An overview of drug discovery and development
Affiliation.
- 1 Department of biomedical Science, Nazarbayev University School of Medicine, Nur-Sultan 010000, Kazakhstan.
- PMID: 32270704
- DOI: 10.4155/fmc-2019-0307
A new medicine will take an average of 10-15 years and more than US$2 billion before it can reach the pharmacy shelf. Traditionally, drug discovery relied on natural products as the main source of new drug entities, but was later shifted toward high-throughput synthesis and combinatorial chemistry-based development. New technologies such as ultra-high-throughput drug screening and artificial intelligence are being heavily employed to reduce the cost and the time of early drug discovery, but they remain relatively unchanged. However, are there other potentially faster and cheaper means of drug discovery? Is drug repurposing a viable alternative? In this review, we discuss the different means of drug discovery including their advantages and disadvantages.
Keywords: drug repurposing; high throughput; natural sources; small molecule.

Publication types
- Artificial Intelligence
- Drug Development*
- Drug Evaluation, Preclinical
- Share full article

Chinese Company Under Congressional Scrutiny Makes Key U.S. Drugs
Lawmakers raising national security concerns and seeking to disconnect a major Chinese firm from U.S. pharmaceutical interests have rattled the biotech industry. The firm is deeply involved in development and manufacturing of crucial therapies for cancer, cystic fibrosis, H.I.V. and other illnesses.
A WuXi Biologics facility in Wuxi, China. WuXi AppTec and an affiliated company, WuXi Biologics, have received millions of dollars in tax incentives to build sprawling research and manufacturing sites in Massachusetts and Delaware. Credit... Imaginechina Limited, via Alamy
Supported by

By Christina Jewett
- April 15, 2024
A Chinese company targeted by members of Congress over potential ties to the Chinese government makes blockbuster drugs for the American market that have been hailed as advances in the treatment of cancers, obesity and debilitating illnesses like cystic fibrosis.
WuXi AppTec is one of several companies that lawmakers have identified as potential threats to the security of individual Americans’ genetic information and U.S. intellectual property. A Senate committee approved a bill in March that aides say is intended to push U.S. companies away from doing business with them.
But lawmakers discussing the bill in the Senate and the House have said almost nothing in hearings about the vast scope of work that WuXi does for the U.S. biotech and pharmaceutical industries — and patients. A New York Times review of hundreds of pages of records worldwide shows that WuXi is heavily embedded in the U.S. medicine chest, making some or all of the main ingredients for multibillion-dollar therapies that are highly sought to treat cancers like some types of leukemia and lymphoma as well as obesity and H.I.V.
The Congressional spotlight on the company has rattled the pharmaceutical industry, which is already struggling with widespread drug shortages now at a 20-year high . Some biotech executives have pushed back, trying to impress on Congress that a sudden decoupling could take some drugs out of the pipeline for years.
WuXi AppTec and an affiliated company, WuXi Biologics grew rapidly, offering services to major U.S. drugmakers that were seeking to shed costs and had shifted most manufacturing overseas in the last several decades.
WuXi companies developed a reputation for low-cost and reliable work by thousands of chemists who could create new molecules and operate complex equipment to make them in bulk. By one estimate, WuXi has been involved in developing one-fourth of the drugs used in the United States. WuXi AppTec reported earning about $3.6 billion in revenue for its U.S. work.
“They have become a one-stop shop to a biotech,” said Kevin Lustig, founder of Scientist.com, a clearinghouse that matches drug companies seeking research help with contractors like WuXi.
WuXi AppTec and WuXi Biologics have also received millions of dollars in tax incentives to build sprawling research and manufacturing sites in Massachusetts and Delaware that local government officials have welcomed as job and revenue generators. One WuXi site in Philadelphia was working alongside a U.S. biotech firm to give patients a cutting-edge therapy that would turbocharge their immune cells to treat advanced skin cancers.
The tension has grown since February, when four lawmakers asked the Commerce, Defense and Treasury Departments to investigate WuXi AppTec and affiliated companies, calling WuXi a “giant that threatens U.S. intellectual property and national security.”
A House bill called the Biosecure Act linked the company to the People’s Liberation Army, the military arm of the Chinese Communist Party. The bill claims WuXi AppTec sponsored military-civil events and received military-civil fusion funding.
Richard Connell, the chief operating officer of WuXi AppTec in the United States and Europe, said the company participates in community events, which do not “imply any association with or endorsement of a government institution, political party or policy such as military-civil fusion.” He also said shareholders do not have control over the company or access to nonpublic information.

Last month, after a classified briefing with intelligence staff, the Senate homeland security committee advanced a bill by a vote of 11 to 1: It would bar companies from receiving government contracts for work with Wuxi, but would allow the companies to still obtain contracts for unrelated projects. Government contracts with drugmakers are generally limited, though they were worth billions of dollars in revenue to companies that responded to the Covid-19 pandemic.
Mr. Connell defended the company’s record, saying the proposed legislation “relies on misleading allegations and inaccurate assertions against our company.”
WuXi operates in a highly regulated environment by “multiple U.S. federal agencies — none of which has placed our company on any sanctions list or designated it as posing a national security risk,” Mr. Connell said. WuXi Biologics did not respond to requests for comment.
Smaller biotech companies, which tend to rely on government grants and have fewer reserves, are among the most alarmed. Dr. Jonathan Kil, the chief executive of Seattle-based Sound Pharmaceuticals, said WuXi has worked alongside the company for 16 years to develop a treatment for hearing loss and tinnitus, or ringing in the ear. Finding another contractor to make the drug could set the company back two years, he said.
“What I don’t want to see is that we get very anti-Chinese to the point where we’re not thinking correctly,” Dr. Kil said.
It is unclear whether a bill targeting WuXi will advance at all this year. The Senate version has been amended to protect existing contracts and limit supply disruptions. Still, the scrutiny has prompted some drug and biotechnology companies to begin making backup plans.
Peter Kolchinsky, managing partner of RA Capital Management, estimated that half of the 200 biotech companies in his firm’s investment portfolio work with WuXi.
“Everyone is likely considering moving away from Wuxi and China more broadly,” he said in an email. “Even though the current versions of the bill don’t create that imperative clearly, no one wants to be caught flat-footed in China if the pullback from China accelerates.”
The chill toward China extends beyond drugmakers. U.S. companies are receiving billions of dollars in funding under the CHIPS Act, a federal law aimed at bringing semiconductor manufacturing stateside.
For the last several years, U.S. intelligence agencies have been warning about Chinese biotech companies in general and WuXi in particular. The National Counterintelligence and Security Center, the arm of the intelligence community charged with warning companies about national security issues, raised alarms about WuXi’s acquisition of NextCODE, an American genomic data company.
Though WuXi later spun off that company, a U.S. official said the government remains skeptical of WuXi’s corporate structure, noting that some independent entities have overlapping management and that there were other signs of the Chinese government’s continuing control or influence over WuXi.
Aides from the Senate homeland security committee said their core concerns are about the misuse of Americans’ genomic data, an issue that’s been more closely tied to other companies named in the bill.
Aides said the effort to discourage companies from working with WuXi and others was influenced by the U.S. government’s experience with Huawei, a Chinese telecommunications giant. By the time Congress acted on concerns about Huawei’s access to Americans’ private information, taxpayers had to pay billions of dollars to tear Huawei’s telecommunication equipment out of the ground.
Yet WuXi has far deeper involvement in American health care than has been discussed in Congress. Supply chain analytics firms QYOBO and Pharm3r, and some public records, show that WuXi and its affiliates have made the active ingredients for critical drugs.
They include Imbruvica, a leukemia treatment sold by Janssen Biotech and AbbVie that brought in $5.9 billion in worldwide revenue in 2023. WuXi subsidiary factories in Shanghai and Changzhou were listed in government records as makers of the drug’s core ingredient, ibrutinib.
Dr. Mikkael A. Sekeres, chief of hematology at the University of Miami Health System, called that treatment for chronic lymphocytic leukemia “truly revolutionary” for replacing highly toxic drugs and extending patients’ lives.
Janssen Biotech and AbbVie, partners in selling the drug, declined to comment.
WuXi Biologics also manufactures Jemperli, a GSK treatment approved by the Food and Drug Administration last year for some endometrial cancers. In combination with standard therapies, the drug improves survival in patients with advanced disease, said Dr. Amanda Nickles Fader, president of the Society of Gynecologic Oncology.
“This is particularly important because while most cancers are plateauing or decreasing in incidence and mortality, endometrial cancer is one of the only cancers globally” increasing in both, Dr. Fader said.
GSK declined to comment.
The drug that possibly captures WuXi’s most significant impact is Trikafta, manufactured by an affiliate in Shanghai and Changzhou to treat cystic fibrosis, a deadly disease that clogs the lungs with debilitating, thick mucus. The treatment is credited with clearing the lungs and extending by decades the life expectancy of about 40,000 U.S. residents. It also had manufacturers in Italy, Portugal and Spain.
The treatment has been so effective that the Make-A-Wish Foundation stopped uniformly granting wishes to children with cystic fibrosis. Trikafta costs about $320,000 a year per patient and has been a boon for Boston-based Vertex Pharmaceuticals and its shareholders, with worldwide revenue rising to $8.9 billion last year from $5.7 billion in 2021, according to a securities filing .
Trikafta “completely transformed cystic fibrosis and did it very quickly,” said Dr. Meghan McGarry, a University of California San Francisco pulmonologist who treats children with the condition. “People came off oxygen and from being hospitalized all the time to not being hospitalized and being able to get a job, go to school and start a family.”
Vertex declined to comment.
Two industry sources said WuXi plays a role in making Eli Lilly’s popular obesity drugs. Eli Lilly did not respond to requests for comment. WuXi companies also make an infusion for treatment-resistant H.I.V., a drug for advanced ovarian cancer and a therapy for adults with a rare disorder called Pompe disease.
WuXi is known for helping biotech firms from the idea stage to mass production, Dr. Kolchinsky said. For example, a start-up could hypothesize that a molecule that sticks to a certain protein might cure a disease. The company would then hire WuXi chemists to create or find the molecule and test it in petri dishes and animals to see whether the idea works — and whether it’s safe enough for humans.
“Your U.S. company has the idea and raises the money and owns the rights to the drug,” Dr. Kolchinsky said. “But they may count on WuXi or similar contractors for almost every step of the process.”
WuXi operates large bioreactors and manufactures complex peptide, immunotherapy and antibody drugs at sprawling plants in China.
WuXi AppTec said it has about 1,900 U.S. employees. Officials in Delaware gave the company $19 million in tax funds in 2021 to build a research and drug manufacturing site that is expected to employ about 1,000 people when fully operational next year, public records and company reports show.
Mayor Kenneth L. Branner Jr. of Middletown, Del., called it “one of those once-in-a-lifetime opportunities to land a company like this,” according to a news report when the deal was approved.
In 2022, the lieutenant governor of Massachusetts expressed a similar sentiment when workers placed the final steel beam on a WuXi Biologics research and manufacturing plant in Worcester. Government officials had approved roughly $11.5 million in tax breaks to support the project. The company announced this year that it would double the site’s planned manufacturing capacity in response to customer demand.
And in Philadelphia, a WuXi Advanced Therapies site next to Iovance Biotherapeutics was approved by regulators to help process individualized cell therapies for skin cancer patients. Iovance has said it is capable of meeting demand for the therapies independently.
By revenue, WuXi Biologics is one of the top five drug development and manufacturing companies worldwide, according to Statista , a data analytics company. A WuXi AppTec annual report showed that two-thirds of its revenue came from U.S. work.
Stepping away from WuXi could cause a “substantial slowdown” in drug development for a majority of the 105 biotech companies surveyed by BioCentury , a trade publication. Just over half said it would be “extremely difficult” to replace China-based drug manufacturers.
BIO, a trade group for the biotechnology industry, is also surveying its members about the impact of disconnecting from WuXi companies. John F. Crowley, BIO’s president, said the effects would be most difficult for companies that rely on WuXi to manufacture complex drugs at commercial scale. Moving such an operation could take five to seven years.
“We have to be very thoughtful about this so that we first do no harm to patients,” Mr. Crowley said. “And that we don’t slow or unnecessarily interfere with the advancement of biomedical research.”
Julian E. Barnes contributed reporting, and Susan C. Beachy contributed research.
Christina Jewett covers the Food and Drug Administration, which means keeping a close eye on drugs, medical devices, food safety and tobacco policy. More about Christina Jewett
Advertisement
Emerging Drug Trends

- Emerging drugs, which include designer drugs and new psychoactive substances , are substances that have appeared or become more popular in the drug market in recent years.
- Emerging drugs have unpredictable health effects . They may be as powerful or more powerful than existing drugs, and may be fatal.
- Because drug markets change quickly, NIDA supports the National Drug Early Warning System (NDEWS) , which tracks emerging substances. NIDA also advances the science on emerging drugs by supporting research on their use and on their health effects.
What are emerging drugs?
Emerging drugs are mind-altering substances that have become more common in recent years. They may be sold in drug markets or at convenience stores and online. Since 2013, the United Nations Office on Drugs and Crime has identified more than 1,000 emerging drugs worldwide. 1
These substances, which include designer drugs and new psychoactive substances , come from many sources. Some were first developed as potential treatments or research chemicals. Others originate in illicit labs and are created to mimic the effects of drugs regulated under the Controlled Substances Act . These emerging substances often produce similar effects and/or are chemically similar to illegal or prescription opioids, stimulants, benzodiazapines (“benzos”), or other existing types of drugs.
People may seek out these drugs for recreation or use them to self-medicate without medical supervision. They may also be added to other drugs without a buyer knowing it. As a result, the health effects of emerging drugs are largely unknown, potentially posing a public health threat and contributing to the overdose crisis . 2,3
NIDA monitors emerging drug trends through its Designer Drug Research Unit and through support for the National Drug Early Warning System (NDEWS) , which tracks drug-related emergency calls.
What are the effects of emerging drugs?
An emerging drug’s effects depend on the type of substance it is—for instance, if it is a new type of opioid , depressant , synthetic cannabinoid , psychedelic , or stimulant. Its effects may be unpredictable and unwanted, especially if it is an unknown ingredient in another drug. A person may not know what substance or substances they have really taken. And because these substances are new to the drug market, clinicians or researchers may not know their effects or how potent (powerful) they are until people begin to visit emergency departments or clinics with symptoms of negative health effects. 4
In addition, emerging substances are usually not included in emergency department drug tests and are not routinely included in the toxicology tests used after a fatal overdose. The delay in this data means there is also a delay in understanding how widespread use of the drug is, why and how these drugs have their effects, and how to care for people who experience negative effects of those substances.
NIDA researchers and grantees collaborate to identify how these emerging drugs work and their potential health effects, including those that have the potential to impact the overdose crisis. NIDA also supports the National Drug Early Warning System (NDEWS) to track emerging substances and their impact on drug-related emergency calls.
What are nitazenes?
Nitazenes are a class of lab-made (synthetic) opioids that may be as powerful or more powerful than fentanyl. 4 They were developed in research labs in the 1950s as potential pain relievers but never marketed. Nitazenes are most often sold as a white powder or tablets. People may not be aware that they have taken nitazenes, as they may be added to other substances, including fentanyl, heroin, and benzodiazepines. 5
Nitazenes began to re-emerge in the drug supply in 2019, after the U.S. Drug Enforcement Administration banned fentanyl-related substances. 6,7 Researchers and authorities are monitoring nitazenes, including isotonitazene, protonitazene, etonitazene, N-piperidinyl etonitazene, and metonitazene. Many nitazenes are listed as Schedule 1 drugs under the Controlled Substances Act.
Like all opioids , nitazenes can slow breathing, blood pressure, and heart rate to dangerously low levels, potentially contributing to overdose . Preliminary NIDA-supported research shows that the opioid overdose reversal medication naloxone is effective with isonitazene, metonitazene, and etonitazene, though it may require repeated doses. More research is needed to confirm these findings with additional nitazenes and in larger groups of people. Fentanyl test strips do not detect nitazenes.
What is tianeptine?
Tianeptine is an antidepressant medication that is not approved for use in the United States. NIDA-funded research suggests that most people take tianeptine in dietary supplements marketed as cognitive enhancers or nootropics, often sold in convenience stores and online. It may be blended with or taken at the same time as other nootropics (like phenibut and racetams) and is also used with substances such as kratom , kava, and gabapentin.
Tianeptine is not an opioid but at high doses it can have opioid-like effects, such as dangerous drops in blood pressure, heart rate, or breathing rate. Research shows that other effects include problems with brain, heart, and digestive function.
Research has shown that tianeptine can cause symptoms of a substance use disorder, including tolerance—which is when you need to take more of a drug for it to have the same level of effect—and withdrawal. Withdrawal from tianeptine has been associated with pain and problems with brain, heart, and digestive function. Early evidence suggests that tianeptine-related substance use disorder can be treated with medications for opioid use disorder , such as buprenorphine. 8
What are new psychoactive substances?
“New psychoactive substances” is a term used to describe lab-made compounds created to skirt existing drug laws . The category may include medications created by pharmaceutical companies or researchers that were never meant to reach the public .
These substances belong to a number of drug classes:
- Synthetic opioids. These drugs are chemically different from existing lab-made opioids like fentanyl . They include brorphine and U-47700. Researchers first identified brorphine in the unregulated drug supply in 2018. New synthetic opioids may slow breathing, blood pressure, and heart rate to dangerously low levels, potentially contributing to overdose. Emerging opioids can be as powerful or more powerful than fentanyl, which itself is 50 to 100 times more powerful than morphine.
- Synthetic cannabinoids , sometimes called “K2” or “Spice.” Lab-made cannabinoids are chemically similar to the cannabis plant but may have very different effects. Newer synthetic cannabinoids include ADMB-5,Br-BUTINACA and MDMB-4en-PINACA. MDMB-4en-PINACA has been associated with hallucinations, paranoia, and confusion. These substances have been found in people who died from accidental overdose. 9
- Synthetic cathinones , also known as “Bath Salts.” Lab-made cathinones are stimulants that are chemically related to, but not derived from, the khat plant. People sometimes take synthetic cathinones as a less expensive alternative to other stimulants, but cathinones have also been found as an added ingredient in other recreational drugs. Emerging cathinones include eutylone, N,N-dimethylpentylone (dipentylone), and pentylone. These substances have been found in people who died from overdose. 10
- Synthetic benzodiazapines. Benzodiazapenes are a class of lab-made depressants that include prescription medications such as diazepam (sometimes sold as Valium), alprazolam (sometimes sold as Xanax), and clonazepam (sometimes sold as Klonopin). Recent data show that new versions of recreationally manufactured bezodiazapines include bromazolam, disalkylgidazepam, and flubromazepam. 11
How does NIDA support research into emerging drugs?
NIDA supports research tracking the emergence of new drugs into the unregulated drug supply, including via the National Drug Early Warning System (NDEWS) , collaboration with other researchers, partners around the world, and social media. The Institute studies or supports research on changes in the lab-made drug supply and how these emerging substances work in the brain, as well as their health effects and potential as therapeutic treatments.
NIDA also researches ways to prevent substance use and misuse , and studies whether and how harm reduction methods may prevent, reverse, or reduce rates of overdose.
Latest from NIDA

Law enforcement seizures of psilocybin mushrooms rose dramatically between 2017-2022

Can science keep up with designer drugs?

Xylazine appears to worsen the life-threatening effects of opioids in rats
Find more resources on emerging drugs.
- See recent data on Overdose Rates from the Centers for Disease Control and Prevention (CDC).
- Stay up to date on new and emerging substances at the National Drug Early Warning System website
- Early warning advisory on new psychoactive substances. United Nations Office on Drugs and Crime. Accessed April 15, 2024. https://www.unodc.org/LSS/Page/NPS
- Singh VM, Browne T, Montgomery J. The emerging role of toxic adulterants in street drugs in the US illicit opioid crisis . Public Health Rep . 2020;135(1):6-10. doi:10.1177/0033354919887741
- Gladden RM, Chavez-Gray V, O'Donnell J, Goldberger BA. Notes from the field: overdose deaths involving eutylone (psychoactive bath salts) - United States, 2020 . MMWR Morb Mortal Wkly Rep . 2022;71(32):1032-1034. Published 2022 Aug 12. doi:10.15585/mmwr.mm7132a3
- Pergolizzi J Jr, Raffa R, LeQuang JAK, Breve F, Varrassi G. Old drugs and new challenges: A narrative review of nitazenes . Cureus . 2023;15(6):e40736. Published 2023 Jun 21. doi:10.7759/cureus.40736
- Ujváry I, Christie R, Evans-Brown M, et al. DARK classics in chemical neuroscience: Etonitazene and related benzimidazoles . ACS Chem Neurosci . 2021;12(7):1072-1092. doi:10.1021/acschemneuro.1c00037
- Benzimidazole opioids, other name: nitazenes. Drug Enforcement Agency. Issued January 2024. Accessed April 15, 2024. https://www.deadiversion.usdoj.gov/drug_chem_info/benzimidazole-opioids.pdf
- Papsun DM, Krotulski AJ, Logan BK. Proliferation of novel synthetic opioids in postmortem investigations after core-structure scheduling for fentanyl-related substances . Am J Forensic Med Pathol . 2022;43(4):315-327. doi:10.1097/PAF.0000000000000787
- Trowbridge P, Walley AY. Use of buprenorphine-naloxone in the treatment of tianeptine use disorder . J Addict Med . 2019;13(4):331-333. doi:10.1097/ADM.0000000000000490
- Simon G, Kuzma M, Mayer M, Petrus K, Tóth D. Fatal overdose with the cannabinoid receptor agonists MDMB-4en-PINACA and 4F-ABUTINACA: A case report and review of the literature . Toxics . 2023;11(8):673. Published 2023 Aug 5. doi:10.3390/toxics11080673
- Ehlers PF, Deitche A, Wise LM, et al. Notes from the field: Seizures, hyperthermia, and myocardial injury in three young adults who consumed bromazolam disguised as alprazolam - Chicago, Illinois, February 2023 . MMWR Morb Mortal Wkly Rep . 2024;72(5253):1392-1393. Published 2024 Jan 5. doi:10.15585/mmwr.mm725253a5
How major influenza discoveries and Tamiflu are founded in research by a 'hallucinating' professor at the Great Barrier Reef
When Graeme Laver was strolling along the New South Wales coastline in the 1960s very little was known about the origins of influenza pandemics sweeping the globe.
Professor Laver, alongside virologist Robert Webster, came across a beach littered with dead wedge-tailed shearwaters.
At the time, the virus had been discovered in domestic birds like ducks, but little was known about influenza in wild birds.
Professor Laver set out to test shearwater birds and uncover the structure of the virus, which led to breakthroughs in how influenza was treated, as well as leading to anti-viral drugs such as Tamiflu and vaccine improvements.
"Because mutton birds nest in burrows on barrier islands off the coast … Graeme decided that it would be a 'bit of a joke' to test birds on the Great Barrier Reef for influenza," Professor Webster wrote in a biography.
Professor Laver's "joke" still informs an understanding of and treatment of influenza viruses today.
It comes as Australian health departments are on alert for a possible spike in flu hospitalisations this year, with more than 7,000 cases in Queensland recorded so far this year .
'Laver is hallucinating'
Tryon Island is a remote, tiny coral cay island at the southern end of the Great Barrier Reef, about 86 kilometres from Gladstone.
In 2004, Professor Laver wrote:
"We toyed with the idea of doing this on the coral islands of the Great Barrier Reef. "Why there? Can you think of a more unlikely place to look for flu? "Beautiful islands in an azure sea, hot sand, a baking sun, and a warm coral lagoon. "What better place to do flu research!"
In 1969 he secured $500 in funding from the World Health Organization to begin his expedition.
In later years, he said "this was just as well as my head of department at the Australian National University, when asked for funds for an expedition to look for the flu on the Great Barrier Reef, said 'Laver is hallucinating'".
"He also said that in any case I wouldn't be able to catch the birds," he said.
"But I knew that thousands upon thousands of mutton birds, or shearwaters, nested on the coral cays of the reef in burrows in the sand, and that all you had to do to catch these wild, free-flying sea birds was to bend over and pick them up."
A lifelong obsession
After Professor Webster found birds on Tryon which had been infected with influenza, Professor Laver mixed parts of the human virus with a part of the bird samples to look at how the flu could be prevented.
Senior librarian at the Queensland State Library, Christina Ealing-Godbold, studied their work and said the virologist tested hundreds of birds as part of this study, which proved the virus could be inhibited.
"This was a huge breakthrough because they didn't understand what was causing these pandemics," Ms Ealing-Gobold said.
"It was his lifetime obsession to try and solve the irregular pandemic upheavals that have dominated medical science for so many years."
Professor Laver even sent samples from a black noddy bird to a Russian space station to grow under microgravity conditions.
The endeavour was later abandoned as the crystals were found to grow almost as well on land.
Californian pharmaceutical company Gilead Science used Professor Laver's research to develop the anti-viral drug Tamiflu, which is still available today.
ABC Capricornia — local news in your inbox
- X (formerly Twitter)
Related Stories
Triple whammy of viruses, whooping cough adds pressure to early onset of flu season.
Bird flu has spread to dairy cows in the US. Here's why the world is watching
'This place is HELL': Nurse's diary provides rare insight into deadly Spanish Flu quarantine station
- Avian Influenza
- Epidemics and Pandemics
- Heron Island
- Medical History
- Rockhampton
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 23 September 2021
The champions of drug development
Nature Biotechnology volume 39 , page 1167 ( 2021 ) Cite this article
6004 Accesses
14 Altmetric
Metrics details
Our new podcast series ‘Hope Lies in Dreams’ highlights the importance of visionaries in shepherding drugs through the setbacks of drug development to commercial success.
Drug development is a long game. And when it comes to shepherding an entirely new therapeutic modality to market, it is a very long game indeed. Our current podcast series, Hope Lies in Dreams , focuses on the history of one such therapeutic modality: antisense oligonucleotides (ASOs). It highlights the many highs and lows that drug makers experience bringing a completely new type of drug molecule to market. But most of all, it showcases the importance of an individual personality—and the team around them—in pushing a drug program to commercial success, despite clinical disappointments, investor flight, big pharma skepticism and market failures.
As recounted in our podcast, Ionis had to persevere for decades before attaining commercial success with the ASO Spinraza in spinal muscular atrophy. History shows us this is not unique. New therapeutic modalities—the first recombinant proteins, monoclonal antibodies (mAbs), gene therapies and oligonucleotide therapies—often follow a twisted path to market. They routinely break the backs of the startups founded around them, empty the pockets of investors, and may even end up gathering dust on pharmaceutical company shelves before they ultimately bear fruit.
In the case of replacement proteins, Genentech was the startup that nucleated recombinant DNA technology. Founded in 1976 with venture capital money, it supplied the know-how that created the first recombinant DNA product (Humulin, a recombinant insulin marketed in 1982 by Eli Lilly) and the first commercial drug from a biotech company (Protropin, a recombinant human growth hormone first marketed in 1985). Genentech created an entirely new way of developing drugs. But it went after the lowest-hanging fruit: recombinant protein alternatives to already approved plasma-purified products. Other new therapeutic modalities proved harder to crack.
mAbs, ASOs, small interfering RNAs (siRNAs), gene therapies and gene-editing products have presented drug makers with an added challenge: how to develop and deliver a type of therapeutic molecule that the human body has never encountered. Little wonder that the timelines for maturation and marketing of such drugs are many years longer than the 10–12+ years needed for a typical small-molecule drug. And no surprise that bankrolling the development of such therapeutic modalities can take hundreds of millions, if not billions, of dollars.
Take Centocor, a company founded in 1979. It took nearly 20 years before turning hybridoma technology into market success with the mAb Remicade (infliximab). Along the way, the company faced financial ruin with the failure of its anti-septic shock mAb Centoxin (nebacumab); indeed, for nearly the entirety of its history, Centocor lost money : $194 million in 1992, $74 million the next year, $127 million year the year after that. On and on it went, until the company finally got ReoPro (abciximab) on the market in 1994 and Remicade four years later.
Since Ionis was founded in 1989, the company has eaten through ~$1.5 billion of investor money to fuel its voracious R&D engine. Its first commercialized product, Vitravene (fomivirsen, 1998), was withdrawn; its second approved product, Kynamro (mipomersen, 2013), was a commercial failure. And yet somehow the company still found a way to survive and mature antisense into an established drug modality, curing a disease that had confounded medicine.
What is striking about the ASO story—and of nearly every other successful therapeutic modality—is the importance of a single personality in overcoming the disappointments and setbacks of drug development and finally attaining commercial success. In the case of Ionis, that individual was former CEO Stan Crooke.
For Crooke, the needs of shareholders, although important, always fell somewhere below the needs of patients and the needs of the science. Ionis’s programs faced a bewildering number of obstacles and setbacks over the three decades it took to get to Spinraza. And yet, as CEO, Crooke always somehow found a way through: raising funds when company valuations were low and, in the dark years, surviving on loans, special financing vehicles and debt—anything that kept the science moving and the drugs progressing through the pipeline.
Look at any other successful biotech company developing groundbreaking drugs and one can find similar individuals: Michael Wall, Centocor’s founder, who kept the company afloat despite investor lawsuits and development flops, allowing it to finally emerge to launch Remicade; Alnylam’s John Maraganore, who oversaw development of the first siRNA drug, Onpattro (patisaran), despite clinical disappointments and investor and pharma skepticism; Julian Adams, who kept Velcade (bortezomib) alive at Millennium when executives wanted to kill the drug; Brian Druker, who battled to convince Novartis executives not to shelve Gleevec (imatinib), the drug that launched precision medicine; Vicki Sato, who oversaw the development of Vertex’s key antiviral drugs Agenerase (amprenavir), Lexiva (fosamprenavir) and Incivek (telaprevir) when pharma was abandoning infectious disease; BioNTech’s Katalin Karikó and Uğur Şahin, who persevered with mRNA vaccines when many dismissed the approach; and Andrea van Elsas (now at Third Rock Ventures), who championed Keytruda (pembrolizumab) when Schering-Plough executives wanted to shelve Organon’s checkpoint inhibitor.
These are leaders who believe in their drugs and the teams developing them. These are the visionaries. These are the individuals who refuse to give up on their drug technologies, despite naysayers and skeptics and corporate executives myopically chasing market share.
While every drug development program is characterized by its own unique idiosyncrasies and setbacks, successful drug programs share one thing: a pioneering individual who displays the true grit needed to bring a transformative drug technology to market.
Such individuals may drive their teams too hard, exert too much control and show a persistence that sometimes borders on sheer bloody-mindedness. But they are the ones who make the difference. They are the force behind many of the drugs that change the face of medical practice. Let’s salute them and celebrate them.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
The champions of drug development. Nat Biotechnol 39 , 1167 (2021). https://doi.org/10.1038/s41587-021-01095-z
Download citation
Published : 23 September 2021
Issue Date : October 2021
DOI : https://doi.org/10.1038/s41587-021-01095-z

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
9 facts about Americans and marijuana

The use and possession of marijuana is illegal under U.S. federal law, but about three-quarters of states have legalized the drug for medical or recreational purposes. The changing legal landscape has coincided with a decades-long rise in public support for legalization, which a majority of Americans now favor.
Here are nine facts about Americans’ views of and experiences with marijuana, based on Pew Research Center surveys and other sources.
As more states legalize marijuana, Pew Research Center looked at Americans’ opinions on legalization and how these views have changed over time.
Data comes from surveys by the Center, Gallup , and the 2022 National Survey on Drug Use and Health from the U.S. Substance Abuse and Mental Health Services Administration. Information about the jurisdictions where marijuana is legal at the state level comes from the National Organization for the Reform of Marijuana Laws .
More information about the Center surveys cited in the analysis, including the questions asked and their methodologies, can be found at the links in the text.
Around nine-in-ten Americans say marijuana should be legal for medical or recreational use, according to a January 2024 Pew Research Center survey . An overwhelming majority of U.S. adults (88%) say either that marijuana should be legal for medical use only (32%) or that it should be legal for medical and recreational use (57%). Just 11% say the drug should not be legal in any form. These views have held relatively steady over the past five years.
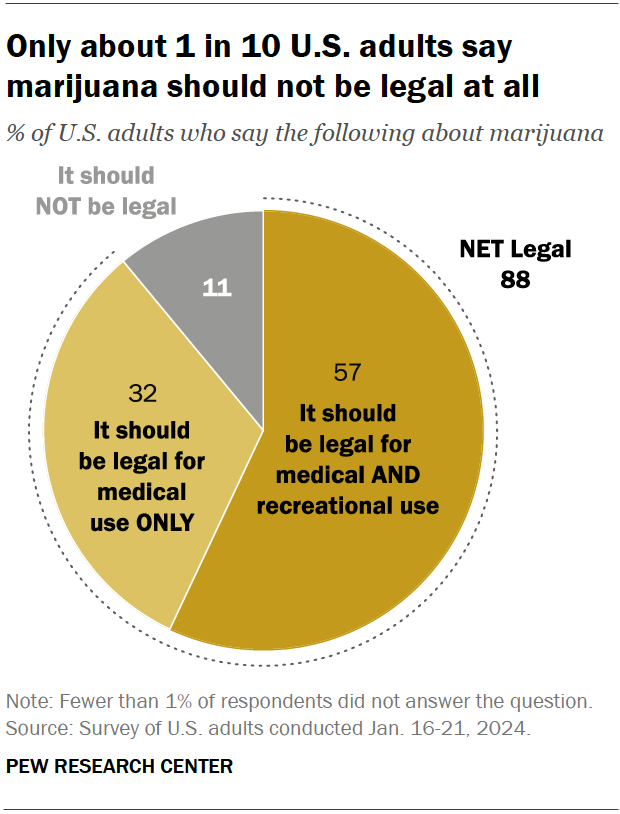
Views on marijuana legalization differ widely by age, political party, and race and ethnicity, the January survey shows.
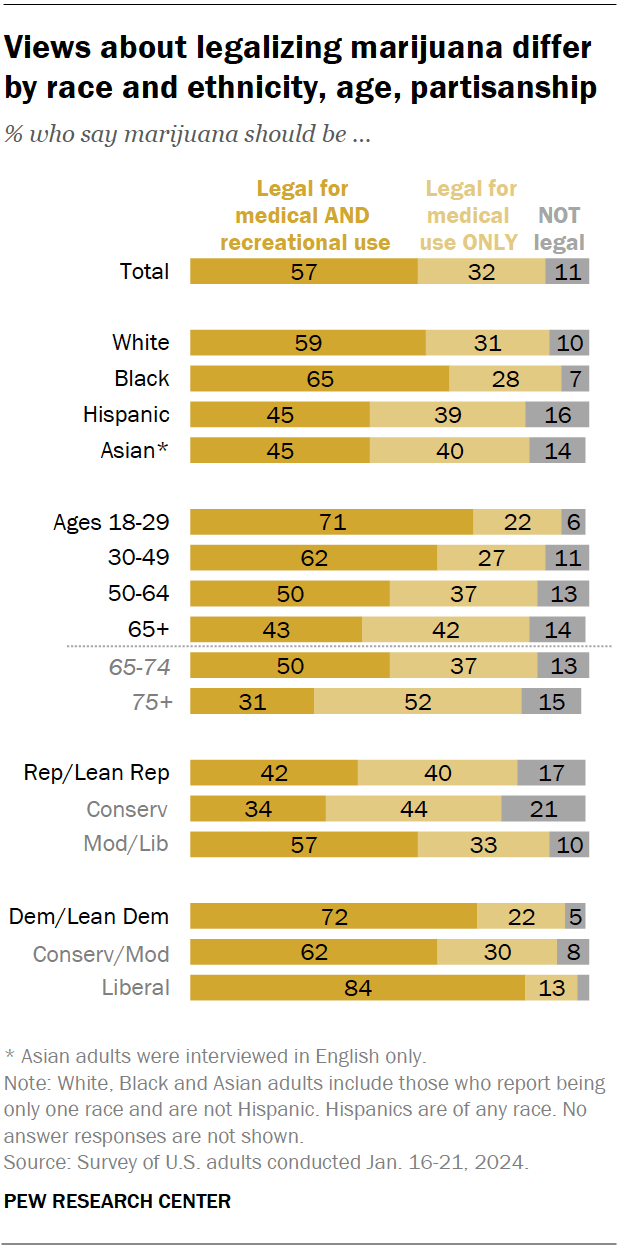
While small shares across demographic groups say marijuana should not be legal at all, those least likely to favor it for both medical and recreational use include:
- Older adults: 31% of adults ages 75 and older support marijuana legalization for medical and recreational purposes, compared with half of those ages 65 to 74, the next youngest age category. By contrast, 71% of adults under 30 support legalization for both uses.
- Republicans and GOP-leaning independents: 42% of Republicans favor legalizing marijuana for both uses, compared with 72% of Democrats and Democratic leaners. Ideological differences exist as well: Within both parties, those who are more conservative are less likely to support legalization.
- Hispanic and Asian Americans: 45% in each group support legalizing the drug for medical and recreational use. Larger shares of Black (65%) and White (59%) adults hold this view.
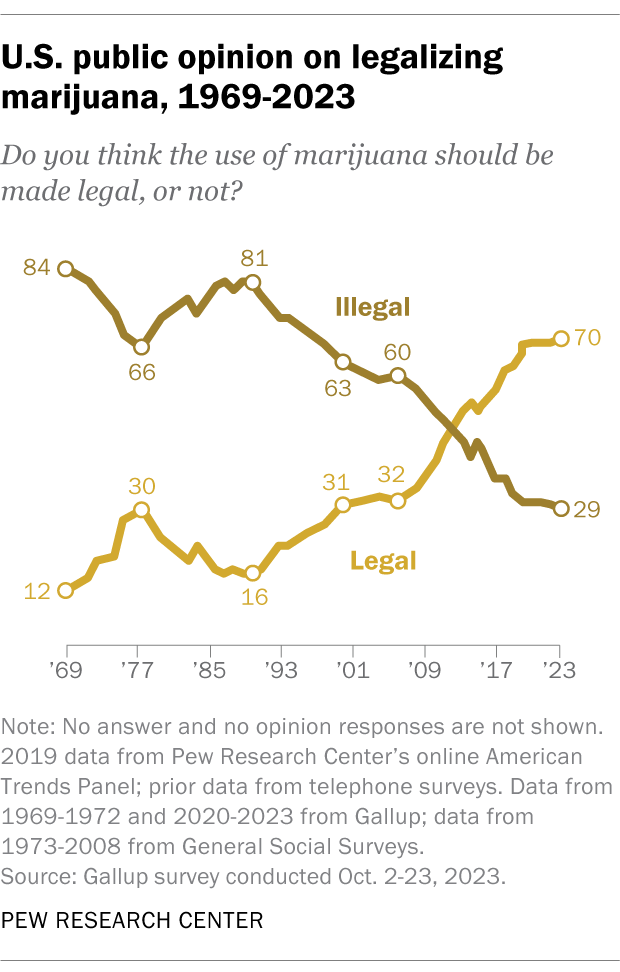
Support for marijuana legalization has increased dramatically over the last two decades. In addition to asking specifically about medical and recreational use of the drug, both the Center and Gallup have asked Americans about legalizing marijuana use in a general way. Gallup asked this question most recently, in 2023. That year, 70% of adults expressed support for legalization, more than double the share who said they favored it in 2000.
Half of U.S. adults (50.3%) say they have ever used marijuana, according to the 2022 National Survey on Drug Use and Health . That is a smaller share than the 84.1% who say they have ever consumed alcohol and the 64.8% who have ever used tobacco products or vaped nicotine.
While many Americans say they have used marijuana in their lifetime, far fewer are current users, according to the same survey. In 2022, 23.0% of adults said they had used the drug in the past year, while 15.9% said they had used it in the past month.
While many Americans say legalizing recreational marijuana has economic and criminal justice benefits, views on these and other impacts vary, the Center’s January survey shows.
- Economic benefits: About half of adults (52%) say that legalizing recreational marijuana is good for local economies, while 17% say it is bad. Another 29% say it has no impact.
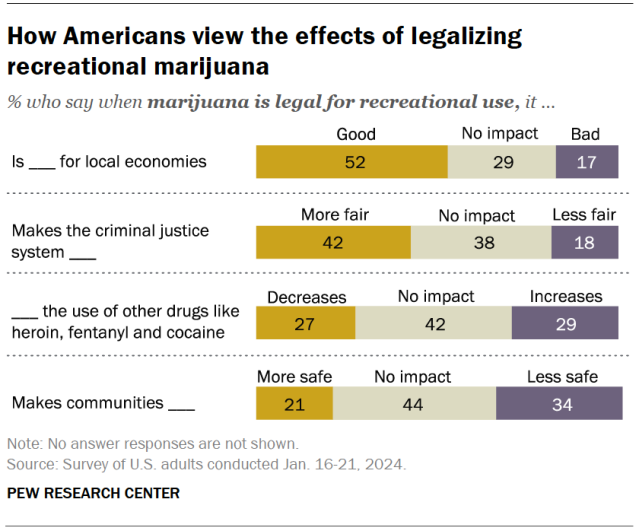
- Criminal justice system fairness: 42% of Americans say legalizing marijuana for recreational use makes the criminal justice system fairer, compared with 18% who say it makes the system less fair. About four-in-ten (38%) say it has no impact.
- Use of other drugs: 27% say this policy decreases the use of other drugs like heroin, fentanyl and cocaine, and 29% say it increases it. But the largest share (42%) say it has no effect on other drug use.
- Community safety: 21% say recreational legalization makes communities safer and 34% say it makes them less safe. Another 44% say it doesn’t impact safety.
Democrats and adults under 50 are more likely than Republicans and those in older age groups to say legalizing marijuana has positive impacts in each of these areas.
Most Americans support easing penalties for people with marijuana convictions, an October 2021 Center survey found . Two-thirds of adults say they favor releasing people from prison who are being held for marijuana-related offenses only, including 41% who strongly favor this. And 61% support removing or expunging marijuana-related offenses from people’s criminal records.
Younger adults, Democrats and Black Americans are especially likely to support these changes. For instance, 74% of Black adults favor releasing people from prison who are being held only for marijuana-related offenses, and just as many favor removing or expunging marijuana-related offenses from criminal records.
Twenty-four states and the District of Columbia have legalized small amounts of marijuana for both medical and recreational use as of March 2024, according to the National Organization for the Reform of Marijuana Laws (NORML), an advocacy group that tracks state-level legislation on the issue. Another 14 states have legalized the drug for medical use only.
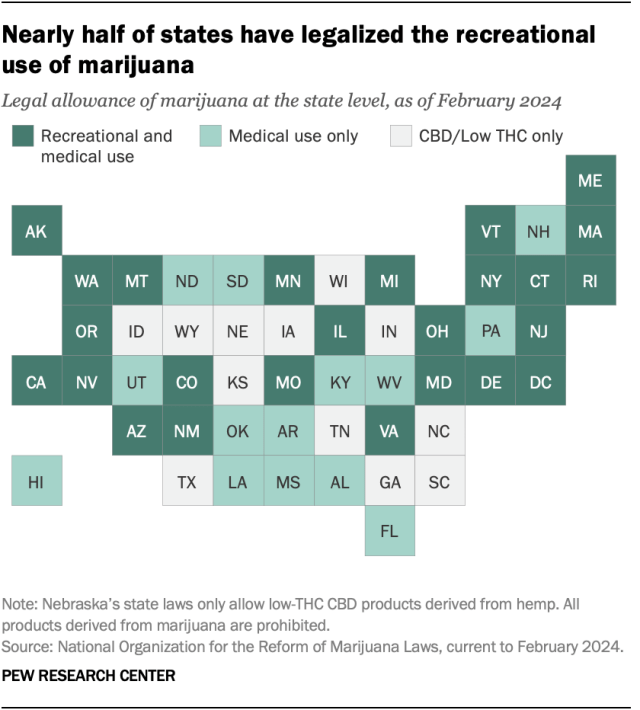
Of the remaining 12 states, all allow limited access to products such as CBD oil that contain little to no THC – the main psychoactive substance in cannabis. And 26 states overall have at least partially decriminalized recreational marijuana use , as has the District of Columbia.
In addition to 24 states and D.C., the U.S. Virgin Islands , Guam and the Northern Mariana Islands have legalized marijuana for medical and recreational use.
More than half of Americans (54%) live in a state where both recreational and medical marijuana are legal, and 74% live in a state where it’s legal either for both purposes or medical use only, according to a February Center analysis of data from the Census Bureau and other outside sources. This analysis looked at state-level legislation in all 50 states and the District of Columbia.
In 2012, Colorado and Washington became the first states to pass legislation legalizing recreational marijuana.
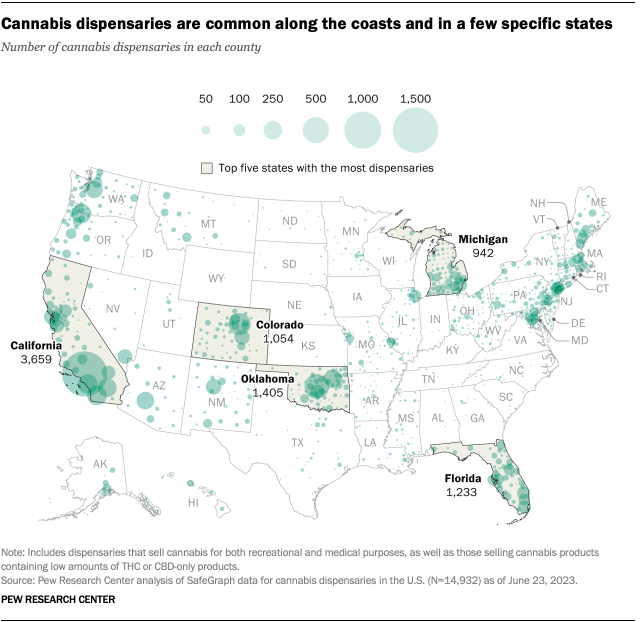
About eight-in-ten Americans (79%) live in a county with at least one cannabis dispensary, according to the February analysis. There are nearly 15,000 marijuana dispensaries nationwide, and 76% are in states (including D.C.) where recreational use is legal. Another 23% are in medical marijuana-only states, and 1% are in states that have made legal allowances for low-percentage THC or CBD-only products.
The states with the largest number of dispensaries include California, Oklahoma, Florida, Colorado and Michigan.
Note: This is an update of a post originally published April 26, 2021, and updated April 13, 2023.
- Drug Policy
- Health Care
- Health Policy
- Medicine & Health
- Political Issues
- Politics & Policy
Majority of U.S. Catholics Express Favorable View of Pope Francis
Americans rate their federal, state and local governments less positively than a few years ago, about 1 in 4 u.s. teachers say their school went into a gun-related lockdown in the last school year, changing partisan coalitions in a politically divided nation, about half of americans say public k-12 education is going in the wrong direction, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmaceuticals (Basel)
- PMC10536713

Research in the Field of Drug Design and Development
Grazyna biala.
1 Chair and Department of Pharmacology with Pharmacodynamics, Medical University of Lublin, Chodźki 4A, 20-093 Lublin, Poland; [email protected] (E.K.); [email protected] (M.K.-S.); [email protected] (J.O.-G.);
Ewa Kedzierska
Marta kruk-slomka, jolanta orzelska-gorka, sara hmaidan, aleksandra skrok, jakub kaminski, eva havrankova.
2 Department of Chemical Drugs, Faculty of Pharmacy, Masaryk University of Brno, 601 77 Brno, Czech Republic; zc.inum.mrahp@eavoknarvah
Dominika Nadaska
3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University Bratislava, 832 32 Bratislava, Slovakia ks.abinu.mrahpf@kilam (I.M.)
Associated Data
Data sharing is not applicable.
The processes used by academic and industrial scientists to discover new drugs have recently experienced a true renaissance, with many new and exciting techniques being developed over the past 5–10 years alone. Drug design and discovery, and the search for new safe and well-tolerated compounds, as well as the ineffectiveness of existing therapies, and society’s insufficient knowledge concerning the prophylactics and pharmacotherapy of the most common diseases today, comprise a serious challenge. This can influence not only the quality of human life, but also the health of whole societies, which became evident during the COVID-19 pandemic. In general, the process of drug development consists of three main stages: drug discovery, preclinical development using cell-based and animal models/tests, clinical trials on humans and, finally, forward moving toward the step of obtaining regulatory approval, in order to market the potential drug. In this review, we will attempt to outline the first three most important consecutive phases in drug design and development, based on the experience of three cooperating and complementary academic centers of the Visegrád group; i.e., Medical University of Lublin, Poland, Masaryk University of Brno, Czech Republic, and Comenius University Bratislava, Slovak Republic.
1. Introduction
Through the process of drug design and discovery, potential new therapeutic agents are identified, using different computational, experimental, and clinical models [ 1 , 2 , 3 ]. Despite advances in biotechnology and pharmacology, and in our understanding of biological mechanisms, drug discovery is still a lengthy, costly, difficult, and sometimes inefficient process. In its first step, drug design involves the outline of compounds that are complementary in shape and structure to the molecular target with which they interact and bind. Nowadays, drug design frequently relies on computer modeling techniques and bioinformatic approaches. That means that a synthetic approach has to already exist, or has to be developed according to the natural physico-chemical properties of the reacting compounds. The environmental burden, production safety, economic point of view, and other principles of green chemistry (see Section 2 ) should be taken into account when designing methods for the synthesis of target compounds.
In general, the process of drug development consists of three main stages: drug discovery, pre-clinical development using cell-based and animal models/tests, clinical trials on humans and, finally, moving forward toward the step of obtaining regulatory approval, in order to market the potential drug (see, e.g., ref. [ 4 ] for drug screening). Modern drug discovery aims to increase the affinity, selectivity (to reduce the potential of side effects), efficacy/potency, metabolic stability (to increase the half-life), and oral bioavailability of new drugs. As stated, drug discovery starts with the finding of a hit molecule that elicits a desired activity in a screening assay [ 1 , 2 , 4 ]. Then, its structure is optimized, in terms of improving its affinity and selectivity, reducing its toxicity, improving its water and lipid solubility, and improving its pharmacokinetic properties in general, which converts the hit molecule into a lead one; i.e., a drug candidate. Next, pre-clinical studies are focused on establishing the mode of action of the drug candidate, and its pharmacokinetics in animals (rodents, zebrafish), including its bioavailability, toxic metabolites, if any, routes of excretion, efficacy on animals, drug formulation, and stability. Thirdly, the clinical trials comprise the longest and the most expensive stage of the process, consisting of three phases; i.e., on healthy volunteers, and then on several hundred patients suffering from the target disease, and then several thousand patients from several clinical centers around the world are involved. The aim of this phase is to evaluate the efficacy and safety of the drug in humans, its pharmacokinetics in the human body, and the immediate side effects, if there are any [ 3 ]. If the drug passes this phase successfully, then it is ready for registration and marketing. However, the drug continues to be observed for its safety and side effects. This last phase is known as post-marketing surveillance, and it is practically endless, continuing until the drug is on the market.
In this review, we will attempt to outline the first three most important consecutive phases in drug design and development, based on the experience of three cooperating and complementary academic centers of the Visegrád group. The elements that make up the legal and legislative regulations aimed at registering a drug and introducing it onto the market may be the subject of other review works in this field. In our paper, we focused especially on small-molecule drugs, due to the significant amount of information collected on this extremely important topic, and the experiences of the authors, and the partners of the international project in this field of research. Several innovative types of therapeutics were also briefly discussed. Our intention was to use our own scientific and academic experiences from three complementary centers and cooperating scientists and, in addition, briefly inform about the design and development of several classes of non-small-molecule drugs, as well. Several types of these innovative therapeutics were discussed as the topics of open lectures organized within the project for students, scientists, and the general public.
2. Principles of Green Chemistry—A New Approach to the Synthesis of Drugs
Chemistry is all around us. It is used in the food industry, materials, electronics, cosmetics, and many other industries. Such a wide use of chemistry, however, can only be sustainable if we try to minimize its negative impact on the environment. With this aim, in the 1990s, Dr. John Warner and Dr. Paul Anastas developed the Twelve Principles of Green Chemistry [ 5 ]. Green chemistry, by definition, is the design of chemical products and processes that reduce and/or eliminate the use or generation of hazardous substances [ 5 , 6 ]. Nowadays, these principles are adapted in the vast majority of chemical laboratories, and they are taken into account when designing new compounds, to minimize the risk from chemical reactions to human health and the environment. The design and production of new pharmaceuticals are no exception ( Figure 1 ).

The Twelve Principles of Green Chemistry, inspired by [ 6 ].
One of the most interesting principles of green chemistry, which was very quickly and effectively applied to the synthesis of organic compounds, is the catalysis of these reactions. The reactions of organic compounds often do not proceed with a 100% conversion of the starting substances, large amounts of undesirable by-products can be formed, reactions require extreme conditions, etc. All these problems are incompatible with green chemistry (atom economy, reducing derivatives, energy efficiency, etc.). However, they can have a relatively simple solution—catalysis [ 6 , 7 , 8 , 9 ].
A catalyst is defined as “a substance that changes the velocity of a reaction without itself being changed in the process” [ 5 ]. This means that the catalyst reduces the activation energy of the reaction, i.e., the minimum energy that the particles of the reacting substances must-have in order for a chemical reaction to occur (for example, to create one new substance from two reacting compounds). At the same time, the catalyst emerges from the entire reaction in its original, unchanged form. Theoretically, this means that the catalyst can be used in small quantities, and be recycled indefinitely; therefore, it does not generate any waste [ 7 , 8 , 9 ]. Of course, theory is not practice. Therefore, catalysts and their use have been studied very intensively in recent decades, to improve the selectivity and yield of the reactions in which they are used, as well as the recyclability, economy, and safety of catalysts.
One of the most common and, at the same time, most widely used ways to obtain highly recyclable, economical, efficient, and selective catalysts is the immobilization of catalysts on heterogeneous supports, such as polymers [ 10 , 11 ], oxides [ 12 , 13 , 14 ], and others [ 15 , 16 , 17 , 18 , 19 ]. For example, enzymes are nowadays widely used in the manufacturing of pharmaceuticals, fine chemicals, flavors, food, fragrances, and other products [ 19 ]. Therefore, scientists have also focused on how best to immobilize them to increase their efficiency and other desirable properties. Recently, some interesting new ways of immobilizing them were published.
Enzymes immobilized on DNA nanostructures can potentially be used in the synthesis of complex biomolecules, which cannot be realized through the conventional synthetic approach [ 20 ]. Very interesting groups of supports comprise agriculture and food waste [ 19 , 21 ]. These materials usually have a high surface area and various functional groups (hydroxyl-, carboxyl-, amino-, thiol-, and others) available for immobilization [ 19 , 21 ]. Examples of successfully used supporters from this group are eggshell [ 21 , 22 ], eggshell membrane [ 21 , 23 , 24 , 25 ], coconut fiber [ 21 , 26 , 27 , 28 ], corn cob [ 21 , 29 ], corn husk [ 21 , 30 ], rice husk [ 21 , 27 , 31 , 32 ], spent coffee grounds [ 21 , 33 , 34 ], spent grains [ 21 , 35 ], and so on.
Another remarkable class of supports for the immobilization of enzymes are MOFs (metal–organic frameworks). These compounds consist of metal ions (or clusters) coordinated with organic ligands, to form a crystalline structure similar to a cage [ 36 ]. The structure of a MOF can be relatively easily modified to reach a very high surface area and a high porosity—this leads to a very high loading of enzymes and, therefore, a very good biocatalytic activity [ 15 , 21 ]. Furthermore, with the right choice of particle size and other related properties, we can also obtain an easily recyclable catalytic system—MOFs can be separated from the reaction mixture via, for example, filtration or centrifugation [ 37 ].
Enzymes supported on magnetic nanoparticles can also be easily separated from the reaction mixture using an external magnetic field. Iron oxide (Fe 3 O 4 ) is most widely used as the magnetic supporter for the following reasons: its nontoxicity, high biocompatibility, and easy-to-handle immobilization [ 38 , 39 , 40 , 41 ]. However, other magnetic particles, such as silica-coated magnetic nanocarriers [ 41 ], magnetic amine-functionalized nanospheres [ 42 ], and chitosan-modified Fe 3 O 4 nanoparticles [ 43 ], can also be used.
3. Brief Insight into Development and Optimization in Drug Discovery, and Fundamental Roles and Importance of Computer-Aided Drug Design in This Process
Early-phase drug discovery is focused on finding highly promising synthetic compounds, semi-synthetic derivatives, or molecules of natural origin that show a notable impact on various phases of a disease, by controlling particular biological signaling cascade(s) in desirable ways. From the point of view of medicinal chemistry, this process is pretty complex, is quite often very time-consuming, and involves many theoretical, as well as experimental investigations, and many optimization steps within particular fields of this scientific discipline [ 44 , 45 ].
The platforms for optimization include the identification and proper characterization of relevant biological targets: systematic screening, the very precise evaluation of ligand (mono- or multi-functional drug)–target interactions, and rational drug design, as well as the detailed investigation and correct interpretation of structure–activity, structure–pharmacokinetics, and/or structure–toxicity relationships [ 44 , 46 ].
The integral part of this optimization is defining the pharmacophore as a very essential feature of the drug responsible for the desired biological activity, as well as suitably chosen bioisosteric modifications with the implementation of the principles and rules of bioisosterism.
Bioisosterism means the replacement of particular groups within the structure of a biologically active compound, in order to improve its biological activity, selectivity toward specific biological target(s), or metabolic stability, or to achieve a decrease in toxicity, if selective toxic properties are not therapeutically required [ 47 ].
Other optimization procedures aim to suitably modify the selectivity profiles of drugs toward chosen biological targets, with regard to the desired biological activity, and property-based design, including the structural, physicochemical, and pharmacokinetic (what the body does to the drug) features of the drugs, or promising drug candidates [ 44 , 45 ]. Furthermore, their biotransformation pathways in vivo, and elimination routes from the organism, as well as strategies for drug repurposing, as identified new therapeutic areas for previously approved or investigational drugs that are outside the scope of the original medical indication [ 44 , 48 ], definitely have to be taken into consideration.
The design and structural optimization of drugs using so-called proteolysis-targeting chimera (PROTAC) technology [ 46 ] marks a remarkably innovative conceptual shift from the views reflecting traditional concepts in drug discovery, based on the inhibition of biological functions by small-molecule drugs.
However, several more- or less-serious obstacles, or even failures, can occur at each level of the drug discovery and development process. Moreover, the financial requirements and excessively long time connected with bringing a biologically effective, selective, and non-toxic compound drug to the pharmaceutical and medicinal market are regarded as other possible areas where the situation can become complicated. Thus, computer-aided drug design (CADD) has been viewed as a very powerful strategy in the drug discovery pipeline. Structure-based and ligand-based drug design techniques via CADD provide essential information for molecular docking (MD), molecular dynamics (MDy), and ADMET (absorption, distribution, metabolism, elimination, and toxicity) modeling, respectively [ 44 , 49 , 50 ].
The first two areas (MD and MDy) may provide a very beneficial look into the efficacy or potency of drugs; the latter is capable of notably influencing the clinical success of the drugs involved in clinical trials. The aim of medicinal chemists is to precisely design and synthesize safe drugs showing a favorable combination of pharmacodynamic (what the drug does to the body), structural, physicochemical, pharmacokinetic (ADME features), and toxicological variables [ 51 , 52 ]. MD is a computational technique aimed at finding the accurate binding pose of a biological target (protein)–ligand (drug) complex, and evaluating and describing the strength of such a complex, by using various scoring functions and parameters to select the best pose generated by each molecule in a rank order [ 49 ].
MDy simulation can shed light on the prediction of atom movement in a molecular/biological system. This timely process is based on intermolecular interactions, following Newtonian physics [ 52 ]. Both the recognition and the capture of the motion and position of each atom in the system provide extremely beneficial information to scientists. For example, the simulations could contribute to uncovering the mystery around progressive neurodegenerative diseases caused by various types of protein misfolding and aggregation. Alzheimer’s disease is characterized by the formation of amyloid plaques both extracellularly (β-amyloid peptide) and intracellularly (tau protein); the hallmark of Parkinson’s disease is an accumulation of aggregates of the α-synuclein protein [ 49 , 53 , 54 ].
Small molecules are (organic) synthetic compounds or molecules of natural origin, which have a molecular weight ( MW ) < 1500 Da (or in g/mol units). The value could not be viewed so strictly, several scientific papers regarded the interval 900–1000 Da as the upper (fixed) limit [ 55 , 56 ], in fact. The MW descriptor correlates with the ability of the compounds:
- (a) rapidly cross biological membranes;
- (b) gain access to the relevant intracellular biological targets (the DNA or RNA of proteins, for example).
The impact of drugs on these biological targets is often selective, dose-dependent, and associated with strongly required pharmacotherapeutic interventions (the treatment of particular diseases) or unfavorable effects (carcinogenic, teratogenic, etc.).
Not only are CADD approaches frequently utilized in evaluating structurally relatively simple small-molecule drugs and drug candidates, but these computational techniques are also employed in the investigation of peptide and protein therapeutics [ 57 , 58 ], PROTACs [ 46 , 59 ], and nucleic-acid-based therapeutics, as small interfering RNAs (siRNAs), for example, are [ 60 , 61 , 62 ].
Peptides contribute notably to advances in the fields of pharmacy and medicine. The successful design of peptide and protein therapeutics is dependent on knowledge about their structure and their biological targets. The proper identification of active sites for the proteins is considered a preliminary task to be fulfilled in the precise design of these (non-small-molecule) therapeutics [ 63 , 64 ]. In general, the computer-aided projection of amino-acid-based therapeutics or candidate peptides [ 57 ] covers:
- (a) peptidomimetics design (de novo design, peptide-driven pharmacophoric method, geometry-similarity method, sequence-based method, fragment-based method, hybrid peptide-driven shape, and pharmacophoric method);
- (b) peptide design (ligand-based design, target-based design, and de novo design);
- (c) the designing of therapeutic proteins (template-based design and de novo design).
CADD methods can be used in the initial structure proposal of these novel biopharmaceuticals, and the prediction of their properties (conformational features, stability, binding affinity, or interaction energies, for example), to study the mechanism(s) of interaction between them and the relevant biological targets (receptors), to predict the binding energies of the bonds formed during ligand–protein interactions, in order to explore the inhibitory activity of various physiological enzymes toward peptides and proteins [ 63 ]. In silico tools are employed in the design and evaluation of peptides showing anticancer, antihypertensive, antimycobacterial, anti-inflammatory, quorum-sensing, or cell-penetrating properties, as well as in the design and evaluation of peptide inhibitors for human immunodeficiency virus (HIV) or Alzheimer’s disease [ 58 , 64 ].
The heterobifunctional PROTAC molecule [ 46 , 59 , 65 ] consists of three parts:
- (a) the target protein ligand (warhead)—the ligand (structural scaffold of a molecule of natural origin or a synthetic compound) targets proteins of interest (POIs); i.e., several nuclear receptors, various protein kinases, proteins involved in transcriptional regulation, neurodegenerative-related proteins, or fusion proteins;
- (b) the E3 ubiquitin ligase ligand (E3-binder)—the ligand can be a structural scaffold of lenalidomide , thalidomide , or pomalidomide (so-called LTP agents), for example. These binders target E3 ubiquitin ligases, such as the Von Hippel–Lindau or cereblon (CRBN);
- (c) a linker of varying size connecting the warhead and E3-binder—the linker contains a so-called anchor point influencing the length and steric properties (spatial arrangement) of a PROTAC therapeutic.
PROTACs facilitate ternary complex formation between POI, which has to be selectively degraded, and an E3 ligase. The process results in polyubiquitination of the POI, and its subsequent degradation within a 26S proteasome. At least 20 PROTACs were involved in ongoing clinical trials by December 2022. Two of the most advanced oral-active PROTAC clinical candidates, ARV-110 and ARV-471 ( Figure 2 ), are nuclear androgen receptor ( ARV-110 ) and estrogen receptor-α ( ARV-471 ) degraders for the treatment of prostate and breast cancer [ 66 , 67 ], respectively.

The structures of the oral-active PROTAC clinical candidates ARV-110 and ARV-471 .
The online PROTAC-DB 2.0 database covers approximately 3300 PROTACs, 1000 ligands of POIs, 80 ligands of E3 ligases, and more than 1500 different linkers [ 68 ].
The designed and synthesized peptide-based PROTACs belonging to the first (older) generation contained a short peptide sequence. However, their activity was quite low, and they were characterized by poor cell permeability. Moreover, the size of such molecules allowed the immune system to recognize them and produce antibodies [ 69 ]. The new generation of PROTACs, i.e., small-molecule-based PROTACs, showed more promise, in terms of being designed and structurally optimized into the drugs. The scaffolds of small molecules incorporated into their structure as the moieties for recognizing an E3 ubiquitin ligase were more easily absorbed into the human body than the peptides were [ 69 ].
Generally, various types of degradation approaches can be considered, including PROTAC, in-cell click-formed proteolysis-targeting chimera (CLIPTAC), photochemically targeting chimera (PHOTAC), semiconducting polymer nano-PROTAC (SPNpro), floate-PROTAC, antibody-PROTAC conjugate, antibody-based PROTAC (AbTAC), ribonuclease-targeting chimera (RIBOTAC), transcription factor PROTAC (TF-PROTAC), chaperone-mediated protein degradation (CHAMP), biological PROTAC (bioPROTAC), or molecular glue [ 66 ].
The rational design and structural optimization of PROTACs with CADD techniques is extremely important, taking into strong consideration the main goal in the design of these innovative types of molecules—a precisely targeted protein degradation [ 46 ].
The virtual screening process requires several phases, including the proper selection of a warhead, E3-ligase, and E3-binder. The combinations of the warheads and E3-binders with specific linkers, available in existing libraries [ 68 ], or designed via generative algorithms, lead to a considerable number of structurally different compounds.
The discovery of suitable warheads and E3-ligands is similar to the process connected with small-molecule drugs. The design of linkers might be, in fact, complicated in the case of PROTACs because a particular POI and E3 ligase cannot interact with each other if an effective PROTAC molecule is not present. Thus, various properties of the linker, i.e., the length, the appropriate attachment site, the eventual incorporation of a photo-switchable group, or the selection of a clickable linker, have to be taken into very strong consideration [ 67 ].
Several docking procedures—more- or less-extensive MDy simulations—were employed to predict and design the ternary complex structures, and evaluate their stability [ 70 ].
PROTACs might be successfully utilized in the treatment of various diseases and disorders, including cancer [ 71 ], infections caused by bacteria [ 72 ] or viruses [ 73 ], neurodegenerative diseases [ 74 ], or inflammation and oxidative stress [ 75 ].
Gene therapy is a very powerful treatment modality for many diseases via the delivery of therapeutic nucleic acids to human cells [ 76 ]. This type of therapy involves the suppression of gene expression, artificially increased expression, or gene modification. The effective suppression of gene expression can be achieved at the mRNA level, i.e., post-transcriptional gene silencing, using RNA interference (RNAi) technology. The factor triggering this process is short dsRNA, in the form of microRNA (miRNA) or siRNA [ 76 , 77 ]. siRNAs, as the most promising type of RNA-based therapeutic oligonucleotide drugs, are double-stranded macromolecules, usually containing approximately 20–21 base pairs. These therapeutics, which confer a multitude of advantages compared to traditional treatment modalities, are used for gene downregulation or complete post-transcriptional silencing [ 61 ].
Proper chemical modification, improved efficacy, and targeted delivery using suitable carrier systems, which could minimize the off-target effects, immune response, and toxicity, are challenges within the development and optimization of siRNA-based therapeutics [ 78 ]. Indeed, in silico approaches could contribute to resolving those quests [ 79 , 80 , 81 , 82 , 83 ]. Various techniques of CADD were very helpful in attempting to find relevant therapeutic answers to coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; Betacoronavirus, Coronaviridae ) [ 84 , 85 , 86 , 87 ], which was first identified in Wuhan (Hubei Province, China) in December 2019 [ 88 ]. Some examples of such in silico investigations are provided in the next section of the paper.
Both the nucleocapsid phosphoprotein gene and the surface glycoprotein gene in almost 140 SARS-CoV-2 strains spread worldwide were relevant biological targets for several siRNAs specifically designed in silico, using virtual modelling and docking analysis. The therapeutics targeted various conserved regions of those genes [ 89 ]. The research predicted that a group of investigated siRNAs might effectively fight a given RNA virus. Other siRNAs, proposed via molecular interaction and dynamics analysis, potently silenced an RNA-dependent RNA polymerase ( RdRp ) gene coding an essential viral RdRp enzyme [ 90 ]. The siRNA-3 molecule, whose structure was proposed within a molecular docking analysis [ 91 ], could also be an effective anti-SARS-CoV-2 agent. In addition, the antiviral activity and safety of siRNA-3 were confirmed in vitro, using a human embryonic kidney 293 cell line [ 91 ].
Thus, the idea to use siRNAs in the treatment of infections caused by various DNA viruses (such as the hepatitis B virus, herpes simplex virus type 1, human papillomaviruses, or murine herpesvirus 68), RNA viruses (such as coronaviruses, dengue virus, Ebola virus, hepatitis C virus, human respiratory syncytial virus, influenza virus A, rotaviruses, or West Nile virus) and retroviruses (such as HIV) is very reasonable [ 92 , 93 , 94 ].
Various siRNAs are currently approved worldwide for the treatment of diverse diseases, including neurodegenerative diseases [ 95 ], polyneuropathy [ 96 ], acute hepatic porphyria [ 97 ], hypercholesterolaemia [ 98 ], and primary hyperoxaluria type 1 [ 99 ]. Other siRNAs are also involved in clinical trials for the treatment of cancer [ 100 ] and immune-mediated diseases [ 101 ].
Traditional concepts in the design and development of drugs or drug candidates previously focused on their potency, without paying as much attention to their other characteristics [ 102 ]. Currently, the ability to predict the pharmacokinetic properties of drugs using appropriate computational techniques is a pivotal task in assisting early ADMET investigations. Properly chosen artificial procedures might considerably reduce the number of in vitro and/or in vivo tests in laboratories.
Solubility in an aqueous environment, permeability via the membranes of a biological system, metabolic stability, and transporter properties are of critical importance to the success of drugs in real life. These features notably influence their ADMET profiles [ 102 ].
The drug-likeness term is defined according to sufficiently acceptable features in clinically available drugs [ 103 , 104 , 105 ]. These characteristics include:
- (a) structural and physicochemical properties— MW , lipophilicity defined via a logarithm of a partition coefficient value estimated/calculated for an octan-1-ol/water partition system (log P ), effective lipophilicity, acid–base properties (acid–base dissociation constant; p K a ), size, flexibility (number of aromatic and non-aromatic cyclic systems, number of double and triple bonds, number of rotatable bonds ( n rotb )), number of carbon atoms, fraction of sp 3 carbon atoms (number of sp 3 hybridized carbons/total carbon count; Fsp 3 ), distribution of electrons, polar surface area value ( PSA ; expressed in Å 2 units), number of hydrogen bond donors ( n OHNH ) and acceptors ( n ON ), shape and stereochemical characteristics, reactivity, number of so-called heavy atoms, presence and number/absence of stereogenic centers, solubility, permeability, or chemical stability;
- (b) biochemical properties—biotransformation, affinity to proteins, tissue binding, transport properties (connected with PSA );
- (c) pharmacodynamics—the proper characteristics of the pharmacophore; the pharmacodynamic profile of a drug is notably influenced by structural and physicochemical properties, as well as its ADMET;
- (d) pharmacokinetics and toxicity (ADMET) features—biological availability, drug–drug interactions, half-life, lethal dose values, proper characteristics of the toxicophore (the qualitative structural feature of a drug that is assumed to be primarily responsible for its toxic properties).
Many of the given parameters can be generated in silico using CADD procedures, various web engines, interactive applets, software, and software packages. For example, the log p value predicted via a computational CLOGP method [ 106 ] for an octan-1-ol/water partition system (CLOGP parameter), n OHNH , n ON , n rotb , as well as the PSA value [ 106 , 107 ] provide extremely valuable insight into the properties of drugs or promising drug candidates, including their ADMET features, and their ability to be passively absorbed when they are administered per os . In addition, this early information can help scientists and scientific teams to properly design their studies, make correct experimental decisions, and avoid wasting precious resources [ 54 , 108 , 109 ] within the synthetic phases of the research, via in vitro and/or in vivo biological assays. Detailed exploration of structure–property relationships is crucial to properly guiding structural modifications in order to make improvements in the considered features of the drugs or drug candidates.
The relevant descriptors defining the key structural, physicochemical, ADMET, and related parameters for individual drugs or various sets of (biologically active) molecules can be calculated through employing commercially available software or free web tools with an attractive design and user-friendly interface. A very good example of one such freely available online platform is the SwissADME predictor [ 110 ]. The limitation of SwissADME is the fact that only small-molecule drugs can be analyzed within its interactive environment.
Lipinski et al. (1997) stated [ 111 ] that the molecules characterized by MW > 500 Da, CLOGP > 5.00, n ON > 10, and n OHNH > 5 would be passively absorbed per os to a (notably) lesser extent. The small-molecule drugs, which were not substrates for active transporters and met the given criteria—rule of five (Ro5), i.e., MW ≤ 500 Da, CLOGP ≤ 5.00, n ON ≤ 10, and n OHNH ≤ 5—might be satisfactorily absorbed. Exceptions from Ro5 can be found mainly (but not only) among biologically active agents of natural origin [ 111 , 112 ]. More recent research [ 113 ] indicated that the MW , n ON , and n OHNH parameters fitted well with how ligand-binding sites were formed in proteins.
Other in silico evaluations considered the appropriate balance between the structural rigidity and the flexibility of drugs whose characteristics were beyond Ro5 (bRo5), a favorable factor in their binding to biological targets with a high affinity. A balance in polarity was maintained if the calculated two-dimensional (2D) PSA was ≤250 Å 2 . The optimal lipophilicity of these bRo5 compounds was defined by a CLOGP of around 4.00. However, if their n OHNH and/or n ON were ≥6, the probability of sufficient passive oral absorption was reduced [ 108 ].
The oral delivery of peptide- and protein-based drugs is quite limited [ 114 , 115 ], due to their enzymatic digestion in the gastrointestinal tract, and insufficient permeation via the intestinal epithelium. The strategies for the oral delivery of those drugs [ 63 , 114 , 116 ], consider:
- (a) their chemical modifications—particular changes are made according to empirical processes (PEGylation, glycosylation, the use of adjuvant molecules to enhance delivery and lipidization), as well as outputs from CADD (the optimization of aqueous solubility, for example);
- (b) their modifications in formulation design—various permeation or absorption enhancers are used, for example;
- (c) the modulation of a pH value of the environment;
- (d) the direct inhibition of the enzymes responsible for therapeutics’ degradation (cleavage of peptide bond(s)).
Cyclic peptides with a limited conformational flexibility showed a better biological availability when being administered per os , compared to the linear ones. However, the criteria applied for the evaluation of the oral bioavailability of small-molecule drugs cannot be efficiently used within peptide drug development [ 117 , 118 ], in fact.
From the descriptors employed for the characterization of peptide therapeutics approved by U.S. Food and Drug Administration in the period 2012–2016, i.e., MW , CLOGP, n OHNH , n ON , n rotb , and Fsp 3 , only the last of these parameters met the relevant criteria for small-molecule drugs [ 118 ]. The most orally available peptides were described with MW ≤ 1200 Da and CLOGP = 5–8. In addition, they contained five times more H -bond donors and acceptors than was acceptable, according to Ro5, for small-molecule drugs [ 118 ].
Looking at the chemical structure of PROTACs indicates that they do not meet Ro5. When considering the MW , CLOGP, n ON , n OHNH , PSA , and n rotb parameters, as well as the number of incorporated aromatic systems, the CRBN group E3 ligands ( LTP ligands) predominate over the others. The reason for such a prioritization is a lower MW and more drug-like characteristics of these LTP agents. In fact, finding a way to achieve optimal pharmacokinetic properties is one of the major tasks in the design and development of PROTACs [ 65 , 119 ]. The newly introduced AB-MPS metric parameter was calculated for these therapeutics [ 65 , 119 , 120 ], using the calculated distribution coefficient (CLOGD) value, the number of aromatic rings, and n rotb . A lower AB-MPS score ( AB-MPS ≤ 14) in a PROTAC indicated a higher probability that it would be orally absorbed.
Computational chemistry methods are also involved in various fields in medicine. Nanomedicine includes the processes of the diagnosis and treatment of a disease or traumatic injury, and its prevention in order to improve human health, using sophisticated medical therapies. These goals are achieved via molecular tools, and molecular knowledge of the human body [ 121 ]. Computational simulations valuably contribute to the design of medicinally utilized nanoparticles, the size of which varies from 1 nm to 100 nm, with optimized biological, functional, and toxicological characteristics. In silico approaches generate relevant computational models, which might help to design biomedical nanocarriers (liposomes, detrimers, gold nanoparticles, micelles, or virus-based nanoparticles, for example), and predict their interactions with the drugs loaded in them. Attractive smart drug delivery systems include nucleic acids, DNA, exosomes, or aptamers [ 122 , 123 , 124 ].
Artificial-intelligence-assisted nanomedicine rational design methods can be used in the detailed investigation or prediction of nanomaterials’ ability to cross biological barriers, and their biological properties. In addition, such design methods might reveal, and help us to understand more clearly, the complex interactions of the nanomaterials with biological systems and environments. A knowledge of the ADMET features of original nanoformulated drugs or drug candidates with high potency, selectivity, and specificity, using computational chemistry techniques, is also a crucial condition [ 122 , 123 , 125 , 126 ]. However, the importance of validation from obtained computational outputs with experimental data should also be mentioned.
There is no doubt that the various in silico approaches listed in this chapter will be increasingly employed in the prediction of human pharmacokinetics in early drug discovery. The accurate computational prediction of pharmacodynamics, ADMET, and pharmaceutical properties for various sets of compounds is currently possible. Such outputs from artificial tools are probably, at least, no worse than those related to the studies in vitro, with several decisive advantages—relatively rapid and dynamic evaluation processes, or the requirement of notably fewer resources [ 127 ], for example.
The most suitable approach to modern drug design and development is to create a properly balanced set of relevant in silico, in vitro, and in vivo descriptors for the simulation of the pharmacodynamic properties of the drugs and pharmacokinetics in specific human populations, to predict most accurately the interindividual variability [ 127 , 128 ].
4. Several Innovations within In Vitro Screening Approaches for Drug Candidates or Drugs
The traditionally established approaches for the proper testing of drug candidates or drugs could be very roughly divided into in vitro 2D culture screenings, in vivo evaluations, and in human studies [ 129 ]. Bioassay techniques can be classified [ 130 ] into two very robust subcategories, i.e., bench-top and primary bioassay screening, and high-throughput screening. The researchers try to continuously implement prospective innovative experimental steps and improved methodologies at each phase of the testing. Thus, several examples of such innovative in vitro approaches can be found in the following sections of the paper.
The conventional procedures for the lead optimization of anti-tuberculosis (anti-TB) drugs within the pre-clinical phase of their development are driven by the so-called three Ms; i.e., the determination of a minimum inhibitory concentration value; the efficacy determination, using murine models; and evaluation of the anti-TB activity in humans [ 131 ]. The way to optimize the in vitro screening of promising anti-TB compounds and, thus, the selection of anti-TB chemotherapeutic modalities, is to use more rational and dynamic non-standard assays, to more clearly understand where specific populations of mycobacteria reside. These compartments will be target sites for precisely selected and screened molecules. When combining in vitro conditions into one model, the researchers have to take into strong consideration the broth-based in vitro composition, changes in the pH value and oxygen levels during particular phases of the infection, or the availability of oxygen [ 132 , 133 ]. Hollow fiber systems allow for in vitro investigations into the pharmacodynamic/pharmacokinetic profile of a drug, or combination(s), and these evaluations are more consistent with in vivo pharmacokinetic studies [ 133 ].
The use of proper combination therapy, according to the data obtained from in vitro and in vivo experiments with optimally established conditions, can lead to the treatment of the disease in multiple compartments in the body. The result might be savings in the time and resources spent, shortening of the TB treatment, and minimizing the number of anti-TB compounds required to treat patients suffering from TB [ 134 ].
The innovative procedures within anticancer drug discovery and development are based on use of three-dimensional (3D) tumor models. These 3D methods, which more precisely mimic physiological conditions, as well as particular states of the disease, compared to their 2D counterparts, together with more advanced screening techniques and protocols, might lead to considerable improvements in the usefulness of in vitro investigations. In addition, they make the pathway from promising anticancer drug molecule candidates to effective, selective, and safe drugs for ‘real life’ more dynamic and more straightforward [ 135 , 136 , 137 ]. The classification system of 3D cancer models reflects different criteria and conditions. Thus, these models can be divided as follows: scaffold-free systems, organoids, and tumoroids as new avatars for drug screening, scaffold-based tumor models, hydrogel-based 3D cancer models, bioreactors, microcarrier-based models, and cancer-on-chip, respectively. More details regarding the specific characteristics of these 3D models can be found in a paper by Brancato et al. [ 135 ] or Booij et al. [ 136 ].
Human prion diseases are a class of neurodegenerative disorders that are regarded as incurable. The diseases are caused by a misfolded prion protein [ 138 , 139 ]. One of the main obstacles to finding relatively satisfactory therapeutic possibilities for humans has been the lack of well-defined and established screening methodologies. In future, the innovative and effective options within in vitro assays [ 140 ] would be the use of a protein misfolding cyclic amplification procedure, to successfully mimic the pathological process of conversion (misfolding) of a specific physiological prion protein (PrP C ) into the abnormally folded one (PrP Sc ). Another possibility is to carry out a very efficient real-time quaking-induced conversion amplification method, conceptually similar to the previous one.
5. Pre-Clinical In Vitro and In Vivo Studies
Earlier drug discovery was almost exclusively based on animal experiments, clinical observations, and serendipity. Nowadays, in accordance with the rational order of research, before it is approved for human use, a new compound must undergo a series of precise toxicological and pharmacological tests, which constitute a panel called “pre-clinical trials”. The first step is usually in vitro testing on genetically modified cell cultures, to check the drug’s effects on reproduction or carcinogenicity. In recent years, a major input has come from biochemistry. The effect of many drugs in human therapy could be explained biochemically as effects on specific enzymes or receptors. With the detection of more and more receptor subtypes, the activity spectrum of a single compound became more and more complicated. At present, molecular biology provides pharmacologists with human receptors and ion channels expressed in mammalian cells in culture. This avoids the apparent species differences, but the multitude of natural and perhaps artificial subtypes raises the question of physiological and pathological relevance. In recent years, a major input has come from biochemistry and molecular biology. The effect of many drugs in human therapy could be explained biochemically as effects on specific enzymes or receptors. The use of these methods allows for a much faster and more accurate characterization of the drug effect than that via traditional methods. At the same time, substances that cause side effects, or are not active enough, are omitted, resulting in a significant reduction in time and money. Predicting the pharmacokinetics of drugs or their side effects on the basis of a chemical structure is only approximately possible, which is why studies on animals or isolated organs are still a necessity. Similarly, effects seen in tissue cultures are often not typical of the whole intact organism. So, it will always be a challenge for the pharmacologist to correlate in vitro data with in vivo findings; according to the old saying: in vitro simplicitas ; in vivo veritas . The main purpose of preclinical studies is to assess the safety of new drugs, and to confirm the direction of their therapeutic action. In vitro research—computer modeling, or tests on single cells or on cell and tissue cultures—has become more and more popular. Indeed, these studies are very useful in the initial phase, as they provide valuable information about intracellular processes, allow for initial toxicity testing, and help us to understand the mechanisms of action of new drugs. However, they do not cover the complexity of the whole organism, because individual cells react differently to the whole body. Furthermore, the possible side effects of drugs can be better detected in whole animals than in individual cells. Therefore, most of the results obtained in vitro must be replicated in vivo, i.e., in a living organism, which means that animal experiments are necessary for the discovery and evaluation of drugs [ 141 ].
However, they should be performed only if they are necessary and well-conceived. The credibility of the results obtained in pre-clinical in vivo studies depends mainly on the appropriate organization of the research, the selection of animal species, and their numbers and welfare. Well-designed studies makes it possible to extrapolate the results to humans. It is appropriate to conduct research on two different species, one of which is not a rodent. Therefore, in our laboratories, research is usually carried out on zebrafish ( Danio rerio ) and small rodents (mice and rats), also due to their low costs. However, a sufficiently large number of animals is needed in order to obtain a statistical result. In extremely important research, despite the high financial outlay, and other difficulties associated with it, substances are tested on dogs and primates. Another important issue is the selection of appropriate pharmacological tests and models. These tests and models must be relevant; that is, they should predict the intended therapeutic indications. A pharmacological model can be considered relevant or correlational if the effects obtained correlate with the results observed in human therapy. To be significant or “correlated”, a model must meet three basic criteria: firstly, the model must be sensitive in a dose-dependent fashion to standard compounds that are known to offer the desired therapeutic property; secondly, the relative potency of the known active agents in the model should be comparable to their relative potency in clinical use; and, thirdly, the model should be selective; that is, the effects of the known agents in this therapeutic indication should be distinguishable from the effects of drugs for other indications. Positive data with a new compound allow the prediction of a therapeutic effect in patients.
5.1. Rodent Experiments, the 3R Rule
Concerning pre-clinical studies, scientists can employ many models and tests, using rodents, to evaluate, among others, anxiety, depression, schizophrenia-like behavior, antinociception, locomotor activity and motor coordination, stress-induced abnormalities, epilepsy, or rewarding properties of newly-synthesized or natural products, e.g., drug candidates. After a pharmacological and toxicological screening, a non-toxic dose can be chosen for further testing, after many different routes of administration, e.g., intraperitoneally (i.p.) or subcutaneously (s.c.). The use of animals for experiments relating to the development of medical science has been an integral aspect for many years. This fact, however, often raises ethical questions, as, for the most part, interference with a living organism involves a violation of the welfare, i.e., the quality of life, of the animal. An experiment that is not carefully thought out can cause additional suffering, as well as pain, which is a strong stress factor [ 142 ]. Here, there is a conflict between the duty to look after people’s health, improve their social wellbeing, and benefit from science, and the environmental protection associated with preventing animal suffering [ 143 ]. In a report on the number of animals used for scientific purposes in the countries of the European Union, compiled in 1991 by the European Commission, we can find that this was 11.79 million animals in the 10 member countries. A 2008 report stated that this was now 12 million in the 27 member countries, which was followed by 9.58 million in 2017 [ 144 ]. The above data confirm the quantitative and qualitative development of alternative methods to animal models that has taken place in recent years. In some cases, these may even represent a better solution than studies conducted on mice and rats, the main model animals, whose body response often differs from that of humans. However, this is not always possible, and many studies still require the use of laboratory animals [ 145 ].
To reduce the number of unreflective in vivo experiments, and to make them more humane, in 1959, Rex L. Burch and William Russel developed the 3R principle, entitled ‘The principles of humane experimental technique’. It contains three guidelines:
- replacement ,
- reduction ,
- refinement .
These three rules involve increasing the use of alternative research methods to those using animals, reducing the number of animals used for scientific purposes altogether, and minimizing suffering, pain, and injury. At its core is an overall improvement in animal welfare, which can be understood as animals remaining healthy, well-nourished, and living in a friendly environment, possibly free from factors that are a source of stress or fear, and motivated to perform [ 146 ]. Considering the 3R rule, researchers take a more responsible approach to planning and conducting experiments [ 142 ]. Today, this principle is widely used by most countries in the world, and supported by organizations such as the World Organization for Animal Health, and the European Council [ 147 ]. The 3R principle also formed the basis for the European Parliament’s adoption of Directive 2010/63 regarding the protection of the interests of experimental animals. It now represents the standard for information on the use of animals for scientific purposes, as, in addition to members of the European Union, other independent countries have also brought this document into force, so we can say that the directive is implemented in two-thirds of European countries [ 144 ].
The first of the three Rs represents replacement. The aim of this is to use methods that do not require the use of animals, or to replace vertebrates with other research material, such as higher plants, vertebrate embryos, micro-organisms, or cell or tissue cultures. Examples include the use of human- or animal-derived tissues or cells, and the utilization of invertebrates, such as the fruit fly Drosophila melanogaster , or the worm Caenorhabditis elegans . Computer methods and mathematical models are also an alternative [ 142 ]. Nowadays, the possibilities for replacing animal models are increasingly promising. However, the in vitro system is much less complex than the organism, and does not offer the possibility of directly replacing the animal model, so combinations of different methods are often needed. These, however, do not always cover all aspects of the human biological system, with the result that tests conducted in vitro must often be repeated in vivo. Additionally, for the registration of medicinal products, prior animal experiments are required. These make it possible to determine the toxicity, teratogenicity, likely effects on the embryo, fetus, and offspring in subsequent generations. Therefore, although there is a legitimate need to replace laboratory animals in research, a universal solution has still not been developed that offers this possibility for every type of experiment [ 148 , 149 ].
Another rule of thumb is to reduce the number of animals used in a study. The use of too few animals, however, may lead to difficulties in the proper processing of the results, and the correct statistical analysis of the data obtained. Moreover, it is recognized that the suffering of each animal used should be minimized, even if this involves using more animals in the test. The answer to these concerns is to strike a balance between reducing the total number of animals allocated to an experiment, and obtaining reliable results. To achieve this, it is necessary to improve the design of the experiment itself, as well as the statistical analysis, by sharing results and materials between groups of investigators. To facilitate this process, various online tools are being developed, such as the Experimental Design Assistant website and application, which further assist researchers in designing and reporting their experiments. A reduction in the number of animals used by up to several tens of per cent can also be achieved through the use of new research technologies. They provide a source of objective and quantifiable data, as well as support with research [ 142 , 150 ].
A continuous improvement of research methods is at the heart of maintaining adequate welfare for laboratory animals. This includes the use of in vivo technologies that reduce pain and stress in animals. Examples include the ability to collect and analyze smaller volumes of samples, such as blood, plasma, or serum. This is particularly applicable in the case of small rodents, as it allows several samples to be taken from the same animal, thus minimizing the number of individuals in the group, and avoiding the need to kill the animal. Additionally, the use of analgesics, and habituating the animals to certain practices, can improve their welfare. However, improvement concerns not only the conditions under which the experiment is conducted, but also the conditions of maintenance. Therefore, the proper handling of the animals in husbandry and minor procedures is very important, due to the possible occurrence of stress in the animals, which, in further stages, may translate into differentiated experimental results [ 142 , 150 ]. The animals’ habitat itself, and the satisfaction of natural needs, are no less important as factors. For this reason, in the case of rodents, nesting material, toys, tunnels, or wooden elements are placed in cages as their habitat. Due to their herd instinct, if the specifications of the experiment do not indicate otherwise, the animals are reared in groups [ 151 ].
5.2. Zebrafish Model
As stated, various animal species are used in pre-clinical studies. Although mice and rats are the most popular, zebrafish are growing in importance.
Danio rerio (the zebrafish) is a freshwater tropical fish of small size (4–5 cm). Its natural habitat is rivers in India. The body is spindle-shaped and laterally flattened. The name (zebrafish) is associated with the presence of horizontal blue stripes on the sides of the body, resembling zebra stripes. There is sexual dimorphism. The male is slimmer, has golden stripes between the blue ones, and an orange color on the fins. The female has a larger, whitish belly, and silver stripes instead of gold. In addition, in adult females, a genital wart is located in front of the caudal fin [ 152 , 153 , 154 ].
Recently, there has been a tremendous increase in the use of the zebrafish in modelling human diseases, because of the numerous advantages it offers. Changes in the law to reduce the suffering of animals during scientific research have made the zebrafish a popular alternative to previously used mammals [ 150 ]. This is due to the ease of breeding zebrafish, their rapid development, and their ex vivo fertilization.
For scientific purposes, zebrafish are grown in laboratory aquaculture under controlled conditions. These animals, before being sent for scientific research, obtain a health certificate confirming the fact that they are free from disease. Despite this, all fish are quarantined prior to testing [ 155 ]. Following ex vivo fertilization, there is the rapid development of a translucent embryo, with developed segmental muscles, organs, and a brain beginning to form. After 48–72 h, a free-floating larva hatches, transforming into a juvenile, which reaches sexual maturity after about 3–4 months. Danio rerio lay about 200 eggs a week. The eggs develop externally, and are small (ca. 4 mm at 5 days post-fertilization, dpf). This makes it easy to array embryos and larvae in 96-well microtiter plates for high-throughput drug screening [ 152 , 156 ]. The embryos are translucent, which allows the microscopic examination of very early developmental processes that cannot be observed in mammals, due to our development inside the uterus [ 157 ]. As vertebrates, these fish show a similarity to humans in their tissues and organs, and the processes taking place in them. Of particular importance is the analogy in the structure of the brain and the nervous system, and their functions. Recently, the zebrafish has emerged as a valuable and attractive model for various neurological disorders, including schizophrenia [ 158 , 159 ]. These fish have the ability to regenerate cells and tissues: brain, spinal cord, heart, retina, photoreceptors, and fins. Research is being undertaken on the possibility of using this ability in retinal diseases of various etiologies [ 160 ]. Another important feature is the convergence of the zebrafish genome with the human one. Danio rerio has 25 chromosomes with an approximately 70% homology to human genes. The genetic information of zebrafish can be modified. The resulting phenotypically mutated individuals correspond to true human disease states, and include ethology, disease progression, and resolution. This has contributed to the wider use of these fish in pre-clinical studies of various diseases with the use of new chemicals and existing drugs in indications other than the original ones. These studies can be performed on both embryos and larvae. This gives the opportunity to better understand the action of the drug, determine its pharmacokinetics and pharmacodynamics, and obtain the necessary information for the next stages of research [ 156 ]. Numerous advantages of Danio rerio have allowed the creation of models of many diseases. Several cancer models have been created, including leukemia, melanoma, cardiovascular disease, the immune system, infectious diseases, diabetes, obesity, and behavioral disorders [ 161 , 162 ]. This has contributed to progress in many fields of science, including oncology and toxicology. New studies with zebrafish and new substances with medicinal properties are being modelled all the time [ 161 ]. The disadvantages of these models include problems with the standardization of the test and its reproducibility, as well as problems with the administration of the substance. Most often, they are dissolved in the water in which the fish swim. This poses a risk to them, and may also pose a problem with regard to the data obtained for larger vertebrates [ 163 ].
Numerous advantages of zebrafish have made them more and more often used in pre-clinical and toxicological studies on various substances. The versatility of this model has contributed to significant developments in biology, medicine, and pharmacology, as well as the discovery of new drugs, and new applications for those already known [ 164 ].
6. Clinical Trials—General Information
Promising results of pre-clinical studies allow the drug to be admitted to the next stage of testing—clinical trials, which are carried out on human volunteers. The purpose of clinical trials is to assess the pharmacological and/or pharmacodynamic effects, and to determine the adverse events (AEs), of the tested chemical compound. Clinical trials are conducted in accordance with the Good Clinical Practice (GCP) guidelines developed by the International Council for Harmonization (ICH). GCP is a set of legal and ethical standards that strictly defines the method of planning, conducting, monitoring, documenting, and reporting clinical trial results. In the clinical trial planning phase, an independent bioethical committee must issue an opinion on the project, and each subsequent stage of the trial must be carried out with the utmost care for the patient’s wellbeing. The superior document in clinical trials is the clinical study protocol, which defines the organization and course of the trial, as well as all procedures that should be performed as part of it. A patient (referred to as a study participant in a clinical trial) voluntarily joining a clinical trial is required to sign the Informed Consent Form (ICF), the second most important trial document, which defines, among other things, the trial participant’s rights and obligations, as well as the risks and benefits associated with the trial. It should be emphasized that the study participant enters the clinical trial fully consciously, and the new drug (referred to as the investigational product or study drug) may, but does not have to, help to alleviate the symptoms of the disease. Therefore, the study participant has the option to withdraw from the study at any time, without giving a reason [ 165 ].
Most clinical trials are randomized, which means that study participants are randomly assigned to treatment groups: each person has the same probability of being assigned to a study drug or placebo cohort. In open-label clinical trials, both the patient and the physician (referred to as the “Investigator” in clinical trials) know what substance is administered, but this may affect the reliability of the results. Therefore, most studies are double-blind, which, in practice means that neither the study participant nor the investigator has any information as to which treatment group the participant has been assigned to [ 165 ]. There are five main phases in clinical trials ( Figure 3 ).

The phases of clinical trials.
These are preliminary human studies to confirm the results of pre-clinical studies. Subtherapeutic doses of the drug are used on a small group of healthy volunteers. Phase 0 studies are aimed at deepening information on the mechanism of action of the drug, and the processes it undergoes after entering the body. This phase of clinical trials typically involves a group of between 10 and 15 healthy volunteers. However, it is often skipped in favor of Phase I clinical trials [ 166 , 167 ].
If Phase 0 is skipped, this is the first-in-human (FIH) phase. It is carried out on healthy volunteers, usually men. In the event that the study drug may be potentially toxic (e.g., in cancer), the phase I study is carried out on sick people. This part of the study is primarily used to assess the safety of the drug, and determine the expected dosage. Phase I studies also provide valuable pharmacokinetic data on absorption, metabolism, excretion, and potential interactions. Typically, between 50 and 100 healthy volunteers participate in this phase of clinical trials [ 166 , 167 ].
The next stage of clinical trials concerns people diagnosed with the disease. Patients must meet all of the inclusion criteria in the study protocol, and not meet any of the exclusion criteria. The protocol assumes, among other things, what age potential patients should be, what diseases they can/cannot suffer from, and what concomitant medications they can/cannot take. Phase II usually involve a small number of patients, and the main purposes of the trials are to confirm the safety of the investigational product, and to obtain data on its effectiveness. This phase usually involves a group of between 100 and 300 patients who are suffering from the disease [ 166 , 167 ].
The success of Phase II enables the next stage of testing, which is Phase III. Studies are carried out on a larger population, and in many clinical research centers around the world. The duration of Phase III varies, and depends on the information contained in the protocol; usually, the study lasts several years. The purpose of this research is to determine the optimal dose, and to confirm the effectiveness of the study drug in a much larger group of people, from several hundred to several thousand patients. A positive result in Phase III enables the registration of a new drug [ 166 , 167 ].
Phase IV is the time after the drug is placed on the market. This phase of studies allows for the assessment of efficacy and AEs regarding the long-term use of the drug by thousands of patients around the world. During the observation of drug activity, the results of the previous phases of the study are verified. New indications for drug administration are also sought [ 166 , 167 ].
7. Conclusions
Drug research on new, potentially effective drugs, including vaccines, is one of the goals of the medical sciences, and constitutes an integral part of national and transnational health support programs. Analyses across all therapeutic areas indicate that the development of new medicine, from molecule synthesis, to target identification and approval for marketing, takes over 12 years [ 168 ]. Despite advances in biotechnology, this process is lengthy, costly, and difficult. Any advances or progress in science and biotechnology immediately find an application in medicine, in pharmacy, and in drug discovery and development. Investments in drug design are worthwhile, because the better a given drug candidate is designed during the experimental stage, the less likely it is for the drug to fail in the late stages, where the tests are more expensive, especially in clinical trials, and the safer and more effective it will be. The COVID-19 pandemic forced scientists to rethink how to accelerate the timelines of the discovery and development of drugs and vaccines, as an example. Modern, effective, and less costly methods for drug discovery are required, including artificial intelligence, which is able to gather and analyze large amounts of data in a short time, to select appropriate targets and complementary ligands, to design and perform tests. The ultimate goal for future drug development is to be able to design and develop a specific, non-toxic, effective, and patient-tailored drug over a period of several hours. Although this goal seems fantastical at the moment, it is completely achievable in the near future.
Funding Statement
This review is a part of an international project, “Education and research in the field of drug design and development within the V4 region” (no. 22130144), financed by the International Visegrád Fund.
Author Contributions
Conceptualization, G.B., E.K., J.O.-G. and M.K.-S.; methodology, E.H., G.B., E.K., J.O.-G., M.K.-S., D.N. and I.M.; literature review, D.N., I.M., S.H., A.S., J.K. and E.H.; validation, G.B., E.H. and I.M.; formal analysis, D.N., I.M., E.H., G.B., E.K., J.O.-G. and M.K.-S.; investigation, D.N., I.M., E.H., G.B., E.K., J.O.-G. and M.K-S.; resources, G.B.; data curation, D.N., I.M., E.H., G.B., E.K., J.O.-G. and M.K.-S.; writing—original draft preparation, D.N., I.M., E.H., G.B., E.K., J.O.-G., M.K.-S., S.H., A.S. and J.K.; writing—review and editing, D.N., I.M., E.H., G.B., E.K., J.O.-G. and M.K.-S.; visualization, D.N., I.M., E.H., G.B., E.K., J.O.-G. and M.K.-S.; supervision, G.B.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
Drug Development Research is an interdisciplinary pharmacology journal publishing papers and reviews covering all areas of drug development, including medicinal and process chemistry, biotechnology and biopharmaceuticals, toxicology, drug delivery, formulation, pharmacokinetics, and clinical trial reviews. Since 1981, we serve a diverse research community including pharmacologists, pharmacists ...
Drug development articles from across Nature Portfolio. Drug development describes the process of developing a new drug that effectively targets a specific weakness in a cell. This process ...
Drug discovery articles from across Nature Portfolio. Atom; ... Computational models that require very little data could transform biomedical and drug development research in Africa, as long as ...
From target discovery to clinical drug development with human genetics. This Review provides a perspective on the development of non-cancer therapies based on human genetics studies and suggests ...
Drug Development Research will feature Editor's Choice Articles. The Editor-in-Chief will highlight one paper per issue chosen based on the impact of the topic and scientific quality. Each selected article will be freely available to read for a period of 1 month post publication. Find the Editor's Choice Articles here.
Abstract. A new medicine will take an average of 10-15 years and more than US$2 billion before it can reach the pharmacy shelf. Traditionally, drug discovery relied on natural products as the main source of new drug entities, but was later shifted toward high-throughput synthesis and combinatorial chemistry-based development.
Drug development and discovery includes preclinical research on cell-based and animal models and clinical trials on humans, and finally move forward to the step of obtaining regulatory approval in order to market the drug. ... It covered seventeen research articles and one communication contributed from experts all around the world, as briefed ...
AIMS & SCOPE. Drug Development Research publishes research papers and review-type articles covering all area within drug development: from target identification and validation and structure-activity relationship studies, through to post-market clinical reports. Topics covered include but are not limited to medicinal and process chemistry ...
This article provides a brief overview of the processes of drug discovery and development. Our aim is to help scientists whose research may be relevant to drug discovery and/or development to frame their research report in a way that appropriately places their findings within the drug discovery and development process and thereby support effective translation of preclinical research to humans.
The development of drugs (both small molecules and biologics) intended to treat advanced cancer is guided by ICH S9. 1 Analysis of multiple development packages supporting oncology programs has demonstrated that 1-month repeat-dose studies in 2 species are generally adequate to support clinical development of oncology products through phase 2.
The process for a drug/vaccine starts with Research and Development and Preclinical animal testing. Most of the drugs will not progress from this stage or early study phases, as drugs and vaccines must show that they are stable compounds, properly absorbed, delivered to target site, and function for noted indication, while showing positive ...
This process takes an average of 10-15 years. After accounting for the costs of failed trials, the median capitalized research and development investment to bring a new drug to market has been estimated at $985.3 million (95% CI, $683.6-$1228.9 million), and the mean investment was estimated at $1335.9 million (95% CI, $1042.5-$1637.5 ...
Drug discovery and development articles from across Nature Portfolio. Atom; RSS Feed; Definition. Drug discovery and development together are the complete process of identifying a new drug and ...
Read the latest articles of Letters in Drug Design & Discovery at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
The use of artificial intelligence (AI) has been increasing in various sectors of society, particularly the pharmaceutical industry. In this review, we highlight the use of AI in diverse sectors of the pharmaceutical industry, including drug discovery and development, drug repurposing, improving pharmaceutical productivity, and clinical trials, among others; such use reduces the human workload ...
Read the latest Research articles in Drug discovery and development from Nature. ... United Kingdom has cut drug-development research jobs over past decade. Outlook | 11 May 2016.
Therefore, this article focuses on the anti-inflammatory effect of AST, conducts research on relevant literature from 2003 to 2023, and reviews the alleviating effects and molecular mechanisms of AST on various inflammation-related cell and animal models, aiming to provide ideas and references for its subsequent research and the development of ...
The impact of M&A on the pharmaceutical landscape extends far beyond individual companies. mergers can: Increase Consolidation: With fewer players, the industry becomes more concentrated, potentially leading to less competition and higher drug prices. hift Research Focus: M&A can influence what gets prioritized in research and development.
Officials in Delaware gave the company $19 million in tax funds in 2021 to build a research and drug manufacturing site that is expected to employ about 1,000 people when fully operational next ...
Emerging drugs have unpredictable health effects. They may be as powerful or more powerful than existing drugs, and may be fatal. Because drug markets change quickly, NIDA supports the National Drug Early Warning System (NDEWS), which tracks emerging substances. NIDA also advances the science on emerging drugs by supporting research on their ...
Drug Development Research is an interdisciplinary pharmacology journal publishing papers and reviews covering all areas of drug development, including medicinal and process chemistry, biotechnology and biopharmaceuticals, toxicology, drug delivery, formulation, pharmacokinetics, and clinical trial reviews. Since 1981, we serve a diverse research community including pharmacologists, pharmacists ...
Brain Development. Drug experimentation is commonly initiated during adolescence and the risk to addiction is increased with early drug use. The greater vulnerability of adolescents to drug use and experimentation is driven by multiple factors including genetics that are associated with the developmental trajectories of the human brain ...
Professor Graeme Laver was behind a major influenza discovery in the 1970s when he began testing birds on Tryon Island for the flu. His research led to the development of drugs like Tamiflu.
Take Centocor, a company founded in 1979. It took nearly 20 years before turning hybridoma technology into market success with the mAb Remicade (infliximab). Along the way, the company faced ...
While many Americans say they have used marijuana in their lifetime, far fewer are current users, according to the same survey. In 2022, 23.0% of adults said they had used the drug in the past year, while 15.9% said they had used it in the past month. While many Americans say legalizing recreational marijuana has economic and criminal justice ...
Drug research on new, potentially effective drugs, including vaccines, is one of the goals of the medical sciences, and constitutes an integral part of national and transnational health support programs. ... The ultimate goal for future drug development is to be able to design and develop a specific, non-toxic, effective, and patient-tailored ...