

Approval Sheet for Thesis with Examples and Format
- Post author: Rajveer
- Post last modified: March 17, 2023
- Reading time: 13 mins read

Writing thesis and stuck on approval sheet? If yes then don’t worry in this article I will tell you how you can design a perfect approval sheet. I’ll also share a few different examples and the cool thing is that the samples are downloadable in word doc format. So let’s jump into the article.
Approval Sheet for Thesis
1. add university name and logo, 2. start with the heading, 3. write a statement for the approval sheet, 4. add a section for author’s signature and date, 5. add a section for approval committee members, 6. design a signature and date section for approval committee, importance of approval sheet in thesis, check samples and select a format for your approval sheet, thesis approval sheet example 1, thesis approval sheet example 2, thesis approval sheet example 3, final suggestions on thesis approval sheet, where to add the thesis approval sheet, what is the ideal length of the thesis approval sheet statement, who writes the thesis approval sheet, why is an approval sheet necessary in a thesis.
Writing a thesis requires a lot of research and time. It is not an easy task for most of us but in the end, we need to do it. Another important and challenging task is to get approval from the authorities. Most of the researchers are apprehensive about thesis approval rejection because of their bad thesis approval sheet.
After completing the thesis, you will need to create and add the approval sheet pages for your thesis. You can submit the thesis to your college or university after getting approval from the authorities.
The approval letter is usually added on the second page of the thesis. This authorities approval improves the reputation of the thesis in the academic field for submitting it on different platforms.
So now you have understood what is the approval sheet in the thesis. It’s time to understand how you can write and design a perfect approval sheet for a thesis.
How to Write and Design Approval Sheet for Thesis
Writing and designing an approval sheet is not a difficult task. You just need to follow some guidelines and basics and you will be able to create a perfect thesis approval sheet.
Below I have shared a quick guide to creating an approval sheet for the thesis. Create a Microsoft Word document or google doc whatever you are using and get started with the quick guide below .
The first step is to add your university name and logo at the top of the page. It usually comes in the header section. You can add a logo image of your university/college and write the name in bold and center. This is the basic thing you probably already have added in other pages too so you can just copy-paste.
The next step in writing an approval sheet is to create a new MS Document or Google document and start writing with a title. Give the page a bold and focused title, “Approval Sheet.” The title is one of the parameters that will help readers to identify what the page is about.
Write a short introductory statement for thesis approval just after the heading. It includes your thesis title name and your name. Keep the statement short and simple. A maximum of 100 to 150 words statement is enough.
This statement will inform the authorities about your thesis topic. Write in a formal language without grammatical errors. If you are not a grammar expert and do a lot of grammar mistakes then try to use some grammar checker tools to avoid grammar mistakes .
After writing the statement, design a section for the thesis author’s signature and date. You can write the author’s signature on the left and the date on the right, or you can use the left side for just the signature and date.
You need to design it right after the statement. See a quick example below.
Author Signature ………………
Date ……………….
Now add the next section for the members of the approval committee. Give it a title like “Approval Committee” or “Approval Examiners”. Write a brief statement for thesis approval on behalf of the approval committee as they will sign under it.
Now you are almost done. Just add a signature and date section for the members of the approval committee.
Once you have completed all the steps and your approval sheet is ready you can add it to the second page of your thesis file.
An approval sheet is an important page in the thesis. This is an essential page to get approval from the authorities for submitting your thesis to the university/college and various other platforms. An approved thesis is a sign of confidence in your research and paper.
Thesis approval is usually given by the research and approval committee of a university or college. Once your thesis is approved you can submit the papers on any platform and people will trust your papers because of the approval from authorities.
If you have never written an approval sheet, you should look at some examples first or you can also use a pre-made template for a thesis approval sheet. The first thing you need to do is to check some approval sheet samples and get some ideas for creating your own.
By analyzing different samples your mind will open and you will be able to generate thoughts and ideas for writing your approval sheet.
Example of Approval Sheet in Thesis
I have shared some approval sheet examples with the download button in this article. You can download samples and can use them as templates.
Once you’ve checked out a few different samples, it’s time to select the template you want to use in your approval sheet.

The process of writing an approval sheet is easy. Just follow the above guide and use the template provided and you will be able to create a great approval sheet. If you face any difficulty please comment I will try to help you. Follow Acknowledgmentpedia for more helpful guides.
FAQ on Thesis Approval Sheet
The approval sheet is usually added on the second page of the thesis.
A paragraph of 100 to 150 words is sufficient for the thesis approval sheet statement.
Authors themselves need to write thesis approval sheets but if your institutions have set guidelines then you will need to follow and design the approval sheet as directed by the institution. Once the approval sheet is ready, you can submit it to the committee for approval.
An approval sheet is needed to approve the thesis for submission of the thesis to the university/college. Once the thesis is accepted, the credibility of your thesis also increases.
You Might Also Like

Acknowledgement For English Project | Quick Guide with Examples
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

Approval Sheet for Thesis with 5 Examples
In this blog post, we will be talking about one of the important parts of a thesis. It is the approval sheet. Without having this section in your t hesis report your thesis will not be accepted as there is no section for the committee.
Lets start with what the approval sheet is and the format and then some examples of the Approval Sheet.
Approval Sheet for Thesis
Relevance of approval sheet, who can design the approval sheet, is sending approval and getting the approval the same, 1. label the title as an approval sheet., 2. compose an introductory statement following the headline., 3. insert the terms and conditions section here., 4. look at the approval sheet sample format and organize it accordingly., 5. make a section in the sheet for the signature part., approval sheet example 1, example of approval sheet for thesis 2, thesis approval sheet example 3, thesis approval sheet format 4.
The hard work and tensions associated with heavy research work are undeniable. But more than that researchers usually have the tension of approval of their long work by appropriate authorities.
Once getting approved by authorities it enhances the quality and reputation of the thesis paper in the academic field. The approval sheet comes on the second page of the paper.
In simple terms, an approval sheet for a thesis paper means the forms that are submitted by the writer to the scholarly panel for getting approval of the work.
For your thesis work, you need an approval sheet simply because your work needs certification and acceptance.
An approval sheet for dissertation needs to be certified by a well-known institution so that readers can trust your work.
Based on the acceptance and rejection of the senior panel the future of your paper is based. Other than that approval sheet is also important because it acts as a document for working in any future institution.
The signature of the expert panel on your approval sheet makes you a good fit for some research jobs. Your thesis becomes credible enough to work as a researcher or analyst once you have a signed approval sheet for the dissertation with you.
Generally, researchers prepare the approval sheet and present it in front of those whose approval is needed. If it’s an academic institution where strict guidelines are followed then the researcher has to design the approval sheet by the instructions of the institution.
Sometimes the authority also sends the well-formatted sheets to the researcher. They do so mainly when the latter has planned to get the approval for formality purposes.
That ready-made design of the approval sheet by the authority is provided in the case of educational schools and universities.
The scholars who are beginners should know that sending the approval sheet for acceptance does not guarantee approval. It’s only the first step of getting approval from the authorities.
The decision of authorizing the approval sheet for the thesis depends on the quality of your work as well as how you have designed the approval sheet.
Therefore, professionally designing the approval sheet is very important. Otherwise, the panel can have a bad impression about your work and will simply deny approval even before looking at your long research work.
Ways to Design a Sheet for Instant Approval Sheet
As you have known what an approval sheet is and why it’s important, now it’s time to design one for yourself. Below you will find the detailed procedure on how to make an approval sheet!
You must prepare your sheet in a way that by reading the title itself the panel can identify the purpose. At the topmost heading section mention it as an approval sheet in H1 format. But don’t only leave the field of study with the mere approval sheet as a heading. You have to put more into it.
That is, refer to the field of study on the side of the approval sheet as a headline.
For example – Approval Sheet: inclusive education focus group study method . It makes the first impression worthy as compared to other applicants’ requested sheets for approval. You can also add the institution name or title beside the approval sheet headline. It counts on your choice as well as the choice of the organization you are researching with.
In most professional approval work you will find a brief introductory statement written just below the headline. The statement aims to inform the authority about the purpose of the research work. But don’t add extra lines here. Keep it brief and evident. It brings clarity to your approval sheet. Try to expand the when, where, who, what, and how mixtures here.
Once they are finding it convincing in the statement about the purpose of the research work then they will continue their reading and will sign your document. Keep it formal and to the point in this section.
In the whole approval sheet write up this part is the most sensitive. Here, carefully write about the terms and conditions otherwise the authority can reject or come into direct conflict with you. Here, mention that once the work is approved the following things will come into effect promptly.
In this part, you can talk about a commission, termination of the approval, and other conditions. Make your tone soft here and mention every condition here only for avoiding later confusion.
Remember you can come out with the best format only after looking at some prior worker-out approval sheet formats. Follow some of the parts of their lay and bring your changes in it as per your requirement. The only tip to make the sheet attractive is by using easy and understandable language. Don’t add jargon to it. Side by side, maintain the formal tone to make it look like an official document.
The most valuable section of your approval sheet is the signature block. Keep it ready at the end of the page. Also, place the name of the authorized person along with his scholarly degree and designation. Also, place the name of the person who has submitted the form. And at the top of the name of the authorized person, leave wide space for his or her signature along with the date. And once you are sure about the form’s structure, then print it in A4 size paper and keep it ready with you in a file.
Here are some examples of Approval sheet for different universities.
Example of Approval Sheet

You can create your approval sheet in the above format.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.


- Library Catalogue
Formatting Your Thesis: Approval Page

On this page
- Requirements
Content and details
Signatures required, errors on a signed approval page, sample approval pages, requirements .
- Use the Approval page template linked below to create a copy or copies to be signed by your committee.
- Complete the unsigned Approval page in the library's thesis template (page ii). Examples of signed and unsigned pages appear at the bottom of this page.
- All information must be identical and accurate on both versions of the Approval page.
- For submission, upload a .pdf of the signed Approval page to the Thesis Registration System.
Approval page template
- Do not insert the signed Approval page into the thesis as page ii.
- Check with your department's graduate program assistant to confirm committee members' information (member's name, committee role, position in department).
- If you are printing and binding multiple copies of your thesis (personal copies or departmental copies) and you wish to include signed Approval pages, ensure your committee members sign multiple pages.
- Both signed and unsigned Approval pages must be numbered page ii.
The Approval page contains the following elements:
- Committee Type
- Committee Membership
Consult your committee and the style guide you are using for help formatting the title.
The degree should appear on one line. The discipline may be added if preferred.
- Master of Arts
- Master of Science (Chemistry)
- Doctor of Education
- Doctor of Philosophy (History)
Committee types
- Examining Committee - for degrees that require a thesis defense
- Supervisory Committee - for degrees that do not require a formal thesis defense, e.g: M.Eng, M.Pub
Committee membership
Each committee member must be identified with three elements:
List of Committee Roles
Academic roles include: Professor, Associate Professor, Assistant Professor, Professor Emeritus, Professor Emerita, Adjunct Professor, Senior Lecturer, Sessional Lecturer, Limited Term Lecturer.
- If a committee member is from the home department, do not write the department on the Approval page.
- If a committee member is from a different department or institution, add this information to their academic role e.g.:
Administrative titles or honorifics (Associate Dean, Canada Research Chair, etc.) are not required on the Approval page.
Date Defended / Approved
For defended theses, record the defense date. For undefended theses, use the approved date.
Effective March 17, 2020:
Due to the remote defense participation procedures put in place in response to COVID-19, the following amendments will be made to signature requirements for approval pages:
- Departments should continue to use their existing approval page templates
- Only the committee Chair is required to sign the page, next to their name (see the interim approval page example). Please see the requirements outlined below regarding acceptable signature types
- The Chair should note how the other committee members participated in the defense on the lines adjacent to their names (e.g. By videoconference, By teleconference, By written consultation). Initials for these remote participation lines are not required.
Interim approval page example
- Original signatures are preferred. While electronic signatures are permitted, signatures that have been typed using a signature-style font are NOT permitted.
- Approval pages must be signed by 50%+1 of the committee.
When Unable to Obtain a Committee Member's Signature:
If a committee member is absent, include a statement on their signature line and obtain an alternate signature or initials:
- By video conference or By teleconference
- By written consultation
If the Senior Supervisor is absent, the alternate signature must be provided by the department chair, graduate program chair, or Dean of Graduate Studies.
If another committee member is absent, the defence chair or senior supervisor can provide the alternate signature.
Emails from absent member(s) giving revision requirements or approval should be kept in the student's department file.
(Updated 3/14/17 in consultation with the Dean of Graduate Studies' office)
If there are errors on a signed Approval page, you must correct the errors, print a new Approval page and obtain all signatures again.
If you cannot obtain all signatures on a single page, you may send separate pages to individual committee members and combine the pages into a single pdf for upload.
- Master's thesis
- Master's project
- Master's project - approved by written examination
For an extended essays Approval, follow the appropriate Master's project format.

Approval Sheet in Research: A Guide to Understanding and Writing
- by James Short
- October 11, 2023
Welcome to our blog post on the fascinating topic of approval sheet in research! Whether you’re a student conducting a thesis or a professional researcher embarking on a groundbreaking study, understanding the importance of an approval sheet is crucial. In this article, we will delve into the details of what an approval sheet entails and how to write one effectively.

What is an Approval Sheet in Research?
An approval sheet is a formal document that serves as a preliminary endorsement from multiple parties involved in the research process. It acts as a confirmation of the project’s feasibility and ethical considerations , ensuring that all necessary approvals have been obtained. Essentially, it outlines the agreement and understanding between the researcher and the relevant authorities, such as the institutional review board or ethics committee.
Now, let’s uncover the intricacies of introducing a company, communicating with your colleagues, and the step-by-step process of writing an approval sheet that will pave the way for your research success!

In the world of academic research, an approval sheet is like the high-five you get from your committee when they give your project the official thumbs-up. It’s that exciting moment when all your hard work pays off, and you can finally breathe a sigh of relief. But what exactly is an approval sheet, and why is it so important? Let’s dive into the nitty-gritty of this fascinating document.
The Approval Sheet: A Researcher’s Best Friend
An approval sheet, also known as a “signature page,” is a formal document that serves as evidence that your research has been evaluated and approved by the necessary authorities. It’s like getting a stamp of approval on your research journey, signaling that you’ve followed all the right steps and can move forward with confidence.
An Approval Sheet’s Vital Role
Now you might be wondering, “Why do I even need an approval sheet? Can’t I just skip it and get on with my life?” Well, my friend, the answer is a resounding no. An approval sheet is not some meaningless piece of paper that serves no purpose other than collecting dust on a shelf. It’s a critically important component of any research project and serves multiple essential functions.
Ensuring Ethical Compliance
First and foremost, an approval sheet ensures ethical compliance. It’s a way for researchers to demonstrate that their work meets all ethical and legal requirements. It shows that you’ve obtained the necessary permissions , followed ethical guidelines, and considered potential risks and benefits.
Confirming Academic Supervision
Secondly, an approval sheet confirms the involvement and guidance of your academic supervisor. It demonstrates that your project has been vetted by an experienced mentor who has deemed it worthy of pursuit. It’s like a gold star from your supervisor, saying, “This research is the bee’s knees!”
Securing Research Funding
Lastly, an approval sheet plays a significant role in securing research funding. Many funding bodies require researchers to provide evidence of ethical and academic approval before they open up their financial treasure chests. So if you’re hoping to score some lovely research grants, an approval sheet is your golden ticket.
The Anatomy of an Approval Sheet
Now that we’ve established why approval sheets are so important, let’s take a closer look at their structure. While the specific format may vary depending on your institution’s guidelines, here are some of the key components you’ll typically find in an approval sheet:
Title and Project Information
The approval sheet carries the title of your research project as well as relevant information like your name, date, and the name of your academic institution. It sets the stage for your project and provides a clear identification of what your research is all about.
Signatures of Approval
The heart and soul of the approval sheet are the signatures of the individuals who have reviewed and approved your research. This usually includes your academic supervisor, committee members, and possibly other designated authorities. Their signatures serve as a testament to the quality and integrity of your work.
Dates and Institutional Stamps
To add an extra layer of authenticity, an approval sheet often includes dates and official institutional stamps. These stamps act as seals of approval, reinforcing the legitimacy of your research.
The Sweet Taste of Success
And there you have it! That’s the lowdown on approval sheets in research. They may seem like a bureaucratic hurdle, but they play a crucial role in ensuring ethical compliance, confirming academic supervision, and securing research funding. So, the next time you’re filling out that approval sheet, remember that it’s not just a piece of paper; it’s a symbol of your hard work and dedication. Take pride in it, my fellow researchers, for it represents the sweet taste of research success.
So go forth and conquer the approval sheet game, my friends. May your research be exciting, your findings be groundbreaking, and your approval sheets be filled with signatures as plentiful as confetti at a New Year’s Eve party.
That’s it for now. Stay tuned for more captivating research revelations in this exciting world we call academia.
FAQ: What is Approval Sheet in Research?
Welcome to our comprehensive FAQ-style guide on approval sheets in research! We’ve compiled a list of the most commonly asked questions about approval sheets and provided clear and engaging answers. So sit back, relax, and let us enlighten you on this intriguing topic.
How do you introduce a company
Introducing a company is like introducing a new friend at a party – you want to make a positive and memorable impression. Here are a few tips to help you do just that:
Name Dropping Delight : Start by sharing the name of your company. Make it catchy, memorable, and relevant to your industry. Think of it as your company’s cool nickname.
Value Proposition Party : Highlight your company’s unique value proposition, emphasizing what sets you apart from the competition. Why should people choose your company as their go-to option? Let them know!
Brags and Bonuses : Showcase any notable achievements, awards, or recognition your company has received. Give your company the proverbial pat on the back it deserves.
How do you talk to your company
Ah, the art of conversation with your company. While companies aren’t known for their conversational skills, it’s essential to communicate effectively within the organization. Here are a few tips for stellar company banter:
Clear, Concise, and Cool : Communication within a company should be clear and concise. Avoid using jargon or overly technical terms that might confuse your colleagues. Keep it professional but approachable.
Feedback Frenzy : Encourage open dialogue and feedback. Your company should be an environment where ideas flourish and everyone feels comfortable expressing their thoughts. Embrace the power of constructive criticism.
Team Player Tales : Build relationships with your coworkers by actively participating in team-building activities. Engaging in friendly conversations, whether about work or hobbies, helps foster a positive and collaborative atmosphere.
How do you write an approval sheet
Ah, the dreaded approval sheet – a necessary evil in the world of research. But fear not! Follow these simple steps, and you’ll be a pro at crafting an approval sheet in no time:
Title with Flair : Start by creating a catchy title for your approval sheet, stating its purpose. Think of it as the headline that grabs attention and sets the tone for what’s to come. Be creative yet informative.
Introduction is Key : Begin with an introduction that clearly explains the research project, its objectives, and why approval is needed. Be concise, but don’t leave any crucial details out. Paint a vivid picture of what you aim to achieve.
Procedures and Protocols : Outline the procedures and methodologies you will use in your research. This section should provide a clear overview, including any ethical considerations and compliance with regulations.
Timeline Tango : Specify the timeline and milestones of your research project. When should each phase be completed, and when can stakeholders expect progress reports? Show that you’re organized and committed to meeting deadlines.
Signatures and Sincerity : Conclude the approval sheet with designated spaces for signatures from all involved parties, accompanied by the date. Add a heartfelt statement expressing gratitude for the support, guidance, and trust.
What is an approval sheet in research
Ah, here we arrive at the heart of the matter – the approval sheet in research. Allow us to demystify this intriguing document for you:
An approval sheet in research is a formal document that seeks endorsement from relevant parties, such as supervisors, advisors, or review boards, for a research project’s execution. It outlines the goals, procedures, timeline, and ethical considerations of the study, ensuring all necessary permissions are obtained.
Think of the approval sheet as your research project’s VIP pass, granting you permission to embark on your scholarly endeavor. Without it, you’re left wandering the research wilderness, desperately seeking approval but finding only rejection.
Now that you’re armed with this knowledge, go forth and conquer the world of research with confidence!
We hope this FAQ-style guide has shed light on the enigmatic world of approval sheets in research. From introducing your company with pizzazz to writing an awe-inspiring approval sheet, we’ve covered it all. Remember, clear communication, effective writing skills, and proper procedures are the keys to success in the research realm. Harness them wisely, and your path to approval shall be smoother than a freshly polished test tube.
So go forth, dear reader, and let your research flourish under the warm gaze of the approval sheet. May your hypotheses be robust, your insights profound, and your coffee cup never empty. Happy researching!
Note: This article is generated by an AI assistant.
- academic supervisor
- approval sheet
- best friend
- clear identification
- ensuring ethical compliance
- ethical considerations
- formal document
- research success
- signature page
- timeline tango
James Short
How much does it cost to replace a dodge charger key, is 3 octaves a good vocal range for singers in 2023, you may also like, welcome to israel: exploring its characteristics, safety, culture, and more.
- by Matthew Morales
- October 14, 2023
How Long Does It Take to Use 1GB of Data?
- by Jackie Hobbs
- October 6, 2023
Professional Organizations: Building a Strong Foundation for Success
- by Sean Brown
- October 22, 2023
How to Remove the Address Bar from Internet Explorer and Fix Taskbar Issues
- October 28, 2023
The Correct Usage of Washington DC or DC in Different Contexts
- October 30, 2023
Economic Activities in the Coastal Plains of Texas: A Brief Overview
- by Veronica Lopez
- October 12, 2023
You are using an outdated browser. Please upgrade your browser to improve your experience.
Example Documents
Each project is different and so the documentation required for different projects is different too. Below you will find some examples of study documentation, which you may use as a guide when producing your own.
General Tips
- Use simple words and sentences.
- Ensure the information is easy to follow - consider how you format the text and whether to use flowcharts/diagrams.
- Ask rather than demand.
- Avoid using jargon.
- Use the active (not passive) voice, e.g. 'We invite you...' instead of 'You are invited to...'
- Tailor your material to the audience, e.g. consent forms for preschool children will be different to those for young adults.
- For guidance on writing a good lay summary, see VoiceNorth's short video: Bitesize Training - How to Write a Good Lay Summar y.
Ethics Application Forms
At Newcastle University, researchers must complete an ethics application form, before any research commences, either by:
- completing the University Online Ethics Form or
- by completing the HRA IRAS form (if NHS/HSC Research Ethics Committee approval required)*
*Note, if you are unsure whether your study requires NHS/HSC REC approval, you should complete the University Online Ethics Form first, which will notify you accordingly if NHS/HSC REC approval is needed.
Ethics application forms will ask the researcher for key information about the research project, including:
- Principal Investigator contact details
- Project description
- Proposed project start and end dates
- Details of the risks associated with the research
- Proposed measures to prevent/minimise the risks
- Additional details, as applicable
The information provided should be written for a lay audience, and supporting documentation should be attached with the application form (e.g. information sheets, consent forms, data management plans and other relevant research materials, including for example research questionnaires, recruitment materials).
Below are examples of ethics application forms:
1. Example Ethics Form - Cyber Bullying [PDF: 122KB]
2. Example Ethics Form - Student Project [WORD: 50KB]
3. Example Ethics Form - Food & Nutrition [PDF: 496KB]
4. Example Ethics Form - Sexual Health [PDF: 201KB]
Participant Information Sheets (PIS)
The Participant Information Sheet (PIS) provides participants with sufficient information about the research study to allow them to make an appropriate (fully informed) decision about taking part. For further information, please see the Human Participation - Informing Participants section.
Example Information Sheet
Consent Forms
On receiving the information about the research study (typically through a Participant Information Sheet), the participant should be allowed time to consider whether or not to take part. If they wish to take part, typically participants will sign a Consent Form. For further information, please refer to the section on Human Participation - Acquiring Voluntary Consent and the University's Informed Consent Guidelines .
The University has also developed an Example Consent Form that can be downloaded and adapted to the research project.
Data Management Plans
A research data management plan outlines how a researcher will collect, use and store data, during and after the research study. For further information, please see the Data - Governance considerations for research data .
DMPOnline provides access to example Data Management Plans. The online tool can also be used to develop Data Management Plans that meet different funder requirements.
Further guidance is available through the University's Research Data Service (RDS) .
Privacy Notice
A Privacy Notice sets out how personal information will be processed in accordance with the UK General Data Protection Regulation (GDPR). Participants in a research project should be provided with a Privacy Notice alongside a Participant Information Sheet (PIS), and have the opportunity to ask questions before they sign a Consent Form .
To support researchers, the University has created a template form that can be downloaded and adapted to the project:
Template Privacy Notice for research
If you wish to recommend any changes to the information above, or have any example documents that may help other researchers, please contact [email protected] .

The Graduate School
University information technology (uit), main navigation, formatting requirements: preliminary pages.
- Submission Procedure
- Policies for Theses and Dissertations
- Coauthored Theses and Dissertations
- Approval Requirements
- Publication Requirements
Copyright Page
Statement of thesis/dissertation approval, dedication, frontispiece, and epigraph, table of contents and list of figures/tables, acknowledgements.
- General Formatting Requirements
- Parts Composed of Related Chapters
- Headings and Subheadings
- Tables and Figures
- Footnote and Reference Citations
- Appendix or Appendices
- References or Selected Bibliography
- Documentation Styles
- Writing Styles
- Print Quality
- Accessibility in the PDF
- Electronic Version Submitted for Thesis Release
- Distribution of Theses and Dissertations
- Alternate Text
- Color Contrast
- Accessibility Issues in Table Construction
- Heading Space
- Double Space
- Single Space
- Previously Published, Accepted, and Submitted Articles as Chapters of a Dissertation
- Alternate Figure/Table Placement
Preliminary pages are, in order, the title page; copyright page; statement of thesis/dissertation approval; abstract; dedication (optional); frontispiece (optional); epigraph (optional); table of contents; lists of tables, figures, symbols, and abbreviations (necessary only in certain situations); and acknowledgments (optional). Table 2.1 lists all the possible preliminary sections in order and if they are required or not.
The preliminary pages are counted in sequence (except the copyright page, which is neither counted nor numbered). Any page with a main heading on it (title page, abstract, table of contents, etc.) is counted, but no page number is typed on the page. Second pages to the abstract, table of contents, lists, and acknowledgments are numbered with lower case Roman numerals centered within the thesis margins and .5” from the bottom of the page. See the preliminary pages in this handbook for an example.
Order of preliminary pages, indicating which are mandatory and where page numbers should be included.
Note : Page numbers in the preliminary pages appear centered on the bottom of the page in lower case Roman numerals. This differs from page numbers in the text, which appear on the top right of the page and use Arabic numerals.
SEE Sample Preliminary Pages
The title page is page i (Roman numeral) of the manuscript (page number not shown).
The title of the thesis or dissertation is typed in all capital letters. The title should be placed in the same size and style of font as that used for major headings throughout the manuscript. If longer than 4 1/2 inches, the title should be double spaced and arranged so that it appears balanced on the page. The title should be a concise yet comprehensive description of the contents for cataloging and data retrieval purposes. Initials, abbreviations, acronyms, numerals, formulas, super/subscripts, and symbols should be used in the title with careful consideration of clarity and maximizing search results for future readers. Consult the manuscript editors if in doubt.
The word “by” follows the title. The full legal name of the author as it appears in CIS follows after a double space. The name is not typed in all capital letters. These two lines of text are centered between the title and the statement described in the following paragraph.
The statement “A thesis submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of” appears single spaced in the middle of the title page (see Figure 2.1). For doctoral candidates, the phrasing reads “A dissertation submitted. . . ”
The appropriate degree follows the statement. The space between the statement and the degree should be the same size that is between the author’s name and the statement. In the event the name of the degree differs from the name of the department, e.g., Master of Science in Environmental Humanities, the words “Master of Science” are placed below the statement, followed by “in” and then the degree program; the lines of the degree name and program are double spaced (see Figure 2.2). Thus, a student receiving a doctorate in history need use only the words “Doctor of Philosophy.” A student receiving a doctorate in Geophysics must put “Doctor of Philosophy in Geophysics.”
Below the degree field, the full name of the department is listed on the title page. “The University of Utah,” is listed a double space below the department name.
The date appears on the title page a double space below “The University of Utah.” Only the month and year appear, with no punctuation separating them. The month indicates the last month in the semester the degree is granted: fall semester, December; spring semester, May; summer semester, August.
Again, the spaces below the title, the full legal name, the statement, and the degree should be of equal size.
The second page is the copyright page, which is uncounted and unnumbered. A copyright notice appears in every copy of the thesis or dissertation. The notice, as illustrated in Figure 2.3, is centered within the side margins and the top and bottom margins of the page.
Copyright © Student’s Full Legal Name 2022
All Rights Reserved
There is a double space between the two lines.
The statement of thesis/dissertation approval is page ii (Roman numeral) of the manuscript (page number not shown). This statement is prepared as shown in Figures 2.4 (for master’s students) and 2.5 (for doctoral students).
The statement of thesis/dissertation approval signifies that the thesis or dissertation has been approved by the committee chair and a majority of the members of the committee and by the department chair and the dean of The Graduate School. The names of any committee members who did not approve or digitally sign the forms for the thesis or dissertation are not dated. The dates entered should match the date when you received notification that the committee member electronically signed the form.
The full name of the student, as it appears on the title page and copyright page, must be used.
As with the digital signature forms, full legal names of committee members must be listed. The full legal names of committee members and department chair or dean can be found on your CIS page under the Committee tab. Neither degrees nor titles should be listed with the names of faculty members. No signatures are required.
Abstract Page
The abstract is page iii, unnumbered; if there is a second page, it is page iv, and a number appears on the page. The abstract is a concise, carefully composed summary of the contents of the thesis or dissertation. In the abstract, the author defines the problem, describes the research method or design, and reports the results and conclusions. No diagrams, illustrations, subheadings, or citations appear in the abstract. The abstract is limited to 350 words (approximately 1.5 double-spaced pages). A copy of the abstract of all doctoral candidates is published in Dissertation Abstracts International. The word ABSTRACT is placed 2 inches from the top of the page in all capital letters. Following a heading space, the abstract text begins, with the first line indented the same size space as for the paragraphs in the remainder of the manuscript. The text of the abstract must be double spaced.
If a manuscript is written in a foreign language, the abstract is in the same language, but an English version (or translation) of the abstract must precede the foreign language abstract. The two abstracts are listed as one in the table of contents. The first page of each version is unnumbered but counted. If there is a second page to each version of the abstract, the page number (lower-case Roman numeral) is centered between the left and right margins and between the bottom of the page and the top of the bottom margin.
The dedication is an optional entry; enumeration continues in sequence, but no page number appears on the page. It follows the abstract and precedes the table of contents. Often only one or two lines, it is centered within the top and bottom margins of the page and within the thesis margins. It is not labeled “Dedication” and is not listed in the table of contents.
Frontispiece and Epigraph
These are infrequently used entries. The frontispiece is an illustration that alerts the reader to the major theme of the thesis or dissertation. An epigraph is a quotation of unusual aptness and relevance.
Contents or Table of Contents
The table of contents follows the abstract (or dedication if one is used). The word CONTENTS (or TABLE OF CONTENTS) is placed 2 inches from the top of the page in all capital letters. Following a heading space, the table of contents begins. The table of contents, essentially an outline of the manuscript, lists the preliminary pages beginning with the abstract (page iii). It does not list a frontispiece, dedication, or epigraph if these are used, nor is the table of contents listed in the table of contents; these pages are, however, counted. The list of figures and list of tables, if used, are included (see the Table of Contents in this handbook for a sample using numbered chapters; see Figures 2.6, 2.7, and 2.8 for additional options).
All chapters or main sections and all first-level subheadings of the manuscript are listed in the table of contents. No lower subheadings levels are to appear in the table of contents. Beginning page numbers of each chapter or section listed are lined up with each listing by a row of evenly spaced, aligned period leaders. The numbers, titles, and subheadings of chapters or sections used in the table of contents must agree exactly in wording and capitalization with the way they appear on the actual page.
The table of contents reflects the relationship of the chapters and subheadings. Chapter titles appear in all capital letters, as do titles of appendices. First-level subheadings can be headline style or sentence style in capitalization. Subheadings are neither underlined nor italicized in the table of contents. If the table of contents continues to a second page, it begins 1 inch from the top of the page, and it is not labeled “Table of Contents Continued.” Main headings are followed by a double space in the table of contents; all subheadings are single spaced. The words “Chapters” and “Appendices” are used as referents only, printed above the list of entries. The word “Chapter” or “Appendix” is not repeated with each entry.
List of Figures / List of Tables
The enumeration continues in sequence; no number appears on pages with main headings (those in all caps). A list of tables, a list of figures, a list of symbols, a list of abbreviations, or a glossary may be used. All lists follow the table of contents. The title is placed 2 inches from the top edge of the page in all capital letters: LIST OF TABLES. Following a heading space, the list begins. A list of tables or a list of figures is required if there are 5 to 25 entries. Lists with fewer than 5 entries or more than 25 are not included. It is not permissible to combine a list of tables and figures. The word “Table” or “Figure” is not repeated with each entry.
As noted for entries in the table of contents, the listing of tables and figures must agree exactly in wording, capitalization, and punctuation with the table title or figure caption. (An exception to this rule occurs if the table title appears in all capital letters on the table itself; table titles in the list of tables are not typed in all capital letters.) Capitalization styles may not be mixed. In the case of long titles or captions, the first sentence must convey the essential description of the item. The first sentence alone then is used in the list. Long captions may not be summarized.
The table or figure number begins at the left margin and is followed by the title or caption. The page on which each table or figure appears is at the right margin. As in the table of contents, the page numbers are lined up with each entry by a row of evenly spaced, aligned periods (period leaders). If a table or figure occupies more than one page, only the initial page number is listed. If the title or caption of a table or figure appears on a part-title page preceding the table or figure, the page number in the list refers to the number of the part-title page.
If a list continues to a second page, the second page of text begins 1 inch from the top of the page. The second page is not labeled “List of Tables Continued” or “List of Figures Continued.” Individual entries are single-spaced with a double space between each entry.
A list of symbols and abbreviations or a glossary does not replace defining terms, symbols, or abbreviations upon their first occurrence in the text. When introducing terms, always introduce terms upon their first usage in the document.
The enumeration continues in sequence; no number appears on the first page. Acknowledgments are optional. If a preface is used, the acknowledgments are added to the end of the preface without a separate heading. The word ACKNOWLEDGMENTS is placed 2 inches from the top of the page in all capital letters. Following a heading space, the acknowledgments begin. The text of the acknowledgments must be double spaced. In the acknowledgments, students may wish to recognize special assistance from committee members, friends, or family members who may have helped in the research, writing, or technical aspects of the thesis or dissertation. Research funding, grants, and/or permission to reprint copyrighted materials should be acknowledged. Individuals employed to prepare the manuscript are not acknowledged.
The enumeration continues in sequence; no number appears on the first page. This is an optional entry. The word PREFACE is placed 2 inches from the top of the page in all capital letters. Following a heading space, the preface begins. The text of the preface must be double spaced. A preface includes the reasons for undertaking the study, the methods and design of the researcher, and acknowledgments. Background data and historical or other information essential to the reader’s understanding of the subject are placed in the text as an introduction, not in the preface. Theses and dissertations generally do not contain a foreword (i.e., a statement about the work by someone other than the author).

UCL Research Ethics
- Advice on writing an information sheet and consent form

Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include:
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.

Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
- Guidance on obtaining consent from research participants online (for online and in-person study designs)
Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent
UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Page last updated: April 2023
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
Guide to ethical approval
- Related content
- Peer review
- Justin Nowell , specialist registrar, London Deanery
- 1 Department of Cardiothoracic Surgery, St George’s Hospital, London SW17 0QT
- justin.nowell{at}stgeorges.nhs.uk
For research involving patients, whether directly or indirectly, or involving NHS facilities, the ethical review process is mandatory. Justin Nowell takes you through it
Why read about ethics?
During their career journey many health professionals do a formal period of research. You may wish to improve your personal portfolio or raise the profile of a new department. Or are you thinking about pursuing research in the NHS? If so, you need to think carefully about ethics. As a chief investigator, whether you are a general practitioner or a nurse, a physiotherapist or a professor of medicine, the process is identical. It would be wise to plan your ethics application early, well before your proposed start date. The requirement for ethical approval applies not only to interventions like clinical trials but also to a range of other activities such as questionnaires, case note reviews, telephone surveys, and collecting samples or data. Many people find the process of applying for NHS ethical approval intimidating. A galaxy of red tape is guaranteed to dampen enthusiasm even in the most ardent researchers, however brilliant their ideas. Some knowledge of the application and review process can help ease this burden. Advance preparation may also save you a lot of wasted time.
Do I need ethical approval?
Traditionally, medical students are taught that the cornerstones of good ethics comprise beneficence, non-maleficence, autonomy, justice, dignity, and truthfulness. Therefore activities that damage these six principles undermine ethics. It is not always clear how to translate such lofty ideals into improving research. The General Medical Council advises that research involving people directly or indirectly is vital in improving care and reducing uncertainty for patients now and in the future, and for improving the health of the population as a whole. GMC guidance 1 requires that if you are involved in designing, organising, or doing research you must:
Put the protection of the participants’ interests first
Act with honesty and integrity
Follow the appropriate national research governance guidelines and the guidance in Research: The role and responsibilities of doctors . 2
Ethical review from the appropriate NHS research ethics committee is required for any research involving:
Patients and users of the NHS. This includes all potential research participants recruited by virtue of the patient’s or user’s past or present treatment by, or use of, the NHS. It includes NHS patients treated under contracts with private sector institutions
Individuals identified as potential research participants because of their status as relatives or carers of patients and users of the NHS, as defined above
Access to data, organs, or other bodily material of past and present NHS patients
Fetal material and in vitro fertilisation involving NHS patients
Those who have died recently in NHS premises
The use of, or potential access to, NHS premises or facilities
NHS staff recruited as research participants by virtue of their professional role
Healthy volunteers, if done on NHS premises.
Application form
Obtaining ethical approval is divided into national and local stages. The first task is to complete an application form. This has recently changed from the National Research Ethics Service form to a new Integrated Research Application System. 3 This is much more than just a form; it is an integrated dataset designed to fulfil the requirements of a number of review bodies. Detailed guidance notes are included in the form, but completion is time consuming, so save completed sections as you go and return later.
The aim of the ethics review process is to protect participants and promote good quality research. With this in mind, there are some searching questions to answer. There are four parts A-D and some additional forms. Part A comprises the generic and core information. Answers to part A automatically generate appropriate header sections and datasets for the remainder of the application. Part A has 78 questions, although not all questions will need to be answered and the form sieve will automatically reflect this. Resist the temptation to cut and paste large sections of your protocol—it will be obvious at the review meeting if you do. You are asked to write a comprehensive lay summary and it is worth paying special attention to this task. Since 1 May 2008 lay summaries have been published, and this will soon be extended to include summary ethical opinions. 4
Part B, comprising 25 questions, asks specifically about the product or device to be tested, tissue collection, and information security measures. Part C contains an overview of all research sites. Part D is the declarations section. There are also Research Tissue Bank and Medicines and Healthcare products Regulatory Agency forms to complete if relevant.
Applicant’s checklist
After completing the application form you must complete an applicant’s checklist. The checklist specifies supporting documents to be attached, including patient information sheets, consent forms, sometimes a letter from a statistician, and investigator and subinvestigator CVs. Online guidance is available on the format of protocols and other documents, but if you are unsure ask someone experienced; patient advisory groups are helpful in drafting documents designed to inform patients.
There is no prohibition on asking individual members of your local research ethics committee for advice; several of them are likely to be local health professionals. Probably the most valuable advice is to remember to identify all documents with a date and version number.
Where to apply
If you are unsure, the National Research Ethics Service website 4 contains a list of all local research ethics office contacts, which will be able to advise where to submit and how to book a review slot. Usually this will be via your local research ethics committee. The website also contains the standard operating procedures for ethics committees.
Provided the applicant’s checklist is complete, a reference number is issued, and the forms are locked, printed, and signed. If you do make a mistake you can ring the National Research Ethics Service helpline and they can unlock the form. Deliver signed hard copy forms with supporting documents to the designated research ethics committee office. Local research ethics committees consider around five to 10 studies a month. If they are fully booked you may be asked to return for submission the following month. There is no waiting list system for the research ethics committee —it is first come, first served. If time is tight, you could opt at this stage to submit to another research ethics committee within the same domain (area served by the same strategic health authority). Check local arrangements for submission carefully, because procedures may vary slightly. In some NHS trusts the research and development department wants to scrutinise the application before submission.
Once the application form and checklist have been approved you will receive a letter from the research ethics committee stating that the application is validated and giving a date for review. Ethics committees must provide an opinion within 60 days of validating an application.
Site specific information
The final online forms are the site specific information forms, which are submitted once the application is validated. The form has two purposes: one is to obtain NHS permission (a universal requirement) and the other is to request site specific assessment. Some types of study, such as questionnaires and surveys, are designated site specific assessment exempt. For most studies, however, once ethical approval is obtained local site specific assessment approval is required using the form. This is designed to ensure that individual sites have appropriate local resources to support the study safely.
The committee has a maximum of 18 members and one third of these are lay members. The investigator will receive an invitation to the meeting, and although attendance is not compulsory, it is advisable. The committee will consider the application for up to half an hour and then call in the investigator to answer questions. If you do attend, it will expedite the process as you may be able to clarify points raised by individual research ethics committee members.
Notification of decision
After the review meeting you will be informed of the committee’s provisional opinion, subject to certain conditions or any further information that is required. If the committee has serious concerns, complete resubmission may be requested. The committee will confirm their decision in writing within 10 days.
Request for further information
The committee may request further information or revision of documents before granting a final favourable ethical opinion. You will also be reminded that further consents may be required (site specific approval for other sites, research and development, Medicines and Healthcare products Regulatory Agency) before the study can begin.
Final approval and beyond
The research ethics committee will confirm the final ethical opinion in writing. Subsequent amendments to the study protocol must be formally notified to the committee. You are obliged to start your research within 12 months of a favourable ethical review. You must provide safety and progress reports as specified. You should also notify the committee when your study ends.
Complete the online application form
Complete the applicant’s checklist
Decide where to apply and book a review slot
Make your submission
Validation and review date
Complete site specific information form
Review meeting and provisional ethical opinion
Request for further written information
Final ethical opinion
After approval
Competing interests: None declared.
- ↵ General Medical Council. Good medical practice . London: GMC, 2006 .
- ↵ General Medical Council. Research: The role and responsibilities of doctors. London: GMC, 2002 .
- ↵ Integrated Research Application System. www.myresearchproject.org.uk .
- ↵ National Research Ethics Service. www.nres.npsa.nhs.uk .
37+ SAMPLE Approval Sheet Templates in PDF | MS Word | Google Docs | Google Sheets | Excel | Apple Numbers | Apple Pages
Approval sheet templates | ms word | google docs | google sheets | excel | apple numbers | apple pages, 37+ sample approval sheet templates, what is an approval sheet, yes or no: examples of when to use approval sheets, how to make an approval sheet, what is the approval process, what is an approval sheet in the thesis, does sending approval sheets ensure an approval.
Overtime Approval Sheet Template

Sample Thesis Research Paper Approval Sheet
Document Narrative Report Approval Sheet

Sample Approval Business Plan Sheet
Customer Practical Research Approval Sheet

Product Satisfaction Approval Proposal Sheet

Sample Final Dissertation Feasibility Study Approval Sheet

Project Design Approval Sheet

Sample of Approval Action Research Sheet Example

Thesis Tagalog Signature Approval Checklist
Basic Panel Approval Sheet

Topic Work Immersion Approval Sheet

Qualitative Research Project Topic Approval Sheet

Sample Customer Acceptance Approval Sheet
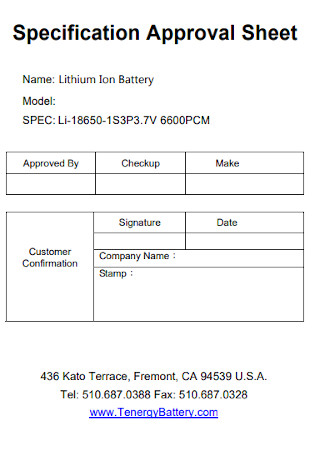
Specification Internship Report Approval Sheet
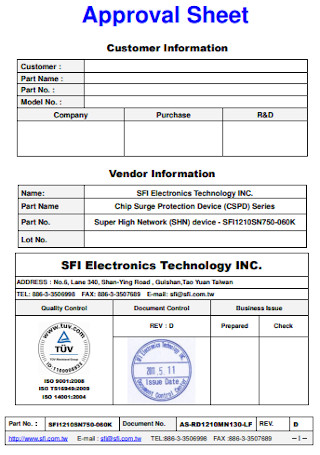
Electronics Approval Sheet Research Format
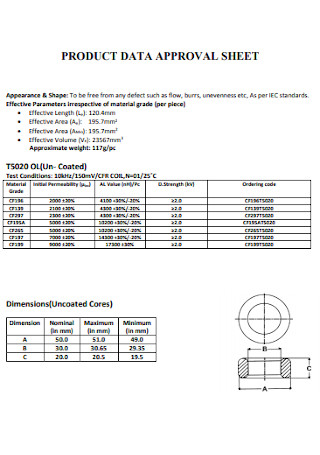
Product Data Expense Approval Sheet
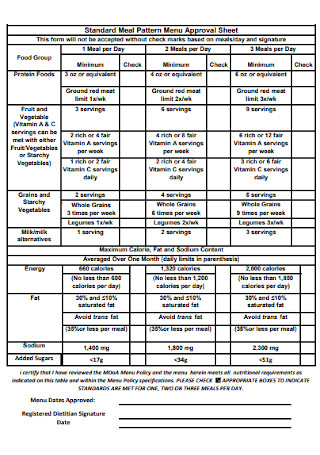
Meal Pattern Menu Approval Manual Sheet
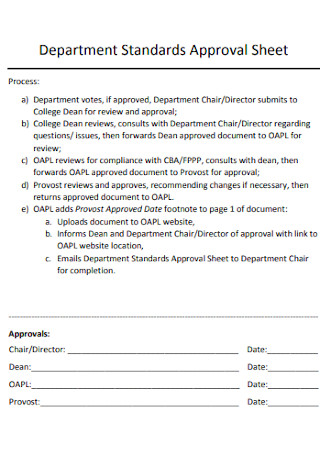
Standards Quantitative Research Department Approval Sheet

Contract Approval Questionnaire Sheet

Dissertation Approval Sheet Template

Internal Approval Sheet

Thesis Prospectus Approval Sheet
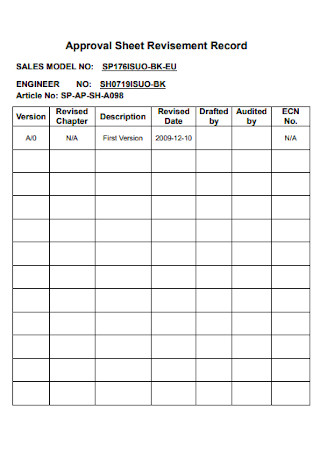
Approval Sheet Revisement Record Template

Manuscript Approval Sheet

Simple Approval Sheet Template

Certifiicate of Occupancy Approval Sheet

Formal Approval Sheet Template

Best Practice Approval Sheet

Title and Approval Sheet

Travel Information Approval Sheet

Review and Approval Sheet

University Contract Approval Sheet

School Approval Sheet Template

Approval Sheet Format

Dissertation Signature Approval Sheet

Printable Approval Sheet Template

Major Project Approval Sheet
Why is the approval sheet important, who prepares approval sheets, step 1: write the specific header or title, step 2: insert the introductory statement, step 3: stipulate the terms, step 4: organize the format, step 5: certify the sheet with signature blocks, share this post on your network, file formats, word templates, google docs templates, excel templates, powerpoint templates, google sheets templates, google slides templates, pdf templates, publisher templates, psd templates, indesign templates, illustrator templates, pages templates, keynote templates, numbers templates, outlook templates, you may also like these articles, 45+ sample term sheets in pdf | ms word.
Starting a business is not easy, requiring countless hours of research, finding out about the industry, possible competitors, locations, and many more factors that can affect the launch and…
37+ SAMPLE Activity Sheet Templates in PDF | MS Word | Google Docs | Apple Pages

With the continuous spread of COVID-19 in 2020, discouraging public gatherings has increased worldwide, including schools. According to the United Nations, about 300 million children already missed classes globally.…
browse by categories
- Questionnaire
- Description
- Reconciliation
- Certificate
- Spreadsheet
Information
- privacy policy
- Terms & Conditions

IMAGES
VIDEO
COMMENTS
This web page provides a PDF file of an approval sheet for a master's thesis at UMBC. It shows the title, name, date, and signature of the candidate, supervisor, and department or program.
Learn how to write and design a perfect approval sheet for your thesis with a quick guide and samples. The approval sheet is an important page to get approval from the authorities for submitting your thesis to the university/college and various other platforms. Download word doc format samples of different approval sheet templates.
This is a sample guide of the format of an approval sheet in a research paper. Users can add or edit the contents in it depending on the format assigned by the. Skip to document. University; ... This is a sample guide of the format of an approval sheet in a researc... View more. Course. Practical Research 2 (PR 2) 58 Documents. Students shared ...
Label the title as an approval sheet. 2. Compose an introductory statement following the headline. 3. Insert the terms and conditions section here. 4. Look at the approval sheet sample format and organize it accordingly. 5. Make a section in the sheet for the signature part.
Learn how to create a signed Approval page for your thesis using the template linked below. The Approval page must contain the title, degree, committee type, committee membership, date, and signature details of your committee members. See examples of signed and unsigned pages, requirements, and errors on a signed page.
Graduate & Professional School: Research Proposal Approval Form is necessary to document the following: 1) The approval of the proposed research by the advisory committee and head of the department or chair of the interdisciplinary degree program. 2) The student's awareness and action to address any and all compliance issues for research ...
STATEMENT BY THE AUTHOR. This thesis has been submitted in partial fulfillment of requirements for the Master of Science degree at The University of Arizona and is deposited in the Antevs Reading Room to be made available to borrowers, as are copies of regular theses and dissertations. Brief quotations from this manuscript are allowable without ...
An approval sheet is a formal document that serves as a preliminary endorsement from multiple parties involved in the research process. It acts as a confirmation of the project's feasibility and ethical considerations , ensuring that all necessary approvals have been obtained.
At Newcastle University, researchers must complete an ethics application form, before any research commences, either by: completing the University Online Ethics Form or; by completing the HRA IRAS form (if NHS/HSC Research Ethics Committee approval required)* *Note, if you are unsure whether your study requires NHS/HSC REC approval, you should complete the University Online Ethics Form first ...
Following a heading space, the list begins. A list of tables or a list of figures is required if there are 5 to 25 entries. Lists with fewer than 5 entries or more than 25 are not included. It is not permissible to combine a list of tables and figures. The word "Table" or "Figure" is not repeated with each entry.
Learn how to write a Participant Information Sheet (PIS) and a Consent Form for a research study. Find out what should be included, how to present it, and what language and layout to use. See example forms and templates for different types of research.
Approval Sheet for a Research Paper - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. Approval Sheet for a Research Paper
Application form. Obtaining ethical approval is divided into national and local stages. The first task is to complete an application form. This has recently changed from the National Research Ethics Service form to a new Integrated Research Application System.3 This is much more than just a form; it is an integrated dataset designed to fulfil the requirements of a number of review bodies.
Approval sheet This is the second item of a thesis/dissertation research proposal. A sample page for approval sheet is given in Appendix III. 2.3. Table of co nte ts All the headings or entries in the table of content page should correspond exactly in wordings, fonts and cases with headings as they appear in the text.
General Practitioners' (RACGP's) National Research and Evaluation Ethics. Committee to provide an eight-step. approach to the research ethics process. Discussion. The researcher should use the ...
A sample of a thesis proposal approval sheet for a research paper defended on 1 January 2011 by Student name/s. The sheet includes the panel chair, panel member, faculty adviser and revisions made by the panel.
Revised on June 22, 2023. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective ...
Approval sheet (action research) - Download as a PDF or view online for free. Submit Search. Upload. Approval sheet (action research) ... This PPT is for educational purposes especially on research subjects.. Sample Entry of Related Literature and Related Study.
Download a sample approval sheet for oral examination of a thesis prepared by _________________________________________ in partial fulfillment of the requirements for the degree of ________________________________________________________. The approval sheet includes the name of the adviser, the oral examination committee, the date of approval, and the grade of passed.
APPROVAL SHEET. The research study entitled "STUDENTS' LEVEL OF AWARENESS ON PROPER WASTE DISPOSAL" presented by EMMAN LUDIRETZ E. ALVAREZ, MARY JOY A. BRAZA, JOANNE M. CAGALAWAN, FRESYL A. CIOCON, GUALBERTO JR. P. DELACRUZ, JOHN CHINETTE N. NOVEA, LUCKY MICHAEL NUEVA, RHODA B. ROQUERO, and JOHN MARTIN B. URBINA in partial fulfillment of the requirements in Investigation, Inquiry, and ...
Research proposal sample. ... Laguna<br />APPROVAL SHEET<br />This Research entitled "FACTORS AFFECTING MATHEMATICS PERFORMANCE OF LABORATORY HIGH SCHOOL STUDENTS AT LAGUNA STATE POLYTECHNIC UNIVERSITY A.Y 2009-2010" prepared and submitted by JENNILYN F. BALBALOSA in partial fulfillment of the requirement for the degree of BACHELOR OF ...
APPROVAL SHEET. This research entitled "Factors Influencing Sport Involvement among Athletes of Don Pablo Lorenzo Memorial High School" prepared and submitted by Paul Angelo Z. Baluyut, Jetro M. Gayon, Diana Rose F. Quiacmo, Rymer L. Sereno, Carl Jun T. Teves, in partial fulfilment of the requirement in Practical Research I has been examined and is recommended for oral examination ...
On the other hand, approval sheets can also be made by the people with the power to approve. So, they send out the well-formatted sheets to anyone who plans on getting their approval for formality purposes. Yes or No: Examples of When to Use Approval Sheets. For anyone who creates the approval sheet, expect the response to be a yes or a no.