- Patient Care & Health Information
- Diseases & Conditions
- Rheumatoid arthritis
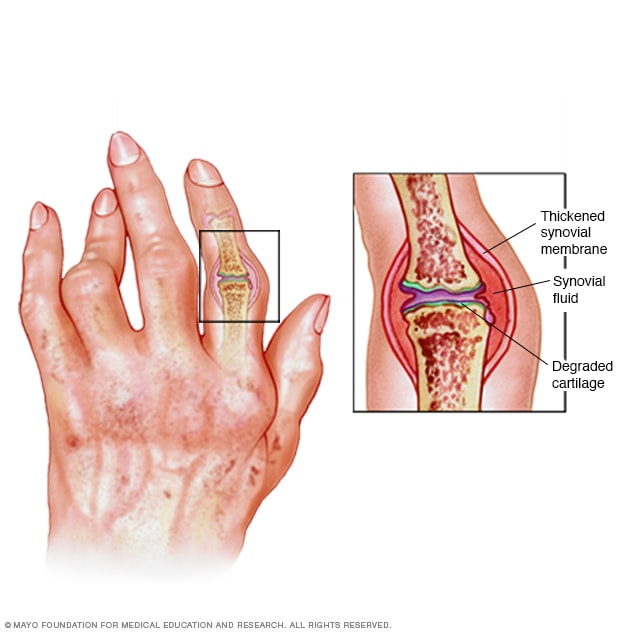
Rheumatoid arthritis can cause pain, swelling and deformity. As the tissue that lines your joints (synovial membrane) becomes inflamed and thickened, fluid builds up and joints erode and degrade.
Rheumatoid arthritis is a chronic inflammatory disorder that can affect more than just your joints. In some people, the condition can damage a wide variety of body systems, including the skin, eyes, lungs, heart and blood vessels.
An autoimmune disorder, rheumatoid arthritis occurs when your immune system mistakenly attacks your own body's tissues.
Unlike the wear-and-tear damage of osteoarthritis, rheumatoid arthritis affects the lining of your joints, causing a painful swelling that can eventually result in bone erosion and joint deformity.
The inflammation associated with rheumatoid arthritis is what can damage other parts of the body as well. While new types of medications have improved treatment options dramatically, severe rheumatoid arthritis can still cause physical disabilities.

Products & Services
- A Book: Mayo Clinic Guide to Arthritis
- Assortment of Products for Independent Living from Mayo Clinic Store
- Products for Mobility and Safety
Signs and symptoms of rheumatoid arthritis may include:
- Tender, warm, swollen joints
- Joint stiffness that is usually worse in the mornings and after inactivity
- Fatigue, fever and loss of appetite
Early rheumatoid arthritis tends to affect your smaller joints first — particularly the joints that attach your fingers to your hands and your toes to your feet.
As the disease progresses, symptoms often spread to the wrists, knees, ankles, elbows, hips and shoulders. In most cases, symptoms occur in the same joints on both sides of your body.
About 40% of people who have rheumatoid arthritis also experience signs and symptoms that don't involve the joints. Areas that may be affected include:
- Salivary glands
- Nerve tissue
- Bone marrow
- Blood vessels
Rheumatoid arthritis signs and symptoms may vary in severity and may even come and go. Periods of increased disease activity, called flares, alternate with periods of relative remission — when the swelling and pain fade or disappear. Over time, rheumatoid arthritis can cause joints to deform and shift out of place.
When to see a doctor
Make an appointment with your doctor if you have persistent discomfort and swelling in your joints.
Vivien Williams: Pain, swelling and stiffness in your joints — all are symptoms of rheumatoid arthritis. But because these symptoms come and go, the condition can sometimes be tricky to diagnose. And it's important to get the right diagnosis because starting treatment early can make a difference.
Virginia Wimmer, has rheumatoid arthritis: Give me your best shot!
Ms. Williams: At first, Virginia Wimmer blamed her painful joints on too much volleyball.
Ms. Wimmer: In my knees and in my wrists.
Ms. Williams: For a couple years, she put up with the pain and swelling that would come and go. Then things got much worse.
Ms. Wimmer: I couldn't have a ball touch my arms.
Ms. Williams: She couldn't do much of anything, let alone play outside with her daughter.
Ms. Wimmer: That was really hard. She'd have to beg me to play with her, and teach her, and help her. And I just had to sit and watch.
Ms. Williams: Virginia was diagnosed with rheumatoid arthritis.
Nisha Manek, M.D., Rheumatology, Mayo Clinic: Rheumatoid arthritis is an inflammatory condition. It's also associated with the immune system.
Ms. Williams: Dr. Nisha Manek says it happens when the immune system becomes deregulated. You see, the joint capsule has a lining of tissue called the synovium. The synovium makes fluid that keeps joints lubricated. When you have rheumatoid arthritis, your immune system sends antibodies to the synovium and causes inflammation. This causes pain and joint damage, especially in small joints in the fingers and wrists. But it can affect any joint.
The good news is that treatment for rheumatoid arthritis has improved dramatically over the last years. Medications, such as methotrexate, help bring the immune system back into balance and steroids can help calm flare-ups. So what was once an often crippling disease can now be controlled for many people — people like Virginia whose disease is pretty severe.
Ms. Wimmer: You can get to the point where you are doing the things that you love and that is the goal.
Ms. Williams: Dr. Manek says if you have pain, swelling and stiffness in your joints that comes and goes and is on both sides of your body, see your doctor to see if it is rheumatoid arthritis.
Rheumatoid is different than osteoarthritis which damages joints because of wear and tear.
For Medical Edge, I'm Vivien Williams.
More Information
- Rheumatoid arthritis: Does pregnancy affect symptoms?
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Rheumatoid arthritis is an autoimmune disease. Normally, your immune system helps protect your body from infection and disease. In rheumatoid arthritis, your immune system attacks healthy tissue in your joints. It can also cause medical problems with your heart, lungs, nerves, eyes and skin.
Doctors don't know what starts this process, although a genetic component appears likely. While your genes don't actually cause rheumatoid arthritis, they can make you more likely to react to environmental factors — such as infection with certain viruses and bacteria — that may trigger the disease.
Risk factors
Factors that may increase your risk of rheumatoid arthritis include:
- Your sex. Women are more likely than men to develop rheumatoid arthritis.
- Age. Rheumatoid arthritis can occur at any age, but it most commonly begins in middle age.
- Family history. If a member of your family has rheumatoid arthritis, you may have an increased risk of the disease.
- Smoking. Cigarette smoking increases your risk of developing rheumatoid arthritis, particularly if you have a genetic predisposition for developing the disease. Smoking also appears to be associated with greater disease severity.
- Excess weight. People who are overweight appear to be at a somewhat higher risk of developing rheumatoid arthritis.
Complications
Rheumatoid arthritis increases your risk of developing:
- Osteoporosis. Rheumatoid arthritis itself, along with some medications used for treating rheumatoid arthritis, can increase your risk of osteoporosis — a condition that weakens your bones and makes them more prone to fracture.
- Rheumatoid nodules. These firm bumps of tissue most commonly form around pressure points, such as the elbows. However, these nodules can form anywhere in the body, including the heart and lungs.
- Dry eyes and mouth. People who have rheumatoid arthritis are much more likely to develop Sjogren's syndrome, a disorder that decreases the amount of moisture in the eyes and mouth.
- Infections. Rheumatoid arthritis itself and many of the medications used to combat it can impair the immune system, leading to increased infections. Protect yourself with vaccinations to prevent diseases such as influenza, pneumonia, shingles and COVID-19.
- Abnormal body composition. The proportion of fat to lean mass is often higher in people who have rheumatoid arthritis, even in those who have a normal body mass index (BMI).
- Carpal tunnel syndrome. If rheumatoid arthritis affects your wrists, the inflammation can compress the nerve that serves most of your hand and fingers.
- Heart problems. Rheumatoid arthritis can increase your risk of hardened and blocked arteries, as well as inflammation of the sac that encloses your heart.
- Lung disease. People with rheumatoid arthritis have an increased risk of inflammation and scarring of the lung tissues, which can lead to progressive shortness of breath.
- Lymphoma. Rheumatoid arthritis increases the risk of lymphoma, a group of blood cancers that develop in the lymph system.
- Is depression a factor in rheumatoid arthritis?
- Rheumatoid arthritis: Can it affect the eyes?
- Rheumatoid arthritis: Can it affect the lungs?
- Rheumatoid arthritis. National Institute of Arthritis and Musculoskeletal and Skin Diseases. https://www.niams.nih.gov/Health_Info/Rheumatic_Disease/default.asp. Accessed Feb. 9, 2021.
- Rheumatoid arthritis. American College of Rheumatology. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis. Accessed Feb. 9, 2021.
- Matteson EL, et al. Overview of the systemic and nonarticular manifestations of rheumatoid arthritis. https://www.uptodate.com/contents/search. Accessed Feb. 9, 2021.
- Goldman L, et al., eds. Rheumatoid arthritis. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Feb. 9, 2021.
- Ferri FF. Rheumatoid arthritis. In: Ferri's Clinical Advisor 2021. Elsevier; 2021. https://www.clinicalkey.com. Accessed Feb. 9, 2021.
- Kellerman RD, et al. Rheumatoid arthritis. In: Conn's Current Therapy 2021. Elsevier; 2021. https://www.clinicalkey.com. Accessed Feb. 9, 2021.
- Moreland LW, et al. General principles and overview of management of rheumatoid arthritis in adults. https://www.uptodate.com/contents/search. Accessed Feb. 9, 2021.
- Xeljanz, Xeljanz XR (tofacitinib): Drug safety communication — Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine. https://www.fda.gov/safety/medical-product-safety-information/xeljanz-xeljanz-xr-tofacitinib-drug-safety-communication-initial-safety-trial-results-find-increased. Accessed Feb. 23, 2021.
- Office of Patient Education. Arthritis: Caring for your joints. Mayo Clinic. 2017.
- Living with arthritis. American Occupational Therapy Association. https://www.aota.org/About-Occupational-Therapy/Patients-Clients/Adults/Arthritis.aspx. Accessed Feb. 23, 2021.
- Renaldi RZ. Total joint replacement for severe rheumatoid arthritis. https://www.uptodate.com/contents/search. Accessed Feb. 9, 2021.
- Rheumatoid arthritis: In depth. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/rheumatoid-arthritis-in-depth. Accessed Feb. 23, 2021.
- Chang-Miller A (expert opinion). Mayo Clinic. Feb. 27, 2021.
- 6 tips to manage rheumatoid arthritis symptoms
- Does stress make rheumatoid arthritis worse?
- Ease rheumatoid arthritis pain when grocery shopping
- How do I reduce fatigue from rheumatoid arthritis?
- Protect your joints while housecleaning
- Rethinking Rheumatoid Arthritis
- Rheumatoid arthritis and exercise
- Tips to make your mornings easier
Associated Procedures
- C-reactive protein test
- Elbow replacement surgery
- Hip replacement
- Knee replacement
- Rheumatoid factor
- Sed rate (erythrocyte sedimentation rate)
- Shoulder replacement surgery
- Spinal fusion
News from Mayo Clinic
- Mayo Clinic researchers identify link between gut bacteria and pre-clinical autoimmunity and aging in rheumatoid arthritis Oct. 07, 2023, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

AMY M. WASSERMAN, MD
Am Fam Physician. 2011;84(11):1245-1252
Patient information : A handout on this topic is available at https://familydoctor.org/familydoctor/en/diseases-conditions/rheumatoid-arthritis.html .
Author disclosure: No relevant financial affiliations to disclose.
Rheumatoid arthritis is the most commonly diagnosed systemic inflammatory arthritis. Women, smokers, and those with a family history of the disease are most often affected. Criteria for diagnosis include having at least one joint with definite swelling that is not explained by another disease. The likelihood of a rheumatoid arthritis diagnosis increases with the number of small joints involved. In a patient with inflammatory arthritis, the presence of a rheumatoid factor or anti-citrullinated protein antibody, or elevated C-reactive protein level or erythrocyte sedimentation rate suggests a diagnosis of rheumatoid arthritis. Initial laboratory evaluation should also include complete blood count with differential and assessment of renal and hepatic function. Patients taking biologic agents should be tested for hepatitis B, hepatitis C, and tuberculosis. Earlier diagnosis of rheumatoid arthritis allows for earlier treatment with disease-modifying antirheumatic agents. Combinations of medications are often used to control the disease. Methotrexate is typically the first-line drug for rheumatoid arthritis. Biologic agents, such as tumor necrosis factor inhibitors, are generally considered second-line agents or can be added for dual therapy. The goals of treatment include minimization of joint pain and swelling, prevention of radiographic damage and visible deformity, and continuation of work and personal activities. Joint replacement is indicated for patients with severe joint damage whose symptoms are poorly controlled by medical management.
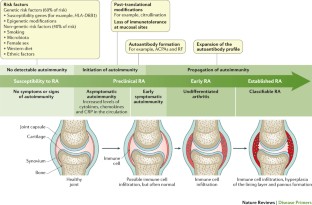
Rheumatoid arthritis (RA) is the most common inflammatory arthritis, with a lifetime prevalence of up to 1 percent worldwide. 1 Onset can occur at any age, but peaks between 30 and 50 years. 2 Disability is common and significant. In a large U.S. cohort, 35 percent of patients with RA had work disability after 10 years. 3
Etiology and Pathophysiology
Like many autoimmune diseases, the etiology of RA is multifactorial. Genetic susceptibility is evident in familial clustering and monozygotic twin studies, with 50 percent of RA risk attributable to genetic factors. 4 Genetic associations for RA include human leukocyte antigen-DR4 5 and -DRB1, and a variety of alleles called the shared epitope. 6 , 7 Genome-wide association studies have identified additional genetic signatures that increase the risk of RA and other autoimmune diseases, including STAT4 gene and CD40 locus. 5 Smoking is the major environmental trigger for RA, especially in those with a genetic predisposition. 8 Although infections may unmask an autoimmune response, no particular pathogen has been proven to cause RA. 9
RA is characterized by inflammatory pathways that lead to proliferation of synovial cells in joints. Subsequent pannus formation may lead to underlying cartilage destruction and bony erosions. Overproduction of proinflammatory cytokines, including tumor necrosis factor (TNF) and interleukin-6, drives the destructive process. 10
RISK FACTORS
Older age, a family history of the disease, and female sex are associated with increased risk of RA, although the sex differential is less prominent in older patients. 1 Both current and prior cigarette smoking increases the risk of RA (relative risk [RR] = 1.4, up to 2.2 for more than 40-pack-year smokers). 11
Pregnancy often causes RA remission, likely because of immunologic tolerance. 12 Parity may have long-lasting impact; RA is less likely to be diagnosed in parous women than in nulliparous women (RR = 0.61). 13 , 14 Breastfeeding decreases the risk of RA (RR = 0.5 in women who breastfeed for at least 24 months), whereas early menarche (RR = 1.3 for those with menarche at 10 years of age or younger) and very irregular menstrual periods (RR = 1.5) increase risk. 14 Use of oral contraceptive pills or vitamin E does not affect RA risk. 15
TYPICAL PRESENTATION
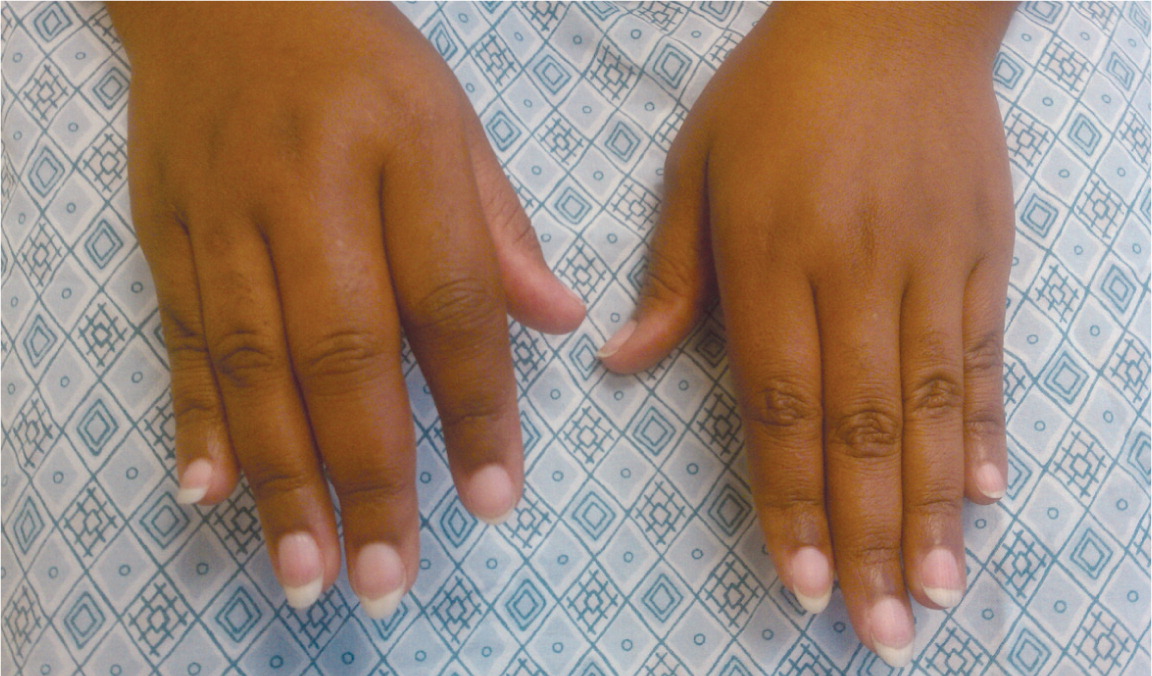
Patients with RA typically present with pain and stiffness in multiple joints. The wrists, proximal interphalangeal joints, and metacarpophalangeal joints are most commonly involved. Morning stiffness lasting more than one hour suggests an inflammatory etiology. Boggy swelling due to synovitis may be visible ( Figure 1 ) , or subtle synovial thickening may be palpable on joint examination. Patients may also present with more indolent arthralgias before the onset of clinically apparent joint swelling. Systemic symptoms of fatigue, weight loss, and low-grade fever may occur with active disease.
DIAGNOSTIC CRITERIA
In 2010, the American College of Rheumatology and European League Against Rheumatism collaborated to create new classification criteria for RA ( Table 1 ) . 16 The new criteria are an effort to diagnose RA earlier in patients who may not meet the 1987 American College of Rheumatology classification criteria. The 2010 criteria do not include presence of rheumatoid nodules or radiographic erosive changes, both of which are less likely in early RA. Symmetric arthritis is also not required in the 2010 criteria, allowing for early asymmetric presentation.
In addition, Dutch researchers have developed and validated a clinical prediction rule for RA ( Table 2 ) . 17 , 18 The purpose of this rule is to help identify patients with undifferentiated arthritis that is most likely to progress to RA, and to guide follow-up and referral.
DIAGNOSTIC TESTS
Autoimmune diseases such as RA are often characterized by the presence of autoantibodies. Rheumatoid factor is not specific for RA and may be present in patients with other diseases, such as hepatitis C, and in healthy older persons. Anti-citrullinated protein antibody is more specific for RA and may play a role in disease pathogenesis. 6 Approximately 50 to 80 percent of persons with RA have rheumatoid factor, anti-citrullinated protein antibody, or both. 10 Patients with RA may have a positive antinuclear antibody test result, and the test is of prognostic importance in juvenile forms of this disease. 19 C-reactive protein levels and erythrocyte sedimentation rate are often increased with active RA, and these acute phase reactants are part of the new RA classification criteria. 16 C-reactive protein levels and erythrocyte sedimentation rate may also be used to follow disease activity and response to medication.
Baseline complete blood count with differential and assessment of renal and hepatic function are helpful because the results may influence treatment options (e.g., a patient with renal insufficiency or significant thrombocytopenia likely would not be prescribed a nonsteroidal anti-inflammatory drug [NSAID]). Mild anemia of chronic disease occurs in 33 to 60 percent of all patients with RA, 20 although gastrointestinal blood loss should also be considered in patients taking corticosteroids or NSAIDs. Methotrexate is contraindicated in patients with hepatic disease, such as hepatitis C, and in patients with significant renal impairment. 21 Biologic therapy, such as a TNF inhibitor, requires a negative tuberculin test or treatment for latent tuberculosis. Hepatitis B reactivation can also occur with TNF inhibitor use. 22 Radiography of hands and feet should be performed to evaluate for characteristic periarticular erosive changes, which may be indicative of a more aggressive RA subtype. 10
DIFFERENTIAL DIAGNOSIS
Skin findings suggest systemic lupus erythematosus, systemic sclerosis, or psoriatic arthritis. Polymyalgia rheumatica should be considered in an older patient with symptoms primarily in the shoulder and hip, and the patient should be asked questions related to associated temporal arteritis. Chest radiography is helpful to evaluate for sarcoidosis as an etiology of arthritis.
Patients with inflammatory back symptoms, a history of inflammatory bowel disease, or inflammatory eye disease may have spondyloarthropathy. Persons with less than six weeks of symptoms may have a viral process, such as parvovirus. Recurrent self-limited episodes of acute joint swelling suggest crystal arthropathy, and arthrocentesis should be performed to evaluate for monosodium urate monohydrate or calcium pyrophosphate dihydrate crystals. The presence of numerous myofascial trigger points and somatic symptoms may suggest fibromyalgia, which can coexist with RA. To help guide diagnosis and determine treatment strategy, patients with inflammatory arthritis should be promptly referred to a rheumatology subspecialist. 16 , 17
After RA has been diagnosed and an initial evaluation performed, treatment should begin. Recent guidelines have addressed the management of RA, 21 , 22 but patient preference also plays an important role. There are special considerations for women of childbearing age because many medications have deleterious effects on pregnancy. Goals of therapy include minimizing joint pain and swelling, preventing deformity (such as ulnar deviation) and radiographic damage (such as erosions), maintaining quality of life (personal and work), and controlling extra-articular manifestations. Disease-modifying antirheumatic drugs (DMARDs) are the mainstay of RA therapy.
DMARDs can be biologic or nonbiologic ( Table 3 ) . 23 Biologic agents include monoclonal antibodies and recombinant receptors to block cytokines that promote the inflammatory cascade responsible for RA symptoms. Methotrexate is recommended as the first-line treatment in patients with active RA, unless contraindicated or not tolerated. 21 Leflunomide (Arava) may be used as an alternative to methotrexate, although gastrointestinal adverse effects are more common. Sulfasalazine (Azulfidine) or hydroxychloroquine (Plaquenil) is recommended as monotherapy in patients with low disease activity or without poor prognostic features (e.g., seronegative, nonerosive RA). 21 , 22
Combination therapy with two or more DMARDs is more effective than monotherapy; however, adverse effects may also be greater. 24 If RA is not well controlled with a nonbiologic DMARD, a biologic DMARD should be initiated. 21 , 22 TNF inhibitors are the first-line biologic therapy and are the most studied of these agents. If TNF inhibitors are ineffective, additional biologic therapies can be considered. Simultaneous use of more than one biologic therapy (e.g., adalimumab [Humira] with abatacept [Orencia]) is not recommended because of an unacceptable rate of adverse effects. 21
NSAIDS AND CORTICOSTEROIDS
Drug therapy for RA may involve NSAIDs and oral, intramuscular, or intra-articular corticosteroids for controlling pain and inflammation. Ideally, NSAIDs and corticosteroids are used only for short-term management. DMARDs are the preferred therapy. 21 , 22
COMPLEMENTARY THERAPIES
Dietary interventions, including vegetarian and Mediterranean diets, have been studied in the treatment of RA without convincing evidence of benefit. 25 , 26 Despite some favorable outcomes, there is a lack of evidence for the effectiveness of acupuncture in placebo-controlled trials of patients with RA. 27 , 28 In addition, thermotherapy and therapeutic ultrasound for RA have not been studied adequately. 29 , 30 A Cochrane review of herbal treatments for RA concluded that gamma-linolenic acid (from evening primrose or black currant seed oil) and Tripterygium wilfordii (thunder god vine) have potential benefits. 31 It is important to inform patients that serious adverse effects have been reported with use of herbal therapy. 31
EXERCISE AND PHYSICAL THERAPY
Results of randomized controlled trials support physical exercise to improve quality of life and muscle strength in patients with RA. 32 , 33 Exercise training programs have not been shown to have deleterious effects on RA disease activity, pain scores, or radiographic joint damage. 34 Tai chi has been shown to improve ankle range of motion in persons with RA, although randomized trials are limited. 35 Randomized controlled trials of Iyengar yoga in young adults with RA are underway. 36
DURATION OF TREATMENT
Remission is obtainable in 10 to 50 percent of patients with RA, depending on how remission is defined and the intensity of therapy. 10 Remission is more likely in males, nonsmokers, persons younger than 40 years, and in those with late-onset disease (patients older than 65 years), with shorter duration of disease, with milder disease activity, without elevated acute phase reactants, and without positive rheumatoid factor or anti- citrullinated protein antibody findings. 37
After the disease is controlled, medication dosages may be cautiously decreased to the minimum amount necessary. Patients will require frequent monitoring to ensure stable symptoms, and prompt increase in medication is recommended with disease flare-ups. 22
JOINT REPLACEMENT
Joint replacement is indicated when there is severe joint damage and unsatisfactory control of symptoms with medical management. Long-term outcomes are good, with only 4 to 13 percent of large joint replacements requiring revision within 10 years. 38 The hip and knee are the most commonly replaced joints.
Long-term Monitoring
Although RA is considered a disease of the joints, it is also a systemic disease capable of involving multiple organ systems. Extra-articular manifestations of RA are included in Table 4 . 1 , 2 , 10
Patients with RA have a twofold increased risk of lymphoma, which is thought to be caused by the underlying inflammatory process, and not a consequence of medical treatment. 39 Patients with RA are also at an increased risk of coronary artery disease, and physicians should work with patients to modify risk factors, such as smoking, high blood pressure, and high cholesterol. 40 , 41 Class III or IV congestive heart failure (CHF) is a contraindication for using TNF inhibitors, which can worsen CHF outcomes. 21 In patients with RA and malignancy, caution is needed with continued use of DMARDs, especially TNF inhibitors. Biologic DMARDs, methotrexate, and leflunomide should not be initiated in patients with active herpes zoster, significant fungal infection, or bacterial infection requiring antibiotics. 21 Complications of RA and its treatments are listed in Table 5 . 1 , 2 , 10
Patients with RA live three to 12 years less than the general population. 40 Increased mortality in these patients is mainly due to accelerated cardiovascular disease, especially in those with high disease activity and chronic inflammation. The relatively new biologic therapies may reverse progression of atherosclerosis and extend life in those with RA. 41
Data Sources: A PubMed search was completed in Clinical Queries using the key terms rheumatoid arthritis, extra-articular manifestations, and disease-modifying antirheumatic agents. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, Essential Evidence, and UpToDate. Search date: September 20, 2010.
Etiology and pathogenesis of rheumatoid arthritis. In: Firestein GS, Kelley WN, eds. Kelley's Textbook of Rheumatology . 8th ed. Philadelphia, Pa.: Saunders/Elsevier; 2009: 1035–1086.
Bathon J, Tehlirian C. Rheumatoid arthritis clinical and laboratory manifestations. In: Klippel JH, Stone JH, Crofford LJ, et al., eds. Primer on the Rheumatic Diseases . 13th ed. New York, NY: Springer; 2008: 114–121.
Allaire S, Wolfe F, Niu J, et al. Current risk factors for work disability associated with rheumatoid arthritis. Arthritis Rheum. 2009;61(3):321-328.
MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43(1):30-37.
Orozco G, Barton A. Update on the genetic risk factors for rheumatoid arthritis. Expert Rev Clin Immunol. 2010;6(1):61-75.
Balsa A, Cabezón A, Orozco G, et al. Influence of HLA DRB1 alleles in the susceptibility of rheumatoid arthritis and the regulation of antibodies against citrullinated proteins and rheumatoid factor. Arthritis Res Ther. 2010;12(2):R62.
McClure A, Lunt M, Eyre S, et al. Investigating the viability of genetic screening/testing for RA susceptibility using combinations of five confirmed risk loci. Rheumatology (Oxford). 2009;48(11):1369-1374.
Bang SY, Lee KH, Cho SK, et al. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum. 2010;62(2):369-377.
Wilder RL, Crofford LJ. Do infectious agents cause rheumatoid arthritis?. Clin Orthop Relat Res. 1991(265):36-41.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094-1108.
Costenbader KH, Feskanich D, Mandl LA, et al. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503.e1-e9.
Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294(21):2751-2757.
Guthrie KA, Dugowson CE, Voigt LF, et al. Does pregnancy provide vaccine-like protection against rheumatoid arthritis?. Arthritis Rheum. 2010;62(7):1842-1848.
Karlson EW, Mandl LA, Hankinson SE, et al. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses' Health Study. Arthritis Rheum. 2004;50(11):3458-3467.
Karlson EW, Shadick NA, Cook NR, et al. Vitamin E in the primary prevention of rheumatoid arthritis: the Women's Health Study. Arthritis Rheum. 2008;59(11):1589-1595.
Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative [published correction appears in Ann Rheum Dis . 2010;69(10):1892]. Ann Rheum Dis. 2010;69(9):1580-1588.
van der Helm-van Mil AH, le Cessie S, van Dongen H, et al. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis. Arthritis Rheum. 2007;56(2):433-440.
Mochan E, Ebell MH. Predicting rheumatoid arthritis risk in adults with undifferentiated arthritis. Am Fam Physician. 2008;77(10):1451-1453.
Ravelli A, Felici E, Magni-Manzoni S, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005;52(3):826-832.
Wilson A, Yu HT, Goodnough LT, et al. Prevalence and outcomes of anemia in rheumatoid arthritis. Am J Med. 2004;116(suppl 7A):50S-57S.
Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
Deighton C, O'Mahony R, Tosh J, et al.; Guideline Development Group. Management of rheumatoid arthritis: summary of NICE guidance. BMJ. 2009;338:b702.
AHRQ. Choosing medications for rheumatoid arthritis. April 9, 2008. http://www.effectivehealthcare.ahrq.gov/ehc/products/14/85/RheumArthritisClinicianGuide.pdf . Accessed June 23, 2011.
Choy EH, Smith C, Doré CJ, et al. A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology (Oxford). 2005;44(11):1414-1421.
Smedslund G, Byfuglien MG, Olsen SU, et al. Effectiveness and safety of dietary interventions for rheumatoid arthritis. J Am Diet Assoc. 2010;110(5):727-735.
Hagen KB, Byfuglien MG, Falzon L, et al. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst Rev. 2009;21(1):CD006400.
Wang C, de Pablo P, Chen X, et al. Acupuncture for pain relief in patients with rheumatoid arthritis: a systematic review. Arthritis Rheum. 2008;59(9):1249-1256.
Kelly RB. Acupuncture for pain. Am Fam Physician. 2009;80(5):481-484.
Robinson V, Brosseau L, Casimiro L, et al. Thermotherapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2002;2(2):CD002826.
Casimiro L, Brosseau L, Robinson V, et al. Therapeutic ultrasound for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev. 2002;3(3):CD003787.
Cameron M, Gagnier JJ, Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2011;2:CD002948.
Brodin N, Eurenius E, Jensen I, et al. Coaching patients with early rheumatoid arthritis to healthy physical activity. Arthritis Rheum. 2008;59(3):325-331.
Baillet A, Payraud E, Niderprim VA, et al. A dynamic exercise programme to improve patients' disability in rheumatoid arthritis: a prospective randomized controlled trial. Rheumatology (Oxford). 2009;48(4):410-415.
Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, et al. Dynamic Exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. 2009(4):CD006853.
Han A, Robinson V, Judd M, et al. Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2004;3:CD004849.
Evans S, Cousins L, Tsao JC, et al. A randomized controlled trial examining Iyengar yoga for young adults with rheumatoid arthritis. Trials. 2011;12:19.
Katchamart W, Johnson S, Lin HJ, et al. Predictors for remission in rheumatoid arthritis patients: a systematic review. Arthritis Care Res (Hoboken). 2010;62(8):1128-1143.
Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum. 1998;41(6):1072-1082.
Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692-701.
Friedewald VE, Ganz P, Kremer JM, et al. AJC editor's consensus: rheumatoid arthritis and atherosclerotic cardiovascular disease. Am J Cardiol. 2010;106(3):442-447.
Atzeni F, Turiel M, Caporali R, et al. The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases. Autoimmun Rev. 2010;9(12):835-839.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2011 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 February 2018
- Rheumatoid arthritis
- Josef S. Smolen 1 ,
- Daniel Aletaha 1 ,
- Anne Barton 2 ,
- Gerd R. Burmester 3 ,
- Paul Emery 4 , 5 ,
- Gary S. Firestein 6 ,
- Arthur Kavanaugh 6 ,
- Iain B. McInnes 7 ,
- Daniel H. Solomon 8 ,
- Vibeke Strand 9 &
- Kazuhiko Yamamoto 10
Nature Reviews Disease Primers volume 4 , Article number: 18001 ( 2018 ) Cite this article
60k Accesses
1366 Citations
271 Altmetric
Metrics details
- Autoimmunity
- Biological therapy
- Small molecules
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disease that primarily affects the joints and is associated with autoantibodies that target various molecules including modified self-epitopes. The identification of novel autoantibodies has improved diagnostic accuracy, and newly developed classification criteria facilitate the recognition and study of the disease early in its course. New clinical assessment tools are able to better characterize disease activity states, which are correlated with progression of damage and disability, and permit improved follow-up. In addition, better understanding of the pathogenesis of RA through recognition of key cells and cytokines has led to the development of targeted disease-modifying antirheumatic drugs. Altogether, the improved understanding of the pathogenetic processes involved, rational use of established drugs and development of new drugs and reliable assessment tools have drastically altered the lives of individuals with RA over the past 2 decades. Current strategies strive for early referral, early diagnosis and early start of effective therapy aimed at remission or, at the least, low disease activity, with rapid adaptation of treatment if this target is not reached. This treat-to-target approach prevents progression of joint damage and optimizes physical functioning, work and social participation. In this Primer, we discuss the epidemiology, pathophysiology, diagnosis and management of RA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
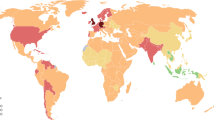
Global epidemiology of rheumatoid arthritis
Juvenile idiopathic arthritis
Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases
Malmstrom, V., Catrina, A. I. & Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol. 17 , 60–75 (2017).
PubMed Google Scholar
Myasoedova, E., Crowson, C. S., Kremers, H. M., Therneau, T. M. & Gabriel, S. E. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 62 , 1576–1582 (2010).
PubMed PubMed Central Google Scholar
Tobon, G. J., Youinou, P. & Saraux, A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J. Autoimmun 35 , 10–14 (2010).
Malemba, J. J. et al . The epidemiology of rheumatoid arthritis in Kinshasa, Democratic Republic of Congo—a population-based study. Rheumatology 51 , 1644–1647 (2012).
Peschken, C. A. & Esdaile, J. M. Rheumatic diseases in North America's indigenous peoples. Semin. Arthritis Rheum. 28 , 368–391 (1999).
CAS PubMed Google Scholar
Kawatkar, A. A., Portugal, C., Chu, L.-H. & Iyer, R. Racial/ethnic trends in incidence and prevalence of rheumatoid arthritis in a large multi-ethnic managed care population [abstract]. Arthritis Rheum. 64 , S1061 (2012).
Google Scholar
MacGregor, A. J. et al . Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 43 , 30–37 (2000).
Stahl, E. A. et al . Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat. Genet. 44 , 483–489 (2012).
CAS PubMed PubMed Central Google Scholar
Padyukov, L. et al . A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann. Rheum. Dis. 70 , 259–265 (2011).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30 , 1205–1213 (1987). This is the single most important paper on the genetics of RA, after the seminal work of Peter Stastny detecting the association between RA and HLA-DR4, and explains the differences in associations found between different populations by a short amino acid sequence common to all these molecules.
Weyand, C. M., Hicok, K. C., Conn, D. L. & Goronzy, J. J. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann. Intern. Med. 117 , 801–806 (1992).
Okada, Y. et al . Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506 , 376–381 (2014).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 , 661–678 (2007).
Stahl, E. A. et al . Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 42 , 508–514 (2010).
Raychaudhuri, S. et al . Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat. Genet. 41 , 1313–1318 (2009).
Eyre, S. et al . High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat. Genet. 44 , 1336–1340 (2012).
Begovich, A. B. et al . A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75 , 330–337 (2004).
Remmers, E. F. et al . STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357 , 977–986 (2007).
Thomson, W. et al . Rheumatoid arthritis association at 6q23. Nat. Genet. 39 , 1431–1433 (2007).
Barton, A. et al . Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat. Genet. 40 , 1156–1159 (2008).
Raychaudhuri, S. Recent advances in the genetics of rheumatoid arthritis. Curr. Opin. Rheumatol 22 , 109–118 (2010).
Karlson, E. W. et al . Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Ann. Rheum. Dis. 69 , 1077–1085 (2010).
Viatte, S. et al . Replication of associations of genetic loci outside the HLA region with susceptibility to anti-cyclic citrullinated peptide-negative rheumatoid arthritis. Arthritis Rheumatol. 68 , 1603–1613 (2016).
Viatte, S. et al . Genetic markers of rheumatoid arthritis susceptibility in anti-citrullinated peptide antibody negative patients. Ann. Rheum. Dis. 71 , 1984–1990 (2012).
Trynka, G. et al . Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat. Genet. 45 , 124–130 (2013).
Martin, P. et al . Capture Hi-C reveals novel candidate genes and complex long-range interactions with related autoimmune risk loci. Nat. Commun. 6 , 10069 (2015).
McGovern, A. et al . Capture Hi-C identifies a novel causal gene, IL20RA, in the pan-autoimmune genetic susceptibility region 6q23. Genome Biol. 17 , 212 (2016).
Viatte, S. et al . Association between genetic variation in FOXO3 and reductions in inflammation and disease activity in inflammatory polyarthritis. Arthritis Rheumatol. 68 , 2629–2636 (2016).
Krabben, A., Huizinga, T. W. & Mil, A. H. Biomarkers for radiographic progression in rheumatoid arthritis. Curr. Pharm. Des. 21 , 147–169 (2015).
Lee, J. C. et al . Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell 155 , 57–69 (2013).
Oliver, J., Plant, D., Webster, A. P. & Barton, A. Genetic and genomic markers of anti-TNF treatment response in rheumatoid arthritis. Biomark Med. 9 , 499–512 (2015).
Gomez-Cabrero, D. et al . High-specificity bioinformatics framework for epigenomic profiling of discordant twins reveals specific and shared markers for ACPA and ACPA-positive rheumatoid arthritis. Genome Med. 8 , 124 (2016).
Liu, Y. et al . Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat. Biotechnol. 31 , 142–147 (2013). This paper describes the importance of epigenetic modifications beyond genetic factors in the pathogenesis of RA.
Meng, W. et al . DNA methylation mediates genotype and smoking interaction in the development of anti-citrullinated peptide antibody-positive rheumatoid arthritis. Arthritis Res. Ther. 19 , 71 (2017).
Frank-Bertoncelj, M. et al . Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nat. Commun. 8 , 14852 (2017).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35 , 347–369 (2014).
Crowson, C. S. et al . The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 63 , 633–639 (2011).
Alpizar-Rodriguez, D., Pluchino, N., Canny, G., Gabay, C. & Finckh, A. The role of female hormonal factors in the development of rheumatoid arthritis. Rheumatology 56 , 1254–1263 (2017).
Alamanos, Y., Voulgari, P. V. & Drosos, A. A. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin. Arthritis Rheum. 36 , 182–188 (2006).
Sugiyama, D. et al . Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 69 , 70–81 (2010).
Vesperini, V. et al . Association of tobacco exposure and reduction of radiographic progression in early rheumatoid arthritis: results from a French multicenter cohort. Arthritis Care Res. 65 , 1899–1906 (2013).
CAS Google Scholar
Kallberg, H. et al . Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann. Rheum. Dis. 70 , 508–511 (2011).
Sokolove, J. et al . Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology 55 , 1969–1977 (2016).
Svendsen, A. J. et al . Differentially methylated DNA regions in monozygotic twin pairs discordant for rheumatoid arthritis: an epigenome-wide study. Front. Immunol. 7 , 510 (2016).
Jiang, X., Alfredsson, L., Klareskog, L. & Bengtsson, C. Smokeless tobacco (moist snuff) use and the risk of developing rheumatoid arthritis: results from a case-control study. Arthritis Care Res. 66 , 1582–1586 (2014).
Naranjo, A. et al . Smokers and non smokers with rheumatoid arthritis have similar clinical status: data from the multinational QUEST-RA database. Clin. Exp. Rheumatol. 28 , 820–827 (2010).
Stolt, P. et al . Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann. Rheum. Dis. 64 , 582–586 (2005).
Webber, M. P. et al . Nested case-control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis Rheumatol. 67 , 1369–1376 (2015).
Too, C. L. et al . Occupational exposure to textile dust increases the risk of rheumatoid arthritis: results from a Malaysian population-based case-control study. Ann. Rheum. Dis. 75 , 997–1002 (2016).
Hajishengallis, G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15 , 30–44 (2015).
Kharlamova, N. et al . Antibodies to Porphyromonas gingivalis indicate interaction between oral infection, smoking, and risk genes in rheumatoid arthritis etiology. Arthritis Rheumatol. 68 , 604–613 (2016).
Konig, M. F. et al . Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl Med. 8 , 369ra176 (2016).
Chen, J. et al . An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 8 , 43 (2016).
Scher, J. U. et al . Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2 , e01202 (2013).
Pianta, A. et al . Two rheumatoid arthritis-specific autoantigens correlate microbial immunity with autoimmune responses in joints. J. Clin. Invest. 127 , 2946–2956 (2017).
Naciute, M. et al . Frequency and significance of parvovirus B19 infection in patients with rheumatoid arthritis. J. Gen. Virol. 97 , 3302–3312 (2016).
Gasque, P., Bandjee, M. C., Reyes, M. M. & Viasus, D. Chikungunya pathogenesis: from the clinics to the bench. J. Infect. Dis. 214 (Suppl. 5), S446–S448 (2016).
Tan, E. M. & Smolen, J. S. Historical observations contributing insights on etiopathogenesis of rheumatoid arthritis and role of rheumatoid factor. J. Exp. Med. 213 , 1937–1950 (2016).
Ljung, L. & Rantapaa-Dahlqvist, S. Abdominal obesity, gender and the risk of rheumatoid arthritis — a nested case-control study. Arthritis Res. Ther. 18 , 277 (2016).
Lu, B., Solomon, D. H., Costenbader, K. H. & Karlson, E. W. Alcohol consumption and risk of incident rheumatoid arthritis in women: a prospective study. Arthritis Rheumatol. 66 , 1998–2005 (2014).
Lee, Y. C. et al . Post-traumatic stress disorder and risk for incident rheumatoid arthritis. Arthritis Care Res. 68 , 292–298 (2016).
Camacho, E. M., Verstappen, S. M. & Symmons, D. P. Association between socioeconomic status, learned helplessness, and disease outcome in patients with inflammatory polyarthritis. Arthritis Care Res. 64 , 1225–1232 (2012).
Radner, H., Lesperance, T., Accortt, N. A. & Solomon, D. H. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res. 69 , 1510–1518 (2017).
Lopez-Mejias, R. et al . Cardiovascular risk assessment in patients with rheumatoid arthritis: the relevance of clinical, genetic and serological markers. Autoimmun. Rev. 15 , 1013–1030 (2016).
Sparks, J. A. et al . Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the Nurses’ Health Study. Arthritis Care Res. 68 , 753–762 (2016).
Markusse, I. M. et al . Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann. Intern. Med. 164 , 523–531 (2016).
Masi, A. T. Articular patterns in the early course of rheumatoid arthritis. Am. J. Med. 75 , 16–26 (1983).
Makrygiannakis, D. et al . Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 67 , 1488–1492 (2008).
Dissick, A. et al . Association of periodontitis with rheumatoid arthritis: a pilot study. J. Periodontol. 81 , 223–230 (2010).
Holers, V. M. Autoimmunity to citrullinated proteins and the initiation of rheumatoid arthritis. Curr. Opin. Immunol. 25 , 728–735 (2013).
Muller, S. & Radic, M. Citrullinated autoantigens: from diagnostic markers to pathogenetic mechanisms. Clin. Rev. Allergy Immunol. 49 , 232–239 (2015).
Trouw, L. A., Huizinga, T. W. & Toes, R. E. Autoimmunity in rheumatoid arthritis: different antigens — common principles. Ann. Rheum. Dis. 72 (Suppl. 2), ii132–ii136 (2013).
Aletaha, D. et al . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69 , 1580–1588 (2010). This article explains that after almost 25 years, new RA classification criteria were developed in a data-driven, consensus process; these criteria also allow classification of patients with early RA who failed to be classified with the previous criteria.
Nielen, M. M. et al . Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50 , 380–386 (2004). After the seminal observations of Kimmo Aho on the presence of RF and anti-keratin antibodies (which later became known as ACPAs), this paper reveals that ACPA (and RF) precedes the clinical symptoms of RA by assessing sera from blood donors.
Aletaha, D., Alasti, F. & Smolen, J. S. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 17 , 229 (2015).
Laurent, L. et al . IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann. Rheum. Dis. 74 , 1425–1431 (2015).
Sokolove, J. et al . Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 66 , 813–821 (2014).
van Beers, J. J. et al . ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res. Ther. 15 , R140 (2013).
Deane, K. D. et al . The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 62 , 3161–3172 (2010).
de Hair, M. J. et al . Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 66 , 513–522 (2014).
Kraan, M. C. et al . Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 41 , 1481–1488 (1998).
Arend, W. P. & Firestein, G. S. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat. Rev. Rheumatol. 8 , 573–586 (2012).
Steiner, G. Auto-antibodies and autoreactive T-cells in rheumatoid arthritis: pathogenetic players and diagnostic tools. Clin. Rev. Allergy Immunol. 32 , 23–36 (2007).
Kinslow, J. D. et al . Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. Arthritis Rheumatol. 68 , 2372–2383 (2016).
Shi, J. et al . Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann. Rheum. Dis. 73 , 780–783 (2014).
Bohler, C., Radner, H., Smolen, J. S. & Aletaha, D. Serological changes in the course of traditional and biological disease modifying therapy of rheumatoid arthritis. Ann. Rheum. Dis. 72 , 241–244 (2013).
Klarenbeek, P. L. et al . Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 71 , 1088–1093 (2012).
Ai, R. et al . DNA methylome signature in synoviocytes from patients with early rheumatoid arthritis compared to synoviocytes from patients with longstanding rheumatoid arthritis. Arthritis Rheumatol. 67 , 1978–1980 (2015).
Castor, C. W. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 3 , 140–151 (1960).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365 , 2205–2219 (2011).
Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233 , 233–255 (2010).
Stanczyk, J. et al . Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 58 , 1001–1009 (2008).
Philippe, L. et al . MiR-20a regulates ASK1 expression and TLR4-dependent cytokine release in rheumatoid fibroblast-like synoviocytes. Ann. Rheum. Dis. 72 , 1071–1079 (2013).
Lefevre, S. et al . Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15 , 1414–1420 (2009).
Ziff, M. Relation of cellular infiltration of rheumatoid synovial membrane to its immune response. Arthritis Rheum. 17 , 313–319 (1974).
Humby, F. et al . Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLOS Med. 6 , e1 (2009).
Randen, I. et al . Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J. Immunol. 148 , 3296–3301 (1992).
Catrina, A. I., Ytterberg, A. J., Reynisdottir, G., Malmstrom, V. & Klareskog, L. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat. Rev. Rheumatol. 10 , 645–653 (2014).
Orr, C. et al . Synovial tissue research: a state-of-the-art review. Nat. Rev. Rheumatol 13 , 463–475 (2017).
Kiener, H. P. et al . Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 60 , 1305–1310 (2009).
Keyszer, G. et al . Differential expression of cathepsins B and L compared with matrix metalloproteinases and their respective inhibitors in rheumatoid arthritis and osteoarthritis: a parallel investigation by semiquantitative reverse transcriptase-polymerase chain reaction and immunohistochemistry. Arthritis Rheum. 41 , 1378–1387 (1998).
Bottini, N. & Firestein, G. S. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9 , 24–33 (2013).
Muller-Ladner, U. et al . Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149 , 1607–1615 (1996).
Tak, P. P., Zvaifler, N. J., Green, D. R. & Firestein, G. S. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol. Today 21 , 78–82 (2000).
Ai, R. et al . Joint-specific DNA methylation and transcriptome signatures in rheumatoid arthritis identify distinct pathogenic processes. Nat. Commun. 7 , 11849 (2016).
Schett, G. & Gravallese, E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 8 , 656–664 (2012).
Redlich, K. & Smolen, J. S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 11 , 234–250 (2012).
Harre, U. et al . Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122 , 1791–1802 (2012).
Krishnamurthy, A. et al . Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 75 , 721–729 (2016).
Hayer, S. et al . Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum. 56 , 79–88 (2007).
Nakano, S. et al . Immunoregulatory role of IL-35 in T cells of patients with rheumatoid arthritis. Rheumatology 54 , 1498–1506 (2015).
Behrens, F. et al . MOR103, a human monoclonal antibody to granulocyte–macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 74 , 1058–1064 (2015).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388 , 2023–2038 (2016).
Genovese, M. C. et al . Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374 , 1243–1252 (2016).
Boyle, D. L. et al . The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann. Rheum. Dis. 74 , 1311–1316 (2015).
Genovese, M. C. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 60 , 317–320 (2009).
Genovese, M. C. et al . A phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study of 2 dosing regimens of fostamatinib in patients with rheumatoid arthritis with an inadequate response to a tumor necrosis factor-alpha antagonist. J. Rheumatol. 41 , 2120–2128 (2014).
Firestein, G. S. The disease formerly known as rheumatoid arthritis. Arthritis Res. Ther. 16 , 114 (2014).
Smolen, J. S. et al . Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthritis Rheum. 38 , 38–43 (1995).
Koduri, G. et al . Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 49 , 1483–1489 (2010).
Bongartz, T. et al . Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 62 , 1583–1591 (2010).
Minichiello, E., Semerano, L. & Boissier, M. C. Time trends in the incidence, prevalence, and severity of rheumatoid arthritis: a systematic literature review. Joint Bone Spine 83 , 625–630 (2016).
Theander, L. et al . Severe extraarticular manifestations in a community-based cohort of patients with rheumatoid arthritis: risk factors and incidence in relation to treatment with tumor necrosis factor inhibitors. J. Rheumatol 44 , 981–987 (2017).
Aggarwal, R. et al . Distinctions between diagnostic and classification criteria? Arthritis Care Res. 67 , 891–897 (2015).
Radner, H., Neogi, T., Smolen, J. S. & Aletaha, D. Performance of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: a systematic literature review. Ann. Rheum. Dis. 73 , 114–123 (2014).
Hazlewood, G. et al . Algorithm for identification of undifferentiated peripheral inflammatory arthritis: a multinational collaboration through the 3e initiative. J. Rheumatol. Suppl. 87 , 54–58 (2011).
Kurko, J. et al . Genetics of rheumatoid arthritis — a comprehensive review. Clin. Rev. Allergy Immunol. 45 , 170–179 (2013).
Aletaha, D. & Smolen, J. S. Joint damage in rheumatoid arthritis progresses in remission according to the Disease Activity Score in 28 joints and is driven by residual swollen joints. Arthritis Rheum. 63 , 3702–3711 (2011).
Gormley, G. J. et al . Can diagnostic triage by general practitioners or rheumatology nurses improve the positive predictive value of referrals to early arthritis clinics? Rheumatology 42 , 763–768 (2003).
Villeneuve, E. et al . A systematic literature review of strategies promoting early referral and reducing delays in the diagnosis and management of inflammatory arthritis. Ann. Rheum. Dis. 72 , 13–22 (2013).
de Rooy, D. P., van der Linden, M. P., Knevel, R., Huizinga, T. W. & van der Helm-van Mil, A. H. Predicting arthritis outcomes — what can be learned from the Leiden Early Arthritis Clinic? Rheumatology 50 , 93–100 (2011).
van der Helm- van Mil, A. H. et al . A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 56 , 433–440 (2007).
van Aken, J. et al . Five-year outcomes of probable rheumatoid arthritis treated with methotrexate or placebo during the first year (the PROMPT study). Ann. Rheum. Dis. 73 , 396–400 (2014).
Weinblatt, M. E. Efficacy of methotrexate in rheumatoid arthritis. Br. J. Rheumatol 34 (Suppl. 2), 43–48 (1995).
Visser, K. & van der Heijde, D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann. Rheum. Dis. 68 , 1094–1099 (2009).
van Ede, A. E. et al . Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 44 , 1515–1524 (2001). This study changed the approach to methotrexate therapy, showing that folate substitution does not reduce the efficacy but highly improves the tolerability of methotrexate, thus allowing optimal dosing of the drug.
Felson, D. T. et al . American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 38 , 727–735 (1995).
van der Heijde, D. M. et al . Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann. Rheum. Dis. 51 , 177–181 (1992). This article presents the first definition of a continuous measure for disease activity; its derivative, DAS28, became more widely used because it is less time-consuming to evaluate and further simplifications of composite measures were developed with CDAI and SDAI.
Smolen, J. S. et al . A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 42 , 244–257 (2003).
van der Heide, A. et al . The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann. Intern. Med. 124 , 699–707 (1996). This is one of the first studies to reveal the importance of the early institution of DMARD therapy and thus the inappropriateness of the pyramid approach, although patients still had relatively long-standing disease.
Huizinga, W. J., Machold, K. P., Breedveld, F. C., Lipsky, P. E. & Smolen, J. S. Criteria for early rheumatoid arthritis: From Bayes’ law revisited to new thoughts on pathogenesis. (Conference summary). Arthritis Rheum. 46 , 1155–1159 (2002).
Nell, V. et al . Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology 43 , 906–914 (2004).
McCarty, D. J. Suppress rheumatoid inflammation early and leave the pyramid to the Egyptians. J. Rheumatol 17 , 1117–1118 (1990).
Grigor, C. et al . Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364 , 263–269 (2004).
Aletaha, D. et al . Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 52 , 2625–2636 (2005).
Felson, D. T. et al . American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann. Rheum. Dis. 70 , 404–413 (2011). This paper provides a new definition for remission that is sufficiently stringent to be associated with lack of considerable residual disease activity, functional impairment and progression of damage.
Smolen, J. S. et al . Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann. Rheum. Dis. 75 , 3–15 (2016). This study defines the pathways to optimizing disease control in RA on the basis of available evidence and consensus finding,
Elliott, M. J. et al . Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 344 , 1105–1110 (1994). This is the first controlled study showing the efficacy of a biological agent in RA — namely, the anti-TNF antibody infliximab.
Maini, R. N. et al . Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 41 , 1552–1563 (1998).
Mierau, M. et al . Assessing remission in clinical practice. Rheumatology 46 , 975–979 (2007).
Verstappen, S. M. M. et al . Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann. Rheum. Dis. 66 , 1443–1449 (2007).
Klarenbeek, N. B. et al . The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann. Rheum. Dis. 70 , 1039–1046 (2011).
Smolen, J. S. et al . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 76 , 960–977 (2017).
Singh, J. A. et al . 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 68 , 1–25 (2016).
Lau, C. S. et al . APLAR rheumatoid arthritis treatment recommendations. Int. J. Rheum. Dis. 18 , 685–713 (2015).
Smolen, J. S., Aletaha, D., Grisar, J. C., Stamm, T. A. & Sharp, J. T. Estimation of a numerical value for joint damage-related physical disability in rheumatoid arthritis clinical trials. Ann. Rheum. Dis. 69 , 1058–1064 (2010).
Aletaha, D., Smolen, J. & Ward, M. M. Measuring function in rheumatoid arthritis: identifying reversible and irreversible components. Arthritis Rheum. 54 , 2784–2792 (2006).
Aletaha, D., Alasti, F. & Smolen, J. S. Optimisation of a treat-to-target approach in rheumatoid arthritis: strategies for the 3-month time point. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2015-208324 (2015).
Haraoui, B. et al . Treating rheumatoid arthritis to target: a Canadian physician survey. J. Rheumatol. 39 , 949–953 (2012).
Pascual-Ramos, V., Contreras-Yanez, I., Villa, A. R., Cabiedes, J. & Rull-Gabayet, M. Medication persistence over 2 years of follow-up in a cohort of early rheumatoid arthritis patients: associated factors and relationship with disease activity and with disability. Arthritis Res. Ther. 11 , R26 (2009).
Schoenthaler, A. M., Schwartz, B. S., Wood, C. & Stewart, W. F. Patient and physician factors associated with adherence to diabetes medications. Diabetes Educ. 38 , 397–408 (2012).
Kuusalo, L. et al . Impact of physicians’ adherence to treat-to-target strategy on outcomes in early rheumatoid arthritis in the NEO-RACo trial. Scand. J. Rheumatol 44 , 449–455 (2015).
Solomon, D. H. et al . Implementation of treat to target in rheumatoid arthritis through a learning collaborative: results of the TRACTION trial. Arthritis Rheumatol. (in press).
Smolen, J. S. & Aletaha, D. Forget personalised medicine and focus on abating disease activity. Ann. Rheum. Dis. 72 , 3–6 (2013).
Van der Heijde, D. M.F. M., van't Hof, M., van Riel, P. L. & van de Putte, L. B. A. Development of a disease activity score based on judgement in clinical practice by rheumatologists. J. Rheumatol 20 , 579–581 (1993).
Prevoo, M. L. L., van't Hof, M. A., Kuper, H. H., van de Putte, L. B. A. & van Riel, P. L.C. M. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38 , 44–48 (1995).
Bakker, M. F., Jacobs, J. W., Verstappen, S. M. & Bijlsma, J. W. Tight control in the treatment of rheumatoid arthritis: efficacy and feasibility. Ann. Rheum. Dis. 66 (Suppl. 3), iii56–iii60 (2007).
Smolen, J. S. & Aletaha, D. The assessment of disease activity in rheumatoid arthritis. Clin. Exp. Rheumatol. 28 (Suppl. 59), S18–S27 (2010).
Smolen, J. S. et al . Brief Report: Remission rates with tofacitinib treatment in rheumatoid arthritis: a comparison of various remission criteria. Arthritis Rheumatol. 69 , 728–734 (2017).
Smolen, J. S. & Aletaha, D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 63 , 43–52 (2011).
Emery, P. et al . IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 67 , 1516–1523 (2008).
Schoels, M., Alasti, F., Smolen, J. S. & Aletaha, D. Evaluation of newly proposed remission cut-points for disease activity score in 28 joints (DAS28) in rheumatoid arthritis patients upon IL-6 pathway inhibition. Arthritis Res. Ther. 19 , 155 (2017).
Studenic, P., Smolen, J. S. & Aletaha, D. Near misses of ACR/EULAR criteria for remission: effects of patient global assessment in Boolean and index-based definitions. Ann. Rheum. Dis. 71 , 1702–1705 (2012).
Aletaha, D., Martinez-Avila, J., Kvien, T. K. & Smolen, J. S. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann. Rheum. Dis. 71 , 1190–1196 (2012).
Dorner, T. et al . The changing landscape of biosimilars in rheumatology. Ann. Rheum. Dis. 75 , 974–982 (2016).
Schneider, C. K. Biosimilars in rheumatology: the wind of change. Ann. Rheum. Dis. 72 , 315–318 (2013).
van der Goes, M. C. et al . Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann. Rheum. Dis. 69 , 1913–1919 (2010).
Verschueren, P. et al . Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate-to-high-dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: the CareRA trial. Ann. Rheum. Dis. 74 , 27–34 (2015).
de Jong, P. H. et al . Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann. Rheum. Dis. 73 , 1331–1339 (2014).
Nam, J. L. et al . Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann. Rheum. Dis. 73 , 75–85 (2014). This is an important study showing that methotrexate plus glucocorticoids is not inferior to methotrexate plus anti-TNF in early RA, thus refuting the use of biological agents before methotrexate.
LaRochelle, G. E. Jr, LaRochelle, A. G., Ratner, R. E. & Borenstein, D. G. Recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients with rheumatic diseases receiving low-dose prednisone. Am. J. Med. 95 , 258–264 (1993).
Pincus, T., Sokka, T., Castrejon, I. & Cutolo, M. Decline of mean initial prednisone dosage from 3 to 3.6 mg/day to treat rheumatoid arthritis between 1980 and 2004 in one clinical setting, with long-term effectiveness of dosages less than 5 mg/day. Arthritis Care Res. 65 , 729–736 (2013).
del Rincón, I., Battafarano, D. F., Restrepo, J. F., Erikson, J. M. & Escalante, A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 66 , 264–272 (2014).
Smolen, J. S. et al . Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate without or with concomitant infliximab. Results from the ASPIRE trial. Arthritis Rheum. 54 , 702–710 (2006).
Kiely, P., Walsh, D., Williams, R. & Young, A. Outcome in rheumatoid arthritis patients with continued conventional therapy for moderate disease activity—the early RA network (ERAN). Rheumatology 50 , 926–931 (2011).
van der Lubbe, P. A., Dijkmans, B. S., Markusse, H., Nassander, U. & Breedveld, F. C. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis [abstract]. Arthritis Rheum. 38 , 1097–1106 (1995).
Blanco, F. J. et al . Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 69 , 1144–1153 (2017).
Bonelli, M. et al . Abatacept (CTLA-4IG) treatment reduces the migratory capacity of monocytes in patients with rheumatoid arthritis. Arthritis Rheum. 65 , 599–607 (2013).
Buch, M. H. et al . Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 70 , 909–920 (2011).
Nam, J. L. et al . Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 76 , 1113–1136 (2017).
Chatzidionysiou, K. et al . Efficacy of glucocorticoids, conventional and targeted synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 76 , 1102–1107 (2017).
Fleischmann, R. et al . Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69 , 506–517 (2017).
Fleischmann, R. et al . Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390 , 457–468 (2017).
Weinblatt, M. E. et al . Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 65 , 28–38 (2013).
Porter, D. et al . Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet 388 , 239–247 (2016).
Smolen, J. S. et al . Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet 388 , 2763–2774 (2016).
Taylor, P. C. et al . Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376 , 652–662 (2017). This is the first study to show any drug being superior to an anti-TNF in RA, here using baricitinib, an oral (small-molecule) DMARD that inhibits JNK1 and JNK2 (to learn more about the clinical implications of these data, more studies are needed).
Smolen, J. S. et al . Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 383 , 321–332 (2014).
Kavanaugh, A. et al . Testing treat-to-target outcomes with initial methotrexate monotherapy compared with initial tumour necrosis factor inhibitor (adalimumab) plus methotrexate in early rheumatoid arthritis. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2017-211871 (2017).
Kavanaugh, A. et al . Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann. Rheum. Dis. 72 , 64–71 (2013).
Quinn, M. A. et al . Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 52 , 27–35 (2005).
Stamm, T. et al . Induction of sustained remission in ealry inflammatory arthritis with the combination of infliximab plus methotrexate, methotrexate alone or placebo: the DINORA trial [abstract]. Ann. Rheum. Dis. 76 (Suppl. 2), 560 (2017).
Emery, P. et al . Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann. Rheum. Dis. 74 , 979–984 (2015).
Holloway, K. & van Dijk, L. The World Medicines Situation 2011, Rational Use of Medicines 3rd edn (World Health Organzization, Geneva, 2011).
Jorgensen, K. K. et al . Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 389 , 2304–2316 (2017).
Kay, J. et al . Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2017-211937 (2017).
Scheiman, J. M. NSAID-induced gastrointestinal injury: a focused update for clinicians. J. Clin. Gastroenterol. 50 , 5–10 (2016).
Nissen, S. E. et al . Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 375 , 2519–2529 (2016).
Strehl, C. et al . Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann. Rheum. Dis. 75 , 952–957 (2016).
Burmester, G. R. et al . Adalimumab long-term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 76 , 414–417 (2017).
DRFZ. RABBIT Risk Score of Infections. RABBIT http://www.biologika-register.de/en/home/risk-score/ (2017).
Strangfeld, A. et al . Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res. Ther. 12 , R5 (2010).
Burmester, G. R., Panaccione, R., Gordon, K. B., McIlraith, M. J. & Lacerda, A. P. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann. Rheum. Dis. 72 , 517–524 (2013).
Strangfeld, A. et al . Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann. Rheum. Dis. 76 , 504–510 (2017).
Molloy, E. S., Calabrese, C. M. & Calabrese, L. H. The risk of progressive multifocal leukoencephalopathy in the biologic era: prevention and management. Rheum. Dis. Clin. North Am. 43 , 95–109 (2017).
Winthrop, K. L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol 13 , 234–243 (2017).
Kubo, S., Nakayamada, S. & Tanaka, Y. Baricitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Immunol. 12 , 911–919 (2016).
Strand, V. et al . Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann. Rheum. Dis. 68 , 1800–1804 (2009).
Matcham, F. et al . The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin. Arthritis Rheum. 44 , 123–130 (2014).
Hewlett, S. et al . Patients’ perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis Rheum. 53 , 697–702 (2005).
West, E. & Jonsson, S. W. Health-related quality of life in rheumatoid arthritis in Northern Sweden: a comparison between patients with early RA, patients with medium-term disease and controls, using SF-36. Clin. Rheumatol 24 , 117–122 (2005).
Buitinga, L. Valuation of quality of life in rheumatoid arthritis. Thesis, Univ. Twente (2012).
Abu al Fadl, E. M., Ismail, M. A., Thabit, M. & El-Serogy, Y. Assessment of health-related quality of life, anxiety, and depression in patients with early rheumatoid arthritis. Egyptian Rheumatol. 36 , 51–56 (2014).
Murillo, Y. A., Almagro, R. M., Campos-Gonzales, I. D. & Cardiel, M. H. Health related quality of life in rheumatoid arthritis, osteoarthritis, diabetes mellitus, end stage renal disease and geriatric subjects. Rheumatol. Clin. 11 , 68–72 (2017).
Shokri, A., Mottaghi, P. & Qolipour, K. Quality of life and its predictors among Iranian patients with rheumatoid arthritis: a systematic review. J. Health Res. 6 , e24636 (2015).
Kwan, Y. H., Koh, E. T., Leong, K. P. & Wee, H. L. Association between helplessness, disability, and disease activity with health-related quality of life among rheumatoid arthritis patients in a multiethnic Asian population. Rheumatol. Int. 34 , 1085–1093 (2014).
Scott, I. C., Ibrahim, F., Lewis, C. M., Scott, D. L. & Strand, V. Impact of intensive treatment and remission on health-related quality of life in early and established rheumatoid arthritis. RMD Open 2 , e000270 (2016).
Gerhold, K. et al . Health-related quality of life in patients with long-standing rheumatoid arthritis in the era of biologics: data from the German biologics register RABBIT. Rheumatology 54 , 1858–1866 (2015).
Strand, V. & Singh, J. in Targeted Treatment of the Rheumatic Diseases 1st edn (ed. Weisman, M. ) (Elsevier, Philadelphia, PA, 2009).
Strand, V. & Singh, J. A. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs 70 , 121–145 (2010).
Chen, J. S., Makovey, J., Lassere, M., Buchbinder, R. & March, L. M. Comparative effectiveness of anti-tumor necrosis factor drugs on health-related quality of life among patients with inflammatory arthritis. Arthritis Care Res. 66 , 464–472 (2014).
Strand, V. & Khanna, D. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin. Exp. Rheumatol. 28 (Suppl. 59), S32–S40 (2010).
Strand, V. et al . It's good to feel better but it's better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J. Rheumatol. 38 , 1720–1727 (2011).
Wolfe, F., Michaud, K. & Strand, V. Expanding the definition of clinical differences: from minimally clinically important differences to really important differences. Analyses in 8931 patients with rheumatoid arthritis. J. Rheumatol. 32 , 583–589 (2005).
Kosinsk, M., Zhao, S. Z., Dedhiya, S., Osterhaus, J. T. & Ware, J. E. Jr. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 43 , 1478–1487 (2000).
Ward, M. M., Guthrie, L. C. & Alba, M. I. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res. 66 , 1783–1789 (2014).
Gossec, L. et al . Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann. Rheum. Dis. 70 , 935–942 (2011).
Dougados, M. et al . Defining cut-off values for disease activity states and improvement scores for patient-reported outcomes: the example of the Rheumatoid Arthritis Impact of Disease (RAID). Arthritis Res. Ther. 14 , R129 (2012).
Pincus, T., Richardson, B., Strand, V. & Bergman, M. J. Relative efficiencies of the 7 rheumatoid arthritis Core Data Set measures to distinguish active from control treatments in 9 comparisons from clinical trials of 5 agents. Clin. Exp. Rheumatol. 32 (Suppl. 85), 47–54 (2014).
Taylor, P. C., Moore, A., Vasilescu, R., Alvir, J. & Tarallo, M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol. Int. 36 , 685–695 (2016).
Franke, L. C., Ament, A. J., van de Laar, M. A., Boonen, A. & Severens, J. L. Cost-of-illness of rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 27 (Suppl. 55), S118–S123 (2009).
Lundkvist, J., Kastäng, F. & Kobelt, G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur. J. Health Econ. 8 (Suppl. 2), S49–S60 (2008).
ter Wee, M. M., Lems, W. F., Usan, H., Gulpen, A. & Boonen, A. The effect of biological agents on work participation in rheumatoid arthritis patients: a systematic review. Ann. Rheum. Dis. 71 , 161–171 (2012).
Bansback, N. et al . Triple therapy versus biologic therapy for active rheumatoid arthritis: a cost-effectiveness analysis. Ann. Intern. Med. 167 , 8–16 (2017).
Dorner, T. et al . The role of biosimilars in the treatment of rheumatic diseases. Ann. Rheum. Dis. 72 , 322–328 (2013).
Smolen, J. S., van der Heijde, D., Machold, K. P., Aletaha, D. & Landewe, R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 73 , 3–5 (2014).
Her, M. & Kavanaugh, A. Critical analysis of economic tools and economic measurement applied to rheumatoid arthritis. Clin. Exp. Rheumatol 30 (Suppl. 73), S107–S111 (2012).
Smolen, J. S. & Steiner, G. Therapeutic strategies for rheumatoid arthritis. Nat. Rev. Drug Discov. 2 , 473–488 (2003).
Gartner, M. et al . Immediate access rheumatology clinic: efficiency and outcomes. Ann. Rheum. Dis. 71 , 363–368 (2012).
Puchner, R. et al . Efficacy and outcome of Rapid Access Rheumatology Consultation: an office-based pilot cohort study. J. Rheumatol. 43 , 1130–1135 (2016).
Smolen, J. S. & Aletaha, D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat. Rev. Rheumatol. 11 , 276–289 (2015).
Deane, K. D. et al . Identification of undiagnosed inflammatory arthritis in a community health fair screen. Arthritis Rheum. 61 , 1642–1649 (2009).
Sparks, J. A. et al . Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study: rationale and design for a randomized controlled trial evaluating rheumatoid arthritis risk education to first-degree relatives. Contemp. Clin. Trials 39 , 145–157 (2014).
Sparks, J. A. et al . Disclosure of personalized rheumatoid arthritis risk using genetics, biomarkers, and lifestyle factors to motivate health behavior improvements: a randomized controlled trial. Arthritis Care Res. https://doi.org/10.1002/acr.23411 (2017).
van Dongen, H. et al . Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 56 , 1424–1432 (2007).
Gerlag, D. M. et al . A single infusion of rituximab delays the onset of arthritis in subjects at high risk of developing RA [abstract]. Arthritis Rheumatol. 68 (Suppl 10), 3028 (2016).
Emery, P. et al . Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann. Rheum. Dis. 69 , 510–516 (2010).
Arnett, F. C. et al . The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31 , 315–324 (1988).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02603146 (2017).
Braun, J. & Rau, R. An update on methotrexate. Curr. Opin. Rheumatol. 21 , 216–223 (2009).
Keen, H. I., Conaghan, P. G. & Tett, S. E. Safety evaluation of leflunomide in rheumatoid arthritis. Expert Opin. Drug Saf. 12 , 581–588 (2013).
Download references
Author information
Authors and affiliations.
Division of Rheumatology, Department of Medicine 3, Medical University of Vienna, Waehringer Guertel 18–20, Vienna, 1090, Austria
Josef S. Smolen & Daniel Aletaha
Arthritis Research UK Centre for Genetics and Genomics and NIHR Manchester Biomedical Research Centre, Manchester Academic Health Sciences Centre, The University of Manchester and Central Manchester Foundation Trust, Manchester, UK
Anne Barton
Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Berlin, Germany
Gerd R. Burmester
Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Chapel Allerton Hospital, Leeds, UK
NIHR Leeds Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, UK
Division of Rheumatology, Allergy and Immunology, University of California–San Diego School of Medicine, La Jolla, CA, USA
Gary S. Firestein & Arthur Kavanaugh
Institute of Infection Immunity and Inflammation, University of Glasgow, Glasgow, UK
Iain B. McInnes
Division of Rheumatology, Brigham and Women's Hospital, Boston, MA, USA
Daniel H. Solomon
Division of Immunology and Rheumatology, Stanford University, Palo Alto, CA, USA
Vibeke Strand
Laboratory for Autoimmune Diseases, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan
Kazuhiko Yamamoto
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (J.S.S.); Epidemiology (K.Y. and A.B.); Mechanisms/pathophysiology (I.B.M. and G.S.F.); Diagnosis, screening and prevention (D.A. and D.H.S.); Management (J.S.S., P.E. and G.R.B.); Quality of life (V.S. and A.K.); Outlook (J.S.S. and I.B.M.); Overview of Primer (J.S.S.).
Corresponding author
Correspondence to Josef S. Smolen .
Ethics declarations
Competing interests.
J.S.S. has received grant support from and/or provided expert advice to AbbVie, Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Celgene, Celltrion, Gilead, Glaxo, ILTOO, Janssen, Lilly, Pfizer, MSD, Roche, Samsung, Novartis-Sandoz and UCB. D.A. served as a consultant and/or speaker for AbbVie, AstraZeneca, BMS, Janssen, Medac, MSD, Pfizer, Roche and UCB and received grant support from BMS. A.B. received grants, speaker fees and/or consultancy fees from Pfizer, Eli Lilly, Janssen, Celgene, Roche-Chugai and Boehringer-Ingelheim. G.R.B. received honoraria for consulting and lectures from AbbVie, BMS, MSD, Pfizer, UCB and Roche. P.E. has undertaken clinical trials and provided expert advice to Pfizer, MSD, AbbVie, BMS, UCB, Roche, Novartis, Samsung, Sandoz and Eli Lilly. G.S.F. has received grant funding from Janssen and Gilead. A.K. has served as a consultant and/or performed clinical research for AbbVie, Amgen, Celgene, Janssen, Novartis and UCB. I.B.M. has received grants, speaker fees and/or consultancy fees from BMS, AbbVie, Pfizer, Eli Lilly, GSK, Janssen, Novartis, Celgene, Roche-Chugai, UCB and Boehringer-Ingelheim. D.H.S. serves in unpaid roles on a clinical trial sponsored by Pfizer. V.S. has served as a consultant to AbbVie, Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Celltrion, Genentech/Roche, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sanofi and UCB and is a founding member of the executive of OMERACT (Outcome Measures in Rheumatology; 1992–present), an organization that develops and validates outcome measures in rheumatology randomized controlled trials and longitudinal observational studies and receives arm's-length funding from 36 sponsors. K.Y. received honoraria for consulting and lectures from AbbVie, AYUMI, BMS, Chugai, Eisai, Janssen, Ono, Pfizer, Tanabe-Mitsubishi and UCB.

PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, powerpoint slide for fig. 8, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Smolen, J., Aletaha, D., Barton, A. et al. Rheumatoid arthritis. Nat Rev Dis Primers 4 , 18001 (2018). https://doi.org/10.1038/nrdp.2018.1
Download citation
Published : 08 February 2018
DOI : https://doi.org/10.1038/nrdp.2018.1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Synovium microenvironment-responsive injectable hydrogel inducing modulation of macrophages and elimination of synovial fibroblasts for enhanced treatment of rheumatoid arthritis.
Journal of Nanobiotechnology (2024)
Development of a complex Interdisciplinary Nurse-coordinated SELf-MAnagement (INSELMA) intervention for patients with inflammatory arthritis
- Jette Primdahl
- Ann Bremander
- Bente Appel Esbensen
BMC Health Services Research (2024)
Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets
- Yuexuan Liu
Molecular Medicine (2024)
The effects of intermittent fasting diet on quality of life, clinical symptoms, inflammation, and oxidative stress in overweight and obese postmenopausal women with rheumatoid arthritis: study protocol of a randomized controlled trial
- Mahsa Ranjbar
- Sakineh Shab-Bidar
- Kurosh Djafarian
Trials (2024)
Causality between rheumatoid arthritis and the risk of cognitive impairment: a Mendelian randomization study
- Lincheng Duan
Arthritis Research & Therapy (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to secondary sidebar
- Skip to footer
Johns Hopkins Arthritis Center
RA Pathophysiology
Note: More up to date information regarding RA pathogenesis may be found in lectures given by the author on this website.
Immune Mediated Inflammatory Disease
Histopathology, disease initiation, propagation of disease, inflammatory mediators in ra.
In the last decade we have significantly increased our knowledge of the underlying pathobiology of rheumatoid arthritis. The introduction of targeted biological therapy has provided experiential rather than experimental evidence that multiple different immunological and inflammatory pathways are operative. Each year there are descriptions of new cytokines, mediators, and pathways that show additional promise in unraveling the complex pathobiological pathways.
Rheumatoid arthritis is best characterized as an immune mediated inflammatory disease (IMID). Within a framework that recognizes both immunological activation and inflammatory pathways, we can begin to evaluate the multiple components of disease initiation and propagation. This framework highlights that once initiated and even after a putative trigger may be eliminated, there are feed forward pathways that result in an auto-perpetuating process.
The synovium, in normal joints, is a thin delicate lining that serves several important functions. The synovium serves as an important source of nutrients for cartilage since cartilage itself is avascular. In addition, synovial cells synthesize joint lubricants such as hyaluronic acid, as well as collagens and fibronectin that constitute the structural framework of the synovial interstitium.
1. Synovial lining or intimal layer: Normally, this layer is only 1-3 cells thick. In RA, this lining is greatly hypertrophied (8-10 cells thick). Primary cell populations in this layer are fibroblasts and macrophages.
2. Subintimal area of synovium: This is where the synovial blood vessels are located; this area normally has very few cells. In RA, however, the subintimal area is heavily infiltrated with inflammatory cells, including T and B lymphocytes, macrophages, mast cells, and mononuclear cells that differentiate into multinucleated osteoclasts. The intense cellular infiltrate is accompanied by new blood vessel growth (angiogenesis). In RA, the hypertrophied synovium (also called pannus) invades and erodes contiguous cartilage and bone. As such, it can be thought of as a tumor-like tissue, although mitotic figures are rare and, of course, metastasis does not occur.
Composed primarily of type II collagen and proteoglycans, this is normally a very resilient tissue that absorbs considerable impact and stress. In RA, its integrity, resilience and water content are all impaired. This appears to be due to elaboration of proteolytic enzymes (collagenase, stromelysin) both by synovial lining cells and by chondrocytes themselves. Cytokines including IL1 and TNF drive the generation of reactive oxygen and nitrogen species and while increasing chondrocyte catabolic pathways and matrix destruction, also inhibit new cartilage formation. Polymorphonuclear leukocytes in the synovial fluid may also contribute to this degradative process.
Composed primarily of type I collagen, bony destruction is a characteristic of RA. This process is primarily driven by the activation of osteoclasts. Osteoclasts differentiate under the influence of cytokines especially the interaction of RANK with its ligand. The expression of these are driven by cytokines including TNF and IL1, as well as other cytokines including IL-17. There may also be a contribution to bony destruction from mediators derived from activated synovial cells.
Synovial Cavity
The synovial cavity is normally only a “potential” space with 1-2ml of highly viscous (due to hyaluronic acid) fluid with few cells. In RA, large collections of fluid (“effusions”) occur which are, in effect, filtrates of plasma (and, therefore, exudative – i.e., high protein content). The synovial fluid is highly inflammatory. However, unlike the rheumatoid synovial tissue in which the infiltrating cells are lymphocytes and macrophages but not neutrophils, in synovial fluid the predominant cell is the neutrophil.
The search for an elusive single trigger for RA has been ongoing for many years. Multiple studies have failed to conclusively demonstrate that any organism or exposure is singly responsible for the disease. However, a number of well done epidemiological studies and genetic studies have provided valuable information to inform our genera, albeit still incomplete, understanding of the dynamic process of disease initiation.
Genetic Susceptibilities
In the early 1980’s an association was described for the association of RA with class II major histocompatability (MHC) antigens, specifically the shared epitope found in HLA-DR4. Class II MHC on the surface of an antigen presenting cell interacts with a T cell receptor in the context of a specific antigen, usually a small peptide sequence from a protein. A sequence of amino acid residues with highly conserved sequence and charge characteristics within the hypervariable region of HLA-DR4 remains the largest genetic risk factor described for RA, estimated to contribute approximately 30% of the genetic risk for the disease. It is hypothesized that a triggering peptide (or peptides) with a tight conformational fit for the pocket formed by these residues is an early event leading to the activation of T lymphocytes. More recently, it has been found that modified citrullinated peptides may have significant binding specificity for shared epitope alleles, with some data now suggesting that citrullinated sequences from different proteins are associated with allelic restriction. (A more detailed discussion of citrullination is below).
Other genetic susceptibilities have been described in RA, but their relative contributions to the disease are still not well defined. These include peptidyl arginine deiminase-4 (PAD-4) which may lead to increased citrullination, PTNP22, STAT4, and CTLA4 which may be involved in T cell activation, TNF receptors, and others.
That RA has a genetic component is also borne out through a number of studies of monozygotic (from the same embryo, thus nearly identical DNA) and dizygotic (from different embryos) twins. In these studies the concordance rates between twins was higher in monozygotic twins ranging from 15-35% compared with dizygotic twins in which the concordance was in the 5% range. Even the dizygotic RA prevalence was higher than the general population estimates of approximately 1%. It is important to emphasize however that even in twins with nearly identical DNA, there was far from perfect correlation of the development of RA, implicating many other factors related to the development of disease than genetic factors.
Triggers of Disease
The fact that there is not perfect genetic concordance implicates other factors in disease development. A search for these elusive triggers has been largely unrevealing. A number of well performed studies have demonstrated that cigarette smoking is a significant risk factor for the development of disease and also with disease severity. Interestingly this relationship is especially strong in individuals who carry the shared epitope, and even more in patients who have RA autoantibodies.
The search for bacterial or viral infections as causes of RA have often been hypothesized, and many patients will relate the onset of their symptoms to an antecedent infection; however, the recovery of organisms or their DNA from blood or joint tissue have been unfruitful in discovering “the” elusive infection responsible for RA. Nonetheless, the ability of an infection to activate a number of immunological and inflammatory pathways may “prime the pump” in combination with other factors.
Perhaps the most exciting developments in the last few years in terms of RA initiation has been the growing research to evaluate the possible role of oral bacteria as a trigger for RA. There has been a longstanding association described between periodontal disease with RA, however cause and effect has been far from proven. Periodontal disease is characterized by significant inflammation of the gums that leads to bone destruction and collagen matrix destruction. Both are inflammatory diseases with many of the same mediators and pathways involved, thus this could simply be an association between two inflammatory processes. However, it is now recognized that a specific species of bacteria, Porphyromonas gingivalis , which colonizes patients with periodontal disease and marks the progression from gingivitis to more aggressive periodontitis has an enzyme that can cause citrullination of proteins. With the growing recognition that protein citrullination is an early event leading to an immune response against these in RA, these data suggest that periodontal infection may precede the development of RA in some patients serving as a disease initiation factor. A number of groups worldwide, including our own, are now investigating these pathways to better understand these processes.
Citrullination
The recognition of antibodies directed against citrullinated peptides in RA has been a major development to improve disease identification and provide prognostic information. Citrulline is a post-translational modification that occurs on arginine residues contained within proteins and peptides. There are a number of enzymes that can cause citrullination to occur, present in various cell types and tissues known as peptidylarginine deiminases (PADs). Citrullination is a normal process, required for normal skin formation and other physiologic functions. However, in rheumatoid arthritis an autoimmune response develops against citrullinated peptides detected as anti-citrullinated peptide antibodies (ACPA). One of tests to detect these antibodies detects anti-cyclic citrullinated peptides (anti-CCP), currently the most commonly used diagnostic test for them. The presence of anti-CCP are >98% specific for the diagnosis of rheumatoid arthritis; however, not all patients with RA will develop anti-CCP antibodies.
Of significant importance is the recognition that these anti-CCP antibodies may be detected up to 15 years before the onset of clinical symptoms of RA indicating a preclinical phase of disease in which immunologic activation is already ongoing. Moreover, it has recently been demonstrated that specific citrullinated peptide sequences bind to shared epitope alleles with high affinity and can lead to T cell activation.
The mechanisms to citrullination that lead to RA remain unclear. A polymorphism in the PAD4 gene which may lead to increased citrullination has been described populations. In RA patients, autoantibody responses also develop against the PAD4 protein, associated with a more aggressive disease course. One species of oral bacteria Porphyromonas gingivalis has a PAD enzyme. Given the relationships described with periodontal disease and RA, it has been hypothesized that this bacteria may also serve to initiate citrullination in the preclinical phases of RA.
T cell activation
Upon encounter with antigen in the context of MHC on an antigen presenting cell, a T lymphocyte is positioned for 3 possible fates: activation, anergy/tolerance, or apoptosis (death). T cell activation is only possible if the T cell receives a “second signal” through engaging additional cellular receptors. One of the most important of these second signals is delivered through the CD28 molecule on the surface of the T cell but many other second signals are involved in this process of “costimulation”. Upon engagement of these receptors, a T cell usually becomes activated. Failure to engage the stimulatory receptors, or engagement of a down-regulator receptor will cause the cell to become tolerant to the antigen (eg does not activate when exposed to the antigen) or to undergo programmed cell death through apoptosis. The process of T-cell costimulation is interrupted by abatacept, a biological therapy used to treat RA.
When T cells become activated, they will in turn proliferate and begin to secrete additional cytokines including IL-2 which furthers their proliferation, and depending on other exposures, cytokines such as IFN-γ, TNF, and IL-4. It is the effect of these T-cell derived cytokines that additional cells become activated. T cells also directly interact through surface receptors with other cells to generate additional activation signals.
B Cell Activation and Autoantibodies
B cells become activated through interactions with T cells and through soluble cytokines that enhance their proliferation and differentiation. B cells express a number of receptors on their surfaces during their differentiation, including the molecule CD20, which is lost upon terminal differentiation to antibody-forming plasma cells. B cells and plasma cells can be found in rheumatoid synovium sometimes as lymphoid aggregates in the subsynovium. The effects of B cells extend beyond their roles in forming plasma cells including cytokine production, direct cellular interactions, and they themselves serve as antigen-presenting cells to T lymphocytes. The role of B cells in RA has been clearly demonstrated with the efficacy of rituximab which eliminates circulating B cells, though with limited impact on autoantibody formation.
One of the features of most autoimmune diseases is the presence of disease-specific autoantibodies that help to define disease phenotypes. Antibodies are made by plasma cells, which represent the terminal stage of differentiation for B lymphocytes. Rheumatoid arthritis is characterized by the presence of autoantibodies known as rheumatoid factors (RF) and anti-citrullinated peptide antibodies (ACPA, which includes the anti-cyclic citrullinated peptide antibody or anti-CCP). Rheumatoid factors have been long recognized as a feature of many patients with RA. These are autoantibodies in the classical sense; they are antibodies directed against native antibodies, most classically described as IgM antibodies that recognize the Fc portion of IgG molecules, but RF may also be of the IgG or IgA isotypes. Rheumatoid factors are not specific for the diagnosis of RA, but are seen in many other inflammatory and autoimmune conditions. These include Sjogren’s syndrome, chronic infections including tuberculosis and endocarditis, hepatitis C, chronic kidney or liver disease, lymphoproliferative diseases including myeloma, and other conditions. While the rheumatoid factor may be seen in other inflammatory conditions, ACPA are highly specific for rheumatoid arthritis and define a more aggressive disease phenotype (discussed more above).
Effector Cell Activation
While T cells and B cells represent the immunological aspects of RA, most of the damage from the disease is driven through effector cells and their products including cytokines and other mediators. The synovial lining in RA represents an expansion of fibroblast like cells and macrophages. It is the macrophage that has been seen as one of the master orchestrators of the effector damage in RA. Macrophages are rich sources and major producers of proinflammatory cytokines including TNF, IL-1, IL-6, IL-8, and GMCSF. These cytokines further stimulate the macrophage, as well as other cells in the microenvironment in a including fibroblasts and osteoclasts, and finally at distant sites in the body through cell surface receptors including the hepatocyte which is responsible for the generation of acute phase response proteins (such as C-reactive protein). Macrophages are also producers of prostaglandins and leukotrienes, nitric oxide, and other pro-inflammatory mediators with local and systemic effects. The synovial fibroblast , also secretes cytokines including IL-6, IL-8 and GM-CSF, and other mediators including destructive proteases and collagenases.
Neutrophils are recruited in very large numbers to the rheumatoid cavity where they can be aspirated in the synovial fluid. The recruitment of neutrophils to the joint is likely driven by IL-8, leukotriene B4, and possibly localized complement activation through C5a. Neutrophils in the synovial fluid are in an activated state, releasing oxygen-derived free radicals that depolymerize hyaluronic acid and inactivate endogenous inhibitors of proteases, thus promoting damage to the joint.
Chondrocytes , like synovial fibroblasts, are activated by IL1 and TNF to secrete proteolytic enzymes. They may, therefore, contribute to the dissolution of their own cartilage matrix, thus explaining the progressive narrowing of joint spaces seen radiographically in this disease.
One of the most important group of mediators in RA are cytokines. The most prominent of these are TNF, IL-1, and IL-6 . These cytokines, released in the synovial microenvironment have autocrine (activating the same cell), paracrine (activating nearby cells), and endocrine (acting at distant sites) effects and accounting for many systemic manifestations of disease. There are many shared functions of TNF, IL-1, and IL-6, and these cytokines in turn upregulate the expression of the others. Among the important effects of these cytokines are:
- Induction of cytokine synthesis
- Upregulation of adhesion molecules
- Activation of osteoclasts
- Induction of other inflammatory mediators including prostaglandins, nitric oxide, mtrix metalloproteinases
- Induction of the acute phase response (e.g. C-reactive protein, increased ESR)
- Systemic features (e.g., fatigue, fever, cachexia)
- Activation of B cells (IL-6)
Other cytokines are increasingly described in RA. These include IL-8 which is involved in cellular recruitment, GM-CSF involved in macrophage development, IL-15 involved in T cell proliferation, IL-17 which has pleiotropic effects on multiple cell types including osteoblast expression of RANK leading to osteoclast activation, and IL-23 involved in increasing TH17 cell differentiation.
Soluble mediators of inflammation that may diffuse in from blood and/or be formed locally within the joint cavity include prostaglandins, leukotrienes, matrix metalloproteinases . Prostaglandins are involved in pain senstitization localized inflammation, and some effects on bone, and leukotrienes play roles in vascular permeability and chemotaxis. Matrix metalloproteinases (MMPs) are potent in their ability to enzymatically degrade the collagen matrix of cartilage. Kinins cause release of prostaglandins from synovial fibroblasts, and are also potent algesic (pain-producing) agents. Complement may be available for interaction with immune complexes to generate additional chemotactic stimuli. The neuropeptide substance P is a potent vasoactive, proinflammatory peptide that has also been implicated in RA.

Receive the Latest News from Johns Hopkins Rheumatology
Join our mailing list to receive the latest news and updates from Johns Hopkins Rheumatology.
Health Care Professional Yes No
Interested In Arthritis Center News Information from the Division of Rheumatology Lupus Center News Lyme Disease News Myositis Center News Scleroderma Center News Sjögren’s Syndrome Center News Vasculitis Center News
You have Successfully Subscribed!
Use of this site.
All information contained within the Johns Hopkins Arthritis Center website is intended for educational purposes only. Physicians and other health care professionals are encouraged to consult other sources and confirm the information contained within this site. Consumers should never disregard medical advice or delay in seeking it because of something they may have read on this website.
- Johns Hopkins Rheumatology
- Johns Hopkins Lupus Center
- Johns Hopkins Lyme Disease Research Center
- Johns Hopkins Myositis Center
- Johns Hopkins Scleroderma Center
- Johns Hopkins Sjögren’s Syndrome Center
- Johns Hopkins Vasculitis Center
Connect With Us

We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 111: Rheumatoid Arthritis
Stephanie Gruber; Bianca Harris; Susan Hylland
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Chapter summary from the pharmacotherapy handbook, key concepts, patient care process, beyond the book.
- INTRODUCTION
- EPIDEMIOLOGY
- PATHOPHYSIOLOGY
- CLINICAL PRESENTATION
- DIAGNOSTIC CRITERIA
- EVALUATION OF THERAPEUTIC OUTCOMES
- ABBREVIATIONS
- SELF-ASSESSMENT QUESTIONS
- SELF-ASSESSMENT QUESTION-ANSWERS
- Full Chapter
- Supplementary Content
For the Chapter in the Schwinghammer Handbook, please go to Chapter 4, Rheumatoid Arthritis .
Patient characteristics (eg, age, sex, pregnancy status, insurance)
Social history (eg, tobacco/alcohol use, activity)
Patient medical history (eg, health conditions, immunizations, recent infections)
Family medical history (eg, autoimmune conditions)
Current medications
Past RA medication trials
Subjective symptom report
Objective data such as blood pressure, labs (eg, ESR, CRP, CBC), imaging (eg, DEXA, x-ray films, ultrasound), physical examination (eg, number of tender/swollen joints)
Patient subjective report (eg, pain score, duration of morning joint stiffness, adherence to therapy, injection technique/medication storage, side effects to drug therapy, disability, fatigue)
Change in number of tender/swollen joints, labs, or imaging
Cardiovascular risk factors
Infection risk and upcoming procedures
Patient treatment preference (utilize motivational interviewing as appropriate)
Drug therapy (see Table 111-2 )
Referrals when appropriate (eg, tobacco treatment clinic, podiatry, mental health, social work, physical and/or occupational therapy)
Patient education (eg, dosing, side effects, infection risk management, symptom self-monitoring)
Order follow-up labs based on therapy chosen (see Table 111-4 )
Implement *
Provide patient education regarding rationale for and follow-up of treatment plan
Provide patient with written medication changes, time frame for follow-up, and clinic/emergency contact information
Coordinate and schedule follow-up
Follow-up: Monitor and Evaluate
Subjective symptom changes and impact on daily activities
Presence of adverse effects and infections
Laboratory results as indicated for therapy
Patient adherence to treatment plan
Time frame dependent on treatment plan (generally every 1-3 months)
* Collaborate with a rheumatologist.
Direct-to-consumer advertising refers to the marketing of products to patients rather than healthcare professionals. This is a common marketing strategy, particularly for pharmaceutical products. Watch the following advertisements for tofacitinib and adalimumab:
https://www.ispot.tv/ad/O1Z1/xeljanz-mornings-raking
https://www.ispot.tv/ad/wrWz/humira-food-drive
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
The clinical features of rheumatoid arthritis
Affiliation.
- 1 Department of Rheumatology, University of Ancona, Italy.
- PMID: 9652497
- DOI: 10.1016/s0720-048x(98)00038-2
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by progressive damage of synovial-lined joints and variable extra-articular manifestations. Tendon and bursal involvement are frequent and often clinically dominant in early disease. RA can affect any joint, but it is usually found in metacarpophalangeal, proximal interphalangeal and metatarsophalangeal joints, as well as in the wrists and knee. Articular and periarticular manifestations include joint swelling and tenderness to palpation, with morning stiffness and severe motion impairment in the involved joints. The clinical presentation of RA varies, but an insidious onset of pain with symmetric swelling of small joints is the most frequent finding. RA onset is acute or subacute in about 25% of patients, but its patterns of presentation also include palindromic onset, monoarticular presentation (both slow and acute forms), extra-articular synovitis (tenosynovitis, bursitis), polymyalgic-like onset, and general symptoms (malaise, fatigue, weight loss, fever). The palindromic onset is characterized by recurrent episodes of oligoarthritis with no residual radiologic damage, while the polymyalgic-like onset may be clinically indistinguishable from polymyalgia rheumatica in elderly subjects. RA is characteristically a symmetric erosive disease. Although any joint, including the cricoarytenoid joint, can be affected, the distal interphalangeal, the sacroiliac, and the lumbar spine joints are rarely involved. The clinical features of synovitis are particularly apparent in the morning. Morning stiffness in and around the joints, lasting at least 1 h before maximal improvement is a typical sign of RA. It is a subjective sign and the patient needs to be carefully informed as to the difference between pain and stiffness. Morning stiffness duration is related to disease activity. Hand involvement is the typical early manifestation of rheumatoid arthritis. Synovitis involving the metacarpophalangeal, proximal interphalangeal and wrist joints causes a characteristic tender swelling on palpation with early severe motion impairment and no radiologic evidence of bone damage. Fatigue, feveret, weight loss, and malaise are frequent clinical signs which can be associated with variable manifestations of extra-articular involvement such as rheumatoid nodules, vasculitis, hematologic abnormalities, Felty's syndrome, and visceral involvement. Although there is no laboratory test to exclude or prove the diagnosis of rheumatoid arthritis, several laboratory abnormalities can be detected. Abnormal values of the tests for evaluation of systemic inflammation are the most typical humoral features of RA. These include: erythrocyte sedimentation rate, acute phase proteins and plasma viscosity. Erythrocyte sedimentation rate and C-reactive protein provide the best information about the acute phase response. The C-reactive protein is strictly correlated with clinical assessment and radiographic changes. Plain film radiography is the standard investigation to assess the extent of anatomic changes in rheumatoid arthritis patients. The radiographic features of the hand joints in early disease are characterized by soft tissue swelling and mild juxtaarticular osteoporosis. In the the past 10 years, ultrasonography has gained acceptance for studying joint, tendon and bursal involvement in RA. It may improve the early clinical assessment and the follow-up of these patients, showing such details as synovial thickening even within finger joints. Other imaging techniques, such as magnetic resonance, computed tomography and scintigraphy may provide useful information about both the features and the extent for anatomic damage in selected rheumatoid arthritis patients. The natural history of the disease is poorly defined; its clinical course is fluctuating and the prognosis unpredictable. RA is an epidemiologically relevant cause of disability. An adequate early treatment of RA may alter the diseas
Publication types
- Arthritis, Rheumatoid / diagnosis*
- Diagnostic Imaging*
- Joints / pathology
- Periarthritis / diagnosis
- Sensitivity and Specificity
- Synovitis / diagnosis
Advertisement
Geiser Sign in a patient with rheumatoid arthritis
- CLINICAL IMAGE
- Published: 04 April 2024
- Volume 43 , pages 1785–1786, ( 2024 )
Cite this article

- Daniela Oliveira ORCID: orcid.org/0000-0001-9734-3162 1 , 2 ,
- Raquel Miriam Ferreira 1 , 3 ,
- Carlos Marques Gomes 1 , 3 &
- Carlos Vaz 1 , 2 , 3
638 Accesses
5 Altmetric
Explore all metrics
Avoid common mistakes on your manuscript.
Presentation
A 69-year-old woman with rheumatoid arthritis (RA) under methotrexate and anti-TNF alpha agent treatment presented with a painful, firm and compressible swelling above the right acromioclavicular joint (ACJ) (Fig. 1 A). Ultrasound revealed a large hypoechoic mass over ACJ and degenerative changes of this joint (Fig. 1 B), complete supraspinous and infraspinous tear and glenohumeral joint (GHJ) synovitis. Magnetic Resonance Imaging confirmed these findings and revealed fluid communication from the GHJ to a large ACJ cyst—Geyser Sign (GS) (Fig. 1 C). Conservative treatment with an anti-inflammatory agent was provided and patient was referred to orthopaedics for evaluation.

a Presence of swelling over the right shoulder (arrow). b Right shoulder ultrasound image (LOGIC S8, MC6-15 MHZ linear transducer, longitudinal view over the acromioclavicular joint—ACJ), revealed a large hypoechoic cyst (arrow) with well-defined borders and 5.5 cm larger diameter above the ACJ. c Right shoulder Magnetic Resonance Imaging (coronal oblique T2 fat-suppressed image), revealing massive rotator cuff tear, moderate volume effusion with synovitis of the glenohumeral joint (GHJ) and a large ACJ cyst with 5.65 × 2.96 cm (arrow). Note the presence of communicating synovial fluid from the GHJ through the ACJ (arrowhead)—Geyser sign
GS is a rare imaging sign initially described by Craig et al. in 1984 following a shoulder arthrography conducted on a patient with ACJ cyst along with a rotator cuff tear [ 1 , 2 ]. GS portrays the accumulation of synovial fluid escaping from the rotator cuff tear across the subacromial bursa and “erupting” through ACJ with capsule distension and cyst formation. Two types of ACJ cysts have been identified: type 1, confined to the ACJ and distinguished by the absence of communication with the GHJ; and type 2, defined by a rotator cuff tear that enables synovial fluid communication to be established between the ACJ and GHJ [ 3 ], as described in this case. This case is an example of a rare manifestation of rotator cuff tear associated with osteoarthritis of the ACJ and synovitis of the GHJ in a patient with RA.
Craig EV (1986) The acromioclavicular joint cyst. An unusual presentation of a rotator cuff tear. Clin Orthop Relat Res 202:189–192
Article Google Scholar
Craig EV (1984) The geyser sign and torn rotator cuff: clinical significance and pathomechanics. Clin Orthop Relat Res 191:213–215
Hiller AD, Miller JD, Zeller JL (2010) Acromioclavicular joint cyst formation. Clin Anat 23:145–152. https://doi.org/10.1002/ca.20918
Article PubMed Google Scholar
Download references
No Funding.
Author information
Authors and affiliations.
Rheumatology Department, University Hospital Center of São João, Alameda Prof. Hernâni Monteiro, 4200-319, Porto, Portugal
Daniela Oliveira, Raquel Miriam Ferreira, Carlos Marques Gomes & Carlos Vaz
Center for Health Technology and Services Research (CINTESIS), Faculty of Medicine, University of Porto, Porto, Portugal
Daniela Oliveira & Carlos Vaz
Department of Medicine of Faculty of Medicine, University of Porto, Porto, Portugal
Raquel Miriam Ferreira, Carlos Marques Gomes & Carlos Vaz
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Daniela Oliveira .
Ethics declarations
Disclosures, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Oliveira, D., Ferreira, R.M., Gomes, C.M. et al. Geiser Sign in a patient with rheumatoid arthritis. Clin Rheumatol 43 , 1785–1786 (2024). https://doi.org/10.1007/s10067-024-06952-1
Download citation
Received : 10 March 2024
Revised : 18 March 2024
Accepted : 25 March 2024
Published : 04 April 2024
Issue Date : May 2024
DOI : https://doi.org/10.1007/s10067-024-06952-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

Modifying PEST for Psoriatic Arthritis Screening (ScreenX)
- Study Details
- Tabular View
- No Results Posted

The purpose of this study is to assess the impact of adding two questions and pictures to the validated PEST on the potential diagnosis of PsA in participants with moderate-to-severe plaque PsO in Canada.
Patients will be enrolled in the study for up to 66 days and will be asked to fill-out a PsA screening questionnaire at their first dermatologist visit. Patients screening positive for PsA will have a second visit with a rheumatologist where a full PsA diagnosis assessment will be performed. A remote 'end of study' (EOS) visit will be conducted by the dermatologist to document the patient's biologic Disease-Modifying Antirheumatic Drugs (bDMARDs) treatment choice and status.

Proportion of patients with a new PsA diagnosis or suspicion of PsA, confirmed by the rheumatologist who scored:
- Negative on PEST and positive on PEST+pictures
- Negative on PEST+2 and positive on PEST+pictures+2 will be evaluated to assess the impact of adding pictures as reference to guide patients answering the screening questions (question 1, 3, and 5)
Proportion of patients with false positive score between each group will be evaluated:
- PEST+2 vs. PEST+pictures+2
- PEST+pictures vs. PEST
- PEST+pictures+2 vs. PEST
Key inclusion criteria:
- Moderate-to-severe plaque PsO patients who are candidates for bDMARDs, as per provincial reimbursement criteria
- Adult patients at the time of informed consent signature
- Patients able to understand and willing to comply with protocol requirements, instructions, and restrictions
- Residents of Canada
Key exclusion criteria:
- Patients who have previously screened positive for PsA through PEST or any other screening method
- Patients who have been diagnosed with PsA and/or followed by a rheumatologist
- Patients who have been diagnosed with inflammatory arthritis unrelated to PsA (rheumatoid arthritis, reactive arthritis, enteropathic arthritis, axial spondyloarthritis)
- Patients treated with a bDMARD for moderate-to-severe plaque PsO or any other medical condition
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
- Find in topic
Please read the Disclaimer at the end of this page.
INTRODUCTION — Rheumatoid arthritis (RA) is a symmetric, inflammatory, peripheral polyarthritis of unknown etiology. It typically leads to joint destruction through the erosion of cartilage and bone. Untreated, it will lead to loss of physical function, inability to carry out daily tasks of living, and difficulties in maintaining employment. Uncontrolled inflammation may have other health risks including higher rates of cardiovascular disease, osteoporosis, and certain types of cancer (eg, lymphoma).
Early recognition and treatment with disease-modifying antirheumatic drugs (DMARDs) are important to prevent joint damage and disability. However, in patients with early disease, the joint manifestations are often difficult to distinguish from other forms of inflammatory polyarthritis; the more distinctive signs of RA, such as joint erosions, rheumatoid nodules, and other extraarticular manifestations, are seen primarily in patients with longstanding, poorly controlled disease and are frequently absent on initial presentation.
This topic will review the approach to the diagnosis and differential diagnosis of RA. The clinical features of this disorder, its extraarticular manifestations, and laboratory markers that are clinically useful in the diagnosis of RA are discussed in detail separately:
● (See "Clinical manifestations of rheumatoid arthritis" .)
● (See "Overview of the systemic and nonarticular manifestations of rheumatoid arthritis" .)
● (See "Biologic markers in the assessment of rheumatoid arthritis" .)
EVALUATION AND DIAGNOSIS — We suspect rheumatoid arthritis (RA) in any patient presenting with an inflammatory polyarthritis. In such a patient, we perform serologic and radiologic tests to look for evidence confirming the presence of RA.
When to suspect — While the clinical features of RA can be diverse, most patients with chronic RA will have the following features:
● Constitutional symptoms – Many patients with RA may present with symptoms associated with an inflammatory process. These symptoms may include unintentional weight loss, asthenia, fatigue, and myalgias.
● Morning stiffness – Morning stiffness is the hallmark of an inflammatory arthritis. Morning stiffness is generally characterized by difficulty mobilizing the joints after a prolonged period of rest. Morning stiffness lasting for longer than one hour implies the presence of an inflammatory joint disease. However, this is not specific to RA, and this finding can be present in a spectrum of inflammatory diagnoses. (See "Clinical manifestations of rheumatoid arthritis", section on 'Symptoms and physical findings' .)
● Joint manifestations – In general, the patient will notice joint pain and swelling that predominantly affects the small joints of the hands and feet (particularly the metacarpophalangeal [MCP], metatarsophalangeal [MTP], and/or proximal interphalangeal [PIP] joints). However, patients may present with other patterns of joint involvement, including an acute monoarthritis. (See "Clinical manifestations of rheumatoid arthritis", section on 'Upper extremity' and "Clinical manifestations of rheumatoid arthritis", section on 'Lower extremity' and "Clinical manifestations of rheumatoid arthritis", section on 'Palindromic rheumatism' .)
Serologic and radiologic tests are sometimes obtained as part of a broad evaluation of nonspecific complaints and/or of an extraarticular feature. Findings such as joint erosions and RA-associated autoantibodies may become the primary basis for suspecting a diagnosis of RA:
● Elevated acute phase reactants – Elevations of the erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) level are consistent with the presence of an inflammatory state, such as RA. Normal acute phase reactants may occur in untreated patients with RA, but such findings are infrequent and should prompt consideration of alternative diagnoses [ 1 ].
The degree of elevation of these acute phase reactants varies with the severity of inflammation and synovitis. As an example, an ESR of 50 to 80 is not uncommon in patients with severely active RA. By comparison, an ESR of 20 to 30 can be observed with only a few mildly to moderately active joints. (See "Acute phase reactants" .)
● Autoantibodies – These include rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies. (See 'Confirmatory testing' below.)
● Radiographic abnormalities – Periarticular osteopenia, joint space narrowing, and bone erosions are late manifestations of RA. When present, these manifestations are most often detected on plain radiographs, although they may also be noted on magnetic resonance imaging (MRI) or ultrasonography. (See 'Confirmatory testing' below.)
Patients who have all or most of the features described above have a high probability of RA. Patients who only have one or two of these features have a low probability of RA and merit a closer examination for alternate diagnoses. (See 'Differential diagnosis' below.)
The presence of extraarticular features (eg, rheumatoid nodules, interstitial lung disease, scleritis) may prompt an evaluation for RA, although these patients should also have many of the features described above. The extraarticular features of RA are described in greater depth elsewhere. (See "Overview of the systemic and nonarticular manifestations of rheumatoid arthritis" .)
Establish the presence of inflammatory arthritis — Diagnosing an inflammatory polyarthritis requires a joint examination of the upper and lower extremities to look for evidence of synovitis. Symptomatology alone is not adequate to establish a diagnosis of an inflammatory arthritis. The joint examination and the hallmarks of synovitis are described in detail separately. (See "Evaluation of the adult with polyarticular pain", section on 'Joint examination' .)
RA has a characteristic distribution of joint involvement. In patients with RA, synovitis is typically present in the MCP and PIP joints of the hands. The wrists are also commonly involved, as are the second to fifth MTP joints in the feet. However, any upper- or lower-extremity joint may be affected.
Other patterns of joint involvement may potentially be associated with RA, but for such patients, it is important to exclude other diagnoses for which those patterns may be more characteristic. For example, distal interphalangeal (DIP) involvement strongly suggests a diagnosis of osteoarthritis (OA) or psoriatic arthritis ( table 1 ). (See "Clinical manifestations of rheumatoid arthritis", section on 'Symptoms and physical findings' and 'Osteoarthritis' below and 'Rheumatic' below.)
Potential challenges to identifying synovitis include:
● Early disease – Synovitis and tenosynovitis may be subtle initially and evolve to involve a greater number of joint areas. Patients with symptoms that are suspicious but that lack obvious joint swelling may need to be followed closely before the diagnosis can be made through careful examination. Imaging studies may be used to identify more subtle abnormalities earlier in the course of disease; however, imaging evidence of synovitis and tenosynovitis is not specific to RA [ 2 ]. (See 'Radiologic studies' below.)
● Body habitus – In some patients, the presence of subtle synovitis may be difficult to ascertain due to body habitus. In such patients, imaging studies may be useful to confirm the presence of an inflammatory arthritis. (See 'Radiologic studies' below.)
Confirmatory testing — In a patient suspected of having RA who has a pattern of synovitis consistent with this diagnosis, we conduct additional serologic and radiologic tests to confirm the diagnosis.
Serology — We suggest obtaining both RF and anti-citrullinated peptide antibodies in all patients suspected of having RA.
Rheumatoid factor
● Sensitivity and specificity – Estimates of the sensitivity, specificity, and positive predictive value of RF vary depending upon the population examined.
The reported sensitivity of RF in RA (ie, the proportion of patients with RA who are RF positive) ranges from 26 to 90 percent. A meta-analysis reported the overall sensitivity to be 69 percent (95% CI 65-73) [ 3 ].
The specificity depends substantially upon the choice of the control group. The overall specificity of RF reported in heterogeneous publications analyzed as part of a meta-analysis was 85 percent [ 3 ]. The prevalence of RF in a young healthy population is approximately 4 percent, consistent with a specificity of 96 percent in this group.
High positive RF (ie, above three times the upper limit of normal) is more specific for a diagnosis of RA than low positive RF. However, the specificity of RF drops substantially when examining patients with other rheumatic or inflammatory diagnoses [ 1 ]. The prevalence of RF in other conditions is discussed separately. (See "Rheumatoid factor: Biology and utility of measurement", section on 'Clinical disorders associated with rheumatoid factor positivity' .)
● Use in patients with a moderate to high pretest probability of rheumatoid arthritis – We suggest against testing patients with joint pain in the absence of synovitis (eg, nonspecific arthralgias, fibromyalgia, OA) because a positive test result is more likely to represent a false-positive result. (See 'When to suspect' above.)
RF testing in a rheumatology clinic practice with a prevalence of RA of 16 percent demonstrated a specificity of 95 to 97 percent, yielding a positive predictive value of approximately 80 percent [ 4 ].
Most studies of patients in early arthritis clinics demonstrate that RF helps predict persistent synovitis and/or RA [ 5 ]. In patients with “undifferentiated” inflammatory arthritis, the presence of RF was helpful in predicting a diagnosis of RA, with a positive odds ratio of approximately 30 [ 6 ].
RF is generally detectable prior to the clinical onset of RA. A retrospective study of stored blood samples collected as part of routine blood donation demonstrated that nearly 30 percent of those who later develop RA have serum RF present for a median of 4.5 years prior to diagnosis [ 7,8 ]
● Use in patients with a low pretest probability of rheumatoid arthritis – We suggest against testing patients with joint pain in the absence of synovitis (eg, nonspecific arthralgias, fibromyalgia, OA) because a positive test result is likely to represent a false-positive result.
RF is a poor screening test for the general population [ 9 ]. Population-based studies have shown that a small number of healthy people with a positive RF develop RA over time, especially if more than one isotype is persistently elevated and if there are high levels of RF [ 9,10 ]. Although RF may be found in the circulation prior to the development of RA, most asymptomatic persons with a positive RF do not progress to RA. (See "Rheumatoid factor: Biology and utility of measurement" .)
As with any diagnostic test, the predictive value of the RF is directly affected by the estimated likelihood of disease prior to ordering the test (ie, the pretest probability) and, with RF, by the proportion of individuals being tested with a nonrheumatic disorder associated with RF production ( table 2 ).
Anti-citrullinated peptide antibodies
● Sensitivity and specificity – Measurement of anti-citrullinated peptide antibodies (ACPA) is useful in the differential diagnosis of early polyarthritis because of the relatively high specificity for RA of these antibodies [ 11,12 ]. The sensitivity of current ACPA assays is approximately 70 to 75 percent, while specificity of ACPA for RA is relatively high, usually over 90 percent [ 3,11,13-19 ]. (See "Undifferentiated systemic rheumatic (connective tissue) disease and overlap syndromes" .)
High positive ACPA (ie, above three times the upper limit of normal) is more specific for a diagnosis of RA than low positive ACPA [ 1 ].
As with RF, ACPA may be present prior to the appearance of symptoms of RA. This phenomenon is discussed separately. (See "Epidemiology of, risk factors for, and possible causes of rheumatoid arthritis" .)
● Use in patients with a moderate to high pretest probability of rheumatoid arthritis – For patients with an inflammatory, small joint arthritis and with a moderate to high pretest probability of RA, the presence of ACPA testing confirms a diagnosis of RA. (See 'When to suspect' above.)
In a meta-analysis of 37 studies of patients with known or suspected RA, anti-CCP antibody had a sensitivity of 67 percent and a specificity of 95 percent for a diagnosis of RA [ 3 ].
● Use in patients with a low pretest probability of rheumatoid arthritis – We suggest against measuring ACPA in patients with a low pretest probability of RA. Although ACPA testing is more specific than RF for RA [ 3,12 ], it may be detected in several autoimmune rheumatic diseases, including systemic lupus erythematosus, Sjögren's disease, and psoriatic arthritis, usually in association with a deforming or erosive arthritis. ACPA have also been reported in association with tuberculosis, and, less commonly, chronic obstructive pulmonary disease and alpha-1-antrypsin disease [ 3,14,18,20-43 ].
Seronegative rheumatoid arthritis — Both RF and ACPA are negative on presentation in up to 50 percent of patients and remain negative during follow-up in 20 percent of patients with RA. Patients who lack both RF and ACPAs may be diagnosed with RA based upon findings otherwise characteristic of RA if appropriate exclusions have been met. For example, presence of a large number of swollen joints in a symmetric, small joint pattern or the presence of other features of RA may help establish the diagnosis in the absence of positive serologic testing.
Ultimately, seronegative RA is a clinical diagnosis and may be difficult to definitively distinguish from other forms of inflammatory arthritis. The diagnosis of seronegative RA may be secure only after monitoring the patient’s response to therapy over an extended period of time. In patients newly diagnosed with seronegative RA, we suggest a careful review of alternate diagnoses prior to initiating pharmacotherapy. (See 'Inflammatory diagnoses' below.)
Radiologic studies
● Plain film radiography – We suggest obtaining plain film radiographs of the hands and feet in all patients suspected of having RA (see 'When to suspect' above). Even if they do not assist in establishing the initial diagnosis of RA, plain film radiographs of the hands and feet may also be useful to assess the progression of joint damage over time.
• Characteristic findings – Plain radiographs are often normal early in disease. Therefore, radiographic abnormalities are not required to establish a diagnosis of RA.
The early changes evident on plain films may include only soft tissue swelling and periarticular osteopenia ( image 1A-C ). To be detected by plain radiography, erosions must have eroded through the cortex of the bone around the margins of the joint.
In some patients, erosions occur first in the ulnar styloid ( image 2A-B ) or MTP joints ( image 3A-B ). Joint space narrowing may also be present.
In a patient with a moderate to high suspicion of RA, the presence of periarticular osteopenia, joint space narrowing, and bone erosions, or joint subluxation confirms the diagnosis of RA.
Radiologic changes are generally symmetric, except in patients with joint injury or hemiplegia [ 44,45 ].
• Frequency of bone erosions – In studies done in the late 1980s and early 1990s, prior to the advent of highly effective biologic therapies, erosions in the MCP ( image 4A-B and image 5 ) and PIP ( image 6A-B ) joints were identified by plain radiography in 15 to 30 percent of patients in the first year of the disease. By the end of the second year of disease in patients who did not respond to therapy, the cumulative incidence of erosions was 90 percent [ 46,47 ].
In a 2024 study of 724 patients suspected of having RA, erosions were found in only 32 patients (4.4 percent) [ 48 ]. Additionally, radiographs led to a change in diagnostic classification for only two patients (0.3 percent). Patients who lacked RA-associated autoantibodies and/or acute phase reactant elevation were less likely to demonstrate RA-associated erosions.
● Ultrasonography – We suggest ultrasonography when the presence of synovitis cannot be determined by physical examination due to body habitus or inaccessibility of the affected joint. In a patient with a moderate to high suspicion of RA, a symmetric polyarthritis, particularly of the MCP, MTP, and/or PIP joints, confirms a diagnosis of RA. (See 'Establish the presence of inflammatory arthritis' above.)
• Synovial hypertrophy – Ultrasonography is a sensitive imaging technique for estimating the degree of inflammation and the volume of inflamed tissue. Direct comparison of color Doppler ultrasonography and contrast-enhanced MRI in one study of 29 patients demonstrated agreement regarding the presence or absence of inflammation between the two techniques in 75 percent of the joints of the hands and wrists [ 49 ]. Both imaging modalities found features of inflammation in joints that were neither tender nor swollen on physical examination.
• Bone erosions – Ultrasonography is more sensitive than conventional radiography for the detection of bone erosions; however, it is not routinely used for this purpose, as the clinical significance of erosions that can be detected only on ultrasound is not clear.
In one study, sonography detected 6.5-fold more MCP joint erosions than radiography in early RA and 3.4-fold more erosions in late disease [ 50 ]. Moreover, the sonographic erosions corresponded to MRI bone abnormalities.
Ultrasound evaluation for bone erosions and synovitis is described in further detail separately. (See "Musculoskeletal ultrasonography: Clinical applications", section on 'Joints' .)
● Magnetic resonance imaging – We suggest MRI when the presence of synovitis cannot be determined by physical examination and ultrasonography is not available. In a patient with a moderate to high suspicion of RA, a symmetric polyarthritis, particularly of the MCP, MTP, and/or PIP joints, supports a diagnosis of RA. (See 'Establish the presence of inflammatory arthritis' above.)
• Bone marrow edema – Decreased signal from the bone marrow on T1-weighted images and enhancement of the marrow with gadolinium administration is interpreted as bone marrow edema. The presence of marrow edema on MRI is predictive of later development of erosive disease [ 51 ].
• Synovial hypertrophy – It is also possible to identify and estimate the quantity of hypertrophic synovial tissue using contrast-enhanced MRI. The presence of MRI-detected synovial proliferation correlates with the later development of bone erosions [ 52 ]. Use of this imaging technique outside of research settings may be hastened by the development of MRI scanners that are designed specifically for imaging the extremities [ 53,54 ].
• Bone erosions – MRI is also a more sensitive technique than plain radiography for identifying bone erosions; however, it is not routinely used for this purpose, as the clinical significance of erosions detected only detected by MRI is unclear [ 55 ].
When radiography and MRI were compared in a group of 55 patients with early arthritis, MRI identified seven times as many erosions in the MCP and PIP joints as plain radiography [ 56 ]. MRI also may detect bone erosions earlier in the course of the disease than is possible with plain films [ 57 ]. As an example, approximately 45 percent of patients with symptoms for only four months were found to have erosions detected by this method [ 58 ].
Diagnosis — Clinicians often use the 2010 American College of Rheumatology/European League Against Rheumatology (ACR/EULAR) classification criteria for RA as a guide to diagnosis. However, many patients with RA will not satisfy these criteria early in the course of disease and may still be appropriate candidates for treatment.
Using these criteria, a classification as “definite RA” is based upon the presence of synovitis in at least one joint, the absence of an alternative diagnosis that better explains the synovitis, and the achievement of a total score of at least 6 (of a possible 10) from the individual scores in four domains [ 59-61 ]. The highest score achieved in a given domain is used for this calculation. These domains and their values are:
● Number and site of involved joints:
• 2 to 10 large joints (from among shoulders, elbows, hips, knees, and ankles) = 1 point
• 1 to 3 small joints (from among the MCP joints, PIP joints, second through fifth MTP joints, thumb IP joints, and wrists) = 2 points
• 4 to 10 small joints = 3 points
• Greater than 10 joints (including at least 1 small joint) = 5 points
● Serological abnormality (RF or ACPA)
• Low positive (above the upper limit of normal) = 2 points
• High positive (greater than three times the upper limit of normal) = 3 points
● Elevated acute phase response (ESR or CRP) above the upper limit of normal = 1 point
● Symptom duration at least six weeks = 1 point
Using the ACR/EULAR classification system to diagnose RA comes with important caveats:
● Ultimately, RA is a clinical diagnosis. A patient with polyarticular gout, for example, could potentially be assigned 6 points using the ACR/EULAR classification system for RA. In this case, it would be incumbent upon the clinician to realize that the presence of tophi and episodic pattern of joint involvement was more consistent with a crystalline arthropathy.
● The diagnosis of seronegative RA may be particularly challenging. A diagnosis of seronegative RA may be secure only after monitoring the patient’s response to therapy over an extended period of time. In patients newly diagnosed with seronegative RA, we suggest a careful review of alternate diagnoses prior to initiating pharmacotherapy. A suggested review of systems to evaluate for alternate diagnoses is presented in the table ( table 1 ).
● The 2010 ACR/EULAR criteria were developed primarily to identify patients with early RA. These criteria may fail to identify patients who have very early presentations of RA or inactive disease. In these cases, a presumptive diagnosis of RA may depend primarily on the patient’s history or imaging studies.
These issues are discussed in greater detail elsewhere. (See "Clinical manifestations of rheumatoid arthritis" and 'Differential diagnosis' below.)
DIFFERENTIAL DIAGNOSIS — Joint pain involving the hands is a common clinical presentation for a variety of conditions. Rheumatologists are frequently consulted by other clinicians to evaluate patients with this complaint but who lack the characteristic features of rheumatoid arthritis (RA; eg, synovitis affecting the small joints of the hands, rheumatoid factor [RF] or anti-citrullinated peptide antibody [ACPA] positivity). In such patients, a thorough history and physical may uncover alternate diagnoses that should be considered prior to initiating therapy.
Additionally, a failure to respond to typical RA therapies may also prompt consideration of alternate diagnoses.
One approach to the differential diagnosis is to consider patients in terms of inflammatory versus noninflammatory diagnoses. Inflammatory diagnoses are characterized by the presence of synovitis on examination and are often accompanied by constitutional symptoms and acute phase reactant elevation. Noninflammatory diagnoses are characterized by joint dysfunction in the absence of synovitis, constitutional symptoms, and acute phase reactant elevation.
Some alternate diagnoses that should be considered, along with their characteristic features, are described below.
A suggested review of systems to evaluate for alternate diagnoses is presented in the table ( table 1 ). A basic overview of the approach to a patient with polyarticular pain is described separately. (See "Evaluation of the adult with polyarticular pain" .)
Inflammatory diagnoses — These diagnoses are typically considered in patients with synovitis on examination who lack autoantibodies specific for RA, such as RF or ACPA ( table 1 ).
● Viral polyarthritis – A viral arthritis is typically suspected in a patient who presents with an inflammatory arthritis and concomitant evidence of a viral syndrome. The approach to establishing a diagnosis of a viral arthritis is discussed elsewhere. (See "Viral arthritis: Causes and approach to evaluation and management" .)
Viral polyarthritis generally resolves spontaneously. However, several viral infections (eg, hepatitis B virus, Chikungunya) can cause a chronic polyarthritis. These topics are discussed in detail elsewhere. (See "Viral arthritis: Causes and approach to evaluation and management" .)
● Lyme arthritis – Lyme arthritis should be suspected in a patient who presents with a mono- or oligoarthritis who lives in an area endemic for Lyme disease. The approach to establishing a diagnosis of Lyme disease is discussed elsewhere. (See "Diagnosis of Lyme disease", section on 'Approach to diagnosis' .)
Lyme arthritis is a late manifestation of Lyme disease; half of patients will not remember having been bitten by a tick. Lyme arthritis is characterized by intermittent or persistent inflammatory arthritis in a few large joints, most typically monoarthritis of the knee. The most commonly involved joints, after the knee, are the shoulder, ankle, elbow, temporomandibular joint, and wrist. Migratory arthralgias without frank arthritis may occur during early localized or early disseminated Lyme disease. (See "Musculoskeletal manifestations of Lyme disease" .)
● Other infectious arthritis – Infectious arthritis should be suspected in a patient presenting with a monoarticular arthritis who is at increased risk of infection (eg, diabetes, chronic immunosuppression). The diagnosis is established by culturing the pathogen from the synovial fluid or from the blood.
An infectious arthritis is usually monoarticular, but polyarthritis can occur. Patients with septic arthritis may or may not appear toxic on examination, depending upon the stage of their infection, the presence of medications that can mask infection (eg, glucocorticoids), and other clinical variables. Peripheral blood leukocytosis with a left shift is common but not invariably present.
A low threshold for suspecting infection is required, particularly in compromised hosts. Patients with RA are at increased risk for joint infections because a damaged joint can serve as a nidus of infection. Synovial fluid changes, including marked granulocytosis and low glucose levels, are similar to those seen in RA. (See "Septic arthritis in adults" .)
● Other systemic rheumatic diseases – Early RA may be difficult to distinguish from the arthritis of systemic lupus erythematosus (SLE), Sjögren's disease, inflammatory myopathy (eg, dermatomyositis), and overlap syndromes such as mixed connective tissue disease. In contrast with RA, these disorders are generally characterized by the presence of other systemic features, such as rashes, dry mouth and dry eyes, myositis, or nephritis, and by various autoantibodies not often seen in RA ( table 1 ).
While diagnostic uncertainty in early RA is expected, the diagnosis should become clearer with time.
Morning stiffness, symmetric, erosive arthritis, subcutaneous (“rheumatoid”) nodules, and ulnar deviation of the digits are highly characteristic of RA. However, these features can also be found with other diagnoses:
• Morning stiffness is common in all inflammatory arthritides. Symmetric arthritis can be seen in patients with SLE. (See "Clinical manifestations and diagnosis of systemic lupus erythematosus in adults", section on 'Arthritis and arthralgias' .)
• An erosive arthritis has been described in some overlap syndromes, particularly those associated with anti-tRNA synthetases and anti-U1 RNP antibodies [ 62 ]. (See "Mixed connective tissue disease" .)
• Infrequently, nodules similar to those seen in RA may occur in patients with SLE, and other nodular lesions (eg, gouty tophi) may mimic rheumatoid nodules. (See "Rheumatoid nodules", section on 'Differential diagnosis' .)
• Ulnar deviation of the digits that can be manually moved back into normal alignment is a Jaccoud’s arthropathy, which is not found with RA. The joint deformities of Jaccoud's arthropathy are caused by loosening and lengthening of periarticular structures and tendons, whereas the joint deformities of RA are caused by cartilage loss and joint destruction. Jaccoud’s arthropathy occurs in up to 5 to 10 percent of patients with Sjögren's disease or SLE and can also occur in sarcoidosis [ 63 ]. (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus" and "Sarcoid arthropathy" and "Clinical manifestations of Sjögren's disease: Extraglandular disease", section on 'Joints' .)
● Crystalline arthritis – Crystalline arthritis (gout and acute or chronic calcium pyrophosphate deposition disease [CPPD; pseudogout]) should be suspected in any patient over the age of 50 presenting with an inflammatory mono- or oligoarthritis. The diagnosis is established by the finding of urate or calcium pyrophosphate crystals, respectively, in synovial fluids.
Crystalline arthritis should also be considered in a patient with a polyarticular arthritis who has a long history of recurrent, self-limited flares that initially affected only one or two joints. The hallmark of a crystalline arthritis is its self-limited nature. Unlike RA, even in the absence of therapy, the flare will remit spontaneously in several weeks. However, since crystalline arthritis is not uncommon, its presence does not exclude the diagnosis of RA.
These diagnoses are discussed in detail separately. (See "Clinical manifestations and diagnosis of gout" and "Clinical manifestations and diagnosis of calcium pyrophosphate crystal deposition (CPPD) disease" .)
● Polymyalgia rheumatica – Polymyalgia rheumatica (PMR) should be suspected in any patient over the age of 50 presenting with an inflammatory arthritis limited to the shoulder and hips. Unlike RA, PMR is usually associated with marked myalgias in the shoulders and hips, and joint involvement tends to be milder [ 64 ]. The diagnosis is established by its rapid response to low-dose glucocorticoids.
In patients initially diagnosed with PMR, persistent or recurrent small joint arthritis with tapering of glucocorticoids and the absence of other findings suggestive of PMR may lead to a change in the diagnosis to RA after several months or even years of treatment.
PMR is discussed in detail separately. (See "Clinical manifestations and diagnosis of polymyalgia rheumatica" .)
● Reactive arthritis – Reactive arthritis should be suspected in any patient who presents with a monoarthritis or oligoarthritis following a recent infection (eg, urethritis, enteric) [ 65 ]. The following findings are more consistent with reactive arthritis than RA:
• Asymmetric pattern of joint involvement
• Symptoms or signs of enthesopathy (inflammation at the site where a tendon inserts into a bone, eg, the insertion point of the Achilles tendon into the heel)
• Keratoderma blennorrhagica ( picture 1 ) or circinate balanitis ( picture 2 and picture 3 ) (see "Reactive arthritis", section on 'Extraarticular signs and symptoms' )
• Radiologic evidence of sacroiliitis and/or spondylitis
• The presence of human leukocyte antigen (HLA) B27
• “Sausage” swelling of the digits, caused by inflammation of the tenosynovium ( picture 4 )
Reactive arthritis is discussed separately. (See "Reactive arthritis" .)
● Inflammatory bowel disease-associated arthritis – Inflammatory bowel disease (IBD)-associated arthritis should be suspected in a patient with an inflammatory arthritis who has a known or suspected diagnosis of IBD. Less commonly, arthritis may be the presenting symptom of IBD.
While IBD-related arthritis is more commonly a large-joint oligoarticular arthritis or spondyloarthritis with sacroiliitis, patients with IBD may infrequently develop a peripheral polyarthritis with prominent involvement of the metacarpophalangeal (MCP) joints that can be mistaken for RA.
As the spectrum of drugs used for IBD-associated arthritis and RA are similar, differentiating between these diagnoses may not be necessary to initiate pharmacotherapy. This diagnosis is discussed separately. (See "Clinical manifestations and diagnosis of arthritis associated with inflammatory bowel disease and other gastrointestinal diseases" .)
● Psoriatic arthritis – Psoriatic arthritis is typically diagnosed in a patient who presents with an oligoarthritis who has psoriasis and is seronegative for RF and ACPA.
However, it can be difficult to distinguish psoriatic arthritis from RA, since the arthritis may precede the rash by several years, and a small number of patients with psoriatic arthritis are RF positive. In such cases, the diagnosis may not be clear until the patient develops features that are highly characteristic of a specific diagnosis (eg, pattern of joint erosions in RA).
Because many therapies for these diagnoses overlap, distinguishing these diagnoses is not necessary prior to initiating pharmacotherapy. Psoriatic arthritis is discussed separately. (See "Clinical manifestations and diagnosis of psoriatic arthritis" .)
● Palindromic rheumatism – Palindromic rheumatism is a form of inflammatory arthritis that sequentially affecting one to several joint areas for hours to days, with symptom-free periods that may last from days to months. A minority of patients with this presentation will eventually evolve into RA [ 66,67 ]. The diagnosis is made clinically, based on a characteristic history of joint involvement.
However, unlike RA, palindromic rheumatism is associated with inflammation of the periarticular rather than intraarticular structures. Furthermore, the intense, episodic nature of palindromic rheumatism is similar to what is observed in autoinflammatory diseases, rather than RA. (See "Clinical manifestations of rheumatoid arthritis", section on 'Palindromic rheumatism' .)
● Sarcoid arthropathy – Sarcoid arthropathy should be considered in a patient who presents with an inflammatory arthritis and has a prior diagnosis of pulmonary sarcoidosis. The approach to establishing a diagnosis of sarcoid arthropathy is discussed separately. (See "Sarcoid arthropathy", section on 'Diagnostic approach' .)
Chronic arthritis in sarcoidosis may be oligo- or polyarticular and can appear similar to RA in some patients. It most frequently affects the ankles, knees, hands, wrist, and MCP and proximal interphalangeal (PIP) joints.
This disorder is distinguished from RA by the following findings:
• Elevated serum concentrations of angiotensin-converting enzyme (ACE) are found in up to 50 percent of patients
• A chest radiograph may reveal characteristic findings of sarcoidosis
• The pattern of acute arthritis with Lofgren’s syndrome in patients with sarcoidosis is not observed in those with RA
This diagnosis is discussed in detail elsewhere. (See "Sarcoid arthropathy" .)
● Multicentric reticulohistiocytosis – Multicentric reticulohistiocytosis should be suspected in a patient who presents with a highly destructive form of arthritis that is unresponsive to immunosuppressive therapies [ 68-73 ]. This diagnosis may be accompanied by smooth, shiny, erythematous nodules located in the periungual region. The diagnosis is established radiographically, or through biopsy of a skin lesion.
The rapid joint destruction of multicentric reticulohistiocytosis resembles the arthritis mutilans occasionally observed in psoriatic arthritis. Binucleated or multinucleated foreign body type giant cells are present on skin or synovial biopsies in multicentric reticulohistiocytosis [ 74,75 ]. In a minority of patients, an underlying malignancy may be present. (See "Cutaneous manifestations of internal malignancy", section on 'Multicentric reticulohistiocytosis' .)
Paraneoplastic and cancer treatment-related disease — Joint pain or frank polyarthritis can occur in association with cancer. The following are some examples:
● Hypertrophic osteoarthropathy – Patients with hypertrophic osteoarthropathy, sometimes termed hypertrophic pulmonary osteoarthropathy, typically demonstrate clubbing of the digits, joint pain, and periosteal new bone formation. Additionally, they give a characteristic history suggestive of bone pain and often describe the pain as deep and achy; nocturnal pain is common. Joint effusions may occur.
Hypertrophic osteoarthropathy is discussed in detail separately. (See "Malignancy and rheumatic disorders", section on 'Hypertrophic osteoarthropathy' .)
● Myelodysplasia – Patients with myelodysplastic syndrome sometimes develop an inflammatory polyarthritis that mimics RA [ 76 ]. The majority of patients are seronegative for RF and few are positive for ACPA or exhibit erosive changes on joint radiography. The arthritis may precede the diagnosis of myelodysplasia in at least half of the patients. In a cohort study of 87 patients with myelodysplastic syndrome, five (6 percent) had inflammatory arthritis that resembled RA [ 77 ]. Persistence of anemia, other cytopenias, or elevated acute phase reactants despite control of the arthritis should heighten suspicion of myelodysplasia [ 76 ].
This diagnosis is discussed in detail elsewhere. (See "Clinical manifestations, diagnosis, and classification of myelodysplastic syndromes (MDS)" .)
● Immune checkpoint inhibitor therapy – Cancer therapy that involves use of immune checkpoint inhibitor (ICI) immunotherapy may result in a number of rheumatologic and other adverse effects, including inflammatory arthritis that resembles RA. The joint symptoms may range from mild to severe and can develop at almost any time during ICI therapy. RF and ACPAs are often absent. This diagnosis is discussed in detail elsewhere. (See "Rheumatologic complications of checkpoint inhibitor immunotherapy", section on 'Inflammatory arthritis' .)
Noninflammatory diagnoses — These diagnoses are typically associated with joint pain or dysfunction in the absence of true synovitis.
Osteoarthritis — Osteoarthritis (OA) can be confused with RA in the middle-aged or older patient when the small joints of the hands are involved. However, different patterns of clinical involvement usually permit the correct diagnosis ( table 3 ). The following are examples:
● Distribution of joint findings – OA of the fingers typically affects the distal interphalangeal (DIP) joints and is frequently associated with Heberden's nodes in this area. By contrast, RA typically affects the MCP and PIP joints and is not associated with Heberden's nodes.
The carpometacarpal joint of the thumb is typically involved in OA.
● Joint examination characteristics – Swelling of the joints is hard and bony in OA. By contrast, soft, warm, boggy, and tender joints are typical of RA.
● Presence of stiffness – Stiffness of the joint is a very common feature of RA but is relatively uncommon in OA. Furthermore, the stiffness of RA is characteristically worse after resting the joint (eg, morning stiffness), while the stiffness of OA, if present, is typically worse after any effort and is often described as evening stiffness. Morning stiffness in OA, when present, is usually transient or lasts no more than a few minutes, unlike the more sustained stiffness typical of RA.
● Radiographs – Radiographs also help distinguish RA from OA. OA is characterized by narrowing of the joint space due to cartilage loss and osteophytes due to bone remodeling, but not erosions or cysts.
Erosive or inflammatory OA can cause severe and rapidly progressive arthritis in the small joints of the hands, often at middle age. Swelling in the PIPs with central joint erosion may be present. Central erosions (found in the center of the joint space) should be distinguished from the marginal erosions of RA, which are found at the margin of the joint space. Distinction from RA can be made by characteristic findings on radiographs as well as a lack of systemic inflammation and serologic markers.
● Rheumatoid factor – OA is classically associated with the absence of RFs and normal levels of acute phase reactants. However, RFs may be present, usually in low titer, consistent with the patient's (older) age.
OA is discussed separately. (See "Clinical manifestations and diagnosis of osteoarthritis" .)
Stenosing tenosynovitis — Stenosing tenosynovitis should be suspected in any patient presenting with hand pain associated with triggering or locking of the digits. Stenosing tenosynovitis may be distinguished from RA by eliciting pain over the flexor tendons at the base of the digit and by the absence of synovitis in the joints of the hand.
Narrowing of the flexor tendon sheaths leading to stenosing tenosynovitis is common among patients with diabetes, thyroid disease, or occupational risk factors. Hand pain may be present even in the absence of overt triggering. (See "Trigger finger (stenosing flexor tenosynovitis)" .)
Diabetic cheiroarthropathy is a related condition that results in difficulty in the extension of the digits of the hand. This diagnosis is discussed separately. (See "Limited joint mobility in diabetes mellitus" .)
Carpal tunnel syndrome — Carpal tunnel syndrome should be suspected in any patient complaining of hand swelling, burning, or numbness, typically at night or in the morning. Carpal tunnel syndrome should also be considered in patients who demonstrate a positive Tinel or Phalen sign, have thenar wasting, and/or demonstrate poor hand dexterity or weakness in the "pinch test." The diagnosis can be confirmed with nerve conduction studies documenting median nerve dysfunction.
Synovitis in the wrist from RA may also cause median nerve entrapment leading to carpal tunnel syndrome. Thus, the presence of carpal tunnel syndrome does not exclude concurrent RA. However, carpal tunnel syndrome is common and observed at higher rates in patients with some comorbid conditions (eg, diabetes).
This diagnosis is discussed separately. (See "Carpal tunnel syndrome: Clinical manifestations and diagnosis" .)
Hypermobility syndrome — Although the hypermobility syndromes can bear superficial resemblances to RA due to the presence of polyarthralgia, there are important distinguishing features:
● The hypermobility syndromes are associated with hyperextensible joints, and patients lack signs of synovitis.
● The hypermobility syndrome is not associated with significant titers of RF or ACPAs or with elevated levels of acute phase reactants.
This diagnosis is discussed in detail elsewhere. (See "Clinical manifestations and diagnosis of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorder" .)
Fibromyalgia — Fibromyalgia may be distinguished from RA by using the following features:
● Fibromyalgia is associated with tender points at nonarticular sites and chronic widespread pain. However, there is no evidence of synovitis, such as swelling, warmth, or diminished joint range of motion, although patients may exhibit joint-line tenderness on examination. (See "Clinical manifestations and diagnosis of fibromyalgia in adults" .)
● Fibromyalgia is not associated with significant titers of RF or ACPAs or with elevated levels of acute phase reactants.
Although RA is usually not difficult to distinguish from fibromyalgia, diagnostic confusion can arise when assessment of serologies or acute phase reactants is performed and modestly elevated results are observed that are of unclear significance. In addition, a significant minority of patients with RA also develop fibromyalgia. The source of complaints in such patients needs to be carefully assessed to distinguish heightened pain sensitivity from pain related to inflammatory joint disease.
SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Rheumatoid arthritis" .)
INFORMATION FOR PATIENTS — UpToDate offers two types of patient education materials, “The Basics” and “Beyond the Basics.” The Basics patient education pieces are written in plain language, at the 5 th to 6 th grade reading level, and they answer the four or five key questions a patient might have about a given condition. These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are written at the 10 th to 12 th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon.
Here are the patient education articles that are relevant to this topic. We encourage you to print or e-mail these topics to your patients. (You can also locate patient education articles on a variety of subjects by searching on “patient info” and the keyword(s) of interest.)
● Basics topics (see "Patient education: Rheumatoid arthritis (The Basics)" )
● Beyond the Basics topics (see "Patient education: Rheumatoid arthritis symptoms and diagnosis (Beyond the Basics)" and "Patient education: Rheumatoid arthritis treatment (Beyond the Basics)" )
SUMMARY AND RECOMMENDATIONS
● When to suspect – A patient with rheumatoid arthritis (RA) will typically have constitutional symptoms, morning stiffness, elevated acute phase reactants, and a small-joint arthritis affecting the hands and feet. Joint erosions appear late in the disease process. (See 'When to suspect' above.).
● Evaluation – A diagnosis of RA requires an examination of the extremities to detect the presence of synovitis, which will typically present as a symmetric polyarthritis affecting the metacarpophalangeal (MCP), metatarsophalangeal (MTP), and proximal interphalangeal (PIP) joints.
In a patient suspected of having RA, both MRI and ultrasound can be used to determine the presence of synovitis when the physical examination is not clear. (See 'Establish the presence of inflammatory arthritis' above.)
● Diagnosis
• Use of serologies – In a patient who presents with a symmetric polyarthritis, the presence of rheumatoid factor (RF) or anti-citrullinated peptide antibodies(ACPA) confirms the diagnosis of RA. However, these tests have limited value as a screening tool or to evaluate patients with a syndrome atypical for RA, since both may appear in patients with other rheumatic or inflammatory diagnoses. (See 'Serology' above.)
• Use or radiologic studies – Plain radiographs demonstrating the presence of joint erosions confirms the diagnosis of RA. However, erosions are a late finding of RA, and their absence does not rule out RA. (See 'Radiologic studies' above.)
• Classification criteria – The 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria were not designed to establish a diagnosis of RA, although they are often used for this purpose. It is important to remember that these criteria may not identify RA in all patients, particularly after the initiation of immunosuppressive therapies. (See 'Diagnosis' above.)
● Differential diagnosis – Some infections, malignancies, and rheumatic diseases may present with synovitis that can mimic RA. Noninflammatory diagnoses, such as osteoarthritis (OA), carpal tunnel syndrome, and hypermobility syndromes, may present with joint pain or dysfunction in the absence of synovitis. (See 'Differential diagnosis' above.)
ACKNOWLEDGMENTS — The UpToDate editorial staff acknowledges Ravinder N Maini, BA, MB BChir, FRCP, FMedSci, FRS and PJW Venables, MA, MB BChir, MD, FRCP, who contributed to earlier versions of this topic review.
- Funovits J, Aletaha D, Bykerk V, et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis 2010; 69:1589.
- Baker JF, Conaghan PG, Gandjbakhch F. Update on magnetic resonance imaging and ultrasound in rheumatoid arthritis. Clin Exp Rheumatol 2018; 36 Suppl 114:16.
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007; 146:797.
- Wolfe F, Cathey MA, Roberts FK. The latex test revisited. Rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis Rheum 1991; 34:951.
- Jansen AL, van der Horst-Bruinsma I, van Schaardenburg D, et al. Rheumatoid factor and antibodies to cyclic citrullinated Peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis. J Rheumatol 2002; 29:2074.
- Quinn MA, Green MJ, Marzo-Ortega H, et al. Prognostic factors in a large cohort of patients with early undifferentiated inflammatory arthritis after application of a structured management protocol. Arthritis Rheum 2003; 48:3039.
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380.
- Tracy A, Buckley CD, Raza K. Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Semin Immunopathol 2017; 39:423.
- Nielsen SF, Bojesen SE, Schnohr P, Nordestgaard BG. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ 2012; 345:e5244.
- Halldórsdóttir HD, Jónsson T, Thorsteinsson J, Valdimarsson H. A prospective study on the incidence of rheumatoid arthritis among people with persistent increase of rheumatoid factor. Ann Rheum Dis 2000; 59:149.
- Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2006; 65:845.
- Whiting PF, Smidt N, Sterne JA, et al. Systematic review: accuracy of anti-citrullinated Peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010; 152:456.
- Schellekens GA, de Jong BA, van den Hoogen FH, et al. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 1998; 101:273.
- Bas S, Genevay S, Meyer O, Gabay C. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology (Oxford) 2003; 42:677.
- Zeng X, Ai M, Tian X, et al. Diagnostic value of anti-cyclic citrullinated Peptide antibody in patients with rheumatoid arthritis. J Rheumatol 2003; 30:1451.
- Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis 2003; 62:870.
- van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 2004; 50:709.
- Fabien N, Olsson NO, Goetz J, et al. Prevalence of autoantibodies to cyclic citrullinated peptide in patients with rheumatic diseases other than rheumatoid arthritis: a French multicenter study. Clin Rev Allergy Immunol 2008; 34:40.
- van Venrooij WJ, Zendman AJ. Anti-CCP2 antibodies: an overview and perspective of the diagnostic abilities of this serological marker for early rheumatoid arthritis. Clin Rev Allergy Immunol 2008; 34:36.
- Qing YF, Zhang QB, Zhou JG, et al. The detecting and clinical value of anti-cyclic citrullinated peptide antibodies in patients with systemic lupus erythematosus. Lupus 2009; 18:713.
- Kakumanu P, Sobel ES, Narain S, et al. Citrulline dependence of anti-cyclic citrullinated peptide antibodies in systemic lupus erythematosus as a marker of deforming/erosive arthritis. J Rheumatol 2009; 36:2682.
- Gottenberg JE, Mignot S, Nicaise-Rolland P, et al. Prevalence of anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with primary Sjögren's syndrome. Ann Rheum Dis 2005; 64:114.
- Mohammed K, Pope J, Le Riche N, et al. Association of severe inflammatory polyarthritis in primary Sjögren's syndrome: clinical, serologic, and HLA analysis. J Rheumatol 2009; 36:1937.
- Atzeni F, Sarzi-Puttini P, Lama N, et al. Anti-cyclic citrullinated peptide antibodies in primary Sjögren syndrome may be associated with non-erosive synovitis. Arthritis Res Ther 2008; 10:R51.
- Chan MT, Owen P, Dunphy J, et al. Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol 2008; 35:77.
- Bogliolo L, Alpini C, Caporali R, et al. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol 2005; 32:511.
- Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis 2005; 64:1145.
- Alenius GM, Berglin E, Rantapää Dahlqvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis 2006; 65:398.
- Böckelmann R, Gollnick H, Bonnekoh B. Anti-cyclic citrullinated peptide antibodies in psoriasis patients without arthritis. Arthritis Rheum 2006; 54:1701.
- Elkayam O, Segal R, Lidgi M, Caspi D. Positive anti-cyclic citrullinated proteins and rheumatoid factor during active lung tuberculosis. Ann Rheum Dis 2006; 65:1110.
- Kakumanu P, Yamagata H, Sobel ES, et al. Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies, but their sera also react with unmodified arginine-containing peptide. Arthritis Rheum 2008; 58:1576.
- Mori S, Naito H, Ohtani S, et al. Diagnostic utility of anti-cyclic citrullinated peptide antibodies for rheumatoid arthritis in patients with active lung tuberculosis. Clin Rheumatol 2009; 28:277.
- Matsui T, Shimada K, Ozawa N, et al. Diagnostic utility of anti-cyclic citrullinated peptide antibodies for very early rheumatoid arthritis. J Rheumatol 2006; 33:2390.
- Wood AM, de Pablo P, Buckley CD, et al. Smoke exposure as a determinant of autoantibody titre in α₁-antitrypsin deficiency and COPD. Eur Respir J 2011; 37:32.
- de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Verpoort KN, et al. Progression of joint damage in early rheumatoid arthritis: association with HLA-DRB1, rheumatoid factor, and anti-citrullinated protein antibodies in relation to different treatment strategies. Arthritis Rheum 2008; 58:1293.
- Lindqvist E, Eberhardt K, Bendtzen K, et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 2005; 64:196.
- Meyer O, Labarre C, Dougados M, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 2003; 62:120.
- Rönnelid J, Wick MC, Lampa J, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005; 64:1744.
- Finckh A, Liang MH. Anti-cyclic citrullinated peptide antibodies in the diagnosis of rheumatoid arthritis: bayes clears the haze. Ann Intern Med 2007; 146:816.
- Jansen LM, van Schaardenburg D, van der Horst-Bruinsma I, et al. The predictive value of anti-cyclic citrullinated peptide antibodies in early arthritis. J Rheumatol 2003; 30:1691.
- van der Helm-van Mil AH, Verpoort KN, Breedveld FC, et al. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005; 7:R949.
- Quinn MA, Gough AK, Green MJ, et al. Anti-CCP antibodies measured at disease onset help identify seronegative rheumatoid arthritis and predict radiological and functional outcome. Rheumatology (Oxford) 2006; 45:478.
- Bukhari M, Thomson W, Naseem H, et al. The performance of anti-cyclic citrullinated peptide antibodies in predicting the severity of radiologic damage in inflammatory polyarthritis: results from the Norfolk Arthritis Register. Arthritis Rheum 2007; 56:2929.
- Koh JH, Jung SM, Lee JJ, et al. Radiographic Structural Damage Is Worse in the Dominant than the Non-Dominant Hand in Individuals with Early Rheumatoid Arthritis. PLoS One 2015; 10:e0135409.
- THOMPSON M, BYWATERS EG. Unilateral rheumatoid arthritis following hemiplegia. Ann Rheum Dis 1962; 21:370.
- van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum 1992; 35:26.
- Fuchs HA, Kaye JJ, Callahan LF, et al. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol 1989; 16:585.
- Ulijn E, den Broeder N, Ten Cate D, et al. Limited Diagnostic and Prognostic Value of Routine Radiographs in Newly Presenting Arthritis Suspected of Rheumatoid Arthritis: A Retrospective Study. Arthritis Care Res (Hoboken) 2024; 76:497.
- Terslev L, Torp-Pedersen S, Savnik A, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum 2003; 48:2434.
- Wakefield RJ, Gibbon WW, Conaghan PG, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum 2000; 43:2762.
- McQueen FM, Benton N, Perry D, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum 2003; 48:1814.
- McQueen FM, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis 1999; 58:156.
- Ejbjerg BJ, Narvestad E, Jacobsen S, et al. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis 2005; 64:1280.
- Naraghi AM, White LM, Patel C, et al. Comparison of 1.0-T extremity MR and 1.5-T conventional high-field-Strength MR in patients with rheumatoid arthritis. Radiology 2009; 251:829.
- Cohen SB, Potter H, Deodhar A, et al. Extremity magnetic resonance imaging in rheumatoid arthritis: Updated literature review. Arthritis Care Res (Hoboken) 2011; 63:660.
- Klarlund M, Ostergaard M, Jensen KE, et al. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. The TIRA Group. Ann Rheum Dis 2000; 59:521.
- McQueen FM. The use of MRI in early RA. Rheumatology (Oxford) 2008; 47:1597.
- McQueen FM, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis 1998; 57:350.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69:1580.
- 2010 ACR/EULAR classification criteria for rheumatoid arthritis. http://www.rheumatology.org/practice/clinical/classification/ra/ra_2010.asp (Accessed on January 30, 2014).
- Venables PJ. Polymyositis-associated overlap syndromes. Br J Rheumatol 1996; 35:305.
- Cronin ME. Musculoskeletal manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am 1988; 14:99.
- Pease CT, Haugeberg G, Montague B, et al. Polymyalgia rheumatica can be distinguished from late onset rheumatoid arthritis at baseline: results of a 5-yr prospective study. Rheumatology (Oxford) 2009; 48:123.
- Toivanen A. Reactive arthritis. In: Rheumatology, Klippel, Dieppe (Eds), Mosby, London 1994. p.491.
- Salvador G, Gomez A, Vinas O, et al. Prevalence and clinical significance of anti-cyclic citrullinated peptide and antikeratin antibodies in palindromic rheumatism. An abortive form of rheumatoid arthritis? Rheumatology (Oxford) 2003; 42:972.
- Russell AS, Devani A, Maksymowych WP. The role of anti-cyclic citrullinated peptide antibodies in predicting progression of palindromic rheumatism to rheumatoid arthritis. J Rheumatol 2006; 33:1240.
- Sanchez-Alvarez C, Sandhu AS, Crowson CS, et al. Multicentric reticulohistiocytosis: the Mayo Clinic experience (1980-2017). Rheumatology (Oxford) 2020; 59:1898.
- Kumar B, Singh N, Rahnama-Moghadam S, et al. Multicentric Reticulohistiocytosis: A Multicenter Case Series and Review of Literature. J Clin Rheumatol 2018; 24:45.
- Matejicka C, Morgan GJ, Schlegelmilch JG. Multicentric reticulohistiocytosis treated successfully with an anti-tumor necrosis factor agent: comment on the article by Gorman et al. Arthritis Rheum 2003; 48:864.
- Calamia KT, Walsh JS, Bradley T, et al. Treatment of multicentric reticulohistiocytosis with etanercept: a case report (abstract). Arthritis Rheum 2003; 48:S618.
- Goto H, Inaba M, Kobayashi K, et al. Successful treatment of multicentric reticulohistiocytosis with alendronate: evidence for a direct effect of bisphosphonate on histiocytes. Arthritis Rheum 2003; 48:3538.
- Codriansky KA, Rünger TM, Bhawan J, et al. Multicentric reticulohistiocytosis: a systemic osteoclastic disease? Arthritis Rheum 2008; 59:444.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. A review of 33 patients. Medicine (Baltimore) 1969; 48:287.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum 2000; 43:930.
- Mekinian A, Braun T, Decaux O, et al. Inflammatory arthritis in patients with myelodysplastic syndromes: a multicenter retrospective study and literature review of 68 cases. Medicine (Baltimore) 2014; 93:1.
- Chandran G, Ahern MJ, Seshadri P, Coghlan D. Rheumatic manifestations of the myelodysplastic syndromes: a comparative study. Aust N Z J Med 1996; 26:683.

Contributor Disclosures
Contributor disclosures are reviewed for conflicts of interest by the editorial group. When found, these are addressed by vetting through a multi-level review process, and through requirements for references to be provided to support the content. Appropriately referenced content is required of all authors and must conform to UpToDate standards of evidence.
Conflict of interest policy
Print Options
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(11); 2023 Nov
- PMC10710847

Diet and Lifestyle Impact on Rheumatoid Arthritis: A Comprehensive Review
Kartikey v shekhar.
1 Medical Education, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, IND
Mrunmayee M Pathak
Gajanan pisulkar.
2 Orthopaedics, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, IND
A systemic, inflammatory illness such as rheumatoid arthritis (RA) causes progressive cartilage and bone degradation in addition to joint involvement. Factors related to genetics and environment determine susceptibility to RA. In recent years, an increasing body of research has illuminated the pivotal role of diet and lifestyle in influencing the risk and progression of illnesses. Some nutrients, like polyunsaturated fatty acids, can combat inflammation. They also act as antioxidants, thus protecting against the onset of RA. Conversely, substances like salt and red meat have adverse effects, promoting the development and progression of RA through indirect mechanisms that impact gut microbiota and body composition. As we look ahead, potential supplementary therapies alongside the existing RA treatment regimen may manifest as specific dietary patterns and supplements. Promising candidates include the Mediterranean Diet (MD), vitamin D, and probiotics, which could potentially confer protective benefits. A poor level of education and low socioeconomic status, as well as smoking, an unhealthy diet, and obesity, have all been linked to an increased risk of RA in large epidemiological studies. Additionally, several lifestyle choices affect how well RA responds to antirheumatic medications. A worse treatment outcome is linked, among other things, to smoking, obesity, and insufficient physical activity. Therefore, RA sufferers must be urged to live a healthy lifestyle and eat well.
Introduction and background
Rheumatoid arthritis (RA) is an inflammatory ailment known for its capacity to inflict significant damage upon synovial joints, often leading to profound disability and heightened mortality rates [ 1 ]. The etiology of RA is intricate, with genetic and environmental elements serving as critical determinants in its commencement and severity [ 1 ]. A well-established risk factor is a genetic predisposition supported by epidemiological data that illustrates an elevated vulnerability in individuals with a familial history of RA [ 2 ]. Gender and age also wield considerable influence, with women and middle-aged individuals being more frequently affected. Lifestyle choices, such as smoking, excessive consumption of red meat, lack of exercise, low intake of dietary fibers and essential fatty acids, consumption of alcohol, and gluten consumption, further augment the risk, while infectious agents like Epstein-Barr virus, retroviruses, and parvovirus B19 can potentially incite or exacerbate the condition [ 1 , 3 ]. Hormonal, dietary, socioeconomic, and ethnic aspects further contribute to the multifaceted nature of RA's pathogenesis. Importantly, many of these elements are interlinked, impacting the ailment's evolution and progression. Understanding the interplay between genetic and environmental factors is paramount in enhancing preventive measures, diagnosis, and management of RA [ 1 ].
Among these factors, dietary choices and nutritional intake have been the subject of extensive research regarding their potential involvement in the onset and progression of autoimmune diseases [ 2 ]. Although some research has suggested that there may be a link between specific dietary patterns - especially when it comes to the amount of fruit, vegetables, and meat consumed and diseases like RA - the findings are still unclear. A growing amount of research has been conducted in recent years to examine the role nutrition and food may have in managing and preventing many illnesses, including RA [ 2 , 3 ]. When considering genetic and other lifestyle variables, the Mediterranean diet has a role in the reduced prevalence of RA in Southern Europe as opposed to Northern Europe and North America [ 4 ]. The main goals of this research are to investigate how nutrition and food affect the development of RA and to evaluate how nutritional choices can potentially influence the disease's activity [ 3 , 5 ].
Methodology
We performed a comprehensive search in the electronic databases PubMed, Medline, Embase, Google Scholar, and ResearchGate and an examination of the English-language literature. Inclusion criteria include peer-reviewed journals produced in English, articles published in the last 15 years, the full text of the publication; type of publication: review articles, systematic review, meta-analysis, or empirical studies published in peer-reviewed scientific journals; compliance with the combinations of keywords: synbiotics, essential fatty acids, human leukocyte antigen, elimination diet, rheumatoid arthritis. Exclusion criteria for the study encompass articles that don't provide the full text of the publication, are not in English, fall outside the specified publication types (review articles, systematic reviews, meta-analyses, or empirical studies in peer-reviewed scientific journals), involve malignancy or co-morbidities impacting the disease's presentation, present treatment protocols other than those under review. Key terms used for the search were "synbiotics"[all fields] or "essential fatty acids"[all fields] and "Human Leukocyte Antigen"[all fields] or "Elimination Diet"[Mesh terms] or "rheumatoid arthritis"[all fields]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method used in the research methodology are depicted in Figure 1 .

Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flowchart for the keywords used in the literature review.
Years before the disease's clinical presentation, a multi-step process involving interactions between hereditary and environmental variables begins the pathogenesis of RA. The human leukocyte antigen (HLA) class II molecule is encoded on chromosome 6p21.3, the most important genetic risk locus linked to RA. A typical amino acid sequence known as the "shared epitope" (SE) is found at locations 70-74 in the third region of the DR-1 chain in several HLA-DRB1 alleles associated with seropositive RA [ 4 ]. In genetically susceptible people, environmental factors can compromise their endurance to autoantigen, such as citrullinated and carbamylated proteins. Long before any detectable symptoms appear, various environmental factors, including cigarette smoking, air pollution, exposure to dust, dietary habits, and susceptibility to infections, significantly contribute to developing autoantibodies and systemic autoimmune responses [ 5 ]. The pathophysiology of RA is supported by several important discoveries that point to the role of environmental, nutritional, reproductive, and lifestyle variables. For example, the fact that two-thirds of RA patients are female may indicate a potential function for female hormones. Furthermore, the incidence and age at the beginning of RA exhibit a discernible latitude gradient effect, and higher educational attainment and socioeconomic level are consistently associated with a higher risk of developing RA [ 6 ]. It's important to emphasize that one's diet significantly influences the makeup of their microbiota, which has been linked to the disease's onset and progression (Figure (Figure2 2 ).

Figure credit: Author (Kartikey Vats Shekhar)
TMAO: Trimethylamine N-oxide
Compared to women, men seem to face a higher level of risk than women of getting RA due to smoking. Remarkably, if you stop smoking, the correlation between your chances of developing RA seems to decrease. To be more precise, the correlation between smoking and the risk of anti-citrullinated peptide antibody-negative RA (ACPA-negative RA) vanished entirely twenty years after stopping. In contrast, the correlation with the risk of ACPA-positive RA remained linked to the quantity of cigarettes smoked. The connection between smoking and the widely recognized genetic susceptibility marker, known as the HLA-DRB1 SE, and how this association impacts the onset of RA in individuals with both antibody-positive and antibody-negative forms is a subject of interest [ 7 ]. Smokers with two copies of the SE had a 21-fold increased risk of getting RA compared to non-smokers without any SE copies. Furthermore, the quantity and severity of smoking raised the likelihood of ACPA-positive RA linked to SE and smoking. In the context of ACPA-positive RA, our findings highlight a strong gene-environment interaction and a dose-response connection between genetic and environmental risk variables [ 8 ].
Exposure to silica is the second most commonly stated environmental risk linked to the onset of RA. Despite accounting for the influence of smoking, various longitudinal and case-control investigations have uncovered associations between RA in males and specific occupations, including those involving granite workers, rock drillers, and stone crushers. Notably, case-control studies have revealed an inverse relationship between silica exposure and the risk of RA in individuals working with sandstone, ceramics, and refractory materials. Like smoking, silica exposure is largely linked to seropositive RA [ 9 ]. Interestingly, smokers exposed to silica had a significant incidence of ACPA-positive RA, suggesting that these exposures may interact. But, as these results don't account for variables like duration or intensity, care should be exercised when interpreting them [ 10 ].
Reproductive factors in women
Studies investigating the complex and multifaceted pathophysiology of RA have emphasized the role of female hormones, including progestogens and estrogen.
Gender Discrepancy
Before age 50, the ratio of women to men diagnosed with RA was 2:4, indicating a higher prevalence in women. This suggests that female hormones may play a role in susceptibility.
Age-Related Shifts
Beyond 60, the proportion of women to men decreases, suggesting that the impact of female hormones on RA risk may vary with age [ 11 ].
Postpartum and Menopause
Given the elevated prevalence of RA during these life stages, it's conceivable that hormonal changes associated with these transitions contribute to the onset of the condition.
Reproductive Years
Approximately 50% of RA cases initiate during a woman's reproductive years, implying a potential connection between the disease's development and female hormones.
Hormonal Fluctuations
Changes in hormonal exposure in females, as seen in events like pregnancy, postpartum, breastfeeding, menopause, and the utilization of external hormones, can induce fluctuations in their hormonal profiles. It is crucial to note that the impact of female hormones on RA can exhibit both pro-inflammatory and anti-inflammatory characteristics. This duality is contingent upon factors like serum hormone concentrations and the particular stage of the reproductive cycle. Ongoing research is actively investigating the precise mechanisms by which these hormones impact the pathophysiology of RA. While these findings suggest a link between female hormones and RA, rheumatologists diligently study the exact role and underlying mechanisms [ 12 ].
Impacts of body composition on RA
An individual's body composition can majorly impact how likely they are to develop RA. Obesity, a higher body mass index (BMI), and an enlarged waist measurement are risk factors for RA. Because white adipose tissue may create pro-inflammatory mediators, including IL-6, interferon (IFN)-alfa, C-reactive protein (CRP), and adipokines, it is classified as an "endocrine organ." The heightened synthesis of pro-inflammatory substances promotes the onset of autoimmunity. Leptin is an inflammatory adipokine that inhibits regulatory T-cell function in addition to inducing the production of inflammatory cytokines by macrophages. Leptin hormone hinders B-cell apoptosis and increases autoreactive cell preservation and growth in the preclinical stage of RA. Another adipokine that has several uses and structural similarities to tumor necrosis factor-alpha (TNF-α) is called adiponectin [ 13 ]. In people with RA, adiponectin contributes to the inflammatory environment that fosters osteoclast formation in the synovial tissue. A disease known as "sarcopenic obesity," which affects up to two-thirds of people with established RA, especially women, is typified by a rise in fat mass and muscle atrophy without a discernible change in body weight. Pro-inflammatory cytokines found in skeletal muscles encourage the breakdown of muscle proteins and cause a decrease in muscle mass. Nonetheless, several lifestyle choices, such as glucocorticoid usage, food habits, and inactivity greatly increase the likelihood of RA patients acquiring sarcopenic obesity [ 14 ].
Elimination diet
Rheumatoid arthritis symptoms might be made worse by several meals and dietary ingredients. An "elimination diet" strategy, which involves removing food-related antigens that may exacerbate illness symptoms, may thus be taken into consideration [ 15 ]. The mucosal immune system and the external environment are shielded from one another by the intestinal epithelium. The immunological response that develops in response to certain food antigens is largely determined by this barrier [ 16 ]. Several investigations have suggested that some foods may function as antigens in the human body. They pass through the epithelium of the gastrointestinal tract, engage the mucosal immune system, and then reach the circulation. Additionally, it has been noted that the gut mucosa of RA patients on non-steroidal anti-inflammatory drugs (NSAIDs) becomes more permeable to allergens [ 17 ]. Two groups of RA patients who tested positive for the antibody were included in the research. While the other group had a diet confined to allergies, including lactoproteins and yellow colors, the first group abstained from additives, allergens, and preservatives. Patients with RA who adhered to these dietary plans achieved similar clinical outcomes [ 18 ].
Two groups of RA patients were included in another study by Karatay et al.: those with a positive skin prick test (SPT) reaction to a minimum of one food item and those having wholly negative SPT findings. Every patient adhered to an exclusion diet for a certain amount of time. Foods that produced positive skin prick reactions were given to the Patient Participation Group (PPG), whereas larger amounts of highly allergic maize and non-allergenic rice- known to cause allergies in RA patients were ingested by the placebo non-allergenic grain (PNG) group. This was followed by a re-elimination phase [ 19 ]. Many indicators showed increases during and after the re-elimination phase among the PPG, including Erythrocyte Sedimentation Rate (ESR), CRP, pain, joint swelling, joint sensitivity, Risk Analysis Index (RAI) score, TNF, and IL-1. According to these studies, food allergies could work as immune system triggers, causing macrophages and other effector cells to become active and causing inflammation. In RA therapy, inflammatory mediators such as TNF-α and IL-1 are addressed. Interestingly, eating foods that trigger allergies seems to increase these inflammatory mediators. Thus, cutting out certain items that cause allergies from RA patients' diets could be advantageous, perhaps lessening the requirement for anti-TNF-α and recombinant human IL-1 receptor antagonists throughout their therapy [ 20 ].
Dietary fibres and whole grains
Previous studies have established a clear inverse correlation between the consumption of dietary fiber and markers of inflammation, such as plasma fibrinogen, TNF-α, high-sensitivity CRP (hs-CRP), and IL-6 levels, which are indicative of rheumatoid arthritis [ 21 ]. Food items are considered "whole" when they contain equal amounts of germ, endosperm, and bran, akin to whole grains. Whole cereals like wheat, corn, rye, whole rice, oats, barley, millets, sorghum, canary seed, fonio, and wild rice are renowned for their high levels of antioxidants, phytic acid, vitamin E, and selenium. These constituents are believed to contribute to reducing inflammation [ 22 ]. The Food and Drug Administration (FDA) has authorized claims for health promotion regarding dietary fibers and whole grains in relation to RA, even in the absence of conclusive evidence. As per the Dietary Reference Intakes recommendations, a dietary fiber intake within the range of 14 grams per 1,000 kilocalories, or 25 grams for adult women and 38 grams for adult men, is deemed beneficial for one's health [ 23 ].
Due to strong phenolic compounds, including shogaols and gingerols, ginger has long been known for its medicinal benefits. Turmeric, abundant in phenolic curcuminoids, has also demonstrated anti-tumor properties [ 24 ]. In research, the adjuvant-induced arthritic rats received a precise combination of ginger and turmeric. This combination showed protective benefits against RA extra-articular complications. In a separate research, the same team discovered that ginger and turmeric, when combined at a dose of 200 mg/kg body weight, might independently reduce RA signs and symptoms in male Wistar albino rats that had been subjected to an adjuvant-induced arthritic condition. The results demonstrated statistical significance, as evidenced by a p-value of 0.05 when compared to the control group that solely received indomethacin [ 25 ].
In an in vitro investigation using synoviocytes generated from RA patients and expressing IL-1 and IL-6, curcumin has also been demonstrated to be a strong anti-inflammatory spice [ 26 ]. For the treatment of RA, methotrexate is a commonly used antirheumatic medication; however, it also causes vascular endothelial dysfunction and elevates oxidative stress. Methotrexate-induced vascular endothelial dysfunctions in male Wistar rats were reported to be reduced by co-administration of curcumin and folic acid [ 27 ]. In many Southeast Asian cuisines, cinnamon bark, or Cinnamomum zeylanicum , is employed. The polyphenolic fraction of cinnamon bark was used by Rathi et al. to treat male Swiss albino mice and Wistar rats used as RA animal models. In their research, it was found that there were inhibitory effects observed in the release of cytokines such as IL-2, IL-4, and IFN, along with a reduction in TNF-α levels [ 28 ].
Essential fatty acids
The ability of omega-3 or omega-6 fatty acids to reduce inflammation and inhibit the immune system has been demonstrated. Gamma-linolenic acid (GLA), an omega-6 fatty acid, is abundant in borage seed oil. Thirty-seven patients with active RA participated in a double-blind experiment where they were given borage seed oil containing 1.4 g of GLA daily, whereas the placebo group received cottonseed oil. The group that got GLA had considerably lower ratings for sore and swollen joints after 24 weeks of intake, whereas the placebo group showed no change [ 25 ]. The medicinal potential of gamma-linolenic acid, omega-3 fatty acids alpha-linolenic and stearidonic acid, and black currant seed oil (BCSO) has also been studied. In a double-blind study, RA patients received 10.5 g of BCSO and soybean oil as a placebo for 24 weeks. Compared to the placebo group, the BCSO-treated group showed significantly better results regarding joint tenderness and pain relief [ 29 ].
High levels of omega-3 fatty acids are found in fish oils, and several controlled studies have examined how well they work to treat RA. In a double-blind study, RA patients were given fish oil containing 3.6 g of omega-3 fatty acids daily. At the same time, the placebo group received a combination of fatty acids for 12 weeks, comparable to the amount found in a typical diet. Compared to the placebo group, the fish oil group had less morning stiffness and a notable gain in grip strength. The capacity of the omega-3 fatty acids eicosapentaenoic and docosahexaenoic acids to lessen the severity of RA has been studied. Compared to the placebo group, which got just maize oil, RA patients who took these derivatives in a dose of 130 mg/kg body weight/day for 26-30 weeks experienced significantly less discomfort, morning stiffness, and painful joints [ 30 ].
There is ongoing discussion over the connection between alcohol use and the onset of RA. While some studies suggest that drinking alcohol might accelerate the course of RA, other studies find no evidence of this relationship. Alcohol usage was shown to be dose-dependently related to a lower incidence of RA as compared to non-drinkers in a recent case-control research conducted in a Scandinavian population. These results were consistent with gender, age, and variations in cyclic citrullinated peptide (CCP) status [ 31 ]. In another study, which concentrated on the frequency of alcohol intake among Caucasian RA patients rather than the quantity, a similar tendency was seen. Researchers found a notable inverse correlation between increased alcohol consumption and the severity indicators of RA, encompassing factors such as CRP levels, the Disease Activity Score in 28 joints (DAS28), the modified Health Assessment Questionnaire, and the Pain Visual Analog Scale (VAS).
The natural substance epigallocatechin-3-gallate (EGCG), which is gaining much interest as a nutraceutical, has demonstrated encouraging therapeutic qualities. Extracted from the dried leaves of Camellia sinensis and Camellia assamica , two members of the Theaceae family of plants, it is the primary phytochemical in green tea. The prophylactic properties of green tea against a range of maladies, such as neurological diseases, inflammatory disorders, cardiovascular ailments, and various malignancies, have been extensively demonstrated [ 32 ]. The resistance of synovial fibroblasts to apoptosis is one of the unique characteristics of RA. This resistance is frequently associated with the overexpression of anti-apoptotic proteins such as Mcl-1 and B-cell lymphoma-2 (Bcl-2) and the ongoing synthesis of proteins like nuclear factor (NF) and protein kinase B (PKB). Research has effectively demonstrated that synovial fibroblasts' Mcl-1 levels may be lowered by EGCG injection, increasing the cells' susceptibility to apoptosis. Furthermore, EGCG effectively inhibits the synthesis of matrix metalloproteinases (MMP-1, MMP-2, and MMP-3) in synovial fibroblasts, thereby halting the degradation of bone and cartilage. EGCG therapy reduces the production of IL-1 and IL-6 by synovial fibroblasts in RA patients while enhancing the activity of the soluble glycoprotein gp130 receptor inhibitor, which in turn inhibits IL-6 trans-signaling [ 33 ].
The first research to look at the relationship between eating red meat and the likelihood of developing inflammatory polyarthritis was conducted by Pattison et al. [ 34 ]. According to their research, consuming more red meat and protein may raise your chance of developing inflammatory polyarthritis. They did admit, though, that it was unclear whether these correlations resulted from other lifestyle variables or causal. Later, several prospective cohort studies investigated the association between meat consumption-including red meat, processed meat, and poultry, and the chance of developing RA. Still, none of them found a statistically significant correlation [ 35 ].
Gliadin and glutenin, the main proteins in wheat grains, form a complex combination called gluten, which can cause an immunological reaction, particularly in celiac disease patients. According to recent studies, gluten may also function as an antigen in RA, changing the immune system's response [ 36 ]. A year of strict adherence to a vegan, gluten-free diet was associated with a substantial drop in antibodies against gliadin and beta-lactoglobulin and a decline in the incidence of disease in individuals with RA. Lower levels of low-density lipoprotein (LDL) and oxidized LDL were seen in another randomized research with 66 RA patients who followed the same dietary pattern, which similarly showed protective benefits against atherosclerosis and inflammation [ 37 ].
Subtotal fasting involves a restricted intake of carbohydrates and energy, primarily through vegetable juice and vitamin and mineral supplements. Fasting can reverse the usual immunological condition associated with RA by lowering the quantity and activity of CD4+ cells. Increased Th1 and Th17 lineage differentiation and activation of CD4+ T cells are common in RA [ 38 ]. T cell activation can be reduced by a brief (7-10 days) fasting period, which can also have a transient immunosuppressive impact. While evidence of decreased pain and inflammation (as assessed by ESR and CRP) has been shown while fasting, these benefits are transient and do not result in long-term adjustments to disease activity [ 39 ].
It has been demonstrated that a vegan diet, rich in dietary fibers, lactobacilli, and antioxidants, can improve the composition of the gut flora and RA disease activity. Twenty-four people with moderate to severe RA participated in a single-blind dietary intervention experiment and followed a low-fat diet (less than 10% fat) for four weeks. Except for the length of the morning stiffness, there were notable decreases in body weight and RA symptoms after this time [ 40 ]. A comparative analysis of all the studies included in the review is described in Table 1 .
Conclusions
In conclusion, eating a diet high in raw or mildly cooked vegetables, especially greens and legumes, as well as adding spices like ginger and turmeric, might be beneficial for treating rheumatoid arthritis (RA). The diet should also include seasonal fruits and probiotic yogurt, which are high in natural antioxidants and anti-inflammatory qualities. On the other hand, meals heavy in salt, oils, butter, sugar, and animal products should be avoided by patients, as well as processed foods. Furthermore, nutritional supplements such as multivitamins, vitamin D, and cod liver oil can help treat RA. This dietary-focused strategy and low-impact aerobic training can be utilized to improve RA self-management at a reasonable cost. Nevertheless, it's important to emphasize that great patient compliance is usually required for effective RA therapy and management. Studies have repeatedly shown that smoking greatly increases the chance of RA, especially in those genetically predisposed to the condition. Exposure to silica is a strong non-smoking inhalant risk factor for RA, and it may interact with smoking. These two variables are linked to a common notion of systemic and pulmonary mucosal inflammation. Among the other inhalants, silica is the most significant inhalant risk factor. Though several research have examined possible links between different reproductive variables and the risk of RA, the impact of female hormones on that risk is still unknown. Inconsistent findings might be ascribed to methodological flaws and biases, including failing to account for smoking. Higher hormonal levels are associated with factors like early menarche, late menopause, parity, postmenopausal hormone therapy (PMH), and oral contraceptive use; lower hormonal levels are associated with postpartum status, early menopause, late menopause, and the use of anti-estrogen medications. A fascinating method for researching hormone exposures in females may be to evaluate total hormonal exposures while considering a woman's lifetime reproductive experiences.
The authors have declared that no competing interests exist.

IMAGES
VIDEO
COMMENTS
initial clinical presentation Rheumatoid arthritis (RA) most typically presents as polyarticular disease and with a gradual onset, but some patients can present with acute onset, with intermittent or migratory joint involvement, or with monoarticular disease.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown cause. The hallmark feature of this condition is persistent symmetric polyarthritis (synovitis) that affects the hands and feet, though any joint lined by a synovial membrane may be involved.
Early rheumatoid arthritis tends to affect your smaller joints first — particularly the joints that attach your fingers to your hands and your toes to your feet. As the disease progresses, symptoms often spread to the wrists, knees, ankles, elbows, hips and shoulders. In most cases, symptoms occur in the same joints on both sides of your body.
Due to the varied clinical presentation and lack of universal pathognomonic testing for RA, diagnosing the disease can be challenging early in the course of the disease. ... Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized ...
The clinical spectrum of rheumatoid arthritis (RA) presentation is heterogeneous, with wide variation in age of onset, degree of joint involvement and severity. Delayed rheumatological referral is associated with less favourable long-term outcomes. ... The clinical presentation of RA is heterogeneous, as is the subsequent disease course. It may ...
Rheumatoid arthritis is a chronic, systemic autoimmune disease manifested primarily as inflammatory arthritis, typically involving the small joints of the hands and feet. It can lead to severe disa...
The course of RA and the initial presentation can be highly variable; most people with RA have definite synovitis on clinical assessment, but sometimes this is not obvious, leading to uncertainty about the diagnosis. ... The 2016 National Clinical Audit for Rheumatoid Arthritis and Early Inflammatory Arthritis reported that 92% of people had ...
Rheumatoid arthritis is the most commonly diagnosed systemic inflammatory arthritis. ... allowing for early asymmetric presentation. ... Tehlirian C. Rheumatoid arthritis clinical and laboratory ...
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by prolonged morning stiffness, synovitis of the small joints of the hands and feet and with extraarticular manifestations. Marginal erosions and periarticular osteopenia are typical radiographic findings. Early diagnosis and initiation of disease-modifying antirheumatic drug therapy are key to the prevention of joint ...
E.M. Gravallese and G.S. FiresteinN Engl J Med 2023;388:529-542. Rheumatoid arthritis is a chronic, systemic autoimmune disease manifested primarily as inflammatory arthritis, typically involving ...
Rheumatoid arthritis is a chronic, inflammatory, autoimmune disease that primarily affects the joints. This Primer by Smolen et al. provides the latest insights into the epidemiology, genetics ...
Rheumatoid arthritis is a chronic, systemic, autoimmune inflammatory disease that mainly affects the joints and periarticular soft tissues. In this Seminar, we provide an overview of the main aspects of rheumatoid arthritis. Epidemiology and advances in the understanding of rheumatoid arthritis pathogenesis will be reviewed. We will discuss the clinical manifestations of rheumatoid arthritis ...
Clinical presentation The clinical presentation of RA is heterogeneous, as is the subsequent disease course. It may begin at any age with a wide spectrum of onset, although patients most commonly present from the sixth decade onwards with a peak incidence between 70 and 80 years of age. Women are affected about twice as commonly as men in the UK.2
multiple potential paths to a common clinical presentation. The disease progress- ... ized alleles associated with rheumatoid arthritis. R620W, a gain-of-function amino acid change in PTPN22 ...
The presence of anti-CCP are >98% specific for the diagnosis of rheumatoid arthritis; however, not all patients with RA will develop anti-CCP antibodies. Of significant importance is the recognition that these anti-CCP antibodies may be detected up to 15 years before the onset of clinical symptoms of RA indicating a preclinical phase of disease ...
The clinical spectrum of rheumatoid arthritis (RA) presentation is heterogeneous, with wide variation in age of onset, degree of joint involvement and severity. Delayed rheumatological referral is associated with less favourable long-term outcomes. Multidisciplinary teamwork is key to the success of a holistic approach to patient care and addressing issues that are important to the person.
Background. Rheumatoid arthritis (RA) is a symmetrical, persistent and destructive chronic inflammatory disease of connective tissue that predominantly affects the synovial membrane of freely movable joints, causing pain and deformity. 1 RA has been noted as a major cause of inflammatory disability. Early detection of disease and initiation of disease-modifying drugs can reduce the progression ...
Rheumatoid arthritis is a systemic autoimmune condition in which inappropriate activation of innate and adaptive immune responses cause inflammation leading to bone, cartilage, and synovium erosion. The primary goal of treatment includes targeting disease remission/low disease activity ultimately aiming at enhancing quality of life.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by progressive damage of synovial-lined joints and variable extra-articular manifestations. Tendon and bursal involvement are frequent and often clinically dominant in early disease. ... The clinical presentation of RA varies, but an insidious onset of pain with symmetric ...
NEW YORK — Patients with rheumatoid arthritis (RA) carry a high risk for cardiovascular events, but mounting clinical evidence suggests they're being undertreated to manage that risk ...
Rheumatoid arthritis (RA) is an immune-mediated multisystem inflammatory disease that predominantly affects the synovial joints. It was first described by Alfred Baring Garrod in the year 1800. The disease can lead to inflammation, joint destruction, deformity, and disability, and may also present with extra-articular manifestations. Inflammatory arthritis involving the small joints of the ...
A 69-year-old woman with rheumatoid arthritis (RA) under methotrexate and anti-TNF alpha agent treatment presented with a painful, firm and compressible swelling above the right acromioclavicular joint (ACJ) (Fig. 1A). Ultrasound revealed a large hypoechoic mass over ACJ and degenerative changes of this joint (Fig. 1B), complete supraspinous and infraspinous tear and glenohumeral joint (GHJ ...
Summary scores of the patient acceptability questionnaire of PsA screening questionnaires and a categorical presentation of question-by-question responses at Visit 1 ... Patients who have been diagnosed with inflammatory arthritis unrelated to PsA (rheumatoid arthritis, reactive arthritis, enteropathic arthritis, axial spondyloarthritis ...
INTRODUCTION — Rheumatoid arthritis (RA) is a symmetric, inflammatory, peripheral polyarthritis of unknown etiology. It typically leads to joint destruction through the erosion of cartilage and bone. ... DIFFERENTIAL DIAGNOSIS — Joint pain involving the hands is a common clinical presentation for a variety of conditions. Rheumatologists are ...
A systemic, inflammatory illness such as rheumatoid arthritis (RA) causes progressive cartilage and bone degradation in addition to joint involvement. ... Years before the disease's clinical presentation, a multi-step process involving interactions between hereditary and environmental variables begins the pathogenesis of RA. The human leukocyte ...