Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 14 December 2023

The most important issue about water is not supply, but how it is used

- Peter Gleick 0
Peter Gleick is co-founder and a senior fellow at the Pacific Institute in Oakland, California. He is the author of The Three Ages of Water: Prehistoric Past, Imperiled Present, and a Hope for the Future (2023)
You can also search for this author in PubMed Google Scholar
Floods, droughts, pollution, water scarcity and conflict — humanity’s relationship with water is deteriorating, and it is threatening our health and well-being, as well as that of the environment that sustains us. The good news is that a transition from the water policies and technologies of past centuries to more effective and equitable ways of using and preserving this vital resource is not only possible, but under way. The challenge is to accelerate and broaden the transition.
Water policies have typically fostered a reliance on centralized, often massive infrastructure, such as big dams for flood and drought protection, and aqueducts and pipelines to move water long distances. Governments have also created narrow institutions focused on water, to the detriment of the interconnected issues of food security, climate, energy and ecosystem health. The key assumption of these ‘hard path’ strategies is that society must find more and more supply to meet what was assumed to be never-ending increases in demand.

Nature Outlook: Water
That focus on supply has brought great benefits to many people, but it has also had unintended and increasingly negative consequences. Among these are the failure to provide safe water and sanitation to all; unsustainable overdraft of ground water to produce the food and fibre that the world’s 8 billion people need; inadequate regulation of water pollutants; massive ecological disruption of aquatic ecosystems; political and violent conflict over water resources; and now, accelerating climate disruption to water systems 1 .
A shift away from the supply-oriented hard path is possible — and necessary. Central to this change will be a transition to a focus on demand, efficiency and reuse, and on protecting and restoring ecosystems harmed by centuries of abuse. Society must move away from thinking about how to take more water from already over-tapped rivers, lakes and aquifers, and instead find ways to do the things we want with less water. These include, water technologies to transform industries and allow people to grow more food; appliances to reduce the amount of water used to flush toilets, and wash clothes and dishes; finding and plugging leaks in water-distribution systems and homes; and collecting, treating and reusing waste water.
Remarkably, and unbeknown to most people, the transition to a more efficient and sustainable future is already under way.
Singapore and Israel, two highly water-stressed regions, use much less water per person than do other high-income countries, and they recycle, treat and reuse more than 80% of their waste water 2 . New technologies, including precision irrigation, real-time soil-moisture monitoring and highly localized weather-forecasting models, allow farmers to boost yields and crop quality while cutting water use. Damaging, costly and dangerous dams are being removed, helping to restore rivers and fisheries.
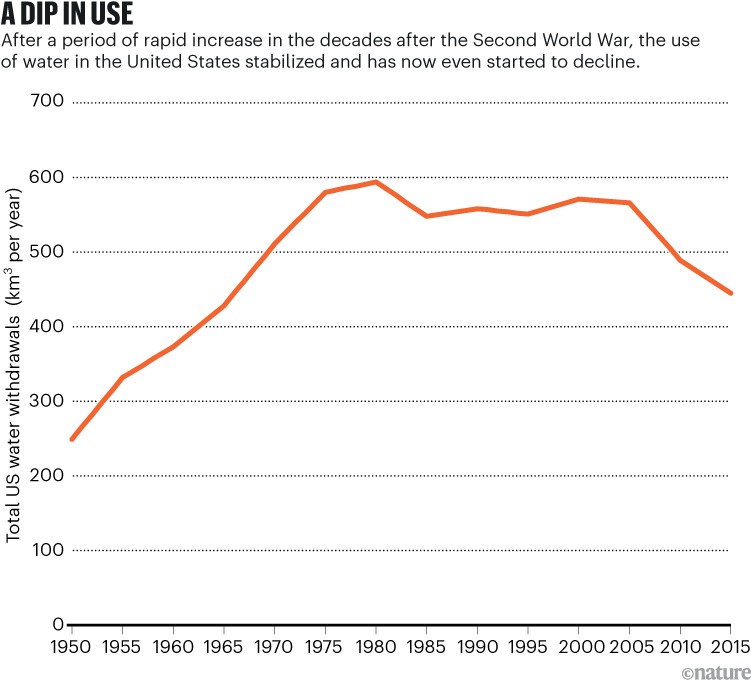
Source: US Geological Survey
In the United States, total water use is decreasing even though the population and the economy are expanding. Water withdrawals are much less today than they were 50 years ago (see ‘A dip in use’) — evidence that an efficiency revolution is under way. And the United States is indeed doing more with less, because during this time, there has been a marked increase in the economic productivity of water use, measured as units of gross domestic product per unit of water used (see ‘Doing more with less’). Similar trends are evident in many other countries.
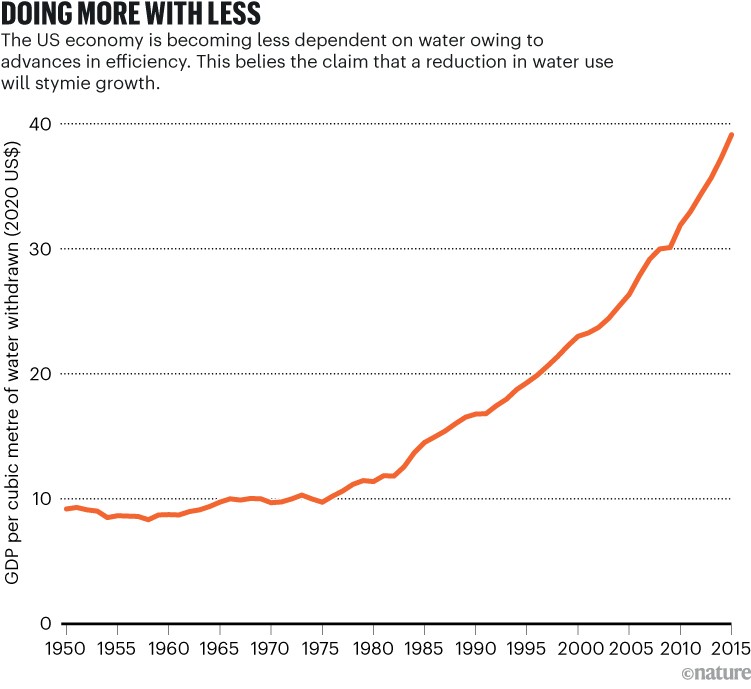
Source: US Geological Survey/US Department of Commerce.
Overcoming barriers
The challenge is how to accelerate this transition and overcome barriers to more sustainable and equitable water systems. One important obstacle is the lack of adequate financing and investment in expanding, upgrading and maintaining water systems. Others are institutional resistance in the form of weak or misdirected regulations, antiquated water-rights laws, and inadequate training of water managers with outdated ideas and tools. Another is blind adherence by authorities to old-fashioned ideas or simple ignorance about both the risks of the hard path and the potential of alternatives.
Funding for the modernization of water systems must be increased. In the United States, President Biden’s Infrastructure Investment and Jobs Act provides US$82.5 billion for water-related programmes, including removing toxic lead pipes and providing water services to long-neglected front-line communities. These communities include those dependent on unregulated rural water systems, farm-worker communities in California’s Central Valley, Indigenous populations and those in low-income urban centres with deteriorating infrastructure. That’s a good start. But more public- and private-investments are needed, especially to provide modern water and sanitation systems globally for those who still lack them, and to improve efficiency and reuse.
Regulations have been helpful in setting standards to cut waste and improve water quality, but further standards — and stronger enforcement — are needed to protect against new pollutants. Providing information on how to cut food waste on farms and in food processing, and how to shift diets to less water-intensive food choices can help producers and consumers to reduce their water footprints 3 . Corporations must expand water stewardship efforts in their operations and supply chains. Water institutions must be reformed and integrated with those that deal with energy and climate challenges. And we must return water to the environment to restore ecological systems that, in turn, protect human health and well-being.
In short, the status quo is not acceptable. Efforts must be made at all levels to accelerate the shift from simply supplying more water to meeting human and ecological water needs as carefully and efficiently as possible. No new technologies need to be invented for this to happen, and the economic costs of the transition are much less than the costs of failing to do so. Individuals, communities, corporations and governments all have a part to play. A sustainable water future is possible if we choose the right path.
doi: https://doi.org/10.1038/d41586-023-03899-2
This article is part of Nature Outlook: Water , a supplement produced with financial support from the FII Institute. Nature maintains full independence in all editorial decisions related to the content. About this content .
Caretta, M. A. et al . In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (eds Portner, H.-O. et al .) 551–712 (Cambridge Univ. Press, 2022)
Google Scholar
Giakoumis, T., Vaghela, C. & Voulvoulis, N. Adv. Chem. Pollut. Environ. Manag. Protect. 5 , 227–252 (2020).
Article Google Scholar
Heller, M. C., Willits-Smith, A., Mahon, T., Keoleian, G. A. & Rose, D. Nature Food 2 , 255–263 (2021).
Article PubMed Google Scholar
Download references
Related Articles

- Water resources
- Sustainability
- Environmental sciences

How to achieve safe water access for all: work with local communities
Comment 22 MAR 24

The Solar System has a new ocean — it’s buried in a small Saturn moon
News 07 FEB 24
Groundwater decline is global but not universal
News & Views 24 JAN 24
Real-world plastic-waste success stories can help to boost global treaty
Correspondence 14 MAY 24
Inequality is bad — but that doesn’t mean the rich are

Diana Wall obituary: ecologist who foresaw the importance of soil biodiversity
Obituary 10 MAY 24

Forestry social science is failing the needs of the people who need it most
Editorial 15 MAY 24
One-third of Southern Ocean productivity is supported by dust deposition
Article 15 MAY 24
Postdoc in CRISPR Meta-Analytics and AI for Therapeutic Target Discovery and Priotisation (OT Grant)
APPLICATION CLOSING DATE: 14/06/2024 Human Technopole (HT) is a new interdisciplinary life science research institute created and supported by the...
Human Technopole
Research Associate - Metabolism
Houston, Texas (US)
Baylor College of Medicine (BCM)
Postdoc Fellowships
Train with world-renowned cancer researchers at NIH? Consider joining the Center for Cancer Research (CCR) at the National Cancer Institute
Bethesda, Maryland
NIH National Cancer Institute (NCI)
Faculty Recruitment, Westlake University School of Medicine
Faculty positions are open at four distinct ranks: Assistant Professor, Associate Professor, Full Professor, and Chair Professor.
Hangzhou, Zhejiang, China
Westlake University
PhD/master's Candidate
PhD/master's Candidate Graduate School of Frontier Science Initiative, Kanazawa University is seeking candidates for PhD and master's students i...
Kanazawa University
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Cape Cod Has a Big Septic Tank Problem
Cape Cod’s water is turning “pea-soup green”—and after decades of scientific detective work, we know why.
Rachel Feltman, Barbara Moran, Kathleen Masterson, Madison Goldberg, Jeffery DelViscio

Strangely Shaped Bubbles Tell the Story of Ice’s Formation and Composition
Bubbles shaped like teardrops, flattened eggs and worms reveal ice’s inner life
Rachel Berkowitz

Imagining a Radical New Relationship with the Mississippi River
The Mississippi River has been manipulated for decades. A new book considers alternative forms of control
Meera Subramanian

Is Cold-Water Swimming Good for You?
Though sometimes overstated, the benefits of cold-water swimming are slowly becoming clearer
Jesse Greenspan

Will Mexico City Run Out of Water?
More huge cities are facing Day Zero—the date water taps go dry—just as Cape Town, South Africa, did
Jordan Kinard

How Will EPA’s New Rule about ‘Forever Chemicals’ Protect Your Drinking Water?
A new EPA rule will limit PFASs, or “forever chemicals,” in your drinking water for the first time. Here’s what that means for you
Katherine Bourzac

Cape Cod Faces a Rising 'Yellow Tide'
Tourism is big business on the cape, but a growing environmental issue could disrupt the lives of tourists and residents, alike.
Duy Linh Tu, Sebastian Tuinder, Barbara Moran, Anaissa Ruiz Tejada, Joseph Polidoro, Jeffery DelViscio

As Israel Floods Gaza’s Tunnels with Seawater, Scientists Worry about Aquifer Contamination
The Israel Defense Forces has confirmed it is dumping seawater into the web of tunnels beneath Gaza, which scientists say may contaminate the aquifer that supplies most of Gaza’s water
Josie Glausiusz, Nature magazine

Groundwater Is Declining Globally, but There Are Hopeful Exceptions
The most detailed global look at groundwater yet shows a lot of loss but also stories of success in restoring some aquifers
Stephanie Pappas

Why Are Alaska’s Rivers Turning Orange?
Streams in Alaska are turning orange with iron and sulfuric acid. Scientists are trying to figure out why

PFAS ‘Forever Chemicals’ Found in Freshwater Fish, Yet Most States Don’t Warn Residents
Staggering amounts of toxic “forever chemicals” have been found in freshwater fish, but there is no federal guidance on what is a safe amount to eat
Hannah Norman, KFF Health News

First-Ever Flood Forecasting Maps Show Houses and Roads at Risk
The National Weather Service has launched the first flood forecasting system with precise, real-time data showing spots that are at imminent risk of inundation
Minho Kim, E&E News
November 30, 2023
Traversing the waterways
In their anniversary editorial, Editors-in-Chief Jenna Davis and Pierre Horwitz and Executive Editor Debora Walker reflect on PLOS Water' s first year and their hopes and expectations for the future.
Image credit: PLOS
Collection: Safe and Sustainable Water in Cities
This collection features articles that contribute new insights to the theme of fresh water in cities..
Water use and management
Water sovereignty for Indigenous Peoples: Pathways to pluralist, legitimate and sustainable water laws in settler colonial states
In this Review, Erin O’Donnell discusses how challenging false assumption of aqua nullius creates novel pathways for reform, enabling pluralist water laws and water governance models that improve both legitimacy and sustainability of settler state water governance.
Image credit: Drop of Water, by ronymichaud, Pixabay License
governance, policy, and politics
Putting diplomacy at the forefront of Water Diplomacy
In this Review, Hussein and colleagues stress the need to emphasize diplomacy and the goals beyond the water field in transboundary water governance.
Image credit: handshake by Gerd Altmann, Pixabay
water use and management
Safe and sustainable water in cities
In this Editorial, Narayan and Davis introduce our latest Collection and take a look at the themes that emerged as worthy research priorities for the urban water community.
Image credit: Tokyo by Telophase, Pixabay
Analysis of Microcystis aeruginosa physiology by spectral flow cytometry: Impact of chemical and light exposure
Brentjens and colleagues examined the impact of H2O2 and light stress on harmful algal bloom-forming cyanobacteria fluorescence using flow cytometry.
Image credit: cyanobacteria by armennano, Pixabay
Health IMPACTS AND SANITATION
A tale of two communities: Comparing user perceptions of condominial and conventional sewer systems in Salvador, Brazil
Palma and colleagues compare user perceptions of sewer systems in two communities in Brazil to inform implementation and long-term sustainability.
Image credit: Sewer Cover, by knavilio, Pixabay License
SOCIAL, CULTURAL, AND BEHAVIOURAL RESEARCH
Nature based solutions for flood risks: What insights do the social representations of experts provide?
Brueder and colleagues consider solutions for flood risks in the context of social representations
Image credit: Breuder et al., CC BY 4.0
Equity AND JUSTICE
Key mechanisms of a gender and socially inclusive community engagement and participatory design approach in the RISE program in Makassar, Indonesia and Suva, Fiji
Francis and colleagues identify mechanisms for engaging diverse residents in safe water access and management programs such as ‘RISE’
Image credit: Splashing, By PublicDomainPictures, Pixabay License
WATER RESOURCES AND HYDROLOGY
Irish surface water response to the 2018 drought
Image credit: Smith et al., cc BY 4.0
WATER AND WASTE WATER TREATMENT
How do water matrices influence QSPR models in wastewater treatment?–A case study on the sonolytic elimination of phenol derivates
Image credit: Glienke et al., CC BY 4.0
Spatiotemporal trends in particle-associated microbial communities in a chlorinated drinking water distribution system
Image credit: Ferrebee et al., CC BY 4.0
WATER AND HYDROLOGY
Improved urban runoff prediction using high-resolution land-use, imperviousness, and stormwater infrastructure data applied to a process-based ecohydrological model
Image credit: Halama et al., CC BY 4.0
Safe and Sustainable Water in Cities
World water day 2023 – accelerating change, explore the latest research making an impact in your field, applying inclusive authorship criteria: two examples from plos water, river restoration, special collection for the 2023 unc water and health conference: science, policy and practice, publish with plos.
- Submission Instructions
- Submit Your Manuscript
Connect with Us
- PLOS Water on Twitter
- PLOS on Facebook
Get new content from PLOS Water in your inbox
Thank you you have successfully subscribed to the plos water newsletter., sorry, an error occurred while sending your subscription. please try again later..
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Article Collection Archive
- Author Guidelines
- Submission Site
- Open Access
- Call for Papers
- Why Publish?
- About Nutrition Reviews
- About International Life Sciences Institute
- Editorial Board
- Early Career Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, physiological effects of dehydration, hydration and chronic diseases, water consumption and requirements and relationships to total energy intake, water requirements: evaluation of the adequacy of water intake, acknowledgments, water, hydration, and health.
- Article contents
- Figures & tables
- Supplementary Data
Barry M Popkin, Kristen E D'Anci, Irwin H Rosenberg, Water, hydration, and health, Nutrition Reviews , Volume 68, Issue 8, 1 August 2010, Pages 439–458, https://doi.org/10.1111/j.1753-4887.2010.00304.x
- Permissions Icon Permissions
This review examines the current knowledge of water intake as it pertains to human health, including overall patterns of intake and some factors linked with intake, the complex mechanisms behind water homeostasis, and the effects of variation in water intake on health and energy intake, weight, and human performance and functioning. Water represents a critical nutrient, the absence of which will be lethal within days. Water's importance for the prevention of nutrition-related noncommunicable diseases has received more attention recently because of the shift toward consumption of large proportions of fluids as caloric beverages. Despite this focus, there are major gaps in knowledge related to the measurement of total fluid intake and hydration status at the population level; there are also few longer-term systematic interventions and no published randomized, controlled longer-term trials. This review provides suggestions for ways to examine water requirements and encourages more dialogue on this important topic.
Water is essential for life. From the time that primeval species ventured from the oceans to live on land, a major key to survival has been the prevention of dehydration. The critical adaptations cross an array of species, including man. Without water, humans can survive only for days. Water comprises from 75% body weight in infants to 55% in the elderly and is essential for cellular homeostasis and life. 1 Nevertheless, there are many unanswered questions about this most essential component of our body and our diet. This review attempts to provide some sense of our current knowledge of water, including overall patterns of intake and some factors linked with intake, the complex mechanisms behind water homeostasis, the effects of variation in water intake on health and energy intake, weight, and human performance and functioning.
Recent statements on water requirements have been based on retrospective recall of water intake from food and beverages among healthy, noninstitutionalized individuals. Provided here are examples of water intake assessment in populations to clarify the need for experimental studies. Beyond these circumstances of dehydration, it is not fully understood how hydration affects health and well-being, even the impact of water intakes on chronic diseases. Recently, Jéquier and Constant 2 addressed this question based on human physiology, but more knowledge is required about the extent to which water intake might be important for disease prevention and health promotion.
As noted later in the text, few countries have developed water requirements and those that exist are based on weak population-level measures of water intake and urine osmolality. 3 , 4 The European Food Safety Authority (EFSA) was recently asked to revise existing recommended intakes of essential substances with a physiological effect, including water since this nutrient is essential for life and health. 5
The US Dietary Recommendations for water are based on median water intakes with no use of measurements of the dehydration status of the population to assist. One-time collection of blood samples for the analysis of serum osmolality has been used by the National Health and Nutrition Examination Survey program. At the population level, there is no accepted method of assessing hydration status, and one measure some scholars use, hypertonicity, is not even linked with hydration in the same direction for all age groups. 6 Urine indices are used often but these reflect the recent volume of fluid consumed rather than a state of hydration. 7 Many scholars use urine osmolality to measure recent hydration status. 8 , – 12 Deuterium dilution techniques (isotopic dilution with D 2 O, or deuterium oxide) allow measurement of total body water but not water balance status. 13 Currently, there are no completely adequate biomarkers to measure hydration status at the population level.
In discussing water, the focus is first and foremost on all types of water, whether it be soft or hard, spring or well, carbonated or distilled. Furthermore, water is not only consumed directly as a beverage; it is also obtained from food and to a very small extent from oxidation of macronutrients (metabolic water). The proportion of water that comes from beverages and food varies according to the proportion of fruits and vegetables in the diet. The ranges of water content in various foods are presented in Table 1 . In the United States it is estimated that about 22% of water intake comes from food while the percentages are much higher in European countries, particularly a country like Greece with its higher intake of fruits and vegetables, or in South Korea. 3 , – 15 The only in-depth study performed in the United States of water use and water intrinsic to food found a 20.7% contribution from food water; 16 , 17 however, as shown below, this research was dependent on poor overall assessment of water intake.
Ranges of water content for selected foods.
Data from the USDA national nutrient database for standard reference, release 21, as provided in Altman. 126
This review considers water requirements in the context of recent efforts to assess water intake in US populations. The relationship between water and calorie intake is explored both for insights into the possible displacement of calories from sweetened beverages by water and to examine the possibility that water requirements would be better expressed in relation to calorie/energy requirements with the dependence of the latter on age, size, gender, and physical activity level. Current understanding of the exquisitely complex and sensitive system that protects land animals against dehydration is covered and commentary is provided on the complications of acute and chronic dehydration in man, against which a better expression of water requirements might complement the physiological control of thirst. Indeed, the fine intrinsic regulation of hydration and water intake in individuals mitigates prevalent underhydration in populations and its effects on function and disease.
Regulation of fluid intake
To prevent dehydration, reptiles, birds, vertebrates, and all land animals have evolved an exquisitely sensitive network of physiological controls to maintain body water and fluid intake by thirst. Humans may drink for various reasons, particularly for hedonic ones, but drinking is most often due to water deficiency that triggers the so-called regulatory or physiological thirst. The mechanism of thirst is quite well understood today and the reason nonregulatory drinking is often encountered is related to the large capacity of the kidneys to rapidly eliminate excesses of water or to reduce urine secretion to temporarily economize on water. 1 But this excretory process can only postpone the necessity of drinking or of ceasing to drink an excess of water. Nonregulatory drinking is often confusing, particularly in wealthy societies that have highly palatable drinks or fluids that contain other substances the drinker seeks. The most common of these are sweeteners or alcohol for which water is used as a vehicle. Drinking these beverages is not due to excessive thirst or hyperdipsia, as can be shown by offering pure water to individuals instead and finding out that the same drinker is in fact hypodipsic (characterized by abnormally diminished thirst). 1
Fluid balance of the two compartments
Maintaining a constant water and mineral balance requires the coordination of sensitive detectors at different sites in the body linked by neural pathways with integrative centers in the brain that process this information. These centers are also sensitive to humoral factors (neurohormones) produced for the adjustment of diuresis, natriuresis, and blood pressure (angiotensin mineralocorticoids, vasopressin, atrial natriuretic factor). Instructions from the integrative centers to the “executive organs” (kidney, sweat glands, and salivary glands) and to the part of the brain responsible for corrective actions such as drinking are conveyed by certain nerves in addition to the above-mentioned substances. 1
Most of the components of fluid balance are controlled by homeostatic mechanisms responding to the state of body water. These mechanisms are sensitive and precise, and are activated with deficits or excesses of water amounting to only a few hundred milliliters. A water deficit produces an increase in the ionic concentration of the extracellular compartment, which takes water from the intracellular compartment causing cells to shrink. This shrinkage is detected by two types of brain sensors, one controlling drinking and the other controlling the excretion of urine by sending a message to the kidneys, mainly via the antidiuretic hormone vasopressin to produce a smaller volume of more concentrated urine. 18 When the body contains an excess of water, the reverse processes occur: the lower ionic concentration of body fluids allows more water to reach the intracellular compartment. The cells imbibe, drinking is inhibited, and the kidneys excrete more water.
The kidneys thus play a key role in regulating fluid balance. As discussed later, the kidneys function more efficiently in the presence of an abundant water supply. If the kidneys economize on water and produce more concentrated urine, they expend a greater amount of energy and incur more wear on their tissues. This is especially likely to occur when the kidneys are under stress, e.g., when the diet contains excessive amounts of salt or toxic substances that need to be eliminated. Consequently, drinking a sufficient amount of water helps protect this vital organ.
Regulatory drinking
Most drinking occurs in response to signals of water deficit. Apart from urinary excretion, the other main fluid regulatory process is drinking, which is mediated through the sensation of thirst. There are two distinct mechanisms of physiological thirst: the intracellular and the extracellular mechanisms. When water alone is lost, ionic concentration increases. As a result, the intracellular space yields some of its water to the extracellular compartment. Once again, the resulting shrinkage of cells is detected by brain receptors that send hormonal messages to induce drinking. This association with receptors that govern extracellular volume is accompanied by an enhancement of appetite for salt. Thus, people who have been sweating copiously prefer drinks that are relatively rich in Na+ salts rather than pure water. When excessive sweating is experienced, it is also important to supplement drinks with additional salt.
The brain's decision to start or stop drinking and to choose the appropriate drink is made before the ingested fluid can reach the intra- and extracellular compartments. The taste buds in the mouth send messages to the brain about the nature, and especially the salt content, of the ingested fluid, and neuronal responses are triggered as if the incoming water had already reached the bloodstream. These are the so-called anticipatory reflexes: they cannot be entirely “cephalic reflexes” because they arise from the gut as well as the mouth. 1
The anterior hypothalamus and pre-optic area are equipped with osmoreceptors related to drinking. Neurons in these regions show enhanced firing when the inner milieu gets hyperosmotic. Their firing decreases when water is loaded in the carotid artery that irrigates the neurons. It is remarkable that the same decrease in firing in the same neurons takes place when the water load is applied on the tongue instead of being injected into the carotid artery. This anticipatory drop in firing is due to communication from neural pathways that depart from the mouth and converge onto neurons that simultaneously sense the blood's inner milieu.
Nonregulatory drinking
Although everyone experiences thirst from time to time, it plays little role in the day-to-day control of water intake in healthy people living in temperate climates. In these regions, people generally consume fluids not to quench thirst, but as components of everyday foods (e.g., soup, milk), as beverages used as mild stimulants (tea, coffee), and for pure pleasure. A common example is alcohol consumption, which can increase individual pleasure and stimulate social interaction. Drinks are also consumed for their energy content, as in soft drinks and milk, and are used in warm weather for cooling and in cold weather for warming. Such drinking seems to also be mediated through the taste buds, which communicate with the brain in a kind of “reward system”, the mechanisms of which are just beginning to be understood. This bias in the way human beings rehydrate themselves may be advantageous because it allows water losses to be replaced before thirst-producing dehydration takes place. Unfortunately, this bias also carries some disadvantages. Drinking fluids other than water can contribute to an intake of caloric nutrients in excess of requirements, or in alcohol consumption that, in some people, may insidiously bring about dependence. For example, total fluid intake increased from 79 fluid ounces in 1989 to 100 fluid ounces in 2002 among US adults, with the difference representing intake of caloric beverages. 19
Effects of aging on fluid intake regulation
The thirst and fluid ingestion responses of older persons to a number of stimuli have been compared to those of younger persons. 20 Following water deprivation, older individuals are less thirsty and drink less fluid compared to younger persons. 21 , 22 The decrease in fluid consumption is predominantly due to a decrease in thirst, as the relationship between thirst and fluid intake is the same in young and old persons. Older persons drink insufficient amounts of water following fluid deprivation to replenish their body water deficit. 23 When dehydrated older persons are offered a highly palatable selection of drinks, this also fails to result in increased fluid intake. 23 The effects of increased thirst in response to an osmotic load have yielded variable responses, with one group reporting reduced osmotic thirst in older individuals 24 and one failing to find a difference. In a third study, young individuals ingested almost twice as much fluid as old persons, even though the older subjects had a much higher serum osmolality. 25
Overall, these studies support small changes in the regulation of thirst and fluid intake with aging. Defects in both osmoreceptors and baroreceptors appear to exist as do changes in the central regulatory mechanisms mediated by opioid receptors. 26 Because the elderly have low water reserves, it may be prudent for them to learn to drink regularly when not thirsty and to moderately increase their salt intake when they sweat. Better education on these principles may help prevent sudden hypotension and stroke or abnormal fatigue, which can lead to a vicious circle and eventually hospitalization.
Thermoregulation
Hydration status is critical to the body's process of temperature control. Body water loss through sweat is an important cooling mechanism in hot climates and in periods of physical activity. Sweat production is dependent upon environmental temperature and humidity, activity levels, and type of clothing worn. Water losses via skin (both insensible perspiration and sweating) can range from 0.3 L/h in sedentary conditions to 2.0 L/h in high activity in the heat, and intake requirements range from 2.5 to just over 3 L/day in adults under normal conditions, and can reach 6 L/day with high extremes of heat and activity. 27 , 28 Evaporation of sweat from the body results in cooling of the skin. However, if sweat loss is not compensated for with fluid intake, especially during vigorous physical activity, a hypohydrated state can occur with concomitant increases in core body temperature. Hypohydration from sweating results in a loss of electrolytes, as well as a reduction in plasma volume, and this can lead to increased plasma osmolality. During this state of reduced plasma volume and increased plasma osmolality, sweat output becomes insufficient to offset increases in core temperature. When fluids are given to maintain euhydration, sweating remains an effective compensation for increased core temperatures. With repeated exposure to hot environments, the body adapts to heat stress and cardiac output and stroke volume return to normal, sodium loss is conserved, and the risk for heat-stress-related illness is reduced. 29 Increasing water intake during this process of heat acclimatization will not shorten the time needed to adapt to the heat, but mild dehydration during this time may be of concern and is associated with elevations in cortisol, increased sweating, and electrolyte imbalances. 29
Children and the elderly have differing responses to ambient temperature and different thermoregulatory concerns than healthy adults. Children in warm climates may be more susceptible to heat illness than adults due to their greater surface area to body mass ratio, lower rate of sweating, and slower rate of acclimatization to heat. 30 , 31 Children may respond to hypohydration during activity with a higher relative increase in core temperature than adults, 32 and with a lower propensity to sweat, thus losing some of the benefits of evaporative cooling. However, it has been argued that children can dissipate a greater proportion of body heat via dry heat loss, and the concomitant lack of sweating provides a beneficial means of conserving water under heat stress. 30 Elders, in response to cold stress, show impairments in thermoregulatory vasoconstriction, and body water is shunted from plasma into the interstitial and intracellular compartments. 33 , 34 With respect to heat stress, water lost through sweating decreases the water content of plasma, and the elderly are less able to compensate for increased blood viscosity. 33 Not only do they have a physiological hypodipsia, but this can be exaggerated by central nervous system disease 35 and by dementia. 36 In addition, illness and limitations in daily living activities can further limit fluid intake. When reduced fluid intake is coupled with advancing age, there is a decrease in total body water. Older individuals have impaired renal fluid conservation mechanisms and, as noted above, have impaired responses to heat and cold stress. 33 , 34 All of these factors contribute to an increased risk of hypohydration and dehydration in the elderly.
With regard to physiology, the role of water in health is generally characterized in terms of deviations from an ideal hydrated state, generally in comparison to dehydration. The concept of dehydration encompasses both the process of losing body water and the state of dehydration. Much of the research on water and physical or mental functioning compares a euhydrated state, usually achieved by provision of water sufficient to overcome water loss, to a dehydrated state, which is achieved via withholding of fluids over time and during periods of heat stress or high activity. In general, provision of water is beneficial in individuals with a water deficit, but little research supports the notion that additional water in adequately hydrated individuals confers any benefit.
Physical performance
The role of water and hydration in physical activity, particularly in athletes and in the military, has been of considerable interest and is well-described in the scientific literature. 37 , – 39 During challenging athletic events, it is not uncommon for athletes to lose 6–10% of body weight through sweat, thus leading to dehydration if fluids have not been replenished. However, decrements in the physical performance of athletes have been observed under much lower levels of dehydration, i.e., as little as 2%. 38 Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort. 40 , 41 Rehydration can reverse these deficits and reduce the oxidative stress induced by exercise and dehydration. 42 Hypohydration appears to have a more significant impact on high-intensity and endurance activity, such as tennis 43 and long-distance running, 44 than on anaerobic activities, 45 such as weight lifting, or on shorter-duration activities, such as rowing. 46
During exercise, individuals may not hydrate adequately when allowed to drink according to thirst. 32 After periods of physical exertion, voluntary fluid intake may be inadequate to offset fluid deficits. 1 Thus, mild-to-moderate dehydration can persist for some hours after the conclusion of physical activity. Research performed on athletes suggests that, principally at the beginning of the training season, they are at particular risk for dehydration due to lack of acclimatization to weather conditions or suddenly increased activity levels. 47 , 48 A number of studies show that performance in temperate and hot climates is affected to a greater degree than performance in cold temperatures. 41 , – 50 Exercise in hot conditions with inadequate fluid replacement is associated with hyperthermia, reduced stroke volume and cardiac output, decreases in blood pressure, and reduced blood flow to muscle. 51
During exercise, children may be at greater risk for voluntary dehydration. Children may not recognize the need to replace lost fluids, and both children as well as coaches need specific guidelines for fluid intake. 52 Additionally, children may require more time to acclimate to increases in environmental temperature than adults. 30 , 31 Recommendations are for child athletes or children in hot climates to begin athletic activities in a well-hydrated state and to drink fluids over and above the thirst threshold.
Cognitive performance
Water, or its lack (dehydration), can influence cognition. Mild levels of dehydration can produce disruptions in mood and cognitive functioning. This may be of special concern in the very young, very old, those in hot climates, and those engaging in vigorous exercise. Mild dehydration produces alterations in a number of important aspects of cognitive function such as concentration, alertness, and short-term memory in children (10–12 y), 32 young adults (18–25 y), 53 , – 56 and the oldest adults (50–82 y). 57 As with physical functioning, mild-to-moderate levels of dehydration can impair performance on tasks such as short-term memory, perceptual discrimination, arithmetic ability, visuomotor tracking, and psychomotor skills. 53 , – 56 However, mild dehydration does not appear to alter cognitive functioning in a consistent manner. 53 , – 58 In some cases, cognitive performance was not significantly affected in ranges from 2% to 2.6% dehydration. 56 , 58 Comparing across studies, performance on similar cognitive tests was divergent under dehydration conditions. 54 , 56 In studies conducted by Cian et al., 53 , 54 participants were dehydrated to approximately 2.8% either through heat exposure or treadmill exercise. In both studies, performance was impaired on tasks examining visual perception, short-term memory, and psychomotor ability. In a series of studies using exercise in conjunction with water restriction as a means of producing dehydration, D'Anci et al. 56 observed only mild decrements in cognitive performance in healthy young men and women athletes. In these experiments, the only consistent effect of mild dehydration was significant elevations of subjective mood score, including fatigue, confusion, anger, and vigor. Finally, in a study using water deprivation alone over a 24-h period, no significant decreases in cognitive performance were seen with 2.6% dehydration. 58 It is therefore possible that heat stress may play a critical role in the effects of dehydration on cognitive performance.
Reintroduction of fluids under conditions of mild dehydration can reasonably be expected to reverse dehydration-induced cognitive deficits. Few studies have examined how fluid reintroduction may alleviate the negative effects of dehydration on cognitive performance and mood. One study 59 examined how water ingestion affected arousal and cognitive performance in young people following a period of 12-h water restriction. While cognitive performance was not affected by either water restriction or water consumption, water ingestion affected self-reported arousal. Participants reported increased alertness as a function of water intake. Rogers et al. 60 observed a similar increase in alertness following water ingestion in both high- and low-thirst participants. Water ingestion, however, had opposite effects on cognitive performance as a function of thirst. High-thirst participants' performance on a cognitively demanding task improved following water ingestion, but low-thirst participants' performance declined. In summary, hydration status consistently affected self-reported alertness, but effects on cognition were less consistent.
Several recent studies have examined the utility of providing water to school children on attentiveness and cognitive functioning in children. 61 , – 63 In these experiments, children were not fluid restricted prior to cognitive testing, but were allowed to drink as usual. Children were then provided with a drink or no drink 20–45 min before the cognitive test sessions. In the absence of fluid restriction and without physiological measures of hydration status, the children in these studies should not be classified as dehydrated. Subjective measures of thirst were reduced in children given water, 62 and voluntary water intake in children varied from 57 mL to 250 mL. In these studies, as in the studies in adults, the findings were divergent and relatively modest. In the research led by Edmonds et al., 61 , 62 children in the groups given water showed improvements in visual attention. However, effects on visual memory were less consistent, with one study showing no effects of drinking water on a spot-the-difference task in 6–7-year-old children 61 and the other showing a significant improvement in a similar task in 7–9-year-old children. 62 In the research described by Benton and Burgess, 63 memory performance was improved by provision of water but sustained attention was not altered with provision of water in the same children.
Taken together, these studies indicate that low-to-moderate dehydration may alter cognitive performance. Rather than indicating that the effects of hydration or water ingestion on cognition are contradictory, many of the studies differ significantly in methodology and in measurement of cognitive behaviors. These variances in methodology underscore the importance of consistency when examining relatively subtle chances in overall cognitive performance. However, in those studies in which dehydration was induced, most combined heat and exercise; this makes it difficult to disentangle the effects of dehydration on cognitive performance in temperate conditions from the effects of heat and exercise. Additionally, relatively little is known about the mechanism of mild dehydration's effects on mental performance. It has been proposed that mild dehydration acts as a physiological stressor that competes with and draws attention from cognitive processes. 64 However, research on this hypothesis is limited and merits further exploration.
Dehydration and delirium
Dehydration is a risk factor for delirium and for delirium presenting as dementia in the elderly and in the very ill. 65 , – 67 Recent work shows that dehydration is one of several predisposing factors for confusion observed in long-term-care residents 67 ; however, in this study, daily water intake was used as a proxy measure for dehydration rather than other, more direct clinical assessments such as urine or plasma osmolality. Older people have been reported as having reduced thirst and hypodipsia relative to younger people. In addition, fluid intake and maintenance of water balance can be complicated by factors such as disease, dementia, incontinence, renal insufficiency, restricted mobility, and drug side effects. In response to primary dehydration, older people have less thirst sensation and reduced fluid intakes in comparison to younger people. However, in response to heat stress, while older people still display a reduced thirst threshold, they do ingest comparable amounts of fluid to younger people. 20
Gastrointestinal function
Fluids in the diet are generally absorbed in the proximal small intestine, and the absorption rate is determined by the rate of gastric emptying to the small intestine. Therefore, the total volume of fluid consumed will eventually be reflected in water balance, but the rate at which rehydration occurs is dependent upon factors affecting the rate of delivery of fluids to the intestinal mucosa. The gastric emptying rate is generally accelerated by the total volume consumed and slowed by higher energy density and osmolality. 68 In addition to water consumed in food (1 L/day) and beverages (circa 2–3 L/day), digestive secretions account for a far greater portion of water that passes through and is absorbed by the gastrointestinal tract (circa 8 L/day). 69 The majority of this water is absorbed by the small intestine, with a capacity of up to 15 L/day with the colon absorbing some 5 L/day. 69
Constipation, characterized by slow gastrointestinal transit, small, hard stools, and difficulty in passing stool, has a number of causes, including medication use, inadequate fiber intake, poor diet, and illness. 70 Inadequate fluid consumption is touted as a common culprit in constipation, and increasing fluid intake is a frequently recommended treatment. Evidence suggests, however, that increasing fluids is only useful to individuals in a hypohydrated state, and is of little utility in euhydrated individuals. 70 In young children with chronic constipation, increasing daily water intake by 50% did not affect constipation scores. 71 For Japanese women with low fiber intake, concomitant low water intake in the diet is associated with increased prevalence of constipation. 72 In older individuals, low fluid intake is a predictor for increased levels of acute constipation, 73 , 74 with those consuming the least amount of fluid having over twice the frequency of constipation episodes than those consuming the most fluid. In one trial, researchers compared the utility of carbonated mineral water in reducing functional dyspepsia and constipation scores to tap water in individuals with functional dyspepsia. 75 When comparing carbonated mineral water to tap water, participants reported improvements in subjective gastric symptoms, but there were no significant improvements in gastric or intestinal function. The authors indicate it is not possible to determine to what degree the mineral content of the two waters contributed to perceived symptom relief, as the mineral water contained greater levels of magnesium and calcium than the tap water. The available evidence suggests that increased fluid intake should only be indicated in individuals in a hypohydrated state. 69 , 71
Significant water loss can occur through the gastrointestinal tract, and this can be of great concern in the very young. In developing countries, diarrheal diseases are a leading cause of death in children, resulting in approximately 1.5–2.5 million deaths per year. 76 Diarrheal illness results not only in a reduction in body water, but also in potentially lethal electrolyte imbalances. Mortality in such cases can many times be prevented with appropriate oral rehydration therapy, by which simple dilute solutions of salt and sugar in water can replace fluid lost by diarrhea. Many consider application of oral rehydration therapy to be one of the significant public health developments of the last century. 77
Kidney function
As noted above, the kidney is crucial in regulating water balance and blood pressure as well as removing waste from the body. Water metabolism by the kidney can be classified into regulated and obligate. Water regulation is hormonally mediated, with the goal of maintaining a tight range of plasma osmolality (between 275 and 290 mOsm/kg). Increases in plasma osmolality and activation of osmoreceptors (intracellular) and baroreceptors (extracellular) stimulate hypothalamic release of arginine vasopressin (AVP). AVP acts at the kidney to decrease urine volume and promote retention of water, and the urine becomes hypertonic. With decreased plasma osmolality, vasopressin release is inhibited, and the kidney increases hypotonic urinary output.
In addition to regulating fluid balance, the kidneys require water for the filtration of waste from the bloodstream and excretion via urine. Water excretion via the kidney removes solutes from the blood, and a minimum obligate urine volume is required to remove the solute load with a maximum output volume of 1 L/h. 78 This obligate volume is not fixed, but is dependent upon the amount of metabolic solutes to be excreted and levels of AVP. Depending on the need for water conservation, basal urine osmolality ranges from 40 mOsm/kg to a maximum of 1,400 mOsm/kg. 78 The ability to both concentrate and dilute urine decreases with age, with a lower value of 92 mOsm/kg and an upper range falling between 500 and 700 mOsm/kg for individuals over the age of 70 years. 79 , – 81 Under typical conditions, in an average adult, urine volume of 1.5 to 2.0 L/day would be sufficient to clear a solute load of 900 to 1,200 mOsm/day. During water conservation and the presence of AVP, this obligate volume can decrease to 0.75–1.0 L/day and during maximal diuresis up to 20 L/day can be required to remove the same solute load. 78 , – 81 In cases of water loading, if the volume of water ingested cannot be compensated for with urine output, having overloaded the kidney's maximal output rate, an individual can enter a hyponatremic state.
Heart function and hemodynamic response
Blood volume, blood pressure, and heart rate are closely linked. Blood volume is normally tightly regulated by matching water intake and water output, as described in the section on kidney function. In healthy individuals, slight changes in heart rate and vasoconstriction act to balance the effect of normal fluctuations in blood volume on blood pressure. 82 Decreases in blood volume can occur, through blood loss (or blood donation), or loss of body water through sweat, as seen with exercise. Blood volume is distributed differently relative to the position of the heart, whether supine or upright, and moving from one position to the other can lead to increased heart rate, a fall in blood pressure, and, in some cases, syncope. This postural hypotension (or orthostatic hypotension) can be mediated by drinking 300–500 mL of water. 83 , 84 Water intake acutely reduces heart rate and increases blood pressure in both normotensive and hypertensive individuals. 85 These effects of water intake on the pressor effect and heart rate occur within 15–20 min of drinking water and can last for up to 60 min. Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope. 86 The effect of water intake in these situations is thought to be due to effects on the sympathetic nervous system rather than to changes in blood volume. 83 , 84 Interestingly, in rare cases, individuals may experience bradycardia and syncope after swallowing cold liquids. 87 , – 89 While swallow syncope can be seen with substances other than water, swallow syncope further supports the notion that the result of water ingestion in the pressor effect has both a neural component as well as a cardiac component.
Water deprivation and dehydration can lead to the development of headache. 90 Although this observation is largely unexplored in the medical literature, some observational studies indicate that water deprivation, in addition to impairing concentration and increasing irritability, can serve as a trigger for migraine and can also prolong migraine. 91 , 92 In those with water deprivation-induced headache, ingestion of water provided relief from headache in most individuals within 30 min to 3 h. 92 It is proposed that water deprivation-induced headache is the result of intracranial dehydration and total plasma volume. Although provision of water may be useful in relieving dehydration-related headache, the utility of increasing water intake for the prevention of headache is less well documented.
The folk wisdom that drinking water can stave off headaches has been relatively unchallenged, and has more traction in the popular press than in the medical literature. Recently, one study examined increased water intake and headache symptoms in headache patients. 93 In this randomized trial, patients with a history of different types of headache, including migraine and tension headache, were either assigned to a placebo condition (a nondrug tablet) or the increased water condition. In the water condition, participants were instructed to consume an additional volume of 1.5 L water/day on top of what they already consumed in foods and fluids. Water intake did not affect the number of headache episodes, but it was modestly associated with reduction in headache intensity and reduced duration of headache. The data from this study suggest that the utility of water as prophylaxis is limited in headache sufferers, and the ability of water to reduce or prevent headache in the broader population remains unknown.
One of the more pervasive myths regarding water intake is its relation to improvements of the skin or complexion. By improvement, it is generally understood that individuals are seeking to have a more “moisturized” look to the surface skin, or to minimize acne or other skin conditions. Numerous lay sources such as beauty and health magazines as well as postings on the Internet suggest that drinking 8–10 glasses of water a day will “flush toxins from the skin” and “give a glowing complexion” despite a general lack of evidence 94 , 95 to support these proposals. The skin, however, is important for maintaining body water levels and preventing water loss into the environment.
The skin contains approximately 30% water, which contributes to plumpness, elasticity, and resiliency. The overlapping cellular structure of the stratum corneum and lipid content of the skin serves as “waterproofing” for the body. 96 Loss of water through sweat is not indiscriminate across the total surface of the skin, but is carried out by eccrine sweat glands, which are evenly distributed over most of the body surface. 97 Skin dryness is usually associated with exposure to dry air, prolonged contact with hot water and scrubbing with soap (both strip oils from the skin), medical conditions, and medications. While more serious levels of dehydration can be reflected in reduced skin turgor, 98 , 99 with tenting of the skin acting as a flag for dehydration, overt skin turgor in individuals with adequate hydration is not altered. Water intake, particularly in individuals with low initial water intake, can improve skin thickness and density as measured by sonogram, 100 offsets transepidermal water loss, and can improve skin hydration. 101 Adequate skin hydration, however, is not sufficient to prevent wrinkles or other signs of aging, which are related to genetics and to sun and environmental damage. Of more utility to individuals already consuming adequate fluids is the use of topical emollients; these will improve skin barrier function and improve the look and feel of dry skin. 102 , 103
Many chronic diseases have multifactorial origins. In particular, differences in lifestyle and the impact of environment are known to be involved and constitute risk factors that are still being evaluated. Water is quantitatively the most important nutrient. In the past, scientific interest with regard to water metabolism was mainly directed toward the extremes of severe dehydration and water intoxication. There is evidence, however, that mild dehydration may also account for some morbidities. 4 , 104 There is currently no consensus on a “gold standard” for hydration markers, particularly for mild dehydration. As a consequence, the effects of mild dehydration on the development of several disorders and diseases have not been well documented.
There is strong evidence showing that good hydration reduces the risk of urolithiasis (see Table 2 for evidence categories). Less strong evidence links good hydration with reduced incidence of constipation, exercise asthma, hypertonic dehydration in the infant, and hyperglycemia in diabetic ketoacidosis. Good hydration is associated with a reduction in urinary tract infections, hypertension, fatal coronary heart disease, venous thromboembolism, and cerebral infarct, but all these effects need to be confirmed by clinical trials. For other conditions such as bladder or colon cancer, evidence of a preventive effect of maintaining good hydration is not consistent (see Table 3 ).
Categories of evidence used in evaluating the quality of reports.
Data adapted from Manz. 104
Summary of evidence for association of hydration status with chronic diseases.
Categories of evidence: described in Table 2 .
Water consumption, water requirements, and energy intake are linked in fairly complex ways. This is partially because physical activity and energy expenditures affect the need for water but also because a large shift in beverage consumption over the past century or more has led to consumption of a significant proportion of our energy intake from caloric beverages. Nonregulatory beverage intake, as noted earlier, has assumed a much greater role for individuals. 19 This section reviews current patterns of water intake and then refers to a full meta-analysis of the effects of added water on energy intake. This includes adding water to the diet and water replacement for a range of caloric and diet beverages, including sugar-sweetened beverages, juice, milk, and diet beverages. The third component is a discussion of water requirements and suggestions for considering the use of mL water/kcal energy intake as a metric.
Patterns and trends of water consumption
Measurement of total fluid water consumption in free-living individuals is fairly new in focus. As a result, the state of the science is poorly developed, data are most likely fairly incomplete, and adequate validation of the measurement techniques used is not available. Presented here are varying patterns and trends of water intake for the United States over the past three decades followed by a brief review of the work on water intake in Europe.
There is really no existing information to support an assumption that consumption of water alone or beverages containing water affects hydration differentially. 3 , 105 Some epidemiological data suggest water might have different metabolic effects when consumed alone rather than as a component of caffeinated or flavored or sweetened beverages; however, these data are at best suggestive of an issue deserving further exploration. 106 , 107 As shown below, the research of Ershow et al. indicates that beverages not consisting solely of water do contain less than 100% water.
One study in the United States has attempted to examine all the dietary sources of water. 16 , 17 These data are cited in Table 4 as the Ershow study and were based on National Food Consumption Survey food and fluid intake data from 1977–1978. These data are presented in Table 4 for children aged 2–18 years (Panel A) and for adults aged 19 years and older (Panel B). Ershow et al. 16 , 17 spent a great deal of time working out ways to convert USDA dietary data into water intake, including water absorbed during the cooking process, water in food, and all sources of drinking water.
Beverage pattern trends in the United States for children aged 2–18 years and adults aged 19 years and older, (nationally representative).
Note: The data are age and sex adjusted to 1965.
Values stem from the Ershow calculations. 16
These researchers created a number of categories and used a range of factors measured in other studies to estimate the water categories. The water that is found in food, based on food composition table data, was 393 mL for children. The water that was added as a result of cooking (e.g., rice) was 95 mL. Water consumed as a beverage directly as water was 624 mL. The water found in other fluids, as noted, comprised the remainder of the milliliters, with the highest levels in whole-fat milk and juices (506 mL). There is a small discrepancy between the Ershow data regarding total fluid intake measures for these children and the normal USDA figures. That is because the USDA does not remove milk fats and solids, fiber, and other food constituents found in beverages, particularly juice and milk.
A key point illustrated by these nationally representative US data is the enormous variability between survey waves in the amount of water consumed (see Figure 1 , which highlights the large variation in water intake as measured in these surveys). Although water intake by adults and children increased and decreased at the same time, for reasons that cannot be explained, the variation was greater among children than adults. This is partly because the questions the surveys posed varied over time and there was no detailed probing for water intake, because the focus was on obtaining measures of macro- and micronutrients. Dietary survey methods used in the past have focused on obtaining data on foods and beverages containing nutrient and non-nutritive sweeteners but not on water. Related to this are the huge differences between the the USDA surveys and the National Health and Nutrition Examination Survey (NHANES) performed in 1988–1994 and in 1999 and later. In addition, even the NHANES 1999–2002 and 2003–2006 surveys differ greatly. These differences reflect a shift in the mode of questioning with questions on water intake being included as part of a standard 24-h recall rather than as stand-alone questions. Water intake was not even measured in 1965, and a review of the questionnaires and the data reveals clear differences in the way the questions have been asked and the limitations on probes regarding water intake. Essentially, in the past people were asked how much water they consumed in a day and now they are asked for this information as part of a 24-h recall survey. However, unlike for other caloric and diet beverages, there are limited probes for water alone. The results must thus be viewed as crude approximations of total water intake without any strong research to show if they are over- or underestimated. From several studies of water and two ongoing randomized controlled trials performed by us, it is clear that probes that include consideration of all beverages and include water as a separate item result in the provision of more complete data.
Water consumption trends from USDA and NHANES surveys (mL/day/capita), nationally representative. Note: this includes water from fluids only, excluding water in foods. Sources for 1965, 1977–1978, 1989–1991, and 1994–1998, are USDA. Others are NHANES and 2005–2006 is joint USDA and NHANES.
Water consumption data for Europe are collected far more selectively than even the crude water intake questions from NHANES. A recent report from the European Food Safety Agency provides measures of water consumption from a range of studies in Europe. 4 , – 109 Essentially, what these studies show is that total water intake is lower across Europe than in the United States. As with the US data, none are based on long-term, carefully measured or even repeated 24-h recall measures of water intake from food and beverages. In an unpublished examination of water intake in UK adults in 1986–1987 and in 2001–2002, Popkin and Jebb have found that although intake increased by 226 mL/day over this time period, it was still only 1,787 mL/day in the latter period (unpublished data available from BP); this level is far below the 2,793 mL/day recorded in the United States for 2005–2006 or the earlier US figures for comparably aged adults.
A few studies have been performed in the United States and Europe utilizing 24-h urine and serum osmolality measures to determine total water turnover and hydration status. Results of these studies suggest that US adults consume over 2,100 mL of water per day while adults in Europe consume less than half a liter. 4 , 110 Data on total urine collection would appear to be another useful measure for examining total water intake. Of course, few studies aside from the Donald Study of an adolescent cohort in Germany have collected such data on population levels for large samples. 109
Effects of water consumption on overall energy intake
There is an extensive body of literature that focuses on the impact of sugar-sweetened beverages on weight and the risk of obesity, diabetes, and heart disease; however, the perspective of providing more water and its impact on health has not been examined. The literature on water does not address portion sizes; instead, it focuses mainly on water ad libitum or in selected portions compared with other caloric beverages. A detailed meta-analysis of the effects of water intake alone (i.e., adding additional water) and as a replacement for sugar-sweetened beverages, juice, milk, and diet beverages appears elsewhere. 111
In general, the results of this review suggest that water, when consumed in place of sugar-sweetened beverages, juice, and milk, is linked with reduced energy intake. This finding is mainly derived from clinical feeding studies but also from one very good randomized, controlled school intervention and several other epidemiological and intervention studies. Aside from the issue of portion size, factors such as the timing of beverage and meal intake (i.e., the delay between consumption of the beverage and consumption of the meal) and types of caloric sweeteners remain to be considered. However, when beverages are consumed in normal free-living conditions in which five to eight daily eating occasions are the norm, the delay between beverage and meal consumption may matter less. 112 , – 114
The literature on the water intake of children is extremely limited. However, the excellent German school intervention with water suggests the effects of water on the overall energy intake of children might be comparable to that of adults. 115 In this German study, children were educated on the value of water and provided with special filtered drinking fountains and water bottles in school. The intervention schoolchildren increased their water intake by 1.1 glasses/day ( P < 0.001) and reduced their risk of overweight by 31% (OR = 0.69, P = 0.40).
Classically, water data are examined in terms of milliliters (or some other measure of water volume consumed per capita per day by age group). This measure does not link fluid intake and caloric intake. Disassociation of fluid and calorie intake is difficult for clinicians dealing with older persons with reduced caloric intake. This milliliter water measure assumes some mean body size (or surface area) and a mean level of physical activity – both of which are determinants of not only energy expenditure but also water balance. Children are dependent on adults for access to water, and studies suggest that their larger surface area to volume ratio makes them susceptible to changes in skin temperatures linked with ambient temperature shifts. 116 One option utilized by some scholars is to explore food and beverage intake in milliliters per kilocalorie (mL/kcal), as was done in the 1989 US recommended dietary allowances. 4 , 117 This is an option that is interpretable for clinicians and which incorporates, in some sense, body size or surface area and activity. Its disadvantage is that water consumed with caloric beverages affects both the numerator and the denominator; however, an alternative measure that could be independent of this direct effect on body weight and/or total caloric intake is not presently known.
Despite its critical importance in health and nutrition, the array of available research that serves as a basis for determining requirements for water or fluid intake, or even rational recommendations for populations, is limited in comparison with most other nutrients. While this deficit may be partly explained by the highly sensitive set of neurophysiological adaptations and adjustments that occur over a large range of fluid intakes to protect body hydration and osmolarity, this deficit remains a challenge for the nutrition and public health community. The latest official effort at recommending water intake for different subpopulations occurred as part of the efforts to establish Dietary Reference Intakes in 2005, as reported by the Institute of Medicine of the National Academies of Science. 3 As a graphic acknowledgment of the limited database upon which to express estimated average requirements for water for different population groups, the Committee and the Institute of Medicine stated: “While it might appear useful to estimate an average requirement (an EAR) for water, an EAR based on data is not possible.” Given the extreme variability in water needs that are not solely based on differences in metabolism, but also on environmental conditions and activities, there is not a single level of water intake that would assure adequate hydration and optimum health for half of all apparently healthy persons in all environmental conditions. Thus, an adequate intake (AI) level was established in place of an EAR for water.
The AIs for different population groups were set as the median water intakes for populations, as reported in the National Health and Nutrition Examination Surveys; however, the intake levels reported in these surveys varied greatly based on the survey years (e.g., NHANES 1988–1994 versus NHANES 1999–2002) and were also much higher than those found in the USDA surveys (e.g., 1989–1991, 1994–1998, or 2005–2006). If the AI for adults, as expressed in Table 5 , is taken as a recommended intake, the wisdom of converting an AI into a recommended water or fluid intake seems questionable. The first problem is the almost certain inaccuracy of the fluid intake information from the national surveys, even though that problem may also exist for other nutrients. More importantly, from the standpoint of translating an AI into a recommended fluid intake for individuals or populations, is the decision that was made when setting the AI to add an additional roughly 20% of water intake, which is derived from some foods in addition to water and beverages. While this may have been a legitimate effort to use total water intake as a basis for setting the AI, the recommendations that derive from the IOM report would be better directed at recommendations for water and other fluid intake on the assumption that the water content of foods would be a “passive” addition to total water intake. In this case, the observations of the dietary reference intake committee that it is necessary for water intake to meet needs imposed by metabolism and environmental conditions must be extended to consider three added factors, namely body size, gender, and physical activity. Those are the well-studied factors that allow a rather precise measurement and determination of energy intake requirements. It is, therefore, logical that those same factors might underlie recommendations to meet water intake needs in the same populations and individuals. Consideration should also be given to the possibility that water intake needs would best be expressed relative to the calorie requirements, as is done regularly in the clinical setting, and data should be gathered to this end through experimental and population research.
Water requirements expressed in relation to energy recommendations.
AI for total fluids derived from dietary reference intakes for water, potassium, sodium, chloride, and sulphate.
Ratios for water intake based on the AI for water in liters/day calculated using EER for each range of physical activity. EER adapted from the Institute of Medicine Dietary Reference Intakes Macronutrients Report, 2002.
It is important to note that only a few countries include water on their list of nutrients. 118 The European Food Safety Authority is developing a standard for all of Europe. 105 At present, only the United States and Germany provide AI values for water. 3 , 119
Another approach to the estimation of water requirements, beyond the limited usefulness of the AI or estimated mean intake, is to express water intake requirements in relation to energy requirements in mL/kcal. An argument for this approach includes the observation that energy requirements for each age and gender group are strongly evidence-based and supported by extensive research taking into account both body size and activity level, which are crucial determinants of energy expenditure that must be met by dietary energy intake. Such measures of expenditure have used highly accurate methods, such as doubly labeled water; thus, estimated energy requirements have been set based on solid data rather than the compromise inherent in the AIs for water. Those same determinants of energy expenditure and recommended intake are also applicable to water utilization and balance, and this provides an argument for pegging water/fluid intake recommendations to the better-studied energy recommendations. The extent to which water intake and requirements are determined by energy intake and expenditure is understudied, but in the clinical setting it has long been practice to supply 1 mL/kcal administered by tube to patients who are unable to take in food or fluids. Factors such as fever or other drivers of increased metabolism affect both energy expenditure and fluid loss and are thus linked in clinical practice. This concept may well deserve consideration in the setting of population intake goals.
Finally, for decades there has been discussion about expressing nutrient requirements per 1,000 kcal so that a single number would apply reasonably across the spectrum of age groups. This idea, which has never been adopted by the Institute of Medicine and the National Academies of Science, may lend itself to an improved expression of water/fluid intake requirements, which must eventually replace the AIs. Table 5 presents the IOM water requirements and then develops a ratio of mL/kcal based on them. The European Food Safety Agency refers positively to the possibility of expressing water intake recommendations in mL/kcal as a function of energy requirements. 105 Outliers in the adult male categories, which reach ratios as high as 1.5, may well be based on the AI data from the United States, which are above those in the more moderate and likely more accurate European recommendations.
The topic of utilizing mL/kcal to examine water intake and water gaps is explored in Table 6 , which takes the full set of water intake AIs for each age-gender grouping and examines total intake. The data suggest a high level of fluid deficiency. Since a large proportion of fluids in the United States is based on caloric beverages and this proportion has changed markedly over the past 30 years, fluid intake increases both the numerator and the denominator of this mL/kcal relationship. Nevertheless, even using 1 mL/kcal as the AI would leave a gap for all children and adolescents. The NHANES physical activity data were also translated into METS/day to categorize all individuals by physical activity level and thus varying caloric requirements. Use of these measures reveals a fairly large fluid gap, particularly for adult males as well as children ( Table 6 ).
Water intake and water intake gaps based on US Water Adequate Intake Recommendations (based on utilization of water and physical activity data from NHANES 2005–2006).
Note: Recommended water intake for actual activity level is the upper end of the range for moderate and active.
A weighted average for the proportion of individuals in each METS-based activity level.
This review has pointed out a number of issues related to water, hydration, and health. Since water is undoubtedly the most important nutrient and the only one for which an absence will prove lethal within days, understanding of water measurement and water requirements is very important. The effects of water on daily performance and short- and long-term health are quite clear. The existing literature indicates there are few negative effects of water intake while the evidence for positive effects is quite clear.
Little work has been done to measure total fluid intake systematically, and there is no understanding of measurement error and best methods of understanding fluid intake. The most definitive US and European documents on total water requirements are based on these extant intake data. 3 , 105 The absence of validation methods for water consumption intake levels and patterns represents a major gap in knowledge. Even varying the methods of probing in order to collect better water recall data has been little explored.
On the other side of the issue is the need to understand total hydration status. There are presently no acceptable biomarkers of hydration status at the population level, and controversy exists about the current knowledge of hydration status among older Americans. 6 , 120 Thus, while scholars are certainly focused on attempting to create biomarkers for measuring hydration status at the population level, the topic is currently understudied.
As noted, the importance of understanding the role of fluid intake on health has emerged as a topic of increasing interest, partially because of the trend toward rising proportions of fluids being consumed in the form of caloric beverages. The clinical, epidemiological, and intervention literature on the effects of added water on health are covered in a related systematic review. 111 The use of water as a replacement for sugar-sweetened beverages, juice, or whole milk has clear effects in that energy intake is reduced by about 10–13% of total energy intake. However, only a few longer-term systematic interventions have investigated this topic and no randomized, controlled, longer-term trials have been published to date. There is thus very minimal evidence on the effects of just adding water to the diet and of replacing water with diet beverages.
There are many limitations to this review. One certainly is the lack of discussion of potential differences in the metabolic functioning of different types of beverages. 121 Since the literature in this area is sparse, however, there is little basis for delving into it at this point. A discussion of the potential effects of fructose (from all caloric sweeteners when consumed in caloric beverages) on abdominal fat and all of the metabolic conditions directly linked with it (e.g., diabetes) is likewise lacking. 122 , – 125 A further limitation is the lack of detailed review of the array of biomarkers being considered to measure hydration status. Since there is no measurement in the field today that covers more than a very short time period, except for 24-hour total urine collection, such a discussion seems premature.
Some ways to examine water requirements have been suggested in this review as a means to encourage more dialogue on this important topic. Given the significance of water to our health and of caloric beverages to our total energy intake, as well as the potential risks of nutrition-related noncommunicable diseases, understanding both the requirements for water in relation to energy requirements, and the differential effects of water versus other caloric beverages, remain important outstanding issues.
This review has attempted to provide some sense of the importance of water to our health, its role in relationship to the rapidly increasing rates of obesity and other related diseases, and the gaps in present understanding of hydration measurement and requirements. Water is essential to our survival. By highlighting its critical role, it is hoped that the focus on water in human health will sharpen.
The authors wish to thank Ms. Frances L. Dancy for administrative assistance, Mr. Tom Swasey for graphics support, Dr. Melissa Daniels for assistance, and Florence Constant (Nestle's Water Research) for advice and references.
This work was supported by the Nestlé Waters, Issy-les-Moulineaux, France, 5ROI AGI0436 from the National Institute on Aging Physical Frailty Program, and NIH R01-CA109831 and R01-CA121152.
Declaration of interest
The authors have no relevant interests to declare.
Nicolaidis S . Physiology of thirst . In: Arnaud MJ , ed. Hydration Throughout Life . Montrouge: John Libbey Eurotext ; 1998 : 247 .
Google Scholar
Jequier E Constant F . Water as an essential nutrient: the physiological basis of hydration . Eur J Clin Nutr. 2010 ; 64 : 115 – 123 .
Institute of Medicine . Panel on Dietary Reference Intakes for Electrolytes and Water. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate . Washington, DC: The National Academies Press ; 2005 .
Manz F Wentz A . Hydration status in the United States and Germany . Nutr Rev. 2005 ; 63 :(Suppl): S55 – S62 .
European Food Safety Authority . Scientific opinion of the Panel on Dietetic Products Nutrition and Allergies. Draft dietary reference values for water . EFSA J. 2008 : 2 – 49 .
Stookey JD . High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III . J Am Diet Assoc. 2005 ; 105 : 1231 – 1239 .
Armstrong LE . Hydration assessment techniques . Nutr Rev. 2005 ; 63 (Suppl): S40 – S54 .
Bar-David Y Urkin J Kozminsky E . The effect of voluntary dehydration on cognitive functions of elementary school children . Acta Paediatr. 2005 ; 94 : 1667 – 1673 .
Shirreffs S . Markers of hydration status . J Sports Med Phys Fitness. 2000 ; 40 : 80 – 84 .
Popowski L Oppliger R Lambert G Johnson R Johnson A Gisolfi C . Blood and urinary measures of hydration status during progressive acute dehydration . Med Sci Sports Exerc. 2001 ; 33 : 747 – 753 .
Bar-David Y Landau D Bar-David Z Pilpel D Philip M . Urine osmolality among elementary schoolchildren living in a hot climate: implications for dehydration . Ambulatory Child Health. 1998 ; 4 : 393 – 397 .
Fadda R Rapinett G Grathwohl D Parisi M Fanari R Schmitt J . The Benefits of Drinking Supplementary Water at School on Cognitive Performance in Children . Washington, DC: International Society for Developmental Psychobiology ; 2008 : Abstract from poster session at the 41st Annual Conference of International Society for Psychobiology. Abstracts published in: Developmental Psychobiology 2008: v. 50(7): 726 . http://www3.interscience.wiley.com/cgi-bin/fulltext/121421361/PDFSTART
Eckhardt CL Adair LS Caballero B , et al . Estimating body fat from anthropometry and isotopic dilution: a four-country comparison . Obes Res. 2003 ; 11 : 1553 – 1562 .
Moreno LA Sarria A Popkin BM . The nutrition transition in Spain: a European Mediterranean country . Eur J Clin Nutr. 2002 ; 56 : 992 – 1003 .
Lee MJ Popkin BM Kim S . The unique aspects of the nutrition transition in South Korea: the retention of healthful elements in their traditional diet . Public Health Nutr. 2002 ; 5 : 197 – 203 .
Ershow AG Brown LM Cantor KP . Intake of tapwater and total water by pregnant and lactating women . Am J Public Health. 1991 ; 81 : 328 – 334 .
Ershow AG Cantor KP . Total Water and Tapwater Intake in the United States: Population-Based Estimates of Quantities and Sources . Bethesda, MD: FASEB/LSRO ; 1989 .
Ramsay DJ . Homeostatic control of water balance . In: Arnaud MJ , ed. Hydration Throughout Life . Montrouge: John Libbey Eurotext ; 1998 : 9 – 18 .
Duffey K Popkin BM . Shifts in patterns and consumption of beverages between 1965 and 2002 . Obesity. 2007 ; 15 : 2739 – 2747 .
Morley JE Miller DK Zdodowski C Guitierrez B Perry IIIHM . Fluid intake, hydration and aging . In: Arnaud MJ , ed. Hydration Throughout Life: International Conference Vittel (France) . Montrouge: John Libbey Eurotext ; 1998 : 247 .
Phillips PA Rolls BJ Ledingham JG , et al . Reduced thirst after water deprivation in healthy elderly men . N Engl J Med. 1984 ; 311 : 753 – 759 .
Mack GW Weseman CA Langhans GW Scherzer H Gillen CM Nadel ER . Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation . J Appl Physiol. 1994 ; 76 : 1615 – 1623 .
Phillips PA Johnston CI Gray L . Disturbed fluid and electrolyte homoeostasis following dehydration in elderly people . Age Ageing. 1993 ; 22 : S26 – S33 .
Phillips PA Bretherton M Johnston CI Gray L . Reduced osmotic thirst in healthy elderly men . Am J Physiol. 1991 ; 261 : R166 – R171 .
Davies I O'Neill PA McLean KA Catania J Bennett D . Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load . Age Ageing. 1995 ; 24 : 151 – 159 .
Silver AJ Morley JE . Role of the opioid system in the hypodipsia associated with aging . J Am Geriatr Soc. 1992 ; 40 : 556 – 560 .
Sawka MN Latzka WA Matott RP Montain SJ . Hydration effects on temperature regulation . Int J Sports Med. 1998 ; 19 (Suppl 2 ): S108 – S110 .
Sawka MN Cheuvront SN Carter R 3rd . Human water needs . Nutr Rev. 2005 ; 63 (Suppl): S30 – S39 .
Armstrong LE. Heat acclimatization . In: TD Fahey, ed. Encyclopedia of Sports Medicine and Science . Internet Society for Sport Science: http://sportsci.org . 10 March 1998 .
Falk B Dotan R . Children's thermoregulation during exercise in the heat: a revisit . Appl Physiol Nutr Metab. 2008 ; 33 : 420 – 427 .
Bytomski JR Squire DL . Heat illness in children . Curr Sports Med Rep. 2003 ; 2 : 320 – 324 .
Bar-Or O Dotan R Inbar O Rotshtein A Zonder H . Voluntary hypohydration in 10- to 12-year-old boys . J Appl Physiol. 1980 ; 48 : 104 – 108 .
Vogelaere P Pereira C . Thermoregulation and aging . Rev Port Cardiol. 2005 ; 24 : 747 – 761 .
Thompson-Torgerson CS Holowatz LA Kenney WL . Altered mechanisms of thermoregulatory vasoconstriction in aged human skin . Exerc Sport Sci Rev. 2008 ; 36 : 122 – 127 .
Miller PD Krebs RA Neal BJ McIntyre DO . Hypodipsia in geriatric patients . Am J Med. 1982 ; 73 : 354 – 356 .
Albert SG Nakra BR Grossberg GT Caminal ER . Drinking behavior and vasopressin responses to hyperosmolality in Alzheimer's disease . Int Psychogeriatr. 1994 ; 6 : 79 – 86 .
Maughan RJ Shirreffs SM Watson P . Exercise, heat, hydration and the brain . J Am Coll Nutr. 2007 ; 26 (Suppl): S604 – S612 .
Murray B . Hydration and physical performance . J Am Coll Nutr. 2007 ; 26 (Suppl): S542 – S548 .
Sawka MN Noakes TD . Does dehydration impair exercise performance? Med Sci Sports Exerc. 2007 ; 39 : 1209 – 1217 .
Montain SJ Coyle EF . Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise . J Appl Physiol. 1992 ; 73 : 1340 – 1350 .
Cheuvront SN . Carter R, 3rd, Sawka MN. Fluid balance and endurance exercise performance . Curr Sports Med Rep. 2003 ; 2 : 202 – 208 .
Paik IY Jeong MH Jin HE , et al . Fluid replacement following dehydration reduces oxidative stress during recovery . Biochem Biophys Res Commun. 2009 ; 383 : 103 – 107 .
Kovacs MS . A review of fluid and hydration in competitive tennis . Int J Sports Physiol Perform. 2008 ; 3 : 413 – 423 .
Cheuvront SN Montain SJ Sawka MN . Fluid replacement and performance during the marathon . Sports Med. 2007 ; 37 : 353 – 357 .
Cheuvront SN . Carter R, 3rd, Haymes EM, Sawka MN. No effect of moderate hypohydration or hyperthermia on anaerobic exercise performance . Med Sci Sports Exerc. 2006 ; 38 : 1093 – 1097 .
Penkman MA Field CJ Sellar CM Harber VJ Bell GJ . Effect of hydration status on high-intensity rowing performance and immune function . Int J Sports Physiol Perform. 2008 ; 3 : 531 – 546 .
Bergeron MF McKeag DB Casa DJ , et al . Youth football: heat stress and injury risk . Med Sci Sports Exerc. 2005 ; 37 : 1421 – 1430 .
Godek SF Godek JJ Bartolozzi AR . Hydration status in college football players during consecutive days of twice-a-day preseason practices . Am J Sports Med. 2005 ; 33 : 843 – 851 .
Cheuvront SN Carter R 3rd Castellani JW Sawka MN . Hypohydration impairs endurance exercise performance in temperate but not cold air . J Appl Physiol. 2005 ; 99 : 1972 – 1976 .
Kenefick RW Mahood NV Hazzard MP Quinn TJ Castellani JW . Hypohydration effects on thermoregulation during moderate exercise in the cold . Eur J Appl Physiol. 2004 ; 92 : 565 – 570 .
Maughan RJ Watson P Shirreffs SM . Heat and cold: what does the environment do to the marathon runner? Sports Med. 2007 ; 37 : 396 – 399 .
American Academy of Pediatrics . Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness . Pediatrics. 2000 ; 106 : 158 – 159 .
Cian C Barraud PA Melin B Raphel C . Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration . Int J Psychophysiol. 2001 ; 42 : 243 – 251 .
Cian C Koulmann PA Barraud PA Raphel C Jimenez C Melin B . Influence of variations of body hydration on cognitive performance . J Psychophysiol. 2000 ; 14 : 29 – 36 .
Gopinathan PM Pichan G Sharma VM . Role of dehydration in heat stress-induced variations in mental performance . Arch Environ Health. 1988 ; 43 : 15 – 17 .
D'Anci KE Vibhakar A Kanter JH Mahoney CR Taylor HA . Voluntary dehydration and cognitive performance in trained college athletes . Percept Mot Skills. 2009 ; 109 : 251 – 269 .
Suhr JA Hall J Patterson SM Niinisto RT . The relation of hydration status to cognitive performance in healthy older adults . Int J Psychophysiol. 2004 ; 53 : 121 – 125 .
Szinnai G Schachinger H Arnaud MJ Linder L Keller U . Effect of water deprivation on cognitive-motor performance in healthy men and women . Am J Physiol Regul Integr Comp Physiol. 2005 ; 289 : R275 – R280 .
Neave N Scholey AB Emmett JR Moss M Kennedy DO Wesnes KA . Water ingestion improves subjective alertness, but has no effect on cognitive performance in dehydrated healthy young volunteers . Appetite. 2001 ; 37 : 255 – 256 .
Rogers PJ Kainth A Smit HJ . A drink of water can improve or impair mental performance depending on small differences in thirst . Appetite. 2001 ; 36 : 57 – 58 .
Edmonds CJ Jeffes B . Does having a drink help you think? 6–7-year-old children show improvements in cognitive performance from baseline to test after having a drink of water . Appetite. 2009 ; 53 : 469 – 472 .
Edmonds CJ Burford D . Should children drink more water?: the effects of drinking water on cognition in children . Appetite. 2009 ; 52 : 776 – 779 .
Benton D Burgess N . The effect of the consumption of water on the memory and attention of children . Appetite. 2009 ; 53 : 143 – 146 .
Cohen S . After effects of stress on human performance during a heat acclimatization regimen . Aviat Space Environ. Med. 1983 ; 54 : 709 – 713 .
Culp KR Wakefield B Dyck MJ Cacchione PZ DeCrane S Decker S . Bioelectrical impedance analysis and other hydration parameters as risk factors for delirium in rural nursing home residents . J Gerontol A Biol Sci Med Sci. 2004 ; 59 : 813 – 817 .
Lawlor PG . Delirium and dehydration: some fluid for thought? Support Care Cancer. 2002 ; 10 : 445 – 454 .
Voyer P Richard S Doucet L Carmichael PH . Predisposing factors associated with delirium among demented long-term care residents . Clin Nurs Res. 2009 ; 18 : 153 – 171 .
Leiper JB . Intestinal water absorption – implications for the formulation of rehydration solutions . Int J Sports Med. 1998 ; 19 (Suppl 2 ): S129 – S132 .
Ritz P Berrut G . The importance of good hydration for day-to-day health . Nutr Rev. 2005 ; 63 : S6 – S13 .
Arnaud MJ . Mild dehydration: a risk factor of constipation? Eur J Clin Nutr. 2003 ; 57 (Suppl): S88 – S95 .
Young RJ Beerman LE Vanderhoof JA . Increasing oral fluids in chronic constipation in children . Gastroenterol Nurs. 1998 ; 21 : 156 – 161 .
Murakami K Sasaki S Okubo H , et al . Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women . Eur J Clin Nutr. 2007 ; 61 : 616 – 622 .
Lindeman RD Romero LJ Liang HC Baumgartner RN Koehler KM Garry PJ . Do elderly persons need to be encouraged to drink more fluids? J Gerontol A Biol Sci Med Sci. 2000 ; 55 : M361 – M365 .
Robson KM Kiely DK Lembo T . Development of constipation in nursing home residents . Dis Colon Rectum. 2000 ; 43 : 940 – 943 .
Cuomo R Grasso R Sarnelli G , et al . Effects of carbonated water on functional dyspepsia and constipation . Eur J Gastroenterol Hepatol. 2002 ; 14 : 991 – 999 .
Kosek M Bern C Guerrant RL . The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000 . Bull World Health Organ. 2003 ; 81 : 197 – 204 .
Atia AN Buchman AL . Oral rehydration solutions in non-cholera diarrhea: a review . Am J Gastroenterol. 2009 ; 104 : 2596 – 2604 , quiz 2605.
Schoen EJ . Minimum urine total solute concentration in response to water loading in normal men . J Appl Physiol. 1957 ; 10 : 267 – 270 .
Sporn IN Lancestremere RG Papper S . Differential diagnosis of oliguria in aged patients . N Engl J Med. 1962 ; 267 : 130 – 132 .
Brenner BM , ed. Brenner and Rector's The Kidney , 8th edn. Philadelphia, PA: Saunders Elsevier ; 2007 .
Lindeman RD Van Buren HC Raisz LG . Osmolar renal concentrating ability in healthy young men and hospitalized patients without renal disease . N Engl J Med. 1960 ; 262 : 1306 – 1309 .
Shirreffs SM Maughan RJ . Control of blood volume: long term and short term regulation . In: Arnaud MJ , ed. Hydration Throughout Life . Montrouge: John Libbey Eurotext ; 1998 : 31 – 39 .
Schroeder C Bush VE Norcliffe LJ , et al . Water drinking acutely improves orthostatic tolerance in healthy subjects . Circulation. 2002 ; 106 : 2806 – 2811 .
Lu CC Diedrich A Tung CS , et al . Water ingestion as prophylaxis against syncope . Circulation. 2003 ; 108 : 2660 – 2665 .
Callegaro CC Moraes RS Negrao CE , et al . Acute water ingestion increases arterial blood pressure in hypertensive and normotensive subjects . J Hum Hypertens. 2007 ; 21 : 564 – 570 .
Ando SI Kawamura N Matsumoto M , et al . Simple standing test predicts and water ingestion prevents vasovagal reaction in the high-risk blood donors . Transfusion. 2009 ; 49 : 1630 – 1636 .
Brick JE Lowther CM Deglin SM . Cold water syncope . South Med J. 1978 ; 71 : 1579 – 1580 .
Farb A Valenti SA . Swallow syncope . Md Med J. 1999 ; 48 : 151 – 154 .
Casella F Diana A Bulgheroni M , et al . When water hurts . Pacing Clin Electrophysiol. 2009 ; 32 : e25 – e27 .
Shirreffs SM Merson SJ Fraser SM Archer DT . The effects of fluid restriction on hydration status and subjective feelings in man . Br J Nutr. 2004 ; 91 : 951 – 958 .
Blau J . Water deprivation: a new migraine precipitant . Headache. 2005 ; 45 : 757 – 759 .
Blau JN Kell CA Sperling JM . Water-deprivation headache: a new headache with two variants . Headache. 2004 ; 44 : 79 – 83 .
Spigt MG Kuijper EC Schayck CP , et al . Increasing the daily water intake for the prophylactic treatment of headache: a pilot trial . Eur J Neurol. 2005 ; 12 : 715 – 718 .
Valtin H . “Drink at least eight glasses of water a day.” Really? Is there scientific evidence for “8 x 8”? Am J Physiol Regul Integr Comp Physiol. 2002 ; 283 : R993 – 1004 .
Negoianu D Goldfarb S . Just add water . J Am Soc Nephrol. 2008 ; 19 : 1041 – 1043 .
Madison KC . Barrier function of the skin: “la raison d'etre” of the epidermis . J Invest Dermatol. 2003 ; 121 : 231 – 241 .
Champion RH Burton JL Ebling FJG , eds. Textbook of Dermatology (Rook) . Oxford: Blackwell ; 1992 .
Vivanti A Harvey K Ash S Battistutta D . Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008 ; 47 : 340 – 355 .
Colletti JE Brown KM Sharieff GQ Barata IA Ishimine P Committee APEM . The management of children with gastroenteritis and dehydration in the emergency department . J Emerg Med. 2009 .
Williams S Krueger N Davids M Kraus D Kerscher M . Effect of fluid intake on skin physiology: distinct differences between drinking mineral water and tap water . Int J Cosmet Sci. 2007 ; 29 : 131 – 138 .
Mac-Mary S Creidi P Marsaut D , et al . Assessment of effects of an additional dietary natural mineral water uptake on skin hydration in healthy subjects by dynamic barrier function measurements and clinic scoring . Skin Res Technol. 2006 ; 12 : 199 – 205 .
Warner RR Stone KJ Boissy YL . Hydration disrupts human stratum corneum ultrastructure . J Invest Dermatol. 2003 ; 120 : 275 – 284 .
Loden M . Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders . Am J Clin Dermatol. 2003 ; 4 : 771 – 788 .
Manz F Wentz A . The importance of good hydration for the prevention of chronic diseases . Nutr Rev. 2005 ; 63 (Suppl): S2 – S5 .
Panel on Dietetic Products Nutrition and Allergies . Dietary reference values for water Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies (Question No EFSA-Q-2005-015a) . EFSA J. 2009 ; 8 ( 3 ): 1459 .
Stookey JD Constant F Gardner C Popkin B . Replacing sweetened caloric beverages with drinking water is associated with lower energy intake . Obesity. 2007 ; 15 : 3013 – 3022 .
Stookey JD Constant F Gardner C Popkin BM . Drinking water is associated with weight loss . Obesity. 2008 ; 16 : 2481 – 2488 .
Turrini A Saba A Perrone D Cialfa E D'Amicis D . Food consumption patterns in Italy: the INN-CA Study 1994–1996 . Eur J Clin Nutr. 2001 ; 55 : 571 – 588 .
Sichert-Hellert W Kersting M Manz F . Fifteen year trends in water intake in German children and adolescents: results of the DONALD Study. Dortmund Nutritional and Anthropometric Longitudinally Designed Study . Acta Paediatr. 2001 ; 90 : 732 – 737 .
Raman A Schoeller DA Subar AF , et al . Water turnover in 458 American adults 40–79 yr of age . Am J Physiol Renal Physiol. 2004 ; 286 : F394 – F401 .
Daniels MC Popkin BM . The impact of water intake on energy intake and weight status: a review . Nutr Rev. 2010 ; 9 :in press.
Piernas C Popkin B . Snacking trends in U.S. adults between 1977 and 2006 . J Nutr . 2010 ; 140 : 325 – 332 .
Piernas C Popkin BM . Trends in snacking among U.S. children . Health Affairs. 2010 ; 29 : 398 – 404 .
Popkin BM Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States . Am J Clin Nutr . 2010 ; 91 : 1342 – 1347 .
Muckelbauer R Libuda L Clausen K Toschke AM Reinehr T Kersting M . Promotion and provision of drinking water in schools for overweight prevention: randomized, controlled cluster trial . Pediatrics. 2009 ; 123 : e661 – e667 .
Bar-or O . Temperature regulation during exercise in children and aolescents . In: Gisolfi C Lamb DR , eds. Youth, Exercise, and Sport: Symposium: Papers and Discussions . Indianapolis: Benchmark ; 1989 : 335 – 367 .
National Research Council . Recommended Dietary Allowances . Washington, DC: National Academy Press ; 1989 .
Prentice A Branca F Decsi T , et al . Energy and nutrient dietary reference values for children in Europe: methodological approaches and current nutritional recommendations . Br J Nutr. 2004 ; 92 (Suppl 2 ): S83 – 146 .
German Nutrition Society, Austrian Nutrition Society, Swiss Society for Nutrition Research Swiss Nutrition Association . Reference Values for Nutrient Intake (in English) , 1st edn. Frankfurt: Umschau Brau ; 2002 .
Stookey JD Pieper CF Cohen HJ . Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70+years . Public Health Nutr. 2005 ; 8 : 1275 – 1285 .
Malik VS Popkin BM Bray G Després J-P Hu FB. Sugar sweetened beverages, obesity, type 2 diabetes and cardiovascular disease risk . Circulation 2010 ; 121 : 1356 – 1364 .
Teff KL Grudziak J Townsend RR , et al . Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses . J Clin Endocrinol Metab. 2009 ; 94 : 1562 – 1569 .
Stanhope KL . Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans . J Clin Investigat. 2009 ; 119 : 1322 – 1334 .
Stanhope KL Havel PJ . Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup . Am J Clin Nutr. 2008 ; 88 (Suppl): S1733 – S1737 .
Stanhope KL Griffen SC Bair BR Swarbrick MM Keim NL Havel PJ . Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals . Am J Clin Nutr. 2008 ; 87 : 1194 – 1203 .
Altman P . Blood and Other Body Fluids . Washington, DC: Federation of American Societies for Experimental Biology ; 1961 .
Kalhoff H . Mild dehydration: a risk factor of broncho-pulmonary disorders? Eur J Clin Nutr. 2003 ; 57 (Suppl 2 ): S81 – S87 .
Taitz LS Byers HD . High calorie-osmolar feeding and hypertonic dehydration . Arch Dis Child. 1972 ; 47 : 257 – 260 .
Burge MR Garcia N Qualls CR Schade DS . Differential effects of fasting and dehydration in the pathogenesis of diabetic ketoacidosis . Metabolism. 2001 ; 50 : 171 – 177 .
Jayashree M Singhi S . Diabetic ketoacidosis: predictors of outcome in a pediatric intensive care unit of a developing country . Pediatr Crit Care Med. 2004 ; 5 : 427 – 433 .
Hebert LA Greene T Levey A Falkenhain ME Klahr S . High urine volume and low urine osmolality are risk factors for faster progression of renal disease . Am J Kidney Dis. 2003 ; 41 : 962 – 971 .
Bankir L Bardoux P Mayaudon H Dupuy O Bauduceau B . [Impaired urinary flow rate during the day: a new factor possibly involved in hypertension and in the lack of nocturnal dipping] . Arch Mal Coeur Vaiss. 2002 ; 95 : 751 – 754 .
Blanker MH Bernsen RM Ruud Bosch JL , et al . Normal values and determinants of circadian urine production in older men: a population based study . J Urol. 2002 ; 168 : 1453 – 1457 .
Chan J Knutsen SF Blix GG Lee JW Fraser GE . Water, other fluids, and fatal coronary heart disease: the Adventist Health Study . Am J Epidemiol. 2002 ; 155 : 827 – 833 .
Kelly J Hunt BJ Lewis RR , et al . Dehydration and venous thromboembolism after acute stroke . QJM. 2004 ; 97 : 293 – 296 .
Bhalla A Sankaralingam S Dundas R Swaminathan R Wolfe CD Rudd AG . Influence of raised plasma osmolality on clinical outcome after acute stroke . Stroke. 2000 ; 31 : 2043 – 2048 .
Longo-Mbenza B Phanzu-Mbete LB M'Buyamba-Kabangu JR , et al . Hematocrit and stroke in black Africans under tropical climate and meteorological influence . Ann Med Interne (Paris). 1999 ; 150 : 171 – 177 .
Diamond PT Gale SD Evans BA . Relationship of initial hematocrit level to discharge destination and resource utilization after ischemic stroke: a pilot study . Arch Phys Med Rehabil. 2003 ; 84 : 964 – 967 .
Mazzola BL von Vigier RO Marchand S Tonz M Bianchetti MG . Behavioral and functional abnormalities linked with recurrent urinary tract infections in girls . J Nephrol. 2003 ; 16 : 133 – 138 .
Wilde MH Carrigan MJ . A chart audit of factors related to urine flow and urinary tract infection . J Adv Nurs. 2003 ; 43 : 254 – 262 .
Altieri A La Vecchia C Negri E . Fluid intake and risk of bladder and other cancers . Eur J Clin Nutr. 2003 ; 57 (Suppl 2 ): S59 – S68 .
Donat SM Bayuga S Herr HW Berwick M . Fluid intake and the risk of tumor recurrence in patients with superficial bladder cancer . J Urol. 2003 ; 170 : 1777 – 1780 .
Radosavljevic V Jankovic S Marinkovic J Djokic M . Fluid intake and bladder cancer. A case control study . Neoplasma. 2003 ; 50 : 234 – 238 .
Math MV Rampal PM Faure XR Delmont JP . Gallbladder emptying after drinking water and its possible role in prevention of gallstone formation . Singapore Med J. 1986 ; 27 : 531 – 532 .
Aufderheide S Lax D Goldberg SJ . Gender differences in dehydration-induced mitral valve prolapse . Am Heart J. 1995 ; 129 : 83 – 86 .
Martin B Harris A Hammel T Malinovsky V . Mechanism of exercise-induced ocular hypotension . Invest Ophthalmol Vis Sci. 1999 ; 40 : 1011 – 1015 .
Brucculeri M Hammel T Harris A Malinovsky V Martin B . Regulation of intraocular pressure after water drinking . J Glaucoma. 1999 ; 8 : 111 – 116 .
- dehydration
- energy intake
- water drinking
- fluid intake
- water requirements
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1753-4887
- Print ISSN 0029-6643
- Copyright © 2024 International Life Sciences Institute
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Discharge from a Chinese fertilizer factory winds its way toward the Yellow River. Like many of the world's rivers, pollution remains an ongoing problem.
Water pollution is a rising global crisis. Here’s what you need to know.
The world's freshwater sources receive contaminants from a wide range of sectors, threatening human and wildlife health.
From big pieces of garbage to invisible chemicals, a wide range of pollutants ends up in our planet's lakes, rivers, streams, groundwater, and eventually the oceans. Water pollution—along with drought, inefficiency, and an exploding population—has contributed to a freshwater crisis , threatening the sources we rely on for drinking water and other critical needs.
Research has revealed that one pollutant in particular is more common in our tap water than anyone had previously thought: PFAS, short for poly and perfluoroalkyl substances. PFAS is used to make everyday items resistant to moisture, heat, and stains; some of these chemicals have such long half-lives that they are known as "the forever chemical."
Safeguarding water supplies is important because even though nearly 70 percent of the world is covered by water, only 2.5 percent of it is fresh. And just one percent of freshwater is easily accessible, with much of it trapped in remote glaciers and snowfields.
Water pollution causes
Water pollution can come from a variety of sources. Pollution can enter water directly, through both legal and illegal discharges from factories, for example, or imperfect water treatment plants. Spills and leaks from oil pipelines or hydraulic fracturing (fracking) operations can degrade water supplies. Wind, storms, and littering—especially of plastic waste —can also send debris into waterways.
Thanks largely to decades of regulation and legal action against big polluters, the main cause of U.S. water quality problems is now " nonpoint source pollution ," when pollutants are carried across or through the ground by rain or melted snow. Such runoff can contain fertilizers, pesticides, and herbicides from farms and homes; oil and toxic chemicals from roads and industry; sediment; bacteria from livestock; pet waste; and other pollutants .
Finally, drinking water pollution can happen via the pipes themselves if the water is not properly treated, as happened in the case of lead contamination in Flint, Michigan , and other towns. Another drinking water contaminant, arsenic , can come from naturally occurring deposits but also from industrial waste.
Freshwater pollution effects

Water pollution can result in human health problems, poisoned wildlife, and long-term ecosystem damage. When agricultural and industrial runoff floods waterways with excess nutrients such as nitrogen and phosphorus, these nutrients often fuel algae blooms that then create dead zones , or low-oxygen areas where fish and other aquatic life can no longer thrive.
Algae blooms can create health and economic effects for humans, causing rashes and other ailments, while eroding tourism revenue for popular lake destinations thanks to their unpleasant looks and odors. High levels of nitrates in water from nutrient pollution can also be particularly harmful to infants , interfering with their ability to deliver oxygen to tissues and potentially causing " blue baby syndrome ." The United Nations Food and Agriculture Organization estimates that 38 percent of the European Union's water bodies are under pressure from agricultural pollution.
Globally, unsanitary water supplies also exact a health toll in the form of disease. At least 2 billion people drink water from sources contaminated by feces, according to the World Health Organization , and that water may transmit dangerous diseases such as cholera and typhoid.
Freshwater pollution solutions
In many countries, regulations have restricted industry and agricultural operations from pouring pollutants into lakes, streams, and rivers, while treatment plants make our drinking water safe to consume. Researchers are working on a variety of other ways to prevent and clean up pollution. National Geographic grantee Africa Flores , for example, has created an artificial intelligence algorithm to better predict when algae blooms will happen. A number of scientists are looking at ways to reduce and cleanup plastic pollution .
There have been setbacks, however. Regulation of pollutants is subject to changing political winds, as has been the case in the United States with the loosening of environmental protections that prevented landowners from polluting the country’s waterways.
Anyone can help protect watersheds by disposing of motor oil, paints, and other toxic products properly , keeping them off pavement and out of the drain. Be careful about what you flush or pour down the sink, as it may find its way into the water. The U.S. Environmental Protection Agency recommends using phosphate-free detergents and washing your car at a commercial car wash, which is required to properly dispose of wastewater. Green roofs and rain gardens can be another way for people in built environments to help restore some of the natural filtering that forests and plants usually provide.
For Hungry Minds
Related topics.
- WATER POLLUTION
- ENVIRONMENT AND CONSERVATION
- FRESH WATER
- GROUNDWATER
- WATER QUALITY
- WATER RESOURCES
You May Also Like

Here’s what worries engineers the most about U.S. infrastructure

Are you drinking water all wrong? Here’s what you need to know about hydrating.

Is tap water safe to drink? Here’s what you really need to know.

England’s chalk streams were millions of years in the making. Can they survive today?

Japan releases nuclear wastewater into the Pacific. How worried should we be?
- Environment
- Perpetual Planet
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Collections
- Sustainability in chemistry
- Simple rules
- Teacher well-being hub
- Women in chemistry
- Global science
- Escape room activities
- Decolonising chemistry teaching
- Teaching science skills
- Post-lockdown teaching support
- Get the print issue
- RSC Education

- More from navigation items
Widespread water contamination

- No comments
Find out how forever chemicals taint water supplies globally

Download this
Use this story and the accompanying summary slide for a real-world context when studying potable water and its treatment with your 14–16 learners.
Download the story as MS Word or PDF and the summary slide as MS PowerPoint or PDF .
A new study has found that a substantial fraction of surface and groundwater around the globe contains levels of per- and polyfluoroalkyl substances ( PFAS ) above the national drinking water standards in the regions where they were collected.

Source: © Jim West/Science Photo Library
The strong carbon–fluorine bonds in PFAS don’t degrade in the environment or in our bodies
What are PFAS?
Forever chemicals.
PFAS are a group of around 14,000 synthetic organic compounds with multiple fluorine atoms attached to alkyl chains. Since the 1950s , companies have widely used PFAS in products including non-stick frying pans, clothing, furniture and firefighting foam due to the chemicals’ resistance to heat, water, grease and stains.
These chemicals have a dark side, though. The fluorine and carbon bond is extremely strong and as a result these compounds don’t degrade – in the environment or in our bodies. Scientists have linked a number of serious health conditions to PFAS in recent years, including testicular cancer, thyroid disease, infertility and developmental defects in unborn children.
These chemicals have a dark side, though. The fluorine and carbon bond is extremely strong and as a result these compounds don’t degrade – in the environment or in our bodies. Scientists have linked a number of serious health conditions ( rsc.li/3wBaWh1 ) to PFAS in recent years, including testicular cancer, thyroid disease, infertility and developmental defects in unborn children.
Exceeding drinking water standards

Source: © Dushlik/Shutterstock
Firefighters battling a blaze with PFAS-containing foam but are there more environmentally-friendly alternatives?
An unpleasant surprise
The team found that 32% of groundwater and 16% of the surface water samples exceeded the national drinking water standards in some countries. ‘Drinking water standards vary around the world. In Australia, EU, the US and Canada,’ explains Denis O’Carroll, whose lab carried out the study, ‘the number of samples that exceeded those [limits] was somewhat surprising to us.’
This does not necessarily mean we are drinking water with such high levels of PFAS , however. Many water treatment plants are designed to reduce PFAS levels in water before it reaches our taps.
This article is adapted from Julia Robinson’s in Chemistry World .
D Ackerman Grunfeld et al , Nat . Geosci . , 2024, 17 , 340–346 ( doi . org /10.1038/ s41561 -024-01402-8)
A starter slide to use with 14–16 year-old learners to provide context when studying potable water and its treatment: rsc.li/3QJ23sr
PFAS in water student sheet
Pfas in water summary slide.

More from Nina Notman

Composite decking can capture carbon dioxide

Enzymes make dyeing denim more sustainable

Revealing blueberries’ nanostructure
No comments yet, only registered users can comment on this article., more from science research.

2024-04-26T08:58:00Z By Nina Notman
Could our choice of patio decking help reduce the global warming effects of building materials?

2024-04-05T08:06:00Z By Nina Notman
Can researchers find a way to make jeans blue without using toxic chemicals?

2024-03-22T11:00:00Z By Nina Notman
Find out how microscopic, self-assembling particles give blueberries their characteristic blue hue
- Contributors
- Print issue
- Email alerts
Site powered by Webvision Cloud
- You are here:
- American Chemical Society
- Discover Chemistry
‘Forever chemicals’ found to rain down on all five Great Lakes
FOR IMMEDIATE RELEASE
“The Ins and Outs of Per- and Polyfluoroalkyl Substances in the Great Lakes: The Role of Atmospheric Deposition” Environmental Science & Technology
Perfluoroalkyl and polyfluoroalkyl substances, also known as PFAS or “forever chemicals,” have become persistent pollutants in the air, water and soil. Because they are so stable, they can be transported throughout the water cycle, making their way into drinking water sources and precipitation. According to findings published in ACS’ Environmental Science & Technology , precipitation introduces similar amounts of PFAS into each of the Great Lakes; however, the lakes eliminate the chemicals at different rates.
Consuming PFAS has been linked to negative health outcomes. And in April 2024, the U.S. Environmental Protection Agency (EPA) designated two forever chemicals — PFOS and PFOA — as hazardous substances, placing limits on their concentrations in drinking water. The Great Lakes are a major freshwater source for both the U.S. and Canada, and the EPA reports that the surrounding basin area is home to roughly 10% and 30% of each country’s population, respectively. Previous studies demonstrated that these lakes contain PFAS. But Marta Venier at Indiana University and colleagues from the U.S. and Canada wanted to understand where the compounds come from and where they go.
Between 2021 and 2022, 207 precipitation samples and 60 air samples were taken from five sites surrounding the Great Lakes in the U.S.: Chicago; Cleveland; Sturgeon Point, N.Y.; Eagle Harbor, Mich.; and Sleeping Bear Dunes, Mich. During the same period, 87 different water samples were collected from the five Great Lakes. The team analyzed all the samples for 41 types of PFAS and found:
- ·In precipitation samples, PFAS concentrations largely remained the same across sites, suggesting that the compounds are present at similar levels regardless of population density.
- In air samples, Cleveland had the highest median concentration of PFAS and Sleeping Bear Dunes the lowest, suggesting a strong connection between population density and airborne PFAS.
- In the lake water samples, the highest concentration of PFAS were in Lake Ontario, followed by Lake Michigan, Lake Erie, Lake Huron and Lake Superior.
- ·The concentration of PFOS and PFOA in lake water decreased compared to data from previous studies as far back as 2005, but the concentration of a replacement PFAS known as PFBA remained high, suggesting that further regulation efforts may be needed.
The team calculated that airborne deposition from precipitation is primarily how PFAS get into the lakes, while they’re removed by sedimentation, attaching to particles as they settle to the lakebed or flowing out through connecting channels. Overall, their calculations showed that the northernmost lakes (Superior, Michigan and Huron) are generally accumulating PFAS. Further south, Lake Ontario is generally eliminating the compounds and levels in Lake Erie remain at a steady state. The researchers say that this work could help inform future actions and policies aimed at mitigating PFAS’ presence in the Great Lakes.
The authors acknowledge funding from the Great Lakes Restoration Initiative from the U.S. Environmental Protection Agency’s Great Lakes National Program Office.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News . ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org .
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom newsroom@acs.org
Related Content
More From This Series

Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Water Microbiology. Bacterial Pathogens and Water
Water is essential to life, but many people do not have access to clean and safe drinking water and many die of waterborne bacterial infections. In this review a general characterization of the most important bacterial diseases transmitted through water—cholera, typhoid fever and bacillary dysentery—is presented, focusing on the biology and ecology of the causal agents and on the diseases’ characteristics and their life cycles in the environment. The importance of pathogenic Escherichia coli strains and emerging pathogens in drinking water-transmitted diseases is also briefly discussed. Microbiological water analysis is mainly based on the concept of fecal indicator bacteria. The main bacteria present in human and animal feces (focusing on their behavior in their hosts and in the environment) and the most important fecal indicator bacteria are presented and discussed (focusing on the advantages and limitations of their use as markers). Important sources of bacterial fecal pollution of environmental waters are also briefly indicated. In the last topic it is discussed which indicators of fecal pollution should be used in current drinking water microbiological analysis. It was concluded that safe drinking water for all is one of the major challenges of the 21st century and that microbiological control of drinking water should be the norm everywhere. Routine basic microbiological analysis of drinking water should be carried out by assaying the presence of Escherichia coli by culture methods. Whenever financial resources are available, fecal coliform determinations should be complemented with the quantification of enterococci. More studies are needed in order to check if ammonia is reliable for a preliminary screening for emergency fecal pollution outbreaks. Financial resources should be devoted to a better understanding of the ecology and behavior of human and animal fecal bacteria in environmental waters.
1. Drinking Water as a Vehicle of Diseases
Water is essential to life. An adequate, safe and accessible supply must be available to all. Improving access to safe drinking-water can result in significant benefits to health. Every effort should be made to achieve a drinking water quality as safe as possible [ 1 ].
Many people struggle to obtain access to safe water. A clean and treated water supply to each house may be the norm in Europe and North America, but in developing countries, access to both clean water and sanitation are not the rule, and waterborne infections are common. Two and a half billion people have no access to improved sanitation, and more than 1.5 million children die each year from diarrheal diseases [ 2 ]. According to the WHO, the mortality of water associated diseases exceeds 5 million people per year. From these, more that 50% are microbial intestinal infections, with cholera standing out in the first place.
In general terms, the greatest microbial risks are associated with ingestion of water that is contaminated with human or animal feces. Wastewater discharges in fresh waters and costal seawaters are the major source of fecal microorganisms, including pathogens [ 1 – 4 ].
Acute microbial diarrheal diseases are a major public health problem in developing countries. People affected by diarrheal diseases are those with the lowest financial resources and poorest hygienic facilities. Children under five, primarily in Asian and African countries, are the most affected by microbial diseases transmitted through water [ 5 ].
Microbial waterborne diseases also affect developed countries. In the USA, it has been estimated that each year 560,000 people suffer from severe waterborne diseases, and 7.1 million suffer from a mild to moderate infections, resulting in estimated 12,000 deaths a year [ 6 ]. The most important bacterial diseases transmitted through water are listed in Table 1 .
The main bacterial diseases transmitted through drinking water.
2.1. The Genus Vibrio
Vibrio are small, curved-shaped Gram-negative rods, with a single polar flagellum. Vibrios are facultative anaerobes capable of both fermentative and respiratory metabolism. Sodium stimulates growth of all species and is an absolute requirement for most. Most species are oxidase-positive and reduce nitrate to nitrite. Cells of certain species ( V. cholerae , V. parahaemolyticus and V. vulnificus ) have pili (fimbriae), structures composed of protein TcpA. TcpA formation is co-regulated with cholera toxin expression and is a key determinant of in vivo colonization (see below) [ 7 , 8 ].
Several Vibrio species can infect humans ( Table 2 ). V. cholerae is, by far, the most important of these species. V. alginolyticus has been isolated from several types of soft tissue infections.
Main species of Vibrio and their occurrence in human clinical specimens a .
V. fluvialis , Grimontia hollisae ( V. hollisae ), and V. mimicus can cause diarrhea or infections of the gastrointestinal tract. V. furnissii has been isolated from a few individuals with diarrhea, but there is no evidence that it can actually cause this pathology. V. parahaemolyticus is a well-documented causal agent of acute food-borne gastroenteritis, particularly in Japan and South East Asia. Cases are associated with the consumption of raw or undercooked shellfish such as oysters, shrimp, crabs, and lobster. V. vulnificus is an important cause of (often fatal) septicemia and wound infections. Other vibrios, namely Allivibrio fischeri ( Vibrio fischeri ) and V. natriegens , have no relation with humans [ 7 , 8 ].
Vibrios are primarily aquatic bacteria. Species distribution depends on sodium concentration and water temperature. Vibrios are very common in marine and estuarine environments, living free or on the surfaces and in the intestinal contents of marine animals. Species with a low sodium requirement are also found in freshwater habitats [ 7 , 8 ].
2.2. The Species Vibrio Cholerae
Vibrio cholerae cells can grow at 40 °C with pH 9–10. The growth is stimulated by the presence of sodium chloride. Vibrio cholerae is a very diverse bacterial species ( Table 3 ). It is divided in ca. 200 serovarieties, characterized by the structure of the lipopolysaccharide (LPS) (O antigens). Only serovarieties O1 and O139 are involved in “true” cholera. Some other serovarieties can cause gastroenteritis, but not cholera. The distinction between Classical and El Tor biotypes is based on biochemical and virological characteristics [ 1 , 7 , 8 , 10 , 11 ].
Subdivision of Vibrio cholerae below the species level a .
2.3. Cholera
2.3.1. characterization of the disease.
The incubation period for cholera is ca. 1–3 days. The disease is characterized by an acute and very intense diarrhea that can exceed one liter per hour. Cholera patients feel thirsty, have muscular pains and general weakness, and show signs of oliguria, hypovolemia, hemoconcentration, followed by anuria. Potassium in blood drops to very low levels. Patients feel lethargic. Finally, circulatory collapse and dehydration with cyanosis occurs [ 7 ].
The severity of the disease depends on several factors: (1) personal immunity: this may be conferred by both previous infections and by vaccines; (2) inoculum: the disease only occurs after ingestion of a minimum amount of cells, ca. 10 8 [ 1 , 7 , 8 , 10 , 11 ]; (3) The gastric barrier: V. cholera cells likes basic media and therefore the stomach, normally very acidic, is an adverse medium for bacterial survival. Patients consuming anti-acidic medications are more susceptible to infection than healthy people; (4) blood group: for still unknown reasons, people with O-group blood are more susceptible than others [ 1 , 7 , 8 , 10 , 11 ].
In the absence of treatment, the mortality of cholera-patients is ca. 50%. It is mandatory to replace not only lost water but also lost salts, mainly potassium. In light dehydrations, water and salts can be orally-administered, but in severe conditions, rapid and intravenous-administration is obligatory. The most efficient antibiotic is currently doxicyclin. If no antibiotic is available for treatment, the administration of water with salts and sugar can, in many cases, save the patient and help in the recovery [ 1 , 7 , 8 , 10 , 11 ].
There are two main determinants of infection: (1) the adhesion of the bacterial cells to the intestinal mucous membrane. This depends on the presence of pili and adesins at the cell’s surface; (2) the production of cholera toxin [ 1 , 7 , 8 , 10 , 11 ].
2.3.2. Cholera toxin
Cholera toxin is an exotoxin with a very precise action on target cells. The toxin attaches to a specific receptor (ganglioside Gl) on the cell membrane of intestinal cells and activates the enzyme adenylate cyclase. This results in a non-stop degradation of internal ATP, with release of cAMP and inorganic phosphate. The rise in the internal concentration of cAMP causes an efflux of water, sodium, potassium, chloride and carbonate ions from the cells of the mucous membrane, and this is the main cause of diarrhea [ 7 ].
2.3.3. Cholera pandemics and the emergence of El Tor biotype and O139 serovariety. New facts about cholera epidemiology
Cholera has been a well known disease since the 19th century. In the 19th and 20th centuries, seven major pandemics are recognized. The first six pandemics occurred during the following periods: 1st: 1816–1826, 2nd: 1829–1851, 3rd: 1852–1860, 4th: 1863–1875, 5th: 1881–1896, 6th: 1899–1923. These pandemics all started in Asia, passed through Europe and then reached South America. The Classical biotype was involved. The seventh pandemic, still in course, started in 1961 in the Celebes Isles, in Asia. In the 1960s, the disease spread through Asia, in the 1970s reached the Middle East and Africa, and in 1991 streaked violently across South America. Now El Tor has replaced the Classical biotype. El Tor biotype had been detected before, in 1905, but only in the development of the seventh pandemic did this biotype replace the Classical one and become dominant [ 1 , 7 , 8 , 10 , 11 ].
In 1992, a new serovariety (O139), which was coined the Bengal serovariety, was detected for the first time in Bangladesh. This new serovariety quickly spread to India and to southeastern Asia, displacing O1. Although serovariety O1 El Tor has reappeared in 1994 and 1995, the Bengal serovariety still remains the dominant one. The illness caused by serovarieties O139 and O1 are indistinguishable [ 8 , 12 , 13 ].
In 1991, the seventh pandemic entered South America through the coastal area of Peru. On 23 January, in Chancay, north Peru, Vibrio cholerae O1 El Tor was isolated from patients with cholera symptoms, confirming the disease. In this region, between 24 January and 9 February, 1,859 people were hospitalized and 66 died. From Peru, the disease spread rapidly to other countries in South America. Two routes have been proposed for the entrance of the bacterium in Peru: (1) ballast water from a boat coming from Asia; (2) the El Niño current may have transported zooplankton harboring V. cholerae cells. Shellfish and fish nourishing on this zooplankton became contaminated and the bacterium was transmitted to humans who ate these marine foods [ 14 – 17 ].
The misfortune of people who died in the first months of this disastrous South American cholera epidemic appeared to have unleashed scientists to study the disease harder and, indeed, important epidemiological studies were carried out during this outbreak. These studies confirmed that contaminated uncooked food and beverages can also be a vehicle for transmission of cholera [ 18 ].

2.3.4. Genes for toxin and pili protein production
The genes responsible for toxin production are harbored in the CTXΦ segment (7–9.7 kb) of the chromosome (only in toxigenic strains). The CTXΦ segment carries at least six genes. In addition to the gene encoding cholera toxin production, this segment (virulence cassette) include an accessory cholera toxin ( ace ), a zonula occludens toxin ( zot ), core encoded pilin ( cep ), and an open reading frame of unknown function. During the replication of the chromosome, the CTXΦ fragment can form an autonomous copy and this can constitute an independent plasmid. The plasmid can give rise to virus-like particles—CTXΦ bacteriophages, which can infect non-toxigenic strains. The CTXΦ segment incorporates into the chromosome of the infected cells which became toxigenic. This process was demonstrated in vitro in cell suspensions and in vivo in the gut of the rat [ 8 , 13 , 19 , 20 ].
Epidemic and pandemic strains of V. cholerae contain another chromosomal segment designated as VPI. VPI is 39.5 kb in size and contains two ToxR-regulated genes: a regulator of virulence genes (ToxT) and a gene cluster containing colonization factors, including the toxin co-regulated pili (TCP). The tcp gene encodes for the 20.5-kDa TcpA pili protein. This VPI segment appears to be transferable from V. cholerae O1 to non-O1 strains. V. cholerae O139 strains, like O1, carry the structural genes encoded by the CTX operon and TCP. V. cholerae strains non-O1 or O139 normally lack cholera toxin genes and have never been found to carry TCP [ 8 ].
2.3.5. Ecology of the bacterium and the cycle of the disease
V. cholerae non-O1 or O139 strains are common in the environment, especially in estuaries. They have been isolated from many estuarine animals such as birds, frogs, fishes and shellfish, and survive and multiply on the surface of phytoplankton and zooplankton cells [ 8 , 21 ].
V. cholerae O1 and O139 strains are isolable from the environment only in epidemic areas. They survive in the cultivable state in water and aquatic and marine organisms for a considerable period of time [ 8 , 12 , 22 – 24 ]. When V. cholerae cells face adverse environmental conditions, they reduce cell size, became coccoid and enter a dormant stage inside exopolysaccharide biofilms. Cells display a certain metabolism, but are not able to growth and multiply on the surface of agarized media and give rise to colonies. Cells in this viable but non-culturable state retain viability as well as the potential for pathogenicity for significant periods of time [ 25 – 27 ].
Viable but non-culturable cells can leave their dormant stage and multiply again, resulting in an explosion of their concentration in the environment. Since the presence of non-toxigenic strains is common in aquatic milieu, especially in estuaries, if a horizontal transfer of cholera exotoxin producing genes occurs between toxigenic and non-toxigenic strains, the number of toxigenic cells in the environment can rise rapidly and pronouncedly. The episodic nature and the sudden appearance of violent cholera outbreaks, followed by a rapid slowing down, are probably related with these phenomena.
3. Salmonellosis
3.1. the genus salmonella. pathogenicity of main serovars.
The genus Salmonella was designated by Lignières in 1900 [ 28 , 29 ]. Antigenic analysis began when Castellani described, in 1902, a method for absorbing antisera. The first antigenic scheme for Salmonella was published by White in 1926, and subsequently developed extensively by Kauffmann, in two classical works published in 1966 and 1978 [ 28 , 29 ]. The Kauffmann-White antigenic scheme contained, by 1988, about 2,250 different serovars [ 28 , 29 ].
The genus Salmonella , a member of the family Enterobacteriaceae , include Gram-negative motile straight rods. Cells are oxidase-negative and catalase-positive, produce gas from D-glucose and utilize citrate as a sole carbon source. Salmonellae have several endotoxins: antigens O, H and Vi [ 28 , 29 ].
The concept “one serovar-one species”, in use for many years, is no longer acceptable. The taxonomy and nomenclature of the genus Salmonella has been subject of debate since Le Minor and Popoff proposed changes in a paper published in 1987. The issue was settled by a decision of the International Committee on the Systematics of Prokaryotes and published in 2005. The current taxonomy of the genus is presented in Table 4 . According to the rules of bacterial nomenclature, the names of the serovars are not italicized and the first letter must be a capital [ 28 – 30 ].
Current taxonomy and nomenclature of the genus Salmonella . Habitat and pathogenicity of main serovars a .
S. enterica subsp. enterica serovar Enteritidis is the most frequently isolated serovar from humans all over the world. However, locally, other serovars can be predominant. In the period 1994–2004, Tunisia was exposed to salmonellosis outbreaks in 1997, 1999, 2002 and 2004. In 1997, salmonellosis outbreak was caused by serovar Mbandaka. In 1999, three salmonellosis outbreaks were reported from hospitals located in three different regions. Each outbreak was associated with a different serotype: Mbandaka, Livingstone and Typhi Vi+. In 2002, a S. enterica subsp. enterica serovar Livingstone infection occurred in the same hospital that reported an outbreak caused by serovar Typhi Vi+ in 1999, but in a different unit. In that year, the Livingstone serovar jumped to the first position in human infection in Tunisia. In 2004, a second outbreak by serovar Typhi Vi+ was reported. The source of isolation was a fermented juice traditionally extracted from palm-tree [ 31 ].
3.2. Characterization of the Diseases
Salmonellae pathogenic to humans can cause two types of salmonellosis: (1) typhoid and paratyphoid fever (do not confuse with typhus, a disease caused by a rickettsia); (2) gastroenteritis [ 28 ]. Low infective doses (less than 1,000 cells) are sufficient to cause clinical symptoms. Salmonellosis of newborns and infants presents diverse clinical symptoms, from a grave typhoid-like illness with septicemia to a mild or asymptomatic infection. In pediatric wards, the infection is usually transmitted by the hands of staff [ 29 ].
Food-borne Salmonella gastroenteritis are frequently caused by ubiquitous Salmonella serovars such as Typhimurium. About 12 h following ingestion of contaminated food, symptoms (diarrhea, vomiting and fever) appear and last 2–5 days. Spontaneous cure usually occurs. Salmonella may be associated with all kinds of food. Prevention of Salmonella food-borne infection relies on avoiding contamination (improvement of hygiene), preventing multiplication of Salmonella in food (constant storage of food at 4 °C), and use of pasteurization (milk) or sterilization when possible (other foods). Vegetables and fruits may carry Salmonella when contaminated with fertilizers of fecal origin, or when washed with polluted water [ 28 ].
The incidence of typhoid fever decreases when the level of development of a country increases ( i.e. , controlled water sewage systems, pasteurization of milk and dairy products). Where these hygienic conditions are missing, the probability of fecal contamination of water and food remains high and so is the incidence of typhoid fever [ 29 ].
3.3. Ecology of Salmonellae and the Cycle of Salmonellosis
The principal habitat of Salmonella is the intestinal tract of humans and animals [ 28 ]. Salmonellae are constantly found in environmental samples, because they are excreted by humans, pets, farm animals, and wild life. Municipal sewage, agriculture pollution, and storm water runoff are the main sources of these pathogens in natural waters [ 1 , 32 ]. Salmonellae do not seem to multiply significantly in the natural environment, but they can survive several weeks in water and in soil if conditions of temperature, humidity, and pH are favorable [ 28 ].
Salmonellae isolated from environmental sources are predominantly non-Typhi or Paratyphi serovars. In a study carried out in Tunisia during 1994–2004, S. enterica subsp. enterica serovars Anatum, Enteritidis and Corvallis were the most common serotypes isolated from food. The great majority of the strains were isolated from poultry, red meat, milk and dairy products, vegetables and fruits. From environmental sources, 73% of the isolates were from tap water. Serovars Corvallis, Enteritidis, and Anatum were the commonest [ 31 ]. Arvanitidou et al. [ 32 ] reported a comparative study carried out in Rivers Aliakmon and Axios, in northern Greece, during a 1-year period, from May 2002 to April 2003. A total of 29 Salmonella species were recovered from the water samples. Many of the isolated Salmonella serovars were of non-human animal origin such as Mbantaka, Virchow, Hadar, Infantis and Senftenberg, commonly isolated from poultry farm.
Unlike cholera, humans infected with salmonellae can carry the bacteria in the gut without signs of disease. Infected humans can harbor the bacteria for considerable periods of time. About 5% of patients clinically cured from typhoid fever remain carriers for months or even years. These people can be chronic holders of the bacterium in the gut, and constitute the main reservoir of the bacteria in the environment [ 29 ].
The salmonellosis cycle in the environment can involve shellfish. Salmonellae survive sewage treatments if suitable germicides are not used in sewage processing. If effluent from the sewage plant passes into a coastal area, edible shellfish (mussels, oysters) can become contaminated. Shellfish concentrate bacteria as they filter several liters of water per hour. Ingestion by humans of these seafoods (uncooked or superficially cooked) may cause typhoid fever or other salmonellosis. Evidence of such a cycle has been obtained by the use of strain markers, including phage typing [ 29 ].
4. Shigellosis or Bacillary Dysentery
4.1. the genus shigella.
Shigella are Gram-negative, non-sporeforming, non-motile, straight rod-like members of the family Enterobacteriaceae . Cells ferment sugars without gas production. Salicin, adonitol and myo-inositol are not fermented. Cells do not utilize citrate, malonate and acetate as sole carbon source and do not produce H 2 S. Lysine is not decarboxylated. Cells are oxidase-negative and catalase-positive. Members of the genus have a complex antigenic pattern, and taxonomy is based on their somatic O antigens [ 1 , 33 , 34 ].
4.2. Characterization of the Disease
The incubation period is 1–4 days. The disease usually begins with fever, anorexia, fatigue and malaise. Patients display frequent bloody stools of small volume (sometimes grossly purulent) and abdominal cramps. Twelve to 36 hours later, diarrhea progresses to dysentery, blood, mucus and pus appearing in feces that decreases in volume (no more than 30 mL of fluid per kg per day) [ 34 – 36 ].
Although the molecular basis of shigellosis is complex, the initial step in pathogenesis is penetration of the colonic mucosa. The resulting focus of Shigella infection is characterized by degeneration of the epithelium and by an acute inflammatory colitis in the lamina propria. Ultimately, desquamation and ulceration of the mucosa cause leakage of blood, inflammatory elements, and mucus into the intestinal lumen. Under these conditions the absorption of water by the colon is inhibited and the volume of stool is dependent upon the ileocecal flow. As a result, the patient will pass frequent, scanty, dysenteric stools [ 37 , 38 ].
In order for Shigella to enter an epithelial cell, the bacterium must first adhere to its target cell. Generally, the bacterium is internalized via an endosome, which it subsequently lyses to gain access to the cytoplasm where multiplication occurs [ 37 , 38 ].
4.3. Virulence Factors
S. dysenteriae serotype 1 produces high levels of a cytotoxic Shiga toxin. S. sonnei and S. flexneri produce much lower amounts of this toxin. Shiga toxin binds to Galotl-4Galp (galabiose) glycolipid receptors and inhibits mammalian protein synthesis by cleaving the N-glycosidic bond at adenine 4324 in 28S rRNA. The toxic mechanism is identical to that of the plant toxin ricin, produced by Ricinus communis . Shigella also release a LPS endotoxin (O antigens), that cause an inflammatory response [ 37 , 38 ].
Shigella 180- to 230-kb plasmids encode genes essential for virulence, namely for: production of adhesins involved in the adherence of bacteria onto the surface of target epithelial cells; production of invasion plasmid antigens (Ipa) that have a direct role in the Shigella invasion process; transport or processing functions that ensure the correct surface expression of the Ipa proteins; induction of endocytic uptake of bacteria and disruption of endocytic vacuoles; regulation of plasmid-encoded virulence genes [ 37 , 38 ].
Shigella emerged from E. coli during evolution. The acquisition and evolution of the pathogenicity island which encodes all of the genes required for cell invasion and phagolysosomal lysis, permitted a major alteration in pathogenesis [ 37 , 38 ].
4.4. Risk Factors
Many studies have identified risk factors and protective effects for shigellosis incidence and fatality. Despite gradual improvements in water supply, shigellosis continues to be endemic among the disadvantaged populations living in the tropics, often among displaced populations following natural disasters and political crises. In Guatemala, young children, the elderly, and 15–44-year-old males were found to be most susceptible to S. dysenteriae serotype 1. In Sierra Leone, the attack rate was higher among children younger than 5 years of age than in the rest of the population. In rural Bangladesh, shigellosis was most common in children aged 1–2 years and in people 60 years or older. In Dhaka, Bangladesh, it was found that shigellosis mortality was most common in severely malnourished people of all ages, in children under 2 who were not being breastfed, and in all children under 1. In a 3-year study carried out in Matlab, Bangladesh, during 1992 to 1994, it was found that the incidence of S. dysenteriae serotype 1 and S. flexneri was highest in children under 2 followed by children from 2 to 5. The location of S. dysenteriae serotype 1 risk varies in time but S. flexneri risk areas were persistent in time. Neighborhoods near bazaars with many non-septic latrines were at highest risk for S. dysenteriae serotype 1. S. flexneri was most common in flood-controlled areas. It was concluded that S. dysenteriae serotype 1 risk was more related to hygiene and sanitation whereas S. flexneri was more related to the environment [ 35 ].
4.5. Shigellosis through the World
The total number of Shigella episodes that occur each year throughout the World is estimated to be 164.7 million, including 163.2 million cases in developing countries, 1.1 million of which result in death. Children under 5 account for 61% of all deaths attributable to shigellosis [ 35 , 36 ].
Shigella species are not uniformly distributed in the world. S. dysenteriae is usually found in densely populated areas of South America, Africa and Asia. Infections usually result in significant epidemic outbreaks. Serotype 1 has been distinguished by both its virulence and its ability to produce ravaging epidemics. It predominates in India, Malaysia and Guatemala. Serotype 2 predominates in Yemen and Nigeria. S. flexneri is usually found in areas where endemic shigellosis occurs. S. boydii occurs sporadically, except in the Indian subcontinent where it was first identified. S. sonnei usually occurs in Western developed countries, such as France and USA [ 35 , 36 ].
Important epidemics were reported in the last decades: (1) in 1970 in Central America where 112,000 people were affected and 13,000 died; (2) in 1985, in Texas (USA), 5,000 people became infected after ingestion of contaminated lettuce; (3) in May–June 1994, domestic cases of S. sonnei infection were detected in several European countries, including Norway, Sweden, and the United Kingdom. Epidemiological evidence incriminated imported iceberg lettuce as the vehicle of transmission; (4) in 1996, in Paris, with 153 reported patients [ 33 ].
4.6. Ecology of Shigellae and the Cycle of Shigellosis
Shigella is typically an inhabitant of the intestinal tract of humans and other primates [ 1 , 33 , 34 , 36 , 39 ]. It is typically spread by fecal-contaminated drinking water or food, or by direct contact with an infected person. In water, shigellae can survive for at least six months at room temperature, and this high survival favors transmission through water. Flies have been implicated on the transmission of Shigella cells from human feces to foods. The hand is an important vehicle for transmission of shigellosis, since S. dysenteriae serotype 1 cells survives for up to one hour on a human’s skin and a very small inoculum is required to unchain infection and disease. Indeed, studies on American volunteers experimentally infected with Shigella have shown that as few as one hundred Shigella cells given orally cause the disease in 25–50% of the cases. Resistance of Shigella to gastric juice certainly accounts, although not exclusively, for this high infectivity [ 36 , 40 ]. Asymptomatic and inappropriately-treated patients with shigellosis can harbor the bacteria in the gut and these appear to be the main reservoirs of the bacteria in the environment [ 41 ].
Recent reports on the ecology of shigellae have brought new elements for the understanding of the cycle of the disease. In environmental waters of regions with high numbers of shigellosis’ cases, it has been found that, although numbers of cultivable cells were low, genetic elements such as plasmids and genetic fragments with bacteriophage origin, could be detected. Many of the genes that code for exotoxin production are precisely found in these genetic elements. These results suggest that the sudden rise of the number of virulent strains in the environment can result from the incorporation, by cells with reduced virulence, of this type of genetic elements present in the waters. If this hypothesis is confirmed, there is a certain similarity between the cholera and the shigellosis cycles in the environment. It remains to be elucidated if shigellae can also exist in environmental waters in a viable but non-culturable state, as vibrios [ 41 ].
5. Pathogenic Escherichia coli Strains
E. coli strains isolated from intestinal diseases have been grouped into at least six different main groups, based on epidemiological evidence, phenotypic traits, clinical features of the disease and specific virulence factors. From these, enterotoxigenic (ETEC, namely O148), enterohemorrhagic (EHEC, namely O157) and enteroinvasive serotypes (EIEC, namely O124) are of outstanding importance and can be transmitted through contaminated water [ 42 , 43 ].
5.1. Enterotoxigenic E. coli (ETEC) Strains
Enterotoxigenic E. coli (ETEC) serotypes can cause infantile gastroenteritis. The number of reports of their occurrence in developed countries is comparatively small, but it is an extremely important cause of diarrhea in the developing world, where there is no adequate clean water and poor sanitation. In developing countries, these strains are the most commonly isolated bacterial enteropathogen in children below 5 years of age, and account for several hundred million cases of diarrhea and several ten of thousand deaths each year [ 42 – 44 ].
Disease caused by ETEC follows ingestion of contaminated food or water and is characterized by profuse watery diarrhea lasting for several days that often leads to dehydration and malnutrition in young children [ 42 – 44 ]. ETEC also are the most common cause of “travelers’ diarrhea” that affects individuals from industrialized countries travelling to developing regions of the World [ 42 – 44 ].
5.2. Enterohemorrhagic E. coli (EHEC) Strains
Reported outbreaks had been associated mainly with the consumption of contaminated foods, such as raw or undercooked ground meat products and raw milk. The primary reservoir of this bacterium has been found to be healthy cattle [ 42 , 45 , 46 ].
E. coli serotype O157:H7 causes abdominal pain, bloody diarrhea, and hemolytic uremic syndrome. This bacterium produces Shiga-like toxins. The incubation period is 3–4 days, and the symptoms occur for 7–10 days. It is estimated that 2–7% of E. coli O157:H7 infections result in acute renal failure [ 42 , 45 , 46 ].
Although E. coli O157:H7 is not usually a concern in treated drinking water, outbreaks involving consumption of drinking water contaminated with human sewage or cattle feces have been documented. An increasing number of outbreaks are associated with the consumption of fruits and vegetables (sprouts, lettuce, coleslaw, salad) contaminated with feces from domestic or wild animals at some stage during cultivation or handling. EHEC has also been isolated from bodies of water (ponds, streams), wells and water troughs, and has been found to survive for months in manure and water-trough sediments [ 45 , 46 ].
Person-to-person contact is an important mode of transmission through the oral-fecal route. An asymptomatic carrier state has been reported, where individuals show no clinical signs of disease but are capable of infecting others [ 45 , 46 ].
5.3. Enteroinvasive E. coli (EIEC) Strains
Enteroinvasive E. coli (EIEC) behave in many respects like shigellae. They are capable of invading and multiplying in the intestinal epithelial cells of the distal large bowel in humans. The illness is characterized by abdominal cramps, diarrhea, vomiting, fever, chills, a generalized malaise, and the appearance of blood and mucus in the stools of infected individuals. [ 42 , 43 , 47 ].
EIEC strains were isolated, for instance, from 28 subjects in the Jesreel district of Israel during a peak period for dysentery. An investigation in Croatia showed that E. coli O124 could frequently be isolated from cases of gastroenteritis, enterocolitis, and dysentery. The dysentery was more common among the older age groups, while the two other types of disease occurred equally in all age groups. A 1985 survey was carried out in Bankok, Thailand in which 410 children with diarrhea and an equal number of control children without diarrhea were examined for the presence of strains of Shigella , EIEC, and other pathogens. It was found that 17 of the children with diarrhea and six without yielded EIEC [ 42 , 43 ].
Any food contaminated with human feces from an ill individual, either directly or via contaminated water, could cause disease in others. Outbreaks have been associated with hamburger meat and unpasteurized milk [ 47 ].
6. Emerging Waterborne Bacterial Pathogens
The emerging pathogenic bacteria of concern outlined here have the potential to be spread through drinking water, but they do not correlate with the presence of E. coli or with other commonly used drinking water quality indicators, such as coliform bacteria. In most cases, there are no satisfactory microbiological indicators of their presence. More studies are needed in order to understand the real significance and dimension of the diseases caused by water contaminated with these bacteria, and the ecology of these pathogens [ 45 ].
6.1. Mycobacterium Avium Complex (Mac)
The Mycobacterium avium complex (Mac) consists of 28 serovars of two distinct species: Mycobacterium avium and Mycobacterium intracellulare. The importance of Mac organisms was recognized with the discovery of disseminated infection in immunocompromised people, particularly people with HIV and AIDS. Members of MAC are considered opportunistic human pathogens [ 45 , 48 ].
Mac organisms have been identified in a broad range of environmental sources, including marine waters, rivers, lakes, streams, ponds, springs, soil, piped water supplies, plants, and house dust. Mac organisms have been isolated from natural water and drinking water distribution systems in the USA [ 45 , 49 , 50 ].
The ubiquitous nature of Mac organisms results from their ability to survive and grow under varied conditions. Mac organisms can proliferate in water at temperatures up to 51 °C and can grow in natural waters over a wide pH range [ 45 ]. These mycobacteria are highly resistant to chlorine and the other chemical disinfectants used for the treatment of drinking-water. Standard drinking-water treatments will not eliminate Mac organisms but, if operating satisfactorily, will significantly reduce the numbers that may be present in the source water to a level that represents a negligible risk to the general population. The entryway of these mycobacteria in distribution systems is through leaks. Growth of Mac organisms in biofilms is probably important for their continuous presence in distribution systems. Slow growing mycobacteria can be found at densities greater than 4,000 per cm 2 in the surface biofilm, creating a potentially high level of exposure [ 48 ].
The symptoms encountered with Mac infections result from colonization of either the respiratory or the gastrointestinal tract, with possible dissemination to other locations in the body. Exposure to Mac organisms may occur through the consumption of contaminated food, the inhalation of air with contaminated soil particles, or contact with or ingestion, aspiration, or aerosolization of potable water containing the organisms [ 45 ].
With respect to water supplies, infection with M. avium and M. intracellulare has been well documented. Unlike gastrointestinal pathogens, where E. coli can be used to indicate their potential presence, no suitable indicators have been identified to signal increasing concentrations of Mac organisms in water systems [ 45 ].
6.2. Helicobacter Pylori
Helicobacter pylori has been cited as a major etiologic agent for gastritis and has been implicated in the pathogenesis of peptic and duodenal ulcer disease and gastric carcinoma. However, most individuals that are infected by this pathogen remain asymptomatic [ 45 ].
Using culture-based methods, H. pylori has not been isolated from environmental sources, including water [ 45 , 51 ]. On the contrary, molecular methods have been successful in detecting this pathogen. Fluorescence in situ hybridization has been successfully used to detect this pathogen in drinking water distribution systems and other water bodies. Polymerase chain reaction has also been used to detect the presence of H. pylori DNA in drinking water, especially associated with biofilms [ 45 , 51 , 52 ]. In drinking-water biofilms, H. pylori cells rapidly lose culturability, entering a viable but non-culturable state. In these biofilms, cells can persist for more than one month, with densities exceeding 10 6 cells per cm 2 [ 51 ].
How the organism is transmitted is still not fully understood. However, the fact that it has been recovered from saliva, dental plaques, the stomach, and fecal samples strongly indicates oral-oral or fecal-oral transmission. Water and food appear to be of lesser direct importance, but they can still play a significant role in situations with improper sanitation and hygiene [ 45 ].
6.3. Aeromonas Hydrophyla
In recent years, A. hydrophila has gained public health recognition as an opportunistic pathogen. It has been implicated as a potential agent of gastroenteritis, septicemia, meningitis, and wound infections. It can play a significant role in intestinal disorders in children under five years old, the elderly, and immunosuppressed people. [ 45 , 53 , 54 ].
Aeromonas hydrophila are Gram-negative, non-sporeforming, rod-shaped, facultative anaerobic bacilli belonging to the family Aeromonadaceae . Although A. hydrophila is usually the dominant species, other aeromonads, such as A. caviae and A. sobria , have also been isolated from human feces and from water sources [ 45 , 54 ].
Aeromonas species, including A. hydrophila , are ubiquitous in the environment. It is frequently isolated from food, drinking water, and aquatic environments [ 45 , 53 , 54 ]. In clean rivers and lakes, concentrations of Aeromonas spp. are usually around 10 2 colony-forming units (CFU)/mL. Groundwaters generally contain less than 1 CFU/mL. Drinking water immediately leaving the treatment plant has been found to contain between 0 and 10 2 CFU/mL. Drinking water in distribution systems can display higher Aeromonas concentrations, due to the growth in biofilms [ 45 , 55 ]. Aeromonas spp. have been found to grow between 5 °C and 45 °C [ 44 , 54 ]. A. hydrophila is resistant to standard chlorine treatments, probably surviving inside biofilms [ 56 ].
The common routes of infection suggested for Aeromonas are the ingestion of contaminated water or food or contact of the organism with a break in the skin. Drinking or natural mineral water can be a possible source of contamination for humans. No person-to-person transmission has been reported [ 45 , 54 ].
7. Microbiological Water Analysis
7.1. the rationale of the use of fecal indicator bacteria.
The most important bacterial gastrointestinal diseases transmitted through water are cholera, salmonellosis and shigellosis. These diseases are mainly transmitted through water (and food) contaminated with feces of patients. Drinking water can be contaminated with these pathogenic bacteria, and this is an issue of great concern. However, the presence of pathogenic bacteria in water is sporadic and erratic, levels are low, and the isolation and culture of these bacteria is not straightforward. For these reasons, routine water microbiological analysis does not include the detection of pathogenic bacteria. However, safe water demands that water is free from pathogenic bacteria [ 57 ].
The conciliation of the two needs was met by the discovery and testing of indicator bacteria. Water contaminated with pathogenic species also has the normal inhabitants of the human intestine. A good bacterial indicator of fecal pollution should fulfill the following criteria: (1) exist in high numbers in the human intestine and feces; (2) not be pathogenic to humans; (3) easily, reliably and cheaply detectable in environmental waters. Additionally, the following requisites should be met if possible: (4) does not multiply outside the enteric environment; (5) in environmental waters, the indicator should exist in greater numbers than eventual pathogenic bacteria; (6) the indicators should have a similar die-off behavior as the pathogens; (7) if human fecal pollution is to be separated from animal pollution, the indicator should not be very common in the intestine of farm and domestic animals [ 1 , 4 , 6 , 57 , 58 ]. The usefulness of indicator bacteria in predicting the presence of pathogens was well illustrated in many studies, namely by Wilkes et al. [ 59 ].
7.2. The Composition of Human and Animal Feces
Microbiological analysis of the human feces was important in order to structure and validate the use of fecal indicator bacteria in environmental waters. Bacteria present in feces are naturally derived from the microbiota of the human gastrointestinal tract.
Although bacteria are distributed throughout the human gastrointestinal tract, the major concentration of microbes and metabolic activity can be found in the large intestine. The upper bowel (stomach, duodenum, and jejunum) has a sparse microbiota with up to 10 5 CFU/ml of contents. From the ileum on, bacterial concentrations gradually increase reaching in the colon 10 10 to 10 11 CFU/g [ 60 ].
It has been estimated that at least 500–1,000 different microbial species exist in the human gastrointestinal microbiota, although on a quantitative basis 10–20 genera usually predominate ( Table 6 ). The total number of microbial genes in the human gastrointestinal tract has been estimated as 2–4 million. This represents an enormous metabolic potential which is far greater than that possessed by the human host [ 60 , 64 ].
Total viable count in feces of healthy humans (children, adults and elderly) a .
The composition of feces from an individual is stable at genus level, but the species composition can vary markedly from day to day. The relative proportion of intestinal bacterial groups can vary between individuals [ 60 , 64 ].
The microflora of the human gastrointestinal tract is dominated by obligate anaerobes, which are ca. 10 3 more abundant than facultative anaerobes. The main anaerobic genera are Bacteroides , Eubacterium and Bifidobacteria. These organisms account for ca. 90% of the cultivable human fecal bacteria. Bacteroides (mainly B. thetaiotaomicron and B. vulgatus ) are the most abundant organism in the human feces and account for 20–30% of cultivable bacteria. The most abundant facultative anaerobes are Enterococci and Enterobacteriaceae . The main Enterobacteriaceae genera are Escherichia , Citrobacter , Klebsiella , Proteus and Enterobacter. Citrobacter and Klebsiella are present in most individuals although in low numbers. Proteus and Enterobacter are only present in a minority of humans [ 64 ].
A variety of molecular techniques have been used to study the microbial composition of the human gastrointestinal tract. Results yielded by these studies have shown that many microbes detected by molecular techniques are not isolable by conventional culture-based methods. The presence of high proportions of bifidobacteria detected by culture-based methods is not supported by the results of molecular-based studies. However, the results of molecular-based approaches support many of the findings derived from culture-based methods: the dominance of the obligate anaerobes over facultative anaerobes; the presence of high counts of Bacteroides , Clostridium and Eubacterium [ 64 ].
Anaerobic bacteria such as Bacteroides and Eubacterium are not easily cultured by conventional techniques since require incubation chambers with nitrogen atmosphere. Bifidobacterium and Lactobacillus tolerate some oxygen but are fastidious bacteria growing very slowly in culture media. Therefore, these four genera are not adequate to be used as indicators of fecal pollution (the introduction of molecular techniques may improve the situation). Citrobacter , Klebsiella and Enterobacter are present in low numbers in the human intestine and are widespread in environmental waters, and therefore are also not suitable as indicators of fecal pollution. Clostridium , Streptococcus and Escherichia do not suffer from these drawbacks. Therefore, their suitability as fecal indicators has been tested since several decades.
7.3. Fecal Bacteria in Their Hosts and in the Environment
7.3.1. bacteroides.
The traditional genus Bacteroides included Gram-negative, non-sporeforming, anaerobic pleiomorphic rods. Many species have been transferred to other genera— Mitsuokella, Porphyromonas, Prevotella, Ruminobacter . Bacteroides are the most abundant bacteria in human feces. In animal feces, on the contrary, Bacteroides are present at low numbers. Although anaerobic, Bacteroides are among the most tolerant to oxygen of all anaerobic human gastrointestinal species. B. thetaiotaomicron is one of the most abundant species in the lower regions of the human gastrointestinal tract. Bacteroides have a high pathogenic potential and account for approximately two-thirds of all anaerobes isolated from clinical specimens. The most frequently isolated species has been B. fragilis . The survival of Bacteroides in environmental waters is usually much lower than the survival of coliforms [ 64 , 65 ].
7.3.2. Eubacterium
The traditional genus Eubacterium included anaerobic non-sporeforming Gram-positive rods. Some species have been transferred to other genera— Actinobaculum , Atopobium , Collinsella , Dorea , Eggerthella , Mogibacterium , Pseudoramibacter and Slackia . Cells are not very aerotolerant. Species isolated from the human gastrointestinal tract include: E. barkeri , E . biforme , E. contortum , E. cylindrioides , E. hadrum , E. limosum , E. moniliforme, E. rectal and E. ventricosum [ 64 ].
7.3.3. Bifidobacterium
Bifidobacteria are Gram-positive, non-sporeforming, pleiomorphic rods. Bifidobacteria are anaerobic (some species tolerate oxygen in the presence of carbon dioxide) or facultative anaerobic. The optimum growth temperature is 35–39 °C. The genus Bifidobacterium contains ca. 25 species, most of which have been detected in the human gastrointestinal tract [ 64 – 66 ].
Bifidobacteria are present in high numbers in the feces of humans and some animals. Several Bifidobacterium species are specific either for humans or for animals. B. cuniculi and B. magnum have only been found in rabbit fecal samples, B. gallinarum and B. pullorum only in the intestine of chickens and B. suis only in piglet feces. In human feces, the species composition changes with the age of the individual. In the intestine of infants B. breve and B. longum generally predominate. In the adult, B. adolescentis , B. catenulatum , B. pseudocatenulatum and B. longum are the dominant species. In both human and animal feces, bifibobacteria are always much more abundant than coliforms [ 64 – 66 ].
Bifidobacteria have been found in sewage and polluted environmental waters, but appears to be absent from unpolluted or pristine environments such as springs and unpolluted soil. This results from the fact that upon introduction into the environment, bifidobacteria decrease appreciably in numbers, probably due to their stringent growth requirements. Bifidobacteria grow poorly below 30 °C and have rigorous nutrient requirements. Reports on the survival of bifidobacteria in environmental waters indicate that their survival is lower than that of coliforms [ 64 – 66 ].
The presence of bifidobacteria in the environment is therefore considered an indicator of fecal contamination. Since some species are specific for humans and animals, the identification of Bifidobacterium species present in the polluted water could, in principle, provide information on the origin of fecal pollution [ 64 – 66 ].
A study carried out in a highly contaminated stream near Bologna, Italy, revealed that B. adolescentis , B. catenulatum , B. longum , B. pseudocatenulatum and B. thermophilum were the most representative species, whereas B. angulatum , B. animalis subsp. animalis (B. animalis) , B. breve , B. choerinum , B. minimum , B. pseudolongum subsp. globosum ( B. globosum ) and B. subtile occurred only in low numbers [ 66 ].
Bifidobacteria are the less studied of all fecal bacteria, due to the technical difficulties in their isolation and cultivation. Other Gram-positive bacteria, such as Streptococcus and Lactobacillus , which may occur in higher numbers than bifidobacteria, can inhibit their growth. Although selective media has been designed for the isolation of bifidobacteria from environmental waters, the outcome is still unsatisfactory, with appreciable numbers of false positives and low recovery percentages [ 64 – 66 ].
7.3.4. Clostridia
The genus Clostridium is one of the largest genera of the prokaryotes containing 168 validly published species. From these, 77 (including C. perfringens ) are considered to belong to a united group— Clostridium sensu stricto [ 64 , 67 , 68 ].
Clostridia are Gram-positive rods, forming endospores. Most of the clostridial species are motile with peritrichous flagellation. Cells are catalase-negative and do not carry out a dissimilatory sulphate reduction. Clostridia usually produce mixtures of organic acids and alcohols from carbohydrates and proteins. Many species are saccharolytic and proteolytic. Some species fix atmospheric dinitrogen [ 64 , 67 , 68 ].
The genus Clostridium includes psychrophilic, mesophilic, and thermophilic species. The major role of these organisms in nature is in the degradation of organic material to acids, alcohols, CO 2 , H 2 , and minerals. Frequently, a butyric acid smell is associated with the proliferation of clostridia. The ability to form spores that resist dryness, heat, and aerobic conditions makes the clostridia ubiquitous [ 64 , 67 , 68 ].
Most species are obligate anaerobic, although tolerance to oxygen occurs. Oxygen sensitivity restricts the habitat of the clostridia to anaerobic areas or areas with low oxygen tensions. Growing and dividing clostridia will, therefore, not be found in air saturated surface layers of lakes and rivers or on the surface of organic material and soil. Clostridial spores, however, are present with high probability in these environments, and will germinate when oxygen is exhausted and when appropriate nutrients are present [ 64 , 67 , 68 ].
C. perfringens ferment lactose, sucrose and inositol with the production of gas, produce a stormy clot fermentation with milk, reduce nitrate, hydrolyze gelatin and produce lecithinase and acid phosphatase. The species is divided into five types, A to E, on the basis of production of major lethal toxins [ 68 , 69 ].
C. perfringens appears to be a universal component of the human and animal intestine, since has been isolated from the intestinal contents of every animal that has been studied. Humans carry C. perfringens as part of the normal endogenous flora. The main site of carriage is the distal gastrointestinal tract. The principal habitats of type A are the soil and the intestines of humans, animals, and birds. Types B, C, D, and E appears to be obligate parasites of animals and occasionally are found in humans [ 68 , 69 ].
Clostridium perfringens is the most frequently isolated Clostridium in clinical microbiology laboratories, although it seldom causes serious infections. C. perfringens is isolated from infections in humans and the organism most commonly found in gas gangrene in humans. C. perfringens is most commonly isolated from infections derived from the colonic flora, namely peritonitis or abdominal abscess [ 68 , 69 ].
This organism is a common cause of food poisoning due to the formation of the enterotoxin in the intestine. C. perfringens food poisoning is seldom fatal, being marked by diarrhea and nausea, with no vomiting and no fever [ 68 , 69 ].
Sources yielding C. perfringens include soil and marine sediment samples worldwide, clothing, raw milk, cheese, semi-preserved meat products, and venison. Like E. coli , C. perfringens does not multiply in most water environments and is a highly specific indicator of fecal pollution. Berzirtzoglou et al. [ 70 ] reported a comparative study on the occurrence of vegetative cells and spores of Clostridium perfringens in a polluted station of the lake Pamvotis, in rural North-West Greece. The numbers of C. perfringens varied according to the water depth. Sporulated forms were found in all sampling sites with the exception of the surface sampling.
7.3.5. Lactobacillus
Lactobacilli are non-sporeforming Gram-positive long rods. There are more than thirty species in the genus. Most are microaerophillic, although some are obligate anaerobes. Cells are catalase-negative and obtain their energy by the fermentation of sugars, producing a variety of acids, alcohol and carbon dioxide. Lactobacilli have complex nutritional requirements and in agarized media may need the supplementation with aminoacids, peptides, fatty-acid esters, salts, nucleic acid derivatives and vitamins. Lactobacilli very rarely cause infections in humans [ 64 ].
7.3.6. Enterococci
Enterococci are Gram-positive, non-sporeforming, catalase-negative ovoid cells. Cells occur singly, in pairs or short chains. Optimal growth for most species is 35–37 °C. Some will grow at 42–45 °C and at 10 °C. Growth requires complex nutrients but is usually abundant on commonly used bacteriological media. Cells are resistant to 40% bile, 0.4% azide, 6.5% sodium chloride, have β-glucosidase and hydrolyze esculin. The enterococci are facultative anaerobic but prefer anaerobic conditions [ 64 , 71 ].
The genus was separated from Streptococcus in the 1980s. Enterococci form relatively distinct groups. Members of such groups exhibit similar phenotypic characteristics and species delimitation can be difficult. The E. faecalis group contains, among others, E. faecalis . The E. avium group contains, among others, E. avium . The E. faecium group contains, among others, E. faecium , E. durans and E. hirae . The E. gallinarum group contains, among others, E. gallinarum [ 64 , 71 ].
Most species are part of the intestinal flora of mammals, reptiles, birds, and other animals. In the human digestive tract, E. faecalis is the prevailing species, although in particular situations, E. faecium may predominate. In poultry, E. cecorum , E. durans , E. faecalis , E. faecium and E. hirae and dominate the intestinal flora [ 64 , 71 ].
Enterococci have been increasingly isolated from a variety of nosocomial and other infections, mainly from the urinary tract and wound infections, bacteremias, and endocarditis [ 64 , 71 ].
Although enterococci are considered only a temporary part of the microflora of plants, in optimal conditions, cells can proliferate on their surfaces. E. casseliflavus , E. faecalis , E. faecium , E. hirae , E. mundtii and E. sulfureus have been isolated from plants. They are generally isolated more often from flowers than from buds or leaves [ 64 , 71 ].
Enterococci are naturally present in many kinds of foods, especially those of animal origin such as milk and milk products, meat and fermented sausages. Enterococci are usually considered secondary contaminants of food, although they often play a positive role in ripening and aroma development of some types of cheeses [ 64 , 71 ]. Although soil is not a natural habitat for enterococci, cells can be found in this habitat due to the transport by rain [ 64 , 71 ].
Environmental waters are not a natural habitat for enterococci and their presence in this milieu is considered the result of fecal pollution. The most common species found in environmental waters are E. durans, E. faecalis , E. faecium and E. hirae , and less commonly, E. avium , E. cecorum , E. columbae and E. gallinarum . However, pristine waters in Finland have been reported to contain E. casseliflavus [ 64 , 71 ].
In environmental samples (compost, sewage effluent, harbor sediments, brackish water and swimming pool water), Pinto et al. [ 72 ] reported the isolation of E. casseliflavus , E. durans , E. faecalis , E. faecium , E. gallinarum and E. hirae. E. durans , E. faecium and E. hirae were isolated from all sources except from harbor sediments. E. raffinosus was only isolated from compost and swimming pool water. E. faecalis and E. faecium accounted for the vast majority of enterococcal strains.
7.3.7. Escherichia
Escherichia , a member of Enterobacteriaceae , are oxidase-negative catalase-positive straight rods that ferment lactose. Cells are positive in the Methyl-Red test, but negative in the Voges-Proskauer assay. Cells do not use citrate, do not produce H 2 S or lipase, and do not hydrolyze urea [ 73 ]. E. coli is a natural and essential part of the bacterial flora in the gut of humans and animals. Most E. coli strains are nonpathogenic and reside harmlessly in the colon. However, certain serotypes do play a role in intestinal and extra-intestinal diseases, such as urinary tract infections [ 43 ]. In a study of the enteric bacteria present in the feces of Australian mammals, Gordon and FitzGibbon [ 74 ] reported that E. coli was the commonest species, being isolated from nearly half of the species studied.
7.3.8. Citrobacter
Citrobacter , a member of Enterobacteriaceae , are motile straight rods. Cells are oxidase-negative, catalase-positive and positive in the Methyl-Red test. Cells use citrate, are negative in the Voges-Proskauer test and do not decarboxylate lysine [ 73 ].
In a study of the enteric bacteria present in the feces of Australian mammals, Gordon and FitzGibbon [ 74 ] reported the isolation of C. amalonaticus, C. freundii and C. koseri ( C. diversus ). Citrobacter species can be isolated from different clinical sites. In particular, C. freundii is an intestinal inhabitant of humans that may sometimes have—or acquire—the ability to produce an enterotoxin and thus become an intestinal pathogen. Citrobacter is reported to occur in environments such as water, sewage, soil and food [ 75 , 76 ].
7.3.9. Klebsiella and Raoultella
Klebsiella and Raoultella are Enterobacteriaceae , oxidase-negative catalase-positive non-motile straight rods, surrounded by a capsule. Cells decarboxylate lysine, but are ornithine and arginine dihydrolase negative. Cells grow on KCN, do not produce H 2 S and ferment most carbohydrates [ 73 ].
In humans, K. pneumoniae is present as commensal in the nasopharynx and in the intestinal tract. Klebsiella spp. can cause human diseases, ranging from asymptomatic colonization of the intestinal, urinary, or respiratory tract to fatal septicemia. Klebsiella are mostly considered nosocomial pathogens. K. pneumoniae and Enterobacter aerogenes ( K. mobilis ) are most frequently involved, although K. oxytoca and R. planticola , and rarely R. terrigena , can be found. In the hospital, the principal reservoir of K. pneumoniae is the gastrointestinal tract of patients. The principal vectors are the hands of personnel [ 77 , 78 ]. In a study of the enteric bacteria present in the feces of Australian mammals, Gordon and FitzGibbon [ 74 ] reported the isolation of K. pneumoniae and K. oxytoca .
Klebsiellae are ubitiquous in the environment. They have been found in a variety of environmental situations, such as soil, vegetation, or water, and they influence many biochemical and geochemical processes. They have been recovered from aquatic environments receiving industrial wastewaters, plant products, fresh vegetables, food with a high content of sugars and acids, frozen orange juice concentrate, sugarcane wastes, living trees, and plants and plant byproducts. They are commonly associated with wood, sawdust, and waters receiving industrial effluents from pulp and paper mills and textile finishing plants (see below). Klebsiella have been isolated from the root surfaces of various plants. K. pneumoniae , K. oxytoca , and R. planticola are all capable of fixing dinitrogen [ 77 , 78 ].
7.3.10. Enterobacter
Enterobacter a member of Enterobacteriaceae , are motile straight rods. Cells are positive in the Voges-Proskauer test VP and in Simmons citrate agar. Cells do not decarboxylate lysine, but are ornithine positive. Malonate is usually utilized and gelatin is slowly liquefied. Cells do not produce H 2 S, deoxyribonuclease and lipase [ 73 ].
In a study of the enteric bacteria present in the feces of Australian mammals, Gordon and FitzGibbon [ 74 ] reported the isolation of Enterobacter cloacae subsp. cloacae ( E. cloacae ), E. cancerogenus ( E. taylorae ) and E. aerogenes ( Klebsiella mobilis ).
Before the widespread use of antibiotics, Enterobacter species were rarely found as pathogens, but these organisms are now increasingly encountered, causing nosocomial infections such as urinary tract infections and bacteremia. In addition, they occasionally cause community-acquired infections [ 79 , 80 ].
In the USA, the Surveillance and Control of Pathogens of Epidemiological Importance project analyzed 24,179 nosocomial bloodstream infections, from 1995–2002. Enterobacter species were the second most common gram-negative organism, behind Pseudomonas aeruginosa . Both bacteria were reported to each represent 4.7% of bloodstream infections in intensive care units. Enterobacter species represented 3.1% of bloodstream infections in non-intensive care units. Of nearly 75,000 gram-negative organisms collected from intensive care units’ patients in the USA, between 1993 and 2004, Enterobacter species comprised 13.5% of the isolates. Multidrug resistance increased over time, especially in infections caused by E. cloacae [ 81 ].
In the USA, the National Healthcare Safety Network reported a study on healthcare-associated infections between 2006 and 2007. They found Enterobacter species to be the eighth most common cause of healthcare-associated infections (5% of all infections) and the fourth most common gram-negative cause of these infections [ 82 ].
Enterobacter cloacae subsp. cloacae ( E. cloacae ) occurs in the intestinal tracts of humans and animals, in hospital environments, the skin, in water, sewage, soil, meat. Nitrogen-fixing strains have been isolated from the roots of rice plants. E. amnigenus has been mostly isolated from water, but some strains were isolated from clinical specimens from the respiratory tract, wounds and feces. E. asburiae strains were isolated from clinical specimens, mostly urine, respiratory tract, feces, wounds, and blood [ 79 , 80 ].
7.4. Origin of the Use of Fecal Indicator Bacteria
Historically, the design and use of indicators of fecal pollution comes from the end of the 19th to beginning of the 20th century. In 1880, von Fritsch described Klebsiella pneumoniae and K. pneumoniae subsp. rhinoscleromatis ( Klebsiella rhinoscleromatis ) as micro-organisms characteristically found in human feces [ 83 ]. In 1885, Escherich described several microorganisms in the feces of newborn and suckling babies. This included a motile, rod-shaped microorganism that caused milk to clot, which was named “ Bacterium coli commune ”. He observed that within a few weeks after birth, this bacterium became the dominant organism in the infant colon [ 6 ]. Also in 1885, Percy and Grace Frankland started the first routine bacteriological examination of water in London, using Robert Koch’s solid gelatin media to count bacteria [ 83 ]. In 1891, Percy and Grace Frankland came up with the concept that organisms characteristic of sewage must be identified to provide evidence of potentially dangerous pollution [ 83 ]. In 1892, Schardinger proposed that since “ Bacterium coli ” was a characteristic component of the fecal flora, its presence in water could be taken as an indication of the presence of fecal pollution and therefore of the potential presence of enteric pathogens [ 6 ]. Soon after the description of “ Bacterium coli ”, other bacteria were isolated from stools and water— Klebsiella in 1882 and Enterobacter in 1890 [ 6 ]. By 1893, the “Wurtz method” of enumerating “ Bacterium coli ”, by direct plating water samples on litmus lactose agar, was being used by sanitary bacteriologists. This was based on the concept of acid and gas production (detected by the Durham tube) from lactose as a diagnostic feature [ 6 ]. In 1905, MacConkey described his now famous MacConkey’s broth, which was diagnostic for lactose-fermenting bacteria tolerant of bile salts. Coliforms were already considered to be a heterogeneous group of organisms, many of which were not of fecal origin. The origins of the critical observation that “ Bacterium coli ” was largely fecal in origin while other coliforms were not, could be claimed by Winslow and Walker in 1907 [ 83 ].
Various classification schemes for coliforms have emerged. The earliest were those of MacConkey in 1909, which recognized 128 different coliform types, while Bergey and Deehan in 1908, identified 256. By the early 1920s, differentiation of coliforms had come to a series of correlations that suggested that indole production, gelatin liquefaction, sucrose fermentation and the Voges–Proskauer reaction were among the more important tests for determining fecal contamination. These developments culminated in the IMViC (Indole, Methyl Red, Voges–Proskauer and Citrate) tests for the differentiation of so-called fecal coliforms, soil coliforms and intermediates [ 83 ].
7.5. Fecal Indicator Bacteria
7.5.1. coliforms.
Total coliforms are Gram-negative, oxidase-negative, non-sporeforming rods, that ferment lactose with gas production at 35–37 °C, after 48h, in a medium with bile salts and detergents [ 1 , 4 , 6 , 57 , 84 ]. When the test of coliforms is carried out with environmental waters, several species of the four Enterobacteriaceae genera Escherichia , Klebsiella , Enterobacter and Citrobacter give positive results and therefore are coliforms according to this definition. However, the environmental significance of these four genera is very disparate as discussed in the present text. Therefore, total coliform counts are not necessarily a measure of fecal pollution and indeed can have no relation with this cause [ 1 , 4 , 6 , 84 ].
Fecal coliforms (or thermotolerant coliforms) are traditionally defined as coliforms that ferment lactose at 44.5 °C in a medium with bile salts [ 1 , 4 , 57 , 84 ]. The range of species detected by the experimental procedure is much lower than that of total coliforms. With environmental polluted waters, only E. coli , and K. oxytoca and K. pneumoniae gave positive results in the test [ 85 ].
Traditional tests for total and fecal coliforms are carried out either by the multiple-tube fermentation technique or by filtration through membrane. The multiple-tube fermentation technique is used for medium or highly contaminated waters, and the filtration through membrane for low or very low contaminated waters. Filtration through membrane is a very sensitive technique since can detect one (culturable) cell in 500 or even 1,000 mL of water. However, both methods take several days to complete and do not detect viable but non-culturable bacteria [ 3 , 57 , 86 ]. These limitations stimulate the discovery of alternative methods, faster and, if possible, less prone to false negative results such as those caused by the viable but non-culturable bacteria.
The detection of β-D-galactosidase activity (at 37 °C) is usually a good marker for total coliforms in environmental waters, since most of these bacteria display this enzymatic activity [ 1 , 3 , 57 , 87 – 91 ]. Most Escherichia , Citrobacter , Enterobacter , Klebsiella and Raoultella strains have galactosidase. Hafnia , Serratia and Yersinia also possess this enzymatic activity. Most Proteus , Salmonella and Edwardsiella strains do not display β-galactosidase [ 92 – 95 ]. Ca. 10% of the coliform strains isolated from the environment do not have an active formic hydrogenolyase (cleaves formate with the formation of CO 2 ) and therefore do not produce gas being undetected by the traditional techniques but are detected by the assay of β-galactosidase activity [ 57 , 96 , 97 ].
β-galactosidase cleaves lactose in glucose and galactose, and can be detected by using colored or fluorescent markers that change color after enzyme action, such as XGAL (5-bromo-4-chloro-3-indol-β-galactopyranoside) and ONPG (O-nitrophenyl-β-D-galactopyranoside) or MUGAL (4-methylumbelliferyl-β-D-galactopyranoside), respectively [ 57 , 96 , 97 ].
In environmental waters, the presence of Aeromonas or Vibrio cholerae can be a source of false positives in the β-D-galactosidase assay, since these bacteria have galactosidase, but are not coliforms [ 93 , 95 , 96 , 98 ]. Additionally, in particular environments, such as estuaries, β-galactosidase activity can overestimate total coliform count due to UV-stimulated enzymatic activity in certain bacteria such as E. coli [ 86 ].
The detection of β-D-glucuronidase activity (at 44.5 °C) is, generally, a good marker for fecal coliforms in environmental polluted waters and very specific for E. coli [ 13 , 85 , 87 – 91 , 97 , 99 – 101 ]. In Gram-negative bacteria, this enzymatic activity if found in most E. coli strains and in some Salmonella and Shigella strains [ 92 – 95 , 97 , 102 ]. Aeromonas , Citrobacter , Enterobacter , non- coli Escherichia , Hafnia , Klebsiella , Proteus , Serratia , Vibrio , Yersinia , and most Salmonella strains do not display β-glucuronidase activity [ 93 – 95 , 101 , 102 ].
β-D-glucuronidase activity can be detected by using colored or fluorescent markers that change color after enzyme action, such as XGLUC (5-bromo-4-chloro-3-indoxyl-β- d -glucuronide), IBDG (indoxil-β-glucuronide), and MUGLU (4-methylumbelliferyl-β- d -glucuronide), respectively [ 57 , 97 , 100 ].
The presence of this enzyme in some strains of Bacteroides , Flavobacterium , Staphylococcus , Streptococcus , in anaerobic corynebacteria and Clostridium , has also been reported [ 93 , 95 – 97 , 102 ]. β-D-glucuronidase activity in fecal bacteria other then E. coli ( Bacteroides , bifidobacteria, clostridia, enterococci and Lactobacillus ) is very limited [ 61 ]. Although all these glucuronidase positive bacteria could lead to false positive detections in the fecal coliform test, experimental results for environmental polluted waters indicate a significant correlation between fecal coliform detection using conventional techniques and the glucuronidase assay, suggesting that false positives are not significant [ 85 , 96 ].
The detection of total coliforms and fecal coliforms by enzymatic methods are much less time consuming than traditional techniques. With fluorescent markers and the use of a spectrofluorimeter the detection of coliforms can be performed in minutes [ 57 , 101 ]. However, in very low contaminated waters, enzymatic methods might not be able to detect coliform cells. Moreover, on-line monitoring of glucuronidase activity is currently too insensitive to replace culture based detection of E. coli . Nevertheless, on-line enzymatic methods can be a valuable complementary tool for high temporal resolution monitoring. More research is needed in order to enhance sensitive and lower detection limits of available on-line glucuronidase techniques.
The seminal work of Leclerc et al. [ 103 ] clarified the diversified roles that coliforms have in the environment and the real meanings of the tests on total coliforms and fecal coliforms. It was shown that Enterobacteriaceae encompass three groups of bacteria with very different roles in the environment. Group I harbored only E. coli . Since this species usually do not survive for long periods outside this environment (but see topic 10), it was considered a good and reliable indicator of fecal pollution (both animal and human). Group II, the “ubiquitary” group, encompassed several species of Klebsiella ( K. pneumoniae and K. oxytoca ), Enterobacter ( Enterobacter cloacae subsp. cloacae , E. aerogenes ) and Citrobacter ( C. amalonaticus , C. koseri and C. freundii ). These bacteria live in the animal and human gut, but also in the environment, and are easily isolated from the soil, polluted water and plants. Their presence in polluted waters does not necessarily indicate fecal pollution. Finally Group III was composed of Raoultella planticola , R. terrigena, Enterobacter amnigenus and Kluyvera intermedia ( Enterobacter intermedius ), Serratia fonticola , and the genera Budvicia , Buttiauxella , Leclercia , Rahnella , Yersinia , and most species of Erwinia and Pantoea . These bacteria live in fresh waters, plants and small animals. They grow at 4 °C, but not at 41 °C. They are not indicators of fecal pollution, although can be detected in the total coliform test. Leclerc et al. concluded that: (1) in the enterobacteria, E. coli is the only true and reliable indicator of fecal pollution in environmental waters; (2) the traditional total coliform test should be abandoned, since it can detect bacteria that have no connection with fecal pollution; (3) the detection of fecal coliforms must be carried out at 44.5 °C, and positive results confirmed by identification to species levels in order to exclude false positives such as K. pneumoniae .
7.5.2. Streptococci and Enterococci
Fecal streptococci also belong to the traditional indicators of fecal pollution. Fecal streptococci are Gram-positive, catalase-negative, non-sporeforming cocci that grow at 35 °C in a medium containing bile salts and sodium azide. Cells hydrolyze esculin [ 1 , 4 , 57 ]. Azide is a strong inhibitor of the respiratory chain. Since streptococci are one of the very few bacteria that have no respiratory chain, the test is very specific for this group, and false positives are rarely found [ 104 , 105 ].
Fecal enterococci ( E. faecalis , E. faecium, E. avium and E. gallinarum ) are fecal streptococci that grow in the presence of 6.5% NaCl at 45 °C. Selective media use these particular characteristics in order to separate enterococci from the other streptococci [ 104 , 105 ].
Several studies [ 104 , 106 ] have reported on the microbiological composition of human and animal (cattle, chicken, deer, dog, fowl, goose, and swine) feces. E. faecalis and E. faecium were present in human and animal feces. However, whereas human feces almost have only these two enterococci, in the animals others species co-occur, like E. avium , E. cecorum , E. durans , E. gallinarum and E. hirae . It was concluded that in urban areas where contamination with dog and chicken feces is not likely, the best marker for human fecal pollution was E. faecalis .
The intestinal enterococci group has been used as an index of fecal pollution. In human feces, the numbers of intestinal enterococci are generally about an order of magnitude lower than those of E. coli ( Table 6 ). Most species do not grow in environmental waters. In this milieu, fecal enterococci are able to survive longer, are more resistant to drying and chlorination, than E. coli [ 1 , 84 ].
However, caution should be taken with interpreting the results obtained by the enterococci procedure in water analysis. Enterococci and other group D-streptococci are present in many foods, especially those of animal origin. The isolation of E. faecalis and E. faecium was used to indicate fecal contamination of food. However, enterococci are now also considered as normal parts of the food microflora and not only as indicators for poor hygiene [ 104 ]. In addition, agricultural soils and crops with added with manure also harbor enterococci [ 105 ].
7.5.3. The use of ratios between indicator counts
The ratio of counts of fecal coliforms to fecal streptococci has been proposed as a means to differentiating between contamination from human and animal sources. Ratios greater than 4 have been suggested to indicate a human source whereas ratios less than 0.7 suggest an animal source. This results from the fact that streptococcal concentrations in human feces are generally less than coliforms ( Table 6 ). In contrast, in animal feces fecal streptococci generally outnumber fecal coliforms ( Table 7 ). In urban sewage, fecal streptococci tend to be present in concentrations 10–100 times less than fecal coliforms [ 65 ].
Bacteria in the feces of farm and domestic warm-blooded animals a .
Geldreich [ 107 ] summarized the information available on the fecal coliforms to fecal streptococci ratios in the feces of warmblooded animals, and reported the following values: human feces, 4.3; cattle, sheep, and poultry, from 0.104 to 0.421; and wild animals (including rabbits, field mice, chipmunks, and birds), 0.0008 to 0.043. Fecal coliforms to fecal streptococci ratios for the feces of wild animals appear to be at least 10-fold lower than those of domestic livestock.
Doran and Linn [ 108 ] reported a study of the runoff from a cow-calf pasture in eastern Nebraska (USA), monitored during a three-year period. It was concluded that the fecal coliforms to fecal streptococci ratio in pasture runoff was useful in identifying the relative contributions of cattle and wildlife and in evaluating the effects of cattle management and distribution on runoff water quality. Ratios below 0.05 were indicative of wildlife sources and ratios above 0.1 were characteristic of grazing cattle. Fecal coliforms to fecal streptococci ratios of diluted cattle waste in excess of 1 were interpreted as the result of differential aftergrowth and die-off between fecal coliforms and fecal streptococci. Ratios between 0.7 and 4.0 may indicate situations where cattle are localized close to sampling or outflow points.
However, the interpretation of this ratio should be cautious. It has been observed a shift in the ratio with time and distance from the fecal pollution source. This resulted from the fact that both in surface and groundwaters, fecal streptococci are more persistent than fecal coliforms. Therefore increasing the distance from the pollution point and with passing time, the ratio tends to decrease without a change in the nature of the pollution source [ 65 ]. For these reasons, this ratio has been considered by some authors as too unreliable to be useful in characterizing pollution sources [ 65 , 84 ].
The ratio of fecal enterococci to fecal streptococci differs among vertebrate species. Humans have a predominance of enterococci, whereas animals contain appreciable amounts of streptococci. However, since enterococci are also present in animals and are more persistent in the environment than other fecal streptococci, the identification of the enterococci and streptococci species present in polluted waters, and the concomitant calculation of this ratio is generally considered unreliable as an indicator of the source of fecal pollution [ 65 ].
7.5.4. Limitations of coliform and enterococcus counts as indicator of fecal pollution
An extreme case of uselessness of the determination of total and fecal coliforms and enterococci in the assessment of fecal pollution has been demonstrated by several authors studying the microbiology of pulp and paper mill effluents.
Caplenas and Kanarek [ 109 ] reported a study of pulp and paper mills located in Wisconsin (USA). Fresh water supplies, re-cycled water within mills, treated effluent wastewater and waters receiving effluent wastes downstream, were assessed for the presence of fecal coliforms and Klebsiella . Wastewaters prior to treatment contained fecal coliforms and Klebsiella . Up to 84% of the fecal coliforms (detected by the standard test procedure) were indeed Klebsiella . In treated effluent wastewaters this value reached 90%. Treatment of the wastewater lowered the concentration of “true” fecal bacterial contamination, but since Klebsiella grew rapidly in the wastewaters, fecal coliform counts were high, although no true fecal contamination was involved. The source of Klebsiella was traced to the early pulping stages in the mills. Klebsiella maintains a wood, bark or soil reservoir. It was concluded that: (1) Klebsiella are ubiquitous in the pulp and paper mill industry processing stages; (2) the standard procedure for fecal coliform estimation is useless to assess the microbiological quality of the effluents of these industries; (3) The assay of E. coli should replace the fecal coliform detection procedure.
Gauthier et al. [ 110 ] and Gauthier and Archibald [ 58 ] reported two studies of seven pulp and paper mills in Ontario and Quebec, Canada. Total and fecal coliforms and enterococci were detected in nearly all the biotreatment, biosolids (sludges), and in-mill water system samples. In the mill samples, the majority of the fecal coliforms (detected by the standard test procedure) were K. pneumoniae , Raoultella terrigena and Raoultella planticola , with E. coli in minority. E. faecalis and E. faecium were detected in relatively large numbers in most samples from all of the seven mills examined. Other coliforms such as Enterobacter spp. and Citrobacter freundii were occasionally recovered from total and fecal coliform tubes. Biofilms established in the piping, tanks, and machinery where thermal and pH conditions permit were the most likely source of these bacteria. Analyses using two independent Salmonella detection/enumeration methods showed no detectable Salmonella cells in the sludges and final effluents of the five mills tested. It was concluded that for these particular systems, the determination of total and fecal coliforms and enterococci is useless and have no relationship with real fecal pollution. These studies also demonstrated the importance of checking the identities of bacteria causing the positive results in the tests. Both Escherichia and Klebsiella can give positive results in the fecal coliform test, but their ecological meaning is opposite.
Another important case of failure of the use of coliforms to detect fecal pollution was the 1993 Cryptosporidium outbreak in Milwaukee (USA).
Cryptosporidium parvum , a protozoan parasite that causes gastrointestinal illness, is transmitted by ingestion of oocysts excreted in human or animal feces. Typical modes of transmission include person to person, animal to person, by exposure to contaminated food or water [ 111 , 112 ].
From 1990 to 2000, at least 10 cryptosporidiosis outbreaks associated with contaminated drinking water were reported in the USA. In 1993, an estimated 403,000 residents of the greater Milwaukee area (Wisconsin, population, ca. 1.61 million) became ill when an ineffective filtration process led to the inadequate removal of Cryptosporidium oocysts in one of two municipal water-treatment plants [ 111 , 112 ].
It was the largest waterborne disease outbreak in documented USA history. Over the span of approximately two weeks, people became ill with stomach cramps, fever, diarrhea and dehydration caused by the pathogen. More than half the people who received residential drinking water from the southern water-treatment plant became ill, which was twice the rate of illness among people whose residential drinking water came mainly from the northern water-treatment plant. Over 54 deaths were attributed to this outbreak, mostly among the elderly and immunocompromised people, such as AIDS patients [ 111 , 112 ].
Standard microbiological water analysis was ineffective in detecting this parasite. Indeed, throughout the period from February to April, samples of treated water from both plants were negative for coliforms. The origin of the contamination was determined as water from Lake Michigan. No specific source of the Cryptosporidium was ever identified but runoff from abnormally heavy spring rains most likely carried the parasite to the lake [ 111 , 112 ].
7.5.5. Clostridium perfringens
Sulphite-reducing clostridia, namely Clostridium perfringens , are spore-forming Gram-positive, non-motile, anaerobic, sulfite-reducing rods. C. perfringens is present in higher numbers in the feces of some animals, such as dogs, than in the feces of humans and less often in the feces of many other warm-blooded animals. The numbers excreted in feces are normally substantially lower than those of E. coli.
Clostridium spores are exceptionally resistant to unfavorable conditions in water environments, including UV irradiation, temperature and pH extremes, and disinfection processes, such as chlorination. Although clostridia probably do not growth in surface waters, the high resistance of their spores makes their presence ubiquitous in environmental waters [ 1 , 4 , 84 , 113 ].
The presence of chlorine in water rapidly inactivates indicator bacteria such as E. coli and coliforms, but it leaves the most resistant pathogens almost unaffected for several hours. This creates a false sense of security by providing negative coliform and negative E. coli results to authorities responsible for water testing. Giardia cysts, Crystosporidium oocysts, and human enteric viruses all have higher resistance to disinfectants and constitute a major public health risk if distribution system integrity is breached. C. perfringens spores are less affected by the residual concentrations of chlorine. Testing for the spores of this bacterium can probably provide an added margin of safety in the evaluation of treatment [ 114 ].
7.5.6. Correlations between parameters used to assess fecal pollution
In environmental waters, several studies have reported significant correlations between indicators of fecal pollution and between indicators and pathogenic gastrointestinal bacteria.
Charriere et al. [ 115 ] reported a study of deep aquifer waters (raw waters and piped chlorinated waters) in Normandy, France. In heavily contaminated raw waters and in slightly contaminated treated waters, fecal coliforms and enterococci were correlated.
Martins et al. [ 116 ] reported a study of 60 public outdoor swimming pools in Sao Paulo city, Brazil. Total coliforms, fecal coliforms and fecal streptococci levels increased with number of bathers and water temperature, and decreased with chlorine levels. All these indicators were significantly correlated with each other.
Ferguson et al. [ 117 ] reported a study carried out in Georges River, in the Sydney region, Australia. In the water column, concentrations of fecal coliforms, fecal streptococci, C. perfringens spores were all positively correlated with each other. Isolation of Salmonella spp. were most frequent during rainfall and sewage overflow events. In the water column, 55% of samples contained Salmonella when fecal coliform densities exceeded 2,000 CFU/100 mL.
Medema et al. [ 118 ] reported a study of seven different fresh water sites normally used for triathlon competitions. Sites were small rivers, channels, lakes and harbors, and were influenced by sewage effluents and agricultural run-off. When data from all triathlons were pooled, geometric mean densities of fecal coliforms and E. coli , of E. coli and fecal enterococci and of fecal coliforms and fecal enterococci, were significantly correlated.
Polo et al. [ 119 ] reported a study of water samples obtained from 213 beaches, eight rivers and 14 freshwaters in north-eastern Spain. In freshwaters and heavily contaminated seawaters, Salmonella and fecal coliforms were correlated, while in less contaminated seawaters, the highest correlation was with between Salmonella and fecal streptococci.
Byamukama et al. [ 99 ] reported a study of the microbiology of Nakivubo channel, Uganda. This channel receives raw sewage from slums, industrial effluents, and discharges from a sewage treatment plant and from a complex of slaughterhouses. Water from eight sampling sites was assessed for the presence of total and fecal coliforms, E. coli and sulphite-reducing clostridia. All microbiological parameters were significantly correlated.
Noble et al. [ 120 ] reported a comparative determination of total coliforms, fecal coliforms and enterococci in 108 sites along the southern California coastline, USA. Results by traditional and enzymatic methods and from all three parameters were correlated.
Harwood et al. [ 121 ] reported a study on indicator and pathogenic microorganisms carried out in six wastewater reclamation facilities in the USA. Data from disinfected effluent (reclaimed water) samples were analyzed separately (by facility) and as a pooled data set (all facilities). Significant correlations between indicator organism concentrations were observed in the pooled data sets, namely for total and fecal coliforms.
Cabral and Marques [ 85 ] reported a microbiological study of a polluted river (Febros) in the Great Oporto area, northwest Portugal. Total and fecal coliforms, fecal streptococci and enterococci were all significantly correlated with each other.
Touron et al. [ 122 ] reported a study carried out in the Seine estuary, France. Water was sampled at nine stations (along an upstream/downstream transect of 156 km), during nine years, for fecal coliforms, E. coli , enterococci and Clostridium perfringens spores. At the upstream part of the estuary (at Poses), Salmonella and fecal coliforms, and E. coli and enterococci counts were correlated. At the mouth of the estuary (at Honfleur), significant correlation was found for Salmonella and enterococci counts. No significant correlation between concentrations of any combination of indicator organism and pathogen was observed.
Wilkes et al. [ 59 ] reported a comparative study on the presence and concentration of several pathogenic and indicator bacteria in the surface water of a Canadian river. Surface water was collected within the South Nation River basin in eastern Ontario, from the river proper, and from several lower stream order tributaries. Using data aggregated during the entire multi-year study, significant correlations were found among all indicator bacteria - total and fecal coliforms, E. coli , Enterococcus , and C. perfringens .
However, in others studies no correlation was found between the different fecal indicator bacteria. Garrido-Pérez et al. [ 123 ] reported a study of the bathing seawater quality in 18 Spanish beaches near the Strait of Gibraltar. Sample locations were selected as a single point located in the area of highest bather density of each beach. No significant correlation was found between fecal coliforms and Clostridium perfringens counts in the bathing seawater.
8. Fecal Indicator Chemical Compounds
Several chemical substances have been used as markers of fecal pollution in environmental waters. Caffeine is present in several beverages and in many pharmaceutical products. It is excreted in the urine of individuals who have ingested the substance. The main source of caffeine in domestic wastewaters is excretion following consumption of coffee, tea, soft drinks, or medication. Levels of caffeine in domestic wastewater have been measured to be between 20 and 300 μg/L. Levels in receiving waters are much lower due to significant dilution. Due to its high solubility, low octanol-water partition coefficient, insignificant volatility and clear anthropogenic origin, the presence of caffeine in environmental waters can be a good marker for human fecal pollution [ 124 – 129 ].
However, relationships between fecal indicator bacteria and caffeine are variable. Wu et al. [ 129 ] reported a study carried out in the Rochor Canal and Marina Bay, Singapore. In Rochor Canal, the highest concentration of caffeine (1.35 ng/mL) was found at downstream, and the lowest (0.68 and 0.37 ng/mL) were determined at middle and upstream points. At Marina Bay, the concentration of caffeine was in the range of 0.41–0.96 ng/mL. Fecal coliform concentrations were very high, exceeding 5,000 CFU/100 mL. Caffeine and fecal coliform concentrations were significantly correlated in Rochor Canal samples, but no significant correlation was observed in Martina Bay water samples.
The use of caffeine as marker of fecal pollution has additional important limitations. Caffeine is often present in the urban environment from numerous plant species debris as well as from human “dumping” of coffee wastes. In addition, the current analytical methods used are relatively complex and expensive [ 124 ].
Coprostanol (5β-cholestan-3β-ol) is a fecal stanol that is formed by indigenous bacteria present in the gut of humans and higher animals, during catabolism of cholesterol. It is the main stanol present in human feces (24 to 89% of total steroids) and in domestic wastewater. Based on these facts, it has been proposed as a chemical indicator of human fecal pollution. Feces from pigs and cats also contain coprostanol, but at much lower levels. Additional fecal stanols, such as 24-ethylcoprostanol, were found to be predominant in herbivores, such as cows, horses, and sheep, suggesting potential use of this chemical as an indicator of fecal pollution from these sources. Reported half-lives of coprostanol in aerobic conditions are generally lower than 10 days at 20 °C. Thus, the presence of coprostanol in an aerobic environment can be considered an indication of recent fecal input to the waters. [ 124 , 128 , 130 ].
Isobe et al. [ 130 ] reported a study carried out in the Mekong Delta (Vietnam) and in the Tokyo metropolitan area. During the wet season in the Mekong Delta, higher bacterial densities were observed in rivers, probably due to the higher bacterial inputs from soil particles with runoff. In Tokyo, higher bacterial densities were usually observed during summer, followed by those in the typhoon aftermath and winter. Significant correlations between the concentrations of E. coli and coprostanol (log scale) were found in all surveys. It was concluded that the determination of coprostanol can improve standard microbiological assays of fecal pollution.
Reports from several regions throughout the world indicate variable quantitative relationships between fecal coliform densities and coprostanol concentrations. In the Derwent Estuary and Sydney region (Australia), coprostanol concentration of 400 ng/L corresponded to 1,000 CFU of fecal coliforms/100 mL. In the Mekong Delta, during the wet season, this coliform density corresponded to 30 ng coprostanol/L, and in the dry seasons, 100 ng/L. In Tokyo metropolitan area, these values were 30 ng coprostanol/L, in summer, and 100 ng coprostanol/L, in a typhoon aftermath. These differences were interpreted as a result of differences in water temperature and soil particle concentration [ 130 ].
Fecal sterol analysis, although expensive and complex, has resolved problems of source attribution in urban and rural environments not possible with use of traditional fecal indicator bacteria [ 124 ]. These chemical indicators are especially useful in environments which allow survival and growth of fecal bacteria. For instance, in tropical regions, characterized by high temperatures and frequent rainstorms that facilitate erosion of soils, fecal bacteria can proliferate in environmental waters reaching densities that are not representative of real sewage inputs in the environment [ 130 ].
9. Sources of Fecal Bacterial Pollution of Environmental Waters
9.1. sources of surface and groundwater contamination.
Determinations carried out in the sewage systems of urbanized areas have confirmed the presence of high numbers of intestinal bacteria. Treatment of sewage reduces the concentration of these bacteria by 1–2 logs, but effluent still contains high levels of intestinal bacteria ( Table 8 ). Effluents from sewage treatment plants can be a source of contamination of surface waters with fecal bacteria.
Typical concentrations of selected bacteria in raw and treated domestic wastewater a .
Septic tanks, cesspools, latrines and other on-site systems are widely used for wastewater storage and treatment. The water percolating from these facilities contains bacteria that may contaminate groundwater supplies.
Many farmers use cellars, tanks or landfills to store manure. Water leaching from these storage sites may also contaminate groundwater, especially during periods of rainfall. The application of animal manure to agricultural lands as fertilizer is common practice throughout the world. Bacteria present in the manure may leach into the groundwater.
The potential for bacteria present in human and animal wastes to contaminate water in nearby wells needs special attention [ 131 ]. An important source of contamination of surface and ground waters is runoff water from agricultural and pasture lands, and urban areas.
Fecal bacteria enter surface water by direct deposit of feces and by overland runoff. The movement of animal wastes into surface waters can be a major factor contributing to the pollution of available water in many regions. Over one-third of the land area of USA is used for grazing livestock and receives 50% of all livestock wastes.
In a study reported by Doran and Linn [ 108 ], runoff from a cow-calf pasture in eastern Nebraska was monitored during a three-year period. Rainfall runoff from the grazed area contained 5 to 10 times more fecal coliforms than runoff from the fenced, ungrazed area. However, fecal streptococci counts were higher in runoff from the ungrazed area and reflected the contributions from wildlife.
Urban and suburban areas are dominated by impervious cover. During storms, rainwater flows across these impervious surfaces, mobilizing contaminants. The pollutants carried in runoff originate from a variety of urban and suburban nonpoint sources. Contaminants commonly found in stormwater runoff include fecal and pathogenic bacteria. Stormwater transports pollutants to water bodies such as lakes and streams [ 132 ].
Enterococci and E. coli can be found in high numbers in most storm drains and creeks. In Southern California (USA), Ferguson et al. [ 133 ] found high levels of enterococci ( Enterococcus faecalis , Enterococcus faecium , Enterococcus hirae , Enterococcus casseliflavus and Enterococcus mundtii ) in intertidal sediments in a seasonal river, and near a storm drain outlet.
9.2. Survival in Surface Water
Most intestinal bacteria that contaminate environmental waters are not able to survive and multiply in this environment. Survival rates vary widely among fecal bacteria introduced in environmental waters. Pathogenic enteric bacteria and E. coli display low survival rates ( Table 9 ).
Reduction times for fecal bacteria in surface waters a .
The ability of fecal bacteria to survive in environmental waters generally increases as the temperature decreases. Others factors that influence survival include dissolved organic carbon concentration, sunlight intensity and the ability to enter the viable but non-culturable state [ 131 ].
In a comparative study on the survival of 10 different coliform species ( E. coli , Citrobacter freundii , Citrobacter youngae , Klebsiella pneumoniae , K. oxytoca , Enterobacter amnigenus , Enterobacter cloacae subsp. cloacae , and Pantoea agglomerans ( Enterobacter agglomerans )) inoculated in sterilized river water with different concentrations of dissolved organic carbon, Boualam et al. [ 134 ] found that only C. freundii , K. pneumoniae and E. cloacae subsp. cloacae remained cultivable after 96 hours of incubation. In a posterior study, using the same bacteria and medium, Boualam et al. [ 135 ] found that after 28 days, only C. freundii and E. cloacae subsp. cloacae survived.
Baudišová [ 136 ] reported a comparative study on the survival of total coliforms, fecal coliforms and E. coli , in sterile and non-sterile river water. In sterile water, all bacteria survived for many months. However, in non-sterile conditions (closer to true environmental conditions), the elimination rate of all bacteria was considerably faster. Total coliforms survived the longest and E. coli the shortest.
9.3. Survival in Groundwater
Survival of bacteria in groundwater is influenced by several factors, namely the survival in soil, since in order to reach the groundwater bacteria have to percolate through the soil. Generally, survival in soil (and concomitantly in groundwater) is enhanced by low temperatures, high soil humidity, neutral or alkaline soil pH and the presence of organic carbon [ 131 ].
10. Which Indicators of Fecal Pollution Should be Used?
Several fecal indicator bacteria in environmental waters are in current use. From these stand out fecal coliforms, E. coli and enterococci [ 1 , 6 , 137 ]. In environmental waters, most fecal coliform strains are E. coli .
In particular situations, the presence of E. coli is definitively not associated with fecal pollution. These situations were firstly detected in some African countries, namely Nigeria, Ivory Coast and New Guinea (although not in others, such as Uganda) [ 42 , 99 ].
Recent studies carried out in temperate zones indicated that E. coli can persist in secondary, nonhost habitats, outside the hot tropical areas, and become naturalized in these habitats. Byappanahalli et al. [ 138 ] reported that E. coli could be isolated from coastal temperate forest soils in Indiana (USA). The aquatic alga Cladophora glomerata (L.) from several Lake Michigan beaches was shown to harbor high densities of E. coli [ 139 ].
Ishii et al. [ 140 ] reported a study on the survival of E. coli in temperate riverine soils of northern Minnesota (USA). Viable E. coli populations were repeatedly isolated from northern temperate soils in three Lake Superior watersheds. Seasonal variation in the population density of soilborne E. coli was observed; the greatest cell densities were found in the summer to fall, and the lowest numbers, occurred during the winter to spring months. Horizontal, fluorophore-enhanced repetitive extragenic palindromic PCR (HFERP) DNA fingerprint analyses indicated that identical soilborne E. coli genotypes, overwintered in frozen soil and were present over time, and that these strains were different from E. coli strains obtained from wildlife commonly found in the studied habitats or river water. Soilborne E. coli strains had HFERP DNA fingerprints that were unique to specific soils and locations. In laboratory studies, naturalized E. coli strains had the ability to grow and replicate to high cell densities, in nonsterile soils when incubated at 30 or 37 °C and survived longer than 1 month when soil temperatures were lower than 25 °C. It was concluded that these E. coli strains became naturalized, autochthonous members of the soil microbial community.
In a latter paper, Ksoll et al. [ 141 ] studied epilithic periphyton communities at three sites on the Minnesota shoreline of Lake Superior (USA). Fecal coliform densities increased up to 4 orders of magnitude in early summer, and decreased during autumn. HFERP DNA fingerprint analyses indicated that waterfowl (geese, terns, and gulls) were the major primary source of periphyton E. coli strains that could be identified. Periphyton and sewage effluent were also major potential sources. Several periphyton E. coli isolates were genotypically identical, repeatedly isolated over time. Inoculated E. coli rapidly colonized natural periphyton in laboratory microcosms and persisted for several weeks, and some cells were released to the overlying water. It was concluded that E. coli had became a naturalized member of the bacterial periphyton communities.
The presence, persistence, and possible naturalization of E. coli in these habitats can confound the use of fecal coliforms as a reliable indicator of recent fecal contamination of environmental waters. Future studies should consider other nonhost habitats as potential sources of fecal coliform bacteria in aquatic environments.
Considering these limitations, it appears to be advisable, in order to check the microbiological quality of drinking water, to complement the determination of Escherichia coli with the assay of enterococci. This rationale that has been followed, for many years, in the making of the drinking-water legislation in the European Union.
However, for many developing countries, where limited financial resources are the norm and reality, the routine determination of these two parameters can be difficult to implement. In these circumstances, it appears common sense that is better to determine a (good) parameter, such as Escherichia coli , than have no analysis done.
In this context, USA legislation emerges as a pragmatic approach to the problem. According to the American legislation, total coliforms are the routine parameter to be determined. Only when these determinations are repeatedly positive, it is mandatory to assess fecal coliforms [ 142 , 143 ]. Although total coliforms are not necessarily fecal bacteria, the rationale behind this system is correct, since: (1) a positive test in fecal coliforms (which is our target) is necessarily positive in the total coliform procedure; (2) the inverse is not necessarily true; (3) total coliforms are easily and cheaply assayed in waters.
As an alternative to the determination of both E. coli and enterococci, the assay of ammonia in environmental waters can be useful and complement the determination of fecal coliforms.
Ammonia is one of the key molecules in the nitrogen cycle. The presence of ammonia in surface waters can be due to direct contamination by agricultural fertilizers, and/or to microbial degradation of proteins, nucleic acids and urea, implying therefore the presence of a considerable concentration of organic matter in the water. Ammonia is rapidly oxidized in the environment and is typically found in natural waters at concentrations less than 0.1 mg/L. Concentrations significantly above this indicate gross contamination by fresh sanitary waste, where ammonia levels are typically very high (tens or hundreds of mg/L) [ 84 ].
Espigares et al. [ 144 ] reported a comparative study of chemical and microbiological indicators (total and fecal coliforms, fecal streptococci and sulphite-reducing clostridia) in a stretch of the Guadalquivir River (Spain) and its affluents. Total coliforms were correlated with fecal coliforms, but were not correlated with fecal streptococci and clostridia. Fecal coliforms were correlated with the other indicators. Fecal streptococci and sulphite-reducing clostridia were correlated with the other indicators except for total coliforms. All these microbiological indicators were correlated with dissolved oxygen (negatively), dissolved organic carbon and ammonia (positively). Cabral and Marques [ 85 ] in a study of a polluted river (Febros) in the Great Oporto area (northwest Portugal) found that ammonia was significantly correlated with all the microbiological assayed parameters—total and fecal coliforms, fecal streptococci and enterococci. These correlations are most probably due to the carry-over of organic matter in wastewaters, and to a high microbial ammonification activity [ 85 ].
Simple and rapid in-field tests and automated and continuous systems are available for the assay of ammonia in environmental waters. More studies are needed in order to confirm the use of ammonia as a reliable parameter in a preliminary screening for emergency fecal pollution outbreaks.
11. Conclusions
- Safe drinking water for all is one of the major challenges of the 21st century.
- Microbiological control of drinking water should be the norm everywhere.
- Routine basic microbiological analysis of drinking water should be carried out by assaying the presence of Escherichia coli by the culture methods. On-line monitoring of glucuronidase activity is currently too insensitive to replace culture based detection of E. coli but is a valuable complementary tool for high temporal resolution monitoring. Whenever financial resources are available, coliform determinations should be complemented with the quantification of enterococci.
- More studies are needed in order to check if ammonia is reliable for a preliminary screening for emergency fecal pollution outbreaks.
- Financial resources should be devoted to a better understanding of the ecology and behavior of human and animal fecal bacteria in environmental waters.
Current taxonomy and nomenclature of the genus Shigella . Habitat and pathogenicity of species a .
Disappearance rates of fecal bacteria in groundwaters a .
ORIGINAL RESEARCH article
The influence of water safety knowledge on adolescents’ drowning risk behaviors: a framework of risk-protect integrated and kap theory.

- 1 School of Physical Education, Southwest University, Chongqing, China
- 2 School of Physical Education, Hubei Minzu University, Enshi, Hubei, China
- 3 School of Physical Education, Huzhou University, Huzhou, China
Introduction: Although previous research has examined the risk factors for drowning behavior among adolescents, it is unclear whether this association is influenced by water safety knowledge. This study aimed to examine whether water safety knowledge is associated with adolescents’ drowning risk behaviors and whether drowning risk perceptions and attitudes could have a chain mediating role in the association between water safety knowledge and adolescents’ drowning risk behaviors.
Methods: This study included 7,485 adolescents from five Chinese provinces and cities. We used the Drowning Risk Behaviors Scales (DRBS) to evaluate the risk of drowning behaviors. The Water Safety Knowledge Scale (WSKS) was used to evaluate the competence level of water safety knowledge. The Drowning Risk Perceptions Scale (DRPS) was used to evaluate the risk level of perceptions, and the Drowning Risk Attitudes Scale (DRAS) was used to evaluate the risk level of attitudes.
Results: The results of the mediating effect test showed that water safety knowledge (WSK) affected drowning risk behaviors (DRB) through three indirect paths. Drowning risk perceptions (DRP) and attitudes (DRA) have significantly mediated the association between WSK and DRB. In conclusion, DRP and DRA can act as mediators between WSK and DRB, not only individually, but also as chain mediators, where the direct effect is-0.301, the total indirect effect is-0.214, and the total mediated indirect effect is 41.5%.
Discussion: Water safety knowledge negatively predicts adolescents’ drowning risk behaviors; water safety knowledge has an inhibitory effect on drowning risk perceptions. Water safety knowledge can directly influence adolescents’ drowning risk perceptions and indirectly affect drowning risk behaviors through the mediation of drowning risk perceptions and attitudes comprising three paths: (1) the drowning risk perceptions mediation path, (2) the drowning risk attitudes mediation path, and (3) the drowning risk perceptions and attitudes mediation paths.
1 Introduction
Drowning claimed more than 2.5 million lives worldwide from 2010 to 2019. According to the WHO’s most recent global health estimates, 236,000 people drowned in 2019, demonstrating that drowning deaths exceeded deaths from either protein-energy malnutrition or maternal conditions ( 1 ). Approximately 57, 000 people die from drowning annually in China, accounting for 20% of all drowning deaths worldwide. 56% of these deaths occur in children aged 5–14, in other words, 88 children die from drowning each day ( 2 , 3 ). Therefore, it is crucial to understand how we can effectively prevent drowning among adolescents.
Drowning accidents of children and adolescents aged 5–14 are more likely to occur in open waters, such as rivers, lakes, ponds, reservoirs, and seas, owing to the hot summers resulting from global warming. Previous studies have found that the main causes of drowning accidents are drowning risk behaviors (DRB) and unintentional falls into water. Drowning risk behaviors refer to individuals putting themselves in risky circumstances on their own initiative, and unintentionally falling into open water refers to individuals’ risk ignorance ( 4 ). However, swimming skills and water safety knowledge (WSK) are key factors in these two causes. Mastering swimming skills and water safety knowledge is greatly significant to water safety education. Adolescents who acquire swimming skills may be able to overcome drowning, but without water safety knowledge, they still face the threat of drowning ( 3 ). Mastery of water safety knowledge not only changes adolescents’ drowning risk attitudes (DRA) and behaviors, but also strengthens their drowning risk perceptions (DRP). However, few studies have examined the mechanisms underlying water safety knowledge, drowning risk perceptions, drowning risk attitudes, and drowning risk behaviors.
Adolescents’ drowning risks are directly related to their drowning risk behaviors ( 5 ). A previous study found that individuals with a higher drowning risk tended to drown more than those with a low risk of drowning ( 6 ). Various types of water activities exist in China, including diving, snorkeling, water skiing, surfboard skiing, planking, catching fish, splashing, and swimming. Drowning accidents during these activities among adolescents are increasingly common because of the lack of guardian supervision and the induction of deviant behaviors from partners.
Mastering water safety knowledge helps adolescents reduce their drowning risk behaviors. Water safety knowledge consists of declarative knowledge about swimming safety and procedural knowledge about swimming rescue, including common sense of water safety, water safety legislative knowledge, and water safety judgments ( 5 ). (1) Common sense of water safety refers to a fundamental understanding of water activities, rescues, equipment, and the production of simple floating devices. (2) Water safety legislative knowledge refers to awareness of the laws and regulations of the water activity area, warning signs, beach flags, etc. (3) Water safety judgments refer to the ability to assess one’s own physical condition, weather, and water environment ( 4 ). In 2007, The International Lifesaving Federation stated that most drowning episodes can be prevented by understanding water safety and swimming skills ( 5 ). In general, a high level of water safety knowledge helps adolescents efficiently identify potential drowning risks. For example, adolescents would have better knowledge of various warning signs, could assess their own health, weather, and water conditions more effectively, and would use life-saving knowledge and skills to approach drowning events more reasonably. Moreover, water safety awareness and behaviors underpinned by water safety knowledge help avoid adolescents’ unintentional falls into open water, unintentional submersions, and drowning ( 4 ). Additionally, empirical studies have demonstrated that water safety knowledge strongly promotes pandemic prevention behaviors and health-related adaptive behaviors ( 7 , 8 ), and prevents risky diving behaviors and unintentional injuries ( 9 , 10 ). Based on this previous literature, we hypothesized that water safety knowledge may reduce the occurrence of drowning risk behaviors in adolescents.
Hypothesis 1 : Water safety knowledge has an inhibition effect on drowning risk behavior.
In addition to directly influencing adolescents’ drowning risk behaviors, water safety knowledge can also indirectly prevent drownings by enhancing risk perceptions. Empirical research has demonstrated that the combination of water safety knowledge and risk perceptions has a substantial impact on drowning risk behaviors ( 8 ). Risk perceptions are used to describe people’s attitudes and intuitive judgments towards risk and play a crucial role in human safety behaviors ( 11 ). Altarawneha et al. ( 12 ) first applied a dual-process approach to the study of risk perception, arguing that public risk perception studies should combine cognitive and affective appraisals to form a dynamic “dual-process” ( Figure 1 ). Risk perceptions are significantly related to the level of subjective knowledge (risk awareness) and the degree of personal familiarity with water environments; thus, they can drive individuals’ behavioral decisions ( 12 ). Furthermore, the dual-process approach model also supports Finucane’s point that “understanding how affect and cognition interact and collaborate in human judgment and decision-making is crucial for understanding risk perceptions” ( 13 ). Adolescents’ understanding of water environments’ risks originate from their water safety knowledge. For example, recognizing water safety signs and beach safety flags may warn adolescents to inhibit some drowning risk behaviors ( 4 ). Moreover, water safety knowledge enhances adolescents’ drowning risk perceptions, alerts them to drowning risks, and encourages persuasion and precautions against drowning risk behaviors. However, it is unclear whether water safety knowledge inhibits drowning risk behaviors through drowning risk perceptions. Accordingly, we predicted that drowning risk perceptions would have an inhibition role in the relationship between water safety knowledge and drowning risk behaviors ( 14 , 15 ).
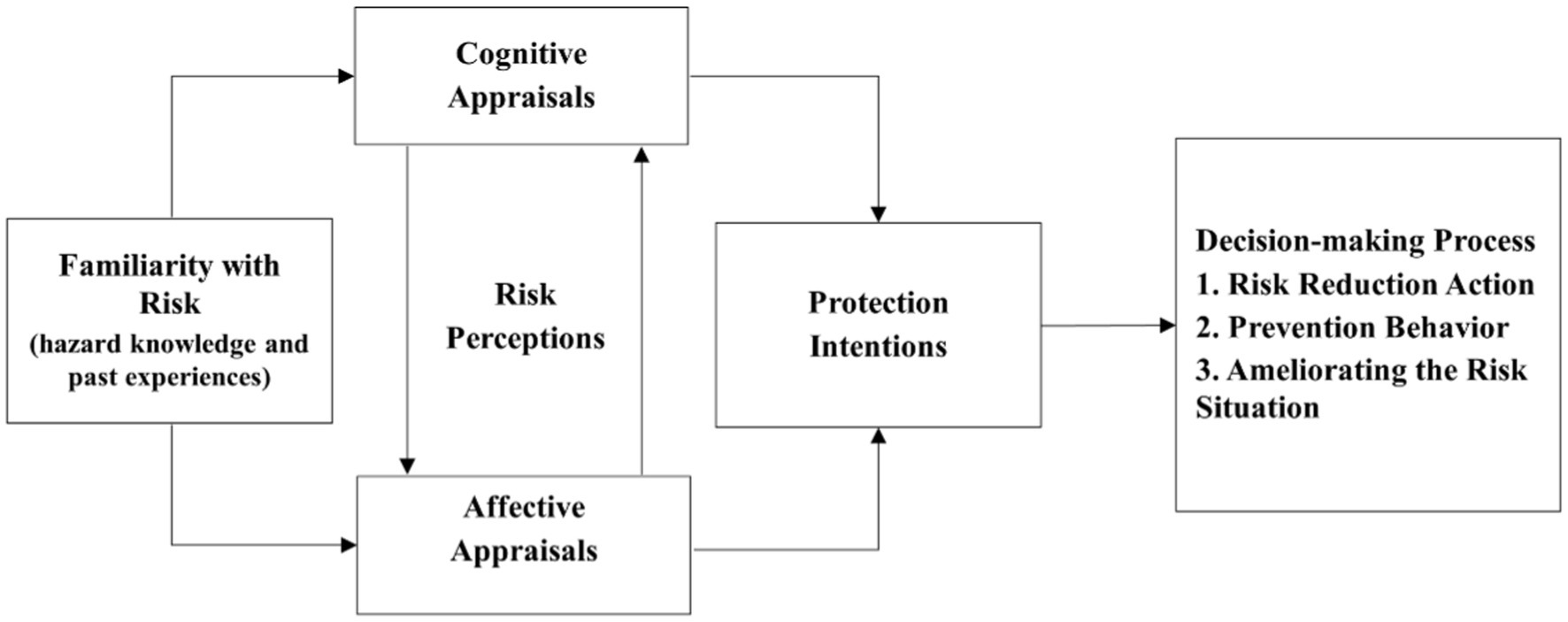
Figure 1 . Conceptual illustration of the cognition-affect-intention (CAI) model.
Hypothesis 2 : Drowning risk perceptions have an inhibition role in the relationship between water safety knowledge and drowning risk behaviors.
The Knowledge, Attitude, and Practice (KAP) theory commonly utilized to study people’s health behavior divides human behavior change into three sequential processes: (1) acquiring knowledge, (2) forming beliefs and positive attitudes, and (3) eventually changing behaviors. In this process, knowledge is the basis for behavioral change, while beliefs and attitudes are the forces behind behavior change ( 16 ). Moreover, the KAP model is one of the most effective methods for drowning prevention because it considers people’s existing knowledge, beliefs, local environments, and social norms to understand why they engage in risky drowning behaviors ( 4 ). However, a study of young surfers in New Zealand found that surfing safety knowledge and attitudes had a significant impact on their high-risk surfing behaviors, which contributed to their high drowning probability ( 17 ). Many studies have demonstrated that the application of KAP theory to mediation models is a reliable predictor of drowning risk for all types of water activities, and that attitudes are important as a mediating variable between knowledge and behavior ( 15 , 18 , 19 ). Therefore, we posit that drowning risk attitudes can work as a mediator in the relationship between water safety knowledge and drowning risk behaviors.
Hypothesis 3 : Drowning risk attitudes will mediate the relationship between water safety knowledge and drowning risk behaviors.
The Knowledge, Risk perception, Attitude, and Practice (KRPAP) theoretical model was created by adding risk perception factors based on the KAP theory. It is conceivable to investigate the potential chain mediation of risk perceptions and attitudes on drowning risk behavior using the KRPAP theory, which holds that people’s behavior undergoes a knowledge-risk perceptions-attitudes-behavior change process. Taking the COVID-19 pandemic as an example, factors such as the administration’s inability to release pertinent information adequately and in a timely manner, the public not being well informed about COVID-19, and information asymmetry all might result in erroneous risk perceptions. This impact has the potential to transform people’s attitudes and behaviors over time, leading to verbal and behavioral radicalization among the public and contributing to societal instability ( 20 ). Compared with the KAP model, the KRPAP model has been used less frequently in previous drowning prevention research. Based on the above analysis, we hypothesized that drowning risk perceptions and attitudes would have a chain mediating effect between water safety knowledge and drowning risk behaviors ( Figure 2 ).
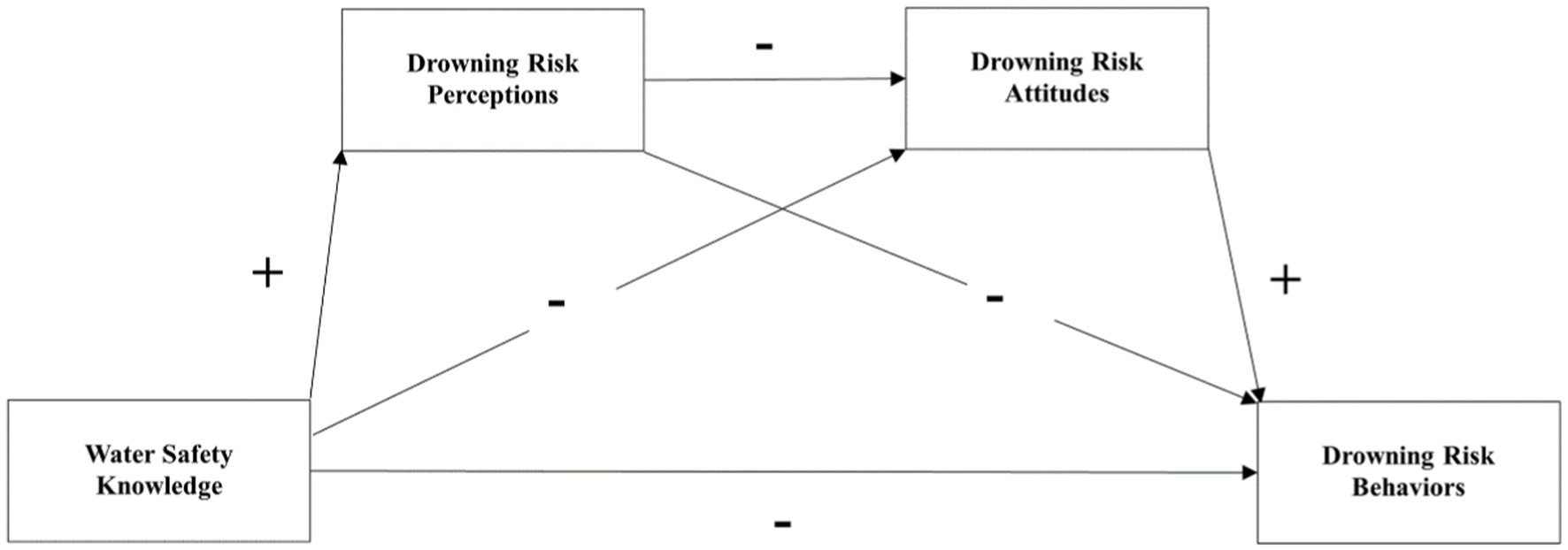
Figure 2 . Theoretical model.
Hypothesis 4 : Drowning risk perceptions and attitudes have a chain-mediating effect on water safety knowledge and drowning risk behaviors.
2 Materials and methods
2.1 participants and procedure.
We used cross-sectional data from primary and secondary school students in China, and conducted pre-test training for local teachers and students to normalize the investigation process. Before completing the formal questionnaire, all primary and secondary school students agreed to participate, and written informed consent forms were obtained from their primary guardians. We distributed and obtained 8,000 questionnaires, of which 7,485 (93.6%) were valid. Finally, this study included 3,663 males (48.9%) and 3,822 females (51.1%). Ethical approval was obtained from the Institutional Review Board of the College of Physical Education at Southwest University (ethical approval number: SWU20180601).
2.2 Measures
2.2.1 drowning risk behaviors scale.
The Drowning Risk Behaviors Scale (DRBS) was used to evaluate an individual’s risk level for drowning behavior ( 15 ). The Chinese version of the DRBS is considered to have good reproducibility and validity ( 21 ). The drowning risk behaviors scale consisted of 10 items (e.g., “Swimming with no adults”; “Swimming in the wild waters without safety protection”; “Diving in the unknown depth of water”; “Playing roughshod with your mates while swimming”; “Swimming when you are sick”) rated on a five-point Likert scale ranging from 1 (Never) to 5 (Always). All items were added to produce a total score, with higher scores indicating a more severe drowning risk. The internal consistency coefficient (Cronbach’s alpha) was 0.934.
2.2.2 Drowning risk perceptions scale
The Drowning Risk Perceptions Scale (DRPS) was used to evaluate an individual’s level of drowning risk perceptions ( 22 ). The reliability and validity of the Chinese version of the DRPS have been previously described ( 6 ). DRPS consists of 13 items and four subscales, including F1 = Susceptibility to drowning (e.g., “Drowning is a leading cause of death among children”; “Every student is at the risk of drowning”; “You and your partners are at the risk of drowning”), F2 = Seriousness of drowning (e.g., “Drowning is a serious problem”; “Drowning students must be taken to hospital”; “Most drowning victims would die”). F3 = Benefits of swimming skills (e.g., “Swimming is a life skill”; “Swimming skills will decrease drowning risk”; “Swimming skills are necessary for a particular professional career”), and F4 = Barriers perceived (e.g., “Lack of swimming instructors”; “Lack of swimming lessons in school”; “Unaffordable swimming lessons”; “Swimming pool is too far”). Each item was rated on a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). All items were summed to produce a total score, with higher scores indicating a higher drowning risk perception. In this study, the internal consistency reliability (Cronbach’s alpha) of the overall scale was 0.839, and the internal consistency coefficients (Cronbach’s alpha) of the four-dimensional scales were 0.703, 0.788, 0.746, and 0.720. From the degree of fit of the four-factor model, CFI, IFI, NNFI, and GFI >0.90, and RMSEA <0.08, indicating that the four-factor structural model fits well.
2.2.3 Water safety knowledge scale
We used the Water Safety Knowledge Scale (WSKS) to evaluate an individual’s level of water safety knowledge ( 23 ). The Chinese version of the WSKS has good reproducibility and validity ( 6 ). The water safety knowledge scale consisted of 10 items: (1) “Do you know about water safety?”; (2) “Do you know the common methods for rescuing those who fall into water?”; (3) “Do you know how to perform self-rescue in water?”; (4) “Do you know how to perform CPR?”; (5) “Can you recognize common water safety signs?”; (6) “Do you know how to react when someone else falls into the water?”; (7) “Do you know the correct use of life jackets and life preservers?”; (8) “Do you know the most effective ways to call for help while drowning?”; (9) “Do you know how to rest when fatigued by swimming?”; and (10) “Do you know how to swim safely?.” These were rated on a five-point Likert-type scale ranging from 1 (very unfamiliar) to 5 (very familiar). All items were summed to produce a total score, with higher scores indicating a higher level of education. The internal consistency coefficient (Cronbach’s alpha) was 0.943.
2.2.4 Drowning risk attitudes scale
The Drowning Risk Attitudes Scale (DRAS) was used to evaluate an individual’s drowning risk attitudes. The Chinese version of the DRA has good reproducibility and validity ( 23 ). The DRAS consists of 10 items: (1) “Before swimming, is there no need to consider whether the waters are safe?”; (2) “Are good swimmers always safe from drowning?”; (3) “Is swimming in rivers safe?”; (4) “Is it best to enter water immediately to help your partner draw?”; (5) “Does swimming in shallow water guarantee that you will not drown?”; (6) “Is it safe to swim with a partner who can swim, instead of an adult?”; (7) “Is it always safe to go swimming with a lifejacket?”; (8) “Is it safe to play near the edge of water without entering it?”; (9) “Is walking on ice safe?”; and (10) “Is it safe to swim while wearing clothing?”. These were rated on a five-point Likert-type scale ranging from 1 (strongly disagree) to 5 (strongly agree). All items were summed to produce a total score, with higher scores indicating higher levels of risk intention. The internal consistency coefficient (Cronbach’s alpha) was 0.964.
2.3 Data analysis
SPSS version 26.0 was used to explore the correlations among water safety knowledge, drowning risk perceptions, drowning risk attitudes, and drowning risk behaviors. Harman’s single-factor test was utilized to examine 42 items for the common method bias test ( 24 ). The results revealed 11 factors with eigenvalues greater than one, and the variance explained by the first factor was less than 40% (19.246%), indicating that there was no common method bias in this study.
Mediating hypotheses were examined based on the mediating effects analysis process ( 25 ). All data were analyzed using Hayes’ SPSS macro program PROCESS ( 26 ). Water safety knowledge was used as an independent variable, drowning risk perceptions and attitudes as mediating variables, and drowning risk behaviors as dependent variables. The mediating effect was tested using bootstrapping (repeated sampling of 5,000 times). If the 95% confidence interval did not include zero, the mediating effect was considered significant.
3.1 Descriptive and Pearson correlation analysis
The results of the correlation analyses for all variables are shown in Table 1 . Drowning risk perceptions are positively correlated with water safety knowledge; drowning risk attitudes and behaviors are negatively correlated with water safety knowledge; drowning risk attitudes and behaviors are negatively correlated with drowning risk perceptions; and drowning risk behaviors are positively correlated with drowning risk attitudes.
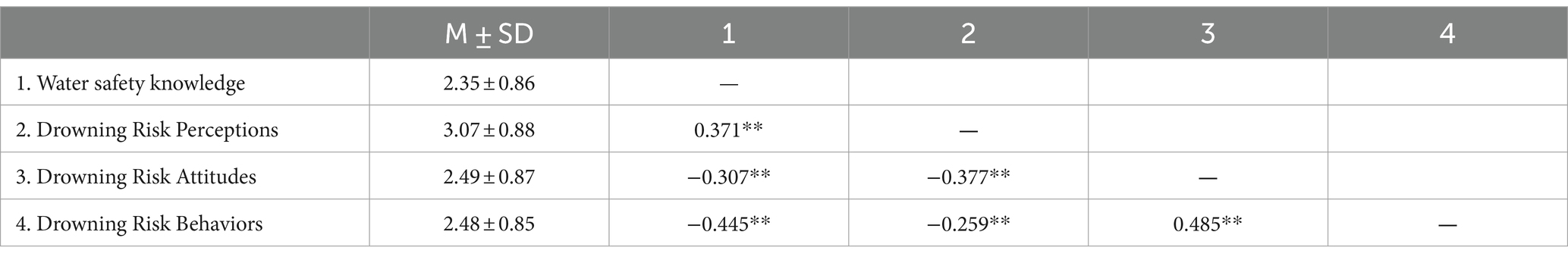
Table 1 . Descriptive statistics and correlations of key variables ( n = 7,485).
3.2 Testing for chain mediating effect
The results of the regression analysis are presented in Table 2 . Water safety knowledge positively predicted drowning risk perceptions (β =0.371, p < 0.001). Water safety knowledge and drowning risk perceptions negatively predicted drowning risk attitudes (β = −0.194, p < 0.001; β = −0.305, p < 0. 001). When incorporating water safety knowledge, drowning risk perceptions and drowning risk attitudes into the equation, their predictive effects were significant. Namely, water safety knowledge and drowning risk perceptions negatively predicted drowning risk behaviors (β = −0.260, p < 0. 001; β = −0.213, p < 0. 001), and drowning risk attitudes positively predicted drowning risk behaviors (β =0.345, p < 0.001).
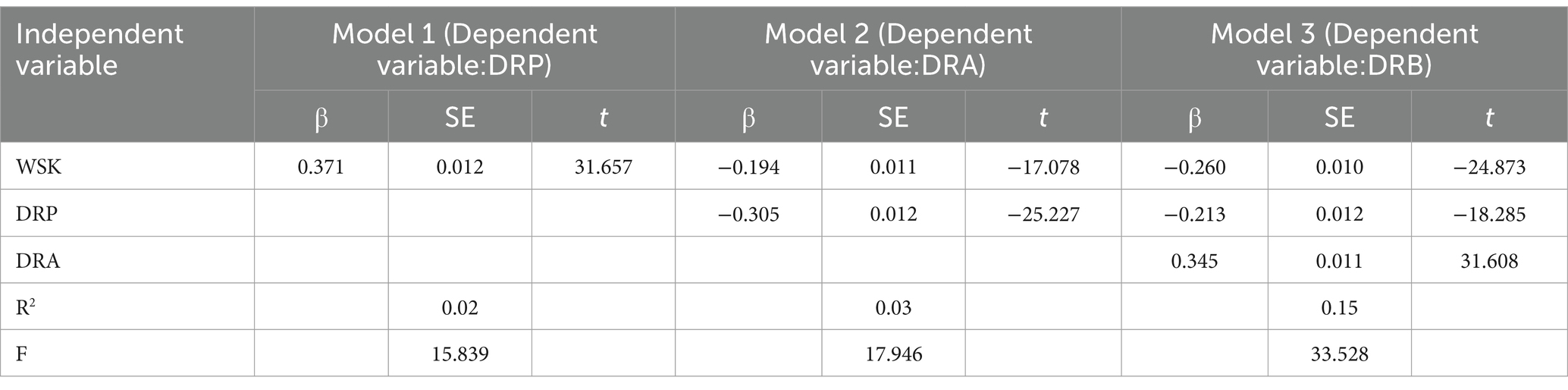
Table 2 . Regression analysis of the mediation model between drowning risk perceptions and drowning risk attitude.
The results of the mediating effects tests are presented in Table 3 and Figure 3 . WSK affected DRB through three indirect paths. The 95% confidence interval (CI) of the indirect effect paths did not include zero, revealing that DRP and DRA had significant mediating effects on the association between WSK and DRB. The first path, WSK → DRP → DRB, accounts for 17.7% of total effect. The second path, WSK → DRA → DRB, accounted for 15.1% of the total effect. The third path, WSK → DRP → DRA → DRB, accounted for 8.7% of the total effect. In conclusion, DRP and DRA can act as mediators between WSK and DRB, not only individually, but also as chain mediators, where the direct effect is-0.301, the total indirect effect is-0.214 (a1b1 + a2b2 + a1a3b2), and the total mediated indirect effect is 41.5%.
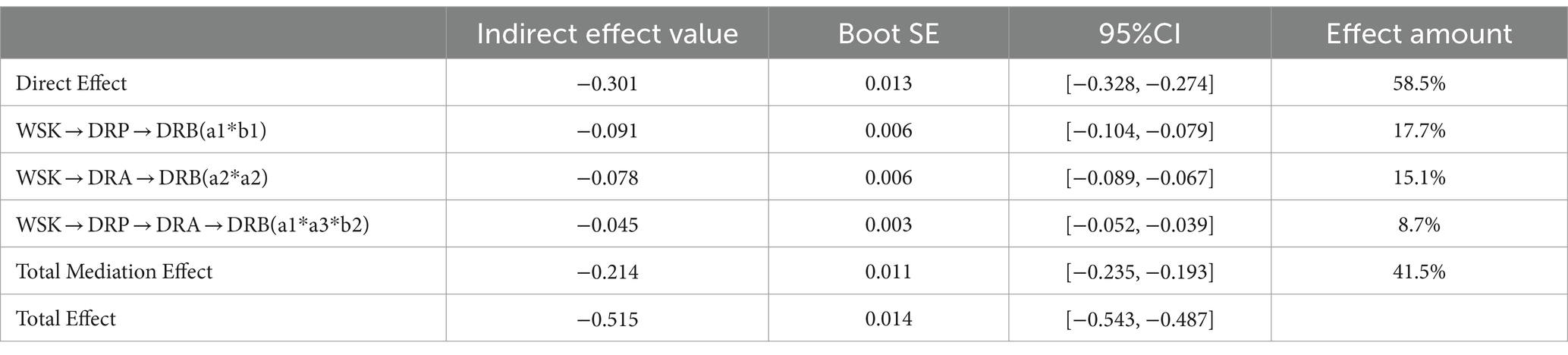
Table 3 . Mediation effect volume analysis.
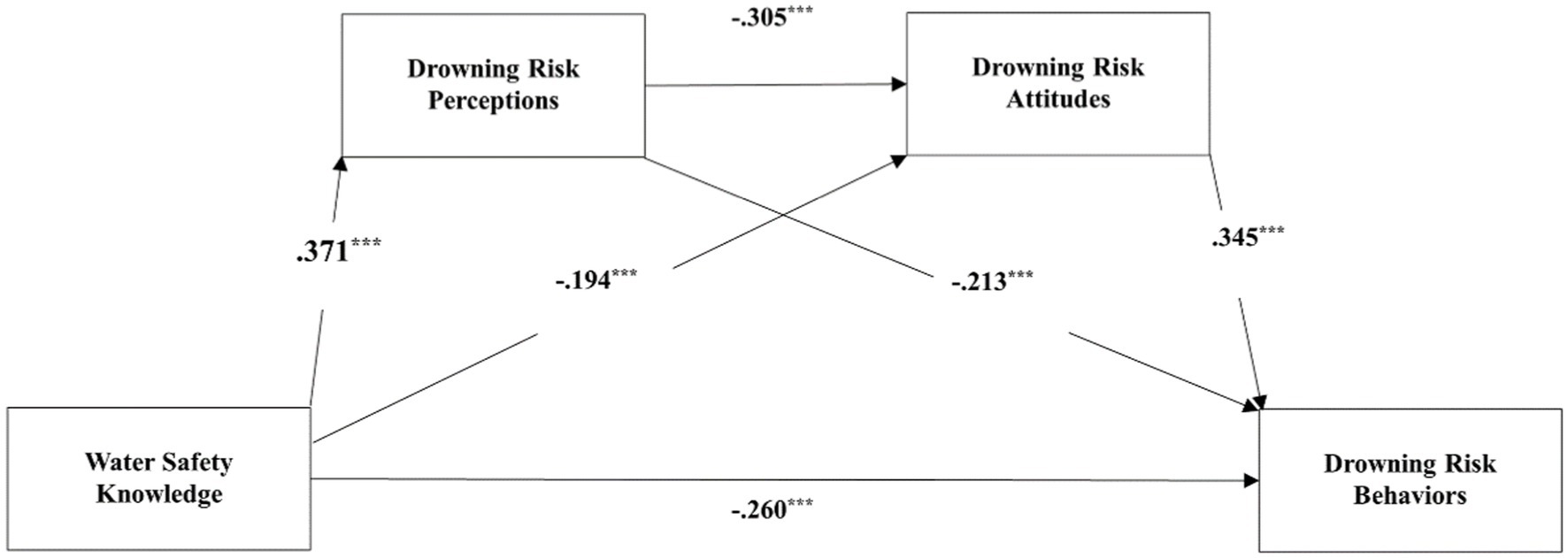
Figure 3 . The chain mediating model. Path coefficients are standardized coefficients. *** p < 0.001.
4 Discussion
The resolution adopted by the General Assembly on April 28, 2021 stresses the need to raise awareness of the importance of drowning prevention and the need for urgent coordinated multisectoral action to improve water safety through education, knowledge sharing, and other activities, with the aim of reducing preventable deaths ( 27 ). Our results support the role of water safety knowledge as a significant negative predictor of drowning risk behaviors and are consistent with previous findings ( 28 , 29 ). Adolescents who have a high level of water safety knowledge can be sensitive to physical conditions, weather, and potential risks of aquatic environments, and can combine the skills they have learned to save themselves and others. For instance, adolescents’ familiarity with water warning signs will enable them to reduce their drowning risk.
China currently ranks first in the world in terms of drowning fatalities, and water safety education lags severely. We found that few primary and secondary schools had teaching materials related to the recognition of water warning signs. It is noteworthy that the proportion of schools and universities with swimming pools was relatively low. Taking the nine top economic districts in Chongqing as an example, only 8 of the 446 (1.79%) primary schools, 18 of the 249 (7.23%) middle schools, and 12 of the 71 (16.9%) universities were equipped with swimming pools. In this context, two factors are extremely important for promoting the rapid development of water safety education in China. The first factor is parental involvement. Morrongiello et al. ( 30 ) found that, in addition to schools, parents were an especially critical element in the development of adolescents’ drowning prevention skills, which was even more apparent in China. Some parental approaches, such as enrolling children in aquatics training, are the most beneficial in helping children develop the water safety knowledge and swimming skills ( 31 ). The second most important factor is the financial support. The WHO ( 3 ) has noted that more than 90% of drowning fatalities occur in low-and middle-income countries (LMIC). However, certain urban and rural parts of China can be compared with both developed countries and LMICs. There are families in China’s rural areas whose parents earn little income and cannot cover the costs for their children to receive water safety education. Therefore, financial support can help reduce the incidence of drowning accidents by providing more adolescents with opportunities to receive water safety education.
Our results support the inhibitory effect of water safety knowledge on drowning risk behaviors through drowning risk perceptions. Meanwhile, regarding Hypothesis 2 of this study, the second path (WSK → DRA → DRB) is consistent with previous research findings ( 32 ) while the first path (WSK → DRP → DRB) is our new finding. However, water safety knowledge strengthens adolescents’ drowning risk perceptions and can thus decrease the probability of adolescents intentionally exposing themselves to risk. Moreover, the dual-process approach model indicated that risk perceptions were significantly related to subjective knowledge and personal familiarity with the water environment, which in turn, helped adolescents avoid drowning risk behaviors. More specifically, individuals develop the ability to identify risk through the collection, comprehension, and application of knowledge, and build up their “antibodies” to self-protect against drowning. These “antibodies” are a stress response to water environments and enable one to remain alert to threats in the aquatic environment.
However, sensitivity to the aquatic environment and the ability to be aware of potential drowning risks in advance also rely on mastering water safety knowledge. Furthermore, a combination of knowledge and risk perception is also essential, especially when drowning prevention and rescue of oneself or others requires the most appropriate judgement and decision-making. However, unintentionally falling into the water and drowning requires extra attention ( 33 ). In China, most children and adolescents do not know how to swim or are not good swimmers. According to a vast number of drowning accidents reported, diving, swimming in deep water, and physical exhaustion are the leading causes of drowning in adults, whereas unintentional falls into water are more common among children and adolescents ( 2 ). In the case of unintentional drowning, it is difficult for a drowning adolescent or adult to survive because of heavy and wet clothing reducing the speed of swimming, countercurrents, and other factors ( 34 ). The strengthening of drowning precautions and the improvement of risk perception and risk identification stem from the continuous learning of water safety knowledge, so that the drowning risk can be reduced.
In this study, we found that drowning risk attitudes partially mediated the relationship between water safety knowledge and drowning risk behaviors, which is consistent with previous studies ( 15 , 17 ). The use of KAP theory in drowning prevention research has been well established, and the WHO ( 4 ) has also specifically highlighted its use of KAP theory ( 4 ). In China, the Ministry of Education, schools, and students are the three key participants in the implementation of water safety education. First, the Ministry of Education plays a general directive role in drowning prevention education in schools by establishing a dedicated educational website and propagating drowning prevention on its official website. Schools are more direct implementers of water safety education, primarily through awareness campaigns and lectures just before summer holidays, as well as through public education on WeChat. However, owing to academic pressure and the paucity of teachers and teaching equipment, water safety courses have limited class time and are not as popular as they should be. Finally, students, who are the target audience for water safety education, participate in courses and lectures to gain water safety knowledge. Nevertheless, many students lack motivation to attend water safety education courses and learn very little about water safety knowledge simply because these courses are not part of the main academic assessment system for students.
In summary, the water safety education program in Chinese schools lacks organization, resulting in a low level of adolescent water safety knowledge and passive drowning risk attitudes. Negative attitudes invariably lead to negative behaviors, which can exacerbate issues such as drowning accidents, shortsightedness, and deteriorating health. Negative attitudes towards health-harming behaviors generated by exam-oriented education tend to result in negative behaviors and exacerbate drowning accidents, shortsightedness, deteriorating physical fitness, and other issues that the Chinese society must address.
Our study also demonstrated that drowning risk perceptions and attitudes act as chain mediators between water safety knowledge and drowning risk behaviors. An increase in water safety knowledge promotes drowning risk perceptions, which in turn can improve drowning risk attitudes and prevent risky behaviors. This pathway, which has not been extensively explored in previous drowning prevention studies, is consistent with findings on the prevention of heat-related illnesses ( 35 ). Negative information refers to information that threatens individuals’ safety and sensitizes them to environmental dangers. However, owing to the limited knowledge volume, water safety knowledge does not contain much negative information. This may easily lead adolescents to lose awareness of water environment dangers, resulting in negative attitudes and riskier behaviors. Chinese transportation authorities have attempted to include negative information in dangerous driving education, such as the use of vehicle accident videos, to make drivers more alert of risky behaviors including drink driving, speeding, and running red lights.
In drowning prevention studies, a similar strategy can be employed to raise awareness of the serious consequences of drowning accidents to prevent high-risk rescue behaviors, such as direct diving, hand-holding, and direct hand rescuing by adolescents. Summarizing and learning from other people’s drowning experiences can help individuals avoid mistakes. Moreover, according to psychological education, we should ensure that the warning bells are always ringing, because risk perceptions, like knowledge and memory, will diminish with time and the influence of other people and events. Currently, China’s birth population is decreasing each year, and the deaths of adolescents due to drowning accidents pose a threat to the young population. Therefore, more emphasis should be placed on water safety education to protect young people as well as the future of families and the country at large.
5 Limitations and future research
This study has several limitations. First, this study followed a cross-sectional design; therefore, we could not establish the causal association between water safety knowledge and drowning risk behaviors. Cross-lagged designs and experimental interventions are needed in future studies. Second, this study was limited to exploring the mediating effect of drowning risk perceptions and attitudes on adolescents’ water safety knowledge and drowning risk behaviors; whether other factors, such as sensation seeking, emotion, swimming overconfidence, verbal persuasion, and behavioral imitation, also play a moderating role needs further examination. Third, future studies may need to apply water competency models and Behavioral Event Interviews (BEI) to identify the characteristics of various water competencies and behaviors to better prevent adolescent drowning accidents.
6 Conclusion
Water safety knowledge negatively predicts adolescent drowning risk behaviors, and has an inhibitory effect on drowning risk perceptions. Water safety knowledge can directly influence adolescents’ drowning risk perceptions and indirectly affect drowning risk behaviors through the mediation of drowning risk perceptions and attitudes comprising three paths. The first was the drowning risk perceptions mediation path. Second was a drowning risk attitudes mediating path. The third chain mediation path involved drowning risk perceptions and attitudes. In conclusion, the establishment of the mediation model reveals the mechanism by which water safety knowledge influences adolescents’ drowning risk behaviors, serving as a reference for the prevention of adolescent drowning risk behaviors. In the future, the level of water safety knowledge can be improved to help adolescents enhance their drowning risk perceptions, avoid negative risk attitudes, and reduce drowning risk behaviors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the College of Physical Education at Southwest University (ethical approval number: SWU20180601). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
ShiL: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. ShuL: Writing – review & editing. ZR: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. HZ: Funding acquisition, Writing – review & editing, Validation, Supervision. XL: Writing – review & editing. LL: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Education of Humanities and Social Science Research on Youth Fund (project 20YJC890016), Fundamental Research Funds for the Central Universities (project SWU2109329), and Higher Education Teaching Reform Project of Hubei Province under Grant Number (project 2021378).
Acknowledgments
We thank all primary and secondary school students who agreed to participate in this study and provided informed consent for data analysis. We also thank the local staff at primary and secondary schools for their dedicated work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Meddings, DR, Scarr, JP, Larson, K, Vaughan, J, and Krug, EG. Drowning prevention: turning the tide on a leading killer. Lancet Public Health . (2021) 6:e692–5. doi: 10.1016/S2468-2667(21)00165-1
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wang, L, Cheng, X, Yin, P, Cheng, P, Liu, Y, Schwebel, DC, et al. Unintentional drowning mortality in China, 2006-2013. Inj Prev . (2019) 25:47–51. doi: 10.1136/injuryprev-2017-042713
3. WHO. (2014). Global report on drowning: preventing a leading killer. Available at: https://apps.who.int/iris/bitstream/handle/10665/143893/9789241564786_eng.pdf
Google Scholar
4. WHO. Preventing drowning: An implementation guide World Health Organization (2017) Available at: https://apps.who.int/iris/bitstream/handle/10665/255196/9789245511939-chi.pdf .
5. Stallman, RK, Moran, K, Quan, L, and Langendorfer, S. From swimming skill to water competence: towards a more inclusive drowning prevention future. Int J Aquatic Res Educ . (2017) 10:3. doi: 10.25035/ijare.10.02.03
Crossref Full Text | Google Scholar
6. Luo, S, Wang, B, Zhang, H, Fang, C, Bu, S, and Shi, K. Effect of water safety skills on water high-risk practices for adolescents: moderated mediating effect. J Shenyang Sport Univ . (2017) 36:66–72. doi: 10.3969/j.issn.1004-0560.2017.01.012
7. Golden, L, Manika, D, and Brockett, P. The importance of personally relevant knowledge for pandemic risk prevention behavior: a multimethod analysis and two-country validation. Health Mark Q . (2021) 38:223–37. doi: 10.1080/07359683.2021.1989746
8. Wang, Y, Zhang, X, Li, Y, Liu, Y, Sun, B, Wang, Y, et al. Knowledge, attitude, risk perception, and health-related adaptive behavior of primary school children towards climate change: a cross-sectional study in China. Int J Environ Res Public Health . (2022) 19:15648. doi: 10.3390/ijerph192315648
9. Ma, X, Zhang, Q, Jiang, R, Lu, J, Wang, H, Xia, Q, et al. Parents' attitudes as mediators between knowledge and behaviours in unintentional injuries at home of children aged 0-3 in Shanghai, eastern China: a cross-sectional study. BMJ Open . (2021) 11:e054228. doi: 10.1136/bmjopen-2021-054228
10. Zhou, D, Chang, M, Gu, G, Sun, X, Xu, H, Wang, W, et al. Analysis of risky driving behavior of Urban Electric bicycle drivers for improving safety. Sustain For . (2022) 14:1243. doi: 10.3390/su14031243
11. Cho, J, and Lee, J. An integrated model of risk and risk-reducing strategies. J Bus Res . (2006) 59:112–20. doi: 10.1016/j.jbusres.2005.03.006
12. Altarawneha, L, Mackee, J, and Gajendran, T. The influence of cognitive and affective risk perceptions on flood preparedness intentions: a dual-process approach. Procedia Eng . (2018) 212:1203–10. doi: 10.1016/j.proeng.2018.01.155
13. Finucane, ML. The role of feelings in perceived risk In: S Roeser, R Hillerbrand, P Sandin, and M Peterson, editors. Essentials of risk theory . Netherlands: Springer (2013). 57–74.
14. McCool, JP, Moran, K, Ameratunga, S, and Robinson, E. New Zealand beachgoers' swimming behaviours, swimming abilities, and perception of drowning risk. Int J Aquatic Res Educ . (2008) 2:7–15. doi: 10.25035/ijare.02.01.02
15. Moran, K. Re-thinking drowning risk: The role of water safety knowledge, attitudes and behaviours in the aquatic recreation of New Zealand youth: A thesis presented in fulfilment of the requirements for the degree of doctor of philosophy at Massey University, Palmerston North, New Zealand (Doctoral Massey University (2006) Available at: http://hdl.handle.net/10179/642 .
16. Cleary, A, and Dowling, M. Knowledge and attitudes of mental health professionals in Ireland to the concept of recovery in mental health: a questionnaire survey. J Psychiatr Ment Health Nurs . (2009) 16:539–45. doi: 10.1111/j.1365-2850.2009.01411.x
17. Willcox-Pidgeon, SM, Kool, B, and Moran, DK. Knowledge, attitudes, and behaviours of new Zealand youth in surf beach environments. Int J Aquatic Res Educ . (2017) 10:6. doi: 10.25035/ijare.10.02.06
18. Williamson, A, Hatfield, J, Sherker, S, Brander, R, and Hayen, A. A comparison of attitudes and knowledge of beach safety in Australia for beachgoers, rural residents and international tourists. Aust N Z J Public Health . (2012) 36:385–91. doi: 10.1111/j.1753-6405.2012.00888.x
19. Woodward, E, Beaumont, E, Russell, P, and MacLeod, R. Public understanding and knowledge of rip currents and beach safety in the UK. Int J Aquatic Res Educ . (2015) 9:49–69. doi: 10.25035/ijare.09.01.06
20. Chen, X, Ren, K, and Shen, Y. The effect of perceived organizational support on the prohibitive voice behavior of knowledgeable talents during the COVID-19 pandemic: exploring moderating role of the digitalization level. Front Psychol . (2022) 13:1020263. doi: 10.3389/fpsyg.2022.1020263
21. Luo, S, Shi, K, Zhang, H, Wang, B, and Hu, Y. Effect of parental behavioral control on water high-risk practices for adolescents: moderated mediating effect. Stud Psychol Behav . (2019) 17:259–67. doi: 10.3969/j.issn.1672-0628.2019.02.016
22. Laosee, O, Khiewyoo, J, and Somrongthong, R. Drowning risk perceptions among rural guardians of Thailand: a community-based household survey. J Child Health Care . (2014) 18:168–77. doi: 10.1177/1367493513485477
23. Xia, W, Wang, B, Zhang, X, Liu, L, and Wang, Y. Preparing of questionnaire about water safety KAP for pupils. China Safety Sci J . (2012) 22:3–9. doi: 10.16265/j.cnki.issn1003-3033.2012.12.004
24. Zhou, H, and Long, L. Statistical remedies for common method biases. Adv Psychol Sci . (2004) 12:942–50. doi: 10.3969/j.issn.1671-3710.2004.06.018
25. Wen, Z, and Ye, B. Analyses of mediating effects: the development of methods and models. Adv Psychol Sci . (2014) 22:731. doi: 10.3724/sp.J.1042.2014.00731
26. Hayes, AF, and Scharkow, M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci . (2013) 24:1918–27. doi: 10.1177/0956797613480187
27. WHO. (2021). WHO guideline on the prevention of drowning through provision of day-care and basic swimming and water safety skills. Available at: https://apps.who.int/iris/bitstream/handle/10665/343075/9789240030008-eng.pdf?sequence=1
28. Morrongiello, BA, Cusimano, M, Barton, BK, Orr, E, Chipman, M, Tyberg, J, et al. Development of the BACKIE questionnaire: a measure of children's behaviors, attitudes, cognitions, knowledge, and injury experiences. Accid Anal Prev . (2010) 42:75–83. doi: 10.1016/j.aap.2009.07.006
29. Petrass, LA, and Blitvich, JD. Preventing adolescent drowning: understanding water safety knowledge, attitudes and swimming ability. The effect of a short water safety intervention. Accid Anal Prev . (2014) 70:188–94. doi: 10.1016/j.aap.2014.04.006
30. Morrongiello, BA, Sandomierski, M, Schwebel, DC, and Hagel, B. Are parents just treading water? The impact of participation in swim lessons on parents' judgments of children's drowning risk, swimming ability, and supervision needs. Accid Anal Prev . (2013) 50:1169–75. doi: 10.1016/j.aap.2012.09.008
31. Farizan, NH, Mani, KKC, Sutan, R, and Hod, R. A concept paper on improving parental knowledge and practices on water safety and their children: a guide for drowning prevention. Int J Acad Res Bus Soc Sci . (2021) 11:262–71. doi: 10.6007/IJARBSS/v11-i15/10651
32. Woods, M, Koon, W, and Brander, RW. Identifying risk factors and implications for beach drowning prevention amongst an Australian multicultural community. PLoS One . (2022) 17:e0262175. doi: 10.1371/journal.pone.0262175
33. Saunders, CJ, Adriaanse, R, Simons, A, and van Niekerk, A. Fatal drowning in the Western cape, South Africa: a 7-year retrospective, epidemiological study. Inj Prev . (2019) 25:529–34. doi: 10.1136/injuryprev-2018-042945
34. Moran, K. Can you swim in clothes? An exploratory investigation of the effect of clothing on water competency. Int J Aquatic Res Educ . (2014) 8:338–50. doi: 10.25035/ijare.08.04.05
35. Arsad, FS, Hod, R, Ahmad, N, Baharom, M, and Tangang, F. The Malay-version knowledge, risk perception, attitude and practice questionnaire on heatwaves: development and construct validation. Int J Environ Res Public Health . (2022) 19:2279. doi: 10.3390/ijerph19042279
Keywords: adolescents, water safety knowledge, drowning risk perceptions, drowning risk attitudes, drowning risk behaviors
Citation: Luo S, Luo S, Ren Z, Zhang H, Li X and Liu L (2024) The influence of water safety knowledge on adolescents’ drowning risk behaviors: a framework of risk-protect integrated and KAP theory. Front. Public Health . 12:1354231. doi: 10.3389/fpubh.2024.1354231
Received: 13 December 2023; Accepted: 29 April 2024; Published: 10 May 2024.
Reviewed by:
Copyright © 2024 Luo, Luo, Ren, Zhang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lian Liu, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supporting IrO x nanosheets on hollow TiO 2 for highly efficient acidic water splitting
- Research Article
- Published: 16 May 2024
Cite this article

- Ge Yu 1 na1 ,
- Ruilong Li 1 na1 ,
- Yanmin Hu 1 ,
- Xingen Lin 1 ,
- Dongyang Wu 1 ,
- Gongming Wang 1 &
- Xun Hong 1
The efficiency of proton exchange membrane water electrolysis (PEM-WE) for hydrogen production is heavily dependent on the noble metal iridium-based catalysts. However, the scarcity of iridium limits the large-scale application of PEM-WE. To address this issue, it is promising to select an appropriate support because it not only enhances the utilization efficiency of noble metals but also improves mass transport under high current. Herein, we supported amorphous IrO x nanosheets onto the hollow TiO 2 sphere (denoted as IrO x ), which demonstrated excellent performance in acidic electrolytic water splitting. Specifically, the annealed IrO x catalyst at 150 °C in air exhibited a mass activity of 1347.5 A·g Ir −1 , which is much higher than that of commercial IrO 2 of 12.33 A·g Ir −1 at the overpotential of 300 mV for oxygen evolution reaction (OER). Meanwhile, the annealed IrO x exhibited good stability for 600 h operating at 10 mA·cm −2 . Moreover, when using IrO x and annealed IrO x catalysts for water splitting, a cell voltage as low as 1.485 V can be achieved at 10 mA·cm −2 . The cell can continuously operate for 200 h with negligible degradation of performance.

This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Zhu, W. X.; Song, X. C.; Liao, F.; Huang, H.; Shao, Q.; Feng, K.; Zhou, Y. J.; Ma, M. J.; Wu, J.; Yang, H. et al. Stable and oxidative charged Ru enhance the acidic oxygen evolution reaction activity in two-dimensional ruthenium-iridium oxide. Nat. Commun. 2023 , 14 , 5365.
Article CAS PubMed PubMed Central Google Scholar
Liu, S. H.; Tan, H.; Huang, Y. C.; Zhang, Q. B.; Lin, H. P.; Li, L.; Hu, Z. W.; Huang, W. H.; Pao, C. W.; Lee, J. F. et al. Structurally-distorted RuIr-based nanoframes for long-duration oxygen evolution catalysis. Adv. Mater. 2023 , 35 , 2305659.
Article CAS Google Scholar
Wu, H.; Huang, Q. X.; Shi, Y. Y.; Chang, J. W.; Lu, S. Y. Electrocatalytic water splitting: Mechanism and electrocatalyst design. Nano Res. 2023 , 16 , 9142–9157.
Article Google Scholar
Zhu, P.; Xiong, X.; Wang, D. S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022 , 15 , 5792–5815.
Zhu, C. X.; Yang, J. R.; Zhang, J. W.; Wang, X. Q.; Gao, Y.; Wang, D. S.; Pan, H. G. Single-atom materials: The application in energy conversion. Interdiscip. Mater. 2024 , 3 , 74–86.
Zhang, D. F.; Li, M. N.; Yong, X.; Song, H. Q.; Waterhouse, G. I. N.; Yi, Y. F.; Xue, B. J.; Zhang, D. L.; Liu, B. Z.; Lu, S. Y. Construction of Zn-doped RuO 2 nanowires for efficient and stable water oxidation in acidic media. Nat. Commun. 2023 , 14 , 2517.
Shi, Z. P.; Li, J.; Wang, Y. B.; Liu, S. W.; Zhu, J. B.; Yang, J. H.; Wang, X.; Ni, J.; Jiang, Z.; Zhang, L. J. et al. Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers. Nat. Commun. 2023 , 14 , 843.
Tian, C.; Liu, R.; Zhang, Y.; Yang, W. X.; Wang, B. Ru-doped functional porous materials for electrocatalytic water splitting. Nano Res. 2024 , 17 , 982–1002.
Zheng, X. B.; Yang, J. R.; Xu, Z. F.; Wang, Q. S.; Wu, J. B.; Zhang, E. H.; Dou, S. X.; Sun, W. P.; Wang, D. S.; Li, Y. D. Ru-Co pair sites catalyst boosts the energetics for the oxygen evolution reaction. Angew. Chem., Int. Ed. 2022 , 61 , e202205946.
Zheng, X. B.; Yang, J. R.; Li, P.; Wang, Q. S.; Wu, J. B.; Zhang, E. H.; Chen, S. H.; Zhuang, Z. C.; Lai, W. H.; Dou, S. X. et al. Ir-Sn pair-site triggers key oxygen radical intermediate for efficient acidic water oxidation. Sci. Adv. 2023 , 9 , eadi8025.
Shi, Y.; Lu, Z. X.; Guo, L. L.; Wang, Z. D.; Guo, C. Q.; Tan, H. Y.; Yan, C. F. Fabrication of IrO 2 decorated vertical aligned self-doped TiO 2 nanotube arrays for oxygen evolution in water electrolysis. Int. J. Hydrogen Energy 2018 , 43 , 9133–9143.
Genova-Koleva, R. V.; Alcaide, F.; Álvarez, G.; Cabot, P. L.; Grande, H. J.; Martínez-Huerta, M. V.; Miguel, O. Supporting IrO 2 and IrRuO nanoparticles on TiO 2 and Nb-doped TiO 2 nanotubes as electrocatalysts for the oxygen evolution reaction. J. Energy Chem. 2019 , 34 , 227–239.
Wang, Y. N.; Zhang, M. C.; Kang, Z. Y.; Shi, L.; Shen, Y. C.; Tian, B. Y.; Zou, Y. C.; Chen, H.; Zou, X. X. Nano-metal diborides-supported anode catalyst with strongly coupled TaO x /IrO 2 catalytic layer for low-iridium-loading proton exchange membrane electrolyzer. Nat. Commun. 2023 , 14 , 5119.
Shi, Z. P.; Li, J.; Jiang, J. D.; Wang, Y. B.; Wang, X.; Li, Y.; Yang, L. T.; Chu, Y. Y.; Bai, J. S.; Yang, J. H. et al. Enhanced acidic water oxidation by dynamic migration of oxygen species at the Ir/Nb 2 O 5− x catalyst/support interfaces. Angew. Chem., Int. Ed. 2022 , 61 , e202212341.
Wang, Y. B.; Ma, R. P.; Shi, Z. P.; Wu, H. X.; Hou, S.; Wang, Y.; Liu, C. P.; Ge, J. J.; Xing, W. Inverse doping IrO x /Ti with weakened Ir–O interaction toward stable and efficient acidic oxygen evolution. Chem 2023 , 9 , 2931–2942.
Lv, H.; Wang, S.; Li, J. K.; Shao, C. F.; Zhou, W.; Shen, X. J.; Xue, M. Z.; Zhang, C. M. Self-assembled RuO 2 @IrO x core–shell nanocomposite as high efficient anode catalyst for PEM water electrolyzer. Appl. Surf. Sci. 2020 , 514 , 145943.
Dong, S.; Zhang, C. Y.; Yue, Z. Y.; Zhang, F. R.; Zhao, H.; Cheng, Q. Q.; Wang, G. L.; Xu, J. F.; Chen, C.; Zou, Z. Q. et al. Overall design of anode with gradient ordered structure with low iridium loading for proton exchange membrane water electrolysis. Nano Lett. 2022 , 22 , 9434–9440.
Article CAS PubMed Google Scholar
Lin, F. X.; Lv, F.; Zhang, Q. H.; Luo, H.; Wang, K.; Zhou, J. H.; Zhang, W. Y.; Zhang, W. S.; Wang, D. W.; Gu, L. et al. Local coordination regulation through tuning atomic-scale cavities of pd metallene toward efficient oxygen reduction electrocatalysis. Adv. Mater. 2022 , 34 , 2202084.
Zhao, Y. F.; Lu, X. F.; Wu, Z. P.; Pei, Z. H.; Luan, D. Y.; Lou, X. W. Supporting trimetallic metal-organic frameworks on S/N-doped carbon macroporous fibers for highly efficient electrocatalytic oxygen evolution. Adv. Mater. 2023 , 35 , 2207888.
Joo, J. B.; Lee, I.; Dahl, M.; Moon, G. D.; Zaera, F.; Yin, Y. D. Controllable synthesis of mesoporous TiO 2 hollow shells: Toward an efficient photocatalyst. Adv. Funct. Mater. 2013 , 23 , 4246–4254.
Wu, G.; Zheng, X. S.; Cui, P. X.; Jiang, H. Y.; Wang, X. Q.; Qu, Y. T.; Chen, W. X.; Lin, Y.; Li, H.; Han, X. et al. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019 , 10 , 4855
Article PubMed PubMed Central Google Scholar
Wen, Y. Z.; Chen, P. N.; Wang, L.; Li, S. Y.; Wang, Z. Y.; Abed, J.; Mao, X. N.; Min, Y. M.; Dinh, C. T.; De Luna, P. et al. Stabilizing highly active Ru sites by suppressing lattice oxygen participation in acidic water oxidation. J. Am. Chem. Soc. 2021 , 143 , 6482–6490
Li, R. L.; Rao, D. W.; Zhou, J. B.; Wu, G.; Wang, G. Z.; Zhu, Z. X.; Han, X.; Sun, R. B.; Li, H.; Wang, C. et al. Amorphization-induced surface electronic states modulation of cobaltous oxide nanosheets for lithium-sulfur batteries. Nat. Commun. 2021 , 12 , 3102.
Gao, J. J.; Xu, C. Q.; Hung, S. F.; Liu, W.; Cai, W. Z.; Zeng, Z. P.; Jia, C. M.; Chen, H. M.; Xiao, H.; Li, J. et al. Breaking long-range order in iridium oxide by alkali ion for efficient water oxidation. J. Am. Chem. Soc. 2019 , 141 , 3014–3023.
Sun, W.; Ma, C. L.; Tian, X. L.; Liao, J. J.; Yang, J.; Ge, C. J.; Huang, W. W. An amorphous lanthanum-iridium solid solution with an open structure for efficient water splitting. J. Mater. Chem. A 2020 , 8 , 12518–12525.
Li, N.; Cai, L.; Wang, C.; Lin, Y.; Huang, J. Z.; Sheng, H. Y.; Pan, H. B.; Zhang, W.; Ji, Q. Q.; Duan, H. L. et al. Identification of the active-layer structures for acidic oxygen evolution from 9R-BaIrO 3 electrocatalyst with enhanced iridium mass activity. J. Am. Chem. Soc. 2021 , 143 , 18001–18009.
Fan, Z. L.; Ji, Y. J.; Shao, Q.; Geng, S. Z.; Zhu, W. X.; Liu, Y.; Liao, F.; Hu, Z. W.; Chang, Y. C.; Pao, C. W. et al. Extraordinary acidic oxygen evolution on new phase 3R-iridium oxide. Joule 2021 , 5 , 3221–3234.
Dang, Q.; Lin, H. P.; Fan, Z. L.; Ma, L.; Shao, Q.; Ji, Y. J.; Zheng, F. F.; Geng, S. Z.; Yang, S. Z.; Kong, N. N. et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021 , 12 , 6007.
Kwon, T.; Hwang, H.; Sa, Y. J.; Park, J.; Baik, H.; Joo, S. H.; Lee, K. Cobalt assisted synthesis of ircu hollow octahedral nanocages as highly active electrocatalysts toward oxygen evolution reaction. Adv. Funct. Mater. 2017 , 27 , 1604688.
Lv, F.; Feng, J. R.; Wang, K.; Dou, Z. P.; Zhang, W. Y.; Zhou, J. H.; Yang, C.; Luo, M. C.; Yang, Y.; Li, Y. J. et al. Iridium-tungsten alloy nanodendrites as pH-universal water-splitting electrocatalysts. ACS Cent. Sci. 2018 , 4 , 1244–1252.
Wang, X.; Qin, Z.; Qian, J. J.; Chen, L. Y.; Shen, K. IrCo nanoparticles encapsulated with carbon nanotubes for efficient and stable acidic water splitting. ACS Catal. 2023 , 13 , 10672–10682.
Mu, X. Q.; Zhang, X. Y.; Chen, Z. Y.; Gao, Y.; Yu, M.; Chen, D.; Pan, H. Z.; Liu, S. L.; Wang, D. S.; Mu, S. C. Constructing symmetry-mismatched Ru x Fe 3− x O 4 heterointerface-supported Ru clusters for efficient hydrogen evolution and oxidation reactions. Nano Lett. 2024 , 24 , 1015–1023.
Mu, X. Q.; Liu, S. L.; Zhang, M. Y.; Zhuang, Z. C.; Chen, D.; Liao, Y. R.; Zhao, H. Y.; Mu, S. C.; Wang, D. S.; Dai, Z. H. Symmetry-broken Ru nanoparticles with parasitic Ru-Co dual-single atoms overcome the volmer step of alkaline hydrogen oxidation. Angew. Chem., Int. Ed. 2024 , 63 , e202319618.
Zhu, L. L.; Lin, H. P.; Li, Y. Y.; Liao, F.; Lifshitz, Y.; Sheng, M. Q.; Lee, S. T.; Shao, M. W. A rhodium/silicon co-electrocatalyst design concept to surpass platinum hydrogen evolution activity at high overpotentials. Nat. Commun. 2016 , 7 , 12272.
Mahmood, J.; Li, F.; Jung, S. M.; Okyay, M. S.; Ahmad, I.; Kim, S. J.; Park, N.; Jeong, H. Y.; Baek, J. B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017 , 12 , 441–446.
Huang, X.; Zeng, Z. Y.; Bao, S. Y.; Wang, M. F.; Qi, X. Y.; Fan, Z. X.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013 , 4 , 1444.
Article PubMed Google Scholar
Cheng, Y. F.; Lu, S. K.; Liao, F.; Liu, L. B.; Li, Y. Q.; Shao, M. W. Rh-MoS 2 nanocomposite catalysts with Pt-like activity for hydrogen evolution reaction. Adv. Funct. Mater. 2017 , 27 , 1700359.
Feng, J. R.; Lv, F.; Zhang, W. Y.; Li, P. H.; Wang, K.; Yang, C.; Wang, B.; Yang, Y.; Zhou, J. H.; Lin, F. et al. Iridium-based multimetallic porous hollow nanocrystals for efficient overall-watersplitting catalysis. Adv. Mater. 2017 , 29 , 1703798.
Pu, Z. H.; Amiinu, I. S.; Kou, Z. K.; Li, W. Q.; Mu, S. C. RuP 2 -based catalysts with platinum-like activity and higher durability for the hydrogen evolution reaction at all pH values. Angew. Chem., Int. Ed. 2017 , 56 , 11559–11564.
Jiang, P.; Yang, Y.; Shi, R. H.; Xia, G. L.; Chen, J. T.; Su, J. W.; Chen, Q. W. Pt-like electrocatalytic behavior of Ru-MoO 2 nanocomposites for the hydrogen evolution reaction. J. Mater. Chem. A 2017 , 5 , 5475–5485.
Download references
Acknowledgements
The National Key R&D Program of China (Nos. 2018YFA0702001 and 2021YFA1500400), the National Natural Science Foundation of China (Nos. 22371268 and 22175163), Fundamental Research Funds for the Central Universities (No. WK2060000016), Anhui Development and Reform Commission (No. AHZDCYCX-2SDT2023-07), and Youth Innovation Promotion Association of the Chinese Academy of Science (No. 2018494) supported this work. We acknowledge USTC Tang Scholar.
Author information
Ge Yu and Ruilong Li contributed equally to this work.
Authors and Affiliations
Center of Advanced Nanocatalysis (CAN), Department of Applied Chemistry, University of Science and Technology of China, Hefei, 230026, China
Ge Yu, Ruilong Li, Yanmin Hu, Xingen Lin, Ze Lin, Dongyang Wu, Gongming Wang & Xun Hong
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Gongming Wang or Xun Hong .
Electronic Supplementary Material
Rights and permissions.
Reprints and permissions
About this article
Yu, G., Li, R., Hu, Y. et al. Supporting IrO x nanosheets on hollow TiO 2 for highly efficient acidic water splitting. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6681-7
Download citation
Received : 11 March 2024
Revised : 01 April 2024
Accepted : 02 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1007/s12274-024-6681-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- oxygen evolution reaction
- hydrogen evolution reaction
- amorphous IrO x
- hollow TiO 2
- proton exchange membrane water electrolysis
- Find a journal
- Publish with us
- Track your research
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Professor Emeritus David Lanning, nuclear engineer and key contributor to the MIT Reactor, dies at 96

Previous image Next image
David Lanning, MIT professor emeritus of nuclear science and engineering and a key contributor to the MIT Reactor project, passed away on April 26 at the Lahey Clinic in Burlington, Massachusetts, at the age of 96.
Born in Baker, Oregon, on March 30, 1928, Lanning graduated in 1951 from the University of Oregon with a BS in physics. While taking night classes in nuclear engineering, in lieu of an available degree program at the time, he started his career path working for General Electric in Richland, Washington. There he conducted critical-mass studies for handling and designing safe plutonium-bearing systems in separation plants at the Hanford Atomic Products Operation, making him a pioneer in nuclear fuel cycle management.
Lanning was then involved in the design, construction, and startup of the Physical Constants Testing Reactor (PCTR). As one of the few people qualified to operate the experimental reactor, he trained others to safely assess and handle its highly radioactive components.
Lanning supervised experiments at the PCTR to find the critical conditions of various lattices in a safe manner and conduct reactivity measurements to determine relative flux distributions. This primed him to be an indispensable asset to the MIT Reactor (MITR), which was being constructed on the opposite side of the country.
An early authority in nuclear engineering comes to MIT
Lanning came to MIT in 1957 to join what was being called the “MIT Reactor Project” after being recruited by the MITR’s designer and first director, Theos “Tommy” J. Thompson, to serve as one of the MITR’s first operating supervisors. With only a handful of people on the operations team at the time, Lanning also completed the emergency plan and startup procedures for the MITR, which achieved criticality on July 21, 1958.
In addition to becoming a faculty member in the Department of Nuclear Engineering in 1962, Lanning’s roles at the MITR went from reactor operations superintendent in the 1950s and early 1960s, to assistant director in 1962, and then acting director in 1963, when Thompson went on sabbatical.
In his faculty position, Lanning took responsibility for supervising lab subjects and research projects at the MITR, including the Heavy Water Lattice Project. This project supported the thesis work of more than 30 students doing experimental studies of sub-critical uranium fuel rods — including Lanning’s own thesis. He received his PhD in nuclear engineering from MIT in fall 1963.
Lanning decided to leave MIT in July 1965 and return to Hanford as the manager of their Reactor Neutronics Section. Despite not having plans to return to work for MIT, Lanning agreed when Thompson requested that he renew his MITR operator’s license shortly after leaving.
“Because of his thorough familiarity with our facility, it is anticipated that Dr. Lanning may be asked to return to MIT for temporary tours of duty at our reactor. It is always possible that there may be changes in the key personnel presently operating the MIT Reactor and the possible availability of Dr. Lanning to fill in, even temporarily, could be a very important factor in maintaining a high level of competence at the reactor during its continued operation,” Theos J. Thompson wrote in a letter to the Atomic Energy Commission on Sept. 21, 1965
One modification, many changes
This was an invaluable decision to continue the MITR’s success as a nuclear research facility. In 1969 Thompson accepted a two-year term appointment as a U.S. atomic energy commissioner and requested Lanning to return to MIT to take his place during his temporary absence. Thompson initiated feasibility studies for a new MITR core design and believed Lanning was the most capable person to continue the task of seeing the MITR redesign to fruition.
Lanning returned to MIT in July 1969 with a faculty appointment to take over the subjects Thompson was teaching, in addition to being co-director of the MITR with Lincoln Clark Jr. during the redesign. Tragically, Thompson was killed in a plane accident in November 1970, just one week after Lanning and his team submitted the application for the redesign’s construction permit.
Thompson’s death meant his responsibilities were now Lanning’s on a permanent basis. Lanning continued to completion the redesign of the MITR, known today as the MITR-II. The redesign increased the neutron flux level by a factor of three without changing its operating power — expanding the reactor’s research capabilities and refreshing its status as a premier research facility.
Construction and startup tests for the MITR-II were completed in 1975 and the MITR-II went critical on Aug. 14, 1975. Management of the MITR-II was transferred the following year from the Nuclear Engineering Department to its own interdepartmental research center, the Nuclear Reactor Laboratory , where Lanning continued to use the MITR-II for research.
Beyond the redesign
In 1970, Lanning combined two reactor design courses he inherited and introduced a new course in which he had students apply their knowledge and critique the design and economic considerations of a reactor presented by a student in a prior term. He taught these courses through the late 1990s, in addition to leading new courses with other faculty for industry professionals on reactor safety.
Co-author of over 70 papers , many on the forefront of nuclear engineering, Lanning’s research included studies to improve the efficiency, cycle management, and design of nuclear fuel, as well as making reactors safer and more economical to operate.
Lanning was part of an ongoing research project team that introduced and demonstrated digital control and automation in nuclear reactor control mechanisms before any of the sort were found in reactors in the United States. Their research improved the regulatory barriers preventing commercial plants from replacing aging analog reactor control components with digital ones. The project also demonstrated that reactor operations would be more reliable, safe, and economical by introducing automation in certain reactor control systems. This led to the MITR being one of the first reactors in the United States licensed to operate using digital technology to control reactor power.
Lanning became professor emeritus in May 1989 and retired in 1994, but continued his passion for teaching through the late 1990s as a thesis advisor and reader. His legacy lives on in the still-operational MITR-II, with his former students following in his footsteps by working on fuel studies for the next version of the MITR core.
Lanning is predeceased by his wife of 60 years, Gloria Lanning, and is survived by his two children, a brother, and his many grandchildren .
Share this news article on:
Related links.
- MIT Nuclear Reactor Laboratory
- Department of Nuclear Science and Engineering
Related Topics
- Nuclear science and engineering
- Nuclear power and reactors
Related Articles

Professor Emeritus Sow-Hsin Chen, global expert in neutron science and devoted mentor, dies at 86

Professor Emeritus Michael Driscoll, leader in nuclear engineering and beloved mentor, dies at 86

Sara Hauptman: Learning to operate a nuclear reactor
Retirement dinner honors 155 community members.
Previous item Next item
More MIT News
Researchers develop a detector for continuously monitoring toxic gases
Read full story →

The beauty of biology

Navigating longevity with industry leaders at MIT AgeLab PLAN Forum

Jeong Min Park earns 2024 Schmidt Science Fellowship

Elaine Liu: Charging ahead
Scientists use generative AI to answer complex questions in physics
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram

IMAGES
COMMENTS
Singapore and Israel, two highly water-stressed regions, use much less water per person than do other high-income countries, and they recycle, treat and reuse more than 80% of their waste water 2 ...
Water coverage from Scientific American, featuring news and articles about advances in the field.
Water Research publishes refereed, original research papers on all aspects of the science and technology of the anthropogenic water cycle, water quality, and its management worldwide. A broad outline of the journal's scope includes: •Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment ...
PLOS Water brings together research of the highest methodological and ethical standards for water as a vital resource for societies in every region of the world. Get Started ... This collection features articles that contribute new insights to the theme of fresh water in cities. Read more. 11/21/2023. Water use and management.
Water Resources Research is an AGU hydrology journal publishing original research articles and commentaries on hydrology, water resources, and the social sciences of water. Abstract Water distinguishes our planet compared to all the others we know about. While the global supply of available freshwater is more than adequate to meet all current ...
Water Resources Research. JOURNAL METRICS >. Online ISSN:1944-7973. Print ISSN:0043-1397. Water Resources Research is an open access journal that publishes original research articles and commentaries on hydrology, water resources, and the social sciences of water that provide a broad understanding of the role of water in Earth's system.
As water itself is known for the phenomenon of water bridge (see Fig. 1), also discussed later in this review, we believe that a bold, open-minded and transdisciplinary approach bridging different views and methodologies is needed when dealing with water.We hope that this review will be a small effort in this direction. We focus on the positive aspects of the various theories and studies put ...
2. Representative Research Evidence. As shown in Table 2, a variety of methods and theoretical approaches have influenced our present understanding and theories regarding human water intake, euhydration, hypohydration, and water requirements.The range of measured or calculated variables includes dietary macronutrients, 24-h TWI (defined above), biomarkers of hydration status, water volumes (i ...
In fact, due to the dedicated efforts of governments and NGOs since the 1992 Earth Summit, safe drinking water has been made available to some 1.7 billion people around the world, with projects ...
1 INTRODUCTION. Safe, affordable, and accessible drinking water is integral to health, dignity, and human rights. Globally, about 2 billion people lack access to safe and affordable drinking water (UNICEF & WHO, 2019).Recent media attention on lead contamination in Flint and wells running dry in California has elevated public awareness of drinking water injustices in the United States (Felton ...
The critical adaptations cross an array of species, including man. Without water, humans can survive only for days. Water comprises from 75% body weight in infants to 55% in elderly and is essential for cellular homeostasis and life. 1 Nevertheless there are many unanswered questions about this most essential component of our body and our diet ...
The selection of these research articles was used to carry out a critical review of the current situation to propose future challenges to achieve efficient, and sustainable water treatment processes. 2. Critical Review: Evaluation of the Current Situation, Perspectives, and Challenges in the Detection of Contaminants, Health Risk Assessment ...
Much of the research on water and physical or mental functioning compares a euhydrated state, usually achieved by provision of water sufficient to overcome water loss, to a dehydrated state, which is achieved via withholding of fluids over time and during periods of heat stress or high activity. In general, provision of water is beneficial in ...
Water pollution can come from a variety of sources. Pollution can enter water directly, through both legal and illegal discharges from factories, for example, or imperfect water treatment plants ...
Water resources are crucial in developing any area as they serve as a major source of potable, agricultural, and industrial water. Water contamination, caused by natural and anthropogenic activities, poses a significant threat to public health globally. This review synthesizes data from various studies published in national and international journals, as well as reports from governmental and ...
The results indicated that annual output of the related scientific articles increased steadily. Water Research, Environmental Science & Technology, and Journal American Water Works Association were the three most common journals in drinking water research. The USA took a leading position out of 168 countries/territories, followed by Japan and ...
The team found that 32% of groundwater and 16% of the surface water samples exceeded the national drinking water standards in some countries. 'Drinking water standards vary around the world. In Australia, EU, the US and Canada,' explains Denis O'Carroll, whose lab carried out the study, 'the number of samples that exceeded those [limits ...
The authors annually evaluated the reduction in the use of water (2.5 10 5 tons), nitrogen (5.6 NH 3 -N tons), and carbon emissions (134 tons) through the reuse of treated sewage. Advances in the management of solid waste and wastewater treatment include landfilling, the composting of organic matter, reductions in greenhouse gas emissions and ...
The five Great Lakes are a major source of fresh drinking water to both the U.S. and Canada, but are also home to PFAS, or "forever chemicals." The compounds are more prevalent in air and water near urban population centers, according to research published in Environmental Science & Technology.
Water contaminated with pathogenic species also has the normal inhabitants of the human intestine. A good bacterial indicator of fecal pollution should fulfill the following criteria: (1) exist in high numbers in the human intestine and feces; (2) not be pathogenic to humans; (3) easily, reliably and cheaply detectable in environmental waters ...
Hypothesis 4: Drowning risk perceptions and attitudes have a chain-mediating effect on water safety knowledge and drowning risk behaviors.. 2 Materials and methods 2.1 Participants and procedure. We used cross-sectional data from primary and secondary school students in China, and conducted pre-test training for local teachers and students to normalize the investigation process.
To enhance oil-water separation efficiency, it is urgent to synthesize novel membrane materials that exhibit high flux and high retention efficiency and excellent antisolvent property. Herein, a seeding-extension-bud protrusion procedure was developed to fabricate superhydrophobic separation membranes through UV-assisted grafting and covalent modification on a microporous polypropylene ...
The efficiency of proton exchange membrane water electrolysis (PEM-WE) for hydrogen production is heavily dependent on the noble metal iridium-based catalysts. However, the scarcity of iridium limits the large-scale application of PEM-WE. To address this issue, it is promising to select an appropriate support because it not only enhances the utilization efficiency of noble metals but also ...
In his faculty position, Lanning took responsibility for supervising lab subjects and research projects at the MITR, including the Heavy Water Lattice Project. This project supported the thesis work of more than 30 students doing experimental studies of sub-critical uranium fuel rods — including Lanning's own thesis.