Nature vs. Nurture: Child Obesity
Introduction.
Child obesity is a major health issue, which is becoming more prominent and severe. It is important to note that one of the most commonly accepted ideas is based on the environment and its related factors. However, the overall impact of one’s genes should not be overlooked because it can create strong propensities and predispositions. On the basis of the given assessment, it is evident that a child’s environment is a stronger influencer than his or her genetic makeup.
One should be aware of the fact that genetic factors and inherited predispositions can be major contributors to childhood obesity. A study suggests that at least 21% of BMI variations are due to the genetic makeup of a person, including a child (Locke et al., 2015). In other words, one cannot dismiss the nature element because a wide range of different genes can dictate the course of BMI alterations in a child. One’s nervous system can significantly contribute to the emergence of obesity, and the pathways and genes linked with genetic components are involved in energy metabolism, adipogenesis, insulin action, glutamate signaling, and synaptic function (Locke et al., 2015). In other words, nature can have a strong influence on a child’s propensity to become obese.
With the understanding that obesity is accompanied by a significant number of various complications, interest in the causes of obesity is growing. In this regard, an active search is underway for biologically active substances and genes encoded at different stages of their synthesis. However, it is not possible to find anyone substance or one gene that would determine the development of obesity. Therefore, at present, among the main causes of obesity, both genetic, hormonal factors and diencephalic factors are considered, and, of course, all this against the background of improper diet and lifestyle.
Studying the genealogy of obese individuals often found that other family members were obese as well. This forced everyone to explore in more detail the genetic aspects of obesity. From the point of view of genetics, obesity can be monogenic and polygenic. Among the monogenic forms of obesity, mutations of the leptin gene, the leptin receptor gene, the convertase gene, the prohormone gene 1, and the 4B-melanocortin receptor gene are the most studied. Obesity is morbid and manifests itself from the first years of life (Locke et al., 2015). In addition, there are syndromes associated with obesity, but monogenic obesity is extremely rare.
Polygenic obesity is most often encountered by general practitioners, as well as specialized specialists such as endocrinologists and cardiologists. In general, the nature of most forms of obesity is, of course, multifactorial. In the development of such forms of obesity, both genetic factors, the contribution of which is at least 25%, and environmental factors, that is, lifestyle and diet, are important (Locke et al., 2015).
Of primary importance in the manifestation of this phenotype is insulin resistance in muscle tissue, accompanied by a decrease in glucose uptake. In accordance with this assumption, it is believed that insulin resistance of muscles will be a limiting glucose utilization by muscles, thus preventing the development of hypoglycemia during fasting (Locke et al., 2015). At the same time, during the period of an abundance of food, such a phenotype will contribute to the development of hyperglycemia and the conservation of energy in adipose tissue.
Environmental factors and the environment itself can be major contributing factors, and it is evident that an excess amount of food is a necessary requirement for the development of obesity among both children and adults. A highly comprehensive study conducted on twins reveals that the nurture element is more predominant during early childhood and puberty, whereas the nature element takes over near adulthood (Silventoinen et al., 2016). It is explained by the fact that as children become older, they gain independence from their parents, which results in a complete separation when they become adults (Silventoinen et al., 2016).
In other words, genetically coded behavior cannot be expressed fully during the childhood years due to the strong influence of parents because they are the ones making dietary decisions. However, as soon as a child, teenager, or adult gains independence, such as during college years, the genes become the dictators of behavior. It means that childhood obesity is likely to be the result of environmental factors rather than genetic makeup since the genes cannot fully control the exhibited behavior.
One of the common nurture-based components involves social aspects, which might be the result of socioeconomic status (SES). Social determinants of childhood obesity include increased food portion sizes in foodservice establishments, advertising of high-calorie foods in the media, the prevalence of instant food, videogame fashion, and augmented television and computer time. A significant effect on the formation of excess body weight in children was revealed by the regularity and length of time spent playing video and computer games. The relationship between time spent watching TV and the development of childhood obesity has been confirmed in a study.
The impact of watching TV and online video platforms on the formation of obesity is not only a decrease in a child’s physical activity. In childhood, advertising promotes the unconscious choice of a certain food brand. A child can watch a huge number of food commercials per year, most of which are high-calorie. The consequence of this influence of the media is an increase in the consumption of foods high in fat and carbohydrates by children. Children from families where one of the parents was obese may prefer more high-calorie meals, given a choice. Obese children of preschool and school-age can choose the most advertised brand of food.
The information presented above reflects the negative impact of the external environment on the formation of an overweight child by promoting unhealthy nutrition. Large portion sizes of food consumed are the leading cause of overweight (Silventoinen et al., 2016). It can be assumed that there is a clear relationship between the amount of food consumed during snacks and the formation of overweight in children. The use of vegetables as a snack in school meals can simultaneously reduce the volume of the main course in addition to increasing their daily consumption. The number of snacks outside of the main meals by school-age children is proportional to the development of overweight and obesity.
In conclusion, from the descriptions and information presented above, it is evident that although genes are strong determinants of the issue, the environment lies at its core. It is important to note that both studies do not dismiss the role of genes in the development of childhood obesity. However, genes’ expression cannot yield a high level of impact due to a lack of independence from parental guidance. Therefore, nature can be a major and even sole cause of obesity among adults, who are mostly independent and can freely exhibit genetic behavior, but children and adolescents are under the control of their environment.
Locke, A. E., Kahali, B., Berndt, S. I., Justice, A. E., Pers, T. H., … Buchkovich, M. L. (2015). Genetic studies of body mass index yield new insights into obesity biology. Nature, 518 (7538), 197-206.
Silventoinen, K., Jelenkovic, A., Sund, R., Hur, Y.-M., Yokoyama, Y., Honda, C., … Aaltonen, S. (2016). Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. The American Journal of Clinical Nutrition, 104 (2), 371-379.
Cite this paper
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2022, June 7). Nature vs. Nurture: Child Obesity. https://studycorgi.com/nature-vs-nurture-child-obesity/
"Nature vs. Nurture: Child Obesity." StudyCorgi , 7 June 2022, studycorgi.com/nature-vs-nurture-child-obesity/.
StudyCorgi . (2022) 'Nature vs. Nurture: Child Obesity'. 7 June.
1. StudyCorgi . "Nature vs. Nurture: Child Obesity." June 7, 2022. https://studycorgi.com/nature-vs-nurture-child-obesity/.
Bibliography
StudyCorgi . "Nature vs. Nurture: Child Obesity." June 7, 2022. https://studycorgi.com/nature-vs-nurture-child-obesity/.
StudyCorgi . 2022. "Nature vs. Nurture: Child Obesity." June 7, 2022. https://studycorgi.com/nature-vs-nurture-child-obesity/.
This paper, “Nature vs. Nurture: Child Obesity”, was written and voluntary submitted to our free essay database by a straight-A student. Please ensure you properly reference the paper if you're using it to write your assignment.
Before publication, the StudyCorgi editorial team proofread and checked the paper to make sure it meets the highest standards in terms of grammar, punctuation, style, fact accuracy, copyright issues, and inclusive language. Last updated: September 24, 2022 .
If you are the author of this paper and no longer wish to have it published on StudyCorgi, request the removal . Please use the “ Donate your paper ” form to submit an essay.

Childhood Disadvantage and Obesity: Is Nurture Trumping Nature?
Obesity has been one of the fastest growing health concerns among children, particularly among disadvantaged children. For children overall, obesity rates have tripled from 5% in the early 1970s to about 15% by the early 2000s. For disadvantaged children, obesity rates are closer to 20%. In this paper, we first examine the impact of various measures of disadvantage on children's weight outcomes over the past 30 years, finding that the disadvantaged have gained weight faster. Over the same period, adult obesity rates have grown, and we expect parental obesity to be closely tied to children's obesity, for reasons of both nature and nurture. Thus, examining changes in the parent-child correlation in BMI should give us some insight into the ways in which the environment that parents and children share has affected children's body mass, or into how the interaction of genes and environment has changed. We find that the elasticity between mothers' and children's BMI has increased since the 1970s, suggesting that shared genetic-environmental factors have become more important in determining obesity. Despite the faster weight gain for the disadvantaged, there appears to be no clear difference for by disadvantaged group in either the parent-child elasticity or in identifiable environmental factors. On average, the increases in parents' BMI between the early 1970s and the early 2000s can explain about 37 percent of the increase in children's BMI. Although common environmental/genetic factors play a larger role now than in earlier time periods, child specific environments such as schools and day care play a potentially important role in determining children's health status.
This research was funded in part by the Annie E. Casey Foundation. We thank them for their support but acknowledge that the findings and conclusions presented in this report are those of the authors alone, and do not necessarily reflect the opinions of the Foundation. We thank Chris Rogers at NCHS for help accessing the confidential NHANES data, Qing Chang and Pauline Yu for helpful research assistance, and Jon Gruber, John Cawley, Doug Staiger, Bruce Sacerdote and participants in the Disadvantaged Youth Conference for helpful comments. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research.
MARC RIS BibTeΧ
Download Citation Data
Published Versions
Childhood Disadvantage and Obesity: Is Nurture Trumping Nature? , Patricia M. Anderson, Kristin F. Butcher, Diane Whitemore Schanzenbach. in The Problems of Disadvantaged Youth: An Economic Perspective , Gruber. 2009
More from NBER
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .

Obesity: Nature or Nurture?
- First Online: 01 January 2010
Cite this chapter

- Robert H. Lustig 2
Part of the book series: Endocrine Updates ((ENDO,volume 30))
2000 Accesses
1 Citations
The debate about the causes of the current obesity epidemic rages on. The issue of “Whose fault is it?” frequently devolves into a related question: “Is it ‘nature’ (i.e., inherent in our genes and biochemistry before birth, and therefore out of our control) or ‘nurture’ (i.e., behaviors learned after birth and within our control to change)?” This chapter explores the phenomena and evidence which support and refute both sides of this argument. A conceptual framework is offered, whereby two of the biochemical mediators of obesity (hyperinsulinemia and glucocorticoids) can be applied throughout the life cycle. In this formulation, the question of “nature” versus “nurture” becomes merely a manifestation of the timing of the query. The results of such a rethinking of the nature–nurture argument argue for adding an “environmental safety” approach to the current “personal responsibility” approach, in order to more effectively combat the obesity epidemic.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
FORESIGHT. Tackling obesity: future choices – project report. 2nd ed. 2007. Government Office for Science http://www.foresight.gov.uk/Obesity/Obesity_final_part1.pdf .
Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy and fat intakes of children and adolescents in the United States: data from the National Health and Nutrition Examination Surveys. Am J Clin Nutr. 2000;72:1343s–53s.
PubMed CAS Google Scholar
Centers for Disease Control. Trends in intake of energy and macronutrients – United States, 1971–2000. Morb Mortal Wkly Rep. 2004;53:80–2.
Google Scholar
Swinburn B. Increased energy intake alone virtually explains all the increase in body weight in the United States from the 1970s to the 2000s. Eur Congr Obes (Amsterdam (Abstr.)). 2009;T1:RS3.3.
Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity. 2007;15:2739–47.
Article PubMed Google Scholar
Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88.
Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97:667–75.
Le KA, Tappy L. Metabolic effects of fructose. Curr Opin Nutr Metab Care. 2006;9:469–75.
Article CAS Google Scholar
Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev. 2007;65:S13–23.
Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906.
Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–57.
Gross LS, Li S, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–9.
Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–22.
Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8.
Brown CM, Dulloo AG, Montani JP. Sugary drinks in the pathogenesis of obesity and cardiovascular diseases. Int J Obes. 2008;32:528–34.
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264.
Article PubMed CAS Google Scholar
Kimm SYS, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls in adolescence. N Engl J Med. 2002;347:709–15.
Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am. 2001;48:931–53.
Li S, Treuth MS, Wang Y. How active are American adolescents and have they become less active? Obes Rev. 2009; [epub Oct 27, 2009].
Marshall SJ, Biddle SJ, Gorely T, Cameron N, Murdey I. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. Int J Obes Relat Metab Disord. 2004;28:1238–46.
Mark AE, Janssen I. Relationship between screen time and metabolic syndrome in adolescents. J Public Health. 2008;30:153–60.
Article Google Scholar
Keith SW, Redden DT, Katzmaryk PT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes. 2006;30:1585–94.
Atkinson RL, Dhurandhar NV, Allison DB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes. 2005;29:281–6.
Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Ann N Y Acad Sci. 2008;1135:265–79.
Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–9.
Kamath CC, Vickers KS, Ehrlich A, et al. Clinical review: behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. J Clin Endocrinol Metab. 2008;93:4606–15.
McGovern L, Johnson JN, Paulo R, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2007;93:4600–5.
Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;CD003817.
Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–9.
Latner JD, Stunkard AJ. Getting worse: the stigmatization of obese children. Obes Res. 2003;11:452–6.
Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–32.
Kim J, Peterson KE, Scanlon KS, et al. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity. 2006;14:1164–71.
Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54.
Mieda M, Yanigasawa M. Sleep feeding, and neuropeptides: roles of orexins and orexin receptors. Curr Opin Neurobiol. 2002;12:339–46.
Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–20.
Franco M, Orduñez P, Caballero B, et al. Impact of energy intake, physical activity, and population-wide weight loss on cardiovascular disease and diabetes mortality in Cuba, 1980–2005. Am J Epidemiol. 2007;166:1374–80.
Epstein LH, Roemmich JN, Raynor HA. Behavioral therapy in the treatment of pediatric obesity. Pediatr Clin North Am. 2001;48:981–93.
Mellin LM, Slinkard LA, Irwin CE. Adolescent obesity intervention: validation of the SHAPEDOWN program. J Am Diet Assoc. 1987;87:333–8.
Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120:669–81.
Center for Weight and Health. Pediatric overweight: a review of the literature June 2001. http://www.cnr.berkeley.edu/cwh/news/announcements.shtml#lit_review (2001). Accessed Dec 1, 2009.
Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15–9.
Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801.
Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:454–8.
Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8.
Champigny O, Ricquier D. Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue: evidence for diet-induced and cold-induced responses. J Nutr. 1990;120:1730–6.
Aronne LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–5.
CAS Google Scholar
Lustig RH. The efferent arm of the energy balance regulatory pathway: neuroendocrinology and pathology. In: Donahoue PA, editor. Obesity and energy metabolism: research and clinical applications. Totowa, NJ: Humana; 2007. pp. 69–86.
Bray GA, Greenway FL. Current and potential drugs for treatment of obesity. Endocr Rev. 1999;20:805–75.
Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–50.
Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–64.
Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–92.
Boden G. Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci. 1998;3:169–75.
Lustig RH. The “skinny” on childhood obesity: how our Western environment starves kids’ brains. Pediatr Ann. 2006;35:898–907.
PubMed Google Scholar
Flier JS. What’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83:1407–13.
Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T-cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103.
Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–75.
Ulijaszek SJ. Secular trend in birthweight among the Purari delta population, Papua New Guinea. Ann Hum Biol. 2001;28:246–55.
Davidson S, Litwin A, Peleg D, Erlich A. Are babies getting bigger? Secular trends in fetal growth in Israel – a retrospective hospital-based cohort study. Isr Med Assoc J. 2007;9:649–51.
Ludwig DS, Currie J. The relationship between pregnancy weight gain and birth weight: a within family comparison. Lancet. 2010 Aug 4. [Epub ahead of print].
Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Frougel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–62.
Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66.
Dina C, Meyre DGS, Durand E, Körner A, Jacobson P, Carlsson LM, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–6.
Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. Insulin and insulin-like growth factors in the CNS. Trends Neurosci. 1988;11:107–11.
Muntzel M, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces non-uniform regional increases in sympathetic nerve activity. Am J Physiol. 1994;267:R1350–5.
Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10.
Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity, and leptin action. Nat Med. 2006;12:917–24.
Mori H, Hanada R, Hanada T, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–43.
Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of SOCS3. Nat Med. 2004;10:734–8.
Kubota N, Terauchi Y, Tobe K, et al. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest. 2004;114:917–27.
Choudhury AI, Heffron H, Smith MA, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–50.
Lin X, Taguchi A, Park S, et al. Dysregulation of insulin receptor substrate 2 in β-cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–16.
Plum L, Ma X, Hampel B, et al. Enhanced PIP(3) signaling in POMC neurons causes K(ATP) channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–901.
Munzberg H, Myers MG. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–70.
Kellerer M, Lammers R, Fritsche A, et al. Insulin inhibits leptin receptor signalling in HEK293 cells at the level of janus kinase-2: a potential mechanism for hyperinsulinaemia-associated leptin resistance. Diabetologia. 2001;44:1125–32.
Hill JW, Williams KW, Ye C, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–805.
Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the first law of thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2:447–58.
Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–4.
Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005;115:3579–86.
Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol. 2004;46:220–9.
Lustig RH, Post SM, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88:611–6.
Harz KJ, Muller HL, Waldeck E, Pudel V, Roth C. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab. 2003;88:5227–31.
Bray GA, Gallagher TF. Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a review of the literature. Medicine. 1975;54:301–33.
Lustig RH, Rose SR, Burghen GA, et al. Hypothalamic obesity in children caused by cranial insult: altered glucose and insulin dynamics, and reversal by a somatostatin agonist. J Pediatr. 1999;135:162–8.
Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88:2586–92.
Velasquez-Mieyer PA, Cowan PA, Arheart KL, et al. Suppression of insulin secretion promotes weight loss and alters macronutrient preference in a subset of obese adults. Int J Obes. 2003;27:219–26.
Lustig RH, Greenway F, Velasquez-Mieyer P, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes. 2006;30(2):331–41.
Lustig RH, Sen S, Soberman JE, Velasquez-Mieyer PA. Obesity, leptin resistance, and the effects of insulin suppression. Int J Obes. 2004;28:1344–8.
Li HJ, Ji CY, Wang W, Hu YH. A twin study for serum leptin, soluble leptin receptor, and free insulin-like growth factor-1 in pubertal females. J Clin Endocrinol Metab. 2005;90:3659–64.
Castracane VD, Hendrickx AG, Henson MC. Serum leptin in nonpregnant and pregnant women and in old and new world nonhuman primates. Exp Biol Med. 2005;230:251–4.
McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFα, leptin, and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diab Metab Res Rev. 2006;22:131–8.
Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10R.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73.
Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–8.
Yajnik CS, Lubree HG, Rege SS, et al. Adiposity and hyperinsulnemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87:5575–80.
Arends NJ, Boonstra VH, Duivenvoorden HJ, Hofman PL, Cutfield WS, Hokken-Koelega AC. Reduced insulin sensitivity and the presence of cardiovascular risk factors in short prepubertal children born small for gestational age (SGA). Clin Endocrinol. 2005;62:44–50.
Potau N, Gussinye M, Sanchez Ufarte C, Rique S, Vicens-Calvet E, Carrascosa A. Hyperinsulinemia in pre- and post-pubertal children born small for gestational age. Horm Res. 2001;56:146–50.
Yajnik CS, Fall CH, Vaidya U, et al. Fetal growth and glucose and insulin metabolism in four-year-old Indian children. Diabet Med. 1995;12:330–6.
Silverman BL, Landsberg L, Metzger BE. Fetal hyperinsulinism in offspring of diabetic mothers: association with the subsequent development of childhood obesity. Ann N Y Acad Sci. 1993;699:36–45.
Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diab Care. 1998;21 Suppl 2:B142–9.
Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes. Pediatrics. 2005;115:e290–6.
Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima Indians: contributions of obesity and parental diabetes. Am J Epidemiol. 1981;113:144–56.
Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risk for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;42:2208–11.
Marceau P, Biron S, Hould FS, et al. Outcome of pregnancies after obesity surgery. In: Guy-Grand B, Ailhaud G, editors. Progress in obesity research. John Libbey Eurotext, Montrouge, France 8th ed. 1999. pp. 795–802.
Kral JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1664–9.
Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–86.
Petry CJ, Ozanne SE, Wang CL, Hales CN. Effects of early protein restriction and adult obesity on rat pancreatic hormone content and glucose tolerance. Horm Metab Res. 2000;32:233–9.
Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–86.
Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–7.
Boloker J, Gertz SJ, Simmons RA. Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes. 2002;51:1499–506.
Franke K, Harder T, Aerts L, et al. ‘Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res. 2005;1031:276–83.
Harder T, Franke K, Fahrenkrog S, et al. Prevention by maternal pancreatic islet transplantation of hypothalamic malformation in offspring of diabetic mother rats is already detectable at weaning. Neurosci Lett. 2003;352(3):163–6.
Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10.
Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5.
Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–6.
Davis M, Whelan PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34.
LeDoux J. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–84.
Sapolsky R, Krey L, McEwen B. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrinol Rev. 1986;7:284–301.
Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc. 1985;44:243–8.
Smagin GN, Dunn AJ. The role of CRF receptor subtypes in stress-induced behavioural responses. Eur J Pharmacol. 2000;405:199–206.
Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum, or CRH1 receptors in amygdala. J Neurosci. 2002;22:7926–35.
La Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–85.
Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci. 2003;100:11696–701.
Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–25.
Adam TC, Epel ES. Stress, eating, and the reward system. Physiol Behav. 2007;91:449–58.
Epel ES, McEwen BS, Seeman T, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–32.
Appelhans BM, Pagoto SL, Peters EN, Spring BJ. HPA axis response to stress predicts short-term snack intake in obese women. Appetite. 2010 Feb;54(1):217–20.
Epel ES, Lapidus R, McEwen BS, Brownell KD. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49.
Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007 Jul 24;91:449–58.
Dallman MF, Pecoraro NC, La Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain, Behav Immun. 2005;19:275–80.
Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic syndrome. Ann. N Y Acad Sci. 1999;892:297–307.
Epel E, McEwen B, Seeman T, et al. Can stress shape your body? Consistently greater stress-induced cortisol secretion among women with abdominal fat. Psychosom Med. 2000;62:623–32.
Thakore J, Richards P, Reznek R, Martin A, Dinan T. Increased intraabdominal fat in major depression. Biol Psychiatry 1997;41:1140–2.
Weber-Hamann B, Hentschel F, Kneist A, et al. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002;64:274–7.
Rosmond R, Dallman M, Bjorntorp P. Stress related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic, and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–9.
Rosmond R, Lapidus L, Marin P, Bjorntorp P. Mental distress, obesity, and body fat distribution in middle-aged men. Obes Res. 1996;4:245–52.
Ahlberg AC, Ljung T, Rosmond R, et al. Depression and anxiety symptoms in relation to anthropometry and metabolism in men. Psychiatry Res. 2002;112:101–10.
Wallerius S, Rosmond R, Ljung T, Holm G, Bjorntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest. 2003;26:616–9.
Vitaliano P, Scanlan J, Zhang J, Savage M, Hirsch I, Siegler I. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–35.
Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–7.
Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–5.
Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–62.
Brunner EJ, Hemingway H, Walker BR, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–65.
Andrew R, Gale CR, Walker BR, Seckl JR, Martyn CN. Glucocorticoid metabolism and the metabolic syndrome: associations in an elderly cohort. Exp Clin Endocrinol Diab. 2002;110:284–90.
Faggiano A, Pivonello R, Spiezia S, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab. 2003;88:2527–33.
Reynolds RM, Walker BR. Human insulin resistance: the role of glucocorticoids. Diab Obes Metab. 2003;5:5–12.
Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–79.
Greenfield EA, Marks NF. Violence from parents in childhood and obesity in adulthood: using food in response to stress as a mediator of risk. Soc Sci Med. 2009;68:791–8.
Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–43.
Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol. Behav. 1999;66:511–5.
Roemmich JN, Wright SM, Epstein LH. Dietary restraint and stress-induced snacking in youth. Obes Res. 2002;10:1120–6.
Johnson JG, Cohen P, Kasen S, Brook JS. Childhood adversities associated with risk for eating disorders or weight problems during adolescence or early adulthood. Am J Psychiatry. 2002;159:394–400.
Reilly JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86:3742–5.
Reilly JJ, Ventham JC, Newell J, Aitchison T, Wallace WH, Gibson BE. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24:1537–41.
French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–21.
Bloom SL, Sheffield JS, McIntire DD, Leveno KJ. Antenatal dexamethasone and decreased birth weight. Obstet Gynecol. 2001;97:485–90.
Entringer S, Wüst S, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199:e1–7.
Phillips DI, Barker DJ, Fall CH, et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–60.
Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106.
Lajic S, Nordenström A, Hirvikoski T. Long-term outcome of prenatal treatment of congenital adrenal hyperplasia. Endocrinol Dev. 2008;13:82–98.
Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–25.
Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–7.
De Vries A, Holmes MC, Heijnis A, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117:1058–67.
Nyirenda MJ, Carter R, Tang JI, et al. Prenatal programming of metabolic syndrome in the common marmoset is associated with increased expression of 11beta-hydroxysteroid dehydrogenase type 1. Diabetes. 2009;58:2873–9.
Download references
Acknowledgments
The author would like to thank Drs. Elissa Epel, Mary Dallman, Clement Cheung, Amanda Drake, Mark Tremblay, and Anastasia Hadjiyannakis for their collegiality and for their intellectual contributions to this treatise.
Author information
Authors and affiliations.
Division of Pediatric Endocrinology, Department of Pediatrics, University of California, San Francisco, CA, 94143-0434, USA
Robert H. Lustig
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Robert H. Lustig .
Editor information
Editors and affiliations.
Director, Weight Assessment for Teen and, Division of Endocrinology, University of California, San Francisco, San Francisco, 94143, USA
Robert H Lustig
Rights and permissions
Reprints and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Lustig, R.H. (2011). Obesity: Nature or Nurture?. In: Lustig, R. (eds) Obesity Before Birth. Endocrine Updates, vol 30. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-7034-3_1
Download citation
DOI : https://doi.org/10.1007/978-1-4419-7034-3_1
Published : 20 September 2010
Publisher Name : Springer, Boston, MA
Print ISBN : 978-1-4419-7033-6
Online ISBN : 978-1-4419-7034-3
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Nurture is more...
Nurture is more important than nature in childhood obesity, study finds
- Related content
- Peer review
- Zosia Kmietowicz
A study comparing the weight of biological and adopted children to that of their parents has found that lifestyles, rather than genes, are primarily responsible for the children being overweight. 1
The researchers, from the Centre for Economic Performance at the London School of Economics and Political Science, concluded that policies to influence parents’ lifestyles could help to tackle overweight in children.
For the study the researchers compiled data from 13 years of the Health Survey for England from 1997 to 2009. This annual survey measures health related behaviours in adults and children including weight, height, and body mass index. A nurse validates the measurements of weight and height, overcoming some of the problems seen in other surveys, said the researchers.
The overall sample included 13 536 observations of children in which both parents were biological and 300 observations in which both parents were adoptive.
The researchers found that, when both adoptive parents were overweight, the likelihood of an adopted child being overweight was as much as 21% higher than when the parents were not overweight. Because these children were adopted, their weight problems could be largely attributed to their parents’ lifestyles rather than to their genes, the researchers wrote.
They added that, in comparison, children with two biological overweight parents were 27% more likely to be overweight, showing the relatively small influence of genetics.
When the researchers looked at the effect of only a mother or father being overweight the results were more mixed. Among adoptees, they found no effect when only the mother was overweight; but when only the father was overweight or obese they found a small effect.
The research also showed that being obese was more strongly influenced by genetics than by lifestyle factors. And the transmission of being overweight or obese from parents to children due to lifestyle factors was not found to be affected by the children having a full time working mother.
The researchers concluded, “Overweight is passed through generations, and the pathway seems to be primarily driven by the children environment. In contrast, and consistently with the behavioural [genetics] literature, obesity exhibits a highly genetic component.”

- Download figure
- Open in new tab
- Download powerpoint
Cite this as: BMJ 2015;350:h817
- ↵ Costa-Font J, Jofre-Bonet M, Le Grand J. Vertical transmission of overweight: evidence form English adoptees. Centre for Economic Performance discussion paper no 1324. January 2015. http://cep.lse.ac.uk/pubs/download/dp1324.pdf .
OPINION article
Pediatric obesity: an economic perspective.

- 1 Department of Pediatrics, University of Florida College of Medicine, Jacksonville, FL, United States
- 2 Department of Family Medicine, Saint Vincent's Health Center, Jacksonville, FL, United States
Introduction
Pediatric obesity is increasingly common in the United States, with over thirty percent of American children considered obese (BMI over the 95th percentile for age); and over forty percent of American adolescents classified as overweight (BMI between the 85th and 95th percentile for age) ( 1 ). The most serious projections estimate that over eighty-five percent of American adults will be overweight or obese by 2030 ( 2 ). Childhood obesity is now considered a growing epidemic requiring intervention and preventative measures, similar to tobacco use ( 3 ).
Childhood obesity affects every single organ system ( 3 ). Obesity is associated with concomitant or increased risk of nearly every chronic disease and condition, from diabetes to dyslipidemia, to cancers and poor mental health ( 4 ). The longer a person is obese the more compounded the costs from obesity's associated comorbidities, hypertension, hyperlipidemia, type 2 diabetes mellitus, obstructive sleep apnea, hepatic steatosis, orthopedic conditions, certain cancer, depression, and social isolation ( 5 ).
In the past, the public health community demonstrated that public smoking bans, advertising bans and increased taxation on cigarettes worked to decrease the general use of tobacco in the United States. The same policies could work when applied to the fast-food industry. These interventions act as economic disincentives and reduce the triggers that influence consumption without outright bans on fast food products.
Behavioral and Classical Economics
John et al. ( 6 ) make the distinction between traditional economic theory and the field of behavioral economics in that behavioral economics attempts to reconcile psychological “errors” such as short-term time preferences, loss aversion etc. with economic theory; “whereas standard economics is premised on a rational choice model and assumes that individuals make decisions optimally, behavioral economics not only acknowledges that behavior is often suboptimal, but also identifies decision errors and judgmental biases that contribute to such departures from optimality” ( 6 ).
The behavior economic field attempts to account for economic irrationality by incorporating psychological factors. Because many people tend to be impulsive, favoring smaller short-term benefits over larger, but more future oriented benefits, the classical economic model fails to accurately predict behavior. People in fact, do not always act in their long-term interests. John et al. ( 6 ) write that behavioral economics, “is emerging as a key discipline in modifying self-destructive behaviors, such as those leading to obesity.”
John et al. ( 6 ) created a study in which a cohort was motivated to lose weight by means of a deposit contract in which participants deposited “between $0.00 and $3.00 per day of their own funds to a deposit contract. During the month, participants accumulated rewards … equal to his deposit, plus a 1:1 match from the researchers … Participants were aware, however, that they would only receive accumulated awards if they weighed at or below their weight loss goal at the end of the month weigh-in. Thus, these participants could earn $84 net ($168 gross) per month (i.e., by making the maximum $3.00 daily deposit, and … attaining their daily weight loss goal (1 lb per week).” In comparison to control subjects who only met with a dietician, intervention subjects in this study lost over 8 times as much weight (average incentive with loss = 8.70 pounds, mean control = 1.17 pounds).
In this study, John et al. ( 6 ) demonstrated that interventions that emphasize small economic rewards, but especially economic loss-aversion can be an important facet in combating obesity. The researchers also demonstrated that even small rewards act as a strong incentive if they occur immediately, demonstrating the tendency to be motivated by a short-term time preference.
Discounting The Future
According to Rasmussen et al. ( 7 ), a formula for delay discounting considering an indifference point at which hyperbolic (short-time preference) discounting occurs may be a useful tool when considering monetary spending which can also be applied to eating behavior. Rasmussen et al. ( 7 ) discuss an experiment in which “researchers pose a series of hypothetical choices to participants in which they choose between a relatively small monetary outcome (e.g., $10) available immediately and a larger delayed monetary outcome (e.g., $100).” The researchers gradually then manipulate the “smaller, sooner” reward until reaching a point where the subject “switches from choosing the larger, delayed amount to choosing the smaller, sooner amount” ( 7 ). This value or pattern of switching can be described using Mazur's hyperbolic discounting equation ( 8 ):
V is the subjective value of the delayed reward, A is the numerical amount of the delayed reward, D is the time delay, and k is “a free parameter that quantifies the rate of decay of the reward value as delay increases, or the relative degree of discounting” ( 7 ).
Rasmussen et al. ( 7 ) report that in general, “the value of the outcome is equal to the amount but loses value with delay… higher k values refer to greater sensitivity to delay, or higher impulsivity.” Rasmussen et al. ( 7 ) believe that understanding discounting may “prove useful in the development of treatments across a wide array of problems in particular, obesity.”
More Than Short Term Time Preference: Genetics, Epigenetics, and Environment
It is true that genetic factors influence obesity. Genetics can be of paramount importance as is seen in Prader–Willi, Beckwith–Wiedemann syndrome and other genetic syndromes that lead to obesity. There are also endocrine disorders that result in a small number of pediatric obesity cases.
Over sixty common genetic markers have now been identified as predisposing factors for an increased susceptibility to obesity with thirty-two of the most common genetic variants responsible for ~ <1.5% of the overall inter-individual variation in BMI ( 9 ). However, genetic markers in general seem to play a small role (<2%) in the development of obesity.
The field of Epigenetics explores the “phenomena and mechanisms that cause chromosome-bound, heritable changes to gene expression that are not dependent on changes to DNA sequence” ( 10 ). Epigenetics is dependent on environmental influences. It is likely that our changing environment may be inducing epigenetic changes that are contributing to higher levels of obesity in the population. “Secular trends in obesity in children, adolescents and adults have shown an increase in obesity with urbanization, clearly indicating the role of the environment” ( 11 ).
Nearly all obesity is related to environmental factors that facilitate excess calorie intake ( 4 ). As researchers develop a deeper understanding of obesity, epigenetics may ultimately resolve the nature vs. nurture debate. By reconciling these two perspectives, epigenetics may offer new solutions for environmental changes which may decrease the prevalence of obesity in the population.
Health and Social Costs
Beyond the obvious correlations of obesity with hypertension, hyperlipidemia, type 2 diabetes mellitus, depression and social isolation, obesity is also associated with functional and anatomical brain changes. When compared to those with a lower BMI, obese adults demonstrate frontal lobe, anterior cingulate gyrus, hippocampal, and thalamic brain atrophy ( 12 ). Obesity is correlated with poorer cognitive performance in executive functions, especially in impulse control ( 13 ).
Obesity increases overall mortality. A study by Mokdad et al. ( 14 ) determined that, in the United States, 15% of deaths were attributable to excess weight. Rome ( 3 ) notes that “…prevention is paramount because (the) morbidly obese individuals…remain at risk for a shortened lifespan if they do not achieve a significant weight loss.” Obesity in middle age may shorten lifespan by up to seven years ( 15 ).
Childhood obesity is particularly dangerous as it sets the pattern for a lifetime. Children who become obese are more likely to remain obese as adults. Adolescents who become obese “…have a 90% chance that their obesity will persist into adulthood” ( 3 ). These children are more likely to eat than to spend time with friends ( 16 ). They miss or have decreased involvement in important life activities including decreased opportunities for dating, marriage, and reproduction.
Advertising and Marketing an Unhealthy Diet
Food companies in the US spend billions of dollars annually advertising food products. In 2006, marketing companies spent $1.6 billion advertising food specifically to children ( 17 ). In 2004, the average child in the USA viewed 15 food advertisements daily ( 17 ). By 2007, American families spent “42% of every food dollar on food prepared by others, up from 25% in 1970.” ( 18 ).
Children typically develop emotional connections between themselves and brands that they see advertised (e.g., McDonald's Happy Meal, Joe Camel, or the Marlboro Man) ( 19 ). When children are exposed to attractive food triggers, they are stimulated to desire the advertised food and increase its intake ( 20 ). Children do not have the sophistication, even with parental guidance, to fully understand the real consequences of consuming what is advertised by the fast-food industries. Children also do not understand obesity's future costs ( 21 ).
A tremendous amount of research has been conducted to explore dietary patterns that seem to be the most important factor in the pathogenesis of obesity ( 22 ). In particular, “…sugar-sweetened beverages, have received considerable attention largely because added sugar consumption has been rising concomitantly with prevalent obesity” ( 23 ). Regulation of sugary beverages provide an example of the possible role of policy interventions in combating the obesity epidemic. Fernandez and Raine ( 24 ) report that “sugar sweetened beverage (SSB) taxation is a viable anti-obesity policy. However, researchers and public health practitioners need to be vigilant of industry tactics to curtail SSB lowering efforts.”
Success in the Past: The Tobacco Model
Since the 1964 release of the Surgeon General's report describing the dangers of tobacco use, smoking prevalence has been cut in half in the United States ( 25 ). Media campaigns and anti-smoking laws have been successful in shifting the public's view of smoking as a benign, even attractive habit to a largely stigmatized and undesirable one. In 2009, the Children's Health Insurance Program Reauthorization Act increased cigarette taxes by $0.62 to a total federal tax of $1.01 per pack. Decreased tobacco use prevalence rates afterwards proved “…that increasing the cost of cigarettes is one of the most powerful interventions we can make to prevent smoking and reduce prevalence” ( 25 ). Increased taxation was part of a dual pronged approach. The Tobacco Control Act, the second prong in the assault on tobacco use, assessed fees to tobacco manufacturers for sustained public media campaigns targeting youth tobacco prevention and cessation ( 25 ).
Beginning Interventions
In 2014, the Mexican government implemented two policy regulations designed to combat and reverse its obesity incidence rate: taxes on high-calorie foods and drinks; and the restriction of television advertising for high-calorie food and soft drinks between 14:30 and 19:30 on weekdays and between 07:30 and 19:30 on weekends ( 26 ). “Overall, 40% of commercials for soft drinks, confectionery and chocolates (will) disappear from TV, in favor of products which “meet nutritional standards,” per the Mexican health ministry.” ( 26 ) Norway, the United Kingdom and Quebec have also banned fast food advertising ( 26 ).
Although no successful legislation interventions have passed in the United States, there have been attempts to limit the sale of large soft drinks in New York City ( 27 ); to increase taxation on fast food, and to eliminate soda sales in schools ( 4 ). Some researchers advocate limiting production and importation of sugary beverages along with increased taxation on fast foods; and advocate for fast food restaurant zoning restrictions ( 4 ).
Conclusion: Pediatric Obesity Economic Policy
Hruby and Hu ( 4 ) write, “That barely one in three people in the USA today are normal weight portends, quite simply, an astounding and frightening future.” If obesity trends could be reversed, “significant reductions in public health and healthcare expenditures could occur” ( 28 ). The most important intervention is the prevention of pediatric obesity itself.
The public health community has demonstrated that public smoking bans, bans on advertising cigarettes to children, and increased taxation on tobacco was successful in decreasing tobacco use in the United States. The same increased taxation and bans on advertising (without outright fast-food bans) could work when applied to the restaurant industry.
While multiple interventions are needed, policies that eliminate problematic environmental triggers (advertising) would likely show an immediate benefit because they combat impulsive/compulsive use and impose a minimal inconvenience ( 21 ). If we were to eliminate the marketing, as has been done in Mexico, Quebec, The United Kingdom and elsewhere, and if taxation on fast food were increased—by following the cigarette model—we would have a means to combat the obesity epidemic.
Author Contributions
NA and BI together conceptualized, collected data, created the formal analysis, methodology, administered the project, and wrote the original and all subsequent drafts.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ms. Susan Harnett, Medical Information Services Librarian at the Borland Health Science Library, University of Florida-Jacksonville who helped with formatting, proofreading, and editing this report.
1. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US Children, 1999–2016. Pediatrics . (2018) 141:e20173459. doi: 10.1542/peds.2017-3459
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity Silver Spring Md . (2008) 16:2323–30. doi: 10.1038/oby.2008.351
CrossRef Full Text | Google Scholar
3. Rome ES. Obesity prevention and treatment. Pediatr Rev . (2011) 32:363–73. doi: 10.1542/pir.32-9-363
4. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics . (2015) 33:673–89. doi: 10.1007/s40273-014-0243-x
5. Güngör NK. Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol . (2014) 6:129–43. doi: 10.4274/jcrpe.1471
6. John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. (2011) 26:621–6. doi: 10.1007/s11606-010-1628-y
7. Rasmussen EB, Robertson SH, Rodriguez LR. The utility of behavioral economics in expanding the free-feed model of obesity. Behav Processes . (2016) 127:25–34. doi: 10.1016/j.beproc.2016.02.014
8. Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analysis of Behavior: Vol. 5. The Effect of Delay and of Intervening Events of Reinforcement Value . Hillsdale, NJ: Erlbaum (1987). p. 55–73.
Google Scholar
9. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet . (2010) 42:937–48. doi: 10.1038/ng.686
10. Deans C, Magert KA. What do you mean, “epigenetic?” Genetics . (2015) 199:887–96. doi: 10.1534/genetics.114.173492
11. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA . (2012) 307:483–90. doi: 10.1001/jama.2012.40
12. Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp . (2009) 31:353–64. doi: 10.1002/hbm.20870
13. Reinert KRS, Po'e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes . (2013) 2013:1–10. doi: 10.1155/2013/820956
14. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. (2004) 291:1238–45. doi: 10.1001/jama.291.10.1238
15. Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med . (2003) 138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008
16. Epstein LH, Roemmich JN, Robinson JL, Paluch RA, Winiewicz DD, Fuerch JH, et al. A randomized trial of the effects of reducing television viewing and computer use on body mas index in young children. Arch Pediatr Adolesc Med . (2008) 162:239–45. doi: 10.1001/archpediatrics.2007.45
17. Institute of Medicine. National academy of sciences, committee on food marketing and the diets of children and youth. In: McGinnis JM, Gootman J, Kraak VI, editors. Food Marketing to Children and Youth: Threat or Opportunity? Washington, DC: Institute of Medicine of the National Academies (2006).
18. Clauson A, Leibtag E. Food CPI, Prices, and Expenditures Briefing Room, Table 12 . Washington, DC: US Department of Agriculture, Economic Research Service (2008).
19. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing tobacco use among youth and young adults: a report of the surgeon general. In: The Tobacco Industry's Influences on the Use of Tobacco among Youth . Atlanta, GA: Centers for Disease Control and Prevention (2012).
PubMed Abstract | Google Scholar
20. Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite . (1997) 28:33–47. doi: 10.1006/appe.1996.0057
21. Heshmat S. Eating Behavior and Obesity. Behavioral Economics Strategies for Health Professionals . New York, NY: Springer Publishing Company LLC (2011).
22. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation . (2012) 125:1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453
23. Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, typeb2 diabetes mellitus, and cardiovascular disease risk. Circulation . (2010) 121:1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185
24. Fernandez MA, Raine KD. Insights on the influence of sugar taxes on obesity prevention efforts. Curr Nutr Rep . (2019) 8:333–9. doi: 10.1007/s13668-019-00282-4
25. US Department of Health and Human Services Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking -−50 Years of Progress: A Report of the Surgeon General . Atlanta, GA: U.S. Department of Health and Human Services (2014).
26. Gallagher J. Mexico Restricts Soft Drink TV ads to Fight Obesity . BBC News (2014).
27. New York City bans supersize sodas. BBC News . (2012). Available online at: http://www.bbc.com/news/world-us-canada-19593012 (accessed June 18, 2020).
28. National Institutes of Health National Heart Lung and Blood Institute. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults . Bethesda, MD: Obesity Education Initiative: Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary; U.S. Department of Health and Human Services (1998).
Keywords: pediatric obesity, health economics, sugar tax, fast food marketing, advertising bans
Citation: Albrecht NM and Iyengar BS (2021) Pediatric Obesity: An Economic Perspective. Front. Public Health 8:619647. doi: 10.3389/fpubh.2020.619647
Received: 20 October 2020; Accepted: 04 December 2020; Published: 08 January 2021.
Reviewed by:
Copyright © 2021 Albrecht and Iyengar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathan Montoya Albrecht, nathan.albrecht@jax.ufl.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
On the Nature vs. Nurture of Obesity
Highlights from the ongoing debate over the factors underlying the epidemic on pace to see 42% of Americans obese within the next 20 years .
joe_13/Flickr
In a head-to-head piece published yesterday in the British Medical Journal , Timothy Frayling, a professor of human genetics at the University of Exeter, argues that genetics outweigh (ahem) environmental factors as we look at causes of obesity. He cites research that has found adiposity between twins is concordant in up to 70 percent of cases. An obesity-related gene has also been identified; people with two copies of the so-called FTO gene are generally heavier compared to those without the gene variant. Moreover -- and not surprisingly -- sedentary people with the obesity-linked gene tended to be heavier than those with the gene who were physically active. Frayling concludes that our DNA may actually be far more responsible for human obesity than our surroundings.
Home — Essay Samples — Nursing & Health — Child Obesity — The Contributions of the Nature Versus Nurture Theories in Child Obesity
The Contributions of The Nature Versus Nurture Theories in Child Obesity
- Categories: Child Obesity Childhood Obesity
About this sample

Words: 1391 |
Published: Feb 8, 2022
Words: 1391 | Pages: 3 | 7 min read
Table of contents
Introduction, nature-genetics and biological factors, nurture-environmental factors, diet and adult influences.
- Chesi, A., & Grant, S. F. (2015). The Genetics of Pediatric Obesity. Trends in Endocrinology & Metabolism, 26(12), 711–721. doi: 10.1016/j.tem.2015.08.008
- Childhood Obesity Facts. (2019, June 24). Retrieved from https://www.cdc.gov/obesity/data/childhood.html.
- Hemmingsson, E. (2018). Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction , Offspring Distress, and Junk Food Self-Medication. Current Obesity Reports, 7(2), 204–209. doi: 10.1007/s13679-018-0310-2
- LEP gene - Genetics Home Reference - NIH. (2013, December). Retrieved from https://ghr.nlm.nih.gov/gene/LEP.
- Nogues, P., Santos, E. D., Jammes, H., Berveiller, P., Arnould, L., Vialard, F., & Dieudonné, M.-N. (2019). Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clinical Epigenetics, 11(1). doi: 10.1186/s13148-019-0612-6
- Obesity. (2014, September 5). Retrieved from https://www.who.int/topics/obesity/en/.
- Staff, A. (2018, November 27). Effects of varying amounts of carbohydrate on metabolism after weight loss. Retrieved from https://www.hsph.harvard.edu/nutritionsource/2018/11/27/effects-of-varying-amounts-of-carbohydrate-on-metabolism-after-weight-loss/.
- Thyroid and Weight. (n.d.). Retrieved from https://www.thyroid.org/thyroid-and-weight/.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Verified writer
- Expert in: Nursing & Health

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
2 pages / 909 words
6 pages / 2514 words
7 pages / 3056 words
2 pages / 716 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Child Obesity
Throughout recent years obesity has been a very important topic in our society. It has continued to rise at high rates especially among children. This causes us to ask what are the causes of childhood obesity? There are many [...]
A heart attack is a frightening experience. If you have had a heart attack, or are close with someone who has, you are not alone: tens of thousands of Americans survive. As you work toward recovery, please use the following [...]
In his article, “Changing Ethics in Life and Death Decision Making,” Peter Singer makes many great points about the sanctity of human life and the religious, scientific, and moral implications of death. Singer brings up [...]
Of the dozens of videos you watch every day, how many do you actually remember? The goal of this PSA video is to be one that you would remember. A good PSA is strong, genuine, and powerful enough to leave an impression . To [...]
Roe vs. Wade was a law established in America on January 22nd, 1973. This court decision allowed women to be able to get abortions in the first trimester. Despite this, the debate on whether abortion should be legal is still [...]
In his Letter to Menoeceus, Epicurus outlines his philosophy of attaining happiness and details the proper attitude that Epicureans should have toward the gods and toward death. In reference to the latter, following his [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 23 September 2021
The genetics of obesity: from discovery to biology
- Ruth J. F. Loos ORCID: orcid.org/0000-0002-8532-5087 1 , 2 , 3 , 4 &
- Giles S. H. Yeo ORCID: orcid.org/0000-0001-8823-3615 5
Nature Reviews Genetics volume 23 , pages 120–133 ( 2022 ) Cite this article
143k Accesses
388 Citations
469 Altmetric
Metrics details
- Disease genetics
- Endocrine system and metabolic diseases
- Genetic association study
- Genetic variation
The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people’s health. Polygenic (or common) obesity and rare, severe, early-onset monogenic obesity are often polarized as distinct diseases. However, gene discovery studies for both forms of obesity show that they have shared genetic and biological underpinnings, pointing to a key role for the brain in the control of body weight. Genome-wide association studies (GWAS) with increasing sample sizes and advances in sequencing technology are the main drivers behind a recent flurry of new discoveries. However, it is the post-GWAS, cross-disciplinary collaborations, which combine new omics technologies and analytical approaches, that have started to facilitate translation of genetic loci into meaningful biology and new avenues for treatment.
Similar content being viewed by others
A phenome-wide comparative analysis of genetic discordance between obesity and type 2 diabetes
Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities


Independent phenotypic plasticity axes define distinct obesity sub-types
Introduction.
Obesity is associated with premature mortality and is a serious public health threat that accounts for a large proportion of the worldwide non-communicable disease burden, including type 2 diabetes, cardiovascular disease, hypertension and certain cancers 1 , 2 . Mechanical issues resulting from substantially increased weight, such as osteoarthritis and sleep apnoea, also affect people’s quality of life 3 . The impact of obesity on communicable disease, in particular viral infection 4 , has recently been highlighted by the discovery that individuals with obesity are at increased risk of hospitalization and severe illness from COVID-19 (refs 5 , 6 , 7 ).
On the basis of the latest data from the NCD Risk Factor Collaboration, in 2016 almost 2 billion adults (39% of the world’s adult population) were estimated to be overweight (defined by a body mass index (BMI) of ≥25 kg m − 2 ), 671 million (12% of the world’s adult population) of whom had obesity (BMI ≥30 kg m − 2 ) — a tripling in the prevalence of obesity since 1975 (ref. 8 ) (Fig. 1 ). Although the rate of increase in obesity seems to be declining in most high-income countries, it continues to rise in many low-income and middle-income countries and prevalence remains high globally 8 . If current trends continue, it is expected that 1 billion adults (nearly 20% of the world population) will have obesity by 2025. Particularly alarming is the global rise in obesity among children and adolescents; more than 7% had obesity in 2016 compared with less than 1% in 1975 (ref. 8 ).
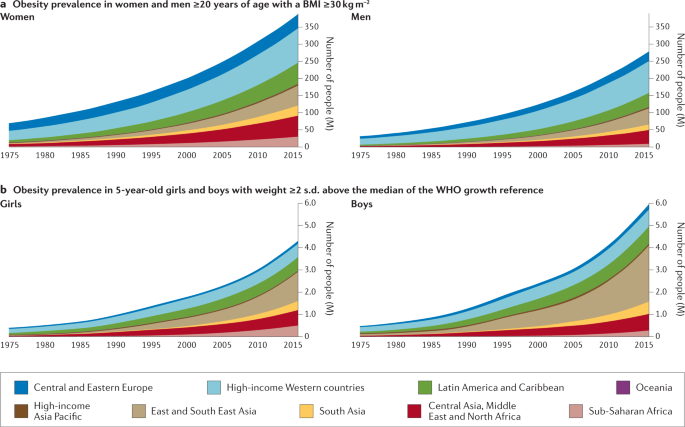
The prevalence of obesity has risen steadily over the past four decades in children, adolescents (not shown) and adults worldwide. a | Prevalence of obesity (body mass index (BMI) ≥30 kg m −2 ) in women and men ≥20 years of age, from 1975 to 2016. b | Prevalence of obesity (weight ≥2 s.d. above the median of the WHO growth reference) in 5-year-old girls and boys from 1975 to 2016. Geographical regions are represented by different colours. Graphs are reproduced from the NCD Risk Factor Collaboration (NCD RisC) website and are generated from data published in ref. 8 .
Although changes in the environment have undoubtedly driven the rapid increase in prevalence, obesity results from an interaction between environmental and innate biological factors. Crucially, there is a strong genetic component underlying the large interindividual variation in body weight that determines people’s response to this ‘obesogenic’ environment . Twin, family and adoption studies have estimated the heritability of obesity to be between 40% and 70% 9 , 10 . As a consequence, genetic approaches can be leveraged to characterize the underlying physiological and molecular mechanisms that control body weight.
Classically, we have considered obesity in two broad categories (Fig. 2 ): so-called monogenic obesity , which is inherited in a Mendelian pattern, is typically rare, early-onset and severe and involves either small or large chromosomal deletions or single-gene defects; and polygenic obesity (also known as common obesity), which is the result of hundreds of polymorphisms that each have a small effect. Polygenic obesity follows a pattern of heritability that is similar to other complex traits and diseases. Although often considered to be two distinct forms, gene discovery studies of monogenic and polygenic obesity have converged on what seems to be broadly similar underlying biology. Specifically, the central nervous system (CNS) and neuronal pathways that control the hedonic aspects of food intake have emerged as the major drivers of body weight for both monogenic and polygenic obesity. Furthermore, early evidence shows that the expression of mutations causing monogenic obesity may — at least in part — be influenced by the individual’s polygenic susceptibility to obesity 11 .
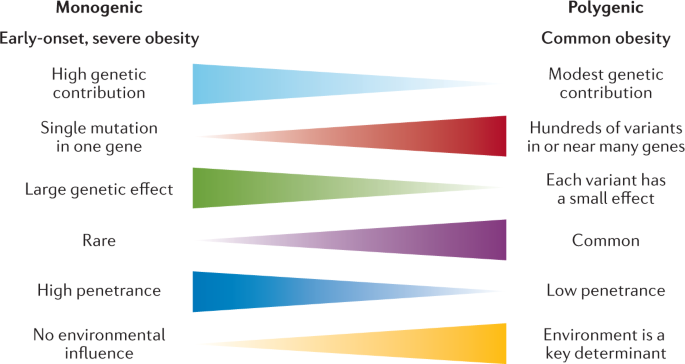
Key features of monogenic and polygenic forms of obesity .
In this Review, we summarize more than 20 years of genetic studies that have characterized the molecules and mechanisms that control body weight, specifically focusing on overall obesity and adiposity, rather than fat distribution or central adiposity. Although most of the current insights into the underlying biology have been derived from monogenic forms of obesity, recent years have witnessed several successful variant-to-function translations for polygenic forms of obesity. We also explore how the ubiquity of whole-exome sequencing (WES) and genome sequencing has begun to blur the line that used to demarcate the monogenic causes of obesity from common polygenic obesity. Syndromic forms of obesity, such as Bardet–Biedl, Prader–Willi, among many others 12 , are not reviewed here. Although obesity is often a dominant feature of these syndromes, the underlying genetic defects are often chromosomal abnormalities and typically encompass multiple genes, making it difficult to decipher the precise mechanisms directly related to body-weight regulation. Finally, as we enter the post-genomic era, we consider the prospects of genotype-informed treatments and the possibility of leveraging genetics to predict and hence prevent obesity.
Gene discovery approaches
The approaches used to identify genes linked to obesity depend on the form of obesity and genotyping technology available at the time. Early gene discovery studies for monogenic forms of obesity had a case-focused design: patients with severe obesity, together with their affected and unaffected family members, were examined for potential gene-disrupting causal mutations via Sanger sequencing. By contrast, genetic variation associated with common forms of obesity have been identified in large-scale population studies, either using a case–control design or continuous traits such as BMI. Gene discovery for both forms of obesity was initially hypothesis driven; that is, restricted to a set of candidate genes that evidence suggests have a role in body-weight regulation. Over the past two decades, however, advances in high-throughput genome-wide genotyping and sequencing technologies, combined with a detailed knowledge of the human genetic architecture, have enabled the interrogation of genetic variants across the whole genome for their role in body-weight regulation using a hypothesis-generating approach.
Gene discovery for monogenic obesity
Many of the candidate genes and pathways linked to body-weight regulation were initially identified in mice, such as the obese ( ob ) 13 and diabetes ( db ) 14 mouse lines, in which severe hyperphagia and obesity spontaneously emerged. Using reverse genetics , the ob gene was shown to encode leptin, a hormone produced from fat, and it was demonstrated that leptin deficiency resulting from a mutation in the ob gene caused the severe obesity seen in the ob/ob mouse 15 (Fig. 3 ). Shortly after the cloning of ob , the db gene was cloned and identified as encoding the leptin receptor (LEPR) 16 . Reverse genetics was also used to reveal that the complex obesity phenotype of Agouti ‘lethal yellow’ mice is caused by a rearrangement in the promoter sequence of the agouti gene that results in ectopic and constitutive expression of the agouti peptide 17 , 18 , which antagonizes the melanocortin 1 and 4 receptors (MC1R and MC4R) 19 , 20 . This finding linked the melanocortin pathway to body-weight regulation, thereby unveiling a whole raft of new candidate genes for obesity.
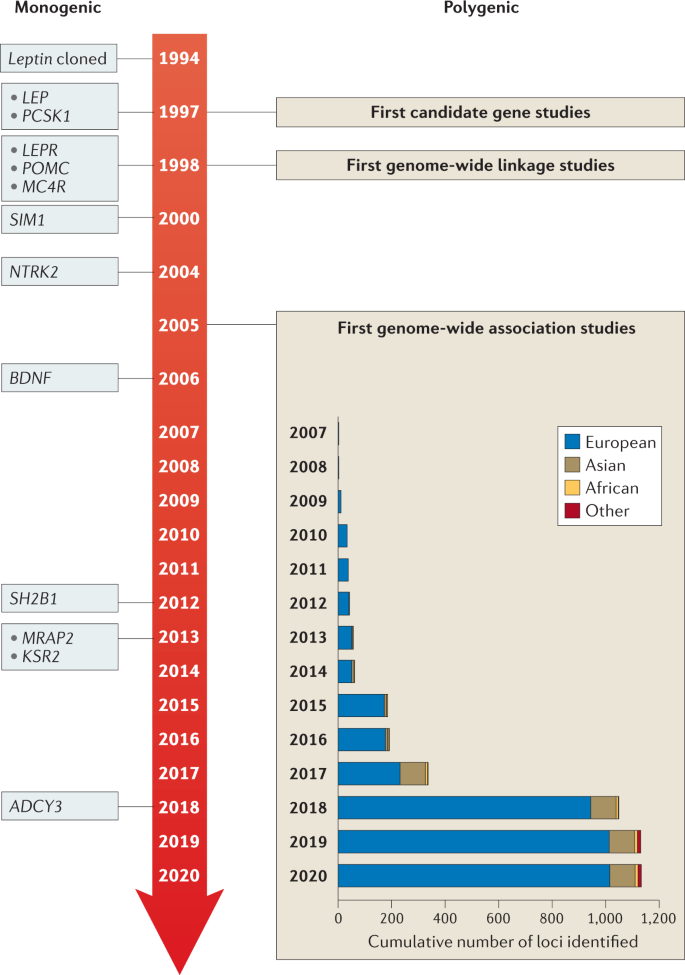
Genes identified for monogenic obesity in a given year are shown on the left. Discoveries made for polygenic obesity are shown on the right, including a cumulative count of newly discovered loci per year and by ancestry. Although candidate gene and genome-wide linkage studies became available in the late 1990s, findings were limited, and these study designs are not as frequently used as genome-wide association studies.
Once the genes for leptin and its receptor were identified, they became candidate genes for human obesity, and in 1997 the first humans with congenital leptin deficiency were identified 21 . This discovery was rapidly followed by the report of humans with mutations in the gene encoding the leptin receptor ( LEPR ) 22 , as well as in genes encoding multiple components of the melanocortin pathway, including PCSK1 (ref. 23 ), MC4R 24 , 25 , 26 and POMC 27 , 28 , 29 , all of which were found to result in severe early-onset obesity (Table 1 ).
Advances in high-throughput DNA sequencing led to candidate gene screening being replaced by WES, an unbiased approach that allows all coding sequences to be screened for mutations. However, it rapidly became clear that, whereas candidate gene studies yielded few mutations, WES identified too many potential obesity-associated variants such that the noise often masked the true causative mutations. However, with improved algorithms to predict the pathogenicity of mutations, as well as a rapidly expanding toolkit of functional assays, it has become easier to filter the likely pathogenic mutations. Several success stories have been reported in which WES has identified novel pathways and genes linked to obesity, such as the class 3 semaphorins (SEMA3A–G), which have been shown to direct the development of certain hypothalamic neurons, including those expressing pro-opiomelanocortin (POMC) 30 (see ‘Other neuronal circuits and molecules linked to severe obesity’).
Most monogenic obesity mutations have been identified in cohorts of patients with severe and early-onset (<10 years old) obesity. Additionally, as monogenic obesity often demonstrates a recessive inheritance pattern 31 , consanguinity in populations has further increased the chance of identifying mutations, owing to greater chances of homozygosity of deleterious mutations 32 . For example, studies have reported that mutations in the genes encoding leptin, LEPR and MC4R explain 30% of cases of severe obesity in children from a consanguineous Pakistani population 33 , and single-gene defects more broadly account for nearly 50% 34 .
Gene discovery for polygenic obesity
The discovery of genes that influence polygenic obesity, which is common in the general population, started off slowly with candidate gene studies and genome-wide linkage studies . The candidate gene approach was first applied in the mid-1990s and aimed to validate genes identified through human and animal models of extreme obesity for a role in common obesity (Fig. 3 ). Common variants in such candidate genes were tested for association with obesity risk, BMI or other body composition traits. Over the subsequent 15 years, hundreds of genes were studied as candidates, but variants in only six ( ADRB3 (ref. 35 ), BDNF 36 , CNR1 (ref. 37 ), MC4R 38 , PCSK1 (ref. 39 ) and PPARG 40 ) showed reproducible association with obesity outcomes. The genome-wide linkage approach made its entrance into the field towards the end of the 1990s (Fig. 3 ). Genome-wide linkage studies rely on the relatedness of individuals and test whether certain chromosomal regions co-segregate with a disease or trait across generations. Even though more than 80 genome-wide linkage studies identified >300 chromosomal loci with suggestive evidence of linkage with obesity traits, few loci were replicated and none was successfully fine-mapped to pinpoint the causal gene or genes 41 . Ultimately, candidate gene and genome-wide linkage studies, constrained by small sample sizes, sparse coverage of genetic variation across the genome and lack of replication, only had a marginal impact on the progression of gene discovery for common obesity outcomes.
However, the pace of gene discovery for common diseases accelerated with the advent of genome-wide association studies (GWAS) (Fig. 3 ). The first GWAS for obesity traits were published in 2007 and identified a cluster of common variants in the first intron of the FTO locus that was convincingly associated with BMI 42 , 43 . Many more GWAS followed and, to date, nearly 60 GWAS have identified more than 1,100 independent loci associated with a range of obesity traits 44 (Supplementary Tables 1 , 2 ).
As sample sizes increase with each consecutive GWAS, the statistical power to identify more loci also increases, in particular for loci that are less common and/or have smaller effects. For example, the first GWAS were relatively small ( n = ~5,000) and identified only the FTO locus 42 , 43 . The BMI-increasing allele of FTO is common, particularly in populations of European ancestry (minor allele frequency (MAF) 40–45%), and has a relatively large effect on BMI (0.35 kg m −2 per allele; equivalent to 1 kg for a person who is 1.7 m tall). Ten years and numerous GWAS later, the most recent GWAS for BMI included nearly 800,000 individuals, identified more than 750 loci, with MAFs as small as 1.6% and per-allele effects as low as 0.04 kg m −2 per allele (equivalent to 120 g for a person who is 1.7 m tall) 45 . Combined, these genome-wide significant loci explained 6% of variation in BMI 45 . Large-scale international collaborations have been formed, such as the Genetic Investigation for Anthropometric Traits (GIANT) consortium , that combine summary statistics of individual GWAS to generate data sets comprising hundreds of thousands of individuals. Furthermore, many GWAS efforts have maximized sample size by focusing on BMI as the primary obesity outcome, an inexpensive and easy-to-obtain measurement that is readily available in most studies. As such, the vast majority of loci have been identified first in GWAS of BMI, but their effects typically transfer to other overall adiposity outcomes.
Even though BMI is widely used, it is considered a crude proxy of overall adiposity because it does not distinguish between lean and fat mass 46 . Therefore, GWAS have been performed for more refined obesity traits, such as body fat percentage 47 , 48 , fat-free mass 49 , imaging-derived adipose tissue 50 , circulating leptin levels 51 and LEPR levels 52 . In addition, two GWAS have focused on persistent healthy thinness, assuming that genes that determine resistance to weight gain may also inform obesity prevention and weight loss maintenance 53 , 54 . Although GWAS of more refined and alternative obesity outcomes are generally much smaller than those for BMI, the phenotypes are often a more accurate representation of body-weight regulation and, as such, the loci identified tend to more often point to relevant biological pathways that underlie obesity.
Almost all GWAS loci for obesity outcomes were first identified in adults. Most of these loci also associate with obesity and/or BMI in children and adolescents, highlighting the fact that the genetic underpinning of obesity is relatively constant across the course of life 55 , 56 , 57 . Similarly to gene discovery for other common diseases, the obesity genetics field has suffered from a strong bias in population representation, with the vast majority of GWAS being performed in populations that are exclusively or predominantly of European ancestry. Nevertheless, some loci have first been discovered in populations of Asian 58 , African 59 , 60 , Hispanic or other ancestry 61 , despite their much smaller sample sizes. Broadly, loci identified in one ancestry demonstrate good transferability (that is, directionally consistent associations) across other ancestries, even though effect sizes and allele frequencies may differ. The modest-to-high genetic correlations across ancestries observed for BMI ( r = 0.78) are consistent with good transferability 62 , but also suggest that ancestry-specific loci remain to be discovered. Besides increasing the sample sizes of GWAS in populations of non-European ancestry, demographic, evolutionary and/or genomic features of specific populations (such as founder, consanguineous or isolated populations) have been leveraged for gene discovery, identifying genetic variants with large effects that are common in the discovery population, such as CREBRF , first identified in Samoan populations, and ADCY3 , first identified in the Greenlandic population, but rare or nonexistent in most others 63 , 64 , 65 , 66 . CREBRF has been shown to play a role in cellular energy storage and use, and may be implicated in cellular and organismal adaptation to nutritional stress 65 . ADCY3 colocalizes with MC4R at the primary cilia of a subset of hypothalamic neurons that have been implicated in body-weight regulation 67 .
GWAS have typically focused on biallelic, common genetic variation (MAF >5%), but have also been used to screen for the role of copy number variants (CNVs) in obesity. So far, only a few CNVs have been identified that have a convincing association with BMI, such as the 1p31.1 45-kb deletion near NEGR1 (ref. 68 ), which encodes a cell-adhesion molecule expressed in the brain 69 ; the 16p12.3 21-kb deletion upstream of GPRC5B 70 , which may modulate insulin secretion 71 ; the 10q11.22 CNV in PPYR1 (also known as NPY4R ) 72 , which encodes a potent anti-obesity agent known to inhibit food intake 73 ; and the 1p21.1 multi-allele CNV encompassing AMY1A 74 , which produces salivary α-amylase, a key enzyme in starch digestion 75 .
To determine the role of other types of variation in obesity, alternative genome-wide screens have been performed. For example, the impact of low-frequency and rare protein-coding variants has been tested using exome sequencing and exome array data 76 , 77 , 78 , 79 . It was speculated that low-frequency (MAF 1–5%) and rare (MAF <1%) variants would have larger effects than common variants, and thus be easier to detect. Nevertheless, even large-scale studies identified only a few robust associations for rare coding variants. For example, exome-wide screening based on array data from more than 400,000 individuals identified p.Tyr35Ter (rs13447324) in MC4R ; p.Arg190Gln (rs139215588) and p.Glu288Gly (rs143430880) in GIPR , which stimulates insulin secretion and mediates fat deposition 80 ; p.Arg95Ter (rs114285050) in GRP151 , which modulates habenular function that controls addiction vulnerability 81 ; and p.Arg769Ter (rs533623778) in PKHD1L1 , which has been involved in cancer development 77 , 78 . A recent study that leveraged WES data for more than 600,000 individuals identified 16 genes for which the burden of rare nonsynonymous variants was associated with BMI, including five brain-expressed G protein-coupled receptors ( CALCR , MC4R , GIPR , GPR151 and GPR75 ) 79 .
As obesity is a complex, multifactorial condition, some GWAS have integrated demographic factors (such as sex and age 82 ) and environmental factors (such as physical activity 83 , diet 84 or smoking 85 ) into their analyses. Despite sample sizes of more than 200,000 individuals, these genome-wide gene-by-environment (G×E) interaction analyses remain challenging and so far only 12 loci have been identified, the effects of which on obesity are attenuated or exacerbated by non-genetic factors. Nevertheless, the G×E interaction between the FTO locus and a healthy lifestyle has been robustly replicated. Specifically, increased physical activity or a healthy diet can attenuate the effect of the FTO locus on obesity risk by 30–40% 86 , 87 .
The increasing availability of large-scale cohorts and biobanks, such as the UK Biobank , the Million Veterans Project , All of Us , Biobank Japan and 23andMe , combined with ongoing work by the GIANT consortium, will boost sample sizes further to easily exceed 4 million participants in meta-analyses, expediting the discovery of many more obesity-associated loci. However, translation of GWAS-identified loci into new biological insights remains a major challenge.
From genes to biology
Despite the difficulties in validating causative mutations and variants, genetic studies into both rare and common obesity over the past two decades have revealed two surprisingly cogent, overarching biological messages: first, the leptin–melanocortin pathway is a key appetitive control circuit 31 , 88 (Fig. 4 ); and second, genes that are either enriched or exclusively expressed within the brain and CNS have a central role in obesity 89 .
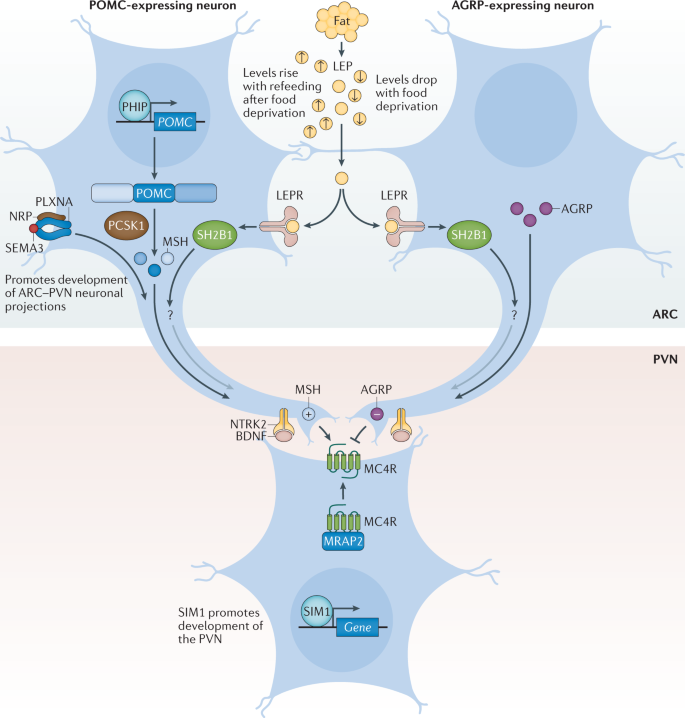
Pro-opiomelanocortin (POMC)-expressing neurons and agouti-related protein (AGRP)-expressing neurons within the arcuate nucleus of the hypothalamus (ARC) act to sense circulating leptin (LEP) levels, which reflect fat mass. These neurons signal to melanocortin 4 receptor (MC4R)-expressing neurons in the paraventricular nucleus of the hypothalamus (PVN), which controls appetite, thus linking long-term energy stores to feeding behaviour. Binding of class 3 semaphorins (SEMA3) to their receptors NRP and PLXNA influences the projection of POMC neurons to the PVN. Binding of brain-derived neurotrophic factor (BDNF) to its receptor neurotrophic receptor tyrosine kinase 2 (NTRK2) is thought to be an effector of leptin-mediated synaptic plasticity of neurons, including those in the ARC and PVN. The transcription factor SIM1 is crucial for the proper development of the PVN. +, agonist; −, antagonist; LEPR, leptin receptor; MRAP2, melanocortin receptor accessory protein 2; MSH, melanocyte-stimulating hormone; SH2B1, SH2B adaptor protein 1.
The leptin–melanocortin pathway and MC4R
Leptin is a key hormone secreted by adipocytes, which circulates at levels in proportion to fat mass 90 . Leptin also responds to acute changes in energy state, as its levels decrease with food deprivation and are restored during re-feeding. Administration of leptin to fasted mice abrogates many of the neuroendocrine consequences of starvation, suggesting that the normal biological role of leptin is to initiate the starvation response 91 . Leptin signals through the LEPR, which exists in several different isoforms. However, obesity-related effects of leptin are predominantly mediated by a long isoform that contains an intracellular domain (LEPRb), which is expressed in various regions of the CNS 90 .
Within the arcuate nucleus (ARC) of the hypothalamus, LEPRb is found on two populations of neurons at the heart of the melanocortin pathway, one of which expresses POMC and the other agouti-related protein (AGRP) 92 (Fig. 4 ). POMC is post-translationally processed by prohormone convertases to produce several biologically active moieties, including β-lipotrophin and β-endorphin, and, crucially, the melanocortin peptides adrenocorticotrophin (ACTH) and α-, β- and γ-melanocyte-stimulating hormone (MSH) 93 . The ARC POMC neurons project to MC4R neurons within the paraventricular nucleus (PVN) where melanocortin peptides signal to decrease food intake 92 . By contrast, AGRP acts as an endogenous antagonist of MC4R to increase food intake 92 , 94 . MC3R is another centrally expressed receptor that binds to both melanocortin peptides and AGRP; however, as mice with targeted deletions in the gene are not obese but instead have altered fat to lean mass ratio, MC3R is less likely to be related to food intake and more likely to be involved in nutrient partitioning 95 , 96 .
We can state with confidence that the fine balance of melanocortinergic agonism and AGRP antagonism of MC4R, in response to peripheral nutritional cues such as leptin, plays a central part in influencing appetitive drive 92 . The genetic evidence clearly supports this contention, with mutations in most genes of the melanocortin pathway resulting in hyperphagia and severe obesity in both humans and mice 31 , 88 . In fact, the vast majority of single-gene disruptions causing severe early-onset obesity in humans fall within this pathway, including LEPR , POMC , AGRP , MCR4R , PCSK1 (ref. 23 ), SH2B1 (ref. 97 ), PHIP 98 , MRAP2 (ref. 99 ) and SIM1 (ref. 100 ) (Fig. 4 ; Table 1 ). Mutations in MC4R in particular, are the most common single-gene defect leading to hyperphagia and obesity. Pathogenic mutations in MC4R are found in up to 5% of cases of severe childhood obesity 101 and up to 0.3% of the general population 101 , 102 . Of note, the degree of receptor dysfunction, as measured by in vitro assays, can predict the amount of food eaten at a test meal by an individual harbouring that particular mutation 101 . Thus MC4R does not act in a binary on/off manner, but as a rheostat; put simply, the melanocortin pathway is a ‘tunable’ system. In addition to regulating food intake, it also regulates food preference, with individuals who carry mutations in MC4R showing a preference for food with higher fat content 103 .
The importance of the melanocortin pathway in regulating feeding behaviour is highlighted by the identification of naturally occurring mutations in pathway genes in a wide range of different species where the appropriate selection pressure has been present (Table 1 ). For example, studies have found that 20–25% of Labrador retrievers, which are known to be more food-motivated than other dog breeds, carry a 14-bp deletion in POMC that disrupts the β-MSH and β-endorphin coding sequences and is associated with greater food motivation and increased body weight 104 . Also, certain breeds of pig have been shown to carry MC4R missense mutations that are associated with fatness, growth and food intake traits 105 . MC4R mutations even contribute to the adaptation and survival of blind Mexican cavefish to the nutrient-poor conditions of their ecosystem 106 .
Other neuronal circuits and molecules linked to severe obesity
It is now clear that in addition to engaging classical neuropeptide–receptor systems within the brain, leptin also rapidly modifies synaptic connections between neurons 107 , and that this structural plasticity is crucial to its downstream functions. One of the ways in which this plasticity is thought to be achieved is via brain-derived neurotrophic factor (BDNF) signalling to its receptor TrkB. BDNF is widely expressed in the CNS where it plays an important part in neuronal development 108 , 109 . In the hippocampus, BDNF contributes to synaptic plasticity and long-term potentiation associated with memory and learning 110 . However, evidence has emerged that implicates BDNF and TrkB in the regulation of mammalian eating behaviour and energy balance 111 . BDNF is downregulated by nutritional deprivation and upregulated by leptin within the ventromedial nucleus (VMN) of the hypothalamus 112 , although this regulation is probably indirect, as very few VMN BDNF neurons express the LEPR 113 (Fig. 4 ) and some evidence indicates that it acts at least in part downstream of melanocortin signalling 112 . Crucially, genetic disruption of BDNF 114 , 115 and TrkB 112 , 116 in both humans and mice results in hyperphagia and severe obesity.
Another group of neuronal proteins important in the development of neuronal circuitry and linked to energy balance are the class 3 semaphorins (SEMA3A–G). A study in humans found that 40 rare loss-of-function variants in SEMA3A–G and their receptors (PLXNA1–4, NRP1 and NRP2) were significantly enriched in 982 individuals with severe obesity compared with 4,449 controls 30 . Disruption of several of these genes in zebrafish caused increased somatic growth and/or adiposity, and experiments with mouse hypothalamic explants suggest that SEMA3 signalling via NRP2 receptors drives the development of POMC projections from the ARC to the PVN 30 . However, given that these results are from a single study, more data are required to confirm the exact role of class 3 semaphorins in energy homeostasis.
Insights from genetic loci linked to common obesity
Unlike candidate gene studies, GWAS make no a priori assumptions about the underlying biology that links genetic variants to a disease of interest. While this agnostic approach allows for new biological insights, the vast majority of GWAS-identified variants map to the non-coding parts of genes or to regions between genes. As such, they do not directly disrupt the protein-coding regions, but instead overlap with regulatory elements that influence expression of genes in close proximity or even over long distances.
However, even if the causative genes are unknown, pathway, tissue and functional enrichment analyses based on the genes located in the GWAS loci can provide insights into potential mechanisms. Since the very first GWAS for BMI 68 , 117 , such analyses have pointed to the CNS being a key player in body-weight regulation, consistent with insights from human and animal models of extreme obesity. Recent analyses that include the latest BMI-associated loci, combined with updated multi-omics databases and advanced computational tools, have further refined these observations. In addition to the hypothalamus and pituitary gland (which are both known appetite regulation sites), other brain areas have been highlighted, including the hippocampus and the limbic system (which are involved in learning, cognition and emotion) and the insula and the substantia nigra (which are related to addiction and reward) 58 , 89 , 118 , 119 . The enrichment of immune-related cells (such as lymphocytes and B cells) and adipose tissue was found to be weaker 58 .
Although enrichment analyses provide preliminary insights into the broad biology represented by genes in the GWAS loci, determining which genes, variants and/or underlying mechanisms are causal has proved an arduous task. For example, the FTO locus, which was identified more than a decade ago and harbours six genes, is the most extensively studied GWAS-identified obesity locus (Fig. 5 ). Despite its highly significant and widely replicated association with obesity 120 , the causal variants and/or genes in the FTO locus have not yet been pinpointed with convincing evidence, and the mechanisms by which the locus affects body weight have not been fully elucidated. Early functional follow-up analyses suggested that FTO itself might be responsible, as Fto deficiency in mice results in a lean phenotype, whereas Fto overexpression is associated with increased body weight 121 , 122 . Studies in mice have suggested that FTO plays a role in cellular nutrient sensing 123 , 124 . Other studies found evidence that FTO influences brain regions that affect appetite, reward processing and incentive motivation by regulating ghrelin levels in humans 125 or by controlling dopaminergic signalling in mice 126 , 127 . In addition, variants in the FTO locus were shown to alter a regulatory element that controls the transcription of Rpgrip1l in mice, a ciliary gene located immediately upstream of Fto 128 , 129 , 130 . Mice with reduced Rpgrip1l activity exhibit hyperphagic obesity, possibly mediated through diminished leptin signalling 128 , 129 , 130 . In recent years, studies in human and animal models have shown that variants in the FTO locus directly interact with the promoter of Irx3 , a gene located 0.5 Mb downstream of FTO . Irx3 -deficient mice were found to exhibit weight loss and increased metabolic rate with browning of white adipose tissue, without changes in physical activity or appetite 131 , 132 . Further in-depth functional characterization showed that rs1421085 in the FTO locus disrupts a conserved binding motif for the transcriptional repressor ARID5B, which leads to a doubling of IRX3 and IRX5 expression during early adipocyte differentiation 132 . The authors argue that increased expression of these genes results in a developmental shift from energy-dissipating beige adipocytes to energy-storing white adipocytes, a fivefold reduction in mitochondrial thermogenesis and increased lipid storage 132 . However, given that multiple studies have shown that the FTO locus is robustly associated with food intake, with no evidence to date linking it to changes in energy expenditure, the relevance of this observation to the actual observed human phenotype still needs to be explored 133 . A recent study reports that the FTO locus affects gene expression in multiple tissues, including adipose tissue and brain, and, more broadly, that the genetic architecture of disease-associated loci may involve extensive pleiotropy and allelic heterogeneity across tissues 134 .
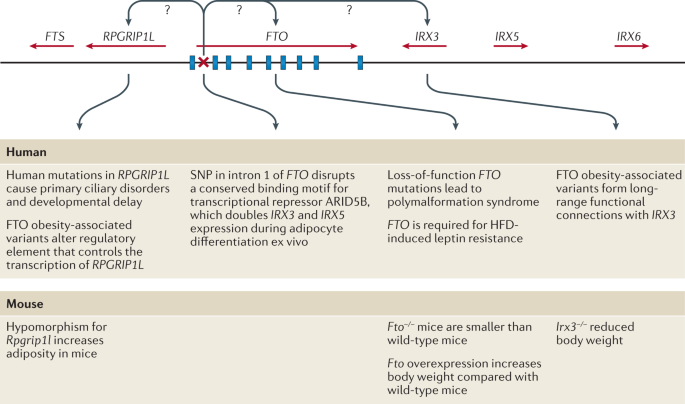
FTO contains nine exons (depicted by blue rectangles) and the body mass index (BMI)-associated SNP identified in genome-wide association studies (depicted by a red ×) maps to intron 1. IRX3 and RPGRIP1L have both been proposed to be the causal genes for obesity within the locus and to act on body weight through distinct mechanisms. HFD, high-fat diet.
Besides the FTO locus, functional follow-up analyses have been performed for only a few obesity-associated GWAS loci. For example, early studies identified a cluster of variants just downstream of TMEM18 (refs 68 , 117 ). TMEM18 encodes a poorly characterized transmembrane protein that is highly conserved across species and widely expressed across tissues, including in several regions of the brain 135 , 136 . Tmem18 deficiency in mice results in a higher body weight owing to increased food intake, whereas Tmem18 overexpression reduces food intake and limits weight gain 136 . A knockdown experiment in Drosophila melanogaster suggests that TMEM18 affects carbohydrate and lipid levels by disrupting insulin and glucagon signalling 137 .
Two other GWAS loci for which functional analyses have been performed are located just upstream of CADM1 (ref. 82 ) and in CADM2 (ref. 70 ), genes that encode cell-adhesion proteins of the immunoglobulin superfamily and mediate synaptic assembly in the CNS 138 . The BMI-increasing alleles at each locus are associated with increased expression of CADM1 and CADM2 in the hypothalamus 139 , 140 . Deficiency of either Cadm1 or Cadm2 in mice results in a lower body weight and increased insulin sensitivity, glucose tolerance and energy expenditure without any change in food intake 139 , 140 . Conversely, increased neuronal expression of either Cadm1 or Cadm2 is associated with elevated body weight 139 , 140 . Furthermore, CADM1 is expressed in POMC neurons and Cadm1 deficiency leads to an increase in the number of excitatory synapses, suggestive of an increased synaptic plasticity 140 . Cadm2 -deficient mice exhibit increased locomotor activity and higher core body temperature 139 .
Another GWAS locus, just upstream of NEGR1 , harbours two deletions associated with increased obesity risk 68 , 117 , 141 . These deletions do not overlap with the coding sequence of NEGR1 , but encompass a conserved transcription factor-binding site for NKX6.1 , a potent transcriptional repressor 68 , 141 . Loss of binding of NKX6.1 leads to higher NEGR1 expression 141 , which is consistent with the observation that BMI-increasing alleles (that is, deletions) at this locus are associated with higher NEGR1 expression in the brain. Similar to CADM1 and CADM2, NEGR1 is a cell-adhesion molecule of the immunoglobulin superfamily that is expressed in several regions of the brain and has been shown to have a role in brain connectivity 69 , 142 , a process believed to be important in obesity 143 . NEGR1 deficiency in mice was shown to result in lower body weight, mainly due to reduced lean mass, mediated by lower food intake 144 . However, two other functional studies, one in mice and one in rats, found that knockdown of Negr1 expression resulted in the opposite phenotype — increased body weight and food intake 145 , 146 . While NEGR1 deficiency in mice was found to impair core behaviours, so far, findings and proposed mechanisms are not fully aligned 69 , 147 , 148 , 149 .
Taken together, functional follow-up analyses for these loci are slowly expanding our understanding of the pathophysiology that drives weight gain. However, many more obesity-associated loci are waiting to be translated into new biological insights. A major hurdle in translating GWAS loci into plausible candidate genes and appropriate paradigms for functional research is the annotation of the associated variants in a locus. Defining the regulatory function of the non-coding variants, identifying their putative effector transcripts and determining their tissues of action remains an ongoing challenge. The advent of high-throughput genome-scale technologies for mapping regulatory elements, combined with comprehensive multi-omics databases, advanced computational tools and the latest genetic engineering and molecular phenotyping approaches, is poised to speed up the translation of GWAS loci into meaningful biology 150 .
Converging results from monogenic and polygenic forms of obesity
Gene discovery is often dichotomized by allele frequency and disease prevalence; that is, mutations are sought for monogenic forms of obesity and common variants for polygenic obesity (Fig. 2 ). However, it is increasingly recognized that monogenic and polygenic forms of obesity are not discrete entities. Instead, they lie on a spectrum and share — at least in part — the same biology. As GWAS have continued to discover more obesity-associated loci, an increasing number of these loci harbour genes that were first identified for extreme and early-onset obesity in humans or animal models, including MC4R 151 , 152 , BDNF 117 , SH2B1 (refs 68 , 117 ), POMC 70 , LEP 51 , 153 , LEPR 52 , 154 , NPY 155 , SIM1 (ref. 155 ), NTRK2 (ref. 58 ), PCSK1 (ref. 154 ) and KSR2 (ref. 77 ). In fact, most of these genes encode components of the leptin–melanocortin and BDNF–TrkB signalling pathways (Table 1 ). Thus, whereas genetic disruption of components of these pathways results in severe obesity, genetic variants in or near these same genes that have more subtle effects on their expression will influence where an individual might sit in the normal distribution of BMI.
Although most genes have been first identified for extreme forms of obesity, a locus harbouring ADCY3 was first identified in GWAS for common obesity 77 , and ADCY3 was subsequently confirmed as having a role in extreme obesity 63 , 64 . ADCY3 encodes an adenylate cyclase that catalyses the synthesis of cAMP, an important second messenger in signalling pathways. There is some evidence that ADCY3 (adenylate cyclase) colocalizes with MC4R at the primary cilia of PVN neurons 67 and that cilia are required specifically on MC4R-expressing neurons for the control of energy homeostasis 156 . In mice, disruption of Adcy3 or Mc4r in the cilia of these neurons impairs melanocortin signalling, resulting in hyperphagia and obesity 67 .
As more GWAS loci are reported, we expect that findings across different lines of obesity research will continue to converge, providing accumulating evidence for new biology.
From genes to clinical care
Genetic insights from gene discovery efforts are increasingly being used in the context of precision medicine in ways that directly affect health. Knowing a patient’s genotype may enable a more precise diagnosis of the type of obesity, which in turn allows the prescription of personalized treatment or prevention strategies. Furthermore, knowing an individual’s genetic susceptibility to obesity early in life may help to more accurately predict those most at risk of gaining weight in the future.
Use of genotype information in treatment of obesity
When a disease is caused by a single mutation and the environmental contribution is limited, as is the case for some forms of extreme and early-onset obesity, a genetic test can be instrumental in correctly diagnosing patients. Although no standard genetic testing panel is currently available for extreme and early-onset obesity, some clinics, research centres and pharmaceutical companies sequence well-known candidate genes to identify the functional mutation that may be the cause of a patient’s excess body weight. Such a genetic diagnosis can lessen the feelings of guilt and blame for the patient, and alleviate social stigma and discrimination. Importantly, a genetic diagnosis can inform disease prognosis and, in some cases, it will determine treatment. To date, there are two treatments for obesity that are tailored to patient genotype.
The prototype of genotype-informed treatment for obesity is the administration of recombinant human leptin in patients who are leptin-deficient owing to mutations in the LEP gene 157 , 158 . Although congenital leptin deficiency is exceptionally rare (only 63 cases have been reported to date 28 ), leptin replacement therapy has been remarkably beneficial for these patients by substantially reducing food intake, body weight and fat mass, and normalizing endocrine function 157 , 158 . It has literally transformed their lives.
The second genotype-informed treatment for obesity is setmelanotide, a selective MC4R agonist that was recently approved by the FDA for rare monogenic obesity conditions including LEPR, PCSK1 and POMC deficiency 159 . Setmelanotide acts as a substitute for the absent MSH in patients with POMC deficiency owing to mutations in POMC or PCSK1 , and in patients with LEPR deficiency owing to mutations in LEPR , which is essential for POMC function 160 , 161 , 162 . Daily subcutaneous injection of setmelanotide results in substantial weight loss and in reduction of hunger 160 , 161 , 162 . After a 1-year treatment with setmelanotide in phase III trials, patients with POMC deficiency lost on average 25.6% of their initial weight, with 80% of patients achieving at least a 10% weight loss 162 . The adverse effects of setmelanotide treatment are minor, and include hyperpigmentation, nausea and/or vomiting, penile erection and injection site reactions. Weight loss in patients with LEPR deficiency was less pronounced; on average, they lost 12.5% of their initial weight, with only 45% of patients achieving at least a 10% weight loss 162 . The difference in weight loss between the two patient groups may be because POMC deficiency directly affects the production of MC4R ligands (α-MSH and β-MSH), whereas LEPR deficiency affects signalling upstream of POMC 162 . As such, setmelanotide may be able to completely restore MC4R signalling in POMC deficiency, but only partially in LEPR deficiency. Even though the average weight loss in POMC-deficient patients was twice that in LEPR-deficient patients, the reduction in hunger was substantially larger in LEPR-deficient patients (−43.7%) than in POMC-deficient patients (−27.1%) 162 . The reasons for the discrepancy between weight loss and reduction in hunger remain to be studied in greater depth. It has been estimated that in the USA, >12,800 individuals carry mutations in the melanocortin pathway for whom setmelanotide may be more effective for weight loss than any other treatment 163 . Although 12,800 carriers represent only a fraction (0.004%) of the adult population in the USA, and not all of these mutation carriers are overweight or obese, for the patients for whom setmelanotide is effective, it may end a lifelong battle to lose weight 163 . In patients without genetic defects, neither setmelanotide nor leptin administration have, to date, demonstrated a substantial effect on weight loss 164 , 165 .
These two genotype-informed treatments show how insight into the underlying biological mechanisms can guide the development of molecules and medications that restore impaired pathways, at least in monogenic forms of obesity caused by deficiency of one protein. Nevertheless, there remain substantial obstacles in the transition from conventional to precision medicine for monogenic obesity, which would require the adoption of systematic WES for individuals suspected to be carriers of deleterious mutations, and eventually even standardized screening at birth. We are clearly a long way from such a scenario at present.
Use of genotype information in prediction of obesity
As more variants are being discovered for common obesity, there is a growing expectation that genetic information will soon be used to identify individuals at risk of obesity. Knowing a person’s genetic susceptibility would allow for a more accurate prediction of who is at risk of gaining weight and give an opportunity to intervene earlier to prevent obesity more effectively. Genetic susceptibility to complex disease, including obesity, is assessed using a polygenic score (PGS). PGSs to assess obesity susceptibility are based on GWAS for BMI (PGS BMI ), the latest of which includes data on more than 2 million variants and explains 8.4% of the variation in BMI 166 . The average BMI of individuals with a high PGS BMI (top decile) is 2.9 kg m −2 (equivalent to 8 kg in body weight) higher and their odds of severe obesity (BMI ≥40 kg m −2 ) is 4.2-fold higher than those with a lower PGS BMI (lowest nine deciles) 166 .
Despite these strong associations with BMI and obesity, the predictive performance of the PGS BMI is weak, which is unsurprising given its limited explained variance. For example, using the same PGS BMI and data from the UK Biobank, we estimate that the area under the receiver operating characteristic curve (AUC ROC ) is only 0.64 to predict obesity. This means that the probability that an individual with obesity has a higher PGS BMI than an individual without obesity is 0.64. However, for a PGS to have clinical utility, the AUC ROC needs to be much higher (>0.80). In addition, we calculated the extent to which a PGS BMI ≥90th percentile correctly classifies individuals with obesity (Fig. 6 ). We found that such a predictive test (PGS BMI ≥90th percentile) has a positive predictive value of 0.43, meaning that of those who were predicted to develop obesity, only 43% actually developed obesity. Its sensitivity is 0.19, which means that of the individuals who developed obesity, only 19% had been correctly classified by the PGS BMI . Given that the current treatment options for obesity are low risk, or even generally beneficial, the high false-positive rate is less concerning than the low sensitivity, as some at-risk individuals may miss the opportunity for early prevention.
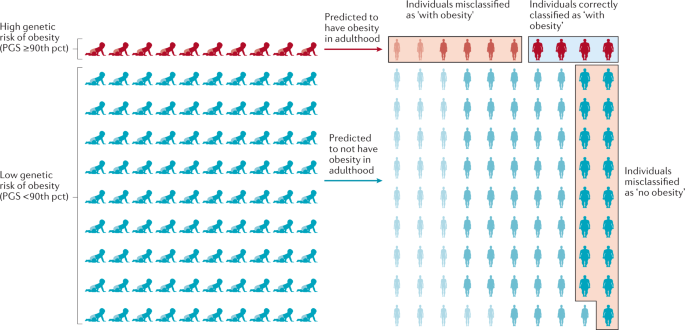
The outcome is illustrated for a polygenic score (PGS) that assumes that individuals with a score in the highest decile (≥90th percentile (pct)) will develop obesity, has a positive predictive value of 0.4 and a sensitivity of 0.19. Of ten individuals with a high score classified by the PGS as ‘with obesity’, four will be classified correctly but the other six will be misclassified and will not develop obesity — a positive predictive value of 0.4. Likewise, 17 of the 90 individuals with a score <90th pct who are predicted to not develop obesity, will develop obesity. Thus, only four of the 21 individuals who developed obesity were correctly classified by the PGS — a sensitivity of 0.19. Misclassified individuals are indicated by the red boxes, individuals correctly classified as ‘with obesity’ are indicated by a blue box. Adapted with permission from ref. 170 , Elsevier.
Thus, the current PGS BMI has a high rate of misclassification and does not reliably predict who is at risk of developing obesity and who is not. The predictive ability of PGSs are expected to improve as GWAS increase in sample size and algorithms to calculate the scores become more refined. Nevertheless, given the importance of socio-demographic, lifestyle and clinical risk factors in the aetiology of obesity, it is unlikely that a PGS BMI will ever be able to accurately predict obesity on its own. Instead, effective prediction models will have to include genetic and non-genetic factors, including a broad spectrum of demographic, environmental, clinical and possibly molecular markers, as well.
Conclusions and future perspectives
What initially began as two apparently distinct approaches, one studying rare Mendelian causes of extreme obesity, and the other exploring complex polygenic influences of population body-weight distribution, have eventually converged on the central role of the brain in regulating body weight. In particular, both approaches have highlighted the roles of the leptin–melanocortin pathway and TrkB–BDNF signalling. Perhaps it seems obvious now, but it was by no means certain that, just because genetic disruption of a pathway resulted in a severe phenotype, polymorphisms within that same pathway would produce a more subtle and nuanced result.
The GWAS approach is hypothesis-free, with the promise to reveal new genes that point to new biology and pathways. However, for the vast majority of the >1,000 GWAS-identified loci, we do not know which genes are causal, what cells, tissues and organs they act in to affect body weight, and we do not understand the underlying mechanisms. The translation from variant to function is a well-known challenge 167 , but with increasing availability of new omics data, high-throughput technologies and advanced analytical approaches, there is an unprecedented opportunity to speed up the translation of hundreds of GWAS loci.
Sample size remains a major driver for gene discovery. In an ongoing collaboration that combines data from more than 3 million individuals of diverse ancestry from the GIANT consortium, the UK Biobank and 23andMe, the number of BMI-associated GWAS loci is set to double. Also, a recent WES effort of more than 640,000 individuals has demonstrated that rare mutations are discoverable when sample sizes are sufficiently large 79 . However, alternative study designs, a focus on more refined phenotypes or a focus on population subgroups (that is, more homogeneous groups of individuals with similar outcomes) could further add to gene discovery.
Translation of only a few dozen of the GWAS-identified loci could tremendously improve our insights into the biology of obesity and possibly reveal new therapeutic targets. It would also take us a little closer to the ‘holy grail’ — the ability to move away from a failed ‘one-size-fits-all’ strategy, and towards true precision medicine for obesity, metabolic disease and other diet-related illnesses.
GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 , 13–27 (2017).
Article Google Scholar
Must, A. et al. The disease burden associated with overweight and obesity. JAMA 282 , 1523–1529 (1999).
Article CAS PubMed Google Scholar
Fontaine, K. R. & Barofsky, I. Obesity and health-related quality of life. Obes. Rev. 2 , 173–182 (2001).
Bhattacharya, I., Ghayor, C., Perez Dominguez, A. & Weber, F. E. From influenza virus to novel corona virus (SARS-CoV-2)—the contribution of obesity. Front. Endocrinol. 11 , 556962 (2020).
Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 369 , m1966 (2020).
Article PubMed PubMed Central Google Scholar
Cummings, M. J. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395 , 1763–1770 (2020).
Article CAS PubMed PubMed Central Google Scholar
Zhao, X. et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front. Endocrinol. 11 , 595109 (2020).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390 , 2627–2642 (2017).
Maes, H. H., Neale, M. C. & Eaves, L. J. Genetic and environmental factors in relative body weight and human obesity. Behav. Genet. 27 , 325–351 (1997).
Elks, C. E. et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front. Endocrinol. 3 , 29 (2012). This paper reports a large-scale meta-analysis of heritability data of twin and family studies .
Chami, N., Preuss, M., Walker, R. W., Moscati, A. & Loos, R. J. F. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS Med. 17 , e1003196 (2020). This study shows that the effect of MC4R mutations on BMI and obesity risk is attenuated by the polygenic background .
Kaur, Y., de Souza, R. J., Gibson, W. T. & Meyre, D. A systematic review of genetic syndromes with obesity. Obes. Rev. 18 , 603–634 (2017).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41 , 317–318 (1950).
Hummel, K. P., Dickie, M. M. & Coleman, D. L. Diabetes, a new mutation in the mouse. Science 153 , 1127–1128 (1966).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372 , 425–432 (1994). This paper reports the cloning of leptin, which provided the first molecular evidence that feeding and regulation of body weight had a hormonal basis .
Article CAS Google Scholar
Chen, H. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db / db mice. Cell 84 , 491–495 (1996). This paper reports the cloning of the LEPR and the localization of its signalling version, which highlights the role of the brain in the control of appetitive behaviour .
Bultman, S. J., Michaud, E. J. & Woychik, R. P. Molecular characterization of the mouse agouti locus. Cell 71 , 1195–1204 (1992).
Miller, M. W. et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 7 , 454–467 (1993).
Lu, D. et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371 , 799–802 (1994).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385 , 165–168 (1997).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387 , 903–908 (1997). This work demonstrates that leptin signalling is also relevant in the human context, and provides early evidence, together with mutations in PCSK1 , of a monogenic cause of severe human obesity .
Clement, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392 , 398–401 (1998). This work confirms the observations from mice about leptin signalling to the brain .
Jackson, R. S. et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16 , 303–306 (1997). This study, together with mutations in the leptin gene, provides early evidence of a monogenic cause of severe human obesity, and also highlights the role of the nascent melanocortin pathway in body-weight regulation .
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88 , 131–141 (1997).
Yeo, G. S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20 , 111–112 (1998).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in MC4R is associated with a dominant form of obesity. Nat. Genet. 20 , 113–114 (1998). This paper, together with Yeo et al. (1998), shows that heterozygous mutations in MC4R result in severe human obesity, establishing the role of the central melanocortin pathway in regulating human appetitive behaviour .
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19 , 155–157 (1998). This paper provides direct evidence that melanocortin peptides play a key role in the regulation of energy homeostasis .
Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5 , 1066–1070 (1999).
Challis, B. G. et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc. Natl Acad. Sci. USA 101 , 4695–4700 (2004).
van der Klaauw, A. A. et al. Human semaphorin 3 variants link melanocortin circuit development and energy balance. Cell 176 , 729–742.e18 (2019).
Farooqi, S. & O’Rahilly, S. Genetics of obesity in humans. Endocr. Rev. 27 , 710–718 (2006).
Saeed, S., Arslan, M. & Froguel, P. Genetics of obesity in consanguineous populations: toward precision medicine and the discovery of novel obesity genes. Obesity 26 , 474–484 (2018).
Article PubMed Google Scholar
Saeed, S. et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity 23 , 1687–1695 (2015).
Saeed, S. et al. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes 69 , 1424–1438 (2020).
Kurokawa, N. et al. The ADRB3 Trp64Arg variant and BMI: a meta-analysis of 44 833 individuals. Int. J. Obes. 32 , 1240–1249 (2008).
Shugart, Y. Y. et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur. J. Hum. Genet. 17 , 1050–1055 (2009).
Benzinou, M. et al. Endocannabinoid receptor 1 gene variations increase risk for obesity and modulate body mass index in European populations. Hum. Mol. Genet. 17 , 1916–1921 (2008).
Wang, D. et al. Association of the MC4R V103I polymorphism with obesity: a Chinese case–control study and meta-analysis in 55,195 individuals. Obesity 18 , 573–579 (2010).
Nead, K. T. et al. Contribution of common non-synonymous variants in PCSK1 to body mass index variation and risk of obesity: a systematic review and meta-analysis with evidence from up to 331 175 individuals. Hum. Mol. Genet. 24 , 3582–3594 (2015).
Tonjes, A., Scholz, M., Loeffler, M. & Stumvoll, M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care 29 , 2489–2497 (2006).
Rankinen, T. et al. The human obesity gene map: the 2005 update. Obesity 14 , 529–644 (2006).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 , 889–894 (2007). This paper describes the first GWAS (for type 2 diabetes) to identify a locus ( FTO ) robustly associated with BMI .
Scuteri, A. et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 3 , e115 (2007).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47 , D1005–D1012 (2019).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700 000 individuals of European ancestry. Hum. Mol. Genet. 27 , 3641–3649 (2018).
Javed, A. et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr. Obes. 10 , 234–244 (2015).
Kilpelainen, T. O. et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 43 , 753–760 (2011).
Lu, Y. et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat. Commun. 7 , 10495 (2016).
Zillikens, M. C. et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat. Commun. 8 , 80 (2017).
Chu, A. Y. et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 49 , 125–130 (2017).
Kilpelainen, T. O. et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 7 , 10494 (2016).
Sun, Q. et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum. Mol. Genet. 19 , 1846–1855 (2010).
Riveros-McKay, F. et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15 , e1007603 (2019).
Orthofer, M. et al. Identification of ALK in thinness. Cell 181 , 1246–1262.e22 (2020).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28 , 3327–3338 (2019).
Felix, J. F. et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 25 , 389–403 (2016).
Vogelezang, S. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16 , e1008718 (2020).
Akiyama, M. et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 49 , 1458–1467 (2017).
Ng, M. C. Y. et al. Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium. PLoS Genet. 13 , e1006719 (2017).
Gurdasani, D. et al. Uganda genome resource enables insights into population history and genomic discovery in Africa. Cell 179 , 984–1002.e36 (2019).
Wojcik, G. L. et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 570 , 514–518 (2019).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51 , 584–591 (2019).
Grarup, N. et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet. 50 , 172–174 (2018).
Saeed, S. et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat. Genet. 50 , 175–179 (2018).
Minster, R. L. et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat. Genet. 48 , 1049–1054 (2016).
Andersen, M. K. et al. The derived allele of a novel intergenic variant at chromosome 11 associates with lower body mass index and a favorable metabolic phenotype in Greenlanders. PLoS Genet. 16 , e1008544 (2020).
Siljee, J. E. et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat. Genet. 50 , 180–185 (2018).
Willer, C. J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41 , 25–34 (2009).
Singh, K. et al. Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders. Sci. Rep. 9 , 5457 (2019).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42 , 937–948 (2010).
Soni, A., Amisten, S., Rorsman, P. & Salehi, A. GPRC5B a putative glutamate-receptor candidate is negative modulator of insulin secretion. Biochem. Biophys. Res. Commun. 441 , 643–648 (2013).
Jarick, I. et al. Novel common copy number variation for early onset extreme obesity on chromosome 11q11 identified by a genome-wide analysis. Hum. Mol. Genet. 20 , 840–852 (2011).
Sainsbury, A. et al. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol. Cell Biol. 23 , 5225–5233 (2003).
Falchi, M. et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat. Genet. 46 , 492–497 (2014).
Meisler, M. H. & Ting, C. N. The remarkable evolutionary history of the human amylase genes. Crit. Rev. Oral Biol. Med. 4 , 503–509 (1993).
Hendricks, A. E. et al. Rare variant analysis of human and rodent obesity genes in individuals with severe childhood obesity. Sci. Rep. 7 , 4394 (2017).
Turcot, V. et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50 , 26–41 (2018). This large-scale exome-wide discovery study reports the use of array data to identify rare coding variations associated with BMI .
Emdin, C. A. et al. Analysis of predicted loss-of-function variants in UK Biobank identifies variants protective for disease. Nat. Commun. 9 , 1613 (2018).
Akbari, P. et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 373 , eabf8683 (2021). This large-scale exome-wide discovery study reports the use of WES data to identify mutations associated with BMI .
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132 , 2131–2157 (2007).
Antolin-Fontes, B. et al. The habenular G-protein-coupled receptor 151 regulates synaptic plasticity and nicotine intake. Proc. Natl Acad. Sci. USA 117 , 5502–5509 (2020).
Winkler, T. W. et al. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 11 , e1005378 (2015).
Graff, M. et al. Genome-wide physical activity interactions in adiposity — a meta-analysis of 200,452 adults. PLoS Genet. 13 , e1006528 (2017).
Smith, C. E. et al. Genome-wide interactions with dairy intake for body mass index in adults of European descent. Mol. Nutr. Food Res. 62 , 1700347 (2018).
Justice, A. E. et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat. Commun. 8 , 14977 (2017).
Qi, Q. et al. Fried food consumption, genetic risk, and body mass index: gene–diet interaction analysis in three US cohort studies. BMJ 348 , g1610 (2014).
Kilpelainen, T. O. et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 8 , e1001116 (2011).
Yeo, G. S. H. Genetics of obesity: can an old dog teach us new tricks? Diabetologia 60 , 778–783 (2017).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015). This large-scale GWAS for BMI shows that BMI-associated loci frequently localize in or near genes that act in the brain .
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395 , 763–770 (1998).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382 , 250–252 (1996).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24 , 155–163 (1999).
Bertagna, X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. North Am. 23 , 467–485 (1994).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278 , 135–138 (1997).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141 , 3518–3521 (2000).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 26 , 97–102 (2000).
Doche, M. E. et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 122 , 4732–4736 (2012).
Marenne, G. et al. Exome sequencing identifies genes and gene sets contributing to severe childhood obesity, linking PHIP variants to repressed POMC transcription. Cell Metab. 31 , 1107–1119.e12 (2020).
Asai, M. et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341 , 275–278 (2013).
Michaud, J. L. et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 10 , 1465–1473 (2001).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348 , 1085–1095 (2003).
Wade, K. H. et al. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort. Nat. Med. 27 , 1088–1096 (2021). This paper establishes the frequency of loss-of-function mutations in MC4R to be 0.3%, much more common than previously appreciated .
van der Klaauw, A. et al. Role of melanocortin signalling in the preference for dietary macronutrients in human beings. Lancet 385 , S12 (2015).
Raffan, E. et al. A deletion in the canine POMC gene is associated with weight and appetite in obesity-prone Labrador retriever dogs. Cell Metab. 23 , 893–900 (2016).
Kim, K. S., Larsen, N., Short, T., Plastow, G. & Rothschild, M. F. A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mamm. Genome 11 , 131–135 (2000).
Aspiras, A. C., Rohner, N., Martineau, B., Borowsky, R. L. & Tabin, C. J. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proc. Natl Acad. Sci. USA 112 , 9668–9673 (2015).
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304 , 110–115 (2004).
Davies, A. M. The role of neurotrophins in the developing nervous system. J. Neurobiol. 25 , 1334–1348 (1994).
McAllister, A. K., Katz, L. C. & Lo, D. C. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18 , 767–778 (1997).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92 , 8856–8860 (1995).
Kernie, S. G., Liebl, D. J. & Parada, L. F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 19 , 1290–1300 (2000).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6 , 736–742 (2003).
Xu, B. & Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 17 , 282–292 (2016).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15 , 1748–1757 (2001).
Gray, J. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55 , 3366–3371 (2006).
Yeo, G. S. et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 7 , 1187–1189 (2004).
Thorleifsson, G. et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 41 , 18–24 (2009).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50 , 621–629 (2018).
Ndiaye, F. K. et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int. J. Obes. 44 , 539–543 (2020).
Loos, R. J. & Yeo, G. S. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10 , 51–61 (2014).
Church, C. et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 42 , 1086–1092 (2010).
Fischer, J. et al. Inactivation of the Fto gene protects from obesity. Nature 458 , 894–898 (2009).
Cheung, M. K., Gulati, P., O’Rahilly, S. & Yeo, G. S. FTO expression is regulated by availability of essential amino acids. Int. J. Obes. 37 , 744–747 (2013).
Gulati, P. et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc. Natl Acad. Sci. USA 110 , 2557–2562 (2013).
Karra, E. et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Invest. 123 , 3539–3551 (2013).
Hess, M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16 , 1042–1048 (2013).
Ruud, J. et al. The fat mass and obesity-associated protein (FTO) regulates locomotor responses to novelty via D2R medium spiny neurons. Cell Rep. 27 , 3182–3198.e9 (2019).
Stratigopoulos, G., LeDuc, C. A., Cremona, M. L., Chung, W. K. & Leibel, R. L. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J. Biol. Chem. 286 , 2155–2170 (2011).
Stratigopoulos, G. et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 19 , 767–779 (2014).
Stratigopoulos, G. et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 126 , 1897–1910 (2016).
Smemo, S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507 , 371–375 (2014).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373 , 895–907 (2015).
O’Rahilly, S., Coll, A. P. & Yeo, G. S. FTO obesity variant and adipocyte browning in humans. N. Engl. J. Med. 374 , 191 (2016).
PubMed Google Scholar
Sobreira, D. R. et al. Extensive pleiotropism and allelic heterogeneity mediate metabolic effects of IRX3 and IRX5. Science 372 , 1085–1091 (2021).
Almen, M. S. et al. The obesity gene, TMEM18, is of ancient origin, found in majority of neuronal cells in all major brain regions and associated with obesity in severely obese children. BMC Med. Genet. 11 , 58 (2010).
Larder, R. et al. Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation. Proc. Natl Acad. Sci. USA 114 , 9421–9426 (2017). Aside from the efforts at characterizing the FTO locus, this paper establishes a plausible role for yet another GWAS-identified candidate gene, TMEM18 , in energy homeostasis .
Wiemerslage, L. et al. The Drosophila ortholog of TMEM18 regulates insulin and glucagon-like signaling. J. Endocrinol. 229 , 233–243 (2016).
Biederer, T. et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297 , 1525–1531 (2002).
Yan, X. et al. Cadm2 regulates body weight and energy homeostasis in mice. Mol. Metab. 8 , 180–188 (2018).
Rathjen, T. et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat. Neurosci. 20 , 1096–1103 (2017).
Wheeler, E. et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat. Genet. 45 , 513–517 (2013).
Schafer, M., Brauer, A. U., Savaskan, N. E., Rathjen, F. G. & Brummendorf, T. Neurotractin/kilon promotes neurite outgrowth and is expressed on reactive astrocytes after entorhinal cortex lesion. Mol. Cell. Neurosci. 29 , 580–590 (2005).
Matikainen-Ankney, B. A. & Kravitz, A. V. Persistent effects of obesity: a neuroplasticity hypothesis. Ann. N. Y. Acad. Sci. 1428 , 221–239 (2018).
Lee, A. W. et al. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE 7 , e41537 (2012).
Joo, Y., Kim, H., Lee, S. & Lee, S. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int. J. Obes. 43 , 1769–1782 (2019).
Boender, A. J., van Gestel, M. A., Garner, K. M., Luijendijk, M. C. & Adan, R. A. The obesity-associated gene Negr1 regulates aspects of energy balance in rat hypothalamic areas. Physiol. Rep. 2 , e12083 (2014).
Singh, K. et al. Neuronal growth and behavioral alterations in mice deficient for the psychiatric disease-associated negr1 gene. Front. Mol. Neurosci. 11 , 30 (2018).
Szczurkowska, J. et al. NEGR1 and FGFR2 cooperatively regulate cortical development and core behaviours related to autism disorders in mice. Brain 141 , 2772–2794 (2018).
PubMed PubMed Central Google Scholar
Noh, K. et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry 24 , 1189–1205 (2019).
Cano-Gamez, E. & Trynka, G. From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 11 , 424 (2020).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40 , 768–775 (2008).
Chambers, J. C. et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat. Genet. 40 , 716–718 (2008).
Yaghootkar, H. et al. Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity. Diabetes 69 , 2806–2818 (2020).
Kichaev, G. et al. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104 , 65–75 (2019).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28 , 166–174 (2019).
Wang, Y. et al. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J. Clin. Invest. 131 , e142064 (2021).
Article CAS PubMed Central Google Scholar
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341 , 879–884 (1999). This paper is the first description of the treatment of leptin deficiency using leptin replacement therapy .
Farooqi, I. S. & O’Rahilly, S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 223 , T63–T70 (2014).
Yeo, G. S. H. et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol. Metab. 48 , 101206 (2021).
Kuhnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375 , 240–246 (2016). This paper is the first to demonstrate that the melanocortin pathway is a relevant and, so far, safe therapeutic target for the treatment of at least rare genetic causes of obesity .
Clement, K. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24 , 551–555 (2018).
Clement, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8 , 960–970 (2020).
Ayers, K. L. et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J. Clin. Endocrinol. Metab. 103 , 2601–2612 (2018).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282 , 1568–1575 (1999).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab. 6 , 1321–1329 (2017).
Khera, A. V. et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177 , 587–596.e9 (2019). This study describes a comprehensive PGS and its association with BMI throughout the life course .
Loos, R. J. F. 15 years of genome-wide association studies and no signs of slowing down. Nat. Commun. 11 , 5900 (2020).
Pearce, L. R. et al. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell 155 , 765–777 (2013).
Greenfield, J. R. et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 360 , 44–52 (2009).
Torkamani, A. & Topol, E. Polygenic risk scores expand to obesity. Cell 177 , 518–520 (2019).
Download references
Acknowledgements
R.J.F.L. is supported by funding from Novo Nordisk Foundation (NNF Laureate Award) and the US National Institutes of Health (R01DK110113; R01DK107786; R01HL142302; R01 DK124097). G.S.H.Y. is supported by the Medical Research Council (MRC Metabolic Diseases Unit (MC_UU_00014/1)). The authors thank M. Guindo Martinez for her help with creating data for Fig. 3 and Supplementary Tables 1 and 2.
Author information
Authors and affiliations.
Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark
Ruth J. F. Loos
Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Mindich Child Health and Development Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA
MRC Metabolic Diseases Unit, University of Cambridge Metabolic Research Laboratories, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK
Giles S. H. Yeo
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Correspondence to Ruth J. F. Loos or Giles S. H. Yeo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Genetics thanks D. Meyre, T. Fall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
23andMe: https://research.23andme.com/collaborate/
All of Us: https://allofus.nih.gov/
Biobank Japan: http://jenger.riken.jp/en/
Genetic Investigation for ANthropometric Traits (GIANT) consortium: https://portals.broadinstitute.org/collaboration/giant/index.php/Main_Page
Million Veterans Project: https://www.research.va.gov/mvp/
NCD Risk Factor Collaboration (NCD RisC): https://www.ncdrisc.org/data-visualisations.html
UK Biobank: https://www.ukbiobank.ac.uk/
Supplementary information
Supplementary information.
An environment that promotes weight gain.
A severe, early-onset form of obesity, caused by a single-gene mutation, with little or no influence of the environment.
A common multifactorial form of obesity, resulting from an interaction between the obesogenic environment and hundreds of genetic variants.
An approach used to understand the function of a gene by analysing the consequences of genetically manipulating specific sequences within the gene.
A hypothesis-driven approach to study the effect of a given gene (chosen based on the current understanding of its biology and pathophysiology) on susceptibility to the phenotype under study.
A method that relies on the relatedness of study participants to test whether certain chromosomal regions co-segregate with a disease or trait across generations.
(GWAS). A hypothesis-generating approach that screens whole genomes for associations between genetic variants and a phenotype of interest at much higher resolution than is possible for genome-wide linkage studies, and is thus better able to narrow down the associated locus.
(PGS). A measure used to assess an individual’s genetic susceptibility to disease, calculated by summing the number of disease-increasing alleles, weighted by each variant’s effect size observed in a genome-wide association study.
(AUC ROC ). A metric used to assess the ability of a predictor to discriminate between individuals with and without a disease. The AUC ranges from 0.50 (equal to tossing a coin) to 1.0 (perfect prediction).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Loos, R.J.F., Yeo, G.S.H. The genetics of obesity: from discovery to biology. Nat Rev Genet 23 , 120–133 (2022). https://doi.org/10.1038/s41576-021-00414-z
Download citation
Accepted : 17 August 2021
Published : 23 September 2021
Issue Date : February 2022
DOI : https://doi.org/10.1038/s41576-021-00414-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genetic effects of sequence-conserved enhancer-like elements on human complex traits.
- Wing Hung Wong
Genome Biology (2024)
Hyperphagia and impulsivity: use of self-administered Dykens’ and in-house impulsivity questionnaires to characterize eating behaviors in children with severe and early-onset obesity
- Lara Arnouk
- Hélène Chantereau
- Béatrice Dubern
Orphanet Journal of Rare Diseases (2024)
Associations between maternal pre-pregnancy BMI and infant striatal mean diffusivity
- Aylin Rosberg
- Harri Merisaari
- Jetro J. Tuulari
BMC Medicine (2024)
Identifying latent genetic interactions in genome-wide association studies using multiple traits
- Andrew J. Bass
- Shijia Bian
- Michael P. Epstein
Genome Medicine (2024)
A genomics perspective of personalized prevention and management of obesity
- Kalliopi K. Gkouskou
- Maria G. Grammatikopoulou
- Aristides G. Eliopoulos
Human Genomics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
The Nature vs. Nurture Debate
Genetic and Environmental Influences and How They Interact
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Verywell / Joshua Seong
- Definitions
- Interaction
- Contemporary Views
Nature refers to how genetics influence an individual's personality, whereas nurture refers to how their environment (including relationships and experiences) impacts their development. Whether nature or nurture plays a bigger role in personality and development is one of the oldest philosophical debates within the field of psychology .
Learn how each is defined, along with why the issue of nature vs. nurture continues to arise. We also share a few examples of when arguments on this topic typically occur, how the two factors interact with each other, and contemporary views that exist in the debate of nature vs. nurture as it stands today.
Nature and Nurture Defined
To better understand the nature vs. nurture argument, it helps to know what each of these terms means.
- Nature refers largely to our genetics . It includes the genes we are born with and other hereditary factors that can impact how our personality is formed and influence the way that we develop from childhood through adulthood.
- Nurture encompasses the environmental factors that impact who we are. This includes our early childhood experiences, the way we were raised , our social relationships, and the surrounding culture.
A few biologically determined characteristics include genetic diseases, eye color, hair color, and skin color. Other characteristics are tied to environmental influences, such as how a person behaves, which can be influenced by parenting styles and learned experiences.
For example, one child might learn through observation and reinforcement to say please and thank you. Another child might learn to behave aggressively by observing older children engage in violent behavior on the playground.
The Debate of Nature vs. Nurture
The nature vs. nurture debate centers on the contributions of genetics and environmental factors to human development. Some philosophers, such as Plato and Descartes, suggested that certain factors are inborn or occur naturally regardless of environmental influences.
Advocates of this point of view believe that all of our characteristics and behaviors are the result of evolution. They contend that genetic traits are handed down from parents to their children and influence the individual differences that make each person unique.
Other well-known thinkers, such as John Locke, believed in what is known as tabula rasa which suggests that the mind begins as a blank slate . According to this notion, everything that we are is determined by our experiences.
Behaviorism is a good example of a theory rooted in this belief as behaviorists feel that all actions and behaviors are the results of conditioning. Theorists such as John B. Watson believed that people could be trained to do and become anything, regardless of their genetic background.
People with extreme views are called nativists and empiricists. Nativists take the position that all or most behaviors and characteristics are the result of inheritance. Empiricists take the position that all or most behaviors and characteristics result from learning.
Examples of Nature vs. Nurture
One example of when the argument of nature vs. nurture arises is when a person achieves a high level of academic success . Did they do so because they are genetically predisposed to elevated levels of intelligence, or is their success a result of an enriched environment?
The argument of nature vs. nurture can also be made when it comes to why a person behaves in a certain way. If a man abuses his wife and kids, for instance, is it because he was born with violent tendencies, or is violence something he learned by observing others in his life when growing up?
Nature vs. Nurture in Psychology
Throughout the history of psychology , the debate of nature vs. nurture has continued to stir up controversy. Eugenics, for example, was a movement heavily influenced by the nativist approach.
Psychologist Francis Galton coined the terms 'nature versus nurture' and 'eugenics' and believed that intelligence resulted from genetics. Galton also felt that intelligent individuals should be encouraged to marry and have many children, while less intelligent individuals should be discouraged from reproducing.
The value placed on nature vs. nurture can even vary between the different branches of psychology , with some branches taking a more one-sided approach. In biopsychology , for example, researchers conduct studies exploring how neurotransmitters influence behavior, emphasizing the role of nature.
In social psychology , on the other hand, researchers might conduct studies looking at how external factors such as peer pressure and social media influence behaviors, stressing the importance of nurture. Behaviorism is another branch that focuses on the impact of the environment on behavior.
Nature vs. Nurture in Child Development
Some psychological theories of child development place more emphasis on nature and others focus more on nurture. An example of a nativist theory involving child development is Chomsky's concept of a language acquisition device (LAD). According to this theory, all children are born with an instinctive mental capacity that allows them to both learn and produce language.
An example of an empiricist child development theory is Albert Bandura's social learning theory . This theory says that people learn by observing the behavior of others. In his famous Bobo doll experiment , Bandura demonstrated that children could learn aggressive behaviors simply by observing another person acting aggressively.
Nature vs. Nurture in Personality Development
There is also some argument as to whether nature or nurture plays a bigger role in the development of one's personality. The answer to this question varies depending on which personality development theory you use.
According to behavioral theories, our personality is a result of the interactions we have with our environment, while biological theories suggest that personality is largely inherited. Then there are psychodynamic theories of personality that emphasize the impact of both.
Nature vs. Nurture in Mental Illness Development
One could argue that either nature or nurture contributes to mental health development. Some causes of mental illness fall on the nature side of the debate, including changes to or imbalances with chemicals in the brain. Genetics can also contribute to mental illness development, increasing one's risk of a certain disorder or disease.
Mental disorders with some type of genetic component include autism , attention-deficit hyperactivity disorder (ADHD), bipolar disorder , major depression , and schizophrenia .
Other explanations for mental illness are environmental. This includes being exposed to environmental toxins, such as drugs or alcohol, while still in utero. Certain life experiences can also influence mental illness development, such as witnessing a traumatic event, leading to the development of post-traumatic stress disorder (PTSD).
Nature vs. Nurture in Mental Health Therapy
Different types of mental health treatment can also rely more heavily on either nature or nurture in their treatment approach. One of the goals of many types of therapy is to uncover any life experiences that may have contributed to mental illness development (nurture).
However, genetics (nature) can play a role in treatment as well. For instance, research indicates that a person's genetic makeup can impact how their body responds to antidepressants. Taking this into consideration is important for getting that person the help they need.
Interaction Between Nature and Nurture
Which is stronger: nature or nurture? Many researchers consider the interaction between heredity and environment—nature with nurture as opposed to nature versus nurture—to be the most important influencing factor of all.
For example, perfect pitch is the ability to detect the pitch of a musical tone without any reference. Researchers have found that this ability tends to run in families and might be tied to a single gene. However, they've also discovered that possessing the gene is not enough as musical training during early childhood is needed for this inherited ability to manifest itself.
Height is another example of a trait influenced by an interaction between nature and nurture. A child might inherit the genes for height. However, if they grow up in a deprived environment where proper nourishment isn't received, they might never attain the height they could have had if they'd grown up in a healthier environment.
A newer field of study that aims to learn more about the interaction between genes and environment is epigenetics . Epigenetics seeks to explain how environment can impact the way in which genes are expressed.
Some characteristics are biologically determined, such as eye color, hair color, and skin color. Other things, like life expectancy and height, have a strong biological component but are also influenced by environmental factors and lifestyle.
Contemporary Views of Nature vs. Nurture
Most experts recognize that neither nature nor nurture is stronger than the other. Instead, both factors play a critical role in who we are and who we become. Not only that but nature and nurture interact with each other in important ways all throughout our lifespan.
As a result, many in this field are interested in seeing how genes modulate environmental influences and vice versa. At the same time, this debate of nature vs. nurture still rages on in some areas, such as in the origins of homosexuality and influences on intelligence .
While a few people take the extreme nativist or radical empiricist approach, the reality is that there is not a simple way to disentangle the multitude of forces that exist in personality and human development. Instead, these influences include genetic factors, environmental factors, and how each intermingles with the other.
Schoneberger T. Three myths from the language acquisition literature . Anal Verbal Behav . 2010;26(1):107-31. doi:10.1007/bf03393086
National Institutes of Health. Common genetic factors found in 5 mental disorders .
Pain O, Hodgson K, Trubetskoy V, et al. Identifying the common genetic basis of antidepressant response . Biol Psychiatry Global Open Sci . 2022;2(2):115-126. doi:10.1016/j.bpsgos.2021.07.008
Moulton C. Perfect pitch reconsidered . Clin Med J . 2014;14(5):517-9 doi:10.7861/clinmedicine.14-5-517
Levitt M. Perceptions of nature, nurture and behaviour . Life Sci Soc Policy . 2013;9:13. doi:10.1186/2195-7819-9-13
Bandura A, Ross D, Ross, SA. Transmission of aggression through the imitation of aggressive models . J Abnorm Soc Psychol. 1961;63(3):575-582. doi:10.1037/h0045925
Chomsky N. Aspects of the Theory of Syntax .
Galton F. Inquiries into Human Faculty and Its Development .
Watson JB. Behaviorism .
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
Nature vs. Nurture Essay
Nature is the influence of genetics or hereditary factors in determining the individual’s behavior. In other words, it is how natural factors shape the behavior or personality of an individual. In most cases, nature determines the physical characteristics which in effect influence the behavior of an individual. Physical characteristics such as physical appearance, type of voice and sex which are determined by hereditary factors influences the way people behave.
Nurture on the other is the upbringing of an individual according to the environmental conditions. That is, the way individuals are socialized. Basically, nurture is the influence of environmental factors on an individual’s behavior.
According to this paradigm, an individual’s behavior can be conditioned depending on the way one would like it to be. Often, individuals’ behaviors are conditioned by the socio-cultural environmental factors. It is because of socio-cultural environmental conditions that the differences in the behavior of individuals occur.
Nature determines individual traits that are hereditary. In other words, human characteristics are determined by genetic predispositions which are largely natural. Hereditary traits are normally being passed from the parents to the offspring. They include characteristics that determine sex and physical make up. According to natural behaviorists, it is the genes that will determine the physical trait an individual will have. These are encoded on the individuals DNA.
Therefore, behavioral traits such as sexual orientation, aggression, personality and intelligence are also encoded in the DNA. However, scientists believe that these characteristics are evolutionary. That is, they change over time depending on the physical environment adaptability. Evolutionary scientists argue that changes in genes are as a result of mutations which are caused by environmental factors. Thus, natural environment determines individual characteristics which are genetically encoded in the DNA.
Conversely, individuals possess traits that are not naturally determined. These are characteristics that are learnt rather than being born with. These are traits which largely determined by the socio-cultural environmental factors or the way the individuals are socialized within the society depending on the societal values.
These traits are learnt as an individual develops and can easily be changed by the socio-cultural environment where the individual is currently staying. These characteristics include temperament, ability to master a language and sense of humor. Behavioral theorists believe that these traits can be conditioned and altered much like the way animal behavior can be conditioned.
From the discussion it can be deduced that individuals’ traits are determined by hereditary genes and at the same time can be natured. There are those traits that cannot be changed in an individual no matter what condition the person is exposed to. These traits are inborn and embed within the individual hereditary factors.
In most cases, they constitute the physical characteristics of an individual. They also determine the physical behaviors such as walking style, physical appearance and eating habits. At the same time there are learned characteristics which are normally being conditioned by the socio-cultural values. Individuals learn these traits from the way they are socialized within the immediate social or cultural environment. In other words, such behaviors are conditioned by the cultural values encouraged by the immediate society.
In conclusion, nature vs. nurture debate still remains controversial. However, all agree that nature and nurture play a crucial role in determining an individual’s behavior. Nature is associated with heredity roles in determining the individuals characteristics where as nurture is associated with the role of socio-cultural environment in determining the individuals behavior.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 28). Nature vs. Nurture. https://ivypanda.com/essays/nature-vs-nurture/
"Nature vs. Nurture." IvyPanda , 28 Feb. 2024, ivypanda.com/essays/nature-vs-nurture/.
IvyPanda . (2024) 'Nature vs. Nurture'. 28 February.
IvyPanda . 2024. "Nature vs. Nurture." February 28, 2024. https://ivypanda.com/essays/nature-vs-nurture/.
1. IvyPanda . "Nature vs. Nurture." February 28, 2024. https://ivypanda.com/essays/nature-vs-nurture/.
Bibliography
IvyPanda . "Nature vs. Nurture." February 28, 2024. https://ivypanda.com/essays/nature-vs-nurture/.
- The Nature-Nurture Controversy
- Language Acquisition: Nature vs. Nurture
- The Difficult Issue of Nature vs. Nurture
- Practical aspects of the field of speech and language development
- Conformity, Groupthink, and Bystander Apathy
- Students Drinking Behavior at HBCU'S
- Seduction and Flirtation Devices
- Motivation Theories in Business Environment

IMAGES
VIDEO
COMMENTS
The interaction between parental obesity and age of the child in the study by Treuth et al does not rule out the combined effect of nature and nurture. Whether it is an expression of nature or the end result of nurture, the causal web of obesity is complex, and we may never be able to uncover the intricate pattern of interlinking threads.
This review summarises evidence on the extent to which genes and the environment influence energy intake and energy expenditure, and as a result, contribute to the ongoing global obesity epidemic. The concept of genetic susceptibility to the environment driving human variation in body weight is discussed. Keywords: Epidemiology, public health ...
Almost 20 years ago, the World Health Organization (WHO) declared the problem of rising levels of obesity a 'global epidemic', 1 yet the prevalence of overweight (body mass index (BMI; a ratio of weight to height commonly used to categorise weight status) ⩾ 25 kg/m 2) and obesity (BMI ⩾ 30 kg/m 2) has continued to rise. 2,3 In 2016, more than 1.9 billion adults (39% of the world's ...
Nature versus nurture is a phrase with which many of us are familiar. Our genes are responsible for many of our traits, but nurture or environmental influences surely have a part to play. When it ...
A number of reviews and meta-analyses agree that children who sleep less have an increased risk for obesity between 56% and 89% 152, 153. Increased BMI in children is associated with reduced sleep duration, which is most likely due to an increase in adipose tissue deposits 154.
Nature. One should be aware of the fact that genetic factors and inherited predispositions can be major contributors to childhood obesity. A study suggests that at least 21% of BMI variations are due to the genetic makeup of a person, including a child (Locke et al., 2015). In other words, one cannot dismiss the nature element because a wide ...
mothers' average BMI over the 30-year period we examine can explain at most 40 percent of the. 7.3 percent increase in children's average BMI. The title of this paper asks whether in determining children's obesity, "nurture" is. trumping "nature" and whether that is different for disadvantaged children.
an interaction between nature and nurture causes this condition. Evidence from family, twin and adoption studies suggests that inheritance has an impact in genetic susceptibility and, although exact mechanisms have not been found to explain common obesity, genome-wide scans have had promising results.
DOI 10.3386/w13479. Issue Date October 2007. Obesity has been one of the fastest growing health concerns among children, particularly among disadvantaged children. For children overall, obesity rates have tripled from 5% in the early 1970s to about 15% by the early 2000s. For disadvantaged children, obesity rates are closer to 20%.
Obesity, particularly in children, is a major health concern in many developed economies, where it presents a costly risk to health services. Any policy response must take into account the inter-generational transmission of overweightness and obesity to children. This column uses evidence from the Health Survey of England to assess the extent to which nature and nurture factors play a role in ...
3.1 Risk Factors for Obesity Ascribable to "Nurture". Aside from the obvious changes in the caloric and exercise milieu in which we find ourselves, numerous other processes associated with increased weight gain have been proffered as examples of environmental change.
Nurture is more important than nature in childhood obesity, study finds. A study comparing the weight of biological and adopted children to that of their parents has found that lifestyles, rather than genes, are primarily responsible for the children being overweight. 1. The researchers, from the Centre for Economic Performance at the London ...
Synergy of nature and nurture in childhood obesity. BE Levin. For example, the increased obesity in offspring of DIO dams that are kept obese during gestation and lactation is associated with ...
It is now quite clear that we are in the midst of an epidemic of childhood obesity in the developed world. 1 Many explanations for this epidemic have been put forward, but the link between ...
As researchers develop a deeper understanding of obesity, epigenetics may ultimately resolve the nature vs. nurture debate. By reconciling these two perspectives, epigenetics may offer new solutions for environmental changes which may decrease the prevalence of obesity in the population. Health and Social Costs
On the Nature vs. Nurture of Obesity. Highlights from the ongoing debate over the factors underlying the epidemic on pace to see 42% of Americans obese within the next 20 years. joe_13/Flickr. In ...
Nature-Genetics and Biological Factors. Child obesity is caused by changes in certain chromosomal genes in the body. The Genome-wide association studies organization (GWAS) has discovered that a mutation of the LEP gene within the hypothalamus aids in the loss of appetite control and has a dominant effect on gaining excessive adiposity especially in children (Chesi et al, 2015).
1510 Words. 7 Pages. Open Document. Ian Duffy Nature v. Nurture in Childhood Adiposity The nature versus nurture debate is one of the most longstanding arguments in the history of psychology and it aims to determine what has greater influence on personal development; one's genes and inherited qualities compared to one's environment.
Nurture- Physical Inactivity, Sociodemographic Features, and Diet. On the other hand, nurture seems to play a much larger role in childhood obesity. As previously mentioned, a child whose parents are obese have an astronomical increased risk of becoming obese (80%), which may reflect environmental influences their parents play on their child ...
Crucially, there is a strong genetic component underlying the large interindividual variation in body weight that determines people's response to this 'obesogenic' environment. Twin, family ...
The Nature vs. Nurture Debate. Nature refers to how genetics influence an individual's personality, whereas nurture refers to how their environment (including relationships and experiences) impacts their development. Whether nature or nurture plays a bigger role in personality and development is one of the oldest philosophical debates within ...
Nature vs. Nurture Essay. Nature is the influence of genetics or hereditary factors in determining the individual's behavior. In other words, it is how natural factors shape the behavior or personality of an individual. In most cases, nature determines the physical characteristics which in effect influence the behavior of an individual.