
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


12.7: Bohr’s Theory of the Hydrogen Atom
- Last updated
- Save as PDF
- Page ID 46970

Learning Objectives
- Describe early atomic models.
- Explain Bohr’s theory of the hydrogen atom.
- Distinguish between correct and incorrect features of the Bohr model, in light of modern quantum mechanics.
The great Danish physicist Niels Bohr (1885–1962) made immediate use of Rutherford’s planetary model of the atom. (Figure \(\PageIndex{1}\)). Bohr became convinced of its validity and spent part of 1912 at Rutherford’s laboratory. In 1913, after returning to Copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on the planetary model of the atom. For decades, many questions had been asked about atomic characteristics. From their sizes to their spectra, much was known about atoms, but little had been explained in terms of the laws of physics. Bohr’s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics.

Atomic Spectra
Atomic and molecular emission and absorption spectra have been known for over a century to be discrete (or quantized). Well before they were understood from first principles, chemists have been using the emission and absorption spectra for identification of elements. Figure \(\PageIndex{2}\) shows iron emission spectrum, for example. No other elements emit the exactly the same set of frequencies of light. With the discovery of substructure of the atom and the discovery of photon (or more precisely, refined understanding of the particle nature of electromagnetic waves where the particle energy is proportional to the frequency of electromagnetic waves), these resonant frequencies of light emitted by atoms could be used to infer an atomic model.
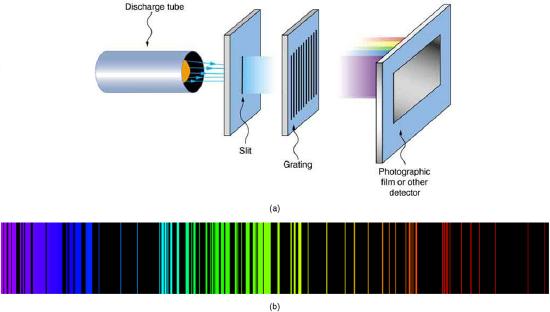
For the hydrogen atom, the lightest element with the simplest atom, a pattern for its line spectrum was noticed by experimentalists (see Figure \(\PageIndex{3}\)). All wavelengths of the line spectrum could be described by a following formula, for the suitable choice of two integers \(n_{i}\) and \(n_{f}\):
\[\frac{1}{\lambda}=R\left(\frac{1}{n_{\mathrm{f}}^{2}}-\frac{1}{n_{\mathrm{i}}^{2}}\right), \label{1}\]
where \(\lambda\) is the wavelength of the emitted EM radiation and \(R\) is the Rydberg constant , determined by the experiment to be
\[R=1.097 \times 10^{7} / \mathrm{m}\left(\text { or } \mathrm{m}^{-1}\right). \nonumber \]
The \(n_{\mathrm{f}}\) is a positive integer associated with a specific series, which are named after their discoverers. For the Lyman series, \(n_{\mathrm{f}}=1\); for the Balmer series, \(n_{\mathrm{f}}=2\); for the Paschen series, \(n_{\mathrm{f}}=3\); and so on. The Lyman series is entirely in the UV, while part of the Balmer series is visible with the remainder UV. The Paschen series and all the rest are entirely IR. There are apparently an unlimited number of series, although they lie progressively farther into the infrared and become difficult to observe as \(\n_{\mathrm{f}}\) increases. The \(n_{\mathrm{i}}\) is a positive integer greater than \(n_{\mathrm{f}}\). So for example, for the Balmer series, \(n_{\mathrm{f}}=2\) and \(n_{\mathrm{i}}=3,4,5,6, \ldots\).
So, before Bohr's model of the hydrogen atom, such was the picture of atomic theory—full of suggestive (and even well-organized) data and no unifying explanation. Ernest Rutherford is quoted as saying, "All science is either physics or stamp-collecting." What he meant is, there are branches of science whose practitioners would be satisfied with a collection of interesting facts (i.e. "stamp-collecting"). But what makes physics physics is the search for the theoretical framework providing explanations based on fundamental principles, not idiosyncratic descriptions. Bohr's model brought the science of spectroscopy into physics.
Bohr's Model for Hydrogen
The planetary model of the atom suggested by Rutherford was in trouble. While the model provided a possible picture of how the very small atomic nucleus might be arranged with the electrons in a stable arrangement, it did not provide for the size of electron orbits (which would be related to the size of the atom), and the arrangement was not actually stable—an orbiting electron is an oscillating charge; an oscillating charge emits electromagnetic waves; electromagnetic waves carry away energy; so as the electron loses energy, it would fall into the proton. By some estimates, this would occur in as short a time as \(10^{-7} \mathrm{~s}\)!
Bohr's starting point for his successful model was this: he proposed that the orbits of electrons in atoms are quantized . To fully understand this statement, we can compare the orbits of electrons in atoms to the orbits of planets in the solar system. The orbits of planets are not quantized. While laws of physics govern how planets move in the solar system (see for example, Kepler's laws, or their derivation by Newton starting with the inverse-square law of gravitation), there is no law of physics dictating how far each body in the solar system must be from the Sun. So the orbits of planets are not quantized.
So what Bohr was proposing was an entirely new law of physics no one had known before. In one sense, it was not completely new (Planck and Einstein already enjoyed some successes from suggesting quantization of energy in thermal oscillators and EM radiation); in another sense, it was a big break from centuries of classical mechanics. This was Bohr's quantization rule: angular momentum of an electron in its orbit is quantized . In mathematical form,
\[L=n \hbar, \nonumber \]
where nn could take on any positive integer value (\(n=1,2,3, \ldots\)), and \(\hbar\) is known as the reduced Planck constant (\(\hbar=h / 2 \pi\)). And angular momentum, \(L\), as you might remember from earlier chapter, is given by the following for a particle in a uniform circular orbit: \(L=m v r\), where \(m\) is the mass of the particle, \(v\) is the speed of the particle in orbit, and rr is the radius of circular orbit. Using this as the starting point, semiclassical analysis of orbital motion yields a whole array of quantized (i.e. allowed) values of orbital distance \(\left(r_{n}\right)\), orbital speed \(\left(v_{n}\right)\), and orbital energy \(\left(E_{n}\right)\), among others (see: Table \(\PageIndex{1}\) for a summary).
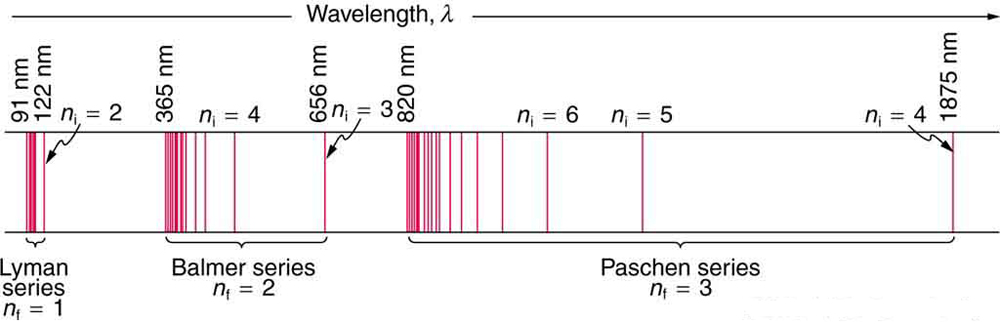
With the quantized orbital energies for the electron, we have a ready explanation for the features of atomic spectra. EM radiation is emitted when an electron transitions from a higher energy level (\(E_{i}\)) to a lower energy level (\(E_{f}\)), with the photon carrying away the energy difference,
\[h f=\Delta E=E_{i}-E_{f}, \label{2} \]
where \(f\) is the frequency of the photon. Figure \(\PageIndex{4}\) shows a schematic representation of this relationship. With only discrete values of energy \(E_{n}\) allowed, there are only discrete values of frequency (\(f\)) and wavelength (\(\lambda\)) allowed also, as shown in the line spectra.
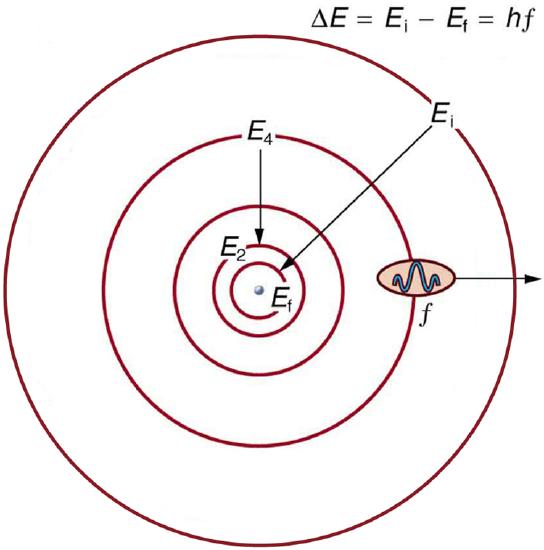
Energy-level diagram , shown in Figure \(\PageIndex{5}\), is another convenient way to illustrate these relationships. Allowed energy levels for the atom are plotted vertically with the lowest state (or ground state ) at the bottom and with excited states above that. The energies of the lines in an atomic spectrum correspond to the differences in energy levels in the level diagram (figure illustrates a transition from \(E_{4}\) to \(E_{2}\), which would show up in the atomic spectrum as one line).
Two key results are worth highlighting. The first is the Bohr radius , or the smallest orbital radius \(a\), given for \(n=1\),
\[\begin{align*} a &=r_{1}=\hbar^{2} / m k e^{2} \\ &=0.529 \times 10^{-10} \mathrm{~m}. \end{align*} \]
This is the Bohr model's prediction for the size of the atom, made with nothing more than electric constants, mass of the electron, and the Planck's constant, and this theoretical prediction matches experimentally measured sizes of atoms fairly well.
The second is the derivation of the Rydberg formula, first given in Equation \(\eqref{1}\). To derive this, we start out with Equation \(\eqref{2}\) and substitute in expressions for hydrogen energies from Table \(\PageIndex{1}\):
\[\begin{align*} h f &=-\frac{m k^{2} e^{4}}{2 n_{i}^{2} \hbar^{2}}-\left(-\frac{m k^{2} e^{4}}{2 n_{f}^{2} \hbar^{2}}\right) \\ &=\frac{m k^{2} e^{4}}{2 \hbar^{2}}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right) \end{align*} \]
Frequency \(f\) is equal to \(c / \lambda\). Plugging this in and solving for \(1 / \lambda\) while also replacing all instances of \(\hbar\) with \(h / 2 \pi\), we get,
\[\frac{1}{\lambda}=\frac{2 \pi^{2} m k^{2} e^{4}}{h^{3} c}\left(\frac{1}{n_{f}}-\frac{1}{n_{i}}\right), \nonumber \]
which yields an analytical expression for the Rydberg constant,
\[R=\frac{2 \pi^{2} m k^{2} e^{4}}{h^{3} c}=1.097 \times 10^{7} \mathrm{~m}^{-1}. \nonumber \]
Figure \(\PageIndex{6}\) shows an energy-level diagram for hydrogen that also illustrates how the various spectral series for hydrogen are related to transitions between energy levels.
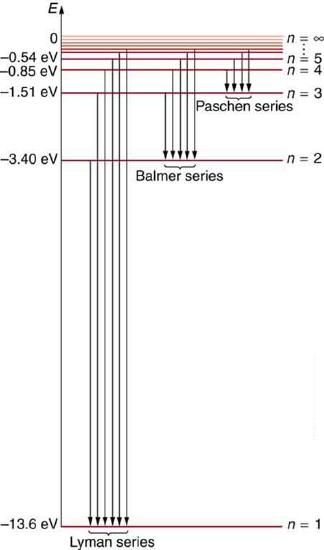
We see that Bohr’s theory of the hydrogen atom answers the question as to why this previously known formula describes the hydrogen spectrum. It is because the energy levels are proportional to \(1 / n^{2}\), where \(n\) is a non-negative integer. A downward transition releases energy, and so \(n_{\mathrm{i}}\) must be greater than \(n_{\mathrm{f}}\). The various series are those where the transitions end on a certain level. For the Lyman series, \(n_{\mathrm{f}}=1\) — that is, all the transitions end in the ground state (see also Figure \(\PageIndex{6}\)). For the Balmer series, \(n_{\mathrm{f}}=2\), or all the transitions end in the first excited state; and so on. What was once a recipe is now based in physics, and something new is emerging—angular momentum is quantized.
Triumphs and Limits of the Bohr Theory
Bohr did what no one had been able to do before. Not only did he explain the spectrum of hydrogen, he correctly calculated the size of the atom from basic physics. Some of his ideas are broadly applicable. Electron orbital energies are quantized in all atoms and molecules. Angular momentum is quantized. The electrons do not spiral into the nucleus, as expected classically. These are major triumphs.
But there are limits to Bohr’s theory. It cannot be applied to multielectron atoms, even one as simple as a two-electron helium atom. Bohr’s model is a semiclassical model. The orbits are quantized (quantum mechanical) but are assumed to be simple circular paths (classical). As quantum mechanics was developed, it became clear that there are no well-defined orbits; rather, there are "clouds" of probability. Bohr’s theory also did not explain that some spectral lines are doublets (split into two) when examined closely. These deficiencies are addressed in later, fully-quantum-mechanical atomic models, but it should be kept in mind that Bohr did not fail. Rather, he made very important steps along the path to greater knowledge and laid the foundation.
Section Summary
\[\frac{1}{\lambda}=R\left(\frac{1}{n_{\mathrm{f}}^{2}}-\frac{1}{n_{\mathrm{i}}^{2}}\right), \nonumber\]
\[R=1.097 \times 10^{7} \mathrm{~m}^{-1}. \nonumber\]
- The constants \(n_{\mathrm{i}}\) and \(n_{\mathrm{f}}\) are positive integers, and \(n_{\mathrm{i}}\) must be greater than \(n_{\mathrm{f}}\).
\[\Delta E=h f=E_{\mathrm{i}}-E_{\mathrm{f}}, \nonumber\]
where \(\Delta E\) is the change in energy between the initial and final orbits and \(hf\) is the energy of an absorbed or emitted photon. It is useful to plot orbital energies on a vertical graph called an energy-level diagram.
\[L=m_{e} v r_{n}=n \frac{h}{2 \pi}(n=1,2,3 \ldots), \nonumber\]
- Additional quantized orbital quantities—orbital radius, orbital speed, and orbital energy—can be derived starting from Bohr's assumption, and they yield predictions consistent with the experimental Rydberg formula.
- While Bohr's semiclassical model of the atom does not account for all experimental facts about the atom, it is an important stepping stone to fully-quantum-mechanical models of the atom.

M-Physics Tutorial
- Class 11 (Physics)
- Class 12 (Physics)
- Classical Mechanics
- Electronics
- Electrostatics
- Modern Physics
- Quantum Mechanics
- Statistical Mechanics
- Thermodynamics
- NET Physics
- B.Sc.+M.Sc. (Physics)
- Class 11 (Chemistry)
- Class 12 (Chemistry)
- Terms of Use
- Buy PDF Notes
Atoms Class 12 notes Physics Chapter 12
Introduction.
In this chapter, we will study various atomic models. Initially, J.J. Thomson proposed an atomic model in which he thought of as electrons embedded in between protons. In 1911, his student Earnest Rutherford proposed a nuclear model, on the basis of a scattering experiment. In spite of strong experimental evidence, Rutherford’s model of the atom was rejected on the ground of the classical theory of electromagnetism.
So in order to rectify the shortcomings of Rutherford’s model, in 1913, Niels Bohr combined the classical and early quantum concepts of Einstein and Plank to explain the stability of an atom.
Thomson Model
According to Thomson, "An atom consists of positively charged matter, into which negatively charged particles are embedded randomly". But this model did not last long as it could not explain the observations of Rutherford's alpha-particle scattering experiment.
Alpha-Particle Scattering
In 1911, Rutherford , along with his assistants, H. Geiger and E. Marsden, performed the Alpha Particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’.
They took a thin gold foil having a thickness of 2.1×10 -7 m and placed it in the center of a rotatable detector made of zinc sulfide and a microscope. Then, they directed a beam of 5.5MeV alpha particles emitted from a radioactive source at the foil. Lead bricks collimated these alpha particles as they passed through them.

After hitting the foil, the scattering of these alpha particles could be studied by the brief flashes on the screen. Rutherford and his team expected to learn more about the structure of the atom from the results of this experiment.
Recommended Books
- NCERT Textbook For Class 12 Physics Part 1 & 2
- CBSE All In One Physics Class 12 2022-23 Edition
- Oswaal CBSE Chapterwise Question Bank Class 12 Physics Book
- Modern's abc Plus of Physics for Class-12 (Part I & II)
Read also: Nuclei Class 12 Physics Notes Chapter 13
Observations
Here is what they found:
Most of the alpha particles passed through the foil without suffering any collisions
Around 0.14% of the incident alpha particles scattered by more than 1 o
Around 1 in 8000 alpha particles deflected by more than 90 o
Rutherford’s Nuclear Model
In 1912, Rutherford proposed his nuclear model of the atom . It is also known as Rutherford's planetary model of the atom. Salient features of Rutherford's atom model are as follows :
Every atom consists of a tiny central core, named nucleus, in which the entire positive charge and the almost whole mass of the atom are concentrated. The size of the nucleus is typically 10 -4 times the size of an atom.
Most of an atom is empty space.
In free space around the nucleus, electrons would be moving in orbits just as the planets do around the sun. The centripetal force needed for the orbital motion of electrons is provided by electrostatic attractive forced experience by electrons due to a positively charged nucleus.
An atom as a whole is electrically neutral. Thus, the total positive charge of the nucleus is exactly equal to the total negative charge of all the electrons orbiting in an atom .
Read also: Aldehydes, Ketones and Carboxylic Acids Chemistry Class 12 Notes Chapter 12
Bohr Model of the Hydrogen Atom
It was Niels Bohr (1885-1962) who used the concept of quantized energy and explained the model of a hydrogen atom in 1913. Bohr combined classical and early quantum concepts and proposed a theory in the form of three postulates. These postulates are:
Postulate I: An electron in an atom could revolve in certain stable orbits without emitting radiant energy. Each atom has certain definite stable orbits. Electrons can exist in these orbits. Each possible orbit has definite total energy. These stable orbits are called the stationary states of the atom.
Postulate II: An electron can revolve around the nucleus in an atom only in those stable orbits whose angular momentum is the integral multiple of h/2π (where h is Planck’s constant). Therefore, the angular momentum (L) of the orbiting electron is quantized.
`mvr=\frac{nh}{2\pi}` where, n = 1, 2, 3, .....
Postulate III: An electron can make a transition from its stable orbit to another lower stable orbit. While doing so, a photon is emitted whose energy is equal to the energy difference between the initial and final states. Therefore, the energy of photon is given by,
hυ = E i – E f
where E i and E f are the energies of the initial and final states.
Ground State and the Excited States
The lowest energy level of an atom is called the “ ground state ” and higher levels are called “ excited states ”. The H-atom has the lowest energy in the state for the principal quantum number n = 1. and all other states (i.e, for n = 2, 3, 4…) are excited states. Thus E 2 , E 3 , E 4 …are called the first, the second, and the third …excited states respectively.
Ionisation Energy and Ionisation Potential
The minimum energy needed to ionize an atom is called “ ionisation energy ”. The potential difference through which an electron should be accelerated to acquire this much energy is called “ ionisation potential ”. Hence, ionisation energy of H-atom in the ground state is 13.6 eV and ionisation potential is 13.6 V.
Read also: Conceptual Questions for Class 12 Physics Chapter 12 Atoms
Binding Energy
The binding energy of a system is defined as the minimum energy needed to separate its constituents over large distances. This may also be defined as the energy released when its constituents are brought from infinity to form the system. The binding energy of H-atom in the ground state is 13.6 eV which is the same as its ionization energy.
Excitation Energy and Excitation Potential
The energy needed to take an atom from its ground state to an excited state is called the “ excitation energy ” of that excited state. The potential through which an electron should be accelerated to acquire this energy is called the “ excitation potential ”.
The Line Spectra of the Hydrogen Atom
Bohr’s model explains the spectral lines of the hydrogen atomic emission spectrum . While the electron of the atom remains in the ground state, its energy is unchanged. When the atom absorbs one or more quanta of energy, the electron moves from the ground state orbit to an excited state orbit that is further away. When the atom relaxes back to a lower energy state, it releases energy that is again equal to the difference in energy of the two orbits.
Based on the wavelengths of the spectral lines, Bohr was able to calculate the energies that the hydrogen electron would have in each of its allowed energy levels . He then mathematically showed which energy level transitions corresponded to the spectral lines in the atomic emission spectrum.
The general formula for wavelength of emitted radiation is given by
`\frac{1}{λ}=R(\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}})`
where n 2 = 2, 3, 4, .... and n 2 > n 1
R = 1.01 x 10 7 m -1 = Rydberg constant

He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level (n = 2). This is called the Balmer series . Transitions ending in the ground state (n = 1) are called the Lyman series , but the energies released are so large that the spectral lines are all in the ultraviolet region of the spectrum. The transitions called the Paschen series and the Brackett series both result in spectral lines in the infrared region because the energies are too small.
de-Broglie’s Explanation of Bohr’s Second Postulate
de-Broglie explained the second postulate of Bohr’s atomic model by assuming an electron to be a particle wave. Therefore, it should form standing waves under resonance conditions.

According to de-Broglie, for an electron moving in n th circular orbit of radius r,
2πr = nλ n = 1, 2, 3, .....
i.e., the circumference of the orbit should be an integral multiple of the de-Broglie wavelength of an electron moving in n th orbit. As we know that de-Broglie wavelength,
`λ=\frac{h}{mv}`
`2\pi r=\frac{nh}{mv}`
`mvr=\frac{nh}{2\pi}`
Impact parameter: Perpendicular distance of initial velocity vector of α-particles from the center of the nucleus.
Distance of closest approach: Distance of a point from the nucleus at which α-particle is nearest to the center of the nucleus.
Bohr radius: The first orbit of a hydrogen atom is called the Bohr radius .
Ground state: Lowest state of an atom, called the ground state , is the state in which the electron revolves in the orbit of the smallest radius, the Bohr radius, a 0 .
Ionization energy: Minimum energy required to free an electron from the ground state of a hydrogen atom is called the ionization energy .
The first atomic model was proposed by J.J. Thomson in 1898, according to this model the positive charge of the atom was uniformly distributed throughout the volume of the atom and negatively charged electrons are embedded in it like seeds in watermelon.
Rutherford’s nuclear model: Entire positive charge and most of the mass of an atom are concentrated in a small volume called the nucleus , with electrons revolving around the nucleus just as planets revolve around the sun. This model could not explain
(i) Stability of atom,
(ii) The line spectra of atoms.
Bohr’s model of hydrogen atom: Bohr proposed a model for hydrogen and hydrogen-like atom, which explained the line spectra emitted by atoms, according to this model only those orbits are stable for which angular momentum of the electron is an integral multiple of h/2π.
de-Broglie’s explanation to Bohr’s postulate of quantization: He assumed electrons to be particle waves, therefore, the circumference of the stable orbit should be an integral multiple of the wavelength of the wave associated with the electron.
Atom, as a whole, is electrically neutral and therefore contains an equal amount of positive and negative charges.
In Thomson’s model , an atom is a spherical cloud of positive charges with electrons embedded in it.
In Rutherford’s model, most of the mass of the atom and all its positive charge is concentrated in a tiny nucleus and the electrons revolve around it.
Atoms of each element are stable and emit a characteristic spectrum. The spectrum consists of a set of isolated parallel lines termed a line spectrum . It provides useful information about the atomic structure .
The atomic hydrogen emits a line spectrum consisting of various series. As Lyman series, Balmer series, Paschen series, Brackett series, Pfund series.
de Broglie’s hypothesis that electrons have a wavelength λ = h/mv gave an explanation for Bohr’s quantized orbits by bringing in the wave-particle duality. The orbits correspond to circular standing waves in which the circumference of the orbit equals a whole number of wavelengths.
Bohr’s model is applicable only to hydrogenic (single electron) atoms. It cannot be extended to even two-electron atoms such as helium. This model is also unable to explain the relative intensities of the frequencies emitted even by hydrogenic atoms.

- Atoms Chapter 12 Physics NCERT PDF Book
- Aldehydes, Ketones and Carboxylic Acids Chemistry Class 12 Notes Chapter-12
- Class 12 Physics All Chapter wise Notes
- Anonymous 9:36 pm, February 27, 2023 These notes are very useful for me thanks a lot
- Anonymous 6:23 am, March 02, 2023 increase your website security defense it is very easy to hack
- Anonymous 11:10 am, February 11, 2024 you give better notes as compared to others.thank you sir. please include important graphs and diagrams which are important.
- Bohr Model of the Hydrogen Atom
Bohr Model of the hydrogen atom attempts to plug in certain gaps as suggested by Rutherford’s model by including ideas from the newly developing Quantum hypothesis. Bohr postulated that in an atom , electrons could revolve in stable orbits without emitting radiant energy.
Suggested Videos
Bohr model of the hydrogen atom attempts to plug in certain gaps as suggested by Rutherford’s model by including ideas from the newly developing Quantum hypothesis. According to Rutherford’s model, an atom has a central nucleus and electron/s revolve around it like the sun-planet system.
However, the fundamental difference between the two is that, while the planetary system is held in place by the gravitational force, the nucleus-electron system interacts by Coulomb’s Law of Force. This is because the nucleus and electrons are charged particles. Also, an object moving in a circle undergoes constant acceleration due to the centripetal force.
Further, electromagnetic theory teaches us that an accelerating charged particle emits radiation in the form of electromagnetic waves. Therefore, the energy of such an electron should constantly decrease and the electron should collapse into the nucleus. This would make the atom unstable.
The classical electromagnetic theory also states that the frequency of the electromagnetic waves emitted by an accelerating electron is equal to the frequency of revolution . This would mean that, as the electron spirals inwards, it would emit electromagnetic waves of changing frequencies. In other words , it would emit a continuous spectrum . However, actual observation tells us that the electron emits a line spectrum.
Watch Modern Atomic Theory –
Bohr model postulates.
Bohr, in an attempt to understand the structure of an atom better, combined classical theory with the early quantum concepts and gave his theory in three postulates:
Postulate I
In a radical departure from the established principles of classical mechanics and electromagnetism, Bohr postulated that in an atom, electron/s could revolve in stable orbits without emitting radiant energy. Further, he stated that each atom can exist in certain stable states. Also, each state has a definite total energy. These are stationary states of the atom.
Postulate II
Bohr defined these stable orbits in his second postulate. According to this postulate:
- An electron revolves around the nucleus in orbits
- The angular momentum of revolution is an integral multiple of h/2p – where hàPlanck’s constant [h = 6.6 x 10 -34 J-s].
- Hence, the angular momentum (L) of the orbiting electron is: L = nh/2p
Postulate III
In this postulate, Bohr incorporated early quantum concepts into the atomic theory. According to this postulate, an electron can transition from a non-radiating orbit to another of a lower energy level. In doing so, a photon is emitted whose energy is equal to the energy difference between the two states. Hence, the frequency of the emitted photon is:
hv = Ei – Ef
(Ei is the energy of the initial state and Ef is the energy of the final state. Also, Ei > Ef).
Some important equations
Radii of Bohr’s stationary orbits

- n – integer
- r n – radius of the n th orbit
- h – Planck’s constant
- ε 0 – Electric constant
- m – Mass of the electron
- Z – the Atomic number of the atom
- e – Elementary charge
Since ε 0 , h, m, e, and p are constants and for a hydrogen atom, Z = 1, r n α n 2
The velocity of Electron in Bohr’s Stationary Orbits

Since ε 0 , h, and e are constants and for a hydrogen atom, Z = 1, r n α (1/n)
Total Energy of Electron in Bohr’s Stationary Orbits

The negative sign means that the electron is bound to the nucleus.
Although these equations were derived under the assumption that electron orbits are circular, subsequent experiments conducted by Arnold Sommerfeld reaffirm the fact that the equations hold true even for elliptical orbits.
Energy Levels
When the electron is revolving in an orbit closest to the nucleus, the energy of the atom is the least or has the largest negative value. In other words, n = 1. For higher values of n, the energy is progressively larger.
The state of the atom wherein the electron is revolving in the orbit of smallest Bohr radius (a 0 ) is the ‘Ground State’. In this state, the atom has the lowest energy. The energy in this state is:
E 1 = -13.6 eV
Hence, the minimum energy required to free an electron from the ground state of an atom is 13.6 eV. This energy is the ‘Ionization Energy’ of the hydrogen atom. This value agrees with the experimental value of ionization energy too.
Now, a hydrogen atom is usually in ‘Ground State’ at room temperature. The atom might receive energy from processes like electron collision and acquire enough energy to raise the electron to higher energy states or orbits. This is an ‘excited’ state of the atom. Therefore, the energy required by the atom to excite an electron to the first excited state is:
E 2 – E 1 = -3.40 eV – (-13.6) eV = 10.2 eV
Similarly, to excite the electron to the second excited state, the energy needed is:
E3 – E1 = -1.51 eV – (-13.6) eV = 12.09 eV
Remember, that the electron can jump to a lower energy state by emitting a photon. Also, note that, as the excitation of the hydrogen atom increases, the minimum energy required to free the electron decreases.
Solved Examples for You
Question: How many postulates are present in the Bohr model of a hydrogen atom? Solution: Bohr model of a hydrogen atom has three postulates. The postulate of the circular orbit, postulate of the selected orbit and postulate of the origin of spectral lines.
Question: According to the Bohr model, what is the energy of the atom in the ground state? Solution: According to the Bohr model, the energy of the atom in the ground state is -13.6 eV.
Customize your course in 30 seconds
Which class are you in.

- Shell Model
- Frank Hertz Experiment
- Effects of Radiation
- Ionizing Radiation
- Quantum Mechanics
- Alpha-Particle Scattering and Rutherford’s Nuclear Model of Atom
- Atomic Spectra
One response to “Atomic Spectra”
i really have learnt alot, but its is difficult for me to register because my country(Nigeria) is not on the listed countries. pls kindly include if you can
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Physics library
Course: physics library > unit 17.
- De Broglie wavelength
- Quantum Wavefunction
- Atomic Energy Levels

Bohr model radii (derivation using physics)
- Bohr model radii
- Bohr model energy levels (derivation using physics)
- Bohr model energy levels
- Absorption and emission
- Emission spectrum of hydrogen
- Bohr's model of hydrogen
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
Talk to our experts
1800-120-456-456
- Bohrs Model

What is Neils Bohr Atomic Model?
Bohr’s Model of an Atom is a framework comprising a little, thick core encompassed by circling electrons—like the structure of the Solar System, yet with fascination given by electrostatic powers instead of gravity. This was an improvement to the Rutherford model and can be considered as a quantum physical explanation for it. The Bohr model's key achievement lay in clarifying the Rydberg equation for the discharge lines of nuclear hydrogen. While the Rydberg equation had been known tentatively, it didn't increase hypothetical support until the Bohr model was presented.
The Bohr model clarified the purpose of the Rydberg equation and justified the basic physical constants of the equation.
The Neil Bohr atomic model and the entirety of its replacements portray the properties of electrons with respect to a bunch of potential values. Atoms gain or discharge energy when the electrons jump between the energy levels or shells.
Postulates of Bohr Atomic Model
The following are the chief postulates explained by the Neils Bohr atomic theory.
The model shows that the electrons in atoms are in orbits of different energy levels around the core (like the planets circling the sun).
Neils used the term energy levels (or shells) to portray these orbits of varying energy. He said that the energy of an electron is quantized, which means electrons can have fixed energy levels, but, nothing in the middle.
An electron typically involves an energy level which is called its ground state. Yet, it can move to a higher-energy, less-stable level, or shell, by gaining energy. This higher-energy, the less-stable state, is known as the electron's energized state.
After the excitement phase is over, the electron can regain its ground state by releasing the energy it gained.
The energy released by electrons has the segment of the electromagnetic range (the scope of frequencies of energy) that people recognize as light. Slight changes in the measure of the energy are viewed as the light of various wavelengths.
Energy Levels
The Bohr atomic theory gives practically accurate outcomes just for a framework where two charged points circle each other at speeds significantly less than that of light. This not just includes a one-electron framework, for example, the hydrogen atom, separately ionized helium, and doubly ionized lithium. However, it incorporates positronium and Rydberg conditions of any molecule where one electron is far away from everything else.
The energy levels of electrons at different orbits are represented by n= 1, 2, 3…. ( integers) and are known as quantum numbers. These numbers are assigned to the sequential shells such as K, L, M, N for 1, 2, 3, 4, respectively. The lowest energy level of an electron is n=1 which is closest to the nucleus and is generally said to be its ground state.
The Rydberg Formula
The Rydberg equation, which was known exactly before Bohr's formula, is found in Bohr's hypothesis as depicting the energies of changes or quantum bounces between orbital energy levels. Bohr's equation gives the mathematical estimation of the known and estimated Rydberg constant, but regarding more central constants of nature, including the electron's charge and the Planck constant.
At the point when the electron gets moved from its ground energy level to a higher one, it starts jumping back each level until it goes to the first position, which leads to the release of energy. Utilizing the inferred equation for the distinctive energy levels of a hydrogen atom, one may decide the frequencies of light that a hydrogen atom can transmit.
The energy transmitted by an atom of hydrogen is given by the difference of two hydrogen energy levels:
Ephoton = E 0 (1/nh 2 − 1/nj 2 )
nh is the last energy level, and nj is the underlying energy level.
The following formula gives the wavelength of a photon.
\[\overline{v}\] = \[\frac{1}{λ}\] = \[R(\frac{1}{n_1^2} - \frac{1}{n_2^2})\]
Limitations of Bohr's Model of an Atom
The following are the main limitations of the Bohr model.
It couldn't clarify the spectra acquired from bigger atoms.
It does not comply with the Heisenberg Uncertainty Principle.
It likewise fails to clarify the Stark impact of the electric field on the spectra of atoms.
Niels Bohr model of an atom fails to clarify the Zeeman Effect on the spectra of atoms.

FAQs on Bohrs Model
1. State how the Bohr Model can explain the Movement of Electrons.
Answer: The hypothesis takes note of the electrons in the atoms to travel around a focal core in roundabout circles and can just revolve steadily at a particular distance from the core in certain fixed circles. Such circles are identified with specific energies and are likewise referred to as energy shells or energy levels. As a rule, electrons fill the lower-energy orbitals before filling the immediate next higher energy orbital until the lower-energy orbital is full.
2. Give the origin of the Bohr Atomic Model.
Answer: Due to the reason that the electrons spiral inwards, the atom may collapse as all electrons will be attracted to the core and release energy. There would be a ceaseless smear, in recurrence, of electromagnetic radiation. In any case, late-nineteenth-century scientists indicated that atoms just discharge light (that is, electromagnetic radiation) at certain discrete frequencies. To explain this concept, Niels Bohr proposed, in 1913, the Bohr model of an atom.
- Structure of Atom
- Bohrs Atomic Model And Its Limitations
Niels Bohr Atomic Model And Limitations
Thomson’s atomic model and Rutherford’s atomic model failed to answer any questions related to the energy of an atom and its stability.
Table of Contents
- Bohr’s Theory
Recommended Videos
Limitations of bohr atomic model theory.
- Frequently Asked Questions – FAQs
Bohr’s Theory – Bohr’s Atomic Model

In the year 1913, Niels Bohr proposed an atomic structure model, describing an atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged nucleus like planets around the sun in our solar system, with attraction provided by electrostatic forces, popularly known as Bohr’s atomic model. It was basically an improved version of Rutherford’s atomic model overcoming its limitations. On most of the points, he is in agreement with him, like concepts of nucleus and electrons orbiting it. Salient features of Niels Bohr atomic model are:
- Electrons revolve around the nucleus in stable orbits without emission of radiant energy. Each orbit has a definite energy and is called an energy shell or energy level.
- An orbit or energy level is designated as K, L, M, N shells. When the electron is in the lowest energy level, it is said to be in the ground state.
- An electron emits or absorbs energy when it jumps from one orbit or energy level to another. When it jumps from a higher energy level to lower energy level it emits energy while it absorbs energy when it jumps from a lower energy level to a higher energy level.
- The energy absorbed or emitted is equal to the difference between the energies of the two energy levels (E 1 , E 2 ) and is determined by Plank’s equation.
ΔE = E 2 -E 1 = h𝜈
ΔE = energy absorbed or emitted h= Plank’s constant 𝜈= frequency of electromagnetic radiation emitted or absorbed
- The angular momentum of an electron revolving in energy shells is given by:
m e v r = n h/ 2 π
Where, n= number of corresponding energy shell; 1, 2, 3 ….. m e = mass of the electron v= velocity r=radius h= Plank’s constant
Bohr’s Model of an Atom

- It violates the Heisenberg Uncertainty Principle . The Bohr atomic model theory considers electrons to have both a known radius and orbit i.e. known position and momentum at the same time, which is impossible according to Heisenberg.
- The Bohr atomic model theory made correct predictions for smaller sized atoms like hydrogen, but poor spectral predictions are obtained when larger atoms are considered.
- It failed to explain the Zeeman effect when the spectral line is split into several components in the presence of a magnetic field.
- It failed to explain the Stark effect when the spectral line gets split up into fine lines in the presence of an electric field.
Frequently Asked Questions – FAQs
What is the limitation of bohr atomic model theory.
Bohr Atomic Model Theory fails to explain the effect of magnetic field on the spectra of atoms. It also failed to explain the Stark effect and Heisenberg Uncertainty Principle.
What is the significance of Bohr Atomic Model Theory?
Bohr was the foremost to find that electrons move around the nucleus in different orbits and we can determine an element’s properties by the number of electrons in the valence shell.
How do electrons move in Bohr’s model?
According to Bohr, electrons move around the central nucleus in a fixed circular orbit. These orbits of specific energies and are also referred to as energy shells or energy levels.
Who was the first scientist to discover electrons?
J. J. Thomson was the first to discover electrons while studying the properties of the cathode ray.
How many electrons are present in the L shell?
There are 8 electrons in the L shell.
To learn more on Rutherford and Thomson’s atomic structure model, please register to BYJU’S – The Learning App.
- Energy level diagram
- Planck’s Quantum Theory

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Thank you very much
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

CBSE 12 Physics
12.10 de broglie’s explanation of bohr’s second postulate of quantisation and limitations of bohr’s atomic model.

Bohr’s Theory Of Hydrogen Atoms

Table of Contents
Bohr model of the hydrogen atom was the first atomic model to effectively make sense of the radiation spectra of atomic hydrogen. Niels Bohr presented the atomic Hydrogen model in the year 1913. Bohr Model of the hydrogen atom endeavours to connect specific holes as proposed by Rutherford’s model. It holds a unique spot in history as it led to quantum mechanics by presenting the quantum hypothesis.
Fill Out the Form for Expert Academic Guidance!
Please indicate your interest Live Classes Books Test Series Self Learning
Verify OTP Code (required)
I agree to the terms and conditions and privacy policy .
Fill complete details
Target Exam ---
Planetary Model of the Atom
- Quantum mechanics arose during the 1920s. Neil Bohr, one of the authors of quantum mechanics, was keen on the much-discussed subject of the time – the construction of the atom. Various atomic models, including the hypothesis proposed by J.J Thompson and the disclosure of core by Ernest Rutherford, had arisen. In any case, Bohr upheld the planetary model, which affirmed that electrons rotated around a decidedly charged core very much like the planets around the sun.
All things considered, researchers actually had numerous unanswered inquiries, for example,
- For what reason didn’t the electrons drop into the core as predicted by old-style material science?
- Where are the electrons and what do they do there?
- How is the discrete emanation lines delivered by invigorated components connected to the interior construction of the atom?
Bohr resolved this large number of inquiries utilizing an apparently basic suspicion: What assuming electron circles and energies, could display just explicit qualities? You can actually look at Atomic Theory to find out about the different atomic hypotheses set forward by researchers in the mid-twentieth century.

Energy Level – Principle Energy Level
Bohr’s atomic model:.
- Thomson’s and Rutherford’s atomic models neglected to address any inquiries connected with the energy of an atom and its solidness. In the year 1913, Niels Bohr proposed an atomic model, portraying an atom as a little, emphatically charged core encompassed by electrons that movement in roundabout circles around the decidedly charged core, like the planets around the sun in our planetary group, with fascination given by the electrostatic powers.
- This model is prominently known as the Bohr model of an atom. Bohr proposed an atomic model of a hydrogen atom.
- Bohr’s model gave a legitimate clarification for the solidness of electrons spinning in circles. He named these circles as energy shells.
Bohr’s Equation
- Bohr Model of the hydrogen atom first proposed the planetary model, yet later a presumption concerning the electrons was made. The supposition that was the quantization of the design of atoms. Bohr’s suggested that electrons circled the core in explicit circles or shells with a decent span. Just those shells with a range given by the situation beneath were permitted, and it was unimaginable for electrons to exist between these shells.
- Numerically, the permitted worth of the atomic range is given by the situation:
r(n)=n 2 x r(1)
- n is a positive whole number
- r(1) is the littlest permitted sweep for the hydrogen atom otherwise called the Bohr’s range
The Bohr’s radius has a value of r(1)=0.529 x 10 -10 m
Bohr determined the energy of an electron in the nth degree of hydrogen by considering the electrons in a roundabout, quantized circles as:
E(n)=-1/n 2 x 13.6eV
- 13.6 eV is the most minimal conceivable energy of a hydrogen electron E(1).
- The energy acquired is generally a negative number and the ground state n = 1, has the most bad worth. The explanation being that the energy of an electron in circle is comparative with the energy of an electron that is completely isolated from its core, and it is perceived to have an energy of 0 eV. Since the electron in a decent circle around the core is more steady than an electron that is incredibly a long way from its core, the energy of the electron in circle is negative all the time.

Absorption and Emission
- As indicated by Bohr’s model, an electron would ingest energy as photons to become eager to a higher energy level. Subsequent to running away to the higher energy level, otherwise called the invigorated express, the energized electron is less steady, and along these lines, would quickly emanate a photon to return to a slower, more steady energy level. The energy of the transmitted photon is equivalent to the distinction in energy between the two energy levels for particular progress. The energy can be determined utilizing the condition:
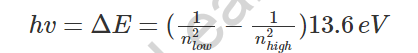
Limitations of the Bohr Model of the Hydrogen Atom:
- Bohr’s model doesn’t function admirably for complex atoms.
- It couldn’t make sense of why a few otherworldly lines are more extraordinary than others.
- It couldn’t make sense of why a few otherworldly lines split into various lines within the sight of an attractive field.
The Heisenberg’s vulnerability standard goes against actual electrons for Bohr existing in explicit circles with a known sweep and speed. Albeit the advanced quantum mechanical model and the Bohr Model of the Hydrogen Atom might appear to be immeasurably changed, the key thought is something very similar in both. Traditional material science isn’t adequate to depict every one of the peculiarities that happen on an atomic level. Yet, Bohr was the first to understand the quantization of electronic shells by melding the possibility of quantization into the electronic design of the hydrogen atom and was effectively ready to make sense of the discharge spectra of hydrogen as well as other one-electron frameworks.
- Bohr recommended that electrons travel in explicit circles, shells around the core. As indicated by Bohr’s computation, the energy for an electron in the shell is given by the articulation:
- E(n)= -1/n 2 x 13.6eV
- Bohr’s Model of the Hydrogen Atom isn’t pertinent for frameworks with more than one electron.
Question: State two limitations of Bohr’s model of the atom.
- It infringes upon the Heisenberg Uncertainty Principle.
- The Bohr Model considers electrons to have both a known radius and orbit, which is unimaginable, as per Heisenberg.
For what reason goes Bohr's model work for hydrogen?
Since hydrogen and hydrogen-like atoms have one electron and, in this manner, don't encounter electron relationship impacts.
How do electrons move in Bohr's model?
The hypothesis noticed that electrons in atoms travel around a focal core in roundabout circles and can circle steadily at an unmistakable arrangement of good ways from the core in specific fixed roundabout circles. Such circles are connected with specific energies and are additionally alluded to as energy shells or energy levels.
How did Sommerfeld modify Bohr's hypothesis?
Numerous changes have been acquainted with the Bohr model, most outstandingly the Sommerfeld model or Bohr - Sommerfeld model, which recommended that electrons move around a core in curved circles as opposed to round circles of the Bohr model. The Bohr - Sommerfeld framework was confused, adding to numerous mysteries.
Related content
Talk to our academic expert!
Language --- English Hindi Marathi Tamil Telugu Malayalam
Get access to free Mock Test and Master Class
Register to Get Free Mock Test and Study Material
Offer Ends in 5:00

IMAGES
VIDEO
COMMENTS
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
Rutherford explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels. Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which ...
The great Danish physicist Niels Bohr (1885-1962) made immediate use of Rutherford's planetary model of the atom. (Figure 12.7.1 12.7. 1 ). Bohr became convinced of its validity and spent part of 1912 at Rutherford's laboratory. In 1913, after returning to Copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on ...
Read also: Aldehydes, Ketones and Carboxylic Acids Chemistry Class 12 Notes Chapter 12. Bohr Model of the Hydrogen Atom. It was Niels Bohr (1885-1962) who used the concept of quantized energy and explained the model of a hydrogen atom in 1913. ... de Broglie's hypothesis that electrons have a wavelength λ = h/mv gave an explanation for Bohr ...
Examples on Bohr Atomic Model. Example 1: Calculate the maximum number of electrons an o shell can hold. Solution: We know that O shell means 5th shell. Therefore, n=5. Applying the formula 2n 2 = 2 x 5 2 = 50. Thus, the maximum number of electrons O shell can hold is 50.
Bohr calculated the energy of an electron in the nth level of hydrogen by considering the electrons in circular, quantized orbits as: \ (\begin {array} {l}E (n)=-\frac {1} {n^2}\times 13.6\,eV\end {array} \) Where, 13.6 eV is the lowest possible energy of a hydrogen electron E (1). The energy obtained is always a negative number and the ground ...
The de-Broglie hypothesis presented Bohr's second postulate on the quantization of electron angular momentum. Only resonant standing waves can persist in electron orbits and energy states because of the electron's wave nature. ... Get all the important information related to the CBSE Class 12 Examination including the process of application ...
E 1 = -13.6 eV. Hence, the minimum energy required to free an electron from the ground state of an atom is 13.6 eV. This energy is the 'Ionization Energy' of the hydrogen atom. This value agrees with the experimental value of ionization energy too. Now, a hydrogen atom is usually in 'Ground State' at room temperature.
️📚👉 Watch Full Free Course Videos: https://www.magnetbrains.com ️📚👉 Grab Notes by Expert Teachers Here: https://www.pabbly.com/out/magnet-brains ️ ...
L is defined to be r x p, which is r*p*sin (theta), where theta is the angle between the radius vector and the momentum vector. Since they are moving in a circle, that means that p and r are perpendicular, so sin (theta) is just 1, leaving rp. Since p is just mv, that means that L=mvr. The way he arrived at the conclusion L=nh/2pi is ...
The great Danish physicist Niels Bohr (1885-1962) made immediate use of Rutherford's planetary model of the atom. Bohr became convinced of its validity and spent part of 1912 at Rutherford's laboratory. In 1913, after returning to Copenhagen, he began publishing his theory of the simplest atom, hydrogen, based on the planetary model of ...
The two theories, de Broglie equation and Bohr's hypothesis of atoms, have a great impact on quantum mechanics. De Broglie equation was the work of Louis de Broglie, a French physicist, and Bohr's hypothesis of atoms belongs to Neil Henrik Bohr is a great scientist who gave lots of contributions to quantum mechanics.
Postulates of Bohr Atomic Model. The following are the chief postulates explained by the Neils Bohr atomic theory. The model shows that the electrons in atoms are in orbits of different energy levels around the core (like the planets circling the sun). Neils used the term energy levels (or shells) to portray these orbits of varying energy.
The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). The orbits in which the electron may travel are shown as grey circles; their ...
Join us in this in-depth video as we explore the de-Broglie explanation of Bohr's 2nd postulate in atoms for class 12 students, especially those preparing fo...
Contents1 What is the Bohr Model? How is the De Broglie Equation Related to Bohr's Model?1.1 Postulates of Bohr's Theory1.2 Bohr's Quantum Condition from de Brog-lie's Hypothesis Physics Topics can also be used to explain the behavior of complex systems, such as the stock market or the dynamics of traffic flow. What is the Bohr […]
Bohr's Theory - Bohr's Atomic Model. In the year 1913, Niels Bohr proposed an atomic structure model, describing an atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged nucleus like planets around the sun in our solar system, with attraction provided by ...
Bohr's 2nd hypothesis by De-Broglie equation // class 12 physics // Atoms.Derivation of Bohr's 2nd hypothesis by De-Broglie equation
This set of Class 12 Physics Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on "DE Broglie's Explanation of Bohr's Second Postulate of Quantisation". ... Explanation: de - Broglie hypothesis did modify Bohr's second postulate. This postulate of Bohr regarding the quantization of the angular momentum of an electron was ...
12.10 De Broglie's Explanation of Bohr's Second Postulate of Quantisation and Limitations of Bohr's Atomic Model. 13 Nuclei. 14 Topics. 13.01 Atomic Masses and Composition of Nucleus. 13.02 Discovery of Neutrons. 13.03 Size of Nucleus. 13.04 Mass-Energy Equivalence and Concept of Binding Energy. 13.05 Binding Energy per Nucleon.
Introduction. De Broglie's theory is the one of the most fundamental theories which gives a direction to quantum mechanics from classical physics. It describes the dual nature of matter, i.e., a matter can behave like both particle and wave. The phenomenon of a beam of light which diffracted just like a wave is explained by this theory.
Numerically, the permitted worth of the atomic range is given by the situation: r (n)=n2 x r (1) Where, n is a positive whole number. r (1) is the littlest permitted sweep for the hydrogen atom otherwise called the Bohr's range. The Bohr's radius has a value of r (1)=0.529 x 10-10m. Bohr determined the energy of an electron in the nth ...
Class 12 Physics https://www.youtube.com/@DynamicVidyapeeth/playlists?view=50&sort=dd&shelf_id=2Chapter 1, Electric Charges and Fields https://youtube.com/pl...