Explore Jobs
- Jobs Near Me
- Remote Jobs
- Full Time Jobs
- Part Time Jobs
- Entry Level Jobs
- Work From Home Jobs
Find Specific Jobs
- $15 Per Hour Jobs
- $20 Per Hour Jobs
- Hiring Immediately Jobs
- High School Jobs
- H1b Visa Jobs
Explore Careers
- Business And Financial
- Architecture And Engineering
- Computer And Mathematical
Explore Professions
- What They Do
- Certifications
- Demographics
Best Companies
- Health Care
- Fortune 500
Explore Companies
- CEO And Executies
- Resume Builder
- Career Advice
- Explore Majors
- Questions And Answers
- Interview Questions

What is a clinical study manager and how to become one
A clinical study manager plans, executes, and finalizes clinical trials. They oversee the entire process, including preparing protocols, recruiting volunteers, and managing budgets and timelines. Clinical study managers work with regulatory affairs specialists, data managers, and statisticians. They ensure compliance with relevant regulations, such as Good Clinical Practice (GCP), and validate that the data collected remains accurate and complete. Overall, clinical study managers contribute to the development of new drugs and medical devices through clinical trials.
How long does it takes to become a clinical study manager?
It takes approximately 6 to 7 years to become a clinical study manager.
Year 1-4: Bachelor's Degree A typical clinical study manager needs a bachelor's degree, which takes about 4 years to complete.
Year 5-6: Experience After obtaining a degree, one to two years of relevant experience is typical for this role.
Year 6-7: Training Additionally, 3 to 6 months of on-site training and 6 to 12 months of on-job training are usually required.
- Salary $78,195
- Growth Rate 6%
- Jobs Number 61,352
- Most Common Skill GCP
- Most Common Degree Bachelor's degree
- Best State California
Clinical Study Manager pros and cons
High level of responsibility and autonomy
Collaborative work with interdisciplinary teams
Competitive salary and benefits
Flexibility in work schedule and location
Varied work tasks and responsibilities
High level of stress and pressure to meet deadlines
Long working hours, including evenings and weekends
Risk of failure or negative outcomes in clinical trials
Limited ability to control study outcomes due to external factors such as patient recruitment or unexpected events
Challenging communication with patients, investigators, and sponsors
Clinical Study Manager career paths
A clinical study manager can pursue various career paths, including project management, senior leadership roles like vice president or chief nursing officer, and even transitioning to similar roles in clinical research, operations, and quality assurance. They can also specialize in managing specific clinical trials or overseeing regional or senior regional management.
Key steps to become a clinical study manager
Explore clinical study manager education requirements, most common clinical study manager degrees.
Bachelor's
Master's
Start to develop specific clinical study manager skills
A clinical study manager's skills are varied and crucial to the success of a clinical trial. They must manage projects related to the commercialization and post-market analysis of medical products. They also plan and manage all aspects of investigational product supply for assigned clinical studies and offer guidance to investigational sites regarding data capture, query resolution, and regulatory processes. They prepare and review documentation for site start-up activities, author clinical study reports and abstracts, and present periodic reports on study timelines, forecasts, study budgets, and working relationships with various departments. Sharolyn Kawakami-Schulz Ph.D. , Director, Office of Professional Development at the University of Minnesota Medical School, adds, "Graduates will need to demonstrate their ability to continue to learn and adapt. Communication skills - written, oral, and to various audiences - will continue to be key in their ability to succeed and do well in all sectors."
Complete relevant clinical study manager training and internships
Research clinical study manager duties and responsibilities.
Clinical study managers oversee clinical studies and ensure that they are conducted in compliance with regulations. They manage the supply of investigational products, prepare regulatory documentation, and author clinical study reports. They also work with vendors and investigators to ensure that studies are conducted in accordance with Good Clinical Practice. They review and negotiate contracts, manage site monitoring, and conduct audits. As one clinical study manager explained, "I managed projects related to commercialization and post market analysis for abdominal aortic aneurysm repair grafts as guided by FDA regulations."
- Lead CRA training for Latin American, Asian and European to review GCP and protocol training.
- Manage projects relate to commercialization and post market analysis for abdominal aortic aneurysm repair grafts as guided by FDA regulations.
- Participate in CRF (EDC) design and CCG preparation.
- Assist patients who suffer from depression, bipolar mood disorders, mental retardation, schizophrenia, post-traumatic disorder.
Prepare your clinical study manager resume
When your background is strong enough, you can start writing your clinical study manager resume.
You can use Zippia's AI resume builder to make the resume writing process easier while also making sure that you include key information that hiring managers expect to see on a clinical study manager resume. You'll find resume tips and examples of skills, responsibilities, and summaries, all provided by Zippi, your career sidekick.
Choose From 10+ Customizable Clinical Study Manager Resume templates

Apply for clinical study manager jobs
Now it's time to start searching for a clinical study manager job. Consider the tips below for a successful job search:
- Browse job boards for relevant postings
- Consult your professional network
- Reach out to companies you're interested in working for directly
- Watch out for job scams

Are you a Clinical Study Manager?
Share your story for a free salary report.
Average clinical study manager salary
The average Clinical Study Manager salary in the United States is $78,195 per year or $38 per hour. Clinical study manager salaries range between $49,000 and $122,000 per year.
What Am I Worth?
How do clinical study managers rate their job?
Updated April 5, 2024
Editorial Staff
The Zippia Research Team has spent countless hours reviewing resumes, job postings, and government data to determine what goes into getting a job in each phase of life. Professional writers and data scientists comprise the Zippia Research Team.
Clinical Study Manager Related Careers
- Clinical Associate
- Clinical Coordinator
- Clinical Director
- Clinical Manager
- Clinical Project Manager
- Clinical Research Assistant
- Clinical Research Associate
- Clinical Research Coordinator
- Clinical Research Manager
- Coordinator And Research Assistant
- Medical Manager
- Practice Manager
- Research Administrator
- Research Coordinator
- Research Nurse
Clinical Study Manager Related Jobs
- Clinical Associate Jobs
- Clinical Coordinator Jobs
- Clinical Director Jobs
- Clinical Manager Jobs
- Clinical Project Manager Jobs
- Clinical Research Assistant Jobs
- Clinical Research Associate Jobs
- Clinical Research Coordinator Jobs
- Clinical Research Manager Jobs
- Coordinator And Research Assistant Jobs
- Medical Manager Jobs
- Practice Manager Jobs
- Research Administrator Jobs
- Research Coordinator Jobs
- Research Nurse Jobs
What Similar Roles Do
- What Does a Clinical Associate Do
- What Does a Clinical Coordinator Do
- What Does a Clinical Director Do
- What Does a Clinical Manager Do
- What Does a Clinical Project Manager Do
- What Does a Clinical Research Assistant Do
- What Does a Clinical Research Associate Do
- What Does a Clinical Research Coordinator Do
- What Does a Clinical Research Manager Do
- What Does a Coordinator And Research Assistant Do
- What Does a Medical Manager Do
- What Does a Practice Manager Do
- What Does a Research Administrator Do
- What Does a Research Coordinator Do
- What Does a Research Nurse Do
Resume For Related Jobs
- Clinical Associate Resume
- Clinical Coordinator Resume
- Clinical Director Resume
- Clinical Manager Resume
- Clinical Research Assistant Resume
- Clinical Research Associate Resume
- Clinical Research Coordinator Resume
- Medical Manager Resume
- Practice Manager Resume
- Research Coordinator Resume
- Research Nurse Resume
- Research Project Coordinator Resume
- Senior Clinical Research Associate Resume
- Senior Research Associate Resume
- Study Coordinator Resume
- Zippia Careers
- Executive Management Industry
- Clinical Study Manager
Browse executive management jobs
Clinical Research Manager

United States

Clinical Research Manager manages the clinical monitoring process and the administration of clinical trials. Supervises CRAs in in-house and on-site monitoring, filing, and clinical trial administration. Being a Clinical Research Manager oversees adherence to SOPs, Good Clinical Practice and FDA regulations. Helps with the development and implementation of clinical processes, procedures, and programs. Additionally, Clinical Research Manager may require a master's degree in nursing. May require ACRP or SOCRA Clinical Research Professional exam completion. Typically reports to a director. The Clinical Research Manager manages subordinate staff in the day-to-day performance of their jobs. True first level manager. Ensures that project/department milestones/goals are met and adhering to approved budgets. Has full authority for personnel actions. To be a Clinical Research Manager typically requires 5 years experience in the related area as an individual contributor. 1-3 years supervisory experience may be required. Extensive knowledge of the function and department processes.
Employers: Find Surveys For This Job
Employers: Job Description Management Tool
Employees: Get a Salary Increase
- Market Research Manager
- Clinical Research Director
- Clinical Research Associate I
- Scientist - Clinical Research
- Clinical Research Coordinator
- Clinical Research Associate II
- Top Clinical Research Executive
- Research and Development Manager
- Research and Development Engineer
- Research and Development Director
- Clinical Research Manager Salaries with a Master's Degree or MBA
- Clinical Research Manager Salaries with a JD, MD, PhD or Equivalent
- Pharmaceuticals
- Biotechnology
- Business Services
- Edu., Gov't. & Nonprofit
- MFG Durable
- MFG Nondurable
- San Diego, CA Clinical Research Manager Salaries
- Boston, MA Clinical Research Manager Salaries
- Raleigh, NC Clinical Research Manager Salaries
- San Jose, CA Clinical Research Manager Salaries
- New York, NY Clinical Research Manager Salaries
- Newark, NJ Clinical Research Manager Salaries
- Washington, DC Clinical Research Manager Salaries
- San Francisco, CA Clinical Research Manager Salaries
- Trenton, NJ Clinical Research Manager Salaries
- Philadelphia, PA Clinical Research Manager Salaries
Next Application Deadline: June 7
Home > Resources > Healthcare Analytics > Clinical Research Manager Job Description
Clinical Research Manager Job Description

- Published July 26, 2017
- Updated August 28, 2023
Clinical research managers hold one of the most critical jobs in the healthcare industry, overseeing all aspects of clinical trials that evaluate new medications and medical devices.
People in these positions carry the responsibility for making sure the trial is conducted under very strict, very specific guidelines.
The job carries a great deal of responsibility. Clinical trials are a vital part of the development of any new medication or medical device, and companies often have their future riding on the outcome. Clinical trial managers not only oversee the trial, but also act as liaison between the trial site and the clinical study sponsor.
It’s a difficult job, but one that can provide a rewarding, stable and often lucrative career.
What Clinical Research Managers Do
When a company develops a new healthcare product, they must meet government regulations before being allowed to sell their product on the market. This means conducting clinical trials that determine whether the product does what its developers say it’s going to do, as well as ensuring that the product is safe for use.
Clinical research managers have a variety of duties associated with such trials. They include:
- Planning and overseeing the trial
- Determining whether a product accomplishes the goal for which it was produced
- Ensuring the product meets all government regulations and standards
- Managing a team of clinical research associates and specialists
- Training clinical research team members and evaluating their performance
In addition, clinical research managers communicate with trial sponsors, keeping them updated on progress.
Skills and Education Needed
Working as a clinical research manager requires a mix of skills. They include leadership in guiding the research team, communication skills with both the team and study sponsors, as well as the technical skills needed to successfully plan and execute a clinical trial.
The people in this field come from various backgrounds. They typically have at least a bachelor’s degree in a field related to their clinical work, ranging from biology to life sciences and bioengineering. Attaining the manager position almost always requires a master’s degree or doctorate.
The ability to write study protocols and other research-related documents is key, as is expert-level knowledge in clinical trial practices and regulations. Project management skills also can prove beneficial for clinical research managers, given the complexity of the job and requirement to lead cross-functional teams.
Typically, those who become research managers also have years of experience at lower levels of the operation, including research specialists.
*National long-term projections may not reflect local and/or short-term economic or job conditions, and do not guarantee actual job growth. Information provided is not intended to represent a complete list of hiring companies or job titles, and program options do not guarantee career or salary outcomes. Students should conduct independent research for specific employment information.
Related Articles

What is Propensity Score Matching in Healthcare?

Using Analytics for Healthcare Inventory Management

Can EHRs Facilitate Improved Medication Reconciliation?
Academic calendar, get our program guide, if you are ready to learn more about our programs, get started by downloading our program guide now..
How can we help you?
Begin your journey with us – join our Early Career Talent Program! Apply today for Early Career Talent Program (Opens in new window)
Saved Jobs (0)

Job Details
Search Jobs
Clinical Research Manager
The Department of Dermatology (Dermatology Operations) within Stanford University, is a dynamic and innovative Department dedicated to excellence in research, medical education, and clinical care. Our Department is driven by over 70 faculty members and a cadre of staff who are the pillar of strength in the Department’s ongoing efforts into the prevention and treatment of various dermatologic diseases, conditions & disorders.
We are seeking a Clinical Research Manager (CRM) who is passionate about clinical research and project management. The CRM will report to the Associate Director, Clinical Research Operations and work with a robust clinical research team, hand in hand with Principal Investigators, Clinical Research Managers, Program Managers, and Associates in support of dermatology patients. The Clinical Research Manager will provide leadership and oversight of studies conducted by several affinity groups within the Department. The Clinical Research Manager will manage clinical research operations, relationships with other affinity groups and staff, study quality management, personnel management and career skill development, regulatory compliance, and fiscal oversight. The CRM will also assist with building automated clinical research workflows and solutions within the Department. The successful applicant will have the ability to supervise multiple clinical research staff, collaborate with external vendors, and enjoy working in a dynamic work environment. Exceptional diplomacy, interpersonal and communication skills are essential, as is a high degree of integrity. Attention to detail and the ability to manage multiple responsibilities simultaneously are also critical attributes.
Our Department strives to find team members who are passionate about their work, are creative and want to deliver results. We place a high priority on equipping our team members to perform their job efficiently, helping them acquire new skills and grow within the organization. We encourage our team members to have a healthy balance between work commitments and life outside of work and provide support to achieve this balance. If you are looking to make a large impact through global-reaching clinical research in a rapidly growing academic research organization, we encourage you to apply!
Note the position will be based on the Stanford Redwood City (SRWC) campus and is hybrid (working on-site and working from home) subject to operational needs.
Dermatology Clinical Research is a growing team that’s dedicated to supporting translational medicine and contributing to Stanford Medicine’s mission. We invite you to join our team!
Duties include:
- Hire, orient, train, and conduct performance reviews for staff handling research administration activities associated with the conducting of clinical trials. Monitor staffing levels and identify adequate coverage for trial workload across teams of study coordinators.
- Supervise the implementation of and adherence to study protocols. Educate research staff on established policies, processes, and procedures.
- Determine effective strategies for promoting/recruiting research participants and retaining participants in long-term clinical trials. Develop consent forms for approval by Human Subjects Panel.
- Coordinate new protocol submissions, renewals, and revisions to Institutional Review Board for multiple studies. Complete annual reports to Institutional Review Board, CSTA, FDA and other regulatory agencies. Submit Investigational New Drug applications to the FDA as required.
- Audit operations, including laboratory procedures, to ensure compliance with applicable regulations; provide leadership in identifying and implementing corrective actions/processes. Monitor Institutional Review Board submissions and respond to requests and questions.
- Provide leadership and expertise in identifying and completing research grants. Oversee financial resources, as needed, create internal and external budgets for research protocols, assure financial accountability, and serve as primary liaison between sponsor, department accounting, and Research Management Group.
- Lead or chair committees or task forces to address and resolve significant issues.
- Engage in high-level outreach and networking opportunities, representing the research program to a variety of internal and external audiences.
- Analyze trends in recruitment and assure there is a limited number of competing trials. Make recommendations for a variety of options within a trial; track physician compliance.
- Assist with analysis of data and preparation of manuscripts and scientific presentations.
* - Other duties may also be assigned
DESIRED QUALIFICATIONS:
- Experience developing reports and dashboards.
- Experience with Smartsheet.
- Experience with Notion.
EDUCATION & EXPERIENCE (REQUIRED):
Bachelor's degree in related field and five years of experience in clinical research, or an equivalent combination of education and relevant experience. Master's degree preferred.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Excellent interpersonal skills.
- Proficiency in Microsoft Office and database applications.
- Experience with research protocols and regulatory or governing bodies, which include HIPAA and FDA regulations, Institutional Review Board requirements, and Good Clinical Practices.
- Knowledge of medical terminology.
- Demonstrated managerial experience.
CERTIFICATIONS & LICENSES:
Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
PHYSICAL REQUIREMENTS*:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
* - Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
WORK STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University's Administrative Guide, http://adminguide.stanford.edu .
The expected pay range for this position is $108,000 to $136,000 per annum/hour. Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website ( https://cardinalatwork.stanford.edu/benefits-rewards ) provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources by submitting a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Share this job
Similar jobs.
- Research Environmental Measurements Facility Laboratory Manager Stanford, California
- Research Process Operations Engineer Stanford, California
- Research Director of Research Data Science Stanford, California
About the Location
Find out what it’s like to live and work in Stanford, California.
Find your Fit
Jobs For You
No Recently Viewed Jobs.
No Jobs Saved.
Join Our Talent Community
Be the first to hear about job openings, upcoming events, and news at Stanford School of Medicine.
Country Code +1 +1242 +1246 +1264 +1268 +1284 +1340 +1441 +1473 +1649 +1664 +1670 +1671 +1684 +1758 +1767 +1784 +1849 +1868 +1869 +1876 +1939 +20 +211 +212 +213 +216 +218 +220 +221 +222 +223 +224 +225 +226 +227 +228 +229 +230 +231 +232 +233 +234 +235 +236 +237 +238 +239 +240 +241 +242 +243 +244 +245 +248 +249 +250 +251 +252 +253 +254 +255 +256 +257 +258 +261 +262 +264 +265 +266 +267 +268 +269 +27 +290 +291 +297 +298 +299 +30 +31 +32 +33 +34 +345 +350 +351 +352 +353 +354 +355 +356 +357 +358 +359 +36 +370 +371 +372 +373 +374 +375 +376 +377 +378 +379 +380 +381 +382 +385 +386 +387 +389 +39 +40 +41 +420 +421 +423 +43 +44 +45 +46 +47 +48 +49 +500 +501 +502 +503 +504 +505 +506 +507 +508 +509 +51 +52 +53 +54 +55 +56 +57 +58 +590 +591 +593 +594 +595 +596 +597 +598 +599 +60 +61 +62 +63 +64 +65 +66 +670 +672 +673 +674 +675 +676 +677 +678 +679 +680 +681 +682 +683 +685 +686 +687 +688 +689 +690 +692 +7 +77 +81 +82 +84 +850 +852 +853 +855 +856 +86 +872 +880 +886 +90 +91 +92 +93 +94 +95 +960 +961 +962 +963 +964 +965 +966 +967 +968 +970 +971 +972 +973 +974 +975 +976 +977 +98 +992 +993 +994 +995 +996 +998 Phone Number
Email Address
Upload Resume Remove
Opt-in Promotion
By signing up, I acknowledge I have read the Stanford Medicine privacy notice , and I wish to receive email communications. I understand I can opt out from receiving email communications at any time.
Confirm Email
Already signed up? Update your profile here .
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
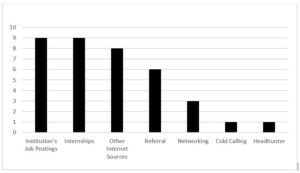
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
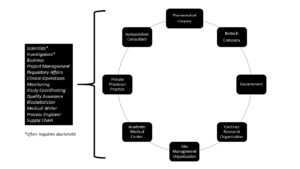
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Clinical Research Coordinator
- Ophthalmology
- Columbia University Medical Center
- Opening on: Apr 23 2024
- Job Type: Officer of Administration
- Regular/Temporary: Regular
- Hours Per Week: 35
- Salary Range: $62,400 - $65,000
Position Summary
Under the direction of the Director of the Clinical Trials Unit (CTU) and Principal Investigators, the Clinical Research Coordinator will conduct clinical research studies (industry-sponsored and investigator-initiated) within the Columbia University Irving Medical Center (CUIMC) Department of Ophthalmology in adherence with assigned study protocols and manuals of operation and in accordance with clinical research principles.
Responsibilities
- Serve as the contact person for those interested in study participation and assist with recruitment activities including pre-screening electronic medical records for eligibility, contacting potential subjects, explaining all study procedures, and consenting eligible subjects or assenting parents or guardians for children enrolled in research studies.
- Coordinate day-to-day aspects of study related procedures, including, but not limited to scheduling visits and procedures, data entry, preparing for research visits, research visit documentation, maintenance of regulatory binders and study files, creation and/or maintenance of source documentation, preparation for monitoring visits, site initiation/closeout visits and audits as needed.
- Be able to coordinate and perform research testing and imaging for clinical research studies including but not limited to visual acuity, refraction, dark adaptation, visual field, microperimetry, fluorescein angiography, fundus photography, optical coherence tomography (OCT), ICG angiography, slit lamp photography, MP1, corneal mapping, specular biomicroscopy including confocal imaging, HRT Analyzer (glaucoma), and ERGs.
- Be able to administer surveys, such as the National Eye Institute Vision Function Questionnaire (NEI-VFQ-25), EuroQOL-5 Dimension, Reading speed, Health Utilities Index.
- Work with the research team and ocular photography department to ensure that all required eye exams and ocular testing are scheduled and completed according to protocol.
- Obtain and maintain study certifications for ETDRS, OCT, and photography for clinical trials.
- Obtain access to sponsors’ electronic data capture (EDC) systems, complete EDC trainings, and enter data into the EDC within 5 days of seeing the study patient.
- Maintain and organize study-related documentation and records using the EDC platforms, including capturing adverse events and serious adverse events and preparing for monitoring visits.
- Respond to all sponsor-related queries in a timely manner.
- Ensure that all aspects of Good Clinical Practice are followed at all times by developing and ensuring adherence with Standard Operating Procedure (SOP) for clinical studies being conducted in the Ophthalmology Clinical Trials Unit.
- Work with the Regulatory Manager to gain CUIMC Institutional Review Board (IRB) approval in a timely manner by creating informed consent forms using sponsors’ templates, responding to IRB correspondents, submitting amendments, renewals, modifications, and other regulatory documents required by the sponsor and FDA, including progress reports.
- Ensure that all appropriate Institutional, State, and Federal regulations are followed throughout the course of the study according to study-related protocols and manuals.
- Work directly with sponsors’ designated Clinical Research Organizations (CRO) to complete all required study start-up documents including FDA 1572 forms, investigator signatures, CVs, medical licenses, Conflict of Interest, HIPAA, and Human Subjects Trainings in a timely manner.
- Complete feasibility forms requested by sponsors in a timely manner to assess ophthalmic equipment and examination rooms to conduct the studies.
Minimum Qualifications
- Bachelor’s degree or equivalent in education and experience, plus minimum of 1 to 2 years of related experience.
- Conform to all applicable HIPAA, billing compliance and safety requirements.
- Must be able to work effectively with minimal supervision.
- Prior research experience to include recruiting study participants, conducting standardized protocol visits and data entry.
- Excellent verbal and written communication skills and attention to detail required.
- Computer skills (Word, Excel) required.
- Excellent interpersonal skills.
- Willingness to travel to different sites.
Preferred Qualifications
- Working knowledge of Spanish
- Phlebotomy license
- Prior experience in ophthalmology
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs

Clinical Research Coordinator - General Medicine
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .

Principal Clinical Trial Manager, Clinical Operations
What you'll do.
- Coordinate and manage production of key deliverables for external research collaborations and clinical studies to study completion
- Identify barriers to timely and successful study execution and propose solutions, with regular reporting of study performance metrics
- Manage internal resources, external partners, consultants, vendors, and internal/external budgets to ensure the timely and cost-effective implementation of the clinical strategy for one or more clinical studies
- Manage all clinical aspects of study: budget and metrics; operations; study document development and review (study specific plans, eCRF Guidelines, lab manuals, etc.)
- Assess and recommend CROs and preferred vendors for implementation of clinical studies, and subsequently manages CRO relationships and contracts for study execution, where applicable
- Ensure audit readiness at all times by assessing and mitigating study compliance with all regulatory requirements (knowledge of GCP, ICH guidelines and regulatory requirements for clinical trial management is required)
- Provide input in the development of clinical trial related documents including but not limited to: protocols, case report forms, informed consents, timelines, monitoring plans, laboratory manuals, training materials, and site initiation visit slide presentation
- Maintain proficiency in understanding of early detection practices within oncology and an in-depth knowledge of molecular testing practices within multiple cancer types
What you'll have accomplished 12 months from now
- You will have established an exemplary rapport and relationship with external partners and vendors
- Assessed and recommended CROs and preferred vendors for implementation of clinical studies, and subsequently managed CRO relationships and contracts for study execution, where applicable
- Managed the study submission to IRB in coordination with CRO, as appropriate
- Overseen the execution of site training, either performed by DELFI Diagnostics or CRO partners, and ongoing educational interventions to assure compliance with study protocols, as appropriate
- Ensured external collaborations and clinical trials are executed in compliance with the protocol and ICH/GCP guidelines/regulations: participate in the planning of quality assurance activities and coordinate resolution of audit findings of vendors and CROs; ensure audit-ready condition of clinical trial documentation
- Consulted with cross-functional teams on data collection, regulatory questions, and protocol execution as it pertains to the DELFI assay
- Established and maintained effective communication and collaboration with functional area peers, including research, clinical lab operations team, regulatory, quality assurance, and commercial, as well as thought leaders to meet program objectives
- You will have taken ownership of the financial aspects of assigned program(s) and trial(s) including collaboration with finance partners to generate and review forecasts and accruals and escalates variances as appropriate
- You will have supported Senior Management by reviewing the unique enrollment requirements for a clinical study and provided a strategy for successful trial enrollment and completion
- You will have assisted with authoring and review of multiple departmental SOPs
- You will be a respected member of the clinical development team, having contributed to the collaborative delivery of key trials and analyses
What you'll bring to DELFI
- Bachelor’s degree (or higher) in science or health-related discipline
- 10 years of direct clinical operations experience in a sponsor (Pharmaceutical or Biotech) or a CRO role and bachelor’s degree; or 8 years with a Master’s degree; or 5 years experience with a PhD; or equivalent experience
- Industry experience within in vitro diagnostics (IVD)
- Demonstrated experience in participating in the development, implementation, management, and completion of large, regulated clinical trials
- Ability to assist in preparation of a clinical development plan, clinical trial design, protocol writing, and essential documents
- Strong understanding of GCPs, ICH, and knowledge of regulatory requirements
- Familiarity with ISO 20916 and/or 14155 standards preferred
- Well developed written and verbal communication skills demonstrated by an ability to present clear instruction/direction to teams at the same level in the organization and influence at higher levels in the organization
- Excellent organizational and interpersonal skills to manage multiple priorities and external relationships within various research collaborations
- Ability and willingness to travel ~10-15% of the time
- Significant CRO Project Management experience (either at a CRO or managing CROs)
- Global clinical trial experience
- Knowledge of medical device clinical trials design, clinical research and best practices from early stage through regulatory submission
Delfi Diagnostics Inc. Home Page

Cancer Clinical Research Project Manager – Data Quality & Study Start- Up 103005 (Hybrid)
🔍 school of medicine, stanford, california, united states.
Cancer Clinical Research Project Manager – Gyn-Onc (Hybrid)
The Stanford Cancer Institute (SCI) is one of an elite number of National Cancer Institute-Designated Comprehensive Cancer Centers in the country, and is a prominent, dynamic, growing and complex Institute within the Stanford University School of Medicine. The SCI actively works to build synergies and collaborations among faculty with cancer-relevant expertise from four Schools and over 30 departments across Stanford University. Given the SCI's mission, breadth, and depth, it employs over 320 staff members in a fast-paced, team-oriented, and forward-thinking environment with tremendous opportunities for personal and professional growth. The Cancer Clinical Trials Office (CCTO) is an integral component of the Stanford Cancer Institute. The vital work performed there enables our adult and pediatric cancer centers to translate research from the laboratory into the clinical setting. You will be working with an unparalleled leading-edge community of faculty and staff who are fundamentally changing the world of health care in the cancer arena.
We seek a Cancer Clinical Research Project Manager (CCRPM) to help us enact our mission to reduce cancer mortality through comprehensive cancer research, treatment, education, and outreach. Our CCRPM will provide oversight for data management integrity and study start-up process across a cancer clinical research group. They will independently manage significant and key aspects of clinical research study data entry, data analysis, and training of team members for all aspects of data quality. This position collaborates closely with physician investigators and clinical research staff to drive successful implementation of cancer clinical trials. Responsibilities include working with the research team(s), clinical staff, and Stanford Health Care departments to support conduct of safe and compliant clinical research.
Reporting to the Gynecological oncology (Gyn-Onc) Clinical Research Manager (CRM), the CCRPM will be conversant in the goals, mission, and priorities of the Institute, and utilize this knowledge to ensure the safety and well-being of trial participants. We seek candidates with excellent interpersonal skills and attention to detail. Our staff run toward challenges, and you will have a demonstrated history of doing the same with a high degree of professionalism, initiative, and flexibility.
Duties include*:
- Oversee data management for research projects. Develop and manage systems to organize, collect, report, and monitor data collection. Extract, analyze, and interpret data.
- Develop project schedules, targets, measurements, and accountabilities, as assigned. Lead team meetings and prepare/approve minutes.
- Formally supervise, train, and/or mentor new staff or students, as assigned, potentially including hiring, preparing or assisting with the preparation of performance evaluations, and performing related duties, in addition to instruction on project work.
- Audit operations, to ensure compliance with applicable regulations; provide leadership in identifying and implementing corrective actions/processes.
- Collaborate with principal investigators and study sponsors, monitor and report serious adverse events, and resolve study queries.
- Provide leadership in determining, recommending, and implementing improvements to policies/processes; define best practices.
- Ensure regulatory compliance. Regularly inspect study document to ensure ongoing regulatory compliance.
*- Other duties may also be assigned.
DESIRED QUALIFICATIONS:
- Knowledge of clinical trials data management and clinical quality management.
- Clinical knowledge in the field of Gynecology Oncology.
- Knowledge of various projects and implementation strategies.
EDUCATION & EXPERIENCE (REQUIRED):
Bachelor's degree in a related field and two years of experience in clinical research, or an equivalent combination of education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office and database applications.
- Experience with research protocols and regulatory or governing bodies, which include HIPAA and FDA regulations, Institutional Review Board requirements, and Good Clinical Practices.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred. May require a valid California Driver’s License.
PHYSICAL REQUIREMENTS*:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
*- Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
WORK STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University's Administrative Guide, http://adminguide.stanford.edu .
The expected pay range for this position is $69,100 to $92,000 per annum.
Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website ( https://cardinalatwork.stanford.edu/benefits-rewards ) provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources by submitting a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
- Schedule: Full-time
- Job Code: 4923
- Employee Status: Regular
- Requisition ID: 103005
- Work Arrangement : Hybrid Eligible
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: Jan 29, 2024
Post Date: Feb 15, 2023
Post Date: Aug 05, 2022
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
Toward Pharmacy-Led Value Based Care Model for Buprenorphine Initiation and Management (PharmValue): Development of a Scalable Pharmacist-Led Collaborative Practice Agreement for Medication for Opioid Use Disorder
Pharmacist involvement in managing medications for opioid use disorder (MOUD), such as buprenorphine, has been previously shown feasible and has the potential for nationwide scalability. To maximize impact, such models must make use of scope of practice enhancing authorities, such as collaborative practice agreements. This project will: (1) engage community stakeholders to co-develop a model collaborative practice agreement for pharmacist-managed buprenorphine care and (2) identify existing legal authorities and advocacy opportunities to expand pharmacist-managed buprenorphine care in all 50 states. This will be accomplished using a community-engaged research approach as well as two 50-state reviews of state statutes, rules, and adopted policies or existing guidelines related to pharmacist-managed MOUD, particularly focused on pharmacist prescriptive authority and, separately, existing state-level legal barriers to MOUD care.
Principal Investigator(s)
Kenneth hohmeier, pharmd.
881 Madison Avenue Memphis , TN 38163 United States
Rachel Barenie, PharmD, J.D., M.P.H.
- Open access
- Published: 24 April 2024
The effect of obesity and subsequent weight reduction on cardiac morphology and function in cats
- Catheryn Partington 1 , 4 ,
- Hannah Hodgkiss-Geere 1 ,
- Georgia R. T. Woods 2 ,
- Joanna Dukes-McEwan 1 ,
- John Flanagan 3 ,
- Vincent Biourge 3 &
- Alexander J. German 2
BMC Veterinary Research volume 20 , Article number: 154 ( 2024 ) Cite this article
Metrics details
In people, obesity is a risk factor for cardiovascular disease, associated with systemic hypertension, cardiac remodelling and systolic and diastolic dysfunction. Weight reduction can reverse myocardial remodelling and reduce risk of subsequent cardiovascular disease. In cats, far less is known regarding the effects of obesity and subsequent weight reduction on cardiovascular morphology and function. This prospective study aimed to assess cardiac morphology and function, heart rate variability, cardiac biomarkers and body composition before and after controlled weight reduction in cats with obesity. Body composition analysis (by dual energy x-ray absorptiometry, DEXA) and cardiovascular assessment (echocardiography, systemic arterial systolic blood pressure, electrocardiography, plasma cardiac biomarkers) were performed prior to weight management in twenty cats with obesity. These investigations were repeated in eleven cats that reached target weight.
At baseline, systemic hypertension was not documented, but the majority of cats with obesity (15 out of 19) showed echocardiographic evidence of diastolic dysfunction. Eleven of 20 cats had increased maximal end-diastolic septal or left ventricular free wall thickness (≥ 6.0 mm) at baseline. Median (interquartile range) percentage of weight lost in the cats reaching target weight was 26% (17–29%), with a median reduction in body fat mass of 45% (26–64%). Both the end-diastolic left ventricular free wall (median magnitude of change -0.85 mm, IQR -0.05 mm to -1.55 mm, P = 0.019; median percentage reduction 14.0%) and end-diastolic interventricular septum (median magnitude of change -0.5 mm, IQR -0.2 mm to -1.225 mm, P = 0.047; median percentage reduction 7.9%) thickness decreased after weight reduction. Following weight reduction, pulsed wave tissue Doppler imaging of the left ventricular free wall was consistent with improved diastolic function in 4 out of 8 cats, however there was no significant difference in overall diastolic function class. Further, there was no change in heart rate variability or cardiac biomarkers with weight reduction.
An increase in left ventricular wall thickness and diastolic dysfunction were common echocardiographic features in cats with obesity within our study and may be reversible with successful weight and fat mass loss. Further studies are required to clarify the clinical consequences of these findings.
Peer Review reports
Introduction
The cardiovascular effects of obesity in people are well documented, not only resulting from the association with atherosclerosis and ischaemic myocardial disease but also from the effects on cardiac morphology and function [ 1 , 2 ]. Obesity is associated with chronic increases in preload and afterload, activation of the renin–angiotensin–aldosterone system and sympathetic nervous system as well as alterations in cellular homeostasis and energy metabolism. One consequence is that systemic hypertension is common amongst people with obesity [ 2 ]. Left ventricular (LV) hypertrophy is commonly reported, with or without concurrent chamber dilatation [ 3 ]. Impaired diastolic and systolic function are also frequently seen [ 2 ]. Consequently, obesity is an independent cardiovascular risk factor in people [ 2 ].
Obesity in pet cats and dogs is also a major health concern, associated with increased morbidity and mortality [ 4 , 5 ]. The cardiovascular effects of obesity reported in dogs share some similarities to those observed in people, although findings amongst reports are variable [ 6 , 7 , 8 , 9 ]. Varying patterns of left ventricular hypertrophy are reported in dogs with obesity [ 6 , 7 ], in addition to diastolic dysfunction in some [ 6 , 8 ], but not all [ 7 ] reports, whilst the presence of systemic hypertension in dogs with obesity remains variable between studies [ 6 , 7 , 8 , 9 ]. In cats, less is known regarding the cardiovascular effects of obesity. Litster and Buchanan [ 10 ] reported no significant echocardiographic changes in cats with obesity compared with cats in ideal weight, besides an increase in precordial distance and increased thickness of the right ventricular free wall. In contrast, De Souza et al. [ 11 ] reported an increase in vertebral heart score, diastolic left ventricular free wall (LVFWd) thickness and blood pressure in 20 cats with obesity, whilst Stepien et al. [ 12 ] similarly reported increased wall thickness but reported no significant change in blood pressure. Diastolic function was not assessed in either report; however, Champion [ 13 ] reported an association between increased left ventricular wall thickness (LVWT) and the presence of diastolic dysfunction in 22 cats that were overweight or had obesity.
Hypertrophic cardiomyopathy (HCM) is the most common acquired heart disease of cats, effecting around 14.7% of the population, and is defined as increased LVWT in the absence of abnormal loading conditions [ 14 ]. Cats with a greater bodyweight are reported to have an increased risk of developing HCM, whilst the association between body condition score (BCS) and risk of developing HCM varies between reports [ 14 , 15 ]. Further, other disease processes may result in increased LVWT, mimicking a HCM phenotype. Thus, uncertainty about the impact of obesity on cardiac structure and function may hinder interpretation of increased LVWT and thus complicate the diagnosis of HCM in cats with obesity.
In humans, obesity is also associated with an increase in heart rate, mediated for the most part by altered sympathovagal balance [ 2 ]. Heart rate variability (HRV) is an indicator of this autonomic tone and has been used to predict risk of cardiovascular disease in people with obesity [ 16 ]. Studies assessing HRV in dogs with obesity show varied results. While Champion [ 13 ] reported no difference in heart rate variability in cats in overweight or obese condition, compared with those in ideal body condition score; however, to the authors’ knowledge, the effect of controlled weight reduction in cats has not been assessed.
Therefore, the first aim of the current study was to assess a cohort of cats with obesity for the presence of systolic or diastolic dysfunction, altered wall thickness and systemic hypertension. A second aim was to monitor for changes in echocardiographic variables, cardiac biomarkers, and systemic blood pressure in response to a controlled weight reduction programme, using dual energy x-ray absorptiometry (DEXA) to quantify changes in body composition. Thirdly, we aimed to monitor for changes in autonomic balance by examining changes in HRV during controlled weight reduction. We hypothesised that cats with obesity would show signs of diastolic dysfunction and increased left ventricular wall thickness, which may improve with weight reduction.
Study animals
Twenty cats were enrolled, of which 11 reached their weight reduction target. All cats were of the domestic shorthair breed, with a median age of 7.4 years (interquartile range [IQR] 5.6 -8.9 years) at time of enrolment (Supplementary Table 1 ). There were 8 females and 12 males (all neutered). Median weight for all cats at enrolment was 7.2 kg (IQR 6.46- 8.38 kg) with a median body condition score (BCS) of 8/9 (IQR 8–9). Two cats did not undergo DEXA post-weight reduction due to lack of consent for sedation. There was no difference in the age ( P = 0.518, r [effect size] = 0.14), bodyweight ( P = 0.820, r = 0.05) or BCS ( P = 0.222, r = 0.27) at time of inclusion between those cats that did and did not achieve target bodyweight.
Baseline cardiovascular variables (all cats)
The baseline data for cardiovascular variables for all cats is shown in Supplementary Tables 1 and 2 . Baseline systolic blood pressure (SBP) was within the reference interval [RI] of < 160 mmHg (median 135 mmHg, IQR 120 – 147 mmHg) in all cats, except the two for which this data were missing (due to measurement not being tolerated). Both N-terminal Pro B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin I (hs-cTnI) were missing for three cats at inclusion. Concentration of NT-proBNP was less than the laboratory upper reference range of 100 pmol/L in 17/17 cats (median 23.9 pmol/L, IQR 23.9–27.5 pmol/L). Of the two cats that had increased hs-cTnI, the concentration was only mildly increased (0.055 ng/mL, RI ≤ 0.04 ng/mL) in one; in this cat, NT-proBNP was 61.0 pmol/L and left atrial (LA) size was normal, but LVWT was mildly increased (diastolic interventricular septum [IVSd] 6.0 mm). In the second cat, hs-cTnI was moderately increased (0.134 ng/mL); in this cat, NT-proBNP was 31.0 pmol/L, LA size was normal but there was a mild increase in LVWT (IVSd 6.3 mm). In both cats, electrocardiography (ECG) revealed normal sinus rhythm. Normal hs-cTnI concentrations were documented at baseline in all other cats (median 0.007 ng/mL, IQR 0.004–0.038 ng/mL). Median vasovagal tonus index (VVTI) was 5.21 (IQR 4.93–5.70).
On echocardiography, LVWT was increased (defined as ≥ 6.0 mm) in most (11/20) cats, with wall thickness being equivocal (defined as 5.5—5.9 mm) in an additional 5/20 cats. The increase in LVWT affected the interventricular septum more often than the free wall, with an IVSd ≥ 6.0 mm in 11/20 cats and an IVSd of 5.5- 5.9 mm in 2/20 (6.05, IQR 5.23–6.58 mm), compared with a LVFWd ≥ 6.0 mm in 5/20 cats and 5.5- 5.9 mm in 7/20 cats (5.55, IQR 5.13–5.98 mm). One cat had systolic anterior motion of the mitral valve, causing mild dynamic left ventricular outflow tract (LVOT) obstruction (LVOT velocity 2.25 m/s). A second cat had mild chordal anterior motion, but no obvious increase in LVOT velocity (1.34 m/s). Most cats had no LA dilation; short axis left atrium to aorta ratio (LA/Ao) < 1.6 in 19/20 cats (1.30; IQR 1.22–1.44) and maximal left atrial diameter (LADmax) < 16 mm in 12/18 cats (15.05 mm, IQR 13.70–16.03 mm).
For one cat, diastolic function class was not determined due to incomplete echocardiographic data. At baseline, diastolic function was considered to be normal in 4/19 cats, whilst 9/19 cats had impaired relaxation and 6/19 had pseudonormal diastolic function. Tissue Doppler imaging of the LVFW was consistent with impaired relaxation (E’/A’ < 1) in 13/17 cats (pulsed wave tissue Doppler imaging [pw-TDI] data incomplete for 2 cats). Transmitral flow showed an impaired relaxation pattern in 7/17 cats and isovolumetric relaxation time (IVRT) was increased in 7/19 cats (median 59.0, IQR 54.0–63.0 ms, RI < 60.0 ms). For the right lateral wall E’/A’ was < 1 in 18/18 cats (pw-TDI of the right wall data incomplete for 2 cats). There was no evidence of systolic dysfunction based on fractional shortening (54%, IQR 48.3–59.5%, RI > 30%) in any cat; however, pw-TDI S’ velocities of the septal and lateral walls were mildly decreased in 8/19 and 9/19 cats, respectively (IVS 6.5 cm/s, IQR 4.9–9.0 cm/s; LVFW 6.6 cm/s, IQR 4.8–9.4 cm/s; RI > 6 cm/s [ 17 ], for the 19 cats).
Six-lead ECG in 19/20 cats showed sinus rhythm, one of which had a left anterior fascicular block pattern and two showed evidence of intraventricular conduction disturbance (as shown by altered QRS morphology). One cat had sinus bradycardia with a heart rate of 120 bpm. The median heart rate for all cats was 180 bpm (IQR 160–215 bpm).
Weight reduction outcomes
Outcomes of weight reduction, for the 11 cats that reached their target weight, are shown in Table 1 . The median duration of weight loss was 190 days (IQR 162–229 days) and median weight lost was 1.45 kg (IQR 1.16–2.35 kg), equating to a decrease of 26% (IQR 17–29%) of starting body weight. Median rate of weight loss was 1.64% per week (IQR 1.07–2.29%). Body condition score decreased by a median of 3 units (IQR 3–3, P = 0.003, r = 0.90), lean mass change was -7.6% (IQR -3.4% to -8.4%; P = 0.008, r = 0.89) and body fat change was -45% (IQR -26% to -64%, P = 0.008, r = 0.89).
Changes in cardiovascular variables with weight reduction
Cardiovascular variables before and after weight reduction are shown in Table 2 . Systolic blood pressure measurement was missing for one cat after weight reduction. Two cats were hypertensive on this second assessment (SBP 170 mmHg and 180 mmHg), whilst all the remaining cats were normotensive. There was no change in SBP ( P = 0.438, r = 0.26), heart rate ( P = 0.932, r = 0.26) or HRV ( P = 0.790, r = 0.08) with weight reduction. Cardiac biomarkers were missing for four cats after weight reduction; for the remaining seven cats, there was no change in NT-proBNP ( P = 0.144, r = 0.55) or hs-cTnI ( P = 0.461, r = 0.28) concentrations. Of the two cats with increased hs-cTnI at baseline, this normalised after weight reduction in one cat, but remained mildly increased in the other (0.045 nmol/L, RI ≤ 0.04); NT-proBNP concentration was increased (110 pmol/L, RI < 100) and an increase in LVWT was also seen in this cat. For all other cats, cardiac biomarkers remained within normal reference range at this second time point.
After weight reduction, 4/11 cats had equivocal LVWT and five increased LVWT (compared with 1/11 and 8/11 respectively, at baseline). Six-lead ECG showed sinus rhythm in all cats; in addition, one cat had a left anterior fascicular block pattern and one had evidence of intraventricular conduction disturbance based on QRS morphology. In one cat, there was progression of LVWT and increased cardiac biomarkers (SBP was within the RI); this cat was considered to have progressive preclinical HCM and was not included in further statistical analysis. In the remaining 10 cats, there were decreases in both the maximal LVFWd (median magnitude of change -0.85 mm, IQR -0.05 mm to -1.55 mm, P = 0.019, r = 0.74; median percentage reduction 14.0%) and maximal IVSd (median magnitude of change -0.5 mm, IQR -0.2 mm to -1.225 mm, P = 0.047, r = 0.63; median percentage reduction 7.9%) after weight reduction (Fig. 1 ). There was no change in any other two-dimensional (2D) echocardiographic variable with weight reduction. There was a trend for the diastolic function class to improve, but this did not reach statistical significance ( P = 0.763). When assessing individual variables of diastolic function, there was an increase in the LVFW pw-TDI derived E’A’ ratio with weight reduction ( P = 0.012, r = 0.89); this ratio normalised in 4/8 cats, remained normal in 2/8 and remained consistent with impaired relaxation in 2/8 cats. There were no changes in the other variables of diastolic function, nor of systolic function, with weight reduction.
Changes in left ventricular wall thickness following controlled weight reduction in 10 cats. Line plots showing the changes in a maximal end-diastolic interventricular thickness and b maximal end-diastolic left ventricular free wall thickness with weight reduction for each cat achieving target weight reduction. The cat with progressive HCM has been excluded. IVSd: end-diastolic interventricular septum thickness, LVFWd: end-diastolic left ventricular free wall thickness
In people, the cardiovascular effects of obesity are well documented [ 2 ]; however, less is known about the cardiovascular effects of obesity in cats. In the current study, cats with obesity frequently had an increase in LVWT and diastolic dysfunction, with some improvement following weight reduction suggesting that these changes might be reversible. DEXA body composition results confirmed loss of fat mass as expected during weight reduction.
Fifty-five percent (11/20) of cats in this study had at least one wall measurement equal to or exceeding 6 mm (IVSd ≥ 6 mm in 11/20; LVFWd ≥ 6 mm in 5/20), consistent with a HCM phenotype [ 20 ]. This corresponds to the changes seen in people and dogs with obesity in which LV hypertrophy is commonly reported [ 2 , 7 , 8 , 9 ]. Our findings are also consistent with those of Stepien et al. [ 12 ] who reported LV hypertrophy in overweight cats. Development of increased LVWT in obesity is likely to be multifactorial involving both myocardial remodelling in response to altered preload and afterload, as well as myocardial and epicardial lipid deposition and altered cellular metabolism [ 1 , 21 , 22 ]. Insulin resistance and increased serum insulin-like growth factor concentration have been identified in some cats with HCM, supporting this role of metabolic changes in the development of increased LVWT [ 23 , 24 ]. Further, an association between both bodyweight and BCS and serum concentrations of insulin, insulin-like growth factor and glucose has been shown in cats with subclinical HCM [ 23 ]. The presence of increased LVWT in cats with obesity brings into question the possible impact on cardiac function and long-term cardiac morbidity and mortality, as well as highlighting the need for clarifying interpretation of LVWT measurements in cats with obesity. Häggström et al. [ 25 ] showed that many echocardiographic variables (including LA size and LVWT) increase with increasing body weight and, therefore, proposed the use of allometric scaling to bodyweight. However, since the BCS of cats in that study were not documented, it was unclear as to whether the increase in cardiac dimensions noted was associated with lean mass, fat mass or both. Most cats in our study had normal LA size, despite above average bodyweight and increased LVWT; this might suggest that increased fat mass and increased lean mass do not have the same effects on cardiac dimensions.
Decreased LVWT with weight reduction is seen in both people and dogs with obesity [ 7 , 9 , 26 ], and was also seen in this study, with a significant decrease in both the LVFWd and IVSd (median reduction of -0.85 mm and -0.5 mm for the LVFWd and IVSd respectively; following exclusion of one cat deemed at follow-up to have primary HCM). In people, improvement in LVWT with weight reduction is in-part a result of reduced afterload secondary to improved systemic hypertension [ 2 , 22 ]. However, systemic hypertension was excluded in cats from the current study, indicating this reverse remodelling must be multifactorial; possible mechanisms could include a decrease in lipid accumulation within cardiomyocytes and metabolic changes [ 1 , 22 ]. In one study, there was a decrease in LVWT in cats with subclinical HCM following diet change (starch restricted, high protein diet supplemented with eicosapentaenoic acid and docosahexaenoic acid), despite no clinically significant change in body weight or BCS [ 27 ]. In the current study, the change in diet may also have influenced LVWT and, therefore, we cannot definitively conclude that the decreased wall thickness was a result of reduced body fat.
Diastolic dysfunction was evident in many of the cats with obesity in the current study, but systolic dysfunction was not observed. Both diastolic and systolic dysfunction are reported in people with obesity, resulting in an increased risk of heart failure [ 2 , 21 ], whilst diastolic dysfunction has also been reported in dogs with obesity [ 6 ]. In our study, although the change in overall diastolic function class did not change with weight reduction, there was a significant change in the pw-TDI E’A’ ratio of the LVFW, which normalised in 4/6 cats (impaired relaxation pre-weight reduction). This suggests an improvement in diastolic function following controlled weight reduction, as reported in humans [ 28 , 29 ]; in contrast, no such improvement was seen in dogs [ 30 ]. It would be necessary to study a larger number of cats in order to assess this further. Development of diastolic dysfunction is multifactorial: triglyceride accumulation increases apoptosis of cardiomyocytes, renin–angiotensin–aldosterone system activation and increased aldosterone concentration contribute to myocardial fibrosis, whilst inflammatory cytokines contribute to fibrosis and increased wall stiffness, all contributing to diastolic dysfunction [ 2 ]. However, many other variables including age and SBP may affect diastolic function, with diastolic dysfunction being a ‘normal’ finding on echocardiography of older animals. Given the small sample size, we did not attempt to correct for these potentially confounding variables in the statistical comparisons. However, our cohort did include young cats in which diastolic dysfunction would not be expected (age range 1.3 – 12.8 years); furthermore, improvement in diastolic function would not be seen if a result of age, we thus concluded that obesity was a more likely cause.
Given that obesity is associated with increased sympathetic drive [ 31 ], we hypothesised that cats with obesity would have a decrease in HRV (an indicator of sympathetic tone), which may improve following controlled weight reduction. However, there were no changes in either heart rate or HRV in the current study. This is likely to reflect the increased sympathetic drive typically seen in cats attending veterinary clinics, which makes it difficult to assess possible effects of obesity on autonomic tone. Use of ambulatory ECG may be a better way to assess heart rate and HRV, although even this approach might be hampered by stress and subsequent increased sympathetic tone. Further, frequency domain analysis of HRV (such as by Holter ECG), instead of the time domain method VVTI, may give different results. Although, in one Holter study, there was no difference between cats that were either overweight or had obesity, compared with cats in ideal body condition [ 13 ].
Cardiac biomarkers were within reference range for all but two cats in the current study, with no significant change following weight reduction. This might suggest that obesity had not contributed to clinically-significant increases in wall stress or myocardial injury, despite the increase in wall thickness. However, in human heart failure patients with obesity, a smaller increase in NT-proBNP occurs compared with those with a normal body mass index [ 32 ] suggesting a more complex interaction between NT-proBNP and obesity. In the one cat with persistently increased hs-cTnI, there was an increase in NT-proBNP, LA size and LVWT at follow up despite weight reduction. This cat was diagnosed with primary HCM.
Obesity-related cardiac dysfunction is associated with increased morbidity and an increased risk of cardiovascular death in people. Considering the worldwide prevalence of both obesity and HCM in the pet cat population, it is important to develop a better understanding of the effects of obesity on feline cardiac structure and function. There is an obesity paradox in human cardiology, whereby patients with obesity have longer survival times once heart failure has developed [ 33 , 34 ] however, human patients with HCM and obesity have more advanced echocardiographic changes, worse heart failure scores and are more symptomatic, than those of ideal bodyweight [ 34 , 35 , 36 ]. These findings suggest that obesity may be an important modifier of HCM penetrance, severity and progression [ 37 ]. A similar obesity paradox has been reported in cats with heart failure in one study [ 38 ] although no association between bodyweight and prognosis was seen in a second study of cats [ 39 ] emphasising the complexity of the relationship between obesity and heart disease. Although we did not examine effects of obesity on cats with HCM in the current study, our results did demonstrate that obesity is associated with increased LVWT and diastolic dysfunction, which could be mistaken for, or mask, feline HCM. Further research is required to understand whether obesity should be considered as a HCM phenocopy, or whether obesity acts as a modifier of HCM penetrance and expression in cats.
The main limitation of the current study is the small number of cats; the study might have been underpowered as a result, meaning that subtle changes in cardiac function might have been missed. A power calculation to determine the required study population size was not performed; instead, the number of cats recruited was instead pragmatic, based on the number of cases likely to be recruited over the study time frame. However, our population size was equivalent to those used in recent canine studies [ 6 , 7 , 9 ]. Given concerns that the study might have been underpowered, the P value was not adjusted for multiple comparisons, which is itself a limitation. As a third study limitation, when comparing echocardiographic findings before and after weight reduction, few echocardiographic differences were identified. This made it inappropriate to explore associations between changes in body composition (based on DEXA) and changes in echocardiographic variables. Further, many of the cardiovascular measurements might have been affected by confounding variables; for example, diastolic function is affected by age, a variable which was not controlled in this study. However, a wide age range was present in the study cats, thereby making age-effects less likely. Heart rate can affect some echocardiographic variables and might also have been a confounder when comparing pre and post weight reduction data; however, such an impact should be minimal as there was no significant change in heart rate.
One challenge of this study, and another inherent limitation, was the difficulty in interpreting the cause of increased LVWT in study cats. Considering the prevalence of subclinical HCM in the feline population of around 14.7% [ 14 ], some cats with increased LVWT at baseline might have had subclinical HCM. However, if this were the sole cause of hypertrophy, this would not be expected to improve with time and thus a reduction in wall thickness would not be expected after weight reduction. The possibility of concomitant subclinical HCM is more likely to have underestimated changes seen with weight reduction, rather than vice versa. Presence of HCM might also have caused diastolic dysfunction although, in such cases, improvement in diastolic function would also not be expected, making obesity the more likely cause. Considering that HCM is common in cats [ 14 ] and that we aimed to assess wall thickness, a HCM phenotype (i.e., increased LVWT) which was not clinically relevant at the time of the study was not an exclusion criterion.
A further limitation is that the changes in LVWT, although statistically significant with a medium-to-large effect size, could be the result of inter-observer and intra-observer variability or daily variation. A control group to monitor for change in wall thickness over time in cats with a stable weight, would have helped eliminate this possibility. However, given that the differences documented were greater than the expected variation of LV wall measurements (both inter-observer and intra-observer coefficients of variation ≤ 5.9% for LV wall measurements by this group) [ 27 ] the changes observed are more likely to reflect genuine changes. The clinical significance of small changes in LVWT could be questioned; not least given that other factors (such as hydration status) might instead be responsible. However, this is likely to be an inherent issue of measuring wall thickness in cats. The changes reported in this study of 0.5–0.85 mm, although small, are clinically relevant when considering such a change could be the difference between a diagnosis of normal or a HCM phenotype. Finally, study investigators could not be blinded as to whether a cat had achieved target weight loss, as only such cats underwent repeat echocardiography; however, they were blinded to the baseline echocardiography results at time of repeat echocardiography.
Conclusions
An increase in LVWT and diastolic dysfunction were common echocardiographic features in cats with obesity within our study and may be reversible with successful weight reduction. This highlights the importance of weight management in these cats. Further research is needed to investigate the clinical importance of these echocardiographic changes in otherwise healthy cats, and in cats with HCM.
Cats referred to the Royal Canin Weight Management Clinic, University of Liverpool, for assessment and management of obesity, were recruited between August 2016 and November 2018. To meet eligibility criteria, cats could not have had significant cardiac or systemic disease, as assessed during initial examinations (as below). To be included in the final assessment, cats had to have reached their weight reduction target by the study end date (November 2019). The study protocol was approved by the University of Liverpool Veterinary Research Ethics Committee (RETH000353 and VREC793) and the Royal Canin Ethical Review Committee (150,720–55). Owners of all participating animals gave written, informed consent.
Weight reduction regimen
Details of the weight reduction protocol used have been previously described [ 40 , 41 ]. In brief, cats were determined to be clinically well with no systemic disease that may affect the ability to lose weight, based on physical examination, haematology, serum biochemistry, urinalysis and free thyroxine, performed during the initial visit. Body condition score was assessed using a nine-point scale [ 42 ]. DEXA was performed under sedation (after cardiac evaluation as follows) with midazolam (0.2 mg/kg), fentanyl (5 mcg/kg) and medetomidine (15 mcg/kg) intramuscular, reversed with atipamezole (37.5 mcg/kg) intramuscular. Body composition was analysed by DEXA, and data used to create individualised weight reduction plans for each cat, establishing both an ideal and a target weight, as previously described [ 40 , 43 ]. In brief, the ideal weight was defined as the weight at which the cat’s body fat mass was optimal, based on DEXA body composition data. Target weight was the goal weight set for the period of controlled weight reduction. In most cases, target weight matched ideal weight; however, in some cases, a partial weight reduction protocol was used whereby the target weight set was greater than the ideal weight, with decisions made based on age and severity of obesity [ 44 ]. In such cases, the aim was for the cat to lose enough weight to lead to improved health and wellbeing, even though the cat would still be in overweight condition at the end of their period of weight reduction.
Commercially available, wet and dry therapeutic diets (Supplementary Table 3 ) were used for the controlled weight reduction protocol, with the choice of formulation dependent on owner and cat preferences. Food allocation was estimated by calculating the metabolic energy requirement based on the ideal weight, as previously described [ 40 ]. Individualised advice on lifestyle and activity alterations were also given to assist in weight reduction by a registered veterinary nurse (GRTW).
Cats were reweighed every two to four weeks to assess progress, with subsequent changes to the weight reduction diet if required. Cats were deemed to have reached the primary endpoint if target weight was achieved within the study period. Full laboratory analysis and DEXA were repeated at time of achieving target weight.
Cardiovascular evaluation
Cardiac evaluation was performed prior to sedation for DEXA at both the initial visit and after reaching target weight. Cats considered to have a mild, preclinical HCM phenotype (stage B1 [ 20 ]) were not excluded based on the aim of assessing LVWT. Presence of congenital heart disease or cardiomyopathies other than HCM were considered exclusion criteria.
Systolic blood pressure
Systolic blood pressure was measured indirectly by the Doppler method (Ultrasonographic Doppler Flow Detector 811-B; Parks Medical Electronics), as previously described [ 45 ], by an experienced operator (cardiology nurse, GRTW, or the echocardiographer). SBP was measured in a quiet room with gentle handling prior to other procedures. Cats were allowed to acclimatise to the environment before five measurements were taken, with the mean value recorded. Values equal to and exceeding 160 mmHg were considered consistent with hypertension [ 45 ].
- Cardiac biomarkers
Blood was collected into EDTA tubes by jugular venepuncture at the initial and last assessments. Samples were immediately centrifuged and separated EDTA-plasma stored at -20 °C until after study completion and sent as a single batch on dry ice to an external laboratory (IDEXX Laboratories, Wetherby, West Yorkshire, UK) for measurement of high sensitivity cardiac troponin I (Beckman Coulter Access hs-cTnI assay; IDEXX Laboratories) and second-generation N-terminal Pro B-type natriuretic peptide (Cardiopet proBNP test, IDEXX Laboratories).
Electrocardiography
Six-lead ECG was obtained from all cats restrained in right lateral recumbency or sternal (dependent on temperament). Routine analysis of the ECG was performed, including rate, rhythm and standard lead II measurements. To assess HRV, the R-R interval for 20 consecutive cardiac cycles was measured. The VVTI was then calculated as the natural logarithm of the variance of these R-R intervals (VVTI = Ln[SD RR ] 2 ) [ 46 ].
- Echocardiography
Complete 2D, M-mode, colour flow, spectral and tissue Doppler echocardiography was performed with a Vivid 7 ultrasound machine (GE Healthcare), using a 10 MHz transducer, by an EBVS® European Veterinary Specialist in Small Animal Cardiology or a resident in training under the direct supervision of such a specialist. Echocardiography was performed without sedation, with cats positioned in both right and left lateral recumbency. Vagal manoeuvres (nasal planum pressure [ 47 ]) were utilised to attempt to separate E and A waves when summation occurred. Simultaneous ECG was used for timing of events during the cardiac cycle. Analysis was performed on a remote, off-line measuring system, Footnote 1 by the operator who performed the echocardiography. The mean value of three cardiac cycles, in sinus rhythm was obtained for each variable and used in analysis.
Left atrial diameter (LADmax) and short axis left atrium to aorta ratio (LA/Ao) were measured as previously described [ 48 , 49 , 50 ]. LA enlargement was defined as LADmax ≥ 16 mm and/or LA/Ao ≥ 1.6. Measurement of end-diastolic interventricular septum (IVSd) and left ventricular free wall (LVFWd) thickness was made on 2D echocardiography as previously described [ 51 ], utilising a leading-edge-to-trailing edge method for measurement of the septum, leading-edge-to-leading edge for the free wall. The IVSd was measured in basal, mid and apical regions and the LVFWd measured in basal and mid regions (on three cardiac cycles and the mean for each region calculated), ensuring inclusion of any regions of focal hypertrophy, but exclusion of points of false tendon attachment or endocardial thickening; the region of maximal thickness for each wall was defined as the maximal IVSd and maximal LVFWd and utilised in statistical analysis. An increase in LVWT, which would be considered consistent with a HCM phenotype, was defined by a maximal diastolic wall thickness ≥ 6 mm; values of 5.5 to 5.9 mm were considered equivocal, and values < 5.5 mm were considered normal [ 20 ]. M-mode of the LV was obtained from a right parasternal short axis view at the level of the chordae tendineae, with the cursor bisecting the LV cavity symmetrically. M-mode fractional shortening was calculated using the standard formula [ 51 ]. Cats were classified as having either normal left ventricular diastolic function, impaired relaxation or pseudonormal diastolic function based on combined assessment of transmitral flow, measurement of E wave and A wave velocities and velocity ratios, IVRT duration and pw-TDI acquired as previously described [ 51 , 52 ]. Transmitral data of cats with complete mitral E and A wave summation were excluded; partial summation was allowed if the A wave started late in E deceleration (when E wave was < 0.2 m/s) [ 53 ]. Pw-TDI was utilised to assess longitudinal motion of the septal and lateral mitral annuli and lateral tricuspid annuli (diastolic E’ and A’ and systolic S’ velocities; E’/A’ ratio) as previously described [ 52 , 54 , 55 ]. Cats with summated E’/A’ underwent vagal manoeuvres [ 47 ] to attempt to transiently separate them, but data was excluded if persistent E’A’ summation was present. Cats were classified as having impaired left ventricular relaxation based on one or more of the following criteria: a decrease in mitral E/A (< 1), pw-TDI LVFW E’A’ (< 1) or increase in IVRT (> 60 ms). If the transmitral E/A and/or IVRT were within reference ranges but pw-TDI LVFW E’A’ < 1, this was considered pseudonormal diastolic function [ 51 , 52 ]. For cats that reached the end point, cardiac evaluations were repeated, allowing comparison between the two time-points.
Statistical analysis was performed with the use of commercially available software (SPSS 28.0). Sample size was based on pragmatic recruitment within the study time frame. For every cat, a mean of each echocardiographic and clinical variable was recorded for each time-point. On account of the small sample size, non-parametric tests were used. The median (and IQR) was reported for all descriptive statistics. Baseline weight, age and BCS were compared between the cats that did and did not achieve weight reduction using a Mann–Whitney U test. For the cats that completed the study, a Wilcoxon-signed rank test was used to compare continuous variables pre- and post- weight reduction and a marginal homogeneity test used to compare categorical variables (diastolic function class). For both the Mann–Whitney U and Wilcoxon-signed ranks test, effect size was calculated from the z statistic, and used to calculate r , as described by Cohen [ 19 ], using the formula r = Z / √N (where Z is the z statistic and N is the sample size). The effect size, r , was interpreted according to the rules of Cohen [ 19 ], whereby values of 0.1, 0.3 and ≥ 0.5 were considered small, medium and large effects respectively [ 18 , 19 ]. The level of statistical significance was set at P < 0.05, for two-sided analyses.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
GE Echopac version 113, GE Medical Systems, Buckinghamshire, UK.
Abbreviations
Two dimensional
Body condition score
Dual energy X-ray absorptiometry
- Hypertrophic cardiomyopathy
Heart rate variability
High-sensitivity cardiac troponin I
Interquartile range
Isovolumetric relaxation time
End-diastolic interventricular septum thickness
Left atrium
Short axis left atrium to aorta ratio
Maximal left atrial diameter
Left ventricle / left ventricular
End-diastolic left ventricular free wall thickness
Left ventricular wall thickness
Left ventricular outflow tract
N-terminal Pro B-type Natriuretic Peptide
Pulsed wave tissue Doppler imaging
Reference interval
Vasovagal tonus index
Lau DCW, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–41.
Article CAS PubMed Google Scholar
Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306.
Article PubMed PubMed Central Google Scholar
Cuspidi C, Rescaldani M, Sala C, et al. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32(1):16–25.
German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136(S7):1940S–1946S.
Salt C, Morris PJ, Wilson D, et al. Association between life span and body condition in neutered client-owned dogs. J Vet Intern Med. 2019;33(1):89–99.
Article PubMed Google Scholar
Mehlman E, Bright JM, Jeckel K, et al. Echocardiographic evidence of left ventricular hypertrophy in obese dogs. J Vet Intern Med. 2013;27:62–8.
Adolphe JL, Silver TI, Childs H, et al. Short-term obesity results in detrimental metabolic and cardiovascular changes that may not be reversed with weight loss in an obese dog model. Br J Nutr. 2014;112:647–56.
Tropf M, Nelson OL, Lee PM, et al. Cardiac and metabolic variables in obese dogs. J Vet Intern Med. 2017;31(4):1000–7.
Article CAS PubMed PubMed Central Google Scholar
Neto GBP, Brunetto MA, Sousa MG, et al. Effects of weight loss on the cardiac parameters of obese dogs. Pesq Vet Bras. 2010;30:167–71.
Article Google Scholar
Litster AL, Buchanan J. Radiographic and echocardiographic measurement of the heart in obese cats. Vet Radiol Ultrasound. 2000;41:320–5.
De Souza FB, Golino D, Bonatelli S, et al. Effect of obesity on echocardiographic parameters and vertebral heart size (VHS) in cats. Semin Cienc Agrar. 2020;41:493–503.
Stepien RL, Jackson MW, Henik RA, et al. Cardiovascular effects of acute weight gain in cats. Proceedings of the ESVIM 5th Annual Conference; 1995 August 31- September 2; Cambridge, UK.
Champion T. Effects of obesity and overweight on cardiovascular and respiratory parameters in cats [paper in Portuguese]. PhD thesis, Universidade Estadual Paulista, 2011.
Payne JR, Brodbelt DC, Luis FV. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol. 2015;17:S244–57.
Novo Matos J, Payne JR, Seo J, et al. Natural history of hypertrophic cardiomyopathy in cats from rehoming centers: The CatScan II study. J Vet Intern Med. 2022;36(6):1900–12.
Latchman PL, Mathur M, Bartels MN, et al. Impaired autonomic function in normotensive obese children. Clin Auton Res. 2011;21:319–23.
Sugimoto K, Fujii Y, Sunahara H, et al. Assessment of left ventricular longitudinal function in cats with subclinical hypertrophic cardiomyopathy using tissue Doppler imaging and speckle tracking echocardiography. J Vet Med Sci. 2015;77(9):1101–8.
Fritz CO, Miller PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Pyschol Gen. 2012;141(1):2–18.
Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. New York: Routledge; 1988.
Google Scholar
Luis Fuentes V, Abbott J, Chetboul V, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med. 2020;34(3):1062–77.
Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–74.
Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension. 2008;51(2):181–7.
Van Hoek I, Hodgkiss-Geere H, Bode E, et al. Associations among echocardiography, cardiac biomarkers, insulin metabolism, morphology and inflammation in cats with asymptomatic hypertrophic cardiomyopathy. J Vet Intern Med. 2020;34:591–9.
Yang VK, Freeman LM, Rush JE. Comparisons of morphometric measurements and serum insulin-like growth factor concentration in healthy cats and cats with hypertrophic cardiomyopathy. Am J Vet Res. 2008;69:1061–6.
Häggström J, Andersson ÅO, Falk T, et al. Effect of body weight on echocardiographic measurements in 19,866 pure-bred cats with or without heart disease. J Vet Intern Med. 2016;30(5):1601–11.
Alpert MA. Management of obesity cardiomyopathy. Am J Med Sci. 2001;321:237–41.
Van Hoek I, Hodgkiss-Geere H, Bode EF, et al. Association of diet with left ventricular wall thickness, troponin I and IGF-1 in cats with subclinical hypertrophic cardiomyopathy. J Vet Intern Med. 2020;34(6):2197–210.
Lee SC, Daimon M, Di Tullio MR, et al. Beneficial effect of body weight control on left ventricular diastolic function in the general population: an analysis of longitudinal data from a health check-up clinic. Eur Heart J Cardiovasc Imaging. 2017;19(1):36–142.
Karimian S, Stein J, Bauer B, et al. Improvement of impaired diastolic left ventricular function after diet-induced weight reduction in severe obesity. Diabetes Metab Syndr Obes. 2017;10:19–25.
Partington C, Hodgkiss-Geere H, Woods GRT, et al. The effect of obesity and subsequent weight reduction on cardiac structure and function in dogs. BMC Vet Res. 2022;18(1):351.
Stein PK, Bosner MS, Kleiger RE, et al. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–81.
Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90.
Harsha DW, Bray GR. Weight loss and blood pressure control (pro). Hypertension. 2008;51:1420–5.
Olivotto I, Maron BJ, Tomberli B, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62:449–57.
Canepa M, Sorensen LL, Pozios I, et al. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:1182–9.
Fumagalli C, Maurizi N, Day SM, et al. Association of obesity with adverse long-term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2019;5:65–72.
Article PubMed Central Google Scholar
Nollet EE, Westenbrink D, Boer RA, et al. Unravelling the genotype-phenotype relationship in hypertrophic cardiomyopathy: obesity-related cardiac defects as a major disease modifier. J Am Heart Assoc. 2020;9(22): e018641.
Finn E, Freeman LM, Rush JE, et al. The relationship between body weight, body condition, and survival in cats with heart failure. J vet Intern Med. 2010;24(6):1369–74.
Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract. 2010;51(10):540–7.
German AJ, Holden SL, Bissot T, et al. Dietary energy restriction and successful weight loss in obese client-owned dogs. J Vet Intern Med. 2007;21:1174–80.
PubMed Google Scholar
German AJ, Holden SL, Bissot T, et al. A high protein high fibre diet improves weight loss in obese dogs. Vet J. 2010;183:294–7.
Laflamme DP. Development and validation of a body condition score system for dogs. Canine Practice. 1997;22:10–155.
Raffan E, Holden SL, Cullingham F, et al. Standardized positioning is essential for precise determination of body composition using dual-energy x-ray absorptiometry in dogs. J Nutr. 2006;136(S7):1976S–1978S.
German AJ, Woods-Lee GRT, Biourge V, et al. Partial weight reduction protocols in cats lead to better weight outcomes, compared with complete protocols, in cats with obesity. Front Vet Sci. 2023;10:1211543.
Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2018;32:1803–22.
Häggström J, Hamlin RL, Hansson K, et al. Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. J Small Anim Pract. 1996;37:69–75.
Smith DN, Schober KE. Effects of vagal manoeuvres on heart rate and Doppler variables of left ventricular filling in healthy cats. J Vet Cardiol. 2013;15(1):33–40.
Maerz I, Schober K, Oechtering G. Echocardiographic measurement of left atrial dimension in healthy cats and cats with left ventricular hypertrophy. Tieraerztl Prax Ausg K Kleintiere Heimtiere. 2006;34:331.
Hansson K, Häggström J, Kvart C, et al. Left atrial to aortic root indices using two dimensional and M-mode echocardiography in Cavalier King Charles Spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 2002;43:568–75.
Chetboul V, Passavin P, Trehiou-Sechi E, et al. Clinical, epidemiological and echocardiographic features and prognostic factors in cats with restrictive cardiomyopathy: a retrospective study of 92 cases (2001–2015). J Vet Intern Med. 2019;33:1222–31.
Boon J. Evaluation of size, function and hemodynamics. In: Boon JA, editor. Veterinary Echocardiography. 2nd ed. Chichester: Wiley-Blackwell; 2011. p. 202–2088.
Schober KE, Chetboul V. Echocardiographic evaluation of left ventricular diastolic function in cats: hemodynamic determinants and pattern recognition. J Vet Cardiol. 2015;17(S1):S102–33.
Oh J, Seward JB, Tajik AJ. Assessment of diastolic function and diastolic heart failure. In: Oh JK, Seward JB, Tajik AJ, editors. The Echo Manual. 3rd ed. Philadelphia: Williams & Wilkins; 2007. p. 120–42.
Koffas H, Dukes-McEwan J, Corcoran BM, et al. Pulsed tissue Doppler imaging in normal cats and cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2006;20:65–77.
Seiler G, Dukes-McEwan J, Gaschen L. Chapter 2: basics of thoracic ultrasonography. In: Schwartz T, Johnson V (eds). BSAVA: manual of canine and feline thoracic imaging. 1st edn. Gloucester: BSAVA, 2008, pp 56–61.t
Download references
Acknowledgements
The authors thank the referring veterinarians for referring cases to the weight management clinic and the clinical staff in the cardiology and anaesthesia departments at the University of Liverpool for assistance with case management.
The study was funded by a grant from Royal Canin, a division of Mars Petcare. The funding body had no role in the design, analysis and reporting of the study, but reviewed the final manuscript.
Author information
Authors and affiliations.
Institute of Infection, Veterinary, Ecological and Sciences, Department of Small Animal Clinical Sciences, Teaching Hospital, University of Liverpool, Neston, UK
Catheryn Partington, Hannah Hodgkiss-Geere & Joanna Dukes-McEwan
Institute of Life Course and Medical Sciences, Department of Small Animal Clinical Sciences, Teaching Hospital, University of Liverpool, Neston, UK
Georgia R. T. Woods & Alexander J. German
Royal Canin Research Center, Aimargues, France
John Flanagan & Vincent Biourge
Present address: Department of Veterinary Medicine, University of Cambridge, Madingley Road, Cambridge, CB3 0ES, UK
Catheryn Partington
You can also search for this author in PubMed Google Scholar
Contributions
AJG and GRTW collated cases and weight data and implemented the weight reduction regimen. JDM and HHG performed cardiac investigations. AJG, JDM and HHG designed the study. CP analysed and interpreted data and was the main writer of the manuscript. AJG, JDM and HHG edited the manuscript. All authors (including JF and VB) read and improved the final manuscript.
Corresponding author
Correspondence to Catheryn Partington .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the University of Liverpool Veterinary Research Ethics Committee (RETH000353 and VREC793) and the Royal Canin Ethical Review Committee (150720–55). Enrolled cats were client-owned pets referred for weight management and all clinical investigations performed, other than non-invasive echocardiography and electrocardiography, were routine investigations (deemed standard of care) for such patients. All diagnostic procedures were clinically indicated, were to the benefit of the patient and were performed to the highest standards of veterinary practice. The intervention (therapeutic diet) was clinically necessary to improve the health and welfare of the patients. Owners of all participating animals gave written, informed consent.
The authors confirm that all methods were carried out in accordance with relevant guidelines and veterinary regulations, and that although the study involved clinical cases (not experimental animals) methods were reported in accordance to the ARRIVE guidelines as applicable.
Consent for publication
Not applicable.
Competing interests
The study was funded by a grant from Royal Canin, a division of Mars Petcare, and this company manufactured the diets fed in this study. Vincent Biourge and John Flanagan are employees of Royal Canin. Alexander J. German and Georgia R.T. Woods are employees of the University of Liverpool but their positions are funded by Royal Canin. Both have received financial remuneration and gifts for providing educational material, speaking at conferences, and consultancy work. Joanna Dukes-McEwan and Hannah Hodgkiss-Geere have also participated in a study funded by Royal Canin investigating a nutritional management for feline hypertrophic cardiomyopathy. Catheryn Partington has no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplementary table 1..
Baseline demographic and cardiovascular data for all enrolled cats. Baseline data for all 20 enrolled cats (sex, age, body weight, body condition score, blood pressure, heart rate, electrocardiographic and echocardiographic diagnosis).
Additional file 2: Supplementary Table 2.
Epidemiological and cardiovascular variables at baseline for all 20 cats. Baseline epidemiological and cardiovascular variables for all 20 cats given as median and interquartile range.
Additional file 3: Supplementary Table 3.
Average composition of the therapeutic diets used for weight reduction. Composition of the two commercially available weight management diets used in the weight management regimen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Partington, C., Hodgkiss-Geere, H., Woods, G.R.T. et al. The effect of obesity and subsequent weight reduction on cardiac morphology and function in cats. BMC Vet Res 20 , 154 (2024). https://doi.org/10.1186/s12917-024-04011-0
Download citation
Received : 02 November 2023
Accepted : 09 April 2024
Published : 24 April 2024
DOI : https://doi.org/10.1186/s12917-024-04011-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diastolic function

IMAGES
VIDEO
COMMENTS
Clinical Research - Regional Manager. NEXTSTAGE CLINICAL RESEARCH LLC. Beaumont, TX. $60,000 - $85,000 a year. Full-time. Monday to Friday + 2. Easily apply. Understanding of budget management and financial planning for clinical research studies. Their primary responsibility is to ensure the smooth functioning and….
Senior Clinical Research Associate- Florida (REMOTE) Merck Sharp & Dohme. Remote in Rahway, NJ 07065. $122,800 - $193,300 a year. Full-time. Excellent understanding and working knowledge of clinical research, phases of clinical trials, current GCP/ICH & country clinical research law & guidelines.
1,050 Clinical study manager jobs in United States. Most relevant. Alliance of Multispeciality Research. 3.3. Experienced Clinical Research Coordinator, RN. Daphne, AL. $32.00 Per Hour (Employer est.) Easy Apply.
Clinical Research - Regional Manager. NEXTSTAGE CLINICAL RESEARCH LLC. Beaumont, TX. $60,000 - $85,000 a year. Full-time. Monday to Friday + 2. Easily apply. A is a crucial role within the field of clinical research, the region manager is responsible for overseeing and managing the operations of a clinical research…. Active 4 days ago.
Clinical Research - Regional Manager. NEXTSTAGE CLINICAL RESEARCH LLC. Beaumont, TX. $60,000 - $85,000 a year. Full-time. Monday to Friday + 2. Easily apply. A is a crucial role within the field of clinical research, the region manager is responsible for overseeing and managing the operations of a clinical research…. Active Today.
Manager of Bio Clinical Statistics - AZ - Office of Research and Sponsored Programs. Glendale, AZ. $58K - $101K (Glassdoor est.) Master's degree from an accredited college or university in Biostatistics or a related field.
Jacobio Pharma. Greater Boston 1 week ago. Today's top 3,000+ Clinical Research Manager jobs in United States. Leverage your professional network, and get hired. New Clinical Research Manager ...
It takes approximately 6 to 7 years to become a clinical study manager. Year 1-4: Bachelor's DegreeA typical clinical study manager needs a bachelor's degree, which takes about 4 years to complete. Year 5-6: ExperienceAfter obtaining a degree, one to two years of relevant experience is typical for this role. Year 6-7: TrainingAdditionally, 3 to ...
A clinical research coordinator reports to a principal investigator, who is responsible for the overall design and management of the study. In contrast, a clinical researcher organizes the day-to-day running of the trials. As a clinical research coordinator, you will also work closely with sponsors and staff within their department responsible ...
Local Trial Manager (LTM) - Multiple Openings. Washington D.C., District Of Columbia. ... lead, manage and co-ordinate the conduct of clinical trials from study start-up to close out (CSR ... progress and local/country level study delivery Planning, management and oversight of clinical study ...
Clinical Research Manager. Clinical Research Manager manages the clinical monitoring process and the administration of clinical trials. Supervises CRAs in in-house and on-site monitoring, filing, and clinical trial administration. Being a Clinical Research Manager oversees adherence to SOPs, Good Clinical Practice and FDA regulations.
Working as a clinical research manager requires a mix of skills. They include leadership in guiding the research team, communication skills with both the team and study sponsors, as well as the technical skills needed to successfully plan and execute a clinical trial. The people in this field come from various backgrounds.
The Clinical Research Manager will provide leadership and oversight of studies conducted by several affinity groups within the Department. The Clinical Research Manager will manage clinical research operations, relationships with other affinity groups and staff, study quality management, personnel management and career skill development ...
Every investigator should have a clear financial management plan to adequately support their research endeavors. The plan should include a defined department process and a responsibility log which outlines the team members accountable for the process. Please review Clinical Trial Budgeting & Billing before starting your study. Feasibility Checklist
For organizations who want to provide their clinical study teams with continuous, ongoing education and professional development resources, Organization Membership is a turnkey and scalable solution. ... ([email protected]) is a Clinical Research Administration Manager at Nationwide Children's Hospital and an Instructor for the Master of ...
Stanford University. 4.3. Cancer Clinical Research Project Manager - EDD Data quality and study Start-Up (Hybrid) Stanford, CA. $72K - $92K (Employer est.) Bachelor's degree in a related field and two years of experience in clinical research, or an equivalent combination of education and relevant experience.…. 24h.
The Clinical Research Manager will manage clinical research operations, relationships with other affinity groups and staff, study quality management, personnel management and career skill development, regulatory compliance, and fiscal oversight. The CRM will also assist with building automated clinical research workflows and solutions within ...
In this clinical research management master's, you're encouraged to innovate and develop entrepreneurial skills to lead clinical research endeavors. In addition to taking six core courses, you'll also complete four approved electives and a clinical research management project. Courses include: Core.
What does a Clinical Research Manager do? Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure ...
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
Position Summary. Under the direction of the Director of the Clinical Trials Unit (CTU) and Principal Investigators, the Clinical Research Coordinator will conduct clinical research studies (industry-sponsored and investigator-initiated) within the Columbia University Irving Medical Center (CUIMC) Department of Ophthalmology in adherence with assigned study protocols and manuals of operation ...
Quality Management Practices. Ideally, every clinical trial should have a Clinical Trial Quality Management Plan (QMP) describing the tools that will be used to ensure study quality. The extent and nature of monitoring may be determined based on various considerations such as trial design, complexity, size, risks to subjects, and endpoints of the trial.
As Principal Clinical Trial Manager (Principal CTM), Clinical Operations you will be responsible for the successful oversight and management of multiple external research collaborations and clinical trials from early development through publication. The Principal CTM will lead completion of study deliverables through active participation in all aspects of study design, execution, analysis and ...
They will independently manage significant and key aspects of clinical research study data entry, data analysis, and training of team members for all aspects of data quality. ... Reporting to the Gynecological oncology (Gyn-Onc) Clinical Research Manager (CRM), the CCRPM will be conversant in the goals, mission, and priorities of the Institute ...
A study manager may manage both new and ongoing clinical trials at one time to enhance the progress of the lab. Hospitals, pharmaceutical companies, private research firms and universities are the typical employers of study managers, as these entities consistently run new research projects that need management.
Manager, Clinical Research. American Gastroenterological Association. Hybrid work in United States. $60,000 - $80,000 a year. Full-time. Easily apply. Contributes to the conceptualization, development and implementation of programs and other activities supporting clinical research in gastroenterology. Posted.
The associations between deprivation and SPC risks could provide clinical management insights. ... The study has provided increased precision in SPC risk estimates in both women and men, further elucidating the factors contributing to the variability in SPC risks. ... CH is supported by a Wellcome Trust Clinical Research Training Fellowship ...
The publication was co-authored by Dr Ally Ibrahim Olotu, who was supported through TDR's Clinical Research and Development Fellowship. In October 2023, WHO updated its recommendation for malaria vaccines to include both the RTS,S and R21 vaccines, stating these should be used for the prevention of P.falciparum malaria in children living in malaria endemic areas. About 80% of malaria deaths ...
This will be accomplished using a community-engaged research approach as well as two 50-state reviews of state statutes, rules, and adopted policies or existing guidelines related to pharmacist-managed MOUD, particularly focused on pharmacist prescriptive authority and, separately, existing state-level legal barriers to MOUD care.
Further studies are required to clarify the clinical consequences of these findings. In people, obesity is a risk factor for cardiovascular disease, associated with systemic hypertension, cardiac remodelling and systolic and diastolic dysfunction. ... This highlights the importance of weight management in these cats. Further research is needed ...