- Undergraduate
- High School
- Architecture
- American History
- Asian History
- Antique Literature
- American Literature
- Asian Literature
- Classic English Literature
- World Literature
- Creative Writing
- Linguistics
- Criminal Justice
- Legal Issues
- Anthropology
- Archaeology
- Political Science
- World Affairs
- African-American Studies
- East European Studies
- Latin-American Studies
- Native-American Studies
- West European Studies
- Family and Consumer Science
- Social Issues
- Women and Gender Studies
- Social Work
- Natural Sciences
- Pharmacology
- Earth science
- Agriculture
- Agricultural Studies
- Computer Science
- IT Management
- Mathematics
- Investments
- Engineering and Technology
- Engineering
- Aeronautics
- Medicine and Health
- Alternative Medicine
- Communications and Media
- Advertising
- Communication Strategies
- Public Relations
- Educational Theories
- Teacher's Career
- Chicago/Turabian
- Company Analysis
- Education Theories
- Shakespeare
- Canadian Studies
- Food Safety
- Relation of Global Warming and Extreme Weather Condition
- Movie Review

Admission Essay
- Annotated Bibliography
- Application Essay
- Article Critique
- Article Review
- Article Writing
- Book Review
- Business Plan
- Business Proposal
- Capstone Project
- Cover Letter
- Creative Essay
- Dissertation
- Dissertation - Abstract
- Dissertation - Conclusion
- Dissertation - Discussion
- Dissertation - Hypothesis
- Dissertation - Introduction
- Dissertation - Literature
- Dissertation - Methodology
- Dissertation - Results
- GCSE Coursework
- Grant Proposal
- Marketing Plan
- Multiple Choice Quiz
- Personal Statement
- Power Point Presentation
- Power Point Presentation With Speaker Notes
- Questionnaire
- Reaction Paper
- Research Paper
- Research Proposal
- SWOT analysis
- Thesis Paper
- Online Quiz
- Literature Review
- Movie Analysis
- Statistics problem
- Math Problem
- All papers examples
- How It Works
- Money Back Policy
- Terms of Use
- Privacy Policy
- We Are Hiring
Medical Lab Science, Admission Essay Example
Pages: 1
Words: 300
Hire a Writer for Custom Admission Essay
Use 10% Off Discount: "custom10" in 1 Click 👇
You are free to use it as an inspiration or a source for your own work.
A career in clinical laboratory science requires dedication, knowledge, commitment, and an analytical approach to conducting research. Clinical laboratory science is a challenging and unique field because it supports the potential development of new discoveries that facilitate clinical medicine. My career path has led me to an interest in clinical laboratory science because it will give me an opportunity to utilize my education and training to advance the field and the greater good of the general public. My desire to gain future employment in clinical laboratory science will support my desire to explore problems and developing solutions using an experimental approach. In this field, I will work collaboratively with other scientists and clinicians to develop new discoveries and facilitate clinical studies in a manner that is consistent with moral and ethical standards. I believe that clinical laboratory science supports an opportunity to establish new perspectives to solve problems and support the advancement of clinical research and science.
I am confident that my education and training will serve me well as I enter the field of clinical laboratory science because I am focused, driven, and motivated to advance practice methods and techniques to support the health and wellbeing of the population. I will also continue to learn and expand my skill set to understand the full scope if clinical laboratory science in the modern era. I consider clinical laboratory science to be one of the most critical areas of medicine because it supports the ongoing development of diagnostic tools and approaches to ensure that healthcare offerings are successful and accurate at all times. The opportunities that are available through clinical research will provide me with a basis for continued growth and knowledge in my career path that will also contribute to the growth and success of the field and its objectives.
Stuck with your Admission Essay?
Get in touch with one of our experts for instant help!
Marilyn Monroe, Essay Example
New York State Practice Act, Essay Example
Time is precious
don’t waste it!
Plagiarism-free guarantee
Privacy guarantee
Secure checkout
Money back guarantee

Related Admission Essay Samples & Examples
Clinical leadership, admission essay example.
Pages: 4
Words: 1153
A Health Care Provider, Admission Essay Example
Pages: 3
Words: 739
London School of Economics, Admission Essay Example
Pages: 2
Words: 570
Family Nurse Practitioner, Admission Essay Example
Words: 479
Academy of Medical Science Technology, Admission Essay Example
Words: 596
St. James School of Medicines, Admission Essay Example
Words: 614
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Clinical laboratory.
Marlon L. Bayot ; John E. Lopes ; Muhammad Zubair ; Prisha Naidoo .
Affiliations
Last Update: January 26, 2024 .
- Definition/Introduction
Clinical laboratories are healthcare facilities providing a wide range of laboratory procedures that aid clinicians in diagnosing, treating, and managing patients. [1] These laboratories are manned by scientists trained to perform and analyze tests on samples of biological specimens collected from patients.
In addition, clinical laboratories may employ pathologists, clinical biochemists, laboratory assistants, laboratory managers, biomedical scientists, medical laboratory technicians, medical laboratory assistants, phlebotomists, and histology technicians. [2] Most clinical laboratories are situated within or near hospital facilities to provide access to clinicians and their patients. [1]
Classifications of clinical laboratories indicated below reveal that these facilities provide quality laboratory tests that are significant for addressing medical and public health needs.
The list below is non-exhaustive as new laboratory models are emerging:
- Government (usually part of hospitals and medical centers under the Department of Pathology or Laboratory Medicine)
- Private (part of a medical or healthcare institution)
- General clinical laboratories provide standard diagnostic laboratory tests
- Specialty laboratories provide less commonly used diagnostic and confirmatory tests
- Clinical chemistry
- Clinical Microbiology
- Blood banking and serology (ie, Immunohematology, Transfusion medicine)
- Histopathology and cytopathology
- Molecular biology
- Public health: providing tests such as water analysis and testing for environmental toxins
- Peripheral laboratories provide routine screening, diagnostic (eg, conventional and rapid diagnostic tests), and follow-up tests for patients, usually within the local community [3]
- May conduct additional tests than those provided in peripheral laboratories and can serve as referral laboratories for special cases.
- Aside from performing tests, they perform management and supervisory tasks under specific areas of jurisdiction. [4]
- Policy and program implementation
- Training and development
- Monitoring, evaluation, and research [1]
In the past, the value of clinical laboratories as an integral part of the healthcare system was unrecognized. [5] Over time, more clinicians have recognized the need for laboratory tests to confirm their diagnoses and support monitoring patient response to therapy. [6] Aside from value to individual patients, clinical laboratories were also used for screening and surveillance of diseases. On a larger scale, program managers used some relevant tests as surrogate indicators to assess the progress of public, international, and global health programs. [7]
Laboratory networks were developed across countries and states to foster coordination and collaboration within the specified geographic areas. [8] Quality management systems within these laboratories have recently become significant issues, including standardization of laboratory services, strengthening laboratory systems, and developing new and rapid diagnostic tools. These issues are continually addressed by local and international health authorities and technical experts employing a patient-centered approach.
Clinical laboratories perform testing logically and strictly. Generally, there are 3 phases of the laboratory testing process that each facility should follow. Standard operating procedure manuals and job aids are written for guidance for each phase step: pre-analytical, analytical, and post-analytical. [9] The pre-analytical phase is critical, with over 60% to 70% of laboratory errors occurring in this phase. [10]
Clinical laboratory professionals have embraced technology over the years to derive answers to clinical questions. Modern clinical laboratories use technologies, including spectrophotometry, atomic absorption spectroscopy, cytometry, flame emission photometry, nephelometry, electrochemical, optical sensors, electrophoresis, and chromatography.
- Spectrophotometry is a technique used to measure the absorbance of colored compounds in solution, helping to identify and quantify various substances in blood and body fluids. [11]
- Atomic absorption spectroscopy (AAS): a vital tool in clinical analysis, enabling the measurement of metallic element concentrations within biological fluids and tissues like whole blood, plasma, urine, saliva, brain tissue, liver, hair, and muscle tissue. [12]
- Cytometry is a technique to measure the properties of individual cells, such as size, shape, and DNA content, which can help diagnose and monitor conditions like cancer or genetic disorders. [13]
- Flame emission photometry: a technique to measure the emission of light from a sample excited by a flame, helping to identify and quantify compounds in blood and body fluids. [14]
- Nephelometry is a technique to measure the turbidity of a solution, which helps diagnose and monitor conditions like liver disease or kidney failure. [15]
- Electrochemical technologies are used to measure the electrical properties of a solution, such as pH, conductivity, and redox potential, which help diagnose and monitor conditions like acid-base disorders or electrolyte imbalances. [16]
- Optical sensor technologies: use sensors that detect and measure various properties of a sample using light, such as refractive index or fluorescence, which helps identify and quantify various substances in biological fluids. [17]
- Electrophoresis is a technique to separate and analyze proteins in a sample, which helps diagnose and monitor conditions like multiple myeloma or amyotrophic lateral sclerosis. [18]
- Chromatography is a technique that helps identify and quantify different components in blood and bodily fluids by separating and analyzing compounds in a sample according to their molecular properties, such as size, charge, or shape. [19]
The landscape of clinical laboratory operations has transformed due to the integration of automation, impacting both the analytical and non-analytical aspects. This transition towards automation commenced over 5 decades ago, focusing on automating laboratory test procedures. [20] However, the true leap occurred in the 1990s when non-analytical automation gained momentum, featuring conveyor systems, interfaced analyzers, and automated specimen processing and storage. Automation in the clinical laboratory is classified into 3 categories: manual, stand-alone automation (modular), and total lab automation (TLA). [21]
Automation has a wide-ranging impact, improving laboratory ordering, testing, and reporting processes while eliminating tedious and time-consuming chores. [22] It has ushered in a new era of heightened productivity by streamlining the use of reagents and materials, standardizing operations, and reducing the occurrence of outliers. The efficiency increases production rates and improves accuracy and precision in test results. Automation is a cornerstone in modern clinical laboratories, revolutionizing operations and elevating the overall quality of laboratory testing. [23]
Clinical laboratory specialists perform an array of tasks, including developing and validating new laboratory tests, assessing and defining the analytical and clinical performance, conveying laboratory results to clinicians, offering valuable education and guidance to the clinical team, evaluating the cost-effectiveness and intrinsic value, ensuring strict compliance with regulatory standards, engaging in quality assurance measures, and participating in both basic and clinical research endeavors. [24]
The laboratory professional must maintain the confidentiality of medical information, use resources appropriately, abide by codes of conduct, follow ethical publishing rules, and manage and disclose conflicts of interest. [25]
- Issues of Concern
Providing high-quality diagnostic testing is the goal of all clinical laboratories. Improving laboratory capacity is crucial to address various issues and problems. Managing resources, training, supervision, planning, budgeting, quality assurance, logistics and supply, and biosafety and equipment management are necessary to optimize laboratory services provided to patients. [25]
In 2018, the World Health Organization developed and released the Essential Diagnostics List (EDL). This list was expected to align the health community to the accessibility and availability of high-quality testing of clinical laboratories, especially in resource-limited settings. [26] Using the EDL with essential medicines list (EML), authorities can now focus their efforts so that people receive the necessary laboratory services. [27]
Accreditation for clinical laboratories was recently relevant due to the emergence of international laboratory standards. Several guidelines for laboratories have been developed to regulate laboratory test procedures and maintain their quality. [28] An example of laboratory accreditation is the ISO 15189 provided by the International Organization for Standardization (ISO), which focuses on meeting the requirements for the quality and competence of medical laboratories. [29] Another example is biosafety guidelines around microbiological agents such as bacteria, viruses, parasites, and microbiological products. [30]
The need for risk management in clinical laboratories was highlighted to maintain the accuracy and reliability of laboratory tests. The Clinical Laboratory Standards Institute (CLSI) developed a guideline to introduce risk management principles specifically in the clinical laboratory. [31] From risk assessment to risk analysis, evaluation, and control to continuous quality improvement, the clinical laboratory should be able to minimize errors along its path of the workflow (ie, preanalytic, analytic, and postanalytic phases). Significant risks such as specimen collection, processing, and disposal of laboratory wastes should be considered. [32]
A laboratory information system (LIS) is valuable in managing results and other pertinent information regarding patients and their samples. [33] The development of a laboratory information system started in the 1960s, concentrating on data reduction, analog-digital conversion, and radioimmunoassay analysis. Recently, the focus has evolved into digital histopathology and genomics, issues about patient access to data, and more. [34] In a rapidly changing environment for the modalities of patient record systems, there is a need for collaboration between clinical systems developers and laboratory-based informaticians to modify and improve the existing technology to meet patient needs.
- Clinical Significance
As the challenges faced by clinical laboratories rise, clinicians should be aware of the impact on their patients. While patients and people in the community are not well aware, the function and mandate of clinical laboratories remain the same: the provision of high-quality laboratory diagnostic tests.
Improving existing laboratory services should not be overlooked when developing newer diagnostic tests. [35] Health authorities at the global level and stakeholders, including clinicians, experts, and other healthcare professionals at the local level, must recognize that clinical laboratories affect the most important clients of healthcare: patients. [36]
- Nursing, Allied Health, and Interprofessional Team Interventions
In the clinical laboratory setting, the collaborative efforts of various healthcare professionals, including physicians, advanced practitioners, nurses, pharmacists, and allied health experts, are instrumental in patient-centered care and outcomes. Nurses play a pivotal role by utilizing their skills in patient advocacy, attention to detail, and specimen collection. They contribute significantly to ethical considerations, ensuring patient confidentiality and dignity in all laboratory procedures. Alongside nurses, allied health professionals and pharmacists exhibit expertise in managing and interpreting laboratory results, informing diagnosis and treatment.
Interprofessional communication is a cornerstone, facilitating seamless critical information exchanges among team members, leading to enhanced care coordination and patient safety. This collaborative approach ensures the timely delivery of laboratory results to clinicians, empowering informed clinical decisions. As a result, the clinical laboratory becomes an integral part of the healthcare ecosystem, promoting patient-centered care, fostering improved team performance, and ultimately elevating patient outcomes.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Marlon Bayot declares no relevant financial relationships with ineligible companies.
Disclosure: John Lopes declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Zubair declares no relevant financial relationships with ineligible companies.
Disclosure: Prisha Naidoo declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Bayot ML, Lopes JE, Zubair M, et al. Clinical Laboratory. [Updated 2024 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Preference of saliva over other body fluids as samples for clinical and laboratory investigations among healthcare workers in Ibadan, Nigeria. [Pan Afr Med J. 2019] Preference of saliva over other body fluids as samples for clinical and laboratory investigations among healthcare workers in Ibadan, Nigeria. Lasisi TJ, Lawal FB. Pan Afr Med J. 2019; 34:191. Epub 2019 Dec 11.
- [Mycobacterial tests]. [Kekkaku. 2008] [Mycobacterial tests]. Takashima T, Higuchi T. Kekkaku. 2008 Jan; 83(1):43-59.
- Assessment of laboratory logistics management information system practice for HIV/AIDS and tuberculosis laboratory commodities in selected public health facilities in Addis Ababa, Ethiopia. [Pan Afr Med J. 2013] Assessment of laboratory logistics management information system practice for HIV/AIDS and tuberculosis laboratory commodities in selected public health facilities in Addis Ababa, Ethiopia. Desale A, Taye B, Belay G, Nigatu A. Pan Afr Med J. 2013; 15:46. Epub 2013 Jun 8.
- Review Value-added laboratory medicine in an era of managed care. [Clin Chem. 1995] Review Value-added laboratory medicine in an era of managed care. McDonald JM, Smith JA. Clin Chem. 1995 Aug; 41(8 Pt 2):1256-62.
- Review [View of a Laboratory Physician on the Present and Future of Clinical Laboratories]. [Rinsho Byori. 2014] Review [View of a Laboratory Physician on the Present and Future of Clinical Laboratories]. Matsuo S. Rinsho Byori. 2014 Oct; 62(10):988-92.
Recent Activity
- Clinical Laboratory - StatPearls Clinical Laboratory - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Importance of Clinical Laboratory Science Research Paper
Clinical laboratory science is very essential in the diagnosis of infections and disorders. It provides information that guides in the collection and examination of a specimen. The results obtained enable doctors and other physicians to make the accurate diagnosis and therefore design the appropriate drug therapy for the patients. Some diseases like rheumatoid arthritis are interesting to learn due to their unique laboratory tests performed. Anti- cyclic citrullinated peptide test is carried out to check presence or absence of anti- cyclic citrullinated peptide antibodies. This test is positive for people who are prone to these diseases. Such diseases have promoted research in this department, enabling more accurate diagnosis and therefore treatment of patients.
Point of Care Testing
This is the laboratory testing of patients in operating rooms, recovery areas, patient’s bedside, or home testing. This has increased the interaction of laboratory physicians, respiratory therapists, nurses, assistant physicians, operating room technologists, and anaesthesiologists. It provides cost effective methods and advancements in medical care and enables an immediate and accurate assessment in intensive care units or parent units, emergency departments, and operating rooms. Tests performed mostly are analytical, such as prothrombin time coagulation test, partial thromboplastin time coagulation, and erythrocyte measurements like hematocrit. The regulations of CLIA’88 are followed to ensure accurate and quality results are obtained as well as quality control and proficiency testing is provided. The laboratory director ensures that all regulations are implemented and adhered to.
The safety of the patients and the laboratory personnel should be maintained. Most of laboratory accidents are usually caused by lack of paying attention to instructions, carelessness, and lack of concise and proper communication during the testing. By use of suitable mechanism, strategies, common sense, and staying alert can be used to minimize such accidents. There are bodies that are responsible for ensuring blood borne pathogen prevention and protection, and ensuring electrical, fire, and chemical safety is guaranteed which Health Administration, and Occupational Safety and other bodies. Musculoskeletal disorders are the most common physical, ergonomic stressors where muscles, nerves, tendons are affected, for example, tendinitis and sciatica.
Workers are vaccinated against hepatitis B, rubella, and measles infections. Conducive working conditions, education on first aid methods, and personal protective equipment are provided. The Joint Commission and National Patient Safety Goals reinforce patient safety methods. Therefore, errors in the pre-analytical phase, for example, in blood sample collection and patient identification, and analytical errors like verifying the accuracy of abnormal results are avoided as well as Post-analytical mistakes like communication of tests results are avoided.
This is the process of making a puncture in a vein, usually in the arm, with a cannula for the purpose of drawing blood; It is also referred as a venipuncture. Factors such as ambient temperature, altitude, humidity, and light are considered during this procedure. This procedure should be carried out by trained personnel to ensure the patient’s safety and that the correct results are obtained, and no complications like iatrogenic anemia arise. Anticoagulants such as ethylene diamine tetra-acetic acid prevent coagulation caused by fibrinogen. Blood cultures should be protected from contamination. Specimens are susceptible to clotting, hemolysis; icterus, and lipemia, in addition, tests performed are mostly pre-analytical. Laboratory and clinical standards institute are responsible for regulating laws and legislations governing phlebotomy.
Clinical Chemistry: Lipids
Lipids are biochemical molecules with cholesterol and triglycerides; they are plasma-bound, forming lipoproteins. They have functions in the body, for example, insulation to allow nerve impulses and heat loss, energy storage, structural components of cell membranes, and they act as hormones.70% of the cholesterol in the human body is from the liver, the rest if from dietary intake. High levels of cholesterol increase the risk of atherosclerotic disease. Cholesterol is an essential structural component of the animal cell membrane and precursor of bile acids and steroidal hormones.
The serum is the specimen used by a patient who has fasted for 12-15 hours. The instrument used is a serum separator evacuated tube, and an enzymatic cholesterol assay is carried out. The desirable amount is less than 180mg/dl, the moderate amount is between 200-239mg/dl, and those at high risk have levels of more than 240mg/dl. Familial lecithin cholesterol acyltransferase deficiency is a disease transmitted as a recessive trait. It is usually associated with hyperphospholipidemia and hypercholesterolemia with underlying conditions of hypertriglyceridemia.
Hematology: Leukemia
This is an uncontrollable and abnormal proliferation and progressive displacement of hematopoietic cells with standard cellular elements.They are classified morphologically into lymphocytic and myelogenous, they termed as acute or chronic, depending on the amount or number of blasts available. Examples include acute myelogenous leukemia, chronic myeloproliferative disorder, and lymphoblastic anemia. Flow cytometry identifies specific cluster designation and cell surface membrane markers. Christmas disease or Hemophilia B, also called factor IX (FIX) deficiency, is a genetic disorder caused by missing or defective factor IX.
Microbiology: Parasitology
This is the study of parasites, their hosts, and their relationship. Parasites such as Trichomonas vaginalis cause infection to their host. The trophozoite is tear-shaped, elongated, and with a jerky undulating motion. It is present in freshly voided urine in wet vaginal preparations and prostatic secretion and can be analyzed and reviewed under the microscope (Turgeon, 2018). A rapid antigen detection test is available; it is very easy and rapid conduct the OSOM Trichomonas Rapid Test which is a dipstick method. The TV system is used for culture and direct microscopic examination in Pouch.
Immunology: Autoimmune Disorders
This is the study of molecules, cells, and systems responsible for recognizing and disposing of non-self-substances. Autoimmune disorders include that body attacks healthy cells; they include systemic lupus and rheumatoid disorders.Rheumatoid arthritis is a chronic inflammatory disorder affecting joints structures and tissues. The manifestations can be articular and extra-articular. Juvenile rheumatoid arthritis is another chronic synovitis condition that affect chidren in their childhood stages.The immunofluorescent technique detects high immunoglobulin G, and immunoglobulin M. Identification of R factor in serum or synovial fluid is used for diagnosis. The higher the R factor, the more joint destruction there is. The R factor is determined by conducting a test known as Rapid Latex Agglutination.
Blood Banking: Platelets
Generally, patients who are bleeding excessively are subjected to platelets concentrates due to low platelet count, abnormal platelets, or after massive transfusions. Platelets are harvested by the process of plateletpheresis or from a single unit of fresh, whole blood. Plateletpheresis allows for a yield of 200 to 500mL of a single donor platelet unit with a minimum of 3.0×1011 platelets. They are prepared by centrifugation and removal of plasma from a new division of donor blood and subsequent separation of platelets from platelet-poor plasma.
They are stored at room temperature (20°C – 24°C) with continuous, gentle agitation for 5days, although bacterial contamination occurs sometimes. Generally, the process of blood banking usually takes place in the lab to ensure that the blood products or any donated blood are saved before they are engaged in medical procedures or blood transfusions. This process can be conducted by testing the presence of diseases in the blood or typing the blood before it is transmitted. Such an approach is fundamental during the patient care unit because it helps the physician avoid any medical-related errors before donating or transfusing the blood.
Coagulation: Therapeutic Anticoagulant Therapy
Coagulation is the process by which coagulation factors, plasma proteins, and calcium are subjected to work together on the platelets’ surface to make or form a strong fibrin clot.Therapeutic anticoagulation therapy uses glycoprotein receptor drugs such as abciximab, eptifibatide, aspirin and clopidogrel, intravenous antiplatelet membrane, and thrombolytics. Direct thrombin inhibitors (DTI) such as new oral anticoagulants, warfarin, a vitamin K antagonist analog, and anti-Xa anticoagulants such as heparin are also used.
Turgeon, M. L. (2018). Linne & Ringsrud’s Clinical Laboratory Science E-Book: Concepts, Procedures, and Clinical Applications . Elsevier Health Sciences.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, December 19). Importance of Clinical Laboratory Science. https://ivypanda.com/essays/importance-of-clinical-laboratory-science/
"Importance of Clinical Laboratory Science." IvyPanda , 19 Dec. 2022, ivypanda.com/essays/importance-of-clinical-laboratory-science/.
IvyPanda . (2022) 'Importance of Clinical Laboratory Science'. 19 December.
IvyPanda . 2022. "Importance of Clinical Laboratory Science." December 19, 2022. https://ivypanda.com/essays/importance-of-clinical-laboratory-science/.
1. IvyPanda . "Importance of Clinical Laboratory Science." December 19, 2022. https://ivypanda.com/essays/importance-of-clinical-laboratory-science/.
Bibliography
IvyPanda . "Importance of Clinical Laboratory Science." December 19, 2022. https://ivypanda.com/essays/importance-of-clinical-laboratory-science/.
- Disseminated Intravascular Coagulation
- Dye Wastewater Purification Using Coagulation
- Management of Rheumatoid Arthritis During Pregnancy
- Stroke: Diagnostic and Treatment
- Rheumatoid Arthritis: Causes, Symptoms, Treatments
- Point of Care and the Peripheral Blood Draw Laboratory-Run Anticoagulant Testing
- Rheumatoid Arthritis: Disease' Biology
- Rheumatoid Arthritis Problem Review
- Blood Disorder: Disease Analysis
- Heparins and Heparin-Induced Thrombocytopenia
- Aspects of Evidence-Based Research
- Prevention Bundle to Reduce Hospital-Acquired Pressure Injuries in Critical Care
- Early Menopause and How to Treat Its Symptoms
- Analysis of Tort in Society and Healthcare
- Comprehensive Assessment in Healthcare
Engineering a Smarter Future Together
Akkodis is your partner in Smart Industry. We combine best-in-class tech expertise and digital engineering talent to drive global innovation.
Akkodis Positioned as Global Leader by Zinnov
The Pivotal Role of Synthetic Data in Generative AI
Technology, Talent and Transformation
Through our four service lines, Akkodis delivers on your technology and talent demands of today, future-proofing your organization for the challenges of tomorrow.
Akkodis Thinkers & Makers Magazine
The pain relief dosing device giving patients control.
This featured Thinkers & Makers magazine article covers the innovative new device for administering pain relief, developed from the ground up by Akkodis engineers specialized in medical technology, can not only empower patients but also ease the workload of hospital staff.
.jpg?h=2774&iar=0&w=4106&hash=8C688B420AB1C9911091FB1B97C70963)
Partnerships
Akkodis Market Leader Among Digital Engineering Service Providers

Writing a strong scientific paper in medicine and the biomedical sciences: a checklist and recommendations for early career researchers
- Open access
- Published: 28 July 2021
- Volume 72 , pages 395–407, ( 2021 )
Cite this article
You have full access to this open access article

- Payam Behzadi 1 &
- Márió Gajdács ORCID: orcid.org/0000-0003-1270-0365 2 , 3
15k Accesses
51 Citations
406 Altmetric
Explore all metrics
Scientific writing is an important skill in both academia and clinical practice. The skills for writing a strong scientific paper are necessary for researchers (comprising academic staff and health-care professionals). The process of a scientific research will be completed by reporting the obtained results in the form of a strong scholarly publication. Therefore, an insufficiency in scientific writing skills may lead to consequential rejections. This feature results in undesirable impact for their academic careers, promotions and credits. Although there are different types of papers, the original article is normally the outcome of experimental/epidemiological research. On the one hand, scientific writing is part of the curricula for many medical programs. On the other hand, not every physician may have adequate knowledge on formulating research results for publication adequately. Hence, the present review aimed to introduce the details of creating a strong original article for publication (especially for novice or early career researchers).
Similar content being viewed by others
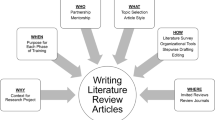
Why, When, Who, What, How, and Where for Trainees Writing Literature Review Articles
Gerry L. Koons, Katja Schenke-Layland & Antonios G. Mikos

How to Write and Publish a Research Paper for a Peer-Reviewed Journal
Clara Busse & Ella August

Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach
Zachary Munn, Micah D. J. Peters, … Edoardo Aromataris
Avoid common mistakes on your manuscript.
Introduction
The writing and editing of scientific papers should be done in parallel with the collection and analysis of epidemiological data or during the performance of laboratory experiments, as it is an integral step of practical research. Indeed, a scholar paper is the figurative product of scientific investigations (Behzadi and Behzadi 2011 ; Singh and Mayer 2014 ). Moreover, the publication of scholarly papers is important from the standpoint of providing relevant information—both locally and internationally—that may influence clinical practice, while in academia, national and international academic metrics (in which the number and quality of papers determine the score and rank of the scientists) are relevant to fulfill employment criteria and to apply for scientific grants (Grech and Cuschieri 2018 ; Singer and Hollander 2009 ). Thus, scientific writing and the publication of quality peer-reviewed papers in prestigious academic journals are an important challenge for medical professionals and biomedical scientists (Ahlstrom 2017 ). Writing a strong scholarly paper is a multi-procedure task, which may be achieved in a right manner by using a balanced and well-designed framework or blueprint (Gemayel 2016 ; Tóth et al. 2020 ). All in all, time needs to be spent of writing a well-designed and thoughtful scientific proposal to support the research, which will subsequently end in the publication of a paper in a prestigious, peer-reviewed, indexed and scholarly journal with an impact factor (IF). A well-designed scientific project encompasses well-supported and strong hypotheses and up-to-date methodology, which may lead to the collection of remarkable (and reproducible!) data. When a study is based on a strong hypothesis, suitable methodology and our studies result in usable data, the next step is the analysis and interpretation of the said data to present a valuable conclusion at the end of our studies. These criteria give you an influent confidence to prepare a robust and prestigious scholarly paper (Ahlstrom 2017 ; Behzadi 2021 ; Kallet 2004 ; Stenson et al. 2019 ). The aim of this review is to highlight all the necessary details for publication of a strong scientific writing of original article, which may especially be useful for novice or early career researchers.
Approaches for writing and formatting manuscripts before submission
In the presence of effective and appropriate items for writing a strong scientific paper, the author must know the key points and the main core of the study. Thus, preparing a blueprint for the paper will be much easier. The blueprint enables you to draft your work in a logical order (Gemayel 2016 ). In this regard, employment of a mass of charge, free or pay-per-use online and offline software tools can be particularly useful (Gemayel 2016 ; Behzadi and Gajdács 2020 ; Behzadi et al. 2021 ; Ebrahim 2018 ; Issakhanian and Behzadi 2019 ; O'Connor and Holmquist 2009 ; Petkau et al. 2012 ; Singh and Mayer 2014 ; Tomasello et al. 2020 ). Today, there are a wide range of diverse software tools which can be used for design and organization of different parts of your manuscript in the correct form and order. Although traditionally, many scientist do not use these softwares to help formulate their paper and deliver their message in the manuscript, they can indeed facilitate some stages of the manuscript preparation process. Some of these online and offline software facilities are shown in Table 1 .
The first step of writing any scientific manuscript is the writing of the first draft. When writing the first draft, the authors do not need to push themselves to write it in it’s determined order (Behzadi and Gajdács 2020 ; Gemayel 2016 ); however, the finalized manuscript should be organized and structured, according to the publisher’s expectations (Berman et al. 2000 ; Behzadi et al. 2016 ). Based on the contents of the manuscripts, there are different types of papers including original articles, review articles, systematic reviews, short communications, case reports, comments and letters to the editor (Behzadi and Gajdács 2020 ; Gemayel 2016 ), but the present paper will only focus on the original articles structured in the IMRAD (Introduction, Methods, Results and Discussion) structure. Materials and methods, results, discussion or introduction sections are all suitable target sections to begin writing the primary draft of the manuscript, although in most cases, the methods section is the one written first, as authors already have a clear sense and grasp on the methodologies utilized during their studies (Ebrahim 2018 ). The final sections of IMRAD papers which should be completed are the abstract (which is basically the mini-version of the paper) and conclusion (Liumbruno et al. 2013 ; Paróczai et al. 2021 ; Ranjbar et al. 2016 ). The authors should be aware that the final draft of the manuscript should clearly express: the reason of performing the study, the individuality (novelty and uniqueness) of the work, the methodology of the study, the specific outcomes examined in this work, the importance, meaning and worth of the study. The lack of any of the items in the manuscript will usually lead to the direct rejection of the manuscript from the journals. During the composition of the manuscript (which corresponds to any and all sections of the IMRAD), some basics of scientific writing should be taken into consideration: scientific language is characterized by short, crisp sentences, as the goal of the publication is to deliver the main message concisely and without confusion. It is a common misconception that scientific writing needs to be “colorful” and “artistic,” which may have the opposite effect on the clarity of the message. As the main goal of publishing is to deliver the message (i.e., the results) of our study, it is preferred that scientific or technical terms (once defined) are used uniformly, with avoiding synonyms. If young scientists have linguistic difficulties (i.e., English is not their first language), it is desirable to seek the help of professional proofreading services to ensure the correct grammar use and clarity. Traditionally, the passive voice was expected to be used in scientific communication, which was intended to strengthen the sense of generalization and universality of research; however, nowadays the active voice is preferred (symbolizing that authors take ownership and accountability of their work) and sentences in passive voice should take up < 10% of the paper (Berman et al. 2000 ; Behzadi et al. 2016 ).
Every scientist should be able to present and discuss their results in their own words, without copy–pasting sentences from other scientists or without referring to the work of others, if it was used in our paper. If an author copies or represents another authors’ intellectual property or words as their own (accidentally or more commonly on purpose) is called plagiarism. Scientific journals use plagiarism checker softwares to cross-check the level of similarity between the submitted works and scientific papers or other materials already published; over a certain threshold of similarity, journals take action to address this issue. Plagiarism is highly unethical and frowned upon in the scientific community, and it is strictly forbidden by all relevant scientific publishers, and if one is caught with plagiarism, the scientific paper is usually rejected immediately (if this occurs during the submission process) or is retracted. There are some freely available online software tools (e.g., iThenticate® ( http://www.ithenticate.com/ ) and SMALL SEO TOOLS ( https://smallseotoolz.net/plagiarism-checker ) for authors to screen their works for similarities with other sources; nevertheless, it is also unethical to use these tools to determine the “acceptable” level of similarity (i.e., cheating) before submitting a paper.
The structure of an IMRAD article includes the title, author’s(s’) name(s), author’s(s’) affiliation(s), author’s(s’) ORCID iD(s) ( https://orcid.org/ ), abstract, keywords, introduction, methods (or materials and methods), results, discussion, conclusion, acknowledgements, conflict of interest and references (Behzadi and Behzadi 2011 ; Singh and Mayer 2014 ). The acronym of ORCID (with a hard pronunciation of C ( https://orcid.org/blog/2013/01/07/how-should-orcid-be-pronounced )) (abbreviation of Open Researcher & Contributor ID) is considered as unique international identifier for researchers (Haak et al. 2012 ; Hoogenboom and Manske 2012 ). The ORCID iD is composed of 16 digits and introduced in the format of https URI ( https://support.orcid.org/hc/en-us/articles/360006897674 ). It is recommended for the authors to register their ORCID iD. The ORCID is important for manuscript submissions, manuscript citations, looking at the works of other researchers among other things (Haak et al. 2012 ; Hoogenboom and Manske 2012 ).
The contents of the IMRAD-structured manuscripts
Although the IMRAD format seems to be a cul-de-sac structure, it can be a suitable mold for both beginners and professional writers and authors. Each manuscript should contain a title page which includes the main and running (shortened) titles, authors’ names, authors’ affiliations (such as research place, e-mail, and academic degree), authors’ ORCID iDs, fund and financial supports (if any), conflicts of interest, corresponding author’s(s’) information, manuscript’s word count and number of figures, tables and graphs (Behzadi and Gajdács 2020 ).
As the title is the first section of your paper which is seen by the readers, it is important for the authors to take time on appropriately formulating it. The nature of title may attract or dismiss the readers (Tullu and Karande 2017 ). In this regard, a title should be the mirror of the paper’s content; hence, a proper title should be attractive, tempting, specific, relevant, simple, readable, clear, brief, concise and comprehensive. Avoid jargons, acronyms, opinions and the introduction of bias . Short and single-sentenced titles have a “magic power” on the readers. Additionally, the use of important and influent keywords could affect the readers and could be easy searchable by the search engines (Cuschieri et al. 2019 ). This can help to increase the citation of a paper. Due to this fact, it is recommended to consider a number of titles for your manuscript and finally select the most appropriate one, which reflects the contents of the paper the best. The number of titles’ and running titles’ characters is limited in a wide range of journals (Cuschieri et al. 2019 ).
The abstract is the vitrine of a manuscript, which should be sequential, arranged, structured and summarized with great effort and special care. This section is the second most important part of a manuscript after title (Behzadi and Gajdács 2020 ). The abstract should be written very carefully, deliberately and comprehensively in perfect English, because a well-written abstract invites the readers (the editors, reviewers, and readers who may cite the paper in the future) to read the paper entirely from A to Z and a rough one discourages readers (the editors and reviewers) from even handling the manuscript (Cuschieri et al. 2019 ). Whether we like it or not, the abstract is the only part of the manuscript that will be read for the most part; thus, the authors should make an effort to show the impressiveness and quality of the paper in this section.
The abstract as an independent structured section of a manuscript stands alone and is the appetizer of your work (Jirge 2017 ). So as mentioned, this part of paper should be written accurately, briefly, clearly, and to be facile and informative. For this section, the word count is often limited (150 to 250/300 words) and includes a format of introduction/background/, aim/goal/objective, methods, results and conclusions. The introduction or background refers to primary observations and the importance of the work, goal/aim/objective should represent the hypothesis of the study (i.e., why did you do what you did?), the methods should cover the experimental procedures (how did you do what you did?), the results should consider the significant and original findings, and finally, the clear message should be reported as the conclusion. It is recommended to use verbs in third person (unless specified by the Journal’s instructions). Moreover, the verbs depicting the facts which already have been recognized should be used in present tense while those verbs describing the outcomes gained by the current work should be used in past tense. For beginners in scientific publishing, it is a common mistake to start the writing of the manuscript with the abstract (which—in fact—should be the finalizing step, after the full text of the paper has already been finished and revised). In fact, abstract ideally is the copy-pasted version of the main messages of the manuscript, until the word limit (defined by the journal) has been reached. Another common mistake by inexperienced authors is forgetting to include/integrate changes in the abstract to reflect the amendments made in the bulk text of the paper. All in all, even a paper with very good contents and significant results may could be rejected because of a poor and weak abstract (Behzadi and Gajdács 2020 ).
Keywords are the key point words and terms of the manuscript which come right after abstract section. The keywords are used for searching papers in the related fields by internet search engines. It is recommended to employ 3 to 10 keywords in this section. The keywords should be selected from the MeSH (Medical Subject Headings) service, NCBI ( https://www.ncbi.nlm.nih.gov/mesh/ ). An appropriate title should involve the most number of keywords (Behzadi and Gajdács 2020 ; Jirge 2017 ).
Introduction section should be framed up to four paragraphs (up to 15% of the paper’s content). This section should be progressed gradually from general to specific information and gaps (in a funnel-formed fashion). In another words, the current condition of the problem and the previous studies should be briefly presented in the first paragraph. More explanation should be brought in discussion section, where the results of the paper should be discussed in light of the other findings in the literature (Ahlstrom 2017 ; Behzadi 2021 ). In this regard, the original articles and some key references should be cited to have a clarified description. The second paragraph should clarify the lack of knowledge regarding the problem at present, the current status of the scientific issue and explain shortly the necessity and the importance of the present investigation. Subsequently, the relevance of this work should be described to fill the current gaps relating to the problem. The questions (hypothesis/purpose) of the study comprising “Why did you do?/What did you do?/So What?” should be clarified as the main goal in the last paragraph (Ahlstrom 2017 ; Behzadi 2021 ; Burian et al. 2010 ; Lilleyman 1995 ; Tahaei et al. 2021 ). A concise and focused introduction lets the readers to have an influent understanding and evaluation for the performance of the study. The importance of the work presented should never be exaggerated, if the readers feel that they have been misled in some form that may damage the credibility of the authors’ reputation. It is recommended to use standard abbreviations in this section by writing the complete word, expression or phrase for the first time and mentioning the related abbreviation within parenthesis in this section. Obviously, the abbreviations will be used in the following sentences throughout the manuscript. The authors should also adhere to international conventions related to writing certain concepts, e.g., taxonomic names or chemical formulas. In brief, the introduction section contains four key points including: previous studies, importance of the subject, the presence of serious gap(s) in current knowledge regarding the subject, the hypothesis of the work (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ). Previously, it was recommended by majority of journals to use verbs in past tense and their passive forms; however, this shows a changing trend, as more and more journals recommend the use of the active voice.
Materials and methods
As the materials and methods section constitutes the skeleton of a paper (being indicative of the quality of the data), this section is known as the keystone of the research. A poor, flawed or incorrect methodology may result in the direct rejection of manuscripts, especially in high IF journals, because it cannot link the introduction section into the results section (Haralambides 2018 ; Meo 2018 ). In other words, the methods are used to test the study’s hypothesis and the readers judge the validity of a research by the released information in this section. This part of manuscript belongs to specialists and researchers; thus, the application of subheadings in a determined and relevant manner will support the readers to follow information in a right order at the earliest. The presentation of the methodologies in a correct and logical order in this section clarifies the direction of the methods used, which can be useful for those who want to replicate these procedures (Haralambides 2018 ; Juhász et al. 2021 ; Meo 2018 ). An effective, accurate, comprehensive and sufficient description guarantees the clarity and transparency of the work and satisfies the skeptical reviewers and readers regarding the basis of the research. The following questions should be answered in this section: “What was done?” and “How was it done?” and “Why was it done?”
The cornerstones of the methods section including defining the type of study, materials (e.g., concentration, dose, generic and manufacturer names of chemicals, antibiotics), participants (e.g., humans, animals, microorganisms), demographic data (e.g., age, gender, race, time, duration, place), the need for and the existence of an ethical approval or waiver (in accordance with the Declaration of Helsinki and its revisions) for humans and animals, experimental designs (e.g., sampling methods, time and duration of the study, place), protocols, procedures, rationale, criteria, devices/tools/techniques (together with their manufacturers and country of origin), calibration plots, measurement parameters, calculations, statistical methods, tests and analyses, statistical software tools and version among many other things should be described here in methods section (Haralambides 2016 ; Stájer et al. 2020 ). If the details of protocols make this section extremely long, mention them in brief and cite the related papers (if they are already published). If the applied protocol was modified by the researcher, the protocol should be mentioned as modified protocol with the related address. Moreover, it is recommended to use flow charts (preferably standard flow charts) and tables to shorten this section, because “a picture paints a thousand words” (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ).
The used online guidelines in accordance with the type of study should be mentioned in the methods section. In this regard, some of these online check lists, including the CONSORT (Consolidated Standards of Reporting Trials) statement ( http://www.consort-statement.org/ ) (to improve the reporting randomized trials), the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement ( http://www.prisma-statement.org/ ) (to improve the reporting of systematic reviews and meta-analyses), the STARD (Standards for Reporting Diagnostic accuracy studies) statement ( http://www.equator-network.org/wp-content/uploads/2015/03/STARD-2015-checklist.pdf ) (to improve the reporting of diagnostic accuracy studies), the STORBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement ( https://www.strobe-statement.org/index.php?id=strobe-home ) (to improve the reporting of observational studies in Epidemiology), should be mentioned and highlighted in medical articles. Normally, the methods section begins with mentioning of exclusion (depicting safe selection) and inclusion (depicting no bias has happened) criteria (regarding the populations studied) and continues by the description of procedures and data collection. This section usually ends by the description of statistical data analyses. As mentioned in a previous section, older recommendations in “Instructions for authors” suggested the use of verbs in past tense, in 3rd person and passive forms, whereas novel guidelines suggest more text written in the active voice (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ).
The results including negative and positive outcomes should be reported clearly in this section with no interpretation (Audisio et al. 2009 ; Behzadi et al. 2013 ). The most original information of an IMRAD paper originates from the results section. Indeed, the reported findings are the main core of the study which answers to the research question (hypothesis) “what was found?” The results section should answer all points brought up in the methods section. Categorization of findings by subheadings from the major to minor results, chronologically or by any logical order, facilitates readers to comprehend the results in an effective and influent manner (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ).
Representing the motive of experiments, the related experimental setups, and the gained outcomes supports the quality and clarity of your results, because these components create logical and influent communications between obtained data, observations and measurements. The results section should represent all types of data (major to minor), variables (dependent and independent), variables effects and even accidental findings. The statistical analyses should be represented at the end of results section. The statistical significance should be represented by an exact amount of p value ( p < 0.05 is usually recognized and set as the threshold for statistical significance, while p > 0.05 depicts no statistical significance). Moreover, the mentioning of the 95% confidence intervals and related statistical parameters is also needed, especially in epidemiological studies (Mišak et al. 2005 ).
It is recommended to use tables, figures, graphs and charts in this section to give an influent representation of results to the readers. Using well-structured tables deeply impresses the readers. Usually the limitation of the number of figures, graphs, tables and charts is represented in the section of instructions for authors of the journal. Remember that well-designed tables and figures act as clean mirrors which transfer a clear and sharp illustration of your work and your efforts in preparing the manuscript. Thus, a well-designed graph, table, charts or figure should be understood easily; in other words, they should be represented as self-explanatory compartments. Avoid repeating the represented data in figures, tables, charts and graphs within the text. Citing figures, graphs, charts and tables in right positions within the text increases the impact and quality of your manuscript (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ). Showing the highest and lowest amounts in tables by bolding or highlighting them is very effective. Normally, the legends are placed under graphs and figures and above the tables. It is recommended to begin the figure legends with conclusion and finish it by important technical key points.
Discussion and conclusion
This section represents the interpretations of results. In other words, discussion describes what these results do mean by the help of mechanistic interpretations of causes and effects. This argument should be achieved sharp and strong in a logical manner (Gajdács 2020 ; Rasko et al. 2016 ). The interpretations should be supported by relevant references and evidences. Usually, the first paragraph of discussion involves the key points of results. The represented data in results section should not be repeated within the discussion section. Magnification and exaggeration of data should never occur! “A good wine needs no bush.” Care about the quality of discussion section, because this part of the manuscript is determinative item for the acceptance of the paper (Ahlstrom 2017 ; Behzadi 2021 ).
Avoid representing new data in discussion, which were not mentioned in the results section. The following paragraphs should represent the novelty, differences and/or similarities of the obtained findings. Unusual and findings not predicted should be highlighted (Gajdács 2020 ; Rasko et al. 2016 ). It is important to interpret the obtained results by the strong references and evidences. Remember that citation of strong and relevant references enforces your evaluations and increases the quality of your points of view (Mack 2018 ; Shakeel et al. 2021 ). The probable weaknesses or strengths of the project should be discussed. This critical view of the results supports the discussion of the manuscript. The discussion section is finished by the final paragraph of conclusion. A critical paragraph in which the potential significance of obtained findings should be represented in brief (Ahlstrom 2017 ; Behzadi 2021 ). The bring/take-home message of the study in conclusion section should be highlighted. For writing a conclusion, it is recommended to use non-technical language in perfect English as it should be done in abstract section (Alexandrov 2004 ). It is suggested to use verbs in present tense and passive forms, if not otherwise mandated by the journal’s instructions. In accordance with policy of journals, the conclusion section could be the last part of discussion or presented within a separate section after discussion section (Ahlstrom 2017 ; Behzadi 2021 ).

Acknowledgements
This section is placed right after discussion and/or conclusion section. The unsaid contributors with pale activities who cannot be recognized as the manuscripts’ authors should be mentioned in acknowledgement section. Financial sponsors, coordinators, colleagues, laboratory staff and technical supporters, scientific writing proof readers, institutions and organizations should be appreciated in this section. The names listed in acknowledgements section will be indexed by some databases like US National Library Medicine (NLM) ( https://www.nlm.nih.gov/ ) (Ahlstrom 2017 ).
Conflict of interest
If the authors have any concerns regarding moral or financial interests, they should declare it unambiguously, because the related interests may lead to biases and suspicions of misconducts (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ). This section usually comes right after acknowledgements and before references.
Application of relevant and pertinent references supports the manuscript’s scientific documentary. Moreover, utilization of related references with high citation helps the quality of the manuscript. For searching references, it is recommended to use search engines like Google Scholar ( https://scholar.google.com/ ), databases such as MEDLINE ( https://www.nlm.nih.gov/bsd/medline.html ) and NCBI ( https://www.ncbi.nlm.nih.gov/ ) and Web sites including SCOPUS ( https://www.scopus.com/ ), etc.; in this regard, the keywords are used for a successful and effective search. Each journal has its own bibliographic system; hence, it is recommended to use reference management software tools, e.g., EndNote®. The most common bibliographic styles are APA American Psychological Association, Harvard and Vancouver. Nevertheless, the authors should aware of retracted articles and making sure not to use them as references (Ahlstrom 2017 ; Behzadi 2021 ; Lilleyman 1995 ; Tahaei et al. 2021 ). Depending on the journal, there are different limitations for the number of references. It is recommended to read carefully the instructions for authors section of the journal.
Conclusions for future biology
From the societal standpoint, the publication of scientific results may lead to important advances in technology and innovation. In medicine, patient care—and the biomedical sciences in general—the publication of scientific research may also lead to substantial benefits to advancing the medical practice, as evidence-based medicine (EBM) is based on the available scientific data at the present time. Additionally, academic institutions and many academic centers require young medical professionals to be active in the scientific scene for promotions and many employment prospects. Although scientific writing is part of the curricula for many medical programs, not every physician may have adequate knowledge on formulating research results for publication adequately. The present review aimed to briefly and concisely summarize the details of creating a favorable original article to aid early career researchers in the submission to peer-reviewed journal and subsequent publication. Although not all concepts have been discussed in detail, the paper allows for current and future authors to grasp the basic ideas regarding scientific writing and the authors hope to encourage everyone to take the “leap of faith” into scientific research in medicine and to submit their first article to international journals.
Data accessibility
Not applicable.
Ahlstrom D (2017) How to publish in academic journals: writing a strong and organized introduction section. J East Eur Cent Asian Res 4(2):1–9
Google Scholar
Alexandrov AV (2004) How to write a research paper. Cerebrovasc Dis 18(2):135–138
Article PubMed Google Scholar
Audisio RA, Stahel RA, Aapro MS, Costa A, Pandey M, Pavlidis N (2009) Successful publishing: how to get your paper accepted. Surg Oncol 18(4):350–356
Behzadi P (2021) Peer review publication skills matter for academicians. Iran J Pathol 16(1):95–96
Behzadi P, Behzadi E (2011) A new aspect on how to write an original article, 1st edn. Persian Science & Research Publisher, Tehran
Behzadi P, Gajdács M (2020) Dos and don’ts of a successfully peer-reviewed publication: from A-Z. Eur J Microbiol Immun 10:125–130
Article Google Scholar
Behzadi E, Behzadi P, Ranjbar R (2013) Abc’s of writing scientific paper. Infectioro 33(1):6–7
Behzadi P, Najafi A, Behzadi E, Ranjbar R (2016) Microarray long oligo probe designing for Escherichia coli: an in-silico DNA marker extraction. Cent Eur J Urol 69(1):105–111
CAS Google Scholar
Behzadi P, García-Perdomo HA, Karpiński TA (2021) Toll-like receptors: general molecular and structural biology. J Immunol Res 2021:e9914854
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucl Acids Res 28(1):235–242
Article CAS PubMed PubMed Central Google Scholar
Burian K, Endresz V, Deak J, Kormanyos Z, Pal A, Nelson D, Virok DP (2010) Transcriptome analysis indicates an enhanced activation of adaptive and innate immunity by chlamydia-infected murine epithelial cells treated with interferon γ. J Infect Dis 202:1405–1414
Cuschieri S, Grech V, Savona-Ventura C (2019) WASP (write a scientific paper): structuring a scientific paper. Early Human Dev 128:114–117
Ebrahim AN (2018) Publishing Procedure and Strategies to Improve Research Visibility and Impact. https://figshare.com/articles/presentation/Publishing_Procedure_and_Strategies_to_Improve_Research_Visibility_and_Impact/7475036
Gajdács M (2020) Taxonomy and nomenclature of bacteria with clinical and scientific importance: current concepts for pharmacists and pharmaceutical scientists. Acta Pharm Hung 89(4):99–108
Gemayel R (2016) How to write a scientific paper. FEBS 283(21):3882–3885
Article CAS Google Scholar
Grech V, Cuschieri S (2018) Write a scientific paper (WASP)-a career-critical skill. Early Human Dev 117:96–97
Haak LL, Fenner M, Paglione L, Pentz E, Ratner H (2012) ORCID: a system to uniquely identify researchers. Learn Publ 25(4):259–264
Haralambides HE (2016) Dos and don’ts in scholarly publishing. Marit Econ Logist 18(2):101–102
Haralambides HE (2018) Dos and don’ts of scholarly publishing (part II). Marit Econ Logist 20(3):321–326
Hoogenboom BJ, Manske RC (2012) How to write a scientific article. Int J Sports Phys Ther 7(5):512–517
PubMed PubMed Central Google Scholar
Issakhanian L, Behzadi P (2019) Antimicrobial Agents and Urinary Tract Infections. Curr Pharm Des 25(12):1409–1423
Article CAS PubMed Google Scholar
Jirge PR (2017) Preparing and publishing a scientific manuscript. J Hum Reprod Sci 10(1):3–9
Juhász J, Ligeti B, Gajdács M, Makra N, Ostorházi E, Farkas FB, Stercz B, Tóth Á, Domokos J, Pongor S, Szabó D (2021) Colonization dynamics of multidrug-resistant Klebsiella pneumoniae are dictated by microbiota-cluster group behavior over individual antibiotic susceptibility: a metataxonomic analysis. Antibiotics 10(3):e268
Kallet RH (2004) How to write the methods section of a research paper. Resp Care 49(10):1229–1232
Lilleyman J (1995) How to write a scientific paper—a rough guide to getting published. Arch Dis Child 72(3):268–270
Liumbruno GM, Velati C, Pasqualetti P, Franchini M (2013) How to write a scientific manuscript for publication. Blood Transf 11(2):217–226
Mack CA (2018) How to write a good scientific paper. The United States of America: Society of Photo-Optical Instrumentation Engineers (SPIE) Press. ISBN 9781510619135
Meo SA (2018) Anatomy and physiology of a scientific paper. Saudi J Biol Sci 25(7):1278–1283
Article PubMed PubMed Central Google Scholar
Mišak A, Marušić M, Marušić A (2005) Manuscript editing as a way of teaching academic writing: experience from a small scientific journal. J Second Lang Writ 14(2):122–131
O’Connor TR, Holmquist GP (2009) Algorithm for writing a scientific manuscript. Biochem Mol Biol Educ 37(6):344–348
Paróczai D, Sejben A, Kókai D, Virók DP, Endrész V, Burián K (2021) Beneficial immunomodulatory effects of fluticasone propionate in chlamydia pneumoniae-infected mice. Pathogens 10(3):e338
Petkau A, Stuart-Edwards M, Stothard P, Van Domselaar G (2012) Interactive microbial genome visualization with GView. Bioinformatics 26(24):3125–3126
Ranjbar R, Behzadi P, Mammina C (2016) Respiratory tularemia: Francisella tularensis and microarray probe designing. Open Microbiol J 10:176–182
Rasko Z, Nagy L, Radnai M, Piffkó J, Baráth Z (2016) Assessing the accuracy of cone-beam computerized tomography in measuring thinning oral and buccal bone. J Oral Implant 42:311–314
Shakeel S, Iffat W, Qamar A, Ghuman F, Yamin R, Ahmad N, Ishaq SM, Gajdács M, Patel I, Jamshed S (2021) Pediatricians’ compliance to the clinical management guidelines for community-acquired pneumonia in infants and young children in Pakistan. Healthcare 9(6):e701
Singer AJ, Hollander JE (2009) How to write a manuscript. J Emerg Med 36(1):89–93
Singh V, Mayer P (2014) Scientific writing: strategies and tools for students and advisors. Biochem Mol Biol Educ 42(5):405–413
Stájer A, Kajári S, Gajdács M, Musah-Eroje A, Baráth Z (2020) Utility of photodynamic therapy in dentistry: current concepts. Dent J 8(2):43
Stenson JF, Foltz C, Lendner M, Vaccaro AR (2019) How to write an effective materials and methods section for clinical studies. Clin Spine Surg 32(5):208–209
Tahaei SAS, Stájer A, Barrak I, Ostorházi E, Szabó D, Gajdács M (2021) Correlation between biofilm-formation and the antibiotic resistant phenotype in staphylococcus aureus isolates: a laboratory-based study in Hungary and a review of the literature. Infect Drug Res 14:1155–1168
Tomasello G, Armenia I, Molla G (2020) The protein imager: a full-featured online molecular viewer interface with server-side HQ-rendering capabilities. Bioinformatics 36(9):2909–2911
Tóth Á, Makai A, Jánvári L, Damjanova I, Gajdács M, Urbán E (2020) Characterization of a rare bla VIM-4 metallo-β-lactamase-producing Serratia marcescens clinical isolate in Hungary. Heliyon 6(6):e04231
Tullu M, Karande S (2017) Writing a model research paper: a roadmap. J Postgrad Med 63(3):143–146
Download references
Payam Behzadi would like to thank the Islamic Azad University, Shahr-e-Qods Branch, Tehran, Iran, for approving the organization of the workshop on “How to write a scientific paper?” Márió Gajdács would also like to acknowledge the support of ESCMID’s “30 under 30” Award.
Open access funding provided by University of Szeged. Márió Gajdács was supported by the János Bolyai Research Scholarship (BO/00144/20/5) of the Hungarian Academy of Sciences and the New National Excellence Programme (ÚNKP-20-5-SZTE-330) of the Ministry of Human Resources.
Author information
Authors and affiliations.
Department of Microbiology, College of Basic Sciences, Shahr-e-Qods Branch, Islamic Azad University, Tehran, 37541-374, Iran
Payam Behzadi
Institute of Medical Microbiology, Faculty of Medicine, Semmelweis University, Budapest, Nagyvárad tér 4, 1089, Hungary
Márió Gajdács
Department of Pharmacodynamics and Biopharmacy, Faculty of Pharmacy, University of Szeged, Szeged, Eötvös utca 6., 6720, Hungary
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Márió Gajdács .
Ethics declarations
The authors declare that they have no competing interests, monetary or otherwise.
Ethical statement
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Behzadi, P., Gajdács, M. Writing a strong scientific paper in medicine and the biomedical sciences: a checklist and recommendations for early career researchers. BIOLOGIA FUTURA 72 , 395–407 (2021). https://doi.org/10.1007/s42977-021-00095-z
Download citation
Received : 08 April 2021
Accepted : 16 July 2021
Published : 28 July 2021
Issue Date : December 2021
DOI : https://doi.org/10.1007/s42977-021-00095-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Scientific research
- Publications
- Medical publications
- Clinical medicine
- Peer review
- Academic training
- Abstracting and indexing
- Find a journal
- Publish with us
- Track your research
- Faculty & Staff
Students admitted to the UW now have until June 1 to commit, a result of FAFSA delays. We anticipate that financial aid offers will be sent in late April or early May.
Medical Laboratory Science
School of Medicine
Medical Laboratory Science, offered by the Department of Laboratory Medicine, is a profession of highly knowledgeable and skilled individuals who perform clinical laboratory tests on patient samples. This is a critical part of healthcare, as the results obtained by these laboratory tests are a vital tool for physicians in their diagnosis, treatment and prevention of disease. The disciplines within Laboratory Medicine that students learn about and train in are: Clinical Chemistry, Hematology/Coagulation, Microbiology, Transfusion Medicine, Urinalysis and Clinical Research.
Major category : Capacity-constrained
Topic(s) : Health, Natural and environmental sciences
Applicant type
Freshmen can apply to the UW to begin autumn quarter or winter quarter (U.S. applicants only)
Read more about applying to the UW as a freshman , including details for programs that provide high school students with college credit (like Running Start).
Quarters of general admission to UW : autumn / winter (U.S. applicants only) / spring / summer
Preparation for the major is a factor in transfer admission.
- Use the information below to help you prepare for this degree
- Visit MyPlan to run a degree audit.
- Use the UW Equivalency Guide to find out how courses taken at a Washington state community or technical college will transfer to the UW.
- UW college and school graduation requirements
Department admission information
Departmental application deadline : February 15 Please contact the department for details about the application process.
- Students must earn a minimum of 90 quarter credits, including both prerequisite courses for medical laboratory science and general education courses required for graduation from the University of Washington
Read more about applying to the UW as a transfer student .
Courses required for the major:
- BIOL 118 NSc – Survey of Physiology
- BIOL 180 NSc – Introductory Biology
- BIOL 200 NSc – Introductory Biology
- BIOL 220 NSc – Introductory Biology
- CHEM 142 NSc RSN – General Chemistry
- CHEM 152 NSc RSN – General Chemistry
- CHEM 162 NSc RSN – General Chemistry
- CHEM 223 NSc – Organic Chemistry Short Program
One of the following:
- STAT 220 NSc RSN – Basic Statistics
- Any basic statistics course
Entering transfer information:
Total undergraduates: 46 Total from Washington community colleges: 16
Entering transfer GPA (from WA community colleges):
3.75 – 4.00: 7 3.50 – 3.74: 3.25 – 3.49: 3.00 – 3.24: 2.75 – 2.99: 2.50 – 2.74: 2.49 and below:
Career outcomes
- Student/Faculty Portal
- Learning Hub (Brightspace)
- Continuous Professional Development

Medical Laboratory Scientist
What does a medical laboratory scientist do.
A medical laboratory scientist (MLS), also known as a medical technologist or clinical laboratory scientist, works to analyze a variety of biological specimens. They are responsible for performing scientific testing on samples and reporting results to physicians.
Medical laboratory scientists perform complex tests on patient samples using sophisticated equipment like microscopes. The data they find plays an important role in identifying and treating cancer, heart disease, diabetes, and other medical conditions. It is estimated 60 to 70 percent of all decisions regarding a patient's diagnosis, treatment, hospital admission, and discharge are based on the results of the tests medical laboratory scientists perform.
Video: Behind the scenes: Medical Laboratory Scientist
Scope of practice
Medical laboratory scientists collaborate very closely with physicians and medical laboratory technicians in diagnosing and monitoring disease processes, as well as monitoring the effectiveness of therapy. Areas of medical laboratory training include microbiology, chemistry, hematology, immunology, transfusion medicine, toxicology, and molecular diagnostics.
Medical laboratory scientists have a wide variety of responsibilities and duties, including:
- Examining and analyzing blood, body fluids, tissues, and cells
- Relaying test results to physicians
- Utilizing microscopes, cell counters, and other high-precision lab equipment
- Cross-matching blood for transfusion
- Monitoring patient outcomes
- Performing differential cell counts looking for abnormal cells to aid in the diagnosis of anemia and leukemia
- Establishing quality assurance programs to monitor and ensure the accuracy of test results
- Overseeing the work of a medical laboratory technician
Medical laboratory scientist vs. medical laboratory technician
While similar, there are a few key differences between a medical lab scientist and a medical lab technician. They both work in the lab and perform tests on biological samples, however, a medical lab scientist typically has more education and is able to perform more involved lab work. A medical lab technician performs more of the routine lab work and is often supervised by a medical lab scientist.
Medical laboratory scientist vs. medical laboratory assistant
A medical laboratory assistant is a subgroup of medical laboratory technician. They are responsible for preparing biological specimens, recording information, and perform more of the lab maintenance tasks such as cleaning equipment and stocking supplies. A medical laboratory scientist will work with a medical laboratory assistant by analyzing their prepared specimens and relaying information for them to record.
Work environment
Medical lab scientists work in hospitals, clinics, forensic or public health laboratories, as well as pharmaceutical industries, biotechnology companies, veterinary clinics, or research institutions. Depending on the setting, their work hours may vary; but typically labs are run 24 hours a day, seven days a week. This allows for flexibility in scheduling.
Medical laboratory scientists spend the majority of their time on their feet, analyzing test results in the lab.
Becoming a medical laboratory scientist
Successful medical lab scientists are effective communicators with a sound intellect and interest in science and technology. Excellent eye-hand coordination, dexterity, and visual acuity are important to skillfully perform and analyze tests.
Individuals who love science and research, but prefer to have little-to-no interaction with patients, would be a good fit for the medical laboratory scientist career.
Higher education requirements
After obtaining a high school diploma (or the equivalent), most will go on to obtain some level of higher education and training in order to become a medical laboratory scientist.
Common higher education requirements for medical laboratory scientist jobs include:
- Completing a bachelor’s degree in medical technology or clinical laboratory science. A bachelor’s degree in a science or health-related field (e.g. chemistry or microbiology) may also be considered.
- Completing a clinical laboratory program or internship through a hospital-based program or as part of their education
- National certification as a medical technologist (MT), clinical laboratory scientist (CLS), or medical laboratory scientist (MLS)
- Previous experience in a healthcare setting
Certification and licensing
Most employers require medical laboratory scientists to obtain certification through an accrediting body, such as the American Society for Clinical Pathology (ASCP) Board of Certification (BOC) . After passing the credentialing exam, medical laboratory scientists (MLS) can practice under the credentials of MLS(ASCP)CM.
Licensure by state may also be required.
Career opportunities and outlook
The median salary for a medical lab scientist is $57,800, though salaries can range between $30,000-$79,000 depending on education, location, and previous experience.
Job growth and security are high for medical laboratory technicians and scientists. According to the Bureau of Labor Statistics , there is currently a shortage of medical lab technicians and scientists in many parts of the country which guarantees ample employment opportunities and sometimes higher salaries for graduates. With the volume of laboratory tests continuing to increase due to both population growth and the development of new types of tests, job opportunities are expected to increase faster than average with over 26,000 new positions expected to be available by 2030.
With additional training and experience, a medical lab scientist can become a department lead or lab manager. Others may seek specializations to advance their careers. Typically, a medical lab technician will progress to a medical lab scientist with more training.
By the numbers
median annual salary
years of higher education
job growth projected from 2020-2030
Medical laboratory scientist programs at Mayo Clinic
Mayo Clinic offers several programs and rotations to further your education and prepare you for a career as a medical laboratory scientist, medical laboratory assistant, or medical laboratory technician.
- Medical Laboratory Science Clinical Rotation (Arizona)
- Medical Laboratory Science Clinical Rotation (Florida)
- Medical Laboratory Science Program (Florida and Minnesota)
- Medical Laboratory Technician Clinical Rotation (Florida)
Browse similar careers

Cytogenetic technologist

Cytotechnologist

Histology technician
Careers in healthcare: Let us help you find your fit
- UNC Chapel Hill
Program Goals and Objectives

MCLS Program Goals
Upon completion of the UNC-Chapel Hill Master’s in Clinical Laboratory Science –Medical Laboratory Science Track, MLS graduates will be prepared to:
- Serve as leaders in the clinical laboratory profession in technical, educational, research and administrative roles.
- Critically analyze professional literature and apply that information in work settings.
- Communicate effectively with health care providers and the public.
- Continue to learn throughout their professional careers.
MCLS Educational Objectives
At the completion of the MCLS-MLS program, students will be able to:
- Explain the principles and methods used in molecular laboratory tests.
- Explain the clinical significance of molecular laboratory procedures used in the diagnosis and treatment of disease and the maintenance of health.
- Use the principles of method evaluation to select new techniques and instruments.
- Apply bio-statistical concepts to data to draw conclusions and evaluate significance of research results.
- Use research methods to design, conduct and disseminate results of studies on new technologies, procedures or diagnostic correlations.
- Assess the quality assurance practices used to ensure the accuracy and reliability of laboratory information.
- Explain and apply the major principles and practices of laboratory administration, supervision and budgeting.
- Explain and apply principles of effective test utilization.
- Interpret, implement, and comply with laws, regulations and accrediting standards and guidelines of relevant governmental and non-governmental agencies.
- Apply principles of management to the acquisition and evaluation of laboratory information systems.
- Design, implement and evaluate resource management strategies to maintain optimal laboratory efficiency.
- Develop a strategic plan to support the professional career development of clinical laboratory personnel.
- Use educational methods to present information and develop instructional materials.
- Communicate effectively with diverse audiences using a variety of formats (e.g. presentations, written communications).
- Develop and complete a Capstone project in advanced clinical laboratory practice, education, or laboratory operations.
- Free Samples
- Premium Essays
- Editing Services Editing Proofreading Rewriting
- Extra Tools Essay Topic Generator Thesis Generator Citation Generator GPA Calculator Study Guides Donate Paper
- Essay Writing Help
- About Us About Us Testimonials FAQ
- Studentshare
- Clinical laboratory science
Clinical laboratory science - Scholarship Essay Example

- Subject: Education
- Type: Scholarship Essay
- Level: Undergraduate
- Pages: 1 (250 words)
- Downloads: 29
- Author: mkoelpin
Extract of sample "Clinical laboratory science"
King’s College pa ment of Purpose. It is my strong believe that our accomplishment in life is measured not just our academic and material success but our contribution to the betterment of humanity. The knowledge we acquire through education and experience should applied addressing the problems facing our modern society so as to positively influence life. This I believe is possible through quality education that emphasizes holistic transformation of the student apart from just academic achievement.
It is on this background I am applying to the Clinical Laboratory Science Program at the King’s College pa. King’s’ mission of preparing graduates intellectually, morally and spiritually for a satisfying future life particularly interests me. My interest in the field of Clinical Laboratory Science started way back in my childhood and is significantly inspired by the family background with parents in the medical field. As a small child I began admiring the work done by my parents of assisting patients and ensuring they lead a quality life.
This made me to work hard in school so to attain grades that would enable get a college opportunity to further my studies in the area. The nationwide deficiency of professionals in the area of Clinical Laboratory Science has also contributed significantly as I feel by graduating in the field I will be able to positively impact by helping in the process of saving lives which will give me great satisfaction. I therefore want to be part of the solution and not the problem thus believes as a professional CLS will be better placed to serve and positively influence humanity.
I strongly believe in the power of knowledge in transforming society and hence participate actively in extracurricular activities such as club clubs, academic forums and games in which I believe lies opportunity for information exchange. I also participate with the intention of growing holistically and maintaining personal health both physically, mentally and psychologically which I believe is very important to my life especially as a student. I will therefore utilize all available facilities on the campus to enrich my graduate experience and emerge as a responsible person in the society.
- Cited: 2 times
- Copy Citation Citation is copied Copy Citation Citation is copied Copy Citation Citation is copied
CHECK THESE SAMPLES OF Clinical laboratory science
The microbial world - use of genus, graduate letter for admission, medical ethics in the clinical laboratory science, health care organizations, mayo clinic case, the field of medical technology, an analysis of a selected genus, safety violations within a medical facility.

- TERMS & CONDITIONS
- PRIVACY POLICY
- COOKIES POLICY

IMAGES
VIDEO
COMMENTS
Words: 300. Admission Essay. Hire a Writer for Custom Admission Essay. Use 10% Off Discount: "custom10" in 1 Click 👇. HIRE A WRITER! You are free to use it as an inspiration or a source for your own work. A career in clinical laboratory science requires dedication, knowledge, commitment, and an analytical approach to conducting research.
Medical Laboratory Science Essay. Sort By: Page 1 of 50 - About 500 essays. Decent Essays. Short Term Goals For Medical Laboratory Science. 688 Words; 3 Pages ... After discovering my passion for science and math, I pursue a career in Clinical Laboratory Science with enthusiasm. My desire to study this subject originates from the exciting and ...
Clinical Laboratory Science Personal Statement. Decent Essays. 703 Words. 3 Pages. Open Document. After discovering my passion for science and math, I pursue a career in Clinical Laboratory Science with enthusiasm. My desire to study this subject originates from the exciting and rapidly-moving subject area that is highly relevant to major ...
The hidden profession that saves lives. Medical Laboratory Science (also called Clinical Laboratory Science) is one of the most under-recognized health professions - with excellent job prospects. Caption: A Clinical Laboratory Science class at Texas State University (Photo by Chandler Prude) As an undergraduate microbiology major and MS ...
A degree in clinical laboratory science provides a plethora of opportunities for an individual. The primary responsibility of a clinical laboratory specialist is to analyze biological specimens by performing testing and reporting the results (Mayo Clinic, n.d.). For example, they can work in the medical field and both in clinical and research ...
Laboratory medicine has undergone a profound evolution in organizational, methodological, and cultural terms in recent decades [].From the organizational point of view, we are living in the era of consolidation, i.e., the formation of networks of consolidated laboratories with marked automation and integration of the various branches of laboratory medicine [].
Definition/Introduction. Clinical laboratories are healthcare facilities providing a wide range of laboratory procedures that aid clinicians in diagnosing, treating, and managing patients. [1] These laboratories are manned by scientists trained to perform and analyze tests on samples of biological specimens collected from patients.
Clinical Laboratory Science (CLS) is the official journal of the American Society for Clinical Laboratory Science.CLS is an online journal published quarterly and features articles on the very latest in research, education, and government actions affecting clinical laboratory science professionals and their integration and impact on the healthcare system.
Clinical Laboratory Science, also called Medical Laboratory Science or Medical Technology, is the health profession that provides laboratory information and services needed for the diagnosis and treatment of disease. Clinical Laboratory Scientists perform a variety of laboratory tests, ensure the quality of the test results, explain the significance of laboratory tests, evaluate new methods ...
This research paper, "Importance of Clinical Laboratory Science" is published exclusively on IvyPanda's free essay examples database. You can use it for research and reference purposes to write your own paper.
Clinical laboratory science (CLS) is an interdisciplinary field that involves setting up, qualifying, validating and performing laboratory assays on human samples to provide the reliable information required to effectively diagnose disease, monitor treatment and assess immune responses (to vaccines, for example). Accurately reporting such practices remains key to the continuous development and ...
HIGHLY RECOMMENDED TO VIEW: Okay, ignore the silly beginning, because this video is a great (and short!) overview on how to select a research topic that's manageable for your assignment.Nice tips on narrowing a huge topic by considering the angles of WHO, WHAT, WHERE, WHY, WHEN, or HOW. Also, tips to keep from making a topic too narrow. (See box on right "Narrowing your topic" for other examples.)
Scientific writing is an important skill in both academia and clinical practice. The skills for writing a strong scientific paper are necessary for researchers (comprising academic staff and health-care professionals). The process of a scientific research will be completed by reporting the obtained results in the form of a strong scholarly publication. Therefore, an insufficiency in scientific ...
School of Medicine. Medical Laboratory Science, offered by the Department of Laboratory Medicine, is a profession of highly knowledgeable and skilled individuals who perform clinical laboratory tests on patient samples. This is a critical part of healthcare, as the results obtained by these laboratory tests are a vital tool for physicians in ...
Healthcare Reimbursement in the Laboratory Tiffanee James November 14, 2016 Clinical Laboratory Science Management Georgia McCauley PhD, MBA, MT(AMT), CRA Introduction One thing that is consistent in healthcare is that healthcare is forever evolving.
ASCLS Today; October, 2012, 26 (9), p.6, 15. Explore the latest full-text research PDFs, articles, conference papers, preprints and more on CLINICAL LABORATORY SCIENCE. Find methods information ...
For more information about submissions to the Dialogue and Discussion section contact: Margaret LeMay-Lewis, Managing Editor, Clinical Laboratory Science Editorial Office, IC Ink, 858 Saint Anne's Drive, Iowa City, IA 52245. (319) 354-3861. [email protected]. of a doctorate in clinical laboratory science (DCLS), an advanced degree that ...
Completing a bachelor's degree in medical technology or clinical laboratory science. A bachelor's degree in a science or health-related field (e.g. chemistry or microbiology) may also be considered. Completing a clinical laboratory program or internship through a hospital-based program or as part of their education
Essay. Applicants will be asked to upload a one-to-two page typed statement describing their interest in CLS, their career goals, and how they think they can contribute to the CLS profession. ... Bachelor of Science in Clinical Laboratory Science (BSCLS):email us: BSCLS Masters of Clinical Laboratory Science (MCLS):email us: MCLS. Find Us. 321 ...
MCLS Program Goals Upon completion of the UNC-Chapel Hill Master's in Clinical Laboratory Science -Medical Laboratory Science Track, MLS graduates will be prepared to: Serve as leaders in the clinical laboratory profession in technical, educational, research and administrative roles. Critically analyze professional literature and apply that information in work settings. Communicate ...
Foremost, my goal is to get accepted into the Clinical Laboratory Science Program. The has been my goal for a while because of my interest in math and science as well as my. Free Essay: Ever since I can remember, I have always possessed passion for science and math. Clinical Laboratory Science provides a platform for the...
Extract of sample "Clinical laboratory science". King's College pa ment of Purpose. It is my strong believe that our accomplishment in life is measured not just our academic and material success but our contribution to the betterment of humanity. The knowledge we acquire through education and experience should applied addressing the problems ...
Satisfactory Essays. 348 Words; ... clinical lab, science and elementary education. The University offers over 10 graduate programs for both master's and doctorate. Therefore, they also have Bachelor of Science degrees that are offered in Clinical Laboratory Science, Exercise Physiology, Health Care Management, and Nursing. The Clinical ...