An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


The Weight-Inclusive versus Weight-Normative Approach to Health: Evaluating the Evidence for Prioritizing Well-Being over Weight Loss
Tracy l tylka, rachel a annunziato, deb burgard, sigrún daníelsdóttir, ellen shuman, rachel m calogero.
- Author information
- Article notes
- Copyright and License information
*Tracy L. Tylka: [email protected]
Academic Editor: Robyn Sysko
Received 2014 Jan 16; Revised 2014 May 31; Accepted 2014 Jun 25; Issue date 2014.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Using an ethical lens, this review evaluates two methods of working within patient care and public health: the weight-normative approach (emphasis on weight and weight loss when defining health and well-being) and the weight-inclusive approach (emphasis on viewing health and well-being as multifaceted while directing efforts toward improving health access and reducing weight stigma). Data reveal that the weight-normative approach is not effective for most people because of high rates of weight regain and cycling from weight loss interventions, which are linked to adverse health and well-being. Its predominant focus on weight may also foster stigma in health care and society, and data show that weight stigma is also linked to adverse health and well-being. In contrast, data support a weight-inclusive approach, which is included in models such as Health at Every Size for improving physical (e.g., blood pressure), behavioral (e.g., binge eating), and psychological (e.g., depression) indices, as well as acceptability of public health messages. Therefore, the weight-inclusive approach upholds nonmaleficience and beneficience, whereas the weight-normative approach does not. We offer a theoretical framework that organizes the research included in this review and discuss how it can guide research efforts and help health professionals intervene with their patients and community.
1. Introduction
Jasmine is waiting in the exam room and her chart shows that her weight today is up five pounds from her last visit two years ago, putting her BMI at 32. Her blood pressure was borderline high in contrast to the normal readings in previous visits. Although Jasmine's labs were normal in past visits, they are out of date. When Dr. Johnson greets her today, Jasmine seems anxious and tells Dr. Johnson, “I almost did not come in today knowing my weight is up from the last time I was here and you suggested a diet. I feel like such a failure. However, I need help for my migraines, so here I am.” Dr. Johnson and Jasmine look at each other, there is a beat of silence, and they both sigh. Dr. Johnson thinks about all the moments like this one. Usually patients are coming in reluctantly, with medical issues that cannot wait any longer. There is a palpable sense of frustration about yet another problem related to high weight. There is a predictably tense discussion about what needs to happen. Promises are made, referrals are given, and patients drop out of sight until the next medical crisis that absolutely cannot be ignored. Dr. Johnson cannot help but think, “Could there be a better way?”
Weight management (i.e., weight loss and weight cycling) is a central component of health improvement and health care regimens in the United States and similarly westernized countries. Regardless of whether or not it is relevant to the presenting concern, patients seeking medical evaluations or treatment are typically evaluated first on the basis of their weight [ 1 – 3 ]. For example, primary care guidelines recommend that higher-weight individuals with a BMI above 30 should be provided with weight loss interventions and nutritional advice automatically even if their presenting concerns are unrelated to body weight [ 4 ], whereas lower-weight individuals may not be given a blood sugar evaluation because they do not fit the “high-risk profile” of a person with type II diabetes [ 2 ]. A weight-centric emphasis in medical care may overshadow patients' health concerns and needs, potentially leading to “false negatives” (i.e., failure to diagnose a true condition because a patient's weight is classified as average) in addition to the “false positives” (i.e., misdiagnosing a healthy higher-weight patient as unhealthy, thus prescribing weight loss).
The vignette above underscores the fact that many practitioners and patients are frustrated and fatigued by this process of pursuing weight loss and weight cycling [ 5 – 8 ], increased patient shame [ 9 – 11 ], and intensified weight bias from the health care provider [ 12 – 18 ]. Health professionals are responsible for adhering to ethical principles in the care of their patients, such as beneficience (i.e., the obligation to benefit and contribute to optimum health for patients and communities) and nonmaleficience (i.e., the obligation to avoid harming patients and communities). Yet, the dominant focus on weight loss and weight management may move health care professionals away from these principles, creating a dilemma in the delivery of ethical care and public health promotion. This dilemma occurs because a weight-normative approach to health emphasizes the pursuit of weight loss, despite extensive evidence demonstrating that weight loss is not sustainable long-term for most people [ 19 – 21 ] and weight cycling (commonly associated with weight loss efforts) is linked to adverse health [ 6 – 8 ].
In this paper, we review evidence that challenges the weight-normative approach for health promotion and offer evidence to support a weight-inclusive approach for health promotion. Instead of imagining that well-being is only possible at a specific weight, a weight-inclusive approach considers empirically supported practices that enhance people's health in patient care and public health settings regardless of where they fall on the weight spectrum [ 1 , 2 , 22 ]. These approaches differ in the emphasis each one places on weight. While health care professionals using either approach may share some commonalities (e.g., recommending similar self-care practices), they contrast in the relative importance they place on body weight in the context of health and medical treatment, their perceptions of the malleability of weight, and how they respond to patients based on their weight.
Far from being radical, we view adopting a weight-inclusive approach as more conservative than a weight-normative approach for facilitating health because it does not recommend a treatment option that shows more documented risks to patients than benefits. Prescribing weight loss carries the risk of adverse outcomes for adherents and lacks evidence for sustainability over time, potentially setting many patients on a path of weight cycling [ 23 , 24 ]. The weight-inclusive approach acknowledges the scientific (albeit unpopular) evidence that people have little choice about what they weigh due to the interplay between involuntary genetic and environmental factors (e.g., lacking access to nutrient-dense foods priced outside of the family food budget) [ 25 – 31 ]. A weight-inclusive approach attempts to improve patient access to health care by recommending that health care providers recognize weight-normative biases (e.g., stereotypes that higher-weight patients must have, and lower-weight patients do not have, diseases often associated with obesity) and practices (e.g., prescribing weight-loss diets to higher-weight patients regardless of their physical health) within health care settings and challenge them in their own interactions with patients [ 1 , 2 , 32 – 39 ]. Emerging over the last four decades, this shift away from a weight-normative approach among many health care professionals acknowledges the failure of weight loss and weight management goals for improving health and recognizes the many factors that do support human health and well-being.
The issue of whether to adopt a weight-normative or weight-inclusive approach to health is not simply a philosophical matter. Large-scale interventions designed to affect masses of people are being implemented on the basis of the weight-normative approach. A recent scopic review of papers on the unintended harm caused by public health interventions found that over a third of the papers covered the possible harmful effects of obesity-related public health efforts [ 40 ]. Obesity-related public heath efforts were identified as potentially harmful because they (a) have been based on limited or poor quality evidence, (b) focus on preventing one extreme outcome at the expense of another extreme outcome (boomerang effects), (c) lack community engagement, and (d) ignore the root cause of the problems. If pursuit of the most ethical and effective pathways to health and well-being is the priority, and health care professionals intend to uphold the principle of doing no harm, we argue an alternative to the weight-normative approach is required. In the following sections, we review the problems and limitations of the weight-normative approach to health and then highlight the weight-inclusive approach as an alternative model for health care and health improvement.
2. The Weight-Normative Approach
We refer to the many principles and practices of health care and health improvement that prioritize weight as a main determinant of health as the weight-normative approach. This approach rests on the assumption that weight and disease are related in a linear fashion, with disease and weight increasing in tandem. Under the weight-normative approach, personal responsibility for “healthy lifestyle choices” and the maintenance of “healthy weights” are emphasized. On the basis of these beliefs, the weight-normative approach focuses on weight loss and weight management to prevent and treat a myriad of health problems. Despite the ubiquitous and pervasive nature of the weight-normative approach, we argue that a critical examination of the evidence does not support such a focus on weight and weight loss to improve health or prevent obesity.
First, despite the widely held belief within the medical community and general population that a higher body mass index (BMI) causes poor health, data do not (and cannot) support this link. The risk for mortality is highest for people with BMIs < 18.5 (underweight) and BMIs > 35 (obese II), but lowest for people with BMIs 25 to <30 (overweight), and the risk of those with BMIs 18.5 to <25 (average weight) and BMIs 30 to 35 (obese I) is comparable to and falls between the other groups [ 41 – 43 ]. Indeed, BMI is a corollary of certain conditions such as osteoarthritis, sleep apnea, hypertension, and coronary heart disease [ 44 – 47 ]. However, the data available cannot confirm that BMI causes these diseases, as causality can only be inferred via experimental designs. Other factors often partially or fully explain the links between BMI and health, such as exercise, nutrition, insulin resistance, and weight stigma [ 45 – 53 ].
Second, the weight-normative approach bestows negative judgments onto higher-weight individuals by promoting the view that (a) higher-weight individuals are unhealthy and thus a burden on society and (b) weight can be controlled through will power and thus if a person is fat, then it is due to poor lifestyle habits [ 2 , 32 , 54 ]. Given these underlying judgments, it is unsurprising that weight bias has been documented in professionals from a wide range of disciplines including physicians, nurses, psychologists, and dieticians [ 55 ]. Yet, genetic and involuntary environmental contributions to body weight outweigh voluntary lifestyle choices [ 25 , 56 – 58 ]. Body weight is defended by a powerful biological system that reacts to a negative energy balance by lowering metabolism and increasing hunger, food preoccupation, and hedonic responses to food [ 26 , 27 ]. Longitudinal research has found that children whose parents used restrictive feeding have a higher likelihood of eating in the absence of hunger and an elevated BMI later in childhood [ 58 – 60 ]. Lower-income families and communities may find it impossible to purchase high-quality nutrient-dense foods such as fresh fruits and vegetables given their limited budget and/or access to such foods [ 28 – 30 , 61 , 62 ]. Instead, refined grains and added sugars, fats, and preservatives are generally inexpensive and readily available in lower-income communities [ 28 – 30 ]. Furthermore, lower-income neighborhoods have fewer physical activity resources, such as parks, green spaces, bike paths, and recreational facilities when compared to higher income neighborhoods [ 63 , 64 ]. Crime, traffic, and unsafe playground equipment are also barriers to physical activity in lower-income communities [ 65 , 66 ]. Thus, there are important limitations placed on the degree to which body weight can be altered through voluntary action, making public health messages to “maintain a healthy weight” appear both uninformed and unfair.
Third, the promotion of “healthy weight” as the key to health and well-being may instill a sense of learned helplessness in the majority of people who will be unable to attain these weight-based goals [ 1 , 2 , 5 , 36 ]. If attempts to reach and maintain a “healthy” weight continually fail or are seen as impossible given available resources, the practice of healthy behaviors may be seen as futile. Overall, there is considerable evidence that the focus on weight and weight loss is linked to diminished health. In the following sections we review a number of failures and aggravating circumstances of the weight-normative approach to elucidate why change is needed.
2.1. The Data behind the Failure of Weight Loss Interventions
Rising weight trends in western societies have created an intense focus on weight loss initiatives, but none have generated long-term results for the majority of participants . As stated by Jeffery and colleagues, despite a plethora of interventions that result in initial weight loss, participants “almost always fail to maintain the behavior changes that brought them these positive results” [ 20 ]. For example, it has been estimated that no more than 20% of participants who complete weight-based lifestyle interventions maintain weight loss one year later [ 21 ], and the percentage of people maintaining weight loss continues to drop by the second year [ 19 ]. A meta-analysis of 29 studies on structured weight loss programs conducted in the United States found that participants regained 77% of their initial weight loss, on average, after five years [ 67 ]. As it stands, these outcomes are disheartening and not encouraging, but if we actually critically evaluate these studies, it is likely that the statistics for maintenance of weight loss are even worse. That is, most of these statistics are taken from published studies and therefore may represent the most “promising” findings in terms of weight maintenance and omit data from the people who drop out and are more likely to have regained weight. Also, these studies tend to be based on rigorous trials of weight loss programs at the exclusion of more commonly employed strategies and have rigid exclusion criteria (e.g., comorbidities such as mood disorders or binge eating disorder).
2.2. The Data behind the Dangers of Weight Cycling
Often diet failure is accompanied by weight cycling or “yo-yo dieting”—repeated periods of weight loss and weight gain [ 23 ]. Twenty years ago, Brownell and Rodin published a foundational paper reviewing adverse medical, metabolic, and psychological health outcomes linked to weight cycling [ 23 , 24 ]. Indeed, a large body of literature has connected weight cycling directly to compromised health, including higher mortality, higher risk of osteoporotic fractures and gallstone attacks, loss of muscle tissue [ 6 – 8 ], hypertension [ 51 ], chronic inflammation [ 52 ], and some forms of cancer such as renal cell carcinoma, endometrial cancer, and non-Hodgkin's lymphoma [ 50 ]. Here, we highlight two seminal contributions to our understanding of this link between weight cycling and compromised health. The landmark Framingham Heart Study was perhaps one of the most jarring indictments of weight cycling [ 6 ]. Using a sophisticated definition of weight cycling (capturing frequency and magnitude of fluctuations), mortality and morbidity were examined in more than 5000 individuals over a 32-year period. Results indicated that weight cycling was strongly linked to overall mortality, as well as mortality and morbidity related to coronary heart disease for both men and women. Similar results were found in the EFFORT cohort study conducted in Germany [ 7 ], which only included men, a generally underrepresented population in the weight cycling literature. In this study, 505 middle-aged men were grouped into the weight categories of stable nonobese, stable obese, weight loss, weight gain, and weight fluctuations. Among these groupings, only the weight fluctuations category was associated with mortality over the 15-year follow-up period. Of greatest interest, the stable obese category was not linked to higher risk of death relative to the stable nonobese category.
Weight cycling also has been shown to be connected to compromised physical health and psychological well-being. In an experimental study, Leibel et al. revealed that prospective weight loss led to reductions in metabolic energy expenditure [ 68 ]. The authors suggested that this reduction would make it difficult for their participants to maintain their newly suppressed weight. Research has shown that in order to maintain current BMI, formerly overweight dieters must eat less than their same-BMI counterparts who were never overweight [ 69 ]. As an illustration, a formerly obese woman with a BMI of 24 might be restricted to 1500 kcal/day, whereas a woman with a BMI of 24 who was never obese might be able to eat as much as 2000 kcal/day. The formerly obese woman might therefore have to employ more rigid dietary habits in order to make sure that her calories do not exceed 1500 kcal/day. Further evidence for a metabolic disruption was demonstrated in a study of 109 Korean women who participated in a community-based weight loss program [ 53 ]. Those with a history of weight cycling (43% of the sample) lost more lean muscle mass but not more body fat and lagged behind in positive changes to body composition and cholesterol, compared to their nonweight cycling counterparts, despite having lost a similar amount of weight overall.
Greater emotional distress was found to be connected to weight cycling among men and women, especially those who expected to have more personal and social success when thin (e.g., “I will be more successful, loved, desired, and healthy once I am thin/lean”), a mindset that the weight-normative approach cultivates [ 70 ]. Similarly, based on participants from the large Nurses' Health Study II, Field and colleagues found that women with a weight cycling history (39% of the sample) gained more weight over time and engaged in less physical activity but more binge eating than their noncycling peers [ 24 ]. Another recent study found that weight cycling is common among African American women (63% of the sample) and is associated with poor psychological outcomes, such as binge eating, thinness expectations, and self-esteem [ 71 ]. Overall, research conducted around the world for the past 25 years has repeatedly shown that weight cycling is inextricably linked to adverse physical health and psychological well-being.
2.3. The Risk of Eating Disorders in the Maintenance of Weight Loss
There is growing evidence that individuals who try to achieve and maintain a weight-suppressed state are at risk for binge eating disorder [ 72 , 73 ] and bulimia nervosa [ 74 , 75 ], likely because of the dietary rigidity needed to maintain a weight-suppressed state and the binge eating that may follow once the diet is “broken.” Leading researchers have found that rigid dieting is usually disrupted by episodes of overeating [ 72 , 76 ] and is associated with eating in the absence of hunger [ 73 ] in experimental research. In some individuals, these temporary losses of control are accompanied by subsequent behaviors employed to compensate for calories consumed during a binge episode (e.g., vomiting, laxative misuse, excessive exercise, and fasting) [ 72 – 74 ]. Abrupt disruptions in caloric expenditures brought on by binge eating and the use of compensatory behaviors may also be linked to weight cycling and the associated negative outcomes described above [ 72 , 76 ].
Furthermore, attempts to suppress weight are associated with poorer outcomes in treating patients with bulimia: those who have bulimia who try to maintain a weight-suppressed state are likely to binge eat [ 77 ], gain weight [ 75 , 78 ], and drop out of psychotherapeutic treatment [ 77 ]. Notably, behavioral weight loss (BWL) has been considered one treatment option for binge eating disorder (BED). A rigorous examination of three approaches to treat BED found that individuals with BED who were randomly assigned to a BWL treatment group experienced a small reduction (Cohen's d = 0.25) in BMI after treatment but did not maintain this reduction one year or two years later [ 79 ]. The other two treatment approaches (interpersonal psychotherapy and cognitive-behavioral therapy) did not reduce participants BMI from baseline to the follow-up periods. While the other two treatment approaches reduced binge eating, BWL did not.
It stands to reason, then, that weight suppression and food restriction should not be goals of treatment. Since dieting has been associated with the onset and maintenance of eating disorders, and the cessation of dieting is a crucial step in the treatment of eating disorders, encouraging higher-weight patients to enter a weight-suppressed state by dieting is likely physically harmful and hence violates professional codes of ethics [ 80 – 84 ].
2.4. Heightened Weight Stigma under the Weight-Normative Approach
The emphasis on achieving a “healthy” weight implies that there is a healthy or normal weight that each of us should be striving to attain and maintain. Moreover, the medical endorsement of normative weights gives credibility to cultural messages prizing thinness (for women), leanness (for men), and weight loss. Internalization of socially prescribed body ideals is related to body shame, body dissatisfaction, eating disorders for women [ 74 , 85 – 88 ], and potentially harmful muscle-enhancing and disordered eating behaviors for men [ 89 ]. The medical and cultural emphasis on “good weights” and “bad weights” produces the opportunity for weight stigma.
Weight stigma refers to negative weight-related attitudes and beliefs that manifest as stereotypes, rejection, prejudice, and discrimination towards individuals of higher weights [ 90 ]. There are many forms of weight stigma [ 91 ], including repeated weight-related teasing, bullying, harassment, violence, hostility, ostracism, pressures to lose weight/be thin, negative appearance commentary, and weight-related microaggressions. Microaggressions are intentional or unintentional verbal, behavioral, or environmental indignities that communicate hostility or negativity toward people who hold less power in society [ 92 ]. For example, suggesting a diet to a patient when the patient came in for a concern unrelated to weight would be a weight-related microaggression. Complimentary weightism , or appearance-related compliments (e.g., Telling a patient, “You've lost weight…looking good!”), is also stigmatizing because although seemingly positive on the surface, it still marks people as good or bad based on their weight [ 93 ].
Weight stigma occurs across a range of life domains, including school settings (higher-weight children are often stigmatized by peers, classmates, teachers, and school administrators) [ 94 – 98 ], health care environments (higher-weight patients are stigmatized by healthcare professionals and insurance companies) [ 12 – 15 ], public health initiatives [ 37 , 99 , 100 ], workplace settings (higher-weight employees are judged negatively by coworkers, supervisors, and employers) [ 101 , 102 ], and interpersonally by loved ones (intimate partners, friends, and parents) [ 90 , 103 ]. Some obstetricians and gynecologists in southern Florida have refused to perform medical services for women over 200 lbs [ 104 ]. In a large sample of women who were classified as overweight or obese, 69% experienced weight bias by a physician (with over half reporting bias on multiple occasions), 46% from nurses, 37% from dietitians, and 21% from health professionals [ 105 ]. Psychologists have been found to ascribe more pathology, greater severity of symptoms, and worse prognosis to obese patients when compared to thinner patients presenting with identical psychological profiles [ 106 ]. Weight stigma is also manifested in sociostructural barriers to accessing medical care (e.g., insurance companies that will not cover higher-weight individuals), and within the medical setting, barriers to appropriately sized equipment [ 3 , 107 ]. Health care professionals' ignorance about the medical needs of higher-weight individuals, such as appropriate surgical procedures or proper dosages of medicine and chemotherapy for higher-weight individuals, is also a form of weight stigma [ 107 ].
Ironically, many professionals who treat obesity [ 16 ] and eating disorders [ 18 ] exhibit weight bias towards their patients. Health professionals who specialize in the field of obesity and weight-loss treatment demonstrate varying degrees of antifat bias, attributing negative stereotypes such as lazy, stupid, and worthless to higher-weight people [ 17 ]. Among professionals treating eating disorders, 56% observed other professionals in their field making negative comments about obese patients, 42% believed that practitioners who treat eating disorders often hold negative stereotypes about obese patients, and 35% indicated that practitioners feel uncomfortable caring for those who are obese [ 18 ]. Eating disorder professionals with stronger weight stigma were more likely to attribute obesity to behavioral causes and perceived poorer treatment outcomes for these patients. When health providers attribute weight-related stereotypes to their patients, it affects the quality of care that patients along the weight spectrum receive. Experiencing weight bias in health care settings may discourage higher-weight patients from making prohealth lifestyle changes and seeking routine or preventative care and encourage lower psychological well-being [ 55 , 100 , 105 ].
Due to the focus on weight evaluation and privileging thinness, even lower-weight individuals could experience weight-related stigma and microaggressions. For example, lower-weight individuals may be told that they are “hated” because they can “eat anything and still be thin,” harming their interpersonal relationships. Health care professionals may ignore lower-weight individuals' symptoms suggestive of sleep apnea and type II diabetes because they do not fit the “weight profile” tied to these conditions. Even patients who are not “flagged” for their weight may be engaging in disordered eating behaviors that are detrimental to their health (e.g., the BMI of those who have bulimia is usually in the average range [ 108 ]).
Using national survey data with a 10-year follow-up, Schafer and Ferraro found that societal weight stigma is linked to internalized weight stigma [ 109 ]. Internalized weight stigma refers to the degree to which individuals personally adopt negative weight-based societal stereotypes and judge themselves and others based on these stereotypes [ 10 , 110 , 111 ]. This self-judgment may foster body blame and body shame (e.g., “If only I wasn't so large, I would not be teased—I am therefore ashamed of my body”) and appearance monitoring (e.g., vigilant about wearing slimming clothing to prevent others' from stigmatizing her body). Internalized weight bias is not related to BMI; thus, a person of any weight can experience and internalize weight bias and discrimination [ 112 ].
It is important to understand the associations between weight stigma and diminished health and well-being. Although research has challenged the assumption that high BMI causes disease, these variables do covary. One explanation for why they might covary is the experience of weight stigma [ 48 ]. Weight stigma is associated with increased caloric consumption, a pattern which challenges the common wisdom that pressures to lose weight will motivate overweight individuals to lose weight [ 49 ]. Across a 4-year longitudinal study of a large, nationally representative study of community adults, those who experienced weight stigma were 2.5 times more likely than those who were not stigmatized to become obese [ 113 ]. Priming overweight women to think about weight-related stereotypes (i.e., inducing weight stigma) led them to report significantly diminished exercise and dietary health intentions [ 114 ]. Further, Schafer and Ferraro found that weight stigma was related to increased health risks that are typically attributed to being obese, such as functional disability and decreased self-rated health, over a 10-year period [ 109 ]. The evidence further indicates that weight stigma is related to elevated ambulatory blood pressure [ 115 ], unhealthy weight control and binge eating behaviors [ 116 – 120 ], bulimic symptoms [ 121 ], negative body image [ 121 – 124 ], low self-esteem [ 121 , 122 ], and depression [ 122 , 125 , 126 ] among children, adolescents, and adults.
3. The Weight-Inclusive Approach
As an alternative to the weight-normative paradigm, the weight-inclusive approach rests on the assumption that everybody is capable of achieving health and well-being independent of weight, given access to nonstigmatizing health care. This approach challenges the belief that a particular BMI reflects a particular set of health practices, health status, or moral character. Under this paradigm, weight is not a focal point for medical treatment or intervention. Weight is not viewed as a behavior, but eating nutritious food when hungry, ceasing to eat when full, and engaging in pleasurable (and thus more sustainable) exercise are self-care behaviors that can be made more accessible for people. In these ways, this approach also tries to minimize weight stigma and thus may help patients feel comfortable in the health care setting, more able to discuss their health concerns, and less likely to experience the health care encounter as stigmatizing by health care providers [ 3 ]. The weight-inclusive approach adheres to an ethical principle held by health care professions [ 80 – 84 ]: “above all, do no harm.” Accordingly, then, there are no set health-related interventions that prioritize BMI reduction as a goal, given that a predominant focus on BMI reduction is linked to weight stigma and internalized weight stigma, which have detrimental connections to physical health and well-being [ 90 , 91 , 100 , 105 , 109 ].
A weight-inclusive approach seeks to (a) eradicate weight-based iatrogenic practices within health care and other health-related industries and (b) end the stigmatization of health problems (i.e., healthism ), thereby facilitating access to health care for all individuals [ 1 , 2 , 32 – 39 ]. In taking this approach, the blame for the failure to lose weight is placed on the deleterious process of weight loss rather than on the individual, which may help minimize internalized weight stigma [ 32 ]. The weight-inclusive approach follows some general a priori principles for health professionals [ 1 , 2 , 32 – 39 ]. These principles combine in various ways and in various applications in terms of policy making, the provision of health care within practice and the community, and the patient's personal decision-making about her or his own well-being [ 2 , 32 , 39 ].
Do no harm.
Appreciate that bodies naturally come in a variety of shapes and sizes, and ensure optimal health and well-being is provided to everyone, regardless of their weight.
Given that health is multidimensional, maintain a holistic focus (i.e., examine a number of behavioral and modifiable health indices rather than a predominant focus on weight/weight loss).
Encourage a process-focus (rather than end-goals) for day-to-day quality of life. For example, people can notice what makes their bodies rested and energetic today and incorporate that into future behavior, but also notice if it changes; they realize that well-being is dynamic rather than fixed. They keep adjusting what they know about their changing bodies.
Critically evaluate the empirical evidence for weight loss treatments and incorporate sustainable, empirically supported practices into prevention and treatment efforts, calling for more research where the evidence is weak or absent.
Create healthful, individualized practices and environments that are sustainable (e.g., regular pleasurable exercise, regular intake of foods high in nutrients, adequate sleep and rest, adequate hydration). Where possible, work with families, schools, and communities to provide safe physical activity resources and ways to improve access to nutrient-dense foods.
Where possible, work to increase health access, autonomy, and social justice for all individuals along the entire weight spectrum. Trust that people move toward greater health when given access to stigma-free health care and opportunities (e.g., gyms with equipment for people of all sizes; trainers who focus on increments in strength, flexibility, V02 Max, and pleasure rather than weight and weight loss).
There are many models which include a weight-inclusive emphasis, some more fragmentary, some more comprehensive, some more focused on research evidence, some more reliant on clinical experience (while proponents lobby for new research conceptualizations and trials), and some more focused on policy and social justice while others target individual health behaviors. Such models include Health at Every Size (HAES) [ 1 , 19 , 32 , 54 , 127 ], Health in Every Respect [ 35 ], and Physical Activity at Every Size [ 37 ]. For the purposes of this paper, we explore one version in more depth, the Health at Every Size (HAES) model, as trademarked and defined by the Association for Size Diversity and Health (ASDAH) [ 54 ].
3.1. Health at Every Size
The HAES model comes out of discussions among healthcare workers, consumers, and activists who reject the use of weight, size, or BMI as a proxy for health and reject the myth that weight is a result of personal choices independent of uncontrollable or involuntary genetic and environmental factors [ 1 , 19 , 32 , 33 , 54 , 127 ]. The HAES model addresses the broad forces that support health, such as safe and affordable access to care. It also helps people find sustainable practices that support individual and community well-being. Grounding itself in a social justice framework, the HAES model honors the healing power of social connections and evolves in response to the experiences and needs of a diverse community.
The HAES model (see Figure 1 ) rests on the evidence that while there are links between extremes of weight and health problems, evidence for the role of factors other than weight in people's health is stronger [ 25 – 31 ]. HAES further affirms a holistic definition of health, which cannot be characterized as simply the absence of physical or mental illness, limitation, or disease, but also the presence of quality of life (e.g., life satisfaction), which is needed for physical health and psychological well-being [ 1 , 32 , 54 ]. Health should be conceived as a resource or capacity available to all regardless of health condition, ability level, or social class, and not as an outcome or objective of living. Pursuing health is neither a moral imperative nor an individual obligation, and health status should never be used to judge, oppress, or determine the value of an individual. Thereby HAES upholds the ethical principles of beneficience and nonmaleficience by focusing on eradicating weight stigma, honoring human differences (size diversity), and pursuing empirically supported interventions that promote physical health and psychological well-being (see https://www.sizediversityandhealth.org/content.asp?id=197/ for HAES Principles [ 32 ]).

Health at Every Size (HAES): a model using a weight-inclusive approach.
Consistent with a weight-inclusive emphasis, HAES offers concrete suggestions for how to manage decisions about food and exercise in the aftermath (or absence) of a dieting mindset. HAES advocates for intuitive eating, based on evidence that demonstrates greater well-being for people who attend and respond to physiological hunger and satiety cues to determine when and how much to eat, and who pay attention to how certain foods affect the body (e.g., in terms of energy level, stamina, and medical issues such as diabetes and food allergies) [ 34 , 128 – 130 ]. Because such individuals eat according to their internal cues the majority of the time, intuitive eating may be able to buffer situational and/or dissociative eating within environments that contain many opportunities to eat less nutritiously (e.g., fast-food restaurants, bakeries, convenience marts, etc.) [ 131 ]. Nevertheless, lack of sleep may disrupt hunger and satiety cues as it interferes with the body's leptin and ghrelin levels [ 132 ], so helping patients ensure they get adequate rest may be a goal for intervention. Years of dieting and/or the experience of clinical eating disorders may also disrupt patients' awareness of and trust in their hunger and satiety cues, and thus interventions may be needed to help patients recognize and rely on these cues [ 34 ]. HAES also argues for pleasurable movement based on evidence that exercising for pleasure in lieu of weight loss is linked to well-being and positive body image [ 133 ]. These two particular recommendations are given because people have been educated to diet and exercise for weight loss and sometimes they need concrete suggestions about how to proceed toward adaptive eating and exercise. Being compliant or rebellious about pursuing weight loss is replaced by a return to a process that honors the body's physiological signals of hunger, satiety, and need for movement.
3.2. The Data behind the Weight-Inclusive Approach
In addition to the data that speak against a weight-normative approach to health, there are also data in support of a weight-inclusive approach. Most of this research has focused on the HAES model and tested it against models which emphasize the weight-normative approach. Bacon and Aphramor reviewed the six existing randomized controlled trials of this research [ 36 ]. The inclusion criteria for the studies included publication in a peer-reviewed journal and an explicit focus on self-acceptance within the HAES intervention. The HAES model resulted in both statistically and clinically significant improvements for the participants on physiological measures (e.g., blood pressure), health practices (e.g., increased physical activity), and psychological measures (e.g., self-esteem and disordered eating). HAES achieved these health improvements more successfully than models that emphasize dieting. The participants within the HAES groups also demonstrated increased adherence (reduced dropout rates) and no adverse outcomes [ 36 ].
To take one illustrative example, a HAES-based program that emphasized intuitive eating and size acceptance was evaluated against a dieting-based weight-loss program with a sample of 30- to 45-year-old women classified as overweight or obese [ 19 , 127 ]. Participants within each program received six months of weekly group interventions followed by six months of monthly aftercare group support. Findings yielded more positive results for the HAES-based program over the 1-year [ 127 ] and 2-year [ 19 ] follow-ups. Specifically, the HAES group decreased total cholesterol, low-density lipoprotein (LDL cholesterol), triglycerides, and systolic blood pressure at the 2-year follow-up and sustained improvements from the 1-year to 2-year follow-ups. Whereas the dieting group lost weight and showed initial improvements on many variables at the 1-year follow up, they had regained weight and did not sustain improvement at the 2-year follow- up [ 19 ]. The HAES group decreased eating restraint, physical hunger rating, disinhibited eating, drive for thinness, bulimic symptomatology, body dissatisfaction, poor interoceptive awareness, depression, and body image avoidance and increased self-esteem at both 1-year and 2-year follow-up. Correspondingly, participants in the dieting-based program only reduced disinhibited eating but reported decreased self-esteem [ 19 ]. Furthermore, attrition was higher in the diet group (41%) compared to the HAES group (8%) [ 19 , 127 ]. These findings suggest that HAES-based interventions demonstrate better adherence to practices that promote physical health and psychological well-being than dieting-based interventions, and these effects can be sustained over time.
The focus on weight loss in the weight-normative approach could be understood by patients as promoting thinness as a goal, whereas the idealization of thinness (i.e., thin-ideal internalization) and pursuit of thinness are challenged within the weight-inclusive approach. Research on secondary eating disorder prevention efforts has also provided evidence in support of the weight-inclusive approach. For example, in their program of research on the Body Project , Stice and Presnell examined whether reducing participants' thin-ideal internalization and focus on weight loss would reduce their dysfunctional eating attitudes and behaviors [ 134 ]. In this program, participants engaged in a series of verbal, written, and behavioral exercises in which they actively critiqued the thin ideal. These exercises were intended to produce cognitive dissonance, such that their original attitudes (e.g., “I want to be thin,” “only if I am thin will I be beautiful”) would conflict with their recent behavior (e.g., role playing where they convince other girls that many body types are beautiful). To decrease their cognitive dissonance, participants changed their original prothinness and proweight loss attitudes to make them fit with their recent behavior of rejecting the thin ideal. Overall, the Body Project has been effective in helping early-to-late adolescent girls reduce their pursuit of the thin ideal, accept their bodies, improve mood, decrease eating disorder symptoms (e.g., binge eating and use of unhealthy weight control behaviors), and lower the risk for developing future symptoms [ 135 ].
3.3. Reducing Weight Stigma under the Weight-Inclusive Approach: A Model and Strategies
Health care professionals need to work to reduce cultural and interpersonal weight stigma within health care and their patients' environments in order to facilitate the processes that bolster physical health and psychological well-being. On the basis of the evidence for the links between weight stigma and adverse health and well-being reviewed previously [ 109 , 113 – 121 ], and the intervening variables that could help explain these links, we devised a theoretical model (see Figure 2 ) that organizes these variables and the associations between them. This model can be used to help health care professionals identify points of intervention to reduce weight stigma and the other model variables that may maintain lower physical health and well-being.
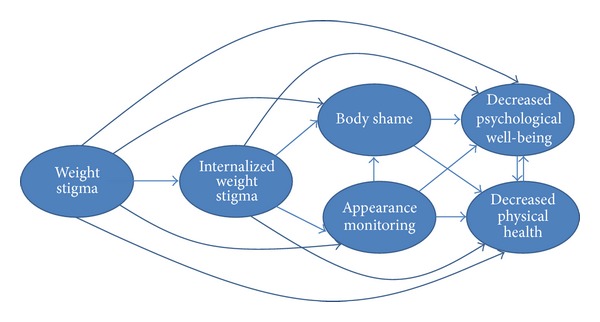
Theoretical model of weight stigma and its associated variables.
Similar to other theoretical models that positioned sociocultural influences as the source for negative body image and dysfunctional self-care behaviors [ 136 – 138 ], we positioned weight stigma as the starting point for negative health. In light of weight stigma's associations with internalized weight stigma [ 109 ], lower physical health [ 109 , 113 – 115 ], lower psychological well-being [ 116 – 121 ], body blame and shame [ 121 – 124 ], and appearance monitoring [ 130 , 139 ], proponents of the weight-inclusive approach challenge health care providers to examine their own biases around weight. These biases are part of a wider cultural climate of weight stigma that pervades health care education and everyday life. It is possible that much of the healing power of the health care relationship is social—in the quality of the connection between health care providers and their patients and their mutual trust and regard [ 140 ]. This connection is threatened for patients by the experience of being stereotyped and reduced to a BMI category. Quality of care for higher-weight patients can be optimized by adopting effective and sensitive strategies to communicate with all patients along the weight continuum [ 55 , 100 ]. Given the enormous social pressures to focus on weight loss and to connect weight loss to health, we know that providers, even those with the best of intentions, may unintentionally give the impression that they are biased against higher-weight patients, leaving their patients feeling unwelcome, invisible, and shamed.
One way health care professionals could engage with higher-weight patients is to view their office environment through a weight-inclusive lens. Does the office set-up communicate to all patients that their healthcare needs will be met there without shame or discrimination? Or is the office stigmatizing from the moment they arrive? For example, do waiting and exam rooms have furniture that fits higher-weight individuals? Do office staff automatically weigh in every patient, on a scale in a public hallway, even if the patient is coming in for an issue totally unrelated to weight, for example, a wart removal? How do nurses respond when a patient says, “no thank you” to being weighed? What is the office culture around weight? Has weight bias ever been addressed by the entire staff, such as through continuing education or sensitivity training? Are gowns and medical equipment (e.g., blood pressure cuffs) stocked to fit higher-weight patients?
By being a source of support and “grounding” against the stigma higher-weight patients regularly face, the weight-inclusive approach may facilitate patient adherence to health promoting practices and the guidance of their health care providers. Health care professionals can offer this support through the provider-patient bond and by connecting individuals via support groups (in person or online) that follow the weight-inclusive approach. For example, HAES has a website that could be useful to recommend for patients ( http://www.haescommunity.org/ ). Table 1 provides a list of weight-inclusive principles and examples of how health care providers can implement them in practice. We recognize that various health care professionals need to work as a team to fully implement these principles, with each professional implementing the principles within her or his boundaries of expertise.
Translating weight-inclusive principles into weight-inclusive practice.
*Health care professionals who may want to take the lead in implementing this principle within their practice. We encourage a team approach whereby physicians, mental health professionals, and nutritionists work together to ensure that a weight-inclusive approach is followed.
In addition to the above strategies, a weight-inclusive approach includes a focus on intrapersonal variables that sustain poor physical health and well-being (see Figure 2 ). For instance, health care professionals can become educated about the links between internalized weight stigma and poor self-care that maintain adverse physical health and negative psychological well-being [ 9 , 110 – 112 , 121 , 127 ], for example, and share this knowledge with their patients. Health care professionals can also inform patients of the rich literature that explicates the bidirectional influences between physical health and well-being [ 141 – 145 ]. That is, if patients begin self-care practices that enhance physical health, they will likely feel better psychologically as well, and these psychological gains are then linked to further increases in self-care practices that enhance health.
Health care professionals can also target internalized weight stigma's links with body shame and appearance monitoring (see Figure 2 ). In particular, patients often blame and shame their bodies for how they look and feel, but body blame and shame are often responses to the wider cultural stigma around weight and their personal experiences of weight discrimination over their lifetimes [ 121 – 124 ]. Body blame and shame can be reframed for patients to communicate that the source is likely internalized societal weight stigma [ 112 , 146 ] from being stigmatized for their weight [ 109 ], and not their bodies' actual weight or size [ 147 ]. Health care professionals can also help patients mentally shift from habitual appearance monitoring, which is associated with lower self-care and ignoring physical health [ 85 , 148 , 149 ], to attending to their bodies in more positive ways that emphasize self-care. There are some interventions (e.g., self-compassion) that can enhance patients' well-being in tandem with improvements in body shame [ 150 ]. The key is for both health care professionals and patients to appreciate the extent to which body loathing and shame is associated with reduced engagement in self-care [ 85 , 120 ]. There is a cultural belief that people have to be dissatisfied with their weight (or any aspect of their appearance) in order to be motivated to improve it. This belief has not found general support in the literature; in fact, the reverse is supported: people are more likely to take care of their bodies when they appreciate and hold positive feelings toward their bodies [ 133 , 147 , 151 ].
In order to encourage self-care behaviors, patients also need to reconnect with their bodies, that is, focus on internal body awareness rather than engage in external appearance monitoring [ 85 , 147 , 148 , 151 ] (see Figure 2 ). Internal body awareness is required to be able to know when something is “not right” with their bodies as well as attend to their bodies' physical and psychological needs [ 147 ]. For example, awareness of hunger and satiety cues is needed to determine when and how much to eat in order to prevent under- or overeating [ 148 , 151 ]. Raising internal bodily awareness could be facilitated by offering unconditional acceptance of people's bodies and bodily experience in lieu of weight stigmatization. Indeed, women who received body acceptance by others (in contrast to weight stigma) reported higher body appreciation and less habitual appearance monitoring [ 148 , 151 ]; thus, they are more connected to the functionality of their bodies and less shameful of their bodies. Moreover, body acceptance by others fully accounted for the link between women's BMI and their body appreciation [ 151 ]. This finding underscores the need to eradicate all health care interventions that foster weight stigma to improve patients' perceptions that the health care environment and health care professionals accept their bodies. Greater internal awareness and appreciating the body are related to higher eating based on physiological hunger and satiety cues and less situational and emotional eating [ 148 , 151 ]—additional reasons for health care professionals to encourage clients to appreciate their bodies and listen to their bodies' internal cues.
3.4. A Weight-Inclusive Approach to Public Health
The current public health model operates through the identification of risk factors and population-based efforts to reduce such risks in order to prevent disease and promote health [ 99 ]. The reduction of risk factors occurs through various forms of public action, including regulatory efforts (e.g., taxing and legislation), community-based universal programs (e.g., Health Promoting Schools), and public health messaging to raise awareness of the risks and benefits associated with certain behaviors (e.g., “ 5-a-day ”). However, this model has been criticized for focusing too heavily on factors that are perceived to be under personal control while neglecting the larger sociocultural and economic conditions that dictate much of people's lived experiences, choices, and opportunities [ 37 , 99 ].
Syme pointed out that the conditions often referred to as “lifestyle diseases,” under which overweight and obesity are named, have been associated with a variety of genetic and environmental factors that occur well outside personal control [ 99 ]. This lack of control is especially true for populations that face most health challenges. Populations with the worst health outcomes tend to also be populations living under the most socioeconomic constraints and have the least amount of personal control over their lives [ 31 ]. Marmot has written extensively about the contributions of social and economic inequalities to public health issues and the critical importance of considering these issues in public health policy [ 152 ]. Disregard for the environment within which people live offers “a rather decontextualized” approach to public health that is unlikely to be effective and may even be unethical due to the potential for harm [ 37 ].
An approach to public health that incorporates a weight-inclusive approach may not only circumvent the adverse health and well-being consequences linked to the weight-normative approach but also may enhance population health. Longitudinal studies have repeatedly shown that, irrespective of actual weight, body satisfaction and freedom from weight-based teasing and stigma are linked to reduced risk for unhealthy dieting practices, sedentary behaviors, eating disturbances, and weight gain among young people [ 95 , 153 , 154 ]. Public health messages that are free of weight focus also appear to be more acceptable to the public and more likely to encourage healthy behaviors than messages emphasizing weight control or obesity prevention. For example, a large nationally representative U.S. survey revealed that participants responded most favorably to public health messages that promoted healthy behaviors without any reference to weight or obesity at all [ 155 ]. The survey further showed that messages perceived as weight stigmatizing were negatively received and rated less likely to foster healthy behavior change. The findings have since replicated in randomized controlled settings [ 156 ].
Several scholars have proposed actions that may be taken at the policy level to prevent and reduce harm associated with a weight-focused sociocultural climate [ 35 , 157 , 158 ]. However, a serious, inbuilt resistance to change appears to be present within health systems. For instance, OReilly and Sixsmith have argued that an overreliance on the dominant position of powerful institutions, such as the World Health Organization, has resulted in a dead-lock situation where public health authorities uncritically accept and maintain the weight-normative approach without scrutinizing its validity, effectiveness, or ethical implications [ 158 ]. Thus, the weight-normative approach becomes a self-perpetuated dogma. The indications of harm associated with this paradigm, however, demand that a closer look be taken and actions to reduce the focus on weight within public health be implemented. Certainly, during this implementation phase, data would be needed to evaluate the outcomes of moving away from a weight-normative toward a weight-inclusive approach.
OReilly and Sixsmith analyzed policy options that could be used to shift the weight-normative approach to a more weight-inclusive approach in public health [ 158 ]. They conducted interviews with key stakeholders who were asked to rank proposed policy changes in terms of estimated effectiveness in challenging the weight-normative approach, likelihood of promoting equity and reducing weight bias, political and public acceptability, and the practicalities of implementation. The policy change that received the most favorable rating was adopting language that did not mention weight in public health messages. This was seen as a very low cost action with a high level of public acceptability and political feasibility. The shift from a weight-normative to a weight-inclusive approach also emerged as a public preference in a recent Canadian report where members of the community were engaged in a discussion, in person and online, about feasible action to promote healthy weight in children. The most popular idea expressed online was to turn away from a weight-normative approach in health promoting efforts as many participants expressed concern with the language on weight and instead preferred a focus on healthy living [ 159 ]. As this is a policy change that can be implemented with relative ease, OReilly and Sixsmith highlighted it as a viable and recommended action for governments to reduce harm caused by weight stigma and weight preoccupation [ 158 ].
Other promising policy options in OReilly and Sixsmith's analysis were implementing antiweight bias training for health professionals and establishing research guidelines that ensure the inclusion of measures of possible third-factor contributions to obesity research, such as socioeconomic status, physical activity, and dietary factors [ 158 ]. Several interventions to reduce weight bias among preservice and practicing health professionals have already been reported in the scientific literature with promising results [ 157 , 160 , 161 ]. In one study, over three hundred public health promoters were offered a single-day workshop on weight bias and related issues which led to significant decreases in antifat prejudice, decreased internalization of media stereotypes on weight and shape, and increased self-efficacy for addressing weight bias after intervention [ 157 ].
4. Summary of the Competing Approaches
The research demonstrates that a focus on weight is associated with adverse physical health and psychological well-being for patients and community members. Dieting is inextricably linked to significant physiological barriers to overall physical health that likely could have been prevented. The strain of unsuccessful weight loss attempts on physical health is not consistent with a beneficent and nonmaleficent approach to clinical practice and public health. Moreover, the weight-normative approach blames the individual rather than the process when weight loss attempts fail, which is then tied to body blame, body shame, internalized weight stigma, and decreased psychological well-being. Under the weight-normative approach, weight stigma likely filters into health care professionals' relationships with their patients, even if it is unintentional.
The weight-inclusive approach supports the health of people across the weight continuum and challenges weight stigma. Data from randomized controlled trials have upheld the efficacy of programs with a weight-inclusive emphasis, such as HAES. Specifically, participants following the HAES model achieved statistically and clinically significant improvements in physiological measures (e.g., blood pressure), behavioral practices (e.g., increased physical activity, decreased binge eating), and psychological measures (e.g., increased self-esteem, decreased depressive symptoms) and did not demonstrate any adverse outcomes, despite the fact that weight remained relatively unchanged. Other research has supported the weight-inclusive approach, such that living in a body-accepting environment (i.e., one without weight stigma) is associated with higher body appreciation and lower habitual appearance monitoring, independent of BMI. The weight-inclusive approach, then, upholds the ethical principles of beneficience and nonmaleficience and can be used as a springboard for generating additional clinical and public health interventions. Points of intervention, based on targeting the variables that are connected to reduced physical health and well-being (see Figure 2 ), as well as the mechanisms of action between the variables, are offered for health professionals who work with patients or within public health settings.
Returning to the vignette in the Introduction, we now frame the health care encounter between the doctor and patient through the lens of weight-inclusion and well-being instead of the pursuit of weight loss, taking into account how little time doctors get to spend with patients during a typical office visit.
Jasmine is waiting in the exam room and her chart shows that her weight today is up five pounds from her last visit two years ago, putting her BMI at 32. Her blood pressure was borderline high in contrast to the normal readings in previous visits. Although Jasmine's labs were normal in past visits, they are out of date. When Dr. Johnson greets her today, Jasmine seems anxious and tells Dr. Johnson, “I almost did not come in today knowing my weight is up from the last time I was here and you suggested a diet. I feel like such a failure. However, I need help for my migraines, so here I am.” Dr. Johnson and Jasmine look at each other, there is a beat of silence, and they both sigh.
Dr. Johnson says, “You know, Jasmine, I have been reading the research on weight loss interventions and weight-cycling and I'm realizing that if the same thing happens to almost everyone, it probably is not the fault of the person, it is probably more about the process itself. So, instead of focusing on weight loss, I'm encouraging my patients to think about what makes them feel better in their everyday lives; emotionally and physically. For example, do you feel better when you eat more fruits and vegetables, drink more water, take a walk with a friend, meditate to relieve stress, and get enough sleep? There's good evidence that those behaviors are going to make you healthier and feel better even if your weight does not change.”
Jasmine is a bit surprised by Dr. Johnson's shift and says, “Well, typically, when my weight loss slows down or stops completely, I stop doing any of those things you mentioned that would help me feel better and be healthier.” Dr. Johnson says, “I understand, but we're going to turn the focus from your weight to your health. Because those behaviors are linked to health, why not do them anyway?”
Jasmine smiles at Dr. Johnson and says, “It sure would be easier to come back and see you the next time I'm supposed to if I did not have to lose weight first.”
Dr. Johnson replies, “I do not want anything to stand in the way of you getting your medical care, including worrying that I might scold you. Now that we have a better plan, I am going to have the nurse retake your blood pressure.” Jasmine and Dr. Johnson then discuss treatment options for Jasmine's migraines.
Right before Dr. Johnson leaves the room, Jasmine shares one more quick concern, “I like the shift from weight to health, but there is this Weight Focusers group at work. If I do not go, I'll get charged a higher premium for my health insurance.”
Dr. Johnson says, “Let me know if I can help with that. The Affordable Care Act is supposed to allow you to follow your doctor's recommendation, and I have no evidence that Weight Focusers is going to make you healthier and lots of evidence that says that weight cycling is linked to poorer health.”
Jasmine leaves the doctor's office feeling hopeful and understood.
As Dr. Johnson finishes the chart note, she realizes that her own body is relaxed, her jaw unclenched. She feels like she has made a better connection with Jasmine and developed a sustainable treatment plan she can follow. Dr. Johnson is curious and maybe even a little eager to see what happens next. However, she does wonder what will happen if the reviewers do not see weight loss in this patient, or a goal of weight loss in the treatment plan.
5. Directions for Future Research
More research on the weight-inclusive versus weight-normative approach is sorely needed as many important questions remain unanswered. Research into the variety of expressions of weight stigma can reveal nuanced associations that advance scholars' understanding of its influence and expression. For example, weight stigma could be operationalized as weight-related teasing, bullying, discrimination, commentary, and objectification, and the source could also be operationalized (e.g., partners, health care system, family, friends, etc.). Similarly, decreased physical health and psychological well-being can be defined in many different ways and these operationalizations may reveal different relationships with weight and body-based variables.
Another alternative conceptualization would be to explore what happens in the absence of weight stigma, which would directly examine weight-inclusive approach. Those who do not experience weight stigma (whether because of their weight or their environment/community/culture) may demonstrate body appreciation and superior health and well-being. Although some research into positive body-accepting environments has begun [ 148 , 151 ], these studies are in their infancy and would benefit from additional research. In addition, it would be useful to know whether individuals who transition out of weight-stigmatizing environments (e.g., away from stigmatizing partners or family members) receive health and well-being related benefits (and the extent of these benefits) or whether memories of being stigmatized continue to influence their health and well-being at a similar level. In the latter instance, perhaps mental health providers could work individually with patients to buffer internalized weight stigma and promote individual empowerment. In particular, interventions that emphasize self-compassion [ 161 ] may be useful for these therapeutic endeavors, as empirical evidence suggests that self-compassion is an adaptive mindset to cultivate in the context of improving body image and eating behavior [ 162 – 167 ]. Indeed, a 3-week online self-compassion intervention reduced body shame and improved body appreciation in community women; these women maintained these outcomes at a 3-month follow-up relative to a wait-list control group [ 150 ]. Among women high in dietary restraint, those who were induced to think self-compassionately after eating a doughnut as part of the experimental task (i.e., they were told that all people eat unhealthy foods at times and asked to not to be hard on themselves because “this little amount of food does not matter anyway”) were able to reduce their distress and disinhibited eating relative to a control group who did not receive the self-compassion induction [ 167 ].
Those working in patient settings and public health should investigate the impact of moving from a weight-normative approach to a weight-inclusive approach on their patients and communities. Researchers could explore the effects on patients' compliance and willingness to address health issues proactively when weight loss is removed from the equation. Qualitative designs could be used to garner rich data on the challenges and benefits of this change to health care, treatment adherence (e.g., more likely go to follow-up appointments for medical concerns), and overall health improvement. In addition, more research is needed to examine which particular components of the weight-inclusive approach, individually or in conjunction with other components, have the strongest connection to health improvement and promotion.
6. Conclusion
The weight-normative approach is not improving health for the majority of individuals across the entire weight continuum. Weight is overemphasized for higher-weight individuals (i.e., assumptions are made that they are unhealthy) and underemphasized for lower- or “average-” weight individuals (i.e., assumptions are made that they are healthy). Furthermore, we know that weight loss through dieting is not sustainable over time for the vast majority of higher-weight individuals and is linked to harmful consequences. Therefore, we argue that it is unethical to continue to prescribe weight loss to patients and communities as a pathway to health, knowing the associated outcomes—weight regain (if weight is even lost) and weight cycling—are connected to further stigmatization, poor health, and well-being. The data suggest that a different approach is needed to foster physical health and well-being within our patients and communities.
Advocates of a weight-inclusive approach assert that we are acting on behalf of our patients' and communities' interests when we centralize health for people at all points along the weight continuum and work to eradicate weight stigma in all settings, including health care and public health. This paper has reviewed the data in support of a weight-inclusive approach to foster physical and psychological well-being. We encourage both scholars and practitioners to study and document what happens when health professionals and their target populations shift their focus to developing sustainable healthy behaviors for every body.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
- 1. Bacon L. End the war on obesity: make peace with your patients. MedGenMed Medscape General Medicine. 2006;8(4, article 40) [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Bacon L. Health at Every Size. Dallas, Tex, USA: Benbella Books; 2010. [ Google Scholar ]
- 3. National Association to Advance Fat Acceptance. Guidelines for Healthcare Providers Who Treat fat Patients. 2012. http://issuu.com/naafa/docs/naafa_healthcarep_guidelines_2011_v06_screencut . [ Google Scholar ]
- 4. National Obesity Forum. Guidelines on Management of Adult Obesity and Overweight in Primary Care. 2006. http://www.nationalobesityforum.org.uk/images/stories/W_M_guidelines/NOF_Adult_Guildelines_Feb_06.pdf . [ Google Scholar ]
- 5. Mann T, Tomiyama AJ, Westling E, Lew A, Samuels B, Chatman J. Medicare’s search for effective obesity treatments: diets are not the answer. American Psychologist. 2007;62(3):220–233. doi: 10.1037/0003-066X.62.3.220. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Lissner L, Odell PM, D’Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. The New England Journal of Medicine. 1991;324(26):1839–1844. doi: 10.1056/NEJM199106273242602. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. European Journal of Epidemiology. 2007;22(10):665–673. doi: 10.1007/s10654-007-9167-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Nilsson PM. Is weight loss beneficial for reduction of morbidity and mortality? What is the controversy about? Diabetes Care. 2008;31(2):S278–S283. doi: 10.2337/dc08-s268. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Puhl RM, Moss-Racusin CA, Schwartz MB. Internalization of weight bias: implications for binge eating and emotional well-being. Obesity. 2007;15(1):19–23. doi: 10.1038/oby.2007.521. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Schwartz MB, Vartanian LR, Nosek BA, Brownell KD. The influence of one’s own body weight on implicit and explicit anti-fat bias. Obesity. 2006;14(3):440–447. doi: 10.1038/oby.2006.58. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Wang SS, Brownell KD, Wadden TA. The influence of the stigma of obesity on overweight individuals. International Journal of Obesity. 2004;28(10):1333–1337. doi: 10.1038/sj.ijo.0802730. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Price JH, Desmond SM, Krol RA, Snyder FF, O’Connell JK. Family practice physicians’ beliefs, attitudes, and practices regarding obesity. The American Journal of Preventive Medicine. 1987;3(6):339–345. [ PubMed ] [ Google Scholar ]
- 13. Kristeller JL, Hoerr RA. Physician attitudes toward managing obesity: differences among six specialty groups. Preventive Medicine. 1997;26(4):542–549. doi: 10.1006/pmed.1997.0171. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Foster GD, Wadden TA, Makris AP, et al. Primary care physicians’ attitudes about obesity and its treatment. Obesity Research. 2003;11(10):1168–1177. doi: 10.1038/oby.2003.161. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Kaminsky J, Gadaleta D. A study of discrimination within the medical community as viewed by obese patients. Obesity Surgery. 2002;12(1):14–18. doi: 10.1381/096089202321144513. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Schwartz MB, O’Neal H, Brownell KD, Blair SN, Billington C. Weight bias among health professionals specializing in obesity. Obesity Research. 2003;11(9):1033–1039. doi: 10.1038/oby.2003.142. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Schwartz MB, Chambliss HO, Brownell KD, Blair SN, Billington C. Weight bias among health professionals specializing in obesity. Obesity Research. 2003;11(9):1033–1039. doi: 10.1038/oby.2003.142. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Puhl RM, Latner JD, King KM, Luedicke J. Weight bias among professionals treating eating disorders: attitudes about treatment and perceived patient outcomes. International Journal of Eating Disorders. 2014;47(1):65–75. doi: 10.1002/eat.22186. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Bacon L, Stern JS, Van Loan MD, Keim NL. Size acceptance and intuitive eating improve health for obese, female chronic dieters. Journal of the American Dietetic Association. 2005;105(6):929–936. doi: 10.1016/j.jada.2005.03.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Jeffery RW, Epstein LH, Wilson GT, et al. Long-term maintenance of weight loss: current status. Health Psychology. 2000;19(1):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Wing RR, Phelan S. Long-term weight loss maintenance. The American Journal of Clinical Nutrition. 2005;82(1):222S–225S. doi: 10.1093/ajcn/82.1.222S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Matheson EM, King DE, Everett CJ. Healthy lifestyle habits and mortality in overweight and obese individuals. Journal of the American Board of Family Medicine. 2012;25(1):9–15. doi: 10.3122/jabfm.2012.01.110164. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Brownell KD, Rodin J. Medical, metabolic, and psychological effects of weight cycling. Archives of Internal Medicine. 1994;154(12):1325–1330. [ PubMed ] [ Google Scholar ]
- 24. Field AE, Manson JE, Taylor CB, Willett WC, Colditz GA. Association of weight change, weight control practices, and weight cycling among women in the Nurses’ Health Study II. International Journal of Obesity. 2004;28(9):1134–1142. doi: 10.1038/sj.ijo.0802728. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Silventoinen K, Rokholm B, Kaprio J, Sørensen TIA. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. International Journal of Obesity. 2010;34(1):29–40. doi: 10.1038/ijo.2009.177. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. MacLean PS, Bergouignan A, Cornier M, Jackman MR. Biology’s response to dieting: the impetus for weight regain. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2011;301(3):R581–R600. doi: 10.1152/ajpregu.00755.2010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Sumithran P, Proietto J. The defence of body weight: a physiological basis: for weight regain after weight loss. Clinical Science. 2013;124(4):231–241. doi: 10.1042/CS20120223. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Monsivais P, Drewnowski A. Lower-energy-density diets are associated with higher monetary costs per kilocalorie and are consumed by women of higher socioeconomic status. Journal of the American Dietetic Association. 2009;109(5):814–822. doi: 10.1016/j.jada.2009.02.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Drewnowski A. The cost of US foods as related to their nutritive value. The American Journal of Clinical Nutrition. 2010;92(5):1181–1188. doi: 10.3945/ajcn.2010.29300. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Monsivais P, Drewnowski A. The rising cost of low-energy-density foods. Journal of the American Dietetic Association. 2007;107(12):2071–2076. doi: 10.1016/j.jada.2007.09.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Andreyeva T, Blumenthal DM, Schwartz MB, Long MW, Brownell KD. Availability and prices of foods across stores and neighborhoods: the case of New Haven, Connecticut. Health Affairs. 2008;27(5):1381–1388. doi: 10.1377/hlthaff.27.5.1381. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Burgard D. What is health at every size? In: Rothblum E, Solovay S, editors. The Fat Studies Reader. New York, NY, USA: New York University Press; 2009. pp. 41–53. [ Google Scholar ]
- 33. Watkins PL. Health at every size: an end to the war on obesity? The European Health Psychologist. 2013;15(1):1–5. [ Google Scholar ]
- 34. Tribole E, Resch E. Intuitive Eating. New York, NY, USA: St. Martin’s Press; 2012. [ Google Scholar ]
- 35. Aphramor L, Gingras J. Helping people change: promoting politicised practice in the health care professions. In: Rich E, Monaghan LF, Aphramor L, editors. Debating Obesity. London, UK: Palgrave MacMillan; 2011. pp. 192–218. [ Google Scholar ]
- 36. Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutrition Journal. 2011;10(1, article 9) doi: 10.1186/1475-2891-10-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Mansfield L, Rich E. Public health pedagogy, border crossings and physical activity at every size. Critical Public Health. 2013;23(3):356–370. [ Google Scholar ]
- 38. Cohen L, Perales DP, Steadman C. The O word: why the focus on obesity is harmful to community health. Californian Journal of Health Promotion. 2005;3:154–161. [ Google Scholar ]
- 39. Miller WC. Fitness and fatness in relation to health: implications for a paradigm shift. Journal of Social Issues. 1999;55(2):207–219. [ Google Scholar ]
- 40. Allen-Scott LK, Hatfield JM, McIntyre L. A scoping review of unintended harm associated with public health interventions: towards a typology and an understanding of underlying factors. International Journal of Public Health. 2014;59(1):3–14. doi: 10.1007/s00038-013-0526-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories a systematic review and meta-analysis. Journal of the American Medical Association. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Orpana HM, Berthelot J, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of canadian adults. Obesity. 2010;18(1):214–218. doi: 10.1038/oby.2009.191. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Mehta NK, Chang VW. Mortality attributable to obesity among middle-aged adults in the United States. Demography. 2009;46(4):851–872. doi: 10.1353/dem.0.0077. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. International Journal of Obesity. 2001;25(5):622–627. doi: 10.1038/sj.ijo.0801585. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. de Gonzalez AB, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. New England Journal of Medicine. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. Journal of Clinical Endocrinology and Metabolism. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Hoerger TJ. Controversies in obesity mortality: a tale of two studies. Health Promotion Economics. 2006;1(1):1–4. [ Google Scholar ]
- 48. Muennig P. The body politic: the relationship between stigma and obesity-associated disease. BMC Public Health. 2008;8, article 128:128–138. doi: 10.1186/1471-2458-8-128. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Schvey NA, Puhl RM, Brownell KD. The impact of weight stigma on caloric consumption. Obesity. 2011;19(10):1957–1962. doi: 10.1038/oby.2011.204. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Thompson HJ, McTiernan A. Weight cycling and cancer: weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prevention Research. 2011;4(11):1736–1742. doi: 10.1158/1940-6207.CAPR-11-0133. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Guagnano MT, Ballone E, Pace-Palitti V, et al. Risk factors for hypertension in obese women. The role of weight cycling. European Journal of Clinical Nutrition. 2000;54(4):356–360. doi: 10.1038/sj.ejcn.1600963. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Strohacker K, McFarlin BK. Influence of obesity, physical inactivity, and weight cycling on chronic inflammation. Frontiers in Bioscience. 2010;2(2):98–104. doi: 10.2741/e70. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Yoo HJ, Kim BT, Park YW, Park KH, Kim CW, Joo N. Difference of body compositional changes according to the presence of weight cycling in a community-based weight control program. Journal of Korean Medical Science. 2010;25(1):49–53. doi: 10.3346/jkms.2010.25.1.49. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Association for Size Diversity and Health (ASDAH) Health at Every Size (HAES) Principles. 2014. https://sizediversityandhealth.org/ [ Google Scholar ]
- 55. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity. 2009;17(5):941–964. doi: 10.1038/oby.2008.636. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Schwartz MW. An inconvenient truth about obesity. Molecular Metabolism. 2012;1(1-2):2–4. doi: 10.1016/j.molmet.2012.07.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. The New England Journal of Medicine. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. The American Journal of Clinical Nutrition. 2002;76(1):226–231. doi: 10.1093/ajcn/76.1.226. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. American Journal of Clinical Nutrition. 1999;69(6):1264–1272. doi: 10.1093/ajcn/69.6.1264. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Carper JL, Orlet Fisher J, Birch LL. Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite. 2000;35(2):121–129. doi: 10.1006/appe.2000.0343. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Beaulac J, Kristjansson E, Cummins S. A systematic review of food deserts, 1966–2007. Preventing Chronic Disease: Public Health Research, Practice, and Policy. 2009;6(3):1–10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Larson NI, Story MT, Nelson MC. Neighborhood Environments: disparities in access to healthy foods in the U.S. American Journal of Preventive Medicine. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Estabrooks PA, Lee RE, Gyurcsik NC. Resources for physical activity participation: does availability and accessibility differ by neighborhood socioeconomic status? Annals of Behavioral Medicine. 2003;25(2):100–104. doi: 10.1207/S15324796ABM2502_05. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Crawford D, Timperio A, Giles-Corti B, et al. Do features of public open spaces vary according to neighbourhood socio-economic status? Health & Place. 2008;14(4):889–893. doi: 10.1016/j.healthplace.2007.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Lumeng JC, Appugliese D, Cabral HJ, Bradley RH, Zuckerman B. Neighborhood safety and overweight status in children. Archives of Pediatrics and Adolescent Medicine. 2006;160(1):25–31. doi: 10.1001/archpedi.160.1.25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Neckerman KM, Lovasi GS, Davies S, et al. Disparities in urban neighborhood conditions: evidence from GIS measures and field observation in New York city. Journal of Public Health Policy. 2009;30(supplement 1):S264–S285. doi: 10.1057/jphp.2008.47. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. The American Journal of Clinical Nutrition. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England Journal of Medicine. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Leibel RL, Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism: Clinical and Experimental. 1984;33(2):164–170. doi: 10.1016/0026-0495(84)90130-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Olson EA, Visek AJ, McDonnell KA, DiPietro L. Thinness expectations and weight cycling in a sample of middle-aged adults. Eating Behaviors. 2012;13(2):142–145. doi: 10.1016/j.eatbeh.2011.11.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Osborn RL, Forys KL, Psota TL, Sbrocco T. Yo-yo dieting in African American women: weight cycling and health. Ethnicity and Disease. 2011;21(3):274–280. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Polivy J, Herman CP. Dieting and Binging. A Causal Analysis. American Psychologist. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Mitchell JE, Devlin MJ, de Zwann M, et al. Binge-Eating Disorder: Clinical Foundations and Treatment. New York, NY, USA: Guilford Press; 2008. [ Google Scholar ]
- 74. Stice E. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychological Bulletin. 2002;128(5):825–848. doi: 10.1037/0033-2909.128.5.825. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Lowe MR, Davis W, Lucks D, Annunziato R, Butryn M. Weight suppression predicts weight gain during inpatient treatment of bulimia nervosa. Physiology and Behavior. 2006;87(3):487–492. doi: 10.1016/j.physbeh.2005.11.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. New York, NY, USA: Guilford Press; 2008. [ Google Scholar ]
- 77. Butryn ML, Juarascio A, Lowe MR. The relation of weight suppression and BMI to bulimic symptoms. International Journal of Eating Disorders. 2011;44(7):612–617. doi: 10.1002/eat.20881. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Herzog DB, Thomas JG, Kass AE, Eddy KT, Franko DL, Lowe MR. Weight suppression predicts weight change over 5 years in bulimia nervosa. Psychiatry Research. 2010;177(3):330–334. doi: 10.1016/j.psychres.2010.03.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Archives of General Psychiatry. 2010;67(1):94–101. doi: 10.1001/archgenpsychiatry.2009.170. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. American Medical Association. AMA Code of Medical Ethics. 2010. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/ [ Google Scholar ]
- 81. American Nurses Association. ANA short definitions of ethical principles and theories. http://www.nursingworld.org/MainMenuCategories/EthicsStandards/Resources/Ethics-Definitions.pdf .
- 82. American Dietetics Association. American Dietetic Association/commission on dietetic registration code of ethics for the profession of dietetics and process for consideration of ethics issues. 2009, http://www.nut.tcu.edu/forms/2010_ADA_Code_of_Ethics_for_Profession_of_Dietetics.pdf . [ DOI ] [ PubMed ]
- 83. American Psychological Association. Ethical principles of psychologists and code of conduct. 2010, http://apa.org/ethics/code/index.aspx .
- 84. Gillon R. Medical ethics: four principles plus attention to scope. British Medical Journal. 1994;309(6948):184–188. doi: 10.1136/bmj.309.6948.184. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Moradi B, Dirks D, Matteson AV. Roles of sexual objectification experiences and internalization of standards of beauty in eating disorder symptomatology: a test and extension of objectification theory. Journal of Counseling Psychology. 2005;52(3):420–428. [ Google Scholar ]
- 86. Stice E. A prospective test of the dual-pathway model of bulimic pathology: mediating effects of dieting and negative affect. Journal of Abnormal Psychology. 2001;110(1):124–135. doi: 10.1037//0021-843x.110.1.124. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Stice E, Agras WS. Predicting onset and cessation of bulimic behaviors during adolescence: a longitudinal grouping analysis. Behavior Therapy. 1998;29(2):257–276. [ Google Scholar ]
- 88. Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: a 2-year prospective investigation. Health Psychology. 2002;21(2):131–138. [ PubMed ] [ Google Scholar ]
- 89. Tylka TL. Refinement of the tripartite influence model for men: dual body image pathways to body change behaviors. Body Image. 2011;8(3):199–207. doi: 10.1016/j.bodyim.2011.04.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Education Research. 2008;23(2):347–358. doi: 10.1093/her/cym052. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Neumark-Sztainer D, Story M, Faibisch L. Perceived stigmatization among overweight African-American and Caucasian adolescent girls. Journal of Adolescent Health. 1998;23(5):264–270. doi: 10.1016/s1054-139x(98)00044-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. American Psychologist. 2007;62(4):271–286. doi: 10.1037/0003-066X.62.4.271. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Calogero RM, Herbozo S, Thompson JK. Complimentary weightism: the potential costs of appearance-related commentary for women’s self-objectification. Psychology of Women Quarterly. 2009;33(1):120–132. [ Google Scholar ]
- 94. Lumeng JC, Forrest P, Appugliese DP, Kaciroti N, Corwyn RF, Bradley RH. Weight status as a predictor of being bullied in third through sixth grades. Pediatrics. 2010;125(6):e1301–e1307. doi: 10.1542/peds.2009-0774. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Archives of Pediatrics and Adolescent Medicine. 2003;157(8):733–738. doi: 10.1001/archpedi.157.8.733. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Neumark-Sztainer D, Story M, Harris T. Beliefs and attitudes about obesity among teachers and school health care providers working with adolescents. Journal of Nutrition Education and Behavior. 1999;31(1):3–9. [ Google Scholar ]
- 97. Bauer KW, Yang YW, Austin SB. “How can we stay healthy when you’re throwing all of this in front of us?” Findings from focus groups and interviews in middle schools on environmental influences on nutrition and physical activity. Health Education and Behavior. 2004;31(1):34–46. doi: 10.1177/1090198103255372. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Puhl RM, Latner JD. Stigma, obesity, and the health of the nation’s children. Psychological Bulletin. 2007;133(4):557–580. doi: 10.1037/0033-2909.133.4.557. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Syme SL. The prevention of disease and promotion of health: the need for a new approach. European Journal of Public Health. 2007;17(4):329–330. doi: 10.1093/eurpub/ckm081. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Puhl RM, Heuer CA. Obesity stigma: important considerations for public health. The American Journal of Public Health. 2010;100(6):1019–1028. doi: 10.2105/AJPH.2009.159491. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Rudolph CW, Wells CL, Weller MD, Baltes BB. A meta-analysis of empirical studies of weight-based bias in the workplace. Journal of Vocational Behavior. 2009;74(1):1–10. [ Google Scholar ]
- 102. Giel KE, Zipfel S, Alizadeh M, et al. Stigmatization of obese individuals by human resource professionals: an experimental study. BMC Public Health. 2012;12(1, article 525) doi: 10.1186/1471-2458-12-525. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Davison KK, Birch LL. Predictors of fat stereotypes among 9-year-old girls and their parents. Obesity Research. 2004;12(1):86–94. doi: 10.1038/oby.2004.12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. McGee G. Fat chance getting an obstetrician in South Florida? Ethics and discrimination in obstetrics and gynecology. The American Journal of Bioethics. 2011;11(6):1–2. doi: 10.1080/15265161.2011.591210. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Puhl RM, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity. 2006;14(10):1802–1815. doi: 10.1038/oby.2006.208. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Davis-Coelho K, Waltz J, Davis-Coelho B. Awareness and prevention of bias against fat clients in psychotherapy. Professional Psychology: Research and Practice. 2000;31(6):682–684. [ Google Scholar ]
- 107. Puhl R, Brownell KD. Bias, discrimination, and obesity. Obesity Research. 2001;9(12):788–805. doi: 10.1038/oby.2001.108. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. National Eating Disorder Association. Health Consequences of Eating Disorders. 2014. http://www.nationaleatingdisorders.org/health-consequences-eating-disorders . [ Google Scholar ]
- 109. Schafer MH, Ferraro KF. The stigma of obesity: does perceived weight discrimination affect identity and physical health? Social Psychology Quarterly. 2011;74(1):76–97. [ Google Scholar ]
- 110. Durso LE, Latner JD, White MA, et al. Internalized weight bias in obese patients with binge eating disorder: associations with eating disturbances and psychological functioning. International Journal of Eating Disorders. 2012;45(3):423–427. doi: 10.1002/eat.20933. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Carels RA, Burmeister J, Oehlhof MW, et al. Internalized weight bias: ratings of the self, normal weight, and obese individuals and psychological maladjustment. Journal of Behavioral Medicine. 2013;36(1):86–94. doi: 10.1007/s10865-012-9402-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Durso LE, Latner JD. Understanding self-directed stigma: development of the weight bias internalization scale. Obesity. 2008;16(2):S80–S86. doi: 10.1038/oby.2008.448. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Sutin AR, Terracciano A. Perceived weight discrimination and obesity. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0070048.e70048 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Seacat JD, Mickelson KD. Stereotype threat and the exercise/ dietary health intentions of overweight women. Journal of Health Psychology. 2009;14(4):556–567. doi: 10.1177/1359105309103575. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Matthews KA, Salomon K, Kenyon K, Zhou F. Unfair treatment, discrimination, and ambulatory blood pressure in black and white adolescents. Health Psychology. 2005;24(3):258–265. doi: 10.1037/0278-6133.24.3.258. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Almeida L, Savoy S, Boxer P. The role of weight stigmatization in cumulative risk for binge eating. Journal of Clinical Psychology. 2011;67(3):278–292. doi: 10.1002/jclp.20749. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Ashmore JA, Friedman KE, Reichmann SK, Musante GJ. Weight-based stigmatization, psychological distress, & binge eating behavior among obese treatment-seeking adults. Eating Behaviors. 2008;9(2):203–209. doi: 10.1016/j.eatbeh.2007.09.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Haines J, Neumark-Sztainer D, Eisenberg ME, Hannan PJ. Weight teasing and disordered eating behaviors in adolescents: longitudinal findings from project EAT (Eating Among Teens) Pediatrics. 2006;117(2):e209–e215. doi: 10.1542/peds.2005-1242. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Neumark-Sztainer D, Falkner N, Story M, Perry C, Hannan PJ, Mulert S. Weight-teasing among adolescents: correlations with weight status and disordered eating behaviors. International Journal of Obesity. 2002;26(1):123–131. doi: 10.1038/sj.ijo.0801853. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Thompson JK, Coovert MD, Richards KJ, Johnson S, Cattarin J. Development of body image, eating disturbance, and general psychological functioning in female adolescents: covariance structure modeling and longitudinal investigations. International Journal of Eating Disorders. 1995;18(3):221–236. doi: 10.1002/1098-108x(199511)18:3<221::aid-eat2260180304>3.0.co;2-d. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Vartanian LR, Novak SA. Internalized societal attitudes moderate the impact of weight stigma on avoidance of exercise. Obesity. 2011;19(4):757–762. doi: 10.1038/oby.2010.234. [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Friedman KE, Reichmann SK, Costanzo PR, Zelli A, Ashmore JA, Musante GJ. Weight stigmatization and ideological. Beliefs: relation to psychological functioning in obese adults. Obesity Research. 2005;13(5):907–916. doi: 10.1038/oby.2005.105. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Cattarin JA, Thompson JK. A three-year longitudinal study of body image, eating disturbance, and general psychological functioning in adolescent females. Eating Disorders. 1994;2(2):114–125. [ Google Scholar ]
- 124. Grilo CM, Wilfley DE, Brownell KD, Rodin J. Teasing, body image, and self-esteem in a clinical sample of obese women. Addictive Behaviors. 1994;19(4):443–450. doi: 10.1016/0306-4603(94)90066-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 125. Crocker J, Cornwell B, Major B. The stigma of overweight: affective consequences of attributional ambiguity. Journal of Personality and Social Psychology. 1993;64(1):60–70. doi: 10.1037//0022-3514.64.1.60. [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Myers A, Rosen JC. Obesity stigmatization and coping: relation to mental health symptoms, body image, and self-esteem. International Journal of Obesity. 1999;23(3):221–230. doi: 10.1038/sj.ijo.0800765. [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Bacon L, Keim NL, van Loan MD, et al. Evaluating a “non-diet” wellness intervention for improvement of metabolic fitness, psychological well-being and eating and activity behaviors. International Journal of Obesity. 2002;26(6):854–865. doi: 10.1038/sj.ijo.0802012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Tylka TL. Development and psychometric evaluation of a measure of intuitive eating. Journal of Counseling Psychology. 2006;53(2):226–240. [ Google Scholar ]
- 129. Tylka TL, Wilcox JA. Are intuitive eating and eating disorder symptomatology opposite poles of the same construct? Journal of Counseling Psychology. 2006;53(4):474–485. [ Google Scholar ]
- 130. Tylka TL, Kroon Van Diest AM. The Intuitive Eating Scale-2: item refinement and psychometric evaluation with college women and men. Journal of Counseling Psychology. 2013;60(1):137–153. doi: 10.1037/a0030893. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Wansink B. Mindless Eating: Why We Eat more than We Think. New York, NY, USA: Bantam Books; 2006. [ Google Scholar ]
- 132. Spiegel K, Tasali E, Penev P, van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Homan KJ, Tylka TL. Appearance-based exercise motivation moderates the relationship between exercise frequency and positive body image. Body Image. 2014;11(2):101–108. doi: 10.1016/j.bodyim.2014.01.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Stice E, Presnell K. The Body Project: Promoting Body Acceptance and Preventing Eating Disorders: Facilitator’s Guide. New York, NY, USA: Oxford University Press; 2007. [ Google Scholar ]
- 135. Stice E, Rohde P, Gau J, Shaw H. An effectiveness trial of a dissonance-based eating disorder prevention program for high-risk adolescent girls. Journal of Consulting and Clinical Psychology. 2009;77(5):825–834. doi: 10.1037/a0016132. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Thompson JK, Coovert MD, Stormer S. Body image social comparison and eating disturbance: a covariance structure modeling investigation. International Journal of Eating Disorders. 1999;26(1):43–53. doi: 10.1002/(sici)1098-108x(199907)26:1<43::aid-eat6>3.0.co;2-r. [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Thompson JK, Heinberg LJ, Altabe M, Tantleff-Dunn S. Exacting Beauty: Theory, Assessment and Treatment of Body Image Disturbance. Washington, Wash , USA: American Psychological Association; 1999. [ Google Scholar ]
- 138. Fredrickson BL, Roberts T-A. Objectification theory: toward understanding women’s lived experiences and mental health risks. Psychology of Women Quarterly. 1997;21(2):173–206. [ Google Scholar ]
- 139. Tylka TL, Hill MS. Objectification theory as it relates to disordered eating among college women. Sex Roles. 2004;51(11-12):719–730. [ Google Scholar ]
- 140. Fischer AR, Jome LM, Atkinson DR. Reconceptualizing multicultural counseling: universal healing conditions in a culturally specific context. The Counseling Psychologist. 1998;26(4):525–588. [ Google Scholar ]
- 141. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. The American Journal of Clinical Nutrition. 2014;99(1):181–197. doi: 10.3945/ajcn.113.069880. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database of Systematic Reviews. 2008;8(4) doi: 10.1002/14651858.CD004366.pub3.CD004366 [ DOI ] [ PubMed ] [ Google Scholar ]
- 143. Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: a meta-analysis. Journal of Sport and Exercise Psychology. 1998;20(4):339–357. [ Google Scholar ]
- 144. Scheier MF, Carver CS. Dispositional optimism and physical well-being: the influence of generalized outcome expectancies on health. Journal of Personality. 1987;55(2):169–210. doi: 10.1111/j.1467-6494.1987.tb00434.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 145. Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: a meta-analytic review. Annals of Behavioral Medicine. 2009;37(3):239–256. doi: 10.1007/s12160-009-9111-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Carels RA, Wott CB, Young KM, Gumble A, Koball A, Oehlhof MW. Implicit, explicit, and internalized weight bias and psychosocial maladjustment among treatment-seeking adults. Eating Behaviors. 2010;11(3):180–185. doi: 10.1016/j.eatbeh.2010.03.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 147. Tylka TL, Augustus-Horvath CL. Fighting self-objectification in prevention and intervention contexts. In: Calogero RM, Tantleff-Dunn S, Thompson JK, editors. Self-Objectification in Women: Causes, Consequences, and Counteractions. 2011. pp. 187–214. [ Google Scholar ]
- 148. Avalos LC, Tylka TL. Exploring a model of intuitive eating with college women. Journal of Counseling Psychology. 2006;53(4):486–497. [ Google Scholar ]
- 149. Fiissel DL, Lafreniere KD. Weight control motives for cigarette smoking: further consequences of the sexual objectification of women? Feminism and Psychology. 2006;16(3):327–344. [ Google Scholar ]
- 150. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2014 [ Google Scholar ]
- 151. Augustus-Horvath CL, Tylka TL. The acceptance model of intuitive eating: a comparison of women in emerging adulthood, early adulthood, and middle adulthood. Journal of Counseling Psychology. 2011;58(1):110–125. doi: 10.1037/a0022129. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Marmot M. Social determinants of health inequalities. The Lancet. 2005;365(9464):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 153. Neumark-Sztainer D, Paxton SJ, Hannan PJ, Haines J, Story M. Does body satisfaction matter? Five-year longitudinal associations between body satisfaction and health behaviors in adolescent females and males. Journal of Adolescent Health. 2006;39(2):244–251. doi: 10.1016/j.jadohealth.2005.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. van den Berg P, Neumark-Sztainer D. Fat ′n happy 5 years later: is it bad for overweight girls to like their bodies? The Journal of Adolescent Health. 2007;41(4):415–417. doi: 10.1016/j.jadohealth.2007.06.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 155. Puhl R, Peterson JL, Luedicke J. Fighting obesity or obese persons? Public perceptions of obesity-related health messages. International Journal of Obesity. 2013;37(6):774–782. doi: 10.1038/ijo.2012.156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 156. Puhl R, Luedicke J, Lee Peterson J. Public reactions to obesity-related health campaigns: a randomized controlled trial. The American Journal of Preventive Medicine. 2013;45(1):36–48. doi: 10.1016/j.amepre.2013.02.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 157. McVey GL, Walker KS, Beyers J, Harrison HL, Simkins SW, Russell-Mayhew S. Integrating weight bias awareness and mental health promotion into obesity prevention delivery: a public health pilot study. Preventing Chronic Disease. 2013;10(article E46) doi: 10.5888/pcd10.120185. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 158. OReilly CO, Sixsmith J. From theory to policy: reducing harms associated with the weight-centered health paradigm. Fat Studies. 2012;1(1):97–113. [ Google Scholar ]
- 159. Public Health Agency of Canada. Our health, our future—a national dialogue on healthy weights dialogue report. 2011, http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/ohof-nsna/index-eng.php .
- 160. O’Brien KS, Puhl RM, Latner JD, Mir AS, Hunter JA. Reducing anti-fat prejudice in preservice health students: a randomized trial. Obesity. 2010;18(11):2138–2144. doi: 10.1038/oby.2010.79. [ DOI ] [ PubMed ] [ Google Scholar ]
- 161. Diedrichs PC, Barlow FK. How to lose weight bias fast! Evaluating a brief anti-weight bias intervention. British Journal of Health Psychology. 2011;16(4):846–861. doi: 10.1111/j.2044-8287.2011.02022.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 162. Neff K. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self and Identity. 2003;2:85–101. [ Google Scholar ]
- 163. Schoenefeld SJ, Webb JB. Self-compassion and intuitive eating in college women: examining the contributions of distress tolerance and body image acceptance and action. Eating Behaviors. 2013;14(4):493–496. doi: 10.1016/j.eatbeh.2013.09.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eating Behaviors. 2013;14(2):207–210. doi: 10.1016/j.eatbeh.2013.01.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236–245. doi: 10.1016/j.bodyim.2012.01.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 166. Breines J, Toole A, Tu C, Chen S. Self-compassion, body image, and self-reported disordered eating. Self and Identity. 2014;13(4):432–448. [ Google Scholar ]
- 167. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. Journal of Social and Clinical Psychology. 2007;26(10):1120–1144. [ Google Scholar ]
- View on publisher site
- PDF (779.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 06 December 2017
Food for thought: how nutrition impacts cognition and emotion
- Sarah J. Spencer 1 ,
- Aniko Korosi 2 ,
- Sophie Layé 3 ,
- Barbara Shukitt-Hale 4 &
- Ruth M. Barrientos 5
npj Science of Food volume 1 , Article number: 7 ( 2017 ) Cite this article
158k Accesses
161 Citations
189 Altmetric
Metrics details
- Neuroendocrine diseases
More than one-third of American adults are obese and statistics are similar worldwide. Caloric intake and diet composition have large and lasting effects on cognition and emotion, especially during critical periods in development, but the neural mechanisms for these effects are not well understood. A clear understanding of the cognitive–emotional processes underpinning desires to over-consume foods can assist more effective prevention and treatments of obesity. This review addresses recent work linking dietary fat intake and omega-3 polyunsaturated fatty acid dietary imbalance with inflammation in developing, adult, and aged brains. Thus, early-life diet and exposure to stress can lead to cognitive dysfunction throughout life and there is potential for early nutritional interventions (e.g., with essential micronutrients) for preventing these deficits. Likewise, acute consumption of a high-fat diet primes the hippocampus to produce a potentiated neuroinflammatory response to a mild immune challenge, causing memory deficits. Low dietary intake of omega-3 polyunsaturated fatty acids can also contribute to depression through its effects on endocannabinoid and inflammatory pathways in specific brain regions leading to synaptic phagocytosis by microglia in the hippocampus, contributing to memory loss. However, encouragingly, consumption of fruits and vegetables high in polyphenolics can prevent and even reverse age-related cognitive deficits by lowering oxidative stress and inflammation. Understanding relationships between diet, cognition, and emotion is necessary to uncover mechanisms involved in and strategies to prevent or attenuate comorbid neurological conditions in obese individuals.
Similar content being viewed by others
Feeding gut microbes to nourish the brain: unravelling the diet–microbiota–gut–brain axis
Investigating nutrient biomarkers of healthy brain aging: a multimodal brain imaging study
Neural correlates of obesity across the lifespan
Introduction.
Cognitive and emotional dysfunctions are an increasing burden in our society. The exact factors and underlying mechanisms precipitating these disorders have not yet been elucidated. Next to our genetic makeup, the interplay between specific environmental challenges occurring during well-defined developmental periods seems to play an important role. Interestingly, such brain dysfunction most often co-occurs with metabolic disorders (e.g., obesity) and/or poor dietary habits; obesity and poor diet can lead to negative health implications including cognitive and mood dysfunctions, suggesting a strong interaction between these elements (Fig. 1 ). Obesity is a global phenomenon, with around 38% of adults and 18% of children and adolescents worldwide classified as either overweight or obese. 1 Even in the absence of obesity, poor diet is commonplace, 2 with, for instance, many eating foods that are highly processed and lacking in important polyphenols and anti-oxidants or that contain well-below the recommended levels of omega-3 polyunsaturated fatty acids (PUFA). In this review, we will discuss the extent of, and mechanisms for, diet’s influence on mood and cognition during different stages of life, with a focus on microglial activation, glucocorticoids and endocannabinoids (eCBs).
Schematic depiction of how nutrition influences cognition and emotion. Overeating, obesity, acute high-fat diet consumption, poor early-life diet or early life adversity can produce an inflammatory response in peripheral immune cells and centrally as well as having impact upon the blood–brain interface and circulating factors that regulate satiety. Peripheral pro-inflammatory molecules (cytokines, chemokines, danger signals, fatty acids) can signal the immune cells of the brain (most likely microglia) via blood-borne, humoral, and/or lymphatic routes. These signals can either sensitize or activate microglia leading to de novo production of pro-inflammatory molecules such as interleukin-1beta (IL1β), IL-6, and tumor necrosis factor alpha (TNFα) within brain structures that are known to mediate cognition (hippocampus) and emotion (hypothalamus, amygdala, prefrontal cortex and others). Amplified inflammation in these regions impairs proper functioning leading to memory impairments and/or depressive-like behaviors. Polyunsaturated fatty acids (PUFA), polyphenolics, and a positive (+ve) early life environment (appropriate nutrition and absence of significant stress or adversity) can prevent these negative outcomes by regulating peripheral and central immune cell activity. Images are adapted from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 Unported License https://creativecommons.org/licenses/by/3.0/ . Salmon and hamburger images were downloaded from Bing.com with the License filter set to “free to share, and use commercially”. The blueberry image is courtesy of author Assistant Prof. Ruth Barrientos
Perinatal diet disrupts cognitive function long-term, a role for microglia
Poor diet in utero and during early postnatal life can cause lasting changes in many aspects of metabolic and central functions, including impairments in cognition and accelerated brain aging, 3 but see. 4 Maternal gestational diabetes and even a junk food diet in the non-diabetic can lead to metabolic complications, including diabetes and obesity in the offspring. 5 , 6 It can also cause changes in reward processing in the offspring brain such that they grow to prefer foods high in fat and sucrose. 7 , 8 Similarly, early introduction of solid food in children and high childhood consumption of fatty foods and sweetened drinks can accelerate weight gain and lead to metabolic complications long-term that may be associated with poorer executive function. 9 On the other hand, some dietary supplements can positively influence cognition, as is seen with supplementation of baby formula with long chain omega-3 PUFA improving cognition in babies. 10 In these randomized control trials (RCTs), an omega-3 PUFA-enriched formula beginning shortly after birth, or 6 weeks’ breast feeding, significantly improved performance of 9-month old babies on a problem solving task (a two-step task to retrieve a rattle, known to correlate with performance on IQ tasks).
From animal models, it is clear that the effects of diet in early life are far-reaching. Even obesity in rat sires (that play no part in rearing the offspring) leads to pancreatic beta cell dysfunction in female offspring, which can be passed on to the next generation. 11 Obesity and high-fat diet feeding in rat and mouse dams during pregnancy and lactation leads to impairments in several tests of mood, including those modeling depressive and anxious behaviors, as well as negatively impacting cognition. 12 Diet in the post-partum to weaning period can impact similar behaviors. 13
Additional to the impact of a prenatal diet, over-consumption of the mother’s milk during the first 3 weeks of a rat’s life leads to lasting obesity in males and females. 14 This neonatal overfeeding also disrupts cognitive function. For example, neonatally overfed rats perform poorly in the novel object recognition test and in the delayed spatial win-shift radial arm maze, as adults, compared with control rats. 15 These findings are interesting to compare with the effects of poor diet in adults where a longer-term high-fat diet (around 20 weeks in the rat) 16 , 17 , 18 and / or high-fat diet in conjunction with a pre-diabetic phenotype 19 is necessary to induce cognitive dysfunction. While there are no differences in post-learning synaptogenesis (synaptophysin) or apoptosis (caspase-3) to explain the effects seen in the neonatally overfed, these rats do have an impaired microglial response to the learning task. 15
Microglia are one of the major immune cell populations in the brain. In development, they are essential for synaptic pruning, while in a mature animal their major role is in mounting a pro-inflammatory immune response and phagocytosing pathogens and injured brain cells. 20 Hyper-activated microglia can lead to cognitive dysfunction through excess pro-inflammatory cytokine production causing impaired long-term potentiation-induction, reduced production of plasticity-related molecules including brain-derived neurotrophic factor and insulin-like growth factor-1, and reduced synaptic plasticity 20 However, an appropriate microglial response may also be essential for effective learning.
Neonatally overfed rats have more microglia in the CA1 region of the hippocampus at postnatal day 14, i.e., while they still have access to excess maternal milk and are undergoing accelerated weight gain. These microglia also have larger soma and retracted processes, indicative of a more activated phenotype. By the time these rats reach adulthood, there persists an increase in the area immunolabelled with microglial marker Iba1 in the dentate gyrus. In the neonatally overfed, the microglial response to a learning task is less robust than in controls. This effect is associated with a suppression of cell proliferation in control animals relative to the neonatally overfed, potentially to preserve existing neuronal networks and minimize novel inputs while learning takes place. 21 Interestingly, global inducible microglial and monocyte depletion can lead to improved performance in the Barnes maze, 22 suggesting withdrawal of microglial activity at specific learning phases is important for learning. These findings implicate microglia in the long-term effects of early life overfeeding on cognition suggesting normal microglia must be able to robustly respond to learning tasks and neonatal overfeeding impairs their ability to do so.
Neuroinflammatory processes, including the role of microglia, can clearly be impacted by neonatal diet and represent at least one contributing mechanism for how cognitive function is affected. Neuroinflammation and microglia can also be impacted by other early life events and play a significant role in how stress during development alters long-term physiology.
Early-life stress (ES) programs vulnerability to cognitive disorders
ES alters brain structure and function life-long, leading to increased vulnerability to develop emotional and cognitive disorders as is evident from several preclinical and clinical studies. 23 , 24 , 25 The exact underlying mechanisms for such programming remain elusive. There is extensive seminal work indicating a key role for sensory stimuli from the mother and neuroendocrine factors (e.g., stress hormones) in this programming, 26 , 27 however it has been recently suggested that these factors might act synergistically with metabolic and nutritional elements. 28 In fact, ES is associated with increased vulnerability to develop metabolic disorders such as obesity, which mostly co-occur with cognitive deficits, 29 , 30 and both ES and an adverse early nutritional environment lead to strikingly similar cognitive impairments later in life, 28 , 31 suggesting that metabolic factors and nutritional elements might mediate some of the ES effects on brain structure and function.
The brain has a very high demand for nutrients in this early period and nutritional imbalances affect normal neurodevelopment resulting in lasting cognitive deficits. 32 Understanding the role of metabolic factors and specific nutrients in this context is key to develop effective peripheral (e.g., nutritional) intervention strategies. A mouse model of the chronic ES of limited nesting and bedding material during the first postnatal week has been shown to lead to aberrant maternal care, which leads to cognitive decline in the ES offspring. 24 , 33 , 34
The hippocampus, a brain region key for cognitive functions, is permanently altered in its structure and function in these ES-exposed offspring. The hippocampus is in fact particularly sensitive to the early-life environment as it continues its development into the postnatal period. 35 Adult neurogenesis (AN) is a unique form of plasticity, which takes place in the hippocampus, consisting of the proliferation of neuronal progenitor cells that differentiate and mature into fully functional neurons that subsequently integrate into the existing hippocampal circuitry. These newly formed neurons are involved in various aspects of hippocampus-dependent learning and memory. 36 AN is affected persistently by ES 24 , 37 and, more precisely, while ES exposure initially increases neurogenesis (i.e., proliferation and differentiation of newborn cells) at postnatal day 9, at later time points (postnatal day 150), the survival of the newly born cells is reduced. 24 In addition, ES affects the neuroinflammatory profile in a lasting manner, with, for example, increased CD68 (phagocytic microglia expression) in adulthood. 38
Importantly, ES persistently affects peripheral adipose tissue metabolism as well. White adipose mass (WAT), plasma leptin (the adipokine released from the WAT) and leptin mRNA expression in WAT are persistently reduced in ES-exposed offspring. 39 In addition, exposure of ES mice to an unhealthy western style diet, leads to a higher increase in adiposity in these mice when compared to controls. These findings suggest that ES exposure leads to metabolic dysregulation and a greater vulnerability to develop obesity in a moderately obesogenic environment. Whether these metabolic alterations contribute to the ES-induced cognitive deficits warrants further investigation. 39
In addition to peripheral metabolism, ES-induced alterations in the nutritional composition of the dam’s milk, and/or nutrient intake/absorption by the pup 25 , 28 , 40 could have lasting consequences for brain structure and function. Indeed, the essential micronutrient, methionine, a critical component of the one-carbon (1-C) metabolism that is required for methylation, and for synthesis of proteins, phospholipids and neurotransmitters, is reduced after ES exposure in plasma and hippocampus of postnatal day 9 offspring. Importantly, a short supplementation of the maternal diet only during ES exposure with essential 1-C metabolism-associated micronutrients not only restores methionine levels peripherally as well as centrally, but rescues (some of) the effects of ES on hippocampal cognitive measures in adulthood and prevents the ES-induced hypothalamic-pituitary–adrenal axis hyperactivity at postnatal day 9. 25
These studies highlight the importance of studying metabolic factors and nutrients in the ES-induced effects on the brain. In the near future, it will be key to further understand the exact mechanisms mediating the effects of nutrients and metabolic factors and the windows of opportunity for interventions on brain function, as this will open entirely new avenues for targeted nutrition for vulnerable populations. However, while the early life period is a window of particular vulnerability to the programming effects of diet and other environmental influences, diet at other phases of life is also important in dictating mood and cognition.
Adult consumption of a high-fat diet: a vulnerability factor for hippocampal-dependent memory
Adults in developed countries are consuming diets higher in saturated fats and/or refined sugars than ever before. Indeed, recent reports show that approximately 12% of American adults’ daily energy intake comes from saturated fats and 13% from added sugars, 41 significantly more than what is recommended (5–10%) by the US Department of Agriculture and the Department of Health and Human Services. Not surprisingly, these dietary habits have contributed to the increasing prevalence of obesity among adults, which is currently approximately 37% in the US, a sharp rise from the 13% prevalence rate of 1960. 42
These statistics are alarming because aside from its well-known provocation of cardiovascular disease, metabolic syndrome, and type 2 diabetes, obesity has now also been associated with mild cognitive impairments and dementia. There is growing evidence that neuroinflammation may underlie obesity-induced cognitive deficits. 9 Recently, studies have demonstrated that short-term consumption (1–7 days) of an unhealthy diet (e.g., high saturated fat and/or high sugar) triggers neuroinflammatory processes, suggesting that obesity per se may not be necessary to cause cognitive disruptions. 43 , 44 For the last 10–15 years, the hypothalamus has received the vast majority of the attention with regard to obesity-induced neuroinflammatory responses and functional declines, 45 perhaps due to its close proximity to the third ventricle, circumventricular organs, and mediobasal eminence, where inflammatory signals from the periphery have easier entry into the brain. Indeed, long chain saturated fatty acids have been shown to directly pass into the hypothalamus producing an inflammatory response there through activation of toll-like receptor 4 signaling. 46 , 47 This active passage of saturated fatty acids, however, has not been observed in the hippocampus, a key brain region that mediates learning and memory. 46 Nonetheless, high-fat diet consumption has been demonstrated to impair hippocampus-dependent memory function in humans and rodents. For example, compared to rodents that consumed a control diet, those that consumed a high-fat and/or high-sugar diet exhibited robust impairments in various types of memory (e.g., spatial, contextual), as indicated by weaker performances in the Y-maze, 48 radial arm maze, 15 novel object recognition task, 15 novel place recognition task, 44 , 49 Morris water maze, 50 and contextual fear conditioning. 18 , 51 Also, adult humans who consumed a high-fat diet for 5 days exhibited significantly reduced focused attention and reduced retrieval speed of information from working and episodic memory, compared with those who consumed a standard diet. 52
Many of these studies, and others, have shown that high-fat diet-induced cognitive deteriorations are accompanied by elevated neuroinflammatory markers or responses in the hippocampus. 15 , 18 , 44 , 48 , 49 , 50 , 51 , 53 However, the mechanisms by which these neuroinflammatory processes signal and/or affect the hippocampus are not entirely clear. There is growing evidence that high-fat diets may compromise the hippocampus by sensitizing the immune cells (most likely microglia) of this brain structure, thus priming the inflammatory response to subsequent challenging stimuli. 18 , 50 , 51 For example, one study demonstrated that adult rats that had eaten a high-fat diet for 5 months exhibited a sensitized hippocampus such that when they received a relatively mild stressor (a single, 2 s, 1.5 mA footshock) following a learning session the neuroinflammatory response in the hippocampus was potentiated compared to the response of rats that had eaten the regular chow, and this response led to deficits in long-term contextual memory. 18 Another study showed that just 3 days of consuming a high-fat diet was sufficient to sensitize the hippocampus of adult rats. Here, a low-dose peripheral immune challenge (with lipopolysaccharide; LPS) produced an exaggerated neuroinflammatory response in the hippocampus of these rats compared to those that consumed the regular chow, and also led to contextual memory deficits. 51
Significantly elevated pro-inflammatory cytokines in the hippocampus have been shown to deteriorate various mechanisms that enable synaptic plasticity (such as long-term potentiation), and thus long-term memory. 54 Sobesky et al. 51 demonstrated that high-fat diet consumption primes the cells of the hippocampus by elevating the glucocorticoid steroid hormone corticosterone in this region. Despite its classic role as an immunosuppressant, there is increasing evidence demonstrating that corticosterone can prime hippocampal microglia and potentiate the inflammatory response to a subsequent challenge. 55 , 56 , 57 For example, Frank et al. 55 elegantly showed that when corticosterone was elevated prior to a peripheral immune challenge (LPS), the resulting inflammatory response in the hippocampus was potentiated. In contrast, when corticosterone was elevated after the immune challenge, the neuroinflammatory response was suppressed. These findings suggest that the temporal relationship between the corticosterone increase and the immune challenge dictates whether a pro-inflammatory or anti-inflammatory response will result. 55 Sobesky et al. 51 found that rats that consumed the high-fat diet for 3 days exhibited significantly increased levels of corticosterone in their hippocampus compared to rats that consumed the regular chow or a novel macronutrient-matched control diet. This high-fat diet-induced corticosterone rise was accompanied by increases in the endogenous danger-associated molecular pattern high-mobility group box 1 (HMGB1), the interleukin (IL)-1 inflammasome-associated protein NLRP3, and the microglial activation marker cd11b. high-fat diet alone did not, however, elevate the pro-inflammatory cytokine IL-1β unless rats were subsequently challenged with a low-dose of LPS. Thus, LPS challenge potentiated the pro-inflammatory response in the hippocampus of high-fat diet-fed rats compared to the response to LPS in chow-fed rats. To evaluate the role of corticosterone signaling in neuroinflammatory priming caused by consumption of high-fat diet, Sobesky et al. 51 administered the glucocorticoid receptor antagonist, mifepristone, prior to high-fat diet consumption. This resulted in a normalized hippocampal IL-1β response to low-dose LPS. Furthermore, mifepristone significantly reduced the high-fat diet + LPS-induced expression of HMGB1, IκBα, and NLRP3. Moreover, mifepristone treatment effectively prevented contextual memory deficits caused by high-fat diet consumption combined with LPS challenge. These data provide strong evidence for the idea that (a) high-fat diet consumption increases corticosterone within the hippocampus, and (b) this corticosterone is a key mediator in sensitizing microglia or other immune cells of the hippocampus; (c) sensitized microglia produce a potentiated neuroinflammatory response to subsequent immune or stressful challenges, thus producing cognitive deficits. Notably, though, while high-fat diet per se can have significant detrimental impact on cognitive processes, specific dietary components may be able to reverse these effects, omega-3 PUFA are one such potentially beneficial component.
Dietary omega-3 PUFA regulate neuroinflammation and eCBs: role in mood and cognitive disorders
Since their discovery in the early 20th century, considerable attention has been paid to the roles of PUFA in brain functions. Omega-3 and omega-6 PUFA are essential fatty acids, meaning that they have to be provided by the diet. Western diet contains excessive amounts of omega-6 PUFA as compared to omega-3 leading to an unbalanced ratio between these two fatty acids with cardiovascular and brain health consequences. Essential omega-3 and omega-6 fatty acids are found in green vegetables, seeds and nuts although coming from different sources with linolenic acid (LA, 18:2 omega-6) found in most plants, coconut and palm and α-linolenic acid (ALA, 18:3 omega-3) in green leafy vegetables, flax and walnuts. Once consumed, LA and ALA are metabolized into arachidonic acid (AA, 20:4 omega-6) and docosahexaenoic acid (DHA, 22:6 omega-3), respectively.
AA and DHA are the main omega-6 and omega-3 long chain PUFA found in the brain. Both long chain PUFA have pivotal roles in brain physiology as they regulate fundamental neurobiological processes, in particular the ones involved in cognition and mood. 58 , 59 AA and DHA are esterified to the phospholipid of neuronal and glial cell membranes with a total brain phospholipid proportion of around 10% for AA and 20% for DHA. Due to the limited capacity of the brain to synthesize long chain PUFA, preformed DHA can be provided by dietary supply of oily fishes. Hence, increased consumption of DHA-rich products results in a partial replacement of AA by DHA in brain cell membranes. 60 Conversely, a lower omega-3 PUFA intake leads to lower brain levels of DHA with increased AA levels. Higher AA and DHA are reported in women as compared to men, suggesting a gender difference in PUFA levels. 61 These differences could be linked to sex hormones as they differentially influence PUFA metabolism with estrogen stimulating, and testosterone inhibiting, the conversion of both omega-3 and omega-6 precursors into their respective long chain metabolites. However, whether these differences in PUFA have a role in specific brain diseases with a gender component has been poorly questioned and requires further investigation.
After its direct consumption and/or metabolization in the liver, DHA is increased in the blood and is likely to freely enter into the brain as non-esterified fatty acid. 58 More recently, Mfsd2a (major facilitator superfamily domain-containing protein 2a), which is expressed by brain endothelial cells and adiponectin receptor 1 in the retina, has been revealed to be important to DHA uptake and retention. 62
Abnormal omega-3 PUFA levels have been extensively described in both the peripheral tissues and in the brain of patients with mood disorders or cognitive decline, leading to a large number of RCTs aiming at evaluating the effectiveness of long chain omega-3 PUFA dietary supplementation on mood and cognitive disorders. 58 , 63 Overall, the results are discordant, due to the heterogeneity of methods used to evaluate the depressive and/or cognitive symptoms, the form, dose and duration of the omega-3 PUFA supplementation, the lack of evaluation of nutritional intake and metabolism of PUFA prior to starting the supplementation, or the lack of evaluation of genotype-associated risk factors. 64 However, despite the discrepancies in the results, it is important to note that several RCTs performed in patients with depressive disorders revealed an additional effect of long chain omega-3 PUFA supplementation to antidepressant treatments. 65 Of note, a recent study identifies that depressive patients presenting a high level of inflammatory markers are more responsive to long chain omega-3 PUFA supplementation. 66 This observation is highly relevant as these PUFA are potent regulators of inflammation 58 and inflammation is a crucial component of mood disorders. Concerning cognitive decline, despite poor positive results of PUFA dietary supplementation in Alzheimer’s disease (AD) patients, RCTs using DHA supplementation in subjects carrying the apolipoprotein E ε4 (APOE4) allele, a risk factor for AD, reveal an improvement of pre-dementia. 64 Overall, discrepancies in clinical studies strongly support the need for preclinical studies aimed at depicting the mechanisms of omega-3 PUFA on brain dysfunctions, which should help to better target populations at risk of cognitive and mood disorders. In addition, the consideration of omega-3 PUFA levels in food to cover the physiological requirement of these PUFA for an optimal brain function is a challenge for the food industry.
Through direct or indirect effects, DHA and AA modulate neurotransmission and neuroinflammation, which are key processes in cognition and mood. 58 , 59 Unesterified long chain PUFA are released from cell membranes upon the activation of phospholipase A2 (PLA2) to exert their effects. 67 Once released, AA and DHA are metabolized into bioactive mediators through cyclooxygenase (COX), lipoxygenases (LOX) and cytochrome P450. 68 The conversion of AA into several prostanoids, including prostaglandins (PG), leukotrienes (LT), thromboxanes (TX) and lipoxins (LX), is crucial in the progression of inflammation, including in the brain. 58 DHA is also metabolized through the COX/LOX pathways to generate metabolites with anti-inflammatory and pro-resolutive properties. 68 In the brain, LOX-derived specialized proresolving mediators (SPMs), neuroprotectin D1 (NPD1), resolvin D5 (RvD5), and maresin 1 (MaR1) are detected. 68 , 69 Some of these SPMs potently modulate neuroinflammation in vivo and in vitro, through their direct effect on microglia. 70 , 71 DHA and SPMs are impaired at the periphery and in the brains of AD patients. 72 , 73 Interestingly, decreased DHA distribution in AD patient brains correlates with synaptic loss rather than amyloid beta (Aβ) deposition. 74 In addition, DHA or SPMs promote phagocytosis of Aβ42 by microglia 75 and modulate microglia number and activation in vivo. 76 Whether SPMs play a role in the protective activity of long chain omega-3 PUFA in mood and cognitive disorders associated to neuroinflammation remains to be established.
eCBs are other key PUFA-derived lipid mediators in the brain. The main brain AA-derived eCBs are the fatty acid ethanolamides anandamide (AEA) and 2-arachidonoylglycerol (2-AG), while docosahexaenoylethanolamide (DHEA or synaptamide) is an eCB-like derived from DHA. 77 ECBs half-life in the brain is regulated by specific catabolizing enzymes fatty acid amide hydrolase for AEA and DHEA and monoacylglycerol lipase for 2-AG. Regarding neuroinflammatory processes, AA-derived eCBs are oxidized into bioactive PG by COX and LOX, which promote inflammation. 78 AEA and 2-AG bind to at least two cannabinoid receptors, type 1 (CB1) and type 2 (CB2), which are Gi/o protein-coupled with numerous signaling pathways in the brain. 79 , 80 DHEA has a lower binding affinity for CB1 and CB2 receptors as compared to AEA and 2-AG and rather bind GPR receptors, in particular GPR110 in the brain. The dietary omega-3/omega-6 PUFA ratio directly influences the proportion of ethanolamides derived from AA and DHA. 81 The modulation of eCB is accompanied by the impairment of neuronal CB1R activity and synaptic activity in several brain structures. 82 , 83 2-AG and AEA regulate synaptic function by suppressing excitatory and inhibitory synapse neurotransmitter release by acting as retrograde messengers at presynaptic CB1. 84 The importance of brain eCB signaling in the understanding of how altered dietary intake of PUFA correlates with a range of neurological disorders is of high interest. 81 However, other dietary factors may also contribute to improved cognition and prevention of cognitive disorders. Polyphenolic-rich foods are a further example that have been shown to have benefit, particularly in the context of aging.
Dietary interventions with polyphenolic-rich foods can improve neuronal and behavior deficits associated with aging
It is estimated that approximately 20% of the US total population will be older than 65 by the year 2050, which is almost double what it is today. 85 Additionally, the US is faced with an increasingly overweight/obese population that is at heightened risk for metabolic disorders, resulting in diabetes and cardiovascular disease, and concomitant behavioral impairment. Aging and metabolic dysregulation are both associated with numerous cognitive and motor deficits on tasks that require fine motor control, balance, short-term and long-term memory, or executive function. Studies in both humans and animal models have demonstrated that oxidative stress and inflammation, as well as impaired insulin resistance, are common features in cardio-metabolic and vascular disease, obesity, and age-related declines in cognitive and motor function. 86 Neuroinflammation occurs locally in the brain; however, peripheral inflammatory cells and circulating inflammatory mediators (e.g., cytokines) can also infiltrate the brain, and this occurs more readily as we age. 87 Therefore, strategies must be found to reduce oxidative and inflammatory vulnerability to age-related changes and reverse deficits in motor and cognitive function.
Targeting peripheral inflammation and insulin signaling could reduce insulin resistance and infiltration of inflammatory mediators into the brain and, as a result, reduce the incidence of a variety of age-related deficits. Studies have shown that plants, particularly colorful fruit or vegetable-bearing plants, contain polyphenolic compounds that have potent antioxidant and anti-inflammatory activities, 88 and increased fruit and vegetable intake has been associated with reduced fasting insulin levels. 89 Evidence is accumulating that consumption of these polyphenol-rich foods, particularly berry fruit, may be a strategy to forestall or even reverse age-related neuronal deficits resulting from neuroinflammation. 90 Recently this evidence has been extended to double-blind, placebo-controlled, randomized human intervention studies that have demonstrated that the consumption of flavonoid/polyphenols is associated with benefits to cognitive function. 91
Preclinical studies have led to the hypothesis that the key to reducing the incidence of age-related deficits in behavior is to alter the neuronal environment with polyphenolic-rich foods like berry fruit, such that neuroinflammation and oxidative stress, and the vulnerability to them, would be reduced. In early studies with animal models, crude blueberry (BB) or strawberry extracts significantly attenuated 92 and reversed 93 age-related motor and cognitive deficits in senescent rodents. BB supplementation also protected 9 month old C57Bl/6 mice against the damaging effects of consuming a high-fat diet. 94 Novel object recognition memory was impaired by the high-fat diet, but blueberry supplementation prevented recognition memory deficits in a time-dependent manner. Spatial memory, as measured by the Morris water maze, was also improved after 5 months on the diets. 94 Subsequent research suggested that berry fruit polyphenols may possess a multiplicity of actions in addition to their anti-inflammatory and antioxidant activities. 90 Additionally, the anthocyanins contained in blueberries have been shown to enter the brain, and their concentrations were correlated with cognitive performance. 95
Epidemiological studies that have focused on fruit and vegetable intake and cognitive function have also largely found that adequate consumption can prevent cognitive decline, while low intake is associated with increased cognitive decline. 85 Specifically, increased intake of blueberries and strawberries, as well as increased intakes of anthocyanidins and total flavanoids, were associated with slowing the rate of cognitive decline by up to 2.5 years. 96
The ability of berry fruit to protect against age-related cognitive decline has also been examined in a growing number of double-blind, placebo-controlled, randomized, human intervention studies. Thus, blueberry juice significantly improved word list recall and paired associate learning in older men and women with age-related memory decline that consumed it, relative to baseline, with paired associate learning also significantly improved relative to placebo controls. 97 A recent study 98 that measured similar cognitive tasks as those in the rodent studies, showed that freeze-dried blueberries (24 g/day, equivalent to one cup of fresh blueberries) for 90 days improved two measures of executive function in older adults (ages 60–75). Participants in the blueberry group showed significantly fewer repetition errors in the California Verbal Learning test as well as reduced switch cost on a task-switching test across study visits, relative to controls who consumed placebo powder. However, no improvement in gait or balance was observed following blueberry intake. 98 Finally, 12 weeks of blueberry concentrate supplementation improved brain perfusion, task-related activation, and cognitive function (i.e., working memory) in healthy older adults who consumed 30 mL blueberry concentrate providing 387 mg anthocyanidins. 99 These studies suggest that berry fruit might be an effective strategy to prevent, delay, or reverse cognitive dysfunction during aging.
Cognitive aging does not occur simultaneously across cognitive domains, with various domains peaking in early adulthood before reaching a plateau or declining. Therefore, interventions early in life may yield health benefits that are only measureable in later life. Blueberries have been shown to have positive cognitive benefits in two acute, cross-over designed studies in school-aged children (ages 7–10). The first study 100 showed that consumption of a flavonoid-rich blueberry (200 g) drink led to significantly better delayed word-list recall, compared to a matched vehicle group, on the Rey auditory-verbal learning test, suggesting more effective coding of memory items. However, there was no benefit of blueberry intervention on measures of attention, response inhibition, or visuospatial memory, and a negative impact on proactive interference. 100 The second study 100 by the same group examined cognition at baseline, and then 1.15, 3, and 6 h after consuming placebo (vehicle) or blueberry drinks containing 15 or 30 g freeze-dried wild blueberry (WBB) powder. Consumption of WBB powder improved recall at 1.15 h, improved delayed word recognition, which was sustained at each time point measured, and improved accuracy on a challenging interference task at 3 h. The best cognitive performance was seen after the 30 g dose, and particularly on those tasks with a higher cognitive demand. 100
As humans age, their ability to defend against the effects of oxidative stress and inflammation weakens, putting elderly people at increased risk for neuronal disease and degradation. Neuroprotective foods, such as berries and other dark-colored fruits, represent one way to protect aging brains against this damage by reducing inflammation and oxidative stress in the brain, thereby protecting against cognitive declines in aged populations.
This review has highlighted the latest advances in how foods and patterns of consumption at different times of development affect the brain, and the behavioral manifestations that may result from these effects. For example, early life overfeeding can permanently sensitize the brain’s neuroinflammatory response to challenging stimuli resulting in cognitive and immune dysfunctions throughout life. ES alters brain function, via metabolic and nutritional factors, to increase vulnerability to develop emotional and cognitive disorders. Long-term and short-term consumption of high saturated fatty foods during adulthood produces a sensitized inflammatory phenotype, via a glucocorticoid rise, in the hippocampus, leading to learning and memory vulnerabilities. Imbalance of omega-3 and omega-6 PUFA contribute to neurodevelopmental disorders by altering microglial activation resulting in abnormal formation of neuronal networks and activity. Finally, consumption of fruits and vegetables high in polyphenolics can prevent and reverse age-related cognitive deficits by lowering oxidative stress and inflammation. Collectively these data show that attention to dietary composition is important for lasting impact beyond the metabolic and highlight the promising likelihood that we may improve our cognition throughout life and into the aging period with simple dietary interventions. These data highlight the need for food industries and science, alike, to focus on research and development of nutritional strategies that are most appropriate to support our cognitive and emotional health; foods that are high in omega-3 PUFA and polyphenolics may be a promising place to start.
Data availability
No data sets were generated or analyzed during the current study.
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384 , 766–781 (2014).
Article Google Scholar
Imamura, F. et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob. Health 3 , e132–e142 (2015).
de Rooij, S. R., Wouters, H., Yonker, J. E., Painter, R. C. & Roseboom, T. J. Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl. Acad. Sci. USA 107 , 16881–16886 (2010).
de Groot, R. H. et al. Prenatal famine exposure and cognition at age 59 years. Int. J. Epidemiol. 40 , 327–337 (2011).
Dabelea, D. et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49 , 2208–2211 (2000).
Article CAS Google Scholar
Boney, C. M., Verma, A., Tucker, R. & Vohr, B. R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115 , e290–296 (2005).
Ong, Z. Y. & Muhlhausler, B. S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB. J. 25 , 2167–2179 (2011).
Gugusheff, J. R., Ong, Z. Y. & Muhlhausler, B. S. A maternal “junk-food” diet reduces sensitivity to the opioid antagonist naloxone in offspring postweaning. FASEB. J. 27 , 1275–1284 (2013).
Miller, A. A. & Spencer, S. J. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav. Immun. 42 , 10–21 (2014).
Drover, J., Hoffman, D. R., Castaneda, Y. S., Morale, S. E. & Birch, E. E. Three randomized controlled trials of early long-chain polyunsaturated fatty acid supplementation on means-end problem solving in 9-month-olds. Child Dev. 80 , 1376–1384 (2009).
Ng, S. F. et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467 , 963–966 (2010).
Rivera, H. M., Christiansen, K. J. & Sullivan, E. L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 9 , 194 (2015).
Bolton, J. L. & Bilbo, S. D. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialogues Clin. Neurosci. 16 , 307–320 (2014).
Google Scholar
Stefanidis, A. & Spencer, S. J. Effects of neonatal overfeeding on juvenile and adult feeding and energy expenditure in the rat. PLoS One 7 , e52130 (2012).
De Luca, S. N. et al. Early life overfeeding impairs spatial memory performance by reducing microglial sensitivity to learning. J. Neuroinflammation 13 , 112 (2016).
Jeon, B. T. et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 61 , 1444–1454 (2012).
Lu, J. et al. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IkappaB kinase beta/nuclear factor-kappaB-mediated inflammatory pathways in mice. Brain Behav. Immun. 25 , 1658–1667 (2011).
Sobesky, J. L. et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav. Immun. 42 , 22–32 (2014).
Soares, E. et al. Spatial memory impairments in a prediabetic rat model. Neuroscience 250 , 565–577 (2013).
Di Benedetto, S., Muller, L., Wenger, E., Duzel, S. & Pawelec, G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 75 , 114–128 (2017).
Dupret, D. et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 5 , e214 (2007).
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82 , 380–397 (2014).
Heim, C., Newport, D. J., Mletzko, T., Miller, A. H. & Nemeroff, C. B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33 , 693–710 (2008).
Naninck, E. F. et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 25 , 309–328 (2015).
Naninck, E. F. et al. Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB. J. 31 , 505–518 (2017).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7 , 847–854 (2004).
Ivy, A. S. et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 30 , 13005–13015 (2010).
Lucassen, P. J. et al. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 36 , 621–631 (2013).
Danese, A. & Tan, M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol. Psychiatry 19 , 544–554 (2014).
Maniam, J., Antoniadis, C. P., Wang, K. W. & Morris, M. J. Early life stress induced by limited nesting material produces metabolic resilience in response to a high-fat and high-sugar diet in male rats. Front. Endocrinol. 6 , 138 (2015).
Monk, C., Georgieff, M. K. & Osterholm, E. A. Research review: maternal prenatal distress and poor nutrition—mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry 54 , 115–130 (2013).
Brown, A. S., van Os, J., Driessens, C., Hoek, H. W. & Susser, E. S. Further evidence of relation between prenatal famine and major affective disorder. Am. J. Psychiatry 157 , 190–195 (2000).
Ivy, A. S., Brunson, K. L., Sandman, C. & Baram, T. Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154 , 1132–1142 (2008).
Rice, C. J., Sandman, C. A., Lenjavi, M. R. & Baram, T. Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149 , 4892–4900 (2008).
Pleasure, S. J., Collins, A. E. & Lowenstein, D. H. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J. Neurosci. 20 , 6095–6105 (2000).
CAS Google Scholar
Oomen, C. A., Bekinschtein, P., Kent, B. A., Saksida, L. M. & Bussey, T. J. Adult hippocampal neurogenesis and its role in cognition. Wiley Interdiscip. Rev. Cogn. Sci. 5 , 573–587 (2014).
Korosi, A. et al. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 227 , 400–409 (2012).
Hoeijmakers, L. et al. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain Behav. Immun. 63 , 160–175 (2017).
Yam, K. Y. et al. Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system and changes the vulnerability to western-style diet later in life in mice. Psychoneuroendocrinology 77 , 186–195 (2017).
Yam, K. Y., Naninck, E. F., Schmidt, M. V., Lucassen, P. J. & Korosi, A. Early-life adversity programs emotional functions and the neuroendocrine stress system: the contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress 18 , 328–342 (2015).
Micha, R. et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ 348 , g2272 (2014).
Fryar, C. D., Carroll, M. D. & Ogden, C. L. Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2013–2014. NCHS Data Brief , 1–6 (2016).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122 , 153–162 (2012).
Beilharz, J. E., Maniam, J. & Morris, M. J. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 306 , 1–7 (2016).
De Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146 , 4192–4199 (2005).
Milanski, M. et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29 , 359–370 (2009).
Maric, T., Woodside, B. & Luheshi, G. N. The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav. Immun. 36 , 35–45 (2014).
Almeida-Suhett, C. P., Graham, A., Chen, Y. & Deuster, P. Behavioral changes in male mice fed a high-fat diet are associated with IL-1beta expression in specific brain regions. Physiol. Behav. 169 , 130–140 (2017).
Tran, D. M. & Westbrook, R. F. A high-fat high-sugar diet-induced impairment in place-recognition memory is reversible and training-dependent. Appetite 110 , 61–71 (2017).
Boitard, C. et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 40 , 9–17 (2014).
Sobesky, J. L. et al. Glucocorticoids mediate short-term high-fat diet induction of neuroinflammatory priming, the NLRP3 inflammasome, and the danger signal HMGB1. eNeuro 3 , 1–17 (2016).
Holloway, C. J. et al. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am. J. Clin. Nutr. 93 , 748–755 (2011).
Tantot, F. et al. The effect of high-fat diet consumption on appetitive instrumental behavior in rats. Appetite 108 , 203–211 (2017).
Barrientos, R. M., Kitt, M. M., Watkins, L. R. & Maier, S. F. Neuroinflammation in the normal aging hippocampus. Neuroscience 309 , 84–99 (2015).
Frank, M. G., Miguel, Z. D., Watkins, L. R. & Maier, S. F. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav. Immun. 24 , 19–30 (2010).
Barrientos, R. M. et al. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol. Aging 36 , 1483–1495 (2015).
Frank, M. G., Hershman, S. A., Weber, M. D., Watkins, L. R. & Maier, S. F. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology 40 , 191–200 (2014).
Bazinet, R. P. & Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 15 , 771–785 (2014).
Joffre, C., Nadjar, A., Lebbadi, M., Calon, F. & Laye, S. n-3 LCPUFA improves cognition: the young, the old and the sick. Prostaglandins Leukot. Essent. Fat. Acids 91 , 1–20 (2014).
Joffre, C. et al. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot. Essent. Fat. Acids 114 , 1–10 (2016).
Kitson, A. P., Stroud, C. K. & Stark, K. D. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids 45 , 209–224 (2010).
Nguyen, L. N. et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509 , 503–506 (2014).
Saunders, E. F. et al. Omega-3 and Omega-6 polyunsaturated fatty acids in bipolar disorder: a review of biomarker and treatment studies. J. Clin. Psychiatry 77 , e1301–e1308 (2016).
Yassine, H. N. et al. Association of docosahexaenoic acid supplementation with alzheimer disease stage in apolipoprotein E epsilon4 carriers: a review. JAMA. Neurol. 74 , 339–347 (2017).
Bozzatello, P., Brignolo, E., De Grandi, E. & Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J. Clin. Med. 5 , 1–43 (2016).
Rapaport, M. H. et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol. Psychiatry 21 , 71–79 (2016).
Burke, J. E. & Dennis, E. A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50 Suppl, S237–242 (2009).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510 , 92–101 (2014).
Bazan, N. G. The docosanoid neuroprotectin D1 induces homeostatic regulation of neuroinflammation and cell survival. Prostaglandins Leukot. Essent. Fat. Acids 88 , 127–129 (2013).
Rey, C. et al. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 55 , 249–259 (2016).
Lukiw, W. J. & Bazan, N. G. Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem. Res. 25 , 1173–1184 (2000).
Zhu, M. et al. Pro-resolving lipid mediators improve neuronal survival and increase Abeta42 phagocytosis. Mol. Neurobiol. 53 , 2733–2749 (2016).
Freund-Levi, Y. et al. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer’s disease: the OmegAD study. Dement. Geriatr. Cogn. Disord. 27 , 481–490 (2009).
Yuki, D. et al. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer’s disease. Sci. Rep. 4 , 7130 (2014).
Hjorth, E. et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 35 , 697–713 (2013).
Hopperton, K. E., Trepanier, M. O., Giuliano, V. & Bazinet, R. P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-beta 1-40 in mice. J. Neuroinflammation 13 , 257 (2016).
Kim, H. Y. & Spector, A. A. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot. Essent. Fat. Acids 88 , 121–125 (2013).
Alhouayek, M. & Muccioli, G. G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 35 , 284–292 (2014).
Marsicano, G. & Lutz, B. Neuromodulatory functions of the endocannabinoid system. J. Endocrinol. Invest. 29 , 27–46 (2006).
Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58 , 1017–1030 (2010).
Bosch-Bouju, C., Layé, S. Dietary Omega-6/Omega-3 and Endocannabinoids: Implications for Brain Health and Diseases in Cannabinoids in Health and Disease (ed Meccariello, R.) InTech. https://doi.org/10.5772/62498 (2016).
Lafourcade, M. et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 14 , 345–350 (2011).
Thomazeau, A., Bosch-Bouju, C., Manzoni, O. & Laye, S. Nutritional n-3 PUFA deficiency abolishes endocannabinoid gating of hippocampal long-term potentiation. Cereb. Cortex 27 , 2571–2579 (2017).
Castillo, P. E., Younts, T. J., Chavez, A. E. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76 , 70–81 (2012).
Miller, M. G., Thangthaeng, N., Poulose, S. M. & Shukitt-Hale, B. Role of fruits, nuts, and vegetables in maintaining cognitive health. Exp. Gerontol. 94 , 24–28 (2016).
Panza, F. et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J. Alzheimers Dis. 21 , 691–724 (2010).
McGeer, P. L. & McGeer, E. G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Brain Res. Rev. 21 , 195–218 (1995).
Stevenson, D. E. & Hurst, R. D. Polyphenolic phytochemicals—just antioxidants or much more? Cell. Mol. Life Sci. 64 , 2900–2916 (2007).
Cavallo, D. N., Horino, M. & McCarthy, W. J. Adult intake of minimally processed fruits and vegetables: associations with cardiometabolic disease risk factors. J. Acad. Nutr. Diet. 116 , 1387–1394 (2016).
Miller, M. G. & Shukitt-Hale, B. Berry fruit enhances beneficial signaling in the brain. J. Agric. Food Chem. 60 , 5709–5715 (2012).
Lamport, D. J., Saunders, C., Butler, L. T. & Spencer, J. P. Fruits, vegetables, 100% juices, and cognitive function. Nutr. Rev. 72 , 774–789 (2014).
Joseph, J. A. et al. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J. Neurosci. 18 , 8047–8055 (1998).
Joseph, J. A. et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 19 , 8114–8121 (1999).
Carey, A. N., Gomes, S. M. & Shukitt-Hale, B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J. Agric. Food Chem. 62 , 3972–3978 (2014).
Andres-Lacueva, C. et al. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 8 , 111–120 (2005).
Devore, E. E., Kang, J. H., Breteler, M. M. & Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 72 , 135–143 (2012).
Krikorian, R. et al. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 58 , 3996–4000 (2010).
Miller, M. G., Hamilton, D. A., Joseph, J. A. & Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. https://doi.org/10.1007/s00394-017-1400-8 (2017).
Bowtell, J. L., Aboo-Bakkar, Z., Conway, M., Adlam, A. R. & Fulford, J. Enhanced task related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metabol. 42 , 773–779 (2017).
Whyte, A. R. & Williams, C. M. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition 31 , 531–534 (2015).
Download references
Acknowledgements
This work was supported by funding from by a National Health and Medical Research Council Career Development Fellowship, a Club Melbourne Fellowship and a Brain Foundation Research Gift to S.J.S., an NWO Meervoud and NWO Food Cognition and Behavior (NWO-FCB), JPI-Nutricog to A.K., and funding from the USDA Intramural, U.S. Highbush Blueberry Council, and California Strawberry Commission to B.SH.
Author information
Authors and affiliations.
School of Health and Biomedical Sciences, RMIT University, Melbourne, VIC, 3788, Australia
Sarah J. Spencer
Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, Amsterdam, 1098 XH, Netherlands
Aniko Korosi
Nutrition et Neurobiologie Intégrée, INRA, Bordeaux University, Bordeaux, UMR1286, France
Sophie Layé
USDA-ARS, Human Nutrition Research Center On Aging at Tufts University, Boston, MA, 02111-1524, USA
Barbara Shukitt-Hale
Department of Psychology & Neuroscience, and Center for Neuroscience, University of Colorado, Campus Box 345, Boulder, CO, 80309-0345, USA
Ruth M. Barrientos
You can also search for this author in PubMed Google Scholar
Contributions
All authors made substantial contributions to the design and conception of the work, drafting it, revising it critically for important intellectual content. All authors approve the completed version.
Corresponding author
Correspondence to Sarah J. Spencer .
Ethics declarations
Competing interests.
The authors declare that they have no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Spencer, S.J., Korosi, A., Layé, S. et al. Food for thought: how nutrition impacts cognition and emotion. npj Sci Food 1 , 7 (2017). https://doi.org/10.1038/s41538-017-0008-y
Download citation
Received : 30 April 2017
Revised : 24 July 2017
Accepted : 10 August 2017
Published : 06 December 2017
DOI : https://doi.org/10.1038/s41538-017-0008-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Epigenetic orchestration of neurodegenerative disorders: a possible target for curcumin as a therapeutic.
- Shweta Tripathi
Neurochemical Research (2024)
Identifying distinct subtypes of mother-to-infant bonding using latent profile analysis in a nationwide Japanese study
- Kosuke Hagiwara
- Takahiro Tabuchi
Archives of Women's Mental Health (2024)
Nutritional strategies cause memory damage and alter biochemical parameters without causing neuroinflammation
- Keila Rufatto de Souza
- Nicole Alessandra Engel
- Gislaine Tezza Rezin
Metabolic Brain Disease (2024)
Exploring the effects of health behaviors and mental health on students’ academic achievement: a cross-sectional study on lebanese university students
- Dalal Hammoudi Halat
- Souheil Hallit
- Mohamad Rahal
BMC Public Health (2023)
Human nutritional relevance and suggested nutritional guidelines for vitamin A5/X and provitamin A5/X
- Torsten Bohn
- Julian Hellman-Regen
Nutrition & Metabolism (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Foster Care and Child Well-Being
- First Online: 22 February 2022
Cite this chapter

- Heather N. Taussig 5 &
- Tali Raviv 6
Part of the book series: Child Maltreatment ((MALT,volume 14))
1966 Accesses
The promotion of well-being among youth in foster care, over and above safety and permanency, has become an important focus of the child welfare system over the past decade. This chapter briefly reviews the history of foster care in the United States using a child well-being lens, reviews the efficacy of programs designed to promote well-being for youth in foster care, and discusses the challenges and successes of adapting existing evidence-based programs for this population. The chapter concludes that although there have been some programs which have demonstrated efficacy in improving social, emotional, and behavioral functioning among children in care, there are not nearly enough evidence-based interventions to meet the needs of these youth and their families. Recommendations include the continued development and rigorous testing of innovative programs designed for this population as well as the testing of sensitive adaptations of evidence-based interventions designed for other populations. There is also the need for programs that work across the developmental spectrum and in different placement settings, and programs which aim to promote positive youth development, not just ameliorate problems. Children who are placed in foster care deserve contextually- and culturally-sensitive programming with demonstrated efficacy in promoting their well-being.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Foster Care and Child Well-Being: A Promise Whose Time Has Come

Wellbeing of Children in the Foster Care System

Multidimensional Treatment Foster Care for Preschoolers: A Program for Maltreated Children in the Child Welfare System
Administration on Children, Youth and Families, Administration for Children, Youth and Families (ACYF). (2012). Information Memorandum: Promoting social and emotional well-being for children and youth receiving Child Welfare services (ACYF-CB-IM-12-04). U.S. Department of Health and Human Services.
Google Scholar
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Author.
Bellamy, J. L., Gopalan, G., & Traube, D. E. (2010). A national study of the impact of outpatient mental health services for children in long-term foster care. Clinical Child Psychology and Psychiatry, 15 (4), 467–479.
Bernard, K., Dozier, M., Bick, J., Lewis-Morrarty, E., Lindhiem, O., & Carlson, E. (2012). Enhancing attachment organization among maltreated infants: Results of a randomized clinical trial. Child Development, 83 (2), 623–636.
Bick, J., & Dozier, M. (2013). The effectiveness of an attachment-based intervention in promoting foster mothers’ sensitivity toward foster infants. Infant Mental Health Journal, 34 (2), 95–103.
Bilaver, L. A., Javlicek, J., & Davis, M. M. (2020). Prevalence of special health care needs among foster youth in a nationally representative survey. JAMA Pediatrics, 174 (7), 727–729.
Blair, K., Topitzes, J., & Mersky, J. P. (2019). Brief, group-based parent-child interaction therapy: Examination of treatment attrition, non-adherence, and non-response. Children and Youth Services Review, 106 , 1–8.
Blumberg, E., Landsverk, J., Ellis-MacLeod, E., Ganger, W., & Culver, S. (1996). Use of the public mental health system by children in foster care: Client characteristics and service use patterns. Journal of Behavioral Health Services and Research, 23 (4), 389–405.
Burns, B. J., Phillips, S. D., Wagner, H. R., Barth, R. P., Kolko, D. J., Campbell, Y., & Landsverk, J. (2004). Mental health need and access to mental health services by youth involved with Child Welfare: A national survey. Journal of the American Academy of Child and Adolescent Psychiatry, 43 (8), 960–970.
Bywater, T., Hutchings, J., Linck, P., Whitaker, C., Daley, D., Yeo, S. T., & Edwards, R. T. (2010). Incredible Years parent training support for foster carers in Wales: A multi-centre feasibility study. Child: Care, Health, and Development, 37 (2), 233–243.
California Evidence-Based Clearinghouse. (2020, November 4). www.cebc4cw.org .
Chaffin, M., Silovsky, J. F., Funderburk, B., Valle, L. A., Brestan, E., Balachova, T., Jackson, S., Lensgraf, J., & Bonner, B. L. (2004). Parent-child interaction therapy with physically abusive parents. Efficacy for reducing future abuse reports. Journal of Consulting and Clinical Psychology, 72 (3), 500–510.
Chamberlain, P., & Reid, J. B. (1998). Comparison of two community alternatives to incarceration for chronic juvenile offenders. Journal of Consulting and Clinical Psychology, 66 (4), 624–633.
Chamberlain, P., Price, J., Leve, L. D., Laurent, H., Landsverk, J., & Reid, J. B. (2008). Prevention of behavior problems for children in foster care: Outcomes and mediation effects. Prevention Science, 9 (1), 17–27.
Child Trends Databank. (2019). Foster care. https://www.childtrends.org/?indicators=foster-care
Child Welfare Information Gateway. (2012). Major federal legislation concerned with child protection, child welfare and adoption . [Fact sheet]. Children’s Bureau/Administration for Children,.
Child Welfare Information Gateway. (2020). Foster care statistics 2018. U.S. Department of Health and Human Services, Administration for Children and Families, Children’s Bureau.
Child Welfare League of America. (2018). The President’s fiscal year 2019 budget request. https://www.cwla.org/wp-content/uploads/2018/02/CWLA-Summary-of-Presidents-FY-2019-Childrens-Child-Welfare-Budget.pdf
Cohen, J. A., Mannarino, A. P., & Deblinger, E. (2006). Treating trauma and traumatic grief in children and adolescents. Guilford Press.
Conn, A., Szilagy, M. A., Alpert-Gillis, L., Webster-Stratton, C., Manly, J. T., Goldstein, N., & Lee, S. H. (2018). Pilot randomized controlled trial of foster parent training: A mixed-methods evaluation of parent and child outcomes. Children and Youth Services Review, 89 , 188–197.
Cook, A., Spinazzola, J., Ford, J., Lanktree, C., Blaustein, M., Cloitre, M., DeRosa, R., Hubbard, R., Kagan, R., Liautaud, J., Mallah, K., Olafson, E., & van der Kolk, B. (2005). Complex trauma in children and adolescents. Psychiatric Annals, 35 (5), 390–398.
Cooley, M. E., Veldorale-Griffin, A., Petren, R. E., & Mullis, A. K. (2014). Parent-child interaction therapy: A meta-analysis of child behavior outcomes and parent stress. Journal of Family Social Work, 17 , 191–208.
Courtney, M. E., Dworsky, A., Lee, J., & Raap, M. (2009). Midwest evaluation of the adult functioning of former foster youth: Outcomes at age 23 and 24 . Chapin Hall at the University of Chicago.
Courtney, M., Zinn, A., Johnson, H., & Malm, K. (2011). Evaluation of the massachusetts adolescent outreach program for youths in intensive foster care: Final Report (OPRE Report # 2011–14). Office of Planning, Research, and Evaluation, Administration for Children and Families, U.S. Department of Health and Human Services. https://www.acf.hhs.gov/sites/default/files/opre/eval_mass.pdf
Courtney, M. E., Okpych, N. J., Park, K., Harty, J., Feng, H., Torres-Garcia, A., & Sayed, S. (2018). Findings from the California Youth Transitions to Adulthood Study (CalYOUTH): Conditions of youth at age 21 . Chapin Hall at the University of Chicago.
Deblinger, E., Lippman, J., & Steer, R. (1996). Sexually abused children suffering posttraumatic stress symptoms: Initial treatment outcome findings. Child Maltreatment, 1 (4), 310–321.
DeRosa, R., Habib, M., Pelcovitz, D., Rathus, J., Sonnenklar, J., Ford, J., Sunday, S., … Kaplan, S. (2006). Structured psychotherapy for adolescents responding to stress [Brochure].
Donkoh, C., Underhill, K., & Montgomery, P. (2006). Independent living programmes for improving outcomes for young people leaving the care system. Children and Youth Services Review, 28 (12), 1435–1448.
Dorsey, S., Pullman, M. D., Berliner, L., Koschmann, E., McKay, M., & Deblinger, E. (2014a). Engaging foster parents in treatment: A randomized trial of supplementing Trauma-Focused Cognitive Behavioral Therapy with evidence-based engagement strategies. Child Abuse & Neglect, 38 , 1508–1520.
Dorsey, S., Conover, K. L., & Cox, J. R. (2014b). Improving foster parent engagement: Using qualitative methods to guide tailoring of evidence-based engagement strategies. Journal of Clinical Child & Adolescent Psychology, 43 (6), 877–889.
Dozier, M., Peloso, E., Lindhiem, O., Gordon, M. K., Manni, M., Sepulveda, S., Ackerman, J., Bernier, A., & Levine, S. (2006). Developing evidence-based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. Journal of Social Issues, 62 (4), 767–785.
Dozier, M., Peloso, E., Lewis, E., Laurenceau, J. P., & Levine, S. (2008). Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology, 20 (3), 845–859.
Dozier, M., Lindhiem, O., Lewis, E., Bick, J., Bernard, K., & Peloso, E. (2009). Effects of a foster parent training program on young children’s attachment behaviors: Preliminary evidence from a randomized clinical trial. Child & Adolescent Social Work Journal, 26 (4), 321–332.
Eastman, A. L., Foust, R., Prindle, J., Palmer, L., Erlich, J., Giannella, E., & Putnam-Hornstein, E. (2019). A descriptive analysis of the child protection histories of youth and young adults arrested in California. Child Maltreatment, 24 (3), 324–329.
Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., Koss, M. P., & Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14 (4), 245–258.
Fisher, P. A., & Kim, H. K. (2007). Intervention effects on foster preschoolers’ attachment-related behaviors from a randomized trial. Prevention Science, 8 (2), 161–170.
Fisher, P. A., Burraston, B., & Pears, K. C. (2005). The early intervention foster care program: Permanent placement outcomes from a randomized trial. Child Maltreatment, 10 (1), 61–71.
Fisher, P. A., Stoolmiller, M., Gunnar, M. R., & Burraston, B. (2007). Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology, 32 (8–10), 892–905.
Fisher, P. A., Kim, H. K., & Pears, K. C. (2009). Effects of Multidimentional Treatment Foster Care for Preschoolers (MTFC-P) on reducing permanent failures among children with placement instability. Child and Youth Services Review, 31 (5), 541–546.
Fisher, P. A., Van Ryzin, M. J., & Gunnar, M. R. (2011). Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology, 36 (4), 531.
Font, S. A., & Gershoff, E. T. (2020). Foster care: How we can, and should, do more for maltreated children. SRCD Social Policy Report, 33 (3), 1–40. https://doi.org/10.1002/sop2.10
Article Google Scholar
Garland, A. F., Hough, R. L., Landsverk, J. A., McCabe, K. M., Yeh, M., Ganger, W., & Reynolds, B. J. (2000). Racial and ethnic variations in mental health care utilization among children in foster care. Children’s Services: Social Policy, Research, & Practice, 3 (3), 133–146.
Garland, A. F., Hough, R. L., McCabe, K. M., Yeh, M. Y., Wood, P. A., & Aarons, G. A. (2001). Prevalence of psychiatric disorders in youths across five sectors of care. Journal of the American Academy of Child and Adolescent Psychiatry, 40 (4), 409–418.
Goldman, F.J., Lloyd, S.W., Murphy, R.A., Crowson, M.M., Casanueva, C., Zolotor, A., Coker-Schwimmer, M., Letourneau, K., Gilbert, A., Swinson Evans, T., Crotty, T., & Viswanathan, M. (2013). Child exposure to trauma: Comparative effectiveness of interventions addressing maltreatment (Comparative Effectiveness Review No. 89 AHRQ Publication No. 13-EHC002-EF). Agency for Healthcare Research and Quality.
Goodkind, S., Shook, J., Kolivoski, K., Pohlig, R., Little, A., & Kim, K. (2020). From child welfare to jail: Mediating effects of juvenile justice placement and other system involvement. Child Maltreatment, 25 (4), 410–425.
Greeson, J. K. P., Garcia, A. R., Kim, M., & Courtney, M. E. (2015). Foster youth and social support: The first RCT of Independent Living Services. Research on Social Work Practice, 25 (3), 349–357.
Hacsi, T. (1995). From indenture to family foster care: A brief history of child placing. Child Welfare, 74 (1), 162–180.
Hadley, A.M., Mbwana, K., & Hair, E.C. (2010). What works for older youth during transition to adulthood: Lessons from experimental evaluations of programs and interventions (Report No. 2010–05). Child Trends .
Halfon, N., Berkowitz, G., & Klee, L. (1992). Mental health service utilization by children in foster are in California. Pediatrics, 89 (6), 1238–1244.
Hambrick, E. P., Oppenheim-Weller, S., N’zi, A. M., & Taussig, H. N. (2016). Mental health interventions for children in foster care: A systematic review. Children and Youth Services Review, 70 , 65–77.
Jones, A. S., LaLiberte, T., & Piescher, K. N. (2015). Defining and strengthening child well-being in child protection. Children and Youth Services Review, 54 , 57–70.
Justice Policy Institute. (2014). Sticker shock: Calculating the full price tag for youth incarceration. http://www.justicepolicy.org/uploads/justicepolicy/documents/sticker_shock_final_v2.pdf
Kempe, C. H., Silverman, F. N., Steele, B. F., Droegemueller, W., & Silver, H. K. (1962). The battered-child syndrome. Journal of the American Medical Association, 181 (1), 17–24.
Kim, H. K., & Leve, L. D. (2011). Substance use and delinquency among middle school girls in foster care: A three-year-follow-up of a randomized controlled trial. Journal of Consulting and Clinical Psychology, 79 (6), 740–750.
Knott, T., & Donovan, K. (2010). Disproportionate representation of African-American children in foster care: Secondary analysis of the national child abuse and neglect data system, 2005. Children and Youth Services Review, 32 (5), 679–684.
LaLiberte , T. Crudo, T., & Skallet, H.O. (2014) . Spring 2014 CW360°—Attending to well-being in child welfare . University of Minnesota . https://cascw.umn.edu/wp-content/uploads/2014/04/CW360_Spring2014_WEB.pdf
Letarte, M., Normandeau, S., & Allard, J. (2010). Effectiveness of a parent training program “Incredible Years” in a child protection service. Child Abuse & Neglect, 34 (4), 253–261.
Leve, L. D., Chamberlain, P., & Reid, J. B. (2005). Intervention outcomes for girls referred from juvenile justice: Effects on delinquency. Journal of Consulting and Clinical Psychology, 73 (6), 1181–1185.
Leve, L. D., Fisher, P. A., & Chamberlain, P. (2009). Multidimensional Treatment Foster Care as a preventive intervention to promote resilience among youth in the Child Welfare System. Journal of Personality, 77 (6), 1869–1902.
Lewis-Morrarty, E., Dozier, M., Bernard, K., Terracciano, S. M., & Moore, S. V. (2012). Cognitive flexibility and theory of mind outcomes among foster children: Preschool follow-up results of a randomized clinical trial. Journal of Adolescent Health, 51 (2), S17–S22.
Lieberman, A. F., Van Horn, P., & Ippen, C. G. (2005). Toward evidence-based treatment: Child-parent psychotherapy with preschoolers exposed to marital violence. Journal of American Academy of Child and Adolescent Psychiatry, 44 (12), 1241–1248.
Linares, L. O., Montalto, D., Li, M., & Oza, V. S. (2006). A promising parenting intervention in foster care. Journal of Consulting and Clinical Psychology, 74 (1), 32–41.
Lou, C., Anthony, E. K., Stone, S., Vu, C. M., & Austin, M. J. (2008). Assessing child and youth well-being: Implications for child welfare practice. Journal of Evidence-Based Social Work, 5 (1–2), 91–133.
Lu, Y. E., Landsverk, J., Ellis-Macleod, E., Newton, R., Ganger, W., & Johnson, I. (2004). Race, ethnicity, and case outcomes in child protective services. Children and Youth Services Review, 26 (5), 447–461.
McCrae, J. S., Barth, R. P., & Guo, S. (2010). Changes in maltreated children’s emotional-behavioral problems following typically provided mental health services. American Journal of Orthopsychiatry, 80 (3), 350–361.
McDonald, T. P., Allen, R. I., Westerfelt, A., & Piliavin, I. (1996). Assessing the long-term effects of foster care: A research synthesis . CWLA Press.
McKay, M. M., & Bannon, W. M., Jr. (2004). Engaging families in child mental health services. Child and Adolescent Psychiatric Clinics of North America, 13 (4), 905–921.
McKay, M. M., Hibbert, R., Hoagwood, K., Rodriguez, J., Murray, L., Legerski, J., & Fernandez, D. (2004). Integrating evidence-based engagement interventions into “real world” child mental health settings. Brief Treatment and Crisis Intervention, 4 (2), 177–186.
McKindon, A. (2019). Applying the research and evaluation provisions of the Family First Prevention Services Act. Child Trends. https://www.childtrends.org/wp-content/uploads/2019/10/FamilyFirstAct_ChildTrends_October2019.pdf
McNeil, C. B., Herschell, A. D., Gurwitch, R. H., & Clemens-Mowrer, L. (2005). Training foster parents in parent-child interaction therapy. Education and Treatment of Children, 28 (2), 182–196.
Mersky, J. P., Topitzes, J., Janczewski, C. E., & McNeil, C. B. (2015). Enhancing foster parent training with parent-child interaction therapy: Evidence from a randomized field experiment. Journal of the Society for Social Work and Research, 6 (4), 591–616.
Mersky, J. P., Topitzes, J., Grant-Savela, S. D., Brondino, M. J., & McNeil, C. B. (2016). Adapting parent-child interaction therapy to foster care: Outcomes from a randomized trial. Research on Social Work Practice, 26 (2), 157–167.
Murray, K. O., & Gesiriech, S. (2004). A brief legislative history of the child welfare system. Pew Charitable Trust.
Pears, K. C., Kim, H. K., & Fisher, P. A. (2012). Effects of a school readiness intervention for children in foster care on oppositional and aggressive behaviors in kindergarten. Children in Youth Services Review, 34 (12), 2361–2366.
Pears, C. K., Fisher, A. P., Kim, H. K., Bruce, J., Healey, V. C., & Yoerger, K. (2013). Immediate effects of a school readiness intervention for children in foster care. Early Education and Development, 24 (6), 771–791.
Pears, K. C., Kim, H. K., & Fisher, P. A. (2016). Decreasing risk factors for later alcohol use and antisocial behaviors in children in foster care by increasing early promotive factors. Children and Youth Services Review, 65 , 156–165.
Petrenko, C. L. M., Culhane, S. E., Garrido, E. F., & Taussig, H. N. (2011). Do youth in out-of-home care receive recommended mental health and educational services following screening evaluations? Children and Youth Services Review, 33 (10), 1911–1918.
Price, J. M., Chamberlain, P., Landsverk, J., Reid, J. B., Leve, L. D., & Laurent, H. (2008). Effects of a foster parent training intervention on placement changes of children in foster care. Child Maltreatment, 13 (1), 64–75.
Smith, D. K., Leve, L. D., & Chamberlain, P. (2011). Preventing internalizing and externalizing problems in girls in foster care as they enter middle school: Impact of an intervention. Prevention Science, 12 (3), 269–277.
Somers, C. L., Goutman, R. L., Day, A., Enright, O., Crosby, S., & Taussig, H. (2020). Academic achievement among a sample of youth in foster care: The role of school connectedness. Psychology in the Schools, 57 (12), 1845–1863.
Sprang, G. (2009). The efficacy of a relational treatment of maltreated children and their families. Child and Adolescent Mental Health, 14 (2), 81–88.
Stouthamer-Loeber, M., Loeber, R., Homish, D. L., & Wei, E. (2001). Maltreatment of boys and the development of disruptive and delinquent behavior. Development and Psychopathology, 13 (4), 941–944.
Tarren-Sweeney, M. (2010). It’s time to re-think mental health services for children in care, and those adopted from care. Clinical Child Psychology and Psychiatry, 15 (4), 613–626.
Taussig, H.N., & Culhane, S.E. (2005). Foster care as an intervention for abused and neglected children. In K.A. Kendall-Tackett, & S.M. Giacomoni (Eds.), Child victimization: Maltreatment, bullying and dating violence, prevention and intervention (pp. 20:1–20:25). Civic Research Institute.
Taussig, H. N., & Culhane, S. E. (2010). Impact of a mentoring and skills group program on mental health outcomes for maltreated children in foster care. Archives of Pediatrics & Adolescent Medicine, 164 (8), 739–746.
Taussig, H. N., & Raviv, T. (2014). Foster care and child well-being: A promise whose time has come. In J. E. Korbin, & R. D. Krugman (Eds.), Handbook of child maltreatment (pp. 393–410). Springer.
Taussig, H. T., Culhane, S. E., & Hettleman, D. H. (2007). Fostering Healthy Futures: An innovative preventive intervention for preadolescent youth in out-of-home care. Child Welfare, 86 (5), 113–131.
Taussig, H. T., Culhane, S. E., Garrido, E., & Knudtson, M. D. (2012). RCT of a mentoring and skills group program: Placement and permanency outcomes for foster youth. Pediatrics, 130 (1), e33–e39.
Taussig, H. N., Harpin, S. B., & Maguire, S. A. (2014). Suicidality among preadolescent maltreated children in foster care. Child Maltreatment, 19 (1), 17–26.
Taussig, H. N., Weiler, L., Rhodes, T., Hambrick, E., Wertheimer, R., Fireman, O., & Combs, M. (2015). Fostering Healthy Futures for Teens: Adaptation of an evidence-based program. Journal of the Society for Social Work and Research, 6 (4), 617–642.
Taussig, H. N., Weiler, L. M., Garrido, E. F., Rhodes, T., Boat, A., & Fadell, M. (2019). A positive youth development approach to improving mental health outcomes for maltreated children in foster care: Replication and extension of an RCT of the Fostering Healthy Futures program. American Journal of Community Psychology, 64 (3–4), 405–417.
Taussig, H. N., Bender, K., Bennett, R., Massey Combs, K., Fireman, O., & Wertheimer, R. (2020). Mentoring for teens with child welfare involvement: Permanency outcomes from a randomized controlled trial of the Fostering Healthy Futures for Teens program. Child Welfare, 97 (5), 405–417.
Taussig, H.N., Dmitrieva, J., Garrido, E.F, & Cooley, J.L., & Crites, E. (under review). Fostering healthy futures preventive intervention for children in foster care: Long-term delinquency outcomes from a randomized controlled trial.
Thomas, R., & Zimmer-Gembeck, M. J. (2007). Behavioral outcomes of Parent-Child Interaction Therapy and Triple P—Positive Parenting Program: A review and meta-analysis. Journal of Abnormal Child Psychology, 25 (3), 475–495.
Thornberry, T. P., Huizinga, D., & Loeber, R. (2004). The causes and correlates studies: Findings and policy implications. Journal of the Office of Juvenile Justice and Delinquency Prevention, 9 , 3–19. Reprinted in T. J. Bernard (Ed.), (2006), Serious delinquency: An anthology (pp. 39–52). Roxbury.
Timmer, S. G., Urquiza, A. J., Zebell, N. M., & McGrath, J. M. (2005). Parent-child interaction therapy: Application to maltreating parent-child dyads. Child Abuse and Neglect, 29 , 825–842.
Timmer, S. G., Urquiza, A. J., Herschell, A. D., McGrath, J. M., Zebell, N. M., Porter, A. L., & Vargas, E. C. (2006a). Parent-child interaction therapy: An application of empirically supported treatment to maltreated children in foster care. Child Welfare League of America, 85 (6), 919–939.
Timmer, S. G., Urquiza, A. J., & Zebell, N. M. (2006b). Challenging foster caregiver-maltreated child relationships: The effectiveness of parent-child interaction therapy. Children and Youth Services Review, 28 (1), 1–19.
United States Department of Health and Human Services, Administration for Children and Families, Administration for Children, Youth, and Families. (2012). AFCARS data: Trends in foster care and adoption – FY 2002–FY 2011 . www.acf.hhs.gov/programs/cb
Vázquez, A. L., & Villodas, M. T. (2019). Racial/ethnic differences in caregivers’ perceptions of the need for and utilization of adolescent psychological counseling and support services. Cultural Diversity and Ethnic Minority Psychology, 25 (3), 323.
Wade, R., Cronholm, P. F., Fein, J. A., Forke, C. M., Davis, M. B., Harkins-Schwarz, M., Pachter, L. M., & Bair-Merritt, M. H. (2016). Household and community-level Adverse Childhood Experiences and adult health outcomes in a diverse urban population. Child Abuse & Neglect, 52 , 135–145.
Webster-Stratton, C. (1984). Randomized trial of two parent-training programs for families with conduct-disordered children. Journal of Consulting and Clinical Psychology, 52 (4), 666–678.
Weiler, L. M., Garrido, E. F., & Taussig, H. N. (2016). Well-being of children in the foster care system. In M. R. Korin (Eds.), Health promotion for children and adolescents (pp. 371–388). Springer.
Weiner, D. A., Schneider, A., & Lyons, J. S. (2009). Evidence-based treatments for trauma among culturally-diverse foster care youth: Treatment retention and outcomes. Children and Youth Services Review, 31 (11), 1199–1205.
Wesley, B. C., Pryce, J., & Samuels, G. M. (2020). Meaning and essence of child well-being according to child welfare professionals. Child and Adolescent Social Work Journal, 37 , 425–441.
Widom, C. S. (1991). The role of placement experiences in mediating the criminal consequences of early childhood victimization. American Journal of Orthopsychiatry, 6 (2), 195–209.
Williams, S. C., & Sepulveda, K. (2019). In 2017, the rate of children in foster care rose in 39 states . Child Trends. https://www.childtrends.org/2017-the-number-of-children-in-foster-care-rose-in-39-states
Winoker, M. & Crawford, G. (2014). Fostering healthy futures child welfare cost study . Prepared for the Colorado Department of Human Services.
Woodgate, R. L., Morakinyo, O., & Martin, K. (2017). Interventions for youth aging out of care: A scoping review. Children and Youth Services Review, 82 , 280–300.
Zetlin, A., Weinberg, L., & Kimm, C. (2004). Improving education outcomes for children in foster care: Intervention by an education liaison. Journal of Education for Students Placed at Risk, 9 (4), 421–429.
Download references
Author information
Authors and affiliations.
University of Denver, Denver, CO, USA
Heather N. Taussig
Northwestern University, Feinberg School of Medicine, Chicago, IL, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Heather N. Taussig .

Editor information
Editors and affiliations.
Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO, USA
Richard D. Krugman
Department of Anthropology, Case Western Reserve University, Cleveland, OH, USA
Jill E. Korbin
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Taussig, H.N., Raviv, T. (2022). Foster Care and Child Well-Being. In: Krugman, R.D., Korbin, J.E. (eds) Handbook of Child Maltreatment. Child Maltreatment, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-030-82479-2_26
Download citation
DOI : https://doi.org/10.1007/978-3-030-82479-2_26
Published : 22 February 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-82478-5
Online ISBN : 978-3-030-82479-2
eBook Packages : Social Sciences Social Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
New research shows 'profound' link between dietary choices and brain health
New research has highlighted the profound link between dietary choices and brain health.
New research has highlighted the profound link between dietary choices and brain health.
Published in Nature , the research showed that a healthy, balanced diet was linked to superior brain health, cognitive function and mental wellbeing. The study, involving researchers at the University of Warwick, sheds light on how our food preferences not only influence physical health but also significantly impact brain health.
The dietary choices of a large sample of 181,990 participants from the UK Biobank were analysed against and a range of physical evaluations, including cognitive function, blood metabolic biomarkers, brain imaging, and genetics -- unveiling new insights into the relationship between nutrition and overall wellbeing.
The food preferences of each participant were collected via an online questionnaire, which the team catagorised into 10 groups (such as alcohol, fruits and meats). A type of AI called machine learning helped the researchers analyse the large dataset.
A balanced diet was associated with better mental health, superior cognitive functions and even higher amounts of grey matter in the brain -- linked to intelligence -- compared with those with a less varied diet.
The study also highlighted the need for gradual dietary modifications, particularly for individuals accustomed to highly palatable but nutritionally deficient foods. By slowly reducing sugar and fat intake over time, individuals may find themselves naturally gravitating towards healthier food choices.
Genetic factors may also contribute to the association between diet and brain health, the scientists believe, showing how a combination of genetic predispositions and lifestyle choices shape wellbeing.
Lead Author Professor Jianfeng Feng, University of Warwick, emphasised the importance of establishing healthy food preferences early in life. He said: "Developing a healthy balanced diet from an early age is crucial for healthy growth. To foster the development of a healthy balanced diet, both families and schools should offer a diverse range of nutritious meals and cultivate an environment that supports their physical and mental health."
Addressing the broader implications of the research, Prof Feng emphasized the role of public policy in promoting accessible and affordable healthy eating options. "Since dietary choices can be influenced by socioeconomic status, it's crucial to ensure that this does not hinder individuals from adopting a healthy balanced dietary profile," he stated. "Implementing affordable nutritious food policies is essential for governments to empower the general public to make informed and healthier dietary choices, thereby promoting overall public health."
Co-Auhtor Wei Cheng, Fudan University, added: "Our findings underscore the associations between dietary patterns and brain health, urging for concerted efforts in promoting nutritional awareness and fostering healthier eating habits across diverse populations."
Dr Richard Pemberton, Certified Lifestyle Physician and GP, Hexagon Health, who was not involved in the stud, commented: "This exciting research further demonstrates that a poor diet detrimentally impacts not only our physical health but also our mental and brain health. This study supports the need for urgent government action to optimise health in our children, protecting future generations. We also hope this provides further evidence to motivate us all to make better lifestyle choices, to improve our health and reduce the risk of developing chronic disease."
- Diet and Weight Loss
- Staying Healthy
- Dieting and Weight Control
- Nutrition Research
- Mental Health
- Animal Learning and Intelligence
- Origin of life
- Dietary mineral
- Health science
- Epidemiology
- Healthy diet
- Public health
- Antioxidant
Story Source:
Materials provided by University of Warwick . Note: Content may be edited for style and length.
Journal Reference :
- Ruohan Zhang, Bei Zhang, Chun Shen, Barbara J. Sahakian, Zeyu Li, Wei Zhang, Yujie Zhao, Yuzhu Li, Jianfeng Feng, Wei Cheng. Associations of dietary patterns with brain health from behavioral, neuroimaging, biochemical and genetic analyses . Nature Mental Health , 2024; DOI: 10.1038/s44220-024-00226-0
Cite This Page :
Explore More
- Fire-Risk Blocking Hydrogen Production System
- Tiny Electric Currents May Cut Skin Infections
- Grain-Sized Soft Robots for Drug Delivery
- PFAS: US Drinking Water Contamination
- Chronic Pain Predicted Within 3 Days of Injury
- Electric Cars: Long-Lasting Renewable Batteries
- Wildfires Becoming Faster, More Dangerous
- Beyond Superheavy Element Livermorium
- Weight-Loss Drug May Cut Alzheimer's Risk
- Human Actions Cause Insect Color Change
Trending Topics
Strange & offbeat.
- Study protocol
- Open access
- Published: 12 September 2012
Understanding determinants of nutrition, physical activity and quality of life among older adults: the Wellbeing, Eating and Exercise for a Long Life (WELL) study
- Sarah A McNaughton 1 ,
- David Crawford 1 ,
- Kylie Ball 1 &
- Jo Salmon 1
Health and Quality of Life Outcomes volume 10 , Article number: 109 ( 2012 ) Cite this article
48k Accesses
63 Citations
5 Altmetric
Metrics details
Nutrition and physical activity are major determinants of health and quality of life; however, there exists little research focusing on determinants of these behaviours in older adults. This is important, since just as these behaviours vary according to subpopulation, it is likely that the determinants also vary. An understanding of the modifiable determinants of nutrition and physical activity behaviours among older adults to take into account the specific life-stage context is required in order to develop effective interventions to promote health and well-being and prevent chronic disease and improve quality of life.
The aim of this work is to identify how intrapersonal, social and environmental factors influence nutrition and physical activity behaviours among older adults living in urban and rural areas. This study is a cohort study of adults aged 55-65 years across urban and rural Victoria, Australia. Participants completed questionnaires at baseline in 2010 and will complete follow-up questionnaires in 2012 and 2014. Self-report questionnaires will be used to assess outcomes such as food intake, physical activity and sedentary behaviours, anthropometry and quality of life. Explanatory variables include socioeconomic position, and measures of the three levels of influence on older adults’ nutrition and physical activity behaviours (intrapersonal, social and perceived environmental influences).
Obesity and its determinant behaviours, physical inactivity and poor diet are major public health concerns and are significant determinants of the quality of life among the ageing population. There is a critical need for a better understanding of the determinants of nutrition and physical activity in this important target group. This research will provide evidence for the development of effective policies and programs to promote and support increased physical activity and healthy eating behaviours among older adults.
Worldwide, it is well-recognised that the population is ageing and that this will have significant economic and social impacts. In 2009, 21% of the population in developed countries were aged>60 years and it is projected that by 2050, the proportion aged >60 years will have increased to 33%, double that of children under 15 years of age [ 1 ]. Since 1995, Australia’s estimated population aged 45 years and over has increased by 30%. While life expectancies are increasing, there is also an awareness of the need for improved quality of life at older ages. The disease burden attributable to chronic disease increases substantially from age 45 onwards, however an estimated 80% of health problems associated with old age can be prevented or delayed primarily by lifestyle changes implemented in the 55-65 year age group [ 2 ].
Nutrition, physical activity and ageing
Nutrition and physical activity are major determinants of health and disease and are associated with risk of premature mortality, coronary heart disease, hypertension, colon cancer, type 2 diabetes, osteoporosis and weight gain [ 3 ]. Promoting physical activity and a healthy diet thus has the potential to substantially reduce the burden of disease and improve quality of life. Currently older adults consume too few fruits and vegetables, and have lower than recommended intakes of a range of nutrients important for prevention of chronic disease [ 4 ]. It is also estimated that approximately 45% of adults are not sufficiently active to achieve health benefits and older adults are less likely to participate in ‘sufficient’ physical activity than younger adults [ 5 ].
There are a number of specific issues relevant to nutrition and physical activity behaviours of older adults. Nutritional needs change during older age with the required intakes of many nutrients increasing alongside a decreased energy requirement [ 6 ]. Therefore, the quality of diet with food choices based on nutrient-dense foods becomes increasingly important, particularly for the avoidance of weight gain. In addition, there is an increasing use of medications with potential for interactions with dietary intake and nutritional status [ 6 ]. Of particular significance with respect to physical activity, age-associated loss of muscle mass can result in reduced muscle strength in older persons [ 7 ] and is a major cause of their increased disability prevalence [ 8 ]. Increased physical activity is a potentially important strategy among older adults for maintaining functional status and independence [ 9 ]. Later adulthood is a critical period for promotion of nutrition and physical activity, as chronic disease will typically present during this life-stage, there are immediate benefits to improving chronic disease risk profiles and there is an ability to maximize health by avoiding or delaying preventable disability [ 3 ].
As well as the biological changes that accompany ageing, it is a period of social and psychological transition. During older adult life, there are a number of transitions that can lead to substantial lifestyle changes which may directly or indirectly impact on health including retirement, relationship breakdown or partner loss and changing household structures (“empty nest”). Populations undergoing transitional life-stages are at increased risk of poor health due to potential changes to lifestyle that impact negatively on nutrition and physical activity behaviours [ 10 , 11 ]. A life-course approach to prevention is necessary to develop interventions that are relevant to each stage of life, with strategies that are age-appropriate [ 3 ]. Existing research in the area of health and well-being of older adults focuses on the predictors or risk factors for chronic disease and use of health services [ 12 ] and there is little research focusing on the influences of nutrition and physical activity behaviours. In addition, there are few studies assessing nutrition and physical activity behaviours longitudinally among older adults. Longitudinal research is important for enabling tracking of behaviours and their determinants during this period of potential transition and the development of causal theoretical models of health behaviour.
Socioeconomic and geographic variations in nutrition and physical activity
Socioeconomic differentials in health including those relating to obesity are well recognised [ 13 ]. Similarly, nutrition and physical activity behaviours are known to vary according to socioeconomic position. There is little research on the mechanisms underlying socioeconomic variations in nutrition and physical activity behaviours specific to the older adult population group and how socioeconomic differentials in these behaviours are impacted by the life events typical in this life-stage, such as retirement [ 14 , 15 ].
In addition, rural populations suffer higher rates of socioeconomic disadvantage with lower incomes, and lower levels of educational attainment. Older adults living in rural areas have worse health compared with those in cities with lower life expectancies and higher rates of illness and disease [ 16 ]. People living in rural areas face particular challenges which impact upon health, including social isolation, limited access to transport, facilities and services [ 16 ]. The rural population is particularly susceptible to the problems associated with an ageing population since rural areas have a higher proportion of older adults compared to urban areas, driven by a combination of inward migration of older adults and outward migration of young people [ 16 ].
Understanding nutrition and physical activity behaviours in ageing
A variety of models have been applied to the study of health behaviour, such as the theory of planned behaviour, social cognitive theory and the transtheoretical or “stages of change” model [ 17 ]. A broader framework is the social ecological model [ 18 ] which acknowledges the environment in which the behaviours occur [ 19 ] and that there is a need to consider the influence of factors in the social and physical environment, the inter-relationships between environmental and intrapersonal influences, and the ability of the individual to adapt to these influences.
Intrapersonal factors such as self-efficacy, enjoyment, barriers and intentions in relation to nutrition and physical activity and social influences such as social support and sabotage are thought to be important influences on nutrition and physical activity behaviours [ 20 ]. However, there is little research concerning these influences among older adults and just as nutrition and physical activity behaviours vary according to subpopulation, it is likely that the determinants also vary. For example, in cross-sectional studies of mid-aged and older adults, nutrition knowledge [ 21 ], self-efficacy, family support factors [ 22 ] and aspects of the environment have been shown to be associated with eating behaviours [ 23 ]. However existing studies focus on broad age ranges (>40 years) and are not specifically focused on older adults in the peri-retirement phase and therefore it is necessary for research on the influences on nutrition and physical activity behaviours to take into account the specific life-stage context [ 24 ]. Furthermore, conducting research in the Australian context is important for the development of appropriate strategies and interventions and may be particularly important when trying to understand interactions between intrapersonal, social and environmental influences as important cross-country variations in some determinants have been demonstrated [ 25 ].
To examine nutrition and physical activity behaviours, obesity and quality of life among older adults aged 55-65 years and track changes in these behaviours and outcomes over 2 and 4 year periods.
To examine the intrapersonal, social and environmental influences on nutrition and physical activity behaviours and changes in these behaviours among older adults.
To assess variations in nutrition and physical activity behaviours and obesity across urban and rural areas among older adults.
To assess variations in nutrition and physical activity behaviours and obesity according to socioeconomic position and investigate the mechanisms through which socioeconomic position influences nutrition, physical activity and obesity among older adults.
The study was designed as a prospective cohort study of older adults aged 55-65 years at baseline, with baseline data collection in 2010 and follow-up at two-year intervals at Time 2 (T2, 2012) and Time 3 (T3, 2014). Data at T2 and T3 will be collected at the same time of year as T1 to negate any potential seasonal effects. Data is collected using a self-administered postal questionnaire. Adults aged 55-65 years were the focus of this study as they are an important group with respect to chronic disease prevention and they are potentially going through a number of life-stage transitions such as retirement.
Participants
Participants were selected from the Australian Electoral Commission (AEC) using a stratified random sampling process. In Australia, voting is compulsory for persons aged 18 years and over, and the AEC estimates that the electoral role represents 89.7% of those who were eligible to enrol and vote [ 26 ]. Suburbs in Victoria were classified as urban or rural using a classification consistent with the Australian Regional Infrastructure Development Fund Act 1999 [ 27 ] and suburbs with populations of less than 1000 or less than 200 55-65 year olds were excluded. All suburbs in urban and rural areas were then classified according to the socioeconomic Index for Areas score (SEIFA) which is assigned by the Australian Bureau of Statistics [ 28 ], and divided into tertiles (i.e low, medium and high SEIFA). Fourteen postcodes from each SEIFA tertile (i.e low, medium and high SEIFA) were randomly selected and an equal number of participants from areas from each tertile of SEIFA score selected. From each suburb, 134 participants (equal numbers of men and women) were selected, resulting in a total sampling pool of 11256. Of the surveys distributed, 380 were returned as undeliverable and 95 were returned from individuals outside of the 55-65 year age bracket. In total, 4,082 completed surveys were returned (38% response rate). Table 1 shows the sociodemographic characteristics of our final sample.
Participants selected from the electoral role were sent a letter inviting them to participate in the study and one week later were sent the survey and a reply-paid envelope for survey return. After three weeks, non-respondents received a reminder letter encouraging them to return their questionnaire. After a further three weeks, the remaining non-respondents received a second reminder letter and a replacement questionnaire and reply-paid envelope. This process of sending two reminders is standard practice [ 29 , 30 ].
Participants will be re-contacted at T2 and T3 and the same procedures and protocols for postal survey administration will be used. Follow-up after two and four years will allow sufficient time to detect changes in weight, nutrition and physical activity during this life-stage [ 31 ]. Recruitment and retention are promoted via media releases in the local survey areas, personalised survey letters, newsletters to participants with details of study results, birthday cards and access to a study website and phone number for information and change of address.
Questionnaire
The questionnaire was designed to include measures of the outcome variables (nutrition, physical activity, sedentary behaviour, obesity, quality of life), potential determinants of these outcomes and relevant covariates. The social ecological model was used as a framework for development of the questionnaire and selection of the range of potential determinants of nutrition and physical activity behaviours [ 24 ]. Items on the questionnaire examined all three levels of influence on nutrition and physical behaviours (intrapersonal, social and neighbourhood environmental influences). Where possible, established questionnaire items from the literature with known reliability and validity were used. The full range of measures included in the questionnaire are shown in Table 2 and key variables are summarised here.
Quality of life
The Medical Outcomes Study Short-Form General Health Survey (SF-36) is included as a measure of quality of life [ 32 – 34 ]. Scores for General Health, Physical Health, and Mental Health are computed. The Physical Health Component includes physical functioning, role-physical, bodily pain, and general health. The Mental Health Component includes vitality, social functioning, role-emotional and mental health. The questions were altered to Australian conditions in line with the Australian Longitudinal Study on Women’s Health [ 33 , 35 , 36 ].
Anthropometry
Measures of self-reported height and weight were collected. Self-reported height and weight data are strongly correlated with measured height and weight r > 0.9 [ 37 ]. Self-reported weight and height information is adequate for use in large epidemiological studies examining weight or body mass index [ 38 – 40 ] and has been used in several large cohorts in Australia and worldwide to investigate weight change [ 41 , 42 ].
Dietary intake
Diet was measured using a 111-item food frequency questionnaire assessing usual frequency of intake of food and beverages over the last 6 months previously developed for use with Australian adults in the National Nutrition Survey and other national surveys [ 43 – 45 ]. Additional validated short questions on food habits concerning breakfast consumption, salt use, type of milk consumed, trimming the fat from meat, daily fruit and vegetable consumption and food security were also included [ 45 , 46 ].
Physical activity and sedentary behaviours
Physical activity in the past week was assessed using the long version of the self-administered International Physical Activity Questionnaire (IPAQ-L). This survey demonstrated excellent one-week test-retest reliability (pooled r = 0.81) and acceptable validity (pooled r = 0.33) when compared to accelerometer-measured physical activity in a 12-country, 14-site study [ 47 ]. The IPAQ-L assesses duration, frequency and intensity of leisure, work, commuting and household/yard activities. Data on total sitting time were also collected from the IPAQ-long with respondents asked to report time spent sitting while at work, at home, while doing study, and in leisure-time during the last 7 days [ 47 , 48 ] Respondents were also asked to report sitting time while doing specific activities (watching tv and during computer activities) [ 49 ].
Sociodemographic factors
Demographic variables that were considered to be important potential moderators or confounders of the associations between behavioural predictors and outcomes were measured. These included age, country of birth, marital status, measures of socioeconomic position (education, employment, own and household income, postcode as an area level measure of socioeconomic position) [ 50 , 51 ], retirement status, household composition and living arrangements.
Intrapersonal factors
In relation to nutrition and physical activity, the questionnaire included measures of self-efficacy [ 52 ], enjoyment, barriers and intentions [ 53 , 54 ], outcome expectancies[ 55 ], perceived behavioural control [ 54 ] and nutrition knowledge [ 56 ]. It also included measures of perceptions of ageing and retirement [ 57 ].
Social factors
The questionnaire assessed support and sabotage for nutrition and physical activity behaviours (i.e. family and friend support and sabotage) [ 58 ], social participation [ 59 ], social capital [ 60 ] and social cohesion [ 61 ] using established measures.
Environmental influences (home and neighbourhood): Participants were asked about their perceptions of their local environment including safety, aesthetics, walking environment [ 62 ], and the cost, availability, and convenience of food and food stores. Home availability of fruits, vegetables and high energy foods and beverages and the number of televisions in the house were also assessed.
Data will be initially analysed using univariate statistics to examine the distribution of key variables. Based on the initial descriptive analyses, we will employ multivariate procedures where appropriate to examine the correlates of nutrition and physical activity behaviours. We will systematically examine associations between the different domains of intrapersonal, social and environmental characteristics; and physical activity and food intake behaviours. Urban-rural, and socioeconomic comparisons in key outcomes and determinants and their associations will be examined using t-tests, ANOVA and regression models with interaction terms. We will conduct longitudinal regression analysis using baseline measures of intrapersonal, social and neighbourhood environmental factors to test predictive models of behaviour and investigate the effect of changes in nutrition and physical activity behaviours on weight status and quality of life. Multilevel modelling will be used to take into account the effect of area-level measures of socioeconomic status and environment. In addition, the mediating relationships among intrapersonal, social and environmental factors and nutrition and physical activity behaviours and obesity will be examined using structural equation modelling and mediational techniques based on regression analyses [ 63 ].
Ethics and study funding
Ethical approval to conduct the study was granted by the Deakin University Human Research Ethics Committee (2009-105). This project was awarded funding from the Diabetes Australia Research Trust for the baseline measures in the urban sample of participants. Funding was also received from the Australian Research Council to establish the rural sample and for the two-year follow-up (T2) and the four-year follow-up (T3) of both groups (Project Grant ID: DP1095595, FT100100581).
Obesity and its determinant behaviours, physical inactivity and poor diet are major public health concerns and are significant determinants of the quality of life among the ageing population. However, influences on eating and physical activity behaviours among older adults are currently not well understood. This cohort has a number of unique features that will allow the development of a thorough understanding of the determinants of nutrition and physical activity behaviour, obesity and quality of life among older adults. For example, it will focus on adults aged 55-65 years, a sub-group of older adults likely to be undergoing a number of life transitions, particularly retirement, and therefore, are at risk of weight gain. In addition, longitudinal data on physical activity and food intake in older adults will be gathered allowing changes in diet and physical activity to be tracked in order to understand the changes in nutrition and physical activity behaviours during this stage of transition.
The promotion of nutrition and physical activity is a key strategy for the prevention of a range of chronic diseases including cardiovascular disease, obesity, diabetes mellitus and cancer, as well as osteoporosis, asthma and poor mental health, and has the potential to substantially reduce the burden of disease in Australia. Improving nutrition and physical activity is likely to have significant economic benefits for Australia, with long-term gains in productivity and reductions in both direct and indirect healthcare costs [ 64 ]. While much is known about the importance of these lifestyle behaviours in health and disease, little is known about the optimal strategies for their promotion among older adults. This research will contribute evidence on key behavioural determinants which is required in order to inform the development of effective policies and programs to promote and support increased physical activity and healthy eating behaviours among older adults.
Department of Economic and Social Affairs; United Nations: World Population Ageing: 1950–2050 . United Nations, New York; 2009.
Google Scholar
Department of Health and Ageing: National Strategy for an Ageing Australia An Older Australia, Challenges and Opportunities for all . Commonwealth of Australia, Canberra; 2001.
WHO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation . WHO Technical Report Series 916, Geneva; 2003.
Magarey A, McKean S, Daniels LA: Evaluation of fruit and vegetable intakes of Australian adults: the National Nutrition Survey 1995. Aust N Z J Public Health 2006, 30(1):32–37. 10.1111/j.1467-842X.2006.tb00083.x
Article PubMed Google Scholar
Armstrong T, Bauman A, Davies J: Physical activity patterns of Australian adults . Australian Institute of Health and Welfare, Canberra; 2000.
National Health and Medical Research Council: Dietary guidelines for Older Australians . Commonwealth of Australia, Canberra; 1999.
Wannamethee SG, Shaper AG, Lennon L, Whincup PH: Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 2007, 86: 1339–1346.
CAS PubMed Google Scholar
Australian Institute of Health and Welfare: Older Australia at a glance. Cat. No. 52 . 4th edition. AIHW, Canberra; 2007.
Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al .: Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007, 39(8):1435–1445. 10.1249/mss.0b013e3180616aa2
Edstrom KM, Devine CM: Consistency in women’s orientations to food and nutrition in midlife and older age: a 10-year qualitative follow-up. J Nutr Educ 2001, 33(4):215–223. 10.1016/S1499-4046(06)60034-1
Article CAS PubMed Google Scholar
Kremers SP, Visscher TL, Brug J, Paw MJ CA, Schouten EG, Schuit AJ: Netherlands research programme weight gain prevention (NHF-NRG): rationale, objectives and strategies. Eur J Clin Nutr 2005, 59(4):498–507. 10.1038/sj.ejcn.1602100
45 and Up Study Collaborators. Cohort Profile: The 45 and Up Study. Int J Epidemiol 2008, 37(5):941–947.
Article Google Scholar
Ball K, Crawford D: Socioeconomic status and weight change in adults: a review. Soc Sci Med 2005, 60(9):1987–2010. 10.1016/j.socscimed.2004.08.056
Mishra GD, Ball K, Arbuckle J, Crawford D: Dietary patterns of Australian adults and their association with socioeconomic status: results from the 1995 National Nutrition Survey. Eur J Clin Nutr 2002, 56: 687–693. 10.1038/sj.ejcn.1601391
Chinn DJ, White M, Drinkwater C, Raybould S: Barriers to physical activity and socioeconomic position: implications for health promotion. J Epidemiol Comm Health 1999, 53: 191–192. 10.1136/jech.53.3.191
Article CAS Google Scholar
National Rural Health Alliance, Aged and Community Services Australia: Older people and aged care in rural, regional and remote Australia . Aged and Community Services Australia, Melbourne; 2004.
Baranowski T, Weber Cullen K, Baranowski J: Psychosocial correlates of dietary intake: advancing dietary intervention. Ann Rev Nutr 1999, 19: 17–40. 10.1146/annurev.nutr.19.1.17
Stokols D: Translating social ecological theory into guidelines for community health promotion. Am J Health Promot 1996, 10(4):282–298. 10.4278/0890-1171-10.4.282
Glanz K, Bishop DB: The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health 2010, 31: 399–418. 10.1146/annurev.publhealth.012809.103604
Nestle M, Wing R, Birch L, DiSogra L, Drewnowski A, Middleton S, et al .: Behavioral and social influences on food choice. Nutr Rev 1998, 56(5 Pt 2):S50-S64.
Dallongeville J, Marecaux N, Cottel D, Bingham A: Association between nutrition knowledge and nutritional intake in middle-aged men from northern France. Public Health Nutr 2001, 4(1):27–33.
Hermstad AK, Swan DW, Kegler MC, Barnette JK, Glanz K: Individual and environmental correlates of dietary fat intake in rural communities: a structural equation model analysis. Soc Sci Med 2010, 71(1):93–101. 10.1016/j.socscimed.2010.03.028
Sharkey JR, Johnson CM, Dean WR: Food access and perceptions of the community and household food environment as correlates of fruit and vegetable intake among rural seniors. BMC Geriatr 2010, 10: 32. 10.1186/1471-2318-10-32
Article PubMed Central PubMed Google Scholar
Crawford D, Ball K: Behavioural determinants of the obesity epidemic. Asia Pacific J Clin Nutr 2002, 11(Suppl):S718-S721.
Ball K, Timperio AF, Crawford DA: Understanding environmental influences on nutrition and physical activity behaviors: where should we look and what should we count? Int J Behav Nutr Phys Act 2006, 3: 33. 10.1186/1479-5868-3-33
Australian Electoral Commission (AEC): AEC Annual Report 2009–2010 . Commonwealth of Australia, Canberra; 2010.
State Government of Victoria: Regional Infrastructure Development Fund Act 1999 Act No. 64/19991999 . State Government of Victoria, Melbourne; 1999.
Australia Bureau of Statistics: 2001 Census of Population and Housing: Information Paper – Socio Economic Indexes for Areas (Cat. No. 2039.0) . Commonwealth of Australia, Canberra; 2003.
Dillman DA: Mail and Telephone Surveys: The Total Design Method . Wiley, New York; 1978.
Dillman DA: Mail and internet surveys: The tailored design method . John Wiley & sons, Hoboken; 2007.
Chung S, Popkin BM, Domino ME, Stearns SC: Effect of retirement on eating out and weight change: an analysis of gender differences. Obesity 2007, 15(4):1053–1060. 10.1038/oby.2007.538
Ware JE, Sherbourne CD: The MOS 36-ltem Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Medical Care 1992, 30(6):473–483. 10.1097/00005650-199206000-00002
Schofield MJ, Mishra G: Validity of the sf-12 compared with the sf-36 health survey in pilot studies of the Australian Longitudinal Study on Women’s Health. J Health Psychol 1998, 3(2):259–271. 10.1177/135910539800300209
Mishra GD, Gale CR, Sayer AA, Cooper C, Dennison EM, Whalley LJ, et al .: How useful are the sf-36 sub-scales in older people? mokken scaling of data from the halcyon programme. Qual Life Res 2011, 20(7):1005–1010. 10.1007/s11136-010-9838-7
Lee C, Dobson AJ, Brown WJ, Bryson L, Byles J, Warner-Smith P: Cohort profile: the Australian Longitudinal Study on Women’s Health. Int J Epidemiol 2005, 34(5):987–991. 10.1093/ije/dyi098
Dobson AJ, Byles J, Brown WJ: Womens Health Australia: Fifth survey for mid age women March 2007 . The University of Newcastle, Newcastle; 2007.
Rowland M: Self-reported weight and height. Am J Clin Nutr 1990, 52(6):1125–1133.
McAdams MA, Van Dam RM, Hu FB: Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity 2007, 15(1):188–196. 10.1038/oby.2007.504
Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC: Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990, 1(6):466–473. 10.1097/00001648-199011000-00009
Burton NW, Brown W, Dobson A: Accuracy of body mass index estimated from self-reported height and weight in mid-aged australian women. Aust N Z J Public Health 2010, 34(6):620–623. 10.1111/j.1753-6405.2010.00618.x
Ball K, Crawford D, Ireland P, Hodge A: Patterns and demographic predictors of 5-year weight change in a multi-ethnic cohort of men and women in Australia. Public Health Nutr 2003, 6(3):269–81.
Field AE, Willett WC, Lissner L, Colditz GA: Dietary fat and weight gain among women in the nurses’ health study. Obesity 2007, 15(4):967–976. 10.1038/oby.2007.616
Smith KJ, McNaughton SA, Gall SL, Blizzard L, Dwyer T, Venn AJ: Involvement of young australian adults in meal preparation: cross-sectional associations with sociodemographic factors and diet quality. J Am Diet Assoc 2010, 110(9):1363–1367. 10.1016/j.jada.2010.06.011
Smith K, Gall SL, McNaughton SA, Blizzard L, Dwyer T, Venn AJ: Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the childhood determinants of adult health study. Am J Clin Nutr 2010, 92: 1316–1325. 10.3945/ajcn.2010.30101
McLennan W, Podger A: National Nutrition Survey Users’ Guide Australian Bureau of Statistics Catalogue No. 4801.0 . AGPS, Canberra; 1998.
Rutishauser IHE, Webb K, Abraham B, Allsopp R: Evaluation of short dietary questions from the 1995 National Nutrition Survey. National Food and Nutrition Monitoring and Surveillance Project . Commonwealth Department of Health and Aged Care, Canberra; 2001.
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al .: International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003, 35(8):1381–1395. 10.1249/01.MSS.0000078924.61453.FB
Bauman A, Ainsworth BE, Sallis JF, Hagströmer M, Craig CL, Bull FC, et al .: The descriptive epidemiology of sitting a 20-country comparison using the international physical activity questionnaire (ipaq). Am J Prev Med 2011, 41(2):228–235. 10.1016/j.amepre.2011.05.003
Salmon J, Owen N, Crawford D, Bauman A, Sallis JF: Physical activity and sedentary behavior: a population-based study of barriers, enjoyment, and preference. Heal Psychol 2003, 22(2):178–188.
Ball K, Mishra G, Crawford D: Which aspects of socioeconomic status are related to obesity among men and women? Int J Obes 2002, 26(4):559–565. 10.1038/sj.ijo.0801960
Mishra G, Ball K, Dobson A, Byles J, Warner-Smith P: The measurement of socioeconomic status: investigation of gender-and age-specific indicators in australia: national health survey ’95. Soc Indic Res 2001, 56: 73–89. 10.1023/A:1011834621663
Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR: The development of self-efficacy scales for healthrelated diet and exercise behaviors. Health Educ Res 1988, 3(3):283–292. 10.1093/her/3.3.283
Andajani-Sutjahjo S, Ball K, Warren N, Inglis V, Crawford D: Perceived personal, social and environmental barriers to weight maintenance among young women: a community survey. Int J Behav Nutr Phys Act 2004, 1(1):15. 10.1186/1479-5868-1-15
Giles-Corti R, Macintyre S, Clarkson JP, Pilora T, Donovan RJ: Environmental and lifestyle factors associated with overweight and obesity in perth, australia. Am J Heal Promot 2003, 18(1):93–102. 10.4278/0890-1171-18.1.93
Baranowski T, Watson K, Missaghian M, Broadfoot A, Baranowski J, Cullen K, et al .: Parent outcome expectancies for purchasing fruit and vegetables: a validation. Public Health Nutr 2007, 10(3):280–291.
Parmenter K, Wardle J: Development of a general nutrition knowledge questionnaire for adults. Eur J Clin Nutr 1999, 53(4):298–308. 10.1038/sj.ejcn.1600726
Demakakos P, Hacker E, Gjonça E: Perceptions of ageing. In Retirement, health and relationships of the older population in England: The 2004 English Longitudinal Study of Ageing (Wave 2) . Edited by: Banks J, Breeze E, Lessof C, Nazroo J. Institute for Fiscal Studies, London; 2006.
Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR: The development of scales to measure social support for diet and exercise behaviors. Prev Med 1987, 16(6):825–836. 10.1016/0091-7435(87)90022-3
Baum FE, Bush RA, Modra CC, Murray CJ, Cox EM, Alexander KM: Epidemiology of participation: an Australian community study. J Epidemiol Community Health 2000, 54(6):414–423. 10.1136/jech.54.6.414
Article PubMed Central CAS PubMed Google Scholar
Lochner K, Kawachi I, Kennedy BP: Social capital: a guide to its measurement. Health Place 1999, 5(4):259–270. 10.1016/S1353-8292(99)00016-7
Sampson RJ, Raudenbush SW, Earls F: Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 1997, 277(5328):918–924. 10.1126/science.277.5328.918
Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T: Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol 2007, 165(8):858–867. 10.1093/aje/kwm040
MacKinnon DP, Fairchild AJ, Fritz MS: Mediation analysis. Annu Rev Psychol 2007, 58: 593–614. 10.1146/annurev.psych.58.110405.085542
Tam CS, Garnett SP, Cowell CT, Campbell K, Cabrera G, Baur LA: Soft drink consumption and excess weight gain in Australian school students: results from the nepean study. Int J Obes 2006, 30(7):1091–3. 10.1038/sj.ijo.0803328
Download references
Acknowledgements
SAM is supported by an Australian Research Council Future Fellowship (FT100100581), KB is supported by a NHMRC Senior Research Fellowship (ID479513). JS is supported by a NHMRC Senior Research Fellowship (ID1026216).
Author information
Authors and affiliations.
Centre for Physical Activity & Nutrition Research, School of Exercise & Nutrition Sciences, Deakin University, 221 Burwood Hwy, 3125, Burwood, VIC, Australia
Sarah A McNaughton, David Crawford, Kylie Ball & Jo Salmon
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sarah A McNaughton .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
SAM took a leading role in writing the grant that was subsequently funded by the Diabetes Australia Research Trust and the Australian Research Council and drafting the study protocol for publication. All authors contributed to the study design, grant preparation, questionnaire and measures development and contributed to the drafting and editing of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
McNaughton, S.A., Crawford, D., Ball, K. et al. Understanding determinants of nutrition, physical activity and quality of life among older adults: the Wellbeing, Eating and Exercise for a Long Life (WELL) study. Health Qual Life Outcomes 10 , 109 (2012). https://doi.org/10.1186/1477-7525-10-109
Download citation
Received : 05 March 2012
Accepted : 04 September 2012
Published : 12 September 2012
DOI : https://doi.org/10.1186/1477-7525-10-109
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Physical Activity
- Sedentary Behaviour
- Physical Activity Behaviour
- Socioeconomic Position
- Breakfast Consumption
Health and Quality of Life Outcomes
ISSN: 1477-7525
- Submission enquiries: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Evidence on the contribution of community gardens to promote physical and mental health and well-being of non-institutionalized individuals: A systematic review
Roles Conceptualization, Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Instituto de Saúde Ambiental, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
Roles Conceptualization, Methodology, Writing – original draft, Writing – review & editing
Affiliation EnviHeB Lab, Instituto de Saúde Ambiental, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
Roles Conceptualization, Methodology, Writing – review & editing
Affiliations Instituto de Saúde Ambiental, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal, EnviHeB Lab, Instituto de Saúde Ambiental, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal, Unbreakable Idea Research, Lisboa, Portugal
Roles Writing – review & editing
Affiliations Instituto de Saúde Ambiental, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal, Laboratório de Nutrição, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
Affiliation Câmara Municipal de Cascais, Lisboa, Portugal
Affiliation Departamento das Ciências Sociais do Território, Faculdade de Arquitectura, Universidade de Lisboa, Lisboa, Portugal
- Tarsila Lampert,
- Joana Costa,
- Osvaldo Santos,
- Joana Sousa,
- Teresa Ribeiro,
- Elisabete Freire

- Published: August 6, 2021
- https://doi.org/10.1371/journal.pone.0255621
- Reader Comments
Introduction
There has been growing interest in community gardens as an effective and affordable health promotion strategy. However, most available evidence is derived from qualitative studies, whereas quantitative research on this subject is limited.
To synthetize the literature about physical and mental health outcomes associated with community gardening. Two main questions were addressed: a) is there evidence, from quantitative studies, that community gardening is associated to physical and mental health and well-being of non-institutionalized individuals? b) Does community gardening provokes any discomfort in terms of physical health, i.e., bodily pain, to their beneficiaries?
A systematic review of the literature was carried out following PRISMA guidelines by searching relevant electronic databases (PubMed, Scopus, and Web of Science). Empirical, quantitative studies published in English with no restrictions concerning the date of publication were considered eligible. The quality of the evidence was appraised using the tool developed by the National Heart, Lung, and Blood Institute of the National Institutes of Health for Observational Cohort and Cross-Sectional Studies.
Overall, 8 studies were considered eligible, of which seven studies were rated as having good methodological quality (one scored as fair). Community gardeners had significantly better health outcomes than their neighbours not engaged in gardening activities in terms of life satisfaction, happiness, general health, mental health, and social cohesion.
Community gardens are associated to health gains for their users, irrespective of age, being an affordable and efficient way of promoting physical and mental health and well-being. To encourage the design, maintenance, and prospective evaluation of supportive urban environments promoting healthy and, at the same time, sustainable lifestyles, is essential to achieve public health gains and environmental sustainability.
Citation: Lampert T, Costa J, Santos O, Sousa J, Ribeiro T, Freire E (2021) Evidence on the contribution of community gardens to promote physical and mental health and well-being of non-institutionalized individuals: A systematic review. PLoS ONE 16(8): e0255621. https://doi.org/10.1371/journal.pone.0255621
Editor: Marcel Pikhart, University of Hradec Kralove: Univerzita Hradec Kralove, CZECH REPUBLIC
Received: February 9, 2021; Accepted: July 20, 2021; Published: August 6, 2021
Copyright: © 2021 Lampert et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This literature review was funded by the Fundação para a Ciência e a Tecnologia and by the Empresa Municipal de Ambiente de Cascais through a doctoral grant to the first author of this paper (PDE/BDE/122672/2016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: We declare that the Author OS is the CEO of the company Unbreakable Idea Research, which is a for-profit organization in the areas of health services, health-related training and applied health research. This affiliation does not alter our adherence to PLOS ONE policies on sharing data and materials. We also declare that the company does not have any direct benefit from the involvement of its CEO in this project.
The global burden of mental illness is considerable, and it encompasses individual, family, social and economic impacts [ 1 ]. At the individual level, people suffering from (transient or chronic) mental illness also experience impaired quality of life characterized by distress-related feelings, lack of control, low self-esteem and confidence, among others [ 2 , 3 ]. This condition strongly affects their everyday living [ 4 ], including their social interactions [ 5 ] and performance at the workplace [ 6 ]. Moreover, stigma and discrimination towards people with mental illness still prevails. with negative consequences for those mentally ill [ 7 ], who might refrain from seeking professional help [ 8 ].
A recently published literature review concluded that the global burden of mental illness in terms of years lived with disabilities (YLDs) has been underestimated, and placed mental illness at the top of the list accounting for 32.4% of YLDs [ 1 ]. Concerning disability-adjusted life-years (DALYs), mental illness is at the same level as cardiovascular and circulatory diseases, accounting for 13.0% of DALYs [ 1 ]. These pictures call for action against the high burden of mental illness and gain urgency in the context of the current COVID-19 pandemic. The available literature addressing the impact of COVID-19 on mental health supports psychological suffering (e.g., anxiety, depression, post-traumatic disorder, psychological distress) from lockdowns, social distancing measures, being diagnosed with COVID-19 or being a health professional working at the frontline [ 9 – 11 ]. Now more than ever before, mental health promotion should be the main avenue to tackle the burden of mental illness.
Human contact with nature has been highly valued in health promotion over the last years. As such, there has been a growing interest on the health benefits from greenspace exposure, i.e., parks, gardens and forests, with evidence in favour of positive health outcomes (e.g. [ 12 – 17 ]). Interestingly, some authors argue that the mental health benefits arising from the contact with nature should embody the list of services provided by the natural ecosystems [ 13 ], which include crop pollination and climate regulation, among others. Empirical evidence supports the beneficial influence of greenspace exposure on several health outcomes. These include physical and general health [ 18 ]; disease prevention [ 19 – 21 ]; restoration of the individuals’ psychological resources by providing them with an environment free from physical and social stressors [ 22 ]; and improvement of the cognitive function, including memory, attention, concentration and impulse inhibition [ 23 ].
Contact with nature in urban areas is challenging, because outdoor greenspaces are much reduced compared to non-urban, rural areas. Cox et al. (2017) investigated which natural characteristics of selected neighbourhoods in British urban areas contributed the most for mental health gains of the nearby residents. These authors concluded that vegetation cover and the abundance of birds in the afternoon were the most relevant factors contributing for mental health benefits measured as decreased prevalence of depression, anxiety, and stress. Another study concluded that the prevalence of mental health conditions can be reduced if minimum values of vegetation cover are maintained [ 20 ]. Thus, green spaces can also function as a promotion strategy for mental health [ 24 ]. These findings are highly relevant to inform strategic public health interventions and support urban planning solutions that ease the interaction between city dwellers and nature [ 25 ].
In 2019, approximately 57% of the world population lived in cities [ 26 ] and spent the great majority of the time indoors (e.g., at home, school, workplace); pre-COVID-19 pandemic estimates pointed out that humans spend, on average, 85–90% of their time indoors [ 27 ]. Then, the great challenge is to integrate nature within the urban infrastructure. One avenue to tackle this issue is by promoting citizens’ participation in community gardens [ 28 ]. Community gardens are also known as urban gardens, allotment gardens, allotments, community agriculture, agricultural allotments, roof top gardens, roof top agriculture, roof top farms, all these terms referring to a greenspace located in an urban area, where community residents mainly grow vegetables for their own consumption, although border flower beds are also commonly grown, while profiting from it in the company of other members from the neighbourhood and/or their family with no imposed frequency schedule [ 29 ]. Community gardens serve various relevant functions at multiple levels. At the environmental level, they can add to climate change mitigation by sequestrating atmospheric carbon, thus contributing for reducing the amount of greenhouse gases [ 30 ]. As previously mentioned, community gardens are also considered a sustainable way to improve the quality of life of city dwellers [ 31 , 32 ], namely by providing citizens with the opportunity to be in close contact with nature [ 33 , 34 ] while supporting healthy lifestyles [ 35 ].
Horticultural therapy, i.e., the engagement of individuals in horticultural activities with live plants to improve their health and well-being [ 36 ], has produced health benefits on people with various mental health conditions in different settings (e.g., [ 37 – 39 ]. However, less is known about the mental health outcomes for non-clinical populations engaging in gardening activities. A study carried out in The Netherlands provided support for a positive effect of gardening activities on relief from acute stress [ 40 ]. In another study, community gardeners were induced some stress and randomly assigned to a 30-min outdoors gardening session or indoors reading. The levels of stress measured as salivary cortisol and self-reported positive mood were significantly lower in those assigned to gardening activities versus the reading group [ 40 ]. There is also some evidence that engaging in community gardening improves well-being by encouraging healthy behaviours, such as physical activity [ 41 ] and the consumption of locally grown healthy foods [ 42 , 43 ]. Moreover, a qualitative study conducted in the United States pointed out that gardening is considered a moderate intensity activity that can provide older adults with the health benefits of regular moderate intensity physical activity [ 44 ]. On the other hand, some body positions during gardening can be uncomfortable or even cause pain when the target audience is the elderly [ 44 ].
Despite increased attention that community gardening has received in recent years, most available evidence on health and well-being promotion comes from qualitative studies [ 45 , 46 ]. As such, this study aims to review quantitative evidence about physical and mental health outcomes of community gardening. More specifically, this literature review addresses two main questions. First, is there evidence, from quantitative studies, that community gardening contributes to increased physical and mental health and well-being of non-institutionalized individuals? Second, does community gardening provokes any discomfort in terms of physical health, i.e., bodily pain, to their users? To answer these questions, a systematic literature review following PRISMA guidelines [ 47 ] was conducted.
Search strategy and inclusion criteria
A systematic literature review was performed following PRISMA guidelines [ 47 ] through a search of studies contained in PubMed, Scopus and Web of Science electronic databases with no restrictions concerning publication date (PRISMA Checklist is provided as S1 Checklist ). The search was conducted on July 2–4, 2019, and updated on November 17–19, 2020, by using a pairwise combination of two blocks of both free-text and medical subject headings (MeSH) terms. The search strategy followed for PubMed is provided as S1 File . The following keywords were used as alternatives: (“Community garden*” OR “Urban garden*” OR “Allotment garden*” OR Allotment OR “Community agriculture” OR “Agricultural allotment” OR “Roof*top garden*” OR “Roof*top agriculture” OR “Roof*top farm*”) AND (“Mental health” OR “Quality of life” OR *happiness OR “Well*being” OR “Life satisfaction” OR “Satisfaction with life” OR “Psychological well*being” OR “Subjective well*being” OR Depression OR Anxiety OR Dysthymia OR Loneliness OR “Musculoskeletal injur*” OR “Musculoskeletal condition*” OR “Osteo*articular injur*” OR “Osteo*articular disease*”).
Citations retrieved were downloaded, duplicates were removed, titles and abstracts were independently screened for eligibility by two authors of this review (TL and JC). In case of disagreement, a third researcher (OS) independently assessed the article for eligibility. Articles were assessed for eligibility based on the following criteria: a) empirical cross-sectional quantitative studies; b) community-based studies; c) data on subjective or psychological well-being and/or physical well-being reported in the study; d) the gardens referred to in the studies were exclusively community gardens; and e) full texts available in English. Documents reporting data from studies conducted in home gardens, also referred to as household gardens, as well as qualitative studies, literature reviews and grey literature were excluded.
Data extraction and analysis
Data were independently extracted by two authors of this review (TL and JC) into a standardized table, and a third researcher (OS) checked data for consensus. Data extracted from each article were as follows: authors, year of publication, title of the paper, country of data collection, setting (rural versus urban), target population, sample size of the participants, sample size of gardens, inclusion criteria, exclusion criteria, characteristics of the gardens (e.g., area, number of plots), motivation(s) for selecting those gardens, health outcomes under study (i.e., subjective or psychological well-being and/or physical well-being), instruments of data collection, main conclusions, and direction of the association between community gardening and health outcomes.
Quality assessment
The quality of the evidence was appraised using the tool by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) for Observational Cohort and Cross-Sectional Studies [ 48 ]. This was done independently by two authors of the paper (TL and JC); in case of disagreement, an independent evaluation was made by a third researcher (OS).
Fig 1 depicts the selection process of articles included in this systematic literature review. Eight articles were considered eligible from the initial list of 262 potentially relevant titles. Main methodological characteristics of the articles are summarized in Table 1 . Studies included in this literature review were conducted in the United States of America (n = 3), United Kingdom (n = 1), the Netherlands (n = 1), Japan (n = 1), Singapore (n = 1), and Portugal (n = 1). All studies were conducted in an urban setting and had a cross-sectional design; one of them used a mixed-methods approach by combining cross-sectional quantitative data collection and qualitative semi-structured interviews [ 49 ]. Target population was composed of adult gardeners and non-gardeners residing in the cities where the studies were carried out; one study targeted Bhutanese refugees living in the United States [ 49 ]. In all studies, outcomes of interest were compared between gardeners and non-gardeners. With regard to inclusion and exclusion criteria, these were generally not provided in the articles, with three exceptions in which specific inclusion criteria for the target population were defined: a) individuals aged 50+ years [ 50 ], b) Nepali Bhutanese Refugees [ 49 ], and c) gardeners from the urban organic allotment garden at Devesa Park, Portugal [ 51 ]. Only the study carried out in Singapore referred to exclusion criteria: participants who did not complete the survey; individuals under the age of 18 and over the age of 100; and residents who engaged in physical activities outdoors, alone and not in a group, were excluded from the study [ 52 ]. The number of community gardens analyzed in the studies ranged from 1 to 64; however, not all studies reported this information. Community gardens were variable in terms of their characteristics, including size and facilities offered to gardeners. Detailed information regarding garden characteristics was generally not provided in the papers ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0255621.g001
https://doi.org/10.1371/journal.pone.0255621.t001
Characterization of the participants
Characterization of the participants (gardeners and non-gardeners) and data on the association between gardening and mental and physical well-being are provided in Table 2 . The sample size of community gardeners ranged from 16 [ 53 ] to 165 [ 19 ], whereas the number of participants enrolled in the studies who were not engaged in gardening activities ranged from 28 [ 49 , 52 ] to 167 [ 19 ]. One study considered two groups of participants, i.e., regular and occasional gardeners, based on the frequency they engaged in gardening activities [ 53 ]. Two studies also included a group of people who performed their gardening activities within their home gardens [ 50 , 52 ]. With regard to non-gardeners, one study addressed community gardening and other leisure activities for stress reduction, and the latter group included home gardeners, walkers and people who engaged in physical activity indoors [ 50 ].
https://doi.org/10.1371/journal.pone.0255621.t002
No study targeted only men or women, though gender representation within groups (gardeners versus non-gardeners) was highly variable among studies ( Table 2 ). Only two studies indicated the range of participants’ age: 50+ years old [ 50 ] and between 18 and 100 years old [ 52 ]. The remaining studies provided the average age of the participants, usually above 40 years old for both gardeners and non-gardeners ( Table 2 ).
Community gardens and mental and physical well-being
Studies included in this literature review addressed two types of outcomes: physical and mental health and well-being. These were measured by asking participants to fill in specific questionnaires ( Table 2 ). All studies assessed mental health and well-being, whereas physical health and well-being was covered in five out of the eight studies. Regarding physical health and well-being, respondents were generally asked to rate their general health status [ 19 , 40 , 49 – 51 , 53 , 54 ]. In one study, they were also asked about chronic conditions [ 40 ]. No study investigated musculoskeletal or osteoarticular injuries related to community gardening. Concerning mental health and well-being, gardeners and non-gardeners where asked about life satisfaction [ 40 , 51 , 53 , 54 ], perceived stress [ 40 , 50 , 52 ], anxiety symptoms [ 49 ], depression symptoms [ 49 ], perceived social support [ 49 , 50 ], health-related quality of life [ 50 ], and social contacts [ 19 , 40 ]. The study conducted in Singapore also assessed connection with nature, resilience, subjective well-being, self-esteem, optimism and openness [ 52 ]. One study targeted Bhutanese refugees living in the USA and asked participants about posttraumatic stress and adjustment to the new country [ 49 ].
According to our quality assessment criteria, seven studies included in this literature review were rated as “good” and only one scored “fair” ( Fig 2 ). Regarding the article scored as "fair", its results pointed out to a positive association between community gardens and physical and mental well-being [ 51 ]. Overall, a positive association between engaging in community gardening and physical and mental health and well-being was found in all studies included in this literature. A study addressing the mental health outcomes of community gardening among Nepali Bhutanese refugees living in the United States found perceived social support to be higher among gardeners than non-gardeners. However, no significant effect of community gardening on symptoms of depression, anxiety, somatic complains and adjustment to life in a new country was detected among these participants [ 49 ].
https://doi.org/10.1371/journal.pone.0255621.g002
Main findings
In this study, quantitative evidence on physical and mental health outcomes arising from engaging in community gardening was reviewed. Despite only eight studies met our inclusion criteria, their conclusions support the association between community gardening and positive physical (general health) and mental health (life satisfaction, happiness, mental health and social cohesion) outcomes among non-institutionalized individuals. No data about physical injuries (i.e., osteoarticular and/or musculoskeletal injuries) associated with engaging in community gardening activities were retrieved in the literature search.
Positive health outcomes associated to community gardening activity
Overall, results here in provide evidence on the association between community gardening and positive health outcomes, irrespectively of participants’ gender, age, ethnicity, and country of residence. With regard to physical health, gardeners perceived their general health status to be better than community dwellers not involved in gardening activities [ 19 , 40 ]. This might be due to the influence of gardening in health behaviors, namely regular physical activity [ 55 ], which is associated to a risk reduction for chronic conditions, such as cardiovascular disease, hypertension, cancer, obesity, but also to a reduction in the risk of premature death [ 56 ]. Indeed, gardening is considered to be a moderate intensity activity [ 41 , 57 ], involving low to moderate intensity tasks [ 58 ] that proved sufficient for older adults to meet the recommendations on 30 minutes moderate intensity physical activity sessions, five (or more) days a week, if regularly undertaken [ 59 ]. Interestingly, one study included in this literature review reported differences in health outcomes between gardeners and non-gardeners only for those aged 62+ years—gardeners scored significantly better than non-gardeners, whereas no statistically significant differences were detected between younger gardeners and non-gardeners [ 40 ]. In a world getting older and characterized by an inverted age pyramid [ 60 ], community gardening seems a promising avenue to tackle age-related disability and promote healthy aging [ 61 ].
Apart from likely influencing health behaviors through increased physical activity, community gardening potentially impacts diet via increased consumption of fruit and vegetables [ 29 , 62 , 63 ]. Four studies included in this literature review provided data on the frequency of fruit and/or vegetable intake, which was higher for gardeners compared to non-gardeners [ 19 , 40 , 53 , 54 ]. Moreover, growing vegetables for own consumption rated second concerning the motivations of Japanese community dwellers to engage in community gardening [ 19 ]. By successfully improving nutrition, community gardens not only contribute to reduce the risk of chronic diseases, such as cardiovascular disease and some cancers [ 64 ], but are also highly relevant to reduce inequalities in urban food systems [ 65 ]. As such, there has been growing interest in the role of these green spaces to increase access to nutritious food in the so-called ‘food deserts’, i.e., areas with limited access to affordable and nutritious food [ 29 , 66 , 67 ]. Evidence available from Rockford, Illinois, shows that community gardens also encompass diet benefits for non-gardeners because these individuals also have increased access to fruit and vegetables via shared production surplus from individual plots [ 66 , 68 ]. Moreover, production from the cultivation of communal plots by volunteers engaged in local neighbourhood networks is also donated to social service organisations and deprived families, thus contributing to increase their access to nutritious food and reduce food inequalities [ 66 ].
All studies included in this literature review support a positive association between community gardening and mental health and well-being among non-institutionalized individuals. Overall, gardeners reported higher levels of life satisfaction [ 40 , 51 , 54 ], less perceived stress [ 40 , 50 ], increased perceived social support [ 49 ] and social contacts [ 19 , 40 ] than non-gardeners. Interestingly, perceived stress and social contacts were moderated by age among Dutch gardeners: community dwellers aged 62+ years engaged in gardening activities reported significantly lower stress levels and increased social contacts than non-gardeners (same age range), whereas no differences were found between younger gardeners and non-gardeners (62+ years) [ 40 ]. This finding is highly relevant under the context of healthy aging. As people age, their social network becomes narrower due to the combined effects of their reliance on stable and close relationships plus a decline in the establishment of new relationships [ 69 ]. As such, increased social contact by active participation in activities within the local neighbourhood, such as community gardening, has the potential to reduce loneliness feelings and increase mental health and well-being of older adults, although not restricted to this age group [ 30 , 61 , 70 ]. Community gardens provide a place for individuals to interact with other gardeners, neighbours, friends and family, thus contributing for broadening and strengthening of individual social networks, sometimes promoting intergenerational contacts [ 71 ] and social cohesion [ 72 ]. This encompasses positive impacts for mental health and well-being [ 73 ], in particular for vulnerable populations, such as older people [ 74 ] as previously considered. One study included in this literature review addressed the experiences of Bhutanese refugees during resettlement in the United States, by investigating and comparing several indicators of mental health and well-being between gardeners and non-gardeners [ 49 ]. Despite the two groups did not differ in levels of self-reported distress, symptoms of depression, anxiety and somatic complaints, gardeners reported significantly greater social support than non-gardeners [ 49 ]. Increased social support has been previously reported by refugees engaged in community gardening [ 75 , 76 ], although only a few studies have been conducted up to now [ 74 ]. By gathering to grow vegetables and fruits, refugees interact with individuals with the same cultural background, which allows them to maintain ties to their culture of origin, but they are also provided with the opportunity for a smoothly inclusion process in the country of arrival by interacting with natives who also gather to gardening [ 75 – 77 ]. Interestingly, no differences for self-reported social support between community gardeners and home gardeners were found in one study included in this literature review (50). Further understanding on the association between engaging in community gardening versus home gardening and self-perceived social support will benefit from future comparative studies of these two activities.
Findings from this literature review are especially relevant given the current COVID-19 pandemic situation. The rapid spread of the SARS-CoV-2 virus brought a sudden change in the routine of the world population, and the year 2020 was characterized by lockdowns in several countries, as well as social containment and restrictions to mobility. Such abrupt disruptions in everyday life might negatively impact physical and mental health and well-being [ 78 ]. During periods of social isolation, easily accessible natural environments, such as community gardens, provide an adequate environment for individuals to engage in physical activity while relaxing [ 79 , 80 ]. Outdoor green spaces in the neighbourhood where individuals can go, in a safer manner and complying with the recommendations from the health authorities, for time slots of 30–40 min everyday have an enormous potential to help build resilience and maintain physical and mental health and well-being [ 78 ]. Moreover, their role in complementing food shortages during crisis, such as during the World War II, is well known [ 81 ]. As such, community gardens potentially play a role in improving food security during the COVID-19 pandemic, which undoubtedly affected food systems [ 82 ].
Community gardening-related physical injuries
No study assessing and/or reporting community gardening-related physical injuries, namely musculoskeletal and osteoarticular injuries, was retrieved in our literature search. This finding is quite striking given the large body of evidence available in the literature concerning physical injuries associated to agricultural practices and farming (e.g., [ 83 – 86 ]). For example, a systematic literature review addressing the prevalence of musculoskeletal disorders among farmers found that low back pain was the most frequently reported musculoskeletal disorder [ 87 ]. Injuries caused by hand tools manipulation, such as finger cuts, have also been frequently reported among farmers [ 86 , 88 ]. Except for machinery, the types of hand tools used in farming and community gardening are potentially the same, e.g., shovel and sickle, which suggests that community gardeners might be exposed to the same types of injuries that farmers are. More research in this area is needed to disentangle between the physical health benefits versus potential risks of community gardening.
Community gardens: A sustainable health promotion strategy
Human development and urbanization have generated a series of environmental problems, such as overconsumption of natural resources, water and air pollution, waste production [ 89 , 90 ], and reduction of green spaces [ 89 , 91 ]. These encompass major challenges and threats to human health and environmental sustainability [ 92 – 94 ]. Community gardening has the potential to contribute to achieve gains in human health and environmental sustainability, as pointed out in a growing body of literature (e.g., [ 95 , 96 ] and also supported by results herein. By creating urban spaces where community dwellers gather to grow fruits and vegetables, public authorities are empowering the local communities and providing them with safer, enjoyable, all-inclusive settings that ease healthier choices, while fostering active participation in health and promoting the contact with nature in a sustainable manner, as envisaged in the Ottawa Charter for Health Promotion [ 97 ].
At the European level, one of the various actions under the European Green Deal, an action plan by the European Commission aimed at making the EU’s economy sustainable, is to ensure more sustainable food systems [ 98 ].To accomplish this, the creation of supportive food environments making easier to choose healthy and sustainable diets is central to achieve human health gains, thus reducing the economic burden of disease and the environmental impacts from food production [ 99 ]. This “Farm to Fork Strategy” establishes key goals to improve healthy lifestyles, health, and the environment by building a food chain that benefits both the consumer and the environment. Indeed, the recommendations under this H2020 Green Deal initiative aims at stimulating sustainable food production and processing practices; reducing the distance of the power chain between the source and the consumer; and increasing organic food production and food safety [ 99 ]. Under this context, community gardens potentially add valuable contributes to a more sustainable Europe concerning food system with focus in production and consumption.
Community gardens are an affordable and efficient, yet challenging, way to bring nature back to cities and potentially contribute to the provision of ecosystem services [ 100 ]. As green spaces, community gardens serve as a habitat for fauna and flora [ 101 ], being considered a potential reservoir of urban biodiversity [ 31 , 102 ]. They also contribute to increase the proportion of permeable soil surface [ 103 ], filtering and storing water from the rain, thus contributing for floods’ prevention (Quayle, 2008). In addition, community gardens promote environmental education in urban areas [ 31 , 100 ], offering a hands-on experience on ecological processes [ 104 ]. Thus, it is not surprising that interest in these green spaces has boomed in recent years, which often leaves community dwellers in waiting lists for a couple of years before being provided with a patch for them to cultivate [ 105 ]. Therefore, a great challenge in urban planning is now to increase the availability of these spaces. However, this cannot be done without considering the motivations that lead community dwellers to engage in community gardening [ 106 ], as well as to design and equip these green spaces with the infrastructures and tools that are needed for users to successfully profit from it [ 106 ].
Strengths and limitations
This manuscript reviews quantitative evidence from cross-sectional studies on the association between community gardens and physical and mental health and well-being of the non-institutionalized population. However, given the cross-sectional study designs no causality relations can be ascertained.
To our knowledge, musculoskeletal and osteoarticular injuries have not been previously addressed in literature reviews. Despite no data was obtained on community gardening-related injuries, this is a relevant finding and indicates that more research in this realm is needed. However, since only a few articles were retrieved and are not representative of community gardening from any specific geographic region, any conclusions and generalizations should be taken cautiously. The few articles retrieved might be due to the language filter used—only studies published in English were considered. Nevertheless, considering that the great majority of the scientific peer-reviewed journals are published in English, we are confident that this methodological option did not significantly affect our results.
Supporting information
S1 checklist. prisma 2009 checklist..
https://doi.org/10.1371/journal.pone.0255621.s001
S1 File. Strategy to search for studies that investigate the evidence that community gardening contributes to increased physical and mental health and well-being of non-institutionalized persons.
Details are provided only for one (PubMed) of the three databases surveyed (Web of Science and SCOPUS). The same search strategy was used for the remaining databases, and to meet the search requirements of each database required, some modifications were necessary in relation to the field tags.
https://doi.org/10.1371/journal.pone.0255621.s002
Acknowledgments
The Authors thank Instituto de Saúde Ambiental for providing the required support for this investigation, in particular to Dr. Ana Virgolino for logistical and administrative support, and to Professor António Vaz Carneiro, for his support and encouragement.
- View Article
- PubMed/NCBI
- Google Scholar
- 22. Hartig T, Mitchell R, Vries S De, Frumkin H. Nature and Health. Nat Heal Terry Hartig,1 Richard Mitchell,2 Sjerp Vries,3 Howard Frumkin4 1Institute Hous Urban Res Uppsala Univ SE-75120 Uppsala, Sweden; email [email protected] 2Centre Res Environ Soc an. 2014. https://doi.org/10.1146/annurev-publhealth-032013-182443
- 25. World Health Organization (WHO). Espaços Verde Urbanos. Um Manual para a Ação. 2017.
- 26. The world Bank Group. Urban population (% of total population). In: The world Bank Group. 2020 pp. 1–4.
- 27. European Commission. Indoor air pollution: new EU research reveals higher risks than previously thought. 2020 pp. 1–6.
- 32. Thompson S, Corkery L, Judd B. The Role of Community Gardens in Sustaining Healthy Communities. ISBN 978-0-646-48194-4. 2007; 161–171.
- 37. Yasukawa M. International Handbook of Occupational Therapy Interventions. Springer NY, editor. 2009.
- 48. US National Heart L and BIQAT for OC and C-SSA online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tool . Study Quality Assessment Tools. 2020 pp. 1–58.
- 56. Warburton DER, Nicol CW, Bredin SSD. Review Health benefits of physical activity: the evidence. C • March 14, 2006 • 174(6) | 801 © 2006 C Media Inc or its Licens. 2006.
- 97. Ottawa. A promoção da saúde a carta de Ottawa. World Heal Organ Ottawa Chart Heal Promot Proc First Int Conf Heal Promot Ottawa, ON, Canada, 21 Novemb 1986. 1986.
- 98. European Commission. A European Green Deal Striving to be the first climate-neutral continent. 2020.
- 99. European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the regions. A Farm to Fork Strategy for a fair, healthy and environmentally-friendly food system. 2020.
- 101. Duží B, Tóth A, Bihuňová M SR. Challenges of Urban Agriculture: Highlights on the Czech and Slovak Republic Specific. In: Vávra J, Lapka M, Cudlínová E (eds.) Current Challenges of Central Europe: Society and Environment,. 2014.
- 102. Lorraine Weller Clarke; G. Darrel Jenerette. Biodiversity and direct ecosystem service regulation in the community gardens of Los Angeles, CA. Landsc Ecol 30637–653. 2015; 637–653. https://doi.org/10.1007/s10980-014-0143-7
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The Psychological Benefits of Breastfeeding: Fostering Maternal Well-Being and Child Development
Anushree modak, vaishnavi ronghe, kavita p gomase.
- Author information
- Article notes
- Copyright and License information
Anushree Modak [email protected]
Corresponding author.
Received 2023 Aug 29; Accepted 2023 Oct 9; Collection date 2023 Oct.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The value of breastfeeding surpasses its utilitarian role in nourishing, encompassing profound psychological advantages for mothers and children. The orchestration of emotional bonds relies on the interplay of oxytocin and prolactin, fundamental hormones that underpin maternal attachment, mitigate postpartum depression, and cultivate self-confidence. Simultaneously, breastfeeding promotes infant development by fostering robust brain growth, bolstering immune defenses, and nurturing cognitive and emotional maturation - all of which are nurtured through maternal interactions. We must respond to the call for heightened advocacy of breastfeeding. This entails delivering education, easily accessible support, and creating an environment where breastfeeding is normalized. By dispelling misconceptions and eradicating stigmatization associated with breastfeeding, we can amplify awareness and empower mothers to make well-informed decisions for their newborns. These implications reverberate extensively. Enhanced maternal mental well-being and self-assurance form the bedrock of healthier family dynamics. At the same time, the dividends of cognitive, emotional, and immunological enrichment in children represent a more promising future. At a societal level, the embrace and promotion of breastfeeding cultivate an environment that places immense value on the health and happiness of both mothers and children. This journey is more profound than mere sustenance; it signifies a complex web of advantages. Elevating awareness and support for breastfeeding solidifies the global commitment to comprehensive maternal and child welfare and the flourishing of meaningful relationships.
Keywords: awareness, oxytocin, child development, maternal well-being, psychological benefits, breastfeeding
Introduction and background
Breastfeeding plays a pivotal role in ensuring the health and well-being of mothers and children. Beyond its extensively documented nutritional advantages, breastfeeding is central to establishing the foundation of robust maternal and child health. The act of breastfeeding not only supplies essential nutrients but also nurtures a profound emotional and psychological connection between the mother and her infant. Therefore, understanding the broader implications of breastfeeding for maternal well-being and child development is crucial in advancing comprehensive healthcare strategies [ 1 , 2 ].
While the physical merits of breastfeeding are firmly established, there has been a growing focus in recent years on its psychological dimensions. This comprehensive review delves deeply into the psychological facets of breastfeeding and how this intimate and nurturing practice significantly contributes to the emotional health and development of both mothers and their children. By exploring the intricate interplay between hormones, neural pathways, and emotional bonds, we aim to illuminate the substantial and multifaceted benefits beyond breastfeeding's nutritional content [ 3 ].
This review article's primary aim is to thoroughly examine the psychological advantages linked to breastfeeding, encompassing maternal well-being and child development. By synthesizing current research, we strive to spotlight the intricate mechanisms that underlie the positive influence of breastfeeding on maternal mental health, the cultivation of bonding and attachment, and its contribution to optimal child development. Moreover, we seek to underscore the importance of supportive environments, societal attitudes, and healthcare policies in fostering breastfeeding as a holistic approach that enhances the overall health and happiness of mothers and their children.
Psychological benefits of breastfeeding for mothers
Release of Oxytocin and Bonding With the Baby
The release of oxytocin, often called the "bonding hormone," represents one of the most profound psychological benefits of breastfeeding. Oxytocin is a neurochemical released during breastfeeding and skin-to-skin contact, playing a pivotal role in fostering a deep emotional connection between the mother and her baby. This hormone is instrumental in promoting love, trust, and attachment, forming the cornerstone of a secure and nurturing relationship between mother and child. As mothers breastfeed, they engage in moments of close physical proximity and intimacy, contributing to the establishment of a robust maternal-infant bond. Research indicates that oxytocin's influence on bonding enhances the mother's and child's overall emotional well-being [ 4 ].
Reduction of Postpartum Depression and Anxiety
Breastfeeding's positive impact on maternal mental health is noteworthy, with a demonstrated reduction in the risk of postpartum depression and anxiety. The release of oxytocin during breastfeeding strengthens the maternal-infant bond and aids in regulating stress and mood. Breastfeeding promotes focused attention and relaxation, akin to a meditative experience, which can effectively alleviate sadness and anxiety. Additionally, the structured routine established through breastfeeding can contribute to a sense of predictability and stability, essential components in mitigating postpartum emotional challenges [ 5 ].
Increased Self-Esteem and Maternal Confidence
Breastfeeding often leads to an increase in self-esteem and maternal confidence. Directly providing nourishment and sustenance from a mother's body generates a profound sense of accomplishment and capability. Conquering the obstacles associated with breastfeeding, such as perfecting latch techniques and ensuring an adequate milk supply, cultivates a newfound sense of mastery and self-assuredness. Significantly, this elevated confidence level extends beyond breastfeeding, positively influencing mothers' perceptions of their parenting capabilities [ 6 ].
Sense of Accomplishment and Empowerment
Breastfeeding is a fundamental and intrinsic facet of motherhood, giving women a sense of accomplishment and empowerment as they nourish their infants in this distinct manner. The ability to sustain and nurture a growing baby through their bodies reinforces a profound connection to the elemental roles of motherhood across human history. This shared human experience fosters a sense of purpose and empowerment, equipping mothers with the ability to navigate the challenges of early parenthood while experiencing a heightened sense of fulfillment [ 2 ].
Child developmental benefits of breastfeeding
Nutritional Advantages for Optimal Brain Development
Breast milk as a reservoir of essential nutrients: breast milk is often called "liquid gold" due to its incredible composition of essential nutrients tailored to meet an infant's growth and development needs. It contains many vitamins, minerals, proteins, fats, and carbohydrates that support the baby's overall health, significantly contributing to brain development [ 2 ].
Docosahexaenoic acid (DHA) and arachidonic acid (ARA): two crucial components found in breast milk are DHA and ARA. These are omega-3 and omega-6 fatty acids, respectively, that play a pivotal role in cognitive growth and visual acuity. DHA, in particular, is a major structural component of the brain and the retina of the eyes. It is crucial for the development and maintenance of neural pathways, synapses, and cell membranes in the brain, thereby influencing learning, memory, and overall cognitive function. ARA also contributes to brain and nervous system development and supports various cellular processes [ 2 ].
Building blocks for brain tissue: both DHA and ARA act as building blocks for brain tissue. During the rapid growth phase of infancy and early childhood, the brain undergoes significant structural and functional development. These fatty acids form new neural connections and intricate pathways that enable the brain to process information, make decisions, and engage in problem-solving.
Cognitive growth and visual acuity: cognitive growth refers to the development of various cognitive abilities, including language, attention, memory, and problem-solving skills. Visual acuity, on the other hand, relates to the sharpness and clarity of vision. The presence of DHA and ARA in breast milk helps ensure that the infant's brain and visual system develop optimally, setting the stage for a child's ability to perceive the world, understand it, and interact with it effectively [ 3 - 4 ].
Support for learning and problem-solving: the neural pathways established during early brain development lay the foundation for a child's capacity to learn, retain information, and engage in effective problem-solving throughout life. Adequate intake of DHA and ARA during infancy through breastfeeding can contribute to enhanced cognitive abilities, which can have a lasting impact on a child's academic performance, social interactions, and overall success [ 5 ].
Robust nutritional bedrock: breast milk's unique composition provides infants with a robust nutritional foundation that supports their growth and development during the critical early years of life. While formula milk attempts to replicate some of these nutritional components, breast milk remains the gold standard due to its tailored and dynamic nature that adapts to the growing baby's changing needs [ 6 - 7 ].
Role of Breast Milk in Building a Strong Immune System
Breast milk is a remarkable substance and its benefits go beyond mere nutrition. It is a complex fluid that provides many immunological benefits crucial for developing a strong immune system in infants. This is due to breast milk's multifaceted immune-boosting attributes, which actively contribute to fortifying the infant's immune system. A newborn's immune system is not fully developed, making them particularly susceptible to various infections and illnesses. However, nature has equipped mothers with a powerful tool to help their infants combat these challenges: breast milk. Breast milk is a dynamic source of passive immunity, transferring various immunological factors from mother to child. These factors are crucial in bolstering the infant's immunity during their initial months of life [ 6 ].
One of the key components transmitted through breast milk is antibodies. Antibodies are proteins the mother's immune system produces in response to infections or vaccinations. When these antibodies are passed to the infant through breast milk, they offer protection against various pathogens. This early protection can be vital in preventing severe infections and reducing the severity of illnesses. White blood cells, another essential immune system element, are also present in breast milk. These cells are fundamental in detecting and fighting off harmful microorganisms. By providing these cells through breast milk, mothers are effectively arming their infants with an added layer of defense against potential threats [ 7 ].
Beyond antibodies and white blood cells, breast milk contains a diverse array of bioactive compounds. These compounds include cytokines, enzymes, growth factors, and other molecules in immune modulation, inflammation regulation, and tissue development. Collectively, these compounds contribute to the overall immune-boosting properties of breast milk. The process of breastfeeding itself offers more than just immunological benefits. Skin-to-skin contact during breastfeeding, for instance, promotes the transfer of beneficial bacteria from the mother to the infant, thereby shaping the infant's developing gut microbiota. A balanced gut microbiome is increasingly recognized as critical in immune system development and overall health. The culmination of all these immune-boosting attributes results in breastfed infants having a significant advantage in immune protection. Breast milk not only helps prevent infections but also contributes to the overall health and well-being of the infant. Studies have shown that breastfed infants experience fewer respiratory infections, gastrointestinal illnesses, and allergies than formula-fed infants [ 8 ].
Cognitive and Emotional Development Through Maternal Interaction
The cognitive and emotional development process in infants is a complex and fascinating journey, heavily influenced by the interactions between mothers and their children. One particularly significant aspect of this development is the act of breastfeeding, which serves as a dynamic platform for nurturing cognitive and emotional growth. Breastfeeding is not just about providing essential nutrition; it is a multifaceted experience beyond transferring nutrients. It is a time when the mother and child come together uniquely and intimately. The quoted text beautifully describes this experience as a "symphony" orchestrated by various elements [ 3 ].
Physical closeness: breastfeeding creates a close physical bond between the mother and the infant. The infant feels the warmth and comfort of the mother's body, enhancing feelings of security and closeness. This physical proximity triggers a cascade of physiological responses contributing to emotional well-being [ 4 ].
Direct eye contact: mothers and infants often use direct eye contact during breastfeeding. This visual exchange is crucial not only for ensuring proper latching but also for promoting emotional connection. Eye contact is a powerful means of conveying affection, love, and attention. This interaction lays the foundation for developing trust and emotional reciprocity [ 5 ].
Skin-to-skin touch: the gentle skin-to-skin touch that accompanies breastfeeding has numerous benefits. It regulates the infant's body temperature, heart rate, and breathing. But beyond these physiological effects, touch is a primal form of communication. It fosters a sense of security, soothing the infant and creating positive associations with feeding [ 6 ].
All these elements of maternal interaction during breastfeeding trigger the release of hormones such as oxytocin and prolactin. Oxytocin, often called the "love hormone," enhances social bonding, trust, and emotional attachment. Prolactin supports milk production and is associated with feelings of nurturing and maternal care. These hormones, acting as "architects," as described in the text, are crucial in shaping the infant's brain development and emotional balance [ 7 ].
Long-Term Effects on Reducing the Risk of Chronic Diseases
Nutritional content: breast milk is uniquely tailored to meet an infant's nutritional needs. It contains a balanced combination of essential nutrients, vitamins, and minerals that contribute to the healthy growth and development of the child. These early nutritional experiences could potentially influence metabolic processes and set the foundation for better health in the long term [ 8 ].
Immunological factors: breast milk contains many immunological components such as antibodies, white blood cells, and enzymes that help bolster the infant's immune system. This enhanced immune response during infancy might improve immune function, potentially reducing the risk of chronic inflammatory conditions linked to diseases like obesity and cardiovascular issues [ 8 ].
Metabolic programming: early life experiences, including nutrition during infancy, can profoundly impact metabolic programming. Breastfeeding might influence gene expression, hormonal regulation, and metabolic pathways, affecting how the body processes energy and manages metabolic functions later in life. This programming could play a role in reducing the risk of obesity and related conditions [ 8 - 9 ].
Microbiota and gut health: breast milk contributes to the establishment of a healthy gut microbiota in infants. A balanced gut microbiome is increasingly recognized as crucial for overall health, including metabolic and immune function. Through breastfeeding, positive effects on gut health might contribute to long-term health benefits [ 9 ].
Bonding and attachment
Skin-to-Skin Contact and Its Impact on Mother-Infant Attachment
Skin-to-skin contact holds remarkable significance in shaping the attachment between mothers and infants. This intimate practice, often intertwined with breastfeeding, wields the capacity to initiate a profound emotional bond. Through skin-to-skin moments, the physical closeness triggers the release of oxytocin, commonly known as the "bonding hormone." This biological response elicits comfort, security, and a profound connection. A critical outcome of such interactions is the amplification of the mother's receptiveness to her baby's cues and requirements. The physical closeness and direct skin contact facilitate a heightened awareness of the infant's nonverbal signals, fostering an intuitive understanding of their needs. This sensitized responsiveness becomes pivotal in building trust and emotional intimacy as the mother learns to promptly interpret and meet her baby's needs [ 10 ].
Beyond its emotional dimensions, skin-to-skin contact assumes a practical role in facilitating successful breastfeeding. The proximity during these moments can trigger the baby's natural feeding reflexes, often leading to a smoother latch and more effective breastfeeding sessions. This supports the baby's nutritional needs and bolsters the emotional bond between mother and child. The enduring impact of skin-to-skin contact is the establishment of a robust and enduring mother-infant bond. The foundation of trust and intimacy cultivated through these interactions is a cornerstone for secure attachment. Research has shown that skin-to-skin practices in the early postpartum can contribute to more positive developmental outcomes, including enhanced cognitive and emotional growth [ 10 ].
Development of Secure Attachment and Emotional Regulation
Secure attachment: secure attachment refers to the emotional connection and bond between an infant and their primary caregiver, usually the mother. This attachment is characterized by the child feeling safe, loved, and confident in the caregiver's presence. Securely attached infants actively explore their environment and develop healthy social and emotional skills. They have a foundation of trust that allows them to seek comfort and support from their caregiver when needed. Breastfeeding contributes to the development of secure attachment through consistent and responsive interactions between the caregiver and the infant. When a mother breastfeeds, she holds the baby close, providing physical closeness, eye contact, and skin-to-skin contact. These interactions foster a sense of intimacy and connection between the caregiver and the infant, helping the infant build a strong emotional bond [ 9 ]. The act of breastfeeding also involves the release of hormones like oxytocin, often referred to as the "love hormone." Oxytocin promotes feelings of warmth and connection between the mother and the baby. These positive interactions and emotional experiences create a foundation for the infant's understanding of relationships, forming the basis for future social interactions [ 10 ].
Emotional regulation: emotional regulation refers to managing and controlling one's emotions in response to different situations. It is an essential skill for overall emotional well-being and healthy social interactions. Infants are born with limited emotional regulation abilities and rely heavily on their caregivers to help them regulate their emotions [ 10 ]. Breastfeeding plays a role in developing emotional regulation skills by providing a source of comfort and soothing during times of distress. When a baby is breastfed, they experience physical closeness, warmth, and nourishment, which can help reduce stress and anxiety. Sucking during breastfeeding also has a calming effect on the baby's nervous system. Through consistent breastfeeding interactions, infants learn that their caregiver is a reliable source of comfort and support. This understanding fosters a sense of security, which allows the infant to develop the ability to self-soothe and regulate their emotions gradually. Over time, the infant begins to internalize these experiences and strategies, contributing to their growing emotional resilience and self-regulation skills [ 11 ].
Role of Breastfeeding in Promoting Responsive Parenting
The role of breastfeeding in promoting responsive parenting is multifaceted and significant. Breastfeeding goes beyond being a means of providing essential nutrition; it plays a crucial role in fostering a nurturing and attentive caregiving approach that benefits both the mother and the infant. This process involves a deep connection between the physical act of breastfeeding, the emotional bonding it facilitates, and establishing a responsive parenting style [ 9 ].
Recognition of infant's needs: breastfeeding requires frequent and intimate interactions between the mother and the infant. Nursing encourages the mother to be consistently present and attuned to her baby's cues. As she spends time feeding and nourishing her baby, she becomes more adept at recognizing subtle signs and signals that indicate the infant's needs - hunger, discomfort, or a need for comfort. This heightened awareness forms the basis of responsive parenting, where the mother is better equipped to promptly address her baby's needs [ 10 ].
Enhanced bonding and attachment: the physical closeness that breastfeeding entails, including skin-to-skin contact, triggers the release of oxytocin - often referred to as the "love hormone" or "bonding hormone." Oxytocin fosters emotional connection, trust, and attachment between the mother and the infant. This bond is crucial for developing a secure and healthy attachment-based relationship. This secure attachment is a foundation for the child's emotional and psychological development, influencing their future relationships and well-being [ 11 ].
Modeling effective communication: breastfeeding establishes a communication dynamic between the mother and the infant. During nursing sessions, the baby's cues are met with the mother's timely responses - whether through feeding, soothing, or comforting. This interaction models a form of communication in which the baby's nonverbal cues are acknowledged and appropriately addressed. This experience of being understood and responded to lays the groundwork for effective communication and empathy in the child's later interactions [ 12 ].
Emotional regulation: responsive parenting nurtures emotional regulation in infants. When a mother consistently responds to her baby's needs, the infant learns that their emotions and distress will be addressed. This sense of security and predictability fosters emotional regulation, enabling the child to learn how to manage their emotions healthily. This skill is vital for their well-being and social development [ 13 ].
Nurturing a caregiving approach: breastfeeding mothers often adopt a caregiving approach characterized by sensitivity, warmth, and attentiveness. The act of nurturing through breastfeeding extends to other aspects of caregiving, where mothers are more likely to respond to their baby's signals promptly and empathetically. This approach creates a nurturing environment where the baby feels valued and understood [ 14 ].
Hormonal and neurological influences
Oxytocin's Role in Fostering Maternal Caregiving and Bonding
Physiological aspects: oxytocin is primarily known for stimulating uterine contractions during labor and promoting the milk ejection reflex during breastfeeding. These physiological effects are crucial for childbirth and maternal care. When a baby suckles at the mother's breast, sensory receptors in the nipple are stimulated, releasing oxytocin from the brain's hypothalamus. This release causes the milk to flow through the ducts, allowing the baby to feed [ 14 ].
Emotional aspects: beyond its physiological effects, oxytocin has profound emotional implications as well. It has been dubbed the "love hormone" or "bonding hormone" because of its involvement in social and emotional behaviors. Oxytocin is released during positive social interactions, such as hugging, touching, and intimate moments. It plays a role in creating feelings of attachment, trust, and emotional closeness. In maternal caregiving, oxytocin fosters a strong emotional bond between the mother and her baby [ 14 ].
Maternal caregiving and bonding: oxytocin's influence on maternal caregiving is remarkable. When oxytocin is released during breastfeeding, it not only aids in milk ejection but also has a calming and soothing effect on the mother. This can create a positive feedback loop: the mother's relaxation releases more oxytocin, reinforcing her positive emotional state. This cycle can significantly contribute to the mother's feelings of warmth and affection toward her baby [ 14 ].
Attachment and trust: oxytocin is closely tied to developing a secure attachment between a mother and her baby. Attachment refers to the emotional bond that forms between an infant and their primary caregiver. Oxytocin encourages the mother to respond sensitively to her baby's cues and needs. When the mother consistently meets the baby's needs and responds with affection and care, the baby develops a sense of security and trust. This lays the foundation for healthy emotional and social development [ 14 ].
Empathy and emotional regulation: oxytocin also promotes empathy, which is essential for understanding and responding to the emotions of others. This is crucial in caregiving, as it helps the mother attune to her baby's emotional state. Additionally, oxytocin is linked to emotional regulation, helping the mother and the baby manage stress and anxiety. This regulation is vital for maintaining a nurturing and secure environment for the baby's growth [ 14 ].
Prolactin and Its Effects on Maternal Behavior and Nurturing Instincts
Prolactin is a hormone produced by the pituitary gland, a small gland located at the base of the brain. Its primary role is to stimulate and regulate milk production in the mammary glands of mammals, including humans. During pregnancy, prolactin levels rise, preparing the breasts for milk production. After childbirth, when a mother starts breastfeeding, prolactin levels experience a surge. This surge in prolactin is essential for initiating and maintaining lactation - the process of producing and releasing milk to nourish the newborn [ 15 ].
Broader effects on maternal behavior: while prolactin's primary function is related to milk production, it also has a broader range of effects on maternal behavior and emotions. This hormone has been found to influence various aspects of a mother's behavior and psychology, contributing to her ability to nurture and care for her baby. Some of the notable effects of prolactin are described below.
Elevated prolactin levels during breastfeeding encompass a range of effects that profoundly influence maternal behavior and nurturing instincts. These effects extend beyond the hormone's primary role in milk production, encompassing emotional and behavioral dimensions that enhance the mother-child relationship. Mothers experience a dual benefit of relaxation and contentment due to increased prolactin levels. This tranquil state creates an ideal setting for bonding and caregiving as mothers become more attuned to their baby's cues and needs, establishing a strong emotional connection [ 15 ].
Simultaneously, prolactin enhances a mother's focus on caregiving tasks. This heightened attentiveness enables swift responses to the baby's demands - from feeding to providing comfort. The augmented dedication to these tasks fosters a nurturing environment vital for the infant's holistic development. Prolactin's role in activating and amplifying nurturing instincts is crucial. These instincts drive behaviors that promote bonding, including a heightened preference for physical closeness. Holding, cuddling, and skin-to-skin contact become exceptionally rewarding and essential for both mother and baby under the influence of prolactin. This hormone also cultivates more attentive interactions. Mothers with elevated prolactin levels exhibit a remarkable sensitivity to their baby's cues, expressions, and vocalizations, allowing them to deliver well-timed and appropriate care [ 15 ].
Impact of Breastfeeding on Stress Reduction for Both Mother and Child
Breastfeeding yields a tranquilizing influence that benefits both the mother and the child, leading to stress reduction through a range of mechanisms. In the mother's case, breastfeeding initiates the release of oxytocin, a hormone that counteracts the impact of stress hormones such as cortisol. This hormonal reaction fosters a state of relaxation and overall well-being for the mother. Conversely, breastfeeding serves as a source of solace and pacification for the infant through skin-to-skin contact, rhythmic suckling, and the sensory experience of breast milk's taste and aroma. These pacifying effects are crucial in regulating the baby's stress response and cultivating a sense of security and ease [ 16 ].
Psychological well-being of mothers
Positive Effects on Maternal Mental Health and Overall Happiness
Breastfeeding has many beneficial impacts on maternal mental health and overall well-being. The secretion of hormones such as oxytocin and prolactin during breastfeeding plays a pivotal role in inducing sensations of calmness, joy, and emotional harmony. These hormones act as natural stress mitigators and facilitators of a positive emotional state. Furthermore, breastfeeding cultivates a sense of accomplishment and contentment, elevating a mother's self-regard and contentment with her role as a nurturer. Breastfeeding engenders a profound sense of fulfillment, bolstering the mother's self-esteem and fostering a deep satisfaction with her caregiving responsibilities. The intimate and nurturing moments shared during breastfeeding can forge enduring positive memories, contributing to a mother's happiness [ 17 ]. The tender bonds formed in these moments nurture the child and serve as sources of profound joy and contentment for the mother, enriching her emotional experience and overall well-being.
Promotion of Body Positivity and Accepting Postpartum
Breastfeeding catalyzes fostering body positivity and self-acceptance within the postpartum phase. This transformative experience empowers women to embrace the remarkable capacity of their bodies to nourish and support their newborns, leading to the cultivation of a newfound admiration for their physical forms. Breastfeeding establishes a palpable and intimate link between a mother's body and the thriving of her child, redirecting attention from societal ideals of beauty towards the empowerment found in nurturing and sustaining life. This shift in perspective can positively influence body image perceptions and bolster self-assurance amid the significant physical transformations accompanying motherhood. Mothers can find a path toward appreciating their bodies for their functional and life-sustaining role by prioritizing nurturing and nourishing through breastfeeding. This shift aligns with a broader movement of celebrating diverse body shapes and sizes, and it can contribute to an enhanced sense of self-esteem and body positivity during a period of remarkable bodily change [ 18 ].
Peer Support and Sense of Community Among Breastfeeding Mothers
Breastfeeding frequently catalyzes the formation of nurturing communities among mothers who share similar journeys. Through peer support groups, virtual discussion forums, and physical gatherings, breastfeeding mothers find a platform to connect, exchange insights, and narrate their narratives. These exchanges culminate in cultivating camaraderie and a profound sense of inclusion, addressing potential sentiments of seclusion that can manifest in new mothers. The common threads of shared experiences, encompassing challenges and triumphs, knit together a robust community fabric. This communal tapestry offers a sanctuary where mothers can gain understanding, empathy, and encouragement from those who comprehend the nuances of breastfeeding. By engaging in these networks, mothers collectively navigate the intricacies of motherhood, providing a valuable lifeline of support that bolsters their emotional and mental well-being [ 19 ]. These peer connections provide a platform for practical advice and forge a deeper connection that extends beyond breastfeeding. The shared experiences create bonds that validate the emotions and struggles that come with motherhood, ultimately contributing to an enhanced sense of belonging and improved psychological resilience. The network's ability to foster mutual understanding and shared growth significantly enhances the holistic well-being of breastfeeding mothers.
Challenges and strategies
Addressing Common Breastfeeding Difficulties and Their Impact
Breastfeeding can come with various challenges, including sore nipples, engorgement, latch problems, and low milk supply. These challenges, if not adequately addressed, can significantly impact a mother's breastfeeding experience and overall well-being. Persistent difficulties can lead to frustration, stress, and a sense of failure. It is important to acknowledge these challenges, seek timely support from healthcare professionals or lactation consultants, and explore strategies to overcome them. Addressing breastfeeding difficulties effectively can help prevent adverse psychological outcomes and foster a more positive experience for both mother and child [ 20 ].
Importance of Education and Lactation Support for Successful Breastfeeding
Education and access to lactation support are crucial factors in ensuring successful breastfeeding and promoting maternal psychological well-being. Providing expectant mothers with accurate information about breastfeeding during prenatal care equips them with the knowledge and confidence to navigate potential challenges. Lactation consultants and breastfeeding support groups offer practical guidance and emotional encouragement, helping mothers overcome obstacles and build positive breastfeeding relationships. By offering education and ongoing support, healthcare providers can empower mothers to make informed decisions and feel more capable in their breastfeeding journey [ 21 ].
Overcoming Societal Barriers and Promoting Breastfeeding-Friendly Environments
Societal barriers, such as cultural norms, workplace policies, and lack of public support, can hinder breastfeeding success and contribute to maternal stress. Creating breastfeeding-friendly environments is essential to alleviate these barriers. Implementing workplace policies that support breastfeeding mothers, ensuring access to private and comfortable spaces for pumping, and promoting public acceptance of breastfeeding can significantly improve the breastfeeding experience. By actively addressing societal challenges, communities can foster an environment where mothers feel empowered to breastfeed confidently and without fear of judgment [ 22 ].
Cultural and societal influences
Cultural Perspectives on Breastfeeding and Maternal Well-being
Cultural beliefs and practices play a significant role in shaping attitudes toward breastfeeding and maternal well-being. In some cultures, breastfeeding is viewed as a natural and integral aspect of motherhood, closely linked to a woman's identity and sense of purpose. In others, cultural norms and misconceptions may influence perceptions of breastfeeding, affecting a mother's confidence and self-esteem. Recognizing and respecting diverse cultural perspectives is essential for promoting positive maternal well-being. Educating communities about the psychological benefits of breastfeeding while acknowledging cultural values can contribute to a more inclusive and supportive environment for mothers [ 23 ].
Shifting Societal Attitude Towards Breastfeeding in Public
Societal attitudes toward breastfeeding in public spaces have evolved, with advocacy efforts aiming to normalize this natural behavior. Despite progress, lingering discomfort or stigma associated with breastfeeding in public can contribute to a mother's stress and anxiety. Shifting societal attitudes requires public education campaigns that emphasize the importance of breastfeeding and create awareness about the rights of breastfeeding mothers. Encouraging open conversations and fostering an atmosphere of acceptance can alleviate mothers' concerns and contribute to their overall well-being [ 24 ].
Policy Implications for Supporting Breastfeeding Mothers
Government policies and institutional support play a vital role in supporting breastfeeding mothers. Policies that mandate reasonable accommodations for breastfeeding mothers in the workplace, such as designated pumping spaces and flexible break times, can alleviate stress and enable mothers to continue breastfeeding while returning to work. Additionally, policies that support maternity leave and access to lactation consultants ensure that mothers have the time and resources they need to establish successful breastfeeding practices. Collaborative efforts between governments, healthcare institutions, and workplaces are essential for creating an environment prioritizing maternal well-being through breastfeeding-friendly policies [ 25 ].
Weaning and transition
Emotional Worlds of Weaning for Both Mother and Child
Weaning, transitioning a baby from breastfeeding to other forms of nourishment, is an emotionally significant phase for both mother and child. For mothers, weaning can evoke emotions ranging from pride and accomplishment to sadness and nostalgia as a cherished chapter ends. Similarly, children may experience various emotions as the familiar source of comfort and nourishment changes. Understanding and acknowledging these emotions is crucial in ensuring both parties' supportive and empathetic transition [ 26 ].
Strategies for Facilitating a Smooth Transition From Breastfeeding
To facilitate a smooth transition from breastfeeding, several strategies can be employed. Gradual weaning, wherein breastfeeding sessions are gradually reduced over time, can help the mother and child adjust to the change. Introducing alternative feeding methods, such as bottle-feeding or introducing solid foods, can help the child adapt to new ways of receiving nourishment. Creating consistent routines and maintaining nurturing interactions, even without breastfeeding, can provide a sense of continuity and security for the child. Clear communication and transparency with the child, especially for older infants and toddlers, can help them understand the process and cope with the changes [ 27 ].
Maintaining Emotional Connection Beyond Breastfeeding
While breastfeeding may transition, the emotional connection between mother and child remains vital. Engaging in skin-to-skin contact, cuddling, and maintaining eye contact during feedings can continue to provide emotional intimacy and reassurance for both parties. Finding alternative bonding activities, such as reading, playing, and spending quality time together, can help strengthen the emotional bond beyond breastfeeding. The emotional connection formed during breastfeeding can extend and evolve into other forms of closeness and attachment as the child grows [ 13 ].
Conclusions
The psychological advantages of breastfeeding are extensive and profound. The interaction of hormones, facilitated by oxytocin and prolactin, fosters a strong maternal bond, reduces the risk of postpartum depression and anxiety, and enhances self-confidence. Additionally, breastfeeding simultaneously facilitates optimal brain development, a robust immune system, and cognitive-emotional growth in children through maternal interaction. This mutual relationship fosters secure attachment and emotional well-being. Addressing this matter requires immediate action to support breastfeeding and enhance awareness. This involves prioritizing lactation education, offering accessible assistance, and cultivating environments that normalize breastfeeding as a cherished practice. Elevating public awareness about the comprehensive benefits of breastfeeding helps dispel misconceptions, eliminate stigma, and empower mothers to make informed choices in nurturing their infants. The far-reaching implications of breastfeeding involve maternal well-being, child development, and society. They fortify maternal mental health and confidence, contributing to healthier family dynamics. The cognitive, emotional, and immunological advantages gained by children establish a promising foundation for their future. On a societal level, endorsing and fostering breastfeeding create a nurturing environment that values the health and happiness of both mothers and their children. Breastfeeding represents a transformative journey that encompasses more than just nutrition - it represents a complex array of interconnected benefits that resonate throughout our lives. By championing awareness and support for breastfeeding, we contribute to a world that prioritizes the comprehensive health of mothers and their children, fostering thriving relationships.
The authors have declared that no competing interests exist.
- 1. Breastfeeding: crucially important, but increasingly challenged in a market-driven world. Pérez-Escamilla R, Tomori C, Hernández-Cordero S, et al. Lancet. 2023;401:472–485. doi: 10.1016/S0140-6736(22)01932-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Breastfeeding and health outcomes for the mother-infant dyad. Dieterich CM, Felice JP, O'Sullivan E, Rasmussen KM. Pediatr Clin North Am. 2013;60:31–48. doi: 10.1016/j.pcl.2012.09.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Psychological effects of breastfeeding on children and mothers. Krol KM, Grossmann T. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61:977–985. doi: 10.1007/s00103-018-2769-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Smith AS, Tabbaa M, Lei K, et al. Psychoneuroendocrinology. 2016;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Breastfeeding and postpartum depression: an overview and methodological recommendations for future research. Pope CJ, Mazmanian D. Depress Res Treat. 2016;2016:4765310. doi: 10.1155/2016/4765310. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Breastfeeding support - the importance of self-efficacy for low-income women. Entwistle F, Kendall S, Mead M. Matern Child Nutr. 2010;6:228–242. doi: 10.1111/j.1740-8709.2009.00202.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Maternal supply of both arachidonic and docosahexaenoic acids is required for optimal neurodevelopment. Basak S, Mallick R, Banerjee A, Pathak S, Duttaroy AK. Nutrients. 2021;13:3–7. doi: 10.3390/nu13062061. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Human breast milk: from food to active immune response with disease protection in infants and mothers. Lokossou GA, Kouakanou L, Schumacher A, Zenclussen AC. Front Immunol. 2022;13:849012. doi: 10.3389/fimmu.2022.849012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Exclusive breastfeeding for at least four months is associated with a lower prevalence of overweight and obesity in mothers and their children after 2-5 years from delivery. Mantzorou M, Papandreou D, Vasios GK, et al. Nutrients. 2022;14:1–5. doi: 10.3390/nu14173599. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Mother-infant skin-to-skin contact: short- and long-term effects for mothers and their children born full-term. Bigelow AE, Power M. Front Psychol. 2020;11:1921. doi: 10.3389/fpsyg.2020.01921. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Infant-parent attachment: definition, types, antecedents, measurement and outcome. Benoit D. Paediatr Child Health. 2004;9:541–545. doi: 10.1093/pch/9.8.541. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Associations between breastfeeding and maternal responsiveness: a systematic review of the literature. Ventura AK. Adv Nutr. 2017;8:495–510. doi: 10.3945/an.116.014753. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Breastfeeding and active bonding protects against children's internalizing behavior problems. Liu J, Leung P, Yang A. Nutrients. 2013;6:76–89. doi: 10.3390/nu6010076. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. The evolved nest, oxytocin functioning, and prosocial development. Tarsha MS, Narvaez D. Front Psychol. 2023;14:1113944. doi: 10.3389/fpsyg.2023.1113944. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. World Health Organisation. Geneva: World Health Organization; [ Aug; 2023 ]. 2009. WHO: the physiological basis of breastfeeding. In: infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. [ PubMed ] [ Google Scholar ]
- 16. Oxytocin and why it is important for breastfeeding. [ Aug; 2023 ]. 2023. https://www.verywellfamily.com/oxytocin-and-breastfeeding-3574977 https://www.verywellfamily.com/oxytocin-and-breastfeeding-3574977
- 17. Mental health benefits of breastfeeding: a literature review. Tucker Z, O'Malley C. Cureus. 2022;14:0. doi: 10.7759/cureus.29199. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Breastfeeding, body image, and weight control behavior among postpartum women. Gillen MM, Markey CH, Rosenbaum DL, Dunaev JL. Body Image. 2021;38:201–209. doi: 10.1016/j.bodyim.2021.04.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Giving me hope: women's reflections on a breastfeeding peer support service. Thomson G, Crossland N, Dykes F. Matern Child Nutr. 2012;8:340–353. doi: 10.1111/j.1740-8709.2011.00358.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Common breastfeeding problems experienced by lactating mothers during the first six months in Kinshasa. Babakazo P, Bosonkie M, Mafuta E, Mvuama N, Mapatano MA. PLoS One. 2022;17:0. doi: 10.1371/journal.pone.0275477. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Interventions for promoting the initiation of breastfeeding. Balogun OO, O'Sullivan EJ, McFadden A, et al. Cochrane Database Syst Rev. 2016;11:0. doi: 10.1002/14651858.CD001688.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. The Surgeon General's Call to Action to Support Breastfeeding. Rockville, MD: Office of the Surgeon General (US); 2011. The Surgeon General's Call to Action to Support Breastfeeding. [ PubMed ] [ Google Scholar ]
- 23. Cultural beliefs, attitudes and perceptions of lactating mothers on exclusive breastfeeding in The Gambia: an ethnographic study. Sosseh SA, Barrow A, Lu ZJ. BMC Womens Health. 2023;23:18. doi: 10.1186/s12905-023-02163-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Public attitudes toward breastfeeding in public places in Ottawa, Canada. Russell K, Ali A. J Hum Lact. 2017;33:401–408. doi: 10.1177/0890334417695203. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Assessing workplace breastfeeding support among working mothers in the United States. McCardel RE, Padilla HM. Workplace Health Saf. 2020;68:182–189. doi: 10.1177/2165079919890358. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Recognizing the reversible nature of child-feeding decisions: breastfeeding, weaning, and relactation patterns in a shanty town community of Lima, Peru. Marquis GS, Díaz J, Bartolini R, Creed de Kanashiro H, Rasmussen KM. Soc Sci Med. 1998;47:645–656. doi: 10.1016/s0277-9536(98)00130-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. CDC: Weaning. [ Aug; 2023 ]. 2021. https://www.cdc.gov/nutrition/infantandtoddlernutrition/breastfeeding/weaning.html https://www.cdc.gov/nutrition/infantandtoddlernutrition/breastfeeding/weaning.html
- View on publisher site
- PDF (153.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Please note: This website has recently moved from www.health.gov to odphp.health.gov. www.health.gov is now the official website of ODPHP’s parent organization, the Office of the Assistant Secretary for Health (OASH). Please update your bookmarks for easy access to all our resources.
A Concerted Focus on Hunger, Nutrition, and Health Will Help Ensure Well-Being

Health and Well-Being Matter is the monthly blog of the Director of the Office of Disease Prevention and Health Promotion.
The recent White House Conference on Hunger, Nutrition, and Health renewed national attention and inspired action to end hunger and reduce the prevalence of chronic disease in the United States by 2030 — with emphasis on enhancing equitable opportunities for healthy eating and increased physical activity. Realizing these goals requires a far-reaching, cross-sector mobilization of efforts — what the National Strategy on Hunger, Nutrition, and Health [PDF - 795 KB] refers to as “a whole-of-government and whole-of-America approach” to these challenges. That charge acknowledges that the way forward is found across all sectors of society and through organizations collectively working to foster equity and eliminate disparities — especially in the areas of hunger, nutrition, physical activity, and chronic disease.
As home of the Healthy People initiative , the Physical Activity Guidelines for Americans , the Dietary Guidelines for Americans , the President’s Council on Sports, Fitness & Nutrition , and the National Youth Sports Strategy , ODPHP is heavily invested in this cause. It could not be more imperative for the nation. We’re also uniquely positioned to help further the necessary efforts in partnership with many communities inside and outside of government that support better nutrition and increased physical activity.
We can only achieve the greatest impact if we intentionally expand our dialogue and further coordinate action. This is especially important in addressing those inequities that contribute to disparities in accessing nutritious foods and opportunities to be physically active.
Social determinants of health play a critical role in our overall well-being. As I’ve written on multiple occasions, health doesn’t belong solely to the realm of public health or health care — rather, we find opportunities for health and well-being in every aspect of our daily lives.
Policymakers, private industry, educators, and community service organizations — to name just a few — must be active participants to make real progress. Everyone from individual people to the largest corporations and public organizations can make a commitment to the effort to eliminate hunger and improve the health of the nation. As detailed on odphp.health.gov , this list includes:
- The food industry (including manufacturers, distributors, retailers, restaurants, and delivery companies)
- The health care industry (including insurance companies, health systems, and providers)
- Fitness companies (including outdoor recreational organizations and athletic equipment and apparel makers)
- Advertising and marketing companies
- Technology companies
- The transportation sector (including infrastructure developers, mobility service providers, public transit, and charter companies)
- Nonprofit organizations (including advocacy, consumer, and public health groups)
- Philanthropic organizations
- Trade associations
- State, local, territorial, and tribal governments
- Schools and universities
- Community-based and neighborhood organizations
And more! By “everyone”, we mean everyone . All hands are called for. As we engage across these sectors, we must be especially deliberate in including perspectives and solutions from communities and individuals that have lived experience with these challenges. Their ideas are critical to success. They should not only have a place at the table where needs are identified, problems debated, and solutions proposed — they should also have empowered voices to effect change.
Given that the pandemic further exposed the depth and breadth of inequities in social determinants, there’s a new and urgent awareness to more fully and systematically enhance the vital conditions that ensure both individual and collective well-being. It’s an urgency that we haven’t known before. We need to capitalize on that urgency in this moment.
As I write this, I keep thinking about the Closing Plenary Session of the White House Conference . If you haven’t watched it, I urge you to. It clearly summarized all that the Conference sought to achieve, and the session brought out the voices of those who are continuing — and may yet complete — the work underway. At that session, Avani Rai — a Healthy Living Advocate, National 4-H Council Member, and one of many youth participants — eloquently reminded us: “We don’t have a quantity problem in our country. We have a quality problem in our country.”
For me, that sentiment — quality notwithstanding quantity — drives to the heart of what’s before us. We have the tools and the resources to end hunger, improve access to nutritious foods, provide space for increased physical activity, and make enormous strides in reducing chronic disease. But resources must be marshalled equitably and organized intelligently to ensure success. It’s really not a matter of whether we can achieve these things — but rather if we have the will to.
Another message from the Conference that sticks with me, also courtesy of Ms. Rai: “We live in a country where hunger absolutely does not need to be a problem.” She’s right. Hunger doesn’t need to be a problem. The same could be said of rampant poor nutrition and lack of adequate physical activity. As detailed in the Current Federal Programming and Coordination Efforts Related to Food and Nutrition Insecurity and Diet-Related Diseases [PDF - 744 KB] , many efforts are already underway. But as is readily apparent in this report and by the overwhelming need across the nation, these efforts are only the beginning.
Ms. Rai’s words reflect a deep understanding of the issues and the belief — the optimism — that we can do better. To hear such insight and optimism from our nation’s youth gives me hope. Her words convey for me the sense that we may be on the cusp of breaking through. She, and many others like her who have been working on these issues from such a young age, have also witnessed these challenges firsthand. They’ve been moved to action by what they’ve experienced. They understand what’s at stake and what’s possible. We should all share in their understanding and perspective — and we can do better.
If we hear the message from those who’ve experienced these challenges, engage the broadest coalition to effect change, and work collaboratively in our efforts, we can meet the goals espoused at the White House Conference on Hunger, Nutrition, and Health — and promote greater health and well-being for all.
To learn more about how you or your organization can commit to end hunger, help reduce chronic disease, and improve healthy eating and physical activity, please visit the Conference Commitments page on odphp.health.gov .
Yours in health, Paul
Paul Reed, MD Rear Admiral, U.S. Public Health Service Deputy Assistant Secretary for Health Director, Office of Disease Prevention and Health Promotion
In Officio Salutis — In the Service of Health
The Office of Disease Prevention and Health Promotion (ODPHP) cannot attest to the accuracy of a non-federal website.
Linking to a non-federal website does not constitute an endorsement by ODPHP or any of its employees of the sponsors or the information and products presented on the website.
You will be subject to the destination website's privacy policy when you follow the link.

IMAGES
VIDEO
COMMENTS
vegetables, and other plant foods such as wh ole grains, but also fish, meat, and dairy. products constitute natural sources (table 2). Nutrition, Well-Being and Health. 6. dietary antioxidants ...
Evidence suggests that lifestyle, behaviour patterns and eating habits adopted during this age persist throughout adulthood and can have a significant influence on health and wellbeing in later life [3,4]. Furthermore, the transition from childhood into adolescence is often associated with unhealthy dietary changes.
Fostering the growth, development, health and wellbeing of children has been recognised as a global priority. At the heart of achieving the ambitious and transformational vision of the 2030 Agenda for Sustainable Development is the goal for all children to have the best start in life, irrespective of where they are born, their ethnicity, and ...
Other reported benefits included positive biometric changes such as improvements in lipid profile, weight reduction, positive change in blood glucose, and reduction in HbA1c. These benefits may facilitate the management of comorbidities and improve overall health and well-being . This highlights that advances in nutritional psychiatry are ...
Here we aggregated data across 40 unique meta-analyses, which collectively included more than 2,000 studies and 1 million participants to examine the cross-sectional association between self-esteem and overall health/well-being. Results indicated that self-esteem has a robust overall association with health/well-being (r = .31). Moderator ...
The data suggest that a different approach is needed to foster physical health and well-being within our patients and communities. ... Findings from focus groups and interviews in middle schools on environmental influences on nutrition and physical activity. Health Education and Behavior. 2004; 31 (1):34-46. [Google Scholar] 98.
This chapter discusses contemporary theoretical basis for dietary interventions for disease prevention and management and their applications in practice. This chapter (1) introduces key concepts related to the application of theory in understanding and improving diet and eating-related behaviors, (2) reviews behavioral issues related to ...
Ralph Rühl. Nutrition & Metabolism (2023) More than one-third of American adults are obese and statistics are similar worldwide. Caloric intake and diet composition have large and lasting effects ...
Food insecurity and the lack of access to affordable, nutritious food are associated with poor dietary quality and an increased risk of diet-related diseases, including cardiovascular disease, diabetes, and certain types of cancer. Those of lower socioeconomic status and racial and ethnic minority groups experience higher rates of food insecurity, are more likely to live in under-resourced ...
One-to-three times a week, 77 % of foster parents reported. that their children ate a meal or snack from a fast food. restaurant, including dining in or carry out/delivery, and. only 14 % reported ...
The promotion of well-being among children, adolescents, and emerging adults who are in foster care or have a history of out-of-home placement is not a novel idea, yet the emergence of "evidence-based" treatments, the burgeoning of implementation science, and the understanding that safety and permanency (two longstanding central tenets of child welfare practice) are not sufficient for ...
New research has highlighted the profound link between dietary choices and brain health. Published in Nature, the research showed that a healthy, balanced diet was linked to superior brain health ...
This article explores the paradigm of Food Well-Being (FWB), "a positive psychological, physical, emotional, and social relationship with food," for those who experience hunger. ... "Food Insufficiency Exists in the United States: Results from the Third National Health and Nutrition Examination Survey," American Journal of Public ...
Abstract. Ensuring healthy lives and promoting wellbeing across the age spectrum are essential to sustainable development. Nutrition is at the heart of the World Health Organization (WHO) Sustainable Development Goals, particularly for Sustainable Development Goal 2/Subgoal 2, which is to End all forms of malnutrition by 2030.This subgoal addresses people of all ages, including targeted groups ...
Background Nutrition and physical activity are major determinants of health and quality of life; however, there exists little research focusing on determinants of these behaviours in older adults. This is important, since just as these behaviours vary according to subpopulation, it is likely that the determinants also vary. An understanding of the modifiable determinants of nutrition and ...
In this paper, we explore observed and potential connections among nature, biodiversity, ecosystem services and human health and well-being, through biodiversity-ecosystem services linkages, associations of nature with human health, and recent limited evidence relating biodiversity to some human health outcomes based on a review of selected ...
Introduction There has been growing interest in community gardens as an effective and affordable health promotion strategy. However, most available evidence is derived from qualitative studies, whereas quantitative research on this subject is limited. Objectives To synthetize the literature about physical and mental health outcomes associated with community gardening. Two main questions were ...
Cultural Perspectives on Breastfeeding and Maternal Well-being. Cultural beliefs and practices play a significant role in shaping attitudes toward breastfeeding and maternal well-being. In some cultures, breastfeeding is viewed as a natural and integral aspect of motherhood, closely linked to a woman's identity and sense of purpose.
Health and Well-Being Matter is the monthly blog of the Director of the Office of Disease Prevention and Health Promotion. The recent White House Conference on Hunger, Nutrition, and Health renewed national attention and inspired action to end hunger and reduce the prevalence of chronic disease in the United States by 2030 — with emphasis on enhancing equitable opportunities for healthy ...