An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications
Ashok kumar mandal, saurav katuwal, felix tettey, aakash gupta, salyan bhattarai, shankar jaisi, devi prasad bhandari, ajay kumar shah, narayan bhattarai, niranjan parajuli.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] (N.B.); [email protected] (N.P.)
Received 2022 Aug 13; Accepted 2022 Sep 2; Collection date 2022 Sep.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Zinc oxide nanoparticles (ZnO-NPs) have piqued the curiosity of researchers all over the world due to their extensive biological activity. They are less toxic and biodegradable with the capacity to greatly boost pharmacophore bioactivity. ZnO-NPs are the most extensively used metal oxide nanoparticles in electronic and optoelectronics because of their distinctive optical and chemical properties which can be readily modified by altering the morphology and the wide bandgap. The biosynthesis of nanoparticles using extracts of therapeutic plants, fungi, bacteria, algae, etc., improves their stability and biocompatibility in many biological settings, and its biofabrication alters its physiochemical behavior, contributing to biological potency. As such, ZnO-NPs can be used as an effective nanocarrier for conventional drugs due to their cost-effectiveness and benefits of being biodegradable and biocompatible. This article covers a comprehensive review of different synthesis approaches of ZnO-NPs including physical, chemical, biochemical, and green synthesis techniques, and also emphasizes their biopotency through antibacterial, antifungal, anticancer, anti-inflammatory, antidiabetic, antioxidant, antiviral, wound healing, and cardioprotective activity. Green synthesis from plants, bacteria, and fungus is given special attention, with a particular emphasis on extraction techniques, precursors used for the synthesis and reaction conditions, characterization techniques, and surface morphology of the particles.
Keywords: zinc oxide nanoparticles, green synthesis, biological activities
1. Introduction
A diverse application of nanomaterial-based technology has opened a new horizon in material science over the past decades because nanomaterials offer a high surface area and other very distinctive physical, chemical, and biological properties compared to their bulk counterparts [ 1 ]. Nanoparticle (NP) research has gained distinct interest due to the enhanced electrochemical reactivity, thermal conductivity, and nonlinear optical properties of nanoparticles which offer unique applications [ 2 ]. Zinc oxide nanoparticles (ZnO-NPs) are the most commonly used metal oxide nanoparticles because their distinctive optical and chemical properties can be easily modified by altering the morphology and the wide bandgap (3.37 eV) and high excitation binding energy (60 meV) to simulate the ZnO-NPs to be a potent photocatalytic and photo-oxidizing moiety against chemical and biological species [ 3 , 4 ]. They are less toxic to the human body and offer biocompatibility as the Zn ion (Zn 2+ ), a soluble form of ZnO, is a trace element found in the human physiological system. ZnO-based structures have been proven to exhibit biodegradability both in the bulk phase and in the form of nanoparticles [ 5 ]. Zn ions also act as the principal mediators of intracellular bacterial toxicity, disrupting their cell membranes [ 6 ].
Some potential applications where ZnO-NPs have been researched are: therapeutic carriers, biological sensing, gene transfer, nanomedicine discovery, biological labeling, medical implant coatings, electronic sensors, wastewater treatment, and communication [ 4 , 7 , 8 ]. The medical implant coating with zinc oxide and hydroxyapatite exhibited antibacterial and osteoconductive properties, emphasizing the potential of ZnO-NPs in therapeutic diagnostics. ZnO-NPs exhibited cytotoxicity in human cancer cells, resulting in cell death via the apoptotic pathway [ 9 ]. They also promoted antiproliferative activity in triple-negative breast cancer cells [ 10 ], nonautophagic cell death in human lung adenocarcinoma cells with an epidermal growth factor receptor (EGFR) mutation [ 11 ], and anticancer activity via apoptosis in chronic myeloid leukemia cells using a transcriptomic approach [ 12 ]. It has also been shown to induce cytotoxicity in the A549 epithelium and cancer cells [ 13 ]. Recent investigations on the ZnO-Au nanocomposite have developed an electrochemical DNA biosensor [ 14 ], ZnO-NPs for tracing studies in plants [ 15 ], and material in the development of electrochemical sensors in the detection of food additive aspartame [ 16 ]. ZnO-NPs have been shown to influence horizontal gene transfer where it impacts the transformation efficiency of Bacillus subtilis [ 17 ], and the ZnO-Ag NPs have decreased the rate of biofilm formation and gene expression in Staphylococcus aureus at a subminimum inhibitory concentration [ 18 ]. ZnO-NPs have been shown to reduce the parameters responsible for hepatic fibrosis (hydroxyproline) and nephrotoxicity (creatinine, urea, and uric acid) [ 19 ], also attenuating the gonadal toxicity which is induced by cyclophosphamide (an anticancer and immunosuppressant drug) through their antioxidant and antiapoptotic function [ 20 ], and cancer cell death through autophagy induction which supports the release of zinc ions and the generation of reactive oxygen species (ROS) [ 21 ].
In a critical study, zinc ions and ZnO-NPs both showed cytotoxic effects in the earthworm GI tract where it affected the gut epithelium and chlorogenic tissues [ 22 ]. However, ZnO-NPs dissolve slowly in human physiological conditions (pH 6–8), and the United States Food and Drug Administration (USFDA) safety datasheet indicates ZnO as a “Generally Recognized as Safe” (GRAS) substance and nonhemolytic against human red blood cells [ 23 ]. ZnO could be discovered to be a useful nanocarrier to facilitate the drug-delivering and release processes [ 24 , 25 ]. Much research endorses ZnO-NPs as the most beneficial metal nanoparticles, with minimal toxicity and excellent biocompatibility. The structural atom allocation mimics the most bioactive agent, emphasizing its pharmacological effectiveness against various ailments. With all this potential, the objective of this review article is to explore the various synthesis approaches and characterization techniques of ZnO-NPs with a comprehensive mechanistic approach to its biological activity. Although there is an increased number of studies revealing the mutually exclusive and exhaustive area of ZnO-NPs, this review is a comprehensive compilation of recent advances with clear illustrations for a better understanding of the importance of ZnO-NPs in biomedical research.
2. Biological Activities of ZnO-NPs
2.1. antibacterial action of zno-nps.
Bacteria portray a severe threat to human life as the world grapples with escalating antibiotic resistance and bacterial infection. ZnO-NPs have remarkable photo-oxidation and photocatalytic characteristics, and their exceptional antimicrobial properties have led to their recognition as potent agents against MDR [ 26 ]. Although the mechanism of antimicrobial action of ZnO-NPs is not well established, its properties, such as zinc ions and ROS generation, are widely assumed to result in oxidative stress and DNA damage, as well as photocatalytic activity, contributing to antibacterial efficacy ( Figure 1 ). According to Sirelkhatim et al., the oxygen annealing of ZnO increases the number of oxygen atoms on the surface, resulting in increased oxygen atom adsorption and the generation of more ROS, resulting in enhanced oxidation, and hence, a facilitated antimicrobial property [ 27 ]. Moreover, ZnO-NPs cause cytoplasmic shrinkage and the disruption of cell walls leading to cytoplasmic spillage ( Figure 2 ). ZnO-NPs act as an effective bactericidal agent against both Gram-positive as well as Gram-negative bacteria and are found to have direct interaction with the cell wall of bacteria leading to the disruption of its integrity [ 28 ].
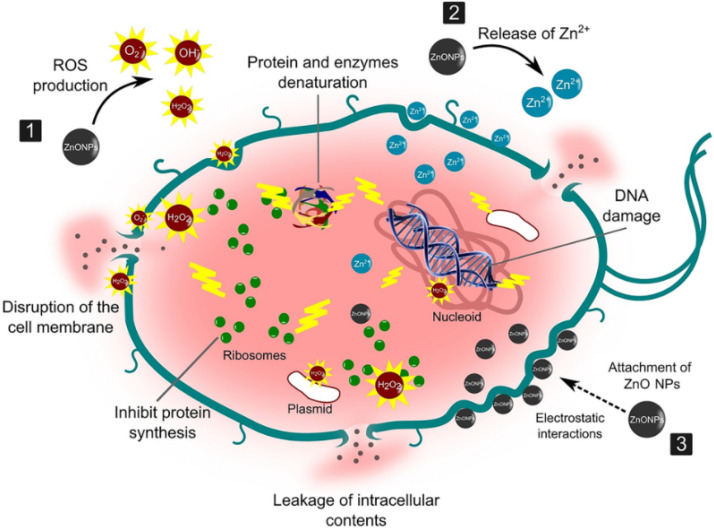
Illustration of the antimicrobial property of ZnO-NPs against the bacterial cell wall. They act as potent antibacterial agents through these possible steps: (1) production of reactive oxygen species (ROS) causing oxidative stress, and membrane and DNA damage leading to bacterial death; (2) dissolution of ZnO-NPs into Zn 2+ and interference with bacterial enzymes, proteins, and amino acids; and (3) electrostatic interaction between ZnO-NPs and cell membrane, resulting in membrane plasma damage and intracellular content leakage. (Reprinted from [ 29 ]; open access under CC BY).
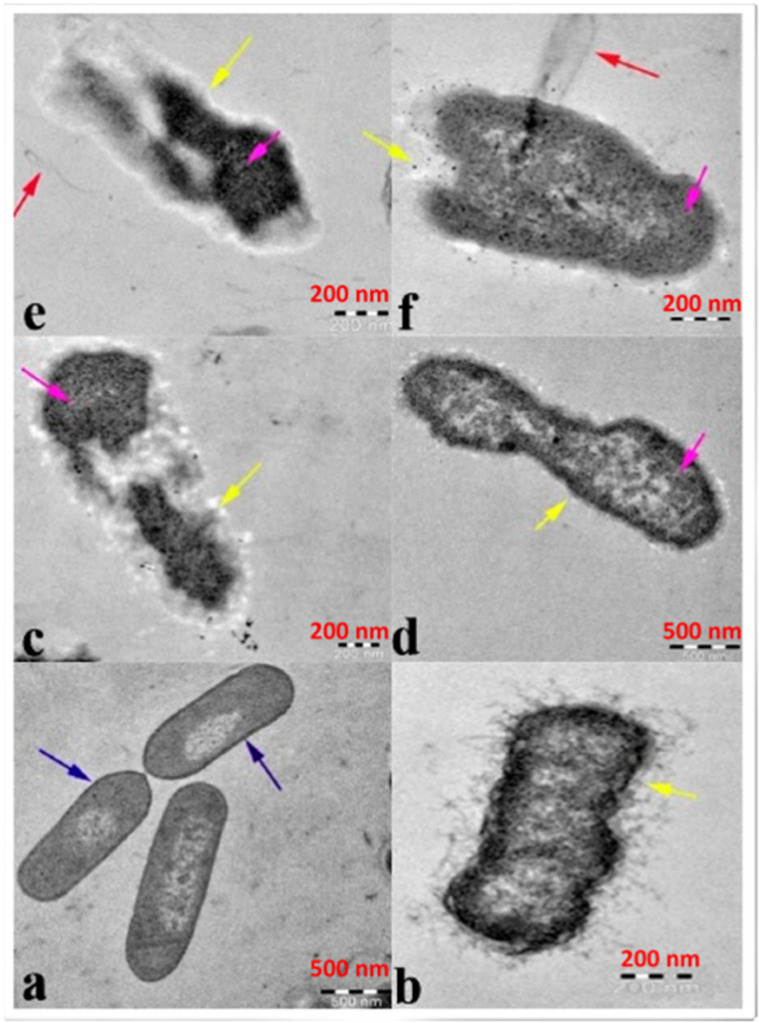
Image illustrating antibacterial efficacy against β-lactam-resistant K. pneumoniae obtained using transmission electron microscopy: ( a ) ZnO-NPs in the untreated state and ZnO-NPs in the treated state ( b – e ). Cytoplasmic shrinkage ( b ) disrupted cell wall and membrane ( c ), denatured protein shows as a dark electron-dense patch ( d ), and cytoplasmic spillage ( e , f ). The blue arrow represents an intact cell wall, the yellow arrow represents a disintegrating cell wall and cell membrane, and the violet arrow represents a denatured protein. (Reprinted from [ 30 ]; open access under CC BY).
2.2. Antifungal Action of ZnO-NPs
The antifungal properties of ZnO-NPs have been discovered in various studies in the literature. Their fungicidal activity varies depending on their structure, size, and concentration. The antifungal potency of biofabricated ZnO-NPs against Candida albicans isolates was investigated, and it was revealed that they were more effective against drug-resistant C. albicans isolates, demonstrating ZnO-NPs’ antifungal potency. Furthermore, it was shown that prophylactic treatment with lower concentrations of ZnO-NPs protects G. mellonella from the infection of C. albicans [ 31 , 32 ]. Similarly, the antifungal resistance of a 2% ZnO-NP-based cold cream exceeded the activity compared to a commercial antifungal cream at 2% on clinical isolates of Candida sp. [ 33 ]. ZnO-NPs have antifungal activity against both Aspergillus and Penicillium and have been investigated for their antidermatophytic activity on Trichophyton mentagrophytes and Trichophyton verrucosum [ 34 , 35 ]. Likewise, the bionanocomposite film of the soy protein isolate (SPI), cinnamaldehyde (CIN), and ZnO-NPs exhibited the highest antifungal activity among SPI, SPI-CIN, and SPI-ZnO-NPs films, where it was 1.56-fold stronger compared to the SPI-ZnO film and 1.24-fold stronger compared to the SPI-CIN film [ 36 ]. The antifungal activity studied against two pathogenic fungi— Botrytis cinerea and Penicillium expansum —revealed that activity is also dependent on nanoparticle concentrations, with the efficacy of the ZnO-NP treatment increasing as the concentration of ZnO-NPs rose from 3 to 12 mM. By affecting cellular functions, ZnO-NPs cause deformation in fungal hyphae, inhibiting the growth of B. cinerea . Similarly, P. expansum prevents the formation of conidiophores and conidia, resulting in the death of fungal hyphae, explaining the fact that P. expansum is found to be more sensitive than B. cinerea , i.e., microbe dependent. The activity detected in B. cinerea revealed the stronger the photo-activation, the greater the activity [ 37 , 38 , 39 ].
2.3. Cytotoxic Effect of ZnO-NPs
ZnO-NPs, compared to other metal oxide NPs, have a significant effect on cancer cells. The anticancer potential of ZnO-NPs is strongly influenced by their shape, size, and concentration. It has been discovered that the smaller the size and higher the concentration of NPs, the greater the anticancer activity [ 40 , 41 ]. They showed concentration-dependent anticancer activity against MCF7 human breast cancer cells, where 93% inhibition of proliferation of cells was noted at 100 µg/mL [ 40 ]. Similarly, fabricated ZnO-NPs exhibited concentration-dependent growth inhibition in human pancreatic cancer cell lines, PNAC-1, and AsPC-1, although they were shown to have a relatively smaller effect on the human normal fibroblast cell line (Hu02), which was found by an MTT assay [ 42 ]. The mechanistic approach ( Figure 3 ) underlying its anticancerous activity includes the production of sufficient ROS to cause substantial oxidative stress and DNA damage, disturbances on lipids and proteins in cells, and other cellular components due to their large semiconductor band gap [ 43 ]. Moreover, the establishment of a redox reaction system and the pro-inflammatory response of cells against ZnO-NPs induce cellular apoptosis. Discrimination between cancerous and normal cells has been a major challenge for a drug to be categorized as anticancerous. Failure to achieve selectivity results in systemic toxic effects. Several studies have revealed the selectivity of ZnO-NPs toward cancerous cells. ZnO-NPs have been demonstrated to be selective to Jurkat cancer cells with minimal toxicity toward normal CD4 + T cells [ 44 ]. Similarly, Hanley and the group proposed that ZnO-NPs had 28–35 times the specific cytotoxicity against cancer carcinoma cells compared to normal cells [ 45 ]. Selective localization by enhanced permeability and retention (EPR) time via extravasation toward tumor cells assists in selective activities affecting tumor cells rather than the normal cells. The electrostatic property of ZnO-NPs facilitates the targeting of tumor sites [ 46 ]. Thus, there is ample evidence that ZnO-NPs can exhibit anticancer effects in specific types of tumor cells in the body, which is depicted in Figure 3 .
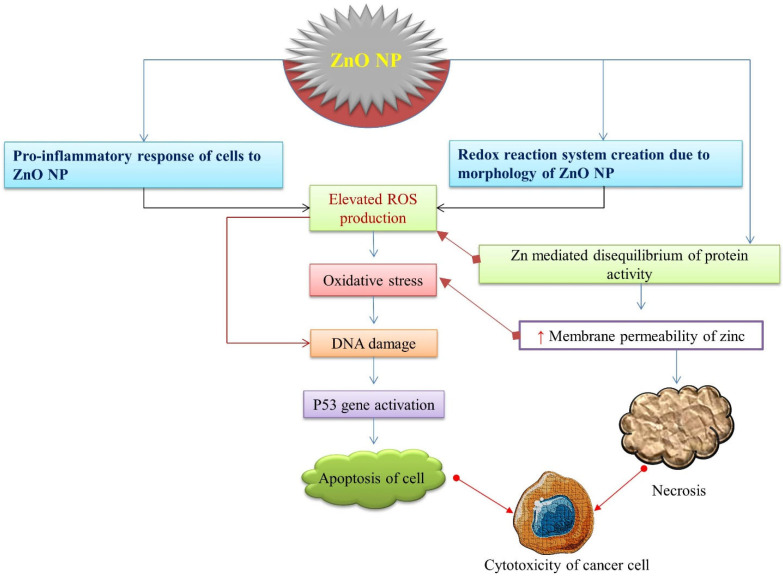
A schematic representation of cytotoxicity potency of ZnO-NPs leading to the death of cancer cells. ZnO-NPs induce ROS production sequentially, leading to oxidative stress, DNA damage, p53 activation, and apoptosis of cancerous cells.
Despite various biomedical applications such as anticancer therapy, drug delivery, gene therapy, and tumor imaging, ZnO-NPs might have deleterious effects on several key organs including the lungs, kidneys, liver, CNS, reproductive system, and fetal development in animal models. However, the ZnO-NP-induced toxicity is multifactorial, and it is yet unknown just how toxic ZnO-NPs are for these organs [ 47 ].
2.4. Wound Healing Activity of ZnO-NPs
Wound healing is the phenomenon of cell injury responses, involving the activation of fibroblasts, endothelial cells, and macrophages where fibroblasts proliferate; an important step in wound healing for tissue regeneration [ 48 ]. It has been predicted that the delivery of ZnO via poly (lactide-co-glycolic acid) (PLGA)/silk fibroin (SF) nanofibers retains the bioavailability of NPs on the wound area and integrates with the unique structural features of electrospun nanofibers, which stimulate wound closure, re-epithelialization, collagen deposition, cellular migration, and angiogenesis [ 49 ]. Besides this, the ZnO-NPs loaded on bromelain-immobilized silk fibroin (SF-Br) reduced inflammation and promoted wound healing on a second-degree burn dressing [ 50 ]. During the healing process, the low doses of ZnO-NPs favored attachment and proliferation of fibroblasts, but the trend reversed at high doses. Metallic particles in nanocrystalline forms reduce wound infection along with promoting wound healing, as observed in adult male albino Wistar rats [ 51 ] and albino rats [ 52 ]. It was found that the functionalization of ZnO-NPs into triethoxysilane poly(amidoamine) dendrimer to generate a cross-linked collagen scaffold enhances re-epithelization and speedier collagen deposition than other scaffolds, which resulted in instantaneous wound healing [ 53 ]. In addition, the biodegradable thiolated bandage with implanted ZnO-NPs demonstrated an enhanced therapeutic agent for treating surgical site infections, satisfying the criteria for the optimal surgical dressing [ 54 ].
Similarly, the functionalization of bacterial nanocellulose (BNC) grafted with aminoalkyl silane and doped with Pullan-ZnO-NPs electrospun nanofibers (A-g-BNC/Pul-ZnO) exhibited superior performance in blood clotting and antibacterial activity that had a 5 log value higher than BNC, and was found to be safe in terms of cytotoxicity as tested in L929 fibroblast cells. It offers growth and proliferation, which was corroborated by the rat model where the scaffolds revealed rapid wound healing due to re-epithelization, and blood vessel and collagen formation [ 55 ]. An in vitro study reported that the bionanocomposite-based 3D chitosan/pectin/ZnO-NP porous films demonstrated no cytotoxicity (biocompatibility) and cell growth and migration (proliferation) for primary human dermal fibroblast cells (HFCs), suggesting a benign biomaterial for promoting wound healing [ 56 ].
Moreover, 3D-printed alginate-ZnO-NP hydrogels exhibited enhanced pore sizes, stiffness, and no detrimental effect on STO-fibroblasts or cell viability, making them a suitable scaffold for wound healing [ 57 ]. Generally, hydrogels are preferred with ZnO-NPs because they have a slow release of nanoparticles from the preparation, which reduces the cytotoxicity from ROS formation and improves wound healing. The above analyses support the findings of Saddik et al., where it was demonstrated that azithromycin-ZnO-NPs impregnated into an HPMC gel enhanced bacterial clearance and epidermal regeneration, which eventually stimulated tissue formation, leading to the rapid healing of the infected wound [ 58 , 59 ]. Another bioscaffold made from sodium alginate gum acacia ZnO-NP hydrogels showed a similar potential in expediting healing in terms of reducing inflammation and produced no scar at the excision wound on rabbit skin [ 60 ]. Thus, topical zinc application has been shown to improve the process of re-epithelialization, reduce inflammation, and inhibit the growth of bacteria in the case of foot ulcers and other topical wounds [ 61 ].
2.5. Anti-Inflammatory Activity of ZnO-NPs
The inflammatory response in the human body is a complicated process that involves immune system activation and the release of pro-inflammatory cytokines such as interleukin (IL)-1, -6, -12, -18, TNF-α, INFγ, and granulocyte-macrophage colony-stimulating factor (GMS-CF) [ 62 ] ( Figure 4 ). Nuclear factor-kappa b (NF-κβ) is a key transcription factor that regulates the expression of many genes that encode pro-inflammatory mediators, such as COX-2 and iNOS, which increase the synthesis of pro-inflammatory mediators such as PGE2 and nitric oxide [ 63 ]. The ZnO-NPs act as anti-inflammatory agents as they have been shown to inhibit the release of pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS) expression, myeloperoxidase, the NF-κβ pathway, and mast cell degranulation [ 64 ]. The mRNA expression of pro-inflammatory cytokines was suppressed by the ZnO-NPs synthesized using Polygala tenuifolia in a dose-dependent manner [ 65 ]. In addition, ZnO-NPs, when doped with aluminum, have been shown to reduce the production of thymic stromal lymphopoietin (TSLP) and caspase-1 activation in mast cells, leading to lowering the expression of pro-inflammatory cytokines, IL-1, IL-6, and TNF-α [ 66 ]. In a comparative study of ZnO-NPs and the ZnO standard form, it was revealed that ZnO-NPs relatively lowered the carrageenan-induced paw edema and amplified the anti-inflammatory activity of the nonsteroidal anti-inflammatory drug, ketoprofen, when administered intraperitoneally [ 67 ]. However, both forms were ineffective when administered per os (po) and guarded the gastric mucosa against the gastric ulcer induced by the administration of ketoprofen. ZnO-NPs have been discovered to have an excellent capping of flavones such as isoorientin, orientin, isovitexin, and vitexin, which have a potent anti-inflammatory response in a variety of ways, including the inhibition of cyclooxygenase, phospholipase A2, and lipoxygenases (enzymes that produce eicosanoids), resulting in a decline in leukotrienes and prostanoids [ 68 ].
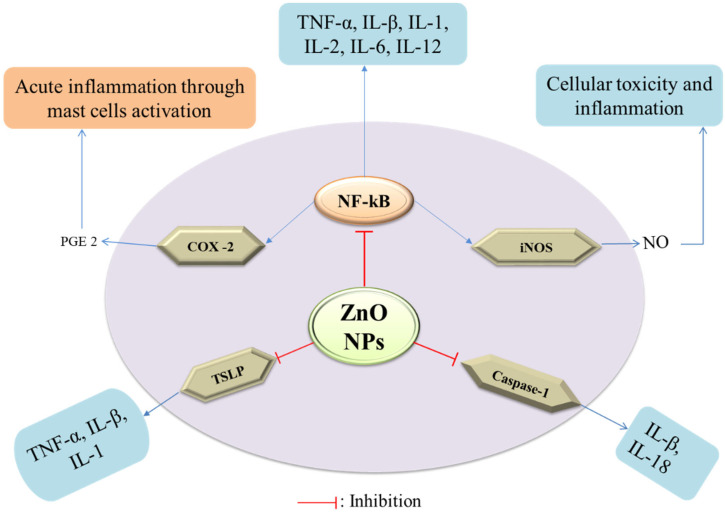
Mechanism of anti-inflammatory potency of ZnO-NPs.
2.6. Orthopedic Implants and Bone Healing Activity of ZnO-NPs
Diseases such as osteoporosis, arthritis, and fibrous dysplasia can cause bone abnormalities and lasting disability. The implantation of orthopedic implants and scaffolds has significantly aided in the treatment of these bone diseases and abnormalities since they consist of materials with positive effects on the bone regeneration process [ 69 ]. Orthopedic implants are usually made of metals and alloys such as titanium, nitinol, stainless steel, and Co-Cr alloys [ 70 ]. Over the last several decades, these metals have been excessively utilized for deformity correction, joint replacements, fracture fixation, soft tissue anchorage, and most importantly, for accelerating bone growth [ 71 ]. Unfortunately, orthopedic implants are not free from side effects once placed in the body, leading to infections, limited corrosion resistance, low cell proliferation, excessive inflammation, and poor osseointegration [ 72 , 73 ]. If infection occurs, the implant loosens, bones take longer to heal, and sometimes prolonged suffering leads to death [ 74 ]. If corrosion occurs, toxicity incites, weakening the implant [ 70 ]. Metal oxide nanoparticles such as ZnO, magnesium oxide (MgO), iron oxide, zirconium oxide, titanium oxide, and silver oxide, when used with orthopedic implants, provide a wide range of solutions for the issues mentioned earlier. Figure 5 highlights how the ZnO coating on the implant helps in osteointegration, the prevention of biofilm formation, and the prevention of premature corrosion of the implant.
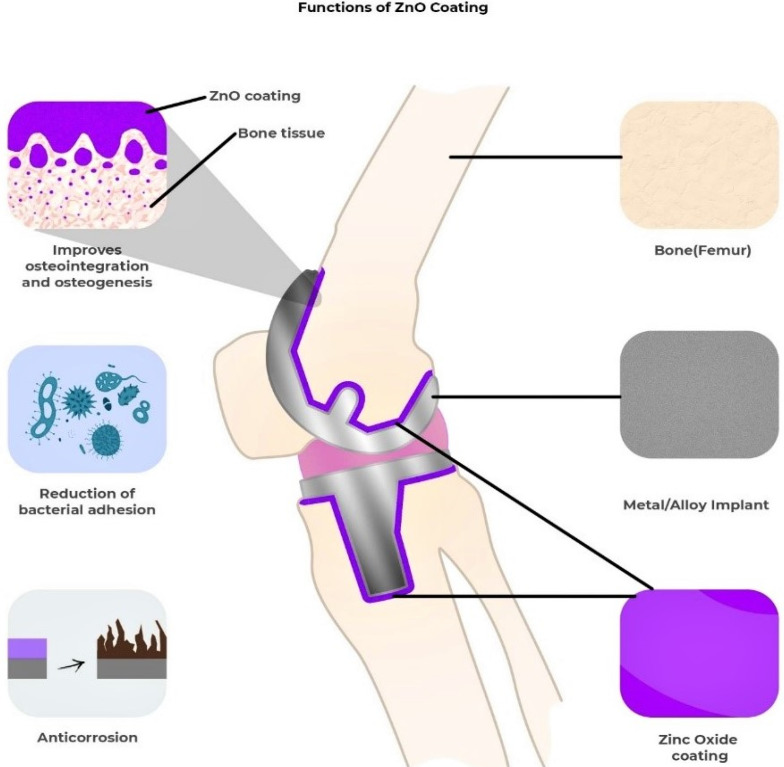
A diagram showing the effects of metal oxide (e.g., ZnO) coating on the orthopedic implant and bone.
Biodegradable metals (BMs) such as Zn, Mg, Ca, and Fe have additional desirable properties for their applications in orthopedics [ 75 , 76 ]. During biodegradation, these metals release metal ions, metal oxides, and hydroxides. The close interaction between the degraded by-product and the stem-progenitor cells at the interface is what gives bone tissue implants their bioactivity [ 77 ]. Therefore, altering the implant’s chemical composition can have a significant impact on the treatment’s effectiveness [ 77 ]. The integration of growth factors into bone tissue scaffolds and implants is a prominent area of interest in the research. Protein growth factors such as insulin-like growth factors and bone morphogenetic proteins can activate cellular signaling cascades to stimulate active healing [ 78 ], including angiogenesis, a crucial step in bone tissue regeneration [ 79 ].
Zn and ZnO have emerged as a recent alternative among these BMs and are commonly employed in combination with other biomaterials to gain diverse qualities in antibacterial ability, cytocompatibility, and corrosion resistance [ 80 , 81 ] due to their customizable size manipulation from micro to nano [ 82 ]. Bone is the principal repository for Zn since it stores about 30% [ 83 ], and Zn helps in the maintenance of bone mass [ 84 ]. It maintains the shape of cell membranes [ 83 ] and is crucial for bone quality. In osteoblastic cells, Zn can directly activate aminoacyl-tRNA synthetase, a rate-limiting enzyme during protein translation [ 85 ], accelerate cellular protein synthesis [ 86 ] and increase the gene expression of the transcription factor Runx2, which is connected to osteoblast differentiation. Zn also prevents the production of osteoclast-like cells from marrow cells, which minimizes osteoclastic bone resorption [ 87 ]. Bone mineralization is aided by the enzyme alkaline phosphatase, which employs zinc as a co-factor [ 88 , 89 , 90 ]. In an in vitro experiment, Zn doses between 7 and 20 nM enhanced alkaline phosphatase activity, but Zn concentrations over 5 µM decreased alkaline phosphatase activity [ 88 , 91 , 92 ]. These findings imply that a Zn shortage may affect bone growth by impairing osteoid mineralization or calcified cartilage production linked to endochondral ossification. Many distinct types of skeletal defects in prenatal and postnatal development are linked to Zn deficiency, and a study demonstrated that osteoporotic patients had lower skeletal Zn levels than the control [ 93 ]. By promoting collagen production, alkaline phosphatase (ALP) activity, and mineralization of bone nodules, Zn can improve osteogenesis ( Figure 6 ).
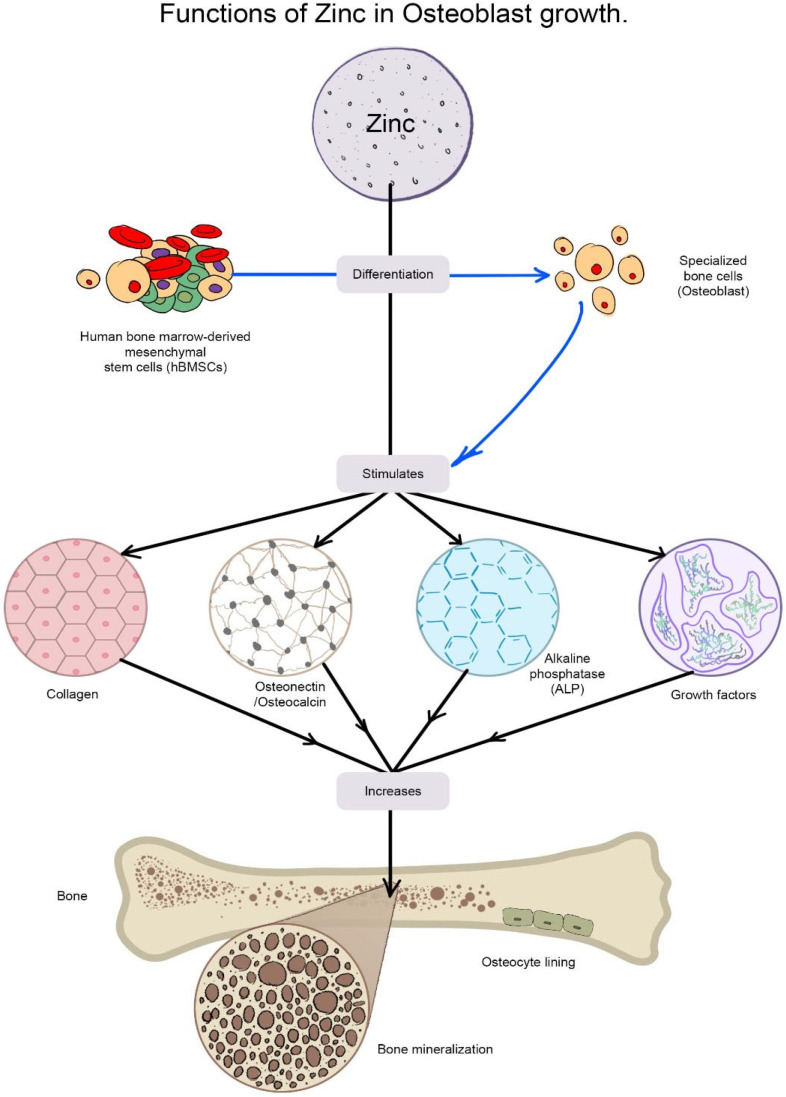
The diagram shows the functions of Zn in stimulating osteoblastic bone formation and mineralization. Zinc stimulates gene expression of various proteins including type I collagen, alkaline phosphatase, and osteocalcin in the cells. Zn is also known to increase the production of growth factors such as IGF-I and TGF-β1 in osteoblastic cells.
Yusa et al. showed that eluted Zn ions from Ti surfaces promoted osteoblast activities in human bone marrow-derived mesenchymal stem cells (hBMSCs) and dental pulp stem cells (hDPSCs) [ 94 ]. In both cell types, the eluted Zn ions stimulated the expression of osteoblast marker genes (collagen type I, ALP, and osteocalcin) and calcium deposition. In hDPSCs, Zn ions further stimulated the expression of Runx2, vascular endothelial growth factor A, and transforming growth factor-beta. Additionally, apoptosis rates in MC3T3-E1 cells increased from 7% in normal media to 75% and 90% when the cells were grown in Zn-deficient or Zn-free media, respectively [ 95 ]. Numerous studies have shown that increasing ZnO content improved antibacterial capacity [ 96 , 97 , 98 ], and nanocoating with ZnO may minimize S. epidermidis adherence, thus enhancing the efficacy of orthopedic implants [ 99 ]. Lin, M.-H. et al. detected that the chitosan/ZnO-NP coating showed 1.2-fold stronger antibacterial activity against E. coli than the chitosan coating alone and actively prevented the formation of biofilm [ 100 ].
Similar to Zn and ZnO, another degradable metal such as Mg provides similar benefits for tissue healing [ 101 ]. Adhikari, U. et al. mimicked the nanostructured architecture and chemical makeup of natural bone tissue matrices with a 3D scaffold made from chitosan, carboxymethyl chitosan, calcium phosphate monobasic, and magnesium oxide. This scaffold also served as a source for soluble metal ions that are beneficial to osteoblast cells and offers a favorable background to promote biomineralization [ 102 ]. Pure Mg corrodes too quickly in physiological pH and produces excessive hydrogen gas, which is its biggest drawback; thus, efforts to use the metal oxide coating in orthopedic applications have been limited [ 101 ]. In addition, the inclusion of biodegradable ZnO-NPs in polycaprolactone enables the gradual release of zinc, which has the potential to improve mesenchymal stem cell (MSC) differentiation as an added advantage. Although osteogenic differentiation was improved on scaffolds with an increased concentration of ZnO, MSC chondrogenic differentiation was boosted on scaffolds with a reduced proportion of ZnO [ 103 ].
2.7. Antidiabetic Action of ZnO-NPs
Diabetes is a metabolic disorder characterized by persistent hyperglycemia. Zinc has been discovered to have an important role in the production, storage, and secretion of insulin [ 104 ]. Furthermore, it improves insulin signaling through pathways, such as elevated PI3K activity, insulin receptor tyrosine phosphorylation, and the inhibition of glycogen synthase kinase [ 105 ]. It has been reported that zinc’s insulin-mimicking activity leads to enhanced lipogenesis and decreased nonesterified fatty acid release from adipocytes [ 106 ]. ZnO-NPs are more frequently chosen for antidiabetic effects over other metal nanoparticles because they increase the expression of GLUT-4 and INS genes due to the confluence of factors such as the enhanced cellular permeation of biosynthesized ZnO-NPs, the promotion of glycolysis via hepatic glycogenesis, and the elevation of insulin levels. Moreover, it imposes synergistic effects on the expression and activity of increased glucokinase and the expression levels of IRA and GLUT-2 [ 107 ].
A study revealed that zinc combined with insulin acts as an autocrine molecule, increasing GSIS from rat-isolated pancreatic islets [ 108 ], and interacts with several components of the insulin transduction system, facilitating glucose metabolism and insulin mRNA expression in hepatic tissue of diabetic rats [ 109 ]. In an alloxan-induced diabetic model, rats administered with 96 mg/dL of ZnO-NPs synthesized from the seed extract of Silybum marianum L. had considerably lower fasting blood sugar (FBS) levels than rats fed with 117 mg/dL of insulin, 110 mg/dL of zinc oxide, and 120 mg/dL of crude extract, implying the potent antidiabetic activity of ZnO-NPs. Antidiabetic medicinal plants have also been used to synthesize ZnO-NPs and studied for antidiabetic effects, such as Rheum ribes [ 110 ] and Cosus igneus [ 111 ]. Similarly, the antidiabetic effect of ZnO-NPs synthesized from the flower extract of Senna auriculata [ 112 ] and leaf extract of Andrographis paniculata was studied in terms of α-amylase inhibitory activity, where it showed a lower IC 50 value (121.42 µg/mL) than the leaf extract of A. paniculata (149.65 µg/mL) and ZnNO 3 (178.84 µg/mL) [ 113 ]. Moreover, the antidiabetic activity of ZnO-NPs synthesized from Withania somnifera was monitored in terms of inhibition of α-amylase and α-glucosidase, showing 90% and 95% inhibition, respectively, at 100 µg/mL [ 114 ]. According to the findings of these studies, ZnO-NPs have a substantial antidiabetic effect in terms of glucose and insulin levels, glucose tolerance, and diabetic dyslipidemia.
2.8. Antioxidant Activity of ZnO-NPs
In the modern world, the ingestion of some oxidized meals is associated with numerous serious ailments, such as hepatomegaly or necrosis of epithelial tissues, because they are capable of producing lipid peroxides and other toxic-free radicals [ 115 , 116 , 117 ]. Various natural and synthetic antioxidants are utilized to neutralize these damaging free radicals, but they have drawbacks such as high reactivity and toxicity when compared to the nanoparticles synthesized these days [ 118 , 119 ]. Das et al. investigated the antioxidant potential of ZnO-NPs and revealed that the antioxidant activity of ZnO-NPs is due to the transfer of electron density from oxygen to the odd electron located at the nitrogen atom in DPPH (2,2-diphenyl-1-picrylhydrazyl), resulting in a reduction in the intensity of the n→π* transition at the 517 nm wavelength [ 120 ].
The previous finding showed that the percentage of inhibition of free radicals by ZnO-NPs on DPPH increases along with that of the concentration, explaining the ZnO-NPs’ promising antioxidant potential [ 121 ]. Similarly, the antioxidant activity of ZnO-NPs synthesized using the Aquilegia pubiflora leaf extract was monitored through four different assays (total antioxidant capacity—TAC, total reducing power—TRP, free radical scavenging assay—FRSA (DPPH), and Trolox antioxidant assay—ABTS) for a better evaluation, and the obtained results in terms of ascorbic acid equivalent per milligram (µg AAE/mg) were directly proportional to the concentration of ZnO-NPs in each assay [ 68 ]. In addition to that, similar studies were carried out using ABTS, DPPH, hydrogen peroxide, and super peroxide scavenging assays, where the DPPH assay exhibited direct dose-dependent behavior and the order of antioxidant activity was as follows: ABTS > DPPH > SOR > H 2 O 2 [ 122 ]. Furthermore, several plant sources such as Salvia hispanica [ 123 ], Borassus flabellifer [ 124 ], and Punica granatum [ 125 ] have been utilized for evaluation of the antioxidant activity of ZnO-NPs. Generally, the antioxidant behavior of ZnO-NPs is due to the reducing ability of NPs and the phytochemicals adsorbed/capped on the surface of ZnO-NPs [ 126 ]. This reveals the unparalleled antioxidant capacity of ZnO-NPs.
2.9. Antiviral Action of ZnO-NPs
ZnO-NPs have been reported to exhibit significant antiviral activities against a plethora of viruses, such as herpes simplex virus (HSV), human papillomavirus (HPV), human immunodeficiency virus (HIV), hepatitis C and E virus (HCV, HEV), and severe acute respiratory syndrome coronavirus (SARS-CoV) [ 127 ]. The mechanism of action underlying the antiviral potency of ZnO-NPs is the stimulation of the innate and adaptive immune response via toll-like receptor signaling pathways and proteins down streaming, which results in the production of pro-inflammatory cytokines that inhibit the virus. Zn 2+ ions exhibit antiviral properties by preventing infection, inactivating virus adsorption/entry, blocking coating, impeding replication, assembly, and release during the virus’s life cycle, and producing reactive oxygen species [ 128 , 129 , 130 , 131 , 132 ]. Zinc inhibits the entry of viruses and viral polyprotein translation, as well as inhibiting viral RNA-dependent RNA polymerase activity, and has been shown to modulate the host immune response to limit viral replication. It is a mediator in the LPS (bacterial lipopolysaccharide)-induced TLR4 (toll-like receptor 4)-dependent MyD88 (myeloid differentiation primary response protein 88) signaling cascade, which results in early NF-κβ activation (nuclear factor-kappa b). This triggers the production of pro-inflammatory cytokines such as TNF-α (tumor necrosis factor-α), IL-1 (interleukin-1), and IL-6 to increase (interleukin-6), which plays a crucial role in the control of viral pathogens [ 133 , 134 ]. Moreover, ZnO-NPs can absorb UV–Vis light, dissociate water molecules, and release Zn 2+ ions, generating ROS such as hydrogen peroxide, hydroxyl radicals, and superoxide that disrupt the lipids, proteins, carbohydrates, and DNA of the virus, leading to its death [ 135 ]. According to Jana et al., polysaccharide-encapsulated ZnO-NPs showed exceptional antiviral action against human cytomegalovirus (HCMV), with cell survival rates of 93.6% and 92.4% at 400 µg/mL [ 136 ]. A survey reported that ZnO-NPs and PEGylated ZnO-NPs have inhibitory effects on the H1N1 influenza virus, with PEGylated ZnO-NPs showing higher anti-influenza activity with less cytotoxicity on MDCK-SIAT1 cells than ZnO-NPs, indicating that PEGylation on the surface of ZnO-NPs enhanced antiviral activity while reducing cytotoxicity [ 137 ]. A recent study on ZnO-NPs demonstrated compelling antiviral activity against SARS-CoV-2 at a very low concentration (IC 50 526 ng/mL), and it was found that ZnO-NPs can produce a large number of free radicals which ultimately induce significant damage to the membrane proteins of SARS-CoV-2. However, ZnO-NPs displayed cytotoxic levels (CC 50 292.2 ng/mL) against VERO-E6 cells [ 138 ]. Similarly, they exhibit excellent antiviral activity against the Chikungunya virus [ 139 ], and these findings suggest that ZnO-NPs might be good antiviral agents.
2.10. Cardioprotective Action of ZnO-NPs
As ZnO-NPs possess potent antioxidant activity, this gives us an idea about their use in the scavenging O 2 • — free radicals, which on the other side, possibly have cardioprotective effects. The O 2 • — free radicals are produced from lipid peroxides obtained from today’s fast foods and are made up of several flavoring/bleaching agents such as monosodium glutamate (MSG), which have several adverse effects on the heart, liver, kidney, testis, pancreas, brain, and other various tissues and organs with signs of inflammation [ 140 , 141 , 142 ]. These free radicals must be scavenged using ZnO-NPs to reduce the adverse effects of oxidative stress produced from the heart failure marker, lipid peroxidation (LPO), and lactoperoxidase-like reactive oxygen species free radicals. A study on the alleviation effect of the ZnO-NP/GTE complex on rats, through feeding two dosages of MSG and a dose of ZnO-NP/GTE (10 mg/kg) by oral gavages daily for 30 days, revealed that there was a reduction in LPO markers such as O 2 • — free radicals with a significant improvement in the level of endogenous antioxidants such as SOD, CAT, GSH, and GPx in cardiac tissue, indicating the protection against oxidative stress [ 143 ]. Thus, ZnO-NPs are believed to restore abnormal cardiac myofiber, implying their cardioprotective potential.
2.11. Anthelminthic Action of ZnO-NPs
ZnO-NPs have a strong anthelminthic effect, which is achieved by inducing oxidative stress by producing hydroxyl ions and ROS, which induces helminth membrane damage by electrostatic binding [ 144 , 145 ]. An in vitro study of ZnO-NPs on Gigantocotyle explanatum [ 146 ] revealed that they possess effective anthelminthic properties in higher concentrations. Flukes survive at lower quantities by increasing the activity of their intracellular antioxidant enzymes, SOD and GST, which scavenge reactive oxygen species [ 147 ], whereas with higher concentrations, SOD and GST possibly become saturated due to overproduction of ROS and hydroxyl ions, which leads to detoxification in flukes. These findings demonstrate sufficient evidence for the anthelminthic potential of ZnO-NPs.
3. Approaches for Synthesizing ZnO-NPs
ZnO-NPs are typically synthesized by utilizing physical, chemical, and biological processes that utilize either top-down or bottom-up approaches ( Figure 7 ). The cutting, grinding, or attrition of larger particles, followed by the formation of smaller particles at the nanoscale level, is referred to as a top-down technique. This method is commonly used for nanoparticle synthesis on a small scale [ 148 ]. The bottom-up approach is the process of synthesis of nanoparticles by gathering already miniaturized atoms/molecules through the application of chemical and physical methods. It is a cheaper method and faster than the top-down approach [ 149 ].
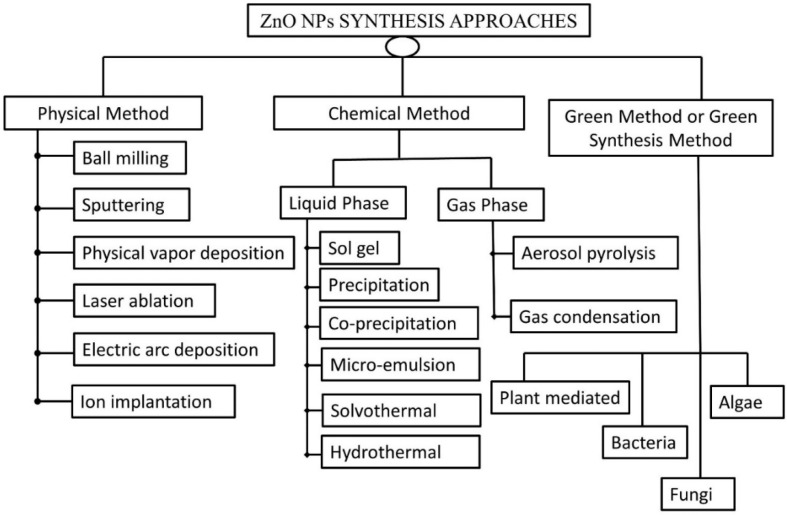
Synthesis approaches for ZnO-NPs.
3.1. Physical Methods
Physical methods are used to synthesize ZnO-NPs by attracting smaller molecules and atoms to produce nanoscale-sized particles that employ physical forces. Physical methods comprise ball milling, sputtering, physical vapor deposition, laser ablation, ion implantation, and electric arc deposition. Ball milling is a nonequilibrium phenomenon in which materials of a larger size are crushed with a ball mill due to collision with high-energy balls. The ball milling process has efficient production rates and is easier and more cost-effective. Salah et al. suggested that 15 spherical balls with a circumference of 20 mm concealed in a 500 mL bowl be used to form nanostructures of ZnO in a study on the antibacterial effectiveness of ZnO-NPs [ 149 ]. Laser ablation methods refer to the process of the removal of particles from the solid and liquid interface using a laser beam as an energy source. A study conducted by Mintcheva et al. provides a piece of evidence that the millisecond-pulsed laser ablation technique produced rod-shaped ZnO-NPs with lengths ranging from 40 to 110 nm and an average diameter of 30 nm [ 150 ]. Physical vapor depositions are a frequently used method in which the deposition of metals coating the surface involves two phenomena, such as evaporation and sputtering. Sputtering is the process of expelling particles from the surface by impacting high-energy particles with plasma ions [ 151 ]. Thermal evaporation is another physical approach in which powdered or condensed products are heated to a higher temperature, evaporation occurs, and the resulting vapors condense to form desirable nanoparticles under controlled conditions such as pressure, temperature, humidity, substrate, and so on [ 152 ].
3.2. Chemical Methods
The chemical methods for synthesizing ZnO-NPs are categorized based on their physical state, which includes solid-phase, liquid-phase, and gas-phase synthesis. Liquid-phase synthesis is a widespread method and a viable alternative to gaseous-phase synthesis. For liquid-phase synthesis, the sol-gel process, colloidal methods, precipitation and co-precipitation methods, microemulsion method, hydrothermal synthesis, and solvothermal and sonothermal methods can be utilized, whereas inert gas condensation methods and pyrolysis can be used for vapor-phase synthesis [ 153 ].
3.2.1. Liquid-Phase Synthesis
The sol-gel process is the process of conversion of prepared colloidal solution (sol) into gel through hydrolyzation, condensation, and polymerization reactions. Zinc acetate hydrate in alcohol is the most used precursor for the synthesis of ZnO-NPs [ 154 ]. Khan and companions synthesized pure and uniform thorn-like ZnO-NPs of a size < 50 nm for the first time by the sol-gel method [ 155 ]. Similarly, precipitation and co-precipitation methods involve the formation of a precipitate when inorganic alkalis act as a reducing agent combined with zinc salt. Sodium hydroxide and zinc sulfate heptahydrate are used as precursors, and by adjusting reaction conditions, these precipitates were washed and calcined at the requisite temperature to produce nanoparticles with the desired shape, size, and characteristics [ 156 ].
Solvothermal synthesis is a technique for facilitating a precursor interaction during synthesis by utilizing a solvent at moderate to high pressure (1–10,000 atm) and temperature (100–1000 °C) [ 157 ]. Hydrothermal synthesis, on the other hand, employs water and is normally performed below the supercritical temperature of the water, i.e., 374 °C. The microemulsion is another technique of synthesizing the thermodynamically stable dispersion of two immiscible liquids, namely, water and hydrocarbons. In general, two forms of microemulsions are utilized, such as oil-in-water (O/W) and water-in-oil (W/O), with the latter being predominantly used for the preparation of NPs by dispersing the metal salt (Zinc salt) precursor in the aqueous phase. Surfactant- and co-surfactant-charged hydrophilic groups aid to minimize interfacial tension between two phases and enhancing colloidal stability [ 158 ].
3.2.2. Gas-Phase Synthesis
The aerosol pyrolysis method is the most commonly used gas-phase synthesis method, in which aerosol droplets dispersed in the gas phase generate aerosol droplets of the precursor zinc salts when heated in a flame. The flame heating causes dehydration, which helps to reduce the size of particles in the nanoscale. The required material decomposes and sinters as a result of the heating over the flame [ 159 ]. Inert gas condensation is another major gas-phase synthesis technique. It involves evaporating zinc inside a heat-resistant compartment using a variety of heat sources, such as electron and laser beams or radio frequencies, and then condensing the vapors by migrating them to cooler chambers containing inert gas. Based on the catalyst, this approach is divided into two categories: physical vapor deposition intrigued without catalytic contact and chemical vapor deposition fascinated with catalytic interaction. It may cause agglomeration and coalescence of nanoparticles, which is a typical demerit of this process. Uhm and coworkers synthesized ZnO-NPs of a better shape and size with a 30 nm diameter by the levitational gas condensation method [ 160 ].
3.3. Green Synthesis
The terms “biological synthesis” and “green synthesis” are often used interchangeably. However, for a biological synthesis to be green, it should comply with the basic principles of green chemistry such as being environmentally friendly, no use of toxic chemicals, reduced derivatization, energy consumption, waste, and so on [ 161 ]. Here, green synthesis is the process of synthesizing nanoparticles by incorporating mainly cell extracts (microbial, plant, fungus, algae, etc.) into the substrate involving biofabrication, i.e., the capping of nanoparticles from natural products such as phytochemicals from plants and proteinous extracts from microorganisms and fungus without using any toxic chemicals. Green synthesis is to be nonhazardous, aligning with the principles of green chemistry. These methods provide merits of biocompatibility, cost-effectiveness, large-scale productivity, ecofriendliness, and being devoid of hazardous chemicals and adverse reaction conditions and are, therefore, an attractive alternative to traditional physical and chemical methods [ 162 ]. As such, microbial and plant extracts release phytochemicals that act as reducing agents as well as fabricating or stabilizing agents; this eliminates the dependence on industrial chemicals. On the contrary, if synthetic chemicals/solvents are employed to assist the reduction-stabilization process or to maintain pH in a green synthesis, such synthesis is better described as biochemical synthesis.
3.3.1. Plant-Mediated Synthesis of ZnO-NPs
A multitude of research supports the synthesis of crystalline ZnO-NPs by chelating a zinc complex with plant extracts. The aerial parts of plants, such as leaves and flowers, are commonly used in green synthesis. To optimize ZnO-NP synthesis, usually, reaction parameters such as temperature, pH, concentration, and time are adjusted. The appearance of a yellow coloration generally indicates the formation of ZnO-NPs, which is further confirmed by qualitative investigations such as UV–visible spectroscopy, SEM, and TEM [ 163 ].
The synthesis of ZnO-NPs with regulated shapes and sizes was accomplished by varying the concentration of plant extracts. Madan et al. synthesized NPs of varied sizes ranging from 9–40 nm and different shapes such as bud, cone, closed pine cone, bullet, and hexagonal disk by altering the concentrations of a plant extract from the leaves of Azadirachta indica [ 164 ]. The possible mechanism of the green synthesis has been explained by several researchers and the result is that the secondary metabolites and proteins present in the plant extracts act as capping and reducing agents which promote nanoparticle synthesis, whereas some studies have proposed that the nanoparticles of metal ions are formed due to the electrostatic interaction of plant proteins and metal ions. Proteins would reduce the metal ions, resulting in a change in the protein secondary structure, as well as in the formation of metal oxide nanoparticle seeds [ 163 , 165 ]. Plant components, from leaf to root, are extensively utilized in metal oxide nanoparticle synthesis because phytochemicals such as polyphenolic compounds, vitamins, polysaccharides, amino acids, alkaloids, terpenoids, etc. extracted from plants aid in the efficient bioreduction of metal ions for the synthesis of NPs that are stable and variable in structure and dimension. Bioreduction is the process of reducing metal ions or metal oxides to zero-valence metal NPs, fascinating in maintaining their stability. These techniques yield a large quantity of very pure nanoparticles that are free of contaminants [ 166 , 167 ]. Table 1 summarizes the key findings of extensive research on several plants employed in the synthesis of ZnO NPs.
Summary of the plant-mediated synthesis of zinc oxide nanoparticles.
3.3.2. Green Synthesis Using Bacterial Extracts
The nanoparticle synthesis using bacterial extracts is a complex and time-consuming technique of green synthesis. It is vital to ensure vigilant monitoring of the culture media throughout the process to avoid contamination. Otherwise, synthesized NPs could be less optimized and ineffective [ 2 ]. A study reported that the synthesis of ZnO-NPs can be carried out using Rhodococcus pyridinivorans and zinc sulfate as the substrate. The synthesized NPs were spherically shaped with a 100–130 nm size range confirmed through FE-SEM and XRD analysis [ 181 ]. The synthesis of nanoflowers (40 nm width and 400 nm height) with potent photocatalytic potency was also performed with B. licheniformis using the green synthesis technique [ 182 ]. The excellent antioxidant activity of NPs synthesized using Pseudomonas aeruginosa was also revealed, indicating that enhanced NP stability was attained due to the rhamnolipid of bacteria used. Thus, it is significant to consider that bacteria can be used as a better capping agent with outstanding stability and potency [ 183 ]. Green synthesis using a bacterial strain is well illustrated in Table 2 .
Summary of the bacteria-mediated synthesis of zinc oxide nanoparticles.
3.3.3. Green Synthesis Using Fungal Extracts
Due to the efficient and large-scale productivity, lower cost, and convenient processing, numerous fungal strains are being used for the green synthesis of ZnO-NPs over bacteria [ 2 ]. Fungi are more tolerable and have better metal bioaccumulative properties than bacterial strains, making them a stronger candidate for nanoparticle synthesis [ 191 ]. A study found that fungal strains such as Candida albicans could be employed to synthesize quasispherical-shaped ZnO-NPs [ 192 ]. Similarly, the mycelia of Aspergillus fumigatus were used to make spherical aggregate-shaped NPs, which agglomerate into a larger size after a few days, indicating the stability and potent capping activity of fungus as a substrate [ 193 ]. Some examples of fungal-mediated synthesis are included in Table 3 .
Summary of the fungal-mediated synthesis of zinc oxide nanoparticles.
3.3.4. Green Synthesis Using Microalgae and Macroalgae
Algae are photosynthetic organisms that are made up of single or multiple cells and lack essential components such as roots, stems, and leaves. Algae are classified into two types, macroalgae, and microalgae, as well as three groups, Rhodophyta (red pigmented), Phaeophyta (brown pigmented), and Chlorophyta (green pigmented). Algae have a limited significance in the synthesis of ZnO-NPs and are better suited for the production of other metal nanoparticles such as silver and gold nanoparticles. Microalgae are commonly employed for the green synthesis of NPs because they have a greater potential to minimize metal toxicity through the biodegradation process [ 198 ]. ZnO-NPs are typically synthesized using algae from the Sargassaceae family. Sargassum muticum was employed to make hexagonal wurtzite-shaped ZnO-NPs [ 199 ]. Similarly, nanoparticles of spherical, radial, triangular, hexagonal, and rod shapes were synthesized from S. myriocystum [ 200 ]. Furthermore, Chlamydomonas reinhardtii , a species of the Chlamydomonaceae family, was used to synthesize various-shaped NPs, such as nanorods, nanoflowers, and porous nanosheets [ 201 ]. Table 4 summarizes the ZnO-NPs synthesized by some of the algae.
Summary of the algal-mediated synthesis of zinc oxide nanoparticles.
4. Characterization of ZnO-NPs
A plethora of studies suggests that the morphology and surface chemistry of nanoparticles influence their biodistribution, safety, and effectiveness in biological systems ( Figure 8 ). Characterization is the core tool for successful applications and the understanding of nanoparticles. Nanoparticle size characterization is complicated by the polydispersity of materials, yet it is important to determine the morphology since the nanoparticle size’s resemblance to biological moieties is assumed to impart many of their distinct nanomedicine capabilities. Optical microscopy cannot resolve nanostructures; therefore, electron microscopy is used to characterize the nanoparticles. SEM and TEM are used to characterize the shapes and sizes, but TEM is used more often because it uses more powerful electrons and presents high resolution and informative image details regarding the atomic scale-like morphology, aggregation state, and distribution, and observes the functionality of capping agents/phytochemicals in enclosing NPs. Some biological molecules such as liposomes and proteins do not deflect the electron beam sufficiently and are invisible to electromagnetic radiation; therefore, dynamic light scattering (DLS), a nondestructive approach that uses a monochromatic laser and is also known as photon correlation spectroscopy, is used to characterize these compounds in suspensions and solutions. Here, small changes in the intensity of scattered laser light in the nanoparticle solution are regulated with a photon detector to analyze the hydrodynamic diameter and morphology of NPs [ 204 ].
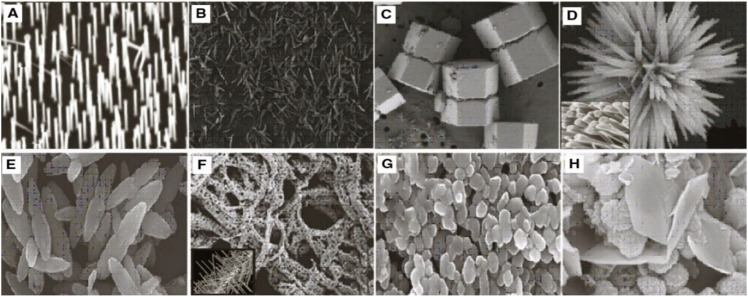
Morphology of ZnO nanostructures: ( A ) needles, rods, and wires; ( B ) helixes and springs; ( C ) nanopellets/nanocapsules; ( D ) flower, snowflake, and dandelion; ( E ) peanut-like; ( F ) interwoven particle hierarchy; ( G ) raspberry, nanosheet/nanoplate; ( H ) circular/round or sphere-shaped. (Reprinted from [ 209 ]; open access under CC BY).
The characterization of nanoparticles in animal tissue is accomplished by energy dispersion X-ray analysis (EDX), which assists in identifying the elemental composition and linkage of metabolites and also facilitates the interpretation of biodistribution of synthesized nanoparticles. Furthermore, atomic force microscopy (AFM) helps in determining the 3D geography (height and volume) of NPs; Fourier transform infrared spectroscopy (FTIR)-attenuated total reflectance (ATR) is an easy and nondestructive technique that contributes metabolites, chemicals, etc. through the synthesis and capping of NPs; UV–visible-diffuse reflectance spectroscopy (UV-DRS) is used to study the optical property of colored samples where the reflectance measurements are utilized to investigate the surface plasmon resonance of metals and hypersensitive biological analysis [ 205 ]; thermal gravimetric-differential thermal analysis (TG-DTA) provides information about the thermal stability, phase transition, and effect of the oxidative as well as reductive environment; photoluminescence (PL) analysis is utilized to determine the band gap, and crystalline purity and impurities; and x-ray photoelectron spectroscopy (XPS) can be used to characterize the morphology, and bioactive surface and material surface chemistry of NPs [ 206 , 207 , 208 ].
ZnO is one of the most significant II-VI compound semiconductor materials in scientific research and technological applications with noncentrosymmetric structures and multiple shape-induced functions. By adjusting the hydrothermal reaction parameters (such as precursor concentration, reaction duration, and pH), several morphologies of ZnO, including microrods, hexagonal pyramid-like rods, and flower-like rod aggregates, have been synthesized, respectively, on glass substrates. The production of ZnO microrods is significantly influenced by the precursor concentration. With longer reaction times, ZnO crystals can change from hexagonal pyramids to rod-like laths. ZnO rod aggregates that resemble flowers are produced at higher pH levels. The findings could provide a strategy for producing ZnO crystals in a certain desirable form [ 210 ]. Similarly, in a recent study, Doustkhah et al. hydrothermally transformed zinc-based metal-organic frameworks into ZnO nanostructures with temperature-dependent tunable structures and catalytic activity, which at an elevated temperature displayed high crystallinity and better dye degradation efficiency than at a lower temperature [ 211 ].
Most of the group II-VI binary compound semiconductors crystallize as hexagonal wurtzite or cubic zinc-blende, with each anion surrounded by four cations at the corners of a tetrahedron. The iconicity of the II-VI compound semiconductor ZnO lies at the interface between covalent and ionic semiconductors. Wurtzite, blende, and rocksalt are potential ZnO crystal formations. Wurtzite is the most thermodynamically stable of these crystal forms at room temperature, but blende is stable when developed on a cubic substrate and rocksalt is stable when synthesized at very high temperatures [ 212 ]. In contrast to the zinc-blende structure, which has two interpenetrating face-centered-cubic (fcc) sublattices that are displaced along the body diagonal by one-quarter of a body diagonal, the wurtzite structure is made up of two interpenetrating hexagonal-closed-packed (hcp) sublattices. Due to the decrease in lattice dimensions, which favors iconicity over a covalent nature, and the structure’s six-fold coordination, wurtzite can undergo the same transformation as other II-VI semiconductors to become rocksalt [ 212 ].
5. Conclusions
This review aimed to explore the synthesis, characterization, and biological activities of ZnO-NPs, illustrating their mechanism of action. Extensive discussion was centered on the green synthesis approach and its biomedical applications. The pathways of different bioactivity were explained, with special emphasis on ZnO-NPs’ biopotency with regard to antibacterial, antifungal, anticancer, anti-inflammatory, antidiabetic, antioxidant, antiviral, wound healing, orthopedic implants, bone healing, and cardioprotective activity, along with the concise interpretation of the green synthesis of nanoparticles using biological sources. The importance and significance of ZnO-NPs in pharmaceutical and biological sectors have attracted scientists to perform an extensive study of their applications in multiple ailments. Green synthesis is an eco-friendly approach that reduces costs, increases production, and improves biocompatibility in humans. Biofabrication with natural compounds helps to stabilize the nanoparticles with reduced toxicity and higher reduction potential. ZnO-NPs possess several compelling pharmacological activities. Special focus should be given to ZnO-NP generation through plant-mediated synthesis, bearing tremendous applications in the fields of pharmaceuticals, food, and cosmetics. The advancement of nanotechnology in the formulation of metal oxide nanoparticles can contribute to the reduction in the dosage used with optimum desired effects and low toxicity.
Acknowledgments
We are thankful to Arpita Roy, Sharda University, India, for her feedback on the manuscript.
Abbreviations
ZnO-NPs: zinc oxide nanoparticles; ROS: reactive oxygen species; SOD: superoxide dismutase; GSTs: glutathione S-transferases; NPs: nanoparticles; SEM: scanning electron microscopy; TEM: transmission electron microscopy; XRD: X-ray diffractometer; DLS: dynamic light scattering; EM: electron microscopy; HRTEM: high-resolution transmission electron microscopy; HRSEM: high-resolution scanning electron microscopy; FE-SEM: filed emission scanning electron microscopy; AFM: atomic force microscopy; GSH: glutathione; GPx: glutathione peroxidase; MSG: monosodium glutamate; DPPH: 2,2-diphenyl-1-picrylhydrazyl; NEFA: nonesterified fatty acid; iNOS: inducible nitric oxide synthase; PGE2: prostaglandin E2; NF-κβ: nuclear factor-kappa b; COX 2: cyclooxygenases 2; IL-1: interleukin-1; TNF: tumor necrosis factor; IL-6: interleukin-6; IL-12: interleukin-12; IL-18: interleukin-18; ATR: attenuated total reflection; EDAX: energy dispersion analysis of X-ray; PL: photoluminescence; XPS: X-ray photoelectron microscopy; TG-DTA: thermal gravimetric-differential thermal analysis; UV-DRS: UV–visible reflectance spectroscopy; BMs: biodegradable metals; ALP: alkaline phosphatase; hBMSCs: human bone marrow-derived mesenchymal stem cells; hDPSCs: human dental pulp stem cells; MDR: multidrug-resistant; HPMC: hydroxypropyl methylcellulose; FBS: fasting blood sugar; CAT: catalase.
Author Contributions
Conceptualization, N.P. and N.B.; methodology, A.K.M.; writing—original draft preparation, A.K.M., S.B., A.G., F.T., N.B., A.K.S. and N.P.; writing—review and editing, S.K., S.J. and D.P.B.; editing images, S.B. and F.T.; supervision and project administration, N.P. and N.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.

Funding Statement
Tettey and Bhattarai acknowledge funding support in part from National Science Foundation (EiR-2100861) for their contribution to this review work.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Jayachandran A., Aswathy T.R., Nair A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021;26:100995. doi: 10.1016/j.bbrep.2021.100995. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Agarwal H., Kumar S.V., Rajeshkumar S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017;3:406–413. doi: 10.1016/j.reffit.2017.03.002. [ DOI ] [ Google Scholar ]
- 3. Rodnyi P.A., Khodyuk I.V. Optical and luminescence properties of zinc oxide (Review) Opt. Spectrosc. 2011;111:776–785. doi: 10.1134/S0030400X11120216. [ DOI ] [ Google Scholar ]
- 4. Shaba E.Y., Jacob J.O., Tijani J.O., Suleiman M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021;11:48. doi: 10.1007/s13201-021-01370-z. [ DOI ] [ Google Scholar ]
- 5. Kielbik P., Kaszewski J., Rosowska J., Wolska E., Witkowski B., Gralak M., Gajewski Z., Godlewski M., Godlewski M.M. Biodegradation of the ZnO:Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomed. Nanotechnol. Biol. Med. 2017;13:843–852. doi: 10.1016/j.nano.2016.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Mandal B.K. Nanobiomaterials in Antimicrobial Therapy. William Andrew Publishing; Cambridge, MA, USA: 2016. Scopes of green synthesized metal and metal oxide nanomaterials in antimicrobial therapy; pp. 313–341. [ DOI ] [ Google Scholar ]
- 7. Wiesmann N., Tremel W., Brieger J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B. 2020;8:4973–4989. doi: 10.1039/D0TB00739K. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Qin X., Zhang J., Wang B., Xu G., Yang X., Zou Z., Yu C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17:4266–4285. doi: 10.1080/15548627.2021.1911016. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Wiesmann N., Kluenker M., Demuth P., Brenner W., Tremel W., Brieger J. Zinc overload mediated by zinc oxide nanoparticles as innovative anti-tumor agent. J. Trace Elem. Med. Biol. 2018;51:226–234. doi: 10.1016/j.jtemb.2018.08.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Stepankova H., Swiatkowski M., Kruszynski R., Svec P., Michalkova H., Smolikova V., Ridoskova A., Splichal Z., Michalek P., Richtera L., et al. The Anti-Proliferative Activity of Coordination Compound-Based ZnO Nanoparticles as a Promising Agent against Triple Negative Breast Cancer Cells. Int. J. Nanomed. 2021;16:4431–4449. doi: 10.2147/IJN.S304902. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Bai K.-J., Chuang K.-J., Ma C.-M., Chang T.-Y., Chuang H.-C. Human lung adenocarcinoma cells with an EGFR mutation are sensitive to non-autophagic cell death induced by zinc oxide and aluminium-doped zinc oxide nanoparticles. J. Toxicol. Sci. 2017;42:437–444. doi: 10.2131/jts.42.437. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Alsagaby S.A., Vijayakumar R., Premanathan M., Mickymaray S., Alturaiki W., Al-Baradie R.S., AlGhamdi S., Aziz M.A., Alhumaydhi F.A., Alzahrani F.A., et al. Transcriptomics-Based Characterization of the Toxicity of ZnO Nanoparticles against Chronic Myeloid Leukemia Cells. Int. J. Nanomed. 2020;15:7901–7921. doi: 10.2147/IJN.S261636. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. He T., Long J., Li J., Liu L., Cao Y. Toxicity of ZnO nanoparticles (NPs) to A549 cells and A549 epithelium in vitro: Interactions with dipalmitoyl phosphatidylcholine (DPPC) Environ. Toxicol. Pharmacol. 2017;56:233–240. doi: 10.1016/j.etap.2017.10.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Hatami Z., Ragheb E., Jalali F., Tabrizi M.A., Shamsipur M. Zinc oxide-gold nanocomposite as a proper platform for label-free DNA biosensor. Bioelectrochemistry. 2020;133:107458. doi: 10.1016/j.bioelechem.2020.107458. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Nath J., Dror I., Landa P., Vanek T., Kaplan-Ashiri I., Berkowitz B. Synthesis and characterization of isotopically-labeled silver, copper and zinc oxide nanoparticles for tracing studies in plants. Environ. Pollut. 2018;242:1827–1837. doi: 10.1016/j.envpol.2018.07.084. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Xu J.W., Cui Z.M., Liu Z.Q., Xu F., Chen Y.S., Luo Y.L. Organic-Inorganic Nanohybrid Electrochemical Sensors from Multi-Walled Carbon Nanotubes Decorated with Zinc Oxide Nanoparticles and In-Situ Wrapped with Poly(2-methacryloyloxyethyl ferrocenecarboxylate) for Detection of the Content of Food Additives. Nanomaterials. 2019;9:1388. doi: 10.3390/nano9101388. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Eymard-Vernain E., Luche S., Rabilloud T., Lelong C. Impact of nanoparticles on the Bacillus subtilis (3610) competence. Sci. Rep. 2018;8:2978. doi: 10.1038/s41598-018-21402-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Shakerimoghaddam A., Razavi D., Rahvar F., Khurshid M., Ostadkelayeh S.M., Esmaeili S.-A., Khaledi A., Eshraghi M. Evaluate the Effect of Zinc Oxide and Silver Nanoparticles on Biofilm and icaA Gene Expression in Methicillin-Resistant Staphylococcus aureus Isolated from Burn Wound Infection. J. Burn Care Res. 2020;41:1253–1259. doi: 10.1093/jbcr/iraa085. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Bashandy S.A., Alaamer A., Moussa S., Omara E.A. Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can. J. Physiol. Pharmacol. 2018;96:337–344. doi: 10.1139/cjpp-2017-0247. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Anan H.H., Zidan R.A., El-Baset S.A.A., Ali M.M. Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell. 2018;54:80–93. doi: 10.1016/j.tice.2018.08.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Hu Y., Zhang H.-R., Dong L., Xu M.-R., Zhang L., Ding W.-P., Zhang J.-Q., Lin J., Zhang Y.-J., Qiu B.-S., et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale. 2019;11:11789–11807. doi: 10.1039/C8NR08442D. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Świątek Z.M., Woźnicka O., Bednarska A.J. Unravelling the ZnO-NPs mechanistic pathway: Cellular changes and altered morphology in the gastrointestinal tract of the earthworm Eisenia andrei. Ecotoxicol. Environ. Saf. 2020;196:110532. doi: 10.1016/j.ecoenv.2020.110532. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Abbasi B.H., Shah M., Hashmi S.S., Nazir M., Naz S., Ahmad W., Khan I.U., Hano C. Green Bio-Assisted Synthesis, Characterization and Biological Evaluation of Biocompatible ZnO NPs Synthesized from Different Tissues of Milk Thistle (Silybum marianum) Nanomaterials. 2019;9:1171. doi: 10.3390/nano9081171. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Lakshmipriya T., Gopinath S.C.B. Nanoparticles in Analytical and Medical Devices. Elsevier; Amsterdam, The Netherlands: 2021. Introduction to nanoparticles and analytical devices; pp. 1–29. [ DOI ] [ Google Scholar ]
- 25. Kalpana V.N., Rajeswari V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018;2018:e3569758. doi: 10.1155/2018/3569758. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Sur D.H., Mukhopadhyay M. Role of zinc oxide nanoparticles for effluent treatment using Pseudomonas putida and Pseudomonas aureofaciens. Bioprocess Biosyst. Eng. 2018;42:187–198. doi: 10.1007/s00449-018-2024-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro. Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Mendes C.R., Dilarri G., Forsan C.F., Sapata V.d.M.R., Lopes P.R.M., de Moraes P.B., Montagnolli R.N., Ferreira H., Bidoia E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022;12:2658. doi: 10.1038/s41598-022-06657-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Yusof H.M., Mohamad R., Zaidan U.H., Rahman N.A.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019;10:57. doi: 10.1186/s40104-019-0368-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Krishnamoorthy R., Athinarayanan J., Periyasamy V.S., Alshuniaber M.A., Alshammari G., Hakeem M.J., Ahmed M.A., Alshatwi A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules. 2022;27:2489. doi: 10.3390/molecules27082489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Abomuti M.A., Danish E.Y., Firoz A., Hasan N., Malik M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Salvia officinalis Leaf Extract and Their Photocatalytic and Antifungal Activities. Biology. 2021;10:1075. doi: 10.3390/biology10111075. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Xu M.-N., Li L., Pan W., Zheng H.-X., Wang M.-L., Peng X.-M., Dai S.-Q., Tang Y.-M., Zeng K., Huang X.-W. Zinc Oxide Nanoparticles Prime a Protective Immune Response in Galleria mellonella to Defend against Candida albicans. Front. Microbiol. 2021;12:766138. doi: 10.3389/fmicb.2021.766138. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Sonia S., Ruckmani K., Sivakumar M. Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: A potent nanocosmeceutical application. Mater. Sci. Eng. C. 2017;79:581–589. doi: 10.1016/j.msec.2017.05.059. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Shobha N., Nanda N., Giresha A.S., Manjappa P., Sophiya P., Dharmappa K., Nagabhushana B. Synthesis and characterization of Zinc oxide nanoparticles utilizing seed source of Ricinus communis and study of its antioxidant, antifungal and anticancer activity. Mater. Sci. Eng. C. 2018;97:842–850. doi: 10.1016/j.msec.2018.12.023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Al-Janabi A.A.H.S., Bashi A.M. Development of a new synthetic xerogel nanoparticles of silver and zinc oxide against causative agents of dermatophytoses. J. Dermatol. Treat. 2018;30:283–287. doi: 10.1080/09546634.2018.1506079. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Wu J., Sun Q., Huang H., Duan Y., Xiao G., Le T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces. 2019;180:31–38. doi: 10.1016/j.colsurfb.2019.04.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. He L., Liu Y., Mustapha A., Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011;166:207–215. doi: 10.1016/j.micres.2010.03.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Kairyte K., Kadys A., Luksiene Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B Biol. 2013;128:78–84. doi: 10.1016/j.jphotobiol.2013.07.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Sharma D., Rajput J., Kaith B., Kaur M., Sharma S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films. 2010;519:1224–1229. doi: 10.1016/j.tsf.2010.08.073. [ DOI ] [ Google Scholar ]
- 40. Motazedi R., Rahaiee S., Zare M. Efficient biogenesis of ZnO nanoparticles using extracellular extract of Saccharomyces cerevisiae: Evaluation of photocatalytic, cytotoxic and other biological activities. Bioorg. Chem. 2020;101:103998. doi: 10.1016/j.bioorg.2020.103998. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Dobrucka R., Dlugaszewska J., Kaczmarek M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices. 2017;20:5. doi: 10.1007/s10544-017-0233-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Zhao C., Zhang X., Zheng Y. Biosynthesis of polyphenols functionalized ZnO nanoparticles: Characterization and their effect on human pancreatic cancer cell line. J. Photochem. Photobiol. B Biol. 2018;183:142–146. doi: 10.1016/j.jphotobiol.2018.04.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Yi G.-C., Wang C., Park W.I. ZnO nanorods: Synthesis, characterization and applications. Semicond. Sci. Technol. 2005;20:S22–S34. doi: 10.1088/0268-1242/20/4/003. [ DOI ] [ Google Scholar ]
- 44. Wang H., Wingett D., Engelhard M., Feris K., Reddy K.M., Turner P., Layne J., Hanley C., Bell J., Tenne D., et al. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J. Mater. Sci. Mater. Electron. 2008;20:11–22. doi: 10.1007/s10856-008-3541-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Hanley C., Layne J., Punnoose A., Reddy K.M., Coombs I., Coombs A., Feris K., Wingett D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19:295103. doi: 10.1088/0957-4484/19/29/295103. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Rasmussen J.W., Martinez E., Louka P., Wingett D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010;7:1063–1077. doi: 10.1517/17425247.2010.502560. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Chong C.L., Fang C.M., Pung S.Y., Ong C.E., Pung Y.F., Kong C., Pan Y. Current Updates On the In vivo Assessment of Zinc Oxide Nanoparticles Toxicity Using Animal Models. BioNanoScience. 2021;11:590–620. doi: 10.1007/s12668-021-00845-2. [ DOI ] [ Google Scholar ]
- 48. Kaur G., Narayanan G., Garg D., Sachdev A., Matai I. Biomaterials-Based Regenerative Strategies for Skin Tissue Wound Healing. ACS Appl. Bio Mater. 2022;5:2069–2106. doi: 10.1021/acsabm.2c00035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Khan A.U.R., Huang K., Jinzhong Z., Zhu T., Morsi Y., Aldalbahi A., El-Newehy M., Yan X., Mo X. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J. Mater. Chem. B. 2021;9:1452–1465. doi: 10.1039/D0TB02822C. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Hasannasab M., Nourmohammadi J., Dehghan M.M., Ghaee A. Immobilization of bromelain and ZnO nanoparticles on silk fibroin nanofibers as an antibacterial and anti-inflammatory burn dressing. Int. J. Pharm. 2021;610:121227. doi: 10.1016/j.ijpharm.2021.121227. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Kantipudi S., Sunkara J.R., Rallabhandi M., Thonangi C.V., Cholla R.D., Kollu P., Parvathaneni M.K., Pammi S.V.N. Enhanced wound healing activity of Ag–ZnO composite NPs in Wistar Albino rats. IET Nanobiotechnol. 2018;12:473–478. doi: 10.1049/iet-nbt.2017.0087. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Metwally A.A., Abdel-Hady A.-N.A.A., Haridy M.A.M., Ebnalwaled K., Saied A.A., Soliman A.S. Wound healing properties of green (using Lawsonia inermis leaf extract) and chemically synthesized ZnO nanoparticles in albino rats. Environ. Sci. Pollut. Res. 2021;29:23975–23987. doi: 10.1007/s11356-021-17670-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Vedhanayagam M., Nair B.U., Sreeram K.J. Collagen-ZnO Scaffolds for Wound Healing Applications: Role of Dendrimer Functionalization and Nanoparticle Morphology. ACS Appl. Bio Mater. 2018;1:1942–1958. doi: 10.1021/acsabm.8b00491. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Arshad R., Sohail M.F., Sarwar H.S., Saeed H., Ali I., Akhtar S., Hussain S.Z., Afzal I., Jahan S., Rehman A.U., et al. ZnO-NPs embedded biodegradable thiolated bandage for postoperative surgical site infection: In vitro and in vivo evaluation. PLoS ONE. 2019;14:e0217079. doi: 10.1371/journal.pone.0217079. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Shahriari-Khalaji M., Hu G., Chen L., Cao Z., Andreeva T., Xiong X., Krastev R., Hong F.F. Functionalization of Aminoalkylsilane-Grafted Bacterial Nanocellulose with ZnO-NPs-Doped Pullulan Electrospun Nanofibers for Multifunctional Wound Dressing. ACS Biomater. Sci. Eng. 2021;7:3933–3946. doi: 10.1021/acsbiomaterials.1c00444. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Soubhagya A., Moorthi A., Prabaharan M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020;157:135–145. doi: 10.1016/j.ijbiomac.2020.04.156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Cleetus C.M., Primo F.A., Fregoso G., Raveendran N.L., Noveron J.C., Spencer C.T., Ramana C.V., Joddar B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020;15:5097–5111. doi: 10.2147/IJN.S255937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Chopra M., Bernela M., Kaur P., Manuja A., Kumar B., Thakur R. Alginate/gum acacia bipolymeric nanohydrogels—Promising carrier for Zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015;72:827–833. doi: 10.1016/j.ijbiomac.2014.09.037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Saddik M.S., Elsayed M.M.A., El-Mokhtar M.A., Sedky H., Abdel-Aleem J.A., Abu-Dief A.M., Al-Hakkani M.F., Hussein H.L., Al-Shelkamy S.A., Meligy F.Y., et al. Tailoring of Novel Azithromycin-Loaded Zinc Oxide Nanoparticles for Wound Healing. Pharmaceutics. 2022;14:111. doi: 10.3390/pharmaceutics14010111. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Manuja A., Raguvaran R., Kumar B., Kalia A., Tripathi B. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020;155:823–833. doi: 10.1016/j.ijbiomac.2020.03.221. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Agren M.S. Studies on zinc in wound healing. Acta Derm. Venereol. Suppl. 1990;154:1–36. [ PubMed ] [ Google Scholar ]
- 62. Idzik M., Poloczek J., Skrzep-Poloczek B., Dróżdż E., Chełmecka E., Czuba Z., Jochem J., Stygar D. The Effects of 21-Day General Rehabilitation after Hip or Knee Surgical Implantation on Plasma Levels of Selected Interleukins, VEGF, TNF-α, PDGF-BB, and Eotaxin-1. Biomolecules. 2022;12:605. doi: 10.3390/biom12050605. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Hanada T., Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/S1359-6101(02)00026-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Agarwal H., Shanmugam V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2019;94:103423. doi: 10.1016/j.bioorg.2019.103423. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Nagajyothi P., Cha S.J., Yang I.J., Sreekanth T., Kim K.J., Shin H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015;146:10–17. doi: 10.1016/j.jphotobiol.2015.02.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Kim M.-H., Seo J.-H., Kim H.-M., Jeong H.-J. Aluminum-doped zinc oxide nanoparticles attenuate the TSLP levels via suppressing caspase-1 in activated mast cells. J. Biomater. Appl. 2016;30:1407–1416. doi: 10.1177/0885328216629822. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Olbert M., Argasińska J.G., Nowak G., Librowski T. Beneficial effect of nanoparticles over standard form of zinc oxide in enhancing the anti-inflammatory activity of ketoprofen in rats. Pharmacol. Rep. 2017;69:679–682. doi: 10.1016/j.pharep.2017.02.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Jan H., Shah M., Andleeb A., Faisal S., Khattak A., Rizwan M., Drouet S., Hano C., Abbasi B.H. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Aqueous Leaf Extract of Aquilegia pubiflora: Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other In Vitro Properties. Oxid. Med. Cell. Longev. 2021;2021:e4786227. doi: 10.1155/2021/4786227. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Basirun W.J., Nasiri-Tabrizi B., Baradaran S. Overview of Hydroxyapatite–Graphene Nanoplatelets Composite as Bone Graft Substitute: Mechanical Behavior and in-vitro Biofunctionality. Crit. Rev. Solid State Mater. Sci. 2017;43:177–212. doi: 10.1080/10408436.2017.1333951. [ DOI ] [ Google Scholar ]
- 70. Kamachimudali U., Sridhar T.M., Raj B. Corrosion of bio implants. Sadhana. 2003;28:601–637. doi: 10.1007/BF02706450. [ DOI ] [ Google Scholar ]
- 71. Filipović U., Dahmane R.G., Ghannouchi S., Zore A., Bohinc K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020;283:102228. doi: 10.1016/j.cis.2020.102228. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Kumar M., Kumar R., Kumar S. Coatings on orthopedic implants to overcome present problems and challenges: A focused review. Mater. Today Proc. 2021;45:5269–5276. doi: 10.1016/j.matpr.2021.01.831. [ DOI ] [ Google Scholar ]
- 73. Wang N., Fuh J.Y.H., Dheen S.T., Kumar A.S. Functions and applications of metallic and metallic oxide nanoparticles in orthopedic implants and scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;109:160–179. doi: 10.1002/jbm.b.34688. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Goodman S.B., Yao Z., Keeney M., Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34:3174–3183. doi: 10.1016/j.biomaterials.2013.01.074. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Yu X., Zhao D., Huang S., Wang B., Zhang X., Wang W., Wei X. Biodegradable magnesium screws and vascularized iliac grafting for displaced femoral neck fracture in young adults. BMC Musculoskelet. Disord. 2015;16:329. doi: 10.1186/s12891-015-0790-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Han P., Cheng P., Zhang S., Zhao C., Ni J., Zhang Y., Zhong W., Hou P., Zhang X., Zheng Y., et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99 wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials. 2015;64:57–69. doi: 10.1016/j.biomaterials.2015.06.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Navarro M., Aparicio C., Charles-Harris M., Ginebra M.P., Engel E., Planell J.A. Development of a Biodegradable Composite Scaffold for Bone Tissue Engineering: Physicochemical, Topographical, Mechanical, Degradation, and Biological Properties. In: Vancso G.J., editor. Ordered Polymeric Nanostructures at Surfaces. Springer; Berlin/Heidelberg, Germany: 2006. pp. 209–231. [ DOI ] [ Google Scholar ]
- 78. Evans C.H. Advances in Regenerative Orthopedics. Mayo Clin. Proc. 2013;88:1323–1339. doi: 10.1016/j.mayocp.2013.04.027. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Day R.M. Bioactive Glass Stimulates the Secretion of Angiogenic Growth Factors and Angiogenesis in Vitro. Tissue Eng. 2005;11:768–777. doi: 10.1089/ten.2005.11.768. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Jin G., Qin H., Cao H., Qian S., Zhao Y., Peng X., Zhang X., Liu X., Chu P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials. 2014;35:7699–7713. doi: 10.1016/j.biomaterials.2014.05.074. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Wang R., He X., Gao Y., Zhang X., Yao X., Tang B. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;75:7–15. doi: 10.1016/j.msec.2017.02.036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Pang S., He Y., Zhong R., Guo Z., He P., Zhou C., Xue B., Wen X., Li H. Multifunctional ZnO/TiO2 nanoarray composite coating with antibacterial activity, cytocompatibility and piezoelectricity. Ceram. Int. 2019;45:12663–12671. doi: 10.1016/j.ceramint.2019.03.076. [ DOI ] [ Google Scholar ]
- 83. Iqbal N., Kadir M.R.A., Mahmood N.H., Salim N., Froemming G., Balaji H., Kamarul T. Characterization, antibacterial and in vitro compatibility of zinc–silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014;40:4507–4513. doi: 10.1016/j.ceramint.2013.08.125. [ DOI ] [ Google Scholar ]
- 84. Yamaguchi M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998;11:119–135. doi: 10.1002/(SICI)1520-670X(1998)11:2/3<119::AID-JTRA5>3.0.CO;2-3. [ DOI ] [ Google Scholar ]
- 85. Seo H.-J., Cho Y.-E., Kim T., Shin H.-I., Kwun I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010;4:356–361. doi: 10.4162/nrp.2010.4.5.356. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Storrie H., Stupp S.I. Cellular response to zinc-containing organoapatite: An in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials. 2005;26:5492–5499. doi: 10.1016/j.biomaterials.2005.01.043. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Frederickson C.J., Koh J.-Y., Bush A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Hove E., Elvehjem C., Hart E. The effect of zinc on alkaline phosphatases. J. Biol. Chem. 1940;134:425–442. doi: 10.1016/S0021-9258(18)73284-5. [ DOI ] [ Google Scholar ]
- 89. Coleman J.E. Structure and Mechanism of Alkaline Phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992;21:441–483. doi: 10.1146/annurev.bb.21.060192.002301. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Anderson H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Clancaglini P., Plzauro J.M., Curti C., Tedesco A.C., Leone F.A. Effect of membrane moiety and magnesium ions on the inhibition of matrix-induced alkaline phosphatase by zinc ions. Int. J. Biochem. 1990;22:747–751. doi: 10.1016/0020-711X(90)90010-Z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Ciancaglini P., Pizauro J., Rezende A., Leone F. Solubilization of membrane-bound matrix-induced alkaline phosphatase with polyoxyethylene 9-lauryl ether (polidocanol): Purification and metalloenzyme properties. Int. J. Biochem. 1990;22:385–392. doi: 10.1016/0020-711X(90)90141-O. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Reginster J.-Y., Strause L.G., Saltman P., Franchimont P. Trace elements and postmenopausal osteoporosis: A preliminary report of decreased serum manganese. [(accessed on 10 August 2022)];Med. Sci. Res. 2022 16:1988. Available online: https://orbi.uliege.be/handle/2268/160100 . [ Google Scholar ]
- 94. Yusa K., Yamamoto O., Iino M., Takano H., Fukuda M., Qiao Z., Sugiyama T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016;71:162–169. doi: 10.1016/j.archoralbio.2016.07.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Guo B., Yang M., Liang D., Yang L., Cao J., Zhang L. Cell apoptosis induced by zinc deficiency in osteoblastic MC3T3-E1 cells via a mitochondrial-mediated pathway. Mol. Cell. Biochem. 2011;361:209–216. doi: 10.1007/s11010-011-1105-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Hu H., Zhang W., Qiao Y., Jiang X., Liu X., Ding C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2011;8:904–915. doi: 10.1016/j.actbio.2011.09.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Chang Y.-Y., Lai C.-H., Hsu J.-T., Tang C.-H., Liao W.-C., Huang H.-L. Antibacterial properties and human gingival fibroblast cell compatibility of TiO2/Ag compound coatings and ZnO films on titanium-based material. Clin. Oral Investig. 2011;16:95–100. doi: 10.1007/s00784-010-0504-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Roknian M., Fattah-Alhosseini A., Gashti S.O., Keshavarz M.K. Study of the effect of ZnO nanoparticles addition to PEO coatings on pure titanium substrate: Microstructural analysis, antibacterial effect and corrosion behavior of coatings in Ringer’s physiological solution. J. Alloys Compd. 2018;740:330–345. doi: 10.1016/j.jallcom.2017.12.366. [ DOI ] [ Google Scholar ]
- 99. Colon G., Ward B.C., Webster T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A. 2006;78A:595–604. doi: 10.1002/jbm.a.30789. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Lin M.-H., Wang Y.-H., Kuo C.-H., Ou S.-F., Huang P.-Z., Song T.-Y., Chen Y.-C., Chen S.-T., Wu C.-H., Hsueh Y.-H., et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021;257:117639. doi: 10.1016/j.carbpol.2021.117639. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Walker J., Shadanbaz S., Woodfield T.B.F., Staiger M.P., Dias G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014;102:1316–1331. doi: 10.1002/jbm.b.33113. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Adhikari U., Rijal N.P., Khanal S., Pai D., Sankar J., Bhattarai N. Magnesium and Calcium-Containing Scaffolds for Bone Tissue Regeneration; Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition; Phoenix, AZ, USA. 11–17 November 2016; [ DOI ] [ Google Scholar ]
- 103. Khader A., Arinzeh T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2019;117:194–209. doi: 10.1002/bit.27173. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Chausmer A.B. Zinc, Insulin and Diabetes. J. Am. Coll. Nutr. 1998;17:109–115. doi: 10.1080/07315724.1998.10718735. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Jansen J., Karges W., Rink L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009;20:399–417. doi: 10.1016/j.jnutbio.2009.01.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Hiromura M., Sakurai H. Action mechanism of metallo-allixin complexes as antidiabetic agents. Pure Appl. Chem. 2008;80:2727–2733. doi: 10.1351/pac200880122727. [ DOI ] [ Google Scholar ]
- 107. Bayrami A., Parvinroo S., Habibi-Yangjeh A., Pouran S.R. Bio-extract-mediated ZnO nanoparticles: Microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif. Cells Nanomed. Biotechnol. 2017;46:730–739. doi: 10.1080/21691401.2017.1337025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Richards-Williams C., Contreras J.L., Berecek K.H., Schwiebert E.M. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal. 2008;4:393–405. doi: 10.1007/s11302-008-9126-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Arvanag F.M., Bayrami A., Habibi-Yangjeh A., Pouran S.R. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L. seed extract. Mater. Sci. Eng. C. 2018;97:397–405. doi: 10.1016/j.msec.2018.12.058. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Meydan I., Burhan H., Gür T., Seçkin H., Tanhaei B., Sen F. Characterization of Rheum ribes with ZnO nanoparticle and its antidiabetic, antibacterial, DNA damage prevention and lipid peroxidation prevention activity of in vitro. Environ. Res. 2021;204:112363. doi: 10.1016/j.envres.2021.112363. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Vinotha V., Iswarya A., Thaya R., Govindarajan M., Alharbi N.S., Kadaikunnan S., Khaled J.M., Al-Anbr M.N., Vaseeharan B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B Biol. 2019;197:111541. doi: 10.1016/j.jphotobiol.2019.111541. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Chandrasekaran S., Anbazhagan V., Anusuya S. Green route synthesis of ZnO nanoparticles using Senna auriculata aqueous flower extract as reducing agent and evaluation of its antimicrobial, antidiabetic and cytotoxic activity. Appl. Biochem. Biotechnol. 2022. in press . [ DOI ] [ PubMed ]
- 113. Rajakumar G., Thiruvengadam M., Mydhili G., Gomathi T., Chung I.-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2017;41:21–30. doi: 10.1007/s00449-017-1840-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Malaikozhundan B., Vinodhini J., Kalanjiam M.A.R., Vinotha V., Palanisamy S., Vijayakumar S., Vaseeharan B., Mariyappan A. High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of Withania somnifera leaf extract-assisted zinc oxide nanoparticle. Bioprocess Biosyst. Eng. 2020;43:1533–1547. doi: 10.1007/s00449-020-02346-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Winston G.W., Regoli F., Dugas A.J., Fong J.H., Blanchard K.A. A Rapid Gas Chromatographic Assay for Determining Oxyradical Scavenging Capacity of Antioxidants and Biological Fluids. Free Radic. Biol. Med. 1998;24:480–493. doi: 10.1016/S0891-5849(97)00277-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Ryu C.S., Kim C.H., Lee S.Y., Lee K.S., Choung K.J., Song G.Y., Kim B.-H., Ryu S.Y., Lee H.S., Kim S.K. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012;132:333–337. doi: 10.1016/j.foodchem.2011.10.086. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Regoli F., Nigro M., Bompadre S., Winston G.W. Total oxidant scavenging capacity (TOSC) of microsomal and cytosolic fractions from Antarctic, Arctic and Mediterranean scallops: Differentiation between three potent oxidants. Aquat. Toxicol. 2000;49:13–25. doi: 10.1016/S0166-445X(99)00070-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Colvin V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003;21:1166–1170. doi: 10.1038/nbt875. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Chen Z., Meng H., Xing G., Chen C., Zhao Y. Toxicological and biological effects of nanomaterials. Int. J. Nanotechnol. 2007;4:179. doi: 10.1504/IJNT.2007.012323. [ DOI ] [ Google Scholar ]
- 120. Das D., Nath B.C., Phukon P., Kalita A., Dolui S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surfaces B Biointerfaces. 2013;111:556–560. doi: 10.1016/j.colsurfb.2013.06.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Loganathan S., Shivakumar M.S., Karthi S., Nathan S.S., Selvam K. Metal oxide nanoparticle synthesis (ZnO-NPs) of Knoxia sumatrensis (Retz.) DC. Aqueous leaf extract and It’s evaluation of their antioxidant, anti-proliferative and larvicidal activities. Toxicol. Rep. 2020;8:64–72. doi: 10.1016/j.toxrep.2020.12.018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Asif N., Fatima S., Aziz N., Shehzadi , Zaki A., Fatma T. Biofabrication and characterization of cyanobacteria derived ZnO NPs for their bioactivity comparison with commercial chemically synthesized nanoparticles. Bioorg. Chem. 2021;113:104999. doi: 10.1016/j.bioorg.2021.104999. [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Rabiee N., Bagherzadeh M., Ghadiri A.M., Kiani M., Aldhaher A., Ramakrishna S., Tahriri M., Tayebi L., Webster T.J. Green Synthesis of ZnO NPs via Salvia hispanica: Evaluation of Potential Antioxidant, Antibacterial, Mammalian Cell Viability, H1N1 Influenza Virus Inhibition and Photocatalytic Activities. J. Biomed. Nanotechnol. 2020;16:456–466. doi: 10.1166/jbn.2020.2916. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Kalaimurugan D., Lalitha K., Durairaj K., Sivasankar P., Park S., Nithya K., Shivakumar M.S., Liu W.-C., Balamuralikrishnan B., Venkatesan S. Biogenic synthesis of ZnO nanoparticles mediated from Borassus flabellifer (Linn): Antioxidant, antimicrobial activity against clinical pathogens, and photocatalytic degradation activity with molecular modeling. Environ. Sci. Pollut. Res. 2022. in press . [ DOI ] [ PubMed ]
- 125. Singh M., Lee K.E., Vinayagam R., Kang S.G. Antioxidant and Antibacterial Profiling of Pomegranate-pericarp Extract Functionalized-zinc Oxide Nanocomposite. Biotechnol. Bioprocess Eng. BBE. 2021;26:728–737. doi: 10.1007/s12257-021-0211-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 126. Moghaddam A.B., Moniri M., Azizi S., Rahim R.A., Ariff A.B., Saad W.Z., Namvar F., Navaderi M., Mohamad R. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules. 2017;22:872. doi: 10.3390/molecules22060872. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Gupta J., Irfan M., Ramgir N., Muthe K.P., Debnath A.K., Ansari S., Gandhi J., Ranjith-Kumar C.T., Surjit M. Antiviral Activity of Zinc Oxide Nanoparticles and Tetrapods against the Hepatitis E and Hepatitis C Viruses. [(accessed on 25 August 2022)];Front. Microbiol. 2022 13:881595. doi: 10.3389/fmicb.2022.881595. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2022.881595 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Briknarová K., Thomas C.J., York J., Nunberg J.H. Structure of a Zinc-binding Domain in the Junín Virus Envelope Glycoprotein. J. Biol. Chem. 2011;286:1528–1536. doi: 10.1074/jbc.M110.166025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 129. Byk L.A., Iglesias N.G., De Maio F.A., Gebhard L.G., Rossi M., Gamarnik A.V. Dengue Virus Genome Uncoating Requires Ubiquitination. mBio. 2016;7:e00804-16. doi: 10.1128/mBio.00804-16. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 130. Te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., Van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Erk I., Huet J.-C., Duarte M., Duquerroy S., Rey F., Cohen J., Lepault J. A Zinc Ion Controls Assembly and Stability of the Major Capsid Protein of Rotavirus. J. Virol. 2003;77:3595–3601. doi: 10.1128/JVI.77.6.3595-3601.2003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Turpin J.A., Terpening S.J., Schaeffer C.A., Yu G., Glover C.J., Felsted R.L., Sausville E.A., Rice W.G. Inhibitors of human immunodeficiency virus type 1 zinc fingers prevent normal processing of gag precursors and result in the release of noninfectious virus particles. J. Virol. 1996;70:6180–6189. doi: 10.1128/jvi.70.9.6180-6189.1996. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 133. Brieger A., Rink L., Haase H. Differential Regulation of TLR-Dependent MyD88 and TRIF Signaling Pathways by Free Zinc Ions. J. Immunol. 2013;191:1808–1817. doi: 10.4049/jimmunol.1301261. [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Haase H., Ober-Blöbaum J.L., Engelhardt G., Hebel S., Heit A., Heine H., Rink L. Zinc Signals Are Essential for Lipopolysaccharide-Induced Signal Transduction in Monocytes. J. Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [ DOI ] [ PubMed ] [ Google Scholar ]
- 135. Lishchynskyi O., Shymborska Y., Stetsyshyn Y., Raczkowska J., Skirtach A.G., Peretiatko T., Budkowski A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022;446:137048. doi: 10.1016/j.cej.2022.137048. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Jana B., Chatterjee A., Roy D., Ghorai S., Pan D., Pramanik S.K., Chakraborty N., Ganguly J. Chitosan/benzyloxy-benzaldehyde modified ZnO nano template having optimized and distinct antiviral potency to human cytomegalovirus. Carbohydr. Polym. 2021;278:118965. doi: 10.1016/j.carbpol.2021.118965. [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. El-Megharbel S., Alsawat M., Al-Salmi F., Hamza R. Utilizing of (Zinc Oxide Nano-Spray) for Disinfection against “SARS-CoV-2” and Testing Its Biological Effectiveness on Some Biochemical Parameters during (COVID-19 Pandemic)—”ZnO Nanoparticles Have Antiviral Activity against (SARS-CoV-2)”. Coatings. 2021;11:388. doi: 10.3390/coatings11040388. [ DOI ] [ Google Scholar ]
- 139. Kumar R., Sahoo G., Pandey K., Nayak M., Topno R., Rabidas V., Das P. Virostatic potential of zinc oxide (ZnO) nanoparticles on capsid protein of cytoplasmic side of chikungunya virus. Int. J. Infect. Dis. 2018;73:368. doi: 10.1016/j.ijid.2018.04.4247. [ DOI ] [ Google Scholar ]
- 140. Hamza R.Z., Al-Salm F.A., El-Shen N.S. Nanoparticles Effects on Zinc Oxide/green Tea Complex on the Lipid Profile and Liver Functions of Rats after Monosodium Glutamate Treatment. J. Appl. Sci. 2018;18:65–70. doi: 10.3923/jas.2018.65.70. [ DOI ] [ Google Scholar ]
- 141. Hamza R.Z., El-Shenawy N.S. The Interaction of Zinc Oxide/Green Tea Extract Complex Nanoparticles and its Effect on Monosodium Glutamate Toxicity in Liver of Rats. Curr. Pharm. Biotechnol. 2019;20:465–475. doi: 10.2174/1389201020666190408120532. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. El-Shenawy N.S., Hamza R.Z., Al-Salmi F.A., Al-Eisa R.A. Evaluation of the Effect of Nanoparticles Zinc Oxide/Camellia sinensis Complex on the Kidney of Rats Treated with Monosodium Glutamate: Antioxidant and Histological Approaches. Curr. Pharm. Biotechnol. 2019;20:542–550. doi: 10.2174/1389201020666190522075928. [ DOI ] [ PubMed ] [ Google Scholar ]
- 143. El-Shenawy N.S., Hamza R.Z., Al-Salmi F.A. Cardioprotective Effect of Zinc Oxide Nanoparticles/Green Tea Extract Complex on Monosodium Glutamate Toxicity. J. Biol. Eng. Med. 2019;2019:1–5. doi: 10.31487/j.JBEM.2019.01.03. [ DOI ] [ Google Scholar ]
- 144. Li X., Xing Y., Jiang Y., Ding Y., Li W. Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens. Int. J. Food Sci. Technol. 2009;44:2161–2168. doi: 10.1111/j.1365-2621.2009.02055.x. [ DOI ] [ Google Scholar ]
- 145. Sawai J., Kawada E., Kanou F., Igarashi H., Hashimoto A., Kokugan T., Shimizu M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996;29:627–633. doi: 10.1252/jcej.29.627. [ DOI ] [ Google Scholar ]
- 146. Khan Y.A., Singh B.R., Ullah R., Shoeb M., Naqvi A.H., Abidi S.M.A. Anthelmintic Effect of Biocompatible Zinc Oxide Nanoparticles (ZnO NPs) on Gigantocotyle explanatum, a Neglected Parasite of Indian Water Buffalo. PLoS ONE. 2015;10:e0133086. doi: 10.1371/journal.pone.0133086. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 147. Kumar A., Pandey A.K., Singh S.S., Shanker R., Dhawan A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011;51:1872–1881. doi: 10.1016/j.freeradbiomed.2011.08.025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Ijaz I., Gilani E., Nazir A., Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020;13:59–81. doi: 10.1080/17518253.2020.1802517. [ DOI ] [ Google Scholar ]
- 149. Rashid M.I., Shahzad T., Shahid M., Ismail I.M., Shah G.M., Almeelbi T. Zinc oxide nanoparticles affect carbon and nitrogen mineralization of Phoenix dactylifera leaf litter in a sandy soil. J. Hazard. Mater. 2017;324:298–305. doi: 10.1016/j.jhazmat.2016.10.063. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Mintcheva N., Aljulaih A.A., Wunderlich W., Kulinich S.A., Iwamori S. Laser-Ablated ZnO Nanoparticles and Their Photocatalytic Activity toward Organic Pollutants. Materials. 2018;11:1127. doi: 10.3390/ma11071127. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 151. Sergievskaya A., Chauvin A., Konstantinidis S. Sputtering onto liquids: A critical review. Beilstein J. Nanotechnol. 2022;13:10–53. doi: 10.3762/bjnano.13.2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 152. Rane A.V., Kanny K., Abitha V.K., Thomas S. Synthesis of Inorganic Nanomaterials. Woodhead Publishing; Cambridge, UK: 2018. Chapter 5—Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites; pp. 121–139. [ DOI ] [ Google Scholar ]
- 153. Islam F., Shohag S., Uddin J., Islam R., Nafady M.H., Akter A., Mitra S., Roy A., Bin Emran T., Cavalu S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials. 2022;15:2160. doi: 10.3390/ma15062160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 154. Navas D., Fuentes S., Castro-Alvarez A., Chavez-Angel E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels. 2021;7:275. doi: 10.3390/gels7040275. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 155. Khan M.F., Ansari A.H., Hameedullah M., Ahmad E., Husain F.M., Zia Q., Baig U., Zaheer M.R., Alam M.M., Khan A.M., et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016;6:27689. doi: 10.1038/srep27689. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 156. Bekele B., Degefa A., Tesgera F., Jule L.T., Shanmugam R., Dwarampudi L.P., Nagaprasad N., Ramasamy K. Green versus Chemical Precipitation Methods of Preparing Zinc Oxide Nanoparticles and Investigation of Antimicrobial Properties. J. Nanomater. 2021;2021:e9210817. doi: 10.1155/2021/9210817. [ DOI ] [ Google Scholar ]
- 157. Gersten B. Solvothermal Synthesis of Nanoparticles—PDF Free Download. 2005. [(accessed on 10 August 2022)]. Available online: https://docplayer.net/35339574-Solvothermal-synthesis-of-nanoparticles.html .
- 158. Singh T.A., Sharma A., Tejwan N., Ghosh N., Das J., Sil P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021;295:102495. doi: 10.1016/j.cis.2021.102495. [ DOI ] [ PubMed ] [ Google Scholar ]
- 159. Iskandar F. Nanoparticle processing for optical applications—A review. Adv. Powder Technol. 2009;20:283–292. doi: 10.1016/j.apt.2009.07.001. [ DOI ] [ Google Scholar ]
- 160. Uhm Y., Han B., Lee M., Hong S., Rhee C. Synthesis and characterization of nanoparticles of ZnO by levitational gas condensation. Mater. Sci. Eng. A. 2007;449–451:813–816. doi: 10.1016/j.msea.2006.02.427. [ DOI ] [ Google Scholar ]
- 161. Anastas P.T., Warner J.C. Green Chemistry: Theory and Practice. Oxford University Press; Oxford, UK: 1998. [(accessed on 12 August 2022)]. Available online: http://catdir.loc.gov/catdir/enhancements/fy0635/98036292-t.html . [ Google Scholar ]
- 162. Bahrulolum H., Nooraei S., Javanshir N., Tarrahimofrad H., Mirbagheri V.S., Easton A.J., Ahmadian G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021;19:86. doi: 10.1186/s12951-021-00834-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Rónavári A., Igaz N., Adamecz D.I., Szerencsés B., Molnar C., Kónya Z., Pfeiffer I., Kiricsi M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules. 2021;26:844. doi: 10.3390/molecules26040844. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 164. Madan H., Sharma S., Udayabhanu , Suresh D., Vidya Y., Nagabhushana H., Rajanaik H., Anantharaju K., Prashantha S., Maiya P.S. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016;152:404–416. doi: 10.1016/j.saa.2015.07.067. [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Rajeshkumar S., Bharath L. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Interact. 2017;273:219–227. doi: 10.1016/j.cbi.2017.06.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 166. Küünal S., Rauwel P., Rauwel E. Plant extract mediated synthesisof nanoparticles. In: Barhoum A., Makhlouf A.S.H., editors. Emerging Applications of Nanoparticles and Architecture Nanostructures. 1st ed. Elsevier; Cambridge, MA, USA: 2018. pp. 411–446. [ DOI ] [ Google Scholar ]
- 167. Augustine R., Hasan A. Phytonanotechnology. Elsevier; Amsterdam, The Netherlands: 2020. Multimodal applications of phytonanoparticles; pp. 195–219. [ DOI ] [ Google Scholar ]
- 168. Umar H., Kavaz D., Rizaner N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2018;14:87–100. doi: 10.2147/IJN.S186888. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 169. Prashanth G.K., Prashanth P.A., Nagabhushana B.M., Ananda S., Krishnaiah G.M., Nagendra H.G., Sathyananda H.M., Singh C.R., Yogisha S., Tejabhiram Y. Comparison of anticancer activity of biocompatible ZnO nanoparticles prepared by solution combustion synthesis using aqueous leaf extracts of Abutilon indicum, Melia azedarach and Indigofera tinctoria as biofuels. Artif. Cells Nanomed. Biotechnol. 2017;46:968–979. doi: 10.1080/21691401.2017.1351982. [ DOI ] [ PubMed ] [ Google Scholar ]
- 170. Chandra H., Patel D., Kumari P., Jangwan J., Yadav S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C. 2019;102:212–220. doi: 10.1016/j.msec.2019.04.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 171. Hassan S.S., Abdel-Shafy H.I., Mansour M.S. Removal of pharmaceutical compounds from urine via chemical coagulation by green synthesized ZnO-nanoparticles followed by microfiltration for safe reuse. Arab. J. Chem. 2019;12:4074–4083. doi: 10.1016/j.arabjc.2016.04.009. [ DOI ] [ Google Scholar ]
- 172. Naseer M., Aslam U., Khalid B., Chen B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020;10:9055. doi: 10.1038/s41598-020-65949-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 173. Salem M.S.E.-D., Mahfouz A.Y., Fathy R.M. The antibacterial and antihemolytic activities assessment of zinc oxide nanoparticles synthesized using plant extracts and gamma irradiation against the uro-pathogenic multidrug resistant Proteus vulgaris. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2020;34:175–196. doi: 10.1007/s10534-020-00271-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 174. Sana S.S., Kumbhakar D.V., Pasha A., Pawar S.C., Grace A.N., Singh R.P., Nguyen V.-H., Van Le Q., Peng W. Crotalaria verrucosa Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles: Assessment of Antimicrobial and Anticancer Activity. Molecules. 2020;25:4896. doi: 10.3390/molecules25214896. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 175. Patil B.N., Taranath T. Limonia acidissima L. leaf mediated synthesis of silver and zinc oxide nanoparticles and their antibacterial activities. Microb. Pathog. 2018;115:227–232. doi: 10.1016/j.micpath.2017.12.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 176. Rad S.S., Sani A.M., Mohseni S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.) Microb. Pathog. 2019;131:239–245. doi: 10.1016/j.micpath.2019.04.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 177. Jayappa M.D., Ramaiah C.K., Kumar M.A.P., Suresh D., Prabhu A., Devasya R.P., Sheikh S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020;10:3057–3074. doi: 10.1007/s13204-020-01382-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 178. Faisal S., Jan H., Shah S.A., Shah S., Khan A., Akbar M.T., Rizwan M., Jan F., Wajidullah , Akhtar N., et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega. 2021;6:9709–9722. doi: 10.1021/acsomega.1c00310. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 179. Al-Mohaimeed A.M., Al-Onazi W.A., El-Tohamy M.F. Multifunctional Eco-Friendly Synthesis of ZnO Nanoparticles in Biomedical Applications. Molecules. 2022;27:579. doi: 10.3390/molecules27020579. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 180. Raja A., Ashokkumar S., Marthandam R.P., Jayachandiran J., Khatiwada C.P., Kaviyarasu K., Raman R.G., Swaminathan M. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018;181:53–58. doi: 10.1016/j.jphotobiol.2018.02.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 181. Kundu D., Hazra C., Chatterjee A., Chaudhari A., Mishra S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: Multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J. Photochem. Photobiol. B Biol. 2014;140:194–204. doi: 10.1016/j.jphotobiol.2014.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 182. Tripathi R., Bhadwal A.S., Gupta R.K., Singh P., Shrivastav A., Shrivastav B. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014;141:288–295. doi: 10.1016/j.jphotobiol.2014.10.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 183. Singh B.N., Rawat A.K.S., Khan W., Naqvi A.H., Singh B.R. Biosynthesis of Stable Antioxidant ZnO Nanoparticles by Pseudomonas aeruginosa Rhamnolipids. PLoS ONE. 2014;9:e106937. doi: 10.1371/journal.pone.0106937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 184. Abdo A.M., Fouda A., Eid A.M., Fahmy N.M., Elsayed A.M., Khalil A.M.A., Alzahrani O.M., Ahmed A.F., Soliman A.M. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and Their Activity against Pathogenic Microbes and Common House Mosquito, Culex pipiens. Materials. 2021;14:6983. doi: 10.3390/ma14226983. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 185. Jayaseelan C., Rahuman A.A., Kirthi A.V., Marimuthu S., Santhoshkumar T., Bagavan A., Gaurav K., Karthik L., Rao K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;90:78–84. doi: 10.1016/j.saa.2012.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 186. Prasad K., Jha A.K. ZnO Nanoparticles: Synthesis and Adsorption Study. Nat. Sci. 2009;1:129–135. doi: 10.4236/ns.2009.12016. [ DOI ] [ Google Scholar ]
- 187. Dhandapani P., Siddarth A.S., Kamalasekaran S., Maruthamuthu S., Rajagopal G. Bio-approach: Ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr. Polym. 2014;103:448–455. doi: 10.1016/j.carbpol.2013.12.074. [ DOI ] [ PubMed ] [ Google Scholar ]
- 188. El-Belely E.F., Farag M.M.S., Said H.A., Amin A.S., Azab E., Gobouri A.A., Fouda A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials. 2021;11:95. doi: 10.3390/nano11010095. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 189. Ebadi M., Zolfaghari M.R., Aghaei S.S., Zargar M., Noghabi K.A. Desertifilum sp. EAZ03 cell extract as a novel natural source for the biosynthesis of zinc oxide nanoparticles and antibacterial, anticancer and antibiofilm characteristics of synthesized zinc oxide nanoparticles. J. Appl. Microbiol. 2021;132:221–236. doi: 10.1111/jam.15177. [ DOI ] [ PubMed ] [ Google Scholar ]
- 190. Barani M., Masoudi M., Mashreghi M., Makhdoumi A., Eshghi H. Cell-free extract assisted synthesis of ZnO nanoparticles using aquatic bacterial strains: Biological activities and toxicological evaluation. Int. J. Pharm. 2021;606:120878. doi: 10.1016/j.ijpharm.2021.120878. [ DOI ] [ PubMed ] [ Google Scholar ]
- 191. Pati R., Mehta R.K., Mohanty S., Padhi A., Sengupta M., Vaseeharan B., Goswami C., Sonawane A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed. Nanotechnol. Biol. Med. 2014;10:1195–1208. doi: 10.1016/j.nano.2014.02.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 192. Shamsuzzaman , Mashrai A., Khanam H., Aljawfi R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017;10:S1530–S1536. doi: 10.1016/j.arabjc.2013.05.004. [ DOI ] [ Google Scholar ]
- 193. Pavani K.V., Kumar N.S., Sangameswaran B.B. Synthesis of lead nanoparticles by Aspergillus species. Pol. J. Microbiol. 2012;61:61–63. doi: 10.33073/pjm-2012-008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 194. Kalpana V., Kataru B.A.S., Sravani N., Vigneshwari T., Panneerselvam A., Rajeswari V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano. 2018;3:48–55. doi: 10.1016/j.onano.2018.06.001. [ DOI ] [ Google Scholar ]
- 195. Raliya R., Tarafdar J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.) Agric. Res. 2013;2:48–57. doi: 10.1007/s40003-012-0049-z. [ DOI ] [ Google Scholar ]
- 196. Pavani K.V., Balakrishna K., Cheemarla N.K. Biosynthesis of zinc nanoparticles by Aspergillus species. Int. J. Nanotechnol. Appl. 2011;5:27–36. [ Google Scholar ]
- 197. Sumanth B., Lakshmeesha T.R., Ansari M.A., Alzohairy M.A., Udayashankar A.C., Shobha B., Niranjana S.R., Srinivas C., Almatroudi A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020;15:8519–8536. doi: 10.2147/IJN.S271743. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 198. Gray D.J.P. Butterworths Medical Dictionary, 2nd ed. J. R. Coll. Gen. Pract. 1978;28:762. [ Google Scholar ]
- 199. Azizi S., Ahmad M.B., Namvar F., Mohamad R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014;116:275–277. doi: 10.1016/j.matlet.2013.11.038. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 200. Nagarajan S., Kuppusamy K.A. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J. Nanobiotechnol. 2013;11:39. doi: 10.1186/1477-3155-11-39. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 201. Rao M.D., Gautam P. Synthesis and characterization of ZnO nanoflowers using Chlamydomonas reinhardtii: A green approach. Environ. Prog. Sustain. Energy. 2016;35:1020–1026. doi: 10.1002/ep.12315. [ DOI ] [ Google Scholar ]
- 202. Subramanian H., Krishnan M., Mahalingam A. Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts. RSC Adv. 2021;12:985–997. doi: 10.1039/D1RA08196A. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 203. Ishwarya R., Vaseeharan B., Kalyani S., Banumathi B., Govindarajan M., Alharbi N.S., Kadaikunnan S., Al-Anbr M.N., Khaled J.M., Benelli G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018;178:249–258. doi: 10.1016/j.jphotobiol.2017.11.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 204. Hall J.B., Dobrovolskaia M.A., Patri A.K., McNeil S.E. Characterization of nanoparticles for therapeutics. Nanomedicine. 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [ DOI ] [ PubMed ] [ Google Scholar ]
- 205. Fang S., Lee H.J., Wark A.W., Corn R.M. Attomole Microarray Detection of MicroRNAs by Nanoparticle-Amplified SPR Imaging Measurements of Surface Polyadenylation Reactions. J. Am. Chem. Soc. 2006;128:14044–14046. doi: 10.1021/ja065223p. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 206. Rajeshkumar S., Malarkodi C., Vanaja M., Annadurai G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016;1116:165–173. doi: 10.1016/j.molstruc.2016.03.044. [ DOI ] [ Google Scholar ]
- 207. Yasmin A., Ramesh K., Rajeshkumar S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014;1:12. doi: 10.1186/s40580-014-0012-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 208. Arfat Y.A., Benjakul S., Prodpran T., Sumpavapol P., Songtipya P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014;41:265–273. doi: 10.1016/j.foodhyd.2014.04.023. [ DOI ] [ Google Scholar ]
- 209. Ali A., Phull A.-R., Zia M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018;7:413–441. doi: 10.1515/ntrev-2018-0067. [ DOI ] [ Google Scholar ]
- 210. Wang H., Xie J., Yan K., Duan M. Growth Mechanism of Different Morphologies of ZnO Crystals Prepared by Hydrothermal Method. J. Mater. Sci. Technol. 2011;27:153–158. doi: 10.1016/S1005-0302(11)60041-8. [ DOI ] [ Google Scholar ]
- 211. Doustkhah E., Esmat M., Fukata N., Ide Y., Hanaor D.A., Assadi M.H.N. MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere. 2022;303:134932. doi: 10.1016/j.chemosphere.2022.134932. [ DOI ] [ PubMed ] [ Google Scholar ]
- 212. Ozgür Ü., Alivov Y.I., Liu C., Teke A., Reshchikov M.A., Doğan S., Avrutin V., Cho S.-J., Morkoç H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005;98:041301. doi: 10.1063/1.1992666. [ DOI ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (3.4 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Advertisement
Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes
- Nano Review
- Open access
- Published: 08 May 2018
- Volume 13 , article number 141 , ( 2018 )
Cite this article
You have full access to this open access article

- Khwaja Salahuddin Siddiqi 1 ,
- Aziz ur Rahman 2 ,
- Tajuddin 2 &
- Azamal Husen ORCID: orcid.org/0000-0002-9120-5540 3
82k Accesses
700 Citations
107 Altmetric
15 Mentions
Explore all metrics
Zinc oxide is an essential ingredient of many enzymes, sun screens, and ointments for pain and itch relief. Its microcrystals are very efficient light absorbers in the UVA and UVB region of spectra due to wide bandgap. Impact of zinc oxide on biological functions depends on its morphology, particle size, exposure time, concentration, pH, and biocompatibility. They are more effective against microorganisms such as Bacillus subtilis , Bacillus megaterium , Staphylococcus aureus , Sarcina lutea , Escherichia coli , Pseudomonas aeruginosa , Klebsiella pneumonia , Pseudomonas vulgaris , Candida albicans , and Aspergillus niger . Mechanism of action has been ascribed to the activation of zinc oxide nanoparticles by light, which penetrate the bacterial cell wall via diffusion. It has been confirmed from SEM and TEM images of the bacterial cells that zinc oxide nanoparticles disintegrate the cell membrane and accumulate in the cytoplasm where they interact with biomolecules causing cell apoptosis leading to cell death.
Similar content being viewed by others

Effect of Zn salts and hydrolyzing agents on the morphology and antibacterial activity of zinc oxide nanoparticles
Zinc Oxide Nanoparticles with Mangiferin: Optical Properties, In Vitro Release Studies, and Antibacterial Activity
Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism.
Avoid common mistakes on your manuscript.
Nanotechnology deals with the manufacture and application of materials with size of up to 100 nm. They are widely used in a number of processes that include material science, agriculture, food industry, cosmetic, medical, and diagnostic applications [ 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 ]. Nanosize inorganic compounds have shown remarkable antibacterial activity at very low concentration due to their high surface area to volume ratio and unique chemical and physical features [ 11 ]. In addition, these particles are also more stable at high temperature and pressure [ 12 ]. Some of them are recognized as nontoxic and even contain mineral elements which are vital for human body [ 13 ]. It has been reported that the most antibacterial inorganic materials are metallic nanoparticles and metal oxide nanoparticles such as silver, gold, copper, titanium oxide, and zinc oxide [ 14 , 15 ].
Zinc is an essential trace element for human system without which many enzymes such as carbonic anhydrase, carboxypeptidase, and alcohol dehydrogenase become inactive, while the other two members, cadmium and mercury belonging to the same group of elements having the same electronic configuration, are toxic. It is essential for eukaryotes because it modulates many physiological functions [ 16 , 17 ]. Bamboo salt, containing zinc, is used as herbal medicine for the treatment of inflammation by regulating caspase-1 activity. Zinc oxide nanoparticles have been shown to reduce mRNA expression of inflammatory cytokines by inhibiting the activation of NF-kB (nuclear factor kappa B cells) [ 18 ].
Globally, bacterial infections are recognized as serious health issue. New bacterial mutation, antibiotic resistance, outbreaks of pathogenic strains, etc. are increasing, and thus, development of more efficient antibacterial agents is demand of the time. Zinc oxide is known for its antibacterial properties from the time immemorial [ 19 ]. It had been in use during the regime of Pharaohs, and historical records show that zinc oxide was used in many ointments for the treatment of injuries and boils even in 2000 BC [ 20 ]. It is still used in sun screen lotion, as a supplement, photoconductive material, LED, transparent transistors, solar cells, memory devices [ 21 , 22 ], cosmetics [ 23 , 24 ], and catalysis [ 25 ]. Although considerable amount of ZnO is produced every year, very small quantity is used as medicine [ 26 ]. The US Food and Drug Administration has recognized (21 CFR 182.8991) zinc oxide as safe [ 27 ]. It is characterized by photocatalytic and photooxidizing properties against biochemicals [ 28 ].
Zinc oxide has been classified by EU hazard classification as N; R50-53 (ecotoxic). Compounds of zinc are ecotoxic for mammals and plants in traces [ 29 , 30 ]. Human body contains about 2–3 g of zinc, and the daily requirement is 10–15 mg [ 29 , 31 ]. No report has demonstrated carcinogenicity, genotoxicity, and reproduction toxicity in humans [ 29 , 32 ]. However, zinc powder inhaled or ingested may produce a condition called zinc fever, which is followed by chill, fever, cough, etc.
Morphology of zinc oxide nanoparticles depends on the process of synthesis. They may be nanorods, nanoplates [ 33 , 34 , 35 ], nanospheres [ 36 ], nanoboxes [ 35 ], hexagonal, tripods [ 37 ], tetrapods [ 38 ], nanowires, nanotubes, nanorings [ 39 , 40 , 41 ], nanocages, and nanoflowers [ 42 , 43 ]. Zinc oxide nanoparticles are more active against gram-positive bacteria relative to other NPs of the same group of elements. Ready to eat food is more prone to infection by Salmonella , Staphylococcus aureus , and E. coli which pose a great challenge to food safety and quality. The antimicrobial compounds are incorporated in the packed food to prevent them from damage. Antimicrobial packaging contains a nontoxic material which inhibits or slows down the growth of microbes present in food or packaging material [ 44 ]. An antimicrobial substance for human consumption must possess the following properties.
It should be nontoxic.
It should not react with food or container.
It should be of good taste or tasteless.
It should not have disagreeable smell.
Zinc oxide nanoparticle is one such inorganic metal oxide which fulfills all the above requirements, and hence, it can safely be used as medicine, preservative in packaging, and an antimicrobial agent [ 45 , 46 ]. It easily diffuses into the food material, kill the microbes, and prevent human being from falling ill. In accordance with the regulations 1935/2004/EC and 450/2009/EC of the European Union, active packaging is defined as active material in contact with food with ability to change the composition of the food or the atmosphere around it [ 47 ]. Therefore, it is commonly used as preservative and incorporated in polymeric packaging material to prevent food material from damage by microbes [ 48 ]. Zinc oxide nanoparticles have been used as an antibacterial substance against Salmonella typhi and S. aureus in vitro. Of all the metal oxide nanoparticles studied thus far, zinc oxide nanoparticles exhibited the highest toxicity against microorganisms [ 49 ]. It has also been demonstrated from SEM and TEM images that zinc oxide nanoparticles first damage the bacterial cell wall, then penetrate, and finally accumulate in the cell membrane. They interfere with metabolic functions of the microbes causing their death. All the characteristics of the zinc oxide nanoparticles depend on their particle size, shape, concentration, and exposure time to the bacterial cell. Further, biodistribution studies of zinc oxide nanoparticles have also been examined. For instance, Wang et al. [ 50 ] have investigated the effect of long-term exposure of zinc oxide nanoparticle on biodistribution and zinc metabolism in mice over 3 to 35 weeks. Their results showed minimum toxicity to mice when they were exposed to 50 and 500 mg/kg zinc oxide nanoparticle in diet. At higher dose of 5000 mg/kg, zinc oxide nanoparticle decreased body weight but increased the weight of the pancreas, brain, and lung. Also, it increased the serum glutamic-pyruvic transaminase activity and mRNA expression of zinc metabolism-related genes such as metallothionein. Biodistribution studies showed the accumulation of sufficient quantity of zinc in the liver, pancreas, kidney, and bones. Absorption and distribution of zinc oxide nanoparticle/zinc oxide microparticles are largely dependent on the particle size. Li et al. [ 51 ] have studied biodistribution of zinc oxide nanoparticles fed orally or through intraperitoneal injection to 6 weeks old mice. No obvious adverse effect was detected in zinc oxide nanoparticles orally treated mice in 14 days study. However, intraperitoneal injection of 2.5 g/kg body weight given to mice showed accumulation of zinc in the heart, liver, spleen, lung, kidney, and testes. Nearly ninefold increase in zinc oxide nanoparticle in the liver was observed after 72 h. Zinc oxide nanoparticles have been shown to have better efficiency in liver, spleen, and kidney biodistribution than in orally fed mice. Since zinc oxide nanoparticles are innocuous in low concentrations, they stimulate certain enzymes in man and plants and suppress diseases. Singh et al. [ 52 ] have also been recently reviewed the biosynthesis of zinc oxide nanoparticle, their uptake, translocation, and biotransformation in plant system.
In this review, we have attempted to consolidate all the information regarding zinc oxide nanoparticles as antibacterial agent. The mechanism of interaction of zinc oxide nanoparticles against a variety of microbes has also been discussed in detail.
Antimicrobial Activity of Zinc Oxide Nanoparticles
It is universally known that zinc oxide nanoparticles are antibacterial and inhibit the growth of microorganisms by permeating into the cell membrane. The oxidative stress damages lipids, carbohydrates, proteins, and DNA [ 53 ]. Lipid peroxidation is obviously the most crucial that leads to alteration in cell membrane which eventually disrupt vital cellular functions [ 54 ]. It has been supported by oxidative stress mechanism involving zinc oxide nanoparticle in Escherichia coli [ 55 ]. However, for bulk zinc oxide suspension, external generation of H 2 O 2 has been suggested to describe the anti-bacterial properties [ 56 ]. Also, the toxicity of nanoparticles, releasing toxic ions, has been considered. Since zinc oxide is amphoteric in nature, it reacts with both acids and alkalis giving Zn 2+ ions.
The free Zn 2+ ions immediately bind with the biomolecules such as proteins and carbohydrates, and all vital functions of bacteria cease to continue. The toxicity of zinc oxide, zinc nanoparticles, and ZnSO 4 ·7H 2 O has been tested (Table 1 ) against Vibrio fischeri . It was found that ZnSO 4 ·7H 2 O is six times more toxic than zinc oxide nanoparticles and zinc oxide. The nanoparticles are actually dispersed in the solvent, not dissolved, and therefore, they cannot release Zn 2+ ions. The bioavailability of Zn 2+ ions is not always 100% and may invariably change with physiological pH, redox potential, and the anions associated with it such as Cl − or SO 4 2− .
Solubility of zinc oxide (1.6–5.0 mg/L) in aqueous medium is higher than that of zinc oxide nanoparticles (0.3–3.6 mg/L) in the same medium [ 57 ] which is toxic to algae and crustaceans. Both nano-zinc oxide and bulk zinc oxide are 40–80-fold less toxic than ZnSO 4 against V. fischeri . The higher antibacterial activity of ZnSO 4 is directly proportional to its solubility releasing Zn 2+ ions, which has higher mobility and greater affinity [ 58 ] toward biomolecules in the bacterial cell due to positive charge on the Zn 2+ and negative charge on the biomolecules.
Since zinc oxide and its nanoparticles have limited solubility, they are less toxic to the microbes than highly soluble ZnSO 4 ·7H 2 O. However, it is not essential for metal oxide nanoparticles to enter the bacterial cell to cause toxicity [ 59 ]. Contact between nanoparticles and the cell wall is sufficient to cause toxicity. If it is correct, then large amounts of metal nanoparticles are required so that the bacterial cells are completely enveloped and shielded from its environment leaving no chance for nutrition to be absorbed to continue life process. Since nanoparticles and metal ions are smaller than the bacterial cells, it is more likely that they disrupt the cell membrane and inhibit their growth.
A number of nanosized metal oxides such as ZnO, CuO, Al 2 O 3 , La 2 O 3 , Fe 2 O 3 , SnO 2 , and TiO 2 have been shown to exhibit the highest toxicity against E. coli [ 49 ]. Zinc oxide nanoparticles are externally used for the treatment of mild bacterial infections, but the zinc ion is an essential trace element for some viruses and human beings which increase enzymatic activity of viral integrase [ 45 , 60 , 61 ]. It has also been supported by an increase in the infectious pancreatic necrosis virus by 69.6% when treated with 10 mg/L of Zn [ 46 ]. It may be due to greater solubility of Zn ions relative to ZnO alone. The SEM and TEM images have shown that zinc oxide nanoparticles damage the bacterial cell wall [ 55 , 62 ] and increase permeability followed by their accumulation in E. coli preventing their multiplication [ 63 ].
In the recent past, antibacterial activity of zinc oxide nanoparticle has been investigated against four known gram-positive and gram-negative bacteria, namely Staphylococcus aureus , E. coli , Salmonella typhimurium , and Klebsiella pneumoniae . It was observed that the growth-inhibiting dose of the zinc oxide nanoparticles was 15 μg/ml, although in the case of K. pneumoniae , it was as low as 5 μg/ml [ 63 , 64 ]. It has been noticed that with increasing concentration of nanoparticles, growth inhibition of microbes increases. When they were incubated over a period of 4–5 h with a maximum concentration of zinc oxide nanoparticles of 45 μg/ml, the growth was strongly inhibited. It is expected that if the incubation time is increased, the growth inhibition would also increase without much alteration in the mechanism of action [ 63 ].
It has been reported that the metal oxide nanoparticles first damage the bacterial cell membrane and then permeate into it [ 64 ]. It has also been proposed that the release of H 2 O 2 may be an alternative to anti-bacterial activity [ 65 ]. This proposal, however, requires experimental proof because the mere presence of zinc oxide nanoparticle is not enough to produce H 2 O 2 . Zinc nanoparticles or zinc oxide nanoparticles of extremely low concentration cannot cause toxicity in human system. Daily intake of zinc via food is needed to carry out the regular metabolic functions. Zinc oxide is known to protect the stomach and intestinal tract from damage by E. coli [ 65 ]. The pH in the stomach varies between 2 to 5, and hence, zinc oxide in the stomach can react with acid to produce Zn 2+ ions. They can help in activating the enzyme carboxy peptidase, carbonic anhydrase, and alcohol dehydrogenase which help in the digestion of carbohydrate and alcohol. Premanathan et al. [ 66 ] have reported the toxicity of zinc oxide nanoparticles against prokaryotic and eukaryotic cells. The MIC of zinc oxide nanoparticles against E. coli , Pseudomonas aeruginosa , and S. aureus were found to be 500 and 125 μg/ml, respectively. Two mechanisms of action have been proposed for the toxicity of zinc oxide nanoparticles, namely (1) generation of ROS and (2) induction of apoptosis. Metal oxide nanoparticles induce ROS production and put the cells under oxidative stress causing damage to cellular components, i.e., lipids, proteins, and DNA [ 67 , 68 , 69 ]. Zinc oxide nanoparticles, therefore, induce toxicity through apoptosis. They are relatively more toxic to cancer cells than normal cells, although they cannot distinguish between them.
Recently, Pati et al. [ 70 ] have shown that zinc oxide nanoparticles disrupt bacterial cell membrane integrity, reduce cell surface hydrophobicity, and downregulate the transcription of oxidative stress-resistance genes in bacteria. They enhance intracellular bacterial killing by inducing ROS production. These nanoparticles disrupt biofilm formation and inhibit hemolysis by hemolysin toxin produced by pathogens. Intradermal administration of zinc oxide nanoparticles was found to significantly reduce the skin infection and inflammation in mice and also improved infected skin architecture.
Solubility and Concentration-Dependent Activity of Zinc Oxide Nanoparticle
Nanoparticles have also been used as a carrier to deliver therapeutic agents to treat bacterial infection [ 1 , 9 ]. Since zinc oxide nanoparticles up to a concentration of 100 μg/ml are harmless to normal body cells, they can be used as an alternative to antibiotics. It was found that 90% bacterial colonies perished after exposing them to a dose of 500–1000 μg/ml of zinc oxide nanoparticles only for 6 h. Even the drug-resistant S. aureus , Mycobacterium smegmatis , and Mycobacterium bovis when treated with zinc oxide nanoparticles in combination with a low dose of anti-tuberculosis drug, rifampicin (0.7 μg/ml), a significant reduction in their growth was observed. These pathogens were completely destroyed when incubated for 24 h with 1000 μg/ml of zinc oxide nanoparticles. It is, therefore, concluded that if the same dose is repeated, the patient with such infective diseases may be completely cured. It was also noted that the size of zinc oxide nanoparticles ranging between 50 and 500 nm have identical effect on bacterial growth inhibition.
Cytotoxicity of zinc oxide has been studied by many researchers in a variety of microbes and plant systems [ 71 , 72 , 73 , 74 ]. Toxicity of zinc oxide nanoparticles is concentration and solubility dependent. It has been shown that maximum exposure concentration of zinc oxide (125 mg/l) suspension released 6.8 mg/l of Zn 2+ ions. Toxicity is a combined effect of zinc oxide nanoparticles and Zn 2+ ions released in the aqueous medium. However, minimal effect of metal ions was detected which suggests that the bacterial growth inhibition is mainly due to interaction of zinc oxide nanoparticles with microorganisms. The cytotoxic effect of a particular metal oxide nanoparticle is species sensitive which is reflected by the growth inhibition zone for several bacteria [ 75 ].
It has been suggested that growth inhibition of bacterial cells occurs mainly by Zn 2+ ions which are produced by extracellular dissolution of zinc oxide nanoparticles [ 76 ]. Cho et al. [ 77 ] have concluded from their studies on rats that zinc oxide nanoparticles remain intact at around neutral or biological pH but rapidly dissolve under acidic conditions (pH 4.5) in the lysosome of the microbes leading to their death. This is true because in acidic condition, zinc oxide dissolves and Zn 2+ ions are produced, which bind to the biomolecules inside the bacterial cell inhibiting their growth.
The zinc oxide nanoparticles have been shown to be cytotoxic to different primary immune-competent cells. The transcriptomics analysis showed that nanoparticles had a common gene signature with upregulation of metallothionein genes ascribed to the dissolution of the nanoparticles [ 78 ]. However, it could not be ascertained if the absorbed zinc was Zn 2+ or zinc oxide or both, although smaller sized zinc oxide nanoparticles have greater concentration in the blood than larger ones (19 and > 100 nm). The efficiency of zinc oxide nanoparticles depends mainly on the medium of reaction to form Zn 2+ and their penetration into the cell.
Chiang et al. [ 79 ] have reported that dissociation of zinc oxide nanoparticles results in destruction of cellular Zn homeostasis. The characteristic properties of nanoparticles and their impact on biological functions are entirely different from those of the bulk material [ 80 ]. Aggregation of nanoparticles influences cytotoxicity of macrophages, and their concentration helps in modulation of nanoparticle aggregation. Low concentration of zinc oxide nanoparticles is ineffective, but at higher concentration (100 μg/ml), they exhibited cytotoxicity which varies from one pathogen to another.
The inadvertent use of zinc oxide nanoparticles may sometime adversely affect the living system. Their apoptosis and genotoxic potential in human liver cells and cellular toxicity has been studied. It was found that a decrease in liver cell viability occurs when they are exposed to 14–20 μg/ml of zinc oxide nanoparticles for 12 h. It also induced DNA damage by oxidative stress. Sawai et al. [ 56 ] have demonstrated that ROS generation is directly proportional to the concentration of zinc oxide powder. ROS triggered a decrease in mitochondria membrane potential leading to apoptosis [ 81 ]. Cellular uptake of nanoparticles is not mandatory for cytotoxicity to occur.
Size-Dependent Antibacterial Activity of Zinc Oxide Nanoparticles
In a study, Azam et al. [ 82 ] have reported that the antimicrobial activity against both gram-negative ( E. coli and P. aeruginosa ) and gram-positive ( S. and Bacillus subtilis ) bacteria increased with increase in surface-to-volume ratio due to a decrease in particle size of zinc oxide nanoparticles. Moreover, in this investigation, zinc oxide nanoparticles have shown maximum (25 mm) bacterial growth inhibition against B. subtilis (Fig. 1 ).
Antibacterial activity and/or zone of inhibition produced by zinc oxide nanoparticles against gram-positive and gram-negative bacterial strains namely a Escherichia coli , b Staphylococcus aureus , c Pseudomonas aeruginosa , and d Bacillus subtilis [ 82 ]
It has been reported that the smaller size of zinc oxide nanoparticles exhibits greater antibacterial activity than microscale particles [ 83 ]. For instance, Au 55 nanoparticles of 1.4-nm size have been demonstrated to interact with the major grooves of DNA which accounts for its toxicity [ 84 ]. Although contradictory results have been reported, many workers showed positive effect of zinc oxide nanoparticles on bacterial cells. However, Brayner et al. [ 63 ] from TEM images have shown that zinc oxide nanoparticle of 10–14 nm were internalized (when exposed to microbes) and damaged the bacterial cell membrane. It is also essential that the zinc/zinc oxide nanoparticles must not be toxic to human being since they are toxic to T cells above 5 mM [ 85 ] and to neuroblastoma cells above 1.2 mM [ 86 ]. Nair et al. [ 87 ] have exclusively explored the size effect of zinc oxide nanoparticles on bacterial and human cell toxicity. They have studied the influence of zinc oxide nanoparticles on both gram-positive and gram-negative bacteria and osteoblast cancer cell lines (MG-63).
It is known that antibacterial activity of zinc oxide nanoparticle is inversely proportional to their size and directly proportional to their concentration [ 88 ]. It has also been noticed that it does not require UV light for activation; it functions under normal or even diffused sunlight. Cytotoxic activity perhaps involves both the production of ROS and accumulation of nanoparticles in the cytoplasm or on the outer cell membrane. However, the production of H 2 O 2 and its involvement in the activation of nanoparticles cannot be ignored. Raghupathi et al. [ 88 ] have synthesized zinc oxide nanoparticles from different zinc salts and observed that nanoparticles obtained from Zn(NO 3 ) 2 were smallest in size (12 nm) and largest in surface area (90.4). Authors have shown that the growth inhibition of S. aureus at a concentration of 6 mM of zinc oxide nanoparticles is size dependent. It has also been indicated from the viable cell determination during the exposure of bacterial cells to zinc oxide nanoparticles that the number of cells recovered decreased significantly with decrease in size of zinc oxide nanoparticles. Jones et al. [ 89 ] have shown that zinc oxide nanoparticles of 8-nm diameter inhibited the growth of S. aureus , E. coli , and B. subtilis. Zinc oxide nanoparticles ranging between 12 and 307 nm were selected and confirmed the relationship between antibacterial activity and their size. Their toxicity to microbes has been ascribed to the formation of Zn 2+ ions from zinc oxide when it is suspended in water and also to some extent to a slight change in pH. Since Zn 2+ ions are scarcely released from zinc oxide nanoparticles, the antibacterial activity is mainly owing to smaller zinc oxide nanoparticles. When the size is 12 nm, it inhibits the growth of S. aureus , but when the size exceeds 100 nm, the inhibitory effect is minimal [ 89 ].
Shape, Composition, and Cytotoxicity of Zinc Oxide Nanoparticles
Zinc oxide nanoparticles have shown cytotoxicity in concentration-dependent manner and type of cells exposed due to different sensitivity [ 90 , 91 ]. Sahu et al. [ 90 ] have highlighted the difference of cytotoxicity between particle size and different sensitivity of cells toward the particles of the same composition. In another recent study, Ng et al. [ 91 ] examined the concentration-dependent cytotoxicity in human lung MRC5 cells. Authors have reported the uptake and internalization of zinc oxide nanoparticles into the human lung MRC5 cells by using TEM investigation. These particles were noticed in the cytoplasm of the cells in the form of electron dense clusters, which are further observed to be enclosed by vesicles, while zinc oxide nanoparticles were not found in untreated control cells. Papavlassopoulos et al. [ 92 ] have synthesized zinc oxide nanoparticle tetrapods by entirely a novel route known as “Flame transport synthesis approach”. Tetrapods have different morphology compared to the conventionally synthesized zinc oxide nanoparticles. Their interaction with mammalian fibroblast cells in vitro has indicated that their toxicity is significantly lower than those of the spherical zinc oxide nanoparticles. Tetrapods exhibited hexagonal wurtzite crystal structure with alternating Zn 2+ and O 2− ions with three-dimensional geometry. They block the entry of viruses into living cells which is further enhanced by precisely illuminating them with UV radiation. Since zinc oxide tetrapods have oxygen vacancies in their structure, the Herpes simplex viruses are attached via heparan sulfate and denied entry into body cells. Thus, they prevent HSV-1 and HSV-2 infection in vitro. Zinc oxide tetrapods may therefore be used as prophylactic agent against these viral infections. The cytotoxicity of zinc oxide nanoparticles also depends on the proliferation rate of mammalian cells [ 66 , 93 ]. The surface reactivity and toxicity may also be varied by controlling the oxygen vacancy in zinc oxide tetrapods. When they are exposed to UV light, the oxygen vacancy in tetrapods is readily increased. Alternatively, the oxygen vacancy can be decreased by heating them in oxygen-rich environment. Thus, it is the unique property of zinc oxide tetrapods that can be changed at will which consequently alter their antimicrobial efficiency.
Animal studies have indicated an increase in pulmonary inflammation, oxidative stress, etc. on respiratory exposure to nanoparticles [ 94 ]. Yang et al. [ 95 ] have investigated the cytotoxicity, genotoxicity, and oxidative stress of zinc oxide nanoparticles on primary mouse embryo fibroblast cells. It was observed that zinc oxide nanoparticles induced significantly greater cytotoxicity than that induced by carbon and SiO 2 nanoparticles. It was further confirmed by measuring glutathione depletion, malondialdehyde production, superoxide dismutase inhibition, and ROS generation. The potential cytotoxic effects of different nanoparticles have been attributed to their shape.
Polymer-Coated Nanoparticles
Many bacterial infections are transmitted by contact with door knobs, key boards, water taps, bath tubs, and telephones; therefore, it is essential to develop and coat such surfaces with inexpensive advanced antibacterial substances so that their growth is inhibited. It is important to use such concentrations of antibacterial substances that they may kill the pathogens but spare the human beings. It may happen only if they are coated with a biocompatible hydrophilic polymer of low cost. Schwartz et al. [ 96 ] have reported the preparation of a novel antimicrobial composite material hydrogel by mixing a biocompatible poly ( N -isopropylacrylamide) with zinc oxide nanoparticles. The SEM image of the composite film showed uniform distribution of zinc oxide nanoparticles. It exhibited antibacterial activity against E. coli at a very low zinc oxide concentration (1.33 mM). Also, the coating was found to be nontoxic toward mammalian cell line (N1H/3T3) for a period of 1 week. Zinc oxide/hydrogel nanocomposite may safely be used as biomedical coating to prevent people from contracting bacterial infections.
Although zinc oxide nanoparticles are stable, they have been further stabilized by coating them with different polymers such as polyvinyl pyrolidone (PVP), polyvinyl alcohol ( PVA ), poly (α, γ, l -glutamic acid) (PGA), polyethylene glycol (PEG), chitosan, and dextran [ 97 , 98 ]. The antibacterial activity of engineered zinc oxide nanoparticles was examined against gram-negative and gram-positive pathogens, namely E. coli and S. aureus and compared with commercial zinc oxide powder. The polymer-coated spherical zinc oxide nanoparticles showed maximum bacterial cell destruction compared to bulk zinc oxide powder [ 99 ]. Since nanoparticles coated with polymers are less toxic due to their low solubility and sustained release, their cytotoxicity can be controlled by coating them with a suitable polymer.
Effect of Particle Size and Shape of Polymer-Coated Nanoparticles on Antibacterial Activity
E. coli and S. aureus exposed to different concentrations of poly ethylene glycol (PEG)-coated zinc oxide nanoparticles (1–7 mM) of varying size (401 nm–1.2 μm) showed that the antimicrobial activity increases with decreasing size and increasing concentration of nanoparticles. However, the effective concentration in all these cases was above 5 mM. There occurs a drastic change in cell morphology of E. coli surface which can be seen from the SEM images of bacteria before and after their exposure to zinc oxide nanoparticles [ 84 ]. It has been nicely demonstrated by Nair et al. [ 87 ] that PEG-capped zinc oxide particles and zinc oxide nanorods are toxic to human osteoblast cancer cells (MG-63) at concentration above 100 μM. The PEG starch-coated nanorods/nanoparticles do not damage the healthy cells.
In Vivo and In Vitro Antimicrobial Activity for Wound Dressing
Of all natural and synthetic wound dressing materials, the chitosan hydrogel microporous bandages laced with zinc oxide nanoparticles developed by Kumar et al. [ 100 ] are highly effective in treating burns, wounds, and diabetic foot ulcers. The nanoparticles of approximately 70–120 nm are dispersed on the surface of the bandage. The degradation products of chitosan were identified as d -glucosamine and glycosamine glycan. They are nontoxic to the cells because they are already present in our body for the healing of injury. The wound generally contains P. aeruginosa , S. intermedicus , and S. hyicus which were also identified from the swab of mice wound and successfully treated with chitosan zinc oxide bandage in about 3 weeks [ 100 ].
Effect of Doping on Toxicity of Zinc Oxide Nanoparticles
Doping of zinc oxide nanoparticles with iron reduces the toxicity. The concentration of Zn 2+ and zinc oxide nanoparticles is also an important factor for toxicity. The concentration that reduced 50% viability in microbial cells exposed to nano- and microsize zinc oxide is very close to the concentration of Zn 2+ that induced 50% reduction in viability in Zn 2+ -treated cells [ 101 , 102 ].
Coating of zinc oxide nanoparticles with mercaptopropyl trimethoxysilane or SiO 2 reduces their cytotoxicity [ 103 ]. On the contrary, Gilbert et al. [ 104 ] showed that in BEAS-2B cells, uptake of zinc oxide nanoparticles is the main mechanism of zinc accumulation. Also, they have suggested that zinc oxide nanoparticles dissolve completely generating Zn 2+ ions which are bonded to biomolecules of the target cells. However, the toxicity of zinc oxide nanoparticles depends on the uptake and their subsequent interaction with target cells.
Interaction Mechanism of Zinc Oxide Nanoparticles
Nanoparticles may be toxic to some microorganisms, but they may be essential nutrients to some of them [ 55 , 105 ]. Nanotoxicity is essentially related to the microbial cell membrane damage leading to the entry of nanoparticles into the cytoplasm and their accumulation [ 55 ]. The impact of nanoparticles on the growth of bacteria and viruses largely depends on particle size, shape, concentration, agglomeration, colloidal formulation, and pH of the media [ 106 , 107 , 108 ]. The mechanism of antimicrobial activity of zinc oxide nanoparticles has been depicted in Fig. 2 .
Mechanisms of zinc oxide nanoparticle antimicrobial activity
Zinc oxide nanoparticles are generally less toxic than silver nanoparticles in a broad range of concentrations (20 to 100 mg/l) with average particle size of 480 nm [ 55 , 62 , 63 ]. Metal oxide nanoparticles damage the cell membrane and DNA [ 63 , 109 , 110 , 111 ] of microbes via diffusion. However, the production of ROS through photocatalysis causing bacterial cell death cannot be ignored [ 112 ]. UV-Vis spectrum of zinc oxide nanoparticle suspension in aqueous medium exhibits peaks between 370 and 385 nm [ 113 ]. It has been shown that it produces ROS (hydroxyl radicals, superoxides, and hydrogen peroxide) in the presence of moisture which ostensibly react with bacterial cell material such as protein, lipids, and DNA, eventually causing apoptosis. Xie et al. [ 114 ] have examined the influence of zinc oxide nanoparticles on Campylobacter jejuni cell morphology using SEM images (Fig. 3 ). After a 12-h treatment (0.5 mg/ml), C. jejuni was found to be extremely sensitive and cells transformed from spiral shape to coccoid forms. SEM studies showed the ascendency of coccoid forms in the treated cells and display the formation of irregular cell surfaces and cell wall blebs (Fig. 3a ). Moreover, these coccoid cells remained intact and possessed sheathed polar flagella. However, SEM image of the untreated cells clearly showed spiral shapes (Fig. 3b ). In general, it has been demonstrated from SEM and TEM images of bacterial cells treated with zinc oxide nanoparticles that they get ruptured and, in many cases, the nanoparticles damage the cell wall forcing their entry into it [ 114 , 115 ].
SEM images of Campylobacter jejuni . a Untreated cells from the same growth conditions were used as a control. b C. jejuni cells in the mid-log phase of growth were treated with 0.5 mg/ml of zinc oxide nanoparticles for 12 h under microaerobic conditions [ 114 ]
Zinc oxide nanoparticles have high impact on the cell surface and may be activated when exposed to UV-Vis light to generate ROS (H 2 O 2 ) which permeate into the cell body while the negatively charged ROS species such as O 2 2− remain on the cell surface and affect their integrity [ 116 , 117 ]. Anti-bacterial activity of zinc oxide nanoparticles against many other bacteria has also been reported [ 1 , 5 , 114 , 115 ]. It has been shown from TEM images that the nanoparticles have high impact on the cell surface (Fig. 4 ).
a TEM images of untreated normal Salmonella typhimurium cells. b Effects of nanoparticles on the cells (marked with arrows). c , d Micrograph of deteriorated and ruptured S. typhimurium cells treated with zinc oxide nanoparticles [ 115 ]
Sinha et al. [ 118 ] have also shown the influence of zinc oxide nanoparticles and silver nanoparticles on the growth, membrane structure, and their accumulation in cytoplasm of (a) mesophiles: Enterobacter sp. (gram negative) and B. subtilis (gram positive) and (b) halophiles: halophilic bacterium sp. (gram positive) and Marinobacter sp. (gram negative). Nanotoxicity of zinc oxide nanoparticles against halophilic gram-negative Marinobacter species and gram-positive halophilic bacterial species showed 80% growth inhibition. It was demonstrated that zinc oxide nanoparticles below 5 mM concentration are ineffective against bacteria. The bulk zinc oxide also did not affect the growth rate and viable counts, although they showed substantial decrease in these parameters. Enterobacter species showed dramatic alterations in cell morphology and reduction in size when treated with zinc oxide.
TEM images shown by Akbar and Anal [ 115 ] revealed the disrupted cell membrane and accumulation of zinc oxide nanoparticles in the cytoplasm (Fig. 4 ) which was further confirmed by FTIR, XRD, and SEM. It has been suggested that Zn 2+ ions are attached to the biomolecules in the bacterial cell via electrostatic forces. They are actually coordinated with the protein molecules through the lone pair of electrons on the nitrogen atom of protein part. Although there is significant impact of zinc oxide nanoparticles on both the aquatic and terrestrial microorganisms and human system, it is yet to be established whether it is due to nanoparticles alone or is a combined effect of the zinc oxide nanoparticles and Zn 2+ ions [ 55 , 106 , 109 , 119 ]. Antibacterial influence of metal oxide nanoparticles includes its diffusion into the bacterial cell, followed by release of metal ions and DNA damage leading to cell death [ 63 , 109 , 110 , 111 ]. The generation of ROS through photocatalysis is also a reason of antibacterial activity [ 62 , 112 ]. Wahab et al. [ 120 ] have shown that when zinc oxide nanoparticles are ingested, their surface area is increased followed by increased absorption and interaction with both the pathogens and the enzymes. Zinc oxide nanoparticles can therefore be used in preventing the biological system from infections. It is clear from TEM images (Fig. 5a, b ) of E. coli incubated for 18 h with MIC of zinc oxide nanoparticles that they had adhered to the bacterial cell wall. The outer cell membrane was ruptured leading to cell lysis. In some cases, the cell cleavage of the microbes has not been noticed, but the zinc oxide nanoparticles can yet be seen entering the inner cell wall (Fig. 5c, d ). As a consequence of it, the intracellular material leaks out leading to cell death, regardless of the thickness of bacterial cell wall.
TEM images of Escherichia coli ( a ), zinc oxide nanoparticles with E. coli at different stages ( b and inset), Klebsiella pneumoniae ( c ), and zinc oxide nanoparticles with K. pneumoniae ( d and inset) [ 120 ]
Mechanism of interaction of zinc oxide nanoparticles with bacterial cells has been outlined below [ 120 ]. Zinc oxide absorbs UV-Vis light from the sun and splits the elements of water.
Dissolved oxygen molecules are transformed into superoxide, O 2 − , which in turn reacts with H + to generate HO 2 radical and after collision with electrons produces hydrogen peroxide anion, HO 2 − . They subsequently react with H + ions to produce H 2 O 2 .
It has been suggested that negatively charged hydroxyl radicals and superoxide ions cannot penetrate into the cell membrane. The free radicals are so reactive that they cannot stay in free and, therefore, they can either form a molecule or react with a counter ion to give another molecule. However, it is true that zinc oxide can absorb sun light and help in cleaving water molecules which may combine in many ways to give oxygen. Mechanism of oxygen production in the presence of zinc oxide nanoparticles still needs experimental evidence.
Zinc oxide at a dose of 5 μg/ml has been found to be highly effective for all the microorganisms which can be taken as minimum inhibitory dose.
Conclusions
Zinc is an indispensable inorganic element universally used in medicine, biology, and industry. Its daily intake in an adult is 8–15 mg/day, of which approximately 5–6 mg/day is lost through urine and sweat. Also, it is an essential constituent of bones, teeth, enzymes, and many functional proteins. Zinc metal is an essential trace element for man, animal, plant, and bacterial growth while zinc oxide nanoparticles are toxic to many fungi, viruses, and bacteria. People with inherent genetic deficiency of soluble zinc-binding protein suffer from acrodermatitis enteropathica, a genetic disease indicated by python like rough and scaly skin. Although conflicting reports have been received about nanoparticles due to their inadvertent use and disposal, some metal oxide nanoparticles are useful to men, animals, and plants. The essential nutrients become harmful when they are taken in excess. Mutagenic potential of zinc oxide has not been thoroughly studied in bacteria even though DNA-damaging potential has been reported. It is true that zinc oxide nanoparticles are activated by absorption of UV light without disturbing the other rays. If zinc oxide nanoparticles produce ROS, they can damage the skin and cannot be used as sun screen. Antibacterial activity may be catalyzed by sunlight, but hopefully, it can prevent the formation of ROS. Zinc oxide nanoparticles and zinc nanoparticles coated with soluble polymeric material may be used for treating wounds, ulcers, and many microbial infections besides being used as drug carrier in cancer therapy. It has great potential as a safe antibacterial drug which may replace antibiotics in future. Application of zinc oxide nanoparticles in different areas of science, medicine, and technology suggests that it is an indispensable substance which is equally important to man and animals. However, longtime exposure with higher concentration may be harmful to living system.
Husen A, Siddiqi KS (2014) Phytosynthesis of nanoparticles: concept, controversy and application. Nano Res Lett 9:229
Article CAS Google Scholar
Husen A, Siddiqi KS (2014) Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol 12:28
Husen A, Siddiqi KS (2014) Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol 12:16
Siddiqi KS, Husen A (2016) Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nano Res Lett 11:98
Siddiqi KS, Husen A (2016) Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nano Res Lett 11:363
Siddiqi KS, Husen A (2016) Engineered gold nanoparticles and plant adaptation potential. Nano Res Lett 11:400
Siddiqi KS, Husen A (2016) Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nano Res Lett 11:482
Siddiqi KS, Rahman A, Tajuddin, Husen A (2016) Biogenic fabrication of iron/iron oxide nanoparticles and their application. Nano Res Lett 11:498
Siddiqi KS, Husen A (2017) Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J Trace Elements Med Biol 40:10–23
Siddiqi KS, Husen A, Rao RAK (2018) A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnol 16:14
Article Google Scholar
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Sawai J (2003) Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 54:177–182
Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E (2003) Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli . J Nutr 133:4077–4082
Husen A (2017) Gold nanoparticles from plant system: Synthesis, characterization and their application, In: Nanoscience and Plant–Soil Systems Vol.–48 (Eds. Ghorbanpourn M, Manika K, Varma A) Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham, Switzerland, pp.455–479
Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Aitken R, Watkins R (2008) Applications and implications of nanotechnologies for the food sector. Food Add Cont: Part A 25:241–258
Jansen J, Karges W, Rink L (2009) Zinc and diabetes—clinical links and molecular mechanisms. J Nutr Biochem 20:399–417
Maremanda KP, Khan S, Jena G (2014) Zinc protects cyclophosphamide-induced testicular damage in rat: involvement of metallothionein, tesmin and Nrf2. Biochem Biophys Res Commun 445:591–596
Kim MH, Seo JH, Kim HM, Jeong HJ (2014) Zinc oxide nanoparticles, a novel candidate for the treatment of allergic inflammatory diseases. Eur J Pharmacol 738:31–39
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Na Rev Neurosci 6:449–462
Halioua B, Ziskind B (2005) Medicine in the days of the pharaohs. Press of Harvard University Press, Belknap
Google Scholar
Ozgur U, Ya IA, Liu C, Teke A, Reshchikov MA, Doğan S, Avrutin V, Cho SJ, Morkoç H (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:041301
Klingshirn C ZnO: from basics towards applications. Phys Status Solidi 244:3027–3073
De Graaf TP, Galley E, Butcher KE (1999) Use of an antimicrobial agent. European patent, p EP1079799
Brahms J, Mattai J, Jacoby R, Chopra S, Guenin E (2005) Dry deodorant containinga sesquiterpene alcohol and zinc oxide. U.S. Patent 20050191257 A1
Speight JG (2002) Chemical and process design handbook. McGraw Hill, Inc., New York
Brown HE (1976) Zinc oxide: properties and applications. International Lead Zinc Research Organization, New York
Lopes de Romana D, Brown KH, Guinard JX (2002) Sensory trial to assess the acceptability of zinc fortificants added to iron-fortified wheat products. J Food Sci 67:461–465
Szabo T, Nemeth J, Dekany I (2003) Zinc oxide nanoparticles incorporated in ultrathin layer silicate films and their photocatalytic properties. Coll Surf A 230:23–35
Auer G, Griebler WD, Jahn B (2005) Industrial inorganic pigments, 3rd edn. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Heideman G, Noordermeer JWM, Datta RN, Noordermeer WM, van Baarle B (2006) Various ways to reduce zinc oxide levels in S-SBR rubber compounds. Macromol Symp 245-246:657–667
Patnaik P (2003) Handbook of inorganic chemicals. McGraw Hill, New York
Araujo-Lima CF, Nunes RJM, Carpes RM, Aiub FAF, Felzenszwalb I (2017) Pharmacokinetic and toxicological evaluation of a zinc gluconate-based chemical sterilant using in vitro and in silico approaches. BioMed Res Inter 2017:5746768
Wang TX, Lou TJ (2008) Solvothermal synthesis and photoluminescence properties of ZnO nanorods and nanorod assemblies from ZnO2 nanoparticles. Mater Lett 62:2329–2331
Jang JS, Yu CJ, Choi SH, Ji SM, Kim ES, Lee JS (2008) Topotactic synthesis of mesoporous ZnS and ZnO nanoplates and their photocatalytic activity. J Catal 254:144–155
Mahmud S, Johar M, Abdullah PGA, Chong J, Mohamad AK (2006) Nanostructure of ZnO fabricated via french process and its correlation to electrical properties of semiconducting varistors. Synth React Inorg Met Org Chem Nano-Met Chem 36:155–159
Kakiuchi K, Hosono E, Kimura T, Imai H, Fujihara S (2006) Fabrication of mesoporous ZnO nanosheets from precursor templates grown in aqueous solutions. J Sol-Gel Sci Technol 39:63–72
Mahmud S, Abdullah MJ (2006) Nanotripods of zinc oxide, IEEE Conf. Emerging Technol.—Nanoelectron pp. 442–446
Shen L, Zhang H, Guo S (2009) Control on the morphologies of tetrapod ZnO nanocrystals. Mater Chem Phys 114:580–583
Ding Y, Wang ZL (2009) Structures of planar defects in ZnO nanobelts and nanowires. Micron 40:335–342
Wang ZL (2004) Nanostructures of zinc oxide. Mater Tod 7:26–33
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Mat 16:R829–R858
Moezzi A, Cortie M, McDonagh A (2011) Aqueous pathways for the formation of zinc oxide nanoparticles. Dalton Trans 40:4871–4878
Xie J, Li P, Li Y, Wang Y, Wei Y (2009) Morphology control of ZnO particles via aqueous solution route at low temperature. Mater Chem Phys 114:943–947
Soares NFF, Silva CAS, Santiago-Silva P, Espitia PJP, Gonçalves MPJC, Lopez MJG, Miltz J, Cerqueira MA, Vicente AA, Teixeira J, da Silva WA, Botrel DA (2009) Active and intelligent packaging for milk and milk products. In: Coimbra JSR, Teixeira JA (eds) Engineering aspects of milk and dairy products. CRC Press Taylor & Francis Group pp, New York, pp 175–199
Chapter Google Scholar
Baum MK, Shor-Posner G, Campa A (2000) Zinc status in human immunodeficiency virus infection. J Nutr 130:1421S–1423S
Hiller JM, Perlmutter A (1971) Effect of zinc on viral-host interactions in a rainbow trout cell line, RTG-2. Water Res 5:703–710
Restuccia D, Spizzirri UG, Parisi OI, Giuseppe Cirillo G, Iemma F, Puoci F, Vinci G, Picci N (2010) New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 21:1425–1435
Espitia PJP, Soares NFF, Coimbra JSR, Andrade NJ, Cruz RS, Medeiros EAA (2015) Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging application. Food Bioprocess Technol 5:1447–1464
Hu X, Cook S, Wang P, Hwang HM (2009) In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Sci Total Environ 407:3070–3072
Wang C, Lu J, Zhou L, Li J, Xu J, Li W, Zhang L, Zhong X, Wang T (2016) Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS One 11:e0164434
Li CH, Shen CC, Cheng YW, Huang SH, Wu CC, Kao CC, Liao JW, Kang JJ (2012) Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology 6:746–756
Singh A, Singh NB, Afzal S, Singh T, Hussain I (2017) Zinc oxide nanoparticles: a review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J Mater Sci. https://doi.org/10.1007/s10853-017-1544-1
Kelly SA, Havrilla CM, Brady TC, Abramo KH, Levin ED (1998) Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ Health Perspect 106:375–384
Rikans LE, Hornbrook KR (1997) Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta 1362:116–127
Zhang L, Jiang Y, Ding Y, Povey M, York D (2007) Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res 9:479–489
Sawai J, Shoji S, Igarashi H, Hashimoto A, Kokugan T, Shimizu M, Kojima H (1998) Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J Ferment Bioeng 86:521–522
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Kahru A, Ivask A, Kasemets K, Pollumaa L, Kurvet I, François M, Dubourguier HC (2005) Bio-tests and biosensors in ecotoxicological risk assessment of field soils polluted with zinc, lead and cadmium. Environ Toxicol Chem 24:2973–2982
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus . Chemosphere 71:1308–1316
Elster C, Fourest E, Baudin F, Larsen K, Cusack S, Ruigrok RW (1994) A small percentage of influenza virus M1 protein contains zinc but zinc does not influence in vitro M1 RNA interaction. Gen J Virol 75:37–42
Lee SP, Xiao J, Knutson JR, Lewis MS, Han MK (1997) Zn2+ promotes the self-association of human immunodeficiency virus type-1 integrase in vitro. Biochemistry 36:173–180
Adams LK, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO 2 , SiO 2 , and ZnO water suspensions. Water Res 40:3527–3532
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18:6679–6686
Yamamoto O, Komatsu M, Sawai J, Nakagawa ZE (2004) Effect of lattice constant of zinc oxide on antibacterial characteristics. J Mater Sci Mater Med 15:847–851
Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine 7:184–192
Lovric J, Cho SJ, Winnik FM, Maysinger D (2005) Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol 12:1227–1234
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807
Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B (2006) Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol 40:4346–4352
Pati R, Mehta RK, Mohanty S, Padhi A, Sengupta M, Vaseeharan B, Goswami C, Sonawane A (2014) Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomedicine 10:1195–1208
Siddiqi KS, Husen A (2017) Plant response to engineered metal oxide nanoparticles. Nano Res Lett 12:92
Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M (2009) Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J Appl Microbiol 107:1193–1201
Dutta RK, Sharma PK, Bhargave R, Kumar N, Pandey AC (2010) Differential susceptibility of Escherichia coli cells toward transition metal-doped and matrix-embedded ZnO nanoparticles. J Phys Chem B 114:5594–5599
Banoee M, Seif S, Nazari ZE, Jafari-Fesharaki P, Shahverdi HR, Moballegh A, Moghaddam KM, Shahverdi AR (2010) ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli . J Biomed Mater Res B 93B:557–561
Baek YW, An YJ (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608
Karlsson HL, Toprak MS, Fadeel B (2014) Toxicity of metal and metal oxide nanoparticle. In: Nordberg GF, Fowler BA, Nordberg M (eds) Handbook on the toxicology of metals, 4th edn. Academic Press pp, London, pp 75–112
Cho WS, Duffin R, Howie SE, Scotton CJ, Wallace WA, Macnee W, Bradley M, Megson IL, Donaldson K (2011) Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol 8:27
Tuomela S, Autio R, Buerki-Thurnherr T, Arslan O, Kunzmann A, Andersson-Willman B, Wick P, Mathur S, Scheynius A, Krug HF, Fadeel B, Lahesmaa R (2013) Gene expression profiling of immune-competent human cells exposed to engineered zinc oxide or titanium dioxide nanoparticles. PLoS One 8:e68415
Chiang HM, Xia Q, Zou X, Wang C, Wang S, Miller BJ, Howard PC, Yin JJ, Beland FA, Yu H, Fu PP (2012) Nanoscale ZnO induces cytotoxicity and DNA damage in human cell lines and rat primary neuronal cells. J Nanosci Nanotechnol 12:2126–2135
Seabra AB, Haddad P, Duran N (2013) Biogenic synthsis of nanostructured iron compound: applications and perspectives. IET Nanobiotechnol 7:90–99
Sharma V, Anderson D, Dhawan A (2012) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17:852–870
Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A (2012) Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine 7:6003–6009
Yamamoto O (2013) Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorg Mater 3:643–646
Tsoli M, Kuhn H, Brandau W, Esche H, Schmid G (2005) Cellular uptake and toxicity of Au55 clusters. Small 1:841–844
Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A (2007) Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett 90:2139021–2139023
CAS Google Scholar
Jeng HA, Swanson J (2006) Toxicity of metal oxide nanoparticles in mammalian cells. J Enviorn Sci Health 41:2699–2711
Nair S, Sasidharan A, Divya Rani VV, Menon D, Nair S, Manzoor K, Raina S (2009) Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med 20:S235–S241
Raghupathi KR, Koodali RT, Manna AC (2011) Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020–4028
Jones N, Ray B, Koodali RT, Manna AC (2008) Antibacterial activity of ZnO nanoparticles suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Sahu D, Kannan GM, Tailang M, Vijayaraghavan R (2016) In vitro cytotoxicity of nanoparticles: a comparison between particle size and cell type. J Nanosci 2016:4023852
Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, Baeg GH (2017) Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster . Int J Nanomedicine 12:1621–1637
Papavlassopoulos H, Mishra YK, Kaps S, Paulowicz I, Abdelaziz R, Elbahri M, Maser E, Adelung R, Röhl C (2014) Toxicity of functional nano-micro zinc oxide tetrapods: impact of cell culture conditions, cellular age and material properties. PLoS One 9:e84983
Taccola L, Raffa V, Riggio C, Vittorio O, Iorio MC, Vanacore R, Pietrabissa A, Cuschieri A (2011) Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomedicine 6:1129–1140
Zhou YM, Zhong CY, Kennedy IM, Leppert VJ, Pinkerton KE (2003) Oxidative stress and NFkappaB activation in the lungs of rats: a synergistic interaction between soot and iron particles. Toxicol Appl Pharmacol 190:157–169
Yang H, Liu C, Yang D, Zhang H, Xi Z (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29:69–78
Schwartz VB, Thétiot F, Ritz S, Pütz S, Choritz L, Lappas A, Förch R, Landfester K, Jonas U (2012) Antibacterial surface coatings from zinc oxide nanoparticles embedded in poly( N- isopropylacrylamide) hydrogel surface layers. Adv Funct Mater 22:2376–2386
Stankovic A, Dimitrijevic S, Uskokovic D (2013) Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothermally synthesized using different surface stabilizing agents. Colloids Surf B 102:21–28
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110
Yamamoto O, Hotta M, Sawai J, Sawai J, Sasamoto T, Kojima H (1998) Influence of powder characteristic of ZnO on antibacterial activity: effect of specific surface area. J Ceram Soc Jpn 106:1007–1011
Kumar PTS, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG, Nair SV, Jayakumar R (2012) Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces 4:2618–2629
George S, Pokhrel S, Xia T, Gilbert B, Ji Z, Schowalter M, Rosenauer A, Damoiseaux R, Bradley KA, Mädler L, Nel AE (2010) Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 4:15–12
Song W, Zhang J, Guo J, Zhang J, Ding F, Li L, Sun Z (2010) Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol Lett 199:389–339
Buerki-Thurnherr T, Xiao L, Diener L, Arslan O, Hirsch C, Maeder-Althaus X, Grieder K, Wampfler B, Mathur S, Wick P, Krug HF (2013) In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology 7:402–416
Gilbert B, Fakra SC, Xia T, Pokhrel S, Mädler L, Nel AE (2012) The fate of ZnO nanoparticles administered to human bronchial epithelial cells. ACS Nano 6:4921–4930
Raffi M, Hussain F, Bhatti TM, Akhter JI, Hameed A, Hasan MM (2008) Antibacterial characterization of silver nanoparticles against E. Coli ATCC-15224. J Mater Sci Technol 24:2192–2196
Choi OK, Hu ZQ (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM (2005) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. Proteome Res 5:916–924
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli . Appl Environ Microbiol 73:1712–1720
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci 275:177–182
Elechiguerra J, Burt J, Morones J, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol 3:6
Huang Z, Zheng X, Yan D, Yin G, Liao X, Kang Y, Yao Y, Huang D, Hao B (2008) Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 24:4140–4144
Zan L, Fa W, Peng T, Gong ZK (2007) Photocatalysis effect of nanometer TiO2 and TiO2-coated ceramic plate on hepatitis B virus. J Photochem Photobiol B 86:165–169
Bajpai KS, Chand N, Chaurasia V (2012) Nano zinc oxide-loaded calcium alginate films with potential antibacterial properties. Food Bioprocess Technol 5:1871–1881
Xie Y, He Y, Irwin LP, Jin T, Shi X (2011) Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni . Appl Environ Microbiol 77:2325–2331
Akbar A, Anal AK (2014) Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control 38:88–95
Akhtar MJ, Ahamed M, Kumar S, Majeed Khan MA, Ahmad J, Alrokayan SA (2012) Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine 7:845–857
Leung YH, Xu X, Ma APY, Liu F, Ng AMC, Shen Z, Gethings LA, Guo MY, Djurišić AB, Lee PKH, Lee HK, Chan WK, Leung FCC (2016) Toxicity of ZnO and TiO 2 to Escherichia coli cells. Sci Rep 6:35243
Sinha R, Karan R, Sinha A, Khare SK (2011) Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour Technol 102:1516–1520
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Wahab R, Mishra A, Yun SI, Kim YS, Shin HS (2010) Antibacterial activity of ZnO nanoparticles prepared via non-hydrolytic solution route. Appl Microbiol Biotechnol 87:1917–1925
Download references
Acknowledgements
The authors are thankful to publishers for the permission to adopt the table and figures in this review.
Author information
Authors and affiliations.
Department of Chemistry, Aligarh Muslim University, Aligarh, Uttar Pradesh, 202002, India
Khwaja Salahuddin Siddiqi
Department of Saidla (Unani Pharmacy), Aligarh Muslim University, Aligarh, Uttar Pradesh, 202002, India
Aziz ur Rahman & Tajuddin
Department of Biology, College of Natural and Computational Sciences, University of Gondar, P.O. Box #196, Gondar, Ethiopia
Azamal Husen
You can also search for this author in PubMed Google Scholar
Contributions
AH, KSS, AR, and T gathered the research data. AH and KSS analyzed these data and wrote this review paper. All the authors read and approved the final manuscript.
Corresponding author
Correspondence to Azamal Husen .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Siddiqi, K.S., ur Rahman, A., Tajuddin et al. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res Lett 13 , 141 (2018). https://doi.org/10.1186/s11671-018-2532-3
Download citation
Received : 04 October 2017
Accepted : 16 April 2018
Published : 08 May 2018
DOI : https://doi.org/10.1186/s11671-018-2532-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Zinc oxide nanoparticles
- Microorganisms
- Antimicrobial
- Biodistribution
- Find a journal
- Publish with us
- Track your research
Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks
Affiliations.
- 1 Advance School of Chemical Sciences, Faculty of Basic Sciences, Shoolini University, Solan, Himachal Pradesh 173212, India.
- 2 Advance School of Chemical Sciences, Faculty of Basic Sciences, Shoolini University, Solan, Himachal Pradesh 173212, India. Electronic address: [email protected].
- 3 Division of Molecular Medicine, Bose Institute, P-1/12, CIT Scheme VII M, Kolkata 700054, India. Electronic address: [email protected].
- PMID: 33212389
- DOI: 10.1016/j.cis.2020.102317
In recent years, zinc oxide nanoparticles (ZnONPs) emerged as an excellent candidate in the field of optical, electrical, food packaging and particularly in biomedical research. ZnONPs show cancer cell specific toxicity via the pH-dependent (low pH) dissolution into Zn 2+ ions, which generate reactive oxygen species and induce cytotoxicity in cancer cells. Further, ZnONPs have also been used as an effective carrier for the targeted delivery of several anticancer drugs into tumor cells. The increasing focus on ZnONPs resulted in the development of various synthesis approaches including chemical, pHysical, and green or biological for the manufacturing of ZnONPs. In this article, at first we have discussed the various synthesis methods of ZnONPs and secondly its biomedical applications. We have extensively reviewed the anticancer mechanism of ZnONPs on different types of cancers considering its size, shape and surface charge dependent cytotoxicity. Photoirradiation with UV light or NIR laser further increase its anticancer activity via synergistic chemo-photodynamic effect. The drug delivery applications of ZnONPs with special emphasis on drug loading mechanism, stimuli-responsive controlled release and therapeutic effects have also been discussed in this review. Finally, its side effects to vital body organs with mechanism via different exposure routes, the future direction of the ZnONPs research and application are also discussed.
Keywords: Adverse effects; Anticancer; Drug delivery; Synthesis, zinc oxide nanoparticles.
Copyright © 2020 Elsevier B.V. All rights reserved.
Publication types
- Antineoplastic Agents / adverse effects
- Antineoplastic Agents / chemical synthesis
- Antineoplastic Agents / chemistry
- Antineoplastic Agents / pharmacology
- Chemistry Techniques, Synthetic
- Drug Carriers / adverse effects
- Drug Carriers / chemical synthesis
- Drug Carriers / chemistry
- Drug Carriers / pharmacology
- Nanoparticles*
- Zinc Oxide / adverse effects
- Zinc Oxide / chemistry*
- Zinc Oxide / pharmacology*
- Antineoplastic Agents
- Drug Carriers

COMMENTS
Zinc oxide nanoparticles (ZnO-NPs) have piqued the curiosity of researchers all over the world due to their extensive biological activity. They are less toxic and biodegradable with the capacity to greatly boost pharmacophore bioactivity.
Zinc oxide (ZnO) - based nanomaterials have been recognized to be of countless uses for numerous important requests from the beginning of nanoscience as a result of the great quantity of zinc...
Zinc oxide nanoparticles have been shown to have better efficiency in liver, spleen, and kidney biodistribution than in orally fed mice. Since zinc oxide nanoparticles are innocuous in low concentrations, they stimulate certain enzymes in man and plants and suppress diseases.
Zinc oxide nanoparticles (ZnO-NPs) have gained significant interest in the agricultural and food industry as a means of killing or reducing the activity of microorganisms. The antibacterial properties of ZnO-NPs may improve food quality, which has a direct impact on human health.
In recent years, zinc oxide nanoparticles (ZnONPs) emerged as an excellent candidate in the field of optical, electrical, food packaging and particularly in biomedical research. ZnONPs show cancer cell specific toxicity via the pH-dependent (low pH) dissolution into Zn 2+ ions, which gener …
Among the widely used nanomaterials, zinc oxide (ZnO) is a promising candidate that finds wide applications in nanostructured material systems owing to its favourable indirect band gap, large free-exciton binding energy, excellent photocatalytic and antibacterial properties. 11–14 Due to this wide range of properties and applications, ZnO is considered as a multifunctional material with ...