Antidepressant Drugs and Health-Related Quality of Life: A Reader's Guide on How to Examine a "Viral" Research Paper With a Critical Eye
Affiliation.
- 1 Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
- PMID: 35617606
- DOI: 10.4088/JCP.22f14527
Antidepressant drugs are effective against depression. They also improve subjective and functional outcomes such as disability, work functioning, social functioning, well-being, and health-related quality of life (HRQoL) in depressed patients. However, a recent large retrospective cohort study found that depressed subjects who received vs did not receive antidepressants did not differ in improvement in HRQoL, as measured using the 12-item Short Form (SF-12) Health Survey at the start and at the end of a 2-year period. The authors of the study therefore questioned the benefits of continuation of antidepressant drugs, suggesting a role for nonpharmacological interventions, instead. The study "went viral"; its findings were widely disseminated in the mass media and at medical and health care websites for physicians and for the lay public. The study, however, suffered from serious methodological shortcomings. These shortcomings are systematically explained so that readers understand how to critically read a research paper. This is important because uncritical acceptance of the findings of the study can negatively impact attitudes toward antidepressant medication among patients and health care professionals and may even result in decreased medication adherence in patients receiving antidepressant maintenance therapy.
© Copyright 2022 Physicians Postgraduate Press, Inc.
- Antidepressive Agents* / therapeutic use
- Depression / drug therapy
- Medication Adherence
- Quality of Life*
- Retrospective Studies
- Antidepressive Agents
- Research article
- Open access
- Published: 08 March 2018

Antidepressant use and risk of adverse outcomes in people aged 20–64 years: cohort study using a primary care database
- Carol Coupland ORCID: orcid.org/0000-0002-2327-3306 1 ,
- Trevor Hill 1 ,
- Richard Morriss 2 ,
- Michael Moore 3 ,
- Antony Arthur 4 &
- Julia Hippisley-Cox 1
BMC Medicine volume 16 , Article number: 36 ( 2018 ) Cite this article
14k Accesses
53 Citations
64 Altmetric
Metrics details
Antidepressants are one of the most commonly prescribed medications in young and middle-aged adults, but there is relatively little information on their safety across a range of adverse outcomes in this age group. This study aimed to assess associations between antidepressant treatment and several adverse outcomes in people aged 20–64 years diagnosed with depression.
We conducted a cohort study in 238,963 patients aged 20–64 years registered with practices across the UK contributing to the QResearch primary care database. Only patients with a first diagnosis of depression were included. Outcomes were falls, fractures, upper gastrointestinal bleed, road traffic accidents, adverse drug reactions and all-cause mortality recorded during follow-up. Cox proportional hazards models were used to estimate hazard ratios associated with antidepressant exposure adjusting for potential confounding variables.
During 5 years of follow-up, 4651 patients had experienced a fall, 4796 had fractures, 1066 had upper gastrointestinal bleeds, 3690 had road traffic accidents, 1058 had experienced adverse drug reactions, and 3181 patients died. Fracture rates were significantly increased for selective serotonin reuptake inhibitors (adjusted hazard ratio 1.30, 95% CI 1.21–1.39) and other antidepressants (1.28, 1.11–1.48) compared with periods when antidepressants were not used. All antidepressant drug classes were associated with significantly increased rates of falls. Rates of adverse drug reactions were significantly higher for tricyclic and related antidepressants (1.54, 1.25–1.88) and other antidepressants (1.61, 1.22–2.12) compared with selective serotonin reuptake inhibitors. Trazodone was associated with a significantly increased risk of upper gastrointestinal bleed. All-cause mortality rates were significantly higher for tricyclic and related antidepressants (1.39, 1.22–1.59) and other antidepressants (1.26, 1.08–1.47) than for selective serotonin reuptake inhibitors over 5 years but not 1 year, and were significantly reduced after 85 or more days of treatment with selective serotonin reuptake inhibitors. Mirtazapine was associated with significantly increased mortality rates over 1 and 5 years of follow-up.
Conclusions
Selective serotonin reuptake inhibitors had higher rates of fracture than tricyclic and related antidepressants but lower mortality and adverse drug reaction rates than the other antidepressant drug classes. The association between mirtazapine and increased mortality merits further investigation. These risks should be carefully considered and balanced against potential benefits for individual patients when the decision to prescribe an antidepressant is made.
Peer Review reports
Depression is a serious condition, common in adults of all ages worldwide [ 1 , 2 ]. It is frequently treated with antidepressant drugs, with many countries reporting substantial increases in the prescribing rates of these drugs in recent decades [ 3 , 4 , 5 ]. Reports from the US, Canada and UK have shown that antidepressants are one of the most commonly prescribed types of medication in young and middle-aged adults [ 6 , 7 , 8 , 9 ], taken by 7% of adults aged 18–39 years and by 14% of adults aged 40–59 in the US [ 9 ]. Several different types of antidepressant drug are available, with broadly equal efficacy, although there is ongoing debate over their effectiveness compared to placebo [ 10 , 11 , 12 ]. Therefore, the choice of an antidepressant largely depends on the consideration of potential adverse effects. Guidelines recommend that selective serotonin reuptake inhibitors should generally be considered as the first-line treatment for depression [ 13 ].
Despite the widespread use of antidepressants, there is relatively little information on their safety across a range of serious adverse outcomes in young and middle-aged adults. Adverse effects have been evaluated in randomised controlled trials, but these trials are usually in select groups and are relatively small and short term, therefore lacking the power to detect rare but serious adverse effects. Observational studies have shown associations between the use of selective serotonin reuptake inhibitors and increased risks of fractures and falls [ 14 , 15 ], but these studies have either been carried out only in older people or have been dominated by outcomes occurring in older people where event rates are higher. There is some indication that antidepressants may impair the ability to drive in older people, but the evidence in younger drivers is equivocal [ 16 ]. Similarly studies have found increased risks of gastrointestinal bleeds [ 17 ], adverse drug reactions [ 18 ] and all-cause mortality [ 19 ] associated with antidepressant use, but there is a lack of evidence in young and middle-aged adults, where patterns of risk may differ compared with older people due to greater levels of comorbidity, interactions with other prescribed medications, and increased susceptibility to adverse effects in older populations [ 20 , 21 ].
Given the lack of evidence regarding antidepressant safety in a younger population despite the large numbers of prescriptions issued to this group for increasingly long durations, we performed a large cohort study in people aged 20–64 years in order to investigate the associations between different antidepressant drugs and the risks of several potential adverse outcomes. We aimed to provide a comprehensive assessment of risks by drug class, and for the most commonly prescribed individual antidepressant drugs.
The cohort study was designed to assess associations between antidepressant treatment and several different adverse outcomes, including falls, fractures, upper gastrointestinal bleed, road traffic accident, adverse drug reaction, and all-cause mortality. Findings for suicide, self-harm, epilepsy and cardiovascular outcomes have been previously reported [ 22 , 23 , 24 ]. Full details of the study design and methods can be found in the study protocol [ 25 ].
Study cohort
A large primary care database (QResearch, version 34) was used to select the study cohort. At the time of the study the QResearch database included health records of over 12 million patients from more than 600 general practices across the United Kingdom which record data using the Egton Medical Information Systems (EMIS) medical records computer system. The information recorded includes patient characteristics, clinical diagnoses, symptoms and prescribed medications.
The study cohort included patients aged 20–64 years with a first recorded diagnosis of depression between January 1, 2000, until July 31, 2011. Diagnostic Read codes, which are the standard clinical codes used in general practice in the United Kingdom, were used to identify patients with a diagnosis of depression, using codes employed in previous studies [ 26 , 27 , 28 ]. Patients were only eligible for inclusion if their diagnosis of depression occurred at least 12 months after their registration with a study practice and the installation date of their practice’s EMIS computer system to ensure it was a new diagnosis and not a retrospective recording of a previous diagnosis.
Patients were excluded if they had a previous recorded diagnosis of depression, or if they had received prescriptions for an antidepressant either before the study start date (January 1, 2000), before their registration date, before the age of 20, or more than 36 months before their first recorded diagnosis of depression. We excluded patients with a previous diagnosis of depression or prescriptions for antidepressants more than 36 months prior to diagnosis so that antidepressant prescribing during follow-up would not be influenced by any prior experiences or preferences that would be difficult to account for in the analyses. Where patients were prescribed antidepressants within the 36 months before their recorded diagnosis of depression we assumed that these were being prescribed for depression. Patients were also excluded if they were temporary residents due to lack of follow-up data or if they had a diagnosis of schizophrenia, bipolar disorder or another type of psychosis, or had been prescribed lithium or antimanic drugs to reduce indication bias.
Each patient’s study entry date was defined as the date of the first recorded diagnosis of depression, or the date of the first prescription for an antidepressant if that was earlier. Patients were then followed up until the earliest date of leaving the practice, death or end of the follow-up period (August 1, 2012).
The outcomes for these analyses were falls, fractures (including vertebral, rib, pelvis, upper limb, lower limb, distal radius, hip and skull fractures), upper gastrointestinal bleed, road traffic accidents, adverse drug reactions and all-cause mortality. Patients with these outcomes were identified if they were recorded either on the patients’ general practice record using the relevant Read codes or on their linked Office of National Statistics cause of death record using International Classification of Diseases diagnostic codes, employing codes similar to those used in previous studies [ 29 , 30 , 31 ]. The adverse drug reactions outcome included specific codes for adverse reactions to antidepressants, codes for adverse drug reactions where the drug was not specified and codes for bullous eruption. Patients with a previous diagnosis of an outcome were excluded from the analysis of the respective outcome.
Antidepressant poisoning and sudden death were two further pre-specified outcomes [ 25 ], but numbers of patients with these outcomes recorded were too small for further analysis.
Information was extracted from all prescriptions for antidepressants issued during follow-up. We calculated the duration of each prescription in days by dividing the number of tablets prescribed by the number of tablets to be taken each day. If the information on tablets per day was missing or not sufficiently detailed (< 5% of total prescriptions) we estimated the duration of the prescription based on the number of tablets prescribed, as in our previous study [ 29 ]. Antidepressant drugs were grouped according to the four main classes in the British National Formulary: tricyclic and related antidepressants, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors and other antidepressants. If different antidepressant drugs were prescribed on the same date these were classified as combined prescriptions. Patients were classified as continually exposed to an antidepressant during periods where there were no gaps of more than 90 days between the end of one prescription and the start of the next. Patients were also classified as exposed for the first 90 days after the estimated date of stopping an antidepressant in order to account for any delays in starting the prescription or accumulation of tablets as well as to attribute the outcomes occurring during withdrawal periods to the antidepressant.
The daily dose of each prescription was calculated by multiplying the number of tablets to be taken each day by the dose of each tablet, and then converted to a defined daily dose using values assigned by the World Health Organization’s Collaborating Centre for Drug Statistics Methodology ( www.whocc.no/atc_ddd_index ). The 11 most commonly prescribed individual antidepressant drugs were also assessed separately, as in our previous study [ 26 ].
Confounding variables
Confounders were variables considered to be potential risk factors for the outcomes or associated with the likelihood of receiving a particular antidepressant treatment, based on our previous study of antidepressants in people aged 65 or over [ 26 ]. These were age at study entry; sex; year of diagnosis of depression; severity of index diagnosis of depression (categorised as mild, moderate or severe, using codes published by Martinez et al. [ 27 ] and some further classification of additional codes included in this study by a member of the study team); deprivation (Townsend deprivation score corresponding to the patients postcode, in fifths); smoking status (non-smoker, ex-smoker, light smoker: 1–9 cigarettes/day, moderate smoker: 10–19 cigarettes/day, heavy smoker: ≥ 20 cigarettes/day, not recorded); alcohol intake (none, trivial: < 1 unit/day, light: 1–2 units/day, medium: 3–6 units/day, heavy: 7–9 units/day, very heavy: > 9 units/day, not recorded); ethnic group (categorised as either white/not recorded or non-white (Indian, Pakistani, Bangladeshi, other Asian, black African, black Caribbean, Chinese, other including mixed)); comorbidities at baseline (binary variables for each of coronary heart disease, stroke/transient ischaemic attack, diabetes, hypertension, cancer, epilepsy/seizures, hypothyroidism, osteoarthritis, rheumatoid arthritis, asthma/chronic obstructive airways disease, osteoporosis, liver disease, renal disease, obsessive-compulsive disorder); and use of other drugs at baseline (binary variables for each of antihypertensive drugs, aspirin, statins, anticoagulants, non-steroidal anti-inflammatory drugs, anticonvulsants, hypnotics/anxiolytics, anti-psychotics, bisphosphonates, oral contraceptives, hormone replacement therapy). In addition, a record of falls at baseline was included as a confounding variable for the fracture outcome.
Statistical analysis
Cox’s proportional hazards model was used to estimate associations between each of the outcomes and antidepressant drug exposure, using robust standard errors to allow for clustering of patients within practices and excluding patients from the analysis if they had the outcome recorded at baseline. The main analyses were based on the first 5 years of follow-up after study entry. We selected 5 years of follow-up for the main analyses as this includes periods of long-term treatment and allows more events to accrue to increase the power of the study compared with a shorter period. Patients prescribed monoamine oxidase inhibitors at any time were excluded from the analyses due to small numbers.
The analysis calculated unadjusted and adjusted hazard ratios (HRs) by antidepressant class treated as a time-varying exposure to allow for patients starting and stopping and also changing between treatments during follow-up. The reference category for these analyses was no current use of antidepressant treatment. This category included both unexposed time in patients treated with antidepressants at other time points during follow-up, as well as unexposed time from the group of patients who did not receive any prescriptions for antidepressants during follow-up. In an additional analysis we used treatment with selective serotonin reuptake inhibitors as the reference category. We used Wald’s significance tests to identify significant differences between the antidepressant classes.
Analyses were also performed for antidepressant dose with separate dose categories within each class (≤ 0.5, > 0.5 and ≤ 1.0, and > 1.0 defined daily doses). We carried out tests for trend for each class using dose as a continuous variable. Analyses were performed for time-varying exposures of time since starting treatment (categorised as no use, 1–28 days, 29–84 days, 85 or more days) and since stopping (1–28 days, 29–84 days and 85–182 days after stopping treatment) within each antidepressant class. The 11 most commonly prescribed antidepressants were also analysed separately, first using periods of time with no antidepressant treatment as the reference category then using citalopram (the most commonly prescribed antidepressant) as the reference category, and we used Wald’s significance tests to identify significant differences between these 11 drugs. We tested for interactions between antidepressant class and age and also for the upper gastrointestinal bleed outcome we tested for interactions between antidepressant class and non-steroidal anti-inflammatory drugs and aspirin. We assessed the proportional hazards assumption using log minus log plots.
Three sensitivity analyses were performed [ 22 ]. In the first, we restricted the analyses to the first year of follow-up, since baseline characteristics are less likely to change within this period, and fewer switches occur between different antidepressant drugs, so the results are less susceptible to residual confounding. In the second sensitivity analysis, the entire follow-up period was included to increase power and encompass long durations of antidepressant use. The third sensitivity analysis used 5 years of follow-up and excluded patients who had not received any antidepressant prescriptions during follow-up. We carried out this third analysis because patients who were untreated during follow-up might differ systematically from treated patients (such as having a dislike of taking tablets, a preference for non-drug treatments or less severe depression), and these differences could distort comparisons with the untreated reference category.
We calculated absolute risks of the outcomes over 1 year based on the method described by Altman et al. [ 32 ], accounting for the confounding variables by using the adjusted hazard ratios (aHRs) from the analyses based on 1 year of follow-up.
We used all eligible patients in the database to maximise power. We used a P value of less than 0.01 (two-tailed) to determine statistical significance. Analyses were carried out using Stata (v12.1).
The initial cohort included 327,235 patients with a first diagnosis of depression made during the study period and between the ages of 20 and 64 years. A total of 88,272 (27.0%) patients were excluded because they had been prescribed an antidepressant either before the study entry date, before age 20 or more than 36 months before their date of diagnosis of depression, or had schizophrenia, bipolar disorder or other psychoses, or had been prescribed lithium or antimanic drugs. This left 238,963 eligible patients in the final study cohort (Fig. 1 ).
Flow chart for selection of patients included in study cohort
The total length of follow-up was 1,307,326 person-years, with a median of 5.2 years per person. Characteristics of the study cohort at baseline are shown in Table 1 . The cohort included 146,028 (61%) women and the mean age was 39.5 (SD 11.1) years.
Antidepressant treatment during follow-up
The majority of patients in the cohort (209,476, 87.7%) were treated with antidepressants during follow-up. The median duration of treatment was 221 days (interquartile range 79–590 days), with 36.6% of treated patients having 1 or more years of treatment and 5.5% having 5 or more years of treatment. Selective serotonin reuptake inhibitors were the most frequently prescribed antidepressant class (189,968 patients had 2,379,668 prescriptions), followed by tricyclic and related antidepressants (61,901 patients had 533,798 prescriptions), other antidepressants (33,631 patients had 422,079 prescriptions), and monoamine oxidase inhibitors (156 patients had a total of 1791 prescriptions). There were 83,784 combined prescriptions where two or more different antidepressant drugs were prescribed on the same day.
The three most commonly prescribed antidepressants were citalopram (1,023,255 prescriptions; 31.5%), fluoxetine (778,285; 23.9%) and amitriptyline (236,416; 7.3%), out of a total of 3,252,633 prescriptions (with combined prescriptions counting as single prescriptions). Numbers of prescriptions overall and by prescribed daily dose category for the 11 most commonly prescribed antidepressants are shown in Additional file 1 : Table S1. Prescribed doses tended to be lowest for tricyclic and related antidepressants, with the exception of lofepramine, which had the highest prescribed doses.
Incidence rates
At baseline, 4321 patients had a previous fall recorded, 23,746 had a prior fracture recorded, 1600 had a prior upper gastrointestinal bleed, 9372 a previous road traffic accident, and 1114 a previous adverse drug reaction. These patients were excluded from analyses of each respective outcome, along with the 156 patients prescribed monoamine oxidase inhibitors.
During the first 5 years of follow-up, 4651 patients experienced one or more falls (incidence rate of 529 per 100,000 person-years), 4796 had fractures (596 per 100,000), 1066 had an upper gastrointestinal bleed (119 per 100,000), 3690 had a road traffic accident (428 per 100,000), and 1058 experienced an adverse drug reaction (118 per 100,000); further, there were 3181 deaths from all causes (351 per 100,000). In addition, 74 patients had antidepressant poisoning recorded (8 per 100,000), and there were 16 sudden deaths (2 per 100,000).
Results of analyses for falls
Table 2 shows hazard ratios for each antidepressant class compared with periods of time when these drugs were not being used over the 5 years of follow-up. There were significantly increased rates of falls in all antidepressant classes compared with untreated periods of time. Table 3 presents HRs with selective serotonin reuptake inhibitor treatment as the reference category and shows that, in a direct comparison of fall rates between the antidepressant classes, there were no significant differences overall ( P = 0.59). There were significant trends in fall rates by dose in each of the drug classes (Table 4 ).
Eight of the 11 most commonly prescribed antidepressants were associated with significantly increased fall rates (at P < 0.01) when compared with non-use over 5 years of follow-up (Fig. 2 ); for dosulepin, the association was significant at P = 0.013. Table 5 presents HRs with citalopram as the reference category and shows that there were no overall significant differences between the rates for these 11 drugs.
Adjusted hazard ratios for falls, fracture, upper gastrointestinal bleed, road traffic accident, adverse drug reaction, and all-cause mortality for individual antidepressant drugs over 5 years follow-up. TCA tricyclic and related antidepressant, SSRI selective serotonin reuptake inhibitor
In the analysis restricted to the first year of follow-up, aHRs for the antidepressant drug classes compared with untreated periods were smaller than in the analysis with 5 years of follow-up (Table 6 ).
Results of analyses for fracture
Over 5 years of follow-up, the fracture rate was significantly increased for selective serotonin reuptake inhibitors (aHR 1.30, 95% CI 1.21–1.39) and other antidepressants (1.28, 95% CI 1.11–1.48), but not tricyclic and related antidepressants (0.92, 95% CI 0.80–1.06) when compared with periods of time when antidepressants were not being used (Table 2 ). There was a significantly lower fracture rate for tricyclic and related antidepressants when directly compared with selective serotonin reuptake inhibitors (aHR 0.71, 95% CI 0.61–0.82) with significant differences ( P < 0.001) between the antidepressant drug classes overall (Table 3 ). Fracture rates increased significantly with dose only for selective serotonin reuptake inhibitors (Table 4 ).
There were significantly increased fracture rates for citalopram, escitalopram, fluoxetine, sertraline and venlafaxine when compared with no use of antidepressants over 5 years of follow-up (Fig. 2 ). In a direct comparison with citalopram as the reference category, fracture rates were significantly reduced for amitriptyline (aHR 0.68, 95% CI 0.55–0.83) and dosulepin (aHR 0.67, 95% CI 0.51–0.87) (Table 5 ), with some indication of significant differences between the 11 most commonly prescribed antidepressants ( P = 0.015).
aHRs for the antidepressant drug classes compared with untreated periods in two separate age groups (20–44, 45–64 years) are shown in Additional file 1 : Table S2; there was a significant interaction between drug class and age ( P = 0.01). For the group of other antidepressants there was a significantly increased risk in people aged 20–44 (aHR 1.50, 95% CI 1.25–1.80) but no significant association in people aged 45–64 (aHR 1.04, 95% CI 0.82–1.32). Selective serotonin reuptake inhibitors were associated with a significantly increased risk of fracture in both age groups. aHRs for selective serotonin reuptake inhibitors compared with untreated periods were similar in men (1.31, 95% CI 1.18–1.45) and women (1.30, 95% CI 1.19–1.42).
Results of analyses for upper gastrointestinal bleed
Rates of upper gastrointestinal bleed over 5 years of follow-up were significantly increased for tricyclic and related antidepressants and combined antidepressants compared with no use of antidepressants (Table 2 ), although they were not significantly increased when directly compared with selective serotonin reuptake inhibitors (Table 3 ). Further, there were no significant differences between the antidepressant drug classes overall ( P = 0.07) and no significant trends with dose (Table 4 ).
Trazodone was associated with a significantly increased rate of upper gastrointestinal bleeds compared with no use of antidepressants over 5 years of follow-up (Fig. 2 ). In a direct comparison with citalopram as the reference group, the aHR for trazodone was 2.73 (95% CI 1.44–5.17), and there was also some indication of an increased risk for venlafaxine (aHR 1.53, 95% CI 1.03–2.27), although there were no significant overall differences between the 11 most commonly prescribed antidepressants ( P = 0.08) (Table 5 ).
HRs for selective serotonin reuptake inhibitors, other antidepressants and combined antidepressants compared with untreated periods were higher in people aged 20–44 than people aged 45–64 (Additional file 1 : Table S2), but the interaction between drug class and age was not statistically significant ( P = 0.11). There were no significant interactions between antidepressant drug class and use of either non-steroidal anti-inflammatory drugs or aspirin.
In the analysis restricted to the first year of follow-up, the aHR for the group of other antidepressants compared with untreated periods was higher than in the 5-year analysis (aHR 2.42, 95% CI 1.56–3.76 for 1 year analysis) but the aHRs for the other drug classes were similar in both analyses (Table 6 ). HRs for the individual drugs when compared with citalopram were similar in the 1- and 5-year analyses, except for venlafaxine, where the aHR was higher in the analysis restricted to the first year of follow-up than in the 5-year analysis (aHR 3.10, 95% CI 1.88–5.11) (Additional file 1 : Table S3), and there were significant differences between the 11 most commonly prescribed antidepressants ( P = 0.002).
Results of analyses for road traffic accidents
There were no significant associations between road traffic accidents and the antidepressant drug classes (Table 2 ) or individual drugs (Fig. 2 and Table 5 ), and no significant trends with dose (Table 4 ).
Results of analyses for adverse drug reactions
Rates of adverse drug reactions over 5 years of follow-up were significantly increased for all classes of antidepressants compared with non-use (Table 2 ). There were significant differences between the classes overall, with higher rates for tricyclic and related antidepressants and other antidepressants when directly compared with selective serotonin reuptake inhibitors (aHRs 1.54 (95% CI 1.25–1.88) and 1.61 (1.22–2.12), respectively; Table 3 ), but no significant trends with dose in any of the drug classes (Table 4 ).
Most of the 11 most commonly prescribed antidepressants were associated with significantly increased risks (at P < 0.01) compared with non-use over 5 years of follow-up (Fig. 2 ), with the exception of trazodone ( P = 0.82) and escitalopram ( P = 0.03). There were significant overall differences between the most commonly prescribed antidepressants with significantly higher rates for amitriptyline, lofepramine and venlafaxine when compared with citalopram as the reference category (Table 5 ).
There was a significant interaction between antidepressant drug class and age ( P < 0.001) with higher aHRs in people aged 20–44 than those aged 45–64 years for all drug classes when compared with untreated periods over 5 years of follow-up (Additional file 1 : Table S2).
Results of analyses for all-cause mortality
In the analysis of 5 years of follow-up, all-cause mortality rates were significantly increased for all classes of antidepressants compared with non-use (Table 2 ). The reductions in HRs comparing unadjusted and adjusted results were mainly due to adjustment for age, with some additional decreases from adjusting for use of other drugs. When directly compared with selective serotonin reuptake inhibitors, mortality rates were significantly higher for tricyclic and related antidepressants, other antidepressants and combined antidepressants (aHRs 1.39 (95% CI 1.22–1.59), 1.26 (1.08–1.47) and 1.58 (1.16–2.17), respectively; Table 3 ) with significant differences between the drug classes. There were no significant trends with dose in any of the drug classes (Table 4 ).
HRs for the 11 most commonly prescribed antidepressants compared with non-use are shown in Fig. 2 . In the analysis with citalopram as the reference group, there were significantly higher mortality rates for amitriptyline (aHR 1.77, 95% CI 1.49–2.10) and mirtazapine (aHR 1.67, 95% CI 1.33–2.09; Table 5 ), with significant differences between the 11 most commonly prescribed antidepressants overall ( P < 0.001).
In the analysis restricted to the first year of follow-up, there were no significant increases in mortality rates for any of the drug classes compared with non-use (at P < 0.01; Table 6 ), and HRs for tricyclic and related antidepressants, other antidepressants and combined antidepressants were no longer significantly increased when compared with selective serotonin reuptake inhibitors (aHRs 1.03 (95% CI 0.80–1.32), 1.28 (0.97–1.68) and 1.67 (0.80–3.49), respectively).
For individual drugs during the first year of follow-up the HR for amitriptyline was lower than in the 5-year analysis and was not associated with a significantly increased mortality rate compared with citalopram (1.36, 95% CI 0.99–1.86), but the HR for mirtazapine was almost the same as in the 5-year analysis (aHR 1.63, 95% CI 1.12–2.38, P = 0.011; Additional file 1 : Table S3), although numbers were smaller.
Analyses of duration of use
HRs according to time since starting and stopping treatment for each antidepressant class over 5 years of follow-up are shown in Additional file 1 : Table S4. These show that, generally, rates remained increased throughout treatment for all classes of antidepressants for falls. For fractures, the rates were significantly increased after 28 days of starting selective serotonin reuptake inhibitor treatment and during the 28–84 days after starting treatment with the group of other antidepressants. For adverse drug reactions, rates were highest during the first 28 days of treatment but remained increased throughout treatment for all antidepressant classes. All-cause mortality rates were only significantly increased during the first 28 days of treatment for all antidepressant classes, and were significantly reduced after treatment of 85 or more days with selective serotonin reuptake inhibitors. All-cause mortality rates were highest during the first 1–28 days after stopping treatment.
Sensitivity analyses
There were some differences between the results of analyses restricted to 1 year of follow-up and the main 5-year analyses as described above. When the entire follow-up period was used, and when patients who had not received any antidepressant prescriptions during follow-up were removed from the analysis, the aHRs comparing antidepressant classes with untreated periods were similar to those in the main 5-year analyses for all outcomes (Additional file 1 : Table S5).
Absolute risks
Table 7 shows absolute risks of the six outcomes over 1 year by antidepressant class and for the individual drugs. Absolute risks were mostly less than 60 per 10,000 patients over 1 year and were highest overall for falls and fractures. The absolute risk of fracture associated with selective serotonin reuptake inhibitors was 20 per 10,000 higher than for tricyclic and related antidepressants, and for other antidepressants it was 27 per 10,000 higher. The absolute risk of a gastrointestinal bleed was 29 per 10,000 higher for venlafaxine compared with citalopram. Mirtazapine was associated with an excess risk of 23 per 10,000 for all-cause mortality compared with citalopram.
This large study found several differences in the rates of adverse outcomes between different antidepressant classes and individual drugs in people aged 20–64 years with a diagnosis of depression. Our key findings were that selective serotonin reuptake inhibitors and other antidepressants were associated with significantly increased fracture rates. All drug classes were associated with significantly increased risks of falls. Rates of adverse drug reaction were significantly higher for tricyclic and related antidepressants and other antidepressants than for selective serotonin reuptake inhibitors. Mortality rates were significantly higher for tricyclic antidepressants and other antidepressants than with selective serotonin reuptake inhibitor treatment over 5 years of follow-up, but not during the first year of follow-up.
Among individual antidepressant drugs, fracture rates were significantly increased for citalopram, escitalopram, fluoxetine, sertraline and venlafaxine compared with periods of non-use of antidepressants. Amitriptyline, lofepramine and venlafaxine were associated with significantly higher rates of adverse drug reactions compared with citalopram. Trazodone was associated with a significantly higher rate of upper gastrointestinal bleeding over 5 years of follow-up. Mirtazapine and amitriptyline were associated with highest mortality rates over 5 years of follow-up, but only mirtazapine was associated with a significantly increased risk during the first year of follow-up.
In this cohort of adults aged 20–64 years, the absolute risks of the adverse outcomes were mostly less than 0.6% per year, and for falls, fractures, upper gastrointestinal bleeding and all-cause mortality they were considerably lower than the equivalent risks in older people [ 26 ]. Whilst for individuals the excess risks associated with antidepressant use are low, given the widespread use of these drugs in adults, the population effects could be considerable.
Additional analyses examining patterns of risk according to duration of use found the increases in all-cause mortality rates across all antidepressant classes were only apparent during the first 28 days of treatment, after which they declined rapidly. This is a period during which depressive symptoms can be most severe, and we have previously shown that rates of suicide and self-harm in this cohort were highest in the first 28 days after starting antidepressant treatment [ 22 ]. Mortality rates were also substantially increased in the first 28 days after stopping antidepressants, which may reflect patients stopping medication due to onset of illness or hospital or hospice admission rather than a direct effect of drug withdrawal. Although amitriptyline was associated with the highest increase in mortality rates over 5 years of follow-up it was not associated with a significantly increased risk during the first year of follow-up. The increased risk over 5 years of follow-up might occur due to amitriptyline being initiated to relieve neuropathic pain in patients who developed cancer although it is not specifically licenced for this in the UK [ 33 ]. However, the increased mortality rate for mirtazapine was similar in magnitude in the 1- and 5-year analyses.
Strengths and limitations
This study included a large representative sample of 238,963 people aged 20–64 diagnosed with depression in the general UK population. All eligible patients were included, so there is no selection bias arising from non-response. Data on prescriptions and confounding variables were recorded prospectively before the outcomes occurred, thereby avoiding recall bias.
To reduce indication bias we only included patients with a diagnosis of depression, since antidepressants, and particularly tricyclic antidepressants, are prescribed for a range of indications, including off-label conditions such as insomnia and pain, and these indications will be associated with the outcomes considered in this study to a varying degree [ 34 ]. Depression itself is an established risk factor for several of the outcomes considered here, including falls, fracture and all-cause mortality [ 35 , 36 , 37 , 38 , 39 ], and restricting the cohort to patients with a diagnosis of depression helped distinguish the effects of antidepressant treatment from those of depression itself. We also adjusted for severity of depression at first diagnosis, although we were not able to account for changes in severity of depression over time as depression severity scores are not routinely recorded in general practice. Our cohort only included people with a first diagnosis of depression who had not previously been prescribed antidepressants to avoid biases associated with prevalent use or prior experiences during previous treatment episodes [ 40 ]. The results of the sensitivity analyses excluding patients who did not receive antidepressant prescriptions during follow-up were very similar to the main analyses where these patients contributed follow-up time to the unexposed reference category. This indicates that including these patients who may differ in terms of treatment preferences and other personal characteristics did not distort the results.
We carried out analyses directly comparing event rates during treatment with different antidepressant classes as well as including comparisons with untreated periods. Comparisons with untreated periods of time are still susceptible to indication bias since the depression may have resolved or be less severe during these periods, leading to a reduced incidence of the events. Further, this could explain the increased rates of mortality during periods of treatment with all classes of antidepressant compared with untreated periods, particularly in the 5-year analysis, where patients receiving antidepressant treatment after 1 year are likely to have more severe or treatment-resistant depression. The analyses directly comparing treated groups with each other are less vulnerable to these biases.
We accounted for a large number of potential confounding variables in the analysis, including other comorbidities and use of other medications; however, as with any observational study, the findings are still susceptible to residual confounding due to lack of adjustment for certain potential risk factors such as dietary factors and physical activity, which are not routinely recorded in primary care. Similarly, we did not adjust for chronic pain, since it is inconsistently recorded in primary care, or for conditions such as multiple sclerosis and fibromyalgia, but these would likely have a low prevalence in this age group.
Our outcomes were restricted to medical outcomes recorded in GP records or on death certificates, and we were not able to include pertinent outcomes such as interpersonal and psychological effects as they are seldom included in these records [ 41 ]. A further limitation is that the outcomes were not formally adjudicated in this study, and some more minor events, such as might occur for falls, adverse drug reactions or road traffic accidents, would not be medically reported or recorded so there will be some misclassification of the outcomes; this also means our findings for these outcomes relate to more severe, medically reported events. Validation studies in other UK primary care databases have shown high levels of validity across a range of diseases; for example, Khan [ 42 ] reported high positive predictive values for validation studies of upper gastrointestinal bleeding and hip fracture. We included information from death certificates to identify additional patients with the outcomes, which will have increased ascertainment and reduced misclassification. However, most codes used to identify road traffic accidents did not indicate whether the person was driving or a passenger, or whether they were responsible for the crash; therefore, findings for this outcome are particularly susceptible to misclassification biases. We excluded patients with a prior history of each adverse outcome from the analysis of that outcome to ensure that only new events were included and to remove potential biases arising from changes to treatments and behaviours as a consequence of prior events.
There is likely to be some misclassification of the antidepressant exposure variables as patients may not have actually taken their prescribed antidepressant medication, or may not have taken it at the prescribed dose. This misclassification could underestimate associations with drug use. Furthermore, although the cohort was large, the number of events was small for some of the antidepressant exposure categories and some of the stratified analyses.
Comparison with other studies
Many observational studies have consistently found increased risks of falls in older people taking antidepressants [ 26 , 43 , 44 , 45 ]. Fewer studies have examined the risks in younger people. Our findings show that rates of falls are also increased in younger people taking antidepressants, and increase with dose. A review of studies in older people found that the increased risks of falls were similar for selective serotonin reuptake inhibitors and tricyclic antidepressants [ 14 ]; likewise, we found associations were similar for these drug classes in younger people. A number of factors are likely to explain the increased risk of falls associated with antidepressants, including effects on concentration, balance and reaction times, and orthostatic hypertension and sedative effects, particularly for the tricyclic antidepressants and mirtazapine, and sleep disturbance with selective serotonin reuptake inhibitors resulting in drowsiness and dizziness [ 14 ].
Our finding of an increased risk of fracture for selective serotonin reuptake inhibitors concurs with the findings of many other observational studies [ 15 , 46 ], though these have predominantly been conducted in older populations, whilst we found an increased risk even in people aged 20–44. These increases may be due to decreased bone density since use of selective serotonin reuptake inhibitors has been shown to be associated with a reduction in bone mineral density and bone loss, even in adolescent boys [ 47 ]. We did not find an increased fracture risk for tricyclic antidepressants, which contrasts with the findings of two systematic reviews [ 15 , 48 ], although these found smaller increases for tricyclic antidepressants than for selective serotonin reuptake inhibitors [ 46 ]. A Danish case-control study [ 49 ] that examined age and dose effects for selective serotonin reuptake inhibitors and tricyclic antidepressants found that the fracture risk associated with selective serotonin reuptake inhibitors increased with age but only in medium- and high-dose users, whilst for tricyclic antidepressants there was only an increased fracture risk in the oldest age group (> 60 years) for the highest dose. Few studies have examined other antidepressants or individual antidepressants, yet a recent cohort study of middle-aged and older adults found similar fracture risks when comparing serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) with selective serotonin reuptake inhibitors [ 50 ].
Several studies have found upper gastrointestinal bleeding to be more common among patients taking selective serotonin reuptake inhibitors, particularly when used in combination with non-steroidal anti-inflammatory drugs [ 17 , 51 ]. A number of mechanisms have been proposed for this increased risk, including depletion of platelet serotonin content causing an inhibition of platelet plug formation or direct toxicity on the gastrointestinal mucosa [ 52 ]. In our study, we found a higher risk for tricyclic and related antidepressants than for selective serotonin reuptake inhibitors, although this was only in the lowest dose category and may therefore reflect preferential prescribing of low-dose tricyclic antidepressants rather than selective serotonin reuptake inhibitors in people with suspected risk factors for bleeding. We did not find a stronger association when selective serotonin reuptake inhibitors were used in combination with non-steroidal anti-inflammatory drugs, although, as our study was in a younger age group than most previous studies, these differences may be due to smaller numbers prescribed this drug combination. Our finding that venlafaxine and trazodone were associated with the highest risks has been found in other studies [ 26 , 53 , 54 , 55 ].
Increased tolerability of selective serotonin reuptake inhibitors in comparison with tricyclic antidepressants is long established [ 56 , 57 , 58 ], with selective serotonin reuptake inhibitors having fewer side effects and adverse reactions, particularly for anticholinergic and sedating effects. Lofepramine had the highest rate of adverse drug reactions in this study, as we also found in our study of older people [ 26 ], though this drug was prescribed at higher doses than the other antidepressants. Venlafaxine was also associated with an increased risk of adverse drug reactions compared with citalopram, which concurs with the findings of a meta-analysis of double-blind randomized trials that reported higher rates of discontinuation due to adverse events for venlafaxine compared with selective serotonin reuptake inhibitors [ 59 ]. Amitriptyline was mainly prescribed at low doses, but still showed an increased risk of adverse drug reactions.
We found no evidence of associations with antidepressant treatment for road traffic accidents, although our outcome was broad and not specific to drivers of vehicles. Findings from other studies are inconclusive, with some showing increased risks for selective serotonin reuptake inhibitors and some for tricyclic antidepressants particularly in older people, whilst others have shown no associations with antidepressant use [ 16 , 60 , 61 , 62 , 63 , 64 ]. Many of these studies have not accounted for depression, which itself can impair driving performance [ 65 , 66 ].
Our findings of increased mortality rates over 5 years for all antidepressant classes are similar to those of a cohort study in postmenopausal women that found increased mortality rates among users of selective serotonin reuptake inhibitors, tricyclic and related antidepressants, and other antidepressants with a mean follow-up of 5.9 years [ 19 ]. The authors proposed possible mechanisms for these associations but also suggested they could be due to antidepressant use reflecting other causes of increased mortality, such as residual depressive symptoms, that may not have been fully controlled. Previous observational studies in people aged 65 and over have found mirtazapine to be associated with the highest increases in mortality rates [ 26 , 67 ]. A study of FDA Summary Basis of Approval reports was carried out to assess whether medication may worsen the already increased mortality risk for patients with severe psychiatric illness [ 38 ]. This study combined mortality rates across short- or medium-term randomised clinical trials of psychotropic drugs in patients with psychiatric illness, and found that, among patients with depression, the overall mortality risk was similar for selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors compared to placebo, but there was a significantly higher risk for the group of heterocyclic antidepressants, which included amitryptiline, imipramine, maprotiline and mirtazapine. Suicide was the most common cause of death. This study of trial data, which is not susceptible to residual confounding, provides some support for our findings of increased mortality rates for amitryptiline and mirtazapine in comparison with citalopram, although the study did not assess these drugs individually and the number of deaths was low. Herein, we have not investigated specific causes of death, although in an analysis of suicide we found a 3.7-fold increased risk for mirtazapine but no association for amitryptiline [ 22 ], although suicide only accounted for a relatively small proportion of all deaths.
Clinical implications and future research
Antidepressants are one of the most commonly prescribed medications in younger and middle-aged adults, although their benefits in the treatment of depression may be relatively small, especially for mild and moderate depression [ 10 , 68 ]. These small beneficial effects could be outweighed by harmful effects, but there is limited evidence on their safety in younger and middle-aged adults, particularly for outcomes such as falls and fracture. Although susceptible to indication bias and residual confounding, this study has found increased rates of falls, fractures, upper gastrointestinal bleeds, adverse drug reactions and all-cause mortality during periods of antidepressant use compared with non-use for most classes of antidepressant. This study also found that, over 5 years, selective serotonin reuptake inhibitors and other antidepressants were associated with significantly increased fracture rates compared with tricyclic and related antidepressants, whereas rates of all-cause mortality and adverse drug reaction were significantly higher for tricyclic and related antidepressants and other antidepressants than for selective serotonin reuptake inhibitors. These risks should be carefully considered and balanced against potential benefits for individual patients when the decision to prescribe an antidepressant is made so as to avoid unnecessary treatment or to help select the most appropriate treatment where required.
Of particular concern is the association of mirtazapine with increased suicide and mortality rates in each of the observational studies that we have performed [ 22 , 26 ] and in the US study of randomised controlled trial reports [ 38 ], whereas other antidepressant drugs have shown inconsistent relationships with mortality risk. This relationship, along with the increased risk for amitriptyline over 5 years, requires further investigation to ascertain how much of the increased risk for mortality is associated with suicide and other specific causes of death, how much due to selective prescribing in people with life threatening illness (such as because of the sedation it produces to aid sleep in people who might have pain as well as depression) and how much is due to some other mechanism.
This large study of potential adverse outcomes in combination with the findings for cardiovascular outcomes [ 24 ], suicide and self-harm [ 22 ], and epilepsy [ 23 ] has provided a comprehensive assessment of antidepressant safety in people aged 20–64 years diagnosed with depression. Although the findings are from an observational study design and are therefore susceptible to residual confounding, our results do indicate potential increased risks for some adverse outcomes for consideration when antidepressants are prescribed. Thus, even though they are quite rare outcomes, these adverse effects of antidepressants need to be considered alongside the benefits in working age adults as well as in older people.
Abbreviations
Adjusted hazard ratio
Confidence interval
Egton Medical Information Systems
Hazard ratio
Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84.
Article PubMed PubMed Central Google Scholar
Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study. Lancet. 2013;386:743–800.
Article Google Scholar
Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, et al. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ. 2009;339:b3999.
Stephenson CP, Karanges E, McGregor IS. Trends in the utilisation of psychotropic medications in Australia from 2000 to 2011. Aust N Z J Psychiatry. 2013;47:74–87.
Article PubMed Google Scholar
Pratt L, Brody D, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. NCHS Data Brief. 2011;76:1–8.
Google Scholar
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the united states from 1999-2012. JAMA. 2015;314:1818–30.
Article CAS PubMed PubMed Central Google Scholar
Scholes S, Faulding S, Mindell J. Use of prescribed medicines. NHS Health Survey for England. 2013; Chapter 5. https://digital.nhs.uk/media/25813/Health-Survey-for-England-2013-Chapter-5-Prescribed-medicines/Any/HSE2013-Ch5-pres-meds . Accessed 21 Feb 2018.
Rotermann M, Sanmartin C, Hennessy D, Arthur M. Prescription medication use by Canadians aged 6 to 79. Health Rep. 2014;25(6):3–9.
Moore TJ, Mattison DR. Adult utilization of psychiatric drugs and differences by sex, age, and race. JAMA Intern Med. 2017;177:274–5.
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med. 2008;5:e45.
Khan A, Brown WA. Antidepressants versus placebo in major depression: an overview. World Psychiatry. 2015;14:294–300.
Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry. 2017;17:58.
NICE. Depression: The Treatment and Management of Depression in Adults (Updated Edition). Leicester: British Psychological Society; 2010.
Darowski A, Chambers SACF, Chambers DJ. Antidepressants and falls in the elderly. Drugs Aging. 2009;26:381–94.
Article CAS PubMed Google Scholar
Rabenda V, Nicolet D, Beaudart C, Bruyère O, Reginster J-Y. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporos Int. 2013;24:121–37.
Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving. Drug Saf. 2011;34:125–56.
Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811–9.
Degner D, Grohmann R, Kropp S, Ruther E, Bender S, et al. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry. 2004;37(Suppl 1):S39–45.
CAS PubMed Google Scholar
Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women's Health Initiative Study. Arch Intern Med. 2009;169:2128–39.
Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol. 2003;38:843–53.
Corsonello A, Pedone C, Antonelli IR. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem. 2010;17:571–84.
Coupland C, Hill T, Morriss R, Arthur A, Moore M, et al. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517.
Hill T, Coupland C, Morriss R, Arthur A, Moore M, et al. Antidepressant use and risk of epilepsy and seizures in people aged 20 to 64 years: cohort study using a primary care database. BMC Psychiatry. 2015;15:1–13.
Coupland C, Hill T, Morriss R, Moore M, Arthur A, et al. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ. 2016;352:i1350.
Coupland C, Morriss R, Arthur A, Moore M, Hill T, et al. Safety of antidepressants in adults aged under 65: protocol for a cohort study using a large primary care database. BMC Psychiatry. 2013;13:135.
Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551.
Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ. 2005;330:389.
Hippisley-Cox J, Pringle M, Hammersley V, Crown N, Wynn A, et al. Antidepressants as risk factor for ischaemic heart disease: case-control study in primary care. BMJ. 2001;323:666–9.
Coupland C, Dhiman P, Barton G, Morriss R, Arthur A, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study analysis using a large primary care database. Health Technol Assess. 2011;15(28):1–202. iii-iv
Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients on Cox 2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case control analysis. BMJ. 2005;366:1366–74.
Hippisley-Cox J, Coupland C, Brindle P. Derivation and validation of QStroke score for predicting risk of ischaemic stroke in primary care and comparison with other risk scores: a prospective open cohort study. BMJ. 2013;346:f2573.
Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492–5.
Fallon MT. Neuropathic pain in cancer. Br J Anaesth. 2013;111:105–11.
Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, et al. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603.
Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, et al. Depression, falls, and risk of fracture in older women. Arch Intern Med. 1999;159:484–90.
Whooley MA, Browner WS. for the Study of Osteoporotic Fractures Research Group. Association between depressive symptoms and mortality in older women. Arch Intern Med. 1998;158:2129–35.
Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatr. 2014;171(4):453–62.
Khan A, Faucett J, Morrison S, Brown WA. Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention-deficit/hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiat. 2013;70:1091–9.
Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiat. 2015;72:334–41.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20.
Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216:67–73.
Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60:e128–36.
Kerse N, Flicker L, Pfaff JJ, Draper B, Lautenschlager NT, et al. Falls, depression and antidepressants in later life: a large primary care appraisal. PLoS One. 2008;3:e2423.
Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA. Antidepressants and the risk of falls among nursing home residents. N Engl J Med. 1998;339:875–82.
Ensrud KE, Blackwell TL, Mangione CM, Bowman PJ, Whooley MA, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50:1629–37.
Wu Q, Bencaz AF, Hentz JG, Crowell MD. Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case–control studies. Osteoporos Int. 2012;23:365–75.
Rizzoli R, Cooper C, Reginster JY, Abrahamsen B, Adachi JD, et al. Antidepressant medications and osteoporosis. Bone. 2012;51:606–13.
Wu Q, Qu W, Crowell MD, Hentz JG, Frey KA. Tricyclic antidepressant use and risk of fractures: A meta-analysis of cohort and case-control studies. J Bone Miner Res. 2013;28:753–63.
Vestergaard P, Prieto-Alhambra D, Javaid MK, Cooper C. Fractures in users of antidepressants and anxiolytics and sedatives: effects of age and dose. Osteoporos Int. 2013;24:671–80.
Lanteigne A, Y-h S, Stürmer T, Pate V, Azrael D, et al. Serotonin–norepinephrine reuptake inhibitor and selective serotonin reuptake inhibitor use and risk of fractures: a new-user cohort study among US adults aged 50 years and older. CNS Drugs. 2015;29:245–52.
Jiang H-Y, Chen H-Z, Hu X-J, Yu Z-H, Yang W, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13:42–50. e43
de Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function. Drugs Aging. 2011;28:345–67.
Opatrny L, Delaney JAC, Suissa S. Gastro-intestinal haemorrhage risks of selective serotonin receptor antagonist therapy: a new look. Br J Clin Pharmacol. 2008;66:76–81.
de Abajo FJ, Garcia-Rodriguez LA. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry. 2008;65:795–803.
de Abajo FJ, Rodriguez LAG, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319:1106–9.
Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand. 2000;101:17–25.
Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry. 2004;16:15–25.
Anderson IM, Tomenson BM. Treatment discontinuation with selective serotonin reuptake inhibitors compared with tricyclic antidepressants: a meta-analysis. BMJ. 1995;310:1433–8.
de Silva VA, Hanwella R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: a meta-analysis of published studies. Int Clin Psychopharmacol. 2012;27:8–16.
Bramness JG, Skurtveit S, Neutel CI, Morland J, Engeland A. Minor increase in risk of road traffic accidents after prescriptions of antidepressants: a study of population registry data in Norway. J Clin Psychiatry. 2008;69:1099–103.
Ravera S, van Rein N, de Gier JJ, de Jong-van den Berg LTW. Road traffic accidents and psychotropic medication use in the Netherlands: a case–control study. Br J Clin Pharmacol. 2011;72:505–13.
Ray WA, Fought RL, Decker MD. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. Am J Epidemiol. 1992;136:873–83.
Gibson JE, Hubbard RB, Smith CJP, Tata LJ, Britton JR, et al. Use of self-controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol. 2009;169:761–8.
Movig KLL, Mathijssen MPM, Nagel PHA, van Egmond T, de Gier JJ, et al. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev. 2004;36:631–6.
Wingen M, Ramaekers JG, Schmitt JAJ. Driving impairment in depressed patients receiving long-term antidepressant treatment. Psychopharmacology. 2006;188:84–91.
Orriols L, Wilchesky M, Lagarde E, Suissa S. Prescription of antidepressants and the risk of road traffic crash in the elderly: a case–crossover study. Br J Clin Pharmacol. 2013;76:810–5.
Danielsson B, Collin J, Jonasdottir Bergman G, Borg N, Salmi P, et al. Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults - a Swedish nationwide study. Br J Clin Pharmacol. 2016;81:773–83.
Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53.
Download references
Acknowledgements
We acknowledge the contribution of practices who contribute to the QResearch® database and acknowledge Egton Medical Information Systems (EMIS) and the University of Nottingham for expertise in establishing, developing and supporting the database. We thank members of the QResearch® Advisory Board for their input into discussions on implications and dissemination. We acknowledge the Office of National Statistics (ONS) for providing mortality data. ONS bear no responsibility for the analysis or interpretation of the data.
The project was funded by the National Institute for Health Research (NIHR) School for Primary Care Research (project number 81). The funding body did not play a role in the study design, writing of the manuscript or in the decision to submit the manuscript for publication. RM’s contribution to the study has been funded through the NIHR Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC EM).
This paper presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Availability of data and materials
The patient level data from the QResearch are specifically licensed according to its governance framework. Researchers may apply for data access at www.qresearch.org . See www.qresearch.org for further details.
Author information
Authors and affiliations.
Division of Primary Care, University of Nottingham, 13th floor, Tower Building, University Park, Nottingham, NG7 2RD, UK
Carol Coupland, Trevor Hill & Julia Hippisley-Cox
Institute of Mental Health, Jubilee Campus, Wollaton Road, Nottingham, NG8 1BB, UK
Richard Morriss
University of Southampton Medical School, Primary Care and Population Sciences, Aldermoor Health Centre, Aldermoor Close, Southampton, SO16 5ST, UK
Michael Moore
School of Nursing Sciences, Faculty of Medicine and Health Sciences, Edith Cavell Building, University of East Anglia, Norwich Research Park, Norwich, NR4 7TJ, UK
Antony Arthur
You can also search for this author in PubMed Google Scholar
Contributions
CC, JHC, RM, AA and MM contributed to the overall conception and design of the study. CC wrote the first draft of this manuscript. JHC undertook the data extraction. TH and CC carried out statistical analyses. All authors contributed to the interpretation of results and drafting of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Carol Coupland .
Ethics declarations
Ethics approval and consent to participate.
The project was independently peer reviewed and accepted by the QResearch Scientific board and approved in accordance with the agreed procedure with the Trent Research Ethics Committee (reference number: MREC/03/4/021).
Consent for publication
Not applicable.
Competing interests
The authors of this manuscript have the following competing interests: financial support from the National Institute for Health Research (NIHR) for the submitted work; JHC is director of QResearch, which is a not-for-profit venture between the University of Nottingham and EMIS (commercial supplier of GP clinical systems). RM’s contribution to the study has been funded through the NIHR Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC EM).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Table S1. Numbers of prescriptions for different antidepressant drugs by dose category. Table S2. Adjusted hazard ratios for six adverse outcomes (falls, fracture, upper gastrointestinal bleed, adverse drug reaction, road traffic crash and all-cause mortality) by antidepressant class compared with no use of antidepressants in (A) ages 20–44 years and (B) 45–64 years over 5 years of follow-up. Table S3. Unadjusted and adjusted hazard ratios for six adverse outcomes (falls, fracture, upper gastrointestinal bleed, adverse drug reaction, road traffic crash and all-cause mortality) by antidepressant drug, compared with citalopram over 1 year of follow-up. Table S4. Adjusted hazard ratios for six adverse outcomes (falls, fracture, upper gastrointestinal bleed, adverse drug reaction, road traffic crash and all-cause mortality) by antidepressant class compared with no use of antidepressants according to duration of use and time since stopping for each antidepressant class over 5 years of follow-up. Table S5. Adjusted hazard ratios for six adverse outcomes (falls, fracture, upper gastrointestinal bleed, adverse drug reaction, road traffic crash and all-cause mortality) by antidepressant class compared with no use of antidepressants, over (A) total follow-up time and (B) 5 years of follow-up, excluding untreated patients. (DOCX 115 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Coupland, C., Hill, T., Morriss, R. et al. Antidepressant use and risk of adverse outcomes in people aged 20–64 years: cohort study using a primary care database. BMC Med 16 , 36 (2018). https://doi.org/10.1186/s12916-018-1022-x
Download citation
Received : 13 July 2017
Accepted : 09 February 2018
Published : 08 March 2018
DOI : https://doi.org/10.1186/s12916-018-1022-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antidepressants
- Gastrointestinal bleed
- Adverse effects
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- The trouble with...
The trouble with antidepressants: why the evidence overplays benefits and underplays risks—an essay by John B Warren
- Related content
- Peer review
- John B Warren , director
- Medicines Assessment, Ipswich, Suffolk, UK
- jbwarren5{at}gmail.com
Widespread prescribing has not reduced mental disability or suicide, raising questions about the assessment of evidence on effectiveness and safety of antidepressants, writes John Warren
Depression can be severe and reduce life expectancy. Antidepressant prescribing has increased substantially in recent years so that one in eight UK adults, some 7.3 million people, now receive a prescription for antidepressants each year, and many take them long term. 1 More than 60% of US residents taking antidepressants do so for more than two years. 2
Although meta-analyses seem to support widespread use, concerns have been raised about the effectiveness and safety of the drugs. The conclusions of meta-analyses have been criticised because of manufacturers’ influence on trials, 3 4 under-recognition of the placebo effect, inadequate attention to negative data, different methods used to assess risk and benefit, and lack of benefit on suicide. There are also concerns about limited safety databases and the huge commercial promotion of these drugs.
Analysis of the benefits and risks of drugs in psychiatry differs from other therapeutic areas. There are no reliable biomarkers of psychiatric disease and no primary endpoint to summarise safety and efficacy (an equivalent to mortality in cardiovascular or oncology trials). Psychiatry therefore depends on composite scales for diagnosis and to assess drug efficacy. As composite measures are rarely used for adverse events, trials are likely to overestimate benefit and underestimate risks, with serious implications for public health. Although prescribers will often see patients improve over time, questions remain about how much antidepressants contribute to this and whether long term treatment is safe.
Unclear mechanism of action
A common justification for using antidepressants is that they correct a chemical deficiency in the brain. The monoamine hypothesis, over 50 years old, implicates serotonergic, noradrenergic, and dopaminergic neurotransmission in the pathogenesis of depression.
Deficiency of the neurotransmitter dopamine explains Parkinson’s disease, but no similar chemical deficiency has been shown in the human brain for depression, the biochemistry of which remains complex and unexplained. 5 6 Depression has no subclassification depending on which of the three amines is deficient, even though each amine differs in its pharmacology and physiology.
The limitations of the monoamine hypothesis are widely accepted in terms of drug efficacy, though altered monoamine neurotransmission remains relevant to much of the safety profile of antidepressants. But composite endpoint safety data from long term trials does not have sufficient sensitivity to fully document the effect of these alterations in brain biochemistry on the psyche. This includes quantifying neurophysiological adaptation to long term treatment.
Promotion of small effects
Symptom severity fluctuates spontaneously during depression, and antidepressants started during exacerbations can appear to be more successful than they are. In a typical 6-12 week trial, scores among participants in the placebo arm fall from a mean of roughly 25 to 12-15 on the widely used Hamilton Depression Rating Scale. Any additional effect of active treatment is usually of questionable clinical importance.
A cycle of enthusiasm for the latest drug, big pharma’s large promotional budgets, and the delayed recognition of risk recurs throughout the history of pharmacology. Past examples in psychiatry include morphine, heroin, insulin, metronidazole, chloral hydrate, bromides, hyoscine, barbiturates, amphetamines, and major tranquilisers.
Esketamine, although not a typical antidepressant, is a recent example of how limited evidence for a new drug can attract favourable publicity. 7 The trials used the Montgomery-Åsberg Depression Rating Scale (score range 0-60), which is more sensitive to changes induced by antidepressants than the Hamilton scale. The US Food and Drug Administration approved esketamine in 2019 8 based on a finding of a 20 points reduction with esketamine compared with 16 points with placebo in the first 28 days of treatment. 8 Most of the reduction in the esketamine arm was also seen with placebo. The four point difference between drug and placebo reached a significance of P<0.05 in some trials only if a one sided P value was used. 9 Despite these small changes and no evidence of a persistent, clinically relevant, benefit, the FDA approval was accompanied by press coverage 7 and the drug heralded as a “first in class” treatment in the New England Journal of Medicine . 9
The small effect sizes reported for antidepressants are often further reduced after a drug is marketed. This was the fate of reboxetine, 10 11 authorised in the UK in 1997. The effectiveness of reboxetine was analysed by remission and responder rates, which do not translate directly into clinical significance. Reboxetine was ineffective in mild or moderate depression. A post-hoc analysis showed a statistically significant treatment effect on response rate for severe depression, though not a clinically significant benefit in symptom rating scales. 10 A former FDA employee noted the full set of data, on which the FDA had based a negative opinion for reboxetine, is not publicly available. 12
Meta-analyses and mean differences
Two recent systematic reviews and meta-analyses—one examining 21 common antidepressants 13 and the other selective serotonin reuptake inhibitors (SSRIs) 14 —found statistically, but not clinically, significant effects. Both attracted publicity that promoted antidepressant use 15 despite criticisms of the analyses. 16 17 18 19 20
Unblinding from adverse events may have contributed to the 0.3 standardised mean difference in effect size seen with antidepressants 13 and the mean difference of <2 on the Hamilton scale with SSRIs. 14 Standardised mean difference compensates for different rating scales, and a minimum of difference of 0.5 has been recommended for clinical significance. 21 A 2004 guideline from the National Institute for Health and Care Excellence (NICE) proposed a minimum of three points for a clinically significant difference on the 52 point Hamilton scale. 22 Even a three point change may be too low a threshold, 23 because it is undetectable by clinicians. 24
The spontaneous variability of the disease within the study population means that both placebo and active treatment patients can sometimes be classified as responders. It is the mean difference between the two groups that defines the treatment effect; it is not valid to say some individuals have responded to treatment, as this cannot be distinguished from background fluctuation in symptoms.
No evidence shows that increasing antidepressant dose increases the response in severe depression (standardised mean difference 0.05; 95% confidence interval −0.14 to 0.25). 25 Higher doses have been linked to violence, suicidality, homicide, mania, and psychosis. 26
The Oxford meta-analysis of 21 antidepressants did not sufficiently account for bias, selective outcome reporting, or reasons for attrition. 16 17 18 19 20 Efficacy analysis was restricted to 8-12 weeks’ treatment, though treatment for years is common. For the SSRI meta-analysis, there were almost no data on suicidal behaviour, quality of life, and long-term effects. 14 A reasonable conclusion of systematic meta-analyses is that antidepressants do not cause clinically significant improvements in depression.
Missing negative data
Meta-analyses depend on data from systematic reviews, 27 but to be reliable they need to dig into regulatory datasets to find the data. 3 21
The more compounds that are developed, the greater the chance of a false positive result. Less than half of antidepressant trials submitted to the FDA have positive results, 3 28 but many more trials with negative findings are not submitted. Journals rarely check protocol endpoints, and published claims of efficacy are often greater than the effects observed on the protocol specified primary endpoint. 29 When antidepressants are approved, negative data can be overlooked, as with vilazodone in 2011, when two positive trials were mentioned in the FDA label and five negative trials omitted. 29 Negative results must be included in risk-benefit analyses, as shown by the case of reboxetine. 10 11
It is a challenge to ensure meta-analyses consider all available data. Many negative trials of antidepressants are not publicly available, 25 26 28 and about half of trials do not comply with EU requirements to register their results. 30
Negative trials may be submitted to regulators under conditions of confidentiality. Trials from failed developments may never be submitted. 17 Some drugs developed for depression, such as sibutramine or varenicline, have been approved for other indications after trials in depression failed. Meta-analyses consider the influence of multiplicity on statistical significance; this is important as more than 20 P values can be reported for a single trial. 31 But meta-analyses cannot consider the influence of multiplicity for trials whose results are not available, or for drugs where a failed development means they are no longer classified as antidepressants, even though their results may cast doubt on a class benefit.
Unbalanced methods for assessing benefit and risk
Psychiatry depends on composite endpoints for the diagnosis and monitoring of mental illness. The Montgomery-Åsberg scale measures 10 symptoms, and the Hamilton scale a minimum of 17. Were these symptoms assessed separately, no trial of efficacy would reach statistical significance. By contrast, adverse events are categorised separately and rarely made into a composite.
The safety database submitted for esketamine approval was unusual as it included a composite to assess dissociation, the Clinician-Administered Dissociative Symptoms Scale. The assessment showed that 61%-75% of patients with current treatment developed dissociative or perceptual changes. Other safety indicators, including misuse, suicidal thoughts and behaviours, increased blood pressure, cognitive impairment, and cystitis were measured separately and not summarised in a composite. An incidence of at least 5% and at least twice that of placebo was reported for dissociation, dizziness, nausea, sedation, vertigo, hypoaesthesia, anxiety, lethargy, blood pressure increase, vomiting, and feeling drunk. 8 Whereas one primary endpoint was used to summarise benefit, safety was analysed as a collection of symptoms with no single endpoint, mitigating against finding statistical significance 32 and leading to the asymmetrical analysis of risk and benefit. 33
Limited safety data
Concerns raised by patient representatives about dependence, safety, and the level of prescribing led the UK All-Party Parliamentary Group for Prescribed Drug Dependence to examine the evidence on withdrawal problems with antidepressants. 34 35 More than half of people who attempt to stop taking antidepressants reported withdrawal effects; almost half of the reported effects were severe and some lasted for weeks or months. Many patients have made individual reports of withdrawal adverse events, though such reports have been criticised for a lack methodological rigour. 36 Nonetheless, there is concern that patients’ experiences of withdrawal problems are not fully reflected in drug information labels. 37 38
Data from the US, UK, Australia, Denmark, Iceland, and Sweden suggest a correlation between the use of antidepressants and the increasing prevalence of mental disability. Antidepressants might increase the risk of chronic depression, the incidence of conversion from a unipolar to bipolar disorder, and the chance of being classified disabled. 39
Long term use may propel the illness to a more malignant and unresponsive course, 40 possibly triggered by changes in brain biochemistry. 41 Since 1966 there has been concern that antidepressants might shorten the intervals between depressive episodes; about 6% of patients are in remission after 12 months’ treatment compared with about 85% in control groups. 39 The involvement of the cholinergic system in several types of dementia raises concern about the long term anticholinergic effects of many antidepressants. 42
An observational study of antidepressant use among older people found increases in serious adverse events such as death, falls, fractures, strokes and seizures. 43 Despite the study’s weakness, serious morbidity and mortality cannot be excluded as a consequence of antidepressant treatment of older people.
Inconsistencies
Many prescribers consider their accumulated clinical observations of individual cases proves the efficacy of antidepressants. Given the long established use of these drugs, it might be expected that the level of prescribing would now be stable. Yet the use of antidepressants in the UK has doubled in the past decade, and most are taken for more than a year. This has not reduced the rate of mental disability, or suicide—the most serious outcome in depression. If antidepressants were effective it might be expected that treatment would reduce the suicide rate, or at least suicide ideation.
A review of esketamine describes how a third of patients with treatment resistant depression attempt suicide 9 but does not discuss the effect of the drug on this endpoint. This is despite the fact that three patients who received the drug died by suicide during trials, compared with none in the control group. 7 The FDA label describes a non-significant increase in suicidality, recommending that patients who experience emergent suicidal thoughts or behaviours should stop treatment. 8
Public health concern
Given limited efficacy and long term safety concerns, the current level of UK prescribing is a major public health concern. Widespread prescribing is supported by short duration trials using composite endpoints designed to detect minor changes in efficacy, enthusiastic interpretation of meta-analyses, 13 and large promotion budgets.
The antidepressant market globally has exceeded $10bn (£7.6bn; €8.5bn) a year for several years. Fluoxetine (Prozac) reached blockbuster status, and esketamine is also predicted to reach sales over $1bn a year. Substantial sums are spent on marketing, advertising, public relations, and support of medical education and academia. Direct-to-consumer advertising for SSRIs has cited the monoamine hypothesis, even though it has no scientific support. 44
Money for independent reviews is negligible by comparison. The case to reduce prescribing is driven largely by patient advocates and a safety database devoid of the composites capable of summarising long term safety or identifying dependence. Considerable expertise is needed to select patients with more severe depression who will benefit from treatment and to determine how best to wean off patients for whom the long term risk-benefit balance is not favourable.
Teaching prescribers which drug to choose, when to prescribe it, and to which patient, needs to be expanded to include when not to prescribe and how to deprescribe. Prescribers in busy practice with limited consultation time should be cautious about starting patients on antidepressants for mild or moderate depression.
John Warren is a clinical pharmacologist whose interest in antidepressants developed during 16 years spent as an expert medical assessor at the Medicines and Healthcare Products Regulatory Agency. He represented the UK on the scientific advice working party of the European Medicines Agency, which highlighted the limitations of data used to justify new antidepressant authorisations, particularly an imbalance in the use of risk-benefit composite endpoints.
Acknowledgments
I thank S Dimmitt, R Ferner, Y Looke, J Ritter, H Stamfer, and P Feldschreiber for their help.
Competing interests: I have read and understood BMJ policy on declaration of interests and declare I am paid dividends from the medical consultancy Medicines Assessment. I am a member of the joint specialty committee for clinical pharmacology and therapeutics at the Royal College of Physicians and acted as an RCP representative at the APPG for Prescribed Drug Dependence, Westminster. I advise the Cure Parkinson’s Trust.
Provenance and peer review: Commissioned; externally peer reviewed.
- ↵ Taylor S, Annand F, Burkinshaw P, et al. Dependence and withdrawal associated with some prescribed medicines: an evidence review. Public Health England, 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/829777/PHE_PMR_report.pdf
- Turner EH ,
- Matthews AM ,
- Linardatos E ,
- Rosenthal R
- Ebrahim S ,
- Malachowski C ,
- Ioannidis JP
- Chávez-Castillo M ,
- ↵ Huetteman E. FDA Overlooked red flags in drugmaker’s testing of new depression medicine. Kaiser Health News 2019. https://khn.org/news/fdas-approval-of-new-depression-drug-overlooked-red-flags-in-its-testing/
- ↵ FDA. Esketamine—full prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf
- Farchione T ,
- ↵ Medicines and Healthcare Products Regulatory Authority. Public assessment report—reboxetine: a review of the benefits and risks. 2011. http://www.mhra.gov.uk/home/groups/s-par/documents/websiteresources/con129107.pdf
- Lelgemann M ,
- Grouven U ,
- Cipriani A ,
- Furukawa TA ,
- Salanti G ,
- Jakobsen JC ,
- Katakam KK ,
- Adlington K
- Irving Kirsch I ,
- Jakobsen JC
- Paludan-Müller AS ,
- Moncrieff J ,
- Gøtzche P ,
- ↵ National Institute for Clinical Excellence. Depression: management of depression. primary and secondary care clinical practice guideline no 23. 2004. https://www.nice.org.uk/guidance/CG23 .
- Fennema H ,
- Kaspers-Janssen M ,
- Lepping P ,
- Henssler J ,
- Franklin J ,
- Liberati A ,
- Altman DG ,
- Tetzlaff J ,
- Laughren TP
- Goldacre B ,
- DeVito NJ ,
- Heneghan C ,
- Harrington D ,
- D’Agostino RB Sr . ,
- Gatsonis C ,
- Warren JB ,
- Feldschreiber P
- ↵ Davies J, Pauli R, Montagu L. Antidepressant withdrawal. Survey of patients’ experience by the all-party parliamentary group for prescribed drug dependence. 2018. http://prescribeddrug.org/wp-content/uploads/2018/09/APPG-PDD-Antidepressant-Withdrawal-Patient-Survey.pdf
- Andrews PW ,
- Thomson JA Jr . ,
- Amstadter A ,
- ↵ Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times 2018 Apr 7. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html
- El-Mallakh RS ,
- Coupland CAC ,
- Morriss R ,
- Hippisley-Cox J
- Coupland C ,
- Lacasse JR ,
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
April 23, 2024
Research in Context: Treating depression
Finding better approaches.
While effective treatments for major depression are available, there is still room for improvement. This special Research in Context feature explores the development of more effective ways to treat depression, including personalized treatment approaches and both old and new drugs.

Everyone has a bad day sometimes. People experience various types of stress in the course of everyday life. These stressors can cause sadness, anxiety, hopelessness, frustration, or guilt. You may not enjoy the activities you usually do. These feelings tend to be only temporary. Once circumstances change, and the source of stress goes away, your mood usually improves. But sometimes, these feelings don’t go away. When these feelings stick around for at least two weeks and interfere with your daily activities, it’s called major depression, or clinical depression.
In 2021, 8.3% of U.S. adults experienced major depression. That’s about 21 million people. Among adolescents, the prevalence was much greater—more than 20%. Major depression can bring decreased energy, difficulty thinking straight, sleep problems, loss of appetite, and even physical pain. People with major depression may become unable to meet their responsibilities at work or home. Depression can also lead people to use alcohol or drugs or engage in high-risk activities. In the most extreme cases, depression can drive people to self-harm or even suicide.
The good news is that effective treatments are available. But current treatments have limitations. That’s why NIH-funded researchers have been working to develop more effective ways to treat depression. These include finding ways to predict whether certain treatments will help a given patient. They're also trying to develop more effective drugs or, in some cases, find new uses for existing drugs.
Finding the right treatments
The most common treatments for depression include psychotherapy, medications, or a combination. Mild depression may be treated with psychotherapy. Moderate to severe depression often requires the addition of medication.
Several types of psychotherapy have been shown to help relieve depression symptoms. For example, cognitive behavioral therapy helps people to recognize harmful ways of thinking and teaches them how to change these. Some researchers are working to develop new therapies to enhance people’s positive emotions. But good psychotherapy can be hard to access due to the cost, scheduling difficulties, or lack of available providers. The recent growth of telehealth services for mental health has improved access in some cases.
There are many antidepressant drugs on the market. Different drugs will work best on different patients. But it can be challenging to predict which drugs will work for a given patient. And it can take anywhere from 6 to 12 weeks to know whether a drug is working. Finding an effective drug can involve a long period of trial and error, with no guarantee of results.
If depression doesn’t improve with psychotherapy or medications, brain stimulation therapies could be used. Electroconvulsive therapy, or ECT, uses electrodes to send electric current into the brain. A newer technique, transcranial magnetic stimulation (TMS), stimulates the brain using magnetic fields. These treatments must be administered by specially trained health professionals.
“A lot of patients, they kind of muddle along, treatment after treatment, with little idea whether something’s going to work,” says psychiatric researcher Dr. Amit Etkin.
One reason it’s difficult to know which antidepressant medications will work is that there are likely different biological mechanisms that can cause depression. Two people with similar symptoms may both be diagnosed with depression, but the causes of their symptoms could be different. As NIH depression researcher Dr. Carlos Zarate explains, “we believe that there’s not one depression, but hundreds of depressions.”
Depression may be due to many factors. Genetics can put certain people at risk for depression. Stressful situations, physical health conditions, and medications may contribute. And depression can also be part of a more complicated mental disorder, such as bipolar disorder. All of these can affect which treatment would be best to use.
Etkin has been developing methods to distinguish patients with different types of depression based on measurable biological features, or biomarkers. The idea is that different types of patients would respond differently to various treatments. Etkin calls this approach “precision psychiatry.”
One such type of biomarker is electrical activity in the brain. A technique called electroencephalography, or EEG, measures electrical activity using electrodes placed on the scalp. When Etkin was at Stanford University, he led a research team that developed a machine-learning algorithm to predict treatment response based on EEG signals. The team applied the algorithm to data from a clinical trial of the antidepressant sertraline (Zoloft) involving more than 300 people.

EEG data for the participants were collected at the outset. Participants were then randomly assigned to take either sertraline or an inactive placebo for eight weeks. The team found a specific set of signals that predicted the participants’ responses to sertraline. The same neural “signature” also predicted which patients with depression responded to medication in a separate group.
Etkin’s team also examined this neural signature in a set of patients who were treated with TMS and psychotherapy. People who were predicted to respond less to sertraline had a greater response to the TMS/psychotherapy combination.
Etkin continues to develop methods for personalized depression treatment through his company, Alto Neuroscience. He notes that EEG has the advantage of being low-cost and accessible; data can even be collected in a patient’s home. That’s important for being able to get personalized treatments to the large number of people they could help. He’s also working on developing antidepressant drugs targeted to specific EEG profiles. Candidate drugs are in clinical trials now.
“It’s not like a pie-in-the-sky future thing, 20-30 years from now,” Etkin explains. “This is something that could be in people's hands within the next five years.”

New tricks for old drugs
While some researchers focus on matching patients with their optimal treatments, others aim to find treatments that can work for many different patients. It turns out that some drugs we’ve known about for decades might be very effective antidepressants, but we didn’t recognize their antidepressant properties until recently.
One such drug is ketamine. Ketamine has been used as an anesthetic for more than 50 years. Around the turn of this century, researchers started to discover its potential as an antidepressant. Zarate and others have found that, unlike traditional antidepressants that can take weeks to take effect, ketamine can improve depression in as little as one day. And a single dose can have an effect for a week or more. In 2019, the FDA approved a form of ketamine for treating depression that is resistant to other treatments.
But ketamine has drawbacks of its own. It’s a dissociative drug, meaning that it can make people feel disconnected from their body and environment. It also has the potential for addiction and misuse. For these reasons, it’s a controlled substance and can only be administered in a doctor’s office or clinic.
Another class of drugs being studied as possible antidepressants are psychedelics. These include lysergic acid diethylamide (LSD) and psilocybin, the active ingredient in magic mushrooms. These drugs can temporarily alter a person’s mood, thoughts, and perceptions of reality. Some have historically been used for religious rituals, but they are also used recreationally.
In clinical studies, psychedelics are typically administered in combination with psychotherapy. This includes several preparatory sessions with a therapist in the weeks before getting the drug, and several sessions in the weeks following to help people process their experiences. The drugs are administered in a controlled setting.
Dr. Stephen Ross, co-director of the New York University Langone Health Center for Psychedelic Medicine, describes a typical session: “It takes place in a living room-like setting. The person is prepared, and they state their intention. They take the drug, they lie supine, they put on eye shades and preselected music, and two therapists monitor them.” Sessions last for as long as the acute effects of the drug last, which is typically several hours. This is a healthcare-intensive intervention given the time and personnel needed.
In 2016, Ross led a clinical trial examining whether psilocybin-assisted therapy could reduce depression and anxiety in people with cancer. According to Ross, as many as 40% of people with cancer have clinically significant anxiety and depression. The study showed that a single psilocybin session led to substantial reductions in anxiety and depression compared with a placebo. These reductions were evident as soon as one day after psilocybin administration. Six months later, 60-80% of participants still had reduced depression and anxiety.
Psychedelic drugs frequently trigger mystical experiences in the people who take them. “People can feel a sense…that their consciousness is part of a greater consciousness or that all energy is one,” Ross explains. “People can have an experience that for them feels more ‘real’ than regular reality. They can feel transported to a different dimension of reality.”
About three out of four participants in Ross’s study said it was among the most meaningful experiences of their lives. And the degree of mystical experience correlated with the drug’s therapeutic effect. A long-term follow-up study found that the effects of the treatment continued more than four years later.
If these results seem too good to be true, Ross is quick to point out that it was a small study, with only 29 participants, although similar studies from other groups have yielded similar results. Psychedelics haven’t yet been shown to be effective in a large, controlled clinical trial. Ross is now conducting a trial with 200 people to see if the results of his earlier study pan out in this larger group. For now, though, psychedelics remain experimental drugs—approved for testing, but not for routine medical use.
Unlike ketamine, psychedelics aren’t considered addictive. But they, too, carry risks, which certain conditions may increase. Psychedelics can cause cardiovascular complications. They can cause psychosis in people who are predisposed to it. In uncontrolled settings, they have the risk of causing anxiety, confusion, and paranoia—a so-called “bad trip”—that can lead the person taking the drug to harm themself or others. This is why psychedelic-assisted therapy takes place in such tightly controlled settings. That increases the cost and complexity of the therapy, which may prevent many people from having access to it.
Better, safer drugs
Despite the promise of ketamine or psychedelics, their drawbacks have led some researchers to look for drugs that work like them but with fewer side effects.
Depression is thought to be caused by the loss of connections between nerve cells, or neurons, in certain regions of the brain. Ketamine and psychedelics both promote the brain’s ability to repair these connections, a quality called plasticity. If we could understand how these drugs encourage plasticity, we might be able to design drugs that can do so without the side effects.

Dr. David Olson at the University of California, Davis studies how psychedelics work at the cellular and molecular levels. The drugs appear to promote plasticity by binding to a receptor in cells called the 5-hydroxytryptamine 2A receptor (5-HT2AR). But many other compounds also bind 5-HT2AR without promoting plasticity. In a recent NIH-funded study, Olson showed that 5-HT2AR can be found both inside and on the surface of the cell. Only compounds that bound to the receptor inside the cells promoted plasticity. This suggests that a drug has to be able to get into the cell to promote plasticity.
Moreover, not all drugs that bind 5-HT2AR have psychedelic effects. Olson’s team has developed a molecular sensor, called psychLight, that can identify which compounds that bind 5-HT2AR have psychedelic effects. Using psychLight, they identified compounds that are not psychedelic but still have rapid and long-lasting antidepressant effects in animal models. He’s founded a company, Delix Therapeutics, to further develop drugs that promote plasticity.
Meanwhile, Zarate and his colleagues have been investigating a compound related to ketamine called hydroxynorketamine (HNK). Ketamine is converted to HNK in the body, and this process appears to be required for ketamine’s antidepressant effects. Administering HNK directly produced antidepressant-like effects in mice. At the same time, it did not cause the dissociative side effects and addiction caused by ketamine. Zarate’s team has already completed phase I trials of HNK in people showing that it’s safe. Phase II trials to find out whether it’s effective are scheduled to begin soon.
“What [ketamine and psychedelics] are doing for the field is they’re helping us realize that it is possible to move toward a repair model versus a symptom mitigation model,” Olson says. Unlike existing antidepressants, which just relieve the symptoms of depression, these drugs appear to fix the underlying causes. That’s likely why they work faster and produce longer-lasting effects. This research is bringing us closer to having safer antidepressants that only need to be taken once in a while, instead of every day.
—by Brian Doctrow, Ph.D.
Related Links
- How Psychedelic Drugs May Help with Depression
- Biosensor Advances Drug Discovery
- Neural Signature Predicts Antidepressant Response
- How Ketamine Relieves Symptoms of Depression
- Protein Structure Reveals How LSD Affects the Brain
- Predicting The Usefulness of Antidepressants
- Depression Screening and Treatment in Adults
- Serotonin Transporter Structure Revealed
- Placebo Effect in Depression Treatment
- When Sadness Lingers: Understanding and Treating Depression
- Psychedelic and Dissociative Drugs
References: An electroencephalographic signature predicts antidepressant response in major depression. Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo GA, Rolle CE, Cooper C, Chin-Fatt C, Krepel N, Cornelssen CA, Wright R, Toll RT, Trivedi HM, Monuszko K, Caudle TL, Sarhadi K, Jha MK, Trombello JM, Deckersbach T, Adams P, McGrath PJ, Weissman MM, Fava M, Pizzagalli DA, Arns M, Trivedi MH, Etkin A. Nat Biotechnol. 2020 Feb 10. doi: 10.1038/s41587-019-0397-3. Epub 2020 Feb 10. PMID: 32042166. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, Mennenga SE, Belser A, Kalliontzi K, Babb J, Su Z, Corby P, Schmidt BL. J Psychopharmacol . 2016 Dec;30(12):1165-1180. doi: 10.1177/0269881116675512. PMID: 27909164. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, Bossis AP, Grigsby J, Fischer S, Ross S. J Psychopharmacol . 2020 Feb;34(2):155-166. doi: 10.1177/0269881119897615. Epub 2020 Jan 9. PMID: 31916890. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE. Science . 2023 Feb 17;379(6633):700-706. doi: 10.1126/science.adf0435. Epub 2023 Feb 16. PMID: 36795823. Psychedelic-inspired drug discovery using an engineered biosensor. Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, Azinfar A, Oh WC, Wetsel WC, Olson DE, Tian L. Cell . 2021 Apr 8: S0092-8674(21)00374-3. doi: 10.1016/j.cell.2021.03.043. Epub 2021 Apr 28. PMID: 33915107. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD. Nature . 2016 May 26;533(7604):481-6. doi: 10.1038/nature17998. Epub 2016 May 4. PMID: 27144355.
Connect with Us
- More Social Media from NIH

Can Genetic Testing Reveal the Right Antidepressant?
Precision medicine: how your dna can determine the best antidepressant..
Updated April 27, 2024 | Reviewed by Lybi Ma
- Find a therapist to overcome depression or anxiety
- Pharmacogenetic testing helps identify the best medications for patients based on their unique genetic makeup.
- This testing reduces trial and error with psychiatric medications
- Testing can lead to reduced side effects and a faster response in depression treatment.
By Joshua Plutchik and Lori Plutchik, M.D.
The treatment of clinical depression presents unique challenges, with many patients voicing concerns that echo a disheartening struggle: "I have tried every medication for depression, and nothing works." Or, "I cannot tolerate the side effects of any antidepressants ." Such sentiments underscore the acute need for more tailored therapeutic strategies in mental health care.
Depression is a deeply debilitating disorder that affects over 21 million American adults annually. The repercussions are severe, leading to an astonishing loss of approximately 200 million workdays and inflicting an economic burden of approximately $20 billion each year. Beyond these stark statistics, the human cost is even more alarming. Depression profoundly erodes the quality of life, severely strains personal relationships, and, in severe cases, can culminate in suicide .
Regarding the need for personalized treatment approaches in mental health, consider a parallel with another medical specialty. Imagine seeking treatment for a swollen, painful knee. How would an orthopedist ensure that treatment effectively addresses the underlying cause? Typically, before suggesting surgery, they would perform a comprehensive assessment, including a physical exam, blood tests, and imaging. Why should psychiatric treatment be any different? Just as we would not rush to surgery for a swollen knee without thorough diagnostics, prescribing antidepressants without a comprehensive understanding of a patient’s genetic profile seems increasingly outdated. Pharmacogenetic testing offers critical data, guiding the selection of treatment strategies with precision.
The last decade has seen transformative advancements in psychiatric pharmacogenetics, thus potentially revolutionizing psychiatric care. Dr. Seema Patel, PharmD, BCPP, a medical science liaison at Genomind, elucidates the profound effect of this testing: "The way that pharmacogenetic testing can help you with your medications is being able to identify based on your unique genetics how you might respond to certain medications and what the risk of side effects would be with certain medications" (S. Patel, personal communication, March 24, 2024).
The Mechanics and Merits of Pharmacogenetic Testing
Pharmacogenetic testing is easily administered through a cheek swab in a clinical setting or at home. Many commercial insurance plans and Medicare now cover this. The concept is both simple and profoundly effective: by examining how individual genetic profiles influence drug metabolism and response, this testing pinpoints which class of antidepressants, such as SSRIs or SNRIs, are likely to be most effective for an individual. In addition, it can determine which specific medications are least likely to cause side effects.
Pharmacogenetic testing is also insightful about how non-pharmaceutical interventions, such as dietary supplements like l-methylfolate or magnesium, might benefit patients. The test even offers personalized insight into the benefits of exercise for mental health based on patients' genetic variants.
Scientific Validation of Pharmacogenetic Testing
The promise of pharmacogenetic testing extends beyond the theoretical realm, with robust research substantiating its efficacy. Dr. Patel cited a meta-analysis by Bousman and colleagues (2019) that indicated patients receiving pharmacogenetic-guided treatment exhibited a 70 percent higher probability of achieving remission when compared with those treated under standard care protocols. This supports earlier findings by Rosenblat and colleagues (2018), whose research demonstrated significantly enhanced response and remission rates in the treatment of depression when treatment was informed by genetic testing.
Further, Dr. Patel referenced a study by Swen and colleagues (2023), which found that pharmacogenetic-guided care reduced the risk of adverse drug reactions by 30 percent compared to traditional methods. Another compelling study by David and colleagues (2021) indicated that pharmacogenetic-guided patients were 50 percent less likely to be hospitalized than those under standard care.
The Future of Pharmacogenetic Testing in Psychiatry: A Holistic and Personalized Approach
It is crucial to note that while pharmacogenetic testing is a powerful tool, it should not be the sole basis for psychiatric treatment decisions. It is a component of a multifaceted approach that includes comprehensive clinical evaluations and ongoing patient monitoring. As research continues to advance and more psychiatrists adopt this technology, the hope is that fewer patients will have to endure the often debilitating journey through ineffective treatments. Pharmacogenetic testing promises a deeper understanding of individual responses to psychiatric medications but also paves the way for more personalized, effective mental health treatment.
Bousman, C. A., Arandjelovic, K., Mancuso, S. G., Eyre, H. A., & Dunlop, B. W. (2019). Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics, 20(1), 37–47. https://doi.org/10.2217/pgs-2018-0142
David, V., Fylan, B., Bryant, E., Smith, H., Sagoo, G. S., & Rattray, M. (2021). An Analysis of Pharmacogenomic-Guided Pathways and Their Effect on Medication Changes and Hospital Admissions: A Systematic Review and Meta-Analysis. Frontiers in Genetics, 12. https://doi.org/10.3389/fgene.2021.698148
Rosenblat, J. D., Lee, Y., & McIntyre, R. S. (2018). The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis. Journal of Affective Disorders, 241, 484–491. https://doi.org/10.1016/j.jad.2018.08.056
Swen, J. J., van der Wouden, C. H., Manson, L. E., Abdullah-Koolmees, H., Blagec, K., Blagus, T., Böhringer, S., Cambon-Thomsen, A., Cecchin, E., Cheung, K.-C., Deneer, V. H., Dupui, M., Ingelman-Sundberg, M., Jonsson, S., Joefield-Roka, C., Just, K. S., Karlsson, M. O., Konta, L., Koopmann, R., & Kriek, M. (2023). A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet (London, England), 401(10374), 347–356. https://doi.org/10.1016/S0140-6736(22)01841-4

Lori Plutchik, M.D., is a distinguished board-certified psychiatrist in New York City, who has been in practice for over 25 years.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Teletherapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience
Sean Goddard on Spravato: The Fast-Acting Antidepressant Changing Lives Viking Psychiatry Podcast
- Mental Health
How does Spravato work? When you're caught in the relentless grip of depression, waiting weeks for a medication to work isn't just frustrating—it can feel like an eternity. Sean Goddard of Viking Psychiatry shares his personal and professional journey with Spravato, offering an intimate understanding of why swift treatment can be life-saving. As a dual-certified nurse practitioner and someone who has walked through the shadowed valleys of depression himself, Sean uncovers the potent effects of Spravato on the brain's neurotransmitters, particularly glutamate, and discusses how this revolutionary drug can foster neural growth within hours, not weeks. Join us as we explore how Spravato is rewriting the narrative of mental health treatment. Sean illustrates the science behind this rapid-acting antidepressant with clarity, likening brain-derived neurotrophic factor (BDNF) to 'miracle grow for the brain.' In a field where slow-acting medications have been the norm, hearing about a treatment that can forge new neural pathways so quickly is nothing short of hopeful. This episode isn't just about the mechanics of medicine—it's a testament to human resilience and the transformative power of innovative care that offers more than a glimmer of hope—it offers a rapid route back to life. To learn more about Viking Psychiatry go to: https://www.vikingpsychiatry.com/ Viking Psychiatry 9025 Coldwater Rd Suite 100 Fort Wayne, IN 46825 260-459-9225
- More Episodes
- © 2024 Viking Psychiatry Podcast
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 20 July 2022
The serotonin theory of depression: a systematic umbrella review of the evidence
- Joanna Moncrieff 1 , 2 ,
- Ruth E. Cooper 3 ,
- Tom Stockmann 4 ,
- Simone Amendola 5 ,
- Michael P. Hengartner 6 &
- Mark A. Horowitz 1 , 2
Molecular Psychiatry volume 28 , pages 3243–3256 ( 2023 ) Cite this article
1.26m Accesses
233 Citations
9427 Altmetric
Metrics details
- Diagnostic markers
The serotonin hypothesis of depression is still influential. We aimed to synthesise and evaluate evidence on whether depression is associated with lowered serotonin concentration or activity in a systematic umbrella review of the principal relevant areas of research. PubMed, EMBASE and PsycINFO were searched using terms appropriate to each area of research, from their inception until December 2020. Systematic reviews, meta-analyses and large data-set analyses in the following areas were identified: serotonin and serotonin metabolite, 5-HIAA, concentrations in body fluids; serotonin 5-HT 1A receptor binding; serotonin transporter (SERT) levels measured by imaging or at post-mortem; tryptophan depletion studies; SERT gene associations and SERT gene-environment interactions. Studies of depression associated with physical conditions and specific subtypes of depression (e.g. bipolar depression) were excluded. Two independent reviewers extracted the data and assessed the quality of included studies using the AMSTAR-2, an adapted AMSTAR-2, or the STREGA for a large genetic study. The certainty of study results was assessed using a modified version of the GRADE. We did not synthesise results of individual meta-analyses because they included overlapping studies. The review was registered with PROSPERO (CRD42020207203). 17 studies were included: 12 systematic reviews and meta-analyses, 1 collaborative meta-analysis, 1 meta-analysis of large cohort studies, 1 systematic review and narrative synthesis, 1 genetic association study and 1 umbrella review. Quality of reviews was variable with some genetic studies of high quality. Two meta-analyses of overlapping studies examining the serotonin metabolite, 5-HIAA, showed no association with depression (largest n = 1002). One meta-analysis of cohort studies of plasma serotonin showed no relationship with depression, and evidence that lowered serotonin concentration was associated with antidepressant use ( n = 1869). Two meta-analyses of overlapping studies examining the 5-HT 1A receptor (largest n = 561), and three meta-analyses of overlapping studies examining SERT binding (largest n = 1845) showed weak and inconsistent evidence of reduced binding in some areas, which would be consistent with increased synaptic availability of serotonin in people with depression, if this was the original, causal abnormaly. However, effects of prior antidepressant use were not reliably excluded. One meta-analysis of tryptophan depletion studies found no effect in most healthy volunteers ( n = 566), but weak evidence of an effect in those with a family history of depression ( n = 75). Another systematic review ( n = 342) and a sample of ten subsequent studies ( n = 407) found no effect in volunteers. No systematic review of tryptophan depletion studies has been performed since 2007. The two largest and highest quality studies of the SERT gene, one genetic association study ( n = 115,257) and one collaborative meta-analysis ( n = 43,165), revealed no evidence of an association with depression, or of an interaction between genotype, stress and depression. The main areas of serotonin research provide no consistent evidence of there being an association between serotonin and depression, and no support for the hypothesis that depression is caused by lowered serotonin activity or concentrations. Some evidence was consistent with the possibility that long-term antidepressant use reduces serotonin concentration.
Similar content being viewed by others
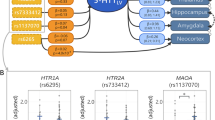
Genetic contributions to brain serotonin transporter levels in healthy adults
The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies
The complex clinical response to selective serotonin reuptake inhibitors in depression: a network perspective
Introduction.
The idea that depression is the result of abnormalities in brain chemicals, particularly serotonin (5-hydroxytryptamine or 5-HT), has been influential for decades, and provides an important justification for the use of antidepressants. A link between lowered serotonin and depression was first suggested in the 1960s [ 1 ], and widely publicised from the 1990s with the advent of the Selective Serotonin Reuptake Inhibitor (SSRI) antidepressants [ 2 , 3 , 4 ]. Although it has been questioned more recently [ 5 , 6 ], the serotonin theory of depression remains influential, with principal English language textbooks still giving it qualified support [ 7 , 8 ], leading researchers endorsing it [ 9 , 10 , 11 ], and much empirical research based on it [ 11 , 12 , 13 , 14 ]. Surveys suggest that 80% or more of the general public now believe it is established that depression is caused by a ‘chemical imbalance’ [ 15 , 16 ]. Many general practitioners also subscribe to this view [ 17 ] and popular websites commonly cite the theory [ 18 ].
It is often assumed that the effects of antidepressants demonstrate that depression must be at least partially caused by a brain-based chemical abnormality, and that the apparent efficacy of SSRIs shows that serotonin is implicated. Other explanations for the effects of antidepressants have been put forward, however, including the idea that they work via an amplified placebo effect or through their ability to restrict or blunt emotions in general [ 19 , 20 ].
Despite the fact that the serotonin theory of depression has been so influential, no comprehensive review has yet synthesised the relevant evidence. We conducted an ‘umbrella’ review of the principal areas of relevant research, following the model of a similar review examining prospective biomarkers of major depressive disorder [ 21 ]. We sought to establish whether the current evidence supports a role for serotonin in the aetiology of depression, and specifically whether depression is associated with indications of lowered serotonin concentrations or activity.
Search strategy and selection criteria
The present umbrella review was reported in accordance with the 2009 PRISMA statement [ 22 ]. The protocol was registered with PROSPERO in December 2020 (registration number CRD42020207203) ( https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=207203 ). This was subsequently updated to reflect our decision to modify the quality rating system for some studies to more appropriately appraise their quality, and to include a modified GRADE to assess the overall certainty of the findings in each category of the umbrella review.
In order to cover the different areas and to manage the large volume of research that has been conducted on the serotonin system, we conducted an ‘umbrella’ review. Umbrella reviews survey existing systematic reviews and meta-analyses relevant to a research question and represent one of the highest levels of evidence synthesis available [ 23 ]. Although they are traditionally restricted to systematic reviews and meta-analyses, we aimed to identify the best evidence available. Therefore, we also included some large studies that combined data from individual studies but did not employ conventional systematic review methods, and one large genetic study. The latter used nationwide databases to capture more individuals than entire meta-analyses, so is likely to provide even more reliable evidence than syntheses of individual studies.
We first conducted a scoping review to identify areas of research consistently held to provide support for the serotonin hypothesis of depression. Six areas were identified, addressing the following questions: (1) Serotonin and the serotonin metabolite 5-HIAA–whether there are lower levels of serotonin and 5-HIAA in body fluids in depression; (2) Receptors - whether serotonin receptor levels are altered in people with depression; (3) The serotonin transporter (SERT) - whether there are higher levels of the serotonin transporter in people with depression (which would lower synaptic levels of serotonin); (4) Depletion studies - whether tryptophan depletion (which lowers available serotonin) can induce depression; (5) SERT gene – whether there are higher levels of the serotonin transporter gene in people with depression; (6) Whether there is an interaction between the SERT gene and stress in depression.
We searched for systematic reviews, meta-analyses, and large database studies in these six areas in PubMed, EMBASE and PsycINFO using the Healthcare Databases Advanced Search tool provided by Health Education England and NICE (National Institute for Health and Care Excellence). Searches were conducted until December 2020.
We used the following terms in all searches: (depress* OR affective OR mood) AND (systematic OR meta-analysis), and limited searches to title and abstract, since not doing so produced numerous irrelevant hits. In addition, we used terms specific to each area of research (full details are provided in Table S1 , Supplement). We also searched citations and consulted with experts.
Inclusion criteria were designed to identify the best available evidence in each research area and consisted of:
Research synthesis including systematic reviews, meta-analysis, umbrella reviews, individual patient meta-analysis and large dataset analysis.
Studies that involve people with depressive disorders or, for experimental studies (tryptophan depletion), those in which mood symptoms are measured as an outcome.
Studies of experimental procedures (tryptophan depletion) involving a sham or control condition.
Studies published in full in peer reviewed literature.
Where more than five systematic reviews or large analyses exist, the most recent five are included.
Exclusion criteria consisted of:
Animal studies.
Studies exclusively concerned with depression in physical conditions (e.g. post stroke or Parkinson’s disease) or exclusively focusing on specific subtypes of depression such as postpartum depression, depression in children, or depression in bipolar disorder.
No language or date restrictions were applied. In areas in which no systematic review or meta-analysis had been done within the last 10 years, we also selected the ten most recent studies at the time of searching (December 2020) for illustration of more recent findings. We performed this search using the same search string for this domain, without restricting it to systematic reviews and meta-analyses.
Data analysis
Each member of the team was allocated one to three domains of serotonin research to search and screen for eligible studies using abstract and full text review. In case of uncertainty, the entire team discussed eligibility to reach consensus.
For included studies, data were extracted by two reviewers working independently, and disagreement was resolved by consensus. Authors of papers were contacted for clarification when data was missing or unclear.
We extracted summary effects, confidence intervals and measures of statistical significance where these were reported, and, where relevant, we extracted data on heterogeneity. For summary effects in the non-genetic studies, preference was given to the extraction and reporting of effect sizes. Mean differences were converted to effect sizes where appropriate data were available.
We did not perform a meta-analysis of the individual meta-analyses in each area because they included overlapping studies [ 24 ]. All extracted data is presented in Table 1 . Sensitivity analyses were reported where they had substantial bearing on interpretation of findings.
The quality rating of systematic reviews and meta-analyses was assessed using AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews) [ 25 ]. For two studies that did not employ conventional systematic review methods [ 26 , 27 ] we used a modified version of the AMSTAR-2 (see Table S3 ). For the genetic association study based on a large database analysis we used the STREGA assessment (STrengthening the REporting of Genetic Association Studies) (Table S4 ) [ 28 ]. Each study was rated independently by at least two authors. We report ratings of individual items on the relevant measure, and the percentage of items that were adequately addressed by each study (Table 1 , with further detail in Tables S3 and S4 ).
Alongside quality ratings, two team members (JM, MAH) rated the certainty of the results of each study using a modified version of the GRADE guidelines [ 29 ]. Following the approach of Kennis et al. [ 21 ], we devised six criteria relevant to the included studies: whether a unified analysis was conducted on original data; whether confounding by antidepressant use was adequately addressed; whether outcomes were pre-specified; whether results were consistent or heterogeneity was adequately addressed if present; whether there was a likelihood of publication bias; and sample size. The importance of confounding by effects of current or past antidepressant use has been highlighted in several studies [ 30 , 31 ]. The results of each study were scored 1 or 0 according to whether they fulfilled each criteria, and based on these ratings an overall judgement was made about the certainty of evidence across studies in each of the six areas of research examined. The certainty of each study was based on an algorithm that prioritised sample size and uniform analysis using original data (explained more fully in the supplementary material), following suggestions that these are the key aspects of reliability [ 27 , 32 ]. An assessment of the overall certainty of each domain of research examining the role of serotonin was determined by consensus of at least two authors and a direction of effect indicated.
Search results and quality rating
Searching identified 361 publications across the 6 different areas of research, among which seventeen studies fulfilled inclusion criteria (see Fig. 1 and Table S1 for details of the selection process). Included studies, their characteristics and results are shown in Table 1 . As no systematic review or meta-analysis had been performed within the last 10 years on serotonin depletion, we also identified the 10 latest studies for illustration of more recent research findings (Table 2 ).
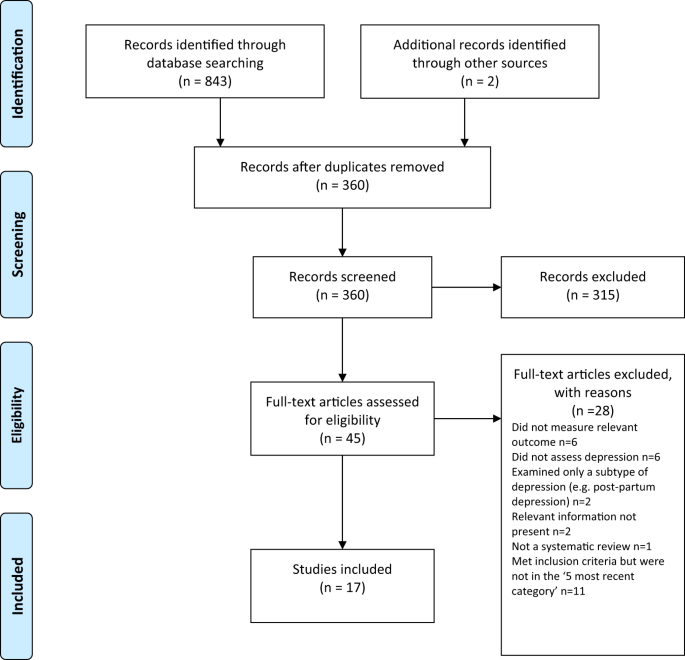
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagramme.
Quality ratings are summarised in Table 1 and reported in detail in Tables S2 – S3 . The majority (11/17) of systematic reviews and meta-analyses satisfied less than 50% of criteria. Only 31% adequately assessed risk of bias in individual studies (a further 44% partially assessed this), and only 50% adequately accounted for risk of bias when interpreting the results of the review. One collaborative meta-analysis of genetic studies was considered to be of high quality due to the inclusion of several measures to ensure consistency and reliability [ 27 ]. The large genetic analysis of the effect of SERT polymorphisms on depression, satisfied 88% of the STREGA quality criteria [ 32 ].
Serotonin and 5-HIAA
Serotonin can be measured in blood, plasma, urine and CSF, but it is rapidly metabolised to 5-hydroxyindoleacetic acid (5-HIAA). CSF is thought to be the ideal resource for the study of biomarkers of putative brain diseases, since it is in contact with brain interstitial fluid [ 33 ]. However, collecting CSF samples is invasive and carries some risk, hence large-scale studies are scarce.
Three studies fulfilled inclusion criteria (Table 1 ). One meta-analysis of three large observational cohort studies of post-menopausal women, revealed lower levels of plasma 5-HT in women with depression, which did not, however, reach statistical significance of p < 0.05 after adjusting for multiple comparisons. Sensitivity analyses revealed that antidepressants were strongly associated with lower serotonin levels independently of depression.
Two meta-analyses of a total of 19 studies of 5-HIAA in CSF (seven studies were included in both) found no evidence of an association between 5-HIAA concentrations and depression.
Fourteen different serotonin receptors have been identified, with most research on depression focusing on the 5-HT 1A receptor [ 11 , 34 ]. Since the functions of other 5-HT receptors and their relationship to depression have not been well characterised, we restricted our analysis to data on 5-HT 1A receptors [ 11 , 34 ]. 5-HT 1A receptors, known as auto-receptors, inhibit the release of serotonin pre-synaptically [ 35 ], therefore, if depression is the result of reduced serotonin activity caused by abnormalities in the 5-HT 1A receptor, people with depression would be expected to show increased activity of 5-HT 1A receptors compared to those without [ 36 ].
Two meta-analyses satisfied inclusion criteria, involving five of the same studies [ 37 , 38 ] (see Table 1 ). The majority of results across the two analyses suggested either no difference in 5-HT 1A receptors between people with depression and controls, or a lower level of these inhibitory receptors, which would imply higher concentrations or activity of serotonin in people with depression. Both meta-analyses were based on studies that predominantly involved patients who were taking or had recently taken (within 1–3 weeks of scanning) antidepressants or other types of psychiatric medication, and both sets of authors commented on the possible influence of prior or current medication on findings. In addition, one analysis was of very low quality [ 37 ], including not reporting on the numbers involved in each analysis and using one-sided p-values, and one was strongly influenced by three studies and publication bias was present [ 38 ].
The serotonin transporter (SERT)
The serotonin transporter protein (SERT) transports serotonin out of the synapse, thereby lowering the availability of serotonin in the synapse [ 39 , 40 ]. Animals with an inactivated gene for SERT have higher levels of extra-cellular serotonin in the brain than normal [ 41 , 42 , 43 ] and SSRIs are thought to work by inhibiting the action of SERT, and thus increasing levels of serotonin in the synaptic cleft [ 44 ]. Although changes in SERT may be a marker for other abnormalities, if depression is caused by low serotonin availability or activity, and if SERT is the origin of that deficit, then the amount or activity of SERT would be expected to be higher in people with depression compared to those without [ 40 ]. SERT binding potential is an index of the concentration of the serotonin transporter protein and SERT concentrations can also be measured post-mortem.
Three overlapping meta-analyses based on a total of 40 individual studies fulfilled inclusion criteria (See Table 1 ) [ 37 , 39 , 45 ]. Overall, the data indicated possible reductions in SERT binding in some brain areas, although areas in which effects were detected were not consistent across the reviews. In addition, effects of antidepressants and other medication cannot be ruled out, since most included studies mainly or exclusively involved people who had a history of taking antidepressants or other psychiatric medications. Only one meta-analysis tested effects of antidepressants, and although results were not influenced by the percentage of drug-naïve patients in each study, numbers were small so it is unlikely that medication-related effects would have been reliably detected [ 45 ]. All three reviews cited evidence from animal studies that antidepressant treatment reduces SERT [ 46 , 47 , 48 ]. None of the analyses corrected for multiple testing, and one review was of very low quality [ 37 ]. If the results do represent a positive finding that is independent of medication, they would suggest that depression is associated with higher concentrations or activity of serotonin.
Depletion studies
Tryptophan depletion using dietary means or chemicals, such as parachlorophenylalanine (PCPA), is thought to reduce serotonin levels. Since PCPA is potentially toxic, reversible tryptophan depletion using an amino acid drink that lacks tryptophan is the most commonly used method and is thought to affect serotonin within 5–7 h of ingestion. Questions remain, however, about whether either method reliably reduces brain serotonin, and about other effects including changes in brain nitrous oxide, cerebrovascular changes, reduced BDNF and amino acid imbalances that may be produced by the manipulations and might explain observed effects independent of possible changes in serotonin activity [ 49 ].
One meta-analysis and one systematic review fulfilled inclusion criteria (see Table 1 ). Data from studies involving volunteers mostly showed no effect, including a meta-analysis of parallel group studies [ 50 ]. In a small meta-analysis of within-subject studies involving 75 people with a positive family history, a minor effect was found, with people given the active depletion showing a larger decrease in mood than those who had a sham procedure [ 50 ]. Across both reviews, studies involving people diagnosed with depression showed slightly greater mood reduction following tryptophan depletion than sham treatment overall, but most participants had taken or were taking antidepressants and participant numbers were small [ 50 , 51 ].
Since these research syntheses were conducted more than 10 years ago, we searched for a systematic sample of ten recently published studies (Table 2 ). Eight studies conducted with healthy volunteers showed no effects of tryptophan depletion on mood, including the only two parallel group studies. One study presented effects in people with and without a family history of depression, and no differences were apparent in either group [ 52 ]. Two cross-over studies involving people with depression and current or recent use of antidepressants showed no convincing effects of a depletion drink [ 53 , 54 ], although one study is reported as positive mainly due to finding an improvement in mood in the group given the sham drink [ 54 ].
SERT gene and gene-stress interactions
A possible link between depression and the repeat length polymorphism in the promoter region of the SERT gene (5-HTTLPR), specifically the presence of the short repeats version, which causes lower SERT mRNA expression, has been proposed [ 55 ]. Interestingly, lower levels of SERT would produce higher levels of synaptic serotonin. However, more recently, this hypothesis has been superseded by a focus on the interaction effect between this polymorphism, depression and stress, with the idea that the short version of the polymorphism may only give rise to depression in the presence of stressful life events [ 55 , 56 ]. Unlike other areas of serotonin research, numerous systematic reviews and meta-analyses of genetic studies have been conducted, and most recently a very large analysis based on a sample from two genetic databanks. Details of the five most recent studies that have addressed the association between the SERT gene and depression, and the interaction effect are detailed in Table 1 .
Although some earlier meta-analyses of case-control studies showed a statistically significant association between the 5-HTTLPR and depression in some ethnic groups [ 57 , 58 ], two recent large, high quality studies did not find an association between the SERT gene polymorphism and depression [ 27 , 32 ]. These two studies consist of by far the largest and most comprehensive study to date [ 32 ] and a high-quality meta-analysis that involved a consistent re-analysis of primary data across all conducted studies, including previously unpublished data, and other comprehensive quality checks [ 27 , 59 ] (see Table 1 ).
Similarly, early studies based on tens of thousands of participants suggested a statistically significant interaction between the SERT gene, forms of stress or maltreatment and depression [ 60 , 61 , 62 ], with a small odds ratio in the only study that reported this (1.18, 95% CI 1.09 to 1.28) [ 62 ]. However, the two recent large, high-quality studies did not find an interaction between the SERT gene and stress in depression (Border et al [ 32 ] and Culverhouse et al.) [ 27 ] (see Table 1 ).
Overall results
Table 3 presents the modified GRADE ratings for each study and the overall rating of the strength of evidence in each area. Areas of research that provided moderate or high certainty of evidence such as the studies of plasma serotonin and metabolites and the genetic and gene-stress interaction studies all showed no association between markers of serotonin activity and depression. Some other areas suggested findings consistent with increased serotonin activity, but evidence was of very low certainty, mainly due to small sample sizes and possible residual confounding by current or past antidepressant use. One area - the tryptophan depletion studies - showed very low certainty evidence of lowered serotonin activity or availability in a subgroup of volunteers with a family history of depression. This evidence was considered very low certainty as it derived from a subgroup of within-subject studies, numbers were small, and there was no information on medication use, which may have influenced results. Subsequent research has not confirmed an effect with numerous negative studies in volunteers.
Our comprehensive review of the major strands of research on serotonin shows there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity. Most studies found no evidence of reduced serotonin activity in people with depression compared to people without, and methods to reduce serotonin availability using tryptophan depletion do not consistently lower mood in volunteers. High quality, well-powered genetic studies effectively exclude an association between genotypes related to the serotonin system and depression, including a proposed interaction with stress. Weak evidence from some studies of serotonin 5-HT 1A receptors and levels of SERT points towards a possible association between increased serotonin activity and depression. However, these results are likely to be influenced by prior use of antidepressants and its effects on the serotonin system [ 30 , 31 ]. The effects of tryptophan depletion in some cross-over studies involving people with depression may also be mediated by antidepressants, although these are not consistently found [ 63 ].
The chemical imbalance theory of depression is still put forward by professionals [ 17 ], and the serotonin theory, in particular, has formed the basis of a considerable research effort over the last few decades [ 14 ]. The general public widely believes that depression has been convincingly demonstrated to be the result of serotonin or other chemical abnormalities [ 15 , 16 ], and this belief shapes how people understand their moods, leading to a pessimistic outlook on the outcome of depression and negative expectancies about the possibility of self-regulation of mood [ 64 , 65 , 66 ]. The idea that depression is the result of a chemical imbalance also influences decisions about whether to take or continue antidepressant medication and may discourage people from discontinuing treatment, potentially leading to lifelong dependence on these drugs [ 67 , 68 ].
As with all research synthesis, the findings of this umbrella review are dependent on the quality of the included studies, and susceptible to their limitations. Most of the included studies were rated as low quality on the AMSTAR-2, but the GRADE approach suggested some findings were reasonably robust. Most of the non-genetic studies did not reliably exclude the potential effects of previous antidepressant use and were based on relatively small numbers of participants. The genetic studies, in particular, illustrate the importance of methodological rigour and sample size. Whereas some earlier, lower quality, mostly smaller studies produced marginally positive findings, these were not confirmed in better-conducted, larger and more recent studies [ 27 , 32 ]. The identification of depression and assessment of confounders and interaction effects were limited by the data available in the original studies on which the included reviews and meta-analyses were based. Common methods such as the categorisation of continuous measures and application of linear models to non-linear data may have led to over-estimation or under-estimation of effects [ 69 , 70 ], including the interaction between stress and the SERT gene. The latest systematic review of tryptophan depletion studies was conducted in 2007, and there has been considerable research produced since then. Hence, we provided a snapshot of the most recent evidence at the time of writing, but this area requires an up to date, comprehensive data synthesis. However, the recent studies were consistent with the earlier meta-analysis with little evidence for an effect of tryptophan depletion on mood.
Although umbrella reviews typically restrict themselves to systematic reviews and meta-analyses, we aimed to provide the most comprehensive possible overview. Therefore, we chose to include meta-analyses that did not involve a systematic review and a large genetic association study on the premise that these studies contribute important data on the question of whether the serotonin hypothesis of depression is supported. As a result, the AMSTAR-2 quality rating scale, designed to evaluate the quality of conventional systematic reviews, was not easily applicable to all studies and had to be modified or replaced in some cases.
One study in this review found that antidepressant use was associated with a reduction of plasma serotonin [ 26 ], and it is possible that the evidence for reductions in SERT density and 5-HT 1A receptors in some of the included imaging study reviews may reflect compensatory adaptations to serotonin-lowering effects of prior antidepressant use. Authors of one meta-analysis also highlighted evidence of 5-HIAA levels being reduced after long-term antidepressant treatment [ 71 ]. These findings suggest that in the long-term antidepressants might produce compensatory changes [ 72 ] that are opposite to their acute effects [ 73 , 74 ]. Lowered serotonin availability has also been demonstrated in animal studies following prolonged antidepressant administration [ 75 ]. Further research is required to clarify the effects of different drugs on neurochemical systems, including the serotonin system, especially during and after long-term use, as well as the physical and psychological consequences of such effects.
This review suggests that the huge research effort based on the serotonin hypothesis has not produced convincing evidence of a biochemical basis to depression. This is consistent with research on many other biological markers [ 21 ]. We suggest it is time to acknowledge that the serotonin theory of depression is not empirically substantiated.
Data availability
All extracted data is available in the paper and supplementary materials. Further information about the decision-making for each rating for categories of the AMSTAR-2 and STREGA are available on request.
Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–64.
CAS PubMed Google Scholar
American Psychiatric Association. What Is Psychiatry? 2021. https://www.psychiatry.org/patients-families/what-is-psychiatry-menu .
GlaxoSmithKline. Paxil XR. 2009. www.Paxilcr.com (site no longer available). Last accessed 27th Jan 2009.
Eli Lilly. Prozac - How it works. 2006. www.prozac.com/how_prozac/how_it_works.jsp?reqNavId=2.2 . (site no longer available). Last accessed 10th Feb 2006.
Healy D. Serotonin and depression. BMJ: Br Med J. 2015;350:h1771.
Google Scholar
Pies R. Psychiatry’s New Brain-Mind and the Legend of the “Chemical Imbalance.” 2011. https://www.psychiatrictimes.com/view/psychiatrys-new-brain-mind-and-legend-chemical-imbalance . Accessed March 2, 2021.
Geddes JR, Andreasen NC, Goodwin GM. New Oxford Textbook of Psychiatry. Oxford, UK: Oxford University Press; 2020.
Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 10th Editi. Lippincott Williams & Wilkins (LWW); 2017.
Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14:158–60.
PubMed PubMed Central Google Scholar
Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–18.
Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10:28.
Hahn A, Haeusler D, Kraus C, Höflich AS, Kranz GS, Baldinger P, et al. Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression. Hum Brain Mapp. 2014;35:3857–66.
Amidfar M, Colic L, Kim MWAY-K. Biomarkers of major depression related to serotonin receptors. Curr Psychiatry Rev. 2018;14:239–44.
CAS Google Scholar
Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci. 2012;367:2378–81.
CAS PubMed PubMed Central Google Scholar
Pilkington PD, Reavley NJ, Jorm AF. The Australian public’s beliefs about the causes of depression: associated factors and changes over 16 years. J Affect Disord. 2013;150:356–62.
PubMed Google Scholar
Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. A disease like any other? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. Am J Psychiatry. 2010;167:1321–30.
Read J, Renton J, Harrop C, Geekie J, Dowrick C. A survey of UK general practitioners about depression, antidepressants and withdrawal: implementing the 2019 Public Health England report. Therapeutic Advances in. Psychopharmacology. 2020;10:204512532095012.
Demasi M, Gøtzsche PC. Presentation of benefits and harms of antidepressants on websites: A cross-sectional study. Int J Risk Saf Med. 2020;31:53–65.
Jakobsen JC, Gluud C, Kirsch I. Should antidepressants be used for major depressive disorder? BMJ Evidence-Based. Medicine. 2020;25:130–130.
Moncrieff J, Cohen D. Do antidepressants cure or create abnormal brain states? PLoS Med. 2006;3:e240.
Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:321–38.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21:95–100.
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2,. version 6.Cochrane; 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Huang T, Balasubramanian R, Yao Y, Clish CB, Shadyab AH, Liu B, et al. Associations of depression status with plasma levels of candidate lipid and amino acid metabolites: a meta-analysis of individual data from three independent samples of US postmenopausal women. Mol Psychiatry. 2020;2020. https://doi.org/10.1038/s41380-020-00870-9 .
Culverhouse RC, Saccone NL, Horton AC, Ma Y, Anstey KJ, Banaschewski T, et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol Psychiatry. 2018;23:133–42.
Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E, et al. STrengthening the REporting of Genetic Association Studies (STREGA)— An Extension of the STROBE Statement. PLoS Med. 2009;6:e1000022.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is quality of evidence and why is it important to clinicians? BMJ. 2008;336:995–8.
Yoon HS, Hattori K, Ogawa S, Sasayama D, Ota M, Teraishi T, et al. Relationships of cerebrospinal fluid monoamine metabolite levels with clinical variables in major depressive disorder. J Clin Psychiatry. 2017;78:e947–56.
Kugaya A, Seneca NM, Snyder PJ, Williams SA, Malison RT, Baldwin RM, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology. 2003;28:413–20.
Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, et al. No support for historical candidate gene or candidate gene-by-interaction hypotheses for major depression across multiple large samples. Am J Psychiatry. 2019;176:376–87.
Ogawa S, Tsuchimine S, Kunugi H. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta-analysis of historic evidence. J Psychiatr Res. 2018;105:137–46.
Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123.
Rojas PS, Neira D, Muñoz M, Lavandero S, Fiedler JL. Serotonin (5‐HT) regulates neurite outgrowth through 5‐HT1A and 5‐HT7 receptors in cultured hippocampal neurons. J Neurosci Res. 2014;92:1000–9.
Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol. 2016;26:397–410.
Nikolaus S, Müller H-W, Hautzel H. Different patterns of 5-HT receptor and transporter dysfunction in neuropsychiatric disorders – a comparative analysis of in vivo imaging findings. Rev Neurosci. 2016;27:27–59.
Wang L, Zhou C, Zhu D, Wang X, Fang L, Zhong J, et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry. 2016;16:1–9.
Kambeitz JP, Howes OD. The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. J Affect Disord. 2015;186:358–66.
Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102.
Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–81.
Shen H-W, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–9.
Hagino Y, Takamatsu Y, Yamamoto H, Iwamura T, Murphy DL, Uhl GR, et al. Effects of MDMA on extracellular dopamine and serotonin levels in mice lacking dopamine and/or serotonin transporters. Curr Neuropharmacol. 2011;9:91–5.
Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith MEA, Wang D-N. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–7.
Gryglewski G, Lanzenberger R, Kranz GS, Cumming P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab. 2014;34:1096–103.
Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–72.
Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–501.
Horschitz S, Hummerich R, Schloss P. Down-regulation of the rat serotonin transporter upon exposure to a selective serotonin reuptake inhibitor. Neuroreport. 2001;12:2181–4.
Young SN. Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects. J Psychiatry Neurosci. 2013;38:294–305.
Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59.
Fusar-Poli P, Allen P, McGuire P, Placentino A, Cortesi M, Perez J. Neuroimaging and electrophysiological studies of the effects of acute tryptophan depletion: A systematic review of the literature. Psychopharmacology. 2006;188:131–43.
Hogenelst K, Schoevers RA, Kema IP, Sweep FCGJ, aan het Rot M. Empathic accuracy and oxytocin after tryptophan depletion in adults at risk for depression. Psychopharmacology. 2016;233:111–20.
Weinstein JJ, Rogers BP, Taylor WD, Boyd BD, Cowan RL, Shelton KM, et al. Effects of acute tryptophan depletion on raphé functional connectivity in depression. Psychiatry Res. 2015;234:164–71.
Moreno FA, Erickson RP, Garriock HA, Gelernter J, Mintz J, Oas-Terpstra J, et al. Association study of genotype by depressive response during tryptophan depletion in subjects recovered from major depression. Mol. Neuropsychiatry. 2015;1:165–74.
Munafò MR. The serotonin transporter gene and depression. Depress Anxiety. 2012;29:915–7.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386–9.
ADS CAS PubMed Google Scholar
Kiyohara C, Yoshimasu K. Association between major depressive disorder and a functional polymorphism of the 5-hydroxytryptamine (serotonin) transporter gene: A meta-analysis. Psychiatr Genet. 2010;20:49–58.
Oo KZ, Aung YK, Jenkins MA, Win AK. Associations of 5HTTLPR polymorphism with major depressive disorder and alcohol dependence: A systematic review and meta-analysis. Aust N. Z J Psychiatry. 2016;50:842–57.
Culverhouse RC, Bowes L, Breslau N, Nurnberger JI, Burmeister M, Fergusson DM, et al. Protocol for a collaborative meta-analysis of 5-HTTLPR, stress, and depression. BMC Psychiatry. 2013;13:1–12.
Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited. Arch Gen Psychiatry. 2011;68:444.
Sharpley CF, Palanisamy SKA, Glyde NS, Dillingham PW, Agnew LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav Brain Res. 2014;273:89–105.
Bleys D, Luyten P, Soenens B, Claes S. Gene-environment interactions between stress and 5-HTTLPR in depression: A meta-analytic update. J Affect Disord. 2018;226:339–45.
Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67:22–26.
Kemp JJ, Lickel JJ, Deacon BJ. Effects of a chemical imbalance causal explanation on individuals’ perceptions of their depressive symptoms. Behav Res Ther. 2014;56:47–52.
Lebowitz MS, Ahn W-K, Nolen-Hoeksema S. Fixable or fate? Perceptions of the biology of depression. J Consult Clin Psychol. 2013;81:518.
Zimmermann M, Papa A. Causal explanations of depression and treatment credibility in adults with untreated depression: Examining attribution theory. Psychol Psychother. 2020;93:537–54.
Maund E, Dewar-Haggart R, Williams S, Bowers H, Geraghty AWA, Leydon G, et al. Barriers and facilitators to discontinuing antidepressant use: A systematic review and thematic synthesis. J Affect Disord. 2019;245:38–62.
Eveleigh R, Speckens A, van Weel C, Oude Voshaar R, Lucassen P. Patients’ attitudes to discontinuing not-indicated long-term antidepressant use: barriers and facilitators. Therapeutic Advances in. Psychopharmacology. 2019;9:204512531987234.
Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer, Cham; 2015.
Schafer JL, Kang J. Average causal effects from nonrandomized studies: a practical guide and simulated example. Psychol Methods. 2008;13:279–313.
Pech J, Forman J, Kessing LV, Knorr U. Poor evidence for putative abnormalities in cerebrospinal fluid neurotransmitters in patients with depression versus healthy non-psychiatric individuals: A systematic review and meta-analyses of 23 studies. J Affect Disord. 2018;240:6–16.
Fava GA. May antidepressant drugs worsen the conditions they are supposed to treat? The clinical foundations of the oppositional model of tolerance. Therapeutic Adv Psychopharmacol. 2020;10:2045125320970325.
Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharm. 2010;647:90–6.
Gartside SE, Umbers V, Hajós M, Sharp T. Interaction between a selective 5‐HT1Areceptor antagonist and an SSRI in vivo: effects on 5‐HT cell firing and extracellular 5‐HT. Br J Pharmacol. 1995;115:1064–70.
Bosker FJ, Tanke MAC, Jongsma ME, Cremers TIFH, Jagtman E, Pietersen CY, et al. Biochemical and behavioral effects of long-term citalopram administration and discontinuation in rats: role of serotonin synthesis. Neurochem Int. 2010;57:948–57.
Download references
There was no specific funding for this review. MAH is supported by a Clinical Research Fellowship from North East London NHS Foundation Trust (NELFT). This funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and affiliations.
Division of Psychiatry, University College London, London, UK
Joanna Moncrieff & Mark A. Horowitz
Research and Development Department, Goodmayes Hospital, North East London NHS Foundation Trust, Essex, UK
Faculty of Education, Health and Human Sciences, University of Greenwich, London, UK
Ruth E. Cooper
Psychiatry-UK, Cornwall, UK
Tom Stockmann
Department of Dynamic and Clinical Psychology, and Health Studies, Faculty of Medicine and Psychology, Sapienza University of Rome, Rome, Italy
Simone Amendola
Department of Applied Psychology, Zurich University of Applied Sciences, Zurich, Switzerland
Michael P. Hengartner
You can also search for this author in PubMed Google Scholar
Contributions
JM conceived the idea for the study. JM, MAH, MPH, TS and SA designed the study. JM, MAH, MPH, TS, and SA screened articles and abstracted data. JM drafted the first version of the manuscript. JM, MAH, MPH, TS, SA, and REC contributed to the manuscript’s revision and interpretation of findings. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Correspondence to Joanna Moncrieff .
Ethics declarations
Competing interests.
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). SA declares no conflicts of interest. MAH reports being co-founder of a company in April 2022, aiming to help people safely stop antidepressants in Canada. MPH reports royalties from Palgrave Macmillan, London, UK for his book published in December, 2021, called “Evidence-biased Antidepressant Prescription.” JM receives royalties for books about psychiatric drugs, reports grants from the National Institute of Health Research outside the submitted work, that she is co-chairperson of the Critical Psychiatry Network (an informal group of psychiatrists) and a board member of the unfunded organisation, the Council for Evidence-based Psychiatry. Both are unpaid positions. TS is co-chairperson of the Critical Psychiatry Network. RC is an unpaid board member of the International Institute for Psychiatric Drug Withdrawal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary tables, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Moncrieff, J., Cooper, R.E., Stockmann, T. et al. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry 28 , 3243–3256 (2023). https://doi.org/10.1038/s41380-022-01661-0
Download citation
Received : 21 June 2021
Revised : 31 May 2022
Accepted : 07 June 2022
Published : 20 July 2022
Issue Date : August 2023
DOI : https://doi.org/10.1038/s41380-022-01661-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The involvement of serotonin in major depression: nescience in disguise.
- Danilo Arnone
- Catherine J. Harmer
Molecular Psychiatry (2024)
Serotonin effects on human iPSC-derived neural cell functions: from mitochondria to depression
- Iseline Cardon
- Sonja Grobecker
- Christian H. Wetzel
Neither serotonin disorder is at the core of depression nor dopamine at the core of schizophrenia; still these are biologically based mental disorders
- Konstantinos N. Fountoulakis
- Eva Maria Tsapakis
The impact of adult neurogenesis on affective functions: of mice and men
- Mariana Alonso
- Anne-Cécile Petit
- Pierre-Marie Lledo
Biallelic variants identified in 36 Pakistani families and trios with autism spectrum disorder
- Ricardo Harripaul
- John B. Vincent
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Advertisement
Supported by
These Books Might Make You Happier
Three new arrivals help readers make sense of our mental health crisis. They also offer solidarity.
- Share full article

By Judith Newman
With the pandemic receding and a fraught election season looming, Americans seem more concerned than ever about mental health — yours, mine and that of the next leader of the free world. According to the C.D.C., a whopping 57.2 million Americans a year make visits to the physician where the primary diagnosis turns out to be a mental disorder . That’s a whole lot of anxiety and depression .
Not surprisingly, there’s a small library of titles that touch on these subjects in different ways. Most are pretty bad; I don’t need to spend $30 for someone to tell me that the secret to stress reduction is a combination of affirmations, nature walks and journaling. But a few new books offer fresh approaches to seeking contentment and peace.
“You are what you eat” has never been my favorite expression, since it means I’m essentially a bag of Tootsie Rolls. Nevertheless, nutrition affects our brains as well as our bodies. The question is: How much? Can we really blame our global mental health crisis on ultra-processed foods? Georgia Ede, a Harvard-trained nutritional psychiatrist, can and does. In her book, CHANGE YOUR DIET, CHANGE YOUR MIND (Balance, 464 pp., $32.50), Ede posits that even the best antidepressant medications are only moderately effective — and that the modern diet leads to a host of horrors, including brain inflammation, hormonal imbalances, emotional instability, depression and dementia. Ede suggests that diet can have a bigger impact than medication and, she writes, “If it’s optimal mental health you seek, you will need to start grounding your food choices in brain biology rather than dietary ideology.”
I’ll admit, I flipped to the back of this book, silently screaming Just tell me what to eat. It turns out, there isn’t a single brain-healthy approach; there are several that can be effective. (Ede is a proponent of the ketogenic diet.) The idea is to eliminate foods that cause inflammation — processed sugars, refined vegetable oils, refined carbohydrates. My scared-straight moment: A 2022 study at Wake Forest University showed that Alzheimer’s patients had linoleic acid blood levels that were 56 percent higher than people without cognitive impairment. I may be confused by much of this book, but you can bet I went into the kitchen and chucked the Pringles.
Joshua Fletcher is a British psychotherapist whose new book, AND HOW DOES THAT MAKE YOU FEEL? (Morrow, 320 pp., $29.99), is a chronicle of his own thoughts while treating patients. It includes case studies and slightly-too-whimsical checklists of Fletcher’s emotions during sessions. But that’s not what makes it useful. The book serves as a primer on common mental health ailments — including O.C.D., depression and panic and anxiety disorders — interspersed with information on different kinds of therapy.
The reason so many people believe therapy isn’t for them, Fletcher insists, is that they haven’t found the right one. I would argue that it’s not so much the modality, but the practitioner. “Therapists are some of the most amazing people I’ve ever met, both personally and professionally,” Fletcher writes. “However, they can also be nauseatingly self-righteous.”
I assumed that nothing in a book of essays about people struggling with their mental health could surprise me. Then I read about Tonie Dreher in Patrick J. Kennedy’s PROFILES IN MENTAL HEALTH COURAGE (Dutton, 336 pp., $30), written with Stephen Fried . Dreher was so strung out after years of crack addiction, and so in denial about her various diagnoses — PTSD, schizophrenia — that she lost track of years of her life; she wasn’t even sure how many children she had given birth to. Today she counsels people who are hoping to be diverted from prison into mental health programs. Dreher’s story is a reminder about the complicated relationship between addiction and mental health: So many people seek drugs to relieve the pain of being mentally ill, thus exacerbating mental illness by becoming addicted to drugs.
Kennedy, a former congressman with his own history of substance abuse and bipolar disorder, introduces readers to Gabrielle Anwar, an actor whose bipolar disorder blew up her career; Justin Maffett, an attorney and impassioned activist in the Black Lives Matter movement who became convinced, during a psychotic break, that he was “an angel sent by God to save the country from racism, Russia and China”; and Ashley Dunlop, a health care aide whose answer to auditory hallucinations was heroin. While many of the stories here involve people living with mental illness who are not only functioning, but helping others, Kennedy makes sure to include one who lost the struggle: his cousin Harry McMurrey, a seemingly happy-go-lucky guy who ended his own life during his senior year of college. At the funeral, Kennedy (who is Edward M. Kennedy’s son) hugged McMurrey’s father and whispered, “There was nothing wrong with Harry. He died of a disease. It manifests itself in a terrible way, but it’s still a disease.”
Explore More in Books
Want to know about the best books to read and the latest news start here..
How did fan culture take over? And why is it so scary? Justin Taylor’s novel “Reboot” examines the convergence of entertainment , online arcana and conspiracy theory.
Jamaica Kincaid and Kara Walker unearth botany’s buried history to figure out how our gardens grow.
A new photo book reorients dusty notions of a classic American pastime with a stunning visual celebration of black rodeo.
Two hundred years after his death, this Romantic poet is still worth reading . Here’s what made Lord Byron so great.
Harvard’s recent decision to remove the binding of a notorious volume in its library has thrown fresh light on a shadowy corner of the rare book world.
Bus stations. Traffic stops. Beaches. There’s no telling where you’ll find the next story based in Accra, Ghana’s capital . Peace Adzo Medie shares some of her favorites.
Each week, top authors and critics join the Book Review’s podcast to talk about the latest news in the literary world. Listen here .
Use of acid reflux drugs linked to higher risk of migraine
People who take acid-reducing drugs may have a higher risk of migraine and other severe headache than people who do not take these medications, according to a study published in the April 24, 2024, online issue of Neurology ® Clinical Practice , an official journal of the American Academy of Neurology. The acid-reducing drugs includeproton pump inhibitors such as omeprazole and esomeprazole, histamine H2-receptor antagonists, or H2 blockers, such as cimetidine and famotidine, and antacid supplements.
The study does not prove that acid-reducing drugs cause migraine; it only shows an association.
Acid reflux is when stomach acid flows into the esophagus, usually after a meal or when lying down. People with acid reflux may experience heartburn and ulcers. People with frequent acid reflux may develop gastroesophageal reflux disease, or GERD, which can lead to cancer of the esophagus.
"Given the wide usage of acid-reducing drugs and these potential implications with migraine, these results warrant further investigation," said study author Margaret Slavin, PhD, RDN, of the University of Maryland in College Park. "These drugs are often considered to be overprescribed, and new research has shown other risks tied to long-term use of proton pump inhibitors, such as an increased risk of dementia."
For the study, researchers looked at data on 11,818 people who provided information on use of acid-reducing drugs and whether they had migraine or severe headache in the past three months.
A total of 25% of participants taking proton pump inhibitors had migraine or severe headache, compared to 19% of those who were not taking the drugs. A total of 25% of those taking H2 blockers had severe headache, compared to 20% of those who were not taking those drugs. And 22% of those taking antacid supplements had severe headache, compared to 20% of those not taking antacids.
When researchers adjusted for other factors that could affect the risk of migraine, such as age, sex and use of caffeine and alcohol, they found that people taking proton pump inhibitors were 70% more likely to have migraine than people not taking proton pump inhibitors. Those taking H2 blockers were 40% more likely and those taking antacid supplements were 30% more likely.
"It's important to note that many people do need acid-reducing medications to manage acid reflux or other conditions, and people with migraine or severe headache who are taking these drugs or supplements should talk with their doctors about whether they should continue," Slavin said.
Slavin noted that the study looked only at prescription drugs. Some of the drugs became available for over-the-counter use at non-prescription strength during the study period, but use of these over-the-counter drugs was not included in this study.
Other studies have shown that people with gastrointestinal conditions may be more likely to have migraine, but Slavin said that relationship is not likely to fully explain the tie between acid-reducing drugs and migraine found in the study.
A limitation of the study is that a small number of people were taking the drugs, especially the H2 blockers.
- Headache Research
- Gastrointestinal Problems
- Disorders and Syndromes
- Anti-obesity drug
- Antiretroviral drug
- Antioxidant
- Cells of the stomach
- Antihistamine
Story Source:
Materials provided by American Academy of Neurology . Note: Content may be edited for style and length.
Journal Reference :
- Margaret Slavin, Cara L. Frankenfeld, Alexander B. Guirguis, Elizabeth K. Seng. Use of Acid-Suppression Therapy and Odds of Migraine and Severe Headache in the National Health and Nutrition Examination Survey . Neurology Clinical Practice , 2024; 14 (3) DOI: 10.1212/CPJ.0000000000200302
Cite This Page :
Explore More
- Mice Given Mouse-Rat Brains Can Smell Again
- New Circuit Boards Can Be Repeatedly Recycled
- Collisions of Neutron Stars and Black Holes
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Antidepressants.
Zachary M. Sheffler ; Preeti Patel ; Sara Abdijadid .
Affiliations
Last Update: May 26, 2023 .
- Continuing Education Activity
While antidepressants may be the drug of choice for depression, they also have FDA approval as treatments for other medical disorders. For example, antidepressants help treat obsessive-compulsive disorder, social phobia, panic disorder, generalized anxiety disorder (GAD), and post-traumatic stress disorder (PTSD). Antidepressants also have non-FDA-approved, off-label indications. This activity reviews the indications, contraindications, action, adverse events, and other key elements of antidepressant therapy in the clinical setting as it relates to the essential points needed by members of an interprofessional team managing the care of patients receiving antidepressant medications for conditions that respond to this medication class.
- Identify the approved and off-label indications for antidepressant medications.
- Summarize the mechanism of action of the various class members in the antidepressant drug class.
- Outline the adverse events of various antidepressant medications.
- Explain the importance of antidepressant therapy and how it affects therapeutic strategy as a component of care coordination and communication among the interprofessional team when using these agents to achieve therapeutic outcomes.
- Indications
Depressive disorders include unipolar major depression, persistent depressive disorder (dysthymia), premenstrual dysphoric disorder, and depressive disorder due to another medical condition. Major depressive disorder (MDD) is one of the most disabling mental illnesses and has significant morbidity and mortality. The lifetime prevalence of MDD ranges from 2 to 21% worldwide. The main sociodemographic correlates were divorced marital status and female gender. [1] With appropriate treatment, 70 to 80% of individuals with major depressive disorder can significantly reduce symptoms. Drugs used for the treatment of depression include the following.
Selective Serotonin Reuptake Inhibitors (SSRIs)
Fluvoxamine
Escitalopram
Serotonin/Norepinephrine Reuptake inhibitors (SNRIs)
- Venlafaxine
Desvenlafaxine
Milnacipran
Levomilnacipran
Atypical Antidepressants
Mirtazapine
- Agomelatine
Serotonin Modulators
Vortioxetine
Tricyclic Antidepressants (TCAs)
Amitriptyline
- Clomipramine
- Trimipramine
Desipramine
Nortriptyline
- Protriptyline
- Maprotiline
Monoamine Oxidase Inhibitors (MAOIs)
- Moclobemide
- Tranylcypromine
Isocarboxazid
NMDA Antagonists
- Esketamine- Intranasal esketamine is FDA approved for treatment-resistant depression in adults, in combination with an oral antidepressant. It is also indicated for treating major depressive disorder with suicidal ideation or behavior in adults. [2]
- The FDA has approved dextromethorphan/bupropion fixed drug combination(FDC) for major depressive disorder. FDA granted dextromethorphan/bupropion breakthrough therapy designation under priority review. [3]
- Meta-analysis of the comparative efficacy of antidepressant results indicates that sertraline and escitalopram have good efficacy with minimal adverse drug reactions than other drugs. Hence sertraline or escitalopram is the initial drug of choice for unipolar major depression. [4]
- Antidepressants are the drug of choice for depression, but they also have FDA approval as treatments for other medical disorders. For example, antidepressants are helpful in treating obsessive-compulsive disorder, social phobia, panic disorder, generalized anxiety disorder (GAD), and post-traumatic stress disorder (PTSD). [5]
- Antidepressants also have non-FDA-approved, off-label indications. For example, tricyclic antidepressants are prescribed for pain, insomnia, and migraine. Trazodone, a serotonin modulator, is used off-label for insomnia. [6]
- Mechanism of Action
The antidepressants all work slightly differently and target certain neurotransmitters to modulate mood and behavior. All currently licensed antidepressants are believed to increase serotonin, norepinephrine, or both in the synapse. The mechanisms to increase these neurotransmitters vary, though antidepressant drugs target reuptake by the nerve terminals. [7]
- The reuptake of 5HT(5-hydroxytryptamine/serotonin) into presynaptic terminals is mediated by SERT; neuronal uptake is the primary process by which neurotransmission via 5HT is terminated. SSRIs block reuptake and enhance and prolong serotonergic neurotransmission. With continuous administration of SSRI, there are sustained increases in cyclic AMP signaling and phosphorylation of the nuclear transcription factors and increases in the expression of trophic factors such as BDNF and increased neurogenesis. [8]
- SSRIs are currently the first-line agents for the treatment of depression. [9]
- Serotonin and norepinephrine reuptake inhibitors (SNRIs) block serotonin and norepinephrine reuptake in the synapse, increasing postsynaptic receptors' stimulation. SNRIs differ in their affinity for the serotonin and norepinephrine transporter.
- In contrast with other selective serotonin-norepinephrine reuptake inhibitors like duloxetine, venlafaxine, and desvenlafaxine; milnacipran and levomilnacipran has higher selectivity for inhibiting norepinephrine reuptake than serotonin reuptake [10] [11]
- Atypical antidepressants have various mechanisms of action.
- Bupropion, for example, works by inhibiting the reuptake of dopamine and norepinephrine at the presynaptic cleft. [12]
- Agomelatine works as an agonist at melatonin receptors MT1 and MT2. It also antagonizes serotonergic 5-HT2C receptors, promoting dopamine and norepinephrine release. [13]
- Mirtazapine works by blocking alpha-2 adrenergic receptors on the cell bodies and nerve terminals, promoting the release of norepinephrine into the synapse. Furthermore, mirtazapine antagonizes the 5-HT receptor, which has been shown to increase norepinephrine and dopamine in the brain's cortical regions. [7]
- Serotonin modulators such as vilazodone inhibit the presynaptic reuptake of serotonin. It is also a partial agonist at the postsynaptic serotonin 5-HT1A receptor.
- Trazodone acts upon postsynaptic serotonin 5-HT2A and 5-HT2C receptors and weakly inhibits presynaptic serotonin reuptake. In addition, Trazodone has additional postsynaptic alpha-adrenergic receptors and histamine receptors blocking activity.
- Nefazodone antagonizes postsynaptic serotonin 5-HT2A receptors and inhibits presynaptic serotonin and norepinephrine reuptake; these actions increase serotonergic transmission at 5-HT1A receptors. [12]
Tricyclic Antidepressants
- TCA, like amitriptyline, inhibits the reuptake of norepinephrine and serotonin at the presynaptic neuronal membrane. Amitriptyline also has an affinity for muscarinic M1 receptors and histamine H1 receptors. TCA thus can cause sedation and anticholinergic side effects. [14]
Monoamine Oxidase Inhibitors
- MAOIs inhibit the monoamine oxidase enzyme responsible for catabolizing serotonin, norepinephrine, and dopamine. Monoamine oxidase inhibitors were the first antidepressants discovered. MAOIs are not recognized as the first-line treatment for depression because of the adverse effects and drug-drug interactions. [15]
- Dysregulation in glutamatergic neurotransmission is implied in the pathophysiology of depression. In clinical research of depression, alteration of glutamate and gamma-aminobutyric acid (GABA) activity have been recognized. Glutamate is an excitatory neurotransmitter that binds to NMDA(N-methyl-D-aspartate receptors). Consequently, NMDA antagonists are useful in the treatment of depression.
- Esketamine- Esketamine, the S-enantiomer of racemic ketamine, is a non-selective, non-competitive N-methyl-D-aspartate (NMDA) antagonist. It is indicated in treatment-resistant depression. [16]
- Dextromethorphan is an uncompetitive NMDA receptor antagonist and opioid σ receptor agonist. Bupropion, as discussed above, works by inhibiting the uptake of dopamine and norepinephrine. The fixed drug combination of dextromethorphan-bupropion has a rapid onset of action(approximately one week) in patients with major depressive disorder. [3] [17]
BDNF Hypothesis
- The initial increase in synaptic serotonin eventually leads to increased neuroprotective proteins such as brain-derived neurotrophic factor (BDNF). BDNF concentrations in depression normalize in response to pharmacological treatment. An increase in BDNF level and enhanced neuroplasticity leads to the remission of depression. [18]
- Administration
Commercially available antidepressants are currently available for administration in various dosage forms, including oral tablets, oral extended-release tablets, oral suspensions, topical creams, and transdermal patches. Studies examine alternative administration routes via inhalation, intranasal, sublingual, and rectal forms, and success was obtained in intranasal esketamine. [19] According to the manufacturer's prescribing information, the usual starting and maintenance doses of commonly used antidepressants appear below. Total daily oral doses may need to be given as two or three equally divided doses per day, depending on antidepressants and comorbidities.
- The starting dose is 20 mg daily, and the usual maintenance dose is 20 to 40 mg daily.
- The starting dose is 5-10 mg daily, and the usual maintenance dose is 10 to 20 mg daily.
- The starting dose is 50 mg daily, and the usual maintenance dose is 100 to 200 mg daily.
- The starting dose is 20 mg daily, and the usual maintenance dose is 20 to 60 mg daily.
- The starting dose is 50 mg daily, and the usual maintenance dose is 50 to 200 mg daily.
SNRIs
Venlafaxine
- The starting dose is 75 mg daily, and the usual maintenance dose is 225 to 375 mg daily.
- The starting dose is 25 to 50 mg daily; the usual maintenance dose is 50 mg daily.
Duloxetine
- The starting dose is 30 mg daily, and the usual maintenance dose is 60 mg daily.
- The starting dose is 12.5 mg daily, and the usual maintenance dose is 100 mg daily.
- The starting dose is 20 mg daily, and the usual maintenance dose is 40 to 120 mg daily.
- The starting dose is 150 mg daily, and the usual maintenance dose is 300 mg daily.
- The starting dose is 15 mg daily, and the usual maintenance dose is 15 to 45 mg daily.
- The starting dose is 200 mg daily, and the usual maintenance dose is 200 to 600 mg daily.
- The starting dose is 150 mg daily, and the usual maintenance dose is 200 to 400 mg daily.
- The starting dose is 10 mg daily, and the usual maintenance dose is 20 mg daily.
- The starting dose is 25 mg daily, and the usual maintenance dose is 50 to 150 mg daily.
- The starting dose is 75 mg daily, and the usual maintenance dose is 150 mg daily.
Clomipramine(off-label use)
- The starting dose is 25 mg daily, and the usual maintenance dose is 100 to 250 mg daily.
- The starting dose is 100 mg daily, and the usual maintenance dose is 100 to 300 mg daily.
- The starting dose is 20 mg daily, and the usual maintenance dose is 20 to 60 mg daily.
- The starting dose is 45 mg daily, and the usual maintenance dose is 60 to 90 mg daily.
Selegiline transdermal patch
- The starting dose is 6 mg/24 hours; the usual maintenance dose is 6 to 12 mg/24 hours.
NMDA antagonists
Esketamine (nasal spray)
- The induction phase(week 1 to 4) dose is 56 mg/84 mg twice weekly, maintenance phase(week 5 to 8) dose is 56 mg/84 mg once a week. Dosing should be individualized( least frequent dosing) according to the clinical response.
Dextromethorphan/bupropion(extended-release tablets)
- The starting dose is 45 mg dextromethorphan/105 mg bupropion once daily in the morning. After three days, increase the dose to the maximum recommended dosage of one tablet twice daily, separated by at least 8 hours. It is not recommended to exceed two doses within the same day. [3]
Switching Antidepressants
- A washout period of 2–5 half-lives (most frequently 2–5 days) between cessation of the previous drug and the introduction of a new drug is the safest switching strategy from the point of view of drug interactions. [20]
Treatment-Resistant Depression
- According to the FDA and EMA, patients are considered to have treatment-resistant depression (TRD) when their major depressive disorder fails to respond sufficiently to ≥2 consecutive antidepressants in a single episode. [21] Treatment-resistant depression requires augmentation with another antidepressant or atypical antipsychotic agent.
Psychotherapy
- The combination of pharmacotherapy and psychotherapy is more effective than pharmacotherapy alone. [22]
- Adverse Effects
The most prevalent side effects of antidepressants include sexual dysfunction, drowsiness, weight gain, insomnia, anxiety, dizziness, headache, dry mouth, blurred vision, nausea, rash, and tremor. Patients may also describe asthenia and malaise while on antidepressant therapy. Clinicians may note symptoms of hyperprolactinemia, syndrome of inappropriate antidiuretic hormone (SIADH), and hyponatremia in patients taking antidepressants. [23]
- Sexual dysfunction
- QTc prolongation [24]
SNRI
- Hypertension
- Diaphoresis
- Bone resorption [25]
Atypical Antidepressants
- Agomelatine- hepatotoxicity
- Mirtazapine-Sedation, Weight gain [26]
- Bupropion- Seizures [27]
Serotonin Modulators
- Nefazodone- Hepatotoxicity( acute hepatitis with cholestasis and variable degrees of centrilobular necrosis) [28]
- Vilazodone- Diarrhea
- Vortioxetine- Nausea
- Trazodone- Sedation, Priapism [29] [30]
- Urinary Retention
- Constipation
- QRS prolongation
- Orthostatic Hypotension [31]
MAO Inhibitors
- Potential for serotonin syndrome [32]
Esketamine
- Significant potential for misuse.
- Dissociative or perceptual changes and sedation [33]
Dextromethorphan/Bupropion
- Dizziness, headache, somnolence, and dry mouth [3]
US Boxed Warning(FDA)
- Suicidal thoughts and behaviors: Antidepressants increased the risk of suicidal thoughts and behavior in pediatric and young adult patients. Closely monitor all antidepressant-treated patients for clinical worsening and the emergence of suicidal thoughts and behaviors. [34]
- Contraindications
- There are several scenarios where antidepressant use may be contraindicated. These scenarios vary between and within classes.
- Antidepressants should be used cautiously in patients with known hypersensitivities or taking other psychotropic medications.
- Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), for example, should not be taken with other SSRIs, monoamine oxidase inhibitors, tricyclic antidepressants, and other psychotropics; this is due to the risk of serotonin syndrome, which can lead to severe neuromuscular and autonomic symptoms. [35]
- Tricyclic antidepressants can provide another good example of relative contraindications in antidepressant therapy. Clinicians should be mindful when prescribing tricyclic antidepressants to individuals with cardiovascular disease. Tricyclic antidepressants have been shown to cause orthostatic hypotension. Additionally, tricyclic antidepressants may lead to heart block in patients with preexisting bundle-branch disease. [36]
- Buproprion, an atypical antidepressant, has seizure disorder listed as a major contraindication. This contraindication applies to patients with an active seizure diagnosis or prior seizure activity history. Like other antidepressants, bupropion should not be used in patients taking monoamine oxidase inhibitors or drugs that can lower the seizure threshold. [37]
- Liver injury due to previous treatment is a contraindication to nefazodone therapy. [28]
- Esketamine is contraindicated in aneurysmal vascular disease(thoracic and abdominal aorta, intracranial, and peripheral arterial vessels) and arteriovenous malformations, according to the product labeling.
- Dextromethorphan/Bupropion is contraindicated in patients with a seizure disorder, bulimia/anorexia nervosa, and concomitant Use with MAO inhibitors or within 14 days of stopping treatment with dextromethorphan/bupropion. [38] [39] [40]
Therapeutic Drug Monitoring
- Clinicians may find utility in monitoring antidepressant levels in their patients. This therapeutic drug monitoring strategy is based on serum or plasma concentrations of antidepressants, which researchers believe is a more reliable index than dosage. Therapeutic drug monitoring of antidepressants is beneficial with agents that have a reliable therapeutic range established.
- Nonetheless, it may also be helpful in patients who are refractory to treatment, have adverse effects, or have a history of noncompliance. Therapeutic drug monitoring is expensive, so clinicians must weigh the benefits to the cost of the study. [37]
Psychiatric Assessment
- Various scales in clinical practice can assist in trending a patient’s symptoms to determine therapeutic response.
- Patient Health Questionnaire (PHQ-9). [41]
- Hamilton Rating Scale for Depression(HDRS-17)
- Montgomery-Asberg Depression Rating Scale(MADRS) [42]
- In addition, monitoring for suicidal ideation is of paramount importance.
- Clinicians should monitor for adverse drug reactions, coexisting anxiety, or medical disorders at each visit.
- Clinicians should also assess the response to therapy and consider augmenting or switching antidepressants in an inadequate response.
- The toxicity of antidepressants varies greatly not only between classes but within them as well. Antidepressants are frequently used to self-poison in an attempt to commit suicide, particularly in women. Older tricyclic antidepressants (TCAs) are more toxic than newer antidepressant classes. Such as selective serotonin reuptake inhibitors (SSRIs). Researchers can track drug toxicity using the fatal toxicity index, a ratio of self-poisoning mortality rates to prescription rates. Researchers may also employ a case fatality index, which compares fatal versus non-fatal self-poisoning attempts. With that said, clinicians may wish to alter treatment strategies depending on a patient’s suicide risk. [43]
- According to the literature review, toxicity is higher for TCAs and MAO inhibitors followed by venlafaxine, bupropion, and mirtazapine and is lower for SSRIs. Among the selective serotonin reuptake inhibitors, citalopram and fluvoxamine appear to be associated with higher case fatality rates in overdose. [44]
SSRI Poisoning
Clinical Features
- CNS- drowsiness, tremor
- CVS- QRS and QTc interval prolongation(especially with citalopram and escitalopram)
- Potential serotonin syndrome: hyperthermia, hypertonia, hyperreflexia, clonus.
- Secure airway, breathing, and circulation; intubate as clinically indicated.
- Treat prolonged QRS intervals with sodium bicarbonate
- Prolonged QTc leading to torsades- Administer magnesium sulfate 2 g IV.
- Treat seizures with benzodiazepines (e.g., lorazepam 1 to 2 mg IV) as needed.
- SSRIs are relatively safe, although serotonin syndrome is common in overdose. The exception is citalopram, which is significantly associated with QTc prolongation. [45]
SNRI Poisoning
- Tachycardia
- Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia
- Changes in the level of consciousness (ranging from somnolence to coma)
- Serotonin syndrome
- Rhabdomyolysis
- Liver necrosis
- Death
- In case of acute overdose with SNRI, the clinician should ensure an adequate airway, breathing, and circulation.
- For serotonin syndrome, specific treatment (such as with cyproheptadine may be considered)
- Consider extracorporeal life support in severe poisoning with venlafaxine. [46]
Atypical Antidepressants Poisoning
Clinical features
- Seizures [47]
- Ensure an adequate airway, oxygenation, and ventilation.
- EEG monitoring is recommended for the first 48 hours post-ingestion.
- Administer intravenous benzodiazepine for seizures. [48] [49]
- Disorientation
- Impaired memory
- Bradyarrhythmias [50]
- Ensure an adequate airway, oxygenation, and ventilation
- Monitor cardiac rhythm and vital signs.
- Treat arrhythmias according to ACLS and PALS protocol.
Serotonin Modulators Poisoning
- Arrhythmias
- Respiratory arrest
- Priapism [51]
- Treatment should be symptomatic and supportive in the case of hypotension or excessive sedation.
- Priapism requires urgent evaluation by a urologist.
- In patients with ischemic priapism, intracavernosal injection such as phenylephrine. [52]
- tachycardia
- serotonin syndrome(altered mental status, autonomic instability, and neuromuscular abnormalities) [53]
- Ensure an adequate airway, breathing, and circulation.
- Serotonin syndrome- Vilazodone has up to 30-fold higher potency for serotonin reuptake inhibition than conventional SSRIs. Consequently, management of serotonin syndrome is the mainstay of therapy.
- Administer benzodiazepines (e.g., lorazepam 1 to 2 mg IV per dose) till the patient is asymptomatic.
- Administer IV fluids.
- Consider sedation, paralysis, and endotracheal intubation for severe hyperthermia.
- Administer antidote cyproheptadine(antagonist at 5-HT1A and 5-HT2A receptors). [54]
TCAs Poisoning
- Anticholinergic - Dilated pupils, absent bowel sounds, constipation, urinary retention
- Cardiac- Tachycardia, hypotension, conduction abnormalities, QRS duration >100 msec
- Neurologic- Sedation, seizures
- Maintain airway, breathing, circulation
- Treat hypotension with intravenous crystalloid. Administer vasopressors such as norepinephrine in refractory hypotension.
- If QRS >100 msec, administer IV sodium bicarbonate.
- Administer activated charcoal(1g/kg) if the patient presents within 2 hours of ingestion; often, charcoal is avoided due to the presence of ileus.
- Administer benzodiazepines (lorazepam 2 mg IV) for seizures.
- QRS interval longer than 100 ms is a reliable predictor of serious complications. [55]
MAOI Poisoning
- Hypertensive crisis
- Establish adequate airway, breathing, and circulation.
- Administer parenteral agents for hypertensive crisis.
- Serotonin syndrome- Administer IV fluids, benzodiazepines, and cyproheptadine. [56]
NMDA antagonist (esketamine) Poisoning
- Dissociation
- Ulcerative or Interstitial Cystitis
- Embryo-fetal Toxicity
- There is no specific antidote for esketamine overdose. In the case of overdose, clinicians should consider the possibility of multiple drug involvement. Contact a certified poison control center for the most up-to-date information on managing overdosage.
Dextromethorphan/bupropion poisoning
- Serotonin Syndrome
- Psychosis [57]
- There is no specific antidote. Provide supportive care.
- Administer benzodiazepines for seizures. [49]
- Consult a medical toxicologist or a certified poison control center. [58]
- Enhancing Healthcare Team Outcomes
While antidepressants are beneficial in treating depression and its other indications, many patients fail to receive adequate treatment. To effectively manage depression, a clinician must employ an interprofessional team-centered approach to effectively detect and diagnose the depression, provide patient education, use evidence-based pharmacotherapy, provide close-follow up for compliance, identify side effects, and determine treatment effectiveness. [59] Studies show multiple factors contribute to patient compliance with antidepressant medications. Generally, concerns about drug side effects were predictive of adherence. [60]
Patient comorbidities can also contribute to compliance with antidepressant medications. Particularly, conditions that impact one’s cognitive status can lead to non-compliance. [61] Alcohol or substance abuse, cardiovascular disease, metabolic disorders, young age, low-income residents, and old-generation antidepressant medication usage were predictive of lower adherence, particularly in the acute phase. [62]
Identifying and addressing these concerns is pivotal in the management of depression and the prescription of antidepressant medications. Several randomized controlled trials support the collaborative care approach in treating depression. Suggestions are that the program includes a depression care manager, psychiatric consultant, prescribing physician, and the patient. The depression care manager will manage the antidepressants, provide education, and coordinate referrals if necessary. The psychiatric consultant will be responsible for improving treatment strategies in patients who are not meeting expectations. [63] Patients may be receiving more than one antidepressant medication at a time. Hence it is essential to identify all the drugs involved in poisoning for the emergency physicians and triage nurses.
Other healthcare team members who must contribute to antidepressant care include the pharmacist and the nursing staff. Psychiatric specialty nurses are best equipped to recognize treatment failure, counsel patients on the medication, monitor adverse events, and assess compliance. Pharmacists can verify agent selection and dosing and perform medication reconciliation for drug interactions. Both pharmacists and nurses need open access to the prescriber in case of concern.
In overdose of antidepressants, emergency department physicians should rapidly stabilize the patient ensuring adequate airway, breathing, and circulation. Cardiac arrhythmias, serotonin syndrome, and seizures require ICU care under the supervision of a critical care physician. Medical toxicologists should be consulted for severe poisoning. Deliberate overdose requires consultation with a psychiatrist. As illustrated above, clinicians (MDs, DOs, NPs, PAs), specialists, pharmacists, nurses, and other healthcare providers are involved in taking care of the patient receiving antidepressant therapy. All interprofessional team members functioning as one unit can maximize efficacy and minimize adverse reactions, translating to optimal patient outcomes. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Zachary Sheffler declares no relevant financial relationships with ineligible companies.
Disclosure: Preeti Patel declares no relevant financial relationships with ineligible companies.
Disclosure: Sara Abdijadid declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Sheffler ZM, Patel P, Abdijadid S. Antidepressants. [Updated 2023 May 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Prescription of Controlled Substances: Benefits and Risks. [StatPearls. 2024] Prescription of Controlled Substances: Benefits and Risks. Preuss CV, Kalava A, King KC. StatPearls. 2024 Jan
- Review Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. [J Manag Care Pharm. 2012] Review Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. Maher AR, Theodore G. J Manag Care Pharm. 2012 Jun; 18(5 Suppl B):S1-20.
- Initial severity and antidepressant efficacy for anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder: An individual patient data meta-analysis. [Depress Anxiety. 2018] Initial severity and antidepressant efficacy for anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder: An individual patient data meta-analysis. de Vries YA, Roest AM, Burgerhof JGM, de Jonge P. Depress Anxiety. 2018 Jun; 35(6):515-522. Epub 2018 Apr 16.
- Amitriptyline. [StatPearls. 2024] Amitriptyline. Thour A, Marwaha R. StatPearls. 2024 Jan
- Review Obsessive-compulsive disorder has a reduced placebo (and antidepressant) response compared to other anxiety disorders: A meta-analysis. [J Affect Disord. 2017] Review Obsessive-compulsive disorder has a reduced placebo (and antidepressant) response compared to other anxiety disorders: A meta-analysis. Sugarman MA, Kirsch I, Huppert JD. J Affect Disord. 2017 Aug 15; 218:217-226. Epub 2017 Apr 29.
Recent Activity
- Antidepressants - StatPearls Antidepressants - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
2.2.1. Inclusion Criteria . Inclusion criteria are the following: (1) literatures of varying methodologies such as observational, cross-sectional, and retrospective studies, survey, and case reports, (2) studies conducted on all patients aged ≥18 years with a primary diagnosis of depression and prescription of at least one antidepressant drug, (3) studies that mainly focused on ...
Monoamine oxidase inhibitors. Iproniazid was the first drug defined as an antidepressant; it was later classified as a monoamine oxidase inhibitor (MAOI)[6,7].Several other MAOIs have been introduced since 1957[].Due to their irreversible inhibition of monoamine oxidase, MOAIs have numerous side effects, such as hepatotoxicity and hypertensive crises, that can lead to lethal intracranial ...
Depression is a potentially life-threatening disorder affecting millions of people across the globe. It is a huge burden to both the individual and society, costing over £9 billion in 2000 alone: the World Health Organisation (WHO) cited it as the third leading cause of global disability in 2004 (first in the developed world), and project it will be the leading cause by 2030.
All antidepressants were more efficacious than placebo in adults with major depressive disorder. Smaller differences between active drugs were found when placebo-controlled trials were included in the analysis, whereas there was more variability in efficacy and acceptability in head-to-head trials. These results should serve evidence-based practice and inform patients, physicians, guideline ...
A systematic review and random-effects model network meta-analysis were conducted to compare the efficacy, acceptability, tolerability, and safety of antidepressants to treat adults with major ...
The average AROC for predicting propensity of medications was 82%. 15 models were constructed to predict remission for 15 different antidepressants. The average prevalence of remission ranged from 3.1% to 49.3%. The cross-validated AROC ranged from 69.2% to 78.5%, with an average of 72.0%.
Depression is a growing global crisis, with females at a higher rate of diagnosis than males. While the percentage of patients on prescribed antidepressants have tripled over the last two decades ...
Side effects to antidepressant medications are common and can impact the prognosis of successful treatment outcome in people with major depressive disorder (MDD). However, few studies have ...
Major depressive disorder is a severe mood disorder associated with a marked decrease in quality of life and social functioning, accompanied by a risk of suicidal behavior. Therefore, seeking out and adhering to effective treatment is of great personal and society-wide importance. Weight changes associated with antidepressant therapy are often cited as the reason for treatment withdrawal and ...
Since then, every 30 years, the use of antidepressants had made a pulsatile leap. Selective serotonin reuptake inhibitors (SSRIs) are the most widely-prescribed psychiatric drugs for the treatment of depression. However, the efficacy was variable and incomplete: 60%-70% of the patients do not experience remission, while 30%-40% do not show a ...
Background: This paper examines the current status of research on the efficacy and effectiveness of antidepressants. Methods: This paper reviews four meta-analyses of efficacy trials submitted to America's Food and Drug Administration (FDA) and analyzes STAR*D (Sequenced Treatment Alternatives to Relieve Depression), the largest antidepressant effectiveness trial ever conducted.
Antidepressant drugs are effective against depression. They also improve subjective and functional outcomes such as disability, work functioning, social functioning, well-being, and health-related quality of life (HRQoL) in depressed patients. ... A Reader's Guide on How to Examine a "Viral" Research Paper With a Critical Eye J Clin Psychiatry ...
Of these patients, 478 were enrolled in the trial (238 in the maintenance group and 240 in the discontinuation group). The average age of the patients was 54 years; 73% were women. Adherence to ...
Depressive disorders are common, costly, have a strong effect on quality of life, and are associated with considerable morbidity and mortality. Effective treatments are available: antidepressant medication and talking therapies are included in most guidelines as first-line treatments. These treatments have changed the lives of countless patients worldwide for the better and will continue to do ...
Depression is a serious condition, common in adults of all ages worldwide [1, 2].It is frequently treated with antidepressant drugs, with many countries reporting substantial increases in the prescribing rates of these drugs in recent decades [3,4,5].Reports from the US, Canada and UK have shown that antidepressants are one of the most commonly prescribed types of medication in young and ...
Widespread prescribing has not reduced mental disability or suicide, raising questions about the assessment of evidence on effectiveness and safety of antidepressants, writes John Warren Depression can be severe and reduce life expectancy. Antidepressant prescribing has increased substantially in recent years so that one in eight UK adults, some 7.3 million people, now receive a prescription ...
Abstract. This review article aims to provide insight into the mechanisms of action, pharmacokinetics, clinical efficacy, safety and tolerability of four novel antidepressants including desvenlafaxine, vortioxetine, vilazodone, and levomilnacipran. Following keywords are used in PubMed and Scopus to search for relevant articles: (depression ...
This special Research in Context feature explores the development of more effective ways to treat depression, including personalized treatment approaches and both old and new drugs. Choat / Adobe Stock. Everyone has a bad day sometimes. People experience various types of stress in the course of everyday life. These stressors can cause sadness ...
Cell Research 31 , 489-490 ( 2021) Cite this article. The mode of action of drugs used to treat depression has long remained mysterious. A new study now shows that unexpectedly, this diverse ...
A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet (London, England), 401(10374 ...
Facts and common misconceptions about some of America's most widely used drugs. By Christina Caron Antidepressants are among the most prescribed medications in the United States. This is, in ...
Neurocrine landed the oral asset as part of a 2020 deal worth up to $2 billion with Takeda, which allowed the biotech to develop certain compounds in the pharma's early-to-mid-stage psychiatry ...
When you're caught in the relentless grip of depression, waiting weeks for a medication to work isn't just frustrating—it can feel like an eternity. Sean Goddard of Viking Psychiatry shares his personal and professional journey with Spravato, offering an intimate understanding of why swift treatment can be life-saving.
In other words, antidepressants improved symptoms in about an extra 20 out of 100 people. Antidepressants can also relieve long-term symptoms of chronic depressive disorder (dysthymia) and chronic depression, and help make them go away completely. An antidepressant can already have an effect within one or two weeks.
Voltaire's famous aphorism of "doctors prescribing drugs of which they know little to patients of whom they know less" may seem unduly cynical but the sheer number of medicines available ...
MSN
Authors of papers were contacted for clarification when data was missing or unclear. ... with most research on depression focusing on ... potentially leading to lifelong dependence on these drugs ...
The book serves as a primer on common mental health ailments — including O.C.D., depression and panic and anxiety disorders — interspersed with information on different kinds of therapy.
People who take acid-reducing drugs may have a higher risk of migraine and other severe headache than people who do not take these medications, according to a study published in the April 24, 2024 ...
While antidepressants may be the drug of choice for depression, they also have FDA approval as treatments for other medical disorders. For example, antidepressants help treat obsessive-compulsive disorder, social phobia, panic disorder, generalized anxiety disorder (GAD), and post-traumatic stress disorder (PTSD). Antidepressants also have non-FDA-approved, off-label indications. This activity ...