An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Stress and hormones
Salam ranabir.
- Author information
- Copyright and License information
Corresponding Author: Dr. Salam Ranabir, Singjamei, Chingamakha, Liwa Road, Imphal, India. E-mail: [email protected]
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In the modern environment one is exposed to various stressful conditions. Stress can lead to changes in the serum level of many hormones including glucocorticoids, catecholamines, growth hormone and prolactin. Some of these changes are necessary for the fight or flight response to protect oneself. Some of these stressful responses can lead to endocrine disorders like Graves’ disease, gonadal dysfunction, psychosexual dwarfism and obesity. Stress can also alter the clinical status of many preexisting endocrine disorders such as precipitation of adrenal crisis and thyroid storm.
Keywords: Graves’ disease, hormones, stress
I NTRODUCTION
‘Stress’ may be defined as any situation which tends to disturb the equilibrium between a living organism and its environment. In day-to-day life there are many stressful situations such as stress of work pressure, examinations, psychosocial stress and physical stresses due to trauma, surgery and various medical disorders. In this review, we will highlight in brief the hormonal changes in stress and its impact on the endocrine system with particular emphasis on Graves’ disease.
H ORMONAL C HANGES D URING S TRESS
In response to stress, the level of various hormones changes. Reactions to stress are associated with enhanced secretion of a number of hormones including glucocorticoids, catecholamines, growth hormone and prolactin, the effect of which is to increase mobilization of energy sources and adapt the individual to its new circumstance.
Activation of the pituitary-adrenal axis is a prominent neuroendocrine response to stress, promoting survival. Stimulation of this axis results in hypothalamic secretion of corticotrophin-releasing factor (CRF). CRF then stimulates the pituitary to adrenocorticotropin (ACTH), 8-lipotropin and 3-endorphin. Plasma levels of these hormones can increase two- to fivefold during stress in humans.[ 1 ] The paraventricular nucleus of the hypothalamus is responsible for the integrated response to stress.[ 2 ] Norepinephrine, serotonin and acetylcholine mediate much of the neurogenic stimulation of CRF production.[ 3 ]
C ATECHOLAMINES
Stimulation of the pituitary-adrenal axis is associated with release of catecholamines. This leads to increased cardiac output, skeletal muscle blood flow, sodium retention, reduced intestinal motility, cutaneous vasoconstriction, increased glucose, bronchiolar dilatation and behavioral activation.[ 4 ] Timio et al .,[ 5 ] have reported increased activation of the adrenosympathetic system during occupational stress.
V ASOPRESSIN
Acute stress leads to rapid release of vasopressin from the paraventricular nucleus of the hypothalamus along with corticotrophin releasing hormone CRH. Vasopressin can stimulate secretion of ACTH from the pituitary by acting on the V1b receptor, potentiating the effect of CRH. During chronic stress with corticotroph responsiveness there is preferential expression of hypothalamic vasopressin over CRH.[ 6 ]
G ONADOTROPINS
In stress there is suppression of circulating gonadotropins and gonadal steroid hormones leading to disruption of the normal menstrual cycle.[ 7 ] Prolonged exposure to stress can lead to complete impairment of reproductive function.[ 8 ] Gonadotrophin releasing hormone GnRH drive to the pituitary is decreased, probably due to increased endogenous CRH secretion.
T HYROID H ORMONES
Thyroid function is usually down-regulated during stressful conditions. T3 and T4 levels decrease with stress. Stress inhibits the thyroid-stimulating hormone (TSH) secretion through the action of glucocorticoids on the central nervous system.[ 9 ]
G ROWTH H ORMONE
The growth hormone (GH) level is increased during acute physical stress. The level can increase up to two- to tenfold. Because of its insulin-antagonistic effect, GH may enhance metabolic activity. In psychological stress, however, GH responses are rarely seen.[ 10 ] Rather there is GH secretory defect with prolonged psychosocial stress.[ 11 ]
Depending on the local regulatory environment at the time of stress, prolactin level can either increase or decrease. Vasopressin and peptide histidine isoleucine may be involved in the secretion of prolactin during stress.[ 12 ] However, the teleological significance of change in the prolactin level is uncertain. It may affect the immune system or some aspect of homeostasis.
Insulin may decrease during stress. This along with increase in its antagonistic hormones can contribute to stress-induced hyperglycemia.[ 13 ]
S TRESS AS A P RECIPITATING F ACTOR /C AUSE OF E NDOCRINE D ISORDERS
Hyperthyroidism.
The relationship between stressful life events and the onset of Graves′ disease (GD) was initially documented by Parry in 1825. There is data available on the high incidence of thyrotoxicosis among refugees from Nazi prison camps. Psychological distress has been reported in up to 65% of younger patients with hyperthyroidism and physical stress in many older patients.[ 14 ] The term ′Kriegsbasedow′ was coined following the observation of increased incidence of GD during major wars. Many epidemiological studies have demonstrated that patients with GD had more stressful life events than control subjects prior to the onset or diagnosis of Graves′ hyperthyroidism and that stress had an unfavorable effect on the prognosis of GD. A study by Winsa et al ., has indicated that negative life events may be a risk factor for GD. Compared with controls, newly diagnosed Graves′ patients claimed to have had more negative life events in the 12 months preceding the diagnosis, and negative life-event scores were also significantly higher (odds ratio 6.3, 95% confidence interval 2.7-14.7, for the category with the highest negative score).[ 15 ] Sonino et al ., in Italy examined 70 patients with GD and a control group of 70 healthy subjects and reported that patients with GD had significantly more positive and negative life events than controls (patients 1.51 total events, controls 0.54; P < 0.001). They investigated the occurrence of stressful life events in the year before the first sign of disease onset.[ 16 ] Kung et al .,[ 17 ] from Hong Kong and Radosavljevi΄c et al .,[ 18 ] from Yugoslavia also reported association of negative life events with GD. In the study by Yoshiuchi et al ., a positive correlation between stress and GD was found in female patients, but not in male patients.[ 19 ] Patients with GD not only had a significantly greater number of stressful life events but also a higher number and greater impact of negative stressful life events compared to patients with toxic nodules and normal controls.[ 20 ] Paunkovic et al ., reported a significant increase in the incidence of GD in Eastern Serbia during the civil war.[ 21 ] However, most of the studies are retrospective case-control studies and it is quite difficult to evaluate the effect of a given stressful event in different individuals. Moreover, the accuracy in filling self-rated questionnaires or answering standardized interviews may vary widely among patients due to different emotional impact. Therefore, it is difficult to definitely rule out the effect of possible mild, still undiagnosed thyroid hyperfunction already present in the examination period.
Genetic factors such as HLA (Human leukocyte antigen) and CTLA-4 (Cytotoxic T lymphocyte antigen – 4) determine the susceptibility to GD.[ 22 ] Stress may lead to immunologic perturbations and may affect the immune response to TSH receptor through modulation of hormones, neurotransmitters and cytokines. A defect of antigen-specific suppressor T-lymphocytes has been proposed to be partially responsible for the initiation of GD.[ 23 ] Stress may result in a defect in the immunologic surveillance leading to production of TSH receptor antibodies.[ 24 ] In genetically susceptible individuals stress favors the development of GD by shifting the Th1-Th2 immune balance away from Th1 towards Th2.[ 25 ] This shifting may affect the onset or course of GD.
However, there are many studies which failed to show any relationship between stress and GD. No significant difference was seen in the number and nature of stressful life events up to six months before the onset of thyrotoxicosis between patients with thyrotoxicosis and nontoxic goiters in the study by Gray and Hoffenberg.[ 26 ] Chiovato et al ., could not find past or present Graves′ hyperthyroidism in patients with panic disorder.[ 27 ]
Diabetes Mellitus
Severe stress may be a risk factor for diabetes. Children aged five to nine years with stress were significantly more likely to be diabetic.[ 28 ] However, recent-onset Type 1 diabetics, 15-34 years old reported no major stress factors within the year before diagnosis.[ 29 ] Thus stress in early life may be a risk factor for diabetes, but not in young adults.
Gonadal dysfunction
In females stress can lead to anovulation, amennorhea and other menstrual irregularities. Among newly incarcerated women with stress 9% had amenorrhea and 33% had menstrual irregularity.[ 30 ]
In males, there can be decreased sperm count, motility and altered morphology.[ 31 ] Ejaculatory disorders, impotence and oligospermia may be associated with psychological factors in male infertility.[ 32 ]
Psychosocial dwarfism
This is an extreme form of failure to thrive and may be associated with dramatic behavioral abnormalities. Defective GH secretion has been reported with stimulation test. Reversal of GH insufficiency within three weeks of removal from hostile environment has been reported.[ 11 ] Munoz-Hoyos et al ., observed a conspicuous reduction in the levels of neuroendocrine markers (melatonin, serotonin, β-endorphins and ACTH) in children suffering from affective deficiency, a diminution which was even more noticeable in the children presenting delayed growth. The organic incapability of confronting stress on a genetic basis, and/or the fact of repeated stresses, from exhaustion of the homeostatic mechanisms, could make some groups of patients liable to suffer depressive symptoms associated with a wide range of deleterious consequences in the endocrine system leading to delayed growth.[ 33 ]
Mental stress leads to chronic activation of the neuroendocrine systems. Cortisol favors central fat deposition, a decrease in the adipostatic signal leptin and an increase in the orexogenic signal ghrelin, inducing increased appetite and food intake. This phenomenon contributes to the current epidemic of obesity. The “stress” genes which have been selected under pressure in ancient environments may have not adapted to the rapid environmental changes of today.[ 34 ]
I MPACT OF S TRESS ON P REEXISTING E NDOCRINE D ISORDERS
Poor glycemic control.
In adults the relationship between stress and poor diabetic control is well established.[ 35 ] Poor metabolic control has also been reported in children and adolescents with Type 1 diabetes with stress.[ 36 ]
Addisonian crisis
Patients with adrenal insufficiency because of various etiologies may develop adrenal crisis on exposure to stress. To prevent this, the replacement doses of steroid need to be doubled during the period of stress.[ 37 ]
Thyroid crisis
Thyroid storm may be precipitated by physical stress. Acute emotional stress can also precipitate thyroid storm.[ 38 ] Yoshiuchi et al ., observed that those patients with GD who were stressed for six months after beginning of therapy were significantly and independently associated with the hyperthyroid state 12 months after beginning therapy.[ 39 ] Fukao et al ., studied the effects of emotional stress and patients′ personality traits on the prognosis of hyperthyroidism in 69 antithyroid drug-treated euthyroid patients with Graves′ hyperthyroidism. They observed a higher frequency of relapse in those who had stress.[ 40 ] A retrospective study by Benvenga on GD found that those who had taken benzodiazepine only in the acute phase of thyrotoxicosis relapsed more compared to those who had taken benzodiazepine for a longer period.[ 41 ] Vos et al ., observed that stress exposure is not related to the biochemical severity of GD, but is directly related to the clinical severity of GD.[ 42 ]
C ONCLUSION
In today's competitive modern world one encounters stress in various aspects of life. As an adaptive response to stress, there is a change in the serum level of various hormones including CRH, cortisol, catecholamines and thyroid hormone. These changes may be required for the fight or flight response of the individual to stress. However, long-term exposure to stress may lead to many deleterious consequences leading to various endocrine disorders. Also, stress leads to change in the clinical course or status of many endocrine conditions.
Source of Support: Nil
Conflict of Interest: None declared.
R EFERENCES
- 1. Hargreaves KM. Neuroendocrine markers of stress. Anesth Prog. 1990;37:99–105. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Herman JP, Figuerierdo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Black PH. Central nervous system-immune system interactions: Psychoneuroendocrinology of stress and its immune consequences. Antimicrob Agents Chemother. 1994;38:1–6. doi: 10.1128/aac.38.1.1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Goldstein D. Stress-induced activation of the sympathetic nervous system. Balliere's Clin Endocr Metab. 1987;1:253–78. doi: 10.1016/s0950-351x(87)80063-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Timio M, Gentili S, Pede S. Free adrenaline and noradrenaline excretion related to occupational stress. BMJ. 1979;42:471–4. doi: 10.1136/hrt.42.4.471. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Aguilera G, Subburaju S, Young S, Chen J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res. 2008;170:29–39. doi: 10.1016/S0079-6123(08)00403-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Reprod Endocrinol. 1997;15:37–45. doi: 10.1055/s-2008-1067966. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Lachelin GC, Yen SS. Hypothalamic chronic anovulation. Am J Obstet Gynecol. 1978;130:825–31. doi: 10.1016/0002-9378(78)90017-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183–92. doi: 10.1159/000087001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Delitala G, Tomasi P, Virdis R. Prolactin, growth hormone and thyrotropin-thyroid hormone secretion during stress in man. Baillieres Clin Endocr Metab. 1987;1:391–414. doi: 10.1016/s0950-351x(87)80069-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Skuse D, Albanese A, Stanhope R, Gilmour J, Gilmour L, Voss L. A new stress-related syndrome of growth failure and hyperphagia in children, associated with reversibility of growth hormone insufficiency. Lancet. 1996;348:353–8. doi: 10.1016/s0140-6736(96)01358-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304:547–9. doi: 10.1038/304547a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Halter JB, Beard JC, Porte D., Jr Islet functions and stress hyperglycemia: Plasma glucose and epinephrine interaction. Am J Physiol. 1984;247:E47–52. doi: 10.1152/ajpendo.1984.247.1.E47. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Hoffenberg R. Aetiology of hyperthyroidism-II. BMJ. 1974;3:508–10. doi: 10.1136/bmj.3.5929.508. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Winsa B, Adani H, Bergstrom R, Gamstedt A, Dahlberg PA, Adamsen U, et al. Stressful life events and Graves’ disease. Lancet. 1991;338:1745–79. doi: 10.1016/0140-6736(91)92298-g. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Sonino N, Girelli ME, Boscaro M, Fallo F, Busnardo B, Fava GA. Life events in the pathogenesis of Graves’ disease: A controlled study. Acta Endocrinol (Copenh) 1993;28:293–6. doi: 10.1530/acta.0.1280293. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Kung AW. Life events, daily stresses and coping in patients with Graves′ disease. Clin Endocrinol (Oxf) 1995;42:303–8. doi: 10.1111/j.1365-2265.1995.tb01879.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Radosavljevi´c VR, Jankovic SM, Marinkovic JM. Stressful life events in the pathogenesis of Graves′ disease. Eur J Endocrinol. 1996;134:699–701. doi: 10.1530/eje.0.1340699. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Yoshiuchi K, Kumano H, Nomura S, Yoshimura H, Ito K, Kanaji Y, et al. Stressful life events and smoking were associated with Graves’ disease in women, but not in men. Psychosom Med. 1998;60:182–5. doi: 10.1097/00006842-199803000-00013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Matos-Santos A, Nobre EL, Costa JG, Nogueira PJ, Macedo A, Galvao-Teles A, et al. Relationship between the number and impact of stressful life events and the onset of Graves′ disease and toxic nodular goitre. Clin Endocrinol (Oxf) 2001;55:15–9. doi: 10.1046/j.1365-2265.2001.01332.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Paunkovic N, Paukovic J, Pavlovic O, Paunovic Z. The significant increase in incidence of Graves’ disease in Eastern Serbia during the Civil War in the Former Yugoslavia (1992 to 1995) Thyroid. 1998;8:37–41. doi: 10.1089/thy.1998.8.37. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Mizokami T, Li AW, El-Kaissi S, Wall JR. Stress and thyroid autoimmunity. Thyroid. 2004;12:1047–55. doi: 10.1089/thy.2004.14.1047. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Davies TF. Pathogenesis of Graves’ disease. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's The Thyroid: A fundamental and clinical text. Philadelphia USA: Lippincott Williams and Wilkins; 2005. pp. 457–73. [ Google Scholar ]
- 25. Tsatsoulis A. The role of stress in the clinical expression of thyroid autoimmunity. Ann N Y Acad Sci. 2006;1088:382–95. doi: 10.1196/annals.1366.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Gray J, Hoffenberg R. Thyrotoxicosis and stress. Q J Med. 1985;54:153–60. [ PubMed ] [ Google Scholar ]
- 27. Chiovato L, Marinò M, Perugi G, Fiore E, Montanelli L, Lapi P, et al. Chronic recurrent stress due to panic disorder does not precipitate Graves’ disease. J Endocrinol Invest. 1998;21:758–64. doi: 10.1007/BF03348042. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Thernlund GM, Dahlquist G, Hannsson K, Ivarsson SA, Ludwigsson J, Sjoblad S, et al. Psychological stress and onset of IDDM in children. Diabetes Care. 1995;18:1323–39. doi: 10.2337/diacare.18.10.1323. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Littorin B, Sundkvist G, Nystrom L, Carlson A, Landin-Olsson M, Ostamn J, et al. Family characteristics and life events before onset of autoimmune type1 diabetes in young adults; a nationwide study. Diabetes Care. 2001;24:1033–7. doi: 10.2337/diacare.24.6.1033. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Allsworth JE, Clarke J, Peipert JF, Hebert R, Cooper A, Boardman LA. The influence of stress on the menstrual cycle among newly incarcerated women. Womens Health Issues. 2007;17:202–9. doi: 10.1016/j.whi.2007.02.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. McGrady AV. Effects of psychological stress on male reproduction: a review. Arch Androl. 1984;131:1–10. doi: 10.3109/01485018408987495. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Palti Z. Psychogenic male infertility. Psychosom Med. 1969;31:326–330. doi: 10.1097/00006842-196907000-00005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Munoz-Hoyos A, Molina-Carballo A, Augustin-Morales MC, Contreras-Chava K, Naranjo-Gomez A, Justicia-Martinez F, et al. Psychosocial dwarfism: Psychopathological aspects and putative neuroendocrine markers. Psychiatr Res. 2010 doi: 10.1016/j.psychres.2010.10.004. in press. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Siervo M, Wells JC, Cizza G. The contribution of psychosocial stress to the obesity epidemic: An evolutionary approach. Horm Metab Res. 2009;41:261–70. doi: 10.1055/s-0028-1119377. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Griffith L, Field B, Lustman P. Life stress and social support in diabetes: Association with glycemic control. Int J Psychiatr Med. 1990;20:365–72. doi: 10.2190/APH4-YMBG-NVRL-VLWD. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Viner S, McGrath M, Trudinger P. Family stress and metabolic control in diabetes. Arch Dis Child. 1996;74:418–21. doi: 10.1136/adc.74.5.418. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Stewart PM. The adrenal cortex. In: Kronenberg HM, et al., editors. Williams Textbook of Endocrinology. Philadelphia: Saunders Elsevier; 2008. pp. 445–503. [ Google Scholar ]
- 38. Wartofsky L. Thyrotoxic Storm. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's The Thyroid: A fundamental and clinical text. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 651–7. [ Google Scholar ]
- 39. Yoshiuchi K, Kumano H, Nomura S, Yoshimura H, Ito K, Kanaji Y, et al. Psychosocial factors influencing the short-term outcome of antithyroid drug therapy in Graves′ disease. Psychosom Med. 1998;60:592–6. doi: 10.1097/00006842-199809000-00014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Fukao A, Takamatsu J, Murakami Y, Sakane S, Miyauchi A, Kuma K, et al. The relationship of psychological factors to the prognosis of hyperthyroidism in antithyroid drug-treated patients with Graves′ disease. Clin Endocrinol (Oxf) 2003;58:550–5. doi: 10.1046/j.1365-2265.2003.01625.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Benvenga S. Benzodiazepine and remission of Graves’ disease. Thyroid. 1996;6:659–60. doi: 10.1089/thy.1996.6.659. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Vos XG, Smit N, Endert E, Brosschot JF, Tijssen JG, Wiersinga WM. Age and stress as determinants of the severity of hyperthyroidism caused by Graves′ disease in newly diagnosed patients. Eur J Endocrinol. 2009;160:193e–9e. doi: 10.1530/EJE-08-0573. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (462.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Physiology, stress reaction.
Brianna Chu ; Komal Marwaha ; Terrence Sanvictores ; Ayoola O. Awosika ; Derek Ayers .
Affiliations
Last Update: May 7, 2024 .
- Introduction
Any physical or psychological stimuli that disrupt homeostasis result in a stress response. The stimuli are called stressors, and physiological and behavioral changes in response to exposure to stressors constitute the stress response. A stress response is mediated through a complex interplay of nervous, endocrine, and immune mechanisms, activating the sympathetic-adreno-medullar (SAM) axis, the hypothalamic-pituitary-adrenal (HPA) axis, and the immune system. [1] The stress response is adaptive to prepare the body to handle the challenges presented by an internal or external environmental challenge, such as stressors. For example, the body's physiological responses to trauma and invasive surgery serve to attenuate further tissue damage. Suppose the exposure to a stressor is actually or perceived as intense, repetitive (repeated acute stress), or prolonged (chronic stress). In that case, the stress response is maladaptive and detrimental to physiology. Exposure to chronic stressors can cause maladaptive reactions, including depression, anxiety, cognitive impairment, and heart disease. [2]
Not all forms of stress are detrimental. Some stressors are enjoyable, stimulating, and inspiring. Termed eustress, these positive stressors replenish our energy, enhance cardiovascular health, boost endurance, and sharpen cognitive function. Eustress fosters mental acuity and motivation. In contrast, distress is characterized by adverse effects on the body and mind.
Stress is categorized into various types based on duration, source, and response.
- Acute stress: The short-term stress that typically results from immediate stressors or challenging situations. The body's fight-or-flight response leads to temporary physiological changes such as increased heart rate and adrenaline release.
- Chronic stress: This occurs when the stressor persists over an extended period. Prolonged exposure to chronic stress can lead to cumulative physiological and psychological effects, increasing the risk of health problems such as cardiovascular disease, anxiety, and depression.
- Episodic acute stress: The stress occurs when individuals experience frequent episodes of acute stress. This pattern may be characteristic of individuals who lead chaotic or disorganized lifestyles, constantly facing deadlines, commitments, or interpersonal conflicts. The cycle of stress exacerbates health issues and impairs daily functioning.
- Traumatic stress: This type results from exposure to traumatic events, such as natural disasters, accidents, or violent acts. The trauma overwhelms an individual's ability to cope and may lead to symptoms of posttraumatic stress disorder (PTSD), including intrusive memories, avoidance behaviors, and hyperarousal.
- Environmental stress: This type arises from adverse or challenging conditions in one's surroundings, including noise, pollution, overcrowding, or unsafe living conditions. These stressors can have detrimental effects on physical and mental health, contributing to a sense of discomfort or unease.
- Psychological stress: The stress stems from cognitive or emotional factors, such as perceived threats, worries, or negative thoughts. Typical stressors include work-related pressures, academic expectations, social comparisons, or self-imposed demands. Manifestations include anxiety, rumination, or perfectionism.
- Physiological stress: Physiological stress refers to the body's response to internal or external stressors that disrupt homeostasis. Examples include illness, injury, sleep deprivation, or nutritional deficiencies, which activate physiological stress pathways and compromise health and well-being. [3] [4] [5] [6]
- Cellular Level
The physiology of stress response has 2 components—a slow response mediated by the HPA axis and a fast response mediated by the SAM axis.
Sympathetic-Adreno-Medullar System
The quick response triggered by SAM activation leads to increased secretion of norepinephrine and epinephrine from the adrenal medulla into the circulation and increased secretion of norepinephrine from the sympathetic nerves, resulting in elevated levels of norepinephrine in the brain. [7] The released epinephrine and norepinephrine interact with α- and β-adrenergic receptors in the central nervous system and on the cell membrane of smooth muscles and other organs throughout the body. When released, norepinephrine and epinephrine bind to specific membrane-bound G-protein receptors to initiate an intracellular cyclic adenosine monophosphate (cAMP) signaling pathway that rapidly activates cellular responses. The activation of these receptors results in the contraction of smooth and cardiac muscle cells, leading to vasoconstriction, increased blood pressure, heart rate, cardiac output, skeletal muscle blood flow, increased sodium retention, increased levels of glucose due to glycogenolysis and gluconeogenesis, lipolysis, increased oxygen consumption, and thermogenesis. The physiological response also reduces intestinal motility, cutaneous vasoconstriction, and bronchiolar dilatation. In addition, SAM activation causes behavioral activation, such as enhanced arousal, alertness, vigilance, cognition, focused attention, and analgesia.
Hypothalamic-Pituitary-Adrenal System
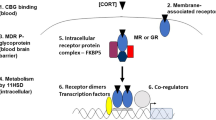
The slow response is due to the activation of the HPA axis, releasing corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus into the circulation. The CRH released from the hypothalamus acts on 2 receptors—CRH-R1 and CRH-R2. CRH-R1, widely distributed in the mammalian brain, is the key receptor for the stress-induced adrenocorticotropic hormone (ACTH) release from the anterior pituitary. CRH-R2 is expressed primarily in peripheral tissues, including skeletal muscles, gastrointestinal tract, and heart, and in subcortical structures of the brain. CRH-binding protein (CRH-BP) has a greater binding affinity with CRH compared to receptors. CRH-BP is expressed in the liver, pituitary gland, brain, and placenta. [8] The role of CRH-BP as a controller of the bioavailability of CRH is supported by studies finding that CRH-BP binds 40% to 60% of CRH in the brain. [9] In exposure to stress, the expression of CRH-BP increases in a time-dependent manner, a negative feedback mechanism to decrease the interaction of CRH with CRH-R1. [2] Serum cortisol level describes the body's total cortisol level, of which 80% is bound to cortisol-binding globulin and 10% is bound to albumin. Unbound cortisol is biologically active.
The released CRH then stimulates the anterior pituitary gland to release ACTH into the bloodstream. ACTH stimulates the adrenal cortex to secrete glucocorticoid hormones, such as cortisol, into the circulation. The inactive form of cortisol, cortisone, is catalyzed to the active form, cortisol, by 11-β-hydroxysteroid dehydrogenases (see Image. The Hypothalamic-Pituitary-Adrenal Axis). [10]
The HPA axis is regulated by pituitary adenylate cyclase-activating polypeptide. Pituitary adenylate cyclase-activating polypeptide may play a role in the production of CRH and modulate multiple levels of the HPA axis. Evidence also points to the involvement in the autonomic response to stress through increased secretion of catecholamines. [11] The pituitary adenylate cyclase-activating polypeptide receptors are G-protein coupled, and the R1 receptor is the most abundant in both central and peripheral tissues. This polypeptide may also modulate estrogen's role in potentiating the acute stress response. [12]
After CRH is released, the hormone binds with CRH-BP because CRH has a higher affinity with CRH-BP compared to the receptors. CRH-BP is expressed in the liver, pituitary gland, brain, and placenta. [5] The role of CRH-BP as a controller of the bioavailability of CRH is supported by studies finding that CRH-BP binds 40% to 60% of CRH in the brain. [6] In exposure to stress, the expression of CRH-BP increases in a time-dependent manner, which is believed to be a negative feedback mechanism that decreases the interaction of CRH with CRH-R1. [2] Serum cortisol level describes the body's total cortisol level, of which 80% is bound to cortisol-binding globulin and 10% is bound to albumin. Unbound cortisol is biologically active.
- Organ Systems Involved
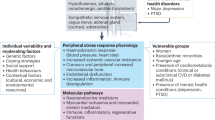
Stress generally affects all body systems, including cardiovascular, respiratory, endocrine, gastrointestinal, nervous, muscular, and reproductive systems. The endocrine system increases the production of steroid hormones, including cortisol, to activate the body's stress response. In the nervous system, stress triggers the sympathetic nervous system, prompting the adrenal glands to release catecholamines. Once the acute stress-induced crisis subsides, the parasympathetic nervous system aids in the body's recovery.
Cardiovascular System
Acute stress causes an increase in heart rate, stronger heart muscle contractions, dilation of the heart, and redirection of blood to large muscles. In contrast, chronic stress induces sustained activation of the sympathetic nervous system and HPA axis, leading to elevated levels of stress hormones such as cortisol and epinephrine. [13] The presence of these stress hormones promotes oxidative stress, endothelial dysfunction, and inflammation, thereby promoting the development of atherosclerosis and compromising vascular function. Moreover, stress-related alterations in lipid metabolism also contribute to dyslipidemia, exacerbating cardiovascular risk. [14]
Respiratory System
The respiratory and cardiovascular systems play crucial roles in supplying oxygen to the body's cells and eliminating carbon dioxide waste. Acute or chronic stress triggers dysregulation of the autonomic nervous system. This dysregulation can lead to a cascade of physiological effects, inducing bronchial hyperresponsiveness and inflammation. More importantly, acute stress can result in changes in breathing patterns due to airway constriction, leading to shortness of breath and rapid shallow breathing, exacerbating respiratory symptoms. Chronic stress also compromises immune function, increasing susceptibility to respiratory infections and exacerbating conditions such as asthma and chronic obstructive pulmonary disease. Furthermore, stress-induced alterations in inflammatory cytokines contribute to airway inflammation and mucus production.
Gastrointestinal System
Catecholamines, such as epinephrine and norepinephrine, released during stress profoundly affect the gastrointestinal system. These hormones bind to adrenergic receptors distributed throughout the gastrointestinal tract, influencing various physiological processes. By activating α-adrenergic receptors in the smooth muscle of the intestines, they cause delayed gastric emptying and reduced intestinal transit (motility). [15] Vasoconstriction in the gastrointestinal vasculature through α-adrenergic receptor activation reduces blood flow to the gut, thus inhibiting gastrointestinal secretions and nutrient absorption. [16]
Stress-induced changes in gut motility can manifest as diarrhea or constipation, whereas increased visceral sensitivity may contribute to symptoms such as irritable bowel syndrome. In addition, stress impairs the integrity of the gastrointestinal mucosal barrier, leading to increased permeability and susceptibility to inflammation and infection. Dysregulation of the gut-brain axis, mediated by stress, exacerbates gastrointestinal disorders and can worsen symptoms through bidirectional communication between the central nervous system and the gut microbiota. [17] Furthermore, stress-related alterations in gut microbiota composition and diversity can further contribute to gastrointestinal dysfunction, highlighting the intricate interplay between stress and gastrointestinal health.
Musculoskeletal System
Chronic stress elicits a cascade of physiological responses, including increased secretion of stress hormones such as cortisol and catecholamines, which impact the musculoskeletal system. Prolonged exposure to elevated levels of cortisol can lead to muscle wasting and decreased bone density by inhibiting osteoblast activity and promoting osteoclast function. In addition, activation of the stress-induced sympathetic nervous system can exacerbate musculoskeletal tension and contribute to conditions such as tension headaches, temporomandibular joint disorders, prolonged recovery from musculoskeletal injuries, and risk of developing conditions, including fibromyalgia and low back pain.
Immune System
When exposure to stress is chronic, the sympathetic nervous system, including the HPA axis, is activated, which can suppress innate and adaptive immune responses. [18] Prolonged elevation of cortisol levels suppresses immune function by inhibiting the production of pro-inflammatory cytokines and reducing the activity of immune cells, particularly lymphocytes. This immunosuppressive effect can increase infection susceptibility, delay wound healing, and exacerbate inflammatory conditions. In addition, chronic stress promotes systemic inflammation through the upregulation of inflammatory mediators, contributing to the pathogenesis of autoimmune diseases and chronic inflammatory disorders. [19]
Reproductive System
Chronic stress can disrupt the delicate balance of the reproductive axis by suppressing the secretion of gonadotropin-releasing hormone from the hypothalamus, which subsequently reduces the release of luteinizing hormone and follicle-stimulating hormone from the pituitary gland. Consequently, this disruption impairs ovarian function in women and reduces testosterone production in men. Chronic stress can also lead to menstrual irregularities, anovulation, and infertility in women, and impaired sexual desire, erectile dysfunction, and decreased sperm quality in men. In addition, stress-induced alterations in sex hormone levels and impaired reproductive function may contribute to conditions such as polycystic ovary syndrome and male hypogonadism. [20]
The heightened autonomic response causes an increase in heart rate and blood pressure. During critical illness, the release of catecholamine decreases blood circulation to the gastrointestinal tract. During stress, plasma levels of norepinephrine and epinephrine redistribute blood volume to conserve the brain's blood supply. Stimulation of the sympathetic nervous system is varied but includes threats to the body such as hypoglycemia, hemorrhagic shock, exercise beyond the anaerobic threshold, and asphyxiation. [21] Epinephrine is also associated with active escape, attack, and fear.
A stressful situation, whether environmental or psychological, can activate a cascade of stress hormones that produce physiological changes. Activating the sympathetic nervous system in this manner triggers an acute stress response called the fight-or-flight response. This response enables an individual to either fight the threat or flee the situation. The rush of adrenaline and noradrenaline secreted from the adrenal medulla leads to a widespread discharge of almost all portions of the sympathetic system throughout the body. Physiological changes of this mass discharge effect include increased arterial pressure, more blood flow to active muscles, less blood flow to organs not needed for rapid motor activity, increased rate of blood coagulation, increased rates of cellular metabolism through the body, increased muscle strength, increased mental activity, increased blood glucose concentration, and increased glycolysis in the liver and muscle. The net effect of all these effects allows a person to perform more strenuous activity than usual. After the perceived threat disappears, the body returns to basal levels.
Physical stress stimulates the HPA and sympathetic nervous systems. Cortisol has various physiological effects, including catecholamine release, insulin suppression, mobilization of energy stores through gluconeogenesis and glycogenolysis, suppression of the immune-inflammatory response, and delayed wound healing. [22] B-cell apoptosis is an effect of the downregulation of the immune response. [23] [24] Wound healing is also delayed due to the effects of collagen synthesis. [25] Aldosterone is a mineralocorticoid hormone that preserves blood pressure through sodium and water retention.
Glucocorticoid-binding receptors exist in the brain as mineralocorticoid and glucocorticoid receptors. The brain's first response to glucocorticoids is to preserve function. Glucocorticoid hormones such as cortisol, corticosterone, and dexamethasone have various effects of conserving energy and maintaining energy supply, such as reducing inflammation, restricting growth, producing power, and removing unnecessary or malfunctioning cellular components. [26]
Stress response is a nuanced interplay among diverse brain centers, particularly the neural mechanisms responsible for triggering stress reactions, which include the locus coeruleus, limbic system, and hypothalamic efferent activation complex. [7] These components are interconnected through various pathways, including ventral and dorsal adrenergic and serotonergic projections. The complex includes the locus coeruleus, hippocampus, septal-hippocampal-amygdaloid complexes, and anterior and posterior hypothalamic nuclei, serving as pivotal anatomical hubs for visceral and somatic efferent responses to emotional stimuli. [27] Essentially, the amygdala, particularly the central nucleus, plays a crucial role in processing emotional aspects of stress and initiating fear responses. The hippocampus, critical for memory formation, regulates the stress response by providing negative feedback to the hypothalamus, thus modulating cortisol release. The prefrontal cortex, involved in executive functions, including decision-making and impulse control, regulates stress responses through top-down inhibition of the amygdala and hypothalamus. [28] The dysregulation of these brain centers is implicated in stress-related disorders such as anxiety, depression, and PTSD.
- Related Testing
Various testing techniques are used to measure stress response in humans.
- Biological markers: Assessing stress hormones such as cortisol, epinephrine, and norepinephrine levels in the blood, saliva, and urine provides objective indicators of the physiological stress response. These markers reflect the activity of the HPA axis and the SAM system. Sympathetic responses are also measurable through microneurography. The microneurography technique involves the insertion of an electrode into a peripheral nerve to measure sympathetic activity in the skin and muscles of the upper or lower limbs.
- Heart rate variability: Heart rate variability analysis assesses the variation in the time interval between consecutive heartbeats, reflecting the balance between the sympathetic (fight-or-flight) and parasympathetic (relaxation) nervous systems. Decreased heart rate variability is associated with sympathetic dominance and increased stress levels, whereas higher heart rate variability is associated with stress resilience and improved cardiovascular health.
- Electroencephalography: This method measures brainwave activity and can accurately gauge stress response. The findings of a study conducted in 2020 suggest that alpha asymmetry, an imbalance in alpha brainwave activity between brain hemispheres, is a potential stress biomarker. Mental health clinicians use neurofeedback to measure brainwaves and provide positive feedback during treatment.
- Electrodermal activity: This is measured by skin conductance or galvanic skin response and reflects changes in sweat gland activity in response to sympathetic arousal. Increased electrodermal activity indicates heightened physiological arousal and stress.
- Blood pressure and heart rate: Monitoring changes in blood pressure and heart rate provides insights into cardiovascular responses to stress, including increased sympathetic activation and vasoconstriction. [29] [30] [31] [32]
- Pathophysiology
General Adaptation Syndrome
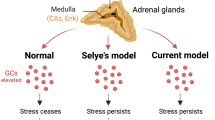
General adaptation syndrome provides a framework for understanding the physiological responses to stress and the potential consequences of chronic stress on health and well-being. The syndrome describes the different stress-induced physiological changes through 3 different stages, with the last 2 stages showing the pathological changes of extended stress. [33] This syndrome is divided into the alarm reaction stage, resistance stage, and exhaustion stage.
The alarm reaction stage refers to the initial symptoms of the body under acute stress and the fight-or-flight response. After the initial shock of the stressful event, the body begins to repair itself by lowering cortisol levels and normalizing the physiologic reactions such as blood pressure and heart rate. During this recovery phase, the body remains alert until the stressful event is no longer triggering. However, if the stressful event persists for extended periods, the body adapts to cope with higher stress levels. [34] The body continues to secrete stress hormones, which maintain the body's elevated physical response to stress. This mechanism induces the resistance stage and includes symptoms such as poor concentration, irritability, and frustration. If the stressful event persists, the body enters the exhaustion stage. Symptoms of this stage include burnout, fatigue, depression, anxiety, and reduced stress tolerance. As the stressful event persists, the body's immune system weakens due to the suppressive effects of stress hormones on immune system cells.
Systemic Effects of Stress
Although the restoration of homeostasis is the goal of the stress response, chronic stress leads to dysfunctional responses, resulting in heart disease, stomach ulcers, sleep dysregulation, and psychiatric disorders. The HPA axis may become suppressed or dysregulated in these maladaptive responses to stress. Stress causes the cardiovascular system to respond with elevated blood pressure and heart rate; chronic activation of this response is a significant cause of cardiovascular disease. Coronary artery disease, stroke, and hypertension occur at a greater incidence in individuals with stress-related psychological disorders. The release of catecholamines in the stress response can have maladaptive effects on the gastrointestinal tract through decreased local blood flow. Chronic stress weakens the immune system, increasing the probability of developing Helicobacter pylori gastric ulcers and bleeding. [35] Sleep quality and quantity affect cortisol response to acute stress. Self-reported high sleep quality showed a strong cortisol stress response, whereas relatively good sleep quality showed a significantly weaker cortisol response in men. Regardless of gender, a blunted cortisol response to stress was observed in individuals experiencing difficulty staying awake and maintaining enthusiasm. [36]
Furthermore, diseases affecting the adrenal system, such as Addison's disease, Cushing's syndrome, and pheochromocytoma, influence the body's stress mechanisms by releasing cortisol and epinephrine. Addison's disease is characterized by a lack of glucocorticoid and/or mineralocorticoid hormones. [37] Conversely, Cushing's syndrome is marked by hypercortisolism due to endogenous or exogenous causes. [38] Pheochromocytomas are catecholamine-secreting tumors of the adrenal glands. [39]
- Clinical Significance
The physiological responses of the body to stress have significant implications in various clinical applications, such as managing healthy patients, patients with hypoadrenalism undergoing surgery, and understanding the relationship between lifestyle changes and the stress response. The physiological stress of surgery causes cortisol levels to rise in a positive correlation to the severity of the surgery. In patients undergoing major surgeries as defined by the POSSUM scale, cortisol levels return to baseline on postoperative days 1 to 5. Postoperative pain severity does not correlate with cortisol levels after cardiac surgery. [21] A study examining cortisol levels during minor, moderate, and major surgeries found that postoperative opiate analgesia does not influence the stress cortisol response. For patients with hypoadrenalism who require cortisol replacement during surgery, the varied level of cortisol secretion correlated to the stress of surgical operations has implications.
For patients with hypoadrenalism undergoing surgery, hydrocortisone injections are administered to mimic cortisol levels observed in individuals with normal adrenal function during surgery; this is believed to help patients with hypoadrenalism withstand the physiological stress of surgery. Recommendations for dosage and supplementation methods vary. European guidelines suggest 100 mg of hydrocortisone intramuscularly before anesthesia, regardless of surgery type. Endocrine Society recommendations suggest 100 mg of hydrocortisone intravenously followed by infusion, which is the basis of the severity of the surgery. Testing cortisol levels in surgeries of varying severity shows that peak cortisol correlates with surgical severity, but peak cortisol levels are lower than previously suggested. [22]
Patients in the intensive care unit are subject to physical and environmental stress, and efforts are made to investigate the link between cortisol levels and illness recovery and to facilitate stressors during the stay to study outcomes. Subjective patient perception of relaxation is heightened with sleep adjuncts such as earplugs, eye masks, and relaxing music. However, these interventions did not influence nocturnal melatonin or cortisol levels. [40]
Long-term exercise can help prevent cardiovascular disease by adapting baseline cardiac performance. Long-term moderate exercise helps relieve stress-induced cardiovascular response by changing baroreflex set points in the nucleus of the tractus solitarius, thereby regulating blood pressure control and blood volume homeostasis regulated by the paraventricular nucleus.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
The Hypothalamic-Pituitary-Adrenal Axis. The HPA axis is the slow response to stressors. Hine J, Schwell A, Kairys N. An unlikely cause of hypokalemia. J Emerg Med. 2017;52(5):e187-e191. doi: 10.1016/j.jemermed.2016.12.011. PMID: 28139270.
Disclosure: Brianna Chu declares no relevant financial relationships with ineligible companies.
Disclosure: Komal Marwaha declares no relevant financial relationships with ineligible companies.
Disclosure: Terrence Sanvictores declares no relevant financial relationships with ineligible companies.
Disclosure: Ayoola Awosika declares no relevant financial relationships with ineligible companies.
Disclosure: Derek Ayers declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Chu B, Marwaha K, Sanvictores T, et al. Physiology, Stress Reaction. [Updated 2024 May 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP

Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Acute and Chronic Mental Health Trauma. [StatPearls. 2024] Acute and Chronic Mental Health Trauma. Feriante J, Sharma NP. StatPearls. 2024 Jan
- Lifestyle Mindfulness In Clinical Practice. [StatPearls. 2024] Lifestyle Mindfulness In Clinical Practice. Srour RA, Keyes D. StatPearls. 2024 Jan
- Review [Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context]. [Encephale. 2012] Review [Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context]. Auxéméry Y. Encephale. 2012 Oct; 38(5):373-80. Epub 2012 Jan 24.
- Review The organization of the stress system and its dysregulation in depressive illness. [Mol Psychiatry. 2015] Review The organization of the stress system and its dysregulation in depressive illness. Gold PW. Mol Psychiatry. 2015 Feb; 20(1):32-47. Epub 2014 Dec 9.
- Social Susceptibility to Multiple Air Pollutants in Cardiovascular Disease. [Res Rep Health Eff Inst. 2021] Social Susceptibility to Multiple Air Pollutants in Cardiovascular Disease. Clougherty JE, Humphrey JL, Kinnee EJ, Robinson LF, McClure LA, Kubzansky LD, Reid CE. Res Rep Health Eff Inst. 2021 Jul; 2021(206):1-71.
Recent Activity
- Physiology, Stress Reaction - StatPearls Physiology, Stress Reaction - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Expert Review
- Published: 21 July 2021
The neuroendocrinology of stress: the stress-related continuum of chronic disease development
- Agorastos Agorastos ORCID: orcid.org/0000-0003-4801-4957 1 , 2 &
- George P. Chrousos 3
Molecular Psychiatry volume 27 , pages 502–513 ( 2022 ) Cite this article
7945 Accesses
149 Citations
16 Altmetric
Metrics details
- Neuroscience
- Psychiatric disorders
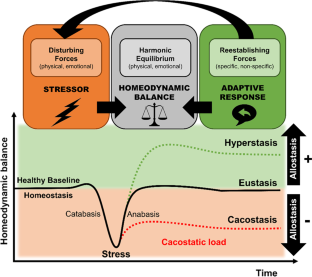
Stress is defined as a state of threatened homeodynamic balance by a wide range of intrinsic or extrinsic, real or perceived challenges or stimuli, defined as stressors. To preserve this optimal homeodynamic state within a physiologic range, organisms have developed a highly sophisticated system, the stress system, which serves self-regulation and adaptability of the organism by energy redirection according to the current needs. Repeated, ephemeral, and motivating stress states lead to adaptive responses and response habituations, being fairly beneficial; in contrast, inadequate, aversive, excessive, or prolonged stress may surpass the regulatory capacity and adjustive resources of the organism and produce maladaptive responses and a chronically altered homeodynamic state associated with compromised mental and physical health and life expectancy. Neuroendocrine responses to stress depend on developmental timing, duration, time of day and nature of stressors leading to a vulnerable phenotype with disrupted stress reactivity (i.e., hyper- or hypoactivation of the stress system), impaired glucocorticoid signaling, and accumulated cacostatic load with cumulatively elevated long-term risk of mental and physical morbidity. This article offers a brief overview on the organization and physiology of the human stress system and its (re)activity, refreshes the plethora of somatic effects of acute and chronic stress and discusses a conceptual model of acute and chronic stress pathophysiology as a continuum in chronic disease development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Stress and cardiovascular disease: an update
The cortisol switch between vulnerability and resilience
Neurocognitive effects of stress: a metaparadigm perspective
Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overv Phys Behav Homeost JAMA. 1992;267:1244–52.
CAS Google Scholar
Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81.
Article CAS PubMed Google Scholar
McEwen BS. Protective and damaging effects of stress mediators. N. Engl J Med. 1998;338:171–9.
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409.
Article CAS PubMed PubMed Central Google Scholar
Elenkov IJ, Chrousos GP. Stress system organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–67.
Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66.
McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30:315–8.
PubMed PubMed Central Google Scholar
McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15.
Article PubMed Google Scholar
Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–301.
Agorastos A, Nicolaides NC, Bozikas VP, Chrousos GP, Pervanidou P. Multilevel interactions of stress and circadian system: implications for traumatic stress. Front Psychiatry. 2019;10:1003.
Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N. Y Acad Sci. 2006;1088:361–72.
Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015;22:6–19.
Chrousos GP, Charmandari E, Kino T. Glucocorticoid action networks—an introduction to systems biology. J Clin Endocrinol Metab. 2004;89:563–4.
Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10:466–75.
Article PubMed PubMed Central Google Scholar
Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog Brain Res. 2010;181:111–26.
Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–83.
Charmandari E, Chrousos GP, Lambrou GI, Pavlaki A, Koide H, Ng SS, et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS ONE. 2011;6:e25612.
Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34:271–92.
Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84.
ter Heegde F, De Rijk RH, Vinkers CH. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92–110.
Wingenfeld K, Otte C. Mineralocorticoid receptor function and cognition in health and disease. Psychoneuroendocrinology. 2019;105:25–35. https://doi.org/10.1016/j.psyneuen.2018.09.010 .
Kino T, Chrousos GP. Circadian CLOCK-mediated regulation of target-tissue sensitivity to glucocorticoids: implications for cardiometabolic diseases. Endocr Dev. 2011;20:116–26.
Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, et al. Psychological stress reactivity and future health and disease outcomes: a systematic review of prospective evidence. Psychoneuroendocrinology. 2020;114:104599.
Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–6.
McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Article CAS Google Scholar
Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45.
Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol. 2020;1191:141–53.
Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35.
Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–65.
Herane-Vives A, Papadopoulos A, de Angel V, Chua KC, Soto L, Chalder T, et al. Cortisol levels in chronic fatigue syndrome and atypical depression measured using hair and saliva specimens. J Affect Disord. 2020;267:307–14.
Tak LM, Cleare AJ, Ormel J, Manoharan A, Kok IC, Wessely S, et al. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87:183–94.
Herane-Vives A, Young AH, Wise T, Aguirre J, de Angel V, Arnone D, et al. Comparison of short-term (saliva) and long-term (hair) cortisol levels in out-patients with melancholic and non-melancholic major depression. BJPsych Open. 2020;6:e41.
Rohleder N. Stress and inflammation - The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. 2019;105:164–71.
Zannas AS, Chrousos GP. Epigenetic programming by stress and glucocorticoids along the human lifespan. Mol Psychiatry. 2017;22:640–6.
Agorastos A, Pervanidou P, Chrousos GP, Baker DG. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front Psychiatry. 2019;10:118.
Youngoung ES, Doom JR, Farrell AK, Carlson EA, Englund MM, Miller GE, et al. Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Dev Psychopathol. 2020;33:1–12.
Google Scholar
Desantis AS, Kuzawa CW, Adam EK. Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. Am J Hum Biol. 2015;27:458–67.
Kajantie E, Feldt K, Räikkönen K, Phillips DI, Osmond C, Heinonen K, et al. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. J Clin Endocrinol Metab. 2007;92:4094–100.
Maercker A, Michael T, Fehm L, Becker ES, Margraf J. Age of traumatisation as a predictor of post-traumatic stress disorder or major depression in young women. Br J Psychiatry. 2004;184:482–7.
Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N. Y Acad Sci. 2009;1179:153–66.
Tsigos C, Stefanaki C, Lambrou GI, Boschiero D, Chrousos GP. Stress and inflammatory biomarkers and symptoms are associated with bioimpedance measures. Eur J Clin Invest. 2015;45:126–34.
Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7.
Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316.
Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51.
Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9:A271–6.
Geiker NRW, Astrup A, Hjorth MF, Sjodin A, Pijls L, Markus CR. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes Rev. 2018;19:81–97.
Download references
Author information
Authors and affiliations.
II. Department of Psychiatry, Division of Neurosciences, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Agorastos Agorastos
VA Center of Excellence for Stress and Mental Health (CESAMH), VA San Diego Healthcare System, San Diego, CA, USA
University Research Institute of Maternal and Child Health and Precision Medicine and UNESCO Chair on Adolescent Health Care, National and Kapodistrian University of Athens, Medical School, Aghia Sophia Children’s Hospital, Athens, Greece
George P. Chrousos
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Agorastos Agorastos .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Agorastos, A., Chrousos, G.P. The neuroendocrinology of stress: the stress-related continuum of chronic disease development. Mol Psychiatry 27 , 502–513 (2022). https://doi.org/10.1038/s41380-021-01224-9
Download citation
Received : 08 April 2021
Revised : 24 June 2021
Accepted : 30 June 2021
Published : 21 July 2021
Issue Date : January 2022
DOI : https://doi.org/10.1038/s41380-021-01224-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Association between residential proximity to major roadways and chronic multimorbidity among chinese older adults: a nationwide cross-sectional study.
- Shuang Zang
BMC Geriatrics (2024)
GLUT1-mediated microglial proinflammatory activation contributes to the development of stress-induced spatial learning and memory dysfunction in mice
- Ling-Jia Qian
Cell & Bioscience (2024)
Hair cortisol and self-perceived stress in adolescents with multi-system functional somatic disorders
- Rebecca Nyengaard
- Karen Hansen Kallesøe
- Charlotte Ulrikka Rask
BMC Psychiatry (2024)
Cumulative lifetime stressor exposure impairs stimulus–response but not contextual learning
- Mario Rosero-Pahi
- Jamila Andoh
- George M. Slavich
Scientific Reports (2024)
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
- María R. Aburto
- John F. Cryan
Nature Reviews Gastroenterology & Hepatology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

COMMENTS
First, stress hormones are released to make energy stores available for the body’s immediate use. Second, a new pattern of energy distribution emerges. Energy is diverted to the tissues that become more active during stress, primarily the skeletal muscles and the brain.
High concentrations of stress hormones can cause declarative memory disorders (Lupien and Lepage, 2001). Animal studies have shown that stress can cause a reversible reduction in spatial memory as a result of atrophy of the hippocampus (Luine et al., 1994).
Abstract. The human stress response has evolved to maintain homeostasis under conditions of real or perceived stress. This objective is achieved through autoregulatory neural and hormonal systems...
This chapter describes the organization and physiology of the stress system, focusing on its interactions with other CNS centers and endocrine axes, and reviews the existing evidence linking stress to pathophysiologic mechanisms implicated in the development of stress-related diseases affecting the endocrine, metabolic, gastrointestinal, and ...
Reactions to stress are associated with enhanced secretion of a number of hormones including glucocorticoids, catecholamines, growth hormone and prolactin, the effect of which is to increase mobilization of energy sources and adapt the individual to its new circumstance.
The body continues to secrete stress hormones, which maintain the body's elevated physical response to stress. This mechanism induces the resistance stage and includes symptoms such as poor concentration, irritability, and frustration.
Acute and chronic stress exposure can disrupt optimal neuroendocrine reactivity, resulting in enhanced vulnerability of the organism to stressors, thus, mediating a repeatedly well-documented...
In recent years, the application of cortisol and stress hormone measurements has given rise to an alternative, quantifiable approach for the psychological evaluation of stress and depression. This review comprehensively evaluates the current state-of-the-art technology for measuring cortisol and dehydroepiandrosterone (DHEA) and their ...
Research suggests that chronic stress contributes to high blood pressure, promotes the formation of artery-clogging deposits, and causes brain changes that may contribute to anxiety, depression, and addiction.
Here we review pertinent topics and research within the social neuroendocrine study of stress, including acute versus chronic stress, and how stress influences social behavior and status.