
- Games & Quizzes
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Why does physics work in SI units?
- Is mathematics a physical science?
- Where was Max Planck educated?


Planck’s radiation law
Our editors will review what you’ve submitted and determine whether to revise the article.
- Chemistry LibreTexts - Planck's Quantum Theory
- University of Texas at Austin - Professor of Physics - The Planck radiation law
- Academia - Planck's radiation law: A many body theory perspective
Planck’s radiation law , a mathematical relationship formulated in 1900 by German physicist Max Planck to explain the spectral-energy distribution of radiation emitted by a blackbody (a hypothetical body that completely absorbs all radiant energy falling upon it, reaches some equilibrium temperature , and then reemits that energy as quickly as it absorbs it). Planck assumed that the sources of radiation are atoms in a state of oscillation and that the vibrational energy of each oscillator may have any of a series of discrete values but never any value between. Planck further assumed that when an oscillator changes from a state of energy E 1 to a state of lower energy E 2 , the discrete amount of energy E 1 − E 2 , or quantum of radiation, is equal to the product of the frequency of the radiation, symbolized by the Greek letter ν and a constant h , now called Planck’s constant , that he determined from blackbody radiation data; i.e., E 1 − E 2 = h ν.

The wavelength of the emitted radiation is inversely proportional to its frequency, or λ = c /ν. The value of Planck’s constant is defined as 6.62607015 × 10 −34 joule∙second.
For a blackbody at temperatures up to several hundred degrees, the majority of the radiation is in the infrared radiation region of the electromagnetic spectrum . At higher temperatures, the total radiated energy increases, and the intensity peak of the emitted spectrum shifts to shorter wavelengths so that a significant portion is radiated as visible light .

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.2: Blackbody Radiation
- Last updated
- Save as PDF
- Page ID 4520

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section you will be able to:
- Apply Wien’s and Stefan’s laws to analyze radiation emitted by a blackbody
- Explain Planck’s hypothesis of energy quanta
All bodies emit electromagnetic radiation over a range of wavelengths. In an earlier chapter, we learned that a cooler body radiates less energy than a warmer body. We also know by observation that when a body is heated and its temperature rises, the perceived wavelength of its emitted radiation changes from infrared to red, and then from red to orange, and so forth. As its temperature rises, the body glows with the colors corresponding to ever-smaller wavelengths of the electromagnetic spectrum. This is the underlying principle of the incandescent light bulb: A hot metal filament glows red, and when heating continues, its glow eventually covers the entire visible portion of the electromagnetic spectrum. The temperature ( T ) of the object that emits radiation, or the emitter , determines the wavelength at which the radiated energy is at its maximum. For example, the Sun, whose surface temperature is in the range between 5000 K and 6000 K, radiates most strongly in a range of wavelengths about 560 nm in the visible part of the electromagnetic spectrum. Your body, when at its normal temperature of about 300 K, radiates most strongly in the infrared part of the spectrum.
Radiation that is incident on an object is partially absorbed and partially reflected. At thermodynamic equilibrium, the rate at which an object absorbs radiation is the same as the rate at which it emits it. Therefore, a good absorber of radiation (any object that absorbs radiation) is also a good emitter. A perfect absorber absorbs all electromagnetic radiation incident on it; such an object is called a blackbody .
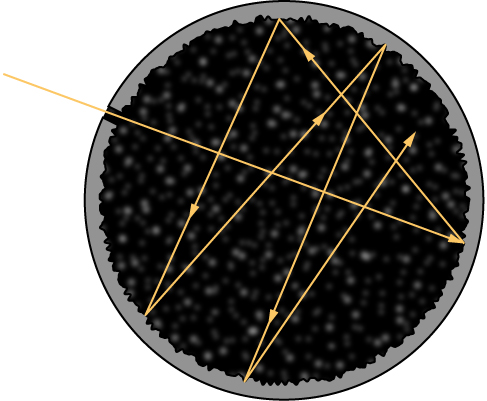
Although the blackbody is an idealization, because no physical object absorbs 100% of incident radiation, we can construct a close realization of a blackbody in the form of a small hole in the wall of a sealed enclosure known as a cavity radiator, as shown in Figure \(\PageIndex{1}\). The inside walls of a cavity radiator are rough and blackened so that any radiation that enters through a tiny hole in the cavity wall becomes trapped inside the cavity. At thermodynamic equilibrium (at temperature T ), the cavity walls absorb exactly as much radiation as they emit. Furthermore, inside the cavity, the radiation entering the hole is balanced by the radiation leaving it. The emission spectrum of a blackbody can be obtained by analyzing the light radiating from the hole. Electromagnetic waves emitted by a blackbody are called blackbody radiation .
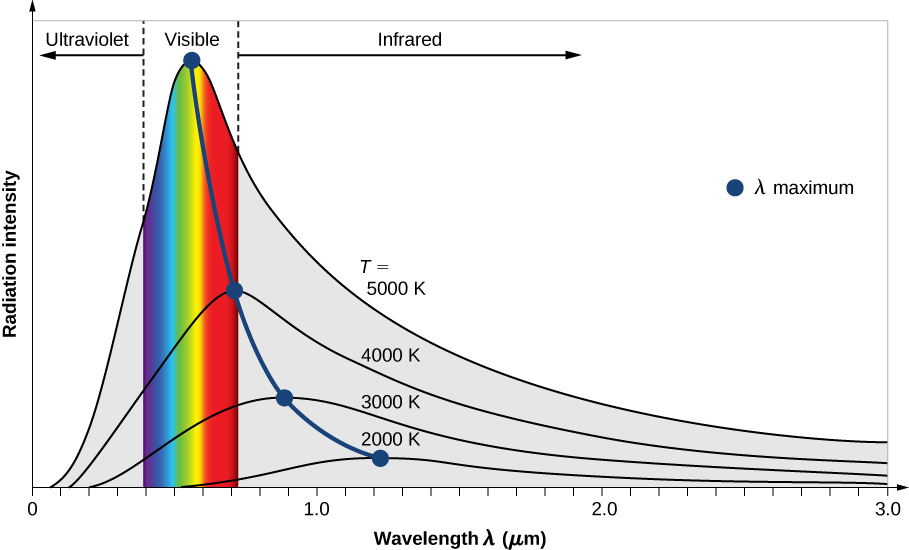
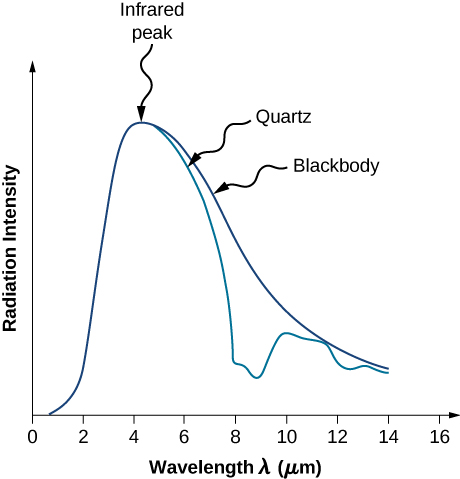
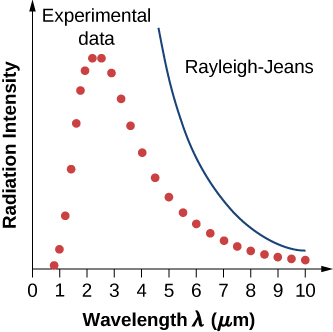
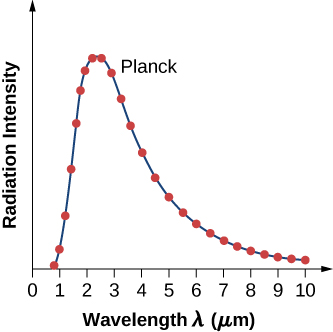
The intensity \(I(\lambda, T)\) of blackbody radiation depends on the wavelength \(\lambda\) of the emitted radiation and on the temperature T of the blackbody (Figure \(\PageIndex{2}\)). The function \(I(\lambda, T)\) is the power intensity that is radiated per unit wavelength; in other words, it is the power radiated per unit area of the hole in a cavity radiator per unit wavelength. According to this definition, \(I(\lambda, T)d\lambda\) is the power per unit area that is emitted in the wavelength interval from \(\lambda\) to \(\lambda + d\lambda\). The intensity distribution among wavelengths of radiation emitted by cavities was studied experimentally at the end of the nineteenth century. Generally, radiation emitted by materials only approximately follows the blackbody radiation curve (Figure \(\PageIndex{3}\)); however, spectra of common stars do follow the blackbody radiation curve very closely.
Two important laws summarize the experimental findings of blackbody radiation: Wien’s displacement law and Stefan’s law . Wien’s displacement law is illustrated in Figure \(\PageIndex{2}\) by the curve connecting the maxima on the intensity curves. In these curves, we see that the hotter the body, the shorter the wavelength corresponding to the emission peak in the radiation curve. Quantitatively, Wien’s law reads
\[\lambda_{max}T = 2.898 \times 10^{-3} m \cdot K \label{Wien} \]
where \(\lambda_{max}\) is the position of the maximum in the radiation curve. In other words, \(\lambda_{max}\) is the wavelength at which a blackbody radiates most strongly at a given temperature T . Note that in Equation \ref{Wien}, the temperature is in kelvins. Wien’s displacement law allows us to estimate the temperatures of distant stars by measuring the wavelength of radiation they emit.
Example \(\PageIndex{1}\): Temperatures of Distant Stars
On a clear evening during the winter months, if you happen to be in the Northern Hemisphere and look up at the sky, you can see the constellation Orion (The Hunter). One star in this constellation, Rigel, flickers in a blue color and another star, Betelgeuse, has a reddish color, as shown in Figure \(\PageIndex{4}\). Which of these two stars is cooler, Betelgeuse or Rigel?
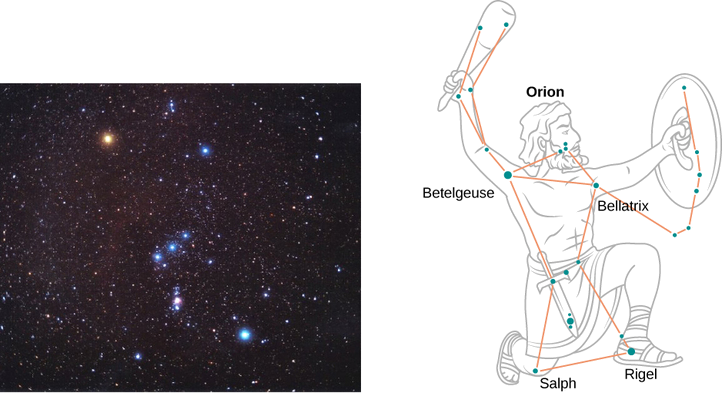
We treat each star as a blackbody. Then according to Wien’s law, its temperature is inversely proportional to the wavelength of its peak intensity. The wavelength \(\lambda_{max}^{(blue)}\) of blue light is shorter than the wavelength \(\lambda_{max}^{(red)}\) of red light. Even if we do not know the precise wavelengths, we can still set up a proportion.
Writing Wien’s law for the blue star and for the red star, we have
\[\begin {align*} \lambda_{max}^{(red)}T_{(red)} &= 2.898 \times 10^{-3} m \cdot K \\[5pt] &= \lambda_{max}^{(blue)} T_{(blue)} \end{align*} \nonumber \]
When simplified, this gives
\[T_{(red)} = \dfrac{\lambda_{max}^{(blue)}}{\lambda_{max}^{(red)}}T_{(blue)} < T_{(blue)} \nonumber \]
Therefore, Betelgeuse is cooler than Rigel.
Significance
Note that Wien’s displacement law tells us that the higher the temperature of an emitting body, the shorter the wavelength of the radiation it emits. The qualitative analysis presented in this example is generally valid for any emitting body, whether it is a big object such as a star or a small object such as the glowing filament in an incandescent lightbulb.
Exercise \(\PageIndex{1}\)
The flame of a peach-scented candle has a yellowish color and the flame of a Bunsen’s burner in a chemistry lab has a bluish color. Which flame has a higher temperature?
Bunsen’s burner
The second experimental relation is Stefan’s law, which concerns the total power of blackbody radiation emitted across the entire spectrum of wavelengths at a given temperature. In \(\PageIndex{2}\) , this total power is represented by the area under the blackbody radiation curve for a given T . As the temperature of a blackbody increases, the total emitted power also increases. Quantitatively, Stefan’s law expresses this relation as
\[P(T) = \sigma AT^4 \nonumber \]
where \(A\) is the surface area of a blackbody, \(T\) is its temperature (in kelvins), and \(σ\) is the Stefan–Boltzmann constant , \(\sigma = 5.670 \times 10^{-8} W/(m^2 \cdot K^4)\). Stefan’s law enables us to estimate how much energy a star is radiating by remotely measuring its temperature.
Example \(\PageIndex{2}\): Power Radiated by Stars
A star such as our Sun will eventually evolve to a “red giant” star and then to a “white dwarf” star. A typical white dwarf is approximately the size of Earth, and its surface temperature is about \(2.5 \times 10^4 K\). A typical red giant has a surface temperature of \(3.0 \times 10^3 K\) and a radius ~100,000 times larger than that of a white dwarf. What is the average radiated power per unit area and the total power radiated by each of these types of stars? How do they compare?
If we treat the star as a blackbody, then according to Stefan’s law, the total power that the star radiates is proportional to the fourth power of its temperature. To find the power radiated per unit area of the surface, we do not need to make any assumptions about the shape of the star because P / A depends only on temperature. However, to compute the total power, we need to make an assumption that the energy radiates through a spherical surface enclosing the star, so that the surface area is \(A = 4\pi R^2\), where R is its radius.
A simple proportion based on Stefan’s law gives
\[\dfrac{P_{dwarf}/A_{dwarf}}{P_{giant}/A_{giant}} = \dfrac{\sigma T_{dwarf}^4}{\sigma T_{giant}^4} = \left(\dfrac{T_{dwarf}}{T_{giant}}\right)^4 = 4820 \label{6.5} \]
The power emitted per unit area by a white dwarf is about 5000 times that the power emitted by a red giant. Denoting this ratio by \(a=4.8×10^3\), Equation \ref{6.5} gives
\[\dfrac{P_{dwarf}}{P_{giant}} = \alpha \dfrac{A_{dwarf}}{A_{giant}} = \alpha \dfrac{4\pi R_{dwarf}^2}{4\pi R_{giant}^2} = \alpha \left( \dfrac{R_{dwarf}}{R_{giant}} \right)^2 = 4.8 \times 10^{−7} \nonumber \]
We see that the total power emitted by a white dwarf is a tiny fraction of the total power emitted by a red giant. Despite its relatively lower temperature, the overall power radiated by a red giant far exceeds that of the white dwarf because the red giant has a much larger surface area. To estimate the absolute value of the emitted power per unit area, we again use Stefan’s law. For the white dwarf, we obtain
\[\dfrac{P_{dwarf}}{A_{dwarf}} = \sigma T_{dwarf}^4 = 5.670 \times 10^{-8} \dfrac{W}{m^2 \cdot K^4} (2.5 \times 10^4 \, K)^4 = 2.2 \times 10^{10} \dfrac{W}{m^2} \label{6.7} \]
The analogous result for the red giant is obtained by scaling the result for a white dwarf:
\[\dfrac{P_{giant}}{A_{giant}} = \dfrac{2.2 \times 10^{10}}{4.82 \times 10^3} \dfrac{W}{m^2} = 4.56 \times 10^6 \dfrac{W}{m^2} \cong 4.6 \times 10^{-6} \dfrac{W}{m^2} \nonumber \]
To estimate the total power emitted by a white dwarf, in principle, we could use Equation \ref{6.7}. However, to find its surface area, we need to know the average radius, which is not given in this example. Therefore, the solution stops here. The same is also true for the red giant star.
Exercise \(\PageIndex{2A}\)
An iron poker is being heated. As its temperature rises, the poker begins to glow—first dull red, then bright red, then orange, and then yellow. Use either the blackbody radiation curve or Wien’s law to explain these changes in the color of the glow.
The wavelength of the radiation maximum decreases with increasing temperature.
Exercise \(\PageIndex{2B}\)
Suppose that two stars, \(α\) and \(β\), radiate exactly the same total power. If the radius of star \(α\) is three times that of star \(β\), what is the ratio of the surface temperatures of these stars? Which one is hotter?
\(T_{\alpha}/T_{\beta} = 1/\sqrt{3} \cong 0.58\), so the star \(\beta\) is hotter.
The term “blackbody” was coined by Gustav R. Kirchhoff in 1862. The blackbody radiation curve was known experimentally, but its shape eluded physical explanation until the year 1900. The physical model of a blackbody at temperature T is that of the electromagnetic waves enclosed in a cavity (Figure \(\PageIndex{1}\)) and at thermodynamic equilibrium with the cavity walls. The waves can exchange energy with the walls. The objective here is to find the energy density distribution among various modes of vibration at various wavelengths (or frequencies). In other words, we want to know how much energy is carried by a single wavelength or a band of wavelengths. Once we know the energy distribution, we can use standard statistical methods (similar to those studied in a previous chapter) to obtain the blackbody radiation curve, Stefan’s law, and Wien’s displacement law. When the physical model is correct, the theoretical predictions should be the same as the experimental curves.
In a classical approach to the blackbody radiation problem, in which radiation is treated as waves (as you have studied in previous chapters), the modes of electromagnetic waves trapped in the cavity are in equilibrium and continually exchange their energies with the cavity walls. There is no physical reason why a wave should do otherwise: Any amount of energy can be exchanged, either by being transferred from the wave to the material in the wall or by being received by the wave from the material in the wall. This classical picture is the basis of the model developed by Lord Rayleigh and, independently, by Sir James Jeans. The result of this classical model for blackbody radiation curves is known as the Rayleigh–Jeans law . However, as shown in Figure \(\PageIndex{5}\), the Rayleigh–Jeans law fails to correctly reproduce experimental results. In the limit of short wavelengths, the Rayleigh–Jeans law predicts infinite radiation intensity, which is inconsistent with the experimental results in which radiation intensity has finite values in the ultraviolet region of the spectrum. This divergence between the results of classical theory and experiments, which came to be called the ultraviolet catastrophe , shows how classical physics fails to explain the mechanism of blackbody radiation.
The blackbody radiation problem was solved in 1900 by Max Planck . Planck used the same idea as the Rayleigh–Jeans model in the sense that he treated the electromagnetic waves between the walls inside the cavity classically, and assumed that the radiation is in equilibrium with the cavity walls. The innovative idea that Planck introduced in his model is the assumption that the cavity radiation originates from atomic oscillations inside the cavity walls, and that these oscillations can have only discrete values of energy. Therefore, the radiation trapped inside the cavity walls can exchange energy with the walls only in discrete amounts. Planck’s hypothesis of discrete energy values, which he called quanta , assumes that the oscillators inside the cavity walls have quantized energies . This was a brand new idea that went beyond the classical physics of the nineteenth century because, as you learned in a previous chapter, in the classical picture, the energy of an oscillator can take on any continuous value. Planck assumed that the energy of an oscillator (\(E_n\)) can have only discrete, or quantized, values:
\[E_n = nhf, \, where \, n = 1,2,3, \ldots \label{eq30} \]
In Equation \ref{eq30}, \(f\) is the frequency of Planck’s oscillator. The natural number \(n\) that enumerates these discrete energies is called a quantum number. The physical constant \(h\) is called Planck’s constant :
\[h = 6.626 \times 10^{-34} J \cdot s = 4.136 \times 10^{-15} eV \cdot s \label{6.10} \]
Each discrete energy value corresponds to a quantum state of a Planck oscillator . Quantum states are enumerated by quantum numbers. For example, when Planck’s oscillator is in its first \(n 1\) quantum state, its energy is \(E_1 = hf\); when it is in the \(n = 2\) quantum state, its energy is \(E_2 = 2hf\); when it is in the \(n = 3\) quantum state, \(E_3 = 3hf\); and so on.
Note that Equation \ref{eq30} shows that there are infinitely many quantum states, which can be represented as a sequence { hf , 2 hf , 3 hf ,…, ( n – 1) hf , nhf , ( n + 1) hf ,…}. Each two consecutive quantum states in this sequence are separated by an energy jump, \(\delta E = hf\). An oscillator in the wall can receive energy from the radiation in the cavity (absorption), or it can give away energy to the radiation in the cavity (emission). The absorption process sends the oscillator to a higher quantum state, and the emission process sends the oscillator to a lower quantum state. Whichever way this exchange of energy goes, the smallest amount of energy that can be exchanged is hf . There is no upper limit to how much energy can be exchanged, but whatever is exchanged must be an integer multiple of hf . If the energy packet does not have this exact amount, it is neither absorbed nor emitted at the wall of the blackbody.
PLANCK’S QUANTUM HYPOTHESIS
Planck’s hypothesis of energy quanta states that the amount of energy emitted by the oscillator is carried by the quantum of radiation, \(\Delta E\):
\[\Delta E = hf \label{planck} \]
Recall that the frequency of electromagnetic radiation is related to its wavelength and to the speed of light by the fundamental relation \(f\lambda = c\). This means that we can express Equation \ref{6.10} equivalently in terms of wavelength \(\lambda\). When included in the computation of the energy density of a blackbody, Planck’s hypothesis gives the following theoretical expression for the power intensity of emitted radiation per unit wavelength:
\[I(\lambda, T) = \dfrac{2\pi hc^2}{\lambda^5} \dfrac{1}{e^{hc/\lambda k_BT} - 1} \label{6.11} \]
where c is the speed of light in vacuum and kBkB is Boltzmann’s constant, \(k_B = 1.380 \times 10^{-23} J/K\). The theoretical formula expressed in Equation \ref{6.11} is called Planck’s blackbody radiation law . This law is in agreement with the experimental blackbody radiation curve (Figure \(\PageIndex{2}\)). In addition, Wien’s displacement law and Stefan’s law can both be derived from Equation \ref{6.11}. To derive Wien’s displacement law, we use differential calculus to find the maximum of the radiation intensity curve \(I(\lambda, T)\). To derive Stefan’s law and find the value of the Stefan–Boltzmann constant, we use integral calculus and integrate \(I(\lambda, T)\) to find the total power radiated by a blackbody at one temperature in the entire spectrum of wavelengths from \(\lambda = 0\) to \(\lambda = \infty\). This derivation is left as an exercise later in this chapter.
Example \(\PageIndex{3}\): Planck’s Quantum Oscillator
A quantum oscillator in the cavity wall in Figure \(\PageIndex{1}\) is vibrating at a frequency of \(5.0 \times 10^{14} Hz\). Calculate the spacing between its energy levels.
Energy states of a quantum oscillator are given by Equation \ref{eq30}. The energy spacing \(\Delta E\) is obtained by finding the energy difference between two adjacent quantum states for quantum numbers n + 1 and n .
We can substitute the given frequency and Planck’s constant directly into the equation:
\[\begin {align*} \Delta E &= E_{n + 1} − En = (n + 1)hf − nhf \\[5pt] &= hf \\[5pt] &= (6.626 \times 10^{−34} \, J \cdot s)(5.0 \times 10^{14} \, Hz) \\[5pt] &= 3.3 \times 10^{− 19} \, J \end{align*} \nonumber \]
Note that we do not specify what kind of material was used to build the cavity. Here, a quantum oscillator is a theoretical model of an atom or molecule of material in the wall.
Exercise \(\PageIndex{3}\)
A molecule is vibrating at a frequency of \(5.0 \times 10^{14}\, Hz\). What is the smallest spacing between its vibrational energy levels?
\(3.3 \times 10^{-19} J\)
Example \(\PageIndex{4}\): Quantum Theory Applied to a Classical Oscillator
A 1.0-kg mass oscillates at the end of a spring with a spring constant of 1000 N/m. The amplitude of these oscillations is 0.10 m. Use the concept of quantization to find the energy spacing for this classical oscillator. Is the energy quantization significant for macroscopic systems, such as this oscillator?
We use Equation \ref{planck} as though the system were a quantum oscillator, but with the frequency f of the mass vibrating on a spring. To evaluate whether or not quantization has a significant effect, we compare the quantum energy spacing with the macroscopic total energy of this classical oscillator.
For the spring constant, \(k = 1.0 \times 10^3 N/m\), the frequency f of the mass, \(m = 1.0 \, kg\), is
\[f = \dfrac{1}{2\pi} \sqrt{\dfrac{k}{m}} = \dfrac{1}{2\pi} \sqrt{\dfrac{1.0 \times 10^3 N/m}{1.0 \, kg}} \simeq 5.0 \, Hz \nonumber \]
The energy quantum that corresponds to this frequency is
\[\Delta E = hf = (6.626 \times 10^{-34} J \cdot s)(5.0 \, Hz) = 3.3 \times 10^{-33} J \nonumber \]
When vibrations have amplitude \(A = 0.10 \, m\), the energy of oscillations is
\[E = \dfrac{1}{2} kA^2 = \dfrac{1}{2}(1000 \, N/m)(0.1 \, m)^2 = 5.0 \, J \nonumber \]
Thus, for a classical oscillator, we have \(\Delta E/E \approx 10^{-34}\). We see that the separation of the energy levels is immeasurably small. Therefore, for all practical purposes, the energy of a classical oscillator takes on continuous values. This is why classical principles may be applied to macroscopic systems encountered in everyday life without loss of accuracy.
Exercise \(\PageIndex{4}\)
Would the result in Example \(\PageIndex{4}\) be different if the mass were not 1.0 kg but a tiny mass of 1.0 µ g, and the amplitude of vibrations were 0.10 µ m?
No, because then \(\Delta E /E \approx 10^{-21}\)
When Planck first published his result, the hypothesis of energy quanta was not taken seriously by the physics community because it did not follow from any established physics theory at that time. It was perceived, even by Planck himself, as a useful mathematical trick that led to a good theoretical “fit” to the experimental curve. This perception was changed in 1905 when Einstein published his explanation of the photoelectric effect, in which he gave Planck’s energy quantum a new meaning: that of a particle of light.
Blackbody Radiation
Introduction.
Newton's laws of motion and universal gravitation, the laws of conservation of energy and momentum, the laws of thermodynamics, and Maxwell's equations for electricity and magnetism were all more or less nearly complete at the end of the 19th century. They describe a universe consisting of bodies moving with clockwork predictability on a stage of absolute space and time. They were used to create the machines that launched two waves of industrial revolution — the first one powered by steam and the second one powered by electric current. They can be used to deliver spacecraft to the ends of the solar system with hyper-pinpoint accuracy. They are mathematically consistent in the sense that no one rule would ever violate another. They agree with reality to a high degree of accuracy as tested in experiment after experiment.
At the end of the 19th century, physics appeared to be at an apex. Several people are reported to have said something like this
There is nothing new to be discovered in physics now. All that remains is more and more precise measurement.
This has been attributed to William Thomson, Lord Kelvin (1824–1907) in an address to the British Association for the Advancement of Science in 1900, but I haven't been able to find the primary source. A similar statement was made twice by the German-American scientist Albert Michelson (1852–1931) as was discussed earlier in this book . It is often reported that Michelson got the idea from Kelvin, but there is little evidence to back this claim up.
At the turn of the century, Kelvin wasn't saying that physics was finished. In fact, I think he was saying quite the opposite. There were two clouds hanging over 19th century physics.
The beauty and clearness of the dynamical theory, which asserts heat and light to be modes of motion, is at present obscured by two clouds. I. The first came into existence with the undulatory theory of light, and was dealt with by Fresnel and Dr Thomas Young; it involved the question, How could the Earth move through an elastic solid, such as essentially is the luminiferous ether? II. The second is the Maxwell-Boltzmann doctrine regarding the partition of energy.
Kelvin is describing two problems with the physics of his time. They are highly technical in nature and not something you could easily describe to your grandmother (unless she had some training in physics). The first one refers to the now discredited theory of the luminiferous ether. The second one describes the inability of electromagnetic theory to adequately predict the characteristics of thermal radiation.
In essence, the first argument went like this. Light is a wave. Waves require a medium. The medium for light was called the luminiferous ether. It must be extremely rigid (since light travels so quickly) and extremely tenuous (since we can't detect its drag). Rigid and tenuous are adjectives that are incompatible (strong yet soft). 19th century physics cannot handle this, therefore 19th century physics is in trouble.
The ray of sunshine that dispersed this dark cloud was the theory of relativity devised by Albert Einstein. The major revelations of this theory were that there is no ether, there is no absolute space, there is no absolute time, mass is not conserved, energy is not conserved, and nothing travels faster than light. For a while, this was the most revolutionary theory in all of physics.
The second dark cloud identified by Kelvin is the subject of the rest of this section and (essentially) most of the rest of this book. I won't breeze through it like I did with the first dark cloud, but I will tell you this, the solution to the problem Kelvin called "the Maxwell-Boltzmann doctrine" lead to the most revolutionary theory in all of physics — quantum mechanics . The major revelations of this theory are that all things are both particles and waves at the same time and that nothing can be predicted or known with absolute certainty.
The arrival of these two revolutionary theories divided physics up into two domains. All theories developed before the arrival of relativity and quantum mechanics and any work derived from them are called classical physics . All theories derived from the basic principles of relativity and quantum mechanics are called modern physics . The word modern was chosen since the foundations of these theories were laid in the first three decades of the 20th century. This the era of modern architecture, modern dance, modern jazz, and modern literature. Modern technologies were starting to appear like electric lights, toasters, refrigerators, sewing machines, radios, telephones, movies, phonograph records, airplanes, automobiles, subways, elevators, skyscrapers, synthetic dyes, nylon, celluloid, machine guns, dynamite, aspirin, and psychology. The early 20th century was filled with revolutionary ideas and inventions. Life now seems unimaginable without them. Modern physics was just one aspect of the modern era.
the failures of classical physics
Frayed edges on the tapestry of classical physics leading to modern physics…
- no apparent motion through the ether
- precession of the perihelion of mercury
- blackbody radiation and the ultraviolet catastrophe
- photoelectric effect
- discrete atomic spectra and the problem of how atoms manage to exist
- radioactive decay
What we know about blackbody radiation
- the shape of the distribution
- the peak shifts according to Wien's law
- the total power output is described by the Stefan-Boltzmann law
John Strutt, Lord Rayleigh and James Jeans Ultraviolet Catastrophe
A blackbody is an idealized object which absorbs and emits all frequencies. Classical physics can be used to derive an equation which describes the intensity of blackbody radiation as a function of frequency for a fixed temperature — the result is known as the Rayleigh-Jeans law. Although the Rayleigh-Jeans law works for low frequencies, it diverges as f 2 ; this divergence for high frequencies is called the ultraviolet catastrophe.
Wilhelm Wien Infrared Catastrophe
In 1896 Wien derived a distribution law of radiation. Planck, who was a colleague of Wien's when he was carrying out this work, later, in 1900, based quantum theory on the fact that Wien's law, while valid at high frequencies, broke down completely at low frequencies.
energy is quantized
Max Planck (1858–1947) Germany. On the Law of Distribution of Energy in the Normal Spectrum. Max Planck. Annalen der Physik 4 (1901): 553.
Proposition…
E = hf
And also (from Einstein later, I think)…
| = | |
| λ |
Let's try to derive the blackbody spectrum.
Planck's law is a formula for the spectral radiance of an object at a given temperature as a function of frequency ( L f ) or wavelength ( L λ ). It has dimensions of power per solid angle per area per frequency or power per solid angle per area per wavelength. (Yuck!)
| = | 2 | 1 | ⎡ ⎢ ⎣ | W | ⎤ ⎥ ⎦ | ||
| / − 1 | sr m Hz | ||||||
| = | 2 | 1 | ⎡ ⎢ ⎣ | W | ⎤ ⎥ ⎦ | ||
| λ | /λ − 1 | sr m m | |||||
When these functions are multiplied by the total solid angle of a sphere (4π steradian) we get the spectral irradiance ( E f or E λ ). This function describes the power per area per frequency or the power per area per wavelength.
| = | 8π | 1 | ⎡ ⎢ ⎣ | W | ⎤ ⎥ ⎦ | ||
| / − 1 | m Hz | ||||||
| = | 8π | 1 | ⎡ ⎢ ⎣ | W | ⎤ ⎥ ⎦ | ||
| λ | /λ − 1 | m m | |||||
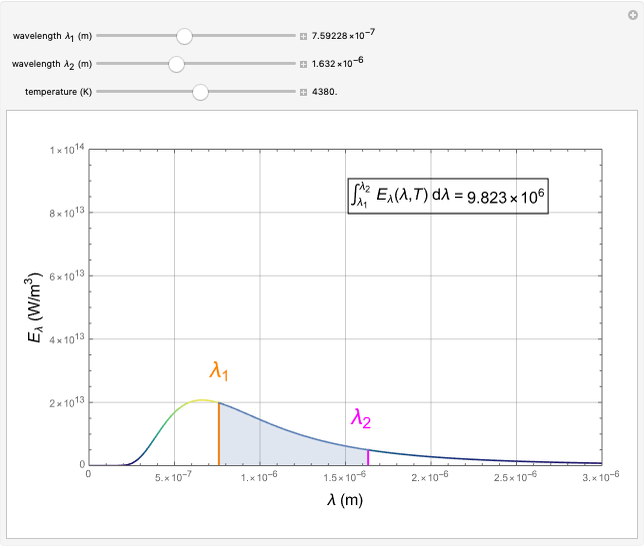
When either of these functions is integrated over all possible values from zero to infinity, the result is the irradiance or the power per area.
| ∞ | ∞ | |||||
| = | ⌠ ⎮ ⌡ | dλ = | ⌠ ⎮ ⌡ | dλ | = | |
| 0 | 0 |
Trust me, the solution looks like this…
| = | 2π | ||
| 15 |
The pile of constants in front of the temperature is known as Stefan's constant .
| σ = |
Multiplying the irradiance by the area gives us the essence of the Stefan-Boltzmann law .
Apply the first derivative test to the wavelength form of Planck's law to determine the peak wavelength as a function of temperature.
where x is the solution of the transcendental equation…
x = 4.965 114 231 744 276 303 698 759 131 322 893 944 055 584 986 797 250 972 814… Combine all the constants together into Wien's constant (or Wien's wavelength constant to distinguish it from the next constant)…
and we get Wien's displacement law (or Wien's wavelength displacement law to distinguish it from the variant that follows it)…
Repeat the first derivative test but this time use the frequency form of Planck's law to determine the peak frequency as a function of temperature.
Trust me again, the solution looks like this…
x = 2.821 439 372 122 078 893 403 191 330 294 485 195 345 881 744 073 114 092 279… Combine all the constants together into Wien's frequency constant …
and we get Wien's frequency displacement law … f max = b ′ T Discuss effective temperature . No object emits a mathematically perfect blackbody radiation spectrum. There will always be lumps in the curve. Set the area under intensity-wavelength curve for a real source of radiation equal to the area under the intensity-wavelength curve for an ideal blackbody and solve for temperature. The effective temperature of an object is the temperature of an ideal blackbody that would radiate energy at the same rate as the real body. Different parts of the Sun are at different temperatures. When combined, the Sun has an effective temperature of 5,772 K . biographical resource Hierzu ist es notwendig, U N nicht als eine stetige, unbeschränkt teilbare, sondern als eine discrete, aus einer ganzen Zahl von endlichen gleichen Teilen zusammengesetzte Grösse aufzufassen. Moreover, it is necessary to interpret U N [the total energy of a blackbody radiator] not as a continuous, infinitely divisible quantity, but as a discrete quantity composed of an integral number of finite equal parts. the whole procedure was an act of despair because a theoretical interpretation had to be found at any price, no matter how high that might be. Nobel Prize in Physics 1918 Presentation Speech by A.G. Ekstrand, President of the Royal Swedish Academy of Sciences Ladies and Gentlemen. The Royal Academy of Sciences has decided to award the Nobel Prize for Physics, for the year 1918, to Geheimrat Dr. Max Planck, professor at Berlin University, for his work on the establishment and development of the theory of elementary quanta. From the time that Kirchhoff enunciated the principle "that the intensity of radiation from a black body is dependent only upon the wavelength of the radiation and the temperature of the radiating body, a relationship worth while investigation", the theoretical treatment of the radiation problem has provided a rich, fertile source of fresh discoveries. It is only necessary here to recall the fertile Doppler principle, and further, the transformation of our - concept of the nature of light as seen now in the electromagnetic theory of light formulated by that great man, Maxwell, the deduction of Stefan's Law by Boltzmann, and Wien's Law of Radiation. Since it was clear, however, that this did not correspond exactly with the reality, but was rather, like a radiation law propounded by Lord Rayleigh, only a special case of the general radiation law, Planck sought for, and in 1900 found, a mathematical formula for the latter, which he derived theoretically later on. The formula contained two constants; one, as was demonstrated, gave the number of molecules in a gram molecule of matter. Planck was also the first to succeed in getting, by means of the said relation, a highly accurate value for the number in question, the so-called Avogadro constant. The other constant, the so-called Planck constant, proved, as it turned out, to be of still greater significance, perhaps, than the first. The product hν, where ν is the frequency of vibration of a radiation, is actually the smallest amount of heat which can be radiated at the vibration frequency ν. This theoretical conclusion stands in very sharp opposition to our earlier concept of the radiation phenomenon. Experience had to provide powerful confirmation, therefore, before Planck's radiation theory could be accepted. In the meantime this theory has had unheard-of success….
There's h and then there's ℏ.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||






















IMAGES
VIDEO
COMMENTS
In Planck's words, "I considered the [quantum hypothesis] a purely formal assumption, and I did not give it much thought except for this: that I had obtained a positive result under any circumstances and at whatever cost." ... In contrast to Planck's and Einstein's formulas, Bohr's formula referred explicitly and categorically to energy levels ...
The wavelength of the emitted radiation is inversely proportional to its frequency, or λ = c /ν. The value of Planck's constant is defined as 6.62607015 × 10 −34 joule∙second. For a blackbody at temperatures up to several hundred degrees, the majority of the radiation is in the infrared radiation region of the electromagnetic spectrum.
Planck's hypothesis of discrete energy values, which he called quanta, ... The theoretical formula expressed in Equation \ref{6.11} is called Planck's blackbody radiation law. This law is in agreement with the experimental blackbody radiation curve (Figure \(\PageIndex{2}\)). In addition, Wien's displacement law and Stefan's law can ...
Planck's constant is currently calculated by scientists to be 6.62607015 x 10 -34 joule-seconds. In 1900, Planck identified his game-changing constant by describing how the smallest bits of matter release energy in discrete bundles called quanta, essentially placing the "quanta" in quantum mechanics. To learn more about the quantum theory ...
The formula contained two constants; one, as was demonstrated, gave the number of molecules in a gram molecule of matter. Planck was also the first to succeed in getting, by means of the said relation, a highly accurate value for the number in question, the so-called Avogadro constant. ... Planck's notion of a natural unit system — one ...
The Planck radiation formula is an example of the distribution of energy according to Bose-Einstein statistics.The above expressions are obtained by multiplying the density of states in terms of frequency or wavelength times the photon energy times the Bose-Einstein distribution function with normalization constant A=1.. To find the radiated power per unit area from a surface at this ...
Wien-Planck's formula. In 1900, Planck gave two different derivations of another, new formula that matched the latest experimental data. 13.1.2 Planck's discovery In 1900, Max Planck was 42 years old and had an established name in thermodynamics. In particular, it was he who stated the Second Law of Thermodynamics in the for well-known
Initially, his hypothesis explained only one set of experimental data—blackbody radiation. If quantization were observed for a large number of different phenomena, then quantization would become a law. In time, a theory might be developed to explain that law. As things turned out, Planck's hypothesis was the seed from which modern physics grew.
The mathematics implied that the energy given off by a blackbody was not continuous, but given off at certain specific wavelengths, in regular increments. If Planck assumed that the energy of blackbody radiation was in the form. E = nhν (1.2.7) (1.2.7) E = n h ν. where n n is an integer (now called a quantum number), then he could explain ...
Through this equation, Planck's probability factor can be more clearly understood. Each frequency of light provides a specific quantized amount of energy. Low frequency light, associated with longer wavelengths would provide a smaller amount of energy, while high frequency light, associated with shorter wavelengths, would provide a larger ...
λmaxT = 2.898 × 10−3m ⋅ K λ max T = 2.898 × 10 −3 m · K. 6.1. where λmax λ max is the position of the maximum in the radiation curve. In other words, λmax λ max is the wavelength at which a blackbody radiates most strongly at a given temperature T. Note that in Equation 6.1, the temperature is in kelvins.
This law is named after a German theoretical physicist Max Planck, who proposed it in 1900. Planck's law is a pioneering result of modern physics and quantum theory. Planck's hypothesis that energy is radiated and absorbed in discrete "quanta" (or energy packets) precisely matched the observed patterns of blackbody radiation and ...
Snapshot 1: quantum mechanics: Planck's law is applied to the energy quantization; the resulting curve follows the blackbody radiation prediction. ... The first theoretical analysis resulted in the Rayleigh-Jeans formula, based on the classical hypothesis that the energies of the photons inside the blackbody could have any values from an ...
Summary. Planck immediately set about attempting to understand the significance of his formula for black-body radiation. He began by using Boltzmann's procedure in statistical mechanics, an approach he had previously rejected, but then adopted empirically a definition of the entropy of the oscillators which introduced the concept of quantisation.
Well, there's one simple way: ∂. 2 S / ∂ U. 2 = − k . U ( hf + U ) (I've put in the correct values for the two parameters here: k is Boltzmann's constant, necessary to match the low frequency equation, the constant β in Wien's formula turns out to be Planck's constant divided by Boltzmann's constant, β = h / k .)
Planck's Hypothesis. Assumptions used: 1. The particles/oscillators near the surface of the blackbody which emits the blackbody radiation can only have discrete values of energy, E n: E n =nhf, where n is a positive interger, f is the frequency of the oscillating particle, h is the Planck's constant. Particles can only have discrete values ...
Planck's Hypothesis. In 1900 Max Planck proposed a formula for the intensity curve which did fit the experimental data quite well. He then set out to find a set of assumptions -- a model -- that would produce his formula. Instead of allowing energy to be continuously distributed among all frequencies, Planck's model required that the energy in ...
Planck's quantum theory has the following presuppositions: Discrete packets or bundles of energy are emitted or absorbed intermittently by matter. Quantum is the name given to the smallest bundle or packet of energy. Using light as an example, a photon is a quantum of light. The absorb quantum energy is proportional to the radiation frequency.
Planck's hypothesis establishes some postulates, which are as follows: ... Planck's Quantum Formula. Planck's Quantum Formula, also sometimes referred to as Planck's equation provides a quantitative description of the amount of spectral radiance at a specific frequency radiated by a Black body in the equilibrium state. As per the given ...
Planck's law is a pioneering result of modern physics and quantum theory.Planck's hypothesis that energy is radiated and absorbed in discrete "quanta" (or energy packets) precisely matched the observed patterns of blackbody radiation and resolved the ultraviolet catastrophe.. Using this hypothesis, Planck showed that the spectral radiance of a body for frequency ν at absolute ...
Quantizing Electrons in the Radiator. In 1900, the German physicist Max Planck (1858-1947) explained the ultraviolet catastrophe by proposing that the energy of electromagnetic waves is quantized rather than continuous. This means that for each temperature, there is a maximum intensity of radiation that is emitted in a blackbody object, corresponding to the peaks in Figure 1.2.1 , so the ...
What is Planck's Equation? Max Planck discovered a theory that energy is transferred in the form of chunks called quanta, assigned as h. The variable h holds the constant value of 6.63 x 10-34 J.s based on the International System of Units, and the variable describes the frequency in s-1. Planck's law helps us calculate the energy of photons when their frequency is known.
Planck's constant relates photon energy to frequency. Planck's constant is one of the fundamental constants in physics that sets the scale for quantum effects. It is the proportionality constant that relates the energy of a photon to the frequency of its corresponding electromagnetic wave. The symbol for Planck's constant is h.It is also known as the Planck constant.