- Open access
- Published: 07 May 2020

Zebrafish as an alternative animal model in human and animal vaccination research
- Ricardo Lacava Bailone 1 , 2 ,
- Hirla Costa Silva Fukushima 3 ,
- Bianca Helena Ventura Fernandes 4 ,
- Luís Kluwe De Aguiar 5 ,
- Tatiana Corrêa 6 ,
- Helena Janke 6 ,
- Princia Grejo Setti 6 ,
- Roberto De Oliveira Roça 2 &
- Ricardo Carneiro Borra 6
Laboratory Animal Research volume 36 , Article number: 13 ( 2020 ) Cite this article
19k Accesses
60 Citations
1 Altmetric
Metrics details
Much of medical research relies on animal models to deepen knowledge of the causes of animal and human diseases, as well as to enable the development of innovative therapies. Despite rodents being the most widely used research model worldwide, in recent decades, the use of the zebrafish ( Danio rerio ) model has exponentially been adopted among the scientific community. This is because such a small tropical freshwater teleost fish has crucial genetic, anatomical and physiological homology with mammals. Therefore, zebrafish constitutes an excellent experimental model for behavioral, genetic and toxicological studies which unravels the mechanism of various human diseases. Furthermore, it serves well to test new therapeutic agents, such as the safety of new vaccines. The aim of this review was to provide a systematic literature review on the most recent studies carried out on the topic. It presents numerous advantages of this type of animal model in tests of efficacy and safety of both animal and human vaccines, thus highlighting gains in time and cost reduction of research and analyzes.
Introduction
The role of the immune system is to protect a body against bacterial, viral, or any foreign antigen invasions. In order to improve protection, vaccination is used to boost immunity against diseases caused by microorganisms. It typically contains a less virulent agent that triggers a reaction, thus, stimulating a body’s immune system to recognize it as foreign. In the process, a body’s defense mechanism learns to recognize and destroy a microorganism, its toxins or surface proteins [ 94 ] every time an invasion is identified. The use of vaccination is important because it promotes the stimulation of the body’s defense mechanisms and the development of both individual and collective immunity. Vaccination can act on specific (adaptive) and nonspecific (innate) immune responses unlike immunostimulants which only act on innate response. In addition, it should be noted the role vaccines play in controlling diseases as preventative as well as non-therapeutic measures. Therefore, the body is able to produce antibodies that recognize, signal and neutralize pathogens or particular cellular responses which detect the specific antigens with high efficiency and affinity. As a result, vaccines protect the body against future infections [ 27 ] thus reducing the need for the use of antibiotics and other types of drugs.
Despite the study of immunology in fish being more recent compared to those of humans and in animals, the concepts and techniques used are similar [ 60 ]. The study of the use of vaccines in fish is an area of fast-growing. As aquaculture expands and the need to control pathogens becomes more pressing, the commercial vaccination of different varieties of fish is already a reality in many countries. It aids in the prevention of diseases that could pose health risks to the shoal as well as in avoiding the economic losses due to mortality caused by infection. It reduces the contamination of water bodies by the excessive use of antibiotics, and the reduction of final fish product quality [ 5 , 24 , 42 , 79 , 100 ].
The Zebrafish model has been widely used in both animal and human health research and, more recently, in aquaculture too. In spite of rodents being the most widely used research model in the world, in recent decades the use of the zebrafish ( Danio rerio ) model has exponentially increased among the scientific community. It follows the principle of 3Rs (replacement, reduction, and refinement) as required by a multiplicity of national and international regulatory bodies. Furthermore, the use of zebrafish model results in a reduction of time and use of resources when compared to those more established animals’ models. It also provides a greater informational and predictive capacity when compared to in vitro results [ 53 ]. Thus, using the zebrafish model, it is possible to replace and reduce the use of mammals in research as well as mitigate problems related to the welfare of those animals. Furthermore, zebrafish is used as confirmatory models of the positive previously obtained results, thus, having the ability to refine the findings [ 2 ]. A review of the literature was carried out aiming at presenting the most recent information on vaccination of fish, which brings to light the advantages of this animal model in tests of efficacy and safety of both animal and human vaccines.
Material and methods
The present study was based on a systematic literature review carried out using databases such as Science Direct, Google Scholar and SciELO (Scientific Electronic Library Online). Emphasis was given on identifying publications using search words and terms containing ‘human vaccination’ and ‘animal vaccination’. Particularly, the main key-words searched included ‘Zebrafish model’, ‘vaccine safety’, ‘diseases’, ‘infection’ and ‘toxicology’. Initially, 99 publications were identified which included books, rulings and articles published by international scientific journals of high impact factor. The publications were selected according to relevance and timeliness. 19% of the articles used were published in the last year, 65% in the last 5 years, and 89% published in the last 10 years.
Zebrafish model and vaccines testing
Vaccination safety.
When devising immunization experiments, challenge trials for vaccine development evaluate the efficacy and safety of the vaccine against different pathogens. These are normally assessed using animal models, mainly mammals, which are often imprecise in reflecting human diseases [ 93 ], not to mention time consuming, and require a large number of animals. Moreover, the mortality and clinical signs as well as laboratory tests are usually analyzed to evaluate the innate (non-specific) or adaptive (specific) immune system response. As in mammals, Zebrafish has a well-maintained adaptive immune system composed of T and B lymphocytes that develop from the thymus and kidneys respectively. However, in relation to the development of memory lymphocytes, fish seem to have memory cells of the type B and T [ 78 ]. Yet, there has not been enough data to confirm that in Zebrafish. Zebrafish also presents the enzyme system involved in the process of genetic rearrangement that originates the B (BCR) and T (TCR) lymphocyte receptors. As in humans, Zebrafish has recombination activator genes that control the rearrangement of gene segments V, D and J to produce the diversity of antibodies and lymphocyte receptors. In addition, the zebrafish’s immune system has only approximately 300,000 antibody-producing B cells, making it three orders of magnitude smaller than mice and five orders simpler than humans [ 48 ].
The efficiency of the humoral response increases due to the increased affinity of the antibodies. Affinity maturation of antibody responses is less efficient in cold-blooded vertebrates compared to mammals. Despite this, in zebrafish, data revealed that specific nucleotides in regions of the BCR receptor were target of directed mutations. Therefore it was suggested that activation-induced deaminase and affinity maturation contributed to the diversification of antibodies also in fish [ 56 ]. Immunization of teleost fish with the TNP-KLH antigen (linked to trinitrophenyl to keyhole limpet hemocyanin), for example, induced the production of specific low affinity antibodies, which were replaced in 5 weeks by antibodies of intermediate affinity, and after 15 weeks, by antibodies with greater affinity for the antigen [ 28 , 97 ].
Among the immunological tests, the most frequent ones are: complete hematological analysis by counting erythrocytes; thrombocytes and leukocytes; differential white cell count; hematocrit; glucose; organ histology, and immunological essays such as serology, specific antibody titration, and agglutination [ 4 , 29 , 57 ]. Furthermore, toxicity tests can be also conducted using zebrafish such as embryotoxicity, hepatotoxicity, neurotoxicity, endocrine toxicity, genotoxicity, among others as proposed by Bailone et al. [ 3 ].
Up to now, these tests have been conducted using rodents, but in recent decades, the Zebrafish model has proved to be an important tool in the studies of infections and immunological responses. This model has the advantage of having OECD-specific guidelines for safety evaluation of chemical compounds (acute toxicity), which is performed within 96 h [ 65 ]. In addition, observations can be made in real-time allowing for the monitoring of embryogenesis (Fig. 1 ) as well as regarding the effects of vaccines in relation to cardiovascular, hepatic, nervous, and endocrine, not to mention, behavioral aspects too [ 18 , 40 ].

Embryos of zebrafish 0, 6, 24 and 48 h’ post-fertilization. Larvae of zebrafish 72 and 96 h post-fertilization
Prior to vaccines being tested on humans, livestock or pets, these should be assessed using animal models to avoid causing them harm, including death, especially in the case of immunosuppressed organisms, children and the elderly [ 26 ]. As for vaccination in humans, for example, about 0.4 to 1.9 people per million who had been vaccinated with BCG against tuberculosis may have developed the disease through vaccine contagion. For hepatitis B, 1 in 600,000 people vaccinated may have presented a severe allergic reaction (anaphylaxis). In the case of vaccine against poliomyelitis, vaccine contagion happened to 1 in every 3.6 million vaccinated. Moreover, to combat yellow fever, the vaccine contagion and seizures happened to 1 in 22 million and internal hemorrhages happened to 1 in 450,000. Thence, the occurrence of side effects is very rare. Side effect reactions in humans may also be observed to be caused by other vaccines such as yellow fever, measles, mumps, rubella, chicken pox, diphtheria and tetanus. The most common symptoms are seizures, severe allergic reactions, meningitis, encephalitis [ 26 ]. Although these risks are irrelevant when compared to damages that could be caused by the non-use of a vaccine, the toxicology, the side effects and immunization at different concentrations ought to be adequately tested.
Thus, the Zebrafish model has the advantage of a researcher to follow in real-time the fish’s development from its embryogenesis to full organ development which is reached about 36 h after fertilization. This allows for a vaccine’s effect on all the major organs precursors to be closely studied [ 53 ] such as using immunohistology (Fig. 2 ).
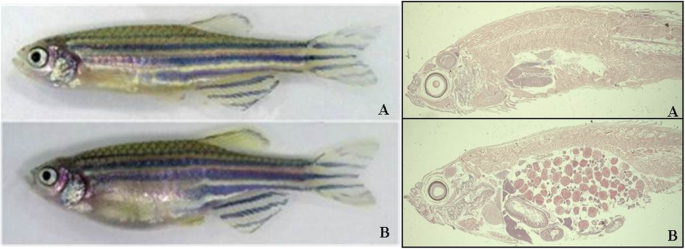
Histology of adult zebrafish (hematoxylin eosin). a Male. b Female
Zebrafish and mammalian toxicity (Lethal concentration – LC 50 ) profiles are surprisingly similar for a range of substances specified in Table 1 below. Therefore, toxicity studies support the effectiveness of using the zebrafish model for the purpose of testing these substances. Furthermore, they can be extrapolated to the active ingredients present in the vaccine, and enabling quick parallel studies of vaccine reactions in humans and zebrafish.
Advantages of zebrafish model in vaccination tests
Compared to other vertebrates, zebrafish have extra biological advantages including high fecundity, external fertilization, optical transparency and rapid development. Moreover, Zebrafish possess a highly developed immune system that is remarkably similar to the human one. Therefore, it is expected that the majority of the signaling pathways and molecules involved in the immune response of mammals would also exist and behave similarly in fish [ 89 ]. Consequently, the presence in fish of elements of innate and adaptive immunity enables research in infectious processes, being susceptible to infections by gram-negative and gram-positive bacteria, protozoa, viruses, fungi and mycobacteria.
The development of special cloning, mutagenesis and transgenesis techniques allowed the identification of a significant number of mutants. Commercial mutant zebrafish lines and the recently developed CRISPR/Cas9 genome modification system provide the means to create knockout zebrafish for studying individual genes at a whole organism level [ 66 ]. Non-pigmenting mutants such as Casper zebrafish have also helped improve visibility of internal organs [ 92 ]. In addition it is easy to generate transgenic zebrafish with ‘reporter genes’ to facilitate analysis in live fish [ 87 ]. Because the zebrafish genome is conserved in humans, information obtained from zebrafish studies may lead to translational results in humans [ 38 ].
Examples of mutant animals displaying human-like diseases are numerous such as: sapje, which has the gene homologous to that of Duchenne muscular dystrophy; dracula , related to erythropoietic protoporphyria; van Gogh, model of the DiGeorge syndrome; and gridlock , which induces coarctation of the aorta [ 47 ]. Research in tumor suppressor genes p53 and apc ( adenomatous polyposis coli) is another area of great interest . The importance of the p53 gene in human carcinogenesis is well recognized and recent studies have shown zebrafish as an excellent model for assessing the presence (or not) of gene stability. Lymphoid leukemia, melanoma and hepato-carcinoma have already been described in zebrafish thus confirming that the molecular mechanisms involved are similar to those of humans [ 49 ].
Regarding the administration of vaccines, in view of the different routes of applications presented in animals and humans, the zebrafish model still allows the immunization of embryos, facilitated by its transparency, using glass needles (Figs. 3 and 4 ). Interestingly, the fact that the fish’s adaptive immune system does not reach maturity up to 4 weeks after fertilization allows them to be used without the need for immunosuppression in the embryonic stages [ 32 ] in the case, for example, of tumor xenograft experiments.

a Vitelline Yolk Injection (24 HPF), Magnifying Glass Nikon SMZ745, 50X; B) Vitelline Yolk Injection (24 h.p.f.), Magnifying Glass Nikon SMZ745, 50X
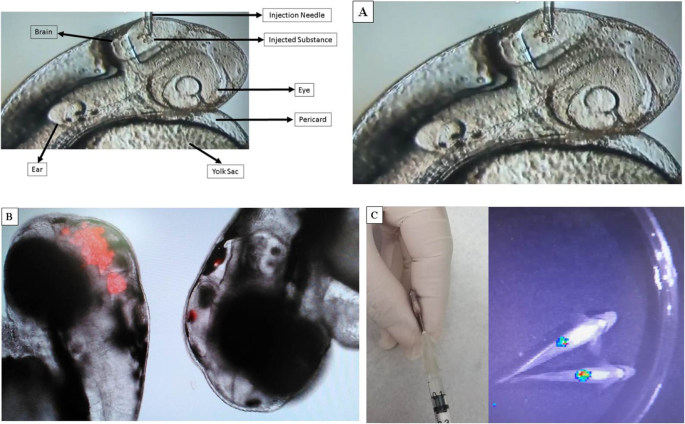
a 24 HPF Zebrafish Embryo Brain Injection, Nikon Microscope; b Brain injection of turbo-red substance into a 24 HPF zebrafish embryo; c Luciferin-labeled 4 T1 tumor cell bioluminescence in 3-month-old animals
In zebrafish larvae, a rapid systemic infection can be initiated by direct microinjection of a bacterial suspension into the bloodstream. Alternatively, a more localized infection may be induced by the injection of microbes into the muscle tail or the hindbrain ventricle [ 6 ]. For high transfer rate, the microbes can be readily injected into the yolk for the first few hours after fertilization. However, it is important to keep in mind that the yolk lacks immune cells, and therefore the bacteria are able to grow freely before invading the larval tissues [ 51 ].
Several transgenic zebrafish lines containing fluorescent markers in different cells of the immune system have been developed to visualize host-microbe interactions in the transparent larvae. For example, recruitment of fluorescent neutrophils to the site of bacterial infection (which can also be labeled with fluorescence) could be easily followed and quantified in real time. Yet, so far, researchers have focused primarily on larval infection patterns [ 51 ].
Fish vaccines
In the prevention of disease outbreaks causing mortalities in aquaculture, similarly to any other animal production system, vaccination is essential. Thus, the use of vaccines for that purpose could be improved based on the results from the studies performed in zebrafish [ 89 ]. The development of vaccines for aquaculture has been an important milestone for guaranteeing a continuous safe and high standard animal health production system. In recent years, zebrafish models have been chosen as the preferred model in the production of fish vaccination experiments against several pathogens that cause losses in aquaculture around the world such as bacteriosis and viruses. One of the most important pathogen studies applied to fishing production is attributed to Guo et al. [ 35 ]. They analyzed the protective efficacy of four iron-related recombinant proteins and their single-walled carbon nanotube encapsulated counterparts against the Aeromonas hydrophila infection in zebrafish. They observed that the immune response was increased after vaccination. Guo et al. [ 34 ] also studied Edwardsiella tarda which is an important intracellular pathogenic bacterium that causes the infectious disease Edwardsiellosis in fish. They proved that live E. tarda vaccine enhanced innate immunity by metabolic modulation in zebrafish.
Vibrio anguillarum , a bacterium that causes vibriosis, was also studied by Ye et al. [ 98 ] who observed the maternal transfer and protection role in zebrafish offspring following vaccination of the brood stock with a live attenuated V. anguillarum vaccine. They proved that the development of immune cells was enhanced and the maternally-derived antibody could protect early embryos and larvae from the attack of specific pathogens via vaccination with a live attenuated vaccine. Furthermore, Liu et al. [ 50 ] analyzed the profiling immune response in zebrafish intestine, skin, spleen and kidney when immersion vaccinated was used with a live attenuated V. anguillarum vaccine. Immersion, or bath vaccination, is a common practice in aquaculture, because of it being convenient as mass vaccination giving sufficient protection. The fish is submerged in water with a sub lethal concentration of the bacteria for a specific time. Liu et al. [ 50 ] observed that antibodies were either produced at antigen-contact tissues or in immune organs. Zhang et al. [ 101 ] studied Th17-like immune response in fish mucosal tissues after administration of live attenuated V. anguillarum via different vaccination routes. When compared to injection vaccination, immersion vaccination elicited intense Th17-like immune responses in the gut tissue of zebrafish. Vibrio vulnificus , that is an aquatic pathogen that can cause primary sepsis and soft tissue infection, was also tested during an experimentation of zebrafish’s reaction to vaccine. It was concluded that CpG oligodeoxynucleotides, a type of essential immunomodulators, protected zebrafish against Vibrio vulnificus induced infection [ 15 ].
Francisella noatunensis is a bacterium that causes granulomatous disease in freshwater and marine fish, and remains an unsolved problem for the aquaculture sector as no efficient vaccines are yet available. Lagos et al. [ 46 ] studied the immunomodulatory properties of Concholepas concholepas hemocyanin against francisellosis in a zebrafish model, proving that his adjuvant was a potential one for aquaculture vaccines. Moreover, Brudal et al. [ 11 ] observed that vaccination with outer membrane vesicles from F. noatunensis reduced the development of francisellosis in a zebrafish model.
Streptococcus sp. has also been studied with the Zebrafish model. Streptococcus parauberis is the major infectious agent of streptococcosis in olive flounder ( Paralichthys olivaceus ). Kim et al. [ 45 ], studying the identification of novel immunogenic proteins against S. parauberis by reverse vaccinology using zebrafish model, identified 41 vaccine candidates against S. parauberis. Furthermore, Streptococcus iniae was studied by Membrebe et al. [ 58 ] testing the protective efficacy of Streptococcus iniae derived enolase against Streptococcal infection in zebrafish model. In that study, enolase protein was evaluated to induce cross-protective immunity against S. iniae and S. parauberis which are major pathogens causing streptococcosis in fish.
Further to the aforementioned examples, many other diseases have been investigated with the Zebrafish model. For example, Rhabdovirus, which is one of the most important diseases in salmonids, is a virus that causes hemorrhagic viral septicemia [ 44 , 64 ]. Listeria monocytogenes [ 19 , 20 ]; Piscirickettsia salmonis which causes salmonid rickettsia sepsis (Tandberg et al. [ 83 ]); and in adjuvant test to improve the efficacy of vaccines [ 44 ], among others [ 82 ].
Animals and human vaccines
The zebrafish model has been used not only in aquaculture, but also in veterinary and human medicine. So far, it has become one of the major model systems used in modern biomedical research [ 51 ]. According to Torraca et al. [ 86 ], zebrafish can be also used as a model for pathogenesis and host defense, modeling many human diseases, such as tuberculosis, Staphylococcus aureus and Shigella infection, among others, as well as model to investigate immune cells, infection and inflammation of different kind of human diseases.
Torraca et al. [ 86 ] posited that zebrafish could also be used as a model for Tuberculosis which is a devastating infectious disease worldwide and with no current prospect of efficient prevention. Tuberculosis is an infectious disease caused by bacilli from the Mycobacterium tuberculosis complex. It is estimated that up to one third of the world’s population is infected with M. tuberculosis and have active tuberculosis, which often develops decades after the primary infection. Annually about two million people perish of tuberculosis and, so far, due to the lack of well-established animal models, such a disease has been difficult to study [ 51 ].
An infection by Mycobacterium marinum in adult zebrafish resembles that of human tuberculosis, as demonstrated by Myllymäki et al. [ 62 ]. Those authors proved that the M. marinum infection model in adult zebrafish was suitable for preclinical screening of tuberculosis immune’s responses and vaccines. It was also a promising new model for tuberculosis vaccine research, including the pre-clinical identification of vaccine antigens [ 16 , 17 , 36 , 41 , 61 , 67 ];). Other species of Mycobacterium have also been studied, such as M. bovis [ 52 , 73 ] and M. abscessos [ 7 ]. M. bovis is most common in cattle, but also affects humans. M. bovis Bacillus Calmette-Guérin vaccine is currently available as a prophylactic tool for preventing the disease. It has been shown to be efficient in preventing disseminated forms of tuberculosis in children; however, its efficiency is limited in areas where individuals have had prior exposure to environmental mycobacteria, and its efficacy decreased with a host’s age [ 55 ].
Moreover, teleost models offer an expanding platform for the understanding of mycobacterial infections and those mechanisms that offer the greatest potential to enhance host protection [ 37 ]. The models make it possible to screen the host and bacterial factors that modify the disease and facilitate the search for new therapeutic agents. It has recently been shown that zebrafish can also be used for the potential screening of DNA-based vaccines and, in particular, for identifying novel antigens protecting against mycobacteria [ 67 ]. Therefore, using the Zebrafish model is expected to accelerate the understanding of the pathogenesis of tuberculosis which would lead to the development of better vaccines. Yet, the usefulness of this model is not limited to tuberculosis, which as seen before it could benefit research for many other important infectious diseases [ 51 ].
Similarly, this model also helps to elucidate bacterial infections in animals and humans by Aeromonas hydrophila [ 91 ], Pseudomonas aeruginosa [ 74 ], Escherichia coli nonpathogenic [ 63 ], E. coli CFT073 [ 95 ], Listeria monocytogenes [ 80 , 81 ], Myroides odoratimimus [ 72 ], Cronobacter turicensis [ 25 ], Streptococcus agalactiae [ 70 , 96 ], Streptococcus iniae and Streptococcus pyogenes [ 59 , 76 , 77 ], among others [ 12 , 85 ].
Shigella is a major cause of dysentery worldwide, accounting for up to 165 million cases of shigellosis each year [ 23 ]. Yet, despite there not existing vaccine available as yet, the human and animal challenge–rechallenge trials with virulent Shigella as well as observational studies in Shigella-endemic areas are promising. The incidence of the disease decreased following Shigella’s infection which pointsto a biological feasibility of a vaccine [ 54 ]. Phalipon et al. [ 71 ] as well as Mani et al. [ 54 ] proposed that adult zebrafish could be used to study the immune response to Shigella, which is crucial to understanding the crosstalk between Shigella and T-lymphocytes [ 75 ] thus this being relevant in the development of vaccine strategies. Studies have also been conducted with Zebrafish model to promote a vaccine against Salmonella, which produces gastroenteritis that causes massive morbidity and mortality in adults and children in developing countries. Howlader et al. [ 39 ] proved that zebrafish was an excellent model for the study of vaccines using successive immersion triple vaccines with the single serotype Salmonella. Typhimurium and Salmonella entereditis induced protective efficacy against a high dose (10 8 CFU/ml) of infection by these pathogens.
Other microorganisms of importance such as fungi which can cause pathologies in humans, such as Candida albicans [ 10 ], Cryptococcus neoformans [ 8 , 84 ] and Mucor circinelloides [ 90 ] have also been the subject of study with teleosts. In addition, viruses such as Herpes simplex [ 13 , 31 ]; human norovirus [ 88 ]; Vesicular stomatitis [ 33 ]; hepatite C [ 21 , 22 ]; Chikungunya [ 1 , 9 , 14 , 68 ]; Sindib [ 69 ] and Influenza A [ 30 ] are some of the human viruses already studied by the zebrafish model in both embryos and larvae.
Conclusions
The use of the Zebrafish model for the production of vaccines with application for both animals and humans, despite already being a reality, is still underused. This model is an important tool for the development of new safe vaccines against diseases which do not yet have preventive treatment, or for which the existing vaccines are not so effective. Thus, previous screening tests with zebrafish have been proven to be effective in preliminary phases prior to testing with mammalians. Despite the evidence from the literature indicating that science in this field is in its infancy, when compared to other animal models used in research, teleost models have proved to be effective in the elucidation of the infection and immunological responses to the diverse animal and human pathogens. In addition, the reduced financial cost and time frame needed for testing are another attractive regarding the use of zebrafish. Thus, it is expected its use would expand in the coming years.
Availability of data and materials
See Materials and Methods section;
Aleksejeva E, Houel A, Briolat V, Levraud JP, Langevin C, Boudinot P. Zebrafish Plzf transcription factors enhance early type I IFN response induced by two non-enveloped RNA viruses. Dev Comp Immunol. 2016;57:48–56. https://doi.org/10.1016/j.dci.2015.12.016 .
Article CAS PubMed Google Scholar
Bailone RL, Aguiar LK, Roça RO, Borra RC, Corrêa T, Janke H, et al. Zebrafish as an animal model for food safety research: trends in the animal research. Food Biotechnol. 2019a;33(4):283–302. https://doi.org/10.1080/08905436.2019.1673173 .
Article CAS Google Scholar
Bailone RL, Fukushima H, Roça R, Corrêa T, Janke H, Setti P, et al. Potenciais usos do Modelo animal zebrafish Danio rerio em pesquisas na medicina veterinária. Rev Educ Cont Med Vet e Zoo CRMV-SP. 2019b; Just Accepted.
Bailone RL, Martins ML, Mouriño JLP, Vieira FN, Pedrotti FS, Nunes GC, et al. Hematology and agglutination titer after polyvalent immunization and subsequent challenge with Aeromonas hydrophila in Nile tilapia ( Oreochromis niloticus ). Arch Med Vet. 2010;42(3):221–7 https://www.redalyc.org/pdf/1730/173016376015.pdf Accessed 27 Feb 2020.
Article Google Scholar
Bao P, Sun X, Liu Q, Zhang Y, Liu X. Synergistic effect of a combined live Vibrio anguillarum and Edwardsiella piscicida vaccine in turbot. Fish Shellfish Immunol. 2019;88:84–90. https://doi.org/10.1016/j.fsi.2019.02.014 .
Benard EL, Van der Sar AM, Ellett F, Lieschke GJ, Spaink HP, Meijer AH. Infection of zebrafish embryos with intracellular bacterial pathogens. JoVE (J Vis Exp). 2012;61:e3781. https://doi.org/10.3791/3781 .
Bernut A, Dupont C, Sahuquet A, Herrmann JL, Lutfalla G, Kremer L. Deciphering and imaging pathogenesis and cording of Mycobacterium abscessus in zebrafish embryos. JoVE (J Vis Exp). 2015;103:e53130. https://doi.org/10.3791/53130 .
Bojarczuk A, Miller KA, Hotham R, Lewis A, Ogryzko NV, Kamuyango AA, et al. Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci Rep. 2016;5(1):1–6. https://doi.org/10.1038/srep2148 .
Briolat V, Jouneau L, Carvalho R, Palha N, Langevin C, Herbomel P, et al. Contrasted innate responses to two viruses in zebrafish: insights into the ancestral repertoire of vertebrate IFN-stimulated genes. J Immunol. 2014;192(9):4328–41. https://doi.org/10.4049/jimmunol.1302611 .
Brothers KM, Newman ZR, Wheeler RT. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell. 2011;10(7):932–44. https://doi.org/10.1128/EC.05005-11 .
Article CAS PubMed PubMed Central Google Scholar
Brudal E, Lampe EO, Reubsaet L, Roos N, Hegna IK, Thrane IM, et al. Vaccination with outer membrane vesicles from Francisella noatunensis reduces development of francisellosis in a zebrafish model. Fish Shellfish Immunol. 2015;42(1):50–7. https://doi.org/10.1016/j.fsi.2014.10.025 .
Brudal E, Ulanova LS, Lampe EO, Rishovd AL, Griffiths G, Winther-Larsen HC. Establishment of three Francisella infections in zebrafish embryos at different temperatures. Infect Immun. 2014;82(6):2180–94. https://doi.org/10.1128/IAI.00077-14 .
Burgos JS, Ripoll-Gomez J, Alfaro JM, Sastre I, Valdivieso F. Zebrafish as a new model for herpes simplex virus type 1 infection. Zebrafish. 2008;5(4):323–33. https://doi.org/10.1089/zeb.2008.0552 .
Burnham LA, Jaishankar D, Thompson JM, Jones KS, Shukla D, Tiwari V. Liposome-mediated herpes simplex virus uptake is glycoprotein-D receptor-independent but requires heparan sulfate. Front Microbiol. 2016;7:973. https://doi.org/10.3389/fmicb.2016.00973 .
Article PubMed PubMed Central Google Scholar
Chen H, Zhang L, Li S, Ling K, Chen X, Lin C. CpG-ODN 2007 protects zebrafish against Vibrio vulnificus -induced infection. bioRxiv. 2019:780742. https://doi.org/10.1101/780742 .
Cen J, Jia ZL, Zhu CY, Wang XF, Zhang F, Chen WY, Liu KC, Li, SY, Zhang, Y. Particulate matter (PM10) induces cardiovascular developmental toxicity in zebrafish embryos and larvae via the ERS, Nrf2 and Wnt pathways. Chemosphere. 2020. p. 126288.
Cheng T, Kam JY, Johansen MD, Oehlers SH. High content analysis of granuloma histology and neutrophilic inflammation in adult zebrafish infected with Mycobacterium marinum . Micron. 2020;129:102782. https://doi.org/10.1016/j.micron.2019.102782 .
Article PubMed Google Scholar
Cornet C, Calzolari S, Miñana-Prieto R, Dyballa S, Van Doornmalen E, Rutjes H, et al. ZeGlobalTox: an innovative approach to address organ drug toxicity using zebrafish. Int J Mol Sci. 2017;18(4):864. https://doi.org/10.3390/ijms18040864 .
Article CAS PubMed Central Google Scholar
Ding C, Fan E, Wang S, Guo L, Li J, Liu Q. A potential aquaculture vaccine vector: evaluation of a double-gene attenuated Listeria monocytogenes in zebrafish ( Danio rerio ). Aquaculture. 2017;479:311–20. https://doi.org/10.1016/j.aquaculture.2017.04.018 .
Ding C, Liu Q, Li J, Ma J, Wang S, Dong Q, et al. Attenuated Listeria monocytogenes protecting zebrafish ( Danio rerio ) against vibrio species challenge. Microb Pathog. 2019;132:38–44. https://doi.org/10.1016/j.micpath.2019.03.040 .
Ding CB, Zhang JP, Zhao Y, Peng ZG, Song DQ, Jiang JD. Zebrafish as a potential model organism for drug test against hepatitis C virus. PLoS One. 2011;6(8):e22921. https://doi.org/10.1371/journal.pone.0022921 .
Ding CB, Zhao Y, Zhang JP, Peng ZG, Song DQ, Jiang JD. A zebrafish model for subgenomic hepatitis C virus replication. Int J Mol Med. 2015;35(3):791–7. https://doi.org/10.3892/ijmm.2015.2063 .
Duggan GM, Mostowy S. Use of zebrafish to study Shigella infection. Dis Model Mech. 2018;11(2):dmm032151. https://doi.org/10.1242/dmm.032151 .
Faber MN, Holland JW, Secombes CJ. Vaccination strategies and IgM responses against PKD in rainbow trout. Fish Shellfish Immunol. 2019;91:423. https://doi.org/10.1016/j.fsi.2019.04.159 .
Fehr A, Eshwar AK, Neuhauss SC, Ruetten M, Lehner A, Vaughan L. Evaluation of zebrafish as a model to study the pathogenesis of the opportunistic pathogen Cronobacter turicensis . Emerg Microbes Infect. 2015;4(1):1–9. https://doi.org/10.1038/emi.2015.29 .
Ferrairo F. Os riscos reais da vacina. Revista Superinteressante. 2015; https://super.abril.com.br/saude/os-riscos-reais-da-vacina/ Accessed 27 Feb 2020.
Figueiredo HCP, Castro GAC, Leal CAG, Netto LN. Uso de vacinas na piscicultura: verdades, mitos e perspectivas. Panorama da Aquicultura. 2009;19(115):22–31 https://panoramadaaquicultura.com.br/uso-de-vacinas-na-piscicultura-verdades-mitos-e-perspectivas/ Accessed 27 Feb 2020.
Google Scholar
Fillatreau S, Six A, Magadan S, Castro R, Sunyer JO, Boudinot P. The astonishing diversity of Ig classes and B cell repertoires in teleost fish. Front Immunol. 2013;4:28. https://doi.org/10.3389/fimmu.2013.00028 .
Fukushima HCS, Leal CAG, Cavalcante RB, Figueiredo HCP, Arijo S, Moriñigo MA, et al. Lactococcus garvieae outbreaks in Brazilian farms Lactococcosis in Pseudoplatystoma sp.–development of an autogenous vaccine as a control strategy. J Fish Dis. 2017;40(2):263–72.
Gabor KA, Goody MF, Mowel WK, Breitbach ME, Gratacap RL, Witten PE, et al. Influenza a virus infection in zebrafish recapitulates mammalian infection and sensitivity to anti-influenza drug treatment. Dis Model Mech. 2014;7(11):1227–37. https://doi.org/10.1242/dmm.014746 .
Ge R, Zhou Y, Peng R, Wang R, Li M, Zhang Y, et al. Conservation of the STING-mediated cytosolic DNA sensing pathway in zebrafish. J Virol. 2015;89(15):7696–706. https://doi.org/10.1128/JVI.01049-15 .
Granato M, Nüsslein-Volhard C. Fishing for genes controlling development. Curr Opin Genet Dev. 1996;6(4):461–8. https://doi.org/10.1016/S0959-437X(96)80068-2 .
Guerra-Varela J, Baz-Martinez M, Da Silva-Alvarez S, Losada AP, Quiroga MI, Collado M, et al. Susceptibility of zebrafish to vesicular stomatitis virus infection. Zebrafish. 2018;15(2):124e132. https://doi.org/10.1089/zeb.2017.1499 .
Guo C, Peng B, Song M, Wu CW, Yang MJ, Zhang JY, et al. Live Edwardsiella tarda vaccine enhances innate immunity by metabolic modulation in zebrafish. Fish Shellfish Immunol. 2015;47(2):664–73. https://doi.org/10.1016/j.fsi.2015.09.034 .
Guo Z, Lin Y, Wang X, Fu Y, Lin W, Lin X. The protective efficacy of four iron-related recombinant proteins and their single-walled carbon nanotube encapsulated counterparts against Aeromonas hydrophila infection in zebrafish. Fish Shellfish Immunol. 2018;82:50–9. https://doi.org/10.1016/j.fsi.2018.08.009 .
Harjula SKE, Saralahti AK, Ojanen MJ, Rantapero T, Uusi-Mäkelä MI, Nykter M, et al. Characterization of immune response against Mycobacterium marinum infection in the main hematopoietic organ of adult zebrafish ( Danio rerio ). Dev Comp Immunol. 2020;103:103523. https://doi.org/10.1016/j.dci.2019.103523 .
Hodgkinson JW, Belosevic M, Elks PM, Barreda DR. Teleost contributions to the understanding of mycobacterial diseases. Dev Comp Immunol. 2019. https://doi.org/10.1016/j.dci.2019.02.011 .
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498. https://doi.org/10.1038/nature12111 .
Howlader DR, Sinha R, Nag D, Majumder N, Mukherjee P, Bhaumik U, et al. Zebrafish as a novel model for non-typhoidal salmonella pathogenesis, transmission and vaccine efficacy. Vaccine. 2016;34(42):5099–106. https://doi.org/10.1016/j.vaccine.2016.08.077 .
Jarque S, Ibarra J, Rubio-Brotons M, García-Fernández J, Terriente J. Multiplex analysis platform for endocrine disruption prediction using zebrafish. Int J Mol Sci. 2019;20(7):1739. https://doi.org/10.3390/ijms20071739 .
Ji J, Torrealba D, Thwaite R, Gomez AC, Parra D, Roher N. Nanostructured TNFα protein targets the zebrafish ( Danio rerio ) immune system through mucosal surfaces and improves the survival after Mycobacterium marinum lethal infection. Aquaculture. 2019;510:138–49. https://doi.org/10.1016/j.aquaculture.2019.05.050 .
Kamalii A, Prabu E, Ruby P, Ahilan B. Advanced developments in fish vaccination. J Aquacult Trop. 2018;33(1/2):101–9 https://search.proquest.com/openview/78c8d674d1ff36d2c136f6dc355bf1b8/1?pq-origsite=gscholar&cbl=506338 Accessed 27 Feb 2020.
Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharm Ther. 2007;82(1):70–80. https://doi.org/10.1038/sj.clpt.6100223 .
Kavaliauskis A, Arnemo M, Speth M, Lagos L, Rishovd AL, Estepa A, et al. Protective effect of a recombinant VHSV-G vaccine using poly (I: C) loaded nanoparticles as an adjuvant in zebrafish ( Danio rerio ) infection model. Dev Comp Immunol. 2016;61:248–57. https://doi.org/10.1016/j.dci.2016.04.010 .
Kim YS, Yoon NK, Karisa N, Seo SH, Lee JS, Yoo SS, et al. Identification of novel immunogenic proteins against Streptococcus parauberis in a zebrafish model by reverse vaccinology. Microb Pathog. 2019;127:56–9. https://doi.org/10.1016/j.micpath.2018.11.053 .
Lagos L, Tandberg JI, Becker MI, Winther-Larsen HC. Immunomodulatory properties of Concholepas concholepas hemocyanin against francisellosis in a zebrafish model. Fish Shellfish Immunol. 2017;67:571–4. https://doi.org/10.1016/j.fsi.2017.06.046 .
Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8(5):353. https://doi.org/10.1038/nrg2091 .
Litman GW, Cannon JP, Dishaw JL. Reconstructing immune phylogeny: new perspectives. Nat Rev Immunol. 2005;5(11):866–79. https://doi.org/10.1038/nri1712 .
Liu S, Steven DL. Zebrafish models for cancer. Ann Rev Pathol: Mech. 2011. https://doi.org/10.1146/annurev-pathol-011110-130330 .
Liu X, Wu H, Liu Q, Wang Q, Xiao J, Chang X, et al. Profiling immune response in zebrafish intestine, skin, spleen and kidney bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol. 2015;45(2):342. https://doi.org/10.1016/j.fsi.2015.04.028 .
Lohi O, Parikka M, Rämet M. The zebrafish as a model for paediatric diseases. Acta Paediatr. 2013;102(2):104–10. https://doi.org/10.1111/j.1651-2227.2012.02835.x .
López V, Risalde MA, Contreras M, Mateos-Hernández L, Vicente J, Gortázar C, et al. Heat-inactivated Mycobacterium bovis protects zebrafish against mycobacteriosis. J Fish Dis. 2018;41(10):1515–28. https://doi.org/10.1111/jfd.12847 .
MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov. 2015;14(10):721. https://doi.org/10.1038/nrd4627 .
Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34:2887–94. https://doi.org/10.1016/j.vaccine.2016.02.075 .
Mantilla Galindo A, Ocampo M, Patarroyo MA. Experimental models used in evaluating anti-tuberculosis vaccines: the latest advances in the field. Expert Rev Vaccines. 2019;18(4):365–77.
Marianes AE, Zimmerman AM. Targets of somatic hypermutation within immunoglobulin light chain genes in zebrafish. Immunology. 2011;132(2):240–55. https://doi.org/10.1111/j.1365-2567.2010.03358.x .
McFetridge R, Sobanjo-ter Meulen A, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2015;33(24):2793–9. https://doi.org/10.1016/j.vaccine.2015.04.025 .
Membrebe JD, Yoon NK, Hong M, Lee J, Lee H, Park K, et al. Protective efficacy of Streptococcus iniae derived enolase against streptococcal infection in a zebrafish model. Vet Immunol Immunopathol. 2016;170:25–9. https://doi.org/10.1016/j.vetimm.2016.01.004 .
Miller JD, Neely MN. Zebrafish as a model host for streptococcal pathogenesis. Acta Trop. 2004;91(1):53–68. https://doi.org/10.1016/j.actatropica.2003.10.020 .
Muktar Y, Tesfaye S, Tesfaye B. Present status and future prospects of fish vaccination: a review. J Vet Sci Technol. 2016;2:299. https://doi.org/10.4172/2157-7579.1000299 .
Myllymäki H, Niskanen M, Luukinen H, Parikka M, Rämet M. Identification of protective postexposure mycobacterial vaccine antigens using an immunosuppression-based reactivation model in the zebrafish. Dis Model Mech. 2018;11(3):dmm033175. https://doi.org/10.1242/dmm.033175 .
Myllymäki H, Niskanen M, Oksanen KE, Sherwood E, Ahava M, Parikka M, et al. Identification of novel antigen candidates for a tuberculosis vaccine in the adult zebrafish ( Danio rerio ). PLoS One. 2017;12(7):e0181942. https://doi.org/10.1371/journal.pone.0181942 .
Nguyen-Chi M, Phan QT, Gonzalez C, Dubremetz JF, Levraud JP, Lutfalla G. Transient infection of the zebrafish notochord with E. coli induces chronic inflammation. Dis Model Mech. 2014;7(7):871–82. https://doi.org/10.1242/dmm.014498 .
Novoa B, Romero A, Mulero V, Rodriguez I, Fernandez I, Figueras A. Zebrafish ( Danio rerio ) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV). Vaccine. 2006;24:5806–16. https://doi.org/10.1016/j.vaccine.2006.05.015 .
OECD. Test no. 236: fish embryo acute toxicity OECD guidelines for the testing of chemicals; 2013. OECD/OCDE N236 Last Modified July 26, 2013. https://www.oecd-ilibrary.org/docserver/9789264203709-en.pdf?expires=1554216347&id=id&accname=guest&checksum=98B3CA87CA423D51D70FAF3B708EA660 . Accessed 27 Feb 2020.
Ojanen M. Reverse genetics to study immunity against mycobacteria in zebrafish ( Danio rerio ); 2019. https://trepo.tuni.fi/bitstream/handle/ 10024/105069/978-952-03-0995-4.pdf?sequence= 1&isAllowed=y Accessed 27 Feb 2020.
Oksanen KE, Halfpenny NJ, Sherwood E, Harjula SKE, Hammarén MM, Ahava MJ, et al. An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine. 2013;31(45):5202–9. https://doi.org/10.1016/j.vaccine.2013.08.093 .
Palha N, Guivel-Benhassine F, Briolat V, Lutfalla G, Sourisseau M, Ellett F, et al. Real-time whole-body visualization of chikungunya virus infection and host interferon response in zebrafish. PLoS Pathog. 2013;9(9):e1003619. https://doi.org/10.1371/journal.ppat.1003619 .
Passoni G, Langevin C, Palha N, Mounce BC, Briolat V, Affaticati P, et al. Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes. Dis Model Mech. 2017;10(7):847e857. https://doi.org/10.1242/dmm.029231 .
Patterson H, Saralahti A, Parikka M, Dramsi S, Trieu-Cuot P, Poyart C, et al. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev Comp Immunol. 2012;38(3):447–55. https://doi.org/10.1016/j.dci.2012.07.007 .
Phalipon A, Mulard LA, Sansonetti PJ. Vaccination against shigellosis: is it the path that is difficult or is it the difficult that is the path? Microbes Infect. 2008;10(9):1057–62. https://doi.org/10.1016/j.micinf.2008.07.016 .
Ravindran C, Varatharajan GR, Raju R, Vasudevan L, Anantha SR. Infection and pathogenecity of Myroides odoratimimus (NIOCR-12) isolated from the gut of grey mullet ( Mugil cephalus (Linnaeus, 1758)). Microb Pathog. 2015;88:22–8. https://doi.org/10.1016/j.micpath.2015.08.001 .
Risalde MA, López V, Contreras M, Mateos-Hernández L, Gortázar C, de la Fuente J. Control of mycobacteriosis in zebrafish ( Danio rerio ) mucosally vaccinated with heat-inactivated Mycobacterium bovis . Vaccine. 2018;36(30):4447–53. https://doi.org/10.1016/j.vaccine.2018.06.042 .
Rocker AJ, Weiss AR, Lam JS, Van Raay TJ, Khursigara CM. Visualizing and quantifying Pseudomonas aeruginosa infection in the hindbrain ventricle of zebrafish using confocal laser scanning microscopy. J Microbiol Methods. 2015;117:85–94. https://doi.org/10.1016/j.mimet.2015.07.013 .
Salgado-Pabón W, Konradt C, Sansonetti PJ, Phalipon A. New insights into the crosstalk between Shigella and T lymphocytes. Trends Microbiol. 2014;22(4):192–8. https://doi.org/10.1016/j.tim.2014.02.002 .
Saralahti A. A zebrafish model for host-pathogen interactions in streptococcal infections; 2019. https://trepo.tuni.fi/bitstream/handle/10024/105436/978-952-03-0981-7.pdf?sequence=1 Accessed 27 Feb 2020.
Saralahti A, Rämet M. Zebrafish and streptococcal infections. Scand J Immunol. 2015;82(3):174–83. https://doi.org/10.1111/sji.12320 .
Scapigliati G, Fausto AM, Picchietti S. Fish lymphocytes: an evolutionary equivalent of mammalian innate-like lymphocytes? Front Immunol. 2018;9:971. https://doi.org/10.3389/fimmu.2018.00971 .
Shahin K, Shinn AP, Metselaar M, Ramirez-Paredes JG, Monaghan SJ, Thompson KD, et al. Efficacy of an inactivated whole-cell injection vaccine for nile tilapia, Oreochromis niloticus (L), against multiple isolates of Francisella noatunensis subsp. orientalis from diverse geographical regions. Fish Shellfish Immunol. 2019;89:217–27. https://doi.org/10.1016/j.fsi.2019.03.071 .
Shan Y, Fang C, Cheng C, Wang Y, Peng J, Fang W. Immersion infection of germ-free zebrafish with Listeria monocytogenes induces transient expression of innate immune response genes. Front Microbiol. 2015;6:373. https://doi.org/10.3389/fmicb.2015.00373 .
Shan Y, Zhang Y, Zhuo X, Li X, Peng J, Fang W. Matrix metalloproteinase-9 plays a role in protecting zebrafish from lethal infection with Listeria monocytogenes by enhancing macrophage migration. Fish Shellfish Immunol. 2016;54:179–87. https://doi.org/10.1016/j.fsi.2016.04.003 .
Sullivan C, Matty MA, Jurczyszak D, Gabor KA, Millard PJ, Tobin DM, Kim CH. Infectious disease models in zebrafish. In: Methods in cell biology, vol. 138: Academic; 2017. p. 101–36. https://doi.org/10.1016/bs.mcb.2016.10.005 .
Tandberg J, Oliver C, Lagos L, Gaarder M, Yáñez AJ, Ropstad E, Winther-Larsen HC. (2017). Membrane vesicles from Piscirickettsia salmonis induce protective immunity and reduce development of salmonid rickettsial septicemia in an adult zebrafish model. Fish Shellfish Immu. 2017;67:189–98. https://doi.org/10.1016/j.fsi.2017.06.015 .
Tenor JL, Oehlers SH, Yang JL, Tobin DM, Perfect JR. Live imaging of host-parasite interactions in a zebrafish infection model reveals cryptococcal determinants of virulence and central nervous system invasion. MBio. 2015;6(5):e01425–15. https://doi.org/10.1128/mBio.01425-15 .
Toh MC, Goodyear M, Daigneault M, Allen-Vercoe E, Van Raay TJ. Colonizing the embryonic zebrafish gut with anaerobic bacteria derived from the human gastrointestinal tract. Zebrafish. 2013;10(2):194–8. https://doi.org/10.1089/zeb.2012.0814 .
Torraca V, Gomes MC, Sarris M, Mostowy S. Meeting report: zebrafish infection and immunity 2019. Lab Anim. 2019;48(10):284–7. https://doi.org/10.1038/s41684-019-0397-4 .
Tsang M. Zebrafish: a tool for chemical screens. Birth Defects Res C Embryo Today. 2010;90(3):185–92. https://doi.org/10.1002/bdrc.20183 .
Van Dycke J, Ny A, Conceição-Neto N, Maes J, Hosmillo M, Cuvry A, et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019;15(9):e1008009. https://doi.org/10.1371/journal.ppat.1008009 .
Varela M, Figueras A, Novoa B. Modelling viral infections using zebrafish: innate immune response and antiviral research. Antivir Res. 2017;139:59–68. https://doi.org/10.1016/j.antiviral.2016.12.013 .
Voelz K, Gratacap RL, Wheeler RT. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides . Dis Model Mech. 2015;8(11):1375–88. https://doi.org/10.1242/dmm.019992 .
Wang Y, Ren Z, Fu L, Su X. Two highly adhesive lactic acid bacteria strains are protective in zebrafish infected with Aeromonas hydrophila by evocation of gut mucosal immunity. J Appl Microbiol. 2016;120(2):441–51. https://doi.org/10.1111/jam.13002 .
White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2(2):183–9. https://doi.org/10.1016/j.stem.2007.11.002 .
WHO. 2016. Human challenge trials for vaccine development: regulatory considerations. Expert Committee on Biological Standardisation. October. Geneva. https://www.who.int/biologicals/expert_committee/Human_challenge_Trials_IK_final.pdf . Accessed 27 Feb 2020.
WHO. Health topics: vaccines; 2019. https://www.who.int/topics/vaccines/en/ > Accessed 27 Feb 2020.
Wiles TJ, Norton JP, Smith SN, Lewis AJ, Mobley HL, Casjens SR, et al. A phyletically rare gene promotes the niche-specific fitness of an E. coli pathogen during bacteremia. PLoS Pathog. 2013;9(2):e1003175. https://doi.org/10.1371/journal.ppat.1003175 .
Wu XM, Cao L, Hu YW, Chang MX. Transcriptomic characterization of adult zebrafish infected with Streptococcus agalactiae . Fish Shellfish Immunol. 2019;94:355–72. https://doi.org/10.1016/j.fsi.2019.09.040 .
Ye J, Kaattari IM, Kaattari SL. The differential dynamics of antibody subpopulation expression during affinity maturation in a teleost. Fish Shellfish Immunol. 2011;30(1):372–7. https://doi.org/10.3389/fimmu.2018.00971 .
Ye N, Wu H, Zhang Y. Maternal transfer and protection role in zebrafish ( Danio rerio ) offspring following vaccination of the brood stock with a live attenuated Vibrio anguillarum vaccine. Aquac Res. 2016;47(11):3667–78. https://doi.org/10.1111/are.12821 .
Zhang C, Willett C, Fremgen T. Zebrafish: an animal model for toxicological studies. Curr Protoc Toxicol. 2003;17(1):1–7. https://doi.org/10.1002/0471140856.tx0107s17 .
Zhang D, Thongda W, Li C, Zhao H, Beck BH, Mohammed H, et al. More than just antibodies: protective mechanisms of a mucosal vaccine against fish pathogen Flavobacterium columnare . Fish Shellfish Immunol. 2017;71:160–70. https://doi.org/10.1016/j.fsi.2017.10.001 .
Zhang H, Shen B, Wu H, Gao L, Liu Q, Wang Q, et al. Th17-like immune response in fish mucosal tissues after administration of live attenuated Vibrio anguillarum via different vaccination routes. Fish Shellfish Immunol. 2014;37(2):229–38. https://doi.org/10.1016/j.fsi.2014.02.007 .
Download references
Acknowledgements
Not applicable.
There was no funding;
Author information
Authors and affiliations.
Ministry of Agriculture, Livestock and Supply, Federal Inspection Service, São Carlos, SP, Brazil
Ricardo Lacava Bailone
São Paulo State University, Botucatu, SP, Brazil
Ricardo Lacava Bailone & Roberto De Oliveira Roça
Health and Biological Sciences Center, Federal University, Federal University of São Carlos, São Carlos, SP, Brazil
Hirla Costa Silva Fukushima
Technical Directorate for Teaching and Research Support, São Paulo University, São Paulo, SP, Brazil
Bianca Helena Ventura Fernandes
Department of Food Technology and Innovation, Harper Adams University, Newport, UK
Luís Kluwe De Aguiar
Department of Genetic and Evolution, Federal University of São Carlos, São Carlos, SP, Brazil
Tatiana Corrêa, Helena Janke, Princia Grejo Setti & Ricardo Carneiro Borra
You can also search for this author in PubMed Google Scholar
Contributions
Bailone (Mean author); Fukushima (Zebrafish review); Fernandes (Photographies); Aguiar (Translator); Corrêa, Junke and Setti (Bibliographic review help, vaccine review, team group of Laboratory of Applied Immunology); Roça (Bailone’s Supervisor, review final corrections); Borra (Bailone’s Co-supervisor, review final corrections). The authors read and approved the final manuscript.
Corresponding author
Correspondence to Ricardo Lacava Bailone .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bailone, R.L., Fukushima, H.C.S., Ventura Fernandes, B. et al. Zebrafish as an alternative animal model in human and animal vaccination research. Lab Anim Res 36 , 13 (2020). https://doi.org/10.1186/s42826-020-00042-4
Download citation
Received : 06 January 2020
Accepted : 19 March 2020
Published : 07 May 2020
DOI : https://doi.org/10.1186/s42826-020-00042-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- 3Rs, Animal health
- Human health
- Vaccine safety
Laboratory Animal Research
ISSN: 2233-7660
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Original Articles
- Communications
- High-Impact Collection
- Author Guidelines
- Submission Site
- Open Access Policy
- Self-Archiving Policy
- Why Publish with Us?
- About Toxicology Research
- Editorial Board
- Authorship Guidelines
- Advertising and Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, materials and methods, the benefits of moderate selenium, toxicity of excessive selenium, the effect of form of selenium on zebrafish, conclusions and future research directions, acknowledgments, author contributions.
- < Previous
The beneficial and toxic effects of selenium on zebrafish. A systematic review of the literature
- Article contents
- Figures & tables
- Supplementary Data
Yuanshan Lin, Liyun Hu, Xinhang Li, Jie Ma, Qipeng Li, Xiaofan Yuan, Yuan Zhang, The beneficial and toxic effects of selenium on zebrafish. A systematic review of the literature, Toxicology Research , Volume 13, Issue 2, April 2024, tfae062, https://doi.org/10.1093/toxres/tfae062
- Permissions Icon Permissions
Selenium is an important and essential trace element in organisms, but its effects on organisms are also a “double-edged sword”. Selenium deficiency or excess can endanger the health of humans and animals. In order to thoroughly understand the nutritional value and toxicity hazards of selenium, researchers have conducted many studies on the model animal zebrafish. However, there is a lack of induction and summary of relevant research on which selenium acts on zebrafish. This paper provides a review of the reported studies. Firstly, this article summarizes the benefits of selenium on zebrafish from three aspects: Promoting growth, Enhancing immune function and anti-tumor ability, Antagonizing some pollutants, such as mercury. Then, three aspects of selenium toxicity to zebrafish are introduced: nervous system and behavior, reproductive system and growth, and damage to some organs. This article also describes how different forms of selenium compounds have different effects on zebrafish health. Finally, prospects for future research directions are presented.
Selenium is an important biologically essential trace element, and its value for human and animal health has been recognized. Selenium participates in the various basic life processes of organisms in the form of the selenoaminoacid incorporated in selenoproteins. 1 The impact of selenium on humans and animals is characterized by a typical “U” shape, and long-term insufficient intake can cause many serious health problems. 2 Selenium deficiency is clearly related to the occurrence of cardiovascular diseases, with a typical example being Keshan disease discovered in selenium deficient areas. It has been proven that oral selenium can reverse selenium deficiency and improve Keshan disease. 3 Severe selenium deficiency can also cause diseases like Kashin-beck disease and other joint diseases. 4 Meanwhile, selenium is also an essential element for maintaining thyroid function, and selenium deficiency is closely related to immune thyroid disease. 5 , 6 Insufficient selenium intake can also lead to liver dysfunction, increased cancer risk, increased mortality rate and so on. 7 However, the excessive addition of selenium to livestock feed by humans indirectly causes selenium poisoning in humans and animals, leading to severe anemia, skeletal stiffness, hair loss, blindness, and more. 8 Current research shows that moderate and high selenium exposure increases the risk of type 2 diabetes and the incidence of cardiovascular disease. 9–11 It is worth noting that selenium slightly above the nutritional level can exhibit severe neurotoxicity, not only damaging various neurotransmitters, key proteins, and even affecting signaling molecules involved in behavior and cognitive function. 12 The safe nutritional range of selenium is relatively narrow, and it is easy for humans and animals to experience deficiency or excess. Just as selenium has an impact on the nervous system, the deficiency of selenoprotein highly affects the function of the central nervous system, 10 but excessive exposure to selenium at similar nutritional levels can produce neurotoxicity. 13 Therefore, researchers need to conduct extensive research to determine the required amount and toxicity.
Fish are sensitive models for detecting the exposure of various toxic substances in the ecological environment, 14 based on the fact that most toxins enter the aquatic environment naturally or unnaturally, and fish living in the aquatic environment easily produce various perceptible toxic reactions to these toxins. 15 Selenium compounds naturally exist in the ecological environment, but they cause harm to fish due to various human interventions flowing into the aquatic environment. 16 The model animal zebrafish is known as the “lab rats in the water” due to its high homology of 87% with human genes, transparent embryos and larvae, rapid reproduction and development, and low breeding costs; it has a wide range of applications in drug toxicology. 17 For this reason, researchers have conducted many studies on selenium using zebrafish models. This article reviews the current reported studies on the beneficial and toxic effects of selenium on zebrafish, providing reference for the research of selenium in Fish and zebrafish.
This review retrieves articles published in English from Pubmed, Web of Science, and Google Scholar databases. Search using the following keywords: “zebrafish”, “selenium”, “selenoprotein”, “selenomethionine”, “selenocysteine”, “sodium selenite”, “selenate”, “selenium nanoparticles”. The retrieval strategy is to use "zebrafish" to search one by one with other keywords in an “and” manner, and exclude duplicate articles. The inclusion criteria include relevant studies on the effects of selenium on zebrafish, and exclude papers unrelated to the toxicity and benefits of selenium. Further read qualified papers to determine the requirements that meet the topic. When reading qualified literature, pay attention to articles that are similar to the topic, and judge whether the content can be used as evidence to support the opinion on the topic.
Selenoprotein promotes growth
The normal growth and various physiological and biochemical functions of fish are inseparable from selenium. Inorganic or organic compounds containing selenium are absorbed by fish and used to synthesize selenoprotein, participating in physiological metabolism. Activating appropriate amounts of selenoenzyme and selenoprotein is important in protecting fish from oxidative damage, promoting fish growth, enhancing fish immunity, and other metabolic regulation. 18 Currently, 14 types of selenoproteins have been detected in zebrafish, of which 11 are highly homologous to human selenoproteins. 19 Most selenoproteins have antioxidant properties, and it has been confirmed that selenoproteins with antioxidant capacity include glutathione peroxidase, thioredoxin reductase, and thyroxine deiodinase, which play an important role in eliminating oxidative stress. 20 Glutathione peroxidase catalyzes the conversion of reduced glutathione to oxidized glutathione. During the catalytic transformation, reduced glutathione converts toxic peroxides or oxygen radicals into non-toxic alcohols or water. 7 Thioredoxin reductase catalyzes the reaction of oxidized thioredoxin with the reducing agent and consumes the electrons provided by NADPH, thereby achieving the purpose of scavenging oxygen free radicals. 21 At the same time, thyroxine deiodinase also regulates the process of thyroxine production, and the physiological metabolism of many tissues and organs is affected by thyroid hormone. 5 Although other selenium proteins’ functions are unclear, they also have antioxidant potential and participate in other important metabolic processes. 22 Selenoprotein P is a plasma selenium transporter protein that plays an important role in maintaining stable selenium levels and antioxidant balance in the brain, reproductive, and other systems. 23 Selenoprotein H is a recently discovered nucleolar oxidoreductase inhibiting tumor activity and regulating redox balance, inflammatory response, and DNA damage during zebrafish embryonic development. Selenoprotein H deficiency in zebrafish disrupts redox balance and causes inflammation, thereby accelerating the development of gastrointestinal tumors. 24 Selenoprotein N is present in the muscle tissue of zebrafish and is highly expressed during the early development of zebrafish somatic cells and notochord. During the development of zebrafish muscles, the lack of selenoprotein N causes a decrease in slow muscle fiber production and abnormal myofibrils, leading to muscular dystrophy. It also leads to impaired Ryanodine receptor/calcium release channel activity in zebrafish muscles. 25
Fish in areas close to human industrial activities or selenium mines can easily ingest selenium to maintain basic life needs due to their proximity to selenium sources. 26 But artificially farmed fish require the addition of selenium-enriched yeast to their feed to maintain selenium balance. 18 Moderate selenium intake can support the expression of various Selenoproteins in important organs and enhance the antioxidant activity of selenium proteases to keep the body’s redox balance and increase the growth rate of fish. 27 Long-term selenium deficiency leads to the continuous accumulation of reactive oxygen species (ROS) in physiological metabolism, leading to oxidative stress and affecting the growth of zebrafish. Through dietary experiments with selenium-enriched yeast added from low to high, it was concluded that adding 0.34 mg/kg selenium to feed is the most suitable for the hypertrophy and growth of zebrafish skeletal muscles while maintaining high antioxidant activity. At this concentration, the protein synthesis rate in zebrafish muscle is the highest, and the degradation rate is the lowest. In contrast, low or high selenium reduces the protein synthesis rate and increases the decomposition rate. 28
Selenium enhances immune function and anti-tumor ability
The importance of selenium in immune function has been confirmed in animal experiments and cell model experiments. 29 Selenium deficiency leads to weakened immunity and increases the probability of tumor occurrence. Selenoprotein expression is limited in low selenium conditions, which affects the differentiation, development, and maturation of immune cells and hinders the activation and function of mature immune cells. 30 Generally speaking, antioxidants also have anti-inflammatory effects. During the inflammatory process, NADPH oxidase is influenced by pro-inflammatory factors and promotes synthesis, which can catalyze the production of a large amount of reactive oxygen species. Reactive oxygen species kill invading microorganisms, but excessive accumulation of reactive oxygen species can also damage normal cells, including immune cells. 31 In selenium deficiency, the redox equilibrium is imbalanced, and the ROS produced by immune cells cannot be removed in time, which affects immune function. Therefore, appropriate selenium supplementation can improve the function of the immune system, thereby reducing the susceptibility to pathogenic microbial infections and cancer. With the rise of nanotechnology, the potential of nanoparticles in medical applications has been gradually explored, especially in cancer treatment. Selenium nanoparticles can be synthesized through chemical, physical, and biological methods, all committed to reducing the cost of synthesis, environmental pollution, toxicity, and improving bioavailability. 32 Compared with ordinary selenium, selenium nanoparticles have higher bioavailability, biological activity, and controllable release, and their antioxidant, immune, and anti-cancer effects are stronger than ordinary selenium. 32 A study 33 comparing the growth, immunity, and oxidation status of tilapia after 65 days of feeding diets containing different forms of selenium found that organic selenium and selenium nanoparticles had the best growth promotion effect, and selenium nanoparticles had the strongest effect on immunity, immune gene expression, and antioxidant capacity. Unfortunately, similar studies have not yet been conducted on zebrafish. Combining inulin fructan with moderate anti-tumor ability and selenium to form selenium nanoparticles has been confirmed to enhance the anti-tumor ability of in vitro experiments and zebrafish tumor xenotransplantation experiments. 34 These research results fully demonstrate the potential of selenium nanoparticles in immune and anti-tumor fields.
Selenium can antagonize mercury and other pollutants poisoning
Mercury is one of the most common toxic elements in aquatic organisms and humans. As a result of human overexploitation of natural resources and industrial pollution, the mercury contents in polluted marine environments increase and are converted by microorganisms into methylmercury, which fish more easily absorb. Methylmercury has strong neurotoxicity and can penetrate the blood–brain barrier to damage nerve cells, causing sensory and behavioral disorders. 35 The interaction between selenium and mercury is relatively complex, and selenium can serve as an antagonist to methylmercury poisoning. Some studies suggest that the neurotoxicity caused by methylmercury may be due to selenium deficiency caused by methylmercury. Methylmercury can chelate with selenium to form a nonbioavailable form, and this selenium deficiency state can be prevented by supplementing selenium. 36 Dietary experiments with selenomethionine and methylmercury in zebrafish have confirmed that increasing dietary selenium can reduce the accumulation of methylmercury in zebrafish muscles and enhance zebrafish’s ability to eliminate methylmercury. 37 When exposed to mercury, methylmercury absorbed by zebrafish preferentially binds to selenoaminoacid in the active center of selenoprotein, thereby affecting the function of selenoprotein and causing redox imbalance. Experiments have shown that methylmercury inhibits the antioxidant selenoproteinase activity and gene expression in zebrafish, and supplementing selenium can partially rescue the inhibitory effect caused by methylmercury. 38
Common pollutants in the aquatic environment include heavy metals, pesticides, etc., which are highly toxic, and most of them can promote oxidation, causing oxidative stress to marine organisms and damaging their health. 39 As an antioxidant, selenium can effectively combat oxidative stress caused by environmental pollutants. Exposure studies of paraquat, a highly toxic quaternary ammonium herbicide, have demonstrated that dietary pretreatment with sodium selenite in zebrafish prevented the increased levels of carbonylated protein, reactive oxygen species, and nitrites/nitrates, and the decrease in non-protein thiols levels caused by paraquat, thereby improving the behavioral and biochemical abnormalities associated with paraquat exposure. 40 In another experiment, 41 zebrafish embryos were exposed to fluoride, which led to oxidative damage and apoptosis, resulting in developmental toxicity and inflammation. Selenomethionine treatment not only alleviates the adverse effects caused by fluoride but also gradually eliminates fluorine-induced oxidative stress and inflammatory responses and relieves fluorine-induced liver and intestinal damage. Recently, it has been reported that selenium selenite can alleviate the inhibitory effects of perfluorooctane sulfonic acid and cadmium on antioxidant enzymes in zebrafish liver and reduce toxicity to zebrafish liver. 42 These studies have demonstrated that selenium can reduce the toxic effects caused by environmental pollutants.
Although selenium was already known to have certain toxic effects before it was recognized as a necessary trace element, humans initially did not pay attention to its adverse effects on natural ecosystems. Selenium in nature is mainly found in carbonate rocks, volcanic soils, and sedimentary soils. However, 40% of selenium in the atmosphere and aquatic environment is caused by the intervention of various industrial activities such as mining and mineral refining. 43 Selenium pollution poses a serious health hazard to marine organisms. The most typical example is the selenium pollution of Lake Belus due to the discharge of wastewater from coal-fired power plants. The fish community in this lake continued to suffer chronic selenium poisoning, causing multi-system and multi-organ damage as well as obvious malformations. 44 Lower selenium concentrations have an antioxidant effect, but high selenium levels produce a prooxidant effect. In the state of high selenium, selenium can directly form intramolecular disulfide bonds, selenium trisulfide bonds, and selenium sulfur bonds with essential thiol groups or cysteine residues in the active center of the enzyme protein to inactivate enzyme protein activity. Selenium can replace sulfur during protein synthesis, causing protein folding errors. In addition, selenium can also consume glutathione to produce superoxide anions, causing accumulation of ROS, leading to a decrease in intracellular reduction status and cell damage. 45 A study on zebrafish embryos exposed to selenium showed that when the concentration of selenomethionine reached 100 ug/L, the mortality and deformity rates of embryos significantly increased. The glutathione level significantly decreased at 400 ug/L, and the TGSH: GSSG ratio decrease indicates that the embryo was under oxidative stress. The addition of antioxidant N-acetylcysteine significantly improved mortality and deformity rates and alleviated low levels of glutathione. 46
The effects of selenium on the nervous system and ethology of zebrafish
Most environmental pollutants have neurotoxic effects on zebrafish, affecting the development of the central nervous system, synthesis and release of neurotransmitters, etc. 47 Excessive selenium also has neurotoxic effects and involves behavioral changes in zebrafish. Regardless of the exposure method, the basic toxic mechanism of excessive selenium is through the induction of oxidative stress. Zebrafish embryos exposed to selenomethionine to 96 h post fertilization (hpf) showed that the number of brain cells decreased significantly and the number of apoptotic cells increased. Mitochondria in brain cells are reduced and destroyed, and the nuclear membrane structure is loosely folded. The inflammatory response of brain tissue is enhanced. 48 In addition, embryos exposed to sodium selenite to 48 hpf through immunofluorescence detection of acetylation α-tubulin found that tubulin in axons was absent or abnormally arranged, resulting in abnormal development of neural tubes and neurons in the trunk and tail. 49 Under selenium exposure, oxidative damage occurs within neuronal cells, disrupting the stability of the mitochondrial inner membrane, producing more ROS, and further damaging other organelles, including the endoplasmic reticulum, impairing protein synthesis function. This may be the reason for the blockage of the synthesis of many neurotransmitters in the brain. The experiment also confirmed that related markers and gene transcription of dopamine, serotonin, γ-aminobutyric acid, acetylcholine, and histamine were affected. 50 Under the influence of this series of pathological changes, zebrafish also have adverse consequences in ethology. Behavioral analysis of zebrafish exposed to selenium revealed that its basic movement ability was impaired. 49 Its locomotor activity weakens with increasing exposure concentration, and touch experiments show that larvae exposed to high concentrations have slower reactions and poorer escape ability. 48 The maze experiment of more complex visual stimuli and food rewards reflects the severe impairment of associative learning behavior in adults exposed to high selenium. 50 , 51
Moreover, the neurological and behavioral effects of selenium exposure can even affect offspring through intra-ovo accumulation. Embryos born from long-term selenium-exposed mothers are no longer exposed to selenium since fertilization. Still, the embryos exhibit the same diminished clustering, decreased social learning ability, and dysregulated neurotransmitter transmission as their mother in adulthood. 52 Peroxisome proliferator-activated receptor (PPAR) is a nonsteroidal nuclear receptor involved in the development and functioning of the nervous system. Activating PPAR can alleviate neurological dysfunction and improve exercise capacity. 53 A study reported that KEGG analysis of zebrafish with neurobehavioral disorders after selenium exposure revealed significant downregulation of PPAR signaling pathway-related genes, confirmed on qPCR. However, using PPAR agonists can only improve the downregulation of related genes but not behavioral defects. 48 The PPAR signaling pathway is involved in the neurotoxic process of selenium exposure, but it may not be the main damage mechanism. The specific mechanism of the nervous system and behavioral changes in zebrafish damaged by high selenium concentrations is still not fully understood.
The effects of selenium on reproduction and growth of zebrafish
Long-term exposure to selenium contamination can lead to the continuous accumulation of selenium levels in fish organs, mostly in the liver, skeletal muscle, heart, and ovary. Although the ovaries are not the most severe selenium residual organs, the reproductive toxicity induced by selenium poses the most harm to the continuation of fish populations. 44 According to reports, under high concentration selenium stress, the primary oocytes of female zebrafish delay maturation and partially undergo apoptosis due to oxidative stress, resulting in a decrease in zebrafish egg production. Due to the biological enrichment of selenium, the hatching rate decreases, and the mortality rate increases in offspring. 44 , 54 The maturation of oocytes is also regulated by hormones in the hypothalamic–pituitary gonadal axis. However, high selenium exposure reduces the expression of this axis hormone in zebrafish through endoplasmic reticulum stress, especially estradiol, which is involved in inhibiting oocyte maturation. 55 Long-term selenium exposure inhibits the growth of parent zebrafish and limits offspring development due to selenium stress transmitted by parents through fertilized eggs. Studies have shown that gene expression and content of the thyroid hormone axis and somatotropin axis are significantly inhibited in both selenium-exposed parents and their offspring born but not exposed to selenium. 56 Moreover, the deformity rate of offspring is considerably higher than that of parents; the degree and types of malformations are also diverse, including craniofacial malformations, eye malformations, pigment loss, pericardial and abdominal edema, spinal cord malformations, spleen and gallbladder lesions, etc. 57
The behavior of fish is crucial in their growth, especially their swimming ability. Excess selenium negatively affects fish behavior through neurotoxicity and can interfere with fish energy metabolism and impair fish swimming ability. Experiments have shown that zebrafish fed excessive organic selenium have a lower critical swimming speed and impaired swimming ability. In maintaining the same regular exercise and balance, the high-selenium group needs to consume more energy and oxygen, i.e. the metabolic rate increases. Biochemical tests showed that zebrafish’s blood sugar and triglyceride levels in the high selenium group increased. 58 The changes in blood sugar and triglycerides have been explained in the latest report: excessive selenium inhibits the expression of genes related to fat breakdown but has no significant effect on genes related to fat synthesis. 59 Excessive selenium also inhibits the glycolytic pathway and tricarboxylic acid cycle and overactivates the gluconeogenesis pathway. The report also found that excessive selenium damaged pancreatic function, inhibited insulin secretion, and ultimately disrupted glycometabolism in zebrafish. 59 In addition, excessive selenium in the endoplasmic reticulum causes protein folding errors and hinders some protein synthesis. 60 Abnormal metabolism of the three major nutrients is bound to have adverse consequences for the growth of zebrafish.
Toxic effects of selenium on other organs
Whether it is environmental or dietary exposure when the selenium concentration exceeds the nutritional range, the fish’s liver is one of the organs with a severe accumulation of selenium. 18 It is also the most sensitive organ to changes in the antioxidant enzyme system. 59 After acute selenium exposure in zebrafish, histopathological observations showed a decrease in liver volume, slow development, and impaired liver function 59 ; Focal necrosis, hemorrhage, and interstitial edema in liver tissue; Hepatocytes have irregular shapes, swelling, nuclear pyknosis, and the formation of fat large vacuoles. 61 Moderate lipid vacuolar degeneration, macrophage aggregation, and glycogen depletion were also observed in the chronic dietary selenium sublethal exposure of Pogonichthys macrolepidotus . 62 Unfortunately, the report did not detect changes in blood glucose levels in P. macrolepidotus . Both reports 59 , 61 tested the antioxidant enzyme system in zebrafish liver and confirmed that the mechanism of liver damage caused by excessive selenium is still driven by oxidative damage. The production of a large number of ROS inhibits the autophagy function of hepatocytes so that hepatocytes cannot remove harmful substances in time, resulting in hepatocyte damage. The autophagy inducer rapamycin restored the autophagic function of hepatocytes and saved some liver function and developmental malformations in zebrafish embryos. 59
The protective effect of selenium on cardiovascular diseases has been studied to a certain extent. It is negatively correlated with the occurrence of cardiovascular diseases in the range of nutrient concentrations. Selenoprotein plays an important role in the heart’s development and the myocardium’s protection from oxidative damage. 63 However, exceeding the nutritional range of selenium poses a serious burden on the cardiovascular system, which has been confirmed in zebrafish. Zebrafish embryos exposed to sodium selenite at 4 to 120 hpf, abnormal cardiac development, and pericardial edema can be seen. Atrioventricular staining shows atrial and ventricular cavity hypoplasia and is smaller than the normal group. 49 Specific fluorescent expression genes labeled myocardial cells in transgenic zebrafish embryos were used to observe cardiac development. After exposure to selenomethionine, smaller ventricles and larger atria were observed, with a significant increase in atrioventricular area ratio. HE staining showed loose myocardial cells. 64 Embryos exposed to more toxic selenium nanoparticles can even see crookedly circular and linear chambers and the ventricles in front of the atrium. 65 Selenium, in either form, caused a statistically significant decrease in the heart rate of zebrafish embryos. 49 , 64 , 65 The oxidative stress caused by excessive selenium leading to cell apoptosis is the fundamental mechanism of cardiac developmental abnormalities, and the antioxidant folic acid can prevent heart defects caused by sodium selenite. 49 Research has found that after exposure to selenomethionine, the expression of the heart development-related gene lrp2b in zebrafish is downregulated. After silencing the lrp 2b gene, zebrafish exhibited similar characteristics to selenomethionine exposure; Overexpression of the lrp 2b gene protects embryos exposed to selenomethionine. 64 The abnormal expression of genes related to cardiac development may be due to selenium interference with DNA methylation in the early developmental genome. 49 Still, it has not been confirmed whether the inhibition of DNA methylation affects the expression of lrp 2b.
One of the little-known symptoms of selenium poisoning in fish is the occurrence of visual impairment, which may be due to the accumulation of selenium in the eyes and damage to the development and function of the eyes. 66 Research has found that excessive selenium exposure leads to incomplete and delayed eye development, smaller eye radius, and abnormal expression of genes related to the eyes and optic nerve in zebrafish. 67 The study also found that oxidative stress caused by high selenium induces apoptosis and ferroptosis. Salvage experiments were conducted using antioxidant, ferroptosis inhibitors, and apoptosis and ferroptosis activator cisplatin. The results showed that antioxidants could not completely antagonize selenium-induced eye defects, while low-dose cisplatin can improve. 67 In addition, confocal X-ray fluorescence imaging technology was used to observe embryos produced by female fish fed a high selenium diet. It was found that selenium preferentially accumulates in the lens of embryos through parental transmission, ultimately leading to lens opacities or cataracts in zebrafish. 68
The different forms of selenium have varying degrees of beneficial and toxic effects on zebrafish, which may be related to the utilization rates of the various forms of selenium in zebrafish. 69 One study treated zebrafish embryos with different forms of selenium and analyzed their accumulation levels, which found the dosage of organic selenium was much lower than that of inorganic selenium. Still, the accumulation concentration of organic selenium in juvenile zebrafish was higher, 70 indicating that organic selenium has greater toxicity and higher bioavailability to fish. So, dietary exposure experiments most commonly use selenomethionine as the source of selenium intake. The inorganic selenium used in the embryo exposure experiment is sodium selenite, with an exposure concentration of 10 umol/L, 49 while the commonly used organic selenium is selenomethionine, with an exposure concentration of 0.5 umol/L. 48 , 59 , 67 The increase in exposure concentration leads to an increase in the embryonic mortality rate and a decrease in the embryonic hatching rate, which affects the experimental results. Lower concentrations must be used for long-term chronic exposure to reduce mortality rates. In addition, the form of selenium also includes simple selenium nanoparticles (SeNPs) synthesized through biological or chemical methods and composite selenium nanoparticles combined with other substances. Embryo exposure experiments have shown that chemically synthesized SeNPs’ antioxidant capacity is stronger than sodium selenite at the same dose. 71 Still, its toxicity is greater than sodium selenite. 65 The toxicity of biosynthetic SeNPs is several times lower than that of sodium selenate, and it has more stable dissolution kinetics and characterization than chemically synthesized SeNPs. 72 However, there has been no study comparing the toxicity of SeNPs with organic selenium. Composite SeNPs cannot be reached in toxicity with other forms of selenium due to their combination with other substances. Still, their significant advantage is that they greatly enhance selenium’s beneficial effects. For example, a composite SeNPs diet composed of chitosan, which also has immune-enhancing properties, can significantly improve the immune function of zebrafish compared to a diet consisting solely of chitosan and sodium selenite. After intraabdominal injection of Aeromonas hydrophila , the survival rate of zebrafish fed with composite SeNPs was significantly higher than that of other groups. 73 Inulin fructan and selenium, which both have anti-tumor effects composed of SeNPs, are also the same. 34
Selenium is an essential trace element for animals; we must truly understand the nutritional value and toxicity to provide a reference for fish to supplement selenium reasonably. An appropriate amount of selenium can provide fish with necessary beneficial effects, such as participating in important physiological processes, promoting growth, enhancing antioxidant effects, enhancing immune response, and preventing related diseases. However, it is also necessary to be cautious of excessive intake, which can cause neurotoxicity and damage to internal organs. Although some achievements have been made in studying selenium on zebrafish, further research is still needed. The following aspects may provide ideas for further research on the benefits and toxicity of selenium to zebrafish: (1) At present, most of the selenoproteins in humans and zebrafish have been identified, but some of the identified selenoproteins have not fully understood their functions and roles in physiological processes. (2) The safe nutritional range of zebrafish selenium is narrow and has a "double-edged sword" characteristic. An authoritative, safe concentration range should be explored, and this range should vary with the chemical form of selenium and different stages of zebrafish development. (3) Selenides and SeNPs can be used as potential therapeutic drugs for some diseases, such as diabetes, 74 cancer, etc., and zebrafish can be used to establish disease models to explore the therapeutic effects of selenium-containing drugs. (4) Excessive selenium can cause body axis deformities in zebrafish, such as lordosis, kyphosis, and scoliosis. Is body axis deformity related to abnormal gene expression or myogenic? There is currently a lack of relevant research in this area. The only study on Japanese medaka has shown that skeletal abnormalities caused by selenium in craniofacial and caudal fin deformity are associated with abnormal gene expression related to cartilage and bone formation. 75 (5) After selenium poisoning, fish can exhibit multiple organ damage, and further research on the pathological changes and damage mechanisms of other organs such as gills, kidneys, and pancreas can be carried out on zebrafish. (6) Most research on organ damage remains focused on the abnormal expression of related genes caused by oxidative stress, and the specific mechanisms of damage or related pathways activated through ROS still need to be further explored.
Yuan Zhang and Yuanshan Lin contributed to the study conception and design. Data collection and analysis were performed by Liyun Hu, Xinhang Li and Jie Ma. The first draft of the manuscript was written by Yuanshan Lin. The manuscript was reviewed and corrected by Qipeng Li and Xiaofan Yuan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
This work was funded by the National Natural Science Foundation of China (grant number 81560366) and the Major Science and Technology Project of Yunnan Provincial Department of Science and Technology, Yunnan Provincial Orthopedic and Sports Rehabilitation Clinical Medicine Research Center (grant number 202102AA310068).
Conflict of interest statement . The authors declare that they have no financial interests.
Mangiapane E , Pessione A , Pessione E . Selenium and selenoproteins: an overview on different biological systems . Curr Protein Pept Sci . 2014 : 15 ( 6 ): 598 – 607 .
Google Scholar
Rayman MP . Selenium intake, status, and health: a complex relationship . Hormones (Athens) . 2020 : 19 ( 1 ): 9 – 14 .
Shimada BK , Alfulaij N , Seale LA . The impact of selenium deficiency on cardiovascular function . Int J Mol Sci . 2021 : 22 ( 19 ): 10713 .
Deng H , Liu H , Yang Z , Bao M , Lin X , Han J , Qu C . Progress of selenium deficiency in the pathogenesis of Arthropathies and selenium supplement for their treatment . Biol Trace Elem Res . 2022 : 200 ( 10 ): 4238 – 4249 .
Gorini F , Sabatino L , Pingitore A , Vassalle C . Selenium: an element of life essential for thyroid function . Molecules . 2021 : 26 ( 23 ): 7084 .
Wang F , Li C , Li S , Cui L , Zhao J , Liao L . Selenium and thyroid diseases . Front Endocrinol (Lausanne) . 2023 : 14 : 1133000 .
Kieliszek M , Błażejak S . Current knowledge on the importance of selenium in food for living organisms: a review . Molecules . 2016 : 21 ( 5 ): 609 .
Kieliszek M . Selenium-fascinating microelement, properties and sources in food . Molecules . 2019 : 24 ( 7 ): 1298 .
Vinceti M , Filippini T , Wise LA , Rothman KJ . A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies . Environ Res . 2021 : 197 : 111210 .
Vinceti M , Mandrioli J , Borella P , Michalke B , Tsatsakis A , Finkelstein Y . Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies . Toxicol Lett . 2014 : 230 ( 2 ): 295 – 303 .
Lundin KK , Qadeer YK , Wang Z , Virani S , Leischik R , Lavie CJ , Strauss M , Krittanawong C . Contaminant metals and cardiovascular health . J Cardiovasc Dev Dis . 2023 : 10 ( 11 ): 450 .
Naderi M , Puar P , Zonouzi-Marand M , Chivers DP , Niyogi S , Kwong RWM . A comprehensive review on the neuropathophysiology of selenium . Sci Total Environ . 2021 : 767 : 144329 .
Vinceti M , Filippini T , Jablonska E , Saito Y , Wise LA . Safety of selenium exposure and limitations of selenoprotein maximization: molecular and epidemiologic perspectives . Environ Res . 2022 : 211 : 113092 .
de Paula Gutiérrez BF , Agudelo CAR . Fish as bioindicators: coal and mercury pollution in Colombia’s ecosystems . Environ Sci Pollut Res Int . 2020 : 27 ( 22 ): 27541 – 27562 .
Aich A , Goswami AR , Roy US , Mukhopadhyay SK . Ecotoxicological assessment of tannery effluent using guppy fish (Poecilia reticulata) as an experimental model: a biomarker study . J Toxicol Environ Health A . 2015 : 78 ( 4 ): 278 – 286 .
Sumana SL , Chen H , Shui Y , Zhang C , Yu F , Zhu J , Su S . Effect of dietary selenium on the growth and immune systems of fish . Animals (Basel) . 2023 : 13 ( 18 ): 2978 .
MacRae CA , Peterson RT . Zebrafish as tools for drug discovery . Nat Rev Drug Discov . 2015 : 14 ( 10 ): 721 – 731 .
Khan KU , Zuberi A , Fernandes JBK , Ullah I , Sarwar H . An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health . Fish Physiol Biochem . 2017 : 43 ( 6 ): 1689 – 1705 .
Kryukov GV , Gladyshev VN . Selenium metabolism in zebrafish: multiplicity of selenoprotein genes and expression of a protein containing 17 selenocysteine residues . Genes Cells . 2000 : 5 ( 12 ): 1049 – 1060 .
Bjørklund G , Shanaida M , Lysiuk R , Antonyak H , Klishch I , Shanaida V , Peana M . Selenium: an antioxidant with a critical role in anti-aging . Molecules . 2022 : 27 ( 19 ): 6613 .
Lu J , Holmgren A . The thioredoxin antioxidant system . Free Radic Biol Med . 2014 : 66 : 75 – 87 .
Labunskyy VM , Hatfield DL , Gladyshev VN . Selenoproteins: molecular pathways and physiological roles . Physiol Rev . 2014 : 94 ( 3 ): 739 – 777 .
Shetty S , Marsicano JR , Copeland PR . Uptake and utilization of selenium from selenoprotein P . Biol Trace Elem Res . 2018 : 181 ( 1 ): 54 – 61 .
Cox AG , Tsomides A , Kim AJ , Saunders D , Hwang KL , Evason KJ , Heidel J , Brown KK , Yuan M , Lien EC , et al. Selenoprotein H is an essential regulator of redox homeostasis that cooperates with p53 in development and tumorigenesis . Proc Natl Acad Sci USA . 2016 : 113 ( 38 ): E5562 – E5571 .
Jurynec MJ , Xia R , Mackrill JJ , Gunther D , Crawford T , Flanigan KM , Abramson JJ , Howard MT , Grunwald DJ . Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle . Proc Natl Acad Sci USA . 2008 : 105 ( 34 ): 12485 – 12490 .
Ullah H , Liu G , Yousaf B , Ali MU , Irshad S , Abbas Q , Ahmad R . A comprehensive review on environmental transformation of selenium: recent advances and research perspectives . Environ Geochem Health . 2019 : 41 ( 2 ): 1003 – 1035 .
Penglase S , Hamre K , Rasinger JD , Ellingsen S . Selenium status affects selenoprotein expression, reproduction, and F 1 generation locomotor activity in zebrafish (Danio rerio) . Br J Nutr . 2014 : 111 ( 11 ): 1918 – 1931 .
Wang L , Yin J , Liao C , Cheng R , Chen F , Yu H , Zhang X . Selenium deficiency-induced high concentration of reactive oxygen species restricts hypertrophic growth of skeletal muscle in juvenile zebrafish by suppressing TORC1-mediated protein synthesis . Br J Nutr . 2023: 130 (11):1841–1851.
Hoffmann PR . Mechanisms by which selenium influences immune responses . Arch Immunol Ther Exp . 2007 : 55 ( 5 ): 289 – 297 .
Avery JC , Hoffmann PR . Selenium, selenoproteins, and immunity . Nutrients . 2018 : 10 ( 9 ): 1203 .
Puertollano MA , Puertollano E , de Cienfuegos GÁ , de Pablo MA . Dietary antioxidants: immunity and host defense . Curr Top Med Chem . 2011 : 11 ( 14 ): 1752 – 1766 .
Kumar A , Prasad KS . Role of nano-selenium in health and environment . J Biotechnol . 2021 : 325 : 152 – 163 .
Ghaniem S , Nassef E , Zaineldin AI , Bakr A , Hegazi S . A comparison of the beneficial effects of inorganic, organic, and elemental Nano-selenium on Nile tilapia: growth, immunity, oxidative status, gut morphology, and immune gene expression . Biol Trace Elem Res . 2022 : 200 ( 12 ): 5226 – 5241 .
Zhang S , Song Z , Shi L , Zhou L , Zhang J , Cui J , Li Y , Jin DQ , Ohizumi Y , Xu J , et al. A dandelion polysaccharide and its selenium nanoparticles: structure features and evaluation of anti-tumor activity in zebrafish models . Carbohydr Polym . 2021 : 270 : 118365 .
Rasinger JD , Lundebye AK , Penglase SJ , Ellingsen S , Amlund H . Methylmercury induced neurotoxicity and the influence of selenium in the brains of adult zebrafish (Danio rerio) . Int J Mol Sci . 2017 : 18 ( 4 ): 725 .
Ralston NVC , Raymond LJ . Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry . Biochim Biophys Acta Gen Subj . 2018 : 1862 ( 11 ): 2405 – 2416 .
Amlund H , Lundebye AK , Boyle D , Ellingsen S . Dietary selenomethionine influences the accumulation and depuration of dietary methylmercury in zebrafish (Danio rerio) . Aquat Toxicol . 2015 : 158 : 211 – 217 .
Penglase S , Hamre K , Ellingsen S . Selenium prevents downregulation of antioxidant selenoprotein genes by methylmercury . Free Radic Biol Med . 2014 : 75 : 95 – 104 .
Valavanidis A , Vlahogianni T , Dassenakis M , Scoullos M . Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants . Ecotoxicol Environ Saf . 2006 : 64 ( 2 ): 178 – 189 .
Müller TE , Nunes ME , Menezes CC , Marins AT , Leitemperger J , Gressler ACL , Carvalho FB , de Freitas CM , Quadros VA , Fachinetto R , et al. Sodium selenite prevents paraquat-induced neurotoxicity in zebrafish . Mol Neurobiol . 2018 : 55 ( 3 ): 1928 – 1941 .
Zhang X , Chen J , Wang G , Chen H , Cao J , Xie L , Luo Y . Interactive effects of fluoride and seleno-l-methionine at environmental related concentrations on zebrafish (Danio rerio) liver via the gut-liver axis . Fish Shellfish Immunol . 2022 : 127 : 690 – 702 .
Lu W , Ahmed W , Mahmood M , Wenjie O , Jiannan L , Yunting W , Jie Y , Wenxin X , Xiuxian F , Zhao H , et al. A study on the effectiveness of sodium selenite in treating cadmium and perfluoro octane sulfonic (PFOS) poisoned zebrafish (Danio rerio) . Biol Trace Elem Res . 2023 : 202 ( 1 ): 319 – 331 .
Tan LC , Nancharaiah YV , van Hullebusch ED , Lens PNL . Selenium: environmental significance, pollution, and biological treatment technologies . Biotechnol Adv . 2016 : 34 ( 5 ): 886 – 907 .
Lemly AD . Symptoms and implications of selenium toxicity in fish: the Belews Lake case example . Aquat Toxicol . 2002 : 57 ( 1–2 ): 39 – 49 .
Lee KH , Jeong D . Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: the selenium paradox (review) . Mol Med Rep . 2012 : 5 ( 2 ): 299 – 304 .
Arnold MC , Forte JE , Osterberg JS , Di Giulio RT . Antioxidant Rescue of selenomethionine-induced teratogenesis in zebrafish embryos . Arch Environ Contam Toxicol . 2016 : 70 ( 2 ): 311 – 320 .
Lin W , Huang Z , Zhang W , Ren Y . Investigating the neurotoxicity of environmental pollutants using zebrafish as a model organism: a review and recommendations for future work . Neurotoxicology . 2023 : 94 : 235 – 244 .
Zhao G , Hu J , Gao M , Zhu Y , Hong Y . Excessive selenium affects neural development and locomotor behavior of zebrafish embryos . Ecotoxicol Environ Saf . 2022 : 238 : 113611 .
Ma Y , Wu M , Li D , Li XQ , Li P , Zhao J , Luo MN , Guo CL , Gao XB , Lu CL , et al. Embryonic developmental toxicity of selenite in zebrafish (Danio rerio) and prevention with folic acid . Food Chem Toxicol . 2012 : 50 ( 8 ): 2854 – 2863 .
Li X , Liu H , Li D , Lei H , Wei X , Schlenk D , Mu J , Chen H , Yan B , Xie L . Dietary Seleno-l-methionine causes alterations in neurotransmitters, ultrastructure of the brain, and Behaviors in zebrafish (Danio rerio) . Environ Sci Technol . 2021 : 55 ( 17 ): 11894 – 11905 .
Naderi M , Salahinejad A , Ferrari MCO , Niyogi S , Chivers DP . Dopaminergic dysregulation and impaired associative learning behavior in zebrafish during chronic dietary exposure to selenium . Environ Pollut . 2018 : 237 : 174 – 185 .
Attaran A , Salahinejad A , Naderi M , Crane AL , Chivers DP , Niyogi S . Transgenerational effects of selenomethionine on behaviour, social cognition, and the expression of genes in the serotonergic pathway in zebrafish . Environ Pollut . 2021 : 286 : 117289 .
Khera R , Mehan S , Kumar S , Sethi P , Bhalla S , Prajapati A . Role of JAK-STAT and PPAR-gamma signalling modulators in the prevention of autism and neurological dysfunctions . Mol Neurobiol . 2022 : 59 ( 6 ): 3888 – 3912 .
Mo A , Wang X , Yuan Y , Liu C , Wang J . Effects of waterborne exposure to environmentally relevant concentrations of selenite on reproductive function of female zebrafish: a life cycle assessment . Environ Pollut . 2021 : 270 : 116237 .
Cheng R , Zhang Z , Zhan C , Qin T , Wang L , Zhang X . Environmentally relevant concentrations of selenite trigger reproductive toxicity by affecting oocyte development and promoting larval apoptosis . Environ Pollut . 2023 : 316 ( Pt 1 ): 120648 .
Cheng R , Zhang J , He Y , Liao C , Wang L , Zhang X . Parental exposure to waterborne selenite induces transgenerational development toxicity in zebrafish offspring . Chemosphere . 2022 : 303 ( Pt 1 ): 134838 .
Chernick M , Ware M , Albright E , Kwok KWH , Dong W , Zheng N , Hinton DE . Parental dietary seleno-L-methionine exposure and resultant offspring developmental toxicity . Aquat Toxicol . 2016 : 170 : 187 – 198 .
Thomas JK , Wiseman S , Giesy JP , Janz DM . Effects of chronic dietary selenomethionine exposure on repeat swimming performance, aerobic metabolism and methionine catabolism in adult zebrafish (Danio rerio) . Aquat Toxicol . 2013 : 130-131 : 112 – 122 .
Zhu Y , Hu J , Zeng S , Gao M , Guo S , Wang M , Hong Y , Zhao G . L-selenomethionine affects liver development and glucolipid metabolism by inhibiting autophagy in zebrafish embryos . Ecotoxicol Environ Saf . 2023 : 252 : 114589 .
Kupsco A , Schlenk D . Molecular mechanisms of selenium-induced spinal deformities in fish . Aquat Toxicol . 2016 : 179 : 143 – 150 .
Kumar N , Krishnani KK , Singh NP . Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish . Environ Sci Pollut Res Int . 2018 : 25 ( 9 ): 8914 – 8927 .
Teh SJ , Deng X , Deng DF , Teh FC , Hung SS , Fan TW , Liu J , Higashi RM . Chronic effects of dietary selenium on juvenile Sacramento splittail (Pogonichthys macrolepidotus) . Environ Sci Technol . 2004 : 38 ( 22 ): 6085 – 6093 .
Handy DE , Joseph J , Loscalzo J . Selenium, a micronutrient that modulates cardiovascular health via redox enzymology . Nutrients . 2021 : 13 ( 9 ): 3238 .
Zhao G , Zhu Y , Hu J , Gao M , Hong Y . L-selenomethionine induces zebrafish embryo cardiovascular defects via down-regulating expression of lrp2b . Chemosphere . 2022 : 290 : 133351 .
Kalishwaralal K , Jeyabharathi S , Sundar K , Muthukumaran A . Comparative analysis of cardiovascular effects of selenium nanoparticles and sodium selenite in zebrafish embryos . Artif Cells Nanomed Biotechnol . 2016 : 44 ( 3 ): 990 – 996 .
Raine JC , Lallemand L , Pettem CM , Janz DM . Effects of chronic dietary selenomethionine exposure on the visual system of adult and F1 generation zebrafish (Danio rerio) . Bull Environ Contam Toxicol . 2016 : 97 ( 3 ): 331 – 336 .
Gao M , Hu J , Zhu Y , Wang X , Zeng S , Hong Y , Zhao G . Ferroptosis and apoptosis are involved in the formation of L-selenomethionine-induced ocular defects in zebrafish embryos . Int J Mol Sci . 2022 : 23 ( 9 ): 4783 .
Choudhury S , Thomas JK , Sylvain NJ , Ponomarenko O , Gordon RA , Heald SM , Janz DM , Krone PH , Coulthard I , George GN , et al. Selenium preferentially accumulates in the eye lens following embryonic exposure: a confocal X-ray fluorescence imaging study . Environ Sci Technol . 2015 : 49 ( 4 ): 2255 – 2261 .
Niimi AJ , Laham QN . Relative toxicity of organic and inorganic compounds of selenium to newly hatched zebrafish (Brachydanio rerio) . Can J Zool . 1976 : 54 ( 4 ): 501 – 509 .
Dolgova NV , Hackett MJ , MacDonald TC , Nehzati S , James AK , Krone PH , George GN , Pickering IJ . Distribution of selenium in zebrafish larvae after exposure to organic and inorganic selenium forms . Metallomics . 2016 : 8 ( 3 ): 305 – 312 .
Kalishwaralal K , Jeyabharathi S , Sundar K , Muthukumaran A . Sodium selenite/selenium nanoparticles (SeNPs) protect cardiomyoblasts and zebrafish embryos against ethanol induced oxidative stress . J Trace Elem Med Biol . 2015 : 32 : 135 – 144 .
Mal J , Veneman WJ , Nancharaiah YV , van Hullebusch ED , Peijnenburg WJ , Vijver MG , Lens PN . A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis . Nanotoxicology . 2017 : 11 ( 1 ): 87 – 97 .
Xia IF , Cheung JS , Wu M , Wong KS , Kong HK , Zheng XT , Wong KH , Kwok KW . Dietary chitosan-selenium nanoparticle (CTS-SeNP) enhance immunity and disease resistance in zebrafish . Fish Shellfish Immunol . 2019 : 87 : 449 – 459 .
dos Santos MM , de Macedo GT , Prestes AS , Ecker A , Müller TE , Leitemperger J , Fontana BD , Ardisson-Araújo DMP , Rosemberg DB , Barbosa NV . Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish . Free Radic Biol Med . 2020 : 158 : 20 – 31 .
Wang H , Chen H , Chernick M , Li D , Ying GG , Yang J , Zheng N , Xie L , Hinton DE , Dong W . Selenomethionine exposure affects chondrogenic differentiation and bone formation in Japanese medaka (Oryzias latipes) . J Hazard Mater . 2020 : 387 : 121720 .
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Journals Career Network
Affiliations
- Online ISSN 2045-4538
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 14 March 2015
The value of zebrafish as an integrative model in effect-directed analysis - a review
- Carolina Di Paolo 1 ,
- Thomas-Benjamin Seiler 1 ,
- Steffen Keiter 1 , 2 ,
- Meng Hu 3 ,
- Melis Muz 3 ,
- Werner Brack 3 &
- Henner Hollert 1 , 4 , 5 , 6
Environmental Sciences Europe volume 27 , Article number: 8 ( 2015 ) Cite this article
8851 Accesses
45 Citations
Metrics details
Bioassays play a central role in effect-directed analysis (EDA), and their selection and application have to consider rather specific aspects of this approach. Meanwhile, bioassays with zebrafish, an established model organism in different research areas, are increasingly being utilized in EDA. Aiming to contribute for the optimal application of zebrafish bioassays in EDA, this review provides a critical overview of previous EDA investigations that applied zebrafish bioassays, discusses the potential contribution of such methods for EDA and proposes strategies to improve future studies. Over the last 10 years, zebrafish bioassays have guided EDA of natural products and environmental samples. The great majority of studies performed bioassays with embryos and early larvae, which allowed small-scale and low-volume experimental setups, minimized sample use and reduced workload. Biotesting strategies applied zebrafish bioassays as either the only method guiding EDA or instead integrated into multiple bioassay approaches. Furthermore, tiered biotesting applied zebrafish methods in both screening phase as well as for further investigations. For dosing, most of the studies performed solvent exchange of extracts and fractions to dimethyl sulfoxide (DMSO) as carrier. However, high DMSO concentrations were required for the testing of complex matrix extracts, indicating that future studies might benefit from the evaluation of alternative carrier solvents or passive dosing. Surprisingly, only a few studies reported the evaluation of process blanks, indicating a need to improve and standardize methods for blank preparation and biotesting. Regarding evaluated endpoints, while acute toxicity brought limited information, the assessment of specific endpoints was of strong value for bioactivity identification. Therefore, the bioassay specificity and sensitivity to identify the investigated bioactivity are important criteria in EDA. Additionally, it might be necessary to characterize the most adequate exposure windows and assessment setups for bioactivity identification. Finally, a great advantage of zebrafish bioassays in EDA of environmental samples is the availability of mechanism- and endpoint-specific methods for the identification of important classes of contaminants. The evaluation of mechanism-specific endpoints in EDA is considered to be a promising strategy to facilitate the integration of EDA into weight-of-evidence approaches, ultimately contributing for the identification of environmental contaminants causing bioassay and ecological effects.
Introduction
Zebrafish is a model vertebrate organism broadly applied in biological sciences, being one of the most important organisms that is used in different research areas as genetics, developmental biology and ecotoxicology [ 1 ]. More recently, its versatility has also been recognized by chemists, which provides an opportunity to enhance interdisciplinary studies involving biology and chemistry [ 2 ] as in effect-directed analysis (EDA) [ 3 ].
Bioassays in EDA
EDA, bioassay-guided fractionation and similar approaches are testing procedures applied to identify the individual bioactive compounds contained in highly complex matrices, such as natural products and environmental samples. Bioassays play a central role in EDA since biological activity directs the chemical fractionation and analysis steps as well as the testing strategy. Since fractionation of the sample is required to reduce the complexity of the original mixture, bioassays are needed to identify the active fractions and to guide further fractionation steps. Target and non-target chemical analyses are applied to select candidates and identify bioactive substances. Bioassays again play an important role in the confirmation phase, for biotesting of the pure substance identified as the bioactive compound [ 3 - 5 ].
Therefore, bioassay selection for EDA studies has to consider aspects that are rather specific to this application. For accurate identification of bioactive fractions, the bioassays should present high sensitivity and low internal test variability and be able to detect different chemicals that address similar endpoints or modes of action. Furthermore, due to limited sample amounts and large numbers of fractions to be tested, high-throughput low-volume bioassays are required [ 5 ].
Thus, in vitro bioassays are often selected for EDA studies; however, certain bioactivities require the organ or organism level for their proper identification, as for compounds in which metabolism plays an important role by interfering with formation or transformation of bioactive metabolites and bioaccumulation profiles [ 6 ]. These are the cases when bioassays with zebrafish early-life stages are considered to be of great value since they combine the organism-level endpoints with advantages of the in vitro format. Furthermore, biotesting strategies integrating organism-based and in vitro bioassays are expected to cover a broad range of bioeffects and related toxicants. The resulting diagnostic power strongly supports the identification of specific toxicants in EDA case studies [ 7 ].
Zebrafish model and bioassays in EDA
The zebrafish Danio rerio exhibits characteristics that make it a very attractive research model, including small size, ease of culture, high fecundity, rapid development, external fertilization and development, and transparency of the embryo. Bioassays with zebrafish embryos and larvae have further advantages that fit very well to EDA requirements. While these tests are relevant to evaluate acute [ 8 ] and chronic [ 9 ] effects in later life stages, the experimental setup exhibits several in vitro test characteristics, including a reduced volume of sample for testing and potential for high-throughput applications. Experiments with early life stages often do not require animal test authorization, and no external feeding is needed by embryos and larvae [ 1 ].
The zebrafish success as a model organism is in great part due to the work of pioneer scientists between the late 1960s and mid-1990s, as George Streisinger, who established the first zebrafish models and performed pioneer works on its genetics and developmental biology [ 10 - 12 ]; Charles Kimmel’s descriptions of the cellular fate map [ 13 ] and the stages of development [ 14 ] in embryos; and Christiane Nüsslein-Volhard, who performed a large-scale mutant screen to identify genes for vertebrate development control [ 15 , 16 ]. Following these ground-breaking studies, there was evident increase in the use of zebrafish in research [ 17 ], resulting in the sequencing of its genome [ 18 ], extensive information on its genetics, genomics, phenotypic and developmental biology [ 19 ], and the establishment of thousands of wild-type and transgenic zebrafish lines [ 20 , 21 ].
Importantly, zebrafish embryos and early larvae might be used to replace or refine experiments with adult fish, being increasingly applied in ecotoxicology to evaluate the toxicity of chemicals, plant protection products, biocides, pharmaceuticals, wastewater effluents and various aqueous environmental samples, and to assess sediment toxicity [ 1 , 22 - 24 ]. Recently, zebrafish embryo toxicity assays have been integrated in biotest batteries in environmental monitoring programmes, as the Joint Danube Survey [ 25 ] and the working group on bioassays of the NORMAN network [ 26 ]. Fish bioassays also play an important role in the implementation of the European Water Framework Directive (WFD) since they provide data for the derivation of environmental quality standards (EQS) and might represent a sensitive taxon for substances with specific modes of action [ 27 ]. Besides, biotests with fish are also included among recommended bioeffect-based tools for environmental assessment in the context of the WFD and the European Marine Strategy Framework Directive (MSFD) [ 7 ]. Consequently, current EU projects are investigating the contribution of zebrafish bioassays for water quality assessment and EDA of environmental samples, with focus on specific modes of action, mechanism-specific endpoints and adverse-outcome pathways [ 28 , 29 ]. Such initiatives are supported by the proposal that EDA contributes as an additional line of evidence in weight-of-evidence frameworks, such as the triad approach, ultimately leading to the identification of the contaminants responsible for the toxic effects observed in bioassays and the environment [ 30 , 31 ].
Context and objectives of this review
This review was developed in the context of the Marie Curie Initial Training Network ‘EDA-EMERGE - Novel tools in effect-directed analysis for identifying and monitoring emerging toxicants on a European scale’, funded by the European Commission within the Seventh Framework Programme for Research [ 28 ]. The literature review aimed to provide an overview of previous EDA investigations that applied bioassays with zebrafish, critically evaluating their objectives, methods, biotesting strategy and outcomes; discuss the potential contribution of further zebrafish bioassays for EDA; and propose strategies that might help optimizing the integration of such biotools into future EDA studies investigating environmental samples.
In order to meet these objectives, the literature was searched using the online tools Thomson Reuters Web of Science (WoS), ScienceDirect (SD) and Google Scholar (GS). In WoS, the terms were searched by topic (searching the fields Title, Abstract, Author Keywords and Keywords Plus® per record) in all databases, and in SD and GS, the terms were searched in all fields. The searches were done for publications in all years, except where indicated. The zebrafish terms used for search or filtration were a combination of ‘zebrafish’ or ‘zebra fish’ or ‘ Danio rerio ’. The EDA and life stage search terms are detailed below.
Zebrafish potential for EDA application
Zebrafish in eda-relevant research areas.
The application of the zebrafish model in EDA-related research areas was verified by search in WoS for the zebrafish terms as keywords in topic/all databases, followed by classification per research area (Figure 1 ). The search period was limited to between 2004 and 2014, to be in agreement with the publication years of EDA studies evaluated in this review. Outcomes are in good agreement with a recent review that applied much more sophisticated search strategy [ 17 ], indicating the usefulness of WoS for a first evaluation of research areas. Among the research areas strongly related to EDA, toxicology (8.9%) and pharmacology (9.0%) were each referred by circa 9% of the publications, while environmental sciences and ecology (3.0%) and chemistry (1.9%) were referred by a lower percentage. The prevalent research fields addressed by more than 20% of publications were mostly those that traditionally apply zebrafish, as genetics and heredity (40.6%), biochemistry and molecular biology (33.9%), developmental biology (30.7%), and zoology (24.6%)
Records for the zebrafish terms, filtered by publication period (2004 to 2014), classified according to research area. Total number of records per period: 63,851. Search done in October 2014 (Web of Science).
Life stages referred to by research studies
The use of different life stages in studies with zebrafish was estimated by search for the zebrafish terms filtered by the life stage terms ‘embryo*’ , ‘larva*’ , ‘juvenile*’ , ‘adult*’ and combinations of those. Again, the search period was limited to the publication years of reviewed EDA studies (2004 to 2014). As illustrated in Figure 2 , more than half of the studies with zebrafish refer to embryos (52.6%) and almost one fourth of these mentioned also either adults or larvae, corresponding to 4.0% and 7.4% of total publications, respectively. The occurrence of studies mentioning adults (13.4%) and larvae (11.9%) was also representative, while circa 1% only referred to juvenile life stages.
Records for the zebrafish and life stage terms, filtered by publication period (2004 to 2014). The life stage terms are ‘embryo*’ , ‘larva*’ , ‘juvenile*’ , ‘adult*’ or combinations of these. Total number of records per period: 63,851. Search done in October 2014 (Web of Science).
EDA studies integrating zebrafish bioassays
Due to the heterogeneous nomenclature found in the literature, different EDA terms as listed in Weller 2012 [ 4 ] plus the term ‘fractionation’ were searched in quotation marks, using the different search tools. After filtering by the zebrafish terms mentioned above, resulting publications were screened for confirmation of the searched content. Review papers, or studies that did not include the EDA procedure or zebrafish bioassays, were excluded. Two studies that followed procedures for toxicity identification evaluation (TIE) [ 32 , 33 ] instead of EDA were included since the similarities between both approaches [ 5 ] make them relevant for this review. In total, 29 publications were found (Table 1 ), which were carefully evaluated for research area, objective, investigated matrix, bioassay endpoint and setup, biotesting strategy, and study outcomes.
Research areas and investigated matrices
Two main fields were prevalent among EDA studies using zebrafish bioassays: drug discovery from natural products and environmental toxicology (Figure 3 ). Natural product studies aimed to identify bioactive compounds for pharmacological applications, investigating mostly plant extracts [ 19 , 34 - 45 ] but also extracts of bacteria [ 46 ], cyanobacteria and algae [ 47 ], seaweed [ 48 ] and marine organisms [ 49 ]. Environmental toxicology studies aimed to identify the toxic compounds in various environmental samples, including marine and fluvial sediments [ 50 - 52 ], soil [ 53 ], cyanobacteria and algae [ 54 , 55 ], industrial effluent [ 33 ], rubber tyre leachates [ 32 ], oil sand process waters [ 56 , 57 ] and river pore water [ 58 ]. Finally, fish skin extracts were investigated in a behavioural sciences study [ 59 ].
EDA studies applying zebrafish bioassays for the investigation of natural products and environmental samples. Number of studies that were evaluated in the literature review identified per main research area and year of publication (Web of Science, ScienceDirect and Google Scholar, October 2014).
Prevalent life stages and exposure setups
EDA studies applied mostly bioassays with early embryos and larvae, following exposure to chemical extracts and fractions in multiwell-plates, often with exposure of several individuals in the same well (Table 2 ). Zebrafish up to 5 days post fertilization (dpf) were the life stages mostly applied, except for experiments that extended the assays up to 6 to 7 dpf [ 34 , 49 , 53 , 56 , 57 ] or a few studies with adults [ 33 , 47 , 59 ]. Environmental toxicology studies for the most part performed exposure not only in 24-well plates (200 μL to 2 mL per embryo or larva) but also in crystallization dishes, scintillation vials or beakers (450 μL to 5 mL per embryo or larva, 40 to 300 mL per adult), while natural product studies were performed exclusively in multiwell-plate setup (<100 to 250 μL per embryo or larva). The exposure of several individuals in the same well or vessel was observed for most of the studies, reflecting the need to reduce workload for EDA biotesting.
Biotesting strategy
The EDA investigations guided only by zebrafish bioassays followed either a single test setup (e.g. [ 36 , 53 , 55 - 57 ]) or a combination of methods (e.g. [ 44 , 52 ]) to evaluate endpoints in zebrafish. Other studies applied methods with additional experimental models, mostly cell-based (e.g. [ 19 , 45 , 51 ]) but also bacteria [ 46 , 50 ] and rodent [ 39 ] assays. When the application of multiple biossays aimed to evaluate distinct bioactivities, the tests were mostly performed in parallel. For instance, bioassay batteries evaluated the occurrence of different toxicity mechanisms [ 50 , 51 ] or effects on different trophic levels in the two TIE studies [ 32 , 33 ]. When instead the aim of multiple methods was to analyse different aspects of the same bioactivity or toxicity mechanism, there was the prevalence of tiered approach biotesting [ 19 , 39 , 45 ]. When applied in screening phase, zebrafish bioassays aimed to identify active fractions by organism-level endpoints, which were later further investigated by additional methods with zebrafish [ 52 ] or with other experimental models [ 19 , 45 ]. As an example, zebrafish bioassays were applied to screen extracts and fractions for anti-angiogenic effects, followed by further investigations on human cells and transgenic zebrafish embryos [ 19 ]. On the other hand, zebrafish bioassays applied only as secondary tests aimed mostly at the confirmation of bioeffect occurrence at the organism level [ 49 ] or to evaluate the occurrence of acute toxicity in fish [ 46 , 47 ].
Use of solvents in bioassays
In biotesting, solvents were used for transference of samples into exposure vessels or as carriers. The first approach was applied using acetonitrile [ 55 ] or ethanol [ 54 ], including also solvent control conditions, and proceeding to solvent evaporation before adding exposure media. The use of solvents as carriers in bioassays showed the prevalence of dimethyl sulfoxide (DMSO), in concentrations ranging from 0.01% [ 35 ], 0.1% [ 31 , 45 ], 0.2% [ 26 ], 0.5% [ 46 , 47 ], 1% [ 33 , 48 - 50 ] up to 2% [ 51 ]. In addition, ethanol was also used as a carrier by a few studies, in concentrations of 0.001% for experiments with larvae [ 40 , 41 ] or 0.435% for experiments with zebrafish adults [ 42 ].
It is relevant to mention that the different procedures for extraction, cleanup, pre-concentration and fractionation of samples, already extensively reviewed elsewhere [ 3 , 4 , 60 ], also involve the use of different solvents and chemicals. Criteria for the use of solvents in EDA studies are the following: low or lack of toxicity in the biotest, the capacity of the solvent to dissolve complex extracts and fractions and the possibility to use the solvent in chemical analysis. The latter is the precondition to make sure that the chemical mixture tested in the bioassay resembles the mixture evaluated in chemical analysis. While DMSO excellently meets the first criterion, it is less suitable for dissolving complex mixtures when compared to other possible alternatives, and it completely fails the criterion related to the use in chemical analysis. Thus, the investigation of other solvents as possible carriers for exposure in zebrafish embryo testing might help to reduce possible artefacts during solvent exchange to DMSO. The evaluation of process blanks in order to exclude artefact toxicity is crucial for successful EDA and will be discussed below.
Positive/negative controls and biotesting of blanks
Positive control conditions that were specific to the evaluated endpoints were often described. For that, there was exposure of the zebrafish to compounds known to cause specific effects such as anti-convulsant activity [ 39 ], glucose uptake [ 45 ], pro-angiogenesis [ 36 , 37 ], anti-angiogenesis [ 19 , 44 ], or estrogenic effects [ 52 , 56 ]. Regarding negative control conditions, most of the studies reported the testing of solvent controls in the same concentration as for the respective sample testing [ 19 , 32 , 34 , 35 , 38 - 40 , 43 , 44 , 47 , 49 - 51 , 53 , 55 ]. Some studies have additionally evaluated a medium only condition in addition to the solvent control [ 36 , 37 , 42 , 54 , 56 , 57 ].
The preparation and biotesting of blanks was described only in few of the evaluated EDA studies and in the two TIE studies. In the EDA studies, there was submission of the respective solvents [ 51 , 53 , 56 , 57 ] or of HPLC-grade water [ 58 ] through the same or part of the procedures that were applied to samples (i.e. sample preparation, extraction, fractionation). The TIE studies described blank preparation by treatment of milliQ water [ 32 ] or 0.1 M KCl solution [ 33 ] in the same way as samples for all procedures. In all these studies, the prepared blanks were evaluated in bioassays in the same way as done for samples and fractions. Another strategy was the use of a fraction that showed to be negative for the evaluated effect as a blank condition [ 45 ]. The exchange between elution solvents and DMSO was identified as a critical step since solvent traces might interfere with bioassays; therefore, blank testing was suggested to always be performed [ 51 ].
Investigated endpoints and studies outcomes
The specificity and sensitivity of bioassays and endpoints in identifying the bioactivity or adverse effects in fractions were considered to be a key issue for the relevance of zebrafish bioassays in EDA. Therefore, it is recommended to identify the critical aspects for endpoint assessment, to optimize bioassays accordingly and to demonstrate the validity of the bioassay by testing known bioactive compounds [ 61 ]. The endpoints and bioassays described in the different studies are summarized in Table 3 and discussed in the context of respective study objectives and outcomes.
Acute toxicity and lethality
Bioactive sediment fractions [ 51 ] and components partially responsible for toxicity in oil sand process water fractions [ 57 ] have been identified by acute toxicity bioassays. The two TIE studies reported inconsistent acute toxicity of industrial effluents [ 33 ] and rubber tyre leachates [ 32 ]. One study investigating seaweed hydrolysates evaluated in vivo toxic potential through acute toxicity testing [ 48 ].
It may be summarized that EDA studies that focused on acute toxicity and lethality had only modest success in determining active compounds. These are unspecific responses that might occur due to exposure to very broad range of compounds; therefore, fractionation typically results in the distribution of toxicity over many different fractions. However, also other unrelated factors might have been involved, as for example, the high complexity of investigated matrices in the reviewed studies. Nevertheless, acute toxicity testing might be a powerful tool in TIE, when applied to evaluate highly contaminated sites with acute toxicity caused by compounds that are well characterized [ 62 ].
Teratogenesis and developmental toxicity
Assessment of teratogenesis and developmental effects was done in studies that identified the bioactive compounds from microalgae, cyanobacteria and plant [ 41 , 54 , 55 ], river pore water [ 58 ] and developmental toxicants in soil [ 53 ]. Most studies evaluated traditionally assessed morphological endpoints, while one investigation of plant fractions focused on ectopic tail formation [ 41 ]. One study identified embryotoxicity in sediment extracts but not in respective fractions, which was attributed to losses of active compound or of synergistic effect during fractionation [ 50 ].
An aspect shared by the successful studies was the meticulous experimental characterization of the original matrices and respective fractions regarding their teratogenic effects and developmental toxicity potential. For instance, there was the determination of the optimal exposure period to identify a phenotype of interest that caused minimal acute toxicity [ 41 ]. Characteristic phenotypical effects were identified for specific fractions [ 53 ], also on a dose-dependent manner [ 54 , 55 ]. Two studies also investigated if additive or synergistic effects occurred between different fractions [ 55 ] or between aryl hydrocarbon receptor (AhR) agonists by co-exposure to a CYP1A inhibitor [ 58 ].
Angiogenesis modulation
Bioassays investigating pro- and anti-angiogenesis modulation by different bioactive plants were the most frequent studies in natural products. To this end, studies applied wild-type zebrafish [ 30 , 42 , 43 ] or the transgenic fli1:EGFP [ 63 ] zebrafish line [ 19 , 36 - 38 , 40 ], in which the zebrafish fli1 promoter drives the expression of enhanced green fluorescent protein in blood vessels. In wild-type zebrafish, staining of the vessels was applied to facilitate scoring [ 30 , 43 ], while the transgenic line allowed in vivo observation of the embryonic vasculature. Selected endpoints evaluated specific cellular-morphological phenotypes, as intersegmental vessel formation. In these assays, the exposure start and duration were set to the most sensitive developmental windows related to the assessed endpoints.
All of the evaluated studies were successful in identifying at least one bioactive compound causing angiogenesis modulation, indicating that the identification of highly specific endpoints on the organism level might be a good requirement for the efficient use of zebrafish bioassays in EDA. The use of transgenic zebrafish lines is also considered to be a great asset for studies that evaluate specific morphological effects since it can facilitate endpoint observation and increase sensitivity of bioassays.
Energy uptake and storage
EDA was successful in identifying known and novel insulin-mimetic compounds in plants [ 45 ] with the contribution of zebrafish bioassays to characterize glucose uptake modulation. The study applied fluorescein-tagged glucose bioprobes and measured fluorescence by microscopy imaging and microplate reader, obtaining dose- and time-dependent responses. Another study applied a fluorescent fatty acid analogue to evaluate fatty acid storage modulation in zebrafish embryos by extracts from marine sponge [ 49 ]. In this case, the characterization of effects was done by extraction of zebrafish lipids followed by thin-layer chromatography. These studies demonstrated that the use of fluorescent bioprobes is a good tool to evaluate effects on the uptake and storage capacity of zebrafish, allowing not only for qualitative but also quantitative analysis of effects.
Antioxidant effects
Zebrafish embryos were integrated into an EDA study that identified and purified aloe vera polysaccharide with protective effects against oxidative stress [ 35 ]. Tests with zebrafish bioassays provided valuable information on organism-level responses regarding the generation of reactive oxygen species and oxidative stress-induced cell death, which were observed in a dose response manner.

Estrogenicity assessment by gene expression
Estrogenic effects were investigated in extracts and fractions of oil sand process waters by vitellogenin gene expression ( vtg1 ) through quantitative polymerase chain reaction (qPCR) in zebrafish early larvae [ 56 ]. Estrogenicity was also assessed by the use of transgenic zebrafish embryos that exhibit green fluorescence protein expression in response to aromatase ( cyp19a1b ) gene induction, with confirmation of results by qPCR [ 52 ].
Gene expression analysis by qPCR showed to be a useful EDA endpoint in zebrafish embryos and larvae when background information allows the selection of specific biomarker genes for the studied effect, as for estrogenicity. The evaluation of sets of genes by qPCR is considered to be a promising strategy for endocrine disruption investigation, when following optimized experimental design regarding exposure intervals and evaluated zebrafish developmental stages [ 64 ]. The transgenic zebrafish embryos were also considered to be experimental models compatible with EDA, and their integration in future studies is expected to be facilitated by automated image analysis procedures [ 52 ].
Neuroactivity and behaviour
EDA was applied to identify anticonvulsant compounds present in plants, by co-exposure of evaluated samples with a convulsant compound, followed by the analysis of larvae total locomotor activity. Effects were assessed with video-tracking and software analysis and with electroencephalogram recording analysis [ 39 ]. Another EDA study of plant neuroactivity applied similar bioassays, in combination with larvae whole-mount in situ hybridization to assess increased brain c-fos gene expression as an indicator of seizure onset and brain damage [ 34 ]. Both studies identified anticonvulsant compounds, demonstrating the usefulness of the zebrafish model to identify neuroactivity in EDA. Also for neuroactivity and behaviour, the assessment of specific endpoints and setting the bioassay accordingly demonstrated to be an effective EDA biotesting approach.
The identification of neuroactive compound extracts of a mixture of red algae and cyanobacteria was investigated by a biotest battery including in vitro and organism-level methods [ 47 ]. Bioassays with zebrafish adults aimed at evaluating the neurotoxic potential of the matrix. However, evaluated endpoints were non-specific acute toxicity and mortality, which provided only minor contribution to the overall study outcomes.
The bioactive compounds responsible for fear behaviour response in fish were investigated by the exposure of zebrafish adults to fish skin extracts and fractions [ 59 ]. Video tracking was used to quantify alarm behaviour by measuring swimming speed and vertical position. The study identified the bioactive compound and proposed a new class of odorants that trigger alarm behaviour in fish. This study required the development of experimental setup and endpoint assessment that were specific to the evaluated behavioural alteration, confirming the importance of this step also for behavioural assessment.
Summary and discussion
Over the last 10 years, EDA studies guided by zebrafish bioassays have successfully identified bioactive or toxic compounds present in diverse biological matrices or environmental samples. Embryos and early larvae were the prevalent zebrafish life stages in these studies, with exposure being done in multiwell-plates, often with several individuals in the same well. In consequence, the sample volume for biotesting was minimized and the workload was reduced, which are important aspects in EDA. Zebrafish bioassays showed also versatility in terms of biotesting strategy, being applied alone or as a part of biotest batteries and in both screening phase as well as for further investigation of active fractions in tiered biotesting.
In spite of its limited capacity to dissolve complex matrix extracts, DMSO was the main carrier solvent applied in zebrafish bioassays. As a result, it was used in concentrations up to two orders of magnitude higher than the recommended for single compound biotesting (0.01%) [ 65 ]. Additionally, DMSO is not suitable for chemical analysis, which restricts the characterization of samples evaluated in biotesting. Therefore, the investigation of alternative carrier solvents would be an asset for zebrafish bioassays in EDA. Passive dosing methods are also promising options, as recently done in EDA investigation of sediments through the use of silicone rods for dosing of extracts and fractions in algae bioassay [ 66 ]. In fact, a loaded polymer silicone cast has successfully been integrated in zebrafish embryo assay for dosing of polycyclic aromatic hydrocarbons [ 67 ].
The EDA procedures for sample extraction, cleanup, pre-concentration and fractionation involve the use of different solvents and chemicals. Nevertheless, while most of the studies evaluated solvent and medium control conditions, the investigation of process blanks in bioassays was reported only by a small number of studies. In addition, methods for blank preparation varied considerably between these studies. Since the biotesting of process blanks is crucial for effective EDA, there is a need to improve and standardize the procedures for their preparation and biotesting in future studies.
Most of the successful EDA studies applied specific and sensitive bioassays evaluating molecular, morphological or behavioural endpoints. Some studies optimized bioassays by identifying the most adequate exposure windows and assessment setups to maximize the specific endpoint response and minimize the interference of acute toxicity [ 41 ]. Further improvements might be achieved by advancing methods for the analysis of endpoints. For instance, the automated analysis of morphological phenotypes in transgenic or wild-type zebrafish would reduce workload and increase reliability in EDA [ 52 ]. Also, EDA of environmental samples would benefit from the analysis of bioassay results in correlation with previously characterized responses to specific classes of pollutants. That is the case of gene expression analysis of biomarker genes for specific mechanisms and modes of action. When analysed in correlation with respective gene modulation by known classes of compounds [ 68 ], biomarker gene responses might indicate the presence of certain classes of chemicals [ 69 ]. Similarly, EDA studies evaluating behavioural phenotypes to identify neuroactivity might rely in the near future on databases of behavioural profiles for different classes of compounds [ 70 , 71 ].
Such outcomes support the idea that EDA investigations of toxic environmental samples would benefit of the application of endpoint- and mechanism-specific methods with zebrafish. That is in fact a great advantage since mechanism-specific toxicity methods with zebrafish are broadly developed, as for AhR-mediated toxicity [ 72 ], genotoxicity [ 73 ] and neurotoxicity [ 74 ]. Furthermore, EDA guided by such zebrafish bioassays could integrate broader environmental assessment strategies, complementing effect-based approaches [ 7 ] and weight-of-evidence frameworks [ 30 ]. In this way, EDA would support the identification of contaminants causing bioassay and ecological effects, and the clarification of links between ecosystem functioning and the responses at different biological levels [ 30 , 62 ]. Finally, the evaluation of toxic aquatic contaminants through EDA guided by zebrafish bioassays might improve the protection of water bodies in the context of the European WFD and MSFD [ 7 , 27 ]. In conclusion, endpoint- and mechanism-specific zebrafish bioassays are considered of great relevance not only for guiding EDA studies but also for integrating EDA into environmental assessment and monitoring, ultimately contributing for environmental quality improvement [ 7 , 75 ].
Conclusions
Zebrafish bioassays have successfully guided different EDA studies; however, further method developments are still needed. Alternative dosing procedures should be investigated, and process blank preparation and biotesting should be standardized. Endpoint- and mechanism-specific bioassays with embryos and larvae are considered to be the most promising zebrafish biotests for future EDA of environmental samples. When integrated into broader environmental assessment strategies, EDA guided by specific zebrafish bioassays might support the identification of compounds causing bioassay and ecological effects, ultimately contributing for environmental quality improvement.
Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, et al. Zebrafish embryos as an alternative to animal experiments—a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol. 2012;33:128–32.
Article Google Scholar
Basu S, Sachidanandan C. Zebrafish: a multifaceted tool for chemical biologists. Chem Rev. 2013;113:7952–80.
Article CAS Google Scholar
Brack W. Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures? Anal Bioanal Chem. 2003;377:397–407.
Weller MG. A unifying review of bioassay-guided fractionation, effect-directed analysis and related techniques. Sensors. 2012;12:9181–209.
Burgess RM, Ho KT, Brack W, Lamoree M. Effects-directed analysis (EDA) and toxicity identification evaluation (TIE): complementary but different approaches for diagnosing causes of environmental toxicity. Environ Toxicol Chem. 2013;32:1935–45.
Di Paolo C, Gandhi N, Bhavsar SP, Van den Heuvel-Greve M, Koelmans AA. Black carbon inclusive multichemical modeling of PBDE and PCB biomagnification and transformation in estuarine food webs. Environ Sci Technol. 2010;44:7548–54.
Wernersson A S CM, Maggi C, Tusil P, Soldan P, James A, Sanchez W, et al. Technical report on aquatic effect-based monitoring tools technical report 2014–077 EU Commission. doi:102779/7260. 2014.
Knobel M, Busser FJ, Rico-Rico A, Kramer NI, Hermens JL, Hafner C, et al. Predicting adult fish acute lethality with the zebrafish embryo: relevance of test duration, endpoints, compound properties, and exposure concentration analysis. Environ Sci Technol. 2012;46:9690–700.
Volz DC, Belanger S, Embry M, Padilla S, Sanderson H, Schirmer K, et al. Adverse outcome pathways during early fish development: a conceptual framework for identification of chemical screening and prioritization strategies. Toxicol Sci. 2011;123:349–58.
George Streisinger. Biographical Memoirs V.68: 353-362. Washington, DC: The National Academies Press; 1995.
Google Scholar
Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature. 1981;291:293–6.
Streisinger G, Coale F, Taggart C, Walker C, Grunwald DJ. Clonal origins of cells in the pigmented retina of the zebrafish eye. Dev Biol. 1989;131:60–9.
Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–94.
CAS Google Scholar
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310.
Nüsslein-Volhard C. The zebrafish issue of development. Development. 2012;139:4099–103.
Nüsslein-Volhard C. The identification of genes controlling development in flies and fishes (Nobel Lecture). Angewandte Chemie Int Ed Eng. 1996;35:2176–87.
Kinth P, Mahesh G, Panwar Y. Mapping of zebrafish research: a global outlook. Zebrafish. 2013;10:510–7.
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503.
Bradford Y, Conlin T, Dunn N, Fashena D, Frazer K, Howe DG, et al. ZFIN: enhancements and updates to the zebrafish model organism database. Nucleic Acids Res. 2010;39(1):D822–9.
Zebrafish International Resource Center - ZIRC [ http://www.zebrafish.org ]
EZRC - European Zebrafish Resource Center [ http://www.ezrc.kit.edu ]
Braunbeck T, Boettcher M, Hollert H, Kosmehl T, Lammer E, Leist E, et al. Towards an alternative for the acute fish LC(50) test in chemical assessment: the fish embryo toxicity test goes multi-species – an update. Altex. 2005;22:87–102.
Hallare A, Seiler T-B, Hollert H. The versatile, changing, and advancing roles of fish in sediment toxicity assessment—a review. J Soils Sediments. 2011;11:141–73.
EURL-ECVAM. Recommendation on the zebrafish embryo acute toxicity test method (ZFET) for acute aquatic toxicity testing. Report EUR 26710. 35p. In: Recommendation on the zebrafish embryo acute toxicity test method (ZFET) for acute aquatic toxicity testing. Report EUR 26710. 35p. 2014.
Joint Danube Survey [ http://www.danubesurvey.org ]
NORMAN Network [ http://www.norman-network.net ]
European Commission. Technical guidance for deriving environmental quality standard. Guidance document n°27, technical report 2011–055, European Communities, 203 p. In: Technical guidance for deriving environmental quality standard. Guidance document n°27, technical report 2011–055, European Communities, 203 p. 2011.
Brack W, Govender S, Schulze T, Krauss M, Hu M, Muz M, et al. EDA-EMERGE: an FP7 initial training network to equip the next generation of young scientists with the skills to address the complexity of environmental contamination with emerging pollutants. Environ Sci Eur. 2013;25:18.
Brack W, Altenburger R, Schüürmann G, Krauss M, López Herráez D, van Gils J, et al. The SOLUTIONS project: challenges and responses for present and future emerging pollutants in land and water resources management. Sci Total Environ. 2014;503–504:22–31.
Hecker M, Hollert H. Effect-directed analysis (EDA) in aquatic ecotoxicology: state of the art and future challenges. Environ Sci Pollut Res. 2009;16:607–13.
Chapman PM, Hollert H. Should the sediment quality triad become a tetrad, a pentad, or possibly even a hexad? J Soils Sediments. 2006;6:4–8.
Wik A, Nilsson E, Källqvist T, Tobiesen A, Dave G. Toxicity assessment of sequential leachates of tire powder using a battery of toxicity tests and toxicity identification evaluations. Chemosphere. 2009;77:922–7.
Fang Y-X, Ying G-G, Zhang L-J, Zhao J-L, Su H-C, Yang B, et al. Use of TIE techniques to characterize industrial effluents in the Pearl River Delta region. Ecotoxicol Environ Saf. 2012;76:143–52.
Buenafe OE, Orellana-Paucar A, Maes J, Huang H, Ying X, De Borggraeve W, et al. Tanshinone IIA exhibits anticonvulsant activity in zebrafish and mouse seizure models. ACS Chem Neurosci. 2013;4:1479–87.
Kang MC, Kim SY, Kim YT, Kim EA, Lee SH, Ko SC, et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr Polym. 2014;99:365–71.
Liu C-L, Cheng L, Kwok H-F, Ko C-H, Lau T-W, Koon C-M, et al. Bioassay-guided isolation of norviburtinal from the root of Rehmannia glutinosa, exhibited angiogenesis effect in zebrafish embryo model. J Ethnopharmacol. 2011;137:1323–7.
Liu C-L, Kwok H-F, Cheng L, Ko C-H, Wong C-W, Wai Fong Ho T, et al. Molecular mechanisms of angiogenesis effect of active sub-fraction from root of Rehmannia glutinosa by zebrafish sprout angiogenesis-guided fractionation. J Ethnopharmacol. 2014;151:565–75.
Crawford AD, Liekens S, Kamuhabwa AR, Maes J, Munck S, Busson R, et al. Zebrafish bioassay-guided natural product discovery: isolation of angiogenesis inhibitors from East African medicinal plants. PLoS One. 2011;6:e14694.
Orellana-Paucar AM, Serruys A-SK, Afrikanova T, Maes J, De Borggraeve W, Alen J, et al. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav. 2012;24:14–22.
Bohni N, Cordero-Maldonado ML, Maes J, Siverio-Mota D, Marcourt L, Munck S, et al. Integration of Microfractionation, qNMR and zebrafish screening for the in vivo bioassay-guided isolation and quantitative bioactivity analysis of natural products. PLoS One. 2013;8:e64006.
Gebruers E, Cordero-Maldonado ML, Gray AI, Clements C, Harvey AL, Edrada-Ebel R, et al. A phenotypic screen in zebrafish identifies a novel small-molecule inducer of ectopic tail formation suggestive of alterations in non-canonical Wnt/PCP signaling. PLoS One. 2013;8:e83293.
Krill D, Madden J, Huncik K, Moeller PD. Induced thyme product prevents VEGF-induced migration in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2010;403:275–81.
Han L, Yuan Y, Zhao L, He Q, Li Y, Chen X, et al. Tracking antiangiogenic components from Glycyrrhiza uralensis Fisch. based on zebrafish assays using high-speed countercurrent chromatography. J Sep Sci. 2012;35:1167–72.
He MF, Liu L, Ge W, Shaw PC, Jiang R, Wu LW, et al. Antiangiogenic activity of Tripterygium wilfordii and its terpenoids. J Ethnopharmacol. 2009;121:61–8.
Lee J, Jung D-W, Kim W-H, Um J-I, Yim S-H, Oh WK, et al. Development of a highly visual, simple, and rapid test for the discovery of novel insulin mimetics in living vertebrates. ACS Chem Biol. 2013;8:1803–14.
Dash S, Nogata Y, Zhou X, Zhang Y, Xu Y, Guo X, et al. Poly-ethers from Winogradskyella poriferorum: antifouling potential, time-course study of production and natural abundance. Bioresour Technol. 2011;102:7532–7.
Suyama TL, Cao Z, Murray TF, Gerwick WH. Ichthyotoxic brominated diphenyl ethers from a mixed assemblage of a red alga and cyanobacterium: structure clarification and biological properties. Toxicon. 2010;55:204–10.
Fitzgerald C, Gallagher E, O'Connor P, Prieto J, Mora-Soler L, Grealy M, et al. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides. 2013;50:119–24.
Rae J, Fontaine F, Salim AA, Lo HP, Capon RJ, Parton RG, et al. High-throughput screening of Australian marine organism extracts for bioactive molecules affecting the cellular storage of neutral lipids. PLoS One. 2011;6:e22868.
Higley E, Grund S, Jones P, Schulze T, Seiler T, Lubcke-von VU, et al. Endocrine disrupting, mutagenic, and teratogenic effects of upper Danube River sediments using effect-directed analysis. Environ Toxicol Chem/SETAC. 2012;31(5):1053–62. Epub 2012 Mar 2023.
Kammann U, Biselli S, Hühnerfuss H, Reineke N, Theobald N, Vobach M, et al. Genotoxic and teratogenic potential of marine sediment extracts investigated with comet assay and zebrafish test. Environ Pollut. 2004;132:279–87.
Fetter E, Krauss M, Brion F, Kah O, Scholz S, Brack W. Effect-directed analysis for estrogenic compounds in a fluvial sediment sample using transgenic cyp19a1b-GFP zebrafish embryos. Aquat Toxicol. 2014;154:221–9.
Legler J, van Velzen M, Cenijn PH, Houtman CJ, Lamoree MH, Wegener JW. Effect-directed analysis of municipal landfill soil reveals novel developmental toxicants in the zebrafish Danio rerio. Environ Sci Technol. 2011;45:8552–8.
Berry JP, Gantar M, Gibbs PDL, Schmale MC. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp Biochem PhysiolPart C Toxicol Pharmacol. 2007;145:61–72.
Jaja-Chimedza A, Gantar M, Gibbs PDL, Schmale MC, Berry JP. Polymethoxy-1-alkenes from aphanizomenon ovalisporum inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Mar Drugs. 2012;10:2322–36.
Reinardy HC, Scarlett AG, Henry TB, West CE, Hewitt LM, Frank RA, et al. Aromatic naphthenic acids in oil sands process-affected water, resolved by GCxGC-MS, only weakly induce the gene for vitellogenin production in zebrafish (Danio rerio) larvae. Environ Sci Technol. 2013;47:6614–20.
Scarlett AG, Reinardy HC, Henry TB, West CE, Frank RA, Hewitt LM, et al. Acute toxicity of aromatic and non-aromatic fractions of naphthenic acids extracted from oil sands process-affected water to larval zebrafish. Chemosphere. 2013;93:415–20.
Fang M, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Giulio RTD, et al. Effect-directed analysis of Elizabeth river pore water: developmental toxicity in zebrafish (Danio rerio). Environ Toxicol Chem. 2014:n/a-n/a.
Mathuru Ajay S, Kibat C, Cheong Wei F, Shui G, Wenk Markus R, Friedrich Rainer W, et al. Chondroitin fragments are odorants that trigger fear behavior in fish. Curr Biol. 2012;22:538–44.
Brack W, editor. Effect-directed analysis of complex environmental contamination, Handbook of environmental chemistry series 15. Berlin, Heidelberg: Springer; 2011. p. 345.
Cordero-Maldonado ML, Siverio-Mota D, Vicet-Muro L, Wilches-Arizábala IM, Esguerra CV, de Witte PAM, et al. Optimization and pharmacological validation of a leukocyte migration assay in zebrafish larvae for the rapid In Vivo bioactivity analysis of anti-inflammatory secondary metabolites. PLoS One. 2013;8:e75404.
Connon RE, Geist J, Werner I. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors. 2012;12:12741–71.
Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–18.
Schiller V, Wichmann A, Kriehuber R, Muth-Kohne E, Giesy JP, Hecker M, et al. Studying the effects of genistein on gene expression of fish embryos as an alternative testing approach for endocrine disruption. Comp Biochem Physiol Toxicol Pharmacol. 2013;157:41–53.
OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. Paris: OECD Publishing; 2013.
Book Google Scholar
Bandow N, Altenburger R, Streck G, Brack W. Effect-directed analysis of contaminated sediments with partition-based dosing using green algae cell multiplication inhibition. Environ Sci Technol. 2009;43:7343–9.
Seiler TB, Best N, Fernqvist MM, Hercht H, Smith KE, Braunbeck T, et al. PAH toxicity at aqueous solubility in the fish embryo test with Danio rerio using passive dosing. Chemosphere. 2014;112:77–84.
Kosmehl T, Otte JC, Yang L, Legradi J, Bluhm K, Zinsmeister C, et al. A combined DNA-microarray and mechanism-specific toxicity approach with zebrafish embryos to investigate the pollution of river sediments. Reprod Toxicol. 2012;33:245–53.
Keiter S, Peddinghaus S, Feiler U, von der Goltz B, Hafner C, Ho N, et al. DanTox—a novel joint research project using zebrafish (Danio rerio) to identify specific toxicity and molecular modes of action of sediment-bound pollutants. J Soils Sediments. 2010;10:714–7.
Ali S, Champagne DL, Richardson MK. Behavioral profiling of zebrafish embryos exposed to a panel of 60 water-soluble compounds. Behav Brain Res. 2012;228:272–83.
Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–7.
Schiwy S, Braunig J, Alert H, Hollert H, Keiter SH. A novel contact assay for testing aryl hydrocarbon receptor (AhR)-mediated toxicity of chemicals and whole sediments in zebrafish (Danio rerio) embryos. Environ Sci Pollut Res Int. 2014.
Kosmehl T, Hallare AV, Reifferscheid G, Manz W, Braunbeck T, Hollert H. A novel contact assay for testing genotoxicity of chemicals and whole sediments in zebrafish embryos. Environ Toxicol Chem/SETAC. 2006;25:2097–106.
de Esch C, Slieker R, Wolterbeek A, Woutersen R, de Groot D. Zebrafish as potential model for developmental neurotoxicity testing: a mini review. Neurotoxicol Teratol. 2012;34:545–53.
Brack W, Klamer HJ, Lopez De Alda M, Barcelo D. Effect-directed analysis of key toxicants in European river basins a review. Environ Sci Pollut Res Int. 2007;14:30–8.
Download references
Acknowledgements
The EDA-EMERGE project is supported by the EU Seventh Framework Programme (FP7-PEOPLE-2011-ITN) under the grant agreement number 290100.
Author information
Authors and affiliations.
Department of Ecosystem Analysis, Institute for Environmental Research, ABBt - Aachen Biology and Biotechnology, RWTH Aachen University, Worringerweg 1, Aachen, 52074, Germany
Carolina Di Paolo, Thomas-Benjamin Seiler, Steffen Keiter & Henner Hollert
Man-Technology-Environment Research Centre, School of Science and Technology, Örebro University, SE-701 82, Örebro, Sweden
Steffen Keiter
UFZ-Helmholtz Centre for Environmental Research, Permoserstrasse 15, Leipzig, 04318, Germany
Meng Hu, Melis Muz & Werner Brack
College of Resources and Environmental Science, Chongqing University, 1 Tiansheng Road, Beibei, Chongqing, 400715, China
Henner Hollert
College of Environmental Science and Engineering and State Key Laboratory of Pollution Control and Resource Reuse, Tongji University, 1239 Siping Road, Shanghai, China
State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Carolina Di Paolo .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
CDP was responsible for the general design of the review and wrote the first draft of the manuscript. The other authors - TBS, SK, MH, MM, WB and HH - contributed with specific information concerning their respective expertise. All authors helped revise the draft of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( https://creativecommons.org/licenses/by/4.0 ), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Di Paolo, C., Seiler, TB., Keiter, S. et al. The value of zebrafish as an integrative model in effect-directed analysis - a review. Environ Sci Eur 27 , 8 (2015). https://doi.org/10.1186/s12302-015-0040-y
Download citation
Received : 01 August 2014
Accepted : 24 February 2015
Published : 14 March 2015
DOI : https://doi.org/10.1186/s12302-015-0040-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effect-directed analysis
- Bioassay-guided fractionation
Review of the zebrafish as a model to investigate per- and polyfluoroalkyl substance toxicity
Affiliations.
- 1 Environmental & Molecular Toxicology Department, College of Agricultural Sciences, Oregon State University, Corvallis, Oregon, USA.
- 2 Sinnhuber Aquatic Research Laboratory, Oregon State University, Corvallis, Oregon, USA.
- PMID: 37220906
- PMCID: PMC10375317
- DOI: 10.1093/toxsci/kfad051
The existence of thousands of per- and polyfluoroalkyl substances (PFAS) and evidence that some cause adverse health effects has created immense need to better understand PFAS toxicity and to move beyond one-chemical-at-a-time approaches to hazard assessment for this chemical class. The zebrafish model enables rapid assessment of large libraries of PFAS, powerful comparison of compounds in a single in vivo system, and evaluation across life stages and generations, and has led to significant advances in PFAS research in recent years. The focus of this review is to assess contemporary findings regarding PFAS toxicokinetics, toxicity and apical adverse health outcomes, and potential modes of action using the zebrafish model. Much of the peer-reviewed literature has focused on a small subset of PFAS structural subclasses, such as the perfluoroalkyl sulfonic acids and perfluoroalkyl carboxylic acids. However, recent data on more diverse PFAS structures are enabling prioritization of compounds of concern. Structure-activity comparisons and the utilization of modeling and 'omics technologies in zebrafish have greatly contributed to our understanding of the hazard potential for a growing number of PFAS and will surely inform our understanding and predictive capabilities for many more PFAS in the future.
Keywords: PFAS; developmental toxicity; mechanisms; mode of action; toxicokinetics.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society of Toxicology.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, U.S. Gov't, Non-P.H.S.
- Alkanesulfonic Acids*
- Carboxylic Acids
- Fluorocarbons* / toxicity
- Sulfonic Acids
- Toxicokinetics
- Fluorocarbons
- Alkanesulfonic Acids
Grants and funding
- T32 ES007060/ES/NIEHS NIH HHS/United States
- P30 ES030287/NH/NIH HHS/United States
Effects of pollutant toxicity on the eyes of aquatic life monitored by visual dysfunction in zebrafish: a review
- ReviewPaper
- Published: 14 October 2022
- Volume 21 , pages 1177–1201, ( 2023 )
Cite this article

- Xiao-Fan Chen 1 ,
- Zhi-Cheng Lin 1 ,
- Zenghua Qi 1 , 2 ,
- Zongwei Cai 1 , 3 &
- Zhi-Feng Chen ORCID: orcid.org/0000-0003-3662-0992 1 , 2
984 Accesses
6 Citations
Explore all metrics
The eyes of aquatic organisms may be damaged by exposure to pollutants. Zebrafish is a common laboratory model to study ocular toxicity, combining both fish and vertebrate characteristics. The toxic effects on zebrafish eyes caused by pollutants include morphological changes and damage to the retina at the molecular, cellular, and tissue levels; and abnormalities in the visual phototransduction and electrical signal transmission processes. Such damage induces functional disorders of vision-related behaviors. The underlying mechanisms include thyroid hormone signaling, retinoic acid signaling, and retinal glucose metabolism. Here, we review the ocular toxicity phenotypes and related signaling pathways induced by contaminants. We present detection methods and emerging tools for studying ocular toxicity. We also propose a model to predict the potential ocular toxicity of contaminants.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Fish to learn: insights into the effects of environmental chemicals on eye development and visual function in zebrafish

Zebrafish (Danio rerio) as an excellent vertebrate model for the development, reproductive, cardiovascular, and neural and ocular development toxicity study of hazardous chemicals

Biochemistry and physiology of zebrafish photoreceptors
Ablonczy Z, Higbee D, Anderson DM, Dahrouj M, Grey AC, Gutierrez D et al (2013a) Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Investig Ophthalmol vis Sci 54:5535–5542. https://doi.org/10.1167/iovs.13-12250
Article CAS Google Scholar
Ablonczy Z, Higbee D, Grey AC, Koutalos Y, Schey KL, Crouch RK (2013b) Similar molecules spatially correlate with lipofuscin and N-retinylidene-N-retinylethanolamine in the mouse but not in the human retinal pigment epithelium. Arch Biochem Biophys 539:196–202. https://doi.org/10.1016/j.abb.2013.08.005
Aït-Ali N, Fridlich R, Millet-Puel G, Clérin E, Delalande F, Jaillard C et al (2015) Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161:817–832. https://doi.org/10.1016/j.cell.2015.03.023
Albalawi A, Alhasani RHA, Biswas L, Reilly J, Akhtar S, Shu X (2018) Carnosic acid attenuates acrylamide-induced retinal toxicity in zebrafish embryos. Exp Eye Res 175:103–114. https://doi.org/10.1016/j.exer.2018.06.018
Anderson DM, Ablonczy Z, Koutalos Y, Spraggins J, Crouch RK, Caprioli RM et al (2014) High resolution MALDI imaging mass spectrometry of retinal tissue lipids. J Am Soc Mass Spectrom 25:1394–1403. https://doi.org/10.1007/s13361-014-0883-2
Anderson DM, Nye-Wood MG, Rose KL, Donaldson PJ, Grey AC, Schey KL (2020) MALDI imaging mass spectrometry of β- and γ-crystallins in the ocular lens. J Mass Spectrom 55:e4473. https://doi.org/10.1002/jms.4473
Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. https://doi.org/10.1002/etc.34
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776. https://doi.org/10.1126/science.284.5415.770
Asson-Batres M, Rochette-Egly C (2016) The biochemistry of retinoid signaling II: the physiology of vitamin A–uptake, transport, metabolism and signaling
Baonza A, Garcia-Bellido A (2000) Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc Natl Acad Sci USA 97:2609–2614. https://doi.org/10.1073/pnas.040576497
Barbagallo S, Baldauf C, Orosco E, Roy NM (2022) Di-butyl phthalate (DBP) induces defects during embryonic eye development in zebrafish. Ecotoxicology 31:178–185. https://doi.org/10.1007/s10646-021-02468-5
Baumann L, Ros A, Rehberger K, Neuhauss SC, Segner H (2016) Thyroid disruption in zebrafish ( Danio rerio ) larvae: different molecular response patterns lead to impaired eye development and visual functions. Aquat Toxicol 172:44–55. https://doi.org/10.1016/j.aquatox.2015.12.015
Baumann L, Segner H, Ros A, Knapen D, Vergauwen L (2019) Thyroid hormone disruptors interfere with molecular pathways of eye development and function in zebrafish. Int J Mol Sci 20:1543. https://doi.org/10.3390/ijms20071543
Bilotta J (2000) Effects of abnormal lighting on the development of zebrafish visual behavior. Behav Brain Res 116:81–87. https://doi.org/10.1016/S0166-4328(00)00264-3
Bilotta J, Saszik S (2001) The zebrafish as a model visual system. Int J Dev Neurosci 19:621–629. https://doi.org/10.1016/s0736-5748(01)00050-8
Bilotta J, Saszik S, Givin CM, Hardesty HR, Sutherland SE (2002) Effects of embryonic exposure to ethanol on zebrafish visual function. Neurotoxicol Teratol 24:759–766. https://doi.org/10.1016/S0892-0362(02)00319-7
Bock KW (2019) Aryl hydrocarbon receptor (AHR): from selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol 168:65–70. https://doi.org/10.1016/j.bcp.2019.06.015
Bollmann JH (2019) The zebrafish visual system: from circuits to behavior. Annu Rev vis Sci 5:269–293. https://doi.org/10.1146/annurev-vision-091718-014723
Article Google Scholar
Boughton BA, Thomas ORB, Demarais NJ, Trede D, Swearer SE, Grey AC (2020) Detection of small molecule concentration gradients in ocular tissues and humours. J Mass Spectrom 55:e4460. https://doi.org/10.1002/jms.4460
Bridges KN, Magnuson JT, Curran TE, Barker A, Roberts AP, Venables BJ (2019) Alterations to the vision-associated transcriptome of zebrafish ( Danio rerio ) following developmental norethindrone exposure. Environ Toxicol Pharmacol 69:137–142. https://doi.org/10.1016/j.etap.2019.04.011
Bridi D, Altenhofen S, Gonzalez JB, Reolon GK, Bonan CD (2017) Glyphosate and Roundup (R) alter morphology and behavior in zebrafish. Toxicology 392:32–39. https://doi.org/10.1016/j.tox.2017.10.007
Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE (1995) A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA 92:10545–10549. https://doi.org/10.1073/pnas.92.23.10545
Browne P, Noyes PD, Casey WM, Dix DJ (2017) Application of adverse outcome pathways to U.S. EPA’s endocrine disruptor screening program. Environ Health Perspect 125:096001. https://doi.org/10.1289/EHP1304
Burreau S, Broman D, Örn U (2000) Tissue distribution of 2,2’,4,4’-tetrabromo[ 14 C]diphenyl ether ([ 14 C]-PBDE 47) in pike ( Esox lucius ) after dietary exposure-a time series study using whole body autoradiography. Chemosphere 40:977–985. https://doi.org/10.1016/S0045-6535(99)00342-2
Caionix G, d’Angelo M, Panella G, Merola C, Cimini A, Amorena M et al (2021) Environmentally relevant concentrations of triclocarban affect morphological traits and melanogenesis in zebrafish larvae. Aquat Toxicol 236:105842. https://doi.org/10.1016/j.aquatox.2021.105842
Carvalho PSM, Tillitt DE (2004) 2,3,7,8-TCDD effects on visual structure and function in swim-up rainbow trout. Environ Sci Technol 38:6300–6306. https://doi.org/10.1021/es034857i
Carvalho PSM, Noltie DB, Tillitt DE (2002) Ontogenetic improvement of visual function in the medaka Oryzias latipes based on an optomotor testing system for larval and adult fish. Anim Behav 64:1–10. https://doi.org/10.1006/anbe.2002.3028
Cassar S, Dunn C, Olson A, Buck W, Fossey S, Ramos MF et al (2018) Inhibitors of nicotinamide phosphoribosyltransferase cause retinal damage in larval zebrafish. Toxicol Sci 161:300–309. https://doi.org/10.1093/toxsci/kfx212
Cavodeassi F, Wilson SW (2019) Looking to the future of zebrafish as a model to understand the genetic basis of eye disease. Hum Genet 138:993–1000. https://doi.org/10.1007/s00439-019-02055-z
Chen X, Emerson MM (2021) Notch signaling represses cone photoreceptor formation through the regulation of retinal progenitor cell states. Sci Rep 11:14525. https://doi.org/10.1038/s41598-021-93692-w
Chen J, Stahl A, Krah NM, Seaward MR, Joyal JS, Juan AM et al (2012) Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS ONE 7:e30203. https://doi.org/10.1371/journal.pone.0030203
Chen LG, Huang YB, Huang CJ, Hu B, Hu CY, Zhou BS (2013) Acute exposure to DE-71 causes alterations in visual behavior in zebrafish larvae. Environ Toxicol Chem 32:1370–1375. https://doi.org/10.1002/etc.2168
Chen LG, Tsui MMP, Shi QP, Hu CY, Wang Q, Zhou BS et al (2018) Accumulation of perfluorobutane sulfonate (PFBS) and impairment of visual function in the eyes of marine medaka after a life-cycle exposure. Aquat Toxicol 201:1–10. https://doi.org/10.1016/j.aquatox.2018.05.018
Chen XF, Chen ZF, Lin ZC, Liao XL, Zou T, Qi Z et al (2021) Toxic effects of triclocarban on larval zebrafish: a focus on visual dysfunction. Aquat Toxicol 241:106013. https://doi.org/10.1016/j.aquatox.2021.106013
Chen ZF, Lin ZC, Lu SQ, Chen XF, Liao XL, Qi Z et al (2022) Azole-induced color vision deficiency associated with thyroid hormone signaling: an integrated in vivo, in vitro, and in silico study. Environ Sci Technol 56:13264–13273. https://doi.org/10.1021/acs.est.2c05328
Chong SJ, Low IC, Pervaiz S (2014) Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 19:39–48. https://doi.org/10.1016/j.mito.2014.06.002
Choudhury S, Thomas JK, Sylvain NJ, Ponomarenko O, Gordon RA, Heald SM et al (2015) Selenium preferentially accumulates in the eye lens following embryonic exposure: a confocal X-ray fluorescence imaging study. Environ Sci Technol 49:2255–2261. https://doi.org/10.1021/es503848s
Chow ES, Hui MN, Cheng CW, Cheng SH (2009) Cadmium affects retinogenesis during zebrafish embryonic development. Toxicol Appl Pharmacol 235:68–76. https://doi.org/10.1016/j.taap.2008.11.013
Chrispell JD, Rebrik TI, Weiss ER (2015) Electroretinogram analysis of the visual response in zebrafish larvae. J vis Exp 16:52662. https://doi.org/10.3791/52662
Chung HY, Chang CT, Young HW, Hu SP, Tzou WS, Hu CH (2013) Ethanol inhibits retinal and CNS differentiation due to failure of cell cycle exit via an apoptosis-independent pathway. Neurotoxicol Teratol 38:92–103. https://doi.org/10.1016/j.ntt.2013.05.006
de Souza CF, Kalloniatis M, Polkinghorne PJ, McGhee CN, Acosta ML (2012) Functional activation of glutamate ionotropic receptors in the human peripheral retina. Exp Eye Res 94:71–84. https://doi.org/10.1016/j.exer.2011.11.008
Dehnert GK, Karasov WH, Wolman MA (2019) 2,4-Dichlorophenoxyacetic acid containing herbicide impairs essential visually guided behaviors of larval fish. Aquat Toxicol 209:1–12. https://doi.org/10.1016/j.aquatox.2019.01.015
Dejana E (2010) The role of wnt signaling in physiological and pathological angiogenesis. Circ Res 107:943–952. https://doi.org/10.1161/circresaha.110.223750
Desroches A, Boucher D, Denault JB (2016) Caspase family. In: Choi S (ed) Encyclopedia of signaling molecules. Springer, New York, pp 1–20. https://doi.org/10.1007/978-1-4614-6438-9_176-1
Chapter Google Scholar
Dong W, Macaulay LJ, Kwok KW, Hinton DE, Ferguson PL, Stapleton HM (2014) The PBDE metabolite 6-OH-BDE 47 affects melanin pigmentation and THRβ MRNA expression in the eye of zebrafish embryos. Endocr Disruptors 2:e969072. https://doi.org/10.4161/23273739.2014.969072
Duan Y, Sun H, Yao Y, Han L, Chen L (2021) Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ Int 155:106609. https://doi.org/10.1016/j.envint.2021.106609
Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931. https://doi.org/10.1016/j.cell.2008.09.002
Easter SS Jr, Malicki JJ (2002) The zebrafish eye: developmental and genetic analysis. Results Probl Cell Differ 40:346–370. https://doi.org/10.1007/978-3-540-46041-1_17
Easter SS Jr, Nicola GN (1997) The development of eye movements in the zebrafish ( Danio rerio ). Dev Psychol 31:267–276. https://doi.org/10.1002/(sici)1098-2302(199712)31:4%3c267::aid-dev4%3e3.0.co;2-p
Eldred KC, Hadyniak SE, Hussey KA, Brenerman B, Zhang PW, Chamling X et al (2018) Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 362:eaau6348. https://doi.org/10.1126/science.aau6348
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Fields S (2001) Proteomics in genomeland. Science 291:1221–1224. https://doi.org/10.1126/science.291.5507.1221
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122:877–902. https://doi.org/10.1161/CIRCRESAHA.117.311401
Germain P, Iyer J, Zechel C, Gronemeyer H (2002) Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415:187–192. https://doi.org/10.1038/415187a
Grey AC (2016) MALDI imaging of the eye: Mapping lipid, protein and metabolite distributions in aging and ocular disease. Int J Mass Spectrom 401:31–38. https://doi.org/10.1016/j.ijms.2016.02.017
Grey AC, Crouch RK, Koutalos Y, Schey KL, Ablonczy Z (2011) Spatial localization of A2E in the retinal pigment epithelium. Investig Ophthalmol vis Sci 52:3926–3933. https://doi.org/10.1167/iovs.10-7020
Gu J, Zhang JY, Chen YY, Wang HY, Guo M, Wang L et al (2019) Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae ( Danio rerio ). Chemosphere 217:629–635. https://doi.org/10.1016/j.chemosphere.2018.10.218
Heijlen M, Houbrechts AM, Bagci E, Van Herck SL, Kersseboom S, Esguerra CV et al (2014) Knockdown of type 3 iodothyronine deiodinase severely perturbs both embryonic and early larval development in zebrafish. Endocrinology 155:1547–1559. https://doi.org/10.1210/en.2013-1660
Heikkinen EM, Auriola S, Ranta VP, Demarais NJ, Grey AC, Del Amo EM et al (2019) Distribution of small molecular weight drugs into the porcine lens: studies on imaging mass spectrometry, partition coefficients, and implications in ocular pharmacokinetics. Mol Pharm 16:3968–3976. https://doi.org/10.1021/acs.molpharmaceut.9b00585
Hollbach N, Tappeiner C, Jazwinska A, Enzmann V, Tschopp M (2015) Photopic and scotopic spatiotemporal tuning of adult zebrafish vision. Front Syst Neurosci 9:20. https://doi.org/10.3389/fnsys.2015.00020
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M et al (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. https://doi.org/10.1038/nature12111
Huang LX, Wang CG, Zhang YY, Wu MF, Zuo ZH (2013) Phenanthrene causes ocular developmental toxicity in zebrafish embryos and the possible mechanisms involved. J Hazard Mater 261:172–180. https://doi.org/10.1016/j.jhazmat.2013.07.030
Huang LX, Zuo ZH, Zhang YY, Wu MF, Lin JJ, Wang CG (2014) Use of toxicogenomics to predict the potential toxic effect of Benzo(a)pyrene on zebrafish embryos: ocular developmental toxicity. Chemosphere 108:55–61. https://doi.org/10.1016/j.chemosphere.2014.02.078
Hurley JB, Lindsay KJ, Du J (2015) Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res 93:1079–1092. https://doi.org/10.1002/jnr.23583
Hyatt GA, Schmitt EA, Marsh-Armstrong NR, Dowling JE (1992) Retinoic acid-induced duplication of the zebrafish retina. Proc Natl Acad Sci USA 89:8293–8297. https://doi.org/10.1073/pnas.89.17.8293
Jaroszynska N, Harding P, Moosajee M (2021) Metabolism in the zebrafish retina. J Dev Biol 9:10. https://doi.org/10.3390/jdb9010010
Jiang Y, Zhang S, Zhang X, Li N, Zhang Q, Guo X et al (2019) Peptidomic analysis of zebrafish embryos exposed to polychlorinated biphenyls and their impact on eye development. Ecotoxicol Environ Saf 175:164–172. https://doi.org/10.1016/j.ecoenv.2019.03.015
Johnson RL, Scott MP (1998) New players and puzzles in the Hedgehog signaling pathway. Curr Opin Genet Dev 8:450–456. https://doi.org/10.1016/S0959-437X(98)80117-2
Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N et al (2016) Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med 22:439–445. https://doi.org/10.1038/nm.4059
Kanow MA, Giarmarco MM, Jankowski CS, Tsantilas K, Engel AL, Du J et al (2017) Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6:e28899. https://doi.org/10.7554/eLife.28899
Kim J, Kim Cy OhH, Ryu B, Kim U, Lee JM et al (2019) Trimethyltin chloride induces reactive oxygen species-mediated apoptosis in retinal cells during zebrafish eye development. Sci Total Environ 653:36–44. https://doi.org/10.1016/j.scitotenv.2018.10.317
Kirla KT, Groh KJ, Steuer AE, Poetzsch M, Banote RK, Stadnicka-Michalak J et al (2016) Zebrafish larvae are insensitive to stimulation by cocaine: importance of exposure route and toxicokinetics. Toxicol Sci 154:183–193. https://doi.org/10.1093/toxsci/kfw156
Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34:19–48. https://doi.org/10.1016/j.preteyeres.2013.02.001
Knapen D, Stinckens E, Cavallin JE, Ankley GT, Holbech H, Villeneuve DL et al (2020) Toward an AOP network-based tiered testing strategy for the assessment of thyroid hormone disruption. Environ Sci Technol 54:8491–8499. https://doi.org/10.1021/acs.est.9b07205
Ko E, Kim D, Kim K, Choi M, Shin S (2020) The action of low doses of persistent organic pollutants (POPs) on mitochondrial function in zebrafish eyes and comparison with hyperglycemia to identify a link between POPs and diabetes. Toxicol Mech Methods 30:275–283. https://doi.org/10.1080/15376516.2020.1717704
Kou Z, Dai W (2021) Aryl hydrocarbon receptor: its roles in physiology. Biochem Pharmacol 185:114428. https://doi.org/10.1016/j.bcp.2021.114428
Lagnado L (1998) Retinal processing: amacrine cells keep it short and sweet. Curr Biol 8:R598–R600. https://doi.org/10.1016/s0960-9822(98)70385-9
Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131:965–973. https://doi.org/10.1242/dev.01074
Li X, Li Y, Li S, Li H, Yang C, Lin J (2021a) The role of Shh signalling pathway in central nervous system development and related diseases. Cell Biochem Funct 39:180–189. https://doi.org/10.1002/cbf.3582
Li Y, Ma H, Chen R, Zhang H, Nakanishi T, Hu J (2021b) Maternal transfer of 2-ethylhexyl diphenyl phosphate leads to developmental toxicity possibly by blocking the retinoic acid Receptor and retinoic X receptor in Japanese medaka ( Oryzias latipes ). Environ Sci Technol 55:5056–5064. https://doi.org/10.1021/acs.est.0c06809
Liang L, Rasmussen MH, Piening B, Shen X, Chen S, Rost H et al (2020) Metabolic dynamics and prediction of gestational age and time to delivery in pregnant women. Cell 181:1680–1692. https://doi.org/10.1016/j.cell.2020.05.002
Liao XL, Chen ZF, Zou T, Lin ZC, Chen XF, Wang YJ et al (2021) Chronic exposure to climbazole induces oxidative stress and sex hormone imbalance in the testes of male zebrafish. Chem Res Toxicol 34:2558–2566. https://doi.org/10.1021/acs.chemrestox.1c00326
Liu WM, Zhang XN, Wei PH, Tian H, Wang W, Ru SG (2018) Long-term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish ( Danio rerio ). J Appl Toxicol 38:248–258. https://doi.org/10.1002/jat.3519
Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK et al (2005) WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437:417–421. https://doi.org/10.1038/nature03928
Maaswinkel H, Li L (2003) Spatio-temporal frequency characteristics of the optomotor response in zebrafish. Vision Res 43:21–30. https://doi.org/10.1016/S0042-6989(02)00395-4
Mahajan N, Arora P, Sandhir R (2019) Perturbed biochemical pathways and associated oxidative stress lead to vascular dysfunctions in diabetic retinopathy. Oxid Med Cell Longev 2019:8458472. https://doi.org/10.1155/2019/8458472
Malicki J, Neuhauss SC, Schier AF, Solnica-Krezel L, Stemple DL, Stainier DY et al (1996) Mutations affecting development of the zebrafish retina. Development 123:263–273. https://doi.org/10.1242/dev.123.1.263
Malicki J, Jo H, Pujic Z (2003) Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev Biol 259:95–108. https://doi.org/10.1016/s0012-1606(03)00181-7
Marelli F, Carra S, Agostini M, Cotelli F, Peeters R, Chatterjee K et al (2016) Patterns of thyroid hormone receptor expression in zebrafish and generation of a novel model of resistance to thyroid hormone action. Mol Cell Endocrinol 424:102–117. https://doi.org/10.1016/j.mce.2016.01.020
Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y et al (2003) N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development 130:2479–2494. https://doi.org/10.1242/dev.00465
Matsuno Y, Kiwamoto T, Morishima Y, Ishii Y, Hizawa N, Hogaboam CM (2018) Notch signaling regulates cell density-dependent apoptosis of NIH 3T3 through an IL-6/STAT3 dependent mechanism. Eur J Cell Biol 97:512–522. https://doi.org/10.1016/j.ejcb.2018.09.001
Meng Q, Yeung K, Kwok ML, Chung CT, Hu XL, Chan KM (2020) Toxic effects and transcriptome analyses of zebrafish ( Danio rerio ) larvae exposed to benzophenones. Environ Pollut 265:114857. https://doi.org/10.1016/j.envpol.2020.114857
Moreno-Marmol T, Cavodeassi F, Bovolenta P (2018) Setting eyes on the retinal pigment epithelium. Front Cell Dev Biol 6:145. https://doi.org/10.3389/fcell.2018.00145
Mueller KP, Neuhauss SC (2010) Quantitative measurements of the optokinetic response in adult fish. J Neurosci Meth 186:29–34. https://doi.org/10.1016/j.jneumeth.2009.10.020
Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS et al (2005) Forward genetic analysis of visual behavior in zebrafish. PLoS Genet 1:575–588. https://doi.org/10.1371/journal.pgen.0010066
Narayan DS, Chidlow G, Wood JP, Casson RJ (2017) Glucose metabolism in mammalian photoreceptor inner and outer segments. Clin Exp Ophthalmol 45:730–741. https://doi.org/10.1111/ceo.12952
Nazifova-Tasinova N, Radeva M, Galunska B, Grupcheva C (2020) Metabolomic analysis in ophthalmology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 164:236–246. https://doi.org/10.5507/bp.2020.028
Neuhauss SCF (2003) Behavioral genetic approaches to visual system development and function in zebrafish. J Neurosci 54:148–160. https://doi.org/10.1002/neu.10165
Neuhauss SCF, Biehlmaier O, Seeliger MW, Das T, Kohler K, Harris WA et al (1999) Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci 19:8603–8615. https://doi.org/10.1523/JNEUROSCI.19-19-08603.1999
Nishimura Y, Hara H (2016) Integrated approaches to drug discovery for oxidative stress-related retinal diseases. Oxid Med Cell Longev 2016:1–9. https://doi.org/10.1155/2016/2370252
Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK et al (2007) Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest 87:499–514. https://doi.org/10.1038/labinvest.3700537
Orger M, Baier H (2005) Channeling of red and green cone inputs to the zebrafish optomotor response. Vis Neurosci 22:275–281. https://doi.org/10.1017/S0952523805223039
Paravani EV, Simoniello MF, Poletta GL, Zolessi FR, Casco VH (2018) Cypermethrin: oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat Res Genet Toxicol Environ Mutagen 826:25–32. https://doi.org/10.1016/j.mrgentox.2017.12.010
Parker GH, Porter H (1934) The control of the dermal melanophores in elasmobranch fishes. Biol Bull Russ Acad Sci 66:30–37. https://doi.org/10.2307/1537459
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104. https://doi.org/10.1038/sj.cdd.4400476
Portugues R, Engert F (2009) The neural basis of visual behaviors in the larval zebrafish. Curr Opin Neurobiol 19:644–647. https://doi.org/10.1016/j.conb.2009.10.007
Prabhudesai SN, Cameron DA, Stenkamp DL (2005) Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Dev Biol 287:157–167. https://doi.org/10.1016/j.ydbio.2005.08.045
Punzo C, Xiong W, Cepko CL (2012) Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem 287:1642–1648. https://doi.org/10.1074/jbc.R111.304428
Purushothaman K, Das PP, Presslauer C, Lim TK, Johansen SD, Lin Q et al (2019) Proteomics analysis of early developmental stages of zebrafish embryos. Int J Mol Sci 20:6359. https://doi.org/10.3390/ijms20246359
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990. https://doi.org/10.1016/j.cellsig.2012.01.008
Richardson R, Tracey-White D, Webster A, Moosajee M (2017) The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 31:68–86. https://doi.org/10.1038/eye.2016.198
Rinner O, Rick JM, Neuhauss SC (2005) Contrast sensitivity, spatial and temporal tuning of the larval zebrafish optokinetic response. Investig Ophthalmol vis Sci 46:137–142. https://doi.org/10.1167/iovs.04-0682
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V et al (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9. https://doi.org/10.1046/j.1600-079X.2003.00092.x
Roy NM, Carneiro B, Ochs J (2016) Glyphosate induces neurotoxicity in zebrafish. Environ Toxicol Pharmacol 42:45–54. https://doi.org/10.1016/j.etap.2016.01.003
Sadamoto K, Yamagiwa Y, Sakaki H, Kurata M (2018) Absence of histopathological changes in the retina of zebrafish treated with sodium iodate. J Vet Sci 80:901–908. https://doi.org/10.1292/jvms.17-0613
Sahel JA, Leveillard T (2018) Maintaining cone function in rod-cone dystrophies. Adv Exp Med Biol 1074:499–509. https://doi.org/10.1007/978-3-319-75402-4_62
Sasagawa S, Nishimura Y, Kon T, Yamanaka Y, Murakami S, Ashikawa Y et al (2016) DNA damage response is involved in the developmental toxicity of mebendazole in zebrafish retina. Front Pharmacol 7:57. https://doi.org/10.3389/fphar.2016.00057
Saszik S, Bilotta J, Givin CM (1999) ERG assessment of zebrafish retinal development. Vis Neurosci 16:881–888. https://doi.org/10.1017/s0952523899165076
Savitskaya MA, Onishchenko GE (2015) Mechanisms of apoptosis. Biochemistry 80:1393–1405. https://doi.org/10.1134/s0006297915110012
Scheer N, Groth A, Hans S, Campos-Ortega JA (2001) An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development 128:1099–1107. https://doi.org/10.1242/dev.128.7.1099
Schmitt EA, Dowling JE (1994) Early eye morphogenesis in the zebrafish, Brachydanio rerio . J Comp Neurol 344:532–542. https://doi.org/10.1002/cne.903440404
Schmitt EA, Dowling JE (1999) Early retinal development in the zebrafish, Danio rerio : light and electron microscopic analyses. J Comp Neurol 404:515–536. https://doi.org/10.1002/(SICI)1096-9861(19990222)404:4%3c515::AID-CNE8%3e3.0.CO;2-A
Shi QP, Tsui MMP, Hu CY, Lam JCW, Zhou BS, Chen LG (2019a) Acute exposure to triphenyl phosphate (TPhP) disturbs ocular development and muscular organization in zebrafish larvae. Ecotoxicol Environ Saf 179:119–126. https://doi.org/10.1016/j.ecoenv.2019.04.056
Shi QP, Wang ZY, Chen LG, Fu JJ, Han J, Hu B et al (2019b) Optical toxicity of triphenyl phosphate in zebrafish larvae. Aquat Toxicol 210:139–147. https://doi.org/10.1016/j.aquatox.2019.02.024
Shi WJ, Jiang YX, Ma DD, Huang GY, Xie L, Chen HX et al (2019c) Dydrogesterone affects the transcription of genes in visual cycle and circadian rhythm network in the eye of zebrafish. Ecotoxicol Environ Saf 183:109556. https://doi.org/10.1016/j.ecoenv.2019.109556
Shi WJ, Huang GY, Jiang YX, Ma DD, Chen HX, Huang MZ et al (2020) Medroxyprogesterone acetate affects eye growth and the transcription of associated genes in zebrafish. Ecotoxicol Environ Saf 193:110371. https://doi.org/10.1016/j.ecoenv.2020.110371
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418. https://doi.org/10.1023/a:1009616228304
Stenkamp DL (2007) Neurogenesis in the fish retina. Int Rev Cytol 259:173–224. https://doi.org/10.1016/s0074-7696(06)59005-9
Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA (2000) Function for Hedgehog genes in zebrafish retinal development. Dev Biol 220:238–252. https://doi.org/10.1006/dbio.2000.9629
Stenkamp DL (2015) Development of the vertebrate eye and retina. In: Hejtmancik JF, Nickerson JM (ed) Molecular biology of eye disease. 397–414
Sun C, Galicia C, Stenkamp DL (2018) Transcripts within rod photoreceptors of the zebrafish retina. BMC Genom 19:127. https://doi.org/10.1186/s12864-018-4499-y
Tail M, Zhang H, Zheng G, Hatami M, Skutella T, Unterberg A et al (2022) The Sonic Hedgehog pathway modulates survival, proliferation, and differentiation of neural progenitor cells under inflammatory stress in vitro. Cells 11:736. https://doi.org/10.3390/cells11040736
Trikha P, Lee DA (2020) The role of AhR in transcriptional regulation of immune cell development and function. Biochim Biophys Acta Rev Cancer 1873:188335. https://doi.org/10.1016/j.bbcan.2019.188335
Trivedi CA, Bollmann JH (2013) Visually driven chaining of elementary swim patterns into a goal-directed motor sequence: a virtual reality study of zebrafish prey capture. Front Neural Circuits 7:86. https://doi.org/10.3389/fncir.2013.00086
Viets K, Eldred KC, Johnston RJ (2016) Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends Genet 32:638–659. https://doi.org/10.1016/j.tig.2016.07.004
Vihtelic TS, Hyde DR (2002) Zebrafish mutagenesis yields eye morphological mutants with retinal and lens defects. Vision Res 42:535–540. https://doi.org/10.1016/S0042-6989(01)00261-9
Volkov LI, Kim-Han JS, Saunders LM, Poria D, Hughes AEO, Kefalov VJ et al (2020) Thyroid hormone receptors mediate two distinct mechanisms of long-wavelength vision. Proc Natl Acad Sci USA 117:15262–15269. https://doi.org/10.1073/pnas.1920086117
Wang YP, Hong Q, Dn Q, Kou CZ, Zhang CM, Guo M et al (2012) Effects of embryonic exposure to polychlorinated biphenyls on zebrafish ( Danio rerio ) retinal development. J Appl Toxicol 32:186–193. https://doi.org/10.1002/jat.1650
Wang WD, Hsu HJ, Li YF, Wu CY (2017) Retinoic acid protects and rescues the development of zebrafish embryonic retinal photoreceptor cells from exposure to paclobutrazol. Int J Mol Sci 18:130. https://doi.org/10.3390/ijms18010130
Wang W, Kini A, Wang Y, Liu T, Chen Y, Vukmanic E et al (2019a) Metabolic deregulation of the blood-outer retinal barrier in retinitis pigmentosa. Cell Rep 28:1323–1334. https://doi.org/10.1016/j.celrep.2019.06.093
Wang Z, Liu CH, Huang S, Chen J (2019b) Wnt signaling in vascular eye diseases. Prog Retin Eye Res 70:110–133. https://doi.org/10.1016/j.preteyeres.2018.11.008
Watanabe K, Nishimura Y, Oka T, Nomoto T, Kon T, Shintou T et al (2010) In vivo imaging of zebrafish retinal cells using fluorescent coumarin derivatives. BMC Neurosci 11:116. https://doi.org/10.1186/1471-2202-11-116
Watanabe K, Nishimura Y, Nomoto T, Umemoto N, Zhang Z, Zhang B et al (2012) In vivo assessment of the permeability of the blood-brain barrier and blood-retinal barrier to fluorescent indoline derivatives in zebrafish. BMC Neurosci 13:101. https://doi.org/10.1186/1471-2202-13-101
Wei N, Zhang X, Hong Q, Jiang Y, Zhang Q, Guo X et al (2019) The sonic hedgehog signaling pathway is suppressed following PCB 1254 exposure during retinal development. Environ Toxicol 34:340–347. https://doi.org/10.1002/tox.22689
Wei S, Chen F, Xu T, Cao M, Yang X, Zhang B et al (2022) BDE-99 disrupts the photoreceptor patterning of zebrafish larvae via transcription factor six7 . Environ Sci Technol 56:5673–5683. https://doi.org/10.1021/acs.est.1c08914
Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y (1995) Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol 79:728–731. https://doi.org/10.1136/bjo.79.8.728
Witkovsky P (2004) Dopamine and retinal function. Doc Ophthalmol 108:17–40. https://doi.org/10.1023/b:doop.0000019487.88486.0a
Xiao Y, Jiang J, Hu W, Zhao Y, Hu J (2017) Toxicity of triphenyltin on the development of retinal axons in zebrafish at low dose. Aquat Toxicol 189:9–15. https://doi.org/10.1016/j.aquatox.2017.05.009
Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C et al (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883–895. https://doi.org/10.1016/S0092-8674(04)00216-8
Xu T, Chen LG, Hu CY, Zhou BS (2013) Effects of acute exposure to polybrominated diphenyl ethers on retinoid signaling in zebrafish larvae. Environ Toxicol Pharmacol 35:13–20. https://doi.org/10.1016/j.etap.2012.10.004
Xu T, Zhao J, Yin DQ, Zhao QS, Dong BZ (2015) High-throughput RNA sequencing reveals the effects of 2,2’,4,4’ -tetrabromodiphenyl ether on retina and bone development of zebrafish larvae. BMC Genom 16:23. https://doi.org/10.1186/s12864-014-1194-5
Xu T, Liu Y, Pan RJ, Zhang B, Yin DQ, Zhao J et al (2017) Vision, color vision, and visually guided behavior: the novel toxicological targets of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47). Environ Sci Technol Lett 4:132–136. https://doi.org/10.1021/acs.estlett.7b00010
Yang XL (2004) Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol 73:127–150. https://doi.org/10.1016/j.pneurobio.2004.04.002
Yemanyi F, Bora K, Blomfield AK, Wang Z, Chen J (2021) Wnt signaling in inner blood-retinal barrier maintenance. Int J Mol Sci 22:11877. https://doi.org/10.3390/ijms222111877
Ying YH, Neuhauss SCF (2008) The optokinetic response in zebrafish and its applications. Front Biosci 13:1899–1916. https://doi.org/10.2741/2810
Zhang X, Hong Q, Yang L, Zhang M, Guo X, Chi X et al (2015) PCB 1254 exposure contributes to the abnormalities of optomotor responses and influence of the photoreceptor cell development in zebrafish larvae. Ecotoxicol Environ Saf 118:133–138. https://doi.org/10.1016/j.ecoenv.2015.04.026
Zhao Y, Fent K (2016) Progestins alter phototransduction cascade and circadian rhythm network in eyes of zebrafish ( Danio rerio ). Sci Rep 6:21559. https://doi.org/10.1038/srep21559
Zimmermann MJY, Nevala NE, Yoshimatsu T, Osorio D, Nilsson DE, Berens P et al (2018) Zebrafish differentially process color across visual space to match natural scenes. Curr Biol 28:2018–2032. https://doi.org/10.1016/j.cub.2018.04.075
Zindler F, Tisler S, Loerracher AK, Zwiener C, Braunbeck T (2020) Norfluoxetine is the only metabolite of fluoxetine in zebrafish ( Danio rerio ) embryos that accumulates at environmentally relevant exposure scenarios. Environ Sci Technol 54:4200–4209. https://doi.org/10.1021/acs.est.9b07618
Zou T, Liang YQ, Liao XL, Chen XF, Wang T, Song YY et al (2021) Metabolomics reveals the reproductive abnormality in female zebrafish exposed to environmentally relevant levels of climbazole. Environ Pollut 275:116665. https://doi.org/10.1016/j.envpol.2021.116665
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (42177254) and the Natural Science Foundation of Guangdong Province, China (2021A1515010018).
Author information
Authors and affiliations.
Guangdong Key Laboratory of Environmental Catalysis and Health Risk Control, Guangdong-Hong Kong-Macao Joint Laboratory for Contaminants Exposure and Health, School of Environmental Science and Engineering, Guangdong University of Technology, Guangzhou, 510006, China
Xiao-Fan Chen, Zhi-Cheng Lin, Zenghua Qi, Zongwei Cai & Zhi-Feng Chen
Key Laboratory for City Cluster Environmental Safety and Green Development of the Ministry of Education, Guangdong University of Technology, Guangzhou, 510006, China
Zenghua Qi & Zhi-Feng Chen
State Key Laboratory of Environmental and Biological Analysis, Department of Chemistry, Hong Kong Baptist University, Hong Kong, 999077, China
Zongwei Cai
You can also search for this author in PubMed Google Scholar
Contributions
XFC and ZCL searched the relevant literature. XFC, ZCL, and ZFC complied with the first draft. ZFC, ZHQ, and ZWC critically analyzed the first draft and designed the final manuscript. All authors discussed and commented on the manuscript.
Corresponding author
Correspondence to Zhi-Feng Chen .
Ethics declarations
Conflict of interest.
The authors declare no conflict of interest.
Consent for publication
All the authors approved the final manuscript and provided their consent for publication.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chen, XF., Lin, ZC., Qi, Z. et al. Effects of pollutant toxicity on the eyes of aquatic life monitored by visual dysfunction in zebrafish: a review. Environ Chem Lett 21 , 1177–1201 (2023). https://doi.org/10.1007/s10311-022-01531-9
Download citation
Received : 28 June 2022
Accepted : 06 October 2022
Published : 14 October 2022
Issue Date : April 2023
DOI : https://doi.org/10.1007/s10311-022-01531-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ocular toxicity
- Environmental pollutant
- Signaling pathway
- Research technique
- Adverse outcome pathway
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 28 November 2023
A systematic review of the impact of environmental enrichment in zebrafish
- Matheus Gallas-Lopes ORCID: orcid.org/0000-0001-5375-2335 1 ,
- Radharani Benvenutti 2 ,
- Nayne I. Z. Donzelli 3 , 4 &
- Matheus Marcon ORCID: orcid.org/0000-0002-1486-688X 3 , 4 , 5
Lab Animal volume 52 , pages 332–343 ( 2023 ) Cite this article
712 Accesses
12 Altmetric
Metrics details
- Behavioural ecology
Environmental enrichment (EE) consists of a series of interventions carried out in the home environment to promote greater exposure to sensory stimuli and mimic the natural habitat of laboratory-housed animals, providing environments closer to those found in nature. Some studies have shown the positive effects of EE in zebrafish housed in a laboratory environment. However, this evidence is still recent and accompanied by contradictory results. Furthermore, there is great variability in the protocols applied and in the conditions of the tests, tanks and materials used to generate an enriched environment. This substantial variability can bring many uncertainties to the development of future studies and hinder the reproducibility and replicability of research. Here, in this context, we carried out a systematic review of the literature, aiming to provide an overview of the EE protocols used in zebrafish research. The literature search was performed in PubMed, Scopus and Web of Science and the studies were selected on the basis of predefined inclusion/exclusion criteria. A total of 901 articles were identified in the databases, and 27 of those studies were included in this review. We conducted data extraction and risk-of-bias analysis in the included studies. Among these studies, the effect of EE was evaluated in two different ways: (1) for animal welfare and (2) as an intervention to prevent behavioral, biochemical, molecular, developmental and breeding dysfunctions. Although the EE protocols in zebrafish presented a series of experimental differences, the results showed that the benefits of the EE for zebrafish were consistent. According to the results described here, the use of EE in the zebrafish home tank improves welfare and may reduce sources of bias in scientific research. However, it is still necessary to develop standardized protocols to improve the application of EE in scientific studies using zebrafish.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
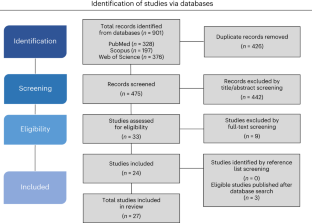
Similar content being viewed by others

Predator-induced fear causes PTSD-like changes in the brains and behaviour of wild animals

Pervasive environmental chemicals impair oligodendrocyte development
The gut microbiota–brain axis in behaviour and brain disorders
Data availability.
Supporting data is available in Open Science Framework ( https://osf.io/2gdey/ ).
Ellenbroek, B. & Youn, J. Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech. 9 , 1079–1087 (2016).
Article CAS PubMed PubMed Central Google Scholar
Dawson, T. M., Golde, T. E. & Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 21 , 1370–1379 (2018).
Spence, R., Gerlach, G., Lawrence, C. & Smith, C. The behaviour and ecology of the zebrafish, Danio rerio . Biol. Rev. 83 , 13–34 (2008).
Article Google Scholar
Kalueff, A. V., Stewart, A. M. & Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 35 , 63–75 (2014).
Stewart, A. M., Braubach, O., Spitsbergen, J., Gerlai, R. & Kalueff, A. V. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 37 , 264–278 (2014).
Lee, C. J., Paull, G. C. & Tyler, C. R. Improving zebrafish laboratory welfare and scientific research through understanding their natural history. Biol. Rev. 97 , 1038–1056 (2022).
Article PubMed Google Scholar
Sert, N. Pdu et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18 , e3000410 (2020).
Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 20 , 235–245 (2019).
Article CAS PubMed Google Scholar
Yerkes, R. M. Almost Human (Century Company, 1925).
Hancocks, D. in Third International Symposium on Zoo Design and Construction 166–173 (Whitley Wildlife Trust, 1980).
Hutchins, M., Hancocks, D. & Crockett, C. Naturalistic solutions to the behavioral problems of captive animals. Zool. Gart. 54 , 28–42 (1984).
Google Scholar
Beaver, B. V. Environmental enrichment for laboratory animals. ILAR J. 31 , 5–11 (1989).
Nithianantharajah, J. & Hannan, A. J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7 , 697–709 (2006).
von Krogh, K., Sørensen, C., Nilsson, G. E. & Øverli, Ø. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio , kept in enriched or barren environments. Physiol. Behav. 101 , 32–39 (2010).
DePasquale, C., Neuberger, T., Hirrlinger, A. M. & Braithwaite, V. A. The influence of complex and threatening environments in early life on brain size and behaviour. Proc. R. Soc. B 283 , 20152564 (2016).
Article PubMed PubMed Central Google Scholar
Hirase, H. & Shinohara, Y. Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 280 , 282–298 (2014).
Mahati, K., Bhagya, V., Christofer, T., Sneha, A. & Shankaranarayana Rao, B. S. Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 134 , 379–391 (2016).
Zhang, T.-Y. et al. Environmental enrichment increases transcriptional and epigenetic differentiation between mouse dorsal and ventral dentate gyrus. Nat. Commun. 9 , 298 (2018).
Hegde, A., Suresh, S. & Mitra, R. Early-life short-term environmental enrichment counteracts the effects of stress on anxiety-like behavior, brain-derived neurotrophic factor and nuclear translocation of glucocorticoid receptors in the basolateral amygdala. Sci. Rep. 10 , 14053 (2020).
Huang, H. et al. Effects of enriched environment on depression and anxiety-like behavior induced by early life stress: a comparison between different periods. Behav. Brain Res. 411 , 113389 (2021).
Jha, S., Dong, B. & Sakata, K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl. Psychiatry 1 , e40 (2011).
Seong, H.-H., Park, J.-M. & Kim, Y.-J. Antidepressive effects of environmental enrichment in chronic stress–induced depression in rats. Biol. Res. Nurs. 20 , 40–48 (2018).
Griñán-Ferré, C. et al. Environmental enrichment improves cognitive deficits, AD hallmarks and epigenetic alterations presented in 5xFAD mouse model. Front. Cell. Neurosci. 12 , 224 (2018).
Article PubMed Central Google Scholar
Jankowsky, J. L. et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci. 25 , 5217–5224 (2005).
Seo, J. H. et al. Environmental enrichment attenuates oxidative stress and alters detoxifying enzymes in an A53T α-synuclein transgenic mouse model of Parkinson’s disease. Antioxidants 9 , 928 (2020).
Wassouf, Z. et al. Environmental enrichment prevents transcriptional disturbances induced by alpha-synuclein overexpression. Front. Cell. Neurosci. 12 , 112 (2018).
DePasquale, C., Fettrow, S., Sturgill, J. & Braithwaite, V. A. The impact of flow and physical enrichment on preferences in zebrafish. Appl. Anim. Behav. Sci. 215 , 77–81 (2019).
Kistler, C., Hegglin, D., Würbel, H. & König, B. Preference for structured environment in zebrafish ( Danio rerio ) and checker barbs ( Puntius oligolepis ). Appl. Anim. Behav. Sci. 135 , 318–327 (2011).
Tan, S. L. T., Handasyde, K. A., Rault, J.-L. & Mendl, M. Insensitivity to reward shifts in zebrafish ( Danio rerio ) and implications for assessing affective states. Anim. Cogn. 23 , 87–100 (2020).
Maia, C. M., Volpato, G. L. & Braithwaite, V. A. A psychological aversive condition does not change individual zebrafish preference for background color or artificial plant enrichments. J. Appl. Anim. Welf. Sci. 25 , 427–438 (2022).
Schroeder, P., Jones, S., Young, I. S. & Sneddon, L. U. What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Lab. Anim. 48 , 328–337 (2014).
Jones, N. A. R., Spence, R., Jones, F. A. M. & Spence-Jones, H. C. Shade as enrichment: testing preferences for shelter in two model fish species. J. Fish Biol. 95 , 1161–1165 (2019).
Krueger, L. D. et al. Enrichment preferences of singly housed zebrafish ( Danio rerio ). J. Am. Assoc. Lab. Anim. Sci. 59 , 148–155 (2020).
Keck, V. A. et al. Effects of habitat complexity on pair-housed zebrafish. J. Am. Assoc. Lab. Anim. Sci. 54 , 378–383 (2015).
PubMed Central Google Scholar
Spence, R., Magurran, A. E. & Smith, C. Spatial cognition in zebrafish: the role of strain and rearing environment. Anim. Cogn. 14 , 607–612 (2011).
Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13 , 93–110 (2012).
Article CAS Google Scholar
DePasquale, C. et al. The influence of an enriched environment in enhancing recognition memory in zebrafish ( Danio rerio ). Front. Vet. Sci. 8 , 749746 (2021).
Gatto, E., Bruzzone, M., Maschio, M. D. & Dadda, M. Effects of environmental enrichment on recognition memory in zebrafish larvae. Appl. Anim. Behav. Sci. 247 , 105552 (2022).
Gerlai, R. Social behavior of zebrafish: from synthetic images to biological mechanisms of shoaling. J. Neurosci. Methods 234 , 59–65 (2014).
Suriyampola, P. S. et al. Zebrafish social behavior in the wild. Zebrafish 13 , 1–8 (2016).
Sykes, D. J., Suriyampola, P. S. & Martins, E. P. Recent experience impacts social behavior in a novel context by adult zebrafish ( Danio rerio ). PLoS ONE 13 , e0204994 (2018).
Levin, E. D., Bencan, Z. & Cerutti, D. T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 90 , 54–58 (2007).
Lee, C. J., Paull, G. C. & Tyler, C. R. Effects of environmental enrichment on survivorship, growth, sex ratio and behaviour in laboratory maintained zebrafish Danio rerio . J. Fish Biol. 94 , 86–95 (2019).
dos Santos, T. G., Mussulini, B. H. M., Frangipani, L. A. & de Oliveira, D. L. Differential impact of shorter and longer periods of environmental enrichment on adult zebrafish exploratory activity ( Danio rerio ) in the novel tank paradigm. Behav. Processes 181 , 104278 (2020).
Gatto, E. et al. Environmental enrichment decreases anxiety-like behavior in zebrafish larvae. Dev. Psychobiol. 64 , e22255 (2022).
Teles, M. C. & Oliveira, R. F. in Zebrafish: Methods and Protocols (eds. Kawakami, K., Patton, E. E. & Orger, M.) 293–305 (Springer, 2016).
Woodward, M. A., Winder, L. A. & Watt, P. J. Enrichment increases aggression in zebrafish. Fishes 4 , 22 (2019).
Wafer, L. N. et al. Effects of environmental enrichment on the fertility and fecundity of zebrafish ( Danio rerio ). J. Am. Assoc. Lab. Anim. Sci. 55 , 291–294 (2016).
PubMed PubMed Central Google Scholar
Collymore, C., Tolwani, R. J. & Rasmussen, S. The behavioral effects of single housing and environmental enrichment on adult zebrafish ( Danio rerio ). J. Am. Assoc. Lab. Anim. Sci. 54 , 280–285 (2015).
Marcon, M. et al. Environmental enrichment modulates the response to chronic stress in zebrafish. J. Exp. Biol. 221 , jeb176735 (2018).
Menezes, F. P., Amorim, R. R., Silva, P. F. & Luchiari, A. C. Alcohol exposure and environmental enrichment effects on contextual fear conditioning in zebrafish. Behav. Processes 197 , 104608 (2022).
Maximino, C., da Silva, A. W. B., Gouveia, A. & Herculano, A. M. Pharmacological analysis of zebrafish ( Danio rerio ) scototaxis. Prog. Neuropsychopharmacol. Biol. Psychiatry 35 , 624–631 (2011).
Sackerman, J. et al. Zebrafish behavior in novel environments: effects of acute exposure to anxiolytic compounds and choice of Danio rerio Line. Int. J. Comp. Psychol. 23 , 43–61 (2010).
Manuel, R. et al. The effects of environmental enrichment and age-related differences on inhibitory avoidance in zebrafish ( Danio rerio Hamilton). Zebrafish 12 , 152–165 (2015).
Atucha, E. & Roozendaal, B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Front. Behav. Neurosci. 9 , 60 (2015).
Small, W. S. An experimental study of the mental processes of the rat. Am. J. Psychol. 11 , 133–165 (1900).
Benvenutti, R. et al. Swimming in the maze: an overview of maze apparatuses and protocols to assess zebrafish behavior. Neurosci. Biobehav. Rev. 127 , 761–778 (2021).
Giacomini, A. C. V. V. et al. Environmental and pharmacological manipulations blunt the stress response of zebrafish in a similar manner. Sci. Rep. 6 , 28986 (2016).
Article CAS PubMed Central Google Scholar
Estes, J. M., Altemara, M. L., Crim, M. J., Fletcher, C. A. & Whitaker, J. W. Behavioral and reproductive effects of environmental enrichment and Pseudoloma neurophilia infection on adult zebrafish ( Danio rerio ). J. Am. Assoc. Lab. Anim. Sci. 60 , 249–258 (2021).
Marcon, M. et al. Enriched environment prevents oxidative stress in zebrafish submitted to unpredictable chronic stress. PeerJ 6 , e5136 (2018).
Strzalka, W. & Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann. Bot. 107 , 1127–1140 (2011).
Seki, T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J. Neurosci. Res. 70 , 327–334 (2002).
Smith, S. M. et al. Cocaine- and amphetamine-regulated transcript activates the hypothalamic–pituitary–adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology 145 , 5202–5209 (2004).
Finsterwald, C. & Alberini, C. M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 112 , 17–29 (2014).
Karoglu-Eravsar, E. T., Tuz-Sasik, M. U. & Adams, M. M. Environmental enrichment applied with sensory components prevents age-related decline in synaptic dynamics: evidence from the zebrafish model organism. Exp. Gerontol. 149 , 111346 (2021).
Masliah, E., Terry, R. D., DeTeresa, R. M. & Hansen, L. A. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci. Lett. 103 , 234–239 (1989).
Vreugdenhil, E. et al. Doublecortin-like, a microtubule-associated protein expressed in radial glia, is crucial for neuronal precursor division and radial process stability. Eur. J. Neurosci. 25 , 635–648 (2007).
Groeneweg, F. L., Trattnig, C., Kuhse, J., Nawrotzki, R. A. & Kirsch, J. Gephyrin: a key regulatory protein of inhibitory synapses and beyond. Histochem. Cell Biol. 150 , 489–508 (2018).
Parichy, D. M. Advancing biology through a deeper understanding of zebrafish ecology and evolution. eLife 4 , e05635 (2015).
Roy, C. et al. Effects of enrichment type, presentation and social status on enrichment use and behavior of sows—part 2: free access stall feeding. Anim. Open Access J. MDPI 12 , 1768 (2022).
Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) (Univ. Oregon Press, 2007).
Kopp, R., Legler, J. & Legradi, J. Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors. Environ. Sci. Pollut. Res. Int. 25 , 4085–4093 (2018).
Krylov, V. V., Izvekov, E. I., Pavlova, V. V., Pankova, N. A. & Osipova, E. A. Circadian rhythms in zebrafish ( Danio rerio ) behaviour and the sources of their variability. Biol. Rev. 96 , 785–797 (2021).
European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. J. Eur Union 50 , 33–79 (2010).
André, V. et al. Laboratory mouse housing conditions can be improved using common environmental enrichment without compromising data. PLoS Biol. 16 , e2005019 (2018).
Russell, W. M. S. & Burch, R. L. The principles of humane experimental technique. Med. J. Aust. 1 , 500–500 (1960).
Gerlai, R. Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol. Biochem. Behav. 178 , 30–38 (2019).
Macleod, M. R. et al. Risk of bias in reports of in vivo research: a focus for improvement. PLoS Biol. 13 , e1002273 (2015).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br. Med. J. 372 , n71 (2021).
Gallas-Lopes, M., Benvenutti, R., Marcon, M. Environmental enrichment strategies for zebrafish: a systematic review. Open Science Framework https://osf.io/2gdey/ (2023).
van Eck, N. J. & Waltman, L. VO in Advances in Data Analysis (eds Decker, R. & Lenz, H.-J.) 299–306 (Springer, 2007).
van Eck, N. J. & Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 , 523–538 (2010).
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14 , 43 (2014).
Download references
Acknowledgements
We thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (FAPEMIG, Proc. APQ-00151-22) and Pró-Reitoria de Pesquisa e Pós-Graduação (PROPPG) at Universidade Federal do Triângulo Mineiro (UFTM) for funding and support. N.I.Z.D. received a fellowship from Fundação de Amparo à Pesquisa do Estado de Minas Gerais. M.G.-L. is recipient of a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). R.B. is recipient of a fellowship from Irish Research Council (IRS) and Epilepsy Ireland.
Author information
Authors and affiliations.
Departamento de Farmacologia, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Matheus Gallas-Lopes
School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland
Radharani Benvenutti
Departamento de Bioquímica, Farmacologia e Fisiologia, Instituto de Ciências Biológicas e Naturais, Universidade Federal do Triângulo Mineiro, Uberaba, Brazil
Nayne I. Z. Donzelli & Matheus Marcon
Laboratório de Zebrafish (ZebLab), Instituto de Ciências Biológicas e Naturais, Universidade Federal do Triângulo Mineiro, Uberaba, Brazil
Programa de Pós-graduação em Ciências da Saúde, Instituto de Ciências da Saúde, Universidade Federal do Triângulo Mineiro, Uberaba, Brazil
Matheus Marcon
You can also search for this author in PubMed Google Scholar
Contributions
M.G.-L., R.B. and M.M. conceived and designed the project. M.M. supervised the project. M.G.-L., R.B., N.I.Z.D. and M.M. collaborated on data acquisition and visualization. M.G.-L., R.B. and M.M. contributed to the investigation and methodology. M.G.-L., R.B. and M.M. drafted the original article.
Corresponding author
Correspondence to Matheus Marcon .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Lab Animal thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Gallas-Lopes, M., Benvenutti, R., Donzelli, N.I.Z. et al. A systematic review of the impact of environmental enrichment in zebrafish. Lab Anim 52 , 332–343 (2023). https://doi.org/10.1038/s41684-023-01288-w
Download citation
Received : 01 March 2023
Accepted : 12 October 2023
Published : 28 November 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s41684-023-01288-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Introduction. For use in genetic studies as an animal model, zebrafish was initially introduced by Streisinger and colleagues 1 in the early 1980s. Large-scale N-ethyl-N-nitrosourea (ENU) mutagenesis was conducted in combination with extensive phenotypic screening 2,3.Further phenotypic characterization of these ENU mutations in most of the major organ systems was performed 4.
Zebrafish is a fascinating animal model for understanding the human pathogenesis of metabolic diseases and identifying potential therapeutic options 21. However, all animal models have unique ...
Zebrafish (Danio rerio) is emerging as an increasingly successful model for translational research on human neurological disorders. In this review, we appraise the high degree of neurological and ...
Therefore, zebrafish constitutes an excellent experimental model for behavioral, genetic and toxicological studies which unravels the mechanism of various human diseases. Furthermore, it serves well to test new therapeutic agents, such as the safety of new vaccines. The aim of this review was to provide a systematic literature review on the ...
Much of medical research relies on animal models to deepen knowledge of the causes of animal and human diseases, as well as to enable the development of innovative therapies. Despite rodents being the most widely used research model worldwide, in recent decades, the use of the zebrafish (Danio rerio) model has exponentially been adopted among the scientific community. This is because such a ...
fi. This review addresses the use of zebra fish as an animal model for biomedical research, mainly in developmental disorders, mental disorders, and communication between the brain and organs. In ...
The growth of its use in translational research can be extrapolated from Fig. 1 A-B, which shows the increase of the use in zebrafish disease models over the years with the data being collected over the available literature from PubMed and Scopus databases (2000-2021). Download : Download high-res image (474KB) Download : Download full-size image
The zebrafish is increasingly recognized as a model organism for translational research into human neuropathology. The zebrafish brain exhibits fundamental resemblance with human neuroanatomical and neurochemical pathways, and hallmarks of human brain pathology such as protein aggregation, neuronal degeneration and activation of glial cells, for example, can be modeled and recapitulated in the ...
The use of zebrafish (Danio rerio) in biomedical research has expanded at a tremendous rate over the last two decades.Along with increases in laboratories using this model, we are discovering new and important diseases. We review here the important pathogens and diseases based on some 20 years of research and findings from our diagnostic service at the NIH-funded Zebrafish International ...
This review discusses the advantages of zebrafish as an animal model, visual system of zebrafish with its similarities and differences from the human eye, embryology of the eye in zebrafish, visual behavioural assays, and role of zebrafish as an animal model in several ocular diseases, including aniridia, retinitis pigmentosa, Leber congenital ...
The literature review clearly shows that many biotic and abiotic factors can affect zebrafish FI. Drugs and toxicants administration, as well as rearing conditions and genetic manipulations, can affect the dietary intake of zebrafish. ... Information gathered from the articles included in the review. (A) zebrafish genotype; (B) ...
Zebrafish offer sophisticated imaging techniques and allow simple and fast genetic and pharmacological approaches with a high throughput. In this review, we highlight achievements and mechanisms concerning microvascular complications discovered in zebrafish, and we discuss the advantages and disadvantages of zebrafish as a model for studying ...
The zebrafish, Danio rerio, has emerged as a major model organism for biomedical research, yet little is known about its natural history. We review the literature pertaining to the geographic range, biotic and abiotic habitats, and life cycle of the zebrafish. We also report our own field study to document several aspects of zebrafish natural ...
A systematic review of the literature Yuanshan Lin, Yuanshan Lin Department of Orthopedic Surgery, First Affiliated Hospital of Kunming Medical University ... researchers have conducted many studies on selenium using zebrafish models. This article reviews the current reported studies on the beneficial and toxic effects of selenium on zebrafish ...
Zebrafish are useful model organisms for drug discovery, particularly in screening, disease modelling and toxicity assays. Here, Patton, Zon and Langenau discuss key recent successes in which ...
The literature review aimed to provide an overview of previous EDA investigations that applied bioassays with zebrafish, critically evaluating their objectives, methods, biotesting strategy and outcomes; discuss the potential contribution of further zebrafish bioassays for EDA; and propose strategies that might help optimizing the integration ...
of zebrafish natural history. We present a hy-pothesis for zebrafish life history in the wild, suggest how studies of natural populations can inform research in the laboratory, and make recommendations for future work in this area. LITERATURE REVIEW An extensive literature exists on Central Asian fishes and specifically on Indian fishes.7-13 Yet,
The present review updates the literature, critically evaluates each of the available models of Parkinson's Disease in zebrafish and compares them with similar models in invertebrates and mammals to determine their advantages and disadvantages. ... As mentioned in the reviews of gene-based models, zebrafish may have one or two homologous ...
The focus of this review is to assess contemporary findings regarding PFAS toxicokinetics, toxicity and apical adverse health outcomes, and potential modes of action using the zebrafish model. Much of the peer-reviewed literature has focused on a small subset of PFAS structural subclasses, such as the perfluoroalkyl sulfonic acids and ...
This study gives an overview of the most commonly examined biomarkers for TH disruption in zebrafish embryos. The literature review about effects seen with 25 known TH disruptors, indicates that besides morphological effects mostly effects on transcript expression where assessed. There was no biomarker/endpoint that gave a response in all ...
The eyes of aquatic organisms may be damaged by exposure to pollutants. Zebrafish is a common laboratory model to study ocular toxicity, combining both fish and vertebrate characteristics. The toxic effects on zebrafish eyes caused by pollutants include morphological changes and damage to the retina at the molecular, cellular, and tissue levels; and abnormalities in the visual ...
A literature review. Hans W. Laale, Hans W. Laale. Department of Zoology, University of Manitoba, Winnipeg, Manitoba, Canada. Search for more papers by this author. ... The Zebrafish, Brachydanio rerio (Hamilton-Buchanan, 1822, 1823) is considered to be the most likely candidate. It is relatively easy to maintain and breed in laboratory aquaria ...
In zebrafish, until this date, the studies in the scientific literature have evaluated the effects of EE on two different types of proposals: (1) to improve zebrafish welfare and (2) for ...
© 2024 Mary Ann Liebert, Inc., publishers. All rights reserved, USA and worldwide. Call us toll free at (800) M-LIEBERT (800-654-3237).
Lipid metabolism and adipose biology in zebrafish. Obesity is a consequence of positive energy balance. Regulation of energy intake and expenditure involves many organ systems including the brain, intestines, skeletal muscle, and adipose tissue (Cai, 2013; Dailey, 2014; Periasamy et al., 2017).Therefore, whole animal models are essential for better understanding of the development and ...
An SLR for a particular topic is a recognised method to conduct a review of research in order to capture all relevant sources of research, analyse it to produce a complete interpretation of research results and identify research gaps (Hulland, 2020; Pittaway & Cope, 2007; Poklepović Peričić & Tanveer, 2019).An SLR method was chosen as it is an effective method of synthesizing ...