- En español – ExME
- Em português – EME

An introduction to different types of study design
Posted on 6th April 2021 by Hadi Abbas

Study designs are the set of methods and procedures used to collect and analyze data in a study.
Broadly speaking, there are 2 types of study designs: descriptive studies and analytical studies.
Descriptive studies
- Describes specific characteristics in a population of interest
- The most common forms are case reports and case series
- In a case report, we discuss our experience with the patient’s symptoms, signs, diagnosis, and treatment
- In a case series, several patients with similar experiences are grouped.
Analytical Studies
Analytical studies are of 2 types: observational and experimental.
Observational studies are studies that we conduct without any intervention or experiment. In those studies, we purely observe the outcomes. On the other hand, in experimental studies, we conduct experiments and interventions.
Observational studies
Observational studies include many subtypes. Below, I will discuss the most common designs.
Cross-sectional study:
- This design is transverse where we take a specific sample at a specific time without any follow-up
- It allows us to calculate the frequency of disease ( p revalence ) or the frequency of a risk factor
- This design is easy to conduct
- For example – if we want to know the prevalence of migraine in a population, we can conduct a cross-sectional study whereby we take a sample from the population and calculate the number of patients with migraine headaches.
Cohort study:
- We conduct this study by comparing two samples from the population: one sample with a risk factor while the other lacks this risk factor
- It shows us the risk of developing the disease in individuals with the risk factor compared to those without the risk factor ( RR = relative risk )
- Prospective : we follow the individuals in the future to know who will develop the disease
- Retrospective : we look to the past to know who developed the disease (e.g. using medical records)
- This design is the strongest among the observational studies
- For example – to find out the relative risk of developing chronic obstructive pulmonary disease (COPD) among smokers, we take a sample including smokers and non-smokers. Then, we calculate the number of individuals with COPD among both.
Case-Control Study:
- We conduct this study by comparing 2 groups: one group with the disease (cases) and another group without the disease (controls)
- This design is always retrospective
- We aim to find out the odds of having a risk factor or an exposure if an individual has a specific disease (Odds ratio)
- Relatively easy to conduct
- For example – we want to study the odds of being a smoker among hypertensive patients compared to normotensive ones. To do so, we choose a group of patients diagnosed with hypertension and another group that serves as the control (normal blood pressure). Then we study their smoking history to find out if there is a correlation.
Experimental Studies
- Also known as interventional studies
- Can involve animals and humans
- Pre-clinical trials involve animals
- Clinical trials are experimental studies involving humans
- In clinical trials, we study the effect of an intervention compared to another intervention or placebo. As an example, I have listed the four phases of a drug trial:
I: We aim to assess the safety of the drug ( is it safe ? )
II: We aim to assess the efficacy of the drug ( does it work ? )
III: We want to know if this drug is better than the old treatment ( is it better ? )
IV: We follow-up to detect long-term side effects ( can it stay in the market ? )
- In randomized controlled trials, one group of participants receives the control, while the other receives the tested drug/intervention. Those studies are the best way to evaluate the efficacy of a treatment.
Finally, the figure below will help you with your understanding of different types of study designs.

References (pdf)
You may also be interested in the following blogs for further reading:
An introduction to randomized controlled trials
Case-control and cohort studies: a brief overview
Cohort studies: prospective and retrospective designs
Prevalence vs Incidence: what is the difference?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on An introduction to different types of study design
you are amazing one!! if I get you I’m working with you! I’m student from Ethiopian higher education. health sciences student
Very informative and easy understandable
You are my kind of doctor. Do not lose sight of your objective.
Wow very erll explained and easy to understand
I’m Khamisu Habibu community health officer student from Abubakar Tafawa Balewa university teaching hospital Bauchi, Nigeria, I really appreciate your write up and you have make it clear for the learner. thank you
well understood,thank you so much
Well understood…thanks
Simply explained. Thank You.
Thanks a lot for this nice informative article which help me to understand different study designs that I felt difficult before
That’s lovely to hear, Mona, thank you for letting the author know how useful this was. If there are any other particular topics you think would be useful to you, and are not already on the website, please do let us know.
it is very informative and useful.
thank you statistician
Fabulous to hear, thank you John.
Thanks for this information
Thanks so much for this information….I have clearly known the types of study design Thanks
That’s so good to hear, Mirembe, thank you for letting the author know.
Very helpful article!! U have simplified everything for easy understanding
I’m a health science major currently taking statistics for health care workers…this is a challenging class…thanks for the simified feedback.
That’s good to hear this has helped you. Hopefully you will find some of the other blogs useful too. If you see any topics that are missing from the website, please do let us know!
Hello. I liked your presentation, the fact that you ranked them clearly is very helpful to understand for people like me who is a novelist researcher. However, I was expecting to read much more about the Experimental studies. So please direct me if you already have or will one day. Thank you
Dear Ay. My sincere apologies for not responding to your comment sooner. You may find it useful to filter the blogs by the topic of ‘Study design and research methods’ – here is a link to that filter: https://s4be.cochrane.org/blog/topic/study-design/ This will cover more detail about experimental studies. Or have a look on our library page for further resources there – you’ll find that on the ‘Resources’ drop down from the home page.
However, if there are specific things you feel you would like to learn about experimental studies, that are missing from the website, it would be great if you could let me know too. Thank you, and best of luck. Emma
Great job Mr Hadi. I advise you to prepare and study for the Australian Medical Board Exams as soon as you finish your undergrad study in Lebanon. Good luck and hope we can meet sometime in the future. Regards ;)
You have give a good explaination of what am looking for. However, references am not sure of where to get them from.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

A well-designed cohort study can provide powerful results. This blog introduces prospective and retrospective cohort studies, discussing the advantages, disadvantages and use of these type of study designs.
News alert: UC Berkeley has announced its next university librarian
Secondary menu
- Log in to your Library account
- Hours and Maps
- Connect from Off Campus
- UC Berkeley Home
Search form
Research methods--quantitative, qualitative, and more: overview.
- Quantitative Research
- Qualitative Research
- Data Science Methods (Machine Learning, AI, Big Data)
- Text Mining and Computational Text Analysis
- Evidence Synthesis/Systematic Reviews
- Get Data, Get Help!
About Research Methods
This guide provides an overview of research methods, how to choose and use them, and supports and resources at UC Berkeley.
As Patten and Newhart note in the book Understanding Research Methods , "Research methods are the building blocks of the scientific enterprise. They are the "how" for building systematic knowledge. The accumulation of knowledge through research is by its nature a collective endeavor. Each well-designed study provides evidence that may support, amend, refute, or deepen the understanding of existing knowledge...Decisions are important throughout the practice of research and are designed to help researchers collect evidence that includes the full spectrum of the phenomenon under study, to maintain logical rules, and to mitigate or account for possible sources of bias. In many ways, learning research methods is learning how to see and make these decisions."
The choice of methods varies by discipline, by the kind of phenomenon being studied and the data being used to study it, by the technology available, and more. This guide is an introduction, but if you don't see what you need here, always contact your subject librarian, and/or take a look to see if there's a library research guide that will answer your question.
Suggestions for changes and additions to this guide are welcome!
START HERE: SAGE Research Methods
Without question, the most comprehensive resource available from the library is SAGE Research Methods. HERE IS THE ONLINE GUIDE to this one-stop shopping collection, and some helpful links are below:
- SAGE Research Methods
- Little Green Books (Quantitative Methods)
- Little Blue Books (Qualitative Methods)
- Dictionaries and Encyclopedias
- Case studies of real research projects
- Sample datasets for hands-on practice
- Streaming video--see methods come to life
- Methodspace- -a community for researchers
- SAGE Research Methods Course Mapping
Library Data Services at UC Berkeley
Library Data Services Program and Digital Scholarship Services
The LDSP offers a variety of services and tools ! From this link, check out pages for each of the following topics: discovering data, managing data, collecting data, GIS data, text data mining, publishing data, digital scholarship, open science, and the Research Data Management Program.
Be sure also to check out the visual guide to where to seek assistance on campus with any research question you may have!
Library GIS Services
Other Data Services at Berkeley
D-Lab Supports Berkeley faculty, staff, and graduate students with research in data intensive social science, including a wide range of training and workshop offerings Dryad Dryad is a simple self-service tool for researchers to use in publishing their datasets. It provides tools for the effective publication of and access to research data. Geospatial Innovation Facility (GIF) Provides leadership and training across a broad array of integrated mapping technologies on campu Research Data Management A UC Berkeley guide and consulting service for research data management issues
General Research Methods Resources
Here are some general resources for assistance:
- Assistance from ICPSR (must create an account to access): Getting Help with Data , and Resources for Students
- Wiley Stats Ref for background information on statistics topics
- Survey Documentation and Analysis (SDA) . Program for easy web-based analysis of survey data.
Consultants
- D-Lab/Data Science Discovery Consultants Request help with your research project from peer consultants.
- Research data (RDM) consulting Meet with RDM consultants before designing the data security, storage, and sharing aspects of your qualitative project.
- Statistics Department Consulting Services A service in which advanced graduate students, under faculty supervision, are available to consult during specified hours in the Fall and Spring semesters.
Related Resourcex
- IRB / CPHS Qualitative research projects with human subjects often require that you go through an ethics review.
- OURS (Office of Undergraduate Research and Scholarships) OURS supports undergraduates who want to embark on research projects and assistantships. In particular, check out their "Getting Started in Research" workshops
- Sponsored Projects Sponsored projects works with researchers applying for major external grants.
- Next: Quantitative Research >>
- Last Updated: Apr 3, 2023 3:14 PM
- URL: https://guides.lib.berkeley.edu/researchmethods
Research Study Types
There are many different types of research studies, and each has distinct strengths and weaknesses. In general, randomized trials and cohort studies provide the best information when looking at the link between a certain factor (like diet) and a health outcome (like heart disease).
Laboratory and Animal Studies
These are studies done in laboratories on cells, tissue, or animals.
- Strengths: Laboratories provide strictly controlled conditions and are often the genesis of scientific ideas that go on to have a broad impact on human health. They can help understand the mechanisms of disease.
- Weaknesses: Laboratory and animal studies are only a starting point. Animals or cells are not a substitute for humans.
Cross-Sectional Surveys
These studies examine the incidence of a certain outcome (disease or other health characteristic) in a specific group of people at one point in time. Surveys are often sent to participants to gather data about the outcome of interest.
- Strengths: Inexpensive and easy to perform.
- Weaknesses: Can only establish an association in that one specific time period.
Case-Control Studies
These studies look at the characteristics of one group of people who already have a certain health outcome (the cases) and compare them with a similar group of people who do not have the outcome (the controls). An example may be looking at a group of people with heart disease and another group without heart disease who are similar in age, sex, and economic status, and comparing their intakes of fruits and vegetables to see if this exposure could be associated with heart disease risk.
- Strengths: Case-control studies can be done quickly and relatively cheaply.
- Weaknesses: Not ideal for studying diet because they gather information from the past, which can be difficult for most people to recall accurately. Furthermore, people with illnesses often recall past behaviors differently from those without illness. This opens such studies to potential inaccuracy and bias in the information they gather.
Cohort Studies
These are observational studies that follow large groups of people over a long period of time, years or even decades, to find associations of an exposure(s) with disease outcomes. Researchers regularly gather information from the people in the study on several variables (like meat intake, physical activity level, and weight). Once a specified amount of time has elapsed, the characteristics of people in the group are compared to test specific hypotheses (such as a link between high versus low intake of carotenoid-rich foods and glaucoma, or high versus low meat intake and prostate cancer).
- Strengths: Participants are not required to change their diets or lifestyle as may be with randomized controlled studies. Study sizes may be larger than other study types. They generally provide more reliable information than case-control studies because they don’t rely on information from the past. Cohort studies gather information from participants at the beginning and throughout the study, long before they may develop the disease being studied. As a group, many of these types of studies have provided valuable information about the link between lifestyle factors and disease.
- Weaknesses: A longer duration of following participants make these studies time-consuming and expensive. Results cannot suggest cause-and-effect, only associations. Evaluation of dietary intake is self-reported.
Two of the largest and longest-running cohort studies of diet are the Harvard-based Nurses’ Health Study and the Health Professionals Follow-up Study.
If you follow nutrition news, chances are you have come across findings from a cohort called the Nurses’ Health Study . The Nurses’ Health Study (NHS) began in 1976, spearheaded by researchers from the Channing Laboratory at the Brigham and Women’s Hospital, Harvard Medical School, and the Harvard T.H. Chan School of Public Health, with funding from the National Institutes of Health. It gathered registered nurses ages 30-55 years from across the U.S. to respond to a series of questionnaires. Nurses were specifically chosen because of their ability to complete the health-related, often very technical, questionnaires thoroughly and accurately. They showed motivation to participate in the long-term study that required ongoing questionnaires every two years. Furthermore, the group provided blood, urine, and other samples over the course of the study.
The NHS is a prospective cohort study, meaning a group of people who are followed forward in time to examine lifestyle habits or other characteristics to see if they develop a disease, death, or some other indicated outcome. In comparison, a retrospective cohort study would specify a disease or outcome and look back in time at the group to see if there were common factors leading to the disease or outcome. A benefit of prospective studies over retrospective studies is greater accuracy in reporting details, such as food intake, that is not distorted by the diagnosis of illness.
To date, there are three NHS cohorts: NHS original cohort, NHS II, and NHS 3. Below are some features unique to each cohort.
NHS – Original Cohort
- Started in 1976 by Frank Speizer, M.D.
- Participants: 121,700 married women, ages 30 to 55 in 1976.
- Outcomes studied: Impact of contraceptive methods and smoking on breast cancer; later this was expanded to observe other lifestyle factors and behaviors in relation to 30 diseases.
- A food frequency questionnaire was added in 1980 to collect information on dietary intake, and continues to be collected every four years.
- Started in 1989 by Walter Willett, M.D., M.P.H., Dr.P.H., and colleagues.
- Participants: 116,430 single and married women, ages 25 to 42 in 1989.
- Outcomes studied: Impact on women’s health of oral contraceptives initiated during adolescence, diet and physical activity in adolescence, and lifestyle risk factors in a younger population than the NHS Original Cohort. The wide range of diseases examined in the original NHS is now also being studied in NHSII.
- The first food frequency questionnaire was collected in 1991, and is collected every four years.
- Started in 2010 by Jorge Chavarro, M.D., Sc.M., Sc.D, Walter Willett, M.D., M.P.H., Dr.P.H., Janet Rich-Edwards, Sc.D., M.P.H, and Stacey Missmer, Sc.D.
- Participants: Expanded to include not just registered nurses but licensed practical nurses (LPN) and licensed vocational nurses (LVN), ages 19 to 46. Enrollment is currently open.
- Inclusion of more diverse population of nurses, including male nurses and nurses from Canada.
- Outcomes studied: Dietary patterns, lifestyle, environment, and nursing occupational exposures that may impact men’s and women’s health; the impact of new hormone preparations and fertility/pregnancy on women’s health; relationship of diet in adolescence on breast cancer risk.
From these three cohorts, extensive research has been published regarding the association of diet, smoking, physical activity levels, overweight and obesity, oral contraceptive use, hormone therapy, endogenous hormones, dietary factors, sleep, genetics, and other behaviors and characteristics with various diseases. In 2016, in celebration of the 40 th Anniversary of NHS, the American Journal of Public Health’s September issue was dedicated to featuring the many contributions of the Nurses’ Health Studies to public health.
Growing Up Today Study (GUTS)
In 1996, recruitment began for a new cross-generational cohort called GUTS (Growing Up Today Study) —children of nurses from the NHS II. GUTS is composed of 27,802 girls and boys who were between the ages of 9 and 17 at the time of enrollment. As the entire cohort has entered adulthood, they complete annual questionnaires including information on dietary intake, weight changes, exercise level, substance and alcohol use, body image, and environmental factors. Researchers are looking at conditions more common in young adults such as asthma, skin cancer, eating disorders, and sports injuries.
Randomized Trials
Like cohort studies, these studies follow a group of people over time. However, with randomized trials, the researchers intervene with a specific behavior change or treatment (such as following a specific diet or taking a supplement) to see how it affects a health outcome. They are called “randomized trials” because people in the study are randomly assigned to either receive or not receive the intervention. This randomization helps researchers determine the true effect the intervention has on the health outcome. Those who do not receive the intervention or labelled the “control group,” which means these participants do not change their behavior, or if the study is examining the effects of a vitamin supplement, the control group participants receive a placebo supplement that contains no active ingredients.
- Strengths: Considered the “gold standard” and best for determining the effectiveness of an intervention (e.g., dietary pattern, supplement) on an endpoint such as cancer or heart disease. Conducted in a highly controlled setting with limited variables that could affect the outcome. They determine cause-and-effect relationships.
- Weaknesses: High cost, potentially low long-term compliance with prescribed diets, and possible ethical issues. Due to expense, the study size may be small.
Meta-Analyses and Systematic Reviews
A meta-analysis collects data from several previous studies on one topic to analyze and combine the results using statistical methods to provide a summary conclusion. Meta-analyses are usually conducted using randomized controlled trials and cohort studies that have higher quality of evidence than other designs. A systematic review also examines past literature related to a specific topic and design, analyzing the quality of studies and results but may not pool the data. Sometimes a systematic review is followed by conducting a meta-analysis if the quality of the studies is good and the data can be combined.
- Strengths: Inexpensive and provides a general comprehensive summary of existing research on a topic. This can create an explanation or assumption to be used for further investigation.
- Weaknesses: Prone to selection bias, as the authors can choose or exclude certain studies, which can change the resulting outcome. Combining data that includes lower-quality studies can also skew the results.
A primer on systematic review and meta-analysis in diabetes research
Terms of use.
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.

Community Blog
Keep up-to-date on postgraduate related issues with our quick reads written by students, postdocs, professors and industry leaders.
Types of Research – Explained with Examples
- By DiscoverPhDs
- October 2, 2020

Types of Research
Research is about using established methods to investigate a problem or question in detail with the aim of generating new knowledge about it.
It is a vital tool for scientific advancement because it allows researchers to prove or refute hypotheses based on clearly defined parameters, environments and assumptions. Due to this, it enables us to confidently contribute to knowledge as it allows research to be verified and replicated.
Knowing the types of research and what each of them focuses on will allow you to better plan your project, utilises the most appropriate methodologies and techniques and better communicate your findings to other researchers and supervisors.
Classification of Types of Research
There are various types of research that are classified according to their objective, depth of study, analysed data, time required to study the phenomenon and other factors. It’s important to note that a research project will not be limited to one type of research, but will likely use several.
According to its Purpose
Theoretical research.
Theoretical research, also referred to as pure or basic research, focuses on generating knowledge , regardless of its practical application. Here, data collection is used to generate new general concepts for a better understanding of a particular field or to answer a theoretical research question.
Results of this kind are usually oriented towards the formulation of theories and are usually based on documentary analysis, the development of mathematical formulas and the reflection of high-level researchers.
Applied Research
Here, the goal is to find strategies that can be used to address a specific research problem. Applied research draws on theory to generate practical scientific knowledge, and its use is very common in STEM fields such as engineering, computer science and medicine.
This type of research is subdivided into two types:
- Technological applied research : looks towards improving efficiency in a particular productive sector through the improvement of processes or machinery related to said productive processes.
- Scientific applied research : has predictive purposes. Through this type of research design, we can measure certain variables to predict behaviours useful to the goods and services sector, such as consumption patterns and viability of commercial projects.

According to your Depth of Scope
Exploratory research.
Exploratory research is used for the preliminary investigation of a subject that is not yet well understood or sufficiently researched. It serves to establish a frame of reference and a hypothesis from which an in-depth study can be developed that will enable conclusive results to be generated.
Because exploratory research is based on the study of little-studied phenomena, it relies less on theory and more on the collection of data to identify patterns that explain these phenomena.
Descriptive Research
The primary objective of descriptive research is to define the characteristics of a particular phenomenon without necessarily investigating the causes that produce it.
In this type of research, the researcher must take particular care not to intervene in the observed object or phenomenon, as its behaviour may change if an external factor is involved.
Explanatory Research
Explanatory research is the most common type of research method and is responsible for establishing cause-and-effect relationships that allow generalisations to be extended to similar realities. It is closely related to descriptive research, although it provides additional information about the observed object and its interactions with the environment.
Correlational Research
The purpose of this type of scientific research is to identify the relationship between two or more variables. A correlational study aims to determine whether a variable changes, how much the other elements of the observed system change.
According to the Type of Data Used
Qualitative research.
Qualitative methods are often used in the social sciences to collect, compare and interpret information, has a linguistic-semiotic basis and is used in techniques such as discourse analysis, interviews, surveys, records and participant observations.
In order to use statistical methods to validate their results, the observations collected must be evaluated numerically. Qualitative research, however, tends to be subjective, since not all data can be fully controlled. Therefore, this type of research design is better suited to extracting meaning from an event or phenomenon (the ‘why’) than its cause (the ‘how’).
Quantitative Research
Quantitative research study delves into a phenomena through quantitative data collection and using mathematical, statistical and computer-aided tools to measure them . This allows generalised conclusions to be projected over time.

According to the Degree of Manipulation of Variables
Experimental research.
It is about designing or replicating a phenomenon whose variables are manipulated under strictly controlled conditions in order to identify or discover its effect on another independent variable or object. The phenomenon to be studied is measured through study and control groups, and according to the guidelines of the scientific method.
Non-Experimental Research
Also known as an observational study, it focuses on the analysis of a phenomenon in its natural context. As such, the researcher does not intervene directly, but limits their involvement to measuring the variables required for the study. Due to its observational nature, it is often used in descriptive research.
Quasi-Experimental Research
It controls only some variables of the phenomenon under investigation and is therefore not entirely experimental. In this case, the study and the focus group cannot be randomly selected, but are chosen from existing groups or populations . This is to ensure the collected data is relevant and that the knowledge, perspectives and opinions of the population can be incorporated into the study.
According to the Type of Inference
Deductive investigation.
In this type of research, reality is explained by general laws that point to certain conclusions; conclusions are expected to be part of the premise of the research problem and considered correct if the premise is valid and the inductive method is applied correctly.
Inductive Research
In this type of research, knowledge is generated from an observation to achieve a generalisation. It is based on the collection of specific data to develop new theories.
Hypothetical-Deductive Investigation
It is based on observing reality to make a hypothesis, then use deduction to obtain a conclusion and finally verify or reject it through experience.

According to the Time in Which it is Carried Out
Longitudinal study (also referred to as diachronic research).
It is the monitoring of the same event, individual or group over a defined period of time. It aims to track changes in a number of variables and see how they evolve over time. It is often used in medical, psychological and social areas .
Cross-Sectional Study (also referred to as Synchronous Research)
Cross-sectional research design is used to observe phenomena, an individual or a group of research subjects at a given time.
According to The Sources of Information
Primary research.
This fundamental research type is defined by the fact that the data is collected directly from the source, that is, it consists of primary, first-hand information.
Secondary research
Unlike primary research, secondary research is developed with information from secondary sources, which are generally based on scientific literature and other documents compiled by another researcher.

According to How the Data is Obtained
Documentary (cabinet).
Documentary research, or secondary sources, is based on a systematic review of existing sources of information on a particular subject. This type of scientific research is commonly used when undertaking literature reviews or producing a case study.
Field research study involves the direct collection of information at the location where the observed phenomenon occurs.
From Laboratory
Laboratory research is carried out in a controlled environment in order to isolate a dependent variable and establish its relationship with other variables through scientific methods.
Mixed-Method: Documentary, Field and/or Laboratory
Mixed research methodologies combine results from both secondary (documentary) sources and primary sources through field or laboratory research.

In this post you’ll learn what the significance of the study means, why it’s important, where and how to write one in your paper or thesis with an example.

An abstract and introduction are the first two sections of your paper or thesis. This guide explains the differences between them and how to write them.

Academic conferences are expensive and it can be tough finding the funds to go; this naturally leads to the question of are academic conferences worth it?
Join thousands of other students and stay up to date with the latest PhD programmes, funding opportunities and advice.

Browse PhDs Now

Are you always finding yourself working on sections of your research tasks right up until your deadlines? Are you still finding yourself distracted the moment

Frances recently completed her PhD at the University of Bristol. Her research investigated the causes and consequences of hazardous lava-water interactions.

Dr Ayres completed her PhD at the University of Warwick in 2017, researching the use of diamond to make electrochemical sensors. She is now a research scientists in the water industry, developing different analytical techniques and sensors to help keep our water systems safe.
Join Thousands of Students
Cookies on this website
We use cookies to ensure that we give you the best experience on our website. If you click 'Accept all cookies' we'll assume that you are happy to receive all cookies and you won't see this message again. If you click 'Reject all non-essential cookies' only necessary cookies providing core functionality such as security, network management, and accessibility will be enabled. Click 'Find out more' for information on how to change your cookie settings.

Study designs
This short article gives a brief guide to the different study types and a comparison of the advantages and disadvantages.
See also Levels of Evidence
These study designs all have similar components (as we’d expect from the PICO):
- A defined population (P) from which groups of subjects are studied
- Outcomes (O) that are measured
And for experimental and analytic observational studies:
- Interventions (I) or exposures (E) that are applied to different groups of subjects
Overview of the design tree
Figure 1 shows the tree of possible designs, branching into subgroups of study designs by whether the studies are descriptive or analytic and by whether the analytic studies are experimental or observational. The list is not completely exhaustive but covers most basics designs.

Figure: Tree of different types of studies (Q1, 2, and 3 refer to the three questions below)
> Download a PDF by Jeremy Howick about study designs
Our first distinction is whether the study is analytic or non-analytic. A non-analytic or descriptive study does not try to quantify the relationship but tries to give us a picture of what is happening in a population, e.g., the prevalence, incidence, or experience of a group. Descriptive studies include case reports, case-series, qualitative studies and surveys (cross-sectional) studies, which measure the frequency of several factors, and hence the size of the problem. They may sometimes also include analytic work (comparing factors “” see below).
An analytic study attempts to quantify the relationship between two factors, that is, the effect of an intervention (I) or exposure (E) on an outcome (O). To quantify the effect we will need to know the rate of outcomes in a comparison (C) group as well as the intervention or exposed group. Whether the researcher actively changes a factor or imposes uses an intervention determines whether the study is considered to be observational (passive involvement of researcher), or experimental (active involvement of researcher).
In experimental studies, the researcher manipulates the exposure, that is he or she allocates subjects to the intervention or exposure group. Experimental studies, or randomised controlled trials (RCTs), are similar to experiments in other areas of science. That is, subjects are allocated to two or more groups to receive an intervention or exposure and then followed up under carefully controlled conditions. Such studies controlled trials, particularly if randomised and blinded, have the potential to control for most of the biases that can occur in scientific studies but whether this actually occurs depends on the quality of the study design and implementation.
In analytic observational studies, the researcher simply measures the exposure or treatments of the groups. Analytical observational studies include case””control studies, cohort studies and some population (cross-sectional) studies. These studies all include matched groups of subjects and assess of associations between exposures and outcomes.
Observational studies investigate and record exposures (such as interventions or risk factors) and observe outcomes (such as disease) as they occur. Such studies may be purely descriptive or more analytical.
We should finally note that studies can incorporate several design elements. For example, a the control arm of a randomised trial may also be used as a cohort study; and the baseline measures of a cohort study may be used as a cross-sectional study.
Spotting the study design
The type of study can generally be worked at by looking at three issues (as per the Tree of design in Figure 1):
Q1. What was the aim of the study?
- To simply describe a population (PO questions) descriptive
- To quantify the relationship between factors (PICO questions) analytic.
Q2. If analytic, was the intervention randomly allocated?
- No? Observational study
For observational study the main types will then depend on the timing of the measurement of outcome, so our third question is:
Q3. When were the outcomes determined?
- Some time after the exposure or intervention? cohort study (‘prospective study’)
- At the same time as the exposure or intervention? cross sectional study or survey
- Before the exposure was determined? case-control study (‘retrospective study’ based on recall of the exposure)
Advantages and Disadvantages of the Designs
Randomised Controlled Trial
An experimental comparison study in which participants are allocated to treatment/intervention or control/placebo groups using a random mechanism (see randomisation). Best for study the effect of an intervention.
Advantages:
- unbiased distribution of confounders;
- blinding more likely;
- randomisation facilitates statistical analysis.
Disadvantages:
- expensive: time and money;
- volunteer bias;
- ethically problematic at times.
Crossover Design
A controlled trial where each study participant has both therapies, e.g, is randomised to treatment A first, at the crossover point they then start treatment B. Only relevant if the outcome is reversible with time, e.g, symptoms.
- all subjects serve as own controls and error variance is reduced thus reducing sample size needed;
- all subjects receive treatment (at least some of the time);
- statistical tests assuming randomisation can be used;
- blinding can be maintained.
- all subjects receive placebo or alternative treatment at some point;
- washout period lengthy or unknown;
- cannot be used for treatments with permanent effects
Cohort Study
Data are obtained from groups who have been exposed, or not exposed, to the new technology or factor of interest (eg from databases). No allocation of exposure is made by the researcher. Best for study the effect of predictive risk factors on an outcome.
- ethically safe;
- subjects can be matched;
- can establish timing and directionality of events;
- eligibility criteria and outcome assessments can be standardised;
- administratively easier and cheaper than RCT.
- controls may be difficult to identify;
- exposure may be linked to a hidden confounder;
- blinding is difficult;
- randomisation not present;
- for rare disease, large sample sizes or long follow-up necessary.
Case-Control Studies
Patients with a certain outcome or disease and an appropriate group of controls without the outcome or disease are selected (usually with careful consideration of appropriate choice of controls, matching, etc) and then information is obtained on whether the subjects have been exposed to the factor under investigation.
- quick and cheap;
- only feasible method for very rare disorders or those with long lag between exposure and outcome;
- fewer subjects needed than cross-sectional studies.
- reliance on recall or records to determine exposure status;
- confounders;
- selection of control groups is difficult;
- potential bias: recall, selection.
Cross-Sectional Survey
A study that examines the relationship between diseases (or other health-related characteristics) and other variables of interest as they exist in a defined population at one particular time (ie exposure and outcomes are both measured at the same time). Best for quantifying the prevalence of a disease or risk factor, and for quantifying the accuracy of a diagnostic test.
- cheap and simple;
- ethically safe.
- establishes association at most, not causality;
- recall bias susceptibility;
- confounders may be unequally distributed;
- Neyman bias;
- group sizes may be unequal.
Thanks for visiting! GoodRx is not available outside of the United States. If you are trying to access this site from the United States and believe you have received this message in error, please reach out to [email protected] and let us know.
- Find Studies
For Researchers
- Learn about Research
What are some different types of research studies?

There are many different types of research studies. Generally, there are two major types of studies available on Research for Me @UNC: research studies and clinical trials . When a research study is about disease or human health, it is called a clinical research study. When a research study involves drugs or other therapies that aim to slow or stop a disease, then it is called a clinical trial. Volunteers are an important part of all of these research studies! Explore other types of research studies below.
Survey - Survey studies ask people questions about their knowledge, attitudes, and feelings about a wide range of topics. You can complete these surveys online, over the phone, or by mail. Sometimes, these studies might also be in-person interviews or group discussions.
Lifestyle - Lifestyle studies look at what happens when people participate in different types of activities over a set period of time. You may attend activity sessions in a center or clinic or be asked to change the way that you do something in your daily activities. Often, these studies are interested in how changes in behavior can affect our health or other parts of our lives.
Drug - Drug studies are heavily regulated by the US Government. Studies often involve medications that are not currently available to the general public. They are called “investigational” drugs and have not yet been approved by the FDA (US Food and Drug Administration) for your normal health care provider to prescribe. Other drug studies may involve comparisons between two or more FDA-approved medications.
Device - Device studies are done to learn if a new medical device helps relieve a certain medical condition. Devices you may be familiar with are pacemakers, diabetes testing meters, and hearing aids. These studies usually involve devices that are not currently available to the general public and have not been approved for use by the FDA. Sometimes, they may be studying an FDA-approved device, but for use in treating a new condition.
Procedure - Procedure studies learn about the safety and effectiveness of certain medical procedures. Sometimes they compare a new medical procedure to one already in use. Procedures might include things like imaging (x-rays), stitches, blood tests, and surgeries.
Medical Outcomes - Outcomes research studies the end results (outcomes) of the structure and processes of the health care system on the health and well-being of patients and populations. These studies look at clinical practices to see if there are better ways for doctors to help patients manage their medical care. Outcomes research often considers patients’ experiences, preferences, and values – all of which may affect whether or not a medical treatment is best for them.
Community-based - Community-based research is done through a true partnership of community leaders and organizations with a UNC researcher or research team. The ideas are driven by community members and the research incorporates voices of all involved. These studies aim to understand problems impacting communities and contribute to solutions through policy or social change.
Copyright © 2013-2022 The NC TraCS Institute, the integrated home of the NIH Clinical and Translational Science Awards (CTSA) Program at UNC-CH. This website is made possible by CTSA Grant UL1TR002489 and the National Center for Advancing Translational Sciences.
Helpful Links
- My UNC Chart
- Find a Doctor
- Accessibility
- Privacy Policy

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Clinical research is the study of health and illness in people.
Scientists may have many reasons for doing a clinical study, such as:
- To explore the cause of a disease or a set of symptoms
- To test if a treatment will help with a symptom or condition
- To learn how a certain behavior affects people’s health
Different types of clinical studies are used in different circumstances. Depending on what is known and what isn’t, scientists may even study the same research question using different kinds of studies and in different groups of people. Here are different types of clinical studies and why they might be used.
Observational Studies
In many studies, researchers do not do experiments or test new treatments; they observe. Observational studies help researchers understand a situation and come up with hypotheses that can be put to the test in clinical trials.
Observational studies can find associations between things but can’t prove that one thing causes another. Types include:
Case Study/Case Series
A detailed description of one or more patients. By documenting new and unusual cases, researchers start to generate hypotheses about causes or risk factors.
- Epidemiological Study
Compares the rate of a disease or condition for groups of people, such as towns in different climates or with different average incomes.
- Cross-Sectional Study
A snapshot of many people at one moment in time. These studies can show how common a condition is and help identify factors associated with it.
Case-Control Study
A group of people who have a condition is compared to a control group of people who don’t. Possible causes or risk factors can emerge.
Cohort Study
A large group of people is observed over time. Some eventually develop a disease or condition. Researchers can learn how often the condition occurs and find possible causes or risk factors.
Clinical Trials
In these studies, researchers test new ways to prevent, detect, or treat disease. Treatments might be new drugs or combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. Clinical trials can also test other aspects of care, such as ways to improve the quality of life for people with chronic illnesses.
A well-designed clinical trial is the gold standard for proving that a treatment or medical approach works, but clinical trials can’t always be used. For example,
scientists can’t randomly assign people to live in different places, or ask people to start smoking or eating an unhealthy diet. Clinical trials are conducted in phases:
- Purpose: Find out whether a medical approach (e.g., drug, diagnostic test, device) is safe, identify side effects, and figure out appropriate doses.
- Number of people: Typically, fewer than 100
- Purpose: Start testing whether a medical approach works. Continue monitoring for side effects; get information that goes into designing a large, phase III trial.
- Number of people: Typically, 100-300
- Purpose: Prove whether a medical approach works; continue monitoring side effects.
- Number of people: As many as needed or able to enroll—can be 1,000 or more
- Purpose: When a medical approach is being marketed, continue gathering information on its effects.
- Number of people: Thousands
How good are these kinds of studies at showing cause and effect?
The strength of a study depends on its size and design. New results may confirm earlier findings, contradict them, or add new aspects to scientists’ understanding. In the end, cause and effect are usually hard to establish without a well-designed clinical trial.
Least Effective to Most Effective:
- Case-Control Study/Cohort Study
- Case Study/Series
- Clinical Trial
What can I do to help?
You’ve begun! Learning about what results mean will help you make good choices with your health care provider.
You could also consider volunteering either as a healthy volunteer or as a participant who has a particular disease or condition.
For more information about clinical trials:
ClinicalResearchTrials.nih.gov
Produced by the National Institutes of Health, the largest source of public funding for medical research in the world. NIH’s mission is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability.
Back to Clinical Research

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.3: Types of Research Studies and How To Interpret Them
- Last updated
- Save as PDF
- Page ID 59269

- Alice Callahan, Heather Leonard, & Tamberly Powell
- Lane Community College via OpenOregon
The field of nutrition is dynamic, and our understanding and practices are always evolving. Nutrition scientists are continuously conducting new research and publishing their findings in peer-reviewed journals. This adds to scientific knowledge, but it’s also of great interest to the public, so nutrition research often shows up in the news and other media sources. You might be interested in nutrition research to inform your own eating habits, or if you work in a health profession, so that you can give evidence-based advice to others. Making sense of science requires that you understand the types of research studies used and their limitations.
The Hierarchy of Nutrition Evidence
Researchers use many different types of study designs depending on the question they are trying to answer, as well as factors such as time, funding, and ethical considerations. The study design affects how we interpret the results and the strength of the evidence as it relates to real-life nutrition decisions. It can be helpful to think about the types of studies within a pyramid representing a hierarchy of evidence, where the studies at the bottom of the pyramid usually give us the weakest evidence with the least relevance to real-life nutrition decisions, and the studies at the top offer the strongest evidence, with the most relevance to real-life nutrition decisions .

Figure 2.1. Hierarchy of research design and levels of scientific evidence with the strongest studies at the top and the weakest at the bottom.
The pyramid also represents a few other general ideas. There tend to be more studies published using the methods at the bottom of the pyramid, because they require less time, money, and other resources. When researchers want to test a new hypothesis , they often start with the study designs at the bottom of the pyramid , such as in vitro, animal, or observational studies. Intervention studies are more expensive and resource-intensive, so there are fewer of these types of studies conducted. But they also give us higher quality evidence, so they’re an important next step if observational and non-human studies have shown promising results. Meta-analyses and systematic reviews combine the results of many studies already conducted, so they help researchers summarize scientific knowledge on a topic.
Non-Human Studies: In Vitro & Animal Studies
The simplest form of nutrition research is an in vitro study . In vitro means “within glass,” (although plastic is used more commonly today) and these experiments are conducted within flasks, dishes, plates, and test tubes. One common form of in vitro research is cell culture. This involves growing cells in flasks and dishes. In order for cells to grow, they need a nutrient source. For cell culture, the nutrient source is referred to as media. Media supplies nutrients to the cells in vitro similarly to how blood performs this function within the body. Most cells adhere to the bottom of the flask and are so small that a microscope is needed to see them. The cells are grown inside an incubator, which is a device that provides the optimal temperature, humidity, and carbon dioxide (CO2CO2) concentrations for cells and microorganisms. By imitating the body's temperature and CO2CO2 levels (37 degrees Celsius, 5% CO2CO2), the incubator allows cells to grow even though they are outside the body.
A limitation of in vitro research compared to in vivo research is that it typically does not take digestion or bioavailability into account. This means that the concentration used might not be physiologically possible (it might be much higher) and that digestion and metabolism of what is being provided to cells may not be taken into account. Cell-based in vitro research is not as complex of a biological system as animals or people that have tissues, organs, etc. working together as well.
Since these studies are performed on isolated cells or tissue samples, they are less expensive and time-intensive than animal or human studies. In vitro studies are vital for zooming in on biological mechanisms, to see how things work at the cellular or molecular level. However, these studies shouldn’t be used to draw conclusions about how things work in humans (or even animals), because we can’t assume that the results will apply to a whole, living organism.

Animal studies are one form of in vivo research, which translates to “within the living.” Rats and mice are the most common animals used in nutrition research. Animals are often used in research that would be unethical to conduct in humans. Another advantage of animal dietary studies is that researchers can control exactly what the animals eat. In human studies, researchers can tell subjects what to eat and even provide them with the food, but they may not stick to the planned diet. People are also not very good at estimating, recording, or reporting what they eat and in what quantities. In addition, animal studies typically do not cost as much as human studies.
There are some important limitations of animal research. First, an animal’s metabolism and physiology are different from humans. Plus, animal models of disease (cancer, cardiovascular disease, etc.), although similar, are different from human diseases. Animal research is considered preliminary, and while it can be very important to the process of building scientific understanding and informing the types of studies that should be conducted in humans, animal studies shouldn’t be considered relevant to real-life decisions about how people eat.
Observational Studies
Observational studies in human nutrition collect information on people’s dietary patterns or nutrient intake and look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health. These types of study designs can only identify correlations (relationships) between nutrition and health; they can’t show that one factor causes another. (For that, we need intervention studies, which we’ll discuss in a moment.) Observational studies that describe factors correlated with human health are also called epidemiological studies . 1
Epidemiology is defined as the study of human populations. These studies often investigate the relationship between dietary consumption and disease development. There are three main types of epidemiological studies: cross-sectional, case-control, and prospective cohort studies.

One example of a nutrition hypothesis that has been investigated using observational studies is that eating a Mediterranean diet reduces the risk of developing cardiovascular disease. (A Mediterranean diet focuses on whole grains, fruits and vegetables, beans and other legumes, nuts, olive oil, herbs, and spices. It includes small amounts of animal protein (mostly fish), dairy, and red wine. 2 ) There are three main types of observational studies, all of which could be used to test hypotheses about the Mediterranean diet:
- Cohort studies follow a group of people (a cohort) over time, measuring factors such as diet and health outcomes. A cohort study of the Mediterranean diet would ask a group of people to describe their diet, and then researchers would track them over time to see if those eating a Mediterranean diet had a lower incidence of cardiovascular disease.
- Case-control studies compare a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes. For example, researchers might compare a group of people with cardiovascular disease with a group of healthy controls to see whether there were more controls or cases that followed a Mediterranean diet.
- Cross-sectional studies collect information about a population of people at one point in time. For example, a cross-sectional study might compare the dietary patterns of people from different countries to see if diet correlates with the prevalence of cardiovascular disease in the different countries.
There are two types of cohort studies: retrospective and prospective. Retrospective studies look at what happened in the past, and they’re considered weaker because they rely on people’s memory of what they ate or how they felt in the past. Prospective cohort studies, which enroll a cohort and follow them into the future, are usually considered the strongest type of observational study design.
Most cohort studies are prospective. Initial information is collected (usually by food frequency questionnaires) on the intake of a cohort of people at baseline, or the beginning. This cohort is then followed over time (normally many years) to quantify health outcomes of the individual within it. Cohort studies are normally considered to be more robust than case-control studies, because these studies do not start with diseased people and normally do not require people to remember their dietary habits in the distant past or before they developed a disease. An example of a prospective cohort study would be if you filled out a questionnaire on your current dietary habits and are then followed into the future to see if you develop osteoporosis. As shown below, instead of separating based on disease versus disease-free, individuals are separated based on exposure. In this example, those who are exposed are more likely to be diseased than those who were not exposed.

Using trans-fat intake again as the exposure and cardiovascular disease as the disease, the figure would be expected to look like this:

There are several well-known examples of prospective cohort studies that have described important correlations between diet and disease:
- Framingham Heart Study : Beginning in 1948, this study has followed the residents of Framingham, Massachusetts to identify risk factors for heart disease.
- Health Professionals Follow-Up Study : This study started in 1986 and enrolled 51,529 male health professionals (dentists, pharmacists, optometrists, osteopathic physicians, podiatrists, and veterinarians), who complete diet questionnaires every 2 years.
- Nurses Health Studies : Beginning in 1976, these studies have enrolled three large cohorts of nurses with a total of 280,000 participants. Participants have completed detailed questionnaires about diet, other lifestyle factors (smoking and exercise, for example), and health outcomes.
Observational studies have the advantage of allowing researchers to study large groups of people in the real world, looking at the frequency and pattern of health outcomes and identifying factors that correlate with them. But even very large observational studies may not apply to the population as a whole. For example, the Health Professionals Follow-Up Study and the Nurses Health Studies include people with above-average knowledge of health. In many ways, this makes them ideal study subjects, because they may be more motivated to be part of the study and to fill out detailed questionnaires for years. However, the findings of these studies may not apply to people with less baseline knowledge of health.
We’ve already mentioned another important limitation of observational studies—that they can only determine correlation, not causation. A prospective cohort study that finds that people eating a Mediterranean diet have a lower incidence of heart disease can only show that the Mediterranean diet is correlated with lowered risk of heart disease. It can’t show that the Mediterranean diet directly prevents heart disease. Why? There are a huge number of factors that determine health outcomes such as heart disease, and other factors might explain a correlation found in an observational study. For example, people who eat a Mediterranean diet might also be the same kind of people who exercise more, sleep more, have a higher income (fish and nuts can be expensive!), or be less stressed. These are called confounding factors ; they’re factors that can affect the outcome in question (i.e., heart disease) and also vary with the factor being studied (i.e., Mediterranean diet).
Intervention Studies
Intervention studies , also sometimes called experimental studies or clinical trials, include some type of treatment or change imposed by the researcher. Examples of interventions in nutrition research include asking participants to change their diet, take a supplement, or change the time of day that they eat. Unlike observational studies, intervention studies can provide evidence of cause and effect , so they are higher in the hierarchy of evidence pyramid.
Randomization: The gold standard for intervention studies is the randomized controlled trial (RCT) . In an RCT, study subjects are recruited to participate in the study. They are then randomly assigned into one of at least two groups, one of which is a control group (this is what makes the study controlled ).
Randomization is the process of randomly assigning subjects to groups to decrease bias. Bias is a systematic error that may influence results. Bias can occur in assigning subjects to groups in a way that will influence the results. An example of bias in a study of an antidepressant drug is shown below. In this nonrandomized antidepressant drug example, researchers (who know what the subjects are receiving) put depressed subjects into the placebo group, while "less depressed" subjects are put into the antidepressant drug group. As a result, even if the drug isn't effective, the group assignment may make the drug appear effective, thus biasing the results as shown below.

This is a bit of an extreme example, but even if the researchers are trying to prevent bias, sometimes bias can still occur. However, if the subjects are randomized, the sick and the healthy people will ideally be equally distributed between the groups. Thus, the trial will be unbiased and a true test of whether or not the drug is effective.

Here is another example. In an RCT to study the effects of the Mediterranean diet on cardiovascular disease development, researchers might ask the control group to follow a low-fat diet (typically recommended for heart disease prevention) and the intervention group to eat a Mediterranean diet. The study would continue for a defined period of time (usually years to study an outcome like heart disease), at which point the researchers would analyze their data to see if more people in the control or Mediterranean diet had heart attacks or strokes. Because the treatment and control groups were randomly assigned, they should be alike in every other way except for diet, so differences in heart disease could be attributed to the diet. This eliminates the problem of confounding factors found in observational research, and it’s why RCTs can provide evidence of causation, not just correlation.
Imagine for a moment what would happen if the two groups weren’t randomly assigned. What if the researchers let study participants choose which diet they’d like to adopt for the study? They might, for whatever reason, end up with more overweight people who smoke and have high blood pressure in the low-fat diet group, and more people who exercised regularly and had already been eating lots of olive oil and nuts for years in the Mediterranean diet group. If they found that the Mediterranean diet group had fewer heart attacks by the end of the study, they would have no way of knowing if this was because of the diet or because of the underlying differences in the groups. In other words, without randomization, their results would be compromised by confounding factors, with many of the same limitations as observational studies.
Placebo: In an RCT of a supplement, the control group would receive a placebo—a “fake” treatment that contains no active ingredients, such as a sugar pill. The use of a placebo is necessary in medical research because of a phenomenon known as the placebo effect. The placebo effect results in a beneficial effect because of a subject’s belief in the treatment, even though there is no treatment actually being administered. An example would be an athlete who consumes a sports drink and runs the 100-meter dash in 11.00 seconds. The athlete then, under the exact same conditions, drinks what he is told is "Super Duper Sports Drink" and runs the 100-meter dash in 10.50 seconds. But what the athlete didn't know was that Super Duper Sports Drink was the Sports Drink + Food Coloring. There was nothing different between the drinks, but the athlete believed that the "Super Duper Sports Drink" was going to help him run faster, so he did. This improvement is due to the placebo effect.

Blinding is a technique to prevent bias in intervention studies. In a study without blinding, the subject and the researchers both know what treatment the subject is receiving. This can lead to bias if the subject or researcher has expectations about the treatment working, so these types of trials are used less frequently. It’s best if a study is double-blind , meaning that neither the researcher nor the subject knows what treatment the subject is receiving. It’s relatively simple to double-blind a study where subjects are receiving a placebo or treatment pill because they could be formulated to look and taste the same. In a single-blind study , either the researcher or the subject knows what treatment they’re receiving, but not both. Studies of diets—such as the Mediterranean diet example—often can’t be double-blinded because the study subjects know whether or not they’re eating a lot of olive oil and nuts. However, the researchers who are checking participants’ blood pressure or evaluating their medical records could be blinded to their treatment group, reducing the chance of bias.
Open-label study:

Single-blinded study:

Double-blinded study:

Like all studies, RCTs and other intervention studies do have some limitations. They can be difficult to carry on for long periods of time and require that participants remain compliant with the intervention. They’re also costly and often have smaller sample sizes. Furthermore, it is unethical to study certain interventions. (An example of an unethical intervention would be to advise one group of pregnant mothers to drink alcohol to determine its effects on pregnancy outcomes because we know that alcohol consumption during pregnancy damages the developing fetus.)
VIDEO: “ Not all scientific studies are created equal ” by David H. Schwartz, YouTube (April 28, 2014), 4:26.
Meta-Analyses and Systematic Reviews
At the top of the hierarchy of evidence pyramid are systematic reviews and meta-analyses . You can think of these as “studies of studies.” They attempt to combine all of the relevant studies that have been conducted on a research question and summarize their overall conclusions. Researchers conducting a systematic review formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence that relates to the research question. Since systematic reviews combine the results of many studies, they help researchers produce more reliable findings. A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies . 4
However, even systematic reviews and meta-analyses aren’t the final word on scientific questions. For one thing, they’re only as good as the studies that they include. The Cochrane Collaboration is an international consortium of researchers who conduct systematic reviews in order to inform evidence-based healthcare, including nutrition, and their reviews are among the most well-regarded and rigorous in science. For the most recent Cochrane review of the Mediterranean diet and cardiovascular disease, two authors independently reviewed studies published on this question. Based on their inclusion criteria, 30 RCTs with a total of 12,461 participants were included in the final analysis. However, after evaluating and combining the data, the authors concluded that “despite the large number of included trials, there is still uncertainty regarding the effects of a Mediterranean‐style diet on cardiovascular disease occurrence and risk factors in people both with and without cardiovascular disease already.” Part of the reason for this uncertainty is that different trials found different results, and the quality of the studies was low to moderate. Some had problems with their randomization procedures, for example, and others were judged to have unreliable data. That doesn’t make them useless, but it adds to the uncertainty about this question, and uncertainty pushes the field forward towards more and better studies. The Cochrane review authors noted that they found seven ongoing trials of the Mediterranean diet, so we can hope that they’ll add more clarity to this question in the future. 5
Science is an ongoing process. It’s often a slow process, and it contains a lot of uncertainty, but it’s our best method of building knowledge of how the world and human life works. Many different types of studies can contribute to scientific knowledge. None are perfect—all have limitations—and a single study is never the final word on a scientific question. Part of what advances science is that researchers are constantly checking each other’s work, asking how it can be improved and what new questions it raises.
Attributions:
- “Chapter 1: The Basics” from Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR , CC BY-NC-SA 4.0
- “The Broad Role of Nutritional Science,” section 1.3 from the book An Introduction to Nutrition (v. 1.0), CC BY-NC-SA 3.0
References:
- 1 Thiese, M. S. (2014). Observational and interventional study design types; an overview. Biochemia Medica , 24 (2), 199–210. https://doi.org/10.11613/BM.2014.022
- 2 Harvard T.H. Chan School of Public Health. (2018, January 16). Diet Review: Mediterranean Diet . The Nutrition Source. https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet/
- 3 Ross, R., Gray, C. M., & Gill, J. M. R. (2015). Effects of an Injected Placebo on Endurance Running Performance. Medicine and Science in Sports and Exercise , 47 (8), 1672–1681. https://doi.org/10.1249/MSS.0000000000000584
- 4 Hooper, A. (n.d.). LibGuides: Systematic Review Resources: Systematic Reviews vs Other Types of Reviews . Retrieved February 7, 2020, from //libguides.sph.uth.tmc.edu/c.php?g=543382&p=5370369
- 5 Rees, K., Takeda, A., Martin, N., Ellis, L., Wijesekara, D., Vepa, A., Das, A., Hartley, L., & Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews , 3 . doi.org/10.1002/14651858.CD009825.pub3
- 6Levin K. (2006) Study design III: Cross-sectional studies. Evidence - Based Dentistry 7(1): 24.
- Figure 2.3. The hierarchy of evidence by Alice Callahan, is licensed under CC BY 4.0
- Research lab photo by National Cancer Institute on Unsplas h ; mouse photo by vaun0815 on Unsplash
- Figure 2.4. “Placebo effect example” by Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR

Types of Research Studies and How To Interpret Them
The field of nutrition is dynamic, and our understanding and practices are always evolving. Nutrition scientists are continuously conducting new research and publishing their findings in peer-reviewed journals. This adds to scientific knowledge, but it’s also of great interest to the public, so nutrition research often shows up in the news and other media sources. You might be interested in nutrition research to inform your own eating habits, or if you work in a health profession, so that you can give evidence-based advice to others. Making sense of science requires that you understand the types of research studies used and their limitations.
The Hierarchy of Nutrition Evidence
Researchers use many different types of study designs depending on the question they are trying to answer, as well as factors such as time, funding, and ethical considerations. The study design affects how we interpret the results and the strength of the evidence as it relates to real-life nutrition decisions. It can be helpful to think about the types of studies within a pyramid representing a hierarchy of evidence, where the studies at the bottom of the pyramid usually give us the weakest evidence with the least relevance to real-life nutrition decisions, and the studies at the top offer the strongest evidence, with the most relevance to real-life nutrition decisions .
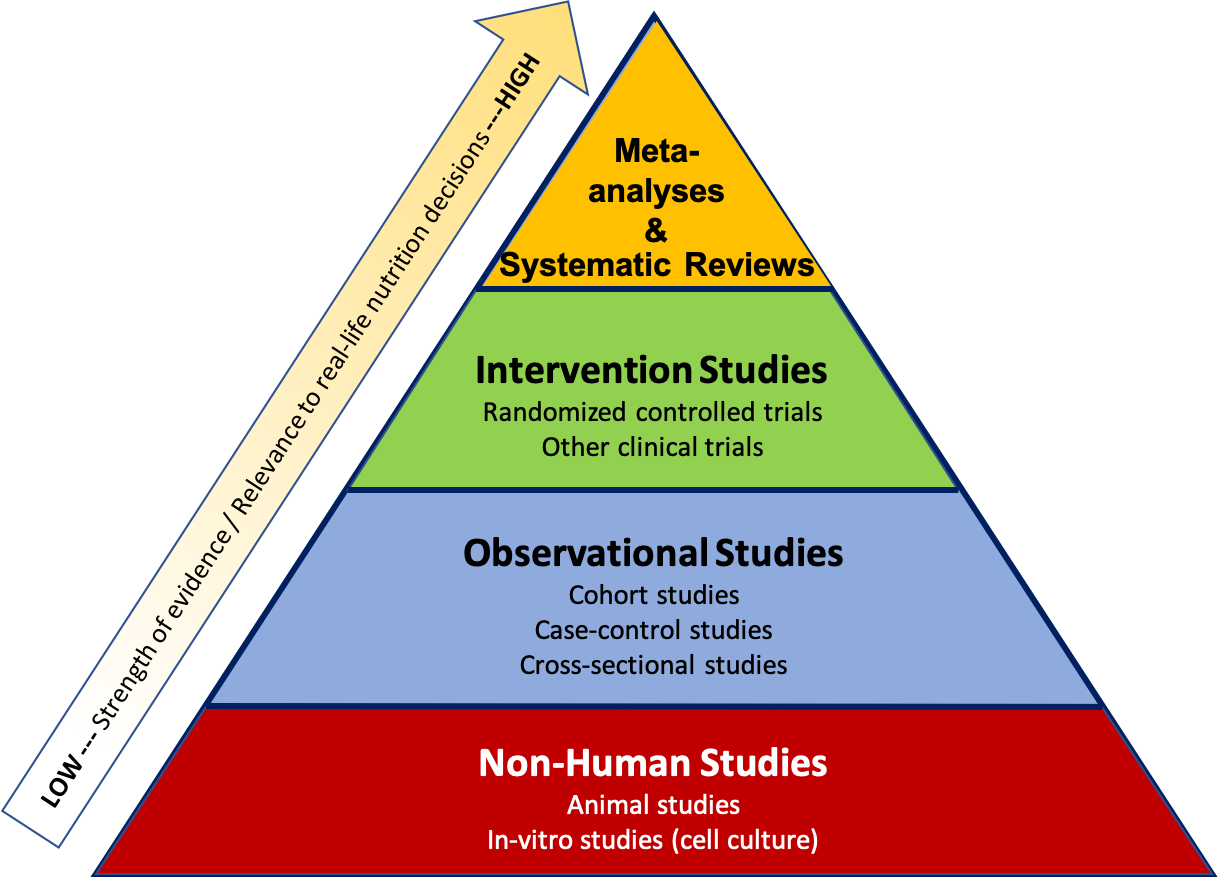
Figure 2.3. The hierarchy of evidence shows types of research studies relative to their strength of evidence and relevance to real-life nutrition decisions, with the strongest studies at the top and the weakest at the bottom.
The pyramid also represents a few other general ideas. There tend to be more studies published using the methods at the bottom of the pyramid, because they require less time, money, and other resources. When researchers want to test a new hypothesis , they often start with the study designs at the bottom of the pyramid , such as in vitro, animal, or observational studies. Intervention studies are more expensive and resource-intensive, so there are fewer of these types of studies conducted. But they also give us higher quality evidence, so they’re an important next step if observational and non-human studies have shown promising results. Meta-analyses and systematic reviews combine the results of many studies already conducted, so they help researchers summarize scientific knowledge on a topic.
Non-Human Studies: In Vitro & Animal Studies
The simplest form of nutrition research is an in vitro study . In vitro means “within glass,” (although plastic is used more commonly today) and these experiments are conducted within flasks, dishes, plates, and test tubes. These studies are performed on isolated cells or tissue samples, so they’re less expensive and time-intensive than animal or human studies. In vitro studies are vital for zooming in on biological mechanisms, to see how things work at the cellular or molecular level. However, these studies shouldn’t be used to draw conclusions about how things work in humans (or even animals), because we can’t assume that the results will apply to a whole, living organism.
Animal studies are one form of in vivo research, which translates to “within the living.” Rats and mice are the most common animals used in nutrition research. Animals are often used in research that would be unethical to conduct in humans. Another advantage of animal dietary studies is that researchers can control exactly what the animals eat. In human studies, researchers can tell subjects what to eat and even provide them with the food, but they may not stick to the planned diet. People are also not very good at estimating, recording, or reporting what they eat and in what quantities. In addition, animal studies typically do not cost as much as human studies.
There are some important limitations of animal research. First, an animal’s metabolism and physiology are different from humans. Plus, animal models of disease (cancer, cardiovascular disease, etc.), although similar, are different from human diseases. Animal research is considered preliminary, and while it can be very important to the process of building scientific understanding and informing the types of studies that should be conducted in humans, animal studies shouldn’t be considered relevant to real-life decisions about how people eat.
Observational Studies
Observational studies in human nutrition collect information on people’s dietary patterns or nutrient intake and look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health. These types of study designs can only identify correlations (relationships) between nutrition and health; they can’t show that one factor causes another. (For that, we need intervention studies, which we’ll discuss in a moment.) Observational studies that describe factors correlated with human health are also called epidemiological studies . 1
One example of a nutrition hypothesis that has been investigated using observational studies is that eating a Mediterranean diet reduces the risk of developing cardiovascular disease. (A Mediterranean diet focuses on whole grains, fruits and vegetables, beans and other legumes, nuts, olive oil, herbs, and spices. It includes small amounts of animal protein (mostly fish), dairy, and red wine. 2 ) There are three main types of observational studies, all of which could be used to test hypotheses about the Mediterranean diet:
- Cohort studies follow a group of people (a cohort) over time, measuring factors such as diet and health outcomes. A cohort study of the Mediterranean diet would ask a group of people to describe their diet, and then researchers would track them over time to see if those eating a Mediterranean diet had a lower incidence of cardiovascular disease.
- Case-control studies compare a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes. For example, researchers might compare a group of people with cardiovascular disease with a group of healthy controls to see whether there were more controls or cases that followed a Mediterranean diet.
- Cross-sectional studies collect information about a population of people at one point in time. For example, a cross-sectional study might compare the dietary patterns of people from different countries to see if diet correlates with the prevalence of cardiovascular disease in the different countries.
Prospective cohort studies, which enroll a cohort and follow them into the future, are usually considered the strongest type of observational study design. Retrospective studies look at what happened in the past, and they’re considered weaker because they rely on people’s memory of what they ate or how they felt in the past. There are several well-known examples of prospective cohort studies that have described important correlations between diet and disease:
- Framingham Heart Study : Beginning in 1948, this study has followed the residents of Framingham, Massachusetts to identify risk factors for heart disease.
- Health Professionals Follow-Up Study : This study started in 1986 and enrolled 51,529 male health professionals (dentists, pharmacists, optometrists, osteopathic physicians, podiatrists, and veterinarians), who complete diet questionnaires every 2 years.
- Nurses Health Studies : Beginning in 1976, these studies have enrolled three large cohorts of nurses with a total of 280,000 participants. Participants have completed detailed questionnaires about diet, other lifestyle factors (smoking and exercise, for example), and health outcomes.
Observational studies have the advantage of allowing researchers to study large groups of people in the real world, looking at the frequency and pattern of health outcomes and identifying factors that correlate with them. But even very large observational studies may not apply to the population as a whole. For example, the Health Professionals Follow-Up Study and the Nurses Health Studies include people with above-average knowledge of health. In many ways, this makes them ideal study subjects, because they may be more motivated to be part of the study and to fill out detailed questionnaires for years. However, the findings of these studies may not apply to people with less baseline knowledge of health.
We’ve already mentioned another important limitation of observational studies—that they can only determine correlation, not causation. A prospective cohort study that finds that people eating a Mediterranean diet have a lower incidence of heart disease can only show that the Mediterranean diet is correlated with lowered risk of heart disease. It can’t show that the Mediterranean diet directly prevents heart disease. Why? There are a huge number of factors that determine health outcomes such as heart disease, and other factors might explain a correlation found in an observational study. For example, people who eat a Mediterranean diet might also be the same kind of people who exercise more, sleep more, have higher income (fish and nuts can be expensive!), or be less stressed. These are called confounding factors ; they’re factors that can affect the outcome in question (i.e., heart disease) and also vary with the factor being studied (i.e., Mediterranean diet).
Intervention Studies
Intervention studies , also sometimes called experimental studies or clinical trials, include some type of treatment or change imposed by the researcher. Examples of interventions in nutrition research include asking participants to change their diet, take a supplement, or change the time of day that they eat. Unlike observational studies, intervention studies can provide evidence of cause and effect , so they are higher in the hierarchy of evidence pyramid.
The gold standard for intervention studies is the randomized controlled trial (RCT) . In an RCT, study subjects are recruited to participate in the study. They are then randomly assigned into one of at least two groups, one of which is a control group (this is what makes the study controlled ). In an RCT to study the effects of the Mediterranean diet on cardiovascular disease development, researchers might ask the control group to follow a low-fat diet (typically recommended for heart disease prevention) and the intervention group to eat a Mediterrean diet. The study would continue for a defined period of time (usually years to study an outcome like heart disease), at which point the researchers would analyze their data to see if more people in the control or Mediterranean diet had heart attacks or strokes. Because the treatment and control groups were randomly assigned, they should be alike in every other way except for diet, so differences in heart disease could be attributed to the diet. This eliminates the problem of confounding factors found in observational research, and it’s why RCTs can provide evidence of causation, not just correlation.
Imagine for a moment what would happen if the two groups weren’t randomly assigned. What if the researchers let study participants choose which diet they’d like to adopt for the study? They might, for whatever reason, end up with more overweight people who smoke and have high blood pressure in the low-fat diet group, and more people who exercised regularly and had already been eating lots of olive oil and nuts for years in the Mediterranean diet group. If they found that the Mediterranean diet group had fewer heart attacks by the end of the study, they would have no way of knowing if this was because of the diet or because of the underlying differences in the groups. In other words, without randomization, their results would be compromised by confounding factors, with many of the same limitations as observational studies.
In an RCT of a supplement, the control group would receive a placebo —a “fake” treatment that contains no active ingredients, such as a sugar pill. The use of a placebo is necessary in medical research because of a phenomenon known as the placebo effect. The placebo effect results in a beneficial effect because of a subject’s belief in the treatment, even though there is no treatment actually being administered.
For example, imagine an athlete who consumes a sports drink and then runs 100 meters in 11.0 seconds. On a different day, under the exact same conditions, the athlete is given a Super Duper Sports Drink and again runs 100 meters, this time in 10.5 seconds. But what the athlete didn’t know was that the Super Duper Sports Drink was the same as the regular sports drink—it just had a bit of food coloring added. There was nothing different between the drinks, but the athlete believed that the Super Duper Sports Drink was going to help him run faster, so he did. This improvement is due to the placebo effect. Ironically, a study similar to this example was published in 2015, demonstrating the power of the placebo effect on athletic performance. 3
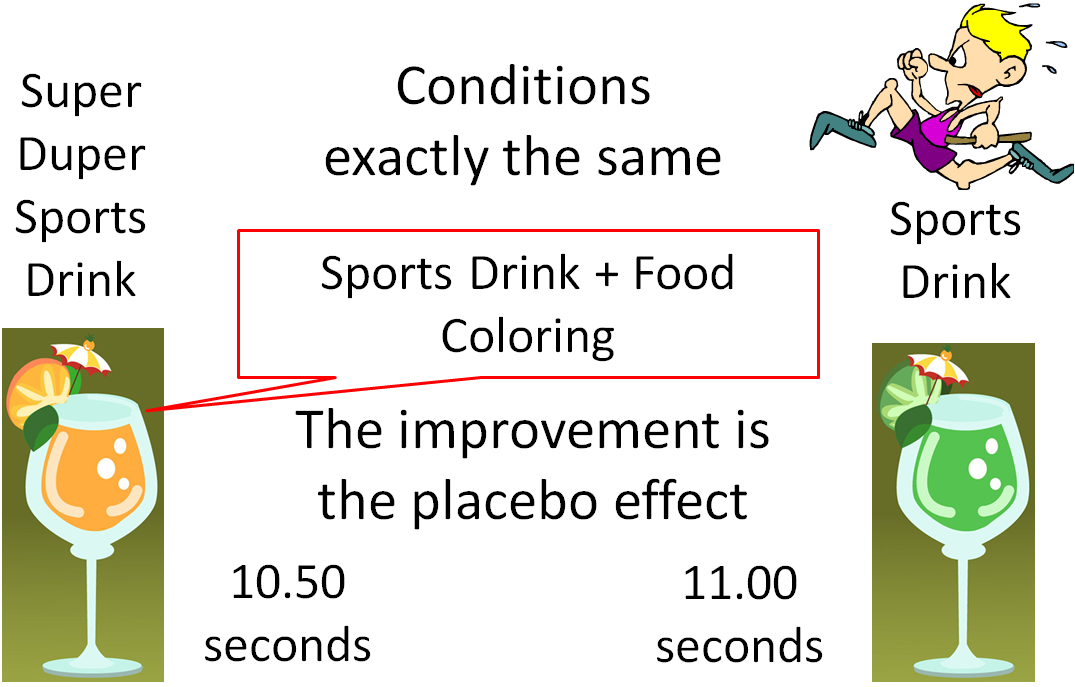
Figure 2.4. An example of the placebo effect
Blinding is a technique to prevent bias in intervention studies. In a study without blinding, the subject and the researchers both know what treatment the subject is receiving. This can lead to bias if the subject or researcher have expectations about the treatment working, so these types of trials are used less frequently. It’s best if a study is double-blind , meaning that neither the researcher nor the subject know what treatment the subject is receiving. It’s relatively simple to double-blind a study where subjects are receiving a placebo or treatment pill, because they could be formulated to look and taste the same. In a single-blind study , either the researcher or the subject knows what treatment they’re receiving, but not both. Studies of diets—such as the Mediterranean diet example—often can’t be double-blinded because the study subjects know whether or not they’re eating a lot of olive oil and nuts. However, the researchers who are checking participants’ blood pressure or evaluating their medical records could be blinded to their treatment group, reducing the chance of bias.
Like all studies, RCTs and other intervention studies do have some limitations. They can be difficult to carry on for long periods of time and require that participants remain compliant with the intervention. They’re also costly and often have smaller sample sizes. Furthermore, it is unethical to study certain interventions. (An example of an unethical intervention would be to advise one group of pregnant mothers to drink alcohol to determine its effects on pregnancy outcomes, because we know that alcohol consumption during pregnancy damages the developing fetus.)
VIDEO: “ Not all scientific studies are created equal ” by David H. Schwartz, YouTube (April 28, 2014), 4:26.
Meta-Analyses and Systematic Reviews
At the top of the hierarchy of evidence pyramid are systematic reviews and meta-analyses . You can think of these as “studies of studies.” They attempt to combine all of the relevant studies that have been conducted on a research question and summarize their overall conclusions. Researchers conducting a systematic review formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence that relates to the research question. Since systematic reviews combine the results of many studies, they help researchers produce more reliable findings. A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies . 4
However, even systematic reviews and meta-analyses aren’t the final word on scientific questions. For one thing, they’re only as good as the studies that they include. The Cochrane Collaboration is an international consortium of researchers who conduct systematic reviews in order to inform evidence-based healthcare, including nutrition, and their reviews are among the most well-regarded and rigorous in science. For the most recent Cochrane review of the Mediterranean diet and cardiovascular disease, two authors independently reviewed studies published on this question. Based on their inclusion criteria, 30 RCTs with a total of 12,461 participants were included in the final analysis. However, after evaluating and combining the data, the authors concluded that “despite the large number of included trials, there is still uncertainty regarding the effects of a Mediterranean‐style diet on cardiovascular disease occurrence and risk factors in people both with and without cardiovascular disease already.” Part of the reason for this uncertainty is that different trials found different results, and the quality of the studies was low to moderate. Some had problems with their randomization procedures, for example, and others were judged to have unreliable data. That doesn’t make them useless, but it adds to the uncertainty about this question, and uncertainty pushes the field forward towards more and better studies. The Cochrane review authors noted that they found seven ongoing trials of the Mediterranean diet, so we can hope that they’ll add more clarity to this question in the future. 5
Science is an ongoing process. It’s often a slow process, and it contains a lot of uncertainty, but it’s our best method of building knowledge of how the world and human life works. Many different types of studies can contribute to scientific knowledge. None are perfect—all have limitations—and a single study is never the final word on a scientific question. Part of what advances science is that researchers are constantly checking each other’s work, asking how it can be improved and what new questions it raises.
Self-Check:
Attributions:
- “Chapter 1: The Basics” from Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR , CC BY-NC-SA 4.0
- “ The Broad Role of Nutritional Science ,” section 1.3 from the book An Introduction to Nutrition (v. 1.0), CC BY-NC-SA 3.0
References:
- 1 Thiese, M. S. (2014). Observational and interventional study design types; an overview. Biochemia Medica , 24 (2), 199–210. https://doi.org/10.11613/BM.2014.022
- 2 Harvard T.H. Chan School of Public Health. (2018, January 16). Diet Review: Mediterranean Diet . The Nutrition Source. https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet/
- 3 Ross, R., Gray, C. M., & Gill, J. M. R. (2015). Effects of an Injected Placebo on Endurance Running Performance. Medicine and Science in Sports and Exercise , 47 (8), 1672–1681. https://doi.org/10.1249/MSS.0000000000000584
- 4 Hooper, A. (n.d.). LibGuides: Systematic Review Resources: Systematic Reviews vs Other Types of Reviews . Retrieved February 7, 2020, from //libguides.sph.uth.tmc.edu/c.php?g=543382&p=5370369
- 5 Rees, K., Takeda, A., Martin, N., Ellis, L., Wijesekara, D., Vepa, A., Das, A., Hartley, L., & Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews , 3 . https://doi.org/10.1002/14651858.CD009825.pub3
- Figure 2.3. The hierarchy of evidence by Alice Callahan, is licensed under CC BY 4.0
- Research lab photo by National Cancer Institute on Unsplas h ; mouse photo by vaun0815 on Unsplash
- Figure 2.4. “Placebo effect example” by Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR
Experiments that are conducted outside of living organisms, within flasks, dishes, plates, or test tubes.
Research that is conducted in living organisms, such as rats and mice.
In nutrition, research that is conducted by collecting information on people’s dietary patterns or nutrient intake to look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health.
Relationships between two factors (e.g., nutrition and health).
Research that follows a group of people (a cohort) over time, measuring factors such as diet and health outcomes.
Research that compares a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes.
Research that collects information about a population of people at one point in time.
Looking into the future.
Looking at what happened in the past.
Factors that can affect the outcome in question.
Research that includes some type of treatment or change imposed by the researchers; sometimes called experimental studies or clinical trials.
The gold standard for intervention studies, because the research involves a control group and participants are randomized.
A “fake” treatment that contains no active ingredients, such as a sugar pill.
The beneficial effect that results from a subject's belief in a treatment, not from the treatment itself.
technique to prevent bias in intervention studies, where either the research team, the subject, or both don’t know what treatment the subject is receiving.
Neither the research team nor the subject know what treatment the subject is receiving.
Either the research team or the subject know what treatment is being given, but not both.
Researchers formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence from previous research that relates to the research question.
A type of systematic review that combines data from multiple studies and uses statistical methods to summarize it, as if creating a mega-study from many smaller studies.
Nutrition: Science and Everyday Application, v. 1.0 Copyright © 2020 by Alice Callahan, PhD; Heather Leonard, MEd, RDN; and Tamberly Powell, MS, RDN is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
Exploring Types of Research Methods: A Comprehensive Guide

Grasping the concept of research method is essential for anyone engaged in research or assessing the outcomes of studies. Whether you're an academic student, a dedicated researcher, or just inquisitive about the world, a thorough understanding of the diverse research methods will assist you in sifting through the extensive array of information at your disposal.
Our detailed guide will walk you through the types of research design, including qualitative and quantitative approaches, as well as descriptive, correlational, experimental, and mixed methods research. We will also touch upon the different types of research methodology, ensuring a comprehensive understanding of the various types of methods in research.
This article will also highlight the pivotal factors to consider when crafting a study and the inherent strengths and limitations of different type of research methods.Whether you're embarking on your own research project or looking to enhance your critical thinking skills Armed with the research methods definition, this guide will equip you with the essential knowledge to make well-informed decisions and formulate significant conclusions in the field of research.
Qualitative vs. Quantitative Research Methods
Qualitative and quantitative research methods represent two fundamentally different approaches to data collection and analysis. Qualitative observation delves into non-numerical data, while quantitative observation involves the scrutiny of data that is numerical and quantifiable.
Qualitative Research:
- Involves gathering and interpreting non-numerical data, such as text, video, photographs, or audio recordings
- Uses sources like interviews, focus groups, documents, personal accounts, cultural records, and observation
- Unstructured or semi-structured format
- Open-ended questions
- Comprehensive perspective on individuals' experiences
- Comparison of participants' feedback and input
- Focus on answering the "why" behind a phenomenon, correlation, or behavior
- Ethnography, for instance, seeks to gain insights into phenomena, groups, or experiences that cannot be objectively measured or quantified, offering a deep dive into the cultural fabric of a community.
- This method is used to understand how an individual subjectively perceives and imparts meaning to their social reality, often revealing underlying bias that can influence the interpretation of social phenomena.
- Data analysis techniques include content analysis, grounded theory, thematic analysis, or discourse analysis
Quantitative Research:
- Focuses on numerical or measurable data
- Uses sources such as experiments, questionnaires, surveys, and database reports
- Multiple-choice format
- Countable answers (e.g., "yes" or "no")
- Numerical analysis
- Statistical picture of a trend or connection
- To define research methods, one must focus on answering the 'what' or 'how' in relation to a particular phenomenon, correlation, or behavior. This foundational approach is crucial in the realm of empirical inquiry.
- Provides precise causal explanations that can be measured and communicated mathematically
- The objectives of scientific inquiry often include hypothesis testing to examine causal relationships between variables, making accurate predictions, and generalizing findings to broader populations.
- Aims to establish general laws of behavior and phenomenon across different settings/contexts
- Used to test a theory and ultimately support or reject it
- Empirical research in psychology utilizes examples of quantitative data such as standardized psychological assessments, neuroimaging data, and clinical outcome measures to inform its findings.
- Data analysis techniques include descriptive and inferential statistics
When selecting research methodology types, it's important to consider various factors such as the study's primary goal, the nature of the research questions and conceptual framework, the variables involved, the context of the study, ethical issues, and whether the focus is on individuals or groups, or on comparing groups and understanding their relationships.
Research design methods play a pivotal role in determining the appropriateness of qualitative methods for studies involving individuals or groups, while quantitative methods are often chosen for studies aimed at comparing groups or deciphering the relationship between variables.
Descriptive Research
Descriptive research is a methodological approach that aims to accurately and systematically depict a population, situation, or phenomenon. It adeptly addresses 'what', 'where', 'when', and 'how' questions, although it steers clear of exploring 'why'. Employing a descriptive research design means observing and documenting variables without exerting control or manipulation, which is particularly beneficial when exploring new topics or problems to identify characteristics, frequencies, trends, and categories.
Descriptive research methods include:
- Surveys: Survey research is a powerful tool that enables researchers to collect extensive data sets, which can then be meticulously analyzed to uncover frequencies, averages, and emerging patterns.
- Observations: Utilizing observation allows researchers to collect data on behaviors and phenomena, ensuring the gathered information is not tainted by the honesty or accuracy of respondents.
- Case studies: Case study research delves into detailed data to pinpoint the unique characteristics of a narrowly defined subject, providing in-depth insights.
Descriptive research can be conducted in different ways:
- Cross-sectional : Observing a population at a single point in time.
- Longitudinal : Following a population over a period of time.
- Surveys or interviews : When the researcher interacts with the participant.
- Observational studies or data collection using existing records : When the researcher does not interact with the participant.
Advantages of descriptive research include:
- Varied data collection methods
- A natural environment for respondents
- Quick and cheap data collection
- A holistic understanding of the research topic
Limitations of descriptive research studies:
- They cannot establish cause and effect relationships.
- The reliability and validity of survey responses can be compromised if respondents are not truthful or tend to provide socially desirable answers.
- The choice and wording of questions on a questionnaire may influence the descriptive findings.
Correlational Research
Correlational research, a non-experimental method, delves into the dynamics between two variables, focusing on the strength and direction of their relationship without manipulating any factors, which is pivotal in understanding associations rather than causality.
Researchers may choose correlational research in the following situations
- When manipulating the independent variable is impractical, impossible, or unethical
- When exploring non-causal relationships between variables
- When testing new measurement tools
In correlational research, the correlation coefficient is measured, which can range from -1 to +1, indicating the relationship's direction and strength. A comprehensive meta-analysis can further elucidate these types of correlations.
- Positive correlation: Both variables change in the same direction
- Negative correlation: Variables change in opposite directions
- Zero correlation: No relationship exists between the variables
Data collection methods for correlational research include
- Naturalistic observation
- Archival research or secondary data
Analytical research methods, such as correlation or regression analyses, are employed to analyze correlational data, with the former yielding a coefficient that clarifies the relationship's intensity and direction, and the latter forecasting the impact of variable changes.
Experimental Research
Experimental research, a methodical scientific approach, manipulates variables to observe their effects and is indispensable for establishing cause-and-effect relationships and making informed decisions in the face of inadequate data.
- Pre-experimental research design : Includes One-shot Case Study Research Design, One-group Pretest-posttest Research Design, and Static-group Comparison.
- True experimental research design Statistical analysis, a cornerstone in testing hypotheses, is pivotal in research for its accuracy in proving or disproving a hypothesis. It's uniquely capable of establishing a cause-effect relationship within a group, making it an indispensable tool for researchers.
- Quasi-experimental design : Similar to an experimental design but assigns participants to groups non-randomly.
Experimental research is essential for various fields, such as:
- Developing new drugs and medical treatments
- Understanding human behavior in psychology
- Improving educational outcomes
- Identifying opportunities for businesses and organizations
To conduct experimental research effectively, researchers must consider three key factors:
- A Control Group and an Experimental Group
- A variable that can be manipulated by the researcher
- Random distribution of participants
Experimental research, whether conducted in laboratory settings with high control variables and internal validity or in field settings that boast both internal and external validity, presents a spectrum of advantages and challenges. Researchers must navigate potential threats to internal validity, including history, maturation, testing, instrumentation, mortality, and regression threats.
Mixed Methods Research
Mixed methods research, an approach that synergizes the rigor of quantitative and qualitative research methods, capitalizes on the strengths of each to provide a comprehensive analysis. This integration, which can occur during data collection, analysis, or presentation of results, is a hallmark of mixed methods research designs.
- Convergent design
- Explanatory sequential design
- Exploratory sequential design
- Embedded design
The practice of triangulation in mixed methods research enhances the integration of quantitative and qualitative data, offering multiple perspectives and a more comprehensive understanding. It also allows for a deeper explanation of statistical results, as exemplified by the EQUALITY study's exploratory sequential design for patient-centered data collection.
In mixed methods research, the intricate research design and methodology combine qualitative and quantitative data collection and analysis. This purposeful mixing of methods and data integration at strategic stages of the research process can reveal relationships between complex layers of research questions, although it demands significant resources and specialized training.
- Explanatory
- Exploratory
- Nested (embedded) designs
Mixed methods research, characterized by its diverse research design and methods, integrates quantitative and qualitative approaches within a single study. Grounded in positivism and interpretivism, it provides a multifaceted understanding of research topics, despite the challenges of mastering both methodologies and collaborating with multidisciplinary teams.
In sum, a thorough grasp of the various research methodologies is crucial for conducting robust research and critically assessing others' findings. From qualitative to quantitative, descriptive, correlational, experimental, and mixed methods research, each approach offers distinct strengths and limitations, guiding researchers to the most suitable methods for effective data collection and analysis.
As we navigate the vast landscape of information available, understanding what are research methods empowers us to make informed decisions, draw meaningful conclusions, and contribute to the advancement of knowledge across various fields. Embracing the diversity of research methods, whether you're a student, researcher, or simply curious, will enhance your critical thinking skills and enable you to uncover valuable insights that shape our understanding of the world.
What are the seven most commonly used research methods? The seven most commonly used research methods are:
- Observation / Participant Observation
- Focus Groups
- Experiments
- Secondary Data Analysis / Archival Study
- Mixed Methods (a combination of some of the above)
What does comprehensive research methodology entail?
Comprehensive research methodology involves conducting a thorough and exhaustive investigation on a specific topic, subject, or issue. This approach is characterized by the meticulous collection, analysis, and evaluation of a wide array of information, data, and sources, with the objective of achieving a deep and comprehensive understanding of the subject matter.
What are the three primary methods to investigate a specific research question?
To investigate a specific research question, you can use:
- Quantitative methods for measuring something or testing a hypothesis.
- Qualitative methods for exploring ideas, thoughts, and meanings.
- Secondary data analysis for examining a large volume of readily-available data.
What does exploration mean in the context of research methodology?
Exploration in research methodology signifies a research approach that aims to delve into questions that have not been extensively explored before. Exploratory research, often qualitative and primary in nature, is focused on uncovering new insights and understanding. Nonetheless, it can also adopt a quantitative stance, particularly when it involves analyzing a large sample size, to further the scope of exploratory research.
Sign up for more like this.

- SMU Libraries
- Scholarship & Research
- Teaching & Learning
- Bridwell Library
- Business Library
- DeGolyer Library
- Fondren Library
- Hamon Arts Library
- Underwood Law Library
- Fort Burgwin Library
- Exhibits & Digital Collections
- SMU Scholar
- Special Collections & Archives
- Connect With Us
- Research Guides by Subject
- How Do I . . . ? Guides
- Find Your Librarian
- Writing Support
Types of Research Papers: Overview
A research paper is simply a piece of writing that uses outside sources. There are different types of research papers with varying purposes and expectations for sourcing.
While this guide explains those differences broadly, disciplines and assignments vary. Ask your professor for clarification on the purpose and types of appropriate research questions and sources.
Need More Help?
Related guides.
- Literature Reviews
- Annotated Bibliographies
- Starting Your Research

Research and Writing Lab
Need last minute help but didn't book an appointment? Every week we offer online drop-in labs.
Tuesdays 3:00pm - 4:30pm via Zoom @ https://smu.zoom.us/j/92637892352 and in-person, Fondren Red 1st floor (near elevators)
- Last Updated: Apr 12, 2024 1:00 PM
- URL: https://guides.smu.edu/researchpapertypes
Research Objectives: The Compass of Your Study
Table of contents
- 1 Definition and Purpose of Setting Clear Research Objectives
- 2 How Research Objectives Fit into the Overall Research Framework
- 3 Types of Research Objectives
- 4 Aligning Objectives with Research Questions and Hypotheses
- 5 Role of Research Objectives in Various Research Phases
- 6.1 Key characteristics of well-defined research objectives
- 6.2 Step-by-Step Guide on How to Formulate Both General and Specific Research Objectives
- 6.3 How to Know When Your Objectives Need Refinement
- 7 Research Objectives Examples in Different Fields
- 8 Conclusion
Embarking on a research journey without clear objectives is like navigating the sea without a compass. This article delves into the essence of establishing precise research objectives, serving as the guiding star for your scholarly exploration.
We will unfold the layers of how the objective of study not only defines the scope of your research but also directs every phase of the research process, from formulating research questions to interpreting research findings. By bridging theory with practical examples, we aim to illuminate the path to crafting effective research objectives that are both ambitious and attainable. Let’s chart the course to a successful research voyage, exploring the significance, types, and formulation of research paper objectives.
Definition and Purpose of Setting Clear Research Objectives
Defining the research objectives includes which two tasks? Research objectives are clear and concise statements that outline what you aim to achieve through your study. They are the foundation for determining your research scope, guiding your data collection methods, and shaping your analysis. The purpose of research proposal and setting clear objectives in it is to ensure that your research efforts are focused and efficient, and to provide a roadmap that keeps your study aligned with its intended outcomes.
To define the research objective at the outset, researchers can avoid the pitfalls of scope creep, where the study’s focus gradually broadens beyond its initial boundaries, leading to wasted resources and time. Clear objectives facilitate communication with stakeholders, such as funding bodies, academic supervisors, and the broader academic community, by succinctly conveying the study’s goals and significance. Furthermore, they help in the formulation of precise research questions and hypotheses, making the research process more systematic and organized. Yet, it is not always easy. For this reason, PapersOwl is always ready to help. Lastly, clear research objectives enable the researcher to critically assess the study’s progress and outcomes against predefined benchmarks, ensuring the research stays on track and delivers meaningful results.
How Research Objectives Fit into the Overall Research Framework
Research objectives are integral to the research framework as the nexus between the research problem, questions, and hypotheses. They translate the broad goals of your study into actionable steps, ensuring every aspect of your research is purposefully aligned towards addressing the research problem. This alignment helps in structuring the research design and methodology, ensuring that each component of the study is geared towards answering the core questions derived from the objectives. Creating such a difficult piece may take a lot of time. If you need it to be accurate yet fast delivered, consider getting professional research paper writing help whenever the time comes. It also aids in the identification and justification of the research methods and tools used for data collection and analysis, aligning them with the objectives to enhance the validity and reliability of the findings.
Furthermore, by setting clear objectives, researchers can more effectively evaluate the impact and significance of their work in contributing to existing knowledge. Additionally, research objectives guide literature review, enabling researchers to focus their examination on relevant studies and theoretical frameworks that directly inform their research goals.
Types of Research Objectives
In the landscape of research, setting objectives is akin to laying down the tracks for a train’s journey, guiding it towards its destination. Constructing these tracks involves defining two main types of objectives: general and specific. Each serves a unique purpose in guiding the research towards its ultimate goals, with general objectives providing the broad vision and specific objectives outlining the concrete steps needed to fulfill that vision. Together, they form a cohesive blueprint that directs the focus of the study, ensuring that every effort contributes meaningfully to the overarching research aims.
- General objectives articulate the overarching goals of your study. They are broad, setting the direction for your research without delving into specifics. These objectives capture what you wish to explore or contribute to existing knowledge.
- Specific objectives break down the general objectives into measurable outcomes. They are precise, detailing the steps needed to achieve the broader goals of your study. They often correspond to different aspects of your research question , ensuring a comprehensive approach to your study.
To illustrate, consider a research project on the impact of digital marketing on consumer behavior. A general objective might be “to explore the influence of digital marketing on consumer purchasing decisions.” Specific objectives could include “to assess the effectiveness of social media advertising in enhancing brand awareness” and “to evaluate the impact of email marketing on customer loyalty.”
Aligning Objectives with Research Questions and Hypotheses
The harmony between what research objectives should be, questions, and hypotheses is critical. Objectives define what you aim to achieve; research questions specify what you seek to understand, and hypotheses predict the expected outcomes.
This alignment ensures a coherent and focused research endeavor. Achieving it necessitates a thoughtful consideration of how each component interrelates, ensuring that the objectives are not only ambitious but also directly answerable through the research questions and testable via the hypotheses. This interconnectedness facilitates a streamlined approach to the research process, enabling researchers to systematically address each aspect of their study in a logical sequence. Moreover, it enhances the clarity and precision of the research, making it easier for peers and stakeholders to grasp the study’s direction and potential contributions.
Role of Research Objectives in Various Research Phases
Throughout the research process, objectives guide your choices and strategies – from selecting the appropriate research design and methods to analyzing data and interpreting results. They are the criteria against which you measure the success of your study. In the initial stages, research objectives inform the selection of a topic, helping to narrow down a broad area of interest into a focused question that can be explored in depth. During the methodology phase, they dictate the type of data needed and the best methods for obtaining that data, ensuring that every step taken is purposeful and aligned with the study’s goals. As the research progresses, objectives provide a framework for analyzing the collected data, guiding the researcher in identifying patterns, drawing conclusions, and making informed decisions.
Crafting Effective Research Objectives

The effective objective of research is pivotal in laying the groundwork for a successful investigation. These objectives clarify the focus of your study and determine its direction and scope. Ensuring that your objectives are well-defined and aligned with the SMART criteria is crucial for setting a strong foundation for your research.
Key characteristics of well-defined research objectives
Well-defined research objectives are characterized by the SMART criteria – Specific, Measurable, Achievable, Relevant, and Time-bound. Specific objectives clearly define what you plan to achieve, eliminating any ambiguity. Measurable objectives allow you to track progress and assess the outcome. Achievable objectives are realistic, considering the research sources and time available. Relevant objectives align with the broader goals of your field or research question. Finally, Time-bound objectives have a clear timeline for completion, adding urgency and a schedule to your work.
Step-by-Step Guide on How to Formulate Both General and Specific Research Objectives
So lets get to the part, how to write research objectives properly?
- Understand the issue or gap in existing knowledge your study aims to address.
- Gain insights into how similar challenges have been approached to refine your objectives.
- Articulate the broad goal of research based on your understanding of the problem.
- Detail the specific aspects of your research, ensuring they are actionable and measurable.
How to Know When Your Objectives Need Refinement
Your objectives of research may require refinement if they lack clarity, feasibility, or alignment with the research problem. If you find yourself struggling to design experiments or methods that directly address your objectives, or if the objectives seem too broad or not directly related to your research question, it’s likely time for refinement. Additionally, objectives in research proposal that do not facilitate a clear measurement of success indicate a need for a more precise definition. Refinement involves ensuring that each objective is specific, measurable, achievable, relevant, and time-bound, enhancing your research’s overall focus and impact.
Research Objectives Examples in Different Fields
The application of research objectives spans various academic disciplines, each with its unique focus and methodologies. To illustrate how the objectives of the study guide a research paper across different fields, here are some research objective examples:
- In Health Sciences , a research aim may be to “determine the efficacy of a new vaccine in reducing the incidence of a specific disease among a target population within one year.” This objective is specific (efficacy of a new vaccine), measurable (reduction in disease incidence), achievable (with the right study design and sample size), relevant (to public health), and time-bound (within one year).
- In Environmental Studies , the study objectives could be “to assess the impact of air pollution on urban biodiversity over a decade.” This reflects a commitment to understanding the long-term effects of human activities on urban ecosystems, emphasizing the need for sustainable urban planning.
- In Economics , an example objective of a study might be “to analyze the relationship between fiscal policies and unemployment rates in developing countries over the past twenty years.” This seeks to explore macroeconomic trends and inform policymaking, highlighting the role of economic research study in societal development.
These examples of research objectives describe the versatility and significance of research objectives in guiding scholarly inquiry across different domains. By setting clear, well-defined objectives, researchers can ensure their studies are focused and impactful and contribute valuable knowledge to their respective fields.
Defining research studies objectives and problem statement is not just a preliminary step, but a continuous guiding force throughout the research journey. These goals of research illuminate the path forward and ensure that every stride taken is meaningful and aligned with the ultimate goals of the inquiry. Whether through the meticulous application of the SMART criteria or the strategic alignment with research questions and hypotheses, the rigor in crafting and refining these objectives underscores the integrity and relevance of the research. As scholars venture into the vast terrains of knowledge, the clarity, and precision of their objectives serve as beacons of light, steering their explorations toward discoveries that advance academic discourse and resonate with the broader societal needs.
Readers also enjoyed

WHY WAIT? PLACE AN ORDER RIGHT NOW!
Just fill out the form, press the button, and have no worries!
We use cookies to give you the best experience possible. By continuing we’ll assume you board with our cookie policy.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
Healthy Living with Diabetes
- Español
On this page:
How can I plan what to eat or drink when I have diabetes?
How can physical activity help manage my diabetes, what can i do to reach or maintain a healthy weight, should i quit smoking, how can i take care of my mental health, clinical trials for healthy living with diabetes.
Healthy living is a way to manage diabetes . To have a healthy lifestyle, take steps now to plan healthy meals and snacks, do physical activities, get enough sleep, and quit smoking or using tobacco products.
Healthy living may help keep your body’s blood pressure , cholesterol , and blood glucose level, also called blood sugar level, in the range your primary health care professional recommends. Your primary health care professional may be a doctor, a physician assistant, or a nurse practitioner. Healthy living may also help prevent or delay health problems from diabetes that can affect your heart, kidneys, eyes, brain, and other parts of your body.
Making lifestyle changes can be hard, but starting with small changes and building from there may benefit your health. You may want to get help from family, loved ones, friends, and other trusted people in your community. You can also get information from your health care professionals.
What you choose to eat, how much you eat, and when you eat are parts of a meal plan. Having healthy foods and drinks can help keep your blood glucose, blood pressure, and cholesterol levels in the ranges your health care professional recommends. If you have overweight or obesity, a healthy meal plan—along with regular physical activity, getting enough sleep, and other healthy behaviors—may help you reach and maintain a healthy weight. In some cases, health care professionals may also recommend diabetes medicines that may help you lose weight, or weight-loss surgery, also called metabolic and bariatric surgery.
Choose healthy foods and drinks
There is no right or wrong way to choose healthy foods and drinks that may help manage your diabetes. Healthy meal plans for people who have diabetes may include
- dairy or plant-based dairy products
- nonstarchy vegetables
- protein foods
- whole grains
Try to choose foods that include nutrients such as vitamins, calcium , fiber , and healthy fats . Also try to choose drinks with little or no added sugar , such as tap or bottled water, low-fat or non-fat milk, and unsweetened tea, coffee, or sparkling water.
Try to plan meals and snacks that have fewer
- foods high in saturated fat
- foods high in sodium, a mineral found in salt
- sugary foods , such as cookies and cakes, and sweet drinks, such as soda, juice, flavored coffee, and sports drinks
Your body turns carbohydrates , or carbs, from food into glucose, which can raise your blood glucose level. Some fruits, beans, and starchy vegetables—such as potatoes and corn—have more carbs than other foods. Keep carbs in mind when planning your meals.
You should also limit how much alcohol you drink. If you take insulin or certain diabetes medicines , drinking alcohol can make your blood glucose level drop too low, which is called hypoglycemia . If you do drink alcohol, be sure to eat food when you drink and remember to check your blood glucose level after drinking. Talk with your health care team about your alcohol-drinking habits.

Find the best times to eat or drink
Talk with your health care professional or health care team about when you should eat or drink. The best time to have meals and snacks may depend on
- what medicines you take for diabetes
- what your level of physical activity or your work schedule is
- whether you have other health conditions or diseases
Ask your health care team if you should eat before, during, or after physical activity. Some diabetes medicines, such as sulfonylureas or insulin, may make your blood glucose level drop too low during exercise or if you skip or delay a meal.
Plan how much to eat or drink
You may worry that having diabetes means giving up foods and drinks you enjoy. The good news is you can still have your favorite foods and drinks, but you might need to have them in smaller portions or enjoy them less often.
For people who have diabetes, carb counting and the plate method are two common ways to plan how much to eat or drink. Talk with your health care professional or health care team to find a method that works for you.
Carb counting
Carbohydrate counting , or carb counting, means planning and keeping track of the amount of carbs you eat and drink in each meal or snack. Not all people with diabetes need to count carbs. However, if you take insulin, counting carbs can help you know how much insulin to take.
Plate method
The plate method helps you control portion sizes without counting and measuring. This method divides a 9-inch plate into the following three sections to help you choose the types and amounts of foods to eat for each meal.
- Nonstarchy vegetables—such as leafy greens, peppers, carrots, or green beans—should make up half of your plate.
- Carb foods that are high in fiber—such as brown rice, whole grains, beans, or fruits—should make up one-quarter of your plate.
- Protein foods—such as lean meats, fish, dairy, or tofu or other soy products—should make up one quarter of your plate.
If you are not taking insulin, you may not need to count carbs when using the plate method.

Work with your health care team to create a meal plan that works for you. You may want to have a diabetes educator or a registered dietitian on your team. A registered dietitian can provide medical nutrition therapy , which includes counseling to help you create and follow a meal plan. Your health care team may be able to recommend other resources, such as a healthy lifestyle coach, to help you with making changes. Ask your health care team or your insurance company if your benefits include medical nutrition therapy or other diabetes care resources.
Talk with your health care professional before taking dietary supplements
There is no clear proof that specific foods, herbs, spices, or dietary supplements —such as vitamins or minerals—can help manage diabetes. Your health care professional may ask you to take vitamins or minerals if you can’t get enough from foods. Talk with your health care professional before you take any supplements, because some may cause side effects or affect how well your diabetes medicines work.
Research shows that regular physical activity helps people manage their diabetes and stay healthy. Benefits of physical activity may include
- lower blood glucose, blood pressure, and cholesterol levels
- better heart health
- healthier weight
- better mood and sleep
- better balance and memory
Talk with your health care professional before starting a new physical activity or changing how much physical activity you do. They may suggest types of activities based on your ability, schedule, meal plan, interests, and diabetes medicines. Your health care professional may also tell you the best times of day to be active or what to do if your blood glucose level goes out of the range recommended for you.

Do different types of physical activity
People with diabetes can be active, even if they take insulin or use technology such as insulin pumps .
Try to do different kinds of activities . While being more active may have more health benefits, any physical activity is better than none. Start slowly with activities you enjoy. You may be able to change your level of effort and try other activities over time. Having a friend or family member join you may help you stick to your routine.
The physical activities you do may need to be different if you are age 65 or older , are pregnant , or have a disability or health condition . Physical activities may also need to be different for children and teens . Ask your health care professional or health care team about activities that are safe for you.
Aerobic activities
Aerobic activities make you breathe harder and make your heart beat faster. You can try walking, dancing, wheelchair rolling, or swimming. Most adults should try to get at least 150 minutes of moderate-intensity physical activity each week. Aim to do 30 minutes a day on most days of the week. You don’t have to do all 30 minutes at one time. You can break up physical activity into small amounts during your day and still get the benefit. 1
Strength training or resistance training
Strength training or resistance training may make your muscles and bones stronger. You can try lifting weights or doing other exercises such as wall pushups or arm raises. Try to do this kind of training two times a week. 1
Balance and stretching activities
Balance and stretching activities may help you move better and have stronger muscles and bones. You may want to try standing on one leg or stretching your legs when sitting on the floor. Try to do these kinds of activities two or three times a week. 1
Some activities that need balance may be unsafe for people with nerve damage or vision problems caused by diabetes. Ask your health care professional or health care team about activities that are safe for you.

Stay safe during physical activity
Staying safe during physical activity is important. Here are some tips to keep in mind.
Drink liquids
Drinking liquids helps prevent dehydration , or the loss of too much water in your body. Drinking water is a way to stay hydrated. Sports drinks often have a lot of sugar and calories , and you don’t need them for most moderate physical activities.
Avoid low blood glucose
Check your blood glucose level before, during, and right after physical activity. Physical activity often lowers the level of glucose in your blood. Low blood glucose levels may last for hours or days after physical activity. You are most likely to have low blood glucose if you take insulin or some other diabetes medicines, such as sulfonylureas.
Ask your health care professional if you should take less insulin or eat carbs before, during, or after physical activity. Low blood glucose can be a serious medical emergency that must be treated right away. Take steps to protect yourself. You can learn how to treat low blood glucose , let other people know what to do if you need help, and use a medical alert bracelet.
Avoid high blood glucose and ketoacidosis
Taking less insulin before physical activity may help prevent low blood glucose, but it may also make you more likely to have high blood glucose. If your body does not have enough insulin, it can’t use glucose as a source of energy and will use fat instead. When your body uses fat for energy, your body makes chemicals called ketones .
High levels of ketones in your blood can lead to a condition called diabetic ketoacidosis (DKA) . DKA is a medical emergency that should be treated right away. DKA is most common in people with type 1 diabetes . Occasionally, DKA may affect people with type 2 diabetes who have lost their ability to produce insulin. Ask your health care professional how much insulin you should take before physical activity, whether you need to test your urine for ketones, and what level of ketones is dangerous for you.
Take care of your feet
People with diabetes may have problems with their feet because high blood glucose levels can damage blood vessels and nerves. To help prevent foot problems, wear comfortable and supportive shoes and take care of your feet before, during, and after physical activity.

If you have diabetes, managing your weight may bring you several health benefits. Ask your health care professional or health care team if you are at a healthy weight or if you should try to lose weight.
If you are an adult with overweight or obesity, work with your health care team to create a weight-loss plan. Losing 5% to 7% of your current weight may help you prevent or improve some health problems and manage your blood glucose, cholesterol, and blood pressure levels. 2 If you are worried about your child’s weight and they have diabetes, talk with their health care professional before your child starts a new weight-loss plan.
You may be able to reach and maintain a healthy weight by
- following a healthy meal plan
- consuming fewer calories
- being physically active
- getting 7 to 8 hours of sleep each night 3
If you have type 2 diabetes, your health care professional may recommend diabetes medicines that may help you lose weight.
Online tools such as the Body Weight Planner may help you create eating and physical activity plans. You may want to talk with your health care professional about other options for managing your weight, including joining a weight-loss program that can provide helpful information, support, and behavioral or lifestyle counseling. These options may have a cost, so make sure to check the details of the programs.
Your health care professional may recommend weight-loss surgery if you aren’t able to reach a healthy weight with meal planning, physical activity, and taking diabetes medicines that help with weight loss.
If you are pregnant , trying to lose weight may not be healthy. However, you should ask your health care professional whether it makes sense to monitor or limit your weight gain during pregnancy.
Both diabetes and smoking —including using tobacco products and e-cigarettes—cause your blood vessels to narrow. Both diabetes and smoking increase your risk of having a heart attack or stroke , nerve damage , kidney disease , eye disease , or amputation . Secondhand smoke can also affect the health of your family or others who live with you.
If you smoke or use other tobacco products, stop. Ask for help . You don’t have to do it alone.
Feeling stressed, sad, or angry can be common for people with diabetes. Managing diabetes or learning to cope with new information about your health can be hard. People with chronic illnesses such as diabetes may develop anxiety or other mental health conditions .
Learn healthy ways to lower your stress , and ask for help from your health care team or a mental health professional. While it may be uncomfortable to talk about your feelings, finding a health care professional whom you trust and want to talk with may help you
- lower your feelings of stress, depression, or anxiety
- manage problems sleeping or remembering things
- see how diabetes affects your family, school, work, or financial situation
Ask your health care team for mental health resources for people with diabetes.
Sleeping too much or too little may raise your blood glucose levels. Your sleep habits may also affect your mental health and vice versa. People with diabetes and overweight or obesity can also have other health conditions that affect sleep, such as sleep apnea , which can raise your blood pressure and risk of heart disease.

NIDDK conducts and supports clinical trials in many diseases and conditions, including diabetes. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for healthy living with diabetes?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care professionals and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of healthy living for people with diabetes, such as
- how changing when you eat may affect body weight and metabolism
- how less access to healthy foods may affect diabetes management, other health problems, and risk of dying
- whether low-carbohydrate meal plans can help lower blood glucose levels
- which diabetes medicines are more likely to help people lose weight
Find out if clinical trials are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical trials for healthy living with diabetes are looking for participants?
You can view a filtered list of clinical studies on healthy living with diabetes that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe for you. Always talk with your primary health care professional before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
NIDDK would like to thank: Elizabeth M. Venditti, Ph.D., University of Pittsburgh School of Medicine.

Main Navigation
/prod01/channel_8/media/scu-dep/current-students/images/Coffs-harbour_student-group_20220616_33.jpg)
- Accept offer and enrol
- Current Students
Personalise your experience
Did you mean..., diploma of arts and social sciences, art/science collaboration wins waterhouse natural science art prize, unit of study scin4003 scientific research context, perspective and methods 2 (2025).
Future students: T: 1800 626 481 E: Email your enquiry here
Current students: Contact: Faculty of Science and Engineering
Students studying at an education collaboration: Please contact your relevant institution
updated - DO NOT REMOVE THIS LINE 6:05 AM on Fri, 12 April
Show me unit information for year
Unit snapshot, credit points, faculty & college.
Faculty of Science and Engineering
Co-requisites
Students must have either completed, or enrol concurrently in, SCIN4002 - Scientific Research Context, Perspective and Methods 1
Unit description
Introduces science Honours students to the range of theoretical frameworks which may inform different types of scientific research and to the methods and methodologies which may be employed in the scientific research process. Encourages students to acquire the skills necessary to carry out, produce and report well designed and articulated research proposals and projects.
Unit content
Orientation: the nature of research; types of research.
The processes of developing a research project: planning and design; identifying the scope and range of a research project; the research design; research methods; formulating research questions; articulating research aims; the ethics of research.
Writing a research proposal: identification and articulation of theoretical frameworks and knowledge gaps relevant to your topic; literature reviews, identification and articulation of methodology; writing the text; citation and referencing; articulating expected outcomes; budget justification.
Introduction to research dissemination skills (scientific writing).
Availabilities
Learning outcomes.
Unit Learning Outcomes express learning achievement in terms of what a student should know, understand and be able to do on completion of a unit. These outcomes are aligned with the graduate attributes . The unit learning outcomes and graduate attributes are also the basis of evaluating prior learning.
On completion of this unit, students should be able to:
critically evaluate techniques and methods used in scientific research
demonstrate awareness of current scientific issues and methods
integrate scientific and management concepts and theories
develop a well designed and articulated research proposal including project summary, project description and budget.
Teaching and assessment
Gold coast (term), lismore (term), national marine science centre coffs harbour (term), online (term), prescribed learning resources, summer term.
- Prescribed text information is not currently available.
- Prescribed resources/equipment information is not currently available.
Prescribed Learning Resources may change in future Teaching Periods.
Fee information
Commonwealth Supported courses For information regarding Student Contribution Amounts please visit the Student Contribution Amounts .
Fee paying courses For postgraduate or undergraduate full-fee paying courses please check Domestic Postgraduate Fees OR Domestic Undergraduate Fees .
International
Please check the international course and fee list to determine the relevant fees.
Courses that offer this unit
Bachelor of science with honours (2025), bachelor of science with honours (2024), any questions we'd love to help.
Estrogen receptors alpha and beta expression in different canine cancer types with an emphasis on hematopoietic malignancies
- Published: 10 April 2024
Cite this article
- Katarzyna Bugiel-Stabla ORCID: orcid.org/0000-0002-7192-7714 1 ,
- Chiara Agnoli ORCID: orcid.org/0000-0002-9004-3834 2 &
- Aleksandra Pawlak ORCID: orcid.org/0000-0002-7449-1661 1
40 Accesses
1 Altmetric
Explore all metrics
Estrogen receptors (ERs) are located in both healthy and neoplastic tissues. The type of estrogen receptor expressed varies depending on its location, tumor type, and species. Estrogen action is mediated by binding to ER and activating the transcriptional and signaling processes that result in the control of gene expression. There are two main types of estrogen receptors: ER alpha (ERα) and ER beta (ERβ). Both receptors are functionally different, they may act antagonistically and are distributed in different tissues but their structure is similar – as they are composed of 5 different domains: A/B, C, D, E, and F. The signaling pathway and hence regulation of the gene expression by ERs is a complex and multifactorial process that involves both genomic and nongenomic actions. In the human reproductive tract, both ERα and β are present, with predominant expression of ERβ, while there are no satisfactory data distinguishing the type of ERs expressed in the canine reproductive tract. In mammary gland neoplasia, a decreased or lacking ERα expression in humans is associated with a poorer prognosis. This is similar to dogs, where higher ERα expression intensity was noted in benign tumors than in carcinomas. In human hematopoietic malignancies, ERβ is a predominant receptor. Selective and non-selective ERβ agonists have an antiproliferative and pro-apoptotic effect on human lymphoma cell lines and may be effective in the therapy of ERβ positive lymphomas and leukemias. In canine lymphoma tissues, none or only marginal expression of ERs was detected over the decades. Considering available data, we conducted preliminary studies proving that, in contrast to humans, the dominant ER expressed in canine hematopoietic tumors is ERα.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
No datasets were generated or analysed during the current study.
Anderson WF, Chen BE, Jatoi I, Rosenberg PS (2006) Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res Treat 100(1):121–126. https://doi.org/10.1007/s10549-006-9231-y
Article CAS PubMed Google Scholar
Arpino G, Weiss H, Lee Av, Schiff R, de Placido S, Osborne CK, Elledge RM (2005) Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97(17):1254–1261. https://doi.org/10.1093/jnci/dji249
Bailey ST, Shin H, Westerling T, Liu XS, Brown M (2012) Estrogen receptor prevents p53-dependent apoptosis in breast cancer. Proc Natl Acad Sci USA 109(44):18060–18065. https://doi.org/10.1073/pnas.1018858109
Article PubMed PubMed Central Google Scholar
Bonkhoff H, Fixemer T, Hunsicker I, Remberger K (1999a) Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 155(2):641–647. https://doi.org/10.1016/S0002-9440(10)65160-7
Article CAS PubMed PubMed Central Google Scholar
Bonkhoff H, Fixemer T, Hunsicker I, Remberger K (1999b) Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 155(2):641–647. https://doi.org/10.1016/S0002-9440(10)65160-7
Bryan JN, Keeler MR, Henry CJ, Bryan ME, Hahn AW, Caldwell CW (2007) A population study of neutering status as a risk factor for canine prostate cancer. Prostate 67(11):1174–1181. https://doi.org/10.1002/pros.20590
Article PubMed Google Scholar
Bugiel-Stabla K, Suarez H, Agnoli B, Obmińska-Mrukowicz C, B., Pawlak A (2021) Sensitivity of canine lymphoma/leukaemia cells to oestrogen receptor beta agonist. Vet Comp Oncol 20(2):13. https://doi.org/10.1111/VCO.12764
Article Google Scholar
Bugiel-Stabla K, Comazzi S, Idziak M, Messina G, Obmińska-Mrukowicz B, Pawlak A (2022) Oestrogen receptor alpha expression in different canine lymphoma subtypes. Abstracts ESVONC Annual Congress May 26–28, 2022, Siracusa, Sicily, 14–14. https://doi.org/10.1111/vco.12850
Canadas-Sousa A, Santos M, Leal B, Medeiros R, Dias-Pereira P (2019) Estrogen receptors genotypes and canine mammary neoplasia. BMC Vet Res 15(1). https://doi.org/10.1186/s12917-019-2062-y
Cerhan JR, Habermann TM, Vachon CM, Putnam SD, Zheng W, Potter JD, Folsom AR (2002) Menstrual and reproductive factors and risk of non-hodgkin lymphoma: the Iowa women’s Health Study (United States). Cancer Causes Control 13(2):131–136. https://doi.org/10.1023/a:1014354105164
Cooke T, George D, Shields R, Maynard P, Griffiths K (1979) Œstrogen receptors and prognosis in early breast cancer. Lancet 313(8124):995–997. https://doi.org/10.1016/S0140-6736(79)92752-1
Couse JF, Lindzey J, Kaj G, Gustafsson J-A, Korach KS (1997) Tissue Distribution and Quantitative Analysis of Estrogen Receptor-a(ERa) and estrogen Receptor-b(ERb) Messenger Ribonucleic Acid in the wild-type andERa-Knockout mouse. Endocrinology 138(11):4613–4621. https://doi.org/10.1210/endo.138.11.5496
De JM, Mulas L, Milla´n Y, Milla´n M, Dios R (2005) A Prospective Analysis of Immunohistochemically Determined Estrogen Receptor and Progesterone Receptor Expression and Host and Tumor Factors as Predictors of Disease-free Period in Mammary Tumors of the Dog. In Vet Pathol 42.
De Andrés PJ, Cáceres S, Clemente M, Pérez-Alenza MD, Illera JC, Peña L (2016) Profile of Steroid receptors and increased aromatase immunoexpression in Canine Inflammatory Mammary Cancer as a potential therapeutic target. Reprod Domest Anim 51(2):269–275. https://doi.org/10.1111/rda.12676
de Cock H, Ducatelle R, Logghe JP (1997) Immunohistochemical lozalization of estrogen receptor in the normal canine female genital tract. Domest Anim Endocrinol 14(3):133–147. https://doi.org/10.1016/S0739-7240(97)00001-5
De Martín J, Ordás J, Millán MY, Chacón F, de Lara M, De Los Monteros E, Reymundo A, C., Jover A (2004) Immunohistochemical expression of estrogen receptor β in normal and tumoral canine mammary glands. Vet Pathol 41(3):269–272. https://doi.org/10.1354/vp.41-3-269
Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson J-A (1997) Human estrogen receptor β-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metabolism 82(12):4258–4265. https://doi.org/10.1210/jcem.82.12.4470
Article CAS Google Scholar
Frank LA, Mullins R, Rohrbach BW (2010) Variability of estradiol concentration in normal dogs. Vet Dermatol 21(5):490–493. https://doi.org/10.1111/j.1365-3164.2010.00896.x
Fuentes N, Silveyra P (2019) Estrogen receptor signaling mechanisms. In Advances in Protein Chemistry and Structural Biology 116, pp. 135–170. Academic Press Inc. https://doi.org/10.1016/bs.apcsb.2019.01.001
Galuszka A, Pawlicki P, Pardyak L, Chmurska-Gąsowska M, Pietsch-Fulbiszewska A, Duliban M, Turek W, Dubniewicz K, Ramisz G, Kotula-Balak M (2021) Abundance of estrogen receptors involved in non-canonical signaling in the dog testis. Anim Reprod Sci 235:106888. https://doi.org/10.1016/j.anireprosci.2021.106888
Hall JM, Couse JF, Korach KS (2001) The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem (Vol 276:36869–36872. https://doi.org/10.1074/jbc.R100029200
Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson J-Å (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87(3):905–931. https://doi.org/10.1152/physrev.00026.2006
Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL (2001) Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res 61(14):5331–5335. http://www.ncbi.nlm.nih.gov/pubmed/11454669
CAS PubMed Google Scholar
Jiang J, Chang H-L, Sugimoto Y, Lin YC (2005) Effects of age on growth and ERbeta mRNA expression of canine prostatic cells. Anticancer Res 25(6B):4081–4090. http://www.ncbi.nlm.nih.gov/pubmed/16312047
Jung HY, Yoo DY, Jo YK, Kim GA, Chung JY, Choi JH, Jang G, Hwang IK (2016) Differential expression of estrogen receptor α and progesterone receptor in the normal and cryptorchid testis of a dog. Lab Anim Res 32(2):128. https://doi.org/10.5625/lar.2016.32.2.128
Khan D, Cowan C, Ahmed SA (2012) Estrogen and signaling in the cells of immune system. Adv Neuroimmune Biology 3(1):73–93. https://doi.org/10.3233/NIB-2012-012039
Kong EH, Pike ACW, Hubbard RE (2003) Structure and mechanism of the oestrogen receptor. Biochem Soc Trans 31(1):56–59. https://doi.org/10.1042/bst0310056
Konturek SJ (2013) Konturek Fizjologia człowieka: Podre̜cznik dla studentów medycyny, 2nd edn. Elsevier LTD
Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-A (1997) Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 138(3):863–870. https://doi.org/10.1210/endo.138.3.4979
Kumar V, Green S, Stack G, Berry M, Jin J-R, Chambon P (1987) Functional domains of the human estrogen receptor. Cell 51(6):941–951. https://doi.org/10.1016/0092-8674(87)90581-2
Lang TJ (2004a) Estrogen as an immunomodulator. In Clinical Immunology (Vol. 113, Issue 3, pp. 224–230). Academic Press Inc. https://doi.org/10.1016/j.clim.2004.05.011
Lang TJ (2004b) Estrogen as an immunomodulator. In Clinical Immunology (Vol. 113, Issue 3, pp.). Academic Press Inc. 113(3);224–230 https://doi.org/10.1016/j.clim.2004.05.011
Lee H-R, Kim T-H, Choi K-C (2012) Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res 28(2):71. https://doi.org/10.5625/lar.2012.28.2.71
Li LC, Chui R, Nakajima K, Oh BR, Au HC, Dahiya R (2000) Frequent methylation of estrogen receptor in prostate cancer: correlation with tumor progression. Cancer Res 60(3):702–706. http://www.ncbi.nlm.nih.gov/pubmed/10676656
Madhu Krishna B, Chaudhary S, Mishra DR, Naik SK, Suklabaidya S, Adhya AK, Mishra SK (2018) Estrogen receptor α dependent regulation of estrogen related receptor β and its role in cell cycle in breast cancer. BMC Cancer 18(1). https://doi.org/10.1186/s12885-018-4528-x
Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, Li Y, Younes M (2001) Estrogen receptor beta expression in invasive breast cancer. Hum Pathol 32(1):113–118. https://doi.org/10.1053/hupa.2001.21506
Millanta F, Calandrella M, Bari G, Niccolini M, Vannozzi I, Poli A (2005) Comparison of steroid receptor expression in normal, dysplastic, and neoplastic canine and feline mammary tissues. Res Vet Sci 79(3):225–232. https://doi.org/10.1016/j.rvsc.2005.02.002
Nativa O, Umehara T, Colvard DS, Therneau TM, Farrow GM, Spelsberg TC, Lieber MM (1997) Relationship between DNA ploidy and functional estrogen receptors in operable prostate cancer. Eur Urol 32(1):96–99. http://www.ncbi.nlm.nih.gov/pubmed/9266239
Nelson RW, Couto CG (2019) Small animal internal medicine, 6th edn. Elsevier LTD
Nelson AW, Tilley WD, Neal DE, Carroll JS (2014) Estrogen receptor beta in prostate cancer: friend or foe? Endocrine-related Cancer 21(4):T219–T234. https://doi.org/10.1530/ERC-13-0508
Nie R, Zhou Q, Jassim E, Saunders PTK, Hess RA (2002) Differential expression of Estrogen Receptors and in the Reproductive Tracts of Adult Male Dogs and cats 1. Biol Reprod 66(4):1161–1168. https://doi.org/10.1095/biolreprod66.4.1161
Nieto A, Peña L, Pérez-Alenza MD, Sánchez MA, Flores JM, Castaño M (2000) Immunohistologic Detection of Estrogen Receptor Alpha in Canine Mammary tumors: clinical and pathologic associations and Prognostic significance. Vet Pathol 37(3):239–247. https://doi.org/10.1354/vp.37-3-239
Osborne CK (1998) Tamoxifen in the treatment of breast Cancer. N Engl J Med 339(22):1609–1618. https://doi.org/10.1056/NEJM199811263392207
Pierdominici M, Maselli A, Locatelli SL, Ciarlo L, Careddu G, Patrizio M, Ascione B, Tinari A, Carlo-Stella C, Malorni W, Matarrese P, Ortona E (2017) Estrogen receptor β ligation inhibits Hodgkin lymphoma growth by inducing autophagy. Oncotarget 8(5):8522–8535. https://doi.org/10.18632/oncotarget.14338
Queiroga FL, Pérez-Alenza MD, Silvan G, Peña L, Lopes C, Illera JC (2005) Role of steroid hormones and prolactin in canine mammary cancer. J Steroid Biochem Mol Biol 94(1–3 SPEC ISS):181–187. https://doi.org/10.1016/j.jsbmb.2004.12.014
Rao J, Jiang X, Wang Y, Chen B (2011) Advances in the understanding of the structure and function of ER-α36,a novel variant of human estrogen receptor-alpha. In Journal of Steroid Biochemistry and Molecular Biology. Elsevier Ltd. 127(3–5):231–237 https://doi.org/10.1016/j.jsbmb.2011.08.004
Reed BG, Carr BR (2000) The Normal Menstrual Cycle and the Control of Ovulation. In Endotext . MDText.com, Inc. http://www.ncbi.nlm.nih.gov/pubmed/25905282
Rutteman G, Misdorp W, Blankenstein M, van den Brom W (1988) Oestrogen (ER) and progestin receptors (PR) in mammary tissue of the female dog: different receptor profile in non-malignant and malignant states. Br J Cancer 58(5):594–599. https://doi.org/10.1038/bjc.1988.266
Tavares WLF, Lavalle GE, Figueiredo MS, Souza AG, Bertagnolli AC, Viana FAB, Paes PRO, Carneiro RA, Cavalcanti GAO, Melo MM, Cassali GD (2010) Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Vet Scand 52(1). https://doi.org/10.1186/1751-0147-52-67
Teske E, Besselink CM, Blankenstein MA, Rutteman GR, Misdorp W (1987) The occurrence of estrogen and progestin receptors and anti-estrogen binding sites (AEBS) in canine non-hodgkin’s lymphomas. Anticancer Res 7(4B):857–860. http://www.ncbi.nlm.nih.gov/pubmed/3674774
Trachtenberg J, Hicks LL, Walsh PC (1980) Androgen- and estrogen-receptor content in spontaneous and experimentally induced canine prostatic hyperplasia. J Clin Invest 65(5):1051–1059. https://doi.org/10.1172/JCI109757
Villamil JA, Henry CJ, Hahn AW, Bryan JN, Tyler JW, Caldwell CW (2009) Hormonal and sex impact on the Epidemiology of Canine Lymphoma. J Cancer Epidemiol 2009:1–7. https://doi.org/10.1155/2009/591753
Weihua Z, Andersson S, Cheng G, Simpson ER, Warner M, Gustafsson JÅ (2003) Update on estrogen signaling. FEBS Lett 546(1):17–24. https://doi.org/10.1016/S0014-5793(03)00436-8
Withrow SJ, Vail DM (2020) Withrow and MacEwen’s Small Animal Clinical Oncology. Withrow & MacEwen’s Small Animal Clinical Oncology. Elsevier. https://doi.org/10.1016/C2016-0-01939-3
Yakimchuk K, Iravani M, Hasni MS, Rhönnstad P, Nilsson S, Jondal M, Okret S (2011) Effect of ligand-activated estrogen receptor Β on lymphoma growth in vitro and in vivo. Leukemia 25(7):1103–1110. https://doi.org/10.1038/leu.2011.68
Yakimchuk K, Jondal M, Okret S (2013) Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. In Molecular and Cellular Endocrinology 375(1–2):121–129. https://doi.org/10.1016/j.mce.2013.05.016
Yang SS, Warner HR (1993) The Underlying Molecular, Cellular and Immunological Factors in Cancer and Aging (S. S. Yang & H. R. Warner, Eds.). Springer US. 330 https://doi.org/10.1007/978-1-4615-2926-2
Yaşar P, Ayaz G, User SD, Güpür G, Muyan M (2017) Molecular mechanism of estrogen–estrogen receptor signaling. Reproductive Medicine and Biology, vol 16. John Wiley and Sons Ltd, pp 4–20. 1 https://doi.org/10.1002/rmb2.12006
Zhang Y, Holford TR, Leaderer B, Boyle P, Zahm SH, Zhang B, Zou K, Morton LM, Owens PH, Flynn S, Tallini G, Zheng T (2004) Menstrual and reproductive factors and risk of non-hodgkin’s lymphoma among connecticut women. Am J Epidemiol 160(8):766–773. https://doi.org/10.1093/aje/kwh278
Download references
This work was supported by “UPWr 2.0: International and interdisciplinary development programme” for Wroclaw University of Environmental and Life Sciences”, co-financed by the European Social Fund under the Operational Program Knowledge Education Development, under contract No. POWR.03.05.00-00-Z062 / 18 of June 4, 2019.
Author information
Authors and affiliations.
Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Wroclaw University of Environmental and Life Sciences, Wrocław, Poland
Katarzyna Bugiel-Stabla & Aleksandra Pawlak
Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy
Chiara Agnoli
You can also search for this author in PubMed Google Scholar
Contributions
A.P. & K.BS. conceived an idea of presented manuscript. K.BS. wrote the main manuscript. prepared figures and tables.A.P. & C.A. suggested literature to review and reviewed the manuscript All authors discussed the results of reviewed literature and contributed to the final manuscript.
Corresponding author
Correspondence to Katarzyna Bugiel-Stabla .
Ethics declarations
Ethics approval and consent to participate.
No approval of research ethics committees was required for the purpose of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Bugiel-Stabla, K., Agnoli, C. & Pawlak, A. Estrogen receptors alpha and beta expression in different canine cancer types with an emphasis on hematopoietic malignancies. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10368-2
Download citation
Received : 01 December 2023
Accepted : 26 March 2024
Published : 10 April 2024
DOI : https://doi.org/10.1007/s11259-024-10368-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Canine lymphoma
- Canine mammary carcinoma
- Canine prostatic carcinoma
- Find a journal
- Publish with us
- Track your research
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Research bias
Types of Bias in Research | Definition & Examples
Research bias results from any deviation from the truth, causing distorted results and wrong conclusions. Bias can occur at any phase of your research, including during data collection , data analysis , interpretation, or publication. Research bias can occur in both qualitative and quantitative research .
Understanding research bias is important for several reasons.
- Bias exists in all research, across research designs , and is difficult to eliminate.
- Bias can occur at any stage of the research process .
- Bias impacts the validity and reliability of your findings, leading to misinterpretation of data.
It is almost impossible to conduct a study without some degree of research bias. It’s crucial for you to be aware of the potential types of bias, so you can minimize them.
For example, the success rate of the program will likely be affected if participants start to drop out ( attrition ). Participants who become disillusioned due to not losing weight may drop out, while those who succeed in losing weight are more likely to continue. This in turn may bias the findings towards more favorable results.
Table of contents
Information bias, interviewer bias.
- Publication bias
Researcher bias
Response bias.
Selection bias
Cognitive bias
How to avoid bias in research
Other types of research bias, frequently asked questions about research bias.
Information bias , also called measurement bias, arises when key study variables are inaccurately measured or classified. Information bias occurs during the data collection step and is common in research studies that involve self-reporting and retrospective data collection. It can also result from poor interviewing techniques or differing levels of recall from participants.
The main types of information bias are:
- Recall bias
- Observer bias
Performance bias
Regression to the mean (rtm).
Over a period of four weeks, you ask students to keep a journal, noting how much time they spent on their smartphones along with any symptoms like muscle twitches, aches, or fatigue.
Recall bias is a type of information bias. It occurs when respondents are asked to recall events in the past and is common in studies that involve self-reporting.
As a rule of thumb, infrequent events (e.g., buying a house or a car) will be memorable for longer periods of time than routine events (e.g., daily use of public transportation). You can reduce recall bias by running a pilot survey and carefully testing recall periods. If possible, test both shorter and longer periods, checking for differences in recall.
- A group of children who have been diagnosed, called the case group
- A group of children who have not been diagnosed, called the control group
Since the parents are being asked to recall what their children generally ate over a period of several years, there is high potential for recall bias in the case group.
The best way to reduce recall bias is by ensuring your control group will have similar levels of recall bias to your case group. Parents of children who have childhood cancer, which is a serious health problem, are likely to be quite concerned about what may have contributed to the cancer.
Thus, if asked by researchers, these parents are likely to think very hard about what their child ate or did not eat in their first years of life. Parents of children with other serious health problems (aside from cancer) are also likely to be quite concerned about any diet-related question that researchers ask about.
Observer bias is the tendency of research participants to see what they expect or want to see, rather than what is actually occurring. Observer bias can affect the results in observationa l and experimental studies, where subjective judgment (such as assessing a medical image) or measurement (such as rounding blood pressure readings up or down) is part of the d ata collection process.
Observer bias leads to over- or underestimation of true values, which in turn compromise the validity of your findings. You can reduce observer bias by using double-blinded and single-blinded research methods.
Based on discussions you had with other researchers before starting your observations , you are inclined to think that medical staff tend to simply call each other when they need specific patient details or have questions about treatments.
At the end of the observation period, you compare notes with your colleague. Your conclusion was that medical staff tend to favor phone calls when seeking information, while your colleague noted down that medical staff mostly rely on face-to-face discussions. Seeing that your expectations may have influenced your observations, you and your colleague decide to conduct semi-structured interviews with medical staff to clarify the observed events. Note: Observer bias and actor–observer bias are not the same thing.
Performance bias is unequal care between study groups. Performance bias occurs mainly in medical research experiments, if participants have knowledge of the planned intervention, therapy, or drug trial before it begins.
Studies about nutrition, exercise outcomes, or surgical interventions are very susceptible to this type of bias. It can be minimized by using blinding , which prevents participants and/or researchers from knowing who is in the control or treatment groups. If blinding is not possible, then using objective outcomes (such as hospital admission data) is the best approach.
When the subjects of an experimental study change or improve their behavior because they are aware they are being studied, this is called the Hawthorne effect (or observer effect). Similarly, the John Henry effect occurs when members of a control group are aware they are being compared to the experimental group. This causes them to alter their behavior in an effort to compensate for their perceived disadvantage.
Regression to the mean (RTM) is a statistical phenomenon that refers to the fact that a variable that shows an extreme value on its first measurement will tend to be closer to the center of its distribution on a second measurement.
Medical research is particularly sensitive to RTM. Here, interventions aimed at a group or a characteristic that is very different from the average (e.g., people with high blood pressure) will appear to be successful because of the regression to the mean. This can lead researchers to misinterpret results, describing a specific intervention as causal when the change in the extreme groups would have happened anyway.
In general, among people with depression, certain physical and mental characteristics have been observed to deviate from the population mean .
This could lead you to think that the intervention was effective when those treated showed improvement on measured post-treatment indicators, such as reduced severity of depressive episodes.
However, given that such characteristics deviate more from the population mean in people with depression than in people without depression, this improvement could be attributed to RTM.
Interviewer bias stems from the person conducting the research study. It can result from the way they ask questions or react to responses, but also from any aspect of their identity, such as their sex, ethnicity, social class, or perceived attractiveness.
Interviewer bias distorts responses, especially when the characteristics relate in some way to the research topic. Interviewer bias can also affect the interviewer’s ability to establish rapport with the interviewees, causing them to feel less comfortable giving their honest opinions about sensitive or personal topics.
Participant: “I like to solve puzzles, or sometimes do some gardening.”
You: “I love gardening, too!”
In this case, seeing your enthusiastic reaction could lead the participant to talk more about gardening.
Establishing trust between you and your interviewees is crucial in order to ensure that they feel comfortable opening up and revealing their true thoughts and feelings. At the same time, being overly empathetic can influence the responses of your interviewees, as seen above.
Publication bias occurs when the decision to publish research findings is based on their nature or the direction of their results. Studies reporting results that are perceived as positive, statistically significant , or favoring the study hypotheses are more likely to be published due to publication bias.
Publication bias is related to data dredging (also called p -hacking ), where statistical tests on a set of data are run until something statistically significant happens. As academic journals tend to prefer publishing statistically significant results, this can pressure researchers to only submit statistically significant results. P -hacking can also involve excluding participants or stopping data collection once a p value of 0.05 is reached. However, this leads to false positive results and an overrepresentation of positive results in published academic literature.
Researcher bias occurs when the researcher’s beliefs or expectations influence the research design or data collection process. Researcher bias can be deliberate (such as claiming that an intervention worked even if it didn’t) or unconscious (such as letting personal feelings, stereotypes, or assumptions influence research questions ).
The unconscious form of researcher bias is associated with the Pygmalion effect (or Rosenthal effect ), where the researcher’s high expectations (e.g., that patients assigned to a treatment group will succeed) lead to better performance and better outcomes.
Researcher bias is also sometimes called experimenter bias, but it applies to all types of investigative projects, rather than only to experimental designs .
- Good question: What are your views on alcohol consumption among your peers?
- Bad question: Do you think it’s okay for young people to drink so much?
Response bias is a general term used to describe a number of different situations where respondents tend to provide inaccurate or false answers to self-report questions, such as those asked on surveys or in structured interviews .
This happens because when people are asked a question (e.g., during an interview ), they integrate multiple sources of information to generate their responses. Because of that, any aspect of a research study may potentially bias a respondent. Examples include the phrasing of questions in surveys, how participants perceive the researcher, or the desire of the participant to please the researcher and to provide socially desirable responses.
Response bias also occurs in experimental medical research. When outcomes are based on patients’ reports, a placebo effect can occur. Here, patients report an improvement despite having received a placebo, not an active medical treatment.
While interviewing a student, you ask them:
“Do you think it’s okay to cheat on an exam?”
Common types of response bias are:
Acquiescence bias
Demand characteristics.
- Social desirability bias
Courtesy bias
- Question-order bias
Extreme responding
Acquiescence bias is the tendency of respondents to agree with a statement when faced with binary response options like “agree/disagree,” “yes/no,” or “true/false.” Acquiescence is sometimes referred to as “yea-saying.”
This type of bias occurs either due to the participant’s personality (i.e., some people are more likely to agree with statements than disagree, regardless of their content) or because participants perceive the researcher as an expert and are more inclined to agree with the statements presented to them.
Q: Are you a social person?
People who are inclined to agree with statements presented to them are at risk of selecting the first option, even if it isn’t fully supported by their lived experiences.
In order to control for acquiescence, consider tweaking your phrasing to encourage respondents to make a choice truly based on their preferences. Here’s an example:
Q: What would you prefer?
- A quiet night in
- A night out with friends
Demand characteristics are cues that could reveal the research agenda to participants, risking a change in their behaviors or views. Ensuring that participants are not aware of the research objectives is the best way to avoid this type of bias.
On each occasion, patients reported their pain as being less than prior to the operation. While at face value this seems to suggest that the operation does indeed lead to less pain, there is a demand characteristic at play. During the interviews, the researcher would unconsciously frown whenever patients reported more post-op pain. This increased the risk of patients figuring out that the researcher was hoping that the operation would have an advantageous effect.
Social desirability bias is the tendency of participants to give responses that they believe will be viewed favorably by the researcher or other participants. It often affects studies that focus on sensitive topics, such as alcohol consumption or sexual behavior.
You are conducting face-to-face semi-structured interviews with a number of employees from different departments. When asked whether they would be interested in a smoking cessation program, there was widespread enthusiasm for the idea.
Note that while social desirability and demand characteristics may sound similar, there is a key difference between them. Social desirability is about conforming to social norms, while demand characteristics revolve around the purpose of the research.
Courtesy bias stems from a reluctance to give negative feedback, so as to be polite to the person asking the question. Small-group interviewing where participants relate in some way to each other (e.g., a student, a teacher, and a dean) is especially prone to this type of bias.
Question order bias
Question order bias occurs when the order in which interview questions are asked influences the way the respondent interprets and evaluates them. This occurs especially when previous questions provide context for subsequent questions.
When answering subsequent questions, respondents may orient their answers to previous questions (called a halo effect ), which can lead to systematic distortion of the responses.
Extreme responding is the tendency of a respondent to answer in the extreme, choosing the lowest or highest response available, even if that is not their true opinion. Extreme responding is common in surveys using Likert scales , and it distorts people’s true attitudes and opinions.
Disposition towards the survey can be a source of extreme responding, as well as cultural components. For example, people coming from collectivist cultures tend to exhibit extreme responses in terms of agreement, while respondents indifferent to the questions asked may exhibit extreme responses in terms of disagreement.
Selection bias is a general term describing situations where bias is introduced into the research from factors affecting the study population.
Common types of selection bias are:
Sampling or ascertainment bias
- Attrition bias
- Self-selection (or volunteer) bias
- Survivorship bias
- Nonresponse bias
- Undercoverage bias
Sampling bias occurs when your sample (the individuals, groups, or data you obtain for your research) is selected in a way that is not representative of the population you are analyzing. Sampling bias threatens the external validity of your findings and influences the generalizability of your results.
The easiest way to prevent sampling bias is to use a probability sampling method . This way, each member of the population you are studying has an equal chance of being included in your sample.
Sampling bias is often referred to as ascertainment bias in the medical field.
Attrition bias occurs when participants who drop out of a study systematically differ from those who remain in the study. Attrition bias is especially problematic in randomized controlled trials for medical research because participants who do not like the experience or have unwanted side effects can drop out and affect your results.
You can minimize attrition bias by offering incentives for participants to complete the study (e.g., a gift card if they successfully attend every session). It’s also a good practice to recruit more participants than you need, or minimize the number of follow-up sessions or questions.
You provide a treatment group with weekly one-hour sessions over a two-month period, while a control group attends sessions on an unrelated topic. You complete five waves of data collection to compare outcomes: a pretest survey, three surveys during the program, and a posttest survey.
Self-selection or volunteer bias
Self-selection bias (also called volunteer bias ) occurs when individuals who volunteer for a study have particular characteristics that matter for the purposes of the study.
Volunteer bias leads to biased data, as the respondents who choose to participate will not represent your entire target population. You can avoid this type of bias by using random assignment —i.e., placing participants in a control group or a treatment group after they have volunteered to participate in the study.
Closely related to volunteer bias is nonresponse bias , which occurs when a research subject declines to participate in a particular study or drops out before the study’s completion.
Considering that the hospital is located in an affluent part of the city, volunteers are more likely to have a higher socioeconomic standing, higher education, and better nutrition than the general population.
Survivorship bias occurs when you do not evaluate your data set in its entirety: for example, by only analyzing the patients who survived a clinical trial.
This strongly increases the likelihood that you draw (incorrect) conclusions based upon those who have passed some sort of selection process—focusing on “survivors” and forgetting those who went through a similar process and did not survive.
Note that “survival” does not always mean that participants died! Rather, it signifies that participants did not successfully complete the intervention.
However, most college dropouts do not become billionaires. In fact, there are many more aspiring entrepreneurs who dropped out of college to start companies and failed than succeeded.
Nonresponse bias occurs when those who do not respond to a survey or research project are different from those who do in ways that are critical to the goals of the research. This is very common in survey research, when participants are unable or unwilling to participate due to factors like lack of the necessary skills, lack of time, or guilt or shame related to the topic.
You can mitigate nonresponse bias by offering the survey in different formats (e.g., an online survey, but also a paper version sent via post), ensuring confidentiality , and sending them reminders to complete the survey.
You notice that your surveys were conducted during business hours, when the working-age residents were less likely to be home.
Undercoverage bias occurs when you only sample from a subset of the population you are interested in. Online surveys can be particularly susceptible to undercoverage bias. Despite being more cost-effective than other methods, they can introduce undercoverage bias as a result of excluding people who do not use the internet.
Cognitive bias refers to a set of predictable (i.e., nonrandom) errors in thinking that arise from our limited ability to process information objectively. Rather, our judgment is influenced by our values, memories, and other personal traits. These create “ mental shortcuts” that help us process information intuitively and decide faster. However, cognitive bias can also cause us to misunderstand or misinterpret situations, information, or other people.
Because of cognitive bias, people often perceive events to be more predictable after they happen.
Although there is no general agreement on how many types of cognitive bias exist, some common types are:
- Anchoring bias
- Framing effect
- Actor-observer bias
- Availability heuristic (or availability bias)
- Confirmation bias
- Halo effect
- The Baader-Meinhof phenomenon
Anchoring bias
Anchoring bias is people’s tendency to fixate on the first piece of information they receive, especially when it concerns numbers. This piece of information becomes a reference point or anchor. Because of that, people base all subsequent decisions on this anchor. For example, initial offers have a stronger influence on the outcome of negotiations than subsequent ones.
- Framing effect
Framing effect refers to our tendency to decide based on how the information about the decision is presented to us. In other words, our response depends on whether the option is presented in a negative or positive light, e.g., gain or loss, reward or punishment, etc. This means that the same information can be more or less attractive depending on the wording or what features are highlighted.
Actor–observer bias
Actor–observer bias occurs when you attribute the behavior of others to internal factors, like skill or personality, but attribute your own behavior to external or situational factors.
In other words, when you are the actor in a situation, you are more likely to link events to external factors, such as your surroundings or environment. However, when you are observing the behavior of others, you are more likely to associate behavior with their personality, nature, or temperament.
One interviewee recalls a morning when it was raining heavily. They were rushing to drop off their kids at school in order to get to work on time. As they were driving down the highway, another car cut them off as they were trying to merge. They tell you how frustrated they felt and exclaim that the other driver must have been a very rude person.
At another point, the same interviewee recalls that they did something similar: accidentally cutting off another driver while trying to take the correct exit. However, this time, the interviewee claimed that they always drive very carefully, blaming their mistake on poor visibility due to the rain.
- Availability heuristic
Availability heuristic (or availability bias) describes the tendency to evaluate a topic using the information we can quickly recall to our mind, i.e., that is available to us. However, this is not necessarily the best information, rather it’s the most vivid or recent. Even so, due to this mental shortcut, we tend to think that what we can recall must be right and ignore any other information.
- Confirmation bias
Confirmation bias is the tendency to seek out information in a way that supports our existing beliefs while also rejecting any information that contradicts those beliefs. Confirmation bias is often unintentional but still results in skewed results and poor decision-making.
Let’s say you grew up with a parent in the military. Chances are that you have a lot of complex emotions around overseas deployments. This can lead you to over-emphasize findings that “prove” that your lived experience is the case for most families, neglecting other explanations and experiences.
The halo effect refers to situations whereby our general impression about a person, a brand, or a product is shaped by a single trait. It happens, for instance, when we automatically make positive assumptions about people based on something positive we notice, while in reality, we know little about them.
The Baader-Meinhof phenomenon
The Baader-Meinhof phenomenon (or frequency illusion) occurs when something that you recently learned seems to appear “everywhere” soon after it was first brought to your attention. However, this is not the case. What has increased is your awareness of something, such as a new word or an old song you never knew existed, not their frequency.
While very difficult to eliminate entirely, research bias can be mitigated through proper study design and implementation. Here are some tips to keep in mind as you get started.
- Clearly explain in your methodology section how your research design will help you meet the research objectives and why this is the most appropriate research design.
- In quantitative studies , make sure that you use probability sampling to select the participants. If you’re running an experiment, make sure you use random assignment to assign your control and treatment groups.
- Account for participants who withdraw or are lost to follow-up during the study. If they are withdrawing for a particular reason, it could bias your results. This applies especially to longer-term or longitudinal studies .
- Use triangulation to enhance the validity and credibility of your findings.
- Phrase your survey or interview questions in a neutral, non-judgmental tone. Be very careful that your questions do not steer your participants in any particular direction.
- Consider using a reflexive journal. Here, you can log the details of each interview , paying special attention to any influence you may have had on participants. You can include these in your final analysis.
- Baader–Meinhof phenomenon
- Sampling bias
- Ascertainment bias
- Self-selection bias
- Hawthorne effect
- Omitted variable bias
- Pygmalion effect
- Placebo effect
Research bias affects the validity and reliability of your research findings , leading to false conclusions and a misinterpretation of the truth. This can have serious implications in areas like medical research where, for example, a new form of treatment may be evaluated.
Observer bias occurs when the researcher’s assumptions, views, or preconceptions influence what they see and record in a study, while actor–observer bias refers to situations where respondents attribute internal factors (e.g., bad character) to justify other’s behavior and external factors (difficult circumstances) to justify the same behavior in themselves.
Response bias is a general term used to describe a number of different conditions or factors that cue respondents to provide inaccurate or false answers during surveys or interviews. These factors range from the interviewer’s perceived social position or appearance to the the phrasing of questions in surveys.
Nonresponse bias occurs when the people who complete a survey are different from those who did not, in ways that are relevant to the research topic. Nonresponse can happen because people are either not willing or not able to participate.
Is this article helpful?
Other students also liked.
- Attrition Bias | Examples, Explanation, Prevention
- Observer Bias | Definition, Examples, Prevention
- What Is Social Desirability Bias? | Definition & Examples
More interesting articles
- Demand Characteristics | Definition, Examples & Control
- Hostile Attribution Bias | Definition & Examples
- Regression to the Mean | Definition & Examples
- Representativeness Heuristic | Example & Definition
- Sampling Bias and How to Avoid It | Types & Examples
- Self-Fulfilling Prophecy | Definition & Examples
- The Availability Heuristic | Example & Definition
- The Baader–Meinhof Phenomenon Explained
- What Is a Ceiling Effect? | Definition & Examples
- What Is Actor-Observer Bias? | Definition & Examples
- What Is Affinity Bias? | Definition & Examples
- What Is Anchoring Bias? | Definition & Examples
- What Is Ascertainment Bias? | Definition & Examples
- What Is Belief Bias? | Definition & Examples
- What Is Bias for Action? | Definition & Examples
- What Is Cognitive Bias? | Definition, Types, & Examples
- What Is Confirmation Bias? | Definition & Examples
- What Is Conformity Bias? | Definition & Examples
- What Is Correspondence Bias? | Definition & Example
- What Is Explicit Bias? | Definition & Examples
- What Is Generalizability? | Definition & Examples
- What Is Hindsight Bias? | Definition & Examples
- What Is Implicit Bias? | Definition & Examples
- What Is Information Bias? | Definition & Examples
- What Is Ingroup Bias? | Definition & Examples
- What Is Negativity Bias? | Definition & Examples
- What Is Nonresponse Bias? | Definition & Example
- What Is Normalcy Bias? | Definition & Example
- What Is Omitted Variable Bias? | Definition & Examples
- What Is Optimism Bias? | Definition & Examples
- What Is Outgroup Bias? | Definition & Examples
- What Is Overconfidence Bias? | Definition & Examples
- What Is Perception Bias? | Definition & Examples
- What Is Primacy Bias? | Definition & Example
- What Is Publication Bias? | Definition & Examples
- What Is Recall Bias? | Definition & Examples
- What Is Recency Bias? | Definition & Examples
- What Is Response Bias? | Definition & Examples
- What Is Selection Bias? | Definition & Examples
- What Is Self-Selection Bias? | Definition & Example
- What Is Self-Serving Bias? | Definition & Example
- What Is Status Quo Bias? | Definition & Examples
- What Is Survivorship Bias? | Definition & Examples
- What Is the Affect Heuristic? | Example & Definition
- What Is the Egocentric Bias? | Definition & Examples
- What Is the Framing Effect? | Definition & Examples
- What Is the Halo Effect? | Definition & Examples
- What Is the Hawthorne Effect? | Definition & Examples
- What Is the Placebo Effect? | Definition & Examples
- What Is the Pygmalion Effect? | Definition & Examples
- What Is Unconscious Bias? | Definition & Examples
- What Is Undercoverage Bias? | Definition & Example
- What Is Vividness Bias? | Definition & Examples
Research could unlock more precise prognoses and targeted treatments for children with cancer
Neuroblastoma study identifies new subgroups with distinct prognoses and potential vulnerabilities to therapies.
Researchers have identified new variations in neuroblastoma that could lead to a more accurate prognosis and better-targeted treatments for this devastating childhood cancer.
A study published in the British Journal of Cancer reveals three new subgroups of the most common type of neuroblastoma, each with different genetic traits, expected outcomes, and distinguishing features that offer clues as to which treatments may be most effective.
Dr Yihua Wang from the University of Southampton, a senior author on the paper said: "This research represents a pivotal advancement in our understanding of MYCN non-amplified neuroblastomas. The results are striking. These kinds of neuroblastomas can be classified into three distinct subgroups, each demonstrating unique prognostic implications and varying vulnerabilities to investigational therapies."
Around 100 children are diagnosed with neuroblastoma each year in the UK, representing six to ten per cent of all childhood cancers. Neuroblastoma is a cancer that starts in a type of nerve cell called a neuroblast. It can present in the abdomen, chest neck or pelvis and can spread to other parts of the body.
The overall prognosis of the disease is poor, with just 20 per cent of patients still alive at 5 years after diagnosis, but the likelihood of the cancer being cured varies widely, with some tumours spontaneously regressing and others proving resistant to therapy and progressing.
One of the key indicators of risk is the amplification of a gene called MYCN , where tumours have too many of this type of gene. This occurs in around 20 per cent of cases and accounts for about 40 per cent of high-risk neuroblastomas.
Researchers from the University of Southampton and China wanted to find out more about cases where the MYCN gene isn't amplified to better understand the diversity of outcomes within these cases. Using advanced analytical techniques, the research team analysed over 1,500 biopsy samples from 16 different datasets sourced from Gene Expression Omnibus (GEO) and ArrayExpress.
The team were able to identify three distinct subtypes of these MYCN non-amplified cases based on their transcriptional signatures -- patterns of gene expression that can provide valuable insights into biological processes.
The first subgroup makes up around half of MYCN non-amplified cases and has the best prognosis, with a long-term survival rate of over 85 per cent, despite some cases being clinically classified as high risk.
Subgroup 2, representing a quarter of MYCN non-amplified cases, had the worst outcomes with a long-term survival rate of 50%. Interestingly, this group had a similar genetic signature to cases where MYCN is amplified. Researchers found a protein called Aurora Kinase A (AURKA) was expressed at significantly higher levels than in the other two subgroups. On further analysis, they found that AURKA mRNA levels alone could predict overall survival. This suggests that patients within the subgroup may benefit from treatment with AURKA inhibitors.
Meanwhile, Subgroup 3, which made up another quarter of MYCN non-amplified cases, is characterised by an 'inflamed' gene signature, with significantly higher levels of activity in immune cells. Further analysis indicates that patients in this subgroup were predicted to respond better to immunotherapy.
Dr Wang added: "This research opens new avenues for personalised medicine in the treatment of neuroblastomas. By leveraging transcriptional subtyping, we are now equipped to offer more precise prognosis and tailor therapies accordingly for patients with MYCN non-amplified neuroblastomas, potentially improving outcomes and quality of life."
The project was supported by the UK Medical Research Council and the Natural Science Foundation of China.
- Personalized Medicine
- Breast Cancer
- Diseases and Conditions
- Multiple Sclerosis
- Alzheimer's
- Huntington's Disease
- Stem cell treatments
- Breast cancer
- Personalized medicine
- Alzheimer's disease
- Sleep disorder
- Renal cell carcinoma
Story Source:
Materials provided by University of Southampton . Note: Content may be edited for style and length.
Journal Reference :
- Xiaoxiao Hu, Yilu Zhou, Charlotte Hill, Kai Chen, Cheng Cheng, Xiaowei Liu, Peiwen Duan, Yaoyao Gu, Yeming Wu, Rob M. Ewing, Zhongrong Li, Zhixiang Wu, Yihua Wang. Identification of MYCN non-amplified neuroblastoma subgroups points towards molecular signatures for precision prognosis and therapy stratification . British Journal of Cancer , 2024; DOI: 10.1038/s41416-024-02666-y
Cite This Page :
Explore More
- Genes for Strong Muscles: Healthy Long Life
- Brightest Gamma-Ray Burst
- Stellar Winds of Three Sun-Like Stars Detected
- Fences Causing Genetic Problems for Mammals
- Ozone Removes Mating Barriers Between Fly ...
- Parkinson's: New Theory On Origins and Spread
- Clash of Stars Solves Stellar Mystery
- Secure Quantum Computing at Home
- Ocean Currents: Collapse of Antarctic Ice ...
- Pacific Cities Much Older Than Previously ...
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pediatr Investig
- v.3(4); 2019 Dec

Clinical research study designs: The essentials
Ambika g. chidambaram.
1 Children's Hospital of Philadelphia, Philadelphia Pennsylvania, USA
Maureen Josephson
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and governed by ethical clinical principles. The purpose of this review is to provide the readers an overview of the basic study designs and its applicability in clinical research.
Introduction
In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the “real world” setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of the population being studied. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and is governed by ethical principles. 2
From an epidemiological standpoint, there are two major types of clinical study designs, observational and experimental. 3 Observational studies are hypothesis‐generating studies, and they can be further divided into descriptive and analytic. Descriptive observational studies provide a description of the exposure and/or the outcome, and analytic observational studies provide a measurement of the association between the exposure and the outcome. Experimental studies, on the other hand, are hypothesis testing studies. It involves an intervention that tests the association between the exposure and outcome. Each study design is different, and so it would be important to choose a design that would most appropriately answer the question in mind and provide the most valuable information. We will be reviewing each study design in detail (Figure 1 ).

Overview of clinical research study designs
Observational study designs
Observational studies ask the following questions: what, who, where and when. There are many study designs that fall under the umbrella of descriptive study designs, and they include, case reports, case series, ecologic study, cross‐sectional study, cohort study and case‐control study (Figure 2 ).

Classification of observational study designs
Case reports and case series
Every now and then during clinical practice, we come across a case that is atypical or ‘out of the norm’ type of clinical presentation. This atypical presentation is usually described as case reports which provides a detailed and comprehensive description of the case. 4 It is one of the earliest forms of research and provides an opportunity for the investigator to describe the observations that make a case unique. There are no inferences obtained and therefore cannot be generalized to the population which is a limitation. Most often than not, a series of case reports make a case series which is an atypical presentation found in a group of patients. This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore. However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity.
Ecologic study
Ecological studies are observational studies that provide a description of population group characteristics. That is, it describes characteristics to all individuals within a group. For example, Prentice et al 5 measured incidence of breast cancer and per capita intake of dietary fat, and found a correlation that higher per capita intake of dietary fat was associated with an increased incidence of breast cancer. But the study does not conclude specifically which subjects with breast cancer had a higher dietary intake of fat. Thus, one of the limitations with ecologic study designs is that the characteristics are attributed to the whole group and so the individual characteristics are unknown.
Cross‐sectional study
Cross‐sectional studies are study designs used to evaluate an association between an exposure and outcome at the same time. It can be classified under either descriptive or analytic, and therefore depends on the question being answered by the investigator. Since, cross‐sectional studies are designed to collect information at the same point of time, this provides an opportunity to measure prevalence of the exposure or the outcome. For example, a cross‐sectional study design was adopted to estimate the global need for palliative care for children based on representative sample of countries from all regions of the world and all World Bank income groups. 6 The limitation of cross‐sectional study design is that temporal association cannot be established as the information is collected at the same point of time. If a study involves a questionnaire, then the investigator can ask questions to onset of symptoms or risk factors in relation to onset of disease. This would help in obtaining a temporal sequence between the exposure and outcome. 7
Case‐control study
Case‐control studies are study designs that compare two groups, such as the subjects with disease (cases) to the subjects without disease (controls), and to look for differences in risk factors. 8 This study is used to study risk factors or etiologies for a disease, especially if the disease is rare. Thus, case‐control studies can also be hypothesis testing studies and therefore can suggest a causal relationship but cannot prove. It is less expensive and less time‐consuming than cohort studies (described in section “Cohort study”). An example of a case‐control study was performed in Pakistan evaluating the risk factors for neonatal tetanus. They retrospectively reviewed a defined cohort for cases with and without neonatal tetanus. 9 They found a strong association of the application of ghee (clarified butter) as a risk factor for neonatal tetanus. Although this suggests a causal relationship, cause cannot be proven by this methodology (Figure 3 ).

Case‐control study design
One of the limitations of case‐control studies is that they cannot estimate prevalence of a disease accurately as a proportion of cases and controls are studied at a time. Case‐control studies are also prone to biases such as recall bias, as the subjects are providing information based on their memory. Hence, the subjects with disease are likely to remember the presence of risk factors compared to the subjects without disease.
One of the aspects that is often overlooked is the selection of cases and controls. It is important to select the cases and controls appropriately to obtain a meaningful and scientifically sound conclusion and this can be achieved by implementing matching. Matching is defined by Gordis et al as ‘the process of selecting the controls so that they are similar to the cases in certain characteristics such as age, race, sex, socioeconomic status and occupation’ 7 This would help identify risk factors or probable etiologies that are not due to differences between the cases and controls.
Cohort study
Cohort studies are study designs that compare two groups, such as the subjects with exposure/risk factor to the subjects without exposure/risk factor, for differences in incidence of outcome/disease. Most often, cohort study designs are used to study outcome(s) from a single exposure/risk factor. Thus, cohort studies can also be hypothesis testing studies and can infer and interpret a causal relationship between an exposure and a proposed outcome, but cannot establish it (Figure 4 ).

Cohort study design
Cohort studies can be classified as prospective and retrospective. 7 Prospective cohort studies follow subjects from presence of risk factors/exposure to development of disease/outcome. This could take up to years before development of disease/outcome, and therefore is time consuming and expensive. On the other hand, retrospective cohort studies identify a population with and without the risk factor/exposure based on past records and then assess if they had developed the disease/outcome at the time of study. Thus, the study design for prospective and retrospective cohort studies are similar as we are comparing populations with and without exposure/risk factor to development of outcome/disease.
Cohort studies are typically chosen as a study design when the suspected exposure is known and rare, and the incidence of disease/outcome in the exposure group is suspected to be high. The choice between prospective and retrospective cohort study design would depend on the accuracy and reliability of the past records regarding the exposure/risk factor.
Some of the biases observed with cohort studies include selection bias and information bias. Some individuals who have the exposure may refuse to participate in the study or would be lost to follow‐up, and in those instances, it becomes difficult to interpret the association between an exposure and outcome. Also, if the information is inaccurate when past records are used to evaluate for exposure status, then again, the association between the exposure and outcome becomes difficult to interpret.
Case‐control studies based within a defined cohort
Case‐control studies based within a defined cohort is a form of study design that combines some of the features of a cohort study design and a case‐control study design. When a defined cohort is embedded in a case‐control study design, all the baseline information collected before the onset of disease like interviews, surveys, blood or urine specimens, then the cohort is followed onset of disease. One of the advantages of following the above design is that it eliminates recall bias as the information regarding risk factors is collected before onset of disease. Case‐control studies based within a defined cohort can be further classified into two types: Nested case‐control study and Case‐cohort study.
Nested case‐control study
A nested case‐control study consists of defining a cohort with suspected risk factors and assigning a control within a cohort to the subject who develops the disease. 10 Over a period, cases and controls are identified and followed as per the investigator's protocol. Hence, the case and control are matched on calendar time and length of follow‐up. When this study design is implemented, it is possible for the control that was selected early in the study to develop the disease and become a case in the latter part of the study.
Case‐cohort Study
A case‐cohort study is similar to a nested case‐control study except that there is a defined sub‐cohort which forms the groups of individuals without the disease (control), and the cases are not matched on calendar time or length of follow‐up with the control. 11 With these modifications, it is possible to compare different disease groups with the same sub‐cohort group of controls and eliminates matching between the case and control. However, these differences will need to be accounted during analysis of results.
Experimental study design
The basic concept of experimental study design is to study the effect of an intervention. In this study design, the risk factor/exposure of interest/treatment is controlled by the investigator. Therefore, these are hypothesis testing studies and can provide the most convincing demonstration of evidence for causality. As a result, the design of the study requires meticulous planning and resources to provide an accurate result.
The experimental study design can be classified into 2 groups, that is, controlled (with comparison) and uncontrolled (without comparison). 1 In the group without controls, the outcome is directly attributed to the treatment received in one group. This fails to prove if the outcome was truly due to the intervention implemented or due to chance. This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion.
Experimental study designs can be divided into 3 broad categories: clinical trial, community trial, field trial. The specifics of each study design are explained below (Figure 5 ).

Experimental study designs
Clinical trial
Clinical trials are also known as therapeutic trials, which involve subjects with disease and are placed in different treatment groups. It is considered a gold standard approach for epidemiological research. One of the earliest clinical trial studies was performed by James Lind et al in 1747 on sailors with scurvy. 12 Lind divided twelve scorbutic sailors into six groups of two. Each group received the same diet, in addition to a quart of cider (group 1), twenty‐five drops of elixir of vitriol which is sulfuric acid (group 2), two spoonfuls of vinegar (group 3), half a pint of seawater (group 4), two oranges and one lemon (group 5), and a spicy paste plus a drink of barley water (group 6). The group who ate two oranges and one lemon had shown the most sudden and visible clinical effects and were taken back at the end of 6 days as being fit for duty. During Lind's time, this was not accepted but was shown to have similar results when repeated 47 years later in an entire fleet of ships. Based on the above results, in 1795 lemon juice was made a required part of the diet of sailors. Thus, clinical trials can be used to evaluate new therapies, such as new drug or new indication, new drug combination, new surgical procedure or device, new dosing schedule or mode of administration, or a new prevention therapy.
While designing a clinical trial, it is important to select the population that is best representative of the general population. Therefore, the results obtained from the study can be generalized to the population from which the sample population was selected. It is also as important to select appropriate endpoints while designing a trial. Endpoints need to be well‐defined, reproducible, clinically relevant and achievable. The types of endpoints include continuous, ordinal, rates and time‐to‐event, and it is typically classified as primary, secondary or tertiary. 2 An ideal endpoint is a purely clinical outcome, for example, cure/survival, and thus, the clinical trials will become very long and expensive trials. Therefore, surrogate endpoints are used that are biologically related to the ideal endpoint. Surrogate endpoints need to be reproducible, easily measured, related to the clinical outcome, affected by treatment and occurring earlier than clinical outcome. 2
Clinical trials are further divided into randomized clinical trial, non‐randomized clinical trial, cross‐over clinical trial and factorial clinical trial.
Randomized clinical trial
A randomized clinical trial is also known as parallel group randomized trials or randomized controlled trials. Randomized clinical trials involve randomizing subjects with similar characteristics to two groups (or multiple groups): the group that receives the intervention/experimental therapy and the other group that received the placebo (or standard of care). 13 This is typically performed by using a computer software, manually or by other methods. Hence, we can measure the outcomes and efficacy of the intervention/experimental therapy being studied without bias as subjects have been randomized to their respective groups with similar baseline characteristics. This type of study design is considered gold standard for epidemiological research. However, this study design is generally not applicable to rare and serious disease process as it would unethical to treat that group with a placebo. Please see section “Randomization” for detailed explanation regarding randomization and placebo.
Non‐randomized clinical trial
A non‐randomized clinical trial involves an approach to selecting controls without randomization. With this type of study design a pattern is usually adopted, such as, selection of subjects and controls on certain days of the week. Depending on the approach adopted, the selection of subjects becomes predictable and therefore, there is bias with regards to selection of subjects and controls that would question the validity of the results obtained.
Historically controlled studies can be considered as a subtype of non‐randomized clinical trial. In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature. 1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Cross‐over clinical trial
In cross‐over clinical trial study design, there are two groups who undergoes the same intervention/experiment at different time periods of the study. That is, each group serves as a control while the other group is undergoing the intervention/experiment. 14 Depending on the intervention/experiment, a ‘washout’ period is recommended. This would help eliminate residuals effects of the intervention/experiment when the experiment group transitions to be the control group. Hence, the outcomes of the intervention/experiment will need to be reversible as this type of study design would not be possible if the subject is undergoing a surgical procedure.
Factorial trial
A factorial trial study design is adopted when the researcher wishes to test two different drugs with independent effects on the same population. Typically, the population is divided into 4 groups, the first with drug A, the second with drug B, the third with drug A and B, and the fourth with neither drug A nor drug B. The outcomes for drug A are compared to those on drug A, drug A and B and to those who were on drug B and neither drug A nor drug B. 15 The advantages of this study design that it saves time and helps to study two different drugs on the same study population at the same time. However, this study design would not be applicable if either of the drugs or interventions overlaps with each other on modes of action or effects, as the results obtained would not attribute to a particular drug or intervention.
Community trial
Community trials are also known as cluster‐randomized trials, involve groups of individuals with and without disease who are assigned to different intervention/experiment groups. Hence, groups of individuals from a certain area, such as a town or city, or a certain group such as school or college, will undergo the same intervention/experiment. 16 Hence, the results will be obtained at a larger scale; however, will not be able to account for inter‐individual and intra‐individual variability.
Field trial
Field trials are also known as preventive or prophylactic trials, and the subjects without the disease are placed in different preventive intervention groups. 16 One of the hypothetical examples for a field trial would be to randomly assign to groups of a healthy population and to provide an intervention to a group such as a vitamin and following through to measure certain outcomes. Hence, the subjects are monitored over a period of time for occurrence of a particular disease process.
Overview of methodologies used within a study design
Randomization.
Randomization is a well‐established methodology adopted in research to prevent bias due to subject selection, which may impact the result of the intervention/experiment being studied. It is one of the fundamental principles of an experimental study designs and ensures scientific validity. It provides a way to avoid predicting which subjects are assigned to a certain group and therefore, prevent bias on the final results due to subject selection. This also ensures comparability between groups as most baseline characteristics are similar prior to randomization and therefore helps to interpret the results regarding the intervention/experiment group without bias.
There are various ways to randomize and it can be as simple as a ‘flip of a coin’ to use computer software and statistical methods. To better describe randomization, there are three types of randomization: simple randomization, block randomization and stratified randomization.
Simple randomization
In simple randomization, the subjects are randomly allocated to experiment/intervention groups based on a constant probability. That is, if there are two groups A and B, the subject has a 0.5 probability of being allocated to either group. This can be performed in multiple ways, and one of which being as simple as a ‘flip of a coin’ to using random tables or numbers. 17 The advantage of using this methodology is that it eliminates selection bias. However, the disadvantage with this methodology is that an imbalance in the number allocated to each group as well as the prognostic factors between groups. Hence, it is more challenging in studies with a small sample size.
Block randomization
In block randomization, the subjects of similar characteristics are classified into blocks. The aim of block randomization is to balance the number of subjects allocated to each experiment/intervention group. For example, let's assume that there are four subjects in each block, and two of the four subjects in each block will be randomly allotted to each group. Therefore, there will be two subjects in one group and two subjects in the other group. 17 The disadvantage with this methodology is that there is still a component of predictability in the selection of subjects and the randomization of prognostic factors is not performed. However, it helps to control the balance between the experiment/intervention groups.
Stratified randomization
In stratified randomization, the subjects are defined based on certain strata, which are covariates. 18 For example, prognostic factors like age can be considered as a covariate, and then the specified population can be randomized within each age group related to an experiment/intervention group. The advantage with this methodology is that it enables comparability between experiment/intervention groups and thus makes result analysis more efficient. But, with this methodology the covariates will need to be measured and determined before the randomization process. The sample size will help determine the number of strata that would need to be chosen for a study.
Blinding is a methodology adopted in a study design to intentionally not provide information related to the allocation of the groups to the subject participants, investigators and/or data analysts. 19 The purpose of blinding is to decrease influence associated with the knowledge of being in a particular group on the study result. There are 3 forms of blinding: single‐blinded, double‐blinded and triple‐blinded. 1 In single‐blinded studies, otherwise called as open‐label studies, the subject participants are not revealed which group that they have been allocated to. However, the investigator and data analyst will be aware of the allocation of the groups. In double‐blinded studies, both the study participants and the investigator will be unaware of the group to which they were allocated to. Double‐blinded studies are typically used in clinical trials to test the safety and efficacy of the drugs. In triple‐blinded studies, the subject participants, investigators and data analysts will not be aware of the group allocation. Thus, triple‐blinded studies are more difficult and expensive to design but the results obtained will exclude confounding effects from knowledge of group allocation.
Blinding is especially important in studies where subjective response are considered as outcomes. This is because certain responses can be modified based on the knowledge of the experiment group that they are in. For example, a group allocated in the non‐intervention group may not feel better as they are not getting the treatment, or an investigator may pay more attention to the group receiving treatment, and thereby potentially affecting the final results. However, certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention such as quitting smoking.
Placebo is defined in the Merriam‐Webster dictionary as ‘an inert or innocuous substance used especially in controlled experiments testing the efficacy of another substance (such as drug)’. 20 A placebo is typically used in a clinical research study to evaluate the safety and efficacy of a drug/intervention. This is especially useful if the outcome measured is subjective. In clinical drug trials, a placebo is typically a drug that resembles the drug to be tested in certain characteristics such as color, size, shape and taste, but without the active substance. This helps to measure effects of just taking the drug, such as pain relief, compared to the drug with the active substance. If the effect is positive, for example, improvement in mood/pain, then it is called placebo effect. If the effect is negative, for example, worsening of mood/pain, then it is called nocebo effect. 21
The ethics of placebo‐controlled studies is complex and remains a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances:
Where no proven intervention exists, the use of placebo, or no intervention, is acceptable; or
Where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the patients who receive any intervention less effective than the best proven one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention.
Extreme care must be taken to avoid abuse of this option”. 22
Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Bias has been defined as “any systematic error in the design, conduct or analysis of a study that results in a mistaken estimate of an exposure's effect on the risk of disease”. 23 There are multiple types of biases and so, in this review we will focus on the following types: selection bias, information bias and observer bias. Selection bias is when a systematic error is committed while selecting subjects for the study. Selection bias will affect the external validity of the study if the study subjects are not representative of the population being studied and therefore, the results of the study will not be generalizable. Selection bias will affect the internal validity of the study if the selection of study subjects in each group is influenced by certain factors, such as, based on the treatment of the group assigned. One of the ways to decrease selection bias is to select the study population that would representative of the population being studied, or to randomize (discussed in section “Randomization”).
Information bias is when a systematic error is committed while obtaining data from the study subjects. This can be in the form of recall bias when subject is required to remember certain events from the past. Typically, subjects with the disease tend to remember certain events compared to subjects without the disease. Observer bias is a systematic error when the study investigator is influenced by the certain characteristics of the group, that is, an investigator may pay closer attention to the group receiving the treatment versus the group not receiving the treatment. This may influence the results of the study. One of the ways to decrease observer bias is to use blinding (discussed in section “Blinding”).
Thus, while designing a study it is important to take measure to limit bias as much as possible so that the scientific validity of the study results is preserved to its maximum.
Overview of drug development in the United States of America
Now that we have reviewed the various clinical designs, clinical trials form a major part in development of a drug. In the United States, the Food and Drug Administration (FDA) plays an important role in getting a drug approved for clinical use. It includes a robust process that involves four different phases before a drug can be made available to the public. Phase I is conducted to determine a safe dose. The study subjects consist of normal volunteers and/or subjects with disease of interest, and the sample size is typically small and not more than 30 subjects. The primary endpoint consists of toxicity and adverse events. Phase II is conducted to evaluate of safety of dose selected in Phase I, to collect preliminary information on efficacy and to determine factors to plan a randomized controlled trial. The study subjects consist of subjects with disease of interest and the sample size is also small but more that Phase I (40–100 subjects). The primary endpoint is the measure of response. Phase III is conducted as a definitive trial to prove efficacy and establish safety of a drug. Phase III studies are randomized controlled trials and depending on the drug being studied, it can be placebo‐controlled, equivalence, superiority or non‐inferiority trials. The study subjects consist of subjects with disease of interest, and the sample size is typically large but no larger than 300 to 3000. Phase IV is performed after a drug is approved by the FDA and it is also called the post‐marketing clinical trial. This phase is conducted to evaluate new indications, to determine safety and efficacy in long‐term follow‐up and new dosing regimens. This phase helps to detect rare adverse events that would not be picked up during phase III studies and decrease in the delay in the release of the drug in the market. Hence, this phase depends heavily on voluntary reporting of side effects and/or adverse events by physicians, non‐physicians or drug companies. 2
We have discussed various clinical research study designs in this comprehensive review. Though there are various designs available, one must consider various ethical aspects of the study. Hence, each study will require thorough review of the protocol by the institutional review board before approval and implementation.
CONFLICT OF INTEREST
Chidambaram AG, Josephson M. Clinical research study designs: The essentials . Pediatr Invest . 2019; 3 :245‐252. 10.1002/ped4.12166 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Here are six common types of research studies, along with examples that help explain the advantages and disadvantages of each: 1. Meta-analysis. A meta-analysis study helps researchers compile the quantitative data available from previous studies. It's an observational study in which the researchers don't manipulate variables.
Created: June 15, 2016; Last Update: September 8, 2016; Next update: 2020. There are various types of scientific studies such as experiments and comparative analyses, observational studies, surveys, or interviews. The choice of study type will mainly depend on the research question being asked. When making decisions, patients and doctors need ...
Types of study design. Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. ... In graphic form, data of different trials can be plotted with the point ...
The type of study design used to answer a particular research question is determined by the nature of question, the goal of research, and the availability of resources. Since the design of a study can affect the validity of its results, it is important to understand the different types of study designs and their strengths and limitations.
Learn the two types of study designs: descriptive and analytical, and their subtypes: cross-sectional, cohort, case-control and experimental. See examples of each design and how they are used to collect and analyze data in biomedical research.
Choosing between all these different research types is part of the process of creating your research design, which determines exactly how your research will be conducted. But the type of research is only the first step: next, you have to make more concrete decisions about your research methods and the details of the study. Read more about ...
About Research Methods. This guide provides an overview of research methods, how to choose and use them, and supports and resources at UC Berkeley. As Patten and Newhart note in the book Understanding Research Methods, "Research methods are the building blocks of the scientific enterprise. They are the "how" for building systematic knowledge.
Research Study Types There are many different types of research studies, and each has distinct strengths and weaknesses. In general, randomized trials and cohort studies provide the best information when looking at the link between a certain factor (like diet) and a health outcome (like heart disease).
Classification of Types of Research. There are various types of research that are classified according to their objective, depth of study, analysed data, time required to study the phenomenon and other factors. It's important to note that a research project will not be limited to one type of research, but will likely use several.
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about: Your overall research objectives and approach. Whether you'll rely on primary research or secondary research. Your sampling methods or criteria for selecting subjects. Your data collection methods.
These study designs all have similar components (as we'd expect from the PICO): A defined population (P) from which groups of subjects are studied. Outcomes (O) that are measured. And for experimental and analytic observational studies: Interventions (I) or exposures (E) that are applied to different groups of subjects.
A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies.4. However, even systematic reviews and meta-analyses aren't the final word on scientific questions.
Research methods are specific procedures for collecting and analyzing data. Developing your research methods is an integral part of your research design. When planning your methods, there are two key decisions you will make. First, decide how you will collect data. Your methods depend on what type of data you need to answer your research question:
Making sense of this new information requires us to understand the different kinds of research. There are three main kinds of health research studies. These are preclinical, experimental, and epidemiologic research. Epidemiologic and preclinical studies often come first, followed by clinical trials involving human participants.
There are many different types of research studies. Generally, there are two major types of studies available on Research for Me @UNC: research studies and clinical trials. When a research study is about disease or human health, it is called a clinical research study. When a research study involves drugs or other therapies that aim to slow or ...
Email. Clinical research is the study of health and illness in people. Scientists may have many reasons for doing a clinical study, such as: To explore the cause of a disease or a set of symptoms. To test if a treatment will help with a symptom or condition. To learn how a certain behavior affects people's health.
This article covers the classification of individual study types. The conception, implementation, advantages, disadvantages and possibilities of using the different study types are illustrated by examples. The article is based on a selective literature research on study types in medical research, as well as the authors' own experience.
A randomised controlled trial (RCT) is an important study design commonly used in medical research to determine the effectiveness of a treatment or intervention. It is considered the gold standard in research design because it allows researchers to draw cause-and-effect conclusions about the effects of an intervention.
Epidemiology is defined as the study of human populations. These studies often investigate the relationship between dietary consumption and disease development. There are three main types of epidemiological studies: cross-sectional, case-control, and prospective cohort studies. Figure 2.2: Types of epidemiology.
Figure 2.3. The hierarchy of evidence shows types of research studies relative to their strength of evidence and relevance to real-life nutrition decisions, with the strongest studies at the top and the weakest at the bottom. The pyramid also represents a few other general ideas.
Case studies: Case study research delves into detailed data to pinpoint the unique characteristics of a narrowly defined subject, providing in-depth insights. Descriptive research can be conducted in different ways: Cross-sectional: Observing a population at a single point in time. Longitudinal: Following a population over a period of time.
A research paper is simply a piece of writing that uses outside sources. There are different types of research papers with varying purposes and expectations for sourcing. While this guide explains those differences broadly, ask your professor about specific disciplinary conventions.
Research Objectives Examples in Different Fields. The application of research objectives spans various academic disciplines, each with its unique focus and methodologies. To illustrate how the objectives of the study guide a research paper across different fields, here are some research objective examples:
Researchers need to understand the features of different study designs, with their advantages and limitations so that the most appropriate design can be chosen for a particular research question. The Centre for Evidence Based Medicine offers an useful tool to determine the type of research design used in a particular study. 7
Do different types of physical activity. People with diabetes can be active, even if they take insulin or use technology such as insulin pumps. ... Clinical trials—and other types of clinical studies—are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care ...
Introduces science Honours students to the range of theoretical frameworks which may inform different types of scientific research and to the methods and methodologies which may be employed in the scientific research process. Encourages students to acquire the skills necessary to carry out, produce and report well designed and articulated research proposals and projects.
Estrogen receptors (ERs) are located in both healthy and neoplastic tissues. The type of estrogen receptor expressed varies depending on its location, tumor type, and species. Estrogen action is mediated by binding to ER and activating the transcriptional and signaling processes that result in the control of gene expression. There are two main types of estrogen receptors: ER alpha (ERα) and ...
Information bias occurs during the data collection step and is common in research studies that involve self-reporting and retrospective data collection. It can also result from poor interviewing techniques or differing levels of recall from participants. The main types of information bias are: Recall bias. Observer bias.
A study published in the British Journal of Cancer reveals three new subgroups of the most common type of neuroblastoma, each with different genetic traits, expected outcomes, and distinguishing ...
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...