SPM TRIAL PAPERS 2021/Kimia (Chemistry)/
SPM Study Group | Feel free to join our whatsapp study group for latest SPM study resources ❤️ 😊
🔥🔥🔥 MAGIC AI TOOLS 🔥🔥🔥 MAGIC AI TOOLS was down on 10/4/2024 - 20/4/2024 due to lack of funding. Problem is fixed, AI model is upgraded and should work even better now. Try it out, it is FREE 😊
- Kimia (Chemistry)
SPM Study Group | Feel free to join our whatsapp study group for discussion 😊, and to get latest SPM study tips and resources ❤️

1. Who developed SPM Trial Paper website?
Few years ago, we were also form 5 students taking spm..
Just like you, we were also stressed and lost sleep because of it. After all, it is one of the most important exams that can potentially affect our future.
Having gone through SPM ourselves, we know how tough it is to do well in so many subjects at the same time.
Thus, we had developed this website, in hopes of assisting our Form 5 juniors to score well in SPM.
You might ask, why a website specifically for SPM Trial Papers?
Because doing tons of SPM Trial Papers had help us score 9A+ in SPM , and received full JPA scholarships for our further studies .
In the next section, we will further explain how SPM Trial Papers can help you score well in SPM. So please keep reading.
2. Why do SPM Trial Questions?
Spm tips are very accurate, they said..
A lot of people out there are selling "SPM TIPS" at a very high price. They claim that their predictions on SPM questions are "PASTI KENA" and "100% SKOR"
But let me tell you the truth after spending hundreds on SPM TIPS.
Having spent lots of cash myself to buy those "SPM TIPS" , I can tell you the truth about it.
Have you ever wondered how those people predict what will come out in SPM? Do you think they have some "insider information" ?
Leaking question is a criminal offence , so it is highly unlikely they would do that.
Then how on earth did they predict the questions with such accuracy?
They actually analyse SPM Trial Question of all states, as well as past year questions. From there, they try to come out with an educated guess on what is going to come out in exam.
If you had look into the thick book of "SPM TIPS", there will be some sample questions that it said is going to appear in the real exam.
But don't be surprised, those questions are nothing but some SPM Trial Questions. They actually do this for good reason.
SPM Trial Papers are similar to real SPM questions.
SPM Trial Papers are set by experienced teachers from various states of Malaysia.
Some teachers who set SPM Trial Paper Questions are also involved in setting the real SPM questions. This means that the questions in SPM Trial Papers may be similar to those in the actual SPM exam.
Finish all Trial and Past Questions, and you will score.
Lastly, I want to emphasize that I cannot guarantee the accuracy of those SPM Tips . Personally, I am not against it, because it at least gives some idea what to study at the last minute.
However, bear in mind that SPM Trial Questions and Past Year Questions are the bases of their predictions. So, finishing all Trial questions and Past Year Questions will likely prepare you well for the exam.
Good Luck for your SPM exam!!
3. Who should I contact for any enquiries regarding the SPM Trial Paper Website?
If you are stuck on a question, feel free to ask us on FB Messenger . 🙂
We will be more than willing to guide you on your questions. 😊
Should you discover any issues in this website, or intend to request for papers not on the website, please do not hesitate to contact us.
Any feedback is highly appreciated on this website. It can help us improve it and serve you better. You can provide us with feedback anonymously with google form .
- FB Messenger: https://m.me/spmpaper.me
- Email: [email protected]
- Google Form: https://forms.gle/Cq8GzRRdYNp9W3oz9
4. Where to get the latest SPM Trial Paper and Soalan Percubaan resources?
This website is continuously updated with the latest SPM Trial Papers and Soalan Percubaan SPM from 2011-2021. Also, we share the latest SPM tips regularly on Facebook. Please like our Facebook Page to stay updated.
Here are a list of our social media handles:
- Facebook: https://www.facebook.com/spmpaper.me
- Instagram: @spmpaper
- Twitter: https://twitter.com/spmpaper
- Blog: https://spmpaper.me
- YouTube: https://www.youtube.com/channel/UCo5-hz-aF77DvSXD5_mYiIA
- Medium: https://medium.com/spmpaper
5. List of states of SPM Trial Papers and Soalan Percubaan SPM
We have collected SPM Trial Papers and Soalan Percubaan from year 2011 to 2021 from following states of Malaysia .
- Negeri Sembilan
- Kuala Lumpur
6. List of subjects of SPM Trial Papers and Soalan Percubaan SPM
Following subjects of SPM Trial Papers are available on this website.
- English language / Bahasa Inggeris
- Malay language / Bahasa Malaysia
- History / Sejarah
- Physics / Fizik
- Chemistry / Kimia
- Math / Matematik
- AddMath / Matematik Tambahan
- Economics / Ekonomi Asas
- Biology / Biologi
- Commerce / Perdagangan
- Science / Sains
- Geography / Geografi

Choose Your Test
Sat / act prep online guides and tips, every ap chemistry practice test available: free and official.
Advanced Placement (AP)

What's the best way to study for AP Chemistry? Practice, practice, practice. This article will provide you with links to every practice test and quiz for AP Chemistry that's available online , including full official and unofficial tests, shorter quizzes that cover each topic area, and other prep services you can access with a subscription!
Official AP Chemistry Practice Exams
Official exams are the best practice materials because they help you make accurate predictions of your performance on the real test. They will also get you used to the test format so that you're not caught off guard by the structure of the final exam.
Unfortunately, for AP Chemistry, most of the available official practice materials are for the old version of the test (pre-2013), but these can still be useful for practice. You should be able to get newer practice tests from your teacher or through review books.
I'd recommend starting with the unofficial practice materials listed later on in this article and then using official tests in the final stages of your studying. That way you'll be in the best position to estimate your ultimate AP score, and you won't squander limited resources.
Old Official Released Exams:
- 2008 AP Chemistry Exam
- 2002 AP Chemistry Exam (multiple choice only)
- 1999 AP Chemistry Exam
- 1994 AP Chemistry Exam
These official exams come from before 2013 (when significant changes were made to the AP Chemistry curriculum), so they're formatted slightly differently from the current test. They have 75 multiple-choice questions (there are now 60) and six free-response questions (there are now seven). There are also five answer choices for each multiple-choice question, whereas now there are only four.
The old AP Chemistry exam emphasized calculations and factual knowledge over a strong understanding of fundamental concepts and mastery of scientific practices. The questions on these tests will still help you practice your skills; just make sure you also use more recent materials for an accurate preview of what to expect on test day.
Current AP Chemistry Course and Exam Description (multiple choice practice included)
Go to page 216 of this course description to review sample multiple-choice and free-response questions for the current exam. This is not a full practice test (it only has 15 multiple-choice questions and two free-response questions total), but it's directly from the College Board, so it's the most accurate and up-to-date representation of the format and content of the test.
Free-Response Questions 2014-2021
These free response questions are from the most up-to-date version of the test. I would advise you to save most of them for later on in the year when you're more serious about practicing for the real AP exam. There are seven questions from each year.
Practice Tests from Your Teacher
Since there aren't any full AP Chemistry practice tests available online that reflect the current format of the exam (well, any that we can legally link to in this blog post), you can also ask your teacher for additional practice materials. AP teachers often have access to extra practice tests from the College Board that are available for classroom use.

Unofficial Free AP Chemistry Practice Exams
There are also a bunch of unofficial resources for AP Chemistry practice questions on various online learning platforms and independent sites. Few of these offer complete tests in the same format as the real exam, but they do provide a large repository of practice questions (mainly multiple-choice). These are great if you're looking for questions in specific topic areas or are studying early on in the year and want to avoid certain concepts that you haven't learned in class yet.
Just be wary of using these resources too much in your studying, and make sure you supplement them with official College Board materials at regular intervals. Unofficial practice questions often lack many of the nuances of real test questions. In a lot of cases, they will test straightforward factual recall whereas on the real test you'll have to do more complex analyses of unfamiliar experimental scenarios.
Varsity Tutors Diagnostic Tests
There are six diagnostic tests here with 50-60 questions each at varying difficulty levels. You'll also be timed as you take the tests so you can get a better sense of your pacing. Questions are multiple-choice only , so this won't give you any free-response practice. There are also tons of practice quizzes divided by topic, so you can use those to tackle tricky subject areas. We'd also recommend trying out their AP Chemistry practice app (it's free).

Albert Quizzes
This site includes quizzes for each concept broken down according to the major units of the course. This site will track your progress and tell you what percentage of questions you got right from each difficulty level (questions are organized into easy, medium, and hard categories). You can also access additional questions, including free-response, if you pay $25 to set up an account.
ScienceGeek
Here you'll find tons of review questions and activities, with lengthy practice quizzes for each unit of the course. This is one of the few resources that has non-multiple-choice questions that you can check automatically online.
This website offers 46 different multiple-choice practice tests (each is quite a bit shorter than the actual exam), and lots more downloadable practice questions organized by topic. You’ll be able to see whether or not you’ve answered correctly, as well as explanations of each question and answer.
PracticeQuiz
This quiz includes 58 free AP Chemistry practice multiple-choice questions.
There's a lot of stuff here, but if you're just looking for practice tests, you can find them at the end of the list of resources for each unit. There are multiple-choice and free-response tests for most units with accompanying answer keys.

Unofficial Paid/Subscription AP Chemistry Practice Exams
Here are some additional resources that will cost you some money, but they might be worth it because they provide full properly-formatted AP Chemistry practice tests.
Peterson's ($39-$49 per month)
- Two full-length practice tests (up to date format and content)
- Answer explanations
- Automatically tells you what you still need to study based on your results
- Also includes test prep for other AP exams
Sterling Test Prep (price varies)
On this site, you can buy individual practice tests for each topic in AP Chemistry. All of them together cost almost $100, so that might not be feasible, but you can get each specialized practice test for about $3 each (most have around 60 questions). You can also just get the Sterling book of practice questions , which many students seem to find helpful.
Review Books (price varies)
Review books can be great resources because many of them include instructions for how to structure your studying in addition to focused content overviews. For AP Chemistry, we recommend the 5 Steps to a 5 and Crash Course books. You can click on the link in the title of this section to read my full article on the best review books for this course.
Barron’s AP Chemistry , and The Princeton Review AP Chemistry Prep book are also excellent resources. In addition, each of these includes several different practice tests you can use to help you be prepared for test day!

How to Use AP Chemistry Practice Tests
Practice tests are great study tools for AP tests, and they're especially helpful for a subject like Chemistry that involves a lot of calculations and experimental analysis. In the next couple of subsections, we'll tell you how to use practice tests throughout the school year to prepare for the AP Chemistry exam.
First Semester: Using Practice Tests for Your Class
It's not practical to take full practice tests during the first semester of AP Chemistry because you haven't covered enough of the course material yet. Focus on official free-response questions and unofficial topic-specific practice tests that address aspects of the curriculum that you've learned already. It's a great idea to start early and do consistent reviews so that your knowledge base remains strong throughout the year.
Since chemistry is a subject that builds on the fundamental concepts learned in the first few months of class, it's vital that those early lessons are solidified in your memory. This way, more complex material that you learn in the second semester won't fly over your head. You can also consider getting a prep book; most of them have practice questions organized by chapter for selective review of different concepts.
Second Semester: Preparing for the AP Test
During your second semester, you can start to take full practice tests to predict your AP score-range. At this point, you've learned most of the material that will be covered in the class, so your scores should accurately reflect your abilities. Every time you take a full practice test, keep track of the areas where you need more practice.
As we mentioned at the beginning of this article, we would recommend saving the most up-to-date official practice materials for later in the semester so that the format of the current test stays fresh in your mind. As you take each test, circle any questions where you were unsure about your answer. Even if your choice ends up being correct, you should still plan to go over these concepts, so you don't feel shaky about them on the real AP test.
After you've finished taking the test (with realistic time constraints!), categorize your mistakes by topic area, and use their distribution to inform the rest of your studying. The purpose of taking practice tests is to diagnose your weaknesses so you can address them as efficiently as possible. DON'T go from one test to the next without taking a deeper look at what went wrong! You'll end up wasting your time, and your second practice test is unlikely to demonstrate much improvement.
Spend at least a couple of hours after each practice test doing practice problems and reviewing concepts that you didn't quite understand when they came up on the test. When you feel satisfied that you have a better handle on the background information and solution methods, you can take a second practice test to see how much you've improved.
The process as a whole should work like this:
- Take and score first practice test (4 hours)
- Evaluate mistakes (1.5 hours)
- Practice problems and study content to improve weak areas (2.5 hours)
- Take and score second practice test (4 hours)
- Reevaluate your progress and repeat steps if necessary!
One cycle through all of these steps will take around 8-10 hours, but you can repeat the steps ad infinitum until you're satisfied with your scores. If you find that you're not improving between practice tests, you'll need to reevaluate your study strategy. To master a complex subject like chemistry, you need to have a strong grasp of the fundamental concepts. Then, you can build on that understanding for more difficult problems. Be sure to do lots of practice problems where you're required to justify your answers!

Practice tests are essential study tools, especially for AP Chemistry. Doing practice problems that align with the format and content of the real exam will help you to gain familiarity with the material and feel less stressed on test day.
Try to start your studying with unofficial practice tests to build up a strong knowledge base, and then move onto official practice tests when you're ready to estimate your real AP score level.
As you take practice tests, assess your mistakes and plan out your study time according to which areas need the most work. Make sure you start with basic concepts and then work your way up to more complex problems. Use these practice materials to detect gaps in your knowledge, and fill them before you take the test!

What's Next?
Want to learn a bit more about the test before you start practicing? Read our expert guide to the AP Chemistry exam , which includes sample questions and study tips!
If you want a complete overview of the concepts that will be covered on the test, check out our ultimate study guide for AP Chemistry . We also have a specific guide to balancing chemical equations , if that's something you need extra help with.
Wondering how you can see chemistry in action in your day-to-day life? If you're looking for chemistry you can taste, we recommend this article on vegetable oil substitutes . If you're thinking more along the lines of something to play with, we have three different recipes for homemade slime . And if you need to clean things up afterwards, be sure to read our article on muriatic acid and how to safely use it .
These recommendations are based solely on our knowledge and experience. If you purchase an item through one of our links, PrepScholar may receive a commission.
Samantha is a blog content writer for PrepScholar. Her goal is to help students adopt a less stressful view of standardized testing and other academic challenges through her articles. Samantha is also passionate about art and graduated with honors from Dartmouth College as a Studio Art major in 2014. In high school, she earned a 2400 on the SAT, 5's on all seven of her AP tests, and was named a National Merit Scholar.
Student and Parent Forum
Our new student and parent forum, at ExpertHub.PrepScholar.com , allow you to interact with your peers and the PrepScholar staff. See how other students and parents are navigating high school, college, and the college admissions process. Ask questions; get answers.

Ask a Question Below
Have any questions about this article or other topics? Ask below and we'll reply!
Improve With Our Famous Guides
- For All Students
The 5 Strategies You Must Be Using to Improve 160+ SAT Points
How to Get a Perfect 1600, by a Perfect Scorer
Series: How to Get 800 on Each SAT Section:
Score 800 on SAT Math
Score 800 on SAT Reading
Score 800 on SAT Writing
Series: How to Get to 600 on Each SAT Section:
Score 600 on SAT Math
Score 600 on SAT Reading
Score 600 on SAT Writing
Free Complete Official SAT Practice Tests
What SAT Target Score Should You Be Aiming For?
15 Strategies to Improve Your SAT Essay
The 5 Strategies You Must Be Using to Improve 4+ ACT Points
How to Get a Perfect 36 ACT, by a Perfect Scorer
Series: How to Get 36 on Each ACT Section:
36 on ACT English
36 on ACT Math
36 on ACT Reading
36 on ACT Science
Series: How to Get to 24 on Each ACT Section:
24 on ACT English
24 on ACT Math
24 on ACT Reading
24 on ACT Science
What ACT target score should you be aiming for?
ACT Vocabulary You Must Know
ACT Writing: 15 Tips to Raise Your Essay Score
How to Get Into Harvard and the Ivy League
How to Get a Perfect 4.0 GPA
How to Write an Amazing College Essay
What Exactly Are Colleges Looking For?
Is the ACT easier than the SAT? A Comprehensive Guide
Should you retake your SAT or ACT?
When should you take the SAT or ACT?
Stay Informed
Get the latest articles and test prep tips!
Looking for Graduate School Test Prep?
Check out our top-rated graduate blogs here:
GRE Online Prep Blog
GMAT Online Prep Blog
TOEFL Online Prep Blog
Holly R. "I am absolutely overjoyed and cannot thank you enough for helping me!”
Sign up now
Fill in the form below to sign up:.
By clicking on the "Sign up" button, you agree to our Terms and Privacy Policy
Already a member? Login
Password Reset
Enter your phone number to reset password:.
- Reset by Phone Number
- Reset by Email
A 6-digit reset code will be sent to your phone number
Cancel? Return to login
A 6-digit reset code will be sent to your email
Login to your account
Forgot Password?
Sign up as a new member

Paper 1 | Objectives | 50 Questions
WASSCE/WAEC MAY/JUNE
Type: Question Paper
Answers provided
- DESCRIPTION
- STUDENTS REVIEWS (0)
No description provided
This paper is yet to be rated
Yet to recieve reviews
Add a review
Share This Paper
Recent posts, mena scholarship for africa 2022.
Middle East and North Africa MENA Scholarship Program (MSP) initiative provides scholarships
8 Proven Tips to Pass Any Exams Without Studying Hard
A guide to passing your exams without studying hard, how to pass any test and top your class
Free Online IT Courses With Certificates For You in 2021
Get free IT courses with certificates online for your skills upgrade in this era of Covid-19.
Healthy Eating Tips For Exam Diet
Apply tips for stress-free eating before and during exams and what to avoid for good grades.
Eating Exam Diet For Good Grades
Eat well during in test days for good grades. How can you plan your exam diet with good grades in mind?
Paper 1 | Objectives
Recommended for you.
Agricultural Science
NECO - BECE
English Language
Mathematics (Core)
Write to us
Past Questions
Daily Grove
Year :
Title : .
Preview displays only 10 out of the 50 Questions

- Form 1 Mathematics Notes
- Form 2 Mathematics Notes
- Form 3 Mathematics Notes
- Form 4 Mathematics Notes
- Form 1 Mathematics Topical Questions and Answers
- Form 2 Mathematics Topical Questions and Answers
- Form 3 Mathematics Topical Questions and Answers
- Form 4 Mathematics Topical Questions and Answers
- Form 1 Functional Writing Notes
- Form 2 Functional Writing Notes
- Form 3 Functional Writing Notes
- Form 4 Functional Writing Notes
- Poetry Notes
- Grammar Notes
- Oral Literature Notes
- Oral Skills Notes
- Guide to Blossoms of the Savannah Summarized Notes - Easy Elimu
- A Doll's House
- The Pearl Study Guide
- Memories We Lost and Other Stories Study Guide
- Inheritance Study Guide
- A Silent song and Other Stories Guide
- Fathers of Nations Guide
- An Artist of the Floating World Guide
- The Samaritan Guide
- Sarufi na Matumizi ya Lugha
- Isimu Jamii Notes
- Fasihi Notes
- Ushairi Notes
- Mwongozo wa Kuandika Insha
- Tumbo Lililoshiba na Hadithi Nyingine
- Mwongozo wa Kigogo
- Mwongozo wa Chozi La Heri - Chozi la Heri Notes PDF
- Mwongozo wa Bembea ya Maisha - Bembea ya Maisha Notes PDF
- Mwongozo wa Nguu za Jadi
- Mwongozo wa Mapambazuko ya Machweo na Hadithi Nyingine
- Biology Form 1 Notes
- Biology Form 2 Notes
- Biology Form 3 Notes
- Biology Form 4 Notes
- Biology Essays
- Form 1 Biology Topical Revision Questions and Answers
- Form 2 Biology Topical Revision Questions and Answers
- Form 3 Biology Topical Revision Questions and Answers
- Form 4 Biology Topical Revision Questions and Answers
- Form 1 Chemistry Notes
- Form 2 Chemistry Notes
- Form 3 Chemistry Notes
- Form 4 Chemistry Notes
- All Chemistry Practicals Notes for KCSE and MOCKS
- Form 1 Chemistry Topical Revision Questions and Answers
- Form 2 Chemistry Topical Revision Questions and Answers
- Form 3 Chemistry Topical Revision Questions and Answers
- Form 4 Chemistry Topical Revision Questions and Answers
- IRE Form 1 Notes
- IRE Form 2 Notes
- IRE Form 3 Notes
- IRE Form 4 Notes
- Physics Form 1 Notes
- Physics Form 2 Notes
- Physics Form 3 Notes
- Physics Form 4 Notes
- CRE Form 1 Notes
- CRE Form 2 Notes
- CRE Form 3 Notes
- CRE Form 4 Notes
- Geography Form 1 Notes
- Geography Form 2 Notes
- Geography Form 3 Notes
- Geography Form 4 Notes
- History Form 1 Notes
- History Form 2 Notes
- History Form 3 Notes
- History Form 4 Notes
- Business Studies Form 1 Notes
- Business Studies Form 2 Notes
- Business Studies Form 3 Notes
- Business Studies Form 4 Notes
- Home Science Form 2 Notes
- Home Science Form 3 Notes
- Home Science Form 4 Notes
- Home Science Form 1 Notes
- Agriculture Form 1 Notes
- Agriculture Form 2 Notes
- Agriculture Form 3 Notes
- Agriculture Form 4 Notes
- Agriculture KCSE 2019 Project
- Computer Studies Form 1 Notes
- Computer Studies Form 2 Notes
- Computer Studies Form 3 Notes
- Computer Studies Form 4 Notes
- KCSE 2017 Reports
- 2018 Pre-Mocks
- 2019 Pre-Mocks
- 2022 Pre Mocks
- 2021/2022 Pre-Mock Past Papers
- 2023 Pre Mocks
- 2017 Mock Past Papers
- 2019 Mock Past Papers
- 2020 Mock Past Papers
- Mock Exam Papers 2021/2022 - Easy Elimu
- Mock Exam 2022 Questions and Answers
- Alliance Boys High School
- Maranda High School
- Form 1 Past Papers
- Form 2 Past Papers
- Form 3 Past Papers
- Form 4 Past Papers
- 2019 KCSE Prediction Papers
- 2020 KCSE Prediction Papers
- 2021 KCSE Prediction Papers
- 2022 KCSE Prediction Questions and Answers - EasyElimu
- KCSE Prediction 2023
- 2020 Post Mock Past Papers
- 2021/2022 Post Mocks
- 2023 Post Mocks
- Play Group: Activities, Homework and Syllabus
- 2023 PP1 Exams
- 2023 PP2 Exams
- Grade 1 Notes
- 2023 Grade 1 Exams
- Grade 2 Notes
- 2023 Grade 2 Exams
- Grade 3 Notes
- 2023 Grade 3 Exams
- Grade 4 Notes
- 2023 Grade 4 Exams
- Grade 5 Notes
- 2023 Grade 5 Exams
- Grade 6 Notes
- KPSEA Exams
- 2023 Grade 6 Exams
- Class 6 : Notes, Revision Papers and Syllabus
- Class 7 : Notes, Revision Papers and Syllabus
- Class 8 Notes
- 2023 Class 8 Exams
- 2023 Kcpe Prediction
- Grade 7 Notes
- 2023 Grade 7 Exams
- Pre Mock Exams 2024
- The New EasyElimu Website
- Form 4 End Term 1 Exams
- Form 3 Exams 2024
- Form 2 End Term 1 Exams
- Form 1 End Term 1 Exams
- All Kiswahili setbook guides
- All English setbook guides
- Form 1 - 4 High School Notes
Chemistry Paper 3 Questions and Answers - KCSE 2020 past papers
« Previous Topic Chemistry Paper 1 Questions and Answers - KCSE 2020 past papers
Next Topic » Chemistry Paper 2 Questions and Answers - KCSE 2020 past papers
THE KENYA NATIONAL EXAMINATIONS COUNCIL Kenya Certificate of Secondary Education 233/3 CHEMISTRY Paper 3 (PRACTICAL)
Instructions to candidates
- Write your name and index number in the spaces provided above
- Sign and write the date of examination in the spaces provided above
- Answer all the questions in the spaces provided in the question paper
- You are not allowed to start working with the apparatus for the first 15 minutes of the 2¼ hours allowed for this paper. This time is to enable you to read the question paper and make sure you have all the chemicals and apparatus that you may need.
- All working must be clearly shown where necessary
- Non-programmable silent electronic calculators and KNEC mathematical tables may be used.
- This paper consists of 8 printed pages.
- Candidates should check the question paper to ascertain that all the pages are printed as indicated and that no questions are missing.
- Candidates should answer the questions in English.
- 5.3g solid A , sodium carbonate;
- Solution B , hydrochloric acid. You are required to determine the:
- Molar heat of the solution of solid A;
- Concentration of the hydrochloric acid, solution B.
- PROCEDURE I
- Using a burette, place 30.0 cm 3 of distilled water in a 100ml plastic beaker. Stir the water with a thermometer and measure its temperature after every half-minute interval. Record the readings in Table 1.
- On the grid provided, plot a graph of temperature (vertical axis) against time.(3 marks)
- Determine from the graph, the temperature change, AT ( mark)
- number of moles of solid A used. (RFM = 106)
- molar enthalpy of solution, ΔH soln and show the sign of ΔH soln (Assume that for the solution, density = 1.0gcm 3 and specific heat capacity = 4.2 Jg -1 K -1 ) (2 marks)
- Fill a burette with solution B.
- Transfer all of the mixture in the 100 ml plastic beaker from procedure I into a 250 ml volumetric flask. Add distilled water to make up to the mark and shake. Label the mixture as solution A.
- Repeat Procedure II. step (iii) and complete Tables 2 and 3.
- concentration, in moles per litre, of sodium carbonate in solution A. RFM 106 (1 mark )
- number of moles of sodium carbonate in 25.0 cm 3 of solution A (1 mark)
- number of moles of hydrochloric acid in the total volume, V 1 + V 2 of solution B. (1 mark)
- concentration, in moles per litre, of hydrochloric acid in solution B. (1 mark)
- Describe the appearance of solid D. (1 mark)
MARKING SCHEME
- Penalize ½ mk ONCE for any space not filled subject to at least 5 readings given, otherwise penalize Fully, ie, award 0 mk
- Penalize ½ mk ONCE for unrealistic temperature readings of less than 10ºC and /or greater than 40°C for t=0 to t = 1½ minutes and for temperature reading(s) of greater than 50°C for readings t= 21⁄2 to t= 5 minutes
- Penalize ½ mk if ALL temperature readings are consistent
- Penalize ½ mk ONCE if temperature reading at t=2½ minutes is below or equal to the initial temperature reading at t = 1½ minutes
- If two or more rows of temperature readings are given, Penalize ½ mk on Complete table. However, for use of decimal, accuracy and trend to be credited, the two or more sets of rows MUST meet the two criteria provided for each case. NOTE! Any reading after t=2½ minutes falls below room temperature(t=1½ mins) Penalize ½ mk
- All temperature readings MUST be recorded consistently either as a whole number, to 1dp or 2 dps, otherwise, penalize fully.
- If readings are recorded to 2 dps, then it should be .00, .025, .50,0.75, otherwise, penalize fully
- If readings are recorded to 1 dp then it should be .0 or .5, otherwise, penalize fully
- If Within +2.0ºC of s.v award ½ otherwise award O mk for accuracy NOTE . If the Candidate's Value earns the mark, tick (v) the reading on the table.
- If no school value is given by the teacher or where the S.V given is unrealistic, Sample and average the candidates' values at t= 1½ mins Per Session that are close. However, if Candidates' Values are too varied, then use KNEC value of 22.5°C as the S.v (at t=1½ Mins)
- Award the first ½mk if temperature reachings from t=½ min to t= 1½ mins are constant
- a Continuous rise to a Maximum followed by a constant at maximum and then a continuous drop.
- a Continuous rise up to a maximum followed by a continous drop
- a Constant at the maximum followed by a continuous drop or
- a sudden rise followed by a Continuous drop
- Penalize. Fully for inverted axes.
- units may or may not be used but if given MUST be Correct, otherwise Penalize Fully for wrong units. NOTE : Both axes. Must be marked accordingly before posting the mark for labelling axes.
- Area Covered by the actual plots must be at least half the grid provided ie, 7 big squares vertically and 9 big squares horizontally.
- Scale intervals must be consistent ON EACH 9 the axes:
- Scale chosen MUST be able to accommodate all the readings whether plotted or not.
- Award for correct scale even if the axes are inverted.
- Penalize Fully if any of the above conditions is not met.
- If 10 or 9 are correctly plotted, award 1mk.
- If only 8 to 5 are correctly plotted, award ½mk.
- Accept correct plots even if axes are inverted and award accordingly
- If any scale intervals are inconsistent, mark the plots, if any - within the first correct interval and treat all the other plots as wrong and award accordingly.
- Mark all the plots on the graph with either a tick (√) or a Cross (X).
- Accept 2 Straight lines Correctly extrapolated up to t = 2 minutes with the initial line being horizontal and the other line a dropping one for 1mk.
- Accept 2 lines not extrapolated and not joined with the initial line being horizontal and the other a dropping one for ½ mk.
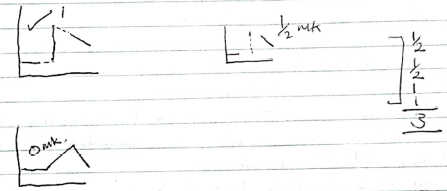
- Accept the correct value of ΔT from the correct graph with or without showing on the graph even if the axes are inverted for 1mk.
- if shown on the graph Correctly but Δt is either missing or wrong award ½mk for correct showing on the graph.
- Reject reading and showing from a wrong graph ie, a graph that has score 0 mk for shape/lines.
- The units may or may not be shown but it shown Must be correct, otherwise Penalize ½ for wrong unit.
- If the expression is NOT shown but answer is correct, award only ½
- The units may or may not be shown, but if shown MUST be correct, otherwise penalize ½mk for wrong units used.
- The 5.3 and lo6 MUST be transferred intact, otherwise penalize FULLY
- Accept correct transfer of ans (C) and d(i) even if rejected in (C) and d(i) above.
- Penalize ½mk for wrong transfer of either ans. (C) or ans.d(i) or BOTH otherwise Penalize FULLY for strange figures used.
- Penalize ½ mk on final correct answer if the negative sign and for the unit is or are wrong. or missing
- Penalize ½mk for wrong answer if arithmetic error is outside ±2 units in the 3rd digit.
- Penalize FULLY for unrealistic find answer if outside the range of -12.6 kJmol -1 to -28.1.kjmol -1 PROCEDURE II
- Complete table with BOTH titrations done, award( 1 mk)
- Wrong arithmetic
- Inverted table
- unrealistic titre(s) (below 1.0cm 3 or in hundreds.)
- Penalize ½mk for each of the above mistakes to a maximum of ½mk Cile (Penalize ½ mk once)
- If No TITRATION is done, award 0mk for complete table as well as for EACH of the other Marking points.
- Accept either 1 or 2 dps used consistently otherwise Penalize FULLY
- If 2 dps are used the second dip should be a "0" or "5" otherwise Penalize Fully.
- Accept inconsistency in the use of Zeros as INITIAL burette reading(s) i.e 0,0.0,0.00
- If at least one titre is within ±0.10cm 3 of the S.V award 1mk
- If no titre is within ±0.10cm 3 to±0.10cm 3 of the S.V award but at least one is within ±0.20cm 3 of the s.v award ½mk
- If there was wrong arithmetic / subtraction in the table, compare the S.v with the worked out CORRECT titre(s) and award accordingly.
- write down all the candidate's' average titres per session and sample those that are close and average them to get the s.v.
- If the candidates' average titres are too varied then use the KNEC Value 16.5cm 3 as the s.v.
- Tick (√) the candidate's chosen titre on the table, if it earns a mark before posting the mark.
- If both titrations are done are consistent and are averaged correctly, award ½mk
- If both titrations are done, are inconsistent and yet averaged, award 0 MK for both Principles of averaging and final accuracy.
- Answer should be expressed to at least 2d.ps unless it works out exactly to 1dp or whole number otherwise Penalize Fully.
- If no working is shown but answer given is correct, credit FULLY
- Penalize FULLY for wrong arithmetic it error is outside ± 2 units in the 2nd dp
- Penalize Fully if no working is shown and answer given is wrong!
- If average titre is within ±0.1 cm 3 F S.V award look
- If the average titre is not within ±0.1cm 3 of s.v but within ±0.20cm 3 of s.v award ½
- If the average titre is beyond ±0.2cm3 of the s.v, award 0 mk.
- Calculations Molarity of soln A = Ans. d(i) above x 1000 250 =correct ans. ½ OR Conc of Solution A = 1000 x 5.3 = 21.2gdm 3 250 Molarity of solution A = 21.2 = 0.2 M 106 NOTE If there is arithmetic error in the intermediate answer of 21.2 g/dm 3 then award ½mk for the correct expression and penalize FULLY for the final answer . OR Molarity of solution A = 1000 x 5.3 = 0.2M 25 x 106
- Moles of Na 2 CO 3 in 25 cm3 of solution A = Ans. f(i) above x 25 = correct ans 1000 OR 5.3 x 25 = 0.05moles 250x106 OR Ans d(i) above x 25 = correct ans 250
- Mols of HCl solution B in V 1 +V 2 = Ans. f(ii) above x 2 = Correct ans
- Answer d(i) and f(i) to f(iii) MUST be transferred INTACT otherwise Penalize back for wrong transfer in each case. However, Penalize FULLY for Strange figure in each case.
- Answer f(i) and f(iv) should be expressed to at least 3dps, unless they work out exactly to 2d.p or 1.d.p. Otherwise, penalize ½mk for round off in each case.
- In answer f(i) to f(iv) units may or may not be given but if given must be Correct otherwise penalize ½ in each case for wrong units used.
- Ans. f(iv) should be in the range of 0.1M to 0.5M otherwise Penalize FULLY for unrealistic answer.
- Accept white solid/ white powder for only ½mk
- Reject solid on its own
- Colourless crystals ( reject)
- Reject white ppt or white solution
- Credit fully for inference in b(i) and b(ii) even if observation has only scored ½
- Penalize fully for any contradictory functional group in the inferences in each case.
Download Chemistry Paper 3 Questions and Answers - KCSE 2020 past papers .
Why download.
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
Related items
- Sign Language Paper 3 Questions and Answers - KCSE 2022 Past Papers
- Electricity Paper 2 Questions and Answers - KCSE 2021 Past Papers
- Music Paper 3 Questions and Answers - KCSE 2022 Past Papers
- Biology Paper 3 Questions - Kapsabet Boys Post Mock 2023 Exams
- Physics Paper 3 Questions - Kapsabet Boys Post Mock 2023 Exams
access all the content at an affordable rate or Buy any individual paper or notes as a pdf via MPESA and get it sent to you via WhatsApp
What does our community say about us?
Join our community on:.

- KCSE Revision Questions
- Privacy Policy
- Mobile App Privacy Policy
- High Schools in Kenya
- Teacher Resources
- Questions and Answers
- Online Tuition and Classes in Kenya
Copyright © 2022 EasyElimu
- Download Our App
- Alevel Schemes
- O-Level Schemes
- English Medium School
- Swahili Medium School
- Nursery / Awali
- All Schemes Links
- Lesson Plan
- Log Book For Secondary
- Log Book For Primary
- Secondary Notes
- Primary Notes
- Form Six NECTA Exams
- Form Four NECTA Exams
- Form Two NECTA Exams
- STD VII NECTA Exams
- STD IV NECTA Exams
- Secondary Exams
- Primary Exams
- Secondary Topical Qns.
- Secondary Topical Exams
- Primary Topical Qns.
- Secondary Regional Exams
- Primary Regional Exams
- Practical Exams & Notes
- LITERARY WORKS
- New Format Exams
- Brain Ignaiter
- Literary Works
- Methali Za Kiswahili
- Vitendawili Vya Kiswahili
- FORM VI NECTA RESULTS 2023
- Form Five Selection 2023
- Form One Selection 2022
- Form V Selecton-2022
- Form Four Necta Results 2022
- Form Two Necta Results 2022
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL OF TANZANIA
CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
032/1 CHEMISTRY 1
(For Both School and Private Candidates)
Time: 3 Hours Year : 2021
Instructions
l. This paper consists of sections A, B and C with a total of fourteen (14) questions.
2. Answer all questions in sections A and B and one (1) question from section C.
3. Sections A and C carry fifteen (15) marks each and section B carries seventy (70) marks.
4. Cellular phones and any unauthorised materials are not allowed in the examination room.
5. Write your Examination Number on every page of your answer booklet(s).
6. The following constants may be used.
Atomic masses: H = 1, O =16, C =12, N = 14, Na =23, Ca = 40, Cl = 35.5, Pb= 207.
1 Faraday = 96,500 coulombs.
Standard pressure = 760 mm Hg.
Standard temperature = 273 K.
SECTION A ( 15 Marks)
Answer all questions in this section.
l. For each of the items (i) — (x) , choose the correct answer from among the given alternatives and write its letter beside the item number in the answer booklet provided.
(i) Which among the following sets of materials can cause fire outbreak?
- Oxygen, carbon dioxide and fuel
- Oxygen, heat and fuel
- Oxygen, foam and fuel
- Oxygen, heat and foam
(ii)What type of fire occurs in vapour air mixture over the surface of flammable liquids?
- Class A
- Class B
- Class D
(iii)Which one of the following processes is a chemical change?
- Butter melts on warm toast
- Water evaporates from the surface
- Juice in a bottle freezes
- Food scrap turns into compost
- Wet cloth dries
(iv) The simplest formula of a compound formed when combining 36 g of magnesium and 14 g of nitrogen is:
- Mg 3 N 2
(v)What is the IUPAC name for H 2 S0 4 ?
- Sulphuric acid
- Sulphuric (VI) acid
- Hydrogen sulphate
- Dihydrogen sulphate
- Hydrogen tetrasulphate
(vi) What type of chemical reaction is represented by the equation Zn(s) + 2HCl(aq) + H 2 (g)?
- Displacement reaction
- Combination reaction
- Precipitation reaction
- Decomposition reaction
- Redox reaction
(vii) What does the random movement of pollen grains suspended in air demonstrates?
- Matter is lighter in nature.
- Matter is solid in nature.
- Matter is particulate in nature.
- Matter is gaseous in nature.
- Matter is wave in nature.
(viii)"Organic matter is among the components of soil." Which role does it play?
- Improving water infiltration of the soil.
- Accelerating break down of organic matter.
- Reserving nutrients thus providing soil fertility.
- Converting of nitrogen into nitrates.
- Providing a room for organic material such as nylons.
(ix) Which of the following sets represents isotopes of an element?
(x)What is to be considered when choosing the best method to extract a particular metal from its ore?
- The metal 's economic value.
- Its availability in an area.
- The metal's ore impurities.
- How it reacts with other materials.
- The metal's shininess.
2. Match the uses of First Aid Kit items in List A with the respective items in List B by writing the letter of the correct response besides the item number in the answer booklet provided.
SECTION B ( 70 Marks)
3. (a) Different salts behave differently when heated. Use balanced chemical equations to show how carbonates and sulphates behave when subjected to heat.
(b) Ammonium nitrate does not react like other nitrates (with exception of the alkali metal nitrates). Explain this fact with the aid of chemical equations. (7 marks)
4. (a) A Form IV student was asked to react phosphate ion and sodium ion forming compound W. Suggest the IUPAC name of W and find the oxidation state of phosphorous in W.
(b) Calculate the percentage composition of lead in the compound Pb(N03)2. (7 marks)
5. (a) How can the society minimize the energy loss encountered in the use of charcoal and fire wood? Give two points.
(b) State whether the following processes are exothermic or endothermic.
(i) Dissolving ammonium chloride in water.
(ii) Photosynthesis.
(iii) Combustion reactions.
(iv) Mixing water and potassium chloride.
(v) Mixing water and strong acids such as concentrated sulphuric acid.
6. (a) Briefly explain the concept of scientific procedure.
(b) What is the importance of the scientific procedure in daily life? Give two points.(7 marks)
7. Use the following components to construct a diagram of water cycle: clouds, animal, water in the soil, rain, plants, water spring, rivers, lakes and water vapour in the atmosphere.(7 marks)
8. Suppose that two gas jars; one containing gas "A" and another one containing gas "B" are made available to you. Gas "A" is used in hardening of margarine whereas gas "B" is used by mountain climbers.
(a) What tests will you conduct to identify each of the two gases?
(b) Give two physical properties and three chemical properties that can be used to distinguish gas "A" from gas "B". (7 marks)
(a) Briefly explain the differences in the results of experiments A and B.
(b) What factors can be adjusted to increase the yield of the product? (7 marks)
10. If 2.0 g of CaC0 3 were reacted with excess dilute HCI acid; (a) what volume of C02 would be given out at s.t.p?
(b) Calculate the mass of C0 2 produced. (7 marks)
11. (a) In three points, differentiate homogenous mixtures from heterogeneous mixtures.
(b) By giving four points, justify the fact that common salt is a compound. (7 marks)
12. (a) Give three ways in which environmental destruction is likely to occur during extraction of metals.
(b) The following equations represent the steps involved in the conversion stages of iron extraction in Bussener converter. Arrange the equations in chronological order from the first step to the last by writing the respective letter so as to get a complete explanation of the conversion stage.
V: 2Cu 2 0(S) + Cu 2 S(S)→ 6Cu (l) + S0 2 (g)
W: FeO (l) + SiO 2 (g) → FeSiO 3
X: 2Cus(s) +30 2 (g) → 2Cu20(s) +2S0 2 (g)
SECTION C ( 15 Marks)
Answer one (1) question from this section.
13. By giving six points, explain how to maintain soil fertility of a particular area.(15 marks)
14. How electrolysis is applied in industries? Describe by giving six points. (15 marks
For Call,Sms&WhatsApp: 255769929722 / 255754805256
Whatsapp us now for any query.
Get Schemes Of Work In Zero Waiting Time
Biology Made Simple Step 4 Ready On The Market! Get Your Copy Now
Chemistry Made Simple Step 1
Chemistry Made Simple Step 2
Chemistry Made Simple Step 3
Chemistry Made Simple Step 4
- Grade 5 Scholarship |
- O/L Past papers |
- 2024 O/L Model Papers |
- Royal College |
- Western Province |
- Online Book Shop
- Combined Maths
- Agricultural Science
- Business Studies
- Business Statistics
- Christianity
- Buddhist Civilization
- Drama and Theatre
- Political Science
- General English
- Agriculture
- Home Economics
- Indian History
- Sri Lankan History
- Grade 11 Papers
- Grade 10 Papers
- Grade 09 Papers
- Grade 08 Papers
- Grade 07 Papers
- Civic Education
- English Language
- Mathematics
- Second Language
- Sinhala Language
- Tamil Language
- Western Music
- Scholarship Exam Past Papers
- Scholarship Model Papers
- Environment
- Catholicism
- Grade 11 English Medium
- Grade 10 English Medium
- Grade 09 English Medium
- Grade 08 English Medium
- Grade 07 English Medium
- Grade 06 English Medium
- Sinhala Medium
- Sinhala Medium Answers
- English Medium
- English Medium Answers
- Tamil Medium
- Sinhala Medim Papers
- English Medium Papers
- Tamil Medium Papers
- Sinhala Medium Marking
- English Medium Marking
- Tamil Medium Marking
- Western Province
- North Western Province
- Southern Province
- North Central Province
- Central Province
- Sabaragamuwa Province
- Royal College
- Ananda College
- D.S.Senanayake
- Devi Balika
- Nalanda College
- Rathnavali Balika
- Visakha College
- Grade 11 Textbooks
- Grade 10 Textbooks
- Grade 9 Textbooks
- Grade 8 Textbooks
- Grade 7 Textbooks
- Grade 6 Textbooks
- WIKI Forum! Join
2021 A/L Chemistry Past Paper | Sinhala Medium
Gce advanced level 2021 chemistry sinhala medium paper quick download.

2021 AL Chemistry Past Paper Sinhala Medium

2021 A/L Chemistry Past Paper in Sinhala Medium
Download 2021 A/L Chemistry paper in Sinhala Medium. You can download Sinhala medium 2021 AL Chemistry past exam paper as a PDF File.
Exam – Advanced Level Year – 2021 Medium – Sinhala Medium Exam Date – 2022.02.17
Download the Previous A/L Chemistry exam papers :
- 2020 Exam Paper 2020 Paper Marking
- 2019 Exam Paper 2019 Paper Marking
- 2018 Paper Marking
- 2017 Exam Paper 2017 Paper Marking
- 2016 Exam Paper 2016 Paper Marking
- 2015 Exam Paper 2015 Paper Marking
- 2014 Exam Paper 2014 Paper Marking
- 2013 Exam Paper 2013 Paper Marking
- 2012 Exam Paper 2012 Paper Marking
- 2011 Exam Paper
- 2010 Exam Paper 2010 Paper Marking
- 2009 Exam Paper
- 2008 Exam Paper
- 2007 Exam Paper
- 2006 Exam Paper
- 2005 Exam Paper
- 2004 Exam Paper
- 2003 Exam Paper
- 2002 Exam Paper
- 2001 Exam Paper
- 2000 Exam Paper
- 1999 Exam Paper
- 1998 Exam Paper
- 1997 Exam Paper
- 1996 Exam Paper
- 1995 Exam Paper
- 1994 Exam Paper
- 1993 Exam Paper
- 1992 Exam Paper
- 1991 Exam Paper
- 1990 Exam Paper
- Join Forum: https://forum.pastpapers.wiki/
- Contribute Us: https://forms.gle/Z7ZV8vixJBhujz1x8
Past Papers WIKI has been the most reliable source of A/L previous papers and other educational materials in Sri Lanka for over four years. The website includes Provincial Papers, Province Educational Materials, and Resources that includes syllabus, question papers, Teacher’s resources, Notes, and a lot more. All of the stuff available here is completely free, and it is presented in the most user-friendly manner possible to ensure that you have no problems.
Download more past papers from the Department of Examinations website.
You can add a comment below or contact us on Facebook . Share this resource with your friends!
Last Update : 2020/11/26
2021 A/L BC Past Paper | Sinhala Medium
2021 a/l biology past paper | english medium.
Pastpapers WIKI
Pastpapers wiki is a free resource site for O/L and A/L Students In Sri Lanka. Pastpapers wiki was founded in October 2019 by Education Resources.lk. The main goal of this site is to provide Past Papers, Marking Schemes, Notes, and other resources that allow students to improve their knowledge.

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Buy Books Online

Pastpapers wiki is a free resource site for O/L and A/L Students In Sri Lanka. Past Papers WiKi was founded in October 2019 by Education Resources.lk. The main goal of this site is to provide Past Papers, Marking Schemes, Notes, and other resources that allow students to improve their knowledge.
https://forum.pastpapers.wiki/ Email: [email protected]
Become a Volunteer – Help Others 🙋♂️
We are currently looking for volunteers who are willing to share their expertise and contribute to our community by sharing educational materials in our forum. We’re looking for individuals who can help us create and share educational materials such as articles, videos, and social media posts that will inspire and educate others.
Share Past Papers 📝 | Help Others 🤝 Join With: https://forum.pastpapers.wiki/

Disclaimer of Past Papers WiKi
This website is continued for your personal appreciation or educational purposes only . All Content of this website is published by extracting the information from online sources such as official government websites, social media, other websites, etc. The copyrights of these contents belong to the responsible owners . If a modification will happen in this information, our website does not assume any responsibility. If you have any questions or suggestions, please contact us.
– Mobile No: 071-8540371 – Email: [email protected]
- Science stream
- Commerce stream
- Technology stream
- Arts Stream
- Common Subjects
- WIKI Forum!
Copyright 2019 -2021 © All rights reserved.
Inorganic Chemistry Essay Questions and Answers in Sinhala Medium
You can browse new model questions for inorganic chemistry here. We will add new questions continuously. Check the page daily to see more questions. Answers and explanations are given with the answer.
Questions are presented according to the added date. So, new questions are listed first.
If you have a problem with these questions or any other question in chemistry, email it me. [email protected]

- N = MgO + MgS
- O = Mg(OH) 2

- X = Ca(NO 3 ) 2 or Sr(NO 3 ) 2

- A = Mg(OH) 2

- A = H 2 O 2

- Login / Register

- International
- Entrepreneurship
- Find Scholarships
- Inter Studies
Join Our Newsletter
Join our subscribers list to get the latest news, updates and special offers directly in your inbox
2021 NABTEB GCE Chemistry (Essay & OBJ) Answers [2nd December]
Get free live 2021 nabteb gce chemistry (chem) objectives and essay/theory questions and answers free of charge | nabteb nov/dec gce free chemistry (chem) obj & essay questions and answers expo room (2nd december, 2021). nabteb nov/dec gce 2021 free chemistry obj & essay (chem) question and answer room thursday 2nd december, 2021chemistry (9:00am-11:30am) a. nabteb nov/dec gce chemistry theory (essay) answers 2021: answers loading........................................ b. nabteb nov/dec gce chemistry theory (essay) answers 2021: answers loading........................................ to subscribe for nabteb nov/dec gce chemistry answers via link only just go out and buy mtn cards of n600 (200 + 200 + 200 = 600) go to your message, type the card pins correctly and send to 08107431933. don't call, just text, if the cards pins are valid, a reply will be sent to you confirming that you have been subscribed. relax and wait for your answers 30minutes before exam starts or after exam starts. nb: do not send used card pins or your number will be blacklisted. nb: online answers comes 1hr after exam commences (keep refreshing this page)click here to join our facebook group. nb: only share this page with trusted students, we will be hiding this page immediately exam ends and a new page will be created for the upcoming exam. kindly do well to bookmark the site and check back later. ===============================================daily subscription - per subjects*******link payment per subject: n600***** [gets answers on time]******link payment per practical: n400***** [gets answers on time]===========================================.
Get Free Live 2021 NABTEB GCE Chemistry (CHEM) Objectives and Essay/Theory Questions and Answers Free of Charge | NABTEB Nov/Dec GCE Free Chemistry (CHEM) OBJ & Essay Questions and Answers EXPO Room (2nd December, 2021).
A. nabteb nov/dec gce chemistry theory (essay) answers 2021:, b. nabteb nov/dec gce chemistry theory (essay) answers 2021:.
Answers Loading........................................
- JUST GO OUT AND BUY MTN CARDS OF N600 ( 200 + 200 + 200 = 600 )
- GO TO YOUR MESSAGE, TYPE THE CARD PINS CORRECTLY AND SEND TO 08107431933.
- DON'T CALL, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU CONFIRMING THAT YOU HAVE BEEN SUBSCRIBED.
- RELAX AND WAIT FOR YOUR ANSWERS 30MINUTES BEFORE EXAM STARTS OR AFTER EXAM STARTS.
- NB: DO NOT SEND USED CARD PINS OR YOUR NUMBER WILL BE BLACKLISTED.
- Click Here to Join Our Facebook Group.
Previous Article
OOU Post-UTME & DE Screening Form 2021/2022 is Out
Next Article
Gombe State University Academic Calendar for 2021/2022 Session
myschoolnews
Related Posts
Islamic teachers under fire for brutalizing students over...
myschoolnews Oct 11, 2021 0 25
2021 Diplomats Scholarships at California Baptist University,...
myschoolnews Oct 12, 2021 0 26
UNILAG Registration For Fresh Students Has Not Commenced...
myschoolnews Oct 14, 2021 0 17
LASU Foundation (JUPEB) Admission List Out – 2021/2022
myschoolnews Oct 18, 2021 0 24
100 level COOU student commits suicide over stress
myschoolnews Oct 13, 2021 0 24
BENPOLY lectures Timetable for second semester, 2020/2021
myschoolnews Oct 25, 2021 0 23
Popular Posts
The Life Changer: 195 JAMB Novel Questions & Answers [PDF]
myschoolnews Jan 21, 2022 0 121
Download JAMB Past Questions & Answers In PDF [All Subjects]
myschoolnews Dec 13, 2021 0 98
National Common Entrance Past Questions & Answers [2011...
myschoolnews Dec 1, 2021 0 91
All About Saksham Scholarship
myschoolnews Aug 2, 2023 0 77
Download BECE Past Questions and Answers PDF for All Subjects
myschoolnews Jan 5, 2022 0 50
Recommended Posts
Canadian Diploma College Scholarships in 2022 – Get Your...
myschoolnews May 7, 2022 0 434
Big Scholarship Programs Without IELTS 2022 | Fully Funded
myschoolnews May 7, 2022 0 428
10 Scholarships to Study in UK - Application Still in Progress..
myschoolnews May 7, 2022 0 545
Check How Students From Nigeria Study in UK Universities...
myschoolnews May 7, 2022 0 446
Top 8 Internship Abroad Scholarships & Grants to Apply...
myschoolnews May 7, 2022 0 395
Random Posts
How to check national examinations council (neco) result....
myschoolnews Oct 15, 2023 0 74
Latest Update On How to check neco result: This page will discuss about How to Check...
President Tinubu Directs Inclusion Of NOUN Graduates In...
myschoolnews Apr 18, 2024 0 3
President Bola Tinubu has directed the Ministry of Education to include graduates...
Apply for the France Excellence PSL Scholarship 2024.
myschoolnews Apr 19, 2024 0 2
Spread the loveApplications are now open for the France Excellence PSL Scholarships...
BOSU Orientation Proramme For Prospective Corps Members...
myschoolnews Mar 28, 2024 0 7
Borno State University, BOSU Orientation Proramme For Prospective Corps Members...
ABSA SAICA Trainee Accountant 2025 for Graduate South Africans
myschoolnews Apr 8, 2024 0 2
Spread the loveApplications are open for the SAICA Trainee Accountant Program Application...
TWAS-ICCBS Postdoctoral Fellowship Program for Developing...
myschoolnews Apr 19, 2024 0 1
The International Centre for Chemical and Biological Sciences (ICCBS) — comprising...
University of East Anglia International High Achiever Scholarship...
The UEA China Postgraduate High Achiever Award is for high achieving students from...
Cyber Innovates Cyber Security Scholarship with MTU 2024
myschoolnews Apr 17, 2024 0 4
Spread the loveCyber Innovates Cyber Security Scholarship with MTU 2024. Apply below....
Latest Npower News Today 17 April, 2023 on Aug Payment,...
myschoolnews Apr 17, 2023 0 214
Read the Latest Npower News Today 2022 on batch c stream 2 deployment update, stipend...
South Korea Scholarships Without IELTS 2024 | Study Free...
South Korea Scholarships Without IELTS 2024 for International Students: Study in...
Voting Poll
How would you rate your teacher.
Vote View Results
Total Vote: 163
View Options
- +2349152153136
- [email protected]
- Wuse, Abuja, Nigeria

CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Chemistry Theory And Objective: Questions & Answers For 2022/2023 WAEC Exam. Are you a student who wants to make a good grade in Chemistry? Here are the WAEC Chemistry questions and everything you need to know about 2021 WAEC Chemistry. This post provides provided for you with all you need to know in Chemistry Paper 1 (Objective) and Chemistry 2 (Essay).
Table of Contents
Welcome to the Chemistry Theory and Objective: Questions & Answers for the 2022/2023 WAEC Exam. This comprehensive guide has been designed to assist students in their preparation for the upcoming WAEC Chemistry examination. It presents a collection of theory and objective questions along with their corresponding answers, covering the essential topics and concepts outlined in the WAEC syllabus. By studying and practicing these questions, you will gain a solid foundation in chemistry and improve your chances of achieving excellent results in the exam. So let’s delve into the world of chemistry and embark on a journey of learning and success.
The Question & Answers: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Furthermore, below are the WAEC Chemistry questions. Read them properly. In fact, they will make you ready to score high in your WAEC Chemistry exam.
The West Africa Examination Council (WAEC) is an examination body in Nigeria. It has the statutory power to conduct the Senior Secondary Certificate Examination in May/June and the General Certificate in Education in November/December.
THE OBJECTIVE OF POST: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Because people are asking about WAEC Chemistry and objective questions and answer 2022/2023. In fact, they are also asking for JAMB 2022 CHEMISTRY questions and answers pdf, The solutions are the objectives of this post. This post, therefore, takes care of those students who plan to do well in the WAEC exams this year.
The best way to read this post is to read it by clicking the highlighted topics for referencing. So check out these related topics that follow.
- Canada Visa Status
- Netherlands International Students guide
- 3 most demanded undergraduate courses in the Netherlands
- Most Popular Degree courses in Australia
- UK-approved graduate schools
- Most popular degree courses in the UK
- Approved Undergraduate – in UK
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
General tips and advice to help you prepare for your exams effectively..
- Study the syllabus: Familiarize yourself with the syllabus for your respective exams. Understand the topics and concepts that are likely to be covered and focus your preparation accordingly.
- Review past papers: Practice solving past exam papers to get an idea of the types of questions that may be asked. This will help you become familiar with the exam format and identify any areas where you need further improvement.
- Understand the concepts: Chemistry involves understanding fundamental concepts and principles. Ensure you have a strong foundation by studying the theory thoroughly. Take notes, create summaries, and use diagrams or visual aids to help you grasp complex concepts.
- Practice problem-solving: Chemistry often requires application and problem-solving skills. Practice solving numerical problems and chemical equations regularly to strengthen your problem-solving abilities. This will also help you become more familiar with the calculations and techniques needed for the exam.
- Seek clarification: If you come across any challenging topics or concepts, don’t hesitate to seek clarification from your teachers, classmates, or online resources. Understanding the material thoroughly will boost your confidence during the exam. CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM..
- Create a study schedule: Plan your study sessions and allocate sufficient time to each topic based on its importance and your level of understanding. Break down your study material into manageable chunks and set realistic goals to stay motivated.
- Collaborate with peers: Consider forming study groups with classmates or friends who are also preparing for the exams. Discussing and explaining concepts to each other can enhance your understanding and retention of the material.
- Utilize available resources: Take advantage of textbooks, reference materials, online resources, and educational platforms that provide study materials, practice questions, and tutorials specific to your exam. These resources can provide valuable insights and additional practice opportunities. CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
- Practice time management: During the exam, time management is crucial. Practice solving questions within the allocated time limits to improve your speed and accuracy. This will ensure that you can complete the exam within the given time frame.
- Stay focused and maintain a healthy lifestyle: Avoid distractions and create a conducive study environment. Take regular breaks, exercise, eat nutritious meals, and get enough sleep to keep your mind and body in optimal condition for studying.
Remember, success in any exam is a result of consistent effort, effective preparation, and a positive mindset. Good luck with your exams!
WAEC Chemistry Questions and Answers 2022:
Furthermore, the questions below are the WAEC Chemistry Questions. Going through them will make you ready to score high in your WAEC 2021 Chemistry Examination. Congratulations.
- How many alkoxy alkanes can be obtained from the molecular formula C4 H4O4
ANSWER: C (3)
- Element Y has two isotopes Y and Y present in the ratio 1:3. The relative atomic mass of Y would be
ANSWER: C (21.5)
- What condition favors the formation of the product for the endothermic reaction, N2O4(g) —><—– 2NO2(g)
A. Decrease in pressure
B. A decrease in volume
C. An increase in pressure
D. A constant volume
ANSWER: A ( Decrease in pressure)
- Elements X and Y have electronic configurations 1S22S22P4 and 1S22S22P63S23P1 respectively. When they combine the formula of the compound formed is
ANSWER: B (Y2X3)
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM
- A solution of 0.20 mole of NaBr and 0.20 mole of MgBr2 in 2.0 dm3 of water is to be analyzed. How many moles of Pb(NO3 )2 must be added to precipitate all the bromide as insoluble PbBr2
A. 0.30 mol
B. 0.10 mol
C. 0.20 mol
D. 0.40 mol
ANSWER: A (0.30 mol)
- Na2CO3 + HCl —-> NaHCO3 + NaCl. The indicator most suitable for this reaction should have pH equal to.
ANSWER: D (9)
- A saturated solution of silver trioxocarbonate (IV), was found to have a concentration of 1.30 x 10-5 moldm-3 . The solubility product of the trioxocarbonate (IV) is
A. 8.79 x 10-15
B. 1.69 x 10-10
C. 1.82 x 10-11
D. 9.84 x 10-10
ANSWER: A (8.79 x 10-15)
QUESTIONS & ANSWERS FOR THE 2022/2023 WAEC EXAM
- 100.0g of KClO3 was added to 40.0 cm^3 of water to give a saturated solution at 298K. If the solubility of the salt is 20.0 moldm^-3 at 298K, what percentage of the salt is left undissolved? {K= 39, Cl = 35.5, O = 16}
- Tetraoxosulphate (VI) ions are the final test using
A. acidified silver nitrate
B. acidic barium chloride
C. lime water
D. dilute hydrochloric acid
ANSWER: D (dilute hydrochloric acid)
- When platinum electrodes are used during the electrolysis of copper (II) tetraoxosulphate (IV) solution, the solution gets progressive
D. Atmospheric
ANSWER: A (Acidic)
WAEC 2022/2023 Chemistry Theory Questions
PAPER 2 (ESSAY) SECTION A
- (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
- Calculate the percentage by mass of silicon tetrachloride. [2 marks]
- Ammonia, NH3, and phosphine, Ph3, are the hydrides of the first two elements in group 5. (a) Draw a dot and cross diagram for the ammonia molecule. [2 marks] (b) Sketch and explain the shape of the ammonia molecule. [3 marks]
- The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
- Compound A consists of carbon and hydrogen only. The compound was found to contain 80% carbon by mass. (a) Calculate the empirical formula of compound A using the data above. (b) The relative molecular mass of compound A was found to be 30. Use this information to deduce the molecular formula of compound A. [H = 1.00 C = 12.00]
- State two factors other than a change in temperature or the use of a catalyst that influence the rate of a chemical reaction.
- Identify the solid remaining when each of the following is heated. (a) lithium trioxonitrate (V) (b) potassium trioxonitrate (V) (c) calcium trioxonitrate (V)
CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For the 2022/2023 WAEC EXAM
- An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
- (a) (i) Explain briefly the fermentation process. (ii) Write a balanced equation for the fermentation of glucose. (iii) What substance must be added to glucose solution to ferment it? (iv) Explain briefly why tightly corked glass filled to the brim with palm wine shatters on standing. (b) State one industrial application of each of the following methods of separation: (i) Crystallization; (ii) Fractional distillation. (c) Explain the following terms: (i) Saponification; (ii) Esterification. (d) Write a balanced equation to illustrate each of the terms in (c). (e) i) What is hydrocarbon compound? (ii) Name two principal sources of hydrocarbons.

QUESTIONS & ANSWERS FOR 2022/2023 WAEC EXAM
- (a) Two elements represented by the letters Q and R have atomic numbers 9 and 12 respectively. (i) Write the electron configuration of R. (ii) To what group does Q belong in the periodic table? (iii) Write the formula of the compound formed when Q combines with R. (iv) Explain briefly, why Q is a good oxidizing agent. (v) State whether R would be expected to form acidic or basic oxide. (b) (i) State two assumptions of the kinetic theory of gases. (ii) When some solids are heated, they change directly into the gaseous state. What name is given to this phenomenon? (iii) List two substances that exhibit the phenomenon mentioned in (ii). (iv) Write an expression to show the mathematical relationship between the rate of diffusion of a gas and its vapor.
READ ALSO FOR CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
- Latest Secondary Education updates
- Physics 2021 WAEC Questions & Answers
- English Language for WAEC May/ June 2021
- How to Become a professional
- Secondary Education List of Subjects – WAEC approved
- Tips for Successful Professionals
- Tertiary Education updates
- Latest professional tips
- How the basic primary education works
- Professionals and Recruitment
HOW TO PARTNER WITH US: CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
In conclusion, mastering the Chemistry theory and objectives is crucial for success in the upcoming 2022/2023 WAEC exam. By diligently studying and understanding the key concepts, practicing with a wide range of questions, and seeking clarification when needed, students can enhance their understanding of this fascinating subject. Remember, a strong foundation in Chemistry will not only boost your performance in the exam but also equip you with valuable knowledge for future academic pursuits. Stay focused, stay determined, and embark on this journey with confidence. Best of luck in your preparations and may you excel in the upcoming WAEC exam! CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Finally, for those who would want to win a scholarship and at times part-time studies, this website is for you. For career professional tutorials for ICAN, CITN, NBA exams, etc. this site is available for your current information. Therefore, send us your questions through the comment box. And keep in touch with us by filling in the email list box below for further updates. Our social media buttons will also help if you like us on then or follow us. Thanks Get ready with CHEMISTRY THEORY And OBJECTIVE: QUESTIONS & ANSWERS For 2022/2023 WAEC EXAM.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Career & Training
- Career Guide
- Loans & Grants
- Professional Tips
- Real Estate
- Scholarship
- Travel Visas
St Charles Edu Services
Genuine Exam Past Questions and Answers Online Bookshop – PDF and MS Word Download
WAEC Chemistry Past Questions and Answers in 2023 PDF Download Objective & Theory
Are you writing the West Africa Examination Council WAEC Internal or External examination, if yes you need the WAEC Past Questions on Chemistry
we at stcharlesedu.com has compiled a good number of Chemistry WAEC Past Questions and Answers in Pdf Chemistry 2 – Theory/Essay Questions. Chemistry 1 – Objective Test Questions.
Our research has confirm that candidate that uses WASSCE Chemistry past questions to prepare is ten times better than those who do not.
Table of Contents
- 1.1 Chemistry WAEC Objective Questions
- 2 SSCE WAEC Chemistry Theory Questions
- 3 Chemistry WAEC Essay Questions
- 4 Free WAEC Chemistry Exam Past Questions Download
- 5 How to Get WASSCE Chemistry Exam Past Questions and Answers
SSCE WAEC Chemistry Objective Questions and Answers
CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout.
Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen. Give only one answer to each question. An example is given below
What others are downloading WAEC Past Questions for all Subjects
Chemistry WAEC Objective Questions
Which of the following elements reacts with water? A. Carbon B. Iodine C. Sodium D. Sulphur
The correct answer is Sodium, which is lettered C and therefore answer space C would be shaded. [A] [ B ] [C] [ D ]
Think carefully before you shade the answer spaces; erase completely any answer you wish to change.
Which of the following raw materials is used in the plastic industry? A. Ethene B. Methane C. Sulphur D. Hydrogen
Which of the following organic compounds can undergo both addition and substitution reactions? A. Petane B. Benzene C. Propane D. Hexane
Which of the following equations represents a redox reaction? A. AgNO 3 (aq) + KCl(ag)->AgCl(s)+ KNO 3 (aq) B. HNO 3 (aq)+ NaOH(aq) -> NaNO 3 (aq) + H 2 O(l) C. CaCO 3 (s) -> CaO(s) + CO 2 (g) D. 2H 2 S(g) + SO 2 (g) -> 2H 2 O(I) + 3S(g)
T he process of extraction of iron from its ore is A. decomposition. B. oxidation. C. reduction. D. sublimation.
What is the solubility of a salt if 0.4 g of it is obtained on evaporating 200 cm3 of its saturated solution to dryness? A. 0.08 gdm -3 B. 2.00 gdm -3 C. 8.00 gdm -3 D. 80.00 gdm -3
An acidic salt has A. double anions in its aqueous solution. B. a single cation in its aqueous solution. C. hydrogen ions in its aqueous solution. D. hydrogen atoms in its aqueous solution.
A reaction is endothermic if the A. reaction vessel feels cool during the reaction. B. enthalpy change is negative. C. bond forming energy exceeds bond breaking energy. D. heat of formation of reactants exceeds heat of formation of products.
In which of the following compounds does hydrogen form ionic compounds? A. CH 4 B. HCl C. NH 3 D. NaH
Consider the following reaction equation: Br 2 + 2KI -> 2KBr + I 2 . Bromine is acting as A. an oxidizing agent. B. a reducing agent. C. an acid. D. a base.
An organic compound has the empirical formula CH 2 . If its molar mass is 42 gmol-1 what is its molecular formula? [H = 1.0, C = 12.0] A. C 2 H 4 B. C 3 H 4 C. C 3 H 6 D. C 4 H 8
Ethene is produced from ethanol by A. decomposition. B. hydrolysis. C. ozonolysis. D. dehydration.
Consider the following equilibrium reaction: 2 AB(g) + B 2 (g) -><- 2AB 3 (g) AH = -XkJmol -1 The backward reaction will be favored by A. a decrease in pressure. B. an increase in pressure. C. a decrease in temperature. D. an introduction of a positive catalyst.
What is the mass of solute in 500 cm 3 of 0.005 moldm -3 H 2 SO 4 ? [H =1.0, O = 16.0, S = 32.0] A. 0.490 g B. 0.049 g C. 0.245 g D. 0.0245 g
Pure water can be made to boil at a temperature lower than 100 °C by A. reducing its quantity. B. decreasing the external pressure. C. distilling it. D. increasing the external pressure.
Consider the following sketch of the solubility curve of some substances. Note: scroll down to download the free chemistry waec questions in pdf copy to view the sketch
At what temperature does the solubility of KNO 3, equal that of NaNO 3 ? A. 0°C B. 20 °C C. 30 °C D. 40 °C
When a salt is added to its saturated solution, the salt A. dissolves and the solution becomes super saturated. B. dissolves and the solution becomes unsaturated. C. precipitates and the solution remains unchanged. D. dissolves and crystals are formed.
When substance X was added to a solution of bromine water, the solution became colorless. X is likely to be A. propane. B. propanoic acid. C. propyne. D. propanol.
The preferential discharge of ions during electrolysis is influenced by the A. mechanism of electrolysis. B. electrolytic reactions. C. nature of the electrode. D. type of electrolytic cell.
The valence electrons of 12 Mg are in the A. 3s orbital. B. 2px orbital. C. 2s orbital. D. 1s orbital.
Stainless Steel is an alloy comprising of A. Fe and C. B. Fe and Ni. C. Fe, C and Ni. D. Fe, C and Al.
The number of hydrogen ions in 1.0 dm 3 of 0.02 moldm -3 tetraoxosulphate(VI) acid is [NA = 6.02 x 1023] A. 1.2 x 10 22 B. 1.2 x 10 23 . C. 2.4 x 10 22 . D. 2.4 x 10 23 .
The most suitable substance for putting out petrol fire is A. water. B. carbon(IV)oxide. C. fire blanket. D. sand.
The following factors would contribute to environmental pollution except A. production of ammonia. B. manufacture of cement. C. photosynthesis. D. combustion.
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants. B. vigorous stirring of the reaction mixture. C. presence of a catalyst. D. change in concentration of the reactants.
The diagram below illustrates a conical flask containing water and ice.
NOTE: scroll down and download the free chemistry pdf past questions to see the diagram
Which of the following statements about the diagram is correct? A. The water is at a lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point
Which of the following statements best explains the differences between a gas and a vapor? A. Unlike gases, vapors are liquids at room temperature B. Unlike gases, vapor can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases
Consider the following reaction equation: 2HCl + Ca(OH) 2 –> CaCl 2 + H 2 O. What is the volume of 0.1 moldrn -3 HCl that would completely neutralize 25cm 3 of 0.3 moldm -3 Ca(OH) 2 ? A. 150 cm 3 B. 75 cm 3 C. 30 cm 3 D. 25 cm 3
Cu and HNO 3 are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively. B. conductivity and corrosiveness respectively. C. melting point and reduction respectively. D. electro negativity and solubility respectively.
Which of the following formulae cannot be an empirical formula? A. CH B. CH2 C. P2O5 D. N204
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity. B. boiling point. C. mass. D. colour.
Want more Chemistry Objective Test Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answers (Obj and Essay) in PDF Format from us.
SSCE WAEC Chemistry Theory Questions
Chemistry Paper 2 Paper 2 will last for 2 hours This paper consists of two sections A and B. Answer one questions from Section A and three questions from Section B.
Credit will be given for clarity of expression and orderly presentation of material.
SECTION A (1ai) Define the term fermentation. (1aii) Name the catalyst that can be used for this process.
(b) Name two factors which determines the choice of an indicator for an acid-base titration. (c) Consider the following reaction equation: [Fe + H2S04 ] FeS04 + H2. Calculate the mass of unreacted iron when 5.0g of iron reacts with 10cm3 of 1.0 moldrrv3 H SO [Fe = 56.0] (d) Name one: (di) Heavy chemical used in electrolytic cells; (dii) Fine chemical used in textile industries.
(e) Explain briefly how a catalyst increases the rate of a chemical reaction. (f) (i) Write the chemical formula for the product formed when ethanoic acid reacts with ammonia. (ii) Give the name of the product formed in 1 (f) (i)..
(g) List three properties of aluminum that makes it suitable for the manufacture of drink can. (h) State two industrial uses of alkylalkanoates. (i) List two effects of global warming. (j) Name two steps involved in the crystallization of a salt from its solution.
Chemistry WAEC Essay Questions
SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
bi. Sketch a graphical representation of Charles’s law. bii. Calculate the volume of oxygen that would be required for the complete combustion of 2.5moles of ethanol at s.t.p. [ molar volume at s.t.p = 22.4dm3]
ci. Define esterification. cii. Give two uses of alkanoates. ciii. Give the products of the alkaline hydrolysis of ethyl ethanoate.
d. A tin coated plate and a galvanized plate were exposed for the same length of time. di. Which of the two plates corrodes faster? dii. Explain briefly your answer in 2 (d) (i).
Want more Chemistry Theory Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answer (Obj and Essay) in PDF Format from us.
Free WAEC Chemistry Exam Past Questions Download
Click to Download your free NECO Past Question on Painting and Decorating Paper 2 and 3
Link 1: WASSCE Chemistry Questions Booklet Link 2: WASSCE Chemistry Questions Booklet
How to Get WASSCE Chemistry Exam Past Questions and Answers
To get the complete and more recent copy of the West Africa Examination Council WAEC Past Questions and answer
Take Note of the following step
Make a Call Call or whatsapp us on 08051311885 for the account number to make payment and how to received your complete copy of the past questions to be sent directly to your email address or whatsapp number.
Mode of Payment. Mobile Transfer or Direct Bank Deposit.
After Payment send us the following Depositor Name: Name of Product Paid for: Valid email address.
DELIVERY ASSURANCE We will deliver the past question to you 10 mins after confirmation of payment to the email you will send to us.
Related Posts:
- WAEC Technical Drawing Past Questions PDF Download – Objective, Essay, Building Plan/Practical Drawing
- WAEC Government Past Questions and Answers in 2023 PDF Download Objective & Theory
- WAEC Financial Accounting Past Questions and Answer 2023 – Objective & Essay
- WAEC Visual Art Past Questions and Answers – Objective, Theory in 2023
- WASSCE/WAEC Electrical Installation & Maintenance Past Questions PDF – Objective/Essay
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here
WAEC GCE Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
WAEC GCE Chemistry Questions and Answers 2023 . Welcome to 2023 WAEC Chemistry Questions and Answers. You will find WAEC GCE Chemistry Objective Answers, WAEC Chemistry Essay 2023, WAEC GCE 2023 Chemistry, and the tips you need to pass your WAEC GCE Chemistry examination with ease.

Table of Contents
WAEC GCE Chemistry Questions and Answers 2023 (Expo)
The 2023 WAEC GCE Chemistry expo will be posted here during the WAEC GCE Chemistry examination. Keep checking and reloading this page for the answers.
WAEC GCE 2023 Chemistry Answers Loading.. .
Today’s WAEC GCE Chemistry OBJ Answers:
———————————————————————————–
Note: The answers below are the 2020 Nov/Dec answers.
NOTE: Pls Trace It From Your Objective. [If you see any options here pick it from your objectives]
1 Involves the loss and gain of electrons 2 Polymerisation 3 Global warming 4 3 5 Zinc ions 6 +1.56V 7 Ethene 8 NH3 9 Propanol 10 28 11 Hydrolysis 12 d-orbital 13 Lowering the activation energy 14 Closeness between reactant particles 15 Remaining the same with time 16 Reaction vessel Fels cool during the reaction 17 Faster 18 Solvent extraction 19 Saturated Solution 20 2.75mol/dm³ 21 Partially dissociates in aqueous solution 22 138g 23 HI 24 2.00cm³ 25 hydrogen chloride 26 strong electrovalent bond between ions 27 is not ductile 28 Electrons 29 C2H4 30 Aluminium 31 0.010mol/dm³ 32 PbCO3 33 Linear 34 HCL and HOCL 35 +1 36 Ionic bond 37 have relatively low ionization energy 38 sour to taste 39 I,III and IV only 40 chrometography 41 2.00 dm 42 mole of solvent in 1dm³ of solution 43 does not contain neutron 44 1s²2s²2p⁶ 45 Ammonium chloride 46 IV 47 I,II and IV only 48 Quantum numbers of Electrons 49 -273⁰C 50 Mass number
1-10: DDDADBBABA
11-20: CDABDCCDDC
21-30: ACDBDCCDAB
31-40: BCADABDBAC
More Answers loading…
WAEC GCE Chemistry Theory Answers:
(i) Sodium trioxonitrate (v) decahydrate
NaNO₃ . 10H₂O
(ii) Sodium Oxide –> Na₂O
(iii) Potassium tetraoxophospate (v) –> K₃PO₄
Products formed are:
Hydrogen gas (a+ cathode)
Chlorine gas (a+ anode)
it decrease down the group
As the atomic radius increases down the group the attraction of the positive nucleus of the electron outer most electron, thus the ionization energy decreases
Nitrogen and carbon (ii) oxide
it is used to heat furnace
it is a source of nitrogen for the manufacture of ammonia
Sodium hydrogen – used in qualitative analysis
– Purification of bauxite
Sodium trioxocarbonate (iv)
-Manufacture of glass
-As a water soften
2-amino propane
(2ai) Percentage C5H12 of mass m = 7.2g Volume of O2 = 20.0dm³ (i) from the general combustion equation CxHy(g) + (x+y/4)O2 –> XCO2 + y/2H2O C5H12(l) + 802(g) –> 5CO2(g) + 6H2O(l)
(2aii) 1 mole of C5H12(72g) = 5 moles of CO2 At stop 7.2gC5H12 = x volume of CO2 X = 7.2g×5×22.4dm³/72g X = 5×2.224 = 11.2dm³ of CO2
(2aiii) Volume of oxygen left after the reaction from the equation of reaction 1 mole of C5H12(72g) = 8(22.4)dm³ 7.2g = x X = 7.2×8×22.4/72 = 17.92dm³ Volume of O2 left after the reaction = 20.0dm³ – 17.92dm³ = 2.08dm³
(2b) When molecules collide with one another they possess kinetic energy. As most energetic molecules (those with greater kinetic energy) try to escape. Their escape may be facilitated by heat or by passing a wave of air over the container or by increasing the surface area of the container. As they try to do this, some molecules will loose energy on collision and fall back to the container; as such the average kinetic of the molecules of the liquid in the container reduces which results to cooling effect.
(2ci) Avogadro’s Law states that the total number of atoms/molecules of a gas (i.e. the amount of gaseous substance) is directly proportional to the volume occupied by the gas at constant temperature and pressure.
(2cii) N2(g) + 3H2—> 2NH3 Where 1 mole = 30cm³ of gas
At constant temperature, the volume of a fixed mass of gas. When the volume of a cylinder or a container is increased, the gases have more space to travel and collide hence the pressure is reduced but as the volume is decreased or compressed the gases have less space to travel therefore more pressure is built up.
No (3ai) ¹³R, ⁸Q ¹³R=1s²,2s²,2p⁶,3s²,3p¹ ⁸Q=1s²,2s²,2p⁴
(3aii) ¹³R=2,8,3 Valency of ¹³R is 3 ⁸Q= 2,6 Valency of ⁸Q=2
(3di) 2H² SO4(aq)+4NaOH(aq)—>2Na² SO4(aq)+4H²O(s)
(3dii) Sodium teraoxosulphate (iv) salt and water
(3diii) The resulting solution NaSO4 is basic and will have no effect on litmus paper
(3div) When heated to dryness it can be used as a dehydrating agent
Typing….
—————————————————————————————————————–
The questions below are strictly for practice.
1. Which of the following statements best explains the difference between a gas and a vapour?
(a) Unlike gases, vapours are liquids at room temperature
(b) Unlike gases, vapour can easily be condensed into liquids
(c) Unlike gases, vapour is readily converted into solids
(d) Vapours are generally denser than gases
2. Consider the following reaction equation: 2HCI + Ca(OH) 2 → CaCI 2 + H 2 O. what is the volume of 0.1 moldm -3 , HCI that would completely neutralize 25 cm 3 or 0.3 moldm -3 Ca(OH) 2 ?
(a) 150 cm 3
(b) 75 cm 3
(c) 30 cm 3
(d) 25 cm 3
3. Cu and HNO 3 are not suitable for preparing hydrogen gas because of their
(a) Reactivity and oxidation respectively
(b) conductivity and corrosiveness respectively
(c) melting point and reduction respectively
(d) electronegativity and solubility respectively
4. Which of the following formulae cannot be an empirical formula?
(c) P 2 O 5
(d) N 2 O 4
5. One of the criteria for confirming the purity of benzene is to determine its
(a) Heat capacity
(b) boiling point
6. When chlorine is passed through a sample of water, the pH of the water sample would be (a) <7
(a) 1.20 x 10 23
(b) 2.41 x 10 23
(c) 3.62 x 10 23
(d) 4.82 x 10 23
8. The strength of metallic bonds depends on the
(a) charge density of the atoms
(b) ductility of the metal
(c) number of valence electrons
(d) total number of electrons in the atoms
9. When zinc is added to AgNO 3 solution, crystals of silver forms on the zinc surface. This indicates that zinc is
(a) oxidized
(b) reduced
(c) decomposed
(d) dissociated
(d) Cu 2 O 2
- WAEC GCE Mathematics Questions and Answers
- WAEC GCE Physics Questions and Answers
11. The change in the oxidation state of iron in the reaction represented by the equation below is 2FeCI 3 + H 2 S →2FeCI 2 + 2HCI + S
(a) +2 to +3
(b) +3 to +2
(c) 0 to +2
(d) +3 to 0
12. Which of the following methods can be used to separate blood cells from plasma?
(a) Centrifugation
(b) Filtration
(c) Chromatography
(d) Distillation
13. Which of the following statements about ionic radius is correct? ‘Ionic radius
(a) Increases as nuclear charge increases
(b) decreases as nuclear charge increases
(c) decreases as nuclear charges decreases.
(d) remains constant as nuclear charge increases
14. Analysis of a hydrocarbon shows that it contains 0.93 g of carbon per gram of the compound. The mole ratio of carbon to hydrogen in the compound is [H=1.0, C=12.0]
15. The law of definite proportions states that
(a) pure samples of same compound contain the same elements combined in the same proportion by mass
(b) pure samples of substances are in the same proportion by mass
(c) chemical compounds are pure because they contain the same elements
(d) matter can neither be created nor destroyed
17. Atoms are electrically neutral because they
(a) don not conduct electricity
(b) contain equal number of protons and electrons
(c) are composed of neutrons and electrons
(d) cannot be attracted by electromagnetic field
18. Common salt (NaC1) is used for preserving foods. Which of the following properties could be used to determine its purity before use?
(a) Solubility in water
(b) melting point
(c) Relative density
(d) Crystalline nature
19. Which of the following electron configurations represents the transition element chromium ( 24 Cr)?
(a) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4
(b) 1s 2 2 2s 2 2p 6 3s 2 3p 6 3d 6
(c) 1s 2 2s 2 2p 6 2s 2 3d 4 4s 1 (d) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5
20. The atomic number of an isotope of hydrogen is equal to its mass number because it
(a) has a totally filled valence shell
(b) has a high charge to mass ratio
(c) does not contain neutrons (d) exhibits isotopy
21. The total number of shared pair of electrons in the compound below is
22. The bonding pair of electrons in a hydrogen chloride molecule is pulled towards the chlorine atom because
(a) Chlorine has a larger atomic size
(b) chlorine has a large atomic mass
(c) chlorine is more electronegative
(d) there is no bonding orbitals within the hydrogen atom
23. The solubility of CO 2 in water can be accounted for by
(a) van der waal’s forces
(b) ionic attraction
(c) dipole attraction
(d) covalent bonding
24. Which of the following properties would not influence electrovalent bond information?
(a) Electronegativity
(b) Electron affinity
(c) Ionization potential
(d) Catalytic ability
25. Particles in a solid exhibit
(a) Vibrational motion only
(b) vibrational and translational motion
(c) vibrational and random motion
(d) random and translational motion
WAEC GCE Chemistry Essay 2023
The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Physics repeated questions and answers.
These questions are for practice. The 2023 WAEC GCE Chemistry expo will be posted on this page 30 minutes before the WAEC GCE Chemistry examination starts. Keep checking and refreshing this page for the answers.
If you have any questions about the WAEC GCE Chemistry questions and answers, kindly drop your questions in the comment box.
Last Updated on October 2, 2023 by Admin
Related posts:

8 thoughts on “WAEC GCE Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
How can I slove hard question in question
Pls i’m a candidate for gce advance level and i need chemistry papers 2023
Please I need mathematics, chemistry, physics and biology questions
Pls always post me from 2021till 2023 in 9 subject which is Econ, maths Igbo chemistry physics, Agric civic bio, Eng
I need waec English for today
I need past questions on all science subjects please
Pls I need the question for chemistry, physics, biology, agricultural science, english and mathematics
Okay heard you
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.


IMAGES
VIDEO
COMMENTS
Chemistry 2021 Free-Response Questions . Begin your response to . QUESTION 1 . on this page. CHEMISTRY . SECTION II . Time—1 hour and 45 minutes 7 Questions . YOU MAY USE YOUR CALCULATOR FOR THIS SECTION. Directions: Questions 1-3 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each.
Score Distributions. Sample Responses Q1. Sample Responses Q2. Sample Responses Q3. Sample Responses Q4. Sample Responses Q5. Sample Responses Q6. Sample Responses Q7. Download free-response questions from past AP Chemistry exams, along with scoring guidelines, sample responses from exam takers, and scoring distributions.
For the correct answer: 1 point. Electron flow should be indicated only in a counter-clockwise direction in the external circuit, from the Cl2 anode to the Mg cathode. (b) For the correct answer and calculated value: No, because 2.0 V is less than 3.73 V, which is the minimum voltage needed for electrolysis to occur.
SEPTEMBER 2021 EXAMINATIONS CHEMISTRY Time Allowed: 3 Hours SECTION A: MULTIPLE CHOICE QUESTIONS Answer all questions in this section. Use the OMR answer sheet provided to answer the questions. ... ESSAY QUESTIONS Answer FOUR questions; ONE question from each course. Ensure you read and follow all the Instructions on the cover page of the ...
Following subjects of SPM Trial Papers are available on this website. 2021/Kimia (Chemistry)/. We provide SPM Trial Paper Questions with answers and Soalan Percubaan SPM from 2011-2022. Subjects included are BM, Sejarah, English, Moral, Physics, Chemistry, Math, AddMath, Biology, Perdagangan, Ekonomi Asas, Science.
These official exams come from before 2013 (when significant changes were made to the AP Chemistry curriculum), so they're formatted slightly differently from the current test. They have 75 multiple-choice questions (there are now 60) and six free-response questions (there are now seven). There are also five answer choices for each multiple ...
10. Which of the graphs illustrates the variation of the p H of a given volume of strong acid solution (y-axis) with the volume of strong base titrated against its (x-axis)? A. B. II. C. III. D. IV. A. Chemistry, 2021, WASSCE/WAEC MAY/JUNE, Exam, Paper 1 | Objectives Past Question Papers Download.
Candidates should answer the questions in English. Element A has mass number 40 and 21 neutrons. Write the electron arrangement of element A. (1 mark) Give the formula of the compound formed when element A reacts with sulphur. (S = 16.0) (1 mark) Study the setup in Figure 1 and then answer the questions that follow.
Chemistry Paper 3 Questions and Answers - KCSE 2021 Past Papers. Download PDF for future reference Get on Whatsapp for 50/-. QUESTIONS. You are provided with: Solution A: 0.10 M solution of a monobasic acid A; Solution B: Sodium hydroxide solution: Solution C: containing 10.0g of acid C per litre of solution. You are required to:
Fill a burette with solution B. Transfer all of the mixture in the 100 ml plastic beaker from procedure I into a 250 ml volumetric flask. Add distilled water to make up to the mark and shake. Label the mixture as solution A. Using a pipette and pipette tiller, place 25.0cm 3 of solution A into a 250 ml conical flask.
Time: 3 Hours Year : 2021. Instructions. l. This paper consists of sections A, B and C with a total of fourteen (14) questions. 2. Answer all questions in sections A and B and one (1) question from section C. 3. Sections A and C carry fifteen (15) marks each and section B carries seventy (70) marks. 4.
To download an updated list of Chemistry past papers Click Here Both Part I (MCQ) and Part II (Essay) question papers of the 2021 GCE Advanced Level Chemistry Examination can be downloaded here for free. This GCE Advanced Level Question Paper was related to the Official Advanced Level Chemistry Examination held on 17th February 2022.
2024 WAEC Chemistry Questions & Answers for Essay and Objective Released. The Waec chemistry answers 2024 essay and objective questions for the West African Examination Council (WAEC) Chemistry SSCE exam paper scheduled to be written on Tuesday, 12th December, 2023 can now be studied here.. The 2023 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective ...
Inorganic Chemistry Essay Questions and Answers in Sinhala Medium. You can browse new model questions for inorganic chemistry here. We will add new questions continuously. Check the page daily to see more questions. Answers and explanations are given with the answer. Questions are presented according to the added date.
Question 1 presents a suite of questions on the reactions and structure of methanoic acid, HCOOH. Part (a) asks the student to write the equilibrium constant expression for the acid ionization reaction of HCOOH. This question addresses Learning Objective SAP-9.C and Science Practice 5.B from the AP Chemistry Course and Exam Description.
CLICK TO SEE ANSWERS. (Chemistry - NECO 2021) Direct Mobile/SMS: N800 MTN CARD. Password/Link: N500 MTN CARD. Forward Subject Name, MTN CARD PINS, Phone number to: 08074021548 via TEXT MESSAGE ...
The 2023 answers will be posted here on 24th May during the exam. WAEC Chemistry OBJ Answers Loading…. 1-10: ACADABBDAC. 11-20: BCAABBCCBB. 21-30: ACBABDACBB. 31-40: ABBDAADCDD. 41-50: BDCDDCDDCD. (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
Get Free Live 2021 NABTEB GCE Chemistry (CHEM) Objectives and Essay/Theory Questions and Answers Free of Charge | NABTEB Nov/Dec GCE Free Chemistry (CHEM) OBJ & Essay Questions and Answers EXPO Room (2nd December, 2021). NABTEB NOV/DEC GCE 2021 FREE CHEMISTRY OBJ & ESSAY (CHEM) QUESTION AND ANSWER ROOM Thursday 2nd December, 2021Chemistry (9:00am-11:30am) A. NABTEB NOV/DEC GCE CHEMISTRY THEORY ...
WAEC 2022/2023 Chemistry Theory Questions. PAPER 2 (ESSAY) SECTION A. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction. (b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne.
SSCE WAEC Chemistry Objective Questions and Answers. CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout. Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen.
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. A) Activation energy. B) Bond energy. C) Kinetic energy. D) Potential energy. 2.
WAEC GCE Chemistry Essay 2023. The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Physics repeated questions and answers. These questions are for practice. The 2023 WAEC GCE Chemistry expo will be posted on this page 30 minutes before the WAEC GCE Chemistry examination starts.