the loess plateau of China in relation to plantation age. PLoS One 12, 1–12. doi: 10.1371/journal.pone.0168890
Keywords: water level, sediment type, growth rate hypothesis, plant stoichiometry, Carex brevicuspis , Polygonum hydropiper
Citation: Hu C, Li F, Yang N, Xie Y-h, Chen X-s and Deng Z-m (2020) Testing the Growth Rate Hypothesis in Two Wetland Macrophytes Under Different Water Level and Sediment Type Conditions. Front. Plant Sci. 11:1191. doi: 10.3389/fpls.2020.01191
Received: 23 March 2020; Accepted: 22 July 2020; Published: 05 August 2020.
Reviewed by:
Copyright © 2020 Hu, Li, Yang, Xie, Chen and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Yang, [email protected] ; Yong-hong Xie, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 June 2024
Nutrient levels control root growth responses to high ambient temperature in plants
- Sanghwa Lee ORCID: orcid.org/0000-0002-6032-2525 1 ,
- Julia Showalter 1 ,
- Ling Zhang 1 ,
- Gaëlle Cassin-Ross ORCID: orcid.org/0000-0001-7862-3281 2 ,
- Hatem Rouached ORCID: orcid.org/0000-0001-6243-1488 2 &
- Wolfgang Busch ORCID: orcid.org/0000-0003-2042-7290 1
Nature Communications volume 15 , Article number: 4689 ( 2024 ) Cite this article
1049 Accesses
97 Altmetric
Metrics details
- Plant molecular biology
- Plant morphogenesis
Global warming will lead to significantly increased temperatures on earth. Plants respond to high ambient temperature with altered developmental and growth programs, termed thermomorphogenesis. Here we show that thermomorphogenesis is conserved in Arabidopsis, soybean, and rice and that it is linked to a decrease in the levels of the two macronutrients nitrogen and phosphorus. We also find that low external levels of these nutrients abolish root growth responses to high ambient temperature. We show that in Arabidopsis, this suppression is due to the function of the transcription factor ELONGATED HYPOCOTYL 5 ( HY5 ) and its transcriptional regulation of the transceptor NITRATE TRANSPORTER 1.1 ( NRT1.1 ). Soybean and Rice homologs of these genes are expressed consistently with a conserved role in regulating temperature responses in a nitrogen and phosphorus level dependent manner. Overall, our data show that root thermomorphogenesis is a conserved feature in species of the two major groups of angiosperms, monocots and dicots, that it leads to a reduction of nutrient levels in the plant, and that it is dependent on environmental nitrogen and phosphorus supply, a regulatory process mediated by the HY5-NRT1.1 module.
Similar content being viewed by others
Spatial regulation of thermomorphogenesis by HY5 and PIF4 in Arabidopsis

Root-derived GA12 contributes to temperature-induced shoot growth in Arabidopsis
Root architecture and hydraulics converge for acclimation to changing water availability
Introduction.
The recent rise in global temperature is largely due to human activities and is predicted to continue. Most optimistic scenarios predict a 1.5 °C increase of global average temperatures by mid-century, while the middle of the road scenarios predict 2.7 °C increase by the end of the century 1 . Temperature profoundly affects biological systems due to its effect on the free energy for biochemical reactions according to the basic principles of thermodynamics 2 , 3 . Plants do not regulate their internal temperature and due to their sessile nature, they are very sensitive to climate change 4 . Plants respond to high ambient temperature with a developmental program termed thermomorphogenesis. The hall-mark phenotypes of thermomorphogenesis are elongated tissues including hypocotyl, petiole and root, hyponastic growth, stomatal development, and early flowering 5 , 6 , 7 , 8 .
There are two transcription factors serving as central hub in thermomorphogenesis, PHYTOCHROME INTERACTING FACTOR 4 (PIF4) and ELONGATED HYPOCOTYL 5 (HY5) both of which were originally identified as light signaling components 6 , 9 , 10 , 11 . PIF4 has a major role in the thermomorphogenesis of the shoot, which also involves other PIFs such as PIF1, 3, 5, and 7 10 , 12 , 13 , 14 , 15 , 16 . HY5 plays a major role in root thermomorphogenesis, which regulates primary root length at the early seedling stage 5 , 6 .
Another key factor for growth and development is nutrient availability. There is a strong interaction of nutrient availability and temperature for determining growth 17 . Conversely, nutrient content is affected by elevated temperature and increased CO 2 levels 18 , 19 , 20 . Nitrogen (N), Phosphorus (P), and Potassium (K) are three macronutrients which are commonly used for agricultural fertilizer and to enhance growth. Due to the importance of macronutrient uptake, nutrient signaling has been widely studied. One of the most well-studied genes in nitrogen uptake is NITRATE TRANSPORTER 1.1 ( NRT1.1 ), which encodes for a dual-affinity nitrate transporter 21 and nitrate sensor 22 , 23 , 24 , 25 . Furthermore, OsNRT1.1B , which is a functional homolog of AtNRT1.1 in rice, has been showed to integrate N and P signaling 26 , suggesting that the role of NRT1.1 as a master regulator of N and P might be conserved across the plant kingdom.
Here, we show that shoot and root thermomorphogenesis are conserved among Arabidopsis ( Arabidopsis thaliana ), soybean ( Glycine max ), and rice ( Oryza sativa ). We find that this is linked to decreased N and P levels in plant tissues at higher temperatures. Conversely, low levels of N and P in the growth medium abolished thermomorphogenesis in Arabidopsis. We found that a module constituted by the thermomorphogenesis key regulator HY5 and the nitrogen transceptor NRT1.1 is in involved in this regulation process.
Plants show conserved shoot and root thermomorphogenesis that goes along with decreased N and P levels in plant tissues
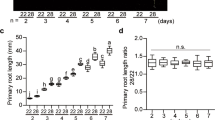
Root thermomorphogenesis studies have been largely restricted to Arabidopsis. We therefore wanted to compare this to the response in other species. For this, we grew Arabidopsis, rice, and soybean seedlings at ambient and elevated temperatures. For Arabidopsis, Col-0 wild-type seedlings were grown at either 21 °C or 28 °C for 5 days after 4 days of germination at 21°C (Fig. 1a, b ). Soybean (Williams 82 variety) and rice (Kitaake ecotype) were grown at either 28 °C or 33 °C for 1 or 2 weeks for soybean and rice, respectively, after 7 days of germination at 28 °C (Fig. 1c-f ). Similar to the reported Arabidopsis shoot and root thermomorphogenesis 6 , 10 (Fig. 1 a, b ), both soybean and rice showed longer shoots and roots at higher temperatures, indicating that plants have a conserved elongation mechanism at higher temperatures.

a – f Phenotypes of Arabidopsis (Col-0; a , b ), soybean (Williams82; c , d ), and rice (Kitaake; e , f ) at normal and higher temperatures. Arabidopsis seedlings were grown for 4 days on 1/2 ms plates at 21 °C and then kept either at 21 °C or 28 °C for 5 additional days. Rice and soybean seedlings were grown for 1 week at 28 °C and then either kept at 28 °C or 33 °C for additional 2 weeks for rice and 1 week for soybean, respectively. Scatter dot plot shows average difference in primary root length for Arabidopsis, and total root length for soybean and rice, and the number of plants. p-Value from one-sided Student’s t test. g, h Nitrogen ( g ) and C/N ratio ( h ) in Arabidopsis shoots (Col-0, hy5-215 , and pifQ ), soybean shoots, and rice shoots using CN analysis. i Phosphorus in Arabidopsis shoots, Soybean shoots, and rice shoots using MP-AES. For ( g–i ), n = 3 biologically independent samples were used. p -Values for the corresponding GxE interactions determined through ANOVA are shown on top of each graph. Asterisks indicate statistically significant difference either 2-way ANOVA or one-sided Student’s t test; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. Average fold difference of each group is indicated in the top region of the plot. Shoot parts from 4-week-old plants from Arabidopsis, soybean, and rice plants were used for the nutrient analyses. Plots indicate mean (horizontal line) and standard deviation (error bars).
Previous studies had exposed temperature-dependent gene expression changes of gene clusters related to nitrogen and other nutrient-related processes 5 , 6 (Supplementary Data 1 ) Furthermore, HY5 has been found to regulate nitrogen 27 , 28 and iron signaling 29 , 30 . We therefore hypothesized that nutrient composition or uptake could be changed at higher temperature. To test this hypothesis, we analyzed the nutrient composition of shoots of 4 week-grown plants of Arabidopsis, rice, and soybean (Fig. 1g–i , Supplementary Fig. 1 ). We also included Arabidopsis hy5-215 11 and pifQ 31 mutants in this analysis. Interestingly, levels of N and P were decreased in Arabidopsis, soybean, and rice at higher temperatures (Fig. 1g–i ), while other nutrients showed less consistent patterns across the species at higher temperatures (Supplementary Fig. 1 ). As previous transcriptome experiments in hy5 mutants had shown nitrogen related processes to be altered 5 , 6 (Supplementary Data 1 ), we hypothesized that the observed temperature-dependent changes in N-levels were mediated by HY5 . However, N-levels in the hy5-215 mutant were not statistically different from Col-0 (Fig. 1g ). This might suggest that while HY5 is involved in transcriptional changes of genes involved in nitrogen-related processes, it isn’t required for the changes in N levels that are observed at high ambient temperature. Because of the prominence of P-level changes that we had observed at higher ambient temperatures, we also measured P in these genotypes. In contrast to N, the hy5-215 mutant plants showed a significant, opposite change of P level changes in response to higher ambient temperature compared to the WT (Fig. 1i ). pifQ was similar to Col-0 at high ambient temperature (Fig. 1g–i ) indicating that PIFs are not required for this. Overall, our results showed that HY5 is involved in the alteration of P levels of mature plant shoots that were observed at high ambient temperatures. As HY5’s impact in thermomorphogenesis can be observed in young seedlings, we wanted to investigate whether similar patterns of nutrient changes among genotypes and temperatures were detectable in the roots of young seedlings. For this, we measured nutrient contents of 9-day-old seedling roots from Arabidopsis, soybean, and rice. However, in these samples we didn’t detect the same trends as observed in older plant roots (Supplementary Fig 2 ). For example, we observed decreased N-levels and increased P-levels in rice, while Arabidopsis and soybean did not show any differences at higher temperature. These data suggest that changes of nutrients accumulate over time in plants and then cumulatively affect nutrient contents in the shoot part of more mature plants. Alternatively, it is also possible that this is a time-dependent regulatory process that starts later than 9 days after germination. Taken together our data show that the levels of N and P in plants are regulated in response to elevated temperature, and that in Arabidopsis HY5 is required for the regulation of temperature-dependent P level changes.
HY5 integrates temperature and N–P signaling and directly represses NRT1.1 transcription
Since we found HY5 to be involved in temperature-dependent P-level changes in Arabidopsis and at least at the transcriptional level affected genes involved in nitrogen-related processes at high ambient temperature, we searched for a target downstream of HY5 that could explain its function. HY5 is a bZIP protein transcription factor, which binds to several DNA sequence motifs including G-box (CACGTG) and CACGT motifs 32 , 33 . Published ChIP-seq data showed that HY5 binds to the promoter region of genes that are involved in nutrient-related responses such as those that we had identified using our RNAseq to be related to nitrogen and organic acids 6 , 32 , 33 ; Supplementary Data 1 ). We identified genes that were bound by HY5 according to the ChIP-seq data 32 and that are in the N–P signaling pathway. These genes included N signaling pathway genes such as NIGT1.1, HHO2, NLP7, NRT1.1, NRT1.5 , NRT2.1 , NIA1 , and LBD37 23 , 34 , 35 , 36 , 37 , 38 , 39 . Interestingly, promoter regions of P signaling pathway genes including PHO1, PHT1;8 , and IPS1 did not show high enrichment in the HY5 ChIP-seq data (Supplementary Fig. 3 ). To examine whether HY5 directly binds to the promoter of the genes from the ChIP-seq analysis in our growth conditions (the published ChIP-seq data were obtained under different light conditions), we performed Chromatin Immunoprecipitation qPCR (ChIP-qPCR) using 4 days 21 °C grown pHY5:HY5-GFP whole seedlings with an additional 5 days growth at either 21 °C or 28 °C (Fig. 2a, b , supplementary Fig. 4a, b ). Interestingly, we detected enrichment at high ambient temperature for only a subset of target promoters, including NIGT1.1, HHO2, NLP7 , and NRT1.1 . This indicates that HY5 directly binds to N signaling genes, with NRT1.1 also being involved in the integration of N and P signaling 26 , 40 . This might indicate that HY5 might directly regulate expression in a temperature dependent manner and thereby exert influence on N–P signaling. To test whether the transcription levels of those genes are altered at high ambient temperature, we performed qPCR of root and shoot tissues (Fig. 2c , supplementary Fig. 4c ). Consistent with our ChIP-qPCR data, temperature-dependent HY5 enriched target genes such as NIGT1.1, HHO2, NLP7 , and NRT1.1 were significantly downregulated while the genes for which we hadn’t found ChIP-qPCR enrichment, such as NRT1.5 , NRT2.1, NIA1, and LBD37 were not altered at high ambient temperatures. Among the genes that were bound by HY5 and downregulated, NRT1.1 stood out as its transcript levels were strongly downregulated at high ambient temperature in the roots of Col-0, but this change of its expression level was abolished in the roots of hy5-215 mutant plants. This was different in the shoot, as downregulation of NRT1.1 upon high ambient temperatures and its dependency on HY5 was much less pronounced there. Overall, this suggested that the transcript level of NRT1.1 is tightly and directly regulated by HY5 in the root in a temperature-dependent manner. To further test whether HY5 directly binds to the promoter region of NRT1.1 , we performed an Electrophoretic Mobility Shift Assay (EMSA) (Fig. 2d ). Consistent with our hypothesis that HY5 binds to the NRT1.1 promoter region, HY5 was able to bind to the G-box motif in the NRT1.1 promoter region. Furthermore, we tested whether HY5 acts as a transcription repressor of NRT1.1 transcription by using a dual luciferase assay in Nicotiana benthamiana (Fig. 2e ). Consistent with our hypothesis that HY5 directly binds to the NRT1.1 promoter and represses its transcription, transcription of the reporter was reduced in the presence of HY5, suggesting that HY5 represses NRT1.1 transcription through direct binding to its promoter region. Taken together, our data strongly suggest that HY5 directly binds to the promoter region of NRT1.1 and represses its transcription at high ambient temperature in the root. However, as we performed ChIP-qPCR in whole seedlings, EMSA in vitro, and the dual luciferase assay in Nicotiana benthamiana , it can’t be fully excluded that the direct binding of HY5 to the NRT1.1 promoter, for some unknown reason (even through HY5 and NRT1.1 are expressed in the root), might not occur in the root.

a IGV image of HY5 ChIP-seq data from Burko et al. 32 of selected N–P signaling genes with transcription direction and binding motif. b Scatter dot plot of ChIP-qPCR results at normal and high ambient temperature of promoter regions of five different genes. p -Values for the two-sided Student’s t test. c Scatter dot plot of qPCR results at normal and high ambient temperature of four different genes using Col-0 and hy5-215 shoot and root samples of seedlings. Relative transcript level was normalized using PP2A as a control and to the expression levels in the shoot. For ( b and c ), n = 3 biologically independent samples were used. p-Values for the corresponding GxE interactions determined through ANOVA are shown on top of each graph. Asterisks indicate statistically significant difference either 2-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. d Electrophoretic Mobility Shift Assay (EMSA) showing GST-HY5 binds to G-box motif of NRT1.1 promoter region. G-box motif containing nucleotides were biotin labeled. Competitor is the same sequence but biotin unlabeled. mCompetitor is mutated version of G-box motif (CACATG to CCCATG) without biotin label. Two independent experiments were repeated with similar results. e dual luciferase assay using Nicotiana benthamiana . (upper) Effector and reporter constructs are described. (lower) Relative FLUC/RLUC showing HY5 as a transcriptional repressor. n = 12 biologically independent samples were used. p -Value from two-sided Student’s t test. Plots from ( b , c , and e ) indicate mean (horizontal line) and standard deviation (error bars).
In contrast to NRT1.1, further investigation of other candidate genes did not provide strong support of their direct regulation by HY5. While the transcript level of NIA1 was altered in hy5-215 mutant, there was no indication of a change in HY5 binding at high ambient temperature according to our ChIP-qPCR (Supplementary Fig. 4a–c ), suggesting that NIA1 transcript level is altered indirectly. Furthermore, the transcript level of NRT1.5 decreased at high ambient temperature both in Col-0 and hy5-215 mutant (Supplementary Fig. 4a-c ), indicating that other components might be responsible for regulating NRT1.5 transcript levels at high ambient temperature. Taken together, our data suggest that HY5 regulates root thermomophogenesis, transcriptional programs relating to nitrogen, and phosphate levels by repressing the N–P signaling genes and by directly regulating key genes such as NRT1.1 .
Root thermomorphogenesis depends on external N–P levels and phosphorylation but shoot to root mobility of HY5 is not required for this
Because HY5 is involved in the regulation of genes that play a role in nitrogen and nutrient-related processes and is required for the appropriate P level changes in response to high ambient temperature (Supplementary Data 1 , Fig. 1g–i ), we hypothesized that HY5 levels might be affected by N–P deficient conditions at high ambient temperature. To test this hypothesis, Col-0 plants were grown for 5 days at either 21 °C or 28 °C after 4 days for germination in 21 °C in three different media conditions: 1/2MS (N:11400 μM, P: 625 μM), mildly nitrogen deficient (N: 550 μM), and mildly phosphorus deficient (P: 100 μM) 41 . HY5 transcript levels only changed in ½ MS medium in response to high ambient temperature, but not in -N or -P conditions (Fig. 3a ). Similar to a short time of exposure to high ambient temperature for 4 h 6 , HY5 protein levels were elevated even after a long time of high-temperature exposure for 5 days in nutrient sufficient ½ MS media. Consistent with the transcript levels, HY5 protein levels did not change in N or P deficient media under high temperature (Fig. 3b ).

a , b HY5 transcript ( a ) and protein ( b ) level grown on different media at control and high ambient temperature. Root samples were analyzed separately. For ( a ), n = 3 biologically independent samples were used. Native HY5 antibody was used for Western blot. Red number indicates the relative signal intensity divided by HY5 signal to Tubulin. c Phenotypes of Col-0, hy5-215 , and 3 different forms or HY5 overexpression lines (WT, A: phospho-dead, D: phospho-mimic) in hy5-215 grown on different media at control and high ambient temperatures. d Scatter dot plot of ( c ). e Phenotypes of excised roots grown on different media at control and high ambient temperature with the number of plants indicated. Average fold difference of each group is indicated in the top region of the plot. Scatter dot plots indicate mean (horizontal line) and standard deviation (error bars). p -Values for the corresponding GxE interactions determined through ANOVA are shown on top of each graph. Asterisks indicate statistically significant difference either 2-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
We then set out to test whether the altered HY5 level affected root thermomorphogenesis in these three different conditions. As it had been shown that SPA mediated HY5 phosphorylation is crucial for root thermomorphogenesis 6 , we not only utilized the hy5-215 mutant line but also three transgenic overexpression lines in the hy5-215 mutant background: 35 S:HY5-GFP, 35 S:HY5 S36A-GFP , and 35 S:HY5 S36D-GFP . In wildtype, root thermomorphogenesis was observed only in ½ MS grown seedlings, which displayed increased HY5 protein levels at high ambient temperature (Fig. 3a, b ). Grown on N or P deficient media, wildtype seedlings did not show a longer primary root at high ambient temperature. This was similar to the response of the hy5-215 mutant, in which there is no HY5 protein produced and that didn’t even display increased root length in nutrient sufficient conditions (Fig. 3b, c ). HY5 plants overexpressing HY5 or a HY5 phospho-mimic S36D version could not only complement the hy5-215 mutant phenotype in nutrient sufficient conditions, but in addition led to thermomorphogenesis in -N, and -P media. In contrast, overexpression of the phospho-dead S36A HY5 version did not complement the hy5-215 mutant phenotype in any of the conditions. This shows that phosphorylation of HY5 is necessary for HY5 activation and stabilization during thermomorphogenesis and HY5 phosphorylation is sufficient for eliciting thermomorphogenesis under otherwise restrictive condition such as low environmental levels of N or P. It also might suggest that SPA dependent phosphorylation is involved in this. Overall, these data suggest that phosphorylation of HY5 is essential for temperature mediated primary root elongation and that the lack of temperature dependent root growth response in N or P deficient medium is due to the lack of increased HY5 levels.
We then tested the effect of excessive amount of N or P on root thermomorphogenesis. For this, Col-0 seedlings were grown in N or P excessive media at high ambient temperature (Supplementary Fig. 5 ). Col-0 seedlings did not display exaggerated root thermomorphogenesis in N or P excessive media at high ambient temperature when comparing it to 1/2MS conditions, indicating that excessive amounts of N or P do not affect root elongation at high ambient temperature. Taken together, these data suggest that sufficient amounts of N and P are required for HY5 mediated root thermomorphogenesis.
While it is not yet fully resolved whether HY5 movement from shoot to root is always necessary for root thermomorphogenesis, we tested this with regards to the inhibition of root thermomorphogenesis by low N and P levels. For this, we excised the roots of 4-day-old Col-0 and hy5-215 seedlings and grew them for additional 5 days in N or P deficient media at high ambient temperature (Fig. 3e ). Excised Col-0 roots were able to respond to high ambient temperature in ½ MS but not in N or P deficient media. Excised hy5-215 roots were not able to respond to the elevated temperature in any of the conditions. Overall, this is supportive of a major root autonomous function of HY5 in root thermomorphogenesis and its alteration by low N and P levels.
NRT1.1 integrates N and P dependent root thermomorphogenesis
NRT1.1 has been shown to be a key component in nitrate signaling and is regulated by nitrate and phosphate level, as well as by auxin in root development 23 , 40 , 42 . NRT1.1 protein level is destabilized during P starvation in Arabidopsis 40 and the OsNRT1.1B-OsSPX4 module has been identified to integrate nitrate and phosphate signaling in rice 26 . As NRT1.1 transcript levels were decreased at high ambient temperature and were tightly regulated by HY5 in a root specific manner via its direct transcriptional suppression (Fig. 2c-f ), we performed qPCR using roots of Col-0 and a HY5 overexpressing line ( 35 S:HY5-GFP / hy5-215 ) grown on nutrient sufficient or deficient media (Fig. 4a ). These plants were grown at either 21 °C or 28 °C for 5 days after they had germinated and grown for 4 days at 21 °C. Seedlings grown on nutrient sufficient medium displayed significantly decreased transcript levels of NRT1.1 at high ambient temperatures. Seedlings grown on N or P deficient media showed a decreased level of NRT1.1 transcript at 21 °C (albeit not as low as those grown on at 28 °C and nutrient sufficient medium) and this was not further decreased in high ambient temperature. This might suggest that sufficient amounts of N and P are necessary for the high level of NRT1.1 transcripts at 21 °C and that nutrient deficiency and high ambient temperature independently reduces NRT1.1 transcript levels to the lowest level. Furthermore, the HY5 overexpression line showed decreased transcript levels of NRT1.1 in all tested nutrient conditions (½ MS, -N, and -P media) at high ambient temperature, which is in line with the thermomorphogenesis phenotypes in these plants (Fig. 3b , c). Overall, these results indicate that while NRT1.1 transcript levels might be independent from HY5 at 21 °C, HY5 is able to repress NRT1.1 transcription at high ambient temperature to trigger root thermomorphogenesis.

a Scatter dot plot of qPCR results at normal and high ambient temperature grown Col-0 and 35S:HY5-GFP / hy5-215 seedlings grown on different media (1/2MS, -N, -P). Only root samples were used for the analysis. The relative transcript level of NRT1.1 was normalized by the expression levels of PP2A and to the expression levels in the shoot. For ( a ), n = 3 biologically independent samples were used. b Confocal microscopy images of pNRT1.1:GFP transgenic line at normal and high ambient temperature grown on different media (1/2MS, -N, -P). c Scatter dot plot of the signals quantified from confocal microscopy images of ( b ). Quantification of the signal intensity. d Western blot analysis of Col-0 and 35S:HY5-GFP / hy5-215 seedling roots using native NRT1.1 antibody. Red number indicates the relative signal intensity divided by NRT1.1 signal to Tubulin. Two independent experiments were repeated with similar results. e Confocal microscopy images of pNRT1.1:NRT1.1-GFP transgenic line at normal and high ambient temperature grown on different media (1/2 ms, -N, -P). Scale bar indicates 50 μm. f Scatter dot plot of the signals quantified from confocal microscopy images of ( e ). Media concentrations includes: 1/2MS (N:11400 μM, P: 625 μM), mildly nitrogen deficient (N: 550 μM, P: 625 μM), and mildly phosphorus deficient (N:11400 μM, P: 100 μM). Scatter dot plots indicate mean (horizontal line) and standard deviation (error bars). For ( c and f ), n = 5 biologically independent samples were used. p -Values for the corresponding ExE interactions determined through ANOVA are shown on top of each graph. Asterisks indicate statistically significant difference either 2-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
To investigate these transcriptional changes in higher detail, we analyzed seedlings of the pNRT1.1:GFP reporter line 43 grown at 21 °C or 28 °C, to assess tissue specific expression changes in different nutrient conditions using confocal microscopy (Fig. 4b, c ). Reflecting the data from the qPCR, the pNRT1.1:GFP signal was decreased in the root apex at high ambient temperature in roots grown on nutrient sufficient medium. The GFP signal was much lower in roots grown on N or P deficient media at both temperatures (Fig. 4b, c ). Overall, these data suggest that sufficient amounts of N and P are necessary for the suppression of NRT1.1 transcript level at high ambient temperature.
As NRT1.1 transcript level is altered in different media and temperature conditions (Fig. 4a–c ), we measured total root protein levels of NRT1.1 using a native NRT1.1 antibody (Fig. 4d ). Similar to the transcriptional response, NRT1.1 protein levels decreased at high ambient temperature under nutrient sufficient conditions (Fig. 2c , Fig. 4a ). This indicates that both transcription and protein level of NRT1.1 are decreased at high ambient temperature. Importantly, the decrease of NRT1.1 protein level observed at high ambient temperature, was less pronounced in -N and -P media (Fig. 4a, d ). The HY5 overexpression line showed strongly decreased NRT1.1 protein levels in all nutrient conditions (½ MS, -N, and -P media) at high ambient temperature (Fig. 4d ) showing that transcriptional downregulation strongly affects NRT1.1 protein levels at high ambient temperature. To assess whether protein degradation also contributes to the observed decrease of NRT1.1 protein level at high ambient temperatures, we treated the samples with cycloheximide (CHX) and ES9-17 for 4 h, which are protein synthesis and clathrin-mediated endocytosis inhibitors, respectively (Supplementary Fig. 6 ). We reasoned that any NRT1.1 level change that is observed between CHX treatment and concomitant treatment with CHX and ES9-17 would be indicate of inhibiting NRT1.1 degradation (through inhibiting clathrin-mediated endocytosis). Concomitant treatment with CHX and ES9-17 resulted in increased levels of NRT1.1 in all treatments when compared to CHX treatments alone, indicating that the observed change of NRT1.1 protein levels at high ambient temperature and in -N and -P conditions are at least partly due to post-translational regulation and degradation. To further corroborate NRT1.1 regulation at the protein level, we examined it in the root apex at cellular resolution. For this, we utilized pNRT1.1:NRT1.1-GFP transgenic plants 23 (Fig. 4e, f ). Consistent with the data from the western blots, NRT1.1 protein level was decreased in the root apex in ½ MS media at high ambient temperature, while N or P deficient media grown seedlings did not show decreased NRT1.1-GFP signal in the root tip (Fig. 4e, f ). Overall, the data suggest that changes in NRT1.1 transcript and protein level might be necessary for root thermomorphogenesis.
The HY5 - NRT1.1 regulatory module is required for the interaction of root thermomorphogenesis and P level
As NRT1.1 transcript level was tightly regulated by HY5 in the root at high ambient temperatures (Fig. 2c–f ), we examined if there is a HY5 feedback loop from NRT1.1 on HY5 . HY5 transcript levels showed similar patterns in the NRT1.1 loss of function mutant line ( chl1-5) compared to Col-0 in the root, where HY5 transcript level is induced at high ambient temperature, and a slightly increased response to high temperature in the shoot (Fig. 5a ). This indicated that there is no significant feedback from NRT1.1 on the HY5 transcript level in the root. To test this at the protein level we performed western blots (Fig. 5b ). Interestingly, HY5 protein level was more accumulated in chl1-5 mutant roots in all our conditions, suggesting that NRT1.1 might regulate HY5 protein stability directly or indirectly via unknown mechanisms. Furthermore, NRT1.1 protein accumulated to a higher extent in hy5-215 mutant roots at high ambient temperature, which was consistent with its transcript level (Fig. 2c ), indicating that HY5 has an important role to suppress NRT1.1 transcript level and thus regulates NRT1.1 protein level at high ambient temperature.

a qPCR results of HY5 transcript level at normal and high ambient temperature using Col-0 and chl1-5 seedlings with separated samples of shoot and root. Relative transcript level was normalized using PP2A as a control and to the expression levels in the shoot. Shoot and root samples were analyzed separately. n = 3 biologically independent samples were used. b Western blot analyses of NRT1.1 and HY5 using Col-0, hy5-215 , and chl1-5 root. Red number indicates the relative signal intensity divided by NRT1.1 or HY5 signal to Tubulin. c Phenotypic analyses of Col-0, hy5-215 , chl1-5 , hy5-215 chl1-5 double mutant at high ambient temperature. d – g Scatter dot plot of phenotypic analyses, hypocotyl length ( d ), root length ( e ), nitrate and nitrite composition ( f ), and phosphate composition ( g ). For nitrate/nitrite and phosphate composition analyses, seeds of each genotype were grown on soil for 2 weeks at 21 °C and transferred into either 21 °C or 28 °C for additional 2 weeks. Then the leaves were used for the analyses. For ( f and g ), n = 3 biologically independent samples were used. p -Values for the corresponding GxE interactions determined through ANOVA are shown on top of each graph. Asterisks indicate statistically significant difference either 2-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. Average fold difference of each group is indicated in the top region of the plot. Scatter dot plots indicate mean (horizontal line) and standard deviation (error bars).
The direct binding of HY5 to the NRT1.1 promoter and the transcriptional regulation of NRT1.1 expression level by HY5 (Fig. 2a–e ) had indicated that HY5 is upstream of NRT1.1. To genetically test this, we obtained mutant lines of HY5 ( hy5-215) , NRT1.1 ( chl1-5) , double mutant ( hy5-215 chl1-5 ). We then assessed hypocotyl and primary root length, as well as N–P composition at standard and high ambient temperatures (Fig. 5c–g ). As reported previously 5 , 6 , hy5-215 showed a thermo-insensitive root phenotype. The chl1-5 mutant showed an increased root elongation phenotype at high ambient temperature compared to that of Col-0, while displaying a similar pattern of shoot thermomorphogenesis (Fig. 5c, d ). Consistent with our data that HY5 represses NRT1.1 expression in a root specific manner at high ambient temperature (Fig. 2c ), hy5-215 chl1-5 double mutant plants showed similar phenotypes to hy5-215 in the shoot and chl1.5 in the root, respectively (Fig. 5c–e ). This indicates that chl1-5 and hy5-215 are additive towards hypocotyl and primary root length in response to temperature. To further support our model, we also checked whether overexpressed NRT1.1 could repress root thermomorphogenesis using a 35S:NRT1.1-MYC line 44 (supplementary Fig. 7 ). As expected, the NRT1.1 overexpression line did not show root thermomorphogenesis indicating that overexpressing NRT1.1 overrides the transcriptional repression of NRT1.1 by HY5 and thus shows a similarity to hy5-215 . To assess whether the root thermomorphogenesis phenotypes were linked to N–P level alterations at high ambient temperature, we measured nitrate/nitrite and phosphate levels in the mutants (Fig. 5f, g ). Consistent with the MP-AES results (Fig. 1g, i ), Col-0 and hy5-215 showed different patterns of P level alteration at high ambient temperature, while all the genotypes showed similar patterns of decreased N level at high ambient temperature. Interestingly, chl1-5 and hy5-215 chl1-5 double mutant plants showed a similar P pattern with hy5-215 , which indicates that the NRT1.1 alteration upon temperature is critical for P level alteration at high ambient temperature. To investigate whether the temperature dependent alteration of phosphate levels is a general part of thermomorphogenesis or tied to the HY5-NRT1.1 module, we utilized the pils6-1 mutant and the 35S:PILS6-GFP , as PILS6 is known regulator of root thermomorphogenesis 45 . Interestingly, both pils6-1 mutant and PILS6 overexpression line showed similar phosphate levels to Col-0, indicating that P level regulation is not a general part of thermomorphogenesis but is specifically regulated by the HY5-NRT1.1 module (Supplementary Fig. 8 ). Taken together, these data suggest that HY5-NRT1.1 regulatory mechanism has an important role to regulate root thermomorphogenesis and P level alteration at high ambient temperature.
The HY5 - NRT1.1 regulatory mechanism alters global gene expression at high ambient temperature
Because the transcript level of NRT1.1 was down-regulated by HY5 in a root specific manner at high ambient temperature, we performed RNAseq to examine the global gene expression pattern at high ambient temperature using Col-0, hy5-215 , and chl1-5 plants. These plants were grown at either 21 °C or 28 °C for additional 5 days after 4 days of germination at 21 °C. Consistent with our qPCR results, NRT1.1 was among the most downregulated gene in Col-0 roots when comparing 28 °C to 21 °C (Fig. 6a ).

a Volcano plot of RNAseq using Col-0 root samples. Col-0 seedlings were grown for 4 days in 21 °C and then transferred to either 21 °C or 28 °C for additional 5 days and collected. Threshold of two sided p -value is 0.05, and Log2 Fold change threshold is −1 and 1. NRT1.1 is labeled with purple dot. b Venn diagram of root Differentially Expressed Genes (DEGs) in Col-0, chl1-5 , and hy5-215 mutant at high ambient temperature. Gene Ontology (GO) analysis clusters within each group are described next to the Venn diagram. c Heatmap analysis shows that Col-0, chl1-5 , and hy5-215 mutant have different expression pattern. Representative GO analyses of each cluster are noted. Three biological repeats were performed for RNAseq analysis.
Many genes were differentially expressed in these three genotypes in response to temperature: 1512, 1761, and 1295 DEGs in Col-0, chl1-5 , and hy5-215 , respectively (Fig. 6b ). More than 83% of root Col-0 DEGs were shared between Col-0 and chl1-5 , indicating that despite the hypersensitive root thermomorphogenesis chl1-5 , the mutant mounts a very similar response. When conducting a Gene Ontology (GO) analysis of the 249 NRT1.1 dependent genes (DEGs in Col-0 but not in chl1-5 ), the significant GO-term “response to chemical” was significantly enriched. Also, almost half of the root Col-0 DEGs were HY5 dependent (755 genes out of 1512 DEGs, >49.9%). This set of genes was enriched for the GO categories related to response to nutrients such as nitrate and sucrose. We reasoned that set of thermomorphogenesis genes would be genes that were not differentially expressed in hy5-215 mutants (as there is no root growth response to temperature) but still occurs in chl1-5 (this mutant as well as the hy5-215/chl1-5 double mutant still shows a root growth response to elevated temperature). This was a set of 593 genes, and it was enriched for nutrients responses such as nitrate and sucrose, similar to the 755 DEGs that were HY5 dependent.
To conduct a more fine-grained analysis of gene expression patterns, we conducted hierarchical clustering and a subsequent GO enrichment analysis of the resulting clusters (Fig. 6c ). Cluster 1 contained genes which increased their expression at high ambient temperature for all genotypes, and these were enriched for circadian rhythm and response to heat. This might indicate that circadian rhythm and heat sensing might be less involved downstream of HY5 / NRT1.1 to affect the root thermomorphogenesis and nutrient alteration at high ambient temperature. Cluster 6 contained genes that decrease their expression at high ambient temperature in Col-0 and chl1-5 , but not in hy5-215 . This gene set was enriched for response to nitrate and inorganic substances. Cluster 7 and 8 contained genes that decrease their expression at high ambient temperature more in chl1-5 but less in hy5-215 . This gene set was enriched for suberin biosynthetic process, metabolic processes such as terpenoid or organic acid, and response to water deprivation or abscisic acid. Overall, these data suggest that the HY5-NRT1.1 regulatory mechanism controls root thermomorphogenesis and N–P alteration at high ambient temperature through the control of distinct biological processes.
The HY5-NRT1.1 regulatory mechanism might be conserved in soybean and rice at higher temperature
It was previously reported that HY5 and NRT1.1 homologs share similar functions in other species 26 , 46 , 47 . Moreover, we showed that root growth responses to higher temperatures are conserved in Arabidopsis, soybean and rice (Fig. 1c, e ). To explore whether the HY5-NRT1.1 regulatory mechanism that we had discovered to be responsible for this in Arabidopsis, is conserved in soybean and rice at higher temperatures, and potentially responsible for the root growth response to higher temperature, we analyzed homologs of HY5 and NRT1.1 in soy and rice (Fig. 7a, b , Supplementary Fig. 9 , 10 ). While soy has 4 homologs of both genes, HY5 and NRT1.1 , rice has 3 and 2 homologs of HY5 and NRT1.1 , respectively. To explore the expression pattern of these homologs in response to high temperature, we performed qPCR (Fig. 7c, d ). While all of the 4 HY5 homologs in soybean showed a higher transcript level in the root at higher temperature, in rice, only OsbZIP48 ( OsHY5L2 ) showed increased transcript levels in the root at higher temperatures. This might suggest that while all of 4 HY5 homologs in soy have a role at higher temperature, only OsbZIP48 might be involved in the response to higher temperature response in rice. Intriguingly, 2 NRT1 .1 homologs in soy showed decreased transcript levels in the root at higher temperature, and the other 2 NRT1.1 homologs in soy showed increased transcript levels in the root at higher temperatures. This might suggest that the function of these genes might have diverged or neo-functionalized with regards to their role at higher temperatures. In rice, only one of 2 NRT1.1 homologs showed a decreased transcript level in the root at higher temperature. Overall, this suggests that the role of HY5-NRT1.1 homologs in soy is more complex, whereas OsbZIP48-OsNRT1.1A might be responsible for higher temperature response in rice. We also performed Western blot analysis using native Arabidopsis NRT1.1 and HY5 antibodies in soy and rice roots (Fig. 7e ). Multiple protein bands were detected in soy and rice roots by these antibodies, suggesting that these bands were NRT1.1 and HY5 homolog proteins. Two of the HY5 protein bands in soy and rice, and one of the NRT1.1 protein bands in soy and rice showed a similar pattern to Arabidopsis: NRT1.1 decreasing at higher temperatures and HY5 accumulating at higher temperature. Taken together, our data suggest that HY5-NRT1.1 regulatory mechanism might be conserved in plants and may contribute to regulate thermomorphogenesis.
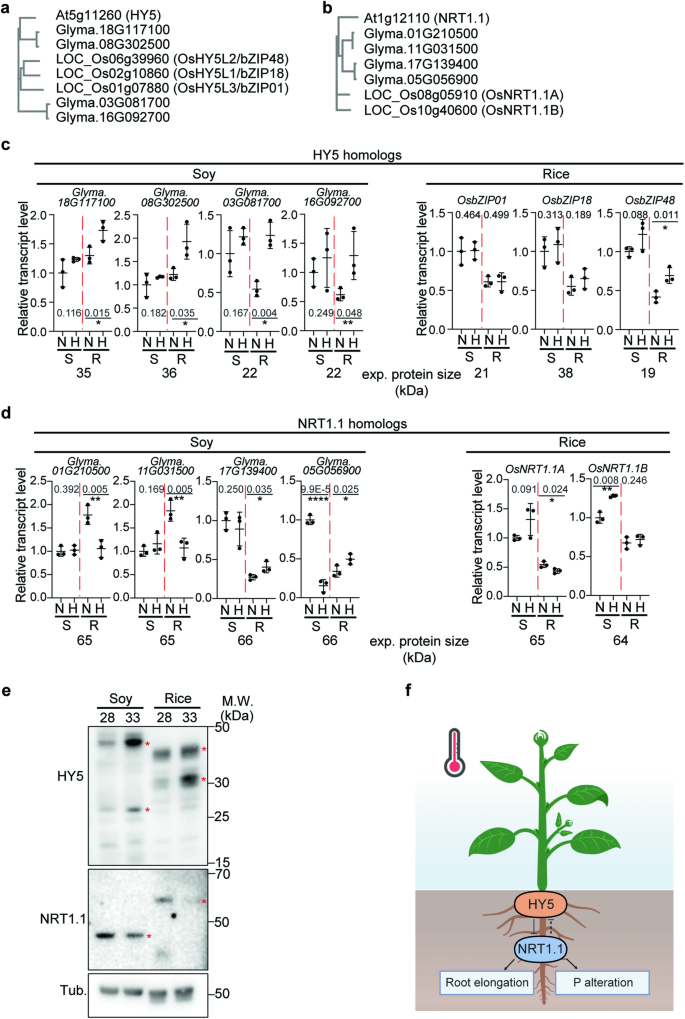
a , b Phylogram of HY5 ( a ) and NRT1.1 ( b ) homologs in Arabidopsis, soy, and rice. c , d Relative transcript level of HY5 ( c ) and NRT1.1 ( d ) homologs in soy and rice. Relative transcript level is normalized by house-keeping genes such as rice ubiquitin and soy tubulin 4 . N and H stand for Normal temperature (28 °C) and Higher temperature (33 °C), respectively. S and R stand for Shoot and Root, respectively. For ( c and d ), n = 3 biologically independent samples were used. Asterisks indicate statistically significant difference using one-sided Student’s t test; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. e Western blot analysis using native Arabidopsis NRT1.1 and HY5 antibodies. Soybean and rice root samples were used. Red asterisks are temperature dependent bands which might be potential HY5 and NRT1.1 bands in soybean and rice. Two independent experiments were repeated with similar results. f Simplified model showing root specific HY5 accumulation inhibiting NRT1.1 transcription to promote root elongation and the HY5-NRT1.1 regulatory mechanism altering N and P uptake at higher temperatures in plants. Figure 7f, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. Scatter dot plots indicate mean (horizontal line) and standard deviation (error bars).
Thermomorphogenesis has been extensively studied in Arabidopsis. We have now shown that this response is conserved in other plant species, covering the major groups of angiosperms, monocots and dicots. We have also found a conserved interaction of thermomorphogenesis and nutrient levels. This interaction is multifaceted: thermomorphogenesis affects the nitrogen and phosphate content of plants, and it is itself dependent on sufficient levels of external nitrogen and phosphate. While the dependence of thermomorphogenesis on external nitrogen and phosphate levels is clearly governed by a HY5 - NRT1.1 regulatory mechanism, only the effect of thermomorphogenesis on phosphate contents of plants seems to be dependent on the HY5 - NRT1.1 mechanism. It is therefore not yet clear how and to which extent the thermomorphogenesis related changes of phosphate and nitrogen tissue levels and the modulation of thermomorphogenesis by the external levels of phosphate and nitrogen are related.
The bZIP transcription factor HY5 was originally identified as a positive regulator of light signaling, and only recently emerged as a major factor to regulate temperature dependent signaling in the root. HY5 functions as both, transcription activator and repressor 11 , 32 , 38 , 48 , and regulates numerous genes by binding to its target promoter region; its binding to a distinct set of target promoters is increased at high ambient temperature 6 (Fig. 2a–e ). Mutation of HY5 has wide ranging consequences for nutrient levels, not only for the levels of N and P, but also for those of Ca, Carbon, K, Na, Mn, and Zn (Supplementary Fig. 1 ). It will be interesting to further elucidate how HY5 changes its preferred direct targets at high ambient temperature.
Downstream of HY5, NRT1.1 acts as a main player in the interaction of temperature responses and nutrients. NRT1.1 was originally discovered as a dual-affinity nitrate transporter and has been shown to integrate nitrate and phosphate signaling in plants 23 , 24 , 25 , 26 . Our data show that decreases in both transcript and protein level of NRT1.1 are critical to trigger root responses and a P level alteration at higher temperatures. In a somehow surprising manner, the HY5-NRT1 regulatory module seems to affect plant tissue P-levels in response to higher temperatures but not N-levels. This is even more surprising as we found that HY5 directly transcriptionally regulates NRT1 and other N-homeostasis genes, but we found no clear signature of a notable direct regulation of P-homeostatic genes.
The root response and alterations in N–P levels are conserved in Arabidopsis, soybean, and rice (Figs. 2 , 4 , 5 , 7 ). Therefore, it might be interesting to further investigate the genetic relationship among the homologs between NRT1.1 and HY5 in soybean and rice. It seems reasonable to assume that since NRT1.1 shares high homology among plants 49 , the role of NRT1.1 might be conserved among plant species at higher temperatures. Because Arabidopsis NRT1 family members have similar but diverged functions 49 , 50 , 51 , it is possible that different NRT1s or even other NRTs might have distinct roles at higher temperature. For example, NRT2.1 has been known as a direct target of HY5 27 , however, while NRT1.1 is strongly regulated (bound and altered transcription level) by HY5 at high ambient temperature in the root (Fig. 2a-c , supplementary Fig. 4 ), NRT2.1 is not. This seems to be a similar situation as found in the PIFs. All PIFs interact with PHYTOCHROME B (PhyB) and share a redundant role in the red-light signaling pathway. However, PIF1 has a dominant role in seed germination and chlorophyll biosynthesis 9 , 52 , while PIF4 and PIF7 have dominant roles in shoot thermomorphogenesis 10 , 13 , and PIF4 and PIF5 in shade avoidance response 53 .
Overall, the observed conserved interactions of nutrient levels and temperature responses are relevant in several ways when it comes to the ongoing climate change. Global warming will affect soil temperatures profoundly, and it has been shown that soil warming affects nutrient availability, especially N and P 54 , 55 . It will therefore be important to better understand the interactions of N, P and temperature that relate to plant growth. This becomes even more important as fertilizer production and application (in particular for N) causes significant emissions of greenhouse gases 56 , 57 . While our nutrient analysis is from 9-day media grown plants 4-week soil grown plants (Fig. 1g-i ; Supplementary Fig. 1 , 2 ), longer growth and analysis of nutrients in other plant parts such as seeds might reveal additional nutrient level changes, some of which might be relevant for the nutritional value of crops. Also, effects on other nutrients at higher temperatures might differ from plant species to plant species, perhaps associated with crop-specific fertilizer formulations 58 , 59 . Taken together, biotechnology or breeding strategies for taking into considerations future temperature regimes and nutrient levels will be important for overcoming the challenges that global warming will pose for the production of nutritious food and feed at the scale that is needed for a growing global population.
Plant materials, growth conditions, and phenotypic analyses
Arabidopsis plants were grown at LED light with light intensity of 100 μmol and diurnal condition of 16L:8D. Soy and rice plants were grown at LED light with light intensity of 200 μmol and diurnal condition of 16L:8D. Arabidopsis seedlings were plated in the media and grown vertically at either 21 °C or 28 °C for additional 5 days after 4 days of germination at 21 °C. For the media preparation, MS full media (Caisson lab, Cat. MSP33) Nitrogen-deficient media (Caisson lab, Cat. MSP19), or Phosphate deficient media (Caisson lab, Cat. MSP21) were used with pH 5.7, 0.8% micropropagation Type 1 phytoagar (Caisson), and supplement of nitrogen or phosphate source according to the previous report 41 (N550: 50μM NH 4 NO 3 , 450μM KNO 3 , and 8900μM KCl, P125: 100μM KH 2 PO 4 and 525μM KCl). And then plates were scanned through the scanner (Epson, Perfection v600) for further analysis using imageJ. The total root length of soy and rice roots was measured using Rhizovision with the parameter: broken roots mode with image threshold 200 60 .
Nutrient analyses
C/N analysis and MP-AES analysis were conducted in this study. For C/N analysis, tissue samples were dried in 70 °C oven for 48 h. Then Genogrinder (Spex SamplePrep 2010-115) was used for milling the samples. Powder samples were sent to NuMega Resonance Labs (San Diego, CA) for Perkin Elmer PE2400-Series II, CHNS/O analyzer analysis. For MP-AES analysis, Dry samples (~5 mg) were digested with nitric acid 65% (EMD Millipore Cat. 1.00456.2500) and hydrogen peroxide 30% (Sigma-Aldrich Cat. H3410-1L) using an Environmental Express® Hotblock digestion system (Cat. SC196). After diluting the samples with miiliQ water, they were quantified by 4210 MP-AES (Agilent). The following wavelengths were used: 202.548 nm for Zn, 214.915 nm for P, 280.271 nm for Mg, 327.395 nm for Cu, 371.993 for Fe, 403.076 nm for Mn, 589.592 nm for Na, 616.217 nm for Ca, 769.897 nm for K. The final concentration was determined using a standard curve.
Confocal microscopy
For the confocal microscopy experiments, plants with two genotypes, pNRT1.1: GFP and pNRT1.1:NRT1.1-GFP transgenic lines were grown at either 21 °C or 28 °C for an additional 5 days after 4 days of germination at 21 °C. Zeiss LSM710 confocal microscope was used for the experiment using the 10x or 20x. Software Zeiss Zen was used for the analysis. For the PI staining, Propidium Iodide (Sigma-Aldrich, Cat. 4170) was used. Samples were gently placed on the solution and stained. For the laser and the filter, a 514 nm laser and a 520/570 nm filter for GFP. The PI signal is excited with either 488 or 514 nm laser and fluorescence emission was filtered by a 600/650 nm filter.
Protein extraction and Western blot analyses
Total protein extracts were made from 50 seedlings of root sample using 50 μL protein extraction buffer, consisting of 0.35 M Tris-Cl pH 7.5, 10x NuPAGE Sample Reducing Agent (Thermofisher, Cat. NP0009), and 4x NuPAGE LDS Sample Buffer (Thermofisher, Cat. NP0008), and 1× protease inhibitor cocktail. After boiling the samples, samples were centrifuged at 20,200 × g for 5 min and loaded into SDS-PAGE gels (Invitrogen, Cat. NP0323BOX). Separated proteins were transferred using transfer stacks (Invitrogen, Cat. IB23002) and then immunoblotted using anti-HY5 (Abiocode, Cat. R1245-2, dilution 1:3000), anti-NRT1.1 (Agrisera, Cat. AS12 2611, dilution 1:2000), or anti-Tubulin (Invitrogen, Cat. 32-2500, dilution 1:5000) antibodies. For the secondary antibodies, anti-mouse (Biorad, Cat. 170-6516, dilution 1:5000), anti-rabbit (Agrisera, Cat. AS09 602, dilution 1:5000), or were used. SuperSignal West Femto Chemiluminescent substrate (ThermoFisher Scientific, Cat. PI34094) was used for the detection of signals.
RNA extraction, cDNA synthesis, and qRT-PCR
For RNA sample preparation, plants with three genotypes, Col-0, hy5-215 , and chl1-5 mutant, were grown at either 21 °C or 28 °C for additional 5 days after 4 days of germination at 21 °C. Shoot and root separated samples were collected with three independent replicates. Samples were extracted with the RNeasy Plant Mini Kit (Qiagen, Cat. 74904). Thermo Scientific™ Maxima H Minus First Strand cDNA Synthesis Kit (ThermoFisher, Cat. FERK1652) was used for cDNA synthesis. Luna® Universal qPCR Master Mix (NEB, Cat. M3003L) and qPCR machine (Biorad, CFX Opus 384 Real-Time PCR System) were used for qPCR analysis. Primer list is in Supplementary Data 2 .
RNA-seq analyses
For RNAseq sample preparation, same condition was used as qRT-PCR. The RNA quality and quantity were analyzed using a 2100 Bioanalyzer tape station (Agilent Technologies) and Qubit Fluorometer (Invitrogen). The sequencing libraries were generated by the Salk Next Generation Sequencing Core according to Illumina manufacturer’s instructions. Sequencing was performed using the Illumina Novaseq6000 platform. For RNAseq analysis, we mapped the short-reads using Arabidopsis Information resource web site ( http://www.arabidopsis.org ) 61 combined with the Splice Transcripts Alignments to Reference (STAR) version 2.7.0a method 62 . Differentially Expressed Genes (DEG) analysis was performed using edgeR 63 . Critical values for the analysis are a false discovery rate (FDR < 0.05) and log2FC (> 0 or <0). DEGs were visualized and k-means clustering method was used to classify DEGs (FDR < 0.05 and |log2FC | >1) by comparing the 21 °C and 28 °C treatment for Col-0 in roots via the ComplexHeatmap 64 package in R. The cluster number (k = 8) was determined by sum of squared error and Bayesian information criterion. The volcano plot was created using Enhanced Volcano R package ( https://github.com/kevinblighe/EnhancedVolcano ). Raw data and processed data for RNA-Seq in Col-0, hy5-215 , and chl1.5 can be accessed from the Gene Expression Omnibus database under accession number GSE262197 .
Chromatin immunoprecipitation (ChIP) assays
Chromatin Immunoprecipitation (ChIP) assays were conducted as described previously with minor modifications 6 . For ChIP assay samples, 4-day-old seedlings of pHY5:HY5-GFP were transferred to 21 °C or 28 °C for additional 5 days and then harvested. Samples were crosslinked using 1% formaldehyde under 30 min of vacuum and 1 M glycine was added for an additional 5 min for quenching. Samples were gently washed with distilled water for five times and ground thoroughly with mortar and pestle using liquid nitrogen. All the buffers for ChIP were from ChIP assay kit (Millipore, Cat. 17-295). Ground samples were placed into 1.5 mL microtube with nuclei isolation buffer for 15 min and then centrifuged at 20,200 × g for 10 min at 4 °C. 1 mL lysis buffer was used for resuspension of the pellet. Then, sonication of chromatin pellet was performed using digital sonifier (Fisher Scientific, Sonic Dismembrator Model 500). For immunoprecipitation, ChIP grade anti-GFP (Abcam, Cat. ab6556) and dynabeads were used. After washing series of low salt wash buffer, high salt wash buffer, LiCl wash buffer, and TE buffer, we add elution buffer for elution. Samples were incubated overnight at 65 °C for reverse crosslinking after adding NaCl with a final 0.2 M concentration. Finally, PCR purification kit (QIAGEN) was used for DNA purification after proteinase K treatment for 2 h. Samples without IP were used as input DNA. Enrichment (% of input) was calculated from each sample relative to their corresponding input. Primer list is in Supplementary Data 2 .
EMSA and dual luciferase assay
Electrophoretic Mobility Shift Assay (EMSA) was conducted according to manufacturer’s protocol (Thermofisher). The Arabidopsis HY5 coding sequence (CDS) was cloned into pDEST15 and transformed into E. coli strain BL21. After induction with 0.5 mM IPTG, protein was purified using GST purification kit (Thermofisher). G-box containing primer were labeled with biotin in the 3’end through Eton Bioscience.
Dual luciferase assay was conducted following the manufacturer’s protocol (Promega, Cat. N1610). NRT1.1 promoter region and HY5 CDS region were cloned into pGreenII 0800-LUC and pGreenII 62-SK, respectively. After transformation into Agrobacterium strain GV3101, culture medium was resuspended into infiltration buffer (10 mM MgCl 2 , 10 mM MES and 200 μM acetosyringone) with adjustment of Optical Density (O.D.) 0.2 and incubated for 2 additional h. Subsequently, medium was infiltrated into Nicotiana Benthamiana leaves for transient expression. After 48 hrs, leaves were collected and FLUC and RLUC were measured using the Tecan Safire 2 platereader. The primer list is in Supplementary Data 2 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA sequencing data were deposited into the Gene Expression Omnibus database (accession number GSE262197 ). Source data are provided with this paper.
IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Working Group II Contribution to the IPCC Sixth Assessment Report (IPCC, 2022).
Held, C. & Sadowski, G. Thermodynamics of bioreactions. Annu. Rev. Chem. Biomol. Eng. 7 , 395–414 (2016).
Article CAS PubMed Google Scholar
Ibanez, C. et al. Ambient temperature and genotype differentially affect developmental and phenotypic plasticity in Arabidopsis thaliana. BMC Plant Biol. 17 , 114 (2017).
Article PubMed PubMed Central Google Scholar
Schleuning, M. et al. Ecological networks are more sensitive to plant than to animal extinction under climate change. Nat. Commun. 7 , 13965 (2016).
Article CAS PubMed PubMed Central ADS Google Scholar
Gaillochet, C. et al. HY5 and phytochrome activity modulate shoot-to-root coordination during thermomorphogenesis in Arabidopsis. Development 147 , dev192625 (2020).
Article CAS PubMed PubMed Central Google Scholar
Lee, S., Wang, W. & Huq, E. Spatial regulation of thermomorphogenesis by HY5 and PIF4 in Arabidopsis. Nat. Commun. 12 , 3656 (2021).
Martins, S. et al. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat. Commun. 8 , 309 (2017).
Article PubMed PubMed Central ADS Google Scholar
Vu, L. D., Xu, X., Gevaert, K. & De Smet, I. Developmental plasticity at high temperature. Plant Physiol. 181 , 399–411 (2019).
Huq, E. & Quail, P. H. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21 , 2441–2450 (2002).
Koini, M. A. et al. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 19 , 408–413 (2009).
Oyama, T., Shimura, Y. & Okada, K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11 , 2983–2995 (1997).
Burko, Y. et al. PIF7 is a master regulator of thermomorphogenesis in shade. Nat. Commun. 13 , 4942 (2022).
Fiorucci, A. S. et al. PHYTOCHROME INTERACTING FACTOR 7 is important for early responses to elevated temperature in Arabidopsis seedlings. New Phytol. 226 , 50–58 (2019).
Kumar, S. V. et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484 , 242–245 (2012).
Lau, O. S. et al. Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr. Biol. 28 , 1273–1280.e1273 (2018).
Lee, S., Zhu, L. & Huq, E. An autoregulatory negative feedback loop controls thermomorphogenesis in Arabidopsis. PLoS Genet. 17 , e1009595 (2021).
Rhee, G-Y. & Gotham I. J. The effect of environmental factors on phytoplankton growth: temperature and the interactions of temperature with nutrient limitation. Limnol. Oceanogr. https://doi.org/10.4319/lo.1981.26.4.0635 (1981).
Bouain, N. et al. Plant growth stimulation by high CO(2) depends on phosphorus homeostasis in chloroplasts. Curr. Biol. 32 , 4493–4500.e4494 (2022).
Carvalho, J. M. et al. Elevated CO 2 and warming change the nutrient status and use efficiency of Panicum maximum Jacq. PLoS ONE 15 , e0223937 (2020).
Cassan, O. et al. A gene regulatory network in Arabidopsis roots reveals features and regulators of the plant response to elevated CO(2). New Phytol. (2023).
Liu, K. H. & Tsay, Y. F. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 22 , 1005–1013 (2003).
Ho, C. H., Lin, S. H., Hu, H. C. & Tsay, Y. F. CHL1 functions as a nitrate sensor in plants. Cell 138 , 1184–1194 (2009).
Krouk, G. et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18 , 927–937 (2010).
Tsay, Y. F., Schroeder, J. I., Feldmann, K. A. & Crawford, N. M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72 , 705–713 (1993).
Ye, J. Y., Tian, W. H. & Jin, C. W. A reevaluation of the contribution of NRT1.1 to nitrate uptake in Arabidopsis under low-nitrate supply. FEBS Lett. 593 , 2051–2059 (2019).
Hu, B. et al. Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 5 , 401–413 (2019).
Chen, X. et al. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 26 , 640–646 (2016).
Jonassen, E. M., Lea, U. S. & Lillo, C. HY5 and HYH are positive regulators of nitrate reductase in seedlings and rosette stage plants. Planta 227 , 559–564 (2008).
Guo, Z. et al. The phyB-dependent induction of HY5 promotes iron uptake by systemically activating FER expression. EMBO Rep. 22 , e51944 (2021).
Huang, L., Zhang, H., Zhang, H., Deng, X. W. & Wei, N. HY5 regulates nitrite reductase 1 (NIR1) and ammonium transporter1;2 (AMT1;2) in Arabidopsis seedlings. Plant Sci. 238 , 330–339 (2015).
Leivar, P. et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18 , 1815–1823 (2008).
Burko, Y. et al. Chimeric activators and repressors define HY5 activity and reveal a light-regulated feedback mechanism. Plant Cell 32 , 967–983 (2020).
Lee, J. et al. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19 , 731–749 (2007).
Alvarez, J. M. et al. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 11 , 1157 (2020).
Chen, C. Z., Lv, X. F., Li, J. Y., Yi, H. Y. & Gong, J. M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 159 , 1582–1590 (2012).
Remans, T. et al. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 140 , 909–921 (2006).
Yu, X., Sukumaran, S. & Mrton, L. Differential expression of the arabidopsis nia1 and nia2 genes. cytokinin-induced nitrate reductase activity is correlated with increased nia1 transcription and mrna levels. Plant Physiol. 116 , 1091–1096 (1998).
Wang, X. et al. The transcription factor NIGT1.2 modulates both phosphate uptake and nitrate influx during phosphate starvation in Arabidopsis and maize. Plant Cell 32 , 3519–3534 (2020).
Maeda, Y. et al. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 9 , 1376 (2018).
Medici, A. et al. AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip. Nat. Commun. 6 , 6274 (2015).
Article CAS PubMed ADS Google Scholar
Gruber, B. D., Giehl, R. F., Friedel, S. & von Wiren, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 163 , 161–179 (2013).
Fang, X. Z. et al. NRT1.1 dual-affinity nitrate transport/signalling and its roles in plant abiotic stress resistance. Front. Plant Sci. 12 , 715694 (2021).
Guo, F. Q., Wang, R., Chen, M. & Crawford, N. M. The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell 13 , 1761–1777 (2001).
Sakuraba, Y., Chaganzhana, Mabuchi, A., Iba, K. & Yanagisawa, S. Enhanced NRT1.1/NPF6.3 expression in shoots improves growth under nitrogen deficiency stress in Arabidopsis. Commun. Biol. 4 , 256 (2021).
Feraru, E. et al. PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 116 , 3893–3898 (2019).
Burman, N., Bhatnagar, A. & Khurana, J. P. OsbZIP48, a HY5 transcription factor ortholog, exerts pleiotropic effects in light-regulated development. Plant Physiol. 176 , 1262–1285 (2018).
Ji, H. et al. Differential light-dependent regulation of soybean nodulation by papilionoid-specific HY5 homologs. Curr. Biol. 32 , 783–795 e785 (2022).
Yang, C. et al. HY5-HDA9 module transcriptionally regulates plant autophagy in response to light-to-dark conversion and nitrogen starvation. Mol. Plant 13 , 515–531 (2020).
Sun, J. et al. Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 507 , 73–77 (2014).
Li, J. Y. et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22 , 1633–1646 (2010).
Lin, S. H. et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20 , 2514–2528 (2008).
Oh, E. et al. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 47 , 124–139 (2006).
Franklin, K. A. Shade avoidance. N. Phytol. 179 , 930–944 (2008).
Article CAS Google Scholar
Kastovska, E. et al. Soil warming during winter period enhanced soil N and P availability and leaching in alpine grasslands: a transplant study. PLoS ONE 17 , e0272143 (2022).
Tian, Y. et al. Long-term soil warming decreases microbial phosphorus utilization by increasing abiotic phosphorus sorption and phosphorus losses. Nat. Commun. 14 , 864 (2023).
Gao, Y. & Cabrera Serrenho, A. Greenhouse gas emissions from nitrogen fertilizers could be reduced by up to one-fifth of current levels by 2050 with combined interventions. Nat. Food 4 , 170–178 (2023).
Article PubMed Google Scholar
Schoumans, O. F., Bouraoui, F., Kabbe, C., Oenema, O. & van Dijk, K. C. Phosphorus management in Europe in a changing world. Ambio 44 , S180–S192 (2015).
Article PubMed ADS Google Scholar
Hoeft F. in Illinois Agronomy Handbook (University of Illinois Urbana-Campaign, 2009).
Warncke D., Dahl, J. & Jacobs, L. Nutrient Recommendations for Field Crops in Michigan Bulletin E2904 (Michigan State University Extension, 2009).
Seethepalli, A. et al. RhizoVision crown: an integrated hardware and software platform for root crown phenotyping. Plant Phenomics 2020 , 3074916 (2020).
Berardini, T. Z. et al. The Arabidopsis information resource: making and mining the “gold standard” annotated reference plant genome. Genesis 53 , 474–485 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29 , 15–21 (2013).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 , 139–140 (2010).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32 , 2847–2849 (2016).
Download references
Acknowledgements
The authors thank all the Busch lab members for critical discussions. We also thank Dr. Gabriel Krouk for providing pNRT1:NRT1-GFP seeds and helpful suggestions. We also thank Drs. Jűrgen Kleine-Vehn and Shuichi Yanagisawa for providing pils6-1 mutant and PILS6-OE, and NRT1.1-OX seeds, respectively. The research was supported by funds from the Salk Harnessing Plants Initiative to W.B. and funds from Michigan State University to H.R.
Author information
Authors and affiliations.
Plant Molecular and Cellular Biology Laboratory, Salk Institute for Biological Studies, 10010 N Torrey Pines Rd, La Jolla, CA, 92037, USA
Sanghwa Lee, Julia Showalter, Ling Zhang & Wolfgang Busch
Department of Plant, Soil, and Microbial Sciences, Michigan State University, East Lansing, MI, 48823, USA
Gaëlle Cassin-Ross & Hatem Rouached
You can also search for this author in PubMed Google Scholar
Contributions
S.L. and W.B. conceived the study and designed the experiments. S.L. and J.S. performed root phenotypic analyses. MP-AES analyses performed by G.C. RNAseq analyses performed by S.L. and L.Z. S.L. was responsible for all other experiments. All the authors analyzed the data. W.B. and H.R. supervised work and provided funds and resources. S.L. and W.B. wrote the manuscript with input of all the authors. All the authors discussed the results and commented on the manuscript.
Corresponding author
Correspondence to Wolfgang Busch .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, supplementary data 2, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lee, S., Showalter, J., Zhang, L. et al. Nutrient levels control root growth responses to high ambient temperature in plants. Nat Commun 15 , 4689 (2024). https://doi.org/10.1038/s41467-024-49180-6
Download citation
Received : 04 August 2023
Accepted : 24 May 2024
Published : 01 June 2024
DOI : https://doi.org/10.1038/s41467-024-49180-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Make a hypothesis about which color in the visible spectrum causes the most plant growth and which color in the visible spectrum causes the least plant growth.
How did you test your hypothesis? Which variables did you control in your experiment and which variable did you change in order to compare your growth results?
Analyze the results of your experiment. Did your data support your hypothesis? Explain. If you conducted tests with more than one type of seed, explain any differences or similarities you found among the types of seeds.
What conclusions can you draw about which color in the visible spectrum causes the most plant growth?
Given that white light contains all colors of the spectrum, what growth results would you expect under white light?
- Carry out an experiment to determine which colors of the light spectrum are used in photosynthesis as evidenced by plant growth.
- Measure plant growth under lights of different colors of the spectrum.

- Review Paper
- Open access
- Published: 28 April 2021
The relationship between plant growth and water consumption: a history from the classical four elements to modern stable isotopes
- Oliver Brendel ORCID: orcid.org/0000-0003-3252-0273 1
Annals of Forest Science volume 78 , Article number: 47 ( 2021 ) Cite this article
35k Accesses
18 Citations
15 Altmetric
Metrics details
A Correction to this article was published on 17 June 2021
This article has been updated
Key message
The history of the relationship between plant growth and water consumption is retraced by following the progression of scientific thought through the centuries: from a purely philosophical question, to conceptual and methodological developments, towards a research interest in plant functioning and the interaction with the environment.
The relationship between plant growth and water consumption has for a long time occupied the minds of philosophers and natural scientists. The ratio between biomass accumulation and water consumption is known as water use efficiency and is widely relevant today in fields as diverse as plant improvement, forest ecology and climate change. Defined at scales varying from single leaf physiology to whole plants, it shows how botanical investigations changed through time, generally in tandem with developing disciplines and improving methods. The history started as a purely philosophical question by Greek philosophers of how plants grow, progressed through thought and actual experiments, towards an interest in the functioning of plants and the relationship to the environment.
This article retraces this history by following the progression of scientific questions posed through the centuries, and presents not only the main methodological and conceptual developments on biomass growth and transpiration but also the development of the carbon isotopic method of estimation. The history of research on photosynthesis is only touched briefly, but the development of research on transpiration and stomatal conductance is presented with more detail.
Research on water use efficiency, following a path from the whole plant to leaf-level functioning, was strongly involved in the historical development of the discipline of plant ecophysiology and is still a very active research field across nearly all levels of botanical research.
1 Introduction
The ratio of biomass accumulation per unit water consumption is known today as water use efficiency (WUE) and is widely relevant to agriculture (e.g. Blum 2009 ; Tallec et al. 2013 ; Vadez et al. 2014 ), to forest ecology (e.g. Linares and Camarero 2012 ; Lévesque et al. 2014 ) and in the context of global climate change (Cernusak et al. 2019 ). This ratio can be defined at various levels, from the physiological functioning of a leaf to the whole plant and at the ecosystem level. This historical review starts at the whole plant level, where WUE can be simply measured by quantifying the amount of water given to a plant and the plant’s increase in biomass during the experiment. The ratio of biomass produced divided by the cumulative water lost during growth is termed whole plant transpiration efficiency (TE= biomass produced/water lost). Historically, the ratio has also been calculated in its inverted form (water lost/biomass produced) and various terms have been used to denote these ratios (see Box 1). As knowledge, concepts and technology advanced, it became desirable to measure TE also at the leaf level, where it is defined either as the ratio of net CO 2 assimilation rate to transpiration (or to the stomatal conductance for water vapour). Therefore, some history of the two leaf-level components of WUE is included here. Numerous articles have been published on the history of the development of research on photosynthesis, and other than the reviews cited in this article, the publications by Govindjee are notable, especially Govindjee and Krogmann ( 2004 ), as they include a long list of other writings on the history of photosynthesis. On the other hand, little has been written about the history of research on transpiration and stomatal conductance. Notable is Brown ( 2013 ), who wrote specifically on the cohesion-tension theory of the rise of sap in trees, including many writings from the late nineteenth century. Consequently, here, photosynthesis research is only broached briefly, whereas transpiration research is more detailed.
As the development of the research on WUE spans a very long period, starting with Greek philosophers, publications are in several languages. Classical writings were in Greek or in Latin, and for these translations are available. However, from the mid-seventeenth century onwards, national languages were more and more used, which can be seen in the number of French- and German-language publications. This review is also a tribute to these nowadays less known seventeenth, eighteenth and nineteenth century French and German natural philosophers and their contribution to the development of the science of plant ecophysiology. Also, towards the beginning of the twentieth century, publications became too numerous to allow a comprehensive review; thus, the author focussed on the use of the carbon stable isotopes methodology and on tree ecology.
Box 1 Short history of names for whole plant transpiration efficiency (TE)
2 what is plant matter made of.
Various Greek philosophers were interested in how substances can change from one thing into another. Thales (624–c. 546 BC) thought that all things come from water, whereas Anaximenes argued that “pneuma” (air) should be the basis of all things (Egerton 2001a ). These assertions were the basis of more than 2000 years of philosophical dispute.
In “De Causis Plantarum”, Theophrastos (371–287 BC) assumed that plants draw nutrition, which consisted of varying amounts of the four elementary humours, from the earth through their roots (Morton 1981 ). Some centuries later, in a Christian work translated in 400 AD from Greek into Latin and known as “Pseudo-Clement’s Recognitions”, an apparent thought experiment was described to “prove that nothing is supplied to seeds from the substance of the earth, but that they are entirely derived from the element of water and the spirit (spiritus) that is in it” (Egerton 2004c ). The author of this thought experiment suggested putting earth into big barrels, growing herbaceous plants in it for several years, then harvesting them and weighing them. His hypothesis was that the weight of the earth would not have changed, and the author used this as an argument that the vegetation biomass could have come only from water. This thought experiment revealed a progress in scientific thinking because the question was posed more precisely than before. It stood out at a time when botany mainly consisted of naming plants and “theoretical botany effectually went out of existence” (Morton 1981 ).
It appears that the question of how plant matter is produced was not pursued in Roman or Arabic writings, which were more concerned with agricultural (the former) and medical (the latter) aspects of plant sciences (Egerton 2001b , 2002 ). Not until the High Middle Ages was a renewed interest shown in plant growth. Adelard of Bath, a twelfth century English natural philosopher, devoted the first four chapters of “Questiones Naturales” (c. 1130–1140; Morton 1981 ) to the question of what plant matter is made of. He argued, within the concepts of the four elements theory, “by just as much as water differs from earth, by so much does it afford less nourishment to roots, I mean than earth does”, clearly being in favour of earth as the source for plant nourishment. His arguments were only theoretical and speculative.
A major step occurred in botanical sciences between the fifteenth and sixteenth centuries; scholars began making experiments to test antique and medieval hypotheses against observations in nature (Egerton 2003 ). In the mid-fifteenth century, and probably related to the translation and printing of the botanical books by Theophrastus (Morton 1981 ), the thought experiment from “Recognitions...” was taken up by Nicholas of Cusa in the fourth part of his “Idiota de mente”, “De staticis experiments”. At a time when the naming of plants for pharmacology was the major interest of savants, he proposed experimental investigations. Nicholas of Cusa described the same thought experiment as did Pseudo-Clement’s Recognitions ; he concluded similarly that “the collected herbs have weight mainly from water” (1450; translation into English by Hopkins 1996 ). Cusa additionally suggested that the plants should be burned at the end of the experiment and the ash weight be taken into account. It is not clear whether the thought experiment was ever physically done.
In the sixteenth century, botanical science began to separate from medical sciences, with the establishment of lectureships in universities (e.g. Padua in 1533) and the establishment of botanical gardens (Egerton 2003 ). The bases existed for advancing science in the seventeenth century of Enlightenment. Francis Bacon, an influential philosopher of his time, conducted a series of plant growth experiments which are reported in his “de Augmentis Scientiarum” (1623; Spedding et al. 1900 ). Bacon discovered that some plants sprouted more quickly in water than in soil (Egerton 2004b ). He concluded that “for nourishment the water is almost all in all, and that the earth doth but keep the plant upright, and save it from over-heat and over-cold” (Hershey 2003 ), thus still upholding the theory proposed by Thales and Nicholas of Cusa. In “The History of the Propagation and Improvement of Vegetable”, Robert Sharrock ( 1660 ) reported that some plants both rooted and grew entirely in water. Although he noted different amounts of transpiration over time, he did not discuss this in relation to plant growth.
In 1662, Johannes Baptista van Helmont published his now-famous willow experiments (van Helmont 1662 ). This may be the first report of an experiment that was based on the thought experiment of Nicholas of Cusa (Hershey 2003 ) with the minor differences of beginning with dried soil and not using herbaceous plants, but rather a willow tree. After weighing the soil, he irrigated it with rain water and planted the weighed stem of a willow tree. The experiment ran for 5 years. At the end, the tree was weighed again, as was the dried soil. He found the soil weighed about 2 ounces less than at the beginning of the experiment, whereas 164 pounds of wood, bark and roots was produced. He concluded that the organic matter could only have come out of the water. Helmont was unaware of the existence of carbon dioxide, but he did know of “gas sylvestre”. He also knew that burning oak charcoal would produce nearly the same amount of gas sylvestre and ash. However, he did not connect this information with the plant growth he had observed (Hershey 2003 ). Robert Boyle published similar experiments in “The sceptical Chymist” (Boyle 1661 ). Boyle claimed that he had done his experiments before he knew of Helmont’s (Egerton 2004c ), although he discussed Helmont’s results and arguments in detail in his book. Boyle doubted the direct transformation of water into plant matter. He admitted, however, that it might be possible that other substances contained in the water could generate new matter (Boyle 1661 ). In the 1660’s, Edme Mariotte also criticised van Helmont’s theory that water alone constituted the only element to produce plant matter. He thought similarly to Boyle that elements in the water could contribute to the plant matter. He also showed that nitrogen compounds were important for plant growth (Bugler 1950 ).
John Woodward, in his “Some Thoughts and Experiments Concerning Vegetation” (Woodward 1699 ), took up again the question of what comprised the source of plant growth. Woodward criticised Helmont’s and Boyle’s experiments, mainly on the precision of weighing the dry soil before and after the experiment, but also the contamination of the irrigation water by terrestrial vegetable or mineral matter. Consequently, he developed a series of hydroponics experiments, where by growing plants in sealed vials, in different types of water and weighing them regularly over the same time period, he could calculate how much biomass was gained over a set time period. He was able to draw a series of conclusions from these experiments by calculating the ratio of water lost to plant mass gained in the same period of time, thereby calculating the inverse of transpiration efficiency. This was probably the first time that the inverse of transpiration efficiency was calculated using experimental data. He showed that 50 to 700 times as much water was lost than biomass gained. He also reported that plants grown in water containing more terrestrial matter grew more and with less water consumed. From these observations, he concluded that water serves only as a vehicle for the terrestrial matter that forms vegetables and that vegetable matter is not formed out of water. He is still remembered more for his geological publications (Porter 1979 ) than for his contributions to botany (Stanhill 1986 ).
In his “history of ecology” series, Egerton ( 2004c ) nicely sums this period thusly: “each of these authors (Bacon, Boyle, Helmont, Sharrock) built upon the work of his predecessors and improved somewhat the understanding of plant growth and how to study it. However, they still fell short of a basic understanding of plant growth. Before that could be achieved, chemists would have to identify the gases in the air”. This series of studies shows that from the end of the seventeenth century onwards, experiments replaced speculation (Morton 1981 ), in botany as well as in many other areas of science.
From the end of the seventeenth century, the question of how plants grow was still unresolved, although it was known that nutrients were conducted from the roots in the ascending sap to the leaves. A major improvement in the understanding of how transpiration and its variations work was the discovery of cells by Robert Hooke towards the middle of the seventeenth century (Egerton 2005 ) and subsequently the discovery of stomata on leaf surfaces. One of the first to describe stomata may have been Malpighi in “Anatomy of Plants” (Malpighi 1675 in Möbius 1901 ). Based on Malpighi’s and Grew’s ( 1682 ) studies, John Ray suggested in “Historia Plantarum” (Ray 1686 in Lazenby 1995 ) that the apertures in the leaves, when open, would give off either breath or liquid. Ray may have been the first to have connected stomata with transpiration. He also suggested that the loss of water by evaporation is compensated constantly by water from the stem, and thus transpiration results from a constant water flux. He also observed that sap ascends the stems of trees in sap-bearing vessels which do not contain valves. He did, however, admit that it cannot be capillary forces that make water go up tall trees.
Ideas on photosynthesis developed slowly from the middle of the seventeenth century onwards. Malpighi ( 1675 ) suggested that leaves produce (“concoct and prepare”) the food of plants and from leaves this food passes to all parts of the plant. Similarly, Claude Perrault in “Essais de Physique” (Perrault 1680 ) defended the hypothesis that the root acts as the mouth of the plant and that the leaves serve to prepare the food arriving with the sap from the root so that it can be used in the rest of the plant. John Ray in “History Plantarum” (Ray 1686 in Lazenby 1995 ) concurs with this, however adding in “The wisdom of God” (Ray 1691 in Lazenby 1995 ) that “not only that which ascends from the Root, but that which they take in from without, from the Dew, moist Air, and Rain”. He also thought that light could play a role in this preparation of the plant sap. At this time, most authors (Malpighi, Perrault, Mariotte, Ray) knew about the circulation of sap, up as well as down, and that leaves served somehow to transform the upcoming sap into food for the plant.
In 1770 , Lavoisier published “Sur la nature de l’eau” (“On the nature of water”, translation by the author) and reviewed the literature on the possibility of water changing into earth to nourish plants. Lavoisier cited the Van Helmont experiment and later works which tested Van Helmont’s idea by growing plants in water (e.g. Boyle, however he did not cite Woodward). He was critical of the idea that it could be a transformation of water that would constitute plant material. This was based mainly on experiments by himself and others, showing even distilled water would contain traces of “soil”. However, he also defended the idea, based mainly on Charles Bonnet’s observations, that leaves absorb vapours from the atmosphere that contribute to plant growth.
Helmont had coined the term “gaz” in the mid-seventeenth century and had been able to distinguish different gazes from air (Egerton 2004a ). It was only in the middle of the eighteenth century that gases were studied in the laboratory and several observations by different researchers would finally lead to an understanding of respiration and photosynthesis (Tomic et al. 2005 ; Nickelsen 2007 ). Richard Bradley seems to be one of the first to clearly state (in letters from 1721 to 1724) that plant nourishment can be drawn from the air. Hales ( 1727 ) agreed with this theory, which was not yet widely accepted (Morton 1981 ), and suggested that light might be involved, which helped to pave the way for the discovery of photosynthesis. Black ( 1756 ) was able to identify carbon dioxide (which he called fixed air) using a lime water precipitation test. He demonstrated that this “fixed air” did not support animal life or a candle flame (Egerton 2008 ). Charles Bonnet ( 1754 ) made an important observation, i.e. branches with leaves that were submerged under water would produce air bubbles on their surfaces when sunlight shone on them, but not after sunset. Senebier refined these experiments in 1781 (Morton 1981 ), by showing that the leaves produced no oxygen in the sunlight when the surrounding water was free of carbon dioxide and that the rate of oxygen production was higher with carbon dioxide-saturated water. Tomic et al. ( 2005 ) present nicely the steps leading up to the term photosynthesis. This began with Priestley ( 1775 ) demonstrating that the air given off by animals and by plants was not the same, Ingen-Housz ( 1779 ) observed the important role of light, and the dispute between Senebier and Ingen-Housz from 1783 to 1789 resolved more clearly the functions of carbon dioxide emission (respiration) and absorption (photosynthesis). Based on these results and his own very detailed observations, de Saussure reported in 1804 that the carbon necessary for plant growth is absorbed mainly by green leaves from atmospheric carbon dioxide and he estimated that the largest part of the accumulated dry matter of plants is made of this carbon. Thus, the dispute of what the plant matter is made of that began in antique Greece was resolved at the end of the eighteenth century.
3 How much water do plants need to grow?
The late eighteenth century marked the beginning of applied agricultural science and the rise of plant physiology (Morton 1981 ). Work continued on transpiration and stomata, with a large number of experiments. Burgerstein ( 1887 , 1889 ) managed to assemble 236 publications on transpiration of plants from 1672 to 1886, citing short abstracts of each and comparing them critically. Also, Unger published in 1862 a major review article covering such subjects as the relationship of transpiration to temperature and humidity; daily cycles, including night; differences in adaxial and abaxial leaf surfaces; the impact on transpiration of type, number, size and distribution of stomata; the structure of the epidermis (cell layers, cuticle, hairs and wax); development of the mesophyll; size of intercellular spaces and cell turgor; and the impact of plant transpiration on the atmosphere (Unger 1862 in Burgerstein 1887 ). Scientists started to reflect on the interaction of plants, or more specifically their leaves, with their environment, and experimentation included the responses of stomata to light quantity (Möldenhawer 1812 ) and quality (Daubeny 1836 in Burgerstein 1887 ). Based on inconsistent observations by e.g. Banks, Möldenhawer and Amici, advances were also made on the functioning of stomata (Mohl 1856 ). However, progress was mainly based on a comment in von Schleiden ( 1849 ) that the state of the stomata would be the result of the water in- or outflow of the pore cells (called “Schliesszellen”) and he showed experimentally that stomata close when the pore cells lose water. As knowledge of transpiration, stomatal opening and their dependence on environmental variables increased, new questions arose about the water consumption of plants.
Another milestone along the way to understanding the transpiration of plants in the nineteenth century was the publication by Sir John Bennet Lawes ( 1850 ), “Experimental investigation into the amount of water given off by plants during their growth; especially in relation to the fixation and source of their various constituents”. He described experiments on wheat, barley, beans, peas and clover using differently fertilised soils. He was using plants in closed containers and an especially designed balance to “estimate the amounts of water given off” (Fig. 1 ). He observed increased evapotranspiration with higher temperatures during the growing season, and asked whether “this increased passage of water through the plants, carrying with it in its course many important materials of growth from the soil, and probably also influencing the changes in the leaves of these, as well as of those derived from the atmosphere, will not be accompanied with an equivalently increased growth and development of the substance of the plant”. This was followed by an important discussion of the influence of temperature on evaporation and growth as well as the resultant ratio. He discussed in the introduction “the relationship of the water given off to the matter fixed in the plants”; he gave his results in this ratio and in the inverse ratio, and applied these ratios to different scientific questions. The first ratio (transpired water divided by plant matter, the inverse of today’s TE) was used to interpret his results in terms of water use compared to field available water, and the latter’s ratio (plant matter divided by transpired water, equivalent to today’s TE) was used to discuss his results in terms of functional differences among species. From the observed functional differences, he concluded that there was “some definite relationship between the passage of water through the plants and the fixation in it of some of its constituents”. He was, thereby, introducing a new question about the link between dry matter accumulation and transpiration, which will be treated in the next chapter.
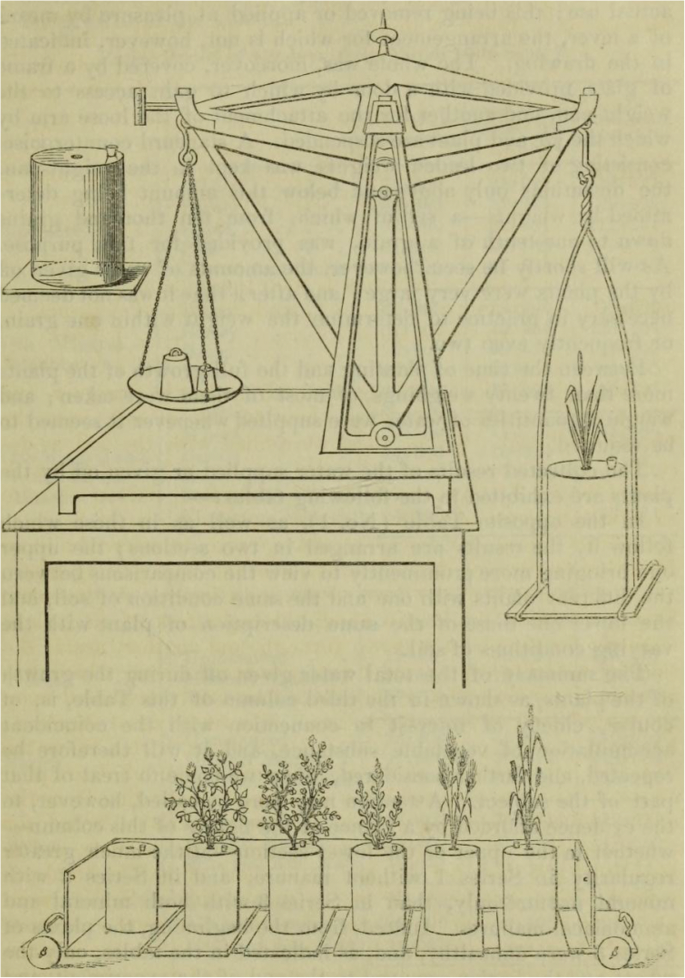
Illustration from Lawes ( 1850 , p. 43) of the special balance constructed for weighing plants in their “jars” to estimate the amounts of water given off and also the “truck” on which a series of jars was moved to the balance
Towards the end of the nineteenth century, research interest started to include agricultural questions of water use. Marié-Davy ( 1869 ) measured transpiration (standardised by leaf surface) of over 30 plant species, including eight tree or shrub species as well as herbaceous and agricultural plants. He estimated transpiration per soil area, thereby establishing that a prairie would transpire more than trees. von Höhnel ( 1879 ) estimated long-term transpiration of branches of 15 tree species (standardised on leaf surface or leaf dry weight). He used these data of branch transpiration to upscale to whole trees and concluded that compared to agricultural plants, the amount of rain seemed sufficient for tree growth. Hellriegel ( 1871 ) had already similarly concluded for cereals in the Mark Brandenburg (Germany) region that rainfall would not be sufficient, as had Marié-Davy ( 1874 ) for wheat in the Paris (France) region. In parallel with these more quantitative interrogations about water use, from the mid-nineteenth century, scientists started to ask more functional questions about the relationship between transpiration and dry matter accumulation, in a context of vigorous growth of botanical sciences and the complex relation between organisms and their environment (Morton 1981 ).
4 Are transpiration and dry matter accumulation linked?
Lawes ( 1850 ) had already reflected on a functional relationship between water flux and plant matter accumulation. In the following years, there were several publications on the transpiration of trees, and although no transpiration efficiency was estimated, the understanding of tree transpiration advanced. Many comparative studies were published. Lawes ( 1851 ) on “Comparative evaporating properties of evergreen and deciduous trees” considered twelve different tree species. He provided measurements of the variation in transpiration with temperature and hygrometry data. With these, he concluded that “evaporation is not a mere index of temperature but that it depends on vitality influenced by heat, light and other causes”. In the late nineteenth century, several researchers estimated and compared values of the ratio of transpiration and dry matter accumulation for different plants (Burgerstein 1887 ). With the growing evidence of variation in this ratio, scientists started to reflect on the relationship between transpiration and dry matter accumulation, aided by the development of new measurement techniques. A major question was if there would be a tight coupling between transpiration and dry matter accumulation, resulting in a constant transpiration efficiency, or if variation could be observed.
Dehérain ( 1869 ) studied evaporation and the decomposition of carbonic acid in leaves of wheat and barley. Using an ingenious apparatus, he was probably the first to directly measure evaporation of water in parallel with carbonic acid decomposition. He studied the effect of variously coloured light, and although he did not calculate the ratio between evaporation and carbonic acid decomposition, he did conclude that light of different colours had a similar effect on carbonic acid decomposition and on water evaporation from the leaves. His final conclusion was that “it is likely that there is existing between the two main functions of plants, evaporation and carbonic acid decomposition, a link, of which we need to determine its nature” (translation from the original French by the author). Several other scientists also commented on the relationship between transpiration and dry matter production. Fittbogen ( 1871 ) supposed, similarly to Lawes ( 1850 ) before him, but with more experimental evidence, that there should be a positive relationship between transpiration and production of dry matter. Dietrich ( 1872 in Burgerstein 1887 ) supposed that this relationship would be linear, whereas Tschaplowitz ( 1878 in Burgerstein 1887 ) introduced the idea that there should be an optimum transpiration at the maximum production of matter. Therefore, when the transpiration would increase over this optimum, this would lead to a decrease in assimilation rate. He was one of the first to suggest a non-linear relationship between transpiration and assimilation. Sorauer in “Studies on evaporation” ( 1880 ) defended the hypothesis that transpiration was not only a physical phenomenon but was also physiological. He stated that “It is not possible as yet to study the plant internal processes which regulate the transpiration, however it is possible to quantify the relationship between dry-matter and transpiration” (translation from German by the author), suggesting thereby TE as a means to advance the understanding of plant internal processes. Sorauer was probably at the cutting edge of science of his time. He pointed out specifically that variability among plants of one species was due to genetics (German, “erbliche Anlagen”), a startling and even daring assertion for his time. He asserted that for comparative studies, genetic variability needed to be minimised. To achieve this, he used, when possible, seeds from the same mother plant, grown in the same environmental conditions and a large number of repetitions. Using these protocols, he was probably one of the first to estimate TE on tree seedlings, showing that there was within species diversity in transpiration and growth, but that their ratio was more constant. He concluded from experiments on pear and apple trees that the pear trees used less water for the same biomass growth. He was able to go one step further and demonstrate that this difference was due to less transpiration per leaf area. By comparing different woody and herbaceous plants with different growth types, he postulated that when plants had a small leaf area combined with high transpiration, they had either a very strong growth increment, a high dry matter percentage, or a large root system. Overall, he observed relationships between dry matter production and transpiration; he concluded that there must be some regulation of the transpiration per unit leaf area by the co-occurring dry matter production.
Hellriegel ( 1883 ) argued that one cannot estimate a constant ratio between transpiration and production as there were factors which influence each independently. He also commented that it might make sense to estimate mean values of transpiration for various agricultural plants, as this would be for practical and scientific value. He thought that the most logical standardisation would be by the mass of the dry matter produced during the same time period. He called this “relative Verdunstungsgrösse” which can be translated into English as “relative transpiration”. He was probably one of the first to give a name to the ratio between whole plant transpiration and dry matter production. He proposed a theory that for a long-term drought, plants would acclimate their morphology to decrease their “relative transpiration”. He provided additional experimental evidence that barley had decreased in relative transpiration over as many as seven levels of soil water deficit, relative to field capacity. Using his own observations, he proposed that when calculating a mean “relative transpiration” for a single species, variation of transpiration should be minimised and that plants should be tested together only under optimal conditions. Given the relatively small differences in relative transpiration that he observed among different crops, Hellriegel suggested that these differences would not explain why some crops grow better in wet locations and others on dry locations. Hellriegel was thus probably one of the first scientists to point out that the relationship between drought adaptation and “relative transpiration” might not be straightforward.
Understanding how biomass and water loss were connected was studied by Iljin ( 1916 ) on a newly detailed level. He measured simultaneously water loss and carbon dioxide decomposition and reported his data as grammes of water lost per cubic centimetre of carbon dioxide decomposed. He concluded from studying more than 20 plant species that “...it is generally agreed that the rates of water loss and of CO 2 assimilation are directly proportionate to stomatal aperture, and that consequently there exists a close connection between these two processes”.
At the end of the nineteenth century, the ratio of transpiration versus dry matter accumulation was recognised as an important plant trait, which varies among and within species in a complex interaction of each component with the other and with environmental factors.
5 How do plants differ in water requirement and how do they respond to variations in environmental factors?
In the late nineteenth century, several researchers estimated and compared values of the ratio of transpiration and dry matter accumulation for a range of cultivated plants (Fittbogen 1871 ; Dietrich 1872 ; Farsky 1877 , cited in Burgerstein 1887 ), giving evidence of the growing interest of agricultural scientists. The number of studies of transpiration efficiency greatly increased, thereby driving a new standardisation in terminology. King ( 1889 ) studied the inverse of transpiration efficiency and described it as “the amount of water required for a ton of dry matter”, and promulgated this terminology by using it in the titles of his publications between 1892 and 1895. Similarly, Leather ( 1910 ) published “Water requirements of the crops of India”, in which he defined the “transpiration ratio” as “the water transpired to the weight of dry plant produced”. The shift from a purely descriptive use of “water requirement” to a clearly defined one was provided by Kearney and Shantz ( 1911 ) as “… the degree to which a plant is economical in its use of water is expressed in its water requirement, or the total quantity of water which it expends in producing a pound of dry matter”. The term “water requirement” is the inverse of the modern transpiration efficiency, and was used by a rapidly increasing number of publications which were published on the water use of crops in the early twentieth century. Montgomery ( 1911 ) may have been the first to use the term for a plant trait in “Methods of determining the water requirements of crops”.
At the beginning of the twentieth century, the importance of gaining knowledge on the water requirements of plants can be seen in the technical effort that went into the measuring equipment. von Seelhorst ( 1902 ) presented a system of growing boxes on rails, placed belowground, including the balance, so that the top of the growing boxes was at the same level as the surrounding soil (Fig. 2 ). In the now well-known studies on “The water requirement of plants. I. Investigations in the Great Plains in 1910 and 1911”, Briggs and Shantz ( 1913a ) measured the water requirement for 21 crop and weed species, sometimes for different varieties of the same crop and under controlled and field conditions. In the same year, they reviewed the available literature on water requirement (Briggs and Shantz 1913b ), increasing their dataset to 31 different crop species. They discussed in detail studies from 29 different authors, many of which had only published once or twice on this subject. A few researchers were notable for their number of publications on the water requirement of crop plants: King with 6 publications between 1889 and 1905, and von Seelhorst with 9 publications between 1899 and 1907. The largest contributions came from Hellriegel ( 1883 ; 10 species) and Leather ( 1911 ; 15 species). Kiesselbach ( 1916 ) also reviewed 59 publications from 1850 to 1915 “which had studied transpiration in relation to crop yield, based upon plants grown beyond the seedling stage”. There were regular publications of original work from 1870s onwards, with more than one publication per year from 1890 onwards. The difference among species and the impact of environmental factors on water requirement was one of the main questions raised. These reviews and the increasing amount of newly published work per year are evidence of the growing interest in the “water requirement” of plants as a trait of increasing importance in agricultural sciences.
Illustration from von Seelhorst ( 1902 ), showing the quite sophisticated outdoor installation “Vegetationskasten” (growing boxes, translations by the author) to weigh plants in small waggons, with a “Kastenwagen” (boxwaggon), b “Waagebalken” (scale beam), c “Deckbretter” (cover board) and d “Waagentisch” (weighing table)
With regard to species differences in water requirement among crops, Schröder ( 1895 , cited in Maximov 1929 ) found two groups, among seven cereals, which differed in water requirement by a factor of 2. Millet, sorghum and maize were known to be drought resistant, and showed a lower water requirement than the remaining plants. These differences were confirmed by Kolkunov ( 1905 , cited in Maximov 1929 ), Briggs and Shantz ( 1914 ), Briggs and Shantz ( 1917 ) and Shantz ( 1927 ). Millet, sorghum and maize are now known to use the C4 carbon pathway of photosynthesis.
With regard to external environmental influences on plants, Briggs and Shantz ( 1913b ) distinguished between soil, atmosphere and plant factors. Soil factors which were investigated were soil moisture content, soil type, cultivation, soil volume, soil temperature, effect of fertilisers in soil or water cultures and effect of previous crops. Weather factors considered were air temperature and humidity, shade and carbon dioxide content. Other factors studied in direct relationship to the plants were parasite attacks, relative leaf area, cutting frequency, defoliation, planting density and the age of plants.
A critique of the term “water requirement” was not long in coming. Dachnowski ( 1914 ) wrote, “It is assumed by many writers that a definite and quantitative relation exists between transpiration and growth, and that hence the ratio of the weight of water absorbed and transpired by a plant during its growth to the green or dry substance produced is an adequate and simple measure of growth.”, followed by an argument why this was not the case.
6 Why do plants differ in transpiration efficiency?
The adaptations of plants to dry environments were an important ecological topic at the beginning of the twentieth century, as the discipline of “physiological ecology” (Iljin 1916 ; Moore 1924 ) began to develop. Iljin ( 1916 ) studied more than 20 different plant species in situ from different ecological locations, e.g. wet bottom soils and variously facing slopes of ravines with different aspects. Iljin proposed that “the water requirements of the different species should be very different, and consequently the amounts of water available should differently affect their processes of life”. Using his observations, he was able to show that “… in no case was the water loss per unit of decomposed CO 2 found to be equal to or more in xerophytes than in mesophytes”, thus suggesting a higher transpiration efficiency. He argued that mesophytes would have to close stomata “… in dry places in order to reduce evaporation, thus diminishing the rate of assimilation as well, whereas in the case of xerophytes, which are adapted to extreme conditions of existence, assimilation in similar circumstances proceeds actively”. He then tried to confirm his hypothesis by transplanting mesophytes from wetter sites to the drier environment of xerophytes. Iljin showed experimentally that in all cases, a higher water requirement was measured for mesophytes transferred to a drier site compared to their original site and compared to xerophytes at the dry site. He interpreted his observations as “plants growing in dry places are adapted to a more economical consumption of water”. He held this to be true for among- and within-species variation.
A milestone in forest “physiological ecology” was Bates’ ( 1923 ) study of the physiological requirements of Rocky Mountain trees. Bates wrote that for foresters, knowledge of demands of tree seedlings for moisture, light, heat and soil fertility was important for planning reforestation. He started a large investigation of six forest tree species, combining field studies to describe ecosystems, with experiments in controlled environments in order to determine species differences in relative transpiration and other water flow-related traits. Bates concluded from the comparison among species that trees of low water requirement would be trees that have a superior control over their water supply. He was however critical of a direct relationship between water requirement and drought resistance in trees. Moore ( 1924 ) commented that in correlating physiological measurements with the habitat characterisation of the species, Bates “... has opened new fields to forest investigations”. He also stressed that the results were counterintuitive in that the most xerophytic species had the highest water requirement, whereas the most mesophytic species had the lowest water requirement.
A similar discrepancy was observed by Maximov ( 1929 ) in the chapter “Efficiency of transpiration” in his book The Plant in relation to water , which was translated from Russian into English rapidly after its publication. Maximov preferred “efficiency of transpiration” to “water requirement”, arguing that the former would be more logically correct, because the determining process (transpiration) should be in the denominator, which also would have the effect that “… an increase in the figure denoting the value of the ratio actually corresponds to an increase of the efficiency per unit of water used”.
In his book, Maximov ( 1929 ) described experiments done at Tiflis Botanic garden (today in Georgia) by Maximov and Alexandrov ( 1917 ), where they studied local xerophytes for 3 years. They found xerophytes with a high efficiency of transpiration, particularly drought-resistant annuals. They also found that plants with a low efficiency of transpiration appeared to be the most typical semi-arid xerophytes. The mesophytes all displayed a medium efficiency. Maximov noted from other observations on the same plants that the “… majority of xerophytes with a low efficiency of water expenditure possess very extensive root systems, far exceeding in length the sub-aerial portions of the plant”. He also observed that these plants showed a strong transpiration and that this transpiration might constitute the “pump” which could draw water through such an extensive root system. He also observed that “members of the group of annual xerophytes with a high efficiency of transpiration are characterised by a relatively large leaf surface, which develops very rapidly”. He argued that this would confer a high intensity of assimilation. From these observations, he concluded a “lack of direct proportionality between efficiency of transpiration and the degree of drought resistance”, but also that “the magnitude of the efficiency of transpiration affords one of the most satisfactory tests of the ecological status of a plant”. Maximov applied the ecological classification developed by Kearney and Shantz ( 1911 ), which they had based on plants of the arid and semi-arid regions of North America: (1) drought-escaping with an annual growth cycle restricted to favourable conditions; (2) drought-evading, delay by various means the exhaustion of soil moisture; (3) drought-enduring, can wilt or dry but remains alive; and (4) drought-resisting, can store a water supply. It should be noted that the ecological definitions behind these concepts have changed with time and are used slightly differently today. Shantz ( 1927 ) argued that many of the drought-evading plants had a low water requirement and Maximov noted that this group included the highly efficient xerophytes with a large leaf area. Maximov also observed that xerophytes from the third group (drought-enduring) could show a very low efficiency of transpiration and belonged to the group of xerophytes with large root systems. Without concluding directly, he suggested a relationship between the transpiration efficiency of a xerophyte and its ecological strategy when facing limited soil water content. These studies by Maximov are among the most complete concerning the relationship between a plants’ resistance to drought and their transpiration efficiency, reflecting the interest of scientists in ecological questions of plant functioning, especially in relation to drought.
Although work on crop plants advanced greatly in the early twentieth century, results were scarcer for tree species. Raber ( 1937 ) concluded his book on “Water utilization by trees, with special reference to the economic forest species of the north temperate zone” with detailed discussions of available data for forest trees. He commented that “much more work on the water requirements of trees of all ages and under varying site conditions is needed”. And he continued that “In view of the importance of planting drought-resistant species in regions where the water supply is below the optimum for most tree species, it is extremely urgent to know more about what qualities make for drought resistance and what species possess these qualities to the greater degree.” These conclusions by Raber show that from the beginning of the twentieth century, the estimation of transpiration efficiency had taken an important place in ecological studies on forest tree species, however not without some critical thoughts on the subject.
7 What is the functional importance of transpiration?
Already in the 1870s and 1880s, the role of stomata in the diffusion of carbon dioxide into the leaf (during the day) and out of the leaf (during the night) was discussed in the scientific literature, as shown by the extensive literature review by Blackman ( 1895 ) (see also section 4 above). Especially the functional importance of transpiration was an open question. There were two opposing lines of thought. As summarised by Iljin ( 1916 ), one defended the line of inquiry that transpiration was important only in the process of transporting mineral salts from roots to leaves; the other held that the opening of stomata was necessary for absorbing the carbonic acid from the atmosphere, which leads to a loss of water and is described as an “inevitable evil”. Iljin ( 1916 ) preferred the second line of investigation and attributed a major role to the stomatal aperture, which controlled both the absorption of carbonic acid from the atmosphere and the loss of water. He concluded that in “physiologico-ecological” investigations, assimilation should be studied together with transpiration. Maskell published a series of papers in 1928, where especially “XVIII.—The relation between stomatal opening and assimilation.” (Maskell and Blackman 1928 ) used an apparatus to estimate apparent CO 2 assimilation and transpiration rate in parallel (Fig. 3 ), and was therefore able to study in detail their interdependence, developing the first mathematical descriptions, based on the development of the theories about the diffusion of gases (Brown and Escombe 1900 ). Methodological advances intensified research on the leaf-level relationship between assimilation and transpiration and allowed the study of plant functioning in more detail. The major step forward was the construction of an infrared gas analyser (URAS: in German “Ultrarotabsorptionsschreiber”, IRGA, infrared gas analyser) by Lehrer and Luft in 1938 (Luft 1943 ) at a laboratory of BASF, IG Farbenindustrie. Normally used in industry and mining, Egle and Ernst ( 1949 ) may have been the first to describe the use of the URAS for plant physiological measurements. By 1959, the URAS was routinely used for measuring stomatal resistance or transpiration in parallel and simultaneously with CO 2 assimilation, on the same leaf (Rüsch 1959 ). This was a great improvement on previous methods and led rapidly to a set of equations for calculating assimilation and stomatal conductance (Gaastra 1959 ).
Two figures taken from Maskell and Blackman ( 1928 ): on the top, Figure 1 (p. 489) showing a “Combined assimilation chamber and porometer for simultaneous investigation of apparent assimilation and stomatal behaviour. A. Section of leaf chamber passing through porometer chamber. B. Back view of leaf chamber showing also air-flow meter attached by pressure tubing to porometer and to leaf chamber”. On the bottom, Figure 5 (p. 497) “Relation between porometer rate and apparent assimilation at ‘high’ light, December 1920.” Exp t LI and LII correspond to 2 days of continuous measurements to what Maskell called “diurnal march”
Scarth ( 1927 ) argued that there would be little advantage for a plant to have a high rate of transpiration, but stressed the “... advantage of maintaining the fullest diffusive capacity of the stomata and the highest possible pressure of CO 2 in the intercellular spaces”. He concluded that the principal function of stomata “... is to regulate that very factor which is presumed to regulate them, viz. the concentration of CO 2 in the leaf or, respectively, in the guard cells”. Maskell and Blackman ( 1928 ) tested this hypothesis experimentally and concluded that the rate of uptake of carbon dioxide was determined by variations in stomatal resistance and by resistances within the leaf, thereby introducing the importance of the CO 2 concentrations in the chloroplasts. The suggestion of a strong link between the leaf internal carbon dioxide concentration and leaf-level WUE represented a large advance in the theoretical understanding of WUE.
Penman and Schofield ( 1951 ) proposed, perhaps, the first theoretical link between the leaf-level transpiration ratio (leaf transpiration divided by assimilation) and the ratio of the coefficients of diffusion of water vapour and carbon dioxide in air, and the water vapour and carbon dioxide air-to-leaf pressure gradients. Gaastra ( 1959 ) suggested that the leaf internal conductance to carbon dioxide is a pivotal point of the ratio of assimilation to transpiration and of the water economy of crop plants. Bierhuizen and Slatyer ( 1965 ) showed that the transpiration ratio could be predicted from water vapour and carbon dioxide gradients over a range of light intensities, temperatures and relative humidities. These studies were the first to suggest that whole plant transpiration efficiency might be regulated directly by leaf functioning and would be therefore a trait in its own right and not only the ratio of two plant traits.
8 How can the transpiration ratio be improved?
Because water is increasingly scarce in a warming world, Rüsch ( 1959 ) queried whether the luxury of highly transpiring tree species could be justified. He argued for selective breeding of tree species varieties with low transpiration-to-assimilation ratio T/A by means of minimising transpiration while maximising assimilation. Also Polster et al. ( 1960 ) assessed the potential suitability of tree species to sites by their dry matter production and transpiration ratio. Troughton ( 1969 ) and Cowan and Troughton ( 1971 ) suggested that genetic selection of plant varieties could be used to improve the transpiration ratio by decreasing leaf internal resistance to carbon dioxide diffusion. Cowan and Farquhar ( 1977 ) built on this theme by proposing that stomata might optimise carbon gain to water lost by varying the conductances to diffusion and thereby maximising the ratio of the mean assimilation rate to mean rate of evaporation in a fluctuating environment. Approaches which target photosynthesis, stomatal opening, leaf internal resistance to carbon dioxide diffusion or stomatal optimisation in order to improve plants performance have since been followed in plant breeding and have largely been reviewed elsewhere (e.g. Condon et al. 2004 ; Cregg 2004 ; Vadez et al. 2014 ).
9 Intrinsic water use efficiency and carbon stable isotopes
Another milestone towards contemporary research on water use efficiency was the use of stomatal conductance to water vapour rather than transpiration by Farquhar and Rashke ( 1978 ) and to calculate water use efficiency as assimilation divided by stomatal conductance. This definition allowed an estimation of water use efficiency resulting only from plant functioning, without a direct impact from leaf-to-air vapour pressure difference and was named by Meinzer et al. ( 1991 ) “intrinsic water use efficiency” (W i ). Knowledge of W i facilitated the search for a genetic basis of within species variation, e.g. Brendel et al. ( 2002 ), Condon et al. ( 2002 ) and Chen et al. ( 2011 ).
Development of the stable carbon isotope method for estimating W i resulted in a widely applicable screening method, and a large increase of publications around plant water use efficiency. Based on the two-step fractionation model (atmospheric CO 2 – leaf internal CO 2 – plant carbon) proposed by Park and Epstein ( 1960 ), various models explaining the difference in carbon isotope composition between atmospheric CO 2 and plant carbon were developed in the late 1970s and early 1980s, e.g. Grinsted ( 1977 ), Schmidt and Winkler ( 1979 ) and Vogel ( 1980 ). Vogel’s model contained many theoretical aspects which, however, lacked experimental understanding. In parallel, Farquhar ( 1980 ) developed a similar model, but which resulted in a simple, elegant mathematical equation relating plant natural abundance carbon isotope discrimination, relative to atmosphere, to the ratio of leaf internal to atmospheric CO 2 concentration. This was, in turn, related to W i . Experimental evidence showed that carbon isotope measurements, in wheat, reflected long-term water use efficiency (Farquhar et al. 1982 ) as well as whole plant transpiration efficiency (Farquhar and Richards 1984 ). They concluded that carbon isotope discrimination may provide an effective means to assess and improve WUE of water-limited crops. Strong correlations between whole plant TE and stable carbon isotope measurements of plant organic material were shown in a host of papers to be. Some of these papers were (1) for crops and other annuals (Hubick et al. 1986 ; Ehleringer et al. 1990 ; Virgona et al. 1990 ) and (2) for trees (Zhang and Marshall 1994 ; Picon et al. 1996 ; Roupsard et al. 1998 ). The isotopic method has spread rapidly as a general estimator of WUE and continues to be used widely in screening programmes for plant improvement as well as in ecological research, e.g. Rundel et al. ( 1989 ) and notably used in tree rings (McCarroll and Loader 2004 ).
10 Closing remarks
Water use efficiency is probably one of the oldest of plant traits to stimulate across the centuries the interest of philosophers, theologians, Middle Age savants, natural philosophers and modern plant scientists across different disciplines (plant physiology, ecophysiology, ecology, genetics, agronomy). The interest began as a purely philosophical one, progressed to thought experiments, towards an interest in plant functioning and its relationship to the environment.
Already in the early Renaissance (mid-fifteenth century), an experimentation was proposed, in a time when botany consisted mainly of naming plants (Morton 1981 ). It is then also an early example of an actually performed experimentation, the famous willow experiment by Van Helmont ( 1662 ) as well as of early “in laboratory” experimentation on plants (hydroponics experiments by Woodward 1699 ). The question of what makes plants grow, between water and soil, kept natural philosophers busy up to the end of the eighteenth century, when the assimilation of CO 2 was discovered and the question finally solved.
Early in the nineteenth century, the interest and experimentation turned to the amount of water that plants would need to grow, in the context of a developing research on agricultural practices (Morton 1981 ). Biomass was used to standardise the water losses which allowed comparisons among species (crops as well as trees) and a beginning study of the impact of different environmental variables.
At the end of the nineteenth century, knowledge on the physiological aspects of CO 2 assimilation and the control of transpiration by stomata had sufficiently advanced, so that scientists started to reflect on their inter-dependency. Was transpiration only a physical process or was there a physiological control? Was transpiration regulated by the dry matter production? Or does the stomatal opening determine the rate of CO 2 assimilation?
At the turn of the twentieth century, the study of species differences led to questioning why these differences did exist. As the discipline of “physiological ecology” developed, “water requirement” was inverted into an “efficiency”, reflecting an evolution from standardising transpiration to a trait in its own right. This introduced ecological questions about the adaptation of plants to dry environments and the relation to transpiration efficiency. Counterintuitive results stimulated the discussion and linked differences in WUE to different ecological strategies.
Methodological and theoretical advances in the description of leaf gas exchange in the mid-twentieth century showed the central role of stomata in the control of transpiration and CO 2 assimilation, leading to the idea that stomata might optimise water losses versus carbon gain. The development of carbon stable isotopes as an estimator of leaf-level WUE was an important step not only to further develop these theoretical considerations, but also towards large-scale studies. In parallel, modelling approaches were developed to scale from leaf-level WUE to whole plant TE, e.g. Cernusak et al. ( 2007 ), and to the field or canopy, e.g. Tanner and Sinclair ( 1983 ).
At least from the beginning of the twentieth century onwards, also critical views on the relationship between water requirement and its relation to growth mostly in terms of yield were published (Dachnowski 1914 ). Viets ( 1962 ) asked “Is maximum water use efficiency desirable?”, especially in terms of crop production. Sinclair et al. ( 1984 ) considered different options for improving water use efficiency, however concluding that most of these have important limitations or drawbacks. This discussion is ongoing, as can be seen by the article published by Blum ( 2009 ): “Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress”.
Exploration and application of transpiration efficiency at the whole plant level, and its derivatives at other levels, are still a very active research field across nearly all levels of forest science: concerning very rapid processes at the leaf level (Vialet-Chabrand et al. 2016 ), up-to-date genetic and genomic approaches for breeding (Plomion et al. 2016 ; De La Torre et al. 2019 ; Vivas et al. 2019 ), studying local adaptation of trees to their environment in a population genetic context (Eckert et al. 2015 ) or an ecological context (Pellizzari et al. 2016 ), water use efficiency from the plant to the ecosystem (Medlyn et al. 2017 ), estimated at the population level (Rötzer et al. 2013 ; Dekker et al. 2016 ) or modelling up to the global earth level (Cernusak et al. 2019 ), just to name a few. Thus, the first curiosity of Greek philosophers has motivated scientists through history, with many exciting discoveries still to come.
Change history
17 june 2021.
A Correction to this paper has been published: https://doi.org/10.1007/s13595-021-01073-0
Bates CG (1923) Physiological requirements of Rocky Mountain trees. J Agric Res 24:97–164
Google Scholar
Bierhuizen J, Slatyer R (1965) Effect of atmospheric concentration of water vapour and CO 2 in determining transpiration-photosynthesis relationships of cotton leaves. Agric Meteorol 2:259–270
Article Google Scholar
Black J (1756) Experiments upon magnesia alba, quicklime, and some other alkaline substances. Essays Obs Phys Lit 2:157–225
Blackman F (1895) XI. Experimental researches on vegetable assimilation and respiration.—No. II. On the paths of gaseous exchange between aerial leaves and the atmosphere. Philos Trans R Soc B 186:503–562
Blum A (2009) Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. F Crop Res 112:119–123
Bonnet C (1754) Recherches sur l’Usage des feuilles dans les plantes. Elie Luzac, Fils, Göttingen
Boyle R (1661) The sceptical chymist. J. Cadweill for J. Crooke
Brendel O, Pot D, Plomion C, Rozenberg P, Guehl JM (2002) Genetic parameters and QTL analysis of ẟ 13 C and ring width in maritime pine. Plant Cell Environ 25:945–953
Article CAS Google Scholar
Briggs LJ, Shantz HL (1913a) The water requirement of plants. I. Investigations in the Great Plains in 1910 and 1911. US Dep Agric Bur Plant Ind Bull 284:1–48
Briggs LJ, Shantz HL (1913b) The water requirement of plants. II. A review of the literature. US Dep Agric Bur Plant Ind Bull 285:1–96
Briggs LJ, Shantz HL (1914) Relative Water Requirement of Plants. J Agric Res 3:1–64
CAS Google Scholar
Briggs LJ, Shantz HL (1917) The water requirement of plants as influenced by environment. In: Proceedings of the Second Pan American Scientific Congress. Pp 95–107
Brown HR (2013) The theory of the rise of sap in trees: some historical and conceptual remarks. Phys Perspect 15:320–358
Brown H, Escombe F (1900) VIII. Static Diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Philos Trans R Soc B 193:223–291
Brown P, Shrader W (1959) Grain yields, evapotranspiration, and water use efficiency of grain sorghum under different cultural practices. Agron J 51:339–343
Bugler G (1950) Un précurseur de la biologie expérimentale: Edme Mariotte. Rev Hist Sci (Paris) 3:242–250
Burgerstein A (1887) Materialien zu einer Monographie betreffend die Erscheinungen der Transpiration der Pflanzen. Verhandlungen der Zool Gesellschaft Wien 37:691–782
Burgerstein A (1889) Materialien zu einer Monographie, betreffend die Erscheinungen der Transpiration der Pflanzen. II. Theil. Verhandlungen der Zool Gesellschaft Wien 39:399–464
Cernusak LA, Aranda J, Marshall JD, Winter K (2007) Large variation in whole-plant water-use efficiency among tropical tree species. New Phytol 173:294–305
Article PubMed Google Scholar
Cernusak LA, Haverd V, Brendel O et al (2019) Robust response of terrestrial plants to rising CO 2 . Trends Plant Sci 24(7):578–586 1–9
Article CAS PubMed Google Scholar
Chen J, Chang SX, Anyia AO (2011) Gene discovery in cereals through quantitative trait loci and expression analysis in water-use efficiency measured by carbon isotope discrimination. Plant Cell Environ 34:2009–2023
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water-use efficiency and crop yield. Crop Sci 42:122–131
PubMed Google Scholar
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2004) Breeding for high water-use efficiency. J Exp Bot 55:2447–2460
Cowan IR, Farquhar GD (1977) Stomatal function in relation to leaf metabolism and environment. In: Integration of activity in the higher plant. University Press, pp 471–505
Cowan IR, Troughton J (1971) The relative role of stomata in transpiration and assimilation. Planta 97:325–336
Cregg B (2004) Improving drought tolerance of trees: theoretical and practical considerations. In: Acta Horticulturae Evaluation, Production and Use, XXVI International Horticultural Congress: Nursery Crops; Development. Aug 11-17, 2002, pp 147–158
Dachnowski A (1914) Transpiration in relation to growth and to the successional and geographical distribution of plants. Ohio Nat 14:241–251
Daubeny C (1836) On the action of light upon the atmosphere. Philos Trans R Soc 126:149–175
De La Torre A, Puiu D, Langley CH et al (2019) Genomic architecture of complex traits in loblolly pine. New Phytol 221:1789–1801
de Saussure N (1804) Chemische Untersuchungen über die Vegetation. Leipzig, 1890
Dehérain M (1869) L’évaporation de l’eau et la decomposition de l’acide carbonique par les feuilles des végétaux. Aannales des Sci Nat–Bot 5(XVII):5–23
Dekker SC, Groenendijk M, Booth BBB, Huntingford C, Cox PM (2016) Spatial and temporal variations in plant water-use efficiency inferred from tree-ring, eddy covariance and atmospheric observations. Earth Syst Dyn 7:525–533
Dietrich T (1872) Ueber die durch unsere Culturpflanzen verdunsteten Wassermengen. Mitth des landw Cent für den Regierungsbezirk Cassel 1872:343
Dreibelbis F, Harrold L (1958) Water-use efficiency of corn, wheat, and meadow crops. Agron J 50:500–5003
Eckert AJ, Maloney PE, Vogler DR, Jensen CE, Mix AD, Neale DB (2015) Local adaptation at fine spatial scales: an example from sugar pine ( Pinus lambertiana , Pinaceae ). Tree Genet Genomes 11:42
Egerton FN (2001a) A history of the ecological sciences: early Greek origins. Bull Ecol Soc Am 82:93–97
Egerton FN (2001b) A history of the ecological sciences, part 4: Roman natural history. Bull Ecol Soc Am 82:243–246
Egerton FN (2002) A history of the ecological sciences, part 7: Arabic language science: botany, geography, and decline. Bull Ecol Soc Am 83:261–266
Egerton FN (2003) A history of the ecological sciences, part 10: botany during the Italian Renaissance and beginnings of the scientific revolution. Bull Ecol Soc Am 84:130–137
Egerton FN (2004a) A history of the ecological sciences, part 12: invertebrate zoology and parasitology during the 1500s. Bull Ecol Soc Am 85:27–31
Egerton FN (2004b) A history of the ecological sciences, part 13: broadening science in Italy and England, 1600–1650. Bull Ecol Soc Am 85:110–119
Egerton FN (2004c) A history of the ecological sciences, part 14: plant growth studies in the 1600s. Bull Ecol Soc Am 85:208–213
Egerton FN (2005) A history of the ecological sciences, part 16: Robert Hooke and the Royal Society of London. Bull Ecol Soc Am 86:93–101
Egerton FN (2008) A history of the ecological sciences, part 28: plant growth studies during the 1700s. Bull Ecol Soc Am 89:159–175
Egle K, Ernst A (1949) Die Verwendung des Ultrarotabsorptionsschreibers für die vollautomatische und fortlaufende CO 2 -Analyse bei Assimilations-und Atmungsmessungen an Pflanzen. Zeitschrift für Naturforsch B 4:351–360
Ehleringer J, White J, Johnson D, Brick M (1990) Carbon isotope discrimination, photosynthetic gas exchange, and transpiration efficiency in beans and range grasses. Acta Oecol 11:611–625
Farquhar G (1980) Carbon isotope discrimination by plants: effects of carbon dioxide concentration and temperature via the ratio of intercellular and atmospheric CO 2 concentrations. In: Carbon dioxide and climate: Australian research. Australian Academy of Science, Canberra, pp 105–110
Farquhar GD, Rashke K (1978) On the resistance to transpiration of the sites of evaporation within the leaf. Plant Physiol 61:1000–1005
Article CAS PubMed PubMed Central Google Scholar
Farquhar GD, Richards PA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular CO2-concentration in leaves. Aust J Plant Physiol 9:121–137
Farsky F (1877) Ueber die Wasserverdunstung von Korn, Gerste und Erbse. Chem List [Chemische Blätter] tom I
Fittbogen J (1871) Altes und Neues aus dem Leben der Gerstenpflanze. Landwirtsch Versuchs-Stationen 13:81–136
Gaastra P (1959) Photosynthesis of crop plants as influenced by light, carbon dioxide, temperature, and stomatal diffusion resistance. Meded van Landbouwhoogeschool Wageningen 59:1–68
Govindjee, Krogmann D (2004) Discoveries in oxygenic photosynthesis (1727-2003): a perspective. Photosynth Res 80:15–57
Grew N (1682) The anatomy of plants. W. Rawlins, London
Grinsted M (1977) A study of the relationships between climate and stable isotope ratios in tree rings. University of Waikato PhD Thesis
Hales S (1727) Vegetable staticks, or, an account of some statical experiments on the sap in vegetables : being an essay towards a natural history of vegetation : also, a specimen of an attempt to analyse the air, by a great variety of chymio-statical experiments. W. and J Innys and T Woodward, London
Hellriegel (1871) Wie viel Wasser beanspruchen unsere Getreidearten zur Production einer vollen Ernte? Amtliches Vereinsblatt des landwirtlischaftlichen Prov fuer die Mark Brand und Niederlausitz 3:60–62
Hellriegel H (1883) Beiträge zu den Naturwiss. Grundlagen des Ackerbaus. F, Vieweg und Sohn, Braunschweig
Hershey D (2003) Misconceptions about Helmont’s willow experiment. Plant Sci Bull 49:78–83
Hobart C, Harris K (1946) Fitting cropping systems to water supplies in Central Arizona. College of Agriculture, University of Arizona, Tucson, AZ, USA
Hopkins J (1996) Nicholas of Cusa on wisdom and knowledge. Arthur Banning Press, Minneapolis
Hubick K, Farquhar G, Shorter R (1986) Correlation between water-use efficiency and carbon isotope discrimination in diverse peanut ( Arachis ) germplasm. Aust J Plant Physiol 13:803–816
Iljin V (1916) Relation of transpiration to assimilation in steppe plants. J Ecol 4:65–82
Ingen-Housz J (1779) Experiments upon vegetables, discovering their great power of purifying the common air in the sunshine and of injuring it in the shade and at night. P. Elmsly, and H. Payne, London
Kearney TH, Shantz HL (1911) The water economy of dry-land crops. Yearb United States Dep Agric 10:351–362
Kiesselbach T (1916) Transpiration as a factor in crop production. Bull Agric Exp Stn Nebraska 6:19–38
King F (1889) Soil physics. Annu Rep Agric Exp Stn Univ Wisconsin 6:189–206
Kolkunov W (1905) Contributions to the problem of breeding drought resistant crop plants. I. Anatomical and Physiological investigations of the degree of xerophily of certain cereals. Mém Polytech Inst Kiev 5(4):
Lavoisier A-L (1770) Sur la nature de l’eau et sur les expériences par lesquelles on a prétendu prouver la possibilité de son changement en terre. Mémoires l’Académie des Sci:73–82
Lawes JB (1850) Experimental investigation into the amount of water given. J Hortic Soc London 5:38–63
Lawes JB (1851) Report upon some experiments undertaken at the suggestion of Professor Lindley, to ascertain the comparative evaporating properties of evergreen and deciduous trees. J Hortic Soc London 6:227–242
Lazenby EM (1995) The historia plantarum generalis of John Ray: book i - a translation and commentary. Newcastle University PhD thesis
Leather JW (1910) Water requirements of crops in India. Mem Dep Agric India Chem Ser 1(3):133–154
Leather JW (1911) Water requirements of crops in India. -II. Mem Dep Agric India Chem Ser 1:205–281
Lévesque M, Siegwolf R, Saurer M, Eilmann B, Rigling A (2014) Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New Phytol 203:94–109
Linares J, Camarero J (2012) From pattern to process: linking intrinsic water-use efficiency to drought-induced forest decline. Glob Chang Biol 18:1000–1015
Luft K (1943) Über eine neue Methode der registrierenden Gasanalyse mit Hilfe der Absorption ultraroter Strahlen ohne spektrale Zerlegung. Z Tech Phys 24:97–104
Malpighi M (1675) Anatome Plantarum. Johannis Martyn, London
Marié-Davy H (1869) Evaporation du sol et des plantes. J d’Agriculture Prat 2:234–239
Marié-Davy H (1874) Note sur la quantité d’eau consommée par le froment pendant sa croissance. Comptes rendus Hebd des séances l’Académie des Sci 79:208–212
Maskell EJ, Blackman FF (1928) Experimental researches on vegetable assimilation and respiration. XVIII.—The relation between stomatal opening and assimilation.—A critical study of assimilation rates and porometer rates in leaves of Cherry Laurel. Proc R Soc Lond Ser B 102:488–533
Maximov NA (1929) The plant in relation to water. George Allen & Unwin LTD, London
Maximov NA, Alexandrov V (1917) The water requirement and drought resistance of plants. Trav du Jard Bot Tiflis 19:139–194
McCarroll D, Loader NJ (2004) Stable isotopes in tree rings. Quat Sci Rev 23:771–801
Medlyn BE, De Kauwe MG, Lin YS et al (2017) How do leaf and ecosystem measures of water-use efficiency compare? New Phytol 216:758–770
Meinzer FC, Ingamells JL, Crisosto C (1991) Carbon isotope discrimination correlates with bean yield of diverse coffee seedling populations. Hort Sci 26:1413–1414
Möbius M (1901) Die Anatomie der Pflanzen I: and II. Theil. Engelmann, W, Leipzig
Mohl H (1856) Welche Ursachen bewirken die Erweiterung und Verengung der Spaltöffnungen? Bot Zeitung 14:697–704
Möldenhawer J (1812) Beyträge zur Anatomie der Pflanzen. CL Wäser, Kiel
Montgomery E (1911) Methods of determining the water requirements of crops. Proc Am Soc Agron 3:261–283
Moore B (1924) Reviewed work: Physiological requirements of Rocky Mountain trees by Carlos G. Bates Ecology 5:298–302
Morton A (1981) History of botanical science: an account of the development of botany from ancient times to the present day. Academic Press, London
Nickelsen K (2007) From leaves to molecules: botany and the development of photosynthesis research. Ann Hist Philos Biol 12:1–40
Park R, Epstein S (1960) Carbon isotope fractionation during photosynthesis. Geochim Cosmochim Acta 21:110–126
Pellizzari E, Camarero JJ, Gazol A, Sangüesa-Barreda G, Carrer M (2016) Wood anatomy and carbon-isotope discrimination support long-term hydraulic deterioration as a major cause of drought-induced dieback. Glob Chang Biol 22:2125–2137
Penman HT, Schofield RK (1951) Some physical aspects of assimilation and transpiration. Symp Soc Exp Biol 5:115–129
Perrault C (1680) Essais de Physique. Jean Baptiste Coignard, Paris
Picon C, Guehl J-M, Aussenac G (1996) Growth dynamics, transpiration and water-use efficiency in Quercus robur plants submitted to elevated CO 2 and drought. Ann des Sci For 53:431–446
Plomion C, Bartholomé J, Bouffier L et al (2016) Understanding the genetic bases of adaptation to soil water deficit in trees through the examination of water use efficiency and cavitation resistance: maritime pine as a case study. J Plant Hyd 3:008
Polster H, Weise G, Neuwirth G (1960) Ecological researches on the CO 2 balance [net assimilation] and water economy of some tree species in sand and alkali soils in Hungary. Arch für Forstwes 9:947–1014
Porter R (1979) John Woodward; ‘A droll sort of philosopher’. Geol Mag 116:335–343
Priestley J (1775) Experiments and observations on different kinds of air. J.Johnson, London
Raber O (1937) Water utilization by trees, with special reference to the economic forest species of the north temperate zone. USDA Misc Pub No 257, Washington DC
Ray J (1686) Historia Plantarum, I edn. The Royal Society, London
Ray J (1691) The wisdom of God manifested in the works of creation ; first published in 1691: reprinted by the Wernerian Club, London 1844-1846
Roeser J (1940) The water requirement of Rocky Mountain conifers. J For 38:24–26
Rötzer T, Liao Y, Goergen K, Schüler G, Pretzsch H (2013) Modelling the impact of climate change on the productivity and water-use efficiency of a central European beech forest. Clim Res 58:81–95
Roupsard O, Joly HI, Dreyer E (1998) Variability of initial growth, water-use efficiency and carbon isotope discrimination in seedlings of Faidherbia albida (Del.) A. Chev., a multipurpose tree of semi-arid Africa. provenance and drought effects. Ann des Sci For 55:329–348
Rundel P, Ehleringer J, Nagy K (1989) Stable isotopes in ecological research. Springer-Verlag, New York
Book Google Scholar
Rüsch J (1959) Das Verhältnis von Transpiration und Assimilation als physiologische Kenngröße, untersucht an Pappelklonen. Theor Appl Genet 29:348–354
Scarth GW (1927) Stomatal movement: its regulation and regulatory role - a review. Protoplasma 2:498–511
Schmidt H-L, Winkler F (1979) Einige Ursachen der Variationsbreite von ẟ 13 C-Werten bei C3- und C4-Pflanzen. Ber Dtsch Bot Ges 92:S 185–S 191
Schröder M (1895) The transpiration of various crop plants. Agric For 10:320–336
Shantz HL (1927) Drought resistance and soil moisture. Ecology 8:145–157
Sharrock R (1660) The history of the propagation & improvement of vegetables. A. Lichfield, Oxford
Sinclair TR, Tanner CB, Bennett JM (1984) Water-use efficiency crop production. Bioscience 34:36–40
Sorauer P (1880) Studien über Verdunstung. Wollny - Forschungen auf dem Gebiete der Agrik tom 3:351–490
Spedding J, Ellis R, Heath D (1900) The works of Francis Bacon, Houghton, Mifflin and Company
Stanhill G (1986) John Woodward - a neglected 17th century pioneer of experimental botany. Isr J Bot 35:225–231
Tallec T, Béziat P, Jarosz N, Rivalland V, Ceschia E (2013) Crops’ water use efficiencies in temperate climate: comparison of stand, ecosystem and agronomical approaches. Agric For Meteorol 168:69–81
Tanner CB, Sinclair TR (1983) Efficient water use in crop production: research or re-search? In: Taylor HM, Jordan WR, Sinclair TR (eds) Limitations to efficient water use crop production. American Society of Agronomy, Madison, pp 1–27
Thornthwaite C (1947) Climate and moisture conservation. Ann Assoc Am Geogr 37:87–100
Tomic S, Cussenot M, Dreyer E (2005) La lumiére et les plantes : histoire de la découverte de la « photosynthése », 1779-1804. In: Changeux J-P (ed) La lumière au siècle des lumières et aujourd’hui: Art et science. Odile Jacob, Paris, pp 145–161
Troughton J (1969) Plant water status and carbon dioxide exchange of cotton leaves. Aust J Biol Sci 22:289–302
Tschaplowitz (1878) Ueber die Verdunstung und Substanzzunahme der Pflanzen. Berichte der Sect für Landwirtsch Versuchswes auf der Naturforscherversammlung zu München 1877(tome XX):74
Unger F (1862) Neue Untersuchungen über die Transpiration der Pflanzen. Sitzungsberichte der Kais Akad der Wissenschaften Wien 44:181–217 and 327-368
Vadez V, Kholova J, Medina S, Kakkera A, Anderberg H (2014) Transpiration efficiency: new insights into an old story. J Exp Bot 65:6141–6153
van Helmont J (1662) Oriatrike or Physick Refined. Lodowick Loyyd, London
Vialet-Chabrand S, Matthews JSA, Brendel O, Blatt MR, Wang Y, Hills A, Griffiths H, Rogers S, Lawson T (2016) Modelling water use efficiency in a dynamic environment: an example using Arabidopsis thaliana. Plant Sci 251:65–74
Viets FG (1962) Fertilizers and the efficient use of water. Adv Agron 14:223–264
Virgona J, Hubick K, Rawson H et al (1990) Genotypic variation in transpiration efficiency, carbon-isotype discrimination and carbon allocation during early growth in sunflower. Aust J Plant Physiol 17:207–214
Vivas M, Rolo V, Wingfield MJ, Slippers B (2019) Maternal environment regulates morphological and physiological traits in Eucalyptus grandis . For Ecol Man 432:631–636
Vogel JC (1980) Fractionation of the carbon isotopes during photosynthesis. In: Sitzungsberichte der Heidelberger Akademie der Wissenschaften. Springer, New York, pp 111–135
von Höhnel FR (1879) Ueber die Wasserverbrauchsmengen unserer Forstbäume mit Beziehung auf die forstlich-meteorologischen Verhältnisse. Wollny - Forschungen aus dem Gebiet der Agric tom II:398–421
von Schleiden MJ (1849) Grundzüge der wissenschaftlichen Botanik, 3rd edn. Verlag von Wilhelm Engelmann, Leipzig
von Seelhorst C (1902) Vegetationskästen zum Studium des Wasserhaushaltes im Boden. J Landwirtsch 50:277–280
Woodward J (1699) Some thoughts and experiments concerning vegetation. Philos Trans R Soc Lond A 21:193–227
Zhang J, Marshall JD (1994) Population differences in water-use efficiency of well-watered and water-stressed western larch seedlings. Can J For Res 24:92–99
Download references
Acknowledgements
Much of the historical background is based on A.G. Morton’s “History of Botanical Sciences” as well as to Frank N. Egerton’s “A History of the Ecological Sciences” series in the “Bulletin of the Ecological Society of America”. The author is also largely indebted to C. Schuchardt from the Library of the Staatsbetrieb Sachsenforst for help with the quest for rare German publications. The author would also like to thank E. Dreyer and J.M. Guehl (both from the SILVA Unit at INRAE Nancy, France) who commented extensively on an earlier version of the draft and J. Williams (University of Sussex), L. Handley and J. Raven (University of Dundee) who made many valuable suggestions and improved language.
Author information
Authors and affiliations.
Université de Lorraine, AgroParisTech, INRAE, UMR Silva, Nancy, France
Oliver Brendel
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Oliver Brendel .
Ethics declarations
Conflict of interest.
The author declares no competing interests.
Additional information
Handling Editor: Andrew Merchant
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Brendel, O. The relationship between plant growth and water consumption: a history from the classical four elements to modern stable isotopes. Annals of Forest Science 78 , 47 (2021). https://doi.org/10.1007/s13595-021-01063-2
Download citation
Received : 26 November 2020
Accepted : 14 April 2021
Published : 28 April 2021
DOI : https://doi.org/10.1007/s13595-021-01063-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Transpiration efficiency
- Water use efficiency
- Plant ecophysiology
- Botanical history
Annals of Forest Science
ISSN: 1297-966X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
How light and temperature work together to affect plant growth
The findings may help scientists develop more resilient plants to help withstand climate change.
Plants lengthen and bend to secure access to sunlight. Despite observing this phenomenon for centuries, scientists do not fully understand it. Now, Salk scientists have discovered that two plant factors -- the protein PIF7 and the growth hormone auxin -- are the triggers that accelerate growth when plants are shaded by canopy and exposed to warm temperatures at the same time.
The findings, published in Nature Communications on August 29, 2022, will help scientists predict how plants will respond to climate change -- and increase crop productivity despite the yield-harming global temperature rise.
"Right now, we grow crops in certain densities, but our findings indicate that we will need to lower these densities to optimize growth as our climate changes," says senior author Professor Joanne Chory, director of Salk's Plant Molecular and Cellular Biology Laboratory and Howard Hughes Medical Institute investigator. "Understanding the molecular basis of how plants respond to light and temperature will allow us to fine-tune crop density in a specific way that leads to the best yields."
During sprouting, seedlings rapidly elongate their stems to break through the covering soil to capture sunlight as fast as possible. Normally, the stem slows down its growth after exposure to sunlight. But the stem can lengthen rapidly again if the plant is competing with surrounding plants for sunlight, or in response to warm temperatures to increase distance between the hot ground and the plant's leaves. While both environmental conditions -- canopy shade and warm temperatures -- induce stem growth, they also reduce yield.
In this study, the scientists compared plants growing in canopy shade and warm temperatures at the same time -- a condition that mimics high crop density and climate change. The scientists used the model plant Arabidopsis thaliana, as well as tomato and a close relative of tobacco, because they were interested to see if all three plant species were affected similarly by this environmental condition.
Across all three species, the team found that the plants grew extremely tall when simultaneously trying to avoid the shade created by neighboring plants and being exposed to warmer temperatures. On a molecular level, the researchers discovered that transcription factor PIF7, a protein that helps turn genes "on" and "off," was the dominant player driving the increased rapid growth. They also found that the growth hormone auxin increased when the crops detected neighboring plants, which fostered growth in response to simultaneous warmer temperatures. This synergistic PIF7-auxin pathway allowed the plants to respond to their environments and adapt to seek the best growing conditions.
A related transcription factor, PIF4, also stimulated stem elongation during warm temperatures. However, when shade and increased temperatures were combined, this factor no longer played an important role.
"We were surprised to find that PIF4 did not play a major role because prior studies have shown the importance of this factor in related growth situations," says first author Yogev Burko, a Salk staff researcher and assistant professor at the Agriculture Research Organization at the Volcani Institute in Israel. "The fact that PIF7 is the dominant driving force behind this plant growth was a real surprise. With this new knowledge, we hope to fine-tune this growth response in different crop plants to help them adapt to climate change."
The researchers believe that there is another player, yet to be discovered, that is boosting the effect of PIF7 and auxin. They hope to explore this unknown factor in future studies. Burko's lab will also be studying how this pathway can be optimized in crop plants.
"Global temperatures are increasing, so we need food crops that can thrive in these new conditions," says Chory, who co-directs Salk's Harnessing Plants Initiative and holds the Howard H. and Maryam R. Newman Chair in Plant Biology. "We've identified key factors that regulate plant growth during warm temperatures, which will help us to develop better-performing crops to feed future generations."
The work was funded by the National Institutes of Health (5R35GM122604-05_05), Howard Hughes Medical Institute, Knut and Alice Wallenberg Foundation (KAW 2016.0341 and KAW 2016.0352), Swedish Governmental Agency for Innovation Systems (VINNOVA 2016-00504), EMBO Fellowships (ALTF 785-2013 and ALTF 1514-2012), BARD (FI-488-13), Human Frontier Science Program (LT000222/2013-L) and Salk's Pioneer Postdoctoral Endowment Fund.
- Endangered Plants
- Agriculture and Food
- Rainforests
- Global Warming
- Sustainability
- Temperature record of the past 1000 years
- Global warming
- Hydroponics
- Paleoclimatology
- Global warming controversy
- Attribution of recent climate change
- Climate engineering
Story Source:
Materials provided by Salk Institute . Note: Content may be edited for style and length.
Journal Reference :
- Yogev Burko, Björn Christopher Willige, Adam Seluzicki, Ondřej Novák, Karin Ljung, Joanne Chory. PIF7 is a master regulator of thermomorphogenesis in shade . Nature Communications , 2022; 13 (1) DOI: 10.1038/s41467-022-32585-6
Cite This Page :
Explore More
- Giant Viruses Found On Greenland Ice Sheet
- Using AI to Decode Dog Vocalizations
- Humans and Woolly Rhinoceros' Extinction
- More Summer Droughts for Northern Hemisphere
- All Electrical Needs from Floating Solar Panels?
- Over 60% of US People Likely to Have CVD by 2050
- Gigantic Jurassic Pterosaur Fossil Unearthed
- Bringing Back an Ancient Bird
- How Quantum Field Theories Decay and Fission
- River Nile's Evolution During Ancient Egypt
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Plants (Basel)

Reaching Natural Growth: Light Quality Effects on Plant Performance in Indoor Growth Facilities
Camilo chiang.
1 Department of Environmental Sciences—Botany, University of Basel, Schönbeinstrasse 6, 4056 Basel, Switzerland; [email protected]
2 Department of Research and Development, Heliospectra, Fiskhamnsgatan 2, 414 58 Gothenburg, Sweden; [email protected]
Daniel Bånkestad
Günter hoch, associated data.
To transfer experimental findings in plant research to natural ecosystems it is imperative to reach near to natural-like plant performance. Previous studies propose differences in temperature and light quantity as main sources of deviations between indoor and outdoor plant growth. With increasing implementation of light emitting diodes (LED) in plant growth facilities, light quality is yet another factor that can be optimised to prevent unnatural plant performance. We investigated the effects of different wavelength combinations in phytotrons (i.e., indoor growth chambers) on plant growth and physiology in seven different plant species from different plant functional types (herbs, grasses and trees). The results from these experiments were compared against a previous field trial with the same set of species. While different proportions of blue (B) and red (R) light were applied in the phytotrons, the mean environmental conditions (photoperiod, total radiation, red to far red ratio and day/night temperature and air humidity) from the field trial were used in the phytotrons in order to assess which wavelength combinations result in the most natural-like plant performance. Different plant traits and physiological parameters, including biomass productivity, specific leaf area (SLA), leaf pigmentation, photosynthesis under a standardised light, and the respective growing light and chlorophyll fluorescence, were measured at the end of each treatment. The exposure to different B percentages induced species-specific dose response reactions for most of the analysed parameters. Compared with intermediate B light treatments (25 and/or 35% B light), extreme R or B light enriched treatments (6% and 62% of B respectively) significantly affected the height, biomass, biomass allocation, chlorophyll content, and photosynthesis parameters, differently among species. Principal component analyses (PCA) confirmed that 6% and 62% B light quality combinations induce more extreme plant performance in most cases, indicating that light quality needs to be adjusted to mitigate unnatural plant responses under indoor conditions.
1. Introduction
Temperature and light are principal determinants of plant growth, as plants react to environmental conditions in their development. With improvements in controlled environment facilities, the use of indoor cultivation systems has increased worldwide, both for research and plant production. One of the problems, that especially plant researchers are confronted with, is a clear difference between plants grown under indoor versus outdoor conditions. These differences are limiting the transferability of results from indoor experiments to natural systems. Several experiments have tried to replicate outdoor growth in indoor facilities, but low correlations have been found [ 1 , 2 ]. Poorter et al., [ 3 ] suggested that this difference comes mainly from the different photothermal ratio (PTR), the ratio between the daily light integral and the daily mean temperature, which is generally much lower in growth chambers. The low PTR in indoor experiments mainly derives from the low and constant irradiances used, compared with the higher and variable sunlight conditions found in nature. In general, conditions in indoor facilities lead to higher specific leaf area (SLA), leaf nitrogen content, and relative growth rate. While maximum photosynthesis (A max ), plant height, and shoot dry weight (SDW), are lower compared with outdoor experiments [ 3 ].
Due to the high photosynthetic efficiency of blue (B) and red (R) light, high electrical efficiency of B and R LEDs, as well as the high technical requirements to create sun-like LED spectra [ 4 , 5 ], most existing indoor plant growth facilities with LED lighting systems use mixtures of mainly B and R light. However, different LED lamps use different proportions of B and R LEDs, or B and R in combination with other LED types, such as white and far-red. This results in very different lighting environments among different indoor growth facilities. In addition, the lack of a common protocol for reporting and measuring LED light irradiance further limits the comparability between experiments [ 6 ]. Many studies have investigated plant response to different B to R ratios. These studies revealed that independent of light intensity, a required minimum percentage of B light is necessary to maintain the activities of photosystem II and I [ 7 ]. Hogewoning et al., [ 8 ] suggested that at least 7% B light is necessary to reproduce near-natural plant growth. In addition, it has been observed that long exposures of monochromatic light can have drastic effects, including non-natural morphologies. With parameters such as shoot elongation, specific leaf area (SLA), chlorophyll concentration and photosynthetic performance being affected [ 9 , 10 , 11 , 12 ].
The vast majority of studies related to light quality effects on plants have been conducted under low light levels, varying between 20 to 330 µmol m −2 s −1 [ 13 , 14 , 15 , 16 , 17 , 18 ], with a few exceptions (for example 550 µmol m −2 s −1 [ 19 ]), even though interactions between light quantity and quality have been reported previously [ 9 ]. Finally, it is also important to consider other light quality related parameters, for example, the effect of red to far red ratio (R:FR). The applied light conditions in indoor cultivation typically has a much higher R:FR ratio (or a complete absence of FR) compared with sunlight conditions. This affects plant photosynthesis, morphology, and development (for example [ 8 , 10 , 14 , 15 , 18 , 19 , 20 ]). Once the R:FR ratio is corrected to more natural values, a more natural-like growth may be achieved, despite the large deviations from natural sunlight in other parts of plant biologically active radiation (280–800 nm; for example [ 21 ])
The aim of this study is to provide the first step in a series of experiments with the overall goal of reaching nature-like growth of plants under indoor conditions. Specifically, we investigate the effects of varying proportions of B and R light within walk-in growth chambers (phytotrons) on growth and physiological traits of plants from different functional groups. We also compared our findings to the same species grown in a natural-light field trial, where we expected more “natural-like” growth in our indoor treatments that applied a closer to natural light spectra. The inclusion of seven different species from different functional plant types further enabled us to identify if light quality affects plant performance differently among species and plant types. In contrast to many previous studies, we explicitly applied more natural-like R:FR ratios and light intensities [ 8 , 9 , 10 , 11 , 12 , 13 , 14 ], and the plants were exposed to temperatures and air humidity based on the pre-measured field trial.
2.1. Light Treatments
Four different treatments were obtained through calibrating the phytotrons for the desired spectra as indicated in Table 1 and Figure 1 .

Applied spectra for the field trial and each of the different light treatment where 6%, 25%, 35% and 62% refers to the percentage of blue light as percentage of the photosynthetic photon flux density (PPFD) (In other words, excluding far-red). The integrated area between 400 and 700 nm corresponds to an approximate 575 μmol m −2 s −1 of photosynthetic photon flux density in each case.
Spectral characteristics of sunlight and of the indoor light treatments, based on the measured spectra shown in Figure 1 .
2.2. Plant Growth and Biomass Allocation
There was a significant interaction between the light treatments and the different species on the total plant height at the end of the experiments ( Table 2 ), where the relationship with the field trial was species dependent. Some species, for example, Alnus and Melissa , were significantly smaller independent of the light treatment, while others, for example, Ocimum , were taller than the same species in the field trial.
p -values derived from the full-factorial ANOVA analyses of the different measured plant traits, with light treatment and species as fixed factors, and the replicates of the individual light treatments as random factors. Non-significant p -values (≥0.05) are indicated as “-”.
* Lettuce was removed from these analyses. ** Interactions or factors were removed from the analysis due non-significance.
Comparing only among the phytotron treatments, all species had shorter individuals at higher percentages of blue (B) light (62%), which was most pronounced in Alnus and Melissa (58 and 52% lower height respectively, compared with the 6% B treatment; Figure 2 A). Other species like Ocimum and Triticum were less affected by changes in B light, but follow the same trend (20 and 15% lower height respectively, compared with the 6% B treatment; Figure 2 A). In several of the tested species, there was a significant difference in plant height between the two intermediate B treatments (25 and 35% B). Averaged across species, 6% B light produced 22% taller plants that were statistically significantly different from the two intermediate treatments. While in the other extreme, 62% B light yielded a statistically significant shortening of plants by approximately 20% compared with the average across treatments ( Figure 2 A). A dose response was obtained for specific leaf area in several species (SLA, Figure 2 B). Unlike the height results, and due to the species-specific reactions to the light treatments, the average response across species did not significantly differ, neither within the light treatments, nor between the light treatments and the outdoor control. However, Lactuca and Alnus, for example, had significant higher SLA at 6% B compared with other light treatments, while other species, for example, Raphanus and Triticum, had higher values at 25 or 35% B light compared with 6 or 62% B light.

Fold change on: plant height ( A ) and SLA ( B ), relative to the average field trial (dotted line). Coloured dots are the average of each species in both experiment runs ( n = 18), the black dots are the average values across all 7 species ( n = 126). Error bars indicate the standard errors. The grey area corresponds to the standard error of the field trial. Different letters indicate statistical difference between groups with experiment replicate and species as a random effect.
There were significant interactions between the light treatments and species for the dry biomass of leaves, shoots, roots and the total dry biomass ( Table 2 ). Similar to plant height and SLA, the relationship between plant biomass and light, under the different light treatments, with the field control was species dependent, yet averaged across all species. Leaf biomass did not significantly differ from the outdoor control in any of the light treatments.
If only the phytotron treatments are compared, there was a lower leaf biomass under 62% B light compared with 6% B light in all investigated species. This was especially the case for the two tree species tested, where Alnus and Ulmus were most sensitive to high percentages of B light ( Figure 3 A). On average, plants exposed to 6% B had 35% higher leaf biomass than plants exposed to 62% B ( Figure 3 A). Similar results were obtained for shoot biomass where, across all species, plants grown at 62% B had a significantly lower shoot biomass compared with all the other light treatments, and yet similar values as in the field trial (except for Ulmus and Ocimum , Figure 3 B). In contrast to the aboveground biomass, the effects of light quality on root biomass were different among all species ( Figure 3 C). In comparison to the field trial, four species ( Ulmus , Lactuca , Ocimum , Triticum ) had significantly higher root biomass in the phytotron treatments, while in three species ( Raphanus , Alnus , Melissa ) it was similar compared to the field trial ( Figure 3 C).

Fold change on: leaves ( A ), shoot ( B ), roots ( C ) and root to shoot ratio ( D ), as dry weight relative to the average value of the field trial (dotted line). Coloured dots are the average of each species in both experiments runs ( n = 18), the black dots are the average values across all 7 species ( n = 126). Error bars indicate the standard errors. The grey area corresponds to the standard error of the field trial. Different letters indicate statistical difference between groups with experiment replicate and species as a random effect.
Across all species, there was no strong effect of light quality on root biomass, but a trend to higher root biomass at 6% B ( Figure 3 C). Total biomass production followed the same trend as found for the individual plant organs, with a significant interaction between light treatment and species ( Table 2 ); higher values under indoor conditions independent of the light treatment, compared to the field trial and increasing biomass with increasing percentage of blue light (data not shown).
With respect to the effect of light quality on the allocation of biomass, there was a significant interaction between light treatment and species for the root to shoot (r:s) mass ratio ( Table 2 ). Almost all species had significantly higher r:s values in the phytotrons compared to the field trial independent of the light treatment, with Triticum showing a four to eight times higher investment in roots compared with the field control ( Figure 3 D). In some species (e.g., Alnus and Ocimum ), 6% and 62% B light induced higher r:s ratios than 25 and 35% B light, while other species (e.g., Melissa and Ulmus ) were almost indifferent with respect to light quality ( Figure 3 D).
2.3. Leaf Pigmentation
There were significant interactions between the different treatments and species in the pigment concentrations of the leaves ( Table 2 ). Furthermore, the difference between the field trial and the different light treatments was species dependent, but all investigated species exhibited higher Chl a concentration in leaves at 62% B light compared to the other light treatments (strongest effect in Lactuca ) and several species exhibited the lowest Chl a concentrations at 6% B light ( Figure 4 A).

Fold change on Chlorophyll a ( A ), Chlorophyll a:b ratio ( B ), carotenoids content ( C ) and Fv/Fm values ( D ) relative to the average value of the field trial (dotted line). Coloured dots are the average of each species in both experiments runs ( n = 18), the black dots are the average values across all 7 species ( n = 126). Error bars indicate the standard errors. The grey area corresponds to the standard error of the field trial. Different letters indicate statistically difference between groups with experiment replicate and species as a random effect.
On average across all species, 6% B was the only treatment significantly different from the field trial, with 24% lower concentration of Chl a. The effect on Chl b was similar to that of Chl a, with a smaller effect of the light quality on the total amount of Chl b (data not shown). As a result, the average a:b ratio across all species was not significantly different among the light treatments, but significantly higher than in the field trial ( Table 2 , Figure 4 B). The concentrations of carotenoids in leaves, showed overall very similar reactions to light quality as chlorophyll, with increasing concentrations at higher proportions of blue, and an interaction between the light treatment and species ( Figure 4 C, Table 2 ). Like chlorophyll and carotenoids, the Fv/Fm values, showed significant interaction between the species and the light treatments ( Table 2 ). Almost all species in the phytotron treatments with 25, 35 and 62% B had Fv/Fm values close to the field trial ( Figure 4 D), except Ocimum , which revealed higher Fv/Fm values indoors than in the field. Averaged across all species, Fv/Fm was significantly lower than in the field at 6% B ( Figure 4 D). Performance index (Pi) absolute values followed the same trend as Fv/Fm (data not shown, Supplementary Table S1 ).
2.4. Photosynthesis and Leaf Respiration
In contrast to the other plant traits tested, all species reacted uniformly to the light treatments in all measured photosynthesis and leaf gas exchange parameters, with no significant interaction between treatment and species effect found ( Table 2 ). When measured with the standardised light of the gas exchange chamber, the average maximum photosynthesis (A max ) across all species was significantly higher in plants raised at 62% B compared with the field trial ( Figure 5 A). Meanwhile, when the same parameter was measured under the in situ light, higher values were reached at either 25% or 35% B light compared with the field trial ( Figure 5 B). The quantum yield of the CO 2 fixation (α) had similar trends to A max , where on average no light treatment was significantly higher than the field trial when the standardised light was used. The 62% B light was the only treatment to induce higher α values than the other light treatments ( Figure 5 C). When α was measured using the in situ light, higher values were reached at either 6%, 25% or 35% B compared to the field trial ( Figure 5 D).

Fold change on maximum photosynthesis ( A max , A , B ), quantum yield of the CO 2 fixation curve (α, C , D ) and dark respiration (DR, E , F ) relative to the average value of the field trial (dotted line). Values were measured with either a standard light with 70% B light and 30% R light (‘standardised light’) or the actual ‘in situ’ light (see methods for details). Coloured dots are the average of each species in both experiments runs ( n = 18), the black dots are the average values across all 7 species ( n = 126). Error bars indicate the standard errors. The grey area corresponds to the standard error of the field trial. Different letters indicate statistical difference between groups with experiment replicate and species as a random effect.
The photosynthetic light compensation point (CP) and the dark respiration of leaves (DR) were significantly different among species ( Table 2 ). Averaged across all species, there were no significant effects of the treatments on CP when the standardised light was used. However, with in situ light significantly lower values were reached under 6 and 25% B conditions, compared with 35 and 62% B and the field trial (data not shown). DR was on average significantly lower in plants exposed to 62% B light compared with other light treatments and the field trial when the standardised light was used ( Figure 5 E). This was not the case for the in situ light, where although several species had higher DR values than the field trial, no significant difference was found between the treatments for the average across species ( Figure 5 F).
2.5. Principal Component Analysis (PCA)
Principal component analysis (PCA) for each species revealed a clustering of each treatment with varying degrees of overlap ( Figure 6 ); from easily differentiable groups between light treatments in some species, for example, Alnus , Lactuca and Triticum , to a more continuous gradient among treatments.

Principal component analysis (PCA) of the measured traits of each species: ( A ) Alnus , ( B ) Ulmus , ( C ) Ocimum , ( D ) Lactuca , ( E ) Melissa , ( F ) Raphanus and ( G ) Triticum , grown under 6% B, 25% B, 35% B and 62% B light. Each lighter point ( n = 18) corresponds to a plant and solid ones to the average weighted centroids of each light treatment, where the name of each species is mentioned in the respective upper right corner. Ellipses correspond to the standard error of the weighted centroids with a confidence interval of 95%.
Melissa , Raphanus , Alnus , Ocimum , Lactuca , and Triticum showed a large variability between treatments from outdoor (field trial) to indoor conditions, while the different light treatments tended to cluster. This was not the case for Melissa , Raphanus , and Ulmus , where the field trial was not clearly separated from the phytotron treatments ( Figure 6 ). The two intermediate treatments (25% and 35% B) yielded responses closer to the average (i.e., the centre of the figure) in most species. The loadings for score calculations were also plotted to determine the importance of each factor. No single parameter was specifically responsible for the variation across treatments and between species, except for CP in Ocimum growing in the field trial ( Figure S1 ). Independent of the species the first two components explained between 31% and 43% of the total variability.
3. Discussion
Previous studies investigating the effect of the spectral light quality on plant performance were mainly focused on single species, and they generally did not directly compare findings with natural conditions. In the present study, we deliberately investigated a suite of species from different functional plant types to determine if, and how, they react to the different treatments. Through application of the same mean climatic conditions indoors, as in the initial field trial, we could better assess which LED light conditions are generating the most natural-like plant performance. Our results showed clear differences within and between the light treatments when compared to the field trial on most measured plant traits. The effect sizes were highly species-specific, while effect directions were similar among species, with the clear exception of SLA and root biomass production. As expected, light treatments with very extreme blue: red (B:R) ratios (6 and 62% B) induced more extreme (‘unnatural’) values in most plant traits than treatments with a more balanced B:R ratio (25 and 35% B).
3.1. Light Quality Effects on Morphology
Studies that compared indoor with outdoor plant growth were previously often biased by a higher plant density in the indoor condition [ 3 ]. In our study, we deliberately kept the exact same plant densities between the field and the phytotron trials to avoid any stand density bias on plant morphology. The effects of B light percentages on plant morphology have been previously reported in several studies [ 8 , 11 , 12 , 21 , 22 , 23 , 24 , 25 ]. In general, B light is sensed by the cryptochrome system, where under high irradiances or high levels of B light, plants exhibit shorter and stunted growth (For example [ 8 , 14 , 26 ]). It is also known that a total lack of B or R light negatively affects plant performance, including growth rate, height, photosynthesis and several other parameters. For example, Hernandez et al. [ 10 ] found that tomato plants grew shorter under either B or R light mixtures compared with only B or R light.
Previous studies have shown that under high levels of B light, there is an increase in the palisade cell area, which can lead to an increase in leaf thickness (For example [ 8 , 10 , 12 ]). However, this B light-induced increase in leaf thickness does not necessarily have to translate into a lower SLA [ 27 ]. Dougher and Budgee [ 22 ] identified that the direction of the effect of B light on SLA is very species dependent. Independent of the applied light quality, Poorter et al. [ 3 ] found that on average, indoor experiments tend to produce plants with higher SLA compared to field grown plants, mainly due to higher temperatures and lower light quantity in indoor facilities. In our study, which applied the average temperature and light quantity as in the field trial, the SLA of most species was similar between plants growing in the phytotrons and in the field.
Under the different treatments stem, leaf, root, and total dry biomass largely followed the trend in plant height. The lower biomass at high B% can thus be explained by a stronger inhibition of stem elongation by B light due to an increased cryptochrome activity [ 14 ], exposing the plants to lower irradiance due to larger distances to the light source compared with plants treated under a lower percentage of B light. In addition, the stunted growth of plants at high B% leads to an increased self-shading of leaves and decrease in light interception, which has been proposed to result in negative consequences for the whole plant productivity [ 21 ]. Although the individual species reacted differently between phytotrons and the field trial, on average, a significantly higher plant biomass within our phytotron treatments compared with the field was found (except for the 62% B treatment). In contrast, Poorter et al. [ 3 ] reported lower biomass under indoor conditions compared with field grown plants depending on species and functional group. Again, this apparent contradiction could be explained by the fact that in contrast to other indoor experiments, we deliberately applied the same average temperatures and light strength in the phytotrons as were measured in the field trial. Poorter et al. [ 3 ] demonstrated that indoor experiments often use low levels of light, which might reduce plant biomass in comparison with outdoor-grown plants.
While the effect of light quality on the aboveground organs was quite similar among species in the current study, the direction of the effect on roots was clearly species dependent. With species such as Alnus and Ocimum exhibiting higher root growth at very low and high B%, and species such as Raphanus and Ulmus showing increased root production at intermediate B percentages (25 and 35% B). To date, scarce information is available on the effects of light quality on belowground plant productivity. A previous study by Yorio et al. [ 28 ] reported that under 10% B mixed with 90% R light there was a higher root production in Lactuca, Raphanus, and Spinacia, compared with plants grown under pure R light. Nhut et al. [ 29 ] found that mixtures of B and R light stimulate the production of roots compared with pure R light in strawberry plantlets. Independent of light quality, we found a significantly enhanced root production in the phytotron treatments compared to the field grown plants, except for the 62% B treatment. As indicated by Poorter et al. [ 3 ], indoor climatization might induce root zone conditions that differ markedly from field conditions, leading to altered root production and consequently profoundly changed plant growth. As all plants in our experiment were regularly watered in both field and phytotron treatments, we can exclude that the observed higher root productivity in the phytotrons results from different water availability between indoor and field trials. However, pot soil temperature was not monitored, and it is possible that it differed significantly between indoor and field conditions, partly due to the lack of infrared radiation from the LED lamps.
3.2. Light Quality Effect on Leaf Pigmentation
The concentration of chlorophyll and carotenoids changed strongly with light quality in our study. Under natural sunlight, cryptochrome activity is reduced at high radiation, thereby signalling strong light conditions in the plant. The same effect can be achieved under experimental conditions by exposing plants to high percentages of B light [ 30 ]. The high proportion of B light in our 62% B treatment thus triggered the enhanced production of photosynthetic pigments despite the fact that the other treatments with lower B% had the same PPFD. In fact, the low concentrations of Chl a and b in plants that have been treated with low levels of B light or monochromatic R light in previous studies, have even led to photo-oxidative stress in plants due to an increase of O 2 - and H 2 O 2 radicals that induce cellular damage [ 8 , 19 ]. Barnes and Bugbee [ 30 ] proposed that a minimum of 20−30 μmol m −2 s −1 of B light is necessary to reach natural-like growth and morphologies, even if such a minimum requirement for B light appears to be highly species-specific [ 31 ]. It is likely that due to all of our light treatments including at least 6% of B light, we did not observe light quality related stress effects in our experiment. However, we identify that even with over 30 μmol m −2 s −1 of B light (at 6% B), higher percentages of B can increase the photosynthetic maximum capacity in several species, indicating that it is not just the quantity of B light, but also its relationship with other wavebands in the spectrum. Interestingly, most species showed higher Chl a:b ratios in the phytotrons compared to the field trial. This effect has been observed previously in indoor-grown plants [ 32 ], where it is attributed to the lack of fluctuating light conditions in indoor facilities.
Like chlorophyll, the production of carotenoids was also significantly increased with 62% of B light compared to 6% B (and 35% B), yet only the 25% B and the 62% B treatments induced higher carotenoid concentrations than in the field trial. Hogewoning et al. [ 8 ] reported an increase of carotenoids in cucumber plants when B was increased to 50% in the light spectra. An increase of carotenoids has been shown to work as an accumulative protection mechanism correlating with high light intensities or high B ratios. For example, the authors of [ 12 ] found that Fv/Fm of rapeseed leaves was reduced under monochromatic B or R light treatments, compared with mixtures of B and R. They attributed this to a higher PS II damage and linked the higher concentrations of carotenoids to a protection mechanism against oxygen radical formation. This is in line with our Fv/Fm results, where lower percentages of B in the applied spectra induce small but significant differences of the Fv/Fm values in almost all investigated species.
3.3. Light Quality Effects on Photosynthesis
When A max was measured under the same standardised light conditions (30% B and 70% R) in the current study, plants under 63% B showed, on average, significantly higher A max compared to plants under 25% B and the field trial. This could be partially explained by the increased chlorophyll concentrations in 63% B treated plants (see above). Previously, higher A max have been linked to higher levels of stomatal conductance and nitrogen concentration, where the latter is correlated to Rubisco, cytochrome, proteins and chlorophyll content [ 33 ]. A higher A max has also been suggested to partially derive from an instantaneous stimulation of photosynthesis (i.e., during the exposure to the light within the gas-exchange chamber) due to the lack of adaptation to the standardised light condition [ 8 ]. In our case, using 70% R in plants adapted to 62% B may promote a higher A max , meanwhile this may not be the case in plants adapted to lower percentages of B light, and therefore higher percentages of R light. Kim et al. [ 15 ] have shown that in Pisum sativum about four days were necessary to reach full photosynthetic acclimation after a transition from a PSI to a PSII stimulating light environment and vice versa. Similarly, Hogewoning et al. [ 34 ] showed in duckweed, that six days were needed to fully acclimate to different light conditions, using the Chl a:b ratio as the control parameter.
In contrast to the measurements of standardised light, when measured under the respective in situ light conditions, A max was significantly lower at very low (6%) or very high (62%) B light conditions, despite the higher concentration of chlorophyll at 62% B or small differences in SLA ( Figure 2 B). In a similar but more extreme experiment, several long-term studies reported lower net photosynthesis or A max in plants raised under monochromatic B or R light [ 8 , 11 , 12 ]. Hogewoning et al. [ 8 ], also reported dysfunctional photosynthesis in cucumber plants, grown under pure R light and a dose response curve in A max when the B% was increased up to 50% B, with no further increase of A max beyond 50% B. The increase of A max with B percentages was associated with a reduction of the SLA, an increase of N and chlorophyll per leaf area, and higher stomatal conductance under mixtures of B and R light compared with only B or R [ 8 ]. Matsuda et al. [ 35 ] reported an increase of A max in spinach plants exposed to a 1:1 B: R radiation compared with just B light, associated with increased leaf N concentration. Shengxin et al. [ 12 ] showed that dark adapted Fv/Fm values were higher (as an indicator for less photo-stress) under mixtures of B and R light compared with monochromatic B or R light.
The effects of treatments on photosynthesis were also visible in the quantum yield of the CO 2 fixation curve (α) of the investigated species. Similar to A max , a more natural level of B light may explain a higher efficiency when an ‘in situ’ light was used for our gas-exchange measurements, with significantly higher values indoor than in the field trial. Similar results have been reported at 15–30% B compared with 50% B [ 8 ]. This effect may indicate the evolutionary adaption of species to the natural sunlight spectrum, with higher quantum yield under a more natural B:R ratio (circa 33% of B in the sunlight spectrum [ 36 ]). Other conditions with extreme levels of B or R light may require the adaptation to each light condition, where CO 2 fixation may have a wavelength dependence related to absorption properties of the different pigments involved. Terashima et al. [ 37 ], described three major causes for the wavelength dependency of the quantum yield: absorption by photosynthetic carotenoids, absorption by non-photosynthetic pigments and an imbalanced excitation of the two photosystems, where an imbalance in excitation will result in quantum yield losses [ 27 , 38 ]. It has been shown that a correct light stimulus, with light qualities matching the species-specific ratio of PSII and PSI, is key to high quantum efficiency of photosynthesis [ 39 ]. The light compensation point of photosynthesis (CP) was generally not affected by light quality. Similar results have been observed in previous cases [ 9 , 12 ].
In the current study, the average dark respiration (DR) using the standardised light, independent of the species, was relatively lower at 62% B compared with the other light treatments or the field trial. Atkin et al. [ 40 ] described in tobacco that observed changes in DR were dependent on the previously applied irradiance (tested between 0 to 300 μmol photons m −2 s −1 ). An instantaneous stimulation of the photosystems in low light adapted plants due the stimulus of an intensity radiation burst was hypothesised. Although the total photon flux was the same between treatments in our study, similar short time effects on DR might have occurred when plants were exposed to a high intensities and light spectrum that they were not adapted to.
3.4. Principal Component Analysis
The PCA analyses performed in this study confirmed that the effects of light quality on plant performance are highly species dependent, and adjustments of the light spectra may help to promote more natural like growth, where more natural growth like plants tend to group closer to the field trial in the PCA. Applying a light spectrum with similar B and R light proportions to sunlight is proposed to avoid physiological plant responses to a lack or excess of B light (which might also differ among species). Although 7% B has been recommended to avoid dysfunctional photosynthesis [ 8 ], this study indicates that levels of 25 to 35% B light in the spectrum are needed in indoor conditions to avoid undesired (i.e., unnatural) effects of the light spectrum on plant growth. This was demonstrated with higher distances of the 6%B light treated plants from the field trial plants in the PCA. No specific trait was identified across the different species to have a higher importance than others ( Figure S1 ), where the ranking of importance of each measured parameter was species dependent. Independent of this, the PCA clearly indicated that other environmental variables should be controlled (e.g., air flux, soil temperature) or more precisely mimicked in indoor growth facilities if natural-like growth is required. A similar approach has been previously used [ 41 ] to understand the difference between indoor and outdoor experiments, with a focus on Arabidopsis ’s metabolism where a clearer clustering of the indoor and outdoor conditions was obtained. Similar values of the first and second component to the ones presented here (first and second component explaining 28 and 15% of the variance, respectively compared with 24 and 15% average across species in our study).
4. Materials and Methods
4.1. plant material and pre-growing conditions.
In this study, we investigated young plants of 7 species from different functional plant types to include the species as the source of variation: trees represented by black alder ( Alnus glutinosa (L.) Gearth, provenance HG4, Zurich, Switzerland), Scotch elm ( Ulmus glabra Huds., provenance Merenschwand, Aargau, Switzerland), herbs represented by basil ( Ocimum basilicum ‘Adriana’), lettuce ( Lactuca sativa ), melissa ( Melissa officinalis ), radish ( Raphanus raphanistrum subsp. sativus (L.) Domin), and grasses represented by winter wheat ( Triticum aestivum ). For the experiments, all plants were raised from seeds. The seeds of both tree species were purchased from the Swiss federal institute for forest, snow and landscape research, WSL, Birmensdorf, Switzerland. All herb seeds were provided from Wyss Samen und Pflanzen AG, Zuchwil, Switzerland, and Triticum seeds were supplied form Sativa AG, Rheinau, Switzerland. Hereinafter, the species will be referred to by their scientific genus name for clearness. Due to the different germination speeds the timing of sowing was different for the species as follows: seeds of Alnus and Ulmus were sown in 20 × 40 × 2 cm trays with commercial substrate (pH 5.8, 250 mg L −1 N, 180 P 2 O 5 mg L −1 , K 2 O 480 mg L −1 , Ökohum, Herrenhof, Switzerland) 43 days before the start of the experiments and were left to germinate under 190 μmols m −2 s −1 of photosynthetic photon flux density (PPFD: 400–700 nm) with 25% Blue (B: 400–500 nm), 32% Green (G: 500–600 nm) and 41% Red (R: 600–700 nm) light and an R to far red (FR: 700–800 nm) ratio (R:FR. 655–665 nm and 725–735 nm; according to [ 42 ]) of 5.1 for 23 days, using LED lighting with a day length of 16 h. Twenty days before the start of the experiment, the light was increased to 240 μmols m −2 s −1 PPFD, with a R: FR of 5.1, to acclimate the plants to higher intensity levels. Thirteen days before the start of the experiment Melissa seeds were sown in the same type of trays and keeping the last-mentioned environmental conditions. Six days before the start of the experiments the remaining species were sown in the same type of trays and under the same environmental conditions, with the exception of Triticum, which was sown immediately in round 2 L pots with a density of 15 seeds per pot (13.5 cm diameter, Poppelmann, Lohne, Germany). All light measurements were done using a using a spectrometer (STS, OceanOptics, Florida, United States). During the germination and the pre-treatment period, the different seedlings were raised at 25 °C/50% relative humidity (RH) during daytime and 15 °C/83% RH during night, with 10 h per day and one-hour light/temperature/humidity ramping pre and post day.
At the start of the experiment, all species, excluding Triticum , were transplanted to the same type of 2 L pot previously used for Triticum, with a single individual in each pot. Moreover, Triticum was thinned to 10 plants per pot. The pots were filled with the same substrate as used in the germination trays, and 4 g of Osmocote slow release fertiliser (Osmocote exact standard 3–4, Scotts, Marysville, OH, USA), containing 16% total N, 9% P2O5, 12% K2O and 2.5% MgO, was added to each plot. All plants were watered daily in the morning throughout the experiment.
The pre-growing procedure was repeated 3 times for this study: First, for the field-trial that was used as reference for the phytotron experiments, and then twice for the different light treatments of the phytotron experiment. (See control and light quality treatments below). No significant difference in initial height or biomass was found at the start of the experiments within species for the different replications (data not shown).
4.2. Control and Light Quality Treatments
To establish a control treatment as a reference point for natural growth, all seven target species were grown in a field trial for 35 days (4 August 2017–7 September 2017) at the botanical garden of the University of Basel, Switzerland. Throughout the field trial, the in situ climate and the natural sunlight spectrum was recorded ( Figure S2 and below). Following the field trial, we exposed plants from the seven different species to four mixtures of B and R light, which can be expressed as a B/R ratio, or as percentage of B light in four walk-in Phytotrons (1.5 m × 2.5 m) with full control of temperature, air humidity and light quality and quantity (prototypes, Enersign GmbH, Basel, Switzerland). To unify nomenclature with previous studies, the four different light treatments will be referred to by their respective B light proportion ( Table 1 ). The light treatments were chosen based on previous literature (e.g., Hogewoning et al. [ 8 ]), measurements of natural light completed in situ [ 36 ], and technical capacities of the phytotrons at the average light intensity of the outdoor treatment. For each treatment, the replication per species was 9 pots (with either one or more individuals per pot depending on species; see above). In all light treatments, the average PPFD from the field trial (575 μmol m −2 s −1 ) was provided at the average height of the different species using 18 LED panels for each chamber consisting of a mixture of B (400–500 nm) , White (2500 K), R (600–700 nm) and FR (700–800 nm) LEDs per panel (prototypes, DHL-Licht, Hanover, Germany). The LED lighting system of each chamber was mounted on movable ceilings, the height of which can be adjusted through the environmental control software of the chambers. To preserve similar light levels at average plant height, the height of the lamps was adjusted twice during the experiment. Based on the field trial conditions, the day length was set to 13 h and 5 min, giving a constant daily light integral (DLI) of 27.1 mol m −2 day −1 in all light treatments. Similar to the light conditions, temperature and humidity during day and night were set to average field trial conditions: 22 °C/66% RH and 18 °C/79% RH, for day and night, respectively, with a period of one-hour ramping before and after daytime. A uniform temperature and humidity distribution within each chamber was ensured by a constant vertical air stream from below. To avoid border and space effects, all plants were randomly distributed within each phytotron on two tables. The tables were rotated by 90° every day. Each light treatment was replicated twice (two separate runs of all four light combinations), where the distribution of the chambers was random between the two runs.
At the end of the 35-day experimental period, a suite of measurements was conducted in the field trial and the phytotron experiments. A description of the measured parameters is given in the following paragraphs. Due to limitations imposed by the lamp characteristics at high intensities, a higher R:FR ratio compared with outdoor (1.8 vs. 1.1) was applied in order to reach the targeted light intensities. No UV light was applied in the phytotrons.
4.3. Climatic Growth Conditions
In order to apply the most natural conditions within the phytotrons, the climate from the field trial at the botanical garden of the University of Basel, Switzerland, was recorded throughout the 35-day growth period ( Figure S2 ). Relative humidity, temperature, and PPFD were measured every 5 min with a weather station (Vantage pro2, Davis, Haywards, CA, USA). In addition, sunlight spectra in the waveband 350–800 nm were recorded every minute using a spectrometer (STS) that was equipped with an optical fiber and a cosine corrector (180º field-of-view; CC-3-UV-S, OceanOptics) placed by the weather station’s PAR sensor facing upwards. The spectrometer was connected to a Raspberry Pi 2 computer for automatic sampling, integration time adjustments and data storage. A posteriori, the spectra were used to calculate photon flux densities within specific wavebands: PAR, B, G, R and FR. The PAR light measurements were verified by comparing the data from the weather station with the data from the spectrometer readings. The data from the field trial were used to calculate average diurnal and nocturnal temperature, air humidity and PAR conditions for the phytotron treatments.
4.4. Morphological Parameters
By the end of the 35-day growth period, plant height was measured as total height from the substrate to the apical tip. In the case of long inflorescences ( Raphanus ) or plants without a clear stem ( Triticum ), extended leaf length was recorded as height, and in the case of Lactuca , no height was recorded. Two full-grown leaves from the top three mature leaves were collected from each plant to measure leaf area (LI-3100, Licor, Lincoln, NE, USA) and calculate the specific leaf area (SLA) in cm 2 g −1 on a dry leaf weight basis. Dry weight (DW) was measured separately for leaves, stems and roots after 10 days drying at 80ºC in a drying oven (UF 260, Memmert, Schwabach, Germany). Due to the lack of a clear stem, only total aboveground and root biomass were measured for Lactuca , Melissa and Triticum . All reported organ weights and the below to above ground biomass ratio (root:shoot-ratio) refer to plant dry mass.
4.5. Chlorophyll Fluorescence and Chlorophyll Content
One night before the end of the experiment, fast chlorophyll fluorescence induction was measured on one of the top three leaves in four randomly chosen plants of each species and treatment by using a continuous excitation fluorometer with an intensity of 3500 μmol m −2 s −1 centred at 627 nm (Pocket PEA, Hansatech instruments Ltd., Norfolk, UK). The plants were dark adapted for at least 20 min before recording photosynthetic maximum quantum yield (Fv/Fm) and the absolute performance index (PI) of the leaves, which has been correlated previously to stress (for calculations and details, see [ 43 ]).
During harvest, two discs of 1.13 cm 2 area from the top four leaves were punched and stored in a 1.5 mL Eppendorf tube together with four to six glass beads of 0.1 mm diameter for later chlorophyll analysis. The tubes were quickly frozen in liquid nitrogen and then kept at −80 °C until analysis. During the day of chlorophyll measurement, the tubes were agitated two times for 10 s to triturate the tissue using a mixing device (Silamat S6, Ivoclar Vivadent, Schaan, Liechtenstein). After adding 0.7 mL of acetone to each tube, they were agitated again for 10 s and then centrifuged at 13,000 rpm at 4ºC for 2 min. A total of 0.25 mL of the supernatant was dissolved in 0.75 mL of acetone, and the sample absorption spectra were measured using a spectrometer (Ultrospec 2100 pro, Biochrom, Holliston, MA, USA). Chlorophyll a and b concentrations, chlorophyll a to b ratio (Chl a, Chl b and a:b ratio, respectively) and total carotenoid concentrations as mg g −1 , were calculated from the spectra using the values at 470, 646 and 663 nm as described in [ 44 ].
4.6. Leaf Gas Exchange
Six days before the end of the experiment, a light response curve of net CO 2 leaf-exchange was measured in one of the top three leaves in three randomly chosen plants per species and treatment using a LI-6800 photosynthesis system (LI-COR, Lincoln, NE, USA). The light response curves were measured under two different light spectra: (i) a standardised artificial light spectrum, composed of 70% R and 30% B (in the following referred to as ‘standardised light’) provided by the chamber head light source to study photosynthesis of the different species under a uniform light spectrum, and, (ii) the respective growing light spectrum (in the following referred to as ‘in situ spectrum’) provided by using a transparent, clear-top chamber head (Clear-top leaf chamber 6800-12A, LI-COR) to study photosynthesis of the different species under their respective growing spectra and avoid any bias on photosynthesis from a non-adapted spectrum. Twelve different light intensities: 2000, 1500, 1000, 800, 600, 400, 200, 100, 50, 25, 10 and 0 μmol m −2 s −1 of PPFD were used for light response curves with the ‘standardised light’ spectrum. Due to lower maximum irradiance in the phytotrons limited by the light quality being applied (see above), the light response curves for the ‘in situ’ growing light were measured only up to a maximum radiation of 700 μmol m −2 s −1 of PPFD (700, 480, 380, 200, 100, 60, 30, 20, 17, 15 and 0 μmol m −2 s −1 of PPFD). All leaf CO 2 -exchange measurements were conducted at 400 ppm CO 2 , 60% relative air humidity and 20 °C leaf temperature, with 60 to 120 s as the threshold for stability after each light change intensity. Stability of readings was assumed when the difference of the slopes between IRGA’s were smaller than 0.5 μmol mol −1 sec −1 and 1 for CO 2 and H 2 O, respectively.
For each light curve, 12 different light models were fitted accordingly [ 45 ], including a model for photo-inhibition [ 46 ]. For each species and treatment, the model with the best fit (lowest sum of squares) was selected (details in [ 45 ]). The selected model was then used to calculate the following four values from the light response curve: maximum photosynthesis within the range of measured light (A max ), quantum yield of the CO 2 fixation (α) as the slope of the linear curve between 0 and 100 μmol m −2 s −1 of PPFD, dark respiration (DR) and the light compensation point (CP) of photosynthesis.
4.7. Statistical Analysis
To evaluate the effect of the light treatments, a two-way analysis of variance (ANOVA) was performed for all measured parameters, considering the species and different treatments as fixed factors and the two replicates of each treatment as a random factor. The significance of the random factor was evaluated using a restricted likelihood ratio test. The data were checked for normal distribution, independence and homogeneity of the variance.
To enable the direct visible and statistical comparison of the treatment effects across species, each measured trait was normalised relative to its mean value on the field trial for each species (the original trait average values per species and treatments are available in Table S1 ). The normalised values were used to perform a one-way ANOVA, considering the treatments as fix factor and species as random factors ( Table S2 ). A Tukey pairwise multiple comparison test was used as post hoc analysis to identify significant differences ( p < 0.05) among treatments. In several cases when all indoor light treatments differed from the field trial, an additional one-way ANOVA was performed without the field trial to highlight the individual response differences to the different light treatments (Data non shown).
Finally, to identify the specific traits that have the maximum variation between treatments and to quantify which treatment gave the overall most similar response compared to the outdoor trial, a principal component analysis (PCA) was performed separately for each species, using the different measured traits as input values. To perform a PCA analysis, the same number of observations is required for each variable but due to fewer photosynthesis measurements, chlorophyll measurements and fluorescence measurements than the number of plants used for biomass measurements, in each species and treatment, the missing values of chlorophyll content and light parameters were imputed using normal distribution with the same average and standard deviation of the available data. All analyses were performed using R [ 47 ] and the package plyr for data processing and lm4, car, RLRsim, emmeans for data analysis and multicomp and vegan for statistically significant representations.
5. Conclusions
The applied light spectra in this study significantly influenced plant morphology, pigment concentration and photosynthesis. Less deviating responses compared to the field trial were reached with either 25% or 35% of B light in almost all species. Hence, if natural like plant growth is desired in indoor plant cultivation, the application of a balanced light spectrum is generally recommended. Despite this, spectral quality of the light source is only one of many factors that can potentially bias plant performance. In this study, we thus aimed to apply similar climatic conditions within the growth chambers as were measured in the field trial to compare outdoor with indoor growth. Nevertheless, we still found significant differences between phytotron and field grown plants in most of the investigated plant traits. This highlights the difficulties to exactly reproduce natural plant performance in indoor growth facilities, as well as the necessity to include the simulation of additional environmental factors (e.g., replication of natural minimum and maximum temperature, humidity and irradiance changes, wind speed and direction) in indoor experiments with plants.
Acknowledgments
We thank Georges Grun and the gardeners at the botanical garden of the University of Basel for their technical support for the phytotron experiments and climate measurements. We also thank Sarah Newberry for proofreading the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/10/1273/s1 , Figure S1: Principal component analysis (PCA) of the measured traits of each specie grown under 6% B, 25% B, 35% B and 62% B light, Figure S2. Environmental conditions of temperature (A), air relative humidity (B) and light intensity as PPFD (C) from the field trial, Table S1: Raw average values by measured trial for each treatment and species; Table S2: p -values for the different measured traits in both experiments using normalised data.
Author Contributions
Conceptualization, C.C., D.B. and G.H.; methodology, C.C., D.B. and G.H.; validation, C.C.; formal analysis, C.C.; investigation, C.C.; resources, D.B. and G.H.; data curation, C.C.; writing—original draft preparation, C.C.; writing—review and editing, D.B. and G.H.; visualization, C.C.; supervision, G.H.; project administration, G.H.; funding acquisition, D.B. and G.H. All authors have read and agreed to the published version of the manuscript.
The presented work was supported by PlantHUB-European Industrial Doctorate funded by the H2020 PROGRAMME Marie Curie Action—People, Initial Training Networks (H2020-MSCA-ITN-2016). The programme is managed by the Zurich-Basel Plant Science Center.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Hypothesis Examples

A hypothesis is a prediction of the outcome of a test. It forms the basis for designing an experiment in the scientific method . A good hypothesis is testable, meaning it makes a prediction you can check with observation or experimentation. Here are different hypothesis examples.
Null Hypothesis Examples
The null hypothesis (H 0 ) is also known as the zero-difference or no-difference hypothesis. It predicts that changing one variable ( independent variable ) will have no effect on the variable being measured ( dependent variable ). Here are null hypothesis examples:
- Plant growth is unaffected by temperature.
- If you increase temperature, then solubility of salt will increase.
- Incidence of skin cancer is unrelated to ultraviolet light exposure.
- All brands of light bulb last equally long.
- Cats have no preference for the color of cat food.
- All daisies have the same number of petals.
Sometimes the null hypothesis shows there is a suspected correlation between two variables. For example, if you think plant growth is affected by temperature, you state the null hypothesis: “Plant growth is not affected by temperature.” Why do you do this, rather than say “If you change temperature, plant growth will be affected”? The answer is because it’s easier applying a statistical test that shows, with a high level of confidence, a null hypothesis is correct or incorrect.
Research Hypothesis Examples
A research hypothesis (H 1 ) is a type of hypothesis used to design an experiment. This type of hypothesis is often written as an if-then statement because it’s easy identifying the independent and dependent variables and seeing how one affects the other. If-then statements explore cause and effect. In other cases, the hypothesis shows a correlation between two variables. Here are some research hypothesis examples:
- If you leave the lights on, then it takes longer for people to fall asleep.
- If you refrigerate apples, they last longer before going bad.
- If you keep the curtains closed, then you need less electricity to heat or cool the house (the electric bill is lower).
- If you leave a bucket of water uncovered, then it evaporates more quickly.
- Goldfish lose their color if they are not exposed to light.
- Workers who take vacations are more productive than those who never take time off.
Is It Okay to Disprove a Hypothesis?
Yes! You may even choose to write your hypothesis in such a way that it can be disproved because it’s easier to prove a statement is wrong than to prove it is right. In other cases, if your prediction is incorrect, that doesn’t mean the science is bad. Revising a hypothesis is common. It demonstrates you learned something you did not know before you conducted the experiment.
Test yourself with a Scientific Method Quiz .
- Mellenbergh, G.J. (2008). Chapter 8: Research designs: Testing of research hypotheses. In H.J. Adèr & G.J. Mellenbergh (eds.), Advising on Research Methods: A Consultant’s Companion . Huizen, The Netherlands: Johannes van Kessel Publishing.
- Popper, Karl R. (1959). The Logic of Scientific Discovery . Hutchinson & Co. ISBN 3-1614-8410-X.
- Schick, Theodore; Vaughn, Lewis (2002). How to think about weird things: critical thinking for a New Age . Boston: McGraw-Hill Higher Education. ISBN 0-7674-2048-9.
- Tobi, Hilde; Kampen, Jarl K. (2018). “Research design: the methodology for interdisciplinary research framework”. Quality & Quantity . 52 (3): 1209–1225. doi: 10.1007/s11135-017-0513-8
Related Posts

OSU Extension Service
- Gardening, lawn and landscape
- Gardening techniques
Environmental factors affecting plant growth
Plant growth and geographic distribution (where the plant can grow) are greatly affected by the environment. If any environmental factor is less than ideal, it limits a plant's growth and/or distribution. For example, only plants adapted to limited amounts of water can live in deserts.
Either directly or indirectly, most plant problems are caused by environmental stress.
Either directly or indirectly, most plant problems are caused by environmental stress. In some cases, poor environmental conditions (e.g., too little water) damage a plant directly. In other cases, environmental stress weakens a plant and makes it more susceptible to disease or insect attack.
Environmental factors that affect plant growth include light, temperature, water, humidity and nutrition. It's important to understand how these factors affect plant growth and development. With a basic understanding of these factors, you may be able to manipulate plants to meet your needs, whether for increased leaf, flower or fruit production. By recognizing the roles of these factors, you'll also be better able to diagnose plant problems caused by environmental stress.
Three principal characteristics of light affect plant growth: quantity , quality and duration .
Light quantity refers to the intensity, or concentration, of sunlight. It varies with the seasons. The maximum amount of light is present in summer, and the minimum in winter. Up to a point, the more sunlight a plant receives, the greater its capacity for producing food via photosynthesis.
You can manipulate light quantity to achieve different plant growth patterns.
You can manipulate light quantity to achieve different plant growth patterns. Increase light by surrounding plants with reflective materials, a white background or supplemental lights. Decrease it by shading plants with cheesecloth or woven shade cloths.
Light quality refers to the color (wavelength) of light. Sunlight supplies the complete range of wavelengths and can be broken up by a prism into bands of red, orange, yellow, green, blue, indigo and violet.
Blue and red light, which plants absorb, have the greatest effect on plant growth. Blue light is responsible primarily for vegetative (leaf) growth. Red light, when combined with blue light, encourages flowering. Plants look green to us because they reflect, rather than absorb, green light.
Knowing which light source to use is important for manipulating plant growth. For example, fluorescent (cool white) light is high in the blue wavelength. It encourages leafy growth and is excellent for starting seedlings. Incandescent light is high in the red or orange range, but generally produces too much heat to be a valuable light source for plants. Fluorescent grow-lights attempt to imitate sunlight with a mixture of red and blue wavelengths, but they are costly and generally no better than regular fluorescent lights.
Duration, or photoperiod , refers to the amount of time a plant is exposed to light. Photoperiod controls flowering in many plants (Figure 1). Scientists used to think that the length of light period triggered flowering and other responses within plants. Thus, they describe plants as short-day or long-day, depending on what conditions they flower under. We now know that it is not the length of the light period, but rather the length of uninterrupted darkness, that is critical to floral development.
Plants are classified into three categories: short-day (long-night), long-day (short-night), or day-neutral, depending on their response to the duration of light or darkness. Short-day plants form flowers only when day length is less than about 12 hours. Many spring- and fall-flowering plants, such as chrysanthemum, poinsettia and Christmas cactus, are in this category.
In contrast, long-day plants form flowers only when day length exceeds 12 hours. Most summer-flowering plants (e.g., rudbeckia, California poppy and aster), as well as many vegetables (beet, radish, lettuce, spinach and potato), are in this category.
Day-neutral plants form flowers regardless of day length. Examples are tomato, corn, cucumber and some strawberry cultivars. Some plants do not fit into any category, but may respond to combinations of day lengths. Petunias, for example, flower regardless of day length, but flower earlier and more profusely with long days.
Environmental factors that affect plant growth include light, temperature, water, humidity and nutrition.
You can easily manipulate photoperiod to stimulate flowering. For example, chrysanthemums normally flower in the short days of spring or fall, but you can get them to bloom in midsummer by covering them with a cloth that completely blocks out light for 12 hours each day. After several weeks of this treatment, the artificial dark period no longer is needed, and the plants will bloom as if it were spring or fall. This method also is used to make poinsettias flower in time for Christmas.
To bring a long-day plant into flower when day length is less than 12 hours, expose the plant to supplemental light. After a few weeks, flower buds will form.
Temperature
Temperature influences most plant processes, including photosynthesis, transpiration, respiration, germination and flowering. As temperature increases (up to a point), photosynthesis, transpiration and respiration increase. When combined with day length, temperature also affects the change from vegetative (leafy) to reproductive (flowering) growth. Depending on the situation and the specific plant, the effect of temperature can either speed up or slow down this transition.
Germination
The temperature required for germination varies by species. Generally, cool-season crops (e.g., spinach, radish and lettuce) germinate best at 55° to 65°F, while warm-season crops (e.g., tomato, petunia and lobelia) germinate best at 65° to 75°F.
Sometimes horticulturists use temperature in combination with day length to manipulate flowering. For example, a Christmas cactus forms flowers as a result of short days and low temperatures (Figure 1). To encourage a Christmas cactus to bloom, place it in a room with more than 12 hours of darkness each day and a temperature of 50° to 55°F until flower buds form.
If temperatures are high and days are long, cool-season crops such as spinach will flower (bolt). However, if temperatures are too cool, fruit will not set on warm-season crops such as tomato.
Crop quality
Low temperatures reduce energy use and increase sugar storage. Thus, leaving crops such as ripe winter squash on the vine during cool, fall nights increases their sweetness.
Adverse temperatures, however, cause stunted growth and poor-quality vegetables. For example, high temperatures cause bitter lettuce.
Photosynthesis and respiration
Thermoperiod refers to daily temperature change. Plants grow best when daytime temperature is about 10 to 15 degrees higher than nighttime temperature. Under these conditions, plants photosynthesize (build up) and respire (break down) during optimum daytime temperatures and then curtail respiration at night. However, not all plants grow best under the same range between nighttime and daytime temperatures. For example, snapdragons grow best at nighttime temperatures of 55°F; poinsettias, at 62°F.
Temperatures higher than needed increase respiration, sometimes above the rate of photosynthesis. Thus, photosynthates are used faster than they are produced. For growth to occur, photosynthesis must be greater than respiration.
Daytime temperatures that are too low often produce poor growth by slowing down photosynthesis. The result is reduced yield (i.e., fruit or grain production).
Breaking dormancy
Some plants that grow in cold regions need a certain number of days of low temperature (dormancy). Knowing the period of low temperature required by a plant, if any, is essential in getting it to grow to its potential.
Peaches are a prime example; most varieties require 700 to 1,000 hours between 32° and 45°F before breaking their rest period and beginning growth. Lilies need six weeks of temperatures at or slightly below 33°F before blooming.
Daffodils can be forced to flower by storing the bulbs at 35° to 40°F in October. The cold temperature allows the bulbs to mature. When transferred to a greenhouse in midwinter, they begin to grow, and flowers are ready to cut in three to four weeks.
Plants are classified as hardy or nonhardy depending on their ability to withstand cold temperatures. Hardy plants are those that are adapted to the cold temperatures of their growing environment.
Woody plants in the temperate zone have very sophisticated means for sensing the progression from fall to winter. Decreasing day length and temperature trigger hormonal changes that cause leaves to stop photosynthesizing and to ship nutrients to twigs, buds, stems and roots. An abscission layer forms where each petiole joins a stem, and the leaves eventually fall off. Changes within the trunk and stem tissues over a relatively short period of time "freeze-proof" the plant.
Winter injury to hardy plants generally occurs when temperatures drop too quickly in the fall before a plant has progressed to full dormancy. In other cases, a plant may break dormancy in mid- or late winter if the weather is unseasonably warm. If a sudden, severe cold snap follows the warm spell, otherwise hardy plants can be seriously damaged.
It is worth noting that the tops of hardy plants are much more cold-tolerant than the roots. Plants that normally are hardy to 10°F may be killed if they are in containers and the roots are exposed to 20°F.
People often forget that plants need water even during winter.
Winter injury also may occur because of desiccation (drying out) of plant tissues. People often forget that plants need water even during winter. When the soil is frozen, water movement into a plant is severely restricted. On a windy winter day, broadleaf evergreens can become water-deficient in a few minutes, and the leaves or needles then turn brown. To minimize the risk of this type of injury, make sure your plants go into the winter well watered.
Water and humidity
Most growing plants contain about 90 percent water. Water plays many roles in plants. It is:
- A primary component in photosynthesis and respiration
- Responsible for turgor pressure in cells (Like air in an inflated balloon, water is responsible for the fullness and firmness of plant tissue. Turgor is needed to maintain cell shape and ensure cell growth.)
- A solvent for minerals and carbohydrates moving through the plant
- Responsible for cooling leaves as it evaporates from leaf tissue during transpiration
- A regulator of stomatal opening and closing, thus controlling transpiration and, to some degree, photosynthesis
- The source of pressure to move roots through the soil
- The medium in which most biochemical reactions take place
Relative humidity is the ratio of water vapor in the air to the amount of water the air could hold at the current temperature and pressure. Warm air can hold more water vapor than cold air. Relative humidity (RH) is expressed by the following equation:
RH = water in air ÷ water air could hold (at constant temperature and pressure)
Relative humidity is given as a percent. For example, if a pound of air at 75°F could hold 4 grams of water vapor, and there are only 3 grams of water in the air, then the relative humidity (RH) is:
3 ÷ 4 = 0.75 = 75%
Water vapor moves from an area of high relative humidity to one of low relative humidity. The greater the difference in humidity, the faster water moves. This factor is important because the rate of water movement directly affects a plant's transpiration rate.
The relative humidity in the air spaces between leaf cells approaches 100 percent. When a stoma opens, water vapor inside the leaf rushes out into the surrounding air (Figure 2), and a bubble of high humidity forms around the stoma. By saturating this small area of air, the bubble reduces the difference in relative humidity between the air spaces within the leaf and the air adjacent to the leaf. As a result, transpiration slows down.
If wind blows the humidity bubble away, however, transpiration increases. Thus, transpiration usually is at its peak on hot, dry, windy days. On the other hand, transpiration generally is quite slow when temperatures are cool, humidity is high, and there is no wind.
Hot, dry conditions generally occur during the summer, which partially explains why plants wilt quickly in the summer. If a constant supply of water is not available to be absorbed by the roots and moved to the leaves, turgor pressure is lost and leaves go limp.
Plant nutrition
Plant nutrition often is confused with fertilization. Plant nutrition refers to a plant's need for and use of basic chemical elements. Fertilization is the term used when these materials are added to the environment around a plant. A lot must happen before a chemical element in a fertilizer can be used by a plant.
Plants need 17 elements for normal growth. Three of them--carbon, hydrogen and oxygen--are found in air and water. The rest are found in the soil.
Six soil elements are called macronutrients because they are used in relatively large amounts by plants. They are nitrogen, potassium, magnesium, calcium, phosphorus and sulfur.
Eight other soil elements are used in much smaller amounts and are called micronutrients or trace elements. They are iron, zinc, molybdenum, manganese, boron, copper, cobalt and chlorine.
Most of the nutrients a plant needs are dissolved in water and then absorbed by its roots. In fact, 98 percent are absorbed from the soil-water solution, and only about 2 percent are actually extracted from soil particles.
Fertilizers
Fertilizers are materials containing plant nutrients that are added to the environment around a plant. Generally, they are added to the water or soil, but some can be sprayed on leaves. This method is called foliar fertilization . It should be done carefully with a dilute solution, because a high fertilizer concentration can injure leaf cells. The nutrient, however, does need to pass through the thin layer of wax (cutin) on the leaf surface.
Fertilizers are not plant food! Plants produce their own food from water, carbon dioxide and solar energy through photosynthesis. This food (sugars and carbohydrates) is combined with plant nutrients to produce proteins, enzymes, vitamins and other elements essential to growth.
Nutrient absorption
Anything that reduces or stops sugar production in leaves can lower nutrient absorption. Thus, if a plant is under stress because of low light or extreme temperatures, nutrient deficiency may develop.
A plant's developmental stage or rate of growth also may affect the amount of nutrients absorbed. Many plants have a rest (dormant) period during part of the year. During this time, few nutrients are absorbed. Plants also may absorb different nutrients as flower buds begin to develop than they do during periods of rapid vegetative growth.

Was this page helpful?
Related content from osu extension.

How to create a slug trap using bread dough slurry
This tutorial walks you through the steps and ingredients needed to create a slug trap using bread dough as an attractant. The steps shown here are an interpretation of the OSU field research, made approachable for a home ...
LeAnn Locher, Rory Mc Donnell | Jun 2024 | Educational gallery Peer reviewed (Gray level)

Gardening with (and without) slugs and snails
Gardening in what's referred to as a global gastropod biodiversity hotspot (Oregon), means dealing with slugs and snails. There are a variety of ways to help prevent damage to your plants. Here is a collection of resources from OSU Extension.
May 2024 | Collection

Collecting and storing seeds from your garden
At some point, home gardeners might want to try saving seeds from their own gardens for future crops instead of buying them. But there are some do's and don'ts that home gardeners should know before giving it a try.
Bill Mansour, Duane Hatch | May 2003 | Article Peer reviewed (Gray level)

Invasive Species: What Gardeners Need to Know
Many of the invasive plant species introduced to the United States originated as garden plants. Learn what you need to know to prevent future invasions.
Linda R. McMahan, Joy Jones, Robert Emanuel | Jul 2011 | Extension Catalog publication Peer reviewed (Orange level)

Firewise Landscaping Basics
Learn about plants that can help you reduce the risk of fire while also creating an attractive environment. See why landscape maintenance and plant placement are just as critical as plant selection.
Amy Jo Detweiler | May 2024 | Video

Get Actionable Results from a Soil, Plant or Environmental Testing Lab
Learn how to work with an analytical laboratory to get useful results from your plant tissue, soil or water samples.
Shannon Cappellazzi, Clare Sullivan, Gordon B. Jones, Linda Brewer | May 2024 | Extension Catalog publication Peer reviewed (Orange level)

Growing School Gardens Summit: Part 2: More fabulous guests!: Kaitlyn Scheuermann with Waukee Community School District in Waukee, IA; Slema Sims with Gardeneers in Chicago, Ill; and Pam Hosimer with University of Maryland Extension
Join Rick "on assignment" as he travels to the Growing School Gardens Summit, in San Diego California. Rick interviews a dozen people with amazing stories. This is part TWO of a four-part series.
Michelle Markesteyn, PhD, MSEL, Rick Sherman | Apr 2024 | Podcast episode Peer reviewed (Gray level)

Can essential oils control slugs?
I've heard essentials oils may be useful in keeping slugs away from my plants and vegetables. Is this true?
Rory Mc Donnell | May 2024 | Featured question

Fall in love with colorful, dazzling dahlias
Pompoms, collarettes, mignons, waterlilies: The names are as colorful as the blooms they describe.
Kym Pokorny | Apr 2, 2021 | News story
5 Steps to Healthy Produce During Wildfire Smoke
Learn the five steps you can take to keep produce safe for consumption during a smoke event due to wildfire.
Glenda Hyde, Diana Rohlman, Julia Van Soelen Kim | Sep 2023 | Extension Catalog publication Peer reviewed (Orange level)

Ivy removal in a home landscape
Linda McMahan tackles ivy and shows how you can remove it from trees and fences.
Linda R. McMahan | Sep 2008 | Article Peer reviewed (Gray level)

Slug chat with OSU slug expert Rory McDonnell
Watch the 45-minute lunchtime SLUG CHAT with Associate Professor and Primary Investigator of OSU’s Invertebrate Crop Pest Lab, Rory McDonnell. Dr. McDonnell’s work focuses on understanding the ecology of invasive slugs and snails,...
LeAnn Locher, Rory Mc Donnell | May 2024 | Video

An Educator's Guide to Vegetable Gardening
This publication is a primer on vegetable gardening written specifically for educators, including those who use gardens as part of a nutrition education curriculum. It outlines a full-circle approach to educational ...
Weston Miller, Beret Halverson, Gail Langellotto | Sep 2011 | Extension Catalog publication Peer reviewed (Orange level)

The ABCs of NPK: A fertilizer guide
Nitrogen, phosphorus and potassium aren't just an alphabet soup of chemicals. They are essential plant nutrients that, when used correctly, help to grow a healthy garden. Learn what fertilizers to apply when in this handy guide.
Lisa Ehle | Jun 2018 | Article

What should I be doing in the winter for weed control?
The straw mulch I used at the end of summer to suppress the weeds doesn't seem to be working. Should I till it and cover with plastic? black or clear? Is there something better. Or should I add more straw. I could probably bring some cardboard home from work. Is this a healthy option?
Ann Kinkley | Dec 2017 | Featured question

Will ladybugs ruin my garden?
Can ladybugs kill plants in my vegetable garden?
Steve Renquist | Jul 2014 | Featured question

Slugs and snails, destructors of crops and gardens, could be controlled by bread dough
Bread dough is a nontoxic, generic and effective tool that could be used in the detection and management of gastropods worldwide.
Kym Pokorny | Aug 18, 2021 | News story

Have a question? Ask Extension!
Ask Extension is a way for you to get answers from the Oregon State University Extension Service. We have experts in family and health, community development, food and agriculture, coastal issues, forestry, programs for young people, and gardening.
Science Project Ideas
Does Music Affect Plant Growth
Though it is still a debatable topic, experiments conducted all over the world indicate that music can affect plant growth. While soothing classical music, Beethoven, Brahms have been seen to help in stimulating growth, certain other music hindered their growth rate. Here is an experiment that can help you in the research and arrive at a conclusion.
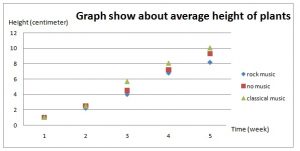
How Does Music Affect Plant Growth: An Experiment
The pot having mustard seeds exposed to music germinates and grows faster than those without music.
- Packet of radish seeds
- 2 plastic pots
- Classical music CD
- 1-meter ruler
- The 2 pots are filled with the same amount of soil and labeled A and B.
- Maintaining a distance of 20 mm in between them, 10 radish seeds are placed in the soil of each pot.
- The pots are kept in such a place that they receive the same amount of sunlight every day.
- They are also watered equally twice every day.
- Pot A is placed beside the CD player playing classical music for 3 hours every day.
- Pot B is kept away from any source of sound.
- Their height is recorded every day for 15 days and tabulated.
It is seen that the plants under the effect of music record a greater increase in average height than the ones placed away from music. The relation between music and plant growth be studied better by plotting the no. of days as the independent variable on a graph paper and the average plant height as the dependent variable. You should have 2 different graphs for the data pertaining to plants growing with and without music on the same graph paper for a good comparative study. In fact, the absence of music does nothing to the normal growth rate.
You Can Also Try
- Check out the influence of rap, rock and heavy metal music on the growing plants.
Music Affecting Plant Growth Video
Possible explanation.
Music has been observed to improve the germination process and enhance growth in plants albeit without a proper scientific explanation. Plants, as such cannot hear sound, but they can feel the vibration of the sound waves in air. The living matter within plants, protoplasm, is in a state of perpetual motion. The sound vibrations add to it, speeding up the transfer of nutrients and resulting in faster growth. However, loud music like rock can be detrimental for development as they increase the vibrations to intolerable levels.
Get all the requisite background information before demonstrating the scope of music for an accelerated growth of plants at science fairs. Serve an eco-friendly purpose by using music therapy to promote healthy greenery in nurseries, gardens, etc.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Biotic plant-soil feedbacks alone do not explain why more diverse plant communities are less invasible
- Research Article
- Published: 27 May 2024
Cite this article

- Hao-Ming Yuan ORCID: orcid.org/0009-0000-3683-7249 1 , 2 na1 ,
- Xiao-Mei Zhang 2 , 3 na1 ,
- Peter Alpert 4 , 5 ,
- Lin-Xuan He 2 ,
- Wei Xue 2 ,
- Lin Huang 2 ,
- Ling Peng 2 &
- Fei-Hai Yu 2
41 Accesses
Explore all metrics
An increase number of studies suggests that more diverse communities of native plants more strongly resist invasion by introduced plants. Here we tested whether biotic plant-soil feedbacks can explain this relationship independently of other factors, via either soil richness, as based on the number of different plant species conditioning the soil; or soil heterogeneity, the degree to which plant-soil feedbacks involving different plant species are spatially separated. No previous study appears to have tested both soil richness and heterogeneity as components of biotic plant-soil feedbacks that might explain why more diverse native plant communities are less invasible.
We conditioned soils with monocultures of six native plant species and grew five introduced plant species individually in sterilized soil inoculated with one, two, or four of the conditioned soils, keeping the conditioned soils separate or mixing them.
Soil richness had little effect on the final dry mass of any introduced species. Higher soil heterogeneity did not decrease final mass in any introduced species and instead increased it in one.
Results suggest that biotic plant-soil feedbacks are not in themselves an important mechanism by which diversity limits invasibility but do not rule out the possibility that such feedbacks play a role in combination with other mechanisms such as abiotic feedbacks or plant competition.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
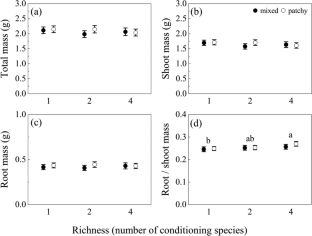
Similar content being viewed by others
Interactions between soil microbes and native species drive a diversity-invasibility relationship
Spatial heterogeneity of plant–soil feedbacks increases per capita reproductive biomass of species at an establishment disadvantage, native and non-native ruderals experience similar plant–soil feedbacks and neighbor effects in a system where they coexist, data availability.
Data deposited in the Dryad Digital Repository https://doi.org/10.5061/dryad.vq83bk40m .
Abbott KC, Karst J, Biederman LA, Borrett SR, Hastings A, Walsh V, Bever JD (2015) Spatial heterogeneity in soil microbes alters outcomes of plant competition. PLoS ONE 10:e0125788
Article PubMed PubMed Central Google Scholar
Bever JD, Westover KM, Antonovics J (1997) Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J Ecol 85:561–573
Article Google Scholar
Burns JH, Brandt AJ, Murphy JE, Kaczowka AM, Burke DJ (2017) Spatial heterogeneity of plant–soil feedbacks increases per capita reproductive biomass of species at an establishment disadvantage. Oecologia 183:1077–1086
Article PubMed Google Scholar
Cavieres LA (2021) Facilitation and the invasibility of plant communities. J Ecol 109:2019–2028
Chen PD, Huang QQ, Zhuge YH, Li CW, Zhu P, Hou YP (2021) The effects of plant-soil feedback on invasion resistance are soil context dependent. Oecologia 197:213–222
Early R, Bradley BA, Dukes JS, Lawler JJ, Olden JD, Blumenthal DM, Gonzalez P, Grosholz ED, Ibanez I, Miller LP, Sorte CJB, Tatem AJ (2016) Global threats from invasive alien species in the twenty-first century and national response capacities. Nat Commun 7:12485
Elton C (1958) The Ecology of invasions by animals and plants. The University of Chicago, Chicago
Book Google Scholar
Fargione JE, Tilman D (2005) Diversity decreases invasion via both sampling and complementarity effects. Ecol Lett 8:604–611
Hassani MA, Özkurt E, Franzenburg S, Stukenbrock EH (2020) Ecological assembly processes of the bacterial and fungal microbiota of wild and domesticated wheat species. Phytobiomes J 4:217–224
Hector A, Dobson K, Minns A, Bazeley-White E, Hartley Lawton J (2001) Community diversity and invasion resistance: an experimental test in a grassland ecosystem and a review of comparable studies. Ecol Res 16:819–831
Hendriks M, Ravenek JM, Smit-Tiekstra AE, van der Paauw JW, de Caluwe H, van der Putten WH, de Kroon H, Mommer L (2015a) Spatial heterogeneity of plant–soil feedback affects root interactions and interspecific competition. New Phytol 207:830–840
Hendriks M, Visser EJ, Visschers IG, Aarts BH, de Caluwe H, Smit-Tiekstra AE, van der Putten WH, de Kroon H, Mommer L (2015b) Root responses of grassland species to spatial heterogeneity of plant–soil feedback. Funct Ecol 29:177–186
Huang L, Chen RY, Xue W, Yu FH (2023) Effects of scale and contrast of spatial heterogeneity in plant-soil feedbacks on plant growth. Sci Total Environ 878:163159
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385
Keesing F, Holt RD, Ostfeld RS (2006) Effects of species diversity on disease risk. Ecol Lett 9:485–498
Article CAS PubMed Google Scholar
Kennedy TA, Naeem S, Howe KM, Knops JM, Tilman D, Reich P (2002) Biodiversity as a barrier to ecological invasion. Nature 417:636–638
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant-soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992
Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, Mellado-Vázquez PG, Malik AA, Roy J, Scheu S (2015) Plant diversity increases soil microbial activity and soil carbon storage. Nat Commun 6:6707
Levine JM, D’Antonio CM (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Maron J, Marler M (2007) Native plant diversity resists invasion at both low and high resource levels. Ecology 88:2651–2661
Naeem S, Knops JM, Tilman D, Howe KM, Kennedy T, Gale S (2000) Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 91:97–108
Paini DR, Sheppard AW, Cook DC, De Barro PJ, Worner SP, Thomas MB (2016) Global threat to agriculture from invasive species. Proc Natl Acad Sci U S A 113:7575–7579
Article CAS PubMed PubMed Central Google Scholar
Petruzzella A, Manschot J, van Leeuwen CHA, Grutters BMC, Bakker ES (2018) Mechanisms of invasion resistance of aquatic plant communities. Front Plant Sci 9:134
Prieur-Richard AH, Lavorel S, Grigulis K, Dos Santos A (2000) Plant community diversity and invasibility by exotics: invasion of Mediterranean old fields by Conyza bonariensis and Conyza canadensis . Ecol Lett 3:412–422
Pysek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kuhn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vila M, Wingfield MJ, Richardson DM (2020) Scientists’ warning on invasive alien species. Biol Rev 95:1511–1534
Rai PK, Singh JS (2020) Invasive alien plant species: their impact on environment, ecosystem services and human health. Ecol Ind 111:106020
Rottstock T, Joshi J, Kummer V, Fischer M (2014) Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology 95:1907–1917
Sardain A, Sardain E, Leung B (2019) Global forecasts of shipping traffic and biological invasions to 2050. Nat Sustain 2:274–282
Speißer B, van Kleunen M (2023) Plants forage for soil patches free of plastic pollution but cannot bag the profits. Sci Rep 13:18506
Teste FP, Kardol P, Turner BL, Wardle DA, Zemunik G, Renton M, Laliberté E (2017) Plant-soil feedback and the maintenance of diversity in Mediterranean-climate shrublands. Science 355:173–176
Tilman D (1997) Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 78:81–92
Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci 101:10854–10861
Van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA (2013) Plant–soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276
van Ruijven J, De Deyn GB, Berendse F (2003) Diversity reduces invasibility in experimental plant communities: the role of plant species. Ecol Lett 6:910–918
Wang XY, Gao S, Chen T, Wang J, Yu FH (2023) Interactions between soil microbes and native species drive a diversity-invasibility relationship. Biol Invasions 25:1461–1472
Wei GW, van Kleunen M (2022) Soil heterogeneity tends to promote the growth of naturalized aliens when competing with native plant communities. J Ecol 110:1161–1173
Wilschut RA, Hume BCC, Mamonova E, van Kleunen M (2023) Plant–soil feedback effects on conspecific and heterospecific successors of annual and perennial central European grassland plants are correlated. Nat Plants 9:1057–1066
Wubs ERJ, Bezemer TM (2016) Effects of spatial plant-soil feedback heterogeneity on plant performance in monocultures. J Ecol 104:364–376
Wubs EJ, Bezemer TM (2018) Plant community evenness responds to spatial plant–soil feedback heterogeneity primarily through the diversity of soil conditioning. Funct Ecol 32:509–521
Xue W, Berendse F, Bezemer TM (2018) Spatial heterogeneity in plant–soil feedbacks alters competitive interactions between two grassland plant species. Funct Ecol 32:2085–2094
Xue W, Yao S-M, Huang L, Roiloa SR, Ji B-M, Yu F-H (2021) Current plant diversity but not its soil legacy influences exotic plant invasion. J Plant Ecol 15:639–649
Xue W, Huang L, Sheng WJ, Zhu JT, Li SQ, Yu FH (2022) Contrasting effects of plant-soil feedbacks on growth and morphology of physically-connected daughter and mother ramets in two clonal plants. Plant Soil 472:479–489
Article CAS Google Scholar
Zeiter M, Stampfli A (2012) Positive diversity–invasibility relationship in species-rich semi-natural grassland at the neighbourhood scale. Ann Bot 110:1385–1393
Zhang ZJ, Liu YJ, Brunel C, van Kleunen M (2020) Evidence for Elton’s diversity–invasibility hypothesis from belowground. Ecology 101:e03187
Zimmermann H, Brandt P, Fischer J, Welk E, von Wehrden H (2014) The human release hypothesis for biological invasions: human activity as a determinant of the abundance of invasive plant species. F1000Research 3:109
Download references
Acknowledgements
We thank Yao-Le Ma and Wei-Long Li for assistance with the experiment.
Research was supported by the National Natural Science Foundation of China (Grant 32001122) and Zhejiang Provincial Natural Science Foundation (Grant LQ21C030003).
Author information
Hao-Ming Yuan and Xiao-Mei Zhang contributed equally to this work.
Authors and Affiliations
College of Ecology and Environment, Chengdu University of Technology, Chengdu, 610059, Sichuan, China
Hao-Ming Yuan
Institute of Wetland Ecology & Clone Ecology, Taizhou University, Taizhou, 318000, Zhejiang, China
Hao-Ming Yuan, Xiao-Mei Zhang, Lin-Xuan He, Wei Xue, Lin Huang, Ling Peng & Fei-Hai Yu
School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, 100083, China
Xiao-Mei Zhang
Department of Biology, University of Massachusetts, Amherst, MA, 01003, USA
Peter Alpert
University and Jepson Herbaria, University of California, Berkeley, CA, 94720, USA
You can also search for this author in PubMed Google Scholar
Contributions
Wei Xue conceived the idea and designed methodology. Hao-Ming Yuan, Xiao-Mei Zhang, Lin-Xuan He, Wei Xue, Lin Huang and Ling Peng conducted the experiment and collected the data. Hao-Ming Yuan and Xiao-Mei Zhang analyzed the data with guidance of Wei Xue. Hao-Ming Yuan, Xiao-Mei Zhang and Wei Xue wrote the first version of the manuscript. Peter Alpert and Fei-Hai Yu improved the manuscript. All authors discussed the results, contributed substantially to the draft and gave final approval for publication.
Corresponding authors
Correspondence to Wei Xue or Fei-Hai Yu .
Ethics declarations
Competing interests.
The authors have no conflicts of interests to declare.
Additional information
Responsible Editor: Emilia Hannula.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
(DOCX 31.6 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Yuan, HM., Zhang, XM., Alpert, P. et al. Biotic plant-soil feedbacks alone do not explain why more diverse plant communities are less invasible. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06759-8
Download citation
Received : 14 December 2023
Accepted : 17 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1007/s11104-024-06759-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biomass allocation
- Introduced invasive species
- Native plant diversity
- Plant-soil interactions
- Spatial heterogeneity
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 31 May 2024
A comprehensive review of in planta stable transformation strategies
- Jérôme Gélinas Bélanger 1 , 2 ,
- Tanya Rose Copley 1 ,
- Valerio Hoyos-Villegas 2 ,
- Jean-Benoit Charron 2 &
- Louise O’Donoughue 1
Plant Methods volume 20 , Article number: 79 ( 2024 ) Cite this article
548 Accesses
7 Altmetric
Metrics details
Plant transformation remains a major bottleneck to the improvement of plant science, both on fundamental and practical levels. The recalcitrant nature of most commercial and minor crops to genetic transformation slows scientific progress for a large range of crops that are essential for food security on a global scale. Over the years, novel stable transformation strategies loosely grouped under the term “in planta” have been proposed and validated in a large number of model (e.g. Arabidopsis and rice), major (e.g. wheat and soybean) and minor (e.g. chickpea and lablab bean) species. The in planta approach is revolutionary as it is considered genotype-independent, technically simple (i.e. devoid of or with minimal tissue culture steps), affordable, and easy to implement in a broad range of experimental settings. In this article, we reviewed and categorized over 300 research articles, patents, theses, and videos demonstrating the applicability of different in planta transformation strategies in 105 different genera across 139 plant species. To support this review process, we propose a classification system for the in planta techniques based on five categories and a new nomenclature for more than 30 different in planta techniques. In complement to this, we clarified some grey areas regarding the in planta conceptual framework and provided insights regarding the past, current, and future scientific impacts of these techniques. To support the diffusion of this concept across the community, this review article will serve as an introductory point for an online compendium about in planta transformation strategies that will be available to all scientists. By expanding our knowledge about in planta transformation, we can find innovative approaches to unlock the full potential of plants, support the growth of scientific knowledge, and stimulate an equitable development of plant research in all countries and institutions.
Introduction
Although it has been more than 40 years since the first publications concerning transgenic plants, plant transformation remains a major bottleneck in most commercially important and underutilized crops [ 1 ]. The recalcitrant nature of many plant species and genotypes to in vitro regeneration is a significant barrier to plant improvement, thus slowing scientific progress and contributing to an overreliance on the same species and genotypes that are more easily amenable to transformation. However, several transformation strategies devoid of or with minimal tissue culture steps have been developed over the years. Altogether these methods offer a promising alternative to the laborious tissue culture steps associated with in vitro techniques. Such transformation strategies are loosely termed “in planta” and have been proven efficient in a breadth of monocot and dicot species. Generally, most in planta methods are also often considered genotype-independent since they do not rely heavily on hormone supplementation and often omit the callus regeneration step. As such, in planta strategies are less prone to somaclonal variations and offer an alternative to circumvent the challenges associated with these long-lasting genetic changes. The simple and affordable nature of these protocols in comparison to in vitro methods makes them particularly suited for minor crops. This feature can allow labs to manage simultaneous genetic transformation projects using various species, genotypes, and constructs with minimal financial requirements and trained personnel. On a global level, these aspects can guarantee an equitable development of plant research in all countries, institutions, and budgets. Moreover, the negligible financial inputs required by labs to undergo in planta projects signifies that riskier projects can be undertaken.
To this day, the only in planta method that has received widespread attention is the Arabidopsis thaliana floral dip method. The floral dip method is one of the most cited protocols in plant molecular biology and is one of the main factors that has contributed to propelling Arabidopsis to the honorable status of “most important model organism in plant biology” [ 2 , 3 , 4 ]. As a whole, the success of this technique clearly depicts the potential of development for universal in planta methods, particularly in the era of CRISPR-Cas9 and high-throughput genome editing. Over the years, several review papers have been written on the topic of in planta transformation, thus demonstrating the importance of the concept [ 5 , 6 , 7 , 8 , 9 , 10 , 11 ]. Largely, papers focused on specific in planta methods, such as the floral dip and the shoot apical meristem (SAM) injury techniques, and do not include the most recent scientific developments in an area that is rapidly evolving. This article aims at complementing these past literature reviews and framing them into the bigger context of in planta transformation as a topic. Overall, we start this review by drawing the conceptual framework of in planta stable transformation and classifying the different in planta strategies. Subsequently, we describe several in planta experimental approaches with a focus on recent advances and finally discuss the future avenues and possibilities in this field of research.
Approaches for data collection and building of the in planta compendium
For the collection of data required to build our in planta transformation compendium, a systematic review was conducted using Google Scholar and Scopus search engines to identify the bulk of research articles. Following the use of these tools, we complemented the compendium using articles initially found on ResearchGate and several other online web references such as EuropePMC. Due to the large number of research articles available for specific techniques (e.g. floral dip and pollen-tube pathway), we focused on identifying research articles that demonstrate the efficiency of these approaches in understudied plant species (i.e. all plants that are not considered commercial or model crops) to improve our global understanding of the applicability of these transformation strategies. On the whole, we manually curated, annotated, and reviewed 323 references (research articles, thesis, patents, etc.) tackling the topic of in planta transformation using this classification scheme (Table S1 ). In total, this compendium includes a total of 139 different species, 105 genera, and a broad range of techniques for each type of explant (Fig. 1 ; Table S1 ). All of the sections referring to specific in planta transformation techniques de facto refer to this compendium to limit the number of in-text references. For visualization, ggplot2 package version 3.3.5 with R version 4.0.4 [ 12 ] was used to build Fig. 1 , whereas Figs. 2 , 3 , 4 , 5 , 6 , 7 , 8 and 9 were created with www.BioRender.com .

Distribution of the publications found in the in planta compendium. This graph shows the distribution of the publications associated with each type of explant

Classification of the four de novo organogenesis pathways. Regeneration-dependent de novo organogenesis strategies can be performed under in vivo (in planta) or in vitro (not in planta) conditions. The direct regeneration mechanism has many advantages over the indirect mechanism as it is simpler and quicker to perform; however, it leads to the formation of chimeric T 0 mutants that require segregation in the T 1 generation to obtain non-chimeric offspring. Moreover, the direct regeneration mechanism does not suffer from somaclonal variation, unlike callus-based methods. Callus-based methods are generally more challenging to perform but can be useful for specific crops (e.g. plants with a long juvenile phase such as trees) that cannot be transformed efficiently using the direct regeneration mechanism. The in vitro indirect regeneration pathway is generally considered highly genotype-dependent due to the use of multiple growing media, whereas direct regeneration methods are more universal due to their use of simple cultivation medium that are suitable for a larger spectrum of genotypes. The classification of these pathways was inspired by the comparative scheme of bud regeneration avenues developed by Shi et al. [ 54 ]

Gamete-based transformation techniques. ( A ) Strategies targeting the female gamete (ovule). Several in planta techniques (e.g. the floral dip [ 59 ], vacuum-infiltration [ 60 ], floral spray [ 76 ], and floral painting [ 67 ]) targeting the female gametes have been developed and validated. In Arabidopsis , in planta strategies targeting the ovules often lead to the generation of hemizygous offspring in the T 1 generation as the male reproductive organs (i.e. pollen and pollen tubes) remain untouched [ 137 , 243 ]. A thorough screening must be performed in the T 1 generation and further to identify positive mutants using a selection marker or reporter gene [ 65 , 66 ]. ( B ) Male gametes-based in planta approaches. In these strategies, the pollen grains are transformed through various methods such as sonication [ 83 ], vacuum infiltration [ 82 ], magnetofection [ 85 , 86 ], Agrobacterium [ 82 , 84 ], particle bombardment [ 80 , 81 ], and electroporation [ 79 ]. Subsequently, these pollen grains are used to pollinate the recipient plant’s ovules and lead to the generation of putatively transformed T 1 offspring. Following this, screening is performed in the T 1 generation to identify positive transformants
Definition of in planta stable transformation
The act of generating stable plant transformants is a combination of two indissociable and interdependent steps: (i) the transformation of a plant cell; and (ii) the development of this cell into a whole plant [ 13 ]. In planta stable transformation, also called in situ transformation, techniques form a heterogeneous group of methods all aiming at performing the direct and stable integration of foreign T-DNA into a plant’s genome and regenerating the transformed cells into whole plants [ 5 , 6 , 7 , 8 , 9 , 10 , 11 ]. Unlike in planta transient transformation strategies, such as agroinfiltration, in planta stable transformation aims at generating heritable modifications using exogenous genetic material. In opposition to in planta strategies, in vitro indirect transformation/regeneration techniques, often called conventional transformation/regeneration methods, aim at regenerating an explant that produces a callus (i.e. a more or less developed unorganized plant structure made of parenchyma cells) under strictly sterile conditions [ 8 ].
Historically, the most common definitions of in planta transformation have been (i) a means of transformation without tissue culture step [ 5 , 7 ] and (ii) a means of transformation of intact plants or plant tissues without callus culture or regeneration [ 14 , 15 ]. In our opinion, these definitions are incomplete and not nuanced enough to take into account the broad diversity of available in planta methods. The challenging aspect of most in vitro indirect transformation/regeneration techniques stems from the combination of the hard-to-maintain micropropagation conditions and the callus regeneration step, more than the singular features of each aspect taken alone. As such, multiple highly efficient in planta research articles performing callus regeneration under in vivo conditions have been published over the years [ 16 , 17 , 18 , 19 , 20 ]. Similarly, several effective in planta protocols using minimal in vitro steps have also been published [ 21 , 22 , 23 , 24 , 25 ]. Conceptually speaking, these published methods all fall within the scope of in planta transformation and were self-described as in planta by their authors; however, their methods do not strictly follow the definitions mentioned above. Furthermore, several articles with major in vitro (e.g [ 26 , 27 , 28 ]) components have been published and were also self-described as in planta by their authors. These contrasting definitions underline the grey zone concerning the use of micropropagation within the realm of in planta transformation. For the purposes of this article, we redefined the in planta concept as the following: a means of plant genetic transformation with no or minimal tissue culture steps. To be considered minimal, the tissue culture steps should meet the following pivotal criteria: (i) short duration with a limited number of medium transfers; (ii) high technical simplicity (i.e. simple medium composition with a limited list of hormones); and (iii) regeneration using a differentiated explant that does not undergo a callus development stage and thus relies on direct regeneration.
Classification of in planta transformation methods
In contrast to conventional transformation methods, in planta strategies are extremely heterogeneous in their modes of action and types of organ targeted. At present, there are hundreds of in planta protocols available in the literature. The classification of these protocols into a structured system is challenging due to numerous factors, including: (i) heterogeneous mode of action; (ii) skewed distribution of the publications between the methods (i.e. some methods have dozens of publications, while others have only one or a few); (iii) specific methods that have been reviewed thoroughly in the past while others are nearly absent from the literature; and (iv) the scientific pertinence/novelty versus the number of publications that are often uncorrelated.
This article has been written with the intent of finding a balance between all of these aspects, with an emphasis on techniques not thoroughly reviewed in the past. A large number of techniques presented in this paper were named/renamed by ourselves to distinguish them from similar techniques. As such, the names found in this paper might differ in other references. To build this review paper, we classified the references based on their explant of choice using the following nomenclature: (i) germline [female (ovule) and male (pollen) gametes]; (ii) embryo (aka zygotes); (iii) shoot apical meristem and adventitious meristems; (iv) vegetative tissues; and (v) novel systems (Table S2 ).
Germline transformation techniques are regeneration-independent strategies that target the haploid female (egg) or male (sperm) gametophytic cells before their fusion and the subsequent generation of a diploid zygote [ 29 , 30 ]. Germline-based transformation techniques can be divided into two categories based on the nature of the targeted sexual organ: (i) ovule (female organ) and (ii) pollen (male organ).
Plant zygotes are progenitor stem cells generated from the fusion of two haploid gametes, the egg and the sperm cells, from which all of the embryonic and post-embryonic organs are generated [ 31 ]. The zygote is divided into two parts, a small apical and a large basal cells [ 32 ]. Through the development of the embryo, the small apical part will give rise to the shoot meristem [ 32 ]. In this paper, the embryo section includes all the methods performed at the post-pollination stage until the emergence of the shoot apical meristem from the seed upon germination.
The shoot and root meristems are highly organized structures composed of proliferating embryonic-type cells involved in the continuous generation of aerial and underground plant organs through mitosis [ 33 ]. A portion of the stem cells present in these meristems are activated upon germination to produce primordia of lateral organs, while a pluripotent undifferentiated population is maintained at its center to ensure self-renewal and integrity [ 33 ]. Unlike the floral meristem, the stem cell features of the shoot apical meristem are maintained throughout the whole life cycle of the plant [ 34 ]. The protocols included in the pre-formed meristem sections include those that target different types of meristems (apical, axillary, or adventitious) upon their emergence from the seed until their senescence.
Callus refers to the accumulation of disorganized cell masses generally associated with the wounding of vegetative tissues [ 35 ]. These pluripotent cell masses either form roots or shoots through cellular reprogramming upon inductive cues (e.g. presence of light) [ 36 ]. Monocots and dicots have important biological differences that influence their respective abilities to form new meristems from a pluripotent callus mass [ 37 , 38 ]. In dicots, most anatomical organs display the ability to generate calluses during the whole life of the plant, whereas monocots do not have a true vascular cambium with the ability to undergo cell rearrangement [ 37 , 38 ]. Callus generation in monocots is limited to the base segment of leaves and the lateral and tip regions of roots [ 37 ]. As such, dicots are much more amenable to in vivo regeneration and propagation (e.g. grafting and cuttings) than monocots [ 37 , 39 ].
Two transformation techniques (i.e. grafting-mediated transformation and transformation using viral-based vectors) have been classified in the “ novel systems ” section because they harbor special features that limit their classification using the four other different types of explants. At present, the scope of these methods remains more limited than all of the other in planta strategies presented here due to specific experimental requirements.
Means of in planta transformation
Plant transformation techniques can be divided into two main gene transfer categories: (i) direct gene transfer; and (ii) indirect gene transfer [ 40 ]. The former transfer strategy aims at introducing naked DNA into a plant genome through chemical or physical means (e.g. biolistics, electroporation, and polyethylene glycol), whereas the latter involves the introduction of DNA using biological vectors (e.g. Agrobacterium spp ., Ochrobactrum haywardense , or viral vectors) [ 40 ]. Agrobacterium tumefaciens -mediated transformation is by far the most used method among the different in planta approaches as it is a simple and cost-effective option that generates few copy numbers in the generated transformants [ 11 ]. In addition, Agrobacterium is effective in a wide range of plant genotypes and species and can be used with various types of in planta strategies, thus making it a robust, reliable, and versatile transformation system [ 11 ]. Agrobacterium rhizogenes is generally used to perform in planta transformation that results in non-heritable changes through the formation of hairy roots in composite plants; however, the recently developed cut-dip-budding [ 41 ] and vine-cutting node inoculation [ 42 ] methods have demonstrated that A. rhizogenes can be used to perform stable transformation in asexually propagated plant species such as sweet potato ( Ipomoea batatas ). Although more marginal in their use, several other methods, such as direct DNA uptake [ 43 ] and biolistics [ 14 , 44 ], are now sometimes used in diverse in planta protocols and are alternatives to Agrobacterium -based methods.
Types of regeneration pathways
In most genetic transformation experiments, the regeneration of a positive somatic mutant cell into a whole plant is the rate-limiting step that is associated with the recalcitrant features of most hard-to-transform species [ 45 ]. In plants, this step can be undertaken using two strategies that are based on totipotency (i.e. a cell’s feature that enables it to dedifferentiate and redifferentiate into different tissues, organs, or whole organisms): (i) somatic embryogenesis; or (ii) de novo organogenesis [ 46 ]. Over the years, fertilization-based transformation techniques based on the transfer of exogenous DNA to male/female haploid gametes (e.g. floral dip) or fertilized diploid zygotes (e.g. pollen-tube pathway) have also been developed and are considered regeneration-independent [ 47 ]. In general, regeneration-independent techniques are often considered more efficient than their dependent counterparts due to their omission of the regeneration step; however, these approaches also have their own set of disadvantages including the generation of hemizygous (i.e. only one copy of a transgene at a given locus in an otherwise diploid cell) individuals when targeting haploid gametes [ 47 ].
Somatic embryogenesis
Somatic embryogenesis is a mechanism in which differentiated cells undergo dedifferentiation to become embryonic stem cells [ 48 , 49 ]. Following this step, embryonic stem cells can differentiate into meristematic cells to become a single and viable plant [ 48 ]. In the literature, the main difference between somatic embryogenesis and indirect de novo organogenesis is the presence of a somatic embryo formation step in the former, whereas the latter undergoes a callus generation step [ 48 ]. As such, both mechanisms require a regeneration step to form a new plant. To our knowledge, somatic embryogenesis, either through the direct or indirect pathways, is not a mechanism used for in planta transformation due to its extensive tissue culture requirements. In consequence, the term regeneration-dependent strategies will refer herein to only methods using a de novo organogenesis mechanism.
De novo organogenesis
Plant regeneration occurs upon cell wounding and aims at repairing or replacing the damaged anatomical structures using totipotency and pluripotency, which will lead to the subsequent generation of adventitious organs [ 48 ]. Adventitious organs are defined as either root or shoot meristematic buds that arise from growing areas that typically do not contain such organs [ 50 ]. In the literature, no specific terms distinguish the adventitious organs which are obtained either from indirect or direct de novo organogenesis [ 48 ]. In addition, indirect and direct shoot regeneration events often occur simultaneously upon wounding [ 16 ], a phenomenon that can generate some confusion between the mechanisms in the literature. However, the distinction between both types of adventitious shoot formation pathways is important due to major differences in their underlying biological mechanisms and impacts on the transformation event. For instance, direct regeneration strategies, both under in vivo and in vitro conditions, can instigate a varying degree of chimerism in the transformants, thereby creating heterogenomic mutants that will require subsequent segregation to recover non-chimeric plants [ 51 , 52 ]. In plants obtained with indirect organogenesis, chimerism is less concerning because single-cell regeneration can be undergone using a selection marker (e.g. antibiotics or herbicides), but somaclonal variations are typically more prevalent [ 53 ].
Overall, techniques using a de novo organogenesis approach can be classified based on their use of tissue culture (i.e. in vivo/tissue culture-independent vs. in vitro/tissue culture-dependent) and methods of regeneration (i.e. direct regeneration vs. indirect regeneration) [ 54 ] (Fig. 2 ). In general, in planta strategies aim at limiting tissue culture to a minimum and consequently either use in vivo direct regeneration or in vivo indirect regeneration strategies. From a technical standpoint, the in vitro direct regeneration pathway can be considered a crossover between the in vitro direct regeneration and in vivo indirect regeneration concepts as the explants are micropropagated under sterile conditions but regenerated through direct organogenesis. Although not considered in planta per se , the protocols using the in vitro direct regeneration pathway generally have a faster regeneration rate (often between 4 and 8 weeks), lessened use of hormones, higher success rates, greater genotype-independency, and decreased technical skills requirements [ 55 , 56 ]. A short section of this paper will be dedicated to the methods using this pathway since those offer a promising alternative to the in vitro indirect regeneration pathway, particularly in monocots [ 57 , 58 ].
Germline transformation
Floral dip and similar methods (ovule).
The most important contributor to the spread of the in planta conceptual framework is undoubtedly the floral dip method in Arabidopsis [ 59 ]. At its essence, the floral dip method is a simple and reliable method that aims at performing germline transformation through the dipping of developing floral tissues into resuspended Agrobacterium inoculum [ 59 ] (Fig. 3 a). The first iteration of this method was developed by Bechtold and Pelletier [ 60 ] using vacuum-infiltration of the floral organs. Despite its high transformation rates, this protocol was largely supplanted by the protocol proposed by Clough and Bent [ 59 ] which removed the vacuum-infiltration step and replaced it with a simple dip into a solution containing Agrobacterium , sucrose, and a surfactant (i.e. Silwet L-77), thus streamlining the technical aspect of the method and increasing the speed of the procedure. As such, the approach developed by Clough and Bent [ 59 ] is now the mainstay for transforming Arabidopsis , a popularity largely due to its high transformation efficiency as rates between 0.1 and 3% are typical Footnote 1 [ 61 ]. Over the years, other iterations of the technique, such as the floral dip with low inoculum density [ 62 ], vacuum-infiltration of closed floral buds [ 63 ], and simplified floral dip [ 64 ], have been proposed to upgrade specific aspects of the method. Although the floral dip approach is a common technique for plant transformation, two factors still readily limit its development on a broader scale: (i) the generation of hemizygous offspring; and (ii) a narrow range of species amenable to the method.

In planta approaches targeting the embryos at an early stage of development. ( A ) Pollen-tube pathway [ 92 ]. To perform the pollen-tube pathway, the plant’s stigmas are removed and the styles are severed shortly after pollination. Subsequently, exogenous donor DNA is applied to the severed styles and delivered to the recipient plant’s ovaries via the growth of the pollen tube. Following the seed set, the putative transformants are screened to identify positive mutants. ( B ) Ovary-drip [ 92 ]. In this approach, the ovary sac is incised using a sterile scalpel, and exogenous DNA is directly delivered to the ovule drop-by-drop using a micropipette. ( C ) Pollen-tube agroinjection [ 113 ]. In this method, a solution of resuspended Agrobacterium is injected into the plant’s pollen tube using injector needles. To do so, the carina is punctured with the needles and the solution is injected until the wing petals are soaked. ( D ) Ovary injection [ 115 , 116 , 118 ]. To apply the ovary injection strategy, a solution of resuspended Agrobacterium is injected into the ovaries (i.e. soybean pods in this case) at an early stage of development to infect the developing embryos. Following this step, the mature seeds are further screened to identify positive mutants
Hemizygous offspring are generated with the floral dip method since the transformation event happens after the divergence of anther and ovary cell lineages in Arabidopsis [ 47 ]. In Arabidopsis , the stigmatic cap forms over the top of the gynoecium, enclosing the locules 3 days before anthesis [ 47 ]. As a consequence, the primary targets of the floral dip method are the female reproductive organs, the ovules, and embryo sacs, whereas the pollen or pollen tubes remain untouched [ 47 ]. To segregate all hemizygous progenies and recover only offspring with homozygous genotypes, a thorough screening must be performed until the T 3 generation as the progenies from the T 2 generation are not stable [ 65 , 66 ].
Although tremendous research has been pursued on the floral dip method, the number of species amenable to this technique remains modest in comparison to other techniques, such as the shoot apical meristem injury approach. At present, the bulk of the floral dip protocols have been developed for species belonging to the Brassicaceae family, but transformation procedures based on this approach have also been demonstrated to be efficient for 12 other families (e.g. Linaceae and Solanaceae) (Table S1 ). Still, the protocols targeting species belonging to families other than Brassicaceae are sparse and generally less efficient due to lower transformation rates, cumbersome manipulations, and complicated technical requirements (e.g. tomato/ Solanum lycopersicum [ 67 ]). Numerous biological and morphological factors have been suggested to explain the limited expansion of the floral dip technique to other plant species, including physical barriers associated with flower morphology [ 61 ], necrotic reaction to the presence of Agrobacterium causing abortions in the flowers [ 61 ], lower seed set [ 68 ], reduced susceptibility to Agrobacterium [ 68 ], and bigger size of the plant and/or flower structures [ 5 ]. Over the years, modifications to the floral dip method have been developed to increase its efficiency with plant species that are not members of the Brassicaceae, while retaining the core concepts of the strategy. Amongst these innovative strategies are the floral bud injection (tomato, poplar/ Populus sp. , chickpea/ Cicer arietinum and sunflower/ Helianthus annuus ) [ 69 , 70 , 71 , 72 ], floral bud painting (maize/ Zea mays and tomato) [ 67 , 73 ], and floral bud spray ( Arabidopsis , wheat/ Triticum aestivum , and Indian mustard/ Brassica juncea ) strategies [ 74 , 75 , 76 ].
Pollen transformation
In the pollen transformation method, the desired foreign gene is introduced into the pollen grains via Agrobacterium or directly with naked DNA [ 77 ] (Fig. 3 b). Following this step, the transformed pollen grains are subsequently used to pollinate the stigma and fertilize the recipient egg in vivo. Pollen grains are an interesting target for transformation as they can be easily isolated, occur in large numbers, and can be easily transformed [ 77 ]. Pollen grains harbor a coat derived from the anther tapetum (the pollenkitt/tryphine), an outer thick cell wall (the exine), and a thin inner cell wall (the intine), that block the integration of exogenous DNA [ 77 ]. In addition, germinating pollen grains release nucleases that catalyze the cleavage of phosphodiester bonds between nucleotides of nucleic acids [ 78 ]. In combination, the thick wall/coat and release of nucleases limit the use of conventional transformation methods to integrate the transgene into the pollen grain [ 77 , 78 ]. To circumvent this problem, various methods such as electroporation [ 79 ], particle bombardment [ 80 , 81 ], vacuum infiltration [ 82 ], sonication [ 83 ], Agrobacterium [ 82 , 84 ], and magnetofection [ 85 , 86 ] have been used to facilitate the introduction of transgenes into pollen grains or microspores, with varying degrees of success. Several transformation methods based on pollen incorporate a short in vitro period at the beginning of the experiment as in the case of the male germline transformation (MAGELITR) system [ 81 ], which can be a limiting factor for labs without access to micropropagation facilities. Overall, pollen transformation has been demonstrated to be efficient in several species, including tobacco [ 79 , 80 , 81 , 87 ], cotton ( Gossypium hirsutum ) [ 82 ], sorghum ( Sorghum bicolor ) [ 88 ], petunia ( Petunia x hybrida ) [ 89 ], Indian mustard [ 83 ], and maize [ 90 ], but its implementation remains challenging in a large number of species, with contrasting results between different labs (e.g. magnetofection was reported to be inefficient in monocots [ 91 ]).
Pollen-tube pathway
The pollen-tube pathway strategy aims at applying exogenous donor DNA onto the severed style of the recipient plant, which will be transported via the growth of the pollen tube to the ovary [ 92 ] (Fig. 4 a). Reaching the ovary, the foreign DNA will be integrated into the undivided recipient zygote, thus leading to the generation of a transformed embryo [ 92 ]. To improve the rates of transformation, researchers often cut the styles of the recipient plant [ 92 ]. The pollen-tube pathway transfer technique is one of the oldest transformation techniques that has been investigated, with reports dating back to 1983 in cotton [ 93 ] and 1989 in rice ( Oryza sativa ) [ 94 ]. Although beneficial in many aspects (e.g. no regeneration step and fast preparation), this method has also demonstrated some limitations in the past, such as poor transformation efficiency [ 95 , 96 ] and a lack of reproducibility [ 97 , 98 , 99 ], which led to a rise in skepticism regarding some of its claimed benefits (e.g. universal application) [ 92 ]. For instance, Li et al. [ 99 ] have observed many inconsistencies with soybean ( Glycine max ) plants treated with the pollen-tube pathway technique. In their experiments, all the plants exhibiting positive β-glucuronidase (GUS) activity were found to be untransformed when analyzed using polymerase chain reaction (PCR). Similarly, morphological variation was observed in the first generation of some plants, but not in the subsequent generations. As a consequence of these inconsistent results, there has been a disinterest in this transformation system in the Western hemisphere [ 92 ]. In the meantime, China continued to improve the procedure and has now developed broad expertise with this transformation strategy, resulting in a significant proportion of the research articles only being available in Mandarin [ 100 ]. When compiling the research articles for this review, we found that a broad selection of protocols is now available for this strategy with dozens of research articles published for major commercial crops, including cotton [ 101 , 102 , 103 ], maize [ 104 ], rice [ 105 ], and wheat [ 106 ], as well as for at least 24 other species.

In planta strategies targeting the embryos at a later stage of development. ( A ) Infection of pre-imbibed embryos with Agrobacterium . The seeds are imbibed with sterile water and either (i) kept uninjured [ 122 ] or (ii) injured using pricking, sonication, or vacuum infiltration [ 121 ]. Following this treatment, the seeds are infected with a solution of Agrobacterium and grown until the T 1 generation for selection. ( B ) Agro-imbibition [ 124 ]. In this approach, seeds are imbibed with a solution of Agrobacterium instead of sterile water and further selected in the T1 generation. ( C ) Imbibition of desiccated embryos [ 125 ]. To perform this method, seeds are first imbibed with sterile water and subsequently desiccated at room temperature for 9–36 h. The seeds are subsequently infected for 2 h with a solution of Agrobacterium and cultivated until the T 1 generation for selection
The ovary-drip method differs from the pollen-tube pathway as the exogenous DNA (i.e. which is supplied under the form of a minimal linear gene cassette) is directly delivered to the ovule after pollination with the complete removal of the style [ 107 ] (Fig. 4 b). Generally, the ovary-drip method has higher transformation rates than the pollen-tube pathway (e.g. 3.38% transformation frequency with the ovary-drip method vs. 0.86% with the pollen-tube pathway [ 108 ]), but requires careful manipulation to limit the risk of mechanical damage to the ovule [ 92 , 109 ]. This method has been used successfully to transform soybean [ 107 , 110 ] and maize [ 111 , 114 ]. One of the key factors influencing the success rate of this method is the length of the style. Liu et al. [ 112 ] investigated the optimal length of the soybean style and found that the complete removal of the style without ovary wounding generated the highest proportion of transformants, 11%.
Pollen-tube agroinjection
At its core, the pollen-tube agroinjection method combines the principles of the pollen-tube injection pathway with A. tumefaciens -mediated transformation (Fig. 4 c) [ 113 ]. In this method, carinas (i.e. two conjoined lower petals of a legume flower that enclose the stamen and style) of freshly opened flowers (in this case peanut) need to be punctured using injector needles and injected with 0.1 mL of resuspended Agrobacterium solution. The method was used to generate transgenic peanut lines encoding the peanut BAX INHIBITOR-1 gene with an overall transformation rate of 50%. To the best of our knowledge, only one research article using this approach has been published, but the high transformation rates suggest that it might be an efficient alternative to the conventional pollen-tube pathway technique.
Ovary injection transformation
The ovary injection method aims at injecting Agrobacterium directly into the locule of a plant’s ovary to reach the embryo using a micro-injector or a syringe after pollination (e.g. cotton [ 114 ]) (Fig. 4 d). This method has been used with success in about ten species, but has been demonstrated to be particularly effective in tomato [ 115 , 116 , 117 ] and, to a minor extent, soybean [ 118 ]. In tomato, Hasan et al. [ 116 ] developed a protocol in which mature and ripe fruits were injected with 1 mL of an Agrobacterium solution containing a GUS reporter and incubated at 28 °C for 48, 72, and 96 h. The highest number of stable transformed plants was obtained with a 48 h incubation period, with 88% being positive for the GUS assay. Using a similar protocol, Yasmeen et al. [ 115 ] obtained transformation rates of 35–42% in tomato depending on the construct. When injecting the Agrobacterium solution at stage I (i.e. 2–3 days after pod formation) in soybean, transformation efficiencies between 6.45 and 14.2% and 28.75–35.48% were respectively obtained using GUS assays on plants and seeds [ 118 ]. To improve the transformation rates of the ovary injection method, a similar method using micro-vibration was developed by Liou [ 119 ]. In this approach, the stigma of the flower is removed and exogenous DNA is injected through the cut-off position and toward the locule inside the ovary. Following this step, a micro-vibration treatment will be performed with an ultra-sonic device to favor the placement of DNA around the ovule and improve integration.
Infection of pre-imbibed embryos with agrobacterium
The infection of pre-imbibed embryos with Agrobacterium is a simple technique in which a seed is injured (e.g. seed pricking, tip cutting, sonication, or puncturation) and then imbibed to facilitate the infection of the embryo by Agrobacterium (Fig. 5 a). This technique was first developed by Graves and Goldman [ 121 ] by pricking four-day-old germinating maize seeds four times in an area extending from the scutellar node through the mesocotyl to infect the cells located in this zone with Agrobacterium . Subsequently, a method for the transformation of soybean was developed using a similar approach [ 121 ]. In the Chee, Fober, and Slightom [ 121 ] protocol, imbibed soybean seeds with one cotyledon removed were pricked at three different points into the plumule, cotyledonary node, and adjacent regions and injected with 30 µL of Agrobacterium culture at each injured point. The observed transformation rates obtained with this method were 0.7% in the R 0 plant and 0.07% in the R 1 generation. Although the rates of transformation were low for both of these protocols, they paved the way to more performing protocols in a large number of species. Following the development of the Graves and Goldman [ 120 ] method in maize, a variant involving the use of uninjured seeds was developed in 1987 using Arabidopsis [ 122 ]. In this protocol developed by Feldmann and David Marks [ 122 ], Arabidopsis seeds were imbibed for 6, 12, or 24 h following a one-step or two-step imbibition protocol, infected with 3 mL of an overnight culture of Agrobacterium and co-cultivated during 24 h before being washed with sterile water. Subsequently, the seeds were sown on vermiculite pre-soaked with a complete nutrient solution. Although the transformation efficiencies were rather low (0.0015-0.3200%), the protocol still demonstrated that it was possible to generate transformants without causing any injuries to the pre-imbibed seeds.
Transformation approaches targeting the apical and adventitious meristems. ( A ) Shoot apical meristem injury under in vivo conditions [ 129 ]. The apical meristematic region is pricked with a needle and subsequently infected with resuspended Agrobacterium . Chimeric T 0 plants are grown under in vivo conditions until seed set. Non-chimeric lines are further selected in the T 1 generation. ( B ) Plumular meristem approach [ 22 , 148 ]. In the plumular meristem approach, young seedlings are decapitated and their radicules excised with a sterile scalpel. Following this treatment, the explants are infected with Agrobacterium and co-cultivated on a sterile medium under in vitro conditions. After co-cultivation, the seedlings are moved to greenhouse conditions and allowed to set seeds. The T 1 offspring are then screened to identify positive mutants
Agro-imbibition
The agro-imbibition technique is a relatively new approach that aims at fully imbibing whole seeds with an Agrobacterium solution to infect them (Fig. 5 b). The method is simple and has a reduced workload; however, seven patents have been deposited for this method, suggesting that a license might be required to use it [ 123 ]. In their recent article, Kharb et al. [ 123 ] detailed the core principles of this genotype-independent in planta strategy. In their protocol, seeds are surface sterilized using a 0.1% HgCl2 solution for 10 min, imbibed in a resuspended culture of Agrobacterium (O.D. = 0.6) with shaking at 100 revolutions per minute (RPM), and then germinated on a simple germination medium containing 250 mg/L cefotaxime or on soil. According to the authors, many species (e.g. chickpea, pigeon pea/ Cajanus cajan , wheat, soybean, and rice) are amenable to this approach, with efficiencies ranging from 14.3% in chickpea up to 93.8% in rice.
Imbibition of desiccated embryos
This approach aims at rehydrating desiccated zygotic embryos with an Agrobacterium solution [ 125 ] (Fig. 5 c). Upon desiccation, several physiological modifications (e.g. bursting of the cell walls) occur which facilitate the integration of DNA in the zygotic embryo [ 126 ]. Consequently, dry cells become permeable to large plasmid DNA molecules and transformation can happen without relying on Agrobacterium [ 126 ]. In addition, cellular permeabilization agents (e.g. toluenes) can be used to improve the proportion of DNA intake [ 127 ]. Arias et al. [ 125 ] developed a protocol in which soybean embryonic axes (i.e. zygotic embryos) were imbibed in an aqueous solution for 18 h and subsequently desiccated at room temperature until reaching a moisture content of 10–25%. After desiccation, the zygotic embryos were imbibed again with an Agrobacterium solution for approximately 2 h at room temperature. Arias et al. [ 125 ] indicated transformation rates between 0 and 80% in T 0 mutants using GUS assays and mentioned that T 3 transformants were generated for the pBPSLM003 and pCAMBIA3301 plasmids with this method, thus indicating that the method can be efficiently used to generate stable transformants. In addition, the method has also been proven to be compatible with Arabidopsis [ 125 ].
Shoot apical and adventitious meristems
Shoot apical meristem injury under in vivo conditions.
The shoot apical meristem is one of the primary targets of in planta transformation, and an extensive literature targeting this organ under in vivo growing conditions is available. All plant species display at least one form of shoot apical meristem [ 128 ], and the transformation of this organ can be performed at almost any stage of a plant’s life, from the seedling to the adult stages [ 8 ]. Together, these two characteristics (i.e. all stages of growth and all plant species) contribute to the universal applicability of the shoot apical meristem injury transformation approach [ 8 , 128 ]. On the whole, the strategies grouped under this approach loosely share four core concepts that are: (i) wounding the apical meristem region using a needle, scalpel, syringe, or another method (e.g. sonication); (ii) infecting the meristem with Agrobacterium ; (iii) growth of the seedlings under in vivo conditions for most of their lifecycle; and (iv) chimeric T 0 generation with selection in the T 0 (rare) or T 1 (standard) generation [ 129 ] (Fig. 6 a). A standardized protocol named apical meristem targeted in planta transformation, which was first validated in safflower ( Carthamus tinctorius ) and peanut respectively by Rohini and Sankara Rao [ 130 ] and Rohini and Sakanra Rao [ 131 ], was proposed as a low-tech efficient transformation method that can be applied to both dicots and monocots. In this standardized method, the differentiating apical meristem region of two-day-old seedlings is injured using a needle and subsequently infected using an Agrobacterium solution supplemented with Winans’ AB minimal and wounded tobacco leaf extract [ 129 , 132 ]. After the infection, the plants are transferred to autoclaved soilrite and allowed to grow for ≈ 1 week under a 16 h photoperiod [ 129 ]. Following this step, the plant is transferred to pots and allowed to set seed. The T 1 offspring of these chimeric plants are subsequently screened using a selectable marker such as antibiotic resistance and/or PCR amplification [ 129 ]. Overall, the transformation efficiencies can be quite high considering the simplicity of the approach. For example, the transformation efficiencies were respectively evaluated to be 5.3% and 1.3% in the cultivars ‘A-1’ and ‘A-300’ using histochemical assays, PCR amplification, and Southern blot analyses in T 0 and T 1 safflower plants [ 130 ]. In peanut, the transformation frequencies were evaluated to be 3.3% based on histochemical assay and by PCR analysis of the GUS gene [ 131 ].

Additional in planta techniques targeting the shoot apical and adventitious meristems. ( A ) Direct organogenesis of propagules (cut-dip-budding technique) [ 41 ]. To perform this method, plants with a high asexual reproduction capacity (e.g. sweet potato) are decapitated and their wounds are treated with a solution of resuspended Agrobacterium rhizogenes . Due to the root-suckering features of these plants, transgenic hairy roots will slowly develop and generate a newly transformed plant. ( B ) Direct organogenesis of propagules (Regenerative activity-dependent in planta injection delivery technique) [ 150 ]. In the RAPID method, a solution of resuspended A. tumefaciens is injected into the stem of plants with a high asexual reproduction capacity such as sweet potato. The plant is subsequently transplanted and transformed roots (pathway #1) or shoots (pathway #2) will subsequently emerge from the wound sites. ( C ) Direct delivery of exogenous morphogenic regulators [ 175 , 244 ]. In the Direct delivery approach, the recipient plants’ meristems are removed using a sterile scalpel, and developmental regulators (e.g. WUSCHEL/WUSCHEL2 ) are subsequently delivered by injecting a solution of resuspended A. tumefaciens into the wound sites. Following this, the wild-type abnormal transgenic offshoots are culled, whereas the normal transgenic shoots are identified for further propagation
Over the years, several variations have been incorporated into this standard protocol to improve the rate of transformation. For example, the generation of mosaic plants in the T 0 generation requires a stringent screening of the transformants to be performed in the T 1 generation. In some protocols, a selection step under in vivo conditions (e.g. maize [ 133 ]) or in vitro conditions using soilrite as a medium (e.g. roselle/ Hibiscus sabdariffa [ 134 ]) has been added after inoculation to select the best-performing T 0 chimeric plants. The addition of this selection step limits the number of plants that will be cultivated until the T 1 generation and improves the overall rate of transformation. Similarly, some protocols have incorporated steps to improve the injury step by adding sonication (e.g. horse gram/ Macrotyloma uniflorum [ 25 ]), electroporation (e.g. pea/ Pisum sativum , soybean, cowpea/ Vigna unguiculata , and lentil/ Lens culinaris [ 135 , 136 ]), and/or vacuum infiltration (e.g. Arabidopsis [ 137 ], barrel clover/ Medicago truncatula [ 138 ], cumin/ Cuminum cyminum [ 139 ], mung bean/ Vigna radiata [ 140 ] and horse gram [ 25 ]) procedures. Additional modifications include: (i) optimization of the Agrobacterium inoculum optical density (e.g. pigeon pea [ 141 ]); (ii) optimization of the acetosyringone concentration (e.g. tuberose/ Polianthes tuberosa [ 142 ]); (iii) addition of a pre-culture step on Murashige and Skoog (MS) medium before inoculation (e.g. chickpea [ 143 ]); (iv) addition of a co-cultivation step on MS medium after inoculation (e.g. radish/ Raphanus sativus [ 144 ]); and (v) use of a germination medium under in vitro conditions (e.g. sesame/ Sesamum indicum [ 145 ]).
Plumular meristem strategy
Amongst the different protocols using direct de novo shoot organogenesis, the plumular meristem strategy was proposed as a time-efficient direct regeneration-based transformation approach with high transformation rates for chickpea [ 22 , 146 ] and pigeon pea [ 147 , 148 ]. In this system, three-day-old seedlings are decapitated at the shoot apex and pricked in the apical portion and cotyledonary nodes [ 22 , 147 ] (Fig. 6 b). After co-cultivation with A. tumefaciens , multiple shoot induction is performed through the transfer of the explants on a sterile MS medium containing 6-benzyl amino purine (BAP) and 1-naphthaleneacetic acid (NAA) for three days. Following this step, the plants are moved to pots and grown under greenhouse conditions until reaching the T 1 generation. The transformation rates using the plumular meristem strategy method were 44% and 72% in the T 1 generation of chickpea [ 22 ] and pigeon pea [ 147 ], respectively. A similar protocol to the plumular meristem method was developed for alfalfa ( Medicago sativa ) [ 149 ]. In this protocol, three-day-old alfalfa seedlings are excised at the cotyledonary attachment region of the hypocotyl and wounded by vortexing with sterile sand. Following the excisions, the plants are transferred to a hormone-free medium for a short recovery time and cultivated in vitro for 14 days in a half-strength MS medium containing timentin. After this cultivation step, plants are transferred to greenhouse conditions for further growth. When performing this protocol, Weeks et al. [ 149 ] observed that excisions performed below the unifoliate leaf base eliminated the potential for shoot recovery, whereas those performed at or above the apical node resulted in the growth of new shoots in 95% of the cases. Using this protocol, about 7% of the seedlings produced progenies segregating for the T-DNA [ 149 ].
Propagule transformation
Several specialized vegetative plant organs involved in asexual reproduction, often called vegetative propagules, are ideal targets for in planta transformation due to the rapid development of growing permanent plant tissues from actively dividing meristematic cells through mitosis [ 150 ]. Propagules include stem tubers (e.g. potato and yams), tuberous roots (e.g. sweet potato and dahlia), root suckers (e.g. apple, pear, blackberries, and raspberries), runners (e.g. strawberries), bulbs (e.g. onions, tulips, and lilies), and plantlets (e.g. mother of thousands/ Kalanchoe daigremontianum ) [ 151 ]. The cut–dip–budding delivery approach aims at actively regenerating shoots from adventitious buds developed from root suckers transformed with Agrobacterium rhizogenes under in vivo conditions [ 41 ] (Fig. 7 a). This strategy has been demonstrated to be efficient with ten cultivars of sweet potato, two herbaceous plants (i.e. rubber dandelion/ Taraxacum kok-saghyz and crown vetch/ Coronilla varia ), and three woody plants (i.e. Chinese sumac/ Ailanthus altissima , Japanese angelica tree/ Aralia elata , and glorybower/ Clerodendrum chinense ). Using this approach, the observed transformation efficiencies were 10–47% for sweet potato, 40–50% for T. kok-saghyz , 3% for C. varia , 39% for A. altissima , 2% for A. elata , and 48% for C. chinense [ 41 ]. A similar in vitro protocol based on the regeneration of shoots from A. rhizogenes -infected hairy roots has been demonstrated to be efficient in apple ( Malus pumila ) and kiwi ( Actinidia chinensis ), with a short regeneration time of about 9–11 weeks [ 152 ]. The Regenerative activity-dependent in planta injection delivery (RAPID) method aims at generating transformants from infected renascent tissues of sweet potato, potato ( Solanum tuberosum ), and bayhops ( Ipomoea pes-caprae ) under in vivo conditions [ 150 ] (Fig. 7 b). In this protocol, stable transformation is obtained through the delivery of A. tumefaciens to the stem by injection and subsequent vegetative propagation of the emerging positive tissues from the wound site. Selection of the positive tissues is performed through molecular detection and/or phenotypic analysis if using a visual selection marker. Overall, the RAPID protocol displayed a short transformation time, between three to ten weeks, with a high transformant acquisition rate of 28–40%. Additional systems for propagule transformation have been developed for banana ( Musa. sp.) suckers [ 153 ], gemmae of umbrella liverwort ( Marchantia polymorphya ) [ 154 ], leaf notches of cathedral bells ( Kalanchoe pinnata ) [ 155 ], sugarcane ( Saccharum spp. ) setts [ 156 ], and bulbs of the Notocactus scopa and Hylocereus trigonus cacti [ 157 ].

In planta transformation using in vitro direct organogenesis and in vivo callus-based approaches. ( A ) In vitro direct organogenesis. The shoot apical meristems (SAM) are excised from the growing seedlings and inoculated with resuspended Agrobacterium [ 57 , 58 ]. Following inoculation, the putatively transformed shoot apical meristems are grown and screened under in vitro conditions to identify positive T 0 mutants. Following the screening process, mutants are rooted and then transferred to in vivo conditions for seed setting. Optionally, embryonic axes from imbibed seeds can be used similarly to shoot apical meristems (details not shown in the figure) [ 55 , 56 , 245 ]. ( B ) In vivo callus regeneration [ 16 , 17 , 19 , 210 ]. Dicot plants are decapitated and their wound sites are injected or rubbed with a solution of Agrobacterium . Subsequently, the wound sites are covered with parafilm and/or aluminum foil to retain moisture and keep the sites under dark conditions to favor callus formation. Optionally, the wounds can be treated with different hormones to promote the formation of a callus. Before or after callus formation, the sites can be treated with a selection marker such as an antibiotic or herbicide to eliminate untransformed calli cells. After the callus is formed, shoot formation is favored by cultivating the callus site under a regular photoperiodic regime. Under these conditions, transformed shoots will emerge from the calli cells surviving the screening process. ( C ) Shoot apical meristem removal and direct regeneration of adventitious meristems [ 16 , 19 , 210 ]. Plants are decapitated and the wound site is inoculated with Agrobacterium through injection and/or rubbing. The wound site is subsequently covered with parafilm and/or aluminum foil to retain moisture and keep it under dark conditions. Chimeric plants regenerate from the wound site and the adventitious shoot can be maintained on the same plant, grafted on another plant, or rooted in a separate container. Selection is performed in the T 1 generation to retrieve non-chimeric offspring
Exogenous morphogenic regulators and direct delivery
In recent years, the use of exogenous morphogenic regulators has also been explored as an efficient option to induce de novo shoot organogenesis. Morphogenic regulators, such as LEAFY COTYLEDON 1 [ 158 , 159 ], LEAFY COTYLEDON 2 [ 160 ], BABY BOOM [ 161 ] and WUSCHEL [ 162 ], are key genes involved in a plethora of functions such as plant morphogenesis and regeneration [ 163 ], de novo establishment of shoot stem cell niche [ 164 ], shoot and root meristem homeostasis [ 165 ] and shoot apical establishment [ 166 ]. As such, their expression is critical for de novo shoot organogenesis. Morphogenic regulators promote the production of somatic embryos or embryo-like structures on vegetative or callus explants, an effect that is increased upon overexpression [ 167 , 171 , 169 ]. Current reports have demonstrated that ectopicly expressed morphogenic regulators can be harnessed to improve the in vitro recovery rates of transgenic calli from hard-to-transform genotypes of at least 12 commercially important monocot species (e.g. rice) [ 170 , 171 , 172 , 173 , 174 ]. Despite the observed increase in the regeneration rates of transgenic calli [ 170 ], the in vitro use of ectopically expressed morphogenic regulators still remains challenging on a technical level.
To overcome these limitations, Maher et al. [ 175 ] and Cody et al. [ 176 ] developed an exogenous morphogenic regulator-based in vivo transformation method called Direct Delivery. In opposition to the Fast-treated Agrobacterium co-culture (Fast-TrACC) method (i.e. a similar method with an in vitro phase), the Direct Delivery entirely sidesteps tissue culture [ 176 ]. In the Direct Delivery method, developmental regulators, such as maize WUSCHEL/WUSCHEL 2 ( Wus2 ), cytokinin ISOPENTYL TRANSFERASE ( ipt ), and A. thaliana SHOOT MERISTEMLESS ( STM ), and gene-editing reagents are directly delivered with Agrobacterium to somatic cells of whole plants to induce the formation of de novo meristems [ 175 , 176 ] (Fig. 7 c). Following the injection of Agrobacterium , visible meristems are removed and shoot formation occurs at the wound sites after 38–48 days [ 175 , 176 ]. Maher et al. [ 175 ] demonstrated that this approach generates high transformation rates with tobacco/ Nicotiana benthamiana (i.e. gene editing efficiencies ranging from 30 to 95%) and observed positive results with potato and grapevine ( Vitis vinifera ) under in vitro conditions. Lian et al. [ 177 ] successfully regenerated snapdragon ( Antirrhinum majus ) and tomato shoots using a protocol similar to Direct Delivery under in vivo conditions but with the PLETHORA (PLT5) developmental regulator. With this ectopic expression approach, transformation efficiencies up to 11.25% and 13.3% were obtained for snapdragon and tomato, respectively [ 177 ]. The same test was performed on cabbage ( Brassica rapa ) and sweet pepper ( Capsicum spp. ) in vivo, but possibly failed due to the rapid deposition of suberin and lignin in response to wounding [ 177 ]. Direct delivery was also performed on apple ( Malus pumila ) and grapevine by Spicer [ 178 ], but without observing gene edits in the generated shoots.
Nodal agroinjection
The nodal agroinjection approach is a simple method that aims at injecting resuspended Agrobacterium in the first and second nodes of cotyledonary branches. This strategy was first validated by Wang et al. [ 179 ] in peanut and subsequently used by Han et al. [ 180 ] to generate CRISPR-Cas9 knockout peanut mutants for the FATTY ACID DESATURASE 2B ( AhFAD2B ) gene. In the original protocol, 5 µL of Agrobacterium was injected into the nodal sections of 30-day-old peanut plants. From the 820 plants recovered with this method, a total of 371 (45.24%) were PCR-positive.
Direct regeneration of embryos and shoot apical meristems under in vitro conditions
This direct regeneration strategy aims at regenerating the meristematic cells of a differentiated explant under in vitro conditions (Fig. 8 a). Both embryonic axes and developed shoot apical meristems have been demonstrated to be suitable explants for direct organogenesis under in vitro conditions. The use of a differentiated explant typically hastens the shoot regeneration rate, diminishes the requirements in hormones, simplifies medium composition (i.e. often only sucrose), and increases the resilience of the explant toward Agrobacterium overgrowth [ 55 , 56 , 181 ]. A large literature search has demonstrated the efficiency of several transformation/regeneration systems for the embryonic axes of watermelon [ 182 ], field bean [ 183 ], cowpea [ 184 ], chickpea [ 185 , 186 ], common bean [ 184 , 187 ], black gram ( Vigna mungo ) [ 188 , 189 ], purslane [ 190 ], eggplant [ 191 ], and snake gourd ( Tricosanthes cucumerina ) [ 27 ]. Two of the most commonly transformed species using the in vitro embryonic axis method are soybean and cotton, sometimes with innovative technical aspects. For example, Paes de Melo et al. [ 55 ] and Ribeiro et al. [ 56 ] have respectively proposed protocols in which soybean and cotton embryonic axes are injured using biolistics and subsequently infected with Agrobacterium . In their protocols, shooting, rooting, and selection are subsequently performed simultaneously in a medium containing 6-benzylaminopurine (BAP) and activated charcoal. In this system, transformants are selected with the selection marker gene AHAS which confers resistance to the systemic herbicide Imazapyr. Using these protocols, Paes de Melo et al. [ 55 ] and Ribeiro et al. [ 56 ] have obtained transformation efficiencies averaging 9.84% for soybean and 60% for cotton. Similarly, several shoot apical meristem-based transformation/regeneration systems have been demonstrated in many dicots (e.g. cucumber [ 192 ], petunia [ 193 ], camelina/ Camelina sativa [ 194 ], Dalmatian chrysanthemum/ Tanacetum cinerariifolium [ 195 ] and cotton [ 196 , 197 ]) and monocots (e.g. wheat [ 14 , 44 , 198 ], finger millet/ Eleusine coracana [ 199 ], foxtail millet/ Setaria italica [ 200 ], pearl millet/ Pennisetum glaucum [ 201 ] and rice [ 57 , 58 , 202 , 203 ]). In addition, an extensive literature dedicated to the in vitro regeneration of embryonic axes or excised shoot apical meristem without transformation is available for a large number of species (e.g. finger millet [ 204 , 205 ], maize [ 206 ] and rice [ 207 ]). These regeneration protocols serve as a basis for the development of new transformation methods as those could be converted with minimal effort. Overall, these in vitro systems offer numerous benefits over many of the in planta systems and are one of the most interesting alternatives to streamline transformation in monocots. However, these methods require access to micropropagation facilities and are technically more challenging than most in planta techniques.

Novel transformation techniques used for in planta transformation. ( A ) Grafting-mediated transformation [ 227 ]. Wild-type scion is grafted to a transgenic rootstock containing Cas9 and gRNA sequences. The grafting procedure leads to the formation of chimeric scions containing Cas9 and gRNA sequences due to the movement of tRNA-like sequence motifs that ensure transcript mobility across the plant. The rootstock to scion movement of these sequences causes heritable edits in the germline cells and edited offspring can be retrieved upon selection in the T 1 generation. ( B ) Viral-based vector using a mobile FT cassette [ 238 ]. The leaves of mutant plants overexpressing Cas9 are agroinfiltrated with a viral vector (e.g. tobacco rattle virus vector) containing a gRNA sequence fused to mobile FT sequences. The gRNA sequence reach the germline cells of the Cas9 overexpressing mutants upon the transcription of FT due to its endogenous natural movement to the shoot apical meristem and the edited offspring are retrieved in the T 1 generation upon selection
Vegetative tissues
Callus-based transformation system.
In transformation systems using an in vivo callus-based approach, seedlings or mature plants are injured and their wounds are treated using a solution of Agrobacterium [ 16 , 54 ] (Fig. 8 b). Following this step, the injuries are subjected to hormone treatment, if necessary, to promote the development of a callus and/or adventitious buds [ 16 ]. In some cases, selection by treating the wounded area using a selection marker (i.e. antibiotic or herbicide) is performed to identify the putative transformants [ 16 ]. In transformed tomatoes, Pozueta-Romero et al. [ 208 ] observed that proper kanamycin selection favors the competition of transformed over untransformed cells during de novo shoot organogenesis, thus increasing significantly the number of regenerated transformed shoots. To promote callus growth, inoculated wounds can be covered with parafilm, aluminum foil, mud, or plastic to maintain proper humidity, and adequate temperature and to provide a dark treatment, as darkness has been demonstrated to favor the development of callus mass [ 16 , 17 ]. Over the years, in vivo callus transformation and/or regeneration has been demonstrated to be feasible in a broad range of fruit trees (e.g. orange/ Citrus sinensis [ 20 , 209 ], longan [ 19 , 210 ], and pomelo/ Citrus maxima [ 17 , 209 ]), vines [passionfruit [ 16 ]], shrubs/trees (e.g. poplar [ 211 , 212 , 213 ] and eucalyptus/ Eucalyptus sp. [ 211 ]) and perennial dicots cultivated as annual (e.g. tomato [ 18 , 208 ]). In their patent, Mily et al. [ 18 ] also mentioned that soybean and coffee ( Coffea sp. ) generate new shoots upon decapitation and that chili pepper, eggplant ( Solanum melongena ), and common bean also display excellent regeneration and GUS expression abilities. Often, plants regenerated using this system will concomitantly undergo direct regeneration events (e.g. tomato and several relatives [ 214 , 215 , 216 , 217 ], soybean [ 218 ], and peanut [ 51 ]) which can lead to some form of mosaicism in the transformed plant (Fig. 8 c). Although the literature for this technique is relatively sparse in comparison to other transformation strategies, a plethora of protocols using indirect de novo shoot induction without transformation are currently available for species such as poinsettia ( Euphorbia pulcherrima ) [ 219 ], tomato [ 216 , 220 , 221 , 222 ], and chili pepper [ 208 ]. In addition, indirect de novo shoot induction without transformation has been validated in lignified woody jujube [ 54 , 223 , 224 , 225 ] and pomegranate ( Punica granatum ) [ 226 ] trees under field conditions for colchicine mutagenesis treatments, thus demonstrating its versatility and potential.
Novel systems
Grafting-mediated transformation.
At present, only one technique, named grafting-mediated genome editing, has been developed as a systematic in planta transformation tool to induce precise modifications in the genome [ 227 ] (Fig. 9 a). In grafted plants, the formation of a successful graft union requires several steps, including the (i) lining of the vascular cambium; (ii) wound healing; (iii) formation of a callus bridge between the rootstock and the scion; (iv) generation of vascular cambium; and (v) development of the secondary xylem and phloem [ 228 ]. The formation of a callus bridge enables the horizontal gene transfer of phloem-mobile protein-coding RNAs through the phloem vasculature of grafted plants [ 229 ]. In 2016, Zhang et al. [ 230 ] demonstrated that transcripts harboring distinctive tRNA-like structures can move from a transgenic rootstock to a wild-type scion and be translated into proteins after transport. Taking advantage of this discovery, Yang et al. [ 227 ] investigated the generation of stable gene-edited plant lines using intraspecific and interspecific grafting in wild-type Arabidopsis and Brassica rapa to generate heritable modifications. To do so, phloem-mobile tRNA-like sequences were fused to Cas9 and guide RNA (gRNA) sequences to induce transport from the provider transgenic rootstock to the recipient scion through root-to-shoot movement. Using this system, the inheritance of deletion edits was 1.6% for heterozygotic and 0.1% for homozygotic genotypes, although the authors underline that these numbers were probably underestimated because the seedlings were screened in pools using PCR. As the T 0 generation is chimeric, segregation must be performed in the subsequent generation to recover non-chimeric lines. To circumvent the step involving the generation of the mutant rootstock in recalcitrant species, the authors suggest using A. thaliana and Nicotiana sp. as rootstocks due to their simple and reliable transformation protocols and their very wide range of compatible distantly related species, including soybean and fava bean [ 39 ].
Viral-based vectors
Virus-induced gene silencing (VIGS) is a method that uses modified viral vectors to induce transient gene silencing in plants [ 231 , 232 ]. This technique allows for efficient gene function analysis but is generally not considered as a reliable method to generate stable mutations in plants although some shreds of evidence suggest that the silencing effect can be transmitted to the next generation [ 233 ]. To circumvent this issue, the virus-induced genome editing (VIGE) method was developed as a means to generate permanent mutations for the production of true-breeding lines [ 234 ]. The scope of action of viral-based vectors significantly increased with the development of genome editing technologies as the expression of short RNA sequences (e.g. gRNA) can be readily performed with the use of in planta Agrobacterium transient transformation strategies (e.g. agroinfiltration, agroinjection, agrospray, agrodrench, and rub inoculation) [ 235 , 236 ]; however, heritable mutations are challenging to generate due to the seclusion of viruses from the meristematic cells of the shoot apical meristem but have been reported on rare occasions (e.g. Tobacco rattle virus [ 234 ] and Barley stripe mosaic virus [ 237 ]). To obtain a greater efficiency at generating heritable genome editing events, Ellison et al. [ 238 ] fused gRNA sequences to mobile FLOWERING LOCUS T ( FT ) sequences and cloned them into a Tobacco rattle virus vector (Fig. 9 b). The resulting vector was subsequently inserted into the cells of Cas9-overexpressing tobacco plants via agroinfiltration. In its natural state, endogenous FT sequences move to the shoot apical meristem to induce flowering via the phloem upon transcription in the leaf tissues [ 239 ]. This characteristic enables the gRNAs to enter the shoot apical meristem upon the transcription of FT , thus generating stable mutations in the future offspring without relying on tissue culture. Following the publication of Ellison et al. [ 238 ], this versatile editing system has been confirmed to be also compatible with the Barley yellow striate mosaic virus [ 240 ] and Cotton leaf crumple virus [ 241 , 242 ].
Since the first reports of in planta transformation in the 1980s [ 122 ], hundreds of in planta protocols have been developed for a large number of species. The classification of these protocols into a structured system is challenging due to the broad range of approaches. However, much of the strength of the in planta concept lies in this heterogeneity and high diversity since it aims to work with the natural biological and morphological features of each species instead of trying to “force” the transformation process through challenging regeneration steps. The high level of versatility, decreased upfront cost, and reduced technical requirements of many of these techniques demonstrate the importance of this field of research for the progress of plant science. Still, many of these techniques require more extended research to validate their use in a broad range of species. For instance, de novo shoot induction using tissue culture-independent approaches seems to be a promising strategy for dicot transformation, particularly for species with a long juvenile period (e.g. fruit trees). The methods are simple, cost and time-efficient, mostly genotype-independent, reliable, and based on prior knowledge from tissue culture-based de novo shoot induction methods. Furthermore, the protocols can be adapted for a wide range of experimental settings (e.g. lab vs. field conditions) and plant developmental stages (e.g. younger seedlings vs. lignified woody plants). Theoretically, this approach boasts all the most important features for a transformation method; however, it seems largely unexplored in the literature in comparison to its in vitro counterpart. The same observations can be made for several methods cited in this article such as embryo desiccation or the shoot apical meristem methods.
At present, the specific reasons slowing a wider adoption of these in planta approaches in the scientific community remain elusive as many of these techniques were demonstrated to be efficient in a large number of species. On the whole, this paper tried to review as many sources as possible, including those hard-to-access research articles, to build a compendium of references and provide the most accurate picture of a field that is rapidly evolving. In their reviews, Kaur and Devi [ 5 ] suggested that the field of in planta research is still in its early stages of development. While we understand the reasons underlying this standpoint, we would like to add some nuances. In planta research has always been at the core of transformation research since its beginning, and the floral dip approach in Arabidopsis is still a major propeller for the development of plant molecular biology. Several approaches are now in their mature phases, especially for dicots, with standardized protocols for a large number of species. In the longer term, many strategies targeting dicots, such as the tissue culture-independent de novo shoot induction method, clearly have the potential to become a mainstay of plant transformation. On the other hand, in planta techniques for monocots are less advanced, less diversified, and often more challenging to operate. Nonetheless, several approaches (e.g. pollen-tube pathway and shoot apical meristem injury methods) have already demonstrated their potential and are used regularly by several labs across the world. In conclusion, the in planta transformation concept offers important contributions to plant biotechnology by offering an alternative to traditional transformation/regeneration techniques and will surely become an increasingly important player in the field of plant transformation in the future.
Online compendium
To further strengthen the content of this compendium, we solicit the support and help of the community to add additional references to the online version of this document available at https://github.com/Inplanta/In_planta_transformation . To do so, people can send their annotated references to the ‘’Issue’’ section of the GitHub page under the following format: (i) Family; (ii) Genera; (iii) Species; (iv) Common name; (v) Type of explant; (vi) Method; (vii) Notes; and (viii) Complete reference. The references should be in an Excel format and need to be submitted along with the original document. The compendium was built to limit in-text citations and provide a user-friendly versatile document to group and annotate in planta references. Overall, video footage showing specific methodological aspects is considered to be particularly helpful for the understanding and replicability of the techniques. To maximize the understanding of this paper, readers are invited to consult the compendium as they are reading.
Data availability
A free, online, and up-to-date version of the in planta compendium is available at https://github.com/Inplanta/In_planta_transformation .
The transformation rates provided in the article have been often calculated using different methods and cannot, in a large number of cases, be directly used for comparison.
Somssich M. A short history of plant transformation. PeerJ Prepr [Internet]. 2019;1:1–28. https://peerj.com/preprints/27556/ .
Woodward AW, Bartel B. Biology in bloom: a primer on the arabidopsis thaliana model system. Genetics. 2018;208(4):1337–49.
Article CAS PubMed PubMed Central Google Scholar
Padole D. Arabidopsis-a model plant. Trends Biosci. 2019;10(February):557–9.
Google Scholar
Koornneef M, Meinke D. The development of Arabidopsis as a model plant. Plant J. 2010;61(6):909–21.
Article CAS PubMed Google Scholar
Kaur RP, Devi S. In planta transformation in plants: a review. Agric Rev. 2019;40(03):159–74.
Jan SA, Shinwari ZK, Shah SH, Shahzad A, Zia MA, Ahmad N. In-planta transformation: recent advances. Rom Biotechnol Lett. 2016;21(1):11085–91.
CAS Google Scholar
Saifi SK, Passricha N, Tuteja R, Kharb P, Tuteja N. In planta transformation: A smart way of crop improvement. In: Tuteja N, Tuteja R, Passricha N, Saifi SKBTA in CIT, editors. Advancement in Crop Improvement Techniques [Internet]. Woodhead Publishing; 2020. pp. 351–62. https://doi.org/10.1016/B978-0-12-818581-0.00021-8 .
Zlobin NE, Lebedeva MV, Taranov VV. CRISPR/Cas9 genome editing through in planta transformation. Crit Rev Biotechnol [Internet]. 2020;40(2):153–68. https://doi.org/10.1080/07388551.2019.1709795 .
Chumakov MI, Moiseeva EM. Technologies of Agrobacterium plant transformation in planta. Appl Biochem Microbiol. 2012;48(8):657–66.
Article CAS Google Scholar
Fan L, Guoying W, Mingqing C. Research progress on in planta transformation mediated by Agrobacterium. Mol plant Breed = Fen zi zhi wu yu zhong [Internet]. 2003;1(1):108–16. http://europepmc.org/abstract/CBA/482234 .
Hao S, Zhang Y, Li R, Qu P, Cheng C. Agrobacterium-mediated in planta transformation of horticultural plants: Current status and future prospects. Sci Hortic (Amsterdam) [Internet]. 2024;325:112693. https://www.sciencedirect.com/science/article/pii/S0304423823008610 .
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. 2010. http://www.gnu.org/copyleft/gpl.html .
Somers DA, Samac DA, Olhoft PM. Recent advances in legume transformation. Plant Physiol. 2003;131(3):892–9.
Imai R, Hamada H, Liu Y, Linghu Q, Kumagai Y, Nagira Y, et al. In planta particle bombardment (IPB): a new method for plant transformation and genome editing. Plant Biotechnol. 2020;37(2):171–6.
Bellido AM, Souza Canadá ED, Permingeat HR, Echenique V. Genetic Transformation of Apomictic grasses: Progress and constraints. Front Plant Sci. 2021;12(November):768393.
Article PubMed PubMed Central Google Scholar
Rizwan HM, Yang Q, Yousef AF, Zhang X, Sharif Y, Kaijie J, et al. Establishment of a novel and efficient agrobacterium-mediated in planta transformation system for passion fruit (Passiflora edulis). Plants. 2021;10(11):2459.
Zhang Y, Zhang D, Zhong Y, Chang X, Hu M, Cheng C. A simple and efficient in planta transformation method for pommelo (Citrus maxima) using Agrobacterium tumefaciens. Sci Hortic (Amsterdam) [Internet]. 2017;214:174–9. https://doi.org/10.1016/j.scienta.2016.11.033 .
Mily R, Neelima S, Anne B, Moran F. Generation of heritably gene-edited plants without tissue culture (Patent US 201862727431) [Internet]. Vol. 1. WO, United States of America: UNIV CALIFORNIA OP - US 201862727431 P; 2020. Available from: https://lens.org/011-524-499-432-378 .
Chen YK, Cheng CZ, Zhang ZH, Lai Z. Establishment of a rapid transgenic method on longan seedling. Chin J Appl Env Biol. 2020;26(6):1540–5.
Xie X, Yang L, Liu F, Tian N, Che J, Jin S, et al. Establishment and optimization of valencia sweet orange in planta transformation system. Acta Hortic Sin. 2020;47(1):111–9.
Rao KS, Sreevathsa R, Sharma PD, Keshamma E, Kumar MU. In planta transformation of pigeon pea: a method to overcome recalcitrancy of the crop to regeneration in vitro. Physiol Mol Biol Plants. 2008;14(4):321–8.
Ganguly S, Ghosh G, Ghosh S, Purohit A, Chaudhuri RK, Das S et al. Plumular meristem transformation system for chickpea: an efficient method to overcome recalcitrant tissue culture responses. Plant Cell Tissue Organ Cult [Internet]. 2020;142(3):493–504. https://doi.org/10.1007/s11240-020-01873-8 .
Svabova L, Smykal P, Griga M. May. Agrobacterium-mediated transformation of pea (Pisum sativum L.): Transformant production in vitro and by non-tissue culture approach. Food Legum Nutr Secur Sustain Agric IS-GPB [Internet]. 2007;(2014):208–20. https://www.researchgate.net/publication/235652958 .
Švábová L, Smýkal P, Griga M, Ondřej V. Agrobacterium-mediated transformation of Pisum sativum in vitro and in vivo. Biol Plant. 2005;49(3):361–70.
Article Google Scholar
Amal TC, Karthika P, Dhandapani G, Selvakumar S, Vasanth K. A simple and efficient Agrobacterium-mediated in planta transformation protocol for horse gram (Macrotyloma uniflorum Lam. Verdc). J Genet Eng Biotechnol. 2020;18(1):1–9.
Karthik S, Pavan G, Prasanth A, Sathish S, Appunu C, Manickavasagam M. Improved in planta genetic transformation efficiency in bitter gourd (Momordica charantia L). Vitr Cell Dev Biol - Plant. 2021;57(2):190–201.
Subramanyam K, Arunachalam C, Thaneswari RM, Sulaiman AA, Manickavasagam M, Ganapathi A. Highly efficient Agrobacterium-mediated in planta genetic transformation of snake gourd (Tricosanthes Cucumerina L). Plant Cell Tissue Organ Cult. 2015;123(1):133–42.
Karthik S, Pavan G, Sathish S, Siva R, Kumar PS, Manickavasagam M. Genotype-independent and enhanced in planta Agrobacterium tumefaciens-mediated genetic transformation of peanut [Arachis hypogaea (L.)]. 3 Biotech [Internet]. 2018;8(4). https://doi.org/10.1007/s13205-018-1231-1 .
Heberle-Bors E, Moreno RMB, Alwen A, Stöger E, Vicente O. Transformation of Pollen. In: Nijkamp HJJ, Van Der Plas LHW, Van Aartrijk J, editors. Dordrecht: Springer Netherlands; 1990. pp. 244–51. https://doi.org/10.1007/978-94-009-2103-0_37 .
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–6.
Khanday I, Sundaresan V. Plant zygote development: recent insights and applications to clonal seeds. Curr Opin Plant Biol [Internet]. 2021;59:101993. https://doi.org/10.1016/j.pbi.2020.101993 .
Jürgens G. Axis formation in plant embryogenesis: cues and clues. Cell. 1995;81(4):467–70.
Article PubMed Google Scholar
Kwiatkowska D. Flowering and apical meristem growth dynamics. J Exp Bot. 2008;59(2):187–201.
Chang W, Guo Y, Zhang H, Liu X, Guo L. Same actor in different stages: genes in shoot apical Meristem Maintenance and Floral Meristem Determinacy in Arabidopsis. Front Ecol Evol. 2020;8(April):1–12.
Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25(9):3159–73.
Kareem A, Radhakrishnan D, Sondhi Y, Aiyaz M, Roy MV, Sugimoto K, et al. De novo assembly of plant body plan: a step ahead of Deadpool. Regeneration. 2016;3(4):182–97.
Hu B, Zhang G, Liu W, Shi J, Wang H, Qi M, et al. Divergent regeneration-competent cells adopt a common mechanism for callus initiation in angiosperms. Regeneration. 2017;4(3):132–9.
Jura-Morawiec J, Oskolski A, Simpson P. Revisiting the anatomy of the monocot cambium, a novel meristem. Planta [Internet]. 2021;254(1):1–10. https://doi.org/10.1007/s00425-021-03654-9 .
Notaguchi M, Kurotani KI, Sato Y, Tabata R, Kawakatsu Y, Okayasu K, et al. Cell-cell adhesion in plant grafting is facilitated by b-1,4-glucanases. Sci (80-). 2020;369(6504):698–702.
Low LY, Yang SK, Kok DXA, Abdullah JO, Tan NP, Lai KS. Transgenic Plants: Gene Constructs, Vector and Transformation Method. In: Çelik Ö, editor. Rijeka: IntechOpen; 2018. p. Ch. 3. https://doi.org/10.5772/intechopen.79369 .
Cao X, Xie H, Song M, Lu J, Ma P, Huang B et al. Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. Innovation [Internet]. 2023;4(1):100345. https://doi.org/10.1016/j.xinn.2022.100345 .
Zhang W, Zuo Z, Zhu Y, Feng Y, Wang Y, Zhao H, et al. Fast track to obtain heritable transgenic sweet potato inspired by its evolutionary history as a naturally transgenic plant. Plant Biotechnol J [Internet]. 2023;21(4):671–3. https://doi.org/10.1111/pbi.13986 .
Davey MR, Rech EL, Mulligan BJ. Direct DNA transfer to plant cells. Plant Mol Biol [Internet]. 1989;13(3):273–85. https://doi.org/10.1007/BF00025315 .
Hamada H, Linghu Q, Nagira Y, Miki R, Taoka N, Imai R. An in planta biolistic method for stable wheat transformation. Sci Rep [Internet]. 2017;7(1):2–9. https://doi.org/10.1038/s41598-017-11936-0 .
Lee K, Wang K. Strategies for genotype-flexible plant transformation. Curr Opin Biotechnol [Internet]. 2023;79:102848. https://www.sciencedirect.com/science/article/pii/S0958166922001823 .
Su YH, Tang LP, Zhao XY, Zhang XS. Plant cell totipotency: Insights into cellular reprogramming. J Integr Plant Biol [Internet]. 2021;63(1):228–43. https://doi.org/10.1111/jipb.12972 .
Desfeux C, Clough SJ, Bent AF. Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method. Plant Physiol. 2000;123(3):895–904.
Long Y, Yang Y, Pan G, Shen Y. New insights into tissue culture plant-regeneration mechanisms. Front Plant Sci. 2022;13:926752.
Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci [Internet]. 2007;12(6):245–52. https://www.sciencedirect.com/science/article/pii/S1360138507000970 .
Xu L, Huang H. Genetic and Epigenetic Controls of Plant Regeneration. In: Galliot BBTCT in DB, editor. Current Topics in Developmental Biology [Internet]. Academic Press; 2014. pp. 1–33. https://www.sciencedirect.com/science/article/pii/B9780123914989000097 .
Zhai Q, Chen R, Liang X, Zeng C, Hu B, Li L et al. Establishment and Application of a Rapid Genetic Transformation Method for Peanut. Chinese Bull Bot [Internet]. 2022;57(3):327–39. https://www.chinbullbotany.com .
Ye X, Shrawat A, Williams E, Rivlin A, Vaghchhipawala Z, Moeller L, et al. Commercial scale genetic transformation of mature seed embryo explants in maize. Front Plant Sci. 2022;13(November):1–17.
Bhatia S, Sharma K. Chapter 13 - Technical Glitches in Micropropagation. In: Bhatia S, Sharma K, Dahiya R, Bera TBTMA of PB in PS, editors. Boston: Academic Press; 2015. pp. 393–404. https://www.sciencedirect.com/science/article/pii/B9780128022214000133 .
Shi Q, Liu P, Wang J, Xu J, Ning Q, Liu M. A novel in vivo shoot regeneration system via callus in woody fruit tree Chinese jujube (Ziziphus jujuba Mill.). Sci Hortic (Amsterdam) [Internet]. 2015;188:30–5. https://doi.org/10.1016/j.scienta.2015.03.013 .
Paes de Melo B, Lourenço-Tessutti IT, Morgante CV, Santos NC, Pinheiro LB, de Jesus Lins CB, et al. Soybean Embryonic Axis Transformation: combining Biolistic and Agrobacterium-mediated protocols to overcome typical complications of in Vitro Plant Regeneration. Front Plant Sci. 2020;11(August):1–14.
Ribeiro TP, Lourenço-Tessutti IT, de Melo BP, Morgante CV, Filho AS, Lins CBJ et al. Improved cotton transformation protocol mediated by Agrobacterium and biolistic combined-methods. Planta [Internet]. 2021;254(2):1–14. https://doi.org/10.1007/s00425-021-03666-5 .
Dey M, Bakshi S, Galiba G, Sahoo L, Panda SK. Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. indica) using TDZ. 3 Biotech. 2012;2(3):233–40.
Article PubMed Central Google Scholar
Yookongkaew N, Srivatanakul M, Narangajavana J. Development of genotype-independent regeneration system for transformation of rice (Oryza sativa ssp. indica). J Plant Res. 2007;120(2):237–45.
Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43.
Bechtold N, Pelletier G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. In: Martinez-Zapater JM, Salinas J, editors. Methods in molecular biology (Clifton, NJ) [Internet]. Totowa, NJ: Humana Press; 1998. pp. 259–66. https://doi.org/10.1385/0-89603-391-0:259 .
Bent A. Arabidopsis thaliana floral dip transformation method. Methods Mol Biol. 2006;343:87–103.
CAS PubMed Google Scholar
Wang Y, Yaghmaiean H, Zhang Y. High transformation efficiency in Arabidopsis using extremely low Agrobacterium inoculum. F1000Research. 2020;9:356.
Das P, Joshi NC. Minor modifications in obtainable Arabidopsis floral dip method enhances transformation efficiency and production of homozygous transgenic lines harboring a single copy of transgene. Adv Biosci Biotechnol. 2011;02(02):59–67.
Ali I, Sher H, Ali A, Hussain S, Ullah Z. Simplified floral dip transformation method of Arabidopsis thaliana. J Microbiol Methods. 2022;197(October):106492.
Wijerathna-Yapa A. Progeny test for generating single copy homozygous transgenic lines in Arabidopsis thaliana. [Internet]. 2019 [cited 2023 Aug 18]. https://www.protocols.io/view/progeny-test-for-generating-single-copy-homozygous-5xrg7m6.html .
Weise S. Selecting Homozygous Transformed Plants [Internet]. 2013 [cited 2023 Aug 20]. pp. 1–5. https://bmb.natsci.msu.edu/sites/_bmb/assets/File/Sharkey_lab/Selecting Homozygous Transformed Plants.pdf.
Honda C, Ohkawa K, Kusano H, Teramura H, Shimada H. A simple method for in planta tomato transformation by inoculating floral buds with a sticky agrobacterium tumefaciens suspension. Plant Biotechnol. 2021;38(1):153–6.
Bent AF. Arabidopsis in Planta Transformation. Uses, Mechanisms, and Prospects for Transformation of Other Species1. Plant Physiol [Internet]. 2000;124(4):1540–7. https://doi.org/10.1104/pp.124.4.1540 .
Cosic M, Laurans F, Grand-Perret C, Laine-Prade V, Descauses MCL, Pilate G et al. Development of an innovative in planta transformation method in poplar. In: IUFRO Tree Biotech 2017. 2017. pp. 1–2.
Shahid S, Hassan MU, Iqbal I, Fatima N, Kousar T, Ikram Z. Development of a simple and efficient protocol for chickpea (Cicer arietinum L.) transformation by floral injection method. Int J Biosci. 2016;8(December):50–9.
Sharada MS, Kumari A, Pandey AK, Sharma S, Sharma P, Sreelakshmi Y, et al. Generation of genetically stable transformants by Agrobacterium using tomato floral buds. Plant Cell Tissue Organ Cult. 2017;129(2):299–312.
Tishchenko OM, Komisarenko AG, Mykhalska SI, Sergeeva LE, Adamenko NI, Morgun BV, et al. Agrobacterium-mediated transformation of sunflower (Helianthus annuus L.) in vitro and in planta using Lba4404 strain harboring binary vector pBi2E with dsRNA-suppressor of proline dehydrogenase gene. Cytol Genet. 2014;48(4):218–26.
Listanto E, Riyanti EI. Agrobacterium tumefaciens -mediated in-planta transformation of Indonesian maize using pIG121Hm-Cs plasmid containing npt II AND hpt genes. Indones J Agric Sci. 2016;17(2):49–56.
Aminedi R, Dhatwalia D, Jain V, Bhattacharya R. High efficiency in planta transformation of Indian mustard (Brassica juncea) based on spraying of floral buds. Plant Cell, Tissue Organ Cult [Internet]. 2019;138(2):229–37. https://doi.org/10.1007/s11240-019-01618-2 .
Singh P, Kumar K. Agrobacterium-mediated In-planta transformation of bread wheat (Triticum aestivum L.). J Plant Biochem Biotechnol [Internet]. 2022;31(1):206–12. https://doi.org/10.1007/s13562-021-00669-x .
Chung MH, Chen MK, Pan SM. Floral spray transformation can efficiently generate Arabidopsis transgenic plants. Transgenic Res. 2000;9(6):471–6.
Eapen S. Pollen grains as a target for introduction of foreign genes into plants: an assessment. Physiol Mol Biol Plants. 2011;17(1):1–8.
Matoušek J, Tupý J. The Release and Some Properties of Nuclease from Various Pollen Species. J Plant Physiol [Internet]. 1985;119(2):169–78. https://www.sciencedirect.com/science/article/pii/S0176161785801759 .
Shi HZ, Wang J, Yang HY, Zhou C, Chen ZH. Exine-detached pollen of Nicotiana tabacum as an electroporation target for gene transfer. Acta Bot Sin. 1996;38(8):626–30.
Aziz N, Machray GC. Efficient male germ line transformation for transgenic tobacco production without selection. Plant Mol Biol. 2003;51(2):203–11.
Touraev A, Stöger E, Voronin V, Heberle-Bors E. Plant male germ line transformation. Plant J. 1997;12(4):949–56.
Li X, Wang XD, Zhao X, Dutt Y. Improvement of cotton fiber quality by transforming the acsA and acsB genes into Gossypium hirsutum L. by means of vacuum infiltration. Plant Cell Rep. 2004;22(9):691–7.
Wang J, Li Y, Liang C. Recovery of transgenic plants by pollen-mediated transformation in Brassica juncea. Transgenic Res. 2008;17(3):417–24.
Hess D, Dressler K, Nimmrichter R. Transformation experiments by pipetting Agrobacterium into the spikelets of wheat (Triticum aestivum L). Plant Sci. 1990;72(2):233–44.
Zhang R, Meng Z, Abid MA, Zhao X. Novel pollen magnetofection system for transformation of cotton plant with magnetic nanoparticles as gene carriers. In: Methods in Molecular Biology. 2019. pp. 47–54.
Zhao X, Meng Z, Wang Y, Chen W, Sun C, Cui B et al. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat Plants [Internet]. 2017;3(12):956–64. https://doi.org/10.1038/s41477-017-0063-z .
Smith CR, Saunders JA, Van Wert S, Cheng J, Matthews BF. Expression of GUS and CAT activities using electrotransformed pollen. Plant Sci. 1994;104(1):49–58.
Wang W, Wang J, Yang C, Li Y, Liu L, Xu J. Pollen-mediated transformation of Sorghum bicolor plants. Biotechnol Appl Biochem. 2007;48(2):79.
Tjokrokusumo D, Heinrich T, Wylie S, Potter R, McComb J. Vacuum infiltration of Petunia hybrida pollen with Agrobacterium tumefaciens to achieve plant transformation. Plant Cell Rep. 2000;19(8):792–7.
Wang ZP, Zhang ZB, Zheng DY, Zhang TT, Li XL, Zhang C, et al. Efficient and genotype independent maize transformation using pollen transfected by DNA-coated magnetic nanoparticles. J Integr Plant Biol. 2022;64(6):1145–56.
Vejlupkova Z, Warman C, Sharma R, Scheller HV, Mortimer JC, Fowler JE. No evidence for transient transformation via pollen magnetofection in several monocot species. Nat Plants [Internet]. 2020;6(11):1323–4. https://doi.org/10.1038/s41477-020-00798-6 .
Ali A, Bang SW, Chung SM, Staub JE. Plant Transformation via Pollen tube-mediated gene transfer. Plant Mol Biol Rep. 2015;33(3):742–7.
Zhou GYyu, Weng J, Zeng Y, Huang J, Qian S, Liu G. Introduction of exogenous DNA into cotton embryos. Methods in enzymology. United States: Elsevier; 1983. pp. 433–81.
Luo Zxun, Wu R. A simple method for the transformation of rice via the pollen-tube pathway. Plant Mol Biol Rep. 1989;7(3):240.
Dhaliwal HS, Tyagi BR, White FW, Gill BS. Attempted Transformation of Wheat (Triticum aestivum) and Tobacco (Nicotiana tabacum) by Plasmid DNA Uptake Via the Pollen-tube Pathway. J Plant Biochem Biotechnol [Internet]. 1992;1(2):127–8. https://doi.org/10.1007/BF03262911 .
Martin N, Forgeois P, Picard E. Investigations on transforming Triticum aestivum via the pollen tube pathway. Agronomie. 1992;12(7):537–44.
Shou H, Palmer RG, Wang K. Irreproducibility of the soybean pollen-tube pathway transformation procedure. Plant Mol Biol Rep. 2002;20(4):325–34.
Langridge P, Brettschneider R, Lazzeri P, Lörz H. Transformation of cereals via Agrobacterium and the pollen pathway: a critical assessment. Plant J. 1992;2(4):631–8.
Li Z, Nelson RL, Widholm JM, Bent A. Soybean Transformation via the Pollen Tube Pathway. Soybean Genet Newslett [Internet]. 2002;1985:1–11. https://www.soybase.org/sgn/articleFiles/36TransSGN.pdf .
Wang M, Sun R, Zhang B, Wang Q. Pollen tube pathway-mediated cotton transformation. In: Zhang B, editor. Methods in Molecular Biology [Internet]. New York, NY: Springer New York; 2019. pp. 67–73. https://doi.org/10.1007/978-1-4939-8952-2_6 .
Zhang H, Zhao F, Zhao Y, Guo C, Li C, Xiao K. Establishment of transgenic cotton lines with high efficiency via pollen-tube pathway. Front Agric China. 2009;3(4):359–65.
Zhang T, Chen T. Cotton pistil drip transformation method. In: Dunwell JM, Wetten AC, editors. Transgenic Plants: Methods and Protocols [Internet]. Totowa, NJ: Springer; 2012. pp. 237–43. https://doi.org/10.1007/978-1-61779-558-9_20 .
TianZi C, ShenJie W, Jun Z, WangZhen G, TianZhen Z. Pistil drip following pollination: a simple in planta Agrobacterium-mediated transformation in cotton. Biotechnol Lett. 2010;32(4):547–55.
Zhang Y, Yin X, Yang A, Li G, Zhang J. Stability of inheritance of transgenes in maize (Zea mays L.) lines produced using different transformation methods. Euphytica. 2005;144(1–2):11–22.
Duan XL, Chen SB. Variation of the characters in rice (Oryza sativa) induced by foreign DNA uptake. Sci Agric Sin. 1985;3:6–10.
Xi Y, Lin Y, Zhang Q, Hou W, Lu M. Studies on introduction of leaf senescence-inhibition gene PSAG12-IPT into common wheat through pollen-tube pathway. Acta Agron Sin [Internet]. 2004;30(6):608–12. https://www.cabdirect.org/cabdirect/abstract/20043109744 .
Liu J, Su Q, An L, Yang A. Transfer of a minimal linear marker-free and vector-free smGFP cassette into soybean via ovary-drip transformation. Biotechnol Lett. 2009;31(2):295–303.
Yang A, Su Q, An L. Ovary-drip transformation: a simple method for directly generating vector- and marker-free transgenic maize (Zea mays L.) with a linear GFP cassette transformation. Planta. 2009;229(4):793–801.
Shi Xxin, Du G, qiang1, Wang X man, Pei D. Studies on Gene Transformation via Pollen Tube Pathway in Walnut. Acta Hortic Sin. 2012;39(7):1243–52.
Gao XR, Wang GK, Su Q, Wang Y, An LJ. Phytase expression in transgenic soybeans: stable transformation with a vector-less construct. Biotechnol Lett. 2007;29(11):1781–7.
Yang A, Su Q, An L, Liu J, Wu W, Qiu Z. Detection of vector- and selectable marker-free transgenic maize with a linear GFP cassette transformation via the pollen-tube pathway. J Biotechnol. 2009;139(1):1–5.
Liu M, Yang J, Cheng YQ, An LJ. Optimization of soybean (Glycine max (L.) Merrill) in planta ovary transformation using a linear minimal gus gene cassette. J Zhejiang Univ Sci B. 2009;10(12):870–6.
Zhou M, Luo J, Xiao D, Wang A, He L, Zhan J. An efficient method for the production of transgenic peanut plants by pollen tube transformation mediated by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. 2023;152(1):207–14.
Zhen-Zi P, Yan Z, Qian Z, Li-Hua H, Hao-Dong C, Yu-Qiang LI et al. In Planta Transformation of Gossypium hirsutum by Ovary-injection of Agrobacterium. Cott Sci [Internet]. 2011;23(4):311–6. https://journal.cricaas.com.cn/Jweb_mhxb/EN/ https://doi.org/10.11963/cs110404 .
Yasmeen A, Mirza B, Inayatullah S, Safdar N, Jamil M, Ali S, et al. In planta transformation of tomato. Plant Mol Biol Rep. 2009;27(1):20–8.
Hasan M, Khan AJ, Khan S, Shah AH, Khan AR, Mirza B. Transformation of tomato (Lycopersicon esculentum Mill.) With Arabidopsis early flowering gene Apetalai (API) through Agrobacterium infiltration of ripened fruits. Pakistan J Bot. 2008;40(1):161–73.
Safdar N, Mirza B. An experimental comparison of different transformation procedures assessed in tomato cv Rio Grande with yeast HAL 1 gene. Turkish J Biochem. 2014;39(3):245–52.
Zia M, Arshad W, Bibi Y, Nisa S, Chaudhary MF. Does agro-injection to soybean pods transform embryos? Plant Omics. 2011;4(7):384–90.
Liou PC. Method for plant gene transferring by micro-vibration and ovary injection (U.S. Patent Application No. 10/228,366) [Internet]. US: LIOU PAN-CHI OP - US 22836602 A; 2004. Available from: https://lens.org/119-782-950-563-355 .
Graves ACF, Goldman SL. The transformation of Zea mays seedlings with Agrobacterium tumefaciens. Plant Mol Biol. 1986;7(1):43–50.
Chee PP, Fober KA, Slightom JL. Transformation of soybean (Glycine max) by infecting germinating seeds with Agrobacterium tumefaciens. Plant Physiol. 1989;91(3):1212–8.
Feldmann KA, David Marks M. Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. MGG Mol Gen Genet. 1987;208(1–2):1–9.
Singh R, Sharma S, Kharb P, Saifi S, Tuteja N. OsRuvB transgene induces salt tolerance in pigeon pea. J Plant Interact. 2020;15(1):17–26.
Kharb P, Chaudhary R, Tuteja N, Kaushik P. A genotype-independent, simple, effective and efficient in planta agrobacterium-mediated genetic transformation protocol. Methods Protoc. 2022;5(5):69.
Arias D, Mckersie B, Taylor J, SCIENCE GMBH OP - US 31478001 P OP - US 0227164 W. In planta transformation by embryo imbibition of Agrobacterium (Patent US 31478001) [Internet]. CA: BASF PLANT; 2003. Available from: https://lens.org/144-522-898-736-153 .
Rao AQ, Ali MA, Khan MAU, Bajwa KS, Iqbal A, Iqbal T et al. Science Behind Cotton Transformation. In: Abdurakhmonov IY, editor. Cotton Research [Internet]. Rijeka: IntechOpen; 2016. p. Ch. 10. https://doi.org/10.5772/64888 .
Mahalakshmi A, Chugh A, Khurana P. Exogenous DNA uptake via cellular permeabilization and expression of foreign gene in wheat zygotic embryos. Plant Biotechnol. 2000;17(3):235–40.
Fletcher JC. Coordination of cell proliferation and cell fate decisions in the angiosperm shoot apical meristem. BioEssays. 2002;24(1):27–37.
Kesiraju K, Sreevathsa R. Apical meristem-targeted in planta transformation strategy: an overview on its utility in crop improvement. Agri Res Technol Open Access J. 2017;8(555734):10–19080.
Rohini VK, Sankara Rao K. Embryo transformation, a practical approach for realizing transgenic plants of safflower (Carthamus tinctorius L). Ann Bot. 2000;86(5):1043–9.
Rohini VK, Sankara Rao K. Transformation of peanut (Arachis hypogaea L.): a non-tissue culture based approach for generating transgenic plants. Plant Sci. 2000;150(1):41–9.
Arthikala MK, Nanjareddy K, Lara M, Sreevathsa R. Utility of a tissue culture-independent Agrobacterium-mediated in planta transformation strategy in bell pepper to develop fungal disease resistant plants. Sci Hortic (Amsterdam) [Internet]. 2014;170:61–9. https://doi.org/10.1016/j.scienta.2014.02.034 .
Abhishek A, Kumari R, Karjagi CG, Kumar P, Kumar B, Dass S et al. Tissue Culture Independent Agrobacterium tumefaciens Mediated In Planta Transformation Method for Tropical Maize (Zea mays.L). Proc Natl Acad Sci India Sect B - Biol Sci [Internet]. 2016;86(2):375–84. https://doi.org/10.1007/s40011-014-0454-0 .
Gassama-Dia YK, Sané D, Ndoye M. Direct genetic transformation of Hibiscus sabdariffa L. Afr J Biotechnol. 2004;3(4):226–8.
Chowrira GM, Akella V, Lurquin PF. Electroporation-mediated gene transfer into intact nodal meristems in Planta - Generating transgenic paclants without in vitro tissue culture. Mol Biotechnol. 1995;3(1):17–23.
Chowrira GM, Akella V, Fuerst PE, Lurquin PF. Transgenic grain legumes obtained by in Planta Electroporation-mediated gene transfer. Appl Biochem Biotechnol - Part B Mol Biotechnol. 1996;5(2):85–96.
Betchtold N. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris Life Sci. 1993;316:1194–9.
Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA, et al. Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. Plant J. 2000;22(6):531–41.
Pandey S, Patel MK, Mishra A, Jha B. In planta transformed cumin (Cuminum cyminum L.) plants, overexpressing the SbNHX1 gene showed enhanced salt endurance. PLoS ONE. 2016;11(7):1–18.
Hasan MM, Bhajan SK, Hoque MI, Sarker RRH, Islam MN, Gene M, et al. In Planta Genetic Transformation of Mungbean (Vigna radiata (L.) Wilczek) with Marker Gene. Plant Tissue Cult Biotechnol. 2019;29(2):245–55.
Parray RA, Kaldate R, Chavan R. Optimization of In-planta Method of Genetic Transformation in Pigeon pea (Cajanus cajan L. Millsp). Int J Curr Microbiol Appl Sci. 2019;8(06):50–62.
Singh KBMM, Madhavan J, Chandra S, Rao U, Mandal PK. In planta transformation of Polianthes tuberosa for concomitant knockdown of flp-1, flp-12 and flp-18 genes induced root-knot nematode resistance. Sci Hortic (Amsterdam). 2023;311:111764.
Reddy YS. In planta transformation studies in chick pea (cicer arietinum l.). Bengaluru: University of Agricultural Science; 2007.
Pervitasari AN, Nugroho ABD, Jung WH, Kim DH, Kim J. An efficient Agrobacterium tumefaciens-mediated transformation of apical meristem in radish (Raphanus sativus L.) using a needle perforation. Plant Cell Tissue Organ Cult [Internet]. 2022;148(2):305–18. https://doi.org/10.1007/s11240-021-02190-4 .
Sultan EAA, Tawfik MS. Stable In-Planta Transformation System For Egyptian Sesame (Sesamum indicum L.) cv. Sohag 1. GM Crop Food [Internet]. 2023;14(1):21–31. https://doi.org/10.1080/21645698.2022.2150041 .
Shriti S, Paul S, Das S. Overexpression of CaMYB78 transcription factor enhances resistance response in chickpea against Fusarium oxysporum and negatively regulates anthocyanin biosynthetic pathway. Protoplasma [Internet]. 2023;260(2):589–605. https://doi.org/10.1007/s00709-022-01797-4 .
Ganguly S, Ghosh G, Purohit A, Kundu Chaudhuri R, Chakraborti D. Development of transgenic pigeonpea using high throughput plumular meristem transformation method. Plant Cell Tissue Organ Cult [Internet]. 2018;135(1):73–83. https://doi.org/10.1007/s11240-018-1444-3 .
Ganguly S, Purohit A, Chaudhuri RK, Das S, Chakraborti D. Embryonic explant and plumular meristem transformation methods for development of transgenic pigeon pea. Methods in Molecular Biology. Springer; 2020. pp. 317–33.
Weeks JT, Ye J, Rommens CM. Development of an in planta method for transformation of alfalfa (Medicago sativa). Transgenic Res. 2008;17(4):587–97.
Mei G, Chen A, Wang Y, Li S, Wu M, Liu X et al. A simple and efficient in planta transformation method based on the active regeneration capacity of plants. bioRxiv [Internet]. 2023;2023.01.02.522521. https://doi.org/10.1101/2023.01.02.522521 .
Megersa HG. Propagation methods of selected Horticultural crops by Specialized organs: review. J Hortic. 2017;04(02):4–7.
Liu L, Qu J, Wang C, Liu M, Zhang C, Zhang X et al. An efficient genetic transformation system mediated by Rhizobium rhizogenes in fruit trees based on the transgenic hairy root to shoot conversion. Plant Biotechnol J. 2024;1–11.
Ramasamy S, Chelliah A, R RM, Mohanraj MS, Velusamy B. Agrobacterium-mediated in planta Transformation of Hill Banana for developing resistance against Banana Bunchy Top Virus. Madras Agric J. 2011;98(December):374–8.
Xu N, ming Man J, Luo R. A non-tissue culture dependent genetic transformation of Marchantia polymorphya L. (Pre-print). ResearchSquare [Internet]. 2023; https://www.researchsquare.com/article/rs-2556225/v1 .
Jung Y, Rhee Y, Auh CK, Shim H, Choi JJ, Kwon ST, et al. Production off recombinant single chain antibodies (scFv) in vegetatively reproductive Kalanchoe pinnata by in planta transformation. Plant Cell Rep. 2009;28(10):1593–602.
Mayavan S, Subramanyam K, Jaganath B, Sathish D, Manickavasagam M, Ganapathi A. Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep. 2015;34(10):1835–48.
Seol E, Jung Y, Lee J, Cho C, Kim T, Rhee Y, et al. In planta transformation of Notocactus scopa cv. Soonjung by Agrobacterium tumefaciens. Plant Cell Rep. 2008;27(7):1197–206.
Lotan T, Ohto MA, Matsudaira Yee K, West MAL, Lo R, Kwong RW, et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93(7):1195–205.
Lowe K, Hoerster G, Sun X, Rasco-Gaunt S, Lazerri P, Ellis S et al. Maize LEC1 Improves Transformation in Both Maize and Wheat. In: Vasil IK, editor. Plant Biotechnology 2002 and Beyond [Internet]. Dordrecht: Springer Netherlands; 2003. pp. 283–4. https://doi.org/10.1007/978-94-017-2679-5_57 .
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, et al. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci U S A. 2001;98(20):11806–11.
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14(8):1737–49.
Zuo J, Niu QW, Frugis G, Chua NH. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002;30(3):349–59.
Nalapalli S, Tunc-Ozdemir M, Sun Y, Elumalai S, Que Q. Morphogenic regulators and their application in improving plant transformation. Rice Genome Eng Gene Ed Methods Protoc. 2021;2238:37–61.
van der Graaff E, Laux T, Rensing SA. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009;10(12):1–9.
Gaillochet C, Lohmann JU. The never-ending story: from pluripotency to plant developmental plasticity. Development. 2015;142(13):2237–49.
Cheng ZJ, Wang L, Sun W, Zhang Y, Zhou C, Su YH, et al. Pattern of auxin and cytokinin responses for shoot meristem induction results from the regulation of cytokinin biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol. 2013;161(1):240–51.
Brand A, Quimbaya M, Tohme J, Chavarriaga-Aguirre P. Arabidopsis LEC1 and LEC2 orthologous genes are key regulators of somatic embryogenesis in cassava. Front Plant Sci. 2019;10(May):1–14.
Purwestri YA, Lee YS, Meehan C, Mose W, Susanto FA, Wijayanti P et al. RWP-RK Domain 3 (OsRKD3) induces somatic embryogenesis in black rice. BMC Plant Biol [Internet]. 2023;23(1):1–15. https://doi.org/10.1186/s12870-023-04220-z .
Lou H, Huang Y, Wang W, Cai Z, Cai H, Liu Z et al. Overexpression of the AtWUSCHEL gene promotes somatic embryogenesis and lateral branch formation in birch (Betula platyphylla Suk.). Plant Cell Tissue Organ Cult [Internet]. 2022;150(2):371–83. https://doi.org/10.1007/s11240-022-02290-9 .
Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho MJ, et al. Morphogenic regulators baby boom and Wuschel improve monocot transformation. Plant Cell. 2016;28(9):1998–2015.
Aesaert S, Impens L, Coussens G, Van Lerberge E, Vanderhaeghen R, Desmet L et al. Optimized Transformation and Gene Editing of the B104 public maize inbred by improved tissue culture and use of morphogenic regulators. Front Plant Sci. 2022;13(April).
Chen Z, Debernardi JM, Dubcovsky J, Gallavotti A. The combination of morphogenic regulators BABY BOOM and GRF-GIF improves maize transformation efficiency. bioRxiv [Internet]. 2022;2022.09.02.506370. https://doi.org/10.1101/2022.09.02.506370 .
Wang N, Ryan L, Sardesai N, Wu E, Lenderts B, Lowe K, et al. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat Plants. 2023;9(2):255–70.
Debernardi JM, Tricoli DM, Ercoli MF, Hayta S, Ronald P, Palatnik JF, et al. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat Biotechnol. 2020;38(11):1274–9.
Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF. Plant gene editing through de novo induction of meristems. Nat Biotechnol [Internet]. 2020;38(1):84–9. https://doi.org/10.1038/s41587-019-0337-2 .
Cody JP, Maher MF, Nasti RA, Starker CG, James C, Chamness JC, et al. Direct delivery and fast-treated Agrobacterium co-culture (Fast-TrACC) plant transformation methods for Nicotiana Benthamiana. Nat Protoc. 2023;18(1):81–107.
Lian Z, Nguyen CD, Liu L, Wang G, Chen J, Wang S, et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol J. 2022;20(8):1622–35.
Spicer RL. Investigating Novel De Novo Meristem induction through a direct delivery gene editing technique in greenhouse grown apple and grape. University of Minnesota; 2023.
Wang C, Wang X, Tang Y, Wu Q, Li G, Song G, et al. Transforming peanut (Arachis hypogaea L.): a simple in planta method. Res Crop. 2013;14(3):850–4.
Han HW, Yu ST, Wang ZW, Yang Z, Jiang CJ, Wang XZ et al. In planta genetic transformation to produce CRISPRed high-oleic peanut. Plant Growth Regul [Internet]. 2023;101(2):443–51. https://doi.org/10.1007/s10725-023-01031-y .
Sutradhar M, Mandal N. Reasons and riddance of Agrobacterium tumefaciens overgrowth in plant transformation. Transgenic Res [Internet]. 2023;32(1–2):33–52. https://doi.org/10.1007/s11248-023-00338-w .
Vasudevan V, Sathish D, Ajithan C, Sathish S, Manickavasagam M. Efficient Agrobacterium-mediated in planta genetic transformation of watermelon [Citrullus lanatus Thunb.]. Plant Biotechnol Rep [Internet]. 2021;15(4):447–57. https://doi.org/10.1007/s11816-021-00691-4 .
Kshirsagar JK, Sawardekar SV, Bhave SG, Gokhale NB, Narangalkar AL, Burondkar MM, et al. Genetic Transformation for Pod Borer Resistance in Dolichos Bean [Lablab purpureus (L.) Sweet]. Legum Res - Int J. 2021;I(Of):1–7.
Che P, Chang S, Simon MK, Zhang Z, Shaharyar A, Ourada J, et al. Developing a rapid and highly efficient cowpea regeneration, transformation and genome editing system using embryonic axis explants. Plant J. 2021;106(3):817–30.
Sadhu SK, Jogam P, Gande K, Banoth R, Penna S, Peddaboina V. Optimization of different factors for an Agrobacterium-mediated genetic transformation system using embryo axis explants of chickpea (Cicer arietinum L). J Plant Biotechnol. 2022;49(1):61–73.
Das Bhowmik SS, Cheng AY, Long H, Tan GZH, Hoang TML, Karbaschi MR, et al. Robust genetic transformation system to obtain non-chimeric transgenic chickpea. Front Plant Sci. 2019;10(April 2019):1–14.
Rech EL, Vianna GR, Aragão FJL. High-efficiency transformation by biolistics of soybean, common bean and cotton transgenic plants. Nat Protoc [Internet]. 2008;3(3):410–8. https://doi.org/10.1038/nprot.2008.9 .
Kapildev G, Chinnathambi A, Sivanandhan G, Rajesh M, Vasudevan V, Mayavan S et al. High-efficient Agrobacterium-mediated in planta transformation in black gram (Vigna mungo (L.) Hepper). Acta Physiol Plant. 2016;38(8).
Muruganantham M, Ganapathi A, Amutha S, Vengadesan G, Selvaraj N. Shoot regeneration from immature cotyledonary nodes in black gram [Vigna mungo (L.) Hepper]. Indian J Biotechnol. 2005;4(4):551–5.
Sedaghati B, Haddad R, Bandehpour M. Development of an efficient in-planta Agrobacterium-mediated transformation method for Iranian purslane (Portulaca oleracea L.) using sonication and vacuum infiltration. Acta Physiol Plant [Internet]. 2021;43(2):1–9. https://doi.org/10.1007/s11738-020-03185-y .
Subramanyam K, Rajesh M, Jaganath B, Vasuki A, Theboral J, Elayaraja D, et al. Assessment of factors influencing the Agrobacterium-mediated in planta seed transformation of brinjal (Solanum melongena L). Appl Biochem Biotechnol. 2013;171(2):450–68.
Baskaran P, Soós V, Balázs E, Van Staden J. Shoot apical meristem injection: A novel and efficient method to obtain transformed cucumber plants. South African J Bot [Internet]. 2016;103(March 2016):210–5. https://doi.org/10.1016/j.sajb.2015.09.006 .
Ulian EC, Smith RH, Gould JH, McKnight TD. Transformation of plants via the shoot apex. Vitr Cell Dev Biol. 1988;24:951–4.
Sitther V, Tabatabai B, Enitan O, Fathabad SG, Dhekney S. Production of transgenic Camelina sativa plants via Agrobacterium-mediated Transformation of shoot apical meristems. Am J Plant Sci. 2019;10(01):1–11.
Li J, Xu Z, Zeng T, Zhou L, Li J, Hu H, et al. Overexpression of TcCHS increases pyrethrin content when using a genotype-independent Transformation System in Pyrethrum (Tanacetum cinerariifolium). Plants. 2022;11(12):1575.
Ganesan M, Bhanumathi P, Kumari KG, Prabha AL, Song P, soon, Jayabalan N. Transgenic Indian Cotton (Gossypium hirsutum) Harboring Rice Chitinase Gene (Chi II) Confers Resistance to Two Fungal Pathogens Faculty of Biotechnology, Cheju National University, 66 Jeju Daehak-Ro, Jeju 690–756, Environmental Biotechnology Natio. Am J Biochem Biotechnol. 2009;5(2):63–74.
Lei J, Wang D, Shao L, Wei X, Huang L. Agrobacterium-mediated transformation of SNC1 gene into cotton shoot apex and Fusarium wilt resistance in T1 plants. Mol Plant Breed. 2010;8(2):252–8.
Hamada H, Liu Y, Nagira Y, Miki R, Taoka N, Imai R. Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci Rep [Internet]. 2018;8(1):6–12. https://doi.org/10.1038/s41598-018-32714-6 .
Satish L, Ceasar SA, Ramesh M. Improved Agrobacterium-mediated transformation and direct plant regeneration in four cultivars of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell Tissue Organ Cult. 2017;131(3):547–65.
Ceasar SA, Baker A, Ignacimuthu S. Functional characterization of the PHT1 family transporters of foxtail millet with development of a novel Agrobacterium-mediated transformation procedure. Sci Rep [Internet]. 2017;7(1):1–17. https://doi.org/10.1038/s41598-017-14447-0 .
Jha P, Shashi, Rustagi A, Agnihotri PK, Kulkarni VM, Bhat V. Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. Using shoot apices as explant source. Plant Cell Tissue Organ Cult. 2011;107(3):501–12.
Sundararajan S, Nayeem S, Sivakumar HP, Ramalingam S. Agrobacterium-mediated rapid and efficient development of transgenics using shoot apex explants in two elite Indica rice cultivars. Cereal Res Commun [Internet]. 2023;1:1–13. https://doi.org/10.1007/s42976-023-00366-6 .
Arockiasamy S, Ignacimuthu S. Regeneration of transgenic plants from two indica rice (Oryza sativa L.) cultivars using shoot apex explants. Plant Cell Rep. 2007;26(10):1745–53.
Asunta M, Alex N, Cecilia M, Mutemi M, Richard OO, Mathew N, et al. Rapid and efficient plant regeneration from shoot apical meristems of finger millet [Eleusine coracana (L.) Gaertn.] Via direct organogenesis. Afr J Biotechnol. 2018;17(29):898–905.
Satish L, Ceasar SA, Shilpha J, Rency AS, Rathinapriya P, Ramesh M. Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn). Vitr Cell Dev Biol - Plant. 2015;51(2):192–200.
Ramakrishnan M, Ceasar SA, Duraipandiyan V, Ignacimuthu S. Efficient plant regeneration from shoot apex explants of maize (Zea mays) and analysis of genetic fidelity of regenerated plants by ISSR markers. Plant Cell Tissue Organ Cult. 2014;119(1):183–96.
Teh CY, Maziah M, Shaharuddin NA, Ho C. Efficient in vitro multiple shoots regeneration system from rice shoot apex in recalcitrant Malaysian Indica rice cultivars (Oryza sativa L). Indian J Biotechnol. 2018;17(3):504–13.
Pozueta-Romero J, Houlné G, Cañas L, Schantz R, Chamarro J. Enhanced regeneration of tomato and pepper seedling explants for Agrobacterium-mediated transformation. Plant Cell Tissue Organ Cult. 2001;67(2):173–80.
Yong H, Yongrui H, Shanchun C, Yixiong T, Jinren Z, Shirong J. New gene transformation technique for commercial citrus cultivars. Chin J Trop Crop. 2000;15:37–41.
Yukun C, Chunzen C, Zhongxiong L, Yuling L, Shengcai L, Zihao Z. Rapid transgenic method for longan (Patent CN 105505991 A) [Internet]. CN: UNIV FUJIAN AGRICULTURE & FORESTRY OP - CN 201610059720 A; 2016. Available from: https://lens.org/029-715-019-242-790. https://lens.org/029-715-019-242-790 .
Ma C, Goralogia GS, Strauss SH, Ma C, Tran H, Rivera AN, De et al. In Planta Transformation in Populus and Eucalyptus. In: In vitro cellular & developmental biology. SPRINGER ONE NEW YORK PLAZA, SUITE 4600, NEW YORK, NY, UNITED STATES; 2021. pp. S51–S51.
Nagle MF, Yuan J, Kaur D, Ma C, Peremyslova E, Jiang Y et al. GWAS identifies candidate genes controlling adventitious rooting in Populus trichocarpa (Pre-print). bioRxiv. 2022;496209.
Yuan J, Kaur D, Zhou Z, Nagle M, Kiddle NG, Doshi NA, et al. Robust high-throughput phenotyping with deep segmentation enabled by a web-based annotator. Plant Phenomics. 2022;2022:1–17.
Steinitz B, Amitay A, Gaba V, Tabib Y, Keller M, Levin I. A simple plant regeneration-ability assay in a range of Lycopersicon species. Plant Cell Tissue Organ Cult. 2006;84(3):269–78.
Collette AT, Gillette NJ. A Histological Study of Regeneration in the Epicotyl of Tomato. Am Midl Nat [Internet]. 1955;54(1):52–60. http://www.jstor.org.proxy3.library.mcgill.ca/stable/2422176 .
Cao H, Zhang X, Ruan Y, Zhang L, Cui Z, Li X et al. MiRNA expression profiling and zeatin dynamic changes in a new model system of in vivo indirect regeneration of tomato. PLoS One [Internet]. 2020;15(12 December):5–7. https://doi.org/10.1371/journal.pone.0237690 .
Johkan M, Mori G, Imahori Y, Mitsukuri K, Yamasaki S, Mishiba K, et al. Shading the cut stems of tomato plants promotes in vivo shoot regeneration via control of the phenolic metabolism. Environ Control Biol. 2008;46(3):203–9.
Amutha S, Kathiravan K, Singer S, Jashi L, Shomer I, Steinitz B, et al. Adventitious shoot formation in decapitated dicotyledonous seedlings starts with regeneration of abnormal leaves from cells not located in a shoot apical meristem. Vitr Cell Dev Biol - Plant. 2009;45(6):758–68.
Nielsen MD, Farestveit B, Andersen AS. Adventitious shoot development from Decapitated Plants of Periclinal Chimeric Poinsettia Plants (Euphorbia pulcherrima Willd ex Klotsch). Eur J Hortic Sci. 2003;68(4):161–8.
Harada M, Oda M, Mori G, Ikeda H. Mass regeneration of shoots from cut surfaces of stems in tomato stock plants. J Japanese Soc Hortic Sci. 2005;74(6):478–81.
Tezuka T, Harada M, Johkan M, Yamasaki S, Tanaka H, Oda M. Effects of auxin and cytokinin on in vivo adventitious shoot regeneration from decapitated tomato plants. HortScience. 2011;46(12):1661–5.
Johkan M, Ono M, Tanaka H, Furukawa H, Tezuka T, Oda M. Morphological Variation, Growth, and Yield of Tomato Plants Vegetatively Propagated by the Complete Decapitation Method. Int J Veg Sci [Internet]. 2016;22(1):58–65. https://doi.org/10.1080/19315260.2014.937022 .
Shi QH, Liu P, Liu MJ, Wang JR, Xu J. A novel method for rapid in vivo induction of homogeneous polyploids via calluses in a woody fruit tree (Ziziphus jujuba Mill). Plant Cell Tissue Organ Cult. 2015;121(2):423–33.
Wang LH, Hu L, Li DK, Liu P, Liu MJ, wanglihu et al. In vivo bud regeneration ability and homogeneous polyploid induction via callus of different jujube genotypes. J Plant Genet Resour [Internet]. 2017;18(1):164–70. https://doi.org/10.1016/j.hpj.2016.08.002 .
Shi Q, Liu P, Liu M, Wang J, Zhao J, Zhao Z et al. In Vivo Fast Induction of Homogeneous Autopolyploids via Callus in Sour Jujube (Ziziphus acidojujuba Cheng et Liu). Hortic Plant J [Internet]. 2016;2(3):147–53. https://doi.org/10.1016/j.hpj.2016.08.002 .
Niu J, Luo X, Chen L, Li H, Liu B, Wang Q, et al. Callus induction in the field and establishment of a bud regeneration system for pomegranate. J Fruit Sci. 2018;35(10):1225–34.
Yang L, Machin F, Wang S, Saplaoura E, Kragler F. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat Biotechnol. 2023;41:958–67.
Rasool A, Mansoor S, Bhat KM, Hassan GI, Baba TR, Alyemeni MN et al. Mechanisms underlying Graft Union formation and Rootstock Scion Interaction in Horticultural plants. 11, Frontiers in Plant Science. 2020. p. 590847.
Ham BK, Lucas WJ. Phloem-Mobile RNAs as systemic signaling agents. Annu Rev Plant Biol. 2017;68:173–95.
Zhang W, Thieme CJ, Kollwig G, Apelt F, Yang L, Winter N, et al. TRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell. 2016;28(6):1237–49.
Ramegowda V, Mysore KS, Senthil-Kumar M. Virus-induced gene silencing is a versatile tool for unraveling the functional relevance of multiple abiotic-stress-responsive genes in crop plants. Front Plant Sci. 2014;5(JUL):1–12.
Burch-Smith TM, Schiff M, Liu Y, Dinesh-Kumar SP. Efficient virus-induced gene silencing in Arabidopsis. Plant Physiol. 2006;142(1):21–7.
Senthil-Kumar M, Mysore KS. Virus-induced gene silencing can persist for more than 2years and also be transmitted to progeny seedlings in Nicotiana benthamiana and tomato. Plant Biotechnol J. 2011;9(7):797–806.
Ali Z, Abul-Faraj A, Li L, Ghosh N, Piatek M, Mahjoub A, et al. Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol Plant. 2015;8(8):1288–91.
Oh Y, Kim H, Kim SG. Virus-induced plant genome editing. Curr Opin Plant Biol [Internet]. 2021;60:101992. https://doi.org/10.1016/j.pbi.2020.101992 .
Abrahamian P, Hammond RW, Hammond J. Plant virus-derived vectors: applications in Agricultural and Medical Biotechnology. Annu Rev Virol. 2020;7:513–35.
Tamilselvan-Nattar-Amutha S, Hiekel S, Hartmann F, Lorenz J, Dabhi RV, Dreissig S et al. Barley stripe mosaic virus-mediated somatic and heritable gene editing in barley (Hordeum vulgare L.). Front Plant Sci [Internet]. 2023;14. https://www.frontiersin.org/articles/ https://doi.org/10.3389/fpls.2023.1201446 .
Ellison EE, Nagalakshmi U, Gamo ME, Huang P, jui, Dinesh-Kumar S, Voytas DF. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat Plants [Internet]. 2020;6(6):620–4. https://doi.org/10.1038/s41477-020-0670-y .
Jackson SD, Hong Y. Systemic movement of FT mRNA and a possible role in floral induction. Front Plant Sci. 2012;3(JUN):3–6.
Chen H, Su Z, Tian B, Liu Y, Pang Y, Kavetskyi V, et al. Development and optimization of a barley stripe mosaic virus-mediated gene editing system to improve Fusarium head blight resistance in wheat. Plant Biotechnol J. 2022;20(6):1018–20.
Lei J, Dai P, Li Y, Zhang W, Zhou G, Liu C et al. Heritable gene editing using FT mobile guide RNAs and DNA viruses. Plant Methods [Internet]. 2021;17(1):1–11. https://doi.org/10.1186/s13007-021-00719-4 .
Lei J, Li Y, Dai P, Liu C, Zhao Y, You Y, et al. Efficient virus-mediated genome editing in cotton using the CRISPR/Cas9 system. Front Plant Sci. 2022;13(November):1–11.
Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: Seed infection/transformation. In: Methods in Arabidopsis Research [Internet]. World Scientific Publishing Co. Pte. Ltd.; 1992. pp. 274–89. https://doi.org/10.1142/9789814439701_0010 .
Cody JP, Maher MF, Nasti RA, Starker CG, James C. Direct delivery and fast treated Agrobacterium co-culture (Fast TrACC) Plant Transformation methods in Nicotiana Benthamiana. Unpublished. 2021;1–27.
Cho HJ, Moy Y, Rudnick NA, Klein TM, Yin J, Bolar J, et al. Development of an efficient marker-free soybean transformation method using the novel bacterium Ochrobactrum haywardense H1. Plant Biotechnol J. 2022;20(5):977–90.
Download references
Acknowledgements
Author JGB is thankful to Éric Fortier for the review of the article.
JGB was supported by the Natural Sciences and Engineering Research Council of Canada, les Fonds de recherche du Québec volet Nature et Technologie, Centre SÈVE, MITACS, and Seed World Group.
Author information
Authors and affiliations.
Centre de recherche sur les grains (CÉROM) Inc., 740 Chemin Trudeau, St-Mathieu-de-Beloeil, Québec, J3G 0E2, Canada
Jérôme Gélinas Bélanger, Tanya Rose Copley & Louise O’Donoughue
Department of Plant Science, McGill University, 21111 Lakeshore Road, St-Mathieu-de-Beloeil, Montréal, Québec, H9X 3V9, Canada
Jérôme Gélinas Bélanger, Valerio Hoyos-Villegas & Jean-Benoit Charron
You can also search for this author in PubMed Google Scholar
Contributions
JGB: conceptualization, analyzed the data, generated the figures and database, and wrote the original draft. All authors reviewed the manuscript.
Corresponding authors
Correspondence to Jérôme Gélinas Bélanger or Louise O’Donoughue .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Rights and permissions
Open Access: This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article unless otherwise stated in a credit line to the data. Reprints and Permissions.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bélanger, J.G., Copley, T.R., Hoyos-Villegas, V. et al. A comprehensive review of in planta stable transformation strategies. Plant Methods 20 , 79 (2024). https://doi.org/10.1186/s13007-024-01200-8
Download citation
Received : 05 March 2024
Accepted : 01 May 2024
Published : 31 May 2024
DOI : https://doi.org/10.1186/s13007-024-01200-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- In planta transformation
- In situ transformation
- Direct organogenesis
- Indirect organogenesis
- Recalcitrant species
- In vivo regeneration
Plant Methods
ISSN: 1746-4811
- Submission enquiries: [email protected]
How to Grow and Care for Polka Dot Plant
:max_bytes(150000):strip_icc():format(webp)/alexandra_jones-5d61400cc7c44bb690abbaefb2c0cd19.jpg)
Debra LaGattuta is a Master Gardener with 30+ years of experience in perennial and flowering plants, container gardening, and raised bed vegetable gardening. She is a lead gardener in a Plant-A-Row, which is a program that offers thousands of pounds of organically-grown vegetables to local food banks. Debra is a member of The Spruce Garden Review Board.
:max_bytes(150000):strip_icc():format(webp)/headshots_FINAL_debra-lagattuta-f269b4b46e0149d8b5aa5bbd9badc187.png)
- Propagating
- Growing From Seed
- Growing in Pots
Overwintering
- Common Pests
- Common Problems
Polka dot plant ( Hypoestes phyllostachya ), sometimes called freckle face plant, is an herbaceous warm-climate perennial with brightly variegated leaves . The most common polka dot plants feature green foliage flecked with pink, but varieties with purple, white, or red variegation are also available. Polka dot plant grows best in warm, humid conditions with bright, indirect light or partial shade.
The Spruce / Leticia Almeida
Polka dot plants are easy to grow with the proper conditions. They have a moderate growth rate and remain relatively small once mature, especially when grown indoors as houseplants. Because they are native to warm climates, many gardeners treat them as annuals when planted outdoors. Polka dot plants are not considered invasive plants in temperate climates, but they are considered invasive in Australia and some other tropical areas, including Hawaii.
The Spruce / Photo Illustration by Amy Sheehan / Leticia Almeida
Polka Dot Plant Care
- Plant polka dot plant in rich, well-drained potting mix.
- Place polka dot plants in a warm location with bright, indirect light indoors or part sun outdoors.
- Water your polka dot plant when the top half-inch of soil has dried out.
- Fertilize plants once per month during spring and summer.
- Polka dot plants complete their growth cycle after flowering, giving them a lifespan of one to two years in most environments.
Polka dot plants have become a problematic, aggressive grower in Queensland and New South Wales, Australia. In the continental US, the plant is not considered invasive and is safe to plant in-ground.
Outdoors, plant polka dot plants in a location that receives some shade. Too much light can cause the plant's variegation to fade. Bright, indirect light from an east- or south-facing window is ideal indoors.
Polka dot plants prefer soil rich in organic matter with good drainage. An all-purpose organic potting mix is typically suitable for these plants. Mix in some pumice or perlite to improve soil drainage.
Keep the soil evenly moist. Water the plant when the top half-inch of soil has dried out. Cut back slightly on watering in the winter, then resume watering once you see new growth appear in the spring.
Temperature and Humidity
Keep your polka dot plant in a warm place with at least 50 percent humidity. They can be a great bathroom plant , if your bathroom has a window. Polka dot plants prefer temperatures over 60 degrees Fahrenheit, so they're only hardy outdoors in USDA growing zones 10 and 11. Move container plants outdoors in the spring after any danger of frost has passed, then bring them back indoors well before the first frost in fall if you plan to overwinter them.
Feed container plants with an organic fertilizer designed for houseplants once a month during the warm growing season. If planting in-ground, mix organic compost into the soil each spring before planting.
Want more gardening tips? Sign up for our free gardening newsletter for our best-growing tips, troubleshooting hacks, and more!
Types of Polka Dot Plants
Different varieties of Hypoestes phyllostachya are bred for their leaf coloration, including:
- ‘Carmina’: has dark green and red-spotted leaves
- ‘Confetti’: offers green leaves with spots of white, pink, rose, red, or burgundy
- ‘Pink Brocade’: features green leaves with mottled pink spots
- ‘Splash’ series: boasts leaves in mixes of greens with splotches of pinks, reds, or whites
Cut or pinch back the top two leaves on each stem every week to promote bushier growth and keep your polka dot plant from becoming leggy . When the plant flowers, clip off the flower spike with clean, sharp shears because the plant will enter dormancy after it flowers. Removing the flowers prevents the plant from going into dormancy.
Pruning Tip
Not sure where to find the best pruners for your polka dot plant? We tested the best pruners on the market, whether you're looking for adjustable pruners, heavy duty, pruners for small hands, and more.
Propagating Polka Dot Plants
You can propagate polka dot plants from stem cuttings . You'll have the most success in spring or summer. Here's how to propagate your polka dot plant from a stem cutting rooted in water. You'll need a small glass or jar and clean, sharp pruners or scissors.
- Cut a stem tip from the mother plant. Make sure it's at least two and ideally four inches long. Remove the leaves on the lower half of the stem.
- Put the cutting in the glass or jar. Add water so that the lower portion of the stem is submerged.
- Put the cutting in a warm place with bright, indirect light. Top off the water to keep the level consistent, and change the water every two weeks or so to keep algae from forming.
- When roots are about two inches long, the cutting is ready to pot up in soil. This can take anywhere from a few weeks to a few months.
How to Grow Polka Dot Plant From Seed
Sow seeds on the surface of warm, moist soil in early spring. Place the plant in a sunny location. The seeds should sprout in a few days. Once the seedling has grown several inches—usually in a couple of weeks—it is ready to transplant into a larger container or plant outdoors. Only plant outdoors after the threat of frost is over.
Potting and Repotting Polka Dot Plant
The best time to repot a polka dot plant is in the spring after its winter dormant period. Your polka dot plant is pot bound when the roots start growing out of the drainage holes in its container. The new pot should be no more than two inches wider and deeper than the old pot. Avoid terra cotta pots, which wick away moisture and can cause the soil to dry out too quickly.
Bring outdoor container plants indoors before night temperatures drop below 60 degrees Fahrenheit in late summer or early fall. You can bring them outdoors again the following spring when night temperatures are consistently above 60 degrees.
Common Pests and Plant Diseases
Pests like mealybugs , aphids , and whiteflies can affect polka dot plants. Typical diseases associated with polka dot plants are root rot, leaf-spot diseases, and powdery mildew. Telltale signs of infestations or disease include discolored or damaged foliage and insects crawling or feeding on leaves and stems.
How to Get Polka Dot Plant to Bloom
Unlike most flowering plants, gardeners typically want to prevent polka dot plant from blooming because flowering causes the plant to go dormant. If you want your plant to last longer, it's best to clip off the flower spike when it forms.
Bloom Months
Polka dot plants typically bloom in late summer or early fall as days begin to shorten.
What Do Polka Dot Plant Flowers Look and Smell Like?
Polka dot plants bloom by sending up a small spike with tiny pink or purple flowers. They're not showy or aromatic.
Common Problems With Polka Dot Plants
Leaves losing their color.
Fading leaf color is typically caused by too much or too little sun. Polka dot plants need bright, indirect light to maintain their color, but hot, direct sun can cause variegation to fade.
Leaves Turning Brown or Drooping
Insufficient water and humidity can cause the polka dot plant's leaves to turn brown or start drooping. Also, too much sunlight can burn the leaves. Hard water and overfertilization are other reasons for a polka dot plant's leaves turning brown. Adjust your humidity or watering habits to revive the plant.
Leaves Turning Yellow or Dropping Off
Overwatering can cause leaves to yellow and even drop. If you notice yellowing, reduce the amount of water you give the plant and make sure you're using potting soil with good drainage.
Polka dot plants can be grown indoors, and outdoors in the right climates. If you live in USDA Hardiness Zones 10 through 11, you can grow polka dot plant outdoors.
Polka dot plants will grow best in a warm, humid place with bright, indirect light or dappled sunlight.
Polka dot plants do not spread very much. Polka dot plants usually grow to 16 to 22 inches tall and 18 to 24 inches wide.
Polka dot plants don't always flower, but when they do, it's in the summer months. Their flowers are small and typically lilac or pink in color.
Jon VanZile was a writer for The Spruce covering houseplants and indoor gardening for almost a decade. He is a professional writer whose articles on plants and horticulture have appeared in national and regional newspapers and magazines.
Polka Dot Plant . Brisbane City Council Weed Identification.
Hypoestes phyllostachya. PlantPono.org.
Polka Dot Plant, Hypoestes phyllostachya . University of Wisconsin, Extension of Horticulture.
More from The Spruce
- How to Grow and Care for an Urn Plant Like a Pro
- Growing Ficus Ruby Can Add a Pop of Color to Your Home—Here's How
- How to Grow and Care for Ti Plant (Good Luck Plant)
- 25 Cat-Safe Plants That Grow Well In Low-Light Conditions
- How to Grow and Care for Coffee Plant
- How to Grow and Care for Alocasia
- How to Grow & Care for Alocasia Polly
- How to Grow and Care for Peperomia Plants
- 26 Indoor Plants Safe for Cats and Dogs
- How to Grow Guzmania Bromeliads for Perfect Blooms At Home
- How to Grow and Care for Corn Plant (Dracaena)
- How to Grow and Care for Pink Princess Philodendron
- Inch Plant Is One of the Easiest Houseplants to Grow—Here's How to Do It
- How to Grow and Care for the African Spear Plant
- The Rare Hoya Australis Is the Perfect Addition to Your Houseplant Collection
- How to Grow and Care for Ficus Shivereana

IMAGES
VIDEO
COMMENTS
Different facets of plant growth and how they are coupled. Growth sensu lato (total area of the Venn diagram) is the change in biomass, or volume. Growth sensu stricto (area contained within solid lines in the Venn diagram) is an irreversible increase in cell number, structural biomass (structural growth), or plant volume (expansive growth). Cell production is part of structural growth, as it ...
Testing a Hypothesis—Plant Growth. Charles Darwin believed that there were hereditary advantages in having two sexes for both the plant and animal kingdoms. Some time after he wrote Origin of Species, he performed an experiment in his garden. He raised two large beds of snapdragons, one from cross-pollinated seeds, the other from self ...
The experiment can be carried out in a team. Your task is to examine and estimate the effects of seed type and amount of water on the growth of a particular type of plant. You will have to design the experiment, collect the data, enter the data into SPSS, carry out the statistical analysis, and formulate your conclusions.
Introduction. The growth rate hypothesis (GRH) proposes that fast-growing organisms have low N:P and C:P ratios due to the relatively high demand for phosphorus-rich RNA to support rapid protein synthesis (Acharya et al., 2004).Various comprehensive reviews confirmed that nutrient-rich plants tend to have low N:P ratios, and supported the validity of GRH in the realm of vascular plants, as N ...
To test this hypothesis, Col-0 plants were grown for 5 days at either 21 °C or 28 °C after 4 days for germination in 21 °C in three different ... N. et al. Plant growth stimulation by high CO(2 ...
or sink-limited plant growth, which ultimately inform plant and vegetation growth models (the 'why'). II. The What: a clarification of various growth facets 1. Plant growth sensu stricto and sensu lato The concept of growth is widely used in the plant science community. However, it is ambiguous, as it encompasses many
Make a hypothesis about which part of the light spectrum causes the most plant growth and which part of the light spectrum causes the least plant growth. Assume that all conditions (soil content, moisture availability, and seed viability) are the same for each seed as it grows. The only variable is the color of the light. State your hypothesis ...
The history of the relationship between plant growth and water consumption is retraced by following the progression of scientific thought through the centuries: from a purely philosophical question, to conceptual and methodological developments, towards a research interest in plant functioning and the interaction with the environment. The relationship between plant growth and water consumption ...
The resource availability hypothesis (RAH), also called the growth rate hypothesis (Coley 1987; Stamp 2003), accepted Feeny's premise that long-lived species (apparent) invested heavily in defences and short-lived species ... Effect of plant growth on investment in defences between species in habitats with different nutrient availability.
Date: August 29, 2022. Source: Salk Institute. Summary: Plants lengthen and bend to secure access to sunlight. Despite observing this phenomenon for centuries, scientists do not fully understand ...
The growth-differentiation balance hypothesis (GDBH) presented in Herms & Mattson , as an extension of Loomis , starts from the assumption that photosynthetic production is not adequate to optimally provision both G and D, thus forcing the evolution of tradeoffs. The GDBH was originally developed from economic perspectives applied to plant ...
In addition, the stunted growth of plants at high B% leads to an increased self-shading of leaves and decrease in light interception, which has been proposed to result in negative consequences for the whole plant productivity . Although the individual species reacted differently between phytotrons and the field trial, on average, a ...
Abstract According to the growth-defense hypothesis in ecology, faster-growing plant species should suffer more from herbivores and pathogens than slower-growing species. Tests of this hypothesis have focused on aboveground plant tissues, herbivores, and pathogens; however, it should also apply to root defense. To test whether faster-growing species suffer more negatively from soil biota than ...
According to the growth-defense hypothesis in ecology, faster-growing plant species should suffer more from herbivores and pathogens than slower-growing species. Tests of this hypothesis have focused on aboveground plant tissues, herbivores, and pathogens; however, it should also apply to root defen …
Here are null hypothesis examples: Plant growth is unaffected by temperature. If you increase temperature, then solubility of salt will increase. Incidence of skin cancer is unrelated to ultraviolet light exposure. All brands of light bulb last equally long. Cats have no preference for the color of cat food. All daisies have the same number of ...
Environmental factors that affect plant growth include light, temperature, water, humidity and nutrition. It's important to understand how these factors affect plant growth and development. With a basic understanding of these factors, you may be able to manipulate plants to meet your needs, whether for increased leaf, flower or fruit production ...
In his perspective "Growth by auxin: when a weed needs acid" (7 Oct. 2005, p. 60), M. Grebe asserts that the acid-growth theory describes how the plant hormone auxin (indole-3-acetic acid, IAA) stimulates cell elongation in developing organs such as leaves and roots. On the basis of this statement, he presents the hypothesis that the proton pump AVP1 of the model plant Arabidopsis thaliana ...
Plants grow more quickly when fertilizer is used than when it's not used. You can start to test your hypothesis by getting two, near identical plants and growing one with fertilizer and one ...
Experiment with Plant Growth Science Projects. (26 results) Garden and grow plants in all sorts of ways-- in different light, soils, water, and more. Test how fruits ripen, plant seeds, grow a garden in water, or start with plantlets rather than seed. Learn to measure plant growth accurately. Hydroponics: Gardening Without Soil.
Sometimes you will see hypothesis written as an "as-the" statement. You would write the same hypothesis but use the words "as-the" instead of "if-then." For example, If the amount of fertilizer applied to plants is increased, then plant growth (height) will increase. As the amount of fertilizer increases the plant growth increases.
LA JOLLA—Plants lengthen and bend to secure access to sunlight. Despite observing this phenomenon for centuries, scientists do not fully understand it. Now, Salk scientists have discovered that two plant factors—the protein PIF7 and the growth hormone auxin—are the triggers that accelerate growth when plants are shaded by canopy and exposed to warm temperatures at the same time.
Nov 30, 2016. —. by. Papiya Dutta. in Science Fair Projects. Though it is still a debatable topic, experiments conducted all over the world indicate that music can affect plant growth. While soothing classical music, Beethoven, Brahms have been seen to help in stimulating growth, certain other music hindered their growth rate.
Hypothesis: I predict that plants will grow better under blue, red and yellow lights than they will under white and green lights. Background: The relationship between light and plant growth can be demonstrated by exposing leaves to various colors of light. Light supplies the power to carry on photosynthesis, the food-making process in leaves.
Different studies have found evidence for this hypothesis (e.g., Fargione and Tilman 2005; ... Xue W, Huang L, Sheng WJ, Zhu JT, Li SQ, Yu FH (2022) Contrasting effects of plant-soil feedbacks on growth and morphology of physically-connected daughter and mother ramets in two clonal plants. Plant Soil 472:479-489. Article CAS Google ...
Plant transformation remains a major bottleneck to the improvement of plant science, both on fundamental and practical levels. The recalcitrant nature of most commercial and minor crops to genetic transformation slows scientific progress for a large range of crops that are essential for food security on a global scale. Over the years, novel stable transformation strategies loosely grouped ...
Polka Dot Plant Care. Plant polka dot plant in rich, well-drained potting mix. Place polka dot plants in a warm location with bright, indirect light indoors or part sun outdoors. Water your polka dot plant when the top half-inch of soil has dried out. Fertilize plants once per month during spring and summer.
The number of big genera has increased from 57 to 86; today one of every four plant species is classified as a member of a big genus, with 14% in just 28 megadiverse genera. Most (71%) of the growth in big genera since 2000 is the result of new species description, not generic re-circumscription.
Plants respond to high ambient temperature with altered developmental and growth programs, termed thermomorphogenesis. Here we show that thermomorphogenesis is conserved in Arabidopsis, soybean, and rice and that it is linked to a decrease in the levels of the two macronutrients nitrogen and phosphorus. We also find that low external levels of ...
Plant microbiota contributes to plant growth and health, including enhancing plant resistance to various diseases. Despite remarkable progress in understanding diseases resistance in plants, the precise role of rhizosphere microbiota in enhancing watermelon resistance against soil-borne diseases remains unclear. Here, we constructed a synthetic community (SynCom) of 16 core bacterial strains ...
TFC (10% thifluzamide-fludioxonil-clothianidin) is a novel wheat seed-coating agent. In the field, we confirmed that 10% TFC plays a positive role in preventing soil-borne diseases and promoting wheat seedling growth. However, its effects on rhizosphere microecology and the underlying molecular mechanism are not fully understood. Field trials revealed a positive effect on the biomass ...