An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cambridge Open


The Flaws and Human Harms of Animal Experimentation
Nonhuman animal (“animal”) experimentation is typically defended by arguments that it is reliable, that animals provide sufficiently good models of human biology and diseases to yield relevant information, and that, consequently, its use provides major human health benefits. I demonstrate that a growing body of scientific literature critically assessing the validity of animal experimentation generally (and animal modeling specifically) raises important concerns about its reliability and predictive value for human outcomes and for understanding human physiology. The unreliability of animal experimentation across a wide range of areas undermines scientific arguments in favor of the practice. Additionally, I show how animal experimentation often significantly harms humans through misleading safety studies, potential abandonment of effective therapeutics, and direction of resources away from more effective testing methods. The resulting evidence suggests that the collective harms and costs to humans from animal experimentation outweigh potential benefits and that resources would be better invested in developing human-based testing methods.
Introduction
Annually, more than 115 million animals are used worldwide in experimentation or to supply the biomedical industry. 1 Nonhuman animal (hereafter “animal”) experimentation falls under two categories: basic (i.e., investigation of basic biology and human disease) and applied (i.e., drug research and development and toxicity and safety testing). Regardless of its categorization, animal experimentation is intended to inform human biology and health sciences and to promote the safety and efficacy of potential treatments. Despite its use of immense resources, the animal suffering involved, and its impact on human health, the question of animal experimentation’s efficacy has been subjected to little systematic scrutiny. 2
Although it is widely accepted that medicine should be evidence based , animal experimentation as a means of informing human health has generally not been held, in practice, to this standard. This fact makes it surprising that animal experimentation is typically viewed as the default and gold standard of preclinical testing and is generally supported without critical examination of its validity. A survey published in 2008 of anecdotal cases and statements given in support of animal experimentation demonstrates how it has not and could not be validated as a necessary step in biomedical research, and the survey casts doubt on its predictive value. 3 I show that animal experimentation is poorly predictive of human outcomes, 4 that it is unreliable across a wide category of disease areas, 5 and that existing literature demonstrates the unreliability of animal experimentation, thereby undermining scientific arguments in its favor. I further show that the collective harms that result from an unreliable practice tip the ethical scale of harms and benefits against continuation in much, if not all, of experimentation involving animals. 6
Problems of Successful Translation to Humans of Data from Animal Experimentation
Although the unreliability and limitations of animal experimentation have increasingly been acknowledged, there remains a general confidence within much of the biomedical community that they can be overcome. 7 However, three major conditions undermine this confidence and explain why animal experimentation, regardless of the disease category studied, fails to reliably inform human health: (1) the effects of the laboratory environment and other variables on study outcomes, (2) disparities between animal models of disease and human diseases, and (3) species differences in physiology and genetics. I argue for the critical importance of each of these conditions.
The Influence of Laboratory Procedures and Environments on Experimental Results
Laboratory procedures and conditions exert influences on animals’ physiology and behaviors that are difficult to control and that can ultimately impact research outcomes. Animals in laboratories are involuntarily placed in artificial environments, usually in windowless rooms, for the duration of their lives. Captivity and the common features of biomedical laboratories—such as artificial lighting, human-produced noises, and restricted housing environments—can prevent species-typical behaviors, causing distress and abnormal behaviors among animals. 8 Among the types of laboratory-generated distress is the phenomenon of contagious anxiety. 9 Cortisone levels rise in monkeys watching other monkeys being restrained for blood collection. 10 Blood pressure and heart rates elevate in rats watching other rats being decapitated. 11 Routine laboratory procedures, such as catching an animal and removing him or her from the cage, in addition to the experimental procedures, cause significant and prolonged elevations in animals’ stress markers. 12 These stress-related changes in physiological parameters caused by the laboratory procedures and environments can have significant effects on test results. 13 Stressed rats, for example, develop chronic inflammatory conditions and intestinal leakage, which add variables that can confound data. 14
A variety of conditions in the laboratory cause changes in neurochemistry, genetic expression, and nerve regeneration. 15 In one study, for example, mice were genetically altered to develop aortic defects. Yet, when the mice were housed in larger cages, those defects almost completely disappeared. 16 Providing further examples, typical noise levels in laboratories can damage blood vessels in animals, and even the type of flooring on which animals are tested in spinal cord injury experiments can affect whether a drug shows a benefit. 17
In order to control for potential confounders, some investigators have called for standardization of laboratory settings and procedures. 18 One notable effort was made by Crabbe et al. in their investigation of the potential confounding influences of the laboratory environment on six mouse behaviors that are commonly studied in neurobehavioral experiments. Despite their “extraordinary lengths to equate test apparatus, testing protocols, and all possible features of animal husbandry” across three laboratories, there were systematic differences in test results in these labs. 19 Additionally, different mouse strains varied markedly in all behavioral tests, and for some tests the magnitude of genetic differences depended on the specific testing laboratory. The results suggest that there are important influences of environmental conditions and procedures specific to individual laboratories that can be difficult—perhaps even impossible—to eliminate. These influences can confound research results and impede extrapolation to humans.
The Discordance between Human Diseases and Animal Models of Diseases
The lack of sufficient congruence between animal models and human diseases is another significant obstacle to translational reliability. Human diseases are typically artificially induced in animals, but the enormous difficulty of reproducing anything approaching the complexity of human diseases in animal models limits their usefulness. 20 Even if the design and conduct of an animal experiment are sound and standardized, the translation of its results to the clinic may fail because of disparities between the animal experimental model and the human condition. 21
Stroke research presents one salient example of the difficulties in modeling human diseases in animals. Stroke is relatively well understood in its underlying pathology. Yet accurately modeling the disease in animals has proven to be an exercise in futility. To address the inability to replicate human stroke in animals, many assert the need to use more standardized animal study design protocols. This includes the use of animals who represent both genders and wide age ranges, who have comorbidities and preexisting conditions that occur naturally in humans, and who are consequently given medications that are indicated for human patients. 22 In fact, a set of guidelines, named STAIR, was implemented by a stroke roundtable in 1999 (and updated in 2009) to standardize protocols, limit the discrepancies, and improve the applicability of animal stroke experiments to humans. 23 One of the most promising stroke treatments later to emerge was NXY-059, which proved effective in animal experiments. However, the drug failed in clinical trials, despite the fact that the set of animal experiments on this drug was considered the poster child for the new experimental standards. 24 Despite such vigorous efforts, the development of STAIR and other criteria has yet to make a recognizable impact in clinical translation. 25
Under closer scrutiny, it is not difficult to surmise why animal stroke experiments fail to successfully translate to humans even with new guidelines. Standard stroke medications will likely affect different species differently. There is little evidence to suggest that a female rat, dog, or monkey sufficiently reproduces the physiology of a human female. Perhaps most importantly, reproducing the preexisting conditions of stroke in animals proves just as difficult as reproducing stroke pathology and outcomes. For example, most animals don’t naturally develop significant atherosclerosis, a leading contributor to ischemic stroke. In order to reproduce the effects of atherosclerosis in animals, researchers clamp their blood vessels or artificially insert blood clots. These interventions, however, do not replicate the elaborate pathology of atherosclerosis and its underlying causes. Reproducing human diseases in animals requires reproducing the predisposing diseases, also a formidable challenge. The inability to reproduce the disease in animals so that it is congruent in relevant respects with human stroke has contributed to a high failure rate in drug development. More than 114 potential therapies initially tested in animals failed in human trials. 26
Further examples of repeated failures based on animal models include drug development in cancer, amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI), Alzheimer’s disease (AD), and inflammatory conditions. Animal cancer models in which tumors are artificially induced have been the basic translational model used to study key physiological and biochemical properties in cancer onset and propagation and to evaluate novel treatments. Nevertheless, significant limitations exist in the models’ ability to faithfully mirror the complex process of human carcinogenesis. 27 These limitations are evidenced by the high (among the highest of any disease category) clinical failure rate of cancer drugs. 28 Analyses of common mice ALS models demonstrate significant differences from human ALS. 29 The inability of animal ALS models to predict beneficial effects in humans with ALS is recognized. 30 More than twenty drugs have failed in clinical trials, and the only U.S. Food and Drug Administration (FDA)–approved drug to treat ALS is Riluzole, which shows notably marginal benefit on patient survival. 31 Animal models have also been unable to reproduce the complexities of human TBI. 32 In 2010, Maas et al. reported on 27 large Phase 3 clinical trials and 6 unpublished trials in TBI that all failed to show human benefit after showing benefit in animals. 33 Additionally, even after success in animals, around 172 and 150 drug development failures have been identified in the treatment of human AD 34 and inflammatory diseases, 35 respectively.
The high clinical failure rate in drug development across all disease categories is based, at least in part, on the inability to adequately model human diseases in animals and the poor predictability of animal models. 36 A notable systematic review, published in 2007, compared animal experimentation results with clinical trial findings across interventions aimed at the treatment of head injury, respiratory distress syndrome, osteoporosis, stroke, and hemorrhage. 37 The study found that the human and animal results were in accordance only half of the time. In other words, the animal experiments were no more likely than a flip of the coin to predict whether those interventions would benefit humans.
In 2004, the FDA estimated that 92 percent of drugs that pass preclinical tests, including “pivotal” animal tests, fail to proceed to the market. 38 More recent analysis suggests that, despite efforts to improve the predictability of animal testing, the failure rate has actually increased and is now closer to 96 percent. 39 The main causes of failure are lack of effectiveness and safety problems that were not predicted by animal tests. 40
Usually, when an animal model is found wanting, various reasons are proffered to explain what went wrong—poor methodology, publication bias, lack of preexisting disease and medications, wrong gender or age, and so on. These factors certainly require consideration, and recognition of each potential difference between the animal model and the human disease motivates renewed efforts to eliminate these differences. As a result, scientific progress is sometimes made by such efforts. However, the high failure rate in drug testing and development, despite attempts to improve animal testing, suggests that these efforts remain insufficient to overcome the obstacles to successful translation that are inherent to the use of animals. Too often ignored is the well-substantiated idea that these models are, for reasons summarized here, intrinsically lacking in relevance to, and thus highly unlikely to yield useful information about, human diseases. 41
Interspecies Differences in Physiology and Genetics
Ultimately, even if considerable congruence were shown between an animal model and its corresponding human disease, interspecies differences in physiology, behavior, pharmacokinetics, and genetics would significantly limit the reliability of animal studies, even after a substantial investment to improve such studies. In spinal cord injury, for example, drug testing results vary according to which species and even which strain within a species is used, because of numerous interspecies and interstrain differences in neurophysiology, anatomy, and behavior. 42 The micropathology of spinal cord injury, injury repair mechanisms, and recovery from injury varies greatly among different strains of rats and mice. A systematic review found that even among the most standardized and methodologically superior animal experiments, testing results assessing the effectiveness of methylprednisolone for spinal cord injury treatment varied considerably among species. 43 This suggests that factors inherent to the use of animals account for some of the major differences in results.
Even rats from the same strain but purchased from different suppliers produce different test results. 44 In one study, responses to 12 different behavioral measures of pain sensitivity, which are important markers of spinal cord injury, varied among 11 strains of mice, with no clear-cut patterns that allowed prediction of how each strain would respond. 45 These differences influenced how the animals responded to the injury and to experimental therapies. A drug might be shown to help one strain of mice recover but not another. Despite decades of using animal models, not a single neuroprotective agent that ameliorated spinal cord injury in animal tests has proven efficacious in clinical trials to date. 46
Further exemplifying the importance of physiological differences among species, a 2013 study reported that the mouse models used extensively to study human inflammatory diseases (in sepsis, burns, infection, and trauma) have been misleading. The study found that mice differ greatly from humans in their responses to inflammatory conditions. Mice differed from humans in what genes were turned on and off and in the timing and duration of gene expression. The mouse models even differed from one another in their responses. The investigators concluded that “our study supports higher priority to focus on the more complex human conditions rather than relying on mouse models to study human inflammatory disease.” 47 The different genetic responses between mice and humans are likely responsible, at least in part, for the high drug failure rate. The authors stated that every one of almost 150 clinical trials that tested candidate agents’ ability to block inflammatory responses in critically ill patients failed.
Wide differences have also become apparent in the regulation of the same genes, a point that is readily seen when observing differences between human and mouse livers. 48 Consistent phenotypes (observable physical or biochemical characteristics) are rarely obtained by modification of the same gene, even among different strains of mice. 49 Gene regulation can substantially differ among species and may be as important as the presence or absence of a specific gene. Despite the high degree of genome conservation, there are critical differences in the order and function of genes among species. To use an analogy: as pianos have the same keys, humans and other animals share (largely) the same genes. Where we mostly differ is in the way the genes or keys are expressed. For example, if we play the keys in a certain order, we hear Chopin; in a different order, we hear Ray Charles; and in yet a different order, it’s Jerry Lee Lewis. In other words, the same keys or genes are expressed, but their different orders result in markedly different outcomes.
Recognizing the inherent genetic differences among species as a barrier to translation, researches have expressed considerable enthusiasm for genetically modified (GM) animals, including transgenic mice models, wherein human genes are inserted into the mouse genome. However, if a human gene is expressed in mice, it will likely function differently from the way it functions in humans, being affected by physiological mechanisms that are unique in mice. For example, a crucial protein that controls blood sugar in humans is missing in mice. 50 When the human gene that makes this protein was expressed in genetically altered mice, it had the opposite effect from that in humans: it caused loss of blood sugar control in mice. Use of GM mice has failed to successfully model human diseases and to translate into clinical benefit across many disease categories. 51 Perhaps the primary reason why GM animals are unlikely to be much more successful than other animal models in translational medicine is the fact that the “humanized” or altered genes are still in nonhuman animals.
In many instances, nonhuman primates (NHPs) are used instead of mice or other animals, with the expectation that NHPs will better mimic human results. However, there have been sufficient failures in translation to undermine this optimism. For example, NHP models have failed to reproduce key features of Parkinson’s disease, both in function and in pathology. 52 Several therapies that appeared promising in both NHPs and rat models of Parkinson’s disease showed disappointing results in humans. 53 The campaign to prescribe hormone replacement therapy (HRT) in millions of women to prevent cardiovascular disease was based in large part on experiments on NHPs. HRT is now known to increase the risk of these diseases in women. 54
HIV/AIDS vaccine research using NHPs represents one of the most notable failures in animal experimentation translation. Immense resources and decades of time have been devoted to creating NHP (including chimpanzee) models of HIV. Yet all of about 90 HIV vaccines that succeeded in animals failed in humans. 55 After HIV vaccine gp120 failed in clinical trials, despite positive outcomes in chimpanzees, a BMJ article commented that important differences between NHPs and humans with HIV misled researchers, taking them down unproductive experimental paths. 56 Gp120 failed to neutralize HIV grown and tested in cell culture. However, because the serum protected chimpanzees from HIV infection, two Phase 3 clinical trials were undertaken 57 —a clear example of how expectations that NHP data are more predictive than data from other (in this case, cell culture) testing methods are unproductive and harmful. Despite the repeated failures, NHPs (though not chimpanzees or other great apes) remain widely used for HIV research.
The implicit assumption that NHP (and indeed any animal) data are reliable has also led to significant and unjustifiable human suffering. For example, clinical trial volunteers for gp120 were placed at unnecessary risk of harm because of unfounded confidence in NHP experiments. Two landmark studies involving thousands of menopausal women being treated with HRT were terminated early because of increased stroke and breast cancer risk. 58 In 2003, Elan Pharmaceuticals was forced to prematurely terminate a Phase 2 clinical trial when an investigational AD vaccine was found to cause brain swelling in human subjects. No significant adverse effects were detected in GM mice or NHPs. 59
In another example of human suffering resulting from animal experimentation, six human volunteers were injected with an immunomodulatory drug, TGN 1412, in 2006. 60 Within minutes of receiving the experimental drug, all volunteers suffered a severe adverse reaction resulting from a life-threatening cytokine storm that led to catastrophic systemic organ failure. The compound was designed to dampen the immune system, but it had the opposite effect in humans. Prior to this first human trial, TGN 1412 was tested in mice, rabbits, rats, and NHPs with no ill effects. NHPs also underwent repeat-dose toxicity studies and were given 500 times the human dose for at least four consecutive weeks. 61 None of the NHPs manifested the ill effects that humans showed almost immediately after receiving minute amounts of the test drug. Cynomolgus and rhesus monkeys were specifically chosen because their CD28 receptors demonstrated similar affinity to TGN 1412 as human CD28 receptors. Based on such data as these, it was confidently concluded that results obtained from these NHPs would most reliably predict drug responses in humans—a conclusion that proved devastatingly wrong.
As exemplified by the study of HIV/AIDS, TGN 1412, and other experiences, 62 experiments with NHPs are not necessarily any more predictive of human responses than experiments with other animals. The repeated failures in translation from studies with NHPs belie arguments favoring use of any nonhuman species to study human physiology and diseases and to test potential treatments. If experimentation using chimpanzees and other NHPs, our closest genetic cousins, are unreliable, how can we expect research using other animals to be reliable? The bottom line is that animal experiments, no matter the species used or the type of disease research undertaken, are highly unreliable—and they have too little predictive value to justify the resultant risks of harms for humans, for reasons I now explain.
The Collective Harms That Result from Misleading Animal Experiments
As medical research has explored the complexities and subtle nuances of biological systems, problems have arisen because the differences among species along these subtler biological dimensions far outweigh the similarities , as a growing body of evidence attests. These profoundly important—and often undetected—differences are likely one of the main reasons human clinical trials fail. 63
“Appreciation of differences” and “caution” about extrapolating results from animals to humans are now almost universally recommended. But, in practice, how does one take into account differences in drug metabolism, genetics, expression of diseases, anatomy, influences of laboratory environments, and species- and strain-specific physiologic mechanisms—and, in view of these differences, discern what is applicable to humans and what is not? If we cannot determine which physiological mechanisms in which species and strains of species are applicable to humans (even setting aside the complicating factors of different caging systems and types of flooring), the usefulness of the experiments must be questioned.
It has been argued that some information obtained from animal experiments is better than no information. 64 This thesis neglects how misleading information can be worse than no information from animal tests. The use of nonpredictive animal experiments can cause human suffering in at least two ways: (1) by producing misleading safety and efficacy data and (2) by causing potential abandonment of useful medical treatments and misdirecting resources away from more effective testing methods.
Humans are harmed because of misleading animal testing results. Imprecise results from animal experiments may result in clinical trials of biologically faulty or even harmful substances, thereby exposing patients to unnecessary risk and wasting scarce research resources. 65 Animal toxicity studies are poor predictors of toxic effects of drugs in humans. 66 As seen in some of the preceding examples (in particular, stroke, HRT, and TGN1412), humans have been significantly harmed because investigators were misled by the safety and efficacy profile of a new drug based on animal experiments. 67 Clinical trial volunteers are thus provided with raised hopes and a false sense of security because of a misguided confidence in efficacy and safety testing using animals.
An equal if indirect source of human suffering is the opportunity cost of abandoning promising drugs because of misleading animal tests. 68 As candidate drugs generally proceed down the development pipeline and to human testing based largely on successful results in animals 69 (i.e., positive efficacy and negative adverse effects), drugs are sometimes not further developed due to unsuccessful results in animals (i.e., negative efficacy and/or positive adverse effects). Because much pharmaceutical company preclinical data are proprietary and thus publicly unavailable, it is difficult to know the number of missed opportunities due to misleading animal experiments. However, of every 5,000–10,000 potential drugs investigated, only about 5 proceed to Phase 1 clinical trials. 70 Potential therapeutics may be abandoned because of results in animal tests that do not apply to humans. 71 Treatments that fail to work or show some adverse effect in animals because of species-specific influences may be abandoned in preclinical testing even if they may have proved effective and safe in humans if allowed to continue through the drug development pipeline.
An editorial in Nature Reviews Drug Discovery describes cases involving two drugs in which animal test results from species-specific influences could have derailed their development. In particular, it describes how tamoxifen, one of the most effective drugs for certain types of breast cancer, “would most certainly have been withdrawn from the pipeline” if its propensity to cause liver tumor in rats had been discovered in preclinical testing rather than after the drug had been on the market for years. 72 Gleevec provides another example of effective drugs that could have been abandoned based on misleading animal tests: this drug, which is used to treat chronic myelogenous leukemia (CML), showed serious adverse effects in at least five species tested, including severe liver damage in dogs. However, liver toxicity was not detected in human cell assays, and clinical trials proceeded, which confirmed the absence of significant liver toxicity in humans. 73 Fortunately for CML patients, Gleevec is a success story of predictive human-based testing. Many useful drugs that have safely been used by humans for decades, such as aspirin and penicillin, may not have been available today if the current animal testing regulatory requirements were in practice during their development. 74
A further example of near-missed opportunities is provided by experiments on animals that delayed the acceptance of cyclosporine, a drug widely and successfully used to treat autoimmune disorders and prevent organ transplant rejection. 75 Its immunosuppressive effects differed so markedly among species that researchers judged that the animal results limited any direct inferences that could be made to humans. Providing further examples, PharmaInformatic released a report describing how several blockbuster drugs, including aripiprazole (Abilify) and esomeprazole (Nexium), showed low oral bioavailability in animals. They would likely not be available on the market today if animal tests were solely relied on. Understanding the implications of its findings for drug development in general, PharmaInformatic asked, “Which other blockbuster drugs would be on the market today, if animal trials would have not been used to preselect compounds and drug-candidates for further development?” 76 These near-missed opportunities and the overall 96 percent failure rate in clinical drug testing strongly suggest the unsoundness of animal testing as a precondition of human clinical trials and provide powerful evidence for the need for a new, human-based paradigm in medical research and drug development.
In addition to potentially causing abandonment of useful treatments, use of an invalid animal disease model can lead researchers and the industry in the wrong research direction, wasting time and significant investment. 77 Repeatedly, researchers have been lured down the wrong line of investigation because of information gleaned from animal experiments that later proved to be inaccurate, irrelevant, or discordant with human biology. Some claim that we do not know which benefits animal experiments, particularly in basic research, may provide down the road. Yet human lives remain in the balance, waiting for effective therapies. Funding must be strategically invested in the research areas that offer the most promise.
The opportunity costs of continuing to fund unreliable animal tests may impede development of more accurate testing methods. Human organs grown in the lab, human organs on a chip, cognitive computing technologies, 3D printing of human living tissues, and the Human Toxome Project are examples of new human-based technologies that are garnering widespread enthusiasm. The benefit of using these testing methods in the preclinical setting over animal experiments is that they are based on human biology. Thus their use eliminates much of the guesswork required when attempting to extrapolate physiological data from other species to humans. Additionally, these tests offer whole-systems biology, in contrast to traditional in vitro techniques. Although they are gaining momentum, these human-based tests are still in their relative infancy, and funding must be prioritized for their further development. The recent advancements made in the development of more predictive, human-based systems and biological approaches in chemical toxicological testing are an example of how newer and improved tests have been developed because of a shift in prioritization. 78 Apart from toxicology, though, financial investment in the development of human-based technologies generally falls far short of investment in animal experimentation. 79
The unreliability of applying animal experimental results to human biology and diseases is increasingly recognized. Animals are in many respects biologically and psychologically similar to humans, perhaps most notably in the shared characteristics of pain, fear, and suffering. 80 In contrast, evidence demonstrates that critically important physiological and genetic differences between humans and other animals can invalidate the use of animals to study human diseases, treatments, pharmaceuticals, and the like. In significant measure, animal models specifically, and animal experimentation generally, are inadequate bases for predicting clinical outcomes in human beings in the great bulk of biomedical science. As a result, humans can be subject to significant and avoidable harm.
The data showing the unreliability of animal experimentation and the resultant harms to humans (and nonhumans) undermine long-standing claims that animal experimentation is necessary to enhance human health and therefore ethically justified. Rather, they demonstrate that animal experimentation poses significant costs and harms to human beings. It is possible—as I have argued elsewhere—that animal research is more costly and harmful, on the whole, than it is beneficial to human health. 81 When considering the ethical justifiability of animal experiments, we should ask if it is ethically acceptable to deprive humans of resources, opportunity, hope, and even their lives by seeking answers in what may be the wrong place. In my view, it would be better to direct resources away from animal experimentation and into developing more accurate, human-based technologies.
Aysha Akhtar , M.D., M.P.H., is a neurologist and preventive medicine specialist and Fellow at the Oxford Centre for Animal Ethics, Oxford, United Kingdom.
1. Taylor K, Gordon N, Langley G, Higgins W. Estimates for worldwide laboratory animal use in 2005 . Alternatives to Laboratory Animals 2008; 36 :327–42. [ PubMed ] [ Google Scholar ]
2. Systematic reviews that have been conducted generally reveal the unreliability and poor predictability of animal tests. See Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007;334:197. See also Pound P, Bracken MB. Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ 2014;348:g3387. See Godlee F. How predictive and productive is animal research? BMJ 2014;348:g3719. See Benatar M. Lost in translation: Treatment trials in the SOD 1 mouse and in human ALS. Neurobiology Disease 2007; 26 :1–13 [ PubMed ] [ Google Scholar ] . And see Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: A systematic review of methylprednisolone . Alternatives to Laboratory Animals 2009; 37 :43–62. [ PubMed ] [ Google Scholar ]
3. Mathews RAJ. Medical progress depends on animal models—doesn’t it? Journal of the Royal Society of Medicine 2008; 101 :95–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
4. See Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philosophy, Ethics, and Humanities in Medicine 2009; 4 :2 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See also Wall RJ, Shani M. Are animal models as good as we think? Theriogenology 2008; 69 :2–9. [ PubMed ] [ Google Scholar ]
5. See note 3, Mathews 2008. See also Hartung T, Zurlo J. Food for thought… alternative approaches for medical countermeasures to biological and chemical terrorism and warfare . ALTEX 2012; 29 :251–60 [ PubMed ] [ Google Scholar ] . See Leist M, Hartung T. Inflammatory findings on species extrapolations: Humans are definitely no 70-kg mice . Archives in Toxicology 2013; 87 :563–7 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See Mak IWY, Evaniew N, Ghert M. Lost in translation: Animal models and clinical trials in cancer treatment . American Journal in Translational Research 2014; 6 :114–18 [ PMC free article ] [ PubMed ] [ Google Scholar ] . And see Pippin J. Animal research in medical sciences: Seeking a convergence of science, medicine, and animal law . South Texas Law Review 2013; 54 :469–511. [ Google Scholar ]
6. For an overview of the harms-versus-benefits argument, see LaFollette H. Animal experimentation in biomedical research In: Beauchamp TL, Frey RG, eds. The Oxford Handbook of Animal Ethics . Oxford: Oxford University Press; 2011:812–18. [ Google Scholar ]
7. See Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases . Nature Medicine 2010; 16 :1210–14 [ PubMed ] [ Google Scholar ] . See Institute of Medicine. Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary. Washington, DC: The National Academies Press; 2013. And see Degryse AL, Lawson WE. Progress towards improving animal models for IPF . American Journal of Medical Science 2011; 341 :444–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
8. See Morgan KN, Tromborg CT. Sources of stress in captivity . Applied Animal Behaviour Science 2007; 102 :262–302 [ Google Scholar ] . See Hart PC, Bergner CL, Dufour BD, Smolinsky AN, Egan RJ, LaPorte L, et al. Analysis of abnormal repetitive behaviors in experimental animal models In Warrick JE, Kauleff AV, eds. Translational Neuroscience and Its Advancement of Animal Research Ethics . New York: Nova Science; 2009:71–82 [ Google Scholar ] . See Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience . American Journal of Primatology 2003; 60 :1–15 [ PubMed ] [ Google Scholar ] . And see Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress . Contemporary Topics in Laboratory Animal Science 2004; 43 :42–51. [ PubMed ] [ Google Scholar ]
9. Suckow MA, Weisbroth SH, Franklin CL. The Laboratory Rat . 2nd ed. Burlington, MA: Elsevier Academic Press; 2006, at 323.
10. Flow BL, Jaques JT. Effect of room arrangement and blood sample collection sequence on serum thyroid hormone and cortisol concentrations in cynomolgus macaques ( Macaca fascicularis ). Contemporary Topics in Laboratory Animal Science 1997;36:65–8.
11. See note 8, Balcombe et al. 2004.
12. See note 8, Balcombe et al. 2004.
13. Baldwin A, Bekoff M. Too stressed to work. New Scientist 2007;194:24.
14. See note 13, Baldwin, Bekoff 2007.
15. Akhtar A, Pippin JJ, Sandusky CB. Animal models in spinal cord injury: A review . Reviews in the Neurosciences 2008; 19 :47–60. [ PubMed ] [ Google Scholar ]
16. See note 13, Baldwin, Bekoff 2007.
17. See note 15, Akhtar et al. 2008.
18. See Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias . Stroke 2004; 35 :1203–8 [ PubMed ] [ Google Scholar ] . See also O’ Neil BJ, Kline JA, Burkhart K, Younger J. Research fundamentals: V. The use of laboratory animal models in research. Academic Emergency Medicine 1999;6:75–82.
19. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment . Science 1999; 284 :1670–2, at 1670. [ PubMed ] [ Google Scholar ]
20. See Curry SH. Why have so many drugs with stellar results in laboratory stroke models failed in clinical trials? A theory based on allometric relationships. Annals of the New York Academy of Sciences 2003;993:69–74. See also Dirnagl U. Bench to bedside: The quest for quality in experimental stroke research . Journal of Cerebral Blood Flow & Metabolism 2006; 26 :1465–78 [ PubMed ] [ Google Scholar ] .
21. van der Worp HB, Howells DW, Sena ES, Poritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Medicine 2010; 7 :e1000245. [ PMC free article ] [ PubMed ] [ Google Scholar ] .
22. See note 20, Dirnagl 2006. See also Sena E, van der Worp B, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends in Neurosciences 2007; 30 :433–9. [ PubMed ] [ Google Scholar ]
23. See Gawrylewski A. The trouble with animal models: Why did human trials fail? The Scientist 2007;21:44. See also Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations . Stroke 2009; 40 :2244–50. [ PMC free article ] [ PubMed ] [ Google Scholar ]
24. See note 23, Gawrylewski 2007. There is some dispute as to how vigorously investigators adhered to the suggested criteria. Nevertheless, NXY-059 animal studies were considered an example of preclinical studies that most faithfully adhered to the STAIR criteria. For further discussion see also Wang MM, Guohua X, Keep RF. Should the STAIR criteria be modified for preconditioning studies? Translational Stroke Research 2013; 4 :3–14 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
25. See note 24, Wang et al. 2013.
26. O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke . Annals of Neurology 2006; 59 :467–7 [ PubMed ] [ Google Scholar ] .
27. See note 5, Mak et al. 2014.
28. See note 5, Mak et al. 2014.
29. See Perrin S. Preclinical research: Make mouse studies work . Nature 2014; 507 :423–5 [ PubMed ] [ Google Scholar ] . See also, generally, Wilkins HM, Bouchard RJ, Lorenzon NM, Linseman DA. Poor correlation between drug efficacies in the mutant SOD1 mouse mode versus clinical trials of ALS necessitates the development of novel animal models for sporadic motor neuron disease. In: Costa A, Villalba E, eds. Horizons in Neuroscience Research. Vol. 5. Hauppauge, NY: Nova Science; 2011:1–39.
30. Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, et al. Neuroprotective agents for clinical trials in ALS: A systematic assessment . Neurology 2006; 67 :20–7 [ PubMed ] [ Google Scholar ] .
31. Sinha G. Another blow for ALS . Nature Biotechnology 2013; 31 :185 [ Google Scholar ] . See also note 30, Traynor et al. 2006.
32. See Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, et al. Experimental models of traumatic brain injury: Do we really need a better mousetrap? Neuroscience 2005; 136 :971–89 [ PubMed ] [ Google Scholar ] . See also Xiong YE, Mahmood A, Chopp M. Animal models of traumatic brain injury . Nature Reviews Neuroscience 2013; 14 :128–42 [ PMC free article ] [ PubMed ] [ Google Scholar ] . And see commentary by Farber: Farber N. Drug development in brain injury. International Brain Injury Association ; available at http://www.internationalbrain.org/articles/drug-development-in-traumatic-brain-injury/ (last accessed 7 Dec 2014).
33. Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: Past experience and current developments . Neurotherapeutics 2010; 7 :115–26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
34. Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development in Alzheimer’s disease: An appraisal from 1984 to 2014 . Journal of Internal Medicine 2014; 275 :251–83 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
35. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases . Proceedings of the National Academy of Sciences USA 2013; 110 :3507–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
36. Palfreyman MG, Charles V, Blander J. The importance of using human-based models in gene and drug discovery . Drug Discovery World 2002. Fall:33–40 [ Google Scholar ] .
37. See note 2, Perel et al. 2007.
38. Harding A. More compounds failing phase I. The Scientist 2004 Sept 13; available at http://www.the-scientist.com/?articles.view/articleNo/23003/title/More-compounds-failing-Phase-I/ (last accessed 2 June 2014).
39. See note 5, Pippin 2013.
40. See note 5, Hartung, Zurlo 2012.
41. Wiebers DO, Adams HP, Whisnant JP. Animal models of stroke: Are they relevant to human disease? Stroke 1990; 21 :1–3. [ PubMed ] [ Google Scholar ]
42. See note 15, Akhtar et al. 2008.
43. See note 2, Akhtar et al. 2009.
44. Lonjon N, Prieto M, Haton H, Brøchner CB, Bauchet L, Costalat V, et al. Minimum information about animal experiments: Supplier is also important . Journal of Neuroscience Research 2009; 87 :403–7. [ PubMed ] [ Google Scholar ]
45. Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception . Pain 1999; 80 :67–82. [ PubMed ] [ Google Scholar ]
46. Tator H, Hashimoto R, Raich A, Norvell D, Fehling MG, Harrop JS, et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury . Journal of Neurosurgery: Spine 2012; 17 :157–229. [ PubMed ] [ Google Scholar ]
47. See note 35, Seok et al. 2013, at 3507.
48. Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, et al. Tissue-specific transcriptional regulation has diverged significantly between human and mouse . Nature Genetics 2007; 39 :730–2 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
49. Horrobin DF. Modern biomedical research: An internally self-consistent universe with little contact with medical reality? Nature Reviews Drug Discovery 2003; 2 :151–4. [ PubMed ] [ Google Scholar ]
50. Vassilopoulous S, Esk C, Hoshino S, Funke BH, Chen CY, Plocik AM, et al. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism . Science 2009; 324 :1192–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
51. See Guttman-Yassky E, Krueger JG. Psoriasis: Evolution of pathogenic concepts and new therapies through phases of translational research . British Journal of Dermatology 2007; 157 :1103–15 [ PubMed ] [ Google Scholar ] . See also The mouse model: Less than perfect, still invaluable. Johns Hopkins Medicine ; available at http://www.hopkinsmedicine.org/institute_basic_biomedical_sciences/news_events/articles_and_stories/model_organisms/201010_mouse_model.html (last accessed 10 Dec 2014). See note 23, Gawrylewski 2007. See note 2, Benatar 2007. See note 29, Perrin 2014 and Wilkins et al. 2011. See Cavanaugh S, Pippin J, Barnard N. Animal models of Alzheimer disease: Historical pitfalls and a path forward. ALTEX online first; 2014 Apr 10. And see Woodroofe A, Coleman RA. ServiceNote: Human tissue research for drug discovery . Genetic Engineering and Biotechnology News 2007; 27 :18 [ Google Scholar ] .
52. Lane E, Dunnett S. Animal models of Parkinson’s disease and L-dopa induced dyskinesia: How close are we to the clinic? Psychopharmacology 2008; 199 :303–12. [ PubMed ] [ Google Scholar ]
53. See note 52, Lane, Dunnett 2008.
54. See note 5, Pippin 2013.
55. Bailey J. An assessment of the role of chimpanzees in AIDS vaccine research . Alternatives to Laboratory Animals 2008; 36 :381–428. [ PubMed ] [ Google Scholar ]
56. Tonks A. The quest for the AIDs vaccine . BMJ 2007; 334 :1346–8 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
57. Johnston MI, Fauci AS. An HIV vaccine—evolving concepts . New England Journal of Medicine 2007; 356 :2073–81. [ PubMed ] [ Google Scholar ]
58. See Rossouw JE, Andersen GL, Prentice RL, LaCroix AZ, Kooperberf C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy menopausal women: Principle results from the Women’s Health Initiative randomized controlled trial . JAMA 2002; 288 :321–33 [ PubMed ] [ Google Scholar ] . See also Andersen GL, Limacher A, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial . JAMA 2004; 291 :1701–12 [ PubMed ] [ Google Scholar ] .
59. Lemere CA. Developing novel immunogens for a safe and effective Alzheimer’s disease vaccine . Progress in Brain Research 2009; 175 :83. [ PMC free article ] [ PubMed ] [ Google Scholar ] .
60. Allen A. Of mice and men: The problems with animal testing. Slate 2006 June 1; available at http://www.slate.com/articles/health_and_science/medical_examiner/2006/06/of_mice_or_men.html (last accessed 10 Dec 2014).
61. Attarwala H. TGN1412: From discovery to disaster . Journal of Young Pharmacists 2010; 2 :332–6 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
62. See Hogan RJ. Are nonhuman primates good models for SARS? PLoS Medicine 2006; 3 :1656–7 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See also Bailey J. Non-human primates in medical research and drug development: A critical review . Biogenic Amines 2005; 19 :235–55. [ Google Scholar ]
63. See note 4, Wall, Shani 2008.
64. Lemon R, Dunnett SB. Surveying the literature from animal experiments: Critical reviews may be helpful—not systematic ones . BMJ 2005; 330 :977–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
65. Roberts I, Kwan I, Evans P, Haig S. Does animal experimentation inform human health care? Observations from a systematic review of international animal experiments on fluid resuscitation . BMJ 2002; 324 :474–6 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
66. See note 60,Allen 2006. See also Heywood R. Target organ toxicity . Toxicology Letters 1981; 8 :349–58 [ PubMed ] [ Google Scholar ] . See Fletcher AP. Drug safety tests and subsequent clinical experience . Journal of the Royal Society of Medicine 1978; 71 :693–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
67. See note 60, Allen 2006. See note 5, Pippin 2013. See also Greek R, Greek J. Animal research and human disease . JAMA 2000; 283 :743–4 [ PubMed ] [ Google Scholar ] .
68. See note 60, Allen 2006. See also note 5, Leist, Hartung 2013.
69. Food and Drug Administration. Development & approval process (drugs); available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ (last accessed 7 Dec 2014). See also http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm (last accessed 7 Dec 2014).
70. Drug discovery pipeline. IRSF; available at http://www.rettsyndrome.org/research-programs/programmatic-overview/drug-discovery-pipeline (last accessed 24 Sept 2014).
71. See note 60, Allen 2006.
72. Follow the yellow brick road. Nature Reviews Drug Discovery 2003;2:167, at 167.
73. See note 5, Pippin 2013.
74. For data on aspirin, see Hartung T. Per aspirin as astra … Alternatives to Laboratory Animals 2009; 37 (Suppl 2 ):45–7 [ PubMed ] [ Google Scholar ] . See also note 5, Pippin 2013. For data on penicillin, see Koppanyi T, Avery MA. Species differences and the clinical trial of new drugs: A review . Clinical Pharmacology and Therapeutics 1966; 7 :250–70 [ PubMed ] [ Google Scholar ] . See also Schneierson SS, Perlman E. Toxicity of penicillin for the Syrian hamster . Proceedings of the Society for Experimental Biology and Medicine 1956; 91 :229–30. [ PubMed ] [ Google Scholar ]
75. See note 67, Greek, Greek 2000.
76. Oral bioavailability of blockbuster drugs in humans and animals. PharmaInformatic . available at http://www.pharmainformatic.com/html/blockbuster_drugs.html (last accessed 19 Sept 2014).
77. Sams-Dodd F. Strategies to optimize the validity of disease models in the drug discovery process . Drug Discovery Today 2006; 11 :355–63 [ PubMed ] [ Google Scholar ] .
78. Zurlo J. No animals harmed: Toward a paradigm shift in toxicity testing . Hastings Center Report 2012;42. Suppl:s23–6 [ PubMed ] [ Google Scholar ] .
79. There is no direct analysis of the amount of money spent on animal testing versus alternatives across all categories; however, in 2008 the Chronicle of Higher Education reported that funding of research involving animals (under basic research) of the National Institute of Health (NIH) remained steady at about 42 percent since 1990. See Monastersky R. Protesters fail to slow animal research. Chronicle of Higher Education 2008:54. In 2012, NIH director Francis Collins noted that the NIH’s support for basic research has held steady at 54 percent of the agency’s budget for decades. The remainder of the NIH’s budget is heavily funded toward clinical research, suggesting that preclinical human-based testing methods are much less funded. See also Wadman M. NIH director grilled over translational research centre. Nature News Blog 2012 Mar 20. Available at http://blogs.nature.com/news/2012/03/nih-director-grilled-over-translational-research-center.html (last accessed 5 Mar 2015). There is no data that suggests that the NIH’s funding of animal experimentation has decreased. A 2010 analysis estimates that at least 50 percent of the NIH’s extramural funding is directed into animal research; see Greek R, Greek J. Is the use of sentient animals in basic research justifiable? Philosophy, Ethics, and Humanities in Medicine 2010; 5 :14 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
80. For a helpful discussion on animal pain, fear, and suffering, see DeGrazia D. Taking Animals Seriously: Mental Lives and Moral Status . New York: Cambridge University Press; 1996:116–23. [ Google Scholar ]
81. See Akhtar A. Animals and Public Health: Why Treating Animals Better Is Critical to Human Welfare . Hampshire, UK: Palgrave Macmillan; 2012:chap. 5.
- Share full article
Advertisement
Supported by
student opinion
Is Animal Testing Ever Justified?
The E.P.A. recently said it would move away from requiring the testing of potentially harmful chemicals on animals. Do you support the decision?

By Natalie Proulx
Find all our Student Opinion questions here.
On Sept. 10, the Environmental Protection Agency said it would move away from requiring the testing of potentially harmful chemicals on animals, a decision that was hailed by animal rights groups but criticized by environmentalists and researchers who said the practice was necessary to rigorously safeguard human health.
What are your thoughts on animal testing? Do you think it is ever justified? Why or why not?
In “ E.P.A. Says It Will Drastically Reduce Animal Testing ,” Mihir Zaveri, Mariel Padilla and Jaclyn Peiser write about the decision:
The E.P.A. Administrator Andrew Wheeler said the agency plans to reduce the amount of studies that involve mammal testing by 30 percent by 2025, and to eliminate the studies entirely by 2035, though some may still be approved on a case-by-case basis. The agency said it would also invest $4.25 million in projects at four universities and a medical center that are developing alternate ways of testing chemicals that do not involve animals. “We can protect human health and the environment by using cutting-edge, ethically sound science in our decision-making that efficiently and cost-effectively evaluates potential effects without animal testing,” Mr. Wheeler said in a memo announcing the changes. The E.P.A. has for decades required testing on a variety of animals — including rats, dogs, birds and fish — to gauge their toxicity before the chemicals can be bought, sold or used in the environment.
The article continues:
The practice of testing with animals has long prompted complex debates driven by passionate views on morality and scientific imperative. Reaction to Tuesday’s announcement was no different. “We are really excited as this has been something we’ve wanted for quite some time,” said Kitty Block, the president and chief executive of the Humane Society of the United States, an animal protection organization. “The alternatives are the future. They’re more efficient and save lives.” Kathleen Conlee, the vice president of animal research issues at the Humane Society, said the E.P.A.’s move is “broad-sweeping and significant.” “This is the first time a government agency has made such a commitment and timelined its specific goals along the way,” Ms. Conlee said. “There’s been a lot of positive action among other federal agencies, but we want to see all government agencies take this step.” Tracey Woodruff, a professor at the University of California, San Francisco’s school of medicine, said current alternatives to animal testing are somewhat useful. But Dr. Woodruff, who worked at the E.P.A. from 1994 to 2007, said only animal testing — a process honed over decades — was robust enough to gauge chemicals’ impacts on people of various ages, genetics and health backgrounds. “I definitely think we should be investing more in this research,” she said, referring to alternative testing. “But it’s really not ready for making decisions yet — at least the way that E.P.A. is making decisions.” Jennifer Sass, a senior scientist at Natural Resources Defense Council, an environmental advocacy group, said she was very concerned by the announcement. Dr. Sass said animals were still necessary to study chronic conditions, like cancer and infertility. Cells in a petri dish cannot yet replace whole living systems, she said. “The E.P.A.’s deadline is arbitrary,” Dr. Sass said. “Our interest isn’t in speed, it’s getting it right. We want proper animal testing because we don’t want harmful chemicals to end up in our food, air and water.”
Students, read the entire article, then tell us:
Do you support the decision by the E.P.A. to move away from requiring the testing of potentially harmful chemicals on animals? Or do you think animal testing is still necessary to regulate harmful substances that can have adverse effects on humans?
How important is it to you that the toxicity of chemicals and other environmental contaminants is rigorously studied and regulated? Why? Do you think not testing on animals hinders those efforts?
The Food and Drug Administration, the National Institutes of Health and the Department of Veterans Affairs are among the government agencies that still rely on animal testing. Do you think animal testing is important in these sectors or any others? Why or why not?
Do you think animal testing is ever justified? If so, what should be the criteria for when, how and on what animals testing is done?
Students 13 and older are invited to comment. All comments are moderated by the Learning Network staff, but please keep in mind that once your comment is accepted, it will be made public.
Natalie Proulx joined The Learning Network as a staff editor in 2017 after working as an English language arts teacher and curriculum writer. More about Natalie Proulx

Cruelty Free International
subtitle: Working to create a world where no animals suffer in a laboratory
breadcrumb navigation:
- Latest news and updates /
- current page Ten reasons why we should turn our backs on animal testing
Ten reasons why we should turn our backs on animal testing
Published on 24 August 2017
Updated: 17 December 2021
Alternatives report calls on scientists to end cosmetics tests on animals
This week, our new report on the alternatives available to animal testing was presented to scientists at the 10th World Congress on Alternatives and Animal Use in the Life Sciences in Seattle.
The report, funded by The Body Shop, lists alternatives to cosmetics testing on animals, and outlines how they are not only more ethical but also more reliable, faster and cheaper than the animal tests they replace.
According to our report, here are TEN reasons why making animals suffer to bring a new eye shadow or cologne into the world belongs in the past:
- Animal tests are already being replaced . Every year the development of alternatives to animal testing is growing. Thanks to advancements in science, animals are being replaced in the testing of cosmetics as well as chemicals and drugs.
- There are simple alternatives available . Alternatives to animal testing for cosmetics include tests on simple organisms like bacteria, or tissue and skin cells donated from people. Some tests can even be carried out on computers or by using chemicals.
- Millions of animals are suffering and dying . Half a million animals are used in cosmetics testing globally every year. It’s time for the cruelty to stop.
- People want animal testing to end . This year Cruelty Free International and The Body Shop joined forces to launch a global campaign for a worldwide ban on animal testing on cosmetics products and ingredients. So far over two million people have signed our petition calling for an end to the cruelty everywhere and forever.
- There is a global trend towards cruelty free cosmetics . Countries all around the world are phasing out animal testing for cosmetics and switching to innovative alternatives. Bans already exist in 39 countries (including the EU) and are being considered in Australia, Brazil and the US.
- Shoppers prefer to buy cosmetics that have not been tested on animals . A whopping 79% of people said they would be likely to swap to a different brand if they discovered that animals were forced to suffer for the cosmetics they used.
- The alternatives to animal testing are much quicker . Tests on tissue and cells in laboratories for skin and eye irritation can be carried out in one day. The same tests carried out on live rabbits takes from two to three weeks.
- Alternatives to animal testing are much cheaper. Tests using computer models can be run at very little cost, saving thousands of pounds. Some cell-based tests cost as little as £500 compared to cruel tests on animals which take two years and cost approximately £1 million.
- Alternatives to animal experiments are more effective . The harmful use of animals in experiments is not only cruel but also often ineffective. While a combination of chemical and human cell tests has been shown to accurately predict human skin reactions 90% of the time, skin irritation tests on rabbits only predict human reactions 60% of the time.
- Celebrities have joined the fight against animal testing . Our high-profile friends are telling the world it’s time to end cosmetics testing on animals, including Game of Thrones star Maisie Williams who is backing our campaign with the Body Shop for a global ban on cosmetics animal testing.
Dr Katy Taylor , Director of Science & Regulatory Affairs at Cruelty Free International , said: “The public desperately wants to see an end to the suffering of animals used to test cosmetics. With better and more sophisticated alternatives now available, governments and decision-makers should take action to end the horrific practice once and for all.”
Cruelty Free International and The Body Shop are aiming to deliver a petition – with a target of 8 million signatures – to the United Nations in 2018, to request an international convention banning cosmetics testing on animals everywhere and forever. If you agree that cosmetics experiments on animals are cruel and it’s time for them to stop, please sign the petition

Recent Posts
- Join NAVS at the Regeneron International Science and Engineering Fair
- Honor World Day for Animals in Laboratories with Your Support
- Unveiling the Hidden Realities of Animal Research: A Call for Transparency and Change
- NAVS and Beagle Freedom Project – Together We’re Making a Difference
- In Memory of Steven Wise
- February 2024
- October 2023
- September 2023
- August 2023
- January 2023
- November 2022
- October 2022
- September 2022
- August 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- February 2020
- Uncategorized
Five Reasons to End Animal Testing

Dr. Thomas Hartung, a well-known toxicologist at Johns Hopkins University and advocate for the development of alternatives to the use of animals, as well as a mentor to a current NAVS/IFER fellowship recipient , recently published an article in ALTEX summarizing the main limitations of experimenting on animals. As Hartung notes, “[Animal experiments] come with shortcomings, and their true contribution is often overrated.”
Following are five reasons why scientists should stop relying so heavily on animal models:
- Animal experiments are not very reproducible . This often stems from lack of bias-reducing measures, poorly planned experiments, inappropriate statistical tests, poor reporting on animal attrition (why animals are dropped from studies) and poor reporting of pain relief in lab animals.
- Animal experiments are expensive and time consuming. Hartung notes that “costs and duration of toxicological studies are clearly prohibitive to satisfy societal safety needs,” in large part because animals are experimented on over a long period of time, during which researchers collect and analyze a lot of data to ensure that they are not missing any harmful effects. Using other approaches, such as cell-based models, could help scientists get results faster and cheaper.
- There are ethical concerns with animal experimentation. What entitles humans to experiment on animals and inflict pain upon them?
- Animal experiments are not even predictive of other animal species, let alone humans. Experiments performed in rats do not predict what happens in mice. Experiments in one strain of mice may not predict what is seen in another strain. Therefore, we shouldn’t assume that animals will be able to accurately predict what happens in humans.
- Animal models do not reflect human diversity . Even if animals could be predictive, to which humans would the data be accurately applied, considering the differences among humans?
Some in the scientific community have suggested investing more time and effort to improve the design of animal experiments, or even the animal models themselves, to address some of the issues raised above. But these resources would pay much higher dividends if they were directed to more human-relevant research, including work with human cell lines, stem cells and tissues, computational models, and even humans themselves.
Help NAVS support the advancement of smarter, human-relevant science that does not harm animals by making a donation today.
Source: Hartung, T. “Opinion versus evidence for the need to move away from animal testing,” ALTEX , 2017

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.10: Animal Testing Should Be Banned
- Last updated
- Save as PDF
- Page ID 35854

- Nathan Nobis
- Morehouse College via Open Philosophy Press
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
“Animal testing” involves experimenting on animals to try to determine whether drugs and medical treatments are safe and effective for humans. It’s wrong and should be banned.
Why? First, and most obviously, drugs and medical procedures treat diseases, injuries, and other health problems. So, to see if a treatment works, a disease or injury must be created in animals. Understatement: this is often unpleasant. Heart attacks in dogs feel awful; bone cancers in mice are painful; pigs being burned, to test burn treatments, is agonizing. Animals living with the induced conditions is unpleasant also. And they are killed at the end of the experiments to study the treatments’ effects.
It’s now easy to see why animal testing is wrong: it violates basic principles of ethical research: it is maleficent, or harmful to the research subjects; it is not beneficial to them; it is forced on them since they don’t consent; and it is unjust in that animals are burdened with problems not their own. Research – at least with animals who are conscious, and so are able to be harmed or made worse off – is wrong for reasons that comparable human research would be wrong.
Some argue that the benefits to humans justify animal testing. But when one group benefits at the major expense of another group, that’s usually wrong. And how exactly might anyone know that humans benefit more than animals are harmed? And there is scientific evidence that animal testing often is not beneficial for humans and that clinical research, public health research, and technology-based research are more useful: see the Physicians Committee for Responsible Medicine and Americans for Medical Advancement for more information.
Some claim there are “no alternatives” to animal testing, that it is “necessary.” But there are alternatives (mentioned above) and it’s not literally necessary that anyone do it: they can refrain. But suppose someone wanted to rob a bank and needed a getaway car: there is “no alternative” to a car and so it is “necessary” for the robbery. Does that make using the car OK? No. Even if something is “necessary” and there are “no alternatives” to doing it to achieve a particular end, that doesn’t make doing the action right: the end determines that.
Finally, some say that this reasoning is all beside the point: if your child was dying and animal testing would save him or her, wouldn’t you want the testing done? Many would and that’s an understandable feeling. But it’s unlikely that animal experimentation would help their child much: other methods are likely more fruitful. And more importantly, if my child were dying and I tried to experiment on my neighbor’s children to try to save my own child, that would be wrong.
Why? Simply because those children would be harmed and treated as mere things to be used (and abused) for my and my child’s benefit, which they are not. Since those reasons apply to many animals experimented upon, animal testing is also wrong.
Home — Essay Samples — Social Issues — Animal Testing — The Reasons Why Animal Testing Should Be Stopped
The Reasons Why Animal Testing Should Be Stopped
- Categories: Animal Cruelty Animal Testing
About this sample

Words: 1186 |
Published: Mar 18, 2021
See expert comments
Words: 1186 | Pages: 3 | 6 min read

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Prof. Kifaru
Verified writer
- Expert in: Law, Crime & Punishment Social Issues

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
1 pages / 642 words
2 pages / 878 words
2 pages / 1002 words
3 pages / 1351 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Animal Testing
Animal testing is a worldwide controversy that is constantly battling between the benefits and drawbacks of using animals for scientific and commercial testing. They have been used for many things like testing make-up products, [...]
The issue of whether animal testing should be banned has sparked intense debate among scientists, ethicists, policymakers, and animal rights advocates. This essay aims to analyze the arguments both for and against banning animal [...]
Animal research has long been a contentious issue, with proponents touting its benefits to scientific progress and detractors decrying its ethical implications. This essay explores both sides of the debate and offers a [...]
Animal testing is a controversial topic that has sparked heated debates among scientists, ethicists, and the general public. The ethical implications of using animals in scientific research are complex and multifaceted, with [...]
Balls, M., & Fabre, I. (2019). Alternatives to Animal Testing: A Review. European Pharmaceutical Review, 24(6), 29-33.Ekwall, B. (2000). The Multicentre Evaluation of In Vitro Cytotoxicity (MEIC) Programme: A Model for [...]
Since long time ago animals starting from mice to cows have been used for research. There are lots of examples of testing these or that phenomena on animals. But is it correct? Is it what a human should do? And what well-known [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free

Essay on Why Animal Testing Should Be Banned
Students are often asked to write an essay on Why Animal Testing Should Be Banned in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Why Animal Testing Should Be Banned
Animal testing is unkind.
Animal testing often causes pain and suffering to animals. They are kept in small cages and are used for experiments that can hurt them. This is not fair because animals feel pain just like we do. We should not make them suffer for our benefits.
Not Always Useful
Many times, tests on animals do not give results that are helpful for humans. This is because animals and humans are different. So, the pain we cause to animals may not even help us in the end.
Better Options Exist
Nowadays, we have other ways to test products that do not involve hurting animals. Scientists can use computer models or grow human cells in labs. These methods can give us good information without causing harm to any living creature.
250 Words Essay on Why Animal Testing Should Be Banned
Animal testing: why it should be banned.
Animal testing refers to scientific experiments using non-human animals as subjects. For many years, animals have been used in experiments to study diseases, test medicines, and explore other scientific questions. However, there are many reasons why this practice should be banned.
Pain and Suffering
Animals experience pain and suffering just like humans do. In animal testing, animals are often subjected to painful procedures, such as surgery, injections, and exposure to harmful chemicals. They may be kept in cramped and unsanitary conditions and denied proper food, water, and veterinary care.
Unreliable Results
Animal testing results are often unreliable when applied to humans. Animals may respond differently to drugs and treatments than humans do, leading to inaccurate or misleading findings. Additionally, the stress and fear experienced by animals during testing can affect the results, making them even less reliable.
Alternatives to Animal Testing
Today, there are many alternative methods available that can replace animal testing. These methods include computer simulations, cell cultures, and human tissue models. These alternatives are not only more humane, but they are often more accurate and reliable than animal testing.
Ethical Concerns
Animal testing raises serious ethical concerns. Many people believe that it is wrong to harm or kill animals for the sake of scientific research. Animals are sentient beings who deserve to be treated with respect and compassion.
In light of the pain and suffering caused to animals, the unreliability of results, the availability of alternatives, and the ethical concerns, it is clear that animal testing should be banned. It is time to move towards a more humane and ethical approach to scientific research.
500 Words Essay on Why Animal Testing Should Be Banned
What is animal testing.
Animal testing is the use of animals in experiments and other scientific studies. Animals are used to test products such as medicines, cosmetics, and chemicals, as well as to study diseases and develop new treatments.
Why is Animal Testing Bad?
Animal testing is bad because it causes pain and suffering to animals. Animals are often subjected to painful procedures, such as surgery, injections, and exposure to toxic chemicals. They may also be deprived of food, water, and sleep.
Animals Are Not Like Humans
The results of animal tests are not always accurate because animals are not like humans. They have different bodies, different metabolisms, and different immune systems. This means that drugs and chemicals that are safe for animals may not be safe for humans.
There Are Alternatives to Animal Testing
There are many alternatives to animal testing that are more accurate and humane. These alternatives include computer models, cell cultures, and human tissue samples. These alternatives are often more cost-effective than animal testing and do not cause pain and suffering to animals.
Animal Testing is Cruel and Unnecessary
Animal testing is a cruel and unnecessary practice. There are many alternatives to animal testing that are more accurate and humane. We should ban animal testing and replace it with these alternatives.
Animal testing is a cruel and unnecessary practice. It causes pain and suffering to animals and is often inaccurate. There are many alternatives to animal testing that are more accurate and humane. We should ban animal testing and replace it with these alternatives.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Which Economic System Is The Best And Why
- Essay on Where Do You See Yourself In The Future
- Essay on Whatsapp Boon Or Bane
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Invertebrates
- Best Dog Collar
- Best Smart Dog Collar
- Halo Dog Collar
- Spoton vs Halo Collar
- Wagz Freedom Dog Collar
- SpotOn Dog Collar
- Best Dog Fence
- Best Invisible Dog Fence
- Dog Accessories
- Dog Training
- Dog Harness
- Dog Leashes
- Dog Grooming
- CBD for Dogs
- Dog Age Calculator
- Dog BMI Calculator
- Dog Chocolate Toxicity Calculator
- Dog Food Calculator
- Dog Harness Size Calculator
- Dog Life Expectancy Calculator
- Puppy Weight Calculator
- Dog Water Intake Calculator
- Dog Crate Size Calculator
- Dog Onion Toxicity Calculator
- Dog Raisin Toxicity Calculator
- Pet Sitter Rates Calculator
- Dog Groomer Tip Calculator
- Dog Quality of Life Calculator
- Cost of Owning a Dog Calculator
- Raw Dog Food Calculator
- Dog Dad Shirts
- Dog Mom Shirts
- Automatic Litter Box
- Cat Accessories
- Cat Collars
- Cat Grooming
- Cat Mom Shirts
- Animal Abuse
- Animal Laws
- Animal Rights
- Animal Testing
- How to Help
- Facts & Stats
- Our Campaigns
Animal Testing Should Be Banned Completely – A Heart-Wrecking Situation
Monika Martyn
January 19, 2023.

WorldAnimalFoundation.org is reader-supported. When you buy through links on our site, we may earn an affiliate commission. Learn More
It’s hard to imagine, as an intelligent species, we’re still discussing animal experimentation. There’s no doubt that animal testing is to the apparent benefit of people. However, that doesn’t make it right that over 100 million innocent animals suffer for our well-being.
Animal experimentation needs to end. It’s not a question of right or wrong. Animals have feelings and the right to live without cruelty inflicted on them for the sake of testing chemicals. Period.
So why do we continue to abuse, cage, cripple, infect, and kill dogs, cats, monkeys, mice, and rats in animal experiments? This is not about thanking the cosmetic industry (Europe, India, Israel) for condemning and banning animal tests. It’s about saving the 100 million abused animals in American laboratories.
Here’s what you need to know to stop the abuse and end animal experiments.
Why Is Animal Testing Bad?
It just is. Animal testing andanimal experiments inflict inhumane suffering on animals. These creatures never consented to have experiments conducted on their bodies, skin, or DNA.
The debate over whether it’s right divides the room. Many people believe animal testing is barbaric and outdated. Others argue that scientific progress has helped save millions of human lives.
It’s Unethical
There are billions of great people in this world. Together, we need to end torturing 100 million animals. Animals experience pain, loneliness, fear, and emotions, just like us. When they have to endure scientific and medical experiments, it’s incomprehensible.
Animal Testing Is Unreliable
Animals and humans share some similarities. It’s one of the main reasons the debate gets so heated: animals have feelings too.
Our differences contribute to the failure of human clinical trials. Many of the pharmaceuticals end up being too dangerous and ineffective for human consumption. That means we subject innocent animals to horrendous suffering for a small return of success.
Up to 95% of experimental drugs that pass animal tests fail in human and clinical trials.
Additionally, some medicines that may work for us are dangerous for animals. For example, aspirin is toxic to animals but is safe for human use. We wouldn’t be able to get it from pharmacies if it had been tested using current animal testing standards.
It’s Bad Science
People believe in science as proof. Therefore, according to the National Institute of Health, 95 out of 100 drugs developed with lab animals fail. In no other facet of life would we accept these results as a ‘good idea.’ It’s proof that animal experiments don’t work.
Animal Testing Is Dangerous for Humans

Sometimes math and science don’t work. A few years ago, big pharma pushed a new wonder drug, Vioxx, to treat arthritis patients. It was a welcomed relief. Lab monkeys and five other animal species showed improvements on paper.
Yet, in the aftermath, patients who took the prescribed pills faced a graver issue. Studies showing 320,000 heart attack and stroke victims proved that lab results didn’t help humans. Sadly, 140,000 people died because of Vioxx.
Another clinical trial ended badly for patients who either suffered severe liver damage or death from Hepatitis B due to a drug experiment conducted on animals.
In 2016, another miracle drug that was reportedly going to treat all kinds of conditions killed a volunteer and left four patients with devastating brain damage. This experimental drug passed muster on mice, rats, dogs, and monkeys. Sadly, nobody reported how those animal subjects felt after having the medication forced on them.
In a new monoclonal antibody treatment tested on monkeys at 500 times the recommended human dose, human volunteers suffered near-fatal allergic reactions.
Human beings often volunteer for human trials to help find cures for human diseases. Animal research facilities should promote the health of animals, not human health.
It’s Wasteful
No doubt, we want our loved ones to receive the best care. Unfortunately, animal experiments only make the grade about half the time. The rest end up in the trash as failed and worthless. The sad part is that research using lab animals takes enormous resources and squanders money, time, human intelligence, and creativity.
All that waste causes human suffering on top of animal suffering. According to Dr. Richard Klausner, “We’ve cured mice of cancer for decades. It simply didn’t work on humans.”
Cancer is nasty. But if animals ultimately fail in medical research or other laboratory experiments, why don’t researchers use advanced technology to test harmful substances?

It’s Archaic
Scientists have compassion too. Many have created modern, effective non-animal testing methods that are cost-effective, fast, and deliver more accurate results without animal testing. These non-animal methods include micro-dosing, in vitro testing, organs-on-chips , simulators, and advanced computer modeling technology.
Humans share genetic information and DNA with plants and animals. For example, cows and humans share about 80%, and common fruit flies about 61%. A banana has 60% human DNA.
It’s possible to look at this debate from two sides. One is to explore the common DNA and use it to our benefit. The other is if they share that much genetic material with us, does it make sense to harm them?

Animals Feel Pain
Evolutionary biologist Marc Bekoff and his many colleagues have done all the necessary research to prove animals feel pain. Mammals share strong similarities in the nervous system, chemical transmitters, emotional states, and perceptual tools to prove animals experience pain. That they experience pain differently is irrelevant.

The Animal Welfare Act should protect animals. Yet lab animals like mice, rats, reptiles, amphibians, and birds used in labs don’t fall under their protection. Instead, lab animals endure inhumane procedures and treatments like scorched skin, immobilization, inhaling toxic fumes, and holes drilled in their skull and spinal cords crushed.
Often, lab animals receive no administered pain relief and are left to suffer intense pain. These institutions, by law or regulations, don’t have to provide any.
Yet, in experiments, when many animals like rats, mice, and chickens trapped in barren cages have access to self-administered pain relief, they use it to reduce the pain. Wild animals also nurse their wounds, show distress, and seek shelter.
They learn to avoid situations that relate to bad experiences with pain. This action indicates that animals are aware of the pain and can associate it with experiences from their past.
It’s Unnecessary
It’s challenging to review pictures of animals used without consent and not form an opinion.
The main reason for banning animal testing, aside from sparing animals the pain, is that we don’t need it. Animal testing should not be part of a university lab experiment paid for by the tax-payer who is against animal torture in the first place.
Animal Testing Is Dangerous for Non-Human Animals
That is the point. It’s not only dangerous, cruel, painful, and inhumane; some of the methods harken back to medieval torture chambers.
Imagine mice, rabbits , rats, and guinea pigs with their eyes burned from drip chemicals or toxic potions smeared into their exposed skin tissue without pain medication.
It’s hard to think about a human consenting to the Draize or LD50 Test. This test measuring toxicity often leads to blindness, scarring, death, and insurmountable pain.
Years from now, the LD50 will be on display in museums as one of the wickedest torture animal tests. This substance test is inflicted on animals to be fatal in 50% of the test subjects. Researchers strap animals to tubes and inject the test substance directly into the sequestered animals’ stomachs. Until they die, which can take days or weeks, animals suffer.
The animals die agonizingly, suffering internal bleeding, diarrhea, vomiting, paralysis, convulsions, and horrendous pain. Death becomes their relief.
Facts About Animal Testing
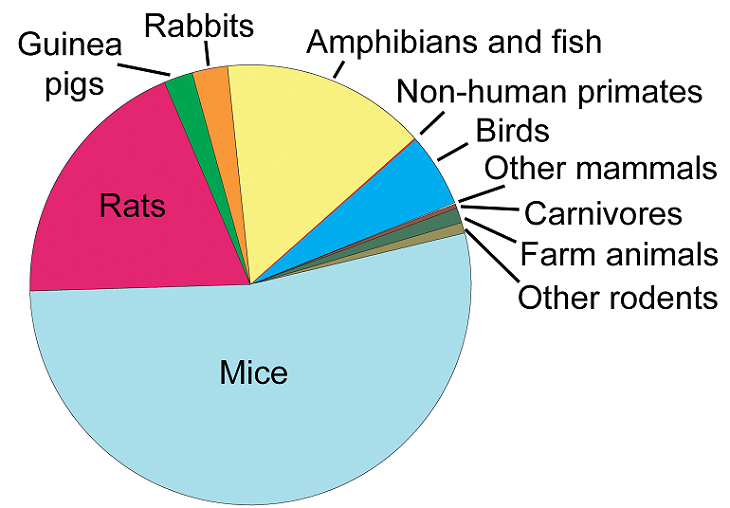
The first fact is that animals suffer. The second is that we must stop animal testing.
The law requires 12,000 animals subjected to over 50 experiments to endure for a company to register a single pesticide. No one argues that pesticides must meet safety standards.
According to the statistics reviewed by the National Institute of Health, only 5% of drugs tested on animals show positive results, while 95% are worthless. That is a bad score. Sixty percent of no-consent animals are exposed to biomedical research and product safety testing.
According to the Humane Society, animals and humans are very different. Animal subjects don’t suffer from the same illnesses as humans. So why are we testing on animals when they don’t contract many human illnesses? HIV, schizophrenia, Parkinson’s disease, heart disease, and certain types of cancer are human diseases.
Substances that cause cancer in people affect animals differently, and only one-third cause cancer in animals. Animal research conducted on over 100 mouse cell types discovered that regulating genes in mice match human DNA. How can you create an exact science and get valid human responses on that foundation?
How to Stop Animal Testing?
Every day, we use products that cost an animal its life or severe pain. Awareness campaigns are only a starting point. Choosing animal cruelty-free products is another. The science supporting animal testing stands on faulty ground. Instead, all industries should concentrate on using new methods and technologies to conduct research that works.
Some industries, like cosmetic companies, are making strides and not testing on animals. However, there’s much room for improvement in the household cleaner, deodorant, fem hygiene, and thousands of pharmaceutical products.

Animal rights activists paved the way to end suffering for endangered species , lab animals, and domesticated animals.
Alternative Ways of Experimenting
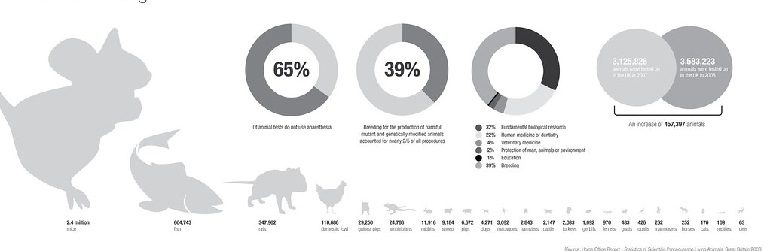
People are smart. Technology has exploded in the last few decades. We can use human cells and tissue, 3D printing, robots, and computer modeling to get more accurate results faster. They’re also cost-effective and don’t subject animals to cruel and unnecessary animal tests.
If this was painful to read, the article has done its job.
In conclusion, animal testing and research must be banned worldwide, as it is against animals’ rights and causes unwanted suffering to lab animals. Also, now there are other available methods to test product toxicity. Cruelty against animals should not be taken lightly just because they are not “humans.”
We’re on the precipice of human evolution and developing a united mindset to stop animal testing once and for all.
Every individual has the power to influence change. Choose cruelty-free products, become involved, champion the cause, and help millions of animals. The USA Government has finally passed a law banning animal testing on cosmetics.
Join the conversation and become an animal advocate. You are the difference!
Leave a comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Latest Posts

7 Types of Hunting Dog Breeds to Join You in the Field

7 Best Guard Dog Breeds in the UK for Personal & Family Protection

9 Most Popular Dog Breeds in UK in 2024

7 Most Popular Dog Breeds in Alabama in 2024

9 Most Popular Dog Breeds in Maryland in 2024

7 Best Guard Dog Breeds in Canada for Protection

10 Most Popular Dog Breeds in Canada in 2024

9 Best Guard Dog Breeds that Love Children and Protect Them

7 Best Guard Dog Breeds for Kids in the United States

7 American Dog Breeds That Don’t Shed Much
Get updates on the latest posts and more from World Animal Foundation straight to your inbox.
Why Animal Testing Should Be Banned: 7 Reasons It Has To Stop

If you’ve landed on this page, chances are you have a pet you love so much or simply adore animals and care about their welfare. For pet lovers, imagine if someone was to subject your furry best friend to months of painful injections. Imagine if your cat was to spend months locked up in a cage just to observe how they react to chemicals. You shudder at that thought, right?
That’s precisely the kind of torture that animals go through during animal experiments. If the disgust you feel right now is not enough to convince you why animal testing should be banned, then keep reading. We explain a few more reasons why we should stop animal testing ASAP.
Learn more: What Is Animal Testing? The Truth Behind Experimenting on Animals
Why Animal Testing Should Be Banned
Despite the continued use of animals in experiments, there are many strong reasons why animal testing should be stopped. Here are some of them:
1. There’s no consent offered
Like you and me, animals can feel, think, and, most importantly, experience pain. But, unlike us, they can’t talk, so they can’t consent to be part of the experiments.
Before a human being can participate in research, they need to sign a consent form that shows they’re aware of what will happen. Can you imagine trying to get an animal to sign a consent form? It’s too silly to even consider. This is why animal research remains popular. There are fewer hurdles to jump through to gain test subjects.
Supporters of animal research say that animals’ lack of knowledge is why they’re perfect for experimentation. But for those of us who believe animal testing should be banned, we think that just because animals aren’t as intelligent as humans doesn’t mean they should be exploited and mistreated. Animals have just as much right to autonomy as we humans do.
2. It produces inaccurate results
92% of drugs that pass animal testing fail human testing. 90% of products that pass animal tests fail to make it to the market. This makes a lot of sense when you think about it. Animals and humans have different physiologies.
We would never test drugs meant for animals on humans. Then why is it a thing to test on animals drugs intended for humans? It definitely shouldn’t be!
3. It’s an old-fashioned and obsolete method
Once upon a time, scientists relied on animals for their research. In the past, the research capabilities of laboratories were not as advanced as they are today. One can argue that the lack of alternatives made animal testing seem necessary. It might have even led to some amazing breakthroughs.
However, in the decades since, scientific innovation has grown. We can grow cells in the labs. In fact, we now have lab-grown body organs. Isn’t that amazing?
This innovation is why we should stop animal testing and move on to the many currently available alternatives.
4. It’s expensive and wasteful
Animal testing isn’t cheap. It can take 10 years of animal tests and $3 million for a single pesticide to get registered. According to the Humane Society International , testing on animals is much more expensive and time-consuming than in-vitro methods. Should animal testing be stopped, we’d have more resources to advance research through alternative methods that don’t require animals or even humans to be tested on.
Think about this for a second: Over 100 million animals are used in experiments yearly, but only 59 new drugs were approved for the market in 2018. Despite the drug industry investing $50 billion annually, we have the same drug approval rate as we did in the 1970s. Is this kind of waste really necessary? I don’t think so.
The cosmetics industry uses animal testing the most . Still, most of the thousands of ingredients found in cosmetics have already been found to be safe. There’s no need to put animals through so much pain and trauma when these ingredients have already been certified safe.
5. There are better alternatives
If there’s one good reason why we should stop animal testing, it’s the fact that alternatives that don’t require animals exist. Thanks to technological advances, many decent alternatives to animal testing exist. In-vitro testing methods work very well and are cheaper. 3-D printing, computer models, and robots can be used instead of animals. Synthetic cellular tissues and eyes can be used to test the irritability of cosmetics.
Should testing on animals be banned, the impact on science and research would be minimal.
6. It’s cruel and painful
Besides being expensive, wasteful, and unnecessary, animal testing is inhumane. According to animal welfare activists, this simple fact is why animal testing should be banned. The animals suffer physically and psychologically even in tests that are described as mild. The different experiments often leave the animal injured or disabled. Some experiments don’t bother with anesthesia or painkillers.
It’s devastating to imagine what those poor creatures are forced to endure. Should animal testing be illegal, we can prevent countless animals from suffering the same fate.

7. It’s simply unethical
It’s unethical to sentence almost 200 million animals to life in a cage and intentionally cause pain, loneliness, and fear just to please human beings.
More Reasons Why Animal Testing Should Be Stopped
Still not convinced? Here are more reasons why animal testing should be banned.
Too many animals are being used in experiments
Globally, over 192 million animals are used in scientific research every year . Over 100 million animals are used in experiments in the United States alone. Truth be told, we don’t know the total number of animals used in animal testing thanks to loopholes in the Animal Welfare Act (AWA).
Animals must be harmed to get proper results
Three types of tests are done: skin and eye irritation, chemical exposure, and lethal dose. In each trial, the animal is harmed to see how it reacts to certain chemicals.
Animal testing is not a legal requirement
The Federal Food, Drug, and Cosmetic Act doesn’t legally require cosmetic animal testing . This means that cosmetics companies don’t need to use animals at all. What’s worse is that most products don’t even need additional testing as they’ve been proven safe. Unbelievable!
Regulated testing is still harmful
Even though The Animal Welfare Act (AWA) allows regulated testing on the animals it protects, 800,000 animals are subjected to regulated tests every year.
While these tests are regulated, and the animals may be subjected to more humane practices, corporations and laboratories are left with injured and traumatized animals once the tests are done. These animals are not given up for adoption due to their disabilities. As such, they’re euthanized.
Banning animal testing worldwide will enhance already existing bans
21% of countries have partial or total bans on animal cosmetic tests. As more people understand the harm caused by these experiments, more and more countries are starting to consider regulating the practice. If the world unanimously agreed to ban animal testing, existing regulations and bans would be taken seriously.
How Can We Stop Animal Testing?
Now that we’ve gone over a good number of reasons why animal testing should be banned let’s delve into how this can be achieved.
To begin with, it’s essential to raise awareness of what a cruel and unnecessary practice of animal testing is. Many people don’t know the true extent of animal testing. By informing them, you can convince them to join the fight against animal testing.
Secondly, it’s vital to use cruelty-free products. These are products that have not been tested on animals. You’re telling corporations that cruelty-free products are the way to go by only buying cruelty-free products. Look for cruelty-free certifications from Cruelty-Free International , Leaping Bunnies , and Beauty Without Bunnies .
Thirdly, we need to push for other alternatives to animal testing . Innovations in science and technology can allow us to develop more humane testing methods that exclude the use of animals as subjects.
Finally, put pressure on your representatives to support legislation that bans animal testing.
The reasons why animal testing should be banned are so many. But the one that should tag your heart the most is just how much pain and torture these beautiful creatures that bring us so much joy go through. Further, the tests cost millions of dollars and can take years to complete. After all of that, there’s a slight chance that the end result is beneficial to humans. Meanwhile, the animals are suffering greatly for something so unnecessary. It is a tragedy, and we should all work together to stop it.
You Might Also Like:
- Pros and Cons of Animal Testing

Stella is a writer and mother from Thika, Kenya.
Her love for nature and the beautiful Kenyan outdoors has inspired Stella to consciously make an effort to lead a more sustainable, eco-friendly lifestyle.

Popular Posts
How To Save Water in the Home
Green Cleaning: The Ultimate Guide
Zero Waste Living Guide
Ethical & Sustainable Clothing Guide
105 Ways To Be More Eco Friendly
Modal title

We’re calling on the FDA to save animals by modernizing drug testing
Wednesday, May 15, 2024
By Sara Amundson and Kitty Block
In an era defined by scientific and technological innovation, testing drugs on dogs, rats, monkeys and other animals is not only becoming increasingly outdated but causes immense animal suffering. Despite publicly indicating a commitment to non-animal test methods, the U.S. Food and Drug Administration’s regulations and guidance documents for pharmaceutical companies are unclear and continue to emphasize the use of animals for drug testing. There is evidence that some companies believe testing on animals is legally required as part of the drug approval process.
That’s why we’ve filed a petition with the FDA today , requesting that the agency take a series of steps to make it plain and clear that animal testing isn’t legally required for drug approval and that the agency encourage companies to use non-animal methods when available. The FDA is legally required to consider and respond to our petition.
Tell the FDA to phase out animal testing >>
The petition asks the FDA to make the following changes to its regulations and guidance documents, which are documents that provide additional information on how to comply with its regulations:
- Amend its regulations to make it clear that the FDA does not require animal testing for drugs.
- Publish a new guidance document describing the non-animal test methods that can be used in place of animal tests. The document should be updated regularly as new non-animal methods become available.
- Commit to adding text to all existing and future guidance documents regarding the regulation of drugs. The new text should encourage companies to use non-animal methods whenever possible and refer them to the new guidance document on accepted non-animal methods.
Without these changes, the lack of clarity in FDA regulations will continue to perpetuate the status quo. That means tens of thousands of animals per year continue to suffer in archaic tests such as those we documented in an undercover investigation at a laboratory in 2022. The ambiguity surrounding whether animal testing is needed for drugs to gain approval is harmful in several ways. It creates confusion and discourages innovation. It may also negatively impact human health.
We all agree that the FDA has a responsibility to make sure that drugs intended for people are safe and effective. This is all the more reason why the agency should promote the use of data from non-animal test methods based in human biology; animal testing is not a reliable predictor of safety or efficacy in humans. Animal testing has acknowledged scientific limitations, but innovative non-animal technologies will only continue to improve. Emerging technologies such as organ-on-a-chip models offer promising approaches that are based on human biology and yield more reliable results.
We are not alone in our demand for the adoption of more humane testing methods. University centers devoted to non-animal methods are making the same case, and a pioneering 2007 National Research Council report spurred the creation of numerous governmental initiatives focused on the ultimate replacement of animals in toxicity testing. As a result of our longstanding political advocacy, Congress has also publicly supported and secured increased funding for alternatives to animal use. The time for change is now. You can help prevent countless animals from suffering by urging the FDA to update its drug testing regulations and embrace non-animal alternatives .
By supporting our petition to modernize the FDA’s drug testing regulations and guidance documents, you can help us create a future where compassion and scientific advancement go hand in hand.
Kitty Block is CEO of the Humane Society of the United States.
Stay connected with news and action alerts:

105 Animal Testing Essay Topic Ideas & Examples
Looking for interesting animal testing topics to research and write about? This field is truly controversial and worth studying!
- 🌶️ Titles: Catchy & Creative
- 🐶 Essay: How to Write
- 🏆 Best Essay Examples
- 📌 Good Topics to Research
- 🎯 Most Interesting Topics to Write about
❓ Animal Testing Research Questions
In your animal testing essay, you might want to explore the historical or legal perspective, focus on the issue of animal rights, or discuss the advantages or disadvantages of animal testing in medicine, pharmacology, or cosmetic industry. We’ve gathered the most creative and catchy animal testing titles and added top animal testing essay examples. There are also useful tips on making and outline, formulating a thesis, and creating a hook sentence for your animal testing essay.
🌶️ Animal Testing Titles: Catchy & Creative
- What would life be like without animal testing?
- Animal testing: the cruelest experiments.
- AWA: why does not it protect all animals?
- What if animals experimented on humans?
- In the skin of a guinea pig: a narrative essay.
- Opposing animal testing: success stories.
- Animal-tested products: should they be destroyed?
- What have we gained from experiments on animals?
- Animal testing and cancer research: past and present.
🐶 Animal Testing Essay: How to Write
Animal testing has been an acute problem for a long time. Scientists and pharmaceutical firms use this approach to test cosmetics, foods, and other products people use daily.
Essays on animal testing are important because they highlight the significance of the problem. Writing outstanding animal testing essays requires extensive research and dedication.
We have prepared some do’s and don’ts for your excellent essay. But first, you should select a topic for your paper. Here are the examples of animal testing essay topics you can choose from:
- The question of animal intelligence from the perspective of animal testing
- Animal testing should (not) be banned
- How animal testing affects endangered species
- The history and consequences of animal testing
- The controversy associated with animal testing
- Animal Bill of Rights: Pros and cons
- Is animal testing necessary?
Remember that these animal testing essay titles are just the ideas for your paper. You are free to select other relevant titles and topics for discussion, too. Once you have selected the problem for your essay, you can start working on the paper. Here are some do’s of writing about animal testing:
- Do extensive preliminary research on the issue you have selected. You should be aware of all the problems associated with your questions, its causes, and consequences. Ask your professor about the sources you can use. Avoid relying on Wikipedia and personal blogs as your primary sources of information.
- Develop a well-organized outline and think of how you will structure your paper. Think of the main animal testing essay points and decide how you can present them in the paper. Remember to include introductory and concluding sections along with several body paragraphs.
- Start your paper with a hooking sentence. An animal testing essay hook should grab the reader’s attention. You can present an interesting question or statistics in this sentence.
- Include a well-defined thesis statement at the end of the introductory section.
- Your reader should understand the issue you are discussing. Explain what animal testing is, provide arguments for your position, and support them with evidence from your research.
- Discuss alternative perspectives on the issue if you are working on a persuasive essay. At the same time, you need to show that your opinion is more reliable than the opposing ones.
- Remember that your paper should not be offensive. Even if you criticize animal testing, stick to the formal language and provide evidence of why this practice is harmful.
There are some important points you should avoid while working on your paper. Here are some important don’ts to remember:
- Avoid making claims if you cannot reference them. Support your arguments with evidence from the literature or credible online sources even if you are writing an opinion piece. References will help the reader to understand that your viewpoint is reliable.
- Do not go over or below the word limit. Stick to your professor’s instructions.
- Avoid copying the essays you will find online. Your paper should be plagiarism-free.
- Avoid making crucial grammatical mistakes. Pay attention to the word choice and sentence structures. Check the paper several times before sending it for approval. If you are not sure whether your grammar is correct, ask a friend to look through the paper for you.
Do not forget to look at some of our free samples that will help you with your paper!
Animal Testing Hook Sentence
Your animal testing essay should start with a hook – an opening statement aiming to grab your reader’s attention. A good idea might be to use an impressive fact or statistics connected to experiments on animals:
- More than 100 million animals are killed in US laboratories each year.
- Animal Welfare Act (AWA) does not cover 99% animals used in experiments: according to it, rats, birds, reptiles, and fish are not animals.
- More than 50% adults in the US are against animal testing.
🏆 Best Animal Testing Essay Examples
- Animal Testing: Should Animal Testing Be Allowed? — Argumentative Essay It is crucial to agree that animal testing might be unethical phenomenon as argued by some groups; nonetheless, it should continue following its merits and contributions to the humankind in the realms of drug investigations […]
- Should Animals Be Used in Medical Research? It is therefore possible to use animals while testing the dangers and the toxicity of new drugs and by so doing; it is possible to protect human beings from the dangers that can emanate from […]
- Cosmetic Testing on Animals The surface of the skin or near the eyes of such animals is meant to simulate that of the average human and, as such, is one of easiest methods of determining whether are particular type […]
- The Debate on Animal Testing The purpose of this paper is to define animal testing within a historical context, establish ethical and legal issues surrounding the acts, discuss animal liberation movements, arguments in support and against the act of animal […]
- Experimentation on Animals However, critics of experimenting with animals argue that animals are subjected to a lot of pain and suffering in the course of coming up with scientific breakthroughs which in the long run may prove futile.
- Animal Testing and Environmental Protection While the proponents of animal use in research argued that the sacrifice of animals’ lives is crucial for advancing the sphere of medicine, the argument this essay will defend relates to the availability of modern […]
- Animal Testing in Medicine and Industry Animal testing is the inescapable reality of medicine and industry. However, between human suffering and animal suffering, the former is more important.
- Preclinical Testing on Animals The authors argue that despite the recent decline in the level of quality and transparency of preclinical trials, the scientific communities should always rely on animal testing before moving to human subjects and the subsequent […]
- Using Animals in Medical Research and Experiments While discussing the use of animals in medical research according to the consequentialist perspective, it is important to state that humans’ preferences cannot be counted higher to cause animals’ suffering; humans and animals’ preferences need […]
- Animal Testing: History and Arguments Nevertheless, that law was more focused on the welfare of animals in laboratories rather than on the prohibition of animal testing.
- Laboratory Experiments on Animals: Argument Against In some cases, the animals are not given any painkillers because their application may alter the effect of the medication which is investigated.
- Animal Testing From Medical and Ethical Viewpoints Striving to discover and explain the peculiarities of body functioning, already ancient Greeks and Romans resorted to vivisecting pigs; the scientific revolution of the Enlightenment era witnessed animal testing becoming the leading trend and a […]
- Negative Impacts of Animal Testing To alter these inhumane laws, we should organize a social movement aiming at the reconsideration of the role of animals in research and improvement of their positions.
- Animal Testing: Long and Unpretty History Nevertheless, that law was more focused on the welfare of animals in laboratories rather than on the prohibition of animal testing.
- Animal Testing as an Unnecessary and Atrocious Practice Such acts of violence could be partially excused by the necessity to test medications that are developed to save human lives however, this kind of testing is even more inhumane as it is ineffective in […]
- Animal Experiments and Inhuman Treatment Although the results of such a laboratory may bring answers to many questions in medicine, genetics, and other vital spheres, it is frequently a case that the treatment of such animals is inhumane and cruel. […]
- Animal Testing for Scientific Research Despite the fact that the present-day science makes no secret of the use of animals for research purposes, not many people know what deprivation, pain, and misery those animals have to experience in laboratories.
- Animal Testing and Ethics I believe it is also difficult to develop efficient legislation on the matter as people have different views on animal research and the line between ethical and unethical is blurred in this area.
- Animal Testing: History and Ethics Moreover, in the twelfth century, another Arabic physician, Avenzoar dissected animals and established animal testing experiment in testing surgical processes prior to their application to man. Trevan in 1927 to evaluate the effectiveness of digitalis […]
- Animal Testing Effects on Psychological Investigation In this context, ethical considerations remain a central theme in psychological research.”Ethics in research refers to the application of moral rules and professional codes of conduct to the collection, analysis, reporting, and publication of information […]
- Genetic Modification and Testing: Ethical Considerations It is done on a molecular level by synthesizing DNA, generating sequences and then inserting the received product into the organism which will be the carrier of the outcome. Another possibility is that the time […]
- Animal Testing: Why It Is Still Being Used The major reason for such “devotion” to animal testing can be explained by the fact that alternative sources of testing are insufficient and too inaccurate to replace conventional way of testing.
- Effects of Animal Testing and Alternatives Another challenge to the proponents of animal testing is related to dosage and the time line for a study. Animal rights values rebuff the notion that animals should have an importance to human beings in […]
- Ethics Problems in Animal Experimentation In spite of the fact that it is possible to find the arguments to support the idea of using animals in experiments, animal experimentation cannot be discussed as the ethical procedure because animals have the […]
- Animal Testing: Ethical Dilemmas in Business This means that both humans and animals have rights that need to be respected, and that is what brings about the many dilemmas that are experienced in this field.
- Should animals be used for scientific research? Therefore, considering the benefits that have been accrued from research activities due to use of animals in scientific research, I support that animals should be used in scientific research.
- Use of Animals in Research Testing: Ethical Justifications Involved The present paper argues that it is ethically justified to use animals in research settings if the goals of the research process are noble and oriented towards the advancement of human life.
- Ethical Problems in Animal Experimentation The banning of companies from testing on animals will force the manufacturers to use conventional methods to test their drugs and products.
- Utilitarianism for Animals: Testing and Experimentation There are alternatives in testing drugs such as tissue culture of human cells and hence this is bound to be more accurate in the findings.
- Use of Animals in Biological Testing Thus, these veterinarians have realized that the results that are realized from the animal research are very crucial in the improvement of the health of human being as well as that of animals.
- Medical Research on Animals Should be Forbidden by Law Vaccines and treatment regimes for various diseases that previously led to the death of humans were all discovered through research on animals.
- Psychoactive Drug Testing on Animals The alterations in behavioral traits of animals due to psychoactive drugs are primarily attributed to the changes in the brain functions or inhibition of certain brain components in animals which ultimately translates to changes in […]
- Negative Impacts of Animal Testing In many instances it can be proofed that drugs have been banned from the market after extensive research on animal testing and consuming a lot of cash, because of the dire effects that they cause […]
📌 Good Animal Testing Topics to Research
- Monkeys Don’t Like Wearing Makeup: Animal Testing In The Cosmetics Industry
- Animal Testing – Should Animal Experimentation Be Permitted
- Essay Animal Testing and In Vitro Testing as a Replacement
- Animal Testing : A Better Knowledge Of Human Body
- The Importance Of Animal Testing For Evaluating Consumer Safety
- The Issues on Animal Testing and the Alternative Procedures to Avoid the Use of the Inhuman Experimentation
- An Alternative to the Harsh and Unnecessary Practices of Animal Testing for Products, Drugs, Chemicals and Other Research
- The Unethical Use of Animals and the Need to Ban Animal Testing for Medical Research Purposes in the United States
- An Argument in Favor of Animal Testing for the Purpose of Clinical Research
- An Argument Against Animal Testing and the Banning of the Practice in the United States
- The Debate About the Ethics of Animal Testing and Its Effects on Us
- An Argument in Favor of Animal Testing as Beneficial to Human Health Research
- Animal Testing and the Reasons Why It Should Be Illegal
- The Principles of the Animal Testing From the Human Perspective
- The Ethical Issues on the Practice of Animal Testing to Test Cosmetics and Drugs
- Stopping Animal Testing and Vivisection by Passing a Bill against Animal Cruelty
🎯 Most Interesting Animal Testing Topics to Write about
- An Argument Against Animal Testing of Consumer Products and Drugs
- The Consequences and Unethical Practice of Animal Testing for Medical Training and Experiments
- How Do The Contributions Of Animal Testing To Global Medical
- Ways To Improve Animal Welfare After Premising The Animal Testing
- Animal Testing – Necessary or Barbaric and Wrong?
- Animal Testing And Its Impact On The Environment
- Animal Testing and Its Contribution to the Advancement of Medicine
- Cosmetics and Animal Testing: The Cause of Death and Mistreatment
- Animal Testing And People For The Ethical Treatment Of Animals
- Animal Rights Activists and the Controversial Issue of Animal Testing
- A History and the Types of Animal Testing in the Medical Area
- Argumentation on Medical Benefits of Animal Testing
- An Analysis of the Concept of Animal Testing Which Lowers the Standard of Human Life
- Is The Humane Society International Gave For Animal Testing
- A Discussion of Whether Animal Testing Is Good for Mankind or Violation of Rights
- The Ethics Of Animal Testing For Vaccine Development And Potential Alternatives
- The Good and Bad of Human Testing and Animal Testing
- What Should the Government Do About Animal Testing?
- Why Does Animal Testing Lower Our Standard of Living?
- Should Animals Be Used in Research?
- Why Should Animal Testing Be Accepted in the World?
- How Does Technology Impact Animal Testing?
- Why Should Animal Testing Be Illegal?
- Should Animal Testing Remain Legal?
- Why Should Animal Testing Be Banned?
- Can the Animal Testing Done to Find Cures for Diseases Be Humane?
- Does Animal Testing Really Work?
- Why Can’t Alternatives Like Computers Replace Research Animals?
- Should Animal Testing Continue to Test Cures for Human Diseases?
- How Does Animal Testing Effect Medicine?
- Should Animal Testing Continue or Be Stopped?
- What Are Advantages and Disadvantages of Animal Testing?
- Why Can Animal Testing Save Our Lives?
- Is Stem Cell Research Beginning of the End of Animal Testing?
- Do Beauty Products Suffer From Negative Publicity if They Conduct Trials on Animals?
- Should Medicine Trials Be Conducted?
- Can Results of Animal Testing Be Generalized to Adults?
- What Are the Origin and History of Animal Testing?
- Why Are Animals Needed to Screen Consumer Products for Safety When Products Tested by Alternative Methods, Are Available?
- How Much Does an Animal Suffer Due to Testing?
- What Is the Effectiveness of Animal Rights Groups in Stopping Animal Testing?
- How Do We Learn From Biomedical Research Using Animals?
- Who Cares for Animals in Research?
- How Do Laboratory Animal Science Professionals Feel About Their Work?
- Why Are There Increasing Numbers of Mice, Rats, and Fish Used in Research?
- How Can We Be Sure Lost or Stolen Pets Are Not Used in Research?
- Why Do Clinical Trials in Humans Require Prior Animal Testing?
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, November 9). 105 Animal Testing Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/animal-testing-essay-examples/
"105 Animal Testing Essay Topic Ideas & Examples." IvyPanda , 9 Nov. 2023, ivypanda.com/essays/topic/animal-testing-essay-examples/.
IvyPanda . (2023) '105 Animal Testing Essay Topic Ideas & Examples'. 9 November.
IvyPanda . 2023. "105 Animal Testing Essay Topic Ideas & Examples." November 9, 2023. https://ivypanda.com/essays/topic/animal-testing-essay-examples/.
1. IvyPanda . "105 Animal Testing Essay Topic Ideas & Examples." November 9, 2023. https://ivypanda.com/essays/topic/animal-testing-essay-examples/.
Bibliography
IvyPanda . "105 Animal Testing Essay Topic Ideas & Examples." November 9, 2023. https://ivypanda.com/essays/topic/animal-testing-essay-examples/.
- Vegetarianism Essay Ideas
- Animal Welfare Ideas
- Bioethics Titles
- Wildlife Ideas
- Extinction Research Topics
- Hunting Questions
- Genetic Engineering Topics
- Zoo Research Ideas

IMAGES
COMMENTS
Arguments against animal testing. Animal experiments are cruel, unreliable, and even dangerous. The harmful use of animals in experiments is not only cruel but also often ineffective. Animals do not naturally get many of the diseases that humans do, such as major types of heart disease, many types of cancer, HIV, Parkinson's disease or ...
Why We Must Stop Animal Testing. If your answer is 'No', now is the time for all of us to know it. Animal testing is not only a research to find cures for human diseases, it is also an experimentation to establish safety of various products such as daily necessities, cosmetic products and medicines. To produce a safe product for us ...
Introduction. Annually, more than 115 million animals are used worldwide in experimentation or to supply the biomedical industry. 1 Nonhuman animal (hereafter "animal") experimentation falls under two categories: basic (i.e., investigation of basic biology and human disease) and applied (i.e., drug research and development and toxicity and safety testing).
Animal Testing: Conclusion. Animal testing is a helpful phenomenon in biological, medical, and other scientific investigations demanding its incorporation. The phenomenon is helpful, viable, and should be embraced despite the opposing opinions. Animal testing helps in developing effective, safe, viable, qualitative, and less toxic drugs.
The E.P.A. Administrator Andrew Wheeler said the agency plans to reduce the amount of studies that involve mammal testing by 30 percent by 2025, and to eliminate the studies entirely by 2035 ...
The first animal protection law was established in Great Britain in 1822. A significant milestone in the history of animal protection legislation was the introduction of the Cruelty to Animals Act in 1876 in Great Britain. This law was promoted by Charles Darwin who, despite being a biologist and a scientist, was against vivisection.
According to our report, here are TEN reasons why making animals suffer to bring a new eye shadow or cologne into the world belongs in the past: Animal tests are already being replaced. Every year the development of alternatives to animal testing is growing. Thanks to advancements in science, animals are being replaced in the testing of ...
Save the Animals: Stop Animal Testing. Using animals in research and to test the safety of products has been a topic of heated debate for decades. According to data collected by F. Barbara Orlans for her book, In the Name of Science: Issues in Responsible Animal Experimentation, sixty percent of all animals used in testing are used in ...
As Hartung notes, " [Animal experiments] come with shortcomings, and their true contribution is often overrated.". Following are five reasons why scientists should stop relying so heavily on animal models: Animal experiments are not very reproducible. This often stems from lack of bias-reducing measures, poorly planned experiments ...
Persuasive Essay Against Animal Testing. Animal testing has been a controversial topic for many years, with strong arguments on both sides. However, the practice of using animals for testing purposes is not only ethically questionable but also scientifically unreliable. In this essay, we will explore the history and debates surrounding animal ...
Nathan Nobis. Morehouse College via Open Philosophy Press. "Animal testing" involves experimenting on animals to try to determine whether drugs and medical treatments are safe and effective for humans. It's wrong and should be banned. Why? First, and most obviously, drugs and medical procedures treat diseases, injuries, and other health ...
We Need to Stop Animal Testing Essay. "Every day in countries around the world, animals are fighting for their lives, these are mutilated and confined to tiny cages so that we can kill them in outdated product tests for cosmetics, personal-care products, and household-cleaning products. These animals are burned, blinded, poisoned ...
Con 1 Animal testing is cruel and inhumane. Animals used in experiments are commonly subjected to force feeding, food and water deprivation, the infliction of burns and other wounds to study the healing process, the infliction of pain to study its effects and remedies, and "killing by carbon dioxide asphyxiation, neck-breaking, decapitation, or other means," according to Humane Society ...
To conclude the essay, animal testing has induced unnecessary pain to innocent animals and should be terminated. Testing has produced some advancements in the past, but it is a barbaric way of trying to advance the world. Every year helpless animals endure procedures that ultimately end in death.
250 Words Essay on Why Animal Testing Should Be Banned Animal Testing: Why It Should Be Banned. Animal testing refers to scientific experiments using non-human animals as subjects. For many years, animals have been used in experiments to study diseases, test medicines, and explore other scientific questions.
The second is that we must stop animal testing. The law requires 12,000 animals subjected to over 50 experiments to endure for a company to register a single pesticide. No one argues that pesticides must meet safety standards. According to the statistics reviewed by the National Institute of Health, only 5% of drugs tested on animals show ...
Sample Speech Against Animal Testing. Good morning, ladies and gentlemen, it is great to be here with you all on this marvellous morning. I am here to convince all of you to oppose, stop and disengage from the cruel, detrimental and unnecessary animal testing. Do you know that the lipstick, the eyeshadow and the mascara we use to make ourselves ...
There are several reasons why the use of animals is critical for biomedical research: • Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us! • Animals are susceptible to many of the same health problems as humans - cancer, diabetes, heart disease, etc. • With a shorter life cycle than humans ...
15. To Kill a Lab Rat. Many animals used in testing are euthanized after experiments by being forced to inhale carbon dioxide. Animals feel distress during this process, and this article reports that labs, because of the pain and discomfort felt by animals, are being asked to use anesthesia.
It also contains many other alternatives such as cell culture, tissue culture, computer models which can easily replace testing on animals. There is a several reasons which shows that animal testing should be banned. First and the foremost reason is that animal suffers a lot from these experimentation as scientist give them injuries without ...
Despite the continued use of animals in experiments, there are many strong reasons why animal testing should be stopped. Here are some of them: 1. There's no consent offered. Like you and me, animals can feel, think, and, most importantly, experience pain. But, unlike us, they can't talk, so they can't consent to be part of the experiments.
We are not alone in our demand for the adoption of more humane testing methods. University centers devoted to non-animal methods are making the same case, and a pioneering 2007 National Research Council report spurred the creation of numerous governmental initiatives focused on the ultimate replacement of animals in toxicity testing. As a result of our longstanding political advocacy, Congress ...
Here are the examples of animal testing essay topics you can choose from: The question of animal intelligence from the perspective of animal testing. Animal testing should (not) be banned. How animal testing affects endangered species. The history and consequences of animal testing.