Clinical trial background and update presentation: The all-in-one guide
Perfect for patients, researchers, and healthcare professionals alike.
Raja Bothra
Building presentations

Welcome to the world of medical research and innovation, the ability to convey complex information clearly and effectively is paramount.
Clinical trials, at the heart of medical advancements, require meticulous presentation to communicate their significance and findings accurately.

What are clinical trials?
Clinical trials are meticulously designed studies that aim to assess the effectiveness and safety of medical treatments, drugs, or procedures. These trials are an indispensable part of the medical research process, guiding us towards breakthroughs in healthcare.
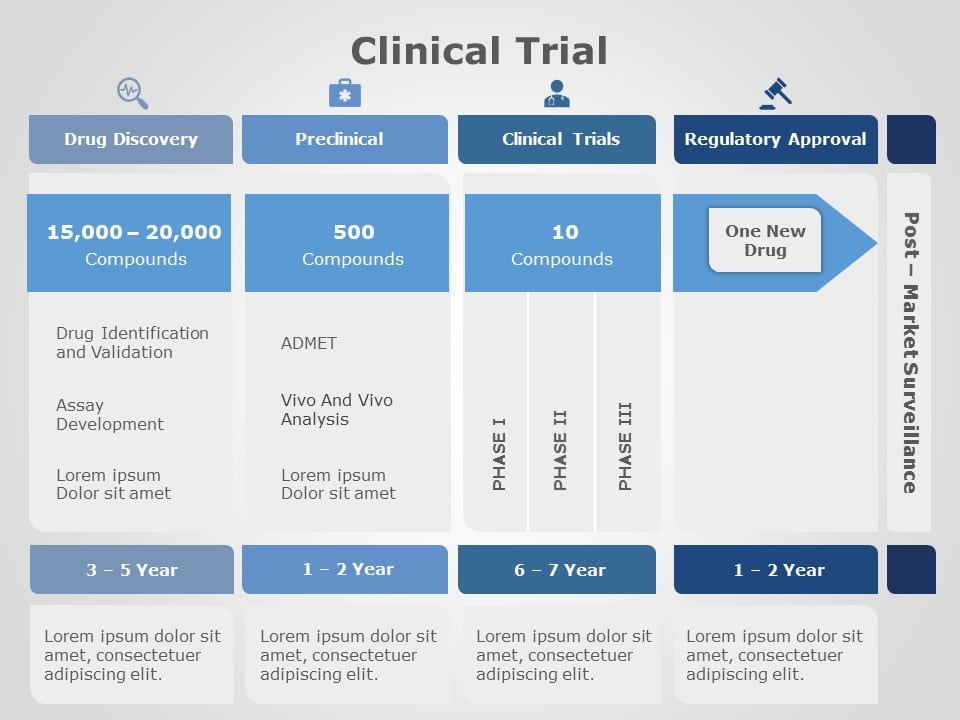
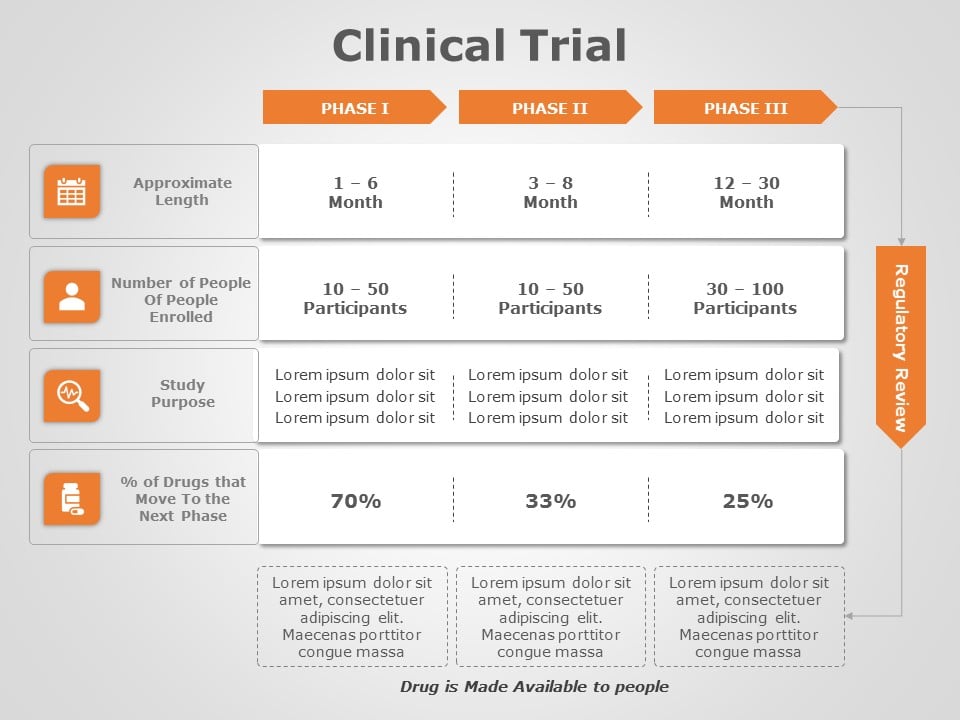
Clinical trial phases
Before diving into the intricacies of creating an outstanding clinical trial presentation, it's crucial to understand the phases of clinical trials.
- Pre-clinical phase : This initial phase involves laboratory testing and research on animals. The focus here is on the safety and efficacy of potential treatments.
- Phase I : The first human testing phase, primarily evaluating safety and dosage.
- Phase II : Expanding the scope, this phase explores the treatment's effectiveness and side effects.
- Phase III : Large-scale trials to confirm efficacy and monitor side effects.
- Phase IV : Post-approval monitoring to ensure ongoing safety and efficacy.
What are the advantages of clinical trials presentation?
Clinical trial presentations are not just about data and statistics; they are a crucial tool for disseminating information effectively. Here are some key advantages:
- Clear communication : A well-structured presentation simplifies complex data, making it accessible to various stakeholders.
- Transparency : Presentations foster trust by openly sharing research methodologies, results, and potential risks.
- Impactful decisions : Stakeholders can make informed decisions based on presentation insights.
- Continual improvement : Feedback from presentations aids in refining research methodologies and treatments.
Now that we've established the importance of clinical trial presentations, let's explore how to structure an effective one.
Here is a guide to clinical trial investment decision presentation
How to structure an effective clinical trial background and update presentation
An effective clinical trial background and update presentation should be well-organized, informative, and tailored to your audience. To ensure your message resonates and your presentation captivates, follow this structured approach:
Introduction
Begin your presentation with a compelling introduction that sets the stage for what's to come. Start with a brief overview of the clinical trial, including its purpose, design, and current status.
Introduce the key topics that you will be covering in your presentation, such as:
- Background of the disease : Provide a comprehensive overview of the disease or condition being studied. Discuss its prevalence, incidence, and risk factors.
- Standard of care : Examine the current standard of care treatment and its limitations. Highlight the gaps or shortcomings that the clinical trial aims to address.
- Rationale for the trial : Explain why the clinical trial is necessary. What are the driving factors behind the development of a new treatment? What do researchers hope to achieve and learn?
Dive deeper into the background of the disease or condition.
- Discuss its prevalence, emphasizing the scope of the problem in the population.
- Explore its incidence, shedding light on how frequently new cases arise.
- Highlight the known risk factors associated with the disease, showcasing the complexity of the issue.
- Elaborate on the limitations of the current standard of care treatment, emphasizing where it falls short in addressing the needs of patients.
- Provide a compelling rationale for the clinical trial. Why is it critical to explore new treatment options? What are the potential benefits for patients and the healthcare system?
Now, bring your audience up to speed on the progress of the clinical trial.
- Discuss the number of participants enrolled in the trial, emphasizing the growing body of evidence.
- Clarify the stage of the trial, whether it's in the early phases or advanced stages of evaluation.
- Share any key findings to date. Highlight any positive results that have been obtained, and transparently address any challenges encountered along the way.
- Explain the next steps for the trial, outlining the roadmap ahead. Discuss the potential implications of the trial's results on patients, healthcare providers, and the broader healthcare system.
In your conclusion, summarize the key points of your presentation to reinforce the main takeaways.
- Reiterate the importance of the clinical trial and its potential to make a significant impact on the lives of patients.
- Be prepared to answer questions from your audience. Engage in thoughtful discussions to showcase your confidence and expertise.
Visual aids
To enhance your presentation's impact, consider using visual aids such as slides, charts, and graphs. These tools can:
- Illustrate complex concepts in a clear and visually appealing manner.
- Present data and results in a format that is easy for your audience to understand.
- Enhance engagement and retention of key information.
By following this structured approach and incorporating visual aids effectively, your clinical trial presentation will not only inform but also captivate your audience, making a lasting impression and advancing the cause of medical research.
Here is a guide on clinical training presentation
Do's and don'ts on a clinical trial background and update presentation
In this section, we'll cover essential guidelines for creating a powerful clinical trial presentation.
- Be transparent : Share your methodologies and any potential conflicts of interest.
- Use visuals : Visual aids improve understanding and retention.
- Engage the audience : Encourage questions and discussions.
- Stay updated : Keep your presentation current with the latest data and trends.
- Practice : Rehearse your presentation to ensure smooth delivery.
Don'ts:
- Overwhelm with data : Avoid excessive technical jargon and data overload.
- Be overconfident : Acknowledge limitations and uncertainties in your research.
- Neglect ethics : Ensure your research adheres to ethical standards.
- Rush through : Allow ample time for questions and discussions.
Summarizing key takeaways
- Clinical trials are vital for medical advancements.
- Structure your presentation: introduction, background, update, conclusion.
- Visual aids enhance understanding.
- Do's: Be transparent, use visuals, engage the audience, stay updated, practice.
- Don'ts: Avoid data overload, overconfidence, neglecting ethics, rushing.
In conclusion, mastering the art of clinical trial presentations is an ongoing journey. By combining your expertise with effective communication, you can contribute significantly to the advancement of medical research worldwide. So, take the stage, present with confidence, and make a difference in the world of healthcare.
1. Where can I find a clinical trial PowerPoint template for my presentation?
You can easily download a clinical trial presentation template from various sources online. These templates are often editable, allowing you to customize them to your needs. Check popular presentation platforms like PowerPoint and Google Slides for options.
2. What's the key aspect to consider when designing clinical trial presentation slides?
The quality of your presentation slides is crucial. Pay careful attention to the details, ensuring clarity and accuracy. Use appropriate icons, clipart, infographics, and visual aids to enhance your content.
3. How can I make my clinical trial presentation engaging for a medical research team?
To engage your team, consider using a Google Slides theme or a PowerPoint template that aligns with your topic. Incorporating graphics and diagrams can also help convey complex information in an accessible way.
4. Are there any specific guidelines for presenting clinical trial results involving a new drug?
Absolutely. It's essential to declare any potential conflicts of interest and to present the data safely and accurately. Utilize an appropriate theme to maintain consistency throughout your presentation.
5. Can you explain the clinical trial process using presentation slides?
Certainly. Presentation slides can be a valuable tool for explaining the clinical trial process. Use a clear and logical process flow to illustrate the journey from idea to treatment. Ensure each aspect is well-defined and supported with images and text.
Create your clinical trial background and update presentation with Prezent
Harness the power of Prezent, the AI presentation software designed to streamline your presentation creation process. With a wide array of clinical trial presentation templates available, you can easily design professional and visually appealing presentations. Don't miss out on this invaluable tool to improve your medical research communication.
Ready to supercharge your clinical trial presentations? Try our free trial or book a demo today with Prezent!
Get the latest from Prezent community
Join thousands of subscribers who receive our best practices on communication, storytelling, presentation design, and more. New tips weekly. (No spam, we promise!)
Interactive PowerPoint Presentation about Clinical Trials
- Format Lessons
- Language/s English
- Target Audience Further education, Self-directed learning
- Difficulty Introductory
- = 1, orange: orangeStars() }, event: { mouseover: isLoggedIn() ? mouseoverStars.bind($data, 1) : null, mouseout: isLoggedIn() ? mouseoutStars.bind($data, 1): null }' >
- = 2, orange: orangeStars() }, event: { mouseover: isLoggedIn() ? mouseoverStars.bind($data, 2) : null, mouseout: isLoggedIn() ? mouseoutStars.bind($data, 2): null }' >
- = 3, orange: orangeStars() }, event: { mouseover: isLoggedIn() ? mouseoverStars.bind($data, 3) : null, mouseout: isLoggedIn() ? mouseoutStars.bind($data, 3): null }' >
- = 4, orange: orangeStars() }, event: { mouseover: isLoggedIn() ? mouseoverStars.bind($data, 4) : null, mouseout: isLoggedIn() ? mouseoutStars.bind($data, 4): null }' >
- = 5, orange: orangeStars() }, event: { mouseover: isLoggedIn() ? mouseoverStars.bind($data, 5) : null, mouseout: isLoggedIn() ? mouseoutStars.bind($data, 5): null }' >
Key Concepts addressed
- 2-1a Comparison groups should be similar
- 2-1d People should not know which treatment they get
- 1-1a Treatments can harm
- 1-2e Comparisons are needed to identify treatment effects
An interactive Powerpoint presentation for people thinking about participating in a clinical trial or interested in learning about them.
The European Communication on Research Awareness Needs (ECRAN) Project has created two lay-friendly resources explaining clinical trials for people who want to know more about them:
- a 5-minute animated film available in 23 languages, and
- an interactive PowerPoint presentation for people considering whether to participate in clinical trials.
In this video, Iain Chalmers introduces the slides, which can be downloaded below.
The slide presentation uses a PowerPoint slide show file format with macros enabled.
- The PPS file (PowerPoint not needed)
- The PPT file (PowerPoint needed)
“Clinical trials” is a PowerPoint slide presentation for patients or a lay audience. The presentation covers:
- What are clinical trials?
- Why are clinical trials important
- Are you considering enrolling in a clinical trial?
- The clinical trial process
- Informed consent
- Rights and protections
- Trials registers
The format works well as a self-directed presentation or as a teaching aid. The presentation uses interactive self-test questions as you go along to make sure that you’ve been paying attention!
Another great feature of this resource is that it is pitched at the right level. It doesn’t overload you with too much information. At the same time, they have managed to cover all the bases.
Leave a Reply Cancel reply

You may also like
Qualitative research.
Finding and appraising qualitative evidence
Clinical Trials Career
For lecture on 3 June 2021
Diagnostic tests
Resources for teaching LR etc
Privacy Overview
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches
infertility
30 templates

16 templates

49 templates

27 templates

frida kahlo
56 templates

el salvador
32 templates
Clinical Trials Day
Clinical trials day presentation, free google slides theme and powerpoint template.
May 20th is a great opportunity to speak about the importance of clinical trials in medicine because it’s the Clinical Trial Day! This day will be celebrated worldwide and promoted with speeches, events, ads and presentations like this one. This template includes a modern design inspired by the modernity of technology and the blue tones that represent medicine. We have included lots of editable resources and visual elements that will help you when sharing your ideas. Download it and celebrate the improvements that clinical trials have made in medicine!
Features of this template
- 100% editable and easy to modify
- 35 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

You are using an outdated browser. Please upgrade your browser to improve your experience.
Clinical Trials PowerPoint Templates
Our vast library of Clinical Trials PowerPoint templates are professionally designed to elevate your presentation style in any professional meeting. The collection provides a comprehensive framework to professionals across industries to streamline their efforts, ideas and overall message. The fully editable Clinical Trials PowerPoint and Google Slides templates breathe life into complex facts, figures and information and present them in an engaging manner to ensure a long lasting impact on your audience’s minds.
Our extensive collection of Clinical Trials PPT templates promote productivity and convenience when it comes to its applications. These templates have been thoughtfully designed to ensure maximum visual impact. Explore our collection of Clinical Trials presentation templates and download the perfect template to take your presentation to new heights!
- Price <= $5.99
- Price > $5.99
Clinical Trials 07 PowerPoint Template
Login to use this feature
Add-to-favs lets you build a list for inspiration and future use.
Log in now to start adding your favs.
If you don't have one. A free account also gives you access to our free templates library
Clinical Trials 06 PowerPoint Template
Clinical Trials 02 PowerPoint Template
Clinical Trials 01 PowerPoint Template
Clinical Trials 04 PowerPoint Template
Clinical Trials 05 PowerPoint Template
Clinical Trials 03 PowerPoint Template
Clinical trials powerpoint templates for presentations:.
The Clinical Trials PowerPoint templates go beyond traditional static slides to make your professional presentations stand out. Given the sleek design and customized features, they can be used as PowerPoint as well as Google Slides templates . Inculcated with visually appealing unique and creative designs, the templates will double your presentation value in front of your audience. You can browse through a vast library of Clinical Trials Google Slides templates, PowerPoint themes and backgrounds to stand out in your next presentation.
What Is A Clinical Trials PowerPoint Template?
A Clinical Trials PowerPoint template is a ready-made presentation template that provides a structured framework for creating professional Clinical Trials presentations. The Clinical Trials PPT presentation template includes design elements, layouts, and fonts that you can customize to fit your content and brand.
What Are the Advantages of Clinical Trials Presentation Templates?
Clinical Trials PPT presentation templates can be beneficial because they:
- Add multiple visual and aesthetic layers to your slides.
- Ensure that complex information, insights and data is presented in a simplistic way.
- Enhance the overall visual appeal of the content.
- Save you a lot of time as you don’t have to start editing from scratch.
- Improve the professional outlook of your presentation.
How To Choose The Best Clinical Trials Presentation Templates?
Keep the following points in mind while choosing a Clinical Trials Presentation template for PowerPoint (PPT) or Google Slides:
- Understand your presentation goals and objectives.
- Make sure the Clinical Trials template aligns with your visual needs and appeal.
- Ensure the template is versatile enough to adapt to various types of content.
- Ensure the template is easily customizable.
Can I Edit The Elements In Clinical Trials PowerPoint Templates?
Yes, our Clinical Trials PowerPoint and Google Slides templates are fully editable. You can easily modify the individual elements including icons, fonts, colors, etc. while making your presentations using professional PowerPoint templates .

Are Clinical Trials PowerPoint Templates Compatible with Google Slides?
Yes, all our Clinical Trials presentation templates are compatible and can be used as Clinical Trials Google Slides templates.
How to Download Clinical Trials PowerPoint Templates for presentations?
To download Clinical Trials presentation templates, you can follow these steps:
- Select the resolution (16*9 or 4*3).
- Select the format you want to download the Clinical Trials template in (Google Slides or PowerPoint).
- Make the payment (SlideUpLift has a collection of paid as well as free Clinical Trials PowerPoint templates).
- You can download the file or open it in Google Slides.
Related Presentation Templates
49 templates
4 templates
2 templates
282 templates
Patient Journey
8 templates
Forgot Password?
Privacy Overview
Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
Home PowerPoint Templates Template Backgrounds Animated Clinical Study PowerPoint Templates
Animated Clinical Study PowerPoint Templates

Animated Clinical Study PowerPoint Templates will help you understand the Clinical Trial Process. It highlights the medical process of carefully testing drugs, medical treatment or hospital intervention before administering them. This is a critical phase in medical treatment to avoid the potential adverse effects and medical risks leading to the alteration of patient care and poor prognosis.
Clinical trials are designed with a number of parameters to generate accurate results. These parameter standards include a series of studies and evaluation. This case study template highlights the three important phases: patient data research, design clinical trials, and post evaluation statistics.
Medical researchers, doctors, and the entire medical team undergo rigorous research and pre-clinical testing before declaring a drug or a method safe for human consumption. This has been strictly regulated by responsible organizations and departments like the FDA itself. This Clinical Trial PowerPoint Template is keenly designed to focus on the following key concepts:
- Study design
- Design Study documents
- Investigator Section
- Regulatory Authority
- Committee Review
- Approval Letter
- Investigator Meeting
- Patient Enrollment
- Clinical Trials
- Site Initiation
- Follow up visits
- Data Management
- Statistics Review
Our Clinical Study PowerPoint Templates and other Medical Research PowerPoint Templates have been carefully reviewed by professionals. All the data from research, testing of methods up to weighing the benefits and identifying inefficiency were carried out. All our PowerPoint templates are user-friendly. You can easily edit the SMART icons, shapes, clipart, column data, statistics, process flow, image placeholders and text boxes.
You must be logged in to download this file.
Favorite Add to Collection
Details (16 slides)

Supported Versions:
Subscribe today and get immediate access to download our PowerPoint templates.
Related PowerPoint Templates
Incident Report PowerPoint Template

Simple Legal Slides PowerPoint Template

Research Paper Presentation Template

Neuroscience PowerPoint Template

STATISTICS 542 Introduction to Clinical Trials
Jul 18, 2012
1.66k likes | 2.06k Views
STATISTICS 542 Introduction to Clinical Trials. David L. DeMets, Ph.D. Dept. of Biostatistics & Medical Informatics 600 Highland Avenue Room K6/446 Phone: 263-1706 E-Mail: [email protected]. STATISTICS 542 - REFERENCES.
Share Presentation
- clinical research enterprise
- case report anecdotal
- statewide access
- clinical research
- clinical research training program

Presentation Transcript
STATISTICS 542Introduction to Clinical Trials David L. DeMets, Ph.D. Dept. of Biostatistics & Medical Informatics 600 Highland Avenue Room K6/446 Phone: 263-1706 E-Mail: [email protected]
STATISTICS 542 - REFERENCES 1. Friedman, Furberg & DeMets (3rd edition, 1998) Fundamentals of Clinical Trials. Springer-Verlag, NY, NY. 2. Pocock (1986) Clinical Trials - A Practical Approach. Wiley and Sons, New York, NY. 3. Meinert (1986) Clinical Trials - Design, Conduct & Analysis. Oxford University Press, New York, NY. 4. Hill (1962) Statistical Methods of Clinical and Preventive Medicine. Oxford University Press, New York, NY. 5. Tygstrup, Lachin & Juhl (1982) The Randomized Clinical Trial and Therapeutics Decisions. Marcell Dekker, New York, NY. 6. Shapiro & Louis (1983) Clinical Trials - Issues and Approaches. Marcell Dekker, New York, NY. 7. Mike & Stanley (1982) Statistics in Medicine Research. Wiley and Sons, New York. 8. Bulpitt (1983) Randomized Controlled Clinical Trials. Martinus Nijhoff, Boston, MA. 9. Peto, Pike, Armitage, et al. (1976) Design and Analysis of Randomized Clinical Trials Requiring Prolonged Observation of Each Patient. British Journal of Cancer.
Rationale for Biostat 542 • Common goal; critical review of the literature throughout our careers • Clinical practice • Clinical research • Special goal: to conduct clinical research, some understanding of study design & analysis is necessary
Clinical Practice & the Literature • Not all published articles of the same quality, regardless of journal • Not all evidence of the same level • Anecdotal • Observational • Experimental • Practicioner needs to sort out the evidence
Clinical Research • Many challenges • Clinical research workforce • Adequate training programs • Funding • Political & financial pressures • Practice vs research time conflicts • NIH initiatives
NIH Road MapZerhouni (2003) Science • New Pathways to Discovery • Research Teams of the Future • Re-engineering the Clinical Research Enterprise • Translational Research • Clinical Research Networks • Clinical Research Workforce Training
Discipline of Clinical Research Clinician Clinical Trialist Common Core Knowledge Statistician Clinical Pharm Behavioral Scientist
Clinical Research Training: a multidisciplinary workforce • Previous training “on the job”, sort of “trial and error” • Rigorous training programs are just starting • Many disciplines now involved in clinical research without formal training in this science • Threat of the “silver tsunami”
Composition of the Physician Workforce in the U.S. - 1980-2003 Ref: JAMA 2005; 294(11):1343-51
Aging of Funded NIH Investigators 1985-2004 Ref: JAMA 2005; 294(11):1343-51
Multidisciplinary Clinical Research Career Development (K12) NIH Clinical Research Career Development Programs College Professional Post-Doc Prof. Advancement Grad School Pre-doctoral Clinical Research Training Program (T32)
Training Pyramid in Patient-Oriented Research PhD MS Degree Capstone Degree Workshops
NIH RPG Funding Success Rates: 1985-2000“Similar Success” Ref: JAMA 2005; 294(11):1343-51
Application Trends for NIH Grants Ref: JAMA 2005; 294(11):1343-51
Physician Researchers • Have similar success rates in obtaining funding as PhD’s • Do not submit as many applications • Have not taken advantage of recent NIH budget increase
Clinical Research • Clinic is a perfectly good laboratory • Clinicians busy in their practice can also participate / lead research efforts • Difficult to be in the clinic mostly & run a competitive wet lab
NIH Initiative Clinical Translational Science Award (CTSA) • NIH grant investment in 60 research institutions • Focus on three areas • Training • Clinical research infrastructure • Community outreach • UW has submitted its application
Wisconsin Network for Health Research (WiNHR) Howard Bailey MD, Dave DeMets Phd, on behalf ofWiNHR InvestigatorsSept, 2005
WiNHR - Basic Idea • Create an efficient organizational network of selected clinical sites statewide who would agree to participate in clinical trials or other human subjects based research • Provide research staff support (infrastructure) at each site to provide quality control • Centralize some aspects of the research, e.g. create a data center at the UW-Madison or central IRB • Trial protocols could be generated by any participating site, with review for scientific merit • Individual protocols funded separately, taking advantage of existing infrastructure
Rationale • National recognition (e.g. NIH, FDA) of need for more and better evidence based medicine • Each sites local population isn’t large enough or diverse enough to perform efficient/representative research • Public forums expressed the following – • Promote expanded community-based clinical research trials • Build collaborations between community- and UW-based researchers
Benefits of WiNHR • Provides population statewide access to clinical trials studying new diagnostic, preventative and therapeutic interventions • Provides statewide practitioners, especially those not involved with larger clinic or med school-based research groups, an opportunity to participate and have more rapid access to advancements in health care • Expands capabilities of Wisconsin researchers focused on human subjects research • More studies and done faster • Possible statewide clinical database of > 3 million lives which would provide a powerful research tool • Improve funding opportunities
WiNHR Nodal Sites
Wausau Madison Green Bay LaCrosse Milwaukee Janesville Freeport, IL Waukesha Potential WiNHRCollaborators Wisconsin Oncology Network (WON) Sites • • • • •
WiNHR Long Range Benefits • Efficiency • A true statewide collaborative health care effort • Well-positioned to compete for health care research • Currently not feasible in single sites, e.g. 100-1,000 subjects per study • In the new post-genomics/informatics era.
Wisconsin Oncology Network (WON) • Proof of Concept / UWCCC • 7 sites with collaborating oncologists • 15-20 protocols completed or underway • Patients being accrued, interventions being given • Dr Ann Traynor, WON Director • Largely volunteer basis – can’t expand
Wausau Madison Green Bay LaCrosse Milwaukee Janesville Freeport, IL Waukesha Wisconsin Oncology Network
Research Cycle Clinical Trials Research (human, comparative) Translational Research Basic Research (bench, animal) Observational Research (human, epidemiological)
Evidence-Based Medicine • Ideally based on data from clinical trials • Need to understand fundamentals of good design and analysis • Not all published data of same quality
TYPES OF EVIDENCE (1) • Randomized Clinical Trial (RCT) is gold standard • RCT minimizes bias • Can’t do RCTs for all important questions (time, funding, ethics) • Must make choices on what evidence to use for clinical guidelines
TYPES OF EVIDENCE (2) • Need to remember limitations of evidence clinical guidelines based on • Continue to strive to improve evidence • Need to read the literature critically
TYPES OF EVIDENCE (3) • Recent history teaches us to be cautious • Not seeking most rigorous evidence has proven to be problematic • Theory and observational studies based evidence have not always led to correct conclusion for important questions
Types of Clinical Research 1. Case Report Anecdotal Problem 2. Observational a. Case-Control/Retrospective (lung cancer) b. Cross Sectional (WESDR) Beaver Dam c. Prospective (Framington) WESDR-II Associations 3. Drug Development Phase 0, Phase I, and Phase II 4. Experimental a. Historical Controls b. Concurrent (Non-randomized) c. Randomized “Effect”
The Cycle of Clinical Therapeutics Discovery RCTs Guidelines Patient Outcomes Performance Indicators Caregiver Performance Califf R et al JACC 2002
Comparison of ESC, ACC/AHA, CCS and HFSA
I IIa IIb III Guidelines:Classes of Recommendation Intervention is useful and effective Evidence conflicts/opinions differ but lean towards efficacy Evidence conflicts/opinions differ but lean against efficacy Intervention is not useful/effective and may be harmful
Comparison of pharmacology therapyESC, ACC/AHA, CCS and HFSA ESC ACC/AHA CCS HFSA Level Class Level Class Level Class Level Class • ACE inhibitor A I A I A I A I • Beta-blocker A I A I A I A IAngiotensin receptor blocker • a) ACE inhibitor intolerant B I A I A I A Ib) ACE inhibitor treated- - B IIb A I A IIa • to reduce mortality B IIa - - - - - - • to reduce hospitalisationA I - - - - - - McMurray JV, Swedberg K. Eur Heart J Aug 2006
Alternatives to Evidence Based Medicine • Eminence-based medicine • Confidence-based medicine • Eloquence-based medicine • Vehemence-based medicine • Providence-based medicine • Diffidence-based medicine • Nervousness-based medicine Isaacs D, Fitzgerald D. Br Med J 1999;319:1618.
Alternatives to Evidence Based Medicine(Ref:Marc Pfeffer) • Last-patient-experience based med • Hot-tip-based med • My-resident-told-me based med • Chat-room based-med • Direct-to-consumer advertising based med
Challenge: Attack on Clinical Trials • Pending Congressional Legislation • NIH/FDA leadership • Wall Street & WSJ • Patient Advocacy Groups • References • SCT (JCT, 2006) • Jacobsen & Parmet (JAMA, Jan 10, 2006) • Cancer Letter (Aug, 2005)
Senate Bill 1956 • An amendment to Federal Food, Drug & Cosmetic Act (Senator Brownback-K) • Known as the ACCESS Act • A three tiered approval system • More responsive to “the needs of seriously ill patients”
Proposed Three Tier Approval • Tier I • Based on Phase I information • Based on clinical, not statistical analysis • May require post approval studies • Tier II • Based on biomarkers & surrogates • Tier III • Standard requirements
Some Issues in Proposed Legislation • Challenge of placebo controlled studies • De-emphasize statistical analysis-no disapprovals solely on the basis of statistical analysis or 95% CIs • Evidence may be based on uncontrolled studies such as case histories, observational studies, mechanism of actions, computer models… • Outcome data may be a surrogate or biological marker
Washington Leadership • Use modern mathematical tools…efficacy derived from large cohort of patients given same treatment • Use Bayesian statistics to glean strong conclusions from large cohort data, just like economists • Develop chemoprevention based on surrogates and biomarkers • RCTs not the best future approach
WSJ Editorials • WSJ against 1962 Food, Drug & Cosmetic Act amendment • Places an unnecessary burden by demanding proof of efficacy • Terminally ill patients should be free to take Phase I tested interventions • An old argument getting renewed attention • Current target is FDA oncology
Some Advocacy Groups • Abigail Alliance for Better Access • Oncologists have allowed statisticians to take over • A cult-like belief….that you can’t learn anything…unless you do a clinical trial • In environmental toxicology, when a statistician walks in, scientists cringe …you are doing battle with a functional idiot • Ref: Cancer Letter August 5, 2005
Court Action • In 2003, Alliance sued for expanded access • August 2004, District Court dismissed case-no fundamental right to INDs • May, 2006 District Court ruling overturned, “access is a constitutional right” • Case probably headed to Supreme Court • Major implications for • Drug safety • Regulatory Authority • Clinical research & practice
Definition of a Clinical Trial A prospective study comparing the effect and value of intervention(s) against a control in human subjects NOTE: • Prospective not retrospective • Intervention/Equipment • preventive -drug • therapeutic -device • diagnostic -procedure • -biologic • Control Group • no intervention -current standard therapy • placebo (Diehl, 1938) -previous standard • Humans not animals • ethics • informed consent
Clinical TrialsNatural Experiment • General Lancaster (1600) • East Indian Shipping Co. • 4 ships - Lancaster’s ship fortuitously had lemon juice on board • Lancaster’s ship remained free of scurvy • Natural Experiment, not planned
- More by User

Introduction to Randomized Clinical Trials
Introduction to Randomized Clinical Trials. Deborah Grady Professor of Medicine University of California, San Francisco. Randomized Trials. Rationale Basic designs Participants Intervention Blinding Outcomes Adherence Follow-up. Rationale. Why do a randomized blinded trial
790 views • 45 slides

Statistics 542 Introduction to Clinical Trials Meta Analysis
Statistics 542 Introduction to Clinical Trials Meta Analysis. Meta-Analysis. Alternatives? Occasionally Complementary? Yes Meta-Analysis Combination of similar studies using similar subjects and similar treatments and similar outcomes.
676 views • 32 slides
Clinical Trials
Clinical Trials. Importance in future therapies. What are the Requirements to Produce New Drugs? Drug must work significantly better than a control treatment
667 views • 25 slides

STATISTICS 542 Introduction to Clinical Trials SAMPLE SIZE ISSUES
STATISTICS 542 Introduction to Clinical Trials SAMPLE SIZE ISSUES. Ref: Lachin, Controlled Clinical Trials 2:93-113, 1981. Sample Size Issues. Fundamental Point Trial must have sufficient statistical power to detect differences of clinical interest
1.54k views • 83 slides

STATISTICS 542 Intro to Clinical Trials Quality of Life Assessment
STATISTICS 542 Intro to Clinical Trials Quality of Life Assessment. Why Are We Interested in the Quality of Life?. The United States Food and Drug Administration has stated that efficacy with respect to overall survival and/or improvements in QOL might provide the basis for drug approval
594 views • 22 slides

STATISTICS 542 Intro to Clinical Trials SURVIVAL ANALYSIS
STATISTICS 542 Intro to Clinical Trials SURVIVAL ANALYSIS. Survival Analysis Terminology. Concerned about time to some event Event is often death Event may also be, for example 1. Cause specific death 2. Non-fatal event or death, whichever comes first death or hospitalization
608 views • 50 slides

Clinical Trials. Jean Bourbeau, MD Respiratory Epidemiology and Clinical Research Unit McGill University Clinical Epidemiology (679) June 17, 2005. Clinical Trial Objectives. Define an experimental study and distinguish the major types
2.38k views • 75 slides

Introduction to Clinical Trials
630 views • 49 slides

Introduction to Randomized Clinical Trials. Stephen Bent Associate Professor of Medicine University of California, San Francisco. Randomized Trials. Rationale Basic designs Participants Intervention Blinding Outcomes Adherence Follow-up. Take Home Messages.
852 views • 65 slides

Introduction to Design of Genomic Clinical Trials
Introduction to Design of Genomic Clinical Trials. Richard Simon, D.Sc. Chief, Biometric Research Branch National Cancer Institute http://brb.nci.nih.gov. Biometric Research Branch Website brb.nci.nih.gov. Powerpoint presentations Reprints & Technical Reports BRB-ArrayTools software
435 views • 36 slides

Statistics for Clinical Trials in Neurotherapeutics
Statistics for Clinical Trials in Neurotherapeutics. Barbara C. Tilley, Ph.D. Medical University of South Carolina. Funding:. NIA Resource Center on Minority Aging 5 P30 AG21677. NINDS Parkinson’s Disease Statistical Center U01NS043127 and U01NS43128. Sample Size.
732 views • 59 slides

Recruitment to Clinical Trials:
Recruitment to Clinical Trials:. What are the Strategies for Success?. Adwoa Hughes-Morley. How easy is it to recruit to clinical trials?. Across all contexts (McDonald et al, 2006) 53% of trials are awarded an extension 45% recruit less than 80% of their target
386 views • 12 slides

Clinical Trials. Common Terminology. Randomization Chance allocation to one of two or more treatments To insure comparability of groups on factors (known and unknown)other than the treatment To control bias To allow valid application of statistical methods.
469 views • 18 slides

An Introduction to Clinical Trials: Design Issues
An Introduction to Clinical Trials: Design Issues. Edgar R Miller III PhD, MD Welch Center for Prevention, Epidemiology and Clinical Research Johns Hopkins University School of Medicine and Bloomberg School of Public Health. Type of Studies. Non-experimental (Observational) Case report
1.85k views • 139 slides

STATISTICS 542 Introduction to Clinical Trials “What’s the Question?”
STATISTICS 542 Introduction to Clinical Trials “What’s the Question?”. Quote by Dr. Max Halperin NHLBI, National Institutes of Health. Primary vs. Secondary Question. Primary most important, central question ideally, only one stated in advance basis for design and sample size
993 views • 83 slides

An Introduction to Clinical Trials
An Introduction to Clinical Trials A lecture for trial site staff and anyone new to clinical research and clinical trials. What is a Clinical Trial?. Data safety and monitoring board Clinical.
468 views • 22 slides

Clinical Trials Aurelius Global masterclasses is providing In house training on Clinical Trials in Europe. Overview clinical trials have increased dramatically in recent years. In this masterclass learn how evolutions in technology and risk management process offer new opportunities to increase clinical trials protocols. Venue Amsterdam, The Neatherlands https://aureliusglobalmasterclass.com/events/2nd-edition-clinical-trial-regulations-with-ich-gcp-e6-r2-workshop/
171 views • 13 slides

1.45k views • 139 slides

Introduction to Clinical Trials. Afshin Ostovar Bushehr University of Medical Sciences Bushehr, 2011. Research Design Epidemiology. Descriptive Studies Case Reports Case Series Cross Sectional Survey Analytic Studies Observational Studies Case-Control or Case-Comparison Cohort Studies
597 views • 50 slides

Powerpoint Templates
Icon Bundle
Kpi Dashboard
Professional
Business Plans
Swot Analysis
Gantt Chart
Business Proposal
Marketing Plan
Project Management
Business Case
Business Model
Cyber Security
Business PPT
Digital Marketing
Digital Transformation
Human Resources
Product Management
Artificial Intelligence
Company Profile
Acknowledgement PPT
PPT Presentation
Reports Brochures
One Page Pitch
Interview PPT
All Categories

Research Design For Clinical Trials Powerpoint Presentation Slides
Clinical trial phases involve various steps that are followed to ensure the safety and efficacy of the newly developed drug by testing it on targeted individuals in a controlled environment. Check out our efficiently designed Research Design for Clinical Trials PowerPoint template. In this presentation, we have covered the process flow of clinical trial phases along with significant milestones. It also includes primary and secondary goals, the number and type of patients, dosage details, and outcomes of each corresponding phase of the clinical trial. This PPT also covers graphs through which the success rate of the trial and the cost involved in each phase can be visually represented. Build a powerful template like this for yourself and book a free demo with our research team now.

These PPT Slides are compatible with Google Slides
Compatible With Google Slides

- Google Slides is a new FREE Presentation software from Google.
- All our content is 100% compatible with Google Slides.
- Just download our designs, and upload them to Google Slides and they will work automatically.
- Amaze your audience with SlideTeam and Google Slides.
Want Changes to This PPT Slide? Check out our Presentation Design Services
Get Presentation Slides in WideScreen
Get This In WideScreen
- WideScreen Aspect ratio is becoming a very popular format. When you download this product, the downloaded ZIP will contain this product in both standard and widescreen format.

- Some older products that we have may only be in standard format, but they can easily be converted to widescreen.
- To do this, please open the SlideTeam product in Powerpoint, and go to
- Design ( On the top bar) -> Page Setup -> and select "On-screen Show (16:9)” in the drop down for "Slides Sized for".
- The slide or theme will change to widescreen, and all graphics will adjust automatically. You can similarly convert our content to any other desired screen aspect ratio.
- Add a user to your subscription for free
You must be logged in to download this presentation.
Do you want to remove this product from your favourites?
PowerPoint presentation slides
This complete presentation has PPT slides on wide range of topics highlighting the core areas of your business needs. It has professionally designed templates with relevant visuals and subject driven content. This presentation deck has total of fifty five slides. Get access to the customizable templates. Our designers have created editable templates for your convenience. You can edit the color, text and font size as per your need. You can add or delete the content if required. You are just a click to away to have this ready-made presentation. Click the download button now.

People who downloaded this PowerPoint presentation also viewed the following :
- Complete Decks , All Decks , Medical
- Drug Development Phase ,
- Clinical research ,
- Drug Testing Phase ,
- Medical Testing And Research ,
- New Drug Clinical Trials
Content of this Powerpoint Presentation
Slide 1 : This slide displays the title Research Design for Clinical Trials. Slide 2 : This slide shows the various steps involved in the clinical trial process. Slide 3 : This slide indicates the key steps involved in the clinical drug investigation process. Slide 4 : This slide highlights the process flow of clinical study for the new drug investigation. Slide 5 : The mentioned slide depicts the steps of the clinical research process. Slide 6 : This slide covers the clinical research trial steps for the successful investigation and launch of the new drug. Slide 7 : This slide highlights the framework for the clinical research trial. Slide 8 : This slide indicates the complete flow of the new drug testing process depicted via multiple steps. Slide 9 : This slide depicts the research process for new drug development. Slide 10 : This slide shows the multiple steps of the clinical trial process with the results of each phase. Slide 11 : This slide covers the different phases of research trial for the testing of new medicine for human consumption. Slide 12 : This slide tabulates the multiples phases of the drug testing process in clinical research trials. Slide 13 : This slide depicts the key and derived goals of multiple phases of the clinical trial process for the synthesis of the new drug. Slide 14 : This slide depicts the primary goal of each phase of the clinical research trial. Slide 15 : This slide shows the tabulation of key objectives of multiple phases of the clinical research process. Slide 16 : This slide indicates the information regarding the multiple stages of the clinical trial process. Slide 17 : This slide covers the costing involved in multiple steps of the new drug investigation in the clinical trial. Slide 18 : This slide covers the detailed description of the multiple stages of the clinical research process to check new drug efficacy. Slide 19 : In this slide, tabulation is done of key objectives of each step of clinical research trials. Slide 20 : This slide shows the multiple steps of the clinical trial to determine if the new drug is safe and effective for human consumption. Slide 21 : This slide tabulates the characteristics of each step of the clinical trial process. Slide 22 : The slide covers the costs involved in each step of the clinical trial procedure. Slide 23 : The slide visually presents the clinical trial procedures. Slide 24 : This slide depicts the framework for the successful completion of the clinical trial to investigate the efficacy of the new drug. Slide 25 : This slide illustrates the multiple steps involved in the clinical research trial to check the safety of the new drug. Slide 26 : This slide covers the clinical research trial phases with major milestones such as IND (Investigational New Drug) and NDA (New Drug Application). Slide 27 : This slide visually presents the clinical research trial stages that are plotted on the graph. Slide 28 : This slide visually presents the success rate of multiple stages of the clinical trial procedure. Slide 29 : This slide showcase Graph Indicating Clinical Trial Phases Probability of Success. Slide 30 : This slide showcase Graph highlighting Cost involved in Multiple Clinical Trial Phases. Slide 31 : This slide depicts the process flow of the clinical trial procedure. Slide 32 : This slide showcase Specialist Giving Medicine Dose in Clinical Trial Phases. Slide 33 : This slide showcase Researcher Working on New Drug Compound in Clinical Trial Phases. Slide 34 : This slide showcase Medicine Compounds in Multiple Phases of Clinical Trial. Slide 35 : This slide showcase Clinical Trial Phases Conduced by Technician in Laboratory. Slide 36 : This slide showcase Drug Specialist Working with Microscope in Clinical Trial Phases. Slide 37 : This slide showcase Clinical Trial Phases Performed by Pharmacist. Slide 38 : This slide showcase Clinical Trial Phases Depicted Via DNA and Patients. Slide 39 : This slide showcase Phase Endpoint Report of New Drug in Clinical Trial. Slide 40 : This slide showcase Medical Reports with Injection for Clinical Trial Phases. Slide 41 : This slide showcase Microscope with Medical Solution in Clinical Trial Phases. Slide 42 : This slide showcase Clinical Trial Phases Poster with Medicines and Health Monitor. Slide 43 : This is the icons slide. Slide 44 : This slide presents title for additional slides. Slide 45 : This slide exhibits yearly timeline of company. Slide 46 : This slide shows puzzle for displaying elements of company. Slide 47 : This slide shows Comparison of male and female user. Slide 48 : This slide shows roadmap of company. Slide 49 : This slide shows Magnifying Glass. Slide 50 : This slide shows Our target. Slide 51 : This slide depicts posts for past experiences of clients. Slide 52 : This slide displays Venn. Slide 53 : This slide exhibits yearly profits stacked line charts for different products. The charts are linked to Excel. Slide 54 : This slide exhibits yearly profits stacked coloumn charts for different products. The charts are linked to Excel. Slide 55 : This is thank you slide & contains contact details of company like office address, phone no., etc.
Research Design For Clinical Trials Powerpoint Presentation Slides with all 60 slides:
Use our Research Design For Clinical Trials Powerpoint Presentation Slides to effectively help you save your valuable time. They are readymade to fit into any presentation structure.

Ratings and Reviews
by Edmund Ortega
October 18, 2022
by Domingo Hawkins
October 17, 2022


Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
One-Month Ticagrelor Monotherapy After PCI in Acute Coronary Syndromes - IVUS-ACS/ULTIMATE-DAPT
Contribution to literature:.
Highlighted text has been updated as of April 8, 2024.
The IVUS-ACS trial showed that IVUS-guided PCI is superior to angiography-guided PCI for 1 year outcomes among patients undergoing PCI for an ACS presentation.
The ULTIMATE-DAPT trial showed that compared with standard 12-month DAPT with aspirin and ticagrelor, 1-month DAPT followed by de-escalation to ticagrelor monotherapy reduces bleeding without an increase in ischemic events at 1 year among patients undergoing PCI for ACS.
Description:
The goal of the trial was to compare the efficacy of intravascular ultrasound (IVUS)-guided vs. angiography-guided percutaneous coronary intervention (PCI) among patients undergoing acute coronary syndrome (ACS) PCI. Further, it sought to assess the safety and efficacy of ticagrelor monotherapy compared with dual antiplatelet therapy (DAPT) with aspirin and ticagrelor for 12 months following completion of 1-month DAPT for ACS PCI.
Study Design
Initially, patients were randomized to IVUS-guided (n = 1,753) or angiography-guided (n = 1,752) PCI for index ACS event. All patients received DAPT with ticagrelor and aspirin for 30 days. If they had no ischemic or bleeding events at the end of 30 days, they were randomized to continuing DAPT for 12 months total (n = 1,700), or stopping aspirin and switching to ticagrelor + placebo (n = 1,700). Ticagrelor dose was 90 mg bid; aspirin dose was 100 mg daily. During follow-up, a reduction in ticagrelor from 90 mg to 60 mg bid was required in 0.8% of patients.
- Total randomized participants: 3,400
- Median duration of follow-up: 1 year
- Median patient age: 63 years
- Percentage female: 26%
Inclusion criteria:
- ≥18 years of age
- Either 1) biomarker-positive non–ST-segment elevation myocardial infarction (NSTEMI) or STEMI, OR 2) biomarker-negative unstable angina (DS ≥90%, ruptured plaque, or thrombotic lesion)
- Had been randomized in the IVUS-ACS trial of IVUS-guided vs. angiography-guided PCI (for ULTIMATE-DAPT)
- Remained event free after PCI with contemporary drug-eluting stents (DES) for 1 month on ticagrelor (90 mg bid) plus aspirin (100 mg qd)
Exclusion criteria:
- Stroke within 3 months or any permanent neurologic deficit
- Previous coronary artery bypass grafting
- Any planned surgery within 12 months
- Estimated glomerular filtration rate <20 mL/min/1.73 m²
- Need for chronic oral anticoagulation
- Life expectancy <1 year
Other salient features/characteristics:
- Chinese race: 88%
- Chronic kidney disease: 7%
- Prior PCI: 10%
- Prior stroke: 9%
- Presentation: unstable angina: 40%, NSTEMI: 32%, STEMI: 28%
- Left ventricular ejection fraction: 63%
- Number of diseased vessels: one-vessel disease (70%), two-vessel disease (23%)
- Type of DES use: Firehawk (52%), Resolute (41%)
Principal Findings:
The primary endpoint of target vessel failure (cardiac death, target vessel MI, or clinically driven target vessel revascularization) for IVUS-guided vs. angiography-guided PCI, was: 4.0% vs. 7.3% (hazard ratio 0.55, 95% confidence interval 0.41-0.74, p = 0.0001).
- Cardiac death: 0.5% vs. 1.1%, p = 0.17
- Spontaneous MI: 0.6% vs. 1.5%, p = 0.0143
- Clinically driven target vessel revascularization: 1.4% vs. 3.2%, p = 0.001
Key secondary outcomes for IVUS-guided vs. angiography-guided PCI:
- Definite or probable stent thrombosis: 0.6% vs. 0.9%, p = 0.64
- Clinically driven target lesion revascularization: 1.3% vs. 2.5%, p = 0.014
ULTIMATE DAPT:
The primary effectiveness endpoint, Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding for ticagrelor + placebo vs. ticagrelor + aspirin, was: 2.1% vs. 4.6% (hazard ratio 0.45, 95% confidence interval 0.30-0.66, p < 0.0001).
- BARC 3 or 5: 0.7% vs. 1.7%, p = 0.009
- TIMI major or minor bleeding: 0.7% vs. 1.6%, p = 0.01
The primary safety endpoint, major adverse cardiovascular or cerebrovascular events (cardiac death, MI, ischemic stroke, definite stent thrombosis, clinically driven target vessel revascularization): 3.6% vs. 3.7%, p < 0.0001 for noninferiority, p = 0.89 for superiority
Key secondary outcomes for ticagrelor + placebo vs. ticagrelor + aspirin:
- All-cause mortality: 0.7% vs. 0.8%, p = 0.84
- Nonprocedure MI: 0.9% vs. 0.7%, p = 0.29
- Repeat revascularization: 2.4% vs. 2.4%, p = 0.95
- Stent thrombosis: 0.3% vs. 0.3%, p = 0.96
Interpretation:
The results of this trial show that IVUS-guided PCI is superior to angiography-guided PCI for 1- year outcomes among patients undergoing PCI for an ACS presentation. Further, 1-month DAPT followed by de-escalation to ticagrelor monotherapy reduces bleeding compared with 12-month DAPT without an increase in ischemic events at 1 year among these patients (including those presenting with STEMI).
The IVUS data are concordant with recent studies suggesting a benefit with IVUS-guided PCI compared with angiography alone guided PCI, especially for complex subsets. This trial provides evidence for the same among ACS patients.
There have been several recent trials (such as TWILIGHT and TICO) suggesting that de-escalation from DAPT to APT monotherapy, typically P2Y12 inhibitor monotherapy, after 3 months post-PCI for ACS is feasible and safe. In the STOPDAPT-2 ACS trial, de-escalating DAPT to clopidogrel monotherapy after 1 month did not meet prespecified noninferiority criteria compared with standard 12 months of DAPT for ACS-PCI. The current trial suggests that DAPT duration of 1 month followed by ticagrelor monotherapy may be sufficient among patients undergoing ACS-PCI. These are important findings and may influence clinical practice. One caveat is that patients in this trial were mostly Chinese; extrapolation to other ethnic/racial groups is unclear. In addition, other trials have suggested some harm with shorter durations among STEMI patients (SMART-DATE, STOPDAPT-2 STEMI subgroup).
In the current trial, although the p-value for interaction was negative, the ischemic event rates appeared somewhat increased with shorter duration of DAPT. This population needs careful study in future studies. Finally, with the advent of high-sensitivity troponin assays, the incidence of true “unstable angina” has diminished in the United States. In the current trial, 40% were enrolled with a diagnosis of unstable angina. It is unclear if some of these represented non-ACS patients.
References:
Li X, Ge Z, Kan J, et al., on behalf of the IVUS-ACS Investigators. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicenter, randomized trial. Lancet 2024;Apr 8:[Epub ahead of print] .
Ge Z, Kan J, Gao X, et al., on behalf of the ULTIMATE-DAPT Investigators. Ticagrelor alone versus ticagrelor plus aspirin from month 1 to month 12 after percutaneous coronary intervention in patients with acute coronary syndromes (ULTIMATE-DAPT): a randomized, placebo-controlled, double-blind clinical trial. Lancet 2024;Apr 7:[Epub ahead of print] .
Presented by Dr. Shaoliang Chen at the American College of Cardiology Annual Scientific Session (ACC.24), Atlanta, GA, April 8, 2024.
Presented by Dr. Gregg W. Stone at the American College of Cardiology Annual Scientific Session (ACC.24), Atlanta, GA, April 7, 2024.
Clinical Topics: Acute Coronary Syndromes, Invasive Cardiovascular Angiography and Intervention, Interventions and ACS
Keywords: ACC24, ACC Annual Scientific Session, Acute Coronary Syndrome, Percutaneous Coronary Intervention
You must be logged in to save to your library.
Jacc journals on acc.org.
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Email: [email protected]
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- ACC.org Quick Start Guide
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.

IMAGES
COMMENTS
Clinical Trials - An Introduction - Download as a PDF or view online for free. Submit Search. Upload. Clinical Trials - An Introduction • 448 likes • 196,630 views. Dr Purnendu Sekhar Das Follow. This presentation will give an overview of the Clinical Research/Drug Development and licensing(US FDA) process. Please feel free to provide ...
Typically, trials progress through distinct phases: Phase 1: Small-scale studies assess safety and dosage in healthy volunteers. Phase 2: Larger groups with the target condition receive the intervention, evaluating its efficacy and safety. Phase 3: Even larger trials confirm effectiveness and compare the intervention to existing treatments or ...
ADAPTIVE PHASE CLINICAL TRIALS • Study that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data (usually interim data) from subjects in the study. • The purpose is to make clinical trials more flexible, efficient and fast.
Clinical trials are research studies involving people (healthy volunteers or patients) that test the safety and efficacy of a new treatment. A 'treatment' in this context could mean: A medicine. A medical device - such as a cardiac stent (used for narrow or weak blood vessels).
Free Google Slides theme and PowerPoint template. These Clinical Trial Infographics are simply great for medical purposes: talking about treatments, steps, and diseases is simple if you use these timelines, arrows, bars and circle charts, banners and text blocks.
Clinical and pharma icons. To make your presentation slides more appealing, this template has medical icons on it so you can use them as visual aids during the explanation of each clinical trial phase. Get your presentation custom designed by us, starting at just $10 per slide. STEP 1. UPLOAD PRESENTATION.
Update. Now, bring your audience up to speed on the progress of the clinical trial. . Discuss the number of participants enrolled in the trial, emphasizing the growing body of evidence. Clarify the stage of the trial, whether it's in the early phases or advanced stages of evaluation. Share any key findings to date.
The European Communication on Research Awareness Needs (ECRAN) Project has created two lay-friendly resources explaining clinical trials for people who want to know more about them: a 5-minute animated film available in 23 languages, and. an interactive PowerPoint presentation for people considering whether to participate in clinical trials.
Cost List. A Cost List (rate schedule) is used for cost determination of negotiated budgets and invoices. The goal in using a Cost List is to standardize department rates to negotiate for Industry (For-Profit) sponsored contracts and to reduce the difference in cost estimates. Tip: Contact DOM CTP for DOM approved Cost List.
Template 8: Project Management Clinical Monitoring Presentation Slide . This PPT layout helps you present your clinical monitoring and clinical trial support needs. It is designed to help you get the most out of your clinical trials, making it easier than ever to track your progress and keep on schedule. Grab it now! Grab this template ...
Free Google Slides theme and PowerPoint template. May 20th is a great opportunity to speak about the importance of clinical trials in medicine because it's the Clinical Trial Day! This day will be celebrated worldwide and promoted with speeches, events, ads and presentations like this one. This template includes a modern design inspired by ...
To download Clinical Trials presentation templates, you can follow these steps: Select the resolution (16*9 or 4*3). Select the format you want to download the Clinical Trials template in (Google Slides or PowerPoint). Make the payment (SlideUpLift has a collection of paid as well as free Clinical Trials PowerPoint templates).
Clinical trials A properly planned experiment and executed clinical trial is a powerful experimental technique for assessing the effectiveness of an intervention. Friedman, Furer and Demets. Definition. Download Presentation. evaluation. most appropriate. only means. response variable. single response variable.
Content of this Powerpoint Presentation. Slide 1: This slide introduces Clinical Research Trial Stages. State your company name and begin. Slide 2: This slide shows the various steps involved in the clinical trial process. Slide 3: This slide indicates the key steps involved in the clinical drug investigation process.
An Introduction to Clinical Trials A lecture for trial site staff and anyone new to clinical research and clinical trials. What is a Clinical Trial? • Data safety and monitoring board • Clinical A properly planned and executed clinical trial is a powerful experimental technique for assessing the effectiveness of an intervention. What makes ...
Clinical Trial Stages Ppt PowerPoint Presentation Complete Deck With Slides. Induce strategic thinking by presenting this complete deck. Enthrall your audience by deploying this thought provoking PPT deck. It can be downloaded in both standard and widescreen aspect ratios, thus making it a complete package to use and deploy.
Animated Clinical Study PowerPoint Templates will help you understand the Clinical Trial Process. It highlights the medical process of carefully testing drugs, medical treatment or hospital intervention before administering them. This is a critical phase in medical treatment to avoid the potential adverse effects and medical risks leading to ...
Slide 1 of 6. Research Design For Clinical Trials Researcher Working On New Drug Compound In Clinical Trial Phases. Slide 1 of 6. Clinical Trial Phases Depicted Via DNA And Patients Research Design For Clinical Trials. Slide 1 of 2. Heart disease clinical trials ppt powerpoint presentation portfolio show.
STATISTICS 542 Introduction to Clinical Trials. David L. DeMets, Ph.D. Dept. of Biostatistics & Medical Informatics 600 Highland Avenue Room K6/446 Phone: 263-1706 E-Mail: [email protected]. STATISTICS 542 - REFERENCES. Slideshow 568088 by mervin.
Content of this Powerpoint Presentation. Slide 1: This slide displays the title Research Design for Clinical Trials. Slide 2: This slide shows the various steps involved in the clinical trial process. Slide 3: This slide indicates the key steps involved in the clinical drug investigation process.
The results of this trial show that IVUS-guided PCI is superior to angiography-guided PCI for 1- year outcomes among patients undergoing PCI for an ACS presentation. Further, 1-month DAPT followed by de-escalation to ticagrelor monotherapy reduces bleeding compared with 12-month DAPT without an increase in ischemic events at 1 year among these ...