Animal cloning

So Dolly was not the first clone, and she looked like any other sheep, so why did she cause so much excitement and concern? Because she was the first mammal to be cloned from an adult cell, rather than an embryo. This was a major scientific achievement, but also raised ethical concerns. Since 1996, when Dolly was born, other sheep have been cloned from adult cells, as have mice, rabbits, horses and donkeys, pigs, goats and cattle. In 2004 a mouse was cloned using a nucleus from an olfactory neuron, showing that the donor nucleus can come from a tissue of the body that does not normally divide.

How was Dolly produced?
Producing an animal clone from an adult cell is obviously much more complex and difficult than growing a plant from a cutting. So when scientists working at the Roslin Institute in Scotland produced Dolly, the only lamb born from 277 attempts, it was a major news story around the world. To produce Dolly, the scientists used the nucleus of an udder cell from a six-year-old Finn Dorset white sheep. The nucleus contains nearly all the cell's genes. They had to find a way to 'reprogram' the udder cells - to keep them alive but stop them growing – which they achieved by altering the growth medium (the ‘soup’ in which the cells were kept alive). Then they injected the cell into an unfertilised egg cell which had had its nucleus removed, and made the cells fuse by using electrical pulses. The unfertilised egg cell came from a Scottish Blackface ewe. When the scientists had managed to fuse the nucleus from the adult white sheep cell with the egg cell from the black-faced sheep, they needed to make sure that the resulting cell would develop into an embryo. They cultured it for six or seven days to see if it divided and developed normally, before implanting it into a surrogate mother, another Scottish Blackface ewe. Dolly had a white face. From 277 cell fusions, 29 early embryos developed and were implanted into 13 surrogate mothers. But only one pregnancy went to full term, and the 6.6kg Finn Dorset lamb 6LLS (alias Dolly) was born after 148 days.
Why are scientists interested in cloning?
The main reason that the scientists at Roslin wanted to be able to clone sheep and other large animals was connected with their research aimed at producing medicines in the milk of such animals. Researchers have managed to transfer human genes that produce useful proteins into sheep and cows, so that they can produce, for instance, the blood clotting agent factor IX to treat haemophilia or alpha-1-antitrypsin to treat cystic fibrosis and other lung conditions. Cloned animals could also be developed that would produce human antibodies against infectious diseases and even cancers. ‘Foreign’ genes have been transplanted into zebra fish, which are widely used in laboratories, and embryos cloned from these fish express the foreign protein. If this technique can be applied to mammalian cells and the cells cultured to produce cloned animals, these could then breed conventionally to form flocks of genetically engineered animals all producing medicines in their milk. There are other medical and scientific reasons for the interest in cloning. It is already being used alongside genetic techniques in the development of animal organs for transplant into humans (xenotransplantation). Combining such genetic techniques with cloning of pigs (achieved for the first time in March 2000) would lead to a reliable supply of suitable donor organs. The use of pig organs has been hampered by the presence of a sugar, alpha gal, on pig cells, but in 2002 scientists succeeded in knocking out the gene that makes it, and these ‘knockout’ pigs could be bred naturally. However, there are still worries about virus transmission. The study of animal clones and cloned cells could lead to greater understanding of the development of the embryo and of ageing and age-related diseases. Cloned mice become obese, with related symptoms such as raised plasma insulin and leptin levels, though their offspring do not and are normal. Cloning could be used to create better animal models of diseases, which could in turn lead to further progress in understanding and treating those diseases. It could even enhance biodiversity by ensuring the continuation of rare breeds and endangered species.
What happened to Dolly?
Dolly, probably the most famous sheep in the world, lived a pampered existence at the Roslin Institute. She mated and produced normal offspring in the normal way, showing that such cloned animals can reproduce. Born on 5 July 1996, she was euthanased on 14 February 2003, aged six and a half. Sheep can live to age 11 or 12, but Dolly suffered from arthritis in a hind leg joint and from sheep pulmonary adenomatosis, a virus-induced lung tumour to which sheep raised indoors are prone. On 2 February 2003, Australia's first cloned sheep died unexpectedly at the age of two years and 10 months. The cause of death was unknown and the carcass was quickly cremated as it was decomposing. Dolly’s chromosomes were are a little shorter than those of other sheep, but in most other ways she was the same as any other sheep of her chronological age. However, her early ageing may reflect that she was raised from the nucleus of a 6-year old sheep. Study of her cells also revealed that the very small amount of DNA outside the nucleus, in the mitochondria of the cells, is all inherited from the donor egg cell, not from the donor nucleus like the rest of her DNA. So she is not a completely identical copy. This finding could be important for sex-linked diseases such as haemophilia, and certain neuromuscular, brain and kidney conditions that are passed on through the mother's side of the family only.
Improving the technology
Scientists are working on ways to improve the technology. For example, when two genetically identical cloned mice embryos are combined, the aggregate embryo is more likely to survive to birth. Improvements in the culture medium may also help.
Ethical concerns and regulation
Most of the ethical concerns about cloning relate to the possibility that it might be used to clone humans. There would be enormous technical difficulties. As the technology stands at present, it would have to involve women willing to donate perhaps hundreds of eggs, surrogate pregnancies with high rates of miscarriage and stillbirth, and the possibility of premature ageing and high cancer rates for any children so produced. However, in 2004 South Korean scientists announced that they had cloned 30 human embryos, grown them in the laboratory until they were a hollow ball of cells, and produced a line of stem cells from them. Further ethical discussion was raised in 2008 when scientists succeeded in cloning mice from tissue that had been frozen for 16 years. In the USA, President Clinton asked the National Bioethics Commission and Congress to examine the issues, and in the UK the House of Commons Science and Technology Committee, the Human Embryology and Fertilisation Authority and the Human Genetics Advisory Commission all consulted widely and advised that human cloning should be banned. The Council of Europe has banned human cloning: in fact most countries have banned the use of cloning to produce human babies (human reproductive cloning). However, there is one important medical aspect of cloning technology that could be applied to humans, which people may find less objectionable. This is therapeutic cloning (or cell nucleus replacement) for tissue engineering, in which tissues, rather than a baby, are created. In therapeutic cloning, single cells would be taken from a person and 'reprogrammed' to create stem cells, which have the potential to develop into any type of cell in the body. When needed, the stem cells could be thawed and then induced to grow into particular types of cell such as heart, liver or brain cells that could be used in medical treatment. Reprogramming cells is likely to prove technically difficult. Therapeutic cloning research is already being conducted in animals, and stem cells have been grown by this method and transplanted back into the original donor animal. In humans, this technique would revolutionise cell and tissue transplantation as a method of treating diseases. However, it is a very new science and has raised ethical concerns. In the UK a group headed by the Chief Medical Officer, Professor Liam Donaldson, has recommended that research on early human embryos should be allowed. The Human Fertilisation and Embryology Act was amended in 2001 to allow the use of embryos for stem cell research and consequently the HFEA has the responsibility for regulating all embryonic stem cell research in the UK. There is a potential supply of early embryos as patients undergoing in-vitro fertilisation usually produce a surplus of fertilised eggs. As far as animal cloning is concerned, all cloning for research or medical purposes in the UK must be approved by the Home Office under the strict controls of the Animals (Scientific Procedures) Act 1986 . This safeguards animal welfare while allowing important scientific and medical research to go ahead.
Further information
The Roslin Institute has lots of information about the research that led to Dolly, and the scientific studies of Dolly, as well as links to many other sites that provide useful information on the scientific and ethical aspects of this research. The report of the Chief Medical Officer's Expert Advisory Group on Therapeutic Cloning: Stem cell research: medical progress with responsibility is available from the UK Department of Health , PO Box 777, London SE1 6XH. Further information on therapeutic cloning and stem cell research is available from the Medical Research Council . Interesting illustrated features on cloning have been published by Time , New Scientist . BBC News Online has a Q&A What is Cloning? IMAGE © THE ROSLIN INSTITUTE
Featured news

Bird flu in cows: is it a human problem?

Openness, a powerful tool to support science

Long Covid, can animals provide the answers?
Subscribe to our newsletter.
Get the latest articles and news from Understanding Animal Research in your email inbox every month. For more information, please see our privacy policy .
- Introduction to Genomics
- Educational Resources
- Policy Issues in Genomics
- The Human Genome Project
- Funding Opportunities
- Funded Programs & Projects
- Division and Program Directors
- Scientific Program Analysts
- Contact by Research Area
- News & Events
- Research Areas
- Research investigators
- Research Projects
- Clinical Research
- Data Tools & Resources
- Genomics & Medicine
- Family Health History
- For Patients & Families
- For Health Professionals
- Jobs at NHGRI
- Training at NHGRI
- Funding for Research Training
- Professional Development Programs
- NHGRI Culture
- Social Media
- Broadcast Media
- Image Gallery
- Press Resources
- Organization
- NHGRI Director
- Mission & Vision
- Policies & Guidance
- Institute Advisors
- Strategic Vision
- Leadership Initiatives
- Diversity, Equity, and Inclusion
- Partner with NHGRI
- Staff Search
Cloning Fact Sheet
The term cloning describes a number of different processes that can be used to produce genetically identical copies of a biological entity. The copied material, which has the same genetic makeup as the original, is referred to as a clone. Researchers have cloned a wide range of biological materials, including genes, cells, tissues and even entire organisms, such as a sheep.
Do clones ever occur naturally?
Yes. In nature, some plants and single-celled organisms, such as bacteria , produce genetically identical offspring through a process called asexual reproduction. In asexual reproduction, a new individual is generated from a copy of a single cell from the parent organism.
Natural clones, also known as identical twins, occur in humans and other mammals. These twins are produced when a fertilized egg splits, creating two or more embryos that carry almost identical DNA . Identical twins have nearly the same genetic makeup as each other, but they are genetically different from either parent.
What are the types of artificial cloning?
There are three different types of artificial cloning: gene cloning, reproductive cloning and therapeutic cloning.
Gene cloning produces copies of genes or segments of DNA. Reproductive cloning produces copies of whole animals. Therapeutic cloning produces embryonic stem cells for experiments aimed at creating tissues to replace injured or diseased tissues.
Gene cloning, also known as DNA cloning, is a very different process from reproductive and therapeutic cloning. Reproductive and therapeutic cloning share many of the same techniques, but are done for different purposes.
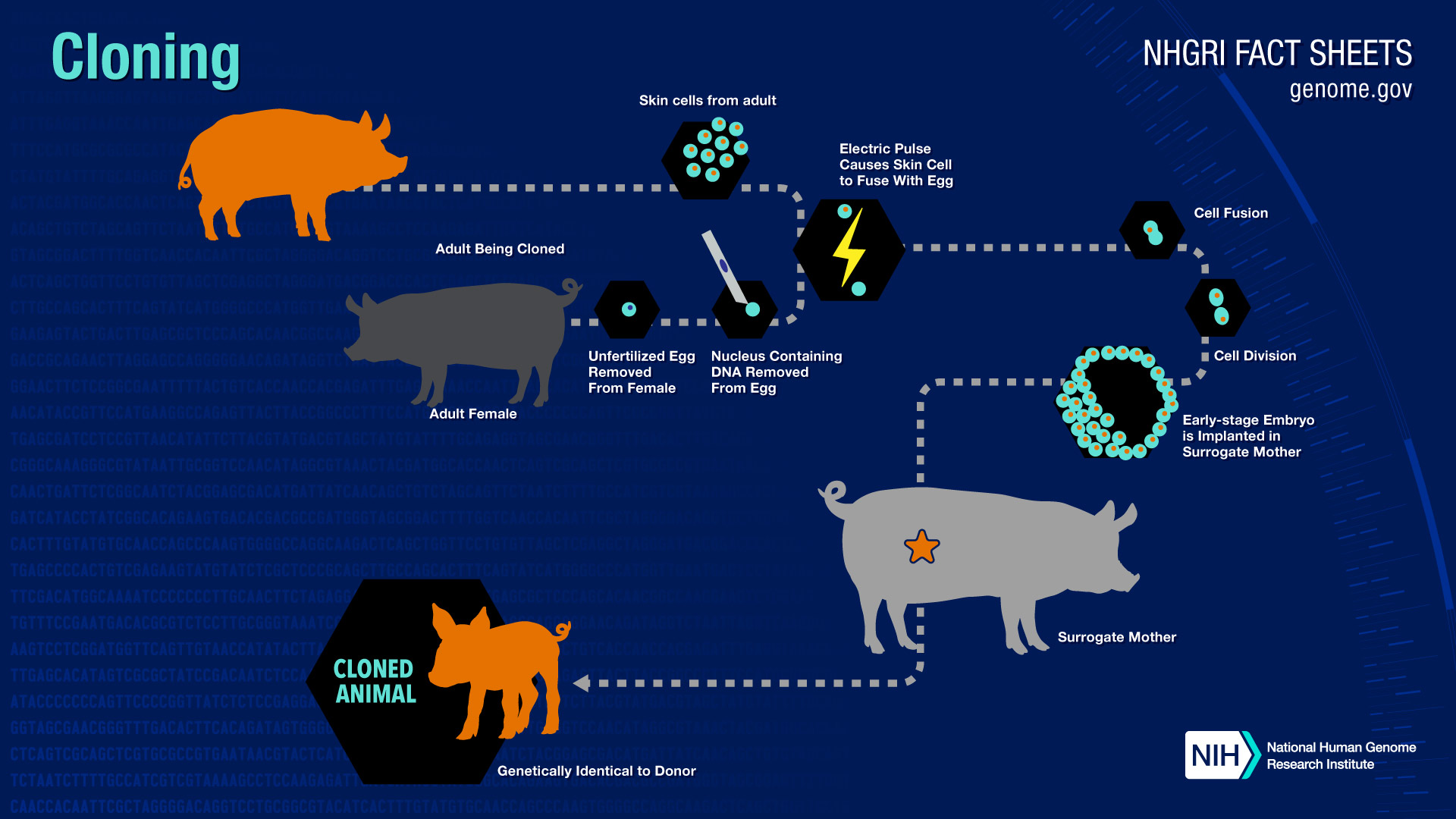
What sort of cloning research is going on at NHGRI?
Gene cloning is the most common type of cloning done by researchers at NHGRI. NHGRI researchers have not cloned any mammals and NHGRI does not clone humans.
How are genes cloned?
Researchers routinely use cloning techniques to make copies of genes that they wish to study. The procedure consists of inserting a gene from one organism, often referred to as "foreign DNA," into the genetic material of a carrier called a vector. Examples of vectors include bacteria, yeast cells, viruses or plasmids, which are small DNA circles carried by bacteria. After the gene is inserted, the vector is placed in laboratory conditions that prompt it to multiply, resulting in the gene being copied many times over.
How are animals cloned?
In reproductive cloning, researchers remove a mature somatic cell , such as a skin cell, from an animal that they wish to copy. They then transfer the DNA of the donor animal's somatic cell into an egg cell, or oocyte, that has had its own DNA-containing nucleus removed.
Researchers can add the DNA from the somatic cell to the empty egg in two different ways. In the first method, they remove the DNA-containing nucleus of the somatic cell with a needle and inject it into the empty egg. In the second approach, they use an electrical current to fuse the entire somatic cell with the empty egg.
In both processes, the egg is allowed to develop into an early-stage embryo in the test-tube and then is implanted into the womb of an adult female animal.
Ultimately, the adult female gives birth to an animal that has the same genetic make up as the animal that donated the somatic cell. This young animal is referred to as a clone. Reproductive cloning may require the use of a surrogate mother to allow development of the cloned embryo, as was the case for the most famous cloned organism, Dolly the sheep.
What animals have been cloned?
Over the last 50 years, scientists have conducted cloning experiments in a wide range of animals using a variety of techniques. In 1979, researchers produced the first genetically identical mice by splitting mouse embryos in the test tube and then implanting the resulting embryos into the wombs of adult female mice. Shortly after that, researchers produced the first genetically identical cows, sheep and chickens by transferring the nucleus of a cell taken from an early embryo into an egg that had been emptied of its nucleus.
It was not until 1996, however, that researchers succeeded in cloning the first mammal from a mature (somatic) cell taken from an adult animal. After 276 attempts, Scottish researchers finally produced Dolly, the lamb from the udder cell of a 6-year-old sheep. Two years later, researchers in Japan cloned eight calves from a single cow, but only four survived.
Besides cattle and sheep, other mammals that have been cloned from somatic cells include: cat, deer, dog, horse, mule, ox, rabbit and rat. In addition, a rhesus monkey has been cloned by embryo splitting.
Have humans been cloned?
Despite several highly publicized claims, human cloning still appears to be fiction. There currently is no solid scientific evidence that anyone has cloned human embryos.
In 1998, scientists in South Korea claimed to have successfully cloned a human embryo, but said the experiment was interrupted very early when the clone was just a group of four cells. In 2002, Clonaid, part of a religious group that believes humans were created by extraterrestrials, held a news conference to announce the birth of what it claimed to be the first cloned human, a girl named Eve. However, despite repeated requests by the research community and the news media, Clonaid never provided any evidence to confirm the existence of this clone or the other 12 human clones it purportedly created.
In 2004, a group led by Woo-Suk Hwang of Seoul National University in South Korea published a paper in the journal Science in which it claimed to have created a cloned human embryo in a test tube. However, an independent scientific committee later found no proof to support the claim and, in January 2006, Science announced that Hwang's paper had been retracted.
From a technical perspective, cloning humans and other primates is more difficult than in other mammals. One reason is that two proteins essential to cell division, known as spindle proteins, are located very close to the chromosomes in primate eggs. Consequently, removal of the egg's nucleus to make room for the donor nucleus also removes the spindle proteins, interfering with cell division. In other mammals, such as cats, rabbits and mice, the two spindle proteins are spread throughout the egg. So, removal of the egg's nucleus does not result in loss of spindle proteins. In addition, some dyes and the ultraviolet light used to remove the egg's nucleus can damage the primate cell and prevent it from growing.
Do cloned animals always look identical?
No. Clones do not always look identical. Although clones share the same genetic material, the environment also plays a big role in how an organism turns out.
For example, the first cat to be cloned, named Cc, is a female calico cat that looks very different from her mother. The explanation for the difference is that the color and pattern of the coats of cats cannot be attributed exclusively to genes. A biological phenomenon involving inactivation of the X chromosome (See sex chromosome ) in every cell of the female cat (which has two X chromosomes) determines which coat color genes are switched off and which are switched on. The distribution of X inactivation, which seems to occur randomly, determines the appearance of the cat's coat.
What are the potential applications of cloned animals?
Reproductive cloning may enable researchers to make copies of animals with the potential benefits for the fields of medicine and agriculture.
For instance, the same Scottish researchers who cloned Dolly have cloned other sheep that have been genetically modified to produce milk that contains a human protein essential for blood clotting. The hope is that someday this protein can be purified from the milk and given to humans whose blood does not clot properly. Another possible use of cloned animals is for testing new drugs and treatment strategies. The great advantage of using cloned animals for drug testing is that they are all genetically identical, which means their responses to the drugs should be uniform rather than variable as seen in animals with different genetic make-ups.
After consulting with many independent scientists and experts in cloning, the U.S. Food and Drug Administration (FDA) decided in January 2008 that meat and milk from cloned animals, such as cattle, pigs and goats, are as safe as those from non-cloned animals. The FDA action means that researchers are now free to using cloning methods to make copies of animals with desirable agricultural traits, such as high milk production or lean meat. However, because cloning is still very expensive, it will likely take many years until food products from cloned animals actually appear in supermarkets.
Another application is to create clones to build populations of endangered, or possibly even extinct, species of animals. In 2001, researchers produced the first clone of an endangered species: a type of Asian ox known as a guar. Sadly, the baby guar, which had developed inside a surrogate cow mother, died just a few days after its birth. In 2003, another endangered type of ox, called the Banteg, was successfully cloned. Soon after, three African wildcats were cloned using frozen embryos as a source of DNA. Although some experts think cloning can save many species that would otherwise disappear, others argue that cloning produces a population of genetically identical individuals that lack the genetic variability necessary for species survival.
Some people also have expressed interest in having their deceased pets cloned in the hope of getting a similar animal to replace the dead one. But as shown by Cc the cloned cat, a clone may not turn out exactly like the original pet whose DNA was used to make the clone.
What are the potential drawbacks of cloning animals?
Reproductive cloning is a very inefficient technique and most cloned animal embryos cannot develop into healthy individuals. For instance, Dolly was the only clone to be born live out of a total of 277 cloned embryos. This very low efficiency, combined with safety concerns, presents a serious obstacle to the application of reproductive cloning.
Researchers have observed some adverse health effects in sheep and other mammals that have been cloned. These include an increase in birth size and a variety of defects in vital organs, such as the liver, brain and heart. Other consequences include premature aging and problems with the immune system. Another potential problem centers on the relative age of the cloned cell's chromosomes. As cells go through their normal rounds of division, the tips of the chromosomes, called telomeres, shrink. Over time, the telomeres become so short that the cell can no longer divide and, consequently, the cell dies. This is part of the natural aging process that seems to happen in all cell types. As a consequence, clones created from a cell taken from an adult might have chromosomes that are already shorter than normal, which may condemn the clones' cells to a shorter life span. Indeed, Dolly, who was cloned from the cell of a 6-year-old sheep, had chromosomes that were shorter than those of other sheep her age. Dolly died when she was six years old, about half the average sheep's 12-year lifespan.
What is therapeutic cloning?
Therapeutic cloning involves creating a cloned embryo for the sole purpose of producing embryonic stem cells with the same DNA as the donor cell. These stem cells can be used in experiments aimed at understanding disease and developing new treatments for disease. To date, there is no evidence that human embryos have been produced for therapeutic cloning.
The richest source of embryonic stem cells is tissue formed during the first five days after the egg has started to divide. At this stage of development, called the blastocyst, the embryo consists of a cluster of about 100 cells that can become any cell type. Stem cells are harvested from cloned embryos at this stage of development, resulting in destruction of the embryo while it is still in the test tube.
What are the potential applications of therapeutic cloning?
Researchers hope to use embryonic stem cells, which have the unique ability to generate virtually all types of cells in an organism, to grow healthy tissues in the laboratory that can be used replace injured or diseased tissues. In addition, it may be possible to learn more about the molecular causes of disease by studying embryonic stem cell lines from cloned embryos derived from the cells of animals or humans with different diseases. Finally, differentiated tissues derived from ES cells are excellent tools to test new therapeutic drugs.
What are the potential drawbacks of therapeutic cloning?
Many researchers think it is worthwhile to explore the use of embryonic stem cells as a path for treating human diseases. However, some experts are concerned about the striking similarities between stem cells and cancer cells. Both cell types have the ability to proliferate indefinitely and some studies show that after 60 cycles of cell division, stem cells can accumulate mutations that could lead to cancer. Therefore, the relationship between stem cells and cancer cells needs to be more clearly understood if stem cells are to be used to treat human disease.
What are some of the ethical issues related to cloning?
Gene cloning is a carefully regulated technique that is largely accepted today and used routinely in many labs worldwide. However, both reproductive and therapeutic cloning raise important ethical issues, especially as related to the potential use of these techniques in humans.
Reproductive cloning would present the potential of creating a human that is genetically identical to another person who has previously existed or who still exists. This may conflict with long-standing religious and societal values about human dignity, possibly infringing upon principles of individual freedom, identity and autonomy. However, some argue that reproductive cloning could help sterile couples fulfill their dream of parenthood. Others see human cloning as a way to avoid passing on a deleterious gene that runs in the family without having to undergo embryo screening or embryo selection.
Therapeutic cloning, while offering the potential for treating humans suffering from disease or injury, would require the destruction of human embryos in the test tube. Consequently, opponents argue that using this technique to collect embryonic stem cells is wrong, regardless of whether such cells are used to benefit sick or injured people.
Last updated: August 15, 2020
ENCYCLOPEDIC ENTRY
Cloning is a technique scientists use to create exact genetic replicas of genes, cells, or animals.
Biology, Genetics, Health, Chemistry
Cloned Beagles
Two Beagle puppies successfully cloned in Seoul, South Korea. These two dogs were cloned by a biopharmaceutical company that specializes in stem cell based therapeutics.
Photograph by Handout

Cloning is a technique scientists use to make exact genetic copies of living things. Genes , cells, tissues, and even whole animals can all be cloned .
Some clones already exist in nature. Single-celled organisms like bacteria make exact copies of themselves each time they reproduce. In humans, identical twins are similar to clones . They share almost the exact same genes . Identical twins are created when a fertilized egg splits in two.
Scientists also make clones in the lab. They often clone genes in order to study and better understand them. To clone a gene , researchers take DNA from a living creature and insert it into a carrier like bacteria or yeast. Every time that carrier reproduces, a new copy of the gene is made.
Animals are cloned in one of two ways. The first is called embryo twinning. Scientists first split an embryo in half. Those two halves are then placed in a mother’s uterus. Each part of the embryo develops into a unique animal, and the two animals share the same genes . The second method is called somatic cell nuclear transfer. Somatic cells are all the cells that make up an organism, but that are not sperm or egg cells. Sperm and egg cells contain only one set of chromosomes , and when they join during fertilization, the mother’s chromosomes merge with the father’s. Somatic cells , on the other hand, already contain two full sets of chromosomes . To make a clone , scientists transfer the DNA from an animal’s somatic cell into an egg cell that has had its nucleus and DNA removed. The egg develops into an embryo that contains the same genes as the cell donor. Then the embryo is implanted into an adult female’s uterus to grow.
In 1996, Scottish scientists cloned the first animal, a sheep they named Dolly. She was cloned using an udder cell taken from an adult sheep. Since then, scientists have cloned cows, cats, deer, horses, and rabbits. They still have not cloned a human, though. In part, this is because it is difficult to produce a viable clone . In each attempt, there can be genetic mistakes that prevent the clone from surviving. It took scientists 276 attempts to get Dolly right. There are also ethical concerns about cloning a human being.
Researchers can use clones in many ways. An embryo made by cloning can be turned into a stem cell factory. Stem cells are an early form of cells that can grow into many different types of cells and tissues. Scientists can turn them into nerve cells to fix a damaged spinal cord or insulin-making cells to treat diabetes.
The cloning of animals has been used in a number of different applications. Animals have been cloned to have gene mutations that help scientists study diseases that develop in the animals. Livestock like cows and pigs have been cloned to produce more milk or meat. Clones can even “resurrect” a beloved pet that has died. In 2001, a cat named CC was the first pet to be created through cloning. Cloning might one day bring back extinct species like the woolly mammoth or giant panda.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, specialist, content production, last updated.
October 19, 2023
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources

U.S. Food and Drug Administration (FDA) - Animal Cloning (05/2021)
Cloning FAQs - The Roslin Institute, University of Edinburgh (06/2021)
National Human Genome Research Institute (NHGRI) - Cloning (08/2020)
Food Standards, Australia/New Zealand - Food from cloned animals (03/2016)
BIO - All About Animal Cloning (09/2010)
Animal Cloning (03/2008)
Are the Progeny of Cloned Animals Safe to Eat? (01/2008)
Is livestock cloning another form of genetic engineering? (01/2008)
Health and Behavioral Studies on Cloned Cattle (6/2005)
Animal Cloning
A clone is an organism that is descended from, and genetically identical to, a single common ancestor. Animals can be cloned by embryo splitting or nuclear transfer . Embryo splitting involves bisecting the multicellular embryo at an early stage of development to generate "twins". This type of cloning occurs naturally and has also been performed in the laboratory with a number of animal species.
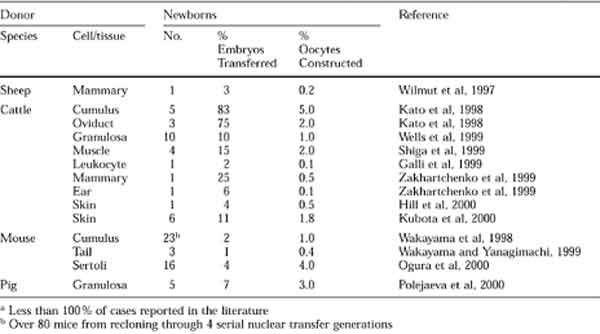
Cloning can also be achieved by nuclear transfer where the genetic material from one cell is placed into a "recipient" unfertilized egg that has had its genetic material removed by a process called enucleation . The first mammals were cloned via nuclear transfer during the early 1980s, almost 30 years after the initial successful experiments with frogs . Numerous mammalian species have been cloned from cells of preimplantation embryos: namely mice, rats, rabbits, pigs, goats, sheep, cattle and even two rhesus monkeys.
Dolly the sheep was the first animal to be cloned via nuclear transfer from a cultured adult cell in 1996. Since then, a diverse range of adult tissues have been successfully cloned in a variety of species including cattle, mice, pigs, cats, rabbits, goats, and zebrafish.
The proportion of adult cell nuclei to develop into live offspring after transfer into an enucleated egg is very low. High rates of abortion have been observed at various stages of pregnancy after placement of the eggs containing the adult cell nuclei into recipient animals . Various abnormalities have been observed in cloned cows and mice after birth and this has been found to be somewhat dependent on the type of tissue that originated the nuclei used to make the clone. The reasons for the low efficiency of cloning by nuclear transfer are currently under investigation but it is thought that it may be related to insufficient nuclear reprogramming as the cloned nuclei goes from directing the production of an adult somatic cell to directing the production of a whole new embryo.
Mammals Cloned From Adult Cells (Table from De Berardino, 2001)
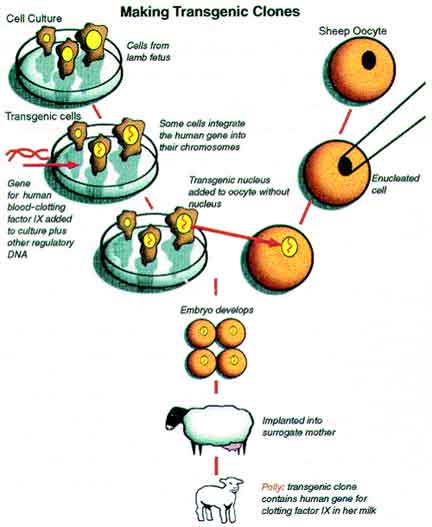
Cloning offers the opportunity to make transgenic animals from cultured cells that have been genetically engineered. The first genetically engineered or transgenic mammalian clones were sheep born in 1997 carrying the coding sequences for human clotting factor IX, which is an important therapeutic for hemophiliacs. One of these transgenic sheep, Polly, expressed this protein in her milk. Cloning may also be useful for the preservation of rare and endangered species. The "Frozen Zoo" developed by the San Diego Zoo partnered with a number of companies including ViaGen Equine to enable the cloning of a Przewalski’s horse in 2020 (https://www.promegaconnections.com/galloping-to-greatness-meet-kurt-the-first-cloned-przewalskis-horse/). In human therapeutics, patients may be able to clone their own nuclei to make healthy tissue that could be used to replace diseased tissue without the risk of immunological rejection.
Making Genetically Engineered Clones (Data from Schnieke et al., 1997; Figure from De Berardino, 2001). Fetal cells in culture were transfected with a DNA sequence containing a selectable marker (neomycin resistance), the human gene for clotting factor IX, and a regulatory sequence to direct the gene to function only in the mammary gland. Following selection for neomycin resistance, nucleus from surviving cells were each transferred to an enucleated egg. Of the three transgenic clones born, one named POLLY survived and later secreted human clotting factor in her milk. Polly is the first transgenic mammalian clone.
Companies Using Cloning Technology
- TransOva Genetics (U.S.)
- Viagen (U.S.)
- Sooam Biotech (South Korea)
1. Briggs,R, King,TJ: Transplantation of living nuclei from blastula cells into enucleated frogs' eggs. Proc.Natl.Acad.Sci.U.S.A 39: 455-463 (1952).
2. Meng,L, Ely,JJ, Stouffer,RL, Wolf,DP: Rhesus monkeys produced by nuclear transfer. Biol Reprod 57: 454-459 (1997).
3. Wilmut,I, Schnieke,AE, McWhir,J, Kind,AJ, Campbell,KH: Viable offspring derived from fetal and adult mammalian cells. Nature 385: 810-813 (1997).
4. Galli,C, Duchi,R, Moor,RM, Lazzari,G: Mammalian leukocytes contain all of the genetic information necessary for the development of a new individual. Cloning 1: 161-170 (1999).
5. Hill,JR, Burghardt,RC, Jones,K, Long,CR, Looney,CR, Shin,T, Spencer,TE, Thompson,JA, Winger,QA, Westhusin,ME: Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol Reprod 63: 1787-1794 (2000).
6. Kato,Y, Tani,T, Sotomaru,Y, Kurokawa,K, Kato,J, Doguchi,H, Yasue,H, Tsunoda,Y: Eight calves cloned from somatic cells of a single adult. Science 282: 2095-2098 (1998).
7. Kubota,C, Yamakuchi,H, Todoroki,J, Mizoshita,K, Tabara,N, Barber,M, Yang,X: Six cloned calves produced from adult fibroblast cells after long-term culture. Proc.Natl.Acad.Sci.U.S.A 97: 990-995 (2000).
8. Shiga,K, Fujita,T, Hirose,K, Sasae,Y, Nagai,T: Production of calves by transfer of nuclei from cultured somatic cells obtained from Japanese black bulls. Theriogenology 52: 527-535 (1999).
9. Wells,DN, Misica,PM, Tervit,HR: Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod 60: 996-1005 (1999).
10. Zakhartchenko,V, Alberio,R, Stojkovic,M, Prelle,K, Schernthaner,W, Stojkovic,P, Wenigerkind,H, Wanke,R, Duchler,M, Steinborn,R, Mueller,M, Brem,G, Wolf,E: Adult cloning in cattle: potential of nuclei from a permanent cell line and from primary cultures. Mol.Reprod Dev. 54: 264-272 (1999).
11. Ogura,A, Inoue,K, Ogonuki,N, Noguchi,A, Takano,K, Nagano,R, Suzuki,O, Lee,J, Ishino,F, Matsuda,J: Production of male cloned mice from fresh, cultured, and cryopreserved immature Sertoli cells. Biol Reprod 62: 1579-1584 (2000).
12. Wakayama,T, Perry,AC, Zuccotti,M, Johnson,KR, Yanagimachi,R: Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394: 369-374 (1998).
13. Wakayama,T, Yanagimachi,R: Cloning of male mice from adult tail-tip cells. Nat.Genet. 22: 127-128 (1999).
14. Polejaeva,IA, Chen,SH, Vaught,TD, Page,RL, Mullins,J, Ball,S, Dai,Y, Boone,J, Walker,S, Ayares,DL, Colman,A, Campbell,KH: Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407: 86-90 (2000).
15. Shin,T, Kraemer,D, Pryor,J, Liu,L, Rugila,J, Howe,L, Buck,S, Murphy,K, Lyons,L, Westhusin,M: A cat cloned by nuclear transplantation. Nature 415: 859 (2002).
16. Chesne,P, Adenot,PG, Viglietta,C, Baratte,M, Boulanger,L, Renard,JP: Cloned rabbits produced by nuclear transfer from adult somatic cells. Nature Biotechnology 20: 366-369 (2002).
17. Keefer,CL, Baldassarre,H, Keyston,R, Wang,B, Bhatia,B, Bilodeau,AS, Zhou,JF, Leduc,M, Downey,BR, Lazaris,A, Karatzas,CN: Generation of dwarf goat (Capra hircus) clones following nuclear transfer with transfected and nontransfected fetal fibroblasts and in vitro-matured oocytes. Biol Reprod 64: 849-856 (2001).
18. Lee,KY, Huang,HG, Ju,BS, Yang,ZG, Lin,S: Cloned zebrafish by nuclear transfer from long-term-cultured cells. Nature Biotechnology 20: 795-799 (2002).
19. Tsunoda,Y, Kato,Y: Recent progress and problems in animal cloning. Differentiation 69: 158-161 (2002).
20. Di Berardino,MA: Animal cloning--the route to new genomics in agriculture and medicine. Differentiation 68: 67-83 (2001).
21. Schnieke,AE, Kind,AJ, Ritchie,WA, Mycock,K, Scott,AR, Ritchie,M, Wilmut,I, Colman,A, Campbell,KH: Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science 278: 2130-2133 (1997).
22. Lanza,RP, Cibelli,JB, Diaz,F, Moraes,CT, Farin,PW, Farin,CE, Hammer.C.J., West,MD, Damiani,P: Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 2: 79-90 (2000).
Animal Cloning ( See Animal Ethics; Animal Research; Cloning)
- First Online: 27 May 2021
Cite this chapter

- Henk ten Have 3 &
- Maria do Céu Patrão Neves 4
80 Accesses
Cloning in the animal world is achieved naturally in several ways. Asexual reproduction is when an organism creates a copy of itself without any contribution of genetic material from another individual. It is the most elementary form of (plant and) animal cloning and happens in nature through fragmentation (a new organism grows from a fragment of the progenitor), gemmulation (aggregates of cells mostly archaeocytes become isolated), and parthenogenesis (an unfertilized egg develops into a new individual). Although not involving genetic material from a second source, parthenogenesis can be considered sexual reproduction because it involves gametes.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Author information
Authors and affiliations.
Center for Healthcare Ethics, Duquesne University, Pittsburgh, PA, USA
Henk ten Have
University of the Azores, Ponta Delgada, Portugal
Maria do Céu Patrão Neves
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Henk ten Have .
Rights and permissions
Reprints and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
ten Have, H., Patrão Neves, M. (2021). Animal Cloning ( See Animal Ethics; Animal Research; Cloning). In: Dictionary of Global Bioethics. Springer, Cham. https://doi.org/10.1007/978-3-030-54161-3_53
Download citation
DOI : https://doi.org/10.1007/978-3-030-54161-3_53
Published : 27 May 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-54160-6
Online ISBN : 978-3-030-54161-3
eBook Packages : Religion and Philosophy Philosophy and Religion (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
March 11, 2013
10 min read
Will Cloning Ever Save Endangered Animals?
Right now, cloning is not a viable conservation strategy. But some researchers remain optimistic that it will help threatened species in the future
By Ferris Jabr

On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
In 2009 the Brazilian Agricultural Research Corp. (Embrapa) and the Brasilia Zoological Garden began scavenging and freezing blood, sperm and umbilical cord cells from roadkill and other wild animals that had died, mostly in the Cerrado savanna—an incredibly diverse collection of tropical forest and grassland ecosystems home to at least 10,000 plant species and more than 800 species of birds and mammals, some of which live nowhere else in the world. Specimens were collected from the bush dog, collared anteater, bison and gray brocket deer, among other species. The idea was to preserve the genetic information of Brazil's endangered wildlife. One day, the organizations reasoned, they might be able to use the collected DNA to clone endangered animals and bolster dwindling populations. So far the two institutions have collected at least 420 tissue samples. Now they are collaborating on a related project that will use the DNA in these specimens to improve breeding and cloning techniques. Current cloning techniques have an average success rate of less than 5 percent, even when working with familiar species; cloning wild animals is usually less than 1 percent successful. Any animals born during Brazil's new undertaking will live in the Brasilia Zoo, says Embrapa researcher Carlos Martins . Expanding captive populations of wild animals, he and his team hope, will discourage zoos and researchers from taking even more wild animals out of their native habitats. Martins and his colleagues have not yet decided which species they will attempt to clone but the maned wolf and jaguar are strong candidates. The International Union for Conservation of Nature classifies both animals as "near threatened" on its Red List of Threatened Species , two levels below "endangered." Many researchers agree that, at present, cloning is not a feasible or effective conservation strategy. First of all, some conservationists point out, cloning does not address the reasons that many animals become endangered in the first place—namely, hunting and habitat destruction. Even if cloning could theoretically help in truly desperate situations, current cloning techniques are simply too ineffective to make much of a difference. Compared with cloning domestic species—particularly cattle, which have been successfully cloned for years to duplicate desirable traits—cloning endangered species is far more difficult for a number of reasons. Successful cloning generally involves at least three essential components: DNA from the animal to be cloned; a viable egg to receive that DNA; and a mother to gestate the resulting embryo. Often, hundreds of embryos and attempted pregnancies are needed to produce even a few clones. Scientists usually have a poor understanding of endangered animals' reproductive physiology, which makes it too risky to extract a sufficient number of eggs from that species or rely on females of that species to give birth to clones. Legal protections sometimes preclude threatened species from such procedures as well. To compensate, researchers fuse the DNA of an endangered species with eggs from a closely related species and select mothers from the latter. Such hybrid embryos often fail to develop properly. Although they are keenly aware of these problems, Martins and his colleagues, as well as a few other scientists around the world, think that efforts to archive the genetic information of endangered wildlife are worthwhile. Some researchers remain optimistic that cloning will become a useful tool for conservation in the future. Optimists point to recent successes cloning wild mammals using closely related domestic species, improved techniques for preventing developmental abnormalities in a cloned embryo, better neonatal care for newborn clones and in vitro fertilization made possible by stem cells derived from frozen tissue. The first clones In the early 1950s, at the Lankenau Hospital Research Institute in Philadelphia, Robert Briggs and Thomas King successfully cloned 27 northern leopard frogs through a process known as nuclear transfer. The nucleus, often called the command center of the cell, contains most of a vertebrate's DNA—except for the DNA within bean-shaped, energy-generating organelles named mitochondria. Briggs and King emptied frog eggs of their nuclei, sucked nuclei out of cells in frog embryos and injected those nuclei into the empty eggs. Many of the eggs developed into tadpoles that were genetically identical to the embryos that had donated their nuclear DNA. In 1958 John Gurdon, then at the University of Oxford, and colleagues cloned frogs with nuclear DNA extracted from the cells of fully formed tadpoles. Unlike embryonic cells, which are genetically flexible enough to become a variety of different tissues, a tadpole's cells are "differentiated"—that is, the patterns of genes they express have changed to fit the profile of a specific cell type: a skin, eye or heart cell, for example. Gurdon demonstrated that, when transplanted into an egg, nuclear DNA from a mature cell reverts to the more versatile state characteristic of DNA in an embryo's cells. This breakthrough encouraged scientists to try cloning far larger animals using DNA from adult cells. In 1996 researchers in Scotland attempted to clone a female Finn-Dorset sheep . They injected nuclei extracted from her udder cells into nearly 300 empty eggs derived from Scottish blackfaces, a different sheep breed. Out of those prepared eggs, the scientists managed to create more than 30 embryos. Only five of those embryos developed into lambs after being implanted in surrogate Scottish blackfaces. And only one of those lambs survived into adulthood. The researchers named her Dolly. Since then some biologists have repeatedly suggested that cloning could help save endangered species, especially in dire situations in which only a few dozen or a handful of animals remain. The smaller, more homogenous and more inbred a population, the more susceptible it is to a single harmful genetic mutation or disease. Clones could theoretically increase the genetic diversity of an endangered population if researchers have access to preserved DNA from many different individuals. At the very least, clones could stabilize a shrinking population. And, some researchers argue, a genetically homogenous but stable population would be better than extinction; some highly inbred groups of wild animals, such as Chillingham cattle in England, have survived just fine for hundreds of years. One species that might benefit from cloning is the northern white rhinoceros, which is native to Africa. In 1960 the global northern white rhino population was more than 2,000 strong, but poaching has reduced their numbers to as few as 11 today. By last count, three live in zoos—two in San Diego and one in the Czech Republic—four live in the Ol Pejeta Conservancy in Kenya and as few as four individuals may still live in the wild based on unconfirmed reports , but they have not been spotted in several years. Most of the captive animals are uninterested in mating or infertile, although two rhinos mated in the summer of 2012. Right now, though, cloning is unlikely to help the white rhino or any other threatened species. To date, the story of cloning endangered animals is one of a few high-profile successes and many, many failures. Since the early 2000s, using the same technique that produced Dolly, researchers have cloned several endangered and even extinct mammals, including a mouflon sheep and a bovine known as a gaur in 2001 ; a kind of wild cattle called a banteng in 2003 ; a wild goat known as the Pyrenean ibex in 2009; and wild coyotes in 2012. In each case many more clones died before birth than survived; in most cases none of the clones survived into adulthood. Mismatched All those attempted clones of endangered or extinct animals died in different ways for different reasons, but they all shared one fundamental problem—they were not exact replicas of their counterparts. In most cases, researchers have combined DNA from the threatened species with eggs from a related domestic species. Each surrogate mother is often implanted with dozens of hybrid embryos in order to achieve at least a few pregnancies, a strategy that requires extracting hundreds of eggs. Because the reproductive physiology of most endangered animals is so poorly understood, researchers are often unsure when the animals ovulate and how best to acquire their eggs. In some cases legal protections prevent scientists from harvesting eggs from threatened species. For all these reasons, they turn to more familiar domestic species instead. Injecting the DNA of one species into the egg of another species—even a closely related one—creates an unusual hybrid embryo that often fails to develop properly in the womb of a surrogate mother. Hybrid embryos have the nuclear DNA of the cloned species and the mitochondrial (mtDNA) DNA of the donor egg. This mismatch becomes problematic as the embryo develops. Nuclear DNA and mtDNA work together; they both contain genetic recipes for proteins with which cells extract energy from food. In a hybrid embryo these proteins do not always fit together properly, which leaves cells starved for energy. Complicating matters further, the surrogate mother often rejects the hybrid embryo because she recognizes some of the embryo's tissues, particularly the placenta, as foreign. Another problem—and the most intractable so far—is that a hybrid embryo created via nuclear transfer is not a genetic blank slate like most embryos. All vertebrates begin life as hollow balls of embryonic stem cells, which can become almost any type of adult cell. Each of those stem cells contains a copy of the exact same genome packaged into chromosomes—tight bundles of DNA and histone proteins. As the embryo develops, the stem cells begin to take on their adult forms: some become skin cells, others heart cells and so on. Different types of cells begin to express different patterns of genes. Inside each cell an assortment of molecules and enzymes interacts with DNA and histones to change gene expression. Some molecules, such as methyl groups, physically block cellular machinery from reading the genetic instructions in certain segments of DNA; some enzymes loosen the bonds between histones and DNA, making particular genes more accessible. Eventually, each cell type—skin cell, liver cell, brain cell—has the same genome, but a different epigenome: a unique pattern of genes that are actively expressed or effectively silenced. Over time, an adult cell's epigenome can change even further, depending on the animal's life experiences. So when researchers inject an adult cell's nucleus into an empty egg, the nucleus brings its unique epigenome with it. As Gurdon's early experiments in the 1950s and subsequent studies have shown, an egg is capable of erasing the epigenome of introduced nuclear DNA, wiping the slate clean—to some extent. This process of "nuclear reprogramming" is poorly understood, and the egg often fails to complete it properly, especially when the egg is from one species and the nuclear DNA from another. Incomplete nuclear reprogramming is one of the main reasons, scientists think, for the many developmental abnormalities that kill clones before birth and for the medical issues common to many survivors, such as extremely high birth weight and organ failure. Some researchers see ways around these problems. Pasqualino Loi of the University of Teramo in Italy was part of a team that successfully cloned endangered mouflon sheep in the early 2000s; the clones died within six months of birth. Loi and his colleagues think they can increase the chances of a hybrid embryo surviving in a surrogate mother's womb. First, they propose, researchers could nurture a hybrid embryo for a short time in the lab until it develops into what is known as a blastocyst—the ball-shaped beginnings of a vertebrate composed of an outer circle of cells, the trophoblast, surrounding a clump of rapidly dividing stem cells known as the inner cell mass. Eventually, the trophoblast becomes the placenta. Researchers could scoop out the inner cell mass from the hybrid blastocyst, Loi suggests, and transplant it into an empty trophoblast derived from the same species as the surrogate mother. Because the surrogate mother is far less likely to reject a trophoblast from her own species, the developing embryo within has a much better chance of surviving. Scientists have also figured out how to encourage nuclear reprogramming by bathing the egg in certain compounds and chemicals, such as trichostatin A, which stimulate or inhibit the enzymes that determine a cell's epigenome. Most recently, Teruhiko Wakayama of the RIKEN Center for Developmental Biology in Kobe, Japan and his colleagues produced 581 cloned mice from a single donor mouse over 25 generations, using trichostatin A to achieve success rates as high as 25 percent in some but not all generations. To solve the mismatch of mtDNA and nuclear DNA, Loi suggests simply removing the egg's native mtDNA and replacing it with mtDNA from the species to be cloned—something that researchers tried in the 1970s and '80s, but have not attempted recently for reasons that are unclear. Some of the most successful attempts to clone endangered animals in recent years have involved two of the most beloved domestic species—cats and dogs. At the Audubon Center for Research of Endangered Species in New Orleans, Martha Gomez and her colleagues have created many African wildcat clones since the mid-2000s, using domestic cats as surrogate mothers. Gomez says eight clones have survived into adulthood so far and are all healthy today. She attributes her success, in part, to the fact that wildcats and domestic cats are much more closely related to each other than are most wild and domestic species paired for the purpose of cloning. She and her team have also learned to increase success rates with caesarian sections—to spare clones the stress of a typical birth—and to keep newborn clones in intensive care for a few weeks, as though they were premature babies. In 2008, B. C. Lee of Seoul National University in Korea and his colleagues achieved similar success using domestic dogs to create three healthy male gray wolf clones . Lee's team had previously created two female gray wolf clones. All five animals survived into adulthood, Lee confirms. Working with black-footed cats, which are native to Africa and listed as "Vulnerable" on the Red List, Gomez is now focusing on a method of cloning that differs from nuclear transfer. She is trying to transform adult cells from black-footed cats into stem cells and subsequently induce those stem cells to become sperm and eggs. Then, through in vitro fertilization or similar techniques, she could impregnate domestic cats with black-footed cat embryos. Alternatively, stem cell-derived sperm and eggs could be used to impregnate females of the endangered species. To say that this approach is technically challenging would be an understatement, but researchers have made impressive progress. In 2011 Jeanne Loring of the Scripps Research Institute in La Jolla, Calif., and her colleagues produced stem cells from the frozen skin cells of two endangered species—the northern white rhino and a baboonlike primate known as a drill. And in 2012 Katsuhiko Hayashi of Kyoto University Graduate School of Medicine and colleagues turned skin cells from adult mice into stem cells, which they then transformed into viable eggs. After fertilizing the eggs with sperm in test tubes, the researchers implanted the embryos in surrogate mother mice that gave birth to healthy and fertile offspring . "I'm not saying cloning is going to save endangered species," Gomez says, "but I am still a believer of cloning as another tool. It's not easy, though. The research moves slow." Teramo’s Loi remains optimistic too. He thinks that scientists should continue to collect and preserve the genetic information of endangered animals, as Brazil has done, creating bio-banks of tissue on ice, such as the "frozen zoo" at the San Diego Zoo’s Institute for Conservation Research. If researchers manage to dramatically increase the efficiency of cloning wild and endangered animals—whether with nuclear transfer or in vitro fertilization—then the DNA they need will be waiting for them. If they do not, bio-banks will still be useful for more basic research. "Once cloning of endangered animals is properly established, it will be a very powerful tool," Loi says. "If something can be done, it will be done in 10 years."
- Our Mission and Values
- Annual Reports
- Diversity and Inclusion
- Message from the Director
- Public Programs
- Careers, Culture and Benefits
- Whitehead Fellows Program
- Campus and Innovation Centers
- Cell Dynamics
- Regeneration, Rejuvenation and Aging
- Genes and Genomes
- Infectious Disease
- Nervous System
- Plant Biology
- Members and Fellows
- Postdoctoral Scholars
- For Journalists
Scientists Show Cloning Leads to Severe Dysregulation of Many Genes
CAMBRIDGE, Mass. — New results from Rudolf Jaenisch’s lab at the Whitehead Institute for Biomedical Research confirmed that the cloning process jeopardizes the integrity of an animal’s whole genome. Scientists had suspected this based on studying a mere dozen genes, but the current study, which will be reported online in the Proceedings of the National Academies of Science this week, expansively surveyed 10,000 genes for abnormalities.
Using DNA arrays, the researchers found that approximately one in every 25 genes were abnormally expressed in placentas from cloned mice, and to a lesser extent, the livers of cloned mice exhibited abnormal gene expression. “Recent studies showing premature death, pneumonia, liver failure, and obesity in aging cloned mice could be a consequence of these gene expression abnormalities,” says Jaenisch.
This study establishes unequivocally that the normalcy of surviving cloned animals should not be based on superficial clinical examinations but rather on detailed molecular analyses of tissues from adult cloned animals. In other words, even seemingly “normal-looking” clones may have serious underlying epigenetic abnormalities. Epigenetic defects are not mutations but are caused by faulty reprogramming of the donor nucleus leading to an atypical conformation that results in abnormal gene expression. Thus, cloning for the purpose of producing another human being, known as reproductive cloning, is completely unsafe and unethical.
The data suggest that many factors may contribute to abnormal gene expression in cloned animals including the cloning procedure itself and epigenetic errors inherited from the cell used in cloning. Epigenetic errors can occur in all genes and lead to dysfunction. Currently it is impossible to test for epigenetic errors during pregnancy, since they don’t affect the base sequence of a gene. This work was done in collaboration with Eric Lander at the Whitehead Center for Genome Research.
The cloning procedure, however, can be safely used to create embryonic stem cells to treat diseases such as Alzheimer’s, diabetes, and autoimmune diseases. The procedure, often called therapeutic cloning, involves removing the nucleus, which contains the DNA, from an egg and replacing it with the nucleus from an adult. For instance, the nucleus from the skin cell of an Alzheimer’s patient can be transferred into an emptied egg. The egg resets the developmental clock of the transferred nucleus and the reprogrammed cell starts developing into an embryo that is genetically identical to the patient.
At the stage when the embryo develops into a hollow ball of approximately a hundred cells it is explanted into a petri dish and can give rise to embryonic stem (ES) cells. These cells have the potential to become any cell in the body, including new neurons, muscle cells, and blood cells. Because the ES cells are derived from the patient, they can be used to treat the patient without the complications associated with foreign donor tissue such as immune rejection.
“It is important to remember that embryonic stem cells when combined with normal cells—as is the case with cell therapy—may function fine. Embryonic stem cells used to make whole animals by nuclear cloning, however, will most likely produce organisms that are abnormal, since many of the abnormally expressed genes have defined roles in fetal development,” says Jaenisch.
Earlier this year Whitehead scientists used a mouse model to establish for the first time that a combination of therapeutic cloning, gene therapy, and embryonic stem cell differentiation could be used to create custom-tailored cellular therapies for genetic disorders.
Communications and Public Affairs Phone: 617-452-4630 Email: [email protected]
Rudolf Jaenisch’s lab studies the genetic and epigenetic basis of diseases such as Parkinson’s, Alzheimer’s, autism and cancer.
Related News
Researchers at Whitehead Institute are uncovering the underlying genetics, mechanisms, and principles of regeneration.

Whitehead Institute researchers investigate methylation, the chemical tags that help define gene expression during development and disease.

Whitehead Institute researchers are uncovering new ways that genes are regulated that upend existing paradigms of gene expression and provide important insights into health and disease. This collection of stories and multimedia explores that research.
Monkey Clones Created in the Lab. Now What?
In a controversial milestone, researchers have cloned a pair of macaques using a method that could, in theory, be used to clone humans.
In a world first, Chinese researchers have successfully cloned macaques using the same technique that yielded the famous clone Dolly the sheep . The milestone, published in Cell on Wednesday , marks the first time that primates have ever been cloned in such a manner.
The years-long effort, led by Chinese Academy of Sciences postdoctoral fellow Zhen Liu, culminated in the recent birth of two female macaques, Zhong Zhong and Hua Hua. The macaques’ names are drawn from the word zhonghua , an adjective for the Chinese people.
The two macaques—eight weeks and six weeks old—are genetically identical, both clones of the same donor culture of fetal monkey cells. The young monkeys are reportedly healthy and currently live in an incubator.
The discovery potentially leads to a brave new world of biomedical research and will unquestionably spark debate over cloning another primate species: humans. Here’s everything you need to know about these potentially controversial clones.
ARE THESE THE FIRST CLONED MONKEYS?
Technically, no. In 1999, researchers “cloned” a rhesus macaque by splitting an early-stage macaque embryo into multiple parts—in effect, creating artificial identical twins. Other research showed that monkey cells could be cloned to create lines of stem cells. However, these efforts yielded only cells confined to petri dishes, not fully developed monkeys.
“It’s about time, because I thought it would never happen,” says Shoukhrat Mitalipov , the head of Oregon Health and Science University’s Center for Embryonic Cell and Gene Therapy. He wasn’t involved with the study, but he worked on previous monkey-cloning efforts.
For Hungry Minds
So why is this a big deal.
In 1996, Dolly the sheep became the first mammal cloned using a technique called somatic cell nuclear transfer. Unlike embryo splitting, which can only yield a few copies, this method can theoretically produce an indefinite number of clones from a single donor. This lets researchers craft customizable, genetically uniform populations of animals with potential for biomedical research.
Since then, scientists have cloned more than 20 species—from cows to rabbits to dogs—using this technique, but the Chinese effort marks the first time that non-human primates have been cloned successfully in the same way. This is a big deal, because the cloning technique in this study may apply to other primates such as humans. However, the study’s authors stress that they have no intention of cloning humans.
HOW DOES THIS CLONING METHOD WORK?
In animals such as sheep and monkeys, individuals have a bundle inside every cell called a nucleus, which contains a copy of their distinct genetic code. Somatic cell nuclear transfer involves delicately transferring the nucleus from one animal’s cell into another animal’s egg.
They then chemically prod the egg into developing, as if it had been naturally fertilized. If this embryo reaches a certain stage of development, scientists can then implant it into the surrogate. If the procedure is successful, the surrogate will get pregnant and give birth to an animal that is genetically identical to the nucleus donor.

WHY DID IT TAKE SO LONG TO CLONE PRIMATES THIS WAY?
The full process isn’t as simple as plucking a nucleus out of a skin cell, plugging it into an egg, and expecting it to form a flawless clone. As embryonic cells differentiate into skin, muscle, and other tissues, their DNA gets spooled, bunched up, and tagged so that only specific genes are expressed within any given cell type. It’s a bit like reading a choose-your-own-adventure book, settling on a particular plot you like, and gluing together the pages you skipped.
To increase the odds of success, researchers must nudge the donor nucleus’s DNA into resembling the DNA of a young embryo. Turning back this genetic clock requires complex chemical protocols that must be fine-tuned for individual species. In part, this is why monkey cloning long proved elusive. The team in China tried several versions of the method before one worked, says study coauthor Qiang Sun , director of the Nonhuman Primate Research Facility at the Chinese Academy of Sciences Institute of Neuroscience.
Sun’s team temporarily bathed the clone eggs in trichostatin A, a compound that helped ensure the donor DNA didn’t bunch up. They also prodded the eggs to make enzymes that snipped certain chemical tags off the donor DNA, freeing up locked embryonic genes. The researchers also tried creating clones from both adult and fetal cells, but only clones derived from fetal cells survived. It’s thought that the fetal cells were less “hardened” into their cell types than the adult cells, but because the fetal cells were differentiated, they too required reprogramming.
WHY CLONE MONKEYS IN THE FIRST PLACE?
The researchers say that they want to use this technique to breed macaques for biomedical research. Exact genetic copies of the same animal would reduce the variability in results when testing new drugs or other therapies.
“For the cloning of primate species, including humans, the technical barrier is now broken,” says study coauthor Mu-Ming Poo , who directs the Center for Excellence in Brain Science and Intelligence Technology at the Chinese Academy of Sciences. “However, the reason we chose to break this barrier is to produce animal models that are useful for human medicine. There’s no intention to apply this method to humans.”
You May Also Like

This 1,700-year-old sacrificial monkey has a surprising tale

U.S. arrests Cambodian official headed to wildlife summit—for monkey smuggling

The Monkey Who Went Into the Cold
Koen Van Rompay , a virologist at the California National Primate Research Center, thinks that such clones would be useful in the long run: “If there would be an efficient way to clone monkeys, that could actually reduce the number of monkeys needed to answer a certain research question,” he says.
Van Rompay and Mitalipov caution, however, that the promised therapeutic benefits may not be right around the corner. For one, the technique doesn’t strike them as particularly efficient. Of the team’s 21 cloning attempts using fetal donor cells, only two resulted in healthy live births. What’s more, Zhong Zhong and Hua Hua are only two months old. Researchers don’t yet know what infirmities, if any, will afflict them later in life as a result of being cloned.
“This is just a step,” says Van Rompay. “We are not ready to clone monkeys large-scale.”
BUT IS IT ETHICAL TO MAKE CLONES?
The use of non-human primates as lab animals has long been contentious. Animal-welfare groups have cast experiments on non-human primates as cruel, exactly because of the animals’ similarities to humans. They also express concern over cloning itself, pointing to miscarriages, sterile social environments, human hand-raising, and other unnatural stressors.
“It gives this sense that animals are disposable and commodities for us to use,” says Kathleen Conlee , vice president of animal research issues at the Humane Society of the United States. “Is this appropriate, to have an animal you can do whatever you want to? ... It creates a poor dynamic about how we treat animals overall.”
China, in particular, faces high scrutiny on matters of animal welfare because it has no comprehensive laws against animal cruelty . The study authors say that their facilities follow animal-welfare regulations set by the U.S. National Institutes of Health and that they are attentive to the macaques’ welfare.
It’s possible that improvements in genetics and computer modeling will limit the need for lab monkeys, says Eliza Bliss-Moreau , a behavioral neuroscientist at the California National Primate Research Center. “Technology has advanced so much in the last decade,” she says. “Some of the questions that you would think of in behavioral neuroscience as being targets for [cloning], we’re already tackling in other ways.”
But many biomedical researchers insist that primate models are still necessary for studying complex human diseases and disorders, from Parkinson’s to HIV/AIDS to autism. “I don’t think there will ever be a way we can avoid non-human primates in biomedical research,” says Van Rompay. “If that happens, that would be great, but right now, in-vitro and computer models are not sufficient.”
Johns Hopkins University bioethicist Jeffrey Kahn , an expert on the use of primates in biomedical research, says that the questions this new study poses are complex: “Should we invest in this or organs on a chip? I don’t think it’s that simple.”
WHAT DOES THIS MEAN FOR CLONING HUMANS?
In short, this study suggests that human cloning could be technically possible in a matter of months to years. “The genie’s out of the bottle now,” says Jose Cibelli , a cloning expert at Michigan State University who wasn’t involved with this study.
Whether reproductive human cloning should proceed, however, is another question entirely. All of the scientists interviewed by National Geographic stressed that at present, human cloning would be unnecessary and irresponsible. “There’s no reason to clone humans at this time,” says Poo. “There must be international discussion on this issue.”
Bioethicist Kahn urges a global discussion, as well: “What should we do about it—the ‘we’ being societies, countries, oversight bodies, governments?” he says. “What kind of governance do we think is necessary to prevent bad things from happening to humans, in the context of technology like this?”
WHAT’S NEXT?
The Chinese research team says that they will monitor the long-term health of Zhong Zhong and Hua Hua, including the pair’s brain development. The coauthors also say that the government of Shanghai strongly supports their research and is underwriting plans to expand their laboratory by more than tenfold. In addition, they expressed hope that Chinese society—which is rapidly shifting its views on animal welfare—will keep an open mind to conducting research on non-human primates.
“With all this improvement, along with the high standards of ethical concerns, I think that Chinese society will accept this,” says Poo. “I hope that societies in Western countries will realize once we demonstrate the cloned monkeys’ usefulness in curing disease, they will gradually change their mind.”

Related Topics

Monkeys of Morocco

A Monkey That Knows No Bounds

Where the World’s Only Grass-Eating Monkeys Thrive

Threatened Species Are Thriving in Yellowstone. Now What?

Heard of Zoroastrianism? The ancient religion still has fervent followers
- Environment
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Coronavirus Coverage
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Animal & Veterinary
- Safety & Health
Animal Cloning

In 2001, when it became apparent that animal cloning may become a commercial venture to help improve the quality of herds, FDA requested livestock producers and researchers to keep food from animal clones or their offspring out of the food supply. Since then, FDA has conducted an intensive evaluation that included examining the safety of food from these animals and the risk to animal health.
Based on a final risk assessment, a report written by FDA scientists and issued in January 2008, FDA has concluded that meat and milk from cow, pig, and goat clones and the offspring of any animal clones are as safe as food we eat every day.
What is FDA Doing?
- Animal Cloning: A Risk Assessment (PDF - 22.3MB) Persons using assistive technology may not be able to fully access information in the Risk Assessment. For assistance, please call 240-402-7002.
- Risk Management Plan
- CVM GFI #179 Use of Animal Clones and Clone Progeny for Human Food/Animal Feed
- FDA's Response to Public Comment on the Animal Cloning Risk Assessment, Risk Management Plan, and Guidance for Industry
- CVM Memorandum I - Draft 5/21/03 Conference Call on Prenatal Care for Animal Clones and their Dams Summary
- CVM Memorandum II - ViaGen Industry Meetings
Consumer Health Information
- Myths about Cloning Responses to the questions provided in this document represent FDA's view in light of the conclusions and recommendations outlined in the Animal Cloning Risk Assessment, Risk Management Plan, and Guidance for Industry #179.
- A Primer on Cloning and Its Use in Livestock Operations Responses to the questions provided in this document represent FDA's view in light of the conclusions and recommendations outlined in the Animal Cloning Risk Assessment, Risk Management Plan, and Guidance for Industry #179.
Frequently Asked Questions
- Consumer FAQs
- Producer FAQs
Related Information
- Biotechnology Products at CVM: Animals and Animal Food
- University of Maryland AgNIC Agricultural Biotechnology Gateway
- Animal Biotechnology: Science Based Concerns
- Glossary of Biotechnology for Food and Agriculture United Nations, Food and Agriculture Organization
- 21 CFR Part 511--New Animal Drugs for Investigational Use
- United States Department of Agriculture
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Published: 28 January 2019
Naturally clonal vertebrates are an untapped resource in ecology and evolution research
- Kate L. Laskowski ORCID: orcid.org/0000-0003-1523-9340 1 ,
- Carolina Doran 1 ,
- David Bierbach ORCID: orcid.org/0000-0001-7049-2299 1 ,
- Jens Krause 1 , 2 &
- Max Wolf 1
Nature Ecology & Evolution volume 3 , pages 161–169 ( 2019 ) Cite this article
2655 Accesses
19 Citations
60 Altmetric
Metrics details
- Behavioural ecology
- Evolutionary developmental biology
- Experimental organisms
- Social evolution
Science requires replication. The development of many cloned or isogenic model organisms is a testament to this. But researchers are reluctant to use these traditional animal model systems for certain questions in evolution or ecology research, because of concerns over relevance or inbreeding. It has largely been overlooked that there are a substantial number of vertebrate species that reproduce clonally in nature. Here we highlight how use of these naturally evolved, phenotypically complex animals can push the boundaries of traditional experimental design and contribute to answering fundamental questions in the fields of ecology and evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Selection-driven trait loss in independently evolved cavefish populations

The population genetics of crypsis in vertebrates: recent insights from mice, hares, and lizards

Contrasting signatures of genomic divergence during sympatric speciation
Edwards, J. L. et al. Cloning adult farm animals: a review of the possibilities and problems associated with somatic cell nuclear transfer. Am. J. Reprod. Immunol. 50 , 113–123 (2003).
CAS PubMed Google Scholar
Bolker, J. Model organisms: there’s more to life than rats and flies. Nature 491 , 31–33 (2012).
Hubbs, C. L. & Hubbs, L. C. Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science 76 , 628–630 (1932).
Vrijenhoek, R. C., Dawley, R. M., Cole, C. J. & Bogart, J. P. in Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogart, J. P.) 19–23 (New York State Museum Bulletin, New York, 1989).
Neaves, W. B. & Baumann, P. Unisexual reproduction among vertebrates. Trends Genet. 27 , 81–88 (2011).
Avise, J. Clonality: The Genetics, Ecology, and Evolution of Sexual Abstinence In Vertebrate Animals (Oxford Univ. Press, Oxford, 2008).
Google Scholar
Vrijenhoek, R. C. Unisexual fish: model systems for studying ecology and evolution. Annu. Rev. Ecol. Syst. 25 , 71–96 (1994).
Crews, D. in Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogart, J. P.) 132–143 (New York State Museum Bulletin, New York, 1989).
Dawley, R. M. & Bogart, J. P. Evolution and Ecology of Unisexual Vertebrates Vol. 466 (New York State Museum Bulletin: New York, 1989).
Sinclair, E. A., Pramuk, J. B., Bezy, R. L., Crandall, K. A. & Sites, J. W. Jr. DNA evidence for nonhybrid origins of parthenogenesis in natural populations of vertebrates. Evolution 64 , 1346–1357 (2010).
PubMed Google Scholar
Warren, W. C. et al. Clonal polymorphism and high heterozygosity in the celibate genome of the Amazon molly. Nat. Ecol. Evol. 2 , 669–679 (2018).
PubMed PubMed Central Google Scholar
Crews, D., Grassman, M. & Lindzey, J. Behavioral facilitation of reproduction in sexual and unisexual whiptail lizards. Proc. Natl Acad. Sci. USA 83 , 9547–9550 (1986).
CAS PubMed PubMed Central Google Scholar
Fujita, M. K. & Moritz, C. Origin and evolution of parthenogenetic genomes in lizards: current state and future directions. Cytogenet. Genome Res. 127 , 261–272 (2009).
Schlupp, I. The evolutionary ecology of gynogenesis. Annu. Rev. Ecol. Evol. Syst. 36 , 399–417 (2005).
Lampert, K. P. & Schartl, M. The origin and evolution of a unisexual hybrid: Poecilia formosa . Phil. Trans. R. Soc. B 363 , 2901–2909 (2008).
Stöck, M., Lampert, K. P., Möller, D., Schlupp, I. & Schartl, M. Monophyletic origin of multiple clonal lineages in an asexual fish ( Poecilia formosa ). Mol. Ecol. 19 , 5204–5215 (2010).
Moritz, C., Donnellan, S., Adams, M. & Baverstock, P. R. The origin and evolution of parthenogenesis in Heteronotia binoei (Gekkonidae): extensive genotypic diversity among parthenogens. Evolution 43 , 994–1003 (1989).
Craig, S. F., Slobodkin, L. B., Wray, G. A. & Biermann, C. H. The ‘paradox’of polyembryony: a review of the cases and a hypothesis for its evolution. Evol. Ecol. 11 , 127–143 (1997).
Tatarenkov, A., Lima, S. M., Taylor, D. S. & Avise, J. C. Long-term retention of self-fertilization in a fish clade. Proc. Natl Acad. Sci. USA 106 , 14456–14459 (2009).
Abbott, J. K. & Morrow, E. H. Obtaining snapshots of genetic variation using hemiclonal analysis. Trends Ecol. Evol. 26 , 359–368 (2011).
Bossdorf, O., Richards, C. L. & Pigliucci, M. Epigenetics for ecologists. Ecol. Lett. 11 , 106–115 (2008).
Ekblom, R. & Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 107 , 1–15 (2011).
Matz, M. V. Fantastic beasts and how to sequence them: ecological genomics for obscure model organisms. Trends Genet. 34 , 121–132 (2018).
Kenkel, C. D. & Matz, M. V. Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat. Ecol. Evol. 1 , 0014 (2016).
Oleksiak, M. F., Churchill, G. A. & Crawford, D. L. Variation in gene expression within and among natural populations. Nat. Genet. 32 , 261–266 (2002).
Crowley, J. J. et al. Analyses of allele-specific gene expression in highly divergent mouse crosses identifies pervasive allelic imbalance. Nat. Genet. 47 , 353–360 (2015).
Brenowitz, E. A. & Zakon, H. H. Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends Neurosci. 38 , 273–278 (2015).
Todd, E. V., Black, M. A. & Gemmell, N. J. The power and promise of RNA-seq in ecology and evolution. Mol. Ecol. 25 , 1224–1241 (2016).
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16 , 6–21 (2002).
Verhoeven, K. J. F. & Preite, V. Epigenetic variation in asexually reproducing organisms. Evolution 68 , 644–655 (2014).
Kalisz, S. & Purugganan, M. D. Epialleles via DNA methylation: consequences for plant evolution. Trends Ecol. Evol. 19 , 309–314 (2004).
Oldach, M. J., Workentine, M., Matz, M. V., Fan, T. Y. & Vize, P. D. Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol. Ecol. 26 , 2514–2526 (2017).
Bierbach, D., Laskowski, K. L. & Wolf, M. Behavioural individuality in clonal fish arises despite near-identical rearing conditions. Nat. Commun. 8 , 15361 (2017).
Edenbrow, M. & Croft, D. P. Behavioural types and life history strategies during ontogeny in the mangrove killifish. Kryptolebias marmoratus. Anim. Behav. 82 , 731–741 (2011).
Schlosser, I. J., Doeringsfeld, M. R., Elder, J. F. & Arzayus, L. F. Niche relationships of clonal and sexual fish in a heterogeneous landscape. Ecology 79 , 953–968 (1998).
Cole, C. J., Taylor, H. L. & Townsend, C. R. Morphological variation in a unisexual whiptail lizard ( Aspidoscelis exsanguis ) and one of its bisexual parental species ( Aspidoscelis inornata ) (Reptilia: Squamata: Teiidae): is the clonal species less variable? Am. Mus. Novit. 3849 , 1–20 (2016).
Dawley, R. M., Schultz, R. J. & Goddard, K. A. Clonal reproduction and polyploidy in unisexual hybrids of Phoxinus eos and Phoxinus neogaeus (Pisces; Cyprinidae). Copeia 1987 , 275–283 (1987).
Massicotte, R., Whitelaw, E. & Angers, B. DNA methylation: a source of random variation in natural populations. Epigenetics 6 , 421–427 (2011).
Massicotte, R. & Angers, B. General-purpose genotype or how epigenetics extend the flexibility of a genotype. Genet. Res. Int. 2012 , 317175 (2012).
Leung, C., Breton, S. & Angers, B. Facing environmental predictability with different sources of epigenetic variation. Ecol. Evol. 6 , 5234–5245 (2016).
Schlupp, I., Parzefall, J. & Schartl, M. Biogeography of the Amazon molly. Poecilia formosa. J. Biogeogr. 29 , 1–6 (2002).
Bi, K. & Bogart, J. P. Time and time again: unisexual salamanders (genus Ambystoma ) are the oldest unisexual vertebrates. BMC Evol. Biol. 10 , 238 (2010).
Castonguay, E. & Angers, B. The key role of epigenetics in the persistence of asexual lineages. Genet. Res. Int. 2012 , 534289 (2012).
Vogt, G. Facilitation of environmental adaptation and evolution by epigenetic phenotype variation: insights from clonal, invasive, polyploid, and domesticated animals. Environ. Epigenet. 3 , dvx002 (2017).
McNamara, J. M., Dall, S. R. X., Hammerstein, P. & Leimar, O. Detection vs. selection: integration of genetic, epigenetic and environmental cues in fluctuating environments. Ecol. Lett. 19 , 1267–1276 (2016).
Stamps, J. A. & Frankenhuis, W. E. Bayesian models of development. Trends Ecol. Evol. 31 , 260–268 (2016).
Dall, S. R., McNamara, J. M. & Leimar, O. Genes as cues: phenotypic integration of genetic and epigenetic information from a Darwinian perspective. Trends Ecol. Evol. 30 , 327–333 (2015).
Mackay, T. F. The genetic architecture of quantitative traits. Annu. Rev. Genet. 35 , 303–339 (2001).
Fisher, D. N., Brachmann, M. & Burant, J. B. Complex dynamics and the development of behavioural individuality. Anim. Behav. 138 , e1–e6 (2018).
Frankenhuis, W. E. & Panchanathan, K. Balancing sampling and specialization: an adaptationist model of incremental development. Proc. R. Soc. B 278 , 3558–3565 (2011).
Freund, J. et al. Emergence of individuality in genetically identical mice. Science 340 , 756–759 (2013).
Vogt, G. et al. Production of different phenotypes from the same genotype in the same environment by developmental variation. J. Exp. Biol. 211 , 510–523 (2008).
Gärtner, K. A third component causing random variability beside environment and genotype. A reason for the limited success of a 30 year long effort to standardize laboratory animals? Lab. Anim. 24 , 71–77 (1990).
McNamara, J. M., Green, R. F. & Olsson, O. Bayes’ theorem and its applications in animal behaviour. Oikos 112 , 243–251 (2006).
Trimmer, P. C. et al. Decision-making under uncertainty: biases and Bayesians. Anim. Cogn. 14 , 465–476 (2011).
Stein, L. R., Bukhari, S. A. & Bell, A. M. Personal and transgenerational cues are nonadditive at the phenotypic and molecular level. Nat. Ecol. Evol. 2 , 1306–1311 (2018).
Stamps, J. A., Biro, P. A., Mitchell, D. J. & Saltz, J. B. Bayesian updating during development predicts genotypic differences in plasticity. Evolution 72 , 2167–2180 (2018).
Mousseau, T. A. & Fox, C. W. The adaptive significance of maternal effects. Trends Ecol. Evol. 13 , 403–407 (1998).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7 , 847–854 (2004).
Dingemanse, N. J. & Wolf, M. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365 , 3947–3958 (2010).
Bolnick, D. I. et al. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161 , 1–28 (2003).
Toscano, B. J., Gownaris, N. J., Heerhartz, S. M. & Monaco, C. J. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia 182 , 55–69 (2016).
Thornton, A. & Lukas, D. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367 , 2773–2783 (2012).
Dingemanse, N. J. et al. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76 , 1128–1138 (2007).
Bell, A. M. & Sih, A. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus ). Ecol. Lett. 10 , 828–834 (2007).
Laskowski, K. L. & Bell, A. M. Competition avoidance drives individual differences in response to a changing food resource in sticklebacks. Ecol. Lett. 16 , 746–753 (2013).
Laskowski, K. L. & Pruitt, J. N. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc. R. Soc. B 281 , 20133166 (2014).
Stamps, J. A. & Krishnan, V. V. Combining information from ancestors and personal experiences to predict individual differences in developmental trajectories. Am. Nat. 184 , 647–657 (2014).
Dall, S. R. X., Houston, A. I. & McNamara, J. M. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7 , 734–739 (2004).
Biro, P. A. & Stamps, J. A. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23 , 361–368 (2008).
Wolf, M. & Weissing, F. J. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365 , 3959–3968 (2010).
Edenbrow, M. & Croft, D. P. Environmental and genetic effects shape the development of personality traits in the mangrove killifish Kryptolebias marmoratus . Oikos 122 , 667–681 (2012).
Bijleveld, A. I. et al. Personality drives physiological adjustments and is not related to survival. Proc. R. Soc. B 281 , 20133135 (2014).
Clark, C. W. Antipredator behavior and the asset-protection principle. Behav. Ecol. 5 , 159–170 (1994).
Wolf, M., van Doorn, G. S., Leimar, O. & Weissing, F. J. Life-history trade-offs favour the evolution of animal personalities. Nature 447 , 581–584 (2007).
Luttbeg, B. & Sih, A. Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365 , 3977–3990 (2010).
Mathot, K. J. & Dall, S. R. Metabolic rates can drive individual differences in information and insurance use under the risk of starvation. Am. Nat. 182 , 611–620 (2013).
Kurvers, R. H. J. M., Krause, J., Croft, D. P., Wilson, A. D. M. & Wolf, M. The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol. Evol. 29 , 326–335 (2014).
Krause, J. & Ruxton, G. D. Living in Groups (Oxford Univ. Press, Oxford, 2002).
Farine, D. R. & Whitehead, H. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84 , 1144–1163 (2015).
Wolf, M. & Krause, J. Why personality differences matter for social functioning and social structure. Trends Ecol. Evol. 29 , 306–308 (2014).
Laskowski, K. L., Wolf, M. & Bierbach, D. The making of winners (and losers): how early dominance interactions determine adult social structure in a clonal fish. Proc. R. Soc. B 283 , 20160183 (2016).
Firth, J. A. & Sheldon, B. C. Experimental manipulation of avian social structure reveals segregation is carried over across contexts. Proc. R. Soc. B 282 , 20142350 (2015).
Liker, A. & Bókony, V. Larger groups are more successful in innovative problem solving in house sparrows. Proc. Natl Acad. Sci. USA 106 , 7893–7898 (2009).
Aplin, L. M. et al. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518 , 538–541 (2015).
Costa, G. C. & Schlupp, I. Biogeography of the Amazon molly: ecological niche and range limits of an asexual hybrid species. Glob. Ecol. Biogeogr. 19 , 442–451 (2010).
Schultz, R. J. in Evolution and Genetics of Life Histories 103–119 (Springer, New York, 1982).
Quattro, J. M., Avise, J. C. & Vrijenhoek, R. C. An ancient clonal lineage in the fish genus Poeciliopsis (Atheriniformes: Poeciliidae). Proc. Natl Acad. Sci. USA 89 , 348–352 (1992).
Bohlen, J. & Ráb, P. Species and hybrid richness in spined loaches of the genus Cobitis (Teleostei: Cobitidae), with a checklist of European forms and suggestions for conservation. J. Fish Biol. 59 , 75–89 (2001).
Janko, K. et al. Diversity of European spined loaches (genus Cobitis L.): an update of the geographic distribution of the Cobitis taenia hybrid complex with a description of new molecular tools for species and hybrid determination. J. Fish Biol. 71 , 387–408 (2007).
CAS Google Scholar
Choleva, L., Apostolou, A., Rab, P. & Janko, K. Making it on their own: sperm-dependent hybrid fishes ( Cobitis ) switch the sexual hosts and expand beyond the ranges of their original sperm donors. Phil. Trans. R. Soc. B 363 , 2911–2919 (2008).
Bogart, J. P., Bi, K., Fu, J., Noble, D. W. & Niedzwiecki, J. Unisexual salamanders (genus Ambystoma ) present a new reproductive mode for eukaryotes. Genome 50 , 119–136 (2007).
Berger, L. in The Reproductive Biology of Amphibians 367–388 (Springer, New York, 1977).
Graf, J.-D. & Polls Pelaz, M. in Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogart, J. P.) 289–301 (New York State Museum Bulletin, New York, 1989).
Taylor, H. L., Cole, C. J., Dessauer, H. C. & Parker, E. Jr. Congruent patterns of genetic and morphological variation in the parthenogenetic lizard Aspidoscelis tesselata (Squamata: Teiidae) and the origins of color pattern classes and genotypic clones in eastern New Mexico. Am. Mus. Novit. 3424 , 1–40 (2003).
Dessauer, H. C. & Cole, C. J. Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogart, J. P.) 49–71 (New York State Museum Bulletin, New York, 1989).
Moritz, C. et al. The material ancestry and approximate age of parthenogenetic species of Caucasian rock lizards ( Lacerta : Lacertidae). Genetica 87 , 53–62 (1992).
Uzzell, T. & Darevsky, I. S. Biochemical evidence for the hybrid origin of the parthenogenetic species of the Lacerta saxicola complex (Sauria: Lacertidae), with a discussion of some ecological and evolutionary implications. Copeia 1975 , 204–222 (1975).
Reeder, T. W., Cole, C. J. & Dessauer, H. C. Phylogenetic relationships of whiptail lizards of the genus Cnemidophorus (Squamata: Teiidae): a test of monophyly, reevaluation of karyotypic evolution, and review of hybrid origins. Am. Mus. Novit. 3365 , 1–61 (2002).
Tucker, D. B. et al. Methodological congruence in phylogenomic analyses with morphological support for teiid lizards (Sauria: Teiidae). Mol. Phylogenet. Evol. 103 , 75–84 (2016).
Moritz, C. et al. in Evolution and Ecology of Unisexual Vertebrates Vol. 466 (eds Dawley, R. M. & Bogart, J. P.) 87–112 (New York State Museum Bulletin, New York, 1989).
Good, D. & Wright, J. Allozymes and the hybrid origin of the parthenogenetic lizard Cnemidophorus exsanguis . Experientia 40 , 1012–1014 (1984).
Lutes, A. A., Baumann, D. P., Neaves, W. B. & Baumann, P. Laboratory synthesis of an independently reproducing vertebrate species. Proc. Natl Acad. Sci. USA 108 , 9910–9915 (2011).
Scharnweber, K., Plath, M., Winemiller, K. O. & Tobler, M. Dietary niche overlap in sympatric asexual and sexual livebearing fishes Poecilia spp . J. Fish Biol. 79 , 1760–1773 (2011).
Tobler, M. & Schlupp, I. Parasites in sexual and asexual mollies ( Poecilia , Poeciliidae, Teleostei): a case for the Red Queen? Biol. Lett. 1 , 166–168 (2005).
Schlupp, I., Marler, C. & Ryan, M. J. Benefit to male sailfin mollies of mating with heterospecific females. Science 263 , 373–374 (1994).
Vrijenhoek, R. C. Animal clones and diversity. Bioscience 48 , 617–628 (1998).
Quattro, J. M., Avise, J. C. & Vrijenhoek, R. C. Molecular evidence for multiple origins of hybridogenetic fish clones (Poeciliidae: Poeciliopsis ). Genetics 127 , 391–398 (1991).
Vrijenhoek, R. in Population Biology and Evolution 217–231 (Springer, New York, 1984).
Gray, M. M. & Weeks, S. C. Niche breadth in clonal and sexual fish ( Poeciliopsis ): a test of the frozen niche variation model. Can. J. Fish. Aquat. Sci. 58 , 1313–1318 (2001).
Semlitsch, R. D., Hotz, H. & Guex, G. D. Competition among tadpoles of coexisting hemiclones of hybridogenetic Rana esculenta : support for the frozen niche variation model. Evolution 51 , 1249–1261 (1997).
Download references
Acknowledgements
We thank M. Schartl and I. Schlupp for constructive conversations. This work was supported in part by the Deutsche Forschungsgemeinschaft (Grant LA 3778/1-1 to K.L.L.; grant BI 1828/2-1 to D.B.) and the Alexander von Humboldt Foundation (Postdoctoral Fellowship to C.D.).
Author information
Authors and affiliations.
Department of Biology & Ecology of Fishes, Leibniz-Institute of Freshwater Ecology & Inland Fisheries, Berlin, Germany
Kate L. Laskowski, Carolina Doran, David Bierbach, Jens Krause & Max Wolf
Faculty of Life Sciences, Humboldt-Universität zu Berlin, Berlin, Germany
Jens Krause
You can also search for this author in PubMed Google Scholar
Contributions
K.L.L., M.W. and J.K. conceived the idea for the manuscript. K.L.L. wrote the initial draft. All authors substantially contributed to revisions and editing of manuscript.
Corresponding author
Correspondence to Kate L. Laskowski .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Laskowski, K.L., Doran, C., Bierbach, D. et al. Naturally clonal vertebrates are an untapped resource in ecology and evolution research. Nat Ecol Evol 3 , 161–169 (2019). https://doi.org/10.1038/s41559-018-0775-0
Download citation
Received : 28 June 2018
Accepted : 29 November 2018
Published : 28 January 2019
Issue Date : February 2019
DOI : https://doi.org/10.1038/s41559-018-0775-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Evolutionary mechanisms and practical significance of reproductive success and clonal diversity in unisexual vertebrate polyploids.
- Jian-Fang Gui
Science China Life Sciences (2024)
The emergence and development of behavioral individuality in clonal fish
- Kate L. Laskowski
- David Bierbach
Nature Communications (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Therapeutic cloning research is already being conducted in animals, and stem cells have been grown by this method and transplanted back into the original donor animal. In humans, this technique would revolutionise cell and tissue transplantation as a method of treating diseases. However, it is a very new science and has raised ethical concerns ...
The first canine was cloned in 2005 and was the 15th animal to be cloned (Fig. 1C) 1.Unlike other species, canine cloning remains comparatively difficult, due to the lack of in vitro oocyte ...
Cloning Fact Sheet. The term cloning describes a number of different processes that can be used to produce genetically identical copies of a biological entity. The copied material, which has the same genetic makeup as the original, is referred to as a clone. Researchers have cloned a wide range of biological materials, including genes, cells ...
Cloning articles from across Nature Portfolio. ... Research Open Access 25 Jan 2022 Communications Biology. Volume: 5, P: 95 ... FDA is the wrong agency to regulate genetically engineered animals.
Cloning is a technique scientists use to make exact genetic copies of living things. Genes, cells, tissues, and even whole animals can all be cloned. Some clones already exist in nature. Single-celled organisms like bacteria make exact copies of themselves each time they reproduce. In humans, identical twins are similar to clones.
Cloning offers the opportunity to make transgenic animals from cultured cells that have been genetically engineered. The first genetically engineered or transgenic mammalian clones were sheep born in 1997 carrying the coding sequences for human clotting factor IX, which is an important therapeutic for hemophiliacs.
Cloning publications over time and breed differences. (A) Mammal cloning as represented by the number of articles published per year.(B) Representation of the number of publications by species per year, upper left indicates the scale difference of small laboratory animals, mice and rats, when compared to the most published of the larger species.. Dotted lines indicate the peak in cloning ...
Animal cloning has gained popularity as a method to produce genetically identical animals or superior animals for research or industrial uses. However, the long-standing question of whether a ...
Some agricultural cloning is used in the U.S. and China to capitalize on the genes of a few extraordinary specimens, scientists say, but the European Parliament voted last year to ban cloning ...
In two cloning studies [ 26; 50], researchers did find that inbred animals showed much poorer cloning success than outbred animals, but even in outbred strains, cloning efficiency was low 0.36-1.8% of the hybrid cloned embryos produced from nuclei of hybrid cells resulted in live births). That suggests that inbreeding, although it plays a role ...
The goal of animal cloning includes the production of genetically modified animal for human consumption. Therefore, this research endeavoured to study animal cloning's current scientific findings, examine the by-product of said process, and determine its permissibility in an Islamic context. This study employed descriptive literature reviews.
The cloning of pets as a way to overcome their short life expectancy is a growing business reinforcing the ethical criticism of human ownership of animals and their commodification. In endangered species and in the future also extinct species cloning opens new horizons for the preservation of biodiversity. This is the only field where cloning ...
The cloned rhesus monkey, named ReTro, is the first to survive to adulthood. Credit: Qiang Sun. For the first time, a cloned rhesus monkey ( Macaca mulatta) has lived into adulthood — surviving ...
Current cloning techniques have an average success rate of less than 5 percent, even when working with familiar species; cloning wild animals is usually less than 1 percent successful. Any animals ...
New results from Rudolf Jaenisch's lab at the Whitehead Institute for Biomedical Research confirmed that the cloning process jeopardizes the integrity of an animal's whole genome. Scientists had suspected this based on studying a mere dozen genes, but the current study, which will be reported online in the Proceedings of the National Academies of Science this week, expansively surveyed ...
January 24, 2018. • 11 min read. In a world first, Chinese researchers have successfully cloned macaques using the same technique that yielded the famous clone Dolly the sheep. The milestone ...
Cloning is the outcome of the hard works on use of genetic engineering in animal breeding, treatment of hereditary diseases in human and replicating organisms. 16 In 1901, transfer of nucleus of a salamander embryonic cell to a enucleated cell was successfully undertaken. During 1940-1950, scientists could clone embryos in mammals.
Animal cloning is a technique for the production of genetically indistinguishable copies of the desired animal. So far, adult animals such as cattle, pigs, rabbits, sheep, and goats have been cloned using nuclear transfer from a somatic cell. The reprogramming of the somatic cell nucleus in developing an early embryo is a major challenge.
The cloning of large farm animals from genetically manipulated donor nuclei will have significant practical benefits. ... Following these reports, cloning research stalled for a couple of years ...
More likely is progress in establishing medical models of human and animal disease for biomedical research. SCNT in domestic animals has been used to study the potential of regenerative medicine. For example, cloned pigs have served as both controls and recipients for neural stem cells, demonstrating the potential for spinal cord repair .
Animal Cloning. In 2001, when it became apparent that animal cloning may become a commercial venture to help improve the quality of herds, FDA requested livestock producers and researchers to keep ...
Science requires replication. The development of many cloned or isogenic model organisms is a testament to this. But researchers are reluctant to use these traditional animal model systems for ...
Animal cloning is becoming a useful technique for producing transgenic farm animals and is likely to be used to produce clones from valuable adults. Other applications will also undoubtedly be discovered in the near future, such as for preserving endangered breeds and species. Although cloning promises great advantages for commerce and research ...