- Research article
- Open access
- Published: 20 September 2021

A study on knowledge, attitudes and practices regarding dengue fever, its prevention and management among dengue patients presenting to a tertiary care hospital in Sri Lanka
- K. P. Jayawickreme ORCID: orcid.org/0000-0001-9503-2854 1 ,
- D. K. Jayaweera 1 ,
- S. Weerasinghe 1 ,
- D. Warapitiya 1 &
- S. Subasinghe 1
BMC Infectious Diseases volume 21 , Article number: 981 ( 2021 ) Cite this article
17k Accesses
6 Citations
1 Altmetric
Metrics details
The World Health Organization (WHO) has ranked dengue as one of the top ten threats to Global health in 2019. Sri Lanka faced a massive dengue epidemic in 2017, the largest outbreak in the country during the last three decades, consisting of 186,101 reported cases, and over 320 deaths. The epidemic was controlled by intense measures taken by the health sector. However, the reported dengue cases and dengue deaths in 2019 were significantly higher than that of 2018. Deaths were mostly due to delay in hospitalization of severe dengue patients. The mortality of dengue hemorrhagic fever is 2–5% if detected early and treated promptly, but is high as 20% if left untreated.
A descriptive cross-sectional study was done among patients with dengue fever presenting to the Sri Jayawardenepura General Hospital during October 2019. Data was collected using a questionnaire comprising 20 questions based on knowledge, attitudes and practices on dengue, which were categorized into questions on awareness of mortality and severity of dengue burden, prevention of dengue vector mosquito breeding and acquiring the infection, patient’s role in dengue management, and warning signs requiring prompt hospitalization.
The mean KAP score on all questions was 55%, while a majority of 65.2% patients scored moderate KAP scores (50–75%) on all questions, and only 7.6% had high KAP scores (> 75%). The highest categorical mean score of 62% was on awareness of dengue prevention, followed by 54% on awareness of dengue burden, and only 51% on dengue management. Only 5.3% patients scored high scores on awareness of dengue management, followed by 28.5%, and 40.9% patients scoring high scores on awareness of dengue burden, and awareness of prevention of dengue respectively. The mean KAP scores on all questions increased with increasing age category.
The population relatively has a better awareness of dengue prevention, as compared to awareness of dengue mortality and dengue management. The identified weak point is patient awareness of the patients’ role in dengue management, and identifying warning signs requiring prompt hospitalization. This results in delay in treatment, which is a major cause for increased mortality. There was a correlation between those who had good knowledge on dengue burden and those who were aware of patients’ role in dengue management. An action plan should be implemented to improve public awareness through education programs on the role of the public and patients in dengue management to drive a better outcome.
Peer Review reports
The World Health Organization (WHO) has ranked dengue as one of the top ten threats to Global health in 2019 [ 1 ]. Brady et al. estimates a 3.9 billion prevalence of people, accounting to 40%-50% of the world’s population being at risk of infection. 128 countries worldwide are at risk of dengue infection, of which 70% of the global burden being in Asia [ 2 , 3 ]. The reported dengue cases to WHO increased from < 0.5 million in 2000 to > 3.34 million in 2016, characterized by a worldwide outbreak [ 4 ]. Although the world-wide numbers declined in 2017, there was a significant rise again in 2019 with 4.3 million cases worldwide. The highest number of dengue cases worldwide in 2019 in descending order were reported in Brazil, Philippines, Vietnam, Mexico, Nicaragua, Malaysia and India respectively, with Sri Lanka being placed in the 8th place worldwide, and in the 5th place in Asia [ 5 ]. Following a steady rise in annual dengue cases, Sri Lanka faced a massive dengue epidemic in 2017, which was the largest outbreak in the country during the last three decades, consisting of 186,101 reported cases, and over 320 deaths. The epidemic was controlled by intense measures taken by the health sector. However, the reported dengue cases rose again in 2019 reaching 102,746, being twice the number of reported cases of 51,659 in 2018, indicating re-emergence of an outbreak in 2019. A majority of cases being in the western province, with 20% in the Colombo district [ 6 ]. The dengue deaths in 2019 were 90; higher than the total dengue deaths in 2018 being 58, albeit with reduced mortality rate per overall cases [ 6 , 7 ]. The mortality of dengue fever is < 1%, and that of dengue hemorrhagic fever is 2–5% if detected early and treated promptly, but is high as 20% if dengue hemorrhagic fever is left untreated [ 8 ].
Dengue virus is a flavivirus transmitted by mosquito vectors, such as Aedes aegypti and Aedes albopictus. Dengue fever was first serologically confirmed in Sri Lanka in 1962 [ 9 ]. All four serotypes of dengue virus, DENV-1 to DENV-4 have been circulating in the country, and each serotype has many genotypes [ 9 ]. The most common cause for occurrence of new epidemics is the shift of the circulating serotype and genotype of the dengue virus, which is predisposed by increased foreign travel introducing new strains [ 9 ]. The dengue outbreak in 2003 was predominantly due to DENV-3 and DENV-4. The outbreaks in 2006, 2009 and 2010 was predominantly due to DENV-1 [ 9 ]. The predominant serotype in the 2017 epidemic was DENV-2 which was infrequent since 2009 [ 10 ]. The outbreak in 2019 was predominantly due to previously latent serotype DENV-3 [ 11 ].
The WHO published and implemented a “Global Strategy for Dengue Prevention And Control” targeting the years from 2012 to 2020, with the goals of improving dengue mortality, and morbidity by the year 2020, and estimating the true disease burden. The main elements of the global strategy were diagnosis and case management, integrated surveillance and outbreak preparedness, sustainable vector control, future vaccine implementation, basic operational and implementation research [ 12 ].This global strategy follows 10 priority areas for planning dengue emergency response, adapted from Rigau-Pérez and Clark in 2005, which also includes Engaging the community and relevant professional groups about dengue control as well as their participation in dengue prevention and control [ 13 ].
A recent study in Malaysia, showed that the population had only an average knowledge, and poor attitudes and practices on dengue prevention. They identified that a significant percentage had erroneous beliefs, such as fogging being the mainstay of dengue vector control. It had led them to a false sense of security, while evading actual measures that should be taken. They also identified that a proportion of people believed they had no responsibility in preventing dengue breeding, which needed urgent attention. They highlighted that it was impossible to reduce dengue prevalence without community participation, and concluded that measures were urgently required to educate the public to change their attitudes. The Communications for behavioral changes program on dengue prevention were subsequently implemented by Health departments of Malaysia to improve dengue awareness and prevention [ 14 ].
Although there had been a few studies on public awareness on dengue prevention, there was limited evidence focused on public awareness on their role in dengue prevention and management. It is therefore very important to take active measures to reduce the incidence and mortality of dengue, for which the responsibility lies not only with health professionals, but also with the general public. The purpose of this study is to identify the level of awareness in patients on preventing and managing dengue infection, and awareness of the patient’s role and responsibility in the above. Our goals were to identify areas in dengue control and management that need improvement, to implement policies that raise patient participation to deliver a better outcome of dengue infection, its complications and its management.
Study design
This is a descriptive cross-sectional study assessing the knowledge, attitudes, and practices on dengue fever, its prevention and the patient’s role in management, among the dengue patients presenting to a tertiary care hospital in Sri Lanka during the month of October 2019.
Study setting
The study was done among a random sample of 132 patients with dengue fever or dengue hemorrhagic fever who were admitted to adult medical wards for treatment at the Sri Jayawardenepura General Hospital during October 2019. These patients comprised people from draining areas of the western province of Sri Lanka.
Sample size
The number of patients who presented to the Sri Jayawardenepura General hospital in the month of October 2019 was 200. A sample size of 132 was calculated with a confidence interval of 95%, to match the population to assess a statistically significant result.
Participants
The study population was randomly selected among adult patients older than 13 years of age admitted with dengue infection to the medical wards of the Sri Jayawardenepura General Hospital during the month of October 2019.
Participants were not selected from the same family who would likely to be influenced by similar knowledge, to avoid bias of pseudo-replication.
Data collection
Data collection was commenced after obtaining the approval from the institutional Ethical Review committee of the Sri Jayawardenepura General Hospital and Postgraduate Training Centre (SJGH/20/ERC/017). Data was collected using a self-administered validated questionnaire regarding Knowledge, Attitudes, and Practices (KAP) on dengue in languages English, Sinhala, and Tamil which were translated and extensively reviewed for validation (Additional file 1 : Appendix S1, Additional file 2 : Appendix S2, Additional file 3 : Appendix S3).
Data was collected from randomly selected participants, only after informed written consent was obtained. The questionnaires were filled by the participants themselves using the validated questionnaire of the language convenient to them. The study investigators were with them while filling the questionnaire in case the participants needed to clarify any questions in order to ensure quality. The data was collected anonymously, while strict confidentiality of the responses and the results was maintained.
The questionnaire consisted of 20 questions which, comprised 5 questions on knowledge, 6 questions on attitudes, and 9 questions on practices on dengue fever and haemorrhagic fever, its prevention and patient’s role in management. Prior to analysis they were then re-categorized into questions on awareness of:
mortality and severity of dengue burden—5 questions
prevention of dengue vector mosquito breeding and acquiring the infection—5 questions
patient’s role in dengue management, and warning signs requiring prompt hospitalization—10 questions
The responses to each question was analyzed with percentage estimated of correct responses. The total marks scored by each participant to the whole questionnaire was estimated as a percentage, which has been defined as the “KAP score”. KAP score is an abbreviation used for the total score of the questions based on K nowledge, A ttitudes, and P ractices regarding dengue burden, dengue prevention and management in this study. The total results were categorized as “low” when KAP were < 50%, “moderate” when KAP scores were 50–75%, and “high” when KAP scores were > 75%.
Statistical methods
Data was analyzed using the SPSS (Statistical Package for the Social Sciences) software. All the questionnaire sheets were filled completely and none of the sheets were excluded. The mean of the KAP score of each category was calculated. The percentage of the population who scored low, moderate and high KAP scores was calculated separately. The responses to each of the 20 questions were analyzed separately to infer the areas which needed further improvement in awareness of the general public on dengue.
The study population comprised 61% males, and 39% females with a male: female ratio of 3:2. When categorizing by age, 42% of the study population was less than 30 years old, 36% were between 30 and 50 years old, and 22% were more than 50 years old. Of those who were between 30 and 50 years, 35% were graduates or diploma holders. Of those who were more than 50 years old, 21% were graduates or diploma holders. When categorizing by level of education, 10% of the population was currently schooling, 8% were adults educated up to less than ordinary level (O/L) at school who were not graduates or diploma holders, 18% were adults educated up to O/L at school who were not graduates or diploma holders, 34% were adults educated up to advanced level (A/L) at school who were not graduates or diploma holders, 24% were adults who had completed school education and were undergraduates, 6% were adults who had completed school education and were graduates or diploma holders (Table 1 ).
The mean KAP score of the sample population from the questionnaire was 55.04%. When categorizing the KAP scores as low (< 50%), moderate (50–75%), and high (> 75%), a majority of 65.2% of the population had moderate KAP scores. 27.3% had low KAP scores, and only 7.6% had high KAP scores (Fig. 1 ).
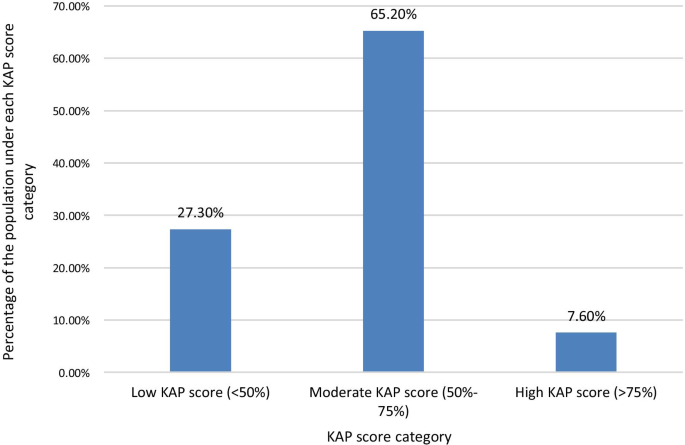
Percentage of the study population who scored under each KAP score level Category. When categorizing the KAP scores as low (< 50%), moderate (50–75%), and high (> 75%) scores, a majority of 65.2% of the population had moderate KAP scores. 27.3% had low KAP scores, and only 7.6% had high KAP scores
The KAP score achieved was higher with increasing age. The highest mean total KAP score of 57.86% was among those > 50 years of age, with those aged < 30 years having a mean KAP score of 53.48% and those aged 30–50 years having a mean KAP score of 55.21% (Fig. 2 ). The mean KAP score on awareness of dengue mortality and burden among the age categories < 30 years, 30–50 years, and > 50 years was 49.29, 56.88, and 58.57% respectively. The mean KAP score on awareness on prevention of dengue vector breeding and acquiring the infection among the age categories < 30 years, 30–50 years, and > 50 years was 63.57, 59.38, and 63.57% respectively. The mean KAP score on awareness of patients’ role in dengue management and warning signs requiring prompt hospital admission among the age categories < 30 years, 30–50 years, and > 50 years was 49.82, 52.08, and 51.79% respectively (Fig. 3 ).
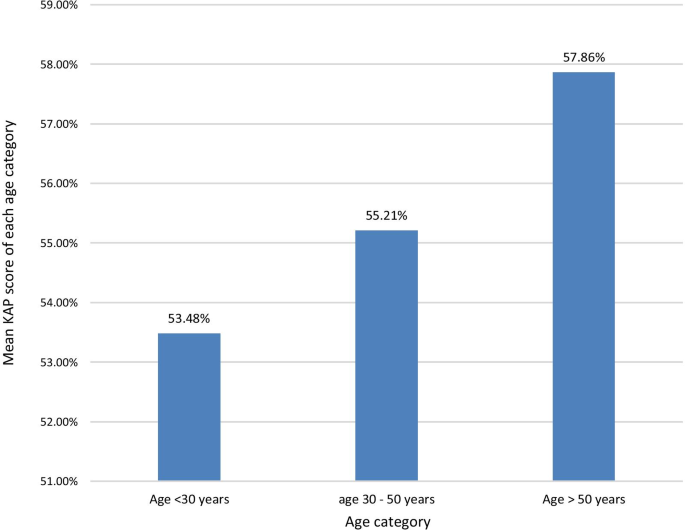
The mean KAP score of each age category. The KAP score achieved was higher with increasing age. The highest mean KAP score of 57.86% was among those > 50 years of age, with those aged < 30 years having a mean KAP score of 53.48% and those aged 30–50 years having a mean KAP score of 55.21%
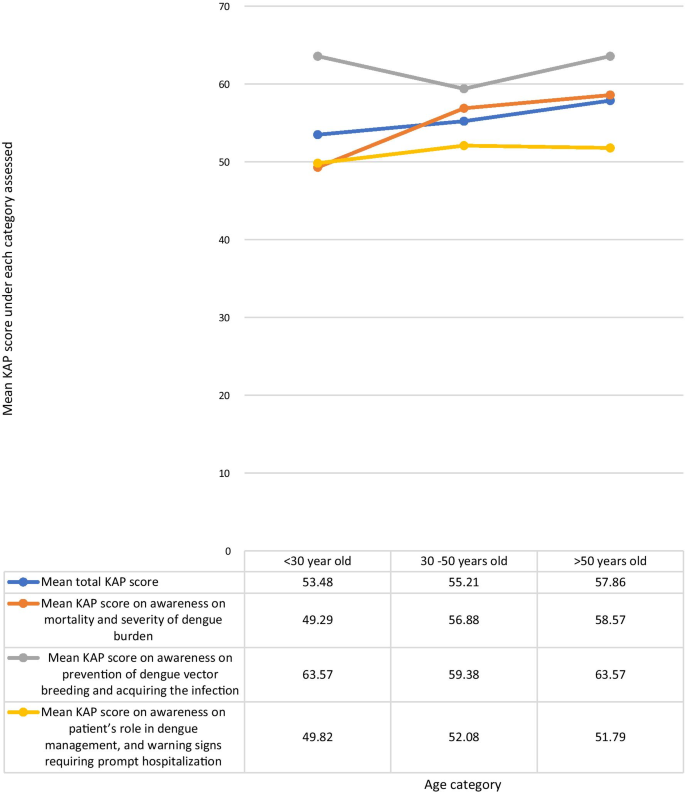
Comparison of the total KAP score, awareness on mortality and severity ofdengue burden, awareness on prevention of dengue vector breeding and acquiring the infection, and awareness on patient’s role in dengue management, and warning signs requiring prompt hospitalization under each age category
The mean KAP score was higher among those with higher educational qualification levels. The highest mean KAP score of 58.13% was among graduates and professional diploma holders of any field, and the lowest score of 49% was among adults educated in school up to below O/L. The mean total KAP score among those currently schooling was 54.62%. Adults who were not undergraduates, graduates, or diploma holders, who were out of school, but were educated at school up to O/L and those who had completed schooling after A/L had mean total KAP scores of 53.96 and 54.67% respectively. The mean KAP score on awareness of dengue mortality and severity of dengue burden among each of the age categories; schooling, adults educated less than O/L, adults educated up to O/L, adults educated up to A/L, under graduates, graduates or diploma holders were 50.77, 42, 60.83, 50.44, 58.75, and 55% respectively. The mean KAP scores on awareness on prevention of dengue vector breeding and acquiring the infection among each of the educational categories in above order were 60, 60, 60, 64, 60.94, 67.5% respectively. The mean KAP scores on awareness of the patient’s role in dengue management and warning signs requiring prompt hospital admission among each of the educational categories in above order were 53.85, 45, 44.58, 51.56, 55, 55% respectively (Fig. 4 ). The mean KAP score among females was 55.48%. and that of males was 54.75%.
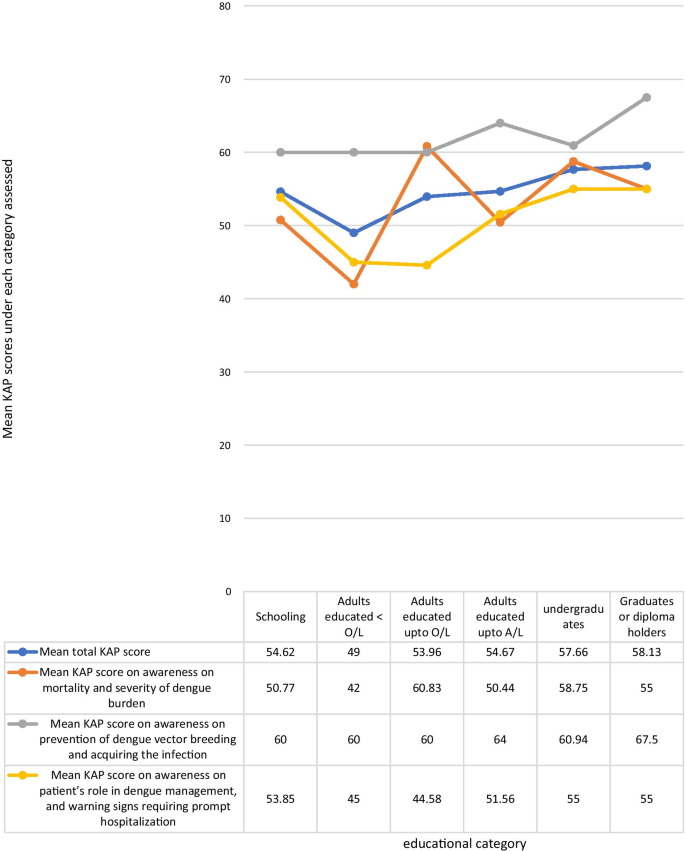
Comparison of the total KAP score, awareness on mortality and severity of dengue burden, awareness on prevention of dengue vector breeding and acquiring the infection, and awareness on patient’s role in dengue management, and warning signs requiring prompt hospitalization under each educational category
When analyzing data by categorizing the questions by the awareness on the area assessed, the highest mean KAP score of 62.05% was on questions on awareness of prevention of dengue vector breeding and acquiring the infection, while the lowest mean KAP score of 51.06% was on questions on awareness of patient’s role in dengue management, and warning signs requiring prompt hospitalization. The mean KAP score on awareness of dengue mortality and severity of burden was 54.02% (Fig. 5 ). On analysis of questions related to awareness of dengue mortality and severity of burden, only 28.8% had high KAP scores, 40.9% had low KAP scores, and 30.3% had moderate KAP scores. On the analysis of questions related to awareness on dengue prevention, an equal percentage of 40.9% had low and high KAP scores respectively, and 18.2% had moderate KAP scores. Analysis of questions related to awareness on patient’s role in dengue management and warning signs prompting hospitalization showed, only 5.3% had high KAP scores, 62.9% had moderate KAP scores, and 31.8% had low KAP scores (Fig. 6 ).
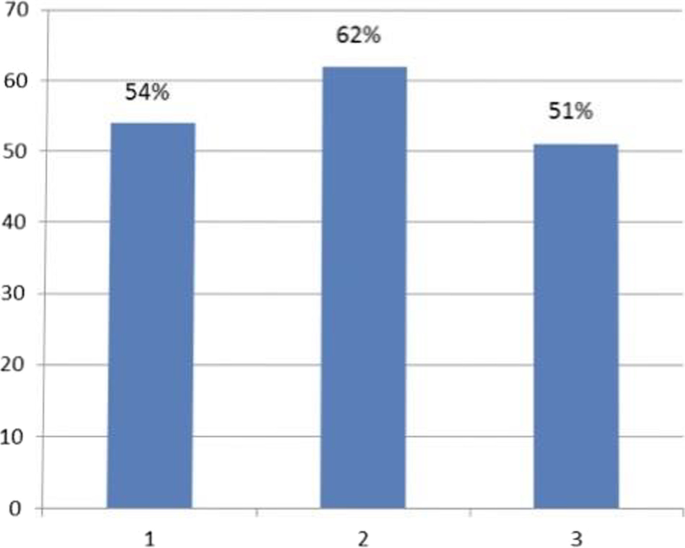
Mean KAP score of each area assessed. 1. Mean KAP score on awareness of mortality and severity of dengue burden- 54%. 2. Mean KAP score on awareness of prevention of dengue breeding and acquiring the infection—62%. 3. Mean KAP score on awareness of patient’s role in dengue management, and warning signs requiring prompt hospitalization—51%
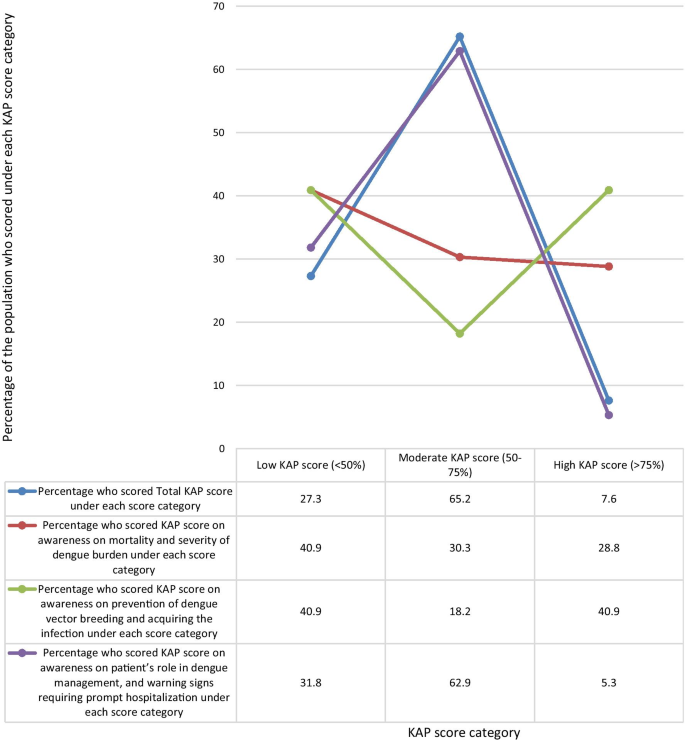
Comparison of percentage of the population who scored low (< 50%), moderate (50%-75%), and high (> 75%) KAP scores under each area assessed
There is no statistically significant correlation between the mean KAP scores on awareness of dengue mortality and severity of dengue burden, and the mean KAP scores on awareness on prevention of dengue vector breeding and acquiring infection according to the spearman’s test (p = 0.084). Although there is a statistically significant correlation between the mean KAP scores on awareness of dengue mortality and severity of dengue burden, and the mean KAP scores on awareness of patient’s role in dengue management and warning signs requiring prompt hospital admission according to the spearman’s test (p = 0.015).
The populations response to each individual question is shown in Table 2 . The percentage of the population who knew the correct answer for the questions on awareness of dengue burden and mortality were as follows: The number of reported dengue cases in Sri Lanka for the year during the outbreak in 2017 was close to 200,000 (42%), The number of reported dengue cases in the year 2019 is higher than that of 2018 (52%), Of 100 persons who get dengue fever only 1 or less persons would die per year when detected early and proper access to medical care (The mortality of dengue fever is < 1%) (60%), The mortality rate of dengue hemorrhagic fever is 2–5%, but is high as 20% if left untreated (60%), The WHO has ranked dengue as one of the top ten threats to Global health in 2019 (56%).
The percentage of the population who knew the correct answer for the questions on awareness of dengue prevention were as follows: all persons with dengue fever do not need to be notified to the Public Health Inspector (PHI) (39%), dengue vector mosquitoes breed in muddy water (52%), The peak biting times of the dengue mosquito is morning and evening (80%), If a person gets dengue fever once in their life, they will be immune to it and will not get dengue fever again (44%), discarded tires, coconut shells, and plastic containers collecting rain water in the garden should be destroyed to prevent dengue vector breeding (96%).
The percentage of the population who knew the correct answer to the questions on awareness of dengue management and warning signs which require prompt hospitalization were as follows: There is a special drug available to treat dengue fever (43%), papaya leaf juice increases the platelet count and thus helps treat dengue fever (33%), dengue patients with a platelet count < 150,000/mm 3 with a rapid drop are recommended to be admitted to hospital (85%), abdominal pain in a dengue patient is not an indication for hospital admission (32%), all pregnant mothers with dengue fever are recommended to be admitted in hospital irrespective of the platelet count (83%), NS1 antigen can be tested on any day since the onset of fever to diagnose dengue fever (23%), a negative report of dengue IgM antibody done on the second day since onset of fever means the patient does not have dengue fever (17%), When a dengue patient has a platelet count > 150,000/mm3 and does not meet criteria which require hospital admission, they should drink 2500 ml of oral fluids per day at home (40%), When a dengue patient has a platelet count > 150,000/mm3 and does not meet criteria which require hospital admission, they should check their Full blood count daily to assess the drop in platelet count (65%), dengue patients should avoid having red or brown drinks (89%).
Dengue virus has four serotypes. Acquisition of dengue infection due to one serotype does not give immunity against a subsequent infection with another serotype, though there is about a two years period of cross-protection [ 15 ]. All four serotypes share only 60–75% identity at amino acid level, and are thus considered as different viruses [ 14 ]. Infection from one serotype gives life-long immunity against that particular serotype [ 10 , 15 ]. Once the cross protection wanes off, secondary dengue infection is more severe than primary dengue infection [ 10 , 15 ]. However only 44% of the study population were aware that occurrence of dengue infection once, does not prevent occurrence of the disease again.
Dengue transmission increases during the rainy season in Sri Lanka, mostly in July, due to increasing dengue vector mosquito breeding places. Other causes for increase in the number of dengue cases is urbanization, climate change, and poor vector control and prevention of disease [ 10 ]. 96% of our cohort were aware of the need to destroy and clean water collecting areas, to prevent breeding of the dengue vector, while 84% of the cohort of a similar study done in the central province of Sri Lanka was aware of this same fact. This is probably because the latter study was done in 2015, prior to the dengue epidemic in 2017 [ 16 ]. Intense measures were taken in the country by which the epidemic in 2017 was controlled. This included clean-up campaigns, awareness programs, National dengue prevention and control, National Strategic framework (2016–2020) to align their action with the WHO Global strategy for dengue prevention and control (2012–2020), The Presidential Task Force on Dengue (PTF) and National dengue control unit of the Ministry of Health launched a rapid inter-sectoral program for prevention and control of dengue [ 7 ]. Awareness programs were held in rural and urban community gatherings, taught in school and institutions, shared on social media, television and radio [ 7 ]. However, data regarding the targeted population for these awareness programs was sparse. Dengue is ranked the third commonest notifiable disease in Sri Lanka, by which means the health sector can implement active vector control measures in the identified areas [ 17 ]. Only 39% of the study population was aware that all persons with dengue fever should be notified to the PHI. The low number of people who were aware of the importance of notifying dengue cases to the PHI, was probably due to the general public being unaware of the PHI’s role in dengue prevention, and lack of awareness of their responsibility in notifying cases, and it’s importance in vector control. Lack of notification of disease hinders action taken for vector control, which gives a falsely lower number of reported cases than the actual number. People should be educated on this to improve notification and vector control. Notification to the PHI of dengue patients managed at home or in the hospital should be made mandatory to avoid negligence in notification. This study population had a relatively good awareness about dengue breeding sites and biting times, probably due to awareness programs during the 2017 epidemic. Literature has shown the importance of improving knowledge on dengue prevention to control dengue outbreaks [ 18 ].
A study in Vietnam during the dengue epidemic in 2017 showed that 91% of the study population considered dengue to be dangerous to very dangerous [ 19 ]. Our study evaluated patients already being admitted for treatment of dengue at the Sri Jayawardenepura general hospital, comprising of patients from the western province, which has the highest dengue burden in the country. A similar study was done in the central province of Sri Lanka by Jayalath et al . among out patients visiting the Peradeniya hospital for reasons other than dengue. Jayalath et al. showed that 95% of their study population knew dengue was a severe disease [ 16 ]. 75% of the cohort of a similar study done among patients being admitted for treatment of dengue fever, in the northern province of Sri Lanka in 2017, knew that dengue was a severe disease [ 20 ]. Our study population had a moderate mean KAP score (54%) on questions on awareness on dengue severity and burden. 40.9% of the population had low awareness on severity and burden of dengue, and only 28.8% had high awareness on its severity and burden. This difference in evidence regarding awareness of severity of dengue in the above studies, could be because the questions by which awareness was evaluated was different in the three studies, and because our study, and the study in the northern province evaluated patients who had already acquired dengue fever and were admitted for treatment at that time. It could also be speculated that these populations acquired dengue infection due to their lack of awareness in prevention of disease.
This lack of awareness on the severity of dengue and it’s burden is probably due to most dengue patients uneventfully recovering from uncomplicated dengue fever, and due to successful dengue management by the healthcare system in the country. This study identified that those who had good awareness on the mortality and severity of the burden of dengue, also had a good awareness about their role in managing dengue, as well as warning signs requiring prompt hospital admission. It can be concluded that there is a strong correlation between those who have an appreciation of the gravity of the symptoms caused by dengue, and the likelihood of them educating themselves on dengue management and their active participation in it. Rozita et al. showed that people who were infected by dengue, or had a family member infected by the disease had better knowledge, attitudes and practices about dengue compared to those who did not [ 21 ]. A study in Singapore in 2017 after the country’s largest dengue epidemic showed that attitudes and practices regarding dengue among primary care physicians significantly improved after experiencing the epidemic [ 22 ]. Chanthalay S et al . showed that those who had better knowledge and attitudes regarding dengue are more likely to take precautions to prevent the disease [ 23 ]. Those who have good awareness will have a good understanding of the gravity and impact of the disease, will know the importance of preventing it, and will be aware of necessary preventive measures.
The mortality of dengue fever is < 1%, and that of dengue hemorrhagic fever is 2–5% if detected early and treated promptly, but is high as 20% if dengue hemorrhagic fever is left untreated [ 8 ]. In 2015 Malhi et al. reported that the presence of comorbidities like diabetes mellitus, hypertension, chronic kidney disease, allergies, asthma, ischemic heart disease and hepatic anomalies, as well as delay in identification and treatment were linked to increased mortality from dengue [ 24 ]. However, in 2017 a study by the same authors showed that 50% of dengue deaths were of previously healthy individuals with no comorbidities [ 25 ]. Therefore, the leading cause for dengue related complications and deaths is delayed identification and treatment of disease. This can be due to delays by the patient or health staff, mostly due to delayed patient presentation to the hospital [ 26 ].Studies have shown that late presentation of dengue fever to the hospital leads to increased development of dengue haemorrhagic fever, dengue shock syndrome, multi-organ involvement like acute kidney injury, and increased mortality [ 26 , 27 , 28 ]. According to the study findings, by identifying areas where the public has misconceptions and misunderstandings about dengue fever, its prevention and management, we can implement steps to improve those loop holes. By following correct practices, avoiding malpractices, and timely hospital admission, his will reduce dengue fatality, improve the outcome, and will also reduce the burden on the healthcare system.
The national Guidelines on dengue management indicates the need for hospital admission in a dengue patient if the platelet count is < 100,000, or platelet count between 100,000- 150,000 with a rapid drop in platelets, fever for three days with any warning signs such as abdominal pain, persistent vomiting, mucosal bleeding, lethargy and restlessness [ 29 ]. Irrespective of the above criteria, admission is required in dengue patients who are pregnant, elderly, obese, with comorbidities, or with adverse social circumstances [ 29 ]. In this study, 85 and 83% patients respectively were aware of the indication for admission as per the platelet count or if pregnant, but only 32% patients knew admission was indicated with warning signs like abdominal pain. Therefore, people need to be educated about warning signs of severe dengue infection. People who do not require admission must be educated about cautious self-management at home until they require admission [ 29 ]. By doing so there will be less likelihood to miss warning signs, will have improved outcome, and there will be less burden to hospital staff. Only 40% of patients knew about fluid management at home, but 89% knew to avoid red drinks.
Serological testing is important to confirm the diagnosis of dengue fever when the presentation is atypical or when unsure of the diagnosis. NS1 antigen is tested in the patient’s blood on the first few days of the disease and has a sensitivity of 60–90%. Dengue IgM antibody will be positive in the patient’s blood only after the 5th day of illness [ 29 ]. Therefore, patients should be educated about the ideal time to do each test to avoid false negatives being reported by doing the test at the wrong time of the illness. However, dengue infection cannot be excluded by a negative serological lab report. Few patients knew about the timing of testing, with only 23% and 17% being aware of the timing of testing, and sensitivity of NS1 antigen and dengue IgM respectively. It is important that health care professionals guide patients on the correct timing to do the serological tests. It would be prudent to do such serological tests only on request by a physician, to avoid patients testing at the wrong time, and getting a report which cannot be interpreted at that time of the illness. False negatives of serological testing can further be avoided by laboratory staff rechecking the patients’ day of the illness, and the physicians request form prior to drawing blood.
This study shows that people had misconceptions about dengue management. Only 43% knew there was no special drug to treat dengue fever. There is no particular drug to treat dengue, but is managed by careful monitoring and fluid tailoring resuscitation [ 29 ]. A tetravalent live attenuated dengue vaccine has been registered for use in several countries [ 15 ]. In sero-negative individuals it is believed that the vaccine mimics a silent natural infection, giving temporary cross-protection against all serotypes, and subsequently causing severe dengue infection when primarily infected [ 15 ]. However, its efficacy varies in different countries and is not currently recommended for use in Sri Lanka [ 15 ]. The use of papaya leaf juice in dengue management had recently gained interest, leading to many people consuming the juice assuming improvement of dengue infection. Research has shown papaya leaf juice to improve platelet counts, but has not shown to prevent or reduce fluid leaking in dengue hemorrhagic fever [ 30 ]. This can adversely cause early rise in platelet count masking the onset of fluid leaking, which can be detrimental in managing dengue hemorrhagic fever. 33% of our cohort believed papaya leaf juice helped treat dengue fever, while 13.4% of the cohort in a study done in Sri Lanka in 2015 believed the same to be true. This is probably because the concept of the effect of papaya leaf juice on platelet count came in to light only later on [ 16 ].
This study demonstrated an increasing trend in awareness on all categories, such as among people with a higher level of education, and maturity by age, indicating that education and maturity are important factors for improved awareness. Kumanan et al. showed a significant association between educational level and knowledge regarding dengue fever, and no significant association between educational level and preventive practices [ 20 ]. The trend in our study demonstrated on Fig. 3 suggests that responses in the awareness on dengue mortality and severity of dengue burden steadily increased with age, and strongly influence the mean total KAP scores. The highest awareness in all age categories was on dengue prevention and the lowest awareness in all categories was on patients’ role in dengue management and warning signs requiring prompt hospitalization (Fig. 3 ).
There was inadequate awareness among adults who dropped out of school prior to completion of the full school education up to advanced level even when they are older. This may demonstrate a population with lower level of understanding of the information given, and those who were not regularly educated at school regarding dengue infection as they dropped out. Those who drop out of school are also those who usually have a poor social background, and they may also have inadequate access to social media and electronic media to receive updates about dengue mortality, prevention and management. This highlights the need for any information to reach the people of all social backgrounds when implementing strategies to improve public awareness on dengue infection. Dissemination of information should be done in various ways targeting different populations of different levels of understanding. People with lower education levels should be the main target group requiring more advice and education regarding the patient’s role in dengue management.
This population has a relatively a better awareness on dengue prevention as compared to awareness of dengue mortality and dengue management. This is possibly due to prior media education of the public on prevention during the previous epidemic in 2017. The identified weak point is patient awareness on the patient’s role in dengue management, as well as identifying warning signs requiring prompt hospitalization. It causes delay in treatment, which is a major cause for increased mortality. The trend demonstrated on Fig. 5 suggests that responses in the dengue management and warning signs prompt hospitalization area strongly influence the total KAP scores. This indicates that patient awareness on the role of the public and patients on dengue management is critical in the outcome of dengue infection. An action plan should be implemented targeting improving public awareness by education programs on the role of the public and patients in dengue management, to improve outcome.
The general public play a major role in prevention and management of dengue fever, and influence the outcome. Jayalath et al. showed that 30% of their population believed the responsibility of dengue prevention lay with the public, while 66% believed both the public and the government were responsible [ 16 ]. In order to improve involvement of patients and the public in dengue prevention, control and management, attention should be paid on educating the public and patients on the disease.
Limitations and recommendations for future research
This study focused on 132 patients from one hospital. Therefore, the conclusions can be relevant only to draining areas in the vicinity of this hospital, and may not represent the knowledge, attitudes and practices in other parts of Sri Lanka. However, since majority of the dengue cases in the country are concentrated in the western province, of which a significant number of patients present to the Sri Jayawardenepura General Hospital, the findings of this study may represent the most dengue dense area in the country. Large scale future research from all parts of the country may be beneficial to infer the knowledge, attitudes, and practices of the country as whole.
The general public was educated about Dengue infection by various means, including messages on social media, electronic media, awareness programs at schools, and village meetings, posters and distribution of leaflets, during the dengue epidemic in 2017. This study did not extensively evaluate whether the study participants had been exposed to these prior teaching about Dengue infection, and if they did, by what means they were educated. However almost all the study participants had access to electronic and social media. This may not be the same when inferring on the population in some rural parts of Sri Lanka who may not have similar access to such media education. Awareness programs and active participation of the general public in dengue prevention and management should be implemented. We suggest future follow up research of the awareness on dengue infection among the public, before and after implementing formal dengue awareness strategies to assess the effectiveness of it. In addition to follow up research before and after implementing disease awareness steps, we also suggest future research to assess an association and comparison of dengue mortality and outcome before and after implementing practices to further educate the public, in order to identify its impact on dengue management and outcome.
The population has relatively a better awareness on dengue prevention, as compared to awareness of dengue mortality and dengue management. The identified weak point is patient awareness on the patient’s role in dengue management, and identifying warning signs requiring prompt hospitalization causing delay in treatment, which is a major cause for increased mortality. There was a correlation between those who had good knowledge on dengue burden and those who were aware of the patients’ role in dengue management. There is also an increasing trend in awareness on all categories, especially among people with a higher level of education, and maturity by age, indicating that education and maturity are important factors for improved awareness. An action plan should be implemented targeting improving public awareness on the role of the public and patients in dengue management to improve outcome.
Availability of data and materials
The raw data sets analyzed during the current study are available on reasonable request from the corresponding author.
Abbreviations
Dengue virus
Knowledge attitudes and practices
Ordinary level at school
Advanced level at school
Ten threats to global health in 2019. World Health Organization. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 . Accessed 4 Jan 2020.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7.
Article CAS Google Scholar
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760.
Article Google Scholar
Dengue and severe dengue. World health organization.4th November 2019. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue . Accessed 4 Nov 2019.
Dengue worldwide overview 2019. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/dengue-monthly . Accessed 4 Jan 2020.
Epidemiology unit, Ministry of Health Sri Lanka. Dengue, disease surveillance trends. http://www.epid.gov.lk . Accessed 4 Jan 2020.
Dengue DREF final report 2017. Dengue Sri Lanka. International federation of red cross and red crescent societies. https://www.chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Freliefweb.int%2Fsites%2Freliefweb.int%2Ffiles%2Fresources%2FMDRLK007dfr.pdf&clen=1774569&chunk=true . Accessed 4 Jan 2020.
Lahiri M, Fisher D, Tambyah PA. Dengue mortality: reassessing the risks in transition countries. Trans R Soc Trop Med Hyg. 2008;102(10):1011–6.
Sirisena PDNN, Noordeen F. Evolution of dengue in Sri Lanka—changes in the virus, vector, and climate. Int J Infect Dis. 2014;19:6–12.
Jayarajah U, Faizer S, de Zoysa I, Senevirathne SL. A large Dengue epidemic affects Sri Lanka in 2017. IJPSAT. 2017;6(1):84–6.
Google Scholar
National Dengue control unit. Ministry of Health, Nutrition, and Indigenous Medicine. http://www.dengue.health.gov.lk . Accessed 4 Jan 2020.
World Health Organization. Global strategy for dengue prevention and control 2012–2020. Geneva: World Health Organization
Rigau-Pérez JG, Clark GG. Còmo responder a una epidemia de dengue: vision global y experiencia en Puerto Rico [How to respond to a dengue outbreak: global vision and experience in Puerto Rico]. Pan Am J Public Health. 2005;17:282–93.
Selvarajoo S, Liew JWK, Tan W, et al. Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: a cross-sectional study. Sci Rep. 2020;10(1):9534. https://doi.org/10.1038/s41598-020-66212-5 .
Article CAS PubMed PubMed Central Google Scholar
Applicability of dengue vaccines. Weekly epidemiological report. Apublication of the Epidemiological unit, Ministry of Health, nutrition and indigenous medicine, Sri Lanka. 18th - 24th March 2017. Volume 44. no. 12. https://www.chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=http%3A%2F%2Fwww.epid.gov.lk%2Fweb%2Fimages%2Fpdf%2Fwer%2F2017%2Fvol_44_no_12-english.pdf&clen=1799202&chunk=true . Accessed 6 Jan 2020.
Jayalath T, Ralapanawa U, Karunaratne S, Dassanayake UKA, Pathirage M, et al. Knowledge and attitude regarding dengue fever among the outdoor patients of the teaching hospital Peradeniya, Sri Lanka. Int J Med Res Health Sci. 2018;7(1):77–84.
Annual health bulletin of Sri Lanka. Department of Health Services, Colombo, Sri Lanka (2002)
Al-Zurfi BM, Fuad MD, Abdelqaderm MA, Baobaidm MF, Elnajehm M, Ghazim HF, Ibrahim MH, Abdullah MR. Knowledge, attitude and practice of dengue fever and health education programme among students of Alam shah science school, Cheras Malaysia Malays. J Public Health Med. 2006;6:62–7.
Nguye HV, Than PQT, Nguyen TH, Vu GT, et al. Knowledge, attitude and practice about dengue fever among patients experiencing the 2017 Outbreak in Vietnam. Int J Environ Res Public Health. 2017;2019(16):976.
Kumanan T, Logeswaran D. A study on knowledge, attitude and practices regarding dengue among hospitalized patients from Northern Sri Lanka. Sri Lankan J Infect Dis. 2018;8(2):127–32. https://doi.org/10.4038/sljid.v8i2.8220 .
Wan Rozita WM, Yap BW, Veronica S, Muhammad AK, Lim KH, Sumarni MG. Knowledge, attitude and practice (KAP) survey on dengue fever in an urban malay residential area in Kuala Lumpur. Malays J Public Health Med. 2006;6:62–7.
Junxiong P, ZoeJane-Lara H, Tun LH, Jing Y, Yee SL. Assessing changes in knowledge, attitude and practices of dengue diagnosis and management among primary care physicians after the largest dengue epidemic in Singapore. BMC Infect Dis. 2017;17:428.
Chanthalay S, Jiraporn C, Somsak W, Cheerwith R. Knowledge, attitudes and preventive behaviours related to dengue vector breeding control measures among adults in communities of Vientiane, capital of Lao PDR. J Infect Public Health. 2015;8:466–73.
Mallhi TH, Khan AH, Adnan AS, et al. Clinico-laboratory spectrum of dengue viral infection and risk factors associated with dengue hemorrhagic fever: a retrospective study. BMC Infect Dis. 2015. https://doi.org/10.1186/s12879-015-1141-3 .
Article PubMed PubMed Central Google Scholar
Mallhi TH, Khan AH, Sarriff A, Adnan AS, Khan YH. Determinants of mortality and prolonged hospital stay among dengue patients attending tertiary care hospital: a cross-sectional retrospective analysis. BMJ Open. 2017;7(7):e016805.
Mallhi TH, Adnan AS, Khan AH, Habib Y, et al. Patients related diagnostic delay in dengue: an important cause of morbidity and mortality. Clin Epidemiol Glob Health. 2016;4(4):200–1.
Yatra IM. Disease history and delayed diagnosis of dengue infection as risk factors for dengue shock syndrome in Wangaya Hospital Denpasar. Public Health Prev Med Arch. 2015. https://doi.org/10.15562/phpma.v3i2.108 .
Nguyen Thi KT, Nguyen Ngoc AT, Khau MT, Nguyen TT, Luong CQ. Epidemiology analysis of deaths associated with dengue hemorrhagic fever in Southern Viet Nam in 1999–2000. Dengue Bull. 2001;25:28–32.
Guidelines on the management of dengue fever and dengue haemorrhagic fever in adults. National Guidelines 2012. Ministry of Health, Sri Lanka.
Rajapakse S, de Silva NL, Weeratunga P, et al. Carica papaya extract in dengue: a systematic review and meta-analysis. BMC Complement Altern Med. 2019;19:265. https://doi.org/10.1186/s12906-019-2678-2 .
Download references
Acknowledgements
We all express our gratitude to all participants who consented to take part in this study.
Authors’ information
SS is a Consultant Physician [MBBS, MD, FRACP] Medical unit, Sri Jayawardenepura General Hospital. KPJ [MBBS], DKJ [MBBS] and DW [MBBS] are Registrars in Internal medicine, and SW is a Senior Registrar in Medicine at the Sri Jayawardenepura General Hospital.
No funding was obtained for this study.
Author information
Authors and affiliations.
Sri Jayewardenepura General Hospital, Kotte, Sri Lanka
K. P. Jayawickreme, D. K. Jayaweera, S. Weerasinghe, D. Warapitiya & S. Subasinghe
You can also search for this author in PubMed Google Scholar
Contributions
Data collection was done by KPJ, DKJ and DW. Analysis, interpretation of data, literature review and writing of the report was done by KPJ. SS and SW guided the study and corrected the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to K. P. Jayawickreme .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was taken from the institutional Ethical Review committee of the Sri Jayawardenepura General Hospital and Postgraduate Training Centre to conduct this study (SJGH/20/ERC/017). Informed written consent was taken from all the participants. All the participants were above the age of 13 years. In the very few participants aged between 13 and 16, informed written consent was obtained from both the participant and the parent or guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix s1..
Questionnaire in English.
Additional file 2: Appendix S2.
Questionnaire in Sinhala.
Additional file 3: Appendix S3.
Questionnaire in Tamil.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jayawickreme, K.P., Jayaweera, D.K., Weerasinghe, S. et al. A study on knowledge, attitudes and practices regarding dengue fever, its prevention and management among dengue patients presenting to a tertiary care hospital in Sri Lanka. BMC Infect Dis 21 , 981 (2021). https://doi.org/10.1186/s12879-021-06685-5
Download citation
Received : 03 April 2020
Accepted : 13 September 2021
Published : 20 September 2021
DOI : https://doi.org/10.1186/s12879-021-06685-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dengue fever
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
A Growing Problem in the United States
Environmental factors contributing to dengue as a public health threat, pathogenesis, clinical considerations, presentation and evaluation, diagnostic testing for symptomatic denv infection, traditional prevention measures, novel vector control efforts, current dengue vaccines, principles of live-attenuated dengue vaccines, history of dengvaxia, safety and efficacy, prevaccination laboratory testing, conclusion and future directions, acknowledgment, dengue: a growing problem with new interventions.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Joshua M. Wong , Laura E. Adams , Anna P. Durbin , Jorge L. Muñoz-Jordán , Katherine A. Poehling , Liliana M. Sánchez-González , Hannah R. Volkman , Gabriela Paz-Bailey; Dengue: A Growing Problem With New Interventions. Pediatrics June 2022; 149 (6): e2021055522. 10.1542/peds.2021-055522
Download citation file:
- Ris (Zotero)
- Reference Manager
Dengue is the disease caused by 1 of 4 distinct, but closely related dengue viruses (DENV-1–4) that are transmitted by Aedes spp. mosquito vectors. It is the most common arboviral disease worldwide, with the greatest burden in tropical and sub-tropical regions. In the absence of effective prevention and control measures, dengue is projected to increase in both disease burden and geographic range. Given its increasing importance as an etiology of fever in the returning traveler or the possibility of local transmission in regions in the United States with competent vectors, as well as the risk for large outbreaks in endemic US territories and associated states, clinicians should understand its clinical presentation and be familiar with appropriate testing, triage, and management of patients with dengue. Control and prevention efforts reached a milestone in June 2021 when the Advisory Committee on Immunization Practices (ACIP) recommended Dengvaxia for routine use in children aged 9 to 16 years living in endemic areas with laboratory confirmation of previous dengue virus infection. Dengvaxia is the first vaccine against dengue to be recommended for use in the United States and one of the first to require laboratory testing of potential recipients to be eligible for vaccination. In this review, we outline dengue pathogenesis, epidemiology, and key clinical features for front-line clinicians evaluating patients presenting with dengue. We also provide a summary of Dengvaxia efficacy, safety, and considerations for use as well as an overview of other potential new tools to control and prevent the growing threat of dengue.
Dengue is the disease caused by 4 closely related but distinct viruses, dengue virus 1–4 (DENV-1–4), referred to as virus types or serotypes. DENVs are most commonly transmitted by the bite of an infected female Aedes spp. mosquito. It is the most common arboviral disease globally, with an estimated 390 million dengue virus infections and 96 million symptomatic cases annually. 1 Global incidence has almost doubled in the last 3 decades and is expected to continue growing in Asia, sub-Saharan Africa, and Latin America. About half of the global population now lives in areas that are suitable for dengue transmission ( Fig 1 ). 2 , 3 Historically, the highest burden of dengue has been in children, adolescents, and young adults. 4 In 2019, countries across the Americas reported more than 3 million dengue cases, the highest number ever recorded, 5 with a greater proportion of severe dengue cases and increased mortality in the pediatric population of children aged 5 to 9 years. 6 Dengue is increasingly common as an etiology of fever in international travelers 7 and has been reported as the leading febrile disease etiology for travelers from some endemic regions during epidemic years. 8 In addition to circulation of all four DENVs worldwide, surveillance of returning travelers with dengue has demonstrated high genetic diversity among circulating DENV genotypes within serotypes, with potential implications for immune or vaccine escape. 9 , 10

Map showing the risk of dengue by country as of 2020. “Frequent or Continuous” risk indicates that there are either frequent outbreaks or ongoing transmission. “Sporadic or Uncertain” indicates that risk is either variable and unpredictable or that data from that country are not available. For updated information, visit https://www.cdc.gov/dengue/areaswithrisk/around-the-world.html .
Increasing numbers of dengue cases in the United States are a growing concern. In parts of the United States and freely associated states with endemic dengue transmission, including American Samoa, Puerto Rico, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau, dengue outbreaks can be explosive, overwhelming the health care system capacity. In Puerto Rico, the largest US territory where dengue is endemic, the highest incidence of dengue cases and hospitalizations from 2010 to 2020 occurred among children aged 10 to 19 years. 11 For the same period, confirmed dengue cases ranged from a minimum of 3 cases in 2018 to a maximum of 10 911 cases in 2010, 11 although suspected case counts during outbreak years were considerably higher. 12
Although local dengue transmission does not occur frequently in most states, increasing numbers of US travelers 13 with dengue have been reported in recent years, with a record 1475 cases in 2019, more than 50% higher than the previous peak in 2016 ( Fig 2 ). 14 Viremia among travel-associated dengue cases can also result in focal outbreaks in nonendemic areas, with competent mosquito vectors for dengue present in approximately half of all US counties. 15 Local dengue cases have been reported in multiple states in recent years, including 70 cases in Florida in 2020, 14 200 cases in Hawaii in 2015, 14 and 53 cases in Texas in 2013. 16
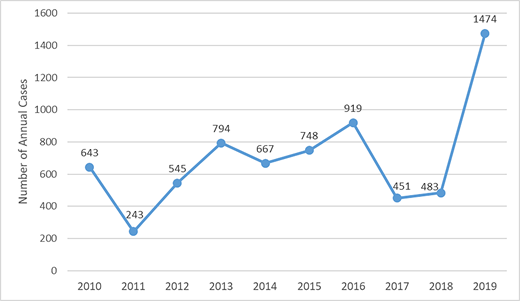
Annual number of travel-associated cases of dengue reported into ArboNET, the national arboviral surveillance system managed by the CDC, from all US jurisdictions from 2010 to 2019 ( n = 6967).
In dengue-endemic areas, environmental factors such as standing water where mosquitoes lay eggs, poor housing quality, lack of air conditioning, and climatic factors (ie, temperature, precipitation, and humidity) increase the abundance, distribution, and risk of exposure to Aedes aegypti , the main vector responsible for dengue transmission, or other Aedes spp. mosquitoes that can also transmit dengue. 2 , 17 – 21 Climate change is predicted to further increase the population at risk for dengue primarily through increased transmission in currently endemic areas and secondarily through expansion of the geographic range of Aedes spp. mosquitoes ( Fig 3 ). 2 , 22 Urbanization, increasing population density, human migration, and growing social and environmental factors associated with poverty and forced displacement are also expected to drive the increase in dengue incidence and force of infection globally. 21 , 23 – 26 Travel is an important driver of dengue expansion by introducing dengue into nonendemic areas with competent vectors 13 , 23 or by introducing new serotypes into endemic areas naïve to the new serotype, thereby increasing the risk for antibody-dependent enhancement (ADE) and severe disease. 27 , 28 Combined environmental effects of poverty and the increased scale and rapidity of human movement can also increase the risk for dengue. 24 , 29 The combined environmental effects of climate change, urbanization, poverty, and human migration together expand the threat of dengue for both individuals and public health systems in the future.
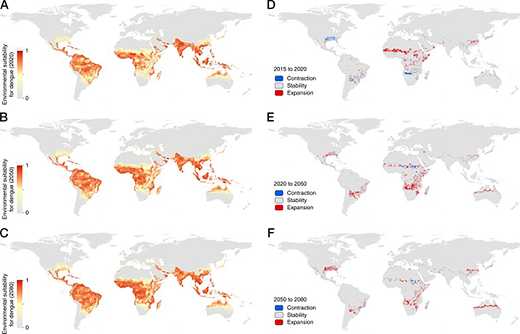
A-C, Projections of average trends in environmental suitability for dengue transmission from 2015 to 2020, 2020 to 2050, and 2050 to 2080. D–F, Areas with expansion or contraction of the Aedes vector range over the same time periods. (Reprinted with permission from Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nature Microbiology. 2019;4(9):1510.)
DENVs belong to the genus Flavivirus in the family Flaviviridae . Because there are 4 dengue serotypes, individuals living in endemic areas can be infected up to 4 times in their life. Although most dengue virus infections are asymptomatic or only cause mild disease, severe disease can occur and is characterized by plasma leakage, a pathophysiologic process by which the protein rich fluid component of blood leaks into the surrounding tissue, leading to extravascular fluid accumulation resulting in shock, coagulopathy, or end organ impairment. 30 , 31
Infection with 1 dengue serotype induces life-long protection against symptomatic infection with that specific serotype (homotypic immunity) 32 , 33 and induces only short-term cross-reactive protection from disease to the other serotypes (heterotypic immunity) for several months to years. 34 , 35 Older children and adults experiencing their second dengue infection are at the highest risk for severe disease because of ADE. ADE has also been observed among infants, in that infants born to mothers with previous dengue virus infection had the lowest risk for dengue shortly after birth and a period of higher risk for severe disease approximately 4 to 12 months after birth, followed by a decrease in risk for severe disease from approximately 12 months after birth. 36 The initial period of lowest risk was correlated with high levels of passively acquired maternal dengue antibodies immediately after birth, and the period of enhanced risk with a decline in these antibodies to subneutralizing levels. After further degradation of these maternal antibodies, there was neither protection from dengue afforded by high levels of antibodies postnatally nor enhanced risk of dengue and severe disease from the intermediate levels of antibodies. 37 Later work showed that lower heterotypic antibody titers are ineffective at neutralizing the virions but still bind them, facilitating binding to Fcγ receptors on circulating monocyte cells, and result in higher viremia than in primary infections ( Fig 4 ). 38 The feared sequela of plasma leakage is believed to be mediated by high levels of DENV nonstructural protein 1 (NS1), a key protein for viral replication and pathogenesis, 39 , 40 that damages endothelial glycocalyces and disrupts endothelial cell junctions. 41 , 42 Cell-mediated immunity through dengue-specific CD8 T cells is thought to protect against ADE and severe disease. 43 , 44

The proposed mechanism of antibody-dependent enhancement with heterotypic antibodies binding to the dengue viruses and entering monocytes through Fcγ receptors. Viral replication occurs in the infected monocyte and releases high levels of virus and dengue virus NS1 protein, which, in turn, lead to increased vascular permeability contributing to severe disease. (Reprinted with permission from Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature Reviews Microbiology. 2007;5(7):524.)
Although ADE occurs in infants due to the interaction between maternal antibodies and primary infection, it is also explanatory for severe disease in older children and adults where the heterotypic antibodies produced after a primary dengue infection will wane over time to subneutralizing levels, resulting in the highest risk for severe disease with secondary infection. Following secondary infection, potent cross-neutralizing/multitypic antibodies are induced that then protect against severe disease in tertiary and quaternary infections. 45 , 46 Although the risk of severe dengue is highest with secondary infection, it can also occur in primary, tertiary, and quaternary infections, and possibly following Zika virus infection. 47 , 48 Identifying cases of severe dengue and understanding the pathogenesis of disease severity is an active area of research with important implications for future vaccines and interventions. 49
DENV infections have a wide range of presentations from asymptomatic infection (approximately 75% of all infections 50 ) to mild to moderate febrile illness to severe disease with associated coagulopathy, shock, or end organ impairment ( Table 1 ). 30 , 31 Symptomatic infections most commonly present with fever accompanied by nonspecific symptoms such as nausea, vomiting, rash, myalgias, arthralgias, retroorbital pain, headache and/or leukopenia. 51 Severe disease develops in as many as 5% of all patients with dengue, although certain populations such as infants aged ≤1 year, pregnant individuals, and adults aged ≥65 years, or individuals with specific underlying conditions such as diabetes, class III obesity, hypertension, asthma, coagulopathy, gastritis or peptic ulcer disease, hemolytic disease, chronic liver disease, anticoagulant therapy, or kidney disease, are at increased risk of severe disease. 52 , 53 In all patients with dengue, warning signs are specific clinical findings that can predict progression to severe disease and are used by the World Health Organization (WHO) to help clinicians in triage and management decisions. Dengue warning signs include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, liver enlargement of >2 cm, and increasing hematocrit concurrent with rapid decrease in platelet count ( Table 1 ). 52
Classification of Dengue Severity and Case Management 51 ,134, 135
Although warning signs are useful for evaluating patients with a high suspicion of dengue (for example, during an outbreak), they are not intended to differentiate dengue from other infectious and noninfectious diseases such as influenza, coronavirus disease 2019, malaria, Zika, measles, leptospirosis, rickettsial disease, typhoid, Kawasaki, or idiopathic thrombocytopenic purpura. Because prompt recognition and early treatment of dengue can greatly reduce morbidity and mortality, 54 , 55 clinicians practicing in the United States and other nonendemic areas should keep dengue in the differential diagnosis for febrile illness in travelers and in areas with competent mosquito vectors.
For symptomatic dengue patients, nucleic acid amplification tests (NAATs) on serum, plasma, or whole blood detect DENV RNA during the first 7 days of illness with high sensitivity and specificity. 56 , 57 Likewise, NS1 antigen can also be detected within the first 7 days and provides confirmatory evidence of DENV infection. 58 For patients with a negative NAAT or patients presenting more than 7 days after symptom onset, a positive anti-DENV immunoblobulin M (IgM) can suggest recent infection, although with less certainty than NAAT or NS1 testing, owing to cross-reactivity with other flaviviruses. Notably, Zika virus is a flavivirus that has been transmitted in most countries where DENV transmission is present. 59 In patients from areas with ongoing transmission of another flavivirus (eg, Zika virus) and whose only evidence of dengue is a positive anti-DENV IgM test, plaque reduction neutralization tests (PRNT) quantifying virus-specific neutralizing antibody titers can distinguish DENV from other flaviviruses, in some but not all cases. PRNTs, however, are rarely available in clinical laboratories and typically do not provide results within a timeframe that is meaningful for clinicians managing acute disease. PRNT’s may be valuable in circumstances where confirming the diagnosis may have important clinical implications, such as distinguishing dengue from a Zika virus infection in a pregnant individual, or epidemiologic implications for a region, such as distinguishing yellow fever from dengue. 60 , 61
The US Food and Drug Administration (FDA) has approved a NAAT for use on serum and whole blood, an NS1 antigen enzyme-linked immunosorbent assay test in serum, and an IgM enzyme-linked immunosorbent assay in serum. 56 , 59 , 62 – 64 Other non–FDA-approved tests for DENV infection are used in clinical practice and are commercially available at accredited laboratories.
Although several medications have been explored as potential therapeutics for dengue, none have demonstrated a reduction in viremia, clinical manifestations, or complications. 30 , 65 As such, dengue treatment focuses on supportive care. Clinicians should evaluate all patients at presentation and in follow-up for warning signs or other signs and symptoms of severe dengue ( Table 1 ). Most patients without warning signs may be treated as outpatients, whereas patients at high risk of progression to severe disease based on age or underlying conditions, patients with warning signs, or patients with challenging social circumstances should be evaluated for observation or inpatient management. 66
For outpatients, fever can be controlled with acetaminophen and physical cooling measures; because of the risk of bleeding and thrombocytopenia, aspirin and nonsteroidal anti-inflammatory drugs are not recommended. Early, abundant oral hydration has been associated with lower hospitalization rates in children with dengue and is a key component of outpatient dengue care. 67 – 69
Early recognition of warning signs or severe dengue is essential for the prompt initiation of systematic intravenous fluid management to restore intravascular volume and avoid related complications and disease progression. 30 , 70 Large-volume resuscitation with isotonic solutions is recommended for patients in shock. 54 , 71 – 73 Fluid management in dengue requires continuous clinical and laboratory monitoring and rate adjustments to maintain adequate volume but also to prevent fluid overload. Mortality for untreated severe dengue can be 13% or higher 74 , 75 but can be reduced to <1% with early diagnosis and appropriate management. 55 Detailed information on systematic fluid management is provided in the current WHO, Pan American Health Organization, and Centers for Disease Control and Prevention (CDC) guidelines. 72 , 73 , 76
Corticosteroids, 77 immunoglobulins, 78 and prophylactic platelet transfusions 79 , 80 have not demonstrated benefits in patients with dengue and are not recommended.
Prevention of dengue involves protection against mosquito bites. Travelers to and residents of endemic areas can prevent mosquito bites by using US Environmental Protection Agency–approved insect repellents ( https://www.epa.gov/insect-repellents ) and wearing clothing that covers arms and legs. The use of screened windows and doors, air conditioning, and bed nets has been associated with protection from dengue infections. 24 , 81 – 87 Sites where mosquitoes lay eggs should be eliminated by emptying and scrubbing, covering, or eliminating standing water receptacles around the house. Mosquito bite prevention measures are important for all persons at risk for dengue, including vaccinated children.
Traditional vector control interventions can be time consuming and inefficient. 88 Furthermore, chemical control is limited by widespread insecticide resistance in endemic areas. 89 In response to these challenges, novel vector control methods have been developed including several strategies employing genetically modified mosquito technology and 2 strategies using Wolbachia pipientis , an intracellular bacterium found in about 60% of all insects but not commonly found in wild Aedes mosquitos. 90 – 92
The first strategy utilizing Wolbachia is Wolbachia -mediated suppression, in which a reduction in wild populations of Aedes mosquitoes is achieved by continuously releasing infected males into the environment. 93 When the infected males mate with wild females, the resultant eggs are inviable, leading to a decline in wild mosquito populations. 94 Some reports have documented reduction of the wild populations that can transmit dengue by more than 80%. 95 , 96
The second strategy is the Wolbachia replacement method, where both Wolbachia -infected male and female mosquitoes are released. Because Wolbachia is transmitted maternally, the mosquitoes that hatch from the eggs of infected females will be infected with Wolbachia from birth. 97 , 98 Wolbachia infection in female mosquitoes taking a bloodmeal reduces transmission of arboviruses, including dengue, chikungunya, and Zika. This method has demonstrated significant reductions of nearly 80% for the outcomes of dengue infection and related hospitalizations in areas where it has been implemented 99 and is currently being deployed in several countries.
Extensive studies have found no evidence of Wolbachia in the plants, soil, or other insects in contact with the Wolbachia -infected mosquitoes or any evidence of Wolbachia transmission to humans from the bites of infected mosquitoes, indicating that safety risks from Wolbachia -based interventions for humans and the environment are low. 100
ACIP made the first recommendation of a dengue vaccine (Dengvaxia) for use in the United States on June 24, 2021, marking an historic moment for dengue control following decades of global efforts to develop a safe and effective vaccine. Two other vaccines, TAK-003 developed by Takeda and TV003 developed by the National Institutes of Health, are in late-stage trials with efficacy results published or expected in 2022.
All 3 are live vaccines and contain 4 different attenuated vaccine viruses (tetravalent) targeting each of the dengue virus serotypes ( Fig 5 ) with the goal of achieving balanced protective immunity against all 4 serotypes, in both those who are DENV naïve and those who have been previously infected with DENV. Vaccine virus replication (infectivity) of each vaccine serotype after immunization will lead to antigenic stimulation, which then results in homotypic immunity. Infectivity by vaccine virus serotype differed among the 3 vaccines ( Table 2 ).

Key features of the 3 live attenuated dengue vaccines. Each DENV serotype is represented by a color (DENV-1 = green, DENV-2 = gray, DENV-3 = crimson, and DENV-4 = blue). Dengvaxia is comprised of 4 chimeric viruses in which the prM and E of each DENV serotype replaces those of yellow fever 17D (yellow). 132 TAK-003 is comprised of 1 full-length DENV-2 and 3 chimeric viruses (prM and E of DENV-1, DENV-3, and DENV-4 on a DENV-2 background). 133 TV003 is comprised of 3 full-length DENV and 1 chimeric virus. 123 The total number of dengue proteins in each vaccine is also shown.
Percentage of Vaccine Recipients with Detectable Vaccine Virus Serotype by RT-PCR after a Single Dose of the Indicated Vaccine in Persons without Previous Dengue Virus Infections
Data are presented as percentage.
These differences in vaccine serotype specific infectivity mirrored the induction of neutralizing homotypic antibody titers. Dengvaxia induced approximately 70% homotypic antibody for DENV-4 but <50% for DENV-1, DENV-2, and DENV-3. 101 Antibodies induced by TAK-003 were 83% homotypic for DENV-2 and 5%, 12%, and 27% homotypic for DENV-1, DENV-3, and DENV-4, respectively. 102 TV003 induced a balanced homotypic antibody response to DENV-1 (62%), DENV-2 (76%), DENV-3 (86%), and DENV-4 (100%). 103 Although homotypic antibody titers are associated with serotype specific vaccine efficacy, immune correlates that reliably predict vaccine efficacy have not yet been identified and remain an area of active research. 46
Dengvaxia uses a 3-dose schedule with each dose given 6 months apart (at months 0, 6, and 12). It was developed by Washington and St Louis Universities and Acambis and licensed to Sanofi Pasteur in the 2000s, entered phase 3 trials in the 2010s, and was first recommended by WHO in 2016 for persons aged 9 years and older living in highly endemic areas. Long-term follow-up data (over 5 years) from the phase 3 trials and further analyses of the efficacy results 104 – 107 demonstrated that children with evidence of previous DENV infection were protected from virologically confirmed dengue illness, including severe dengue if they were vaccinated with Dengvaxia. However, risk of hospitalization for dengue and severe dengue was increased among children without previous dengue infection who were vaccinated with Dengvaxia and had a subsequent dengue infection in the years after vaccination. In children without a previous dengue infection, the vaccine acts as a silent primary dengue infection resulting in a “secondary-like” infection upon their first infection with wild-type DENV and an increased risk of severe disease due to ADE ( Fig 6 ). 108 , 109 After these findings, WHO revised their recommendations for the vaccine to only be given to children with laboratory-confirmed evidence of a past infection. Following WHO’s recommendation, the FDA licensed Dengvaxia in 2019, and in 2021, ACIP recommended routine use of Dengvaxia for children aged 9–16 years with laboratory confirmation of previous DENV infection and living in areas where dengue is endemic. Dengvaxia is the first dengue vaccine recommended for use in the United States.
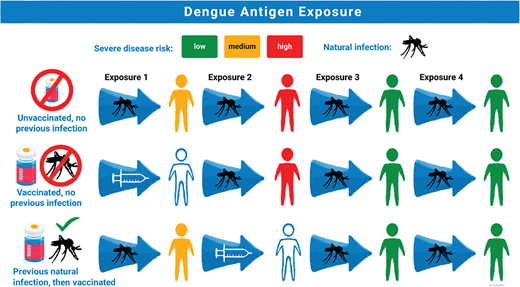
Proposed mechanism of Dengvaxia efficacy based on prior dengue antigen exposure. Risk of severe disease is represented by color (low = green, medium = yellow, and high = red). Exposure to dengue antigens is represented by mosquito figure for wild-type exposure and by a syringe for Dengvaxia exposure. The first row shows an unvaccinated individual exposed to 4 different dengue serotypes in their life with highest risk for severe disease with second infection and low risk of severe disease in the third and fourth infection. The second row shows an individual without previous dengue exposure who receives Dengvaxia, which acts as a silent primary infection, and then has higher risk for severe disease upon their first exposure to wildtype dengue, the equivalent of the second exposure to dengue antigen. The third row shows an individual with previous wild-type infection who receives Dengvaxia which acts as a silent second dengue exposure with lower risk for severe disease in subsequent exposures to wild-type dengue.
For children aged 9 to 16 years with evidence of previous dengue infection, Dengvaxia has an efficacy of about 80% against the outcomes of symptomatic virologically confirmed dengue (VCD) followed over 25 months as well as hospitalization for dengue and severe dengue as defined by criteria set by the trial’s independent data monitoring committee and followed over 60 months ( Table 3 ). 105 , 106 The efficacy by serotype mirrored its induction of a homotypic immune response 101 with highest protection against DENV-4 (89%), followed by DENV-3 (80%), and lowest against DENV-1 (67%) and DENV-2 (67%) ( Table 3 ). 106 Protection against mortality could not be reported because there were no dengue-related deaths in the phase 3 trials.
Dengvaxia Efficacy by Outcome and by Serotype in Persons 9–16 Years Old with Evidence of Previous Dengue Virus Infection
Pooled vaccine efficacy data are from CYD14 and CYD15 (clinical trial registration: NCT01373281, NCT01374516). CI, confidence interval; VE, vaccine efficacy. Data are presented as perentages.
Follow-up over 25 mo.
Follow-up over 60 mo.
The most frequently reported side effects (regardless of the dengue serostatus before vaccination) were headache (40%), injection site pain (32%), malaise (25%), asthenia (25%), and myalgia (29%) ( n = 1333). 108 Serious adverse events (ie, life-threatening events, hospitalization, disability or permanent damage, and death) within 28 days were rare in both vaccinated participants (0.6%) and control participants (0.8%) and were not significantly different. At 6 months, fewer severe adverse events were reported in the vaccine (2.8%) than in the control arm (3.2%). 108
Children who were seronegative for dengue at the time of vaccination had increased risk of severe illness on subsequent dengue infections. Risk of dengue-related hospitalization was approximately 1.5 times higher, and risk of severe dengue was approximately 2.5 times higher among seronegative children aged 9 to 16 years who were vaccinated than control participants over a 5-year period. 106
The requirement for a laboratory test before administration creates a unique challenge for Dengvaxia implementation. In areas with ongoing transmission of flaviviruses other than dengue, qualifying laboratory tests include a positive NAAT or NS1 test performed during an episode of acute dengue or a positive result on prevaccination screening tests for serologic evidence of previous infection that meet specific performance characteristics. In areas without other ongoing flavivirus transmission, a positive dengue IgM assay during an episode of acute dengue is also considered a qualifying laboratory test. 11
Prevaccination screening is critical because many DENV infections are asymptomatic or do not result in medical visits and testing. Thus, a significant proportion of previously infected individuals who could benefit from the vaccine will not be aware of or have laboratory documentation of their previous dengue infection. 110 – 113 One of the most challenging aspects in selecting a prevaccination test is defining benchmarks for test performance, as explored by several international working groups. 114 , 115 To reduce the risk of vaccinating someone without previous DENV infection, test specificity is a priority. Although test specificity and sensitivity are independent of seroprevalence, positive predictive value (PPV) and negative predictive value are dependent on seroprevalence and describe the likelihood of a true positive if a patient tests positive or the likelihood of a true negative if a patient tests negative ( Table 4 ). In areas with moderate or low seroprevalence (eg, 30%–50%), high test specificity (>98%) is required to achieve a PPV of 90% and therefore reduce the risk of misclassifying seronegative individuals. In these settings, near-perfect specificity at the expense of sensitivity is preferred to minimize the risk of vaccinating a misclassified negative individual and subsequently increasing their risk of severe dengue. However, high-prevalence areas (eg, >60%) would benefit from a higher test sensitivity and more moderate specificity (eg, 95%), which would increase identification of children who would benefit from the vaccine. 116
Test Performance for a Dengue Prevaccination Screening Test in Different Seroprevalence Scenarios 11
NPV, negative predictive value; PPV, positive predictive value.
CDC recommends that prevaccination screening tests that determine previous dengue infection have a minimum sensitivity of 75% and a minimum specificity of 98%. The recommendations also specify that the tests should be used in populations where they will achieve a positive predictive value (PPV) of ≥90% and a negative predictive value (NPV) of ≥75%. These rows demonstrate that tests with the same CDC recommended minimum sensitivity and specificity will have different PPV and NPV depending on the seroprevalence of the population in which they are used.
Because dengue seroprevalence at age 9 to 16 years is estimated to be approximately 50% in Puerto Rico 117 , 118 (where most of the eligible population for Dengvaxia in the United States and its territories and freely associated states reside), the CDC recommends that tests have a minimum sensitivity of 75% and a minimum specificity of 98%. The recommendations also specify that the test performance in the population should achieve a PPV of ≥90% and a negative predictive value of ≥75%. 11 These test characteristics were used to model the risks and benefits of implementing Dengvaxia. Using Puerto Rico’s population and an estimated seroprevalence of 50%, the model found that Dengvaxia vaccination would avert approximately 4148 symptomatic disease cases and 2956 hospitalizations over a 10-year period. This implementation would also result in an additional 51 hospitalizations caused by vaccination of people without previous dengue infection who were misclassified by the screening test. 119 The most common cause of hospitalization among vaccinated children will be breakthrough disease because the vaccine is not 100% efficacious.
TAK-003, developed by Takeda, consists of 2 doses given 3 months apart. The clinical trial population was primarily composed of children aged 4 to 16 years. At 18 months after vaccination, vaccine efficacy was found to be 80.2% against VCD, which waned to 62.0% by 3 years after vaccination. 120 , 121 Efficacy against hospitalization for dengue remained higher, at 83.6% at 3 years after vaccination. Differences in efficacy were observed by history of previous dengue infection, with higher efficacy among persons with previous infection compared with those without previous infection (65.0%–54.3%), and by age, with higher efficacy in older children. In contrast to findings from Dengvaxia at 25 months, children who were seronegative at the time of TAK-003 vaccination did not show an overall increased risk for hospitalization and severe disease compared with the placebo group at 3 years, although efficacy varied by DENV serotype and an age effect could not be ruled out ( Table 5 ). 106 , 120 Efficacy against both VCD and hospitalization varied by serotype and corresponded to the homotypic antibody titers, 102 with highest efficacy against DENV-2 and lowest against DENV-3 and DENV-4. Among children without previous DENV infection, there was no observed efficacy for VCD against DENV-3 or DENV-4. In the safety analysis, the number of serious adverse events was similar between vaccine (2.9%) and placebo (3.5%) groups.
TAK-003 Efficacy by Serostatus, Outcome, Serotype, and Age Group in Persons Aged 4–16 Years Over 36 Months of Follow-Up 120
Vaccine efficacy data are from clinical trial NCT02747927. CI, confidence interval; VE, vaccine efficacy. Data presented as percentage.
In March 2021, Takeda submitted TAK-003 to the European Medicines Agency for prevention of dengue from any DENV serotype among people aged 4 to 60 years. 122 The company will also be submitting filings to regulatory agencies in Argentina, Brazil, Colombia, Indonesia, Malaysia, Mexico, Singapore, Sri Lanka, and Thailand during 2021 and has future plans to submit to the FDA.
TV003 was developed by the National Institutes of Health and was formulated by selecting serotype-specific components that were determined to provide the most balanced safety and immunogenicity profile based on an evaluation of multiple monovalent and tetravalent candidates. 123 , 124 Because antibody titers failed to predict the efficacy of Dengvaxia, a human infection model was developed to assess the protective immunity induced by TV003 against DENV-2 challenge. Forty-eight volunteers were enrolled and randomized to receive TV003 (24) or placebo (24). Six months later, volunteers were administered a naturally attenuated DENV-2 challenge virus. 125 The primary efficacy endpoint was protection against detectable viremia after challenge. After challenge, DENV-2 was recovered by culture or reverse transcription-polymerase chain reaction (RT-PCR) from 100% of placebo recipients ( n = 20) and 0% of TV003 recipient ( n = 21) ( P < .0001). Postchallenge, rash was observed in 80% of placebo recipients compared with 0% of TV003 recipients ( P < .0001).
TV003 has been licensed to several manufacturers globally, including Merck & Co in the United States and the Instituto Butantan in Brazil. Phase 3 trials in Brazil are underway with efficacy and safety results expected in late 2022 (Clinical trial registration: NCT02406729).
Dengue is the most common arboviral disease worldwide and is projected to increase in range and global burden of disease. Although advancements in the field have progressed incrementally for decades, the recent approval of Dengvaxia for routine use marks a major step forward for control and prevention efforts in the United States and paves the way for future dengue vaccines.
Dengvaxia has several complexities that necessitate future research, including the possibility of fewer doses in the initial schedule followed by booster doses in later years. 30 Because it is the first vaccine to require laboratory testing before administration, public–private partnerships to develop more specific, sensitive, and accessible tests or testing algorithms will be key to minimize vaccination of persons without previous DENV infection and maximize benefit to those with previous infection. Jurisdictions that wish to use Dengvaxia will need to gather seroprevalence data and ensure that prevaccination screening tests meet the requirements for positive and negative predictive values. Furthermore, behavioral science assessments to elicit community-level perceptions and concerns combined with health systems research on optimal “test-and-vaccinate” strategies will result in dengue vaccination programs that are well accepted, efficient, and tailored to individual communities.
TAK-003 and TV003 are in late-stage trials and could soon be approaching licensure. An indication for use in travelers would offer clinicians in nonendemic areas of the United States a prophylactic therapeutic option for their patients. While awaiting the approval of a vaccine with balanced serotype immunity, a mix-and-match strategy guided by differences in serotype-dominant immune responses in each vaccine (TAK-003 followed by Dengvaxia, for example) could potentially lead to higher levels of protection against dengue, but it has yet to be evaluated for safety and efficacy in clinical trials. 126 For all 3 vaccines, studies evaluating efficacy against emerging DENV serotype variants will be important to assess long-term protection induced by the vaccine strains. 10 , 127
Future vaccines against dengue could also benefit from the lessons learned from the COVID-19 pandemic, namely that new vaccine platform technologies plus political will can result in rapid development of safe and effective vaccines and that clear communication with the public is crucial to successful vaccine implementation. 128 – 130 Dengue vaccines based on an mRNA platform are already under investigation. 131
Vaccines are a powerful new tool in our arsenal against dengue, but they are only 1 of many interventions, including novel vector control strategies, to control a virus with a complex epidemiology, immunopathogenesis, and clinical picture influenced by climate change, urbanization, poverty, and human migration. Clinicians should remain vigilant in recognizing and diagnosing patients with dengue, because early treatment remains the cornerstone for reducing morbidity and mortality. However, with the recent approval of Dengvaxia, we are 1 step closer on the path to dengue elimination and can expect exciting new developments in dengue interventions in the near future.
We thank Ms Alexia E. Rodriguez, MPH, for her review of the manuscript.
Drs Wong, Adams, and Paz-Bailey conceptualized and designed the structure of the review, drafted portions of the initial manuscript, and reviewed and revised the manuscript; Drs Durbin, Muñoz-Jordán, Sánchez-González, and Volkman drafted portions of the initial manuscript and reviewed and revised the manuscript; Dr Poehling reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: No external funding.
CONFLICT OF INTEREST DISCLOSURES: Dr Durbin is a scientific advisor to Merck & Co on dengue vaccine development. The other authors have no conflicts of interest to disclose.
Advisory Committee on Immunization Practices
antibody dependent enhancement
Centers for Disease Control and Prevention
dengue virus
Food and Drug Administration
immunoglobulin M
nucleic acid amplification test
nonstructural protein 1
positive predictive value
plaque reduction neutralization test
virologically confirmed dengue
World Health Organization

Supplementary data
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 09 December 2015
Tackling dengue fever: Current status and challenges
- Taoufik Nedjadi 1 ,
- Sherif El-Kafrawy 2 ,
- Sayed S. Sohrab 2 ,
- Philippe Desprès 3 ,
- Ghazi Damanhouri 1 &
- Esam Azhar 2
Virology Journal volume 12 , Article number: 212 ( 2015 ) Cite this article
19k Accesses
28 Citations
4 Altmetric
Metrics details
According to recent statistics, 96 million apparent dengue infections were estimated worldwide in 2010. This figure is by far greater than the WHO prediction which indicates the rapid spread of this disease posing a growing threat to the economy and a major challenge to clinicians and health care services across the globe particularly in the affected areas.
This article aims at bringing to light the current epidemiological and clinical status of the dengue fever. The relationship between genetic mutations, single nucleotide polymorphism (SNP) and the pathophysiology of disease progression will be put into perspective. It will also highlight the recent advances in dengue vaccine development.
Thus far, a significant progress has been made in unraveling the risk factors and understanding the molecular pathogenesis associated with the disease. However, further insights in molecular features of the disease and the development of animal models will enormously help improving the therapeutic interventions and potentially contribute to finding new preventive measures for population at risk.
Dengue fever is a major cause of illness and death worldwide. The disease is caused by dengue virus which gets transmitted to humans by the bites of infected mosquitoes, Aedes (Ae.) aegypti and Ae. albopictus [ 1 ]. The disease represents a global health issue as it is endemic in around 100 countries, most of which are in tropical and sub-tropical areas. Over the last decades, the incidence rate and the geographic distribution of dengue have rapidly increased (almost 30-fold). Data from the World Health Organization (WHO) estimates up to 100 million cases of dengue fever each year [ 2 ]. However, a recent published work by Bhatt et al . (2013) suggested that the burden of dengue is far more than the WHO estimation and indicated that 390 million infections of dengue virus could have happened every year [ 3 ]. Changes in dengue epidemiology and the increase in incidence rates (with and without co-morbidities) have led the WHO to propose a new dengue classification system according to disease severity (Fig. 1 ) [ 2 ].
WHO dengue case classification ( Adopted from; Dengue Guidelines for diagnosis, treatment, prevention and control, New edn. Geneva: WHO; 2009 )
Etiology and mode of transmission
Dengue fever is caused by infection with dengue virus (DENV). The DENV is a vector-borne virus transmitted to humans primarily by bites from two mosquito species, Ae. aegypti or Ae. albopictus . DENV is a single positive-stranded RNA virus belonging to Flavivirus genus of the Flaviviridae family and has 4 major serotypes (DENV 1–4) that are antigenically distinct from each other. Each DENV serotype is phylogenetically distinct suggesting that each serotype could be considered a separate virus [ 4 ]. Three dengue serotypes out of four (DENV 1–3) have been found in Middle Eastern countries including Saudi Arabia and Yemen. Interestingly, DENV-1 strain isolated in Saudi Arabia exhibited a high genetic similarity with DENV-1 strain isolated from Asian population, suggesting a widespread of the Asian genotype, probably through Asian pilgrims [ 5 , 6 ]. A recently published article has unveiled a new serotype (DENV-5), to be added to the existing ones [ 7 ]. This discovery is still controversial and little-known enough to conclude how the 5 th dengue serotype might add to the burden associated with dengue infection.
Mosquitoes transmit the virus by feeding on blood of infected persons. At first, the virus infects and replicates in the mid-gut epithelium of the mosquito and then spreads to other organs until it reaches the salivary glands after 10–14 days where it can be inoculated to another person during subsequent blood meal. Vertical transmission of DENV in mosquitoes, i.e. from mosquito to larvae has been reported by a number of research groups. In India, Angel & Joshi (2008) reported the detection of dengue virus by indirect fluorescence antibody test (IFAT) in laboratory reared mosquitoes originating from larvae collected from urban and rural areas [ 8 ]. A similar study was conducted in Brazil by Martins et al. (2012) and confirmed the isolation of DENV-type 3 in Ae. albopictus larvae and DENV-type 2 in Ae. aegypti larvae [ 9 ]. Similar findings were also reported in Mexico [ 10 ] and Indonesia [ 11 ]. On the other hand, mother-to-infant transmission of dengue virus via cord blood or breast milk remains controversial [ 12 – 14 ].
Clinical manifestations
Based on the results from several studies, the WHO has launched a new dengue classification. This classification divides dengue cases into a) cases with/without warning signs and b) severe dengue cases [ 2 ]. However, it is important to note that numerous research groups have debated the rational of this classification as it does not fit their unique local settings. The criteria for dengue case classification are presented in Fig. 1 .
Clinically, dengue infection has a broad spectrum of features. The vast majority of cases are asymptomatic and passes unnoticed. Typically, the symptoms start to be prominent after an incubation period of 3–10 days [ 15 ]. The severity of the clinical manifestations varies from mild symptoms to severe life threatening symptoms in the case of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [ 16 ]. Predicting the progression of the mild signs to a severe DHF/DSS remains a challenge due to non-specificity of clinical presentation and the incomplete understanding of pathophysiology of the disease and its underlying molecular mechanisms.
Dengue with warning signs
The early signs of the disease are non-specific. According to the WHO classification (2009), DF is characterized by febrile episode (≥40 °C for 2–7 days) frequently associated with rash, nausea, vomiting, and headache. Although the disease affects people of all ages from infancy through to adulthood [ 17 ], epidemiological data showed that children tend to tolerate this phase of illness better than adults [ 18 ]. The persistence of the aforementioned symptoms and appearance of other symptoms, such as abdominal pain, mucosal bleed, and lethargy and restlessness can be seen 3–7 days later. Laboratory analysis of mild dengue fever cases usually shows abnormal leukocyte counts and moderate elevation of the hepatic amino-transferase enzyme activity [ 19 ]. The emergence of these symptoms is a warning sign for disease progression to severe form (DHF/DSS) if therapeutic intervention is not undertaken. At this stage clinical intervention and continuous surveillance are imperative to prevent vascular leakage, especially in an endemic area.
Severe dengue
This form of dengue infection can be attributed to any of the four known serotypes DENV 1–4. The likelihood of developing DHF/DSS is high in patients who have experienced dengue infection in the past with heterogeneous serotype [ 20 ]. About 5–10 % of patients progress to develop a severe DHF/DSS which can be fatal unless treated promptly [ 21 ]. This form develops at a late stage of DF, where patients may go through defervescence phase characterized by a sudden drop of body’s temperature. This phase is also distinguished by severe bleeding, particularly bleeding from the gastrointestinal tract (black, tarry stool), and thrombocytopenia (<50,000/mm3), which may affect up to 50 % of DHF cases [ 22 ]. Interestingly, there was an observed negative correlation between the severity of DHF and the level of platelets in the blood. The exact mechanism of this correlation has yet to be delineated. The drop of platelet counts and the loss of their functionality lead to a vascular fragility increasing the risk of hemorrhage and plasma leakage [ 23 ]. It has been suggested that during acute phase of the infection DENV replicates quickly in platelets, as this is very critical for virus survival and dissemination [ 24 , 25 ].
The existence of other symptoms such as retro-orbital pain, maculopapular rash, petechiae, or bleeding from the nose or gums will help making definitive diagnosis for DF [ 25 ]. Evidence of plasma leakage in various body cavities such as the pleural cavity and the peritoneal cavity, associated with profuse perspiration, adynamia, and sometimes fainting are signs of rapid progression to shock. Subsidence in systolic pressure and hypotension may result in profound shock, known as dengue shock syndrome (DSS). The duration of DSS for a long time might predispose to further complications such as massive bleeding, disseminated intravascular coagulopathy (DIC), respiratory failure, multi-organ failure, and infrequently encephalopathy leading to death [ 26 , 27 ]. It has been proposed that case fatality related to DHF may reach 15 % of all cases, however, proper medical care and symptomatic management can reduce mortality rate to less than 1 % [ 28 ].
An early and accurate laboratory diagnosis of dengue infection is of paramount importance in the management of the disease. It has been estimated that the number of misdiagnosed dengue cases could reach a record ratio of 50 % of all cases, mainly due to a large disparity of dengue signs and symptoms which overlap with the symptoms of other viral infections, especially for persons living in or traveling to endemic areas of tropical infectious diseases. Dengue fever should be distinguished from other illnesses which share similar symptoms such as chikungunya, Mayaro fever, Ross River fever, West Nile fever, Zika fever, yellow fever and viral hemorrhagic fevers [ 4 ]. Until the antiviral vaccine becomes available, the prevention of severe cases and cut-down of the economic burden of the disease rely enormously on early and accurate diagnosis. The latter is made possible through the availability of several diagnostic laboratory and virological tests.
The onset of later stage symptoms of the illness can be overwhelming and more pathognomonic. Nonetheless, based on WHO classification schemes, the appearance of leukopenia in patients with febrile illness is a major consideration in making diagnosis of dengue infection [ 29 ]. Overall, there is an urgent need to reduce dengue morbidity and mortality by improving the diagnosis and molecular analysis of emerging dengue virus. Thus far, two diagnostic modalities have been applied to detect the disease at an early stage. The first one is a direct method targeting the acute phase of dengue disease, which is based upon detection of genomic RNA by RT-qPCR or soluble NS1 by antigen capture in blood samples from viremic patients. The second is the indirect method that relies on serological tests to detect dengue-related immunoglobulins par Mac-ELISA for the capture of specific IgM or indirect ELISA for the capture of anti-DEN IgGs [ 30 – 34 ].
Genetic alteration/susceptibility to dengue infection
Several risk factors have been associated with dengue infection and its progression to severe DHF/DSS forms. Recent advances in molecular biology have revealed that the genetic makeup of the three elements of dengue infection (the virus, the vector, and the host) plays a primordial role in the pathogenesis of the disease and could potentially contribute to the DHF progression [ 19 , 24 , 35 ]. Hence, an in-depth analysis of genetic variability including polymorphism and mutations could be beneficial in identifying the possible factors and mechanisms of disease development [ 36 ]. The list of host’s genetic factors that confer susceptibility or resistance to dengue infection is summarized in Table 1 .
The mosquito
Like most arboviruses, DENV infect different organs of the mosquito, including the salivary glands and the central nervous system. Mosquito infection elicit behavioral changes including increase of the probing time which lead to host interruption that might lead to wider spread of the virus [ 37 ]. It has been demonstrated that DENV infection induced the expression of cathepsin-B, a putative cystatin, and a hypothetical ankyrin repeat-containing protein genes [ 38 ]. The latter could alter the efficiency of virus replication in the salivary gland. This study has shown that modulation of OBP10 and OBP22 genes expression as well as DENV infection-responsive odorant-binding protein genes increase the time length for initiation of probing before a successful blood meal, resulting in changes in the host seeking behavior of the mosquito. Comparative analysis of the salivary gland transcriptomes of native and DENV-infected Ae. aegypti identified a number of differentially expressed genes related to sugar/protein digestion enzymes, immunity related genes and blood meal acquisition enzymes that might have an impact on the efficiency of viral replication or mosquito feeding behavior. This study showed that DENV infection alter the expression of key host-seeking genes in the mosquito’s main olfactory organs and the antennae [ 38 ].
Recent updates have indicated that resistance of Ae. aegypti to conventional insecticides is related to different mechanisms, one of which is associated with genetic abnormalities within the vector’s genome. Single point mutation in the voltage-gated sodium channel gene at position 1534 ( F1534C ) resulting in phenylalanine to cysteine substitution in Ae. aegypti confers resistance to permethrin. This mutation is widespread in this vector in Southeast Asia and Latin America [ 39 , 40 ]. It has also been reported that a single amino acid substitution Valine to Glycine at position 1016 in domain II, segment 6 of the voltage-gated sodium channel gene was associated with less sensibility of Ae. aegypti to deltamethrin in Thailand [ 41 ].
Human susceptibility to dengue disease
Numerous multi-disciplinary studies confirmed that race, young age, virus strain, female sex and high body-mass index correlate well with increased burden of dengue infection. The observation that people of African background are less likely to develop DHF/DSS compared to their Caucasian counterpart has led to the suggestion that host genetic variability has a major impact on the clinical manifestations of dengue infection [ 42 , 43 ]. Thus, a closer consideration of human genes regulating the severity of dengue infection, especially genes associated with the immune response, might help in controlling disease spread and improve the acute symptoms of the infection. A number of studies have investigated the relationship between the host genetic polymorphisms and DENV infection (Table 1 ).
A single nucleotide polymorphism (SNP) in the promoter of CD209/DC-SIGN was associated to increased risk of developing dengue fever [ 44 ]. Association studies have successfully identified a link between polymorphisms in the human major-histocompatibility-complex (HLA) class I/II genes and non-HLA host genetic factors and severity of dengue disease [ 45 – 47 ]. Polymorphisms of the TAP1 and TAP2 genes could be directly associated with the risk of developing dengue disease among the primary-infected individuals [ 48 ]. Both TAP1 and TAP2 are located within the MHC class II region and homozygosity of the TAP1 at position 1333 and 1637 and for TAP2 at position 2379, respectively, was found to protect against developing severe forms of dengue [ 46 ].
In an independent study [ 49 ], the authors showed that single nucleotide polymorphism of the oligoadenylate synthetase genes ( OAS1, 2 and 3 ), of the OAS/RNase L antiviral immune system, enhance susceptibility to clinical outcomes of dengue infection. An association between the severity of the disease and other genes including human leukocyte antigen class I and class II genes, tumor necrosis factor-alpha, FcGRIIA , vitamin D receptor, transporters associated with antigen presentation, and JAK1 has also been proposed [ 50 ]. The importance of Vit-D in DENV pathogenesis was concluded from newly-gathered data showing that Vit-D impairs DENV replication and polymorphism of Vit-D gene increases the expression of both CD209/DC-SIGN and FcGRIIA receptors that enhance DENV entry in the target cells [ 51 , 52 ].
In another study [ 53 ], the authors have successfully applied genome-wide association study (GWAS) approach to identify loci that confer susceptibility to severe forms of dengue disease. The investigators used samples from 2008 children affected with severe dengue infection against 2018 population control cases in Vietnam. The data showed that SNPs at two loci, MICB and PLCE1 , significantly increased the likelihood of developing DSS in children. This finding was further validated in an independent cohort of 1737 cases and 2934 controls [ 53 ]. A SNP in the MICB gene coding for the MHC class I polypeptide-related sequence B, an inducible activating ligand for the NKG2D type II receptor of immune cells could alter the protective role of natural killer and CD8 + T cells in the host responsiveness to DENV at the early stage of infection [ 54 , 55 ]. On the other hand, PLCE1 plays a primordial role in maintaining intact vascular endothelial cell barrier function, hence, polymorphism of the PLCE1 gene may lead to blood vessels leakage and circulatory hypovolemia during DSS [ 56 ].
Other host candidate genes have also been associated with early onset dengue disease. Among these genes, there were receptors/attachment factors for DENV linked to immune system and inflammatory response. The chemokines CXCL10, CXCL11 and its respective chemokine receptor CXCR3 were reported as biomarkers for severe form of dengue infection [ 57 ]. These results are in agreement with recent emerging data indicating strong association between CXCL10, CXCL11 and CXCR3 and vascular permeability [ 58 ]. The three genes are components of the NF-kB pathway and are involved in the pathogenesis of SARS and West Nile virus encephalitis [ 59 , 60 ]. Cerney et al . (2014) interrogated the effect of DENV on the first point of human contact which is skin cells. The authors demonstrated an increase expression of IFN-β, STAT-1 and CCL5 in a susceptible population of skin dendritic cells (DC) which may facilitate the spread of DENV in the blood [ 61 ]. This process depends enormously on vector-derived salivary factors inoculated on the skin cells [ 62 ].
Current status of dengue vaccine development
Till-date, there is no effective, commercially available, therapy/vaccine for dengue virus. Numerous groups have already made intensive efforts and made good progress to develop a safe, affordable and effective vaccine against all serotypes for global public health [ 63 – 69 ]. Vaccines which are being developed use various approaches such as live attenuated viruses, inactivated viruses, subunit vaccines, DNA vaccines, and chimeric viruses using yellow fever vaccine and attenuated dengue viruses as backbones (Table 2 ).
Live attenuated yellow fever 17D/DENV chimeric vaccine
Currently, only one tetravalent vaccine against dengue virus, developed by Sanofi-Pasteur (France) has reached phase III clinical trial and is expected to be launched in 2015. This vaccine is based on the production of four chimeric live dengue-yellow fever viruses in which the yellow fever (YF) 17D vaccine sequences encoding the envelope proteins prM and E genes were substituted by the prM and E genes from DV of serotype 1, 2, 3, or 4 in a molecular clone of YF-17D [ 69 ]. This vaccine was produced and tested over 6000 people using four dengue virus isolates from Indonesia and Thailand. This candidate vaccine was found to be attenuated and stable in animal models with respect to plaque size and yellow fever virus neurotropism [ 70 ]. Results of the clinical trials showed no adverse effects except moderate injection site pain, headache, and myalgia. Another randomized, controlled trial was launched using a total of 4002 Thai school children to investigate the efficacy of a recombinant, tetravalent vaccine for dengue virus and only 134 dengue cases were reported [ 71 ]. Phase I trial of the vaccine in the Philippines showed that the seropositivity increased gradually (53, 72 & 92 %) after 1–3 vaccinations against all four serotypes as compared to control group. The most promising results were observed in children 2–5 years old who exhibited high levels of reactivity of 91, 100, 96, 100 % for DENV 1–4; respectively [ 72 ].
Another placebo-controlled trial was conducted on 10,275 children from Vietnam (vaccine, n = 6851 Vs placebo, n = 3424) to determine the clinical efficacy and safety of CYD-TDV. The results demonstrated virologically-confirmed cases in 47 % of the vaccine group as compared to the control group (53 %). The efficacy was achieved in up to 56.5 % (95 % CI 43.8–66.4). These findings indicated that the vaccine is highly efficacious with good safety profile when three injections were given to children with age group 2–14 years at 0, 6 and 12 months intervals [ 73 ]. The data emerging from another randomized phase II trial in India indicated that the vaccine has no serious adverse events and the immunogenicity and safety of CYD-TDV were satisfactory [ 74 ]. A pilot study carried out in five Latin American countries where more than 20,000 children aged 9–16 were recruited to receive either the CYD-TDV vaccine or placebo. The results on efficacy (60.8 %) and safety profiles were consistent with the previous findings [ 74 , 75 ]. Interestingly, the vaccine efficacy (80.3 %) against hospitalization for dengue was promising and represented a step forward to developing an effective dengue vaccine [ 75 ].
Live attenuated DENV delta-30 mutation and intertypic DENV chimeric vaccines
Other candidate dengue vaccines have been developed in USA by the Johns Hopkins University and National Institute of Allergy and Infectious Diseases (NIAID) and have reached advanced clinical trials [ 65 ]. Four live-attenuated DENV/delta-30 were generated each containing 30 nucleotides deletion of the 3’-untranslated region of genomic RNA (delta-30). These vaccines efficiently impaired viral growth in human liver carcinoma cells [ 76 ]. To improve the attenuation of DENV-2/delta-30 and DENV-3/delta-30, chimeric DENV were developed by substitution of the prM-E gene region of DENV-4/delta-30 virus with the prM-E genes of DENV-2 and DENV-3 [ 72 , 77 ]. The results from phase I clinical trial showed that all four live-attenuated DENV/delta-30 are safe and immunogenic with minor side effects such as faint rash and transient leucopenia only after higher dose [ 78 , 79 ].
Dengue-measles vaccine
Dengue virus serotype-1 antigen was expressed in a vector based on pediatric live-attenuated Schwarz measles vaccine (MV) by using the envelope domain III (EDIII) fused with the ectodomain of the membrane protein (ectoM). After immunization, long-term production of DENV-1 serotype-specific neutralizing antibodies was observed in measles virus susceptible mice [ 80 ]. A new strategy was evaluated based on single minimal tetravalent DENV antigen expression using viral vector derived from pediatric live-attenuated measles vaccine (MV). A recombinant MV vaccine construct was developed using envelope domain III (EDIII) and ectodomain of the membrane protein. The neutralizing antibodies were induced against all four serotypes of dengue virus after two injections in mice susceptible to MV infection. A strong memory neutralizing response was observed against all four serotypes in immunized mice after inoculation with live DENV from each serotype [ 81 ].
Dengue prM-E DNA vaccine
A naked DNA-based candidate vaccine against DENV has been developed by the Naval Medical Research Center [ 67 , 82 , 83 ]. The genes encoding prM and E of DENV were cloned into a shuttle vector under the transcriptional control of human cytomegalovirus (CMV) promoter. The results of phase I clinical trial showed no adverse effects except mild injection site pain, swelling, and fatigue. After second dose, strong IgM and IgG antibody response was observed which favors the safety profile of this vaccine. To get a better immunogenicity profile, a vaccine based on lipid adjuvant Vaxfectin (Vical Incorporated, San Diego, USA), was developed and the results demonstrated good protection profile against DENV compared to DNA alone [ 84 ]. Based on this technology, different groups have developed other candidate vaccines and achieved good protection in mouse models using envelope glycoproteins prM and E, the non-structural protein NS1 and the helicase/protease NS3 as vaccine antigens [ 85 – 87 ].
Purified inactivated vaccine (PIV)
The first purified inactivated vaccine was developed with aluminum hydroxide (alum) adjuvant and tested in mice and rhesus macaques in the mid-1990s, by Walter Reed Army Institute of Research against dengue 2 serotype and good virus protection was reported after two doses [ 88 , 89 ]. Using similar technology, second generation Japanese encephalitis (JE) PIV vaccine was developed [ 90 , 91 ]. Currently, a new JE vaccine (Ixiaro; Novartis Vaccines) has been approved for use in many countries, including the USA [ 92 ]. Another dengue vaccine (dengue 1 PIV), recombinant subunit dengue E glycoprotein antigen (r80E) was also developed and has entered phase I clinical trial [ 93 – 95 ]. The Centers for Disease Control and Prevention (USA) have also developed a live-attenuated vaccine named DENVax, which was found to be highly immunogenic in both children and adults and has currently entered phase I clinical trial in the United States [ 96 , 97 ]. Recently, a novel third generation approach is being used to develop a vaccine containing recombinant subunit E domain III ( ED3 ) and the results of laboratory tests have shown the development of potent neutralizing antibodies in a mouse model [ 98 – 100 ]. Using the same technology, a tetravalent vaccine was developed and expressed in Pichia pastoris by splicing and using flexible pentaglycyl linkers of the four EDIII. The observed results showed that this antigen elicit specific antibodies against all four DENV serotypes in BALB/c mice [ 101 ].
Lessons from animal models
Animal models are very useful for vaccine test development. The lack of animal models significantly hampered the development and efficacy testing of dengue vaccine. Currently only rhesus macaques and Aotus monkeys are being used for testing the vaccine before clinical trials are initiated [ 62 ]. The D1ME100 vaccine was evaluated in both Aotus monkeys and rhesus monkeys, and found to be immunogenic with 80–95 % protection against dengue infection [ 102 , 103 ]. Porter et al. (2012) demonstrated that injection of non-human primate with three doses on day 1, 28 and 84, with tetravalent dengue DNA vaccine Vaxfectin-adjuvanted, was more efficient against live dengue-2 virus compared to control animals. This finding support initiation of Vaxfectin-adjuvanted phase I clinical trial [ 84 ].
Successful induction of immune response was obtained in mice and rhesus monkeys to the vaccines developed using dengue 4 prM-E, dengue 1 prM-E-nonstructural (NS)1 , and dengue 2 NS3 antigens, and PIV adjuvanted with alum [ 85 , 86 ]. Centers for Disease Control and Prevention (Fort Collins, CO), Hawaii biotech, and Simmons developed different vaccines that showed good immunogenicity in animal models [ 104 ]. Similarly, the psoralen/UV inactivation dengue vaccine was found to be more immunogenic and protective against dengue serotype 1 virus in Aotus monkeys [ 105 ].
Antiviral therapy
Thus far, there are no antiviral drugs available to treat dengue fever; therefore the community will continue to depend on the control of the mosquito vector as the main route to prevent the spread of disease. Alternative approaches have been utilized against flaviviruses by targeting and inhibiting virus entry and the essential elements used in virus replication, nonstructural proteins, RNA polymerase, and proteases. The most important target elements include NS3 helicase nucleoside triphosphatase (NTPase/RNA 5’ triphosphatase (RTPase), NS5 methyl transferase/RNA-dependent RNA polymerase, and NS3/NS2B protease [ 106 – 108 ].
RNA interference (RNAi) technology is also being used to impair virus replication against respiratory syncytial virus, hepatitis viruses, influenza virus, poliovirus and HIV [ 109 , 110 ]. Low molecular weight phenolic compounds such as flavonoids and phytochemicals isolated from plants were previously tested and are being used for anti-dengue therapy [ 111 , 112 ]. An anti-viral inhibitory effect ranging from 50–75 % against DENV replication was observed when methanolic extracts of Momordica charantia and Andrographis paniculata were used in cultured primate cells [ 113 ].
Several attempts have been made in the past to tackle dengue through elimination of Ae. Aegypti. The most successful experiences were related to vector control programs adopted in Cuba and Singapore. The programs were based on intensive insecticidal treatment and reduction of the availability of Aedes larval habitats [ 18 , 114 ]. Unfortunately, lack of sustainability of these stringent measures led to reappearance of dengue outbreaks.
Recently, a novel form of biological control of dengue transmission has been developed and is currently being applied. This is based on the development of genetically modified (GM) mosquitoes infected with a bacterium known as Wolbachia to combat dengue infection. This bacterium blocks replication of the virus inside the mosquito and prevents its transmission to humans [ 115 ]. In 2012, 10 million GM male mosquitoes were released in the wild to decrease the number of Aedes mosquitoes and reduce the rate of dengue transmission. A closer monitoring of the insects revealed that over 85 % of the eggs were Wolbachia-positive which indicated that GM-mosquitoes were overriding wild-mosquitoes resulting in decreased virus transmission [ 116 ]. In an initiative to eradicate dengue fever, scientists from Australia, are leading Eliminate Dengue (ED) program which involves community engagement as a key component in this program. Since the program kicked off in 2011, millions of Wolbachia mosquitoes were released across the North Queensland city—Australia. Based on the promising results obtained from local trial, Eliminate Dengue became an international research program across countries affected by dengue including Australia, Vietnam, Indonesia, Brazil and Colombia [ 117 , 118 ].
Targets of antiviral therapy
Dengue infection can be prevented by alternative approaches. The first one includes blocking virus entry into cells which is mediated by the viral envelope glycoprotein E via receptor-mediated endocytosis [ 119 ]. Dendritic cells, monocytes, and macrophages are the main targets of DENV infectious entry. The second approach involves blocking virus attachment to specific cellular receptors expressed on immune cells, liver cells, and endothelial cells.
Fusion and glycosidase inhibitors
Small molecules and peptides targeting the hydrophobic pocket of the envelope E glycoprotein are characterized as inhibitors of virus entry. Nicholson et al. (2011) explored the inhibitory effects of DN59 and 1OAN1, peptide entry inhibitors. The authors demonstrated that DN59 and 1OAN1 can effectively block antibody dependent enhancement (ADE) in-vitro suggesting that entry inhibitors are potential candidates to prevent development of DHF/DSS [ 120 ]. Two other compounds have also been shown to qualify as potent inhibitors of dengue virus infection are imino-sugars deoxynojirimycin and castanospermine [ 121 ]. These compounds are natural alkaloids derived from the black bean and act as inhibitors against all 4 dengue serotypes by disrupting the folding pathways of the envelope glycoproteins prM and E [ 122 ].
Carbohydrate-binding agents
Various types of carbohydrate-binding agents, isolated from different organisms, have been shown to have anti-viral activities. Three plant lectins, Hippeastrum hybrid agglutinin, Galanthus nivalis agglutinin and Urtica dioica agglutinin isolated from amaryllis, snowdrop and stinging nettle respectively were found to be potent inhibitors of DENV-2 infection by inhibiting viral replication [ 123 ].
Heparan mimetics
Heparan sulfate (HS) is a putative receptor for DENV which interacts with domain III of the E-protein. Virus entry can be blocked by targeting the E-protein-HS interaction with soluble GAGs and other highly charged HS [ 124 ]. Fucoidan was isolated from marine algae and showed antiviral activity against DENV-2 in BHK cells [ 125 ]. Similarly, carrageenan and DL galactan, sulfated polysaccharides from red seaweeds, exhibited strong antiviral activity against DENV-2 and DENV-3 but a very weak activity against DENV-4 and DENV-1. Furthermore, two α -D-glucans were isolated from a Chinese herb and demonstrated high anti-DENV-2 activities in BHK cells [ 112 , 126 ].
Conclusions
Dengue fever represents a real economic burden especially in affected countries. Extensive efforts are needed to tackle disease spread and reduce the mortality rates and the associated healthcare cost. There is a need for more scientific research which we believe is a key route to provide further insight in the pathogenesis of dengue infection and help understanding the underlying molecular mechanisms associated with progression to the severe forms of the disease (DHF/DSS). This will be a step forward to develop an adequate preventive vaccine and effective treatment.
Abbreviations
Dengue virus
Dengue fever
- Dengue hemorrhagic fever
Dengue shock syndrome
Dendritic cells
Tang KF, Ooi EE. Diagnosis of dengue: An update. Expert Rev Anti Infect Ther. 2012;10(8):895–907.
Article CAS PubMed Google Scholar
WHO: Dengue. Guidelines for diagnosis, treatment prevention and control, Geneva, World Health Organization, 2009, WHO/HTM/NTD/DEN/2009. ( http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf )
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7.
Article PubMed Central CAS PubMed Google Scholar
Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Postgrad Med J. 2004;80:588–601.
Madani TA, Abuelzein EL-TM, Al-Bar HM, Azhar EI, Kao M, Alshoeb HO, et al. Outbreak of viral hemorrhagic fever caused by dengue virus type 3 in Al-Mukalla, Yemen. BMC Infect Dis. 2013;14(13):136.
Article Google Scholar
Azhar EI, Hashem AM, El-Kafrawy SA, Abol-Ela S, Abd-Alla AM, Sohrab SS, et al. Complete genome sequencing and phylogenetic analysis of dengue type 1 virus isolated from Jeddah, Saudi Arabia. Virol J. 2015;12:1.
Article PubMed Central PubMed CAS Google Scholar
Normile D. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342(6157):415.
Angel B, Joshi V. Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan. India J Vector Borne Dis. 2008;45:56–9.
PubMed Google Scholar
Martins V, Alencar C, Kamimura M, de Carvalho Arau’jo F, De Simone S, Dutra R, et al. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceara’, Brazil. PLoS One. 2012;7(7):e41386.
Günther J, Martinez-Munoz JP, Pérez-Ishiwara DG, Salas-Benito J. Evidence of vertical transmission of dengue virus in two endemic localities in the State of Oaxaca, Mexico. Intervirology. 2007;50:347–52.
Article PubMed Google Scholar
Mulyatno KC, Yamanaka A, Yotopranoto S, Konishi E. Vertical transmission of dengue virus in Aedes aegypti collected in Surabaya, Indonesia, during 2008–2011. Jpn J Infect Dis. 2012;65:274–6.
Phongsamart W, Yoksan S, Vanaprapa N, Chokephaibulkit K. Dengue virus infection in late pregnancy and transmission to the infants. Pediatr Infect Dis J. 2008;27(6):500–4.
Kariyawasam S, Senanayake H. Dengue infections during pregnancy: Case series from a tertiary care hospital in Sri Lanka. J Infect Dev Ctries. 2010;4(11):767–75.
Barthel A, Gourinat A-C, Cazorla C, Joubert C, Dupont-Rouzeyrol M, Descloux E. Breast milk as a possible route of vertical transmission of dengue virus? Clin Infect Dis. 2013;57(3):415–7.
Chan M, Johansson AM. The incubation periods of dengue viruses. PLoS One. 2012;7(11):e50972.
Guha-Sapir D, Schimmer B. Dengue fever new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1–10.
Article PubMed Central PubMed Google Scholar
Khan NA, Azhar EI, El-Fiky S, Madani HH, Abuljadial MA, Ashshi AM, et al. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop. 2008;105(1):39–44.
Ooi EE, Goh KT, Gubler JD. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–93.
Simmons CP, Farrar JJ, Vinh CN, Wills B. Dengue. N Engl J Med. 2012;366:1423–32.
Low JG, Ooi EE, Tolfvenstam T, Leo YS, Hibberd ML, Ng LC, et al. Early dengue infection and outcome study (EDEN) - study design and preliminary findings. Ann Acad Med Singapore. 2006;35(11):783–9.
Wichmann O, Jelinek T. Dengue in travelers. A Review. J Travel Med. 2004;11:161–70.
Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, et al. Evaluation of diagnostic tests: Dengue. Nat Rev Microbiol. 2010;8(12 Suppl):S30–8.
Schexneider KI, Reedy EA. Thrombocytopenia in dengue fever. Curr Hematol Rep. 2005;4(2):145–8.
Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis. Integr View Clin Microbiol Rev. 2009;22(4):564–81.
Article CAS Google Scholar
Noisakran S, Kulkanya C, Pucharee S, Nattawat O, Hui-Mien H, Francois V, et al. A re-evaluation of the mechanisms leading to dengue hemorrhagic fever. Ann NY Acad Sci. 2009;1171:E24–35.
Marques N, Gan VC, Leo YS. Dengue myocarditis in Singapore: Two case reports. Infection. 2013;41(3):709–14.
Misra UK, Kalita J, Syam UK, Dhole TN. Neurological manifestations of dengue virus infection. J Neurol Sci. 2006;244(1–2):117–22.
Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):3–100.
Low GJ, Ong A, Tan LK, Shera C, Angelia C, Wen YL, et al. The early clinical features of dengue in adults: Challenges for early clinical diagnosis. PLoS Negl Trop Dis. 2011;5(5):e1191.
Alcon-LePoder S, Sivard P, Drouet MT, Talarmin A, Rice C, Flamand M. Secretion of flaviviral non-structural protein NS1: From diagnosis to pathogenesis. Novartis Found Symp. 2006;277:233–47.
Zanluca C, Mazzarotto GA, Bordignon J, Duarte Dos Santos CN. Development, characterization and application of monoclonal antibodies against Brazilian dengue virus isolates. PLoS One. 2014;9(11):e110620.
Dos Santos HW, Poloni TR, Souza KP, Muller VD, Tremeschin F, Nali LC, et al. A simple one-step real-time RT-PCR for diagnosis of dengue virus infection. J Med Virol. 2008;80(8):1426–33.
Article PubMed CAS Google Scholar
Shu PY, Huang JH. Current advances in dengue diagnosis. Clin Vaccine Immunol. 2004;11(4):642–50.
Cordeiro MT, Braga-Neto U, Nogueira RR, Marques TE. Reliable classifier to differentiate primary and secondary acute dengue infection based on IgG ELISA. PloS One. 2009;4(4):e4945.
Vannberg FO, Chapman SJ, Hill AV. Human genetic susceptibility to intracellular pathogens. Immunol Rev. 2011;240(1):105–16.
Lan TN, Hirayama K. Host genetic susceptibility to severe dengue infection. Trop Med Health. 2011;39(4):73–81.
Platt KB, Linthicum KJ, Myint KS, Innis BL, Lerdthusnee K, Vaughn DW. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57(2):119–25.
CAS PubMed Google Scholar
Sim S, Ramirez JL, Dimopoulos G. Dengue virus infection of the Aedes aegypti salivary gland and chemosensory appara tus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog. 2012;8(3):e1002631.
Chang C, Shen WK, Wang TT, Lin YH, Hsu EL, Dai SM. A novel amino acid substitution in a voltage gated sodium channel is associated with knockdown resistance to permethrin in Aedes aegypti. Insect Biochem Mol Biol. 2009;39(4):272–8.
Kawada H, Higa Y, Komagata O, Kasai S, Tomita T, Thi Yen N, et al. Widespread distribution of newly found point mutation in voltage-gated sodium channel in pyrethroid-resistant Aedes aegypti populations in Vietnam. PLoS Negl Trop Dis. 2009;3(10):e527.
Stenhouse SA, Plernsub S, Yanola J, Lumjuan N, Dantrakool A, Choochote W, et al. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasit Vectors. 2013;6(1):253.
De la C Sierra B, Kourí G, Guzmán MG. Race: A risk factor for dengue hemorrhagic fever. Arch Virol. 2007;152(3):533–42.
García G, Sierra B, Pérez AB, Aguirre E, Rosado I, Gonzalez N, et al. Asymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphism. Am J Trop Med Hyg. 2010;82(6):1153–6.
Sakuntabhai A, Turbpaiboon C, Casadémont I, Chuansumrit A, Lowhnoo T, Kajaste-Rudnitski A, et al. A variant in the CD209 promoter is associated with severity of the disease. Nat Genet. 2005;37(5):507–13.
Chaturvedi U, Nagar R, Shrivastava R. Dengue and dengue haemorrhagic fever: Implications of host genetics. FEMS Immunol Med Microbiol. 2006;47(2):155–66.
Soundravally R, Hoti SL. Polymorphisms of the TAP 1 and 2 gene may influence clinical outcome of primary dengue viral infection. Scand J Immunol. 2008;67:618–25.
Othman S, Rahman NA, Yusof R. All serotypes of dengue virus induce HLA-A2 major histocompatibility complex class I promoter activity in human liver cells. Trans R Soc Trop Med Hyg. 2010;104(12):806–8.
Bahram S, Arnold D, Bresnahan M, Strominger JL, Spies T. Two putative subunits of a peptide pump encoded in the human major histocompatibility complex class II region. Proc Natl Acad Sci U S A. 1991;88:10094–8.
Alagarasu K, Honap T, Damle IM, Mulay AP, Shah PS, Cecilia D. Polymorphisms in the oligoadenylate synthetase gene cluster and its association with clinical outcomes of dengue virus infection. Infect Genet Evol. 2013;14:390–5.
Boonnak K, Kaitlyn MD, Gina CD, Tassaneetrithep B, Marovich AM. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J Virol. 2011;85(4):1671–83.
Peurta-Guardo H, de la Cruz Hernendez SI, Rosales VH, Ludert JE, del Angel RM. The 1a, 25-dihydroxy-vitamin D3 reduces dengue virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell lines and cytokine production in the infected monocytes. Antiviral Res. 2012;94(1):57–61.
Alagarasu K, Honap T, Mulay AP, Bachal RV, Shah PS, Cecilia D. Association of vitamin D receptor gene polymorphisms with clinical outcomes of dengue virus infection. Hum Immunol. 2012;73(11):1194–9.
Khor CC, Chau TN, Pang J, Davila S, Long HT, Ong RT, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet. 2011;43(11):1139–41.
Steinle A, Li P, Morris DL, Groh V, Lanier LL, Strong RK, et al. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–87.
Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109(4):1210–5.
Whitehorn J, Chau TN, Nguyet NM, Kien DT, Quyen NT, Trung DT, et al. Genetic variants of MICB and PLCE1 and associations with non-severe dengue. PLoS One. 2013;8(3):e59067.
Fink J, Gu F, Ling L, Tolfvenstam T, Olfat F, Chin KC, et al. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl Trop Dis. 2007;1(2):e86.
Hoh BP, Umi-Shakina H, Zuraihan Z, Zaiharina MZ, Rafidah-Hanim S, Mahiran M, et al. Common variants of chemokine receptor gene CXCR3 and its ligands CXCL10 and CXCL11 associated with vascular permeability of dengue infection in peninsular Malaysia. Hum Immunol. 2015;76(6):421–6.
Tang NL, Chan PK, Wong CK, To KF, Wu AK, et al. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem. 2005;51:2333–40.
Zhang B, Chan YK, Lu B, Diamond MS, Klein RS. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol. 2008;180:2641–9.
Cerny D, Haniffa M, Shin A, Bigliardi P, Tan BK, Lee B, et al. Selective susceptibility of human skin antigen presenting cells to productive dengue virus infection. PLoS Pathog. 2014;10(12):e1004548.
Briant L, Desprès P, Choumet V, Missé D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology. 2014;464–465:26–32.
McArthur MA, Sztein MB, Edelman R. Dengue vaccines: Recent developments, ongoing challenges and current candidates. Expert Rev Vaccines. 2013;12(8):933–53.
Hombach J. Vaccines against dengue: A review of current candidate vaccines at advanced development stages. Rev Panam Salud Publica. 2007;21(4):254–60.
Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5(7):518–28.
Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect Dis. 2009;9(11):678–87.
Raviprakash K, Defang G, Burgess T, Porter K. Advances in dengue vaccine development. Hum Vaccin. 2009;5(8):520–8.
Coller BA, Clements DE. Dengue vaccines: Progress and challenges. Curr Opin Immunol. 2011;23(3):391–8.
Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: Preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29(42):7229–41.
Mantel N, Girerd Y, Geny C, Bernard I, Pontvianne J, Lang J, et al. Genetic stability of a dengue vaccine based on chimeric yellow fever/dengue viruses. Vaccine. 2011;29(38):6629–35.
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet. 2012;380(9853):1559–67.
Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: Randomized controlled phase I trial in the Philippines. Vaccine. 2011;29(22):3863–72.
Huu TN, Quang LC, Vu TQH, Forrat R, Lang J, et al. Safety and immunogenicity of recombinant, live attenuated tetravalent dengue vaccine (CYD- TDV) in healthy Vietnamese adults and children. J Vaccines Vaccin. 2012;3:162.
Google Scholar
Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358–65.
Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. CYD15 study group. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372(2):113–23.
Men R, Bray M, Clark D, Chanock RM, Lai CJ. Dengue type 4 virus mutants containing deletions in the 3’ noncoding region of the RNA genome: Analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J Virol. 1996;70(6):3930–7.
PubMed Central CAS PubMed Google Scholar
Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, Durbin AP, et al. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg. 2001;65(5):414–9.
Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney Jr JE, Thumar B, et al. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidates, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191(5):710–8.
Durbin AP, McArthur J, Marron JA, Blaney Jr JE, Thumar B, Wanionek K, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006;2(4):167–73.
Brandler S, Lucas-Hourani M, Moris A, Frenkiel M-P, Combredet C, et al. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl Trop Dis. 2007;1(3):e96.
Brandler S, Ruffie C, Najburg V, Frenkiel MP, Bedouelle H, Desprès P, et al. Pediatric measles vaccine expressing a dengue tetravalent antigen elicitsneutralizing antibodies against all four dengue viruses. Vaccine. 2010;28(41):6730–9.
Danko JR, Beckett CG, Porter KR. Development of dengue DNA vaccines. Vaccine. 2011;29(42):7261–6.
Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, et al. Evaluation of a prototype dengue-1 DNA vaccine in a phase 1 clinical trial. Vaccine. 2011;29(5):960–8.
Porter KR, Ewing D, Chen L, Wu SJ, Hayes CG, Ferrari M, et al. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine. 2012;30(2):336–41.
Lima DM, de Paula SO, França RF, Palma PV, Morais FR, Gomes-Ruiz AC, et al. A DNA vaccine candidate encoding the structural prM/E proteins elicits a strong immune response and protects mice against dengue-4 virus infection. Vaccine. 2011;29(4):831–8.
Costa SM, Yorio AP, Gonçalves AJ, Vidale MM, Costa EC, Mohana-Borges R, et al. Induction of a protective response in mice by the dengue virus NS3 protein using DNA vaccines. PLoS One. 2011;6(10):e25685.
Zheng Q, Fan D, Gao N, Chen H, Wang J, Ming Y, et al. Evaluation of a DNA vaccine candidate expressing prM-E-NS1 antigens of dengue virus serotype 1 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in immunogenicity and protection. Vaccine. 2011;29(4):763–71.
Putnak R, Barvir DA, Burrous JM, Dubois DR, D’Andrea VM, Hoke CH, et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: Immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996;174(6):1176–84.
Putnak R, Barvir DA, Burrous JM, Dubois DR, D’Andrea VM, Hoke CH, et al. Immunogenic and protective response in mice immunized with a purified, inactivated, Dengue–2 virus vaccine prototype made in fetal rhesus lung cells. Am J Trop Med Hyg. 1996;55(5):504–10.
Srivastava AK, Putnak JR, Lee SH, Hong SP, Moon SB, Barvir DA, et al. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine. 2001;19(31):4557–65.
Eckels KH, Putnak R. Formalin-inactivated whole virus and recombinant subunit flavivirus vaccines. Adv Virus Res. 2003;61:395–418.
Kaltenböck A, Dubischar-Kastner K, Eder G, Jilg W, Klade C, et al. Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese Encephalitis vaccine IC51 and the hepatitis A vaccine HAVRIX1440 in healthy subjects: A single-blind, randomized, controlled Phase 3 study. Vaccine. 2009;27(33):4483–9.
Van Gessel Y, Klade CS, Putnak R, Formica A, Krasaesub S, Spruth M, et al. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO) induced neutralizing antibody titers. Vaccine. 2011;29(35):5925–31.
Coller BA, Barrett AD, Thomas SJ. The development of Dengue vaccines. Introduction. Vaccine. 2011;29(42):7219–20.
Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, et al. Development of a recombinant tetravalent dengue virus vaccine: Immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28(15):2705–15.
Osorio JE, Huang CY, Kinney RM, Stinchcomb DT. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine. 2011;29(42):7251–60.
Brewoo JN, Kinney RM, Powell TD, Arguello JJ, Silengo SJ, Partidos CD, et al. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine. 2012;30(8):1513–20.
Durbin AP, Whitehead SS. Next-generation dengue vaccines: Novel Strategies currently under development. Viruses. 2011;3(10):1800–14.
Schmitz J, Roehrig J, Barrett A, Hombach J. Next generation dengue vaccines: A review of candidates in preclinical development. Vaccine. 2011;29(42):7276–84.
Block OK, Rodrigo WW, Quinn M, Jin X, Rose RC, Schlesinger JJ. A tetravalent recombinant dengue domain III protein vaccine stimulates neutralizing and enhancing antibodies in mice. Vaccine. 2010;28(51):8085–94.
Etemad B, Batra G, Raut R, Dahiya S, Khanam S, Swaminathan S, et al. An envelope domain III–based chimeric antigen produced in pichia pastoris elicits Neutralizing antibodies against all four dengue virus serotypes. Am J Trop Med Hyg. 2008;79(3):353–63.
Kochel TJ, Raviprakash K, Hayes CG, Watts DM, Russell KL, Gozalo AS, et al. A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine. 2000;18(27):3166–73.
Raviprakash K, Kochel TJ, Ewing D, Simmons M, Phillips I, Hayes CG, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. 2000;18(22):2426–34.
Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. 2010;396(2):280–8.
Maves RC, Ore RM, Porter KR, Kochel TJ. Immunogenicity and protective efficacy of a psoralen-inactivated dengue-1 virus vaccine candidate in Aotus nancymaae monkeys. Vaccine. 2011;29(15):2691–96.
Gozdek A, Zhukov I, Polkowska A, Poznanski J, Stankiewicz-Drogon A, Pawlowicz JM, et al. NS3 Peptide, a novel potent hepatitis C virus NS3 helicase inhibitor: Its mechanism of action and antiviral activity in the replicon system. Antimicrob Agents Chemother. 2008;52(2):393–401.
Borowski P, Heising MV, Miranda IB, Liao CL, Choe J, Baier A. ViralNS3 helicase activity is inhibited by peptides reproducing the Arg-rich conserved motif of the enzyme (motif VI). Biochem Pharmacol. 2008;76(1):28–38.
Lim SP, Noble CG, Shi PY. The dengue virus NS5 protein as a target for drug discovery. Antiviral Res. 2015;119:57–67.
Tuschl T. Expanding small RNA interference. Nat Biotechnol. 2002;20(5):446–8.
Alhoot MA, Wang SM, Sekaran SD. Inhibition of dengue virus entry and multiplication into monocytes using RNA interference. PLoS Negl Trop Dis. 2011;5(11):e1410.
Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virology J. 2011;8:560.
Talarico LB, Pujol CA, Zibetti RG, Faría PC, Noseda MD, Duarte ME, et al. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res. 2005;66(2–3):103–10.
Tang LI, Ling AP, Koh RY, Chye SM, Voon KG. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern Med. 2012;12:3.
Monath TP. Dengue: The risk to developed and developing countries. Proc Natl Acad Sci U S A. 1994;91(7):2395–400.
Rossi P, Ricci I, Cappelli A, Damiani C, Ulissi U, Mancini MV, et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit Vectors. 2015;8:278.
Mendes H: Brazil tests GM mosquitoes to fight Dengue. Males with offspring-killing genes are replacing wild insects, say researchers. 11 April 2012. http://www.nature.com/news/brazil-tests-gm-mosquitoes-to-fight-dengue-1.10426 .
Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–7.
McNaughton D, Duong TT. Designing a community engagement framework for a new dengue control method: a case study from central Vietnam. PLoS Negl Trop Dis. 2014;8(5):e2794.
Alen MM, Schols D. Dengue virus entry as target for antiviral therapy. J Trop Med. 2012;2012:628475.
Nicholson CO, Costin JM, Rowe DK, Lin L, Jenwitheesuk E, Samudrala R, et al. Viral entry inhibitors block dengue antibody-dependent enhancement in vitro. Antiviral Res. 2011;89(1):71–4.
Courageot MP, Frenkiel MP, Duarte dos Santos CN, Deubel V, Desprès P. α-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J Virol. 2000;74:564–72.
Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, et al. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol. 2005;79(14):8698–706.
Alen MM, Kaptein SJ, De Burghgraeve T, Balzarini J, Neyts J, Schols D. Antiviral activity of carbohydrate binding agents and the role of DC-SIGN in dengue virus infection. Virology. 2009;387(1):67–75.
Lee E, Pavy M, Young N, Freeman C, Lobigs M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic Flaviviruses. Antiviral Res. 2006;69(1):31–8.
Hidari KI, Takahashi N, Arihara M, Nagaoka M, Morita K, Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophysic Res Comm. 2008;376(1):91–5.
Qiu H, Tang W, Tong X, Ding K, Zuo J. Structure elucidation and sulfated derivatives preparation of two α -dglucans from Gastrodia elata Bl. and their anti-dengue virus bioactivities. Carbohydr Res. 2007;342(15):2230–6.
Download references
Acknowledgements
This Project is funded by the King Abdulaziz City for Science and Technology (KACST) under grant number (ات-33-26) . The authors are also grateful to the kind cooperation provided by the Deanship of Scientific Research (DSR), King Abdulaziz University.
Author information
Authors and affiliations.
King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
Taoufik Nedjadi & Ghazi Damanhouri
Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
Sherif El-Kafrawy, Sayed S. Sohrab & Esam Azhar
UMR PIMIT (I2T team), University of Reunion island, INSERM U1187, CNRS 9192, IRD 249, Technology Platform CYROI, 2 rue Maxime Rivière Saint-Clotilde, La Reunion, 97491, France
Philippe Desprès
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Taoufik Nedjadi .
Additional information
Competing interests.
The authors disclose that there is no conflict of interest.
Authors’ contributions
TN Participated in the review design, coordination and helped to draft the manuscript. SK and SS Participated in the review design and helped to draft the manuscript. PD Participated in the article revision. GD and EA Participated in the review design. Read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Nedjadi, T., El-Kafrawy, S., Sohrab, S.S. et al. Tackling dengue fever: Current status and challenges. Virol J 12 , 212 (2015). https://doi.org/10.1186/s12985-015-0444-8
Download citation
Received : 12 May 2015
Accepted : 01 December 2015
Published : 09 December 2015
DOI : https://doi.org/10.1186/s12985-015-0444-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aedes Aegypti
- Hemorrhagic shock syndrome
- Genetic susceptibility
- Vaccine development
Virology Journal
ISSN: 1743-422X
- Submission enquiries: [email protected]
Dengue Fever: Causes, Complications, and Vaccine Strategies
Affiliations.
- 1 International Centre for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi 110067, India; Department of Biochemistry, University of Delhi, Institute of Home Economics, Hauz Khas, New Delhi 110016, India.
- 2 International Centre for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi 110067, India.
- PMID: 27525287
- PMCID: PMC4971387
- DOI: 10.1155/2016/6803098
Dengue is a highly endemic infectious disease of the tropical countries and is rapidly becoming a global burden. It is caused by any of the 4 serotypes of dengue virus and is transmitted within humans through female Aedes mosquitoes. Dengue disease varies from mild fever to severe conditions of dengue hemorrhagic fever and shock syndrome. Globalization, increased air travel, and unplanned urbanization have led to increase in the rate of infection and helped dengue to expand its geographic and demographic distribution. Dengue vaccine development has been a challenging task due to the existence of four antigenically distinct dengue virus serotypes, each capable of eliciting cross-reactive and disease-enhancing antibody response against the remaining three serotypes. Recently, Sanofi Pasteur's chimeric live-attenuated dengue vaccine candidate has been approved in Mexico, Brazil, and Philippines for usage in adults between 9 and 45 years of age. The impact of its limited application to the public health system needs to be evaluated. Simultaneously, the restricted application of this vaccine candidate warrants continued efforts in developing a dengue vaccine candidate which is additionally efficacious for infants and naïve individuals. In this context, alternative strategies of developing a designed vaccine candidate which does not allow production of enhancing antibodies should be explored, as it may expand the umbrella of efficacy to include infants and naïve individuals.
Publication types
- Clinical Trials as Topic
- Dengue / diagnosis*
- Dengue / epidemiology
- Dengue / etiology
- Dengue / prevention & control*
- Dengue Vaccines / classification
- Dengue Vaccines / genetics
- Dengue Vaccines / immunology
- Dengue Virus / physiology*
- Disease Management
- Outcome Assessment, Health Care
- Dengue Vaccines
ORIGINAL RESEARCH article
Clinical characteristics and risk factors for severe dengue fever in xishuangbanna, during the dengue outbreak in 2019.
A correction has been applied to this article in:
Corrigendum: Clinical Characteristics and Risk Factors for Severe Dengue Fever in Xishuangbanna, During the Dengue Outbreak in 2019
- Read correction

- 1 Institute of Medical Biology, Chinese Academy of Medical Sciences, Peking Union Medical College, Kunming, China
- 2 Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Diseases, Kunming, China
- 3 Yunnan Key Laboratory of Vector-Borne Infectious Disease, Kunming, China
- 4 Xishuangbanna Dai Autonomous Prefecture People’s Hospital, Jinhong, China
- 5 Kunming Medical University, Kunming, China
Background: Dengue poses a large burden on the public health systems worldwide. severe dengue (SD) could lead to more serious clinical symptoms and even death. This study aimed to identify the cause of SD in a clinical trial during the dengue outbreak in Xishuangbanna in 2019, and could provide new insights into the pathogenic mechanisms of SD.
Methods: Mosquito-borne viral (DENV, JEV, and CHIKV) infections were identified. The epidemiological factors and clinical symptoms of inpatients in Xishuangbanna were recorded. The IgG and IgM levels in the serum of dengue inpatients were evaluated, and secondary infections were identified. Then, the structural proteins (C/PrM/E) were sequenced and compared with those of the same type of DENV in the same area as before, and their structures were predicted by the SWISS-MODEL ( expasy.org ). The full-length viral genomes were sequenced and aligned with representative strains by BioEidt or MEGA 5.0.
Results: In this outbreak, the clinical symptoms were more serious in SD. The proportion of SD inpatients of male and Han nationality was larger than that of dengue fever (DF) inpatients ( p < 0.05). DENV-2 infection was the majority in DF, with 45 inpatients. However, DENV-1 infection was the most common SD, with 54 inpatients. There were 3 DENV-3-positive inpatients in the DF group and 6 ZIKV-positive inpatients in the SD group. A secondary infection accounted for 76.47% (78 cases) of SD inpatients, but secondary infections were only in 20% (17 cases) of DF inpatients. In the three-dimensional structure of protein analysis, the C/PrM/E of DENV-1 and DENV-2 showed more stability than previous epidemic strains, while DENV-3 in 2019 showed a looser spatial structure. After a complete genome sequencing and analysis, all six DENV-2 strains belonged to cosmopolitan, five of which clustered into one branch. The GC/AT of the five strains decreased from 2014 to 2018. Compared with DF strains, SD strains had no mutations of commonness.
Conclusions: SD may related to secondary heteromorphic dengue in Xishuangbanna in 2019. The coinfection of ZIKV could be another related factor for SD. The currently datas were very limited and only suggestive.
Introduction
Dengue virus (DENV) is a mosquito-borne flavivirus that is transmitted by the vectors, Aedes aegypti and Aedes albopictus , and is a vector-borne infectious disease virus ( Hawley et al., 1987 ). Dengue virus is a single stranded, positive RNA virus with an envelope genome of approximately 11 kb. The genome encodes a polyprotein, which is processed into three structural proteins [the capsid (C), premembrane (prM), and envelope (E) protein] and seven non-structural proteins (NS1-NS5) ( Guzman and Harris, 2015 ). There are currently four circulating serotypes (DENV-1 to DENV-4) that exhibit up to 70% sequence homology ( Bhatt et al., 2020 ). The incubation period of dengue virus infection is 4–7 days ( Bhatt et al., 2020 ). The disease spectrum ranges from asymptomatic infection and moderate febrile illness (DF) to more severe dengue (SD), such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS; Chaturvedi et al., 2000 ). The clinical symptoms of SD patients mainly include high fever, severe pain in the bones, joints and muscles, headache, skin rash, lymph node enlargement, bleeding, shock and even death ( World Health Organization, 2009 ).
Dengue was listed as a potential threat among ten diseases by the WHO in 2019 ( Norshidah et al., 2021 ). The global incidence has been estimated at 390 million infected individuals each year. In China, no case was reported from 1949 to 1977 until an outbreak occurred in Guangdong Province in 1978 ( Yue et al., 2020 ). In recent years, dengue cases have been reported in almost all provinces (autonomous regions) in China ( Liu, 2020 ). Southeast Asia is an important area for Aedes aegypti and Aedes albopictus and has always been the main epidemic area of dengue disease. Yunnan, as one of the border provinces of China, is adjacent to the Southeast Asian countries Laos, Myanmar and Vietnam. A total of 15,572 dengue cases were recorded in Yunnan Province from 2013 to 2019, as shown in Figure 1 . Dengue cases were concentrated in the border areas, and a total of 8,477 dengue cases were recorded in Xishuangbanna Prefecture (red circle in Figure 1 ), bordering Laos and Myanmar, including 568 imported cases (6.70%) and 7,909 local cases (93.30%) ( Zhang, 2021 ). In Xishuangbanna, few cases of dengue virus infection were reported before 2013. The number of reported dengue virus infections (DENV-3) rose to 1,319 in 2013, 1,132 in 2015 (DENV-2) and 1348 in 2017 (DENV-1). As of November 2019, the number of dengue virus NS1 positive infections exceeded 3,900 ( Zhang et al., 2021 ). With the increase in the number of infections, the number of inpatients with SD increased to 102 in 2019. According to previous reports, 70 of 634 inpatients (11.04%) had SD in 2013 ( Ma et al., 2016 ). Among the 109 inpatients in 2015, 13 (11.9%) had SD ( Cui et al., 2016 ).
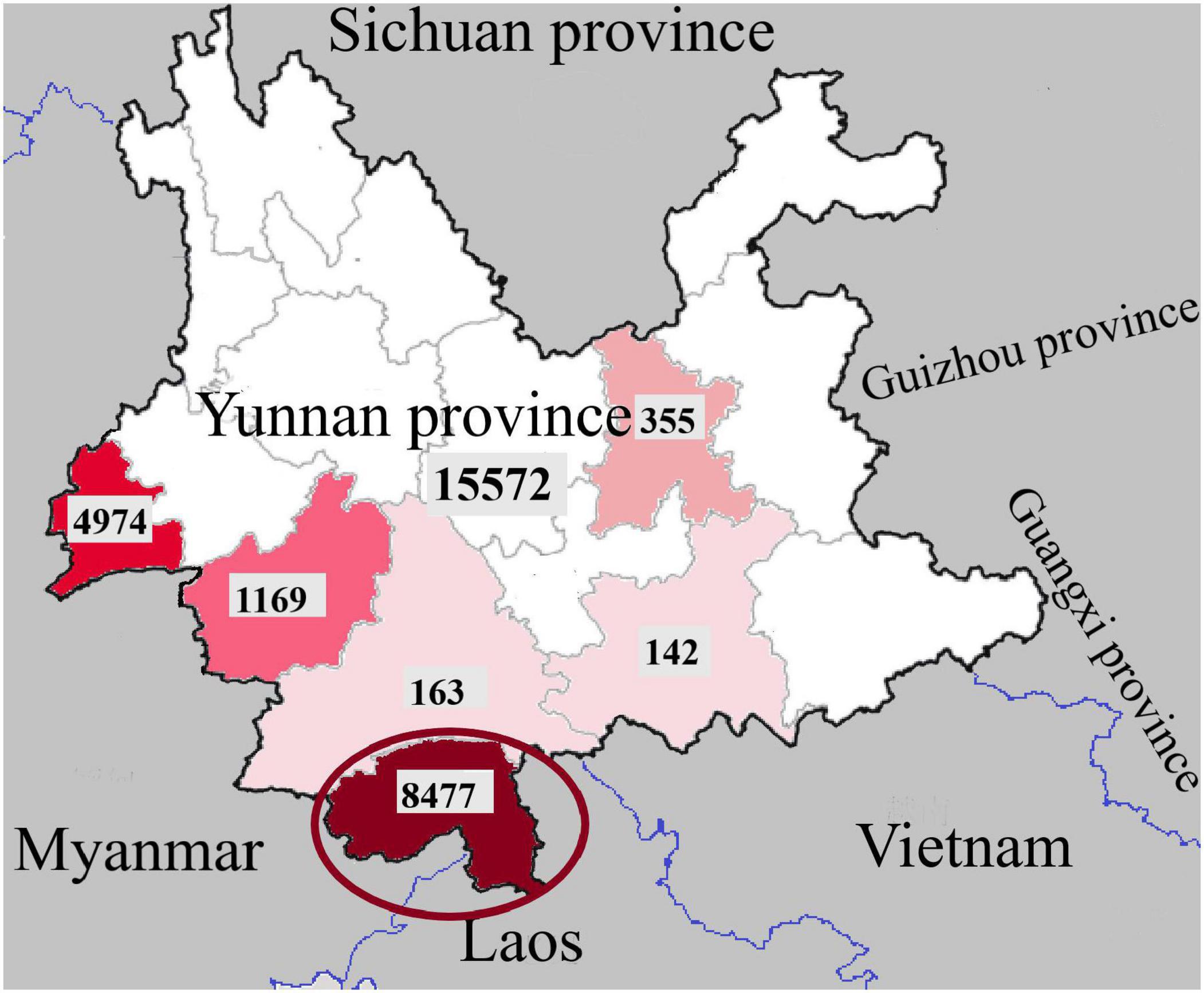
Figure 1. Regional distribution of dengue fever cases in Yunnan Province, China, 2013-2019 (the dates in the picture are from Zhang, 2021 ).
As a more serious form of dengue infection, SD is directly life-threatening. In some areas, the mortality rate of pediatric patients is as high as 5% ( Wang et al., 2016 ). Former studies suggest that age, gender, social status, genetic background, chronic diseases might adversely influence the clinical presentation of dengue infection ( Htun et al., 2015 ). The aim of this article is to study the factors associated with SD. The infection of mosquito-borne viruses [including Zika virus (ZIKV), Japanese encephalitis virus (JEV), and Chikungunya virus (CHIV)] and the serotype of DENV were identified, and the epidemiological factors and clinical symptoms of inpatients in Xishuangbanna were recorded. Then, IgG and IgM in the serum of dengue inpatients were detected, and secondary infections were identified. The structural proteins (C-PrM-E) were sequenced and compared with those of the same type of DENV in the same region. The three-dimensional structure of dengue virus structural proteins was predicted by the SWISS-MODEL ( expasy.org ). Finally, whole genome sequences of 6 inpatients (including 3 SD and 3 DF) were obtained and compared with the sequences of different viruses from different years to detect the homology of the sequence.
Materials and Methods
Study design and participants.
Laboratory-confirmed dengue fever inpatients admitted to the People’s Hospital of Dai Autonomous Prefecture of Xishuangbanna from September to November 2019 were enrolled in this study. Patients were diagnosed based on the Guidelines for the Diagnosis, Treatment, Prevention and Control of Dengue Fever ( World Health Organization, 2009 ). Data on clinical symptoms and laboratory tests were collected for the analysis. Laboratory test data included the white blood cell count (WBC) and the platelet count (PLT). The overall study design is shown in Figure 2 .
Figure 2. Study design and participants.
Mosquito-Borne Virus Identification
A total of 225 DENV-positive serum samples of inpatients were collected from inpatients in the People’s Hospital of Dai Autonomous Prefecture of Xishuangbanna. Dengue NS1 antigen was detected using a DF NS1 test kit (Blue Cross, Beijing, China). Viral RNA was extracted from 140 μL of serum using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and stored at −80°C. Viral RNA was used for PCR the identification of the dengue viral subtypes, Zika virus (ZIKV), Japanese encephalitis virus (JEV) and Chikungunya virus (CHIKV), following the identification of flavivirus. The primers were as Supplementary Table 1 shown ( Wang et al., 2016 ). All dengue virus PCR positive samples were labeled for the next step, which included the amplification of the whole gene or the structural protein nucleic acid sequence.
IgG and IgM Antibodies of DENV Detection
DENV IgG and IgM antibodies were detected by enzyme-linked immunosorbent assay (ELISA) (Order Nr: IB05044 or IB05045, Immuno-Biological Laboratories, Inc., Minneapolis, MN, United States) in 225 DENV-positive serum samples of inpatients, according to the instructions of the manufacturer.
Determination of Primary and Secondary Dengue Virus Infection
The judgment basis of primary and secondary infection was defined as follows ( Wei et al., 2016 ): for specimens taken less than 7 days after the onset, both IgM and IgG antibodies were negative, the judgment could not be made. If the IgM antibody was positive, IgG antibody was negative, the judgment was primary infection. The judgment of secondary infection is that both antibodies are positive, or if IgM antibody was negative, IgG antibody and the DENV RNA are both positive.
Analysis of the Amino Acid Sequence of DENV Structural Proteins (C-PrM-E)
Twenty DENV nucleic acid-positive samples were randomly selected to sequence the nucleic acid sequence of DENV structural proteins and then translated into amino acids with BioEdit 7.0. The C-PrM-E structure was used to build a protein structure model with amino acid sequences by the SWISS-MODEL ( expasy.org ).
Amplification of the Full-Length Genome and Analysis of Isolated DENV Nucleotides
Serum from 3 DF inpatients and 3 SD inpatients was selected for sequencing the full-length genome of DENV, all the six samples were from the same twenty DENV nucleic acid-positive samples used. The primers were used in this study were from our former study ( Jiang et al., 2018 ). Sequences were analyzed using BioEdit 7.0 and compared with sequences available from the BLAST database ( blast.ncbi.nlm.nih.gov/Blast.cgi ). Phylogenetic analyses were performed using the neighbor-joining method with the Tajima-Nei model (MEGA, version 6.0 1 ). The DENV genotype was analyzed using the related reference sequences in NCBI (National Center for Biotechnology Information, Minneapolis, MN, United States) and with known genotypes in the phylogenetic tree. The information of reference sequences were shown in Supplementary Table 2 .
Statistical Analysis
The continuous variables were described by the mean ± standard deviation, and the categorical variables were described by the constituent ratio. Differences or associations with p -values <0.05 were considered significant. All data analyses were performed using SPSS 22.0 software (IBM, Armonk, NY, United States) and GraphPad Prism 7.
Ethical Approval
Institutional Review Board approval was obtained from the Ethics Committee of the Institute of Medical Biology, Chinese academy of Medical Sciences, China. All procedures that were performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Basic Characteristics of Dengue Inpatients
In this study, there were 102 SD patients among the 225 dengue inpatients. Compared with the general dengue patients, the proportions of male and Han nationality inpatients with SD were larger ( p < 0.05) ( Table 1 ). The mean age of DF inpatients was 48.67, the median was 49 (2–97). The youngest is 2 years old and the oldest is 92 years old in DF. The mean age of SD inpatients was 46.14, and the median was 43 (13–88). The youngest is 13 years old and the oldest is 88 years old in SD. The ratio of males to females in SD was 1.76, which was higher than that in DF (0.81). The clinical symptoms were more serious in SD. Compared with DF, the number of low platelet counts (PLT) in SD was greater than that in dengue patients ( p < 0.01). Unexpectedly, other clinical sympt o ms, including fever, vomiting, muscle pain, bleeding, coma, convulsions, and white blood cell counts (WBT), between DF and DHF were no significant differences.
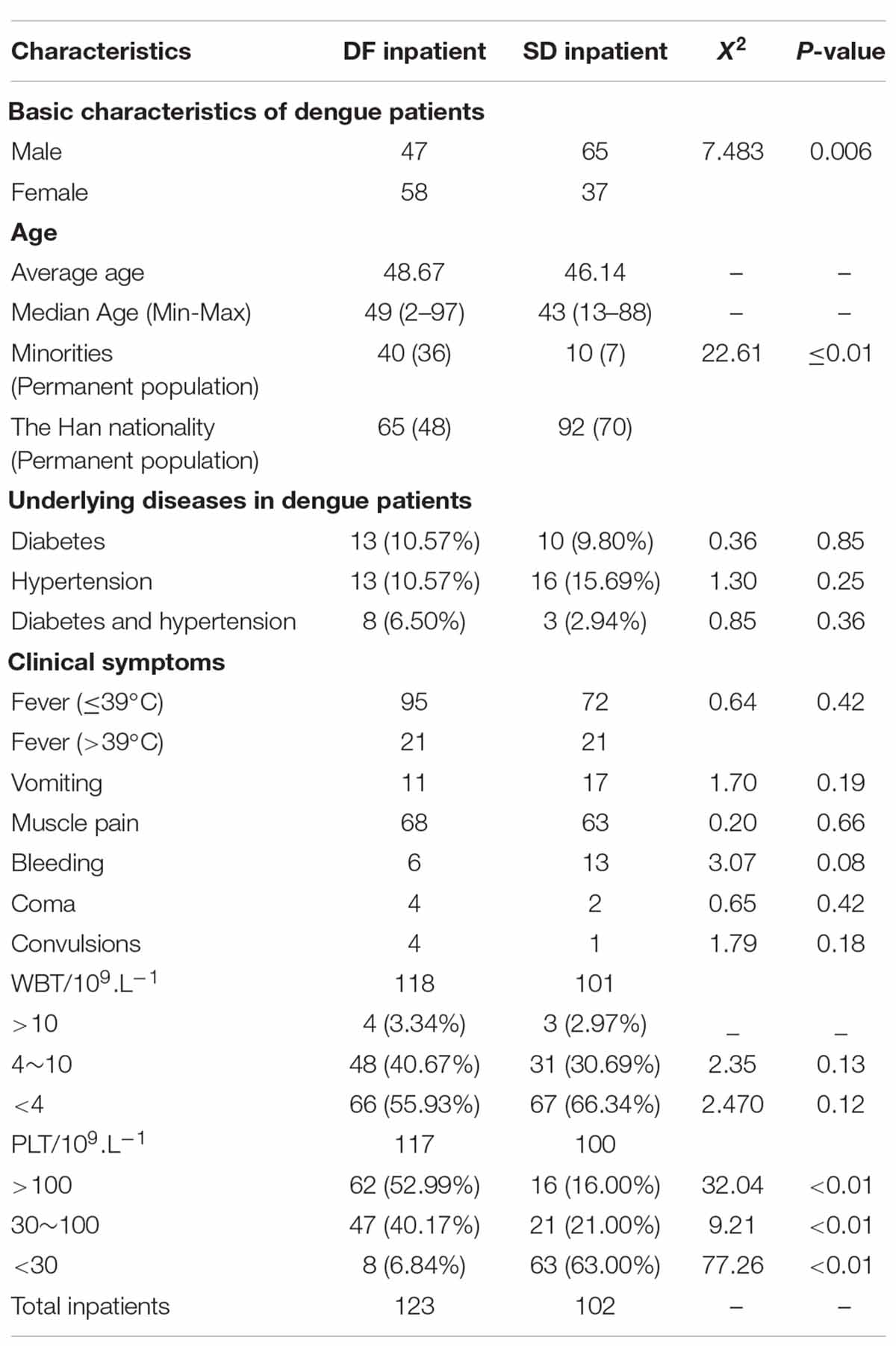
Table 1. Comparison of characteristics of moderate and severe dengue fever inpatients.
A total of 23 diabetics and 29 hypertension patients were found in 225 dengue inpatients. There were 11 inpatients suffering from both two diseases, and 3 of them are SD inpatients. As Table 1 shown, in DF inpatients, 10.57% of patient have diabetes or hypertension, 4.89% have both two diseases. As for SD inpatients, 9.80% of patient have diabetes, 15.69% have hypertension, and only 2.94% have both two diseases. There is no statistical difference between the two groups.
Mosquito-Borne Virus or Virus Coinfection Identification
After nucleic acid samples were extracted and evaluated with specific primers for the presence of different viruses (DENV 1-4, ZIKV, CHIKV, JEV), 54 DENV-1 and 22 DENV-2 were found in 102 samples of sera of SD inpatients. There was one coinfected DENV-1 patient with ZIKV and five coinfected DENV-2 patients with ZIKV. All other viruses were negative. Similarly, among 122 common DF inpatients, there were 19 DENV-1, 45 DENV-2 and 3 DENV-3. The main epidemic serotype of DF inpatients was DENV-2, but the main serotype of SD patients was DENV-1, as shown in Table 2 .
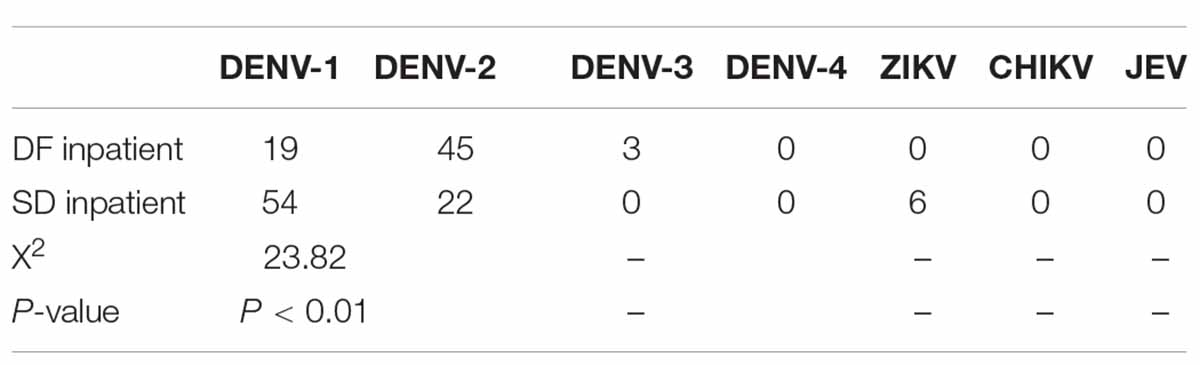
Table 2. Mosquito-borne virus (DENV 1-4, ZIKV, CHIKV, JEV) or virus coinfection identification.
IgG and IgM were detected in 85 DF inpatients, of which 44 were IgM positive and 19 were IgG positive, as shown in Table 3 . In all IgG-positive inpatients, the onset time was less than or equal to 7 days in 17 cases and more than 7 days in 2 cases. IgG and IgM were detected in 95 patients with SD. Among them, 90 patients were IgM positive, and 84 patients were IgG positive. Among all the IgG-positive patients, 78 had an onset time less than or equal to 7 days. According to the judgment basis of primary and secondary infections as previously described in the methods, 78 were secondary infections in SD inpatients and 17 were secondary infections in DF inpatients.
Table 3. IgG and IgM antibodies for DENV detection in dengue inpatients.
Analysis of the Amino Acid Sequence of DENV Structural Proteins (C/PrM/E)
The amino acid sequences of DENV-1, DENV-2 and DENV-3 structural proteins (C/prM/E) were compared by BioEdit. In DENV-1s, compared with the strain KY672931.1 (2015), there were three amino acid mutations of the C protein in strain 5 (2019), from proline(P) to Serine (S), Arginine (R) to Lysine (K) and K to R. Two amino acid mutations were observed in the E protein, including a Leucine (L) to Isoleucine (I), Valine (V) to Alanine (A), and but there were no mutations in the PrM protein. In DENV-2s, compared with strain KY672955.1 (2015), there was one amino acid mutation in the C protein in 15 (2019), K to R. Two amino acid mutations in the PrM protein included K to R, V to A, and one amino acid (L to I) mutation in the E protein. In DENV-3s, compared with strain KR296743.1 (2013), there were two amino acid mutations in the C protein, K to R, asparagine (N) to I, five amino acid mutations in the PrM protein, and ten amino acid mutations in the E protein.
The possible three-dimensional structures of the structural proteins of DENV-1, DENV-2 and DENV-3 in 2019 were later predicted and compared with those of the same type of DENV in Xishuangbanna Prefecture as previously ( Table 4 ). Homology modeling revealed that four strains of DENV-1/DENV-2 had the same three-dimensional structure. However, the two strains of DENV-3 were different. Among the 21 mutation sites in C/prM/E, there were 11 hydrophobic amino acids and 10 hydrophilic amino acids in 2013. However, there were 8 hydrophobic amino acids and 13 hydrophilic amino acids in 2019. The decrease in hydrophobic amino acids in 2019 led to a looser structure than the strain in 2013.
Table 4. The amino acid sequences of DENV-1, DENV-2 and DENV-3 structural proteins (C/prM/E) were compared by BioEdit, and the protein structure was predicted by SWISS-MODEL ( expasy.org ).
Phylogenetic Analysis of Isolated DENV Nucleotide
To analyze whether the sequence is a factor for the severity of dengue patients, we randomly selected three DENV-2 strains from each of the severe and mild patients for whole genome sequencing. After sequencing, nucleic acid and amino acid sequences were analyzed.
In the nucleotide composition analysis, the sequences from DF inpatients (DF10, DF11, and DF15) or SD inpatients (SD9, SD92, and SD106) were compared with other sequences in China from different years, including the strains, MF940237.1 (China Yunnan Province 2015), MN018339.1 (China Guangdong Province 2014), MN018337.1 (China Guangdong Province 2015), MN018340.1 (China Guangdong Province 2016), MN018341.1 (China Guangdong Province 2017), MK783207.1 (China Guangdong Province 2018), and the DENV-2 standard strain NCBI Reference Sequence (NC 001474.2). The results showed that the GC/AT of DENV-2 in China decreased from 2014 to 2018, except the MN018341.1 strain (China Guangdong Province 2017) ( Table 5 ). However, the GC/AT in five of six strains increased in 2019, and the portion rose to the level of 2014. Compared with the other five strains, the GC/AT of DF15 was closer to that of MK783207.1 (China Guangdong Province 2018). Compared with DF strains, SD strains had no mutations of commonness. Although DF15 is different from other virus strains, an evolutionary tree analysis showed that it belongs to the cosmopolitan type, similar to the other five viruses. According to the phylogenetic analysis, DENV-2 of the 2019 dengue outbreak in Yunnan most likely originated from the China Guangdong Province or Thailand, not Yunan Province ( Figure 3 ).
Table 5. Basic information on the DENV-2 sequences was analyzed by BioEdit.
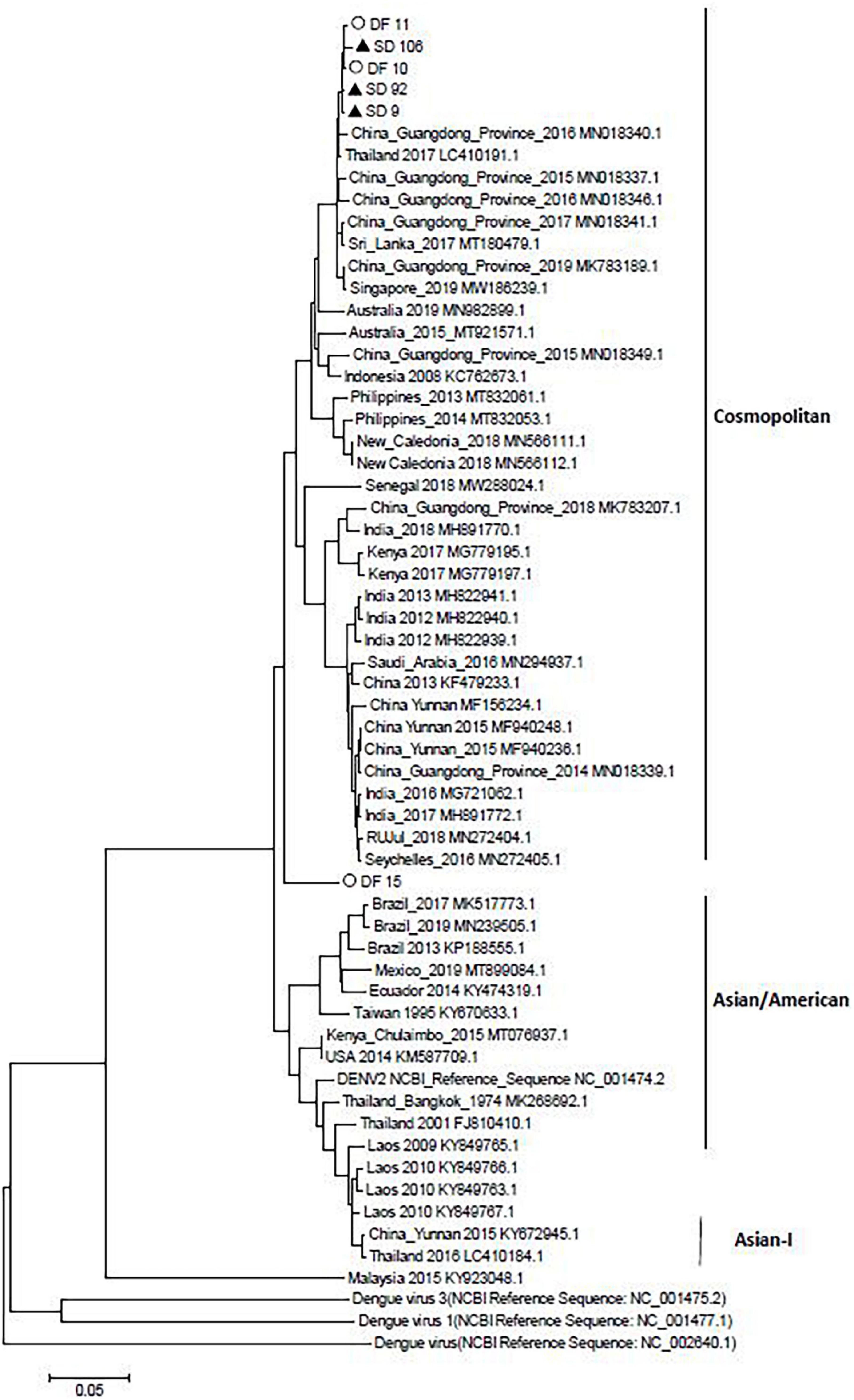
Figure 3. Neighbor-joining phylogenetic tree generated using the nucleotide sequences of complete dengue virus sequences. Study sequences are labeled in black triangles or hollow circles. Others are standard sequences, including sequences of the DENV-2 subgenotype retrieved from the NCBI GenBank. Phylogenetic trees were constructed by the neighbor-joining method and the Kimura 2-parameter model by the MEGA package.
Dengue poses a large burden on the public health systems worldwide. Due to the lack of an ideal animal model, the pathogenesis of dengue has not yet been elucidated. SD is currently believed to be mainly related to secondary heteromorphic DENV infection, coinfection of mosquito-borne viruses, viral variation and host immune response ( Simmons et al., 2012 ; St. John et al., 2013 ). The purpose of this study was to investigate the clinical characteristics and risk factors for severe dengue fever in Xishuangbanna, during the dengue outbreak in 2019.
As a more serious consequence of dengue, SD patients often have more serious clinical symptoms. However, in this outbreak, the only significant difference between SD and dengue patients is the platelet counts. The number of low platelet counts in SD was greater than that in dengue patients ( p < 0.01). But there were no significant differences between DF and DHF in other clinical symptoms. The small sample size was the main reason for those results and the basic characteristics of certain groups were also very important for the pathogenesis of SD. In South America, Southeast Asia and other countries, SD is considered to occur in children and infants ( Sharp et al., 2017 ). However, in this study, there were only two SD inpatients (13 and 18 years old) younger than 18 years old. The mean ages of SD and DF were 46.14 and 48.67, respectively, which were not significantly different. Compared with DF inpatients, SD inpatients were more likely to be male and of Han nationality ( p < 0.05). The formation of SD is not related to age but is related to gender. The reason may be that the spread of dengue is related to the population mobility. Compared with females, males have a larger proportion of migrant workers and are more vulnerable to mosquito bites, which are more likely to lead to SD.
In 2019, there were 22599 cases of dengue fever, with an incidence rate of 1.63/10 million ( Liu, 2020 ). As a typical dengue epidemic area, in 2019, the main epidemic type in Xishuangbanna Prefecture was DENV-1, with an incidence rate of up to 67%. DENV-2 accounted for 32%, and only one patient had DENV-3, which was consistent with our assumption that the epidemic trend was dengue virus. Although DENV-1 was prevalent in Xishuangbanna in 2017, DENV-1 was still prevalent in Xishuangbanna in 2019. According to former studies, DENV-2 and DENV-3 are more likely to cause SD than DENV-1 and DENV-4 ( Fried et al., 2010 ). Among the first infections of this outbreak, 6 SD inpatients were infected with DENV-2, and 3 SD inpatients were infected with DENV-1. After infection with one DENV serotype for the first time, the serogroup cross reactive antibody produced by the host usually can only protect from other serotypes of DENV infection for 3–6 months. When the host is reinfected with heterotypic DENV, the E or PrM antibody produced by the first infection causes a subneutralization titer in the body and forms an immune complex with the virus being infected, increasing the infection rate and replication of the virus ( Murphy and Whitehead, 2011 ). Therefore, we compared the nucleic acid sequence of the PrM protein of the DENV strain in 2019 with that of the dengue virus strain in the same area. Compared with the previous same virus strain genotype, little difference was observed in the primary structure of DENV-1 and DENV-2, but the higher structures were the same. DENV-3 had some differences in the primary structure, which led to different higher structures. These results indicated that DENV-1 and DENV-2 may be more stable than DENV-3. Previous epidemiological studies have shown that the outbreak of DENV-3 and DENV-2 occurred earlier than that of DENV-1 and was more able to lead to subneutralization titers in first infected people. Thus, DENV-1 should be the main serotype in subsequent secondary infections. Among the secondary infection SD inpatients in this study, 50 (%) were DENV-1 and 13 (%) were DENV-2, which was consistent with our study.
ZIKV, CHKIV, and JEV are also transmitted by the mosquito. When there were multiple arboviruses in one place at the same time, humans may be infected with different types of arboviruses at the same time through mosquito bites. In this study, we compared the coinfection of ZIKV, CHIKV, and JEV in SD or DF to explore whether coinfection can lead to an increase in SD. After detection, six inpatients were infected with ZIKV in the SD group, and 0 were infected in the DF group. However, the clinical symptoms of these six coinfected inpatients were not obviously different from those of other SD patients. These results suggested that coinfection may not lead to an aggravation of the symptoms. Unfortunately, among the six ZIKV infected patients, the serum collection time of five of them is longer than 7 days, and the possibility of secondary dengue infection cannot be ruled out. Due to the small number of coinfections in this cohort, more patients needed to be enrolled, to study the role of ZIKV coinfection in SD patients.
The virulence of viruses could influence the occurrence of SD ( Tuiskunen et al., 2011 ). In this study, we selected three DENV-2 epidemic strains from DF or DHF patients and performed whole genome sequencing and analysis. Compared with the sequences before 2019, the GC/AT in five of six strains increased in 2019. However, there was no regularities of the mutations between SD and DF sequences. The results showed that all the sequences from DF and SD belonged to cosmopolitan, and five of them were in a cluster.
The aim of this study was to investigate the causes of SD through the demographic information of SD patients, the co-infection of mosquito-borne viruses, the identification of DENV serotypes, the presence of DENV secondary infections, and the characteristics of the samples of the DENV complete genomes in Xishuangbanna, 2019 ( Zhang et al., 2021 ). The prevalence of three dengue virus serotypes before 2019 might mediate subneutralization titer antibodies and lead to secondary infections, increasing the number of severe dengue patients in Xishuangbanna in 2019. The results of this study might provide insight into early prognostic factors associated with a severe disease progression and improve the rates of early diagnosis and successful treatment. The currently datas were very limited and only suggestive. More dengue patients should be recruited for those study. More other risk factors, especially environmental factors, the basic situation of patients should be included in those studys.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/ , MZ452990-MZ453011.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Institute of Medical Biology, Chinese academy of Medical Sciences, China. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
XW and QS have drafted and revised the manuscript. XW contributed to the sequencing, major experiment and analysis of data. QS and PL have designed and administered the study. TL, YS, JZ, XS, and DL contributed to sample collection. All other co-authors contributed to its finalization and approval for publication.
This research was supported by the National Natural Science Foundation of China (31970868), the Youth Project in Yunnan Province (2019FD082), the Foundation of Yunnan Innovation Team (202105AE160020), Major Projects and Key Research and Development Plans of Yunnan Province (2019ZF004), and Yunnan health training project of high level talents (L-2019030, H-2017052).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.739970/full#supplementary-material
- ^ http://www.megasoftware.net/
Bhatt, P., Sasidharan, S. P., Varma, M., and Arunkumar, G. (2020). Current understanding of the pathogenesis of dengue virus infection. Curr. Microbiol. 78, 17–32. doi: 10.1007/s00284-020-02284-w
PubMed Abstract | CrossRef Full Text | Google Scholar
Chaturvedi, U. C., Agarwal, R., Elbishbishi, E. A., and Mustafa, A. S. (2000). Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol. Med. Microbiol. 28, 183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x
Cui, X. G., Li, L. H., Zhou, H. N., Shan, X. Y., and Bai, C. H. (2016). Clinical characteristics of 109 dengue virus type 2 infected cases in Xishuangbanna. Chin. J. Infect. Dis. 34, 231–232.
Google Scholar
Fried, J. R., Gibbons, R. V., Kalayanarooj, S., Thomas, S. J., Srikiatkhachorn, A., Yoon, I.-K., et al. (2010). Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl. Trop. Dis. 4:e617. doi: 10.1371/journal.pntd.0000617
Guzman, M. G., and Harris, E. (2015). Dengue. Lancet 385, 453–465. doi: 10.1016/S0140-6736(14)60572-9
Hawley, W. A., Reiter, P., Copeland, R. S., Pumpuni, C. B., and Craig, G. B. (1987). Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 236, 1114–1116. doi: 10.1126/science.3576225
Htun, N. S. N., Odermatt, P., Eze, I. C., Boillat-Blanco, N., D’Acremont, V., and Probst-Hensch, N. (2015). Is diabetes a risk factor for a severe clinical presentation of dengue? – review and metaanalysis. PLoS Negl. Trop. Dis. 9:e0003741. doi: 10.1371/journal.pntd.0003741
Jiang, L. M., Ma, D. H., Ye, C., Li, L., Li, X., Yang, J., et al. (2018). Molecular characterization of dengue virus serotype 2 cosmospolitan genotype from 2015 dengue outbreak in Yunnan, China. Front. Cell. Infect. Microbiol. 8:219. doi: 10.3389/fcimb.2018.00219
Liu, Q. Y. (2020). Dengue fever in China: new epidemical trend, challengesand strategies for prevention and control. Chin. J. Vector Biol. Control 31, 1–6.
Ma, D. H., Shan, X. Y., Li, L. H., Li, D., Ma, D., Li, T., et al. (2016). An analysis of clinical features of dengue fever in the border areas of Xishuangbanna, China, Laos and Myanmar. J. Trop. Dis. Parasitol. 14, 231–232.
Murphy, B. R., and Whitehead, S. S. (2011). Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 29, 587–619. doi: 10.1146/annurev-immunol-031210-101315
Norshidah, H., Vignesh, R., and Lai, N. S. (2021). Updates on dengue vaccine and antiviral: where are we heading? Molecules 26, 6768. doi: 10.3390/molecules26226768
Sharp, T. M., Tomashek, K. M., Read, J. S., Margolis, H. S., and Waterman, S. H. (2017). A new look at an old disease: recent insights into the global epidemiology of dengue. Curr. Epidemiol. Rep. 4, 11–21. doi: 10.1007/s40471-017-0095-y
Simmons, C. P., Farrar, J. J., Nguyen, V., and Wills, B. (2012). Dengue. N. Engl. J. Med. 366, 1423–1432. doi: 10.1056/NEJMra1110265
St. John, A. L., Abraham, S. N., and Gubler, D. J. (2013). Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat. Rev. Microbiol. 11, 420–426. doi: 10.1038/nrmicro3030
Tuiskunen, A., Monteil, V., Plumet, S., Boubis, L., Wahlström, M., Duong, V., et al. (2011). Phenotypic and genotypic characterization of dengue virus isolates differentiates dengue fever and dengue hemorrhagic fever from dengue shock syndrome. Arch. Virol. 156, 2023–2032. doi: 10.1007/s00705-011-1100-2
Wang, J., Xie, L., Xie, X. L., Jiang, J. Y., Yang, B., Li, C. M., et al. (2016). Dengue vectors and the natural infection in border with Laos, Jiangcheng County, China. Chin. J. Zoonoses 32, 843–849.
Wei, H. Y., Shu, P. Y., and Hung, M. N. (2016). Characteristics and risk factors for fatality in patients with dengue hemorrhagic fever, Taiwan, 2014. Am. J. Trop. Med. Hyg. 95, 322–327. doi: 10.4269/ajtmh.15-0905
World Health Organization (2009). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control R. Geneva: World Health Organization, 14.
Yue, Y. J., Ren, D. S., Liu, X. B., Wu, H. X., Guo, Y. H., Zhou, N., et al. (2020). A study on spatial characteristics and correlations of different types of dengue cases in mainland China, 2014-2018. Chin. J. Vector Biol. Control 31, 517–520.
Zhang, H. L. (2021). Cross-border spread, indigenous transmission, development trend, and control strategy for dengue fever and chikungunya fever in Yunnan Province, China. Chin. J. Vector Biol. Control 32, 12–18.
Zhang, J., Shu, Y., Shan, X. Y., Li, D., Ma, D., Li, T., et al. (2021). Co-circulation of three dengue virus serotypes led to a severe dengue outbreak in Xishuangbanna, a border area of China, Myanmar, and Laos, in 2019. Int. J. Infect. Dis. 107, 15–17. doi: 10.1016/j.ijid.2021.04.010
Keywords : severe dengue fever, IgG, IgM, dengue inpatients, dengue gene sequence
Citation: Wang X, Li T, Shu Y, Zhang J, Shan X, Li D, Ma D, Long S, Pan Y, Chen J, Liu P and Sun Q (2022) Clinical Characteristics and Risk Factors for Severe Dengue Fever in Xishuangbanna, During the Dengue Outbreak in 2019. Front. Microbiol. 13:739970. doi: 10.3389/fmicb.2022.739970
Received: 12 July 2021; Accepted: 25 January 2022; Published: 10 March 2022.
Reviewed by:
Copyright © 2022 Wang, Li, Shu, Zhang, Shan, Li, Ma, Long, Pan, Chen, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiangming Sun, [email protected] ; Pinghua Liu, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 22 April 2024
Dengue virus pathogenesis and host molecular machineries
- Saumya Sinha 1 ,
- Kinjal Singh 1 ,
- Y. S. Ravi Kumar 2 ,
- Riya Roy 1 ,
- Sushant Phadnis 1 ,
- Varsha Meena 1 ,
- Sankar Bhattacharyya 3 &
- Bhupendra Verma ORCID: orcid.org/0000-0003-1731-5335 1
Journal of Biomedical Science volume 31 , Article number: 43 ( 2024 ) Cite this article
53 Accesses
2 Altmetric
Metrics details
Dengue viruses (DENV) are positive-stranded RNA viruses belonging to the Flaviviridae family. DENV is the causative agent of dengue, the most rapidly spreading viral disease transmitted by mosquitoes. Each year, millions of people contract the virus through bites from infected female mosquitoes of the Aedes species. In the majority of individuals, the infection is asymptomatic, and the immune system successfully manages to control virus replication within a few days. Symptomatic individuals may present with a mild fever (Dengue fever or DF) that may or may not progress to a more critical disease termed Dengue hemorrhagic fever (DHF) or the fatal Dengue shock syndrome (DSS). In the absence of a universally accepted prophylactic vaccine or therapeutic drug, treatment is mostly restricted to supportive measures. Similar to many other viruses that induce acute illness, DENV has developed several ways to modulate host metabolism to create an environment conducive to genome replication and the dissemination of viral progeny. To search for new therapeutic options, understanding the underlying host-virus regulatory system involved in various biological processes of the viral life cycle is essential. This review aims to summarize the complex interaction between DENV and the host cellular machinery, comprising regulatory mechanisms at various molecular levels such as epigenetic modulation of the host genome, transcription of host genes, translation of viral and host mRNAs, post-transcriptional regulation of the host transcriptome, post-translational regulation of viral proteins, and pathways involved in protein degradation.
Introduction
Dengue virus (DENV), a positive-sense (+) single-stranded RNA virus belonging to the Flavivirus family, has spherical-shaped envelope virion particles with surface proteins arranged in icosahedral symmetry [ 1 , 2 ]. The viral genome is about 11 kb long and is translated directly upon entering host cells by the protein synthesis machinery, producing both viral structural and non-structural proteins. The DENV genome synthesizes three structural proteins (Capsid, Membrane, Envelope) which form part of a mature virion particle, and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5), which helps in replication [ 3 ]. The RNA genome and the capsid protein interact to form a complex, while other structural proteins form part of the virion envelope [ 4 ]. Although the NS proteins are absent within the virion, they assist in virus replication and evasion of the immune system within an infected cell [ 3 ]. The structural organization and life cycle of DENV is shown in Fig. 1 .
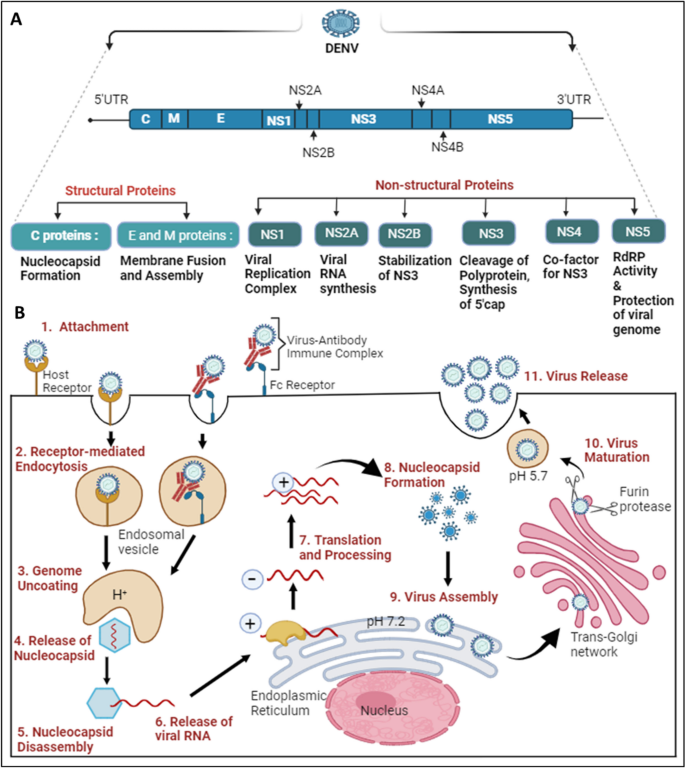
Structural organization and life cycle of DENV: A The Dengue virus genome comprises 5’UTR, ORF, and 3’UTR. The ORF translates into a polyprotein, which is further processed into three structural proteins: C (Capsid), E (Envelope), and M (Membrane), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5). B The initiation of the DENV replication cycle occurs with the entry into the cell via various host cell receptors or through the Fc region of the virus-antibody immune complex, which attaches to Fc receptors present on the target host cell. 1. DENV attaches to host cell receptors and enters the cell. 2. Internalization occurs through receptor-mediated endocytosis, forming an early endosome. 3, 4. Genome uncoating occurs as the pH decreases inside the early endosome; conformational changes take place, releasing the nucleocapsid into the cytoplasm. 5, 6. Disassembly of the nucleocapsid allows viral RNA assembly in the cytoplasm. 7. Viral RNA translocates into the Endoplasmic Reticulum, where translation results in a single polyprotein that is cleaved down by both host and viral proteases. Additionally, a translation switch results in the transcription of viral RNA employing antisense viral RNA. 8. The capsid protein encases the freshly created viral RNA to form the nucleocapsid. 9. Virus assembly occurs on the surface of the Endoplasmic Reticulum. 10. Immature viral particles are transported to the trans-Golgi network, where acidification results in conformational changes, followed by exposure to the furin protease to form mature viral particles. 11. Mature viral particles are exocytosed into the extracellular matrix, completing their replication cycle
The NS1 protein has multiple roles inside and outside the host cell; it also serves as an early indicator to diagnose and assess the level of DENV infection [ 5 , 6 ]. The protein is secreted as a hexamer into the blood circulation of patients, forming an open barrel structure with lipid molecules at the center [ 7 ]. NS1 plays a vital role in severe dengue physiopathology, specifically plasma leakage. A recent report suggested that NS1 activates macrophages via Toll-like receptor 4 (TLR4) and disrupts endothelial cells, resulting in vascular leakage as mentioned in Fig. 2 A [ 8 ]. NS3 acts as a protease using NS2B as a co-factor, cleaving the polyprotein at specific sites and host proteins that would impair dengue infection as depicted in Fig. 2 B [ 9 ]. The importance of the NS3 protein for the survival of the virus makes it a target for antiviral drugs as mentioned in Table 2 . NS4B interacts with NS3 to modulate viral infection, while NS4A indirectly assists in the viral replication process by inducing autophagy, preventing cell death, which is beneficial for viral replication [ 10 ]. NS5 is the most conserved non-structural protein having an RNA-dependent RNA polymerase (RdRp) domain. NS5 is also involved in mRNA capping due to its methyltransferase and guanylyltransferase activities [ 11 ]. The NS5 protein is predominantly located in the nucleus of infected cells, believed to suppress the host anti-viral response [ 12 ]. The DENV envelope protein is involved in attachment and host cell receptor binding for virus entry into the host cell as mentioned in Fig. 2 D [ 13 ]. The newly synthesized viral genome associates with the capsid to form a nucleocapsid, and it buds into the ER lumen together with viral E and prM proteins as shown in Fig. 2 C [ 4 ].
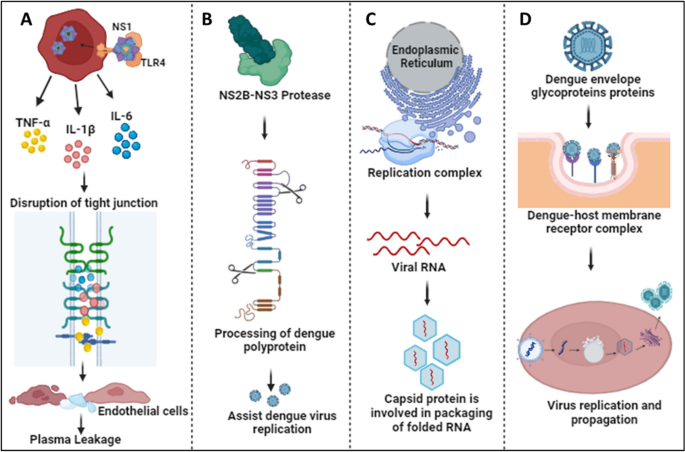
Physiological roles of DENV proteins: A The NS1 protein interacts with TLR4 and activates macrophages, resulting in cytokine release. Cytokines disrupt tight junctions and endothelial barriers, leading to plasma leakage in severe cases of dengue [ 8 ]. B NS3, together with the co-factor NS2B, participates in the processing of the dengue polyprotein, assisting in efficient virus replication [ 9 ]. C The Capsid protein helps in packaging folded RNA released from the DENV replication complex [ 4 ]. D Envelope glycoproteins attach to host receptors and form the dengue-host membrane complex, resulting in virus internalization to process its replication and propagation inside the host cell [ 13 ]
Humans and mosquitoes are the primary hosts and vectors of DENV, respectively. The dengue virus is often undetectable yet prevalent, spreading in cycles of endemic and epidemic disease [ 14 ]. In human, the infection is spread by Aedes species of mosquitoes, specifically Aedes aegypti, Aedes albopictus, and Aedes polynesiensis [ 15 ]. In addition to infected female mosquitoes transmitting the virus to their offspring through eggs, mature female mosquitoes may become infected after feeding on the blood of DENV-infected patients. After ingestion by a mosquito, the virus infects the midgut followed by propagation to other tissues, including the salivary glands. When a mosquito feeds on blood, infectious virus is injected into the skin via salivary gland secretion. Soon after the virus enters the human host’s skin, it initiate its propagation and infects the resident cells [ 16 ]. DENV circulates globally as four serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 [ 17 ]. Approximately 2 to 7 days after a mosquito bite, infection with any serotype of the virus can result in Dengue fever (DF), which may quickly progress to Dengue hemorrhagic fever (DHF), and if neglected or undetected, it can lead to Dengue shock syndrome (DSS) [ 18 , 19 , 20 ]. Dengue has evolved into a serious, potentially fatal consequence for people, as there is currently no vaccine that can effectively reduce the severity of the disease caused by all serotypes, and its prevalence has been steadily increasing [ 21 ].
Whenever the dengue virus infects the human host, both the innate (interferons, complement system, etc.) and adaptive (immunoglobulins and cytotoxic T-cells) immune systems are stimulated to neutralize the virus. In severe Dengue infection, the uncontrolled generation of inflammatory cytokines, leading to phenomenon termed ‘cytokine storm’, has been principally implicated as the causal agent of fatality. Antibodies produced against DENV play a crucial role in disease outcome, as during a secondary heterotypic infection (i.e., an earlier infection by one serotype followed by the current infection by another serotype of DENV), the virus causing the secondary infection cannot be efficiently neutralized by the antibodies against the first serotype. Furthermore, a phenomenon referred to as antibody-dependent enhancement, or ADE, may be enhanced by these non-neutralizing antibodies or low amounts of neutralizing antibodies. Several vaccines have been explored to treat dengue infection, including live attenuated tetravalent vaccines and Dengvaxia. However, developing a vaccine that is effective against all four dengue serotypes remains a challenge [ 22 ]. Importantly, vaccine efficacy varies from person to person, depending on age, serotype, and serostatus [ 23 , 24 ]. Nevertheless, seronegative individuals were advised to avoid vaccination because it was reported that over time, the number of vaccine-induced antibodies decreased [ 25 ]. A potential ADE effect has been a significant restriction for the development of a prophylactic vaccine targeting DENV infection.
In spite of efforts by multiple research groups, there is still no therapeutic drug available in the market that can restrict virus replication or suppress the infection-induced ‘cytokine storm’ to reduce morbidity and/or mortality. Although various drugs, such as Chloroquine, Prednisolone, Balapiravir, Celgosivir, and Lovastatin have been tried, none of them showed promising results during clinical trials. Therefore, supportive medication is mainly used upon the diagnosis of Dengue infection. For example, treatment with paracetamol or pain medications can alleviate or reduce Dengue symptoms, such as muscle soreness, weariness, and fever. Ibuprofen and aspirin, two examples of non-steroidal anti-inflammatory medicines (NSAIDs), are commonly used, but they are not advised because they can exacerbate the prognosis of illnesses, resulting in adverse health conditions. By managing a patient’s fluid content, which is crucial for the management of severe DF, and providing proper medical care, symptoms and mortality can be reduced from more than 20% to less than 1% [ 1 , 26 ]. Notwithstanding supportive therapy, DENV infection may result in endothelial dysfunction, antibody-dependent cellular cytotoxicity (ADCC), cytokine storm (hypercytokinemia), and irregular stimulation of the complement system (CS), leading to a more severe case of Dengue [ 27 ].
Addressing the various host-viral modifications at the molecular level may provide key insights into viral evolution, viral escape mechanisms, host-virus interactions, disease consequences, and public health readiness. This information may advance our understanding of viral infections and facilitate the development of efficient preventative, diagnostic, and therapeutic approaches. The current review will specifically focus on recent advances related to the modulation of epigenomic regulation, transcription, post-transcriptional regulation, as well as the translational and post-translational machinery of the host cell.
DENV and the host epigenetic machinery
Epigenetic plays a vital role as it has the potential to regulate gene expression without altering the genetic sequences. Modulation of epigenetic regulation in the host-DENV interaction is noy yet completely understood. Recent years have seen growing evidence highlighting the pivotal role of epigenetic regulators in gene expression regulation. These regulators encompass various mechanisms such as DNA methylation, chromatin remodeling, histone modification, and regulatory RNA. A comprehensive understanding of the complex interactions among these epigenetic processes is essential for elucidating the consequences they exert on both host cells and DENV during infection. Such insights can aid in the identification of novel therapeutic targets.
Dinakaran et al. provided an overview that at the severe stage of infection, patients show symptoms characterized by inflammation and a cytokine storm resulting in oxidative stress, activating histone deacetylase (HDAC). HDAC factors such as DNA methyltransferase (DNMT) favor the silencing of promoters and slow down host gene transcription processes, as noted in Fig. 3 A. This epigenetic modification is important for investigating mental health issues related to dengue infection. Various HDAC inhibitors like valproate, quetiapine, or carbamazepine are prescribed for treatment as HDAC/Dnmt inhibitors [ 28 ]. Comprehensive transcriptome analysis identified that DENV proteins interact with host genes (CCR10, CNG7, PLXNA3) and lncRNAs (CTBP1-AS, MAFG-AS1) engaged in various biological processes, such as the immune response, inflammation, and the cell cycle, as illustrated in Fig. 3 B. The role of m6A methylation has been implicated in potentially regulating these genes/lncRNAs to enhance DENV replication, as noted in Table 1 [ 29 , 30 ]. Other flaviviruses (YFV, WNV, and ZIKV) also contain m6A methylation, indicating a conserved mechanism within this virus family [ 31 ]. An epitranscriptome analysis showed that various kinds of methylation might be used to regulate flaviviral RNA [ 32 ]. It’s interesting to note that each flavivirus has a unique methylated nucleotide composition. For example, ZIKV vRNA does not include methylated uridines m 3 Um and m 5 Um, while DENV vRNA does [ 32 ]. On the other hand, dimethylcytosines m 5 Cm and m 4 4 C are exclusive to ZIKV vRNA [ 32 ]. Since methylation plays an essential role in the Epstein-Barr virus (RBV) and the murine leukemia virus (MLV) [ 33 , 34 ], it will be interesting to explore the possible roles of these alterations in DENV and ZIKV.
DENV and host epigenetic regulation: A DENV infection results in cytokine storm and leads to oxidative stress conditions in host cell, activating histone deacetylase (HDAC). HDAC activity condenses the chromatin and slows down the host transcription processes [ 28 ]. B DENV protein interacts with various host cellular genes/lnCRNAs and results in m6A RNA methylation. Their localization helps to enhance DENV replication [ 29 , 30 ]. C DENV NS1 interacts with DIDO1, a master epigenetic regulator and somehow elevates DENV replication [ 35 ]. D Capsid protein interacts with core histones (H2A, H2B, H3, and H4) and localizes them in cytoplasm to participate in DENV replication and propagation [ 36 ]
Dengue non-structural genes participate in enhancing virus replication by modulating various epigenetic targets. Localization studies have shown that NS1 binds with the epigenetic regulator DIDO1 (Death Inducer Obligator 1) to promote virus replication, as shown in Fig. 3 C. The silencing of DIDO1 results in reduced virus replication, indicating its significant role [ 35 ]. The dengue structural protein capsid is found to localize in the nucleus and target the four core histones, H2A, H2B, H3, and H4. DENV capsid forms heterodimers with histones and disrupts nucleosome formation for its own amplification, as shown in Fig. 3 D, Table 1 [ 36 ]. Gomes et al. provided an overview that dengue-infected patients with symptoms of haemorrhagic manifestations showed demethylation of the TNFα promoter gene and IFN-γ, leading to acute inflammation resulting in the production of a cytokine storm during dengue infection [ 59 , 60 ].

DENV and the host transcriptional machinery
Human pathogenic viruses have evolved various mechanisms to hijack host cell gene expression at the transcriptional level to facilitate their life cycle. Although the DENV genomic RNA or viral replication complexes are localized exclusively to ER-associated regions of the cytosol, structural and non-structural proteins encoded by the virus migrate to the nucleus and interact with different host proteins. Among DENV proteins, at least two, namely the C and NS5, distribute both in the cytoplasm and nucleus, getting involved in protein-protein interactions that cause the regulation of gene expression [ 61 ]. The C-protein is enriched in the nucleolus, interacting with the host protein nucleolin and facilitating viral morphogenesis [ 62 ]. Additionally, the C-protein has been indicated to mimic histone proteins and interfere with nucleosome formation, thereby influencing host cell transcription [ 36 ]. During virus infection, chemokine levels are elevated as an immune response against viruses. For instance, Interleukin-8 (IL-8) levels are upregulated in patients following infection by different group of RNA viruses such as Coronaviruses and Cytomegaloviruses to promote virus replication [ 63 , 64 ]. Similarly, In DENV-infected patients, higher IL-8 levels have been shown to correlate with severe DHF [ 65 ]. Li and co-workers showed a role for the C-protein in augmenting IL-8 transcription by associating with Positive Transcription Elongation Factor-b (P-TEFb), a complex of CDK9 and Cyclin T1 that promotes gene expression through augmentation of RNA Pol-II processivity [ 66 ]. Since pharmacological inhibition of P-TEFb showed a reversal of IL-8 upregulation, this can be a potential therapeutic strategy to avert the emergence of severe DENV, as illustrated in Fig. 4 D [ 40 , 66 ]. Although the significance of nucleolar enrichment is not clear yet, it is possible that, as observed in other RNA viruses, the DENV C-protein influences host ribosome assembly, prioritizing translation of the viral positive-strand RNA over host mRNAs [ 67 ]. Although there is no evidence that the DENV E-protein migrates to the nucleus, it has been suggested to influence host cell transcription through an interaction with TAL-1, a transcription factor involved in megakaryopoiesis, as shown in Fig. 4 A. In thrombocytopenia, only naive MEG-01 cells could be infected by DENV, and differentiated cells were resistant to virus infection/replication and it slowed the progress of megakaryopoiesis [ 37 ]. It has been observed that DENV infection enhances the activity of the Notch signaling pathway in the human system, and has been shown to limit the process of megakaryopoiesis. As, Notch-1 is known to regulate not only its downstream component RBPjk33, but also the megakaryopoiesis-specific transcription factor TAL-1. The sequestering of TAL-1 in the cytoplasm by DENV E protein may affect its availability for its transcription-related function in the nucleus, thus impeding the megakaryopoiesis. Dengue Virus also dysregulates master transcription factors involved in platelets development and maturation such as NF-E2, GATA-1, and GATA-2 and the PI3K/AKT/mTOR signaling pathway as depicted in Fig. 4 B [ 38 ].
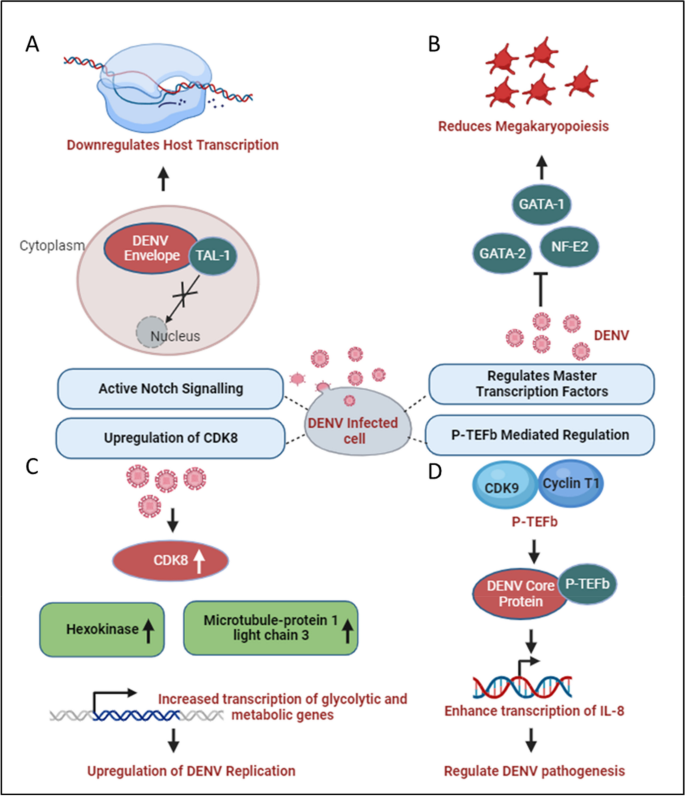
DENV and host transcriptional regulation: A DENV Envelope sequesters the transcription factor TAL-1 in the cytoplasm, affecting its transcriptional function in the nucleus [ 37 ]. B Various factors involved in transcription such as GATA-1, GATA-2, and NF-E2, which participate in the process of megakaryopoiesis, are dysregulated during dengue infection [ 38 ]. C During DENV infection, glucose and metabolic processes increase, enhancing RNA Polymerase II activity. This leads to higher expression of genes like Hexokinase and Microtubule-associated protein 1 light chain 3, promoting increased transcription of metabolic genes and enhancing DENV replication [ 39 ]. D P-TEFb interacts with DENV and activates NF-kB elements within the promoter region of IL-8 gene, enhancing IL-8 transcription which may play a significant role in DENV pathogenesis [ 66 ]
DENV exhibits distinct characteristics among its four serotypes, with DENV2, in particular, showcasing a reliance on increased glucose utilization and enhanced glucose metabolism to produce the necessary metabolic intermediates essential for viral replication [ 68 ]. During DENV-2 infection, there is an elevation in the expression of specific metabolic enzyme-encoding mRNAs, especially glucose-6-phosphate and fructose-6-phosphate in infected cells, through an upregulation in the expression of glucose transporter GLUT1 (which transports glucose from the extracellular environment into the cytoplasm) and hexokinase 2, the first rate-limiting enzyme in the glycolytic pathway [ 69 ]. DENV replication requires high glucose and metabolic intermediate for transactivation of RNA polymerase II [ 39 ]. Butler and co-workers confirmed the transcriptional upregulation of HK2 following DENV infection in a different model system and additionally showed an upregulation of Cyclin-dependent kinase 8 (CDK8) levels in DENV-infected cells to be upstream of HK2 [ 39 ]. Furthermore, the upregulation of CDK8 was shown to be beneficial for virus replication since a reversal led to a reduction in viral titers. As CDK8 expression increases, in addition to that of HK2, there is a concurrent upregulation of crucial genes like microtubule-associated protein 1 light chain 3 (LC3) and other proteins essential for virus replication. This coordinated effect is illustrated in Fig. 4 C [ 39 ].
In a study conducted by A. Carlin, the focus was on interferon-regulatory factors (IRFs), a family of transcription factors known for their role in generating type I interferon (IFN) and eliciting antiviral responses. The investigation involved triple knockout (TKO) mice, genetically engineered to lack three key transcription factors that regulate type I IFN production. Surprisingly, the TKO mice, deficient in the factors controlling type I IFN production, demonstrated increased survival rates when challenged with dengue virus (DENV). This finding strongly suggests that the antiviral effects of IRFs are mediated through interferon responses during DENV infection [ 70 ]. Contrastingly, the study also revealed a strategic countermeasure employed by the dengue virus to facilitate its replication and propagation. Dengue virus actively suppresses interferon-stimulated genes by impeding the recruitment of the transcription factor PAF1C. Additionally, the virus modulates SEC61, a key cellular component, leading to the inhibition of DENV replication. This dual nature of the host-virus interaction underscores the intricate balance between the host’s antiviral defenses, mediated by IRFs and interferon responses, and the virus’s ability to subvert these defenses for its replication advantage [ 61 ].
Depending on the model system used, DENV infection is reported to either augment or decrease the transcriptional activity of NFE2L2, encoded by the NRF2 gene. In monocyte-derived dendritic cells (moDCs), it inhibits NFE2L2 activity, whereas in differentiating megakaryocytes, it seems to augment NFE2L2 activity [ 71 , 72 ]. This manipulation is particularly prominent during instances of dengue fever (DF) and dengue hemorrhagic fever (DHF). DF is characterized by thrombocytopenia, a condition marked by a low platelet count, primarily resulting from the process of megakaryopoiesis. Despite lacking a nucleus, platelets contain essential cellular components such as cytosolic ribosomes, endoplasmic reticulum (ER), Golgi apparatus, and mitochondria, the cell’s powerhouse. These components provide the necessary machinery for viral replication and transcription [ 73 ]. In individuals infected with the dengue virus (DENV), platelets serve as a reservoir for the infectious DENV antigen. However, this aspect is controversial because other studies could not corroborate it. Consequently, severe thrombocytopenia is a prominent symptom of dengue with potential complications leading to dengue shock syndrome (DSS) in certain cases.
DENV and the host post-transcriptional machinery
Modulation of post-transcriptional gene regulation mechanisms within host cells, including alternative splicing, mRNA transport, mRNA degradation, and RNA silencing, plays a crucial role in the Dengue virus (DENV) life cycle. An understanding of these mechanisms is key to unraveling the complex interplay between the virus and its host. Among the non-structural proteins encoded by the DENV genome, NS5 and NS3 have been reported to influence host cell post-transcriptional machinery, facilitating virus replication.
In addition to its unique role in replicating viral genomic RNA (both negative and positive-sense strands) in ER-associated Replication Complexes (RC) and selectively capping positive-sense genomic RNA, NS5 localizes to the host nucleus with the aid of two Nuclear Localization Signals (NLS). This localization influences the splicing of select host cell pre-mRNAs [ 41 ]. Pre-mRNA splicing stitches gene exons together to generate unique mRNAs and is catalyzed by large RNA-protein spliceosomal complexes, termed either major (U2-dependent) or minor (U12-dependent) spliceosomes. The major spliceosome is responsible for removing 99.5% of the introns [ 74 ]. Splicing also affects mRNA transport to the cytoplasm and decoding by the translation machinery. The NS5 interactome includes core components of U5 small nuclear ribonucleoprotein (U5snRNP) complex such as CD2BP2, DDX23, etc. [ 41 ], Fig. 5 A. This modulates alternative splicing of known host antiviral factors like Cystic Fibrosis Transmembrane Regulator (CFTR), EDI (extradomain A of fibronectin), and Bclx (B-cell lymphoma-extra-large), potentially impacting the host’s immune response to Dengue infection [ 41 ]. By modulating the activities of host splicing factors, DENV can orchestrate specific splicing patterns that enhance viral gene expression and protein production [ 75 , 76 , 77 ].
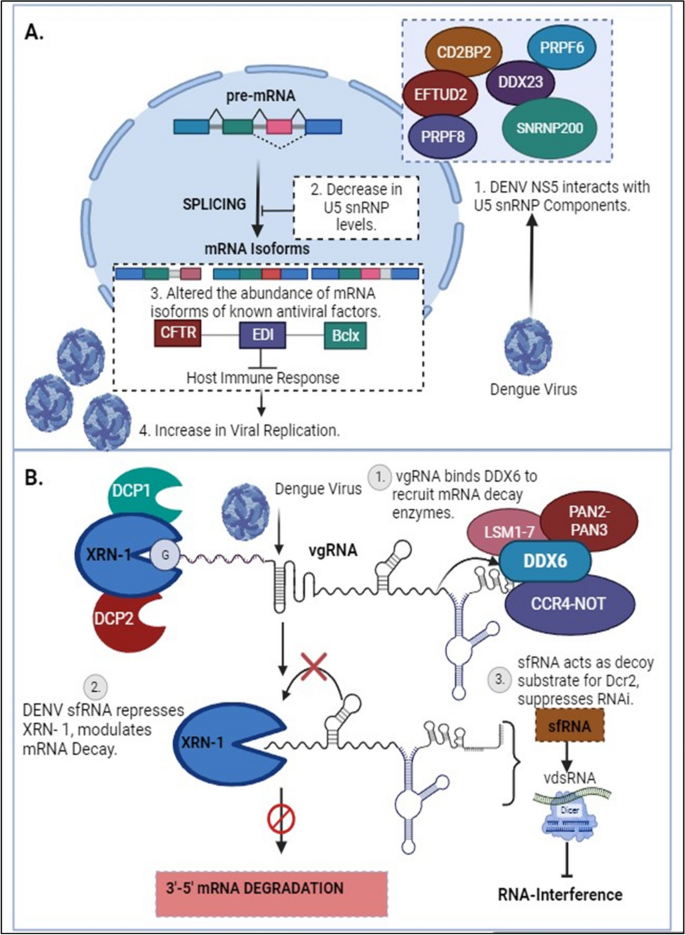
DENV and host post-transcriptional regulations: A Dengue Virus NS5 associates with active spliceosomes, interacting with key components of the U5 snRNP and sequestering them in the cytoplasm. This reduces their levels in the nucleus and alters the events of alternative splicing [ 41 ]. B In the early stages of DENV infection, viral genomic RNA (vgRNA) binds to DDX6 via the 3’UTR and recruits mRNA decay enzymes to the viral replication complex. The exoribonuclease XRN-I initiates the degradation of vgRNA, resulting in the formation of sfRNA. These pseudoknot structures play a role in stalling the XRN1 enzyme near the 5’ border of the 3’ UTR, causing the inhibition of XRN-1 and leading to modulations in mRNA degradation pathways, consequently affecting the RNAi response [ 43 ]
Polyamines (spermidine/spermine) are ubiquitous small, positively charged molecules that can function as chaperones for cellular nucleic acids [ 78 , 79 ]. Multiple viruses are known to depend on this function of polyamines for genome replication [ 80 ]. The enzyme Spermidine/spermine-N1-acetyltransferase (SAT1), an Interferon-sensitive gene (ISG), acetylates polyamines, leading to a drop in intracellular levels and thereby inhibiting viral genome replication [ 81 , 82 ]. However, SAT1 expression is downregulated upon the inclusion of exon-4 of the gene in mature mRNA, an incorporation prevented by the splicing factor RBM10 [ 81 ]. As a counter to SAT1-mediated reduction in intracellular polyamine levels, DENV NS5 interacts with RBM10, mediating its degradation by the proteasome, leading to an increase in the abundance of SAT1 mRNA containing exon-4 and a consequential reduction in SAT1 protein levels [ 42 ]. Additionally, NS5 affects the splicing efficiency of RIG-I, a sensor of viral RNA in the cytoplasm, which functions in inducing an antiviral immune response [ 41 ]. In this context, it is intriguing to note that NS5 reduces the splicing efficiency of endogenous RIG-I mRNA and also increases the expression of dominant-negative forms of IKKε (inhibitor of nuclear factor kappa-B kinase ε) during DENV infection. This, by all means, results in supporting pro-viral conditions in the cell. Hence, it can be appropriately proposed that the interaction of NS5 with the splicing machinery is an immune suppression strategy [ 41 ]. Extending these studies to include other members of the flavivirus family, a study by Michalski et al. suggested that ZIKV sfRNAs also affect host mRNA splicing. ZIKV sfRNAs can sequester splicing factors like PHAX, PPIH, SF3B1/2, and NMP1, which are involved in regulating alternative splicing of host transcripts, significantly contributing to the mis-regulation of cellular RNA splicing, including exon-skipping and intron retention events in various genes during infection [ 83 ].
In response to virus infection, host cells secrete interferons, which bind to cognate receptors on the cell surface and activate the expression of IFN-sensitive genes through signal transduction. STAT2 forms an essential component of this signal transduction pathway, and suppression of this protein leads to a suppression of the innate host antiviral response. While in the cytosol, NS5 associates with the host STAT2 protein, catalyzing its degradation and the consequent silencing of STAT2-dependent gene expression, in the nucleus, NS5 interacts with a multiprotein complex involved in STAT2-independent gene expression and negatively regulates its function [ 53 ]. Petit and coworkers showed that through interaction with PAF1, a component of the Polymerase Associated Factor 1 complex (PAF1C), which is instrumental in the expression of STAT2-independent immune response genes, DENV NS5 inhibits its function, leading to suppression of the antiviral genes targeted by PAF1C [ 84 ].
During dengue infection, the virus interacts with the host cellular machinery responsible for 5’ capping. DENV employs multiple strategies to hijack and manipulate this process. For instance, the virus sequesters essential host factors involved in capping, such as the cap-binding complex and associated enzymes. By doing so, DENV ensures efficient capping of its own viral transcripts, enhancing their stability and translational competence [ 85 ]. During dengue infection, the antibodies produced are generally cross-reactive among DENV serotypes, carrying a higher risk of promoting Antibody-Dependent Enhancement (ADE) [ 86 ]. A study by Narayan and Tripathi, demonstrated that dengue antibody-dependent enhancement (ADE)-mediated virus entry predominantly causes the enrichment of differentially expressed genes (DEGs) that regulate RNA processing. Many of these factors are known to interact with dengue viral RNA, suggesting that dengue ADE modulates the transcriptome of monocytes to upregulate genes responsible for vesicular transport and mRNA processing [ 87 ]. Additionally, the spatial regulation of mRNA transport during dengue infection is mediated by the interaction between viral proteins and host factors involved in mRNA transport processes. For example, RNA-binding proteins (RBPs) are key regulators of mRNA transport and localization. HnRNPs (heterogeneous nuclear ribonucleoproteins), for instance, have been shown to regulate host mRNA expression in response to dengue infection. Modulation of these RBPs by dengue infection can impact mRNA transport and localization, potentially influencing viral replication and pathogenesis. Furthermore, mRNA transport in Dengue infection can influence the host immune response. Viral RNA molecules transported to specific subcellular compartments may interact with immune sensors, such as pattern recognition receptors (PRRs), triggering antiviral immune responses. In addition, the cytosolic localization of viral mRNA molecules can impact the accessibility of viral RNA to host immune factors, influencing the recognition and clearance of the virus [ 88 ].
One pivotal facet of post-transcriptional regulation is mRNA turnover, which involves the mRNA decay machinery to synchronize gene expression by balancing the stability and lifespan of mRNAs through regulated mechanisms. Efficiently evolving viruses exploit the cellular gene expression machinery to escape antiviral responses and control viral replication [ 89 ]. Viral genomic material, in addition to virus-encoded factors and enzymes, interacts with elements of the host mRNA decay pathways to modulate the half-life of both viral and cellular mRNAs. Furthermore, viruses encode ribonucleases to degrade cellular mRNAs, providing multiple advantages, one of which is increased stability of viral transcripts. Additionally, it has been demonstrated that the dengue virus regulates XRN1 (5’-3’ exoribonuclease 1 protein), a key component of the cellular mRNA decay machinery responsible for degrading mRNAs in the 5’ to 3’ direction. DENV undertakes this modulation through subgenomic flavivirus RNA (sfRNA), a non-coding RNA molecule derived from the 3’ untranslated region (UTR) of the viral genome. This sfRNA can bind directly to XRN1, inhibiting its exonuclease activity. By inhibiting XRN1, sfRNA prevents the degradation of viral RNA, including non-coding regions, allowing the virus to maintain a stable pool of genomic RNA for replication. Additionally, by inhibiting XRN1, the virus stabilizes host mRNA, hindering the process of mRNA degradation and potentially dampening the host immune response, as shown in Fig. 5 B [ 43 ]. This particular mechanism of stalling and repression of XRN1 by generating sfRNA appears to be a common strategy by many members of Flavivirus family. Zika virus targets the cytoplasmic 5’-3’-exoribonuclease XRN1, by interacting with the cytoplasmic RNA decay machinery, which results in the suppression of gene expression and the generation of copious amounts of sfRNA in infected cells. Notably, XRN1 targeting is not limited to the Flaviviruses but is employed by RNA viruses from diverse evolutionary backgrounds as part of the molecular arms race to help the infecting virus usurp the innate immune response of the host. Further studies, elucidate that DENV-2 sfRNAs interact with and sequester two viral restriction factors involved in aspects of cellular RNA decay, DDX6 and EDC3, in addition to a deadenylase component CNOT1. Similar results have been obtained with the ZIKIV sfRNA, demonstrating that besides the RNA decay pathway, these viruses also reduce the decapping and deadenylation of transcripts [ 83 ].
During dengue infection, several mRNA degradation pathways have been implicated, including exonucleolytic degradation mediated by the exosome complex, endonucleolytic cleavage by endoribonucleases, and the miRNA-mediated decay pathway [ 90 ]. To aid viruses with their establishment inside the host, these viruses encode several proteins that interact with various host factors. One such class of proteins identified as Viral Suppressors of RNA Silencing (VSR) has been demonstrated to interact with components of the host machinery participating in post-transcriptional gene silencing, a known antiviral defense mechanism. Based on the present study, it has been shown that dengue NS3, a known VSR, interacts with a cellular chaperone, Heat shock protein family A (Hsp70) member 1A (HSPA1A), subsequently modulating its expression levels as well [ 91 ]. Moreover, it was discovered that HSPA1A is linked with the host RNA silencing machinery by coordinating with the Argonaut proteins, Ago1, and Ago2, and translocating to the cytoplasm of the cell along with them. Hence, these outcomes provide evidence for the participation of other host partners in mediating the VSR role of dengue NS3 and in determining the mechanisms of RNA silencing in the case of Dengue virus infection. Furthermore, one of the key mediators of antiviral immunity is Interferon-stimulated genes (ISGs) that play a crucial role in the immune response to DENV infection. IRAV is an RNA-binding protein, which functions as an ISG and localizes to cytoplasmic processing bodies (P bodies) in uninfected cells, where it interacts with the MOV10-RISC complex RNA helicase (MOV10) and Upstream frameshift 1 (UPF1), a key component of nonsense-mediated RNA decay pathways. IRAV and MOV10 are believed to function in conjunction with other proteins like HuR, LARP1, and PABPC (some RNA-binding proteins involved in the mRNA decay pathway) to operate in the disruption of viral RNA by colocalizing with the DENV replicating complex, particularly NS3 and NS4A, thus possessing anti-viral activity. A study by Balinsky et al. in 2017 proposed that IRAV is upregulated during DENV infection [ 44 ].
DENV and the host translational machinery
The Dengue virus, functioning as an intracellular parasite, has developed sophisticated strategies to manipulate the host’s translational machinery, ensuring efficient viral protein synthesis and replication. This intricate interplay between the virus and the host’s translation machinery is crucial for the successful propagation of the virus within the host cell. Upon infection, the Dengue virus genome, a positive-sense single-stranded RNA, serves as a template for the translation of viral proteins. Translation initiation primarily occurs through the cap-dependent translation mechanism [ 92 ]. In this process, the cap-binding protein complex (CBC), specifically the eIF4E factor, recognizes the cap structure at the 5’ end of the viral RNA. Subsequently, the eIF4F complex, comprising eIF4E, the adapter protein eIF4G, and the helicase eIF4A (along with its cofactor eIF4B), binds to the cap and recruits ribosomes to the viral mRNA, facilitating translation initiation [ 45 , 93 ].
In addition to the aforementioned mechanisms, Bidet and Garcia-Blanco identified the DENV-2 NS3 protein as an interacting partner of various translation initiation factors, namely eIF4G, eIF5A, and eIF3L, showcasing the importance of these proteins in upregulating viral RNA translation. This observation underscores DENV-2’s ability to target key components of the host’s translation initiation machinery [ 94 ]. Furthermore, these findings extend beyond DENV; the NS5 protein of the Yellow Fever virus (YFV) was also found to interact with these translation factors in two-hybrid screening assays [ 95 ]. Translation elongation factors, such as eEF1A and eEF2, have also been shown to be essential for WNV and Yellow Fever Virus 17D (YFV17D), respectively, confirming the importance of these protein categories in flaviviral infections, besides the capacity of flaviviruses to manipulate the host’s translational apparatus [ 96 ].
The availability of eIF4E is a key control point for translation. Hypophosphorylation of eIF4E-binding proteins (4E-BPs) prevents their interaction with eIF4E in the cap-binding complex, resulting in the suppression of cap-dependent translation. In such situations, an internal ribosome entry site (IRES) is utilized to initiate cap-independent translation. IRES-mediated translation involves unique sequences present in the Dengue virus 5’-untranslated region (UTR), which recruit ribosomes directly [ 97 , 98 ]. A proposed non-canonical model suggests that during low levels of eIF4E, RNA sequences in the 3’ UTR stabilize translation initiation factors at the 5’ end, subsequently recruiting eIF4G and eIF4A, thereby bypassing the requirement for eIF4E. This process facilitates the formation of a bridge between the 5’ UTR and the 3’ UTR, enhancing translation under these conditions [ 98 ]. A similar phenomenon is observed in other genera within the Flaviviridae family; hepatitis C virus (HCV) contains an IRES to facilitate translation initiation. HCV recognizes the 43S particle primarily through IRES RNA interaction. This direct interaction helps in initiating translation processes [ 99 ]. Interestingly, Polypyrimidine tract-binding protein (PTBP) protein helps other viruses, such coxsackievirus B3 (CVB3), circularise the viral RNA by bridging the UTRs necessary for effective translation of viral RNA [ 100 ].
The Dengue virus 3’ UTR stimulates translation through both cap-dependent and cap-independent mechanisms. Furthermore, the 3’ stem-loop structure complements this process by enhancing polysome formation. While the lack of a 3’ poly(A) tail in the Dengue virus genome is not entirely clear, the 3’ stem-loop domain is functionally analogous to a 3’ poly(A) tail, as it recruits translation initiation factors [ 97 ]. Notably, the 3’ stem-loop functions at both the translation and replication levels, as the Dengue virus RNA serves as both mRNA and a template for negative-strand synthesis [ 101 ]. In contrast CVB3 contains hexa-nucleotide stretch within stem loop C, which is critical for CVB3 IRES mediated translation [ 102 ]. Multifunctional RNA-binding proteins (RBPs) such as G3BP1 (G3BP Stress Granule Assembly Factor 1), G3BP2 (G3BP Stress Granule Assembly Factor 2), and Caprin1 are identified as novel regulators during Dengue virus type 2 (DENV-2) infection. They play a significant role in interferon-stimulated genes (ISGs) translation. It has been reported that DENV-2 sub-genomic flaviviral RNA binds to G3BP1, G3BP2, and Caprin1, leading to the inhibition of ISG mRNA translation, resulting in a proviral effect [ 46 ].
A notable phenomenon observed in Dengue virus translation is the binding of the poly (A)-binding protein (PABP) to the non-polyadenylated 3’ untranslated region (3’ UTR) of the viral RNA. Specifically, PABP interacts internally with the 3’ UTR, positioning itself upstream of the conserved 3’ stem-loop structure near the two dumbbell structures. The PABP-interacting protein 2 (PAIP2), a translation inhibitor specific to PABP, plays a role in interfering with the interaction between the Dengue virus 3’ UTR and PABP. In-vitro experiments using baby hamster kidney cell extracts revealed that PAIP2 inhibits the translation of Dengue virus reporter RNAs in a dose-dependent manner, underscoring its impact on the translational process. These findings shed light on the broader understanding of PABP’s translation mechanism, demonstrating its ability to bind to viral RNA even in the absence of a terminal poly(A) tail. This insight illuminates the intricate regulatory mechanisms governing the translation of Dengue virus RNA and provides potential avenues for therapeutic interventions [ 47 ].
DENV and the host post-translational machinery
The genome of the Dengue virus initiates the formation of a single polypeptide through translation, subsequently processed to yield various functional and non-functional proteins. These proteins undergo post-translational modifications (PTMs), as depicted in Fig. 6 . Given DENV’s lack of its enzymatic machinery for PTMs, it relies on the host’s PTM system for survival [ 103 ]. These modifications involve the covalent addition of small protein molecules or functional groups to specific amino acid residues, including ubiquitination (addition of ubiquitin), lipidification (addition of lipid), glycosylation (addition of carbohydrate), and chemical group modifications such as hydroxylation, phosphorylation, acetylation, and methylation [ 49 ].
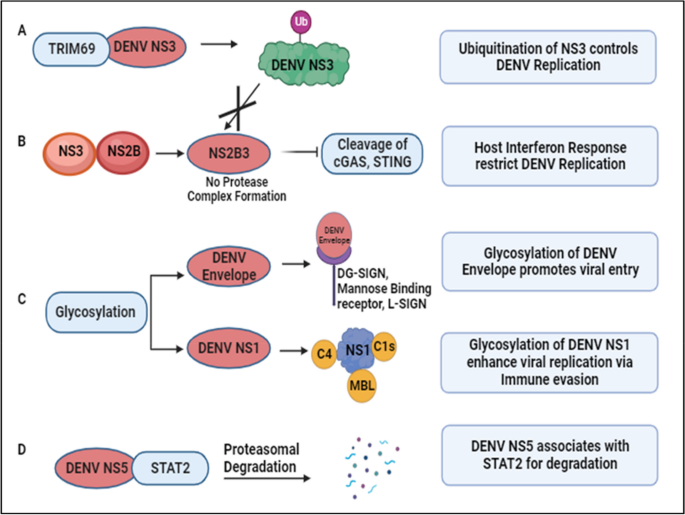
DENV and host post-translational regulation: A DENV NS3 interacts with TRIM69, induced as an Interferon Stimulated Gene (ISG), activating the ubiquitination pathway to restrict DENV replication [ 48 ]. B Conversely, ubiquitination of NS3 prevents the formation of the protease complex (NS2B3), inhibiting the cleavage of Interferon response-related genes, namely cGAS and STING. This results in an elevated host interferon response, targeting DENV replication [ 104 , 105 ]. C Glycosylation of DENV proteins aids attachment to host surface receptors and various complementary proteins, contributing to immune evasion and viral propagation [ 49 , 50 , 51 , 52 ]. D DENV NS5 interacts with STAT2, preventing its phosphorylation and targeting it for proteasomal degradation [ 53 , 106 ]
During infection, these modifications can exert either proviral or antiviral effects by activating or inhibiting proteins or modulating the immune response. An example is the TRIM family protein TRIM69, an interferon-stimulated gene (ISG). TRIM family members, pivotal in the innate immune pathway, play diverse roles from transcription to post-translation. During DENV infection, TRIM69 interacts with DENV NS3, activating the ubiquitination pathway and restricting DENV replication, as illustrated in Fig. 6 A [ 48 ]. Ubiquitination involves covalently attaching ubiquitin to the C-terminal glycine residue of the target protein. DENV NS3, in association with NS2B, forms a protease complex (NS2B3) that cleaves cyclic GMP-AMP synthase (cGAS) and Stimulator of Interferon Genes (STING). Ubiquitination of DENV NS3 prevents its proteolytic degradation, enhancing viral replication. However, reduced degradation of STING and cGAS enhances the interferon response, acting as host defense mechanisms against DENV as noted in Fig. 6 B [ 104 , 105 ]. To target the host cell, DENV utilizes the host’s glycosylation machinery for glycan conjugation on its proteins. Glycosylation, involving attaching glycan moieties to serine or threonine amino acid residues, plays critical roles in DENV entry, assembly, virulence, and pathogenicity. Receptors such as DC-SIGN, mannose-binding receptors, and L-SIGN receptors facilitate DENV entry by recognizing glycans on envelope proteins as shown in Fig. 6 C [ 49 , 50 , 51 ]. Glycosylated DENV NS1 contributes to protein secretion and stability, binding to complement components and preventing lectin complement activation and DENV neutralization, regulating DENV pathogenesis [ 52 ].
Studies reveal novel mechanisms of immune evasion and potential antiviral therapeutic targets. DENV NS5 associates with Signal Transducer and Activator of Transcription 2 (STAT2), a critical component in interferon signaling, preventing its phosphorylation and targeting it for proteasomal degradation, as depicted in Fig. 6 D [ 53 , 106 ]. Additionally, STAT3 upregulation and activation negatively regulate Type I and Type III interferon responses during DENV-2 infection, suggesting STAT3 as a proviral factor for DENV propagation and a potential antiviral target [ 54 ]. Through immunoprecipitation coupled with mass spectrometry analysis, Zhang et al. identified host proteins exhibiting differential ubiquitylation patterns in DENV-infected cells. Notably, they found that the lipid droplet-localized type-III membrane protein AUP1 undergoes deubiquitylation upon DENV infection. This virus-induced deubiquitylation leads to increased AUP1 levels and subsequent accumulation within autophagosomes. Importantly, AUP1 interacts with DENV NS4A protein, promoting viral production. Interestingly, a ubiquitin-modified mutant of AUP1 suppressed the interaction with NS4A, resulting in impaired lipophagy and defective viral replication. These findings implicate ubiquitination as a critical host regulatory mechanism that DENV modulates to facilitate its replication cycle. Further studies are needed to fully elucidate the ubiquitylation changes induced by flaviviral infection [ 107 ].
In a study employing MS-based phosphoproteomics, the global host phosphorylation profile was investigated for DENV [ 108 ], WNV [ 109 ], and ZIKV, revealing shared cellular pathways whose phosphorylation status alters across different flaviviral infections, affecting various RNA processing and metabolic pathways [ 110 ]. Using the same strategy, the authors identified 604 host proteins differentially phosphorylated in JEV infected human U251 glial cells. Importantly, pharmacological inhibition of the JNK1 pathway in mice infected with JEV resulted in reduced inflammatory cytokine secretion, lower viral load, and increased survival rates. This indicates that the JNK1 signaling pathway, modulated by JEV-induced phosphorylation events, plays a critical role in driving the inflammatory response and pathogenesis during JEV infection [ 108 ].
DENV and the host stress responses
DENV relies on host cellular processes for both its replication and translation. Upon infection, DENV intricately interacts with the host’s immune system, triggering a cascade of cellular and molecular responses aimed at combating the invading pathogen. Among these responses, host stress responses play a crucial role in modulating the outcome of DENV infection. Stress Granule (SG) formation is one of the consequences of cellular stress, heat shock, dysregulation of molecular mechanisms, and certain viral infections. Alterations in protein expression mechanisms results in SG assembly [ 111 ].
Molecules such as T cell intracellular antigen (TIA1), TIA-1 related protein (TIAR), and Ras-Gap-SH3 domain-binding protein (G3BP) are among the biomarkers of SG, regulated during cellular stress [ 112 , 113 ]. TIA1 and TIAR interact with DENV NS3, and colocalize in the perinuclear regions to enhance viral RNA formation and prevent SG formation, as shown in Fig. 7 A. In contrast TIA1 and TIAR interacts with WNV 3’ (-) SL RNA to facilitate the virus replication [ 55 ]. Additionally, one study reported that DENV UTRs interact with host cellular proteins such as DDX6, G3BP1, G3BP2, Caprin-1, and USP10 as noted in Table 1 [ 56 ]. These proteins play roles in SG formation, but during DENV infection, they colocalize with DENV RNA, specifically interacting with DENV 3’UTR, significantly influencing DENV replication [ 56 ]. In contrast, G3BP1 and Caprin-1 form a stable complex with the capsid protein of ZIKV to benefit virus replication [ 114 ]. Furthermore, the capsid protein of JEV inhibits (arsenite-induced) SG formation by interacting with the SG protein Caprin-1 [ 115 ]. DENV employs various strategies to hinder SG assembly to enhance its survival. Valosin-containing protein (VCP), a cellular ATPase, reportedly plays a crucial role in DENV replication. VCP, together with Nuclear Protein Localization 4 (NPL4), forms a complex to enhance DENV propagation and disassembles stress granule formation. DENV NS4B activates VCP to evade stress response, preventing SG formation, while simultaneously facilitating cellular translation to enhance DENV replication and translation [ 57 ].
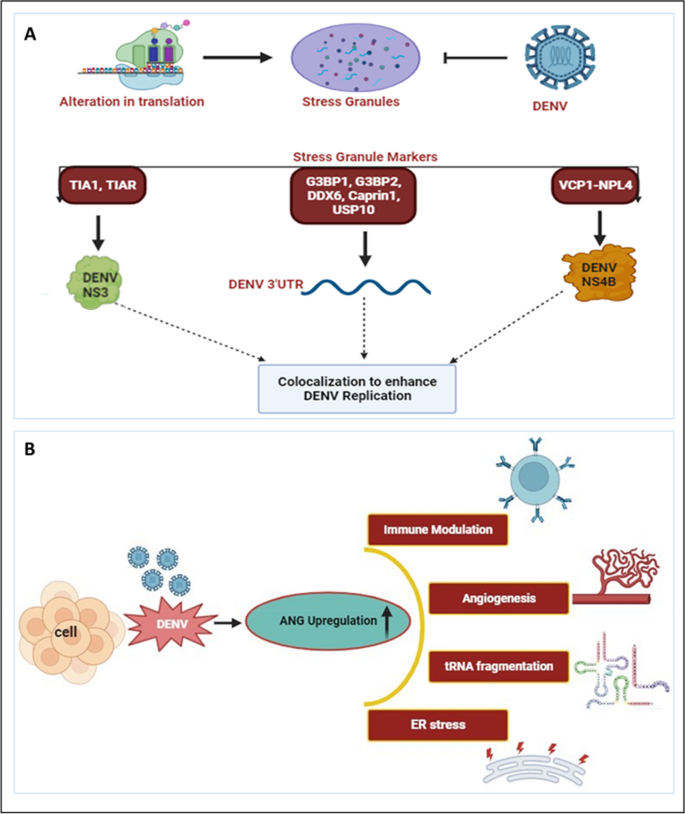
DENV and host stress responses: A Alterations in translation processes result in the assembly of stress granules. However, DENV prevents stress granule assembly by interacting with various stress granule markers. TIA1 and TIAR interact with DENV NS3, while various proteins such as G3BP1, G3BP2, DDX6, Caprin1, and USP10 bind to DENV 3’UTR. Additionally, VCP1, together with NPL4, forms a complex with DENV NS4B. These interactomes colocalize and participate in DENV replication [ 55 , 56 , 57 ]. B During DENV infection, Angiogenin levels have been found to be enhanced compared to uninfected controls. The upregulated Angiogenin may play a crucial role in various processes, including immune modulation, angiogenesis, tRNA fragmentation, and ER stress, among others [ 116 ]
Several studies have indicated the modulation of RNases upon DENV infection. The activity of various ribonucleases (RNases) such as DICER, ELAC2, Angiogenin produced during host stress conditions, including virus infection, results in the formation of various regulatory RNAs [ 117 ]. Recently we have reported the synergistic correlation between host ribonuclease Angiogenin expression and DENV replication. Angiogenin (also known as RNase 5) is a member of the Ribonuclease A superfamily. Our finding revealed that, angiogenin levels are elevated during DENV infection. Interestingly, angiogenin levels remain unaffected by siRNA knockdown, suggesting that dengue infection may directly regulate angiogenin expression. As illustrated in Fig. 7 B [ 116 ], upregulation of angiogenin during DENV infection may have a possible proviral effect, contributing to the replication and propagation of the dengue virus.
Dengue virus infection is known to induce endoplasmic reticulum (ER) stress, triggering signaling pathways associated with ER stress during viral infections. These pathways activate genes that support cell survival and anti-viral defenses. Viruses exploit the host’s cellular machinery, particularly the ER, to produce mature viral offspring and facilitate their replication. In response to the stress induced by alterations in protein production, the ER activates the Unfolded Protein Response (UPR) pathway to maintain ER homeostasis. Upon DENV infection, various UPR elements such as PERK, ATF6, and IRE1α are activated, which in turn activate innate immune factors, such as IRF3, PKR, and NF-κB. This approach might be employed to reduce viral infection. Thus, UPR components could serve as potential target of therapy for reducing DENV replication [ 118 , 119 ].
DENV-NS1 alters cellular behaviour to increase the secretion of extracellular vesicles (EVs) containing miR-148a. These EVs are internalized by human microglial cells, where miR-148a interacts with the 3’UTR region of the ubiquitin-specific peptidase 33 (USP33) protein, leading to its downregulation. Consequently, reduced USP33 expression results in decreased levels of the activating transcription factor 3 (ATF3) protein. ATF3 plays a crucial role in regulating genes involved in proinflammatory pathways, such as TNF-α, NF-κB, and IFN-β. This study elucidates how DENV exploits the EV pathway to deliver miR-148a, thereby modulating USP33 and ATF3 levels in human microglial cells, ultimately contributing to inflammation within the Central Nervous System (CNS) [ 58 ].
DENV and antiviral therapeutics
While there is currently no specific antiviral strategy approved to treat the dengue infection, ongoing research has explored various approaches to combat the infection. These strategies include investigating compounds with the potential to inhibit viral replication, immune modulators intended for enhancing the host’s immune response, and monoclonal antibodies designed to mimic the body’s natural defenses. Additionally, vaccine development has been a focus not only for prevention but also for potential therapeutic interventions. Several candidates are currently undergoing clinical trials as part of significant global efforts toward developing a preventive dengue vaccine. One of these, known as Dengvaxia, has been implemented and licensed in humans in several dengue-endemic countries. However, this vaccine has limitations, including approximately 60% efficacy and an association with antibody-dependent enhancement of disease severity in children, which impede its utilization. As of today, due to certain challenges, drug discovery efforts have not been able to provide any approved therapeutics to treat dengue virus infection [ 120 ].
Recently, Obi JO et al. described a novel small molecule inhibitor, JNJ-1802. JNJ-1802 target the DENV NS4B protein and hinders the NS3 and NS4B interplay, as shown in Fig. 8 A [ 121 ]. JNJ-1802 has demonstrated an effective barrier to resistance, in-vitro antiviral activity at low concentrations, and robust in-vivo efficacy in mice against four DENV serotypes. It has completed a phase I first-in-human clinical study in healthy individuals and was found to be safe [ 122 ]. Additionally, they also reported a compound, JNJ-A07, that exhibits antiviral activity against 21 clinical isolates of the dengue virus, spanning all known serotypes and genotypes of the dengue virus with a similar mechanism of action, but JNJ-1802 was selected over JNJ-A07 due to better preclinical safety profile [ 123 ]. Furthermore, several other DENV NS4B inhibitors have been developed, showing effectiveness as antivirals against dengue virus infection. Xie et al. demonstrated antiviral activity of NITD 618, with EC 50 values ranging from 1.0 to 4.1 µM. This compound functions by inhibiting RNA synthesis, but its poor pharmacodynamics make it challenging for further development as a drug against DENV infections [ 124 ]. Similarly, SDM25N, an opioid receptor antagonist, was identified, and its activity also relies on NS4B, restricting RNA genome replication [ 125 ]. Another group also identified a similar compound, AM404, whose interactions with NS4B are yet to be validated [ 126 ]. Additionally, a spiropyrazolopyridone compound was detected that likely binds to valine 63 (V63) residue of NS4B present in DENV-2 and DENV-3, and restricts virus replication in the stated serotypes [ 127 ]. Hence, this candidate known as compound-14a was considered a potential preclinical contender for future development. Similarly, phenotypic screening of a Novartis compound library with 1.5 million compounds led to the identification of 13,000 potential inhibitors, selecting a hit, NITD-688, that displayed EC 50 values of 8–38 nM inhibiting all DENV serotypes [ 128 ]. The compound’s interaction with NS4B was thoroughly characterized by NMR studies, demonstrating its potential for use in clinical research. Overall, these compounds validate DENV NS4B a validated target for the development of antivirals. It has been established that compounds or inhibitors that target DENV non-structural proteins interfere with viral replication and are possible targets for the development of antiviral treatments. Several drugs, namely Peptide 3, Peptide 4, Peptide 10, and Peptide 11, are involved in the inhibition of the NS1 protein as noted in Table 2 [ 129 ].
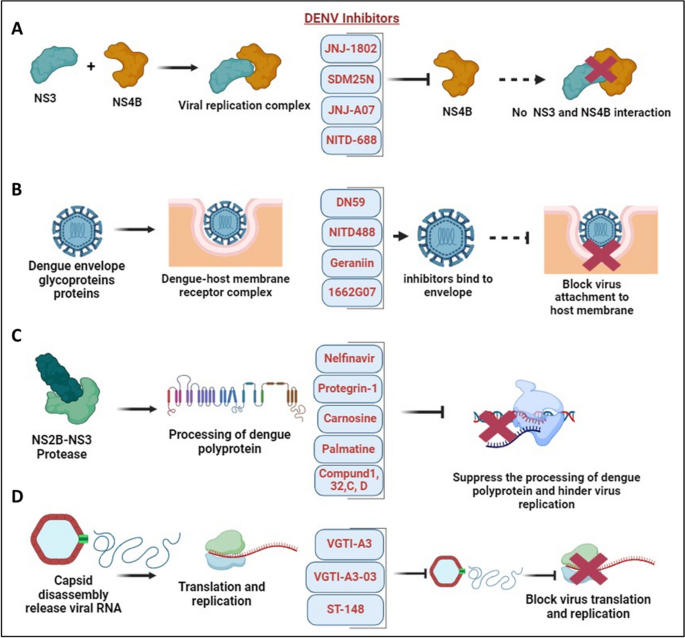
DENV and antiviral therapeutics: A Interaction between DENV NS3-NS4B in the formation of the virus replication complex. Several novel small molecule inhibitors, including JNJ-1802, JNJ-A07, SDM25N, and NITD-688, have been identified to inhibit NS4B, disrupting its interaction with NS3 and thereby suppressing DENV replication [ 122 , 125 , 128 ]. B DENV fusion inhibitors such as Geraniin, DN59, NITD-488, and 1662G07 bind with envelope protein thereby prohibiting virus attachment and entry into the host membrane [ 130 , 131 , 132 , 133 ]. C NS2B-NS3 protease complex participate in the processing of dengue polyprotein and supports virus replication. Inhibitors such as Nelfinavir, Protegrin-1, Carnosine, Palmatine, Compund 1, 32, C, D targets NS2B-NS3 protease complex and hinder virus replication [ 134 , 135 , 136 , 137 ]. D Dengue capsid protein undergoes capsid disassembly, releasing viral RNA for translation and replication. Inhibitors such as VGTI-A3, VGTI-A3-03, and ST-148 interact with the capsid protein, inducing antiviral effects and hindering dengue virus translation and replication [ 138 , 139 ]
In the pursuit of effective therapeutics against DENV, researchers have targeted specific viral proteins crucial for its replication within host cells. Among these targets, the NS3 helicase and NS2B-NS3 protease have garnered considerable attention due to their essential roles in viral replication. Ivermectin, a drug well known for its antiparasitic properties, has emerged as a promising candidate for DENV treatment. Suputtamongkol et al. [ 140 ] reported on a phase 2/3 clinical trial investigating Ivermectin’s efficacy, revealing its ability to suppress NS3 helicase and NS2B-NS3 protease activity, consequently hindering DENV replication in host cells as mentioned in Table 3 . Various compounds have been explored for their potential to inhibit NS3-helicase and NS2B-NS3 protease activity, thereby disrupting viral replication. Lee et al. [ 137 ] outlined several promising candidates, including ST-610 [ 141 ] and Suramin [ 142 ] as NS3-helicase inhibitors, alongside a range of compounds targeting NS2B-NS3 protease such as Protegrin-1 [ 134 ], Retrocyclin-1 [ 135 ], Nelfinavir, Carnosine, Palmatine, and Thiazolidinone-peptide hybrids. Additionally, compounds like Compound 32, Compound 1, 166347, ARDP0006, ARDP0009, Compound 7n, Diaryl(thio)ethers, Compound C, and Compound D have shown potential in inhibiting NS2B-NS3 protease activity [ 136 , 137 ].
In addition to this, several drugs are designed to target structural proteins, as described in Table 2 . For instance, 1662G07 and some analogs target the envelope protein as DENV fusion inhibitors [ 130 ]. Likewise, DN59 [ 131 ], and NITD448 [ 132 ] similarly inhibit the E-protein. Compound like Geraniin target the E-protein to inhibit virus binding and entry as shown in Fig. 8 B [ 133 ]. Furthermore, MLH40 inhibits the interplay between the envelope and the membrane proteins by specifically targeting the prM/M protein [ 143 ]. VGTI-A3 and VGTI-A3-03, by binding with the capsid protein, hinder the interplay between the DENV C protein and host intracellular lipid droplet, thereby disrupting the binding and thus prohibiting virus entry into cells [ 138 ]. A study by Xia et al. demonstrated the effect of a novel compound, ST-148, that inhibits the capsid protein of the dengue virus and interferes in virus replication as mentioned in Fig. 8 D. ST-148 interacts with the α1-α1′ helices region of the capsid protein, resulting in reduced viremia as well as low cytokine levels in the plasma of DENV-infected AG129 mouse model. One structural approach showed that ST-148 promotes two capsid dimers to engage in a “kissing” interaction [ 139 ]. However, a recent investigation reported that ST-148 inhibits DENV-2 but not DENV-1, DENV-3, and DENV-4. The antiviral and resistance mechanisms of ST-148 are yet unclear [ 148 ]. Pre-clinical data of some antiviral agents in the advance stage have shown potential effect against DENV. AT-752, an inhibitor of NS5 RdRp function, is in Phase 2 clinical trial as mentioned in Table 3 [ 145 ]. It reduced the viremia in immunocompromised mouse models infected with DENV. Ketotifen, a drug in Phase 4 clinical trial, reduced vascular leakage in a mouse model infected with DENV [ 147 ]. Zanamivir, and UV-4B as mentioned in Table 3 are still in the early phase of clinical trial [ 144 , 146 ]. Although many of these molecules and compounds are promising candidates for further development, significant limitations of these antivirals involve cytotoxicity and adverse effects. Moreover, clinical trials are crucial for evaluating the safety and efficacy of these antiviral therapies, and their success holds promise for advancing the treatment options available for dengue patients.
The root cause and progression of the disease are significantly influenced by the complex molecular interactions between the dengue virus and the host cellular machinery. In this review, we delve into the interplay between DENV and the host molecular machinery, focusing on epigenetic regulations, transcription, post-transcription, translation, and post-translation processes, including stress granules. These factors play a crucial role in shaping the evolution of an infection, determining the severity of symptoms, and influencing the host body’s defense system response. Epigenetic alterations such as DNA methylation, histone modifications, and involvement of various non-coding RNAs regulate DENV replication, immunological responses, and disease development. The virus exploits these alterations, manipulating host cellular components to facilitate its proliferation and evade immune responses. Transcriptional regulation is another essential aspect of dengue infection, as the virus manipulates the host’s transcriptional machinery to support its development and hinder immune responses. By appropriating key transcription factors and regulators, DENV modifies gene expression patterns, impacting both immune responses and viral pathogenesis.
Similarly, DENV exerts control over its own gene expression and alters the host cell environment through modulating post-transcriptional modifications, including alternative splicing and RNA degradation. Furthermore, at the translational level, DENV competes with host translation factors, employing techniques such as internal ribosome entry sites (IRES) to initiate translation and ensure efficient protein synthesis. Post-translational changes of both DENV and host proteins also play a role in viral replication, immune evasion, and host response regulation. Understanding these complex interactions at the molecular level is imperative for developing efficient diagnostic, therapeutic, and preventive measures against dengue virus infection. Targeting specific elements within these regulatory networks may open new avenues for therapeutic development and intervention, ultimately reducing the impact of this pervasive and often severe dengue infection.
Availability of data and materials
Not applicable.
Abbreviations
Dengue fever
Dengue hemorrhagic fever
Dengue shock syndrome
Non-steroidal anti-inflammatory medicines
Antibody-dependent enhancement
Antibody-dependent cellular cytotoxicity
Complement system
Untranslated Region
Cyclin dependent kinase 8
Hexokinase 2
C-C Motif Chemokine Receptor 10
C-terminal-binding protein antisense 1
MAF BZIP transcription factor G antisense RNA 1
Interferon gamma
Tumour necrosis factor α
CCCTC‐binding factor
Positive transcription elongation factor b
Nuclear factor kappa B
Interleukin-8
T-Cell Acute Lymphocytic Leukemia 1
Small nuclear ribonucleoproteins
CD2 cytoplasmic tail binding protein 2
DEAD box helicase 23
5’-3’ Exoribonuclease 1 protein
Retinoic acid-inducible gene I
Inhibitor of nuclear factor kappa-B kinase ε
Differentially expressed genes (DEGs)
RNA-binding proteins
Heterogeneous nuclear ribonucleoproteins
Sub genomic flavivirus RNA
Interferon-stimulated genes
Viral Suppressors of RNA Silencing
Heat shock protein family A (Hsp70) member 1A
MOV10-RISC complex RNA helicase
Upstream frameshift 1
Viral genomic RNA
RNA Interference
Eukaryotic translation initiation factor 4E
G3BP Stress Granule Assembly Factor 1
G3BP Stress Granule Assembly Factor 2
Cap binding complex
Internal ribosomal entry site
- Post-translational modifications
Tripartite motif family members
Cyclic GMP-AMP synthase
Stimulator of interferon genes
Dendritic cell specific ICAM3 grabbing non-integrin receptor
Mannose binding lectin
Signal transducer and activator of transcription 2
Histone deacetylase
DNA methyltransferase
Death inducer obligator 1
Stress granules
T cell intracellular antigen
TIA-1 related protein
Ras-Gap SH3 domain binding protein
Ubiquitin Specific Peptidase 10
Valosin containing protein
Nuclear protein localization 4
Extradomain A of fibronectin
B-cell lymphoma-extra-large
Cystic fibrosis transmembrane regulator
RNA-binding protein 10
Hu Antigen R
La- Related Protein 1
Cytoplasmic poly(A)-binding protein
Poly(A) Binding Protein Interacting Protein 2
PKR-like ER kinase
Activating transcription factor 6
Activating transcription factor 3
Inositol-requiring enzyme 1 alpha
Protein kinase R
Interferon regulatory transcription factor 3
Ubiquitin carboxyl-terminal hydrolase 33
Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Prim. 2016;2:1–26. https://doi.org/10.1038/nrdp.2016.55 .
Article Google Scholar
Tuiskunen Bäck A, Lundkvist Å. Dengue viruses – an overview. Infect Ecol Epidemiol. 2013;3(1):19839.
Google Scholar
Perera R, Kuhn RJ. Structural proteomics of dengue virus. Curr Opin Microbiol. 2008;11(4):369–77.
Article CAS PubMed PubMed Central Google Scholar
Byk LA, Gamarnik AV. Properties and functions of the dengue virus capsid protein. Annu Rev Virol. 2016;3:263–81.
Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186(8):1165–8.
Article CAS PubMed Google Scholar
Bhatt P, Sabeena SP, Varma M, Arunkumar G. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78(1):17–32. https://doi.org/10.1007/s00284-020-02284-w .
Gutsche I, Coulibaly F, Voss JE, Salmon J, D’Alayer J, Ermonval M, et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci U S A. 2011;108(19):8003–8.
Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7:304.
Lee WHK, Liu W, Fan JS, Yang D. Dengue virus protease activity modulated by dynamics of protease cofactor. Biophys J. 2021;120(12):2444–53. https://doi.org/10.1016/j.bpj.2021.04.015 .
McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J Biol Chem. 2011;286(25):22147–59.
Henderson BR, Saeedi BJ, Campagnola G, Geiss BJ. Analysis of RNA binding by the dengue virus NS5 RNA capping enzyme. PLoS One. 2011;6(10):1–9.
Tay MYF, Fraser JE, Chan WKK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013;99(3):301–6. https://doi.org/10.1016/j.antiviral.2013.06.002 .
Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, et al. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23(4):728–38.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. https://doi.org/10.1038/nature12060 .
Dwivedi VD, Tripathi IP, Tripathi RC, Bharadwaj S, Mishra SK. Genomics, proteomics and evolution of dengue virus. Brief Funct Genomics. 2017;16(4):217–27.
CAS PubMed Google Scholar
Madhry D, Pandey KK, Kaur J, Rawat Y, Sapra L, Kumar RYS, et al. Role of non-coding RNAs in dengue virus-host interaction. Front Biosci Sch. 2021;13(1):44–55.
Article CAS Google Scholar
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Nathan MB, et al. Europe PMC Funders Group Dengue: a continuing global threat Europe PMC Funders Author Manuscripts. Nat Rev Microbiol. 2010;8(12):7–16.
Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role inpathogenesis and application asadiagnostic biomarker. Antiviral Res. 2013;98(2):192–208. https://doi.org/10.1016/j.antiviral.2013.03.008 .
Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619.
Yacoub S, Mongkolsapaya J, Screaton G. The pathogenesis of dengue. Curr Opin Infect Dis. 2013;26(3):284–9.
Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol. 2021;67(10):687–702.
Wong JM, Adams LE, Durbin AP, Muñoz-Jordán JL, Poehling KA, Sánchez-González LM, et al. Dengue: a growing problem with new interventions. Pediatrics. 2022;149(6):e2021055522.
Article PubMed Google Scholar
Diamond MS, Pierson TC. Molecular insight into dengue virus pathogenesis and its implications for disease control. Cell. 2015;162(3):488–92. https://doi.org/10.1016/j.cell.2015.07.005 .
Simmons CP, McPherson K, Van Vinh CN, Hoai Tam DT, Young P, Mackenzie J, et al. Recent advances in dengue pathogenesis and clinical management. Vaccine. 2015;33(50):7061–8.
Yong YK, Wong WF, Vignesh R, Chattopadhyay I, Velu V, Tan HY, et al. Dengue infection - recent advances in disease pathogenesis in the era of COVID-19. Front Immunol. 2022;13(July):1–17.
Palanichamy Kala M, St. John AL, Rathore APS. Dengue: update on clinically relevant therapeutic strategies and vaccines. Curr Treat Options Infect Dis. 2023;15(2):27–52. https://doi.org/10.1007/s40506-023-00263-w .
Article PubMed PubMed Central Google Scholar
Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532–43.
Dinakaran D, Sreeraj VS, Venkatasubramanian G. Dengue and psychiatry: manifestations, mechanisms, and management options. Indian J Psychol Med. 2022;44(5):429–35.
Wang X, Xia H, Liu S, Cao L, You F. Epigenetic regulation in antiviral innate immunity. Eur J Immunol. 2021;51(7):1641–51.
Zhang Y, Guo J, Gao Y, Li S, Pan T, Xu G, et al. Dynamic transcriptome analyses reveal m6A regulated immune non-coding RNAs during dengue disease progression. Heliyon. 2023;9(1):e12690.
Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, et al. N6-methyladenosine in flaviviridae viral RNA genomes regulates infection. Cell Host Microbe. 2016;20(5):654–65. https://doi.org/10.1016/j.chom.2016.09.015 .
Ruggieri A, Helm M, Chatel-Chaix L. An epigenetic ‘extreme makeover’: the methylation of flaviviral RNA (and beyond). RNA Biol. 2021;18(5):696–708. https://doi.org/10.1080/15476286.2020.1868150 .
Henry BA, Kanarek JP, Kotter A, Helm M, Lee N. 5-Methylcytosine modification of an Epstein-Barr virus noncoding RNA decreases its stability. RNA. 2020;26(8):1038–48.
Courtney DG, Chalem A, Bogerd HP, Law BA, Kennedy EM, Holley CL, et al. Extensive epitranscriptomic methylation of A and C residues on murine leukemia virus transcripts enhances viral gene expression. MBio. 2019;10(3):1–12.
Caraballo GI, Rosales R, Viettri M, Castillo JM, Cruz R, Ding S, et al. The dengue virus nonstructural protein 1 (NS1) interacts with the putative epigenetic regulator DIDO1 to promote flavivirus replication in mosquito cells. J Virol. 2022;96(12):1–17.
Colpitts TM, Barthel S, Wang P, Fikrig E. Dengue virus capsid protein binds core histones and inhibits nucleosome formation in human liver cells. PLoS One. 2011;6(9):e24365.
Banerjee A, Tripathi A, Duggal S, Banerjee A, Vrati S. Dengue virus infection impedes megakaryopoiesis in MEG-01 cells where the virus envelope protein interacts with the transcription factor TAL-1. Sci Rep. 2020;10(1):1–12. https://doi.org/10.1038/s41598-020-76350-5 .
Lahon A, Arya RP, Banerjea AC. Dengue virus dysregulates master transcription factors and PI3K/AKT/mTOR signaling pathway in megakaryocytes. Front Cell Infect Microbiol. 2021;11(August):1–11.
Butler M, Chotiwan N, Brewster CD, DiLisio JE, Ackart DF, Podell BK, et al. Cyclin-dependent kinases 8 and 19 regulate host cell metabolism during dengue virus serotype 2 infection. Viruses. 2020;12(6):1–21.
Zaborowska J, Isa NF, Murphy S. P-TEFb goes viral. BioEssays. 2016;38:S75-85.
De Maio FA, Risso G, Iglesias NG, Shah P, Pozzi B, Gebhard LG, et al. The dengue virus NS5 protein intrudes in the cellular spliceosome and modulates splicing. PLoS Pathog. 2016;12(8):1–29.
Pozzi B, Bragado L, Mammi P, Torti MF, Gaioli N, Gebhard LG, et al. Dengue virus targets RBM10 deregulating host cell splicing and innate immune response. Nucleic Acids Res. 2020;48(12):6824–38.
Dickson AM, Wilusz J. Strategies for viral RNA stability: live long and prosper. Trends Genet. 2011;27(7):286–93.
Balinsky CA, Schmeisser H, Wells AI, Ganesan S, Jin T, Singh K, Zoon KC. IRAV (FLJ11286), an interferonstimulated gene with antiviral activity against dengue virus, interacts with MOV10. J Virol. 2017;91(5):10–128.
Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol. 2011;9(12):860–75.
Bidet K, Dadlani D, Garcia-Blanco MA. Correction: G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog. 2017;13(3):e1006295.
Polacek C, Friebe P, Harris E. Poly(A)-binding protein binds to the non-polyadenylated 3′ untranslated region of dengue virus and modulates translation efficiency. J Gen Virol. 2009;90(3):687–92.
Wang K, Zou C, Wang X, Huang C, Feng T, Pan W, et al. Interferon-stimulated TRIM69 interrupts dengue virus replication by ubiquitinating viral nonstructural protein 3. PLoS Pathog. 2018;14(8):1–24.
Pokidysheva E, Zhang Y, Battisti AJ, Bator-Kelly CM, Chipman PR, Xiao C, et al. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124(3):485–93.
Idris F, Muharram SH, Diah S. Glycosylation of dengue virus glycoproteins and their interactions with carbohydrate receptors: possible targets for antiviral therapy. Arch Virol. 2016;161(7):1751–60.
Yap SSL, Nguyen-Khuong T, Rudd PM, Alonso S. Dengue virus glycosylation: what do we know? Front Microbiol. 2017;8(JUL):1–16.
CAS Google Scholar
Thiemmeca S, Tamdet C, Punyadee N, Prommool T, Songjaeng A, Noisakran S, et al. Secreted NS1 protects dengue virus from mannose-binding lectin-mediated neutralization. J Immunol. 2016;197(10):4053–65.
Ashour J, Laurent-Rolle M, Shi P-Y, García-Sastre A. NS5 of dengue virus mediates STAT2 binding and degradation. J Virol. 2009;83(11):5408–18.
Srivastava S, Chaudhary N, Ojha A, Guchhait P, Patel AK. Signal transducer and activator of transcription 3 (STAT3) acts as a proviral factor for dengue virus propagation. Virus Res. 2021;300(March):198436. https://doi.org/10.1016/j.virusres.2021.198436 .
Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A. 2007;104(21):9041–6.
Ward AM, Bidet K, Yinglin A, Ler SG, Hogue K, Blackstock W, et al. Quantitative mass spectrometry of DENV-2 RNA-interacting proteins reveals that the DEAD-box RNA helicase DDX6 binds the DB1 and DB2 3’ UTR structures. RNA Biol. 2011;8(6):1173–86.
Arakawa M, Tabata K, Ishida K, Kobayashi M, Arai A, Ishikawa T, et al. Flavivirus recruits the valosin-containing protein-NPL4 complex to induce stress granule disassembly for efficient viral genome replication. J Biol Chem. 2022;298(3):101597. https://doi.org/10.1016/j.jbc.2022.101597 .
Mishra R, Lahon A, Banerjea AC. Dengue virus degrades USP33–ATF3 axis via extracellular vesicles to activate human microglial cells. J Immunol. 2020;205(7):1787–98.
De Aguiar GPCG, Da Silva Leite CMG, Dias B, Vasconcelos SMM, De Moraes RA, De Moraes MEA, et al. Evidence for host epigenetic signatures arising from arbovirus infections: a systematic review. Front Immunol. 2019;10(MAY):1–14.
Bartholomeusz A, Locarnini S. Associated with antiviral therapy. Antivir Ther. 2006;55(November 2005):52–5.
Shah PS, Link N, Jang GM, Sharp PP, Zhu T, Swaney DL, et al. Comparative flavivirus-host protein interaction mapping reveals mechanisms of dengue and Zika virus pathogenesis. Cell. 2018;175(7):1931-1945.e18.
Balinsky CA, Schmeisser H, Ganesan S, Singh K, Pierson TC, Zoon KC. Nucleolin interacts with the dengue virus capsid protein and plays a role in formation of infectious virus particles. J Virol. 2013;87(24):13094–106.
Li L, Li J, Gao M, Fan H, Wang Y, Xu X, et al. Interleukin-8 as a biomarker for disease prognosis of coronavirus disease-2019 patients. Front Immunol. 2021;11(January):1–10.
Murayama T, Kuno K, Jisaki F, Obuchi M, Sakamuro D, Furukawa T, Mukaida N, Matsushima K. Enhancement human cytomegalovirus replication in a human lung fibroblast cell line by interleukin-8. J Virol. 1994;68(11):7582–5.
Raghupathy R, Chaturvedi UC, Al-Sayer H, Elbishbishi EA, Agarwal R, Nagar R, et al. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol. 1998;56(3):280–5.
Li LL, Hu ST, Wang SH, Lee HH, Wang YT, Ping YH. Positive transcription elongation factor b (P-TEFb) contributes to dengue virus-stimulated induction of interleukin-8 (IL-8). Cell Microbiol. 2010;12(11):1589–603.
Wang X, Zhu J, Zhang D, Liu G. Ribosomal control in RNA virus-infected cells. Front Microbiol. 2022;13(November):1–15.
Weng SC, Tsao PN, Shiao SH. Blood glucose promotes dengue virus infection in the mosquito Aedes aegypti. Parasit Vectors. 2021;14(1):1–9. https://doi.org/10.1186/s13071-021-04877-1 .
Fontaine KA, Sanchez EL, Camarda R, Lagunoff M. Dengue virus induces and requires glycolysis for optimal replication. J Virol. 2015;89(4):2358–66.
Carlin AF, Plummer EM, Vizcarra EA, Sheets N, Joo Y, Tang W, et al. An IRF-3-, IRF-5-, and IRF-7-independent pathway of dengue viral resistance utilizes IRF-1 to stimulate type I and II interferon responses. Cell Rep. 2017;21(6):1600–12. https://doi.org/10.1016/j.celrep.2017.10.054 .
Ferrari M, Zevini A, Palermo E, Muscolini M, Alexandridi M, Etna MP, et al. Dengue virus targets Nrf2 for NS2B3-mediated degradation leading to enhanced oxidative stress and viral replication. J Virol. 2020;94(24):e01551-20.
Kaur J, Rawat Y, Sood V, Periwal N, Rathore DK, Kumar S, et al. Replication of dengue virus in K562-megakaryocytes induces suppression in the accumulation of reactive oxygen species. Front Microbiol. 2022;12(January):1–16.
Simon AY, Sutherland MR, Pryzdial ELG. Dengue virus binding and replication by platelets. Blood. 2015;126(3):378–85.
Han J, Xiong J, Wang D, Fu XD. Pre-mRNA splicing: where and when in the nucleus. Trends Cell Biol. 2011;21(6):336–43. https://doi.org/10.1016/j.tcb.2011.03.003 .
Chauhan K, Kalam H, Dutt R, Kumar D. RNA splicing: a new paradigm in host-pathogen interactions. J Mol Biol. 2019;431(8):1565–75. https://doi.org/10.1016/j.jmb.2019.03.001 .
Ashraf U, Benoit-Pilven C, Lacroix V, Navratil V, Naffakh N. Advances in analyzing virus-induced alterations of host cell splicing. Trends Microbiol. 2019;27(3):268–81. https://doi.org/10.1016/j.tim.2018.11.004 .
Boudreault S, Roy P, Lemay G, Bisaillon M. Viral modulation of cellular RNA alternative splicing: a new key player in virus–host interactions? Wiley Interdiscip Rev RNA. 2019;10(5):1–20.
Lightfoot HL, Hall J. Endogenous polyamine function - the RNA perspective. Nucleic Acids Res. 2014;42(18):11275–90.
Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol. 2015;427(21):3389–406. https://doi.org/10.1016/j.jmb.2015.06.020 .
Mounce BC, Cesaro T, Moratorio G, Hooikaas PJ, Yakovleva A, Werneke SW, et al. Inhibition of polyamine biosynthesis is a broad-spectrum strategy against RNA viruses. J Virol. 2016;90(21):9683–92.
Li MMH, MacDonald MR. Polyamines: small molecules with a big role in promoting virus infection. Cell Host Microbe. 2016;20(2):123–4. https://doi.org/10.1016/j.chom.2016.07.012 .
Mounce BC, Poirier EZ, Passoni G, Simon-Loriere E, Cesaro T, Prot M, et al. Interferon-induced spermidine-spermine acetyltransferase and polyamine depletion restrict Zika and Chikungunya viruses. Cell Host Microbe. 2016;20(2):167–77. https://doi.org/10.1016/j.chom.2016.06.011 .
Michalski D, Gustavo Ontiveros J, Russo J, Charley PA, Anderson JR, Heck AM, et al. Zika virus noncoding sfRNAs sequester multiple host-derived RNA-binding proteins and modulate mRNA decay and splicing during infection. J Biol Chem. 2019;294(44):16282–96.
Petit MJ, Kenaston MW, Pham OH, Nagainis AA, Fishburn AT, Shah PS. Nuclear dengue virus NS5 antagonizes expression of PAF1-dependent immune response genes. PLoS Pathog. 2021;17(11):1–23. https://doi.org/10.1371/journal.ppat.1010100 .
Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80(23):11418–31.
Shukla R, Ramasamy V, Shanmugam RK, Ahuja R, Khanna N. Antibody-dependent enhancement: a challenge for developing a safe dengue vaccine. Front Cell Infect Microbiol. 2020;10(October):1–12.
Narayan R, Tripathi S. Intrinsic ADE: the dark side of antibody dependent enhancement during dengue infection. Front Cell Infect Microbiol. 2020;10(October):1–6.
Viktorovskaya OV, Greco TM, Cristea IM, Thompson SR. Identification of RNA binding proteins associated with dengue virus RNA in infected cells reveals temporally distinct host factor requirements. PLoS Negl Trop Dis. 2016;10(8):1–22.
Abernathy E, Glaunsinger B. Emerging roles for RNA degradation in viral replication and antiviral defense. Virology. 2015;479–480:600–8. https://doi.org/10.1016/j.virol.2015.02.007 .
Ahmed MR, Du Z. Molecular interaction of nonsense-mediated mRNA decay with viruses. Viruses. 2023;15(4):816.
Kakumani PK, Rajgokul KS, Ponia SS, Kaur I, Mahanty S, Medigeshi GR, et al. Dengue NS3, an RNAi suppressor, modulates the human miRNA pathways through its interacting partner. Biochem J. 2015;471(1):89–99.
Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433(7025):477–80.
Villas-Bôas CSA, Conceição TM, Ramírez J, Santoro ABM, Da Poian AT, Montero-Lomelí M. Dengue virus-induced regulation of the host cell translational machinery. Brazilian J Med Biol Res. 2009;42(11):1020–6.
Bidet K, Garcia-Blanco MA. Flaviviral RNAs: weapons and targets in the war between virus and host. Biochem J. 2014;462(2):215–30.
Morais ATS, Terzian ACB, Duarte DVB, Bronzoni RVB, Madrid MCFS, Gavioli AF, et al. The eukaryotic translation initiation factor 3 subunit L protein interacts with Flavivirus NS5 and may modulate yellow fever virus replication. Virol J. 2013;10(1):1.
Davis WG, Blackwell JL, Shi P-Y, Brinton MA. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J Virol. 2007;81(18):10172–87.
Holden KL, Harris E. Enhancement of dengue virus translation: role of the 3′ untranslated region and the terminal 3′ stem-loop domain. Virology. 2004;329(1):119–33.
Edgil D, Polacek C, Harris E. Dengue virus utilizes a novel strategy for translation initiation when cap-dependent translation is inhibited. J Virol. 2006;80(6):2976–86.
Kieft JS, Zhou K, Jubin R, Doudna JA. Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA. 2001;7(2):194–206.
Verma B, Bhattacharyya S, Das S. Polypyrimidine tract-binding protein interacts with coxsackievirus B3 RNA and influences its translation. J Gen Virol. 2010;91(5):1245–55.
Zeng L, Falgout B, Markoff L. Identification of specific nucleotide sequences within the conserved 3′-SL in the dengue type 2 virus genome required for replication. J Virol. 1998;72(9):7510–22.
Bhattacharyya S, Verma B, Pandey G, Das S. The structure and function of a cis-acting element located upstream of the IRES that influences Coxsackievirus B3 RNA translation. Virology. 2008;377(2):345–54.
Kumar R, Mehta D, Mishra N, Nayak D, Sunil S. Role of host-mediated post-translational modifications (PTMS) in RNA virus pathogenesis. Int J Mol Sci. 2021;22(1):1–26.
Aguirre S, Luthra P, Sanchez-Aparicio MT, Maestre AM, Patel J, Lamothe F, et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat Microbiol. 2017;2(March):1–11.
Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8(10):e1002934.
Mazzon M, Jones M, Davidson A, Chain B, Jacobs M. Dengue virus ns5 inhibits interferon-a signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J Infect Dis. 2009;200(8):1261–70.
Zhang J, Lan Y, Li MY, Lamers MM, Fusade-Boyer M, Klemm E, et al. Flaviviruses exploit the lipid droplet protein AUP1 to trigger lipophagy and drive virus production. Cell Host Microbe. 2018;23(6):819-831.e5. https://doi.org/10.1016/j.chom.2018.05.005 .
Ye J, Zhang H, He W, Zhu B, Zhou D, Chen Z, et al. Quantitative phosphoproteomic analysis identifies the critical role of JNK1 in neuroinflammation induced by Japanese encephalitis virus. Sci Signal. 2016;9(448):1–16.
Zhang H, Sun J, Ye J, Ashraf U, Chen Z, Zhu B, et al. Quantitative label-free phosphoproteomics reveals differentially regulated protein phosphorylation involved in West Nile virus-induced host inflammatory response. J Proteome Res. 2015;14(12):5157–68.
Scaturro P, Stukalov A, Haas DA, Cortese M, Draganova K, Płaszczyca A, et al. An orthogonal proteomic survey uncovers novel Zika virus host factors. Nature. 2018;561(7722):253–7.
Guan Y, Wang Y, Fu X, Bai G, Li X, Mao J, et al. Multiple functions of stress granules in viral infection at a glance. Front Microbiol. 2023;14(March):1–11.
Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10(6):430–6. https://doi.org/10.1038/nrm2694 .
Xia J, Chen X, Xu F, Wang Y, Shi Y, Li Y, et al. Dengue virus infection induces formation of G3BP1 granules in human lung epithelial cells. Arch Virol. 2015;160(12):2991–9.
Hou S, Kumar A, Xu Z, Airo AM, Stryapunina I. crossm Zika virus hijacks stress granule proteins. J Virol. 2017;91(16):1–21.
Katoh H, Okamoto T, Fukuhara T, Kambara H, Morita E, Mori Y, et al. Japanese encephalitis virus core protein inhibits stress granule formation through an interaction with Caprin-1 and facilitates viral propagation. J Virol. 2013;87(1):489–502.
Madhry D, Malvankar S, Phadnis S, Srivastava RK, Bhattacharyya S, Verma B. Synergistic correlation between host angiogenin and dengue virus replication. RNA Biol. 2023;20(1):805–16. https://doi.org/10.1080/15476286.2023.2264003 .
Pandey KK, Madhry D, Ravi Kumar YS, Malvankar S, Sapra L, Srivastava RK, et al. Regulatory roles of tRNA-derived RNA fragments in human pathophysiology. Mol Ther - Nucleic Acids. 2021;26(December):161–73.
Diwaker D, Mishra KP, Ganju L. Effect of modulation of unfolded protein response pathway on dengue virus infection. Acta Biochim Biophys Sin (Shanghai). 2015;47(12):960–8.
Peña J, Harris E. Dengue virus modulates the unfolded protein response in a time-dependent manner. J Biol Chem. 2011;286(16):14226–36.
Silva JP, Fernandez-Sesma A. Challenges on the development of a dengue vaccine: a comprehensive review of the state of the art. J Gen Virol. 2023;104(3):1–12.
Obi JO, Gutiérrez-Barbosa H, Chua JV, Deredge DJ. Current trends and limitations in dengue antiviral research. Trop Med Infect Dis. 2021;6(4):1–19.
Goethals O, Kaptein SJF, Kesteleyn B, Bonfanti JF, Van Wesenbeeck L, Bardiot D, et al. Blocking NS3–NS4B interaction inhibits dengue virus in non-human primates. Nature. 2023;615(7953):678–86.
Kaptein SJF, Goethals O, Kiemel D, Marchand A, Kesteleyn B, Bonfanti JF, et al. Publisher Correction: A pan-serotype dengue virus inhibitor targeting the NS3–NS4B interaction (Nature, (2021), 598, 7881, (504-509), 10.1038/s41586-021-03990-6). Nature. 2021;599(7883):E2.
Xie X, Wang Q-Y, Xu HY, Qing M, Kramer L, Yuan Z, et al. Inhibition of dengue virus by targeting viral NS4B protein. J Virol. 2011;85(21):11183–95.
van Cleef KWR, Overheul GJ, Thomassen MC, Kaptein SJF, Davidson AD, Jacobs M, et al. Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antiviral Res. 2013;99(2):165–71. https://doi.org/10.1016/j.antiviral.2013.05.011 .
Van Cleef KWR, Overheul GJ, Thomassen MC, Marjakangas JM, Van Rij RP. Escape mutations in NS4B render dengue virus insensitive to the antiviral activity of the paracetamol metabolite AM404. Antimicrob Agents Chemother. 2016;60(4):2554–7.
Wang Q-Y, Dong H, Zou B, Karuna R, Wan KF, Zou J, et al. Discovery of dengue virus NS4B inhibitors. J Virol. 2015;89(16):8233–44.
Moquin SA, Simon O, Karuna R, Lakshminarayana SB, Yokokawa F, Wang F, et al. NITD-688, a pan-serotype inhibitor ofthe dengue virus NS4B protein, shows favorable pharmacokinetics and efficacy in preclinical animal models. Sci Transl Med. 2021;13(579):1–14.
Songprakhon P, Thaingtamtanha T, Limjindaporn T, Puttikhunt C, Srisawat C, Luangaram P, et al. Peptides targeting dengue viral nonstructural protein 1 inhibit dengue virus production. Sci Rep. 2020;10(1):1–16. https://doi.org/10.1038/s41598-020-69515-9 .
Schmidt AG, Lee K, Yang PL, Harrison SC. Small-molecule inhibitors of dengue-virus entry. PLoS Pathog. 2012;8(4):e1002627.
Hrobowski YM, Garry RF, Michael SF. Peptide inhibitors of dengue virus and West Nile virus infectivity. Virol J. 2005;2:1–10.
Wang QY, Patel SJ, Vangrevelinghe E, Hao YX, Rao R, Jaber D, et al. A small-molecule dengue virus entry inhibitor. Antimicrob Agents Chemother. 2009;53(5):1823–31.
Abdul Ahmad SA, Palanisamy UD, Tejo BA, Chew MF, Tham HW, Syed HS. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol J. 2017;14(1):1–13.
Rothan HA, Abdulrahman AY, Sasikumer PG, Othman S, Abd Rahman N, Yusof R. Protegrin-1 inhibits dengue NS2B-NS3 serine protease and viral replication in MK2 cells. J Biomed Biotechnol. 2012;2012:1–6.
Rothan HA, Han HC, Ramasamy TS, Othman S, Rahman NA, Yusof R. Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect Dis. 2012;12(1):1.
Tomlinson SM, Malmstrom RD, Russo A, Mueller N, Pang YP, Watowich SJ. Structure-based discovery of dengue virus protease inhibitors. Antiviral Res. 2009;82(3):110–4.
Lee MF, Wu YS, Poh CL. Molecular mechanisms of antiviral agents against dengue virus. Viruses. 2023;15(3):705.
Smith JL, Sheridan K, Parkins CJ, Frueh L, Jemison AL, Strode K, et al. Characterization and structure-activity relationship analysis of a class of antiviral compounds that directly bind dengue virus capsid protein and are incorporated into virions. Antiviral Res. 2018;155:12–9.
Xia H, Xie X, Zou J, Noble CG, Russell WK, Holthauzen LMF, et al. A cocrystal structure of dengue capsid protein in complex of inhibitor. Proc Natl Acad Sci U S A. 2020;117(30):17992–8001.
Suputtamongkol Y, Avirutnan P, Mairiang D, Angkasekwinai N, Niwattayakul K, Yamasmith E, et al. Ivermectin accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: a combined phase 2/3 randomized double-blinded placebo controlled trial. Clin Infect Dis. 2021;72(10):E586–93.
Byrd CM, Grosenbach DW, Berhanu A, Dai D, Jones KF, Cardwell KB, et al. Novel benzoxazole inhibitor of dengue virus replication that targets the NS3 helicase. Antimicrob Agents Chemother. 2013;57(4):1902–12.
Basavannacharya C, Vasudevan SG. Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format. Biochem Biophys Res Commun. 2014;453(3):539–44. https://doi.org/10.1016/j.bbrc.2014.09.113 .
Panya A, Sawasdee N, Junking M, Srisawat C, Choowongkomon K, Yenchitsomanus PT. A peptide inhibitor derived from the conserved ectodomain region of DENV membrane (M) protein with activity against dengue virus infection. Chem Biol Drug Des. 2015;86(5):1093–104.
Callahanid M, Trestonid AM, Lin G, Smith M, Kaufman B, Khaliq M, et al. Randomized single oral dose phase 1 study of safety, tolerability, and pharmacokinetics of Iminosugar UV-4 Hydrochloride (UV-4B) in healthy subjects. PLoS Negl Trop Dis. 2022;16(8):1–21. https://doi.org/10.1371/journal.pntd.0010636 .
Good SS, Shannon A, Lin K, Moussa A, Julander JG, La Colla P, et al. Evaluation of AT-752, a double prodrug of a guanosine nucleotide analog with in vitro and in vivo activity against dengue and other flaviviruses. Antimicrob Agents Chemother. 2021;65(11):e0098821.
Glasner DR, Ratnasiri K, Puerta-Guardo H, Espinosa DA, Beatty PR, Harris E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017;13(11):1–22.
St John AL, Rathore APS, Raghavan B, Ng ML, Abraham SN. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife. 2013;2013(2):1–18.
Byrd CM, Dai D, Grosenbach DW, Berhanu A, Jones KF, Cardwell KB, et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob Agents Chemother. 2013;57(1):15–25.
Download references
Acknowledgements
The authors acknowledge All India Institute of Medical Sciences, New Delhi, and Department of Biotechnology, AIIMS, New Delhi for the support.
This work was supported to BV from SERB (EEQ/2022/000362), DBT (BT/PR39859/MED/29/1519/2020). The BV lab is also supported by research grants provided by AIIMS (AC-31, A-948).
Author information
Authors and affiliations.
Department of Biotechnology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, 110029, India
Saumya Sinha, Kinjal Singh, Riya Roy, Sushant Phadnis, Varsha Meena & Bhupendra Verma
Department of Biotechnology, M. S. Ramaiah Institute of Technology, MSR Nagar, Bengaluru, India
Y. S. Ravi Kumar
Translational Health Science and Technology Institute, NCR Biotech Science Cluster, Faridabad, India
Sankar Bhattacharyya
You can also search for this author in PubMed Google Scholar
Contributions
SS, KS, RKYS, and B.V. contributed to the conception, design, analysis, and drafting of the manuscript. RR, SP, VM and SB contributed to the drafting of the manuscript. All authors approved the final version of the manuscript for submission.
Corresponding author
Correspondence to Bhupendra Verma .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sinha, S., Singh, K., Ravi Kumar, Y.S. et al. Dengue virus pathogenesis and host molecular machineries. J Biomed Sci 31 , 43 (2024). https://doi.org/10.1186/s12929-024-01030-9
Download citation
Received : 24 January 2024
Accepted : 14 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s12929-024-01030-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dengue virus
- Epigenomic regulation
- Transcription regulation
- Post-transcriptional modifications
- Translation regulation
- Stress granule formation
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
April 17, 2024
A Dengue Fever Outbreak Is Setting Records in the Americas
At least 2.1 million cases of dengue fever have been reported in North and South America, and this year 1,800 people have died from the mosquito-borne disease
By Francisco "A.J." Camacho & E&E News

A worker fumigates a house against the Aedes aegypti mosquito to prevent the spread of dengue fever in a neighborhood in Piura, northern Peru.
Ernesto Benavides/AFP via Getty Images
CLIMATEWIRE | At least 2.1 million people in North and South America have been infected this year with dengue — a record-setting figure that scientists attribute in part to climate change.
The Pan American Health Organization says there have been about 2.1 million confirmed cases of the potentially fatal disease in the Americas since January. That’s already more than the record-setting mark of 2 million confirmed cases for all of 2023.
And this year’s figure could be much higher. As many as 5.1 million people may have been infected in North and South America, according to the Pan American Health Organization, the United Nations agency in charge of international health cooperation in the Americas.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The outbreak has pushed Puerto Rico, Peru and nine of Brazil’s 26 states to declare states of emergency. More than 1,800 people in the Americas have died this year from dengue.
“We already have a large number of cases this year, not only in Brazil but also Paraguay and Argentina and other countries — even Uruguay and areas where there has been no transmission of dengue for a century,” said Pan American Health Organization director Jarbas Barbosa in a March press briefing.
Dengue typically causes short-term symptoms such as rashes and joint pain but the disease can be life-threatening in severe cases.
Mosquito bites spread the disease to humans, and public health experts say that warmer winters that don’t kill enough mosquitoes are one cause contributing to dengue outbreaks.
A compounding factor this year has been El Niño, a natural, temporary, and occasional warming of part of the Pacific that generates higher precipitation in much of the Americas.
Those elements — higher temperatures and more rain — are foundational for dengue outbreaks because they create the perfect breeding grounds for mosquitoes.
Barbosa, in his March briefing, cited a “combination of climate change and El Niño” as key factors of this year’s outbreak.
The number of dengue cases in North and South America has exploded over the past several decades. Dengue cases in the Americas are roughly five times higher in the 2020s than in the late 1990s.
A March study published in the journal Nature found that mosquito reproduction speed is “strongly influenced” by temperature and rainfall because mosquitoes die off in colder weather and precipitation makes puddles for mosquitoes to lay eggs.
“Every heat wave is a push that builds up dengue transmission,” said Christovam Barcellos, co-author of the Nature paper and senior researcher at Brazil-based Fiocruz research foundation. “There are more heat wave incidents in central Brazil, and that is the zone most affected by dengue now.”
Barcellos said heat waves mean not only more mosquitoes: “People change their behavior when a heat wave comes, they go out on the streets more” which increases their exposure to disease-carrying insects.
“It’s a complementary phenomenon,” he said.
While the United States often sees thousands of dengue cases annually, only about 6 percent are locally acquired while most are picked up during travel, according to data from the Centers for Disease Control and Prevention.
But this year, health experts worry dengue could hit the lower 48 hard.
“If a series of heat waves also come to the U.S., it can augment transmission,” Barcellos said.
Kacey Ernst, chair of the Department of Epidemiology and Biostatistics at the University of Arizona, shares Barcellos’ concern.
“We have had sporadic cases of locally acquired dengue in Florida and Texas for years now, and Arizona saw its first locally acquired case in 2022, so the potential is there,” Ernst said.
“I don’t often use words like explosion of transmission,” Ernst said. “But it seems to be an accurate description of dengue transmission this year.”
Reprinted from E&E News with permission from POLITICO, LLC. Copyright 2024. E&E News provides essential news for energy and environment professionals.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 23, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Dengue fever infections found to have negative impacts on infant health for three years
by University of Birmingham

Dengue infections in pregnant women may have a negative impact on the first years of children's lives, new research has found.
Dengue fever is the most prevalent mosquito-borne disease globally and poses a threat to half of the world's population. There has been a dramatic rise in cases over recent years, with cases in the Americas reaching more than three million cases in 2023. Since January 2024, Brazil has reported more than 3.5 million cases, marking the largest dengue outbreak on record.
The paper, co-authored by Dr. Livia Menezes from the University of Birmingham and Dr. Martin Foureaux Koppensteiner from the University of Surrey, has been published in the American Economic Journal: Applied Economics .
The study looks at a large data set of dengue fever infections in expectant mothers from Minas Gerais, Brazil, and the resulting birth outcomes. It finds that babies born to women who were infected with dengue fever during their pregnancy had lower birth weights, increasing the risk of newborns being classed as having a very and extremely low birth weight by 67% and 133%, respectively.
Dr. Livia Menezes, Assistant Professor in Economics at the University of Birmingham and co-author of the study, said, "Even though dengue is a very common mosquito-borne disease, there has not been much attention given to the impact it has on birth outcomes and as a result, what can be done to improve them and protect pregnant women and their children."
"This paper sets out robust research which shows that being infected with dengue fever, even with a mild case, while pregnant can have a significant impact on the health of the child once born. These birth outcomes can even have longer-term impacts, for example, previous research has shown that low birth weight can negatively affect socio-economic outcomes and health in adulthood."
The researchers also found that children whose mothers were infected with dengue fever while pregnant had a 27% increased risk of being hospitalized from birth to age three. The highest risk of hospitalization for these children comes in their second year of life, where there is a 76% increase.
Dr. Martin Foureaux Koppensteiner, Associate Professor in Economics at the University of Surrey, said, "These negative birth outcomes are not only limited to the health of individual children and mothers, but they have a much wider impact for communities where dengue fever is common. Hospitalizations and ongoing health issues resulting from maternal infections all have a cost, and one that could be avoided, or at least minimized with increased awareness and improved policy.
"We strongly suggest that dengue fever should be considered alongside the TORCH infections to manage and avoid when pregnant, which currently include Toxoplasmosis, Rubella, HIV, syphilis, chicken pox, Zika, and influenza among others."
The study also highlights the possible consequences of climate change expanding the reach of dengue fever. While the disease has historically been limited to tropical and subtropical regions, it now has an established presence in over 120 countries. Aedes mosquitoes, which carry and transmit dengue, have found breeding grounds in countries previously unaffected, including Croatia, France, Portugal, and the southern states of the U.S..
Dr. Menezes concludes, "As the planet heats, we can expect to see dengue fever become even more common in countries that have previously not had high infection rates. This is a problem that needs to be taken seriously and quickly.
"Policy changes and things like vector control, updated risk communication with key groups, and vaccine adoption can all reduce the risk of pregnant women being infected with dengue. Action needs to be taken by governments and health organizations to provide better protection for pregnant women and their children."
Explore further
Feedback to editors

Study highlights increased risk of second cancers among breast cancer survivors
28 minutes ago

Opioids during pregnancy not linked to substantially increased risk of psychiatric disorders in children

Research identifies pitfalls and opportunities for generative AI in patient messaging systems

A closed-loop drug-delivery system could improve chemotherapy
2 hours ago

It's easier now to treat opioid addiction with medication—but use has changed little, study finds

Solving the riddle of the sphingolipids in coronary artery disease
3 hours ago

New AI technology estimates brain age using low-cost EEG device

Alteration of brain network condition could predict painful vaso-occlusive crisis in patients with sickle cell disease
4 hours ago

Trials reveal that internet-based conversations help sustain brain function in older adults

Study finds that a dash of exercise can help students focus and enjoy university lectures
Related stories.

Maternal dengue fever: 'Compelling evidence' that even mild infections impact birth outcomes
Jul 13, 2023

Puerto Rico declares dengue epidemic as cases climb
Mar 28, 2024

Brazil to launch vaccination campaign as dengue surges
Jan 23, 2024
Dengue fever: An expert explains the mosquito-borne infection
Feb 21, 2024

Brazil launches dengue vaccination amid outbreak
Feb 10, 2024
Puerto Rico has declared an epidemic following a spike in dengue cases
Mar 26, 2024
Recommended for you

New algorithm could provide early warning for asthma attacks
5 hours ago

Researchers discover biology behind Fontan-operation-associated liver disease

Gene-based therapy restores cellular development and function in brain cells from people with Timothy syndrome
6 hours ago

COVID-19 pandemic alters view that doctors are obligated to provide care: Study
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Share full article
Advertisement
Supported by
Global Health
The Push for a Better Dengue Vaccine Grows More Urgent
A public research institute in Brazil has proved a new shot protects against the disease, but can’t make it fast enough to stop the huge outbreak sweeping Latin America.

By Stephanie Nolen
Stephanie Nolen has been covering the growing health threat from mosquitoes and the scientific quest for new solutions.
The outbreak of dengue fever that has unfolded in Latin America over the past three months is staggering in its scale — a million cases in Brazil in a matter of weeks, a huge spike in Argentina, a state of emergency declared in Peru and now another in Puerto Rico.
It forewarns of a changing landscape for the disease. The mosquitoes that spread dengue thrive in densely populated cities with weak infrastructure, and in warmer and wetter environments — the type of habitat that is expanding quickly with climate change.
More than 3.5 million cases of dengue have been confirmed by governments in Latin America in the first three months of 2024, compared with 4.5 million in all of 2023. There have been more than 1,000 deaths so far this year. The Pan-American Health Organization is warning that this may be the worst year for dengue ever recorded .
The rapidly shifting disease landscape needs new solutions, and researchers in Brazil delivered the lone shred of good news in this story with the recent announcement that a clinical trial of a new dengue vaccine, delivered in a single shot, had provided strong protection against the disease .
There are two existing vaccines for dengue, but one is an expensive two-shot regimen, while the other can be given only to people who have already had a dengue infection.
The new one-shot vaccine uses live, weakened forms of all four strains of the dengue virus. It was created by scientists at the National Institutes of Health in the United States and licensed for development by the Instituto Butantan, a huge public research institute in São Paulo.
Butantan will make the vaccine. It already produces most of the immunizations used in Brazil, and has the capacity to make tens of millions of doses of this new one. The institute plans to submit the dengue vaccine to Brazil’s regulatory agency for approval in the next few months and could begin producing it next year.
But that won’t help with this outbreak and, by the time the production gears up and a national rollout gets started, it may not be enough to help with the next one, either; dengue typically surges in three- or four-year cycles.
And it won’t necessarily be of help to the rest of Latin America: Butantan will only make the vaccine for Brazil. The multinational drug company Merck & Co., which also licensed the NIH technology, is developing a related vaccine which will be sold in the rest of the world; the efficacy of that vaccine has not yet been tested in a late-stage clinical trial.
And there is, of course, demand for a dengue vaccine beyond the Americas: Mosquitoes are spreading the disease to Croatia, Italy, California and other regions that haven’t seen it before. Places used to handling mild outbreaks now face record-breaking ones: Bangladesh had 300,000 cases last year.
Dengue is commonly known by the name breakbone fever, after the excruciating joint pain it causes. Not everybody experiences that pain: Three-quarters of people infected with dengue don’t have any symptoms at all, and among those who do, most cases resemble only a mild flu.
But about 5 percent of people who become sick will progress to what’s called severe dengue. Plasma, the protein-rich fluid component of blood, can start to leak out of blood vessels, causing patients to go into shock or have organ failure.
When patients with severe dengue are treated with blood transfusions and intravenous fluids, the mortality rate tends to be between 2 and 5 percent. But when they don’t get treatment — because they don’t realize it’s dengue and don’t seek treatment quickly enough, or because health centers are overwhelmed — the mortality rate is 15 percent.
In Brazil, the current dengue outbreak is hitting children hardest; those under 5 have the highest mortality rate of any age group, followed by those age 5 to 9. Adolescents between 10 and 14 have the highest number of confirmed cases, according to the Instituto Oswaldo Cruz , a national public health research center.
As clinics began to be overwhelmed with dengue patients in January, the Brazilian government bought the entire global stock of a Japanese-made vaccine for dengue called Qdenga. Public health nurses are delivering it to children ages 6 to 16, but there will be enough vaccine to fully vaccinate only 3.3 million of Brazil’s 220 million people this year.
This big national effort will protect a few million children, but it won’t contribute anything to its herd immunity.
Qdenga is not cheap: It’s about $115 per dose in Europe and $40 in Indonesia. Brazil is paying $19 per dose, having negotiated a lower price for its huge purchase.
Takeda Pharmaceuticals, which makes Qdenga, announced a deal last month with Biological E, a large Indian generic drug maker, to license and produce up to 50 million doses a year, part of a race to accelerate production. The Indian vaccine should cost considerably less. But Biological E is unlikely to have regulatory approval to market it before 2030; it’s a slow process that involves transferring technology, setting up a production line and getting a new version of even a well-known product approved by regulators.
Dengue costs Brazil at least $1 billion a year in health care treatment and lost productivity. And that figure doesn’t take into account the human suffering involved.
The fact that there are four different strains of the dengue virus complicates more than the process of making a vaccine: The potentially fatal form of the disease is more common when patients have a second infection, with a different strain than they had the first time. Qdenga protects against all four strains of dengue, and the hope is that the new Butantan vaccine does, too, although the data released so far shows it tested against only the two types that were circulating during the first part of the trial; more results are expected in June.
Millions more people will have been exposed to dengue when this outbreak finally passes. But they’re going to need that new vaccine more urgently than ever.
Stephanie Nolen is a global health reporter for The Times. More about Stephanie Nolen
- Open access
- Published: 24 April 2024
Dengue haemorrhagic fever in chronic kidney disease and heart failure: challenges in fluid management
- Manudi Vidanapathirana ORCID: orcid.org/0000-0002-0725-1238 1
Tropical Medicine and Health volume 52 , Article number: 33 ( 2024 ) Cite this article
Metrics details
Dengue haemorrhagic fever (DHF) is recognized to have high mortality in patients with chronic kidney disease (CKD) and heart failure (HF). They are at high risk of shock during the ascending limb of the critical phase of DHF, fluid overload during convalescence and bleeding throughout the entire illness. Physiological changes and medications used in CKD/HF make the diagnosis and monitoring of DHF difficult. Treatment with standard fluid regimens also poses a challenge due to the propensity for fluid overload. As a result, standard dengue guidelines do not provide recommendations on fluid management regimens in DHF with CKD/HF. This article provides a narrative review on the existing evidence for management of DHF in patients with volume-changed states such as HF, CKD and nephrotic/ nephritic syndromes. It will explore the relevant diagnostic and therapeutic dilemmas, acknowledge the challenges for developing guidelines and recommend strategies to improve fluid management in these groups of patients.
Introduction
Dengue haemorrhagic fever (DHF) is a severe presentation of dengue, characterized by plasma leakage and, at times, haemorrhagic manifestations [ 1 ]. The plasma leakage occurs during a 48-h period known as the critical phase, and it is evidenced by a rise in haematocrit of at least 20% [ 1 , 2 ]. Critical phase usually starts approximately 3–5 days after onset of fever [ 1 ]. The critical phase consists of an initial 24-h period called the ascending limb, during which the rate of plasma leakage may gradually increase, and a subsequent 24-h period, during which the rate of leakage gradually declines [ 1 ]. This is followed by a convalescent period characterized by plasma reabsorption and return to homeostasis [ 1 ]. The concern during the critical phase is intravascular volume depletion and shock, and the concern during the convalescence phase is fluid overload [ 1 , 2 ]. Haemorrhagic manifestations may occur at any point of the febrile illness in DHF [ 1 ].
Standard guidelines on the management of DHF highlight the need for adhering to precisely calculated fluid regimens [ 1 , 2 , 3 , 4 , 5 ]. The generally recommended fluid quota for 48 h in DHF is the maintenance fluid combined with 5% of deficit, which usually amounts to 4600 millilitres in an individual weighing 50 kg or above [ 1 ]. The need for such strict fluid regimens in DHF is for the purpose of striking a careful balance between prevention of shock in the leaking phase and prevention of fluid overload in the convalescence phase [ 2 ]. Existing guidelines provide recommendations on the fluid quota and the rate of fluid administration to be followed in healthy individuals with DHF during the critical phase. However, no guideline explores the fluid administration to be followed in DHF in patients with changed intra- and extravascular volume states, such as in heart failure (HF) or chronic kidney disease (CKD). Existing guidelines acknowledge that patients with HF and CKD are in the high-risk category and must be admitted to receive in-patient fluid management, but there is no further guidance given in this regard.
Fluid administration in volume-changed states is problematic due to two reasons. Firstly, CKD and HF are both conditions that are prone to fluid overload, in the form of pulmonary oedema and systemic venous congestion [ 6 ]. This risk is higher with anuric or oliguric patients with CKD. When these individuals are treated with the same fluid regimens as those used for DHF in healthy individuals, they are at high risk of developing fluid overload. Secondly, despite the risk of fluid overload, these individuals are still at risk of shock during the leaking phase, if an adequate fluid quota is not administered. Naturally, the question arises as to how fluid administration should be guided in these patients to achieve this delicate balance.
This article provides a narrative review on the existing evidence for management of DHF in patients with volume-changed states such as CKD, nephrotic and nephritic syndromes and HF. It will explore the relevant diagnostic and therapeutic dilemmas, acknowledge the challenges for developing guidelines and recommend certain strategies to improve fluid management in these groups of patients.
A literature search was conducted on the topic using Google Scholar and Medline from February–March 2024. The following Boolean operators were used to focus the search, and only articles in English were included.
"dengue haemorrhagic fever" AND "chronic kidney disease" NOT "acute-kidney-injury"
“dengue haemorrhagic fever” AND “heart failure”
For the first search item, there were 361 results. Duplicated articles, irrelevant articles, articles on acute kidney injury (AKI) secondary to dengue and articles on dengue in renal transplant recipients were excluded. After exclusion, 13 articles were left for analysis.
The second search item yielded 1660 results. Duplicated articles, irrelevant articles, articles on heart failure/ myocarditis/ cardiomyopathy secondary to dengue and articles without a focus on fluid management were excluded. After exclusion, 1 article was included in the review.
Implications of CKD and HF on the pathophysiology of DHF
Plasma leakage in DHF occurs due to increased capillary permeability secondary to virus-related immune dysregulation [ 2 ]. Antibody-dependent viral enhancement is a recognized mechanism which increases the likelihood of plasma leakage and occurrence of DHF, in a secondary infection [ 7 ]. Once the virus enters macrophages facilitated by previously formed antibodies, a cytokine cascade comprising of interleukins comes in to play [ 7 ]. This is responsible for endothelial injury and vascular permeability that consequently results in leakage.
Cytokines such as tumour necrosis factor (TNF)-alpha and interleukin (IL)-6 are responsible for endothelial injury in DHF, and these are reported to be increased in patients with CKD [ 8 ]. This increase in cytokines, combined with the intrinsic uraemic milieu of CKD which further aggravates endothelial dysfunction, can increase the risk and amount of DHF-related plasma leakage in CKD [ 8 ]. Additionally, uraemia in CKD reduces thrombogenecity through dysfunction of platelets and von Willebrand factor [ 8 ]. This can in turn increase the haemorrhagic manifestations of DHF in patients with CKD.
A study carried out by Lee et al. in 2019 identified that impaired immunological mechanisms occur in end-stage renal disease (ESRD) patients with dengue [ 9 ]. It was found that levels of several cytokines, including IL-8, IL-10, IL-12p40, TNF-α, monocyte chemoattractant protein 1, vascular endothelial growth factor and granulocyte macrophage colony-stimulating factor, were significantly lower in the ESRD population than in the control group. Viral load cycle threshold values were also significantly lower in the ESRD group at 6 h and 24 h post-infection. No significant difference in the viral load cycle threshold values between the two groups was found at 48 h and 72 h post-infection. However, this was conducted as an in vitro study, and its translation into clinical practice, in terms of affecting the clinical progression of DHF in CKD, is not very clear.
Increased mortality in DHF with heart disease and CKD
Several studies conducted among dengue patients, both with and without plasma leakage, have concluded that mortality in those with heart disease and CKD is higher compared to healthy controls [ 10 ]. Reasons for the increased mortality in these groups may be manifold, such as propensity for haemodynamic fluctuations, risk of bleeding and use of drugs such as anti-hypertensives, diuretics and antiplatelet agents that can worsen complications in dengue [ 11 , 12 ]. Improper fluid management regimens may also contribute to increased mortality in these groups, and it may be an important modifiable factor that can be optimized, to target a reduction in mortality.
A study done by Lee et al. published in 2023 assessed characteristics of 138 dengue patients with CKD [ 11 ]. This study included patients with and without plasma leakage and demonstrated a high mortality rate of 46.3% (n = 64) among dengue patients with CKD. The stages of CKD of the patients who died were stages 2 and 3 in 34.4%(n = 22), stages 4 and 5 in 51.5% (n = 33) and end-stage renal disease on dialysis in (4.1% (n = 9)). However, the group consisting of patients with ESRD was underrepresented. There were 57 (41.3%) and 58 patients (42.02%), respectively, in the groups for CKD stages 2–3 and stages 4–5, but only 23 (16.7%) patients for the group containing patients with ESRD. The underrepresentation of ESRD patients in this study may have accounted for the seemingly lower mortality seen in the ESRD group. When fatality is calculated for each group, deaths comprised 38.5% in CKD stages 2–3, 56.9% in stages 4–5 and 39.13% in ESRD. Complications experienced by the patient that died in the CKD group were gastrointestinal bleeding (56.3% (n = 36)), severe hepatitis (40.6% (n = 26)), pneumonia (25%(n = 16)), pulmonary oedema (12.5%(n = 8)) and bacteraemia (9.4% (n = 6)). The median time from symptom onset to death was 6 days. Risk factors associated with high mortality were altered level of consciousness at presentation, pulmonary oedema, leucocytosis and severe hepatitis.
Another study in 2023 corroborates the increased mortality seen in dengue patients with CKD [ 12 ]. This study included 433, 802 dengue patients, both with and without plasma leakage. Of the total number, 0.5% (n = 2134) had a diagnosis of CKD. Details on the stages of CKD in these patients were not given. Characteristics observed more commonly in the CKD group were tachycardia, haemodynamic fluctuations, increased capillary refill time, pulmonary oedema with respiratory failure and higher rates of severe bleeding (e.g. haematemesis).
Both studies described above show that CKD patients with dengue were more at risk of haemodynamic fluctuations and gastrointestinal bleeding. While only the latter study comments on the occurrence of intravascular volume depletion, both studies have noted an increased occurrence of pulmonary oedema in the CKD group.
With regard to heart disease, a study published in 2017 has recognized heart disease as an independent predictor of severe dengue and severe organ involvement in dengue [ 13 ]. Mechanisms hypothesized for this these adverse manifestations are endothelial dysfunction and increased nitrous oxide contributing to the cytokine storm and increased vascular permeability. It is recognized that renal autoregulation is offset, if cardiac output in a patient already having heart disease/ heart failure is further compromised during dengue, thus increasing the risk of kidney injury.
Diagnostic and therapeutic challenges of DHF in CKD and heart disease
Factors that distinguish DHF from uncomplicated dengue are presence of capillary leakage resulting in extravascular fluid accumulation, development of extensive petechae/ ecchymosis, overt bleeding, marked thrombocytopenia, hypoalbuminemia, elevated liver enzymes and hyponatremia [ 8 ]. Several of these factors are seen in CKD patients as part of CKD itself and therefore may not help to characterize the severity of dengue in this population. Thrombocytopenia is seen in CKD patients due to uraemia-related marrow suppression, or heparin-induced thrombocytopenia [ 8 ]. Petechae may be seen in CKD patients due to inherent thrombocytopenia, thrombasthenia or increased vascular fragility [ 8 ]. Hyponatremia is seen in CKD due to dilutional hyponatremia in hypervolaemic states, and hypoalbuminemia is seen in CKD due to an imbalance between synthesis and loss [ 14 , 15 ].
A rise in haematocrit is used in dengue management to identify the onset of leakage in DHF [ 2 ]. However, CKD carries with it certain confounders that limit the utility of haematocrit as a useful marker of leakage in this population. A rise in haematocrit unrelated to leaking may be seen in these patients due to the use of diuretics with a restrictive fluid regimen, for fear of causing fluid overload [ 16 , 17 ]. This may lead to a false diagnosis of DHF. Additionally, as reiterated above, CKD patients are at high risk of bleeding during DHF, which may mask a rise in haematocrit and may lead to leakage being overlooked [ 17 ].
A study carried out by Thomas et al. in 2018 among renal transplant recipients and patients with CKD showed that there was increased haemoconcentration among the CKD group, compared to the transplant group and control group, with normal renal functions [ 18 ]. It is postulated that reluctance for fluid administration in the CKD group may have accounted for this finding.
Nadir WBC count was lowest in renal transplant recipients when compared to CKD patients and control group, hypothesized to be due to bone marrow suppression due to the use of mycofenolate mofetil and azathioprine in their immunosuppressive regime. This may also account for the longer time taken for normalization of platelet count in renal transplant recipients. Occurrence of bleeding manifestations was seen in a total of 7 patients across all groups and was not statistically different between the 3 groups.
A study carried out by Chen et al. in 2019 identified certain other difficulties associated with diagnosis and treatment of dengue in ESRD patients on haemodialysis [ 19 ]. One reported diagnostic problem was dismissal of symptoms like vomiting and abdominal pain, as markers of severe dengue, since these symptoms which are common among ESRD patients on dialysis were likely to be attributed to uraemia-/ dialysis-related symptoms. Treatment problems encountered were the development of hypervolaemia and pulmonary oedema, with the use of the fluid regimen recommended by World Health Organization, and the increased occurrence of intradialytic hypotension. Expectedly, bleeding manifestations were more frequently noted.
According to standard dengue guidelines, acidosis, bleeding, hypocalcaemia and hypoglycaemia are factors that cause refractory shock in DHF and lead to the development of dengue shock syndrome (DSS, 1). All four factors occur more commonly in CKD patients, due to CKD itself, and this must be borne in mind treated accordingly, in CKD patients with shock in dengue.
No studies have commented on the limitations of individual vital parameters in monitoring of DHF in CKD, but these can be anticipated from the physiological changes that occur in CKD. Blood pressure which is high CKD due to arterial calcification and volume overload may be maintained until significant capillary leakage has taken place in DHF and therefore may be an insensitive marker of intravascular volume depletion [ 20 ]. Urine output too is a poor marker due to the oliguric/ anuric state exhibited by some patients with CKD.
There are no studies focused on the diagnostic and therapeutic dilemmas among patients with heart failure in dengue, but some challenges can be estimated from the physiological changes occurring in HF. Factors confounding the diagnosis of DHF in heart failure are the baseline existence of pleural effusions and ascites in congestive cardiac failure and the baseline presence of hypoalbuminemia and hypercholesterolemia [ 21 , 22 ]. Haematocrit may be misleading in heart failure, due to the use of diuretics, fluid restriction as part of management in heart failure, and due to increased risk of bleeding with dengue due to antiplatelet use. Vital parameters such as pulse rate, blood pressure and pulse pressure may be misleading depending on the patient’s baseline ejection fraction and use of medications altering these parameters [ 23 ]. The same applies for urine output.
Various trials have demonstrated the limited clinical utility of haemodynamic parameters such as central venous pressure and pulmonary capillary wedge pressure in guiding fluid responsiveness in any condition requiring fluid resuscitation [ 24 ]. This is primarily due to the varying effects on these parameters by cardiac contractility. As a result, dynamic parameters such as stroke volume variation, pulse pressure variation and change in vena cava diameter have been proposed to guide fluid therapy. Due to the lack of superiority of a single variable in clinical studies, a combination of these is recommended in the context of the clinical picture. However, further assessment of the utility of these parameters in DHF is needed before recommendations can be made.
Use of inferior vena cava (IVC) collapsibility has been shown to correlate with the rise in haematocrit in DHF and dengue shock syndrome, and the use of IVC collapsibility has therefore been reported to be a better marker for guiding fluid therapy than haematocrit [ 25 ]. This may be especially true in instances when haematocrit may misleadingly not show a rise, due to combined leaking and bleeding. The use of the sum of pleural and peritoneal fluid volumes is not recommended to be used to guide fluid therapy, since it is likely to represent an underestimation of the true value [ 26 ].
In terms of fluid administration, the battle between crystalloids and colloids remains age old [ 24 ]. For healthy individuals that develop DHF, it is recommended to use crystalloids for fluid management and reserve the use of colloids for certain special instances [ 2 ]. Colloids are indicated in DHF when there is persistent shock despite the use of two crystalloid boluses, shock in the descending limb of the critical phase with features of fluid overload and signs of shock with near completion of the fluid quota [ 2 ]. However, the use of 0.9% saline, which is the most commonly used crystalloid, is known to be associated with hyperchloraemic metabolic acidosis [ 24 ]. This can worsen the pre-existing metabolic acidosis in renal failure and result in detrimental consequences such as renal vasoconstriction, further decrease in glomerular filtration rate and negative inotropy [ 24 ]. There is currently no research available to recommend alternate crystalloids or colloids over the use of 0.9% saline in dengue in patients with CKD [ 24 ]. The use of crystalloids therefore remains the standard of practice [ 24 ]. A randomized controlled trial carried out among 230 Vietnamese children with DSS has shown that Ringer Lactate (RL) had the longest recovery time, compared to normal saline, gelatin and dextran [ 27 ]. There is a theoretical risk of worsening hyperkalaemia and acidosis with RL in CKD patients due to its constituents [ 27 ]. This makes it a questionable choice for this population. While colloids have been shown to perform better in shock states in some studies, the ‘reverse osmotic’ effect where the osmotically active colloid leaks out and worsens capillary leakage needs to be considered [ 27 ]. If as hypothesized earlier, vascular endothelial dysfunction and risk of leakage are in fact higher in the CKD population, the author infers that colloids may not be a suitable intravenous fluid for this population [ 8 ].
Case reports on the fluid management of DHF in volume-changed states
There are 4 case reports/series which focus on fluid management of DHF in CKD [ 16 , 17 , 28 , 29 ].
A case series by Kuo et al. outlines the progression of 3 patients with CKD who developed DHF and proceeded DSS and death [ 28 ]. Problems with diagnosis existed for all 3 patients since the suspicion of dengue had been low, and initial symptoms had been attributed to uraemia. The diagnosis had only been entertained later and proven serologically. All 3 patients had also developed acute liver injury and gastrointestinal bleeding, as part of DSS. The fluid regimen followed in these 3 patients is not given in the case series, but it could possibly be presumed that since the patients developed DSS, there might have been initial under-filling, due to the general restrictive intake of CKD. Under-replacement of fluids may have continued for a significant duration of the illness due to low suspicion of dengue. All 3 patients had also received blood products in the form of fresh-frozen plasma (FFP) and red cell concentrate. It is possible that transfusion of FFP to these patients with CKD may have served as a risk factor for overfilling and pulmonary oedema during the convalescence phase. Only one patient had been dialyzed, and the risks of worsening haemodynamic stability in an already unstable patient are highlighted through this. While this case series does not reveal much in terms of fluid management, which is the focus of the current review, it is useful since it draws attention to maintaining vigilance for dengue in CKD patients in high-prevalence areas and showcases the detrimental one-way path that ensues when DHF is not recognized and managed properly. On another note, these patients had received desmopressin as a treatment for bleeding. Whether desmopressin is useful in terms of preventing or treating bleeding in dengue with CKD needs more research, and its unpredictable effects in terms of water retention, and potential for fluid overload in the descending limb and convalescent stage make its use questionable.
Another case report by Lim et al. in 2019 outlines the progression of a patient with ESRD developed DF and had signs of intravascular volume depletion at the time of admission [ 16 ]. When he reached a cumulative intake of 2500 ml, he started showing signs of leakage, with development of pleural effusions. Regular haemodialysis was continued, with the ultra-filtrate being guided by volume status and phase of dengue. The ultra-filtrate has had to be increased during the recovery phase, although the exact volumes are not disclosed. The fluid regime that was used was 0.35 mL/kg/h (500 mL) of 0.9% saline over 24 h, 0.9% l saline 3 mL/kg/h (180 mL/h) for 4 h, 0.9% saline 1 mL/kg/h (60 mL/h) for 3 h, then off drip to encourage oral intake of 50–100 mL hourly. It was planned to convert to colloids if intravascular hypovolemia persisted or consider blood transfusion if signs of overt bleeding developed, but neither was required in this patient. Clinical assessment of fluid status was done by regular monitoring of vital signs, charting of input output and assessment of skin turgor and mucosa. Investigations that were used for assessment of volume status were haematocrit, ultrasonic assessment of inferior vena caval collapsibility and ultrasonic assessment of the third spaces to detect pleural effusion and ascites. The article comments on other available invasive methods such as monitoring of central venous pressure, central venous oxygen saturation, cardiac output, pulmonary arterial pressure, mixed venous oxygen saturation and stroke volume variation, which may be required if a patient becomes critically ill requiring micromanagement of fluid and tissue perfusion, but appears to not have used them for the patient reported. This article also recommends temporarily withholding antihypertensive therapy and restarting them during the recovery phase.
Additionally, it makes recommendations on practicing heparin-free dialysis until the platelet count rises to 100, stopping blood thinning agents when the platelet count drops below 100 and temporarily stopping azathioprine/ mycophenolate mofetil due to their myelosuppresive effects. It recommends to double the dose of steroids to prevent Addisonian crisis, in those who are on long-term steroids.
A case report from Sri Lanka outlines the case of a patient with CKD stage 3A due to lupus nephritis [ 17 ]. The patient has presented with nephrotic syndrome and subsequently developed DHF complicated with bleeding and fluid overload. The diagnostic challenges in this case were recognition of DF in a patient with lupus-related cytopenias and identification of leakage in a patient with effusions and ascites due to nephrotic syndrome. The main management challenge was determining the amount and rate of fluid administration. Due to the presence of baseline lupus-related cytopenias, platelet counts could not be used as a marker of severe dengue in this patient. The baseline presence of extravascular fluid in the form of pleural effusions and ascites, and the presence of per vaginal bleeding obscuring a haematocrit rise, made it difficult to diagnose leakage in this patient. In her case, DF was diagnosed with the use of NS1 antigen which is more than 99% specific for dengue, and leakage was diagnosed with the timing of transition from febrile to afebrile status without clinical improvement and tenderness in the right hypochondrium. Fluid management followed a restrictive protocol. However, the total fluid quota of 4600 ml calculated for her ideal body weight was theoretically allowed, in case features of shock developed. Furosemide and albumin which were part of the management of nephrotic syndrome in this patient were continued during the critical phase. A total amount of 3150 ml of crystalloid fluid was used during the critical phase and she did not develop signs of shock. However, despite the use of a restrictive fluid regime and the continuation of diuretics, she developed fluid overload during the descending limb. This required intubation with mechanical ventilation and continuous renal replacement therapy.
A case report from India published in 2024 describes the challenging case of a 16-year-old female with ESRD on regular haemodialysis, who developed dengue fever [ 29 ]. The dengue fever was serologically confirmed. This case was complicated by the rapid development acute respiratory distress syndrome (ARDS) which intensified the already existent challenges of fluid management. It is unclear from the case whether the patient had uncomplicated dengue or DHF. There is mention of bilateral pulmonary shadows with minimal pleural effusions on a chest x-ray, but this could have been accounted for by ARDS. A point-of-care ultrasound has also been performed, but there is no comment on the presence or absence of pleural effusions or ascites. Fluid therapy in this patient has been guided by IVC collapsibility index. Although the exact amounts of fluid used are not mentioned, sufficient intravenous fluid has been administered to reduce the IVC collapsibility index from more than 50% to less than 30%. Intravenous crystalloids had been used for resuscitation. There are two factors that have complicated fluid management in this case, apart from the challenge imposed by ESRD. First is that, there appears to have been additional sepsis confounding the clinical picture. The patient has had dysuria and right sided flank pain, possibly indicating pyelonephritis. The white cell count has been 30,000/mm 3 , which also favours a diagnosis of bacterial sepsis. In fact, the authors themselves have managed the patient for sepsis with intravenous antibiotics and inotropic support. Therefore, the fluid requirements in this case may have been increased by the presence of sepsis. The second additional challenge to fluid management is the development of ARDS. ARDS may worsen the burden of fluid overload due to the migration of fluid into the alveoli. It is thus a dilemma in this case to decide on the approach to fluid therapy. While the exact amounts of fluid given to this patient are not disclosed in the final report, the authors suggest a protocol of goal directed resuscitation during the shock phase, followed by a more restrictive protocol once shock has resolved. This patient has also been given sustained low efficiency dialysis in an ICU, but has succumbed to the illness. The authors recommend to use both IVC parameters in the form of diameter and collapsibility index, and point-of-care lung ultrasound to guide fluid therapy.
These published cases illustrate the importance of being vigilant of dengue and highlight the increased risk of bleeding, DSS and fluid overload that occurs in CKD patients with dengue.
Three of the cases give an insight in to the fluid regimen. It is evident from the third case that while a restrictive regimen was able to circumvent shock, it still resulted in fluid overload [ 17 ]. However, not all patients may be able to circumvent shock with a restrictive approach, and adhering steadfastly to a restrictive protocol in patients with severe leaking during the critical phase may increase the risk of shock and mortality. Accordingly, the fourth case favours a more liberal approach during the period of shock, followed by a more restrictive approach after mitigation of shock [ 29 ]. This carries with a risk of fluid overload during the descending limb and convalescence of the critical phase. The author deduces from this that the fluid quota would need to change in patients with CKD, but hourly administration rates need to take in to account the real-time risk of shock versus overload.
There is only one published study on fluid management of DHF in heart failure [ 30 ].
A case report from Malaysia describes the management of dengue, without capillary leakage in a patient with heart failure [ 30 ]. The ejection fraction of the patient was not included in the report. Dengue management comprised of input output monitoring, daily input of 1 L of fluid and continuation of oral furosemide 40 mg daily. The plan was to continue the same fluid regime if the urine output was more than 500 ml per day and weight gain per day was 1 kg or less. However, it is mentioned that the patient had fluid losses in the form of vomiting and diarrhoea initially.
It is difficult to glean information that can be synthesized into recommendations from this case since it did not have plasma leakage. However, it is reasonable to speculate that an alternate, less-restrictive fluid regimen may have been required in case of plasma leakage. The patient was on diuretics and had volume losses in the form of vomiting and diarrhoea, which if combined with plasma leakage may have resulted in shock and DSS, if continued on a restrictive regimen.
Challenges for establishing guidelines and strategies proposed
The main problem of management of DHF in the volume-shifted state is the difficulty in striking the balance between adequate filling to prevent shock and avoidance of overfilling. Additional factors interfering with this balance are the use of medications such as anti-hypertensive medications and diuretics, and the increased risk of bleeding. The author recognizes the difficulty in having a generic guideline for this population, due to the individual variations in the degree of volume shifting, differences in types and doses of medications used and oliguric/anuric state seen only in some patients.
In spite of these factors, the author proposes the following strategies to better guide fluid management in this population:
Maintenance of vigilance for dengue and DHF in high prevalence areas. One must be careful not to attribute certain symptoms such as vomiting and abdominal pain to uraemia alone, if associated with fever.
Involvement of a multidisciplinary team with physicians, infectious disease specialists, nephrologists, cardiologists, intensivists and transfusion physicians was relevant for management of DHF in CKD and HF.
Recognition of the limitations of diagnosis of plasma leakage in DHF in CKD and HF.
Pleural effusions, ascites, rise in haematocrit, hypoalbuminemia and hypercholesterolemia may not be useful in detecting leakage since these may already exist at baseline in patients with CKD and HF. Therefore, other markers recommended in standard guidelines may have to be relied upon e.g. settling of fever with a platelet drop to less than 1,000,000/μL, tenderness in the right hypochondrium and clinical deterioration with settling of fever [ 2 ].
Recognizing that the guideline recommended fluid quota might be harmful for patients with HF/CKD and deciding on an individual fluid quota using the clinicians’ judgement of risk of shock versus fluid overload
Use of all vital parameters in context of each other and the clinical picture during DHF monitoring, due to the following limitations:
Pulse rate: Inability to mount a tachycardia due to the use of beta blockers
Blood pressure and pulse pressure: Low baseline blood pressure and pulse pressure in heart failure due to low ejection fraction, maintenance of systolic blood pressure till the last stages of shock in CKD due to high baseline blood pressure and arterial calcification
Urine output: Misleading urine output to the use of diuretics and the inherent oliguric/anuric nature
Vital parameters may change due to worsening of the original comorbidity (e.g. worsening of heart failure, nephrotic syndrome) rather than due to severe dengue
Use of investigation markers in context of each other and the clinical picture, due to the following limitations:
Thrombocytopenia may be present at baseline in CKD
Haematocrit rise may not always be present in CKD and heart disease patients with leaking, due to high risk of co-existent bleeding
Haematocrit rise may be seen due to non-leaking related reasons, such as inadequate fluid administration and use of diuretics
Hypoalbuminemia and hypercholesterolemia, which are markers of leaking, may be seen at baseline in heart failure, CKD and nephrotic syndrome
Considering the use of IVC collapsibility in combination with vital parameters, haematocrit and the clinical context to guide fluid therapy
Guiding hourly fluid administration rates bearing the above principles in mind.
Rate of leakage in DHF generally follows the shape of an inverted V, with a rising rate in the ascending limb of the critical phase with maximal leakage at the peak and a decreasing rate in the descending limb with cessation of leakage by 48 hours [ 2 ]. Hourly fluid administration in DHF can vary from 2 to 10 millilitres (ml) per kilogram (kg) per hour (h, 2). The hourly administration rate will need to be adjusted based on the assessment of degree of leakage as indicated by vital parameters and haematocrit [ 2 ].
Stable patients will have normal vital parameters with a minimal rise in haematocrit. They can be managed with 2 ml/kg/h of fluids [ 2 ].
Unstable patients can be in pre-shock or shock [ 2 ]. Pre-shock is indicated by cold extremities, prolonged capillary refill time, tachycardia, tachypnea, narrowing of pulse pressure and postural hypotension [ 2 ]. Compensated shock is indicated by a narrowing of pulse pressure to 20 mmHg or less [ 2 ]. Decompensated shock is indicated by reduced systolic blood pressure to less than 90 mmHg or a reduction of 20% of the baseline systolic blood pressure [ 2 ]. Profound shock is indicated by undetectable blood pressure [ 2 ].
Maximal rate of fluid leakage that occurs in shock is 10 ml/kg which is the amount required by patients with shock [ 2 ]. This can be given over 1 h in compensated shock, or as a fast bolus in decompensated and profound shock [ 2 ].
A rate of administration in between 3 and 7 ml/kg can be used for patients in pre-shock, depending on the degree of derangement of vital parameters, rise in haematocrit and the response to fluids administered in the preceding hour [ 2 ]. It must be borne in mind that rapid leakage only lasts for a short time, and the requirement for fluid in between 5 and 7 ml/kg/h will be quite short [ 2 ]. After the mitigation of this period, stabilization of vital parameters and haematocrit should prompt clinicians to gradually reduce the hourly rate of fluid administration in a stepwise manner [ 2 ]. It should also be noted that all parameters should be assessed collectively, and decisions should not be based on a single parameter individually [ 2 ]. Fluid administration that is increased merely for the purpose of maintaining urine output and reducing haematocrit in patients with otherwise stable vital parameters can lead to fluid overload [ 2 ].
During the descending limb too, monitoring must continue as in the ascending limb [ 2 ]. Provided that parameters remain stable, the author suggests that reduction in fluid administration to less than 2 ml/kg/h can be attempted at the physician’s discretion to prevent fluid overload.
Renal replacement therapy in the form of sustained low efficiency dialysis or continuous renal replacement therapy may be needed during the critical phase, depending on the presence of overload and other indications for dialysis [ 17 , 29 ].
The author speculates that a focus on mitigation of shock rather than fluid overload may be more important during the ascending limb of the critical phase [ 29 ]. Unresolved shock may lead to organ failures including acute liver injury with liver failure, deterioration of renal function, disseminated intravascular coagulation and death [ 2 ]. An observational study in Sri Lanka that assessed fluid requirements of patients with DHF showed that there were some DHF patients who required fluid in excess of the calculated fluid quota (more than maintenance and 5% of deficit), sometimes requiring more than 7.5% of the deficit [ 31 ]. Surprisingly, the incidence of fluid overload was not as frequent as expected, but was seen more in individuals that exceeded more than 7.5% of deficit in terms of fluid administration. The study does not comment on the comorbidities of these individuals, but presumably had been conducted among healthy individuals. Therefore, their reduced tendency for fluid overload cannot be translated in to that of the CKD or HF population. Use of such large amounts of fluid will inevitably result in fluid overload, in these special populations [ 6 ]. But this study is important in showing that some patients with DHF have severe leakage, and therefore, their fluid requirements may exceed calculated or predicted values [ 31 ]. In such patients, care must be taken with fluid management with an aim of preventing shock.
On a final note, the author emphasizes the importance of management of pre-shock and shock using the above principles, hourly monitoring with special attention to trends in vital parameters and use of clinician discretion in reducing hourly fluid administration during the descending limb and the use of renal replacement therapy when necessary.
Being mindful of the occurrence of acidosis, bleeding, hypocalcaemia and hypoglycaemia, which patients with CKD are prone to and may cause refractory shock
Considering withdrawal of anti-hypertensive medications and diuretics during the ascending limb of the leaking phase, if blood pressure is marginal
Considering re-initiation of diuretics during the descending limb of the leaking phase to prevent fluid overload
Considering withdrawal of antiplatelet medications once platelet count is below a certain level according to clinician judgement of bleeding risk versus thrombotic risk. Re-initiation can be considered once platelets are seen to be rising and above a reasonable level deemed by the managing physician.
Transfusion of blood according to the indications given in standard guidelines—consider transfusion under diuretic cover if fluid overload is deemed a valid risk
Adjustment of the ultra-filtrate in the routine dialysis prescription according to the volume status in the patient
Use of heparin free dialysis until the platelet count is seen to be rising and above a reasonable level as per physician discretion.
The author proposes these as suggestions for consideration, rather than recommendations, due to the individual differences in comorbidities and dengue progression.
Gaps in research
There is further evidence required on the clinical utility of invasive modalities of haemodynamic monitoring, choice of intravenous fluid due to the problems associated with use of 0.9% saline in CKD and the clinical utility of desmopressin to treat bleeding due to its effects on intravascular volume.
Conclusions
Diagnosis and management of DHF in CKD and HF pose various challenges. Clinicians should recognize the need for dynamic, individual-specific fluid quotas based on risk of shock versus fluid overload. Volume status of patients must be regularly assessed to guide fluid administration, with recognition of the caveats of standard parameters.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Acute respiratory distress syndrome
- Chronic kidney disease
- Dengue haemorrhagic fever
Dengue shock syndrome
End-stage renal disease
Fresh-frozen plasma
- Heart failure
Interleukin
Inferior vena cava
Millilitres
Ringer lactate
Tumour necrosis factor
Tetralogy of Fallot
Kalayanarooj S. Clinical manifestations and management of Dengue/DHF/DSS. Trop Med Health. 2011;39(4 Suppl):83–7. https://doi.org/10.2149/tmh.2011-S10 .
Article PubMed PubMed Central Google Scholar
National Epidemiology Unit. Guidelines on the Management of Dengue and Dengue Haemorrhagic Fever in Adults 2012. https://www.epid.gov.lk/web/images/pdf/Publication/guidelines_for_the_management_of_df_and_dhf_in_adults.pdf . Accessed 21 Feb 2024.
Kalayanarooj S, Nimmannitya S. Guidelines for dengue hemorrhagic fever case management. Bangkok: Bangkok Medical Publisher; 2004.
Google Scholar
Verdeal JC, Costa Filho R, Vanzillotta C, Macedo GL, Bozza FA, Toscano L, Prata A, Tanner AC, Machado FR. Guidelines for the management of patients with severe forms of dengue. Rev Bras Ter Intensiva. 2011;23(2):125–33.
Article PubMed Google Scholar
Tayal A, Kabra SK, Lodha R. Management of dengue: an updated review. Indian J Pediatr. 2023;90(2):168–77. https://doi.org/10.1007/s12098-022-04394-8 .
Ralapanawa DMPUK, Kularatne SAM. Current management of denguein adults: a review. Int Med J Malaysia. 2015;14(1):29–42.
Bhatt P, Sabeena SP, Varma M, Arunkumar G. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78(1):17–32. https://doi.org/10.1007/s00284-020-02284-w .
Article CAS PubMed Google Scholar
Kuo MC, Lu PL, Chang JM, Lin MY, Tsai JJ, Chen YH, Chang K, Chen HC, Hwang SJ. Impact of renal failure on the outcome of dengue viral infection. Clin J Am Soc Nephrol. 2008;3(5):1350–6. https://doi.org/10.2215/CJN.00020108 .
Lee IK, Yang ZS, Ng HY, et al. Impaired production of immune mediators in dengue virus type 2-infected mononuclear cells of adults with end stage renal disease. Sci Rep. 2019;9:19783. https://doi.org/10.1038/s41598-019-56381-3 .
Article CAS PubMed PubMed Central Google Scholar
Toledo J, George L, Martinez E, Lazaro A, Han WW, Coelho GE, Runge Ranzinger S, Horstick O. Relevance of non-communicable comorbidities for the development of the severe forms of dengue: a systematic literature review. PLoS Negl Trop Dis. 2016;10(1): e0004284. https://doi.org/10.1371/journal.pntd.0004284 .
Lee I, et al. In-hospital mortality predictors among hospitalized adults and those with chronic kidney disease with dengue. J Microbiol Immunol Infect. 2023;56(5):996–1006. https://doi.org/10.1016/j.jmii.2023.08.004 .
Daher EF, et al. WCN23–1247 CKD associated with increased mortality in dengue fever. Kidney Int Rep. 2023;8(3):S39–40. https://doi.org/10.1016/j.ekir.2023.02.090 .
Article Google Scholar
Pang J, Hsu J, Yeo T, et al. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: a matched case-control study. Sci Rep. 2017;7:39872. https://doi.org/10.1038/srep39872 .
Lim LM, Tsai NC, Lin MY, Hwang DY, Lin HY, Lee JJ, Hwang SJ, Hung CC, Chen HC. Hyponatremia is associated with fluid imbalance and adverse renal outcome in chronic kidney disease patients treated with diuretics. Sci Rep. 2016;14(6):36817. https://doi.org/10.1038/srep36817 .
Article CAS Google Scholar
Haller C. Hypoalbuminemia in renal failure: pathogenesis and therapeutic considerations. Kidney Blood Press Res. 2005;28(5–6):307–10. https://doi.org/10.1159/000090185 .
Lim XM, Lim CT. Dengue fever in end-stage renal failure patient: case report and updated literature review in the diagnostic and management challenges. Infect Dis Clin Pract. 2019;27(6):310–4. https://doi.org/10.1097/IPC.0000000000000761 .
Vidanapathirana M, Atukorala I. Dengue hemorrhagic fever with bleeding and fluid overload in a patient with active lupus nephritis: a case report of diagnostic and therapeutic challenges. BMC Infect Dis. 2023;23(1):433. https://doi.org/10.1186/s12879-023-08415-5 .
Arun Thomas ET, George J, Sruthi D, Vineetha NS, Gracious N. Clinical course of dengue fever and its impact on renal function in renal transplant recipients and patients with chronic kidney disease. Nephrology. 2018. https://doi.org/10.1111/nep.13265 .
Chen H-J, Tang H-J, Chin-Li L, Chien C-C. Warning signs and severe dengue in end stage renal disease dialysis patients. J Microbiol Immunol Infect. 2020;53(6):979–85. https://doi.org/10.1016/j.jmii.2019.08.005 .
Palit S, Kendrick J. Vascular calcification in chronic kidney disease: role of disordered mineral metabolism. Curr Pharm Des. 2014;20(37):5829–33. https://doi.org/10.2174/1381612820666140212194926 .
Porcel JM. Pleural effusions from congestive heart failure. Semin Respir Crit Care Med. 2010;31(6):689–97. https://doi.org/10.1055/s-0030-1269828 .
Grodin JL, Lala A, Stevens SR, DeVore AD, Cooper LB, AbouEzzeddine OF, Mentz RJ, Groarke JD, Joyce E, Rosenthal JL, Vader JM, Tang WH. Clinical implications of serum albumin levels in acute heart failure: insights from DOSE-AHF and ROSE-AHF. J Card Fail. 2016;22(11):884–90. https://doi.org/10.1016/j.cardfail.2016.01.015 .
Cautela J, Tartiere JM, Cohen-Solal A, Bellemain-Appaix A, Theron A, Tibi T, Januzzi JL Jr, Roubille F, Girerd N. Management of low blood pressure in ambulatory heart failure with reduced ejection fraction patients. Eur J Heart Fail. 2020;22(8):1357–65. https://doi.org/10.1002/ejhf.1835 .
Srisawat N et al. Critical care in dengue management South East Asian Journal of Tropical Medicine and Public Health. 2017; 48 (Supplement 1). https://www.tm.mahidol.ac.th/seameo/2017-48-suppl-1/SAJ-2017-48-suppl-1-171.pdf .
Chatterjee R, Halder P, Samajdar SS, Ghosal P, Mukherjee S, Jana K, Saha B. Correlation between hematocrit and inferior vena cava collapsibility in adult patients with dengue fever: a prospective, observational study at a tertiary care setup in Eastern India. MRIMS J Health Sci. 2023. https://doi.org/10.4103/mjhs.mjhs_85_23 .
Kodikara I, Gamage DTK. Revisit to sonography in dengue management: a review. Sri Lanka J Radiol. 2018;4(1):1–15. https://doi.org/10.4038/sljr.v4i1.69 .
Ngo NT, Cao XT, Kneen R, Wills B, Nguyen VM, Nguyen TQ, Chu VT, Nguyen TT, Simpson JA, Solomon T, White NJ, Farrar J. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis. 2001;32(2):204–13. https://doi.org/10.1086/318479 .
Kuo M-C, et al. Difficulty in diagnosis and treatment of dengue hemorrhagic fever in patients with chronic renal failure: report of three cases of mortality. Am J Trop Med Hygiene. 2007;76(4):752–6.
Prithvi D, Kumar A, Kumar A. Fluid management strategy in dengue acute respiratory distress syndrome with chronic kidney disease: a case report. J Indira Gandhi Inst Med Sci. 2024;10(1):62–4. https://doi.org/10.4103/jigims.jigims_50_23 .
Devaraj NK. Managing dengue fever in heart failure: a case report. Iranian Red Crescent Med J. 2018;20(2):1–4. https://doi.org/10.5812/ircmj.60822 .
Madanayake P, Jayawardena A, Wijekoon SL, et al. Fluid requirement in adult dengue haemorrhagic fever patients during the critical phase of the illness: an observational study. BMC Infect Dis. 2021;21:286. https://doi.org/10.1186/s12879-021-05971-6 .
Download references
Acknowledgements
Author information, authors and affiliations.
Professorial Medical Unit, National Hospital of Sri Lanka, Colombo, Sri Lanka
Manudi Vidanapathirana
You can also search for this author in PubMed Google Scholar
Contributions
MV involved in conceptualization and writing the main manuscript.
Corresponding author
Correspondence to Manudi Vidanapathirana .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Vidanapathirana, M. Dengue haemorrhagic fever in chronic kidney disease and heart failure: challenges in fluid management. Trop Med Health 52 , 33 (2024). https://doi.org/10.1186/s41182-024-00600-9
Download citation
Received : 25 February 2024
Accepted : 17 April 2024
Published : 24 April 2024
DOI : https://doi.org/10.1186/s41182-024-00600-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fluid management
Tropical Medicine and Health
ISSN: 1349-4147
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS EXPLAINER
- 07 November 2023
Dengue is spreading. Can new vaccines and antivirals halt its rise?
- Mariana Lenharo
You can also search for this author in PubMed Google Scholar
Dengue is on the march. This year, more than 4.2 million cases of the disease, which is caused by a virus transmitted by mosquitoes , had been reported by 2 October, compared with half a million in 2000 . And the disease, which was once confined to the tropics, is spreading to new locations around the world, including southern Europe .
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 623 , 470 (2023)
doi: https://doi.org/10.1038/d41586-023-03453-0
Hadinegoro, S. R. et al. N. Engl. J. Med. 373 , 1195–1206 (2015).
Article PubMed Google Scholar
López-Medina, E. et al. J. Infect. Dis. 225 , 1521–1532 (2022).
Download references
Reprints and permissions
Related Articles

- Public health
- Epidemiology

WHO redefines airborne transmission: what does that mean for future pandemics?
News 24 APR 24
More work is needed to take on the rural wastewater challenge
Correspondence 23 APR 24
Monkeypox virus: dangerous strain gains ability to spread through sex, new data suggest
News 23 APR 24
Bacteria deploy umbrella toxins against their competitors
News & Views 17 APR 24

Bird flu outbreak in US cows: why scientists are concerned
News Explainer 08 APR 24
Deadly diseases and inflatable suits: how I found my niche in virology research
Spotlight 17 APR 24
Technician - Senior Technician in Cell and Molecular Biology
APPLICATION CLOSING DATE: 24.05.2024 Human Technopole (HT) is a distinguished life science research institute founded and supported by the Italian ...
Human Technopole
Postdoctoral Fellow
The Dubal Laboratory of Neuroscience and Aging at the University of California, San Francisco (UCSF) seeks postdoctoral fellows to investigate the ...
San Francisco, California
University of California, San Francsico
Postdoctoral Associate
Houston, Texas (US)
Baylor College of Medicine (BCM)
Postdoctoral Research Fellow
Description Applications are invited for a postdoctoral fellow position at the Lunenfeld-Tanenbaum Research Institute, Sinai Health, to participate...
Toronto (City), Ontario (CA)
Sinai Health
Postdoctoral Research Associate - Surgery
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Dengue fever infections have negative impacts on infant health for three years
Risks include low birth weights and higher numbers of infant hospitalizations.
Dengue infections in pregnant women may have a negative impact on the first years of children's lives, new research has found.
Dengue fever is the most prevalent mosquito-borne disease globally and poses a threat to half of the world's population. There has been a dramatic rise in cases over recent years, with cases in the Americas reaching more than three million cases in 2023. Since January 2024, Brazil has reported more than 3.5 million cases, marking the largest dengue outbreak on record.
The paper, co-authored by Dr Livia Menezes from the University of Birmingham and Dr Martin Foureaux Koppensteiner from the University of Surrey, has been published in the American Economic Journal: Applied Economics.
The study looks at a large data set of dengue fever infections in expectant mothers from Minas Gerais, Brazil, and the resulting birth outcomes. It finds that babies born to women who were infected with dengue fever during their pregnancy had lower birth weights, increasing the risk of newborns being classed as having a very and extremely low birth weight by 67% and 133%, respectively.
Dr Livia Menezes, Assistant Professor in Economics at the University of Birmingham and co-author of the study said: "Even though dengue is a very common mosquito-borne disease, there has not been much attention given to the impact it has on birth outcomes and as a result, what can be done to improve them and protect pregnant women and their children.
"This paper sets out robust research which shows that being infected with dengue fever, even with a mild case, whilst pregnant can have a significant impact on the health of the child once born. These birth outcomes can even have longer-term impacts, for example, previous research has shown that low birth weight can negatively affect socio-economic outcomes and health in adulthood."
The researchers also found that children whose mothers were infected with dengue fever whilst pregnant had a 27% increased risk of being hospitalised from birth to age three. The highest risk of hospitalisation for these children comes in their second year of life, where there is a 76% increase.
Dr Martin Foureaux Koppensteiner, Associate Professor in Economics at the University of Surrey said: "These negative birth outcomes are not only limited to the health of individual children and mothers, but they have a much wider impact for communities where dengue fever is common. Hospitalisations and ongoing health issues resulting from maternal infections all have a cost, and one that could be avoided, or at least minimised with increased awareness and improved policy.
"We strongly suggest that dengue fever should be considered alongside the TORCH infections to manage and avoid when pregnant, which currently include Toxoplasmosis, Rubella, HIV, syphilis, chicken pox, Zika, and influenza among others."
The study also highlights the possible consequences of climate change expanding the reach of dengue fever. While the disease has historically been limited to tropical and subtropical regions, it now has an established presence in over 120 countries. Aedes mosquitoes, which carry and transmit dengue, have found breeding grounds in countries previously unaffected, including Croatia, France, Portugal, and the southern states of the USA.
Dr Menezes concludes: "As the planet heats, we can expect to see dengue fever become even more common in countries that have previously not had high infection rates. This is a problem that needs to be taken seriously, and quickly.
"Policy changes and things like vector control, updated risk communication with key groups and vaccine adoption can all reduce the risk of pregnant women being infected with dengue. Action needs to be taken by governments and health organisations to provide better protection for pregnant women and their children."
- Dengue Fever
- Pregnancy and Childbirth
- Infant's Health
- Endangered Plants
- Dengue fever
- Candidiasis
- Origin of life
- Occupational therapy
- HPV vaccine
- Adult stem cell
Story Source:
Materials provided by University of Birmingham . Note: Content may be edited for style and length.
Journal Reference :
- Martin Foureaux Koppensteiner, Lívia Menezes. Maternal Dengue and Health Outcomes of Children . American Economic Journal: Applied Economics , 2024; 16 (2): 530 DOI: 10.1257/app.20210656
Cite This Page :
Explore More
- Holographic Displays: An Immersive Future
- Harvesting Energy Where River Meets Sea
- Making Diamonds at Ambient Pressure
- Eruption of Mega-Magnetic Star
- Clean Fuel Generation With Simple Twist
- Bioluminescence in Animals 540 Million Years Ago
- Fossil Frogs Share Their Skincare Secrets
- Fussy Eater? Most Parents Play Short Order Cook
- Precise Time Measurement: Superradiant Atoms
- Artificial Cells That Act Like Living Cells
Trending Topics
Strange & offbeat.
We've updated our Privacy Policy to make it clearer how we use your personal data. We use cookies to provide you with a better experience. You can read our Cookie Policy here.
Stay up to date on the topics that matter to you
Dengue Fever Infections Can Impact Infant Health for Three Years
Dengue infections in pregnant women may have a negative impact on the first years of children’s lives..

Complete the form below to unlock access to ALL audio articles.
Dengue fever is the most prevalent mosquito-borne disease globally and poses a threat to half of the world's population. There has been a dramatic rise in cases over recent years, with cases in the Americas reaching more than three million cases in 2023. Since January 2024, Brazil has reported more than 3.5 million cases, marking the largest dengue outbreak on record.
The paper, co-authored by Dr Livia Menezes from the University of Birmingham and Dr Martin Foureaux Koppensteiner from the University of Surrey, has been published in the American Economic Journal: Applied Economics.
This paper sets out robust research which shows that being infected with dengue fever, even with a mild case, whilst pregnant can have a significant impact on the health of the child once born. Dr Livia Menezes, Birmingham Business School
The study looks at a large data set of dengue fever infections in expectant mothers from Minas Gerais, Brazil, and the resulting birth outcomes. It finds that babies born to women who were infected with dengue fever during their pregnancy had lower birth weights, increasing the risk of newborns being classed as having a very and extremely low birth weight by 67% and 133%, respectively.
Want more breaking news?
Subscribe to Technology Networks ’ daily newsletter, delivering breaking science news straight to your inbox every day.
Dr Livia Menezes, Assistant Professor in Economics at the University of Birmingham and co-author of the study said: “Even though dengue is a very common mosquito-borne disease, there has not been much attention given to the impact it has on birth outcomes and as a result, what can be done to improve them and protect pregnant women and their children.
“This paper sets out robust research which shows that being infected with dengue fever, even with a mild case, whilst pregnant can have a significant impact on the health of the child once born. These birth outcomes can even have longer-term impacts, for example, previous research has shown that low birth weight can negatively affect socio-economic outcomes and health in adulthood.”
The researchers also found that children whose mothers were infected with dengue fever whilst pregnant had a 27% increased risk of being hospitalised from birth to age three. The highest risk of hospitalisation for these children comes in their second year of life, where there is a 76% increase.
Dr Martin Foureaux Koppensteiner, Associate Professor in Economics at the University of Surrey said: “These negative birth outcomes are not only limited to the health of individual children and mothers, but they have a much wider impact for communities where dengue fever is common. Hospitalisations and ongoing health issues resulting from maternal infections all have a cost, and one that could be avoided, or at least minimised with increased awareness and improved policy.
“We strongly suggest that dengue fever should be considered alongside the TORCH infections to manage and avoid when pregnant, which currently include Toxoplasmosis, Rubella, HIV, syphilis, chicken pox, Zika, and influenza among others."
As the planet heats, we can expect to see dengue fever become even more common in countries that have previously not had high infection rates. This is a problem that needs to be taken seriously, and quickly. Dr Livia Menezes, Birmingham Business School
The study also highlights the possible consequences of climate change expanding the reach of dengue fever. While the disease has historically been limited to tropical and subtropical regions, it now has an established presence in over 120 countries. Aedes mosquitoes, which carry and transmit dengue, have found breeding grounds in countries previously unaffected, including Croatia, France, Portugal, and the southern states of the USA.
Dr Menezes concludes: “As the planet heats, we can expect to see dengue fever become even more common in countries that have previously not had high infection rates. This is a problem that needs to be taken seriously, and quickly.
“Policy changes and things like vector control, updated risk communication with key groups and vaccine adoption can all reduce the risk of pregnant women being infected with dengue. Action needs to be taken by governments and health organisations to provide better protection for pregnant women and their children.”
Reference: Koppensteiner MF, Menezes L. Maternal dengue and health outcomes of children. Am Econ J: Appl Econ . 2024;16(2):530-553. doi: 10.1257/app.20210656
This article has been republished from the following materials . Note: material may have been edited for length and content. For further information, please contact the cited source. Our press release publishing policy can be accessed here .

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Dengue fever.
Timothy J. Schaefer ; Prasan K. Panda ; Robert W. Wolford .
Affiliations
Last Update: November 14, 2022 .
- Continuing Education Activity
Dengue is a mosquito-transmitted virus and the leading cause of arthropod-borne viral disease in the world. It is also known as breakbone fever due to the severity of muscle spasms and joint pain, dandy fever, or seven-day fever because of the usual duration of symptoms. Although most cases are asymptomatic, severe illness and death may occur. Aedes mosquitoes transmit the virus and are common in tropical and subtropical parts of the world. The incidence of dengue has increased dramatically over the past few decades, and the infection is now endemic in some parts of the world. A few people who were previously infected with one subspecies of the dengue virus develop severe capillary permeability and bleeding after being infected with another subspecies of the virus. This illness is known as dengue hemorrhagic fever. This activity reviews the etiology, presentation, evaluation, and management of dengue fever and examines the role of the interprofessional team in evaluating, diagnosing, and managing the condition.
- Describe the pathophysiology of dengue fever.
- Review the symptomatic presentation of dengue fever along with physical exam findings.
- Discuss the available management options for dengue fever, including prevention strategies.
- Explain the importance of interprofessional team strategies for improving care coordination and communication to aid in prompt diagnosis of dengue fever and improve outcomes in patients diagnosed with the condition.
- Introduction
Dengue is a mosquito-transmitted virus and the leading cause of arthropod-borne viral disease in the world. It is also known as breakbone fever due to the severity of muscle spasms and joint pain, dandy fever, or seven-day fever because of the usual duration of symptoms. Although most cases are asymptomatic, severe illness and death may occur. Aedes mosquitoes transmit the virus and are common in tropical and subtropical parts of the world. The incidence of dengue has increased dramatically over the past few decades. The infection is now endemic in some parts of the world. A few people who were previously infected with one subspecies of the dengue virus develop severe capillary permeability and bleeding after being infected with another subspecies of the virus. This illness is known as dengue hemorrhagic fever. [1] [2] [3]
Dengue fever is caused by any of four distinct serotypes (DENV 1-4) of single-stranded RNA viruses of the genus Flavivirus . Infection by one serotype results in lifelong immunity to that serotype but not to others. [4] [5] [6]
- Epidemiology
It is the fastest spreading mosquito-borne viral disease globally, affecting greater than 100 million humans annually. Dengue also causes 20 to 25,000 deaths, primarily in children, and is found in more than 100 countries. Epidemics occur annually in the Americas, Asia, Africa, and Australia. Two transmission cycles maintain the dengue virus: 1) mosquitos carry the virus from a non-human primate to a non-human primate, and 2) mosquitos carry the virus from human to human. The human-mosquito cycle occurs primarily in urban environments. Whether the virus transmits from human to mosquito depends on the viral load of the mosquito’s blood meal.
The primary vectors of the disease are female mosquitoes of the species Aedes aegypti and Aedes albopictus . Although A. aegypti is associated with most infections, A. albopictus’ range is expanding, tolerates cold environment better, is an aggressive feeder but feeds less frequently, and may be associated with increasing numbers. These species of mosquitoes tend to live indoors and are active during the day. Transmission by perinatally, blood transfusions, breast milk, and organ transplantation have been reported.
After 2010, the mean age of patients was 34 years compared to 27.2 years from 1990 to 2010. The dengue viral serotype causing disease outbreaks have varied with time, as has the occurrence of severe dengue fever. [7] [8]
Transmission of dengue generally follows two patterns - epidemic dengue and hyperendemic dengue. When a single strain of DENV is responsible for introduction and transmission it is referred to as epidemic dengue. Dengue epidemics were more common prior to World War II. During an epidemic, all age groups are affected, but the incidence of dengue hemorrhagic fever is relatively low. Hyperendemicity refers to the co-circulation of various serotypes of DENV in a community. Periodic epidemics in an area are linked to the emergence of hyperendemicity. [9] Children are affected more than adults, and the incidence of DHF is relatively higher.
- Pathophysiology
Part of the Flavivirus family, the dengue virus is a 50 nm virion with three structural and seven nonstructural proteins, a lipid envelope, and a 10.7 kb capped positive-sense single strand of ribonucleic acid. Infections are asymptomatic in up to 75% of infected humans. A spectrum of diseases, from self-limiting dengue fever to hemorrhage and shock, may be seen. A fraction of infections (0.5% to 5%) progress to severe dengue. Without proper treatment, fatality rates may exceed 20%. These occur primarily in children. The typical incubation period for the disease is 4 to 7 days, but it can last from 3 to 10 days. Symptoms more than two weeks after exposure are unlikely to be due to dengue fever.
The exact course of events following the dermal injection of the dengue virus by a mosquito bite is unclear. Skin macrophages and dendritic cells appear to be the first targets. It is thought that the infected cells then move to the lymph nodes and spread through the lymphatic system to other organs. Viremia may be present for 24 to 48 hours before the onset of symptoms. A complex interaction of host and viral factors then occurs and determines whether the infection will be asymptomatic, typical, or severe. Severe dengue fever with increased microvascular permeability and shock syndrome is thought to be associated with infection due to a second dengue virus serotype and the patient's immune response. However, cases of severe dengue do occur in the setting of infection by only a single serotype. Worsening microvascular permeability often transpires even as viral titers fall.
- History and Physical
The three phases of dengue include febrile, critical, and recovery.
During the febrile phase, a sudden high-grade fever of approximately 40 C occurs that usually lasts two to seven days. Saddleback or biphasic fever is seen in approximately 6% of cases, particularly in patients with DHF and severe dengue. It is described as a fever that remits at least for one day, and the next fever spike starts, which lasts at least for one more day. [10] Associated symptoms include facial flushing, skin erythema, myalgias, arthralgias, headache, sore throat, conjunctival injection, anorexia, nausea, and vomiting. For skin erythema, a general blanchable macular rash occurs in the first one to two days of fever and the last day of fever. Or, within 24 hours, a secondary maculopapular rash can develop.
Defervescence characterizes the critical phase with a temperature of approximately 37.5 C to 38 C or less on days three through seven. It is associated with increased capillary permeability. This phase usually lasts one to two days. The onset of the critical phase is heralded by a rapid decline in platelet count, rise in hematocrit (the patient may have leukopenia up to 24 hours before platelet count drops), and the presence of warning signs. It can progress to shock, organ dysfunction, disseminated intravascular coagulation, or hemorrhage.
The recovery phase entails the gradual reabsorption of extravascular fluid in two to three days. The patient will display bradycardia at this time.
Expanded dengue syndrome refers to unusual or atypical manifestations in patients with dengue with neurological, hepatic, renal, and other isolated organ involvement. It could be due to profound shock. Neurological manifestations include febrile seizures in young children, encephalitis, aseptic meningitis, and intracranial bleeding. Gastrointestinal involvement may be seen in the form of hepatitis, liver failure, pancreatitis, or acalculous cholecystitis. It may also manifest as myocarditis, pericarditis, ARDS, acute kidney injury, or hemolytic uremic syndrome.
Common laboratory findings include thrombocytopenia, leukopenia, elevated aspartate aminotransferase. The disease is classified as dengue or severe dengue. [11] [12] [13]
Criteria for Dengue Include
- Probable dengue: The patient lives in or has traveled to a dengue-endemic area. Symptoms include fever and two of the following: nausea, vomiting, rash, myalgias, arthralgias, rash, positive tourniquet test, or leukopenia.
- Warning Signs of Dengue: Abdominal pain, persistent vomiting, clinical fluid accumulation such as ascites or pleural effusion, mucosal bleeding, lethargy, liver enlargement greater than 2 cm, increase in hematocrit, and thrombocytopenia.
- Severe Dengue: Dengue fever with severe plasma leakage, hemorrhage, organ dysfunction including transaminitis greater than 1000 international units per liter, impaired consciousness, myocardial dysfunction, and pulmonary dysfunction
- Dengue shock syndrome clinical warnings: Symptoms include rapidly rising hematocrit, intense abdominal pain, persistent vomiting, and narrowed or absent blood pressure.
The virus antigen is detectable by ELISA, polymerase chain reaction, or virus isolation from body fluids. Serology will reveal a marked increase in immunoglobulins. A confirmed diagnosis is established by culture, antigen detection, polymerase chain reaction, or serologic testing.
It is vital to assess pregnant patients with dengue as the symptoms may be very similar to preeclampsia.
- Treatment / Management
Treatment of dengue depends on the patient's illness phase. Those presenting early without any warning signs can be treated on an outpatient basis with acetaminophen and adequate oral fluids. Such patients should receive an explanation regarding the danger signs and be asked to report to the hospital immediately if they notice any. Patients with warning signs, severe dengue, or other situations like infancy, elderly, pregnancy, diabetes, and those living alone need to be admitted. Those with warning signs can be initiated on IV crystalloids, and the fluid rate is titrated based on the patient's response. Colloids can be started for patients in shock and are also preferred if the patient has already received previous boluses of crystalloid and has not responded. Blood transfusion is warranted in case of severe bleeding or suspected bleeding when the patient remains unstable, and hematocrit falls despite adequate fluid resuscitation. Platelet transfusion is considered when platelet count drops to <20,000 cells/microliter, and there is a high risk of bleeding. Avoid giving aspirin and nonsteroidal anti-inflammatory drugs, and other anticoagulants. No antiviral medications are recommended.
No laboratory tests can predict the progression to severe disease.
- Differential Diagnosis
The clinical diagnosis of dengue can be challenging as many other illnesses can present similarly early in the disease course. Other considerations should include malaria, influenza, Zika, chikungunya, measles, and yellow fever. Obtain a detailed history of immunizations, travel, and exposures.
Rapid laboratory identification of dengue fever includes NS1 antigen detection and serologic tests. Serologic tests are only useful after several days of infection and may be associated with false positives due to other flavivirus infections, such as yellow fever or Zika virus.
Untreated severe dengue fever may have a mortality rate of 10% to 20%. Appropriate supportive care reduces the mortality rate to roughly 1%.
- Complications
- Liver injury
- Cardiomyopathy
- Encephalopathy
- Encephalitis
- Postoperative and Rehabilitation Care
Patients should be encouraged to consume ample liquids. The return of a patient's appetite is a sign that the infection is subsiding.
- Consultations
Consulting an infectious disease specialist is recommended because most clinicians have little experience managing this infection. The Centers for Disease Control and Prevention has a hotline that also offers treatment advice.
- Deterrence and Patient Education
The only way to avoid contracting dengue is to prevent mosquito bites and not travel to endemic areas.
Preventative measures include [14] -
Personal Prophylactic Measures: Use of bed nets while in bed even in the daytime, Insecticide-treated materials (ITMs) like window curtains, application of mosquito repellent creams (containing DEET, IR3535, or Icaridin), coils, developing the habit of wearing full sleeve shirts and pants help prevent mosquito bite.
Biological Control
a) Fish: Viviparous species Poecilia reticulata have been used in confined water bodies like large water tanks, open freshwater wells. Only native larvicidal fishes should be used.
b) Predatory Copepods: These small freshwater crustaceans have proven to be effective only in specific container habitats
c) Endosymbiotic control: Mosquitoes infected with Wolbachia (an intracellular parasite) are less susceptible to DENV infection than wild type A. aegypti . [15]
Chemical Control: Larvicidal use in big breeding containers; Insecticide spray: Space sprays can be applied as thermal fogs and cold aerosols. Oil-based formulations are preferred as it inhibits evaporation. Some of the commonly used insecticides are organophosphorus compounds (fenitrothion, malathion) and pyrethroids (bioresmethrin, cypermethrin).
Environmental Measures: Finding the breeding areas and eliminating the pests; proper management of rooftops and sunshades; appropriate covering of stored water like buckets, pots, etc
Health Education: It is the most important weapon to fight against dengue. Sensitizing the people regarding dengue in detail is necessary for the effective implementation of the dengue control program. The sensitization can be done by audiovisual media or mass awareness campaigns.
Community Participation: It's essential to sensitize the communities for their active participation in dengue control programs.
Vaccination: CYD-TDV: a live recombinant tetravalent dengue vaccine, first to be licensed, is approved for endemic areas in 20 countries. [16]
- Enhancing Healthcare Team Outcomes
The diagnosis and management of dengue are complex, and this is best managed by an interprofessional team that includes an infectious disease expert, a CDC consultant, an emergency department clinician, and an internist. The care is supportive with fluid, acetaminophen for fever, and a blood transfusion for hemorrhage. A confirmed diagnosis is established by culture, antigen detection, polymerase chain reaction, or serologic testing. No laboratory tests can predict the progression to severe disease.
The role of the primary care provider and nurse practitioner is to educate the traveler on the prevention of mosquito bites. This means covering exposed skin and using bed nets, particularly during daytime siestas, using mosquito repellents, and indoor insecticides. One should also eradicate mosquito breeding grounds like standing water. The prognosis for untreated dengue is abysmal, but most patients can survive with supportive care, albeit with residual multisystem organ damage. [17] [18]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Main symptoms of dengue fever Contributed by Wikimedia Commons (Public Domain)
Mosquito carried diseases, Zika virus, Dengue fever, West Nile Fever, Chikungunya, Yellow Fever, Malaria Contributed by National Institutes of Health (NIH)
Disclosure: Timothy Schaefer declares no relevant financial relationships with ineligible companies.
Disclosure: Prasan Panda declares no relevant financial relationships with ineligible companies.
Disclosure: Robert Wolford declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Schaefer TJ, Panda PK, Wolford RW. Dengue Fever. [Updated 2022 Nov 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Dengue virus: A global human threat: Review of literature. [J Int Soc Prev Community Dent....] Review Dengue virus: A global human threat: Review of literature. Hasan S, Jamdar SF, Alalowi M, Al Ageel Al Beaiji SM. J Int Soc Prev Community Dent. 2016 Jan-Feb; 6(1):1-6.
- [Dengue fever--not just a tropical infectious disease]. [Med Monatsschr Pharm. 2016] [Dengue fever--not just a tropical infectious disease]. Stock I. Med Monatsschr Pharm. 2016 Mar; 39(3):117-22.
- Infection of Mosquito Cells (C6/36) by Dengue-2 Virus Interferes with Subsequent Infection by Yellow Fever Virus. [Vector Borne Zoonotic Dis. 2016] Infection of Mosquito Cells (C6/36) by Dengue-2 Virus Interferes with Subsequent Infection by Yellow Fever Virus. Abrao EP, da Fonseca BA. Vector Borne Zoonotic Dis. 2016 Feb; 16(2):124-30. Epub 2016 Jan 25.
- Review Value of routine dengue diagnosis in endemic countries. [World J Virol. 2017] Review Value of routine dengue diagnosis in endemic countries. Ayukekbong JA, Oyero OG, Nnukwu SE, Mesumbe HN, Fobisong CN. World J Virol. 2017 Feb 12; 6(1):9-16.
- A rare presentation of dengue fever: bilateral psoas muscle hematoma, intrahepatic cholestatic hepatitis, pancreatitis and pancytopenia. [Oxf Med Case Reports. 2023] A rare presentation of dengue fever: bilateral psoas muscle hematoma, intrahepatic cholestatic hepatitis, pancreatitis and pancytopenia. Islam J, Mondal K, Ghosh SK, Datta AK, Ghosh S. Oxf Med Case Reports. 2023 Oct; 2023(10):omad115. Epub 2023 Oct 23.
Recent Activity
- Dengue Fever - StatPearls Dengue Fever - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
COMMENTS
Dengue is an acute viral illness caused by RNA virus of the family Flaviviridae and spread by Aedes mosquitoes. Presenting features may range from asymptomatic fever to dreaded complications such as hemorrhagic fever and shock. A cute-onset high fever, muscle and joint pain, myalgia, cutaneous rash, hemorrhagic episodes, and circulatory shock ...
In addition, dengue fever may present with extended and unusual manifestations affecting any organ, including the heart, liver, kidney and brain. Studies on vaccine development and vector control are ongoing to prevent this infection of global importance. In this article, the clinicopathological features and management aspects of dengue are ...
DOI: 10.1056/NEJMra1110265. VOL. 366 NO. 15. Dengue is a self-limited, systemic viral infection transmitted between humans by mosquitoes. The rapidly expanding global footprint of dengue is a ...
Background The World Health Organization (WHO) has ranked dengue as one of the top ten threats to Global health in 2019. Sri Lanka faced a massive dengue epidemic in 2017, the largest outbreak in the country during the last three decades, consisting of 186,101 reported cases, and over 320 deaths. The epidemic was controlled by intense measures taken by the health sector. However, the reported ...
Dengue virus (DENV) is responsible for an estimated 100 million symptomatic cases of infection and 10,000 deaths annually. The incidence of dengue has been doubling every decade since 1990.1 Dengue...
Dengue, caused by four closely related viruses, is a growing global public health concern, with outbreaks capable of overwhelming health-care systems and disrupting economies. Dengue is endemic in more than 100 countries across tropical and subtropical regions worldwide, and the expanding range of the mosquito vector, affected in part by climate change, increases risk in new areas such as ...
Basic research includes a wide range of studies focused on learning how the dengue virus is transmitted and how it infects cells and causes disease. This type of research investigates many aspects ...
Infection with any one of the four dengue virus serotypes can cause disease that ranges from a mild febrile illness through to haemorrhagic fever and shock. This Primer outlines the epidemiology ...
Studies were included if they met the eligibility criteria: (1) peer-reviewed original research articles, (2) quantitative observational time series, case-crossover or case series human studies in the general population with dengue infection cases or incidence as the outcome of interest and (3) the exposures of interest were high temperatures ...
Defeating dengue with Wolbachia. A recent study reports the efficacy of Wolbachia -infected mosquito deployments for the control of dengue fever in Indonesia. Ashley York. Research Highlights 18 ...
Dengue is the disease caused by 1 of 4 distinct, but closely related dengue viruses (DENV-1-4) that are transmitted by Aedes spp. mosquito vectors. It is the most common arboviral disease worldwide, with the greatest burden in tropical and sub-tropical regions. In the absence of effective prevention and control measures, dengue is projected to increase in both disease burden and geographic ...
Dengue, or dengue fever, is a disease caused by infection with the dengue virus (DENV), a member of the Flavivirus genus (Flaviviridae) that also includes yellow fever and Japanese encephalitis. Milder cases can range from asymptomatic to clinical manifestations that include high fever, severe headaches, retro-orbital pain, joint and muscle pains, vomiting and rash. DENV is classified into ...
This article aims at bringing to light the current epidemiological and clinical status of the dengue fever. The relationship between genetic mutations, single nucleotide polymorphism (SNP) and the pathophysiology of disease progression will be put into perspective. ... Eliminate Dengue became an international research program across countries ...
Substances. Dengue Vaccines. Dengue is a highly endemic infectious disease of the tropical countries and is rapidly becoming a global burden. It is caused by any of the 4 serotypes of dengue virus and is transmitted within humans through female Aedes mosquitoes. Dengue disease varies from mild fever to severe conditions of deng ….
2 Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Diseases, Kunming, China; 3 Yunnan Key Laboratory of Vector-Borne Infectious Disease, Kunming, ... In 2019, there were 22599 cases of dengue fever, with an incidence rate of 1.63/10 million . As a typical dengue epidemic area, in 2019, the main epidemic type in ...
Most people with dengue have mild or no symptoms and will get better in 1-2 weeks. Rarely, dengue can be severe and lead to death. If symptoms occur, they usually begin 4-10 days after infection and last for 2-7 days. Symptoms may include: high fever (40°C/104°F) severe headache. pain behind the eyes.
Dengue viruses (DENV) are positive-stranded RNA viruses belonging to the Flaviviridae family. DENV is the causative agent of dengue, the most rapidly spreading viral disease transmitted by mosquitoes. Each year, millions of people contract the virus through bites from infected female mosquitoes of the Aedes species. In the majority of individuals, the infection is asymptomatic, and the immune ...
The adverse outcomes were classified according to three systems commonly used in dengue clinical research: dengue fever vs. dengue haemorrhagic fever grades I-IV 5, dengue fever with or without ...
At least 2.1 million cases of dengue fever have been reported in North and South America, and this year 1,800 people have died from the mosquito-borne disease By Francisco "A.J." Camacho & E&E News
QSNICH has engaged in a long-term collaboration with the Armed Forces Research Institute of Medical Sciences (AFRIMS) on dengue diagnostics and detection using ... admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. ...
Dengue infections in pregnant women may have a negative impact on the first years of children's lives, new research has found. Dengue fever is the most prevalent mosquito-borne disease globally ...
More than 3.5 million cases of dengue have been confirmed by governments in Latin America in the first three months of 2024, compared with 4.5 million in all of 2023. There have been more than ...
Dengue fever (DF) is an acute, self-limiting systemic viral illness caused by the dengue virus (Flaviviridae), ... This research also implicates that virus-induced destruction or suppression of myeloid progenitor cells may cause leukopenia in dengue fever. Bradycardia, one of the major dengue manifestations, was found to occur in 46.15% of ...
Dengue haemorrhagic fever (DHF) is a severe presentation of dengue, characterized by plasma leakage and, at times, haemorrhagic manifestations [].The plasma leakage occurs during a 48-h period known as the critical phase, and it is evidenced by a rise in haematocrit of at least 20% [1, 2].Critical phase usually starts approximately 3-5 days after onset of fever [].
QDenga, a vaccine made by Takeda in Osaka, Japan, has so far been shown to be safe for people regardless of whether they have previously been infected, and has an overall efficacy rate of 73% ...
Dengue infections in pregnant women may have a negative impact on the first years of children's lives, new research has found. Dengue fever is the most prevalent mosquito-borne disease globally ...
This paper sets out robust research which shows that being infected with dengue fever, even with a mild case, whilst pregnant can have a significant impact on the health of the child once born. The study looks at a large data set of dengue fever infections in expectant mothers from Minas Gerais, Brazil, and the resulting birth outcomes.
Dengue is a mosquito-transmitted virus and the leading cause of arthropod-borne viral disease in the world. It is also known as breakbone fever due to the severity of muscle spasms and joint pain, dandy fever, or seven-day fever because of the usual duration of symptoms. Although most cases are asymptomatic, severe illness and death may occur. Aedes mosquitoes transmit the virus and are common ...