Lock-and-key model
strong>Lock-and-key model n., [lɑk ænd ki ˈmɑdl̩] Definition: a model for enzyme-substrate interaction
Table of Contents

Lock-and-key model Definition
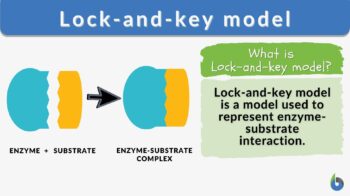
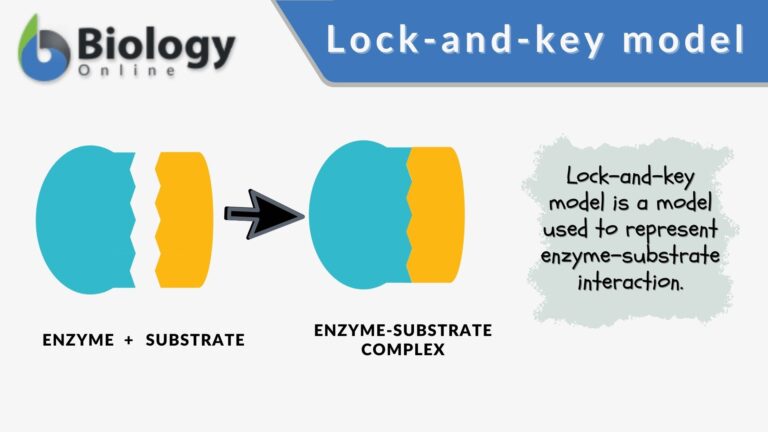
Lock-and-key model is a model for enzyme-substrate interaction suggesting that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. In this model, enzymes are depicted as highly specific. They must bind to specific substrates before they catalyze chemical reactions . The term is a pivotal concept in enzymology to elucidate the intricate interaction between enzymes and substrates at the molecular level. In the lock-and-key model, the enzyme-substrate interaction suggests that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Like a key into a lock , only the correct size and shape of the substrate ( the key ) would fit into the active site ( the keyhole ) of the enzyme ( the lock ).
Compare: Induced fit model See also: enzyme , active site , substrate
Lock-and-key vs. Induced Fit Model
At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model , and the other is the Induced fit model . The lock and key model theory was first postulated by Emil Fischer in 1894. The lock-and-key enzyme action proposes the high specificity of enzymes. However, it does not explain the stabilization of the transition state that the enzymes achieve. The induced fit model (proposed by Daniel Koshland in 1958) suggests that the active site continues to change until the substrate is completely bound to the active site of the enzyme, at which point the final shape and charge are determined. Unlike the lock-and-key model, the induced fit model shows that enzymes are rather flexible structures. Nevertheless, Fischer’s Lock and Key theory laid an important foundation for subsequent research, such as during the refinement of the enzyme-substrate complex mechanism, as ascribed in the induced fit model. The lock-and-key hypothesis has opened ideas where enzyme action is not merely catalytic but incorporates a rather complex process in how they interact with the correct substrates with precision.
Key Components
Components of the lock and key model:
- Enzyme : the enzyme structure is a three-dimensional protein configuration, with an active site from where the substrate binds.
- Substrate : often an organic molecule, a substrate possesses a structural feature that complements the geometry of the enzyme’s active site.
In the lock and key model, both the enzymes and the substrates facilitate the formation of a complex that lowers the activation energy needed for a chemical transformation to occur. Such reduction in the activation energy allows the chemical reaction to proceed at a relatively faster rate, making enzymes crucial in various biological and molecular processes.
Lock-and-key Model Examples
Some of the common examples that are often discussed in the context of the Lock and Key Model are as follows:
- Enzyme lactate dehydrogenase with a specific active site for its substrates, pyruvate and lactate. The complex facilitates the interconversion of pyruvate and lactate during anaerobic respiration
- Enzyme carbonic anhydrase with a specific active site for the substrates carbon dioxide and water. The complex facilitates the hydration of carbon dioxide, forming bicarbonate
- Enzyme lysozyme binding with a bacterial cell wall peptidoglycan, which is a vital immune function
Choose the best answer.
Send Your Results (Optional)

- Aryal, S. and Karki, P. (2023). “Lock and Key Model- Mode of Action of Enzymes”. Microbenotes.com. https://microbenotes.com/lock-and-key-model-mode-of-action-of-enzymes/
- Farhana, A., & Lappin, S. L. (2023, May). Biochemistry, Lactate Dehydrogenase . Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557536/
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on January 11th, 2024
You will also like...
Genetic Engineering Advantages & Disadvantages
This tutorial presents the benefits and the possible adverse eventualities of genetic engineering. Know more about this ..

Nutrients in the soil are essential to the proper growth of a land plant. This tutorial deals with the properties of soi..
Regulation of Organic Metabolism, Growth and Energy Balance
The human body is capable of regulating growth and energy balance through various feedback mechanisms. Get to know the e..

Growth and Plant Hormones
Plants, like animals, produce hormones to regulate plant activities, including growth. They need these hormones to respo..

The arthropods were assumed to be the first taxon of species to possess jointed limbs and exoskeleton, exhibit more adva..

This study guide tackles plant roots in greater detail. It delves into the development of plant roots, the root structur..
Related Articles...
No related articles found

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
From lock and key model to induced fit model
Published by Modified over 5 years ago
Similar presentations
Presentation on theme: "From lock and key model to induced fit model"— Presentation transcript:
Enzymes … what do you know and will find out these next few lessons Lets find out millionaire styley remember the tips from “Mrs B how to answer MCQ”

WHAT IS THE NATURE OF SCIENCE?

Big Idea 2 : The Characteristics of Scientific Knowledge Description A: Scientific knowledge is based on empirical evidence, and is appropriate for understanding.

Chapter 13 Science and Hypothesis. Modern science has had a profound impact on our lives— mostly for the better. The laws and principles of science.

Scientific methodology is the heart of science. The full body of science includes more, as shown:

WHAT IS THE NATURE OF SCIENCE?. SCIENTIFIC WORLD VIEW 1.The Universe Is Understandable. 2.The Universe Is a Vast Single System In Which the Basic Rules.

What do we cover in section C?. Unit 4 research methods Explain the key features of scientific investigation and discuss whether psychology can be defined.

Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body?

The Sciences Natural and Human (Social) Sciences as Areas of Knowledge

WHAT IS THE NATURE OF SCIENCE?. THEORIES ARE THE SCIENTIFIC WORLD VIEW 1.The Universe Is Understandable. 2.The Universe Is a Vast Single System In Which.

False Assumptions 2012/03/25/false-assumptions-lesson/

Enzymes. What are they? Globular Proteins: This is important in explaining how heat can denature them – think tertiary structure Biological catalysts:

Enzymes and How They Work. Enzymes Enzymes are proteins. They are Biological catalysts (speed up the rate of reactions in living things without themselves.

Hypothesis-Based Science The Scientific Method. Science as Inquiry The process of investigation to answer questions about the natural world.
Hydrogen Peroxide Water + Oxygen

Virginia Standard of Learning BIO.1a-m

Section 1: Scientific Methods

Section 2: Scientific Methods

About project
© 2024 SlidePlayer.com Inc. All rights reserved.
Molecular Recognition: Lock-and-Key, Induced Fit, and Conformational Selection
- Living reference work entry
- First Online: 27 October 2020
- Cite this living reference work entry

- Norman Tran 4 &
- Todd Holyoak 4
37 Accesses
In the most general sense, molecular recognition is the mechanism by which two or more molecules come together to form a specific complex. These types of molecular interactions are widespread throughout biology and include diverse processes such as enzyme catalysis, antibody–antigen recognition, protein synthesis, receptor–ligand interactions, and transcriptional regulation, to name a few. Because of the universal importance of molecular recognition in biological function, understanding how molecules unambiguously recognize and interact with one another is fundamentally important to appreciating biological systems as a whole.
Introduction
Just as the field biochemistry grew out of the study of biological fermentation, much of the field of molecular recognition grew out of the study of enzyme selectivity (Voet and Voet 2004 ). Early studies led to the conclusion that substrates combine with enzymes at a specific location on each enzyme’s surface. These conclusions generated...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Beach H, Cole R, Gill ML, Loria JP (2005) Conservation of mus-ms enzyme motions in the apo- and substrate-mimicked state. J Am Chem Soc 127(25):9167–9176
Article CAS Google Scholar
Csermely P, Palotai R, Nussinov R (2010) Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem Sci 35(10):539–546
Dixon M, Webb EC (1979) Enzymes. Academic, New York
Google Scholar
Fischer E (1894) The influence of configuration on enzyme activity. Dtsch Chem Ges 27:2984–2993. (Translated from German)
Gerstein M, Lesk AM, Chothia C (1994) Structural mechanisms for domain movements in proteins. Biochemistry 33(22):6739–6749
Greives N, Zhou HX (2014) Both protein dynamics and ligand concentration can shift the binding mechanism between conformational selection and induced fit. Proc Natl Acad Sci U S A 111(28):10197–10202
Hammes GG, Chang YC, Oas TG (2009) Conformational selection or induced fit: a flux description of reaction mechanism. Proc Natl Acad Sci U S A 106(33):13737–13741
Jencks WP (1975) Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol 43:219–410
CAS PubMed Google Scholar
Koshland DE (1958) Application of a theory of enzyme specificity to protein synthesis. Proc Natl Acad Sci U S A 44(2):98–104
Koshland DE Jr (2004) Crazy, but correct. Nature 432(7016):447
Laidler KH (1951) The influence of pressure on the rates of biological reactions. Arch Biochem 30(2):226–236
Monod J, Wyman J, Changeux JP (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118
Sullivan SM, Holyoak T (2008) Enzymes with lid-gated active sites must operate by an induced fit mechanism instead of conformational selection. Proc Natl Acad Sci U S A 105(37):13829–13834
Tsai CJ, Kumar S, Ma B, Nussinov R (1999a) Folding funnels, binding funnels, and protein function. Protein Sci 8(6):1181–1190
Tsai CJ, Ma B, Nussinov R (1999b) Folding and binding cascades: shifts in energy landscapes. Proc Natl Acad Sci U S A 96(18):9970–9972
Voet D, Voet JG (2004) Biochemistry. Wiley, Hoboken
Weikl TR, Paul F (2014) Conformational selection in protein binding and function. Protein Sci 23(11):1508–1518
Yang J, Gao M, Xiong JW, Su ZD, Huang YQ (2019) Features of molecular recognition of intrinsically disordered proteins via coupled folding and binding. Protein Sci 28(11):1952–1965
Download references
Author information
Authors and affiliations.
Department of Biology, University of Waterloo, Waterloo, ON, Canada
Norman Tran & Todd Holyoak
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Todd Holyoak .
Editor information
Editors and affiliations.
University Leicester MRC Centre, Leicester, UK
Gordon Roberts
Dept Biochemistry, University of Oxford, Oxford, UK
Anthony Watts
Rights and permissions
Reprints and permissions
Copyright information
© 2021 European Biophysical Societies' Association (EBSA)
About this entry
Cite this entry.
Tran, N., Holyoak, T. (2021). Molecular Recognition: Lock-and-Key, Induced Fit, and Conformational Selection. In: Roberts, G., Watts, A. (eds) Encyclopedia of Biophysics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-35943-9_468-1
Download citation
DOI : https://doi.org/10.1007/978-3-642-35943-9_468-1
Received : 02 September 2020
Accepted : 08 September 2020
Published : 27 October 2020
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-35943-9
Online ISBN : 978-3-642-35943-9
eBook Packages : Springer Reference Biomedicine and Life Sciences Reference Module Biomedical and Life Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research

18.6 Enzyme Action
Learning objective.
- Describe the interaction between an enzyme and its substrate.
Enzyme-catalyzed reactions occur in at least two steps. In the first step, an enzyme molecule (E) and the substrate molecule or molecules (S) collide and react to form an intermediate compound called the enzyme-substrate (E–S) complex . (This step is reversible because the complex can break apart into the original substrate or substrates and the free enzyme.) Once the E–S complex forms, the enzyme is able to catalyze the formation of product (P), which is then released from the enzyme surface:
Hydrogen bonding and other electrostatic interactions hold the enzyme and substrate together in the complex. The structural features or functional groups on the enzyme that participate in these interactions are located in a cleft or pocket on the enzyme surface. This pocket, where the enzyme combines with the substrate and transforms the substrate to product is called the active site The location on an enzyme where a substrate binds and is transformed to product. of the enzyme ( Figure 18.10 "Substrate Binding to the Active Site of an Enzyme" ). It possesses a unique conformation (including correctly positioned bonding groups) that is complementary to the structure of the substrate, so that the enzyme and substrate molecules fit together in much the same manner as a key fits into a tumbler lock. In fact, an early model describing the formation of the enzyme-substrate complex was called the lock-and-key model A model that portrays an enzyme as conformationally rigid and able to bond only to a substrate or substrates that exactly fit the active site. ( Figure 18.11 "The Lock-and-Key Model of Enzyme Action" ). This model portrayed the enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site.
Figure 18.10 Substrate Binding to the Active Site of an Enzyme

The enzyme dihydrofolate reductase is shown with one of its substrates: NADP + (a) unbound and (b) bound. The NADP + (shown in red) binds to a pocket that is complementary to it in shape and ionic properties.
Figure 18.11 The Lock-and-Key Model of Enzyme Action

(a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex.
Working out the precise three-dimensional structures of numerous enzymes has enabled chemists to refine the original lock-and-key model of enzyme actions. They discovered that the binding of a substrate often leads to a large conformational change in the enzyme, as well as to changes in the structure of the substrate or substrates. The current theory, known as the induced-fit model A model that says an enzyme can undergo a conformational change when it binds substrate molecules. , says that enzymes can undergo a change in conformation when they bind substrate molecules, and the active site has a shape complementary to that of the substrate only after the substrate is bound, as shown for hexokinase in Figure 18.12 "The Induced-Fit Model of Enzyme Action" . After catalysis, the enzyme resumes its original structure.
Figure 18.12 The Induced-Fit Model of Enzyme Action

(a) The enzyme hexokinase without its substrate (glucose, shown in red) is bound to the active site. (b) The enzyme conformation changes dramatically when the substrate binds to it, resulting in additional interactions between hexokinase and glucose.
The structural changes that occur when an enzyme and a substrate join together bring specific parts of a substrate into alignment with specific parts of the enzyme’s active site. Amino acid side chains in or near the binding site can then act as acid or base catalysts, provide binding sites for the transfer of functional groups from one substrate to another or aid in the rearrangement of a substrate. The participating amino acids, which are usually widely separated in the primary sequence of the protein, are brought close together in the active site as a result of the folding and bending of the polypeptide chain or chains when the protein acquires its tertiary and quaternary structure. Binding to enzymes brings reactants close to each other and aligns them properly, which has the same effect as increasing the concentration of the reacting compounds.
- What type of interaction would occur between an OH group present on a substrate molecule and a functional group in the active site of an enzyme?
Suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you just identified.
- An OH group would most likely engage in hydrogen bonding with an appropriate functional group present in the active site of an enzyme.
- Several amino acid side chains would be able to engage in hydrogen bonding with an OH group. One example would be asparagine, which has an amide functional group.
Skill-Building Exercise
What type of interaction would occur between an COO − group present on a substrate molecule and a functional group in the active site of an enzyme?
One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity . An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. Some enzymes act on a single substrate, while other enzymes act on any of a group of related molecules containing a similar functional group or chemical bond. Some enzymes even distinguish between D- and L-stereoisomers, binding one stereoisomer but not the other. Urease, for example, is an enzyme that catalyzes the hydrolysis of a single substrate—urea—but not the closely related compounds methyl urea, thiourea, or biuret. The enzyme carboxypeptidase, on the other hand, is far less specific. It catalyzes the removal of nearly any amino acid from the carboxyl end of any peptide or protein.

Enzyme specificity results from the uniqueness of the active site in each different enzyme because of the identity, charge, and spatial orientation of the functional groups located there. It regulates cell chemistry so that the proper reactions occur in the proper place at the proper time. Clearly, it is crucial to the proper functioning of the living cell.
Concept Review Exercises
Distinguish between the lock-and-key model and induced-fit model of enzyme action.
Which enzyme has greater specificity—urease or carboxypeptidase? Explain.
The lock-and-key model portrays an enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site. The induced fit model portrays the enzyme structure as more flexible and is complementary to the substrate only after the substrate is bound.
Urease has the greater specificity because it can bind only to a single substrate. Carboxypeptidase, on the other hand, can catalyze the removal of nearly any amino acid from the carboxyl end of a peptide or protein.
Key Takeaways
- A substrate binds to a specific region on an enzyme known as the active site, where the substrate can be converted to product.
- The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions.
- The induced-fit model says that an enzyme can undergo a conformational change when binding a substrate.
- Enzymes exhibit varying degrees of substrate specificity.
What type of interaction would occur between each group present on a substrate molecule and a functional group of the active site in an enzyme?
- CH(CH 3 ) 2
For each functional group in Exercise 1, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
For each functional group in Exercise 2, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
- hydrogen bonding
- ionic bonding
- dispersion forces
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; serine (answers will vary).
- The amino acid has a negatively charged side chain; aspartic acid (answers will vary).
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; asparagine (answers will vary).
- The amino acid has a nonpolar side chain; isoleucine (answers will vary).

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.1: Catalytic Efficiency of Enzymes
- Last updated
- Save as PDF
- Page ID 165287
Introduction
An enzyme's active sites are usually composed of amino acid residues; depending on which amino acid residues are present, the specificity of the substrate can vary greatly. Depending on the pH level, the physical properties (mainly the electric charge) of an enzyme can change. A change in the electric charge can alter the interaction between the active site amino acid residues and the incoming substrate. With that said, the substrate can bind to the active site via hydrogen bonding or van der Waals forces. Once the substrate binds to the active site it forms an enzyme-substrate complex that is then involved in further chemical reactions.
In order for an enzyme to be active and be energetically favorable to allow a chemical reaction to proceed forward, a substrate must bind to an enzyme's "active site". An active site can be thought of as a lock and the substrate as a key; this is known as the lock and key model . A key (substrate) must be inserted and turned (chemical reaction), then the lock (enzyme) opens (production of products). Note that an enzyme might have more than one active site. Another theory on the active site-substrate relationship is the induced fit theory , which is quite opposite of the lock and key theory (where the active site is seemingly inflexible). In the induced fit theory, the active site of the enzyme is very flexible, and only changes its conformation when the substrate binds to it.
Figure 5.1.1: According to the induced fit model, both enzyme and substrate undergo dynamic conformational changes upon binding. The enzyme contorts the substrate into its transition state, thereby increasing the rate of the reaction.
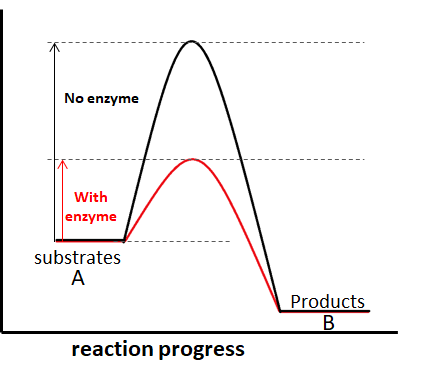
Enzymes work as a catalyst by lowering the Gibbs free energy of activation of the enzyme-substrate complex. Below are two figures showing a basic enzymatic reaction with and without a catalyst.
Enzyme Active Site and Substrate Specificity
Enzymes bind with chemical reactants called substrates. There may be one or more substrates for each type of enzyme, depending on the particular chemical reaction. In some reactions, a single-reactant substrate is broken down into multiple products. In others, two substrates may come together to create one larger molecule. Two reactants might also enter a reaction, both become modified, and leave the reaction as two products.
The enzyme’s active site binds to the substrate. Since enzymes are proteins, this site is composed of a unique combination of amino acid residues (side chains or R groups). Each amino acid residue can be large or small; weakly acidic or basic; hydrophilic or hydrophobic; and positively-charged, negatively-charged, or neutral. The positions, sequences, structures, and properties of these residues create a very specific chemical environment within the active site. A specific chemical substrate matches this site like a jigsaw puzzle piece and makes the enzyme specific to its substrate.
Active Sites and Environmental Conditions
Environmental conditions can affect an enzyme’s active site and, therefore, the rate at which a chemical reaction can proceed. Increasing the environmental temperature generally increases reaction rates because the molecules are moving more quickly and are more likely to come into contact with each other.
However, increasing or decreasing the temperature outside of an optimal range can affect chemical bonds within the enzyme and change its shape. If the enzyme changes shape, the active site may no longer bind to the appropriate substrate and the rate of reaction will decrease. Dramatic changes to the temperature and pH will eventually cause enzymes to denature.
Enzyme-Substrate Complex
When an enzyme binds its substrate, it forms an enzyme-substrate complex. This complex lowers the activation energy of the reaction and promotes its rapid progression by providing certain ions or chemical groups that actually form covalent bonds with molecules as a necessary step of the reaction process. Enzymes also promote chemical reactions by bringing substrates together in an optimal orientation, lining up the atoms and bonds of one molecule with the atoms and bonds of the other molecule. This can contort the substrate molecules and facilitate bond-breaking. The active site of an enzyme also creates an ideal environment, such as a slightly acidic or non-polar environment, for the reaction to occur. The enzyme will always return to its original state at the completion of the reaction. One of the important properties of enzymes is that they remain ultimately unchanged by the reactions they catalyze. After an enzyme is done catalyzing a reaction, it releases its products (substrates).

Home » Science » Chemistry » Biochemistry » Enzymology » What is the Difference Between Induced Fit and Lock and Key
What is the Difference Between Induced Fit and Lock and Key
The main difference between induced fit and lock and key model is that in the induced fit model, the active site of the enzyme does not completely fit to the substrate whereas in the lock and key model, the active site of the enzyme is the complement of the substrate and hence, it precisely fits to the substrate. Furthermore, in the induced fit model, the active site of the enzyme has to undergo a conformational change to improve binding while the lock and key model describes the specificity of the active site of the enzyme to a particular substrate.
Induced fit and lock and key model are the two models of enzyme-substrate interactions. Generally, they describe how the enzymes interact with the substrate.
Key Areas Covered
1. What is Induced Fit Model – Definition, Mechanism of Action, Significance 2. What is Lock and Key Model – Definition, Mechanism of Action, Significance 3. What are the Similarities Between Induced Fit and Lock and Key Model – Outline of Common Features 4. What is the Difference Between Induced Fit and Lock and Key Model – Comparison of Key Differences

Key Terms
Active Site, Enzyme, Induced Fit Model, Lock and Key Model, Substrate

What is Induced Fit Model
The induced fit model is one of the main models , describing the enzyme-substrate interaction. Also, Daniel Koshland suggested this model in 1958. Basically, according to the hypothesis, the active site of the enzyme does not have a rigid conformation. Therefore, the substrate does not completely fit into the active site of the enzyme. Hence, the active site of the enzyme modifies its shape upon the binding of the substrate, becoming complementary to the shape of the substrate. Significantly, this conformational change is possible due to the flexibility of the protein molecule, which serves as the ellnzyme.

Figure 1: Induced Fit Model of Hexokinase
Furthermore, the active site of the enzyme is not static and it requires a separate catalytic group for the action of the enzyme. However, the binding of the catalytic group weakens the bonds formed by the substrate with the active site. Thereby, the induced fit model describes the mechanism of nonaction over competitive inhibitors .
What is Lock and Key Model
Lock and key model is the second model, which describes the enzyme-substrate interaction. However, Emil Fischer suggested this model in 1894. Therefore, it is also called Fisher’s theory. According to the lock and key model, the active site of the enzymes serves as the ‘lock’ while its substrate serves as the ‘key’. On that account, the shape of the active site of the enzyme is complementary to the shape of the substrate. Thereby, the active site of the enzyme can hold the substrate closer to the enzyme by forming an unusable intermediate compound, which is the enzyme-substrate complex.

Figure 2: Induced Fit and Lock and Key Models
Moreover, the close proximity allows the biological reaction to proceed. Therefore, the subsequent dissociation of the enzyme-substrate complex results in the enzyme and the products. Also, the lock and key model does not need a separate catalytic group for the action of the enzyme. In addition to these, the static active site of the enzyme consists of a single entity in the lock and key model.
Similarities Between Induced Fit and Lock and Key Model
- Induced fit and lock and key are the two models, which describe the mechanism of action of the enzyme.
- Both models depend on the degree of precise binding of the substrate to the active site of the enzyme.
- They are important in describing how enzymes increase the rate of a biological reaction through catalysis.
- Both models reduce the activation energy of a specific biochemical reaction .
Difference Between Induced Fit and Lock and Key Model
Definition .
The induced-fit model refers to a model for enzyme-substrate interaction in which the active site of the enzyme does not completely fit to the substrate. On the other hand, the lock and key model refers to a second model for enzyme-substrate interaction in which the active site of the enzyme completely fits with the substrate.
Suggested by
The induced fit model was suggested by Daniel Koshland in 1958 while the lock and key model was suggested by Emil Fischer in 1894.
Fitting of the Active Site of the Enzyme to the Substrate
The active site of the enzyme does not completely fit with the substrate in the induced fit model, while the active site of the enzyme precisely fits with the substrate in the lock and key model.
Significance of the Active Site
In the induced fit model, the active site of the enzyme has to undergo a conformational change to improve binding, while the lock and key model describes the specificity of the active site of the enzyme to a particular substrate.
Active Site Composition
The active site of the enzyme contains two components in the induced fit model, while the active site of the enzyme contains a single entity in the lock and key model.
Catalytic Groups
There is a separate catalytic group in the enzyme in the induced fit model while there is no separate catalytic group in the enzyme in the lock and key model.
Properties of the Active Site
The active site of the enzyme is not static in the induced fit model, while the active site of the enzyme is static in the lock and key model.
Development of a Transition State
A transition state develops before the reactants undergo changes in the induced fit model, while a transition state does not develop before the reactants undergo changes in the lock and key model.
The weakening of the Catalytic Bonds
Catalytic group weakens the substrate bonds either by the nucleophilic or electrophilic attack in the induced fit model, while the catalytic group does not weaken the substrate bonds in the lock and key model.
Nonaction over Competitive Inhibitors
The induced-fit model describes the mechanism of nonaction over competitive inhibitors, while the lock and key model describes the specificity of the active site of the enzyme to a particular substrate.
Conclusion
In brief, the induced fit model is a model for enzyme-substrate interactions in which the substrate does not completely fit into the active site of the enzyme. Hence, the active site of the enzyme has to undergo a conformational change while binding to the substrate. In comparison, the lock and key model is a second model for enzyme-substrate interaction in which the substrate completely fits into the active site of the enzyme. Therefore, it describes the specificity of binding of the active site of the enzyme towards a particular substrate. Therefore, the main difference between induced fit and lock and key model is the mechanism of substrate binding and importance.
References:
1. Cornell, Brent. “Models of Action.” BioNinja , Available Here .
Image Courtesy:
1. “Hexokinase induced fit” By Thomas Shafee – Own work ( CC BY 4.0 ) via Commons Wikimedia 2. “CNX Chem 12 07 Enzyme” By OpenStax ( CC BY 4.0 ) via Commons Wikimedia
About the Author: Lakna
Lakna, a graduate in Molecular Biology and Biochemistry, is a Molecular Biologist and has a broad and keen interest in the discovery of nature related things. She has a keen interest in writing articles regarding science.
You May Also Like These
Leave a reply cancel reply.
Ch. 9 The Development of Russia
Ivan i and the rise of moscow, learning objective.
- Outline the key points that helped Moscow become so powerful and how Ivan I accomplished these major victories
- Moscow was considered a small trading outpost under the principality of Vladimir-Suzdal into the 13th century.
- Power struggles and constant raids under the Mongol Empire’s Golden Horde caused once powerful cities, such as Kiev, to struggle financially and culturally.
- Ivan I utilized the relative calm and safety of the northern city of Moscow to entice a larger population and wealth to move there.
- Alliances between Golden Horde leaders and Ivan I saved Moscow from many of the raids and destruction of other centers, like Tver.
A rival city to Moscow that eventually lost favor under the Golden Horde.
Grand Prince of Vladimir
The title given to the ruler of this northern province, where Moscow was situated.
The Rise of Moscow
Moscow was only a small trading outpost in the principality of Vladimir-Suzdal in Kievan Rus’ before the invasion of Mongol forces during the 13th century. However, due to the unstable environment of the Golden Horde, and the deft leadership of Ivan I at a critical time during the 13th century, Moscow became a safe haven of prosperity during his reign. It also became the new seat of power of the Russian Orthodox Church.
Ivan I (also known as Ivan Kalita) was born around 1288 to the Prince of Moscow, Daniil Aleksandrovich. He was born during a time of devastation and upheaval in Rus’. Kiev had been overtaken by the invading Mongol forces in 1240, and most of the Rus’ principalities had been absorbed into the Golden Horde of the Mongol Empire by the time Ivan was born. He ascended to the seat of Prince of Moscow after the death of his father, and then the death of his older brother Yury.

Ivan I. He was born around 1288 and died in either 1340 or 1341, still holding the title of Grand Prince of Vladimir.
Ivan I stepped into a role that had already been expanded by his predecessors. Both his older brother and his father had captured nearby lands, including Kolomna and Mozhaisk. Yury had also made a successful alliance with the Mongol leader Uzbeg Khan and married his sister, securing more power and advantages within the hierarchy of the Golden Horde.
Ivan I continued the family tradition and petitioned the leaders of the Golden Horde to gain the seat of Grand Prince of Vladimir. His other three rivals, all princes of Tver, had previously been granted the title in prior years. However they were all subsequently deprived of the title and all three aspiring princes also eventually ended up murdered. Ivan I, on the other hand, garnered the title from Khan Muhammad Ozbeg in 1328. This new title, which he kept until his death around 1340, meant he could collect taxes from the Russian lands as a ruling prince and position his tiny city as a major player in the Vladimir region.
Moscow’s Rise
During this time of upheaval, the tiny outpost of Moscow had multiple advantages that repositioned this town and set it up for future prosperity under Ivan I. Three major contributing factors helped Ivan I relocate power to this area:
- It was situated in between other major principalities on the east and west so it was often protected from the more devastating invasions.
- This relative safety, compared to Tver and Ryazan, for example, started to bring in tax-paying citizens who wanted a safe place to build a home and earn a livelihood.
- Finally, Moscow was set up perfectly along the trade route from Novgorod to the Volga River, giving it an economic advantage from the start.
Ivan I also spurred on the growth of Moscow by actively recruiting people to move to the region. In addition, he bought the freedom of people who had been captured by the extensive Mongol raids. These recruits further bolstered the population of Moscow. Finally, he focused his attention on establishing peace and routing out thieves and raiding parties in the region, making for a safe and calm metaphorical island in a storm of unsettled political and military upsets.

Kievan Rus’ 1220-1240. This map illustrates the power dynamics at play during the 13th century shortly before Ivan I was born. Sarai, the capital of the Golden Horde, sat to the southeast, while Moscow (not visible on this map) was tucked up in the northern forests of Vladimir-Suzdal.
Ivan I knew that the peace of his region depended upon keeping up an alliance with the Golden Horde, which he did faithfully. Moscow’s increased wealth during this era also allowed him to loan money to neighboring principalities. These regions then became indebted to Moscow, bolstering its political and financial position.
In addition, a few neighboring cities and villages were subsumed into Moscow during the 1320s and 1330s, including Uglich, Belozero, and Galich. These shifts slowly transformed the tiny trading outpost into a bustling city center in the northern forests of what was once Kievan Rus’.
Russian Orthodox Church and The Center of Moscow
Ivan I committed some of Moscow’s new wealth to building a splendid city center and creating an iconic religious setting. He built stone churches in the center of Moscow with his newly gained wealth. Ivan I also tempted one of the most important religious leaders in Rus’, the Orthodox Metropolitan Peter, to the city of Moscow. Before the rule of the Golden Horde the original Russian Orthodox Church was based in Kiev. After years of devastation, Metropolitan Peter transferred the seat of power to Moscow where a new Renaissance of culture was blossoming. This perfectly timed transformation of Moscow coincided with the decades of devastation in Kiev, effectively transferring power to the north once again.

Peter of Moscow and scenes from his life as depicted in a 15th-century icon. This religious leader helped bring cultural power to Moscow by moving the seat of the Russian Orthodox Church there during Ivan I’s reign.
One of the most lasting accomplishments of Ivan I was to petition the Khan based in Sarai to designate his son, who would become Simeon the Proud, as the heir to the title of Grand Prince of Vladimir. This agreement a line of succession that meant the ruling head of Moscow would almost always hold power over the principality of Vladimir, ensuring Moscow held a powerful position for decades to come.
- Boundless World History. Authored by : Boundless. Located at : https://www.boundless.com/world-history/textbooks/boundless-world-history-textbook/ . License : CC BY-SA: Attribution-ShareAlike
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

frankenstein
22 templates

el salvador
32 templates

summer vacation
19 templates

44 templates

17 templates

pediatrician
27 templates
Travel Guide: Moscow
Travel guide: moscow presentation, free google slides theme and powerpoint template.
Do you know some acquaintances that want to travel to Russia, the biggest country in this planet? Now you can be their own tour guide with this template. Include as much information as possible about tourist attractions, monuments and things to do in Moscow. Let the simplicity of these slides and their cool illustrations speak in favor too!
Features of this template
- 100% editable and easy to modify
- 25 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access


IMAGES
VIDEO
COMMENTS
9. THE LOCK AND KEY MODEL The lock and key hypothesis is focused on the active site The active site of an enzyme has a very unique geometric shape and it is only complementary to a specific substrate molecule. Imagine a puzzle piece. There are only a few pieces that fit with that one piece. Because the active sites are so geometrically unique, an enzyme can only work with a few or just one ...
1 The Lock and Key Hypothesis. Fit between the substrate and the active site of the enzyme is exact Like a key fits into a lock very precisely The key is analogous to the enzyme and the substrate analogous to the lock. Temporary structure called the enzyme-substrate complex formed Products have a different shape from the substrate Once formed ...
Lock and Key Model. A German scientist, Emil Fischer postulated the lock and key model in 1894 to explain the enzyme's mode of action. Fischer's theory hypothesized that enzymes exhibit a high degree of specificity towards the substrate. This model assumes that the active site of the enzyme and the substrate fit perfectly into one another ...
Figure 1 The 'lock and key' model of enzyme action. Fischer's powerful model explained the experimental observations produced by researchers at the time and remained the accepted theory for 60 years. As new experimental techniques allowed researchers to probe enzyme action more closely, a number of experimental observations emerged that ...
Lock-and-key vs. Induced Fit Model. At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model, and the other is the Induced fit model.The lock and key model theory was first postulated by Emil Fischer in 1894.The lock-and-key enzyme action proposes the high specificity of enzymes.
Lock and Key Model. The Lock and Key model is a theory of enzyme action hypothesized by Emil Fischer in 1899. According to Fischer, enzymes exhibit a high degree of specificity to the substances ...
Figure \(\PageIndex{2}\): The Lock-and-Key Model of Enzyme Action. (a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex. ... The current theory ...
The Key-Lock Theory and the Induced Fit Theory. Angew. Chem. Inl. Ed. Engl. 33, Koshland, D. E. (2004). Crazy, but correct. Nature, 432. Download ppt "From lock and key model to induced fit model" Similar presentations . Enzymes … what do you know and will find out these next few lessons Lets find out millionaire styley remember the tips from ...
In 1894, Emil Fisher discovered that glycolytic enzymes are able to distinguish between sugar stereoisomers. Based upon that discovery, he formulated the lock-and-key hypothesis (Fischer 1894), which proposed that enzymes recognize their substrates just as a lock receives a key.That is, only in the case of exact geometric complementarity between the substrate (key) and enzyme (lock) is the ...
Therefore, each enzyme is substrate specific. Models of How Enzymes Work 1. Lock and Key model 2. Induced Fit model Lock and Key Model Substrate (key) fits to the active site (lock) which provides a microenvironment for the specific reaction. ... Enzyme Theory Last modified by: Elementary/Secondary Schools Created Date: 2/13/2006 12:27:42 PM ...
The Lock-and-Key Hypothesis: Evolutionary and Biosystematic Interpretation of Insect Genitalia
Lock and key hypothesis. Lock and key model of Enzymes. The lock and key model was proposed by Emil Fischer in 1898 and is also known as the template model. According to this model, the binding of the substrate and the enzyme takes place at the active site in a manner similar to the one where a key fits a lock and results in the formation of an ...
Enzymes powerpoint for 27th may y12 bio. ... (Lock and Key Model, Induced-Fit Model) • Describe the effects of temperature, pH, and substrate concentration on enzyme activity • Define co-enzymes and co-factors, give examples and describe their role in enzymatic activity. • Uses of Enzymes 3.
Proudly South Africa powerpoint Thorisha.pptx thorishapillay1 ... LOCK AND KEY MODEL Proposed by EMIL FISCHER in 1894. Lock and key hypothesis assumes the active site of an enzymes are rigid in its shape. There is no change in the active site before and after a chemical reaction.
According to the lock and key model, the active site of an enzyme and its substrate have the same shape.So they perfectly fit into each other. The idea was, ...
Figure 18.11 The Lock-and-Key Model of Enzyme Action. (a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex. Working out the precise three-dimensional ...
A key (substrate) must be inserted and turned (chemical reaction), then the lock (enzyme) opens (production of products). Note that an enzyme might have more than one active site. Another theory on the active site-substrate relationship is the induced fit theory , which is quite opposite of the lock and key theory (where the active site is ...
Lock and key model is the second model, which describes the enzyme-substrate interaction. However, Emil Fischer suggested this model in 1894. Therefore, it is also called Fisher's theory. According to the lock and key model, the active site of the enzymes serves as the 'lock' while its substrate serves as the 'key'.
Ivan I (also known as Ivan Kalita) was born around 1288 to the Prince of Moscow, Daniil Aleksandrovich. He was born during a time of devastation and upheaval in Rus'. Kiev had been overtaken by the invading Mongol forces in 1240, and most of the Rus' principalities had been absorbed into the Golden Horde of the Mongol Empire by the time ...
Europe's tallest building, the Federation Tower, is in the "Moscow IBC. The complex also includes the second-tallest, third-tallest, fifth-tallest, sixth-tallest, and seventh-tallest buildings in Europe.
Lock and Key Hypothesis - A New Perspective. Blu Ocean Studios Private Limited is an organization, which has been founded under the firm belief of using research and innovation to drive authentic solutions in the field of Healthcare and wellness. The world has been facing a larger turmoil either due to the environmental factors, the epidemic ...
Free Google Slides theme and PowerPoint template. Do you know some acquaintances that want to travel to Russia, the biggest country in this planet? Now you can be their own tour guide with this template. Include as much information as possible about tourist attractions, monuments and things to do in Moscow. Let the simplicity of these slides ...
S. shubham sharma. Moscow TRANSPORT Strategy. Engineering. 1 of 22. Download Now. Download to read offline. Moscow, comprehensive mobility plan - Download as a PDF or view online for free.